Whey protein supplementation and its potentially adverse effects on health: a systematic review
Affiliations.
- 1 Faculty of Medicine, Drug Research and Development Center, Federal University of Ceara, Fortaleza, Ceará 60430-275, Brazil.
- 2 Health Science Center, Ceará State University, Fortaleza, Ceará 60714-903, Brazil.
- PMID: 32702243
- DOI: 10.1139/apnm-2020-0370
Whey protein comprises soluble whey proteins and its benefits are well described in the literature. However, there are not many studies investigating the potential adverse effect of a diet with indiscriminate use of this supplement. The aim of this study was to perform a systematic review of papers that addressed this theme. A search was conducted in Medline, LILACS, TOXNET, Web of science, and Scopus electronic databases. In the end, 11 documents comprised this review. The majority of the papers associated the damaging effect with the chronic and abusive use of whey protein, with the kidneys and liver being the main organs affected. The other studies related whey protein to aggravation of aggression, presence of acne, and modification of the microbiota. Therefore, excessive consumption over a long period of protein supplementation may have some adverse effects on the body, which is aggravated when associated with sedentary lifestyle. PROSPERO registration no.: CRD42020140466. Novelty: A systematic review of experimental and randomized studies about the use of whey proteins supplements and its impact on physical health. Analysis revealed that chronic and without professional guidance use of whey protein supplementation may cause some adverse effects specially on kidney and liver function. Presented data support a need for future studies co-relating the use of different types of whey protein with and without exercise to better see the impact on human physical health.
Keywords: adverse effects; effets indésirables; effets négatifs; hepatotoxicity; hyperproteic diet; hépatotoxicité; negative effects; protein supplementation; protéine lactosérique; régime hyperprotéique; supplémentation en protéines; whey protein.

Publication types
- Systematic Review
- Diet / adverse effects*
- Diet / methods
- Dietary Supplements / adverse effects*
- Gastrointestinal Microbiome / drug effects
- Health Status*
- Kidney Diseases / etiology
- Liver Diseases / etiology
- Whey Proteins / administration & dosage
- Whey Proteins / adverse effects*
- Whey Proteins
- Search Menu
- Advance Articles
- Editor's Choice
- Supplements
- E-Collections
- Virtual Roundtables
- Author Videos
- Author Guidelines
- Submission Site
- Open Access Options
- About The European Journal of Public Health
- About the European Public Health Association
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Terms and Conditions
- Explore Publishing with EJPH
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
- < Previous
Whey protein supplementation in muscle hypertrophy
- Article contents
- Figures & tables
- Supplementary Data
C Sobral, D Gomes, M Silva, P Martins, A Baltazar, Whey protein supplementation in muscle hypertrophy, European Journal of Public Health , Volume 30, Issue Supplement_2, June 2020, ckaa040.004, https://doi.org/10.1093/eurpub/ckaa040.004
- Permissions Icon Permissions
Introduction The nutritional intake in the context of a hypertrophy training can affect body composition, the increase of muscle mass and strength. Whey protein seems to promote a reduction in body fat, improve hypertrophy, and other potential health benefits. This protein is one of the highest quality proteins due to its amino acid content (especially leucine) and to the rapid digestibility. Whey protein consumption has a robust ability to stimulate muscle protein synthesis. There are 3 types of whey protein: controlled, insulated and hydrolysed.
Objectives The goal of this research is verifying the efficacy of the use of whey protein supplementation in athletes seeking muscle strength and mass gain.
Methodology *
We analysed scientific articles from 2014 to 2019, based on Pubmed and Google scholar. Keywords such as whey protein, supplement, hypertrophy, muscle mass and nutrition were used.
Results From scientific research, six articles were analysed. Thus, it is estimated the analysis of 13 men under the age of 18 years and under 30 years of age. Only men with a percentage of fat mass less than or equal to 23% were selected. Two groups were created: the control group that consumed carbohydrate supplements and the experimental group that consumed 36g/day whey protein. Both groups performed 3 sessions/week of resistance training, and the used dose of protein derived from the diet was 1.4g/Kg/day. Both groups experienced gains in strength, 1 maxi (RM) and fat-free mass (FFM). However, the experimental group had an extra 9% and 27% in 1RM and FFM respectively compared to the control group.
Conclusion In short, supplementation with whey protein contributes to the increase of muscle mass and strength in athletes whose training (frequency, volume and duration) and diet are suitable for this purpose.
- amino acids
- body composition
- muscle proteins
- hypertrophy
- science of nutrition
- fat-free mass
- muscle hypertrophy
- muscle mass
- muscle strength
- whey proteins
- carbohydrate supplement
- strength training
Email alerts
Citing articles via.
- Contact EUPHA
- Recommend to your Library
Affiliations
- Online ISSN 1464-360X
- Print ISSN 1101-1262
- Copyright © 2024 European Public Health Association
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Open access
- Published: 31 May 2019
Effectiveness of whey protein supplements on the serum levels of amino acid, creatinine kinase and myoglobin of athletes: a systematic review and meta-analysis
- Fui-Ching Lam 1 ,
- Tahir Mehmood Khan 1 , 2 ,
- Hani Faidah 3 ,
- Abdul Haseeb 4 , 5 &
- Amer Hayat Khan 5
Systematic Reviews volume 8 , Article number: 130 ( 2019 ) Cite this article
11k Accesses
8 Citations
234 Altmetric
Metrics details
Consuming whey protein supplements, along with physiotherapy and psychotherapy, have been recognised in sports performance. Whey protein supplements (WPS) is one of the commonly used supplements as ergogenic aids for athletes to enhance their muscle performance and recovery during sport-related injuries. The purpose of this systematic review is to investigate the effectiveness of WPS over the blood biochemistry mainly amino acids, creatinine kinase and myoglobin which influence performance and recovery among athletes.
A comprehensive literature search was conducted to identify randomised control trials (RCTs) and non-RCTs that investigated the effectiveness of WPS on amino acids, creatinine kinase and myoglobin among athletes. Risk of Bias in Non-Randomised Studies of Interventions tool (ROBINS-I) and Cochrane Risk of Bias Assessment tool were used to rule out the quality of studies. Meta-analysis was performed using a random effect model with STATA version 14.2. The weighted mean difference was used to estimate the effectiveness of WPS against other supplements.
A total of 333,257 research articles were identified; of these, 15 records were included to proceed with the analysis. Meta-analysis has shown that WPS has significantly overall increased the level of essential amino acids level by 624.03 nmol/L (CI = 169.27, 1078.8; I 2 = 100%; p = 0.00) and branched-chain amino acids level by 458.57 nmol/L (CI = 179.96, 737.18; I 2 = 100%; p = 0.00) compared to the control group (without WPS). Moreover, was observed to decrease myoglobin level by 11.74 ng/ml (CI = − 30.24, 6.76; I 2 = 79.6%; p = 0.007) and creatine kinase level by 47.05 U/L (CI = − 129.47, 35.37; I 2 = 98.4%; p = 0.000) compared to the control group.
The findings revealed that the clinical evidence supports the effectiveness of WPS as a positive ergogenic aid on athletes’ amino acids, creatinine kinase and myoglobin.
Peer Review reports
Introduction
Athletes experience fatigue when they continuously undertake intensive physical training. Both muscular and mental fatigue assist to prevent the body from experiencing muscle damage and fracture injuries [ 1 ]. In some situations, athletes are motivated to carry on their routine exercise, regardless of fatigue [ 2 ]. This will lead them to muscle soreness which also known as delayed onset muscle soreness (DOMS) When inadequate rest and lack of care towards the DOMS, this can further lead to loss of skeletal muscle mass and induce muscle damages and fracture injuries known as sports injuries [ 3 ]. Therefore, observing creatinine kinase and myoglobin level are essential as they are biomarkers for the presence of muscle damage or inflammation after intensive exercise [ 4 , 5 ].
In addition to physiotherapy sessions, athletes consume medications and supplements to boost the recovery process and performance. Often, it happened that some supplements do not disclose the presence of some illegal substances which prohibited by doping agencies—for example, anabolic androgenic steroids, diuretics and epinephrine—which can jeopardise athletes’ careers as they may face penalties or be removed from competitions [ 6 ]. Moreover, due to the lack of quality control, some supplements might contain some substance that is prohibited, or in some case, the concentration of that specific substance may be higher than the allowed dose or limits. In some cases, these substances lead to additional complications that prolong the recovery process and mean opportunities to participate in competitions are lost [ 7 ].
The World Anti-Doping Agency (WADA) is cautious in supplementation consumption among athletes. A WADA-accredited laboratory examined 600 nutritional supplements and found that approximately 15% (%) contained anabolic steroids, which was not disclosed on the bottle label, packaging or leaflets [ 8 ]. One of the most widely used supplements adopted to the WADA recommendations is WPS [ 9 ]. Whey protein has had a large impact on nutritional supplements for the community especially athletes as it contains nearly 50% of essential amino acids (EAA) and about 26% of branched-chain amino acids (BCAA). Moreover, the amino acid composition provided by whey protein has a similar pattern to human skeletal muscle amino acid composition, so it is absorbed more rapidly than other protein sources [ 1 ]. About 60% of the protein can stimulate skeletal muscles in the human body [ 10 ]. Moreover, whey protein can reduce fatigue augmenting muscle protein synthesis and slightly suppresses muscle protein breakdown [ 11 ]. To date, there are few systematic reviews that have explored the impact of whey protein on the body composition and resistance workout-induced improvements in muscle mass and strength [ 12 , 13 ]. However, there is hardly any systematic evidence that investigate the effectiveness of WPS over the blood biochemistry mainly amino acids, creatinine kinase, and myoglobin which influence performance and recovery among athletes. The current systematic review will specifically look into this aspect of WPS and aim to statistically rule out the effect of WPS on the blood biochemistry; amino acids, creatinine kinase and myoglobin of athletes.
A systematic review was conducted to investigate the effectiveness of WPS over the blood biochemistry mainly amino acids, creatinine kinase and myoglobin which influence performance and recovery among athletes. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) were used to perform the systematic search [ 14 ] A protocol of this systematic review is registered in PROSPERO 2016 [CRD42016041842] [ 15 ].
Search terms and search strings
The search strategy used the keyword of “whey*” combined individually with “athlete*”, “injury*”, “muscle*”, “perform*” and “recover*” to find relevant articles from the databases [ 16 ]. Thesaurus terms were applied to medical databases such as PubMed and EMBASE, which were Medical Subject Headings (MeSH) and Embase Subject Headings (EMTREE) [ 17 ].
Proper care was taken to remove the error by resetting filters. For instance, the PubMed database has a filtering function for selected species of human or animal. When filtered on animal species’ studies, studies examined on humans were found, as the WP could originate from cow’s milk. Therefore, when filtered on human species only, studies categorised under the animal species that examined humans may have been omitted. Hence, the databases’ filtering or customising functions were not used as the function would eliminate relevant articles.
Databases selected
Comprehensive literature search was done across medical and health science database such as PubMed, EMBASE via Ovid, Scopus, Cochrane, Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCOhost, SPORTDiscus, Health & Medicine Database via ProQuest, Wiley Online Library, Web of Science, ScienceDirect, Taylor & Francis and SAGE. Manual searches in bibliographies of relevant review articles were also performed to identify any other paper that was not indexed in the selected databases. In addition, all the sport-related journals were individually searched for any potential paper that might meet the inclusion criteria.
Inclusive studies design
Inclusive studies design for the systematic review was randomised controlled trials (RCTs) and non-RCTs designs. No restriction was placed on language. The searched timeframe was from the inception of the databases until 31 January 2017. However, study designs on expert opinions, case reports/series, surveys, review articles, editorials, commercial advertisements, magazine articles, unpublished articles and theses were excluded.
Population intervention comparator and outcomes (PICO)
The population includes active athletes who experienced fatigue and had recovered and/or been hindered in their performance. Studies observed on retired athletes, mixed athletes with non-athletes, animals, cells and gels were excluded.
Intervention
The interventions include whey protein or supplements containing whey protein. The intervention can be found in the form of isolate, concentrate, hydrolysate, denature and protein bars.
The comparators were carbohydrate supplements, protein-containing foods from animal sources (e.g., meat, fish, dairy products, and eggs), protein-containing vegetarian sources (e.g., tofu, legumes, and soy protein), vitamins (e.g., multivitamin, vitamin B, beta-carotene, and folic acid), minerals (e.g., calcium, iron and zinc) and placebos (include no treatment and treatment as usual).
The outcome of interest observed is the effect of WPS amino acids, creatinine kinase and myoglobin.
Conducting the search and selection process
The relevant articles were compiled, and duplicate articles were removed by using EndNote X7. Then, a screening was done on titles and abstracts of the relevant articles based on the inclusion and the exclusion criteria. After that, full-text articles of the screened articles were retrieved. However, in some cases where data was presented as conference abstracts or some additional clarification regarding the data was required, corresponding authors of the specific paper were contacted for further assistance. All the data extraction sheets were piloted, and extraction of all papers was performed by TMK and FCL individually. If there were any variations in the extractions, were resolved by the mutual consensus.
Data extraction
The extracted data was entered into Microsoft Excel 2016, namely [ 18 ]
General information (first author surname, title, and year of publication, journal name)
The article study methods and characteristic (study design)
Participants (age, gender, weight, heights and sporting activity)
Intervention (dose and number times consumed)
Comparators (dose and number times consumed)
The outcome is the data obtained after the participants consumed the intervention or control on amino acids, creatinine kinase and myoglobin. Most of the data are located within the text of the articles and presented in tabular form or graphs. When data was in standard error or standard error mean, it was transformed into a standard deviation [ 19 ].
Assessment of risk of bias for included studies
The inclusive studies were assessed for risk of bias (RoB) by two reviewers independently. Both assessment results were compared and verified for accuracy. A Cochrane Risk of Bias tool criteria was used to assess the RCT studies [ 20 ]. For non-RCT studies, the RoB was assessed using Cochrane Risk of Bias Table and Risk of Bias in Non-Randomised Studies of Interventions tool (ROBINS-I), comparing two or more interventions and presenting a judgement. The ROBINS-I was an upgraded version of the Cochrane Risk of Bias Assessment Tool: for Non-Randomised Studies of Interventions (ACROBAT-NRSI).
Data synthesis
Meta-analysis was performed using a random effect model with STATA version 14.2. The type of data for this analysis was continuous data, which contained mean, standard deviation and sample size [ 21 ]. A random effect model was selected since there were no identical studies throughout all the included studies and the participants were various categories of athletes, which could have had an impact on the intervention effect [ 22 ]. For the meta-analysis arm, the intervention was considered the experimental arm while control arms were alternative supplements or proteins with equivalent quantity and similar visuals such as carbohydrate, placebo, maltodextrin and bovine colostrum. The outcomes were on EAA, BCAA, creatinine kinase and myoglobin.
Two or more eligible studies for an outcome were required to generate weighted mean differences (WMD), 95%confidence intervals (CI), weight percentage, heterogeneity chi-squared, I -squared ( I 2 ) for variation in WMD attributable to heterogeneity, Tau-squared to estimate between-study variance, and forest plot. WMD was preferred as outcome measurements in all studies were made on the same scale [ 23 ]. When the I -square appeared to have more than 50% of heterogeneity, subgroup meta-analyses were conducted by activities or exercises instructed during the study and intervention duration (days). Funnel plots and Egger tests were also computed to examine publication bias.
Inclusive articles selection
For the identified articles, there were 333,257 records from the databases and 1773 records through a manual search. At the screening stage, there were 221,064 records after removing duplicates from the identified stage. After screening the titles and abstract, 169 records were brought to the next stage. Subsequently, 27 records were eligible, as 147 records were excluded given the reading availability of the full text of the articles. Of these 27 papers, 15 papers were found addressing the clinical parameters described in the objectives of this systematic review. The PRISMA flow of these stages is shown in Fig. 1 .
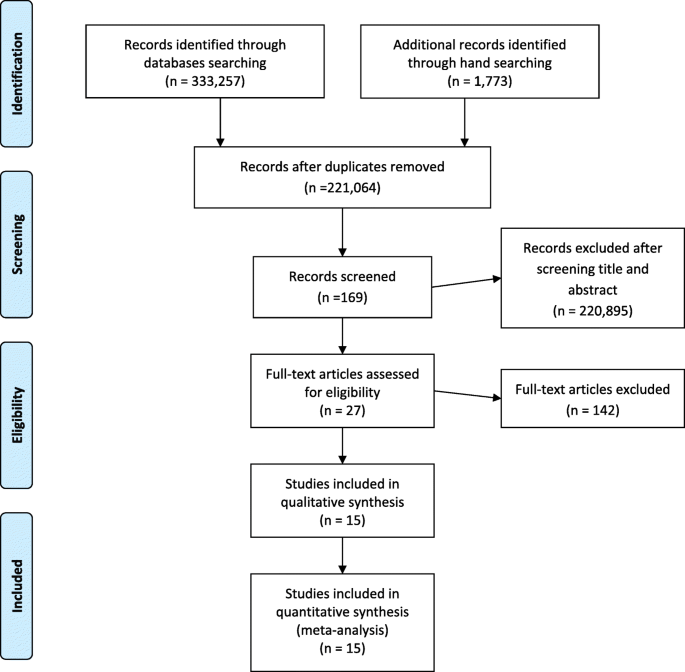
PRISMA flow chart
Study characteristics
The descriptive study characteristics are presented in Table 1 . Of these studies, 13 studies were RCTs [ 26 , 31 , 32 , 33 , 38 ] with crossover [ 35 , 36 , 37 ], blocking [ 27 , 28 ], placebo control, counterbalanced [ 24 , 25 , 29 ] study designs. On the other hand, two studies were non-RCTs with crossover double blinding [ 34 ] and counterbalanced within-group double blinding [ 30 ] study design. The total number of participants was 230, with 207 males and 23 females. Only four studies included both genders. The number of participants ranged from 8 to 24. The participants were from different sports: soccer, badminton, cycling, elite orienting and people from track and field. The intervention duration was from 1 day to 60 days.
Risk of bias
A total of 13 RCT studies were assessed using the Cochrane RoB assessment (see online Additional file 1 : Table S1). The domains and overall assessment are shown in Fig. 2 a. This illustrates that all the studies had a low RoB in “incomplete outcome data” and “other sources of bias”. Eight studies had at least one domain of unclear RoB in “sequence generation”, “allocation concealment”, “blinding of participants and personnel” and “blinding of outcome assessors”. Two studies had high RoB for either “allocation concealment” or “selective outcome reporting” [ 27 , 28 ]. On the other hand, two non-RCTs studies were assessed (see online Additional file 2 : Table S2) based on ROBINS-I, as shown in Fig. 2 b, which had a low RoB in all domains.
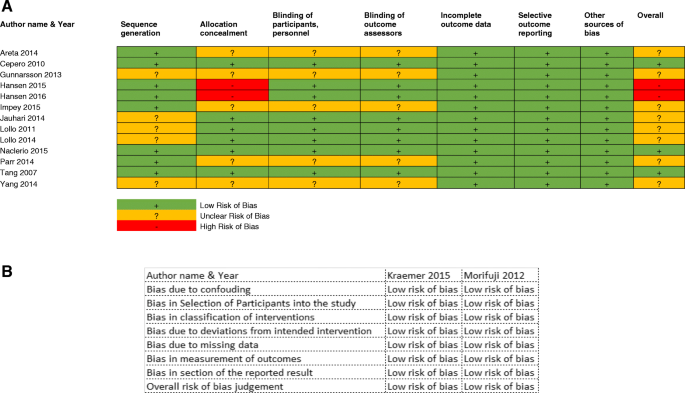
Summary Cochrane ROB assessment for individual RCTs studies ( a ) and summary of ROBINS-I assessment for individual non-RCTs studies ( b )
Meta-analysis
A random effect model of a meta-analysis of 15 studies was conducted to investigate the effectiveness of WPS as compared to other supplements for amino acids, creatinine kinase and myoglobin. Although the studies found at overall of high RoB, they were not removed from the meta-analysis.
Amino acids
A total of six studies have investigated the outcome of WPS over the EAA, and nine studies reported the outcome relevant to BCAA. Overall, it is seen that WPS manage to induce EAA levels among the groups of athletes consuming WPS during the intervention of study period 624.03 nmol/L (CI = 169.27, 1078.8; I 2 = 100%; p = 0.00) compared to the control groups, although high heterogeneity was detected (Fig. 3 a). The individual studies were all favourable to the intervention and their weighted influence of the individual studies was similarly distributed. Similarly, the effect of WPS on BCAA level was significantly better in the intervention group than the control group by 458.57 nmol/L (CI = 179.96, 737.18; I 2 = 100%; p = 0.00) and all studies were favourable to the intervention group (Fig. 3 a). The weighted influence of all the individual studies was equally distributed by 11%. However, both of the outcomes had high heterogeneity between studies, with an I 2 of 100%, which can be mainly due to the diversity in the number of respondents and level of effect which was varying from one study to another. Furthermore, the overall subgroup analyses of EAA and BCAA are merely explained about the heterogeneity as the I 2 value remained high and a standalone study (see online Additional file 3 : Table S3).
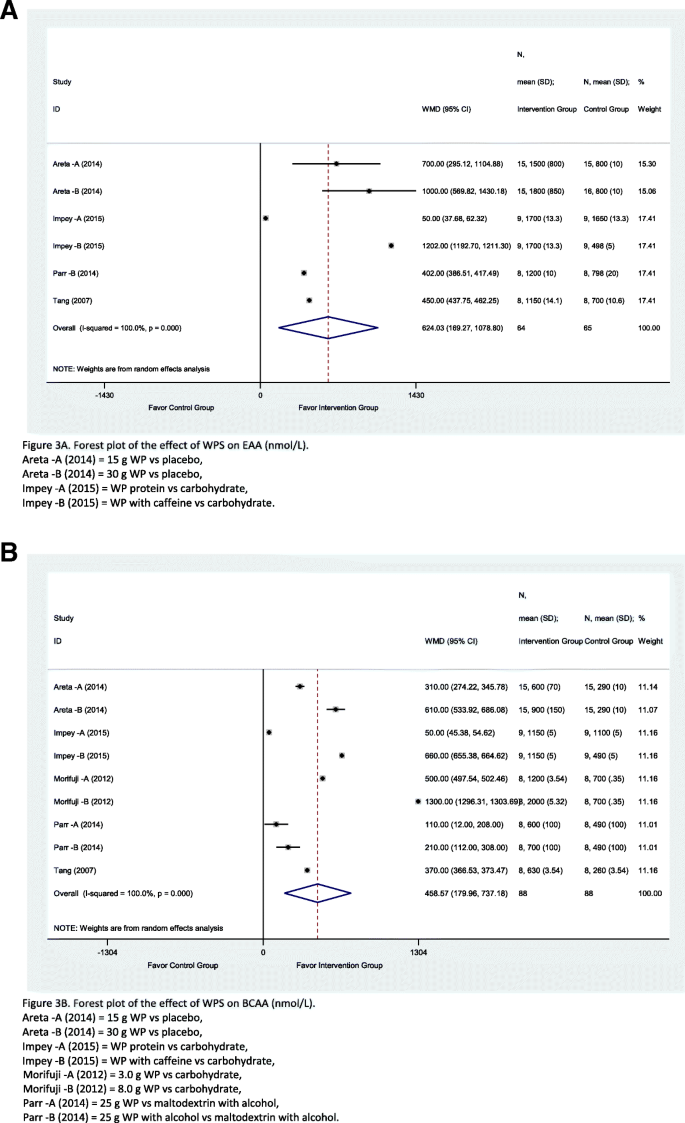
A forest plot of the effect of WPS on EAA (nmol/L). Forest plot of meta-analysis on EAA ( a ) and BCAA ( b )
Three studies were found involved and exploring the effect WPS with myoglobin. Figure 4 a illustrates that the overall WMD of myoglobin level reduces in the intervention group by 11.74 ng/ml (CI = − 30.24, 6.76; I 2 = 79.6%; p = 0.007) compared to the control group, yet it has moderate–high heterogeneity. Two studies were favourable to the control group: Naclerio et al.—A [ 35 ] (weighted = 44.02%) and Naclerio et al.—B [ 35 ] (weighted = 15.03%), while the Gunnarsson et al. [ 26 ] study lie on the no effect line and had the highest weighted influence amount of 40.95%. However, the subgroup analyses did not explain the heterogeneity as the I 2 value remained high and a standalone study (see online Additional file 3 : Table S3).
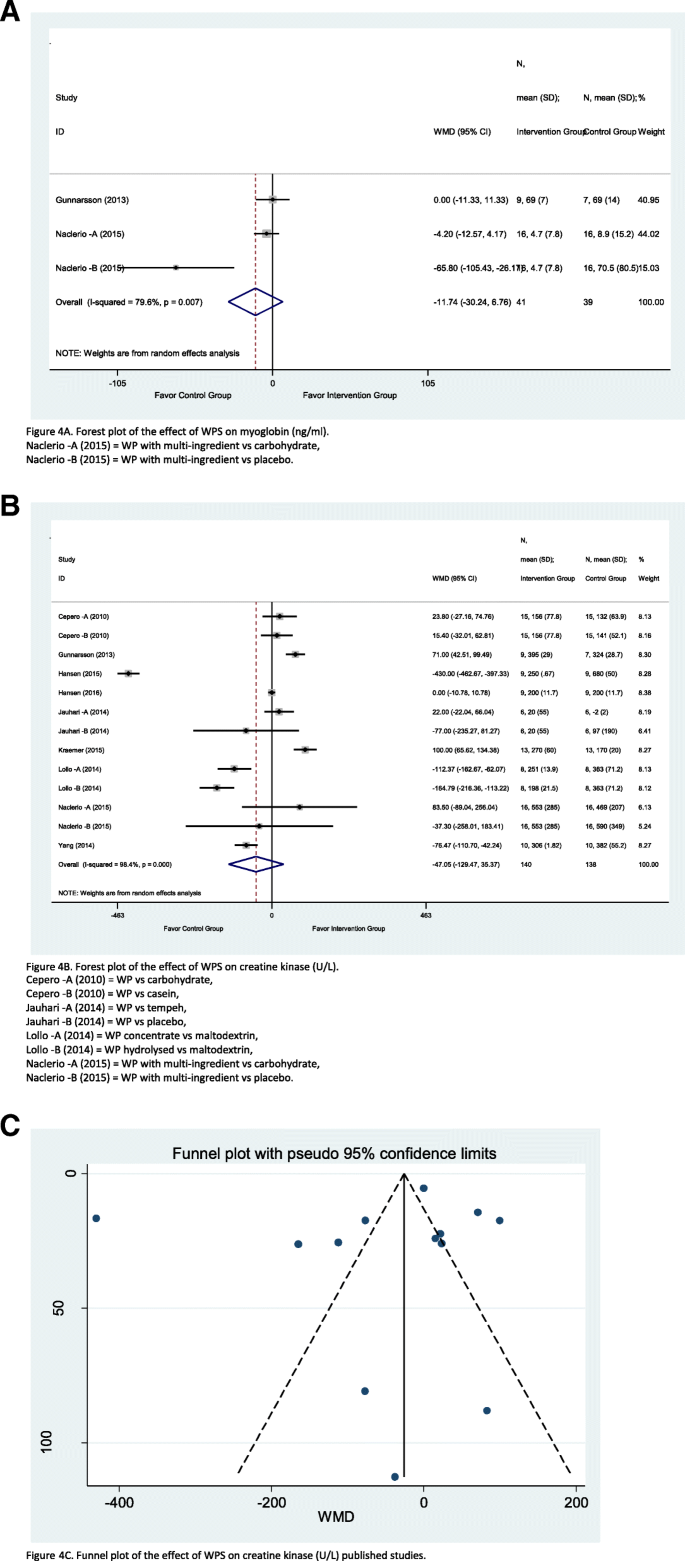
Forest plot of meta-analysis on myoglobin ( a ), creatine kinase ( b ), funnel plot for the studies estimating the effect of whey protein over creatine kinase ( c )
Creatinine kinase
A total of thirteen studies involved WPS with creatinine kinase. Figure 4 b illustrates that the overall creatinine kinase levels were 47.05 U/L (CI = − 129.47, 35.37; I 2 = 98.4%; p = 0.000) significantly lower in the intervention group than in the control group, although with high heterogeneity. Six studies were also favourable to the intervention group: the Gunnarsson et al. [ 26 ] study carried the highest (8.30%) weighted influences and Naclerio et al.—A [ 35 ] study carried the lowest (6.13%) weighted influences. Also, six studies were favourable to the control group: Hansen et al. [ 27 ] study carried the highest (8.28%) weighted influences and Naclerio et al.—B [ 35 ] study carried the lowest (5.24%) weighted influences. The Hansen et al. [ 28 ] study is the only study that lies on the no effect line with a weighed influence of 8.38%. For the publication bias, the funnel plot depicts that there was publication bias as the majority of studies were away from average and outside of the 95% confidence limits (Fig. 4 c), along with the Egger test (see online Additional file 3 : Table S3), where the bias was − 2.1 (CI = − 9.96, 5.75; p = 0.567).
For the subgroup analyses, the physical activities analysis (see online Additional file 3 : Table S3) shows that the cycle group had no heterogeneity ( I 2 = 0%; CI = − 15.42, 54.01) and the resistance exercise subgroup had low evidence and heterogeneity ( I 2 = 28.3%; CI = − 73.71, 79.47). However, the soccer, run, cycle and resistance subgroups have high heterogeneity of 95% and above in I 2 . On the other hand, the heterogeneity for the exercise resistance group was found to be 28.3%. In the intervention duration range (see online Additional file 3 : Table S3), the differences between all subgroups are statistically insignificant. The range period of 1–20 days has high heterogeneity of 98.7% in I 2 , whereas the range of 161–180 days has moderate–low heterogeneity ( I 2 = 50%), and the range of 41–60 days was a stand-alone study (see online Additional file 3 : Table S3).
This is perhaps the first systematic review and meta-analysis to investigate the effectiveness of WPS over the blood biochemistry mainly amino acids, creatinine kinase and myoglobin which influence performance and recovery among athletes. Then again, the intervention was described as WPS, while others as comparators. The search strategy was robust and unlikely to have missed eligible studies. Of the collected studies, 13 (96%) of the included studies were RCTs which many sources of bias had removed from the process [ 23 ]. Two non-RCTs are high quality and the overall assessments had low RoB; this indicated that the two non-RCTs are comparable to RCTs. Meta-analysis is a statistical measurement and procedure for combining data from the multiple studies and developed a statistically single conclusion. The purposes of the meta-analysis are precisely estimate the effect magnitude and identify the reason for the variation and common effect and outcome of data [ 39 ].
Whey protein supplements having high levels of serum amino acids of both EAA and BCAA are well known. Furthermore, the results of the meta-analyses illustrated robust evidence that athletes who consumed WPS had higher levels of serum amino acids than comparators. Essential amino acids of WPS were believed to retain and growth of muscle, while BCAA of WPS was believed to delay the onset of fatigue during prolonged endurance exercise [ 40 , 41 , 42 ]. Moreover, Areta et al. [ 24 ] investigated that amino acids of WPS support muscle protein while Impey et al. [ 29 ] examined WPS enhanced post-exercise muscle protein synthesis rates. Tang et al. [ 37 ] also investigated that a small dose of WP (10 g) was able to stimulate muscle protein synthesis athletes after exercise. Therefore, serum amino acid from WPS absolute ergogenic benefits athletes on delay and recovery from the sports injuries and fatigue [ 40 , 43 ].
In addition, the myoglobin and creatinine kinase levels were lower in the intervention group which indicates that the consumption of WPS can reduce the muscle fatigue or muscle damage than the comparator groups. The release and elevation in myoglobin indicates the presence of muscle damage or inflammation after exercise [ 4 ]. Thus, myoglobin acts as a blood marker for muscle damage [ 44 ]. Moreover, kidneys can be impaired when extreme levels of myoglobin are released, known as rhabdomyolysis [ 45 ]. Subsequently, a lower level of myoglobin would diminish muscle fatigue to prevent muscle damage while athletes drive their strength [ 4 ]. According to the meta-analysis, the overall myoglobin level in the intervention group was lower than that in the control group. Surprisingly, studies have shown that consuming WPS seems to have ergogenic aids as it does lower the myoglobin level [ 26 , 35 ]. Subsequently, a lower level of myoglobin was reflected in athletes’ physical effort: they could go beyond their maximum physical strength while preventing any severe muscle damage [ 44 ].
Creatinine kinase appearing in the blood is considered as a marker of indirect muscle damage [ 5 ]. The level is used to assist in detecting athletes’ body condition of tissue damage. It is reasonable for the creatinine kinase level to elevate temporarily due to strenuous exercise [ 25 , 28 ], but the level should not rise to an extent that could damage skeletal muscles, heart or brain [ 46 ]. Therefore, it is essential for athletes to have a lower creatinine kinase level while driving their physical strength. Based on studies, consuming of WPS does lower creatinine kinase level for active athletes [ 31 , 33 ]. Moreover, Kraemer et al. [ 30 ] observed that the WPS delay muscle soreness and improve the intensity of the physical performance. Lollo et al. [ 33 ] also studied that the positive effect of WPS on attenuated creatine kinase level could be because the properties of WPS have antioxidant capacity. Hence, lower creatine kinase when consuming WPS will aid athletes to prolong time to fatigue and better maintain or improve exercise performance.
Based on evidence and analyses, WPS is found to be effective in improving the serum levels of BCAA and EAA, and on other hand, WPS has shown a substantial effect on reducing myoglobin and creatinine kinase levels that are markers of preventing sports injuries, These result support the consumption of WPS for the athletes during the routine training and muscle injuries to augment the muscle performance and recovery process.
However, there are two main concerns that researchers would like to highlight before any athlete and multidisciplinary team who manages athletes’ health and performance should opt to use WPS; the first one is the higher level of heterogeneity across the compared studies. The subgroup analysis was performed which has shown some declined in heterogeneity for some specific groups. However, for some groups, higher heterogeneity was still there, which is one of the genuine concerns for the researchers while interpreting the results of this meta-analysis. Moreover, the difference in WPS formulation also might have affected the bioavailability and outcome among the studies, and this clinical aspect might have contributed to the heterogeneity in the current meta-analysis.
Recommendation
Future directions for research and conducting research include larger sample sizes, the inclusion of both genders (especially on female athletes), ages, geographical, type of sport and categories of athletes. Interventions that are consumed before, during and/or after sports performances and recovery process also deserve further study, considering the effectiveness of improving athletes’ sports performances and recovery. Additionally, follow-up studies could establish effectiveness for the relation between interventions and long-term performance recovery progress for athletes.
Nonetheless, although WP is recognised as safe supplements for athletes [ 47 , 48 ], concern arises from WADA insight whereby illegal substances can be found in the interventions from the included studies. Two studies reported an intervention containing caffeine [ 26 , 29 ] and a study had an intervention containing alcohol [ 36 ].
The WADA guidelines and recommendations are updated annually and serve as a guide for consuming supplements during the supports and recovery process for athletes. Therefore, it is highly recommended for athletes, and the multidisciplinary team are well-informed and updated themselves on the guidelines and recommendations before using WPS or any supplements.
In conclusion, the current meta-analysis shows the effectiveness of WPS over the blood biochemistry mainly amino acids, creatinine kinase and myoglobin which influence the performance and recovery among athletes and are promising. First of all, the quality of studies has delivered assurance in the validity and reliability of the clinical evidence, whereby most of all the studies were RCTs and, thus, many sources of biases have been omitted. Included studies examined the conditions as close to real life training and competition conditions as possible for athletes. Importantly, athletes need to check, maintain and control the dose as set out by WADA. Moreover, the positive impact of WPS on the essential biomarkers (myoglobin and creatine kinase) aids athletes by delaying or attenuating fatigue and reducing the risk of sports injuries while athletes are reaching beyond their potential aerobic threshold.
Ronghui S. The reasearch on the anti-fatigue effect of whey protein powder in basketball training. Open Biomed Eng J. 2015;9:330–4.
Article Google Scholar
Ferreira HR, et al. Acute oxidative effect and muscle damage after a maximum 4 min test in high performance athletes. PLoS One. 2016;11(4):e0153709.
Cleak MJ, Eston RG. Muscle soreness, swelling, stiffness and strength loss after intense eccentric exercise. Br J Sports Med. 1992;26(4):267–72.
Article CAS Google Scholar
Ramos-Campo DJ, et al. Muscle damage, physiological changes, and energy balance in ultra-endurance mountain-event athletes. Appl Physiol Nutr Metab. 2016;41(8):872–8.
Al-Nawaiseh AM, Pritchett RC, Bishop PA. Enhancing short-term recovery after high-intensity anaerobic exercise. J Strength Cond Res. 2016;30(2):320–5.
Wiese-Bjornstal DM. Psychology and socioculture affect injury risk, response, and recovery in high-intensity athletes: a consensus statement. Scand J Med Sci Sports. 2010;20:103–11.
McInnis KC, Ramey LN. High-risk stress fractures: diagnosis and management. PM&R. 2016;8(3, Supplement):S113–24.
Willick SE, Miller GD, Eichner D. The anti-doping movement. PM&R. 2016;8(3, Supplement):S125–32.
MacKenzie-Shalders KL, et al. The effect of a whey protein supplement dose on satiety and food intake in resistance training athletes. Appetite. 2015;92:178–84.
Poortmans JR, et al. Protein turnover, amino acid requirements and recommendations for athletes and active populations. Braz J Med Biol Res. 2012;45(10):875–90.
Devries MC, Phillips SM. Supplemental protein in support of muscle mass and health: advantage whey. J Food Sci. 2015;80(S1):A8–A15.
Miller PE, Alexander DD, Perez V. Effects of whey protein and resistance exercise on body composition: a meta-analysis of randomized controlled trials. J Am Coll Nutr. 2014;33(2):163–75.
Morton RW, et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. 2017;52(6):376–84.
PubMed PubMed Central Google Scholar
Moher D, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9.
Lam, F.-C., T.M. Khan, and K.-F. Quek. Efficacy and safety of whey protein supplements on performance and recovery among athletes: a systematic review and meta-analysis. 2016; Available from: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016041842 .
Google Scholar
Lemez S, Baker J. Do elite athletes live longer? A systematic review of mortality and longevity in elite athletes. Sports Med Open. 2015;1(1):1–16.
University of York. Centre for Reviews and Dissemination, and Jo Akers. Systematic reviews: CRD's guidance for undertaking reviews in health care. Centre for Reviews and Dissemination; 2009.
Boutron I, et al. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008;148(4):295–309.
Higgins JPT, et al. In: Higgins JPT, Deeks JJ, editors. Selecting studies and collecting data, in Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; 2011.
Higgins JPT, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Saez de Villarreal E, Requena B, Cronin J. The effects of plyometric training on sprint performance: a meta-analysis. J Strength Cond Res. 2012;26(2):575–84.
Borenstein M, et al. Fixed-effect versus random-effects models. Introduction to Meta-analysis 77. Wiley; 2009. p. 85.
Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Updated March. Oxford: The Cochrane Collaboration; 2011.
Areta J, et al. Reduced resting skeletal muscle protein synthesis is rescued by resistance exercise and protein ingestion following short-term energy deficit. Am J Physiol. 2014;306(8):E989.
CAS Google Scholar
Cepero M, et al. Influence of ingesting casein protein and whey protein carbohydrate beverages on recovery and performance of an endurance cycling test. J Hum Sport Exerc. 2010;5(2):158–75.
Gunnarsson TP, et al. Effect of whey protein- and carbohydrate-enriched diet on glycogen resynthesis during the first 48 h after a soccer game. Scand J Med Sci Sports. 2013;23(4):508–15.
Hansen M, et al. Effect of whey protein hydrolysate on performance and recovery of top-class orienteering runners. Int J Sport Nutr Exerc Metab. 2015;25(2):97–109.
Hansen M, et al. Protein intake during training sessions has no effect on performance and recovery during a strenuous training camp for elite cyclists. J Int Soc Sports Nutr. 2016;13:9.
Impey SG, et al. Leucine-enriched protein feeding does not impair exercise-induced free fatty acid availability and lipid oxidation: beneficial implications for training in carbohydrate-restricted states. Amino Acids. 2015;47:407–16. https://doi.org/10.1007/s00726-014-1876-y .
Article CAS PubMed Google Scholar
Kraemer W, et al. The addition of beta-hydroxy-beta-methylbutyrate and isomaltulose to whey protein improves recovery from highly demanding resistance exercise. J Am Coll Nutr. 2015;34(2):91–9.
Jauhari M, et al. Effect of administering tempeh drink on muscle damage recoveries after resistance exercise in student athletes. Pak J Nutr. 2014;12:924–8.
Lollo PC, Amaya-Farfan J, de Carvalho-Silva LB. Physiological and physical effects of different milk protein supplements in elite soccer players. J Hum Kinet. 2011;30:49–57.
Lollo PC, et al. Hydrolysed whey protein reduces muscle damage markers in Brazilian elite soccer players compared with whey protein and maltodextrin. A twelve-week in-championship intervention. Int Dairy J. 2014;34(1):19–24.
Morifuji M, et al. Post-exercise ingestion of different amounts of protein affects plasma insulin concentration in humans. Eur J Sport Sci. 2012;12(2):152–60.
Naclerio F, et al. A multi-ingredient containing carbohydrate, proteins L-glutamine and L-carnitine attenuates fatigue perception with no effect on performance, muscle damage or immunity in soccer players. PLoS One. 2015;10. https://doi.org/10.1371/journal.pone.0125188 .
Parr EB, et al. Alcohol ingestion impairs maximal post-exercise rates of myofibrillar protein synthesis following a single bout of concurrent training. PLoS One. 2014;9(2):E88384.
Tang JE, et al. Minimal whey protein with carbohydrate stimulates muscle protein synthesis following resistance exercise in trained young men. Appl Physiol Nutr Metab. 2007;32:1132–8. https://doi.org/10.1139/H07-076 .
Yang J. Research on application of whey protein in sports drink. Adv J Food Sci Technol. 2014;6(10):1167–70.
Lipsey MW, Wilson DB. Practical meta-analysis, vol. 49. Thousand Oaks: Sage publications; 2001.
Chang C-K, et al. Branched-chain amino acids and arginine improve performance in two consecutive days of simulated handball games in male and female athletes: a randomized trial. PLoS One. 2015;10(3):E0121866.
Tipton KD, et al. Stimulation of net muscle protein synthesis by whey protein ingestion before and after exercise. Am J Physiol Endocrinol Metab. 2007;292(1):E71-E76.
Ha E, Zemel MB. Functional properties of whey, whey components, and essential amino acids: mechanisms underlying health benefits for active people (review). J Nutr Biochem. 2003;14(5):251–8.
Kingsbury KJ, Kay L, Hjelm M. Contrasting plasma free amino acid patterns in elite athletes: association with fatigue and infection. Br J Sports Med. 1998;32(1):25–33.
Ben D, Thomas J, Motley CP. Myoglobinemia and endurance exercise: a study of twenty-five participants in a triathlon competition. Am J Sports Med. 1984;12(2):113–9.
Petejova N, Martinek A. Acute kidney injury due to rhabdomyolysis and renal replacement therapy: a critical review. Crit Care. 2014;18(3):224.
O'Gorman E, et al. Differential effects of creatine depletion on the regulation of enzyme activities and on creatine-stimulated mitochondrial respiration in skeletal muscle, heart, and brain. Biochim Biophys Acta. 1996;1276(2):161–70.
Bolster DR, et al. Dietary protein intake impacts human skeletal muscle protein fractional synthetic rates after endurance exercise. Am J Physiol Endocrinol Metab. 2005;289:E678–83.
Tipton KD, et al. Ingestion of casein and whey proteins result in muscle anabolism after resistance exercise. Med Sci Sports Exerc. 2004;36(12):2073–81.
Download references
Acknowledgements
The authors want to thank Ser Hooi Leng, Anton V Dolzhenko, and Shahrzad Salmasi for translating the articles to English and Inayat Ur Rehman as the second reviewer for RCT RoB assessment.
Availability of data and materials
Detailed searches are available on request (in Endnote etc.)
Author information
Authors and affiliations.
School of Pharmacy, Monash University Malaysia, Jalan Lagoon Selatan, 47500, Bandar Sunway, Selangor Darul Ehsan, Malaysia
Fui-Ching Lam & Tahir Mehmood Khan
The Institute of Pharmaceutical Sciences (IPS), University of Veterinary & Animal Sciences (UVAS), Outfall Road, Lahore, Pakistan
Tahir Mehmood Khan
College of Medicine, Umul Qura University, Makkah, Saudi Arabia
Hani Faidah
College of Pharmacy, Umul Qura University, Makkah, Saudi Arabia
Abdul Haseeb
School of Pharmaceutical Science, University Sains Malaysia, Penang, Malaysia
Abdul Haseeb & Amer Hayat Khan
You can also search for this author in PubMed Google Scholar
Contributions
TMK conceived the idea and formulated the study question. LFC performed the search and data extraction. TMK and LFC devised the methods. TMK, LFC and HF compiled the final analysis and discussion as per the outcomes. All authors equally contributed in the write-up and finalisation of the paper. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Tahir Mehmood Khan .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:.
Risk of Bias. (XLSX 21 kb)
Additional file 2:
The Risk Of Bias In Non-randomized Studies – of Interventions (ROBINS-I) assessment tool. (DOCX 107 kb)
Additional file 3:
Meta-analysis output. (ZIP 162 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Lam, FC., Khan, T.M., Faidah, H. et al. Effectiveness of whey protein supplements on the serum levels of amino acid, creatinine kinase and myoglobin of athletes: a systematic review and meta-analysis. Syst Rev 8 , 130 (2019). https://doi.org/10.1186/s13643-019-1039-z
Download citation
Received : 09 January 2018
Accepted : 10 May 2019
Published : 31 May 2019
DOI : https://doi.org/10.1186/s13643-019-1039-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Supplements
- Evidence-based review
- Performance
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
SYSTEMATIC REVIEW article
Efficacy and safety of whey protein supplements on vital sign and physical performance among athletes: a network meta-analysis.

- 1 School of Pharmacy, Monash University Malaysia, Subang Jaya, Malaysia
- 2 Institute of Pharmaceutical Sciences, University of Veterinary and Animal Sciences, Lahore, Pakistan
- 3 Faculty of Biosciences, University of Veterinary and Animal Sciences, Lahore, Pakistan
- 4 Department of Urology, Bourn Hall Fertility Clinic Dubai, Jumeriah, United Arab Emirates
- 5 Department of Orthopedics, Canadian Specialist Hospital, Abuhail, United Arab Emirates
- 6 Department of Radiology, Emirates Hospital, Jumeriah, United Arab Emirates
Introduction: Athletes train physically to reach beyond their potential maximum aerobic threshold. Whey protein supplements (WPS) are often used in conjunction with physiotherapy and psychotherapy to regain better vital sign and physical performances. This review aimed to explore the clinical evidence on the efficacy and safety of WPS in sports performance and recovery among athletes.
Methodology: A comprehensive literature search was performed to identify relevant randomized control trials (RCTs) that investigated the efficacy and safety of WPS on the vital sign and physical performance among athletes. The Cochrane Risk of Bias (ROB) Assessment tools were used to assess the quality of the studies. Meta-analysis was conducted using the frequentist model with STATA version 14.2 ® .
Results: A total of 333,257 research articles were identified out of which 20 RCTs were included for qualitative synthesis and network meta-analysis with 351 participants. Among the studies, 7 had low ROB and 3 RCTs had high ROB. Of these 20 trials, 16 trials were randomized clinical trials which compared whey protein supplements (WPS) with various comparators i.e., L-alanine, bovine colostrum, carbohydrate, casein, leucine, maltodextrin, rice, protein + caffeine were compared with placebo. Analysis from the pairwise meta-analysis revealed that for respiratory exchange ratio (RER) WPS was found to be significantly improving compared to maltodextrin (WMD = 0.012; 95%CI = 0.001, 0.023). Similarity to RPE (Rate Perceived Exertion), slight difference between WPS and the comparators, however, when the estimation was favorable to the comparators, there was moderate-high heterogeneity. For VO 2max , high heterogeneity appeared when WPS compared to maltodextrin with the I 2 = 97.8% (WMD = 4.064; 95% CI = −4.230, 12.359), meanwhile bovine colostrum (WMD = −2.658; 95%CI = −6.180, 0.865) only comparator that was better than WPS. According to the estimated effect of the supplements on physical performance outcome results, maximum power (8 studies, 185 athletes), highest ranked was bovine colostrum (SUCRA = 70.7%) and the lowest ranked was placebo (SUCRA = 17.9%), yet all insignificant. Then again, on average power (nine studies, 187 athletes), WPS was the highest ranked (SUCRA = 75.4 %) about −112.00 watt (−187.91, −36.08) and most of the estimations were significant. Body mass was reported in 10 studies (171 athletes), carbohydrate may be at the highest ranked (SUCRA = 66.9%) but it is insignificant. Thought the second highest ranked was WPS (SUCRA = 64.7%) and it is significant (WMD = −6.89 kg; CI = −8.24, −5.54).
Conclusion: The findings of this review support the efficacy and safety of WPS as an ergogenic aid on athletes' sports performance and recovery. The overall quality of clinical evidence was found to be valid and reliable from the comprehensive search strategy and ROB assessment.
Introduction
Athletes train to be skilful and physically fit to compete and ensure success against their opponents. The effect of athletes' stamina, body structure and skill development are essential to able to do so, while an effective while an effective nutrition and diet plan to ensure good health and well-being of athletes. It is support from the supplement as ergogenic aids to maintain their performance and to gain a competitive edge. The availability and consumption of supplements, along with physiotherapy and psychotherapy, have been recognized as ergogenic advantages in sports performance and recovery ( Wiese-Bjornstal, 2010 ; Chan et al., 2011 ). supplements might have substances that are harmful and life-threatening effects on athlete health such as alcohol, steroid and caffeine ( Silver, 2001 ).
World Anti-doping Agency (WADA) was established to promote coordinate and monitor illicit drugs use in sports internationally. However, dietary and nutritional supplements have become distressing matters. For many countries and manufacturers of supplements have a lack of quality control, some supplements contain substances that were prohibited such as caffeine and alcohol ( Willick et al., 2016 ). Hence, options for supplements are limited for athletes to compete ethically. One of the popular and easy to purchase protein supplement in sports is whey protein supplements (WPS) as it has shown ergogenic aids which absorbed rapidly, includes all the essential amino acids, and has a high proportion of branched-chain amino acids ( MacKenzie-Shalders et al., 2015 ; Frank et al., 2017 ).
Various systematic review and meta-analysis published that summaries the effect of whey protein (WP) as a dietary supplement ( Nissen and Sharp, 2003 ; Schoenfeld et al., 2013 ; Miller et al., 2014 ). However, there is a lack of consensus over the use of WP, yet, some clinical studies concluded consuming other protein sources or supplements are better than WP ( Taylor et al., 2016 ), which is in contrast with some other studies ( Kraemer et al., 2015 ; Hansen et al., 2016 ) that support WP in comparison to others. Moreover, the quality of studies and risk of bias is another issue that is often neglected while scrutinizing the evidence of other supplements in comparison to WP. The current systematic review and network meta-analysis aim to explore the clinical efficacy and safety of WPS on athletes' vital sign and physical performance.
Study Design and Selection
A systematic review and network meta-analysis were conducted to identify eligible randomized controlled trials (RCTs). The search strategy used the keyword of “whey*” combined individually with “athlete*,” “injur*,” “muscle*,” “perform*,” and “recover*” on databases as well as specific journals: PubMed, EMBASE via Ovid, Scopus, Cochrane, Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCOhost, SPORTDiscus, Health & Medicine Database via ProQuest, Wiley Online Library, Web of Science, ScienceDirect, Taylor & Francis and SAGE. All experimental and observational studies were considered for potential exclusion in this systematic review. No restriction was placed on language. The searched timeframe was from the date of database inception until December 2017. However, studies design on expert opinions, case reports/series, surveys, review articles, editorials, commercial advertisements, magazine articles, unpublished articles were excluded. The protocol of this study was registered in PROSPERO 2016 and the register identification is CRD42016041842 ( Lam et al., 2016 ) and reported the network meta-analysis according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) ( Moher et al., 2009 ). Please see Appendix 1 in the Supplementary Material for detailed PRISMA checklist.
Population of Interest
The participants included were active athletes who experienced fatigue and had recovered and/or had been hindered in their performance. Also, studies that observed on participations who are resistance-trained, trained and physically active were deem be athletes as these participants undertook overpowering physical activities during the intervention that were equivalent to athletes. Regardless of athletes' age and gender. However, studies that observed on retired athletes, mixed athletes with non-athletes, animals, cells, and gels were excluded.
Interventions
The intervention was WP or supplements containing WP. The intervention was found in the form of isolate, concentrate, hydrolysate, denature, and protein bars.
Comparators
Comparators were carbohydrate supplements, protein-containing foods from animal sources (e.g., meat, fish, dairy products, and eggs), protein-containing vegetarian sources (e.g., tofu, legumes, and soy protein), vitamins (e.g., multivitamin, vitamin B, beta-carotene, and folic acid), minerals (e.g., calcium, iron and zinc) and placebos (include no treatment and treatment as usual).

Outcomes Measure
This systematic review and meta-analysis have measured two outcomes which associated with aims: -
a. Vital signs of heart rate, respiratory exchange ratio (RER), rate perceived exertion (RPE), and maximum volume of oxygen ( VO 2max );
b. Strength and body composition which were maximum power, average power, and body mass.
Data Extraction
The extracted data were entered into Microsoft Excel 2016, namely ( Boutron et al., 2008 ):
1. General information (first author surname, title, year of publication, journal name).
2. The article study methods and characteristic (study design).
3. Participants (age, gender, weight, heights, and sporting activity).
4. Intervention (dose of WP consumed and number times consumed).
5. Comparators (type, dose, and number times consumed).
6. Outcomes: -
a. Outcomes that contributed to vital sign and physical performances.
b. The data obtained after the participants consumed the intervention or control.
c. Most of the data located within the text of the articles and presented in tabular form or graphs
d. When data was in standard error or standard error mean, it was transformed into a standard deviation ( Higgins et al., 2011b ).
Assessment of Risk of Bias for Included Studies
The included studies were assessed for their ROB by two reviewers independently. Both assessment results were compared and verified for accuracy. A Cochrane ROB criteria were used to assess the quality of the RCTs ( Higgins et al., 2011a ) ( Appendix 2.1 in Supplementary Material).
Statistical Analysis
Two types of meta-analysis were conducted with STATA version 14.2 ® . Firstly, standard pairwise meta-analysis with a random-effects model ( Borenstein et al., 2009 ) was performed and assessed I-squared ( I 2 ) metrics for heterogeneity. Thus, appearance of heterogeneous when the I 2 appeared to have 50% and above ( Higgins et al., 2003 ). Secondly, random-effect network meta-analysis was performed using frequentist model to compare different interventions with each other. For a common heterogeneity variable for all comparisons was assessed by studies tau-square ( τ 2 ) test ( Turner et al., 2012 ). The type of data for the meta-analysis was continuous data, which contained mean, standard deviation and sample size ( Saez de Villarreal et al., 2012 ). The results pooled estimates of weighted mean difference (WMD) at 95% confidence interval (95% CI).
For indirect and mixed comparisons, network meta-analysis was performed to compare different strategies and the meta-analysis assumes transitivity (i.e., learn about supplement A (vs.) supplement B via supplement B) ( Salanti, 2012 ). The transitivity embraced when the direct comparison between supplements do not differ with respect to the distribution of effect modifiers. For example, studies comparing WPS with placebo were similar to studies comparing carbohydrate with placebo in heart rate parameter. The potential effect modifiers for trials in this setting were the duration of intervention, physical activities during intervention duration and dosage of supplements. On the other hand, disagreement between direct and indirect evidence suggests that the transitivity assumption might not be embraced.
Firstly, investigate a loop-specific approach for consistency within every triangular or quadratic loop as the difference between direct and indirect estimates for a specific comparison in the loop (inconsistency factor) ( Salanti, 2012 ) ( Veroniki et al., 2013 ). Secondly, performed the design-by-treatment interaction model and examined chi-square (χ 2 ) test for a single inference about the plausibility of assuming consistency throughout the entire network ( Higgins et al., 2012 ).
For a better understanding of intervention, surface under the cumulative ranking curve (SUCRA) probabilities conducted to rank the supplements for an outcome. The larger the SUCRA value (express in percentage) was, the better the rank of the intervention would be ( Chaimani et al., 2013 ).
Study Characteristics
The PRISMA flowchart ( Figure 1 ) shows electronic searching processes. Of 169 potentially relevant articles initially screened, 20 articles with 351 participant athletes met the inclusion criteria. The descriptive study characteristics are presented in ( Table 1 ). All the included articles are 16 RCTs are blinding while four articles are non-blinding ( Hoffman et al., 2009 ; Gunnarsson et al., 2013 ; Impey et al., 2015 ; Al-Nawaiseh et al., 2016 ). The most studies demographic were from United States (6 articles) ( Hoffman et al., 2009 ; Smith et al., 2010 ; Joy et al., 2013 ; Schroer et al., 2014 ; Al-Nawaiseh et al., 2016 ; Taylor et al., 2016 ). One study each from China ( Li and Zhao, 2007 ), Finland ( Breen et al., 2011 ), New Zealand ( Macdermid and Stannard, 2006 ), Norway ( Vegge et al., 2012 ), and South Africa ( Oosthuyse and Millen, 2016 ).
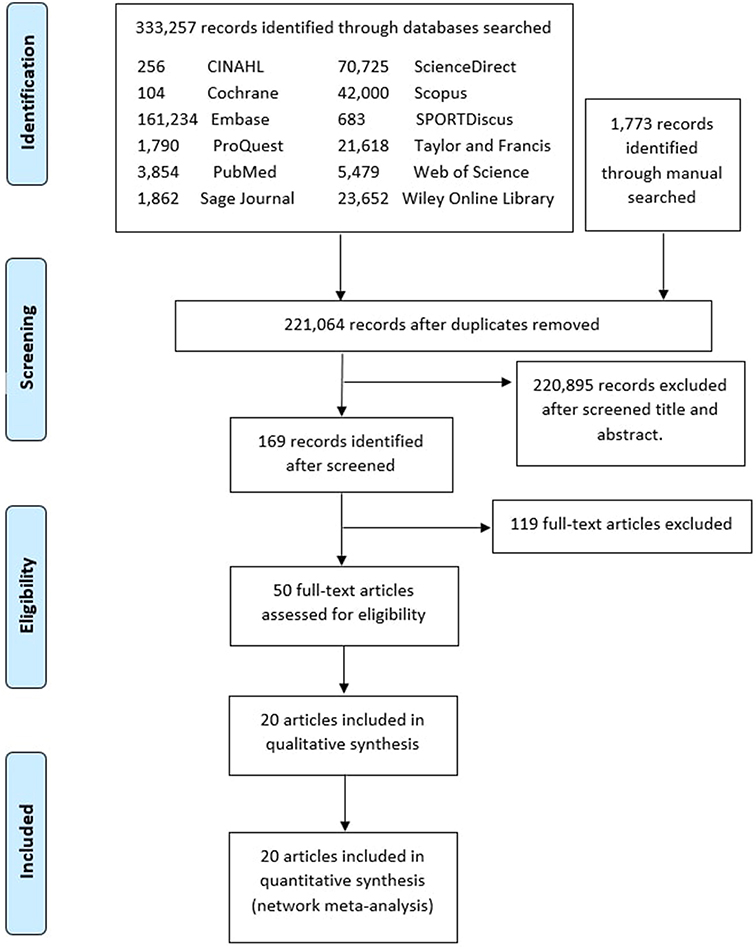
Figure 1 . PRISMA flow diagram.
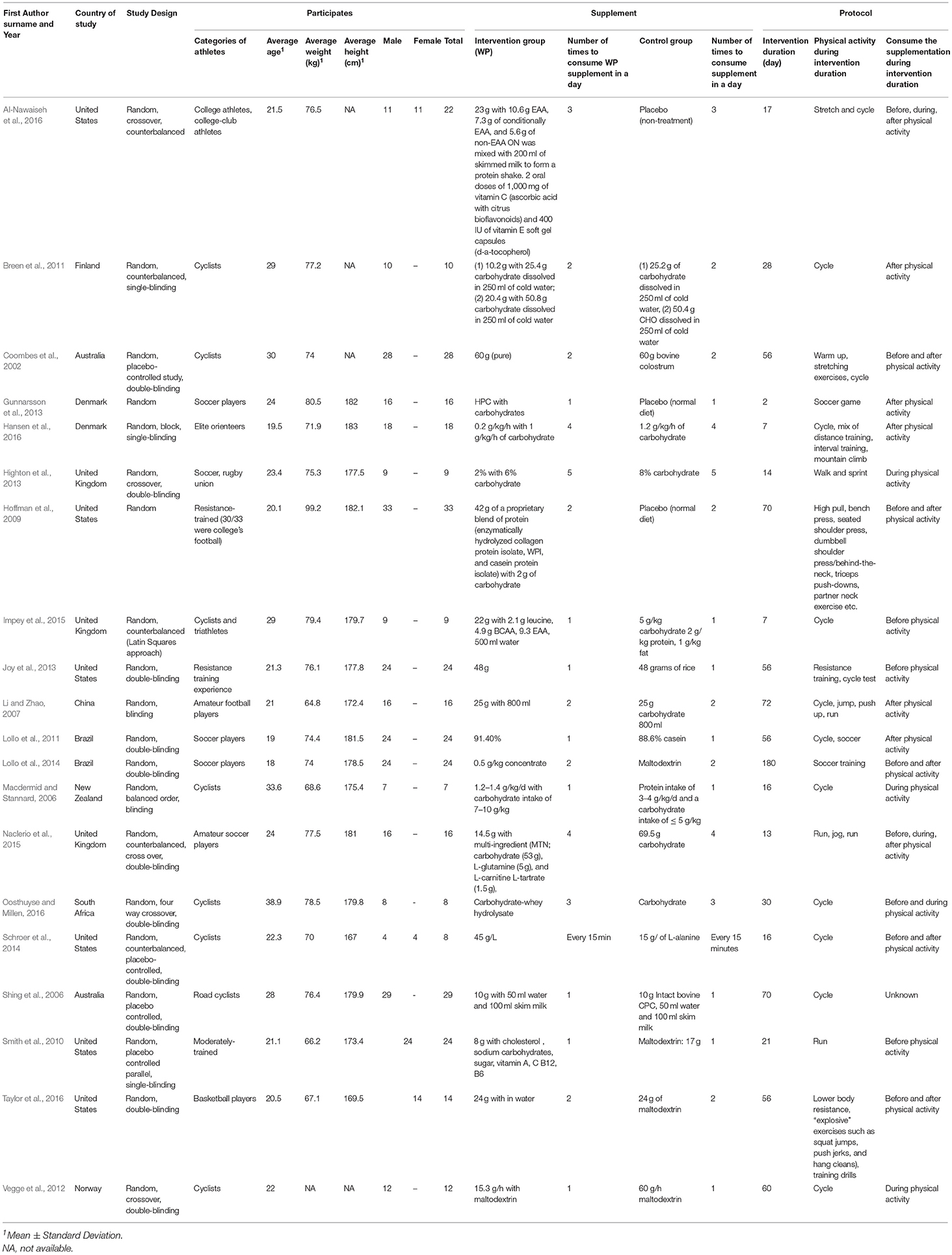
Table 1 . Characteristics of the included studies.
Additionally, the researchers who were consistent in publishing the most regarding WPS for athletes were Lollo and colleagues, had two publications ( Lollo et al., 2011 , 2014 ). Two articles have both males and female athletes ( Schroer et al., 2014 ; Al-Nawaiseh et al., 2016 ), an article has only female athletes ( Taylor et al., 2016 ) while an article was not taken account regarding their gender ( Fukuda et al., 2010 ). In total 351 athletes participated: 298 males, 29 females and 24 athletes did not categories. Furthermore, the minimum number of participants in a study among the included for this review was n = 7 ( Macdermid and Stannard, 2006 ) and the maximum was n = 33 participants ( Hoffman et al., 2009 ).
WPS and the comparators (L-alanine, bovine colostrum, carbohydrate, casein, leucine, maltodextrin, rice, protein + caffeine) were compared with placebo. The number of participants based on the analyses indicated that a total of 351 athletes consumed WPS, followed by carbohydrate (154 athletes), and placebo (137 athletes), respectively. While protein + caffeine (9 athletes) as well as leucine (9 athletes) had the smallest number of participants. The results of network meta-analysis showed in terms of efficacy in Table 2 on the respective outcomes.
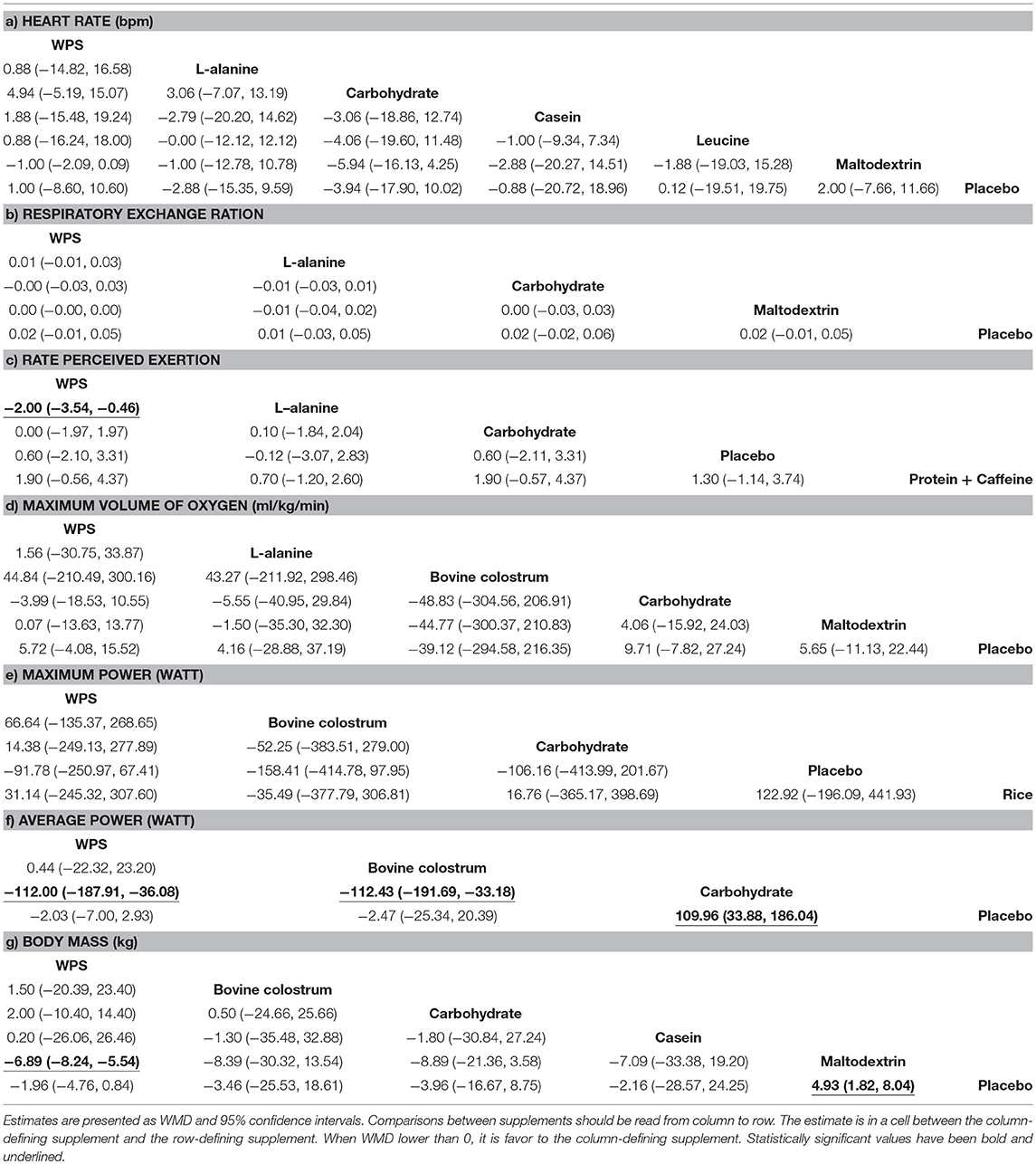
Table 2 . Results of network meta-analyses.
The shortest intervention duration, on average, was 2 days ( Gunnarsson et al., 2013 ) and the longest duration was 180 days ( Lollo et al., 2014 ). Participants consumed supplements, in extreme cases; one study has participants taking supplements every 15 min ( Schroer et al., 2014 ). Participants consumed supplements before, during and/or after physical activities.
Risk of Bias
A total of 20 RCTs were assessed using the Cochrane ROB Tools assessment ( Appendix 2.2 in Supplementary Material). The summary of Cochrane ROB for RCTs ( Figure 2 ) shows 7 studies (35%) have overall low ROB, 10 studies (50%) have overall unclear ROB and 3 studies (15%) have overall high ROB ( Smith et al., 2010 ; Breen et al., 2011 ; Hansen et al., 2016 ).
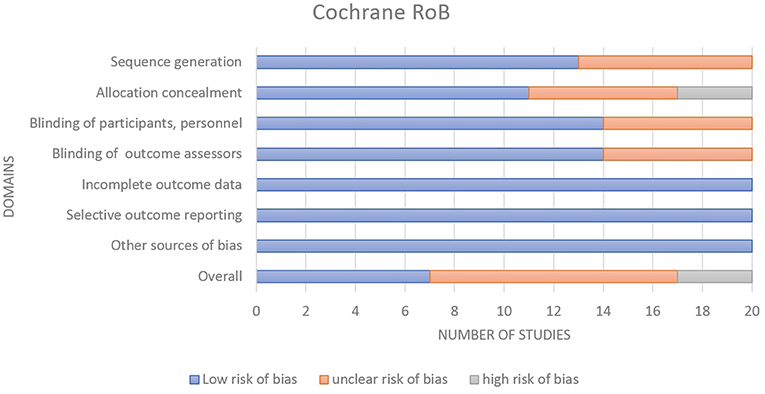
Figure 2 . Summary of Cochrane risk of Bias for the RCTs.
Moreover, the high ROB distributed only at that allocation concealment shows on Figure 3 of the individual studies ROB. The cause of the high ROB was single blinding conducted in the 4 studies, thus, either participants or investigators could possibly foresee assignments and impact on participants' behavior and participation and outcome assessment. While, 20 studies (100%) low ROB for incomplete outcome data, selective outcome reporting and other sources of bias domains.
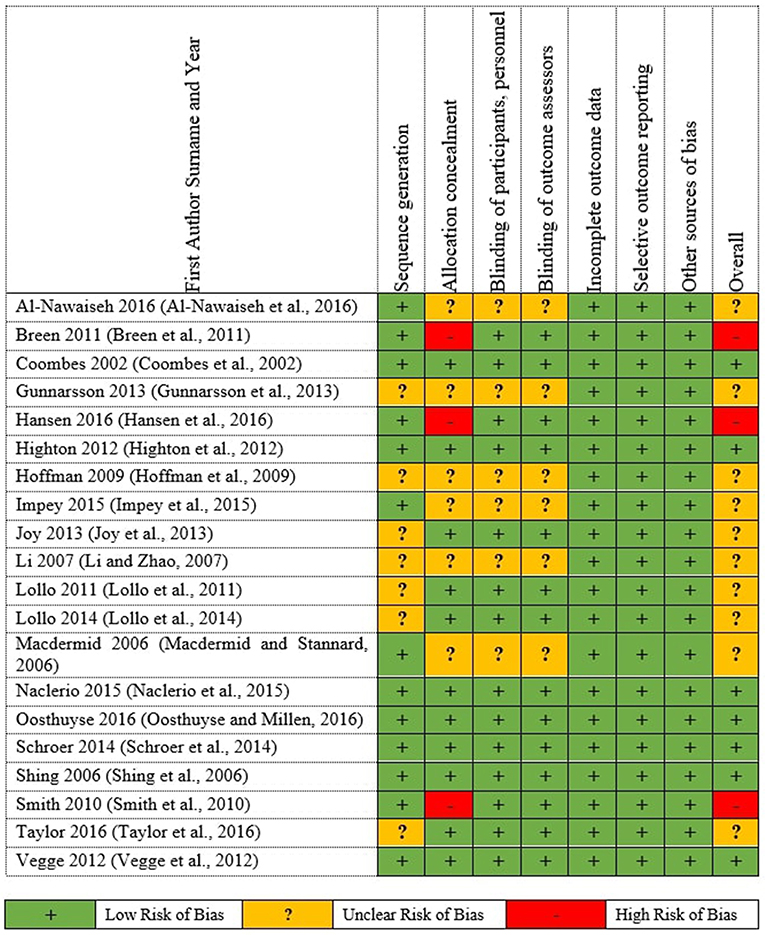
Figure 3 . Summary of Cochrane risk of Bias for the individual RCTs.
Meta-Analysis
The pairwise comparisons of the efficacy of WPS as compared to other supplements on vital sign and physical performances among athletes ( Appendix 3 in Supplementary Material). In terms of the efficacy on vital sign outcome, the analysis result shows heart rate (bpm) slightly increases and decreases. The highest heart rate is 5 bpm (95%CI = −5.231, 15.231) favorable to L-alanine, and lowest heart rate is −1 bpm (95%CI = −2.089, 0.089) favorable to protein + caffeine, yet not significant. For RER, all slightly favorable to WPS compared to the comparators, and it is significant when WPS compared to maltodextrin (WMD = 0.012; 95%CI = 0.001, 0.023). Similarity to RPE, slight difference between WPS and the comparators, however, when the estimation was favorable to the comparators, there was moderate-high heterogeneity. For VO 2max , high heterogeneity appeared when WPS compared to maltodextrin with the I 2 = 97.8% (WMD = 4.064; 95%CI = −4.230, 12.359), meanwhile bovine colostrum (WMD = −2.658; 95%CI = −6.180, 0.865) only comparator that was better than WPS. Apart from RPE and VO 2max on WPS against maltodextrin have heterogeneity, no evidence of heterogeneity was seen in general.
In terms of the efficacy on physical performances outcome, maximum, and average power (watt) results favorable to WPS and show no heterogeneity but favorable to the comparators show moderate-high heterogeneity, yet not significant. Body mass (kg) parameter has slightly different but no significant between WPS and the comparators; the highest body mass was 0.585 kg (95%CI = −6.122, 7.292) compare to bovine colostrum while the lowest body mass was casein of −5.593 kg (95%CI = −8.131, −3.054; I 2 = 86.0%) with high heterogeneity. Detailed on the results of pairwise meta-analyses are given in the Appendix 3 (Supplementary Material).
In the results of network meta-analysis, statistical heterogeneity found moderate only in the network of RPE on a triangular loop of evidence including supplementation comparison between carbohydrate, combined placebo and WPS (carbohydrate—placebo—WPS) (For Network meta-analysis plots please see Appendix 4 in Supplementary Material). Besides that, no appearance of statistical heterogeneity was seen throughout ( Appendixes 5 and 6 in Supplementary Material). The estimates from inconsistency factor (IF) did not show evidence of statistical inconsistency. Moreover, the physical performance outcome shows no triangular or quadratic loops found.
The direct comparisons and network estimates for both vital sign and physical performances outcomes are shown in the league table ( Table 2 ). The comparative efficacy of all supplements ranked with SUCRA probabilities ( Appendix 7 in Supplementary Material). For the vital sign outcome result, heart rate was reported in 12 studies (216 athletes), the highest SUCRA ranked was carbohydrate of 74.9% (WMD = −5.94 bpm; 95%CI = −16.13, 4.25) and the lowest ranked was WPS of 33.1% (WMD = 4.94 bpm; 95%CI = −5.19, 15.07) were not significant. RER was reported in five studies (100 athletes), although L-alanine had the highest (SUCRA = 71.1%) ranked while placebo had the lowest (SUCRA = 42.8%) ranked, the estimations of supplements were generally similar to placebo and non-significant ( Table 2b ). RPE (eight studies, 170 athletes) was superior to protein + caffeine (SUCRA = 93%) yet insignificant, whereas WPS may be at the low ranked (32.7%) but it was significantly lower (WMD = −2.00; 95%CI −3.54, −0.46) of RPE level compared to L-alanine ( Table 2c ). For VO 2max (nine studies, 190 athletes), the highest ranked was placebo (SUCRA = 69.4%) and the lowest ranked was carbohydrate (SUCRA = 29.8%). However, it was revealed from the results of NMA that bovine colostrum had the highest rate of oxygen consumption attainable during the incremental or intensity of physical activities, yet not significant ( Table 2d ).
According to the estimated effect of the supplements on physical performance outcome results (showed in Tables 2e-g ), maximum power (8 studies, 185 athletes), highest ranked was bovine colostrum (SUCRA = 70.7%) and the lowest ranked was placebo (SUCRA = 17.9%), yet all insignificant. Then again, on average power (nine studies, 187 athletes), WPS was the highest ranked (SUCRA = 75.4 %) about −112.00 watt (−187.91, −36.08) and most of the estimations were significant. While, the lowest ranked was carbohydrate (SUCRA = 0.2%). Body mass was reported in 10 studies (171 athletes), carbohydrate may be at the highest ranked (SUCRA = 66.9%) but it is insignificant. Thought the second highest ranked was WPS (SUCRA = 64.7%) and it is significant (WMD = −6.89 kg; CI = −8.24, −5.54). While, the lowest ranked was maltodextrin (12.4%).
Quality of the Studies
The search strategy was robust and unlikely to have missed eligible studies. Majority of the studies had low and unclear ROB. This could due to the methodological difference (may know as methodological heterogeneity), such as binding allocation, a washout period of time and data analysis strategy. For instance, Hoffman et al. (2009) had 70 days of intervention period that was without blinding and had washout period while Breen et al. (2011) on 28 days of intervention period that was single-blinding and no washout period.
Vital Signs Outcome
Heart rate for athletes is an instrument to determine and monitor their daily effort for every training and how hard their body is being trained. A slower increase in heart rate while training sessions act as proof that athletes are physically fit ( Aubert et al., 2003 ; Li and Kim, 2017 ). Although a slower heart rate is preferable, the small differences between the comparators have indicated that WPS is capable and comparable to the comparators.
Rapid absorption of fluids and nutrition assist in better cardiovascular performance in athletes ( Oosthuyse and Millen, 2016 ). These twelve studies have individually shown that WPS and comparators were comparably absorbed rapidly. For WPS, it is known to be absorbed more rapidly than most of the other protein sources, thus it appears to resist coagulation in the stomach and surpass intestines relatively fast ( Frank et al., 2017 ). Whereby, Breen et al. (2011) , Li and Zhao (2007) and Impey et al. (2015) studies have individually examined slower heart rate in WPS as compared to carbohydrate supplements.
Moreover, Oosthuyse and Millen (2016) studied specifically the effect of supplements (WPS and comparators) and placebo. This study had the carbohydrate-casein only supplement that intended to maintain all measures of systolic function, yet, these supplements during the intervention period were parallel consistently ingestion. Thus, the benefits associated with consumption of WPS in the context of heart rate may not be significant in contrast to athletes who consumed comparators (carbohydrate, casein, L-alanine, maltodextrin supplements and placebo).
Based on these findings on examined all supplements had similar heart rate results, therefore, WPS is capable to act as ergogenic aids in athletes' heart rate. Nevertheless, athletes must be mindful about continuous of having low heart rates as their heart enlarged over a prolonged period of time ( Dixon et al., 1992 ; Imai et al., 1994 ). This may lead to suffering from athletic heart syndrome and they may need pacemaker later in their lives.
Respiratory Exchange Ratio (RER)
Respiratory exchange ratio is one of the most metabolic measurements that indicates fuel (mainly carbohydrate or lipid) is being metabolized to supply the body with energy. When RER value is high, carbohydrates are being utilized, whereas when RER value is low, lipid oxidation is being enhanced ( Bergman and Brooks, 1999 ). Furthermore, the individual studies of RER source data are between 0.8 and 0.9 which corresponds to 50% fat and 50% carbohydrate metabolism ( Nelson et al., 2015 ). Vegge et al. (2012) reported that WPS and maltodextrin supplements were associated RER and had similar RER values throughout the prolonged submaximal exercise, while Schroer et al. (2014) found that WPS did not influence RER or performance. Surprisingly, Breen et al. (2011) found that RER value was not extraordinary high with carbohydrate containing supplements, though the study has high ROB ( Figure 3 ). Therefore, athletes consumed WPS has contributed to a higher RER value for better generation of energy.
Rate Perceived Exertion (RPE)
Rate perceived exertion is a method to quantify internal training load or intensity of exercise for athletes. Normally, it is a scale measurement that runs from 0 to 10 rating. Whereby, 0 is no training done and 10 extremely heavy training that athletes are able to cope ( Ekblom and Golobarg, 1971 ; Amtmann et al., 2008 ; Iellamo et al., 2014 ). There is a slight difference between the supplements and a study has high ROB ( Breen et al., 2011 ) ( Figure 3 ). Moreover, Highton et al. (2013) reported that athletes who consumed WPS were exercising at a higher exercise intensity compare to carbohydrate, yet for both groups, RPE value had no great difference. Additionally, Naclerio et al. (2015) examined that WPS provided lower RPE values at the beginning and toward the end of soccer compared to carbohydrates alone or a low calorie placebo. The low RPE, especially at the end of exercising sessions, suggested that availability of glycogen would attenuate the rise in fatigue ( Naclerio et al., 2015 ). This indicates that WPS group had lower RPE compared to comparators with the similar workload done. Hence, athletes who consumed WPS were able to have lower RPE and better coping with the intensity of physical exercise.
Maximum Volume of Oxygen ( VO 2max )
Maximum volume of oxygen is defined as the highest rate of oxygen consumption attainable during the incremental or intensity of physical activities ( Dlugosz et al., 2013 ). It also reflects the cardiorespiratory fitness associated with endurance capacity during the prolonged physical activities ( Ross et al., 2016 ). In general, if athletes are performing more intensely, higher will the VO 2max consumption ( Dlugosz et al., 2013 ). Although the network meta-analysis showed bovine colostrum had better efficacy among all the supplements, Coombes et al. (2002) studied that WPS had similar performance benefits with bovine colostrum alone. Similar to Shing et al. (2006) and Schroer et al. (2014) examined that at the beginning of intensity, there may variance in the intake VO 2max , but, at longer duration, there was no difference in improving intake of oxygen and performance. Thus, this might be one of causes to the huge amount of 95% CI in the network meta-analysis. On the other hand, Smith et al. (2010) studied that 90–115% of VO 2max for higher-intensity exercise, while consuming caffeine supplementation. Although, the study may have increase the performance, caffeine is an illegal substance that prohibited by WADA ( World Anti-Doping Agency, 2017 ). With these findings, WPS has better ergogenic effect in VO 2max that allows athletes to have cardiorespiratory fitness while performing intensively.
Physical Performance
Maximum and average power.
To perform in sport, strength is key performance measurement and one of the main interest that athletes seek for ergogenic aids ( Lemon et al., 1992 ; Tarnopolsky et al., 1992 ; Al-Nawaiseh et al., 2016 ). The ergogenic effect in maximum and average power were higher in placebo and carbohydrates, respectively, as compare bovine colostrum. According to the included studies, WPS is comparable as ergogenic aids in strength for athletes as there are only slight differences between WPS and the comparators ( Coombes et al., 2002 ; Hoffman et al., 2009 ; Joy et al., 2013 ; Hansen et al., 2016 ). Moreover, Shing et al. (2006) examined that athletes who consumed WPS experienced a decrease in strength in the beginning, but they recovered from any residual fatigue and remained unchanged at following the 5–6 days. Moreover, Highton et al. (2013) discovered that WPS ingestion enabled a small increase in exercise intensity in the latter stages of the sports exercise compared to carbohydrate. Al-Nawaiseh et al. (2016) also investigated that average power recovered better and managed about 4 times higher for athletes who consumed WPS than placebo. Hence, WPS would assist athletes in strength at a longer period of consumption with the physical activities.
The key objectives of athletes' development and well-being are body composition. One of the important body composition measurement is body mass ( Anding and Oliver, 2015 ). The analysis had illustrated that WPS improved athletes' body mass by lowering their body mass better than the competitors, though with a marginal difference. Additionally, the individual studies explained that WPS is an ergogenic aid to the body composition as a whole. The relationship of WPS with body mass is well studied and elaborated by Lollo et al. (2011) , Lollo et al. (2014) . Additionally, Lollo et al. (2011) have examined that WPS provided an additional benefit for maintaining and gaining muscle mass in athletes, while Lollo et al. (2014) further assessed that WPS has a net effect on muscle mass gain over prolonged exercise. Moreover, Taylor et al. (2016) reported in particular for female athletes who improved lean body mass and reduction in fat mass. Thus, the results suggested that athletes who need to have achieved Ideal weight by losing their body mass for the sports performance are encouraged to consumed WPS while maintaining or gaining muscle mass ( Brukner and Khan, 2009 ).
There was no relevant data available on the safety, and no side effect was reported in all of the included studies. Therefore, this systematic review and network meta-analysis study are not in the position to discuss it. Nonetheless, WP is recognized as safe supplements for athletes ( Tipton et al., 2004 ; Bolster et al., 2005 ), concern arises from WADA insight whereby illegal substances can be found in the interventions from the included studies which are Smith et al. (2010) contained caffeine. Hence, athletes shall be cautious while taking supplements in the content of not violating WADA rule and regulation ( World Anti-Doping Agency, 2017 ).
Several limitations of this systematic review and meta-analysis are worth considering. Foremost, the study did not overcome problems that were inherent in the primary studies. Also, the review did not correct the biases of the primary studies ( Garg et al., 2008 ). Besides, there would have imprecision related to the impossibility of generalizing diverse characteristics from study to study such as age, gender or geographic factors ( Higgins and Green, 2011 ). On top of that, the discussion and conclusion draw from this systematic review and meta-analysis upon the sports performance and recovery among athletes are at the time they were measured. Therefore, this review cannot establish the causation between the parameters and long-term performances and recovery progress for athletes.
Recommendations
Future directions for research and conducting research that includes larger sample sizes, the inclusion of both gender (especially on female athletes), ages, geographical, type of sport and categories of athletes. Interventions that are consumed before, during and/or after sports performances and recovery process also deserve further considering the effectiveness of improving athletes' vital signs and physical performances. Additionally, follow-up studies could establish effectiveness for the relation between interventions and long-term on vital signs and physical performances progress for athletes. Importantly, it is highly recommended for athletes and their providers are well-inform and updated on WADA guidelines that updated annually before consuming any WPS. These findings are worthy of further inquiry and investigation.
The systematic review and network meta-analysis study has attempted on the clinical evidence efficacy and safety of WPS on performance and recovery among athletes are promising. First of all, the quality of studies has delivered assure validity and reliability of the clinical evidence. Whereby, all the studies were RCTs, thus, many sources of biases have omitted. Therefore, athletes and multidisciplinary team that manages athlete's health and performance can have a clear directive on the evidence with regards WPS as compared to other protein supplements for a vital sign and physical performance. Besides, the included studies examined as close as possible to real life conditions of sports performances and competition for athletes. Therefore, the study can be used as a guide for better decision-making especially when working with a multidisciplinary approach.
Author Contributions
TK performed Statistical analysis and interpretation of results. F-CL wrote the initial draft. AB finalized the paper. HR, MKW, NS, AMK, AE, MK, and AT helped in making revisions and finalization of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.00317/full#supplementary-material
Al-Nawaiseh, A. M., Pritchett, R. C., and Bishop, P. A. (2016). Enhancing short-term recovery after high-intensity anaerobic exercise. Journal of Strength and Conditioning Research 30, 320–325. doi: 10.1519/JSC.0000000000001060
PubMed Abstract | CrossRef Full Text | Google Scholar
Amtmann, J. A., Amtmann, K. A., and Spath, W. K. (2008). Lactate and rate of perceived exertion responses of athletes training for and competing in a mixed martial arts event. J. Strength Conditioning Res. 22, 645–647. doi: 10.1519/JSC.0b013e318166018e
Anding, R., and Oliver, J. M. (2015). Football player body composition: importance of monitoring for performance and health. Sports Sci. Exchang. 28, 1–8.
Google Scholar
Aubert, A. E., Seps, B., and Beckers, F. (2003). Heart rate variability in athletes. Sports Med. 33, 889–919. doi: 10.2165/00007256-200333120-00003
Bergman, B. C., and Brooks, G. A. (1999). Respiratory gas-exchange ratios during graded exercise in fed and fasted trained and untrained men. J. Appl. Physiol. 86, 479–487. doi: 10.1152/jappl.1999.86.2.479
Bolster, D. R., Pikosky, M. A., Gaine, P. C., Martin, W., Wolfe, R. R., Tipton, K. D., et al. (2005). Dietary protein intake impacts human skeletal muscle protein fractional synthetic rates after endurance exercise. Am. J. Physiol. Endocrinol. Metabol. 289, E678–E683. doi: 10.1152/ajpendo.00060.2005
Borenstein, M., Hedges, L. V., Higgins, J. P. T., and Rothstein, H. R. (2009). “Fixed-effect versus random-effects models,” in Introduction to Meta-Analysis , ed M. Borenstein (Chichester: John Wiley & Sons, Ltd), 77–85.
Boutron, I., Moher, D., Altman, D. G., Schulz, K. F., and Ravaud, P. (2008). Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann. Intern. Med. 148, 295–309. doi: 10.7326/0003-4819-148-4-200802190-00008
Breen, L., Philp, A., Witard, O. C., Jackman, S. R., Selby, A., Smith, K., et al. (2011). The influence of carbohydrate-protein co-ingestion following endurance exercise on myofibrillar and mitochondrial protein synthesis. J. Physiol. 589, 4011–4025. doi: 10.1113/jphysiol.2011.211888
Brukner, P., and Khan, K. (2009). Clinical Sports Medicine. North Ryde, NSW: McGraw-Hill
PubMed Abstract | Google Scholar
Chaimani, A., Higgins, J. P. T., Mavridis, D., Spyridonos, P., and Salanti, G. (2013). Graphical tools for network meta-analysis in STATA. PLoS ONE 8:e76654. doi: 10.1371/journal.pone.0076654
Chan, D. K.-C., Hagger, M. S., and Spray, C. M. (2011). Treatment motivation for rehabilitation after a sport injury: application of the trans-contextual model. Psychol. Sport Exerc. 12, 83–92. doi: 10.1016/j.psychsport.2010.08.005
CrossRef Full Text | Google Scholar
Coombes, J. S., Conacher, M., Austen, S. K., and Marshall, P. A. (2002). Dose effects of oral bovine colostrum on physical work capacity in cyclists. Med. Sci. Sports Exer. 34, 1184–1188. doi: 10.1097/00005768-200207000-00020
Dixon, E. M., Kamath, M. V., McCartney, N., and Fallen, E. L. (1992). Neural regulation of heart rate variability in endurance athletes and sedentary controls. Cardiovasc. Res. 26, 713–719. doi: 10.1093/cvr/26.7.713
Dlugosz, E. M., Chappell, M. A., Meek, T. H., Szafranska, P. A., Zub, K., Konarzewski, M., et al. (2013). Phylogenetic analysis of mammalian maximal oxygen consumption during exercise. J. Exp. Biol. 216, 4712–4721. doi: 10.1242/jeb.088914
Ekblom, B., and Golobarg, A. N. (1971). The influence of physical training and other factors on the subjective rating of perceived exertion. Acta Physiol. Scand. 83, 399–406. doi: 10.1111/j.1748-1716.1971.tb05093.x
Frank, K., Patel, K., Lopez, G., and Willis, B. (2017). Whey Protein Research Analysis . Available online at: https://examine.com/supplements/whey-protein/ (accessed September 15, 2018).
Fukuda, D. H., Smith, A. E., Kendall, K. L., and Stout, J. R. (2010). The possible combinatory effects of acute consumption of caffeine, creatine, and amino acids on the improvement of anaerobic running performance in humans. Nutr. Res. 30, 607–614. doi: 10.1016/j.nutres.2010.09.004
Garg, A. X., Hackam, D., and Tonelli, M. (2008). Systematic review and meta-analysis: when one study is just not enough. Clini. J. Am. Soc. Nephrol. 3, 253–260. doi: 10.2215/CJN.01430307
Gunnarsson, T. P., Bendiksen, M., Bischoff, R., Christensen, P. M., Lesivig, B., Madsen, K., et al. (2013). Effect of whey protein- and carbohydrate-enriched diet on glycogen resynthesis during the first 48 h after a soccer game. Scand. J. Med. Sci. Sports 23, 508–515. doi: 10.1111/j.1600-0838.2011.01418.x
Hansen, M., Bangsbo, J., Jensen, J., Bibby, B. M., Madsen, K., Krause-Jensen, M., et al. (2016). Protein intake during training sessions has no effect on performance and recovery during a strenuous training camp for elite cyclists. J. Int. Soc. Sports Nutr. 13:9. doi: 10.1186/s12970-016-0120-4
Higgins, J. P., Jackson, D., Barrett, J. K., Lu, G., Ades, A. E., and White, I. R. (2012). Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res. Synthes. Methods 3, 98–110. doi: 10.1002/jrsm.1044
Higgins, J. P. T., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011a). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343:d5928. doi: 10.1136/bmj.d5928
Higgins, J. P. T., and Green, S. (eds.) (2011). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0. The Cochrane Collaboration . Chichester: John Wiley & Sons Ltd.
Higgins, J. P. T., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327, 557–560. doi: 10.1136/bmj.327.7414.557
Higgins, J. P. T., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2011b). “Selecting studies and collecting data,” in Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 , eds J. P. T. Higgins and J. J. Deeks (Chichester: John Wiley & Sons Ltd.), 151–187.
Highton, J., Twist, C., Lamb, K., and Nicholas, C. (2013). Carbohydrate-protein coingestion improves multiple-sprint running performance. J. Sports Sci. 31, 361–369. doi: 10.1080/02640414.2012.735370
Hoffman, J. R., Ratamess, N. A., Tranchina, C. P., Rashti, S. L., Kang, J., and Faigenbaum, A. D. (2009). Effect of protein-supplement timing on strength, power, and body-composition changes in resistance-trained men. Int. J. Sport Nutr. Exerc. Metab. 19, 172–185. doi: 10.1123/ijsnem.19.2.172
Iellamo, F., Manzi, V., Caminiti, G., Vitale, C., Massaro, M., Cerrito, A., et al. (2014). Validation of rate of perceived exertion-based exercise training in patients with heart failure: insights from autonomic nervous system adaptations. Int. J. Cardiol. 176, 394–398. doi: 10.1016/j.ijcard.2014.07.076
Imai, K., Sato, H., Hori, M., Kusuoka, H., Ozaki, H., Yokoyama, H., et al. (1994). Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J. Am. Coll. Cardiol. 24, 1529–1535. doi: 10.1016/0735-1097(94)90150-3
Impey, S. G., Smith, D., Robinson, A. L., Owens, D. J., Bartlett, J. D., Smith, K., et al. (2015). Leucine-enriched protein feeding does not impair exercise-induced free fatty acid availability and lipid oxidation: beneficial implications for training in carbohydrate-restricted states. Amino Acids 47, 407–416. doi: 10.1007/s00726-014-1876-y
Joy, J. M., Lowery, R. P., Wilson, J. M., Purpura, M., De Souza, E. O., Wilson, S. M., et al. (2013). The effects of 8 weeks of whey or rice protein supplementation on body composition and exercise performance. Nutr. J. 12, 86–93. doi: 10.1186/1475-2891-12-86
Kraemer, W., Hooper, D., Szivak, T., Kupchak, B., Dunn-Lewis, C., Comstock, B., et al. (2015). The addition of beta-hydroxy-beta-methylbutyrate and isomaltulose to whey protein improves recovery from highly demanding resistance exercise. J. Am. Coll. Nutr. 34, 91–99. doi: 10.1080/07315724.2014.938790
Lam, F.-C., Khan, T. M., and Quek, K.-F. (2016). Efficacy and Safety of Whey Protein Supplements on Performance and Recovery Among Athletes: a Systematic Review and Meta-Analysis. University of York . Available online at: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016041842 (accessed September 15, 2018).
Lemon, P. W., Tarnopolsky, M. A., MacDougall, J. D., and Atkinson, S. A. (1992). Protein requirements and muscle mass/strength changes during intensive training in novice bodybuilders. J. Appl. Physiol. 73, 767–775. doi: 10.1152/jappl.1992.73.2.767
Li, M., and Kim, Y. T. (2017). Design of a wireless sensor system with the algorithms of heart rate and agility index for athlete evaluation. Sensors 17, 2373–2387. doi: 10.3390/s17102373
Li, S. C., and Zhao, Y. F. (2007). Effects of carbohydrate and whey protein supplement at appropriate time on physical performance during football game. J. Clini. Rehabil. Tissue Eng. Res. 11, 10304–10307.
Lollo, P. C., Amaya-Farfan, J., and de Carvalho-Silva, L. B. (2011). Physiological and physical effects of different milk protein supplements in elite soccer players. J. Human Kinet. 30, 49–57. doi: 10.2478/v10078-011-0072-3
Lollo, P. C., Amaya-Farfan, J., Faria, I. C., Salgado, J. V. V., Chacon-Mikahil, M. P. T., Cruz, A. G., et al. (2014). Hydrolysed whey protein reduces muscle damage markers in Brazilian elite soccer players compared with whey protein and maltodextrin. A twelve-week in-championship intervention. Int. Dairy J. 34, 19–24. doi: 10.1016/j.idairyj.2013.07.001
Macdermid, P. W., and Stannard, S. R. (2006). A whey-supplemented, high-protein diet versus a high-carbohydrate diet: effects of endurance cycling performance. Int. J. Sport Nutr. Exerc. Metab. 16, 65–77. doi: 10.1123/ijsnem.16.1.65
MacKenzie-Shalders, K. L., Byrne, N. M., Slater, G. J., and King, N. A. (2015). The effect of a whey protein supplement dose on satiety and food intake in resistance training athletes. Appetite 92, 178–184. doi: 10.1016/j.appet.2015.05.007
Miller, P. E., Alexander, D. D., and Perez, V. (2014). Effects of whey protein and resistance exercise on body composition: a meta-analysis of randomized controlled trials. J. Am. Coll. Nutr. 33, 163–175. doi: 10.1080/07315724.2013.875365
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 151, 264–269. doi: 10.7326/0003-4819-151-4-200908180-00135
Naclerio, F., Larumbe-Zabala, E., Cooper, R., Allgrove, J., and Earnest, C. P. (2015). A multi-ingredient containing carbohydrate, proteins L-glutamine and L-carnitine attenuates fatigue perception with no effect on performance, muscle damage or immunity in soccer players. PLoS ONE 10:e0125188. doi: 10.1371/journal.pone.0125188
Nelson, M. T., Biltz, G. R., and Dengel, D. R. (2015). Repeatability of respiratory exchange ratio time series analysis. J. Strength Conditioning Res. 29, 2550–2558. doi: 10.1519/JSC.0000000000000924
Nissen, S. L., and Sharp, R. L. (2003). Effect of dietary supplements on lean mass and strength gains with resistance exercise: a meta-analysis. J. Appl. Physiol. 94, 651–659. doi: 10.1152/japplphysiol.00755.2002
Oosthuyse, T., and Millen, A. M. (2016). Comparison of energy supplements during prolonged exercise for maintenance of cardiac function: carbohydrate only versus carbohydrate plus whey or casein hydrolysate. Appl. Physiol. Nutr. Metab. 41, 674–683. doi: 10.1139/apnm-2015-0491
Ross, R., Blair, S. N., Arena, R., Church, T. S., Després, J.-P., Franklin, B. A., et al. (2016). Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the american heart association. Circulation 134:E653. doi: 10.1161/CIR.0000000000000461
Saez de Villarreal, E., Requena, B., and Cronin, J. (2012). The effects of plyometric training on sprint performance: a meta-analysis. J. Strength Conditioning Res. 26, 575–584. doi: 10.1519/JSC.0b013e318220fd03
Salanti, G. (2012). Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res. Synthes. Methods 3, 80–97. doi: 10.1002/jrsm.1037
Schoenfeld, B. J., Aragon, A. A., and Krieger, J. W. (2013). The effect of protein timing on muscle strength and hypertrophy: a meta-analysis. J. Int. Soc. Sports Nutr. 10, 53–66. doi: 10.1186/1550-2783-10-53
Schroer, A. B., Saunders, M. J., Baur, D. A., Womack, C. J., and Luden, N. D. (2014). Cycling time trial performance may be impaired by whey protein and L-alanine intake during prolonged exercise. Int. J. Sport Nutr. Exerc. Metab. 24, 507–515. doi: 10.1123/ijsnem.2013-0173
Shing, C. M., Jenkins, D. G., Stevenson, L., and Coombes, J. S. (2006). The influence of bovine colostrum supplementation on exercise performance in highly trained cyclists. Br. J. Sports Med. 40, 797–801. doi: 10.1136/bjsm.2006.027946
Silver, M. D. (2001). Use of ergogenic aids by athletes. J. Am. Acad. Orthopaed. Surg. 9, 61–70. doi: 10.5435/00124635-200101000-00007
Smith, A. E., Fukuda, D. H., Kendall, K. L., and Stout, J. R. (2010). The effects of a pre-workout supplement containing caffeine, creatine, and amino acids during three weeks of high-intensity exercise on aerobic and anaerobic performance. J. Int. Soc. Sports Nutr. 7, 10–22. doi: 10.1186/1550-2783-7-10
Tarnopolsky, M. A., Atkinson, S. A., MacDougall, J. D., Chesley, A., Phillips, S., and Schwarcz, H. P. (1992). Evaluation of protein requirements for trained strength athletes. J. Appl. Physiol. 73, 1986–1995. doi: 10.1152/jappl.1992.73.5.1986
Taylor, L. W., Wilborn, C., Roberts, M. D., White, A., and Dugan, K. (2016). Eight weeks of pre- and postexercise whey protein supplementation increases lean body mass and improves performance in Division III collegiate female basketball players. Appl. Physiol. Nutr. Metab. 41, 249–254. doi: 10.1139/apnm-2015-0463
Tipton, K. D., Elliott, T. A., Elliott, T. A., Cree, M. G., Wolf, S. E., Sanford, A. P., et al. (2004). Ingestion of casein and whey proteins result in muscle anabolism after resistance exercise. Med. Sci. Sports Exerc. 36, 2073–2081. doi: 10.1249/01.MSS.0000147582.99810.C5
Turner, R. M., Davey, J., Clarke, M. J., Thompson, S. G., and Higgins, J. P. (2012). Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane database of systematic reviews. Int. J. Epidemiol. 41, 818–827. doi: 10.1093/ije/dys041
Vegge, G., Rønnestad, B. R., and Ellefsen, S. (2012). Improved cycling performance with ingestion of hydrolyzed marine protein depends on performance level. J. Int. Soc. Sports Nutr. 9, 14–24. doi: 10.1186/1550-2783-9-14
Veroniki, A. A., Vasiliadis, H. S., Higgins, J. P., and Salanti, G. (2013). Evaluation of inconsistency in networks of interventions. Int. J. Epidemiol. 42, 332–345. doi: 10.1093/ije/dys222
Wiese-Bjornstal, D. M. (2010). Psychology and socioculture affect injury risk, response, and recovery in high-intensity athletes: a consensus statement. Scand. J. Med. Sci. Sports 20, 103–111. doi: 10.1111/j.1600-0838.2010.01195.x
Willick, S. E., Miller, G. D., and Eichner, D. (2016). The anti-doping movement. PMandR 8(3, Suppl), S125–32. doi: 10.1016/j.pmrj.2015.12.001
World Anti-Doping Agency (2017). Index of Prohibited Substances and Methods . Available online at: https://www.wada-ama.org/en/what-we-do/prohibited-list/index-prohibited-substances-and-methods (accessed September 15, 2018).
Keywords: whey protein supplements, network meta-analysis, athletes, vital sign, physical strength, athlete's health and performance
Citation: Lam F-C, Bukhsh A, Rehman H, Waqas MK, Shahid N, Khaliel AM, Elhanish A, Karoud M, Telb A and Khan TM (2019) Efficacy and Safety of Whey Protein Supplements on Vital Sign and Physical Performance Among Athletes: A Network Meta-Analysis. Front. Pharmacol . 10:317. doi: 10.3389/fphar.2019.00317
Received: 26 September 2018; Accepted: 15 March 2019; Published: 24 April 2019.
Reviewed by:
Copyright © 2019 Lam, Bukhsh, Rehman, Waqas, Shahid, Khaliel, Elhanish, Karoud, Telb and Khan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tahir Mehmood Khan, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
The functionalities and applications of whey/whey protein in fermented foods: a review
- Published: 25 November 2023
- Volume 33 , pages 769–790, ( 2024 )
Cite this article
- Xiaorong Zeng 1 ,
- Yujie Wang 1 ,
- Shuda Yang 1 ,
- Yijun Liu 1 ,
- Xing Li 2 &
- Diru Liu 1
410 Accesses
Explore all metrics
Whey, a major by-product of cheese production, is primarily composed of whey protein (WP). To mitigate environmental pollution, it is crucial to identify effective approaches for fully utilizing the functional components of whey or WP to produce high-value-added products. This review aims to illustrate the active substances with immunomodulatory, metabolic syndrome-regulating, antioxidant, antibacterial, and anti-inflammatory activities produced by whey or WP through fermentation processes, and summarizes the application and the effects of whey or WP on nutritional properties and health promotion in fermented foods. All these findings indicate that whey or WP can serve as a preservative, a source of high-protein dietary, and a source of physiologically active substance in the production of fermented foods. Therefore, expanding the use of whey or WP in fermented foods is of great importance for converting whey into value-added products, as well as reducing whey waste and potential contamination.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Emerging trends in nutraceutical applications of whey protein and its derivatives.
Seema Patel

Whey Production Status, Types, Characterization and Functional Properties
Whey and its derivatives for probiotics, prebiotics, synbiotics, and functional foods: a critical review.
Ourdia Kareb & Mohammed Aïder
Addai FP, Lin F, Wang T, Kosiba AA, Sheng P, Yu F, Gu J, Zhou Y, Shi H. Technical integrative approaches to cheese whey valorization towards sustainable environment. Food & Function. 11: 8407-8423 (2020)
Article CAS Google Scholar
Ali SM, Salem FE, Aboulwafa MM, Shawky RM. Hypolipidemic activity of lactic acid bacteria: Adjunct therapy for potential probiotics. PLoS ONE. 17: e0269953 (2022)
Article CAS PubMed PubMed Central Google Scholar
Arbizu S, Chew B, Mertens-Talcott SU, Noratto G. Commercial whey products promote intestinal barrier function with glycomacropeptide enhanced activity in downregulating bacterial endotoxin lipopolysaccharides (LPS)-induced inflammationin vitro. Food & Function. 11: 5842-5852 (2020)
Aslam H, Green J, Jacka FN, Collier F, Berk M, Pasco J, Dawson SL. Fermented foods, the gut and mental health: a mechanistic overview with implications for depression and anxiety. Nutritional Neuroscience. 23: 659-671 (2020)
Article CAS PubMed Google Scholar
Ayed L, M'Hir S, Asses N. Sustainable whey processing techniques: Innovations in derivative and beverage production. Food Biosciencei. 53: 102642 (2023)
Barba FJ. An integrated approach for the valorization of cheese whey. Foods. 10: 564 (2021)
Barone G, O'Regan J, Kelly AL, O'Mahony JA. Interactions between whey proteins and calcium salts and implications for the formulation of dairy protein-based nutritional beverage products: A review. Comprehensive Reviews in Food Science and Food Safety. 21: 1254-1274 (2022)
Barros CP, Grom LC, Guimarães JT, Balthazar CF, Rocha RS, Silva R, Almada CN, Pimentel TC, Venancio EL, Junior IC. Paraprobiotic obtained by ohmic heating added in whey-grape juice drink is effective to control postprandial glycemia in healthy adults. Food Research International. 140: 109905 (2021)
Baruzzi F, de Candia S, Quintieri L, Caputo L, De Leo F. Development of a synbiotic beverage enriched with bifidobacteria strains and fortified with whey proteins. Frontiers in Microbiology. 8: 640 (2017)
Article PubMed PubMed Central Google Scholar
Baspinar B, Güldaş M. Traditional plain yogurt: a therapeutic food for metabolic syndrome? Critical Reviews in Food Science and Nutrition. 61: 3129-3143 (2021)
Bendelja Ljoljić D, Kalit S, Kazalac J, Dolenčić Špehar I, Mihaljević Žulj M, Maslov Bandić L, Tudor Kalit M. The potential of using Istrian Albumin Cheese Whey in the production of whey distillate. Fermentation. 9: 192 (2023)
Article Google Scholar
Boscaini S, Skuse P, Nilaweera KN, Cryan JF, Cotter PD. The ‘Whey’ to good health: Whey protein and its beneficial effect on metabolism, gut microbiota and mental health. Trends in Food Science & Technology. 133: 1-14 (2023)
Buchanan D, Martindale W, Romeih E, Hebishy E. Recent advances in whey processing and valorisation: Technological and environmental perspectives. International Journal of Dairy Technology. 76: 291-312 (2023)
Chaves FM, Baptista IL, Simabuco FM, Quaresma PGF, Pena FL, Bezerra RMN, Pauli JR, da Cunha DT, Campos-Ferraz PL, Antunes AEC. High-intensity-exercise-induced intestinal damage is protected by fermented milk supplemented with whey protein, probiotic and pomegranate ( Punica granatum L.). British Journal of Nutrition. 119: 896-909 (2018)
Chen M-Y, Wu H-T, Chen F-F, Wang Y-T, Chou D-L, Wang G-H, Chen Y-P. Characterization of Tibetan kefir grain-fermented milk whey and its suppression of melanin synthesis. Journal of Bioscience and Bioengineering. 133: 547-554 (2022)
Chourasia R, Phukon LC, Abedin MM, Padhi S, Singh SP, Rai AK. Whey valorization by microbial and enzymatic bioprocesses for the production of nutraceuticals and value-added products. Bioresource Technology Reports. 19: 101144 (2022)
Cordeiro BF, Oliveira ER, Da Silva SH, Savassi BM, Acurcio LB, Lemos L, Alves JDL, Carvalho Assis H, Vieira AT, Faria AM. Whey protein isolate-supplemented beverage, fermented by Lactobacillus casei BL23 and Propionibacterium freudenreichii 138, in the prevention of mucositis in mice. Frontiers in Microbiology. 9: 2035 (2018)
Daliri EB-M, Lee BH, Park B-J, Kim S-H, Oh D-H. Antihypertensive peptides from whey proteins fermented by lactic acid bacteria. Food Science and Biotechnology. 27: 1781-1789 (2018)
Damar I, Cinar K, Gulec HA. Concentration of whey proteins by ultrafiltration: Comparative evaluation of process effectiveness based on physicochemical properties of membranes. International Dairy Journal. 111: 104823 (2020)
de Souza Pereira AM, Bezerra de Farias DR, de Queiroz BB, de Caldas Nobre MS, Cavalcanti MT, Salles HO, Olbrich dos Santos KM, Dantas de Medeiros AC, Florentino ER, Alonso Buriti FC. Influence of a co-culture of Streptococcus thermophilus and Lactobacillus casei on the proteolysis and ACE-inhibitory activity of a beverage based on reconstituted goat whey powder. Probiotics and Antimicrobial Proteins. 11: 273–282 (2019)
Dimidi E, Cox SR, Rossi M, Whelan K. Fermented foods: definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients. 11: 1806 (2019)
Dineshbhai CK, Basaiawmoit B, Sakure AA, Maurya R, Bishnoi M, Kondepudi KK, Patil G, Mankad M, Liu Z, Hati S. Exploring the potential of Lactobacillus and Saccharomyces for biofunctionalities and the release of bioactive peptides from whey protein fermentate. Food Bioscience. 48: 101758 (2022)
Dopazo V, Illueca F, Luz C, Musto L, Moreno A, Calpe J, Meca G. Evaluation of shelf life and technological properties of bread elaborated with lactic acid bacteria fermented whey as a bio-preservation ingredient. LWT-Food Science and Technology. 174: 114427 (2023)
El Sheikha AF, Hu D-M. Molecular techniques reveal more secrets of fermented foods. Critical Reviews in Food Science and Nutrition. 60: 11-32 (2020)
Article PubMed Google Scholar
Emergen research. Fermented food and ingredients market by food type (fermented dairy products, fermented beverages), by ingredient type (organic acids, amino acids, industrial enzymes), by distribution channel (online stores, supermarkets), forecasts to 2027. https://www.emergenresearch.com/industry-report/fermented-food-and-ingredients-market . Accessed Nov 2020.
Ferreyra LS, Verdini RA, Soazo M, Piccirilli GN. Impact of whey protein addition on wheat bread fermented with a spontaneous sourdough. International Journal of Food Science and Technology. 56 : 4738-4745 (2021)
Foisy Sauvé M, Spahis S, Delvin E, Levy E. Glycomacropeptide: A bioactive milk derivative to alleviate metabolic syndrome outcomes. Antioxidants & Redox Signaling. 34: 201-222 (2020)
Galimberti A, Bruno A, Agostinetto G, Casiraghi M, Guzzetti L, Labra M. Fermented food products in the era of globalization: tradition meets biotechnology innovations. Current Opinion in Biotechnology. 70: 36-41 (2021)
Gantumur M-A, Sukhbaatar N, Qayum A, Bilawal A, Tsembeltsogt B, Oh K-C, Jiang Z, Hou J. Characterization of major volatile compounds in whey spirits produced by different distillation stages of fermented lactose-supplemented whey. Journal of Dairy Science. 105: 83-96 (2022)
Garay PA, Villalva FJ, Paz NF, de Oliveira EG, Ibarguren C, Alcocer JC, Curti CA, Ramón AN. Formulation of a protein fortified drink based on goat milk whey for athletes. Small Ruminant Research. 201: 106418 (2021)
García-Burgos M, Moreno-Fernández J, Alférez MJ, Díaz-Castro J, López-Aliaga I. New perspectives in fermented dairy products and their health relevance. Journal of Functional Foods. 72: 104059 (2020)
García G, Agosto ME, Cavaglieri L, Dogi C. Effect of fermented whey with a probiotic bacterium on gut immune system. Journal of Dairy Research. 87 (1): 134-137 (2020)
Gomaa MA, Allam MG, Haridi AA, Eliwa A-EM, Darwish AM. High-protein concentrated pro-yogurt (Pro-WPI) enriched with whey protein isolate improved athletic anemia and performance in a placebo-controlled study. Frontiers in Nutrition. 8: 788446 (2022)
Hati S, Patel N, Sakure A, Mandal S. Influence of whey protein concentrate on the production of antibacterial peptides derived from fermented milk by lactic acid bacteria. International Journal of Peptide Research and Therapeutics. 24: 87-98 (2018)
Helal A, Nasuti C, Sola L, Sassi G, Tagliazucchi D, Solieri L. Impact of spontaneous fermentation and inoculum with natural whey starter on peptidomic profile and biological activities of cheese whey: A comparative study. Fermentation. 9: 270 (2023)
Hong S-M, Chung E-C, Kim C-H. Anti-obesity effect of fermented whey beverage using lactic acid bacteria in diet-induced obese rats. Korean Journal for Food Science of Animal Resources. 35: 653 (2015)
Hughes P, Risner D, Goddik LM. Whey to vodka. In: Whey-Biological Properties and Alternative Uses. IntechOpen (2018)
Ikarashi N, Fukuda N, Ochiai M, Sasaki M, Kon R, Sakai H, Hatanaka M, Kamei J. Lactobacillus helveticus-fermented milk whey suppresses melanin production by inhibiting tyrosinase through decreasing MITF expression. Nutrients. 12: 2082 (2020)
Iuga M, Boestean O, Ghendov-Mosanu A, Mironeasa S. Impact of dairy ingredients on wheat flour dough rheology and bread properties. Foods. 9: 828 (2020)
Izzo L, Luz C, Ritieni A, Manes J, Meca G. Whey fermented by using Lactobacillus plantarum strains: A promising approach to increase the shelf life of pita bread. Journal of Dairy Science. 103: 5906-5915 (2020a)
Izzo L, Luz C, Ritieni A, Quiles Beses J, Manes J, Meca G. Inhibitory effect of sweet whey fermented by Lactobacillus plantarum strains against fungal growth: A potential application as an antifungal agent. Journal of Food Science. 85: 3920-3926 (2020b)
Jeung WH, Shim J-J, Woo S-W, Sim J-H, Lee J-L. Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 cell extracts inhibit adipogenesis in 3T3-L1 and HepG2 cells. Journal of Medicinal Food. 21: 876-886 (2018)
Jitpakdee J, Kantachote D, Kanzaki H, Nitoda T. Potential of lactic acid bacteria to produce functional fermented whey beverage with putative health promoting attributes. LWT-Food Science and Technology. 160: 113269 (2022)
Kang M, Oh NS, Kim M, Ahn HY, Yoo HJ, Sun M, Kang S-H, Yang HJ, Kwon DY, Lee JH. Supplementation of fermented Maillard-reactive whey protein enhances immunity by increasing NK cell activity. Food & Function. 8: 1718-1725 (2017)
Karimi N, Pourahmad R, Taheri S, Eyvazzadeh O. Isolation and purification of bioactive peptides from yogurt whey: Application as a natural preservative in a model food system. Journal of Food Processing and Preservation. 45: e16086 (2021)
Karwowska M, Kononiuk A. Addition of acid whey improves organic dry-fermented sausage without nitrite production and its nutritional value. International Journal of Food Science and Technology. 53: 246-253 (2018)
Kashyap R, Narayan KS, Vij S. Evaluation of the antimicrobial attribute of bioactive peptides derived from colostrum whey fermented by Lactobacillus against diarrheagenic E. coli strains. Journal of Food Science and Technology. 60: 211-221 (2023)
Kaur H, Gupta T, Kapila S, Kapila R. Protective effects of potential probiotic Lactobacillus rhamnosus (MTCC-5897) fermented whey on reinforcement of intestinal epithelial barrier function in a colitis-induced murine model. Food & Function. 12: 6102-6116 (2021)
Kaur H, Gupta T, Kapila S, Kapila R. Lactobacillus fermentum (MTCC-5898) based fermented whey renders prophylactic action against colitis by strengthening the gut barrier function and maintaining immune homeostasis. Microbial Pathogenesis. 173: 105887 (2022a)
Kaur H, Kaur G, Ali SA. Dairy-based probiotic-fermented functional foods: An update on their health-promoting properties. Fermentation. 8: 425 (2022b)
Kavitake D, Kandasamy S, Devi PB, Shetty PH. Recent developments on encapsulation of lactic acid bacteria as potential starter culture in fermented foods—A review. Food Bioscience. 21: 34-44 (2018)
Kawamata Y, Toyotake Y, Ogiyama D, Takeda Y, Wakayama M. Development of the original whey‐based vinegar using rapeseed meal or wheat bran as a raw material for koji. Journal of Food Processing and Preservation. 45: e16097 (2021)
Kononiuk AD, Karwowska M. Bioactive compounds in fermented sausages prepared from beef and fallow deer meat with acid whey addition. Molecules. 25: 2429 (2020)
Kopec A, Borczak B, Pysz M, Sikora E, Sikora M, Curic D, Novotni D. An addition of sourdough and whey proteins affects the nutritional quality of wholemeal wheat bread. Acta Scientiarum Polonorum Technologia Alimentaria. 13: 43-54 (2014)
Lech M, Niesobska A, Trusek-Holownia A. Dairy wastewater utilization: separation of whey proteins in membrane and chromatographic processes. Desalination and Water Treatment. 57: 23326-23334 (2016)
Lee JS, Hyun IK, Seo H-J, Song D, Kim MY, Kang S-S. Biotransformation of whey by Weissella cibaria suppresses 3T3-L1 adipocyte differentiation. Journal of Dairy Science. 104: 3876-3887 (2021)
Leyva Salas M, Mounier J, Valence F, Coton M, Thierry A, Coton E. Antifungal microbial agents for food biopreservation-a review. Microorganisms. 5: 37 (2017)
Li S, Li P, Feng F, Luo L-X. Microbial diversity and their roles in the vinegar fermentation process. Applied Microbiology and Biotechnology. 99: 4997-5024 (2015)
Londero A, Iraporda C, Garrote GL, Abraham AG. Cheese whey fermented with kefir micro-organisms: Antagonism against Salmonella and immunomodulatory capacity. International Journal of Dairy Technology. 68: 118-126 (2015)
Lopes ACA, Eda SH, Andrade RP, Amorim JC, Duarte WF. New alcoholic fermented beverages—potentials and challenges. Fermented Beverages: 577–603 (2019)
Luz C, Izzo L, Graziani G, Gaspari A, Ritieni A, Manes J, Meca G. Evaluation of biological and antimicrobial properties of freeze-dried whey fermented by different strains of Lactobacillus plantarum. Food & Function. 9: 3688-3697 (2018)
Luz C, Izzo L, Ritieni A, Mañes J, Meca G. Antifungal and antimycotoxigenic activity of hydrolyzed goat whey on Penicillium spp: An application as biopreservation agent in pita bread. LWT-Food Science and Technology. 118: 108717 (2020a)
Luz C, Rodriguez L, Romano R, Manes J, Meca G. A natural strategy to improve the shelf life of the loaf bread against toxigenic fungi: The employment of fermented whey powder. International Journal of Dairy Technology. 73: 88-97 (2020b)
Makwana S, Prajapati JB, Pipaliya R, Hati S. Effects of probiotic fermented milk on management of obesity studied in high-fat-diet induced obese rat model. Food Production, Processing and Nutrition. 5: 3 (2023)
Marco ML, Heeney D, Binda S, Cifelli CJ, Cotter PD, Foligne B, Ganzle M, Kort R, Pasin G, Pihlanto A, Smid EJ, Hutkins R. Health benefits of fermented foods: microbiota and beyond. Current Opinion in Biotechnology. 44: 94-102 (2017)
Mazorra-Manzano MA, Robles-Porchas GR, González-Velázquez DA, Torres-Llanez MJ, Martínez-Porchas M, García-Sifuentes CO, González-Córdova AF, Vallejo-Córdoba B. Cheese whey fermentation by its native microbiota: Proteolysis and bioactive peptides release with ACE-inhibitory activity. Fermentation. 6: 19 (2020)
Mehra R, Kumar H, Kumar N, Ranvir S, Jana A, Buttar HS, Telessy IG, Awuchi CG, Okpala COR, Korzeniowska M, Guiné RPF. Whey proteins processing and emergent derivatives: An insight perspective from constituents, bioactivities, functionalities to therapeutic applications. Journal of Functional Foods. 87: 104760 (2021)
Melini F, Melini V, Luziatelli F, Ficca AG, Ruzzi M. Health-promoting components in fermented foods: An up-to-date systematic review. Nutrients. 11: 1189 (2019)
Meng Y, Liang Z, Zhang C, Hao S, Han H, Du P, Li A, Shao H, Li C, Liu L. Ultrasonic modification of whey protein isolate: Implications for the structural and functional properties. LWT-Food Science and Technology. 152: 112272 (2021)
Mohammadi-Sartang M, Bellissimo N, de Zepetnek JT, Brett N, Mazloomi S, Fararouie M, Bedeltavana A, Famouri M, Mazloom Z. The effect of daily fortified yogurt consumption on weight loss in adults with metabolic syndrome: A 10-week randomized controlled trial. Nutrition, Metabolism and Cardiovascular Diseases. 28: 565-574 (2018)
Moslemi M, Moayedi A, Khomeiri M, Maghsoudlou Y. Development of a whey-based beverage with enhanced levels of conjugated linoleic acid (CLA) as facilitated by endogenous walnut lipase. Food Chemistry. 17: 100547-100547 (2023)
CAS Google Scholar
Nabi X-H, Ma C-Y, Manaer T, Heizati M, Wulazibieke B, Aierken L. Anti-atherosclerotic effect of traditional fermented cheese whey in atherosclerotic rabbits and identification of probiotics. Bmc Complementary and Alternative Medicine. 16: 309 (2016)
Nicolas P, Lujan Ferreira M, Lassalle V. A review of magnetic separation of whey proteins and potential application to whey proteins recovery, isolation and utilization. Journal of Food Engineering. 246: 7-15 (2019)
Olvera-Rosales L, Cruz-Guerrero A, García-Garibay J, Gómez-Ruíz L, Contreras-López E, Guzmán-Rodríguez F, González-Olivares L. Bioactive peptides of whey: Obtaining, activity, mechanism of action, and further applications. Critical Reviews in Food Science and Nutrition. 1–31 (2022)
Park E, Paik H-D, Lee S-M. Combined effects of whey protein hydrolysates and probiotics on oxidative stress induced by an iron-overloaded diet in rats. International Journal of Food Sciences and Nutrition. 69: 298-307 (2018)
Pontonio E, Montemurro M, De Gennaro GV, Miceli V, Rizzello CG. Antihypertensive peptides from ultrafiltration and fermentation of the ricotta cheese exhausted whey: Design and characterization of a functional ricotta cheese. Foods. 10: 2573 (2021)
Rabaioli Rama G, Kuhn D, Beux S, Jachetti Maciel M, Volken de Souza CF. Cheese whey and ricotta whey for the growth and encapsulation of endogenous lactic acid bacteria. Food and Bioprocess Technology. 13: 308–322 (2020)
Ranok A, Kupradit C, Khongla C, Musika S, Mangkalanan S, Suginta W. Effect of whey protein concentrate on probiotic viability and antioxidant properties of yogurt during storage and simulated gastrointestinal transit. International Food Research Journal. 28: 110-119 (2021)
Rebolloso-Padilla ON, Sánchez-Abúndez HF, Ruelas-Chacón X, Fuentes-Lara LO, Corona-Flores JD, Aguilera-Carbó AF. Chapter 10—Development and evaluation of fermented drink, made with sweet cheese whey. In: Kuddus M, Hossain M (eds) Value-Addition in Beverages through Enzyme Technology. pp. 163-176. Academic Press, New York (2023)
Chapter Google Scholar
Reyes-Díaz A, González-Córdova AF, Hernández-Mendoza A, Reyes-Díaz R, Vallejo-Cordoba B. Immunomodulation by hydrolysates and peptides derived from milk proteins. International Journal of Dairy Technology. 71(1): 1-9 (2018)
Ribes S, Fuentes A, Talens P, Manuel Barat J. Prevention of fungal spoilage in food products using natural compounds: A review. Critical Reviews in Food Science and Nutrition. 58: 2002-2016 (2018)
Risner D, Shayevitz A, Haapala K, Meunier-Goddik L, Hughes P. Fermentation and distillation of cheese whey: Carbon dioxide-equivalent emissions and water use in the production of whey spirits and white whiskey. Journal of Dairy Science. 101: 2963-2973 (2018)
Risner D, Tomasino E, Hughes P, Meunier-Goddik L. Volatile aroma composition of distillates produced from fermented sweet and acid whey. Journal of Dairy Science. 102: 202-210 (2019)
Rosa LS, Santos ML, Abreu JP, Rocha RS, Esmerino EA, Freitas MQ, Mársico ET, Campelo PH, Pimentel TC, Silva MC. Probiotic fermented whey-milk beverages: Effect of different probiotic strains on the physicochemical characteristics, biological activity, and bioactive peptides. Food Research International. 164: 112396 (2023)
Ruiz L, Delgado S, Ruas-Madiedo P, Sánchez B, Margolles A. Bifidobacteria and their molecular communication with the immune system. Frontiers in Microbiology. 8: 2345 (2017)
Rul F, Bera-Maillet C, Champomier-Verges MC, El-Mecherfi KE, Foligne B, Michalski MC, Milenkovic D, Savary-Auzeloux I. Underlying evidence for the health benefits of fermented foods in humans. Food & Function. 13: 4804-4824 (2022)
Sah BNP, Vasiljevic T, McKechnie S, Donkor ON. Antioxidative and antibacterial peptides derived from bovine milk proteins. Critical Reviews in Food Science and Nutrition. 58: 726-740 (2018)
Savassi B, Cordeiro BF, Silva SH, Oliveira ER, Belo G, Figueiroa AG, Alves Queiroz MI, Faria AMC, Alves J, Silva TFD. Lyophilized symbiotic mitigates mucositis induced by 5-fluorouracil. Frontiers in Pharmacology. 12: 755871 (2021)
Scalone GLL, Ioannidis AG, Lamichhane P, Devlieghere F, De Kimpe N, Cadwallader K, De Meulenaer B. Impact of whey protein hydrolysates on the formation of 2, 5-dimethylpyrazine in baked food products. Food Research International. 132: 109089 (2020)
Simitzis P, Zikou F, Progoulakis D, Theodorou G, Politis I. A note on the effects of yoghurt acid whey marination on the tenderness and oxidative stability of different meat types. Foods. 10 (11): 2557 (2021)
Skrzypczak K, Fornal E, Wasko A, Gustaw W. Effects of probiotic fermentation of selected milk and whey protein preparations on bioactive and technological properties. Italian Journal of Food Science. 31: 437-450 (2019)
Smith NM, Maloney NG, Shaw S, Horgan GW, Fyfe C, Martin JC, Suter A, Scott KP, Johnstone AM. Daily fermented whey consumption alters the fecal short-chain fatty acid profile in healthy adults. Frontiers in Nutrition. 7: 165 (2020)
Solieri L, Valentini M, Cattivelli A, Sola L, Helal A, Martini S, Tagliazucchi D. Fermentation of whey protein concentrate by Streptococcus thermophilus strains releases peptides with biological activities. Process Biochemistry. 121: 590-600 (2022)
Song D, Bin Lee H, Kim G-B, Kang S-S. Whey fermented by Enterococcus faecalis M157 exhibits antiinflammatory and antibiofilm activities against oral pathogenic bacteria. Journal of Dairy Science. 105: 1900-1912 (2022)
Stadnik J, Stasiak DM. Effect of acid whey on physicochemical characteristics of dry-cured organic pork loins without nitrite. International Journal of Food Science and Technology. 51: 970-977 (2016)
Stiemsma LT, Nakamura RE, Nguyen JG, Michels KB. Does consumption of fermented foods modify the human gut microbiota? The Journal of Nutrition. 150: 1680-1692 (2020)
Stoica M, Antohi VM, Alexe P, Ivan AS, Stanciu S, Stoica D, Zlati ML, Stuparu-Cretu M. New strategies for the total/partial replacement of conventional sodium nitrite in meat products: A review. Food and Bioprocess Technology. 15: 514-538 (2022)
Szabo-Fodor J, Bonai A, Bota B, Egyed LS, Lakatos F, Papai G, Zsolnai A, Glavits R, Horvatovich K, Kovacs M. Physiological effects of whey- and milk-based probiotic yogurt in rats. Polish Journal of Microbiology. 66: 483-490 (2017)
Tsanasidou C, Kosma I, Badeka A, Kontominas M. Quality parameters of wheat bread with the addition of untreated cheese whey. Molecules. 26: 7518 (2021)
Turkmen N, Akal C, Ozer B. Probiotic dairy-based beverages: A review. Journal of Functional Foods. 53: 62-75 (2019)
Wojciak KM, Dolatowski ZJ, Kolozyn-Krajewska D. Use of acid whey and probiotic strains to improve microbiological quality and sensory acceptance of organic fermented sausage. Journal of Food Processing and Preservation. 39: 539-547 (2015a)
Wojciak KM, Krajmas P, Solska E, Dolatowski ZJ. Application of acid whey and set milk to marinate beef with reference to quality parameters and product safety. Acta Scientiarum Polonorum Technologia Alimentaria. 14: 293-302 (2015b)
Wróblewska B, Kaliszewska-Suchodoła A, Markiewicz LH, Szyc A, Wasilewska E. Whey prefermented with beneficial microbes modulates immune response and lowers responsiveness to milk allergens in mouse model. Journal of Functional Foods. 54: 41-52 (2019)
Wronkowska M, Soral-Smietana M, Zdunczyk Z, Juskiewicz J, Jadacka M, Majkowska A, Dajnowiec FJ. Effect of acid whey-fortified breads on caecal fermentation processes and blood lipid profile in rats. British Journal of Nutrition. 118: 169-178 (2017)
Xiang H, Sun-Waterhouse D, Waterhouse GIN, Cui C, Ruan Z. Fermentation-enabled wellness foods: A fresh perspective. Food Science and Human Wellness. 8: 203-243 (2019)
Yao Y, Cai X, Fei W, Ye Y, Zhao M, Zheng C. The role of short-chain fatty acids in immunity, inflammation and metabolism. Critical Reviews in Food Science and Nutrition. 62 (1): 1-12 (2022)
Yoda K, Sun X, Kawase M, Kubota A, Miyazawa K, Harata G, Hosoda M, Hiramatsu M, He F, Zemel MB. A combination of probiotics and whey proteins enhances anti-obesity effects of calcium and dairy products during nutritional energy restriction in aP2-agouti transgenic mice. British Journal of Nutrition. 113: 1689-1696 (2015)
Zandona E, Blazic M, Jambrak AR. Whey utilisation: Sustainable uses and environmental approach. Food Technology and Biotechnology. 59: 147-161 (2021)
Zhang JW, Tong X, Wan Z, Wang Y, Qin LQ, Szeto IMY. Effect of whey protein on blood lipid profiles: a meta-analysis of randomized controlled trials. European Journal of Clinical Nutrition. 70: 879-885 (2016)
Zhao C, Ashaolu TJ. Bioactivity and safety of whey peptides. LWT-Food Science and Technology. 134: 109935 (2020)
Zhao Z, Pan D, Wu Z, Sun Y, Guo Y, Zeng X. Antialcoholic liver activity of whey fermented by Lactobacillus casei isolated from koumiss. Journal of Dairy Science. 97: 4062-4071 (2014)
Zou J, Chang X. Past, present, and future perspectives on whey as a promising feedstock for bioethanol production by yeast. Journal of Fungi. 8: 395 (2022)
Download references
Acknowledgements
This work was supported by the Gansu Science and Technology Program (No. 22JR5RA465 and 23JRRG0020), the Fundamental Research Funds for the Central Universities (lzujbky-2023-33), as well as the National Undergraduate Innovation & Entrepreneurship Training Program of Lanzhou University (202310730217).
Author information
Authors and affiliations.
Institute of Nutrition and Food Hygiene, School of Public Health, Lanzhou University, Lanzhou, 730000, China
Xiaorong Zeng, Yujie Wang, Shuda Yang, Yijun Liu & Diru Liu
Zhangye Water Saving Agricultural Experimental Station, Gansu Academy of Agricultural Sciences, Zhangye, 734000, China
You can also search for this author in PubMed Google Scholar
Contributions
XZ: Methodology, Writing-original draft preparation, Visualization. YW, SY, and YL: Visualization. XL: Conceptualization, Methodology. DL: Conceptualization, Methodology, Writing-review and editing, Supervision.
Corresponding authors
Correspondence to Xing Li or Diru Liu .
Ethics declarations
Conflict of interest.
The authors declare no conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Zeng, X., Wang, Y., Yang, S. et al. The functionalities and applications of whey/whey protein in fermented foods: a review. Food Sci Biotechnol 33 , 769–790 (2024). https://doi.org/10.1007/s10068-023-01460-5
Download citation
Received : 15 May 2023
Revised : 01 October 2023
Accepted : 10 October 2023
Published : 25 November 2023
Issue Date : March 2024
DOI : https://doi.org/10.1007/s10068-023-01460-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Whey protein
- Fermented food
- Physiological function
- Applications
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
Effects of Protein Supplementation on Performance and Recovery in Resistance and Endurance Training
Harry p. cintineo.
1 Center for Health and Human Performance, Rutgers University, New Brunswick, NJ, United States
Michelle A. Arent
Jose antonio.
2 Department of Health and Human Performance, Nova Southeastern University, Davie, FL, United States
Shawn M. Arent
3 Department of Kinesiology and Health, Rutgers University, New Brunswick, NJ, United States
There is robust evidence which shows that consuming protein pre- and/or post-workout induces a significant rise in muscle protein synthesis. It should be noted, however, that total daily caloric and protein intake over the long term play the most crucial dietary roles in facilitating adaptations to exercise. However, once these factors are accounted for, it appears that peri-exercise protein intake, particularly in the post-training period, plays a potentially useful role in terms of optimizing physical performance and positively influencing the subsequent recovery processes for both resistance training and endurance exercise. Factors that affect the utility of pre- or post-workout feeding include but are not necessarily limited to: training status (e.g., novice vs. advanced, or recreational vs. competitive athlete), duration of exercise, the number of training sessions per day, the number of competitive events per day, etc. From a purely pragmatic standpoint, consuming protein post-workout represents an opportunity to feed; this in turn contributes to one's total daily energy and protein intake. Furthermore, despite recent suggestions that one does not “need” to consume protein during the immediate (1 h or less) post-training time frame, it should be emphasized that consuming nothing offers no advantage and perhaps even a disadvantage. Thus, based on performance and recovery effects, it appears that the prudent approach would be to have athletes consume protein post-training and post-competition.
Introduction
Dietary protein plays a critical role in countless physiological processes in the body. The current Recommended Dietary Allowance (RDA) for healthy individuals is 0.8 g/kg/day ( 1 ). It is increasingly evident, however, that protein intake of at least 1.4–1.6 g/kg/day ( 2 ) would be more appropriate for active individuals attempting to optimize training adaptations. In an effort to meet this threshold, protein supplements are often consumed. In 2015, protein powder sales were valued at 4.7 billion U.S. dollars and were second only to sport drinks in the sports nutrition market ( 3 ). The popularity of protein supplements is likely influenced by the claims of increased muscle mass, increased fat loss, improved performance, and improved markers of recovery.
To date several meta-analyses, reviews, and systematic reviews have attempted to quantify and clarify these claims, but with mixed results ( 2 , 4 – 7 ). However, these efforts are complicated by the fact that the populations studied included trained and untrained, healthy normal weight, overweight or obese individuals, as well as injured, movement impaired, and those with metabolic or other diseases states. Additionally, the emphasis of recent reviews has been largely on impacts on muscle protein synthesis (MPS), hypertrophy, and body composition, with most of the outcomes pertaining solely to resistance training ( 2 , 4 – 7 ). Performance and recovery effects have been given secondary consideration at best, and these are areas that would be of particular interest to most athletes or athletic individuals.
Furthermore, performance and recovery outcomes, as well as physiological adaptations, are unique to the modality of training primarily employed. Anaerobic training refers to short bouts of high intensity movements which are often interspersed with longer recovery periods between efforts, with two of the most popular applications being resistance training or interval training ( 8 ). On the other hand, aerobic or endurance training refers to exercise bouts that primarily rely on oxidative phosphorylation and can last from minutes to hours ( 9 ). This latter type of training has received almost no consideration in recent protein reviews. Whether engaging in resistance or endurance training, protein supplementation may have the potential to enhance or complement exercise-induced physiological responses. The purpose of this review is to examine these potential performance and recovery applications of protein supplementation for both resistance and endurance training, with emphasis placed on studies utilizing various “peri-exercise” supplementation protocols within ~60 min pre- or post-training in healthy, exercising individuals.
Protein supplementation and resistance training
A recent comprehensive review by Jager et al. ( 2 ) identified a number of key issues related to protein intake in healthy, exercising individuals. Of particular note, the importance of protein intake during and around a training session for recovery and performance appears to be dependent on total daily protein intake, as well as presence or absence of an energy deficit. While findings do support the effect of post-exercise protein intake on increases in fat free mass (FFM), individuals consuming adequate daily calories and a minimum daily protein intake of 1.6 g/kg may not see any added benefit of immediate post-training protein consumption on muscular strength ( 2 ). However, Morton et al. ( 7 ) suggested that the strength (and hypertrophy) effects of additional post-resistance training protein supplementation may be greater in those with previous resistance training experience and that the magnitude of this effect is somewhat mitigated with aging. Furthermore, it is important to note that resistance-trained individuals in a caloric deficit require significantly more protein to offset any potential loss of lean body mass, with optimal daily protein intake for these individuals potentially being in the range of 2.3–3.1 g/kg FFM ( 10 ). While this recommendation increases total caloric intake from protein, resulting in the necessity to decrease energy intake from fat and carbohydrate, protein appears to have unique characteristics, and overfeeding with protein has been shown to have no negative effects on body composition in trained individuals ( 11 ). Similarly, healthy, older adults also require a greater quantity of total daily protein (0.61 g/kg FFM) compared to their younger counterparts (0.25 g/kg FFM) ( 12 ). Additionally, as a percentage of total daily energy intake, older adults must increase the contribution from protein due to decreases in energy intake, as well as protein's ability to attenuate sarcopenia by increasing muscle hypertrophy, subsequently maintaining or increasing muscular strength and power ( 13 ).
It has previously been demonstrated that ingestion of milk-based protein following a damaging eccentric resistance protocol helps to attenuate the expected decrements in strength and repeated sprint ability from 24 to 72 h following the bout ( 14 – 16 ). Recently, a group of researchers found that whey protein can facilitate muscle recovery following an intense isotonic exercise bout as well and that it is more than just an issue of caloric replacement ( 17 ). They compared the effects of a whey protein supplement (25 g protein, 2.5 g fat, and 3 g CHO) to a calorie-equated carbohydrate drink (32.5 g CHO) in resistance-trained young men performing an acute, total body resistance training protocol, and assessed performance variables at 10- and 24-h post-exercise. A moderate beneficial effect on acute anaerobic power and strength was found in the group that consumed the protein supplement, suggesting that there may have been improvements in rate of recovery over those who consumed the carbohydrate drink ( 17 ). This is particularly notable given that the subjects were already habitually consuming 1.9 g/kg/d of protein and may hold particular relevance for athletes engaging in high-intensity, explosive sports.
It has been suggested that protein quality may have an effect on both acute and chronic adaptations to exercise ( 2 , 18 , 19 ). Protein quality is a measure of a given protein source's ability to provide adequate quantities of the essential amino acids required for protein synthesis ( 20 ). Additionally, leucine, a branched-chain amino acid (BCAA), has been shown to be a prerequisite stimulator of skeletal MPS, which is critical for both the recovery and adaptive processes following a training bout ( 21 ). Given some of the favorable outcomes seen with ingestion of certain complete proteins, particularly milk-based and, more specifically, whey proteins ( 2 ), questions have been raised about the possible application of other protein sources that may be lower in leucine content. Two recent investigations have studied the effects of the quality of a post-exercise protein source on performance and recovery ( 22 , 23 ). Each of these studies took a unique approach to determining the differences in physiological changes following exercise and protein supplementation. Fabre et al. ( 22 ) compared the effects of 20 g of whey protein, 10 g of whey protein plus 10 g of casein protein, and 4 g of whey protein plus 16 g of casein protein consumed post-exercise in 31 recreationally resistance-trained males. Following 9 weeks of resistance training 4 days per week, no differences in changes in body composition, muscular strength, or muscular endurance were found, suggesting all three protein supplements were equally effective. When comparing 16 g of beef protein, 18 g of whey protein, and a calorie-equated carbohydrate drink consumed post-resistance training 3 days per week for 8 weeks in 42 recreationally resistance-trained males, no differences in changes in body composition, muscle thickness, or performance variables were found ( 23 ). One limitation of each of these studies is that they failed to control for total daily energy and macronutrient intake; therefore, subjects may have already been consuming adequate total daily calories and protein so the additional protein, regardless of its source, failed to result in any additional improvements in performance or body composition.
While most protein supplement resistance training studies have used a “post-exercise” administration protocol, it is possible that timing effects extend to the entire peri-workout period. Schoenfeld et al. ( 24 ) examined the effects of consuming 25 g of hydrolyzed whey protein immediately prior to a resistance training session with a 3-h fast post-exercise vs. consuming the same quantity and source of protein immediately following the same training session after having fasted for 3 h in 21 resistance-trained males. All subjects were consuming a 500-kcal surplus and 1.8 g/kg of protein daily. No differences in changes in body composition or one-rep max back squat or bench press were found between the groups after the 8-week intervention. Along with the findings from other studies ( 22 , 23 , 25 ), these data support the idea that protein intake post-workout may not be critical as long as protein is consumed prior to training or total daily protein intake is adequate. However, this does not preclude the possibility that pre- and post-exercise supplementation would be even more beneficial depending on dose.
To interpret the disparate effects of protein supplementation on resistance training performance, a few issues should be taken into account. The training stimulus must be adequate to result in strength improvement, regardless of protein timing, total protein intake, or nutritional status. Protein supplementation by individuals participating in ineffective resistance training programs will be less impactful. The beginning training status of individuals also appears to play a significant a role in any potential benefit seen as a result of protein consumption on strength, hypertrophy, and body composition ( 7 ). While the main focus of this paper is the healthy, trained individual, it is worth noting that protein supplementation for novice individuals may not confer any additional benefit above and beyond that of the training intervention ( 5 ). However, as training status increases, so does the potential effect of protein supplementation for improving performance and recovery.
Alternatively, Reidy and Rasmussen ( 6 ) have proposed the existence of a “protein paradox” wherein well-trained individuals may require less dietary protein due to the increased efficiency of protein turnover in this population. However, it should be noted that this is speculative and has not been fully substantiated by the available research, particularly for performance-related outcomes. Even taking this into account, one factor that appears to be just as important as total daily protein intake in well-trained individuals is the utilization of a specific protein dosing strategy based on body weight or FFM. Additionally, Thomson et al. ( 12 ) showed that healthy, older adults may also benefit from a higher protein intake in addition to a protein dosing strategy to adequately stimulate MPS. Thus, the appropriate timing or pacing of protein intake throughout the day may optimize results from resistance training ( 26 ). While recent critical or meta-analytic reviews have argued that protein timing is inconsequential after accounting for total protein intake ( 6 , 27 ), there are two factors that must be taken into account when considering these conclusions. First, very few “timing” studies have actually been conducted. In most cases, the studies were not designed to compare time of administration, but rather type or quantity of nutrient (or placebo) ingested post-exercise. Second, only a few of the included studies used trained subjects. Most employed novice exercisers. One of the studies that has found a benefit of protein timing ( 28 ) was conducted in experienced resistance-trained males. Again, this may lend credence to the notion that training status matters when considering protein supplementation strategies. Additionally, it should be noted that strength improvements not reaching statistical significance may prove to be significant in areas of individual competition or performance. Very few studies have actually utilized highly trained individuals or athletes, so translating the current findings to this population should be done with caution. Finally, it is worth noting that several studies have shown the addition of carbohydrate and creatine monohydrate to a protein supplement, typically whey protein, results in greater strength and hypertrophy improvements from resistance training programs ( 26 ). Though a detailed discussion of these other macronutrients is beyond the scope of this review, these results do point to an overall “nutrient” impact as well as possible synergistic effects.
Perhaps a driving factor in performance (i.e., strength or power) improvements with peri-workout protein supplementation could be enhanced recovery, which would potentially translate to enhanced capacity for an increased training load stimulus. Recovery from exercise has been measured through many different methods in previous research. Delayed onset muscle soreness (DOMS), which is defined as an aching pain in a given muscle following a novel exercise bout, has been measured subjectively ( 29 ). Though the cause of DOMS is multifaceted and tied to a cascade of events linked to muscle damage, it is not necessarily an indicator of the magnitude of muscle damage and, therefore, cannot be used by itself to determine muscular recovery and adaptations from exercise ( 29 ). Specific biomarkers and MPS rates appear to be the most efficient and widely used methods of objectively determining muscle breakdown, recovery, and adaptation from exercise. Acute elevations of cortisol and creatine kinase (CK) are two biological indicators of muscle damage and the subsequent recovery processes that can be measured through blood sample analysis ( 30 , 31 ). Post-exercise muscle biopsies can be used to determine rates of MPS, which directly measure the magnitude of the recovery process immediately following exercise ( 32 ).
West et al. ( 17 ) measured recovery variables following a total-body resistance training session and found that those subjects who consumed a whey protein supplement (25 g protein, 2.5 g fat, and 3 g CHO) had lower rates of whole body protein breakdown, while those who consumed a carbohydrate supplement (32.5 g CHO) actually had higher rates of whole body protein synthesis. The protein group, however, appeared to improve whole body net protein balance over 24 h post-exercise. As noted previously, the subjects were already consuming 1.9 g/kg/d protein, so additional protein through supplementation may have been less impactful. Interestingly, there was no difference between total body net protein balance between the groups. It should be noted that whole body protein synthesis is not necessarily a reflection of skeletal muscle protein synthesis ( 33 ). Kim et al. ( 33 ) discovered that net protein (whole body) balance was superior with a 70 vs. 40 g dose consumed prior to a resistance-training protocol. However, no differences were found in muscle protein synthesis between the 40 and 70 g dose. Thus, one must not conflate measures of whole body protein metabolism with those of skeletal muscle.
Nevertheless, the recovery of muscle function has been demonstrated in other studies ( 15 , 16 ) of milk protein supplementation after eccentric exercise, perhaps due to myofibrillar protein remodeling. The results of these studies further support the idea that protein consumed post-exercise is crucial for maximizing rates of protein synthesis in skeletal muscle. The effect on total body protein balance, however, is still a bit unclear. Carbohydrates have been shown to have a protein sparing effect, therefore the combination of protein and carbohydrate to decrease rates of muscle protein breakdown (MPB) and increase rates of MPS may be the best strategy for shifting total body protein balance to the net anabolic side ( 34 ), even if carbohydrate itself does not necessarily enhance MPS ( 35 , 36 ). This may partially explain the benefits of the milk supplement used by Cockburn et al. ( 15 ) and Cockburn and Stevenson ( 16 ) as it also contained carbohydrate. Perhaps there is a synergistic effect.
In addition to the investigations discussed earlier regarding post-exercise protein quality and training adaptations, Burd et al. ( 25 ) also measured markers of recovery through protein synthesis. The researchers collected muscle biopsies and measured rates of MPS following resistance training. In the 0–2 h post-exercise window, the group that consumed 30 g of protein in the form of skim milk expressed higher rates of MPS than the group that consumed 30 g of protein from beef ( 25 ). However, rates of MPS in the 2–5 h post-exercise window did not differ. This may be explained by the rate of digestion and absorption of these protein sources. Protein from dairy, specifically the whey portion, appears to be absorbed faster, and elicit a faster MPS response than beef.
The difference between whole egg and protein-equated egg white consumption post-exercise was also studied recently ( 37 ). The researchers measured rates of MPS through muscle biopsies and found that the group that consumed the whole egg exhibited higher rates of MPS. One limitation to this study was the lack of control for total calories and macronutrients. The whole egg treatment consisted of 18 g of protein, 17 g of fat, and 223 kcal, while the egg white treatment consistent of 18 g of protein, 0 g of fat, and only 73 kcal ( 37 ). While the discrepancy in calories between treatment groups may have impacted total daily calories, thus impacting MPS, one cannot ignore the possibility of the role that differences in macronutrients may play.
Lastly, a 2017 investigation looked at the differences between protein-equated native whey protein, whey protein concentrate, and milk ( 38 ). Native whey protein is produced through the filtration of raw milk, while whey protein concentrate is a byproduct of cheese production. Native whey protein consists of undenatured proteins and has a higher leucine content ( 38 ). Each treatment consisted of ~20 g of protein, ~6 g of fat, and ~40 g of carbohydrates but contained 2.7, 2.2, and 2.0 g of leucine, respectively. The supplements were ingested immediately after and again 2 h post-exercise following a moderate intensity lower body resistance training session. Results showed higher blood amino acids concentrations in native whey and whey protein concentrate than in milk. MPS was elevated in the whey protein condition from 1 to 3 h post, while it was elevated 1–5 h post in the native whey condition. There was no difference in MPS 1–5-h post-workout between native whey and whey protein concentrate, though MPS was higher from 1 to 5 h post-workout in the native whey condition compared to milk ( 38 ). Collectively, these data support that whey protein, regardless of its levels of processing (i.e., native whey protein vs. whey protein concentrate), increase MPS by similar magnitudes that are greater than those of milk alone. How this translates to long-term differences remains to be determined.
Protein supplementation and endurance training
While the majority of the literature regarding the effects of protein intake on performance has focused on anaerobic activities, more recent work has examined its role on endurance activities, but this has mostly been absent from the most recent reviews. Similar to resistance training, the impact appears to be at least somewhat dependent on the presence or absence of other nutrients, particularly carbohydrate. A 2010 systematic review and meta-analysis compared 11 studies investigating the effects of consumption of protein and carbohydrate vs. consumption of carbohydrate alone during a bout of cycling on performance during a subsequent bout of cycling ( 39 ). Across the 11 studies, consumption of protein and carbohydrate resulted in an average improvement of 9% in performance (defined as time to exhaustion and time trial performance) compared to consumption of carbohydrate alone ( 39 ). To investigate if the increased caloric intake due to inclusion of protein was responsible for this improved performance, a further analysis of isocarbohydrate and isocaloric conditions was performed. Examination of isocarbohydrate conditions yielded a 10.5% improvement in overall performance, while isocaloric conditions resulted in 3.4% improvement ( 39 ), suggesting that the improvements due to protein inclusion were not simply due to increased calories. When considering only those studies measuring performance by time trial (3), improvements were not statistically significant. However, studies utilizing time to exhaustion protocols (8) did result in statistically significant improvements. It is worth noting that in all studies showing statistically significant improvement, whey protein was the source of protein utilized, though differences between concentrate vs. isolate were not quantified. Again, it is prudent to highlight that performance improvements not reaching statistical significance may have clinical or practical relevance, specifically for athletes. For example, a 1% improvement in performance would have been the difference in winning the Gold Medal instead of the Silver Medal in the men's marathon in the 2016 Olympic Games in Rio. Therefore, even seemingly “trivial” differences do indeed have a significant effect on performance and outcomes at the elite level.
When discussing the impact of protein on performance, it is imperative to include the impact that protein may have on glycogen replenishment and subsequent exercise performance. Standard discussions of glycogen replenishment focus solely on carbohydrate consumption. Recommendations for adequate post-exercise carbohydrate consumption are to consume 0.6–1.0 g/kg carbohydrate within 30 min of cessation of exercise and again every 2 h for the next 4–6 h ( 40 , 41 ). Carbohydrate consumption of 1.2 g/kg every 30 min over 3.5 h also resulted in maximal glycogen replenishment ( 41 , 42 ). In cases of suboptimal post-exercise carbohydrate consumption, the addition of protein can improve glycogen replenishment and decrease symptoms of muscle damage ( 43 ). Practical applications of standard post-exercise carbohydrate consumption recommendations may be limited in real world situations. Moreover, athletes training multiple times daily may have fewer opportunities to consume recovery meals or have an elevated need for “rapid” recovery, including rehydration, to facilitate the subsequent training session. Beyond just glycogen replenishment aspects, it has also been shown that the presence of protein in rehydration beverages can enhance intestinal fluid uptake, aiding in rehydration ( 44 ) and that BCAA consumption during endurance exercise may improve time trial performance and peak power output while improving markers of immune health ( 45 ) and attenuate serotonin levels, subsequently resulting in a delay of central fatigue ( 46 ).
A systematic review by Pasiakos et al. ( 5 ) investigated the relationship between protein, muscle function, and recovery. The authors included studies that measured markers of muscle damage followed by a test of physical performance or muscle function. Populations of the review included healthy individuals with daily dietary protein intake at or above the current RDA of 0.8 g/kg per day. While some of the endurance exercise studies included showed decreases in markers of muscle damage, such as CK, or decreases in muscle soreness in groups consuming protein after initial exercise bout ( 47 – 49 ), many did not ( 50 – 52 ). This may have resulted from the inclusion of studies utilizing both trained and untrained subjects, as well as individuals consuming suboptimal daily protein intakes. Despite the reduced plasma CK levels and muscle soreness, consumption of protein did not result in improvements in subsequent performance measures when repeat performance was tested < 24 h following the initial bout. This evidence suggests that plasma CK levels, perceived level of muscle soreness, and muscle function may only be modestly related or perhaps utilizing a single method of measure paints an inadequate picture of recovery due to individual variability ( 5 ). Without additional studies to clarify these relationships, developing guidelines based on these markers as representing recovery may be ill-advised. Individuals must be cautious when attempting to measure recovery from exercise based on these metrics alone. For example, a recent study of 20 high-level soccer players tested the effects of a milk protein concentrate supplement (80% casein and 20% whey) compared to an isocaloric carbohydrate-containing placebo on high intensity running performance, knee extensor and flexor strength, and antioxidative capacity over the course of a 1-week in-season microcycle consisting of two games separated by 2 days ( 53 ). On game days (days 1 and 4), the supplement was consumed immediately post-, 3 h post-, and 6 h post-match in three different doses of 25, 30, and 25 g, respectively, resulting in a total of 80 g. On training days (days 2, 3, 5, and 6), 20 g of the supplement was consumed with breakfast. High intensity running performance, measured as distance covered at speeds >19 km/h, was greater during the last 15 min of game two following protein supplementation. Additionally, knee extensor concentric strength recovered quicker after the first game following protein supplementation. Endogenous antioxidant concentrations were greater following game two only in the protein-supplemented condition. Though soccer is a “power-endurance” sport rather than simply an endurance sport, these findings hold relevance for understanding the impact of protein intake on recovery and repeated performance in actual athletes.
Since 2014, additional work investigating the impact of protein consumption on biochemical markers of metabolic status, physiological fatigue, and recovery in endurance-trained athletes has been performed ( 54 ). For 5 weeks, elite or experienced marathon runners received either 33.5 g/day of whey protein or maltodextrin 30 min following the completion of each training session leading up to a race covering marathon distance. Blood samples were collected to assess biochemical markers of metabolism, muscle damage, and fatigue and took place prior to beginning the intervention, 1 day following the marathon, and 1 week following the marathon. These markers included CK, lactate dehydrogenase (LDH), AST, and ALT. Runners who supplemented with whey protein displayed decreased AST and ALT compared to maltodextrin-supplemented runners. CK and LDH, biochemical indicators of muscle damage, were significantly greater in the maltodextrin group post-marathon compared to the whey protein-supplemented group. Elevations in CK and LDH were still significant 1-week post-marathon in the maltodextrin group compared to the whey protein group ( 54 ). The whey protein group also showed significantly decreased triglycerides (TG) and total cholesterol (TC) compared to the maltodextrin group post-marathon. The maltodextrin group actually showed increased TC levels. Only the whey protein group showed significant decreases in LDL post-marathon and at 1 week post-marathon ( 54 ). The authors suggested that the decrease in TC seen in whey-supplemented runners may indicate that cholesterol was more efficiently converted to steroid hormones, resulting in improved physiological recovery and adaptations from the strenuous exercise bout. One week post-marathon, most biomarkers of damage and stress were still significantly lower in the whey protein group compared to the maltodextrin group ( 54 ). In addition to the more favorable biomarker profiles in the protein supplemented group, performance in the 12-min run/walk test was also greater in the whey protein-supplemented group 1-week post-marathon. Together, these results indicate that whey protein supplementation during marathon preparation and recovery, and that the supplement aids in attenuating metabolic and muscular damage. Daily dietary assessments were not included in this study ( 54 ), thus limiting possible practical applications or recommendations. As we have addressed previously, caloric deficit or daily protein consumption <1.4–1.6 g/kg may potentiate the effect of peri-workout protein consumption on recovery and subsequent performance. Further studies are necessary to elucidate the potential contribution of peri-workout whey protein ingestion on makers of muscle damage, recovery, and subsequent performance measures in endurance athletes.
In real-world sport performance situations, recovery and performance must be evaluated in the context of an accumulated effect. The ability to train consistently while remaining healthy is critical for continued progression and optimal performance. Endurance athletes in particular are at increased risk for upper respiratory tract infections ( 55 ). Factors contributing to this increased risk may include reduced immune function through low circulation of certain T-lymphocytes, especially during periods of increased volume and/or intensity of training. A diet providing a daily protein intake of 3 g/kg, including 60 g/day of casein protein, has been shown to be sufficient in returning circulating immune cell levels to those seen during lighter training periods, while a diet providing a daily protein intake 1.5 g/kg did not result in enhanced immune cell levels ( 56 , 57 ). Kephart et al. ( 45 ) have also found this beneficial effect on the immune system to extend to BCAA supplementation in doses of 12 g/d in trained cyclists.
Additionally, Rowlands et al. ( 58 ) found that consumption of ~64 g protein over 3 h following intense endurance exercise resulted in gene expression favorable for improving substrate, specifically fatty acid, mobilization and mitochondrial proteins for oxidation, especially in the electron transport chain. Post-exercise consumption of protein at levels thought to maximally stimulate MPS would potentially not have this same impact. Post-exercise protein consumption affects other systems and pathways and should not be considered only in terms of stimulating MPS. As further evidence of this notion, Levenhagen et al. ( 59 ) demonstrated that 10 g of casein protein enhanced MPS following 60 min of moderate intensity endurance exercise. Although this supplementation protocol stimulated MPS, subjects were found to be in negative whole-body protein balance. Because prolonged bouts of endurance exercise (i.e., >2 h) result in considerable oxidation of amino acids, specifically leucine, and intense or prolonged bouts of endurance exercise result in hypoxia-mediated small intestinal injury, negative whole-body protein balance may be common in endurance athletes ( 60 – 62 ). Because of this, protein requirements and recommendations for endurance athletes must consider more than MPS, especially since short-term increases in MPS do not fully explain the dynamics of long-term whole-body net protein balance and various training adaptations.
Conclusions and future direction
Overall, total daily energy and protein intake over the long term play the most crucial dietary roles in facilitating adaptations to exercise. However, once these factors are accounted for, it appears that peri-exercise protein intake plays a potentially useful role in optimizing physical performance and positively influencing the subsequent recovery processes. Challenges surround the definition of “performance” and the appropriate metrics by which to measure it based on desired outcomes. Difficulties also arise in attempting to define and quantify the concept of recovery. Additionally, both performance and recovery must be viewed in context depending on whether the emphasis is an immediate, short-term effect (i.e., 24 h or less) or a long-term training response.
It should also be noted that protein timing, whether it is pre-, during, or post-workout, is often framed within the context of bodybuilding (i.e., the singular goal of increasing skeletal muscle mass). It is evident that to use such a narrow frame of reference ignores the potential utility of protein timing within the context of endurance events (i.e., running, cycling, rowing, swimming, triathlon, etc.), as well as the vast majority of individual and team sports in which skeletal muscle hypertrophy is not a pre-eminent concern. For instance, if one competes in a weight-class sport (e.g., boxing, mixed martial arts, weightlifting, powerlifting, etc.), gains in body weight or lean body mass are often avoided; otherwise, the individual athlete would need to compete in a heavier weight class. In these situations, protein timing in particular may serve a useful role in recovery.
Translating research into practical application requires differentiation between novice or trained individuals, healthy normal weight or healthy overweight individuals, special populations, or those with certain metabolic or disease states. Here, we specifically focus on healthy, exercising individuals and limit our conclusions to these individuals. It is important moving forward that the study populations used are appropriate for the goals of the study and desired applications. For example, it is of little use to have a sample of recreationally-trained individuals if the goal is to understand performance in high-level athletes.
Though protein-containing meals result in increase of MPS on their own, as does resistance training, the timing of ingestion of protein around exercise further enhances this increase of MPS ( 63 , 64 ). It is worth noting that an upper limit for this acute dosing has not really been established, though there is evidence that 40 g of protein stimulates MPS to a greater degree than 20 g following whole-body resistance training ( 65 ). A dose higher than this, however, has not been included using the same timing paradigm. In reality, the “ideal” amount of peri-exercise protein consumption depends on many factors, including total caloric intake, total daily protein intake, training status of the individual, age of the individual, FFM, type of protein consumed, type and amount of other nutrients consumed, and the composition and timing of the most recent pre-training meal.
Much attention has been given to daily protein consumption and thresholds that must be met for peri-training protein consumption to exert additional benefit (>1.6–2.2 g/kg/d). As such, pre-, intra-, and post-training nutrient consumption present additional opportunities for athletes to contribute to their daily protein intake total and can be viewed in the context of ways to meet these “larger” daily needs by optimizing intake.
With regard to endurance exercise, protein consumption during exercise may not confer an immediate ergogenic benefit, especially when carbohydrate consumption is adequate. It may, however, aid in delaying central fatigue, reducing MPB, and contributing to a more positive, whole-body nitrogen balance. Additionally, protein consumption in and around intense or prolonged endurance activity may aid in reduction of upper respiratory tract infection incidence and improved immune system function. It may also aid in upregulating gene expression of proteins necessary for improving bioenergetic pathways. The impact of this on subsequent training sessions should not be dismissed and is an important part of improving performance.
The effect of protein consumption on resistance training is highly dependent on many variables not related to protein. The combination of peri-training protein consumption with inadequate or ineffective resistance training protocols will not maximize improvements in strength or hypertrophy. Resistance training protocol interventions must be of adequate intensity, volume, and frequency with an emphasis on progressive overload to produce results. Additionally, adequate training interventions coupled with calorie-restricted nutrition protocols may require increased protein intake of 2.3–3.1 g/kg FFM to yield desired improvements in strength, hypertrophy, or maintenance of FFM ( 10 ). Consideration must also be made for the age of resistance-trained individuals, as older adults require protein intake over and above that of their younger counterparts to receive the same benefits noted above ( 66 ).
In order to fully understand the role of protein (or any substrate for that matter) on performance, the practical application beyond the contrived training or recovery interventions presented must be addressed. Daily training schedules of athletes require an ongoing ability to recover and perform. As an example, most of the studies included in this area utilized a training protocol that took ~3–4 h per week, typically in moderately-trained individuals. For comparative purposes, a competitive athlete may spend 3–10 times this amount of time training per week (if not more). For this reason, the “window” for recovery should be considered to encompass each and every hour between training and competition. Protein dosing strategies need to take this into account. This becomes even more apparent when considering that the uniform distribution of protein throughout the day results in greater MPS than an uneven distribution even when total daily protein intake is equal ( 67 ). Arciero et al. ( 64 ) demonstrated the combination of resistance training and consumption of 4–6 meals per day containing 20–40 g of protein per meal resulted in positive changes in body composition and physical performance. These results suggest that the pattern of daily protein ingestion may also impact results from resistance training protocols and provides further evidence that we must look beyond the few hours following training to determine the impact that protein may have on performance and recovery. Further evidence in support of extending the “recovery window” concept are results from nighttime protein ingestion studies. Madzima et al. ( 68 ) found that consumption of 30 g of casein, 30 g of whey, or 33 g of carbohydrate 30 min prior to sleep resulted in increased resting energy expenditure and improved VO 2 the following morning. While no statistically significant changes were observed between groups, protein groups trended toward greater increases when compared to the carbohydrate group while morning fat oxidation was greatest in the casein supplemented group.
Taken together, these data demonstrate the need for a more comprehensive view and methods of measuring recovery. Increased sensitization of muscle to protein and nutrients for 24–72 h following training coupled with multiple weekly training sessions results in an on-going state of recovery. Because of this, we need to begin considering this longer stimulus window as an opportunity to maximize feeding, rather than as a reason why immediate post-workout ingestion may not be particularly important. In other words, consuming nothing post-workout would be an unwise strategy if the goal is to potentially optimize the adaptive response to exercise training.
Overall, there appears to be no adaptive advantage to avoiding protein intake in the peri-workout period. Stimulation of MPS in the acute period following training may not result in improvements in strength, hypertrophy, body composition, or performance without deliberate implementation of additional strategies during the prolonged recovery period. As such, this much broader view should be considered with regard to future investigations.
Author contributions
JA and SA conceived the topic. HC, MA, JA, and SA wrote the paper.
Conflict of interest statement
SA is on the Advisory Panel for Dymatize. JA is the CEO of the International Society of Sports Nutrition—an academic non-profit that receives grants in part from companies that sell dietary protein.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer CK declared a past co-authorship with several of the authors SA and JA to the handling Editor.
- Ground Reports
- 50-Word Edit
- National Interest
- Campus Voice
- Security Code
- Off The Cuff
- Democracy Wall
- Around Town
- PastForward
- In Pictures
- Last Laughs
- ThePrint Essential


New Delhi: A first-of-its-kind observational analysis of the most popular protein powders sold and consumed in India has shown that the majority of these supplements falter on quality, labelling or advertised claims.
The findings of the analysis carried out on 36 different brands of protein powders, including those containing herbal and dietary supplements such as vitamins, minerals, and other natural or synthetic ingredients, were published in the peer-reviewed journal Medicine last week.
Protein supplements are extracts or concentrates of high protein foodstuff used for bodybuilding and as a dietary supplement to fulfil protein intake in a lean and pure source of proteins and amino acids (the building blocks of proteins).
The analysis showed that nearly 70 percent of the 36 supplements had inaccurate protein information, with some brands offering only half of what they claimed. Also, around 14 percent of samples contained harmful fungal aflatoxins, while 8 percent showed traces of pesticide residue.
Also, noted the authors — clinical researchers associated with Rajagiri Hospital in Kerala and a technology entrepreneur from the US — “most Indian-made herbal protein-based supplements are poor quality and contain liver toxic botanicals”.
“We demonstrate that the protein-based herbal and dietary supplement industry requires stringent scrutiny, regulation, and basic safety studies before being marketed,” the authors said.
Dr Cyriac Abby Philips, hepatologist from Rajagiri Hospital in Aluva in Kerala, the principal investigator of the self-funded study, told ThePrint that though there is published data from various research groups and clinical units across the world on organ damage, especially liver injury due to herbal and dietary supplements, there has been no proactive and prospective analysis of widely utilised supplements — especially protein-based — in published literature.
“There are occasional published reports that look at the quality of whey protein and amino acids analysis in protein supplements to identify amino acid spiking or ‘doping’ to falsely elevate protein content,” he said.
Philips added that one study also looked at how marketed protein supplements adhered to regulations with respect to quality — but much of this was done on protein supplements sold in the US and there were no such studies done from the Asia Pacific region.
“Our work sheds light on regulatory flaccidity, importance of consumers rights in being privy to transparency regarding choosing safe food or supplement options and general apathy of the medical community towards educating the public regarding food and diet supplements that are potentially beneficial versus potentially harmful,” he said.
ThePrint reached G. Kamala Vardhana Rao, chief executive at the Food Safety and Standards Authority of India (FSSAI), over calls, for comments on the findings in the paper but had received no response by the time of publication. This report will be updated if and when a response is received.
In response to a question in the Lok Sabha in August last year, Union Health Minister Mansukh Mandaviya had informed the Lower House that in 2022-23, as many as 38,053 civil cases and 4,817 criminal cases were lodged by the FSSAI for non-conforming food samples including protein powders and dietary supplements.
Also Read: Govt admits norms on health supplements not adequate, forms high-level panel to suggest framework
The study and the findings
The 36 protein powders analysed were either blended, pure plant-based, and pure whey-based formulations (protein from whey, the watery portion of milk that separates from the curds when making cheese).
The blends included either different blends of proteins or those with herbal extracts.
Of the 14 blended formulations, seven contained herbal extracts, and the rest included various types of protein sources, such as pea, soy, egg, milk (whole, whey, or casein), and peanuts.
Four products were purely plant-based in nature, and 18 powders were purely whey-based and whey-blended (concentrate, hydrolysate, and isolate).
Twenty products were made in India, and the rest were manufactured by multinational companies.
Of the 36 products, nine had less than 40 percent detected protein content, while the rest had above 60 percent. Overall, 25 protein supplements (69.4 percent) were mislabeled about protein content; that is, the protein content per 100 g detected in analysis was less than what was advertised on the product, featuring less than 10 percent to more than 50 percent deficit.
Two products from one manufacturer had 62 percent and 50.4 percent lower protein content while a commonly prescribed protein from a well reputed company also mislabelled protein content of approximately 30 percent deficit than advertised.
Also, according to the authors, certain protein brands were found to contain more than the labelled protein content in the quantification analysis.
Higher protein content could suggest either good quality protein sources used in manufacturing or it could also be part of “protein or amino spiking” where supplement manufacturers intentionally add cheaper protein components such as cheaply available amino acids glycine and taurine to deceptively showcase higher protein content, noted the researchers.
On fungal toxin analysis, five of 36 (13.9 percent) samples were found to be contaminated with aflatoxins — toxins from certain fungi — and in some samples, the aflatoxin content was above 10 μg/kg. In pesticide residue analysis, three samples (8.3 percent) were found to be contaminated by trace amounts.
Based on these results, Philips said on social media that the protein powder by BigMuscles was the “worst brand”, the one by Amway was the “worst plant-based”, and Protinex, Ensure and B-Protin were the “worst brands advertised as the best”.
He also said that brands that need extreme caution include Elements and Nutrilite by Amway as they contain fungal toxins.
Hello, I have a fantastic Saturday morning read for you. In fact, you'll be engrossed in this the whole day. As promised, we have published our disruptive project – the unique public-health project funded by @paraschopra to analyze common/well-known protein supplements sold in… pic.twitter.com/xzb7Gh0G3Q — TheLiverDoc (@theliverdr) April 6, 2024
ThePrint reached the makers of these powders over email for their comments on the findings but had not received a response from Amway, Nestle, Abbott, Danone India and British Biologicals by the time of publication. This report will be updated if and when their response is received.
BigMuscles Nutrition said that the brand and its manufacturing unit are FSSAI-approved and regularly tested for quality assurance by the regulator. The company also said the vegan product tested as part of the project is not being manufactured anymore and added that these products have a shelf life.
“BigMuscles Nutrition is one of the leading nutraceutical brands of the country that has a state-of-the-art manufacturing unit in Faridabad that hardly many brands have. We would like to state that every batch is tested internally for quality assurance, and hence, we maintain a record of each batch that goes to the market,” the firm also said.
It added that to keep the consumers, the company publishes test reports of its products on its website from time to time though it is still a work in progress.
The company claimed to have objected to the study report legally as “these reports are forged and are made a part of the lawsuit the brand has filed for such false claims”.
BigMuscles Nutrition also objected to being dubbed “worst brand”, saying that its quality team would want to cross-check all the tests done based on details such as which samples were picked, how they were transported to the label, which lab carried out the tests, their scope and accreditation, and where was the product bought from.
Philips said the “best” whey brand in the Indian market was One Science Nutrition, and the protein supplement by Nutrabox was the “best medium range” whey. The protein powder by Origin, according to the analysis, was the “best vegan” protein.
Huge implications
The study highlights that similar to the United States Food and Drug Administration, the FSSAI does not approve herbal and dietary supplements but regulates good manufacturing practices.
The safety of contents in protein-based herbal and dietary supplements must be assured by the manufacturer, while the content and labelling are scrutinised by the FSSAI, based on test results submitted by the manufacturer that are not made public and remain non-transparent, according to the study’s authors.
“The implications of our study are important,” said Philips. “One, we need regulatory bodies to come clean and be transparent about food and dietary supplements because these are not tested for efficacy or safety as we do with drugs and medications.”
The only driving factor that makes such products “ready for the market” are good manufacturing practices, the hepatologist and medical researcher explained, adding that the manufacturers cannot be trusted because they are not forthcoming with respect to realistic quality and will do anything to make a profit and enhance promotion.
For example, he said that the new analysis has shown that labelled protein content and identified content were dissimilar in many brands and there were suggestions of “protein spiking or amino acid doping” in some of the brands tested.
“In the absence of regulatory oversight in such situations, it then becomes an ‘every person for themselves’ sort of situation when it comes to choosing a food supplement from a public perspective. This is unfair and dangerous,” he stressed.
Dr Sabine Kapasi, adviser, public health and healthcare services strategist with the United Nations Covid-19 task force who is not directly associated with the study, too, agreed that it underscored the urgent need for stricter regulations and quality control in manufacturing and labelling of protein supplements.
“It stresses the importance of transparent and accurate product information, enabling consumers to make informed decisions about their health,” she said.
Kapase added that the Supreme Court issuing a contempt notice to ayurvedic conglomerate Patanjali for continued dissemination of misleading advertisements further emphasised the necessity for stringent accountability in the health supplement sector.
“These observations reflect a growing worry about misleading information, lack of transparency, and potential health risks posed by such products. As consumers, it ’s vital to be aware of these issues and exercise caution when choosing protein supplements,” she said.
According to Kapase, while protein supplements can offer benefits when used correctly, the study ’s findings highlight the urgent need for increased scrutiny and regulation in the industry.
The report was updated to include the response of BigMuscle Nutrition
(Edited by Nida Fatima Siddiqui)
Also Read: Looking for biotin supplements to reduce hair fall? Multivitamin overdose can worsen it
Subscribe to our channels on YouTube , Telegram & WhatsApp
Support Our Journalism
India needs fair, non-hyphenated and questioning journalism, packed with on-ground reporting. ThePrint – with exceptional reporters, columnists and editors – is doing just that.
Sustaining this needs support from wonderful readers like you.
Whether you live in India or overseas, you can take a paid subscription by clicking here .
- health supplement
- Nutrition study
- protein supplements
It is quite surprising that the famous and reputed companies are indulged in such malpractices and thanks for bringing it to the limelight. At this point of time,it is also worth reporting indigenous and genuine products like Coonvita,a vitamin D prominent product which is selected as one of the top 25 products in India by a woman Agripreneur.Hope your reporting could be stretched to that extent .This is a product which has 4 Times of vitamin D than any of the products now available in the market.It is getting ready for launch and the sampling,patenting ,bioavailability etc being done.It is supported by Kerala State govt,Agri.department and the central govt too.More details are with us and we welcome your reporting about this product
I was shocked to see Amway Protein supplement included in the list of substandard powders. So I queried an Amway distributor and she said the Amway product tested is the one being sold on Amazon, which is fake. It’s not the real Amway protein powder only sold by Amway distributors. Please clarify
You should publish the name of the brand or company name so that public will not purchase such poor product
Pl share your findings…I am worried Thanks
LEAVE A REPLY Cancel
Save my name, email, and website in this browser for the next time I comment.
Most Popular
As modi makes 6th trip to kerala this yr, a look at constituencies where bjp aims to put up tough fight, bjp’s ‘ab ki baar 400 paar’ push isn’t a new idea. it’s a political pledge from 73 years ago, everyone says ‘aayega to modi hi’—some with a drumroll, many with despair.
Required fields are marked *
Copyright © 2024 Printline Media Pvt. Ltd. All rights reserved.
- Terms of Use
- Privacy Policy

IMAGES
VIDEO
COMMENTS
1. Background. Consuming a source of protein after resistance exercise is essential to maximize muscle protein synthesis and net protein balance [1,2], both of which are required to support muscle hypertrophy with training.Current research supports the consumption of a moderate dose (~20-25 g) of rapidly digested, leucine-rich proteins to optimize muscle protein synthesis [3,4,5]; this ...
PDF | On Jan 1, 2011, HemantH Gangurde and others published Whey protein | Find, read and cite all the research you need on ResearchGate
the type of processing. Whey, acidic in nature, will have a pH of approx. 5.1 and is generally produced through direct acidification whereas sweet whey has a pH of around 5.6 and is produced through rennet-coagulation, particularly during the cheese-making process [17]. Table 1. Whey protein constituents and its composition a. Whey Protein ...
Whey protein comprises 20 % of total milk protein and it is rich in branched and essential amino acids, functional peptides, antioxidants and immunoglobulins. It confers benefits against a wide range of metabolic diseases such as cardiovascular complications, hypertension, obesity, diabetes, cancer and phenylketonuria.
One of the popular and easy to purchase protein supplement in sports is whey protein supplements (WPS) as it has shown ergogenic aids which absorbed rapidly, includes all the essential amino acids, and has a high proportion of branched-chain amino acids (MacKenzie-Shalders et al., 2015; Frank et al., 2017).
Key selected reviews involving various aspects of whey protein conducted within the past two decades in terms of aims/objectives and key sections are shown in Table 1.Whereas Minj and Anand (2020) reviewed the bioactive properties, functional characteristics, associated processing limitations, and applications of different whey protein fractions and derivatives involved in the field of food ...
This narrative review critically examines the current research on the health implications of whey protein (WP) supplementation, with a focus on potential risks and adverse effects. WP, commonly consumed for muscle building and weight loss, has been associated with various health concerns. Our comprehensive analysis involved a thorough search of multiple databases, resulting in the inclusion of ...
Nutritional and Medical Perspectives of Whey Protein: A Historical. Overview. Usman Mir Khan, Zeliha Selamoglu 2*. 1 Department of Dairy Technology, Faculty of Animal Production and Technology ...
Whey protein (WP), which is produced from cheese and other dairy products, is a well-known supplemental food with high amounts of -lactoglobulin, -lactalbumin, immunoglobulins, lactoferrin, lactose, minerals, vitamins, and lipids, and is widely used by the food and pharmaceutical businesses [2]. In recent decades, research into the advantages ...
1. Introduction. Derived from the manufacture of cheese and other dairy products, whey protein (WP) is a supplementary food widely used by food and pharmaceutical industries due to its nutritional value with emphasis on the presence of high levels of β-lactoglobulin, α-lactalbumin, immunoglobulins, lactoferrin, lactose, minerals, vitamins, and lipids [1,2,3].
The currently available evidence supports the use of whey protein to optimize muscle mass gain in resistance training individuals. At rest, young individuals may require a minimum threshold amount of >010/kg of protein, providing ~0.060 g/kg of IAA and ~0.010 g/kg of leucine per serving to stimulate MPS.
Whey-derived bioactive components have. antimicrobial and antiviral properties, and enhance immune de fense and bone health, and improve antioxidative activity, and help. protect against cancer ...
Whey proteins are generally marketed in three forms, such as whey protein concentrate, whey protein isolate and whey protein hydrolysate (Sousa et al., 2012). The concentrate has fat and lactose along with the quintessential proteins (29-89%) ( Bounous, 2000 ); the isolate is made of 90% protein ( Hayes & Cribb, 2008 ) and the hydrolysate is ...
Novelty: A systematic review of experimental and randomized studies about the use of whey proteins supplements and its impact on physical health. Analysis revealed that chronic and without professional guidance use of whey protein supplementation may cause some adverse effects specially on kidney and liver function.
There are 3 types of whey protein: controlled, insulated and hydrolysed. Objectives The goal of this research is verifying the efficacy of the use of whey protein supplementation in athletes seeking muscle strength and mass gain. Methodology * We analysed scientific articles from 2014 to 2019, based on Pubmed and Google scholar. Keywords such ...
Whey is one of the high-quality sources of protein with a higher proportion of indispensable amino acids (IAA) compared to other sources. Its high leucine concentration makes whey an optimal ...
Background Consuming whey protein supplements, along with physiotherapy and psychotherapy, have been recognised in sports performance. Whey protein supplements (WPS) is one of the commonly used supplements as ergogenic aids for athletes to enhance their muscle performance and recovery during sport-related injuries. The purpose of this systematic review is to investigate the effectiveness of ...
One of the popular and easy to purchase protein supplement in sports is whey protein supplements (WPS) as it has shown ergogenic aids which absorbed rapidly, includes all the essential amino acids, and has a high proportion of branched-chain amino acids (MacKenzie-Shalders et al., 2015; Frank et al., 2017).
(1) The purpose of this study was to investigate the effect of whey protein supplementation under dietary control on improvements in muscle mass and function following resistance exercise training. (2) Thirty-two men were randomly assigned to a whey protein supplementation group taking whey protein isolate (PSG, n = 17) and a placebo group (CON, n = 15). Participants were provided with three ...
M oreover, c onsuming whey pr otein ha s a. direct effect on increasing th e antioxidant activit y of. body [37,38], the muscular physical ability [39-41], decreasing blood sugar after meal [27,4 ...
Whey, a major by-product of cheese production, is primarily composed of whey protein (WP). To mitigate environmental pollution, it is crucial to identify effective approaches for fully utilizing the functional components of whey or WP to produce high-value-added products. This review aims to illustrate the active substances with immunomodulatory, metabolic syndrome-regulating, antioxidant ...
Keywords: protein bars; whey protein concentrate; organic food; quality; storage 1. Introduction There is a current preference for health-related foods among consumers, including a low-calorie diet containing higher amounts of protein, fiber, and antioxidants, whilst also being easy to handle, store, and consume.
2.2. Protocols for blinded product analyses. All products were subjected to the analysis of total protein content (Kjeldahl method), [] detection and quantification of fungal aflatoxin (high-pressure liquid chromatography and fluorescence detection), [] pesticide residue detection and estimation (gas and liquid chromatography and tandem mass spectrometry), [] detection and quantification of ...
Native whey protein is produced through the filtration of raw milk, while whey protein concentrate is a byproduct of cheese production. Native whey protein consists of undenatured proteins and has a higher leucine content . Each treatment consisted of ~20 g of protein, ~6 g of fat, and ~40 g of carbohydrates but contained 2.7, 2.2, and 2.0 g of ...
New Delhi: A first-of-its-kind observational analysis of the most popular protein powders sold and consumed in India has shown that the majority of these supplements falter on quality, labelling or advertised claims. The findings of the analysis carried out on 36 different brands of protein powders, including those containing herbal and dietary supplements such as vitamins, minerals, and other ...