
- Product Development Strategy
- Medical Device Testing
- Controlled Environmental Regulatory Testing Services
- Biological Safety Consulting
- Clinical Research
- Preclinical Research
- Medical Device Regulatory & Quality
- Medical Writing Solutions
- Medical Device Reimbursement
- NAMSA APEX Program™
- MedTech Market Research Consulting
- Subject Matter Experts
- Therapeutic Expertise
- Resource Library
- MedTech Podcast and Video Series
- NAMSA Medical Device Test Navigators
- EU MDR & IVDR Planning Resources
- COVID-19 IVD Resources
- Monkeypox IVD Resources
- Accreditations & Certifications
- Client Testimonials
- Go to Client Portal

MEDICAL WRITING SERVICES PROVEN TO ACCELERATE COMMERCIALIZATION OBJECTIVES
Our team of world-class clinical medical writers is with you every step way to help support device approval, market introduction and post-market objectives..
CERs/PERs Annually
Medical Writers with Advanced Degrees
Years of Medical Device Experience
PROVEN MEDICAL WRITING SERVICES YOU CAN RELY ON
NAMSA provides a wide range of specialized medical writing, manuscript submission and evidence communication services. Our world-class team of clinical medical writers is expert at identifying, organizing, interpreting and presenting clinical data accurately and professionally for submission to various regulatory bodies. NAMSA’s medical writing services team is also experienced and skilled in developing Clinical Evaluation Plans and Reports to comply with the Medical Device Regulation (MDR) and MEDDEV 2.7.1 rev. 4, as well as Performance Evaluation Plans and Reports to comply with the In Vitro Diagnostic Regulation (IVDR), in collaboration with our EU Regulatory Team.
NAMSA’s global medical writing services team is comprised of dedicated medical writers from a diverse range of clinical and scientific backgrounds of whom >90% hold advanced degrees.
Working in close partnership with NAMSA’s Regulatory , Clinical and Biostatistics Teams, our medical writers are highly responsive to individual needs and are instrumental in helping clients achieve commercial objectives. When working with NAMSA , clients are also provided with a personalized plan to help guide future compliance activities.
Clinical Evaluation Reports & Plans (CERs | CEPs) / Performance Evaluation Reports & Plans (PERs | PEPs)
Clinical Evaluation Reports (CERs) / Performance Evaluation Reports (PERs) are vital technical documents required for market approval of medical devices and In Vitro Diagnostics (IVDs) within a variety of markets. Clinical evaluation is a structured and ongoing process, involving collection, appraisal and analysis of clinical data relating to the device. The purpose is to demonstrate compliance with specific safety and performance requirements as related to its intended purpose. This process involves the evaluation of the clinical benefit-risk profile for the device using the latest available clinical data, e.g., derived from clinical studies, published literature, and active post-market surveillance.
The Clinical Evaluation Plan (CEP) / Performance Evaluation Plan (PEP) describes the scope, methodology and systematic approach to employ within the evaluation. While the CER/PER provides a detailed description of the actual conduct of the clinical evaluation, information analyzed and conclusion(s) reached. Both documents require regular updating throughout the life cycle of the medical device and are subject to review and scrutiny by the regulatory authorities.
NAMSA’s team of CER/PER medical writing experts is highly qualified and skilled, producing approximately 200 CERs/PERs annually in compliance with market-specific requirements. Collaborating with NAMSA’s Regulatory Team, who have worked for various Notified Bodies, our medical writers are equipped with in-depth knowledge of regulatory requirements and guidelines specific to varying global regions and have extensive experience supporting clients with CER/PER submissions.
Together, we ensure the correct match of skills and expertise required to organize, interpret and present data accurately, efficiently and professionally that is highly recognized and trusted by global regulatory entities. We take particular pride in our reputation for speed of delivery, the quality of our reports and our outstanding record for approvals. NAMSA consistently receives positive feedback on our medical writing services from clients and major Notified Bodies, resulting in hundreds of clients passing the approval process without issue.
NAMSA provides CERs/PERs and CEPs/PEPs covering all device classes and therapeutic areas, including combination products. Clinical and Performance Evaluation services offered by NAMSA include:
- Ad-Hoc Medical Writing support
- Medical Writing of plans and reports
- Maintenance or regular updates of plans and reports
- Bundled plans and reports, covering multiple product variants
- Clinical and Performance Evaluation guidance, training and SOP writing/updating
- Gap analyses per international guidance
- Systemic literature review
- Physician or peer review
Clinical Literature Reviews
Clinical literature reviews can be valuable in strengthening a manufacturer’s product portfolio. A thorough search and robust synthesis of available literature relevant to a particular medical device may provide important information to guide further product development, reveal much-needed evidence to substantiate current clinical indications, identify potential ‘off-label’ applications or flag critical adverse event (AE) data.
Depending on the scope, such a review might also consider information gathered from pre-clinical studies for regulatory purposes or to provide information of commercial interest concerning a competitor device. Within the U.S. in particular, both FDA guidance and the Code of Federal Regulations stipulate the need for literature reviews as sources of information regarding safety, efficacy and ‘known use.’
Our medical writers come from diverse clinical, technical and scientific backgrounds to provide you with the objective answers you need to support your desired commercialization and business outcomes.
Labeling, Instructions for Use and User Manuals
Compliance with evolving legal labeling requirements is essential in all countries in which a medical device is distributed. Errors and inaccuracies in labeling and Instructions for Use (IFUs) may threaten patient safety and result in damaging recalls and fines.
Within the United States, the Food and Drug Administration (FDA) administers the Global Unique Device Identification Database (GUDID) and stipulates that from 24 September 2020, all devices—including Class I and unclassified medical devices—must have appropriate UDI labeling. This must include brand name, description, catalog number, handling instructions, expiration date and other pertinent information. NAMSA supports manufacturers in labeling needs by managing UDI exception requests and reviewing labeling and instruction requirements to ensure that all remaining portfolio products are compliant.
A similar, although crucially different, system will operate within the European Union with the rollout of a European Database on Medical Devices (EUDAMED), together with the introduction of European Medical Device Regulation (MDR) EU 2017/745. This will present significant challenges for many manufacturers, as although labeling requirements have been well defined under MDD, with harmonized standards and guidance documents available to aid interpretation, the MDR requirements described in the GSPRs (Annex I) are more complex and descriptive. They include a large number of new elements, especially for implantable and sterile devices, and currently there are no harmonized standards available to help navigate these requirements. Also new in the MDR is the “General requirements regarding the information supplied by the manufacturer” (SPR 23.1), indicating a new format for the presentation user information, more specifically; eLabeling as described in (EU) No 207/2012 on electronic IFUs for medical devices.
NAMSA’s multidisciplinary, cross-functional medical writing service teams understand the criticality of ensuring accuracy, completeness and ergonomic usability of information contained within medical device labels, IFUs and user instruction manuals. Working together, our regulatory and medical writing experts have extensive industry experience to help you design and review these materials to ensure compliance to applicable global regulatory requirements.
Periodic Safety Update Reports and Post-Market Surveillance Reports (PSURs | PMSRs)
The European Medical Device Regulation (MDR) EU 2017/745 requires the preparation of Post-Market Surveillance Reports (PMSR) or Periodic Safety Update Reports (PSUR); (Chapter VII, Article 86), depending on device classification. A PMSR is intended for low-risk class I devices, while a PSUR is intended for moderate and high-risk devices (class IIa, IIb, III, implantable). Irrespective of whether a medical device has a valid certificate under the Medical Device Directive (MDD) 93/42/EEC or MDR, all manufacturers must comply with Post-Market Surveillance requirements outlined in the MDR after the date of application of 26 May 2021.
NAMSA’s highly experienced medical writing team, together with our Clinical and Regulatory experts, support manufacturers in the production and submission of both PMSRs and PSURs. (See Table 1 below)
Table 1 : PMSRs and PSURs—Overview of Reporting Requirements by Risk Category
Scientific Publications & Conference Abstracts
With regular and frequent shifts in global regulatory requirements, reimbursement policies and user requirements, it is becoming increasingly important for manufacturers to demonstrate transparency. Also critical is ensuring that results obtained from Post-Market Surveillance activities and clinical studies are made publicly accessible and adequately described in clinical and scientific literature.
Publishing scientific articles in relevant medical journals and presenting clinical findings at conferences is beneficial for manufacturers. Scientific dissemination of pertinent information helps strengthen awareness and recognition of a product, together with the overall reputation of the manufacturer, among relevant clinical audiences. These activities may support a manufacturer’s marketing efforts and yield direct commercial benefits in terms of sales, especially when the results are favorable. However, even limited data and inconclusive findings, placed in the public domain, may positively influence third-party decisions relating to market access, reimbursement and clinical guidelines when combined with other available data (e.g., in the form of meta-analyses or health economic evaluations).
With solid industry experience and extensive scientific and academic training, NAMSA’s medical writing services can help your business take charge of its global presence and scientific assets. Even if you have not taken the opportunity to involve NAMSA in conducting your clinical research, our medical writers are available and ready to support you in disseminating clinical data regarding your products by preparing ready-to-submit scientific publications and conference abstracts. To achieve this, our medical writers will partner with your organization and study investigators, in addition to NAMSA’s Biostatistics Team (if required) to translate clinical findings into relevant, publishable scientific literature that will support your commercial objectives.
NAMSA’s professional medical writing services include a variety of evidence communication outputs, including:
- Advisory panel presentations
- Conference abstracts, presentations and posters
- Health economic evaluations
- Professional education materials (e.g. clinical summaries)
- Scientific publications, including journal articles (e.g. study protocols, case studies, PMCF studies, clinical trials, etc.)
- Systematic reviews and meta-analyses
- White papers and other manuscripts
Summaries of Safety & Clinical Performance (SSCPs)
The European Medical Device Regulation (MDR) EU 2017/745 requires that manufacturers of implantable and class III devices (other than custom-made or investigational devices) must create a Summary of Safety and Clinical Performance (SSCP). The SSCP document is intended to provide public access to an updated summary of clinical data and other information about a medical device’s safety and clinical performance for healthcare professionals and, if relevant, patients.
This obligation is in addition to the requirement for creating a Periodic Safety Update Report (PSUR). All manufacturers must comply with Post-Market Surveillance (PMS) requirements detailed in the MDR after the date of application on 26 May 2021, irrespective of whether a medical device has a valid certificate under the Medical Device Directive (MDD) 93/42/EEC or MDR.
NAMSA’s experts have carefully analyzed the requirements and recommendations of the MDR and associated guidance documents to help guide clients through this early period of enforcement with ease and confidence. Working closely with our EU Regulatory Team, NAMSA’s medical writers have the expertise and knowledge to create SSCPs tailored towards intended users/healthcare professionals and patient/lay audiences in compliance with these regulatory requirements.
Clinical Study Protocol
A clinical study protocol is a document that describes the objectives, design, methodology, statistical considerations and organization of a clinical trial. Ensuring the quality, validity and ethical conduct of a study involving human subjects is essential. A well-written protocol can ease the approval process by regulatory authorities, ethics committees, and funding agencies, ultimately ensuring the study is conducted as intended, avoiding costly delays and protocol amendments.
NAMSA offers a comprehensive service for developing clinical study protocols. Our experienced medical writers work closely with our clinical, regulatory and biostatistical experts to create protocols tailored to your specific device, indication and target market. We can help you define the study objectives, endpoints, sample size, inclusion and exclusion criteria, randomization and blinding procedures, data collection and analysis methods, safety monitoring and reporting requirements and any other aspects of your trial. We can also assist you with preparing informed consent forms, case report forms, investigator brochures and other study-related documents.
Whether you need a feasibility study, a pilot study, a pivotal trial, or a post-market surveillance study, NAMSA can help you design and execute a clinical study protocol that meets your regulatory and business needs.
Clinical Study Report
Clinical Study Reports (CSRs) are comprehensive documents that summarize the methods, results, and conclusions of a clinical trial. CSRs are essential for regulatory submissions, publications, and marketing activities. They provide the evidence to demonstrate the safety and effectiveness of a medical device in a specific patient population and clinical setting.
A well-written CSR can help speed up the approval process and the market acceptance of a medical device. A poorly written CSR can lead to delays, rejections, or unfavorable reviews. Therefore, qualified medical writers who understand the regulatory requirements, international standards, and the industry best practices, which will help raise the bar of your report development.
NAMSA offers a full range of clinical medical writing services, from protocol development to final report writing. Our medical writers have extensive knowledge and expertise in various therapeutic areas, device types, and study designs. They work closely with our biostatisticians, clinical operations team, and safety team to ensure the accuracy, consistency, and quality of the CSRs. Whether you need a CSR for a pilot study, a pivotal trial, or a post-market surveillance study, NAMSA can deliver it on time and within budget.
Please fill out the form below to download all our exclusive resources.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Your file is being downloaded. Please click here if you are not being redirected automatically.
MDR Clinical Evaluation Reports: Key Elements and Lessons Learned for Successful Compliance
Eu mdr part 1: current opportunities & challenges with clinical data-a namsa panel discussion, eu mdr part 2: how to approach clinical evidence requirements with clinical management & biostatistics, eu mdr 2017/745: optimizing cers within the medical device lifecycle, telling your medical device’s story to regulatory agencies, subscribe to namsa events newsletter.
By submitting this form, you are providing NAMSA consent to contact you directly regarding NAMSA’s services. The information you are providing will be processed and stored by NAMSA to better understand your product needs and interests.
At any time, you can submit a request to withdraw your consent for the use of information provided by you by emailing [email protected] . For additional information, please visit our Privacy Policy or contact us at [email protected] .
Join the NAMSA Network
Update your profile, post comments on the blog; sign-up for events, webinars and classes; receive email alerts and more.
Already have an account? Please Login Here
NAMSA Client Portal
Login to the NAMSA Client Portal for sample submissions, testing reports, project tracking, and more.
WELCOME TO THE NAMSA NETWORK!
Username or Email Address
Remember Me
Looking for Client Portal?
Visit NAMSA Client Portal for sample submissions, testing reports, project tracking, and more.
Not a member yet? Please register here .
Reset Password
Username or email
Get New Password
- Biospecimen Solutions Precision Value & Health Careers

