How to Write About Coronavirus in a College Essay
Students can share how they navigated life during the coronavirus pandemic in a full-length essay or an optional supplement.
Writing About COVID-19 in College Essays

Getty Images
Experts say students should be honest and not limit themselves to merely their experiences with the pandemic.
The global impact of COVID-19, the disease caused by the novel coronavirus, means colleges and prospective students alike are in for an admissions cycle like no other. Both face unprecedented challenges and questions as they grapple with their respective futures amid the ongoing fallout of the pandemic.
Colleges must examine applicants without the aid of standardized test scores for many – a factor that prompted many schools to go test-optional for now . Even grades, a significant component of a college application, may be hard to interpret with some high schools adopting pass-fail classes last spring due to the pandemic. Major college admissions factors are suddenly skewed.
"I can't help but think other (admissions) factors are going to matter more," says Ethan Sawyer, founder of the College Essay Guy, a website that offers free and paid essay-writing resources.
College essays and letters of recommendation , Sawyer says, are likely to carry more weight than ever in this admissions cycle. And many essays will likely focus on how the pandemic shaped students' lives throughout an often tumultuous 2020.
But before writing a college essay focused on the coronavirus, students should explore whether it's the best topic for them.

Writing About COVID-19 for a College Application
Much of daily life has been colored by the coronavirus. Virtual learning is the norm at many colleges and high schools, many extracurriculars have vanished and social lives have stalled for students complying with measures to stop the spread of COVID-19.
"For some young people, the pandemic took away what they envisioned as their senior year," says Robert Alexander, dean of admissions, financial aid and enrollment management at the University of Rochester in New York. "Maybe that's a spot on a varsity athletic team or the lead role in the fall play. And it's OK for them to mourn what should have been and what they feel like they lost, but more important is how are they making the most of the opportunities they do have?"
That question, Alexander says, is what colleges want answered if students choose to address COVID-19 in their college essay.
But the question of whether a student should write about the coronavirus is tricky. The answer depends largely on the student.
"In general, I don't think students should write about COVID-19 in their main personal statement for their application," Robin Miller, master college admissions counselor at IvyWise, a college counseling company, wrote in an email.
"Certainly, there may be exceptions to this based on a student's individual experience, but since the personal essay is the main place in the application where the student can really allow their voice to be heard and share insight into who they are as an individual, there are likely many other topics they can choose to write about that are more distinctive and unique than COVID-19," Miller says.
Opinions among admissions experts vary on whether to write about the likely popular topic of the pandemic.
"If your essay communicates something positive, unique, and compelling about you in an interesting and eloquent way, go for it," Carolyn Pippen, principal college admissions counselor at IvyWise, wrote in an email. She adds that students shouldn't be dissuaded from writing about a topic merely because it's common, noting that "topics are bound to repeat, no matter how hard we try to avoid it."
Above all, she urges honesty.
"If your experience within the context of the pandemic has been truly unique, then write about that experience, and the standing out will take care of itself," Pippen says. "If your experience has been generally the same as most other students in your context, then trying to find a unique angle can easily cross the line into exploiting a tragedy, or at least appearing as though you have."
But focusing entirely on the pandemic can limit a student to a single story and narrow who they are in an application, Sawyer says. "There are so many wonderful possibilities for what you can say about yourself outside of your experience within the pandemic."
He notes that passions, strengths, career interests and personal identity are among the multitude of essay topic options available to applicants and encourages them to probe their values to help determine the topic that matters most to them – and write about it.
That doesn't mean the pandemic experience has to be ignored if applicants feel the need to write about it.
Writing About Coronavirus in Main and Supplemental Essays
Students can choose to write a full-length college essay on the coronavirus or summarize their experience in a shorter form.
To help students explain how the pandemic affected them, The Common App has added an optional section to address this topic. Applicants have 250 words to describe their pandemic experience and the personal and academic impact of COVID-19.
"That's not a trick question, and there's no right or wrong answer," Alexander says. Colleges want to know, he adds, how students navigated the pandemic, how they prioritized their time, what responsibilities they took on and what they learned along the way.
If students can distill all of the above information into 250 words, there's likely no need to write about it in a full-length college essay, experts say. And applicants whose lives were not heavily altered by the pandemic may even choose to skip the optional COVID-19 question.
"This space is best used to discuss hardship and/or significant challenges that the student and/or the student's family experienced as a result of COVID-19 and how they have responded to those difficulties," Miller notes. Using the section to acknowledge a lack of impact, she adds, "could be perceived as trite and lacking insight, despite the good intentions of the applicant."
To guard against this lack of awareness, Sawyer encourages students to tap someone they trust to review their writing , whether it's the 250-word Common App response or the full-length essay.
Experts tend to agree that the short-form approach to this as an essay topic works better, but there are exceptions. And if a student does have a coronavirus story that he or she feels must be told, Alexander encourages the writer to be authentic in the essay.
"My advice for an essay about COVID-19 is the same as my advice about an essay for any topic – and that is, don't write what you think we want to read or hear," Alexander says. "Write what really changed you and that story that now is yours and yours alone to tell."
Sawyer urges students to ask themselves, "What's the sentence that only I can write?" He also encourages students to remember that the pandemic is only a chapter of their lives and not the whole book.
Miller, who cautions against writing a full-length essay on the coronavirus, says that if students choose to do so they should have a conversation with their high school counselor about whether that's the right move. And if students choose to proceed with COVID-19 as a topic, she says they need to be clear, detailed and insightful about what they learned and how they adapted along the way.
"Approaching the essay in this manner will provide important balance while demonstrating personal growth and vulnerability," Miller says.
Pippen encourages students to remember that they are in an unprecedented time for college admissions.
"It is important to keep in mind with all of these (admission) factors that no colleges have ever had to consider them this way in the selection process, if at all," Pippen says. "They have had very little time to calibrate their evaluations of different application components within their offices, let alone across institutions. This means that colleges will all be handling the admissions process a little bit differently, and their approaches may even evolve over the course of the admissions cycle."
Searching for a college? Get our complete rankings of Best Colleges.
10 Ways to Discover College Essay Ideas

Tags: students , colleges , college admissions , college applications , college search , Coronavirus
2024 Best Colleges

Search for your perfect fit with the U.S. News rankings of colleges and universities.
College Admissions: Get a Step Ahead!
Sign up to receive the latest updates from U.S. News & World Report and our trusted partners and sponsors. By clicking submit, you are agreeing to our Terms and Conditions & Privacy Policy .
Ask an Alum: Making the Most Out of College
You May Also Like
Scholarships for lesser-known sports.
Sarah Wood May 15, 2024

Should Students Submit Test Scores?
Sarah Wood May 13, 2024

Poll: Antisemitism a Problem on Campus
Lauren Camera May 13, 2024

Federal vs. Private Parent Student Loans
Erika Giovanetti May 9, 2024

14 Colleges With Great Food Options
Sarah Wood May 8, 2024

Colleges With Religious Affiliations
Anayat Durrani May 8, 2024

Protests Threaten Campus Graduations
Aneeta Mathur-Ashton May 6, 2024

Protesting on Campus: What to Know
Sarah Wood May 6, 2024

Lawmakers Ramp Up Response to Unrest
Aneeta Mathur-Ashton May 3, 2024

University Commencements Must Go On
Eric J. Gertler May 3, 2024

- Research article
- Open access
- Published: 04 June 2021
Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews
- Israel Júnior Borges do Nascimento 1 , 2 ,
- Dónal P. O’Mathúna 3 , 4 ,
- Thilo Caspar von Groote 5 ,
- Hebatullah Mohamed Abdulazeem 6 ,
- Ishanka Weerasekara 7 , 8 ,
- Ana Marusic 9 ,
- Livia Puljak ORCID: orcid.org/0000-0002-8467-6061 10 ,
- Vinicius Tassoni Civile 11 ,
- Irena Zakarija-Grkovic 9 ,
- Tina Poklepovic Pericic 9 ,
- Alvaro Nagib Atallah 11 ,
- Santino Filoso 12 ,
- Nicola Luigi Bragazzi 13 &
- Milena Soriano Marcolino 1
On behalf of the International Network of Coronavirus Disease 2019 (InterNetCOVID-19)
BMC Infectious Diseases volume 21 , Article number: 525 ( 2021 ) Cite this article
16k Accesses
29 Citations
13 Altmetric
Metrics details
Navigating the rapidly growing body of scientific literature on the SARS-CoV-2 pandemic is challenging, and ongoing critical appraisal of this output is essential. We aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Nine databases (Medline, EMBASE, Cochrane Library, CINAHL, Web of Sciences, PDQ-Evidence, WHO’s Global Research, LILACS, and Epistemonikos) were searched from December 1, 2019, to March 24, 2020. Systematic reviews analyzing primary studies of COVID-19 were included. Two authors independently undertook screening, selection, extraction (data on clinical symptoms, prevalence, pharmacological and non-pharmacological interventions, diagnostic test assessment, laboratory, and radiological findings), and quality assessment (AMSTAR 2). A meta-analysis was performed of the prevalence of clinical outcomes.
Eighteen systematic reviews were included; one was empty (did not identify any relevant study). Using AMSTAR 2, confidence in the results of all 18 reviews was rated as “critically low”. Identified symptoms of COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%) and gastrointestinal complaints (5–9%). Severe symptoms were more common in men. Elevated C-reactive protein and lactate dehydrogenase, and slightly elevated aspartate and alanine aminotransferase, were commonly described. Thrombocytopenia and elevated levels of procalcitonin and cardiac troponin I were associated with severe disease. A frequent finding on chest imaging was uni- or bilateral multilobar ground-glass opacity. A single review investigated the impact of medication (chloroquine) but found no verifiable clinical data. All-cause mortality ranged from 0.3 to 13.9%.
Conclusions
In this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic were of questionable usefulness. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards.
Peer Review reports
The spread of the “Severe Acute Respiratory Coronavirus 2” (SARS-CoV-2), the causal agent of COVID-19, was characterized as a pandemic by the World Health Organization (WHO) in March 2020 and has triggered an international public health emergency [ 1 ]. The numbers of confirmed cases and deaths due to COVID-19 are rapidly escalating, counting in millions [ 2 ], causing massive economic strain, and escalating healthcare and public health expenses [ 3 , 4 ].
The research community has responded by publishing an impressive number of scientific reports related to COVID-19. The world was alerted to the new disease at the beginning of 2020 [ 1 ], and by mid-March 2020, more than 2000 articles had been published on COVID-19 in scholarly journals, with 25% of them containing original data [ 5 ]. The living map of COVID-19 evidence, curated by the Evidence for Policy and Practice Information and Co-ordinating Centre (EPPI-Centre), contained more than 40,000 records by February 2021 [ 6 ]. More than 100,000 records on PubMed were labeled as “SARS-CoV-2 literature, sequence, and clinical content” by February 2021 [ 7 ].
Due to publication speed, the research community has voiced concerns regarding the quality and reproducibility of evidence produced during the COVID-19 pandemic, warning of the potential damaging approach of “publish first, retract later” [ 8 ]. It appears that these concerns are not unfounded, as it has been reported that COVID-19 articles were overrepresented in the pool of retracted articles in 2020 [ 9 ]. These concerns about inadequate evidence are of major importance because they can lead to poor clinical practice and inappropriate policies [ 10 ].
Systematic reviews are a cornerstone of today’s evidence-informed decision-making. By synthesizing all relevant evidence regarding a particular topic, systematic reviews reflect the current scientific knowledge. Systematic reviews are considered to be at the highest level in the hierarchy of evidence and should be used to make informed decisions. However, with high numbers of systematic reviews of different scope and methodological quality being published, overviews of multiple systematic reviews that assess their methodological quality are essential [ 11 , 12 , 13 ]. An overview of systematic reviews helps identify and organize the literature and highlights areas of priority in decision-making.
In this overview of systematic reviews, we aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Methodology
Research question.
This overview’s primary objective was to summarize and critically appraise systematic reviews that assessed any type of primary clinical data from patients infected with SARS-CoV-2. Our research question was purposefully broad because we wanted to analyze as many systematic reviews as possible that were available early following the COVID-19 outbreak.
Study design
We conducted an overview of systematic reviews. The idea for this overview originated in a protocol for a systematic review submitted to PROSPERO (CRD42020170623), which indicated a plan to conduct an overview.
Overviews of systematic reviews use explicit and systematic methods for searching and identifying multiple systematic reviews addressing related research questions in the same field to extract and analyze evidence across important outcomes. Overviews of systematic reviews are in principle similar to systematic reviews of interventions, but the unit of analysis is a systematic review [ 14 , 15 , 16 ].
We used the overview methodology instead of other evidence synthesis methods to allow us to collate and appraise multiple systematic reviews on this topic, and to extract and analyze their results across relevant topics [ 17 ]. The overview and meta-analysis of systematic reviews allowed us to investigate the methodological quality of included studies, summarize results, and identify specific areas of available or limited evidence, thereby strengthening the current understanding of this novel disease and guiding future research [ 13 ].
A reporting guideline for overviews of reviews is currently under development, i.e., Preferred Reporting Items for Overviews of Reviews (PRIOR) [ 18 ]. As the PRIOR checklist is still not published, this study was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 statement [ 19 ]. The methodology used in this review was adapted from the Cochrane Handbook for Systematic Reviews of Interventions and also followed established methodological considerations for analyzing existing systematic reviews [ 14 ].
Approval of a research ethics committee was not necessary as the study analyzed only publicly available articles.
Eligibility criteria
Systematic reviews were included if they analyzed primary data from patients infected with SARS-CoV-2 as confirmed by RT-PCR or another pre-specified diagnostic technique. Eligible reviews covered all topics related to COVID-19 including, but not limited to, those that reported clinical symptoms, diagnostic methods, therapeutic interventions, laboratory findings, or radiological results. Both full manuscripts and abbreviated versions, such as letters, were eligible.
No restrictions were imposed on the design of the primary studies included within the systematic reviews, the last search date, whether the review included meta-analyses or language. Reviews related to SARS-CoV-2 and other coronaviruses were eligible, but from those reviews, we analyzed only data related to SARS-CoV-2.
No consensus definition exists for a systematic review [ 20 ], and debates continue about the defining characteristics of a systematic review [ 21 ]. Cochrane’s guidance for overviews of reviews recommends setting pre-established criteria for making decisions around inclusion [ 14 ]. That is supported by a recent scoping review about guidance for overviews of systematic reviews [ 22 ].
Thus, for this study, we defined a systematic review as a research report which searched for primary research studies on a specific topic using an explicit search strategy, had a detailed description of the methods with explicit inclusion criteria provided, and provided a summary of the included studies either in narrative or quantitative format (such as a meta-analysis). Cochrane and non-Cochrane systematic reviews were considered eligible for inclusion, with or without meta-analysis, and regardless of the study design, language restriction and methodology of the included primary studies. To be eligible for inclusion, reviews had to be clearly analyzing data related to SARS-CoV-2 (associated or not with other viruses). We excluded narrative reviews without those characteristics as these are less likely to be replicable and are more prone to bias.
Scoping reviews and rapid reviews were eligible for inclusion in this overview if they met our pre-defined inclusion criteria noted above. We included reviews that addressed SARS-CoV-2 and other coronaviruses if they reported separate data regarding SARS-CoV-2.
Information sources
Nine databases were searched for eligible records published between December 1, 2019, and March 24, 2020: Cochrane Database of Systematic Reviews via Cochrane Library, PubMed, EMBASE, CINAHL (Cumulative Index to Nursing and Allied Health Literature), Web of Sciences, LILACS (Latin American and Caribbean Health Sciences Literature), PDQ-Evidence, WHO’s Global Research on Coronavirus Disease (COVID-19), and Epistemonikos.
The comprehensive search strategy for each database is provided in Additional file 1 and was designed and conducted in collaboration with an information specialist. All retrieved records were primarily processed in EndNote, where duplicates were removed, and records were then imported into the Covidence platform [ 23 ]. In addition to database searches, we screened reference lists of reviews included after screening records retrieved via databases.
Study selection
All searches, screening of titles and abstracts, and record selection, were performed independently by two investigators using the Covidence platform [ 23 ]. Articles deemed potentially eligible were retrieved for full-text screening carried out independently by two investigators. Discrepancies at all stages were resolved by consensus. During the screening, records published in languages other than English were translated by a native/fluent speaker.
Data collection process
We custom designed a data extraction table for this study, which was piloted by two authors independently. Data extraction was performed independently by two authors. Conflicts were resolved by consensus or by consulting a third researcher.
We extracted the following data: article identification data (authors’ name and journal of publication), search period, number of databases searched, population or settings considered, main results and outcomes observed, and number of participants. From Web of Science (Clarivate Analytics, Philadelphia, PA, USA), we extracted journal rank (quartile) and Journal Impact Factor (JIF).
We categorized the following as primary outcomes: all-cause mortality, need for and length of mechanical ventilation, length of hospitalization (in days), admission to intensive care unit (yes/no), and length of stay in the intensive care unit.
The following outcomes were categorized as exploratory: diagnostic methods used for detection of the virus, male to female ratio, clinical symptoms, pharmacological and non-pharmacological interventions, laboratory findings (full blood count, liver enzymes, C-reactive protein, d-dimer, albumin, lipid profile, serum electrolytes, blood vitamin levels, glucose levels, and any other important biomarkers), and radiological findings (using radiography, computed tomography, magnetic resonance imaging or ultrasound).
We also collected data on reporting guidelines and requirements for the publication of systematic reviews and meta-analyses from journal websites where included reviews were published.
Quality assessment in individual reviews
Two researchers independently assessed the reviews’ quality using the “A MeaSurement Tool to Assess Systematic Reviews 2 (AMSTAR 2)”. We acknowledge that the AMSTAR 2 was created as “a critical appraisal tool for systematic reviews that include randomized or non-randomized studies of healthcare interventions, or both” [ 24 ]. However, since AMSTAR 2 was designed for systematic reviews of intervention trials, and we included additional types of systematic reviews, we adjusted some AMSTAR 2 ratings and reported these in Additional file 2 .
Adherence to each item was rated as follows: yes, partial yes, no, or not applicable (such as when a meta-analysis was not conducted). The overall confidence in the results of the review is rated as “critically low”, “low”, “moderate” or “high”, according to the AMSTAR 2 guidance based on seven critical domains, which are items 2, 4, 7, 9, 11, 13, 15 as defined by AMSTAR 2 authors [ 24 ]. We reported our adherence ratings for transparency of our decision with accompanying explanations, for each item, in each included review.
One of the included systematic reviews was conducted by some members of this author team [ 25 ]. This review was initially assessed independently by two authors who were not co-authors of that review to prevent the risk of bias in assessing this study.
Synthesis of results
For data synthesis, we prepared a table summarizing each systematic review. Graphs illustrating the mortality rate and clinical symptoms were created. We then prepared a narrative summary of the methods, findings, study strengths, and limitations.
For analysis of the prevalence of clinical outcomes, we extracted data on the number of events and the total number of patients to perform proportional meta-analysis using RStudio© software, with the “meta” package (version 4.9–6), using the “metaprop” function for reviews that did not perform a meta-analysis, excluding case studies because of the absence of variance. For reviews that did not perform a meta-analysis, we presented pooled results of proportions with their respective confidence intervals (95%) by the inverse variance method with a random-effects model, using the DerSimonian-Laird estimator for τ 2 . We adjusted data using Freeman-Tukey double arcosen transformation. Confidence intervals were calculated using the Clopper-Pearson method for individual studies. We created forest plots using the RStudio© software, with the “metafor” package (version 2.1–0) and “forest” function.
Managing overlapping systematic reviews
Some of the included systematic reviews that address the same or similar research questions may include the same primary studies in overviews. Including such overlapping reviews may introduce bias when outcome data from the same primary study are included in the analyses of an overview multiple times. Thus, in summaries of evidence, multiple-counting of the same outcome data will give data from some primary studies too much influence [ 14 ]. In this overview, we did not exclude overlapping systematic reviews because, according to Cochrane’s guidance, it may be appropriate to include all relevant reviews’ results if the purpose of the overview is to present and describe the current body of evidence on a topic [ 14 ]. To avoid any bias in summary estimates associated with overlapping reviews, we generated forest plots showing data from individual systematic reviews, but the results were not pooled because some primary studies were included in multiple reviews.
Our search retrieved 1063 publications, of which 175 were duplicates. Most publications were excluded after the title and abstract analysis ( n = 860). Among the 28 studies selected for full-text screening, 10 were excluded for the reasons described in Additional file 3 , and 18 were included in the final analysis (Fig. 1 ) [ 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 ]. Reference list screening did not retrieve any additional systematic reviews.
PRISMA flow diagram
Characteristics of included reviews
Summary features of 18 systematic reviews are presented in Table 1 . They were published in 14 different journals. Only four of these journals had specific requirements for systematic reviews (with or without meta-analysis): European Journal of Internal Medicine, Journal of Clinical Medicine, Ultrasound in Obstetrics and Gynecology, and Clinical Research in Cardiology . Two journals reported that they published only invited reviews ( Journal of Medical Virology and Clinica Chimica Acta ). Three systematic reviews in our study were published as letters; one was labeled as a scoping review and another as a rapid review (Table 2 ).
All reviews were published in English, in first quartile (Q1) journals, with JIF ranging from 1.692 to 6.062. One review was empty, meaning that its search did not identify any relevant studies; i.e., no primary studies were included [ 36 ]. The remaining 17 reviews included 269 unique studies; the majority ( N = 211; 78%) were included in only a single review included in our study (range: 1 to 12). Primary studies included in the reviews were published between December 2019 and March 18, 2020, and comprised case reports, case series, cohorts, and other observational studies. We found only one review that included randomized clinical trials [ 38 ]. In the included reviews, systematic literature searches were performed from 2019 (entire year) up to March 9, 2020. Ten systematic reviews included meta-analyses. The list of primary studies found in the included systematic reviews is shown in Additional file 4 , as well as the number of reviews in which each primary study was included.
Population and study designs
Most of the reviews analyzed data from patients with COVID-19 who developed pneumonia, acute respiratory distress syndrome (ARDS), or any other correlated complication. One review aimed to evaluate the effectiveness of using surgical masks on preventing transmission of the virus [ 36 ], one review was focused on pediatric patients [ 34 ], and one review investigated COVID-19 in pregnant women [ 37 ]. Most reviews assessed clinical symptoms, laboratory findings, or radiological results.
Systematic review findings
The summary of findings from individual reviews is shown in Table 2 . Overall, all-cause mortality ranged from 0.3 to 13.9% (Fig. 2 ).
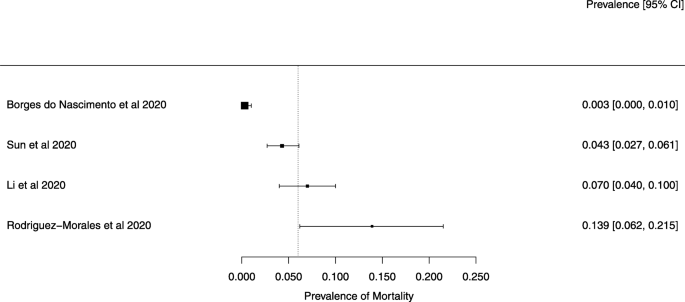
A meta-analysis of the prevalence of mortality
Clinical symptoms
Seven reviews described the main clinical manifestations of COVID-19 [ 26 , 28 , 29 , 34 , 35 , 39 , 41 ]. Three of them provided only a narrative discussion of symptoms [ 26 , 34 , 35 ]. In the reviews that performed a statistical analysis of the incidence of different clinical symptoms, symptoms in patients with COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%), gastrointestinal disorders, such as diarrhea, nausea or vomiting (5.0–9.0%), and others (including, in one study only: dizziness 12.1%) (Figs. 3 , 4 , 5 , 6 , 7 , 8 and 9 ). Three reviews assessed cough with and without sputum together; only one review assessed sputum production itself (28.5%).
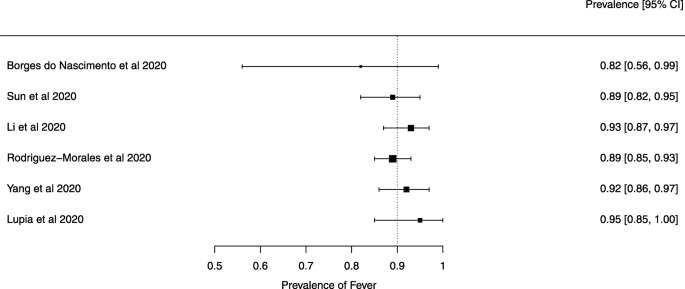
A meta-analysis of the prevalence of fever
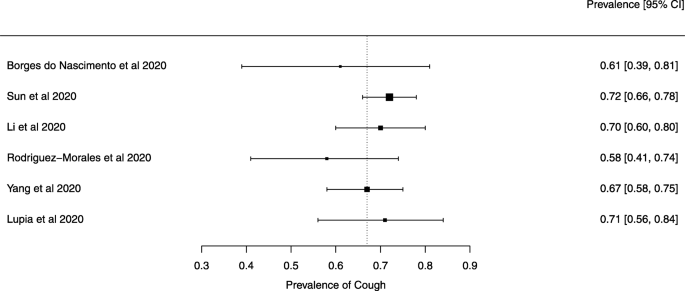
A meta-analysis of the prevalence of cough
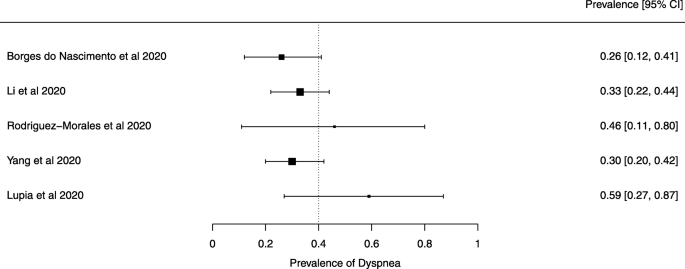
A meta-analysis of the prevalence of dyspnea
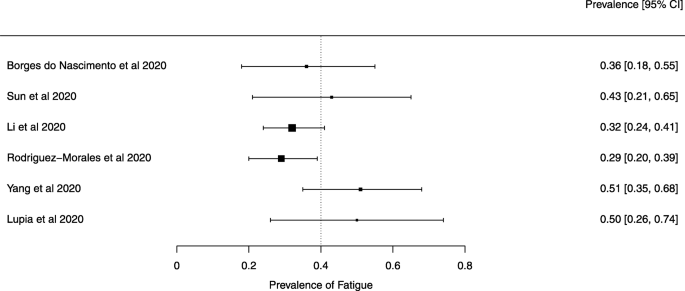
A meta-analysis of the prevalence of fatigue or myalgia
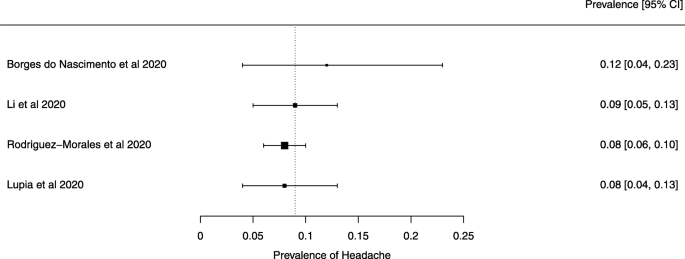
A meta-analysis of the prevalence of headache
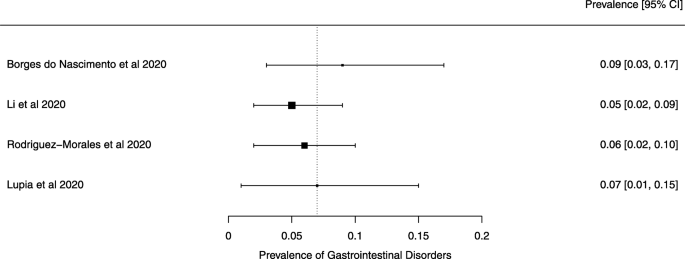
A meta-analysis of the prevalence of gastrointestinal disorders
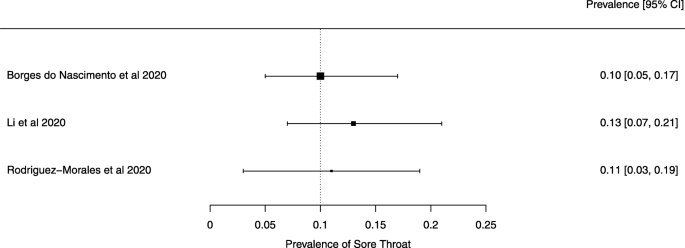
A meta-analysis of the prevalence of sore throat
Diagnostic aspects
Three reviews described methodologies, protocols, and tools used for establishing the diagnosis of COVID-19 [ 26 , 34 , 38 ]. The use of respiratory swabs (nasal or pharyngeal) or blood specimens to assess the presence of SARS-CoV-2 nucleic acid using RT-PCR assays was the most commonly used diagnostic method mentioned in the included studies. These diagnostic tests have been widely used, but their precise sensitivity and specificity remain unknown. One review included a Chinese study with clinical diagnosis with no confirmation of SARS-CoV-2 infection (patients were diagnosed with COVID-19 if they presented with at least two symptoms suggestive of COVID-19, together with laboratory and chest radiography abnormalities) [ 34 ].
Therapeutic possibilities
Pharmacological and non-pharmacological interventions (supportive therapies) used in treating patients with COVID-19 were reported in five reviews [ 25 , 27 , 34 , 35 , 38 ]. Antivirals used empirically for COVID-19 treatment were reported in seven reviews [ 25 , 27 , 34 , 35 , 37 , 38 , 41 ]; most commonly used were protease inhibitors (lopinavir, ritonavir, darunavir), nucleoside reverse transcriptase inhibitor (tenofovir), nucleotide analogs (remdesivir, galidesivir, ganciclovir), and neuraminidase inhibitors (oseltamivir). Umifenovir, a membrane fusion inhibitor, was investigated in two studies [ 25 , 35 ]. Possible supportive interventions analyzed were different types of oxygen supplementation and breathing support (invasive or non-invasive ventilation) [ 25 ]. The use of antibiotics, both empirically and to treat secondary pneumonia, was reported in six studies [ 25 , 26 , 27 , 34 , 35 , 38 ]. One review specifically assessed evidence on the efficacy and safety of the anti-malaria drug chloroquine [ 27 ]. It identified 23 ongoing trials investigating the potential of chloroquine as a therapeutic option for COVID-19, but no verifiable clinical outcomes data. The use of mesenchymal stem cells, antifungals, and glucocorticoids were described in four reviews [ 25 , 34 , 35 , 38 ].
Laboratory and radiological findings
Of the 18 reviews included in this overview, eight analyzed laboratory parameters in patients with COVID-19 [ 25 , 29 , 30 , 32 , 33 , 34 , 35 , 39 ]; elevated C-reactive protein levels, associated with lymphocytopenia, elevated lactate dehydrogenase, as well as slightly elevated aspartate and alanine aminotransferase (AST, ALT) were commonly described in those eight reviews. Lippi et al. assessed cardiac troponin I (cTnI) [ 25 ], procalcitonin [ 32 ], and platelet count [ 33 ] in COVID-19 patients. Elevated levels of procalcitonin [ 32 ] and cTnI [ 30 ] were more likely to be associated with a severe disease course (requiring intensive care unit admission and intubation). Furthermore, thrombocytopenia was frequently observed in patients with complicated COVID-19 infections [ 33 ].
Chest imaging (chest radiography and/or computed tomography) features were assessed in six reviews, all of which described a frequent pattern of local or bilateral multilobar ground-glass opacity [ 25 , 34 , 35 , 39 , 40 , 41 ]. Those six reviews showed that septal thickening, bronchiectasis, pleural and cardiac effusions, halo signs, and pneumothorax were observed in patients suffering from COVID-19.
Quality of evidence in individual systematic reviews
Table 3 shows the detailed results of the quality assessment of 18 systematic reviews, including the assessment of individual items and summary assessment. A detailed explanation for each decision in each review is available in Additional file 5 .
Using AMSTAR 2 criteria, confidence in the results of all 18 reviews was rated as “critically low” (Table 3 ). Common methodological drawbacks were: omission of prospective protocol submission or publication; use of inappropriate search strategy: lack of independent and dual literature screening and data-extraction (or methodology unclear); absence of an explanation for heterogeneity among the studies included; lack of reasons for study exclusion (or rationale unclear).
Risk of bias assessment, based on a reported methodological tool, and quality of evidence appraisal, in line with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method, were reported only in one review [ 25 ]. Five reviews presented a table summarizing bias, using various risk of bias tools [ 25 , 29 , 39 , 40 , 41 ]. One review analyzed “study quality” [ 37 ]. One review mentioned the risk of bias assessment in the methodology but did not provide any related analysis [ 28 ].
This overview of systematic reviews analyzed the first 18 systematic reviews published after the onset of the COVID-19 pandemic, up to March 24, 2020, with primary studies involving more than 60,000 patients. Using AMSTAR-2, we judged that our confidence in all those reviews was “critically low”. Ten reviews included meta-analyses. The reviews presented data on clinical manifestations, laboratory and radiological findings, and interventions. We found no systematic reviews on the utility of diagnostic tests.
Symptoms were reported in seven reviews; most of the patients had a fever, cough, dyspnea, myalgia or muscle fatigue, and gastrointestinal disorders such as diarrhea, nausea, or vomiting. Olfactory dysfunction (anosmia or dysosmia) has been described in patients infected with COVID-19 [ 43 ]; however, this was not reported in any of the reviews included in this overview. During the SARS outbreak in 2002, there were reports of impairment of the sense of smell associated with the disease [ 44 , 45 ].
The reported mortality rates ranged from 0.3 to 14% in the included reviews. Mortality estimates are influenced by the transmissibility rate (basic reproduction number), availability of diagnostic tools, notification policies, asymptomatic presentations of the disease, resources for disease prevention and control, and treatment facilities; variability in the mortality rate fits the pattern of emerging infectious diseases [ 46 ]. Furthermore, the reported cases did not consider asymptomatic cases, mild cases where individuals have not sought medical treatment, and the fact that many countries had limited access to diagnostic tests or have implemented testing policies later than the others. Considering the lack of reviews assessing diagnostic testing (sensitivity, specificity, and predictive values of RT-PCT or immunoglobulin tests), and the preponderance of studies that assessed only symptomatic individuals, considerable imprecision around the calculated mortality rates existed in the early stage of the COVID-19 pandemic.
Few reviews included treatment data. Those reviews described studies considered to be at a very low level of evidence: usually small, retrospective studies with very heterogeneous populations. Seven reviews analyzed laboratory parameters; those reviews could have been useful for clinicians who attend patients suspected of COVID-19 in emergency services worldwide, such as assessing which patients need to be reassessed more frequently.
All systematic reviews scored poorly on the AMSTAR 2 critical appraisal tool for systematic reviews. Most of the original studies included in the reviews were case series and case reports, impacting the quality of evidence. Such evidence has major implications for clinical practice and the use of these reviews in evidence-based practice and policy. Clinicians, patients, and policymakers can only have the highest confidence in systematic review findings if high-quality systematic review methodologies are employed. The urgent need for information during a pandemic does not justify poor quality reporting.
We acknowledge that there are numerous challenges associated with analyzing COVID-19 data during a pandemic [ 47 ]. High-quality evidence syntheses are needed for decision-making, but each type of evidence syntheses is associated with its inherent challenges.
The creation of classic systematic reviews requires considerable time and effort; with massive research output, they quickly become outdated, and preparing updated versions also requires considerable time. A recent study showed that updates of non-Cochrane systematic reviews are published a median of 5 years after the publication of the previous version [ 48 ].
Authors may register a review and then abandon it [ 49 ], but the existence of a public record that is not updated may lead other authors to believe that the review is still ongoing. A quarter of Cochrane review protocols remains unpublished as completed systematic reviews 8 years after protocol publication [ 50 ].
Rapid reviews can be used to summarize the evidence, but they involve methodological sacrifices and simplifications to produce information promptly, with inconsistent methodological approaches [ 51 ]. However, rapid reviews are justified in times of public health emergencies, and even Cochrane has resorted to publishing rapid reviews in response to the COVID-19 crisis [ 52 ]. Rapid reviews were eligible for inclusion in this overview, but only one of the 18 reviews included in this study was labeled as a rapid review.
Ideally, COVID-19 evidence would be continually summarized in a series of high-quality living systematic reviews, types of evidence synthesis defined as “ a systematic review which is continually updated, incorporating relevant new evidence as it becomes available ” [ 53 ]. However, conducting living systematic reviews requires considerable resources, calling into question the sustainability of such evidence synthesis over long periods [ 54 ].
Research reports about COVID-19 will contribute to research waste if they are poorly designed, poorly reported, or simply not necessary. In principle, systematic reviews should help reduce research waste as they usually provide recommendations for further research that is needed or may advise that sufficient evidence exists on a particular topic [ 55 ]. However, systematic reviews can also contribute to growing research waste when they are not needed, or poorly conducted and reported. Our present study clearly shows that most of the systematic reviews that were published early on in the COVID-19 pandemic could be categorized as research waste, as our confidence in their results is critically low.
Our study has some limitations. One is that for AMSTAR 2 assessment we relied on information available in publications; we did not attempt to contact study authors for clarifications or additional data. In three reviews, the methodological quality appraisal was challenging because they were published as letters, or labeled as rapid communications. As a result, various details about their review process were not included, leading to AMSTAR 2 questions being answered as “not reported”, resulting in low confidence scores. Full manuscripts might have provided additional information that could have led to higher confidence in the results. In other words, low scores could reflect incomplete reporting, not necessarily low-quality review methods. To make their review available more rapidly and more concisely, the authors may have omitted methodological details. A general issue during a crisis is that speed and completeness must be balanced. However, maintaining high standards requires proper resourcing and commitment to ensure that the users of systematic reviews can have high confidence in the results.
Furthermore, we used adjusted AMSTAR 2 scoring, as the tool was designed for critical appraisal of reviews of interventions. Some reviews may have received lower scores than actually warranted in spite of these adjustments.
Another limitation of our study may be the inclusion of multiple overlapping reviews, as some included reviews included the same primary studies. According to the Cochrane Handbook, including overlapping reviews may be appropriate when the review’s aim is “ to present and describe the current body of systematic review evidence on a topic ” [ 12 ], which was our aim. To avoid bias with summarizing evidence from overlapping reviews, we presented the forest plots without summary estimates. The forest plots serve to inform readers about the effect sizes for outcomes that were reported in each review.
Several authors from this study have contributed to one of the reviews identified [ 25 ]. To reduce the risk of any bias, two authors who did not co-author the review in question initially assessed its quality and limitations.
Finally, we note that the systematic reviews included in our overview may have had issues that our analysis did not identify because we did not analyze their primary studies to verify the accuracy of the data and information they presented. We give two examples to substantiate this possibility. Lovato et al. wrote a commentary on the review of Sun et al. [ 41 ], in which they criticized the authors’ conclusion that sore throat is rare in COVID-19 patients [ 56 ]. Lovato et al. highlighted that multiple studies included in Sun et al. did not accurately describe participants’ clinical presentations, warning that only three studies clearly reported data on sore throat [ 56 ].
In another example, Leung [ 57 ] warned about the review of Li, L.Q. et al. [ 29 ]: “ it is possible that this statistic was computed using overlapped samples, therefore some patients were double counted ”. Li et al. responded to Leung that it is uncertain whether the data overlapped, as they used data from published articles and did not have access to the original data; they also reported that they requested original data and that they plan to re-do their analyses once they receive them; they also urged readers to treat the data with caution [ 58 ]. This points to the evolving nature of evidence during a crisis.
Our study’s strength is that this overview adds to the current knowledge by providing a comprehensive summary of all the evidence synthesis about COVID-19 available early after the onset of the pandemic. This overview followed strict methodological criteria, including a comprehensive and sensitive search strategy and a standard tool for methodological appraisal of systematic reviews.
In conclusion, in this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all the reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic could be categorized as research waste. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards to provide patients, clinicians, and decision-makers trustworthy evidence.
Availability of data and materials
All data collected and analyzed within this study are available from the corresponding author on reasonable request.
World Health Organization. Timeline - COVID-19: Available at: https://www.who.int/news/item/29-06-2020-covidtimeline . Accessed 1 June 2021.
COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available at: https://coronavirus.jhu.edu/map.html . Accessed 1 June 2021.
Anzai A, Kobayashi T, Linton NM, Kinoshita R, Hayashi K, Suzuki A, et al. Assessing the Impact of Reduced Travel on Exportation Dynamics of Novel Coronavirus Infection (COVID-19). J Clin Med. 2020;9(2):601.
Chinazzi M, Davis JT, Ajelli M, Gioannini C, Litvinova M, Merler S, et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368(6489):395–400. https://doi.org/10.1126/science.aba9757 .
Article CAS PubMed PubMed Central Google Scholar
Fidahic M, Nujic D, Runjic R, Civljak M, Markotic F, Lovric Makaric Z, et al. Research methodology and characteristics of journal articles with original data, preprint articles and registered clinical trial protocols about COVID-19. BMC Med Res Methodol. 2020;20(1):161. https://doi.org/10.1186/s12874-020-01047-2 .
EPPI Centre . COVID-19: a living systematic map of the evidence. Available at: http://eppi.ioe.ac.uk/cms/Projects/DepartmentofHealthandSocialCare/Publishedreviews/COVID-19Livingsystematicmapoftheevidence/tabid/3765/Default.aspx . Accessed 1 June 2021.
NCBI SARS-CoV-2 Resources. Available at: https://www.ncbi.nlm.nih.gov/sars-cov-2/ . Accessed 1 June 2021.
Gustot T. Quality and reproducibility during the COVID-19 pandemic. JHEP Rep. 2020;2(4):100141. https://doi.org/10.1016/j.jhepr.2020.100141 .
Article PubMed PubMed Central Google Scholar
Kodvanj, I., et al., Publishing of COVID-19 Preprints in Peer-reviewed Journals, Preprinting Trends, Public Discussion and Quality Issues. Preprint article. bioRxiv 2020.11.23.394577; doi: https://doi.org/10.1101/2020.11.23.394577 .
Dobler CC. Poor quality research and clinical practice during COVID-19. Breathe (Sheff). 2020;16(2):200112. https://doi.org/10.1183/20734735.0112-2020 .
Article Google Scholar
Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med. 2010;7(9):e1000326. https://doi.org/10.1371/journal.pmed.1000326 .
Lunny C, Brennan SE, McDonald S, McKenzie JE. Toward a comprehensive evidence map of overview of systematic review methods: paper 1-purpose, eligibility, search and data extraction. Syst Rev. 2017;6(1):231. https://doi.org/10.1186/s13643-017-0617-1 .
Pollock M, Fernandes RM, Becker LA, Pieper D, Hartling L. Chapter V: Overviews of Reviews. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane. 2020. Available from www.training.cochrane.org/handbook .
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane. 2020; Available from www.training.cochrane.org/handbook .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. The impact of different inclusion decisions on the comprehensiveness and complexity of overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):18. https://doi.org/10.1186/s13643-018-0914-3 .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. A decision tool to help researchers make decisions about including systematic reviews in overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):29. https://doi.org/10.1186/s13643-018-0768-8 .
Hunt H, Pollock A, Campbell P, Estcourt L, Brunton G. An introduction to overviews of reviews: planning a relevant research question and objective for an overview. Syst Rev. 2018;7(1):39. https://doi.org/10.1186/s13643-018-0695-8 .
Pollock M, Fernandes RM, Pieper D, Tricco AC, Gates M, Gates A, et al. Preferred reporting items for overviews of reviews (PRIOR): a protocol for development of a reporting guideline for overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):335. https://doi.org/10.1186/s13643-019-1252-9 .
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. 2009;3(3):e123–30.
Krnic Martinic M, Pieper D, Glatt A, Puljak L. Definition of a systematic review used in overviews of systematic reviews, meta-epidemiological studies and textbooks. BMC Med Res Methodol. 2019;19(1):203. https://doi.org/10.1186/s12874-019-0855-0 .
Puljak L. If there is only one author or only one database was searched, a study should not be called a systematic review. J Clin Epidemiol. 2017;91:4–5. https://doi.org/10.1016/j.jclinepi.2017.08.002 .
Article PubMed Google Scholar
Gates M, Gates A, Guitard S, Pollock M, Hartling L. Guidance for overviews of reviews continues to accumulate, but important challenges remain: a scoping review. Syst Rev. 2020;9(1):254. https://doi.org/10.1186/s13643-020-01509-0 .
Covidence - systematic review software. Available at: https://www.covidence.org/ . Accessed 1 June 2021.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Borges do Nascimento IJ, et al. Novel Coronavirus Infection (COVID-19) in Humans: A Scoping Review and Meta-Analysis. J Clin Med. 2020;9(4):941.
Article PubMed Central Google Scholar
Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):29. https://doi.org/10.1186/s40249-020-00646-x .
Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279–83. https://doi.org/10.1016/j.jcrc.2020.03.005 .
Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–8. https://doi.org/10.1007/s00392-020-01626-9 .
Article CAS PubMed Google Scholar
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(6):577–83. https://doi.org/10.1002/jmv.25757 .
Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63(3):390–1. https://doi.org/10.1016/j.pcad.2020.03.001 .
Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur J Intern Med. 2020;75:107–8. https://doi.org/10.1016/j.ejim.2020.03.014 .
Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–1. https://doi.org/10.1016/j.cca.2020.03.004 .
Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–8. https://doi.org/10.1016/j.cca.2020.03.022 .
Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–95. https://doi.org/10.1111/apa.15270 .
Lupia T, Scabini S, Mornese Pinna S, di Perri G, de Rosa FG, Corcione S. 2019 novel coronavirus (2019-nCoV) outbreak: a new challenge. J Glob Antimicrob Resist. 2020;21:22–7. https://doi.org/10.1016/j.jgar.2020.02.021 .
Marasinghe, K.M., A systematic review investigating the effectiveness of face mask use in limiting the spread of COVID-19 among medically not diagnosed individuals: shedding light on current recommendations provided to individuals not medically diagnosed with COVID-19. Research Square. Preprint article. doi : https://doi.org/10.21203/rs.3.rs-16701/v1 . 2020 .
Mullins E, Evans D, Viner RM, O’Brien P, Morris E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol. 2020;55(5):586–92. https://doi.org/10.1002/uog.22014 .
Pang J, Wang MX, Ang IYH, Tan SHX, Lewis RF, Chen JIP, et al. Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel coronavirus (2019-nCoV): a systematic review. J Clin Med. 2020;9(3):623.
Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. https://doi.org/10.1016/j.tmaid.2020.101623 .
Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215(1):87–93. https://doi.org/10.2214/AJR.20.23034 .
Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis. J Med Virol. 2020;92(6):612–7. https://doi.org/10.1002/jmv.25735 .
Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–5. https://doi.org/10.1016/j.ijid.2020.03.017 .
Bassetti M, Vena A, Giacobbe DR. The novel Chinese coronavirus (2019-nCoV) infections: challenges for fighting the storm. Eur J Clin Investig. 2020;50(3):e13209. https://doi.org/10.1111/eci.13209 .
Article CAS Google Scholar
Hwang CS. Olfactory neuropathy in severe acute respiratory syndrome: report of a case. Acta Neurol Taiwanica. 2006;15(1):26–8.
Google Scholar
Suzuki M, Saito K, Min WP, Vladau C, Toida K, Itoh H, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117(2):272–7. https://doi.org/10.1097/01.mlg.0000249922.37381.1e .
Rajgor DD, Lee MH, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776–7. https://doi.org/10.1016/S1473-3099(20)30244-9 .
Wolkewitz M, Puljak L. Methodological challenges of analysing COVID-19 data during the pandemic. BMC Med Res Methodol. 2020;20(1):81. https://doi.org/10.1186/s12874-020-00972-6 .
Rombey T, Lochner V, Puljak L, Könsgen N, Mathes T, Pieper D. Epidemiology and reporting characteristics of non-Cochrane updates of systematic reviews: a cross-sectional study. Res Synth Methods. 2020;11(3):471–83. https://doi.org/10.1002/jrsm.1409 .
Runjic E, Rombey T, Pieper D, Puljak L. Half of systematic reviews about pain registered in PROSPERO were not published and the majority had inaccurate status. J Clin Epidemiol. 2019;116:114–21. https://doi.org/10.1016/j.jclinepi.2019.08.010 .
Runjic E, Behmen D, Pieper D, Mathes T, Tricco AC, Moher D, et al. Following Cochrane review protocols to completion 10 years later: a retrospective cohort study and author survey. J Clin Epidemiol. 2019;111:41–8. https://doi.org/10.1016/j.jclinepi.2019.03.006 .
Tricco AC, Antony J, Zarin W, Strifler L, Ghassemi M, Ivory J, et al. A scoping review of rapid review methods. BMC Med. 2015;13(1):224. https://doi.org/10.1186/s12916-015-0465-6 .
COVID-19 Rapid Reviews: Cochrane’s response so far. Available at: https://training.cochrane.org/resource/covid-19-rapid-reviews-cochrane-response-so-far . Accessed 1 June 2021.
Cochrane. Living systematic reviews. Available at: https://community.cochrane.org/review-production/production-resources/living-systematic-reviews . Accessed 1 June 2021.
Millard T, Synnot A, Elliott J, Green S, McDonald S, Turner T. Feasibility and acceptability of living systematic reviews: results from a mixed-methods evaluation. Syst Rev. 2019;8(1):325. https://doi.org/10.1186/s13643-019-1248-5 .
Babic A, Poklepovic Pericic T, Pieper D, Puljak L. How to decide whether a systematic review is stable and not in need of updating: analysis of Cochrane reviews. Res Synth Methods. 2020;11(6):884–90. https://doi.org/10.1002/jrsm.1451 .
Lovato A, Rossettini G, de Filippis C. Sore throat in COVID-19: comment on “clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis”. J Med Virol. 2020;92(7):714–5. https://doi.org/10.1002/jmv.25815 .
Leung C. Comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1431–2. https://doi.org/10.1002/jmv.25912 .
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. Response to Char’s comment: comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1433. https://doi.org/10.1002/jmv.25924 .
Download references
Acknowledgments
We thank Catherine Henderson DPhil from Swanscoe Communications for pro bono medical writing and editing support. We acknowledge support from the Covidence Team, specifically Anneliese Arno. We thank the whole International Network of Coronavirus Disease 2019 (InterNetCOVID-19) for their commitment and involvement. Members of the InterNetCOVID-19 are listed in Additional file 6 . We thank Pavel Cerny and Roger Crosthwaite for guiding the team supervisor (IJBN) on human resources management.
This research received no external funding.
Author information
Authors and affiliations.
University Hospital and School of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil
Israel Júnior Borges do Nascimento & Milena Soriano Marcolino
Medical College of Wisconsin, Milwaukee, WI, USA
Israel Júnior Borges do Nascimento
Helene Fuld Health Trust National Institute for Evidence-based Practice in Nursing and Healthcare, College of Nursing, The Ohio State University, Columbus, OH, USA
Dónal P. O’Mathúna
School of Nursing, Psychotherapy and Community Health, Dublin City University, Dublin, Ireland
Department of Anesthesiology, Intensive Care and Pain Medicine, University of Münster, Münster, Germany
Thilo Caspar von Groote
Department of Sport and Health Science, Technische Universität München, Munich, Germany
Hebatullah Mohamed Abdulazeem
School of Health Sciences, Faculty of Health and Medicine, The University of Newcastle, Callaghan, Australia
Ishanka Weerasekara
Department of Physiotherapy, Faculty of Allied Health Sciences, University of Peradeniya, Peradeniya, Sri Lanka
Cochrane Croatia, University of Split, School of Medicine, Split, Croatia
Ana Marusic, Irena Zakarija-Grkovic & Tina Poklepovic Pericic
Center for Evidence-Based Medicine and Health Care, Catholic University of Croatia, Ilica 242, 10000, Zagreb, Croatia
Livia Puljak
Cochrane Brazil, Evidence-Based Health Program, Universidade Federal de São Paulo, São Paulo, Brazil
Vinicius Tassoni Civile & Alvaro Nagib Atallah
Yorkville University, Fredericton, New Brunswick, Canada
Santino Filoso
Laboratory for Industrial and Applied Mathematics (LIAM), Department of Mathematics and Statistics, York University, Toronto, Ontario, Canada
Nicola Luigi Bragazzi
You can also search for this author in PubMed Google Scholar
Contributions
IJBN conceived the research idea and worked as a project coordinator. DPOM, TCVG, HMA, IW, AM, LP, VTC, IZG, TPP, ANA, SF, NLB and MSM were involved in data curation, formal analysis, investigation, methodology, and initial draft writing. All authors revised the manuscript critically for the content. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Livia Puljak .
Ethics declarations
Ethics approval and consent to participate.
Not required as data was based on published studies.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: appendix 1..
Search strategies used in the study.
Additional file 2: Appendix 2.
Adjusted scoring of AMSTAR 2 used in this study for systematic reviews of studies that did not analyze interventions.
Additional file 3: Appendix 3.
List of excluded studies, with reasons.
Additional file 4: Appendix 4.
Table of overlapping studies, containing the list of primary studies included, their visual overlap in individual systematic reviews, and the number in how many reviews each primary study was included.
Additional file 5: Appendix 5.
A detailed explanation of AMSTAR scoring for each item in each review.
Additional file 6: Appendix 6.
List of members and affiliates of International Network of Coronavirus Disease 2019 (InterNetCOVID-19).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Borges do Nascimento, I.J., O’Mathúna, D.P., von Groote, T.C. et al. Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews. BMC Infect Dis 21 , 525 (2021). https://doi.org/10.1186/s12879-021-06214-4
Download citation
Received : 12 April 2020
Accepted : 19 May 2021
Published : 04 June 2021
DOI : https://doi.org/10.1186/s12879-021-06214-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Coronavirus
- Evidence-based medicine
- Infectious diseases
BMC Infectious Diseases
ISSN: 1471-2334
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Iran J Med Sci
- v.45(4); 2020 Jul
A Narrative Review of COVID-19: The New Pandemic Disease
Kiana shirani, md.
1 Infectious Diseases and Tropical Medicine Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
Erfan Sheikhbahaei, MD
2 Student Research Committee, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
Zahra Torkpour, MD
Mazyar ghadiri nejad, phd.
3 Industrial Engineering Department, Girne American University, Kyrenia, TRNC, Turkey
Bahareh Kamyab Moghadas, PhD
4 Department of Chemical Engineering, Shiraz Branch, Islamic Azad University, Shiraz, Iran
Matina Ghasemi, PhD
5 Faculty of Business and Economics, Business Department, Girne American University, Kyrenia, TRNC, Turkey
Hossein Akbari Aghdam, MD
6 Department of Orthopedic Surgery, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
Athena Ehsani, PhD
7 Department of Biomedical Engineering, Science and Research Branch, Islamic Azad University, Tehran, Iran
Saeed Saber-Samandari, PhD
8 New Technologies Research Center, Amirkabir University of Technology, Tehran, Iran
Amirsalar Khandan, PhD
9 Department of Electrical Engineering, Isfahan (Khorasgan) Branch, Islamic Azad University, Isfahan, Iran
10 0Technology Incubator Center, Isfahan (Khorasgan) Branch, Islamic Azad University, Isfahan, Iran
Nearly every 100 years, humans collectively face a pandemic crisis. After the Spanish flu, now the world is in the grip of coronavirus disease 2019 (COVID-19). First detected in 2019 in the Chinese city of Wuhan, COVID-19 causes severe acute respiratory distress syndrome. Despite the initial evidence indicating a zoonotic origin, the contagion is now known to primarily spread from person to person through respiratory droplets. The precautionary measures recommended by the scientific community to halt the fast transmission of the disease failed to prevent this contagious disease from becoming a pandemic for a whole host of reasons. After an incubation period of about two days to two weeks, a spectrum of clinical manifestations can be seen in individuals afflicted by COVID-19: from an asymptomatic condition that can spread the virus in the environment, to a mild/moderate disease with cold/flu-like symptoms, to deteriorated conditions that need hospitalization and intensive care unit management, and then a fatal respiratory distress syndrome that becomes refractory to oxygenation. Several diagnostic modalities have been advocated and evaluated; however, in some cases, diagnosis is made on the clinical picture in order not to lose time. A consensus on what constitutes special treatment for COVID-19 has yet to emerge. Alongside conservative and supportive care, some potential drugs have been recommended and a considerable number of investigations are ongoing in this regard
What’s Known
- Substantial numbers of articles on COVID-19 have been published, yet there is controversy among clinicians and confusion among the general population in this regard. Furthermore, it is unreasonable to expect physicians to read all the available literature on this subject.
What’s New
- This article reviews high-quality articles on COVID-19 and effectively summarizes them for healthcare providers and the general population.
Introduction
A pathogen from a human-animal virus family, the coronavirus (CoV), which was identified as the main cause of respiratory tract infections, evolved to a novel and wild kind in Wuhan, a city in Hubei Province of China, and spread throughout the world, such that it created a pandemic crisis according to the World Health Organization (WHO). CoV is a large family of viruses that were first discovered in 1960. These viruses cause such diseases as common colds in humans and animals. Sometimes they attack the respiratory system, and sometimes their signs appear in the gastrointestinal tract. There have been different types of human CoV including CoV-229E, CoV-OC43, CoV-NL63, and CoV-HKU1, with the latter two having been discovered in 2004 and 2005, respectively. These types of CoV regularly cause respiratory infections in children and adults. 1 There are also other types of these viruses that are associated with more severe symptoms. The new CoV, scientifically known as “SARS-CoV-2”, causes severe acute respiratory syndrome (SARS). 2 A newer type of the virus was discovered in September 2012 in a 60-year-old man in Saudi Arabia who died of the disease; the man had traveled to Dubai a few days earlier. The second case was a 49-year-old man in Qatar who also passed away. The discovery was first confirmed at the Health Protection Agency’s Laboratory in Colindale, London. The outbreak of this CoV is known as the Middle East Respiratory Syndrome (MERS), commonly referred to as “MERS-CoV”. The virus has infected 2260 people and has killed 912, most of them in the Middle East. 3 - 5 Finally, in December 2019, for the first time in Wuhan, in Hubei Province of China, a new type of CoV was identified that caused pneumonia in humans. 6 SARS-CoV-2 has affected 5404512 people and killed more than 343514 around the world according to the WHO situation report-127 (May 26, 2020). 3 , 7 - 10 The WHO has officially termed the disease “COVID-19”, which refers to corona, the virus, the disease, the year 2019, and its etiology (SARS-CoV-2). This type of CoV had never been seen in humans before. The initial estimates showed a mortality rate ranging from between 1% and 3% in most countries to 5% in the worst-hit areas ( Figure 1 ). 9 Some Chinese researchers succeeded in determining how SARS-CoV-2 affects human cells, which could help to develop techniques of viral detection and had antiviral therapy potential. Via a process termed “cryogenic electron microscopy (cryo-EM)”, these scientists discovered that CoV enters human cells utilizing a kind of cell membrane glycoprotein: angiotensin-converting enzyme 2 (ACE2). Then, the S protein is split into two sub-units: S1 and S2. S1 keeps a receptor-binding domain (RBD); accordingly, SARS-CoV-2 can bind to the peptidase domain of ACE2 directly. It appears that S2 subsequently plays a role in cellular fusion. Chinese researchers used the cryo-EM technique to provide ACE2 when it is linked to an amino acid transporter called “B0AT1”. They also discovered how to connect SARS-CoV-2 to ACE2-B0AT1, which is another complex structure. Given that none of these molecular structures was previously known, the researchers hoped that these studies would lead to the development of an antiviral or vaccine that would help to prevent CoV. Along the way, scientists found that ACE2 has to undergo a molecular process in which it binds to another molecule to be activated. The resulting molecule can bind two SARS-CoV-2 protein molecules simultaneously. The scientists also studied different SARS-CoV-2 RBD binding methods compared with other SARS-CoV-RBDs, which showed how subtle changes in the molecular binding sequence make the coronal structure of the virus stronger.

