
Irritable Bowel Syndrome News
Top headlines, latest headlines.
- Effective Dietary Treatment in IBS
- Fiber Discovery Could Shape Better Gut Health
- Irritable Bowel Syndrome: Low Bacterial ...
- Wearable Device Reveals IBS-Related Changes
- Treating Gut Pain
- Gravity May Cause Irritable Bowel Syndrome?
- Bloating Common Issue Among Americans
- Inactive Inflammatory Bowel Disease and ...
- IBS: Specific Diets Less Important Than Expected
- Treating IBS Pain With Help of Tarantula Venom?
Earlier Headlines
Wednesday, july 27, 2022.
- Histamine-Producing Gut Bacteria Can Trigger Chronic Abdominal Pain
- LATEST NEWS
- Health & Medicine
- Diseases & Conditions
- Alzheimer's Research
- Amyotrophic Lateral Sclerosis
- Attention Deficit Disorder
- Back and Neck Pain
- Birth Defects
- Bladder Disorders
- Blood Clots
- COVID and SARS
- Cervical Cancer
- Bladder Cancer
- Multiple Myeloma
- Pancreatic Cancer
- Brain Tumor
- Colon Cancer
- Breast Cancer
- Ovarian Cancer
- Lung Cancer
- Mesothelioma
- Skin Cancer
- Prostate Cancer
- Cerebral Palsy
- Chikungunya
- Chronic Fatigue Syndrome
- Cold and Flu
- Crohn's Disease
- Cystic Fibrosis
- Dengue Fever
- Down Syndrome
- Eating Disorder Research
- Encephalitis
- Epilepsy Research
- Erectile Dysfunction
- Fibromyalgia
- Gastrointestinal Problems
- HIV and AIDS
- Headache Research
- Hearing Loss
- Heart Health
- Cholesterol
- Stroke Prevention
- Heart Disease
- Hormone Disorders
- Hypertension
- Infectious Diseases
- Insomnia Research
- Irritable Bowel Syndrome
- Kidney Disease
- Liver Disease
- Lung Disease
- Lyme Disease
- Mental Health Research
- Multiple Sclerosis Research
- Mumps, Measles, Rubella
- Muscular Dystrophy
- Osteoporosis
- Parkinson's Research
- Prostate Health
- Restless Leg Syndrome
- Sickle Cell Anemia
- Sleep Disorder Research
- Thyroid Disease
- Triglycerides
- Tuberculosis
- Medical Topics
- Accident and Trauma
- Alternative Medicine
- Birth Control
- Bone and Spine
- Chronic Illness
- Controlled Substances
- Dietary Supplements and Minerals
- Epigenetics
- Food Additives
- Foodborne Illness
- Foot Health
- Gene Therapy
- Health Policy
- Human Biology
- Immune System
- Joint Health
- Medical Imaging
- Nervous System
- Pain Control
- Personalized Medicine
- Pharmacology
- Psychology Research
- Wounds and Healing
- PHYSICAL/TECH
- ENVIRONMENT
- SOCIETY & EDUCATION
- Robotic 'SuperLimbs' Could Help Moonwalkers
- Solving Quantum Many-Body Problems
- When 'Please' Is More Strategic Than Magic
- Autonomous Drones With Animal-Like 'Brains'
- How Practice Forms New Memory Pathways
- Reversing Brain Damage Caused by Ischemic Stroke
- Earth-Sized Planet Orbiting Ultra-Cool Dwarf
- Robots' Sense of Touch as Fast as Humans?
- Avian Flu Detected in NYC Wild Birds
- Metro-Area Quantum Computer Network Demo
Trending Topics
Strange & offbeat.
Recent advances in the treatment of irritable bowel syndrome
Affiliations.
- 1 Department of Medical Sciences, University of Turin, Turin, Italy
- 2 Institute of Biostructure and Bioimaging, National Research Council, Molecular Biotechnology Center, Turin, Italy
- 3 Gastroenterology and Endoscopy Unit, Cardinal Massaia Hospital, Asti, Italy
- 4 Unit of Gastroenterology, Molinette-SGAS Hospital, Turin, Italy
- 5 Institute of Biostructure and Bioimaging, National Research Council, Molecular Biotechnology Center, Turin, Italy; Unit of Gastroenterology, Molinette-SGAS Hospital, Turin, Italy. [email protected]
- PMID: 34463082
- DOI: 10.20452/pamw.16067
Irritable bowel syndrome (IBS) is a chronic functional gastrointestinal disorder which presents with abdominal pain and altered bowel habits. It affects about 20% of the general population, mainly women, and has a considerable impact on the quality of life and health care costs. Four different entities of IBS have been identified: IBS with constipation (IBS‑ C), IBS with diarrhea (IBS D), IBS with a mixed pattern of constipation and diarrhea, and unclassified IBS. Although the precise pathogenesis of IBS remains unclear, its multifactorial nature is evident and includes environmental and host factors. Management of patients with this disease is challenging and a personalized approach is required. A strong, reassuring physician‑ patient relationship is crucial, followed by patient education, dietary advice, and stress reduction. For nonresponding patients, the therapeutic approach may include nonpharmacological therapies and / or pharmacotherapy. The choice of pharmacological treatment is based on the predominant symptom and a prespecified time point should be planned for effectiveness evaluation and dose adjustment. In patients with IBS‑ D, the therapeutic options include mainly antibiotics, such as rifaximin, peripheral opioid agonists, mixed opioid agonists / antagonists, bile acid sequestrants, and antagonists of serotonin 5‑ hydroxytryptamine type 3 receptors. Bulking agents and osmotic laxatives represent the first line therapy for IBS‑ C, while lubiprostone and linaclotide should be reserved for difficult to treat patients. The involvement of gastrointestinal microbiota constitutes a fascinating field of exploration as it offers the potential to be modulated by the use of probiotics, prebiotics, synbiotics as well as fecal microbiota transplantation. This review offers an updated overview on the recent advances in the treatment of IBS.
Publication types
- Abdominal Pain
- Constipation
- Irritable Bowel Syndrome* / therapy
- Quality of Life
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 19, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
Large-scale genetic study finds new link between IBS and the cardiovascular system
by Monash University

New research published in the journal Cellular and Molecular Gastroenterology and Hepatology sheds light on disease mechanisms common to irritable bowel syndrome (IBS) and cardiovascular diseases (CVD).
Led by Dr. Leticia Camargo Tavares, a postdoctoral fellow at the Hypertension Research Laboratory within Monash University's School of Biological Sciences, the study reveals novel insights into the genetic underpinnings of IBS, offering potential avenues for therapeutic intervention.
IBS is one of the most prevalent gastrointestinal disorders globally, affecting up to 10% of the population, with a disproportionate impact on women.
It is characterized by a complex range of symptoms including abdominal pain , bloating, diarrhea and constipation. IBS significantly compromises patients' quality of life. Despite its widespread prevalence, the cause of IBS remains unclear, thus limiting treatment options.
An international consortium of researchers, drawing expertise from Monash University (Australia), CIC bioGUNE (Spain), LUM University, IRGB-CNR, CEINGE, and the University of Naples Federico II (Italy), as well as the University of Groningen (Netherlands), embarked on a comprehensive investigation.
Analyzing data from two large European population cohorts—UK Biobank and Lifelines—the team scrutinized the genetic landscapes of 24,735 people with IBS and 77,149 symptom-free individuals.
Their analysis uncovered four genomic regions, including two previously unidentified loci, associated with increased susceptibility to IBS.
These genetic hotspots implicate pathways central to gastrointestinal motility, intestinal mucosal integrity, and circadian rhythm regulation.
"Although we're yet to conclusively pinpoint specific genes and mechanisms, these findings provide novel insights into IBS pathophysiology, highlighting potential therapeutic targets. So, we expect follow-up research to build on these discoveries," Dr. Tavares said.
Moreover, the researchers found a remarkable link between IBS predisposition and various cardiovascular ailments, encompassing hypertension, ischemic heart disease , and angina pectoris.
Professor Mauro D'Amato, senior author and study supervisor from CIC bioGUNE and LUM University, described this new evidence as the most exciting outcome, underscoring the potential for shared therapeutic modalities.
In another important finding, the study revealed that IBS heritability (the weight of genes in determining one's risk of disease), might be higher than previously thought. This, the authors say, may stem from their adherence to standardized classification criteria in delineating IBS phenotypes, notably the Rome Criteria from the Rome Foundation.
Explore further
Feedback to editors
Study investigates cardiac cell regeneration in search of novel therapeutics
33 minutes ago

Researchers profile clinical, gene and protein changes in 'brain fog' from long COVID
43 minutes ago

Researchers develop theory on traveling waves of activity in the human brain
49 minutes ago

Study indicates the rapid identification of stroke type is key to improving outcomes

Simple learning test may be used to diagnose autism at just six months of age
Researchers identify immunosuppressive pathway that helps newborn hearts regenerate in mouse models

'Trojan Horse' weight loss drug found to be more effective than available therapies
Research explains new method to engineer immune cells that could treat multiple cancer patients

Link between COVID-19 vaccine complication and rare 'common cold' blood disease
Consistent exercise changes how saturated fat is used by the body, study finds
Related stories.
Bowel habits written in the DNA: New clues for irritable bowel syndrome
Dec 8, 2021

Genetic study sheds light on why people get hemorrhoids
Apr 23, 2021
Genetic link to IBS identified in women
Apr 5, 2018

New research shows bowel habits are written in our DNA
Dec 9, 2021

Unveiling gender differences in cancer: New insights into genomic instability
Mar 29, 2024

Study finds women with depression face higher cardiovascular risk than men
Mar 12, 2024
Recommended for you
Infertility treatment found to double the risk of postpartum heart disease
21 hours ago

Researchers identify causative gene in mouse model of inherited lethal arrhythmia
May 15, 2024

Study identifies genetic link between inflammatory bowel disease and Parkinson's disease
May 14, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

Study at Cambridge
About the university, research at cambridge.
- For Cambridge students
- For our researchers
- Business and enterprise
- Colleges and Departments
- Email and phone search
- Give to Cambridge
- Museums and collections
- Events and open days
- Fees and finance
- Postgraduate courses
- How to apply
- Fees and funding
- Postgraduate events
- International students
- Continuing education
- Executive and professional education
- Courses in education
- How the University and Colleges work
- Visiting the University
- Annual reports
- Equality and diversity
- A global university
- Public engagement
Large-scale genetic study reveals new clues for the shared origins of irritable bowel syndrome and mental health disorders
- Research home
- About research overview
- Animal research overview
- Overseeing animal research overview
- The Animal Welfare and Ethical Review Body
- Animal welfare and ethics
- Report on the allegations and matters raised in the BUAV report
- What types of animal do we use? overview
- Guinea pigs
- Equine species
- Naked mole-rats
- Non-human primates (marmosets)
- Other birds
- Non-technical summaries
- Animal Welfare Policy
- Alternatives to animal use
- Further information
- Funding Agency Committee Members
- Research integrity
- Horizons magazine
- Strategic Initiatives & Networks
- Nobel Prize
- Interdisciplinary Research Centres
- Open access
- Energy sector partnerships
- Podcasts overview
- S2 ep1: What is the future?
- S2 ep2: What did the future look like in the past?
- S2 ep3: What is the future of wellbeing?
- S2 ep4 What would a more just future look like?
- Research impact

An international study of more than 50,000 people with irritable bowel syndrome (IBS) has revealed that IBS symptoms may be caused by the same biological processes as conditions such as anxiety. The research highlights the close relationship between brain and gut health and paves the way for development of new treatments.
Although IBS occurs more frequently in those who are prone to anxiety, we don’t believe that one causes the other – our study shows these conditions have shared genetic origins Miles Parkes
IBS is a common condition worldwide, affecting around 1 in 10 people and causing a wide range of symptoms including abdominal pain, bloating and bowel dysfunction that can significantly affect people’s lives. Diagnosis is usually made after considering other possible conditions (such as Crohn’s disease or bowel cancer), with clinical tests coming back ‘normal’. The condition often runs in families and is also more common among people who are prone to anxiety. The causes of IBS are not well understood, but an international team of researchers has now identified several genes that provide clues into the origins of IBS.
The research team, including more than 40 institutions and coordinated by scientists in UK and Spain, looked at genetic data from 40,548 people who suffer with IBS from the UK Biobank and 12,852 from the Bellygenes initiative (a world-wide study aiming to identify genes linked to IBS) and compared them to 433,201 people without IBS (controls), focusing on individuals of European ancestry. The findings were repeated with de-identified data from the genomics company 23andMe Inc., provided by customers who have consented to research, by comparing 205,252 people with IBS to 1,384,055 controls.
The results showed that overall, heritability of IBS (how much your genes influence the likelihood of developing a particular condition) is quite low, indicating the importance of environmental factors such as diet, stress and patterns of behaviour that may also be shared in the family environment.
However, six genetic differences (influencing the genes NCAM1, CADM2, PHF2/FAM120A, DOCK9, CKAP2/TPTE2P3 and BAG6) were more common in people with IBS than in controls. As IBS symptoms affect the gut and bowel, it would be expected that genes associated with increased risk of IBS would be expressed there – but this is not what the researchers found. Instead, most of the altered genes appear to have more clear-cut roles in the brain and possibly the nerves which supply the gut, rather than the gut itself.
Researchers also looked for overlap between susceptibility to IBS and other physical and mental health conditions. They found that the same genetic make-up that puts people at increased risk of IBS also increases the risk for common mood and anxiety disorders such as anxiety, depression, and neuroticism, as well as insomnia. However, the researchers stress that this doesn’t mean that anxiety causes IBS symptoms or vice versa.
Study co-senior investigator and consultant gastroenterologist Professor Miles Parkes from the University of Cambridge explained: “IBS is a common problem, and its symptoms are real and debilitating. Although IBS occurs more frequently in those who are prone to anxiety, we don’t believe that one causes the other – our study shows these conditions have shared genetic origins, with the affected genes possibly leading to physical changes in brain or nerve cells that in turn cause symptoms in the brain and symptoms in the gut.”
The study also found that people with both IBS and anxiety were more likely to have been treated frequently with antibiotics during childhood. The study authors hypothesise that repeated use of antibiotics during childhood might increase the risk of IBS (and perhaps anxiety) by altering the ‘normal’ gut flora (healthy bacteria that normally live in the gut) which in turn influence nerve cell development and mood.
Current treatments for IBS vary widely and include dietary changes, prescription medications targeting the gut or brain, or behavioural interventions. Lead author Chris Eijsbouts from the University of Oxford suggests that discovering genes which contribute to IBS may aid in the development of new treatments in the long term. He said: "Even genetic changes that have only subtle effects on IBS can provide clues about pathways to target therapeutically. Unlike the individual genetic changes themselves, drugs targeting the pathways they tell us about may have a considerable impact on the condition, as we know from other disease areas."
Co-senior investigator Dr Luke Jostins from the University Oxford commented: “We anticipate that future research will build on our discoveries, both by investigating the target genes identified and exploring the shared genetic risk across conditions to improve understanding of the disordered brain-gut interactions which characterise IBS.”
“IBS represents a remarkable challenge for genetic studies. These initial findings have been long awaited, and finally tell us this type of research is worth the struggle,” added Ikerbasque Professor Mauro D’Amato from CIC bioGUNE, co-senior investigator and coordinator of the Bellygenes initiative.
This research received funding and support from National Institute for Health Research (NIHR) Biomedical Research Centres in Cambridge, Oxford, Nottingham and Manchester. Further funding and support was received from the Wellcome Trust, the Li Ka Shing Foundation and the Kennedy Trust for Rheumatology Research in the UK, and the Spanish Ministry of Economy and Competitiveness (Instituto Salud Carlos III), the Health Department of the Basque Government and the Swedish Research Council (Vetenskapsradet).
Reference Eijsbouts, C et al. Genome-wide analysis of 53,400 people with irritable bowel syndrome highlights shared genetic pathways with mood and anxiety disorders. Nature Genetics; 5 Nov 2021; DOI: 10.1038/s41588-021-00950-8
Adapted from a press release by the National Institute for Health Research

Read this next

Over 20,000 people join search for new dementia treatments

Study unpicks why childhood maltreatment continues to impact on mental and physical health into adulthood

UK-wide trials to begin on blood tests for diagnosing dementia

Scientists identify genes linked to DNA damage and human disease
3D image showing irritable bowel syndrome
Credit: Scientific Animations
Search research
Sign up to receive our weekly research email.
Our selection of the week's biggest Cambridge research news sent directly to your inbox. Enter your email address, confirm you're happy to receive our emails and then select 'Subscribe'.
I wish to receive a weekly Cambridge research news summary by email.
The University of Cambridge will use your email address to send you our weekly research news email. We are committed to protecting your personal information and being transparent about what information we hold. Please read our email privacy notice for details.
- Mental health
- Spotlight on neuroscience
- Miles Parkes
- School of Clinical Medicine
Related organisations
- National Institute for Health Research (NIHR)
- NIHR Cambridge Biomedical Research Centre
- Li Ka Shing Foundation
- Kennedy Trust for Rheumatology Research
Connect with us

© 2024 University of Cambridge
- Contact the University
- Accessibility statement
- Freedom of information
- Privacy policy and cookies
- Statement on Modern Slavery
- Terms and conditions
- University A-Z
- Undergraduate
- Postgraduate
- Cambridge University Press & Assessment
- Research news
- About research at Cambridge
- Spotlight on...
- Reference Manager
- Simple TEXT file
People also looked at
Original research article, global research trends in irritable bowel syndrome: a bibliometric and visualized study.