- Clinical Trial Services Overview
- Clinical Trial Management
- Medical Writing
Medical Writing Services
Put the experience, exacting attention to detail, and robust qc processes of our medical writing team to work for your clinical trial. with advanced degrees in the life sciences, our in-house writing team understands the science behind your study and adds an extra layer of experienced data review and messaging that other medical writing teams can’t deliver., medical writing services with precision, our focus on quality, flexibility, and collaboration leads to high-quality deliverables and ensures efficient, accurate documentation.
Clinical Study Report Development Process
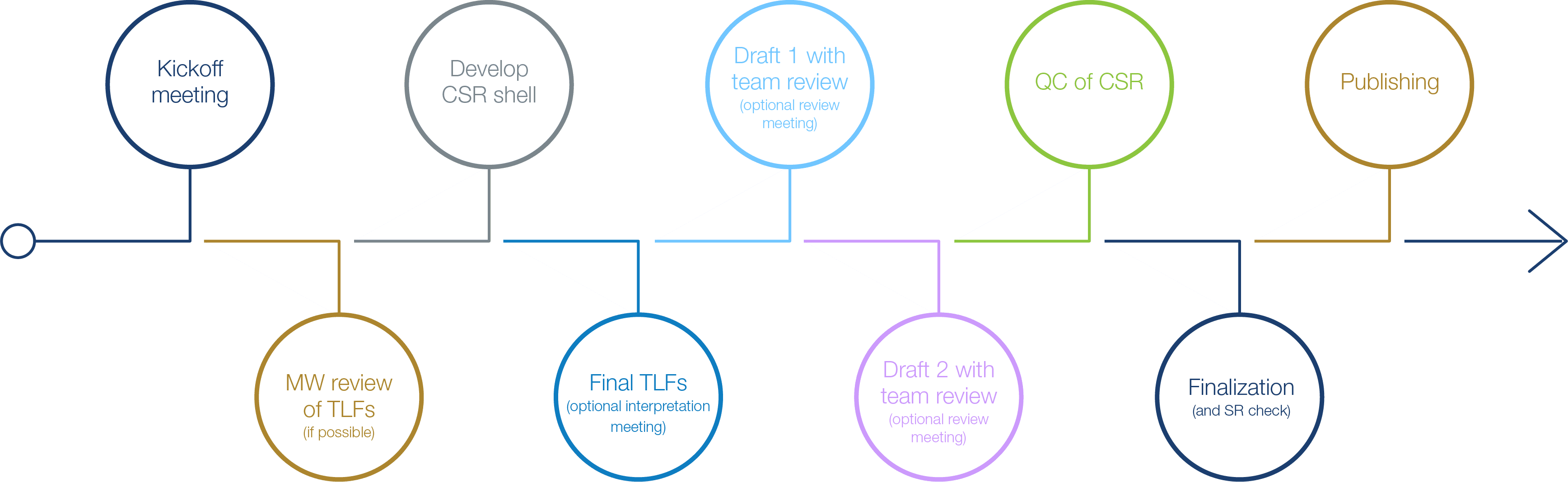
Deep experience
- In-house medical writers with PhDs in organic chemistry and molecular biology and an average of 8 years’ experience in medical writing
- Wide network of specialist medical writing consultants, with a vast array of expertise, each with over 15 years of experience
- Standardized and ICH-compliant processes for document development
- Robust QC review of documents against source data and documents maximizes the integrity of deliverables
Precision results
Our medical writing services include:
- Clinical Study Reports (and addenda/amendments)
- Protocols (and amendments)
- Marketing Application Summary Documents (ie, for NDAs/BLAs)
- Investigator Brochures
- Annual Reports/DSURs
- CSR Patient Narratives
Tell us about your project requirements
An experienced medical writing team is just one of the many reasons to choose Precision for Medicine. Contact us to learn more.
Clinical trial services
Global clinical trial footprint.
Sample processing labs, clinical trial sites and offices in five continents provide the clinical reach and scale to manage complex global programs.
Clinical Development Strategy
Tailored strategies consider the scientific, regulatory, and commercial factors that shape each trial, mitigating risk and advancing the development pathway.
Clinical Trial Design
Advanced trial-design approaches—including basket, umbrella, and adaptive trials—deliver biomarker driven clinical research. Deep experience in these highly complex trial designs maximizes both insights and efficiency.
Biostatistics
Seasoned biostatisticians and statistical programmers deliver insight into every trial phase, from study design to regulatory submissions, all backed by meticulous documentation and data monitoring.
Clinical Sample Management
Sample inventories from a global network of labs supply real-time processing in 55 countries; consolidated data from central labs, screening labs, and specialty labs with clinical data create actionable reports.
Explore other areas of expertise
Precision for Medicine is part of the Precision Medicine Group, an integrated team of experts that extends Precision for Medicine’s therapeutic development capabilities beyond approval and into launch strategies, marketing communication, and payer insights. As one company, the Precision Medicine Group helps pharmaceutical and life-sciences clients conquer product development and commercialization challenges in a rapidly evolving environment.

Medical Writing Services
TransPerfect’s Medical Writing and Publication practice group offers solutions for your clinical and non-clinical documentation needs, providing high-quality and on-time deliverables to keep your projects on track. As a quality service-minded medical writing agency, we’re one of the world’s most renowned global medical writing and translation enterprises.