Most cases with SARS-CoV-2 are asymptomatic or have mild clinical pictures such as influenza and colds. This group of patients should be detected and isolated in their homes to break the transmission chain of the disease and adhere to the precautionary recommendations in order not to infect other people. The screening process will help this group and suppress the outbreak in the community. Patients with the confirmed disease who are admitted to hospitals can contaminate this environment, which should be borne in mind by healthcare providers and policymakers.
Transmission
While the first mode of the transmission of COVID-19 to humans is still unknown, a seafood market where live animals were sold was identified as a potential source at the beginning of the outbreak in the epidemiologic investigations that found some infected patients who had visited or worked in that place. The other viruses in this family, namely MERS and SARS, were both confirmed to be zoonotic viruses. Afterward, the person-to-person spread was established as the main mode of transmission and the reason for the progression of the outbreak. 11 Similar to the influenza virus, SARS-CoV-2 spreads through the population via respiratory droplets. When an infected person coughs, sneezes, or talks, the respiratory secretions, which contain the virus, enter the environment as droplets. These droplets can reach the mucous membranes of individuals directly or indirectly when they touch an infected surface or any other source; the virus, thereafter, finds its ways to the eyes, nose, or mouth as the first incubation places. 11 - 15 It has been reported that droplets cannot travel more than two meters in the air, nor can they remain in the air owing to their high density. Nonetheless, given the other hitherto unknown modes of transmission, routine airborne transmission precautions should be considered in high-risk countries and during high-risk procedures such as manual ventilation with bags and masks, endotracheal intubation, open endotracheal suctioning, bronchoscopy, cardiopulmonary resuscitation, sputum induction, lung surgery, nebulizer therapy, noninvasive positive pressure ventilation (eg, bilevel positive airway pressure and continuous positive airway pressure ), and lung autopsy. In the early stages of the disease, the chances of the spread of the virus to other persons are high because the viral load in the body may be high despite the absence of any symptoms ( Figure 2 ). 11 - 13 The person-to-person transmission rates can be different depending on the location and the infection control intervention; still, according to the latest reports, the secondary COVID-19 infection rate ranges from 1% to 5%. 13 - 23 Although the RNA of the virus has been detected in blood and stool, fecal-oral and blood-borne transmissions are not regarded as significant modes of transmission yet. 19 - 26 There have been no reports of mother-to-fetus transmission in pregnant women. 27

SARS-CoV-2 mode of transmission and clinical manifestations are illustrated in this figure. The potential source of this outbreak was identified to be from animals, similar to MERS and SARS, in epidemiologic studies; nonetheless, person-to-person transmission through droplets is currently the important mode. After reaching mucous membranes by direct or indirect close contact, the virus replicates in the cells and the immune system attacks the body due to its nature. Afterward, the clinical pictures appear, which are much more similar to influenza. However, different patients will have a spectrum of signs and symptoms.
Source Investigation
Recently, the appearance of SARS-CoV-2 in society shocked the healthcare system. 28 - 32 Veterinary corona virologists reported that COVID-19 was isolated from wildlife. Several studies have shown that bats are receptors of the CoV new version in 2019 with variants and changes in the environment featuring various biological characteristics. 33 - 36 The aforementioned mammals are a major source of CoV, which causes mild-to-severe respiratory illness and can even be deadly. In recent years, the virus has killed several thousands of people of all ages. 37 - 39 The mutated alternative of the virus can be transmitted to humans and cause acute respiratory distress. 40 , 41 One of the main causes of the spread of the virus is the exotic and unusual Chinese food in Wuhan: CoV is a direct result of the Chinese food cycle. The virus is found in the body of animals such as bats, 42 and snake or bat soup is a favorite Chinese food. Therefore, this sequence is replicated continuously. Almost everyone who was infected for the first time was directly in the local Wuhan market or had indirectly tried snake or bat soup in a Chinese restaurant. An investigation stated that the Malayan pangolin (Manis javanica) was a possible host for SARS-CoV-2 and recommended that it be removed from the wet market to prevent zoonotic transmissions in the future. 43 , 44
Pathogenesis
The important mechanisms of the severe pathogenesis of SARS-CoV-2 are not fully understood. Extensive lung injury in SARS-CoV-2 has been related to increased virus titers; monocyte, macrophage, and neutrophil infiltrations into the lungs; and elevated levels of pro-inflammatory cytokines and chemokines. Thus, the clinical exacerbation of SARS-CoV-2 infection may be in consequence of a combination of direct virus-induced cytopathic and immunopathological effects due to excessive cytokinesis. Changes in the cytokine/chemokine profile during SARS infection showed increased levels of circulating cytokines such as tumor necrosis factor-α (TNF-α), C–X–C motif chemokine 10 (CXCL10), interleukin (IL)-6, and IL-8 levels, in conjunction with elevated levels of serum pro-inflammatory cytokines such as IL-1, IL-6, IL-12, interferon-gamma (IFN-γ), and transforming growth factor-β (TGF-β). Nevertheless, constant stimulation by the virus creates a cytokine storm that has been related to acute respiratory distress syndrome (ARDS) and multiple organ dysfunction syndromes (MODS) in patients with COVID-19, which may ultimately lead to diminished immunity by lowering the number of CD4+ and CD8+ T cells and natural killer cells (crucial in antiviral immunity) and decreasing cytokine production and functional ability (exhaustion). It has been shown that IL-10, an inhibitory cytokine, is a major player and a potential target for therapeutic aims. 45 - 51 Severe cases of COVID-19 have respiratory distress and failure, which has been linked to the altered metabolism of heme by SARS-CoV-2. Some virus proteins can dissociate iron from porphyrins by attacking the 1-β chain of hemoglobin, which decreases the oxygen-transferring ability of hemoglobin. Research has also indicated that chloroquine and favipiravir might inhibit this process. 52
Clinical Manifestations
SARS-CoV-2, which attacks the respiratory system, has a spectrum of manifestations; nonetheless, it has three main primary symptoms after an incubation period of about two days to two weeks: fever and its associated symptoms such as malaise/fatigue/weakness; cough, which is nonproductive in most of the cases but can be productive indeed; and shortness of breath (dyspnea) due to low blood oxygenation. Although these symptoms appear in the body of the affected person over two to 14 days, patients may refer to the clinic with gastrointestinal symptoms (nausea/vomiting-diarrhea) or decreased sense of smell and/or taste. More devastatingly, however, patients may refer to the emergency room with such coagulopathies as pulmonary thromboembolism, cerebral venous thrombosis, and other related manifestations. The WHO has stated that dry throat and dry cough are other symptoms detected in the early stages of the infection. 53 , 54 The estimations of the severity of the disease are as follows: mild (no or mild pneumonia) in 81%, severe (eg, with dyspnea, hypoxia, or >50% lung involvement on imaging within 24 to 48 hours) in 14%, and critical (eg, with respiratory failure, shock, or multiorgan dysfunction) in 5%. In the early stages, the overall mortality rate was 2.3% and no deaths were observed in non-severe patients. Patients with advanced age or underlying medical comorbidities have more mortality and morbidity. 55 Although adults of middle age and older are most commonly affected by SARS-CoV-2, individuals at any age can be infected. A few studies have reported symptomatic infection in children; still, when it occurs, it has mild symptoms. The vast majority of cases have the infection with no signs and symptoms or mild clinical pictures; they are called “the asymptomatic group”. These patients do not seek medical care and if they come into close contact with others, they can spread the virus. Therefore, quarantine in their home is the best option for the population to break the transmission of the virus. It should be considered that some of these asymptomatic patients have clinical signs such as chest computed tomography scan (CT-Scan) infiltrations. Similar to bacterial pneumonia, lower respiratory signs and symptoms are the most frequent manifestations in serious cases of COVID-19, characterized by fever, cough, dyspnea, and bilateral infiltrates on chest imaging. In a study describing pneumonia in Wuhan, the most common clinical signs and symptoms at the onset of the illness were fever in 99% (although fever might not be a universal finding), fatigue in 70%, dry cough in 59%, anorexia in 40%, myalgia in 35%, dyspnea in 31%, and sputum production in 27%. Headache, sore throat, and rhinorrhea are less common, and gastrointestinal symptoms (eg, nausea and diarrhea) are relatively rare. 7 , 42 , 43 , 45 - 48 , 56 , 57 According to our clinical experience in Iran, anosmia, atypical chest pain, diarrhea, nausea/vomiting, and hemoptysis are other presenting symptoms in the clinic. It should be noted that COVID-19 has some unexplained potential complications such as secondary bacterial infections, myocarditis, central nervous system injury, cerebral edema, MODS, acute demyelinating encephalomyelitis (ADEM), kidney injury, liver injury, new-onset seizure, coagulopathy, and arrhythmias.
Laboratory data : Complete blood counts, which constitute a routine laboratory test, have shown different results in terms of the white blood cell count: from leukopenia and lymphopenia to leukocytosis, although lymphopenia appears to be the most common. Fatal cases have exhibited severe lymphopenia accompanied by an increased level of D-dimer. Liver function enzymes can be increased; however, it is not sufficient to diagnose a disease. The serum procalcitonin level is a marker of infection, especially in bacterial diseases. Patients with COVID-19 who require intensive care unit (ICU) management may have elevated procalcitonin. Increased urea and creatinine, creatinine-phosphokinase, lactate dehydrogenase, and C-reactive protein are other findings in some cases. 7 , 56 , 57
Imaging studies : Routine chest X-ray (CXR) is widely deemed the first-step management to evaluate any respiratory involvement. Although negative findings in CXR do not rule out the viral disease, patients without common findings do not have severe disease and can, consequently, be managed in the outpatient setting. 58 , 59 Another modality is chest CT-Scan. It can be ordered in suspected cases with typical symptoms at the first step, or it can be performed after the detection of any abnormalities in CXR. The most common demonstrations in CT-Scan images are ground-glass opacification, round opacities, and crazy paving with or without bilateral consolidative abnormalities (multilobar involvement) in contrast to most cases of bacterial pneumonia, which have locally limited involvement. Pleural thickening, pleural effusion, and lymphadenopathy are less common. 58 - 61 Tree-in-bud, peribronchial distribution, nodules, and cavity are not in favor of common COVID-19 findings. Although reverse transcriptase-polymerase chain reaction (RT-PCR) is used to confirm the diagnosis, it is a time-consuming procedure and has high false-negative/false-positive findings; hence, in the emergency clinical setting, CT-Scan findings can be a good approach to make the diagnosis. It is deserving of note, however, that false-positive/false-negative cases were reported by one study to be high and other differential diagnoses should be in mind in order not to miss any other cases such as acute pulmonary edema in patients with heart disease.
Suspected cases should be diagnosed as soon as possible to isolate and control the infection immediately. COVID-19 should be considered in any patient with fever and/or lower respiratory tract symptoms with any of the following risk factors in the previous 2 weeks: close contact with confirmed or suspected cases in any environment, especially at work in healthcare places without sufficient protective equipment or long-time standing in those places, and living in or traveling from well-known places where the disease is an epidemic. 61 - 66 Patients with severe lower respiratory tract disease without alternative etiologies and a clear history of exposure should be considered having COVID-19 unless confirmed otherwise. According to the Centers for Disease Control and Prevention (CDC), sending tests to check SARS-CoV-2 in suspected cases is based on physicians’ clinical judgment. Although there are some positive cases without clinical manifestations (ie, fever and/or symptoms of acute respiratory illness such as cough and dyspnea), infectious disease and control centers should take action in society to limit the exposure of such patients to other healthy individuals. The CDC prioritizes the use of the specific test for hospitalized patients, symptomatic patients who are at risk of fatal conditions (eg, age ≥65 y, chronic medical conditions, and immunocompromising conditions) and those who have exposure risks (recent travel, contact with patients with COVID-19, and healthcare workers). 61 - 66 Although treatment should be started after the confirmation of the disease, RT-PCR for highly suspected cases is a time-consuming test; accordingly, a considerable number of clinicians favor the use of a combination of clinical manifestations with imaging modalities (eg, CT-Scan findings) and their clinical judgment regarding the probability of the disease in order not to lose more time. 61 - 66
Treatment of COVID-19
There is no confirmed recommended treatment or vaccine for SARS-CoV-2; prevention is, therefore, better than treatment. Nevertheless, the high contagiousness of COVID-19, combined with the fact that some individuals fail to adhere to precautionary measures or they have significant risk factors, means that this infectious disease is inevitable in some people. Beside supportive treatments, many types of medications have been introduced. These medications come from previous experimental studies on SARS, MERS, influenza, or human immunodeficiency virus (HIV); hence, their efficacy needs further experimental and clinical approval. Patients with mild symptoms who do not have significant risk factors should be managed in their home like a self-made quarantine (in an isolated room); still, prompt hospital admission is required if patients exhibit signs of disease deterioration. 25 , 67 , 68 Isolation from other family members is an important prevention tip. Patients should wear face masks, eat healthy and warm foods similar to when struggling with influenza or colds, do the handwashing process, dispose of the contaminated materials cautiously, and disinfect suspicious surfaces with standard disinfectants. 69 Patients with severe symptoms or admission criteria should be hospitalized with other patients who have the same disease in an isolated department. When the disease is progressed, ICU care is mandatory. 25 , 67 , 68 SARS-CoV-2 attacks the respiratory system, diminishing the oxygenation process and forcing patients with low blood oxygen saturation to take extra oxygen from different modalities. Nasal cannulae, face masks with or without a reservoir, intubation in severe cases, and then extracorporeal membrane oxygenation in refractory hypoxia have been used; however, the safety and efficacy of these measures should be evaluated. As was mentioned above, impaired coagulation is one of the major complications of the disease; consequently, alongside all recommended supportive care and drugs, anticoagulants such as heparin should be administered prophylactically ( Table 1 ). Although it is said that all the clinical signs and symptoms of COVID-19 are induced by the immune system, as other research on influenza and MERS has revealed, glucocorticoids are not recommended in COVID-19 pneumonia unless other indications are present (eg, exacerbation of chronic obstructive pulmonary disease and refractory septic shock) due to the high risk of mortality and delayed viral clearance. Earlier in the national and international guidelines, nonsteroidal anti-inflammatory drugs such as naproxen were recommended on the strength of their antipyretic and anti-inflammatory components; however, the guideline has been revised recently and acetaminophen with or without codeine is currently the favored drug in patients with COVID-19. 25 , 67 , 68 According to the pathogenesis of the disease, whereby cytokine storm and immune-cell exhaustion can be seen in severe cases, selective antibodies against harmful interleukins such as IL-6 and IL-10 or other possible agents can be therapeutic for fatal complications. Tocilizumab, an IL-6 inhibitor, albeit with limited clinical efficacy, has been introduced in China’s National Health Commission treatment guideline for severe infection with profound pulmonary involvement (ie, white lung). 70 , 87
Summary of possible anti-COVID-19 drugs
mg, Milligrams; BD, Every 12 hours; RdRP, RNA-dependent RNA polymerase; TDS, Every 8 hours; IV, Intravenous; IL, Interleukin; μg, Micrograms
RNA synthesis inhibitors (eg, tenofovir disoproxil fumarate and 2’-deoxy-3’-thiacytidine [3TC]), neuraminidase inhibitors (NAIs), nucleoside analogs, lopinavir/ritonavir, atazanavir, remdesivir, favipiravir, INF-β, and Chinese traditional medicine (eg, Shufeng Jiedu and Lianhuaqingwen capsules) are the major candidates for COVID-19. 26 , 70 , 85 , 88 - 96 Antiviral drugs have been investigated for various diseases, but their efficacy in the treatment of COVID-19 is under investigation and several randomized clinical trials are ongoing to release a consensus result on the treatment of this infectious disease. Moderate-to-severe SARS-CoV-2 disease needs drug therapy. Favipiravir, a previously validated drug for influenza, is a drug that has shown promising results for COVID-19 in experimental and clinical studies, but it is under further evaluation. 70 , 79 , 80 Remdesivir, which was developed for Ebola, is an antiviral drug that is under evaluation for moderate-to-severe COVID-19 owing to its promising results in in vitro investigations. 70 , 73 - 75 , 81 Remdesivir was shown to have reduced the virus titer in infected mice with MERS-CoV and improved lung tissue damage with more efficiency compared with a group treated with lopinavir/ritonavir/INF-β. 67 , 70 Another investigation studied the potential efficacy of INF-β-1 in the early stages of COVID-19 as a potential antiviral drug. 86 Although there is some hope, an evidence-based consensus requires further clinical trials. 70 , 77 A combined protease inhibitor, lopinavir/ritonavir, is used for HIV infection and has shown interesting results for SARS and MERS in in vitro studies. 73 - 75 The clinical effectiveness of lopinavir/ritonavir for SARS-CoV-2 was also reported in a case report. 70 , 71 , 74 , 76 Atazanavir, another protease inhibitor, with or without ritonavir is another possible anti-COVID-19 treatment. 77 , 78 NAIs, including oseltamivir, zanamivir, and peramivir, are recommended as antiviral treatment in influenza. 68 Oral oseltamivir was tried for COVID-19 in China and was first recommended in the Iranian guideline for COVID-19 treatment; nevertheless, because of the absence of strong evidence indicating its efficacy for SARS-CoV-2, it was eliminated from the subsequent updates of the guideline. 85 RNA-dependent RNA polymerase inhibitors with anti-hepatitis C effects such as ribavirin have shown satisfactory results against SARS-CoV-2 RNA polymerase; however, they have limited clinical approval. 82 - 84 The well-known drugs for rheumatoid arthritis, systemic lupus erythematosus, and an antimalarial drug, chloroquine 71 and hydroxychloroquine 21 are other potential drugs for moderate-to-severe COVID-19 but with limited or no clinical appraisal. Hydroxychloroquine has exhibited better safety and fewer side effects than chloroquine, which makes it the preferred choice. 70 Furthermore, the immunomodulatory effects of hydroxychloroquine can be used to control the cytokine precipitation in the late phases of SARS-CoV-2 infections. There are numerous mechanisms for the antiviral activity of hydroxychloroquine. A weak base drug, hydroxychloroquine concentrates on such intracellular sections as endosomes and lysosomes, thereby halting viral replication in the phase of fusion and uncoating. Additionally, this immunosuppressive and antiparasitic drug is capable of altering the glycosylation of ACE2 and inhibiting both S-protein binding and phagocytosis. 72 A recent multicenter study showed that regarding the risks of cardiovascular adverse effects and mortality rates, hydroxychloroquine or chloroquine with or without a macrolide (eg, azithromycin) was not beneficial for hospitalized patients, although further research is needed to end such controversies. 97
Disease Duration
It is not easy to quarantine the patients who have fully recovered because there is evidence that they are highly infectious. 81 The recovery time for confirmed cases based on the National Health Commission reports of China’s government was estimated to range between 18 and 22 days. 73 As indicated by the WHO, the healing time seems to be around two weeks for moderate infections and 3 to 6 weeks for the severe/ serious disease. 75 Pan Feng and others studied 21 confirmed cases with COVID-19 pneumonia with about 82 CT-Scan images with a mean interval of four days. Lung abnormalities on chest CT showed the highest severity approximately 10 days after the initial onset of symptoms. All patients became clear after 11 to 26 days of hospitalization. From day zero to day 26, four stages of lung CT were defined as follows: Stage 1 (first 4 days): ground-glass opacities; Stage 2 (second 4 days): crazy-paving patterns; Stage 3 (days 9–13): maximum total CT scores in the consolidations; and Stage 4 (≥14 d): steady improvements in the consolidations with a reduction in the total CT score without any crazy-paving pattern. 74 Nevertheless, there are also rare cases reported from some studies that show the recurrence of COVID-19 after negative preliminary RT-PCR results. For example, Lan and othersstudied one hospitalized and three home-quarantined patients with COVID-19 and evaluated them with RT-PCR tests of the nucleic acid. All the patients with positive RT-PCR test results had CT imaging with ground-glass opacification or mixed ground-glass opacification and consolidation with mild-to-moderate disease. After antiviral treatments, all four patients had two consecutive negative RT-PCR test results within 12 to 32 days. Five to 13 days after hospital discharge or the discontinuation of the quarantine, RT-PCR tests were repeated, and all were positive. An additional RT-PCR test was performed using a kit from a different manufacturer, and the results were also positive. Their findings propose that a minimum percentage of recovered patients may still be infection carriers. 76
Supplements for COVID-19
Since the appearance of SARS-CoV-2 in Wuhan, China, there have been reports of the unreliable and unpredictable use of mysterious therapies. Some recommendations such as the use of certain herbs and extracts including oregano oil, mulberry leaf, garlic, and black sesame may be safe as long as people do not utilize their hands for instance. 98 According to data released by the CDC, vitamin C (VitC) supplements can decrease the risk of colds in people besides preventing CoV from spreading. The aforementioned organization states that frequent consumption of VitC supplements can also decrease the duration of the cold; however, if used only after the cold has risen, its consumption does not influence the disease course. VitC also plays an important role in the body. One of the main reasons for taking VitC is to strengthen the immune system because this vitamin plays a significant part in the immune system. Firstly, VitC can increase the production of white blood cells (lymphocytes and phagocytes) in the bone marrow, which can support and protect the body against infections. Secondly, VitC helps immune cells to function better while preserving white blood cells from damaging molecules such as free oxidative radicals and ions. Thirdly, VitC is an essential part of the skin’s immune system. This vitamin is actively transported to the skin surface, where it serves as an antioxidant and helps to strengthen the skin barrier by optimizing the collagen synthesis process. Patients with pneumonia have lower levels of VitC and have been revealed to have a longer recovery time. 69 , 99 In a randomized investigation, 200 mg/d of VitC was applied to older patients and resulted in improvements in the respiratory symptoms. Another investigation reported 80% fewer mortalities in a controlled group of VitC takers. 73 However, for effective immune system improvement, VitC should be consumed alongside adequate doses of several other supplements. Although VitC plays an important role in the body, often a balanced diet and the consumption of fresh fruits and vegetables can quickly fill the blanks. While taking high amounts of VitC is less risky because it is water-soluble and its waste is eliminated in the urine, it can induce diarrhea, nausea, and abdominal spasms at higher concentrations. Too much VitC may cause calcium-oxalate kidney stones. People with genetic hemochromatosis, an iron deficiency disorder, should consult a physician before taking any VitC supplements as high levels of VitC can lead to tissue damage. Some studies have evaluated the different doses of oral or intravenous VitC for patients admitted to the hospital for COVID-19. Although they used different regimens, all of them demonstrated satisfactory results regarding the resolution of the compilations of the disease, decreased mortality, and shortened lengths of stay in the ICU and/or the hospital. 100 , 101 Immunologists have also recommended 6 000 units of vitamin A (VitA) per day for two weeks, more than twice the recommended limit for VitA, which can create a poisoning environment over time. According to the guidance of the National Institutes of Health (NIH), middle-aged men and women should take 1 and 2 mg of VitA every day, respectively. The safe upper limit of this vitamin is 6000 mg or 5000 units, and overdose can have serious outcomes such as dizziness, nausea, headache, coma, and even death. Extreme consumption of VitA throughout pregnancy can lead to birth anomalies.
Similar to VitC, vitamin D (VitD) has antioxidant, anti-inflammatory, and immune-modulatory effects in our body such as reducing pro-inflammatory cytokines and inhibiting viral replication according to experimental studies. 83 The VitD state of our body is checked through 25 (OH) VitD in the serum. VitD deficiency is pandemic around the world due to multifactorial reasons. It has been shown that VitD deficient patients are prone to SARS-CoV-2 and, accordingly, treating VitD deficiency is not without benefits. Grant and others recommended 10 000 units per day for two weeks and then 5 000 units per day as the maintenance dose to keep the level between 40 and 100 ng/mL. 102 VitD toxicity causes gastrointestinal discomfort (dyspepsia), congestion, hypercalcemia, confusion, positional disorders, dysrhythmia, and kidney dysfunction.
James Robb, 103 a researcher who detected CoV for the first time as a consultant pathologist with the National Cancer Institute of America, suggested the influence of zinc consumption. Oral zinc supplements can be dissolved in the nback of the throat. Short-term therapy with oral zinc can decrease the duration of viral colds in adults. Zinc intake is also associated with the faster resolution of nasal congestion, nasal drainage, sore throats, and coughs. Researchers 104 , 105 have warned that the consumption of more than 1 mg of zinc a day can lead to zinc poisoning and have side effects such as lowered immune function. Children and old people with zinc insufficiency in developing nations are extremely vulnerable to pneumonia and other viral infections. It has also been determined that zinc has a major role in the production and activation of T-cell lymphocytes. 106 , 107
And finally, for high-risk people or those who work in high-risk places such as healthcare providers, hydroxychloroquine has been mentioned to be effective as a prophylactic regimen ( Table 2 ). Although different doses have been investigated so far, Pourdowlat and others recommended 200 mg daily before exposure, and for the post-exposure scenario, a loading dose of 600-800 mg followed by a maintenance dose of 200 mg daily. 74
Possible prophylactic regimens against SARS-CoV-2 infection
IU, International unit; mg, Milligrams; kg, Kilograms; ICU, Intensive care unit; g, Grams; IV, Intravenous; Vit, Vitamin; ng, Nanograms; mL, Milliliter
COVID-19 Kits and Deep Learning
COVID-19 has threatened public health, and its fast global spread has caught the scientific community by surprise. 108 Hence, developing a technique capable of swiftly and reliably detecting the virus in patients is vital to prevent the spreading of the virus. 109 , 110 One of the ways to diagnose this new virus is through RT-PCR, a test that has previously demonstrated its efficacy in detecting such CoV infections as MERS-CoV and SARS-CoV. Consequently, increasing the availability of RT-PCR kits is a worldwide concern. The timing of the RT-PCR test and the type of strain collected are of vital importance in the diagnosis of COVID-19. One of the characteristics of this new virus is that the serum is negative in the early stage, while respiratory specimens are positive. The level of the virus at the early stage of the illness is also high, even though the infected individual experiences mild symptoms. 111 For the management of the emerging situation of COVID-19 in Wuhan, various effective diagnostic kits were urgently made available to markets. While a few different diagnostics kits are used merely for research endeavors, only a single kit developed by the Beijing Genome Institute (BGI) called “Real-Time Fluorescent PCR” has been authenticated for clinical diagnostics. Fluorescent RT-PCR is reliable and able to offer fast results probably within a few hours (usually within two hours). Besides RT-PCR, China has successfully developed a metagenomic-sequencing kit based on combinatorial probe-anchor synthesis that can identify virus-related bacteria, allowing observation and evaluation during the transmission of the virus. Furthermore, the metagenomic-sequencing kit based on combinatorial probe-anchor synthesis is far faster than the abovementioned fluorescent RT-PCR kit. Apart from China, a Singapore-based laboratory, Veredus, developed a virus detection kit (Vere-CoV) in late January. It is a portable Lab-On-Chip used to detect MERS-CoV, SARS-CoV, and SARS-CoV-2, in a single examination. This kit works based on the VereChip™ technology, the lines of code (LOC) program incorporating two different influential molecular biological functions (microarray and PCR) precisely. Several studies have focused on SARS-CoV diagnostic testing. These papers have presented investigative approaches to the identification of the virus using molecular testing (ie, RT-PCR). Researchers probed into the use of a nested PCR technique that contains a pre-amplification step or integrating the N gene as an extra subtle molecular marker to improve on the sensitivity. 112 - 115 CT-Scan is very useful for diagnosing, evaluating, and screening infections caused by COVID-19. One recommendation for scanning the disease is to take a scan every three to five days. According to researchers, most CT-Scan images from patients with COVID-19 are bilateral or peripheral ground-glass opacification, with or without stabilization. Nowadays, because of a paucity of computerized quantification tools, only qualitative reports and sometimes inaccurate analyses of contaminated areas are drawn upon in radiology reports. A categorization system based on the deep learning approach was proposed by a study to automatically measure infected parts and their volumetric ratios in the lung. The functionality of this system was evaluated by making some comparisons between the infected portions and the manually-delineated ones on the CT-Scan images of 300 patients with COVID-19. To increase the manual drawing of training samples and the non-interference in the automated results, researchers adopted a human-based approach in collaboration with radiologists so as to segment the infected region. This approach shortens the time to about four minutes after 3-time updating. The mean Dice similarity coefficient illustrated that the automatically detected infected parts were 91.6% similar to the manually detected ones, and the average of the percentage estimated error was 0.3% for the whole lung. 116 , 117
Prevention Considerations
In the healthcare setting, any individual with the manifestations of COVID-19 (eg, fever, cough, and dyspnea) should wear a face mask, have a separate waiting area, and keep the distance of at least two meters. Symptomatic patients should be asked about recent travel or close contact with a patient in the preceding two weeks to find other possible infected patients. The CDC and WHO have announced special precautions for healthcare providers in the hospital and during different procedures. Wearing tight-fitting face masks with special filters and impermeable face shields is necessary for all of them. 11 , 18 , 65 , 66 , 76 , 118 - 124 Other people should pay attention to the CDC and WHO preventive strategies, which recommend that individuals not touch their eyes, mouth, and nose before washing or disinfecting their hands; wash their hands regularly according to the standard protocol; use effective disinfection solutions (ie, containing at least 60% ethylic alcohol) for contaminated surfaces; cover their mouth when coughing and sneezing; avoid waiting or walking in crowded areas, and observe isolation protocols in their home. Postponing elective work and decreasing non-urgent visits and traveling to areas in the grip of COVID-19 may be useful to lessen the risk of exposure. If suspected individuals with mild symptoms are managed in outpatient settings, an isolated room with minimal exposure to others should be designed. Patients and their caregivers should wear tight-fitting face masks. 11 , 18 , 65 , 66 , 76 , 118 - 124 Substantial numbers of individuals with COVID-19 are asymptomatic with potential exposure; accordingly, a screening tool should be employed to evaluate these cases. In addition to passport checks, corona checks have been incorporated into the protocols in airports and other crowded places. The use of a remote thermometer to measure body temperature leads to an increase in the number of false-negative cases. It is, thus, essential that everyone pay sufficient heed to the WHO and CDC recommendations in their daily life. Traveling is not prohibited, but it should be restricted and passengers from any country should be monitored. 11 , 18 , 65 , 66 , 76 , 118 - 124
SARS-CoV-2 is the new highly contagious CoV, which was first reported in China. While it had a zoonotic origin in the beginning, it subsequently spread throughout the world by human contact. COVID-19 has a spectrum of manifestations, which is not lethal most of the time. To diagnose this condition, physicians can avail themselves of laboratory and imaging findings besides signs and symptoms. RT-PCR is the gold standard, but it lacks sufficient sensitivity and specificity. Although there are some potential drugs for COVID-19 and some vitamins or minerals for prophylaxis, the best preventive strategies are quarantine (staying at home) and the use of personal protective equipment and disinfectants.
Acknowledgement
The authors express their gratitude toward the Supporting Organizations for Foreign Iranian Students, Islamic Azad University Isfahan (Khorasgan) Branch, and Isfahan University of Medical Sciences.
Conflict of Interest: None declared.
Writing about COVID-19 in a college admission essay
by: Venkates Swaminathan | Updated: September 14, 2020
Print article

For students applying to college using the CommonApp, there are several different places where students and counselors can address the pandemic’s impact. The different sections have differing goals. You must understand how to use each section for its appropriate use.
The CommonApp COVID-19 question
First, the CommonApp this year has an additional question specifically about COVID-19 :
Community disruptions such as COVID-19 and natural disasters can have deep and long-lasting impacts. If you need it, this space is yours to describe those impacts. Colleges care about the effects on your health and well-being, safety, family circumstances, future plans, and education, including access to reliable technology and quiet study spaces. Please use this space to describe how these events have impacted you.
This question seeks to understand the adversity that students may have had to face due to the pandemic, the move to online education, or the shelter-in-place rules. You don’t have to answer this question if the impact on you wasn’t particularly severe. Some examples of things students should discuss include:
- The student or a family member had COVID-19 or suffered other illnesses due to confinement during the pandemic.
- The candidate had to deal with personal or family issues, such as abusive living situations or other safety concerns
- The student suffered from a lack of internet access and other online learning challenges.
- Students who dealt with problems registering for or taking standardized tests and AP exams.
Jeff Schiffman of the Tulane University admissions office has a blog about this section. He recommends students ask themselves several questions as they go about answering this section:
- Are my experiences different from others’?
- Are there noticeable changes on my transcript?
- Am I aware of my privilege?
- Am I specific? Am I explaining rather than complaining?
- Is this information being included elsewhere on my application?
If you do answer this section, be brief and to-the-point.
Counselor recommendations and school profiles
Second, counselors will, in their counselor forms and school profiles on the CommonApp, address how the school handled the pandemic and how it might have affected students, specifically as it relates to:
- Grading scales and policies
- Graduation requirements
- Instructional methods
- Schedules and course offerings
- Testing requirements
- Your academic calendar
- Other extenuating circumstances
Students don’t have to mention these matters in their application unless something unusual happened.
Writing about COVID-19 in your main essay
Write about your experiences during the pandemic in your main college essay if your experience is personal, relevant, and the most important thing to discuss in your college admission essay. That you had to stay home and study online isn’t sufficient, as millions of other students faced the same situation. But sometimes, it can be appropriate and helpful to write about something related to the pandemic in your essay. For example:
- One student developed a website for a local comic book store. The store might not have survived without the ability for people to order comic books online. The student had a long-standing relationship with the store, and it was an institution that created a community for students who otherwise felt left out.
- One student started a YouTube channel to help other students with academic subjects he was very familiar with and began tutoring others.
- Some students used their extra time that was the result of the stay-at-home orders to take online courses pursuing topics they are genuinely interested in or developing new interests, like a foreign language or music.
Experiences like this can be good topics for the CommonApp essay as long as they reflect something genuinely important about the student. For many students whose lives have been shaped by this pandemic, it can be a critical part of their college application.
Want more? Read 6 ways to improve a college essay , What the &%$! should I write about in my college essay , and Just how important is a college admissions essay? .
Homes Nearby
Homes for rent and sale near schools

How our schools are (and aren't) addressing race

The truth about homework in America

What should I write my college essay about?
What the #%@!& should I write about in my college essay?
Yes! Sign me up for updates relevant to my child's grade.
Please enter a valid email address
Thank you for signing up!
Server Issue: Please try again later. Sorry for the inconvenience
MINI REVIEW article
Covid-19: emergence, spread, possible treatments, and global burden.

- 1 Department of Pharmacology, Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education, Manipal, India
- 2 Department of Health Sciences, School of Education and Health, Cape Breton University, Sydney, NS, Canada
The Coronavirus (CoV) is a large family of viruses known to cause illnesses ranging from the common cold to acute respiratory tract infection. The severity of the infection may be visible as pneumonia, acute respiratory syndrome, and even death. Until the outbreak of SARS, this group of viruses was greatly overlooked. However, since the SARS and MERS outbreaks, these viruses have been studied in greater detail, propelling the vaccine research. On December 31, 2019, mysterious cases of pneumonia were detected in the city of Wuhan in China's Hubei Province. On January 7, 2020, the causative agent was identified as a new coronavirus (2019-nCoV), and the disease was later named as COVID-19 by the WHO. The virus spread extensively in the Wuhan region of China and has gained entry to over 210 countries and territories. Though experts suspected that the virus is transmitted from animals to humans, there are mixed reports on the origin of the virus. There are no treatment options available for the virus as such, limited to the use of anti-HIV drugs and/or other antivirals such as Remdesivir and Galidesivir. For the containment of the virus, it is recommended to quarantine the infected and to follow good hygiene practices. The virus has had a significant socio-economic impact globally. Economically, China is likely to experience a greater setback than other countries from the pandemic due to added trade war pressure, which have been discussed in this paper.
Introduction
Coronaviridae is a family of viruses with a positive-sense RNA that possess an outer viral coat. When looked at with the help of an electron microscope, there appears to be a unique corona around it. This family of viruses mainly cause respiratory diseases in humans, in the forms of common cold or pneumonia as well as respiratory infections. These viruses can infect animals as well ( 1 , 2 ). Up until the year 2003, coronavirus (CoV) had attracted limited interest from researchers. However, after the SARS (severe acute respiratory syndrome) outbreak caused by the SARS-CoV, the coronavirus was looked at with renewed interest ( 3 , 4 ). This also happened to be the first epidemic of the 21st century originating in the Guangdong province of China. Almost 10 years later, there was a MERS (Middle East respiratory syndrome) outbreak in 2012, which was caused by the MERS-CoV ( 5 , 6 ). Both SARS and MERS have a zoonotic origin and originated from bats. A unique feature of these viruses is the ability to mutate rapidly and adapt to a new host. The zoonotic origin of these viruses allows them to jump from host to host. Coronaviruses are known to use the angiotensin-converting enzyme-2 (ACE-2) receptor or the dipeptidyl peptidase IV (DPP-4) protein to gain entry into cells for replication ( 7 – 10 ).
In December 2019, almost seven years after the MERS 2012 outbreak, a novel Coronavirus (2019-nCoV) surfaced in Wuhan in the Hubei region of China. The outbreak rapidly grew and spread to neighboring countries. However, rapid communication of information and the increasing scale of events led to quick quarantine and screening of travelers, thus containing the spread of the infection. The major part of the infection was restricted to China, and a second cluster was found on a cruise ship called the Diamond Princess docked in Japan ( 11 , 12 ).
The new virus was identified to be a novel Coronavirus and was thus initially named 2019-nCoV; later, it was renamed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) ( 13 ), and the disease it causes is now referred to as Coronavirus Disease-2019 (COVID-19) by the WHO. The virus was suspected to have begun its spread in the Huanan seafood wholesale market in the Wuhan region. It is possible that an animal that was carrying the virus was brought into or sold in the market, causing the spread of the virus in the crowded marketplace. One of the first claims made was in an article published in the Journal of Medical Virology ( 14 ), which identified snakes as the possible host. A second possibility was that pangolins could be the wild host of SARS-CoV-2 ( 15 ), though the most likely possibility is that the virus originated from bats ( 13 , 16 – 19 ). Increasing evidence and experts are now collectively concluding the virus had a natural origin in bats, as with previous such respiratory viruses ( 2 , 20 – 24 ).
Similarly, SARS and MERS were also suspected to originate from bats. In the case of MERS, the dromedary camel is an intermediate host ( 5 , 10 ). Bats have been known to harbor coronaviruses for quite some time now. Just as in the case of avian flu, SARS, MERS, and possibly even HIV, with increasing selection and ecological pressure due to human activities, the virus made the jump from animal to man. Humans have been encroaching increasingly into forests, and this is true over much of China, as in Africa. Combined with additional ecological pressure due to climate change, such zoonotic spillovers are now more common than ever. It is likely that the next disease X will also have such an origin ( 25 ). We have learned the importance of identification of the source organism due to the Ebola virus pandemic. Viruses are unstable organisms genetically, constantly mutating by genetic shift or drift. It is not possible to predict when a cross-species jump may occur and when a seemingly harmless variant form of the virus may turn into a deadly strain. Such an incident occurred in Reston, USA, with the Reston virus ( 26 ), an alarming reminder of this possibility. The identification of the original host helps us to contain future spreads as well as to learn about the mechanism of transmission of viruses. Until the virus is isolated from a wild animal host, in this case, mostly bats, the zoonotic origin will remain hypothetical, though likely. It should further be noted that the virus has acquired several mutations, as noted by a group in China, indicating that there are more than two strains of the virus, which may have had an impact on its pathogenicity. However, this claim remains unproven, and many experts have argued otherwise; data proving this are not yet available ( 27 ). A similar finding was reported from Italy and India independently, where they found two strains ( 28 , 29 ). These findings need to be further cross-verified by similar analyses globally. If true, this finding could effectively explain why some nations are more affected than others.
Transmission
When the spread of COVID-19 began ( Figure 1 ), the virus appeared to be contained within China and the cruise ship “Diamond Princess,” which formed the major clusters of the virus. However, as of April 2020, over 210 countries and territories are affected by the virus, with Europe, the USA, and Iran forming the new cluster of the virus. The USA ( Figure 2 ) has the highest number of confirmed COVID-19 cases, whereas India and China, despite being among the most population-dense countries in the world, have managed to constrain the infection rate by the implementation of a complete lockdown with arrangements in place to manage the confirmed cases. Similarly, the UK has also managed to maintain a low curve of the graph by implementing similar measures, though it was not strictly enforced. Reports have indicated that the presence of different strains or strands of the virus may have had an effect on the management of the infection rate of the virus ( 27 – 29 ). The disease is spread by droplet transmission. As of April 2020, the total number of infected individuals stands at around 3 million, with ~200,000 deaths and more than 1 million recoveries globally ( 30 , 34 ). The virus thus has a fatality rate of around 2% and an R 0 of 3 based on current data. However, a more recent report from the CDC, Atlanta, USA, claims that the R 0 could be as high as 5.7 ( 35 ). It has also been observed from data available from China and India that individuals likely to be infected by the virus from both these countries belong to the age groups of 20–50 years ( 36 , 37 ). In both of these countries, the working class mostly belongs to this age group, making exposure more likely. Germany and Singapore are great examples of countries with a high number of cases but low fatalities as compared to their immediate neighbors. Singapore is one of the few countries that had developed a detailed plan of action after the previous SARS outbreak to deal with a similar situation in the future, and this worked in their favor during this outbreak. Both countries took swift action after the outbreak began, with Singapore banning Chinese travelers and implementing screening and quarantine measures at a time when the WHO recommended none. They ordered the elderly and the vulnerable to strictly stay at home, and they ensured that lifesaving equipment and large-scale testing facilities were available immediately ( 38 , 39 ). Germany took similar measures by ramping up testing capacity quite early and by ensuring that all individuals had equal opportunity to get tested. This meant that young, old, and at-risk people all got tested, thus ensuring positive results early during disease progression and that most cases were mild like in Singapore, thus maintaining a lower death percentage ( 40 ). It allowed infected individuals to be identified and quarantined before they even had symptoms. Testing was carried out at multiple labs, reducing the load and providing massive scale, something which countries such as the USA did quite late and India restricted to select government and private labs. The German government also banned large gatherings and advocated social distancing to further reduce the spread, though unlike India and the USA, this was done quite late. South Korea is another example of how a nation has managed to contain the spread and transmission of the infection. South Korea and the USA both reported their first COVID-19 cases on the same day; however, the US administration downplayed the risks of the disease, unlike South Korean officials, who constantly informed their citizens about the developments of the disease using the media and a centralized messaging system. They also employed the Trace, Test, and Treat protocol to identify and isolate patients fast, whereas the USA restricted this to patients with severe infection and only later broadened this criterion, like many European countries as well as India. Unlike the USA, South Korea also has universal healthcare, ensuring free diagnostic testing.
Figure 1 . Timeline of COVID-19 progression ( 30 – 32 ).
Figure 2 . Total confirmed COVID 19 cases as of May 2020 ( 33 ).
The main mode of transmission of 2019-nCoV is human to human. As of now, animal-to-human transfer has not yet been confirmed. Asymptomatic carriers of the virus are at major risk of being superinfectors with this disease, as all those infected may not develop the disease ( 41 ). This is a concern that has been raised by nations globally, with the Indian government raising concerns on how to identify and contain asymptomatic carriers, who could account for 80% of those infected ( 42 ). Since current resources are directed towards understanding the hospitalized individuals showing symptoms, there is still a vast amount of information about asymptomatic individuals that has yet to be studied. For example, some questions that need to be answered include: Do asymptomatic individuals develop the disease at any point in time at all? Do they eventually develop antibodies? How long do they shed the virus for? Can any tissue of these individuals store the virus in a dormant state? Asymptomatic transmission is a gray area that encompasses major unknowns in COVID-19.
The main route of human-to-human transmission is by droplets, which are generated during coughing, talking, or sneezing and are then inhaled by a healthy individual. They can also be indirectly transmitted to a person when they land on surfaces that are touched by a healthy individual who may then touch their nose, mouth, or eyes, allowing the virus entry into the body. Fomites are also a common issue in such diseases ( 43 ).
Aerosol-based transmission of the virus has not yet been confirmed ( 43 ). Stool-based transmission via the fecal-oral route may also be possible since the SARS-CoV-2 has been found in patient feces ( 44 , 45 ). Some patients with COVID-19 tend to develop diarrhea, which can become a major route of transmission if proper sanitation and personal hygiene needs are not met. There is no evidence currently available to suggest intrauterine vertical transmission of the disease in pregnant women ( 46 ).
More investigation is necessary of whether climate has played any role in the containment of the infection in countries such as India, Singapore, China, and Israel, as these are significantly warmer countries as compared with the UK, the USA, and Canada ( Figure 2 ). Ideally, a warm climate should prevent the virus from surviving for longer periods of time on surfaces, reducing transmissibility.
Pathophysiology
On gaining entry via any of the mucus membranes, the single-stranded RNA-based virus enters the host cell using type 2 transmembrane serine protease (TMPRSS2) and ACE2 receptor protein, leading to fusion and endocytosis with the host cell ( 47 – 49 ). The uncoated RNA is then translated, and viral proteins are synthesized. With the help of RNA-dependant RNA polymerase, new RNA is produced for the new virions. The cell then undergoes lysis, releasing a load of new virions into the patients' body. The resultant infection causes a massive release of pro-inflammatory cytokines that causes a cytokine storm.
Clinical Presentation
The clinical presentation of the disease resembles beta coronavirus infections. The virus has an incubation time of 2–14 days, which is the reason why most patients suspected to have the illness or contact with an individual having the illness remain in quarantine for the said amount of time. Infection with SARS-CoV-2 causes severe pneumonia, intermittent fever, and cough ( 50 , 51 ). Symptoms of rhinorrhoea, pharyngitis, and sneezing have been less commonly seen. Patients often develop acute respiratory distress syndrome within 2 days of hospital admission, requiring ventilatory support. It has been observed that during this phase, the mortality tends to be high. Chest CT will show indicators of pneumonia and ground-glass opacity, a feature that has helped to improve the preliminary diagnosis ( 51 ). The primary method of diagnosis for SARS-CoV-2 is with the help of PCR. For the PCR testing, the US CDC recommends testing for the N gene, whereas the Chinese CDC recommends the use of ORF lab and N gene of the viral genome for testing. Some also rely on the radiological findings for preliminary screening ( 52 ). Additionally, immunodiagnostic tests based on the presence of antibodies can also play a role in testing. While the WHO recommends the use of these tests for research use, many countries have pre-emptively deployed the use of these tests in the hope of ramping up the rate and speed of testing ( 52 – 54 ). Later, they noticed variations among the results, causing them to stop the use of such kits; there was also debate among the experts about the sensitivity and specificity of the tests. For immunological tests, it is beneficial to test for antibodies against the virus produced by the body rather than to test for the presence of the viral proteins, since the antibodies can be present in larger titers for a longer span of time. However, the cross-reactivity of these tests with other coronavirus antibodies is something that needs verification. Biochemical parameters such as D-dimer, C-reactive protein, and variations in neutrophil and lymphocyte counts are some other parameters that can be used to make a preliminary diagnosis; however, these parameters vary in a number of diseases and thus cannot be relied upon conclusively ( 51 ). Patients with pre-existing diseases such as asthma or similar lung disorder are at higher risk, requiring life support, as are those with other diseases such as diabetes, hypertension, or obesity. Those above the age of 60 have displayed the highest mortality rate in China, a finding that is mirrored in other nations as well ( Figure 3 ) ( 55 ). If we cross-verify these findings with the population share that is above the age of 70, we find that Italy, the United Kingdom, Canada, and the USA have one of the highest elderly populations as compared to countries such as India and China ( Figure 4 ), and this also reflects the case fatality rates accordingly ( Figure 5 ) ( 33 ). This is a clear indicator that aside from comorbidities, age is also an independent risk factor for death in those infected by COVID-19. Also, in the US, it was seen that the rates of African American deaths were higher. This is probably due to the fact that the prevalence of hypertension and obesity in this community is higher than in Caucasians ( 56 , 57 ). In late April 2020, there are also claims in the US media that young patients in the US with COVID-19 may be at increased risk of stroke; however, this is yet to be proven. We know that coagulopathy is a feature of COVID-19, and thus stroke is likely in this condition ( 58 , 59 ). The main cause of death in COVID-19 patients was acute respiratory distress due to the inflammation in the linings of the lungs caused by the cytokine storm, which is seen in all non-survival cases and in respiratory failure. The resultant inflammation in the lungs, served as an entry point of further infection, associated with coagulopathy end-organ failure, septic shock, and secondary infections leading to death ( 60 – 63 ).
Figure 3 . Case fatality rate by age in selected countries as of April 2020 ( 33 ).
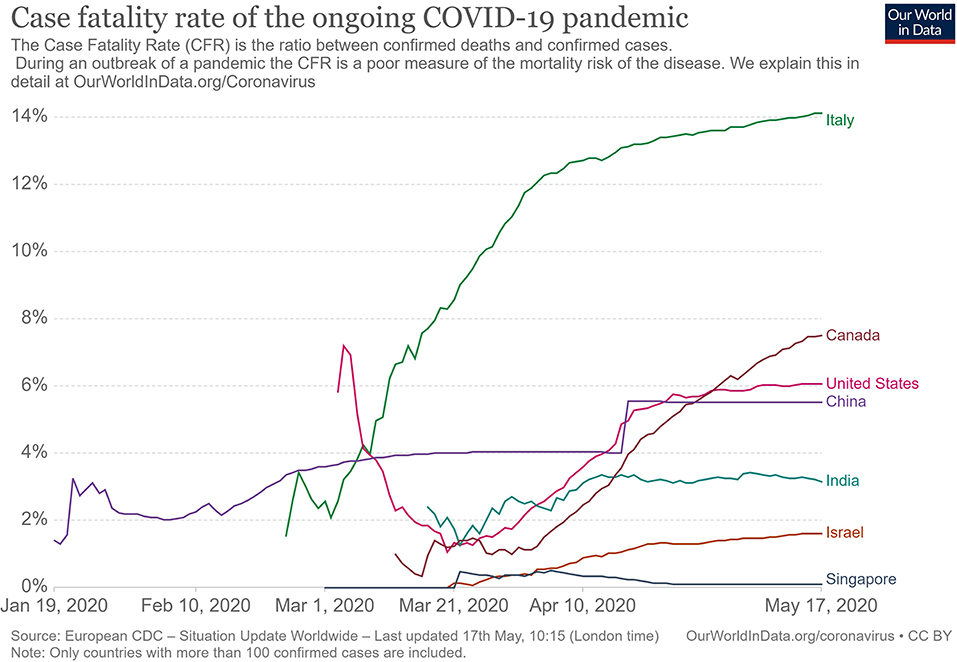
Figure 4 . Case fatality rate in selected countries ( 33 ).
Figure 5 . Population share above 70 years of age ( 33 ).
For COVID-19, there is no specific treatment available. The WHO announced the organization of a trial dubbed the “Solidarity” clinical trial for COVID-19 treatments ( 64 ). This is an international collaborative study that investigates the use of a few prime candidate drugs for use against COVID-19, which are discussed below. The study is designed to reduce the time taken for an RCT by over 80%. There are over 1087 studies ( Supplementary Data 1 ) for COVID-19 registered at clinicaltrials.gov , of which 657 are interventional studies ( Supplementary Data 2 ) ( 65 ). The primary focus of the interventional studies for COVID-19 has been on antimalarial drugs and antiviral agents ( Table 1 ), while over 200 studies deal with the use of different forms of oxygen therapy. Most trials focus on improvement of clinical status, reduction of viral load, time to improvement, and reduction of mortality rates. These studies cover both severe and mild cases.
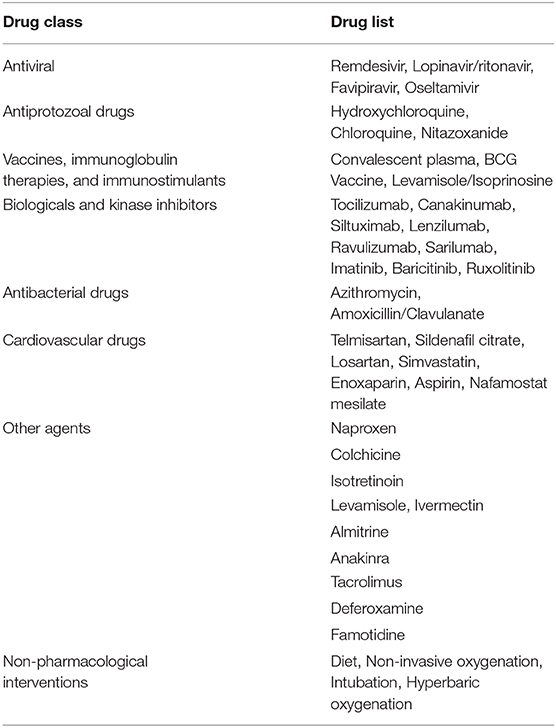
Table 1 . List of therapeutic drugs under study for COVID-19 as per clinical trials registered under clinicaltrials.gov .
Use of Antimalarial Drugs Against SARS-CoV-2
The use of chloroquine for the treatment of corona virus-based infection has shown some benefit in the prevention of viral replication in the cases of SARS and MERS. However, it was not validated on a large scale in the form of a randomized control trial ( 50 , 66 – 68 ). The drugs of choice among antimalarials are Chloroquine (CQ) and Hydroxychloroquine (HCQ). The use of CQ for COVID-19 was brought to light by the Chinese, especially by the publication of a letter to the editor of Bioscience Trends by Gao et al. ( 69 ). The letter claimed that several studies found CQ to be effective against COVID-19; however, the letter did not provide many details. Immediately, over a short span of time, interest in these two agents grew globally. Early in vitro data have revealed that chloroquine can inhibit the viral replication ( 70 , 71 ).
HCQ and CQ work by raising the pH of the lysosome, the cellular organelle that is responsible for phagocytic degradation. Its function is to combine with cell contents that have been phagocytosed and break them down eventually, in some immune cells, as a downstream process to display some of the broken proteins as antigens, thus further enhancing the immune recruitment against an antigen/pathogen. The drug was to be administered alone or with azithromycin. The use of azithromycin may be advocated by the fact that it has been seen previously to have some immunomodulatory role in airway-related disease. It appears to reduce the release of pro-inflammatory cytokines in respiratory illnesses ( 72 ). However, HCQ and azithromycin are known to have a major drug interaction when co-administered, which increases the risk of QT interval prolongation ( 73 ). Quinine-based drugs are known to have adverse effects such as QT prolongation, retinal damage, hypoglycemia, and hemolysis of blood in patients with G-6-PD deficiency ( 66 ). Several preprints, including, a metanalysis now indicate that HCQ may have no benefit for severe or critically ill patients who have COVID-19 where the outcome is need for ventilation or death ( 74 , 75 ). As of April 21, 2020, after having pre-emptively recommended their use for SARS-CoV-2 infection, the US now advocates against the use of these two drugs based on the new data that has become available.
Use of Antiviral Drugs Against SARS-CoV-2
The antiviral agents are mainly those used in the case of HIV/AIDS, these being Lopinavir and Ritonavir. Other agents such as nucleoside analogs like Favipiravir, Ribavirin, Remdesivir, and Galidesivir have been tested for possible activity in the prevention of viral RNA synthesis ( 76 ). Among these drugs, Lopinavir, Ritonavir, and Remdesivir are listed in the Solidarity trial by the WHO.
Remdesivir is a nucleotide analog for adenosine that gets incorporated into the viral RNA, hindering its replication and causing chain termination. This agent was originally developed for Ebola Virus Disease ( 77 ). A study was conducted with rhesus macaques infected with SARS-CoV-2 ( 78 ). In that study, after 12 h of infection, the monkeys were treated with either Remdesivir or vehicle. The drug showed good distribution in the lungs, and the animals treated with the drug showed a better clinical score than the vehicle group. The radiological findings of the study also indicated that the animals treated with Remdesivir have less lung damage. There was a reduction in viral replication but not in virus shedding. Furthermore, there were no mutations found in the RNA polymerase sequences. A randomized clinical control study that became available in late April 2020 ( 79 ), having 158 on the Remdesivir arm and 79 on the placebo arm, found that Remdesivir reduced the time to recovery in the Remdesivir-treated arm to 11 days, while the placebo-arm recovery time was 15 days. Though this was not found to be statistically significant, the agent provided a basis for further studies. The 28-days mortality was found to be similar for both groups. This has now provided us with a basis on which to develop future molecules. The study has been supported by the National Institute of Health, USA. The authors of the study advocated for more clinical trials with Remdesivir with a larger population. Such larger studies are already in progress, and their results are awaited. Remdesivir is currently one of the drugs that hold most promise against COVID-19.
An early trial in China with Lopinavir and Ritonavir showed no benefit compared with standard clinical care ( 80 ). More studies with this drug are currently underway, including one in India ( 81 , 82 ).
Use of Convalescent Patient Plasma
Another possible option would be the use of serum from convalescent individuals, as this is known to contain antibodies that can neutralize the virus and aid in its elimination. This has been tried previously for other coronavirus infections ( 83 ). Early emerging case reports in this aspect look promising compared to other therapies that have been tried ( 84 – 87 ). A report from China indicates that five patients treated with plasma recovered and were eventually weaned off ventilators ( 84 ). They exhibited reductions in fever and viral load and improved oxygenation. The virus was not detected in the patients after 12 days of plasma transfusion. The US FDA has provided detailed recommendations for investigational COVID-19 Convalescent Plasma use ( 88 ). One of the benefits of this approach is that it can also be used for post-exposure prophylaxis. This approach is now beginning to be increasingly adopted in other countries, with over 95 trials registered on clinicaltrials.gov alone, of which at least 75 are interventional ( 89 ). The use of convalescent patient plasma, though mostly for research purposes, appears to be the best and, so far, the only successful option for treatment available.
From a future perspective, the use of monoclonal antibodies for the inhibition of the attachment of the virus to the ACE-2 receptor may be the best bet. Aside from this, ACE-2-like molecules could also be utilized to attach and inactivate the viral proteins, since inhibition of the ACE-2 receptor would not be advisable due to its negative repercussions physiologically. In the absence of drug regimens and a vaccine, the treatment is symptomatic and involves the use of non-invasive ventilation or intubation where necessary for respiratory failure patients. Patients that may go into septic shock should be managed as per existing guidelines with hemodynamic support as well as antibiotics where necessary.
The WHO has recommended that simple personal hygiene practices can be sufficient for the prevention of spread and containment of the disease ( 90 ). Practices such as frequent washing of soiled hands or the use of sanitizer for unsoiled hands help reduce transmission. Covering of mouth while sneezing and coughing, and disinfection of surfaces that are frequently touched, such as tabletops, doorknobs, and switches with 70% isopropyl alcohol or other disinfectants are broadly recommended. It is recommended that all individuals afflicted by the disease, as well as those caring for the infected, wear a mask to avoid transmission. Healthcare works are advised to wear a complete set of personal protective equipment as per WHO-provided guidelines. Fumigation of dormitories, quarantine rooms, and washing of clothes and other fomites with detergent and warm water can help get rid of the virus. Parcels and goods are not known to transmit the virus, as per information provided by the WHO, since the virus is not able to survive sufficiently in an open, exposed environment. Quarantine of infected individuals and those who have come into contact with an infected individual is necessary to further prevent transmission of the virus ( 91 ). Quarantine is an age-old archaic practice that continues to hold relevance even today for disease containment. With the quarantine being implemented on such a large scale in some countries, taking the form of a national lockdown, the question arises of its impact on the mental health of all individuals. This topic needs to be addressed, especially in countries such as India and China, where it is still a matter of partial taboo to talk about it openly within the society.
In India, the Ministry of Ayurveda, Yoga, and Naturopathy, Unani, Siddha and Homeopathy (AYUSH), which deals with the alternative forms of medicine, issued a press release that the homeopathic, drug Arsenicum album 30, can be taken on an empty stomach for 3 days to provide protection against the infection ( 92 ). It also provided a list of herbal drugs in the same press release as per Ayurvedic and Unani systems of medicine that can boost the immune system to deal with the virus. However, there is currently no evidence to support the use of these systems of medicine against COVID-19, and they need to be tested.
The prevention of the disease with the use of a vaccine would provide a more viable solution. There are no vaccines available for any of the coronaviruses, which includes SARS and MERS. The development of a vaccine, however, is in progress at a rapid pace, though it could take about a year or two. As of April 2020, no vaccine has completed the development and testing process. A popular approach has been with the use of mRNA-based vaccine ( 93 – 96 ). mRNA vaccines have the advantage over conventional vaccines in terms of production, since they can be manufactured easily and do not have to be cultured, as a virus would need to be. Alternative conventional approaches to making a vaccine against SARS-CoV-2 would include the use of live attenuated virus as well as using the isolated spike proteins of the virus. Both of these approaches are in progress for vaccine development ( 97 ). Governments across the world have poured in resources and made changes in their legislation to ensure rapid development, testing, and deployment of a vaccine.
Barriers to Treatment
Lack of transparency and poor media relations.
The lack of government transparency and poor reporting by the media have hampered the measures that could have been taken by healthcare systems globally to deal with the COVID-19 threat. The CDC, as well as the US administration, downplayed the threat and thus failed to stock up on essential supplies, ventilators, and test kits. An early warning system, if implemented, would have caused borders to be shut and early lockdowns. The WHO also delayed its response in sounding the alarm regarding the severity of the outbreak to allow nations globally to prepare for a pandemic. Singapore is a prime example where, despite the WHO not raising concerns and banning travel to and from China, a country banned travelers and took early measures, thus managing the outbreak quite well. South Korea is another example of how things may have played out had those measures by agencies been taken with transparency. Increased transparency would have allowed the healthcare sector to better prepare and reduced the load of patients they had to deal with, helping flatten the curve. The increased patient load and confusion among citizens arising from not following these practices has proved to be a barrier to providing effective treatments to patients with the disease elsewhere in the world.
Lack of Preparedness and Protocols
Despite the previous SARS outbreak teaching us important lessons and providing us with data on a potential outbreak, many nations did not take the important measures needed for a future outbreak. There was no allocation of sufficient funds for such an event. Many countries experienced severe lack of PPE, and the lockdown precautions hampered the logistics of supply and manufacturing of such essential equipment. Singapore and South Korea had protocols in place and were able to implement them at a moment's notice. The spurt of cases that Korea experienced was managed well, providing evidence to this effect. The lack of preparedness and lack of protocol in other nations has resulted in confusion as to how the treatment may be administered safely to the large volume of patients while dealing with diagnostics. Both of these factors have limited the accessibility to healthcare services due to sheer volume.
Socio-Economic Impact
During the SARS epidemic, China faced an economic setback, and experts were unsure if any recovery would be made. However, the global and domestic situation was then in China's favor, as it had a lower debt, allowing it to make a speedy recovery. This is not the case now. Global experts have a pessimistic outlook on the outcome of this outbreak ( 98 ). The fear of COVID-19 disease, lack of proper understanding of the dangers of the virus, and the misinformation spread on the social media ( 99 ) have caused a breakdown of the economic flow globally ( 100 ). An example of this is Indonesia, where a great amount of fear was expressed in responses to a survey when the nation was still free of COVID-19 ( 101 ). The pandemic has resulted in over 2.6 billion people being put under lockdown. This lockdown and the cancellation of the lunar year celebration has affected business at the local level. Hundreds of flights have been canceled, and tourism globally has been affected. Japan and Indonesia are estimated to lose over 2.44 billion dollars due to this ( 102 , 103 ). Workers are not able to work in factories, transportation in all forms is restricted, and goods are not produced or moved. The transport of finished products and raw materials out of China is low. The Economist has published US stock market details indicating that companies in the US that have Chinese roots fell, on average, 5 points on the stock market as compared to the S&P 500 index ( 104 ). Companies such as Starbucks have had to close over 4,000 outlets due to the outbreak as a precaution. Tech and pharma companies are at higher risk since they rely on China for the supply of raw materials and active pharmaceutical ingredients. Paracetamol, for one, has reported a price increase of over 40% in India ( 104 – 106 ). Mass hysteria in the market has caused selling of shares of these companies, causing a tumble in the Indian stock market. Though long-term investors will not be significantly affected, short-term traders will find themselves in soup. Politically, however, this has further bolstered support for world leaders in countries such as India, Germany, and the UK, who are achieving good approval ratings, with citizens being satisfied with the government's approach. In contrast, the ratings of US President Donald Trump have dropped due to the manner in which the COVID-19 pandemic was handled. These minor impacts may be of temporary significance, and the worst and direct impact will be on China itself ( 107 – 109 ), as the looming trade war with the USA had a negative impact on the Chinese and Asian markets. The longer production of goods continues to remain suspended, the more adversely it will affect the Chinese economy and the global markets dependent on it ( 110 ). If this disease is not contained, more and more lockdowns by multiple nations will severely affect the economy and lead to many social complications.
The appearance of the 2019 Novel Coronavirus has added and will continue to add to our understanding of viruses. The pandemic has once again tested the world's preparedness for dealing with such outbreaks. It has provided an outlook on how a massive-scale biological event can cause a socio-economic disturbance through misinformation and social media. In the coming months and years, we can expect to gain further insights into SARS-CoV-2 and COVID-19.
Author Contributions
KN: conceptualization. RK, AA, JM, and KN: investigation. RK and AA: writing—original draft preparation. KN, PN, and JM: writing—review and editing. KN: supervision.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to acknowledge the contributions made by Dr. Piya Paul Mudgal, Assistant Professor, Manipal Institute of Virology, Manipal Academy of Higher Education towards inputs provided by her during the drafting of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2020.00216/full#supplementary-material
Supplementary Data 1, 2. List of all studies registered for COVID-19 on clinicaltrials.gov .
1. McIntosh K, Dees JH, Becker WB, Kapikian AZ, Chanock RM. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc Natl Acad Sci USA. (1967) 57:933–40. doi: 10.1073/pnas.57.4.933
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Esper F, Weibel C, Ferguson D, Landry ML, Kahn JS. Evidence of a novel human coronavirus that is associated with respiratory tract disease in infants and young children. J Infect Dis. (2005) 191:492–8. doi: 10.1086/428138
3. Stöhr K. A multicentre collaboration to investigate the cause of severe acute respiratory syndrome. Lancet. (2003) 361:1730–3. doi: 10.1016/S0140-6736(03)13376-4
4. Peiris JSM, Lai ST, Poon LLM, Guan Y, Yam LYC, Lim W, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. (2003) 361:1319–25. doi: 10.1016/S0140-6736(03)13077-2
5. Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet. (2015) 386:995–1007. doi: 10.1016/S0140-6736(15)60454-8
6. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. (2012) 367:1814–20. doi: 10.1056/NEJMoa1211721
7. Perlman S, Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol. (2009) 7:439–50. doi: 10.1038/nrmicro2147
8. Li W, Moore MJ, Vasllieva N, Sui J, Wong SK, Berne MA, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. (2003) 426:450–4. doi: 10.1038/nature02145
9. Ge X-Y, Li J-L, Yang X-L, Chmura AA, Zhu G, Epstein JH, et al. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. (2013) 503:535–8. doi: 10.1038/nature12711
10. Wang M, Hu Z. Bats as animal reservoirs for the SARS coronavirus: Hypothesis proved after 10 years of virus hunting. Virol Sin. (2013) 28:315–7. doi: 10.1007/s12250-013-3402-x
11. Diamond Princess Cruise Ship in Japan Confirms 99 New Coronavirus Cases | World news | The Guardian. Available online at: https://www.theguardian.com/world/2020/feb/17/coronavirus-japan-braces-for-hundreds-more-cases-as-another-china-city-locked-down (accessed February 17, 2020).
Google Scholar
12. Diamond Princess Coronavirus & Quarantine Updates - Notices & Advisories - Princess Cruises. Available online at: https://www.princess.com/news/notices_and_advisories/notices/diamond-princess-update.html (accessed February 17, 2020).
13. Gorbalenya AE, Baker SC, Baric RS, Groot RJ, de Drosten C, Gulyaeva AA, et al. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. (2020) 5:536–44. doi: 10.1038/s41564-020-0695-z
14. Ji W, Wang W, Zhao X, Zai J, Li X. Homologous recombination within the spike glycoprotein of the newly identified coronavirus may boost cross-species transmission from snake to human. J Med Virol . (2020) 7:jmv.25682. doi: 10.1002/jmv.25682
15. Cyranoski D. Did pangolins spread the China coronavirus to people? Nature . (2020) doi: 10.1038/d41586-020-00364-2
CrossRef Full Text | Google Scholar
16. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. (2020) 395:565–74. doi: 10.1016/S0140-6736(20)30251-8
17. Wassenaar TM, Zou Y. 2019_nCoV: Rapid classification of betacoronaviruses and identification of traditional Chinese medicine as potential origin of zoonotic coronaviruses. Lett Appl Microbiol. (2020) 70:324–48. doi: 10.1111/lam.13285
18. Velavan TP, Meyer CG. The Covid-19 epidemic. Trop Med Int Heal . (2020) 25:278–80. doi: 10.1111/tmi.13383
19. Guarner J. Three Emerging Coronaviruses in Two Decades. Am J Clin Pathol . (2020) 153:420–21. doi: 10.1093/ajcp/aqaa029
20. Li W, Shi Z, Yu M, Ren W, Smith C, Epstein JH, et al. Bats are natural reservoirs of SARS-like coronaviruses. Science. (2005) 310:676–9. doi: 10.1126/science.1118391
21. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. (2020) 579:270–3. doi: 10.1038/s41586-020-2012-7
22. Menachery VD, Yount BL, Debbink K, Agnihothram S, Gralinski LE, Plante JA, et al. A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nat Med. (2015) 21:1508–13. doi: 10.1038/nm.3985
23. COVID-19 Coronavirus Epidemic has a Natural Origin—ScienceDaily. Available online at: https://www.sciencedaily.com/releases/2020/03/200317175442.htm (accessed April 22, 2020).
24. Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med . (2020) 26:450–2. doi: 10.1038/s41591-020-0820-9
25. Prioritizing Diseases for Research and Development in Emergency Contexts. Available online at: https://www.who.int/activities/prioritizing-diseases-for-research-and-development-in-emergency-contexts (accessed April 22, 2020).
26. Albariño CG, Guerrero LW, Jenks HM, Chakrabarti AK, Ksiazek TG, Rollin PE, et al. Insights into Reston virus spillovers and adaption from virus whole genome sequences. PLoS ONE. (2017) 12:e0178224. doi: 10.1371/journal.pone.0178224
27. Yao H, Lu X, Chen Q, Xu K, Chen Y, Cheng L, et al. Patient-derived mutations impact pathogenicity of SARS-CoV-2. medRxiv. (2020). doi: 10.2139/ssrn.3578153
28. Stefanelli P, Faggioni G, Lo Presti A, Fiore S, Marchi A, Benedetti E, et al. Whole genome and phylogenetic analysis of two SARS-CoV-2 strains isolated in Italy in January and February 2020: additional clues on multiple introductions and further circulation in Europe. Eurosurveillance. (2020) 25:2000305. doi: 10.2807/1560-7917.ES.2020.25.13.2000305
29. Yadav P, Potdar V, Choudhary M, Nyayanit D, Agrawal M, Jadhav S, et al. Full-genome sequences of the first two SARS-CoV-2 viruses from India. Indian J Med Res. (2020) 151:200–9. doi: 10.4103/ijmr.IJMR_663_20
30. WHO | Coronavirus disease 2019 (COVID-19) Situation Report – 26. Beijing (2020). Available online at: http://www.nhc.gov.cn/xcs/yqtb/list_gzbd.shtml (accessed February 16, 2020).
31. WHO | Middle East Respiratory Syndrome Coronavirus (MERS-CoV). World Health Organization (2020).
32. WHO | Summary of Probable SARS Cases With Onset of Illness From 1 November 2002 to 31 July 2003. World Health Organization (2015).
33. Worldometer. Coronavirus cases. Worldometer. (2020) 1−22.
34. Update on the Outbreak of New Coronavirus Pneumonia as of 24 hours on 15 February. Beijing (2020). Available online at: http://www.nhc.gov.cn/xcs/yqtb/list_gzbd.shtml (accessed February 16, 2020).
35. Sanche S, Lin YT, Xu C, Romero-Severson E, Hengartner N, Ke R. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. (2020) 26. doi: 10.3201/eid2607.200282
36. 83% of India's Coronavirus Patients Are Below the Age of 50: Health ministry data - India News. Available online at: https://www.indiatoday.in/india/story/83-of-india-s-coronavirus-patients-are-below-the-age-of-50-health-ministry-data-1663314-2020-04-04 (accessed April 23, 2020).
37. 42% of Coronavirus Patients in 21-40 age bracket: Govt. Available online at: https://economictimes.indiatimes.com/news/politics-and-nation/42-of-coronavirus-patients-in-21-40-age-bracket-govt/articleshow/74987254.cms (accessed April 23, 2020).
38. Why COVID-19 Case Counts Are so Low in Singapore Hong Kong and Taiwan | Advisory Board Daily Briefing. Available online at: https://www.advisory.com/daily-briefing/2020/03/19/asian-countries (accessed April 29, 2020).
39. Coronavirus: Why so Few Deaths Among Singapore's 14000 Covid-19 Infections? | South China Morning Post. Available online at: https://www.scmp.com/weekasia/health-environment/article/3081772/coronavirus-why-so-few-deaths-among-singapores-14000 (accessed April 29, 2020).
40. Stafford N. Covid-19: Why Germany's case fatality rate seems so low. BMJ. (2020) 7:369. doi: 10.1136/bmj.m1395
41. Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-NCOV infection from an asymptomatic contact in Germany. N Engl J Med . (2020) 382:970–1. doi: 10.1056/NEJMc2001468
42. Coronavirus pandemic | 80% of COVID-19 Cases Either Asymptomatic or Show Mild Symptoms. Health ministry - Moneycontrol.com. Available online at: https://www.moneycontrol.com/news/india/coronavirus-pandemic-80-of-covid-19-cases-either-asymptomatic-or-show-mild-symptoms-health-ministry-5170631.html (accessed April 24, 2020).
43. WHO | Q&A on Coronaviruses. Available online at: https://www.who.int/news-room/q-a-detail/q-a-coronaviruses (accessed February 16, 2020).
44. Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med . (2020) 382:929–36. doi: 10.1056/NEJMoa2001191
45. Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is faecal–oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. (2020) 5:335–37. doi: 10.1016/S2468-1253(20)30048-0
46. Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. (2020) 395:809–15. doi: 10.1016/S0140-6736(20)30360-3
47. Fehr AR, Perlman S. Coronaviruses: An overview of their replication and pathogenesis. In: Coronaviruses: Methods and Protocols (New York: Springer), 1–23. doi: 10.1007/978-1-4939-2438-7_1
48. Chen Y, Liu Q, Guo D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J Med Virol. (2020) 92:418–23. doi: 10.1002/jmv.25681
49. Hoffmann M, Kleine-Weber H, Schroeder S, Mü MA, Drosten C, Pö S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. (2020) 181:271–80. doi: 10.1016/j.cell.2020.02.052
50. Cheng VCC, Chan JFW, To KKW, Yuen KY. Clinical management and infection control of SARS: Lessons learned. Antiviral Res. (2013) 100:407–19. doi: 10.1016/j.antiviral.2013.08.016
51. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
52. Udugama B, Kadhiresan P, Kozlowski HN, Malekjahani A, Osborne M, Li VYC, et al. Diagnosing COVID-19: the disease and tools for detection. ACS Nano . (2020) 14:3822–35. doi: 10.1021/acsnano.0c02624
53. Testing, | CDC,. Available online at: https://www.cdc.gov/coronavirus/2019-ncov/testing/index.html (accessed April 29, 2020).
54. Advice on the Use of Point-of-Care Immunodiagnostic Tests for COVID-19. Available online at: https://www.who.int/news-room/commentaries/detail/advice-on-the-use-of-point-of-care-immunodiagnostic-tests-for-covid-19 (accessed April 29, 2020).
55. Zhang Yanping. The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) — China, 2020. (2020) Available online at: http://weekly.chinacdc.cn/en/article/id/e53946e2-c6c4-41e9-9a9bfea8db1a8f51 (accessed February 18, 2020).
56. Petersen R, Pan L, Blanck HM. Racial and ethnic disparities in adult obesity in the United States: CDC's tracking to inform state and local action. Prev Chronic Dis. (2019) 16:180579. doi: 10.5888/pcd16.180579
57. Wang L, Southerland J, Wang K, Bailey BA, Alamian A, Stevens MA, et al. Ethnic differences in risk factors for obesity among adults in California, the United States. (2017) 2017:2427483. doi: 10.1155/2017/2427483
58. Covid-19 Causes Sudden Strokes in Young Adults Doctors Say - CNN. Available online at: https://edition.cnn.com/2020/04/22/health/strokes-coronavirus-young-adults/index.html (accessed April 24, 2020).
59. Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, et al. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost . (2020) 1–4. doi: 10.1111/jth.14828
60. Lancet T, Medicine R. Comment Understanding pathways to death in patients with. Lancet Respir Med. (2020) 2019:2019–21. doi: 10.1016/S2213-2600(20)30165-X
61. Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA . (2020) 323:1775–6. doi: 10.1001/jama.2020.4683
62. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
63. Doctors Warn COVID-19 Might Cause Strokes in Young Adults. Available online at: https://nypost.com/2020/04/23/doctors-warn-covid-19-might-cause-strokes-in-young-adults/ (accessed April 24, 2020).
64. “Solidarity” Clinical Trial for COVID-19 Treatments. Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments (accessed April 23, 2020).
65. Search, of: COVID-19 - List Results - ClinicalTrials.gov . Available online at: https://www.clinicaltrials.gov/ct2/results?cond=COVID-19&term=&cntry=&state=&city=&dist= (accessed May 1, 2020).
66. De Wilde AH, Jochmans D, Posthuma CC, Zevenhoven-Dobbe JC, Van Nieuwkoop S, Bestebroer TM, et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. (2014) 58:4875–84. doi: 10.1128/AAC.03011-14
67. Tai DYH. Pharmacologic treatment of SARS: current knowledge and recommendations. Ann Acad Med Singapore. (2007) 36:438–43.
PubMed Abstract | Google Scholar
68. Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. (2020) 11:222. doi: 10.1038/s41467-019-13940-6
69. Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. (2020) 14:72–73. doi: 10.5582/bst.2020.01047
70. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. (2020) 30:269–71. doi: 10.1038/s41422-020-0282-0
71. Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. (2005) 2:1–0. doi: 10.1186/1743-422X-2-69
72. Cramer CL, Patterson A, Alchakaki A, Soubani AO. Immunomodulatory indications of azithromycin in respiratory disease: a concise review for the clinician. Postgrad Med. (2017) 129:493–9. doi: 10.1080/00325481.2017.1285677
73. Plaquenil™ Hydroxychloroquine Sulfate Tablets USP Description. Available online at: http://www.cdc.gov/malaria (accessed April 21, 2020).
74. Magagnoli J, Narendran S, Pereira F, Cummings T, Hardin JW, Sutton SS, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv. (2020) doi: 10.1101/2020.04.16.20065920
75. Shamshirian A, Hessami A, Heydari K, Alizadeh-Navaei R, Ebrahimzadeh MA, Ghasemian R, et al. Hydroxychloroquine Versus COVID-19: A Rapid Systematic Review and Meta-Analysis. medRxiv. (2020) doi: 10.1101/2020.04.14.20065276
76. Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov . (2020) 19:149–50. doi: 10.1038/d41573-020-00016-0
77. Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. (2016) 531:381–5. doi: 10.1038/nature17180
78. Williamson BN, Feldmann F, Schwarz B, Meade-White K, Porter DP, Schulz J, et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. bioRxiv. (2020) doi: 10.1101/2020.04.15.043166
79. Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. (2020) 395:1569–78. doi: 10.1016/S0140-6736(20)31022-9
80. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe covid-19. N Engl J Med . (2020) 382:1787–99. doi: 10.1056/NEJMoa2001282
81. Search, of: Lopinavir, Ritonavir, | COVID - List Results - ClinicalTrials.gov . Available online at: https://clinicaltrials.gov/ct2/results?term=Lopinavir%2CRitonavir&cond=COVID&draw=4&rank=22#rowId21 (accessed April 23, 2020).
82. Bhatnagar T, Murhekar M, Soneja M, Gupta N, Giri S, Wig N, et al. Lopinavir/ritonavir combination therapy amongst symptomatic coronavirus disease 2019 patients in India: protocol for restricted public health emergency use. Indian J Med Res. (2020) 151:184–9. doi: 10.4103/ijmr.IJMR_502_20
83. Yeh K-M, Chiueh T-S, Siu LK, Lin J-C, Chan PKS, Peng M-Y, et al. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother. (2005) 56:919–22. doi: 10.1093/jac/dki346
84. Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically Ill patients with COVID-19 with convalescent plasma. JAMA . (2020) 323:1582–9. doi: 10.1001/jama.2020.4783
85. Cao W, Liu X, Bai T, Fan H, Hong K, Song H, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis . (2020) 7:1–6. doi: 10.1093/ofid/ofaa102
86. Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest . (2020) 138745:1–22. doi: 10.1172/JCI138745
87. Ye M, Fu D, Ren Y, Wang F, Wang D, Zhang F, et al. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol. (2 020) doi: 10.1002/jmv.25882
88. Recommendations for Investigational COVID-19 Convalescent Plasma | FDA. Available online at: https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma (accessed April 23, 2020).
89. Search, of: plasma | Interventional Studies | COVID - List Results - ClinicalTrials.gov . Available online at: https://clinicaltrials.gov/ct2/results?term=plasma&cond=COVID&Search=Apply&age_v=&gndr=&type=Intr&rslt= (accessed April 23, 2020).
90. Infection Prevention and Control. Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/infection-prevention-and-control (accessed February 17, 2020).
91. Wilder-Smith A, Freedman DO. Isolation, quarantine, social distancing and community containment: pivotal role for old-style public health measures in the novel coronavirus (2019-nCoV) outbreak. J Travel Med . (2020) 27:taaa020. doi: 10.1093/jtm/taaa020
92. Press Information Bureau AYUSH Advisory for Corona virus. Press Inf Bereau. Available online at: https://pib.gov.in/PressReleasePage.aspx?PRID=1600895 (accessed February 17, 2020).
93. CanSino, Biologics : China Announces First Human Trials of Covid-19 Vaccine | MarketScreener,. Available online at: https://www.marketscreener.com/CANSINO-BIOLOGICS-INC-59318312/news/CanSino-Biologics-China-announces-first-human-trials-of-Covid-19-vaccine-30183232/ (accessed April 7, 2020).
94. Safety, and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) for Prophylaxis SARS CoV-2 Infection - Full Text View - ClinicalTrials.gov . Available online at: https://clinicaltrials.gov/ct2/show/NCT04283461 (accessed April 7, 2020).
95. NIH, Clinical Trial of Investigational Vaccine for COVID-19 Begins | National Institutes of Health (NIH),. Available at: https://www.nih.gov/news-events/news-releases/nih-clinical-trial-investigational-vaccine-covid-19-begins (accessed April 7, 2020).
96. Novel, Coronavirus vaccine manufacturing contract signed — The Jenner Institute,. Available online at: https://www.jenner.ac.uk/about/news/novel-coronavirus-vaccine-manufacturing-contract-signed (accessed April 7, 2020).
97. Xie L, Sun C, Luo C, Zhang Y, Zhang J, Yang J, et al. SARS-CoV-2 and SARS-CoV Spike-RBD structure and receptor binding comparison and potential implications on neutralizing antibody and vaccine development. bioRxiv [Preprint]. (2020). doi: 10.1101/2020.02.16.951723
98. The Global Economic Impact of the Coronavirus Outbreak – Harvard Gazette. Available online at: https://news.harvard.edu/gazette/story/2020/02/the-global-economic-impact-of-the-coronavirus-outbreak/ (accessed February 17, 2020).
99. Shimizu K. 2019-nCoV, fake news, and racism. Lancet . (2020) 395:685–6. doi: 10.1016/S0140-6736(20)30357-3
100. ROHDE RODNEY. 2019 Novel Coronavirus (2019-nCoV) Update: Uncoating the Virus. Am Soc Microbiol. (2020). Available online at: https://asm.org/Articles/2020/January/2019-Novel-Coronavirus-2019-nCoV-Update-Uncoating
101. Virus-free Indonesia more threatened by COVID-19 than Singapore Malaysia: Survey - World - The Jakarta Post. Available online at: https://www.thejakartapost.com/news/2020/02/18/virus-free-indonesia-more-threatened-by-covid-19-than-singapore-malaysia-survey.html (accessed February 18, 2020).
102. Japan, May Lose $1,.29 Billion in Tourism Revenue Due to COVID-19 Outbreak | The Japan Times. Available online at: https://www.japantimes.co.jp/news/2020/02/16/business/economy-business/japan-lose-billion-tourism-revenue-covid19-outbreak/#.XkvxX0fitPY (accessed February 18, 2020).
103. Coronavirus's Effect on Tourism Will Carry Into 2021 Experts Say - Bloomberg. Available online at: https://www.bloomberg.com/news/articles/2020-02-13/coronavirus-s-effect-on-tourism-will-carry-into-2021-experts-say (accessed February 18, 2020).
104. The, week in charts - The cost of covid-19 | Graphic detail | The Economist,. Available online at: https://www.economist.com/graphic-detail/2020/02/14/the-cost-of-covid-19 (accessed February 17, 2020).
105. coronavirus: Covid-19 Impact: Pharma Companies Feel the Pain as Prices of Key Inputs Shoot Up - The Economic Times. Available online at: https://economictimes.indiatimes.com/industry/healthcare/biotech/pharmaceuticals/covid-19-impact-pharma-companies-feel-the-pain-as-prices-of-key-inputs-shoot-up/articleshow/74144044.cms?from=mdr (accessed February 17, 2020).
106. Coronavirus Outbreak: Paracetamol Prices Jump 40% In India As Coronavirus Shuts Down China. Available online at: https://www.ndtv.com/india-news/coronavirus-outbreak-paracetamol-prices-jump-40-in-india-as-coronavirus-shuts-down-china-2181480 (accessed February 18, 2020).
107. The coronavirus could cripple China's economy for longer than Wall Street wants to believe | Business Insider India. Available online at: https://www.businessinsider.in/international/news/the-coronavirus-could-cripple-chinas-economy-for-longer-than-wall-street-wants-to-believe/articleshow/74162183.cms (accessed February 17, 2020).
108. Viral Slowdown - How China's Coronavirus Epidemic Could Hurt the World Economy | Leaders | The Economist. Available online at: https://www.economist.com/leaders/2020/02/13/how-chinas-coronavirus-epidemic-could-hurt-the-world-economy (accessed February 17, 2020).
109. China's Economic Battle With COVID-19 | The ASEAN Post. Available online at: https://theaseanpost.com/article/chinas-economic-battle-covid-19 (accessed February 17, 2020).
110. The Coronavirus Could Cost China's Economy $60 Billion this Quarter. - CNN. Available online at: https://edition.cnn.com/2020/01/31/economy/china-economy-coronavirus/index.html (accessed February 18, 2020).
Keywords: 2019-nCoV, COVID-19, SARS-CoV-2, coronavirus, pandemic, SARS
Citation: Keni R, Alexander A, Nayak PG, Mudgal J and Nandakumar K (2020) COVID-19: Emergence, Spread, Possible Treatments, and Global Burden. Front. Public Health 8:216. doi: 10.3389/fpubh.2020.00216
Received: 21 February 2020; Accepted: 11 May 2020; Published: 28 May 2020.
Reviewed by:
Copyright © 2020 Keni, Alexander, Nayak, Mudgal and Nandakumar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Krishnadas Nandakumar, mailnandakumar77@gmail.com
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Open access
- Published: 04 February 2022