- 1 Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2 Department of Gastroenterology, Xiyuan Hospital, China Academy of Traditional Chinese Medical Sciences, Beijing, China
- 3 National Clinical Research Center for Chinese Medicine Cardiology, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 4 Xiyuan Hospital, Traditional Chinese Medicine Research Institute of Spleen and Stomach Diseases, China Academy of Chinese Medical Sciences, Beijing, China
Background: There are about 10–23% of adults worldwide suffering from irritable bowel syndrome (IBS). Over the past few decades, there are many aspects of uncertainty regarding IBS leading to an ongoing interest in the topic as reflected by a vast number of publications, whose heterogeneity and variable quality may challenge researchers to measure their scientific impact, to identify collaborative networks, and to grasp actively researched themes. Accordingly, with help from bibliometric approaches, our goal is to assess the structure, evolution, and trends of IBS research between 2007 and 2022.
Methods: The documents exclusively focusing on IBS from 2007 to 2022 were retrieved from the Science Citation Index Expanded of the Web of Science Core Collection. The annual productivity of IBS research, and the most prolific countries or regions, authors, journals and resource-, intellectual- and knowledge-sharing in IBS research, as well as co-citation analysis of references and keywords were analyzed through Microsoft Office Excel 2019, CiteSpace, and VOSviewer.
Results: In total, 4,092 publications were reviewed. The USA led the list of countries with the most publications (1,226, 29.96%). Mayo Clinic contributed more publications than any other institution (193, 4.71%). MAGNUS SIMREN stood out as the most active and impactful scholar with the highest number of publications and the greatest betweenness centrality value. The most high-yield journal in this field was Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society (275, 6.72%). Gastroenterology had the most co-citations (3,721, 3.60%). Keywords with the ongoing strong citation bursts were chromogranin A, rat model, peptide YY, gut microbiota, and low-FODMAP diet, etc.
Conclusion: Through bibliometric analysis, we gleaned deep insight into the current status of literature investigating IBS for the first time. These findings will be useful to scholars interested in understanding the key information in the field, as well as identifying possible research frontiers.
Introduction
Associated with abdominal pain, bloating, and altered bowel habits, irritable bowel syndrome (IBS) is a chronic, cyclical and relapsing functional bowel disorder ( 1 ). The global prevalence of IBS is currently estimated at 15%, and IBS symptoms occur in about 10–20% of Westerners ( 2 – 4 ). Irrespective of bowel habit, diagnoses of IBS have traditionally been made by using the Rome diagnostic criteria, which is a symptom-based diagnostic standard that is being updated to Rome IV criteria ( 5 ). Further subtypes of IBS include diarrhea-predominant IBS (IBS-D), constipation-predominant IBS (IBS-C), mixed type of IBS with both diarrhea and constipation (IBS-M), and unclassified IBS ( 6 , 7 ). A key challenge that has faced IBS research to date has been the pathophysiology, which is thought to be multifactorial ( 8 ). There are still no satisfactory treatments for patients with IBS because of its complex pathogenesis. Currently, there is an emphasis on symptomatic management; yet, it involves multiple medications and fails to address the underlying complex pathogenesis of the disease, with approximately one-third of patients failing to respond ( 9 – 12 ).
As a result of the multiple and persistent symptoms of IBS, it contributes to a decline in quality of life, high absenteeism, and high socioeconomic burden. It has been estimated that between 8.5 and 21.6 days a year are taken off work due to IBS. There are approximately 3.6 million physician office visits related to IBS every year, resulting in healthcare costs of more than $30 billion ( 13 – 15 ).
There are many aspects of uncertainty regarding IBS, leading to an ongoing interest in the field as reflected by the huge amount of literature. Thus, it is difficult to characterize the evolution of knowledge components, the current body of knowledge, and the research trends.
The bibliometric analysis utilizes mathematical and statistical methods and involves the use of a series of defined metrics to evaluate the structure, productivity, progress, quality, impact and inter-connectivity of scientific work ( 16 , 17 ). One way to accurately capture and integrate data from disparate sources of heterogeneous information is through a knowledge map, which visualizes the connections between complex data silos ( 18 ). Furthermore, key authors, institutions and countries as well as the structure of scientific collaboration networks can be identified. However, there are few bibliometric studies on IBS research. In this context, the present study aims to use a bibliometric approach to identify, evaluate and visualize all literature published on IBS since 2007 regarding quantitative, semiqualitative, and chronological elements of data collected.
Materials and Methods
Source of the data and search strategy.
The search was performed on the Science Citation Index Expanded of the Web of Science Core Collection (WoSCC) of Clarivate Analytics. All searches were conducted on the same day, February 1, 2022. The literature search was completed by two authors independently for identifying IBS-related publications with the following search strategy: TOPIC:[(adaptive colitis) OR (colon spasm) OR (functional bowel disease) OR (irritable bowel) OR (irritable colon) OR (membranous colitis) OR (mucous colitis) OR (spastic colitis) OR (spastic colon) OR (spastic bowel) OR (functional colonic disease) OR (colon irritable) OR (colon neurosis) OR (bowel neurosis) OR (functional colopathy) OR (functional colonopathy) OR (chronic catarrhal colitis) OR (colica mucosa) OR (colonic enterospasm) OR (dyskinesia of the colon) OR (dyssynergia of the colon) OR (functional enterocolonopathy) OR (Glarry enteritis) OR (glutinous diarrhea) OR (intestinal croup) OR (irritable gut syndrome) OR (lienteric diarrhea) OR (membranous catarrh of the intestine) OR (mucomembranous colic) OR (myxoneurosis) OR (nervous diarrhea) OR (neurogenic mucous) OR (non-specific diarrhea) OR (tubular diarrhea) OR (unhappy colon) OR (unstable colon)] AND Language:(English). Additionally, articles and reviews containing at least one search term in the “title” were included since the aim was to obtain the academic research on the topic of interest. However, the “TOPIC” search enables the inclusion of a considerable amount of off-topic publications with the search terms in abstract, author keywords and keywords plus. The retrieval time was from February 1, 2007 until February 1, 2022. The bibliographic records were collected and saved in plain text. Ultimately, these documents were imported into CiteSpace and VOSviewer for analysis.
Data Analysis
CiteSpace ( 19 ), which is a freely available Java-based software package developed by Professor Chaomei Chen at Drexel University, was applied to (1) perform co-occurrence analysis; (2) visualize key features of literature, such as authors, countries or regions, organizations, and keywords; (3) perform a co-citation analysis of references; (4) depict timeline view of keywords; and (5) capture keywords and references with strong citation bursts. VOSviewer ( 20 ), which is a free software tool based on the Java environment developed by Nees Jan van Eck and Ludo Waltman from Leiden University, was used for creating clusters of keywords. Microsoft Excel 2019 was used to demonstrate the amount of scientific literature published annually.
Publication Output
A total of 4,092 publications were identified, including 3,299 articles (80.62%) and 793 reviews (19.37%). The number of publications per year since 2007 is shown in Figure 1 . There is an overall trend of increased output of scientific research over time, which falls into two stages. The first phase from 2007 to 2016 exhibited a growing trend despite the decrease in 2010, 2013, 2015, and 2016. As the second stage has progressed, the number of documents has increased from 270 in 2016 to 344 in 2019, to 424 in 2021. The volume of papers published during the last 6 years (2016–2021) accounted for 49.82% of all publications.
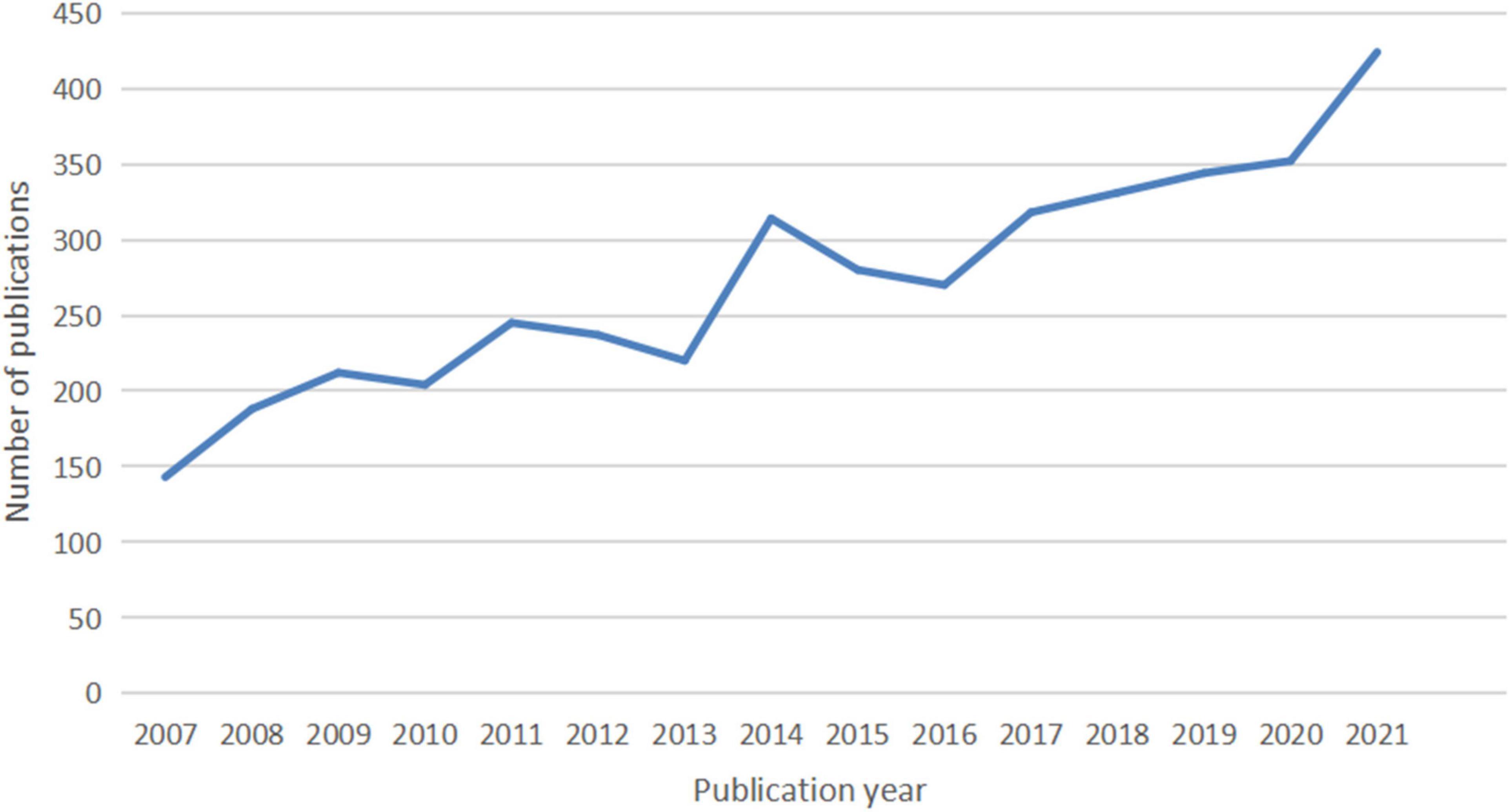
Figure 1. The number of articles published annually in IBS research.
Countries or Regions and Institutions Analysis
In total, IBS articles were published by 407 institutions from 116 countries or regions. As shown in Table 1 , the top 10 countries and institutions are listed. The United Stateswas the leading country in the field, which had an overwhelmingly higher number of publications (1,226, 29.96%). China ranked second (577, 14.10%). In third place is England (421, 10.28%).
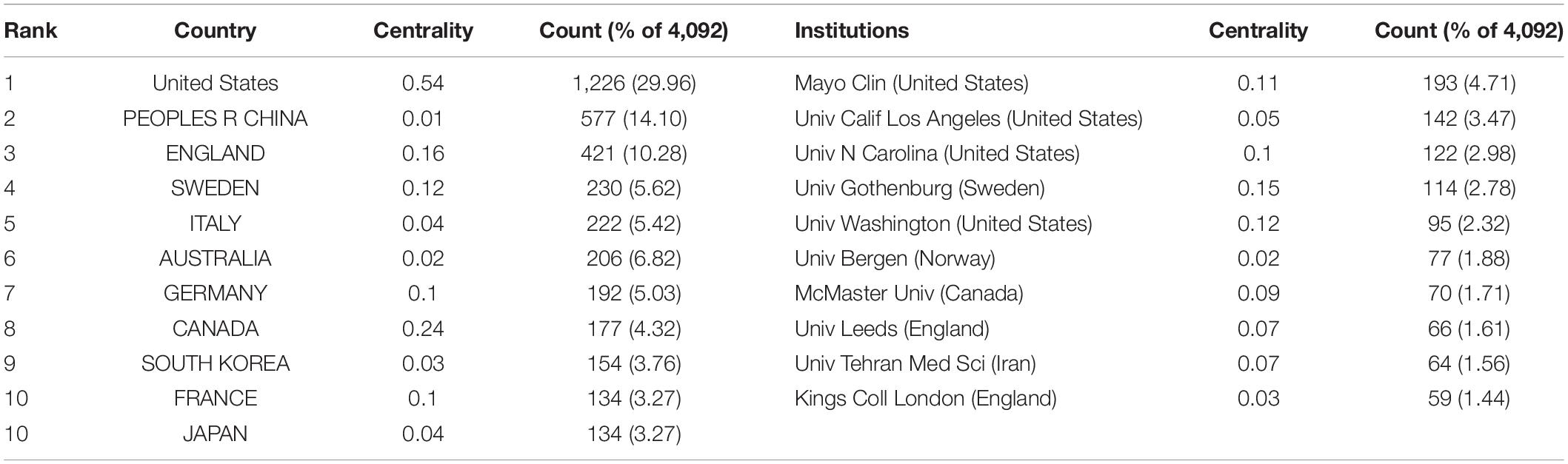
Table 1. The top 10 countries or regions and institutions involved in IBS research.
With regard to contributions of institutions, the majority of the top 10 prolific institutions were from the USA (40%) and England (20%). Among them, Mayo Clin contributed the most publications (193, 4.71%), followed by Univ Calif Los Angeles (142, 3.47%) and Univ N Carolina (122, 2.98%).
In addition, the USA ranked first by the betweenness centrality value (0.54), followed by Canada (0.24), and England (0.16). Univ Gothenburg ranked first by the betweenness centrality value (0.15), followed by Univ Washington (0.12) and Mayo Clin (0.11).
Figure 2 shows the collaboration among the countries or regions. In the map, each node represents a country or territory. The radius of a node increases with its contribution to the research on IBS. The links between nodes represent the collaboration, whereby their thicknesses are proportional to the intensity of the collaboration. A node’s betweenness centrality is calculated in order to identify the node that lies between two or more large groups of nodes. In a network, a node with a betweenness centrality value of more than 0.1 (i.e., one interconnected with more than 10% of the other nodes) exerts substantial influence over others because more information passes through that node. A node with a high betweenness centrality value is marked with a purple ring, while a red ring denotes a burst.
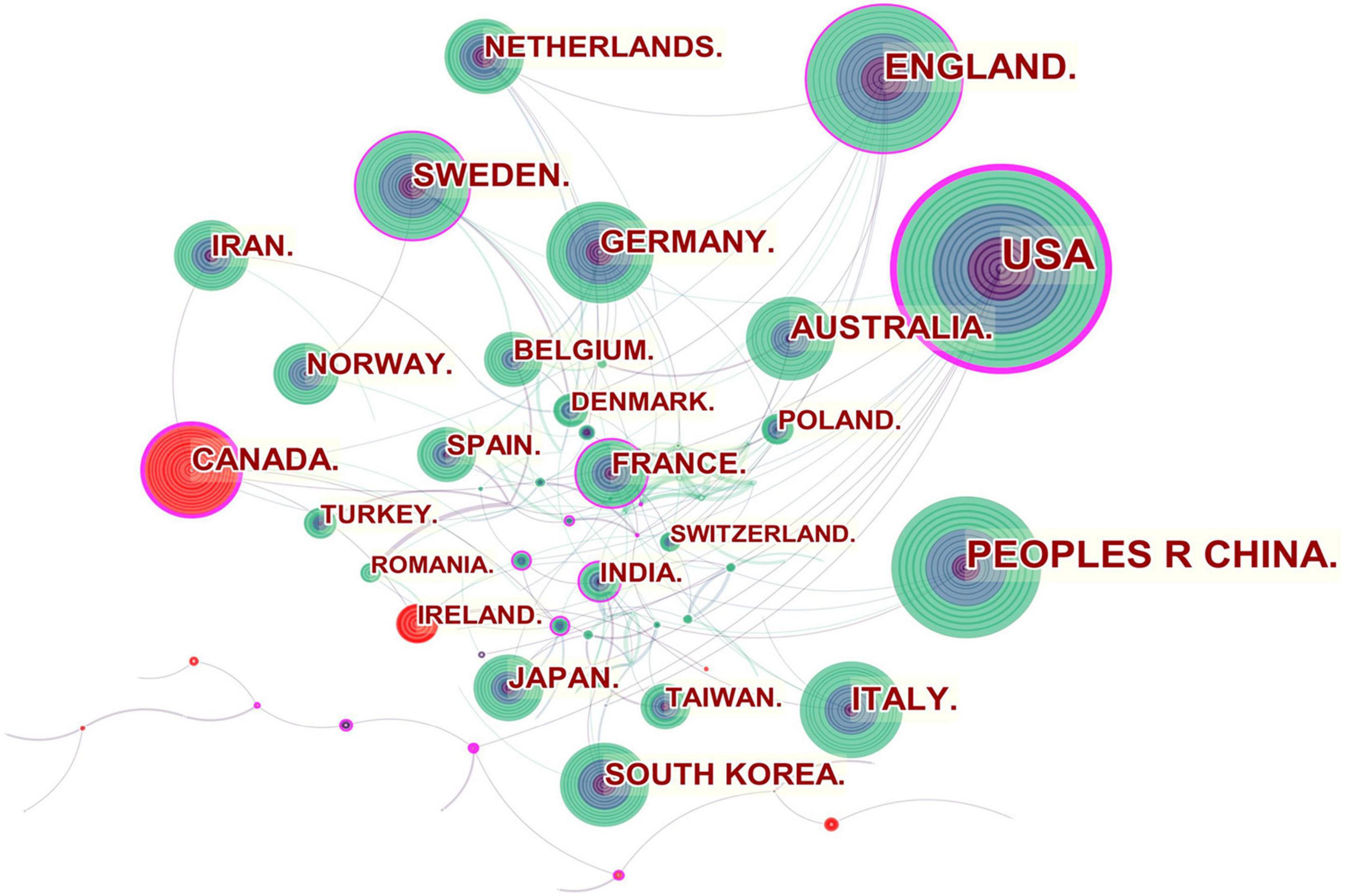
Figure 2. Network of countries and regions engaged in IBS research.
The United States, Canada, England, Sweden, France and India were referred to as central countries for the network owing to their cooperation occurring worldwide. For example, the United States, which possessed the broadest scientific collaboration, worked intensively with Australia, Peru, Israel, Sweden, Canada, Netherlands, Russia, Japan, and South Korea. Canada had close cooperation with Iran, England, the United States, South Africa, France, Argentina, Mexico, and Ireland. The main collaborators with England were the Netherlands, Germany, Australia, New Zealand, Scotland, Jordan, Palestine, Pakistan and Switzerland. Strong bursts were detected for Canada and Ireland.
In the institutional collaboration network shown in Figure 3 , the landmark nodes included Univ Gothenburg, Univ Washington, Mayo Clin, Univ N Carolina, and Univ Nottingham, signifying that they partnered extensively with academic organizations across the globe. The main institutions that collaborated with Univ Gothenburg were Karolinska Inst, Univ N Carolina, Univ North Carolina Chapel Hill, Univ Copenhagen, Sahlgrens Univ Hosp, Sabbatsbergs Hosp, AstraZeneca R&D, Katholieke Univ Leuven, and Univ Leuven. Univ Washington collaborated actively with Keimyung Univ, Ewha Womans Univ, Fred Hutchinson Canc Res Ctr, Broad Inst MIT and Harvard, Harvard Med Sch, Brigham and Womens Hosp, Mayo Clin, Grp Hlth Cooperat Puget Sound, and Campbell Univ. Mayo Clin cooperated frequently with Harvard Med Sch, Baylor Coll Med, Univ Complutense, Univ Sydney, Broad Inst MIT and Harvard, Univ Washington, Montefiore Med Ctr, and Brigham and Womens Hosp. Teheran Univ Med Sci, Univ Calif Los Angeles, Mayo Clin, Univ Washington, McMaster Univ, Univ Manchester, and Cedars Sinai Med Ctr were detected with strong bursts.
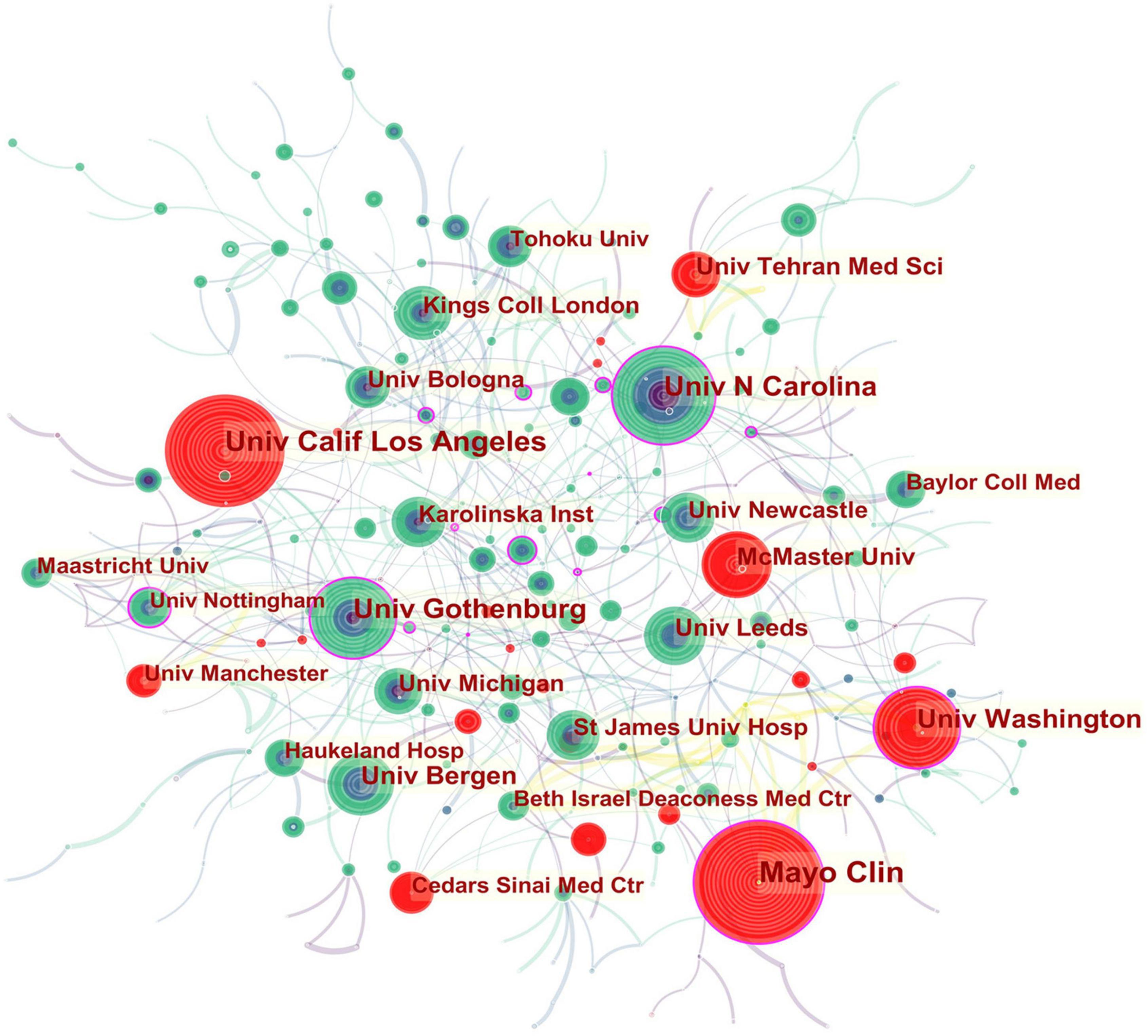
Figure 3. Network of institutions engaged in IBS research.
In all, 423 authors contributed to the IBS studies. As shown in Table 2 , IBS articles were mostly published by authors affiliated with institutions in America (308). MAGNUS SIMREN contributed the most articles (87, 2.12%), followed by MICHAEL CAMILLERI (75, 1.83%), and ALEXANDER C FORD (68, 1.66%). The top authors by the betweenness centrality value were MAGNUS SIMREN (0.13), EMERAN A MAYER (0.12), MICHAEL CAMILLERI (0.09), and NICHOLAS J TALLEY (0.09).
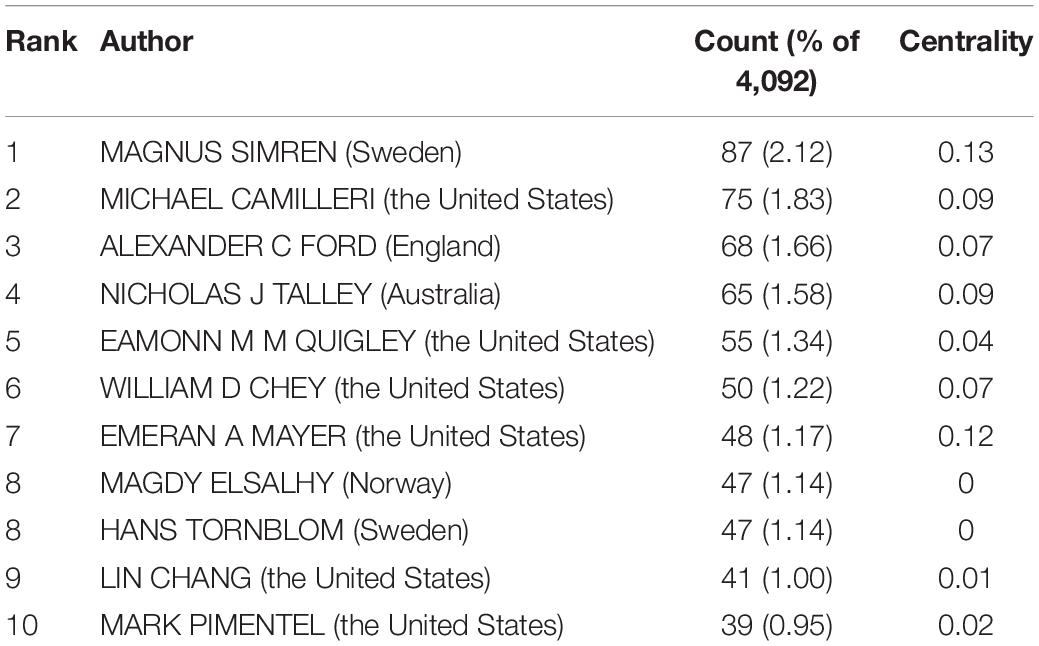
Table 2. The top 10 authors of IBS research.
From the author’s collaboration network, which is presented in Figure 4 , MAGNUS SIMREN and EMERAN A MAYER were located at a central position in the collaboration network. Active collaborations were seen among MAGNUS SIMREN, GUY BOECKXSTAENS (Belgium), LENA OHMAN (Sweden), GISELA RINGSTROM (Sweden), IRIS POSSERUD (Sweden), HANS TORNBLOM (Sweden), EVA JAKOBSSON UNG (Sweden), STINE STORSRUD (Sweden), OLAFUR S PALSSON (the United States), and HASSE ABRAHAMSSON (Sweden). EMERAN A MAYER had close communication with BEATE NIESLER (Germany), BRUCE NALIBOFF (the United States), WENDY SHIH (the United States), ANGELA P PRESSON (Germany), ARPANA GUPTA (the United States), JENNIFER S LABUS (the United States), GUY BOECKXSTAENS (Belgium), and KIRSTEN TILLISCH (the United States).
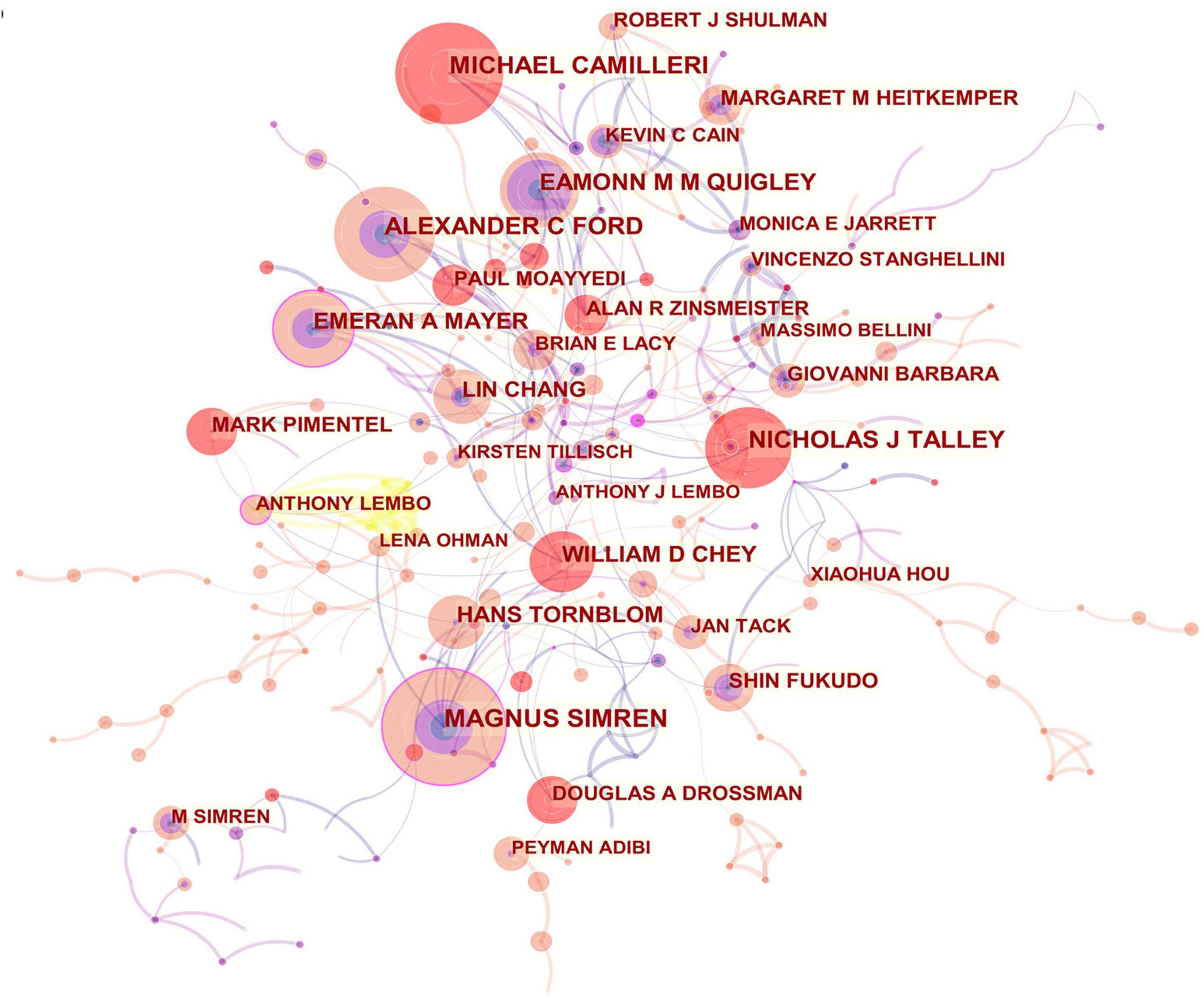
Figure 4. Network of authors in IBS research.
Journals and Co-cited Academic Journals
Publications pertaining to IBS research were found in 799 journals. Below is a brief summary of the 10 most prolific journals as shown in Table 3 . Among them, Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society published the highest number of articles (275, 6.72%), followed by World journal of gastroenterology (171, 4.17%), and Alimentary pharmacology and therapeutics (166, 4.05%). Gastroenterology with the highest impact factor (IF) of 22.682, published 76 articles (1.85%), ranked ninth for the total number of scientific articles. While the journal with the lowest IF of 3.067 was BMC gastroenterology , which ranked eighth with 78 articles (1.90%).
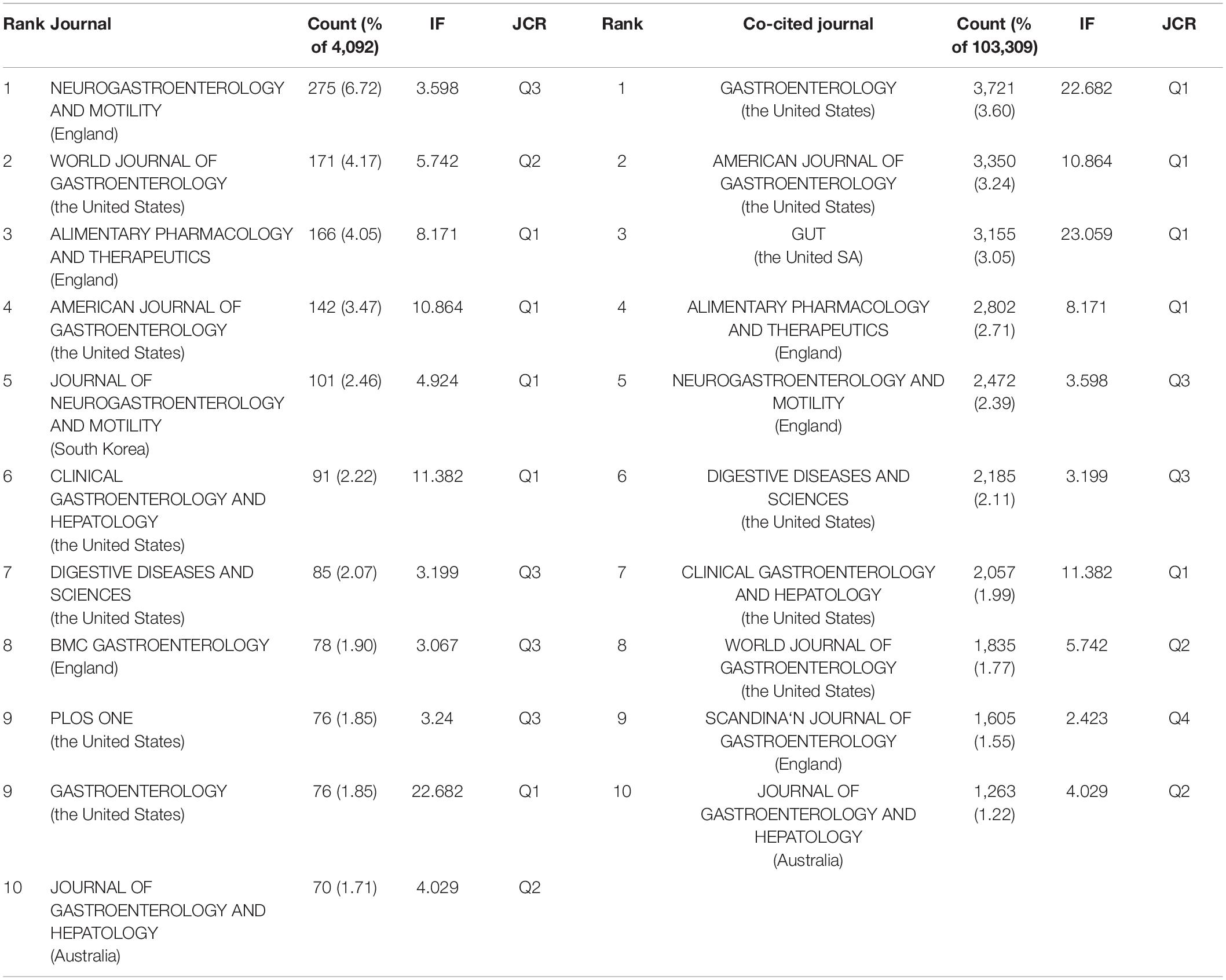
Table 3. Top 10 journal and top 10 co-cited journals in IBS research.
When two or more documents are cited simultaneously by a third paper, the former is termed as “co-cited” ( 21 ). Due to the scientific and objective nature of the co-citation analysis, subjects have been expanded from papers to authors, journals, and disciplines. The frequency at which the documents of two journals are cited together by the documents of another journal is called journal co-citation ( 22 , 23 ). The papers that published research in IBS were co-cited by 1,394 scholarly journals. As shown in Table 3 , Gastroenterology had the most co-citations (3,721, 3.60%), followed by The American journal of gastroenterology (3,350, 3.24%), and Gut (3,155, 3.05%).
There is a concurrence of Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society , World journal of gastroenterology , Alimentary pharmacology and therapeutics , The American journal of gastroenterology , Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association , Digestive diseases and sciences , Gastroenterology , and Journal of gastroenterology and hepatology in the prolific journals and highly co-cited ones.
Co-cited References and References With Citati on Bursts
In the 4,092 IBS publications, there were 1,386 references co-cited. Table 4 provides a list of the top 10 co-cited references. Of the eleven documents, five were published in Gastroenterology , two were published in The New England journal of medicine , one was published in Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association , one was published in JAMA , one was published in The American journal of gastroenterology , and the last one was from Nature reviews. Disease primers . Among them, Mearin et al. ( 24 ) published an article, entitled “ Bowel Disorders ” in Gastroenterology , which was the most frequently co-cited and ranked first (425), followed by “ Functional bowel disorders ”, written by Longstreth et al. ( 7 ) in Gastroenterology (266), “ Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis ,” authored by Lovell and Ford et al. ( 3 ) in Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association (246), and “ Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV ,” published by Drossman ( 25 ) in Gastroenterology (205).
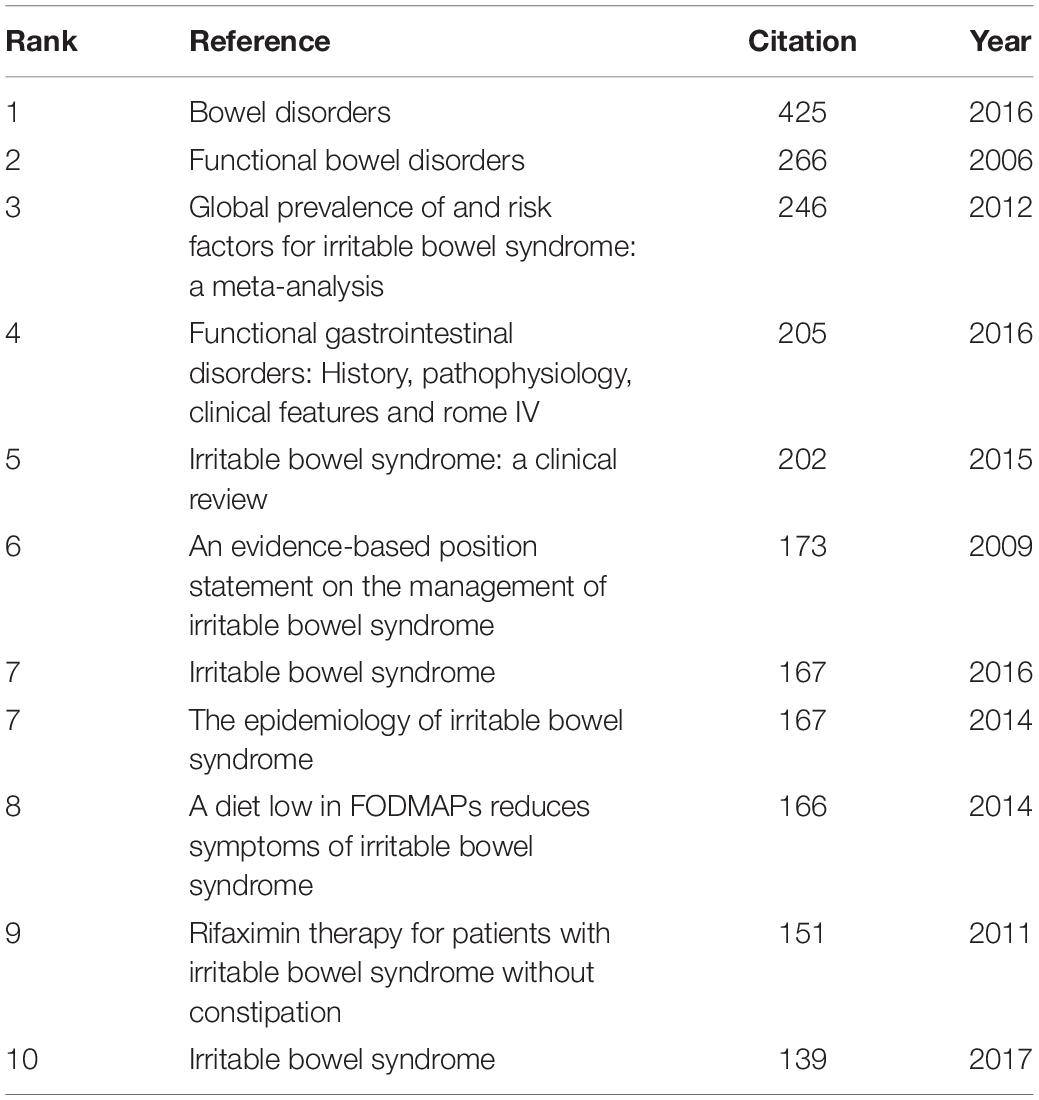
Table 4. Top 10 co-cited references in IBS research.
As shown in Table 5 , the highest-ranked co-cited references by the betweenness centrality value were published from 2008 to 2019. Of the nine references, two were published in Gut , two were published in Alimentary pharmacology and therapeutics , two were published in The American journal of gastroenterology , one was published in World journal of gastroenterology , and the other two were from Gastroenterology and Gut , respectively.
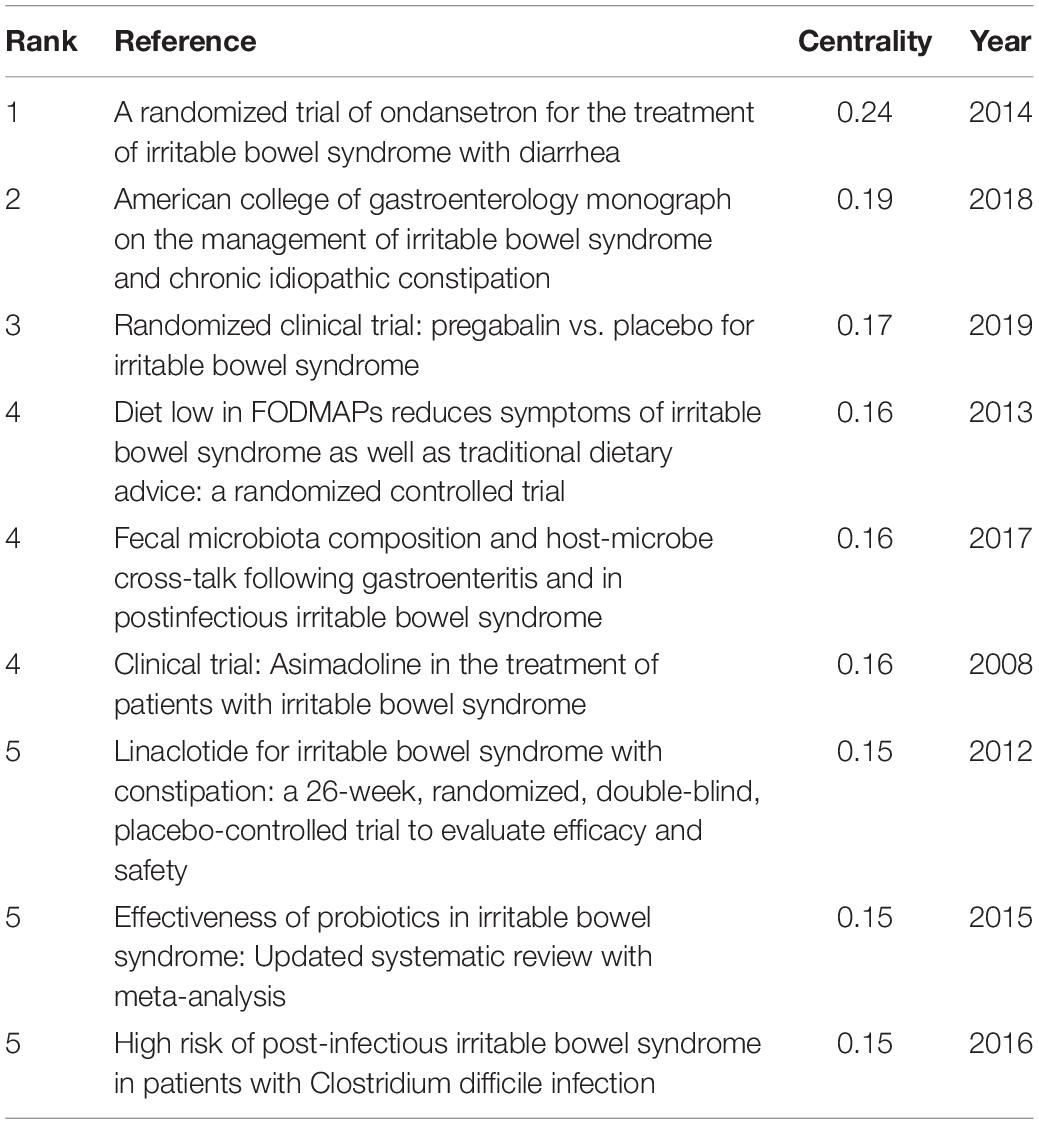
Table 5. Top 5 co-cited references with the highest betweenness centrality in IBS research.
In order to identify literature that has received a lot of attention from peers, the burst detection strategy was applied to publications cited at an increasingly fast rate. In Figure 5 , strong citation bursts for 25 references are shown. Year denotes when the article was published. Strength represents the citation strength. The length of the line corresponds to the period from 2007 to 2022, in which the red segment indicates the time interval of citation bursts. The strongest citation burst was the article entitled “ Functional bowel disorders ” published in Gastroenterology by Longstreth et al. ( 7 ) with a citation burst lasting from 2007 to 2011 (119.61), followed by “ Bowel Disorders ” published by Mearin et al. ( 24 ) with a citation burst spanning from 2017 to 2022 (114.93), and “ Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis ,” published in Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association by Lovell and Ford et al. ( 3 ), which showed a citation burst from 2012 to 2021 (87.08). Those references whose citation bursts ended in 2021 or later deserve special consideration ( 2 , 3 , 24 – 34 ).
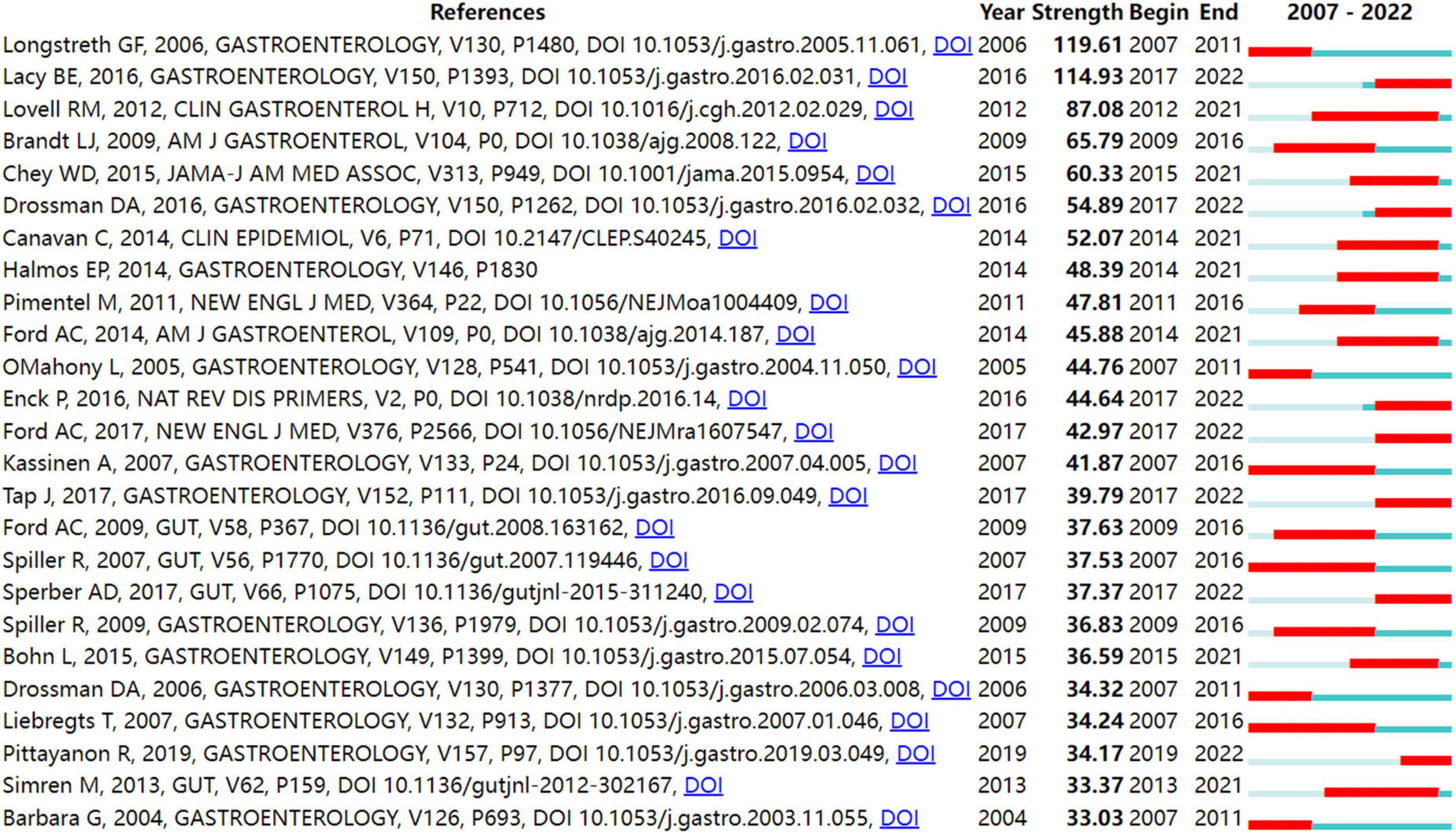
Figure 5. Top 25 references with strong citation bursts in IBS research.
Keywords Analysis
Keyword co-occurrence analysis is derived from the concept of citation coupling as well as co-citation in bibliometrics ( 35 , 36 ). That is, when two keywords that reflect the core research contents of an article appear in the same document, it is considered that there exist the relationship between the two terms. The higher the number of co-occurrences of two terms, the closer their relationship is. A map of keywords co-occurrence is generated based on the frequency of appearance for paired keywords. One of the common methods of identifying hot topics in bibliometrics was co-occurrence analysis of keywords. In the present study, keywords were extracted from 4,092 publications. After excluding irrelevant keywords and merging those with the same semantic meaning, 773 keywords were identified.
Figure 6 shows the map of keywords with highly co-occurrence frequencies that VOSviewer analyzed. Keywords were stratified into four clusters: clinical trials related to IBS (green cluster), post-infectious IBS (purple cluster), the role of the altered composition of intestinal microbiota in IBS (dark blue cluster), pathophysiological mechanisms of IBS (red cluster), IBS or IBS-like symptoms (light blue cluster), and pharmacological and non-pharmacological treatments for IBS (yellow cluster).
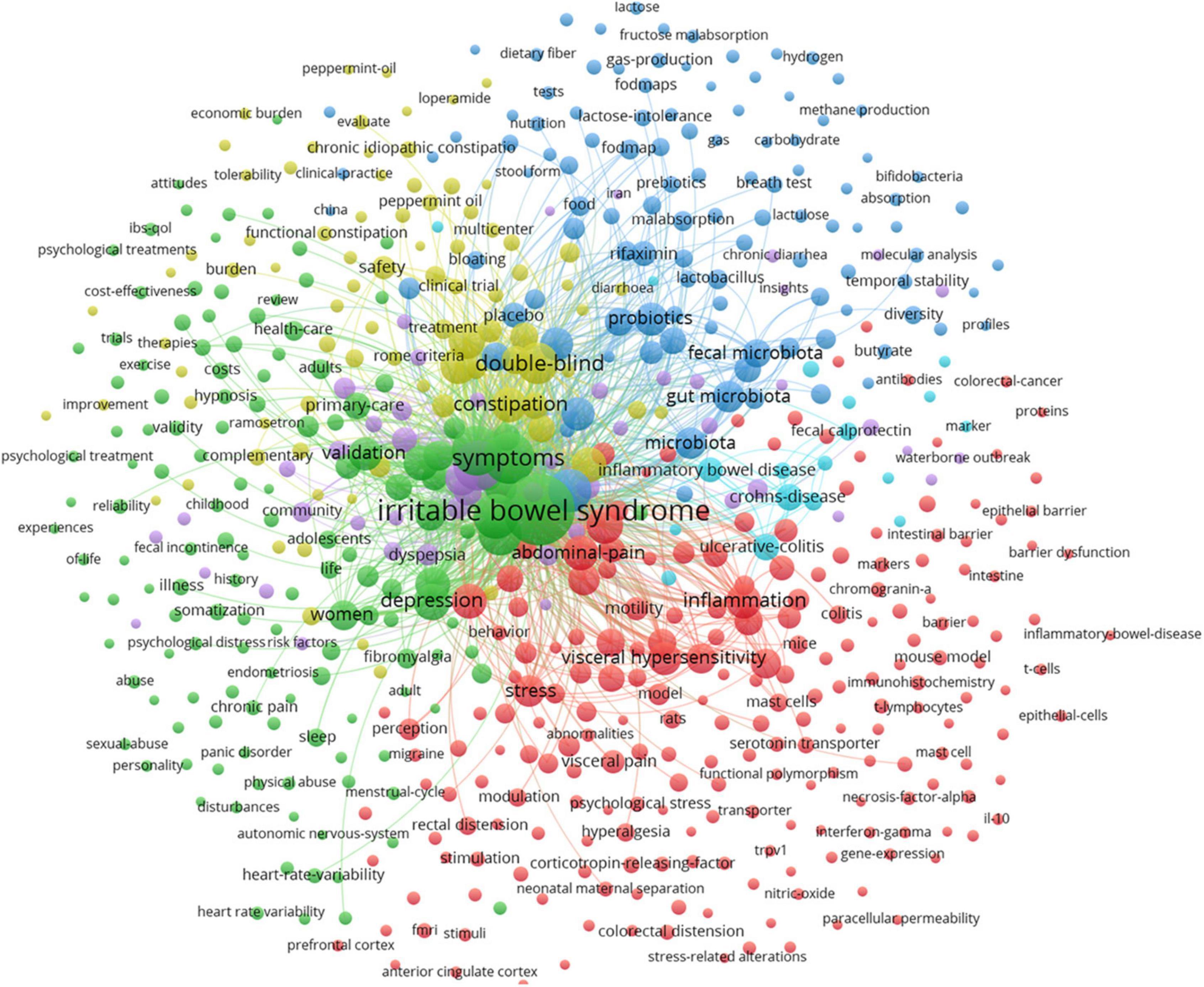
Figure 6. Map of keyword clustering with a minimum of 5 occurrences in IBS.
In Figure 7 , the keywords co-occurrence was visualized in chronologic order. The year placed at the top of the view corresponds to the earliest year when each keyword appeared. Each node in the map represents a keyword. Co-occurrences of keywords are represented by the links.
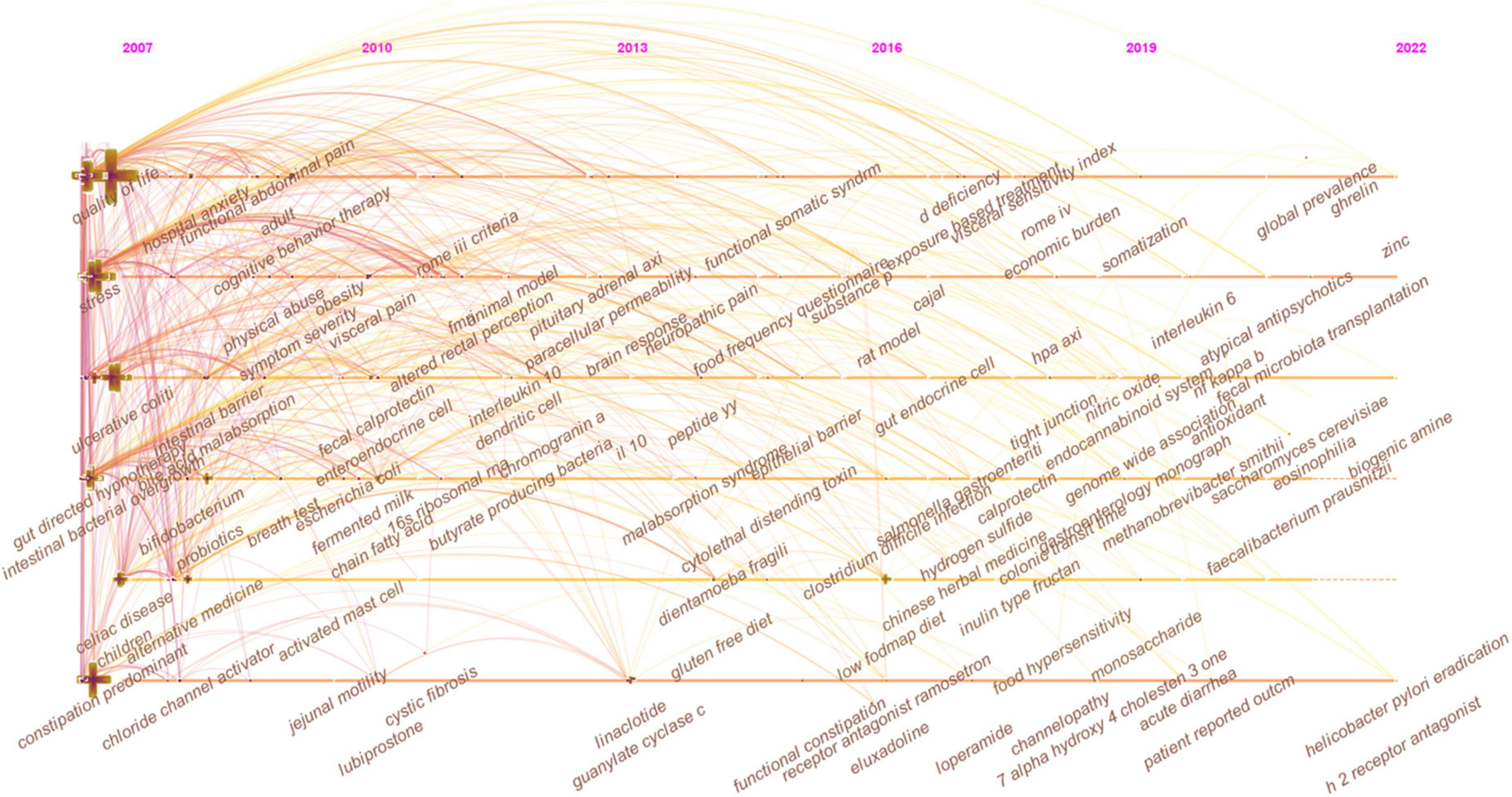
Figure 7. The timeline view of keywords in IBS research.
As shown in Figure 8 , closely related keywords were grouped into different clusters. A cluster is assigned a tag number, and the smaller the number, the more keywords comprise the cluster. The following 10 blocks were presented: #0 visceral pain; #1 primary care; #2 prevalence; #3 small intestinal bacterial overgrowth; #4 diversity, #5 immunohistochemistry; #6 neonatal maternal separation; #7 dyspepsia; #8 food allergy; and #9 serotonin transporter.
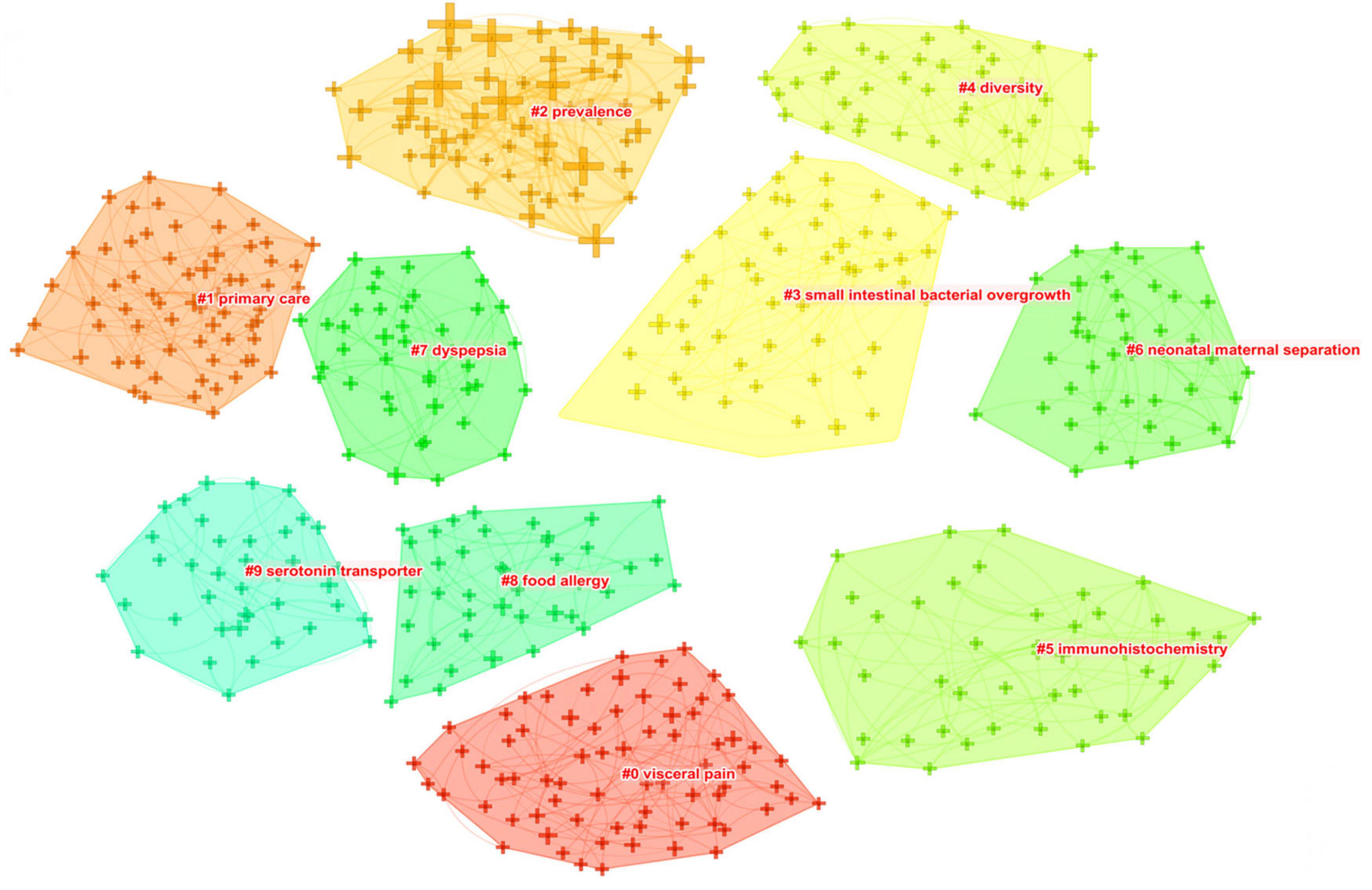
Figure 8. The keyword clustering knowledge map of IBS research.
Table 6 presents the meaningful keywords with high frequency in IBS research. The most frequent keywords were symptom (1,020, 0.01), clinical trial (888, 0.1), quality of life (854, 0.01), epidemiology (819, 0.05), pain (566, 0), gut microbiota (518, 0.05), management (431, 0), hypersensitivity (424, 0.02), efficacy (297, 0.01), and constipation predominant IBS (276, 0.02).
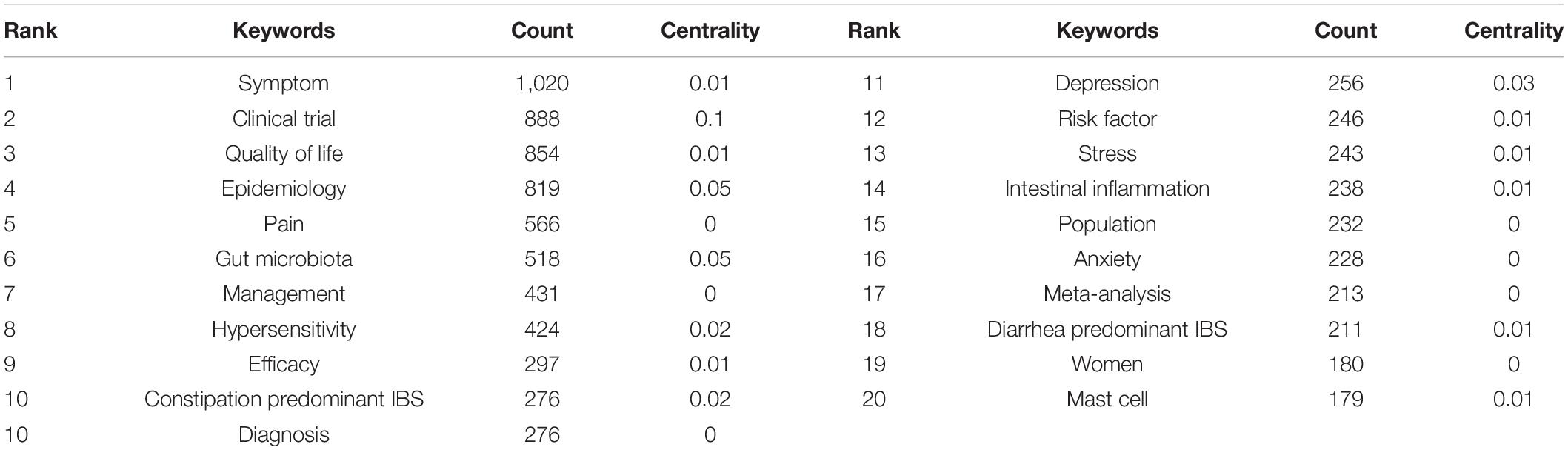
Table 6. Top 20 keywords with the highest count in IBS research.
Strong citation bursts are considered indicators of research frontiers within a particular period of time since the number of citations and occurrences of those terms have surged (19). Figure 9 shows the keywords with strong citation bursts. Some of them exhibited ongoing strong citation bursts, including chromogranin A, rat model, peptide YY (PYY), and gut microbiota, etc.
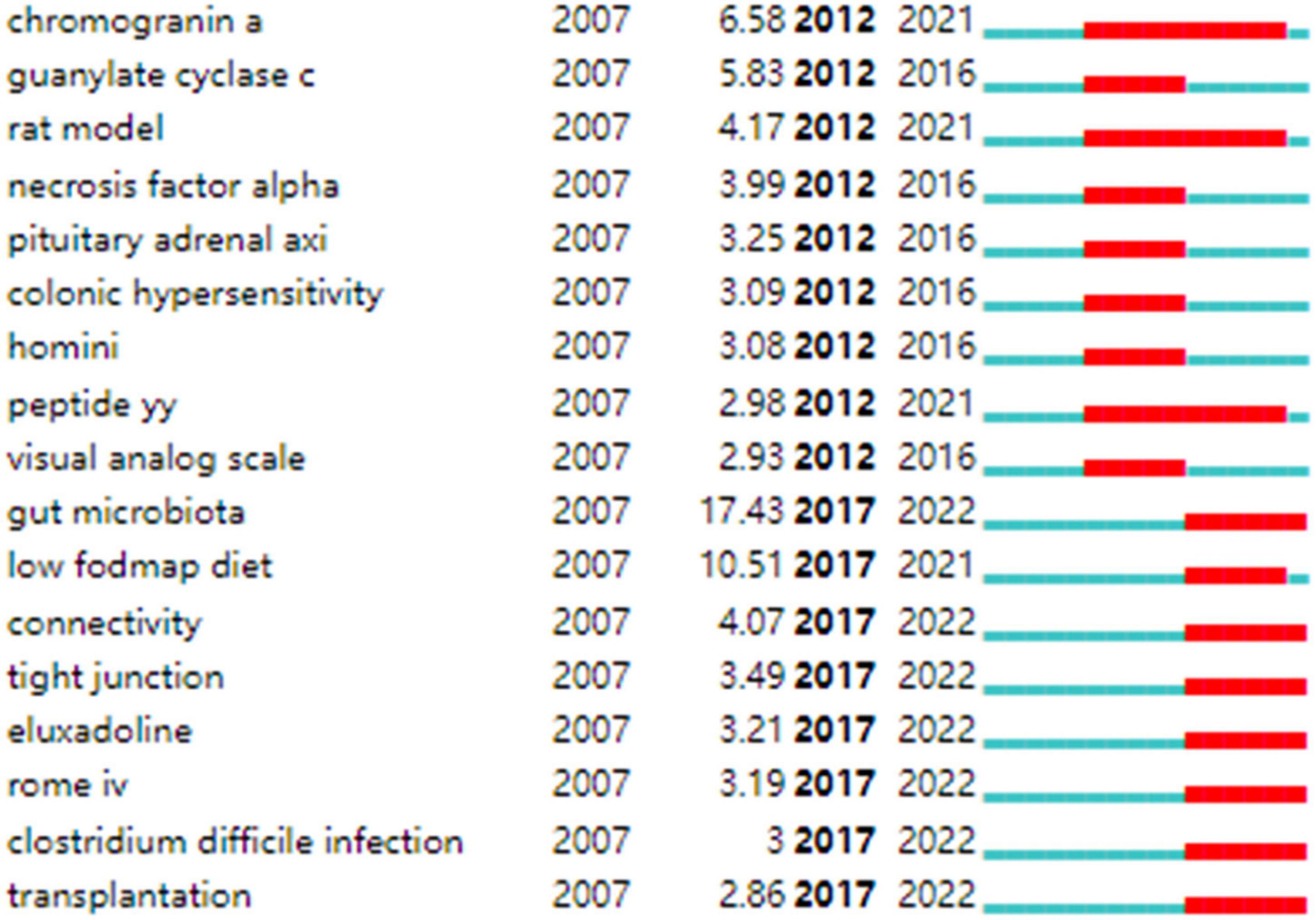
Figure 9. Keywords with strong citation bursts in IBS research.
General Information
The number of academic publications is an important reflection of research activity. As shown in Figure 1 , a total of 2,039 documents were published from 2016 to 2021, a high-yield and rapid growth stage in the field, which has increased significantly compared with the previous 6 years, with 1,500 documents, from 2010 to 2015. Accordingly, it seems possible that the field is about to enter its golden period in the next few years.
Table 1 shows that the highest yielding countries are mostly based in Europe (England, Sweden, Italy, Germany, and France), Asia (China, South Korea, Japan), and North America (the United States and Canada). Any subject with a betweenness centrality value surpassing 0.1 is considered influential in the network. Thus, the highly productive countries in Europe and North America contributed the most impactful research in the field of IBS. Among the high-yield institutions, the top three ranked institutions were in the United States, while there was only one research institution in Asia (Iran). In addition, American and Swedish institutions, including Mayo Clin, Univ N Carolina, Univ Gothenburg, and Univ Washington were prominent in the field with considerable academic influence, given their high volume of publications as well as a high betweenness centrality value. Therefore, the United States and Sweden dominated in research quality and productivity; however, research capacities in Asian regions were generally weak. In the United States, for example, research capacity is likely related to overwhelming support in terms of research, the diversity of researchers with an interest in this field, a wealth of environments well-equipped for research, and the greater availability of a well-trained workforce. In addition, due to the strength of the economy of the United States, significant financial resources are made available to researchers, and scientists enjoy enhanced mobility ( 37 , 38 ). Functional gastrointestinal disorders such as IBS are linked to dysbiosis, and the symptoms triggered is often caused by episodes that affect the microbiome in an environment where the emotional context and enteric nervous system are in synergy ( 39 ). Further, in 2013, the United States launched an innovative program on the gut microbiota-brain axis ( 40 ), which also has led to a surge in publications related to IBS.
As shown in Figure 2 , in general, collaboration exerts positive effects on scientific output, and cooperative research results are of high scientific quality with high academic impacts, particularly those related to transnational collaboration. North American and European countries, which played an influential role in the IBS research, developed cooperative partnerships worldwide. However, collaborations in Asian countries tended to be intra-continental phenomena. It is possible that scientific advances in IBS research in Asian countries were plagued by less transnational cooperation and academic exchange. Notably, Canada and Ireland, which were detected with strong bursts, revealed high scholarly activity over a brief period.
In Figure 3 , collaborations and partnerships among institutions mostly occurred within North America and Europe. Even though some Asian countries have contributed substantially to publications counts, they have not formed a cooperative network, which further confirms that IBS research in Asia lacked intercontinental collaboration. Teheran Univ Med Sci, Univ Calif Los Angeles, Mayo Clin, Univ Washington, McMaster Univ, Univ Manchester, and Cedars Sinai Med Ctr, which exhibited strong bursts, witnessed a large increase in recent publications.
In Table 2 and Figure 4 , the productive authors were mainly from European and North American countries. Swedish and American researchers wielded major influence in IBS research, which also demonstrates the outstanding performance and leading roles of the United States and Sweden in the field. Instead, the academic impacts of Asian scholars were minor. Besides, the overall cooperation and communication still centered on European and American scholars. Hence, Asian nations, including China, South Korea, Japan, India, and Iran, are urged to follow the international pattern of fostering scientific cooperation while raising scientific output, which is directly linked to greater research quality and fortified scientific capability. In fact, a collaborative research project might lead to the participation of experts from various fields, which has been interpreted as positive evidence regarding its impact on the quality of research. As shown in Table 2 , these prolific gastroenterologists who are also likely to initiate collaborations and in most cases provide the central funding or resource support in their community clusters, have interests in neurology, nutrition, and endocrinology (e.g., ALEXANDER C FORD, EMERAN A MAYER, MAGDY ELSALHY, and others). Moreover, the highest-ranked scholar by the betweenness centrality value was MAGNUS SIMREN, indicating that his academic attainments earned him great credibility among peers and had considerable influence in the field. Scholarly contributions from EMERAN A MAYER also occupied an eminent position.
According to Table 3 , IBS research has been published largely in journals from Western countries that specialize in gastroenterology. Studies of high quality and well-designed design are the evidence base for IBS research, as the top prolific journals are typically found in Q1 or Q2. Journals with high co-citations are referred to as mainstream journals, to which researchers are dedicating great attention. Likewise, highly co-cited journals were issued in Western countries, which were classified as Q1 or Q2. This finding enhances the perception of strengthening the construction of scholarly periodicals, especially in Asian nations, for the generation of high-quality scientific outcomes and the dissemination of knowledge in the IBS field. Moreover, Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society , World journal of gastroenterology , Alimentary pharmacology and therapeutics , The American journal of gastroenterology , Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association , Digestive diseases and sciences , Gastroenterology , and Journal of gastroenterology and hepatology were deemed core journals in the field with high publications and co-citations. In addition to serving as reliable references for IBS-related manuscripts, they can also be taken into consideration when submitting manuscripts.
Knowledge Base
Co-cited references are publications that have been cited together by other publications, and are viewed as a knowledge base for a particular field of study. As shown in Table 4 , most literature published in high-impact journals between 2006 and 2017 were reviews or articles describing the epidemiology, risk factors, diagnosis, clinical features, pathophysiology, and management of IBS ( 1 , 3 , 4 , 7 , 24 – 26 , 30 , 41 ). In addtion, Halmos et al. ( 27 ) published the eighth co-cited paper in Gastroenterology in 2014; this study showed that low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAP) diet (LFD) which consisted in reducing the intake of poorly absorbed short-chain carbohydrates, such as lactose or fructo-oligosaccharides, improved gastrointestinal symptoms in IBS. Another co-cited article was published in The New England journal of medicine by Pimentel et al. ( 42 ). The researchers conducted two large Phase III trials of rifaximin in patients with non-constipated IBS, which demonstrated significant relief from symptoms of IBS such as bloating, abdominal pain, and watery or loose stools.
In Table 5 , the top 5 co-cited references with the highest betweenness centrality value, which were considered key in defining the intellectual base of IBS, revolved around (1) therapies that target visceral pain modulation, including 5-hydroxytryptamine 3 (5-HT 3 ) receptor antagonist ( 43 ), the blocker of α2δ subunit on voltage-dependent calcium channels ( 44 ), and peripheral diaryl acetamide kappa-opioid receptor agonist ( 45 ); (2) therapies that increase intestinal secretion for IBS-C, such as agonist of the guanylate cyclase C receptor ( 46 ); (3) treatment targeting the intestinal microbiota ( 47 ); (4) non-pharmacological measure such as dietary modifications ( 32 ); (4) characterization of the intestinal microbiota in especially post-infectious IBS ( 48 ); and (5) risk factors for post-infectious IBS ( 49 ).
As can be seen in Table 6 , the extant studies included in the analysis have primarily addressed IBS-C and IBS-D. The potential explanation for this trend may be that these subtypes are more prevalent. In a recent meta-analysis involving 6,756 participants, it has been reported that, using the Rome IV criteria, the global prevalence for IBS-D is 1.4%, followed by 1.3% for IBS-C, 1.1% for IBS-M, and 0.5% for IBS-U ( 50 ). In spite of the use of the Bristol stool form scale in Rome IV to categorize patients with IBS into subtypes, which results in a lower proportion of patients meeting the criteria for IBS-M or IBS-U, these individuals still comprised more than one third of the patients with IBS according to this meta-analysis ( 50 ). At present, however, there are no licensed therapeutics for use in these patients, which represents a significant unmet need. Therefore, enhanced research is needed.
Additionally, from Figure 7 , in which “depressive symptoms,” “psychological distress,” “FODMAP,” “dysbiosis,” “microbiota,” “visceral hypersensitivity,” “functional magnetic resonance imaging (fMRI),” and “cortex,” are included in each cluster, this demonstrates that there has been a proliferation of research into brain-gut-microbiota (BGM) axis within global IBS research. We can infer that the IBS field will undergo a paradigm shift with the involvement of experts in psychiatry, neurology, microbiology, nutrition, and imaging.
Hot Topics and Frontiers
Figure 5 shows the top 25 references with the strongest bursts of citations, whose research topics scholars followed closely over the past fifteen years. Among them, fourteen references ( 2 , 3 , 24 – 34 ) whose citation bursts continued to 2021 or later have attracted considerable interest from the scientific community, thus reflecting the hot topics and emerging trends in IBS research.
In Figure 7 , the evolution of research topics was identified. In the early years from 2007 to 2013, IBS research began to focus on (1) overlap syndrome; (2) 16S ribosomal RNA gene sequencing; (2) fMRI, glucose breath test, and lactulose breath test; (3) IBS-like symptoms; (4) fibromyalgia, menstrual cycle, endometriosis, and chronic fatigue syndrome; (5) acute gastroenteritis, ischemic colitis, antibiotic-associated diarrhea, Inflammatory bowel disease (IBD), and celiac disease; (6) idiopathic constipation and obstructed defecation syndrome; (7) somatization, sexual abuse, physical abuse, and post-traumatic stress disorder; (8) gluten-free diet; (9) cingulate cortex, dorsal horn neurons, and prefrontal cortex; (10) serine protease activity, lactoferrin, and short chain fatty acids (SCFAs); (11) colonic fermentation, hydrogen sulfide, and methane production; (12) colonic hypersensitivity, altered rectal perception, allodynia, and neuropathic pain; (13) intestinal bacterial overgrowth; (14) butyrate-producing bacteria, lactic acid bacteria, Escherichia coli , Blastocystis hominis , Lactobacillus rhamnosus GG, Lactobacillus reuteri , and Lactobacillus plantarum 299v ; (15) brain-gut axis and hypothalamic-pituitary-adrenal axis; (16) corticotropin-releasing hormone and cortisol; (17) PYY, cholecystokinin, and glutamine; (18) neonatal maternal separation; (19) enteroendocrine cells (EECs), dendritic cells, and T lymphocytes; (20) 5-hydroxytryptamine (5-HT) transporter, cannabinoid receptor, calcium channel, estrogen receptor-β, fibrosis transmembrane conductance regulator, and chloride channel activator; (21) E-cadherin; and (22) tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin (IL)-10, and IL-1β.
From 2013 to 2016, the field focused on (1) early life stress, panic disorder, alexithymia, functional somatic syndrome; (2) collagenous colitis and malabsorption syndrome; (3) functional connectivity, anterior cingulate cortex, and catecholamine; (4) Dientamoeba fragilis and Bifidobacterium infantis 35624; (5) fecal microbiota transplantation (FMT); (6) visceral hyperalgesia and substance P; (7) interstitial cells of Cajal; (8) low-grade inflammation, paracellular permeability, and oxidative stress; and (9) δ opioid receptor and ion transport.
From 2016 to 2022, researchers turned to research on (1) food intake disorder, Brugada syndrome, and channelopathy; (2) Salmonella gastroenteritis and Clostridium difficile infection; (3) uroguanylin, fecal calprotectin, and heat-stable enterotoxin; (4) duodenal microbiome; (5) LFD; (6) water-avoidance stress; (7) bile acid metabolism; (8) Faecalibacterium prausnitzii ( F. prausnitzii ), Akkermansia muciniphila , Methanobrevibacter smithii , and Saccharomyces cerevisiae ; (9) glucagon-like peptide 1, brain derived neurotrophic factor, ghrelin, and neuropeptide Y; (10) eosinophilia and mast cell; and (11) IL-6, nitric oxide, nuclear factor kappa B (NF-κB), and guanylyl cyclase C.
In addition, keywords with the ongoing strong citation bursts shown in Figure 9 were used to identify the hot issues within the field. Of these, gut microbiota is the keyword with the strongest citation burst. Given the hot topics are not separated, but influential and interrelated to each other. We discussed these key hot topics in IBS research under the most popular researched “gut microbiota” framework, highlighting their interrelated aspects as follows:
FODMAP are short-chain carbohydrates that are not readily absorbed in the small intestine, increasing water delivery into the lumen due to osmotic action causing diarrhea. FODMAPs act as a prebiotic for gas-producing bacteria, Clostridium, in the large intestine, increasing gas production ( 51 ). Luminal distension, in turn, is worsened. Among the metabolites fermented from FODMAP are SCFAs like butyrate, acetate, and propionate, as well as carbon dioxide and hydrogen. The presence of these metabolites might also affect microbial colonic environments and IBS symptoms ( 52 , 53 ). The effects of butyrate on visceral sensitivity were demonstrated in healthy volunteers ( 54 ). Through stimulation of 5-HT release from the intestinal mucosa, SCFAs initiate high-amplitude propagated colonic contractions, accelerating intestinal transit ( 55 ).
Over the last decade, research has shown that the FODMAP-restricted diet may be a safe and effective dietary intervention ( 56 ). Several studies have been conducted to conclude that an LFD is effective in relieving overall IBS symptoms and behaves either with non-inferiority or superiority with respect to other comparators ( 27 , 57 , 58 ). The consumption of an LFD has been found to improve symptoms in more than half of IBS patients ( 59 ). However, these trials have been focused on showing their short-term effectiveness, and long-term studies still need to be carried out.
FODMAP restrictions may decrease levels of prebiotics, including fructo-oligosaccharides, galacto-oligosaccharides, and fibers, which are utilized by host microorganisms in a health-enhancing manner. In the end, this results in a reduced amount of highly beneficial bacteria and decreased production of SCFAs that are beneficial to colonocytes. Some studies have consistently reported the effect of an LFD leading to a reduction in Bifidobactrium ( 60 – 63 ), which is believed to be associated with a worse symptom profile, though no studies have yet investigated the detrimental effects of lower bifidobacteria resulting from LFD on long-term health. Twenty-seven IBS patients and six healthy participants were studied with an LFD or a typical Australian diet and researchers found Clostridium Cluster IV and F. prausnitzii levels were reduced in comparison to controls, with the latter known for anti-inflammatory properties due to its ability to produce butyrate, which regulates T helper 17 and T regulatory cells ( 62 ). An impaired level of F. prausnitzii could potentially harm the integrity of the intestinal mucous barrier, which results in dysbiotic microbes causing IBD ( 64 ). In a controlled, single-blind study with forty IBS patients (twenty on an LFD and twenty on a high FODMAP diet) for 3 weeks, researchers found that the LFD increased the richness and diversity of Actinobacteria ( 65 ). A recent review reports changes in gut microbiota composition after an LFD, such as a lower abundance of Bifidobacterium or Bifidobacteriaceae, Lactobacillaceae, Propionibacteriaceae, Clostridium cluster IV , F. prausnitzii , and an increased abundance of Bilophila wadsworthia , Clostridiales family XIII incertae sedis, and Porphyromonas IV .
In conclusion, mixed results were found in research conducted on the effects of LFD on gut microbiota and its metabolites. Inconsistencies between studies may be related to heterogeneity in LFD study designs, and different sample collection, storage, and analysis methodologies. In addition, feces analysis, however, does not reliably present the actual picture of the gastrointestinal tract. Metagenomics, transcriptomics, proteomics, and metabolomics can be more informative.
Another challenge with LFD is that only 50% of IBS patients report symptomatic improvement on an LFD. In an effort to optimize patient selection most likely to respond to the LFD, and to avoid unnecessary dietary restrictions in those less likely to respond, the potential cause of non-response is being investigated in greater detail. The question of whether baseline colonization of microbiota can predict symptomatic response to the diet is receiving increasing attention. In a clinical trial, thirty-one patients with IBS were randomly assigned to follow the LFD and thirty patients to follow National Institute for Health and Care Excellence dietary advice, and over 4 weeks, researchers found the dysbiosis index was higher in non-responders to the diet than in responders ( 63 ). Among responders, Bacteroides stercoris , Pseudomonas , Acinetobacter , Desulfitispora , Parabacteroides , Bacillus , Salmonella ( Citrobacter , Cronobacter , Enterobacter ), Corea , Ruminococcus gnavus , Clostridium , Firmicutes ( Clostridia ), and Streptococcus were lower at baseline ( 63 ).
In another study, 61 IBS adult patients followed the LFD for 4 weeks, with 52% of responders having different microbial composition at baseline when compared with 48% of non-responders ( 66 ). Bacteroides fragilis , Acinetobacter , Ruminiclostridium , Streptococcus , and Eubacterium were revealed to be higher in the responder group compared to the non-responder group and Clostridia/Negativicutes/Bacilli, Actinomycetales , Anaerotruncus, Clostridiales and Shigella / Escherichia were found to be lower in responders than non-responders at baseline ( 66 ). In both studies, the results regarding microbiota differences were noteworthy; it turned out that the results differed even though the intervention, selection criteria, and microbiota test were the same. Only one genus, Streptococcus was identified common in the studies but revealed opposing trends, indicating there is certainly much yet to learn about how symptom response to an LFD could be predicted by fecal bacterial profiles. In addition, fecal volatile organic compounds may serve as predictors of response to the LFD ( 67 ). Hence, gaps of interest include a deeper understanding of how an LFD affects the gut microbiota and research into diagnostic indicators such as bacterial markers and fecal metabolites to help to identify those likely to benefit from this specific intervention.
Enteroendocrine Cells
It has been suggested that cellular components in the gastrointestinal mucosa contribute to IBS pathogenesis; much attention has been devoted to EECs now. The colonic glands are defined by the presence of serotonin-containing (enterochromaffin) cells, peptide YY (PYY)-, oxyntomodulin (enteroglucagon)-containing L cells, pancreatic polypeptide (PP)- and somatostatin-producing cells ( 68 ). Kyösola et al. ( 69 ) and Verity et al. ( 70 ) were among the first to study EECs in the intestinal mucosa of patients with IBS who reported increased numbers of EECs in rectal biopsies from these patients. However, this topic that has felt static for years begins to move.
Chromogranin A, a common marker for EECs, is a granin secreted from secretory granules of all the different types of EECs ( 71 , 72 ). Patients with IBS have a lower density of chromogranin A in their duodenum and colon than healthy subjects, suggesting that their EECs are generally less dense ( 73 ). These abnormalities probably contribute to IBS pathophysiology because low-density EECs are characterized by subsequent low levels of certain hormones, and dysmotility of the gut, visceral hypersensitivity, and abnormal secretion may result from this in patients with IBS ( 74 ). Low cell densities of Musashi 1 (a marker for stem cells and their early progenitors) and neurogenin 3 (a marker for EEC progenitor) in the small and large intestines of IBS patients indicate that intestinal stem cells are low in clonogenic activity and differentiate slowly into endocrine cells ( 75 , 76 ). Thus, it is proposed that the abnormal behavior of stem cells remains a possible cause of the low density of EECs ( 77 ).
Intestinal stem cells are a possible avenue through which factors contributing to the pathophysiology of IBS exert their effects. Specifically, the diet we consume is thought to act as a prebiotic, therefore stimulating certain species of bacteria to grow. In turn, the bacteria ferment the diet, releasing by-products that affect the stem cells and their progeny in a way that reduces their numbers and causes a low differentiation into endocrine cells, which finally results in low density in EECs and the development of IBS symptoms. However, gastrointestinal EECs interact and communicate with each other in complicated ways; it is reported that the higher densities of gastrin-producing cells and lower somatostatin-producing cells observed in the antrum of IBS patients cannot be explained by abnormal stem cells like those that are seen in the small and large intestines, given that the densities of Musashi-1-positive cells don’t differ between IBS patients and healthy controls in the stomach ( 78 ).
Furthermore, it has been established that diet interacts with EECs. This has been supported by the finding that in the stomach and colon of IBS patients, EECs detected by chromogranin A increase toward the values seen in healthy controls following an LFD, probably due to the changes in gastrin-, enterochromaffin-, ghrelin-, and somatostatin-secreting cells in the stomach and enterochromaffin cells and PYY containing L-cells in the colon ( 79 – 82 ).
Moreover, the gut microbiota is able to interact with EECs. FMT is shown to affect the densities of EECs in the duodenum and colon ( 83 , 84 ). To investigate the mechanisms behind the restoration of EECs after receiving FMT, in a study by Mazzawi et al. ( 85 ), patients reported improvements in their IBS symptoms in parallel with changes in their EECs density 3 weeks after FMT. In fact, the changes in the density of EECs do not appear to be caused by an alteration in the stem cells or their early progenitors, rather they may be due to changes in the differentiation progeny, as observed by neurogenin 3 ( 85 ).
PYY which has been captured with a strong citation burst in our study is a hotly researched gut-derived hormone in IBS pathophysiology. This peptide along with enteroglucagon and glucagon-like peptide 1 is co-produced from L cells. The density of EECs, including PYY cells, in the colon and rectum, are lower in IBS patients when compared to healthy controls ( 86 , 87 ). In this way, the presence of low amounts of PYY and low densities of PYY cells in the large intestine will impair the release of PYY, contributing to the dysmotility that is associated with IBS in that this hormone inhibits gastric and pancreatic secretion, delays the emptying of the stomach, and increases water and electrolyte absorption ( 88 ). Moreover, inferred from the fact that PYY modulates 5-HT release, which regulates visceral sensitivity, the low PYY concentration could indirectly contribute to IBS symptoms of visceral hypersensitivity ( 89 ). It has been reported that the consumption of LFD increases the PYY cell density to the normal level in IBS patients and improves the symptoms of IBS as well ( 82 ); in a similar manner, SCFAs, one of the fermentation products of intestinal microbes, have been reported to promote gene expression and stimulate the production of PYY ( 90 ). Hence, it is possible to restore PYY abnormalities by modifying the diet or the microbiota in IBS, and a PYY receptor stimulator could also be helpful for the treatment of IBS.
Taken together, the optimism in research on the links between diet, gut microbiota, stem cells, and EECs permits us to believe that the knowledge of stem cells and EECs (with hormones) will lead to their use in therapeutics and help to elucidate the mechanisms underlying improved symptoms and non-response following FMT and diet modification.
Tight Junctions
While providing nutrients and water to the body, the intestinal barrier protects internal organs from bacteria, luminal antigens, and luminal pro-inflammatory factors ( 91 ). In several disease conditions, the intestinal barrier dysfunction causes bacteria, endotoxins, and other inflammatory mediators to proliferate. In the case of IBS, increased intestinal permeability was highly variable, with 2–62% showing increased permeability compared to 0–15% in controls ( 92 ). The access of noxious substances to submucosa is largely prevented by a network of tight junctions (TJs), adherens junctions, and desmosomes within the intestinal epithelium. A physiological condition allows only water and electrolytes to penetrate the epithelium on the paracellular level. Paracellular permeability is regulated principally by TJs, a network of proteins found at the apex of epithelial lateral membranes, including claudins (CLDN), occludin, junctional adhesion molecule-A, zonula occludens (ZO), etc. A loss of TJs can allow entry of antigenic macromolecules, lipids, peptides from microbes, and even microbes through the epithelium ( 93 ). As a result, the mucosal immune system is over-stimulated, which has been linked to visceral hypersensitivity and symptom generation in IBS.
In IBS, the mechanisms responsible for modulating TJ expression and assembly are complex. It has been suggested that the intestinal microbial changes are a potential driver. When compared with conventional mice, CLDN-1 and occludin levels were higher in germ-free mice with lower paracellular uptake of a standard probe ( 94 ). Based on this, it appears that commensal microbiota affects colonic TJ proteins and paracellular permeability. In germ-free rodents, De Palma et al. ( 95 ) examined the role of the microbiome in regulating intestinal permeability and found that the colonic barrier was disrupted following gavage of fecal slurry from IBS-D. Studies suggest that patients with IBS, particularly those with post-infectious IBS (PI-IBS), exhibit higher levels of fecal proteolytic activity ( 96 ). Dysbiosis-derived proteases contribute to TJ disruption in IBS through the activation of a protease-activated receptor pathway ( 97 ). A study reports that Escherichia coli Nissle 1917 can mitigate the increase in paracellular permeability associated with supernatants obtained from patients with IBS ( 98 ). Furthermore, the activation of toll-like receptor 4 (TLR4)/myeloid differentiation factor 88 (MyD88)/NF-κB pathway in IBS-D is linked to imbalanced inflammatory cytokine expression, finally affecting gastrointestinal motility, secretion, and re-absorption as well as increasing intestinal sensitivity ( 99 , 100 ). More specifically, TLR4 and MyD88 are involved in pro-inflammatory signaling induced by bacterial lipopolysaccharide, whose activation may induce the expression of IFN-γ and TNF-α. In this way, intestinal epithelial barrier function is compromised by IFN-γ and TNF-α, which regulate the organization of several TJ proteins, such as ZO-1, CLDN-1, CLDN-4, and occludin ( 101 ). Therefore, blocking LPS-mediated signaling can be beneficial in protecting against the disruption of gut barriers. The TLR4-MyD88-transforming growth factor β-activated kinase1-NF-kB pathway is induced by wogonin to suppress inflammatory response and the down-regulation of CLDN-1 and ZO-1 in Caco-2 cells ( 102 ). IBS-D rats treated with QingHuaZhiXie prescription, a Chinese herbal compound prescription, showed that occludin, CLDN-1 and ZO-1 expression were restored in colon tissue by inhibiting the TLR4/MyD88/NF-κB pathway, which is accompanied by the improved symptoms of diarrhea and intestinal hypersensitivity ( 103 ).
In addition to the role of bacteria and their structural components in regulating TJ proteins, there may be different ways in which the microbial metabolites play a role. Butyrate, for instance, stimulates adenosine monophosphate-activated protein kinase, resulting in the assembly of TJ proteins which are important for intestinal barrier integrity repair ( 104 ). In response to sodium butyrate treatment, the motif-specific promoter region of CLDN-1 interacts with transcription factor specificity protein 1 to increase the transcription of CLDN-1 ( 105 ). It has been demonstrated that 6-formylindolo (3,2-b) carbazole, a tryptophan ligand, acts by activating the aryl hydrocarbon receptor and prevents TNF-α/IFN-γ-induced decrease in transepithelial electrical resistance and disruption of TJ proteins ( 106 ). Furthermore, polyamines, bile acid metabolites, conjugated fatty acids, and polyphenolic derivatives are examples of microbial metabolites that significantly affect TJ proteins ( 101 ).
Increased intestinal permeability has been observed in 37–62% of patients with IBS-D and 16–50% of patients with PI-IBS ( 92 ). IBS-C studies, however, showed the same level of permeability as controls ( 92 ). The rectosigmoid and descending colon represent the most extensively studied parts of the bowel in IBS, where TJ proteins are still highly heterogeneous. In addition, microbiota abundance and composition are not only related to a particular region within the digestive system, but also to the place where they are sampled. Also, in IBS, remissions are mixed with periods of symptoms escalating. Hence, to identify microbial changes that are missed with cross-sectional sampling, longitudinal sampling strategies, multiple time-point samples, a post-intervention follow-up, and a washout period for cross-over studies are needed; similarly, to better understand target TJ proteins in IBS, it may prove beneficial to sample longitudinally different areas of the gut in the same volunteers.
Even though some promising findings have been made, the intricate relationship between altered microbiota composition with microbial metabolites and TJ proteins modulation should be further explored, particularly in IBS-D and PI-IBS subtypes.
Neuroimaging and Gut Microbiota
Usually, IBS is a medical label used for medically unexplained gastrointestinal symptoms, but it may also reflect disturbances of the BGM axis ( 8 ). Since changes in the composition or functions of gut microbiota are known to affect human behavior and brain physiology, and dysbiosis is often presented by IBS patients, a great deal of attention has been paid to the role that gut microbiota play in this interaction ( 107 ). There is no adequate understanding of how gut microbiota signaling to the brain in humans works, but this process appears to be either modulated by microbial interactions with the host or diet, producing neuroactive compounds that can send signals to the brain via afferent vagal pathways or humoral channels or mediated by bacterial metabolites, which regulates immune function and cytokine production with downstream effects on brain functions through the regulation of neuroinflammation ( 108 ).
The use of neuroimaging as a non-invasive tool to explore the mechanisms of these pathways can be useful in addition to measuring the bioactives. In a new perspective on disorders of the BGM-axis, brain networks (brain connectome) and networks of gut cells and microbiota (gut connectome) are integrated, leading to a significant increase in studies that have combined gut microbiota and neuroimaging to investigate IBS pathophysiology ( 109 ).
Besides identifying structural and functional changes in specific brain regions, neuroimaging studies largely focus on brain connectivity using techniques such as diffusion MRI or fMRI recordings coupled with topological networks ( 110 ). The study by Labus et al. ( 111 ) examined the relationship between gut microbiota and brain structure among IBS patients. An increase in Clostridium members was significantly associated with a larger volume of subcortical areas (putamen, caudate nucleus, nucleus accumbens) and lower insula and prefrontal cortex volumes among the IBS participants ( 111 ). They also focused on Clostridiales, and examined its association with brain function and gastrointestinal sensorimotor function in another study ( 112 ). Lachnospiraceae incertae sedis , Clostridium XIVa and Coprococcus , all within the order Clostridiales, were found to be associated with gastrointestinal sensorimotor function in healthy controls, but not in IBS ( 112 ). Within the subcortical regions, Clostridium XIVa negatively correlated with putamen connectivity, as did Coprococcus and caudate nuclei, which were both related to improved gastrointestinal sensorimotor function ( 112 ). However, in IBS, there was a positive association between Clostridium XIVa and the putamen, the caudate nucleus, and the thalamus connectivity, suggesting that these subcortical areas may be affected by Clostridium XIVa and Coprococcus , and this may contribute to visceral hypersensitivity and pain in IBS ( 112 ). Osadchiy et al. ( 113 ) also reported on fecal metabolites and resting state fMRI. Histidine, glycine, glutamate, spermidine, and anserine variations were significantly correlated with changes in left dorsal part of the posterior cingulate gyrus to the left putamen ( 113 ). In addition, variations in histidine, tryptophan, uracil, 2-deoxyuridine, thymidine, and succinate were related to changes in the right superior frontal gyrus to right putamen. Aberrant tryptophan signaling may be responsible for this interaction in IBS patients ( 113 ). In fact, tryptophan is an important precursor of 5-HT. Several studies have suggested that tryptophan may be an essential amino acid in IBS because 5-HT is important for secretion, absorption, and intestinal transit, as well as mood, pain, and cognitive functions ( 114 ). Recently, Jacobs JP et al. ( 115 ) have shown that among IBS patients, as compared to non-responders, those who responded to cognitive behavioral therapy had increased levels of several members of the Clostridiales order as well as decreased levels of Bacteriodales. Responders showed a reduction in connectivity across multiple cortical networks including sensorimotor, default mode, salience, and emotion regulation networks following treatment, and these brain changes occurred in conjunction with a conversion to Bacteroides-predominant microbiota ( 115 ).
Microbiota together with non-invasive techniques that assess brain function, such as fMRI, have shed light on some aspects of the BGM-axis. The Bergen BGM-study ( 116 ), among others, provides an excellent example of multimodal and interdisciplinary clinical studies designed to verify directionality and causality in the BGM-axis in IBS.
Fecal Microbiota Transplantation
FMT involves the application of a fecal solution from a healthy contributor into the gut of a receiver that is aimed at restoring a dysfunctional microbial composition to a healthy one, and so to improve the function of the gut microbiota. In addition to its commonly recommended use in Clostridioides difficile infection, FMT has also recently gained attention due to the strong evidence linking dysbiosis to IBS pathogenesis ( 117 ). No consensus exists regarding FMT procedure, different routes of FMT delivery (e.g., colonoscopy, nasogastric tube, enema, and oral capsules), the types of formulations (frozen, dried and fresh), and the number and type of donors were examined. Each of these modes of treatment has had varying degrees of clinical success.
According to a meta-analysis of 267 IBS patients, colonoscopic FMT treatment was effective, nasogastric tube treatment was marginally beneficial, and oral capsules failed to deliver benefits ( 118 ). The results of another study investigating the effectiveness of FMT via colonoscopy for the treatment of IBS patients with diarrhea or diarrhea and constipation showed that clinically significant improvement of symptoms was observed in 65% of patients undergoing FMT after 3 months, compared to 43% of control subjects receiving their own feces ( 119 ). In patients with frozen FMT, as opposed to fresh transplants, better results were obtained ( 119 ). In addition, other studies suggest that fresh or frozen donated stool may provide benefits, while capsulized FMT could be harmful ( 118 , 120 ). El-Salhy et al. ( 121 ) conducted a study evaluating the effects of two different doses (30 and 60 g) of FMT. There was a response in 23.6% of the patients who received a placebo, 76.9% who got 30 g FMT, and 89.1% who got 60 g FMT, suggesting a dose-dependent response ( 121 ). Holvoet et al. ( 122 ) suggested a microbiota modulation strategy through FMT could benefit the subgroups with severe bloating and flatulence, in whom there is the most profound disruption to gut microbial composition. FMT also appears to be dependent on the donor, so having a superdonor available would be crucial to ensuring the success of treatment ( 123 ). In addition, having a greater diversity of microbes pre-FMT makes it more likely that the individual will respond positively to FMT ( 122 ). Increasing microbial diversity would seem counterintuitive if FMT succeeded; possibly other transplanted components were responsible for its efficacy.
A Danish trial by Halkjær et al. ( 124 ) presented results that conflicted with the reporting of beneficial effects. During this randomized, double-blind, and placebo-controlled trial, 52 patients with moderate-to-severe IBS were randomized to FMT capsules or placebo for 12 days, with a follow up of 6 months. Although FMT altered gut microbiota composition, patients in the placebo group experienced greater relief of symptoms after 3 months than those in the treatment group. The authors state that altered gut microbiota does not suffice to obtain clinical improvement in IBS ( 121 ). In this regard, it remains to be seen if altered microbiota composition, function, and abundance contribute to rather than result from IBS.
FMT has been accompanied by sparse long-term follow-up data in people with IBS, but it must be stressed that it is not without risks. A study found 20% of the FMT group experienced side effects vs. 2% of the autologous FMT group, including two patients with diverticulitis in the FMT group and none with diverticulitis in the patients with autologous feces ( 121 ). FMT, as currently practiced, is only investigated in a research setting, and the available data on its potential effects is not sufficient to make any conclusive conclusions. Before the FMT can be made available as an openly available treatment option, more large-scale clinical trials are necessary.
Based on the hot topics outlined, we know that there has been a marked improvement in knowledge regarding IBS in recent years, with an increasing understanding of the BGM axis as well as potentially effective therapeutic options. Since IBS is defined as one of disorders of the gut-brain interaction, psychological aspects of IBS require attention.
An estimated 44 and 25% of IBS patients seen in gastroenterology clinics with anxiety and depression constitute the significant cohort of psychiatric comorbidity, respectively ( 125 ). The proposed biopsychological model of IBS implies that gut microbiota influence anxiety and depression secondarily (bottom-up model) and that psychological factors themselves lead to gut microbiota reconfigurations (top-down model) ( 126 ). To date, however, the mechanisms underpinning these psychological comorbidities remain unresolved.
It is interesting to note that most IBS patients with comorbid anxiety and depression manifest gastrointestinal symptoms prior to presenting with psychiatric symptoms ( 127 ) and as described by our analysis, the current landscape of IBS research also appears to primarily focus on the bottom-up approach, with efforts to delineate the roles of gut microbiota in dysregulation of the hypothalamic-pituitary-adrenal axis, the crosstalk between gut microbiota and the host’s immune system (e.g., microbe-associated molecular patterns), the increased intestinal permeability in the setting of inflammation, and the involvement of microbial metabolites, such as SCFAs and neuroactive molecules, in gut-brain communication. The presence of neuroinflammation associated with low-grade intestinal and systemic inflammation in IBS seems to contribute to psychiatric comorbidity ( 128 – 130 ), despite there still being a limited understanding of the nature of this entity that sits at the intersection between IBS, depression, and anxiety. In fact, in few studies, gut microbiota signatures associated with psychiatric comorbidity in IBS have been evaluated ( 95 , 131 , 132 ). In these studies, at least, it appears likely that comorbid patients cluster differently from patients with IBS, depression, or anxiety alone. Research efforts should be intensified to uncover why certain microbiota alterations result in IBS in some cases while in other cases leading to IBS with psychological disorders.
It has also been established but recently further demonstrated in recent preclinical studies that stress, a major contributor to the development of IBS and depression in later life, can also affect the composition and function of the gut microbiota ( 133 , 134 ). BGM axis is a bidirectional pathway; however, research on top-down hypothesis in this subset of patients appears to be lagging.
In this study, based on the 4,092 documents on IBS research retrieved from the WOSCC from 2007 to 2022, we conducted a bibliometric analysis of the knowledge structure, active research topics as well as emerging trends of IBS research. Overall, scientific production showed an upward trend. The United States and Sweden remained dominant in the IBS field with a high number of publications, great scholarly impact, and broad collaboration network in terms of authorship, (intra- and inter-) nationally and institutionally. MAGNUS SIMREN was the predominant contributor to the field with a high academic impact. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society , World journal of gastroenterology , Alimentary pharmacology and therapeutics , The American journal of gastroenterology , Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association , Digestive diseases and sciences , Gastroenterology , and Journal of gastroenterology and hepatology were deemed core journals in the field with high publications and co-citations. In addition, this study identified the LFD, EECs, tight junctions, neuroimaging and gut microbiota, and FMT as the research foci in the IBS field.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
XT, FW, and BZ led the team and were responsible for all aspects of the project. TZ, XM, and WT contributed to the methods, data acquisition, results, and interpretation. WT, JZ, and YW participated in designing and writing the manuscript. TZ, BZ, WT, and JZ revised this manuscript critically for important intellectual content. XT gave final approval of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (No. 81830118), the National Natural Science Foundation of China (No. 81804089), the Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (No: ZYYCXTD-C-202010), and Special Fund for Basic Scientific Research Business of Central Public Welfare Scientific Research Institute (No. ZZ13-YQ-003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Enck P, Aziz Q, Barbara G, Farmer AD, Fukudo S, Mayer EA, et al. Irritable bowel syndrome. Nat Rev Dis Primers. (2016) 2:16014. doi: 10.1038/nrdp.2016.14
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Sperber AD, Dumitrascu D, Fukudo S, Gerson C, Ghoshal UC, Gwee KA, et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome Foundation working team literature review. Gut. (2017) 66:1075–82. doi: 10.1136/gutjnl-2015-311240
3. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. (2012) 10:712–21.e4. doi: 10.1016/j.cgh.2012.02.029
4. Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. (2014) 6:71–80. doi: 10.2147/CLEP.S40245
5. Schmulson MJ, Drossman DA. What Is New in Rome IV. J Neurogastroenterol Motil. (2017) 23:151–63. doi: 10.5056/jnm16214
6. Spiller R, Aziz Q, Creed F, Emmanuel A, Houghton L, Hungin P, et al. Clinical Services Committee of The British Society of Gastroenterology. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut. (2007) 56:1770–98. doi: 10.1136/gut.2007.119446
7. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. (2006) 130:1480–91. doi: 10.1053/j.gastro.2005.11.061
8. Chong PP, Chin VK, Looi CY, Wong WF, Madhavan P, Yong VC. The Microbiome and Irritable Bowel Syndrome - A Review on the Pathophysiology, Current Research and Future Therapy. Front Microbiol. (2019) 10:1136. doi: 10.3389/fmicb.2019.01136
9. Schoenfeld PS. Advances in IBS 2016: A Review of Current and Emerging Data. Gastroenterol Hepatol (N Y). (2016) 12(8 Suppl. 3):1–11.
Google Scholar
10. Saha L. Irritable bowel syndrome: pathogenesis, diagnosis, treatment, and evidence-based medicine. World J Gastroenterol. (2014) 20:6759–73. doi: 10.3748/wjg.v20.i22.6759
11. Thompson WG. The treatment of irritable bowel syndrome. Aliment Pharmacol Ther. (2002) 16:1395–406. doi: 10.1046/j.1365-2036.2002.01312.x
12. Devanarayana NM, Rajindrajith S. Irritable bowel syndrome in children: Current knowledge, challenges and opportunities. World J Gastroenterol. (2018) 24:2211–35. doi: 10.3748/wjg.v24.i21.2211
13. Maxion-Bergemann S, Thielecke F, Abel F, Bergemann R. Costs of irritable bowel syndrome in the UK and US. Pharmacoeconomics. (2006) 24:21–37. doi: 10.2165/00019053-200624010-00002
14. Kwan AC, Hu WH, Chan YK, Yeung YW, Lai TS, Yuen H. Prevalence of irritable bowel syndrome in Hong Kong. J Gastroenterol Hepatol. (2002) 17:1180–6. doi: 10.1046/j.1440-1746.2002.02871.x
15. Cash BD, Lacy BE, Rao T, Earnest DL. Rifaximin and eluxadoline - newly approved treatments for diarrhea-predominant irritable bowel syndrome: what is their role in clinical practice alongside alosetron? Expert Opin Pharmacother. (2016) 17:311–22. doi: 10.1517/14656566.2016.1118052
16. Chen C, Dubin R, Kim MC. Emerging trends and new developments in regenerative medicine: a scientometric update (2000 - 2014). Expert Opin Biol Ther. (2014) 14:1295–317. doi: 10.1517/14712598.2014.920813
17. Thomson Reuters. Using Bibliometrics: A Guide to Evaluating Research Performance with Citation Data. White Paper. Toronto, CA: Thomson Reuters (2008).
18. Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci U S A. (2004) 101(Suppl. 1):5303–10. doi: 10.1073/pnas.0307513100
19. Chen C. CiteSpace II: Detecting and Visualizing Emerging Trends and Transient Patterns in Scientific Literature. J Am Soc Inf Sci Technol (2006) 57:359–77. doi: 10.1002/asi.20317
CrossRef Full Text | Google Scholar
20. van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84:523–38. doi: 10.1007/s11192-009-0146-3
21. Boyack KW, Klavans R. Co-Citation Analysis, Bibliographic Coupling, and Direct Citation: Which Citation Approach Represents the Research Front Most Accurately? J Am Soc Inf Sci Technol (2010) 61:2389–404. doi: 10.1002/asi.21419
22. Tsay M-Y, Xu H, Wu C-W. Journal Co-Citation Analysis of Semiconductor Literature. Scientometrics (2003) 57:7–25. doi: 10.1023/a:1023667318934
23. Trujillo CM, Long TM. Document co-citation analysis to enhance transdisciplinary research. Sci Adv. (2018) 4:e1701130. doi: 10.1126/sciadv.1701130
24. Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, et al. Bowel disorders. Gastroenterology. (2016) 150:1393–407. doi: 10.1053/j.gastro.2016.02.031
25. Drossman DA. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology. (2016). 150:1262–79. doi: 10.1053/j.gastro.2016.02.032
26. Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. (2015) 313:949–58. doi: 10.1001/jama.2015.0954
27. Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. (2014) 146:67–75.e5. doi: 10.1053/j.gastro.2013.09.046
28. Ford AC, Moayyedi P, Lacy BE, Lembo AJ, Saito YA, Schiller LR, et al. Task Force on the Management of Functional Bowel Disorders. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. (2014) 109(Suppl. 1):S2–26. doi: 10.1038/ajg.2014.187
29. Spiller R, Major G. IBS and IBD - separate entities or on a spectrum? Nat Rev Gastroenterol Hepatol. (2016) 13:613–21. doi: 10.1038/nrgastro.2016.141
30. Ford AC, Lacy BE, Talley NJ. Irritable Bowel Syndrome. N Engl J Med. (2017) 376:2566–78. doi: 10.1056/NEJMra1607547
31. Tap J, Derrien M, Törnblom H, Brazeilles R, Cools-Portier S, Doré J, et al. Identification of an Intestinal Microbiota Signature Associated With Severity of Irritable Bowel Syndrome. Gastroenterology. (2017) 152:111–23.e8. doi: 10.1053/j.gastro.2016.09.049
32. Böhn L, Störsrud S, Liljebo T, Collin L, Lindfors P, Törnblom H, et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. (2015) 149:1399–407.e2. doi: 10.1053/j.gastro.2015.07.054
33. Pittayanon R, Lau JT, Yuan Y, Leontiadis GI, Tse F, Surette M, et al. Gut Microbiota in Patients With Irritable Bowel Syndrome-A Systematic Review. Gastroenterology. (2019) 157:97–108. doi: 10.1053/j.gastro.2019.03.049
34. Simrén M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S, et al. Rome Foundation Committee. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. (2013) 62:159–76. doi: 10.1136/gutjnl-2012-302167
35. Li H, An H, Wang Y, Huang J, Gao X. Evolutionary Features of Academic Articles Co-Keyword Network and Keywords Co-Occurrence Network: Based on Two-Mode Affiliation Network. Physica A: Stat Mechanics Its Appl (2016) 450:657–69. doi: 10.1016/j.physa.2016.01.017
36. Lee P-C, Su H-N. Investigating the Structure of Regional Innovation System Research Through Keyword Co-Occurrence and Social Network Analysis. Innovation (2010) 12:26–40. doi: 10.5172/impp.12.1.26
37. Philipson L. Medical research activities, funding, and creativity in Europe: comparison with research in the United States. JAMA. (2005) 294:1394–8. doi: 10.1001/jama.294.11.1394
38. Fontanarosa PB, DeAngelis CD, Hunt N. Medical research–state of the science. JAMA. (2005) 294:1424–5. doi: 10.1001/jama.294.11.1424
39. Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol Stress. (2017) 7:124–36. doi: 10.1016/j.ynstr.2017.03.001
40. Wang HX, Wang YP. Gut Microbiota-brain Axis. Chin Med J (Engl). (2016) 129:2373–80. doi: 10.4103/0366-6999.190667
41. American College of Gastroenterology Task Force on Irritable Bowel Syndrome, Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR, Schoenfeld PS, et al. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. (2009) 104(Suppl. 1):S1–35. doi: 10.1038/ajg.2008.122
42. Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, et al. TARGET Study Group. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. (2011) 364:22–32. doi: 10.1056/NEJMoa1004409
43. Garsed K, Chernova J, Hastings M, Lam C, Marciani L, Singh G, et al. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut. (2014) 63:1617–25. doi: 10.1136/gutjnl-2013-305989
44. Saito YA, Almazar AE, Tilkes KE, Choung RS, Van Norstrand MD, Schleck CD, et al. Randomised clinical trial: pregabalin vs placebo for irritable bowel syndrome. Aliment Pharmacol Ther. (2019) 49:389–97. doi: 10.1111/apt.15077
45. Mangel AW, Bornstein JD, Hamm LR, Buda J, Wang J, Irish W, et al. Clinical trial: asimadoline in the treatment of patients with irritable bowel syndrome. Aliment Pharmacol Ther. (2008) 28:239–49. doi: 10.1111/j.1365-2036.2008.03730.x
46. Chey WD, Lembo AJ, Lavins BJ, Shiff SJ, Kurtz CB, Currie MG, et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. (2012) 107:1702–12. doi: 10.1038/ajg.2012.254
47. Didari T, Mozaffari S, Nikfar S, Abdollahi M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J Gastroenterol. (2015) 21:3072–84. doi: 10.3748/wjg.v21.i10.3072
48. Jalanka-Tuovinen J, Salojärvi J, Salonen A, Immonen O, Garsed K, Kelly FM, et al. Faecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut. (2014) 63:1737–45. doi: 10.1136/gutjnl-2013-305994
49. Wadhwa A, Al Nahhas MF, Dierkhising RA, Patel R, Kashyap P, Pardi DS, et al. High risk of post-infectious irritable bowel syndrome in patients with Clostridium difficile infection. Aliment Pharmacol Ther. (2016) 44:576–82. doi: 10.1111/apt.13737
50. Oka P, Parr H, Barberio B, Black CJ, Savarino EV, Ford AC. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:908-917. doi: 10.1016/S2468-1253(20)30217-X
CrossRef Full Text Epub 2020 Jul 20. Erratum Lancet Gastroenterol Hepatol. (2020) 5:e8. | Google Scholar
51. Rodiño-Janeiro BK, Vicario M, Alonso-Cotoner C, Pascua-García R, Santos JA. Review of Microbiota and Irritable Bowel Syndrome: Future in Therapies. Adv Ther. (2018) 35:289–310. doi: 10.1007/s12325-018-0673-5
52. Muir JG, Gibson PR. The Low FODMAP Diet for Treatment of Irritable Bowel Syndrome and Other Gastrointestinal Disorders. Gastroenterol Hepatol (N Y). (2013) 9:450–2.
53. Murray K, Wilkinson-Smith V, Hoad C, Costigan C, Cox E, Lam C, et al. Differential effects of FODMAPs (fermentable oligo-, di-, mono-saccharides and polyols) on small and large intestinal contents in healthy subjects shown by MRI. Am J Gastroenterol. (2014) 109:110–9. doi: 10.1038/ajg.2013.386
54. Vanhoutvin SA, Troost FJ, Kilkens TO, Lindsey PJ, Hamer HM, Jonkers DM, et al. The effects of butyrate enemas on visceral perception in healthy volunteers. Neurogastroenterol Motil. (2009) 21:952–e76. doi: 10.1111/j.1365-2982.2009.01324.x
55. Hill P, Muir JG, Gibson PR. Controversies and Recent Developments of the Low-FODMAP Diet. Gastroenterol Hepatol (N Y). (2017) 13:36–45.
56. Gibson PR. History of the low FODMAP diet. J Gastroenterol Hepatol. (2017) 32(Suppl. 1):5–7. doi: 10.1111/jgh.13685
57. Staudacher HM, Whelan K, Irving PM, Lomer MC. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J Hum Nutr Diet. (2011) 24:487–95. doi: 10.1111/j.1365-277X.2011.01162.x
58. Chumpitazi BP, Cope JL, Hollister EB, Tsai CM, McMeans AR, Luna RA, et al. Randomised clinical trial: gut microbiome biomarkers are associated with clinical response to a low FODMAP diet in children with the irritable bowel syndrome. Aliment Pharmacol Ther. (2015) 42:418–27. doi: 10.1111/apt.13286
59. Staudacher HM, Whelan K. The low FODMAP diet: recent advances in understanding its mechanisms and efficacy in IBS. Gut. (2017) 66:1517–27. doi: 10.1136/gutjnl-2017-313750
60. Staudacher HM, Lomer MC, Anderson JL, Barrett JS, Muir JG, Irving PM, et al. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J Nutr. (2012) 142:1510–8. doi: 10.3945/jn.112.159285
61. Staudacher HM, Whelan K. Altered gastrointestinal microbiota in irritable bowel syndrome and its modification by diet: probiotics, prebiotics and the low FODMAP diet. Proc Nutr Soc. (2016) 75:306–18. doi: 10.1017/S0029665116000021
62. Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Gibson PR, Muir JG. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut. (2015) 64:93–100. doi: 10.1136/gutjnl-2014-307264
63. Bennet SMP, Böhn L, Störsrud S, Liljebo T, Collin L, Lindfors P, et al. Multivariate modelling of faecal bacterial profiles of patients with IBS predicts responsiveness to a diet low in FODMAPs. Gut. (2018) 67:872–81. doi: 10.1136/gutjnl-2016-313128
64. Cao Y, Shen J, Ran ZH. Association between Faecalibacterium prausnitzii Reduction and Inflammatory Bowel Disease: A Meta-Analysis and Systematic Review of the Literature. Gastroenterol Res Pract. (2014) 2014:872725. doi: 10.1155/2014/872725
65. McIntosh K, Reed DE, Schneider T, Dang F, Keshteli AH, De Palma G, et al. FODMAPs alter symptoms and the metabolome of patients with IBS: a randomised controlled trial. Gut. 2017 Jul;66(7):1241-1251. doi: 10.1136/gutjnl-2015-311339
PubMed Abstract | CrossRef Full Text Epub 2016 Mar 14. Erratum Gut. (2019) 68:1342. | Google Scholar
66. Valeur J, Småstuen MC, Knudsen T, Lied GA, Røseth AG. Exploring Gut Microbiota Composition as an Indicator of Clinical Response to Dietary FODMAP Restriction in Patients with Irritable Bowel Syndrome. Dig Dis Sci. (2018) 63:429–36. doi: 10.1007/s10620-017-4893-3
67. Rossi M, Aggio R, Staudacher HM, Lomer MC, Lindsay JO, Irving P, et al. Volatile Organic Compounds in Feces Associate With Response to Dietary Intervention in Patients With Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. (2018) 16:385–91.e1. doi: 10.1016/j.cgh.2017.09.055
68. Sandström O, el-Salhy M. Human rectal endocrine cells and aging. Mech Ageing Dev. (1999) 108:219–26. doi: 10.1016/s0047-6374(99)00015-9
69. Kyösola K, Penttilä O, Salaspuro M. Rectal mucosal adrenergic innervation and enterochromaffin cells in ulcerative colitis and irritable colon. Scand J Gastroenterol. (1977) 12:363–7. doi: 10.3109/00365527709180942
70. Verity MA, Mellinkoff SM, Frankland M, Greipel M. Serotonin content and argentaffin and Paneth cell changes in ulcerative colitis. Gastroenterology. (1962) 43:24–31.
71. Gunawardene AR, Corfe BM, Staton CA. Classification and functions of enteroendocrine cells of the lower gastrointestinal tract. Int J Exp Pathol. (2011) 92:219–31. doi: 10.1111/j.1365-2613.2011.00767.x
72. Rindi G, Leiter AB, Kopin AS, Bordi C, Solcia E. The “normal” endocrine cell of the gut: changing concepts and new evidences. Ann N Y Acad Sci. (2004) 1014:1–12. doi: 10.1196/annals.1294.001
73. El-Salhy M, Seim I, Chopin L, Gundersen D, Hatlebakk JG, Hausken T. Irritable bowel syndrome: the role of gut neuroendocrine peptides. Front Biosci (Elite Ed). (2012) 4:2783–800. doi: 10.2741/e583
74. El-Salhy M, Gundersen D, Gilja OH, Hatlebakk JG, Hausken T. Is irritable bowel syndrome an organic disorder? World J Gastroenterol. (2014) 20:384–400. doi: 10.3748/wjg.v20.i2.384
75. El-Salhy M, Hausken T, Gilja OH, Hatlebakk JG. The possible role of gastrointestinal endocrine cells in the pathophysiology of irritable bowel syndrome. Expert Rev Gastroenterol Hepatol. (2017) 11:139–48. doi: 10.1080/17474124.2017.1269601
76. Jenny M, Uhl C, Roche C, Duluc I, Guillermin V, Guillemot F, et al. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. (2002) 21:6338–47. doi: 10.1093/emboj/cdf649
77. El-Salhy M. Possible role of intestinal stem cells in the pathophysiology of irritable bowel syndrome. World J Gastroenterol. (2020) 26:1427–38. doi: 10.3748/wjg.v26.i13.1427
78. El-Salhy M, Hausken T, Hatlebakk JG. Density of Musashi-1-positive stem cells in the stomach of patients with irritable bowel syndrome. Mol Med Rep. (2020) 22:3135–40. doi: 10.3892/mmr.2020.11412
79. Mazzawi T, Gundersen D, Hausken T, El-Salhy M. Increased gastric chromogranin A cell density following changes to diets of patients with irritable bowel syndrome. Mol Med Rep. (2014) 10:2322–6. doi: 10.3892/mmr.2014.2498
80. Mazzawi T, Gundersen D, Hausken T, El-Salhy M. Increased chromogranin a cell density in the large intestine of patients with irritable bowel syndrome after receiving dietary guidance. Gastroenterol Res Pract. (2015) 2015:823897. doi: 10.1155/2015/823897
81. Mazzawi T, Hausken T, Gundersen D, El-Salhy M. Effect of dietary management on the gastric endocrine cells in patients with irritable bowel syndrome. Eur J Clin Nutr. (2015) 69:519–24. doi: 10.1038/ejcn.2014.151
82. Mazzawi T, Hausken T, Gundersen D, El-Salhy M. Dietary guidance normalizes large intestinal endocrine cell densities in patients with irritable bowel syndrome. Eur J Clin Nutr. (2016) 70:175–81. doi: 10.1038/ejcn.2015.191
83. Mazzawi T, Lied GA, Sangnes DA, El-Salhy M, Hov JR, Gilja OH, et al. The kinetics of gut microbial community composition in patients with irritable bowel syndrome following fecal microbiota transplantation. PLoS One. (2018) 13:e0194904. doi: 10.1371/journal.pone.0194904
84. Mazzawi T, Hausken T, El-Salhy M. Changes in colonic enteroendocrine cells of patients with irritable bowel syndrome following fecal microbiota transplantation. Scand J Gastroenterol. (2022) 1–5. doi: 10.1080/00365521.2022.2036809 [Epub ahead of print].
85. Mazzawi T, El-Salhy M, Lied GA, Hausken T. The Effects of Fecal Microbiota Transplantation on the Symptoms and the Duodenal Neurogenin 3, Musashi 1, and Enteroendocrine Cells in Patients With Diarrhea-Predominant Irritable Bowel Syndrome. Front Cell Infect Microbiol. (2021) 11:524851. doi: 10.3389/fcimb.2021.524851
86. El-Salhy M, Gundersen D, Hatlebakk JG, Gilja OH, Hausken T. Abnormal rectal endocrine cells in patients with irritable bowel syndrome. Regul Pept. (2014) 188:60–5. doi: 10.1016/j.regpep.2013.11.005
87. El-Salhy M, Gundersen D, Ostgaard H, Lomholt-Beck B, Hatlebakk JG, Hausken T. Low densities of serotonin and peptide YY cells in the colon of patients with irritable bowel syndrome. Dig Dis Sci. (2012) 57:873–8. doi: 10.1007/s10620-011-1948-8
88. Vona-Davis LC, McFadden DW. NPY family of hormones: clinical relevance and potential use in gastrointestinal disease. Curr Top Med Chem. (2007) 7:1710–20. doi: 10.2174/156802607782340966
89. Kojima S, Tohei A, Ikeda M, Anzai N. An Endogenous Tachykinergic NK2/NK3 Receptor Cascade System Controlling the Release of Serotonin from Colonic Mucosa. Curr Neuropharmacol. (2015) 13:830–5. doi: 10.2174/1570159x13666150825220524
90. Zhou J, Martin RJ, Tulley RT, Raggio AM, McCutcheon KL, Shen L, et al. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am J Physiol Endocrinol Metab. (2008) 295:E1160–6. doi: 10.1152/ajpendo.90637.2008
91. Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. (2012) 489:231–41. doi: 10.1038/nature11551
92. Hanning N, Edwinson AL, Ceuleers H, Peters SA, De Man JG, Hassett LC, et al. Intestinal barrier dysfunction in irritable bowel syndrome: a systematic review. Therap Adv Gastroenterol. (2021) 14:1756284821993586. doi: 10.1177/1756284821993586
93. Berkes J, Viswanathan VK, Savkovic SD, Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut. (2003) 52:439–51. doi: 10.1136/gut.52.3.439
94. Hayes CL, Dong J, Galipeau HJ, Jury J, McCarville J, Huang X, et al. Commensal microbiota induces colonic barrier structure and functions that contribute to homeostasis. Sci Rep. (2018) 8:14184. doi: 10.1038/s41598-018-32366-6
95. De Palma G, Lynch MD, Lu J, Dang VT, Deng Y, Jury J, et al. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci Transl Med. (2017) 9:eaaf6397. doi: 10.1126/scitranslmed.aaf6397
96. Barbaro MR, Fuschi D, Cremon C, Carapelle M, Dino P, Marcellini MM, et al. Escherichia coli Nissle 1917 restores epithelial permeability alterations induced by irritable bowel syndrome mediators. Neurogastroenterol Motil. (2018) 30:e13388. doi: 10.1111/nmo.13388
97. Edogawa S, Edwinson AL, Peters SA, Chikkamenahalli LL, Sundt W, Graves S, et al. Serine proteases as luminal mediators of intestinal barrier dysfunction and symptom severity in IBS. Gut. (2020) 69:62–73. doi: 10.1136/gutjnl-2018-317416
98. Maharshak N, Huh EY, Paiboonrungruang C, Shanahan M, Thurlow L, Herzog J, et al. Enterococcus faecalis Gelatinase Mediates Intestinal Permeability via Protease-Activated Receptor 2. Infect Immun. (2015) 83:2762–70. doi: 10.1128/IAI.00425-15
99. He X, Cui LH, Wang XH, Yan ZH, Li C, Gong SD, et al. Modulation of inflammation by toll-like receptor 4/nuclear factor-kappa B in diarrhea-predominant irritable bowel syndrome. Oncotarget . (2017) 8:113957–65. doi: 10.18632/oncotarget.23045
100. Gribar SC, Anand RJ, Sodhi CP, Hackam DJ. The role of epithelial Toll-like receptor signaling in the pathogenesis of intestinal inflammation. J Leukoc Biol. (2008) 83:493–8. doi: 10.1189/jlb.0607358
101. Ghosh S, Whitley CS, Haribabu B, Jala VR. Regulation of Intestinal Barrier Function by Microbial Metabolites. Cell Mol Gastroenterol Hepatol. (2021) 11:1463–82. doi: 10.1016/j.jcmgh.2021.02.007
102. Wang W, Xia T, Yu X. Wogonin suppresses inflammatory response and maintains intestinal barrier function via TLR4-MyD88-TAK1-mediated NF-κB pathway in vitro . Inflamm Res. (2015) 64:423–31. doi: 10.1007/s00011-015-0822-0
103. Huang H, Zhao P, Xi M, Li F, Ji L. Mechanism of QingHuaZhiXie Prescription Regulating TLR4-IECs Pathway in the Intervention of Diarrhea Predominant Irritable Bowel Syndrome. Evid Based Complement Alternat Med. (2021) 2021:5792130. doi: 10.1155/2021/5792130
104. Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. (2009) 139:1619–25. doi: 10.3945/jn.109.104638
105. Wang HB, Wang PY, Wang X, Wan YL, Liu YC. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig Dis Sci. (2012) 57:3126–35. doi: 10.1007/s10620-012-2259-4
106. Yu M, Wang Q, Ma Y, Li L, Yu K, Zhang Z, et al. Aryl Hydrocarbon Receptor Activation Modulates Intestinal Epithelial Barrier Function by Maintaining Tight Junction Integrity. Int J Biol Sci. (2018) 14:69–77. doi: 10.7150/ijbs.22259
107. Casén C, Vebø HC, Sekelja M, Hegge FT, Karlsson MK, Ciemniejewska E, et al. Deviations in human gut microbiota: a novel diagnostic test for determining dysbiosis in patients with IBS or IBD. Aliment Pharmacol Ther. (2015) 42:71–83. doi: 10.1111/apt.13236
108. Martin CR, Osadchiy V, Kalani A, Mayer EA. The Brain-Gut-Microbiome Axis. Cell Mol Gastroenterol Hepatol. (2018) 6:133–48. doi: 10.1016/j.jcmgh.2018.04.003
109. Mayer EA, Labus J, Aziz Q, Tracey I, Kilpatrick L, Elsenbruch S, et al. Role of brain imaging in disorders of brain-gut interaction: a Rome Working Team Report. Gut. (2019) 68:1701–15. doi: 10.1136/gutjnl-2019-318308
110. Kano M, Grinsvall C, Ran Q, Dupont P, Morishita J, Muratsubaki T, et al. Resting state functional connectivity of the pain matrix and default mode network in irritable bowel syndrome: a graph theoretical analysis. Sci Rep. (2020) 10:11015. doi: 10.1038/s41598-020-67048-9
111. Labus JS, Hollister EB, Jacobs J, Kirbach K, Oezguen N, Gupta A, et al. Differences in gut microbial composition correlate with regional brain volumes in irritable bowel syndrome. Microbiome. (2017) 5:49. doi: 10.1186/s40168-017-0260-z
112. Labus JS, Osadchiy V, Hsiao EY, Tap J, Derrien M, Gupta A, et al. Evidence for an association of gut microbial Clostridia with brain functional connectivity and gastrointestinal sensorimotor function in patients with irritable bowel syndrome, based on tripartite network analysis. Microbiome. (2019) 7:45. doi: 10.1186/s40168-019-0656-z
113. Osadchiy V, Mayer EA, Gao K, Labus JS, Naliboff B, Tillisch K, et al. Analysis of brain networks and fecal metabolites reveals brain-gut alterations in premenopausal females with irritable bowel syndrome. Transl Psychiatry. (2020) 10:367. doi: 10.1038/s41398-020-01071-2
114. Mawe GM, Hoffman JM. Serotonin signalling in the gut–functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013 Aug;10(8):473-86. doi: 10.1038/nrgastro.2013.105
PubMed Abstract | CrossRef Full Text Epub 2013 Jun 25. Erratum Nat Rev Gastroenterol Hepatol. (2013) 10:564. | Google Scholar
115. Jacobs JP, Gupta A, Bhatt RR, Brawer J, Gao K, Tillisch K, et al. Cognitive behavioral therapy for irritable bowel syndrome induces bidirectional alterations in the brain-gut-microbiome axis associated with gastrointestinal symptom improvement. Microbiome. (2021) 9:236. doi: 10.1186/s40168-021-01188-6
116. Berentsen B, Nagaraja BH, Teige EP, Lied GA, Lundervold AJ, Lundervold K, et al. Study protocol of the Bergen brain-gut-microbiota-axis study: A prospective case-report characterization and dietary intervention study to evaluate the effects of microbiota alterations on cognition and anatomical and functional brain connectivity in patients with irritable bowel syndrome. Medicine (Baltimore). (2020) 99:e21950. doi: 10.1097/MD.0000000000021950
117. Goldenberg SD, Merrick B. The role of faecal microbiota transplantation: looking beyond Clostridioides difficile infection. Ther Adv Infect Dis. (2021) 8:2049936120981526. doi: 10.1177/2049936120981526
118. Ianiro G, Eusebi LH, Black CJ, Gasbarrini A, Cammarota G, Ford AC. Systematic review with meta-analysis: efficacy of faecal microbiota transplantation for the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. (2019) 50:240–8. doi: 10.1111/apt.15330
119. Johnsen PH, Hilpüsch F, Cavanagh JP, Leikanger IS, Kolstad C, Valle PC, et al. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol Hepatol. (2018) 3:17–24. doi: 10.1016/S2468-1253(17)30338-2
120. Xu D, Chen VL, Steiner CA, Berinstein JA, Eswaran S, Waljee AK, et al. Efficacy of Fecal Microbiota Transplantation in Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Am J Gastroenterol. (2019) 114:1043–50. doi: 10.14309/ajg.0000000000000198
121. El-Salhy M, Hatlebakk JG, Gilja OH, Bråthen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut. (2020) 69:859–67. doi: 10.1136/gutjnl-2019-319630
122. Holvoet T, Joossens M, Vázquez-Castellanos JF, Christiaens E, Heyerick L, Boelens J, et al. Fecal Microbiota Transplantation Reduces Symptoms in Some Patients With Irritable Bowel Syndrome With Predominant Abdominal Bloating: Short- and Long-term Results From a Placebo-Controlled Randomized Trial. Gastroenterology. (2021) 160:145–57.e8.
123. Wilson BC, Vatanen T, Cutfield WS, O’Sullivan JM. The Super-Donor Phenomenon in Fecal Microbiota Transplantation. Front Cell Infect Microbiol. (2019) 9:2. doi: 10.3389/fcimb.2019.00002
124. Halkjær SI, Christensen AH, Lo BZS, Browne PD, Günther S, Hansen LH, et al. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: results from a randomised, double-blind placebo-controlled study. Gut. (2018) 67:2107–15. doi: 10.1136/gutjnl-2018-316434
125. Midenfjord I, Polster A, Sjövall H, Törnblom H, Simrén M. Anxiety and depression in irritable bowel syndrome: Exploring the interaction with other symptoms and pathophysiology using multivariate analyses. Neurogastroenterol Motil. (2019) 31:e13619. doi: 10.1111/nmo.13619
126. Stasi C, Rosselli M, Bellini M, Laffi G, Milani S. Altered neuro-endocrine-immune pathways in the irritable bowel syndrome: the top-down and the bottom-up model. J Gastroenterol. (2012) 47:1177–85. doi: 10.1007/s00535-012-0627-7
127. Koloski NA, Jones M, Talley NJ. Evidence that independent gut-to-brain and brain-to-gut pathways operate in the irritable bowel syndrome and functional dyspepsia: a 1-year population-based prospective study. Aliment Pharmacol Ther. (2016) 44:592–600. doi: 10.1111/apt.13738
128. Maes M, Meltzer HY, Bosmans E, Bergmans R, Vandoolaeghe E, Ranjan R, et al. Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. J Affect Disord. (1995) 34:301–9. doi: 10.1016/0165-0327(95)00028-l
129. Vogelzangs N, Beekman AT, de Jonge P, Penninx BW. Anxiety disorders and inflammation in a large adult cohort. Transl Psychiatry. (2013) 3:e249. doi: 10.1038/tp.2013.27
130. Burns G, Carroll G, Mathe A, Horvat J, Foster P, Walker MM, et al. Evidence for Local and Systemic Immune Activation in Functional Dyspepsia and the Irritable Bowel Syndrome: A Systematic Review. Am J Gastroenterol. (2019) 114:429–36. doi: 10.1038/s41395-018-0377-0
131. Kurokawa S, Kishimoto T, Mizuno S, Masaoka T, Naganuma M, Liang KC, et al. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with irritable bowel syndrome, functional diarrhea and functional constipation: An open-label observational study. J Affect Disord. (2018) 235:506–12. doi: 10.1016/j.jad.2018.04.038
132. Liu Y, Zhang L, Wang X, Wang Z, Zhang J, Jiang R, et al. Similar Fecal Microbiota Signatures in Patients With Diarrhea-Predominant Irritable Bowel Syndrome and Patients With Depression. Clin Gastroenterol Hepatol. (2016) 14:1602–11.e5. doi: 10.1016/j.cgh.2016.05.033
133. Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The Microbiota-Gut-Brain Axis. Physiol Rev. (2019) 99:1877–2013. doi: 10.1152/physrev.00018.2018
134. Tannock GW, Savage DC. Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect Immun. (1974) 9:591–8. doi: 10.1128/iai.9.3.591-598.1974
Keywords : irritable bowel syndrome, VOSviewer, CiteSpace, bibliometrics, hot topics, trends
Citation: Zhang T, Ma X, Tian W, Zhang J, Wei Y, Zhang B, Wang F and Tang X (2022) Global Research Trends in Irritable Bowel Syndrome: A Bibliometric and Visualized Study. Front. Med. 9:922063. doi: 10.3389/fmed.2022.922063
Received: 17 April 2022; Accepted: 09 June 2022; Published: 27 June 2022.
Reviewed by:
Copyright © 2022 Zhang, Ma, Tian, Zhang, Wei, Zhang, Wang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beihua Zhang, [email protected] ; Fengyun Wang, [email protected] ; Xudong Tang, [email protected]
† These authors have contributed equally to this work and share first authorship
This article is part of the Research Topic
Global Excellence in Gastroenterology: Europe
Digestive Diseases
- Current and future treatments for irritable bowel syndrome associated with diarrhea
Sept. 05, 2015

