Adsorption of Oil from Industrial Wastewater with Eucalyptus globulus Labill. Bark: Optimization Study
- Open access
- Published: 20 April 2024
- Volume 235 , article number 273 , ( 2024 )

Cite this article
You have full access to this open access article
- Yasmin I. E. Aboulsoud ORCID: orcid.org/0000-0002-9884-0694 1
Oily wastewater is one of the most hazardous contaminants that can hurt the ecosystem. There is an urgent need to adopt an efficient, eco-friendly, and low-cost material to replace the old traditional treatment methods of oily wastewater that were very expensive in addition to their relatively low efficiency. Eucalyptus bark is considered one of the materials that are rarely used in this field, although it has the characteristics that qualify it to be a distinguished and promising one. The optimum conditions of using Eucalyptus globulus Labill. (Blue gum) bark in the removal of oil from prepared aqueous solutions were concluded before applying in the treatment of real oily industrial wastewater from New Borg El-Arab City, Egypt. The sequential optimization adsorption results were as follows: initial oil concentration, 500 mg/l; adsorbent dosage, 0.5 g/l; pH, 3; exposure time, 45 min; temperature, 20 °C; and shaking rate, 300 rpm. The pretreatment of biomass with H 3 PO 4 proved it to be superior in the oil removal process where the efficacy reached 450.69 mg/g, while the unmodified form came second where the adsorption efficacy reached 395.86 mg/g, after that the NaOH-modified form came third by efficacy reached 315.85 mg/g. The results of SEM elucidated this order of efficacy according to the porosity of the bark surface. FTIR analysis indicated that OH, carboxylic C = O, and carboxylic C-O groups are the contributing groups in the oil adsorption process for the three forms of Eucalyptus bark. The reusability of Eucalyptus bark using n-hexane for one cycle reached 96.34, 97.13, and 95.83% for unmodified, H-modified, and OH-modified forms, respectively, and for five cycles reached 56.29, 58.01, and 55.81% for unmodified, H-modified, and OH-modified forms, respectively. The application of Eucalyptus bark in the H-form in the treatment of real oily wastewater was achieved by efficacy ranging between 91.46 and 96.23% which proves the excellence of Eucalyptus bark in the treatment of oily wastewater.
Avoid common mistakes on your manuscript.
1 Introduction
Like everywhere else in the globe, residential area growth has led to an increase in environmental concerns, foremost among them being environmental pollution (Cetin et al., 2022a ). Oil is one of many pollutants that can seriously harm the ecological system. Municipal and industrial establishments are often the main producers of pollution (Bozdogan et al., 2019 ). Huge quantities of oily effluents are produced from various industries, such as the oil and soap industries, which must be removed before the water can be reused for other purposes or discharged into the environment. The percentages of oily pollutants must be less than the permissible stipulated standard limits when liquid wastes are disposed of in the environment because of the harmful effects they can cause on aquatic organisms and human health. Also, the ecosystem is greatly impacted by the alteration in soil quality and vegetation cover brought on by soil contamination with such wastewater (Cetin et al., 2022b and Cetin et al., 2022c ). As well as, the drainage system may clog when there is a high level of oil and grease present, which may result in an overflow. Using detergents causes an oil emulsion to occur because they contain surfactants with two ends: a hydrophilic end that clings to water molecules and a hydrophobic end that clings to oil particles. However, the removal of emulsified oil is more difficult due to its stability in the aqueous phase (Hamid et al., 2016 , Martini et al., 2020 and Jaber & Alwared, 2019 ).
There are several popular methods for removing oily materials from waterways such as membrane filtration, advanced oxidation, and biological treatment. However, these methods have several disadvantages such as high operating and maintenance costs, toxic sludge generation, and complex procedures. In comparison, adsorption is an effective and economical method (Chai et al., 2015 ). The process of transferring a molecule from a fluid bulk to a solid surface is known as adsorption. Many factors affect the adsorption process of pollutants (adsorbate) onto biomass (adsorbent) such as pH, contact time, temperature, solid:liquid ratio, and adsorbate concentration. Optimization is using a factor in the greatest or most efficient way possible. The optimization may be reached via two methods; the first is the sequential method where the concluded optimum result of the experiment is applied in the subsequent experiment to reach to the maximum efficacy at the last experiment. The second method is the cumulative method, where all experiments are performed at the same time to obtain the optimum results for each, and an additional last experiment is performed under all the concluded optimum conditions to detect the maximum efficacy.
An important factor in the selection and design of an adsorption process is the adsorbent’s cost. In general, absorbent can be assumed to be “low cost” if it requires little processing and is abundant in nature, or waste material either from industry or agricultural by-products with almost no economic value. Plant barks are among the foremost widely applied low-cost biomass materials within the study of pollutant removal from aqueous media or wastewater (Ighalo & Adeniyi, 2020 ). Eucalyptus bark has the potential to replace expensive commercial adsorbents at a lower cost because it is readily available, non-hazardous, and reasonably priced, as well as it was found to be an effective sorbent for the removal of some pollutants (Afroze et al., 2016 , Ghodbane & Hamdaoui, 2008 and Saliba et al., 2002 ).
Eucalyptus globulus (also called the blue gum) is a tall, broadleaf, evergreen shrub, known as one of three globally major fast-growing tree species. It is belonging to the Myrtaceae family, well known and widely grown as ornamental and windshield plant. Different parts of this plant are nutritionally very important and therapeutically highly valuable, as the essential oil (EGEO) is extracted from its leaves, buds, branches, and fruits, and exhibits numerous biological properties, such as wound healing, antidiabetic, antibacterial, anti-inflammatory, and antioxidant. Eucalyptus is primarily indigenous to Tunisia and Australia, but it has also been observed in Africa (including Egypt) and the tropical to temperate southern regions of the USA. Bark of E. globulus is classified as a by-product and it is currently used as fuel (Liu & Li, 2010 , Assaggaf et al., 2022 , Hayat et al., 2015 , El-Kadi et al., 2000 and Mota et al., 2013 ). In developed countries, Eucalyptus is utilized in the pulp and paper industries as the main fiber resource, in addition to essential oil production, manufactured wood products, and green chemical feedstocks (Seng Hua et al., 2024 ).
However, the biomass of Eucalyptus is efficiently applied to remove several heavy metals and dyes from wastewater and aqueous solutions (Anastopoulos et al., 2022 , Zafar et al., 2022 , Alshiteewi, 2023 and Samimi, 2024 ); there are few studies on using this efficient biomass in the adsorption of oily wastewater. This made the goal of the research to deepen the study in removing this pollutant that is difficult to get rid of with this biomass that has not been adequately studied. The exhausted Eucalyptus biomass after biosorption processes can be composted and used as a good substrate in greenhouse experiments (de Carvalho et al., 1991 ).
The objectives of the study were firstly, determining the optimum conditions to adsorb oil from aqueous solutions via batch sequential method using the bark of Eucalyptus globulus trees in its raw form, acidic modified with phosphoric acid and alkali modified with sodium hydroxide; secondly, evaluation the feasibility of the optimum condition application in the treatment of real oily wastewater collected from oil and soap factories in the new Borg El Arab City, Egypt.
2 Material and Methods
2.1 preparation of eucalyptus bark biomass.
Eucalyptus globulus Labill. bark was obtained from the silviculture area in Borg El-Arab region in August 2021. The bark was washed thoroughly with distilled water until the clear color of the leachate was obtained, oven-dried at 40 °C for 24 h, crushed, and sieved through a 2-mm sieve.
Acid-modified biomass (H-modified) was prepared according to Patnukao and Pavasant ( 2008 ), and Eucalyptus bark was impregnated by a ratio (1:1, w:v) into a solution of H 3 PO 4 (A.R grade, 85 wt% in H 2 O) from Sigma-Aldrich Chemical Company, Germany. The mixture was stirred manually for 3 min at room temperature, and washed by hot distilled water until the pH of the leachate was raised to 7; finally, the product was dried at 105 °C for 4 h.
