An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.7(1); 2021 Jan


A cross-sectional study of the prevalence, density, and risk factors associated with malaria transmission in urban communities of Ibadan, Southwestern Nigeria
Oluwaseun bunmi awosolu.
a School of Biological Sciences, Universiti Sains Malaysia USM, 11800, Penang, Malaysia
b Department of Biology, Federal University of Technology, Akure, Nigeria
Zary Shariman Yahaya
Meor termizi farah haziqah, iyabo adepeju simon-oke, comfort fakunle, associated data.
Data included in article/supplementary material/referenced in article.
Malaria is a severe global public health challenge that causes significant morbidity and mortality worldwide, particularly in sub-Saharan Africa. This study was designed to determine the prevalence, parasite density, and risk factors associated with malaria infection transmission among residents of two urban communities of Ibadan, southwestern Nigeria.
Materials and methods
A cross-sectional hospital-based study was carried out on 300 participants. Blood samples were obtained. Thick and thin blood films were prepared and viewed using the standard parasitological technique of microscopy. Moreover, data on sociodemographic and environmental variables were obtained using a pre-tested standard questionnaire.
Of the 300 participants examined, a total of 165 (55.0%) were found positive for Plasmodium falciparum with a mean (S.D) parasite density of 1814.70 (1829.117) parasite/μL of blood. The prevalence and parasite density of malaria infection vary significantly (P < 0.05) with age group. Children <5 years old were more likely to have malaria infection and high parasite densities than adults (p < 0.05). Similarly, in relation to gender, males significantly (P < 0.05) had a higher prevalence (60.2%) and mean (S.D) parasite density of malaria infection [2157.73 (1659.570) parasite/μL of blood] compared to females. Additionally, those without formal education had the highest prevalence (73.0%) and mean (S.D) parasite density of infection [2626.96 (2442.195) parasite/μL of blood]. The bivariate logistic regression analysis shows that age group 6–10 (Crude Odds Ratio, COR 0.066, 95% CI: 0.007–0.635), presence of streams/rivers (COR 0.225, 95% CI: 0.103–0.492), distance from streams/rivers within ≤1 km (COR 0.283, 95% CI: 0.122–0.654) and travel to rural area (COR 4.689, 95% CI: 2.430–9.049) were the significant risk factors.
Conclusions
Malaria infection is prevalent in the study area and was greatly influenced by traveling activities from the rural areas to urban centers and vice versa. Multifaceted and integrated control strategy should be adopted. Health education on mosquito prevention and chemoprophylaxis before and during travel to rural areas are essential.
Ibadan, Malaria infection, Plasmodium falciparum , Prevalence, Risk factors, Urban areas.
1. Introduction
Malaria is an important disease of public health problem caused by Plasmodium parasite belonging to the Apicomplexans [ 1 ]. It is spread when an infected female Anopheles mosquito feeds on human blood [ 2 ]. It is majorly infecting people in the world's tropical and subtropical countries, particularly in sub-Saharan Africa [ 3 ]. The four major malaria parasites causing disease in humans include Plasmodium (P). falciparum , P. vivax , P. malariae, and P. ovale, while P. knowlesi is a zoonotic species found in Southeast Asia [ 4 ]. P. falciparum is considered the most pathogenic of all, and it is most prevalent in Africa [ 5 , 6 ]. Though malaria is a curable and preventable disease, malaria continues to have an overwhelming effect on people's health globally, particularly among pregnant women and children in rural and urban areas [ 3 ]. Globally, it is estimated that 3.2 billion people are at risk of contracting malaria annually [ 1 ]. Furthermore, about 219 million cases which led to approximately 435,000 deaths, were reported in 2017 [ 3 ]. In Nigeria, malaria is transmitted throughout the year, with more than 194 million people predisposed to contracting malaria infection. Thus, Nigeria reported the highest malaria prevalence among all of the world's countries in 2007 [ 7 ]. This has led to an increased level of poverty due to unexpected expenses on treatment, control, and prevention. Moreover, time expected to be at work and school is wasted on ill-health due to malaria infection thereby further aggravating poor conditions in rural and urban areas [ 8 ].
Major risk factors enhancing malaria transmission include demographic factors, environmental factors, and socioeconomic factors. Demographic factors include age and gender, while environmental factors include the presence or absence of bushes and forests which enhance mosquito breeding. Meanwhile, climatic factors include temperature, humidity, and rainfall that may support rapid growth and development of mosquito vectors. Lastly, socioeconomic factors such as education, occupation and income which can directly affect human exposure and treatment pattern. These factors have been well reported, particularly in rural and peri-urban communities in previous studies [ 9 ]. Other studies have compared malaria parasite prevalence in rural and urban areas. Govoetchan and colleagues observed that malaria prevalence was 5.5 times higher in rural Kandi than to urban Kandi in Northeastern Benin [ 10 ]. Despite better conditions in urban areas such as the availability of health facilities and low mosquito breeding sites, studies have shown that malaria parasites are prevalent in urban areas [ 11 , 12 ]. Meanwhile, factors enhancing malaria infections in these urban areas are yet to be fully unraveled. Thus, to align with the target to reduce malaria parasite by 90% between 2016 and 2030 by World Health Organization's Global Technical Strategy for malaria, there is a need to understand and document adequate epidemiological data upon which malaria management and control could be based, particularly in urban settings. Thus, this study sought to investigate the prevalence and risk factors enhancing malaria parasites transmission in two urban areas in Ibadan, Oyo State, Nigeria.
2. Materials and Methods
2.1. study area.
This study was carried out in Adeoyo State Hospital and Oni Memorial Children's Hospital. Both hospitals are in Ibadan South West Local Government Area of Oyo State, Nigeria. Ibadan South West Local Government Area is situated between Latitude 7°21′2.48″N and Longitude 3°51′55.84″E ( Figure 1 ). The population is projected to be 397,700 in 2016 [ 13 ]. Generally, Ibadan city is the third most populous city in Nigeria, with over 6 million people. Thus, Ibadan is categorized as an urban area. An urban area has a high population density with well-designed infrastructure. It could be classified as cities, towns, and suburbs. The climatic condition is typical of tropical regions consisting of rainy and dry seasons that spans through April to October and November to March, respectively. The average annual rainfall is about 2100 mm, while the temperature is about 27 °C [ 14 ]. There are rivers running through the city of Ibadan, which includes Ogunpa, Kudeti, and Ona river, among others. The population consists of civil servants, traders, students, artisans, and farmers. While some of the residents live and settle in the urban City of Ibadan, they still visit rural areas or their home village for some activities. Many come from rural areas to Ibadan, thereby hosting different people from various parts of the country. Nonetheless, Ibadan residents are predominantly of the Yoruba ethnic group.

Map of Adeoyo State Hospital and Oni Memorial Children's Hospital in Ibadan, Nigeria.
2.2. Study design
This is a hospital-based and randomized cross-sectional survey. The study was conducted from May to August 2019 in two different hospitals, which are Adeoyo State Hospital and Oni Memorial Children's Hospital, Ibadan. The quantitative method of data collection was employed, and data was collected with the aid of a pre-tested structured questionnaire from each patient visiting the health facilities. A face-to-face interview was conducted to collect their data such as age, sex, occupation, education, use of mosquito nets, and presence or absence of stream/river within ≤1 km of the participant's home. Other data, such as blood group and haemoglobin genotype were obtained from their medical records. Recruitment of participants was done in the outpatient section of the health facilities. Out of the 310 potential participants initially selected, only 300 eventually participated given a response rate of 96.7%.
2.3. Sample and sampling
The sample size was computed using a previous malaria parasite prevalence of 78% [ 15 ] at a confidence interval (CI) of 95% and a precision of 0.05 (or 5%) following the formula of Araoye, 2004 for calculating sample size [ 16 ]. This gives rise to a total of 300 subjects who were recruited for this study. Criteria for inclusion encompass feelings of headache, fever with temperature ≥38 °C, which was screened at the hospital by one of the health officials, completion of questionnaires, being a resident of Ibadan, blood samples submission, and willingness to provide written or oral informed consent. Those who declined to participate were excluded.
2.4. Blood collection and laboratory procedures
Samples of blood were obtained intravenously with the assistance of a trained Laboratory Technologist. A 3 mL blood was obtained from each participant. After collection, blood samples were transferred into an ethylenediaminetetraacetic acid (EDTA) tube to prevent blood coagulation. Next, thick and thin smears were made on well cleaned and sterilized slides. The thin smear was fixed in absolute ethanol. Subsequently, 3% Giemsa stain was added to the thick and thin smears for 30 min. The slides were later viewed under x100 objective lens of the light microscope to confirm the presence or absence of Plasmodium parasites and the species present. When about 200 microscopic fields have been observed and no parasite discovered, it is considered negative. The mean parasite density was classified according to the recommendations of Atroosh et al. [ 17 ]. Parasite density was recorded as the number of Parasite/μL of blood, assuming an average leucocyte count of 8,000/μL of blood for an average individual [ 1 ]. The formula used is stated as follows:
2.5. Statistical analysis
Data collected were analyzed using SPSS version 20.0 (IBM Corporation, NY, USA). The presence or absence of malaria parasite was computed and the differences in prevalence between age groups and sex were calculated using chi-square test at a 95% level of confidence. The malaria parasite density was computed using the student's t-test for the dichotomous variable while ANOVA was used to determine categorical variables. Malaria-associated risk factors were determined by Bivariate Logistic Model and Multivariate Logistic Regression Analysis. P-values of ≤0.05 were recognized as significant.
2.6. Ethical approval
The protocol for this study was approved by the Ondo State Ministry of Health (protocol number OSHREC/09/04/2018/046), the Ethical Review Committee, Federal University of Technology, Akure, Nigeria. Meanwhile, permission was sought from the hospital management board before study commencement. Written Informed consent was obtained from each adult subject. However, for children, accent was obtained from few while caregivers or guardians provided the informed consent for other younger ones.
Of the total 300 individuals selected for this study, males represent 44.3%, while females represent 55.7%. The mean (S.D) age is 28.03 (17.52). Furthermore, the individuals examined in Adeoyo State hospital and Oni Memorial hospital were 196 (65.3%) and 104 (34.7%), respectively ( Table 1 ). All malaria infections in this study area were observed to be caused by P. falciparum and most (56.4%) of the malaria infections were classified as low (<1000 parasites/μL of blood). Meanwhile, 43.6% were classified as moderate infections, which ranges between 1000 to ≤9999 parasites/μL of blood.
Table 1
Prevalence and density of Plasmodium falciparum infection stratified by sociodemographic variables in Ibadan South West Local Government Area of Oyo State, Nigeria.
In all, a total of 165 participants (55.0%) had malaria infection with mean (S.D) parasite density of 1814.70 (1829.117) parasite/μL of blood. The association between prevalence and density of P. falciparum and sociodemographic factors are presented in Table 1 . Age group ≤5 years has the highest malaria prevalence of 76.7% while the lowest malaria prevalence of 43.2% is noted among the 31–40 year old participants. Generally, malaria infection in this study significantly (P < 0.05) decreases with increasing age and cumulates at 40 years old ( Table 1 ). The highest mean (S.D) parasite density of infection was recorded among participants ≤5 years old [2433.43 (2547.742) parasite/μL of blood] while the least was recorded among participants >50 years old [ 956.42 (1262.708) parasite/μL of blood] (P > 0.05).
In relation to gender, males have a higher prevalence (60.2%) and mean (S.D) parasite density of infection [2157.73 (1659.570) parasite/μL of blood] compared to their female counterparts with malaria prevalence of 50.9% and mean (S.D) parasite density of 1491.85 (209.320) parasite/μL of blood. Infection was significant at P < 0.05.
Furthermore, those without formal education had an infection that almost doubled than those who attained tertiary education (P < 0.05). Similarly travelling to rural areas or villages highly contributed to malaria prevalence and parasite density. Those who travel to rural areas or villages had higher malaria prevalence of 74.4% and mean (S.D) parasite density of 2367.51 (2098.600) parasite/μL of blood compared to those who did not travel to rural areas or villages in the previous month (P < 0.05) ( Table 1 ).
Table 2 details the prevalence and parasite density of P. falciparum infection stratified by haemoglobin genotype and blood group. Genotype HbAA has the highest malaria prevalence of 62.6% and mean (S.D) parasite density of 1937.33 (1627.828) parasite/μL of blood, while genotype HbSS has the least malaria prevalence of 12.5%. The result is statistically significant (P < 0.05). Also, while blood group O significantly (P < 0.05) has the highest prevalence of 68.8%, blood group AB has the least malaria prevalence ( Table 2 ).
Table 2
Prevalence and density of Plasmodium falciparum infection stratified by haemoglobin genotype and blood group in Ibadan South West Local Government Area of Oyo State, Nigeria.
Table 3 presents environmental variables and their association with malaria infection prevalence and density. The presence of streams and distance from streams are significantly (P < 0.05) related to malaria infection prevalence. Those who live nearby rivers/streams within the distance of ≤1 km are more likely to have malaria infection. Similarly, sleeping under the mosquito net could significantly (P < 0.05) reduce malaria infection ( Table 4 ).
Table 3
Prevalence and density of Plasmodium falciparum infection stratified by environmental variables in Ibadan South West Local Government Area of Oyo State, Nigeria.
Table 4
Prevalence and density of Plasmodium falciparum infection stratified by ownership and use of mosquito nets in Ibadan South West Local Government Area of Oyo State, Nigeria.
Additionally, the Bivariate Logistic Regression Model analysis results show the risk factors associated with malaria infection ( Table 5 ). Age group between 6-10 years old, group O blood type, presence of rivers or streams, distance from rivers or streams and travel to rural areas or villages the previous month were observed to be associated with malaria infection prevalence.
Table 5
Bivariate Logistic Regression Rodel for crude odd ratio (CORs) of factors associated with malaria infection prevalence in Ibadan South West Local Government Area of Oyo State, Nigeria.
Multivariate logistic regression analysis of the independent variables is detailed in Table 6 . Presence of rivers or streams, distance of rivers or streams from home, travel to rural areas and having blood group A and AB are significant risk factors.
Table 6
Multivariate Logistic Regression analysis of factors associated with malaria infection prevalence in Ibadan South West Local Government Area of Oyo State, Nigeria.
4. Discussion
This study shows a strong evidence that malaria is still highly prevalent in many urban communities including Ibadan South West Local Government Area of Oyo State, Nigeria. The high prevalence of 55% with mean (S.D) parasite density of 1814.70 (1829.117) parasite/μL of blood is an indication that Ibadan is a high-risk area for malaria transmission, since it falls within the Nigerian malaria risk map estimates of less than 20% in certain zone to more than 70% in other zones [ 18 ]. This is supported by other studies reported from Ibadan [ 8 , 19 , 20 ]. Similarly, the current prevalence of 55% from urban area of Ibadan is significantly (P < 0.05) lower than those reported from many rural areas. This notion is supported by the reports of Wang et al. [ 21 ] and Baragatti et al. [ 22 ] who reported lower malaria prevalence of 24.1% and 26.1% in urban areas of the Republic of Benin and Burkina Faso respectively. In some rural settings, prevalence as high as 74% and 71.4% have been reported [ 9 , 23 ]. Thus, while evidence abounds on malaria prevalence in urban areas, prevalence is generally significantly (P < 0.05) lower than in rural areas [ 11 , 24 ]. This lower malaria prevalence in urban areas could result from better access to health facilities, well-designed houses that can protect against mosquito vectors, improved basic amenities, and reduced mosquito breeding sites [ 25 ].
Our findings on age-specific malaria prevalence patterns and mean parasite density shows that age group ≤5 years has the highest malaria infection. Similar findings have been reported in previous studies [ 26 , 27 ]. The World Health Organization has emphasized the fact that children between the age of 5 years and below are the most vulnerable group of people, particularly in Africa [ 3 ]. This can be attributed to the gradual loss of maternal immunity, coupled with a low level of acquired immunity among children compared to adults. Thus, as age and exposure increase, malaria infection decreases except among the elderly and the immunocompromised. Thus, the focus should be on these children between the age five years and below, even in urban centers. Prevention against mosquito bites should be intensified through the provision of mosquito nets to such households with children. Additionally, the sex pattern of infection in this study shows that males have higher malaria prevalence and mean parasite density than their female colleagues. This is related to previous reports from other studies in malaria-endemic areas such as Ethiopia and Chile [ 28 , 29 ]. This could be because males usually get involved in outdoor activities, stay late until night outside, have a lackadaisical attitude towards malaria prevention and farming, which inadvertently exposes them to high mosquito bites than females. Furthermore, those without formal education had an infection that almost doubled than those who attained tertiary education though no association was reported. This is in line with previous studies which show that people can be acquainted with the knowledge of malaria transmission, prevention and control irrespective of their educational status [ 30 , 31 ]. This is, however, in contrast to the report of Adedotun et al. , [ 32 ] Eteng et al. , [ 33 ] and Dawaki et al. , [ 34 ] who noted that the level of education significantly influences the knowledge, attitude, and practices of people which in turn can lead to reduced malaria infection. Similarly, those who travelled to rural areas or villages in this study area significantly (P < 0.05) had higher malaria prevalence and density. This is corroborated by studies conducted in malaria-endemic zones [ 21 , 35 ]. Generally, people are at greater risks when they travel from urban areas to rural areas due to the high mosquito vectors present in rural areas. This is further aggravated by the low immunity of urban dwellers [ 36 ]. Chemoprophylactic drugs are recommended for use before and during such visits to rural areas to prevent malaria infection.
Furthermore, our findings show that genetic factors such as haemoglobin genotype and blood group also influenced malaria parasite distribution in this study area. Having blood group O is significantly associated with higher malaria infection. This is corroborated by Akhigbe et al. [ 37 ] and Afoakwah et al. [ 38 ], who recorded higher malaria prevalence for blood group O in Ghana. Another study suggested that the ABO blood group does not hinder the development of uncomplicated falciparum malaria but severe malaria [ 39 ]. This variation could be due to the different geographical regions [ 40 ]. In the same vein, haemoglobin AA is significantly associated with malaria infection in this study. This is consistent with previous findings [ 41 , 42 ] but inconsistent with the report by Suchdev et al. [ 43 ] who found no significant association between genotype AA, AS and SS in Kenya.
Finally, the Bivariate Logistic Regression analysis shows that age group 6–10 years, the presence of streams, living near streams within ≤1 km and travel to rural areas were the major risk factors that often increase the odds of malaria infection in this study area. These findings are in agreement with previous studies in Nigeria and other malaria-endemic regions [ 22 , 27 , 35 , 44 , 45 ]. Therefore, the government should provide awareness to urban residents on mosquito breeding site identification and removal from time to time. Additionally, government should ensure the provision of additional mosquito bed net, vaccination, fumigation, indoor residual spray, and enforcement of law on frequent public sanitation. Encouraging travelers to use chemoprophylaxis before and during travel to malaria-endemic zone is highly imperative for management control of malaria in Nigeria.
5. Conclusion
Malaria is endemic in Ibadan city, and this is a glaring evidence indicating malaria as a public health challenge even in urban areas. Major risk factors influencing transmission include age, stream within ≤1 km from home, and, most importantly, travel to rural areas. Health education on mosquito prevention and use of chemoprophylaxis before and during travel to rural areas is important and recommended.
5.1. Limitation
Malaria prevalence in this study area may have been underestimated since the study was a hospital-based study and not a community-based study. Also, the sample size is not large enough, thus likelihood of sampling error. The study did not include vector surveillance to validate the malaria vector species in the study area. In spite of this, the study provides relevant information that can help in making pertinent policies in the study area.
Declarations
Author contribution statement.
O. Awosolu: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Z. Yahaya, M. T. Farah Haziqah and I. Simon-Oke: Analyzed and interpreted the data; Wrote the paper.
C. Fakunle: Performed the experiments; Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Declaration of interests statement.
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Prevalence and associated factors of malaria in children under the age of five years in Wogera district, northwest Ethiopia: A cross-sectional study
Roles Conceptualization, Data curation, Formal analysis, Methodology, Software, Supervision, Writing – original draft
Affiliation Department of Epidemiology and Biostatics, Institute of Public Health, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Roles Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – original draft
Affiliation Wogera District Health Office, North Gondar Zone, Gondar, Ethiopia
Roles Data curation, Formal analysis, Methodology, Software, Writing – review & editing
* E-mail: [email protected]
Affiliation School of Nursing and Midwifery, Haramaya University, Harar, Ethiopia
- Adino Tesfahun Tsegaye,
- Andualem Ayele,
- Simon Birhanu

- Published: October 11, 2021
- https://doi.org/10.1371/journal.pone.0257944
- Reader Comments
Malaria is a major public health problem in sub-Saharan Africa, and children are especially vulnerable. In 2019, an estimated 409,000 people died of malaria, most (274,000) were young children and 94% of the cases and deaths were in Africa. Prior studies in Ethiopia focused on the adult population and high transmission areas. Hence, this study aimed to determine the prevalence and associated factors of malaria in children under five years in low transmission areas.
A facility-based cross-sectional study was conducted among 585 under-five children who attended public health facilities in the Wogera district from September to October, 2017. Health facilities were selected by stratified cluster sampling, and systematic random sampling was held to select study participants from the selected facilities. Multivariable logistic regression was used to identify correlates of malaria.
Of 585 children who provided blood samples, 51 (8.7%) had malaria. The predominant Plasmodium species were P . falciparum 33 (65%) and P . vivax 18 (35%). Regularly sleeping under long-lasting insecticide treated nets (LLIN) was associated with decreased odds of malaria (AOR = 0.08, 95% CI: 0.01–0.09), and an increased odds of malaria was observed among children who live in households with stagnant water in the compound (AOR = 6.7, 95% CI: 3.6–12.6) and children who stay outdoors during the night (AOR = 5.5, 95% CI: 2.7–11.1).
The prevalence of malaria in the study population was high. Environmental and behavioral factors related to LLIN use remain potential determinants of malaria. Continued public health interventions targeting proper utilization of bed nets, drainage of stagnant water, and improved public awareness about reducing the risk of insect bites have the potential to minimize the prevalence of malaria and improve the health of children.
Citation: Tsegaye AT, Ayele A, Birhanu S (2021) Prevalence and associated factors of malaria in children under the age of five years in Wogera district, northwest Ethiopia: A cross-sectional study. PLoS ONE 16(10): e0257944. https://doi.org/10.1371/journal.pone.0257944
Editor: Benedikt Ley, Menzies School of Health Research: Charles Darwin University, AUSTRALIA
Received: December 10, 2020; Accepted: September 14, 2021; Published: October 11, 2021
Copyright: © 2021 Tsegaye et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All data generated or analyzed during this study is included in this published article.