Expert medical writing solutions to create regulated content for patients, HCPs, regulatory approvals and more.
Ensure speed, quality, and compliance for all of your scientific communication, technical writing, regulatory writing, disclosures, and medical education.
Multilingual Medical Writing, Editing, and Quality Control (QC)
We offer in-language medical writing for a variety of areas, including regulatory, scientific communications, disclosure, and medical education and information. TransPerfect’s services currently cover both medical writing for clinical trials and nonclinical documentation for biotech, medical device , life sciences organizations, pharmaceuticals, and CROs. Our medical writer pool specializes in 40+ therapeutic areas and 300+ documentation types.
Clinical Trial Disclosure
TransPerfect Medical Writing provides an end-to-end solution for plain language summaries of clinical trial results, lay language protocol synopses, and plain language publications. We also support protocol and results registry postings to regional registries, such as CT.gov and EudraCT. For those looking for EU CTR and Health Canada PRCI transparency support, we have a team dedicated to redaction and anonymization.
Plain Language Support
We offer a number of services to assist with your plain language initiatives, as this is an ever-growing need across the life science industry. We provide plain language content adaptation, reports, and protocols, readability certificates, and multi-language lay terminology glossaries. And our internal health literacy experts can help your company develop SOPs and WIs to make your plain language initiatives most successful.
Multi-language Meetings and Webinars
We offer both in-person and virtual meeting support. This includes planning, content development, KOL coordination, interpretation, hosting, reporting, and analytics. We can also provide our own virtual platform for meetings and webinars via TransPerfect-owned Trial Interactive.
Training Course Material and e-Learning
Whether for internal employee training or e-learning for patients, TransPerfect can bring your learning to life. We can develop content from scratch to be leveraged in e-learning modules, videos, and offline materials – all deliverables generated by our Learning Solutions team. Content can be translated into 200+ languages.
Transcription and Summaries of Conferences and Meetings
Our medical writers attend both virtual and in-person meetings across the globe to capture and summarize the main findings of investigator meetings, advisory boards, symposia, webinars, and more.
Graphic Design
TransPerfect’s life science designers can develop original scientific illustrations, figures, and infographics. We are also able to provide formatting and desktop publishing. This includes graphic design for presentations, videos, e-learning, medical marketing materials, and more.
Patient and Healthcare Provider Recruitment, Review, and Research
Connect your deliverables with leading HCPs and patients worldwide. Address the extent to which the proposed documentation is well-explained while also giving insight from the perspective of HCPs or patients with select criteria. TransPerfect also offers market research support via recruitment, moderation, data capture, and more.
Publication and Congress Activity Support
TransPerfect provides an end-to-end solution for congress activity and publication planning. We develop a custom plan for each publication with services including writing, author/review facilitation, graphic design, translation, pre-submission inquiries, target journal selection, copyright management, and journal submission. For congress activities, our internal SMEs provide oversight, strategy, and planning to help you achieve optimal reach within the scientific community .
Centralize Your Medical Writing and Regulatory Writing
TransPerfect streamlines the author-to-archive development of non-clinical and clinical documentation for companies such as Pfizer, Roche, and Novartis. We deliver 100% compliant documents while reducing timelines by over 30% and expenses by 25% on average, leveraging our 21 CFR Part 11-compliant collaborative authoring platform and team of over 100 certified medical writing experts.
Controlling Complexity in Clinical Content
Medical writing enables the collection, analysis, and distribution of complex scientific documentation. TransPerfect medical writers come from varying scientific backgrounds and therapeutic areas; most maintaining an advanced degree—MSc, MPH, PhD, or MD.
Departments frequently supported by our solutions include:
Medical/Technical/Regulatory Writing
TransPerfect's medical writing team provides comprehensive support to medical, technical, and regulatory writing departments, offering expert assistance in creating accurate, clear, and regulatory-compliant documents essential for successful clinical trials and product approvals.
Medical/Scientific Information and Education
TransPerfect's medical writing team offers exceptional support in the dissemination of medical and scientific information and education, ensuring that complex scientific data and research findings are accurately and clearly communicated to healthcare professionals and patients alike.
Clinical Trial Disclosure (CTD) or Data Transparency
TransPerfect's medical writing services extend vital support to Clinical Trial Disclosure (CTD) and data transparency departments by ensuring clear, compliant, and accessible communication of clinical trial results and data.
Medical/Scientific Affairs
TransPerfect’s medical writing team helps medical/scientific affairs departments adapt their delivery of important medical information, such as patient outcomes, to effectively reach relevant stakeholders and improve medical product development, the patient experience, and safety standards.
Site and Patient Engagement
TransPerfect’s medical writing team supports site and patient engagement departments with communications that foster stronger relationships and enhance participation and retention in clinical studies, ultimately contributing to the success of clinical trials.
Clinical Development or Research
TransPerfect's medical writing services include essential support to clinical development and research departments, offering expertise in the creation of precise, comprehensive, and regulatory-compliant documentation crucial for the successful progression of clinical trials.
Regulatory Affairs
TransPerfect's medical writing team delivers unmatched support to regulatory affairs departments by producing meticulously crafted documents that meet the stringent standards of global regulatory agencies.
Quality Assurance
TransPerfect's medical writing team provides significant support to QA departments, ensuring QA documentation upholds the highest standards of accuracy and compliance to reinforce the clinical research’s integrity.
Medical Governance
TransPerfect's medical writing services provide pivotal support to medical governance departments by ensuring that all documentation, from policy papers to compliance reports, adheres to the highest standards of clarity and accuracy.
Marketing and Communications
TransPerfect’s medical writing team supports marketing and communication departments by bridging the gap between complex scientific information and engaging marketing communications, creating impactful messages that resonate with diverse audiences.
Publications
TransPerfect’s medical writing team helps authors looking to get published effectively communicate complex research findings, ensuring that the scientific merit and integrity of the work are maintained and facilitating successful publication in esteemed journals.
Benefits of TransPerfect Medical Writing Solutions
Improve content process and efficiency
Eliminate the need for multiple vendors
Scale resources as needed
Support global multilingual content
Ensure 100% compliance
Guarantee quality, timely deliveries
Maintain version control and harmonization across teams to mitigate risk
Reduce delivery timelines
Increase savings from streamlined, end-to-end solutions
Increase patient comprehension and patient engagement
Trusted by Life Sciences Organizations Big and Small
Join TransPerfect's community of over 1,000 life sciences organizations, including pharma, biotech, CROs, IRBs, and agencies.

News and Thought Leadership
Our industry experts have in-depth understanding of the challenges, requirements, and goals for life sciences organizations involved in clinical trial management.
AI and MT "Ask an Expert" Office Hours - 4/10/2024
TransPerfect's experts gather to answer questions from global pharma, biotech, and medical device leaders regarding AI and MT use cases, challenges, opportunities, best practices, and more. ...
AI and MT "Ask an Expert" Office Hours": Clinical and Regulatory Documents and Content
Wed, 05/15/2024 - 12:00 | ...
AI & MT "Ask an Expert" Office Hours: Real World Data
Wed, 04/10/2024 - 12:00 | ...
Engaging Patient Communities: A Pathway to Clinical Trial Success
Tue, 04/16/2024 - 12:00 | ...
Multilingual Labeling: Considerations for Efficient, Cost Effective and Compliance Labeling
Accurate and compliant labeling of an investigational medicinal product (IMP) is an important and integral part of clinical trial operations. A misstep in labeling can lead to significant delays, affecting the entire study’s timeline and potentially incurring costs ranging from ...
An Overview of Generative AI and Machine Translation for Life Sciences
Global life sciences companies are realizing the potential artificial intelligence (AI) and machine learning (ML) has to drive efficiency in content and translation management. To capitalize on this, organizations need to identify the right solutions. Discover important ...
6 Pathways to Take Your MT Strategy to the Next Level in 2024
With 2024 well underway, innovators in the global life sciences and pharmaceutical industries continue to question how to maximize efficiencies and reduce complexities in clinical and regulatory content development and localization. As part of the broader technological roadmap, ...
Ready to Speed Timelines, Improve Quality, and Control Costs?

Medical & Regulatory Writing
Experience the transformative blend of precision and creativity in every regulatory submission, manuscript, and study report. partner with us to convey complex information with simplicity, making your work truly remarkable and impactful to its target audience..

Why partner with a professional medical writer?
"Professional medical writing support was associated with more complete reporting of clinical trial results and higher quality of written English."
Gattrell WT, Hopewell S, Young K, et al. Professional medical writing support and the quality of randomised controlled trial reporting: a cross-sectional study. BMJ Open. 2016 Feb 21;6(2):e010329.
The regulatory reviewers felt that medical writers have a “great and positive influence on document quality; they help keep documents clear, as brief as they can be, and consistent.”
Cooper J, Chamberlain James L, Affleck J, et al. Value of medical writing: the regulator’s perspective. AMWA. 2021;36(4).
"Research can often be judged only by its final written report. A meticulous study can be let down by poor writing, which may lead a reviewer to wonder if lack of attention to detail in the writing indicates lack of attention to detail in the research."
Goodman NW, Edwards MB, Black A. Medical writing: a prescription for clarity. 3rd ed. Cambridge University Press; 2006.

Get in Touch
AccelWrite LLC
United States
+1 (216) 394-4549
We are grateful for your submission!


Bespoke and Quality-Driven Medical Writing Solutions Bespoke and Quality-Driven Medical Writing Solutions
We offer professional medical writing services that are tailored to meet the specific needs of biotech, pharmaceutical, and medical device companies.

Our array of exceptional medical writing services is available on a project-by-project basis, as an integrated solution, or as a functional service provider (FSP).

Our Approach
Each element of our approach fits together perfectly like pieces of a puzzle.
Read more about how we would build a team best suited to your needs.

Therapy Areas
Our range of expertise means we can find the best solution for you.
View our specific experiences that may be relevant to your ongoing projects.

"A great pleasure interacting with Morula Health's project manager and medical writer working on our IMPD. Timely response to the inquiry and 100% on time delivery of the final product. Absolutely loved working with Morula Health and without a doubt will be my first choice if any of the upcoming project(s) might require medical writing"
VP Clinical Operations, US Biotech
45+ countries
35+ therapy areas, 30+ document types, 15+ marketed products.

Redefining Therapeutic Potential

Why Financial Executives Should Consider Outsourcing Medical Writing

Long-term FSP Partnership Facilitates Expansion from China to the US

Medical & Technical Writing Services
On average, our writers have 10 to 15 years of experience in medical writing. We specialize in briefing documents, annual reports, and more. We work with both large and small companies, and can handle any scope of work from full project oversight to supplementing an in-house writing team when you need a little extra help. Take a look at some of the types of writing we can do for you. Or, if you're interested in joining our team, learn more about working at Synterex .
- Briefing documents
- Protocols/amendments (phase 1-4, registries)
- Annual reports
- aNDAs, sNDAs
- NADAs, ANADAs, CNADAs (veterinary/animal health)
- Assessment aids (oncology)
- Breakthrough designations
- Clinical and nonclinical summaries (module 2)
- Clinicaltrials.gov and other trial disclosures
- CMC writing
- Fast-track applications
- Integrated summaries of safety and efficacy
- Literature research
- Manuscripts
- Marketing applications in eCTD format (MAA, NDS, jNDA)
- Nonclinical writing
- Orphan drug applications
- Pharmacovigilance documents (risk management)
- Development safety update reports (DSURs)
- Periodic benefit-risk evaluation reports (PBRERs)
- Periodic safety update reports (PSURs)
- Plain language summaries
- Prescribing information
- Regulatory briefing documents for agency meetings, including pre-IND, end of Phase 1, end of Phase 2/3, pre-NDA and advisory committee
- Regulatory responses
- Responses to agency reviewers
- White papers
- Clinical evidence reports (devices)
Looking for something else? See our other areas of expertise:

Professional Medical Writing Services
Customized high-quality medical writing solutions for healthcare providers. our clinical medical writers are trusted by industry leaders worldwide., proven medical writing solutions.
We are a leading content development company providing medical writing services to our esteemed partners. Medical writing plays an integral part in the healthcare domain, and we have a team of specialized healthcare writers from the pharmaceutical industry, clinical research organizations, and academia who deliver accurate, concise, and timely clinical and regulatory documents. All the documents prepared by Acadecraft go through scientific, statistical, editorial, and quality control reviews. Delivering high-quality writing services for a wide range of medical fields helping business partners achieve business goals.
Our medical writers use their expertise to simplify complex and diverse data with accuracy, creating documents that include but are not limited to-

Types of Medical Writing
Scientific/ publication writing, consumer health writing, regulatory /clinical research writing.
We offer scientific writing and publication services to various educational, research, and publishing houses. Our scientific documentation writing services include conference materials (abstracts, poster presentations, and slide sets), manuscripts, editorial support, journal submission, product website content, educational material for patients, healthcare professionals, and pharmaceutical industry personnel, medical marketing reviews and reports, literature reviews, and publication planning.
We ensure that our furnished writing material is accurate, up-to-date, concise, and uses correct academic terminology.