Analysis of the COVID-19 pandemic: lessons towards a more effective response to public health emergencies
- Yibeltal Assefa ORCID: orcid.org/0000-0003-2393-1492 1 ,
- Charles F. Gilks 1 ,
- Simon Reid 1 ,
- Remco van de Pas 2 ,
- Dereje Gedle Gete 1 &
- Wim Van Damme 2
Globalization and Health volume 18 , Article number: 10 ( 2022 ) Cite this article
39k Accesses
35 Citations
10 Altmetric
Metrics details
The pandemic of Coronavirus Disease 2019 (COVID-19) is a timely reminder of the nature and impact of Public Health Emergencies of International Concern. As of 12 January 2022, there were over 314 million cases and over 5.5 million deaths notified since the start of the pandemic. The COVID-19 pandemic takes variable shapes and forms, in terms of cases and deaths, in different regions and countries of the world. The objective of this study is to analyse the variable expression of COVID-19 pandemic so that lessons can be learned towards an effective public health emergency response.
We conducted a mixed-methods study to understand the heterogeneity of cases and deaths due to the COVID-19 pandemic. Correlation analysis and scatter plot were employed for the quantitative data. We used Spearman’s correlation analysis to determine relationship strength between cases and deaths and socio-economic and health systems. We organized qualitative information from the literature and conducted a thematic analysis to recognize patterns of cases and deaths and explain the findings from the quantitative data.
We have found that regions and countries with high human development index have higher cases and deaths per million population due to COVID-19. This is due to international connectedness and mobility of their population related to trade and tourism, and their vulnerability related to older populations and higher rates of non-communicable diseases. We have also identified that the burden of the pandemic is also variable among high- and middle-income countries due to differences in the governance of the pandemic, fragmentation of health systems, and socio-economic inequities.
The COVID-19 pandemic demonstrates that every country remains vulnerable to public health emergencies. The aspiration towards a healthier and safer society requires that countries develop and implement a coherent and context-specific national strategy, improve governance of public health emergencies, build the capacity of their (public) health systems, minimize fragmentation, and tackle upstream structural issues, including socio-economic inequities. This is possible through a primary health care approach, which ensures provision of universal and equitable promotive, preventive and curative services, through whole-of-government and whole-of-society approaches.
The pandemic of Coronavirus Disease 2019 (COVID-19) is a timely reminder of the nature and impact of emerging infectious diseases that become Public Health Emergency of International Concern (PHEIC) [ 1 ]. The COVID-19 pandemic takes variable shapes and forms in how it affects communities in different regions and countries [ 2 , 3 ]. As of 12 January, 2022, there were over 314 million cases and over 5.5 million deaths notified around the globe since the start of the pandemic. The number of cases per million population ranged from 7410 in Africa to 131,730 in Europe while the number of deaths per million population ranged from 110 in Oceania to 2740 in South America. Case-fatality rates (CFRs) ranged from 0.3% in Oceania to 2.9% in South America [ 4 , 5 ]. Regions and countries with high human development index (HDI), which is a composite index of life expectancy, education, and per capita income indicators [ 6 ], are affected by COVID-19 more than regions with low HDI. North America and Europe together account for 55 and 51% of cases and deaths, respectively. Regions with high HDI are affected by COVID-19 despite their high universal health coverage index (UHCI) and Global Health Security index (GHSI) [ 7 ].
This seems to be a paradox (against the established knowledge that countries with weak (public) health systems capacity will have worse health outcomes) in that the countries with higher UHCI and GHSI have experienced higher burdens of COVID-19 [ 7 ]. The paradox can partially be explained by variations in testing algorithms, capacity for testing, and reporting across different countries. Countries with high HDI have health systems with a high testing capacity; the average testing rate per million population is less than 32, 000 in Africa and 160,000 in Asia while it is more than 800, 000 in HICs (Europe and North America). This enables HICs to identify more confirmed cases that will ostensibly increase the number of reported cases [ 3 ]. Nevertheless, these are insufficient to explain the stark differences between countries with high HDI and those with low HDI. Many countries with high HDI have a high testing rate and a higher proportion of symptomatic and severe cases, which are also associated with higher deaths and CFRs [ 7 ]. On the other hand, there are countries with high HDI that sustain a lower level of the epidemic than others with a similar high HDI. It is, therefore, vital to analyse the heterogeneity of the COVID-19 pandemic and explain why some countries with high HDI, UHCI and GHSI have the highest burden of COVID-19 while others are able to suppress their epidemics and mitigate its impacts.
The objective of this study was to analyse the COVID-19 pandemic and understand its variable expression with the intention to learn lessons for an effective and sustainable response to public health emergencies. We hypothesised that high levels of HDI, UHCI and GHSI are essential but not sufficient to prevent and control COVID-19.
We conducted an explanatory mixed-methods study to understand and explain the heterogeneity of the pandemic around the world. The study integrated quantitative and qualitative secondary data. The following steps were included in the research process: (i) collecting and analysing quantitative epidemiological data, (ii) conducting literature review of qualitative secondary data and (iii) evaluating countries’ pandemic responses to explain the variability in the COVID-19 epidemiological outcomes. The study then illuminated specific factors that were vital towards an effective and sustainable epidemic response.
We used the publicly available secondary data sources from Johns Hopkins University ( https://coronavirus.jhu.edu/data/new-cases ) for COVID-19 and UNDP 2020 HDI report ( http://hdr.undp.org/en/2019-report ) for HDI, demographic and epidemiologic variables. These are open data sources which are regularly updated and utilized by researchers, policy makers and funders. We performed a correlation analysis of the COVID-19 pandemic. We determined the association between COVID-19 cases, severity, deaths and CFRs at the 0.01 and 0.05 levels (2-tailed). We used Spearman’s correlation analysis, as there is no normal distribution of the variables [ 8 ].
The UHCI is calculated as the geometric mean of the coverage of essential services based on 17 tracer indicators from: (1) reproductive, maternal, newborn and child health; (2) infectious diseases; (3) non-communicable diseases; and, (4) service capacity and access and health security [ 9 ]. The GHSI is a composite measure to assess a country’s capability to prevent, detect, and respond to epidemics and pandemics [ 10 ].
We then conducted a document review to explain the epidemic patterns in different countries. Secondary data was obtained from peer-reviewed journals, reputable online news outlets, government reports and publications by public health-related associations, such as the WHO. To explain the variability of COVID-19 across countries, a list of 14 indicators was established to systematically assess country’s preparedness, actual pandemic response, and overall socioeconomic and demographic profile in the context of COVID-19. The indicators used in this study include: 1) Universal Health Coverage Index, 2) public health capacity, 3) Global Health Security Index, 4) International Health Regulation, 5) leadership, governance and coordination of response, 6) community mobilization and engagement, 7) communication, 8) testing, quarantines and social distancing, 9) medical services at primary health care facilities and hospitals, 10) multisectoral actions, 11) social protection services, 12) absolute and relative poverty status, 13) demography, and 14) burden of communicable and non-communicable diseases. These indicators are based on our previous studies and recommendation from the World Health Organization [ 3 , 4 ]. We conducted thematic analysis and synthesis to identify the factors that may explain the heterogeneity of the pandemic.
Heterogeneity of COVID-19 cases and deaths around the world: what can explain it?
Table 1 indicates that the pandemic of COVID-19 is heterogeneous around regions of the world. Figure 1 also shows that there is a strong and significant correlation between HDI and globalisation (with an increase in trade and tourism as proxy indicators) and a corresponding strong and significant correlation with COVID-19 burden.
Human development index and its correlates associated with COVID-19 in 189 countries*
Globalisation and pandemics interact in various ways, including through international trade and mobility, which can lead to multiple waves of infections [ 11 ]. In at least the first waves of the pandemic, countries with high import and export of consumer goods, food products and tourism have high number of cases, severe cases, deaths and CFRs. Countries with high HDI are at a higher risk of importing (and exporting) COVID-19 due to high mobility linked to trade and tourism, which are drivers of the economy. These may have led to multiple introductions of COVID-19 into these countries before border closures.
The COVID-19 pandemic was first identified in China, which is central to the global network of trade, from where it spread to all parts of the world, especially those countries with strong links with China [ 12 ]. The epidemic then spread to Europe. There is very strong regional dimension to manufacturing and trading, which could be facilitate the spread of the virus. China is the heart of ‘Factory Asia’; Italy is in the heart of ‘Factory Europe’; the United States is the heart of ‘Factory North America’; and Brazil is the heart of ‘Factory Latin America’ [ 13 ]. These are the countries most affected by COVID-19 during the first wave of the pandemic [ 2 , 3 , 14 ].
It is also important to note that two-third of the countries currently reporting more than a million cases are middle-income countries (MICs), which are not only major emerging market economies but also regional political powers, including the BRICS countries (Brazil, Russia, India and South Africa) [ 3 , 15 ]. These countries participate in the global economy, with business travellers and tourists. They also have good domestic transportation networks that facilitate the internal spread of the virus. The strategies that helped these countries to become emerging markets also put them at greater risk for importing and spreading COVID-19 due to their connectivity to the rest of the world.
In addition, countries with high HDI may be more significantly impacted by COVID-19 due to the higher proportion of the elderly and higher rates of non-communicable diseases. Figure 1 shows that there is a strong and significant correlation between HDI and demographic transition (high proportion of old-age population) and epidemiologic transition (high proportion of the population with non-communicable diseases). Countries with a higher proportion of people older than 65 years and NCDs (compared to communicable diseases) have higher burden of COVID-19 [ 16 , 17 , 18 , 19 , 20 ]. Evidence has consistently shown a higher risk of severe COVID-19 in older individuals and those with underlying health conditions [ 21 , 22 , 23 , 24 , 25 ]. CFR is age-dependent; it is highest in persons aged ≥85 years (10 to 27%), followed by those among persons aged 65–84 years (3 to 11%), and those among persons aged 55-64 years (1 to 3%) [ 26 ].
On the other hand, regions and countries with low HDI have, to date, experienced less severe epidemics. For instance, as of January 12, 2022, the African region has recorded about 10.3 million cases and 233,000 deaths– far lower than other regions of the world (Table 1 ) [ 27 ]. These might be due to lower testing rates in Africa, where only 6.5% of the population has been tested for the virus [ 14 , 28 ], and a greater proportion of infections may remain asymptomatic [ 29 ]. Indeed, the results from sero-surveys in Africa show that more than 80% of people infected with the virus were asymptomatic compared to an estimated 40-50% asymptomatic infections in HICs [ 30 , 31 ]. Moreover, there is a weak vital registration system in the region indicating that reports might be underestimating and underreporting the disease burden [ 32 ]. However, does this fully explain the differences observed between Africa and Europe or the Americas?
Other possible factors that may explain the lower rates of cases and deaths in Africa include: (1) Africa is less internationally connected than other regions; (2) the imposition of early strict lockdowns in many African countries, at a time when case numbers were relatively small, limited the number of imported cases further [ 2 , 33 , 34 ]; (3) relatively poor road network has also limited the transmission of the virus to and in rural areas [ 35 ]; (4) a significant proportion of the population resides in rural areas while those in urban areas spend a lot of their time mostly outdoors; (5) only about 3% of Africans are over the age of 65 (so only a small proportion are at risk of severe COVID-19) [ 36 ]; (6) lower prevalence of NCDs, as disease burden in Africa comes from infectious causes, including coronaviruses, which may also have cross-immunity that may reduce the risk of developing symptomatic cases [ 37 ]; and (7) relative high temperature (a major source of vitamin D which influences COVID-19 infection and mortality) in the region may limit the spread of the virus [ 38 , 39 ]. We argue that a combination of all these factors might explain the lower COVID-19 burden in Africa.
The early and timely efforts by African leaders should not be underestimated. The African Union, African CDC, and WHO convened an emergency meeting of all African ministers of health to establish an African taskforce to develop and implement a coordinated continent-wide strategy focusing on: laboratory; surveillance; infection prevention and control; clinical treatment of people with severe COVID-19; risk communication; and supply chain management [ 40 ]. In April 2021, African Union and Africa CDC launched the Partnerships for African Vaccine Manufacturing (PAVM), framework to expanding Africa’s vaccine manufacturing capacity for health security [ 41 ].
Heterogeneity of the pandemic among countries with high HDI: what can explain it?
Figures 2 and 3 illustrate the variability of cases and deaths due to the COVID-19 pandemic across high-income countries (HICs). Contrary to the overall positive correlation between high HDI and cases, deaths and fatality rates due to COVID-19, there are outlier HICs, which have been able to control the epidemic. Several HICs, such as New Zealand, Australia, South Korea, Japan, Denmark, Iceland, and Norway, managed to contain their epidemics (Figs. 2 and 3 ) [ 15 , 42 , 43 ]. It is important to note that most of these countries (especially the island states) have far less cross-border mobility than other HICs.
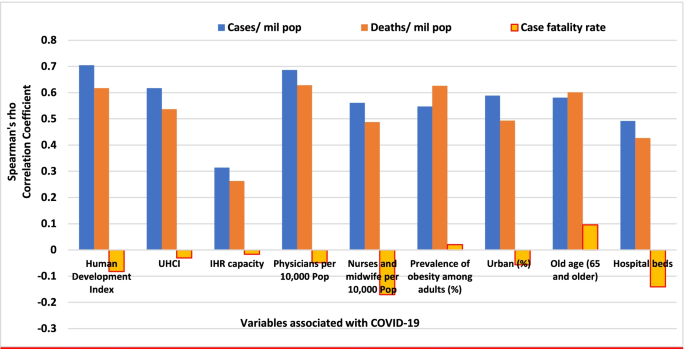
Scatter plot of COVID-19 cases per million population in countries with high human development index (> 0.70)
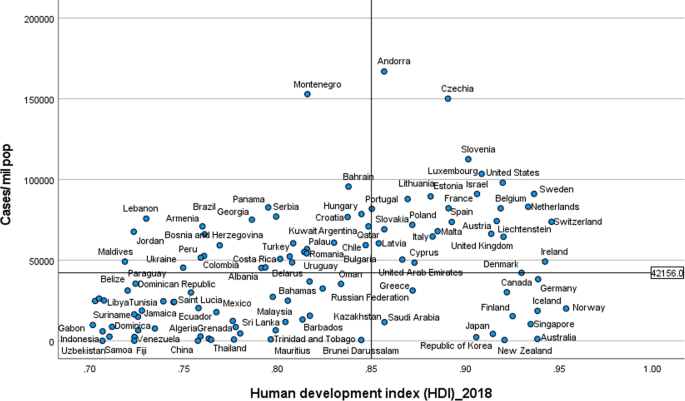
Scatter plot of COVID-19 deaths per million population in countries with high human development index (> 0.70)
HICs that have been successful at controlling their epidemics have similar characteristics, which are related to governance of the response [ 44 ], synergy between UHC and GHS, and existing relative socio-economic equity in the country. Governance and leadership is a crucial factor to explain the heterogeneity of the epidemic among countries with high HDI [ 45 ]. There has been substantial variation in the nature and timing of the public health responses implemented [ 46 ]. Adaptable and agile governments seem better able to respond to their epidemics [ 47 , 48 ]. Countries that have fared the best are the ones with good governance and public support [ 49 ]. Countries with an absence of coherent leadership and social trust have worse outcomes than countries with collective action, whether in a democracy or autocracy, and rapid mobilisation of resources [ 50 ]. The erosion of trust in the United States government has hurt the country’s ability to respond to the COVID-19 crisis [ 51 , 52 ]. The editors of the New England Journal of Medicine argued that the COVID-19 crisis has produced a test of leadership; but, the leaders in the United States had failed that test [ 47 ].
COVID-19 has exposed the fragility of health systems, not only in the public health and primary care, but also in acute and long-term care systems [ 49 ]. Fragmentation of health systems, defined here to mean inadequate synergy and/ or integration between GHS and UHC, is typical of countries most affected by the COVID-19 pandemic. Even though GHS and UHC agendas are convergent and interdependent, they tend to have different policies and practices [ 53 ]. The United States has the highest index for GHS preparedness; however, it has reported the world’s highest number of COVID-19 cases and deaths due to its greatly fragmented health system [ 54 , 55 ]. Countries with health systems and policies that are able to integrate International Health Regulations (IHR) core capacities with primary health care (PHC) services have been effective at mitigating the effects of COVID-19 [ 50 , 53 ]. Australia has been able to control its COVID-19 epidemic through a comprehensive primary care response, including protection of vulnerable people, provision of treatment and support services to affected people, continuity of regular healthcare services, protection and support of PHC workers and primary care services, and provision of mental health services to the community and the primary healthcare workforce [ 56 ]. Strict implementation of public health and social intervention together with UHC systems have ensured swift control of the epidemics in Singapore, South Korea, and Thailand [ 57 ].
The heterogeneity of cases and deaths, due to COVID-19, is also explained by differences in levels of socio-economic inequalities, which increase susceptibility to acquiring the infection and disease progression as well as worsening of health outcomes [ 58 ]. COVID-19 has been a stress test for public services and social protection systems. There is a higher burden of COVID-19 in Black, Asian and Minority Ethnic individuals due to socio-economic inequities in HICs [ 59 , 60 ]. Poor people are more likely to live in overcrowded accommodation, are more likely to have unstable work conditions and incomes, have comorbidities associated with poverty and precarious living conditions, and reduced access to health care [ 59 ].
The epidemiology of COVID-19 is also variable across MICs, with HDI between 0.70 and 0.85, around the world. Overall, the epidemic in MICs is exacerbated by the rapid demographic and epidemiologic transitions as well as high prevalence of obesity. While India and Brazil witnessed rapidly increasing rates of cases and deaths, China, Thailand, Vietnam have experienced a relatively lower disease burden [ 15 ]. This heterogeneity may be attributed to a number of factors, including governance, communication and service delivery. Thailand, China and Vietnam have implemented a national harmonized strategic response with decentralized implementation through provincial and district authorities [ 61 ]. Thailand increased its testing capacity from two to over 200 certified facilities that could process between 10,000 to 100,000 tests per day; moreover, over a million village health volunteers in Thailand supported primary health services [ 62 , 63 ]. China’s swift and decisive actions enabled the country to contain its epidemic though there was an initial delay in detecting the disease. China has been able to contain its epidemic through community-based measures, very high public cooperation and social mobilization, strategic lockdown and isolation, multi-sector action [ 64 ]. Overall, multi-level governance (effective and decisive leadership and accountability) of the response, together with coordination of public health and socio-economic services, and high levels of citizen adherence to personal protection, have enabled these countries to successfully contain their epidemics [ 61 , 65 , 66 ].
On the other hand, the Brazilian leadership was denounced for its failure to establish a national surveillance network early in the pandemic. In March 2020, the health minister was reported to have stated that mass testing was a waste of public funding, and to have advised against it [ 67 ]. This was considered as a sign of a collapse of public health leadership, characterized by ignorance, neoliberal authoritarianism [ 68 ]. There were also gaps in the public health capacity in different municipalities, which varied greatly, with a considerable number of Brazilian regions receiving less funding from the federal government due to political tension [ 69 ]. The epidemic has a disproportionate adverse burden on states and municipalities with high socio-economic vulnerability, exacerbated by the deep social and economic inequalities in Brazil [ 70 ].
India is another middle-income country with a high burden of COVID-19. It was one of the countries to institute strict measures in the early phase of the pandemic [ 71 , 72 ]. However, the government eased restrictions after the claim that India had beaten the pandemic, which lead to a rapid increase in disease incidence. Indeed, on 12 January 2022, India reported 36 million cumulative cases and almost 485,000 total deaths [ 15 ]. The second wave of the epidemic in India exposed weaknesses in governance and inadequacies in the country’s health and other social systems [ 73 ]. The nature of the Indian federation, which is highly centripetal, has prevented state and local governments from tailoring a policy response to suit local needs. A centralized one-size-fits-all strategy has been imposed despite high variations in resources, health systems capacity, and COVID-19 epidemics across states [ 74 ]. There were also loose social distancing and mask wearing, mass political rallies and religious events [ 75 ]. Rapid community transmission driven by high population density and multigenerational households has been a feature of the current wave in India [ 76 ]. In addition, several new variants of the virus, including the UK (B.1.1.7), the South Africa (20H/501Y or B.1.351), and Brazil (P.1), alongside a newly identified Indian variant (B.1.617), are circulating in India and have been implicated as factors in the second wave of the pandemic [ 75 , 76 ].
Heterogeneity of case-fatality rates around the world: what can explain it?
The pandemic is characterized by variable CFRs across regions and countries that are negatively associated with HDI (Fig. 1 ). The results presented in Fig. 4 show that the proportion of elderly population and rate of obesity are important factors which are positively associated with CFR. On the other hand, UHC, IHR capacity and other indicators of health systems capacity (health workforce density and hospital beds) are negatively associated with the CFR (Figs. 1 and 4 ).
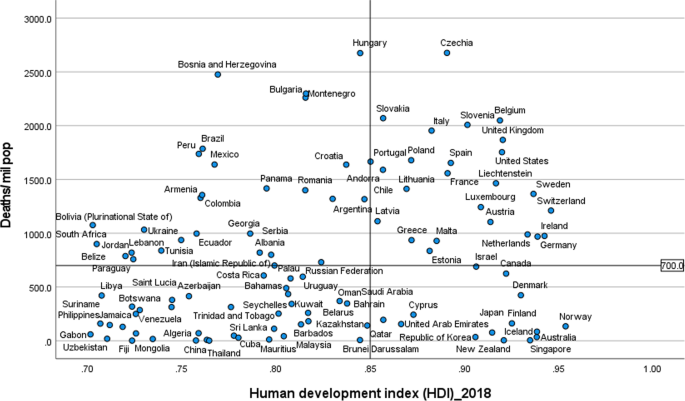
Correlates of COVID-19 cases, deaths and case-fatality rates in 189 countries
The evidence from several research indicates that heterogeneity can be explained by several factors, including differences in age-pyramid, socio-economic status, access to health services, or rates of undiagnosed infections. Differences in age-pyramid may explain some of the observed variation in epidemic severity and CFR between countries [ 77 ]. CFRs across countries look similar when taking age into account [ 78 ]. The elderly and other vulnerable populations in Africa and Asia are at a similar risk as populations in Europe and Americas [ 79 ]. Data from European countries suggest that as high as 57% of all deaths have happened in care homes and many deaths in the US have also occurred in nursing homes. On the other hand, in countries such as Mexico and India, individuals < 65 years contributed the majority of deaths [ 80 ].
Nevertheless, CFR also depends on the quality of hospital care, which can be used to judge the health system capacity, including the availability of healthcare workers, resources, and facilities, which affects outcomes [ 81 ]. The CFR can increase if there is a surge of infected patients, which adds to the strain on the health system [ 82 ]. COVID-19 fatality rates are affected by numerous health systems factors, including bed capacity, existence and capacity of intensive care unit (ICU), and critical care resources (such as oxygen and dexamethasone) in a hospital. Regions and countries with high HDI have a greater number of acute care facilities, ICU, and hospital bed capacities compared to lower HDI regions and countries [ 83 ]. Differences in health systems capacity could explain why North America and Europe, which have experienced much greater number of cases and deaths per million population, reported lower CFRs than the Southern American and the African regions, partly also due to limited testing capacity in these regions (Table 1 ) [ 84 , 85 , 86 ]. The higher CFR in Southern America can be explained by the relatively lower health systems surge capacity that could not adequately respond to the huge demand for health services [ 69 , 86 ]. The COVID-19 pandemic has highlighted existing health systems’ weaknesses, which are not able to effectively prepare for and respond to PHEs [ 87 ]. The high CFRs in the region are also exacerbated by the high social inequalities [ 69 ].
On the other hand, countries in Asia recorded lower CFRs (~ 1.4%) despite sharing many common risk factors (including overcrowding and poverty, weak health system capacity etc) with Africa. The Asian region shares many similar protective factors to the African region. They have been able to minimize their CFR by suppressing the transmission of the virus and flattening the epidemic curve of COVID-19 cases and deaths. Nevertheless, the epidemic in India is likely to be different because it has exceeded the health system capacity to respond and provide basic medical care and medical supplies such as oxygen [ 88 ]. Overall, many Asian countries were able to withstand the transmission of the virus and its effect due to swift action by governments in the early days of the pandemic despite the frequency of travel between China and neighbouring countries such as Hong Kong, Taiwan and Singapore [ 89 ]. This has helped them to contain the pandemic to ensure case numbers remain within their health systems capacity. These countries have benefited from their experience in the past in the prevention and control of epidemics [ 90 ].
There are a number of issues with the use of the CFR to compare the management of the pandemic between countries and regions [ 91 ], as it does not depict the true picture of the mortality burden of the pandemic. A major challenge with accurate calculation of the CFR is the denominator on number of identified cases, as asymptomatic infections and patients with mild symptoms are frequently left untested, and therefore omitted from CFR calculations. Testing might not be widely available, and proactive contact tracing and containment might not be employed, resulting in a smaller denominator, and skewing to a higher CFR [ 82 ]. It is, therefore, far more relevant to estimate infection fatality rate (IFR), the proportion of all infected individuals who have died due to the infection [ 91 ], which is central to understanding the public health impact of the pandemic and the required policies for its prevention and control [ 92 ].
Estimates of prevalence based on sero-surveys, which includes asymptomatic and mildly symptomatic infections, can be used to estimate IFR [ 93 ]. In a systematic review of 17 studies, seroprevalence rates ranged from 0.22% in Brazil to 53% in Argentina [ 94 ]. The review also identified that the seroprevalence estimate was higher than the cumulative reported case incidence, by a factor between 1.5 times in Germany to 717 times in Iran, in all but two studies (0.56 times in Brazil and 0.88 times in Denmark) [ 94 , 95 ]. The difference between seroprevalence and cumulative reported cases might be due to asymptomatic cases, atypical or pauci-symptomatic cases, or the lack of access to and uptake of testing [ 94 ]. There is only a modest gap between the estimated number of infections from seroprevalence surveys and the cumulative reported cases in regions with relatively thorough symptom-based testing. Much of the gap between reported cases and seroprevalence is likely to be due to undiagnosed symptomatic or asymptomatic infections [ 94 ].
Collateral effects of the COVID-19 pandemic
It is important to note that the pandemic has significant collateral effects on the provision of essential health services, in addition to the direct health effects [ 96 ]. Disruptions in the provision of essential health services, due to COVID-19, were reported by nearly all countries, though it is more so in lower-income than higher-income countries [ 97 , 98 ]. The biggest impact reported is on provision of day-to-day primary care to prevent and manage some of the most common health problems [ 99 ].
The causes of disruptions in service delivery were a mix of demand and supply factors [ 100 ]. Countries reported that just over one-third of services were disrupted due to health workforce-related reasons (the most common causes of service disruptions), supply chains, community mistrust and fears of becoming infected, and financial challenge s[ 101 ]. Cognizant of the disruptive effects of the pandemic, countries have reorganized their health system.
Countries with better response to COVID-19 have mobilized, trained and reallocated their health workforce in addition to hiring new staff, using volunteers and medical trainees and mobilizing retirees [ 102 ]. Several strategies have also been implemented to mitigate disruptions in service delivery and utilization, including: triaging to identify the most urgent patient needs, and postponing elective medical procedures; switching to alternative models of care, such as providing more home-based care and telemedicine [ 101 ].
This study identifies that the COVID-19 pandemic, in terms f cases and deaths, is heterogeneous around the world. This variability is explained by differences in vulnerability, preparedness, and response. It confirms that a high level of HDI, UHCI and GHSI are essential but not sufficient to control epidemics [ 103 ]. An effective response to public health emergencies requires a joint and reinforcing implementation of UHC, health emergency and disease control priorities [ 104 , 105 ], as well as good governance and social protection systems [ 106 ]. Important lessons have been learned to cope better with the COVID-19 pandemic and future emerging or re-emerging pandemics. Countries should strengthen health systems, minimize fragmentation of public health, primary care and secondary care, and improve coordination with other sectors. The pandemic has exposed the health effects of longstanding social inequities, which should be addressed through policies and actions to tackle vulnerability in living and working conditions [ 106 ].
The shift in the pandemic epicentre from high-income to MICs was observed in the second global wave of the pandemic. This is due to in part to the large-scale provision of vaccines in HICs [ 15 ] as well as the limitations in the response in LMICs, including inadequate testing, quarantine and isolation, contact tracing, and social distancing. The second wave of the pandemic in low- and middle-income countries spread more rapidly than the first wave and affected younger and healthier populations due to factors, including poor government decision making, citizen behaviour, and the emergence of highly transmissible SARS-CoV-2 variants [ 107 ]. It has become catastrophic in some MICs to prematurely relax key public health measures, such as mask wearing, physical distancing, and hand hygiene [ 108 ].
There is consensus that global vaccination is essential to ending the pandemic. Universal and equitable vaccine delivery, implemented with high volume, speed and quality, is vital for an effective and sustainable response to the current pandemic and future public health emergencies. There is, however, ongoing concern regarding access to COVID-19 vaccines in low-income countries [ 109 ]. Moreover, there is shortage of essential supplies, including oxygen, which has had a major impact on the prevention and control of the pandemic. It is, therefore, vital to transform (through good governance and financing mechanisms) the ACT-A platform to deliver vaccines, therapeutics, diagnostics, and other essential supplies [ 109 , 110 ]. The global health community has the responsibility to address these inequalities so that we can collectively end the pandemic [ 107 ].
The Omicron variant has a huge role in the current wave around the world despite high vaccine coverage [ 111 ]. Omicron appears to spread rapidly around the world ever since it was identified in November 2021 [ 112 ]. It becomes obvious that vaccination alone is inadequate for controlling the infection. This has changed our understanding of the COVID-19 pandemic endgame. The emergence of new variants of concern and their spread around the world has highlighted the importance of combination prevention, including high vaccination coverage in combination with other public health prevention measures [ 112 ].
Overall, the COVID-19 pandemic and the response to it emphasise valuable lessons towards an effective and sustainable response to public health emergencies. We argue that the PHC approach captures the different preparedness and response strategies required towards ensuring health security and UHC [ 113 ]. The PHC approach enables countries to progressively realize universal access to good-quality health services (including essential public health functions) and equity, empower people and communities, strengthen multi-sectoral policy and action for health, and enhance good governance [ 114 ]. These are essential in the prevention and control of public health emergencies, to suppress transmission, and reduce morbidity and mortality [ 115 ]. Access to high-quality primary care is at the foundation of any strong health system [ 116 ], which will, in turn, have effect on containing the epidemic, and reducing mortality and CFR [ 117 ]. Australia is a good example in this regard because it has implemented a comprehensive PHC approach in combination with border restrictions to ensure health system capacity is not exceeded [ 56 ]. The PHC approach will enable countries to develop and implement a context-specific health strategy, enhance governance, strengthen their (public) health systems, minimize segmentation and fragmentation, and tackle upstream structural issues, including discrimination and socio-economic inequities [ 118 ]. This is the type of public health approach (comprehensive, equity-focused and participatory) that will be effective and sustainable to tackle public health emergencies in the twenty-first century [ 119 , 120 ]. In addition, it is vital to transform the global and regional health systems, with a strong IHR and an empowered WHO at the apex [ 121 ]. We contend that this is the way towards a healthier and safer country, region and world.
The COVID-19 pandemic demonstrates that the world remains vulnerable to public health emergencies with significant health and other socio-economic impacts. The pandemic takes variable shapes and forms across regions and countries around the world. The pandemic has impacted countries with inadequate governance of the epidemic, fragmentation of their health systems and higher socio-economic inequities more than others. We argue that adequate response to public health emergencies requires that countries develop and implement a context-specific national strategy, enhance governance of public health emergency, build the capacity of their health systems, minimize fragmentation, and tackle socio-economic inequities. This is possible through a PHC approach that provides universal access to good-quality health services through empowered communities and multi-sectoral policy and action for health development. The pandemic has affected every corner of the world; it has demonstrated that “no country is safe unless other countries are safe”. This should be a call for a strong global health system based on the values of justice and capabilities for health.
Availability of data and materials
Data are available in a public, open access repository: Johns Hopkins University: https://coronavirus.jhu.edu/data/new-cases , and UNDP: http://hdr.undp.org/en/2019-report ; WHO: https://www.who.int/publications/m/item/weekly-epidemiological-update%2D%2D-22-december-2020
Abbreviations
Coronavirus Disease 2019
Case-fatality rates
Human development index
Universal health coverage index
Global Health Security index
High-income countries
Middle-income countries
El Zowalaty ME, Järhult JD. From SARS to COVID-19: A previously unknown SARS-CoV-2 virus of pandemic potential infecting humans–Call for a One Health approach. One Health. 2020;9:100124. https://doi.org/10.1016/j.onehlt.2020.100124 .
Van Damme W, Dahake R, Delamou A, Ingelbeen B, Wouters E, Vanham G, et al. The COVID-19 pandemic: diverse contexts; different epidemics—how and why? BMJ Glob Health. 2020;5(7):e003098.
Article PubMed Google Scholar
World Health Organization (WHO): Coronavirus disease ( COVID-19): situation report, 150. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200618-covid-19-sitrep-150.pdf .
Weekly epidemiological update - 22 December 2020 [ https://www.who.int/publications/m/item/weekly-epidemiological-update%2D%2D-22-december-2020 ].
Worldometer: COVID-19 coronavirus pandemic. 2020. https://www.worldometers.info/coronavirus/ .
Anand S, Sen A. Human Development Index: Methodology and Measurement. 1994. http://hdr.undp.org/en/content/human-development-index-methodology-and-measurement .
De Larochelambert Q, Marc A, Antero J, Le Bourg E, Toussaint J-F. Covid-19 mortality: a matter of vulnerability among nations facing limited margins of adaptation. Public Health. 2020;8. https://doi.org/10.3389/fpubh.2020.604339 .
de Winter JC, Gosling SD, Potter J. Comparing the Pearson and Spearman correlation coefficients across distributions and sample sizes: a tutorial using simulations and empirical data. Psychol Methods. 2016;21(3):273.
World Health Organization. Universal health coverage [ http://www.who.int/universal_health_coverage/en/ ].
Johns Hopkins Center for Health Security. Global Health Security Index [ https://www.ghsindex.org/ ].
Pol Antràs SJR. Esteban Rossi-Hansberg: how do globalisation and pandemics interact? Surprising insights from a new model. In: #LSEThinks | CEP | global development vol. 2020. London: London School of Economics; 2020.
Google Scholar
Cai P. Understanding China’s belt and road initiative; 2017.
Baldwin R, Tomiura E. Thinking ahead about the trade impact of COVID-19. Economics in the Time of COVID-19. 2020;59. https://repository.graduateinstitute.ch/record/298220?ln=en .
Organization WH: Coronavirus disease ( COVID-19): situation report, 182. 2020.
Johns Hopkins University: COVID-19 Map - Johns Hopkins Coronavirus Resource Center. In. Edited by Security JHUCfH; 2021. https://coronavirus.jhu.edu/map.html .
Wang T, Du Z, Zhu F, Cao Z, An Y, Gao Y, et al. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet. 2020;395(10228):e52.
Article CAS PubMed PubMed Central Google Scholar
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061–9.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–13.
Hussain A, Bhowmik B, Do Vale Moreira NC. COVID-19 and diabetes: knowledge in progress. Diabetes Res Clin Pract. 2020;162:108142. https://doi.org/10.1016/j.diabres.2020.108142 . Epub 2020 Apr 9.
Covid C, COVID C, COVID C, Chow N, Fleming-Dutra K, Gierke R, Hall A, et al. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019—United States, February 12–march 28, 2020. Morb Mortal Wkly Rep. 2020;69(13):382.
Group C-S. Characteristics of COVID-19 patients dying in Italy: report based on available data on March 20th, 2020. Rome: Instituto Superiore Di Sanita; 2020. https://www.epicentro.iss.it/coronavirus/bollettino/Report-COVID-2019_20_marzo_eng.pdf .
Guan W-j, Ni Z-y, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20.
Article CAS PubMed Google Scholar
Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. Jama. 2020;323(16):1612–4.
Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the new York City area. JAMA. 2020;323(20):2052–9. https://doi.org/10.1001/jama.2020.6775 .
Covid C, Team R. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–march 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343–6.
Article Google Scholar
Mbow M, Lell B, Jochems SP, Cisse B, Mboup S, Dewals BG, et al. COVID-19 in Africa: dampening the storm? Science. 2020;369(6504):624–6.
Kavanagh MM, Erondu NA, Tomori O, Dzau VJ, Okiro EA, Maleche A, et al. Access to lifesaving medical resources for African countries: COVID-19 testing and response, ethics, and politics. Lancet. 2020;395(10238):1735–8.
Nordling L. Africa's pandemic puzzle: why so few cases and deaths? In: American Association for the Advancement of Science; 2020.
Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med. 2020;173(5):362–7. https://doi.org/10.7326/M20-3012 . Epub 2020 Jun 3.
Nikolai LA, Meyer CG, Kremsner PG, Velavan TP. Asymptomatic SARS coronavirus 2 infection: invisible yet invincible. Int J Infect Dis. 2020;100:112–6. https://doi.org/10.1016/j.ijid.2020.08.076 . Epub 2020 Sep 3.
Rao C, Bradshaw D, Mathers CD. Improving death registration and statistics in developing countries: lessons from sub-Saharan Africa. Southern Afr J Demography. 2004:81–99.
Mehtar S, Preiser W, Lakhe NA, Bousso A, TamFum J-JM, Kallay O, et al. Limiting the spread of COVID-19 in Africa: one size mitigation strategies do not fit all countries. Lancet Glob Health. 2020;8(7):e881–3. Published online 2020 Apr 28. https://doi.org/10.1016/S2214-109X(20)30212-6 .
Nachega J, Seydi M, Zumla A. The late arrival of coronavirus disease 2019 (COVID-19) in Africa: mitigating pan-continental spread. Clin Infect Dis. 2020;71(15):875–8.
Gwilliam K, Foster V, Archondo-Callao R, Briceno-Garmendia C, Nogales A, Sethi K. Africa infrastructure country diagnostic: roads in sub-Saharan Africa: The World Bank; 2008.
Guengant J-P. Africa’s population: history, current status, and projections. In: Africa's Population: In Search of a Demographic Dividend: Springer; 2017. p. 11–31.
Collaborators GOD, Bernabe E, Marcenes W, Hernandez C, Bailey J, Abreu L, et al. Global, regional, and national levels and trends in burden of oral conditions from 1990 to 2017: a systematic analysis for the global burden of disease 2017 study. J Dent Res. 2020;99(4):362–73.
Cambaza EM, Viegas GC, Cambaza C. Manuel a: potential impact of temperature and atmospheric pressure on the number of cases of COVID-19 in Mozambique, southern Africa. J Public Health Epidemiol. 2020;12(3):246–60.
Lawal Y. Africa’s low COVID-19 mortality rate: a paradox? Int J Infect Dis. 2021;102:118–22.
Nkengasong JN, Mankoula W. Looming threat of COVID-19 infection in Africa: act collectively, and fast. Lancet. 2020;395(10227):841–2.
African Union and Africa CDC. African union and Africa CDC launches partnerships for African vaccine manufacturing (PAVM), framework to achieve it and signs 2 MoUs: African Union and Africa CDC; 2021.
Forbes. What Do Countries With The Best Coronavirus Responses Have In Common? Women Leaders [ https://www.forbes.com/sites/avivahwittenbergcox/2020/04/13/what-do-countries-with-the-best-coronavirus-reponses-have-in-common-women-leaders/#603bd9433dec ].
University of Notre Dame. What can we learn from Austria’s response to COVID-19? [ https://keough.nd.edu/what-can-we-learn-from-austrias-response-to-covid-19/ ].
Stoller JK. Reflections on leadership in the time of COVID-19. BMJ Leader. 2020. https://doi.org/10.1136/leader-2020-000244 .
Houston Public Media. What 6 Of The 7 Countries With The Most COVID-19 Cases Have In Common [ https://www.npr.org/sections/goatsandsoda/2020/07/31/896879448/the-nations-with-the-most-to-lose-from-covid-19 ].
Hale T, Petherick A, Phillips T, Webster S. Variation in government responses to COVID-19. Blavatnik school of government working paper. 2020;31. https://en.unesco.org/inclusivepolicylab/sites/default/files/learning/document/2020/4/BSG-WP-2020-031-v3.0.pdf .
The Editors. Dying in a leadership vacuum. N Engl J Med. 2020;383(15):1479–80.
The Cable News Network. US and UK are bottom of the pile in rankings of governments' handling of coronavirus pandemic [ https://edition.cnn.com/2020/08/27/world/global-coronavirus-attitudes-pew-intl/index.html ].
Huston P, Campbell J, Russell G, Goodyear-Smith F, Phillips RL, van Weel C, et al. COVID-19 and primary care in six countries. BJGP Open. 2020;4(4):bjgpopen20X101128. https://doi.org/10.3399/bjgpopen20X101128 .
Goodyear-Smith F, Kinder K, Eden AR, Strydom S, Bazemore A, Phillips R, et al. Primary care perspectives on pandemic politics. Glob Public Health. 2021;16(8-9):1304–19. https://doi.org/10.1080/17441692.2021.1876751 . Epub 2021 Jan 24.
Rutledge PE. Trump, COVID-19, and the war on expertise. Am Rev Public Adm. 2020;50(6-7):505–11.
Ugarte DA, Cumberland WG, Flores L, Young SD. Public attitudes about COVID-19 in response to president trump's social media posts. JAMA Netw Open. 2021;4(2):e210101.
Article PubMed PubMed Central Google Scholar
Lal A, Erondu NA, Heymann DL, Gitahi G, Yates R. Fragmented health systems in COVID-19: rectifying the misalignment between global health security and universal health coverage. Lancet. 2021;397(10268):61–7. https://doi.org/10.1016/S0140-6736(20)32228-5 . Epub 2020 Dec 1.
Tromberg BJ, Schwetz TA, Pérez-Stable EJ, Hodes RJ, Woychik RP, Bright RA, et al. Rapid scaling up of Covid-19 diagnostic testing in the United States—the NIH RADx initiative. N Engl J Med. 2020;383(11):1071–7.
Marwaha J, Halamka J, Brat G. Lifesaving ventilators will sit unused without a national data-sharing effort; 2020.
Kidd MR. Five principles for pandemic preparedness: lessons from the Australian COVID-19 primary care response. Br J Gen Pract. 2020;70(696):316–7. https://doi.org/10.3399/bjgp20X710765 . Print 2020 Jul.
Hsu LY, Tan M-H. What Singapore can teach the US about responding to COVID-19. STAT News. 2020. https://www.statnews.com/2020/03/23/singapore-teach-united-states-about-covid-19-response/ .
Horton R. Offline: COVID-19 is not a pandemic. Lancet (London, England). 2020;396(10255):874.
Article CAS Google Scholar
Raisi-Estabragh Z, McCracken C, Bethell MS, Cooper J, Cooper C, Caulfield MJ, et al. Greater risk of severe COVID-19 in black, Asian and minority ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25 (OH)-vitamin D status: study of 1326 cases from the UK biobank. J Public Health. 2020;42(3):451–60.
Hamidianjahromi A. Why African Americans are a potential target for COVID-19 infection in the United States. J Med Internet Res. 2020;22(6):e19934.
Tangcharoensathien V, Bassett MT, Meng Q, Mills A. Are overwhelmed health systems an inevitable consequence of covid-19? Experiences from China, Thailand, and New York State. BMJ. 2021;372. https://doi.org/10.1136/bmj.n83 .
Organization WH: COVID-19 health system response monitor, Thailand. 2020.
Narkvichien M. Thailand’s 1 million village health volunteers-“unsung heroes”-are helping guard communities nationwide from COVID-19. Nonthaburi: World Health Organization; 2020.
Kupferschmidt K, Cohen J. Can China's COVID-19 strategy work elsewhere? In: American Association for the Advancement of Science; 2020.
Al Saidi AMO, Nur FA, Al-Mandhari AS, El Rabbat M, Hafeez A, Abubakar A. Decisive leadership is a necessity in the COVID-19 response. Lancet. 2020;396(10247):295–8.
Forman R, Atun R, McKee M, Mossialos E. 12 lessons learned from the management of the coronavirus pandemic. Health Policy. 2020;124(6):577–80. https://doi.org/10.1016/j.healthpol.2020.05.008 .
Barberia LG, Gómez EJ. Political and institutional perils of Brazil's COVID-19 crisis. Lancet. 2020;396(10248):367–8.
Ortega F, Orsini M. Governing COVID-19 without government in Brazil: ignorance, neoliberal authoritarianism, and the collapse of public health leadership. Global Public Health. 2020;15(9):1257–77.
Ezequiel GE, Jafet A, Hugo A, Pedro D, Ana Maria M, Carola OV, et al. The COVID-19 pandemic: a call to action for health systems in Latin America to strengthen quality of care. Int J Qual Health Care. 2021;33(1):mzaa062.
Rocha R, Atun R, Massuda A, Rache B, Spinola P, Nunes L, et al. Effect of socioeconomic inequalities and vulnerabilities on health-system preparedness and response to COVID-19 in Brazil: a comprehensive analysis. Lancet Glob Health. 2021;9(6):e782–92. https://doi.org/10.1016/S2214-109X(21)00081-4 . Epub 2021 Apr 12.
Lancet T. India under COVID-19 lockdown. Lancet (London, England). 2020;395(10233):1315.
Siddiqui AF, Wiederkehr M, Rozanova L, Flahault A. Situation of India in the COVID-19 pandemic: India’s initial pandemic experience. Int J Environ Res Public Health. 2020;17(23):8994.
Article CAS PubMed Central Google Scholar
Taneja P, Bali AS. India’s domestic and foreign policy responses to COVID-19. Round Table. 2021;110(1):46–61.
Choutagunta A, Manish G, Rajagopalan S. Battling COVID-19 with dysfunctional federalism: lessons from India. South Econ J. 2021;87(4):1267–99.
Mallapaty S. India's massive COVID surge puzzles scientists. Nature. 2021;592(7856):667–8.
Thiagarajan K. Why is India having a covid-19 surge? In: British Medical Journal Publishing Group; 2021.
Fisman DN, Greer AL, Tuite AR. Age is just a number: a critically important number for COVID-19 case fatality. Ann Intern Med. 2020;173(9):762–3.
Sudharsanan N, Didzun O, Bärnighausen T, Geldsetzer P. The contribution of the age distribution of cases to COVID-19 case fatality across countries: a 9-country demographic study. Ann Intern Med. 2020;173(9):714–20. https://doi.org/10.7326/M20-2973 . Epub 2020 Jul 22.
Think Global Health. The Myth of South Asian Exceptionalism. In: He Myth of South Asian Exceptionalism: South Asia's young population conceals the effects that COVID-19 has on its older and more vulnerable people. vol. 2020; 2020.
Ioannidis JP, Axfors C, Contopoulos-Ioannidis DG. Population-level COVID-19 mortality risk for non-elderly individuals overall and for non-elderly individuals without underlying diseases in pandemic epicenters. Environ Res. 2020;188:109890.
Kim D-H, Choe YJ, Jeong J-Y. Understanding and interpretation of case fatality rate of coronavirus disease 2019. J Korean Med Sci. 2020;35(12):e137. https://doi.org/10.3346/jkms.2020.35.e137 .
Rajgor DD, Lee MH, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776–7.
Sorci G, Faivre B, Morand S. Explaining among-country variation in COVID-19 case fatality rate. Sci Rep. 2020;10(1):1–11.
Sen-Crowe B, Sutherland M, McKenney M, Elkbuli A. A closer look into global hospital beds capacity and resource shortages during the COVID-19 pandemic. J Surg Res. 2021;260:56–63.
Li M, Zhang Z, Cao W, Liu Y, Du B, Chen C, et al. Identifying novel factors associated with COVID-19 transmission and fatality using the machine learning approach. Sci Total Environ. 2021;764:142810.
Undurraga EA, Chowell G, Mizumoto K. COVID-19 case fatality risk by age and gender in a high testing setting in Latin America: Chile, march–august 2020. Infect Dis Poverty. 2021;10(1):1–11.
Taylor L. How Latin America is fighting covid-19, for better and worse. BMJ. 2020;370:m3319. https://doi.org/10.1136/bmj.m3319 .
Bhuyan A. Covid-19: India looks to import oxygen as cases surge, overwhelming hospitals. In: British Medical Journal Publishing Group; 2021.
An BY, Tang S-Y. Lessons from COVID-19 responses in East Asia: institutional infrastructure and enduring policy instruments. Am Rev Public Adm. 2020;50(6-7):790–800.
Chen H, Shi L, Zhang Y, Wang X, Sun G. A cross-country core strategy comparison in China, Japan, Singapore and South Korea during the early COVID-19 pandemic. Glob Health. 2021;17(1):1–10.
Kahathuduwa CN, Dhanasekara CS, Chin S-H. Case fatality rate in COVID-19: a systematic review and meta-analysis. J Prev Med Hyg. 2021;62(2):E311–20. https://doi.org/10.15167/2421-4248/jpmh2021.62.2.1627 . eCollection 2021 Jun.
Meyerowitz-Katz G, Merone L. A systematic review and meta-analysis of published research data on COVID-19 infection-fatality rates. Int J Infect Dis. 2020.
Seoane B. A scaling approach to estimate the COVID-19 infection fatality ratio from incomplete data. PLoS One. 2021;16(2):e0246831. https://doi.org/10.1371/journal.pone.0246831 . eCollection 2021.
Byambasuren O, Dobler CC, Bell K, Rojas DP, Clark J, McLaws M-L, et al. Comparison of seroprevalence of SARS-CoV-2 infections with cumulative and imputed COVID-19 cases: systematic review. PLoS One. 2021;16(4):e0248946.
Shakiba M, Nazemipour M, Salari A, Mehrabian F, Nazari SSH, Rezvani SM, et al. Seroprevalence of SARS-CoV-2 in Guilan Province, Iran, April 2020. Emerg Infect Dis. 2021;27(2):636.
Blanchet K, Alwan A, Antoine C, Cros MJ, Feroz F, Guracha TA, et al. Protecting essential health services in low-income and middle-income countries and humanitarian settings while responding to the COVID-19 pandemic. BMJ Glob Health. 2020;5(10):e003675.
Thome J, Coogan AN, Fischer M, Tucha O, Faltraco F. Challenges for mental health services during the 2020 COVID-19 outbreak in Germany. Psychiatry Clin Neurosci. 2020;74(7):407. https://doi.org/10.1111/pcn.13019 . Epub 2020 May 26.
Riley T, Sully E, Ahmed Z, Biddlecom A. Estimates of the potential impact of the COVID-19 pandemic on sexual and reproductive health in low-and middle-income countries. Int Perspect Sex Reprod Health. 2020;46:73–6.
World Health Organization (WHO): Maintaining essential health services: operational guidance for the COVID-19 context: interim guidance, 1 June 2020. In.: World Health Organization; 2020. https://www.who.int/publications/i/item/WHO-2019-nCoV-essential_health_services-2020.2 .
World Health Organization (WHO): Pulse survey on continuity of essential health services during the COVID-19 pandemic: interim report, 27 August 2020. In.: World Health Organization; 2020. https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-EHS_continuity-survey-2020.1 .
World Health Organization (WHO): Second round of the national pulse survey on continuity of essential health services during the COVID-19 pandemic: interim report, 22 April 2021. In.: World Health Organization; 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-EHS-continuity-survey-2021.1 .
Organization WH. COVID-19: operational guidance for maintaining essential health services during an outbreak: interim guidance, 25 march 2020: World Health Organization; 2020.
Chen Y-Y, Assefa Y. The heterogeneity of the COVID-19 pandemic and national responses: an explanatory mixed-methods study. BMC Public Health. 2021;21(1):1–15.
World Health Organization (WHO). Thirteenth general programme of work 2019–2023. The seventy-first world health assembly. Geneva (Switzerland): World Health Organization; 2018.
Mahjour J, Mirza Z, Rashidian A, Atta H, Hajjeh R, Thieren M, et al. " promote health, keep the world safe, serve the vulnerable" in the eastern Mediterranean region. East Mediterr Health J. 2018;24(4):323–4.
Rollston R, Galea S. COVID-19 and the social determinants of health. Am J Health Promot. 2020;34(6):687–9. https://doi.org/10.1177/0890117120930536b .
Nachega JB, Sam-Agudu NA, Masekela R, van der Zalm MM, Nsanzimana S, Condo J, et al. Addressing challenges to rolling out COVID-19 vaccines in African countries. Lancet Glob Health. 2021;9(6):e746–8. https://doi.org/10.1016/S2214-109X(21)00097-8 . Epub 2021 Mar 10.
Skegg D, Gluckman P, Boulton G, Hackmann H, Karim SSA, Piot P, et al. Future scenarios for the COVID-19 pandemic. Lancet. 2021;397(10276):777–8.
Nhamo G, Chikodzi D, Kunene HP, Mashula N. COVID-19 vaccines and treatments nationalism: challenges for low-income countries and the attainment of the SDGs. Global Public Health. 2021;16(3):319–39.
Figueroa JP, Bottazzi ME, Hotez P, Batista C, Ergonul O, Gilbert S, et al. Urgent needs of low-income and middle-income countries for COVID-19 vaccines and therapeutics. Lancet. 2021;397(10274):562–4.
He X, Hong W, Pan X, Lu G, Wei X. SARS-CoV-2 omicron variant: characteristics and prevention. Med Comm (2020). 2021;2(4):838–45. https://doi.org/10.1002/mco2.110 .
Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;398(10317):2126–8.