Funding: The author(s) received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Abbreviations: AIDS, Acquired Immune Deficiency Syndrome; AOR, Adjusted Odds Ratio; API, Annual Parasite Incidence; CI, Confidence interval; DRC, Democratic Republic of Congo; EMIS, Ethiopia Malaria Indicator Survey; HIV, Human Immune Virus; IRS, Indoor Residual Spraying; LLITN, Long Lasting Insecticide Treated Nets; OPD, Outpatient Department; RDT, Rapid Diagnostic Test; SNNPR, Southern Nation Nationalities and People Region
In sub-Saharan Africa, infectious diseases remain the primary public health threat [ 1 ]. Malaria is one of the commonest infections, disproportionately affecting children and pregnant women. In 2019, an estimated 409,000 people died of malaria. Most (274,000) were young children, and 94% of the infections and deaths occurred in Africa [ 2 , 3 ]. Although several Plasmodium species are responsible for malaria, only a few of them cause most infections.
In 2018, Plasmodium falciparum accounted for 99.7% of estimated malaria cases in the World Health Organization (WHO) African Region, 50% in the WHO South-East Asia Region, 71% in the Eastern Mediterranean, and 65% in the Western Pacific. P . vivax is the predominant parasite in the WHO Region of the Americas, representing 75% of malaria cases [ 3 ]. In Ethiopia, peak malaria transmission occurs between September and December in most parts, following the rainy season from June to August, mainly affecting young children, and P . falciparum and P . vivax are the major malaria parasites [ 4 , 5 ].
Children under five years are one of the most vulnerable groups affected by malaria. Severe anemia, hypoglycemia and cerebral malaria are features of severe malaria more commonly seen in children than in adults [ 6 ]. Children’s susceptibility to diarrhea, respiratory infections, and other illnesses increases when they develop repeated malaria infections [ 7 ]. An estimated 2% of children who recover from cerebral malaria develop learning impairments and disabilities, including epilepsy and spasticity, resulting from the brain damage caused by the infection [ 8 ]. In general, malaria could cause severe outcomes in children in three major ways: First, since children do not usually have acquired immunity, they are more likely to develop severe malaria manifested by seizures or coma (cerebral malaria), which can cause emergency death. Second, through complications related to repeated infections such as anemia. Finally, it causes low birth weight when it happens during pregnancy and increases the risk of death in the first month of life [ 4 ].
According to the WHO 2016 report, the global prevalence of malaria among under-five children was 16% [ 9 ]. In the same year, the prevalence in Ethiopia was 0.6% [ 5 ].
The Ethiopian government developed a National Malaria Control Strategy (NMSP) for the years 2017–2020 that was envisioned to be aligned with the country’s four-year health sector transformation plan (HSTP) 2015/16–2019/20. The proposed goals for the 2017–2020 NMSP include: maintaining near-zero malaria deaths (< = 1 death per 100,000) by 2020, reducing malaria cases by 40% by 2020, and eliminating malaria from Ethiopia by 2030 [ 2 , 5 ].
Even though malaria is one of the leading causes of under-five morbidity and mortality in Ethiopia, prior studies focused only on the adult population and were done in malaria-endemic transmission areas. Nevertheless, it is a potential threat in non-endemic regions [ 5 ]. There has been limited information on the epidemiology of malaria among under-five children living in low malaria transmission areas [ 10 ]. This study aimed to close a critical knowledge gap by assessing the prevalence and determinants of malaria among under-five years old children living in low malaria transmission areas. The findings from this study will inform public health and clinical decision-making and will initiate further investigations.
Methods and materials
Study setting and design.
A health facility-based cross-sectional study was conducted from September to October, 2017 in the Wogera district. Wogera is one of the districts in the North Gondar zone. It has an average altitude of greater than 2050 meters above sea level, with an estimated total population of 274,384, of which 37,152 (13.5%) are children under five years old. The district has 42 rural and one city kebeles (the smallest administrative unit ), of which 15 kebeles (35.7%) are malaria-endemic. In the Wogera district, there was 1 hospital, 10 health centers, 42 health posts, and 4 private health institutions. It shares borders with Dabat and Tach-Armacho in the North, Misrak-Belesa and Janamora in the West, Merab Belesa in the South and Lay-Armacho in the East [ 11 ]. According to the new stratification of malaria risk in the country, the district is under the classification of low transmission areas with expected sporadic epidemics every five years [ 5 ]. Despite that, the report of the district health office indicates that malaria is one of the leading causes of morbidity both in adults and under-five children.
Study participants
All children whose age was five years or below visiting the selected health facilities during the study period were the source population.
Sample size estimation
The calculated sample size was 266 using a single population proportion formula as well as a power approach using a double proportion formula based on previous studies [ 12 ]. Adding a 10% non-response rate and a design effect of two, the final sample size was 585.
Sampling procedure
First, we stratified the health facilities as malaria-endemic and non-endemic based on their altitude. Then, we randomly selected five health centers (Ambagiorgis HC, Gedebgie HC, Selarie HC, Tirgosgia HC, and Chichiki HC) and one hospital (Wogera hospital) from the non-endemic clusters by using a lottery method. The calculated 585 sample size was proportionally allocated to the selected health facilities. Finally, a systematic random sampling technique was used to reach under-five clients who attended the selected health facilities.
Data collection tools and procedures
A structured questionnaire was used for data collection. The tool contained socio-demographic, environmental, and malaria prevention related questions. The questionnaire was initially developed in English and translated into Amharic for data collection. A face-to-face interview of the parents/guardians of the under-five children was conducted to collect the data.
After the interview was completed, following the Federal Democratic Republic of Ethiopia Ministry of Health National Malaria Guidelines, blood was taken from a finger prick to prepare thick and thin blood film smears [ 13 ]. Using a sterile lancet, a finger prick was performed, and 5 micro liters of whole blood was drawn from each child included in the sampling regardless of signs and symptoms of malaria using a capillary tube. The blood smears were prepared on microscope slides and stained using 10% Giemsa to be examined under 100x microscopes for the presence of malaria parasites. The thick smear was used to determine whether the malaria parasites were present or absent and the thin smear was used to identify the type of Plasmodium species. A positive result was defined as the presence of one or more asexual stages (trophozoite, ring stage, merozoite, or gametocyte) of plasmodium [ 14 ].
Data quality assurance
Six laboratory technicians (1 from each health facility) and two supervisors from the district health office were trained for two days by the investigators. Each filled questionnaire was checked thoroughly for completeness and consistency, and the necessary feedback was given to data collectors. Recruitment was preceded by obtaining informed written consent from parents or caregivers of the children. To assure the quality of the microscopic examinations, all positive and randomly selected five percent of the negative slides were checked blindly by another experienced medical laboratory technologist.
Operational definitions
Bed net utilization: was self-reported ownership and regular use of bed nets. A 15-day recall period was used to measure whether each child regularly slept under long lasting insecticide treated nets (LLIN) or not.
Malaria : was defined as a positive thin or thick blood film for the Plasmodium parasite.
Data processing and analysis
After data collection, data were entered using Epi info version 7 and then exported to SPSS version 20 for analysis. The correlates of malaria were identified using bivariate and multivariate logistic regression models. Variables which had a P-value of <0.2 in the bivariable regression were included in the multivariable logistic regression analysis. A P-value <0.05 was considered to determine statistical significance. Finally, adjusted odds ratios (AOR) with a 95% confidence interval (CI) were used to determine the strength of association of variables.
Ethical approval and consent to participate
Ethical approval was obtained from the ethical review committee of the Institute of Public Health, College of Medicine and Health Science, University of Gondar, Ethiopia. Permission was gained from the Amhara Regional Health Bureau, North Gondar health department, and Wogera health office. The caregivers were given detailed explanations about the study’s objectives, procedures, and potential risks and benefits, and written consent was obtained following that. The interview of each study participant took place in a separate room after the children gave blood samples. Appropriate treatment was given to children who tested positive.
Socio-demographic characteristics of study participants
In this study, 585 children from five health centers and one district hospital participated: Gedebgie health center (HC) 178 (30.4%), Ambagiorgis HC 114 (19.5%), Tirgosgia HC 111 (19%), Selarie HC 98 (16.8%), Ambagiorgis hospital 37 (6.3%) and Chichiki HC 47 (8%). Three hundred twenty-three (55.2%) were males and 218 (37.3%) were below 12 months. About 370 (63%) of the respondents live in rural areas, and 305 (54%) of the caregivers can not read and write ( Table 1 ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0257944.t001
Indoor Residual Spraying (IRS), Long Lasting Insecticide Treated Nets (LLIN), and environmental characteristics of study participants
Only 131 (22.4%) of the respondents had LLIN. Of the respondents who possessed LLIN, 90% of respondents reported that their children had regularly slept under LLIN in the last 15 days ( Table 2 ).
https://doi.org/10.1371/journal.pone.0257944.t002
Magnitude of Malaria
In this study, the prevalence of malaria by microscopy among under-five children was 8.7% (51). There was a considerable variation in the prevalence rate between the health facilities, ranging from 0% at Wogera hospital to 21% at Selarie health center ( Table 3 ).
https://doi.org/10.1371/journal.pone.0257944.t003
Factors associated with malaria infection
Both bivariable and multivariable binary logistic regression analyses were done to identify the determinants of malaria infection. In bivariate analysis, factors with a P-value of <0.2 were: place of residence, stagnant water around the home, staying outside during the night, possession of an LLIN and regularly sleeping under an LLIN for the last 2 weeks. However, place of residence, sex of the child, age of the child, age of the mother/guardian, educational status of the mother/guardian, presence of radio/television, child having a regular sleeping area, construction material of the house and incidence of IRS within six months had a P-value of >0.2 in the bivariate analysis and were not included in the final model.
In the final adjusted model, children who stayed outside at night had 5.5 times higher odds of malaria infection than children who did not stay outside at night (AOR = 5.5, 95% CI: 2.7–11.1). Children who regularly slept under a LLIN had 92% lower odds of infection than those who did not sleep regularly (AOR = 0.08, 95% CI: 0.08, 0.09). Children who lived in households with close to stagnant water had—4 times higher odds of malaria infection than children who did not live in those homes with nearby stagnant water (AOR = 4, 95% CI: 1.9, 8.1) ( Table 4 ).
https://doi.org/10.1371/journal.pone.0257944.t004
In this study, we estimated the prevalence of malaria among under-five children in the low-risk area and its determinant factors, and the results showed that the malaria prevalence in under-five children was 8.7%, which is in line with the study conducted in Dilla, Southern Ethiopia, where the prevalence of malaria in under-five children was identified to be 7.1% [ 15 ] and a study of analysis of the five-year trend of malaria at Bichena primary hospital, Amhara Region, Ethiopia, where the overall prevalence of malaria was 9.28% [ 16 ].
This finding is much higher when compared to the national malaria indicator survey in 2015 that identified a prevalence of 0.6% among under-five children [ 5 ] and another study conducted in four regional states in Ethiopia, where the prevalence was 4.6% [ 17 ]. This could be due to the difference in methodology used, and also, it might be due to the season when the studies were conducted. Malaria increases from September to December (major transmission season). However, this finding is lower when compared to the global magnitude of malaria among under-five children, which is about 16% [ 9 ] and studies conducted in East Shewa 18.9% [ 18 ], Tanzania 26.3% [ 19 ], Sudan 22% [ 20 ], Uganda 19.5% [ 21 ], and Mozambique 33% [ 22 ]. Those studies were conducted in low land areas, and the difference could be due to a study population difference in the case of a study conducted in Mozambique in which the study population was people with comorbidity.
In Ethiopia, there is spatial and temporal variability in the occurrence of malaria. The current findings also demonstrated similar spatial variations in the proportion of Plasmodium species, with the predominant occurrence of P . falciparum infections at 65% over P . vivax at 35%. This estimate is approximately similar to the study conducted by the Carter Center in Amhara, Oromia, and Southern Ethiopia, where P . falciparum accounted for 56.5% and P . vivax for 43.5% [ 17 ], and a 7-year trend of malaria study done at primary health facilities in Northwest Ethiopia P . falciparum accounted for 15.6% of the participants, which was threefold higher than P . vivax in the seven-year trend [ 23 ]. However, other studies reported a different proportion, such as those conducted in East Shewa ( P . falciparum = 41.2%, P . vivax = 57.1 and Mixed = 1.8%) [ 19 ]; Hadiya ( P . falciparum = 25.5%, P . vivax = 71.7% and Mixed = 2.8%) [ 24 ] and Dilla town ( P . falciparum = 26.8%, P . vivax = 62.5%, and Mixed = 10.7%) [ 15 ]. The variability could be related to the wide climatic diversity between the areas.
Sleeping under LLIN for the last two weeks was found to be protective against malaria. This evidence is supported by other similar studies conducted in East Shewa [ 18 ], Amhara, Oromia, and SNNRP [ 17 ], Dilla [ 15 ], Ethiopia [ 25 ], Ghana [ 26 ], and Uganda [ 21 ]. It was evident that using ITN properly decreased mosquito bites, and thereby decreased malaria infection.
In this study, malaria was highly prevalent among children living in households with stagnant water in the compound compared to their counterparts. This is consistent with a facility-based cross-sectional study conducted in a low transmission area of the Hadiya zone, south Ethiopia [ 24 ]. This is because water collection is one of the favorable conditions for mosquito breeding, which in turn increases malaria transmission. Staying outside during the night showed a statistically significant association with malaria. Staying outside during the night increases the probability of mosquito bites due to the nocturnal nature of the mosquito.
Limitations of the study
As a limitation of this study, since it is a cross-sectional study, it only captures the point prevalence and can not account for seasonal trends in transmission. All surveys are self-report with no confirmation of bed net ownership or use. RDTs with PCR confirmed were not conducted, nor are there details on the life stages of detected parasites observed–gametocytemia, parasitemia.
The prevalence of malaria in under-five children attending health care facilities in Wogera district was high. Regularly sleeping under a bed net, staying outside during the night, and stagnant water around the household were the main correlates of malaria. Focusing on LLIN distribution, environmental management, and changing attitudes towards malaria prevention and control through health education would help minimize the burden of malaria.
Acknowledgments
We would like to thank the Wogera health bureau, the study participants, data collectors, and supervisors who participated in this study for their commitment and cooperation.
- View Article
- PubMed/NCBI
- Google Scholar
- 2. Communicable disease control (CDC). Malaria Impact of Malaria. 2019. https://www.cdc.gov/malaria/malaria_worldwide/impact.html .
- 3. World health organization (WHO). Fact sheet malaria. 30 November 2020. https://www.who.int/news-room/fact-sheets/detail/malaria .
- 4. President’s Malaria Initiative, Ethiopia. Malaria Operational Plan FY. 2019. https://www.pmi.gov/docs/default-source/default-documentlibrary/malariaoperational-plans/fy19/fy-2019-ethiopia-malaria-operational-plan.pdf?sfvrsn=3 .
- 5. Ethiopia National Malaria Indicator Survey. 2015. https://www.ephi.gov.et/images/pictures/download2009/MIS-2015-FinalReport-December-_2016.pdf .
- 6. World health organization (WHO). Malaria in children under five. 2019. https://www.who.int/malaria/areas/high_risk_groups/children/en/ .
- 9. World health organization (WHO). World malaria report. 2016. https://www.who.int/malaria/publications/world-malaria-report-2016/report/en/ .
- 11. Wogera Woreda health bureau Annual Report. 2016.
- 13. Federal Democratic Republic of Ethiopia Ministry of Health. National Malaria Guidelines fourth edition. November 2017 Addis Ababa. https://www.humanitarianresponse.info/sites/www.humanitarianresponse.info/files/documents/files/eth_national_malaria_guidline_4th_edition.pdf .
- 14. Centres for disease control. Malaria Diagnostic Tests.2017. https://www.cdc.gov/malaria/diagnosis_treatment/diagnostic_tools .
- 17. The Carter Center. Prevalence and Risk Factors for Malaria And Trachoma In Ethiopia.Report of Malaria and Trachoma Survey in Ethiopia.2007.
- 19. Mushashu u. Prevalence of malaria infection among under-fives and the associated factors in muleba district-kagera region tanzania. 2012.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 26 January 2022
Six-year trend analysis of malaria prevalence at University of Gondar Specialized Referral Hospital, Northwest Ethiopia, from 2014 to 2019
- Amanuel Mulugeta 1 ,
- Atsede Assefa 1 ,
- Atsede Eshetie 1 ,
- Birhanie Asmare 1 ,
- Meseret Birhanie 1 &
- Yemataw Gelaw 1
Scientific Reports volume 12 , Article number: 1411 ( 2022 ) Cite this article
4025 Accesses
4 Citations
1 Altmetric
Metrics details
- Medical research
- Microbiology
- Molecular biology
- Molecular medicine
Globally, malaria is the major public health disease caused by plasmodium species and transmitted by the bite of the female anopheles mosquito. Assessment of the trend of malaria prevalence is important in the control and prevention of the disease. Therefore, the objective of this study was to assess the six year trend of malaria prevalence at the University of Gondar Comprehensive Specialized Hospital, northwest Ethiopia, from 2014 to 2019. A retrospective laboratory registration logbook review study was conducted on the malaria blood film examination results at the University of Gondar Comprehensive Specialized Hospital. The data was collected by using a data extraction tool and entered into SPSS version 20 for analysis. Descriptive statistics were used to summarize the socio-demographic characteristics of study participants and presented by graphs, tables and texts. The binary logistic regression was also used to test the association the trend of malaria prevalence and different factors like sex, age, year, and season. From a total of 17,500 malaria blood film examinations, 1341 (7.7%) were confirmed for malaria parasites. Of the confirmed malaria cases, 47.2%, 45.6% and 7.2% were P. vivax, P. falciparum and mixed infection , respectively. The proportion of P. vivax was the predominant species in the first three study years (2014–2016) and P. falciparum became the predominant species in the last three study years (2017–2019). The odds of malaria prevalence was lower by 68%, 60% and 69% in the year 2017, 2018 and 2019 compared to 2014, respectively. It was also 1.41 times higher in males than in females. Moreover, the odds of malaria prevalence were 1.60, 1.64, 2.45 and 1.82 times higher in the age group of < 5, 5–14, 15–24 and 25–54 years old compared to the older age groups (> 54 years old), respectively. Even there was a significant declining in prevalence trend; malaria is still a major public health problem. The study showed that there was high seasonal fluctuation from year to year. Moreover, males and the younger age groups were more affected than females and old age groups, respectively. Therefore, malaria prevention and control activities should be strengthened and require extra efforts by considering these variability.
Similar content being viewed by others

A guide to vaccinology: from basic principles to new developments
Andrew J. Pollard & Else M. Bijker

Infectious disease in an era of global change
Rachel E. Baker, Ayesha S. Mahmud, … C. Jessica E. Metcalf
Drug-resistant tuberculosis: a persistent global health concern
Maha Farhat, Helen Cox, … Madhukar Pai
Introduction
Malaria is one of the protozoan blood parasite that cause morbidity and mortality globally 1 . It is a major public health problem throughout human history, particularly in the tropical and subtropical parts of the world.
According to records from the Ethiopian Federal Ministry of Health, 75% of the country is malarious at which 68% of the total population is living 2 . Malaria is very severe and leading cause of morbidity and mortality for many years in Ethiopia 2 , 3 . There are two peaks seasonal transmissions of malaria in Ethiopia; the months of September to December (autumn) and March to May (spring) 3 , 4 .
In Ethiopia, including the Amhara region, prevention and control activities of the malaria have been implemented as guided by the National Strategic Plan. These prevention and control activities uses a combination intervention strategy including early diagnosis and prompt treatment, selective vector control that involved use of indoor residual spraying (IRS), insecticide-treated mosquito nets (ITNs) and environmental management 4 .
However, malaria control in the country as a whole and in the region particularly continued to experience many problems. Studies have shown that the Plasmodium species compositions and the number of malaria cases vary over time due to different factors, such as change in weather conditions, intervention measures, environmental or human behavioral risk factors 3 , 5 . Some studies in Ethiopia revealed that there was a decrement of Plasmodium species over period of years 5 , 6 . On the other hand, another trend studies showed that there were fluctuation of malaria cases 4 , 7 , 8 . So, it is crucial to assess the current trend of malaria prevalence in the country as well as the study area.
Assessment of the pattern of the current malaria prevalence and understanding how malaria varies in the community as a result of seasonal, environmental, geographical or year-to-year changes will help to evaluate the effectiveness of proven control interventions of the disease in a locality 5 , 9 .
It also gives essential information about achievements of national malaria programs and identifies malaria hot spots. Additionally, it gives important insight into the changing malaria situation, which might guide adjustments of malaria program activities and the prioritization of malaria research and the changing malaria situation requires an updating description of malaria trends 10 , 11 . Therefore, the objectives of this study were to analyze trends of malaria prevalence at University of Gondar Specialized Referral Hospital, northwest Ethiopia to identify trends of Plasmodium species over the time-period.
Study area and study population
The study was conducted at University of Gondar Comprehensive Specialized Hospital located in Gondar town. Gondar is ancient city which is located Northwest direction of Ethiopia, 727 km away from Addis Ababa, the capital city of Ethiopia and 175 km from Bahir Dar, the capital city of Amhara regional state. The town has latitude and longitude 12°361 N 37°281E with an elevation of 2133 m above sea level. According to Central Statistical Agency of Ethiopia 2015 report, it has twelve sub city and 22 urban and 11 rural kebeles with a projected population of 323,900 12 . The city has 8 public health centers and 1 public comprehensive specialized hospital (University of Gondar Comprehensive Specialized Hospital), more than 13 private clinics and 1 general hospital providing health services like diagnosis, treatment, prevention and control of diseases 13 . All malaria examined blood films at the University of Gondar Comprehensive Specialized Hospital and registered at laboratory registration logbook were source of population. On the other hand, the study population in this study were all malaria examined blood films (including both sexes and any age groups) at the University of Gondar Comprehensive Specialized Hospital for the past 6 years (from 2014 to 2019). All registered malaria blood films, except incomplete data and illegible (unreadable) documents, were included from the study.
Study design
A retrospective laboratory registration logbook review study was conducted to determine the 6 years trend of malaria prevalence by reviewing malaria blood film examination laboratory registration logbook at laboratory registration log book of University of Gondar Comprehensive Specialized Hospital (2014–2019).
Sample size and sampling technique
All malaria examined blood films and register at the University of Gondar Comprehensive Specialized Hospital laboratory registration logbook from 2014 to 2019 were the sample size of study. A total of 17,500 malaria examined blood films were included. The malaria examined blood films were selected by the censuses sampling technique.