Irritable bowel syndrome (IBS) is a multifactorial disorder marked by recurrent abdominal pain or discomfort and altered bowel function. It affects between 10 and 20 percent of people in the developed world, about one-third of whom have IBS associated with diarrhea (IBS-D).
Certain factors that alter gastrointestinal function can contribute to IBS symptoms, including stress, prior gastroenteritis, changes in the gut microbiome, and bile acids and short-chain fatty acids, which may stimulate serotonin (5-HT) release and increase colonic permeability and motility.
Still, the underlying cause of IBS in many cases remains unknown. Michael Camilleri, M.D. , of Mayo Clinic in Rochester, Minn., says the ultimate goal "is a better understanding of the mechanisms behind this syndrome so we can foster individualized, specific treatment for IBS patients." So far, that goal remains unrealized.
The only drug currently approved for IBS-D is alosetron, a 5-HT3 antagonist that may relieve abdominal pain and slow colonic and small bowel transit. Alosetron was withdrawn from the market for safety reasons in 2000 and was reintroduced in 2002 with a more restricted indication. Today, incidence rates of adverse events, including ischemic colitis and complications of constipation, are similar to those before the drug was withdrawn.
Non-IBS medications for IBS-D
Given the limited number of drugs marketed specifically for IBS-D, other medications are often used to treat symptoms. They include:
This synthetic mu-opioid agonist decreases intestinal transit while increasing intestinal water and ion absorption. In a small, placebo-controlled study, loperamide improved pain, stool consistency, urgency and overall subjective response, but it must be carefully titrated for individual patients to avoid constipation.
Bile acid binders
Roughly 30 percent of people with IBS-D have diagnosed bile acid malabsorption, and for this subset of patients, bile acid sequestration may relieve the cholerrheic effect of bile acids. Some evidence suggests that certain genetic variants may influence response to the bile sequestrant colesevelam, a medication that may be preferable to cholestyramine.
Antidepressants
Tricyclic agents such as amitriptyline and imipramine were initially prescribed to IBS patients with significant depression. Today, they are frequently used to treat patients with severe or refractory IBS symptoms and may have analgesic and neuromodulatory benefits in addition to their psychotropic effects. In one trial, nearly 70 percent of patients receiving 10 mg of amitriptyline experienced a complete loss of IBS symptoms compared with 28 percent of those on placebo.
Of increasing interest in many gastrointestinal disorders, single or combination probiotics have been investigated for IBS-D in several small trials. In these studies, bloating and distension improved but not diarrhea.
Mast cell stabilizers and 5-aminosalicylic acid (5-ASA)
Gastroenteritis precedes IBS-D in about 25 percent of people. Two anti-inflammatory agents have been used for this subset of patients: mast cell stabilizers such as disodium cromoglycate and ketotifen, and 5-ASA, which has shown mixed results for IBS-D in four small trials.
New drugs for IBS-D
Currently under development or in clinical trials, these drugs are more likely than others to play a role in the future management of IBS-D.
Serotonin synthesis inhibitors
LX-1031 is a tryptophan hydroxylase inhibitor that reduces local 5-HT synthesis and 5-hydroxyindoleacetic acid (5-HIAA) excretion. Unlike previous 5-HT inhibitors, LX-1031 does not cross the blood-brain barrier, thereby reducing the risk of depression and central nervous system disorders. A randomized, placebo-controlled phase II clinical trial in 155 patients showed reductions in urinary 5-HIAA and blood 5-HT as well as improvements in pain and stool consistency.
In two placebo-controlled, parallel-group studies of 1,000 patients with IBS-D, this selective 5-HT3 antagonist increased self-reported global assessment of relief of IBS symptoms. Constipation occurred in roughly 5 percent of participants — less than the rate observed with alosetron.
Spherical carbon adsorbent
AST-120 is a preparation consisting of spherical carbon particles that adsorb bacterial toxins, inflammatory mediators and bile acid products and prevent them from entering systemic circulation. In a phase II randomized, controlled eight-week trial of AST-120 in 115 patients, improvements in pain and bloating were short-lived and there was no significant improvement in stool consistency.
Benzodiazepine receptor modulator
The benzodiazepine receptor modulator dextofisopam binds to benzodiazepine receptors in the brain, not the GI tract, without a sedating effect. In animal studies, it exhibited the potential to reduce colonic motility and visceral sensitivity in response to stress. Further studies are needed to determine the mechanism of action, safety and efficacy in humans.
Peripheral k-agonist
Asimadoline, a kappa-opioid agonist, is being evaluated in clinical trials. So far, it has shown a good safety profile and reduced pain, urgency and stool frequency in IBS-D patients.
In spite of ongoing studies, Dr. Camilleri says several challenges must be met in order to achieve therapeutic advances, including "significant advances in research to understand the pathophysiology and clinical phenotyping of diverse patients with IBS-D, interest and investment by the pharmaceutical companies to develop the next generation of compounds, and greater definition of study endpoints by regulatory agencies to identify a clear path for approval and marketing of those medications."
For more information
Camilleri M. Current and future pharmacological treatments for diarrhea-predominant irritable bowel syndrome . Expert Opinion on Pharmacotherapy. 2013;14:1151.
Receive Mayo Clinic news in your inbox.
Related content.