Our consumer health writing services aim to create informative and accessible documents to help consumers make educated decisions about their health and well-being. Some key documents we offer in our services include health brochures, patient education materials, health pamphlets, health fact sheets, health newsletters, health websites and blogs, health guides and manuals, and health campaign materials.
Our team of experienced medical writers is skilled in creating a wide range of clinical and regulatory documents:
- ICH GCP-compliant Clinical Study Reports (CSRs), including CSR Synopses for public disclosure
- Standard Operating Procedures (SOPs) covering all aspects of drug development, including the design, conduct, and reporting of clinical trials and the outsourcing of Sponsor responsibilities to a CRO
- Clinical and non-clinical sections of the Common Technical Document (CTD), including summaries and overviews for EU and US Regulatory Authorities
- Investigator brochures, study protocols, patient safety narratives
- Pharmacovigilance documents, such as Periodic Safety Update Reports
- Patient information, including informed consent, layperson summaries, patient brochures, and standalone quality checks

Our Clients
We are proud to have served a diverse range of clients, from small businesses to large corporations, in our commitment to accessibility.
What services do you offer under medical writing services?
We offer various services, including clinical research, scientific writing, regulatory writing, marketing writing, social media, manuscript, healthcare, and more.
How do you ensure quality?
We have a streamlined process where clinical writers and quality checkers pay attention to every detail at every step, ensuring that the end product is of the highest quality.
Do you offer niche medical writing services for clinical trials?
Yes, we do. We offer multiple niche-specific medical writing services. Our team of medical and regulatory writers is well-equipped with all the knowledge and resources required to write accurate details of clinical trials. Get an instant response by filling up our inquiry form .
Clients Testimonials
Acadecraft's Voice-Over service was amazing! The team provided accurate and culturally relevant recordings for what we expected. They showed true professionalism and expertise. We highly recommend Acadecraft for their excellent Voiceover services.
- Manav Malhotra
- Sr. Manager – Operations
Always impressed with Acadecraft's expertise! Their translation services play a vital role for our company to drive international growth within our team and clients.
- Alex Capizola
- Business Operations Executive
AcadeCraft's assessment content creation team was able to understand our unique requirements and created customized assessments that fit our needs. The team was prompt and professional, and the quality of their work was good.
Acadecraft have recorded several audiobooks for us. They have a wide range of talented artists with different accents who really bring our stories to life. Their work is of high quality, with good attention to detail.
Acadecraft are reliable, efficient and friendly. Their services are highly recommended by us.
- Mazlini Kirsty Louise
- Editorial Head
As a producer, I've had the pleasure of using Acadecraft for sourcing VO and liaising with artists for several film projects. They offer a wide range of VO profiles and the artists I have collaborated with all were talented and professional. The team at Acadecraft have supported me with great professionalism, responsiveness and creativity. I highly recommend their services.
- Katia Hérault
- Head of Production
Acadecraft has been helpful with connecting our editorial team with subject matter experts (SMEs) who help us QA assessments and create solutions for computational assessments. They have been able to find SMEs to meet our needs and our deadlines. We are happy to continue to partner with Acadecraft.
- Managing Editor
Acadecraft team is always very supportive, and we and Acadecraft corroborate to create educational contents for K12 Students in India.
We appreciate Acadecraft teams' professionality, punctuality, creation skills in each subject.
- Mikiko Matsuoka
- Content Manager
I am thrilled to share my testimonial for Acadecraft which creates interactive and engaging content. Working with this team has been an absolute pleasure from start to finish. Not only did they create outstanding content for our project, but they also went above and beyond to ensure that it was interactive, engaging, and effective.
Throughout the entire process, the team was highly cooperative and communicative, always available to resolve any issues or concerns that arose. They truly made us feel like partners in the project, and their dedication to delivering high-quality content was evident in every interaction.
Thanks to their exceptional work, our project was a huge success, and we couldn't be happier with the results. I highly recommend them to anyone looking for a team that is passionate, professional, and committed to excellence. Wishing them all the best in their future endeavors.
- Hemika Kumar
- Ed-Tech Program Lead
The team at Acadecraft has truly been an end-to-end service provider for us, providing content development services and their commitment, attention to detail and expertise have made the project a success. Their team's dedication, attention to detail, and expertise have been unmatched, making our partnership an absolute pleasure. We highly recommend Acadecraft to anyone looking for a reliable and efficient education solutions provider.
- Yogesh Malhotra
- Senior Manager Team - Program Management
Our experience working with Acadecraft has been great. Their highly knowledgeable team of experts was always available to answer our questions, provide guidance, and ensure we were delighted with the services. Their thorough, accurate assessments provided valuable insights that helped us make informed decisions about our exam performances.
We look forward to continuing our partnership with Acadecraft and leveraging their expertise to help us achieve our business goals.
- Sohail Ahmed
- Senior Manager
I recently used Acadecraft's Video Editing services and I am extremely impressed with the quality of their work. The team at Acadecraft was highly professional, attentive and skilled in delivering my company’s project on time and within budget.
Their attention to detail was impeccable, and they understood my needs and requirements very well. They were able to create a video that not only met my expectations, but far exceeded them.
Throughout the process, they kept me informed and updated on the progress of the project, and were always available to answer any questions I had. Their customer service was excellent, and they were always friendly and easy to work with.
I highly recommend Acadecraft's Video Editing services to anyone who is looking for a high-quality and professional video editing experience. They are truly experts in their field and I look forward to working with them again in the future.
- Senior Executive
The video creation team of Acadecraft is insightful. They understood my requirements carefully and delivered a winning video that perfectly aligned with my business needs.
With a good script, content, sound, and editing – Acadecraft helped me with the best video content to strategize my marketing and promotional campaigns. Their tremendous experience in video editing and professionalism in serving the customer before and after delivering services are commendable.
The passionate team knows great about getting into the details and providing impeccable video services. I am extremely impressed by the work Acadecraft has delivered to me.
I appreciate my collaboration with Acadecraft and look forward to availing of services again.
- Ganesh Sonawane
- Founder & CEO
I required an explainer video for my business, and I am mesmerized by the work Acadecraft’s video editing team delivered to me. The perfectly aligned video elements and superb editing demonstrate the experience, knowledge, and professionalism Acadecraft has.
Acadecraft’s 3d video solutions are amazing. They used a perfect blend of art, color, shape, sound, and editing to create the video, making the video engaging and immersive.
I have always been excited to explore the opportunities of videos in business, and it was my pleasure to make Acadecraft my companion for the best video solutions. I highly recommend this organization and would love to collaborate with them again.
With a holistic approach to creating powerful blended videos, Acadecraft delivered me a well-developed video solution. I appreciate the relentless efforts of the video editing team, whose in-depth knowledge and analytical skills effectively catered to my needs.
The services Acadecraft has given me exceeded my expectations; the team was effective and listened to my requirements carefully, and went the extra mile in researching and creatively developing awesome pieces of video content.
Not only from a quality perspective but on the management and delivery front, Acadecraft’s services are prolific. They stuck to the turnaround time and were constantly in touch with me throughout the creation process.
I recommend Acadecraft for video solutions as they have great hands-on use of animation, graphics, and other creative assets.
- Shweta Patidar
I am thoroughly astounded by Acadecraft's proficient skills! Their exceptional voiceover and translation services were instrumental in amplifying our marketing endeavors and video promotions. They enabled us to communicate effectively with varied audiences and significantly propelled growth across numerous media platforms.
- Sparsh Verma
- Marketing Strategist
Working along with Acadecraft has been an exceptional journey. Their meticulous attention to detail and commitment to maintaining the essence of the content in the transition from English to Arabic was truly impressive. The collaborative spirit and timely communication made the entire process smooth and enjoyable. Without a doubt, I wholeheartedly endorse their services for a remarkable translation experience.
- Yashashwini V Rathod
- Account Director
I am delighted to share my feedback with you on working on the editing and print layout of 9 documents under the KECF project. Overall, I am very happy with the speed with which these documents were edited and the fine attention to details. I also deeply appreciate the quick corrections that were made on our request. Particularly, working with Sanjana was a real pleasure, as I found her very attentive to our requests and her internal quality checks before she sent the documents to us greatly reduced my workload. So many thanks Sanjana! I look forward to working with your agency if such a need arises in future too. Many thanks to Sanjana and your entire team.
- Programme Management Officer
Grab a FREE Accessibility Audit Today!

Expand your website reach.


- How we work
Testimonials
- Case studies
- Services Industries
- Success Stories
- Video Testimonials
- Infographics
- Our Services
- Contact Us
- MORTGAGE SERVICES
- CALL CENTER SERVICES
- DATA SERVICES
- SOFTWARE DEVELOPMENT SERVICES
- HEALTHCARE BPO
- CREATIVE SERVICES
- PHOTO EDITING
- INSURANCE BPO SERVICES
- FINANCE & ACCOUNTING
- ENGINEERING SERVICES
- DATA SCIENCE
- AUTOMATION SERVICES
- RESEARCH & ANALYSIS
- TRANSCRIPTION SERVICES
- LEGAL PROCESS OUTSOURCING
- TRANSLATION SERVICES
- CUSTOMS BROKERAGE
- LOGISTICS SERVICES
- Outsource Services Home
- Research & Analysis
- Pharmaceutical Research
Medical Writing Services

Achieve Precision with our Expert Medical Writing Services.
From Clinical Trials to Peer-Reviewed Publications, Conquer Time Constraints and Complexity, Streamlining Documentation Along the Way.
The resource-intensiveness demanded by in-depth research and the need for specialized expertise exerts immense pressure on healthcare practices. Moreover, time constraints further create complexities in ensuring comprehensive and accurate documentation. As these challenges converge, healthcare practices often find themselves grappling with resource limitations, missed deadlines, and the compromising of documentation quality. This is where our expert medical writing services come to your aid. With a team of seasoned professionals, we offer a strategic partnership that alleviates the strain on your resources, harnesses specialized expertise, ensures timely delivery, and guarantees meticulous quality assurance.
Partner with us to access comprehensive solutions that deliver insightful, thoroughly researched outcomes. Our services ensure the utmost confidentiality, unwavering quality, and remarkably swift turnarounds. Trust us to enhance your communication across a broad spectrum of medical research and analysis domains, including clinical trial documentation, peer-reviewed publications, systematic literature reviews, health economics and outcomes research, pharmacovigilance documents, and more. Our offerings are finely tuned to address the specific requirements of pharmaceutical companies, research institutions, and healthcare organizations, delivering regulatory-compliant content that resonates with your target audience.
Discover How Our Expert Medical Writing Enhances Your Research Reach.
Professional Medical Writing Services We Offer
We have been a pioneer in providing the best quality medical writing support services to clients around the globe. We have the skills and expertise to understand each client's unique business requirements and provide customized medical writing support services. Some of the key professional medical writing support services we offer include -
Scientific Writing Services