Alkali-modified biomass (OH-modified) was prepared according to Afroze et al. ( 2016 ), and Eucalyptus bark was contacted by a ratio (1:10, w:v) with 0.1 M NaOH (ACS reagent, ≥ 97.0%, pellets) from Sigma-Aldrich Chemical Company, Germany. The mixture was stirred with a magnetic stirrer for 12 h, filtered, and rinsed with distilled water until the pH of the leachate was reduced to 7; finally, the product was dried at 90 °C for 12 h.
2.2 Adsorption Experiments (Batch Procedures)
An immiscible oil/water mixture (IOWM) was prepared with the desired concentration via the addition of the required weight of commercial food oil to the specific volume of distilled water (w:v), stirred at 200 rpm for about 5 min, then used immediately for batch procedures. A specific weight of bark biomass was shaken with a desired volume of IOWM. The detailed experimental conditions were shown below each represented data.
Oil concentration was determined gravimetrically according to EPA Method, 1664 ( 2010 ) using n-hexane as extractable material (for analysis EMSURE ACS, from Sigma-Aldrich Chemical Company, Germany).
For desorption experiments, the Eucalyptus bark was oil-loaded under the concluded optimum conditions and then conducted with 0.1 M solution of three types of eluents: NaHCO 3 , CH 3 COOH, and n-hexane. Conditions of adsorption, desorption, and resorption were mentioned in the remarks of each figure.
2.3 Examination of Eucalyptus Bark Surface by Scanning Electron Microscopy
The dried Eucalyptus bark samples (unmodified, modified, and oil-loaded biomasses) were coated using a gold sputter coater (SPI-Module) that was examined by SEM (JSM-5500 LV, JEOL, Japan) under an accelerating voltage of 18 kV.
2.4 Determination of Functional Groups onto Eucalyptus Bark Biomass by Fourier Transform Infrared
Dried Eucalyptus bark samples (unmodified, modified, and oil-loaded biomasses) were finely powdered, pressed into KBr pellets, and analyzed by FTIR Spectrophotometer (8201 DC, Shimadzu, Japan) along wavenumber range 400–4000 cm −1 .
2.5 Application of Biomasses in the Treatment of Real Oily Wastewater
Ten wastewater samples were collected from oil and soap factories in industrial New Borg El-Arab City, in May 2022. Eucalyptus biomasses were conducted to treat oily wastewater samples under the elicited optimum conditions from the batch experiments.
2.6 Calculations and Data Evaluation
2.6.1 adsorption equations.
The amount of adsorbed oil on adsorbents was calculated according to Ighalo and Adeniyi ( 2020 ) and Afroze et al. ( 2016 ) via the equation:
where q is the amount of adsorbed oil onto the unit amount of adsorbent (mg/g), C i is the initial oil concentration (mg/l), C f is the final oil concentration (mg/l), V is the volume of solution (l), and M is the adsorbent mass (g).
2.6.2 Desorption and Resorption Equations
The amount of desorbed oil from Eucalyptus biomasses using NaHCO 3 , CH 3 COOH, and n-hexane as eluents was expressed according to Bulgariu and Bulgariu ( 2014 ) and Kanwal et al. ( 2013 ) via the equation:
The amount of resorbed oil on Eucalyptus biomasses was expressed according to Aboulsoud ( 2008 ) via the equation:
2.6.3 Real Wastewater Treatment Equation
The amount of removed oil from wastewater was calculated according to Shaaban et al. ( 2017 ) via the equation:
where C i is the initial oil concentration before treatment, and C f is the final oil concentration after treatment.
2.6.4 Statistical Analysis
A triplicate of each experiment was subjected to MSTAT-C. Duncan’s test was used to analyze data via an analysis of variance (ANOVA) (Waller & Duncan, 1969 ). At 0.05 significance probability level, means with different alphabetical letters differ significantly.
3 Results and Discussion
3.1 effect of ph.
Because variations in pH cause differences in ionization degree and the surface characteristics of the adsorbent, the efficacy of the adsorption process is influenced by pH (Jaber & Alwared, 2019 ). While maintaining the other parameters constant, pH in the range of 1 to 11 was investigated and the results are shown in Fig. 1 .
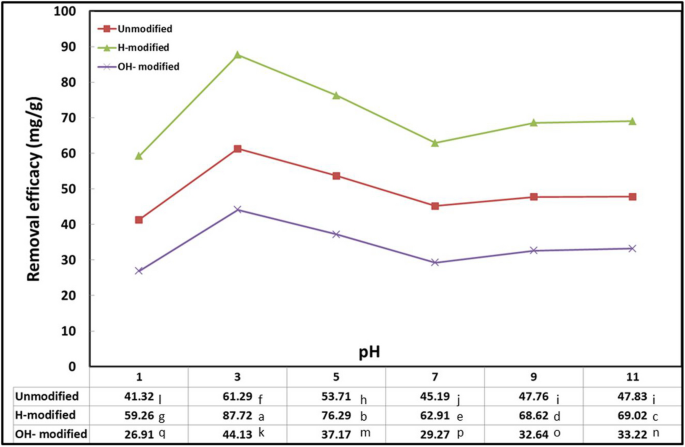
Interaction effect of Eucalyptus bark forms with pH (conditions: room temperature, initial oil concentration, 1 g/l; exposure time, 120 min; adsorbent dosage, 10 g/l; shaking rate, 200 rpm)
All Eucalyptus bark forms followed the same behavior against pH change. Oil removal efficacy increased from 1 to 3; afterward, the efficacy decreased from pH 5 to 7 to give the lowest significant one. The removal efficacy slightly increased again from pH 9 to 11. The influence of acidic pH media can support de-emulsification and the formation of larger oil droplets, which increased oil adsorption onto the sorbent surface. Conversely, in alkaline media, the saponification process took precedence, where the oil undergoes hydrolysis to produce glycerol and fatty acid salts (soap), which is more soluble in water than in n-hexane. This is evident in the change of water clearance and subsequently resulted in the detection of less oil in the solvent and lower oil removal on the adsorbent. The destabilization of organic sorbent materials in a neutral pH media can be linked to the least significant oil removal efficacy at neutral pH than in acidic or alkaline solutions (Martini et al., 2020 and Ahmad et al., 2005 ). As concluded from this experiment, an optimum pH of 3 was applied in the subsequent experiments.
3.2 Effect of Exposure Time
One of the most crucial factors for the effective utilization of the Eucalyptus bark in a practical application is exposure time. Under constant parameters, different time intervals were conducted and results are shown in Fig. 2 . Adsorption occurred in two phases for all bark forms, a primary rapid phase where the oil removal efficacy was greatly increased at a unit time, this phase in observed from 1 to 30 min. It was followed by a secondary slow phase, where the removal efficacy was regressed to give the equilibrium at 45 min and no tangible increase was observed afterward. The abundance of vacant available binding sites on the adsorbent surface, which makes oil particles and bark more likely to interact, contributed to the rapid initial phase. Due to the hindrance effect between oil particles and each other as well as the limited surface area available for the capture of adsorbate after the equilibrium point, removal efficacy tended to level off (Anifah et al., 2022 and Hamid et al., 2016 ). As concluded from this experiment, an optimum exposure time of 45 min was applied in the subsequent experiments.