- Medical Professionals
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
- Share full article
Advertisement
Supported by
Personal Health
Solving the Mystery of I.B.S.
Experts are starting to untangle the biological underpinnings of this common yet perplexing disorder. What they’re finding could offer clues on how to treat it.

By Jane E. Brody
No one with debilitating symptoms likes to be told “it’s all in your head.” Yet, this is often what distressed patients with irritable bowel syndrome hear, implicitly or explicitly, when a medical work-up reveals no apparent explanation for their repeated bouts of abdominal pain, bloating, diarrhea or constipation.
In fact, irritable bowel syndrome, or I.B.S., is a real problem causing real symptoms, no matter how hard its sufferers may wish it gone. But unlike an infection or tumor, I.B.S. is what medicine calls a functional disorder: a condition with no identifiable cause. Patients have no visible signs of damage or disease in their digestive tracts. Rather, the prevailing theory holds that overly sensitive nerves in the patient’s gastrointestinal tract send distress signals to the brain that result in pain and malfunction.
However, as medical science progresses, experts are beginning to find physical explanations for disorders that previously had no known biological cause. For example, conditions like epilepsy, Alzheimer’s disease and migraine were once considered functional disorders, but are now known to have measurable physical or biochemical underpinnings.
And recent research has revealed at least one likely explanation for the symptoms of I.B.S.: an infection in the digestive tract that triggers a localized allergic reaction in the gut. As Dr. Marc E. Rothenberg wrote in The New England Journal of Medicine in June , “Patients with I.B.S. often report that their symptoms started at the time of a gastrointestinal infection.”
Dr. Rothenberg, who is the director of the division of allergy and immunology at Cincinnati Children’s Hospital Medical Center, explained in an interview that the infection can temporarily disrupt the layer of cells that normally lines the bowel. These cells form a barrier that prevents allergy-inducing proteins in foods from being absorbed. When that barrier is penetrated, people can become intolerant to foods that previously caused them no issue.
A study in mice published in the journal Nature in January showed how this might happen. After infecting the rodents’ guts with bacteria, researchers found that the microbes released toxins that initiated an allergic reaction in the intestines, sparking the immune system to create antibodies against specific dietary proteins. When those specific proteins were ingested from foods, an immune reaction caused the rodents’ stomach muscles to contract, mimicking the symptoms of I.B.S., including diarrhea and abdominal pain.
The researchers then showed that a similar immune response occurred in 12 patients with I.B.S. when common food allergens like gluten, wheat, soy or milk were injected into the rectum. Every patient with I.B.S. had a localized reaction to one or more of the allergens, but only two of eight people without I.B.S. reacted to any allergen. Unlike classic food allergies that can produce hives, swelling and other body-wide immune responses, the reaction to allergens in the study was detectable only in the colon.
In describing this intriguing research, Dr. Rothenberg noted that “a great deal remains to be elucidated.” But he added that this and other related research suggests that “common gastrointestinal ailments, such as I.B.S. and functional abdominal pain, may instead be food-induced allergic disorders.” Such findings, the researchers wrote in the January study, hint at “new possibilities for the treatment of irritable bowel syndrome and related abdominal pain disorders,” offering hope that people with I.B.S. may one day find lasting relief.
Such remedies would be a godsend for the 10 to 15 percent of adults in the United States with I.B.S. or other food sensitivities who experience gastrointestinal distress following a meal. Therapeutic possibilities include high doses of antihistamines to counter patients’ sensitivities, as well as targeted treatments that block allergic pathways, Dr. Rothenberg said. He added that there are now drugs in Phase 3 trials — the step before approval — that eliminate the immune cells, known as mast cells, that are responsible for initiating an allergic response in the gut.
How common is I.B.S.?
I.B.S. is the most frequently diagnosed gastrointestinal disorder. Although symptoms can vary from patient to patient, they commonly include cramping, abdominal pain, bloating, intestinal gas, and diarrhea or constipation, or both. The disorder affects more women than men and is most common in people under 50. The annual medical costs of the condition exceed $1 billion in the United States alone.
It’s a chronic condition that requires continual management strategies, like always knowing the location of the nearest bathroom or having to wear diapers when restroom access is limited. The emotional distress it can cause often results in depression and anxiety and may prompt others to think incorrectly that the bowel disorder is self-inflicted.
Can calming therapies help?
There is a known connection between the brain and the gut, and undue stress can certainly aggravate the symptoms of I.B.S. Cognitive behavioral therapy may benefit some patients, and many find it helpful to practice relaxation techniques like positive imagery, progressive muscle relaxation or meditation.
Yoga and other types of physical activity may also diminish symptoms of I.B.S. and improve patients’ quality of life. One clinical trial involving 102 patients found that those who engaged in vigorous physical activity three to five days a week experienced reduced physical and psychological symptoms.
Another soothing technique that can be done anywhere, anytime, to help relieve pain and stress is diaphragmatic breathing, the opposite of sucking in your gut. Instead of pushing out the chest as the lungs fill with air, the diaphragm is pushed down toward the stomach, causing the belly to rise. Practice by placing one hand above your navel to feel your abdomen rise as you inhale slowly through your nose, and then retract as you exhale through your mouth.
Which foods should be avoided if I have I.B.S.?
Patients can also minimize their symptoms by avoiding the foods or drinks that seem to trigger them. Common troublemakers include wheat and other gluten-containing foods, dairy products, citrus fruits, beans, cabbage and related gas-causing vegetables, and carbonated drinks. People may also react badly to spicy or fatty foods, coffee or alcohol.
Some patients find dramatic relief from adopting a strict FODMAP diet that eliminates all fermentable starches and sugars, then gradually adding back one food at a time to determine which ones cause symptoms and are best avoided. The FODMAP diet favorably alters the population of microbes that live in the intestines, reducing gas-producing bacteria that thrive on fermentable foods. (Details of the diet can be found at this website .)
Some evidence suggests that prebiotics or probiotics may be another therapeutic option to manipulate the bacteria that dwell in the intestinal tract, though the findings are limited. In a recent review in JAMA , Dr. Michael Camilleri, a gastroenterologist at the Mayo Clinic, reported that the probiotic Bifidobacterium longum reduced depression and improved quality of life for patients with I.B.S.
An earlier version of this article misstated the name of the journal where a review of I.B.S. appeared. It is JAMA, not JAMA Network.
How we handle corrections
Jane Brody is the Personal Health columnist, a position she has held since 1976. She has written more than a dozen books including the best sellers “Jane Brody’s Nutrition Book” and “Jane Brody’s Good Food Book.” More about Jane E. Brody