Data collection
The six years (2014–2019) malaria blood film examination laboratory registration logbook data was extracted from March to June 2020, at the University of Gondar comprehensive Specialized Hospital laboratory registration log book. The data was collected by laboratory personnel by using data extraction sheet. The data extraction sheet includes result of blood film (Negative and Positive), type of plasmodium species ( P. falciparum , P. vivax and mixed), year of examination, month of examination, season of examination, sex and age of the patient. Data on both negative and positive microscopically confirmed malaria cases were included in the study. At the University of Gondar comprehensive Specialized Hospital, patients presented sign and symptom of malaria (clinical presentation of malaria) were requested by physicians and internists. In Ethiopia, microscopy is the major diagnostic method for malaria, especially in health centers and hospitals 10 . A well-prepared Gimsa stained blood film (both thick and thin smear) was used to diagnose malaria parasites in the laboratory. Unfortunately, complete data regarding clinical presentation of patient, major interventions done against malaria and other environmental factors were not collected.
Data analysis and interpretation
The data were entered into SPSS version 20 for analysis. Descriptive statistics were used to summarize the socio-demographic of study participants and the frequency of malaria on different independent variables and presented by tables, figures and texts. Multivariable binary logistic regression analyses were performed to determine the association between the dependent (malaria prevalence and independent variables (age, sex, and year and season as categorical variable). The multivariable binary logistic regression model was analyzed with enter method and a p value < 0.05 in the multivariable regression model was considered as statistically significant. The model fitness of the final multivariable logistic regression was checked using Hosmer and Lemeshow test.
Data quality assurance
The data were checked for completeness, cleaned, and sorted daily. Moreover, the data quality was assured by following standard operation procedures, double entry. In addition, the quality of blood film staining reagents (Gimsa) was checked for its expiration date and by running the known blood sample. Moreover, the blood film examination was done by laboratory technologist and Medical parasitologist who had taken training on malaria blood film examination and malaria parasite identification. The laboratory personals are also participated in proficiency test.
Ethics approval and consent to participate
All methods were performed following the relevant guidelines and regulations. The University of Gondar has an ethical and review committee in each study field to approve the study on humans. Therefore, the ethical clearance of this study was obtained from the Ethical and Review Committee of the School of Biomedical and Laboratory Sciences, College of Medicine and Health Science, University of Gondar. After discussing the purpose and method of the study, verbal consent was obtained from the Medical Director of the University of Gondar Specialized Referral Hospital before the data collection. Since the study was used secondary data from the registration logbook informed consent for the participants was waived by the Ethical and Review Committee of School of Biomedical and Laboratory Sciences, College of Medicine and Health Science, University of Gondar.
Characteristics of study participants
During 2014 to 2019, a total of 17,500 malaria blood films (in average 2917 blood films per year) were examined microscopically for malaria diagnosis. More than half of the cases were males, 9542 (55.5%) and this was more or less consistent throughout the six years. In the six trends, the most malaria suspected and examined cases were in the age group of 25–54 (7040 (40.2%)) followed by age group of 15–24 (5540 (31.7%)) and the lowest suspected case was examined in the older age groups (> 54 years old) (1485 (8.5)). The trend of suspected cases (malaria blood film examination) was highly fluctuated. The highest blood film examination was performed in the year of 2015 (2789 (24.1%)) followed by year of 2017 (3348 (19.1%)) (Table 1 ).
Annual trends of malaria prevalence and proportion of plasmodium species
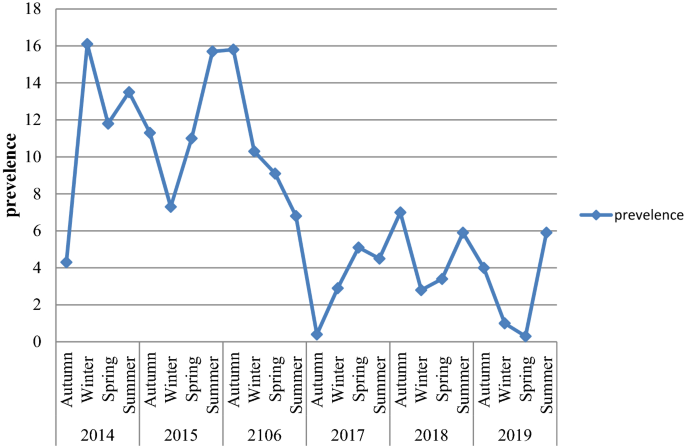
Among a total of 17,500 examined blood films, 1341 (7.7%; 95% CI 7.3–8.1) were positive for plasmodium species during the six year period. There were significant fluctuations and reduction trends of overall malaria during the past 6 years, with a maximum of 11.2% and a minimum of 3.7% of cases in 2016 and 2019, respectively. P. vivax was the predominant plasmodium species. However, the proportion of the plasmodium species was significantly fluctuated in the six years period (chi squared = 62.58, p value < 0.001). In the first 3 study years, the proportion of P. vivax was the predominant plasmodium species and in the last 3 study years P. falcifarum was the predominant plasmodium species Moreover, mixed infection ( P. vivax and P. falcifarum ) showed a significant fluctuating increment trend in the area in the 6 years, with a maximum of 10.9% and a minimum of 3.6% of cases in 2017 and 3 in 2014, respectively (Table 2 , Fig. 1 ).

Annual trend of malaria prevalence and proportion of plasmodium species at University of Gondar specialized referral hospital from 2014 to 2019.
Sex, age and seasonal variations of malaria prevalence
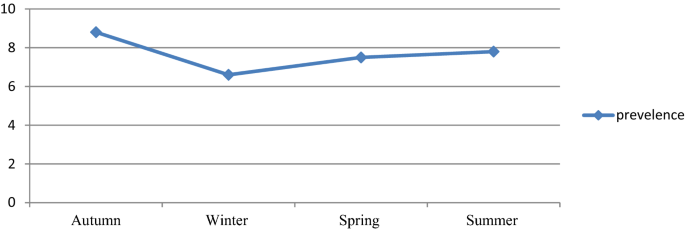
Despite the apparent fluctuation of total malaria trends over 6 years in the study area, malaria cases occurred throughout the year. However, there was a significant variation between the sexes and different age groups. The odds of malaria prevalence among the male was 1.41(95%CI 1.26–1.59) times higher than females. The prevalence of malaria was also higher in lower age groups compare to the older age groups. The odds of malaria prevalence was 1.60 (95%CI 1.14–2.23), 1.64 (95%CI 1.20–2.26), 2.45 (95%CI 1.86–3.22) and 1.82 (95%CI 1.39–2.40) in the age group of < 5 years, 5–14 years, 15–24 years and 25–54 years, respectively compare to age group of > 54 years old. Controlling of the confounding factors of sex and age, the prevalence of malaria also showed significant reduction in the last 3 study years (2017–2019) compare to the first study year (2014). It was decreased by 68% (95%CI 60–75), 60% (95%CI 51–68) and 69% (95%CI 59–77) in the year of 2017, 2018 and 2019, respectively. Moreover, there was a significant seasonal variation in malaria cases. The highest peak of total malaria positivity rate was observed during autumn, (September, October and November; just after the main rainy season) and the minimum positivity rate was observed during winter (the dry season in the months of December, January and February) and showed significant variation. However, controlling of the sex and age group variation in the season, the highest peak of total malaria positivity rate was observed during summer (June, July and August; main rainy season). Moreover, the seasonal variation was not consistent and highly fluctuated in the six years. Even it was the season where the highest malaria case was reported in over all seasonal malaria prevalence, autumn was the season where lowest malaria case was report in 2014 and 2017 (Table 3 , Figs. 2 , 3 ).
Seasonal variations of malaria prevalence among blood smear microscopy at University of Gondar Specialized Referral Hospital from 2014 to 2019.
Seasonal variations of malaria prevalence in each year among patients requested for malaria examination at University of Gondar Specialized Referral Hospital from 2014 to 2019.
The highest prevalence of malaria was seen in August (9.6%) followed by September and November (9.3%) whereas, the lowest prevalence was seen in January (6.1%). The proportion of Plasmodium species highly fluctuated throughout the 12 months. Plasmodium vivax was predominantly high in the winter months (December, January and February), spring months (March, April and May), and the two autumn months (September and November) whereas, Plasmodium falciparum was predominantly high in the summer months (June, July and August) and one of the autumn month (October). The mixed infection was also shoed monthly fluctuation in which the highest peak was observed in March and the lowest peak was observed in December (Table 4 ).
The present study revealed that the average annual malaria prevalence was 7.7% (95% CI 7.3–8.1). This finding was markedly lower than the study conducted elsewhere in Kola Diba, North Gondar, Northwest Ethiopia (39.6%) 4 , Adi Arkay, North Gondar, Northwest Ethiopia (36.1%) 14 , Abeshge, south-central Ethiopia (33.8%) 5 , Woreta Health Center, Northwest Ethiopia (32.6%) 14 , Dembecha Health Center, West Gojjam Zone, Northwest Ethiopia (16.34%) 8 and Halaba special district, Southern Ethiopia (9.5%) 15 . However, the current malaria prevalence was higher than other study finding conducted at Felegehiwot Referral Hospital catchment areas, Bahir Dar, northwest-Ethiopia Ethiopia (5%) 7 . The difference might be due to variations in malaria diagnosis quality and the skills of the laboratory personnel to detect and identify malaria parasites. Moreover, the implementation of malaria prevention and control activities might differ from one area to another. Besides, there might be a difference in demographic characteristics (sex, age), geographic location (altitude, temperature, rainfall) and economical activities differences that also had an effect on the prevalence of malaria. The population awareness about malaria bed net application, its transmission, and health seeking behavior might be also different.
The average annual trend of malaria prevalence revealed that there were slight increments in malaria prevalence in the first two years of the study (2015 and 2016) compared to the year 2014, but statistically, it was insignificant. However, in the last three study years (2017, 2018 and 2019) the trend showed a significant reduction in malaria prevalence. The odds of malaria prevalences were reduced by 68%, 60% and 69% in the year 2017, 2018 and 2019, respectively. The possible reasons for malaria reduction during thes study periods (2017–2019) might be due to the increased attention to malaria control and preventive activities by different responsible bodies, increased awareness of the community on the use of ITNs, IRS, the drainage system of mosquito breeding sites and climate change at national and international level. Integrated control strategies are underway in the local area as part of the nationwide malaria control activities 16 . The finding was similar to the 5-year malaria prevalence trend analysis at Dembecha Health Center, West Gojjam Zone, Northwest Ethiopia which reported that there was fluctuated decline of malaria prevalence 8 . However, the observed prevalence in this study was still considerable and public health problem.
This study demonstrated that on average of the six years of study periods, P. vivax was the predominant species, although there was a species fluctuation from year to year. The proportion of P. vivax , P. falciparum and mixed infections was 47.2%, 45.6%, and 7.2%, respectively. This finding was consistent with the study conducted in Adama City, East Shoa Zone, Oromia, Ethiopia 16 , Halaba health center Southern Ethiopia 15 and Southwest Ethiopia, around Gilgel gibe dam and 10 kilo Metter far from Gilgel gibe dam 3 . The predominance of P. vivax might be due to relapse of dormant liver stages or increased treatment pressure against P. falciparum 17 . However, this finding was in disagreement with the study conducted at two health centers Gorgora and Chuahit in Dembia district 18 , catchment areas of Felegehiwot Referral Hospital 7 and Kola Diba, North Gondar, Northwest Ethiopia 4 which reported that P. falciparum was the predominant species. Moreover, the trend of P. vivax showed reduction whereas, P. falciparum showed an increment trend. In the last three years of the study periods, P. falciparum had become the predominant Plasmodium species. The fluctuated proportion of plasmodium species might be attributed to heterogeneous parasite species and disease distribution include differences in genetic polymorphisms underlying parasite drug resistance and host susceptibility, mosquito vector ecology and transmission seasonality. Plasmodium species interact might have geographical differences and these interactions may even change from year to year in a given locale 19 . The finding also revealed that there was fluctuated increment in the proportion of mixed infection.
The prevalence of malaria was varied among different seasons ranging from 6.6 to 8.8%, and these variations were statistically significant. The highest peak was observed in autumn (8.8%) and the lowest peak was observed in the winter season (6.6%). The malaria prevalence was reduced by 16% in the winter. However, where the sex and age were adjusted, the peak prevalence was observed in summer rather than autumn, in which the prevalence was increased by 32%. The reason might be due to climate change from year to year. In Ethiopia, summer is the season when heavy rainfall is observed and it is not a favorable season for vector spreading 16 . However, there is rainfall variation from year to year 20 . Changes in temperature, rainfall, and relative humidity due to climate change are estimated to influence malaria directly by modifying the behavior and geographical distribution of malaria vectors and by changing the length of the life cycle of the parasite. Climate change is also expected to affect malaria indirectly by changing ecological relationships that are important to the organisms involved in malaria transmission (the vector, parasite, and host) 21 .
The current study revealed that males were more affected by malaria infection than females. The odds of malaria positivity rate among males were 1.41 times higher than females. Similar studies showed that males were more affected than females 22 , 23 , 24 , 25 . The reason behind the high malaria cases in males might be due to the fact that males are involved in outdoor activities. A study conducted in Dembia district, northwest Ethiopia revealed that individuals involved in outdoor activities were more at risk for malaria infection 25 . The other possible reason might be that males are mobile to malaria-endemic areas seeking temporary employment, whereas females do not perform field activities rather they are cookers and stay at home which might reduce the risk of infection.
Age was also contributing factor to the prevalence of malaria. It was higher in younger age groups than the older age groups. The odds of malaria positivity rate among less than five years old children and 5–14 years old were 1.60 and 1.64 times higher than the age group of greater than 55 years old, respectively. The reason might be these age groups may be less immune to commutate than the older age groups (> 55 years old). This was supported by the world health organization report 26 . The study also showed that the odds of malaria positivity rate among the early working groups (15–24) and primarily working groups (25–54) were, 2.45 and 1.82 times higher than the age group of greater than 55 years old, respectively. Another study, conducted on pregnant women in Sherkole district, Benshangul Gumuz regional state, West Ethiopia also revealed that the older age groups were less likely to have malaria infection 27 . The reason behind the high malaria cases in the mentioned age group of 15–24 and 25–54 years old might be the fact that this age group might be involved in outdoor activities and are mobile to malaria-endemic areas seeking temporary employment, whereas the older age group do not perform field activities rather they are staying at home which might reduce the risk of infection. Moreover, the older age groups might frequently expose to malaria previously, which might develop immunity to malaria infection. It was known that natural infection elicits a robust immune response against the blood stage of the parasite, protecting against malaria 28 . However, according to the studies conducted in rural surroundings of Arba Minch Town, south Ethiopia 29 , and Sudan 30 , age had no significant association with malaria infection. Indeed, these studies were focused on a specific study population; under-five children and pregnant women, respectively.
The finding of the current study had its strengths; one it had enough sample size which increased the power of the study; second, it included all age segments of the populations (from children up to the old age groups). However, this study might suffer from the fact that it is secondary data; the reliability of the recorded data could not be ascertained. Moreover, the collected data relayed on the laboratory logbook which lacks participants’ body temperature, clinical presentations and residence. It also lacks information regarding the weather conditions of the month, seasons and years.
The current finding showed that there was a significant declining trend of the of malaria prevalence in the study area. However, the overall prevalence was still a major public health problem and requires extra efforts for further reduction. On average, the highest peak of malaria cases was observed during the autumn seasons. However, there was high fluctuation from year to year. Moreover, males, under-five children and the younger age groups were more affected compare to the older age groups. In addition, even P. vivax was the predominant Plasmodium species in the allover trend, there was a high fluctuation of Plasmodium species from year to year and season to season. Therefore, prevention and control activities should be continued and strengthened in the study area considering these variabilities.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
Adjusted odds ratio
Confidence interval
Plasmodium Falciparum
Plasmodium Vivax
Indoor residual spraying
Insecticide-treated mosquito nets
Kassa, A. & Beyene, B. B. Climate variability and malaria transmission—Fogera district, Ethiopia, 2003–2011. Sci. J. Public Health 2 (3), 234–7 (2014).
Article Google Scholar
Ayele, D. G., Zewotir, T. T. & Mwambi, H. G. Prevalence and risk factors of malaria in Ethiopia. Malar. J. 11 , 195 (2012).
Sena, L. D., Deressa, W. A. & Ali, A. A. Analysis of trend of malaria prevalence in south-west Ethiopia: A retrospective comparative study. Malar. J. 13 , 188 (2014).
Alemu, A., Muluye, D., Mihret, M., Adugna, M. & Gebeyaw, M. Ten year trend analysis of malaria prevalence in Kola Diba, North Gondar, Northwest Ethiopia. Parasites Vectors 5 , 173 (2012).
Yimer, F., Animut, A., Erko, B. & Mamo, H. Past five-year trend, current prevalence and household knowledge, attitude and practice of malaria in Abeshge, south-central Ethiopia. Malar. J. 14 , 230 (2015).
Tesfa, H., Bayih, A. G. & Zeleke, A. J. A 17-year trend analysis of malaria at Adi Arkay, north Gondar zone, Northwest Ethiopia. Malar. J. 17 (1), 155 (2018).
Yimer, M., Hailu, T., Mulu, W., Abera, B. & Ayalew, W. A 5 year trend analysis of malaria prevalence with in the catchment areas of Felegehiwot referral Hospital, Bahir Dar city, northwest-Ethiopia: A retrospective study. BMC. Res. Notes 10 (1), 239 (2017).
Haile, D., Ferede, A., Kassie, B., Abebaw, A. & Million, Y. Five-year trend analysis of malaria prevalence in Dembecha Health Center, West Gojjam Zone, Northwest Ethiopia: A retrospective study. J. Parasitol. Res. 2020 , 8828670 (2020).
Alelign, A., Tekeste, Z. & Petros, B. Prevalence of malaria in Woreta town, Amhara region, Northwest Ethiopia over eight years. BMC Public Health 18 (1), 1–6 (2018).
Federal Democratic Republic of Ethiopia Ministry of Health, National Malaria Guidelines, fourth edition, Federal Democratic Republic of Ethiopia Ministry of Health. https://www.humanitarianresponse.info/sites/www.humanitarianresponse.info/files/documents/files/eth_national_malaria_guidline_4th_edition.pdf . Accessed 1 June 2021.
United State Agency International Development, PRESIDENT’S MALARIA INITIATIVE ETHIOPIA Malaria Operational Plan FY 2018, USA PRESIDENT’S MALARIA INITIATIVE collaboration with Department of Health and Human Science, Center for Disease controll and prevention, United State of America Department of State . https://www.pmi.gov/docs/default-source/default-document-library/malaria-operational-plans/fy-2018/fy-2018-ethiopia-malaria-operational-plan.pdf?sfvrsn=5 . Accessed 1 June 2021.
Getaneh, A., Tegene, B. & Belachew, T. Knowledge, attitude and practices on cervical cancer screening among undergraduate female students in University of Gondar, Northwest Ethiopia: An institution based cross sectional study. BMC Public Health 21 (1), 1–9 (2021).
Levin, K. A. Study design III: Cross-sectional studies. Evid. Based Dent. 7 (1), 24 (2006).
Derbie, A. & Alemu, M. Five years malaria trend analysis in Woreta Health Center, Northwest Ethiopia. Ethiop. J. Health Sci. 27 (5), 465–472 (2017).
Shamebo, T. & Petros, B. Trend analysis of malaria prevalence in Halaba special district, Southern Ethiopia. BMC. Res. Notes 12 (1), 190 (2019).
File, T., Dinka, H. & Golassa, L. A retrospective analysis on the transmission of Plasmodium falciparum and Plasmodium vivax: The case of Adama City, East Shoa Zone, Oromia, Ethiopia. Malar. J. 18 (1), 193 (2019).
Dedgeba, S. & Mamo, H. Malaria trends in Silt’i district from 2009–2015 and current childhood malaria in K’ibbet hospital, south-central Ethiopia. Malar. World J. 8 (22), 1–5 (2017).
Google Scholar
Addisu, A., Tegegne, Y., Mihiret, Y., Setegn, A. & Zeleke, A. J. A 7-year trend of malaria at primary health facilities in Northwest Ethiopia. J. Parasitol. Res. 2020 , 4204987 (2020).
Zimmerman, P. A., Mehlotra, R. K., Kasehagen, L. J. & Kazura, J. W. Why do we need to know more about mixed Plasmodium species infections in humans?. Trends Parasitol. 20 (9), 440–447 (2004).
Simane, B. et al. Review of climate change and health in Ethiopia: Status and gap analysis. Ethiop. J. Health Dev. = Ya’Ityopya tena lemat mashet 30 (1), 28–41 (2016).
Alemu, A., Abebe, G., Tsegaye, W. & Golassa, L. Climatic variables and malaria transmission dynamics in Jimma town, South West Ethiopia. Parasit. Vectors 4 (1), 30 (2011).
Tarekegn, M., Tekie, H., Dugassa, S. & Wolde-Hawariat, Y. Malaria prevalence and associated risk factors in Dembiya district, North-western Ethiopia. Malar. J. 20 (1), 372 (2021).
Tesfahunegn, A., Berhe, G. & Gebregziabher, E. Risk factors associated with malaria outbreak in Laelay Adyabo district northern Ethiopia, 2017: Case-control study design. BMC Public Health 19 (1), 484 (2019).
Alemu, K., Worku, A., Berhane, Y. & Kumie, A. Men traveling away from home are more likely to bring malaria into high altitude villages, Northwest Ethiopia. PLoS ONE 9 (4), e95341 (2014).
Article ADS Google Scholar
Agegnehu, F., Shimeka, A., Berihun, F. & Tamir, M. Determinants of malaria infection in Dembia district, Northwest Ethiopia: A case-control study. BMC Public Health 18 (1), 1–8 (2018).
WHO, malaria fact sheet report; World Health Organization. https://www.who.int/news-room/fact-sheets/detail/malaria , Accessed 18 June 2021.
Gontie, G. B., Wolde, H. F. & Baraki, A. G. Prevalence and associated factors of malaria among pregnant women in Sherkole district, Benishangul Gumuz regional state, West Ethiopia. BMC Infect. Dis. 20 (1), 573 (2020).
Article CAS Google Scholar
Gonzales, S. J. et al. Naturally acquired humoral immunity against plasmodium falciparum malaria. Front. Immunol. 11 (2809), 594653 (2020).
Abossie, A., Yohanes, T., Nedu, A. & Tafesse, W. Prevalence of malaria and associated risk factors among febrile children under five years: A Cross-sectional study in Arba Minch Zuria District, South Ethiopia. Infect. Drug Resist. 7 (13), 363–372 (2020).
Adam, I., Khamis, A. H. & Elbashir, M. I. Prevalence and risk factors for Plasmodium falciparum malaria in pregnant women of eastern Sudan. Malar. J. 4 (1), 1–4 (2005).
Download references
Acknowledgements
We are grateful to the University of Gondar Specialized Referral Hospital managers and laboratory personnel.
Author information
Authors and affiliations.
School Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, The University of Gondar, Gondar, Ethiopia
Amanuel Mulugeta, Atsede Assefa, Atsede Eshetie, Birhanie Asmare, Meseret Birhanie & Yemataw Gelaw
You can also search for this author in PubMed Google Scholar
Contributions
A.M., A.A., A.E. and B.A. participated in the study design, undertook the data collection, analyzed the data. Y.G. analyzed the data, wrote the manuscript and participated on the revision of the manuscript. M.B. participated in the study design, analyzed the data and on the revision of the manuscript. All authors have read the manuscript and approved it to submit for publication.
Corresponding author
Correspondence to Yemataw Gelaw .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Mulugeta, A., Assefa, A., Eshetie, A. et al. Six-year trend analysis of malaria prevalence at University of Gondar Specialized Referral Hospital, Northwest Ethiopia, from 2014 to 2019. Sci Rep 12 , 1411 (2022). https://doi.org/10.1038/s41598-022-05530-2
Download citation
Received : 03 August 2021
Accepted : 13 January 2022
Published : 26 January 2022
DOI : https://doi.org/10.1038/s41598-022-05530-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Analysing the six-year malaria trends at metehara health centre in central ethiopia: the impact of resurgence on the 2030 elimination goals.
- Aynalem Mandefro
- Geletta Tadele
- Lemu Golassa
Malaria Journal (2024)
Molecular investigation of malaria-infected patients in Djibouti city (2018–2021)
- Rahma Abdi Moussa
- Nasserdine Papa Mze
- Hervé Bogreau
Malaria Journal (2023)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
- Brief Report
- Open access
- Published: 23 March 2024
Prevalence of non- falciparum malaria infections among asymptomatic individuals in four regions of Mainland Tanzania
- Zachary R. Popkin-Hall 1 ,
- Misago D. Seth 2 ,
- Rashid A. Madebe 2 ,
- Rule Budodo 2 ,
- Catherine Bakari 2 ,
- Filbert Francis 3 ,
- Dativa Pereus 2 ,
- David J. Giesbrecht 4 ,
- Celine I. Mandara 2 ,
- Daniel Mbwambo 5 ,
- Sijenunu Aaron 5 ,
- Abdallah Lusasi 5 ,
- Samwel Lazaro 5 ,
- Jeffrey A. Bailey 6 , 7 ,
- Jonathan J. Juliano 1 ,
- Julie R. Gutman 8 &
- Deus S. Ishengoma 10 , 2 , 9
Parasites & Vectors volume 17 , Article number: 153 ( 2024 ) Cite this article
321 Accesses
13 Altmetric
Metrics details
Recent studies point to the need to incorporate the detection of non-falciparum species into malaria surveillance activities in sub-Saharan Africa, where 95% of the world’s malaria cases occur. Although malaria caused by infection with Plasmodium falciparum is typically more severe than malaria caused by the non-falciparum Plasmodium species P. malariae , P. ovale spp. and P. vivax , the latter may be more challenging to diagnose, treat, control and ultimately eliminate. The prevalence of non-falciparum species throughout sub-Saharan Africa is poorly defined. Tanzania has geographical heterogeneity in transmission levels but an overall high malaria burden.
To estimate the prevalence of malaria species in Mainland Tanzania, we randomly selected 1428 samples from 6005 asymptomatic isolates collected in previous cross-sectional community surveys across four regions and analyzed these by quantitative PCR to detect and identify the Plasmodium species.
Plasmodium falciparum was the most prevalent species in all samples, with P. malariae and P. ovale spp. detected at a lower prevalence (< 5%) in all four regions; P. vivax was not detected in any sample.
Conclusions
The results of this study indicate that malaria elimination efforts in Tanzania will need to account for and enhance surveillance of these non-falciparum species.
Graphical Abstract
Tanzania has one of the highest malaria burdens in the world, accounting for 3.1% of global malaria cases and 4.1% of global malaria deaths in 2021 [ 1 ]. While most malaria cases in Tanzania and elsewhere in sub-Saharan Africa are caused by Plasmodium falciparum , four other Plasmodium species ( P. vivax , P. malariae , P. ovale curtisi and P. ovale wallikeri ) are present to varying degrees. Current data also suggest that the prevalence of these species is higher than previously believed and that they may become even more prevalent as P. falciparum is controlled and ultimately eliminated [ 2 , 3 , 4 , 5 , 6 ], in line with the WHO goal of a 90% reduction in global malaria burden from 2015 levels by 2030 [ 7 ]. Non-falciparum malaria (i.e. malaria infection due to Plasmodium species other than P. falciparum ) may require different control measures due to major differences in biology, including different Anopheles vectors with different seasonal peaks [ 8 ], relapse and/or chronic infections [ 9 , 10 ], lower parasitemia [ 11 ] and higher rates of asymptomatic infection [ 8 ].
Previous work in Mainland Tanzania has characterized the prevalence of non-falciparum malaria in school children (age: 5–16 years) [ 6 ] and non-falciparum positivity rates among symptomatic patients [ 12 ]. In the school children, the prevalence of P. ovale spp. malaria infections (24%) was similar to that of P. falciparum (22%) [ 6 ], while in symptomatic patients, P. falciparum malaria infections were much more abundant than non-falciparum malaria, although P. ovale spp. positivity rates surpassed 5% in seven of 10 regions [ 12 ]. In both studies, P. malariae was less common than either P. falciparum or P. ovale spp., and P. vivax was rare [ 6 , 12 ]. However, a full characterization of non-falciparum prevalence in Mainland Tanzania requires accounting for asymptomatic individuals of all ages, who may act as potential reservoirs. In the study reported here, we characterized the prevalence of all malaria species among asymptomatic individuals across all ages in three regions with moderate and high malaria transmission intensity, and in children aged < 5 years in one region with high transmission.
The study protocol was approved by the Tanzanian Medical Research Coordinating Committee (MRCC) of the National Institute for Medical Research (NIMR) and involved approved standard procedures for informed consent and sample deidentification. Additional details are described elsewhere [ 13 ]. Deidentified samples were considered non-human subjects’ research at the University of North Carolina and Brown University.
A random subset of 1428 dried blood spot (DBS) samples were drawn from a total of 5860 asymptomatic samples, including 694 samples from a total of 2647 collected from all age groups [ 12 ] during cross-sectional community surveys in the Kigoma ( n = 252/878, high transmission area), Ruvuma ( n = 186/741, high transmission area) and Tanga ( n = 256/1028, moderate transmission area) regions during the Molecular Surveillance of Malaria in Tanzania (MSMT) project in 2021 [ 13 ]. The random subset was representative of the regional sample distribution ( χ 2 = 2.43, df = 2, P = 0.3; Additional file 1 : Table S1), but not representative of the age group distribution ( χ 2 = 10.46, df = 2, P = 0.005; Additional file 1 : Table S2), or the sex distribution ( χ 2 = 43.65, df = 1, P < 0.001; Additional file 1 : Table S3). An additional 734 samples were drawn from 3213 samples collected from children aged < 5 years during cross-sectional household surveys for the Group Antenatal Care project (GANC) [ 14 , 15 ] in Geita in 2021. In all regions, study participants were administered a malaria rapid diagnostic test (mRDT) in their communities. Participants with a positive test result were treated with artemether-lumefantrine and adjunct medications based on the presence of concurrent illnesses.
The molecular analyses used to detect Plasmodium spp. in each sample are described in detail elsewhere [ 12 ]. Briefly, we performed a separate quantitative PCR (qPCR) assay targeting the 18S ribosomal RNA gene ( 18S rRNA) for each species, which allows for both the detection of each species as well as a semi-quantitative estimate of parasitemia. For each region, we calculated prevalence for each species, including both single-species and mixed-species infections. Regional-level maps of prevalence for each species were created using the R package sf (version 1.0.9; R Foundation for Statistical Computing, Vienna, Austria) based on shape files available from GADM.org and naturalearthdata.com accessed via the R package rnaturalearth (version 0.3.2) [ 16 ]. Variation in species-specific prevalence by region and age group was assessed for significance with generalized linear models (GLMs) or analysis of variance (ANOVA), as appropriate, in R.
Excluding the Geita participants who were all aged < 5 years and whose ages were not recorded, the median age of the remaining 694 participants was 20 (interquartile range [IQR] 8–47) years (range 6 months to 87 years). Including the Geita participants, children (≤ 16 years old) constituted 74.2% of participants ( n = 1060), while adults (> 16 years old) constituted the remaining 25.8% ( n = 368). Young children (< 5 years old) comprised 77.9% ( n = 826) of the pediatric participants, while the remaining 22.1% ( n = 234) were school-aged children (5–16 years old). Sex identifications were available for 694 participants and were female-skewed, with 505 female (72.8%) and 189 male participants (27.2%). Of the sampled individuals, 21.1% ( n = 301) were RDT-positive (Additional file 1 : Table S4) using a standard HRP2/Pan RDT (First Response® [Premier Medical Corp., Ltd, Valsad, Gujarat, India]; SD Bioline™ [Abbott Diagnostics Ltd. Korea, Seoul, Republic of Korea]; or Care Start™ [Access Bio, Inc., Somerset, NJ, USA]).
Among all 1428 samples analyzed, P. falciparum was detected in 34.2% ( n = 488, 95% confidence interval [CI] 31.7–36.7%), P. malariae in 1.5% ( n = 22, 95% CI 0.99–2.4%) and P. ovale spp. in 3.4% ( n = 49, 95% CI 2.6–4.5%); P. vivax was not detected in any sample. Plasmodium malariae infections were nearly evenly split between single-species infections (45.5%, n = 10/22) and mixed-species infections with P. falciparum (40.9%, n = 9/22), with the remaining three infections (13.6%) being triple infections with P. falciparum and P. ovale spp. (Table 1 ). In contrast, most P. ovale spp. infections were mixed with P. falciparum (65.3%, n = 32/49), with single-species infections being less common (28.6%, n = 14/49) and triple infections comprising the remainder (Table 1 ). The highest median parasitemia was recorded for Plasmodium malariae at 164,080 parasites/µl blood (IQR 9942–1333,100 p/µl), followed by P. falciparum at 55,200 (IQR 2910–775,000) and P. ovale spp. at 11,868 (IQR 1271–70,840) p/µl. However, there was no significant difference in parasitemia by species ( F (2,556) = 0.085, P = 0.9).
All three Plasmodium species recorded in the samples ( P. vivax was not detected in any sample) were detected in each region (Fig. 1 ). The highest P. falciparum prevalence was found in Geita and Tanga (37.8% [ n = 278/734] and 36.7%, [94/256], respectively; Additional file 1 : Table S5). Plasmodium malariae was relatively rare in all four regions, with the highest prevalence recorded in Tanga (3.1%, n = 8/256) and the lowest in Geita (0.7%, n = 5/734; Additional file 1 : Table S5). Plasmodium ovale spp. prevalence was slightly higher than that of P. malariae (3.2–4.0%, n = 6/186–10/252) in all regions except Tanga (2.3%, n = 6/256; Additional file 1 : Table S5). There was significant variation (by ANOVA) in prevalence between regions for P. falciparum ( F (3,1424) = 6.47, P < 0.001) and P. malariae ( F (3,1424) = 2.87, P = 0.0351), but not for P. ovale spp. ( F (3,1424) = 0.43, P = 0.732).
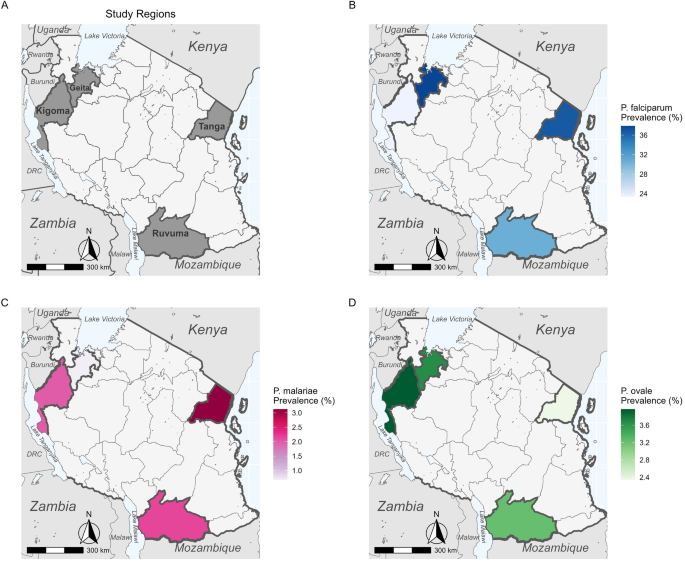
Maps of Tanzania showing the location of study regions ( a ) and the regional prevalence of Plasmodium falciparum ( b ), P. malariae ( c ) and P. ovale spp. ( d ). Plasmodium vivax was not detected in any sample and therefore was not mapped
While age was a significant (GLM: t (1427) = 23.7 , P < 0.001) determinant of P. falciparum infection, there was no significant effect of age for infection with either P. malariae or P. ovale spp. Age group was found to be a significant determinant of infection likelihood for both P. falciparum ( F (2,1418) = 4.92, P = 0.007) and P. malariae ( F (3,1418) = 3.28, P = 0.0381), but not for P. ovale spp. ( F (3,1418) = 0.857, P = 0.425) (Fig. 2 ). While children were significantly (Tukey HSD test: P < 0.05) more likely than adults to have P. falciparum infection, adults were more likely to have P. malariae infection (Tukey HSD test: P < 0.05; Fig. 2 ). There was no significant interaction between age group and region for either P. falciparum or P. malariae infection, but the interaction was nearly significant ( P = 0.055) for P. ovale spp. infection
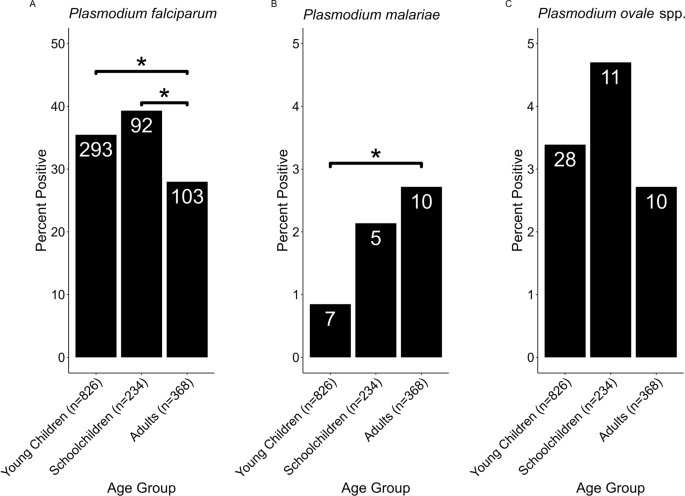
Tukey HSD analysis of malaria species prevalence by age group. A total of 826, 234 and 368 samples were positive for malaria infection in the age groups ‘young children’ (< 5 years), ‘school children’ (5–16 years) and ‘adults’ (> 16 years), respectively. The total number of samples per group for each species is shown on the X -axis under each bar, and the number of positive samples for each group is shown in each bar. a , b , c Prevalence by age group of P. falciparum ( a ) P. malariae ( b ) and P. ovale spp. ( c ). All comparisons marked with an asterisk were significant at the P < 0.05 level; all other comparisons were statistically non-significant
This study builds on previous research with school children and patients with a clinical diagnosis of malaria to describe the prevalence of different malaria species within four regions of Mainland Tanzania. Although P. falciparum is the most prevalent species in Mainland Tanzania, we found that the prevalence of both P. malariae and P. ovale spp. surpassed 3% in at least one region, and could increase as P. falciparum is locally eliminated, as has been seen with non-falciparum species in other contexts [ 1 , 4 ]. In contrast to a 2017 study involving school children, which found that the prevalence of P. ovale spp. was similar to that of P. falciparum [ 6 ], we found P. ovale spp. prevalence to be much lower than that of P. falciparum . In addition, the 2017 survey of school children [ 6 ] mostly identified P. ovale spp. as single-species infections and P. malariae as mixed infections with P. falciparum . In contrast, most of our samples with P. ovale spp. infection were mixed with P. falciparum , and we found similar proportions of single-species and mixed-species P. malariae infections. However, our sample sizes are small and may not necessarily be representative of the full picture, particularly in Geita where samples were only collected from children aged < 5 years. Also, our study included only four regions, of which only one (Tanga) overlaps with those included in the previous study, although we did include a wider age range.
In Tanga, we may have found lower P. ovale spp. prevalence than that reported in the previous study due to the inclusion of adults in our study, who are less likely to test positive for this species, whereas schoolchildren are a major asymptomatic infectious reservoir [ 17 , 18 , 19 ]; however, we did not replicate a significant difference in this study. In addition, the disruptions to malaria control caused by the COVID-19 pandemic, particularly in 2020–2021 [ 20 ], could have caused an increase in P. falciparum prevalence, which might account for the decreased prevalence of P. ovale if there is competition between the two species. Indeed, there was no difference in malaria prevalence in the villages of Magoda, Mamboleo and Mpapayu between 2019 (24.9%) and 2021 (24.5%; unpublished data). However, the prevalence dropped to 6.4% in 2022 following a return to the implementation of normal control activities (unpublished data), so further longitudinal studies may clarify the impact of resumed intense P. falciparum control. However, the malaria prevalence in Tanga dropped from 34.8% to 26.2% between 2020 and 2021 (unpublished data), so P. falciparum control in this region was likely effective during the course of this study, meaning that other factors, such as the inclusion of adults or rainfall levels, were likely a larger determinant of the lower P. ovale spp. prevalence in our study.
We did not find school children to be significantly more likely than adults or young children to test positive for either P. malariae or P. ovale spp. [ 12 ], but this trend, although not significant, was recorded for P. ovale spp. prevalence in this study. Therefore, the lack of significance in these species is likely an artifact of small sample sizes (Table 1 ; Fig. 2 , Additional file 2 : Fig. S1). Our finding that P. malariae was significantly more prevalent among adults likely reflects the presence of chronic infections that are more likely to be found in adults than in children due to the inherently larger number of infection opportunities [ 10 ].
Although P. falciparum remains the most prevalent species in these four regions, P. malariae and P. ovale spp. are present in all four regions, whereas P. vivax was not detected. Achieving malaria elimination in Tanzania will require ongoing surveillance of and targeted interventions for these species. While standard treatments successfully clear P. falciparum , the 3.4% of patients in this study with P. ovale spp. malaria infection may relapse. This study serves as a complement to previous studies focusing on school children and symptomatic patients and provides a full picture of the non-falciparum malaria landscape for communities in Mainland Tanzania. Ongoing analysis of samples collected in 2022 and 2023 will allow us to detect temporal trends in prevalence, and a forthcoming genomic analysis of P. malariae and P. ovale spp. isolates from Tanzania will inform our understanding of population structure and diversity in these species.
Availability of data and materials
Data are available upon reasonable request to the corresponding author.
WHO. World malaria report 2022. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022 . Accessed 24 Mar 2023
Akala HM, Watson OJ, Mitei KK, Juma DW, Verity R, Ingasia LA, et al. Plasmodium interspecies interactions during a period of increasing prevalence of Plasmodium ovale in symptomatic individuals seeking treatment: an observational study. Lancet Microbe. 2021;2:e141–50.
Article CAS PubMed PubMed Central Google Scholar
Betson M, Clifford S, Stanton M, Kabatereine NB, Stothard JR. Emergence of nonfalciparum Plasmodium infection despite regular artemisinin combination therapy in an 18-month longitudinal study of Ugandan children and their mothers. J Infect Dis. 2018;217:1099–109.
Yman V, Wandell G, Mutemi DD, Miglar A, Asghar M, Hammar U, et al. Persistent transmission of Plasmodium malariae and Plasmodium ovale species in an area of declining Plasmodium falciparum transmission in eastern Tanzania. PLOS Negl Trop Dis. 2019;13:e0007414.
Nguiffo-Nguete D, Nongley Nkemngo F, Ndo C, Agbor J-P, Boussougou-Sambe ST, Salako Djogbénou L, et al. Plasmodium malariae contributes to high levels of malaria transmission in a forest–savannah transition area in Cameroon. Parasit Vectors. 2023;16:1–10.
Article Google Scholar
Sendor R, Mitchell CL, Chacky F, Mohamed A, Mhamilawa LE, Molteni F, et al. Similar prevalence of Plasmodium falciparum and non- P falciparum malaria infections among Schoolchildren, Tanzania. Emerg Infect Dis. 2023;29:1143–53.
WHO. Global technical strategy for malaria 2016–2030. 2015. https://www.who.int/docs/default-source/documents/global-technical-strategy-for-malaria-2016-2030.pdf . Accessed 16 Jul 2021
Tarimo BB, Nyasembe VO, Ngasala B, Basham C, Rutagi IJ, Muller M, et al. Seasonality and transmissibility of Plasmodium ovale in Bagamoyo district, Tanzania. Parasit Vectors. 2022;15:56.
Article PubMed PubMed Central Google Scholar
Collins WE, Jeffery GM. Plasmodium ovale : parasite and disease. Clin Microbiol Rev. 2005;18:570–81.
Oriero EC, Amenga-Etego L, Ishengoma DS, Amambua-Ngwa A. Plasmodium malariae , current knowledge and future research opportunities on a neglected malaria parasite species. Crit Rev Microbiol. 2021;0:1–13.
Google Scholar
Roucher C, Rogier C, Sokhna C, Tall A, Trape JF. A 20-year longitudinal study of Plasmodium ovale and Plasmodium malariae prevalence and morbidity in a West African population. PLoS ONE. 2014;9:e87169.
Popkin-Hall ZR, Seth MD, Madebe RA, Budodo R, Bakari C, Francis F, et al. Malaria species positivity rates among symptomatic individuals across regions of differing transmission intensities in Mainland Tanzania. J Infect Dis. 2023. https://doi.org/10.1093/infdis/jiad522 .
Article PubMed Google Scholar
Rogier E, Battle N, Bakari C, Seth MD, Nace D, Herman C, et al. Plasmodium falciparum pfhrp2 and pfhrp3 gene deletions among patients enrolled at 100 health facilities throughout Tanzania: February to July 2021. medRxiv. 2023;223:S81.
Emerson C, Ulimboka S, Lemwayi R, Kinyina A, Nhiga SL, Aaron S, et al. Women attending antenatal care as a sentinel surveillance population for malaria in Geita region, Tanzania: feasibility and acceptability to women and providers. Malar J. 2023;22:1–10.
Gutman JR, Mwesigwa JN, Arnett K, Kangale C, Aaron S, Babarinde D, et al. Using antenatal care as a platform for malaria surveillance data collection: study protocol. Malar J. 2023;22:1–10.
Massicotte P, South A. rnaturalearth: world map data from natural earth. 2023. https://cran.r-project.org/web/packages/rnaturalearth/index.html . Accessed 7 Aug 2023
Abdulraheem MA, Ernest M, Ugwuanyi I, Abkallo HM, Nishikawa S, Adeleke M, et al. High prevalence of Plasmodium malariae and Plasmodium ovale in co-infections with Plasmodium falciparum in asymptomatic malaria parasite carriers in southwestern Nigeria. Int J Parasitol. 2022;52:23–33.