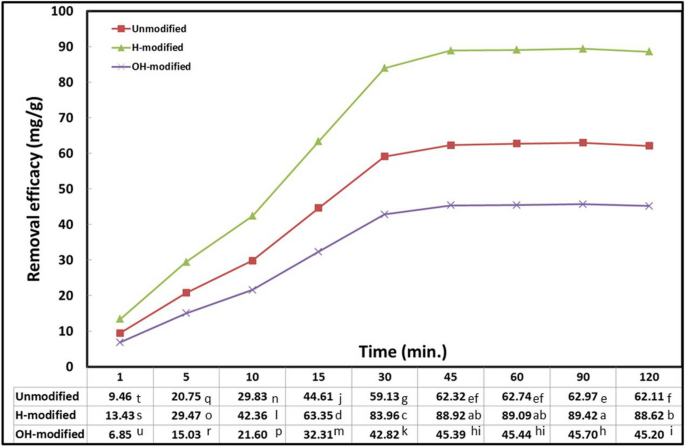
Interaction effect of Eucalyptus bark forms with exposure time (conditions: room temperature, initial oil concentration, 1 g/l; pH, 3; adsorbent dosage, 10 g/l; shaking rate, 200 rpm)
3.3 Effect of Adsorbent Dosage
The assessment of the optimal adsorbent quantity is one of the key elements from an economic perspective. Because it offers the binding sites for oil adsorption, the dosage of the adsorbent has a significant impact on the efficacy of the adsorption process (Jaber & Alwared, 2019 and Badawy et al., 2020 ). To study this parameter, the results were expressed as mg/g and % to detect the highest removal efficacy as well as the saturation level of the adsorbent, respectively (Fig. 3 ).
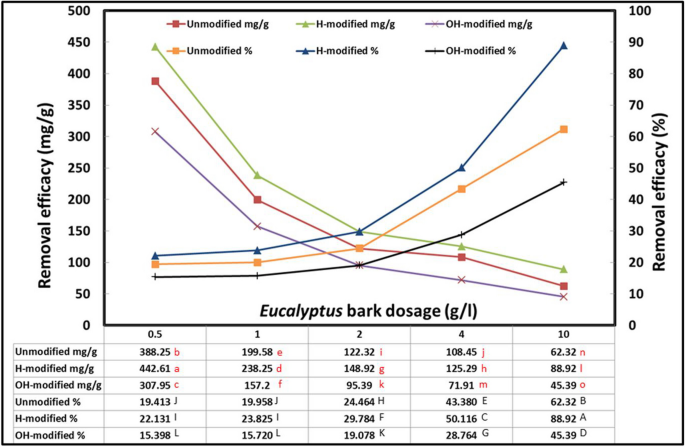
Interaction effect of Eucalyptus bark forms with Eucalyptus bark dosage (conditions: room temperature, initial oil concentration, 1 g/l; pH, 3; exposure time, 45 min; shaking rate, 200 rpm). “Remarks: The results expressed in mg/g are referred to by the lowercase statistical letters, while the results expressed in percent are referred to by the uppercase statistical letters”
While keeping the IOWM volume constant, the Eucalyptus bark dosage was inversely proportional to removal efficacy calculated as mg/g while it was directly proportional to removal efficacy calculated as % to a certain level where the equilibrium was attained. Decreasing the amount of adsorbent from 10 to 0.5 g was associated with increasing the amount of oil adsorbed per unit mass of Eucalyptus bark from 62.32 to 388.25 mg/g for the unmodified form, from 88.92 to 442.61 mg/g for the H-modified form, and from 45.39 to 307.95 mg/g for OH-modified form. On the other hand, this decrease from 10 to 1 g was associated with decreasing the adsorbed % of oil particles from 62.32 to 19.95% for unmodified form, from 88.92 to 23.82% for H-modified form, and from 45.39 to 15.72% for OH-modified form. After that, the decrease was leveled off by decreasing bark dosage to 0.5 g/l and no significant decrease was observed indicating saturation of adsorbent at this ratio. Calculating the efficacy as mg/g referred to the amount of oil adsorbed on the unit mass of Eucalyptus bark, so as the bark dosage increased (as the denominator) while the volume was constant (as the numerator) containing a constant number of oil molecules, caused each unit mass to adsorb fewer oil particles. On the other hand, calculating the efficacy as % refers to the saturation of adsorbent binding sites, as an increase in the Eucalyptus bark dosage increases the oil adsorption due to an increased surface area and consequently the number of available binding sites (Ingole et al., 2014 , Anifah et al., 2022 and Afroze, et al., 2016 ). As concluded from this experiment, an optimum adsorbent dosage of 0.5 g/l was applied in the subsequent experiments.
3.4 Effect of Initial Adsorbate Concentration
This factor represents the change of adsorbate molecules’ number (concentration) while the volume is constant. Conversely, the adsorbent dosage as a factor represents the change in adsorbate volume while the concentration is constant. The initial concentration of adsorbate has a significant impact on the adsorption process, which is crucial for the development of water treatment technology (Afroze, et al., 2016 ). Different oil concentrations were tested with constant parameters, and the results are shown in Fig. 4 . The higher initial oil concentration enhanced the adsorption efficacy until equilibrium was attained at 500 mg/l, at which point there was no significant increase. This is because the solid surfaces of the particles had reached their capacity before adding additional oil content (Jaber & Alwared, 2019 ). The highest significant efficacies were as follows: 444.13, 389.87, and 308.59 mg/g for H-modified, unmodified, and OH-modified forms, respectively. As concluded from this experiment, an optimum initial oil concentration of 500 mg/l was applied in the subsequent experiments.
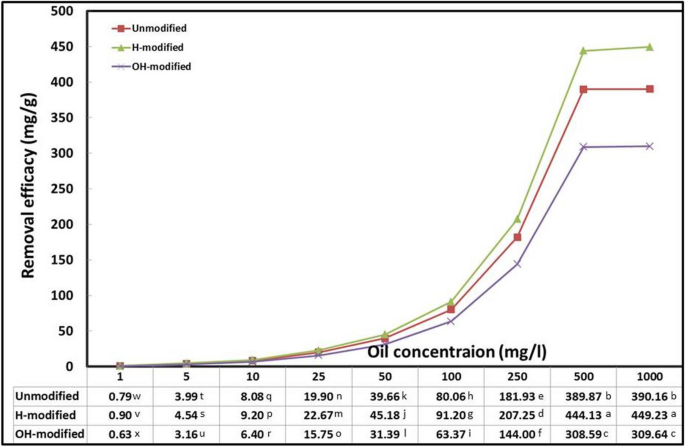
Interaction effect of Eucalyptus bark forms with oil concentration (conditions: room temperature, adsorbent dosage, 0.5 g/l; pH, 3; exposure time, 45 min; shaking rate, 200 rpm)
3.5 Effect of Temperature
In the range of 20–60 °C, the effect of temperature on the oil adsorption process was investigated, and the results are shown in Fig. 5 . Slight decline in oil adsorption was witnessed by temperature elevation from 20 to 40 °C, and the oil adsorption keeps on diminishing discernibly by temperature elevation to 60 °C. This decreasing trend in oil adsorption can be attributed to several factors, including the lower viscosity of IOWM solution, the increase in the random motion of oil molecules suspended in water that is known as Brownian motion, which reduces their entrapment on the sorbent surface, and the increased energy required to attach oil particles. The decrease in adsorption efficacy by increasing temperature means Eucalyptus bark particles acted as exothermic adsorbent (Wahi et al., 2014 , Alaa El-Din et al., 2018 , Jaber & Alwared, 2019 and Martini et al., 2020 ). As concluded from this experiment, an optimum initial oil concentration of 20 °C was applied in the subsequent experiments.