- Current IBS Studies: Help Advance Research
Here are ways that you can help advance research – in person or from the comfort of your home. Take part in these studies.
A Randomized, Double-Blind, Technology-Enabled Trial to Evaluate the Impact of a Multi-Strain Synbiotic (DS-01) on Metagenomic Stability and Metabolic Output of the Gut Microbiota.
Purpose of Study: This study aims to assess the impact of multi-strain consortia of 24 commensal organisms across 12 species with extensive strain-specific in vivo data, assessing a range of gastrointestinal symptoms without negatively altering the naive gut microbiota. High-throughput shotgun DNA sequencing will provide an opportunity for ‘-omics’-based analyses of the gut microbiota, which can be augmented by the metabolite profiles resulting from total microbial activity in the gut. Since many of these metabolites are bioeffector molecules acting upon the host, such analysis can provide a direct measure of the consequences of microbial activity in the gut and provide a novel integrated data set for patients with IBS. Recruited subjects will also use a smart-phone application to report day to day gastrointestinal symptoms, a patient-centric hallmark of this chronic gut condition.
Sponsor: Beth Israel Deaconess Medical Center
Condition : Irritable bowel Syndrome-Constipation (IBS-C) and Irritable Bowel Syndrome-Mixed (IBS-M)
Participation:
- Patient must be willing and able to give informed assent/ consent for participation in the study (see Section 15.2).
- Patient must be willing and able (in the PI’s opinion) to comply with all study requirements.
- Patient must be a premenopausal female aged 18 and older.
- Patient must have a documented history of IBS that is not completely controlled by current IBS drugs.
- Patient must have a score of ≥150 on the IBS-SSS at screening.
- Patient must have no clinically relevant (in the judgment of the PI) abnormal blood laboratory levels at screening or randomization.
- The clinician will assess eligibility as per the Rome IV criteria (Recurrent abdominal pain or discomfort at least 1 day/week in the last 3 months associated with two or more of the following: Improvement with defecation. Onset associated with a change in frequency of stool).
Study Contact: For more information, call Vivian Cheng, MS, MPH at (617)-667-0682, or Email: [email protected]
Added April 2021
Nutrition counseling study for irritable bowel syndrome.
Purpose of study : This study involves free nutrition sessions with a skilled IBS-specializing dietitian.
Sponsor: UCLA Oppenheimer Family Center for Neurobiology of Stress
Participation : Eligible male and female individuals ages 18 and older with a diagnosis of IBS with diarrhea or diarrhea with constipation (IBS-D or IBS-M). To participate, individuals must not have tried the low FODMAP diet before.
Contact : For more information, visit www.uclacns.org/patients/clinical-research or call (310) 206-1656. You may also email Nafessa Islam at [email protected]
Verified May 2018
Irritable Bowel Syndrome and Control Volunteers: Diet Challenge
Purpose of study : The purpose of this study is to study the relationship between the bile acids, short chain fatty acids and bacteria within the intestines. The hypothesis is that changes in the bacterial composition of the stool are associated with the differences in bile acids and short chain fatty acids in patients having irritable bowel syndrome compared to healthy individuals.
Sponsor: Indiana University and NIDDK
Participation : Patients with IBS, ages 18-65 fulfilling Rome IV criteria and asymptomatic controls with no prior history of GI disease or symptoms.
Participants should be on a stable and consistent diet regimen and should not be following an extreme diet intervention such as gluten-free or a low FODMAP diet at the time of study participation.
Contact : For more information, visit clinicaltrials.gov/
Contact: Tonya Hamilton 317-278-9296 [email protected] Contact: Anita Gupta 317-948-9227 ext 3179489227 [email protected]
Added February 2020
Lactobacillus LB as Treatment for Irritable Bowel Syndrome With Predominance of Diarrhea (IBS-D)
Purpose of study : The combination of Lactobacillus fermentum and Lactobacillus delbrueckii (Lactobacillus LB) has proven to be effective and safe for treatment of acute diarrhea in children. Also, a clinical trial in adult patients with chronic diarrhea, showed a reduction in the number of daily stools. However, the evidence is not enough regarding the efficacy and safety of Lactobacillus LB for treatment of patients with irritable bowel syndrome with predominance of diarrhea (IBS-D).
Sponsor: Hospital General de Mexico
Participation : Patients meeting Rome IV criteria for IBS-D, without specific medical treatment for the last 4 weeks prior to inclusion in this study.
Contact : For more information, visit https://clinicaltrials.gov/ or email [email protected]
Purpose of study : The purpose of this study is to examine brain networks at rest in chronic pain conditions compared to healthy controls.
Participation : Men and women between the ages of 18 and 55 who are diagnosed with IBS, right handed, not pregnant and no significant neurological or psychological medical history.
About the study: Participation involves a screening visit, an MRI and one stool sample.
Participants will be compensated up to $100 and get a digital picture of your brain.
Contact : For more information, visit www.uclacns.org/patients/clinical-research or call (310) 206-8545. You can also email Nafeesa Islam at [email protected]
Added May 2018
A Longitudinal Study to Identify IBS Phenotypes Using Fecal Microbiota and Hydrogen Breath Testing
Purpose of study : To provide treatment to patients with IBS-D.
Sponsor: University of Michigan and Michigan Institute for Clinical and Health Research (MICHR)
Participation : Men and women over the age of 18 diagnosed with IBS-D. Prior colonoscopy or sigmoidoscopy within the past 2 years with random colon biopsies to exclude the presence of microscopic colitis.
About the study: Patients will receive either rifaximin or low FODMAP dietary intervention.
Contact : For more information, visit https://clinicaltrials.gov/ct2/ or contact Dr. Allen Lee at (734) 936-9454, [email protected]
Participants Sought for Study on Complementary Approaches to the Treatment of IBS
Purpose of study: The purpose of this study is to find a complementary treatment to help Irritable Bowel Syndrome (IBS) patients in need of relief.
About The Study: The length of this study will be three weeks long, with a short online intervention everyday to help individuals deal with their body’s reactions to their environments. Participants will have additional surveys to assess their overall state at the beginning of the study, at the end of the three week intervention, and again at six weeks for a follow-up.
Participation: Eligible individuals between the ages of 18 – 65 years of age who are experiencing pain or discomfort associated with their gut. Cannot be smokers or have an inflammatory bowel disease.
How to Sign Up: http://bit.ly/IBS_study
There will be a $15 Target gift card for first 30 participants upon completion of study.
Study Contact: Jenna N. Ray, Health Psychologist. Phone: (919) 257 – 7291, Email: [email protected]
Verified August 2017
Canadian IBS and IBD Patients Needed for IMAGINE (Inflammation, Microbiome, and Alimentation: Gastro-Intestinal and Neuropsychiatric Effects) Study
Purpose of study: The overall aim of IMAGINE Network is to understand the interactions between diet-microbiome-host and find new therapies for the treatment of IBS, IBD and associated psychiatric disorders.
Develop innovative therapies (changes in diet, probiotics, fecal transplants or antibiotics) to improve IBD, IBS and mental health Improve outcomes of existing therapies through the assessment of diet-microbiome-host interactions Develop strategies to optimize current therapies to target those who will most benefit from medication as well as identify those in whom medication can be safely discontinued with significant personal benefit and cost savings to the Canadian healthcare system
About The Study: Transform the management of IBD and IBS and associated mental health issues with these disorders.
Sponsor: The IMAGINE (Inflammation, Microbiome, and Alimentation: Gastro-Intestinal and Neuropsychiatric Effects) Chronic Disease Network involves 17 hospitals/universities and 75 researchers across Canada who will study the interactions between the inflammation, microbiome, diet and mental health in patients with inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS). The IMAGINE Network is one of five chronic disease networks in the SPOR (Strategy for Patient Oriented Research) initiative of CIHR (Canadian Institutes of Health Research).
Participation: Persons from across Canada are being invited to participate in the IMAGINE Network. You may be eligible to participate in this study if you have been diagnosed by your physician with Irritable Bowel Syndrome (IBS), Inflammatory Bowel Disease (IBD), including ulcerative colitis or Crohn’s disease or are a healthy individual without gastrointestinal symptoms.
You will not be eligible to participate if you have any of the following criteria:
Age under 4 years. Past gastrointestinal bypass surgery or major bowel resections unrelated to Crohn’s disease. Major concurrent illness such as chronic kidney disease, chronic liver disease other than primary sclerosing cholangitis, chronic immune disease unrelated to IBD. If you have an active eating disorder such anorexia nervosa or bulimia. If you cannot communicate in either French or English.
Due to the specific nature of this research, we require that participants have not had major gastrointestinal surgery (e.g. Roux en y, bowel resection), do not have additional disease(s) that might affect ability to participate (e.g. decompensated liver disease), prescription or non-prescription drug use that is known to cause gastrointestinal (GI) symptoms (e.g. chronic antibiotic use), fad diets or eating disorders that may cause GI symptoms.
Study Contact: Aida Fernandes, Executive Director [email protected] or visit http://imaginespor.com/participate-in-research/
Added May 2020
Share this page
Topics of this article.
- IBS Survey: Share Your Patient Experience (established 2020)
- IBS Patients: Their Illness Experience and Unmet Needs
IFFGD is a nonprofit education and research organization. Our mission is to inform, assist, and support people affected by gastrointestinal disorders.
Our original content is authored specifically for IFFGD readers, in response to your questions and concerns.
If you found this article helpful, please consider supporting IFFGD with a small tax-deductible donation.
Related Information
During Spring 2023, IFFGD worked with PhD and Public Health student Makenna Lenover to develop IBS Infographic visual content for the aboutibs.org website
Overlap of Fibromyalgia and IBS Fibromyalgia (FM) is a condition marked by muscle pain all over the body, sleep problems, and fatigue. FM is often
Any product taken for a therapeutic effect should be considered a drug. Use of medications for IBS, whether prescription, over-the-counter, herbs, or supplements should be
Personal Stories

This information is in no way intended to replace the guidance of your doctor. We advise seeing a physician whenever a health problem arises requiring an expert’s care.
© Copyright 2024 International Foundation for Gastrointestinal Disorders, Inc. (IFFGD). All Rights Reserved.
Our Other Sites
- About Constipation
- About Gastroparesis
- About GI Motility
- About Incontinence
- About Kids GI
- You and Constipation
- You and IBS
- Los Niños y GI
Stay Connected
Keep up-to-date on the latest news, stories, tips, research highlights, and more!
Connect on social media:

IFFGD gratefully acknowledges the following members of our Industry Council.
Patron level:.

Associate Level:

Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- Hosted content
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 73, Issue 6
- Association of healthy lifestyle behaviours with incident irritable bowel syndrome: a large population-based prospective cohort study
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0003-0525-2621 Fai Fai Ho 1 ,
- Hui Sun 2 ,
- Hong Zheng 3 ,
- David C N Wong 3 ,
- Yin-Yan Gao 2 ,
- http://orcid.org/0000-0002-6537-6215 Chen Mao 4 ,
- Yin Ting Cheung 5 ,
- Chun Sing Lam 5 ,
- Maggie H Wang 3 ,
- Irene Xin-Yin Wu 2 ,
- Justin C Y Wu 6 ,
- Vincent C H Chung 3
- 1 School of Chinese Medicine , The Chinese University of Hong Kong , Hong Kong , Hong Kong
- 2 Xiangya School of Public Health , Central South University , Changsha , Hunan , China
- 3 The Jockey Club School of Public Health and Primary Care , The Chinese University of Hong Kong , Hong Kong , Hong Kong
- 4 Department of Epidemiology , Southern Medical University School of Public Health , Guangzhou , Guangdong , China
- 5 School of Pharmacy , The Chinese University of Hong Kong , Hong Kong , Hong Kong
- 6 Department of Medicine and Therapeutics , The Chinese University of Hong Kong , Hong Kong , Hong Kong
- Correspondence to Professor Irene Xin-Yin Wu, Xiangya School of Public Health, Central South University, Changsha, Hunan, China; irenexywu{at}csu.edu.cn
Objectives To evaluate the association between healthy lifestyle behaviours and the incidence of irritable bowel syndrome (IBS).
Design Population-based prospective cohort study.
Setting The UK Biobank.
Participants 64 268 adults aged 37 to 73 years who had no IBS diagnosis at baseline were enrolled between 2006 and 2010 and followed up to 2022.
Main exposure The five healthy lifestyle behaviours studied were never smoking, optimal sleep, high level of vigorous physical activity, high dietary quality and moderate alcohol intake.
Main outcome measure The incidence of IBS.
Results During a mean follow-up of 12.6 years, 961 (1.5%) incident IBS cases were recorded. Among the 64 268 participants (mean age 55.9 years, 35 342 (55.0%) female, 7604 (11.8%) reported none of the five healthy lifestyle behaviours, 20 662 (32.1%) reported 1 behaviour, 21 901 (34.1%) reported 2 behaviours and 14 101 (21.9%) reported 3 to 5 behaviours at baseline. The multivariable adjusted hazard ratios associated with having 1, 2 and 3 to 5 behaviours for IBS incidence were 0.79 (95% confidence intervals 0.65 to 0.96), 0.64 (0.53 to 0.78) and 0.58 (0.46 to 0.72), respectively (P for trend <0.001). Never smoking (0.86, 0.76 to 0.98, P=0.02), high level of vigorous physical activity (0.83, 0.73 to 0.95, P=0.006) and optimal sleep (0.73, 0.60 to 0.88, P=0.001) demonstrated significant independent inverse associations with IBS incidence. No significant interactions were observed between these associations and age, sex, employment status, geographic location, gastrointestinal infection, endometriosis, family history of IBS or lifestyle behaviours.
Conclusions Adhering to a higher number of healthy lifestyle behaviours is significantly associated with a lower incidence of IBS in the general population. Our findings suggest the potential of lifestyle modifications as a primary prevention strategy for IBS.
- IRRITABLE BOWEL SYNDROME
Data availability statement
Data may be obtained from a third party and are not publicly available. The UK Biobank data are available on application at https://www.ukbiobank.ac.uk .
https://doi.org/10.1136/gutjnl-2023-331254
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
WHAT IS ALREADY KNOWN ON THIS TOPIC
Currently, there is no established primary prevention strategy for irritable bowel syndrome (IBS).
Previous studies showed that five modifiable lifestyle factors, including smoking, sleeping, physical activity, diet and alcohol consumption are associated with IBS. However, their combined association with the incidence of IBS is currently unknown.
Current guidelines do not recommend any lifestyle modification interventions as primary prevention measures for IBS.
WHAT THIS STUDY ADDS
Adhering to a higher number of the five healthy lifestyle behaviours is significantly associated with a lower IBS incidence in a middle-aged population.
Our findings suggest the potential of lifestyle modifications as a primary prevention strategy for IBS.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The individual or combined effect of the five healthy lifestyle behaviours on IBS incidence will guide further basic research on discovering potential underlying mechanisms.
Coordinated efforts and support at individual and community levels are required to promote the adoption of the five beneficial lifestyle behaviours among the general population for IBS prevention and for other health benefits.
Introduction
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder characterised by recurrent abdominal pain and disordered bowel habits with abnormal stool form or frequency. 1 IBS affects 5% to 10% of the population worldwide 2 and causes substantial annual direct and indirect expenditure related to disease management in various countries. 3–5
Although the pathophysiology of IBS is not fully understood, the dysfunction of the gut–brain axis, which causes intestinal motility disturbances, visceral hypersensitivity and altered processing in the central nervous system, is well recognised. 6 Current treatments for IBS only aim to improve troublesome symptoms of abdominal pain and bowel habits, but they are either of uncertain efficacy or have adverse events. 6 Patients with IBS often report experiencing comorbid psychiatric disorders, 7 heightened thoughts of suicide because of their symptoms 8 and lower quality of life. 9 Finding a primary prevention strategy for IBS is essential for reducing its disease burden.
In previous studies, some modifiable lifestyle factors, including smoking, 10 sleeping, 11 physical activity, 11 diet 12 and alcohol consumption, 13 14 were found to be independently associated with IBS. It can be hypothesised that the combination of these healthy lifestyle behaviours might also protect against the occurrence of IBS. We assessed the combined association of the above-mentioned five healthy lifestyle behaviours—never smoking, optimal sleep, high level of vigorous physical activity, high dietary quality and moderate alcohol intake—with the incidence of IBS in a large prospective cohort from the UK Biobank.

Study setting and participants
The UK Biobank study is a large-scale prospective cohort study that recruited 502 492 participants aged 37 to 73 years by sending invitation letters to their homes between 2006 and 2010. The participants completed a touchscreen questionnaire and a verbal interview on demographics, health and lifestyles as baseline assessments at one of 22 assessment centres throughout the UK. They also provided biological samples and received physical examinations. The participants were followed up to update their health status. The details of the UK Biobank study have been described elsewhere. 15 16
For the assessment of dietary habits, de Boer et al recommended administering 24-hour dietary recall questionnaires on at least two non-consecutive days, 17 and this approach has shown acceptable reproducibility. 18 Following this suggestion, we included only participants who completed at least two 24-hour dietary recall questionnaires (n=1 26 841, including alcohol intake assessment) from the whole cohort. Participants reporting unrealistic energy intake (outside the range of 800–5000 kcal/day for men and 500–4000 kcal/day for women, n=200), 19 participants who were missing data on any of the five lifestyle behaviours (physical activity, n=17 900; smoking, n=170; sleeping, n=266) or key covariates (employment status, n=131; family history of IBS, n=41 020) and participants with prevalent IBS at baseline (n=2886) were excluded to reduce the probability of inferential bias. Ultimately, 64 286 participants were included in the analysis ( figure 1 ).
- Download figure
- Open in new tab
- Download powerpoint
Flow chart of UK Biobank participants
Ascertainment of exposure
The five healthy lifestyle behaviours were never smoking, optimal sleep, high level of vigorous physical activity, high dietary quality and moderate alcohol intake. The participants were grouped based on how many of the five healthy lifestyle behaviours they had (0, 1, 2 or 3 to 5). The relatively small number of individuals who had 3 (n=11 079), 4 (n=2767) and 5 (n=255) healthy lifestyle behaviours were pooled together as a single group to increase the sample size for analysis. All the lifestyle behaviours were assessed using structured questionnaires on a self-report basis. Details can be found in online supplemental text S1 .
Supplemental material
Ascertainment of outcome.
The outcome of interest of the study was the incidence of IBS, which was defined as code K58 under the International Classification of Diseases, 10th revision (ICD-10) after baseline assessment. The UK Biobank has summarised the dates of the first occurrences of a range of health-related outcomes mapped to ICD-10 codes from various sources, including primary care data, hospital inpatient data, death register records and self-reported medical conditions ( online supplemental text S1 ), which are updated regularly. Therefore, the date when the diagnosis of IBS was first made could be identified.
Ascertainment of covariates
A number of covariates associated with healthy lifestyle behaviours and/or IBS were determined by reviews of relevant observational studies, systematic reviews and consensus reports ( online supplemental table S1 ). These covariates included sociodemographic factors (age, sex, body mass index (BMI), geographic location, marital status and employment status) and medical factors (depression, anxiety, back pain or joint pain, headaches, osteoporosis, asthma, gastrointestinal infection, endometriosis, ectopic pregnancy and family history of IBS). The sociodemographic covariates were assessed using a touchscreen questionnaire at baseline in assessment centres and family history of IBS was collected using a web-based digestive health questionnaire during the follow-up period. The baseline status of existing diseases was confirmed through primary care data, hospital inpatient data, death register records and self-reported medical conditions, with dates of their first occurrence recorded. Details of these assessments and data linkages are available on the UK Biobank website ( www.ukbiobank.ac.uk ).
Patient and public involvement
The analysis was based on data from the UK Biobank, which included extensive public consultation in its design. Patients were not involved in setting the research question or the outcome measures, nor were they involved in developing plans for the design or implementation of the study. No patients were asked to advise on the interpretation or writing up of the results.
Statistical analyses
Baseline characteristics were described across the number of healthy lifestyle behaviours. Continuous variables were presented as the mean (SD) and compared among groups using analysis of variance test or Welch test, as appropriate. Categorical variables were presented as the number (percentage) and compared among groups using Χ 2 test or Fisher’s exact test, as appropriate. At the time of analysis, the UK Biobank data were available up to 1 February 2022, so this date was considered the end of the study. Person years of follow-up were computed from the first interview at baseline until the occurrence of IBS, death, loss of follow-up or the end of the study, whichever occurred first.
Cox proportional hazard models were used to estimate the hazard ratios and 95% confidence intervals for the associations between healthy lifestyle behaviours and the incidence of IBS. To improve confounding control in the analysis process, observational studies often employ data-driven approaches to adjust seemingly related covariates, but inappropriate confounder selection and adjustment (eg, failing to adjust for a confounder or inappropriately adjusting a collider or mediator) might unwittingly introduce more bias and impair the validity of results. 20 To improve the confounder selection process, a graphical tool called directed acyclic graphs (DAGs) has been introduced. 21 DAGs have several advantages over traditional statistical approaches for appropriate confounder selection. First, DAGs provide a visual representation of causal relations between the exposure variable, the outcome variables and other variables that play a role in the causal question of concern. 22 In this way, DAGs enable researchers to identify different types of biases (eg, confounding, selection, overadjustment and detection bias) more efficiently and to consider them early in the study design and subsequent data analysis process. 22 Second, when confounding control is not adequate, DAGs can help researchers identify the bias and assess its direction in specific situations. 23 24 Third, DAGs provide a more comprehensive and transparent process to select confounders. Traditional statistical approaches may not fully capture the intricate interrelationships between variables, which could result in the omission of important confounders and the subsequent introduction of potential bias into the estimate of causal effects. 25 However, DAGs summarise prior knowledge on the complex inter-relationships between variables; they identify and adjust for confounders based on causal relationships rather than relying solely on statistical associations. 26 Each pair of causal relationships is systematically assessed and explicitly documented; supporting evidence is provided when needed, allowing for high transparency and reproducibility. 27
We decided to use the DAG method, given the benefits stated above. We constructed a DAG to summarise prior knowledge of the causal relationships between healthy behaviours, IBS and their covariates to guide potential confounder selection. 28 The construction of the DAG followed the evidence synthesis for constructing directed acyclic graphs protocol, which integrates evidence synthesis strategies and causal inference principles. 27 Briefly, a pool of covariates related to healthy lifestyle behaviours and/or IBS was determined by a series of systematic literature reviews on: (i) observational studies on the healthy lifestyle behaviours–IBS relationship; (ii) systematic reviews on IBS and (iii) consensus reports on IBS from several databases. Furthermore, the relationship between every pair of variables in the DAG was assessed by sequential causal criteria, including temporality, validity and theoretical support. More information about the building of DAGs can be found in online supplemental text S2 and Online supplemental figure S1 .’
The proposed DAG in online supplemental figure S2 was found to be consistent with the UK Biobank data and it indicated that adjustments should be made for the following covariates when estimating the association between healthy behaviours and the incidence of IBS: age (<40, 40–49, 50–59 or ≥60 years), sex (female or male), employment status (in paid employment or self-employed or not employed), geographic location (England area or non-England area, including Wales and Scotland), gastrointestinal infection (yes or no), endometriosis (yes or no) and family history of IBS (yes or no). The proportional hazards assumption of the models was tested using Schoenfeld residuals, and no significant deviation from the assumption was found.
Subgroup analyses by age, sex, employment status, geographic location, gastrointestinal infection and endometriosis were conducted to examine potential modifying effects. Interaction was assessed by including a product term of one of the stratifying variables with healthy lifestyle behaviours in the model.
We also performed several sensitivity analyses. First, we excluded participants whose IBS diagnosis was made solely based on self-report. Second, we applied a different definition for healthy alcohol drinking behaviours: instead of moderate alcohol drinking, only those who abstained from alcohol were considered to be healthy. Third, we used a looser definition for a healthy level of physical activity following the UK Chief Medical Officers’ Physical Activity Guidelines, 29 according to which performing moderate physical activity for 150 min or more per week or vigorous physical activity for 75 min or more per week is recommended. Participants who met either of these recommendations were considered to have a healthy level of physical activity. Fourth, as a sensitivity analysis, we repeated our analysis by including participants who completed at least one dietary questionnaire. Finally, to explore the individual impact of each of the five lifestyle behaviours, we conducted separate analyses for each while adjusting the models for the other behaviours.
All the analyses were performed using IBM SPSS Statistics software version 26.0 (IBM Corporation, Armonk, New York, USA). All the tests were two-sided, with P<0.05 considered statistically significant.
Baseline characteristics
Table 1 presents the baseline characteristics of the study participants across the number of healthy lifestyle behaviours they had. Among the 64 268 participants (mean age 55.9 years, 55.0% female), 7604 (11.8%) had none of the 5 studied healthy lifestyle behaviours, 20 662 (32.1%) had 1 behaviour, 21 901 (34.1%) had 2 behaviours and 14 101 (21.9%) had 3 to 5 behaviours. In comparison with participants who did not perform any of the five healthy lifestyle behaviours, those who adhered to 3 to 5 behaviours were more likely to be younger, female, have a lower BMI, married and in paid employment or self-employed and less likely to have a family history of IBS. In addition, they had a lower prevalence of depression, anxiety, back pain or joint pain, headaches, asthma and gastrointestinal infection. The two comparison groups had similar baseline prevalence of osteoporosis, endometriosis and ectopic pregnancy. Participants excluded from the analysis because of missing information had similar adherences to the five healthy lifestyle behaviours to those of the participants included, with similar age, BMI, percentage of female participants, geographic location (living in England), marital status (being married), IBS family history (yes) and prevalent diseases, although they were less likely to be in paid employment or self-employed ( online supplemental table S2 ).
- View inline
Baseline characteristics of the study participants by the number of healthy lifestyle behaviours*
Healthy lifestyle behaviours and IBS
During a mean follow-up of 12.6 years, 961 (1.5%) incident IBS cases were recorded. In table 2 , after adjusting for age (<40, 40–49, 50–59 or ≥60 years), sex (female or male), employment status (in paid employment or self-employed or not), geographic location (England or non-England area, including Wales and Scotland), gastrointestinal infection (yes or no), endometriosis (yes or no) and family history of IBS (yes or no), we found that adherence to a higher number of healthy lifestyle behaviours was significantly associated with a lower risk of incident IBS (all P<0.05). Compared with not performing any of the five healthy lifestyle behaviours, the adjusted hazard ratios associated with adhering to 1, 2 and 3 to 5 behaviours were 0.79 (0.65 to 0.96), 0.64 (0.53 to 0.78) and 0.58 (0.46 to 0.72), respectively (P for trend <0.001).
Associations between healthy lifestyle behaviours and the risk of incident irritable bowel syndrome (IBS)*
Subgroup, sensitivity and separate analyses
We conducted subgroup analyses by age, sex, employment status, geographic location, gastrointestinal infection, endometriosis and family history of IBS to identify potential effect modifiers, but no significant interactions were found (all P for interaction ≥0.05; table 3 ). In the sensitivity analysis excluding participants whose IBS diagnosis was made solely based on self-report (n=85), the estimates of the association were similar to those of the main analysis shown in Table 2 ( online supplemental table S3 ). However, in both the sensitivity analysis considering zero alcohol consumption as a healthy drinking behaviour ( online supplemental table S4 ) and the sensitivity analysis of relaxing the definition of a healthy level of physical activity for those who met the recommendation of moderate or vigorous physical activity ( online supplemental table S5 ), the estimates of the association were weakened slightly compared with the main analysis. After relaxing the eligibility criteria from completing at least two dietary questionnaires to one, the number of participants included in the analysis increased from 64 268 to 85 937 ( online supplemental figure S3 ). Similar to the main analysis, such sensitivity analysis showed that adherence to a higher number of healthy lifestyle behaviours was significantly associated with a lower risk of incident IBS (all P<0.05; online supplemental table S6 ), although the estimate of association was slightly weakened. Nevertheless, the 95% CIs of all three adjusted hazard ratios (1, 2 and 3 to 5 healthy lifestyle behaviours) in the two samples were overlapping. Finally, in separate analyses of each of the five lifestyle behaviours, never smoking (0.86, 0.76 to 0.98, P=0.02), high level of vigorous physical activity (0.83, 0.73 to 0.95, P=0.006) and optimal sleep (0.73, 0.60 to 0.88, P=0.001) demonstrated significant independent inverse associations with the incidence of IBS, although of a smaller magnitude than adhering to 3 to 5 behaviours ( figure 2 ). No significant independent associations were observed for healthy diet and moderate alcohol consumption, but their effect sizes were very close to being statistically significant ( figure 2 ).
Associations of each healthy lifestyle behaviour with the risk of irritable bowel syndrome (IBS). Healthy lifestyle behaviours included never smoking, a high level of vigorous physical activity (in the highest 50% of the cohort), high dietary quality (in the highest quartile of the dietary approaches to stop hypertension diet score), moderate alcohol intake (5–15 g/day) and optimal sleep (having a sleep duration between 7 and 9 h/day, finding it fairly easy or very easy to get up in the morning, and never or rarely having insomnia and narcolepsy). Hazard ratios were adjusted for age (<40, 40–49, 50–59 or ≥60 years), sex (female or male), employment status (in paid employment or self-employed or not), geographic location (England area or non-England area, including Wales and Scotland), gastrointestinal infection (yes or no), endometriosis (yes or no) and family history of IBS (yes or no). The healthy lifestyle behaviours were also adjusted for each other: never smoking (yes or no), optimal sleep (yes or no), a high level of vigorous physical activity (yes or no), moderate alcohol (yes or no) and high dietary quality (yes or no); ‘no’ was the reference group for each behaviour. For estimates of the association between adhering to 3 to 5 healthy lifestyle behaviours, the reference group comprised those who did not perform any of the behaviours.
Associations between healthy lifestyle behaviours and the risk of incident irritable bowel syndrome (IBS) stratified by subgroups
Our analysis showed that adopting a combination of healthy lifestyle behaviours—never smoking, optimal sleep, high level of vigorous physical activity, high dietary quality and moderate alcohol intake—was significantly associated with a lower risk of subsequent incident IBS even when we adjusted for potential confounders. This primary finding was further supported in subgroup analyses, which indicated that age, sex, employment status, geographic location, gastrointestinal infection and endometriosis did not substantially modify the estimated hazard ratios. To the best of our knowledge, this is one of the first prospective cohort studies to confirm the causal relationship between combinations of healthy lifestyle behaviours and subsequent lower incidence of IBS. These results are consistent with those of a recent cross-sectional study among 3363 Iranian adults, which found that an overall healthy lifestyle was associated with a lower prevalence of IBS. 30
Consensus reports have provided recommendations on the diagnosis and treatment of IBS, but none of them have recommended any preventive measures. 31–33 Although lifestyle modification is recommended as a means of managing IBS symptoms, 6 31–33 its potential role in preventing the onset of the condition has not been given due attention. IBS has a complex aetiology, involving biological, genetic, psychosocial and environmental factors. 6 34 Our findings underscore the value of lifestyle modification in the primary prevention of IBS and suggest that healthy lifestyle choices could significantly attenuate the effects of aetiological factors on the incidence of IBS.
To prevent IBS, it is important for primary healthcare providers to take an active role in delivering appropriate interventions to change health behaviours during routine consultations, as they are often the first point of contact for patients accessing the health system. 35 36 Moreover, support from national and local authorities is required to establish a supportive macroenvironment for changing unhealthy lifestyle behaviours or maintaining healthy behaviours. 37 Coordinated efforts are required at many levels to promote the adoption of the five recommended lifestyle behaviours among the general population for prevention of IBS.
Previous studies have assessed the individual impact of each of the five healthy lifestyle behaviours on the incidence of IBS. The role of smoking in causing IBS is a subject of debate. 38 Our study found that never smoking was independently associated with a lower incidence of IBS. Although existing studies suggest this might be due to smoking’s effect on delaying the gastric emptying of food and mouth–caecum transit time, 39 40 this hypothesis needs to be tested in more up-to-date mechanistic studies. Meanwhile, it is widely acknowledged that the pathogenesis of IBS involves dysregulated communication between the gut and the brain via the gut–brain axis. 6 Several prospective cohort studies found that the gut–brain pathway is bidirectional, showing that anxiety and depression could predict the development of IBS and vice versa. 41 42 Furthermore, previous studies have indicated that individuals with mental illness have significantly higher smoking rates than those without. 43 44 Nearly half of the 148 studies, which included a systematic review, indicated an association between baseline anxiety or depression and subsequent smoking behaviours. 45 According to these findings, smoking may be a surrogate for mental health conditions and mediate the association between mental health conditions and the risk of IBS. While no research to date has provided direct evidence to deal with this question, further research is needed to explore the potential mediating role of smoking in the relationship between mental health and IBS incidence.
Our research revealed that optimal sleep is significantly associated with a reduction in IBS incidence, which is consistent with the findings of an earlier meta-analysis of 36 observational studies. 46 This meta-analysis revealed a high pooled prevalence of sleep disorders (37.6%) among patients with IBS and a strong and significant association between sleep disorders and IBS, with a pooled OR of 2.618. 46 Sleep disturbance could cause an upregulation of inflammatory cytokines such as interleukin-1 and interleuking-6, 47 which can negatively affect the neural control of gastrointestinal motor, sensory and secretory functions. 6 Changes in the levels of these cytokines have been observed in some gastrointestinal diseases, including IBS. 47 This might explain why quality sleep plays a crucial role in preventing the development of IBS.
Although our study found that both moderate and vigorous physical activity were inversely associated with incident IBS, the sensitivity analysis revealed that vigorous intensity outperformed moderate intensity exercise. The benefits of exercise, such as reducing intestinal inflammation and regulating the gut microbiota, might explain the underlying mechanisms of its effect on lowering the risk of IBS. 48 These mechanisms might be more effectively activated by vigorous physical activity; however, these observations require further investigations in future studies.
In contrast to the findings of a cross-sectional study in Iran, 12 our study did not find an independent association between dietary quality and the incidence of IBS, but the result is very close to statistical significance. The DASH diet emphasises a high intake of fruits, vegetables and legumes, which are rich in fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs). 49 The fermentative and osmotic effects of FODMAPs may contribute to IBS symptoms such as diarrhoea and bloating. 49 A low-FODMAP diet has been shown to effectively improve IBS symptoms 50 and has been recommended as an IBS self-management strategy. 32 However, our study participants were IBS-free at baseline, and hence, it is reasonable to assume that they had a relatively healthy gut environment and normal gastrointestinal function relative to patients with IBS. This might partly explain why adherence to the DASH diet did not have a significant protective or harmful effect on the development of IBS in our study.
Research has suggested that alcohol consumption can affect gastrointestinal motility, absorption and intestinal permeability in both animals and humans. 51 However, our results showed that moderate alcohol consumption, similar to the DASH diet, was not associated with IBS incidence. This finding is in agreement with a previous case–control study. 52 Among the five healthy behaviours investigated, DASH diet adherence and moderate alcohol intake had the weakest links to IBS prevention, but their combined effect with the remaining three behaviours was significant. Given the other benefits of these behaviours beyond IBS prevention, adherence to all five positive lifestyle factors is still prudent.
Strengths and limitations
Our study has several strengths. First, the use of a population-based cohort data from the UK Biobank allowed us to demonstrate the positive real-world impact of a healthy lifestyle in reducing IBS incidence. Second, the large sample size and sufficiently long follow-up duration provided adequate statistical power for examining the causal relationship between healthy lifestyle behaviours and incident IBS over a decade. Finally, the use of a DAG provided a transparent and evidence-based approach to guide the appropriate selection of potential confounders based on prior knowledge of the possible causal relationships between healthy lifestyle behaviours, IBS and their covariates.
This study also has some limitations. First, as mentioned previously, only participants who completed at least two dietary questionnaires were included in this study. We compared the baseline characteristics between participants with and without at least two dietary questionnaires. While the two groups were similar in almost all aspects, there were statistically significant differences in most characteristics between these two groups ( online supplemental table S7 ). This difference was clearly due to the very large sample sizes in both groups, of which high statistical power would cause the P values to rapidly approach zero, but indeed, there was no meaningful practical significance between these two groups. In this scenario, we followed existing recommendations to focus on the practical significance rather than the statistical significance of P values when considering their differences. 53 54 Based on the figures presented in online supplemental table S7 ), we observed no substantial practical difference between the two groups, and hence our current sample was not at risk of selection bias within the UK Biobank cohort.
Second, a large portion of participants was excluded owing to missing data on the family history of IBS (n=41 020). This is because the data were collected using a web-based digestive health questionnaire during the follow-up period, which was sent out to participants who provided a valid email address (n=331 832, 66% of the entire UK Biobank cohort) in 2017, and only about half (n=172 949, 52.1%) fully completed the questionnaire as of July 2018. 55 Moreover, this exclusion is supported by a previous study using data from the UK Biobank cohort, which found that people with a family history of IBS had a 3.7-fold increased risk of having IBS compared with those without. 56
Third, the use of self-reported data on lifestyle behaviours might raise concerns about data accuracy. However, the high comprehension and acceptability indicated by participants in pilot studies might dispel that concern. 57 Moreover, it is worth noting that self-reported data have been used to measure lifestyle factors in many similar large-scale population-based studies. 58 59
Fourth, as one of the lifestyle behaviours of interest, sleep quality was self-reported at baseline in our study. However, discrepancies have been found between subjective self-reported sleep experience and objective sleep measurements among patients with sleep problems in previous studies. 60–62 Individuals with only self-reported sleep disturbances are more likely to overestimate sleep onset latency (ie, the time taken to fall asleep) and underestimate total sleep time. 60 63 Therefore, in our analysis, the quality of sleep might be underestimated among participants. Future research should use both self-reported data and objective measurements (eg, polysomnography) to assess sleep quality.
Fifth, when comparing the baseline characteristics of study participants whose IBS diagnosis was made based on different data sources, we found that participants whose IBS diagnosis was made solely based on self-report data were more likely employed and were less likely to have back pain or joint pain and headaches than participants whose IBS diagnosis was retrieved from a trustworthy data source (ie, primary care data, hospital inpatient or death register records), with no significant differences found in other characteristics ( online supplemental table S8 ). However, the sensitivity analysis in which participants whose IBS diagnosis was made solely based on self-report (n=85) were excluded found similar estimates of the association between healthy lifestyle behaviours and the risk of developing IBS ( online supplemental table S3 ), compared with the main analysis in table 2 , which included cases of incident IBS from both self-reported data and the trustworthy data source. The similarity suggested that differences in some of the baseline characteristics between these two groups did not have a substantial impact on the effect estimate of interest.
Sixth, we only used ICD-10 code K58 to identify the outcome of incident IBS, and the symptom-based diagnostic criteria of Rome III were not applied. However, the process of outcome ascertainment can still be considered trustworthy because the majority of incident IBS cases were confirmed based on reliable clinical diagnostic data from primary care, hospital inpatient or death register records. In addition, when compared with the updated Rome IV IBS diagnostic criteria, the benefits of using the Rome III criteria are limited owing to their excessive sensitivity, which would inflate the prevalence of IBS. 64
Seventh, gastrointestinal infection is one of the important risk factors for developing IBS, and our DAG also indicated that it was one of the confounders that should be adjusted when evaluating the association between healthy lifestyle behaviours and incident IBS. In our study, the confounding effect of baseline gastrointestinal infection was eliminated when estimating the effects of healthy lifestyle behaviours on the risk of developing IBS, but subsequent infections were not adjusted, and this might remain a confounder in our analysis.
The eighth limitation is the lack of consideration for changes in healthy lifestyle behaviours during the follow-up period. Future longitudinal studies with repeated measurements are needed to examine the cumulative exposure over time and verify the combined benefit of healthy lifestyle factors in preventing IBS.
Finally, a meta-analysis on IBS found that individuals aged 50 years and older had a lower prevalence of IBS (OR= 0.75; 95% CI 0.62 to 0.92) than those under 50. 65 However, this prospective cohort study was based on the UK Biobank cohort, which only consists of middle-aged and older people with an average age of 55.9 years. Therefore, caution should be exercised when extrapolating the findings to younger populations.
Potential future research
In addition to the focal association between healthy lifestyle behaviours and IBS, we observed some interesting potential associations that require further investigations. First, we found that the percentage of women increased across the number of healthy lifestyle behaviours ( table 1 ). Most women in our study were postmenopausal, and there are several possible health-related reasons motivating them to choose a more healthy lifestyle: (i) improve menopausal symptoms 66 and (ii) reduce the risk of developing breast cancer. 67 It is unlikely that this gender difference is linked to the presence of osteoarthritis, as its prevalence across men and women is similar. 68 Future studies may explore other possible gender-differentiated factors associated with choosing healthy lifestyles in middle-aged populations.
Another interesting finding is that the percentage of baseline depression decreases across the number of healthy behaviours ( table 1 ). The relationship between depression and healthy lifestyle behaviours has been a subject of interest in various studies. Research has demonstrated an inverse relationship between depression and healthy lifestyle behaviours. 69 This observation aligns with a previous prospective cohort study of 667 participants with coronary heart disease, which found that there was a bidirectional relationship between depressive symptoms and lifestyle behaviours. 70 Other studies suggest that individuals with depression might have specific health-related beliefs and behaviours that influence their adherence to a healthy lifestyle. 71 Additionally, studies have found that depression is negatively correlated with health-promoting behaviours, indicating that individuals with depression might engage in fewer health-promoting activities. 72 Further studies should be conducted to confirm or refute the bidirectional relationship between healthy behaviours and depression among the general population.
Moreover, the percentage of participants who had a high level of vigorous physical activity increased while the mean age of participants slightly decreased across the number of healthy behaviours ( table 1 ). If the association truly exists, osteoarthritis (OA), an age-related disease, 73 is probably one of the reasons explaining this observation. An existing study investigating the experience of living with knee OA showed that it can lead to negative attitudes and perceptions about physical activity due to activity limitations and the impact on functional performance. 74 This is further supported by the finding that a large proportion of individuals with OA involving the hips or knees are sedentary, 75 explaining our observation that OA might be a reason for the lower physical activity level among older participants. The potential association between age, vigorous physical activity, and their covariates, including OA, is an area requiring further investigation.
In this study, age, sex, employment status, geographical location, gastrointestinal infection, endometriosis and family history of IBS were treated as confounders and adjusted when evaluating the association between healthy lifestyle behaviours and the risk of developing IBS (ie, the association of interest). Moreover, we assessed if they were effect modifiers on the association of interest, but no significant results were found. However, as they were not our exposure of interest in this study, we did not estimate their effects on the risk of incident IBS. Previous studies with different populations reported these effect estimates. 76–80 Future studies can repeat these analyses using the UK Biobank cohort data to allow comparison.
Conclusions
This study provides evidence that adherence to a higher number of healthy lifestyle behaviours—never smoking, optimal sleep, high level of physical activity, high dietary quality and moderate alcohol intake—is significantly associated with a lower risk of subsequent IBS incidence. These findings suggest that lifestyle modifications should be considered as key primary prevention strategies for IBS. Future research with repeated measurements of lifestyle factors is required to further verify our observations.
Ethics statements
Patient consent for publication.
Not applicable.
Ethics approval
This study involves human participants and was approved by The UK Biobank and received ethical approval from the North West Multi-Centre Research Ethics Committee (REC reference for UK Biobank 11/ NW/0382). Participants gave informed consent to participate in the study before taking part.
Acknowledgments
The study has been conducted using the data from UK Biobank under Application Number 64555.
- Chang L , et al
- Sperber AD ,
- Dumitrascu D ,
- Fukudo S , et al
- Flacco ME ,
- Manzoli L ,
- De Giorgio R , et al
- Li C-Y , et al
- Crockett SD ,
- Murphy CC , et al
- Corsetti M , et al
- Drossman DA ,
- Camilleri M ,
- Mayer EA , et al
- Hopkins L ,
- Whorwell PJ
- Koloski NA ,
- Talley NJ ,
- Fujiwara Y ,
- Kohata Y , et al
- Huang N , et al
- Soltani S ,
- Keshteli AH ,
- Esmaillzadeh A , et al
- van den Brandt PA ,
- Esparza-Juárez F ,
- Chico-Barba G , et al
- Gallacher J ,
- Allen N , et al
- Yévenes-Briones H ,
- Caballero FF ,
- Banegas JR , et al
- de Boer EJ ,
- Slimani N ,
- van ’t Veer P , et al
- Carter JL ,
- Lewington S ,
- Piernas C , et al
- Struijk EA , et al
- Glymour M ,
- Etminan M ,
- Collins GS ,
- Mansournia MA
- VanderWeele TJ ,
- Hernán MA ,
- Flanders WD ,
- Darrow LA , et al
- Greenland S ,
- Williamson EJ ,
- Lawrie J , et al
- Ferguson KD ,
- Katikireddi SV , et al
- Digitale JC ,
- Martin JN ,
- Department of Health and Social Care
- Llwodraeth Cymru Welsh Government
- Department of Health Northern Ireland and the Scottish Government
- Hajishafiee M ,
- Saneei P , et al
- De Schepper H , et al
- Gonlachanvit S ,
- Ghoshal UC , et al
- Ghoshal UC ,
- Sachdeva S ,
- Pratap N , et al
- Drossman DA
- Keyworth C ,
- Goldthorpe J , et al
- Public Health England
- National Health Service England
- Health Education England
- National Institute for Health and Care Excellence
- Palmer KR ,
- Smith B , et al
- Kellow JE ,
- Eckersley GM , et al
- Kalantar J , et al
- Woolhandler S , et al
- Kalapos MP , et al
- Fluharty M ,
- Taylor AE ,
- Grabski M , et al
- Awab A , et al
- Motiani KK ,
- Collado MC ,
- Eskelinen J-J , et al
- Shepherd SJ ,
- Parker FC ,
- Muir JG , et al
- Halmos EP ,
- Shepherd SJ , et al
- Farzaneh N ,
- Ghobaklou M ,
- Moghimi-Dehkordi B , et al
- Lucas Jr HC ,
- Callegaro A ,
- Aris E , et al
- Eijsbouts C ,
- Kennedy NA , et al
- Kværner AS ,
- Birkeland E ,
- Bucher-Johannessen C , et al
- Zhang Y-B ,
- Pan X-F , et al
- Carskadon MA ,
- Dement WC ,
- Mitler MM , et al
- Spinweber CL ,
- Johnson LC ,
- Palsson OS ,
- Whitehead W ,
- Törnblom H , et al
- Lovell RM ,
- Yoshany N ,
- Mazloomy Mahmoodabad SS ,
- Bahri N , et al
- McKenzie F ,
- Ferrari P ,
- Freisling H , et al
- Mazurek Melnyk B ,
- Militello L , et al
- Gehi AK , et al
- Mahalik JR ,
- Harris MP ,
- Di Bianca M
- Shane Anderson A ,
- Wallis JA ,
- Taylor NF ,
- Bunzli S , et al
- Fransen M ,
- Winstanley J , et al
- Johansen SG ,
- Ness-Jensen E
- Schauer B ,
- Ittermann T , et al
- Chiaffarino F ,
- Cipriani S ,
- Ricci E , et al
- Prokop LJ , et al
- Faresjö A ,
- Grodzinsky E ,
- Johansson S , et al
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
Contributors VCHC designed the study. FFH, HS, HZ and DCNW conducted the data analysis. Y-YG, MHW, IX-YW and JW contributed to project administration. FFH and VCHC drafted the manuscript. All authors critically revised the manuscript for important intellectual content and approved the final version of the manuscript. IX-YW is the funding receiver and study guarantor. The corresponding author (IX-YW) attests that all the listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding This work was supported by the National Key R&D Program of China (No. 2020YFC2008601) and National Natural Science Foundation of China (No. 81973709). The funders had no role in the study design or implementation; data collection, management, analysis, or interpretation; manuscript preparation, review, or approval; or the decision to submit the manuscript for publication.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
Irritable Bowel Syndrome
Irritable bowel syndrome (IBS) is a chronic, episodic intestinal disorder characterized by abdominal pain and altered bowel habits. It affects one in seven Americans, although most go undiagnosed. IBS can have a substantial impact on well-being and health, but doctors underestimate the impact the disease can have, particularly the pain and discomfort.
IBS is thought to be caused by a hypersensitivity of the lining of the colon. If you suffer from chronic irritable bowel–type symptoms, such as bloating, abdominal pain, and changes in bowel habits, ask your doctor about getting a formal evaluation for celiac disease. If you have celiac, then go on a strict gluten-free diet. If you do not have the disease, the current recommendation is to first try a healthier diet including more fruits, vegetables, whole grains, and beans , while avoiding processed foods. The reason people may feel better on a gluten-free diet—and therefore conclude they have a problem with gluten —is because they’ve stopped eating so much fast food and other processed junk. If you eat a deep-fried Twinkie and your stomach aches, it may not be the gluten.
If a more healthful diet doesn’t help, try to rule out other causes of chronic gastrointestinal distress. Researchers found that about one-third of people who avoid wheat and/or gluten don’t appear to have gluten sensitivity but, instead, have other conditions, like an overgrowth of bacteria in their small intestines, fructose- or lactose-intolerance, or a neuromuscular disorder like gastroparesis or pelvic floor dysfunction. Only after each of these is ruled out do I suggest people suffering from chronic, suspicious symptoms try a gluten-free diet.
A number of plant foods, such as peppermint oil, kiwi, ginger , cayenne pepper, have been found effective in the treatment of irritable bowel syndrome.
For substantiation of any statements of fact from the peer-reviewed medical literature, please see the associated videos below.
Image Credit: The Clear Communication People / Flickr. This image has been modified.
Subscribe to our free newsletter and receive our Daily Dozen Meal Planning Guide .
Popular Videos for Irritable Bowel Syndrome

Fasting for Irritable Bowel Syndrome

Cayenne Pepper for Irritable Bowel Syndrome & Chronic Indigestion

Ginger for Nausea, Menstrual Cramps, and Irritable Bowel Syndrome

Kiwifruit for Irritable Bowel Syndrome
Peppermint Oil for Irritable Bowel Syndrome
All videos for irritable bowel syndrome.