Sanders D, Nandi S, Labonté R, Vance C, Van Damme W. From primary health care to universal health coverage—one step forward and two steps back. Lancet. 2019;394(10199):619–21.
Hone T, Macinko J, Millett C. Revisiting Alma-Ata: what is the role of primary health care in achieving the sustainable development goals? Lancet. 2018;392(10156):1461–72.
Redwood-Campbell L, Abrahams J. Primary health care and disasters—the current state of the literature: what we know, gaps and next steps. Prehospital Disaster Med. 2011;26(3):184–91.
Bitton A, Ratcliffe HL, Veillard JH, Kress DH, Barkley S, Kimball M, et al. Primary health care as a foundation for strengthening health systems in low-and middle-income countries. J Gen Intern Med. 2017;32(5):566–71.
Dunlop C, Howe A, Li D, Allen LN. The coronavirus outbreak: the central role of primary care in emergency preparedness and response. BJGP Open. 2020;4(1):bjgpopen20X101041. https://doi.org/10.3399/bjgpopen20X101041 .
Assefa Y, Gilks CF, van de Pas R, Reid S, Gete DG, Van Damme W. Reimagining global health systems for the 21st century: lessons from the COVID-19 pandemic. BMJ Glob Health. 2021;6(4):e004882.
Loewenson R, Accoe K, Bajpai N, Buse K, Abi Deivanayagam T, London L, et al. Reclaiming comprehensive public health. BMJ Glob Health. 2020;5(9):e003886.
Rawaf S, Allen LN, Stigler FL, Kringos D, Quezada Yamamoto H, van Weel C, et al. Lessons on the COVID-19 pandemic, for and by primary care professionals worldwide. Eur J Gen Pract. 2020;26(1):129–33.
Gostin LO, Friedman EA. A retrospective and prospective analysis of the west African Ebola virus disease epidemic: robust national health systems at the foundation and an empowered WHO at the apex. Lancet. 2015;385(9980):1902–9.
Download references
Acknowledgements
Not applicable.
No funding was obtained for this study.
Author information
Authors and affiliations.
School of Public Health, the University of Queensland, Brisbane, Australia
Yibeltal Assefa, Charles F. Gilks, Simon Reid & Dereje Gedle Gete
Institute of Tropical Medicine, Antwerp, Belgium
Remco van de Pas & Wim Van Damme
You can also search for this author in PubMed Google Scholar
Contributions
YA conceived the idea of the research, collected and analysed the data, and prepared the first and subsequent drafts. DG participated in data collection and analysis. CFG, SR, RVDP, DG and WVD provided comments during subsequent drafts. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Yibeltal Assefa .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Assefa, Y., Gilks, C.F., Reid, S. et al. Analysis of the COVID-19 pandemic: lessons towards a more effective response to public health emergencies. Global Health 18 , 10 (2022). https://doi.org/10.1186/s12992-022-00805-9
Download citation
Received : 04 August 2021
Accepted : 14 January 2022
Published : 04 February 2022
DOI : https://doi.org/10.1186/s12992-022-00805-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Heterogeneity
Globalization and Health
ISSN: 1744-8603
- Submission enquiries: [email protected]
- General enquiries: [email protected]
REVIEW- Covid-19: Diagnosis, summary of essays and evolving approaches
Affiliations.
- 1 Department of Pharmaceutical Chemistry, College of Pharmacy, Jouf University, Sakaka, Aljouf, Saudi Arabia.
- 2 Faculty of Pharmacy, University of Central Punjab, Lahore, Pakistan.
- 3 Interdisciplinary Research Centre in Biomedical Materials, COMSATS Institute of Information Technology, Lahore, Pakistan.
- 4 School of Textile Science and Engineering, Tiangong University, Tianjin, China.
- 5 Department of Pharmaceutics, College of Pharmacy, Jouf University, Sakaka, Aljouf, Saudi Arabia.
- 6 Department of Clinical Laboratory Sciences, Jouf University, Sakaka, Aljouf, Saudi Arabia.
- 7 Department of Pharmaceutical Chemistry, College of Pharmacy, Jouf University, Sakaka, Aljouf, Saudi Arabia/ Department of Pharmaceutical Chemistry, College of Pharmacy, Omdurman Islamic University, Khartoum, Sudan.
- 8 Shanghai Key Laboratory of Atmospheric Particle Pollution and Prevention, Department of Environmental Science & Engineering, Fudan University, Shanghai, Peoples' Republic of China.
- PMID: 34803020
COVID-19 spread worldwide after its outbreak in December 2019. This review paper aims to educate the readers regarding SARS-CoV-2 diagnostic and detection tools and the issues experienced by researchers. We identify on-the-horizon point-of-care diagnostic tests and inspire scholars to develop their innovations past conception. It will also effectively avoid potential pandemics to establish plug-and-play diagnostic information to handle the SARS infection. The authors agree that arbitrary-access, interconnected systems with flexible functionality accessible at the point-of-care, would enable fast and precise diagnosis and tracking.
Publication types
- COVID-19 / diagnosis*
- COVID-19 Testing / methods
- False Positive Reactions
- Pandemics / prevention & control
- SARS-CoV-2 / pathogenicity
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Supplements
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 5, Issue 7
- The COVID-19 pandemic: diverse contexts; different epidemics—how and why?
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Wim Van Damme 1 ,
- http://orcid.org/0000-0002-4773-5341 Ritwik Dahake 2 ,
- Alexandre Delamou 3 ,
- Brecht Ingelbeen 1 ,
- Edwin Wouters 4 , 5 ,
- Guido Vanham 6 , 7 ,
- Remco van de Pas 1 ,
- http://orcid.org/0000-0003-1681-2604 Jean-Paul Dossou 1 , 8 ,
- http://orcid.org/0000-0003-1294-3850 Seye Abimbola 10 , 11 ,
- Stefaan Van der Borght 12 ,
- Devadasan Narayanan 13 ,
- Gerald Bloom 14 ,
- Ian Van Engelgem 15 ,
- Mohamed Ali Ag Ahmed 16 ,
- http://orcid.org/0000-0002-7000-3712 Joël Arthur Kiendrébéogo 1 , 17 , 18 ,
- Kristien Verdonck 1 ,
- Vincent De Brouwere 1 ,
- Kéfilath Bello 8 ,
- http://orcid.org/0000-0002-5867-971X Helmut Kloos 19 ,
- Peter Aaby 20 ,
- Andreas Kalk 21 ,
- http://orcid.org/0000-0002-2761-3566 Sameh Al-Awlaqi 22 ,
- http://orcid.org/0000-0003-0968-0826 NS Prashanth 23 ,
- Jean-Jacques Muyembe-Tamfum 24 ,
- Placide Mbala 24 ,
- Steve Ahuka-Mundeke 24 ,
- http://orcid.org/0000-0003-2393-1492 Yibeltal Assefa 25
- 1 Department of Public Health , Institute of Tropical Medicine , Antwerpen , Belgium
- 2 Independent Researcher , Bengaluru , India
- 3 Africa Centre of Excellence for Prevention and Control of Transmissible Diseases , Gamal Abdel Nasser University of Conakry , Conakry , Guinea
- 4 Department of Sociology and Centre for Population , University of Antwerp , Antwerpen , Belgium
- 5 Centre for Health Systems Research and Development , University of the Free State—Bloemfontein Campus , Bloemfontein , Free State , South Africa
- 6 Biomedical Department , Institute of Tropical Medicine , Antwerpen , Belgium
- 7 Biomedical Department , University of Antwerp , Antwerpen , Belgium
- 8 Public Health , Centre de recherche en Reproduction Humaine et en Démographie , Cotonou , Benin
- 9 National Institute of Public Health , Phnom Penh , Cambodia
- 10 School of Public Health , University of Sydney , Sydney , New South Wales , Australia
- 11 The George Institute for Global Health , Sydney , New South Wales , Australia
- 12 Board Member , Institute of Tropical Medicine , Antwerpen , Belgium
- 13 Health Systems Transformation Platform , New Delhi , India
- 14 Health and Nutrition Cluster , Institute of Development Studies , Brighton , UK
- 15 European Commission Directorate General for Civil Protection and Humanitarian Aid Operations , Kinshasa , Democratic Republic of Congo
- 16 University of Sherbrooke , Sherbrooke , Quebec , Canada
- 17 Public Health , University of Ouagadougou Health Sciences Training and Research Unit , Ouagadougou , Burkina Faso
- 18 Heidelberg Institute of Global Health, Medical Faculty and University Hospital , Heidelberg University , Heidelberg , Germany
- 19 Department of Epidemiology and Biostatistics , University of California San Francisco , San Francisco , California , USA
- 20 INDEPTH Network , Bandim Health Project , Bissau , Guinea-Bissau
- 21 Bureau GIZ à Kinshasa , Kinshasa , Democratic Republic of Congo
- 22 Center for International Health Protection , Robert Koch Institute , Berlin , Germany
- 23 Health Equity Cluster , Institute of Public Health , Bengaluru , India
- 24 Institut National de Recherche Biomédicale , Kinshasa , Democratic Republic of Congo
- 25 School of Public Health , The University of Queensland , Brisbane , Queensland , Australia
- Correspondence to Professor Wim Van Damme; wvdamme{at}itg.be
It is very exceptional that a new disease becomes a true pandemic. Since its emergence in Wuhan, China, in late 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, has spread to nearly all countries of the world in only a few months. However, in different countries, the COVID-19 epidemic takes variable shapes and forms in how it affects communities. Until now, the insights gained on COVID-19 have been largely dominated by the COVID-19 epidemics and the lockdowns in China, Europe and the USA. But this variety of global trajectories is little described, analysed or understood. In only a few months, an enormous amount of scientific evidence on SARS-CoV-2 and COVID-19 has been uncovered (knowns). But important knowledge gaps remain (unknowns). Learning from the variety of ways the COVID-19 epidemic is unfolding across the globe can potentially contribute to solving the COVID-19 puzzle. This paper tries to make sense of this variability—by exploring the important role that context plays in these different COVID-19 epidemics; by comparing COVID-19 epidemics with other respiratory diseases, including other coronaviruses that circulate continuously; and by highlighting the critical unknowns and uncertainties that remain. These unknowns and uncertainties require a deeper understanding of the variable trajectories of COVID-19. Unravelling them will be important for discerning potential future scenarios, such as the first wave in virgin territories still untouched by COVID-19 and for future waves elsewhere.
- public health
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bmjgh-2020-003098
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Summary box
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, has spread to nearly all countries of the world in only a few months. It is unique that an emerging respiratory virus becomes a pandemic, and can continue human-to-human transmission unabated, probably permanently.
Depending on the context, the trajectory and the impact of the COVID-19 epidemic vary widely across affected countries. This is in fact the case with most infectious diseases.
Despite limited initial knowledge on COVID-19, most societies have deployed draconian measures, including lockdowns, to contain the virus and mitigate its impact. This had variable success, but invariably with profound socioeconomic collateral effects.
Through research and rapid sharing of its findings, progressively more insights on SARS-CoV-2 and COVID-19 have been uncovered (knowns), mainly based on evidence from China, Europe and the USA; however, important knowledge gaps remain (unknowns).
The different COVID-19 epidemics and the responses unfolding in the Global South are little described, analysed or understood. Insights from these less researched contexts are important for discerning potential future scenarios, not only for the first wave in virgin territories still untouched by COVID-19, but also for future waves.
More understanding of lived experiences of people in a variety of contexts is necessary to get a full global picture and allow learning from this variety.
BMJ Global Health and Emerging Voices for Global Health have launched a call for such on-the-ground narratives and analyses on the epidemics of, and responses to, COVID-19.
Introduction
Late in 2019, a cluster of acute respiratory disease in Wuhan, China, was attributed to a new coronavirus, 1–3 later named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). 4 It was soon discovered that the virus is easily transmitted, can cause severe disease and can be quite lethal especially in the elderly and those with comorbidities. 5–8 The new human disease is called COVID-19. 9 Soon it became clear that its global spread was unstoppable. Even with draconian containment measures, such as strict movement restrictions, the so-called lockdown, it spread, and within a few months reached almost all countries and was declared a pandemic by the WHO. 10 Table 1 summarises key events in the unfolding of the COVID-19 pandemic, from December 2019 to May 2020.
- View inline
Key events in the COVID-19 pandemic, December 2019–May 2020
This progression is quite unique. New human pathogens emerge frequently from an animal host, but most cause only a local outbreak. Human-to-human transmission stops at some point, and the virus can only re-emerge as a human pathogen from its animal host. Only very rarely does an emerging pathogen become a pandemic. Over the past decades, a totally new pathogen emerged, caused serious disease, and spread around the globe continuously only once before: the HIV. It seems increasingly likely that SARS-CoV-2 transmission will be continuing. All countries are now facing their own ‘COVID-19 epidemic’.
In only a few months, the scientific community has started to learn the virus’s characteristics and its manifestations in different contexts. 11 But we fail to understand fully why the virus spreads at different speeds and affects populations differently. Our main objective is to make sense of those different expressions of the COVID-19 pandemic, to understand why COVID-19 follows variable trajectories in ways that are often quite different from the collective image created by the mediatisation of the dramatic COVID-19 epidemics in densely populated areas.
We start by exploring the role of context, followed by a brief summary of what is already known at the time of writing about SARS-CoV-2 and COVID-19. We then compare these knowns with what is known of some other viral respiratory pathogens and identify the critical unknowns. We also discuss the coping strategies and collective strategies implemented to contain and mitigate the effect of the epidemic. We finally look ahead to potential future scenarios.
The unfolding COVID-19 pandemic: importance of context
Initially, human-to-human transmission was documented in family/friends clusters. 12–17 Progressively, it became clear that superspreading events, typically during social gatherings such as parties, religious services, weddings, sports events and carnival celebrations, have played an important role. 18–21 Dense transmission has also been documented in hospitals 22 and nursing homes possibly through aerosols. 23 24
SARS-CoV-2 has spread around the world through international travellers. The timing of the introduction of SARS-CoV-2 has largely depended on the intensity of connections with locations with ongoing COVID-19 epidemics; thus, it reached big urban centres first and, within these, often the most affluent groups. From there, the virus has spread at variable speeds to other population groups. 25 26
As of May 2020, the most explosive COVID-19 epidemics observed have been in densely populated areas in temperate climates in relatively affluent countries. 27 The COVID-19 pandemic and the lockdowns have been covered intensively in the media and have shaped our collective image of the COVID-19 epidemic, both in the general public and in the scientific community.
The COVID-19 epidemic has spread more slowly and less intensively in rural areas, in Africa and the Indian subcontinent, and the rural areas of low and lower-middle income countries (LICs/LMICs). Not only the media but also the scientific community has paid much less attention to these realities, emerging later and spreading more slowly.
The dominant thinking has been that it is only a question of time before dramatic epidemics occur everywhere. This thinking, spread globally by international public health networks, has been substantiated by predictive mathematical models based largely on data from the epidemics of the Global North. However, what has been observed elsewhere is quite different although not necessarily less consequential. 28
The effects of the COVID-19 epidemic manifest in peculiar ways in each context. In the early stages of the COVID-19 epidemic in sub-Saharan Africa, the virus first affected the urban elites with international connections. From there, it was seeded to other sections of the society more slowly. In contrast, the collateral effects of a lockdown, even partial in many cases, are mostly felt by the urban poor, as ‘stay home’ orders abruptly intensify hardship for those earning their daily living in the informal urban economy. Governments of LICs/LMICs lack the budgetary space to grant generous benefit packages to counter the socioeconomic consequences. International agencies are very thinly spread, as the pandemic has been concurrent everywhere. Donor countries have focused mainly on their own COVID-19 epidemics.
The epidemic is thus playing out differently in different contexts. Many factors might explain SARS-CoV-2 transmission dynamics. Climate, population structure, social practices, pre-existing immunity and many other variables that have been explored are summarised in table 2 .
Contextual variables potentially influencing transmission of severe acute respiratory syndrome coronavirus 2
Although all these variables probably play some role, many uncertainties remain. It is difficult to assess how much these variables influence transmission in different contexts. It is even more difficult to assess how they interact and change over time and influence transmission among different social groups, resulting in the peculiar COVID-19 epidemic in any particular context.
Insights from other viruses
We do not attempt to give a complete overview of viruses but select only those viruses that emerged recently and caused epidemics such as Ebola, that have obvious similarities in transmission patterns such as influenza and measles, or that are closely related such as other coronaviruses.
Emerging viral respiratory pathogens
Respiratory viruses such as severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV) and avian influenza A and also Ebola have originated from animal hosts and caused human diseases ( table 3 ). These viruses do not continuously circulate from human to human. They create an outbreak only when there is interspecies cross-over transmission, most frequently from bats to another animal host.
Examples of emerging human respiratory viral diseases without sustained human-to-human transmission
The first human case of a disease from an emerging viral pathogen, the ‘index case’ or ‘patient zero’, is invariably someone in close contact with the originating animal host or an intermediary animal host. If this contact occurs in a remote rural community, the spread is usually slow, at low intensity, and could fade out before the pathogen gets a chance to spread to another community. The spread can suddenly intensify if seeded in a densely populated community, frequently in a particular context such as a hospital or during a social event, often referred to as a superspreading event. When the spread reaches a city, it can become a major outbreak, from where it can spread further; this happened with SARS-CoV in Hong Kong in 2003 and with Ebola in Conakry, Freetown and Monrovia in 2014–2016. 29 30 But at some stage human-to-human transmission is interrupted and the outbreak stops.
Only very exceptionally can a new viral pathogen sustain continuous human-to-human transmission. Other viral diseases such as measles and influenza are ‘old’ diseases; they have been studied in great depth. What can we learn from them?
Measles and influenza: the importance of context
It is thought that measles emerged thousands of years ago in the Middle East. It is assumed that a cross-over occurred from the rinderpest virus, 31 to become the human measles virus. Measles has since spread around the globe in continuous human-to-human transmission. When measles, along with other viruses such as smallpox and influenza, was introduced in the Americas by European conquerors, it contributed to a massive die-off of up to 90% of the original population. 32
The transmission dynamics of SARS-CoV-2 can be compared with influenza. Influenza typically causes yearly epidemics in temperate climates during winter with less seasonal patterns in tropical or subtropical regions. 33 34 In hotter climates, such as in sub-Saharan Africa 33 or South and Southeast Asia, 34 it is transmitted year round, often not identified as influenza. Such different epidemic patterns of influenza are still incompletely understood but thought to be associated with temperature and humidity and human behavioural factors such as indoor crowding. 35
But, in contrast to SARS-CoV-2, the influenza virus is not new. Influenza is a very old disease, certainly circulating for several centuries. It has infected most human beings living on the planet already, many of them several times, leaving some immunity but no durable protection. The virus also mutates, giving rise to a new dominant strain every influenza season. Influenza is every year a slightly different virus (due to antigenic drift as a result of progressive mutations) with major differences every few decades (antigenic shift as a result of recombination with novel strains).
One such antigenic shift resulted in the 1918 H1N1 ‘Spanish’ Influenza pandemic, which had an estimated case fatality rate (CFR) of 2%–3%, killing millions. 36 Box 1 summarises some key facts about H1N1, including factors thought to be associated with its high CFR.
Pandemic H1N1 influenza, 1918–2009
The 1918 H1N1 virus probably infected one-third of the world’s population at that time (or ~500 million people). 84
The pandemic had three waves in quick succession; the second wave, in 1919, was worse than the first wave. 84
High mortality, especially in younger persons (5–15 years; ~25% of total deaths) in the 1918 pandemic, may have been due to antibody-dependent enhancement and ‘cytokine storms’. 84 Another possible explanation is that older persons had some protective cross-immunity from previous influenza outbreaks while younger persons did not.
H1N1 continued to circulate along with seasonal influenza viruses, often recombining to produce more severe local outbreaks, including other pandemics between 1918 and 2009, giving it the nickname ‘mother of all pandemics’.
The original 1918 H1N1 strain was replaced by A(H1N1)pdm09 virus that resulted from an antigenic shift and caused the 2009 H1N1 influenza pandemic.
The 2009 H1N1 virus originated in pigs in central Mexico in March 2009 and was responsible for an estimated 284 000 deaths worldwide with an estimated CFR<0.1%. 85 86
During the 2009 pandemic, mortality was much lower than in the 1918 pandemic. Higher mortality in persons younger than 65 years was related to cytokine storms. 87 A role of protective cross-immunity from previous influenza strains in older persons has been suggested.
After August 2010, the A(H1N1)pdm09 virus appeared to have integrated with circulating strains of influenza and continues to cause localised seasonal influenza outbreaks worldwide. 88
A major difference between COVID-19 and influenza is that SARS-CoV-2 is a new pathogen and influenza is not. At the time of writing (May 2020), SARS-CoV-2 has triggered an immune response in over 5 million confirmed infections (and probably in many more), definitely too few to create anything close to herd immunity. Calculations using an estimated reproductive number (R0) for SARS-CoV-2 suggest that herd immunity would require at least 60% of the population to have protective immunity (see box 2 ). 37
On the use of mathematical models during epidemics
A dominant way of studying the transmission dynamics of an infectious disease such as COVID-19, and predicting the amplitude and peak of the epidemic in a population (city, province, country) and analysing the effect of control measures is using mathematical models. Based on available data and several assumptions, a model attempts to predict the course of the epidemic, the expected number of infections, clinical cases and deaths over time. Critical is the effective reproductive number (Rt). When Rt >1, the number of cases in a population increases; when Rt <1, the number of cases decreases. A relatively simple and widely used model is the susceptible-exposed-infectious-recovered model, as used in the two papers recently published in BMJ Global Health on COVID-19 in Africa. 67 89 There are many more types of models, with varying degrees of complexity.
The use of such models has strengths and limitations. Building a mathematical model implies trade-offs between accuracy, transparency, flexibility and timeliness. A difficulty, in general, is that the parameters on which the model is based, the so-called assumptions are frequently uncertain ( table 7 ) and predictions can vary widely if any of the parameters are modestly different. This uncertainty is captured in a sensitivity analysis, leading to various possible quantitative outcomes, usually expressed as a range of plausible possibilities, between ‘worst-case’ and ‘best-case’ scenarios.
With a new disease such as COVID-19, certainly at the start of the outbreak, the parameters had to be based on very limited data from a particular context. However, many variables can widely differ across communities as they critically depend on contextual factors ( table 2 ). In mathematical models, all such uncertainties and unknowns are somehow hidden in the complex formulae of the model, as a quasi ‘black box’. Few people have the knowledge and skill to ‘open up the black box’.
As uncertainties in COVID-19 are large, the range of possibilities produced by a model is wide, with the worst-case scenario typically predicting catastrophic numbers of cases and deaths. Such predictions are often misunderstood by journalists, practitioners and policy-makers, with worst-case estimates getting the most attention, 68 not specifying the huge uncertainties.
Knowns, uncertainties and unknowns about COVID-19, as of May 2020
Like COVID-19, measles and influenza have different epidemic patterns in different contexts. This also is the case for cholera, tuberculosis, HIV/AIDS and most infectious diseases. The difference in patterns is most pronounced and so is easily understood with vector-borne and water-borne diseases. Epidemic patterns are also different for air-borne infections, although they are less easily understood. Transmission of respiratory viruses is influenced by factors related to the virus and the human host but also by factors related to the natural and human environment ( table 4 ).
Factors related to transmission patterns and severity of respiratory viruses
However, we are quite unable to explain fully which factor has which influence, how these factors vary among different social groups and how interdependent or isolated they are. We are certainly unable to fully model all these variables mathematically to explain the epidemic pattern across a variety of different contexts. Too many variables and their interrelations are difficult to quantify, and when all these factors change over time while the pathogen continues to spread in diverse societies, the complexity becomes daunting.
Understanding transmission dynamics is a bit less daunting for measles, as several variables are well known and rather constant across individuals and contexts. The natural transmission pattern of measles, before the introduction of vaccines, has been well described. Measles is mostly a childhood disease, but this is not the case in very remote communities, where measles transmission had been interrupted for extended periods (such as the Faroe Islands). 38 39 Measles affected all age groups when reaching new territories, causing dramatic first-wave epidemics, a phenomenon called ‘virgin soil epidemic’. 40 41 The latest stages of the global dissemination of measles have been well documented, including in Australia, the Fiji islands and the Arctic countries, where such virgin soil epidemics occurred in the 19th and the mid-20th centuries. 32 42 Fortunately, measles infection creates robust protective immunity and after a first wave becomes a typical childhood disease, affecting only those without any prior immunity. 43 Human-to-human transmission of measles virus in a community stops when the virus cannot find new susceptible human hosts and the so-called herd immunity is reached. 44 45 But transmission of measles continues elsewhere on the planet from where it can be reintroduced a few years later when the population without protective immunity has grown large enough to allow human-to-human transmission again.
The epidemic patterns of measles are easily understood as measles is highly infectious, creates disease in almost every infected person and leaves lifelong natural immunity. Measles circulation, prior to vaccination, was continuous only in large urban areas with high birth rates. Everywhere else reintroduction occurred typically every 3–5 years but sometimes only after 10 or 15 years in isolated rural communities (such as among nomadic groups in the Sahel), causing epidemics among all those without acquired immunity and having lost maternal antibodies. 46 These diverse patterns of measles epidemics have been fundamentally changed by variable coverage of measles vaccination. They can still help us make sense of the diversity of COVID-19 epidemics being observed in 2020.
Measles illustrates convincingly that the transmission pattern of a respiratory virus is strongly influenced by the demographic composition, density and mixing pattern of the population and the connectedness to big urban centres. Measles transmission is continuous only in some large urban areas. It presents in short epidemics everywhere else with variable periodicity. This transmission pattern may well be a bit similar for COVID-19. But it took thousands of years for measles to reach all human communities while SARS-CoV-2 spread to all countries in only a few months, despite measles being much more transmissible than SARS-CoV-2. Factors such as increased air travel and more dense community structures play bigger roles for SARS-CoV-2 than they did for measles.
Comparison with other pathogenic coronaviruses
SARS-CoV-2 has many close relatives. Six other human coronaviruses (HCoVs) are known to infect humans. SARS-CoV and MERS-CoV (causing SARS and MERS, respectively) are very rare and do not continuously circulate among humans. The other four (HCoV-229E, HCoV-OC43, HCoV-HKU1 and HCoV-NL63) cause the common cold or diarrhoea and continuously circulate and mutate frequently. 47 48 They can cause disease in the same person repeatedly. The typical coronavirus remains localised to the epithelium of the upper respiratory tract, causes mild disease and elicits a poor immune response, hence the high rate of reinfection (in contrast to SARS-CoV and MERS-CoV, which go deeper into the lungs and hence are relatively less contagious). There is no cross-immunity between HCoV-229E and HCoV-OC43, and new strains arise continually by mutation selection. 49
Coping strategies and collective strategies
How a virus spreads and its disease progresses depend not only on the variables described above ( table 4 ) but also on the human reactions deployed when people are confronted with a disease outbreak or the threat of an outbreak. All these variables combined result in what unfolds as ‘the epidemic’ and the diverse ways it affects communities.
What a population experiences during an epidemic is not fully characterised by the numbers of known infections and deaths at the scale of a country. Such numbers hide regional and local differences, especially in large and diverse countries. The epidemic reaches the different geographical areas of a country at different moments and with different intensities. It affects different communities in variable ways, influencing how these communities perceive it and react to it. What constitutes a local COVID-19 epidemic is thus also characterised by the perceptions and the reactions it triggers in the different sections of the society.
Even before the virus reaches a community, the threat of an epidemic already causes fear, stress and anxiety. Consequently, the threat or arrival of the epidemic also triggers responses, early or late, with various degrees of intensity and effectiveness. The response to an epidemic can be divided into individual and household actions (coping strategies), and collectively organised strategies (collective strategies). Coping strategies are the actions people and families take when disease threatens and sickness occurs, including the ways they try to protect themselves from contagion. Collective strategies are voluntary or mandated measures deployed by organised communities and public authorities in response to an epidemic. These include, among others, isolation of the sick or the healthy, implementation of hygiene practices and physical distancing measures. They can also include mobility restrictions such as quarantine and cordon sanitaire . Coping strategies and collective strategies also include treatment of the sick, which critically depends on the availability and effectiveness of diagnostic and therapeutic tools, and performance of the health system. Collective strategies also include research being deployed to further scientific insight and the development of diagnostic and therapeutic tools, potentially including a vaccine.
Implementation of these measures depends not only on resources available but also on the understanding and interpretation of the disease by both the scientific community and the community at large, influenced by the information people receive from scientists, public authorities and the media. This information is interpreted within belief systems and influenced by rumours, increasingly so over social media, including waves of fake news, recently labelled ‘infodemics’. 50
Coping strategies and collective strategies start immediately, while there are still many unknowns and uncertainties. Progressively, as the pandemic unfolds and scientists interpret observations in the laboratory, in the clinic, and in society, more insights are gained and inform the response.
Table 5 lists measures recommended by the WHO for preventing transmission and slowing down the COVID-19 epidemic. 51–53 ‘Lockdown’ first employed in early 2020 in Wuhan, China, is the label often given to the bundle of containment and mitigation measures promoted or imposed by public authorities, although the specific measures may vary greatly between countries. In China, lockdown was very strictly applied and enforced. It clearly had an impact, resulting in total interruption of transmission locally. 54 55
Measures recommended by the WHO for preventing transmission and slowing down the COVID-19 epidemic, 2020
This list or catalogue of measures is quite comprehensive; it includes all measures that at first sight seem to reduce transmission opportunities for a respiratory virus. However, knowledge is lacking about the effectiveness of each measure in different contexts. As a global health agency, the WHO recommends a ‘generic catalogue’ of measures from which all countries can select an appropriate mix at any one time depending on the phase of the epidemic, categorised in four transmission scenarios (no cases, first cases, first clusters, and community transmission). 52 However, under pressure to act and with little time to consider variable options, public authorities often adopted as ‘blueprint’ with limited consideration for the socioeconomic context. 53 56
The initial lockdown in China thus much inspired the collective strategies elsewhere. This has been referred to as ‘global mimicry’, 57 : the response is somehow partly ‘copy/paste’ from measures observed previously (strong path dependency).
Some epidemiologists in Northern Europe (including the UK, 58 Sweden 59 and the Netherlands 60 ) pleaded against strict containment measures and proposed that building up herd immunity against SARS-CoV-2 might be wiser. Towards early April 2020, it became increasingly clear that reaching herd immunity in the short term was illusive. Most countries thus backed off from the herd immunity approach to combating COVID-19 and implemented lockdowns. 61 The intensity of the lockdowns has been variable, ranging from very strict (‘Chinese, Wuhan style’), over intermediary (‘French/Italian/New York City style’ and ‘Hong Kong style’), to relaxed (‘Swedish style’), or piecemeal.
The effectiveness of lockdowns largely depends on at what stage of the epidemic they are started, and how intensively they are applied. This is quite variable across countries, depending on the understanding and motivation of the population and their perceived risk (‘willingness to adhere’), on the trust they have in government advice (‘willingness to comply’), and on the degree of enforcement by public authorities. The feasibility for different population groups to follow these measures depends largely on their socioeconomic and living conditions. It is obviously more difficult for people living in crowded shacks in urban slums to practise physical distancing measures and strict hand hygiene when water is scarce than for people living in wealthier parts of a city.
Collateral effects of the response
Every intervention against the COVID-19 epidemic has a certain degree of effect and comes at a cost with collateral effects. Each collective strategy (1) has intended and unintended consequences (some are more or less desirable); (2) is more or less feasible and/or acceptable in a given context and for certain subgroups in that society; (3) has a cost, not only in financial terms but in many other ways, such as restrictions on movement and behaviour, stress, uncertainty and others. These costs are more or less acceptable, depending on the perception of the risk and many societal factors; (4) can be implemented with more or less intensity; and (5) can be enforced more or less vigorously.
The balance between benefit and cost is crucial in judging whether measures are appropriate, which is very context specific. Furthermore, benefits and costs are also related to the positionality from which they are analysed: benefits for whom and costs borne by whom? More wealthy societies with strong social safety nets can afford increased temporary unemployment. This is much more consequential in poorer countries, where large proportions of the population live precarious lives and where public authorities cannot implement generous mitigation measures at scale.
The adherence to hygiene and distancing measures depends not only on living conditions but also on risk perception and cultural norms. Mass masking has been readily accepted in some Asian countries, where it was already broadly practised even before the COVID-19 epidemic. It remains more controversial in Western societies, some of which even have legal bans on veiling in public places.
Lockdowns are unprecedented and have triggered intensive public debate. Not surprisingly, the impact of lighter lockdowns on the transmission is much less impressive; they decrease transmission but do not stop it. Quite rapidly, the justification for lockdowns shifted from stopping transmission to ‘flattening the curve’. Also, once a lockdown is started, rationalised, explained and enforced, it is difficult to decide when to stop it. Exit scenarios, usually some form of progressive relaxation, are implemented with the knowledge that transmission will be facilitated again. 62
Knowns and unknowns about SARS-CoV-2/COVID-19
What we already know.
The available information on SARS-CoV-2 and the spectrum of COVID-19 disease is summarised in tables 6 and 7 . It is increasingly becoming clear that most transmission happens indoors and that superspreading events trigger intensive dissemination.
Knowns, uncertainties and unknowns about severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), as of May 2020
Relationship between the dose of the initial infectious inoculum, transmission dynamics and severity of the COVID-19 disease
Hypothesis:
The dose of the virus in the initial inoculum may be a missing link between the variation observed in the transmission dynamics and the spectrum of the COVID-19 disease. It is plausible that:
Viral dose in inoculum is related to severity of disease.
Severity of disease is related to viral shedding and transmission potential.
This hypothesis plays out potentially at three levels:
At individual level: a person infected with a small dose of viral inoculum will on average develop milder disease than a person infected with a high viral inoculum and vice versa.
At cluster level: a person with asymptomatic infection or mild disease will on average spread lower doses of virus in droplets and aerosols and is less likely to transmit disease; when the person transmits, the newly infected person is more likely to have milder disease than if infected by a severely ill person, who spreads on an average higher doses of virus. This causes clusters and chains of milder cases or of more severe cases.
At community level: in certain contexts, such as dense urban centres in moderate climates during the season when people live mostly indoors, the potential for intensive transmission and explosive outbreaks is high, especially during indoor superspreading events. In other contexts, such as in rural areas or in regions with hot and humid climate where people live mostly outdoors, intensive transmission and explosive outbreaks are less likely.
The virology and immunology of SARS-CoV-2/COVID-19 are being studied intensively. This is critical not only to understand what will potentially happen in future waves but also for the development of a vaccine. Some scientists and companies are very upbeat about the possibility of producing a vaccine in record time. Having a vaccine is one thing, but how effective it is, is quite another. As acquired immunity after a natural infection is probably not very robust ( table 6 ), it will also be challenging to trigger robust immunity with a vaccine, but perhaps it is not impossible. Many questions remain, some of which are summarised in table 8 .
Questions and considerations in case a COVID-19 vaccine is developed
Regarding the severity of COVID-19, initial fears of very high mortality have also lessened. It has progressively become clear that many infections remain asymptomatic, that severe disease is rare in children and young adults, and that mortality is heavily concentrated in the very old and those with comorbidities. Table 7 summarises a fuller overview of the present state of knowledge regarding COVID-19.
With COVID-19 epidemics unfolding rapidly, several of the variables in the transmission of SARS-CoV-2 and the disease spectrum of COVID-19 could be quantified. This allows for mathematical modelling. Several models have been quickly developed, leading to predictions of the speed of transmission and the burden of COVID-19 ( box 2 ). Predictive models developed by the Imperial College 63 ; the Center for Disease Dynamics, Economics & Policy and Johns Hopkins University 28 ; the Institute for Health Metrics and Evaluation 64 ; Harvard University 65 ; and the WHO, 66 including an ‘African model’, 67 are a few that are influencing containment strategies around the world.
Critical unknowns and uncertainties
Although the COVID-19 pandemic triggered unprecedented research efforts globally, with over 30 000 scientific papers published between January and April 2020, there are still critical unknowns and many uncertainties.
Tables 6 and 7 summarise many of the knowns, but their relative importance or weight is not clear. For instance, the virus can spread via droplets, hands, aerosols, fomites and possibly through the environment. However, the relative importance of these in various contexts is much less clear. These factors undoubtedly vary between settings, whether in hospitals, in elderly homes, or at mass events. The weight of the variables also probably differs between the seeding and initial spread in a community and the spread when it suddenly amplifies and intensifies. The importance of each variable probably also depends on climatic conditions, not only outdoors, but also on microclimates indoors, influenced by ventilation and air conditioning and built environments.
We summarise the critical unknowns in table 9 along some elements to consider in addressing the unknowns and thoughts on their importance.
Some critical unknowns in SARS-CoV-2 transmission
Uncertainty remains, leading to controversy and directly influencing the choice of containment measures. Controversy continues regarding when and where lockdown or more selective measures are equally effective with lower societal effects.
New evidence is being discovered rapidly. Some evidence comes from field observations and ecological studies; other evidence results from scientific experiments or observations in the laboratory and the clinic. Sense-making by combining insights from different observations and through the lens of various disciplines can lead to hypotheses that can be tested and verified or refuted. One such hypothesis is that there is a relationship between the dose of virus in the infectious inoculum and the severity of COVID-19 disease. Several intriguing observations in the current pandemic could be (partially) explained by such a relationship. We develop this hypothesis in box 3 , as an example of possible further research, to create new insight which may influence control strategies.
This viral inoculum theory is consistent with many observations from the early stages of the COVID-19 pandemic, but it is not easy to test scientifically.
Potential future scenarios of COVID-19
As COVID-19 is a new disease, we should make a distinction between (1) the current 2019–2020 ‘virgin soil pandemic’ caused by SARS-CoV-2, specifically in how it will further spread around the globe in the first wave, and (2) the potential future transmission in subsequent waves. In some countries, transmission will continue at lower levels. In other countries, such as China, the virus may have been eliminated but can be reintroduced in identical or mutated form.
For the current first wave, using influenza and the common cold as reasonable comparisons, it is possible that the major epidemics, as witnessed in Wuhan, northern Italy, or New York, will typically occur in temperate climates in the winter season. Some predict that such epidemics will last between 8 and 10 weeks (but this is just a plausible and reasonable comparison in analogy with seasonal influenza). It is possible that in hotter climates the transmission may become continuous, year round at lower levels. It is increasingly clear that hot climate does not exclude superspreading events as observed in Guayaquil, Ecuador and in various cities in Brazil. Ventilation, air-conditioning and crowded places may still create favourable environments for intensive transmission. It is also quite possible that the more difficult spread of SARS-CoV-2 in such climates may, in certain communities, be compensated for by human factors such as higher population density, closer human contacts and lesser hygiene (as, for instance, exist in urban slums in mega cities in low income countries). How all this plays out in sub-Saharan Africa, in its slums and remote areas, is still largely unknown. With SARS-CoV-2, transmission scenarios are mainly based on mathematical models despite their serious limitations ( box 2 ).
As the virus continues to circulate, it will progressively be less of a ‘new disease’ during subsequent waves. The immunity caused by the first epidemic will influence how the virus spreads and causes disease. Whether later waves will become progressively milder or worse, as observed in the 1918–19 Spanish influenza, is a matter of intense speculation. Both views seem plausible and the two are not necessarily mutually exclusive. Indeed, immunity should be defined on two levels: individual immunity and herd immunity. Individual immunity will dictate how mild or severe the disease will be in subsequent infections. Herd immunity could be defined in different communities/regions/countries that, in theory, could be fenced off, allowing only limited interaction with other areas, impacting the spread of the virus to more vulnerable populations.
The future is unknown, but we can think of likely futures and critical elements therein.
Some obvious critical elements are:
Will there be an effective vaccine? How soon? How effective? How available at scale? How acceptable?
Will there be an effective treatment? How soon? How effective? How available at scale?
The current first wave is unfolding in the absence of effective biomedical tools (no vaccine, no effective antiviral or immune-modulating medicine, only supportive treatment such as oxygen therapy). This comes close to what can be called a ‘natural evolution’ of the COVID-19 pandemic, mostly modified by the containment measures deployed ( table 5 ) and the effect of supportive treatment.
Progressively, we can learn more about the direct health effects of COVID-19 (morbidity and mortality), about appropriate individual and collective measures, 68 the various degrees of societal disruption and the collateral effects on other essential health services (eg, reluctance to use health services for other health problems, because of ‘corona fear’). Our growing knowledge may enable us to progressively improve our response.
Learning from the variety of ways the COVID-19 epidemic is unfolding across the globe provides important ‘ecological evidence’ and creates insights into its epidemiology and impacts. Until now, the insights gained on COVID-19 have been largely dominated by the COVID-19 epidemics in the Global North. More understanding of lived experiences of people in a variety of contexts, where the epidemic is spreading more slowly and with different impacts, is necessary to get a full global picture and allow learning from this variety. This is an important missing piece of the COVID-19 puzzle.
BMJ Global Health and Emerging Voices for Global Health have launched a call ( https://blogs.bmj.com/bmjgh/2020/05/26/from-models-to-narratives-and-back-a-call-for-on-the-ground-analyses-of-covid-19-spread-and-response-in-africa/ ) for such on-the-ground narratives and analyses of the spread of and response to COVID-19, local narratives and analyses that will hopefully help to further enrich our understanding of how and why the COVID-19 pandemic continues to unfold in multiple local epidemics along diverse trajectories around the globe.
Acknowledgments
We would like to thank Johan Leeuwenburg, Piet Kager, and Luc Bonneux for useful comments on a previous draft, the teams of the Riposte corona, INRB, Kinshasa and the Belgian Embassy in Kinshasa for welcoming and hosting WVD during his unscheduled extended stay in Kinshasa during the lockdown, March–June 2020. We are thankful to Mrs. Ann Byers for editing the manuscript at short notice.
- Stratton CW ,
- Tang Yi‐Wei
- Wang W , et al
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses
- Wang Y , et al
- Fan Y , et al
- Hu C , et al
- Wu P , et al
- World Health Organization
- Ko W-C , et al
- Wang Y-J , et al
- Ma AHY , et al
- Epaulard O ,
- Bénet T , et al
- Böhmer MM ,
- Buchholz U ,
- Corman VM , et al
- Chan JF-W ,
- Kok K-H , et al
- Capron I , et al
- Kucharski AJ , et al
- Kupferschmidt K
- The Guardian
- Wang M , et al
- Li Y , et al
- Chen Y , et al
- Wilson ME ,
- ourworldindata.org
- The Center For Disease Dynamics Economics & Policy
- Coltart CEM ,
- Lindsey B ,
- Ghinai I , et al
- Newman LP ,
- Paget J , et al
- Leclerc QJ ,
- Fuller NM ,
- Knight LE , et al
- Taubenberger JK
- Altmann DM ,
- Rhodes CJ ,
- Anderson RM
- FRANCIS JE ,
- Gajdusek DC
- Aaby P , et al
- Krugman S ,
- Friedman H , et al
- van den Driessche P ,
- Yuen K-S , et al
- Shi W , et al
- Burrell C ,
- Zarocostas J
- Ruktanonchai NW ,
- Zhou L , et al
- Kucharski AJ ,
- Russell TW ,
- Diamond C , et al
- Bedford J ,
- Giesecke J , et al
- Pancevski B
- National Geographic
- Gutiérrez P ,
- Ferguson NM ,
- Nedjati-Gilani G , et al
- Institute for Health Metrics and Evaluation
- Kissler SM ,
- Tedijanto C ,
- Goldstein E , et al
- Cabore JW ,
- Karamagi HC ,
- Kipruto H , et al
- Van Damme W ,
- Van Lerberghe W
- Fischetti M ,
- Krzywinski M
- Deslandes A ,
- Tandjaoui-Lambotte Y , et al
- Kumar H , et al
- Centers for Disease Control and Prevention
- Taubenberger JK ,
- Trifonov V ,
- Khiabanian H ,
- Dawood FS ,
- Iuliano AD ,
- Reed C , et al
- Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza ,
- Bautista E ,
- Chotpitayasunondh T , et al
- Pougué Biyong C , et al
- Dorigatti I , et al
- Epidemiology Working Group for NCIP Epidemic Response - Chinese Center for Disease Control and Prevention
- McGoogan JM
- Mizumoto K ,
- Zarebski A , et al
- National Institute of Infectious Diseases (NIID)
- Nishiura H ,
- Kobayashi T ,
- Miyama T , et al
- Heneghan C ,
- Brassey J ,
- Jefferson T , Centre for Evidence-Based Medicine, Nuffield Department of Primary Care Health Sciences, University of Oxford
- Redfield RR
- Hellewell J ,
- Jarvis CI , et al
- Streeck H ,
- Hartmann G ,
- Exner M , et al
- Docherty AB ,
- Harrison EM ,
- Green CA , et al
- Banerjee A ,
- Harris S , et al
- Khafaie MA ,
- Cyranoski D
- Wexler AS ,
- Cappa CD , et al
- Chiew CJ , et al
- van Doremalen N ,
- Bushmaker T ,
- Morris DH , et al
- Zhu B , et al
- Heijnen L ,
- Elsinga G , et al
- Edson J , et al
- de Roda Husman AM
- Cheng H-Y ,
- Liu D-P , et al
- Ferretti L ,
- Kendall M , et al
- Araujo MB ,
- Sajadi MM ,
- Habibzadeh P ,
- Vintzileos A , et al
- Majumder MS ,
- Liu D , et al
- Martelletti L ,
- Martelletti P
- Yang S , et al
- Gao H , et al
- Liang Y , et al
- Ye G , et al
- Bobrovitz N ,
- Yan T , et al
- Müller MA ,
- Li W , et al
- Vitale JN , et al
- Wheeler S , et al
- Britton T ,
- Trapman P ,
- Gomes MGM ,
- Corder RM ,
- King JG , et al
- Wei WI , et al
- Bar-On YM ,
- Flamholz A ,
- Phillips R , et al
- Chen Z , et al
- Achenbach J
- Nextstrain.com
- Larremore DB ,
- Ludvigsson JF
- Mytton OT ,
- Bonell C , et al
- Wei T , et al
- Sothmann P , et al
Twitter @Ingelbeen, @jdossou80, @seyeabimbola, @jarthurk, @@vdbrouwere, @SamehAlawlaqi, @prashanthns
Contributors WVD, RD, EW and YA conceived and designed the study. RD, GV, YA and WVD searched the literature and screened for new emerging evidence. WVD, RD and YA drafted successive versions of the manuscript and coordinated inputs from all coauthors. YA, SA, KV, BI, RvdP and HK contributed to writing the manuscript. AD, J-PD, PI, SVdB, DN, GB, IVE, MAAA, JAK, VDB, KB, PA, AK, SA-A, NSP, J-JM-T, PM and SA-M reviewed successive versions of the manuscript and oriented it, with a field-based and local gaze from Guinea, Benin, Cambodia, Belgium, India, the UK, Mali, Canada, Burkina Faso, Germany, the USA, Guinea-Bissau, the Democratic Republic of Congo, Yemen and Australia. All authors commented on subsequent versions of the manuscript and approved the final version. WVD attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement No additional data are available.
Read the full text or download the PDF:
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 03 October 2022
How COVID-19 shaped mental health: from infection to pandemic effects
- Brenda W. J. H. Penninx ORCID: orcid.org/0000-0001-7779-9672 1 , 2 ,
- Michael E. Benros ORCID: orcid.org/0000-0003-4939-9465 3 , 4 ,
- Robyn S. Klein 5 &
- Christiaan H. Vinkers ORCID: orcid.org/0000-0003-3698-0744 1 , 2
Nature Medicine volume 28 , pages 2027–2037 ( 2022 ) Cite this article
40k Accesses
128 Citations
489 Altmetric
Metrics details
- Epidemiology
- Infectious diseases
- Neurological manifestations
- Psychiatric disorders
The Coronavirus Disease 2019 (COVID-19) pandemic has threatened global mental health, both indirectly via disruptive societal changes and directly via neuropsychiatric sequelae after SARS-CoV-2 infection. Despite a small increase in self-reported mental health problems, this has (so far) not translated into objectively measurable increased rates of mental disorders, self-harm or suicide rates at the population level. This could suggest effective resilience and adaptation, but there is substantial heterogeneity among subgroups, and time-lag effects may also exist. With regard to COVID-19 itself, both acute and post-acute neuropsychiatric sequelae have become apparent, with high prevalence of fatigue, cognitive impairments and anxiety and depressive symptoms, even months after infection. To understand how COVID-19 continues to shape mental health in the longer term, fine-grained, well-controlled longitudinal data at the (neuro)biological, individual and societal levels remain essential. For future pandemics, policymakers and clinicians should prioritize mental health from the outset to identify and protect those at risk and promote long-term resilience.
Similar content being viewed by others
A longitudinal analysis of the impact of the COVID-19 pandemic on the mental health of middle-aged and older adults from the Canadian Longitudinal Study on Aging

Global prevalence of mental health issues among the general population during the coronavirus disease-2019 pandemic: a systematic review and meta-analysis
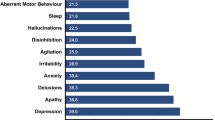
The effects of the COVID-19 pandemic on neuropsychiatric symptoms in dementia and carer mental health: an international multicentre study
In 2019, the COVID-19 outbreak was declared a pandemic by the World Health Organization (WHO), with 590 million confirmed cases and 6.4 million deaths worldwide as of August 2022 (ref. 1 ). To contain the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) across the globe, many national and local governments implemented often drastic restrictions as preventive health measures. Consequently, the pandemic has not only led to potential SARS-CoV-2 exposure, infection and disease but also to a wide range of policies consisting of mask requirements, quarantines, lockdowns, physical distancing and closure of non-essential services, with unprecedented societal and economic consequences.
As the world is slowly gaining control over COVID-19, it is timely and essential to ask how the pandemic has affected global mental health. Indirect effects include stress-evoking and disruptive societal changes, which may detrimentally affect mental health in the general population. Direct effects include SARS-CoV-2-mediated acute and long-lasting neuropsychiatric sequelae in affected individuals that occur during primary infection or as part of post-acute COVID syndrome (PACS) 2 —defined as symptoms lasting beyond 3–4 weeks that can involve multiple organs, including the brain. Several terminologies exist for characterizing the effects of COVID-19. PACS also includes late sequalae that constitute a clinical diagnosis of ‘long COVID’ where persistent symptoms are still present 12 weeks after initial infection and cannot be attributed to other conditions 3 .
Here we review both the direct and indirect effects of COVID-19 on mental health. First, we summarize empirical findings on how the COVID-19 pandemic has impacted population mental health, through mental health symptom reports, mental disorder prevalence and suicide rates. Second, we describe mental health sequalae of SARS-CoV-2 virus infection and COVID-19 disease (for example, cognitive impairment, fatigue and affective symptoms). For this, we use the term PACS for neuropsychiatric consequences beyond the acute period, and will also describe the underlying neurobiological impact on brain structure and function. We conclude with a discussion of the lessons learned and knowledge gaps that need to be further addressed.
Impact of the COVID-19 pandemic on population mental health
Independent of the pandemic, mental disorders are known to be prevalent globally and cause a very high disease burden 4 , 5 , 6 . For most common mental disorders (including major depressive disorder, anxiety disorders and alcohol use disorder), environmental stressors play a major etiological role. Disruptive and unpredictable pandemic circumstances may increase distress levels in many individuals, at least temporarily. However, it should be noted that the pandemic not only resulted in negative stressors but also in positive and potentially buffering changes for some, including a better work–life balance, improved family dynamics and enhanced feelings of closeness 7 .
Awareness of the potential mental health impact of the COVID-19 pandemic is reflected in the more than 35,000 papers published on this topic. However, this rapid research output comes with a cost: conclusions from many papers are limited due to small sample sizes, convenience sampling with unclear generalizability implications and lack of a pre-COVID-19 comparison. More reliable estimates of the pandemic mental health impact come from studies with longitudinal or time-series designs that include a pre-pandemic comparison. In our description of the evidence, we, therefore, explicitly focused on findings from meta-analyses that include longitudinal studies with data before the pandemic, as recently identified through a systematic literature search by the WHO 8 .
Self-reported mental health problems
Most studies examining the pandemic impact on mental health used online data collection methods to measure self-reported common indicators, such as mood, anxiety or general psychological distress. Pooled prevalence estimates of clinically relevant high levels of depression and anxiety symptoms during the COVID-19 pandemic range widely—between 20% and 35% 9 , 10 , 11 , 12 —but are difficult to interpret due to large methodological and sample heterogeneity. It also is important to note that high levels of self-reported mental health problems identify increased vulnerability and signal an increased risk for mental disorders, but they do not equal clinical caseness levels, which are generally much lower.
Three meta-analyses, pooling data from between 11 and 61 studies and involving ~50,000 individuals or more 13 , 14 , 15 , compared levels of self-reported mental health problems during the COVID-19 pandemic with those before the pandemic. Meta-analyses report on pooled effect sizes—that is, weighted averages of study-level effect sizes; these are generally considered small when they are ~0.2, moderate when ~0.5 and large when ~0.8. As shown in Table 1 , meta-analyses on mental health impact of the COVID-19 pandemic reach consistent conclusions and indicate that there has been a heterogeneous, statistically significant but small increase in self-reported mental health problems, with pooled effect sizes ranging from 0.07 to 0.27. The largest symptom increase was found when using specific mental health outcome measures assessing depression or anxiety symptoms. In addition, loneliness—a strong correlate of depression and anxiety—showed a small but significant increase during the pandemic (Table 1 ; effect size = 0.27) 16 . In contrast, self-reported general mental health and well-being indicators did not show significant change, and psychotic symptoms seemed to have decreased slightly 13 . In Europe, alcohol purchase decreased, but high-level drinking patterns solidified among those with pre-pandemic high drinking levels 17 . When compared to pre-COVID levels, no change in self-reported alcohol use (effect size = −0.01) was observed in a recent meta-analysis summarizing 128 studies from 58 (predominantly European and North American) countries 18 .
What is the time trajectory of self-reported mental health problems during the pandemic? Although findings are not uniform, various large-scale studies confirmed that the increase in mental health problems was highest during the first peak months of the pandemic and smaller—but not fully gone—in subsequent months when infection rates declined and social restrictions eased 13 , 19 , 20 . Psychological distress reports in the United Kingdom increased again during the second lockdown period 15 . Direct associations between anxiety and depression symptom levels and the average number of daily COVID-19 cases were confirmed in the US Centers for Disease Control and Prevention (CDC) data 21 . Studies that examined longer-term trajectories of symptoms during the first or even second year of the COVID-19 pandemic are more sparse but revealed stability of symptoms without clear evidence of recovery 15 , 22 . The exception appears to be for loneliness, as some studies confirmed further increasing trends throughout the first COVID-19 pandemic year 22 , 23 . As most published population-based studies were conducted in the early time period in which absolute numbers of SARS-CoV2-infected individuals were still low, the mental health impacts described in such studies are most likely due to indirect rather than direct effects of SARS-CoV-2 infection. However, it is possible that, in longer-term or later studies, these direct and indirect effects may be more intertwined.
The extent to which governmental policies and communication have impacted on population mental health is a relevant question. In cross-country comparisons, the extent of social restrictions showed a dose–response relationship with mental health problems 24 , 25 . In a review of 33 studies worldwide, it was concluded that governments that enacted stringent measures to contain the spread of COVID-19 benefitted not only the physical but also the mental health of their population during the pandemic 26 , even though more stringent policies may lead to more short-term mental distress 25 . It has been suggested that effective communication of risks, choices and policy measures may reduce polarization and conspiracy theories and mitigate the mental health impact of such measures 25 , 27 , 28 .
In sum, the general pattern of results is that of an increase in mental health symptoms in the population, especially during the first pandemic months, that remained elevated throughout 2020 and early 2021. It should be emphasized that this increase has a small effect size. However, even a small upward shift in mental health problems warrants attention as it has not yet shown to be returned to pre-pandemic levels, and it may have meaningful cumulative consequences at the population level. In addition, even a small effect size may mask a substantial heterogeneity in mental health impact, which may have affected vulnerable groups disproportionally (see below).
Mental disorders, self-harm and suicide
Whether the observed increase in mental health problems during the COVID-19 pandemic has translated into more mental disorders or even suicide mortality is not easy to answer. Mental disorders, characterized by more severe, disabling and persistent symptoms than self-reported mental health problems, are usually diagnosed by a clinician based on the International Classification of Diseases, 10th Revision (ICD-10) or the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) criteria or with validated semi-structured clinical interviews. However, during the COVID-19 pandemic, research systematically examining the population prevalence of mental disorders has been sparse. Unfortunately, we can also not strongly rely on healthcare use studies as the pandemic impacted on healthcare provision more broadly, thereby making figures of patient admissions difficult to interpret.
On a global scale and based on imputations and modeling from survey data of self-reported mental health problems, the Global Burden of Disease (GBD) study 29 estimated that the COVID-19 pandemic has led to a 28% (95% uncertainty interval (UI): 25–30) increase in major depressive disorders and a 26% (95% UI: 23–28) increase in anxiety disorders. It should be noted that these estimations come with high uncertainty as the assumption that transient pandemic-related increases in mental symptoms extrapolate into incident mental disorders remains disputable. So far, only four longitudinal population-based studies have measured and compared current mental (that is, depressive and anxiety) disorder prevalence—defined using psychiatric diagnostic criteria—before and during the pandemic. Of these, two found no change 30 , 31 , one found a decrease 32 and one found an increase in prevalence of these disorders 33 . These studies were local, limited to high-income countries, often small-scale and used different modes of assessment (for example, online versus in-person) before and during the pandemic. This renders these observational results uncertain as well, but their contrast to the GBD calculations 29 is striking.
Time-series analysis of monthly suicide trends in 21 middle-income to high-income countries across the globe yielded no evidence for an increase in suicide rates in the first 4 months of the pandemic, and there was evidence of a fall in rates in 12 countries 34 . Also in the United States, there was a significant decrease in suicide mortality in the first pandemic months but a slight increase in mortality due to drug overdose and homicide 35 . A living systematic review 36 also concluded that, throughout 2020, there was no observed increase in suicide rates in 20 studies conducted in North America, Europe and Asia. Analyses of electronic health record data in the primary care setting showed reduced rates of self-harm during the first COVID-19 pandemic year 37 . In contrast, emergency department visits for self-harm behavior were unchanged 38 or increased 39 . Such inconsistent findings across healthcare settings may reflect a reluctance in healthcare-seeking behavior for mental healthcare issues. In the living systematic review, eight of 11 studies that examined service use data found a significant decrease in reported self-harm/suicide attempts after COVID lockdown, which returned to pre-lockdown levels in some studies with longer follow-up (5 months) 36 .
In sum, although calculations based on survey data predict a global increase of mental disorder prevalence, objective and consistent evidence for an increased mental disorder, self-harm or suicide prevalence or incidence during the first pandemic year remains absent. This observation, coupled with the only small increase in mental health symptom levels in the overall population, may suggest that most of the general population has demonstrated remarkable resilience and adaptation. However, alternative interpretations are possible. First, there is a large degree of heterogeneity in the mental health impact of COVID-19, and increased mental health in one group (for example, due to better work–family balance and work flexibility) may have masked mental health problems in others. Various societal responses seen in many countries, such as community support activities and bolstering mental health and crisis services, may have had mitigating effects on the mental health burden. Also, the relationship between mental health symptom increases during stressful periods and its subsequent effects on the incidence of mental disorders may be non-linear or could be less visible due to resulting alternative outcomes, such as drug overdose or homicide. Finally, we cannot rule out a lag-time effect, where disorders may take more time to develop or be picked up, especially because some of the personal financial or social consequences of the COVID pandemic may only become apparent later. It should be noted that data from low-income countries and longer-term studies beyond the first pandemic year are largely absent.
Which individuals are most affected by the COVID-19 pandemic?
There is substantial heterogeneity across studies that evaluated how the COVID pandemic impacted on mental health 13 , 14 , 15 . Although our society as a whole may have the ability to adequately bounce back from pandemic effects, there are vulnerable people who have been affected more than others.
First, women have consistently reported larger increases in mental health problems in response to the COVID-19 pandemic than men 13 , 15 , 29 , 40 , with meta-analytic effect sizes being 44% 15 to 75% 13 higher. This could reflect both higher stress vulnerability or larger daily life disruptions due to, for example, increased childcare responsibilities, exposure to home violence or greater economic impact due to employment disruptions that all disproportionately fell to women 41 , thereby exacerbating the already existing pre-pandemic gender inequalities in depression and anxiety levels. In addition, adolescents and young adults have been disproportionately affected compared to younger children and older adults 12 , 15 , 29 , 40 . This may be the result of unfavorable behavioral and social changes (for example, school closure periods 42 ) during a crucial development phase where social interactions outside the family context are pivotal. Alarmingly, even though suicide rates did not seem to increase at the population level, studies in China 43 and Japan 44 indicated significant increases in suicide rates in children and adolescents.
Existing socio-cultural disparities in mental health may have further widened during the COVID pandemic. Whether the impact is larger for individuals with low socio-economic status remains unclear, with contrasting meta-analyses pointing toward this group being protected 15 or at increased risk 40 . Earlier meta-analyses did not find that the mental health impact of COVID-19 differed across Europe, North America, Asia and Oceania 13 , 14 , but data are lacking from Africa and South America. Nevertheless, a large-scale within-country comparison in the United States found that the mental health of Black, Hispanic and Asian respondents worsened relatively more during the pandemic compared to White respondents. Moreover, White respondents were more likely to receive professional mental healthcare during the pandemic, and, conversely, Black, Hispanic, and Asian respondents demonstrated higher levels of unmet mental healthcare needs during this time 45 .
People with pre-existing somatic conditions represent another vulnerable group in which the pandemic had a greater impact (pooled effect size of 0.25) 13 . This includes people with conditions such as epilepsy, multiple sclerosis or cardiometabolic disease as well as those with multiple comorbidities. The disproportionate impact may reflect this groupʼs elevated COVID-19 risk and, consequently, more perceived stress and fear of infection, but it could also reflect disruptions of regular healthcare services.
Healthcare workers faced increased workload, rapidly changing and challenging work environments and exposure to infections and death, accompanied by fear of infecting themselves and their families. High prevalences of (subthreshold) depression (13% 46 ), depressive symptoms (31% 47 ), (subthreshold) anxiety (16% 46 ), anxiety symptoms (23% 47 ) and post-traumatic stress disorder (~22% 46 , 47 ) have been reported in healthcare workers. However, a meta-analysis did not find a larger mental health impact of the pandemic as compared to the general population 40 , and another meta-analysis (of 206 studies) found that the mental health status of healthcare workers was similar to or even better than that of the general population during the first COVID year 48 . However, it is important to note that these meta-analyses could not differentiate between frontline and non-frontline healthcare workers.
Finally, individuals with pre-existing mental disorders may be at increased risk for exacerbation of mental ill-health during the pandemic, possibly due to disease history—illustrating a higher genetic and/or environmental vulnerability—but also due to discontinuity of mental healthcare. Already before the pandemic, mental health systems were under-resourced and disorganized in most countries 6 , 49 , but a third of all WHO member states reported disruptions to mental and substance use services during the first 18 months of the pandemic 50 , with reduced, shortened or postponed appointments and limited capacity for acute inpatient admissions 51 , 52 . Despite this, there is no clear evidence that individuals with pre-existing mental disorders are disproportionately affected by pandemic-related societal disruptions; the effect size for pandemic impact on self-reported mental health problems was similar in psychiatric patients and the general population 13 . In the United States, emergency visits for ten different mental disorders were generally stable during the pandemic compared to earlier periods 53 . In a large Dutch study 22 , 54 with multiple pre-pandemic and during-pandemic assessments, there was no difference in symptom increase among patients relative to controls (see Fig. 1 for illustration). In absolute terms, however, it is important to note that psychiatric patients show much higher symptom levels of depression, anxiety, loneliness and COVID-fear than healthy controls. Again, variation in mental health changes during the pandemic is large: next to psychiatric patients who showed symptom decrease due to, for example, experiencing relief from social pressures, there certainly have been many patients with symptom increases and relapses during the pandemic.
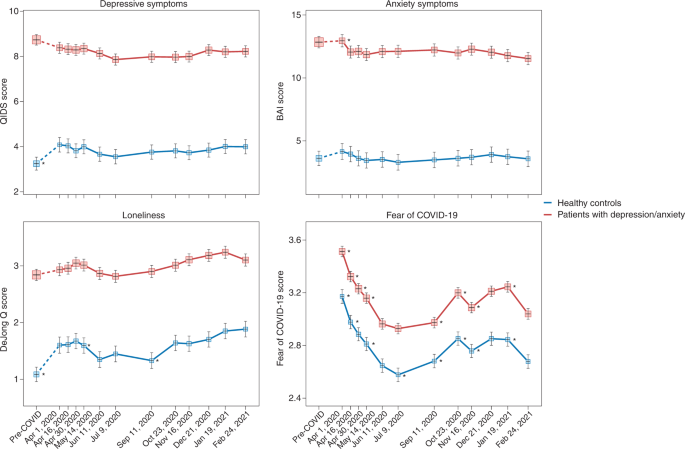
Trajectories of mean depressive symptoms (QIDS score), anxiety symptoms (BAI score), loneliness (De Jong questionnaire score) and Fear of COVID-19 score before and during the first year of the COVID-19 pandemic in healthy controls (blue line, n = 378) and in patients with depressive and/or anxiety disorders (red line, n = 908). The x -axis indicates time with one pre-COVID assessment (averaged over up to five earlier assessments conducted between 2006 and 2019) and 11 online assessments during April 2020 through February 2021. Symbols indicate the mean score during the assessment with 95% CIs. As compared to pre-COVID assessment scores, the figure shows a statistically significant increase of depression and loneliness symptoms during the first pandemic peak (April 2020) in healthy controls but not in patients (for more details, see refs. 22 , 54 ). Asterisks indicate where subsequent wave scores differ from the prior wave scores ( P < 0.05). The figure also illustrates the stability of depressive and anxiety symptoms during the first COVID year, a significant increase in loneliness during this period and fluctuations of Fear of COVID-19 score that positively correlate with infection rates in the Netherlands. Raw data are from the Netherlands Study of Depression and Anxiety (NESDA), which were re-analyzed for the current plots to illustrate differences between two groups (healthy controls versus patients). BAI, Beck Anxiety Inventory; QIDS, Quick Inventory of Depressive Symptoms.
Impact of COVID-19 infection and disease on mental health and the brain
Not only the pandemic but also COVID-19 itself can have severe impact on the mental health of affected individuals and, thus, of the population at large. Below we describe acute and post-acute neuropsychiatric sequelae seen in patients with COVID-19 and link these to neurobiological mechanisms.
Neuropsychiatric sequelae in individuals with COVID-19
Common symptoms associated with acute SARS-CoV-2 infection include headache, anosmia (loss of sense of smell) and dysgeusia (loss of sense of taste). The broader neuropsychiatric impact is dependent on infection severity and is very heterogeneous (Table 2 ). It ranges from no neuropsychiatric symptoms among the large group of asymptomatic COVID-19 cases to milder transient neuropsychiatric symptoms, such as fatigue, sleep disturbance and cognitive impairment, predominantly occurring among symptomatic patients with COVID-19 (ref. 55 ). Cognitive impairment consists of sustained memory impairments and executive dysfunction, including short-term memory loss, concentration problems, word-finding problems and impaired daily problem-solving, colloquially termed ‘brain fog’ by patients and clinicians. A small number of infected individuals become severely ill and require hospitalization. During hospital admission, the predominant neuropsychiatric outcome is delirium 56 . Delirium occurs among one-third of hospitalized patients with COVID-19 and among over half of patients with COVID-19 who require intensive care unit (ICU) treatment. These delirium rates seem similar to those observed among individuals with severe illness hospitalized for other general medical conditions 57 . Delirium is associated with neuropsychiatric sequalae after hospitalization, as part of post-intensive care syndrome 58 , in which sepsis and inflammation are associated with cognitive dysfunction and an increased risk of a broad range of psychiatric symptoms, from anxiety to depression and psychotic symptoms with hallucinations 59 , 60 .
A subset of patients with COVID-19 develop PACS 61 , which can include neuropsychiatric symptoms. A large meta-analysis summarizes 51 studies involving 18,917 patients with a mean follow-up of 77 days (range, 14–182 days) 62 . The most prevalent neuropsychiatric symptom associated with COVID-19 was sleep disturbance, with a pooled prevalence of 27.4%, followed by fatigue (24.4%), cognitive impairment (20.2%), anxiety symptoms (19.1%), post-traumatic stress symptoms (15.7%) and depression symptoms (12.9%) (Table 2 ). Another meta-analysis that assessed patients 12 weeks or more after confirmed COVID-19 diagnosis found that 32% experienced fatigue, and 22% experienced cognitive impairment 63 . To what extent neuropsychiatric symptoms are truly unique for patients with COVID remains unclear from these meta-analyses, as hardly any study included well-matched controls with other types of respiratory infections or inflammatory conditions.
Studies based on electronic health records have examined whether higher levels of neuropsychiatric symptoms truly translate into a higher incidence of clinically overt mental disorders 64 , 65 . In a 1-year follow-up using the US Veterans Affairs database, 153,848 survivors of SARS-CoV-2 infection exhibited an increased incidence of any mental disorder with a relative risk of 1.46 and, specifically, 1.35 for anxiety disorders, 1.39 for depressive disorders and 1.38 for stress and adjustment disorders, compared to a contemporary group and a historical control group ( n = 5,859,251) 65 . In absolute numbers, the incident risk difference attributable to SARS-CoV-2 for mental disorders was 64 per 1,000 individuals. Taquet et al. 64 analyzed electronic health records from the US-based TriNetX network with over 81 million patients and 236,379 COVID-19 survivors followed for 6 months. In absolute numbers, 6-month incidence of hospital contacts related to diagnoses of anxiety, affective disorder or psychotic disorder was 7.0%, 4.5% and 0.4%, respectively. Risks of incident neurological or psychiatric diagnoses were directly correlated with COVID-19 severity and increased by 78% when compared to influenza and by 32% when compared to other respiratory tract infections. In contrast, a medical record study involving 8.3 million adults confirmed that neuropsychiatric disorders were significantly elevated among COVID-19 hospitalized individuals but to a similar extent as in hospitalized patients with other severe respiratory disease 66 . In line with this, a study using language processing of clinical notes in electronic health records did not find an increase in fatigue, mood and anxiety symptoms among COVID-19 hospitalized individuals when compared to hospitalized patients for other indications and adjusted for sociodemographic features and hospital course 67 . It is important to note that research based only on hospital records might be influenced by increased health-seeking behavior that could be differential across care settings or by increased follow-up by hospitals of patients with COVID-19 (compared to patients with other conditions).
Consequently, whether PACS symptoms form a unique pattern due to specific infection with SARS-CoV-2 remains debatable. Prospective case–control studies that do not rely on hospital records but measure the incidence of neuropsychiatric symptoms and diagnoses after COVID-19 are still scarce, but they are critical for distinguishing causation and confounding when characterizing PACS and the uniqueness of neuropsychiatric sequalae after COVID-19 (ref. 68 ). Recent studies with well-matched control groups illustrate that long-term consequences may not be so unique, as they were similar to those observed in patients with other diseases of similar severity, such as after acute myocardial infarction or in ICU patients 56 , 66 . A first prospective follow-up study of COVID-19 survivors and control patients matched on disease severity, age, sex and ICU admission found similar neuropsychiatric outcomes, regarding both new-onset psychiatric diagnosis (19% versus 20%) and neuropsychiatric symptoms (81% versus 93%). However, moderate but significantly worse cognitive outcomes 6 months after symptom onset were found among survivors of COVID-19 (ref. 69 ). In line with this, a longitudinal study of 785 participants from the UK Biobank showed small but significant cognitive impairment among individuals infected with SARS-CoV-2 compared to matched controls 70 .
Numerous psychosocial mechanisms can lead to neuropsychiatric sequalae of COVID-19, including functional impairment; psychological impact due to, for example, fear of dying; stress of being infected with a novel pandemic disease; isolation as part of quarantine and lack of social support; fear/guilt of spreading COVID-19 to family or community; and socioeconomic distress by lost wages 71 . However, there is also ample evidence that neurobiological mechanisms play an important role, which is discussed below.
Neurobiological mechanisms underlying neuropsychiatric sequelae of COVID-19
Acute neuropsychiatric symptoms among patients with severe COVID-19 have been found to correlate with the level of serum inflammatory markers 72 and coincide with neuroimaging findings of immune activation, including leukoencephalopathy, acute disseminated encephalomyelitis, cytotoxic lesions of the corpus callosum or cranial nerve enhancement 73 . Rare presentations, including meningitis, encephalitis, inflammatory demyelination, cerebral infarction and acute hemorrhagic necrotizing encephalopathy, have also been reported 74 . Hospitalized patients with frank encephalopathies display impaired blood-brain barrier (BBB) integrity with leptomeningeal enhancement on brain magnetic resonance images 75 . Studies of postmortem specimens from patients who succumbed to acute COVID-19 reveal significant neuropathology with signs of hypoxic damage and neuroinflammation. These include evidence of BBB permeability with extravasation of fibrinogen, microglial activation, astrogliosis, leukocyte infiltration and microhemorrhages 76 , 77 . However, it is still unclear to what extent these findings differ from patients with similar illness severity due to acute non-COVID illness, as these brain effects might not be virus-specific effects but rather due to cytokine-mediated neuroinflammation and critical illness.
Post-acute neuroimaging studies in SARS-CoV-2-recovered patients, as compared to control patients without COVID-19, reveal numerous alterations in brain structure on a group level, although effect sizes are generally small. These include minor reduction in gray matter thickness in the various regions of the cortex and within the corpus collosum, diffuse edema, increases in markers of tissue damage in regions functionally connected to the olfactory cortex and reductions in overall brain size 70 , 78 . Neuroimaging studies of post-acute COVID-19 patients also report abnormalities consistent with micro-structural and functional alterations, specifically within the hippocampus 79 , 80 , a brain region critical for memory formation and regulating anxiety, mood and stress responses, but also within gray matter areas involving the olfactory system and cingulate cortex 80 . Overall, these findings are in line with ongoing anosmia, tremors, affect problems and cognitive impairment.
Interestingly, despite findings mentioned above, there is little evidence of SARS-CoV-2 neuroinvasion with productive replication, and viral material is rarely found in the central nervous system (CNS) of patients with COVID-19 (refs. 76 , 77 , 81 ). Thus, neurobiological mechanisms of SARS-CoV-2-mediated neuropsychiatric sequelae remain unclear, especially in patients who initially present with milder forms of COVID-19. Symptomatic SARS-CoV-2 infection is associated with hypoxia, cytokine release syndrome (CRS) and dysregulated innate and adaptive immune responses (reviewed in ref. 82 ). All these effects could contribute to neuroinflammation and endothelial cell activation (Fig. 2 ). Examination of cerebrospinal fluid in patients with neuroimaging findings revealed elevated levels of pro-inflammatory, BBB-destabilizing cytokines, including interleukin-6 (IL-6), IL-1, IL-8 and mononuclear cell chemoattractants 83 , 84 . Whether these cytokines arise from the periphery, due to COVID-19-mediated CRS, or from within the CNS, is unclear. As studies generally lack control patients with other severe illnesses, the specificity of such findings to SARS-CoV-2 also remains unclear. Systemic inflammatory processes, including cytokine release, have been linked to glial activation with expression of chemoattractants that recruit immune cells, leading to neuroinflammation and injury 85 . Cerebrospinal fluid concentrations of neurofilament light, a biomarker of neuronal damage, were reportedly elevated in patients hospitalized with COVID-19 regardless of whether they exhibited neurologic diseases 86 . Acute thromboembolic events leading to ischemic infarcts are also common in patients with COVID-19 due to a potentially increased pro-coagulant process secondary to CRS 87 .
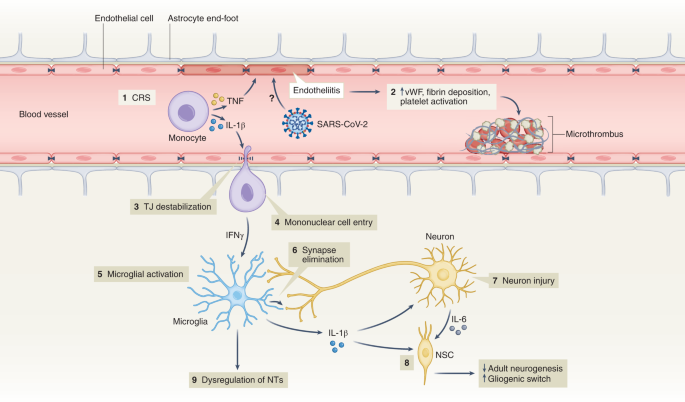
(1) Elevation of BBB-destabilizing cytokines (IL-1β and TNF) within the serum due to CRS or local interactions of mononuclear and endothelial cells. (2) Virus-induced endotheliitis increases susceptibility to microthrombus formation due to platelet activation, elevation of vWF and fibrin deposition. (3) Cytokine, mononuclear and endothelial cell interactions promote disruption of the BBB, which may allow entry of leukocytes expressing IFNg into the CNS (4), leading to microglial activation (5). (6) Activated microglia may eliminate synapses and/or express cytokines that promote neuronal injury. (7) Injured neurons express IL-6 which, together with IL-1β, promote a ‘gliogenic switch’ in NSCs (8), decreasing adult neurogenesis. (9) The combination of microglial (and possibly astrocyte) activation, neuronal injury and synapse loss may lead to dysregulation of NTs and neuronal circuitry. IFNg, interferon-g; NSC, neural stem cell; NT, neurotransmitter; TJ, tight junction; TNF, tumor necrosis factor; vWF, von Willebrand factor.
It is also unclear whether hospitalized patients with COVID-19 may develop brain abnormalities due to hypoxia or CRS rather than as a direct effect of SARS-CoV-2 infection. Hypoxia may cause neuronal dysfunction, cerebral edema, increased BBB permeability, cytokine expression and onset of neurodegenerative diseases 88 , 89 . CRS, with life-threatening levels of serum TNF-α and IL-1 (ref. 90 ) could also impact BBB function, as these cytokines destabilize microvasculature endothelial cell junctional proteins critical for BBB integrity 91 . In mild SARS-CoV-2 infection, circulating immune factors combined with mild hypoxia might impact BBB function and lead to neuroinflammation 92 , as observed during infection with other non-neuroinvasive respiratory pathogens 93 . However, multiple studies suggest that the SARS-CoV-2 spike protein itself may also induce venous and arterial endothelial cell activation and endotheliitis, disrupt BBB integrity or cross the BBB via adoptive transcytosis 94 , 95 , 96 .
Reducing neuropsychiatric sequelae of COVID-19
The increased risk of COVID-19-related neuropsychiatric sequalae was most pronounced during the first pandemic peak but reduced over the subsequent 2 years 64 , 97 . This may be due to reduced impact of newer SARS-CoV-2 strains (that is, Omicron) but also protective effects of vaccination, which limit SARS-CoV-2 spread and may, thus, prevent neuropsychiatric sequalae. Fully vaccinated individuals with breakthrough infections exhibit a 50% reduction in PACS 98 , even though vaccination does not improve PACS-related neuropsychiatric symptoms in patients with a prior history of COVID-19 (ref. 99 ). As patients with pre-existing mental disorders are at increased risk of SARS-CoV-2 infection, they deserve to be among the prioritization groups for vaccination efforts 100 .
Adequate treatment strategies for neuropsychiatric sequelae of COVID-19 are needed. As no specific evidence-based intervention yet exists, the best current treatment approach is that for neuropsychiatric sequelae arising after other severe medical conditions 101 . Stepped care—a staged approach of mental health services comprising a hierarchy of interventions, from least to most intensive, matched to the individual’s need—is efficacious with monitoring of mental health and cognitive problems. Milder symptoms likely benefit from counseling and holistic care, including physiotherapy, psychotherapy and rehabilitation. Individuals with moderate to severe symptoms fulfilling psychiatric diagnoses should receive guideline-concordant care for these disorders 61 . Patients with pre-existing mental disorders also deserve special attention when affected by COVID-19, as they have shown to have an increased risk of COVID-19-related hospitalization, complications and death 102 . This may involve interventions to address their general health, any unfavorable socioenvironmental factors, substance abuse or treatment adherence issues.
Lessons learned, knowledge gaps and future challenges
Ultimately, it is not only the millions of people who have died from COVID-19 worldwide that we remember but also the distress experienced during an unpredictable period with overstretched healthcare systems, lockdowns, school closures and changing work environments. In a world that is more and more globalized, connectivity puts us at risk for future pandemics. What can be learned from the last 2 years of the COVID-19 pandemic about how to handle future and longstanding challenges related to mental health?
Give mental health equal priority to physical health
The COVID-19 pandemic has demonstrated that our population seems quite resilient and adaptive. Nevertheless, even if society as a whole may bounce back, there is a large group of people whose mental health has been and will be disproportionately affected by this and future crises. Although various groups, such as the WHO 8 , the National Health Commission of China 103 , the Asia Pacific Disaster Mental Health Network 104 and a National Taskforce in India 105 , developed mental health policies early on, many countries were late in realizing that a mental health agenda deserves immediate attention in a rapidly evolving pandemic. Implementation of comprehensive and integrated mental health policies was generally inconsistent and suboptimal 106 and often in the shadow of policies directed at containing and reducing the spread of SARS-CoV-2. Leadership is needed to convey the message that mental health is as important as physical health and that we should focus specific attention and early interventions on those at the highest risk. This includes those vulnerable due to factors such as low socioeconomic status, specific developmental life phase (adolescents and young adults), pre-existing risk (poor physical or somatic health and early life trauma) or high exposure to pandemic-related (work) changes—for example, women and healthcare personnel. This means that not only should investment in youth and reducing health inequalities remain at the top of any policy agenda but also that mental health should be explicitly addressed from the start in any future global health crisis situation.
Communication and trust is crucial for mental health
Uncertainty and uncontrollability during the pandemic have challenged rational thinking. Negative news travels fast. Communication that is vague, one-sided and dishonest can negatively impact on mental health and amplify existing distress and anxiety 107 . Media reporting should not overemphasize negative mental health impact—for example, putative suicide rate increases or individual negative experiences—which could make situations worse than they actually are. Instead, communication during crises requires concrete and actionable advice that avoids polarization and strengthens vigilance, to foster resilience and help prevent escalation to severe mental health problems 108 , 109 .
Rapid research should be collaborative and high-quality
Within the scientific community, the topic of mental health during the pandemic led to a multitude of rapid studies that generally had limited methodological quality—for example, cross-sectional designs, small or selective sampling or study designs lacking valid comparison groups. These contributed rather little to our understanding of the mental health impact of the emerging crisis. In future events that have global mental health impact, where possible, collaborative and interdisciplinary efforts with well-powered and well-controlled prospective studies using standardized instruments will be crucial. Only with fine-grained determinants and outcomes can data reliably inform mental health policies and identify who is most at risk.
Do not neglect long-term mental health effects
So far, research has mainly focused on the acute and short-term effects of the pandemic on mental health, usually spanning pandemic effects over several months to 1 year. However, longer follow-up of how a pandemic impacts population mental health is essential. Can societal and economic disruptions after the pandemic increase risk of mental disorders at a later stage when the acute pandemic effects have subsided? Do increased self-reported mental health problems return to pre-pandemic levels, and which groups of individuals remain most affected in the long-term? We need to realize that certain pandemic consequences, particularly those affecting income and school/work careers, may become visible only over the course of several years. Consequently, we should maintain focus and continue to monitor and quantify the effects of the pandemic in the years to come—for example, by monitoring mental healthcare use and suicide. This should include specific at-risk populations (for example, adolescents) and understudied populations in low-income and middle-income countries.
Pay attention to mental health consequences of infectious diseases
Even though our knowledge on PACS is rapidly expanding, there are still many unanswered questions related to who is at risk, the long-term course trajectories and the best ways to intervene early. Consequently, we need to be aware of the neuropsychiatric sequelae of COVID-19 and, for that matter, of any infectious disease. Clinical attention and research should be directed toward alleviating potential neuropsychiatric ramifications of COVID-19. Next to clinical studies, studies using human tissues and appropriate animal models are pivotal to determine the CNS region-specific and neural-cell-specific effects of SARS-CoV-2 infection and the induced immune activation. Indeed, absence of SARS-CoV-2 neuroinvasion is an opportunity to learn and discover how peripheral neuroimmune mechanisms can contribute to neuropsychiatric sequelae in susceptible individuals. This emphasizes the importance of an interdisciplinary approach where somatic and mental health efforts are combined but also the need to integrate clinical parameters after infection with biological parameters (for example, serum, cerebrospinal fluid and/or neuroimaging) to predict who is at risk for PACS and deliver more targeted treatments.
Prepare mental healthcare infrastructure for pandemic times
If we take mental health seriously, we should not only monitor it but also develop the resources and infrastructure necessary for rapid early intervention, particularly for specific vulnerable groups. For adequate mental healthcare to be ready for pandemic times, primary care, community mental health and public mental health should be prepared. In many countries, health services were not able to meet the population’s mental health needs before the pandemic, which substantially worsened during the pandemic. We should ensure rapid access to mental health services but also address the underlying drivers of poor mental health, such as mitigating risks of unemployment, sexual violence and poverty. Collaboration in early stages across disciplines and expertise is essential. Anticipating disruption to face-to-face services, mental healthcare providers should be more prepared for consultations, therapy and follow-up by telephone, video-conferencing platforms and web applications 51 , 52 . The pandemic has shown that an inadequate infrastructure, pre-existing inequalities and low levels of technological literacy hindered the use and uptake of e-health, both in healthcare providers and in patients across different care settings. The necessary investments can ensure rapid upscaling of mental health services during future pandemics for those individuals with a high mental health need due to societal changes, government measures, fear of infection or infection itself.
Even though much attention has been paid to the physical health consequences of COVID-19, mental health has unjustly received less attention. There is an urgent need to prepare our research and healthcare infrastructures not only for adequate monitoring of the long-term mental health effects of the COVID-19 pandemic but also for future crises that will shape mental health. This will require collaboration to ensure interdisciplinary and sound research and to provide attention and care at an early stage for those individuals who are most vulnerable—giving mental health equal priority to physical health from the very start.
WHO Coronavirus (COVID-19) Dashboard (WHO, 2022; https://covid19.who.int/
Rando, H. M. et al. Challenges in defining long COVID: striking differences across literature, electronic health records, and patient-reported information. Preprint at https://www.medrxiv.org/content/10.1101/2021.03.20.21253896v1 (2021).
Nalbandian, A. et al. Post-acute COVID-19 syndrome. Nat. Med. 27 , 601–615 (2021).
Article CAS PubMed PubMed Central Google Scholar
Abbafati, C. et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396 , 1204–1222 (2020).
Article Google Scholar
Penninx, B. W., Pine, D. S., Holmes, E. A. & Reif, A. Anxiety disorders. Lancet 397 , 914–927 (2021).
Article PubMed PubMed Central Google Scholar
Herrman, H. et al. Time for united action on depression: a Lancet –World Psychiatric Association Commission. Lancet 399 , 957–1022 (2022).
Article PubMed Google Scholar
Radka, K., Wyeth, E. H. & Derrett, S. A qualitative study of living through the first New Zealand COVID-19 lockdown: affordances, positive outcomes, and reflections. Prev. Med. Rep. 26 , 101725 (2022).
Mental Health and COVID-19: Early Evidence of the Pandemic’s Impact (WHO, 2022).
Dragioti, E. et al. A large-scale meta-analytic atlas of mental health problems prevalence during the COVID-19 early pandemic. J. Med. Virol. 94 , 1935–1949 (2022).
Zhang, S. X. et al. Mental disorder symptoms during the COVID-19 pandemic in Latin America—a systematic review and meta-analysis. Epidemiol. Psychiatr. Sci. 31 , e23 (2022).
Zhang, S. X. et al. Meta-analytic evidence of depression and anxiety in Eastern Europe during the COVID-19 pandemic. Eur. J. Psychotraumatol . 13 , 2000132 (2022).
Racine, N. et al. Global prevalence of depressive and anxiety symptoms in children and adolescents during COVID-19: a meta-analysis. JAMA Pediatr. 175 , 1142–1150 (2021).
Robinson, E., Sutin, A. R., Daly, M. & Jones, A. A systematic review and meta-analysis of longitudinal cohort studies comparing mental health before versus during the COVID-19 pandemic in 2020. J. Affect. Disord. 296 , 567–576 (2022).
Article CAS PubMed Google Scholar
Prati, G. & Mancini, A. D. The psychological impact of COVID-19 pandemic lockdowns: a review and meta-analysis of longitudinal studies and natural experiments. Psychol. Med. 51 , 201–211 (2021).
Patel, K. et al. Psychological distress before and during the COVID-19 pandemic among adults in the United Kingdom based on coordinated analyses of 11 longitudinal studies. JAMA Netw. Open 5 , e227629 (2022).
Ernst, M. et al. Loneliness before and during the COVID-19 pandemic: a systematic review with meta-analysis. Am. Psychol . 77 , 660–677 (2022).
Kilian, C. et al. Changes in alcohol use during the COVID-19 pandemic in Europe: a meta-analysis of observational studies. Drug Alcohol Rev . 41 , 918–931 (2022).
Acuff, S. F., Strickland, J. C., Tucker, J. A. & Murphy, J. G. Changes in alcohol use during COVID-19 and associations with contextual and individual difference variables: a systematic review and meta-analysis. Psychol. Addict. Behav. 36 , 1–19 (2022).
Varga, T. V. et al. Loneliness, worries, anxiety, and precautionary behaviours in response to the COVID-19 pandemic: a longitudinal analysis of 200,000 Western and Northern Europeans. Lancet Reg. Health Eur . 2 , 100020 (2021).
Fancourt, D., Steptoe, A. & Bu, F. Trajectories of anxiety and depressive symptoms during enforced isolation due to COVID-19 in England: a longitudinal observational study. Lancet Psychiatry 8 , 141–149 (2021).
Jia, H. et al. National and state trends in anxiety and depression severity scores among adults during the COVID-19 pandemic—United States, 2020–2021. MMWR Morb. Mortal. Wkly. Rep. 70 , 1427–1432 (2021).
Kok, A. A. L. et al. Mental health and perceived impact during the first Covid-19 pandemic year: a longitudinal study in Dutch case–control cohorts of persons with and without depressive, anxiety, and obsessive-compulsive disorders. J. Affect. Disord. 305 , 85–93 (2022).
Su, Y. et al. Prevalence of loneliness and social isolation among older adults during the COVID-19 pandemic: a systematic review and meta-analysis. Int. Psychogeriatr. https://doi.org/10.1017/S1041610222000199 (2022).
Knox, L., Karantzas, G. C., Romano, D., Feeney, J. A. & Simpson, J. A. One year on: what we have learned about the psychological effects of COVID-19 social restrictions: a meta-analysis. Curr. Opin. Psychol. 46 , 101315 (2022).
Aknin, L. B. et al. Policy stringency and mental health during the COVID-19 pandemic: a longitudinal analysis of data from 15 countries. Lancet Public Health 7 , e417–e426 (2022).
Lee, Y. et al. Government response moderates the mental health impact of COVID-19: a systematic review and meta-analysis of depression outcomes across countries. J. Affect. Disord. 290 , 364–377 (2021).
Wu, J. T. et al. Nowcasting epidemics of novel pathogens: lessons from COVID-19. Nat. Med. 27 , 388–395 (2021).
Brooks, S. K. et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet 395 , 912–920 (2020).
Santomauro, D. F. et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 398 , 1700–1712 (2021).
Knudsen, A. K. S. et al. Prevalence of mental disorders, suicidal ideation and suicides in the general population before and during the COVID-19 pandemic in Norway: a population-based repeated cross-sectional analysis. Lancet Reg. Health Eur . 4 , 100071 (2021).
Ayuso-Mateos, J. L. et al. Changes in depression and suicidal ideation under severe lockdown restrictions during the first wave of the COVID-19 pandemic in Spain: a longitudinal study in the general population. Epidemiol. Psychiatr. Sci . 30 , e49 (2021).
Vloo, A. et al. Gender differences in the mental health impact of the COVID-19 lockdown: longitudinal evidence from the Netherlands. SSM Popul. Health 15 , 100878 (2021).
Winkler, P. et al. Prevalence of current mental disorders before and during the second wave of COVID-19 pandemic: an analysis of repeated nationwide cross-sectional surveys. J. Psychiatr. Res. 139 , 167–171 (2021).
Pirkis, J. et al. Suicide trends in the early months of the COVID-19 pandemic: an interrupted time-series analysis of preliminary data from 21 countries. Lancet Psychiatry 8 , 579–588 (2021).
Faust, J. S. et al. Mortality from drug overdoses, homicides, unintentional injuries, motor vehicle crashes, and suicides during the pandemic, March–August 2020. JAMA 326 , 84–86 (2021).
John, A. et al. The impact of the COVID-19 pandemic on self-harm and suicidal behaviour: update of living systematic review. F1000Res. 9 , 1097 (2020).
Steeg, S. et al. Temporal trends in primary care-recorded self-harm during and beyond the first year of the COVID-19 pandemic: time series analysis of electronic healthcare records for 2.8 million patients in the Greater Manchester Care Record. EClinicalMedicine 41 , 101175 (2021).
Rømer, T. B. et al. Psychiatric admissions, referrals, and suicidal behavior before and during the COVID-19 pandemic in Denmark: a time-trend study. Acta Psychiatr. Scand. 144 , 553–562 (2021).
Holland, K. M. et al. Trends in US emergency department visits for mental health, overdose, and violence outcomes before and during the COVID-19 pandemic. JAMA Psychiatry 78 , 372–379 (2021).
Kunzler, A. M. et al. Mental burden and its risk and protective factors during the early phase of the SARS-CoV-2 pandemic: systematic review and meta-analyses. Global Health 17 , 34 (2021).
Flor, L. S. et al. Quantifying the effects of the COVID-19 pandemic on gender equality on health, social, and economic indicators: a comprehensive review of data from March, 2020, to September, 2021. Lancet 399 , 2381–2397 (2022).
Viner, R. et al. School closures during social lockdown and mental health, health behaviors, and well-being among children and adolescents during the first COVID-19 wave: a systematic review. JAMA Pediatr. 176 , 400–409 (2022).
Zheng, X. Y. et al. Trends of injury mortality during the COVID-19 period in Guangdong, China: a population-based retrospective analysis. BMJ Open 11 , e045317 (2021).
Tanaka, T. & Okamoto, S. Increase in suicide following an initial decline during the COVID-19 pandemic in Japan. Nat. Hum. Behav. 5 , 229–238 (2021).
Thomeer, M. B., Moody, M. D. & Yahirun, J. Racial and ethnic disparities in mental health and mental health care during the COVID-19 pandemic. J. Racial Ethn. Health Disparities https://doi.org/10.1007/s40615-021-01006-7 (2022).
Hill, J. E. et al. The prevalence of mental health conditions in healthcare workers during and after a pandemic: systematic review and meta-analysis. J. Adv. Nurs. 78 , 1551–1573 (2022).
Marvaldi, M., Mallet, J., Dubertret, C., Moro, M. R. & Guessoum, S. B. Anxiety, depression, trauma-related, and sleep disorders among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 126 , 252–264 (2021).
Phiri, P. et al. An evaluation of the mental health impact of SARS-CoV-2 on patients, general public and healthcare professionals: a systematic review and meta-analysis. EClinicalMedicine 34 , 100806 (2021).
Jorm, A. F., Patten, S. B., Brugha, T. S. & Mojtabai, R. Has increased provision of treatment reduced the prevalence of common mental disorders? Review of the evidence from four countries. World Psychiatry 16 , 90–99 (2017).
Third Round of the Global Pulse Survey on Continuity of Essential Health Services during the COVID-19 Pandemic (WHO, 2021).
Baumgart, J. G. et al. The early impacts of the COVID-19 pandemic on mental health facilities and psychiatric professionals. Int. J. Environ. Res. Public Health 18 , 8034 (2021).
Raphael, J., Winter, R. & Berry, K. Adapting practice in mental healthcare settings during the COVID-19 pandemic and other contagions: systematic review. BJPsych Open 7 , e62 (2021).
Anderson, K. N. et al. Changes and inequities in adult mental health-related emergency department visits during the COVID-19 pandemic in the US. JAMA Psychiatry 79 , 475–485 (2022).
Pan, K. Y. et al. The mental health impact of the COVID-19 pandemic on people with and without depressive, anxiety, or obsessive-compulsive disorders: a longitudinal study of three Dutch case–control cohorts. Lancet Psychiatry 8 , 121–129 (2021).
Dantzer, R., O’Connor, J. C., Freund, G. G., Johnson, R. W. & Kelley, K. W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9 , 46–56 (2008).
Nersesjan, V. et al. Central and peripheral nervous system complications of COVID-19: a prospective tertiary center cohort with 3-month follow-up. J. Neurol. 268 , 3086–3104 (2021).
Wilson, J. E. et al. Delirium. Nat. Rev. Dis. Prim . 6 , 90 (2020).
Rawal, G., Yadav, S. & Kumar, R. Post-intensive care syndrome: an overview. J. Transl. Intern. Med. 5 , 90–92 (2017).
Pandharipande, P. P. et al. Long-term cognitive impairment after critical illness. N. Engl. J. Med. 369 , 1306–1316 (2013).
Girard, T. D. et al. Long-term cognitive impairment after hospitalization for community-acquired pneumonia: a prospective cohort study. J. Gen. Intern. Med. 33 , 929–935 (2018).
Crook, H., Raza, S., Nowell, J., Young, M. & Edison, P. Long covid—mechanisms, risk factors, and management. BMJ 374 , n1648 (2021).
Badenoch, J. B. et al. Persistent neuropsychiatric symptoms after COVID-19: a systematic review and meta-analysis. Brain Commun . 4 , fcab297 (2021).
Ceban, F. et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav. Immun. 101 , 93–135 (2022).
Taquet, M., Geddes, J. R., Husain, M., Luciano, S. & Harrison, P. J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry 8 , 416–427 (2021).
Xie, Y., Xu, E. & Al-Aly, Z. Risks of mental health outcomes in people with covid-19: cohort study. BMJ 376 , e068993 (2022).
Kieran Clift, A. et al. Neuropsychiatric ramifications of severe COVID-19 and other severe acute respiratory infections. JAMA Psychiatry 79 , 690–698 (2022).
Castro, V. M., Rosand, J., Giacino, J. T., McCoy, T. H. & Perlis, R. H. Case–control study of neuropsychiatric symptoms following COVID-19 hospitalization in 2 academic health systems. Mol. Psych. (in the press).
Amin-Chowdhury, Z. & Ladhani, S. N. Causation or confounding: why controls are critical for characterizing long COVID. Nat. Med. 27 , 1129–1130 (2021).
Nersesjan, V. et al. Neuropsychiatric and cognitive outcomes in patients 6 months after COVID-19 requiring hospitalization compared with matched control patients hospitalized for non-COVID-19 illness. JAMA Psychiatry 79 , 486–497 (2022).
Douaud, G. et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 604 , 697–707 (2022).
Zhang, H. et al. Psychological experience of COVID-19 patients: a systematic review and qualitative meta-synthesis. Am. J. Infect. Control 50 , 809–819 (2022).
Mazza, M. G. et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav. Immun. 89 , 594–600 (2020).
Moonis, G. et al. The spectrum of neuroimaging findings on CT and MRI in adults With COVID-19. AJR Am. J. Roentgenol. 217 , 959–974 (2021).
Asadi-Pooya, A. A. & Simani, L. Central nervous system manifestations of COVID-19: a systematic review. J. Neurol. Sci . 413 , 116832 (2020).
Lersy, F. et al. Cerebrospinal fluid features in patients with Coronavirus Disease 2019 and neurological manifestations: correlation with brain magnetic resonance imaging findings in 58 patients. J. Infect. Dis. 223 , 600–609 (2021).
Thakur, K. T. et al. COVID-19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital. Brain 144 , 2696–2708 (2021).
Cosentino, G. et al. Neuropathological findings from COVID-19 patients with neurological symptoms argue against a direct brain invasion of SARS-CoV-2: a critical systematic review. Eur. J. Neurol. 28 , 3856–3865 (2021).
Tian, T. et al. Long-term follow-up of dynamic brain changes in patients recovered from COVID-19 without neurological manifestations. JCI Insight 7 , e155827 (2022).
Lu, Y. et al. Cerebral micro-structural changes in COVID-19 patients—an MRI-based 3-month follow-up study. EClinicalMedicine 25 , 100484 (2020).
Qin, Y. et al . Long-term microstructure and cerebral blood flow changes in patients recovered from COVID-19 without neurological manifestations. J. Clin. Invest . 131 , e147329 (2021).
Matschke, J. et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 19 , 919–929 (2020).
Shivshankar, P. et al. SARS-CoV-2 infection: host response, immunity, and therapeutic targets. Inflammation 45 , 1430–1449 (2022).
Manganotti, P. et al. Cerebrospinal fluid and serum interleukins 6 and 8 during the acute and recovery phase in COVID-19 neuropathy patients. J. Med. Virol. 93 , 5432–5437 (2021).
Farhadian, S. et al. Acute encephalopathy with elevated CSF inflammatory markers as the initial presentation of COVID-19. BMC Neurol . 20 , 248 (2020).
Francistiová, L. et al. Cellular and molecular effects of SARS-CoV-2 linking lung infection to the brain. Front. Immunol . 12 , 730088 (2021).
Paterson, R. W. et al. Serum and cerebrospinal fluid biomarker profiles in acute SARS-CoV-2-associated neurological syndromes. Brain Commun . 3 , fcab099 (2021).
Cryer, M. J. et al. Prothrombotic milieu, thrombotic events and prophylactic anticoagulation in hospitalized COVID-19 positive patients: a review. Clin. Appl. Thromb. Hemost . 28 , 10760296221074353 (2022).
Nalivaeva, N. N. & Rybnikova, E. A. Editorial: Brain hypoxia and ischemia: new insights into neurodegeneration and neuroprotection. Front. Neurosci . 13 , 770 (2019).
Brownlee, N. N. M., Wilson, F. C., Curran, D. B., Lyttle, N. & McCann, J. P. Neurocognitive outcomes in adults following cerebral hypoxia: a systematic literature review. NeuroRehabilitation 47 , 83–97 (2020).
Del Valle, D. M. et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 26 , 1636–1643 (2020).
Daniels, B. P. et al. Viral pathogen-associated molecular patterns regulate blood–brain barrier integrity via competing innate cytokine signals. mBio 5 , e01476-14 (2014).
Reynolds, J. L. & Mahajan, S. D. SARS-COV2 alters blood brain barrier integrity contributing to neuro-inflammation. J. Neuroimmune Pharmacol. 16 , 4–6 (2021).
Bohmwald, K., Gálvez, N. M. S., Ríos, M. & Kalergis, A. M. Neurologic alterations due to respiratory virus infections. Front. Cell. Neurosci . 12 , 386 (2018).
Khaddaj-Mallat, R. et al. SARS-CoV-2 deregulates the vascular and immune functions of brain pericytes via spike protein. Neurobiol. Dis . 161 , 105561 (2021).
Qian, Y. et al. Direct activation of endothelial cells by SARS-CoV-2 nucleocapsid protein is blocked by simvastatin. J Virol. 95 , e0139621 (2021).
Rhea, E. M. et al. The S1 protein of SARS-CoV-2 crosses the blood–brain barrier in mice. Nat. Neurosci. 24 , 368–378 (2021).
Magnúsdóttir, I. et al. Acute COVID-19 severity and mental health morbidity trajectories in patient populations of six nations: an observational study. Lancet Public Health 7 , e406–e416 (2022).
Antonelli, M. et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case–control study. Lancet Infect. Dis. 22 , 43–55 (2022).
Wisnivesky, J. P. et al. Association of vaccination with the persistence of post-COVID symptoms. J. Gen. Intern. Med . 37 , 1748–1753 (2022).
De Picker, L. J. et al. Severe mental illness and European COVID-19 vaccination strategies. Lancet Psychiatry 8 , 356–359 (2021).
Cohen, G. H. et al. Comparison of simulated treatment and cost-effectiveness of a stepped care case-finding intervention vs usual care for posttraumatic stress disorder after a natural disaster. JAMA Psychiatry 74 , 1251–1258 (2017).
Vai, B. et al. Mental disorders and risk of COVID-19-related mortality, hospitalisation, and intensive care unit admission: a systematic review and meta-analysis. Lancet Psychiatry 8 , 797–812 (2021).
Xiang, Y. T. et al. Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. Lancet Psychiatry 7 , 228 (2020).
Newnham, E. A. et al. The Asia Pacific Disaster Mental Health Network: setting a mental health agenda for the region. Int. J. Environ. Res. Public Health 17 , 6144 (2020).
Article CAS PubMed Central Google Scholar
Dandona, R. & Sagar, R. COVID-19 offers an opportunity to reform mental health in India. Lancet Psychiatry 8 , 9–11 (2021).
Qiu, D. et al. Policies to improve the mental health of people influenced by COVID-19 in China: a scoping review. Front. Psychiatry 11 , 588137 (2020).
Su, Z. et al. Mental health consequences of COVID-19 media coverage: the need for effective crisis communication practices. Global Health 17 , 4 (2021).
Petersen, M. B. COVID lesson: trust the public with hard truths. Nature 598 , 237 (2021).
van der Bles, A. M., van der Linden, S., Freeman, A. L. J. & Spiegelhalter, D. J. The effects of communicating uncertainty on public trust in facts and numbers. Proc. Natl Acad. Sci. USA 117 , 7672–7683 (2020).
Titze-de-Almeida, R. et al. Persistent, new-onset symptoms and mental health complaints in Long COVID in a Brazilian cohort of non-hospitalized patients. BMC Infect. Dis. 22 , 133 (2022).
Carfì, A., Bernabei, R. & Landi, F. Persistent symptoms in patients after acute COVID-19. JAMA 324 , 603–605 (2020).
Bliddal, S. et al. Acute and persistent symptoms in non-hospitalized PCR-confirmed COVID-19 patients. Sci. Rep. 11 , 13153 (2021).
Kim, Y. et al. Post-acute COVID-19 syndrome in patients after 12 months from COVID-19 infection in Korea. BMC Infect. Dis . 22 , 93 (2022).
Download references
Acknowledgements
The authors thank E. Giltay for assistance on data analyses and production of Fig. 1 . B.W.J.H.P. discloses support for research and publication of this work from the European Union’s Horizon 2020 research and innovation programme-funded RESPOND project (grant no. 101016127).
Author information
Authors and affiliations.
Department of Psychiatry, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands
Brenda W. J. H. Penninx & Christiaan H. Vinkers
Amsterdam Public Health, Mental Health Program and Amsterdam Neuroscience, Mood, Anxiety, Psychosis, Sleep & Stress Program, Amsterdam, The Netherlands
Biological and Precision Psychiatry, Copenhagen Research Center for Mental Health, Mental Health Center Copenhagen, Copenhagen University Hospital, Copenhagen, Denmark
Michael E. Benros
Department of Immunology and Microbiology, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark
Departments of Medicine, Pathology & Immunology and Neuroscience, Center for Neuroimmunology & Neuroinfectious Diseases, Washington University School of Medicine, St. Louis, MO, USA
Robyn S. Klein
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Brenda W. J. H. Penninx .
Ethics declarations
Competing interests.
The authors declare no conflicts of interest.
Peer review
Peer review information.
Nature Medicine thanks Jane Pirkis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary handling editor: Karen O’Leary, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Penninx, B.W.J.H., Benros, M.E., Klein, R.S. et al. How COVID-19 shaped mental health: from infection to pandemic effects. Nat Med 28 , 2027–2037 (2022). https://doi.org/10.1038/s41591-022-02028-2
Download citation
Received : 06 June 2022
Accepted : 26 August 2022
Published : 03 October 2022
Issue Date : October 2022
DOI : https://doi.org/10.1038/s41591-022-02028-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Mental health disturbance in preclinical medical students and its association with screen time, sleep quality, and depression during the covid-19 pandemic.
- Tjhin Wiguna
- Valerie Josephine Dirjayanto
- Erik Kinzie
BMC Psychiatry (2024)
Protocol for a pilot cluster randomised controlled trial of a multicomponent sustainable return to work IGLOo intervention
- Oliver Davis
- Jeremy Dawson
- Fehmidah Munir
Pilot and Feasibility Studies (2024)
Impact of COVID-19 first wave on the mental health of healthcare workers in a Front-Line Spanish Tertiary Hospital: lessons learned
- Juan D. Molina
- Franco Amigo
- Gabriel Rubio
Scientific Reports (2024)
Long-term risk of psychiatric disorder and psychotropic prescription after SARS-CoV-2 infection among UK general population
- Daniel Prieto-Alhambra
Nature Human Behaviour (2024)
Changes in alcohol consumption and alcohol problems before and after the COVID-19 pandemic: a prospective study in heavy drinking young adults
- Kasey G. Creswell
- Garrett C. Hisler
- Aidan G. C. Wright
Nature Mental Health (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Advertisement
Economic Evaluation of COVID-19 Immunization Strategies: A Systematic Review and Narrative Synthesis
- Systematic Review
- Published: 10 April 2024
Cite this article