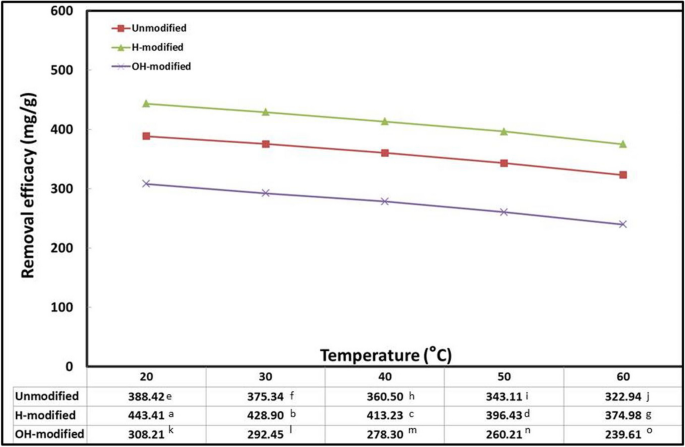
Interaction effect of Eucalyptus bark forms with temperature (conditions: initial oil concentration, 500 mg/l; adsorbent dosage, 0.5 g/l; pH, 3; exposure time, 45 min; shaking rate, 200 rpm)
3.6 Effect of Shaking Rate
Because it affects the likelihood of binding to sorbent binding sites and controls the mobility of sorbate ions, the shaking rate can promote the sorption process (Badawy et al., 2020 ). As shown in Fig. 6 , the adsorption efficacy was directly proportional to the shaking rate. The highest significant adsorption efficacy was attained at the highest shaking rate (300 rpm). Higher stirring speed acts to increase the mass transfer rate and mobility of adsorbent particles and conversely acts to decrease the surface layer resistance, thus acts to increase the adsorption efficacy of the oil (Lin & Liu, 2000 , Garba et al., 2016 , and Ahmad et al., 2005 ). As concluded from this experiment, an optimum shaking rate of 300 rpm was applied in the subsequent experiments.
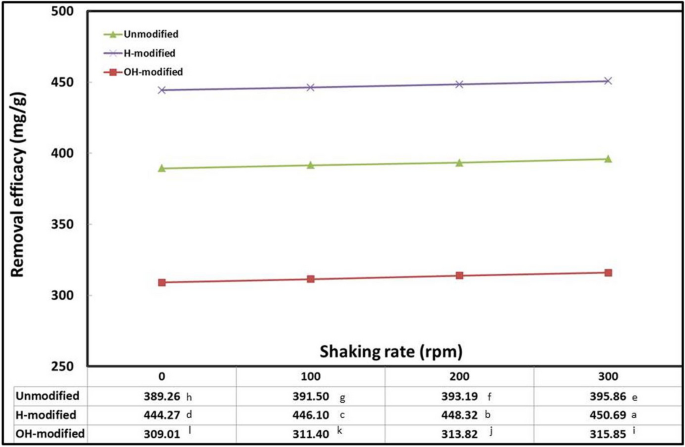
Interaction effect of Eucalyptus bark forms with shaking rate (conditions: initial oil concentration, 500 mg/l; adsorbent dosage, 0.5 g/l; pH, 3; exposure time, 45 min; temperature, 20 °C)

3.7 Effect of Modification Agent
The results of the aforementioned experiments proved that the H-form of Eucalyptus bark is a portentous biological tool able to adsorb oil substances by efficacy reached 450.69 mg/g under the optimum conditions of initial oil concentration, 500 mg/l; adsorbent dosage, 0.5 g/l; pH, 3; exposure time, 45 min; temperature, 20 °C; and shaking rate, 300 rpm. The unmodified form comes second where the adsorption efficacy reached 395.86 mg/g, after that the OH-modified form comes third by efficacy reaching 315.85 mg/g.
H 3 PO 4 is a dependable and effective dehydrating agent that may encourage more cross-linked structure development and enhance the fibrous structure of raw organic materials, which stimulates the creation of pores and consequently expand the biomass structure which is essential for adsorption capacity. Moreover, functional surface group dissociation in an acidic medium contributes to an increase in charge densities. Oppositely, NaOH promotes the saponification of oil particles which decreases the oil adsorption on the bark surface (Daaou & Bendedouch, 2012 , Yorgun & Yıldız, 2015 , Ahmad et al., 2005 and Souza et al., 2016 ).
3.8 Reusability of Adsorbent
The adsorbent should be able to perform a large number of cycles of adsorption, desorption, and resorption to make the process more cost-effective and to secure the safe disposal of sorbent after exhaustion. The adsorbent mass and desorbent solution can both be reused, which makes it possible to use them in continuous systems in industrial processes (Shaaban et al., 2017 ).
Three types of eluents (0.1 M) are examined as alkali, acid, and solvent those are NaHCO 3 , CH 3 COOH, and n-hexane, respectively. The adsorption and resorption processes were conducted under the previously concluded optimum conditions. The highest significant desorption and resorption efficacies were achieved in case of n-hexane followed by NaHCO 3 then CH 3 COOH (Fig. 7 ). This result can be attributed to the type of eluent, as n-hexane is an organic solvent can extract the oil from Eucalyptus bark surface, while NaHCO 3 as an alkali acts to enhance saponification process where the oil undergoes hydrolysis to produce glycerol and fatty acid salts (soap) and these compounds cannot be separated from the eluent, conversely CH 3 COOH as an acid support de-emulsification and the formation of larger oil droplets that makes its elution from bark surface is more difficult (Ahmad et al., 2005 and Martini et al., 2020 ).
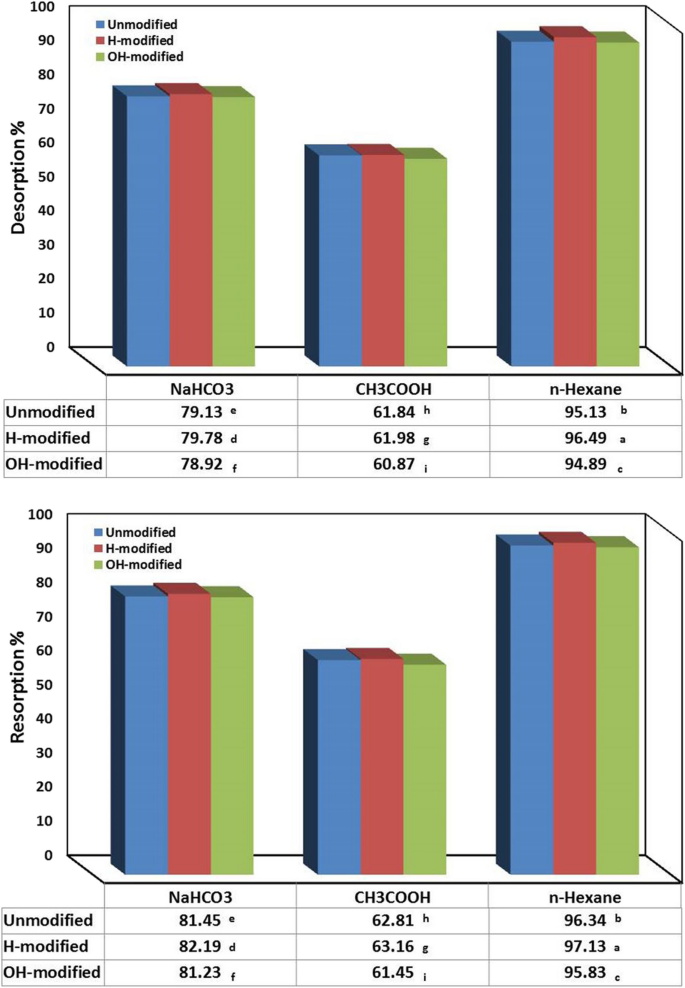
Desorption and resorption efficacies of oil onto Eucalyptus bark forms for one cycle. (Conditions of adsorption and resorption: initial oil concentration, 500 mg/l; adsorbent dosage, 0.5 g/l; pH, 3; exposure time, 45 min; temperature, 20 °C; shaking rate, 300 rpm). (Conditions of desorption: eluent concentration, 0.1 M; adsorbent dosage, 1 g/100 ml; exposure time, 45 min; temperature, 20 °C; shaking rate, 300 rpm)
The reusability of Eucalyptus bark biomass was examined by using 0.1 M n-hexane for five cycles of desorption and resorption (Fig. 8 ). Both the desorption and resorption efficacies decreased with increasing cycle numbers. A potential reason can be credited to the unfavorable impact of the eluent on the biomass binding sites. Additionally, in each cycle, the accumulation of remaining oil molecules within the biomass reduces the efficacy of subsequent resorption (Tüzün et al., 2005 ).