Yoga Put to the Test for IBS, Inflammatory Bowel, Menopause, and Osteoporosis
A study using sham acupuncture underscores the necessity of controlling for expectancy effects.

Ultra-Processed Junk Food Put to the Test
What happened when ultra-processed foods were matched for calories, sugar, fat, and fiber content in the first randomized controlled trial?

Prunes: A Natural Remedy for Constipation
Prunes, figs, and exercise are put to the test as natural home remedies for constipation.

More than half of IBS sufferers appear to have a form of atypical food allergy.

Fiber vs. Low FODMAP for SIBO Symptoms
It may not be the number of bacteria growing in your small intestine, but the type of bacteria, which can be corrected with diet.
Are Small Intestinal Bacterial Overgrowth (SIBO) Tests Valid?
Even if we could accurately diagnose SIBO, if there is no difference in symptoms between those testing positive and those testing negative, then what’s the point?

The World’s Largest Fasting Study
Buchinger modified fasting is put to the test.

Are Ancient Grains Healthier?
Ancient wheats like kamut are put to the test for inflammation, blood sugar, and cholesterol control.

Is Fiber an Effective Anti-Inflammatory?
Most Americans get less than half the recommended minimum fiber intake a day and the benefits of fiber go way beyond bowel regularity.

Ground Ginger to Reduce Muscle Pain
There have been at least eight randomized, double-blind, placebo-controlled trials of ginger for pain.

Is Aloe Effective for Blood Pressure, Inflammatory Bowel, Wound Healing, and Burns?
I discuss the risks and benefits of aloe vera.

How to Strengthen the Mind-Body Connection
Slow-paced breathing at the right frequency can result in a vagal nerve activation, which may have a variety of beneficial effects.

Powdered ginger can be a highly effective, cheap, easy-to-use, safer treatment for nausea, migraine headaches, and menstrual blood loss and pain. Does it also work for IBS intestinal cramping?
Peppermint essential oil should be considered the first-line treatment for IBS.

How to Diagnose Gluten Intolerance
After a formal evaluation to rule out celiac disease, those who suspect they might have gluten sensitivity should first try improving their diet and then have other causes excluded before going on a gluten-free diet, since as many as 1 in 3 people who avoid gluten for symptom control end up having a different disease altogether.

Is Gluten Sensitivity Real?
For more than 30 years, the medical profession has debated the existence of an intolerance to the wheat protein, gluten, unrelated to allergy or celiac disease. What is the evidence pro and con?

Chronic red pepper powder ingestion may be an effective treatment for IBS and chronic dyspepsia (indigestion), both of which can arise from food poisoning.

A kiwifruit intervention was found to improve bowel function in those suffering from irritable bowel syndrome with constipation. This was accomplished without side effects, such as heart attacks and stroke associated with the primary drug prescribed to treat the condition, tegaserod.
Pin It on Pinterest
- Search the site GO Please fill out this field.
- Newsletters
Study: Taking Berberine and Curcumin Together Might Help Alleviate IBS Symptoms
:max_bytes(150000):strip_icc():format(webp)/untitled-5582-Edit-d19315b04e5a4b7e85ec15100d87de46.jpg)
- Taking berberine and curcumin together might help reduce the symptoms of irritable bowel syndrome (IBS), new research suggests.
- For the new study, people with IBS who took a supplement containing both berberine and curcumin reported less abdominal discomfort, bloating, and diarrhea.
- Though the results are promising, more research is needed on berberine, curcumin, and the supplements' effects on IBS symptoms.
New research suggests that taking berberine and curcumin together might help reduce symptoms of irritable bowel syndrome , a common condition that affects the digestive system.
The study, published in Nutrients , found that people with IBS who took a supplement containing berberine and curcumin twice daily for two months reported reduced abdominal discomfort, bloating, and diarrhea.
The authors noted that traditional pharmacological treatments for IBS are often ineffective and that the study shows berberine/ curcumin supplementation could provide a new path for relief.
While the results are promising, Lexi Moriarty, RDN , a registered dietitian at Fueled + Balanced Nutrition, told Health that more rigorous research is needed to assess the supplement’s effect on IBS symptoms .
Miljan ŽivkoviÄ / Getty Images
What Are Berberine and Curcumin?
Berberine is a compound found in certain plants, such as barberry and the Chinese herb Coptis chinensis . Curcumin is the active compound in the spice turmeric.
Both ingredients are considered generally safe and have long been used in traditional medicine to heal numerous ailments, including gastrointestinal conditions. In recent years, they’ve become popular as dietary supplements.
According to the study authors, some modern studies suggest that, when taken individually, the compounds produce effects on the body that could improve IBS symptoms. Both compounds may regulate the gut microbiome and reduce intestinal inflammation. Berberine might also regulate digestion, while curcumin could improve the health of the intestinal lining.
The authors also noted that there's a growing body of evidence from various animal studies that supports the possible therapeutic effect of berberine and curcumin on IBS. More research, however, needs to be done on humans to investigate the safety and efficacy of the supplements and their impact on IBS symptoms.
How the Supplement Affected IBS Symptoms
Researchers wanted to see how a combination of berberine and curcumin would affect IBS patients in a clinical setting.
To do this, they analyzed the outcomes of 146 IBS patients in Belgium, recruited from 38 family physicians and three pharmacies that helped them manage symptoms. The participants first had IBS symptoms before the age of 50 and had no known family history of inflammatory bowel disease and no personal history of colorectal cancer, celiac disease, or other specific gastrointestinal diseases and symptoms.
Researchers divided the participants into two groups based on their clinical diagnosis. Half of the patients took standard IBS medications alone. The rest were instructed to also take a supplement called Enterofytol PLUS (200 milligrams (mg) of berberine and 49 mg of curcumin) after breakfast and dinner for two months.
The team initially took stool samples and assessed the severity of the participants’ IBS systems at the end of the two months.
People taking the berberine/curcumin supplement reported a nearly 50% improvement in abdominal discomfort, bloating, and quality of life. They also had more normal stools. Almost two-thirds of the group participants no longer needed to take medications.
About 7% of participants reported side effects, such as nausea , vomiting, and abdominal pain.
Moriarty said the results would be more convincing if the study included more participants and followed them for longer. “IBS is often a lifelong condition for some patients, and long-term use is something that should be further investigated,” she said.
The inclusion of a randomized, blinded placebo group would also be necessary to eliminate the potential for the placebo effect and other variables, Moriarty added.
Should You Try a Berberine/Curcumin Supplement?
Hadley said that while berberine/curcumin supplements may be beneficial for IBS symptoms, she recommends consulting a healthcare provider before using it.
A medical professional can help you determine if a supplement makes sense for your particular case. They can also recommend the best brand and dosage, as well as assess whether any medications you take may interact with the compounds. Taking antidepressants or antibiotics with curcumin, for example, could raise the risk of adverse side effects.
Also, remember that supplements aren’t the only option for non-pharmaceutical relief. Expert-endorsed natural remedies for treating IBS include eating fiber-rich foods , staying active, limiting caffeine and alcohol, and reducing stress.
Wade U, Pascual-Figal DA, Rabbani F, et al. The Possible Synergistic Pharmacological Effect of an Oral Berberine (BBR) and Curcumin (CUR) Complementary Therapy Alleviates Symptoms of Irritable Bowel Syndrome (IBS): Results from a Real-Life, Routine Clinical Practice Settings-Based Study . Nutrients . 2024;16(8):1204-1204. doi:https://doi.org/10.3390/nu16081204
Related Articles
New gut calming discovery to bring relief to IBS sufferers