- Enxue Chang 1 na1 ,
- Haofei Li 1 na1 ,
- Wanji Zheng 1 na1 ,
- Lan Zhou 1 ,
- Yanni Jia 1 ,
- Yiyin Cao 1 ,
- Xiaoying Zhu 2 , 3 ,
- Juan Xu 4 ,
- Mao You 6 ,
- Kejun Liu 6 ,
- Mingsi Wang 1 &
- Weidong Huang ORCID: orcid.org/0009-0008-9580-6862 1
151 Accesses
1 Altmetric
Explore all metrics
This study aimed to systematically assess global economic evaluation studies on COVID-19 vaccination, offer valuable insights for future economic evaluations, and assist policymakers in making evidence-based decisions regarding the implementation of COVID-19 vaccination.
Searches were performed from January 2020 to September 2023 across seven English databases (PubMed, Web of Science, MEDLINE, EBSCO, KCL-Korean Journal Dataset, SciELO Citation Index, and Derwent Innovations Index) and three Chinese databases (Wanfang Data, China Science and Technology Journal, and CNKI). Rigorous inclusion and exclusion criteria were applied. Data were extracted from eligible studies using a standardized data collection form, with the reporting quality of these studies assessed using the Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022).
Of the 40 studies included in the final review, the overall reporting quality was good, evidenced by a mean score of 22.6 (ranging from 10.5 to 28). Given the significant heterogeneity in fundamental aspects among the studies reviewed, a narrative synthesis was conducted. Most of these studies adopted a health system or societal perspective. They predominantly utilized a composite model, merging dynamic and static methods, within short to medium-term time horizons to simulate various vaccination strategies. The research strategies varied among studies, investigating different doses, dosages, brands, mechanisms, efficacies, vaccination coverage rates, deployment speeds, and priority target groups. Three pivotal parameters notably influenced the evaluation results: the vaccine's effectiveness, its cost, and the basic reproductive number ( R 0 ). Despite variations in model structures, baseline parameters, and assumptions utilized, all studies identified a general trend that COVID-19 vaccination is cost-effective compared to no vaccination or intervention.
Conclusions
The current review confirmed that COVID-19 vaccination is a cost-effective alternative in preventing and controlling COVID-19. In addition, it highlights the profound impact of variables such as dose size, target population, vaccine efficacy, speed of vaccination, and diversity of vaccine brands and mechanisms on cost effectiveness, and also proposes practical and effective strategies for improving COVID-19 vaccination campaigns from the perspective of economic evaluation.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
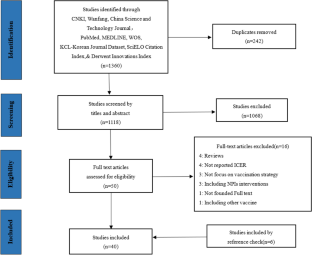
Similar content being viewed by others