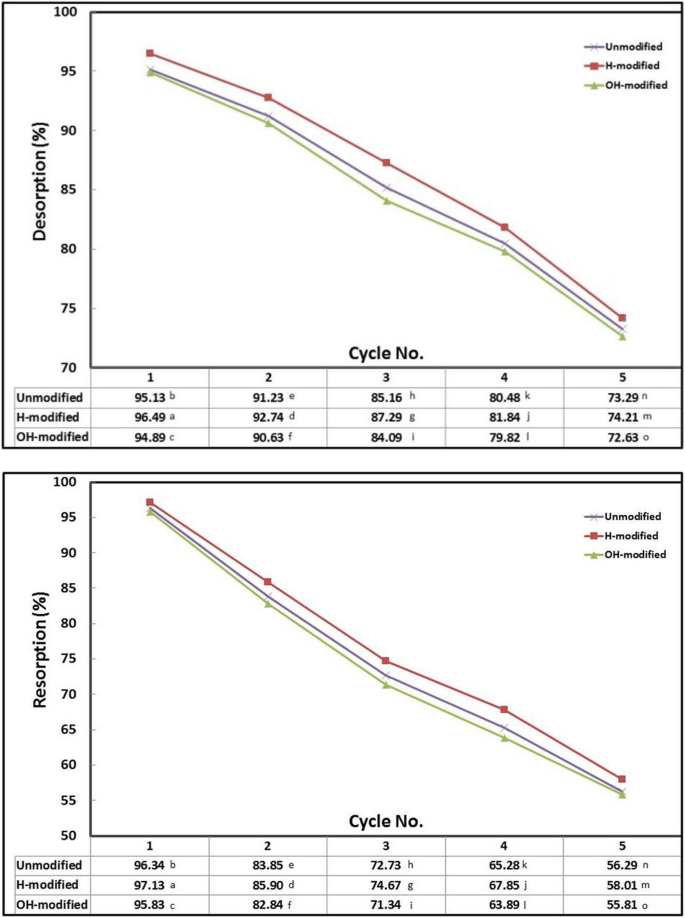
Desorption and resorption efficacies of oil onto Eucalyptus bark forms for five cycles using n-hexane. (Conditions of adsorption and resorption: initial oil concentration, 500 mg/l; adsorbent dosage, 0.5 g/l; pH, 3; exposure time, 45 min; temperature, 20 °C; shaking rate, 300 rpm). (Conditions of desorption: eluent concentration, 0.1 M; adsorbent dosage, 1 g/100 ml; exposure time, 45 min; temperature, 20 °C; shaking rate, 300 rpm)
3.9 Examination of Eucalyptus Bark Surface by Scanning Electron Microscopy (SEM)
SEM was used to study the morphology of Eucalyptus bark biomass and micrographs presented in Fig. 9 . The naturally unmodified biomass contains plenty of pores that are required for oil trapped in the interior surface of the biomass (Martini et al., 2020 ). The biomass surface was changed due to H 3 PO 4 and NaOH modification as well as oil adsorption on these three forms. The H-modified form has a more porous and irregular structure than the unmodified one. Meanwhile, the OH-modified form surface appears smoother and fewer pores are observed. The porous and wrinkled structures provide a more specific surface area available for oil adsorption (Afroze et al., 2016 ). This finding may interpret the superior efficacy of the H-modified form more than the unmodified and the OH-modified form comes third. Oil adsorption onto Eucalyptus forms causes some morphological changes such as a thick cell wall, surface lumps appearing and oil seeming to cover the texture; this may be attributed to the strong affinity and cross-linking between the oil particles and the active groups in the matrix of the cell wall (Mansour et al., 2017 ).
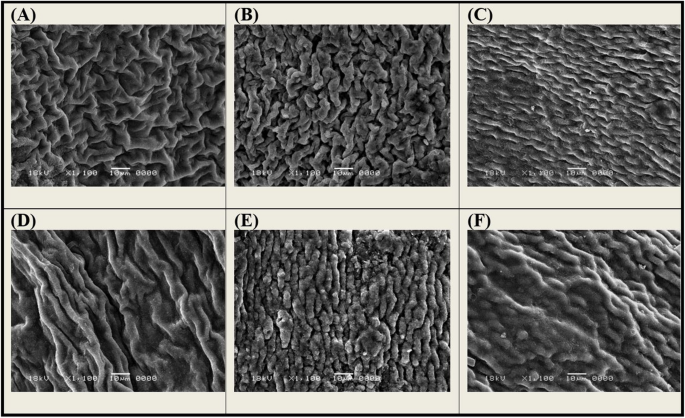
The SEM micrographs (at × 1100 magnification) of the surfaces of Eucalyptus bark forms before and after oil adsorption; A unmodified form, B H-modified form, C OH-modified form, D unmodified oil-loaded form, E H-modified oil-loaded form, F OH-modified oil-loaded form. (Oil adsorption conditions: initial oil concentration, 500 mg/l; adsorbent dosage, 0.5 g/l; pH, 3; exposure time, 45 min; temperature, 20 °C; shaking rate, 300 rpm)
3.9.1 Determination of Functional Groups onto Eucalyptus Bark Biomass by Fourier Transform Infrared
Different forms of Eucalyptus bark under investigation are subjected to FTIR analysis to identify the various groups involved in the oil adsorption process (Table 1 ). The transmittance bands were referred to according to Liu ( 2021 ). The unmodified form was considered as control. Several peaks were observed demonstrating that the surface of Eucalyptus bark is made up of a variety of functional groups. Only the peaks of contributed groups were focused on and discussed. The peaks were corresponding to the hydroxyl group (OH) at 3313.5 cm −1 , C = O in the carboxyl group at 1619.5 cm −1 , C-O in the carboxyl group at 1415.9 cm −1 , nitro group (N = O) at 1316.9 cm −1 , and C-O in the alcoholic group at 1032.3 cm −1 .
The H-modified form exhibited band shifts to higher wavenumber for carboxylic C = O and alcoholic C-O groups; meanwhile, band shifts to lower wavenumber were detected for OH, carboxylic C-O, and N = O groups. A newly developed broadband at 1100–1300 cm −1 for phosphorus compounds due to H 3 PO 4 treatment was detected (Puziy et al., 2002 and Yakout & El-Deen, 2016 ).
The OH-modified form exhibited shifts to higher wavenumber for N = O and alcoholic C-O groups; similar results were detected by Afroze et al. ( 2016 ). Oppositely, a band shift to a lower wavenumber was detected for the carboxylic C = O group. No change was detected in bands corresponding to OH and carboxylic C-O groups.
Regarding the oil-loaded forms, band shifts to higher wavenumbers were exhibited for OH and carboxylic C = O groups; conversely, band shifts to lower wavenumbers were exhibited for the carboxylic C-O group. No change was detected in bands of N = O, alcoholic C-O, and P.
Hence, OH, carboxylic C = O and carboxylic C-O groups are the contributing ones in the oil adsorption process for the three forms of Eucalyptus bark under study.