Through meticulous research synthesis and in-depth understanding, we create research-backed manuscripts that resonate with your target audience, advancing your scientific contributions. We ensure precision, adherence to standards, and impactful content, bolstering your reputation in the medical community.
Medical Dissertation Writing Services

Our experts carefully structure and craft dissertations that adhere to academic standards. By outsourcing your medical dissertation to us, you ensure well-researched and articulate content that reflects your expertise, presenting a polished representation of your research efforts.
Clinical Medical Writer Services

Navigate clinical documentation complexities with our clinical medical writer services. Our writers, experienced in clinical research, synthesize data into precise, regulatory-compliant documentation. We ensure accuracy and coherence from protocols to study reports, facilitating efficient communication with regulatory bodies and peers.
Global Medical Writing and Translation Services

Extend your reach with our global medical writing and translation services. We craft content transcending language barriers, ensuring accurate and culturally relevant communication. Through our expertise in translation and localization, we help you effectively engage diverse audiences while maintaining scientific integrity.
Medical Device Writing Services

Enhance medical device communication with our specialized medical device writing services. Our writers elucidate intricate device functionalities, ensuring clarity for both healthcare professionals and patients. By outsourcing device documentation to us, you present comprehensive, user-friendly materials that facilitate safe and effective device utilization.
Manuscript Writing Services

Advance your research visibility with our manuscript writing services. Our writers transform raw data into polished manuscripts for journals. We create compelling narratives that adhere to journal guidelines through data analysis and interpretation, ensuring your research resonates with peer reviewers.
Scientific Manuscript Writing Services

Elevate your research publication journey with our scientific manuscript writing services. Our experts skillfully structure manuscripts, ensuring coherence and adherence to journal standards. By outsourcing scientific manuscripts to us, you present research-backed content that navigates the intricacies of peer-reviewed publishing.
Drug Brochures Writing Services

Effectively communicate drug information with our drug brochure writing services. Our writers distill complex drug details into accessible content for patients and healthcare professionals. By outsourcing drug brochure creation to us, you ensure informative materials that facilitate understanding and adherence.
Clinical Papers Writing Services

Navigate clinical research communication with our clinical paper writing services. Our writers synthesize complex trial data into concise clinical papers. We create compelling papers that contribute meaningfully to medical literature through expert data interpretation, strengthening your practice's research portfolio.
Multilingual Medical Writing Services

Broaden your global impact with our multilingual medical writing solutions. Our specialists create content that transcends linguistic barriers, ensuring accurate and culturally relevant communication. Through efficient translation and adaptation, we help you effectively engage diverse audiences while maintaining scientific precision.
- Investigator's Brochures Writing Services
- Medical Compliance Writing Services
- Medical Editing for Scientific Materials
- Patient Diaries Writing Services
- Publication Planning Writing Services
- Scientific Information Brochures Writing Services
- Scientific Literature Research Writing Services
- Scientific Research Papers Writing Services
- Medical Conference Coverage Writing Services
- Medical Journalism Writing Services
- TransPerfect Medical Writing Services
- Patient Education Writing Services
Why Choose Us as Your Medical Writing Company?
We offer a comprehensive array of benefits that empower your practice with expertly crafted content and streamlined communication. Here's why we stand out as your ideal medical writing service provider -
High-Quality Services
As an ISO-certified firm, we guarantee regulatory medical writing support of the highest quality, devoid of errors. Our meticulous approach ensures your content reflects precision and compliance.
Short Turnaround Time
Operating across various delivery locations, we ensure quick turnarounds for regulatory medical writing services without compromising quality. Our commitment to punctuality aligns with your time-sensitive needs.
Information Security
Our ISO/IEC 27001:2022 ISMS certification underscores our dedication to maintaining the utmost security and confidentiality in all our professional medical writing support services.
World-class Infrastructure
With access to cutting-edge medical writing tools, technologies, and world-class office spaces, we're primed to deliver excellence in every aspect of our service.
Our team comprises highly skilled and experienced medical writing experts who effortlessly address your diverse requirements, ensuring each piece of content meets the highest standards.

Round the Clock Support
Our customer support team is on standby 24/7, ready to answer your queries, provide guidance, and ensure uninterrupted communication.
Flexible Pricing Packages
Our commitment to affordability is matched by flexibility. Our pricing packages are designed to align with your budget and business requirements, ensuring cost-effectiveness without compromising on quality.
Additional Services We Offer
Content development services.
Our expert writers create tailored content to match your goals, be it patient education, research insights, or informative articles. Outsourcing content development streamlines your messaging, delivering a consistent and impactful narrative that resonates across audiences.
From research papers to clinical study reports, we ensure precision and compelling content that advances your credibility. Outsourcing scientific writing ensures impactful research dissemination and a polished professional image.
Content Rewriting Services
Whether updating patient information or re purposing educational content, we enhance clarity and relevance. Outsourcing content rewriting refreshes your communication approach, effectively conveying information with a professional edge.
Content Editing Services
We fine-tune grammar, structure, and clarity, leaving a lasting impression of your expertise. Outsourcing content editing presents a polished image, engaging your audience effectively and solidifying your professional standing.
Client Success Stories

We Provided Automation of STAT Reports to Barbados-based Radiologists
A Barbados-based group of radiologists approached us with the requirement of automating their STAT reporting process. Our team of teleradiology experts provided the services in no time.

We Helped a US-based Healthcare Client Develop a Forecasting Model
A leading US-based healthcare company was looking for help with the development of a forecasting model for their business. Our team helped them with cost-effective services.
The services that the company and staff have provided thus far are greatly appreciated. Every time I needed something changed, the team was quick to accommodate it. I do not have anything they need to focus on right now, but if anything comes up, I will be sure to let you know. Thank you!
Outsource Medical Writing Support Services
Our specialized medical writing support services go beyond mere documentation. Being a leading service provider with over 18 years of experience in the industry, we understand the pressure of translating intricate data into compliant content. From navigating intricate regulatory requirements to crafting accurate and engaging content, we ensure compliance and quality at every step.
With our team of expert medical writers possessing diverse backgrounds and specializations, you gain access to a wealth of knowledge that enhances the credibility of your practice. Our collaborative approach allows us to create in-depth and well-researched content, providing you with the tools to effectively communicate with patients, peers, and regulatory bodies.
Ready to Optimize Your Medical Communication?

Avail best-in-class services at affordable rates
Get a FREE QUOTE!
Decide in 24 hours whether outsourcing will work for you.
Important Information : We are an offshore firm. All design calculations/permit drawings and submissions are required to comply with your country/region submission norms. Ensure that you have a Professional Engineer to advise and guide on these norms.
Important Note: For all CNC Services : You are required to provide accurate details of the shop floor, tool setup, machine availability and control systems. We base our calculations and drawings based on this input. We deal exclusively with(names of tools).
Read our Privacy Policy .
800-514-7456

Live chat with us
Flatworld Solutions
116 Village Blvd, Suite 200, Princeton, NJ 08540
PHILIPPINES
Aeon Towers, J.P. Laurel Avenue, Bajada, Davao 8000
KSS Building, Buhangin Road Cor Olive Street, Davao City 8000
Flatworld Solutions Pvt. Ltd.
No.6, Banaswadi Main Road, Dodda Banaswadi, Bangalore - 560 043
#81, Survey No.11, Indraprastha, Gubbi Cross, Kothanur P.O., Hennur Bagalur Main Road, Bangalore - 560 077
Corporate Court, #15, Infantry Road, Bangalore - 560 001
Flatworld Mortgage Pvt. Ltd.
No.744, 15th Cross, 24th Main, J P Nagar 6th Phase, Bangalore - 560 078
Frequently Asked Questions (FAQs)
How to choose the right medical writing services company.
Select a medical writing company with a proven track record in producing accurate, compliant, and tailored content. Prioritize providers that demonstrate expertise in the medical field, align with your industry-specific requirements, and have a history of meeting deadlines consistently. A reliable choice will offer a comprehensive range of services, ensuring they can cater to your diverse medical writing needs effectively.
What are the benefits of choosing medical writing services company?
Opting for a medical writing services company ensures access to accurate, compliant, and well-structured medical content. This saves your practice valuable time by outsourcing content creation, enabling healthcare professionals to focus on patient care. Moreover, professionally crafted materials enhance communication with patients, peers, and regulatory bodies, elevating your practice's credibility and authority in the field.
How much does the custom medical writing services cost?
Custom medical writing service costs are influenced by factors like project scope, complexity, and specific requirements. Our competitive pricing model is designed to offer flexibility, tailoring costs to the unique needs of your project. Reach out to us for a personalized quote that aligns with your budget and project goals.
What background and specializations do our medical writers have?
Our medical writers bring diverse backgrounds and specialized expertise to your projects. With professionals well-versed in clinical research, regulatory affairs, medical documentation, and more, we ensure your content is created by experts who understand the nuances of your specific field. This ensures accuracy, relevance, and precision in every piece of content we produce.
Is our team capable of providing in-depth and well-researched content?
Absolutely. Our writers are proficient in conducting comprehensive research to provide in-depth and well-informed content. Each piece we create is backed by credible sources and industry best practices. You can trust that our writers will deliver content that is both accurate and thoroughly researched, meeting the highest standards of quality.

Together for Documenting Results
Medical writing.
Developing and marketing medical products requires a constant output of high quality scientific writing. APCER Life Sciences has an experienced team of writers with clinical, medical, and regulatory expertise to help companies document efficacy and safety and stay in compliance worldwide throughout the product lifecycle. With strengths in project governance and information technology, APCER develops and executes integrated processes that facilitate the transfer of data and knowledge. All content is either written by or written under the close supervision of a physician, always.
Scientific Communication
- Manuscripts
- Conference slide kits
- Review articles
- Drug monographs
- CME slide kits
- Standard response letters and FAQs
- Customized response letters
- Reimbursement dossiers
- Medication guides and questionnaires
- Patient information leaflets
Clinical Writing
- Investigator brochures
- Clinical study protocols
- Informed consent documents
- Clinical study reports
- Patient safety narratives
Regulatory Writing
- Common Technical Documents
- Clinical and non-clinical overviews
- Summary of product characteristics
- Clinical expert statements
- Briefing documents
- Labelling and core data sheets
Benefit/Risk Reports
- Annual updates
- Aggregate reports
- Risk management plans
- Safety update reports
Web Synopses
- Clinicaltrials.gov
- EU clinical trials register
- Company-specific websites

Together , because events require analysis
The apcer advantage.

Domain Expertise
- 100+ physicians, 90% healthcare professionals supporting drug safety and Pharmacovigilance
- Experience and expertise in different product types including Drugs, Vaccines, Biologics, Biosimilars, Cell and Gene Therapy products / Advanced Therapy Medicinal Products, Medical Devices, and Combination products
- Medical and scientific writing to support global product approval and post marketing requirements

Client Focus
- Scalable and flexible operating models
- Strong governance with collaborative approach
- Outcome-driven engagements
- End-to-end Pharmacovigilance, Medical Information, Regulatory Affairs, Quality Assurance and Medical Writing activities throughout the Product lifecycle

Global Presence
- Presence in US, UK, Europe, and Asia
- Safety reporting capabilities in 100+ countries
- Medical Information coverage in 50+ countries and 30+ languages, scalable to 100+ languages
- Developing and contributing to regulatory strategies, to maintain global regulatory compliance across the product life cycle

Quality and Compliance
- Extensive experience in supporting successful Regulatory Inspections
- ISO accreditations received: ISO 9001:2015 (QMS) ISO 27001:2013 (ISMS) ISO 27701:2019 (PIMS)
Explore More

Updating a complex Investigator Brochure (IB) for an oncology product within demanding timelines

Standardization and publication of evidence-based local recommendations for pharmacological treatment of pain

Accelerating product approval by handling complex and voluminous medical writing projects

Efficiently navigating the regulatory submission landscape

Streamlining lifecycle management through proficient eCTD submission roadmap

Medical Writing Services

Pharmaceutical Product Lifecycle Management: A Regulatory Consideration

Personalized Therapy with Advanced Therapy Medicinal Products: A Paradigm Shift
Testimonials.

APCER’s speed, responsiveness and flexibility to meet our safety needs is something we have always valued. APCER has helped harmonize our global pharmacovigilance operations and made us compliant with ever changing regulations.
Medical Director

The audit was carried out professionally and objectively. The scope of the audit was relevant and appropriate, and time efficient. The discussions during the audit were constructive and recommendations where helpful and appropriate. The team members involved in the audit had a positive experience and were made to feel at ease by the auditors.
QPPV and Pharmacovigilance Head

We are thankful to team APCER for bringing in strong practices & helping inspection readiness in our pharmacovigilance framework which led to successful FDA audits.
Senior Director

APCER’s invaluable support with Clinical trial disclosure deadlines helped us in timely submissions of periodic reports.
Head, Clinical Trial Transparency

We appreciate APCER for its efficiency in responding to matters that they get even at the eleventh hour. Working with APCER has made compliance a lot easier.
AVP, Pharmacovigilance
How can we work together for better health.
Upload Document ( Optional ) (Format Supported : PDF, DOC or DOCX | Max File Size : 1 MB)
Please check here to indicate your consent to receive catalogues, service offerings and marketing related emails from APCER Life Sciences.
Please note that by submitting this form, you consent to your personal data being processed in alignment with our Privacy Policy . APCER is committed to ensuring the security and protection of your personal information. Your personal data will be processed for purpose of facilitating your request and may be used for sending you additional marketing and business development-related information about APCER Life Sciences, its affiliates and our services. You can withdraw your consent by writing to us at [email protected] .
- Have any questions?
- (+1) 609 455 1600
- [email protected]

Professional Medical Writing & Editing Services
Top-notch medical writing and editing services from skilled writers, you wear enough hats throughout the day — professional medical writer doesn’t have to be one of them..
Perhaps you’re here because:
- You need to communicate your research and work to others, but you don’t like writing.
- You’ve found a writer but they don’t have the expertise to handle scientific and medical writing requirements, like FDA compliance.
- Your company needs compelling content for patients and consumers, but from writers with industry knowledge and credibility.
- Your in-house writers are overwhelmed with projects and have no time for additional work.

This is where we come in. Let us transform your initial concept into expert medical writing ready for publication.

The Med Writers team can tackle any writing task — from medical manuscripts and grant applications to blog articles and product descriptions. We also offer medical manuscripts editing services to our clients.
While each project is different, we offer the same benefits to every client:
- Some of the fastest service of any medical writing company to meet your tight deadlines.
- Flat-fee pricing that matches any budget and is tailored to your specific project.
- A team of PhD medical scientists and physicians based in the US, who combine their experience in the field with a passion for writing.
- Copy that generates leads and boosts conversions through best practices like SEO optimization and brand voice.
- A money-back satisfaction guarantee.
Medical and scientific topics are inherently complex, but our writing isn’t. We produce highly engaging pieces for lay audiences that are conversational yet informative. But we’re equally proficient at writing high-quality technical documents for specialists. Hire us for your next writing project and cross one more thing off your to-do list!
CONTENT WRITING
Educate and engage your audience with blog posts, social media captions, ebooks, video scripts, newsletters and more
Read More ->
COPYWRITING
Convert your audience with website copy, product descriptions, landing pages, ads, brochures, flyers, webinar scripts and more.
EDITING AND COPY AUDITS
Have a fresh (and professional) set of eyes review your existing content, copy, or documents. We can rewrite, optimize for the web, or simply make suggestions for improvement, depending on your needs.
TECHNICAL & B2B WRITING
Present your work via medical manuscripts, grant proposals, white papers, press releases and more.
Medical Content Writing
Some might say that content is secondary to sales-driving copy. But medical content is integral to your organization’s marketing strategy — it grows your online presence, positions you as an authority in your field, and builds valuable relationships. And it just so happens to be our expertise.
Our medical writers craft informative content that keeps your audience engaged, including:
- Social media captions
- Video scripts
- Newsletters

Our Process:
- 1 Contact us and tell us a little bit about your medical content writing project.
- 2 We’ll get you an initial quote within 2 hours. (Yes, 2!)
- 3 Next, we’ll book a call to fully explore your needs and project details with our project manager and an expert medical writer.
- 4 Sit back while we transform your ideas into appealing educational content.
Medical Copywriting
Browsers, inboxes, and social feeds are flooded with sales pitches 24/7 — you have to compete if you want to convert. Thankfully, our medical writers have a way with words. We’ll convince your audience that your specific service or product solves their exact problem.
Our writers create persuasive copy that turns leads into sales, including:
- Product descriptions
- Landing/sales pages
- Webinar scripts
- Email funnels

- 1 Contact us and tell us a little bit about your medical copywriting project.
- 2 We’ll get you an initial quote within 2 hours. (No, that’s not a typo!)
- 4 Sit back while we transform your ideas into compelling copy.
Technical and B2B Writing
Do you have something to say to specialist or business audiences? Technical writing requires a high degree of accuracy and complicated formatting. Our writers already know the ins and outs of scientific and medical writing, saving you precious time and annoying headaches.
Our medical writers are well-versed in technical and B2B writing, including:
- Medical manuscripts
- Grant proposals
- White papers
- Press releases
PowerPoint presentations

- 1 Contact us and tell us a little bit about your technical or B2B writing project.
We’ll get you an initial quote within 2 hours. (Yes, 2!)
Next, we’ll book a call to fully explore your needs and project details with our project manager and an expert medical writer.
- 4 Sit back while we transform your ideas into ready-to-publish writing.
Editing and Copy Audits
Sometimes all you need is a fresh set of eyes to look at your existing content, copy, or documents. Our medical writers can review what you have and turn it into what you need.
Perhaps your existing copy was written awhile ago and needs to be updated according to new science. Maybe it’s lacking references or is no longer compliant with your industry’s standards. Or you need help with search engine optimization (SEO) keywords and formatting.
We have medical writers with the know-how to get your existing copy working better for you and your organization.
- 1 Contact us and tell us a little bit about why you need editing or a copy audit.
- 2 We’ll get you an initial quote within 2 hours. (Yes, you read that right!)
- 4 Sit back while we elevate your existing copy.

Get your project quote within two hours!
Medical Writing professionals with passion to precision
Emtex offers worldwide professional medical writing and related services to the pharmaceutical (human & veterinary), biotech, medical device and other industries, CROs and academic institutions.
With over 20 years of experience in the field, we guarantee the best possible medical writing and related services.
Explore our services?
Our Services
- Abstract (ABST)
- Ad-hoc Analyses
- Briefing Document (BRD) / Briefing Book
- Briefing Package Review
- Case Report
- Case Report Form (CRF)
- Clinical Development Plan
- Clinical Evaluation Plan (CEP)
- Clinical Evaluation Report (CER)
- Clinical Investigation Plan (CIP)
- Clinical Investigation Report (CIR)
- Clinical Overview (CLO)
- Clinical Study Report (including narratives) (CSR)
- Clinical Summary
- Clinical Trial Protocol (CTP)
- Common Technical Document (CTD)
- CSR statistical Authorship
- Development Safety Update Report (DSUR)
- Digital content and visualization
- Educational or Promotional Materials
- Electronic Informed Consent Form (eICF)
- General Investigational Plan (GIP)
- Informed Consent Form (ICF)
- Investigator’s Brochure (IB)
- Layperson Summary/Plain Language Summary (PLS)
- Lean Writing
- Life Focus Groups
- Literature Review
- Manuscript (MSCT)
- Medical Translations
- Meeting & Conference Report (MCR)
- Narrative (NARR)
- Online Panel Survey
- Pediatric Investigation/Study Plan (PIP/PSP)
- Periodic Benefit-risk Evaluation Report (PBRER)
- Pharmacovigilance Plan (PV Plan)
- Previous Human Experience (PHE)
- Protocol Review
- Quality Control Services (QC)
- Randomization
- Response Documents/Response to Questions (RtQ)
- Review CRF – Case Report Form
- Risk Evaluation and Mitigation Strategies (REMS)
- Risk Management Plan (RMP)
- Sample Size
- Scientific Poster (PSTR)
- Statistical Analysis Plan – Writing & Review
- Statistical Consultancy
- Statistical Expertise and Support
- Study Design
- Study objectives & Endpoints
- Summary of Clinical Efficacy (SCE)
- Summary of Clinical Safety (SCS)
- Summary of Results
- Support DSMB, Interim and DMC meetings (Charter, member, data presentation, meeting report)
- Support publications (Abstracts, Posters)
- Tailored Courses
- Training Sessions
- Transform Data into Graphics and Dashboards
Medical writing & related services
We have the right expertise to deliver high quality and offer cost-effective solutions.
Medical writing and related services. Our passionate and dedicated team has the required know-how and skills to provide you with professional medical writing and related services tailored to your needs. In-depth experience gained from involvement in over 1200 projects ensures an efficient approach to provide optimal support for your project(s).
Our services
Emtex at a glance
Some key facts about Emtex.
Want to find out more?
Value for you
- Emtex delivers high quality
- Emtex helps freeing your time & resources
- Emtex gives cost-effective solutions
- Emtex ensures continuity and long-term relationships
More value for you
This website uses (anonymous) analytical cookies.

- Content Marketing Packages
- Blog Content
- Social Content
- Geo-Location Content
- Press Release
- Landing Page Content
- Email Content
- Ebook Content
- HVAC/Home Services
- Interior Design
- Mental Health
- Real Estate
- For Agencies
- For Businesses
- Frequently Asked Questions
- Get in Touch
(888) 456-2790
(121) 255-53333
[email protected]
Theodore Lowe, Ap #867-859 Sit Rd, Azusa New York
Attract new patients with professional medical writing services
Whether you manage a large health system, local med spa, or innovative cosmetic surgery center, you know firsthand just how competitive the medical sector has become. Fortunately, you can nurture feelings of confidence among your patients and make your brand stand out with Write Collective’s professional medical writing services.

Our work has appeared here:

Our professional medical writing services
Many medical content writing companies specialize in a particular type of copy or serve only a niche segment of the healthcare space. Thanks to our diverse team of talented medical copywriters, Write Collective can offer a broad array of content. By meeting all of your copy needs, we ensure tonal consistency and exceptional content quality when generating material such as:
Blog content
Our medical writing services are the perfect way to keep your blog pages stocked with fresh, captivating copy. You can use this service to discuss your qualifications, educate patients on procedures and treatments, and remove friction from the care delivery process.
Social media copywriting
Do you want to stand out from your competitors and connect with patients across new channels? Hire our medical copywriters and tap into unlimited social media copy for your organization’s social pages.
FAQ pages are a valuable tool in the medical marketing space. By including FAQs on your site, you can clear up misconceptions about services and put your patients at ease before they ever set foot in your facility.
Geo-location pages
Geo-location pages strategically incorporate location-specific keywords to make your content more visible among local audiences.
Press releases
Write Collective’s press release services facilitate clear, confident communication between your healthcare organization and your stakeholders.
Content marketing packages
Are you ready to reinvigorate your digital content? Check out our content marketing packages and let Write Collective’s team of talented medical writers provide you with high-quality copy.

Convey your capabilities with our medical writers
Whether a patient is seeking necessary medical care or pursuing their ideal look by undergoing cosmetic treatments, it is essential that you make them feel confident in your capabilities. Working with a Write Collective medical content writer enables you to form meaningful connections with your audience and show off your skills in a way that helps you attract new clients.
Don’t take our word for it
We work with a handful of insanely talented writers and we keep our business small intentionally with a focus on quality. Sound too good to be true? Check what our clients have to say.

David Thompson
James Bowen

Jared Pomranky

Got any questions?
Are you ready to start? Share your details and we will get in touch.
Do you need something that's not listed here? Contact us and we will do our best to help.

We Provide Writing Support To Medical Education and Pharmaceutical Companies, and Continuing Medical Education Courses through the MiLHO Initiative.
On Time, On Target, On Budget.
Services We Provide
We provide medical writing support of the absolute highest quality. Our work is tailored to meet the needs of medical education, product development, and the dissemination of medical information. We offer continuing medical education courses via our MiLHO platform that translates relevant content to the unique practicing environment of healthcare professionals in some parts of the world.
MEDICAL WRITING SUPPORT

The Edge Medical Writing provides medical writing support to medical education and pharmaceutical companies to prepare documents for healthcare professionals. We understand your need for quality and timely products. That is why we pay close attention to detail in everything we do.
MiLHO COURSES

The practice of medicine is a journey of continuous professional development. The Missing Link to Improved Health Outcomes (MiLHO) platform provides relevant continuing medical education (CME) courses to healthcare professionals practicing in parts of the world with limited access to CME.
Why Use Us?
Personalized to your needs, on time, on target, on budget, quality work backed by experience, committed to the ethical standards of our professional body, the american medical writers association, testimonials, what people are saying, “helen has worked with us in the creation of needs assessments in several different disease states. she listened to our guidance, asked good questions, then researched and wrote creative, fact-based documents that supported the need for educational programs and demonstrated expertise in the disease state. she also provided regular status updates and met all assigned deadlines. she is a joy to work with.”.
— Nancy Lucas, Senior Director, Editorial at Paradigm Medical Communications, LLC
“I have had the privilege to work with Helen Fosam over the past 8 years. Helen has a very strong work ethic, is extremely knowledgeable, a fantastic medical writer and researcher and a great pleasure to work with. Helen is patient, fair and smart. She is a collaborative worker and thus a terrific team player. Her extensive scientific background makes her an asset to any group in the medical industry, whether it is writing, editing, presenting, or brainstorming. I highly recommend Helen to any organization and I am certain she would bring enormous value to any team.”
— Kathryn H. Pucci, VP, US Operations, Prime Medica, Inc.
“My experience working with Helen in the area of oncology has been very positive. She delivers high-quality work on time and on budget. She is an excellent listener, asks smart questions, and is passionate about her work. I highly recommend Helen as a scientific medical writer.”
“i have known helen for a number of years and have been highly impressed with her work. she has the ability to produce swiftly top-class materials that are accurate, attractive and well-written. in addition, helen is very approachable, creative and solution-oriented and has outstanding attention to detail.”, “helen is an accomplished medical writer. she understands the professional education requirements. helen is fast learner. she quickly adapted to the amgen learning approach and brought her medical writing skills to the task of curriculum development. i highly recommend helen for any medical writing situation. she is goal oriented and will accomplish any goal you challenge her to achieve”.
— Angela Morelli, Oncology/ Hematology Senior Regional Medical Liaison
“I recently had the opportunity to work with Helen on a detail- and data-driven manuscript on behalf of one of our company’s most esteemed clients. Helen’s experiential writing and ability to synthesize the material in order to fit the journal’s high-science specifications translated into an exceptional end product. Plus, as drafts were going back and forth, Helen was very receptive to our requests for edits, which made my job as editor that much easier. I know the client was thrilled and I imagine we will call on Helen again for more medical writing. She receives my highest recommendation!”
— Marci F. Adilman, Med/Pharma/Healthcare Editorial Specialist
“Helen is a great asset to our team. She provides timely and highly accurate work. She is professional and asks the right questions to provide the right solutions for our clients. Enjoy working with her!”
— Monchiere Commodore, Training and Development Sr. Manager at Amgen
“I have had the pleasure of working with Helen for over 8 years now. Her expertise in science/medical writing and knowledge is exceptional. She is well tuned in on the latest updates in science discovery and trends and combines all of this with a solid business acumen. Would highly recommend her.”
— Rob McCarry, Senior Director, Educational Strategy at Prova Education, Inc.
“I have worked with Helen on many projects over the past several years. Simply put, Helen is the best. She is adept at understanding and synthesizing large amounts of clinical data and can translate these into relevant and impactful messages for clinicians and educators. In fact, I usually “reserve” complicated topics and/or therapeutic areas for Helen because I know she will handle them expertly. Helen has a tremendous work ethic and typically manages multiple projects and deadlines at any given time. She has an elegant writing style, and her interpersonal skills are fantastic. She takes a “can do” approach to each project and works expertly with all members of a team (from project managers to subject matter experts/KOLs). It’s a pleasure to working with Helen, and I recommend her wholeheartedly for any medical writing project.”
“helen rates among the best of the medical writers i’ve workedwith. she “gets” it and always delivers a comprehensive, well-thought-out product. she is definitely a great asset.”.
— Carolyn Skowronski, PharmD, Associate Director of Scientific Services at Clinical Care Options
“I’ve had the pleasure of working with Helen in my capacity as an executive communication coach. Helen has a wonderful (and rare) combination of being a top expert in her field who also possesses strong soft skills. Her intellectual capacity, personable communication style, and above all dedication to her work are admirable. Helen is a true asset to any group and project in which she belongs. “
— Preston Ni, Organizational Speaker, Trainer, Executive & Private Coach at Preston Ni Communication Coaching , www.nipreston.com

PRO MEDICAL COMMUNICATIONS, LLC
Translating and developing medical and scientific concepts and ideas to produce audience-specific content and materials.
Professional Medical/Science Writing and Communication Services
With a team led by an award-winning nurse practitioner with a doctorate degree in nursing, a graduate certificate in nurse education, and a bachelor’s degree in biochemistry, we can write and produce scientific/medical content or translate complex medical concepts and ideas for different audiences. Our products include research-related documents, disease and drug-related educational, news, and promotional materials, publication materials, scientific posters, abstracts, medical Illustration documents, medical graphic arts and medical website contents. Our target audiences include patients, medical professionals, and the general public. We do not write academic papers for students.

Medical Writing

Medical Illustration

Design and Medical Marketing

Pro Medical Communications, LLC is a professional medical science writing and communications firm that offers digital and print medical and scientific content targeted at medical professionals, researchers, scientists, educators, and the general public.
Our team includes medical and science professionals, medical illustrators/artists, graphic and web designers, printing firms, and digital media experts.

Our Mission
To contribute to advancements in healthcare through translation and development of medical and scientific concepts and ideas to produce audience-specific content and materials.

CUSTOMER SATISFACTION

TEAM MEMBERS

OUR SERVICES
We bring you medical science writing and arts by experts and authorities in the field. Our specialty is medicine and health tinged with arts. We can translate your medical, health, and scientific concepts to provide you audience-specific contents and products that will engage your audience and yield results. We can develop concepts and products for clinicians and health professionals.

Learn More

Learn More About Our Services
Get In Touch
Medical science writing, medical graphic arts, and medical Illustration services.
Contact Us
Subscribe to our eGazette
You're in! Thank you!

- Bahasa Indonesia
- Slovenščina
- Science & Tech
- Russian Kitchen
Khabarovsk: A Mediterranean Dream in Russia

Khabarovsk Bridge across the Amur River. Source: Itar Tass
Sparkling new cathedrals that blend in with fine 19th century architecture, a thriving cultural scene, and the proximity of the Taiga make this city on the great Amur River one of the most diverse in Russia.
With traces of some of the great cities of the Baltic Coast, you’d easily be mistaken for thinking you are 8000 kilometres away from the Russian Far East, but just 30 kilometres separate Khabarovsk from Manchurian China. There is, however, no hint of Asia in this city of 620,000, the last major stop on the Siberian railroad before Vladivostok.
Founded in 1858, everything from its broad tree-lined avenues, pre-Soviet heritage buildings, trams, quaint cafes, squares with fountains and a buzzing riverside, is redolent of Europe.
The city has a laidback Mediterranean feel in the summer, with sun-bathers thronging the riverside beaches to work on their tans. While summers are even hotter and more humid than those in Mumbai, temperatures plunge sharply in the winters and average around minus 30 degrees Celsius. If you like, you can take long walks on the frozen Amur river.

Famous for its ornate fountains, the city’s Lenin Square attracts hordes of skaters, local artists and families in the evenings. In the winters, the square hosts ice sculpture festivals and one of the largest New Year trees in the region.
Khabarovsk is just the perfect city you can explore through leisurely walking. Another relaxed way to savour life in the city is to take its trams, which connect the city centre to many outlying areas.
While walking through the Muravyova-Amurskovo Street, you encounter fine brick buildings from the 19th century as well as pre-Russian Civil War architecture that has been painfully preserved by the proud administration of the city.

Lenin Square in Khabarovsk. Source: Itar Tass
The city literally breathes history. The Muravyova-Amurskovo Street, for example, has five museums. The regional history museum is probably the best in the Russian Far East and showcases the culture of the indigenous people of the region, besides an excellent section dedicated to the Russian Civil War. The brick building was built in 1894 and lived to tell the tale of the brutal war between the Reds and the Whites.
While the city is proud of its Soviet past, with several monuments dedicated to the USSR and the partisans, who fought against foreign armies, there has also been a religious renaissance in Khabarovsk. Viktor Ishaev, a former governor, played a large role in the reconstruction of Russian Orthodox Churches, destroyed during Stalin’s purges. The imposing Uspenya Bozhey Matery (Divine Mother of God) Cathedral is one such replica and is just above the riverfront. The city is home to the largest Orthodox Church in the Russian Far East: the golden-domed Church of the Transfiguration, which is probably the most visible building one sees from the aircraft, while landing in the city.
A stone’s throw away from the Transfiguration church is the majestic World War II memorial. It stands as a testament to the patriotism of the citizens of the Russian Far East, who lost their lives in the war against Nazi Germany, thousands of kilometres away from their hometown.
Most of the city’s attractions are close to the riverside, including a central park that stretches for about 1.5 kilometres to a hill top from where you get the best views of the city. The city owes its existence to Count Nicholai Muravyov-Amurskiy, and there’s a large statue of the founder, who was the governor general of Eastern Siberia. The area around the statue is a favourite spot for couples and when it comes to romantic settings, very few places in the country can match the sight of an autumn full moon over the Amur River, reflecting on the golden domes of a nearby cathedral.
The riverside is buzzing with activity, and has several cafes and restaurants with live rock bands performing late into the night. There are also several hydrofoil services for those that want to cruise the Amur. A 90-minute cruise is a wonderful way to observe the illuminated riverside architecture at night, but there are longer cruises north to the cities of Komsomolsk-na-Amure and Nikolayevsk-na-Amure. These cruises are a great way to explore some of the most stunning and sparsely populated areas in all of Russia.

The park of Khabarovsk. Source: Lori Legion Media
The Great Outdoors
No trip to Khabarovsk is complete without a picnic in the forests near the city. Many residents of the city have summer cottages or ‘dachas’ in an area called Voronezh-1, an ideal place for a barbeque, beer and folk music. There are several forest trails in the area, which is strikingly beautiful in the autumn.
However, those seeking adventure head straight for Peak Heksir, which is only about 970 metres above sea level but is a difficult trek through thick forests. The great Ussuriskaya Taiga begins in this forest, which is home to the Amur Tigers, the largest tigers in the world. The tigers have the advantage of camouflage in the autumn, but can be spotted in the winter, when blankets of snow envelop the mountain range. The forests are also inhabited by the Amur leopards, who are incredibly difficult to spot.
The path to Heksir offers splendid views of Khabarovsk and the domes of the city’s cathedrals are visible from the peak. But the piece de resistance of Heksir is the view of the Russia-China border. The last stretch of Russian territory is at the intersection of the Amur and the Ussuri rivers. All you can see of China is a stretch of forests and there aren’t any human settlements for miles from the border. Special permissions need to be arranged for both Russians and foreigners to climb the peak, since it’s in a politically sensitive area.

Shantar Archipelago. Source: RIA Novosti
Khabarovsk is also close to the isolated Shantar Archipelago, a string of islands, which are only accessible in the non-winter months. This group of 15 islands in the Sea of Okhotsk are famous for their rocky cliffs and spruce forests. The archipelago is a wildlife lover’s paradise in the summer. You can spot Bearded Seals, Bowhead Whales and the endangered Western Grey Whales. Bears are aplenty in the islands, but unfortunately, each summer, there are reports of campers getting killed by bears. Wildlife experts attribute many of these cases to reckless behaviour on the part of tourists from cities. The islands are accessible by helicopter from Khabarovsk as well as via fishing trawlers that make the most of the four months a year they have before the ice flows merge the archipelago with the mainland.
Ajay Kamalakaran was the editor of The Sakhalin Times from 2003 to 2007.
All rights reserved by Rossiyskaya Gazeta.
to our newsletter!
Get the week's best stories straight to your inbox
This website uses cookies. Click here to find out more.

IMAGES
VIDEO
COMMENTS
NAMSA's global medical writing services team is comprised of dedicated medical writers from a diverse range of clinical and scientific backgrounds of whom >90% hold advanced degrees. Working in close partnership with NAMSA's Regulatory, Clinical and Biostatistics Teams, our medical writers are highly responsive to individual needs and are ...
VP OPERATIONS. 10 years experience at The Med Writers managing medical and writing projects. Andrea is your first line of communication with The Med Writers and will make sure our team delivers excellence. Professional medical writing for government, pharmaceutical, biotech, healthcare, scientific, clinical, marketing, research and technical ...
Bioforum offers premium medical writing services across all therapeutic areas and fields within biomedical sciences. All of our writing experts hold doctorates and have many years of writing experience. Documentation styles include study-specific and development program documents. The medical writing service is fully integrated into multi ...
In-house medical writers with PhDs in organic chemistry and molecular biology and an average of 8 years' experience in medical writing. Wide network of specialist medical writing consultants, with a vast array of expertise, each with over 15 years of experience. Standardized and ICH-compliant processes for document development.
TransPerfect's services currently cover both medical writing for clinical trials and nonclinical documentation for biotech, medical device, life sciences organizations, pharmaceuticals, and CROs. Our medical writer pool specializes in 40+ therapeutic areas and 300+ documentation types.
Medical & Regulatory Writing. Experience the transformative blend of precision and creativity in every regulatory submission, manuscript, and study report. Partner with us to convey complex information with simplicity, making your work truly remarkable and impactful to its target audience. Explore our services.
Excelling in Medical Writing. We provide innovative and strategic content support. Professional medical writing services to reach your target audience with us and get help in writing fact-check blogs, review articles, website content, marketing content, grants and much more. Contact us for Medical Writing Projects..
Morula Health offers professional medical writing services that are tailored to meet the specific needs of biotech, pharmaceutical, and medical device companies. ... Our array of exceptional medical writing services is available on a project-by-project basis, as an integrated solution, or as a functional service provider (FSP). Learn More.
Regulatory briefing documents for agency meetings, including pre-IND, end of Phase 1, end of Phase 2/3, pre-NDA and advisory committee. Regulatory responses. Responses to agency reviewers. White papers. Clinical evidence reports (devices) Explore all the types of medical writing Synterex can do for you. Our writers have an average of 10 to 15 ...
Proven Medical Writing Solutions. We are a leading content development company providing medical writing services to our esteemed partners. Medical writing plays an integral part in the healthcare domain, and we have a team of specialized healthcare writers from the pharmaceutical industry, clinical research organizations, and academia who ...
Professional Medical Writing Services We Offer. We have been a pioneer in providing the best quality medical writing support services to clients around the globe. We have the skills and expertise to understand each client's unique business requirements and provide customized medical writing support services. Some of the key professional medical ...
You can withdraw your consent by writing to us at [email protected]. APCER's Global Medical Writing Center provides clinical, medical, and regulatory writing services. Our experienced team of medical writers helps companies document efficacy and safety throughout the product lifecycle. We have offices in United States and Europe.
Our Process: 1. Contact us and tell us a little bit about your medical content writing project. 2. We'll get you an initial quote within 2 hours. (Yes, 2!) 3. Next, we'll book a call to fully explore your needs and project details with our project manager and an expert medical writer. 4.
Professional Medical Writing Services. Become a leader in the industry through our professional medical writing services. From detailed blog posts explaining common illnesses to full-length articles on complex medical topics, our experienced writers can do it all. Through Compose.ly, you receive high-quality medical content made to help your ...
Medical Writing professionals with passion to precision. Emtex offers worldwide professional medical writing and related services to the pharmaceutical (human & veterinary), biotech, medical device and other industries, CROs and academic institutions. With over 20 years of experience in the field, we guarantee the best possible medical writing ...
Our professional medical writing services. Many medical content writing companies specialize in a particular type of copy or serve only a niche segment of the healthcare space. Thanks to our diverse team of talented medical copywriters, Write Collective can offer a broad array of content. By meeting all of your copy needs, we ensure tonal ...
CME writers create the educational resources that makeup CME/CEHP content. Aside from maintaining skills and knowledge, CME/CEHP plays a huge role in quality-of-care standards. Patients and families expect healthcare providers to deliver effective, compassionate, and safe care. Thus, doctors, nurses, and other health professionals must maintain ...
Services We Provide. We provide medical writing support of the absolute highest quality. Our work is tailored to meet the needs of medical education, product development, and the dissemination of medical information. ... "Helen is an accomplished medical writer. She understands the professional education requirements. Helen is fast learner.
Professional Medical/Science Writing and Communication Services With a team led by an award-winning nurse practitioner with a doctorate degree in nursing, a graduate certificate in nurse education, and a bachelor's degree in biochemistry, we can write and produce scientific/medical content or translate complex medical concepts and ideas for ...
Khabarovsk (Russian: Хабаровск [xɐˈbarəfsk] ⓘ) is the largest city and the administrative centre of Khabarovsk Krai, Russia, located 30 kilometers (19 mi) from the China-Russia border, at the confluence of the Amur and Ussuri Rivers, about 800 kilometers (500 mi) north of Vladivostok.As of the 2021 Russian census, it had a population of 617,441.
Khabarovsk (Russian: Хаба́ровск, khah-BAH-ruhvsk) is a city on the Amur river in the Russian Far East, near the Chinese border. Often overlooked due to its proximity to Vladivostok, Khabarovsk could easily be a highlight in the long line of predominately dull cities along the Trans-Siberian. But while most cities look their best when the sun is out, only in few is the effect as ...
The Far-Eastern State Medical University (FESMU) was founded in 1930. At present, the university is headed by the Rector Konstantin V. Zhmerenetsky, Corresponding Member of the Russian Academy of Sciences, M.D., PhD. Education is conducted according to the License and State accreditation in the following specialties: General Medicine, Pediatrics, Dentistry, Pharmacy and Biomedicine,...
Follow Russia Beyond on Twitter. Imagine an autumn full moon over the majestic Amur River, reflecting on the golden domes of a nearby cathedral. Khabarovsk mingles romance, history and fabulous ...