Andolina C, Rek JC, Briggs J, Okoth J, Musiime A, Ramjith J, et al. Sources of persistent malaria transmission in a setting with effective malaria control in eastern Uganda: a longitudinal, observational cohort study. Lancet Infect Dis. 2021;21:1568–78.
Walldorf JA, Cohee LM, Coalson JE, Bauleni A, Nkanaunena K, Kapito-Tembo A, et al. School-age children are a reservoir of malaria infection in Malawi. PLoS ONE. 2015;10:e0134061.
Liu Q, Yan W, Qin C, Du M, Liu M, Liu J. Millions of excess cases and thousands of excess deaths of malaria occurred globally in 2020 during the COVID-19 pandemic. J Glob Health. 2022;12:05045.
Download references
Acknowledgements
The authors wish to thank participants and parents/guardians of all children who took part in the surveillance. We acknowledge the contribution of the following project staff and other colleagues who participated in data collection and/or laboratory processing of samples: Raymond Kitengeso, Ezekiel Malecela, Muhidin Kassim, Athanas Mhina, August Nyaki, Juma Tupa, Anangisye Malabeja, Emmanuel Kessy, George Gesase, Tumaini Kamna, Grace Kanyankole, Oswald Osca, Richard Makono, Ildephonce Mathias, Godbless Msaki, Rashid Mtumba, Gasper Lugela, Gineson Nkya, Daniel Chale, Richard Malisa, Sawaya Msangi, Ally Idrisa, Francis Chambo, Kusa Mchaina, Neema Barua, Christian Msokame, Rogers Msangi, Salome Simba, Hatibu Athumani, Mwanaidi Mtui, Rehema Mtibusa, Jumaa Akida, Ambele Yatinga, and Tilaus Gustav. We also acknowledge the finance, administrative and logistic support team at NIMR: Christopher Masaka, Millen Meena, Beatrice Mwampeta, Gracia Sanga, Neema Manumbu, Halfan Mwanga, Arison Ekoni, Twalipo Mponzi, Pendael Nasary, Denis Byakuzana, Alfred Sezary, Emmanuel Mnzava, John Samwel, Daud Mjema, Seth Nguhu, Thomas Semdoe, Sadiki Yusuph, Alex Mwakibinga, Rodrick Ulomi and Andrea Kimboi. We are also grateful to the management of the National Institute for Medical Research, National Malaria Control Program and President's Office-Regional Administration and Local Government (regional administrative secretaries of the four regions, district officials and Community Health Workers from the four regions). Technical and logistics support from the Bill and Melinda Gates Foundation team is highly appreciated. The following reagents were obtained through BEI Resources, NIAID, NIH: Diagnostic Plasmid Containing the Small Subunit Ribosomal RNA Gene ( 18S ) from Plasmodium falciparum , MRA-177; Plasmodium vivax , MRA-178; Plasmodium malariae , MRA-179; and Plasmodium ovale , MRA-180, contributed by Peter A. Zimmerman. Permission to publish the manuscript was sought and obtained from the Director General of NIMR.
This work was supported, in part, by the Bill & Melinda Gates Foundation [grant number 002202]. Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission. Data collection in Geita was funded by USAID/PMI through Jhpiego and CDC. JJJ also received funding from NIH grant K24AI134990.
Author information
Authors and affiliations.
Institute for Global Health and Infectious Diseases, University of North Carolina, Chapel Hill, NC, USA
Zachary R. Popkin-Hall & Jonathan J. Juliano
National Institute for Medical Research, Dar es Salaam, Tanzania
Misago D. Seth, Rashid A. Madebe, Rule Budodo, Catherine Bakari, Dativa Pereus, Celine I. Mandara & Deus S. Ishengoma
National Institute for Medical Research, Tanga Center, Tanga, Tanzania
Filbert Francis
The Connecticut Agricultural Experiment Station, New Haven, CT, USA
David J. Giesbrecht
National Malaria Control Programme, Dodoma, Tanzania
Daniel Mbwambo, Sijenunu Aaron, Abdallah Lusasi & Samwel Lazaro
Department of Pathology and Laboratory Medicine, Warren Alpert Medical School, Brown University, Providence, RI, USA
Jeffrey A. Bailey
Center for Computational Molecular Biology, Brown University, Providence, RI, USA
Malaria Branch, National Center for Emerging and Zoonotic Infectious Diseases, US Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA
Julie R. Gutman
Harvard T.H. Chan School of Public Health, Boston, MA, USA
Deus S. Ishengoma
Faculty of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia
You can also search for this author in PubMed Google Scholar

Contributions
ZRPH, JAB, JJJ and DSI conceived the study. ZRPH performed computational and epidemiological analyses and wrote the manuscript. MDS, RAM, RB, CB and DP collected samples, extracted DNA and performed qPCR analysis. CIM, JAB, JJJ and DSI oversaw the project. FF and DJG contributed to data analysis. JRG and DSI oversaw data collection in Geita and assisted with the statistical analysis. DM, SA, AL and SL contributed data from NMCP and facilitated data collection. JAB, JJJ, JRG, MDS and DSI edited the manuscript. All authors read and approved the final manuscript.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
Corresponding author
Correspondence to Zachary R. Popkin-Hall .
Ethics declarations
Ethics approval and consent to participate.
The Molecular Surveillance of Malaria in Tanzania (MSMT) study protocol was approved by the Tanzanian Medical Research Coordinating Committee of the National Institute for Medical Research and involved approved standard procedures for informed consent and sample deidentification [ 13 ].
Consent for publication
Not applicable.
Competing interests
We declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: table s1..
Regional distribution of samples in random subset and full dataset. Table S2. Age group distribution of samples in random subset and full dataset. Table S3. Sex distribution of samples in random subset and full dataset. Table S4. Species positivity by region and age group.
Additional file 2: Figure S1.
Samples included in analysis by age group and region.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Popkin-Hall, Z.R., Seth, M.D., Madebe, R.A. et al. Prevalence of non- falciparum malaria infections among asymptomatic individuals in four regions of Mainland Tanzania. Parasites Vectors 17 , 153 (2024). https://doi.org/10.1186/s13071-024-06242-4
Download citation
Received : 29 December 2023
Accepted : 11 March 2024
Published : 23 March 2024
DOI : https://doi.org/10.1186/s13071-024-06242-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Plasmodium malariae
- Plasmodium ovale
- Plasmodium vivax
- Non-falciparum species
- Asymptomatic malaria
Parasites & Vectors
ISSN: 1756-3305
- Submission enquiries: [email protected]
- Open access
- Published: 19 January 2023
Prevalence of malaria and associated clinical manifestations and myeloperoxidase amongst populations living in different altitudes of Mezam division, North West Region, Cameroon
- Ntonifor Helen Ngum 1 , 2 ,
- Ngahbort Belthine Fakeh 1 ,
- Abongwa Edith Lem 1 , 2 &
- Oumar Mahamat 1
Malaria Journal volume 22 , Article number: 20 ( 2023 ) Cite this article
4405 Accesses
3 Citations
2 Altmetric
Metrics details
Malaria is a growing problem in Africa, with prevalence varies from areas to areas due to several factors including the altitude. This study aimed to investigate the malaria distribution and its relationship with level of some blood parameters and plasma myeloperoxidase (MPO) in population of three localities with different altitudes.
A total of 150 participants were recruited in each locality and facial body temperature of each was measured using a forehead digital thermometer. Blood samples were collected and used diagnose malaria parasite using the rapid test followed by Giemsa stain microscopy and have the full blood count and MPO level using a colorimetric method.
The overall prevalence of falciparum malaria was 34.7%, with no difference between the three communities, but Bambili of high altitude had the highest prevalence (70.7%). A majority of the infected persons had mild malaria, with most cases being asymptomatic (temperature < 37.5 ºC). Patients had significant increase of geometric mean malaria parasite density (GMPD) in Bambili (1755 ± 216 parasites/µL) and Bamenda (1060 ± 2515 parasites/µL of blood) than patients in Santa (737 ± 799 parasites/µL). There was a significant risk to have malaria infection in Bambili (OR = 33.367, p = 0.021) than in Santa (OR = 2.309, p = 0.362). Bambili’ participants of 6–10 years showed a high prevalence of malaria (85.7%). GMPD was significantly different between males (p = 0.010) as well as females (p = 0.000). Participants from Santa (11.2 ± 3.2 g/dL) and Bambili (12.6 ± 2.4 g/dL) had a high haemoglobin concentration than those from Bamenda (10.6 ± 2.1 g/dL). There was a significant difference in the WBC counts and platelet counts among infected participants in the study areas. MPO level had an increasing trend among infected participants in Santa (2.378 ± 0.250), Bambili (2.582 ± 0.482) and Bamenda (2.635 ± 0.466).
The results of the present study demonstrated that altitudinal variations significant impact the risk of population to have malaria with high parasitaemia and may contribute to the malaria prevalence and severity by affecting the haemoglobin concentration, WBC and platelet level and plasma MPO in population.
Plasmodium falciparum is the most virulent and deadliest parasite of the human Plasmodium species that causes falciparum malaria [ 1 ]. This disease is highly prevalent in tropical regions especially sub-Saharan Africa (SSA) and the prevalence in Cameroon has been reported to be about 30% [ 2 ]. The occurrence and transmission of malaria parasite in the tropics is influenced by climatic conditions such as temperature and humidity. Changes in altitudinal gradient and attitude towards the use of insecticide-reated bed nets (ITNs) are some underlying factors contributing to the heterogeneity of malaria distribution in endemic communities. The use of ITNs is a World Health Organization (WHO) policy for malaria morbidity control [ 1 , 2 ] and human attitude towards the use of ITNs affects the morbidity and mortalities associated with the disease. Some studies have reported high malaria transmission in lower altitudinal regions in Cameroon [ 3 ], while other studies were of the opinion that altitudinal changes influenced the distribution and density of malaria parasitaemia [ 4 , 5 , 6 ]. However, these data are not enough to reach a general conclusion about the altitudinal implication of malaria transmission and distribution in Cameroon due to lack of data points in other endemic areas of the country with apparent variation in altitude and other related climatic factors like temperature and humidity. The availability of data from poorly explored areas in the country is important in reaching a general conclusion about the dynamics of malaria transmission and distribution based on altitudinal differences.
Haemoglobin levels, white blood cell, and platelet counts are frequently affected by malaria infection, they correlate with the pathological effects of malaria parasite in humans [ 7 ]. Fluctuating levels of these haematological parameters have been suspected to vary among individuals and people in different altitudinal gradient living with malaria parasite. Data related to this view is limited to the South Western Regions of Cameroon mostly along the slope of Mount Cameroon [ 5 , 8 , 9 , 10 ].
In addition, immunity against malaria parasite involves a cascade of immune responses including cellular immunity. One important molecule implicated in cellular innate response against invading pathogens is myeloperoxidase (MPO). This is a leucocyte derived enzyme released mostly by neutrophils and monocytes [ 11 ] that has been implicated in the formation of leucocyte derived-reactive oxygen species (ROS) reported to be potent against malaria parasite [ 12 ]. The direct role of MPO in malaria is still unknown [ 13 ], but it is suspected that MPO plays an indirect role in the clearance of malaria parasite through activation of neutrophils and promoting the recruitment of neutrophils and macrophages leading to enhanced proinflammatory response [ 14 , 15 ] against the parasites.
Information on the distribution of malaria parasites in highlands, semi-highlands, and lowland areas of Mezam division is unknown. Therefore, this study aimed to determine the prevalence of malaria infection using a comprehensive laboratory approach and to identify associated clinical symptoms among a population of different altitudes in the division.
Ethics statement
An administrative authorization for the study was obtained from the Regional Delegate of Public Health for the North West Region. Ethical clearance was obtained from the Institutional Review Board of the Faculty of Health Sciences, The University of Bamenda (N0:2020/0224H/UBa/IRB). The purpose of the study was fully explained to the participants before they took part in the study. They were expected to give their signed informed consent void of coercion. Patients’ results were kept confidential and positive cases referred to meet a medical doctor for treatment (Additional file 1 ).
The study was carried out in Bamenda, Bambili, and Santa localities all found in Mezam division of the North West Region of Cameroon. The North West Region lies along the Western highland area of Cameroon experiencing variations in altitude and climatic conditions, and Mezam just like other divisions in Cameroon is endemic for malaria. Cartographic mapping of Mezam (Arte cartogis Nkwen-Bamenda) presents these three localities with varied climatic and geographic settings. Bamenda has higher environmental temperatures (21.1–22 °C) compared to Bambili (16.1–20.0 °C) and Santa (17.1–20.0 °C). The 3 localities experience the same annual rainfall of about 2001 to 2201 mm per year but the Bambili is located at a higher altitudinal gradient (1600–2, 400 m) compared to Bamenda (1201–1400 m) and Santa (1801–2000 m) [ 16 ]. This cosmopolitan city having a population of above 39,000 inhabitants is found on a latitude of 10 0 10′0''E and longitude 5 0 56′0''N. Bambili is a village in the Tubah Sub Division and a student residential area located on latitude 10 0 13′0''E and longitude 6 0 0′0''N, while Santa characterized by rural and semi urban settings is located on latitude 10 0 12′0''E and longitude 5 0 48′0''N (Fig. 1 ). The main economic activity in the rural areas of Mezam is farming while the semi urban and urban areas are commercial centres.
Map of Bambili, Bamenda and Santa in the Mezam Division (February, 2021). A Location maps. B Map showing climatic conditions of the study area. C Map showing altitudinal differences between the study areas
Study design and period
The study was a hospital based cross-sectional survey conducted in three major health facilities (the Bamenda Regional Hospital, Santa District hospital and the Bambili Community Health Centre) between November 2020 and March 2021. The study involved both laboratory and questionnaire evaluation. The questionnaires were used to collect medical history and demographic information of the participants (Additional file 2 ). Venous blood was collected from consented participants for parasitological, immunological and haematological analysis. This study involved outpatients of both sexes that were three months and older attending malaria consultation in the above health facilities.
Sample size and sampling technique
The population sample size was estimated using current proportion of malaria prevalence in Cameroon of 30.3% [ 17 ] at 95% confident interval (Z-score = 1.96) using the Cochran formula [ 18 ]. The calculated population sample size (323 participants) was adjusted by 40% to 450 participants. The adjusted population was further divided by three based on the three study areas and equal number of participants (150 patients) recruited from each community.
Participants were natives and visitors that had resided in the selected communities for at least three months. All participants gave their written and signed informed consents free of incentives before taking part in the study. Parents and guardians signed the informed consent for minors authorising them to take part in the study. Immunocompromised patients (HIV patience), pregnant women and patients with haemoglobinopathies (sickle cell patients, haemophiliacs, sickle cell carriers), and those on malaria treatment or had taken malaria treatment in the last one month were disqualified from the study.
Data collection
A well-structured questionnaire written in English language was used to screen and collect demographic information (sex, age, and residency) about the participants. At enrolment, patients facial skin temperatures were measured using an infrared forehead digital thermometer (HTD8813C, Hetaida Technology Co. Ltd, China) and the results recorded in degree Celsius. Pyrexia related to malaria was defined as temperatures > 37.5 0 C [ 19 ].
Laboratory sample collection and malaria diagnosis
From each recruited patient, at least 2 mL of venous blood was collected into Ethylenediaminetetraacetic Acid (EDTA) tubes using a vacutainer and transported in a safe flask to the parasitology laboratory of the health facility in the various study areas. Recruited participants were first screened for malaria parasite using a rapid diagnostic test kit (Care Start MR19B69, China) conjugated with P. falciparum histidine protein II (PfHRP II) for specific identification of P. falciparum .
For microscopic examination, both thin and thick blood films were prepared on labelled grease free glass slides using 1µL of blood from each EDTA test tube. The thin films were fixed using methanol for 10 s. Later, both films were stained using 10% Giemsa for 10 min, then carefully washed and the slides air dried using a hair dryer (Xtava, Allure Co. Ltd, USA). The oil immersion (× 100) objective of a light microscope was used to observe the slides. The bench aid for the diagnosis of malaria parasite was used for the identification and confirmation of any malaria parasite [ 20 ]. Malaria parasitic species were appreciated and confirmed on the thin films while the thick films were used for the determination of malaria parasite density per microlitre (µL) of blood. Blood stages (trophoziotes) of the parasite were counted against 200 white blood cells (WBCs) assuming total WBC count of 8, 000 cells/µL of blood [ 21 ]. Up to 100 high power fields were observed before declaring a slide negative. The malaria parasite (MP) density was further classified as mild (MP < 1000/µL of blood), moderate (MP between 1000 and 4999/µL of blood) and severe (MP ≥ 5000/µL of blood) [ 5 ]. The density of the parasite was calculated as follows:
Parasite density = [counted parasites/total WBC counted (200)] x [ 8000WBCs].
Determination of haemoglobin concentration, total white blood cells and platelet counts
Haemoglobin concentration, total WBCs and platelet counts were obtained after performing complete blood count using a haematological analyser machine (URIT-3300, Urit medical Electronic Company Ltd China), following the protocol of the manufacturer. Blood cell indices such as haemoglobin concentration, total platelets count, WBC count were recorded. Haemoglobin concentration < 11 g/dL of blood was used to define anaemia in the patient. Anaemia was further classified as mild (Hb 10.0–10.9 g/dL), moderate (Hb 7–9.9 g/dL) and severe (Hb < 7 g/dL) [ 21 ]. WBC count of > 10,000 cells/μL was classified as Leucocytosis while Leucopenia was WBC count < 4000 cells/μL.
Assessment of plasma myeloperoxidase level
Myeloperoxidase was assessed using a colometric method as adopted by Oumar et al. [ 22 ] with a few modifications. Briefly, one hundred microlitre of phenylenediamine solution and 0.002% hydrogen peroxide in phosphate-citrate buffer (P H = 5.0) were mixed with equal volumes (100 µL) of serum on a microplate. The reaction was stopped after 10 min using 0.1 M sulphuric acid and absorbance was recorded at 490 nm using an ELISA reader (ELISA analyzer, Urit-660, China).
Statistical analysis
The cleaned data was uploaded into the statistical package for social sciences (SPSS) version 23 (IBM SPSS Inc. Chicago, IL, USA) for statistical analysis (Additional file 3 ). The Pearson Chi-Square test was used to explore proportions between groups. One Way ANOVA (Analysis of Variance) and the independent student t-test or the Turkey HSD was used as a post hoc test for multiple comparisons of means between variables such number of blood cells, haemoglobin level and myeloperoxidase level. The Mann–Whitney U test and Kruskal–Wallis Test were used to analyse the mean differences between groups for non-parametric data such as prevalence. The binary logistic regression with non-adjusted odd ratios was used to evaluate some predictors of malaria in the study. The malaria parasite densities were log transformed in base 10 before applying the ANOVA and Turkey HSD multi-comparison tests to explore the difference in the means of the malaria parasite densities between the geographic areas. The cut off point for statistical significance between groups was set at probability level p ≤ 0.05.
Baseline characteristics of the study population
A total of 450 patients were recruited into the study and females recorded the highest participatory rate in all the study areas [60.9% (274/450)]. Most participants [27.6% ( \(124/450)]\) were between the ages of 21–35 years (Table 1 ) and at enrolment, majority of patients in all the study areas were afebrile [88.2% ( \(397/450)]\) with just 11.8% (53/450) of the total population presenting with pyrexia and patients in Bamenda recording the highest pyrexia rate [16.7% ( \(25/150)]\) . The overall mean temperature was 37.0 ± 0.5 0 C. About 19.3% ( \(87/450)\) of the entire population was anaemic and patients in Bamenda recorded more cases of anaemia [28.7% ( \(43/150)]\) .
Spatial distribution of malaria infection
Out of the 450 participants in the study, 156 tested positive for malaria giving a prevalence of 34.7% (Table 2 ). The distribution of Plasmodium spp between the three geographical areas was significantly different (χ 2 = 103.26; p = 0.016). Patients from Bambili registered more positive cases of malaria [70.7% ( \(106/150)]\) while Santa had the least [6.7% \((10/150)]\) . Out of the 43,7% infected patients in the three localities, 87.2% ( \(136/156)\) had asymptomatic malaria though the differences were not statistically significant (p = 0.169) when compared to asymptomatic cases in the three localities. Within the localities, only in patients from Santa, the prevalence of asymptomatic malaria was significantly higher than symptomatic malaria (χ 2 = 10.000, p = 0.019 ). The results (Table 2 ) showed that there was no significant difference in malaria severity level between the different localities, most patients in Bamenda [65.0% (26/40)] and Santa [80.0% (8/10)] were mildly infected with malaria, while in Bambili, the prevalence of moderate malaria [64.4% [ \(68/106)]\) was the highest. Patients in Bambili and Bamenda significantly (F = 6.065; p = 0.003 ) had higher geometric mean parasite density (GMPD ± SEM) of 1755 ± 216 µL and 1060 ± 2515 µL of blood) respectively compared to patients in Santa (Table 2 ).
Relationship between altitude and the prevalence of malaria in the different geographical settings
Table 3 presents the risks to have malaria in Santa and Bambili with reference to the infection in Bamenda. There was a significant risk to have malaria infection in Bambili (OR = 33.367, 95%C. I: 1.691–658.411, p = 0.021) while the risk to have malaria in Santa was not statistically significant (OR = 2.309, 95%C. I: 0.382–13.958, p = 0.362).
Influence of sex and age on the prevalence and density of malaria parasite in the different geographical settings
Table 4 shows the age and gender base prevalence of Malaria in the study areas. There were no significant difference in the distribution of malaria between males and females in each geographical setting, although males recorded a higher prevalence of malaria in the entire study [38.6% (68/176)] than females [32.1% (88/274)]. Heightened malaria prevalence was observed among males in Bamenda [33.8% (22/65)] and Santa [11.1% (6/54)], while in Bambili the prevalence was higher in females [71.0% (66/93)]. There was a sound significant difference (Mann Whitney U test, p = 0.007 ) in the GMPD ± SEM between males and females in the entire study. The density of malaria parasite was higher in females GMPD ± SEM (1691 ± 265 µL) than males (1205 ± 1487 µL). Following the different areas, a significant (Mann Whitney U test, p = 0.009 ) difference was observed only in Bambili, with high prevalence in females (2088 ± 305 µL) than males (1317 ± 237 µL). There was a high significant difference in the mean malaria parasitaemia in each sex group across the three study areas with male patients in Bamenda and Bambili recording higher values (Kruskal Wallis test , p = 0.010 ) than male patients in Santa. Conversely female patients in Santa and Bambili recorded significantly (Kruskal Wallis test , p = 0.000 ) higher parasitaemia than female patients in Bamenda (Table 3 ).
There was a significant distribution of P. falciparum between age groups in the overall study (χ 2 = 267.677, p = 0.027 ). In the different study areas, Bambili had a significant difference in prevalence between the age groups (χ 2 = 238.715 , p = 0.053 ), with patients 6–10 years old showing higher prevalence. For each age group across the three study areas, only patients aged 36–60 years old recorded significant distribution of malaria parasite (χ 2 = 38.513 , p = 0.054 ) with patients in Bambili having 70.0% (14/20), Bamenda 24.4% (11/45), and Santa 5.6% (2/36) Table 4 .
Influence of malaria infection on the haematological symptoms in the population
As shown on Table 5 , the haemoglobin concentration levels of patients residing in Bamenda were lower (11.3 ± 3.4 g/dL) compared to those of Santa and Bambili who recorded significant (F = 1.875, p = 0.145 svy ) higher mean (± SD) haemoglobin concentrations of 11.9 ± 1.5 g/dL and 11.8 ± 3.5 g/dL of blood respectively, even though the post hoc test did not show any significant difference (p = 0.145 svy ). In patients with malaria, a significant difference was observed in Hb in the three study areas (p = 0.004). Patients that tested positive for P. falciparum in Bamenda had lower mean haemoglobin concentrations (Hb 10.6 ± 2.1 g/dL) compared to patients’ resident in Bambili (Hb 12.6 ± 2.4 g/dL). A significant difference was also observed between Hb in the malaria negative patients (p = 0.001 jmp ). Patients that tested negative for P. falciparum in Bambili had lower Hb (Hb 9.9 ± 4.7 g/dL) as compared to patients in Bamenda (Hb 11.5 ± 3.7 g/dL, p = 0.843) and Santa (Hb 11.9 ± 1.3 g/dL, p = 0.000). There was a significant difference in the haemoglobin concentration levels between patients that were positive for P. falciparum as compared to those that tested negative in Santa (t = 1.544, p = 0.000 ) and Bambili (t = − 4.601, p = 0.000 ).
There was equally a significant (F = 87.390, p = 0.000 twz ) difference in the mean white blood cell count among patients resident in the different geographical study areas. Participants in Santa (9.2 ± 3.8 × 10 3 µL, p = 0.000 tw ) and Bamenda (6.1 ± 3.2 × 10 3 µL, p = 0.000 tz ) had higher mean WBC counts than patients in Bambili (4.6 ± 2.1 × 10 3 µL, p = 0.000 wz ). There was a significant difference in the level of WBC in both positive (F = 13.987, p = 0.000 beh ) and negative (F = 49.149, p = 0.000 knq ) patients in all the three study areas. In the positive patients, the WBC level was higher in Santa as compared to Bambili (p = 0.000 eh ) and Bamenda (p = 0.006 eb ). It was also observed that patients from Bamenda had higher level of WBC count compared to those from Bambili (p = 0.018 bh ). Only patients in Santa showed a significant difference (t = − 0.544, p = 0.042 ) in the mean WBC count between patients that tested positive (9.6 ± 6.9 × 10 3 µL) and negative (9.2 ± 3.5 × 10 3 µL for P. falciparum (Table 5 ).
In addition, the results showed a significantly (F = 117.565, p = 0.000 ux σ ) increasing trend in the mean platelet count among patients in Santa (253 ± 99 × 10 3 µL), Bamenda (338 ± 129 u × 10 3 µL) and Bambili (465 ± 132 × 10 3 µL). A similar trend was observed among patients that were positive (F = 20.508, p = 0.000 cfi ) for P. falciparum and among those that were negative (F = 85.828, p = 0.000 lor ) between the study areas (Table 5 ). The post hoc analysis also revealed significant variations in the mean platelet count within the localities among those that had malaria and among those that were not infected (Table 5 ). There were significant differences in the mean platelet counts between patients that were positive for malaria parasite and those that were negative in Bamenda (t = 2.114, p = 0.031 ), Santa (t = − 1.090, p = 0.000 ) and Bambili (t = − 0.027, p = 0.006 ).
Influence of age and sex on the occurrence of anaemia in the population
Out of the 450 patients examined, 87 (19.3%) of them were anaemic and there was a significant (χ 2 = 93.460, p = 0.004 ) variation in anaemia prevalence based on geographical location. Patients from Bamenda recorded the highest prevalence (28.7% [ \(43/150])\) and the least was among patients in Santa (14.0% [ \(21/150])\) . Females of the three study areas showed a significant difference in the prevalence of anaemia (χ 2 = 58.346, p = 0.006 ) with females in Bamenda recording the highest (25.9 [ \(22/85\) ]) prevalence of anaemia compared to Santa and Bambili (Table 6 ). Males however recorded a significantly (χ 2 = 45.765, p = 0.033 ) higher prevalence of anaemia (21.6%) than females (17.9%) in the whole study. Males in Bamenda significantly (χ 2 = 33.662, p = 0.053 ) registered a higher prevalence 32.3% ( \(21/65\) ) of anaemia when compared to females (25.9% [ \(22/65\) ]). With respect to age, there was a significant difference in anaemia prevalence in the entire study (χ2 = 240.679, p = 0.000).
Patients that were ≤ 5 years old and those of 61 years and above registered significantly high anaemia prevalence of (23.8% [ \(25/105\) ]) and (25.9% [ \(22/65\) ]) respectively compared to other age groups in the entire study. A significant difference between age groups was also observed only in Bamenda where patients aged 0–5 years (32.6% [ \(14/43\) ]) and 36–60 years (35.6% [ \(16/45\) ]) were significantly (χ 2 = 164.735, p = 0.001 ) more anaemic.
Influence of malaria status and severity on the level of plasma myeloperoxidase in the different geographical settings
As shown on Table 7 , irrespective of the study area, patients who tested positive for P. falciparum had significantly (t = − 2.803, p = 0.003 ) higher mean myeloperoxidase levels (2.554 ± 0.437) than patients who were negative (2.117 ± 0.862). With respect to study areas, malaria positive and negative patients showed a significant difference in MPO (p = 0.003). Participants in Santa who tested positive for P. falciparum had highly significant (t = − 2.056, p = 0.000 ) MPO levels (2.378 ± 0.250) than participants that tested negative (1.721 ± 0.980).
Comparing the levels of MPO between the study areas, there was a significant variation (F = 7.703, p = 0.001 ghi ) in the MPO levels. The MPO level was significantly lower in Santa (2.007 ± 0.812), as compared to Bamenda (2.604 ± 0.426 g ) and Bambili (2.595 ± 0.461 i ). In malaria positive patients, there was no significant difference (F = 1.112, p = 0.338 abc ) in MPO when comparing the three different study areas. In patients who tested negative for P. falciparum, there was a significant variation (F = 3.734, p = 0.041 def ) in the mean MPO levels among patients in the different geographic area, with a low MPO in participants of Santa than those of Bamenda and Bambili, but the difference was significant only in Bamenda (p = 0.05 de ). Independent of the geographic areas, there was a significant difference between patients with mild, moderate and severe malaria (F = 7.703, p = 0.001 ), but no difference was observed with each area with respect to the malaria categories as well as when comparing participants of the different study areas within the same malaria severity level.
The study aimed at assessing the influence of altitude on malaria prevalence, parasitaemia and comorbidity. The study was carried out in three different localities: Santa, Bambili and Bamenda with altitudes of 1801–2000 m, 1600–2400 m and 1201–1400 m above sea level, respectively. The overall prevalence of malaria among participants in the entire study was 34.7%. A similar community based study conducted along the slope of mount Cameroon in the South West Region showed lower (33.8%) results [ 5 ]. These results were also higher than the national prevalence (30.3%) of malaria in Cameroon [ 2 ]. The overall prevalence of malaria was significant different among participants in Santa, Bamenda, and Bambili with 6.7%, 26.7%, and 70.7% respectively. This variation in malaria prevalence may be due to the differences in the target population notably their knowledge of the disease, but not due to the apparent difference in altitude of the three localities. This was in contrast to results of similar studies conducted in Tanzania [ 4 ] and in the South West Region of Cameroon [ 5 ] that reported high prevalence and transmission of malaria in lowland areas compared to high land areas. The three localities in which this study was conducted are found in the Western Highland area of Cameroon. Though these localities show some variations in climatic conditions (altitude, environmental temperatures), they however experience common intersperse topographic features with overlapping climatic conditions that apparently show no clear cut stiff altitudinal gradient between them. This may be insignificant to influence a bias in the distribution and abundance of the mosquito vector for malaria parasite between these communities hence possibly explain the absence of direct correlation between the malaria prevalence among the populations and the altitude of these localities. However, patients in Bambili of a higher altitude than Bamenda, recorded a higher prevalence of malaria than patients in Santa and this had a similar outcome with the results of [ 23 ] in the South West Region of Cameroon that reported higher prevalence of malaria among populations of higher altitudes. This might suggest that changes in environmental conditions and human activities that favour the adaptation and development of the mosquito vector in higher land areas, Bambili is also a student residential area that is currently experiencing a population boom. Also, Bamenda city that is a transit to this community is marked with social activities in the region coupled with the influx of inhabitants from the different villages in the North West Region due to the Socio-political crises. The attitude of the inhabitants such as poor dressing exposing body parts to the bite of mosquitoes, and keeping late evening hours drinking that coincide with the biting period of mosquitoes maybe contributing factors to the high prevalence of malaria in these communities irrespective of their apparent altitudinal differences.
The malaria parasite density in population in the different areas was significantly different in males as well as in females. In males, the density of malaria parasite was significantly lower in Santa compared to that in Bamenda and Bambili which did not showed a difference. In contrast, in females, Bamenda, localities of low altitude had a low parasite density, followed by Santa and Bambili (locality of high altitude). In female, the results quietly indicate that the altitude may affect the density of parasite in the population. Therefore, the absence of relationship between the malaria parasite density and the altitude of the living localities may due the habit of the males as due to the crisis in the region, males are forced to change regularly the house within the three localities.
Malaria was significantly more prevalent among patients that were 6–10 years in the entire study. Reports of similar studies conducted in Tanzania [ 24 ] and elsewhere in Cameroon [ 23 ] indicated patients of similar age groups with higher prevalence of malaria, respectively. Though the development of immunity to malaria is highly associated with frequency of exposure to the malaria parasite that is independent of altitude, there may be a switch in the age group of highest risk of malaria infection. This may also be due to over focused attention on malaria management among children 0–5 years. Across the three study areas (Bamenda, Santa and Bambili), there was no significant difference in the prevalence of malaria following the age categories. However, the parasite density in population was significantly different among children 0–5 years and adult of 36–60 years in the different localities. The general live condition of participants like type of farming, humidity etc. which vary following the altitude could possibly be the cause of this difference of malaria parasite density among people. Population living in Bambili of high altitude had high malaria parasite density. Majority of the patients in Santa, Bamenda, and in the entire study population suffered mild malaria infection with the exception of patients in Bambili where they were moderately infected. This suggests a possible impact of altitude on malaria severity and the results in the lowland areas of Bamenda were in conformity with a similar study in Tanzania that showed mild malaria prevalence among children in lowland area [ 23 ]. In addition, majority of the patients that tested positive for P. falciparum in the entire study were asymptomatic. An experimental study associated the expression of asymptomatic falciparum malaria and related severities with a switch in the host pro-inflammatory immune response against malaria parasite toward anti-inflammatory response [ 24 ]. This may lead to host tolerance to P. falciparum and possible suppression of the clinical presentation of malaria defined by temperature ≥ 37.5 0 C. This could further explain the significantly higher malaria parasitaemia observed among patients in Bambili and Bamenda though there was no significant variation in the mean malaria parasite density between these localities including the highland area of Santa. Malaria parasitaemia was significantly higher in females than in males in the entire study population. Considering the geographic settings of the study areas, male patients in Bamenda and Bambili recorded significant higher malaria parasitaemia than males in Santa. This similar trend was observed among females where those in Bambili and Santa had higher malaria parasitaemia than females in Bamenda. This suggests a possible gender based impact of altitude and climatic conditions on falciparum malaria parasitaemia.
It was observed that altitudinal variations significantly influenced the distribution of haemoglobin concentration, white blood cells and platelet counts among the populations living in the three different localities. The results revealed that people living in the Santa and Bambili highland areas had higher haemoglobin concentrations than in the Bamenda lowland area. These results were in conformity with reports of a study in the Middle East [ 25 ] that reported higher haemoglobin concentrations among people living at higher attitudes than in people in lowland areas. Generally, patients that tested positive for P. falciparum in the entire study registered lower haemoglobin concentration than patients that were negative and falciparum malaria patients in Bamenda were presented with mild anaemia as a result of lower haemoglobin concentration than patients in Santa and Bambili. Similar observations were recorded among malaria patients in Myanmar [ 20 ], the South West Region of Cameroon [ 22 ] and in Bamenda [ 26 ]. Females and patients 0–5 years significantly recorded high prevalence of anaemia than males and other age groups in all the study areas. This could be related to the biological development of the malaria parasite in parasitized red blood cells that correlated with the high burden of the disease among patients and the monthly physiological loss of blood in females leading to lower haemoglobin concentration in the infected populations. This leads to the suggestion that P. falciparum could be a contributing factor to the burden of anaemia especially in Bamenda and elsewhere.
Participants in Santa recorded higher WBC counts than those in Bamenda and Bambili. There was a similar trend among patients that tested positive for P. falciparum and infected patients also showed elevated levels of WBCs compared to non-infected participants. Though there was a significant increase in leucocyte levels among malaria patients in Santa, the mean WBCs level in all the populations of the different study areas were within normal and below the upper limit of normal range irrespective of their malaria status hence leukocytosis was absent in either of the populations. This suggests that altitudinal variation affects the level of WBCs among malaria patients but may not lead to serious pathologies associated with malaria infection. These suggestions coincide with results of [ 25 ] in the Middle East but conflicting with those of [ 5 ] in Cameroon where falciparum malaria patients were subjected to leucopoenia. This could be related to variations in the measurement scale and cut off points to define pathologies related to abnormal WBC counts in the study designs. In addition, platelet cell counts were significantly higher among malaria patients in highland areas than in lower land areas. Generally, malaria patients in all the study areas had significantly elevated platelet counts compared to non-infected participants. This was contrary to the reports of some studies [ 5 ] that associated malaria with platelet reduction (Thrombocytopenia).
Altitude had a significant impact on the serum myeloperoxidase (MPO) levels among patients infected with P. falciparum across the different study areas. Malaria patients in Bamenda and Bambili recorded higher MPO levels than patients in Santa. With regards to the study areas, patients that tested positive for P. falciparum had elevated levels of MPO than non-infected patients. The implication of MPO in falciparum malaria severity is not well elucidated but [ 13 ] reported that myeloperoxidase production increased during malaria parasite infection and correlated it with parasite clearance. This could possibly explain why majority of the infected patients in this study experienced mild and moderate malaria infection and variation in MPO levels could possibly be used as biomarker for investigating malaria infection in Mezam.
ITNs non-users, increase levels of WBCs and platelet counts were significant predictors of falciparum malaria. Patience presented with pyrexia, anaemia and increased levels of serum MPO levels had increased likelihood of malaria infection. This suggests that these parameters could be used as significant markers of P. falciparum .
Prevalence of malaria infection was high and its distribution varied across altitudinal gradients in Mezam Division, Cameroon. The altitudinal variations had a significant impact on malaria parasitaemia. Clinical symptoms including haemoglobin concentration, WBC counts, platelet cell count and level of plasma MPO in malaria infected patients were significantly related to the altitudes. Therefore, significant attention should be given to malaria infection for both its proper diagnosis and treatment to prevent infection in Mezam Division. The role of environmental factors other than altitudes that facilitate the existence and transmission of malaria infection should also be further investigated.
Availability of data and materials
All datasets on which the conclusions of the manuscript rely are presented in the paper.
CDC. Malaria. Atlanta, Centers for Disease Control and Prevention, 2020. Available from: https://www.cdc.gov/parasites/malaria/index.htmlreviewed 12/05/2020
WHO. WorldMalaria Report 2020.Geneva, World Health Organization;2020. Available from: http//www.who.int/malaria/publications/world-malaria-report-2020
Wanji S, Tanke T, Sali N, Atanga N, Ajonina C, Tendongfor N, et al. Anopheles species of the Mount Cameroon Region: biting habits, feeding behaviour and entomological inoculation rates. Trop Med Int Health. 2003;8:643–9.
Article Google Scholar
Maxwell C, Chambo W, Mwaimu M, Magogo S, Carneiro I, Curtis C. Variation of malaria transmission and morbidity with altitude in Tanzania and with introduction of alphacypermethrin treated nets. Malar J. 2003;2:28.
Kimbi HK, Sumbele I, Malaika N, Anchang J, Lum E, Nana Y, et al. Malaria and haematologic parameters of pupils at different altitudes along the slope of Mount Cameroon: a cross-sectional study. Malar J. 2013;12:193.
Omondi C, Onguru D, Kamau M, Lucy N, Mark OG, Estambale B. Perennial transmission of malaria in the low altitude areas of Baringo County. Kenya Malar J. 2017;16:257.
Maina W, Njenga E, Muchemi E. Knowledge, attitude and practices related to diabetes among community members in four provinces in Kenya: a cross-sectional study. Pan Afr Med J. 2010;7:15–8.
Google Scholar
Nkuo-Akenji TN, Sumbele I, Mankah EN, Njunda AL, Samje M, Kamga L. The burden of malaria and malnutrition among children less than 14 years of age in a rural village of Cameroon. Afr J Food Sci. 2008;8:252–326.
Wanji S, Akotshi DO, Mutro MN, Tepage F, Ukety TO, Diggle PJ, et al. Validation of the rapid assessment procedure for Loiasis (RAPLOA) in the Democratic Republic of Congo. Parasit Vectors. 2012;5:25.
Antonio-Nkondjio C, Ndo C, Njiokou F, Bigoga JD, Awono-Ambene P, Etang J, et al. Review of malaria situation in Cameroon: technical viewpoint on challenges and prospects for disease elimination. Parasit Vectors. 2019;12:501.
Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vascul Biol. 2005;25:1102–11.
Article CAS Google Scholar
Clark IA, Cowden WB, Butcher GA, Hunt NH. Possible roles of tumor necrosis factor in the pathology of malaria. Am J Pathol. 1987;129:192–9.
CAS Google Scholar
Theess W, Sellau J, Steeg C, Klinke A, Baldus S, Cramer JP, et al. Myeloperoxidase attenuates pathogen clearance during Plasmodium yoelii nonlethal infection. Infect Immun. 2016;85:e00475-e516.
Lau D, Mollnau H, Eiserich JP, Freeman BA, Daiber A, Gehling UM, et al. Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. Proc Natl Acad Sci USA. 2005;102:431–6.
Sarr D, Oliveira LJ, Russ BN, Owino SO, Middii JD, Mwalimu S, et al. Myeloperoxidase and other markers of neutrophil activation associate with malaria and malaria/HIV coinfection in the human placenta. Front Immunol. 2021;12: 682668.
Tume SJP, Zetem CC, Nulah SM, Ndzifoin AE, Mbuh BK, Nyuyfoni SR, et al. Climate change and food security in the Bamenda Highlands of Cameroon. In: Squires VR, Gaur MK (eds). Food Security and Land Use Change under Conditions of Climatic Variability. Switzerland, Springer Nature. Chapt. 6:107–24.
Ntonifor HN, Chewa JS, Oumar M, Mbouobda HD. Intestinal helminths as predictors of some malaria clinical outcomes and IL-1β levels in outpatients attending two public hospitals in Bamenda, North West Cameroon. PLoS Negl Trop Dis. 2021;15: e0009174.
Cochran WG. Sampling techniques. 3rd ed. New York: John Wiley & Sons; 1977.
Hasday JD, Thompson C, Singh IS. Fever, immunity, and molecular adaptations. Compr Physiol. 2014;4:109–48.
WHO. Bench aids for the diagnosis of malaria infections, 2nd Edn. Geneva, World Health Organization, 2000. Available from: https://apps.who.int/iris/handle/10665/42195
Cheesbrough M. District laboratory practice in tropical countries. Part 1. 2 nd Edn. New York: Cambridge University Press. 2009.
Oumar M, Chungong NM, Tume C. Alleviated immunological activities of wistar rat peritoneal neutrophils and macrophages by polysaccharide rich extract from the fruit bodies of Microporus vernicipes (Polyporales). J Pharm Res Int. 2020;32:1–11.
Teh RN, Sumbele IUN, Meduke DN. Malaria parasitaemia, anaemia and malnutrition in children less than 15 years residing in different altitudes along the slope of Mount Cameroon: prevalence, intensity and risk factors. Malar J. 2018;17:336.
Khatib RA, Chaki PP, Wang DQ, Mlacha YP, Mihayo MG, Gavana T, et al. Epidemiological characterization of malaria in rural southern Tanzania following China-Tanzania pilot joint malaria control baseline survey. Malar J. 2018;17:292.
Kimenji KM, Wamae K, Ochola-Oyier LI. Understanding P. falciparum asymptomatic infections: a proposition for a transcriptomic approach. Front Immunol. 2019;10:2398.
Kotepui M, Phunphuech B, Phiwklam N. Effect of malaria infection on haematological parameters in a population near Thailand-Myanmar border. Malar J. 2014;13:21.
Download references
Acknowledgements
We would like to acknowledge The General Hospital of Bamenda, the Santa District hospital and the Bambili Community Health Centre, all in the North Cameroon for allowing us to use their laboratories to conduct the parasitological examinations. We would also like to thank the study participants for providing us with their blood sample.
Not applicable. This work was not funded. All research materials were provided by the authors.
Author information
Authors and affiliations.
Department of Biological Sciences, Faculty of Science, The University of Bamenda, BP 39, Bambili, N. W. Region, Cameroon
Ntonifor Helen Ngum, Ngahbort Belthine Fakeh, Abongwa Edith Lem & Oumar Mahamat
African Centre of Excellence for Genomics of Infectious Diseases (ACEGID), Redeemer’s University, Ede, Osun State, Nigeria
Ntonifor Helen Ngum & Abongwa Edith Lem
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: NBF, OM and NHN. Data curation: NBF. Formal analysis: OM and NHN. Investigation: NBF. Supervision: NHN and OM. Original draft: NBF and OM. Review & editing: NHN. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Oumar Mahamat .
Ethics declarations
Consent for publication.
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
Ethical clearance obtained for this study.
Additional file 2.
Questionnaire form used for collection of demographic data.
Additional file 3.
The raw data obtained from the field and the laboratory.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Ngum, N.H., Fakeh, N.B., Lem, A.E. et al. Prevalence of malaria and associated clinical manifestations and myeloperoxidase amongst populations living in different altitudes of Mezam division, North West Region, Cameroon. Malar J 22 , 20 (2023). https://doi.org/10.1186/s12936-022-04438-6
Download citation
Received : 05 November 2022
Accepted : 31 December 2022
Published : 19 January 2023
DOI : https://doi.org/10.1186/s12936-022-04438-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- White blood cells
- Myeloperoxidase
Malaria Journal
ISSN: 1475-2875
- Submission enquiries: [email protected]
- Open access
- Published: 28 March 2024
Malaria infection and predictor factors among Chadian nomads’ children
- Azoukalné Moukénet 1 , 2 ,
- Kebfene Moudiné 3 ,
- Ngarkodje Ngarasta 2 ,
- Clement Kerah Hinzoumbe 4 &
- Ibrahima Seck 1
BMC Public Health volume 24 , Article number: 918 ( 2024 ) Cite this article
Metrics details
In Chad, malaria remains a significant public health concern, particularly among nomadic populations. Geographical factors and the mobility of human populations have shown to be associated with the diversity of Plasmodium species. The study aims to describe the malaria prevalence among nomadic children and to investigate its associated factors.
A cross-sectional study was conducted in February and October 2021 among nomadic communities in Chad. Blood sample were collected and tested from 187 Arab, Fulani and Dazagada nomadic children aged 3–59 months using malaria rapid diagnostic test (RDT). A structured electronic questionnaire was administered to their parents to collect information about the socio‑economic data. Malaria testing results were categorized according to the SD BIOLINE Malaria Ag Pf/Pan RDT procedures. Logistic regression analysis was used to determine key risk factors explaining the prevalence of malaria. STATA version IC 13 was used for statistical analysis.
The overall malaria prevalence in nomadic children was 24.60%, with 65.20% being Plasmodium falciparum species and 34.8% mixed species. Boys were twice as likely (COR = 1.83; 95% CI, 0.92–3.62; p = 0.083) to have malaria than girls. Children whose parents used to seek traditional drugs were five times more likely (AOR = 5.59; 95% CI, 1.40–22.30, p = 0.015) to have malaria than children whose parents used to seek health facilities. Children whose parents reported spending the last night under a mosquito net were one-fifth as likely (AOR = 0.17; 95% CI, 0.03–0.90, p = 0.037) to have malaria compared to children whose parents did not used a mosquito net. Furthermore, Daza children were seventeen times (1/0.06) less likely (AOR = 0.06; 95% CI, 0.01–0.70, p = 0.024) to have malaria than Fulani children and children from households piped water as the main source were seven times more likely (AOR = 7.05; 95% CI, 1.69–29.45; p = 0.007) to have malaria than those using surface water.
Conclusions
Malaria remains a significant public health issue in the nomadic communities of Chad. Community education and sensitization programs within nomad communities are recommended to raise awareness about malaria transmission and control methods, particularly among those living in remote rural areas. The National Malaria Control Program (NMCP) should increase both the coverage and use of long-lasting insecticidal nets (LLINs) and seasonal malaria chemoprevention (SMC) in addition to promoting treatment-seeking behaviors in nomadic communities.
Peer Review reports
Malaria is a public health disease caused by parasites belonging to the Plasmodium genus and transmitted to humans through the bites of infected female Anopheles mosquitoes that breed in aquatic habitats [ 1 , 2 ]. According to the World Health Organization (WHO), an estimated 247 million cases of malaria worldwide in 2021, resulted in 619, 000 deaths. The WHO African Region bears a disproportionately high share of the global malaria burden with 95% of malaria cases and 96% of malaria deaths. Children under 5 accounted for about 80% of all malaria deaths in the Region [ 3 ]. In Chad, malaria is endemic with areas at risk of epidemics. In 2021, the country recorded around 3.5 million cases of malaria and 11, 744 deaths [ 3 ]. Malaria has consistently been the primary health problem reported in health facilities in Chad, with around 50% of malaria cases reported in children under five years old [ 4 , 5 ]. Overall, three Plasmodium species ( falciparum, malariae and ovale ) are incriminated, but 98% of malaria cases are attributable to P. falciparum [ 6 , 7 ].
Well-explored risk factors for malaria infection include biological factors such as age (children under 5), gender [ 8 , 9 ], malnutrition status of children [ 10 ], socioeconomic factors such as poverty [ 11 ], poor awareness and knowledge about malaria prevention and control [ 12 ]. Exposure to mosquito breeding sites [ 11 , 12 , 13 ] increases the risk of malaria infection while the use of preventive interventions such as a new LLIN [ 14 , 15 , 16 , 17 ] reduces this risk. Regarding the diversity of malaria parasites, geographical proximity to areas with various Plasmodium species and the movement of human populations particularly in malaria-endemic areas for a long period have been associated with P. falciparum and P. vivax [ 18 ]..
The Chadian population consists of both mobile and settled populations with around 3.5% being nomadic [ 19 ]. Nomads mainly inhabit the areas between the Saharan and Sahelian zones. Changes in climate [ 20 ], economy [ 21 ] and politics [ 22 ] over the past decades have led to a considerable extension of pastoral mobility toward the Sudanian areas [ 23 ]. Nomads traverse various malaria transmission areas within the country Chad [ 23 ] and sometimes cross the borders in search of pasture and water for their herds. As asymptomatic carriers of malaria parasites, these populations constitute a reservoir of Plasmodium species from other countries.
Numerous malaria control interventions worldwide have been implemented and proven effective [ 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 ]. The combination of some interventions such as the long-lasting insecticide-treated net (LLIN) and the seasonal malaria chemoprevention (SMC) has demonstrated increased effectiveness [ 27 ]. Chad has adopted SMC with sulphadoxine-pyrimethamine and amodiaquine (SPAQ) to prevent seasonal malaria among children aged 3–59 months in the Sahel region [ 36 ]. There is promotion of the use of LLINs distributed through mass distribution and routine basis [ 37 ]. The last mass LLIN distribution campaign in Chad occurred in 2023.
Despite deep understanding of malaria predictive factors, most studies have focused on single factors, thus not providing a holistic recognition of the factors associated with malaria among nomadic communities. Both factors at the individual/household and community levels have been omitted. In Chad, few studies related to malaria in nomadic communities are available. Some of them have focused on knowledge and attitudes toward malaria [ 38 ], and coverage of control interventions among this community [ 37 ]. To address this gap, the objective of this study is to assess the prevalence of malaria and investigate the associated factors among nomadic communities in Chad.
Participants and methods
Study area and design.
This cross-sectional study conducted in February and October 2021 among nomadic communities in the provinces of Hadjer Lamis and Chari Baguirmi in the Sahelian zone of Chad and in Moyen Chari in the Sudanian zone. In comparison to the national level (40.9%), the prevalence of malaria was moderate in Hadjer Lamis (15.9%), high in Chari Baguirmi (37.2%) and very high (68.1%) in Moyen Chari [ 39 ]. In addition to intermittent preventive treatment for pregnant women (IPTp), routine and mass LLIN distribution campaigns have been implemented in all three provinces whereas SMC is being implemented in Hadjer Lamis and Chari Baguirmi provinces targeting children aged 3–59 months.
Each year, transhumant nomads migrate in the endemic area from the Sahelian zone to the Sudanian zone and subsequently return to the northern part of the country with low or no malaria transmission, when the rainy season arrives. The structure of nomad habitat has been described elsewhere [ 38 ]. Accordingly, blood samples were taken twice a year from children aged 3–59 months, and none of these children have been sampled twice. The first samples were taken when the nomads were moving to the south (January– March) and the second set during the high malaria transmission period (July– October) when they reached the Sahelian zone.
Study population
Nomadic children, specifically Arab or Fulani or Dazagada (Daza) children aged 3–59 months at the time of the study whose parents provided consent to blood sample collection were included. To mitigate the inflation of malaria prevalence due to parents selectively designating sick children, in each randomly selected household within the camps, a blood sample was obtained from the oldest child aged 3 to 59 months. If the result from the RDT was positive for Plasmodium , the child received a dose of antimalarial in accordance with the national treatment protocol [ 40 ].
Sampling and recruitment
Within each of three nomad group a multi-stage cluster random sampling technique was employed, with the camp as first stage and the household as second-stage leading to a minimum sample of 180 study subjects. A provision of 4% was added to account for non-response. At the first level, from the list of camps provided by leaders of each three nomad groups, 51 camps were randomly selected using a random number draw. At the second stage, within each selected camp, surveyors used random number draws to select two households (four households for very large camps). For selected household, one member aged older than 18 years was requested for interviews and responded on behalf of the household to which he or she belongs. Additionally, the oldest child of the group of 3–59 months was chosen for blood sampling. Of the 187 children, 81 were blood sampled in January– March and 106 in July– October.
Data collection
After being informed, the parents of the children were required to provide written consent, they indicated their agreement by affixing their signature or fingerprint on two copies of the cards. The demographic characteristics of the child (age and sex), facial temperature by thermoflash for some children and the results of the RDT (SD BIOLINE Malaria Ag Pf/Pan, Standard Diagnostics USA) were entered on the collection sheet with a unique and anonymous identification code for each child.
To record information on the parents’ sociodemographic characteristics, their knowledge and experiences regarding malaria, and their use of mosquito nets, a structured electronic questionnaire developed based on Peto et al. [ 37 , 41 ] was administered to an adult who responded on behalf of the household during blood sample collection. The survey questionnaire was administered in February and October 2021 by three trained data collectors fluent in the local languages and experienced in collecting data for nomad immunization programs. The questionnaire included items on respondents’ socio-demographic characteristics; their knowledge and experiences regarding malaria; and their use of mosquito nets. The questionnaire was implemented in KoBoCollect v2021.2.4 [ 42 ], and was administered offline before responses were uploaded to the server once the WiFi connection was available. While data collectors were administering the questionnaire, health agents were collecting blood sample.
Response variable
The results of malaria test were categorized as either “negative” or “positive” according to the SD BIOLINE Malaria Ag Pf/Pan RDT procedures. The SD Bioline ™ Malaria Ag Pf/Pan test is a rapid, qualitative, and differential test designed to detection of the histidinerich protein 2 (HRP2) antigen of Plasmodium falciparum and the common Plasmodium lactase dehydrogenase (pLDH) of Plasmodium species in human whole blood. The reliability of RDT-based diagnosis is reported elsewhere [ 43 , 44 , 45 , 46 , 47 ].
Explanatory variables
We evaluated the association between malaria prevalence and socioeconomic and contextual factors. The questionnaire included a variety of questions related to socioeconomic and contextual factors, listed below:
characteristics of the individual households and children;
contextual characteristics;
and district and zone variables.
Characteristics of the individual households and children
The main explanatory variables were: (i) the child’s temperature; (ii) the child’s age (3–59 months); (iii) the gender of the child and household characteristics such as (iv) household wealth quintiles, categorized as “lowest”, “middle” and “highest” economic levels; (v) ethnic group (Arab, Dazagada and Fulani); (vi) marital status of the head of household categorized as, “widowed/divorced”, “monogamy” and “polygamy”; (vii) reflex in case of malaria, including seeking a “street drug seller”, “health facility”, “traditional drug”; and (viii) utilization of LLIN and knowledge of malaria.
Common principles used to measure knowledge of malaria include questions on transmission and preventive interventions [ 48 ]. This study used similar principles published elsewhere [ 37 ]. Dimensions included were related to the periods of high transmission (rainy season), the group most at risk (children and pregnant women), means of transmission (mosquito bite) and common symptoms (fever, chills, muscle pain, stomach pain, diarrhea, nausea and vomiting). The dimension of interventions related to sleeping under a LLIN as mean of protection against malaria. Each correct response to question was scored one point and zero for wrong answers. An overall knowledge score was calculated by summing the scores for each respondent across all questions. Those with scores of 2.5 (mid-point between 0 and 5) or above were considered to have good knowledge, while those with lower scores were categorized as having poor knowledge about malaria.
Contextual characteristics
These characteristics were: ix) place of residence categorized as, “urban” or “rural”. Urban residence includes townships, municipalities and cities; x) the season; xi) the malaria control intervention in place (LLINS and SMC).
District and zone variables
These included: viii) all 3 administrative provinces; and ix) all district areas.
Laboratory methods
Rapid diagnostic test
Capillary blood samples were collected by study staff using a finger prick. One drop of blood was used to perform a malaria RDT (SD BIOLINE Malaria Ag PF/Pan, Standard Diagnostics USA). Blood samples were collected by three trained health agents who had previously collected blood sample and perform RDT during the National Malaria Survey (ENIPT) [ 39 ], in addition to participating in nomad immunization programs. Health agents were responsible for collecting children’s blood samples and performing RDT, while data collectors were administered questionnaires to parents. The RDT kits used in this study were obtained from health districts as recommended by the Ministry of health (MOH) and used in public health facilities.
Statistical methods
The data collected during each period were entered into Excel files, and STATA version IC 13 was used for statistical analysis. Percentages, means and standard deviations were calculated. Differences in proportions were assessed with exact Fisher’s tests. Sample means were compared by unpaired Student’s t tests. Values of p < 0.05 were considered significant. The parasitaemia rate was defined as the percentage of children with positive results from RDTs among the total of children surveyed. Few children had their temperature taken; therefore, the malaria prevalence rate was not processed, although children carrying Plasmodium were considered to have malaria.
Principal Component Analysis (PCA) [ 49 ] was used to develop wealth categories for the households based on access to facility including potable water and ownership of durable assets including solar kit, radio, telephone, cart tracked by animal, motorcycle/scooter, and caws/camels and sheep/goats per capita. Access and ownership were coded as 0 or 1 and missing cases were excluded. The first dimension of the PCA was taken as the household wealth score and range into tertiles; households were then placed into socioeconomic categories based on their scores.
We performed a descriptive analysis and presented participants’ social and demographic characteristics stratified by RDT results. We then employed exact Fisher’s tests to assess any difference in malaria prevalence by socioeconomic and contextual factors. Logistic regression analysis was conducted to identify the factors associated with malaria prevalence among nomads in Chad. Crude (COR) and adjusted odds ratios (AOR) were calculated to check statistical associations between the dependent and independent variables using the binary logistic regression and multivariable logistic regression models. All variables in the study were initially tested for association with malaria prevalence using a binary logistic regression model. Those showing a significant statistical association ( p < 0.05) were added into the multivariable analysis model to assess whether the association persisted after controlling against all other variables. A 95% confidence intervals and the 5% significance level were calculated for all odds ratios.
Characteristics of the study population
Overall, 187 children aged 3–59 months were enrolled in this study, distributed across the following districts and provinces: Dourbali (17.6%) and Massenya (13.9%) in Chari Baguirmi province (31.5%); Massaguet (55.1%) in the Hadjer Lamis province (55.1%) and Niellim (13.4%) in Moyen Chari province (13.4%) were enrolled in this study (Table 1 ; Fig. 1 ). Of them, 90 (48.1%) were female, and the enrolled children belonged to Arab (36.4%), Daza (36.4%) and Fulani (27.2%) ethnic groups. Concerning malaria, 86 (46.0%) and 36 (19.2%) participants came from households using street drugs and traditional drugs respectively in case of malaria episodes. Additionally, 154 children (82.4%) were from households with poor utilization of LLINs, and 162 (86.6%) of the participants were living in SMC areas (Table 1 ).
Map of study area
Malaria prevalence according to characteristics of the study participants
Overall 46 (24.60%) children were tested positive for malaria. The malaria prevalence was 21.7% and 28.4% respectively for blood sample collected in October and February 2021 (Fig. 2 ). Fisher’s exact test revealed a notably higher prevalence of malaria in children from the southern regions of the Sahelian zone, specifically Massenya and Dourbali, as well as in Niellim located in the south of the country, compared to the northern part of Sahelian counterparts in Massaguet. Specifically malaria positivity rates were 26.9% in Massenya, 36.4% in Dourbali and 56.0% in Niellim, significantly higher than the 12.6% observed in Massaguet. A significantly higher proportion of participants were tested positive in the area not receiving SMC (56.0%). Regarding the individual characteristics of participants and that of their households, a significant proportion of positive tests were found in boys (29.9%) compared to girls (18.9%). Additionally, positive test results were significantly higher among children from the Fulani (41.2%) and Arab (22.1%) ethnic groups than among those from the Daza (14.7%) ethnic group. A significantly larger proportion of positive tests was also found among children from households seeking traditional drugs (27.8%) and health facilities (32.3%) compared to street drug sellers (17.4%) in the case of malaria episodes (Table 1 ).
Malaria prevalence by month of data collection
Malaria prevalence according to Plasmodium species
The malaria RDT results according to Plasmodium species are presented in Table 2 . Out of 46 positive malaria tests, 16 (34.8% of positive test results) exhibited mixed species ( Plasmodium falciparum, Plasmodium ovale, Plasmodium malaria and/or Plasmodium vivax ). Mixed Plasmodium species were more frequently found in Daza children (70.0%), followed by Fulani children (66.7%). Concerning the use of mosquito nets, mixed Plasmodium species were identified in children whose parents reported not spending the last night under mosquito nets.
Factors associated with a positive test for malaria prevalence
Among all variables, geographic characteristics such as province, SMC area and individual or household characteristics like gender, ethnicity, socioeconomic status and the household’s behavior during malaria episodes, were significantly associated with malaria positivity ( p < 0.05) using the crude logistic regression (Table 3 ). Additionally, the use of mosquito nets (all types and LLINs), knowledge of malaria and the main source of water used by the household were included in the logistic regression adjusting for all other variables and only those significantly associated with malaria were retained.
After adjusting for other individual, household and geographic characteristics, Daza children were seventeen times less likely (AOR = 0.06; 95% CI, 0.01–0.79, p = 0.024) to have malaria than Fulani children. Children whose parents used to seek traditional drugs were five times more likely (AOR = 5.48; 95% CI, 1.38–21.72, p = 0.016) to have malaria than children whose parents used to seek health facilities. Children whose parents reported spending the last night under a mosquito net were one fifth as likely (AOR = 0.17; 95% CI, 0.03–0.93, p = 0.041) to have malaria compared to children whose parents did not spend the last night under a mosquito net. Children from households with piped water as main source were seven times more likely (AOR = 7.05; 95% CI, 1.69–29.45; p = 0.007) to have malaria than children from households using surface water. Children from areas not implementing SMC were twenty times more likely (AOR = 20.61; 95% CI, 2.91–145.77; p = 0.002) to have malaria than children from areas implementing SMC.
Malaria remains a public health challenge in sub-Saharan Africa, including Chad despite ongoing efforts for control and elimination through various interventions and financial investments. It is difficult to reach mobile communities that are more exposed to malaria than the general population. This study aimed to assess the prevalence of malaria and explore individual/household and community factors associated with malaria among nomadic communities in Chad.
This study highlighted the burden of malaria in Chad, with a national prevalence in the general population of 40.9% with high variability according to the endemicity area. The prevalence of malaria is moderate in Hadjer Lamis (15.9%), high in Chari Baguirmi (37.2%) and very high (68.1%) in Moyen Chari [ 39 ]. In this study, the prevalence of malaria was 24.6% which is low compared to the aforementioned study conducted in the general population without specificity for nomad considerations. However, this result is consistent with other studies conducted in the nomadic setting of Chad reporting a malaria prevalence of up to 30% [ 50 ]. The high prevalence documented in this study is also in line with studies in other African countries among nomadic populations such as Fulani pastoralists in southwestern Nigeria, recording a prevalence of 33.6% [ 51 ]. Additionally, in the general population of Ghana, a prevalence of 20.9% was reported [ 9 ].
Among malaria positive tests, 34.8% were found to be a mix of malaria parasites. This result underscores the necessity for further analysis to assess the malaria parasite species in Chad. Studies forming the basis for malaria management protocols in Chad tend to be out dated, reporting 98% of species as Plasmodium falciparum and only 2% as other malaria parasite species [ 6 ]. Furthermore, the results from this study indicate a high percentage of mixed Plasmodium species in Daza children, followed by Fulani children and children whose parents are not accurate users of mosquito nets. This result can be explained by the trajectory followed by both the Daza and Fulani groups during transhumance, with Daza group crossing the country in the east and the Fulani group in the south.
The current study revealed significant variation in the odds of malaria prevalence across provinces. In Chad, approximately 53% of the total land has climatic conditions favorable for malaria transmission (sahelian and sudanian area), covering 98% of the population. Malaria transmission in the Sahelian area spans between 3 and 4 months seasonally, while the Sudanian exhibits high endemicity throughout the year. Other factors, including altitude, temperature, humidity, rainfall, presence of breeding sites, and agricultural activities within provinces may explain variation in the prevalence of malaria between provinces. In this study, the higher malaria prevalence in Moyen Chari (Sudanian area) compared to Chari Baguirmi (Sahelian area) can also be attributed to the impact of malaria intervention in the Sahelian area (SMC) in contrast to the Sudanian area. Therefore, the SMC area was found to be associated with a lower prevalence of malaria.
In the current study, male participants were more likely to test positive for malaria than female. This finding aligns with the results of a study from Ghana and Cameroon establishing a significant association between gender and malaria prevalence, with males having a higher prevalence than females [ 8 , 9 , 12 ]. This could be explained by nomadic boys assisting parents with herds in the early evenings and, therefore, exposing them more to mosquito vectors. Additionally, other studies have suggested that boys tend to play outdoors in the early evenings more frequently than girls, resulting in increased exposure to mosquito vectors [ 8 ].
The study’s findings indicated a higher risk for malaria prevalence for Fulani children compared to Daza children. This result can be attributed to the area of exposure visited by these nomad groups. Fulani nomads ventured further into the Sudanian Chad than Daza who mostly stayed in the Sahelian zone. Furthermore, in recent decades, due to climatic, economic and political changes, a significant increase in pastoral mobility has been recorded in the Sudanian zone [ 23 , 38 ]. Moreover, some nomadic groups, such as the Fulani, have started transitioning to a more sedentary lifestyle in the Sudanese area [ 38 ].
Children whose parents sought traditional drugs for malaria treatment were more likely to have malaria than those whose parents sought health facilities. This finding can be explained by the low efficacy of traditional drugs against malaria and the lack of awareness regarding malaria prevention and treatment. Since these drugs often lack active principles to cure malaria, children may continue to harbor malaria parasites. As indicated elsewhere [ 38 ], traditional drugs in nomadic communities sometimes include ‘koulkul tree leaves’, ‘camel urine’ and ‘beef urine’ or ‘milk butter’. However, these results underscore the imperative need to enhance the availability and accessibility of health services. It has been mentioned elsewhere that the cost of health care and the severity of illness were the primary reasons for selecting health services in nomadic communities [ 38 ]. Furthermore, such interventions should be complemented by health promotion activities, as these factors influence community treatment-seeking patterns and contribute to ongoing malaria transmission.
Among all participants in the study, four to five mentioned spending the last night under a mosquito net of various types. While some may use the mosquito nets to protect themselves from other insect bites [ 38 ], the use of mosquito nets has proven effective in protecting children from malaria. Children whose parents reported spending the last night under a mosquito net were less likely to have malaria than those whose parents had not used a mosquito net. This result underscores the high potential for preventing malaria within this community by utilizing mosquito nets. This impact might have been more significant if nomads had access to LLINs; however, as mentioned elsewhere [ 37 , 38 ], this community has limited access to LLINs.
Children from households with piped water as main source of water were more likely to have malaria than those from households relying on surface water. Typically, piped water and wells are in proximity to nomad camps, enabling women and children to fetch water late at night. In contrast, surface water is far from camps, requiring women and children to seek water during the day with less exposure to mosquito bites. Additionally, queuing for other water sources may be longer than for surface water; resulting in a shorter duration of exposure to mosquito bites. This result appears contrary to findings in Tanzania [ 11 ], which considered piped water as free from stagnant water. However, in the nomad camps, all water sources are used by both humans and animals, meaning stagnant water surrounds all water sources. These stagnant waters serve as breeding ground mosquitoes, facilitating the development of the larvae into adult mosquitoes.
Strengths and limitations
This study has some limitations, including a small size of enrolled children, which may affect its representativeness for the entire nomadic child population. Nevertheless, the results provide insights into the overall descriptive situation of nomadic children. There may be potential bias in measuring LLIN use among children tested for malaria as only member per household responds on behalf of the household without specifying if children were under the mosquito net. Another limitation of this study was the reliance on a cross-sectional survey conducted at the end of the rainy season and the dry season when mosquito density and malaria transmission may be lower than in the rainy season. The study could not delve into parasite typology research through polymerase chain reaction (PCR) analysis due to funding. In terms of recommendations, interventions would be more cost-effective if tailored to the district level rather than at the provincial or national level. However, the study can be valuable for understanding factors associated with an increased likelihood of malaria positivity, guiding stakeholders in the implementation of malaria prevention, control and elimination strategies in nomadic communities, and the entire population in Chad. Furthermore, the study suggests the need for additional research to assess the typology of malaria parasites.
The study findings revealed a malaria prevalence of 24.6% with 34.8% of positive test indicating a mix of malaria parasites. This prevalence is relatively lower as compared to other studies conducted in other settings. Factors influencing malaria infection in nomad communities include the participants’ ethnic group, the reflex of households in malaria cases, the utilization of mosquito nets regardless of type, the main water source used by households and the participants’ living area. The findings underscore that malaria remains a public health concern in Chad, particularly in nomadic communities. The gathered information can guide the implementation of malaria prevention, control and elimination strategies benefiting the entire population in Chad.
The study recommends that the MOH and the NMCP organize community education and sensitization programs within nomadic communities, especially those in remote rural areas, emphasizing the effects of malaria. The NMCP should also enhance both the coverage and use of LLINs and SMC, along with promoting of treatment-seeking behaviors in nomadic communities.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Antenatal care
Adjusted odd ratio
Crude odd ratio
Deoxyribonucleic acid
Intermittent preventive treatment for pregnant women
Long-lasting insecticide-treated net
Ministry of Public Health
National Malaria Control Program ( Programme National de Lutte contre le Paludisme )
Polymerase chain reaction
- Seasonal malaria chemoprevention
Sulphadoxine-pyrimethamine and amodiaquine
United Nations Children’s Fund
World Health Organization
Akpodiete NO, Diabate A, Tripet F. Effect of water source and feed regime on development and phenotypic quality in Anopheles gambiae (s.l.): prospects for improved mass-rearing techniques towards release programmes. Parasit Vectors. 2019;12:210.
Article PubMed PubMed Central Google Scholar
Talapko J, Škrlec I, Alebić T, Jukić M, Včev A. Malaria: the past and the Present. Microorganisms. 2019;7:179.
Article CAS PubMed PubMed Central Google Scholar
WHO. World malaria report 2022. Global Malaria Programme. Geneva: WHO; 2022.
Google Scholar
Ministere de la Sante Publique. Annuaire Des statistiques sanitaires 2017 [Health statistics yearbook 2017]. N’Djamena: Ministry of Health Chad; 2018.
Ministère de la Santé Publique. Annuaire De Statistique sanitaire. Ndjamena: Ministère de la Santé Publique; 2019.
Programme National de Lutte contre le Paludisme. Plan stratégique National de lutte contre le paludisme 2014–2018. 2013.
Traoré B. Epidémiologie moléculaire de la résistance de plasmodium falciparum aux médicaments antipaludiques en République du Tchad. Thesis. USTTB; 2020.
Nyasa RB, Fotabe EL, Ndip RN. Trends in malaria prevalence and risk factors associated with the disease in Nkongho-mbeng; a typical rural setting in the equatorial rainforest of the South West Region of Cameroon. PLoS ONE. 2021;16:e0251380.
Tetteh JA, Djissem PE, Manyeh AK. Prevalence, trends and associated factors of malaria in the Shai-Osudoku District Hospital, Ghana. Malar J. 2023;22:131.
Folarin OF, Kuti BP, Oyelami AO. Prevalence, density and predictors of malaria parasitaemia among ill young Nigerian infants. Pan Afr Med J. 2021;40:25.
Shayo FK, Nakamura K, Al-Sobaihi S, Seino K. Is the source of domestic water associated with the risk of malaria infection? Spatial variability and a mixed-effects multilevel analysis. Int J Infect Dis. 2021;104:224–31.
Article PubMed Google Scholar
Tarekegn M, Tekie H, Dugassa S, Wolde-Hawariat Y. Malaria prevalence and associated risk factors in Dembiya district, North-western Ethiopia. Malar J. 2021;20:372.
Ahmed A, Mulatu K, Elfu B. Prevalence of malaria and associated factors among under-five children in Sherkole refugee camp, Benishangul-Gumuz region, Ethiopia. A cross-sectional study. PLoS ONE. 2021;16:e0246895.
Mkali HR, Reaves EJ, Lalji SM, Al-mafazy A-W, Joseph JJ, Ali AS, et al. Risk factors associated with malaria infection identified through reactive case detection in Zanzibar, 2012–2019. Malar J. 2021;20:485.
Woday A, Mohammed A, Gebre A, Urmale K. Prevalence and Associated Factors of Malaria among Febrile Children in Afar Region, Ethiopia: A Health Facility based study. Ethiop J Health Sci. 2019;29:613–22.
Andronescu LR, Buchwald AG, Coalson JE, Cohee L, Bauleni A, Walldorf JA, et al. Net age, but not integrity, may be associated with decreased protection against Plasmodium falciparum infection in southern Malawi. Malar J. 2019;18:329.
Amare A, Eshetu T, Lemma W. Dry-season transmission and determinants of Plasmodium infections in Jawi district, northwest Ethiopia. Malar J. 2022;21:45.
Khaireh BA, Briolant S, Pascual A, Mokrane M, Machault V, Travaillé C, et al. Plasmodium Vivax and Plasmodium Falciparum infections in the Republic of Djibouti: evaluation of their prevalence and potential determinants. Malar J. 2012;11:395.
INSEED. Deuxième Récensement Général De La Population et de l’Habitat 2009 [Second General Population Census and Habitat 2009]. Rapport De récensement. Ndjamena: Institut National de Statistique, des Etudes Economique et Démographique; 2012.
CTA. Atlas d’élevage Du Bassin Du Lac Tchad. Wageningen. Pays-Bas; 1996.
Clanet J. Géographie Pastorale Au Sahel central. Thèse d’Etat en Géographie Humaine. Paris IV Sorbone; 1994.
Fuchs P. Nomadic society, civil war, and the state in Chad. Nomadic Peoples. 1996;:151–62.
Wiese M. Health-vulnerability in a complex crisis situation. Implications for providing health care to nomadic people in Chad. Verlag für Entwicklungspolitik. Saarbrûcken; 2004.
Bojang K, Akor F, Bittaye O, Conway D, Bottomley C, Milligan P, et al. A randomised trial to compare the safety, tolerability and efficacy of three drug combinations for intermittent preventive treatment in children. PLoS ONE. 2010;5:e11225.
Chatio S, Ansah NA, Awuni DA, Oduro A, Ansah PO. Community acceptability of Seasonal Malaria Chemoprevention of morbidity and mortality in young children: a qualitative study in the Upper West Region of Ghana. PLoS ONE. 2019;14.
Cissé B, Sokhna C, Boulanger D, Milet J, Bâ EH, Richardson K, et al. Seasonal intermittent preventive treatment with artesunate and sulfadoxine-pyrimethamine for prevention of malaria in Senegalese children: a randomised, placebo-controlled, double-blind trial. Lancet. 2006;367:659–67.
Dicko A, Diallo AI, Tembine I, Dicko Y, Dara N, Sidibe Y et al. Intermittent preventive treatment of Malaria provides Substantial Protection against Malaria in Children already protected by an insecticide-treated Bednet in Mali: a Randomised, Double-Blind, placebo-controlled trial. PLoS Med. 2011;8.
Druetz T, Corneau-Tremblay N, Millogo T, Kouanda S, Ly A, Bicaba A, et al. Impact Evaluation of Seasonal Malaria Chemoprevention under Routine Program implementation: a quasi-experimental study in Burkina Faso. Am J Trop Med Hyg. 2018;98:524–33.
Konaté AT, Yaro JB, Ouédraogo AZ, Diarra A, Gansané A, Soulama I, et al. Intermittent preventive treatment of Malaria provides Substantial Protection against Malaria in Children already protected by an insecticide-treated Bednet in Burkina Faso: a Randomised, Double-Blind, placebo-controlled trial. PLoS Med. 2011;8:e1000408.
Sesay S, Milligan P, Touray E, Sowe M, Webb EL, Greenwood BM, et al. A trial of intermittent preventive treatment and home-based management of malaria in a rural area of the Gambia. Malar J. 2011;10:2.
Tagbor H, Antwi GD, Acheampong PR, Bart Plange C, Chandramohan D, Cairns M. Seasonal malaria chemoprevention in an area of extended seasonal transmission in Ashanti, Ghana: an individually randomised clinical trial. Trop Med Int Health. 2016;21:224–35.
Plucinski MM, Chicuecue S, Macete E, Colborn J, Yoon SS, Kachur SP, et al. Evaluation of a universal coverage bed net distribution campaign in four districts in Sofala Province, Mozambique. Malar J. 2014;13:427.
Secrétariat de l’Organisation Mondiale de la Santé. Paludisme: projet de stratégie technique mondiale pour l’après-2015. Rapport Du Secrétariat. Génève: Organisation Mondiale de la Santé; 2015.
Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. The Cochrane Collaboration; 2004.
Gamble CL, Ekwaru JP, ter Kuile FO. Insecticide-treated nets for preventing malaria in pregnancy. Cochrane Database Syst Rev. 2006;2006.
Programme National de Lutte contre le Paludisme. Plan stratégique national de lutte contre le paludisme 2019–2023 [National strategic plan to fight against malaria 2019–2023]. N’Djamena; 2019.
Moukénet A, Richardson S, Moundiné K, Laoukolé J, Ngarasta N, Seck I. Knowledge and practices surrounding malaria and LLIN use among arab, Dazagada and Fulani pastoral nomads in Chad. PLoS ONE. 2022;17:e0266900.
Moukénet A, Honoré B, Smith H, Moundiné K, Djonkamla W-M, Richardson S, et al. Knowledge and social beliefs of malaria and prevention strategies among itinerant nomadic arabs, fulanis and Dagazada groups in Chad: a mixed method study. Malar J. 2022;21:56.
National Malaria Control Program, National Institute of Statistics. Enquête Nationale sur les Indicateurs Du Paludisme Au Tchad De 2017: ENIPT-2017. Ndjamena: National Malaria Control Program Chad; 2018.
Programme National de Lutte contre le Paludisme. Politique Nationale De Lutte Contre Le Paludisme. Politique Nationale. Ndjamena: Ministère de la Santé Publique et de la Solidarité Nationale; 2014.
Peto TJ, Tripura R, Sanann N, Adhikari B, Callery J, Droogleever M, et al. The feasibility and acceptability of mass drug administration for malaria in Cambodia: a mixed-methods study. Trans R Soc Trop Med Hyg. 2018;112:264–71.
The Harvard Humanitarian Initiative. KoBoToolbox. 2021.
Tadesse E, Workalemahu B, Shimelis T. Diagnostic performance evaluation of the SD bioline, malaria antigen AG PF/PAN test. (05FK60) In a malaria endemic area of southern Ethiopia. Rev Inst Med Trop São Paulo. 2016;58:59.
PubMed PubMed Central Google Scholar
Pujo JM, Houcke S, Lemmonier S, Portecop P, Frémery A, Blanchet D, et al. Accuracy of SD Malaria Ag P.f/Pan® as a rapid diagnostic test in French Amazonia. Malar J. 2021;20:369.
Kashosi TM, Mutuga JM, Byadunia DS, Mutendela JK, Mulenda B, Mubagwa K. Performance of SD Bioline Malaria Ag Pf/Pan rapid test in the diagnosis of malaria in South-Kivu, DR Congo. Pan Afr Med J. 2017;27:216.
Malaria rapid diagnostic test performance. Results of WHO product testing of malaria RDTs: Round 8 (2016–2018). https://www.who.int/publications-detail-redirect/9789241514965 . Accessed 12 Mar 2023.
Mfueni E, Devleesschauwer B, Rosas-Aguirre A, Van Malderen C, Brandt PT, Ogutu B, et al. True malaria prevalence in children under five: bayesian estimation using data of malaria household surveys from three sub-saharan countries. Malar J. 2018;17:65.
Fuge TG, Ayanto SY, Gurmamo FL. Assessment of knowledge, attitude and practice about malaria and ITNs utilization among pregnant women in Shashogo District, Southern Ethiopia. Malar J. 2015;14:235.
Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan. 2006;21:459–68.
Moto DD, Daoud S, Tanner M, Zinsstag J, Schelling E. Répartition de la morbidité dans trois communautés nomades du Chari-Baguirmi and Kanem, Chad. Med Trop (Mars). 2004;64:469–73.
Ekpo UF, Omotayo AM, Dipeolu MA. Sendentarization and the prevalence of malaria and anaemia among settled Fulani pastoralists in south-western Nigeria. Niger J Parasitol. 2008;29.
Download references
Acknowledgements
We gratefully thank the nomadic pastoralists of the Arab, Dazagada and Fulani for their hospitality and participation in this study. We acknowledge the medical officers of Massakory, Massaguet, Massenya, Korbol and their teams for their diligent help during this study. This study would not have been possible without the field workers Kabo Karadjom, Bianzoumbé Jonas, Annour Kanika and Issa Younous who helped with the sample and data collection, and performed the rapid diagnostic test. We are thankful to Tsakeu Elyonore Leponkouo for revising the manuscript.
The authors received no specific funding for this work.
Author information
Authors and affiliations.
Cheikh Anta Diop University, Dakar, Senegal
Azoukalné Moukénet & Ibrahima Seck
University of Ndjamena, Ndjamena, Chad
Azoukalné Moukénet & Ngarkodje Ngarasta
Abomey-Calavi University, Abomey-Calavi, Benin
Kebfene Moudiné
UNDP, Ndjamena, Chad
Clement Kerah Hinzoumbe
You can also search for this author in PubMed Google Scholar
Contributions
AM, KM, CKH, NN, and IS conceived the project; KM and AM oversaw the data collection; KM, CKH, and AM analyzed and interpreted the quantitative data, and KK, BJ, AK and IY processed the RDT. AM drafted the manuscript and all authors reviewed subsequent versions and approved the final version for submission.
Corresponding author
Correspondence to Azoukalné Moukénet .
Ethics declarations
Ethics approval and consent to participate.
The aim and objectives of the research were explained to caregivers’ prior consent. Written informed consent was obtained from all participants or their guardians prior to their enrollment. The study protocol was approved by the National Ethics Committee of Chad (Approval No 0193/PR/MESRI/SG/CNBT/2020, 21 September 2020). All experiments were performed in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, supplementary material 3, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Moukénet, A., Moudiné, K., Ngarasta, N. et al. Malaria infection and predictor factors among Chadian nomads’ children. BMC Public Health 24 , 918 (2024). https://doi.org/10.1186/s12889-024-18454-5
Download citation
Received : 23 July 2023
Accepted : 26 March 2024
Published : 28 March 2024
DOI : https://doi.org/10.1186/s12889-024-18454-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Long-lasting insecticidal nets
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
COMMENTS
The high prevalence of 55% with mean (S.D) parasite density of 1814.70 (1829.117) parasite/μL of blood is an indication that Ibadan is a high-risk area for malaria transmission, since it falls within the Nigerian malaria risk map estimates of less than 20% in certain zone to more than 70% in other zones .
5.6eported malaria cases in R HBHI countries since 2018 and comparisons with estimated cases 48 6.vestments in malaria programmes and research In 52 6.1unding trends for malaria control and elimination 52F 6.2vestments in malaria-related R&D 56In 7.istribution and coverage of malaria prevention, diagnosis and treatment D 58
However, there continues to be a significant funding gap for basic research and product development. There is an urgent need to ramp up investment in new malaria tools, to address emerging threats such as urban malaria and the spread of antimalarial drug resistance. We face many challenges, but there are many reasons for hope.
6. Investments in malaria programmes and research 44 6.1 Funding trends for malaria control and elimination 45 6.2 Global trends in real GDP growth and out-of-pocket health expenditure 54 6.3 Investments in malaria-related R&D 58 7. Distribution and coverage of malaria prevention, diagnosis and treatment 60 7.1 Distribution and coverage of ITNs 60
countries. Southern Province, Zambia has maintained a parasite prevalence of <10% since 2012, and the National Malaria Control Center made a goal of creating 5 malaria free zones in the province. As areas approach elimination, better understanding of the changing epidemiology of malaria transmission should be used to inform and determine
researchers and academics to identify priority research areas for malaria. » In 1998, WHO, the World Bank, the United Nations Development Programme and the United Nations Children's Fund created the Roll Back Malaria initiative with the goal of halving the global burden of malaria by 2010. Two years later, leaders of malaria-endemic countries
Malaria case incidence (i.e. cases per 1000 population at risk) reduced from 80 in 2000 to 58 in 2015 and 57 in 2019 (Table 3.1, Fig. 3.2). Between 2000 and 2015, malaria case incidence declined by 27% and then by less than 2% in the period 2015-2019, indicating a slowing of the rate of decline since 2015 (Fig. 3.2).
Prevalence of malaria (symptomatic and asymptomatic malaria combined) This study shows that the overall prevalence estimate of malaria infection in the study area was 5% (95% CI = 3-7) which is comparable to a study conducted on several regions of Ethiopia (4.1%) and the finding from Benna Tsemay district, Southwest Ethiopia (6.1%) .
Previous cross-sectional studies of malaria prevalence in Ethiopia have demonstrated the wide range (2.8 to 39.6%) of malaria prevalence [4,5,6,7,8]. Malaria is one of the major public health problems in Amhara Regional State. In 2012, a total of 1,127,241 cases of malaria were reported in this Region, out of a population of 19,867,817 habitants
Malaria infection during pregnancy is an enormous public health problem, with substantial risks for the mother, her fetus and the neonate. ... • In settings with an HIV prevalence among pregnant women greater than 10%, it is more cost- ... Research to assess the safety, effi cacy and programme
Background Malaria is a major public health problem in sub-Saharan Africa, and children are especially vulnerable. In 2019, an estimated 409,000 people died of malaria, most (274,000) were young children and 94% of the cases and deaths were in Africa. Prior studies in Ethiopia focused on the adult population and high transmission areas. Hence, this study aimed to determine the prevalence and ...
Moreover, the odds of malaria prevalence were 1.60, 1.64, 2.45 and 1.82 times higher in the age group of < 5, 5-14, 15-24 and 25-54 years old compared to the older age groups (> 54 years old ...
The result of this prevalence was lower than the 5.3% prevalence of malaria reported in Gondar Town , the 5.2% malaria prevalence from Jimma town , and 22.1% prevalence among children's less than 5 years in Arba Minch Zuria . This difference could be attributed to the variation in intensity of vector control strategies, altitude, microclimate ...
prevalence rates of both malaria and HIV/AIDS (such as in sub-Saharan Africa), the interaction between the two diseases, including coinfection, worsens the morbidity and mortality of the other. ... including high-priority research in the Global Malaria Programme, with a particular focus on the development of new methods, strategies and tools, and
According to the Ghana demographic and health survey, malaria prevalence ranges from 11.2 to 40.0% . In this study, the prevalence of malaria was found to be 20.9%. This is consistent with a study conducted in Ghana on the prevalence of malaria-positive rapid diagnostic tests and antimalarial treatment which reported a 21.58% prevalence rate .
Overview. Malaria is a life-threatening disease spread to humans by some types of mosquitoes. It is mostly found in tropical countries. It is preventable and curable. The infection is caused by a parasite and does not spread from person to person. Symptoms can be mild or life-threatening. Mild symptoms are fever, chills and headache.
The prevalence of malaria in the study population was high, and environmental and behavioral factors related to LLIN use remain potential determinants of malaria. Background Malaria is a major public health problem in sub-Saharan Africa, and children are especially vulnerable. In 2019, an estimated 409,000 people died of malaria, most (274,000) were young children and 94% of the cases and ...
Background Recent studies point to the need to incorporate the detection of non-falciparum species into malaria surveillance activities in sub-Saharan Africa, where 95% of the world's malaria cases occur. Although malaria caused by infection with Plasmodium falciparum is typically more severe than malaria caused by the non-falciparum Plasmodium species P. malariae, P. ovale spp. and P. vivax ...
Malaria is a growing problem in Africa, with prevalence varies from areas to areas due to several factors including the altitude. This study aimed to investigate the malaria distribution and its relationship with level of some blood parameters and plasma myeloperoxidase (MPO) in population of three localities with different altitudes. A total of 150 participants were recruited in each locality ...
Background In Chad, malaria remains a significant public health concern, particularly among nomadic populations. Geographical factors and the mobility of human populations have shown to be associated with the diversity of Plasmodium species. The study aims to describe the malaria prevalence among nomadic children and to investigate its associated factors. Methods A cross-sectional study was ...
This paper examines the effect of climate change on cereal yields via malaria prevalence in sub-Saharan African (SSA) countries. To achieve this objective, this paper uses a model composed of a system of two equations estimated with the 3SLS method, the semi-parametric approach and the two-stage system GMM method. Panel data from 31 SSA countries over the period 2000-2019 are used. Results ...
US Department of Health and Human Services | Centers for Disease Control and Prevention | MMWR | March 28, 2024 | Vol. 73 | No. 12. Data Analysis. Crude SCD birth prevalence (calculated by dividing the number of newborns with SCD by the total number of live births) and SCD birth prevalence among non-Hispanic Black newborns (calculated by ...