3.9.2 Comparative Evaluation of Eucalyptus Bark Maximum Oil Removal Efficacy with Other Sorbent Tools
The H-form of Eucalyptus bark in the current study was superior to many other sorbent materials in the removal of oily substances, where its removal efficacy reached 450.69 mg/g. For example, Moi et al. ( 2020 ) used the corn cob carbon to remove the emulsified oil from wastewater and achieved an efficacy of 17.1 mg/g. A relatively higher efficacy was recorded by Anifah et al. ( 2022 ) where the sewage sludge was used to prepare an activated carbon that was able to remove oil and grease from restaurant wastewater by efficacy reached 63.75 mg/g. Recently, Alatabe et al. ( 2023 ) used the sedge cane grass for oil adsorption from wastewater; the maximum removal efficacy was 137.40 mg/g.
3.9.3 Treatment of Real Oily Industrial Wastewater from New Borg El-Arab City
Depending on oil concentrations, five of the ten collected wastewater samples from New Borg El-Arab City were chosen to be treated using the H-form of Eucalyptus bark under the optimum extrapolated conditions. According to the Egyptian Environment Law No. 4 of 1994 (amended in Law No. 9 of 2009), the quality of wastewater samples before and after treatment was evaluated, where oil and grease concentration must be less than 15 mg/l (Table 2 ). As expected, the wastewater of oil factories contains higher oil concentrations than soap ones. Oil concentrations ranged between 18.37 and 41.42 mg/l. The removal efficacy ranged between 91.46 and 96.23% which proves the excellence of Eucalyptus bark in the treatment of oily wastewater.
4 Conclusions
As a specialized study that does not have many references and it has not been studied in depth before, the Eucalyptus bark biomass demonstrated promising findings in the treatment of oily wastewater. Moreover, it is a naturalistic, cheap, obtainable, reusable, and environmentally consonant tool. The optimization results were as follows: initial oil concentration, 500 mg/l; adsorbent dosage, 0.5 g/l; pH, 3; exposure time, 45 min; temperature, 20 °C; and shaking rate: 300 rpm. Eucalyptus bark biomass’s ability to be reused ensures environmental preservation when disposed of after use. Pretreatment of biomass with H 3 PO 4 showed higher significant results, while the raw biomass can be used also with reasonable efficacy in case of the requirements of saving time and cost. Eucalyptus bark surface is rich in many functional groups that make it a potential tool for other pollutant removal.
Data Availability
Datasets generated during the current study are available from the corresponding author on reasonable request.
Aboulsoud, Y. I. E. M. (2008). Removal of certain heavy metals by biomaterials derived from some Egyptian algae . Thesis, Faculty of Science, Ain Shams University, Egypt.
Google Scholar
Afroze, S., Sen, T. K., & Ang, H. M. (2016). Adsorption removal of zinc (II) from aqueous phase by raw and base modified Eucalyptus sheathiana bark: Kinetics, mechanism and equilibrium study. Process Safety and Environmental Protection, 102 , 336–352.
Article CAS Google Scholar
Ahmad, A. L., Bhatia, S., Ibrahim, N., & Sumathi, S. (2005). Adsorption of residual oil from palm oil mill effluent using rubber powder. Brazilian Journal of Chemical Engineering, 22 , 371–379.
Alaa El-Din, G. A., Amer, A. A., Malsh, G., & Hussein, M. (2018). Study on the use of banana peels for oil spill removal. Alexandria Engineering Journal, 57 (3), 2061–2068.
Article Google Scholar
Alatabe, M. J. A., Faris, H. A., & Husham, H. (2023). Natural biosorbent for oil adsorption from produced water by sedge cane. Journal of Ecological Engineering , 24 (10).
Alshiteewi, S. (2023). Preparation of biosorbent from Eucalyptus trees and its application for removal of some pollutants in water.
Anastopoulos, I., Ahmed, M. J., & Hummadi, E. H. (2022). Eucalyptus-based materials as adsorbents for heavy metals and dyes removal from (waste) waters. Journal of Molecular Liquids, 356 , 118864.
Anifah, E. M., Ariani, I. K., Hayati, R. N., & Nugraha, S. A. (2022, October). Adsorption of oil and grease in wastewater using activated carbon derived from sewage sludge. In IOP Conference Series: Earth and Environmental Science (Vol. 1098, No. 1, p. 012043). IOP Publishing.
Assaggaf, H. M., Naceiri Mrabti, H., Rajab, B. S., Attar, A. A., Hamed, M., Sheikh, R. A., ... & Bouyahya, A. (2022). Singular and combined effects of essential oil and honey of Eucalyptus globulus on anti-inflammatory, antioxidant, dermatoprotective, and antimicrobial properties: In vitro and in vivo findings. Molecules , 27 (16), 5121.
Badawy, R. K., Shehata, S. M., & Aboulsoud, Y. I. (2020). Assessment of phyto-filtration and biosorption treatment on the removal of contaminant form wastewater. Natural Science, 18 (2), 16–26.
Bozdogan Sert, E., Turkmen, M., & Cetin, M. (2019). Heavy metal accumulation in rosemary leaves and stems exposed to traffic-related pollution near Adana-İskenderun Highway (Hatay, Turkey). Environmental Monitoring and Assessment, 191 , 1–12.
Bulgariu, L., & Bulgariu, D. (2014). Enhancing biosorption characteristics of marine green algae (Ulva lactuca ) for heavy metals removal by alkaline treatment. Journal of Bioprocessing & Biotechniques, 4 (1), 1.
Cetin, M., Aljama, A. M. O., Alrabiti, O. B. M., Adiguzel, F., Sevik, H., & Zeren Cetin, I. (2022a). Determination and mapping of regional change of Pb and Cr pollution in Ankara city center. Water, Air, & Soil Pollution, 233 (5), 163.
Cetin, M., Aljama, A. M. O., Alrabiti, O. B. M., Adiguzel, F., Sevik, H., & Zeren Cetin, I. (2022b). Using topsoil analysis to determine and map changes in Ni Co pollution. Water, Air, & Soil Pollution, 233 (8), 293.
Cetin, M., Isik Pekkan, O., Bilge Ozturk, G., Senyel Kurkcuoglu, M. A., Kucukpehlivan, T., & Cabuk, A. (2022c). Examination of the change in the vegetation around the Kirka Boron mine site by using remote sensing techniques. Water, Air, & Soil Pollution, 233 (7), 254.
Chai, W., Liu, X., Zou, J., Zhang, X., Li, B., & Yin, T. (2015). Pomelo peel modified with acetic anhydride and styrene as new sorbents for removal of oil pollution. Carbohydrate Polymers, 132 , 245–251.
Daaou, M., & Bendedouch, D. (2012). Water pH and surfactant addition effects on the stability of an Algerian crude oil emulsion. Journal of Saudi Chemical Society, 16 (3), 333–337.
de Carvalho, R. G., Beça, C. G., Neves, O. R., & Pereira, M. S. (1991). Composting of pine and eucalyptus barks. Bioresource Technology, 38 (1), 51–63.
El-Kadi, A. M. A., Betana, M. D., & Hussein, M. A. (2000). Evaluation of the herbicidal activity of the active extracts of Eucalyptus globulus . Egyptian Journal of Applied Sciences, 15 (7), 565–576.
EPA METHOD 1664. (2010). N-hexane extractable material (HEM; oil and grease) and silica gel treated n-hexane extractable material (SGT-HEM; non-polar material) by extraction and gravimetry.
Garba, Z. N., Bello, I., Galadima, A., & Lawal, A. Y. (2016). Optimization of adsorption conditions using central composite design for the removal of copper (II) and lead (II) by defatted papaya seed. Karbala International Journal of Modern Science, 2 (1), 20–28.
Ghodbane, I., & Hamdaoui, O. (2008). Removal of mercury (II) from aqueous media using Eucalyptus bark: Kinetic and equilibrium studies. Journal of Hazardous Materials, 160 (2), 301–309.
Hamid, N. A., Malek, N. C., Mokhtar, H., Mazlan, W. S., Tajuddin, R. M., & Alam, S. (2016). Removal of oil and grease from wastewater using natural adsorbents. Jurnal Teknologi, 78 (5–3), 97–102.