Models of COVID-19 vaccine prioritisation: a systematic literature search and narrative review
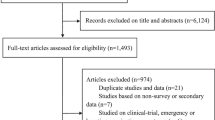
Mapping global acceptance and uptake of COVID-19 vaccination: A systematic review and meta-analysis
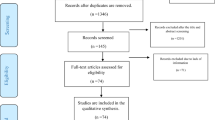
Global COVID-19 vaccine acceptance rate: a systematic review and meta-analysis
Vaezi A, Meysamie A. COVID-19 vaccines cost-effectiveness analysis: a scenario for Iran. Vaccines. 2021;10(1):37.
Article PubMed PubMed Central Google Scholar
WHO. WHO Director-General’s opening remarks at the media briefing on COVID-19–11 March 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-oncovid-19---11-march-2020 .
WHO. Coronavirus disease (COVID-19) Weekly Epidemiological Updates and Monthly Operational Updates; 2023. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports .
Vysochyna A, et al. Impact of coronavirus disease COVID-19 on the relationship between healthcare expenditures and sustainable economic growth. Int J Environ Res Public Health. 2023;20(4):3049.
Di Fusco M, et al. Health outcomes and economic burden of hospitalized COVID-19 patients in the United States. J Med Econ. 2021;24(1):308–17.
Article PubMed Google Scholar
Keogh-Brown MR, et al. The impact of Covid-19, associated behaviours and policies on the UK economy: a computable general equilibrium model. SSM Popul Health. 2020;12: 100651.
John D, et al. Estimation of the economic burden of COVID-19 using disability-adjusted life years (DALYs) and productivity losses in Kerala, India: a model-based analysis. BMJ Open. 2021;11(8): e049619.
An X, et al. Economic burden of public health care and hospitalisation associated with COVID-19 in China. Public Health. 2022;203:65–74.
Article CAS PubMed Google Scholar
Jin H, et al. Economic burden of COVID-19, China, January-March, 2020: a cost-of-illness study. Bull World Health Organ. 2021;99(2):112–24.
Harrison EA, Wu JW. Vaccine confidence in the time of COVID-19. Eur J Epidemiol. 2020;35(4):325–30.
Article CAS PubMed PubMed Central Google Scholar
ANVISA. Bases técnicas para decisão do uso emergencial, Em caráter experimental de vacinas contra a COVID-19 .
Morales-Zamora G, et al. Cost-effectiveness analysis of strategies of COVID-19 vaccination in Colombia: comparison of high-risk prioritization and no prioritization strategies with the absence of a vaccination plan. Value Health Reg Issues. 2022;05(31):101–10.
Article Google Scholar
WHO. COVID-19 vaccine tracker and landscape; 2023. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines .
Group, S. Shiyao Group's COVID-19 mRNA vaccine is included in emergency use in China; 2023. http://www.e-cspc.com/details/details_92_4894.html .
Kirwin E, et al. A net benefit approach for the optimal allocation of a COVID-19 vaccine. Pharmacoeconomics. 2021;39(9):1059–73.
World Health Organization. Adding a vaccine to a national immunization programme: decision and implementation. Geneva: World Health Organization; 2005.
Google Scholar
Walker DG, Hutubessy R, Beutels P. WHO Guide for standardisation of economic evaluations of immunization programmes. Vaccine. 2010;28(11):2356–9.
Utami AM, et al. Cost-effectiveness analysis of COVID-19 vaccination in low- and middle-income countries. J Multidiscip Healthc. 2022;15:2067–76.
Zhao JY, et al. Progress in research of economic evaluation of COVID-19 vaccination strategies. Zhonghua Liu Xing Bing Xue Za Zhi. 2022;43(4):460–5.
CAS PubMed Google Scholar
Utami AM, et al. Economic evaluation of COVID-19 vaccination: a systematic review. J Glob Health. 2023;13:06001.
Santoli G, et al. Incremental net benefit and incremental cost-effectiveness ratio of COVID-19 vaccination campaigns: systematic review of cost-effectiveness evidence. Vaccines (Basel). 2023;11(2):347.
Fu Y, et al. Cost-effectiveness of COVID-19 vaccination: a systematic review. J Evid Based Med. 2023;16(2):152–65.
Aidalina M, Khalsom S. COVID-19 vaccination: a systematic review of vaccination strategies based on economic evaluation studies. Med J Malaysia. 2023;78:411–20.
Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7): e1000097.
International Monetary Fund. Consumer Price Index (CPI). https://data.imf.org/?sk=4ffb52b2-3653-409a-b471-d47b46d904b5&sid=1485878855236 .
International Monetary Fund. Representative exchange rates for selected currencies for December 2023. https://www.imf.org/external/np/fin/data/rms_mth.aspx?SelectDate=2023-12-31&reportType=REP .
Husereau D, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Int J Technol Assess Health Care. 2022;38(1): e13.
Husereau D, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMJ. 2022;376: e067975.
Husereau D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 Explanation and Elaboration: a report of the ISPOR CHEERS II Good Practices Task Force. Value Health. 2022;25(1):10–31.
Du Y, et al. Economic evaluations of 13-valent pneumococcal conjugate vaccine: a systematic review. Expert Rev Vaccines. 2023;22(1):193–206.
Popay J, Roberts H, Sowden A, et al. Guidance on the conduct of narrative synthesis in systematic reviews. 1. A product from the ESRC methods programme version. 2006:b92.
Li R, et al. Cost-effectiveness analysis of BNT162b2 COVID-19 booster vaccination in the United States. Int J Infect Dis. 2022;03(119):87–94.
Kohli M, et al. The potential public health and economic value of a hypothetical COVID-19 vaccine in the United States: use of cost-effectiveness modeling to inform vaccination prioritization. Vaccine. 2021;39(7):1157–64.
Di Fusco M, et al. Public health impact of the Pfizer-BioNTech COVID-19 vaccine (BNT162b2) in the first year of rollout in the United States. J Med Econ. 2022;25(1):605–17.
Bartsch SM, et al. Lives and costs saved by expanding and expediting coronavirus disease 2019 vaccination. J Infect Dis. 2021;224(6):938–48.
Padula WV, et al. Economic value of vaccines to address the COVID-19 pandemic: a U.S. cost-effectiveness and budget impact analysis. J Med Econ. 2021;24(1):1060–9.
Shaker M, Abrams EM, Greenhawt M. A cost-effectiveness evaluation of hospitalizations, fatalities, and economic outcomes associated with universal versus anaphylaxis risk-stratified COVID-19 vaccination strategies. J Allergy Clin Immunol Pract. 2021;9(7):2658-2668.e3.
Fu Y, et al. Effectiveness and cost-effectiveness of inactivated vaccine to address COVID-19 pandemic in China: evidence from randomized control trials and real-world studies. Front Public Health. 2022;07(10): 917732.
Xiong X, et al. Economic value of vaccines to address the COVID-19 pandemic in Hong Kong: a cost-effectiveness analysis. Vaccines (Basel). 2022;10(4):495.
Wang WC, et al. Economic evaluation for mass vaccination against COVID-19. J Formos Med Assoc. 2021;06(120):S95–105.
Zhou H, et al. Cost-effectiveness analysis of vaccination against COVID-19 in China. Front Public Health. 2023;11: 1037556.
Du M, et al. Cost-effectiveness analysis of COVID-19 inactivated vaccines in reducing the economic burden of ischaemic stroke after SARS-CoV-2 infection. Vaccines. 2023;11(5):957.
Fu Y, et al. Cost-effectiveness of COVID-19 sequential vaccination strategies in inactivated vaccinated individuals in China. Vaccines (Basel). 2022;10(10):1712.
Wang Y, et al. Assessing the cost-effectiveness of COVID-19 vaccines in a low incidence and low mortality setting: the case of Thailand at start of the pandemic. Eur J Health Econ. 2022;08:1–14.
Sirison K, et al. Cost-effectiveness analysis of COVID-19 vaccine booster dose in the Thai setting during the period of omicron variant predominance. Trop Med Infect Dis. 2023;8(2):91.
Suphanchaimat R, et al. Prioritization of the target population for coronavirus disease 2019 (COVID-19) vaccination program in Thailand. Int J Environ Res Public Health. 2021;18(20): 10803.
Du Z, et al. Cost effectiveness of fractional doses of COVID-19 vaccine boosters in India. Medicine (N Y). 2023;4(3):182-190.e3.
Du ZW, et al. Modeling comparative cost-effectiveness of SARS-CoV-2 vaccine dose fractionation in India. Nat Med. 2022;28(5):934.
Marco-Franco JE, et al. Simplified mathematical modelling of uncertainty: cost-effectiveness of COVID-19 vaccines in Spain. Mathematics. 2021;9(5):566.
Lopez F, et al. A cost-benefit analysis of COVID-19 vaccination in Catalonia. Vaccines. 2021;10(1):59.
Siedner MJ, et al. Cost-effectiveness of COVID-19 vaccination in low- and middle-income countries. J Infect Dis. 2022;226:1887–96.
Savinkina A, et al. Estimating deaths averted and cost per life saved by scaling up mRNA COVID-19 vaccination in low-income and lower-middle-income countries in the COVID-19 Omicron variant era: a modelling study. BMJ Open. 2022;12(9): e061752.
Jiang Y, Cai D, Shi S. Economic evaluations of inactivated COVID-19 vaccines in six Western Pacific and South East Asian countries and regions: a modeling study. Infect Dis Model. 2021;7(1):109–21.
PubMed PubMed Central Google Scholar
Liu Y, et al. Assessing the impacts of COVID-19 vaccination programme’s timing and speed on health benefits, cost-effectiveness, and relative affordability in 27 African countries. BMC Med. 2023;21(1):85.
Reddy KP, et al. Clinical outcomes and cost-effectiveness of COVID-19 vaccination in South Africa. Nat Commun. 2021;12(1):6238.
Orlewska K, Wierzba W, Sliwczynski A. Cost-effectiveness analysis of COVID-19 vaccination in Poland. Arch Med Sci. 2021;18(4):1021–30.
Edejer TT-T, Baltussen RM, Adam T, Hutubessy R, Acharya A, Evans D, et al. Making choices in health: WHO guide to cost-effective analysis. Geneva: WHO; 2003.
Debrabant K, Grønbæk L, Kronborg C. The cost-effectiveness of a COVID-19 vaccine in a Danish context. Clin Drug Investig. 2021;41(11):975–88.
Mungmunpuntipantip R, Wiwanitkit V. Expected cost effectiveness of the fourth dose of COVID-19 vaccine against the omicron variant of COVID-19: a preliminary report. Int J Physiol Pathophysiol Pharmacol. 2022;14(4):272–5.
CAS PubMed PubMed Central Google Scholar
Fernandes RRA, et al. Cost utility of vaccination against COVID-19 in Brazil. Value Health Reg Issues. 2022;01(31):18–24.
Yaroslavovych TB, et al. Pharmaco economics analysis of COVID-19 vaccines in Ukraine. J Pharm Res Int. 2021;06:140–7.
Orangi S, et al. Epidemiological impact and cost-effectiveness analysis of COVID-19 vaccination in Kenya. BMJ Glob Health. 2022;7(8): e009430.
Sandmann FG, et al. The potential health and economic value of SARS-CoV-2 vaccination alongside physical distancing in the UK: a transmission model-based future scenario analysis and economic evaluation. Lancet Infect Dis. 2021;21(7):962–74.
MacIntyre CR. Navigating post-vaccine COVID-19 futures in the health and economic context. Lancet Infect Dis. 2021;21(7):893–4.
Mahmud I, et al. The health belief model predicts intention to receive the COVID-19 vaccine in Saudi Arabia: results from a cross-sectional survey. Vaccines (Basel). 2021;9(8):864.
Rodrigues CMC, Plotkin SA. Impact of vaccines; health, economic and social perspectives. Front Microbiol. 2020;11:1526.
Yue MA, Ke XT, Liang XY, et al. Application of transmission dynamics model in economic evaluation on HPV vaccine vaccination: a brief introduction. Chin J Public Health. 2021;37(12):1742–5.
Ciotti M, et al. The COVID-19 pandemic: viral variants and vaccine efficacy. Crit Rev Clin Lab Sci. 2021;59(1):66–75.
Mateus J, et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science. 2020;370(6512):89–94.
Mascola JR, Graham BS, Fauci AS. SARS-CoV-2 viral variants-tackling a moving target. JAMA. 2021;325(13):1261–2.
Tregoning JS, et al. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. 2021;21(10):626–36.
Haddad FS, McLawhorn AS. Guidelines for reporting health economics research. Bone Joint J. 2016;98:147–51.
World Health Organization. WHO guide for standardisation of economic evaluations of immunization programmes. Paris: World Health Organization; 2019.
Bertram MY, et al. Methods for the economic evaluation of health care interventions for priority setting in the health system: an update from WHO CHOICE. Int J Health Policy Manag. 2021;10(11):673–7.
Drummond M, Sculpher M, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2015. p. 42–4.
Shields GE, Elvidge J. Challenges in synthesising cost-effectiveness estimates. Syst Rev. 2020;9(1):289.
Download references
Author information
Enxue Chang, Haofei Li and Wanji Zheng have contributed equally to this work and share first authorship.
Authors and Affiliations
School of Health Management, Harbin Medical University, Harbin, China
Enxue Chang, Haofei Li, Wanji Zheng, Lan Zhou, Yanni Jia, Wen Gu, Yiyin Cao, Mingsi Wang & Weidong Huang
School of Elderly Care Services and Management, Nanjing University of Chinese Medicine, Nanjing, Jiangsu, China
Xiaoying Zhu
Nossal Institute for Global Health, School of Population and Global Health, The University of Melbourne, Melbourne, VIC, Australia
Cancer Hospital Chinese Academy of Medical Sciences, Shenzhen Center, Shenzhen, China
Shenzhen Health Capacity Building and Continuing Education Center, Shenzhen, China
National Health Development Research Center, Beijing, 100191, China
Mao You & Kejun Liu
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to Xiaoying Zhu , Kejun Liu , Mingsi Wang or Weidong Huang .
Ethics declarations
This research was funded by the Beijing Natural Science Foundation-Haidian Original Innovation Joint Fund (L212012).
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability
Not applicable.
Ethics Approval
Consent to participate, consent for publication, code availability, author contributions.
Enxue Chang, Weidong Huang, Mao You, Kejun Liu, and Mingsi Wang contributed to the study conception and design. Enxue Chang, Haofei Li, and Wanji Zheng performed screening, full text selection, and data extraction. Enxue Chang, Haofei Li, Wanji Zheng, Lan Zhou, and Yanni Jia conducted the quality appraisal of the studies. Mingsi Wang, Wen Gu, Yiyin Cao, Juan Xu, and Bo Liu contributed to the data interpretation. The first draft of the manuscript was written by Enxue Chang, Weidong Huang, and Xiaoying Zhu, and all authors contributed to the critical revision of the manuscript for intellectual content and approved the final draft submitted for publication.
Additional information
Weidong Huang is the leading corresponding author.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 209 KB)
Rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Chang, E., Li, H., Zheng, W. et al. Economic Evaluation of COVID-19 Immunization Strategies: A Systematic Review and Narrative Synthesis. Appl Health Econ Health Policy (2024). https://doi.org/10.1007/s40258-024-00880-6
Download citation
Accepted : 17 March 2024
Published : 10 April 2024
DOI : https://doi.org/10.1007/s40258-024-00880-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
Students can choose to write a full-length college essay on the coronavirus or summarize their experience in a shorter form. To help students explain how the pandemic affected them, The Common App ...
Coronavirus disease 2019 (COVID-19) is a highly contagious viral illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COVID-19 has had a catastrophic effect on the world, resulting in more than 6 million deaths worldwide. After the first cases of this predominantly respiratory viral illness were reported in Wuhan, Hubei Province, China, in late December 2019, SARS ...
The spread of the "Severe Acute Respiratory Coronavirus 2" (SARS-CoV-2), the causal agent of COVID-19, was characterized as a pandemic by the World Health Organization (WHO) in March 2020 and has triggered an international public health emergency [].The numbers of confirmed cases and deaths due to COVID-19 are rapidly escalating, counting in millions [], causing massive economic strain ...
Favipiravir, a previously validated drug for influenza, is a drug that has shown promising results for COVID-19 in experimental and clinical studies, but it is under further evaluation. 70 , 79 , 80 Remdesivir, which was developed for Ebola, is an antiviral drug that is under evaluation for moderate-to-severe COVID-19 owing to its promising ...
This review will introduce a general overview of coronavirus and describe the clinical features, evaluation, and treatment of COVID-19 patients and provide a means to raise awareness among primary and secondary healthcare providers during the current pandemic. Coronavirus (COVID-19) is an enveloped RNA virus that is diversely found in humans and wild life. A total of six species have been ...
This essay examines key aspects of social relationships that were disrupted by the COVID-19 pandemic. It focuses explicitly on relational mechanisms of health and brings together theory and emerging evidence on the effects of the COVID-19 pandemic to make recommendations for future public health policy and recovery. We first provide an overview of the pandemic in the UK context, outlining the ...
The student or a family member had COVID-19 or suffered other illnesses due to confinement during the pandemic. The student suffered from a lack of internet access and other online learning challenges. Students who dealt with problems registering for or taking standardized tests and AP exams. Jeff Schiffman of the Tulane University admissions ...
COVID-19 can involve persistence, sequelae, and other medical complications that last weeks to months after initial recovery. This systematic review and meta-analysis aims to identify studies ...
The effectiveness of the mRNA vaccines in preventing COVID-19 disease progression in 2021 set new expectations about the role of prevention interventions for the disease. Efficacy observed in the trials was more than 90%.1,2 The efficacy of other vaccines evaluated in large randomised trials, such as the Oxford-AstraZeneca (70%) and Sputnik V (91%) vaccines, have been criticised for elements ...
COVID-19: Emergence, Spread, Possible Treatments, and Global Burden. The Coronavirus (CoV) is a large family of viruses known to cause illnesses ranging from the common cold to acute respiratory tract infection. The severity of the infection may be visible as pneumonia, acute respiratory syndrome, and even death.
1. Introduction. The coronavirus disease 2019 (COVID-19) pandemic has led to unprecedented changes in people's daily lives, with implications for mental health and well-being [1-4], both at the level of a given country's population, and when considering specific vulnerable groups [5-7].In order to mitigate the untoward impact of the pandemic (including lockdown) and support mental health ...
The pandemic of Coronavirus Disease 2019 (COVID-19) is a timely reminder of the nature and impact of Public Health Emergencies of International Concern. As of 12 January 2022, there were over 314 million cases and over 5.5 million deaths notified since the start of the pandemic. The COVID-19 pandemic takes variable shapes and forms, in terms of cases and deaths, in different regions and ...
COVID-19 spread worldwide after its outbreak in December 2019. This review paper aims to educate the readers regarding SARS-CoV-2 diagnostic and detection tools and the issues experienced by researchers. We identify on-the-horizon point-of-care diagnostic tests and inspire scholars to develop their …
The coronavirus disease 2019 (COVID-19) pandemic has led to one of the largest disruptions to learning in history. To a large extent, this is due to school closures, which are estimated to have ...
This essay examines key aspects of social relationships that were disrupted by the COVID-19 pandemic. It focuses explicitly on relational mechanisms of health and brings together theory and emerging evidence on the effects of the COVID-19 pandemic to make recommendations for future public health policy and recovery.
It is very exceptional that a new disease becomes a true pandemic. Since its emergence in Wuhan, China, in late 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, has spread to nearly all countries of the world in only a few months. However, in different countries, the COVID-19 epidemic takes variable shapes and forms in how it affects communities.
This essay examines the implications of the COVID-19 pandemic for health inequalities. It outlines historical and contemporary evidence of inequalities in pandemics—drawing on international research into the Spanish influenza pandemic of 1918, the H1N1 outbreak of 2009 and the emerging international estimates of socio-economic, ethnic and geographical inequalities in COVID-19 infection and ...
While the COVID-19 pandemic has hit different countries with varying intensity, responding to the crisis has presented an unprecedented challenge to most governments. In this context, evaluations provide critical tools to support real time sharing of lessons on what is working, what is not, what could work and for whom. This paper draws lessons from evaluations that governments have carried ...
On a global scale and based on imputations and modeling from survey data of self-reported mental health problems, the Global Burden of Disease (GBD) study 29 estimated that the COVID-19 pandemic ...
To extract practical insight from the large body of COVID-19 modelling literature available, we provide a narrative review with a systematic approach that quantitatively assessed prospective, data-driven modelling studies of COVID-19 in the USA. We analysed 136 papers, and focused on the aspects of models that are essential for decision makers.
Investigating the Prevalence of COVID-19-Related Sleep Disorders Among Individuals Recovering from COVID-19: A Cross-Sectional Analytical Study, Jundishapur Journal of Health Sciences, 15, 4 ...
COVID-19 seems to be more dangerous to those with cardio-vascular disease (CVD) risk factors including behavioural risk factors (eg, ... Essay. investigation of community consequences of prisoner release into the community as a strategy to limit COVID-19 transmis-sion in prisons. Similarly, unpicking the source of ethnic
This study aimed to systematically assess global economic evaluation studies on COVID-19 vaccination, offer valuable insights for future economic evaluations, and assist policymakers in making evidence-based decisions regarding the implementation of COVID-19 vaccination. ... The final 40 papers included in this review consisted of 18 in 2021 ...