9.5 The Kinetic-Molecular Theory
Learning objectives.
- State the postulates of the kinetic-molecular theory
- Use this theory’s postulates to explain the gas laws
The gas laws that we have seen to this point, as well as the ideal gas equation, are empirical, that is, they have been derived from experimental observations. The mathematical forms of these laws closely describe the macroscopic behavior of most gases at pressures less than about 1 or 2 atm. Although the gas laws describe relationships that have been verified by many experiments, they do not tell us why gases follow these relationships.
The kinetic molecular theory (KMT) is a simple microscopic model that effectively explains the gas laws described in previous modules of this chapter. This theory is based on the following five postulates described here. (Note: The term “molecule” will be used to refer to the individual chemical species that compose the gas, although some gases are composed of atomic species, for example, the noble gases.)
- Gases are composed of molecules that are in continuous motion, travelling in straight lines and changing direction only when they collide with other molecules or with the walls of a container.
- The molecules composing the gas are negligibly small compared to the distances between them.
- The pressure exerted by a gas in a container results from collisions between the gas molecules and the container walls.
- Gas molecules exert no attractive or repulsive forces on each other or the container walls; therefore, their collisions are elastic (do not involve a loss of energy).
- The average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas.
The test of the KMT and its postulates is its ability to explain and describe the behavior of a gas. The various gas laws can be derived from the assumptions of the KMT, which have led chemists to believe that the assumptions of the theory accurately represent the properties of gas molecules. We will first look at the individual gas laws (Boyle’s, Charles’s, Amontons’s, Avogadro’s, and Dalton’s laws) conceptually to see how the KMT explains them. Then, we will more carefully consider the relationships between molecular masses, speeds, and kinetic energies with temperature, and explain Graham’s law.

The Kinetic-Molecular Theory Explains the Behavior of Gases, Part I
Recalling that gas pressure is exerted by rapidly moving gas molecules and depends directly on the number of molecules hitting a unit area of the wall per unit of time, we see that the KMT conceptually explains the behavior of a gas as follows:
- Amontons’s law. If the temperature is increased, the average speed and kinetic energy of the gas molecules increase. If the volume is held constant, the increased speed of the gas molecules results in more frequent and more forceful collisions with the walls of the container, therefore increasing the pressure ( Figure 9.31 ).
- Charles’s law. If the temperature of a gas is increased, a constant pressure may be maintained only if the volume occupied by the gas increases. This will result in greater average distances traveled by the molecules to reach the container walls, as well as increased wall surface area. These conditions will decrease the both the frequency of molecule-wall collisions and the number of collisions per unit area, the combined effects of which balance the effect of increased collision forces due to the greater kinetic energy at the higher temperature.
- Boyle’s law. If the gas volume of a given amount of gas at a given temperature is decreased (that is, if the gas is compressed ), the molecules will be exposed to a decreased container wall area. Collisions with the container wall will therefore occur more frequently and the pressure exerted by the gas will increase ( Figure 9.31 ).
- Avogadro’s law. At constant pressure and temperature, the frequency and force of molecule-wall collisions are constant. Under such conditions, increasing the number of gaseous molecules will require a proportional increase in the container volume in order to yield a decrease in the number of collisions per unit area to compensate for the increased frequency of collisions ( Figure 9.31 ).
- Dalton’s Law. Because of the large distances between them, the molecules of one gas in a mixture bombard the container walls with the same frequency whether other gases are present or not, and the total pressure of a gas mixture equals the sum of the (partial) pressures of the individual gases.
molecular speeds and Kinetic Energy
The previous discussion showed that the KMT qualitatively explains the behaviors described by the various gas laws. The postulates of this theory may be applied in a more quantitative fashion to derive these individual laws. To do this, we must first look at speeds and kinetic energies of gas molecules, and the temperature of a gas sample.
In a gas sample, individual molecules have widely varying speeds; however, because of the vast number of molecules and collisions involved, the molecular speed distribution and average speed are constant. This molecular speed distribution is known as a Maxwell-Boltzmann distribution, and it depicts the relative numbers of molecules in a bulk sample of gas that possesses a given speed ( Figure 9.32 ).
The kinetic energy (KE) of a particle of mass ( m ) and speed ( u ) is given by:
Expressing mass in kilograms and speed in meters per second will yield energy values in units of joules (J = kg m 2 s –2 ). To deal with a large number of gas molecules, we use averages for both speed and kinetic energy. In the KMT, the root mean square speed of a particle, u rms , is defined as the square root of the average of the squares of the speeds with n = the number of particles:
The average kinetic energy for a mole of particles, KE avg , is then equal to:
where M is the molar mass expressed in units of kg/mol. The KE avg of a mole of gas molecules is also directly proportional to the temperature of the gas and may be described by the equation:
where R is the gas constant and T is the kelvin temperature. When used in this equation, the appropriate form of the gas constant is 8.314 J/mol⋅K (8.314 kg m 2 s –2 mol –1 K –1 ). These two separate equations for KE avg may be combined and rearranged to yield a relation between molecular speed and temperature:
Example 9.23
Calculation of u rms.
Determine the molar mass of nitrogen in kilograms:
Replace the variables and constants in the root-mean-square speed equation, replacing Joules with the equivalent kg m 2 s –2 :
Check Your Learning
If the temperature of a gas increases, its KE avg increases, more molecules have higher speeds and fewer molecules have lower speeds, and the distribution shifts toward higher speeds overall, that is, to the right. If temperature decreases, KE avg decreases, more molecules have lower speeds and fewer molecules have higher speeds, and the distribution shifts toward lower speeds overall, that is, to the left. This behavior is illustrated for nitrogen gas in Figure 9.33 .
At a given temperature, all gases have the same KE avg for their molecules. Gases composed of lighter molecules have more high-speed particles and a higher u rms , with a speed distribution that peaks at relatively higher speeds. Gases consisting of heavier molecules have more low-speed particles, a lower u rms , and a speed distribution that peaks at relatively lower speeds. This trend is demonstrated by the data for a series of noble gases shown in Figure 9.34 .
Link to Learning
The gas simulator may be used to examine the effect of temperature on molecular speeds. Examine the simulator’s “energy histograms” (molecular speed distributions) and “species information” (which gives average speed values) for molecules of different masses at various temperatures.
The Kinetic-Molecular Theory Explains the Behavior of Gases, Part II
According to Graham’s law, the molecules of a gas are in rapid motion and the molecules themselves are small. The average distance between the molecules of a gas is large compared to the size of the molecules. As a consequence, gas molecules can move past each other easily and diffuse at relatively fast rates.
The rate of effusion of a gas depends directly on the (average) speed of its molecules:
Using this relation, and the equation relating molecular speed to mass, Graham’s law may be easily derived as shown here:
The ratio of the rates of effusion is thus derived to be inversely proportional to the ratio of the square roots of their masses. This is the same relation observed experimentally and expressed as Graham’s law.
As an Amazon Associate we earn from qualifying purchases.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution License and you must attribute OpenStax.
Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction
- Authors: Paul Flowers, Klaus Theopold, Richard Langley, William R. Robinson, PhD
- Publisher/website: OpenStax
- Book title: Chemistry 2e
- Publication date: Feb 14, 2019
- Location: Houston, Texas
- Book URL: https://openstax.org/books/chemistry-2e/pages/1-introduction
- Section URL: https://openstax.org/books/chemistry-2e/pages/9-5-the-kinetic-molecular-theory
© Jan 8, 2024 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.
- Chapter 9: Gases
Kinetic Molecular Theory Practice Questions
Chemistry end of chapter exercises.
Using the postulates of the kinetic molecular theory, explain why a gas uniformly fills a container of any shape.
Can the speed of a given molecule in a gas double at constant temperature? Explain your answer.
Yes. At any given instant, there are a range of values of molecular speeds in a sample of gas. Any single molecule can speed up or slow down as it collides with other molecules. The average velocity of all the molecules is constant at constant temperature.
Describe what happens to the average kinetic energy of ideal gas molecules when the conditions are changed as follows:
(a) The pressure of the gas is increased by reducing the volume at constant temperature.
(b) The pressure of the gas is increased by increasing the temperature at constant volume.
(c) The average velocity of the molecules is increased by a factor of 2.
The distribution of molecular velocities in a sample of helium is shown in [link] . If the sample is cooled, will the distribution of velocities look more like that of H 2 or of H 2 O? Explain your answer.
H 2 O. Cooling slows the velocities of the He atoms, causing them to behave as though they were heavier.
What is the ratio of the average kinetic energy of a SO 2 molecule to that of an O 2 molecule in a mixture of two gases? What is the ratio of the root mean square speeds, u rms , of the two gases?
A 1-L sample of CO initially at STP is heated to 546 K, and its volume is increased to 2 L.
(a) What effect do these changes have on the number of collisions of the molecules of the gas per unit area of the container wall?
(b) What is the effect on the average kinetic energy of the molecules?
(c) What is the effect on the root mean square speed of the molecules?
(a) The number of collisions per unit area of the container wall is constant. (b) The average kinetic energy doubles. (c) The root mean square speed increases to $\sqrt{2}$ times its initial value; $u_{rms}$ is proportional to $\sqrt{KE_{avg}}$
The root mean square speed of H 2 molecules at 25 °C is about 1.6 km/s. What is the root mean square speed of a N 2 molecule at 25 °C?
Answer the following questions:
(a) Is the pressure of the gas in the hot-air balloon shown at the opening of this chapter greater than, less than, or equal to that of the atmosphere outside the balloon?
(b) Is the density of the gas in the hot-air balloon shown at the opening of this chapter greater than, less than, or equal to that of the atmosphere outside the balloon?
(c) At a pressure of 1 atm and a temperature of 20 °C, dry air has a density of 1.2256 g/L. What is the (average) molar mass of dry air?
(d) The average temperature of the gas in a hot-air balloon is $1.30×10^2$ °F. Calculate its density, assuming the molar mass equals that of dry air.
(e) The lifting capacity of a hot-air balloon is equal to the difference in the mass of the cool air displaced by the balloon and the mass of the gas in the balloon. What is the difference in the mass of 1.00 L of the cool air in part (c) and the hot air in part (d)?
(f) An average balloon has a diameter of 60 feet and a volume of $1.1×10^5\;ft^3$. What is the lifting power of such a balloon? If the weight of the balloon and its rigging is 500 pounds, what is its capacity for carrying passengers and cargo?
(g) A balloon carries 40.0 gallons of liquid propane (density 0.5005 g/L). What volume of CO 2 and H 2 O gas is produced by the combustion of this propane?
(h) A balloon flight can last about 90 minutes. If all of the fuel is burned during this time, what is the approximate rate of heat loss (in kJ/min) from the hot air in the bag during the flight?
(a) equal; (b) less than; (c) 29.48 g mol −1 ; (d) 1.0966 g L −1 ; (e) 0.129 g/L; (f) $4.01×10^5$ g; net lifting capacity = 384 lb; (g) 270 L; (h) 39.1 kJ min −1
Show that the ratio of the rate of diffusion of Gas 1 to the rate of diffusion of Gas 2,$\frac{R_1}{R_2}$ is the same at 0 °C and 100 °C.
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
Unit 8: Gases and kinetic molecular theory
About this unit.
This unit is part of the Chemistry library. Browse videos, articles, and exercises by topic.
Ideal gas equation
- The ideal gas law (PV = nRT) (Opens a modal)
- Worked example: Using the ideal gas law to calculate number of moles (Opens a modal)
- Worked example: Using the ideal gas law to calculate a change in volume (Opens a modal)
- Gas mixtures and partial pressures (Opens a modal)
- Dalton's law of partial pressure (Opens a modal)
- Worked example: Calculating partial pressures (Opens a modal)
- Worked example: Vapor pressure and the ideal gas law (Opens a modal)
- Ideal gas law 4 questions Practice
- Calculations using the ideal gas equation 4 questions Practice
Kinetic molecular theory
- The kinetic molecular theory of gases (Opens a modal)
- Kinetic molecular theory and the gas laws (Opens a modal)
- The Maxwell–Boltzmann distribution (Opens a modal)
- Kinetic molecular theory 4 questions Practice

Non-ideal gas behavior
- Introduction to real gases (Opens a modal)
- Real gases: Deviations from ideal behavior (Opens a modal)
- The van der Waals equation (Opens a modal)
- Non-ideal behavior of gases (Opens a modal)
- Deviation from ideal gas law 4 questions Practice
- International
- Schools directory
- Resources Jobs Schools directory News Search

Kinetic Molecular Theory HOMEWORK sets w/ ANSWERS, Sample Test Questions, States of Matter Chemistry
Subject: Chemistry
Age range: 11 - 18
Resource type: Worksheet/Activity
Last updated
22 February 2018
- Share through email
- Share through twitter
- Share through linkedin
- Share through facebook
- Share through pinterest

Tes paid licence How can I reuse this?
Get this resource as part of a bundle and save up to 58%
A bundle is a package of resources grouped together to teach a particular topic, or a series of lessons, in one place.
Chemistry Homework Bundle w/ ANSWERS and Sample Test Questions, editable
Homework sets, worksheets and sample test questions to support the learning of the following units of chemistry: 1) Atomic Structure 2) The Periodic Table, Periodic Trends 3) Ionic Bonding and Metallic Bonding 4) Covalent Bonding, Intermolecular Forces 5) Naming Compounds and Writing Formulas 6) Kinetic Molecular Theory of Matter • Includes over 400 questions in all! • All questions are editable. • All answers are included. Save over 50% with this bundle! This resource is meant to be used by teachers in the US.
Chemistry Homework Bundle w/ ANSWERS and Multiple Choice Exam Practice, editable pack 2
This resource includes 6 units of chemistry homework, worksheets and sample test questions. Unit 1: Kinetic Molecular Theory, States of Matter Unit 2: Gas Properties and the Gas Laws Unit 3: Chemical Names and Formulas (Ionic/Covalent compounds and Acids/Bases) Unit 4: The Chemistry of Water Unit 5: Solutions, Solubility, Concentrations Unit 6: The Periodic Table, Periodic Trends • Includes over 400 questions in all! • All questions are editable. • All answers are included. Months of homework and practice problems for just £5. Save over 50% with this bundle. These resources are meant for teachers in the US.
Your rating is required to reflect your happiness.
It's good to leave some feedback.
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resource to let us know if it violates our terms and conditions. Our customer service team will review your report and will be in touch.
Not quite what you were looking for? Search by keyword to find the right resource:

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

Kinetic Molecular Theory of Gases
- Last updated
- Save as PDF
- Page ID 1406
To better understand the molecular origins of the ideal gas law ,
the basics of the Kinetic Molecular Theory of Gases ( KMT ) should be understood. This model is used to describe the behavior of gases. More specifically, it is used to explain macroscopic properties of a gas, such as pressure and temperature, in terms of its microscopic components, such as atoms. Like the ideal gas law, this theory was developed in reference to ideal gases, although it can be applied reasonably well to real gases.
In order to apply the kinetic model of gases, five assumptions are made:
- Gases are made up of particles with no defined volume but with a defined mass. In other words their volume is miniscule compared to the distance between themselves and other molecules.
- Gas particles undergo no intermolecular attractions or repulsions. This assumption implies that the particles possess no potential energy and thus their total energy is simply equal to their kinetic energies.
- Gas particles are in continuous, random motion.
- Collisions between gas particles are completely elastic. In other words, there is no net loss or gain of kinetic energy when particles collide.
- The average kinetic energy is the same for all gases at a given temperature, regardless of the identity of the gas. Furthermore, this kinetic energy is proportional to the absolute temperature of the gas.
Temperature and KMT
The last assumption can be written in equation form as:
\[KE = \dfrac{1}{2}mv^2 = \dfrac{3}{2}k_BT\]
- \(k_B\) is Boltzmann's constant (k B = 1.381×10 -23 m 2 kg s -2 K -1 ) and
- \(T\) is the absolution temperature (in Kelvin)
This equation says that the speed of gas particles is related to their absolute temperature. In other words, as their temperature increases, their speed increases, and finally their total energy increases as well. However, it is impossible to define the speed of any one gas particle. As such, the speeds of gases are defined in terms of their root-mean-square speed.
Pressure and KMT
The macroscopic phenomena of pressure can be explained in terms of the kinetic molecular theory of gases. Assume the case in which a gas molecule (represented by a sphere) is in a box, length L ( Figure 1). Through using the assumptions laid out above, and considering the sphere is only moving in the x-direction, we can examine the instance of the sphere colliding elastically with one of the walls of the box.
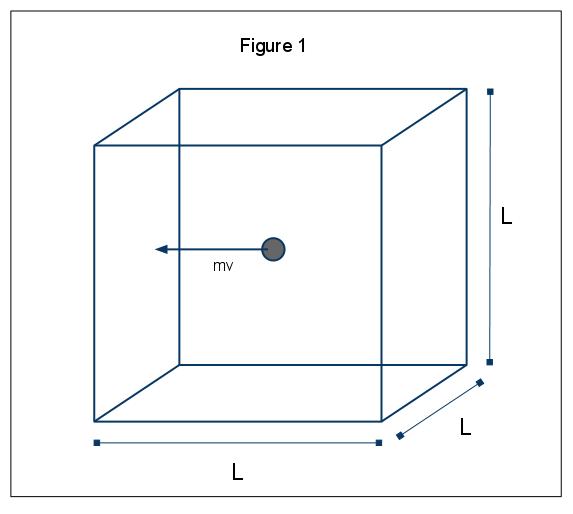
The momentum of this collision is given by p=mv , in this case p=mv x , since we are only considering the x dimension. The total momentum change for this collision is then given by
\[mv_x - m(-v_x) = 2mv_x\]
Given that the amount of time it takes between collisions of the molecule with the wall is L/v x we can give the frequency of collisions of the molecule against a given wall of the box per unit time as v x /2L. One can now solve for the change in momentum per unit of time:
\[(2mv_x)(v_x/2L) = mv_x^2/L\]
Solving for momentum per unit of time gives the force exerted by an object ( F=ma=p/time ). With the expression that F = mv x 2 /L one can now solve for the pressure exerted by the molecular collision, where area is given as the area of one wall of the box, A=L 2 :
\[P=\dfrac{F}{A}\]
\[P=\dfrac{mv_x^2}{[L(L^2)}\]
The expression can now be written in terms of the pressure associated with collisions from N number of molecules:
\[P=\dfrac{Nmv_x^2}{V}\]
This expression can now be adjusted to account for movement in the x, y and z directions by using mean-square velocity for three dimensions and a large value of N . The expression now is written as:
\[P={\dfrac{Nm\overline{v}^2}{3V}}\]
This expression now gives pressure, a macroscopic quality, in terms of atomic motion. The significance of the above relationship is that pressure is proportional to the mean-square velocity of molecules in a given container. Therefore, as molecular velocity increases so does the pressure exerted on the container.
- Oxtoby, Gillis and Campion. Principles of Modern Chemistry . 6th Edition. California: Thomson Brooks/Cole. 2008.
- Chang, Raymond. Physical Chemistry for the Biosciences . California: University Science Books. 2005.
Contributors and Attributions
- Sevini Shahbaz, Andrew Cooley

IMAGES
VIDEO
COMMENTS
See Answer. Question: Kinetic molecular theory explains how the molecular nature of gases contribute to their properties and behavior. Select all of the statements that support the assumptions of the kinetic molecular theory:\\nThere are no attractive forces between the gas particles. \\nThe gas particles are close together in proximity and ...
The kinetic molecular theory (KMT) is a simple microscopic model that effectively explains the gas laws described in previous modules of this chapter. This theory is based on the following five postulates described here. (Note: The term "molecule" will be used to refer to the individual chemical species that compose the gas, although some ...
The five basic tenets of the kinetic-molecular theory are as follows: A gas is composed of molecules that are separated by average distances that are much greater than the sizes of the molecules themselves. The volume occupied by the molecules of the gas is negligible compared to the volume of the gas itself. The molecules of an ideal gas exert ...
The Kinetic-Molecular Theory Explains the Behavior of Gases, Part I. Recalling that gas pressure is exerted by rapidly moving gas molecules and depends directly on the number of molecules hitting a unit area of the wall per unit of time, we see that the KMT conceptually explains the behavior of a gas as follows: Amontons's law.
The kinetic-molecular theory (KMT) is a simple molecular model that effectively explains the physical properties of matter using the motion of the molecules. This theory, focusing on gases, is based on the following five postulates described here. (Note: The term "molecule" will be used to refer to the individual chemical species that compose the gas, although some gases are composed of ...
Explain your answer. Solution. Describe what happens to the average kinetic energy of ideal gas molecules when the conditions are changed as follows: (a) The pressure of the gas is increased by reducing the volume at constant temperature. (b) The pressure of the gas is increased by increasing the temperature at constant volume.
Gas variables and constants: P = pressure V = volume T = temperature n = number of moles m = mass v = velocity R = 8.314 J mol − 1 K − 1 = 0.082 06 L atm mol − 1 K − 1 = 62.36 L Torr mol − 1 K − 1. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more.
Learn. The ideal gas law (PV = nRT) Worked example: Using the ideal gas law to calculate number of moles. Worked example: Using the ideal gas law to calculate a change in volume. Gas mixtures and partial pressures. Dalton's law of partial pressure. Worked example: Calculating partial pressures.
Which of the following is TRUE about the kinetic molecular theory of gases? a) It is a set of rules that all gases follow, all the time. b) it defines the chemical makeup of specific types of gases. c) it helps us make predictions about how a gas may behave. c) it helps us make predictions about how a gas may behave.
Summary: kinetic-molecular theory is a theory that explains the states of matter and is based on the idea that matter is composed of tiny particles that are always in motion. The theory is most easily understood as it applies to gases and it is with gases that we will begin our detailed study. An ideal gas is an imaginary gas whose behavior ...
Ideal Gas Law Equation. PV=nRT. properties of gases. fills any container, easily compressed, diffuses, exerts pressure on its surroundings. ideal gas behavior. minimal interactions with other gas particles. small in size, contain almost no volume. Kinetic Molecular Theory.
Describe the behavior of an ideal gas. The kinetic-molecular theory is a theory that explains the states of matter and is based on the idea that matter is composed of tiny particles that are always in motion. The theory helps explain observable properties and behaviors of solids, liquids, and gases. However, the theory is most easily understood ...
Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. Study how it interacts with gas laws, and view examples. Updated: 11/21/2023
Chemistry chapter 10 homework. A hypothetical gas that perfectly fits all the assumptions of the kinetic- molecular theory is known as. Click the card to flip 👆. Ideal gas. Click the card to flip 👆. 1 / 50.
The kinetic molecular theory (KMT) is a simple microscopic model that effectively explains the gas laws described in previous modules of this chapter. This theory is based on the following five postulates described here. ... [reveal-answer q="997706″]Selected Answers[/reveal-answer] [hidden-answer a="997706″] 2. Yes. At any given ...
Homework sets, worksheet, and sample test questions to support the learning of Kinetic Molecular Theory for high school chemistry students. Includes 71 questions in all. All questions are EDITABLE and ANSWERS are included. This resource is meant to be used by teachers in the US.
To better understand the molecular origins of the ideal gas law, PV = nRT (1) (1) P V = n R T. the basics of the Kinetic Molecular Theory of Gases ( KMT) should be understood. This model is used to describe the behavior of gases. More specifically, it is used to explain macroscopic properties of a gas, such as pressure and temperature, in terms ...