Hayat, U., Jilani, M. I., Rehman, R., & Nadeem, F. (2015). A review on Eucalyptus globulus : A new perspective in therapeutics. International Journal of Chemical and Biochemical Science, 8 , 85–91.
Ighalo, J. O., & Adeniyi, A. G. (2020). Adsorption of pollutants by plant bark derived adsorbents: An empirical review. Journal of Water Process Engineering, 35 , 101228.
Ingole, N. W., Vinchurkar, S. S., & Dharpal, S. V. (2014). Adsorption of oil from waste water by using human hair. Journal of Environmental Science, Computer Science And Engineering & Technology, 3 (1), 207–217.
Jaber, W. S., & Alwared, A. I. (2019). Removal of oil emulsion from aqueous solution by using Ricinus communis leaves as adsorbent. SN Applied Sciences, 1 (8), 1–12.
Kanwal, F., Rehman, R., Mushtaq, M. W., Batool, A., & Naseem, S. (2013). Use of Opuntia dillenii seeds for sorptive removal of acidic textile dyes from water in benign way. Asian Journal of Chemistry, 25 (14), 7710.
Lin, C. C., & Liu, H. S. (2000). Adsorption in a centrifugal field: Basic dye adsorption by activated carbon. Industrial & Engineering Chemistry Research, 39 (1), 161–167.
Liu, X. (2021). IR spectrum and characteristic absorption bands. Organic Chemistry I; Kwantlen Polytechnic University: Surrey, BC, Canada .
Liu, H., & Li, J. (2010). The study of the ecological problems of Eucalyptus plantation and sustainable development in Maoming Xiaoliang. Journal of Sustainable Development, 3 (1), 197.
Mansour, H. A., Badawy, R. K., Shaaban, A. S., Abdel-Rahman, M. E., & Aboulsoud, Y. I. E. (2017). Dyes bio-sorption by two marine algae and their applications on industrial effluents from Borg El-Arab region, Egypt. Egyptian Journal of Botany, 57 (1), 49–73.
Martini, S., Afroze, S., & Roni, K. A. (2020). Modified Eucalyptus bark as a sorbent for simultaneous removal of COD, oil, and Cr (III) from industrial wastewater. Alexandria Engineering Journal, 59 (3), 1637–1648.
Moi, F. Y., Ghazi, R. M., & Alias, M. Z. M. (2020, August). Removal of emulsified oil in wastewater by corn cob carbon. In IOP Conference Series: Earth and Environmental Science (Vol. 549, No. 1, p. 012065). IOP Publishing.
Mota, I., Pinto, P. C. R., Novo, C., Sousa, G., Guerreiro, O., Guerra, Â. R., & Rodrigues, A. E. (2013). Eucalyptus globulus bark as a source of polyphenolic compounds with biological activity. O Papel, 74 (1), 57–64.
Patnukao, P., & Pavasant, P. (2008). Activated carbon from Eucalyptus camaldulensis Dehn bark using phosphoric acid activation. Bioresource Technology, 99 (17), 8540–8543.
Puziy, A. M., Poddubnaya, O. I., Martınez-Alonso, A., Suárez-Garcıa, F., & Tascón, J. M. D. (2002). Synthetic carbons activated with phosphoric acid: I Surface chemistry and ion binding properties. Carbon, 40 (9), 1493–1505.
Saliba, R., Gauthier, H., Gauthier, R., & Petit-Ramel, M. (2002). The use of Eucalyptus barks for the adsorption of heavy metal ions and dyes. Adsorption Science & Technology, 20 (2), 119–129.
Samimi, M. (2024). Efficient biosorption of cadmium by Eucalyptus globulus fruit biomass using process parameters optimization. Global Journal of Environmental Science and Management, 10 (1), 27–38.
CAS Google Scholar
Seng Hua, L.,Wei Chen, L.,Kristak, A. P.,Lubis, L.,Adly, M.& Widya, F., (2024). Eucalyptus : Engineered wood products and other applications. https://doi.org/10.1007/978-981-99-7919-6 .
Shaaban, A. E. S. M., Badawy, R. K., Mansour, H. A., Abdel-Rahman, M. E., & Aboulsoud, Y. I. (2017). Competitive algal biosorption of Al 3+ , Fe 3+ , and Zn 2+ and treatment application of some industrial effluents from Borg El-Arab region, Egypt. Journal of Applied Phycology, 29 , 3221–3234.
Souza, R. S., Porto, P. S., Pintor, A. M., Ruphuy, G., Costa, M. F., Boaventura, R. A., & Vilar, V. J. (2016). New insights on the removal of mineral oil from oil-in-water emulsions using cork by-products: Effect of salt and surfactants content. Chemical Engineering Journal, 285 , 709–717.
Tüzün, I., Bayramoğlu, G., Yalçın, E., Başaran, G., Celik, G., & Arıca, M. Y. (2005). Equilibrium and kinetic studies on biosorption of Hg (II), Cd (II) and Pb (II) ions onto microalgae Chlamydomonas reinhardtii . Journal of Environmental Management, 77 (2), 85–92.
Wahi, R., Chuah, L. A., Ngaini, Z., Nourouzi, M. M., & Choong, T. S. Y. (2014). Esterification of M. sagu bark as an adsorbent for removal of emulsified oil. Journal of Environmental Chemical Engineering, 2 (1), 324–331.
Waller, R. A., & Duncan, D. B. (1969). A Bayes rule for the symmetric multiple comparisons problem. Journal of the American Statistical Association, 64 (328), 1484–1503.
Yakout, S. M., & El-Deen, G. S. (2016). Characterization of activated carbon prepared by phosphoric acid activation of olive stones. Arabian Journal of Chemistry, 9 , S1155–S1162.
Yorgun, S., & Yıldız, D. (2015). Preparation and characterization of activated carbons from Paulownia wood by chemical activation with H 3 PO 4 . Journal of the Taiwan Institute of Chemical Engineers, 53 , 122–131.
Zafar, L., Khan, A., Kamran, U., Park, S. J., & Bhatti, H. N. (2022). Eucalyptus (camaldulensis) bark-based composites for efficient Basic Blue 41 dye biosorption from aqueous stream: Kinetics, isothermal, and thermodynamic studies. Surfaces and Interfaces, 31 , 101897.
Download references
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research was supported and funded by the Desert Research Center, Egypt.
Author information
Authors and affiliations.
Plant Ecology and Range Management Department, Desert Research Center, Cairo, Egypt
Yasmin I. E. Aboulsoud
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Yasmin I. E. Aboulsoud .
Ethics declarations
Competing interests.
The author declares no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Aboulsoud, Y.I.E. Adsorption of Oil from Industrial Wastewater with Eucalyptus globulus Labill. Bark: Optimization Study. Water Air Soil Pollut 235 , 273 (2024). https://doi.org/10.1007/s11270-024-07073-w
Download citation
Received : 12 September 2023
Accepted : 30 March 2024
Published : 20 April 2024
DOI : https://doi.org/10.1007/s11270-024-07073-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Bark pretreatment
- Eucalyptus globulus
- New Borg El-Arab City
- Oily wastewater
- Find a journal
- Publish with us
- Track your research
Unsupervised Clustering-Assisted Method for Consensual Quantitative Analysis of Methanol-Gasoline Blends by Raman Spectroscopy
Affiliations.
- 1 School of Information and Engineering, Suzhou University, Suzhou 234000, China.
- 2 Suzhou Vocational and Technical College, Suzhou 234000, China.
- 3 College of Electrical and Electronic Engineering, Wenzhou University, Wenzhou 325035, China.