Dr Jenny Bailey, CEO of University of Bristol spin out Ferryx.
Newswise — The discovery of a strain of bacteria shown to reduce inflammation in the intestine caused by irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD) could bring relief to millions of sufferers after being turned into an innovative natural food supplement by University of Bristol biotech spin-out Ferryx .
Treatment options for individuals with IBS and IBD are limited and while probiotics are popular, there are currently no conventional probiotics that can function to alleviate symptoms during active flare-ups or if someone is stressed.
The discovery of FX856, a friendly strain of live bacteria, which has shown in animal models with inflammatory bowel disease to survive and thrive during periods of active inflammation delaying disease onset and reducing symptoms, led the Bristol team to create Ferrocalm .
The gut-calming solution, developed over 10 years’ R&D at the University of Bristol, contains FX856 and aims to reduce symptoms such as stomach cramps, bloating, diarrhoea and constipation that people suffer during active flare-ups of IBS, IBD and Crohn’s and ulcerative colitis.
Currently approved for use as a food supplement, Ferrocalm will undergo clinical trials in patients with inflammatory bowel disease in 2024 to test efficacy as a pharmaceutical treatment.
Ferrocalm is the brainchild of Dr Jenny Bailey, a Bristol graduate and award-winning CEO entrepreneur of Ferryx, who has spent 15 years researching how gut inflammation develops to help find a natural solution to improve quality of life for people who suffer from IBS and other gut conditions.
Dr Jenny Bailey, CEO of Ferryx, said: “The launch of Ferrocalm is the culmination of ten years’ of scientific research, all with the aim of helping people to take control of their condition, alleviate their symptoms, and live the life they want to lead using safe, effective bacterial products that prevent and treat inflammatory illness. If we can help one person to feel better, enabling them to go to an event that they would otherwise have missed, take part in sports, go to work, or simply enjoy time with family and friends, then I will be happy. We are already seeing some great results and we look forward to helping many more people achieve relief from gut conditions.”
Dr Tristan Cogan, CTO and Co-founder of FerryX and Senior Lecturer in Infectious Diseases at the University of Bristol Vet School, added: “Both IBS and IBD are lifelong conditions with no cure that can have a significant impact on a sufferer’s day-to-day life.Treatment is mainly based on suppression of symptoms, often with numerous side effects and trials of probiotics in this disease have frequently produced disappointing results as they are rapidly outcompeted during active inflammation or stress. Our discovery of this specific bacteria strain showed promising results in studies including delayed onset of colitis and reduced clinical signs of the disease.”
Ferrocalm is available from www.ferrocalm.com where one month’s supply (30 capsules) costs £19.99 and two months’ supply (60 capsules) is currently available at the discounted rate of £29.98.
Further information
Ferryx is a University of Bristol spin-out biotech company which specialises in scientific innovation in treatment of inflammatory diseases. Formed by CEO Dr Jenny Bailey and Tristan Cogan (CTO), Ferryx researches and produces live biotherapeutic products for human and veterinary use. Incorporated in 2019, the company was the 2020 runner up in the University of Bristol New Enterprise scheme funding awards, and the winners of the Techspark SPARKies ‘Good’ award. Ferryx received investment of £305k to further develop their innovative gut treatments and was named as one of Tech South West’s Scaleup Ones to Watch in February 2023.
Credit: Ferryx (all images)
FX856, a friendly strain of live bacteria: https://fluff.bris.ac.uk/fluff/u1/ficmc/7N7jtD_eC9DNZweOCWHL6gBO7/
https://fluff.bris.ac.uk/fluff/u2/ficmc/AgUZVvHITIhcPCgxYMcHYwBOf/
Dr Jenny Bailey:
https://fluff.bris.ac.uk/fluff/u4/ficmc/TNLzqFHhgB0I_v2PUl4CLQBOb/
Dr Tristan Cogan:
https://fluff.bris.ac.uk/fluff/u3/ficmc/hadAoFu_pEriH_9V6st6ewBOB/
Issued by the University of Bristol Media Team
MEDIA CONTACT
Type of article.
- Experts keyboard_arrow_right Expert Pitch Expert Query Expert Directory
- Journalists
- About keyboard_arrow_right Member Services Accessibility Statement Newswise Live Invoice Lookup Services for Journalists Archived Wires Participating Institutions Media Subscribers Sample Effectiveness Reports Terms of Service Privacy Policy Our Staff Contact Newswise
- Blog FAQ Help
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Expert Review
- Open access
- Published: 02 February 2023
The neurobiology of irritable bowel syndrome
- Emeran A. Mayer ORCID: orcid.org/0000-0003-3923-3349 1 ,
- Hyo Jin Ryu ORCID: orcid.org/0000-0003-4320-2748 2 &
- Ravi R. Bhatt ORCID: orcid.org/0000-0003-2498-8888 3
Molecular Psychiatry volume 28 , pages 1451–1465 ( 2023 ) Cite this article
38k Accesses
33 Citations
71 Altmetric
Metrics details
- Neuroscience
Irritable bowel syndrome (IBS) is the most prevalent disorder of brain-gut interactions that affects between 5 and 10% of the general population worldwide. The current symptom criteria restrict the diagnosis to recurrent abdominal pain associated with altered bowel habits, but the majority of patients also report non-painful abdominal discomfort, associated psychiatric conditions (anxiety and depression), as well as other visceral and somatic pain-related symptoms. For decades, IBS was considered an intestinal motility disorder, and more recently a gut disorder. However, based on an extensive body of reported information about central, peripheral mechanisms and genetic factors involved in the pathophysiology of IBS symptoms, a comprehensive disease model of brain-gut-microbiome interactions has emerged, which can explain altered bowel habits, chronic abdominal pain, and psychiatric comorbidities. In this review, we will first describe novel insights into several key components of brain-gut microbiome interactions, starting with reported alterations in the gut connectome and enteric nervous system, and a list of distinct functional and structural brain signatures, and comparing them to the proposed brain alterations in anxiety disorders. We will then point out the emerging correlations between the brain networks with the genomic, gastrointestinal, immune, and gut microbiome-related parameters. We will incorporate this new information into a systems-based disease model of IBS. Finally, we will discuss the implications of such a model for the improved understanding of the disorder and the development of more effective treatment approaches in the future.
Similar content being viewed by others
Analysis of brain networks and fecal metabolites reveals brain–gut alterations in premenopausal females with irritable bowel syndrome
Global burden of irritable bowel syndrome: trends, predictions and risk factors
Elucidating the putative link between prefrontal neurotransmission, functional connectivity, and affective symptoms in irritable bowel syndrome
Introduction.
IBS is one of the most common disorders of brain-gut interaction globally, with prevalence rates between 1.1 and 45% worldwide, and between 5 and 10% for most Western countries and China [ 1 ]. In contrast to many chronic non-communicable diseases, such as metabolic, neurological, cardiovascular and some forms of cancer, there has been no progressive increase in prevalence during the past 75 years, even though prevalence numbers have been fluctuating due to the periodic changes in official symptom criteria. Based on questionnaire data, women are 1.5–3.0 times more likely to have IBS, reflecting a prevalence in women of 14% and in men of 8.9% [ 2 , 3 ]. However, based on healthcare system utilization, women are up to 2–2.5 times more likely to see a healthcare provider for their symptoms [ 4 ]. Based on the current symptom criteria [ 5 ], IBS is defined by chronically recurring abdominal pain associated with altered bowel habits in the absence of detectable organic disease. IBS symptoms can be debilitating in a small number of patients, but are mild to moderate in the majority of affected individuals [ 6 ]. Based on this definition, other frequently associated somatic or visceral pain and discomfort, as well as anxiety and depression are considered so called comorbid conditions.
The gut-restricted definition of the Rome criteria overlooks the fact that a large number of individuals who meet diagnostic criteria for an anxiety or depressive disorder have IBS and vice versa [ 7 , 8 , 9 , 10 ], and a majority of IBS patients show elevated levels of trait anxiety and neuroticism [ 10 , 11 , 12 , 13 ], or meet diagnostic criteria for an anxiety disorder [ 14 ]. Currently, the commonly associated psychiatric and somatic symptoms are generally referred to as comorbidities, separate from the primary GI diagnosis [ 15 ] and not present in all patients. However, detailed patient histories, frequently reveal symptoms of abdominal discomfort, anxiety and behavioral disturbances starting in early childhood in a majority of patients, and a large recent genetic epidemiological study has provided an intriguing explanation for the co-occurrence of abdominal and psychiatric symptoms in IBS patients on the basis of several shared single nucleotide polymorphisms (see paragraph IBS related genes shared with anxiety disorders below) [ 8 ]. These new findings are consistent with genetic vulnerabilities affecting both the central and the enteric nervous system (ENS), and argue against the long held linear pathophysiological concepts that emotional factors may cause IBS symptoms, or that chronic IBS gut symptoms lead to anxiety and depression Box 1 .
Much of research and drug development in IBS patients has been based on descriptive and symptomatic features, rather than on biology-based disease definitions. These definitions suggest a core abnormality shared by all IBS patients (chronic, recurrent abdominal pain) as well as heterogeneity based on self reports of predominant bowel habit. However, a comprehensive identification of distinct biology-based subgroups of patients including those based on sex, with different underlying pathophysiological components and differential responsiveness to specific therapies, has not been achieved. Subtypes based on bowel habits are generally based on subjective reports of altered bowel habits, without consistent correlates in intestinal transit times, altered regional motility patterns or altered fluid and electrolyte handling by the gut [ 16 ]. Even though some of the most commonly used pharmacological and behavioral therapies are targeted at the level of the brain (low dose tricyclic antidepressants [ 17 ], serotonin reuptake inhibitors [ 18 ], cognitive behavioral therapies [ 19 , 20 ], gut directed hypnosis, stress management [ 21 ]), research and drug development efforts are still predominantly focused on single, usually peripheral targets identified in preclinical models [ 16 ].
Based on such studies and on clinical reports from small samples, an astonishing list of biological abnormalities at various levels of the brain gut axis have been reported in the last 30 years and proposed as potential biomarkers or pathophysiological factors [ 2 ]: smooth muscle cells [ 22 , 23 ], the gut epithelium [ 24 ]; bile acids [ 25 , 26 , 27 , 28 ]; immune system activation [ 29 , 30 ]; neuroendocrine mechanisms [ 31 ]; brain structure and function [ 32 , 33 ]; stress responsiveness [ 34 ]; affective [ 35 , 36 ], cognitive [ 37 , 38 , 39 , 40 ], pain modulation [ 41 , 42 ], gene polymorphisms [ 8 ]; and most recently the gut microbiome [ 43 , 44 , 45 , 46 , 47 ]. In addition, there has been a wealth of comprehensive data and clinical reports demonstrating a strong relationship between psychosocial factors and IBS symptoms [ 48 ]. However, despite the emergent discoveries about possible peripheral [ 29 , 30 ] and central [ 32 , 33 , 35 , 49 , 50 ] components in IBS pathophysiology, the development of animal models with high face and construct validity [ 51 ], the reproduction of visceral hypersensitivity and IBS-relevant features after transplantation of human biospecimen into rodent models, and the recent acceptance of a brain-gut model of IBS [ 52 ], the controversy on the primary role of the nervous system versus peripheral factors still persists in the field [ 33 , 53 ].
In this review, we will discuss the evidence supporting an integrative brain gut microbiome (BGM) model (Fig. 1 ) which incorporates a large body of evidence from studies on peripheral and central neurobiological disease mechanisms, brain and gut targeted influences of the exposome, and results from recently reported large scale genetic analyses with relevance for neuronal dysfunction of the CNS (central nervous system) and ENS (enteric nervous system). This systems biological model is consistent with the frequent comorbidity of IBS with other so-called functional GI disorders, and with other chronic pain and psychiatric disorders, in particular with anxiety. We will use this model to discuss the implications for the pathophysiology of IBS, its association with psychiatric symptoms, and the development of more effective treatment approaches in the future.
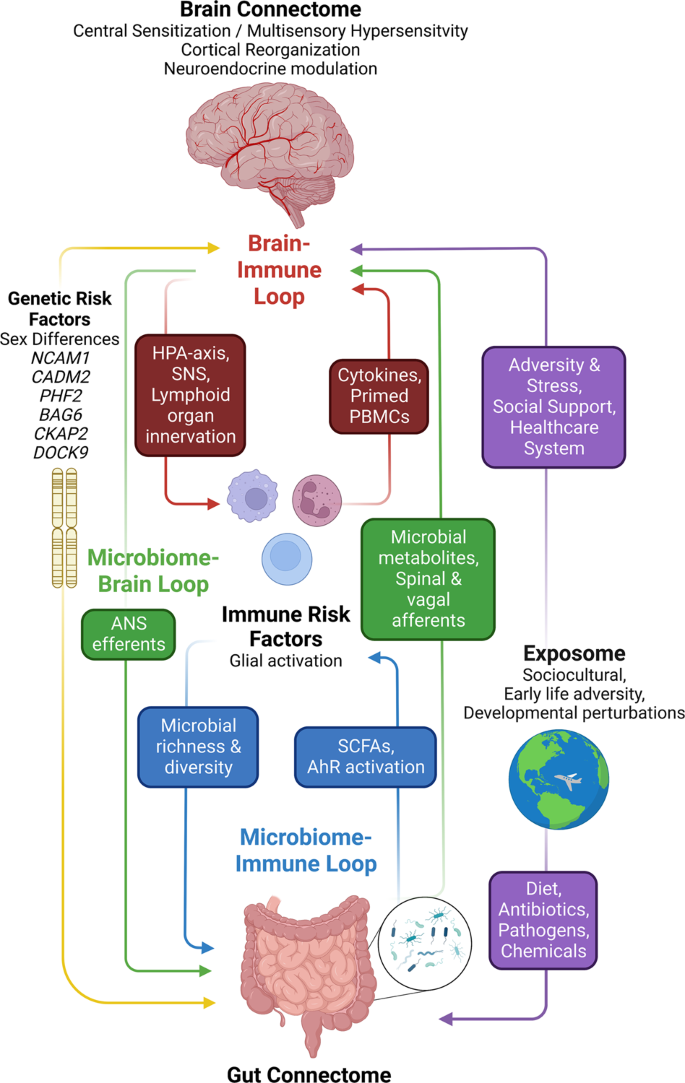
The brain connectome, gut connectome and gut microbiome communicate in a bidirectional way. The response characteristics of the system are determined by vulnerability genes interacting with different influences from the exposome. The different loops use neural, endocrine, paracrine and immune signaling mechanisms. Perturbations (stressors) of the different nodes of the system (brain, gut, immune, microbiota) result in non-linear effects and alterations in response characteristics manifesting as psychiatric and/or gut symptoms. ANS autonomic nervous system, SNS sympathetic nervous system, PBMCs peripheral blood mononuclear cells, SCFAs short chain fatty acids, AhR aryl hydrocarbon receptor.
Box 1 Brain-gut-system abnormalities reported in IBS
Gastrointestinal
Altered intestinal motility and transit time
Altered fluid secretion/absorption
Hypersensitivity of visceral afferents
Altered mucus layer
Gut microenvironment
Altered microbiome composition
Altered fecal bile acid profile
Increased intestinal barrier permeability
Neurological
Structural and functional brain alterations
Alterations in brain receptors for cortical corticotropin release factor (CRF), neurokinin-1 (NRK-1), and cannabinoid-1 receptor systems
Female sex
Gene polymorphisms
PHF2, FAM120AOS
CKAP2, TPTE2P3
The brain-gut-microbiome system
The enteric nervous system and gut connectome.
The ENS is a vast network of different types of intrinsic enteric neurons and glia which are “sandwiched” between the mucosa, and the circular and longitudinal muscle layers of the gut, containing motor neurons, intrinsic primary afferent neurons, and interneurons. Nearly every neurotransmitter class found in the CNS is present in the ENS [ 54 ]. These neurons are organized into two interconnected networks, the myenteric and submucosal plexus, which regulate motility and secretion respectively in a coordinated fashion [ 55 ]. Different classes of neurons are chemically coded by different combinations of neurotransmitters and modulators, many of which are also found in the CNS [ 56 ].
Within the gut, the ENS is closely connected with the gut-based immune system, endocrine system, glial and epithelial cells, making up the gut connectome [ 57 ] (Fig. 2 ). The term connectome reflects close proximity, connectivity, and functional interactions between many cell types and functions in the gut that interact with ENS and CNS.
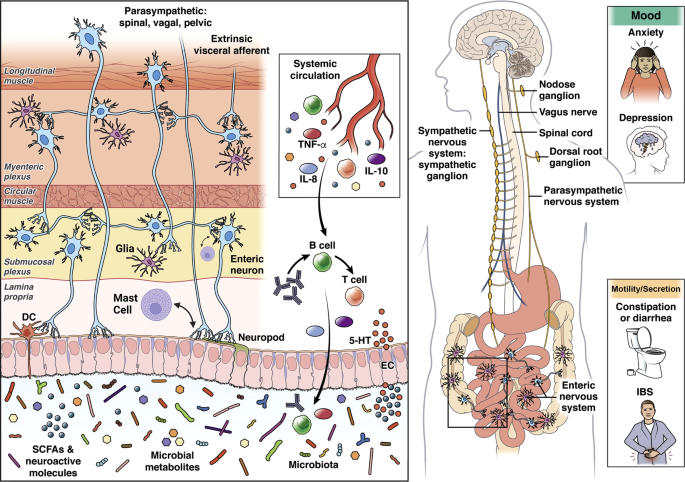
Alterations in these interactions can present as psychiatric and/or IBS symptoms. Modified with permission from [ 79 ].
Beyond the gut, the ENS is connected with the spinal cord, brainstem, and brain via primary spinal and vagal afferents, and postganglionic sympathetic and vagal efferent fibers [ 58 , 59 ]. Although the ENS is capable of regulating all GI functions without input from the CNS, the CNS (brain and spinal cord) has strong modulatory functions in regulating intestinal behaviors [ 60 ] in accordance with the overall state of the organisms and homeostatic perturbations [ 53 ].
Even though the ENS is often being referred to as the “second brain” [ 61 ], evolutionarily speaking, the ENS can be traced back to the cnidaria phylum and epitomized by the hydra genus 650 million years ago [ 62 ]. Historically, it has been classified as a nerve net, but evidence has shown specialized neurons with neurotransmitters such as serotonin, catecholamines, and neuropeptides are also involved [ 63 , 64 ]. In the hydra , the main function of the ENS is peristalsis, mixing movements and expulsion in addition to avoidance behaviors, [ 62 ]. The process of cephalization and the development of bilateria (i.e., organisms through evolution with a head/tail [anterior/posterior axis] and belly/back [dorsal/ventral axis]) led to the development of more complex neuronal systems, most notably the CNS around a central region and highly developed brains. Thus from an evolutionary standpoint, the ENS can be considered “the first brain” [ 56 , 62 ].
ENS related genes
A recent profiling of the human ENS at single-cell resolution highlighted important genes related to neuropathic, inflammatory, and extraintestinal diseases [ 65 ]. Overlapping with the largest GWAS of IBS to date [ 8 ], CADM2 , encoding the cell-adhesion molecule, was highly expressed in myenteric but not mucosal glia [ 65 ]. The known functions of myenteric glia include modulating myenteric neuron activity, regulating oxidative stress and neuroinflammation, providing trophic support, gliogenesis, and neurogenesis [ 66 ]. CADM2 encodes a member of synaptic cell adhesion molecules (SynCAMs) involved in synaptic organization and signaling [ 67 ], and cell adhesion-mediated mechanisms underlying the communication between glia and neurons in the ENS are important in understanding of ENS function in health and disease. For example, perturbed communication between enteric glia and neurons may play a role in dysfunctional ENS circuits in IBS [ 66 ]. The mechanisms underlying neuronal-glia signaling of the ENS in the context of gastrointestinal disorders, IBS, and visceral pain has recently been extensively reviewed [ 66 , 68 ]. It is worth noting that CADM2 has been implicated in a wide range of psychological and neurological traits often observed in IBS patient including, but not limited to psycho-behavioral traits, risk-taking behavior, nervousness-like traits, and neurodevelopmental disorders (e.g., intellectual disability and autism spectrum disorder) [ 69 ]. Moreover, SynCAMs have a large role in synaptogenesis, axon guidance, and synaptic plasticity at a basic neurodevelopmental level which has the potential to affect a variety of disorders [ 70 ].
Similarly, NCAM1 is another gene found in the largest GWAS to date and has been implicated in the development of the ENS. In a similar manner to CADM2 , NCAM1 has been shown to play a role in the ENS regarding cell migration, axon growth, neuronal plasticity and fasciculation [ 71 ], but has not been as thoroughly investigated as CADM2 . A recent cross-tissue atlas applied single-nucleus RNA sequencing from eight healthy human organs showed that a cluster of genes including NCAM1 and CADM2 were involved particularly with cognitive/psychiatric symptoms including general cognitive ability, risk-taking behavior, intelligence, and neuroticism [ 72 ]. Even though the study did not contain tissue samples from the intestinal regions of the ENS, these genes involved in cognitive/psychiatric functions were highly expressed in Schwann cells in the esophagus mucosa, and interstitial cells of Cajal (ICCs) and neurons in the esophagus muscularis [ 72 ].
The gut microbiome
The term gut microbiome refers to the 40 trillion microbial organisms (bacteria, fungi, and archae) and their millions of genes that live throughout the gastrointestinal tract, from the oral cavity to the rectum, with the highest concentration and diversity in the large bowel [ 73 ]. The symbiotic interactions of the 3 groups of microorganisms within the microbiome, and with the extensive gut virome are incompletely understood [ 74 , 75 ]. The characterization of these microorganisms in IBS to date is primarily based on identification of relative abundances and diversity using 16S rRNA sequencing techniques with limited resolution beyond the species level. We refer to several recent review articles on this topic [ 76 , 77 ]. The extensive literature reveals inconsistent findings and a causative relationship of specific microorganisms with IBS symptoms has not been demonstrated. However, both preclinical and some clinical studies have demonstrated a significant effect of psychosocial stress on the relative abundance of gut microbes which is mediated both by stress-induced alterations in regional transit and secretion, and by direct effects of norepinephrine and possibly other signaling molecules released from gut cells on gut microbial gene expression and virulence [ 78 ], suggesting the possibility that the microbiome in subgroups of IBS patients with greater stress reactivity may contribute to certain symptoms [ 79 ].
Brain Connectome alterations in IBS
A growing body of research paired with clinical observations supports a critical role of the brain in the generation and maintenance of IBS symptoms. Regardless of primary symptom triggers, the brain is ultimately responsible for constructing and generating the conscious perception of abdominal pain, discomfort, and anxiety based on sensory input from the gut. Stressful and traumatic events during early life increase chances of developing IBS, and psychosocial stressors in adulthood play a crucial role during the first onset, symptom flare, and perceived severity of the symptoms [ 80 ]; centrally targeted pharmacological treatments and cognitive behavioral strategies have been some of the most effective IBS treatment strategies [ 3 , 16 , 81 ].
Specific brain functions such as sensory processing and modulation, emotion regulation, or cognition are the result of dynamic interactions of distributed brain areas operating in large-scale networks. As summarized in Fig. 3C and Table 1 , these central networks and their properties have been assessed by neuroanatomical and neurophysiological studies in animals [ 51 ], as well as by a wealth of studies using different structural and functional brain imaging techniques and analyses in humans [ 82 , 83 , 84 , 85 , 86 ].
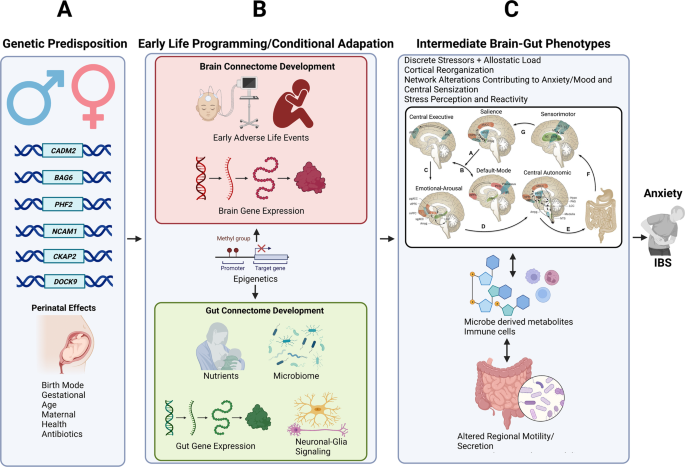
a Vulnerability genes and prenatal influences (including maternal health, nutrition, and stress level) on BGM system development. b the brain and gut transcriptome is influenced by mode of delivery, early adversity, and early nutrition, leading to the development of distinct intermediate brain gut phenotypes c which shape the adult response to influences from the exposome (diet, psychosocial stress).
In humans, several types of networks have been reported [ 33 ] (summarized in Table 1 ): functional brain networks based on evoked responses [ 87 ] or intrinsic connectivity of the brain during rest [ 82 , 83 ]; structural networks based on gray matter parameters [ 88 ] and white matter properties; and anatomical networks based on white matter connectivities [ 89 ]. Both evoked and resting state studies performed in patients with IBS have demonstrated abnormalities in regions and task-related networks linked to salience detection [ 90 , 91 ], emotional arousal [ 92 , 93 , 94 , 95 ], central autonomic control [ 38 , 96 , 97 , 98 ], central executive control [ 90 , 94 , 99 ], and sensorimotor processing [ 38 , 100 , 101 ]. IBS-related alterations in these networks have provided plausible neurobiological substrates for several information-processing abnormalities reported in patients with IBS, such as stress hyperresponsiveness, biased threat appraisal, expectancy of outcomes, cognitive inflexibility, autonomic hyperarousal (emotional arousal and central autonomic networks), symptom-focused attention (central executive network) [ 33 , 53 ] and cognitive inflexibility (central executive network). Supporting the concept of shared pathophysiological factors (so called p-factors), several reported brain network alterations have also been described in other chronic pain conditions [ 102 ] and in anxiety disorders (see Table 1 ).
The Salience Network
The salience network (SN) is integral in mediating the switching of activation between the default mode network (DMN) and central executive network, coordinating and adjusting physiologic/behavioral responses to internal and environmental perturbations of homeostasis [ 103 ]. Visceral inputs to the affective-motivational component of the SN converge onto the anterior insula coordinating response selection and conflict monitoring with the dACC [ 103 ]. Controlled rectal distention in IBS subjects has been shown consistently to result in increased engagement of the core hubs of the SN which are associated with increased affective, emotional, and arousal processes [ 104 , 105 , 106 ]. Reduced neurokinin-1 receptor (NK-1R) availability in the dACC, reflecting NK-1R endocytosis in response to substance P release, was found to be associated with duration of IBS symptoms [ 107 ]. Increased substance P release is thought to result from noxious visceral stimuli and increased engagement of endogenous pain or stress inhibition systems [ 107 ]. In adolescent girls with IBS, lower gray matter volume of the dACC has been observed [ 108 ], and greater salience-sensorimotor connectivity quantified by multiple neuroimaging techniques predicts a lack of symptom alleviation over 3–12 months in patients with IBS [ 109 ].
The default mode network (DMN)
The DMN’s role in pain perception is known to act as an opposite manner to the SN, such that the DMN is suppressed when attention is placed on present sensory stimuli, and is activated when attention is engaged with thoughts away from present sensory stimuli and engaged in mind wandering (i.e., thoughts unrelated to the present sensory environment) [ 110 ]. Studies in chronic pain subjects have shown altered functional connectivity and topological reorganization in various regions, consistent with DMN dysregulation [ 111 ]. Overall neuroimaging research suggests decreased activity of the DMN in patients with IBS [ 112 ]. Lower integrity of anatomical connectivity and resting-state functional connectivity, and lower morphological integrity within the DMN (between the aMPFC and PCC) were found to be predictive of sustained IBS symptom severity over 3–12 months [ 109 ]. Rectal lidocaine administration in IBS subjects was associated with decreased pain perception and with increased coherence in the DMN [ 113 ], supporting an involvement of the DMN in visceral hypersensitivity in patients with IBS.
The Sensorimotor Network
Similar to other chronic pain disorders, imaging studies in IBS subjects have shown alterations of the sensorimotor network (SMN), consistent with alterations in central processing and modulation of viscerosensory and somatosensory information [ 32 , 100 , 109 , 114 , 115 , 116 , 117 ]. This network consists of the primary motor cortex, area 24 of the cingulate cortex, premotor cortex, supplementary motor area (SMA), posterior operculum/insula, as well as primary and sensory cortices in the parietal lobe. In addition, lower gray matter volume in the basal ganglia and thalamus as well as greater functional connectivity within the SMN have been observed in young children with chronic pain [ 108 ]. Greater intrinsic functional connectivity in adults, greater cortical thickness of the posterior insula positively associated with symptom duration, and increasing functional coupling of area 24 and the thalamus, and greater SMN connectivity to the SN predicting sustained symptoms over 3–12 months [ 109 ]. When viewed together, current evidence suggests patients with IBS have functional, morphological, and microstructural SMN alteration, which are likely to play a role in the increased perception of both visceral and somatic stimuli.
The central autonomic network
The central autonomic network (CAN) regulates visceromotor, neuroendocrine, pain, and behavioral responses essential for survival [ 118 ]. Afferents project through the spinal cord and eventually arrive at the main homeostatic processing sites in the brainstem/central autonomic network (including hypothalamus, amygdala, and PAG), and higher cortical processing and modulatory regions [ 119 ]. Historically it has been difficult to non-invasively study the brain stem nuclei in humans due to the limited spatial resolution of neuroimaging methods, but new imaging protocols with a resolution of 1mm 3 and below are allowing new insights [ 120 ].
The CAN is closely connected by vagal and sympathetic efferent projections with the ENS, and afferents from the ENS send viscerosensory signals back to the brain. The hubs of the SN also participate in autonomic control via descending projections to the amygdala (tagging emotional valence and engaging autonomic survival responses to behaviorally relevant stimuli), hypothalamus (regulating homeostasis and a pattern generator for the stress response) and brainstem structures including the periaqueductal gray (PAG) and locus coeruleus (LC). The PAG is a key structure for integrating autonomic, pain modulatory/analgesic, and motor responses to stress [ 121 ], and the LC-norepinephrine system plays a central role in behavioral arousal and stress responses [ 122 , 123 , 124 ].
When viewed together, based on a large number of structural, and functional (resting state and evoked) studies, IBS patients show alterations in several brain networks related to salience assessment, attention, stress perception and responsiveness, and sensory processing. The responsiveness and connectivity of these networks are modulated by several vulnerability genes, which are shared both with ENS genes, and with genes identified in anxiety disorders. Based on these findings, we hypothesize that perturbations of homeostasis arising from the exposome, in the form of psychosocial and gut-targeted stressors interact with genetic factors to a spectrum of clinical phenotypes, ranging from gut symptoms to anxiety.
IBS-related genes shared with anxiety disorders
Prior to the availability of biobank scale data, many candidate gene studies uncovered potential pathways underlying IBS symptoms. These pathways have been extensively reviewed and include the serotonin pathway, SCN5A , and intestinal channelopathy, and sucrase-isomaltase malabsorption [ 125 ]. As serotonin is secreted from enteroendocrine cells and activates enteric sensory and motor neurons, expression level alterations in serotonin receptors and transporters are likely to play a potential role in visceral hypersensitivity, pain, intestinal motility, and secretion. SCN5A encodes the voltage-gated sodium Na v 1.5 channel present on interstitial cells of Cajal (ICCs) in the ENS [ 31 , 126 ]. Genetic mutations on this gene have shown to impair peristalsis and cause constipation, even though slow transit constipation is an uncommon finding in IBS-C [ 127 ]. Lastly, two faulty copies of the SI gene result in reduced disaccharide activity responsible for degradation of sucrose and starch, resulting in diarrhea and gas production in the large intestine from bacterial fermentation and is termed congenital sucrase-isomaltase deficiency (CSID), and should not be considered as IBS [ 125 ]. Even though these findings have established causal relationships between specific genetic abnormalities and non-specific IBS-like GI symptoms in a small number of affected individuals, it is highly unlikely that they play an important role in the great majority of patients.
Recently, the largest genome wide association study with 53,000 cases of IBS across multiple cohorts was completed [ 8 ]. In this study, the strongest risk factors for IBS included long-term or recurring antibiotic exposure in childhood, somatic pain conditions (back pain, limb pain, headaches), psychiatric conditions (anxiety, depression, excessive worrying) and fatigue. The genes included CADM2 , BAG6 , PHF2/FAM120AOS , NCAM1 , CKAP2 / TPTE2P3 , and DOCK9 . Four of the six loci are highly implicated in anxiety/mood disorders and there was a strong genome-wide genetic correlation of IBS with anxiety, neuroticism, depression, insomnia, and schizophrenia. Moreover, the high genetic correlations persisted after taking into account individuals with phenotypic overlap, suggesting common etiological pathways between IBS and anxiety/mood disorders. Implication of the central nervous system was further suggested by the finding that the six identified loci regulate gene expression in many genes primarily expressed in the brain. As already mentioned under ENS above, the genes NCAM1 and CADM2 were two genes which regulate neural circuit formation and influence changes in white matter microstructure in IBS and mood disorders [ 128 , 129 , 130 ]. Specifically, they regulate synaptic cell adhesion molecules, which are present in dorsal root ganglia sensory neurons throughout development, mediate adhesion of sensory axons, and induce neurite outgrowth [ 130 ]. Mechanisms relating to brain development were further implicated by the genes PHF2 (i.e., proper expansion of neural progenitors) and DOCK9 (i.e., dendritic development of the hippocampus), but have not yet been studied in patients with IBS [ 131 , 132 , 133 ].
Importantly, the heritability of IBS was estimated to be a modest 5.8%, suggesting that perturbation of the brain-gut axis by environmental factors arising from the exposome such as early adversity, psychosocial stress, learned behaviors, diet, and possibly dysbiosis play a prominent role.
Considering these new genetic findings and the reported frequent comorbidities of IBS with other chronic pain and psychiatric conditions it is becoming increasingly recognized that IBS is part of a constellation of symptoms that occur on a larger spectrum of altered brain-body interactions [ 134 , 135 ]. This concept is consistent with the “somatic symptom disorder” concept, previously proposed [ 2 ]. The main co-occurring symptoms include hypersensitivity to multiple internal and external sensory stimuli, which could explain the observed association with a variety of seemingly unrelated external and internal factors, previously reported. Other co-occurring symptoms include mood problems, fatigue, and problems with sleep onset and maintenance, as well as memory disturbance [ 134 ]. The neurogenetic basis integrating mood/anxiety and central amplification of sensory inputs (“central sensitization”) based on many of these genetic hits have been well established, which will be discussed below.
Known functions of NCAM1 , DOCK9 , and PHF2 and possible roles in IBS pathophysiology are summarized in Table 2 .
Central sensitization and comorbid chronic pain conditions
The primary mechanism for the core symptom of persistent, chronically recurring abdominal pain that patients with IBS report is thought to result from alterations in the central processing of sensory input from the gut, also referred to as central sensitization [ 134 , 136 ]. The term was originally coined to represent the specific spinal mechanisms responsible for the amplification of nociceptive signaling involving spinal activation of the NMDA receptor [ 137 , 138 ], and is present in various chronic pain disorders such as chronic neuropathic pain, fibromyalgia, headaches, and IBS [ 6 , 134 , 139 , 140 , 141 ]. Today, it is understood that spinal and supraspinal mechanisms both play key roles in the development and maintenance of central sensitization. Based on rodent models of pain, plausible spinal mechanisms include alterations in converging sensory input from different sites on the GI tract and body, temporal and spatial summation, reduced endogenous dorsal horn inhibition, and glial cell activation. Based on human brain imaging studies, supraspinal mechanisms include an altered balance between facilitatory and inhibitory endogenous pain modulation influences, hyperconnectivity between brain networks, alterations of gray matter architecture, elevated CSF glutamate and substance P levels, reduced GABAergic transmission, altered noradrenergic signaling/receptors, and glial cell activation [ 122 , 134 ].
The large overlap - up to a 4.27 odds ratio - between psychiatric phenotypes (primarily anxiety and depression [ 136 , 142 ]) and IBS and other chronic pain disorders, as well as genetic overlap [ 8 , 143 , 144 , 145 ] mentioned earlier, suggests central sensitization as a possible shared pathophysiological factor (p factor) [ 134 , 146 , 147 , 148 ]. The concept of central sensitization was introduced in psychological research in the 1990s based on the observation that highly sensitive persons (HSPs) often share a history of early adversity, psychological profile of introversion (“neuroticism”), and greater emotionality [ 149 ]. Patients with IBS are significantly more likely to exhibit qualities of HSPs, and show central sensitization which is expressed as general sensory hypersensitivity [ 150 ]. The association between chronic pain disorders, psychiatric symptoms, and mechanisms of central sensitization is likely due to the above-mentioned supraspinal alterations, including monoamine neurotransmitter systems (i.e., serotonin, dopamine, noradrenaline), the amino acid GABA, and brain regions underlying both pain transmission/modulation and mood disorders [ 151 , 152 ]. Striato-thalamic-frontal cortical pathways including the prefrontal cortex, amygdala, nucleus accumbens, and thalamic nuclei are key hubs, and alterations in neuronal firing and communication underlie sensory sensitivity and psychiatric symptoms including altered perception, arousal, cognition, and mood [ 152 , 153 , 154 ]. Behaviorally, chronification of central sensitization and negative mood states have been proposed to be in the same continuum of aversion, such that pain motivates the avoidance of further injury, and anxiety promotes behaviors that diminish anticipated danger [ 154 ].
An extensive literature supports the importance of early programming by early adverse life (EAL) events for the development not only of IBS [ 76 ], but also of other chronic pain conditions and psychiatric syndromes [ 155 , 156 ]. Perturbations to the developing brain play a large sole in sensitizing cortical nociceptive circuitry [ 157 ], with the most mechanistic study in humans showing larger event-related potentials (ERPs) to nociceptive stimuli, but not tactical stimuli in infants exposed to many invasive, skin-breaking, painful procedures and morphine [ 158 ]. Moreover, up to 68.4% of children who are exposed to early life traumatic events such as the NICU can develop chronic pain by age 10. Greater amounts of pain-related stressors, painful procedures, and morphine are associated with lower global gray matter volumes throughout childhood [ 159 , 160 ]. In addition to the well documented changes in stress response systems [ 161 , 162 , 163 ], the effect of early-life dietary influences on the gut microbiome and the BGM axis have received increasing attention, even though a direct link with chronic abdominal pain has not been established [ 164 , 165 ].
Clinical and therapeutic implications
Despite a decades-long effort by the pharmaceutical industry, a large number of IBS candidate drugs identified and validated in preclinical models and targeted at both central and gut mechanisms have failed, either due to lack of efficacy or serious side effects [ 16 ]. Of the small number of new drugs obtaining FDA approval, efficacy above placebo has generally not exceeded 10% in phase 3 trials. The great majority of available, FDA approved IBS medications are targeted at intestinal secretion and motility, and the gut microbiome with the goal to improve altered bowel habits and bloating-type symptoms in subgroups of patients [ 16 ].
Pharmacological treatments have been clinically divided into first and second-line approaches [ 16 ], and are aimed at specific symptoms. Moderate quality data has shown low-dose tricyclic antidepressants and SSRIs to be effective for pain (primarily the former) and comorbid anxiety and depression (primarily the latter) [ 16 , 18 ]. As 5-HT receptor-mediated signaling plays important roles both in the brain, as well as in the gut, there is a good rationale for IBS treatments targeted at these receptors. 5-HT released from enterochromaffin cells mediates many GI functions including peristalsis, secretion, pain, and nausea via receptors on ENS and vagal nerve endings [ 31 ]. For example, 5HT-3 receptor antagonists (acting on both gut and brain-located 5HT-3 receptors (such as alosteron, and ramosteron) have shown effectiveness in slowing colonic transit, improving diarrhea, and reducing visceral pain in well-designed randomized controlled trials [ 16 ]. High-quality preclinical data has shown the antagonism of 5HT-3 receptors on the area postrema and vagus nerve have shown a reduction of visceral pain and diarrhea [ 16 , 18 , 166 ], and older data have demonstrated anxiolytic effects [ 167 , 168 , 169 ].
Despite evidence obtained in rodent models of IBS, efforts to develop peripheral visceral analgesics or central stress modulators (antagonists for CRF-1 and NK-1 receptors) have failed to show therapeutic benefits in IBS. This is surprising, as multiple preclinical studies as well as a human brain imaging study had demonstrated effectiveness of the CRF-R1 antagonist Emicerfont (GW876008) on evoked visceral pain and on central stress circuits [ 170 , 171 ]. Because of these disappointing results, increased attention has been shifted to behavioral treatments, including gut-directed hypnosis [ 21 , 81 , 172 , 173 , 174 , 175 ], mindfulness-based stress reduction [ 176 ], and cognitive behavioral approaches [ 19 , 20 , 177 , 178 , 179 ]. Several of these therapeutic approaches have shown promise in improving IBS symptoms, and a few studies have demonstrated associated neurobiological effects on brain mechanisms in salience, emotional arousal, and executive networks [ 172 , 177 ].
As access to therapists specialized in these behavioral IBS treatments is limited, and traditional delivery is time-consuming, web-based versions of these therapies have been evaluated, some of which have been FDA approved and are becoming available to patients [ 180 ]. In addition, several randomized controlled studies have shown some benefits of certain dietary interventions (low FODMAP diet [ 16 ]), and microbiome-targeted treatments (probiotics, antibiotics) [ 181 ].
Summary and conclusions
Even though in subsets of patients, SSRIs and bowel movement targeted therapies are helpful, the model of IBS presented in this review provides precedence for a multidisciplinary therapeutic approach including pharmacological, behavioral, and dietary approaches. Current evidence suggests that there are significant interindividual variations in the response to such therapies, including the predominant bowel habit subtype, severity of gut and psychiatric symptoms, and possibly the presence of gut microbial alterations.
There is growing evidence from clinical, preclinical, and genetic studies supporting the existence of shared p factors in IBS and often comorbid gastrointestinal and non-gastrointestinal pain conditions, as well as psychiatric conditions. Despite shared vulnerability genes, different influences from the environment (exposome) in particular during childhood ultimately shape the specific clinical phenotype. The emerging disease model can explain the failure of reductionistic single mechanism targeted treatment approaches, and is consistent with the evidence for the effectiveness of personalized multidisciplinary approaches involving behavioral, dietary, and pharmacological interventions.
irritable bowel syndrome (IBS); brain-gut-microbiome (BGM); gastrointestinal (GI); enteric nervous system (ENS); central nervous system (CNS); synaptic cell adhesion molecules (SynCAMs); default mode network (DMN); salience network (SAL); sensorimotornNetwork (SMN); central autonomic network (CAN); central executive network (CEN); locus coeruleus (LC); periaqueductal grey (PAG); dorsal anterior cingulate cortex (dACC); posterior cingulate cortex (PCC); N-methyl-D-aspartate (NMDA); gamma-aminobutyric acid (GABA); cerebrospinal fluid (CSF); early adverse life events (EAL); serotonin (5-HT); selective serotonin reuptake inhibitor (SSRI); long-term potentiation (LTP); event-related potentials (ERPs).
Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–21.
Article PubMed Google Scholar
Enck P, Aziz Q, Barbara G, Farmer AD, Fukudo S, Mayer EA, et al. Irritable bowel syndrome. Nat Rev Dis Prim. 2016;2:16014.
Ford AC, Sperber AD, Corsetti M, Camilleri M. Irritable bowel syndrome. Lancet 2020;396:1675–88.
Article CAS PubMed Google Scholar
Drossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Grant Thompson W, et al. U. S. Householder survey of functional gastrointestinal disorders. Dig Dis Sci. 1993;38:1569–80.
Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features, and Rome IV. Gastroenterology 2016;150:1262–79.
Article Google Scholar
Simrén M, Törnblom H, Palsson OS, Van Oudenhove L, Whitehead WE, Tack J. Cumulative effects of psychologic distress, visceral hypersensitivity, and abnormal transit on patient-reported outcomes in irritable bowel Syndrome. Gastroenterology 2019;157:391–402.e2.
Banerjee A, Sarkhel S, Sarkar R, Dhali GK. Anxiety and depression in Irritable Bowel Syndrome. Indian J Psychol Med. 2017;39:741–5.
Article PubMed PubMed Central Google Scholar
Eijsbouts C, Zheng T, Kennedy NA, Bonfiglio F, Anderson CA, Moutsianas L, et al. Genome-wide analysis of 53,400 people with irritable bowel syndrome highlights shared genetic pathways with mood and anxiety disorders. Nat Genet. 2021;53:1543–52.
Article CAS PubMed PubMed Central Google Scholar
Bengtson M-B, Aamodt G, Vatn MH, Harris JR. Co-occurrence of IBS and symptoms of anxiety or depression, among Norwegian twins, is influenced by both heredity and intrauterine growth. BMC Gastroenterol. 2015;15:9.
Lee C, Doo E, Choi JM, Jang S-H, Ryu H-S, Lee JY, et al. The increased level of depression and anxiety in irritable bowel syndrome patients compared with healthy controls: systematic review and meta-analysis. J Neurogastroenterol Motil. 2017;23:349–62.
Muscatello MRA, Bruno A, Mento C, Pandolfo G, Zoccali RA. Personality traits and emotional patterns in irritable bowel syndrome. World J Gastroenterol. 2016;22:6402–15.
Fond G, Loundou A, Hamdani N, Boukouaci W, Dargel A, Oliveira J, et al. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci. 2014;264:651–60.
Hu Z, Li M, Yao L, Wang Y, Wang E, Yuan J, et al. The level and prevalence of depression and anxiety among patients with different subtypes of irritable bowel syndrome: a network meta-analysis. BMC Gastroenterol. 2021;21:23.
Fadgyas-Stanculete M, Buga A-M, Popa-Wagner A, Dumitrascu DL. The relationship between irritable bowel syndrome and psychiatric disorders: from molecular changes to clinical manifestations. J Mol Psychiatry. 2014;2:4.
Mayer EA, Catherine Bushnell M. Functional Pain Syndromes: Presentation and Pathophysiology. Lippincott Williams & Wilkins; 2015.
Camilleri M. Diagnosis and treatment of irritable bowel syndrome: a review. J Am Med Assoc. 2021;325:865–77.
Article CAS Google Scholar
Rahimi R, Nikfar S, Rezaie A, Abdollahi M. Efficacy of tricyclic antidepressants in irritable bowel syndrome: a meta-analysis. World J Gastroenterol. 2009;15:1548–53.
Black CJ, Yuan Y, Selinger CP, Camilleri M, Quigley EMM, Moayyedi P, et al. Efficacy of soluble fibre, antispasmodic drugs, and gut–brain neuromodulators in irritable bowel syndrome: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:117–31.
Kinsinger SW. Cognitive-behavioral therapy for patients with irritable bowel syndrome: current insights. Psychol Res Behav Manag. 2017;10:231–7.
Lackner JM, Jaccard J, Keefer L, Brenner DM, Firth RS, Gudleski GD, et al. Improvement in gastrointestinal symptoms after cognitive behavior therapy for refractory irritable bowel Syndrome. Gastroenterology 2018;155:47–57.
Flik CE, Bakker L, Laan W, van Rood YR, Smout AJPM, de Wit NJ. Systematic review: The placebo effect of psychological interventions in the treatment of irritable bowel syndrome. World J Gastroenterol. 2017;23:2223–33.
Takeshita E, Matsuura B, Dong M, Miller LJ, Matsui H, Onji M. Molecular characterization and distribution of motilin family receptors in the human gastrointestinal tract. J Gastroenterol. 2006;41:223–30.
Miller LJ. Characterization of cholecystokinin receptors on human gastric smooth muscle tumors. Am J Physiol. 1984;247:G402–G410.
CAS PubMed Google Scholar
Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke J-D, Serino M, et al. Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189.
Wei W, Wang H-F, Zhang Y, Zhang Y-L, Niu B-Y, Yao S-K. Altered metabolism of bile acids correlates with clinical parameters and the gut microbiota in patients with diarrhea-predominant irritable bowel syndrome. World J Gastroenterol. 2020;26:7153–72.
Vijayvargiya P, Busciglio I, Burton D, Donato L, Lueke A, Camilleri M. Bile acid deficiency in a subgroup of patients with irritable bowel syndrome with constipation based on biomarkers in serum and fecal samples. Clin Gastroenterol Hepatol. 2018;16:522–7.
Slattery SA, Niaz O, Aziz Q, Ford AC, Farmer AD. Systematic review with meta-analysis: the prevalence of bile acid malabsorption in the irritable bowel syndrome with diarrhoea. Aliment Pharm Ther. 2015;42:3–11.
Bajor A, Törnblom H, Rudling M, Ung K-A, Simrén M. Increased colonic bile acid exposure: a relevant factor for symptoms and treatment in IBS. Gut 2015;64:84–92.
Hughes PA, Zola H, Penttila IA, Blackshaw LA, Andrews JM, Krumbiegel D. Immune activation in irritable bowel syndrome: can neuroimmune interactions explain symptoms? Am J Gastroenterol. 2013;108:1066–74.
Ohman L, Simrén M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol. 2010;7:163–73.
Mawe GM, Hoffman JM. Serotonin signalling in the gut—functions, dysfunctions, and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473–86.
Mayer EA, Gupta A, Kilpatrick LA, Hong JY. Imaging brain mechanisms in chronic visceral pain. Pain 2015;156:S50–S63.
Mayer EA, Labus J, Aziz Q, Tracey I, Kilpatrick L, Elsenbruch S, et al. Role of brain imaging in disorders of brain-gut interaction: a Rome Working Team Report. Gut 2019;68:1701–15.
Larauche M, Mulak A, Taché Y. Stress and visceral pain: from animal models to clinical therapies. Exp Neurol. 2012;233:49–67.
Elsenbruch S. Abdominal pain in Irritable Bowel Syndrome: a review of putative psychological, neural and neuro-immune mechanisms. Brain Behav Immun. 2011;25:386–94.
Elsenbruch S, Schmid J, Bäsler M, Cesko E, Schedlowski M, Benson S. How positive and negative expectations shape the experience of visceral pain: an experimental pilot study in healthy women. Neurogastroenterol Motil. 2012;24:914–e460.
Aizawa E, Sato Y, Kochiyama T, Saito N, Izumiyama M, Morishita J, et al. Altered cognitive function of prefrontal cortex during error feedback in patients with irritable bowel syndrome, based on FMRI and dynamic causal modeling. Gastroenterology 2012;143:1188–98.
Fukudo S. Stress and visceral pain: focusing on irritable bowel syndrome. Pain 2013;154(Suppl 1):S63–S70.
Kennedy PJ, Clarke G, O’Neill A, Groeger JA, Quigley EMM, Shanahan F, et al. Cognitive performance in irritable bowel syndrome: evidence of a stress-related impairment in visuospatial memory. Psychol Med. 2014;44:1553–66.
Tanaka Y, Kanazawa M, Fukudo S, Drossman DA. Biopsychosocial model of irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:131–9.
Piché M, Arsenault M, Poitras P, Rainville P, Bouin M. Widespread hypersensitivity is related to altered pain inhibition processes in irritable bowel syndrome. Pain 2010;148:49–58.
Wilder-Smith CH. The balancing act: endogenous modulation of pain in functional gastrointestinal disorders. Gut 2011;60:1589–99.
Ringel Y, Ringel-Kulka T. The intestinal microbiota and Irritable Bowel Syndrome. J Clin Gastroenterol. 2015;49(Suppl 1):S56–S59.
Ringel Y. The gut microbiome in irritable bowel syndrome and other functional bowel disorders. Gastroenterol Clin North Am. 2017;46:91–101.
Tap J, Derrien M, Törnblom H, Brazeilles R, Cools-Portier S, Doré J, et al. Identification of an intestinal microbiota signature associated with severity of irritable bowel syndrome. Gastroenterology 2017;152:111–23.e8.
Zhuang X, Xiong L, Li L, Li M, Chen M. Alterations of gut microbiota in patients with irritable bowel syndrome: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2017;32:28–38.
Bennet SMP, Ohman L, Simren M. Gut microbiota as potential orchestrators of irritable bowel syndrome. Gut Liver. 2015;9:318–31.
Lackner JM. The role of psychosocial factors in gastrointestinal disorders. Gut. 2014;33:104–16.
Google Scholar
Grundy L, Erickson A, Brierley SM. Visceral Pain. Annu Rev Physiol. 2019;81:261–84.
Al Omran Y, Aziz Q. Functional brain imaging in gastroenterology: to new beginnings. Nat Rev Gastroenterol Hepatol. 2014;11:565–76.
Greenwood-Van Meerveld B, Prusator DK, Johnson AC. Animal models of visceral pain: pathophysiology, translational relevance and challenges/B. Am J Physiol Gastrointest Liver Physiol. 2015;463:G885–G903.
Keefer L, Ballou SK, Drossman DA, Ringstrom G, Elsenbruch S, Ljótsson B. A Rome Working Team Report on brain-gut behavior therapies for disorders of gut-brain interaction. Gastroenterology 2022;162:300–15.
Mayer EA, Labus JS, Tillisch K, Cole SW, Baldi P. Towards a systems view of IBS. Nat Rev Gastroenterol Hepatol. 2015;12:592–605.
Furness JB, Callaghan BP, Rivera LR, Cho H-J. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol. 2014;817:39–71.
Furness JB The Enteric Nervous System. London, England: Blackwell Publishing; 2005.
Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–94.
Bohórquez DV, Liddle RA. The gut connectome: making sense of what you eat. J Clin Invest. 2015;125:888–90.
Margolis KG, Gershon MD, Bogunovic M. Cellular organization of neuroimmune interactions in the gastrointestinal tract. Trends Immunol. 2016;37:487–501.
Rao M, Gershon MD. The bowel and beyond: the enteric nervous system in neurological disorders. Nat Rev Gastroenterol Hepatol. 2016;13:517–28.
Browning KN, Alberto, Travagli R. Central control of gastrointestinal motility. Curr Opin Endocrinol Diabetes Obes. 2019;26:11–6.
Gershon M. The Second Brain: The Scientific Basis of Gut Instinct and a groundbreaking new understanding of nervous disorders of the stomach and intestine. HarperCollins; 1998.
Furness JB, Stebbing MJ. The first brain: Species comparisons and evolutionary implications for the enteric and central nervous systems. Neurogastroenterol Motil. 2018;30. https://doi.org/10.1111/nmo.13234 .
Kass-Simon G, Pierobon P. Cnidarian chemical neurotransmission, an updated overview. Comp Biochem Physiol A Mol Integr Physiol. 2007;146:9–25.
Westfall JA, Elliott SR, MohanKumar PS, Carlin RW. Immunocytochemical evidence for biogenic amines and immunogold labeling of serotonergic synapses in tentacles of Aiptasia pallida (Cnidaria, Anthozoa). Invertebr Biol. 2005;119:370–8.
Drokhlyansky E, Smillie CS, Van Wittenberghe N, Ericsson M, Griffin GK, Eraslan G, et al. The human and mouse enteric nervous system at single-cell resolution. Cell 2020;182:1606–22.e23.
Seguella L, Gulbransen BD. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat Rev Gastroenterol Hepatol. 2021;18:571–87.
Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, et al. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science 2002;297:1525–31.
Morales-Soto W, Gulbransen BD. Enteric Glia: A new player in abdominal pain. Cell Mol Gastroenterol Hepatol. 2019;7:433–45.
Pasman JA, Chen Z, Smit DJA, Vink JM, Van Den Oever MC, Pattij T, et al. The CADM2 gene and behavior: a phenome-wide scan in UK-Biobank. Behav Genet 2022;52:306–14. https://doi.org/10.1007/s10519-022-10109-8 . 22 July 2022.
Frei JA, Stoeckli ET. SynCAMs – From axon guidance to neurodevelopmental disorders. Mol Cell Neurosci. 2017;81:41–8.
Fu M, Vohra BPS, Wind D, Heuckeroth RO. BMP signaling regulates murine enteric nervous system precursor migration, neurite fasciculation, and patterning via altered Ncam1 polysialic acid addition. Dev Biol. 2006;299:137–50.
Eraslan G, Drokhlyansky E, Anand S, Fiskin E, Subramanian A, Slyper M, et al. Single-nucleus cross-tissue molecular reference maps toward understanding disease gene function. Science 2022;376:eabl4290.
de Vos WM, Tilg H, Van Hul M, Cani PD. Gut microbiome and health: mechanistic insights. Gut 2022;71:1020–32.
Liang G, Bushman FD. The human virome: assembly, composition and host interactions. Nat Rev Microbiol. 2021;19:514–27.
Cao Z, Sugimura N, Burgermeister E, Ebert MP, Zuo T, Lan P. The gut virome: A new microbiome component in health and disease. EBioMedicine 2022;81:104113.
Osadchiy V, Martin CR, Mayer EA. The Gut-brain axis and the microbiome: mechanisms and clinical implications. Clin Gastroenterol Hepatol. 2019;17:322–32.
Martin CR, Osadchiy V, Kalani A, Mayer EA. The brain-gut-microbiome axis. Cell Mol Gastroenterol Hepatol. 2018;6:133–48.
Sandrini S, Aldriwesh M, Alruways M. Microbial endocrinology: host–bacteria communication within the gut microbiome. J Endocrinol 2015;225:R21–R34.
Margolis KG, Cryan JF, Mayer EA. The microbiota-gut-brain axis: from motility to mood. Gastroenterology 2021;160:1486–501.
Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut 2000;47:861–9.
Ford AC, Quigley E, Lacy BE, Lembo AJ, Saito YA, Schiller LR, et al. Effect of antidepressants and psychological therapies, including hypnotherapy, in irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1350–65.
Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56.
Guo CC, Kurth F, Zhou J, Mayer EA, Eickhoff SB, Kramer JH, et al. One-year test-retest reliability of intrinsic connectivity network fMRI in older adults. NeuroImage 2012;61:1471–83.
Bullmore E, Sporns O. The economy of brain network organization. Nat Neurosci Rev. 2012;13:336–49.
Grayson DS, Fair DA. Development of large-scale functional networks from birth to adulthood: A guide to the neuroimaging literature. Neuroimage 2017;160:15–31.
Sporns O, Betzel RF. Modular brain networks. Annu Rev Psychol. 2016;67:613–40.
Mayer EA, Aziz Q, Coen S, Kern M, Labus JS, Lane R, et al. Brain imaging approaches to the study of functional GI disorders: A Rome Working Team Report. Neurogastroenterol Motil. 2009;21:579–96.
Tijms BM P, Series, Willshaw DJ, Lawrie SM. Similarity-based extraction of individual networks from gray matter MRI Scans. Cereb Cortex. 2012;22:1530–41.
Hagmann P, Kurant M, Gigandet X, Thiran P, Wedeen VJ, Meuli R, et al. Mapping human whole-brain structural networks with diffusion MRI. PLoS ONE. 2007;2:e597.
Gupta A, Kilpatrick L, Labus J, Tillisch K, Braun A, Hong J-Y, et al. Early adverse life events and resting state neural networks in patients with chronic abdominal pain: evidence for sex differences. Psychosom Med. 2014;76:404–12.
Naliboff BD, Berman S, Suyenobu B, Labus JS, Chang L, Stains J, et al. Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome patients. Gastroenterology 2006;131:352–65.
Bradford K, Shih W, Videlock EJ, Presson AP, Naliboff BD, Mayer EA, et al. Association between early adverse life events and irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2012;10:385–90.
Dickhaus B, Mayer EA, Firooz N, Stains J, Conde F, Olivas TI, et al. Irritable bowel syndrome patients show enhanced modulation of visceral perception by auditory stress. Am J Gastroenterol. 2003;98:135–43.
Labus JS, Gupta A, Coveleskie K, Tillisch K, Kilpatrick L, Jarcho J, et al. Sex differences in emotion-related cognitive processes in irritable bowel syndrome and healthy control subjects. Pain 2013;154:2088–99.
Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology 2011;140:91–100.
Farmer AD, Aziz Q. Visceral pain hypersensitivity in functional gastrointestinal disorders. Br Med Bull. 2009;91:123–36.
Mayer EA, Berman S, Chang L, Naliboff BD. Sex-based differences in gastrointestinal pain. Eur J Pain. 2004;8:451–63.
Tillisch K. Sex specific alterations in autonomic function among patients with irritable bowel syndrome. Gut 2005;54:1396–401.
Hong J-Y, Kilpatrick LA, Labus JS, Gupta A, Katibian D, Ashe-McNalley C, et al. Sex and disease-related alterations of anterior insula functional connectivity in chronic abdominal pain. J Neurosci. 2014;34:14252–9.
Hong J-Y, Kilpatrick LA, Labus J, Gupta A, Jiang Z, Ashe-Mcnalley C, et al. Patients with chronic visceral pain show sex-related alterations in intrinsic oscillations of the resting brain. J Neurosci. 2013;33:11994–2002.
Kilpatrick LA, Ornitz E, Ibrahimovic H, Treanor M, Craske M, Nazarian M, et al. Sex-related differences in prepulse inhibition of startle in irritable bowel syndrome (IBS). Biol Psychol. 2010;84:272–8.
Martucci KT, MacKey SC. Neuroimaging of pain: human evidence and clinical relevance of central nervous system processes and modulation. Anesthesiology 2018;128:1241–54.
Menon V. Salience network. Brain Mapp: Encycl Ref. 2015;2:597–611.
Hall GBC, Kamath MV, Collins S, Ganguli S, Spaziani R, Miranda KL, et al. Heightened central affective response to visceral sensations of pain and discomfort in IBS. Neurogastroenterol Motil. 2010;22:276–e80.
Elsenbruch S, Rosenberger C, Bingel U, Forsting M, Schedlowski M, Gizewski ER. Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology 2010;139:1310–9.
Elsenbruch S, Rosenberger C, Enck P, Forsting M, Schedlowski M, Gizewski ER. Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: an fMRI study. Gut 2010;59:489–95.
Jarcho JM, Feier NA, Bert A, Labus JA, Lee M, Stains J, et al. Diminished neurokinin-1 receptor availability in patients with two forms of chronic visceral pain. Pain 2013;154:987–96.
Bhatt RR, Gupta A, Labus JS, Zeltzer LK, Tsao JC, Shulman RJ, et al. Altered Brain Structure and Functional Connectivity and Its Relation to Pain Perception in Girls With Irritable Bowel Syndrome. Psychosom Med. 2019;81:146–54.
Bhatt RR, Gupta A, Labus JS, Liu C, Vora PP, Jean S, et al. A neuropsychosocial signature predicts longitudinal symptom changes in women with irritable bowel syndrome. Mol Psychiatry. 2022;27:1774–91.
Kucyi A, Davis KD. The dynamic pain connectome. Trends Neurosci. 2015;38:86–95.
Qi R, Ke J, Joseph Schoepf U, Varga-Szemes A, Milliken CM, Liu C, et al. Topological reorganization of the default mode network in irritable bowel syndrome. Mol Neurobiol. 2016;53:6585–93.
Nisticò V, Rossi RE, D’Arrigo AM, Priori A, Gambini O, Demartini B. Functional neuroimaging in irritable bowel syndrome: a systematic review highlights common brain alterations with functional movement disorders. J Neurogastroenterol Motil. 2022;28:185–203.
Letzen JE, Craggs JG, Perlstein WM, Price DD, Robinson ME. Functional connectivity of the default mode network and its association with pain networks in irritable bowel patients assessed via lidocaine treatment. J Pain. 2013;14:1077–87.
Ellingson BM, Mayer E, Harris RJ, Ashe-Mcnally C, Naliboff BD, Labus JS, et al. Diffusion tensor imaging detects microstructural reorganization in the brain associated with chronic irritable bowel syndrome. Pain 2013;154:1528–41.
Jiang Z, Dinov ID, Labus J, Shi Y, Zamanyan A, Gupta A, et al. Sex-related differences of cortical thickness in patients with chronic abdominal pain. PLoS ONE. 2013;8:e73932.
Piché M, Chen JI, Roy M, Poitras P, Bouin M, Rainville P. Thicker posterior insula is associated with disease duration in women with irritable bowel syndrome (IBS) whereas thicker orbitofrontal cortex predicts reduced pain inhibition in both IBS patients and controls. J Pain. 2013;14:1217–26.
Labus J, Dinov I, Jiang Z, Ashe-McNalley C, Zamanyan A, Shi Y, et al. Irritable Bowel Syndrome in female patients is associated with alterations in structural brain networks. Pain 2014;155:137–49.
Benarroch EE. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc. 1993;68:998–1001.
Lamotte G, Shouman K, Benarroch EE. Stress and central autonomic network. Auton Neurosci. 2021;235:102870.
Napadow V, Sclocco R, Henderson LA. Brainstem neuroimaging of nociception and pain circuitries. PAIN Rep. 2019;4:e745.
Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17:379–89.
Suárez-Pereira I, Llorca-Torralba M, Bravo L, Camarena-Delgado C, Soriano-Mas C, Berrocoso E. The role of the Locus Coeruleus in pain and associated stress-related disorders. Biol Psychiatry. 2022;91:786–97.
Valentino RJ, Van, Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharm. 2008;583:194–203.
Taché Y, Mönnikes H, Bonaz B, Rivier J. Role of CRF in stress-related alterations of gastric and colonic motor function. Ann N. Y Acad Sci. 1993;697:233–43.
Camilleri M, Zhernakova A, Bozzarelli I, D’Amato M. Genetics of irritable bowel syndrome: shifting gear via biobank-scale studies. Nat Rev Gastroenterol Hepatol. 2022;19:689–702.
Sanders KM, Ward SM, Koh SD. Interstitial cells: regulators of smooth muscle function. Physiol Rev. 2014;94:859–907.
Beyder A, Mazzone A, Strege PR, Tester DJ, Saito YA, Bernard CE, et al. Loss-of-function of the voltage-gated sodium channel NaV1.5 (channelopathies) in patients with irritable bowel syndrome. Gastroenterology 2014;146:1659–68.
Petrovska J, Coynel D, Fastenrath M, Milnik A, Auschra B, Egli T, et al. The NCAM1 gene set is linked to depressive symptoms and their brain structural correlates in healthy individuals. J Psychiatr Res. 2017;91:116–23.
Kolkova K, Novitskaya V, Pedersen N, Berezin V, Bock E. Neural cell adhesion molecule-stimulated neurite outgrowth depends on activation of protein Kinase c and the RAS–mitogen-activated protein kinase pathway. J Neurosci. 2000;20:2238–46.
Frei JA, Andermatt I, Gesemann M, Stoeckli ET. The SynCAM synaptic cell adhesion molecules are involved in sensory axon pathfinding by regulating axon-axon contacts. Development 2015;142:e0106–e0106.
Kuramoto K, Negishi M, Katoh H. Regulation of dendrite growth by the Cdc42 activator Zizimin1/Dock9 in hippocampal neurons. J Neurosci Res. 2009;87:1794–805.
Pappa S, Padilla N, Iacobucci S, Vicioso M, Álvarez de la Campa E, Navarro C, et al. PHF2 histone demethylase prevents DNA damage and genome instability by controlling cell cycle progression of neural progenitors. Proc Natl Acad Sci USA. 2019;116:19464–73.
Shi L. Dock protein family in brain development and neurological disease. Commun Integr Biol. 2013;6:e26839.
Fitzcharles MA, Cohen SP, Clauw DJ, Littlejohn G, Usui C, Häuser W. Nociplastic pain: towards an understanding of prevalent pain conditions. Lancet 2021;397:2098–110.
Nijs J, George SZ, Clauw DJ, Fernández-de-las-Peñas C, Kosek E, Ickmans K, et al. Central sensitisation in chronic pain conditions: latest discoveries and their potential for precision medicine. Lancet Rheumatol 2021;3:e383–e392.
Midenfjord I, Grinsvall C, Koj P, Carnerup I, Törnblom H, Simrén M. Central sensitization and severity of gastrointestinal symptoms in irritable bowel syndrome, chronic pain syndromes, and inflammatory bowel disease. Neurogastroenterol Motil. 2021;33:e14156.
Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature 1983;306:686–8.
Latremoliere A, Woolf CJ. Central SENSITIZATION: A GENERATOR OF PAIN HYPERSENSITIVITY BY CENTRAL NEURAL PLASTicity. J Pain. 2009;10:895–926.
Verne NG, Himes NC, Robinson ME, Gopinath KS, Briggs RW, Crosson B, et al. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain 2003;103:99–110.
Wilder-Smith CH, Robert-Yap J. Abnormal endogenous pain modulation and somatic and visceral hypersensitivity in female patients with irritable bowel syndrome. World J Gastroenterol. 2007;13:3699–704.
Caldarella MP, Giamberardino MA, Sacco F, Affaitati G, Milano A, Lerza R, et al. Sensitivity disturbances in patients with irritable bowel syndrome and fibromyalgia. Am J Gastroenterol. 2006;101:2782–9.
Iimura S, Takasugi S, Division R, Co M Hsp and gastrointestinal disease symptoms. https://psyarxiv.com/n2c39/download?format=pdf . Accessed 7 August 2022.
Mocci E, Ward K, Dorsey SG, Ament SA, GWAS meta-analysis reveals dual neuronal and immunological etiology for pain susceptibility. medRxiv. 2021:2021.08.23.21262510.
McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain 2003;106:127–33.
Tang J, Gibson SJ. A psychophysical evaluation of the relationship between trait anxiety, pain perception, and induced state anxiety. J Pain. 2005;6:612–9.
Clark JR, Nijs J, Yeowell G, Holmes P, Goodwin PC. Trait sensitivity, anxiety, and personality are predictive of central sensitization symptoms in patients with chronic low back pain. Pain Pr. 2019;19:800–10.
Shigetoh H, Tanaka Y, Koga M, Osumi M, Morioka S. The mediating effect of central sensitization on the relation between pain intensity and psychological factors: a cross-sectional study with mediation analysis. Pain Res Manag. 2019;2019:3916135.
Adams LM, Turk DC. Psychosocial factors and central sensitivity syndromes. Curr Rheumatol Rev. 2015;11:96–108.
Aron EN, Aron A. Sensory-processing sensitivity and its relation to introversion and emotionality. J Pers Soc Psychol. 1997;73:345–68.
Boyce WT. Differential susceptibility of the developing brain to contextual adversity and stress. Neuropsychopharmacology 2016;41:142–62.
Meerwijk EL, Ford JM, Weiss SJ. Brain regions associated with psychological pain: implications for a neural network and its relationship to physical pain. Brain Imaging Behav. 2013;7:1–14.
Elman I, Borsook D. Threat response system: parallel brain processes in pain vis-à-vis fear and anxiety. Front Psychiatry. 2018;9:29.
Belujon P, Grace AA. Regulation of dopamine system responsivity and its adaptive and pathological response to stress. Proc Biol Sci. 2015;282:20142516.
PubMed PubMed Central Google Scholar
Baliki MN, Apkarian AV. Nociception, pain, negative moods, and behavior selection. Neuron 2015;87:474–91.
Zouikr I, Bartholomeusz MD, Hodgson DM. Early life programming of pain: focus on neuroimmune to endocrine communication. J Transl Med. 2016;14:123.
Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, Mccarthy MM, et al. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68:314–9.
Verriotis M, Chang P, Fitzgerald M, Fabrizi L. Development of the Nociceptive brain. Neuroscience 2016;338:207–19.
Slater R, Fabrizi L, Worley A, Meek J, Boyd S, Fitzgerald M. Premature infants display increased noxious-evoked neuronal activity in the brain compared to healthy age-matched term-born infants. Neuroimage 2010;52:583–9.
Van Den Bosch GE, White T, El Marroun H, Simons SHP, Van Der Lugt A, Van Der Geest JN, et al. Prematurity, Opioid exposure and neonatal pain: do they affect the developing brain? Neonatology 2015;108:8–15.
Ranger M, Chau CMY, Garg A, Woodward TS, Beg MF, Bjornson B, et al. Neonatal pain-related stress predicts cortical thickness at age 7 years in children born very preterm. PLoS One. 2013;8:e76702.
Nemeroff CB. Neurobiological consequences of childhood trauma. J Clin Psychiatry. 2004;65(Suppl 1):18–28.
Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–39.
Lippard ETC, Nemeroff CB. The devastating clinical consequences of child abuse and neglect: increased disease vulnerability and poor treatment response in mood disorders. Am J Psychiatry. 2020;177:20–36.
Coley EJL, Hsiao EY. Malnutrition and the microbiome as modifiers of early neurodevelopment. Trends Neurosci. 2021;44:753–64.
Ratsika A, Codagnone MC, O’Mahony S, Stanton C, Cryan JF. Priming for life: early life nutrition and the microbiota-gut-brain axis. Nutrients 2021;13:423.
Andresen V, Montori VM, Keller J, West CP. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in non constipated irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Clin Biomed Res. 2008;6:545–55.
Costall B, Naylor RJ. Anxiolytic potential of 5-HT3 receptor antagonists. Pharm Toxicol. 1992;70:157–62.
Fakhfouri G, Rahimian R, Dyhrfjeld-Johnsen J, Zirak MR, Beaulieu J-M. 5-HT3 receptor antagonists in neurologic and neuropsychiatric disorders: the iceberg still lies beneath the surface. Pharm Rev. 2019;71:383–412.
Olivier B, van Wijngaarden I, Soudijn W. 5-HT(3) receptor antagonists and anxiety; a preclinical and clinical review. Eur Neuropsychopharmacol. 2000;10:77–95.
Hubbard CS, Labus JS, Bueller J, Stains J, Suyenobu B, Dukes GE, et al. Corticotropin-releasing factor receptor 1 antagonist alters regional activation and effective connectivity in an emotional-arousal circuit during expectation of abdominal pain. J Neurosci. 2011;31:12491–500.
Labus JS, Hubbard CS, Bueller J, Ebrat B, Tillisch K, Chen M, et al. Impaired emotional learning and involvement of the corticotropin-releasing factor signaling system in patients with irritable bowel syndrome. Gastroenterology 2013;145:1253–61.e3.
Lowén MBO, Mayer EA, Sjöberg M, Tillisch K, Naliboff B, Labus J, et al. Effect of hypnotherapy and educational intervention on brain response to visceral stimulusin the irritable bowel syndrome. Aliment Pharm Ther. 2013;37:1184–97.
Rutten JMTM, Reitsma JB, Vlieger AM, Benninga MA. Gut-directed hypnotherapy for functional abdominal pain or irritable bowel syndrome in children: a systematic review. Arch Dis Child. 2013;98:252–7.
Rutten JMTM, Vlieger AM, Frankenhuis C, George EK, Groeneweg M, Norbruis OF, et al. Home-based hypnotherapy self-exercises vs individual hypnotherapy with a therapist for treatment of pediatric irritable bowel syndrome, functional abdominal pain, or functional abdominal pain syndrome. JAMA Pediatr. 2017;171:470.
Peters SL, Muir JG, Gibson PR. Review article: gut-directed hypnotherapy in the management of irritable bowel syndrome and inflammatory bowel disease. Aliment Pharm Ther. 2015;41:1104–15.
Naliboff BD, Smith SR, Serpa JG, Laird KT, Stains J, Connolly LS, et al. Mindfulness-based stress reduction improves irritable bowel syndrome (IBS) symptoms via specific aspects of mindfulness. Neurogastroenterol Motil. 2020;32:e13828.
Jacobs JP, Gupta A, Bhatt RR, Brawer J, Gao K, Tillisch K, et al. Cognitive behavioral therapy for irritable bowel syndrome induces bidirectional alterations in the brain-gut-microbiome axis associated with gastrointestinal symptom improvement. Microbiome 2021;9:236.
Lackner JM, Keefer L, Jaccard J, Firth R, Brenner D, Bratten J, et al. The Irritable Bowel Syndrome Outcome Study (IBSOS): Rationale and design of a randomized, placebo-controlled trial with 12 month follow up of self-versus clinician-administered CBT for moderate to severe irritable bowel syndrome. Contemp Clin Trials. 2012;33:1293–310.
Edebol-Carlman H, Ljótsson B, Linton SJ, Boersma K, Schrooten M, Repsilber D, et al. Face-to-face cognitive-behavioral therapy for irritable bowel syndrome: the effects on gastrointestinal and psychiatric symptoms. Gastroenterol Res Pr. 2017;2017:8915872 https://doi.org/10.1155/2017/8915872 .
Owusu JT, Sibelli A, Moss-Morris R, van Tilburg MAL, Levy RL, Oser M. A pilot feasibility study of an unguided, internet-delivered cognitive behavioral therapy program for irritable bowel syndrome. Neurogastroenterol Motil. 2021;33:e14108.
Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharm Ther. 2018;48:1044–60.
Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–8.
Raichle ME, Macleod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–82.
Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76.
Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N. Y Acad Sci. 2008;1124:1–38.
Northoff G. Anxiety disorders and the brain’s resting state networks: from altered spatiotemporal synchronization to psychopathological symptoms. Adv Exp Med Biol. 2020;1191:71–90.
Kim Y-K, Yoon H-K. Common and distinct brain networks underlying panic and social anxiety disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2018;80:115–22.
MacNamara A, DiGangi J, Phan KL. Aberrant spontaneous and task-dependent functional connections in the anxious brain. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:278–87.
Kolesar TA, Bilevicius E, Wilson AD, Kornelsen J. Systematic review and meta-analyses of neural structural and functional differences in generalized anxiety disorder and healthy controls using magnetic resonance imaging. NeuroImage: Clin. 2019;24:102016.
ten Donkelaar HJ, Broman J, van Domburg P The Somatosensory System. In: ten Donkelaar HJ, editor. Clinical Neuroanatomy: Brain Circuitry and Its Disorders, Cham: Springer International Publishing; 2020. p. 171–255.
Woodworth D, Mayer E, Leu K, Ashe-McNalley C, Naliboff BD, Labus JS, et al. Unique microstructural changes in the brain associated with Urological Chronic Pelvic Pain Syndrome (UCPPS) revealed by diffusion tensor MRI, super-resolution track density imaging, and statistical parameter mapping: A MAPP network neuroimaging study. PLoS One. 2015;10:e0140250.
Grinsvall C, Ryu HJ, Van Oudenhove L, Labus JS, Gupta A, Ljungberg M, et al. Association between pain sensitivity and gray matter properties in the sensorimotor network in women with irritable bowel syndrome. Neurogastroenterol Motil. 2020;33:e14027.
Bouziane I, Das M, Friston KJ, Caballero-Gaudes C, Ray D. Enhanced top-down sensorimotor processing in somatic anxiety. Transl Psychiatry. 2022;12:295.
Brandl F, Weise B, Mulej Bratec S, Jassim N, Hoffmann Ayala D, Bertram T, et al. Common and specific large-scale brain changes in major depressive disorder, anxiety disorders, and chronic pain: a transdiagnostic multimodal meta-analysis of structural and functional MRI studies. Neuropsychopharmacology 2022;47:1071–80.
Berman SM, Chang L, Suyenobu B, Derbyshire SW, Stains J, FitzGerald L, et al. Condition-specific deactivation of brain regions by 5-HT3 receptor antagonist Alosetron. Gastroenterology 2002;123:969–77.
Tillisch K, Labus J, Nam B, Bueller J, Smith S, Suyenobu B, et al. Neurokinin-1-receptor antagonism decreases anxiety and emotional arousal circuit response to noxious visceral distension in women with irritable bowel syndrome: a pilot study. Aliment Pharm Ther. 2012;35:360–7.
Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci. 2013;14:488–501.
Hong J-Y, Naliboff BD, Labus JS, Kilpatrick LA, Fling C, Ashe-McNalley C, et al. Sa2014 IBS patients show altered brain responses during uncertain, but not certain expectation of painful stimulation of the abdominal wall. Gastroenterology 2015;148:S–384.
Sylvester CM, Corbetta M, Raichle ME, Rodebaugh TL, Schlaggar BL, Sheline YI, et al. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 2012;35:527–35.
Pessoa L. A network model of the emotional brain. Trends Cogn Sci. 2017;21:357–71.
Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, et al. A validated network of effective amygdala connectivity. Neuroimage 2007;36:736–45.
Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–34.
Labus JS, Mayer EA, Jarcho J, Kilpatrick LA, Kilkens TOC, Evers EAT, et al. Acute tryptophan depletion alters the effective connectivity of emotional arousal circuitry during visceral stimuli in healthy women. Gut 2011;60:1196–203.
Yágüez L, Coen S, Gregory LJ, Amaro E, Altman C, Brammer MJ, et al. Brain response to visceral aversive conditioning: a functional magnetic resonance imaging study. Gastroenterology 2005;128:1819–29.
Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Ohning G, et al. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with Irritable Bowel Syndrome. J Neurosci. 2008;28:349–59.
Kilpatrick LA, Labus JS, Coveleskie K, Hammer C, Rappold G, Tillisch K, et al. The HTR3A Polymorphism c. -42C>T is associated with amygdala responsiveness in patients with irritable bowel syndrome. Gastroenterology. 2011;140:1943–51.
Harrewijn A, Cardinale EM, Groenewold NA, Bas-Hoogendam JM, Aghajani M, Hilbert K, et al. Cortical and subcortical brain structure in generalized anxiety disorder: findings from 28 research sites in the ENIGMA-Anxiety Working Group. Transl Psychiatry. 2021;11:502.
Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–8.
Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–42.
Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12:241–68.
Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506.
Afzal M, Potokar JP, Probert CSJ, Munafò MR. Selective processing of gastrointestinal symptom-related stimuli in irritable bowel syndrome. Psychosom Med. 2006;68:758–61.
Gibbs-Gallagher N, Palsson OS, Levy RL, Meyer K, Drossman DA, Whitehead WE. Selective recall of gastrointestinal-sensation words: evidence for a cognitive-behavioral contribution to irritable bowel syndrome. Am J Gastroenterol. 2001;96:1133–8.
Phillips K, Wright BJ, Kent S. Irritable bowel syndrome and symptom severity: Evidence of negative attention bias, diminished vigour, and autonomic dysregulation. J Psychosom Res. 2014;77:13–9.
Tkalcic M, Domijan D, Pletikosic S, Setic M, Hauser G. Attentional biases in irritable bowel syndrome patients. Clin Res Hepatol Gastroenterol. 2014;38:621–8.
Labus JS, Naliboff BD, Berman SM, Suyenobu B, Vianna EP, Tillisch K, et al. Brain networks underlying perceptual habituation to repeated aversive visceral stimuli in patients with irritable bowel syndrome. NeuroImage 2009;47:952–60.
Seminowicz DA, Shpaner M, Keaser ML, Michael Krauthamer G, Mantegna J, Dumas JA, et al. Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J Pain. 2013;14:1573–84.
Blankstein U, Chen J, Diamant NE, Davis KD. Altered brain structure in irritable bowel syndrome: potential contributions of pre-existing and disease-driven factors. Gastroenterology 2010;138:1783–9.
Qiu C, Liao W, Ding J, Feng Y, Zhu C, Nie X, et al. Regional homogeneity changes in social anxiety disorder: a resting-state fMRI study. Psychiatry Res. 2011;194:47–53.
Naliboff BD, Berman S, Chang L, Derbyshire SWG, Suyenobu B, Vogt BA, et al. Sex-related differences in IBS patients: central processing of visceral stimuli. Gastroenterology 2003;124:1738–47.
Nagel M, Jansen PR, Stringer S, Watanabe K, De Leeuw CA, Bryois J, et al. Meta-analysis of genome-wide association studies for neuroticism in 449,484 individuals identifies novel genetic loci and pathways. Nat Genet. 2018;50:920–7.
Ko H-G, Choi J-H, Park DI, Kang SJ, Lim C-S, Sim S-E, et al. Rapid turnover of Cortical NCAM1 regulates synaptic reorganization after peripheral nerve injury. Cell Rep. 2018;22:748–59.
Ao W, Cheng Y, Chen M, Wei F, Yang G, An Y, et al. Intrinsic brain abnormalities of irritable bowel syndrome with diarrhea: a preliminary resting-state functional magnetic resonance imaging study. BMC Med Imaging. 2021;21:4.
Chen A, Chen Y, Tang Y, Bao C, Cui Z, Xiao M, et al. Hippocampal AMPARs involve the central sensitization of rats with irritable bowel syndrome. Brain Behav. 2017;7:e00650.
Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci. 2016;17:652–66.
Kim H-J, Hur SW, Park JB, Seo J, Shin JJ, Kim S-Y, et al. Histone demethylase PHF2 activates CREB and promotes memory consolidation. EMBO Rep. 2019;20:e45907.
Wei F, Xu ZC, Qu Z, Milbrandt J, Zhuo M. Role of EGR1 in hippocampal synaptic enhancement induced by tetanic stimulation and amputation. J Cell Biol. 2000;149:1325–34.
Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, et al. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 2001;21:9896–903.
Fagerberg L, Hallström BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteom. 2014;13:397–406.
Lee J-G, Ye Y. Bag6/Bat3/Scythe: a novel chaperone activity with diverse regulatory functions in protein biogenesis and degradation. Bioessays 2013;35:377–85.
Kawahara H, Minami R, Yokota N. BAG6/BAT3: emerging roles in quality control for nascent polypeptides. J Biochem. 2013;153:147–60.
Binici J, Koch J. BAG-6, a jack of all trades in health and disease. Cell Mol Life Sci. 2014;71:1829–37.
Case CM, Sackett DL, Wangsa D, Karpova T, McNally JG, Ried T, et al. CKAP2 ensures chromosomal stability by maintaining the integrity of microtubule nucleation sites. PLoS One. 2013;8:e64575.
Zhang S, Wang Y, Chen S, Li J. Silencing of cytoskeleton-associated protein 2 represses cell proliferation and induces cell cycle arrest and cell apoptosis in osteosarcoma cells. Biomed Pharmacother. 2018;106:1396–403.
Download references
Author information
Authors and affiliations.
G. Oppenheimer Center for Neurobiology of Stress and Resilience, Departments of Medicine, Psychiatry and Physiology, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA
Emeran A. Mayer
A.T. Still University School of Osteopathic Medicine in Arizona, Meza, AZ, USA
Hyo Jin Ryu
Imaging Genetics Center, Mark and Mary Stevens Neuroimaging and Informatics Institute, Keck School of Medicine at USC, University of Southern California, Los Angeles, CA, USA
Ravi R. Bhatt
You can also search for this author in PubMed Google Scholar
Contributions
EAM conceptualized the manuscript and played a major role in the writing process and critically reviewed the final manuscript. RRB did an extensive literature review, played a major role in the writing process, and critically reviewed the final manuscript. HJR did an extensive literature review, created the main table, and critically reviewed the final manuscript.
Corresponding author
Correspondence to Emeran A. Mayer .
Ethics declarations
Competing interests.
EAM is a member of the scientific advisory boards of Danone, Axial Therapeutics, Amare, Mahana Therapeutics, Pendulum, Bloom Biosciences, and APC Microbiome Ireland.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Mayer, E.A., Ryu, H.J. & Bhatt, R.R. The neurobiology of irritable bowel syndrome. Mol Psychiatry 28 , 1451–1465 (2023). https://doi.org/10.1038/s41380-023-01972-w
Download citation
Received : 02 December 2022
Revised : 06 January 2023
Accepted : 17 January 2023
Published : 02 February 2023
Issue Date : April 2023
DOI : https://doi.org/10.1038/s41380-023-01972-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Neural circuits regulating visceral pain.
- Xiaoli Chang
- Haiyan Zhang
- Shaozong Chen
Communications Biology (2024)
Non-invasive neuromodulation: an emerging intervention for visceral pain in gastrointestinal disorders
- Md Jahangir Alam
- Jiande D. Z. Chen
Bioelectronic Medicine (2023)
Evaluation of the diagnostic performance of estimated fecal calprotectin and serum intelectin-1 and C-reactive protein solo or in combination for differentiation between patients with query ulcerative colitis and irritable bowel syndrome
- Rizk Sayad R. Sarhan
- Yasmin M. Marei
- Yomna M. Marei
The Egyptian Journal of Internal Medicine (2023)
A CNS circuit that regulates gut motility
- Lisa R. Beutler
Nature Metabolism (2023)
Neuropathic Pain and Spinal Cord Injury: Management, Phenotypes, and Biomarkers
- Eva Widerström-Noga
Drugs (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

Mediterranean diet could help people with irritable bowel symptoms
N ew research looking at the benefits of a Mediterranean diet for people with irritable bowel syndrome (IBS) has yielded surprising results.
Not only did the diet, rich in fruits, vegetables and legumes, improve the mental health of the study participants, but their gastrointestinal symptoms improved, as well.
Dr. Heidi Staudacher, National Health and Medical Research Council Emerging Leadership Fellow at Deakin University's Food & Mood Center, said it was common for people with IBS to avoid some of the foods important in a Mediterranean diet as they are known to trigger a worsening of symptoms.
"Previously we had an understanding that foods such as legumes, certain whole grains and onion, can worsen gut symptoms in some people," Dr. Staudacher said.
"This research suggests there might be a new way to help reduce the burden of IBS symptoms that doesn't focus on cutting out foods that are known to be important for good health."
Dr. Staudacher's research , published in Alimentary Pharmacology & Therapeutics , measured outcomes of 59 people over six weeks who were either following the Mediterranean diet via counseling from a dietitian or eating their usual diet (control group).
"Previous research has shown the Mediterranean diet improves depressive symptoms and we wanted to see whether this type of diet was possible for people with IBS, and also whether it would improve both depressive and gut symptoms in people with IBS," Dr. Staudacher said.
"Many people with IBS also have mental health problems like anxiety and depression. Given the known gut-brain connection, it is plausible that if we can improve people's mental health this might then lead to improvements in the gut symptoms that people with IBS live with."
The study found:
- 83% of participants on the Mediterranean diet had a reduction in their IBS-SSS score (gut symptom severity score) throughout the trial compared with only 37% in the control group,
- Depressive symptoms were lower in the Mediterranean diet compared with controls at the end of the study. This is in line with other research using a Mediterranean diet in people with depression,
- Somewhat surprisingly, gastrointestinal symptoms were also lower in the Mediterranean diet group compared with controls.
"These findings suggest we may be able to look beyond current dietary advice for people with IBS and encourage a broadly health-promoting diet to help manage their symptoms. We now urgently need to conduct a bigger study that compares a Mediterranean diet to a better control diet to give us more clarity about its effect on gut and psychological symptoms," Dr. Staudacher said.
"Dietitians will also need to be involved, to help people increase high fiber and high FODMAP foods gradually into their diets to avoid triggering gut symptoms."
More information: Heidi M. Staudacher et al, Clinical trial: A Mediterranean diet is feasible and improves gastrointestinal and psychological symptoms in irritable bowel syndrome, Alimentary Pharmacology & Therapeutics (2023). DOI: 10.1111/apt.17791
Provided by Deakin University

An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Irritable bowel syndrome.
Nicolas Patel ; Karen B. Shackelford .
Affiliations
Last Update: October 30, 2022 .
- Continuing Education Activity
Irritable bowel syndrome (IBS) is one of the most commonly diagnosed gastrointestinal diseases. IBS, in the absence of any other causative disease, is defined as the presence of abdominal pain or discomfort with altered bowel habits. Diagnosis of IBS has evolved since its first discovery, and today the Rome IV diagnostic criteria are used to diagnose IBS. Depending on the subclass of IBS, symptoms can be managed by a variety of medications and nonpharmaceutical agents. Nonetheless, IBS treatment should be individualized, and a significant factor in management remains a strong patient-clinician relationship. This activity reviews the evaluation and management of and highlights the role of the interprofessional team in the recognition and management of this condition.
- Describe the recommended management of irritable bowel syndrome (IBS).
- Outline the typical presentation for a patient with irritable bowel syndrome (IBS).
- Review the pathophysiology of irritable bowel syndrome (IBS).
- Explain the interprofessional team strategies for improving care coordination and communication regarding the management of patients with IBS.
- Introduction
Irritable bowel syndrome (IBS) is one of the most commonly diagnosed gastrointestinal diseases. IBS, in the absence of any other causative disease, is defined as the presence of abdominal pain or discomfort with altered bowel habits. Diagnosis of IBS has evolved since its first discovery, and today the Rome IV diagnostic criteria are used to diagnose IBS. Depending on the subclass of IBS, symptoms can be managed by a variety of medications and nonpharmaceutical agents. Nonetheless, IBS treatment should be individualized, and a significant factor in management remains a strong patient-physician relationship. [1]
The etiology of IBS is broad and not clearly understood. However, as below in the pathophysiology section, motility, visceral sensation, brain-gut interaction, and psychosocial distress can all play a role in the development of IBS.
- Epidemiology
Nearly 12 percent of patients seek medical care in primary care practices for IBS related complaints. [1] [2] Studies have demonstrated that the prevalence of IBS ranges between ten and fifteen percent; however, the majority of these patients do not seek medical care. [1] IBS is most prevalent in South America at approximately 21 percent and least prevalent in Southeast Asia at 7 percent. [3] [4] In the United States, Canada, and Isreal, IBS symptoms are 1.5 to 2 times more prevalent among women than men. [5] Moreover, women are more likely to report abdominal pain and constipation, whereas men are more likely to report diarrhea. [5] The prevalence of IBS also decreases with age. [3] IBS can also be broken down into more specific diagnoses, which include IBS with diarrhea (IBS-D), IBS with constipation (IBS-C), and IBS with mixed bowel patterns (IBS-M). The prevalence of these three diagnoses differs in the United States versus Europe. In the United States, there is an equal distribution of these diagnoses, whereas, in Europe, IBS-C or IBS-M can be more prevalent. [6]
- Pathophysiology
The pathophysiology of IBS is broad and includes abnormalities involving motility, visceral sensation, brain-gut interaction, and psychosocial distress. [3] One of these can usually be demonstrated in the majority of IBS patients; however, not all symptoms can be attributed to them. [3] Recent studies have also shown altered gut immune activation and intestinal and colonic microbiome are associated with IBS [3] [7] [8] . Environmental contributors to IBS include early life stressors, food intolerance, antibiotics, and enteric infections. [3] Patients often complain that IBS symptoms are related to food intake. However, a true food allergen has a limited contribution to IBS. [3] [9]
- Histopathology
Histopathology examination of the intestinal mucosa in those with IBS can show chronic inflammatory cells, mast cells, enteroendocrine cells, and enteric nerves. [10] . IBS-D is typically associated with a greater increase in mucosal T-lymphocytes than IBS-C. [10] [11] Moreover, there can be an increased number of nerve fibers that stain positive for neuron-specific enolase, substance P, and 5-HT. [10] [12] There also appears to be a significantly increased density of nerve fibers around mast cells. [10] [12]
- History and Physical
IBS typically consists of abdominal pain or discomfort, altered bowel habits along with constipation, diarrhea, or both. Other complaints in patients with IBS include bloating, distention, symptoms brought on by food intake, and a change in pain location and stool pattern with time. [3] . Concerning features would include onset after the age of 50 years old, severe or progressive symptoms, unexplained weight loss, nocturnal diarrhea, rectal bleeding, iron deficiency anemia, or a family history of organic gastrointestinal diseases such as colon cancer, celiac disease, or inflammatory bowel disease. [3] Additional history that would be important would include travel and social history.
The Rome IV criteria are used to diagnose IBD, which requires at least 3 days a month in the last 3 months associated with 2 or more of the following: improvement in abdominal pain or discomfort with defecation, onset associated with a change in frequency of stool, and/or an onset accompanied by a change in form or appearance of stool [3] .
If no alarm findings exist (weight loss, hematochezia, iron deficiency), routine diagnostic testing is not recommended. [1] If symptoms are not typical of IBS or alarm symptoms are present, then a complete blood cell count, comprehensive metabolic panel, inflammatory markers such as erythrocyte sedimentation rate or C-reactive protein, and thyroid stimulating hormone level should be checked. [1] If diarrhea is predominant, fecal leukocytes and stool tests for Clostridium difficile, Giardia, and Cryptosporidium, when appropriate, should be ordered. [1] Testing for celiac disease may be needed as well, and a tissue transglutaminase or TTG-IgA can be obtained. [1] A colonoscopy may be beneficial when there is a family history of inflammatory bowel disease, colon cancer, or alarm symptoms. [1] If the patient has diarrhea, random biopsies should be done on colonoscopy. [1]
- Treatment / Management
One of the most important goals in the management of IBS patients is to develop a trusting patient-physician relationship by actively listening, showing empathy, and setting realistic expectations for treatment. [3] [13] IBS is a symptom-based disorder, and thus treatment goals are aimed at resolving symptoms such as pain, bloating, cramping, and diarrhea or constipation. [3] For constipation, fiber supplements and laxatives can be helpful whereas, in those with diarrhea, medications such as loperamide or probiotics can be helpful. [3] Moreover, increased physical activity can increase colonic transit time and improve symptoms. [3] [14] Patients also often associate food intake with IBS symptoms. Foods such as wheat products, onions, fruits, vegetables, sorbitol, and some dairy can include short-chain, poorly absorbed, highly fermentable carbohydrates, which are known as FODMAPs. FODMAPs have been associated with increased gastrointestinal symptoms in IBS patients. [3] Patients with constant and chronic abdominal symptoms oftentimes can have a response to low dose tricyclic antidepressants (TCAs) or serotonin reuptake inhibitors (SSRIs). [1] Alosetron can be used to treat IBS-D in females but can cause ischemic colitis.
Rifaximin is a nonabsorbable broad-spectrum antibiotic sometimes used to treat patients with irritable bowel syndrome. Patients with irritable bowel syndrome who take rifaximin have less abdominal pain and diarrhea. The effectiveness of rifaximin in patients with IBS is used as evidence to support the idea that bacterial overgrowth plays a role in the etiology of IBS.
- Differential Diagnosis
The differential diagnosis of IBS is broad and ultimately depends on whether the patient has predominant diarrhea or constipation. If a patient has IBS with diarrhea, the differentials includes lactose intolerance, caffeine intake, alcohol intake, gastrointestinal infections (Giardia, Amoeba, HIV), inflammatory bowel disease, medication-induced diarrhea (antibiotic use, proton pump inhibitor, nonsteroidal anti-inflammatory drugs, ACE inhibitor, chemotherapy), celiac disease, malignancies, colorectal cancer, hyperthyroidism, VIPoma, and ischemic colitis. [15] If constipation is the predominant symptoms, then the differentials can include inadequate fiber intake, immobility, Parkinson's disease, multiple sclerosis, spinal injury, diabetes, hypothyroidism, hypercalcemia, medication-induced (opiates, calcium-channel blockers, antidepressants, clonidine), malignancies, bowel obstruction, endometriosis, and diverticular disease. If a patient's history indicates one of these diseases, then appropriate lab testing should be pursued. [15]
IBS has a good prognosis, and the diagnosis is unlikely to change on follow-up. [16] The use of ambulatory health services by IBS patients can be reduced if a positive physician-patient interaction is developed. [16]
- Consultations
Important consults include a gastroenterologist and a nutritionist. Gastroenterologists often sub-specialize in IBS care and are invaluable members of the treatment team. A gastroenterologist can tailor treatment plans for the patient and are also likely to be more aware of advancements in the field of IBS. Given that patients often believe certain food intake is associated with their symptoms, and the findings of FODMAPs association with IBS, nutritionists are vital to providing dietary recommendations for the patient.
- Deterrence and Patient Education
If a patient has concerns about abdominal pain, bloating, cramping, and changes in bowel habits, a visit to a primary care physician is advised. If IBS is diagnosed, a gastroenterology consultation will be needed as they can guide management and treatment.
- Pearls and Other Issues
IBS can be sub-classified into IBS-C, IBS-D, or IBS-M. Although some treatments are the same between the groups, each subclassification is unique and has different treatments focused on the different symptomatologies.
- Enhancing Healthcare Team Outcomes
IBS can be a very disabling syndrome for patients, and it has been shown to be a very common reason for seeking medical attention. It is vital that there is an interprofessional approach when it comes to the care of these patients as it can improve the quality of life, reduce medications needed, and manage the symptoms of IBS much better. [17] The team can include primary care providers, gastroenterologists, specialty-trained nurses, and pharmacists. Nurses provide education to patients and their families, monitor response to treatment, and report patient status to the team. Pharmacists review prescribed medications for dose and interactions, as well as review usage and side effects with patients. [Level 5]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Nicolas Patel declares no relevant financial relationships with ineligible companies.
Disclosure: Karen Shackelford declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Patel N, Shackelford KB. Irritable Bowel Syndrome. [Updated 2022 Oct 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Are bowel symptoms and psychosocial features different in irritable bowel syndrome patients with abdominal discomfort compared to abdominal pain? [World J Gastroenterol. 2022] Are bowel symptoms and psychosocial features different in irritable bowel syndrome patients with abdominal discomfort compared to abdominal pain? Fang XC, Fan WJ, Drossman DD, Han SM, Ke MY. World J Gastroenterol. 2022 Sep 7; 28(33):4861-4874.
- Review Rome Criteria and a Diagnostic Approach to Irritable Bowel Syndrome. [J Clin Med. 2017] Review Rome Criteria and a Diagnostic Approach to Irritable Bowel Syndrome. Lacy BE, Patel NK. J Clin Med. 2017 Oct 26; 6(11). Epub 2017 Oct 26.
- Comparisons of the Rome III and Rome IV criteria for diagnosis of irritable bowel syndrome in Indian and Bangladeshi communities and internal shifts in the diagnostic categories of bowel disorders of gut-brain interactions. [Neurogastroenterol Motil. 2023] Comparisons of the Rome III and Rome IV criteria for diagnosis of irritable bowel syndrome in Indian and Bangladeshi communities and internal shifts in the diagnostic categories of bowel disorders of gut-brain interactions. Ghoshal UC, Rahman MM, Pratap N, Misra A, Sarker SA, Hasan M, Bangdiwala SI, Palsson OS, Sperber AD. Neurogastroenterol Motil. 2023 Jun; 35(6):e14579. Epub 2023 Apr 3.
- Natural History and Disease Impact of Rome IV Vs Rome III Irritable Bowel Syndrome: A Longitudinal Follow-Up Study. [Clin Gastroenterol Hepatol. 2022] Natural History and Disease Impact of Rome IV Vs Rome III Irritable Bowel Syndrome: A Longitudinal Follow-Up Study. Goodoory VC, Houghton LA, Yiannakou Y, Black CJ, Ford AC. Clin Gastroenterol Hepatol. 2022 Mar; 20(3):569-577.e3. Epub 2021 May 3.
- Review Irritable bowel syndrome: update on pathogenesis and management. [Med Princ Pract. 2002] Review Irritable bowel syndrome: update on pathogenesis and management. Alaradi O, Barkin JS. Med Princ Pract. 2002 Jan-Mar; 11(1):2-17.
Recent Activity
- Irritable Bowel Syndrome - StatPearls Irritable Bowel Syndrome - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Food & Function
Research progress in the treatment of inflammatory bowel disease with natural polysaccharides and related structure–activity relationships.

* Corresponding authors
a Affiliated Hospital, Changchun University of Chinese Medicine, Changchun, China E-mail: [email protected]
b College of Pharmacy, Changchun University of Chinese Medicine, Changchun, China
Inflammatory bowel disease (IBD) comprises a group of highly prevalent and chronic inflammatory intestinal tract diseases caused by multiple factors. Despite extensive research into the causes of the disease, IBD's pathogenic mechanisms remain unclear. Moreover, side effects of current IBD therapies restrict their long-term clinical use. In contrast, natural polysaccharides exert beneficial anti-IBD effects and offer advantages over current anti-IBD drugs, including enhanced safety and straightforward isolation from abundant and reliable sources, and thus may serve as components of functional foods and health products for use in IBD prevention and treatment. However, few reviews have explored natural polysaccharides with anti-IBD activities or the relationship between polysaccharide conformation and anti-IBD biological activity. Therefore, this review aims to summarize anti-IBD activities and potential clinical applications of polysaccharides isolated from plant, animal, microorganismal, and algal sources, while also exploring the relationship between polysaccharide conformation and anti-IBD bioactivity for the first time. Furthermore, potential mechanisms underlying polysaccharide anti-IBD effects are summarized, including intestinal microbiota modulation, intestinal inflammation alleviation, and intestinal barrier protection from IBD-induced damage. Ultimately, this review provides a theoretical foundation and valuable insights to guide the development of natural polysaccharide-containing functional foods and nutraceuticals for use as dietary IBD therapies.

- This article is part of the themed collection: Food & Function Review Articles 2023
Article information
Download citation, permissions.
J. Chen, Y. Gao, Y. Zhang and M. Wang, Food Funct. , 2024, Advance Article , DOI: 10.1039/D3FO04919A
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author.
This article has not yet been cited.
Advertisements
- Get 7 Days Free
Biomerica Announces inFoods® IBS Pilot Launch with Prominent 1,100 Member Physician Group Commencing June 2024
- With IBS affecting 10 to 15% of the global population, the associated $21 billion in direct medical costs and absenteeism underscores the urgent need of innovative solutions.
- Recognized as the 7th most common diagnosis by all physicians, IBS represents a significant healthcare burden. inFoods IBS offers a groundbreaking method to identify foods that can trigger IBS symptoms, revolutionizing how IBS symptoms are managed.
- inFoods IBS is backed by extensive clinical research that has demonstrated remarkable efficacy in providing relief to the majority of IBS patients.
- The upcoming presentation of positive inFoods IBS clinical data during the IBS Clinical Session at the Digestive Disease Week 2024 Conference highlights the growing recognition and therapeutic utility of this groundbreaking technology in managing IBS symptoms.
IRVINE, Calif., May 13, 2024 (GLOBE NEWSWIRE) -- Biomerica, Inc. (Nasdaq: BMRA), (the “Company”) a global provider of advanced medical diagnostic and therapeutic products, today announced the initiation of a pilot program for its inFoods ® IBS product with a large 1,100-member physician group. This pilot launch will commence in June 2024.
inFoods ® IBS offers a novel approach by identifying individual patient specific dietary triggers for IBS symptoms, which could revolutionize management of the condition. This technology has demonstrated remarkable success in alleviating symptoms for the majority of patients participating in the most extensive clinical study of its kind.
Irritable Bowel Syndrome (IBS) affects approximately 10-15% of the global population and contributes to an estimated $21 billion in direct medical costs and productivity losses annually. Despite being the seventh most common diagnosis among all physicians, effective treatment solutions have been elusive for most sufferers. The complexity of IBS and the limited therapeutic efficacy of current treatments underlines the critical need for innovative solutions like inFoods ® IBS.
The inFoods IBS product has undergone rigorous testing in a prospective, double-blind, placebo-controlled endpoint study conducted at prestigious institutions including the Mayo Clinic, Beth Israel Deaconess Medical Center (a Harvard Medical School Teaching Hospital), Houston Methodist Hospital, and the University of Michigan. The study results revealed a significant improvement in Abdominal Pain Intensity scores (trial participants with >30% reduction in pain) for IBS patients in the treatment diet arm compared to those in the placebo diet arm (p-value of 0.0246). Notably, this study found that the majority of IBS patients experienced remarkable relief with inFoods IBS.
The inFoods IBS positive clinical data will be presented during the highly anticipated IBS Clinical Session at the upcoming 2024 Digestive Disease Week (DDW) in Washington D.C., the premier international gathering of healthcare professionals specializing in digestive diseases.
About Biomerica (NASDAQ: BMRA ) Biomerica, Inc. ( www.biomerica.com ) is a global biomedical technology company that develops, patents, manufactures and markets advanced diagnostic and therapeutic products used at the point-of-care (in home and in physicians' offices) and in hospital/clinical laboratories for detection and/or treatment of medical conditions and diseases. The Company's products are designed to enhance the health and well-being of people, while reducing total healthcare costs. Biomerica primarily focuses on gastrointestinal and inflammatory diseases where the Company has multiple diagnostic and therapeutic products in development.
About inFoods ® The inFoods IBS test is designed to assess a patient’s above normal immunoreactivity to specific foods utilizing a simple finger-stick blood sample. Instead of difficult to manage broad dietary restrictions, physicians can now use the inFoods IBS information to make targeted, patient-specific recommendations about trigger foods that, when removed from the diet, may alleviate IBS symptoms such as pain, bloating, diarrhea and constipation. The inFoods IBS test and clinical outcomes were studied at several prominent centers including Mayo Clinic, Beth Israel Deaconess Medical Center Inc. - a Harvard Medical School Teaching Hospital, Houston Methodist Hospital, and the University of Michigan. The clinical results for improvement in the Abdominal Pain Intensity (API) responder endpoint of >30% reduction in pain, for IBS patients in the treatment diet arm was greater than patients in the placebo diet arm (p-value of 0.0246). The improvement for patients in the treatment arm versus the placebo arm is considered clinically significant and for certain endpoints is similar and, in some cases, better than the current drugs in the market. Further information about Biomerica’s patented inFoods ® Technology Platform can be found at: https://biomerica.com/inFoods/our-technology/ .
The Private Securities Litigation Reform Act of 1995 provides a "safe harbor" for forward-looking statements. Certain information included in this press release contains statements that are forward-looking, such as statements relating to the Company’s current and future sales, revenues, overhead, expenses, cost of goods, operations, and earnings; the Company's need for raising additional capital; the Company's expected commercialization launch dates and future revenues from the Company's HP Detect product, inFoods IBS product and other products; and diversification of the Company's revenue streams. Such forward-looking information is based upon the current beliefs and expectations of management and involves important risks and uncertainties that could significantly affect anticipated results. In addition, these forward-looking statements are subject to assumptions with respect to future business strategies and decisions that are subject to change. Accordingly, such results may differ materially from those expressed in any forward-looking statements made by or on behalf of Biomerica. Factors that could cause actual results to differ from those expressed in the forward-looking statements are discussed in the "Risk Factors" section of the Company's Annual Report on Form 10-K, Quarterly Reports on Form 10-Q and other reports filed with the SEC and available on the SEC's website (www.sec.gov). The Company is under no obligation to update any forward-looking statements after the date of this release.
Corporate Contact:
Zack Irani 949-645-2111 [email protected]
Source: Biomerica, Inc.
Market Updates
What to invest in during high inflation, never mind market efficiency: are the markets sensible, starbucks stock could use a pick-me-up after big selloff; is it a buy, 5 cheap stocks to buy from an attractive part of the market, markets brief: all eyes on inflation, 5 things we learned from the q1 earnings season, after earnings, is palantir stock a buy, a sell, or fairly valued, what’s happening in the markets this week, stock picks, cisco earnings: positive guidance and splunk inclusion align with our long-term thesis, 3 warren buffett stocks to buy after berkshire hathaway’s just-released 13f filing, going into earnings, is nvidia stock a buy, a sell, or fairly valued, after earnings, is arista stock a buy, a sell, or fairly valued, a cheap dividend aristocrat to buy before it bounces back, alibaba earnings: more positive outlook despite mixed results, after earnings and a 56% rally in 2024, is arm stock a buy, a sell, or fairly valued, how morningstar rates stocks, sponsor center.

IBS Intelligence
Financial Technology News & FinTech Research
Alignment with DORA will deliver a more resilient UK financial ecosystem, research reveals
By puja sharma.
May 15, 2024
Cyberattacks
- Cybersecurity
- Digital Operational Resilience Act
More stringent regulations present an opportunity for businesses to re-evaluate security strategies and identify vulnerabilities The cybersecurity threat landscape in the UK is steadily increasing, with half of businesses reporting either a breach or an attack in the last 12 months. Most recently, a cyberattack on the Ministry of Defence put 270,000 records at risk, including […]
This article can only be read by subscribers. Subscribe to IBSi Premium or Sign in
Get access to IBSi Premium
IBSi Daily News Analysis + monthly IBSi FinTech Journal
Daily insightful Financial Technology news analysis
Weekly snapshots of industry deals, events & insights
Weekly global FinTech use cases
Chart of the Week curated by IBSi’s Research Team
Monthly issues of the iconic IBSi FinTech Journal
Exclusive invitation to a flagship IBSi on-ground event of your choice
Previous Article
May 13, 2024
UK FinTech sector positioned for success with consumer duty regulation, study shows
Next article, is ai the new weapon in chargeback fraud.

Weekly Case Study

Chart of the Week

FinTech insights exclusively curated by the IBSi’s Research Team
Other Related News
account fraud
AI in Cybersecurity
digital payment innovation
May 08, 2024
Alternative Investment
B2B Wealthtech
Family offices embrace Alternative Investments amid growing cybersecurity concerns
Related reports.

Sales League Table Report 2023

Global Digital Banking Vendor & Landscape Report Q1 2024

Wealth Management & Private Banking Systems Report Q1 2024

IBSi Spectrum Report: Supply Chain Finance Platforms Q4 2023

Treasury & Capital Markets Systems Report Q1 2024
Ibsi sales league table.


IMAGES
VIDEO
COMMENTS
Amitriptyline Helps Relieve IBS Symptoms. Oct. 16, 2023 — Amitriptyline can improve symptoms of irritable bowel syndrome in patients seen in GP surgeries, new research has found. The cheap and ...
Irritable bowel syndrome (IBS) is a chronic functional gastrointestinal disorder which presents with abdominal pain and altered bowel habits. It affects about 20% of the general population, mainly women, and has a considerable impact on the quality of life and health care costs. ... National Research Council, Molecular Biotechnology Center ...
New research published in the journal Cellular and Molecular Gastroenterology and Hepatology sheds light on disease mechanisms common to irritable bowel syndrome (IBS) and cardiovascular diseases ...
An international study of more than 50,000 people with irritable bowel syndrome (IBS) has revealed that IBS symptoms may be caused by the same biological processes as conditions such as anxiety. The research highlights the close relationship between brain and gut health and paves the way for development of new treatments.
Introduction. Irritable bowel syndrome (IBS) is a prevalent disorder that greatly reduces patients' quality of life (QOL) and adversely affects the medical economy [].A recent epidemiological survey using the Rome IV criteria revealed that the prevalence of IBS in the general population globally is 4.1% [].In Japan, the prevalence of IBS is 2.2% but that of functional bowel disorders is 25.2 ...
Irritable bowel syndrome is considered a functional bowel disorder with no known organic cause. Latest Research and Reviews Serine proteases and metalloproteases are highly increased in irritable ...
Irritable bowel syndrome (IBS) is one of the most common disorders of gut-brain interaction worldwide, defined according to patterns of gastrointestinal symptoms as described by the Rome ...
Genetic predisposition contributes to disease pathophysiology in irritable bowel syndrome (IBS). This Review provides a comprehensive overview of genetic research in IBS and discusses new concepts ...
Introduction. Associated with abdominal pain, bloating, and altered bowel habits, irritable bowel syndrome (IBS) is a chronic, cyclical and relapsing functional bowel disorder ().The global prevalence of IBS is currently estimated at 15%, and IBS symptoms occur in about 10-20% of Westerners (2-4).Irrespective of bowel habit, diagnoses of IBS have traditionally been made by using the Rome ...
Irritable bowel syndrome (IBS) is a highly prevalent, chronic disorder of gut-brain interaction (DGBI), characterised by abdominal pain in association with change in stool form or frequency.1 It affects 5% of the global population,2 and can be difficult to treat, affecting social functioning, ability to work, and quality of life.1 Impact on quality of life can be worse than other chronic ...
Core Tip: Irritable bowel syndrome (IBS) is a physical and mental illness that is becoming more prevalent, and its impact on society is expanding. Understanding of IBS has changed since the release of the Rome IV diagnosis in 2016, and this paper reviews the literature from the past decade to find that research around the brain-gut axis, diet, and gut microbiota are at the forefront of IBS.
Irritable bowel syndrome (IBS) is a common chronic disorder of gut-brain interaction, characterised by the presence of abdominal pain in association with a change in stool form or frequency.1 Some people experience predominantly constipation (IBS-C), some mostly diarrhoea (IBS-D), and others a mixture of the two (IBS-M). IBS is common, estimated to affect 5% of the global population at any ...
The only drug currently approved for IBS-D is alosetron, a 5-HT3 antagonist that may relieve abdominal pain and slow colonic and small bowel transit. Alosetron was withdrawn from the market for safety reasons in 2000 and was reintroduced in 2002 with a more restricted indication. Today, incidence rates of adverse events, including ischemic ...
In fact, irritable bowel syndrome, or I.B.S., is a real problem causing real symptoms, no matter how hard its sufferers may wish it gone. But unlike an infection or tumor, I.B.S. is what medicine ...
Irritable Bowel Syndrome and Control Volunteers: Diet Challenge. Purpose of study: The purpose of this study is to study the relationship between the bile acids, short chain fatty acids and bacteria within the intestines.The hypothesis is that changes in the bacterial composition of the stool are associated with the differences in bile acids and short chain fatty acids in patients having ...
A total of 24,845 respondents reported current abdominal symptoms meeting standard diagnostic criteria (DHQ Rome III, Fig. 1) at the time of the survey, providing a point prevalence of IBS of 14.5 ...
Reviewed by Emily Henderson, B.Sc. Feb 22 2023. New research from the University of Missouri School of Medicine has established a link between irritable bowel syndrome (IBS) and mental health ...
Objectives To evaluate the association between healthy lifestyle behaviours and the incidence of irritable bowel syndrome (IBS). Design Population-based prospective cohort study. Setting The UK Biobank. Participants 64 268 adults aged 37 to 73 years who had no IBS diagnosis at baseline were enrolled between 2006 and 2010 and followed up to 2022. Main exposure The five healthy lifestyle ...
Irritable Bowel Syndrome. Irritable bowel syndrome (IBS) is a chronic, episodic intestinal disorder characterized by abdominal pain and altered bowel habits. It affects one in seven Americans, although most go undiagnosed. IBS can have a substantial impact on well-being and health, but doctors underestimate the impact the disease can have ...
Associated with abdominal pain, bloating, and altered bowel habits, irritable bowel syndrome (IBS) is a chronic, cyclical and relapsing functional bowel disorder ( 1 ). The global prevalence of IBS is currently estimated at 15%, and IBS symptoms occur in about 10-20% of Westerners ( 2 - 4 ). Irrespective of bowel habit, diagnoses of IBS ...
Published on May 3, 2024. Fact checked by. Nick Blackmer. Taking berberine and curcumin together might help reduce the symptoms of irritable bowel syndrome (IBS), new research suggests. For the ...
The gut-calming solution, developed over 10 years' R&D at the University of Bristol, contains FX856 and aims to reduce symptoms such as stomach cramps, bloating, diarrhoea and constipation that ...
Irritable bowel syndrome (IBS) is the most prevalent disorder of brain-gut interactions that affects between 5 and 10% of the general population worldwide. The current symptom criteria restrict ...
New research looking at the benefits of a Mediterranean diet for people with irritable bowel syndrome (IBS) has yielded surprising results. Not only did the diet, rich in fruits, vegetables and ...
Irritable bowel syndrome (IBS) is one of the most commonly diagnosed gastrointestinal diseases. IBS, in the absence of any other causative disease, is defined as the presence of abdominal pain or discomfort with altered bowel habits. Diagnosis of IBS has evolved since its first discovery, and today the Rome IV diagnostic criteria are used to diagnose IBS. Depending on the subclass of IBS ...
Inflammatory bowel disease (IBD) comprises a group of highly prevalent and chronic inflammatory intestinal tract diseases caused by multiple factors. Despite extensive research into the causes of the disease, IBD's pathogenic mechanisms remain unclear. Moreover, side effects of current IBD therapies restrict their Food &; Function Review Articles 2023
The inFoods IBS positive clinical data will be presented during the highly anticipated IBS Clinical Session at the upcoming 2024 Digestive Disease Week (DDW) in Washington D.C., the premier ...
IBS Intelligence (IBSi) is the world's only pure-play Financial Technology focused research, advisory, and fintech news analysis firm, with a 30-year track record and clients globally. We take pride in covering 400+ technology vendors globally - the largest by any analyst firm in this space.