- PMID: 38611707
- PMCID: PMC11013198
- DOI: 10.3390/molecules29071427
Methanol-gasoline blends have emerged as a promising and environmentally friendly bio-fuel option, garnering widespread attention and promotion globally. The methanol content within these blends significantly influences their quality and combustion performance. This study explores the qualitative and qualitative analysis of methanol-gasoline blends using Raman spectroscopy coupled with machine learning methods. Experimentally, methanol-gasoline blends with varying methanol concentrations were artificially configured, commencing with initial market samples. For qualitative analysis, the partial least squares discriminant analysis (PLS-DA) model was employed to classify the categories of blends, demonstrating high prediction performance with an accuracy of nearly 100% classification. For the quantitative analysis, a consensus model was proposed to accurately predict the methanol content. It integrates member models developed on clustered variables, using the unsupervised clustering method of the self-organizing mapping neural network (SOM) to accomplish the regression prediction. The performance of this consensus model was systemically compared to that of the PLS model and uninformative variable elimination (UVE)-PLS model. Results revealed that the unsupervised consensus model outperformed other models in predicting the methanol content across various types of methanol gasoline blends. The correlation coefficients for prediction sets consistently exceeded 0.98. Consequently, Raman spectroscopy emerges as a suitable choice for both qualitative and quantitative analysis of methanol-gasoline blend quality. This study anticipates an increasing role for Raman spectroscopy in analysis of fuel composition.
Keywords: Raman spectroscopy; consensus model; methanol–gasoline blends; partial least squares discriminant analysis (PLS-DA); self-organizing mapping (SOM).
Grants and funding
- 2020XJGG01/Outstanding Academic and Technical Youths Backbone of Suzhou University
- 2022sdxx029/Anhui Provincial Department of Education Quality Engineering Project
- 2023BSK023/Ph.D. Research Start-up Fund Project
- KJ2021A1110, 2022AH051371, 2022AH051379, 2023AH052957, 2022AH051376/Anhui Provincial Department of Education Natural Science Research Key Project

IMAGES
VIDEO
COMMENTS
Indoor air quality (IAQ) is the air quality within buildings and structures.Poor indoor air quality due to indoor air pollution is known to affect the health, comfort, and well-being of building occupants. It has also been linked to sick building syndrome, reduced productivity, and impaired learning in schools.Common pollutants of indoor air include: secondhand tobacco smoke, air pollutants ...
An air quality index (AQI) is an indicator developed by government agencies to communicate to the public how polluted the air currently is or how polluted it is forecast to become. As air pollution levels rise, so does the AQI, along with the associated public health risk. Children, the elderly and individuals with respiratory or cardiovascular problems are typically the first groups affected ...
In this study, the levels of sulfur dioxide (SO2) and nitrogen dioxide (NO2) were measured indoors and outdoors using passive samplers in Tymar village (20 homes), an industrial area, and Haji Wsu (15 homes), a non-industrial region, in the summer and the winter seasons. In comparison to Haji Wsu village, the results showed that Tymar village had higher and more significant mean SO2 and NO2 ...
However, at the air quality modeling step, the analysis returns to a foundation based on actual historical conditions and data, providing a form of "ground-truthing" of the results. Specifically, actual 2000 historical air quality ... Chapter 4 presents the air quality modeling methodology and results.
Our study builds on evidence linking air pollution to psychosis and depression and provides rare longitudinal evidence linking noise pollution to anxiety. Our findings indicate that air pollution exposure earlier in development (eg, during pregnancy) might be particularly important, and suggest a degree of specificity in terms of pollutant-outcome associations. If causal, our findings suggest ...
This systematic review and meta-analysis aims to consolidate and examine data on the relationship between air pollution and OSA's risk and severity. Methods: A literature search across PubMed, EMBASE, and Web of Science was conducted until January 10, 2024. The selection criteria targeted studies involving OSA participants or those at risk ...
We suggest that researchers harmonize the core insights of the cases by using descriptive and comparative analysis (standardization). Descriptive analysis involves writing narratives or tabulated summaries of the findings from each case, categorized by their epistemological or methodological approach and/or theoretical core concepts.
The approximation of a normal distribution with a Monte Carlo method. Monte Carlo methods, or Monte Carlo experiments, are a broad class of computational algorithms that rely on repeated random sampling to obtain numerical results. The underlying concept is to use randomness to solve problems that might be deterministic in principle. The name comes from the Monte Carlo Casino in Monaco, where ...
Drone delivery in city logistics is gaining attention due to road congestion, environmental threats, etc. However, there are risks associated with using drones which can result in hazardous events, such as conflicts in the air, loss of control, and system failures. It is crucial to assess the risks involved in using different types of drones and choose the option with the lowest risk. The ...
Clean Water Act Methods Update Rule for the Analysis of Effluent (Final) EPA is finalizing changes to its test procedures required to be used by industries and municipalities when analyzing the chemical, physical, and biological properties of wastewater and other samples for reporting under the EPA's National Pollutant Discharge Elimination ...
In the case of the fully quantitative assessment, the central formula is. TIMr = ∑c × Wc × PIMr,c × Sr,c. (1) where TIM is the territorial impact (total or for each dimension: territorial efficiency, quality, identity); r is the region, c is the criterion or sub-criterion in the multicriteria analysis; PIMr,c is the potential impact of a ...
Fiscal efficiency is separate from economic efficiency. Economic analysis of recycling does not include what economists call externalities: unpriced costs and benefits that accrue to individuals outside of private transactions. Examples include less air pollution and greenhouse gases from incineration and less waste leaching from landfills.
Pollutant Time Series Analysis for Improving Air-Quality in Smart Cities Raúl López-Blanco1*, Miguel Chaveinte García2, Ricardo S. Alonso2,3*, Javier Prieto1*, ... environment by different methods and how they influence the quality of life in cities [17]. In most of the occasions, more than knowing the current situation ...
Water, Air, & Soil Pollution - Controlling the structure of cerium dioxide (CeO2)-based photocatalysts for the removal of organic contaminants in water is essential. ... Mineralization of diazinon by low-cost CuO-Kaolin nanocomposite under visible light based RSM methodology: Kinetics, cost analysis, reaction pathway and bioassay. Journal of ...
This work found that rectorite (REC) has a good adsorption effect on cationic pollutants such as ciprofloxacin (CIP), with a maximum adsorption capacity of 79.73 mg/g for CIP. The zeta potential analysis showed that the electronegativity of the REC surface would be weakened after CIP adsorption under acidic conditions. Through adsorption kinetics, isotherm study, XRD, FTIR, and other ...
A rosette sampler is used for collecting water samples in deep water, such as the Great Lakes or oceans, for water quality testing.. Water quality refers to the chemical, physical, and biological characteristics of water based on the standards of its usage. It is most frequently used by reference to a set of standards against which compliance, generally achieved through treatment of the water ...
Water, Air, & Soil Pollution - Oily wastewater is one of the most hazardous contaminants that can hurt the ecosystem. ... FTIR analysis indicated that OH, carboxylic C = O, and carboxylic C-O groups are the contributing groups in the oil adsorption process for the three forms of Eucalyptus bark. The reusability of Eucalyptus bark using n-hexane ...
The correlation coefficients for prediction sets consistently exceeded 0.98. Consequently, Raman spectroscopy emerges as a suitable choice for both qualitative and quantitative analysis of methanol-gasoline blend quality. This study anticipates an increasing role for Raman spectroscopy in analysis of fuel composition.
A 19th-century assay laboratory in Tombstone Courthouse State Historic Park, Arizona. A model of a late 19th-century Canadian seal used to certify the quality of assayed gold.A metallurgical assay is a compositional analysis of an ore, metal, or alloy, usually performed in order to test for purity or quality.. Some assay methods are suitable for raw materials; others are more appropriate for ...
Mass spectrometry. Mass spectrometry ( MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a mass spectrum, a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures ...