

Study at Cambridge
About the university, research at cambridge.
- Undergraduate courses
- Events and open days
- Fees and finance
- Postgraduate courses
- How to apply
- Postgraduate events
- Fees and funding
- International students
- Continuing education
- Executive and professional education
- Courses in education
- How the University and Colleges work
- Term dates and calendars
- Visiting the University
- Annual reports
- Equality and diversity
- A global university
- Public engagement
- Give to Cambridge
- For Cambridge students
- For our researchers
- Business and enterprise
- Colleges & departments
- Email & phone search
- Museums & collections
- Data Management Guide
- Creating your data

- Data Management Guide overview
- Creating your data overview
- Organising your data
- Accessing your data overview
- Looking after your data
- Sharing your data
- Choosing a software licence
- Electronic Research Notebooks overview
- Support overview
- Resources and support at Cambridge overview
- Data Management Plan Support Service
- DMP Pilot for PhDs
- External support
- Data Repository overview
- Upload your data
- Depositor's checklist
- Guidance on the data submission process
- Data Policies overview
- University of Cambridge Research Data Management Policy Framework overview
- Cambridge data-related policies
- Funders Policies overview
- News overview
- Data Champions overview
- Data Champion list
- Data Champion Community
- Data Champions Cartoons
- Alumni Data Champions
- 2024 Call for Data Champions
- Events overview
- Love Data Week 2024
- Past Events
- Contact Us overview
- Our Governance
- Request a Meeting
- Data Management Plan
- Research Data Management
- Choosing Formats
- Intellectual Property Rights
- Data Protection and Ethics
- Accessing your data
- Electronic Research Notebooks

Crafting your data management plan
Most research funders encourage researchers to think about their research data management activities from the beginning of the project. This will often mean a formal plan for managing data (a 'data management plan').
However, even informally setting out your plans and project guidelines can make your life much easier. If you want to be able to reuse your data or manage collaboration with colleagues, it helps to plan for that from the beginning. Decisions you make about which software to use, how to organise, store and manage your data, and the consent agreements you would have to negotiate, will all affect what is possible to do, and what data is shareable in the future.
Planning ahead for your data management needs and activities will help ensure that:
- you have adequate technological resources (e.g. storage space, support staff time)
- your data will be robust and free from versioning errors and gaps in documentation
- your data is backed up and safe from sudden loss or corruption
- you can meet legal and ethical requirements
- you are able to share your finalised data publicly, if you and/or your funder desires
- your data will remain accessible and comprehensible in the near, middle, and distant future.
What do research funders expect?
Most funders expect you to prepare a data management plan when applying for a research grant. Additionally, some funders, for example the Medical Research Council ( MRC ), will require you to regularly review your data management plan and make all necessary amendments while managing your grant. The Economic and Social Research Council (ESRC) provides comprehensive guidelines on how treating personal and sensitive data, as well as on obtaining consent for data collection from participants. The information on funder requirements is available here .
Where do I start?
Much of research data management is simply good research practice so you will already be some way down the line. Data plans are just a way of ensuring (and/or showing) that you have thought about how to create, store, backup, share and preserve your data.
The Digital Curation Centre ( DCC ) has produced an interactive online tool to help researchers create data management plans: DMPOnline . The website records all major UK/European funder requirements, and it automatically tailors the data management plan template to the needs of your funder. You can log in to DMPonline using your Raven account (to do this, simply select the University of Cambridge as your institution, and you will be re-directed to the Raven log-in interface). Data plans that you create are easily exportable to a desired file type (Word, Excel, pdf), so you can simply add them to your grant applications.
What should I include in my data plan?
The best way to start is to look for what your funder expects you to cover in your Data Management Plan. You can either check this on your funder's website or by using the DMPonline tool, which is populated with funder's template and will guide you through your funder's requirements.
Who can help with data planning at the University of Cambridge?
The University has a range of support staff who can help you create a data management plan, including:
- your departmental or college IT staff
- subject and departmental librarians
- your funder - some funders, for example, the Economic and Social Research Council (ESRC), offer support in preparation of data management plans
No matter who you ask for support, please get in touch early, so there is enough time for support staff to help.
Simple data management plan template
Have a look at our simple data management plan template here - if your funder does not provide guidance on data plans, this might be a good starting point.
Related links
- DMPonline - tool to create data management plans
- ESRC - support for data management plans
About this site :
This site is managed by the Research Data Team.
If you have any questions about this site, please e-mail us directly
The project is a joint initiative of Cambridge University Library and the Research Operations Office .
Privacy policy
© 2024 University of Cambridge
- Contact the University
- Accessibility
- Freedom of information
- Privacy policy and cookies
- Statement on Modern Slavery
- Terms and conditions
- University A-Z
- Undergraduate
- Postgraduate
- Research news
- About research at Cambridge
- Spotlight on...
MIT Libraries logo MIT Libraries

Write a data management plan
A data management plan (DMP) will help you manage your data, meet funder requirements, and help others use your data if shared.

Alternatively, you can use the questions below and any specific data management requirements from your funding agency to write your data management plan. Additional resources for creating plans are also provided below.
- What’s the purpose of the research?
- What is the data? How and in what format will the data be collected? Is it numerical data, image data, text sequences, or modeling data?
- How much data will be generated for this research?
- How long will the data be collected and how often will it change?
- Are you using data that someone else produced? If so, where is it from?
- Who is responsible for managing the data? Who will ensure that the data management plan is carried out?
- What documentation will you be creating in order to make the data understandable by other researchers?
- Are you using metadata that is standard to your field? How will the metadata be managed and stored?
- What file formats will be used? Do these formats conform to an open standard and/or are they proprietary?
- Are you using a file format that is standard to your field? If not, how will you document the alternative you are using?
- What directory and file naming convention will be used?
- What are your local storage and backup procedures ? Will this data require secure storage?
- What tools or software are required to read or view the data?
- Who has the right to manage this data? Is it the responsibility of the PI, student, lab, MIT, or funding agency?
- What data will be shared , when, and how?
- Does sharing the data raise privacy, ethical, or confidentiality concerns ? Do you have a plan to protect or anonymize data, if needed?
- Who holds intellectual property rights for the data and other information created by the project? Will any copyrighted or licensed material be used? Do you have permission to use/disseminate this material?
- Are there any patent- or technology-licensing-related restrictions on data sharing associated with this grant? The Technology Licensing Office (TLO) can provide this information.
- Will this research be published in a journal that requires the underlying data to accompany articles?
- Will there be any embargoes on the data?
- Will you permit re-use , redistribution, or the creation of new tools, services, data sets, or products (derivatives)? Will commercial use be allowed?
- How will you be archiving the data? Will you be storing it in an archive or repository for long-term access? If not, how will you preserve access to the data?
- Is a discipline-specific repository available? If not, consider depositing your data into a generalist data repository . Email us at [email protected] if you’re interested in discussing repository options for your data.
- How will you prepare data for preservation or data sharing? Will the data need to be anonymized or converted to more stable file formats?
- Are software or tools needed to use the data? Will these be archived?
- How long should the data be retained? 3-5 years, 10 years, or forever?
Additional resources for creating plans
- Managing your data – Project Start & End Checklists (MIT Data Management Services) : Checklist (PDF) with detailed resources to help researchers set up and maintain robust data management practices for the full life of a project.
- ezDMP : a free web-based tool for creating DMPs specific to a subset of NSF funding requirements.
- Guidelines for Effective Data Management Plans and Data Management Plan Resources and Examples (ICPSR) : Framework for creating a plan and links to examples of data management plans in various scientific disciplines
- Example Plans (University of Minnesota)
- NSF (by the DART project) : assessment rubric and guidance
- NIH (by FASEB)
See other guides to data management for additional guidance on managing data and select information related to particular formats or disciplines.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- CAREER FEATURE
- 13 March 2018
Data management made simple
- Quirin Schiermeier
You can also search for this author in PubMed Google Scholar
When Marjorie Etique learnt that she had to create a data-management plan for her next research project, she was not sure exactly what to do.
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Nature 555 , 403-405 (2018)
doi: https://doi.org/10.1038/d41586-018-03071-1
See Editorial: Everyone needs a data-management plan
Related Articles

The FAIR Guiding Principles for scientific data management and stewardship

How I fled bombed Aleppo to continue my career in science
Career Feature 08 MAY 24

Illuminating ‘the ugly side of science’: fresh incentives for reporting negative results

Hunger on campus: why US PhD students are fighting over food
Career Feature 03 MAY 24

The US Congress is taking on AI —this computer scientist is helping
News Q&A 09 MAY 24
Powerful ‘nanopore’ DNA sequencing method tackles proteins too
Technology Feature 08 MAY 24

Who’s making chips for AI? Chinese manufacturers lag behind US tech giants
News 03 MAY 24
Clinician Researcher/Group Leader in Cancer Cell Therapies
An excellent opportunity is available for a Group Leader with expertise in cellular therapies to join the Cancer Research program at QIMR Berghofer.
Herston, Brisbane (AU)
QIMR Berghofer
Faculty Positions at the Center for Machine Learning Research (CMLR), Peking University
CMLR's goal is to advance machine learning-related research across a wide range of disciplines.
Beijing, China
Center for Machine Learning Research (CMLR), Peking University
Faculty Positions at SUSTech Department of Biomedical Engineering
We seek outstanding applicants for full-time tenure-track/tenured faculty positions. Positions are available for both junior and senior-level.
Shenzhen, Guangdong, China
Southern University of Science and Technology (Biomedical Engineering)
Southeast University Future Technology Institute Recruitment Notice
Professor openings in mechanical engineering, control science and engineering, and integrating emerging interdisciplinary majors
Nanjing, Jiangsu (CN)
Southeast University
Staff Scientist
A Staff Scientist position is available in the laboratory of Drs. Elliot and Glassberg to study translational aspects of lung injury, repair and fibro
Maywood, Illinois
Loyola University Chicago - Department of Medicine
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Ask Yale Library
My Library Accounts
Find, Request, and Use
Help and Research Support
Visit and Study
Explore Collections
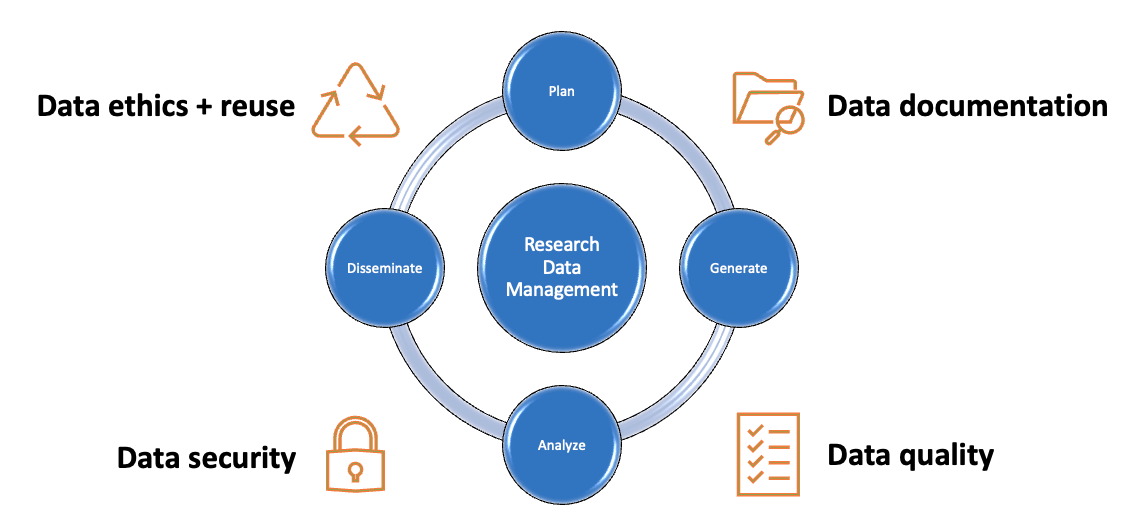
Research Data Management: Plan for Data
- Plan for Data
- Organize & Document Data
- Store & Secure Data
- Validate Data
- Share & Re-use Data
- Data Use Agreements
- Research Data Policies
What is a Data Management Plan?
Data management plans (DMPs) are documents that outline how data will be collected , stored , secured , analyzed , disseminated , and preserved over the lifecycle of a research project. They are typically created in the early stages of a project, and they are typically short documents that may evolve over time. Increasingly, they are required by funders and institutions alike, and they are a recommended best practice in research data management.
Tab through this guide to consider each stage of the research data management process, and each correlated section of a data management plan.
Tools for Data Management Planning
DMPTool is a collaborative effort between several universities to streamline the data management planning process.
The DMPTool supports the majority of federal and many non-profit and private funding agencies that require data management plans as part of a grant proposal application. ( View the list of supported organizations and corresponding templates.) If the funder you're applying to isn't listed or you just want to create one as good practice, there is an option for a generic plan.
Key features:
Data management plan templates from most major funders
Guided creation of a data management plan with click-throughs and helpful questions and examples
Access to public plans , to review ahead of creating your own
Ability to share plans with collaborators as well as copy and reuse existing plans
How to get started:
Log in with your yale.edu email to be directed to a NetID sign-in, and review the quick start guide .
Research Data Lifecycle
Additional Resources for Data Management Planning
- << Previous: Overview
- Next: Organize & Document Data >>
- Last Updated: Sep 27, 2023 1:15 PM
- URL: https://guides.library.yale.edu/datamanagement
Site Navigation
P.O. BOX 208240 New Haven, CT 06250-8240 (203) 432-1775
Yale's Libraries
Bass Library
Beinecke Rare Book and Manuscript Library
Classics Library
Cushing/Whitney Medical Library
Divinity Library
East Asia Library
Gilmore Music Library
Haas Family Arts Library
Lewis Walpole Library
Lillian Goldman Law Library
Marx Science and Social Science Library
Sterling Memorial Library
Yale Center for British Art
SUBSCRIBE TO OUR NEWSLETTER
@YALELIBRARY

Yale Library Instagram
Accessibility Diversity, Equity, and Inclusion Giving Privacy and Data Use Contact Our Web Team
© 2022 Yale University Library • All Rights Reserved
Penn State University Libraries
Data management plans, what this guide covers, data management terms and definitions, components of a dmp, do i need a dmp, quick links.
- Getting Started on Your DMP
- -- 2023 NIH Data Management & Sharing Policy
- Questions to Consider for Your DMP
- Additional Resources
Need Data Management Plan Support?

Image by Gerd Altmann from Pixabay
The Data Learning Center Data Management Support Team is ready to consult with you on your questions about data management plans, including compliance with funding agency mandates. Contact us!
Email: [email protected]
Consultations : schedule here
This guide provides guidance and information for researchers on Data Management Plans (DMPs) including:
- What a DMP is
- Why a DMP is useful and whether one may be required
- How to create an implementable DMP
If you are looking to create a DMP as a requirement for a sponsored project, see this page .
If you are already familiar with DMPs and the requirements for them, see Quick Links at the bottom of this page for frequently used resources and tools.
Throughout this guidance, several terms are used which are defined here:
Data management describes the processes of collecting, organizing, describing, sharing, and preserving data. Data management is vital to any research project to prevent data issues - such as unorganized data or loss of data - from derailing your research project as well as to support making your data findable, accessible, interoperable, and reusable (FAIR).
Research (or scientific) data are the recorded factual material commonly accepted in the scientific community as of sufficient quality to validate and replicate research findings, regardless of whether the data are used to support scholarly publications [adapted from NIH policy (effective January 2023) ]. Research data could be observational, experimental, simulated, or derived. Some examples include tables of numbers, transcripts of interviews, survey results, images, video or audio recordings, genomic data, or code, among others.
A Data Management Plan (DMP) is an outline of what you will do with your data during and after a research project as well as how a researcher will collect, organize, document, describe, share, and preserve your data to make it FAIR. DMPs are often required by funders, but are beneficial to researchers regardless of whether they are required or not. Most funder-required DMPs are submitted as part of the funding proposal as a one to two page narrative document. However, if a DMP is not required, it can be in any format that is helpful to your research team (i.e. an excel sheet, bullet points, etc). A DMP should be a living document which is created before research begins and updated as research progresses.
Data Sharing refers to the practice of making data available to other research stakeholders, including other investigators, research subjects, and the broader public. Various funding agencies, publishers, and other research institutions mandate open data sharing to promote transparency, research reproducibility, and to increase the impact of research data [ NNLM ]. It is important to check the requirements of each of these entities before starting your research. Your plan on how and where you will share your data should be included in the DMP.
FAIR Data Principles are guidelines for making data findable, accessible, interoperable, and reusable (FAIR). Learn more by visiting the Go Fair website .
A DMP is a written document or standard operating procedure outlining what you will do with acquired or generated research data over the course of the project and afterwards, including how you plan to collect, organize, document, describe, share, and preserve your data to make it FAIR . A DMP is a living document that should be created as early as possible, optimally during the planning phase of a project, and updated throughout the project. Things change often, and that's okay - just be sure to update your plan accordingly so it is relevant throughout the life of the project. DMPs are often required for grant-funded research proposals to help ensure that data are properly managed, documented, stored, analyzed, preserved and subsequently shared with other researchers while accounting for legal, privacy, intellectual property and other considerations.
Planning ahead can help you to identify any hurdles to making your data FAIR and to ensure you have the proper resources to fulfill that goal. There are five major questions that a DMP should answer: (adapted from University of Arizona )
- What type of data will be produced?
- How will it be organized and what standards will be used for documentation and metadata describing the data?
- What steps will be taken to protect privacy, security, confidentiality, intellectual property or other rights?
- If others are allowed to reuse the data, how, where and when will the data be accessed and shared ?
- Where will the data be archived and preserved and for how long?
Formalizing the wholistic vision of what data will be produce and how it can be shared with the wider scientific community can help you find gaps and make it easier to communicate the plan with all members of the research team, even if you have considered these aspects of your research already.
Generally, DMPs will address the same topics, but the implementation aspects of the DMP will look different for different researchers, areas of study, and scope of project. The Data Curation Centre (DCC) has developed a checklist for Data Management Plans which outlines further the topics should be addressed in a DMP alongside questions to consider and guidance. These sections include:
- Administrative data : project name, funder, DOI, PI, relevant dates
- Data collection : what data and how is it collected
- Documentation and metadata : metadata schema, form of documentation
- Ethics and legal complianc e: HIPAA, copyright, intellectual property
- Storage and back-up : where will data be stored, who has access, what security precautions will be taken
- Selection and preservation : what data is preserved and how/where will it be preserved
- Data sharing : what data will be shared and how will it be shared, restrictions to sharing
- Responsibilities and resources : who is responsible for each step above, will execution of DMP require additional resources/budget
Whether a DMP is required will depend on institutional and funder policies, but it's always best practice to create a data management plan for each research project. A funder-required DMP must be submitted in the grant application package, and there are specific guidelines on how it should be formatted and what it must include. If a DMP is not funder-required, it can be more informal: a simple text document or spreadsheet containing the relevant details - any format that will assist you and the rest of the research team.
Creating a DMP is considered best practice for everyone
Proper data management provides a lot of benefits to a research team:
- Saves Time: Properly managing data is in your best interest; being able locate past or current data saves time, frustration, and money for the whole research team.
- Increases Citations: When possible to openly, share data, well-managed data can itself be cited and may also lead to more citations for the original paper [ 1 ].
- Enhances Reproducibility: Data management enhances reproducibility by making the methodology more transparent.
- Preserves Data: While data management encourages researchers to consider backup and security measures, it also ensures that data is preserved, not just stored. Preservation focuses on the long-term ability to access and use data, and considers interoperability and open file formats.
A DMP, whatever form it takes, can reduce redundant work, help new members of the research team as they join the project, and keep everyone on the same page about how data will be collected, stored, described, and shared.
And it may be required by your funder.
In February 2013 the Office of Science and Technology Policy (OSTP) issued a call to federal agencies with budgets in excess of $100 million to provide plans for public access to research results from projects funded by them. By fall 2015, most, if not all, federal agencies falling under this requirement have issued their public access plans. Check your funder's website or use the SPARC Research Funder Data Sharing Policies Tool to navigate existing public access policies and find out whether a DMP is required for your project.
If you are still having trouble, contact the Data Learning Center Data Management support team at [email protected].
[1] Heather A. Piwowar and Todd J. Vision, “Data Reuse and the Open Data Citation Advantage,” PeerJ 1 (October 2013): e175, https://dx.doi.org/10.7717/peerj.175 .
If you're already familiar with DMPs and the resources available, here are some quick links that might be useful:
- DMPTool This tool has templates adhering to U.S. funding agency DMP requirements. Steps you through each section of a DMP and generates a plan at the end. Get your plan reviewed by a member of the Research Data Management team.
- SPARC Data Sharing Requirements This is a community resource for tracking, comparing, and understanding current U.S. federal funder research data sharing policies. Originally completed by SPARC & Johns Hopkins University Libraries in 2016, the content of this resource was updated by RDAP and SPARC in 2021.
- Penn State University Libraries Research Data Services University Libraries offer a suite of research data services that are available to all Penn State students, staff, and faculty, including help with consultations, trainings, and links to various tools and services.
- Next: Getting Started on Your DMP >>
- Last Updated: Aug 10, 2023 3:46 PM
- URL: https://guides.libraries.psu.edu/DMP
Research Data Management
- Data Management Plans
What are Data Management Plans?
A Data Management Plan outlines how data will be collected, organized, stored, secured, shared, and preserved in a research project. It covers data collection methods, organization, storage, sharing, preservation, ethics, and researcher responsibilities. Data Management Plans promote transparency and maximize research impact by ensuring your data can be used effectively, by you, your collaborators, and future generations of researchers. They can be a powerful tool for thinking in advance about collaborative research workflows and can help forecast financial costs associated with data so they can be written into budgets and funded.
Data Management Plans are increasingly required by federal grant funding agencies, such as the National Institutes of Health (NIH) , National Science Foundation (NSF) , and National Endowment for the Humanities (NEH) . Different funders have different policies, so it is important to look at the requirements of the granting agency to learn about their Data Policies and Compliance .
What is included in a Data Management Plan?
Data management plans are brief (2-3 page) documents that outline in advance how you will manage your data throughout the life of your project. They often include:
- How the data will be collected
- The type or format of data collected
- The size of the data
- How the data will be described (i.e., will you be using codebooks, logs, specific metadata standards, ontologies, etc.)
- Where the data will be stored, backed up and secured if necessary
- How the data will be analyzed
- How the data will be shared and preserved, or reasons not to do so, including who will have permissions to use the data
What tools can help me write a Data Management Plan?
The DMPTool from the California Digital Library is an online tool for creating data management plans. It has templates and resources to guide you through the process of creating a data management plan that is in compliance with funder requirements.
- << Previous: Plan & Design
- Next: Data Policies & Compliance >>
- Data Policies & Compliance
- Directory Structures
- File Naming Conventions
- Roles & Responsibilities
- Collaborative Tools & Software
- Electronic Lab Notebooks
- Documentation & Metadata
- Reproducibility
- Analysis Ready Datasets
- Image Management
- Version Control
- Data Storage
- Data & Safety Monitoring
- Data Privacy & Confidentiality
- Retention & Preservation
- Data Destruction
- Data Sharing
- Public Access
- Data Transfer Agreements
- Intellectual Property & Copyright
- Unique Identifiers
- Data Repositories
Research Support
Request a data management services consultation.
Email [email protected] to schedule a consultation related to the organization, storage, preservation, and sharing of data.
- Last Updated: Apr 5, 2024 12:02 AM
- URL: https://guides.library.ucdavis.edu/data-management
Research Data Management
- Research Data Management at UVA
- About Research Data Management
- Data Privacy and Human Subjects
- File Management Best Practices
- Metadata and Documentation
- Data Storage, Backup and Security
- Data Citations
- Licensing Data & Code
- Data Rights and Policies
- Open Science and Open Data
Your Research Data Management Team
Research data management librarian.

Senior Research Data Management Librarian

Introduction
A Data Management & Sharing Plan (DMSP), also referred to as a Data Management Plan (DMP), is a formal document that outlines what you will do with your data during the active phase of the research project and after the project ends. This document may also be called a Data Management Plan (DMP) depending on the funding agency. The National Institutes of Health (NIH) refer to it as a DMSP, whereas the National Science Foundation refers to it as a DMP. We use the term DMSP because it suggests both data management and sharing.
DMSPs are typically two-page documents. Most US federal funders and many private foundations require DMSPs to be submitted with their funding applications. Whether you are participating in a funded research project or not, writing a DMSP will help you think about the practices, people, and resources needed to manage your data.
Although funder DMSP requirements may vary, in most cases you will be asked to describe your data, other research products, and relevant software, address metadata, standards, documentation, storage, preservation, and any ethical, legal or other restrictions, and define roles and responsibilities. If applicable, you will need to discuss compliance with federal regulations protecting human subjects and privacy.

The DMP Tool can help with writing your data management plan. It provides customized DMP forms with guidance and examples based on your research funder and research institution selections. You may use this tool to request feedback from collaborators and the UVA Library Data Management Team. If you want to request a consultation or ask a question, email us at [email protected] .
Below are resources to help you write your plan.
- DMP Tool Create Data Management Plans that meet requirements and promote your research.
- Using the DMPTool DMPTool Tutorial
- UVA HSL NIH DMSP Guidance If you are applying to the National Institutes of Health (NIH) for funding, the UVA Health Sciences Library (HSL) provides guidance on six elements required for a NIH Data Management and Sharing Plan (DMSP).
- Checklist for a Data Management Plan. v.4.0 This Digital Curation Centre checklist provides guidance and questions to consider for Storage and Backup and Ethics and Legal Compliance in addition to other aspects of research data management. more... less... DCC. (2013). Checklist for a Data Management Plan. v.4.0. Edinburgh: Digital Curation Centre
- Research Data Management and Sharing This course provides learners with an introduction to research data management and sharing. Topics include practices for the planning, organization, documentation, storage and security, preserving and sharing of data. This course is located on Coursera.org and is offered by The University of North Carolina at Chapel Hill and The University of Edinburgh. Course materials are available upon enrollment.
Data, Standards, and Documentation
Data and other products of research
Describe the data and other products of research to be produced from the research project. Include details about the source (e.g., sensor readings, survey results), forms (e.g., numeric, text, images, audio, video), file types (e.g., csv, txt, png, flac, mp4), and data volume. Also, indicate whether the data will change or grow in size after the research project is finished and data is submitted to a repository and if any specific software is required to analyze the data.
If you are using existing data for secondary analysis, describe the content, source and requirements for obtaining and using that data. If the existing data will be combined with data to be generated from your research project, explain the relationship between the data sets.
- DMP Tool Guidance: Types of Data The DMPTool Help Types of Data section addresses data sources, forms, stability, volume, and file formats.
Funders and data repositories may require specific metadata standards or shared vocabularies to make data easier to find. Relevant data standards may also refer to areas beyond metadata or shared vocabularies, such as file formats for data exchange, guidance for data collection, or requirements for data protection. To find standards appropriate for your discipline, see Choosing and Using Metadata Standards .
Documentation
Describe what documentation will be included with your data to make it understandable. Documentation may be given at three levels. Project level documentation includes the purpose of the study, research questions, hypothesis, methodology, instruments, and measurements used. File and database level documentation describes the datasets and supporting documentation. Variable level documentation defines the variables and values, particularly coded values.
For more details on metadata and documentation, see Metadata and Documentation .
Preservation

Adapted Timbuktu Manuscript image by Mark Fischer with a Creative Commons Attribution-ShareAlike 2.0 license.
You will need to find a place to store your data when it is still being collected, processed, and analyzed at the research institution. Be prepared to discuss how much storage will be needed, how often data will be backed up, how data will be recovered, access control for research team members, secure data transmission from the field, and handling of information with varying degrees of sensitivity. For more details about storage, see Data Storage, Backup, and Security .
Most funders require submission of data to a repository when your research project is finished if there are no restrictions that prohibit submission. For data that will be submitted, indicate which repository will receive your research data. If the funder has its own repository or a preferred repository, use that repository. If not, your discipline may have a commonly used repository. If so, use that repository. If not, use LibraData , the UVA institutional data repository.
When choosing a repository, consider the National Science and Technology Council guidance on Desirable Characteristics of Data Repositories for Federally Funded Research. Also discuss plans for long-term retention of data at your research institution. For more details, see Data Sharing and Preservation .
Find out if your funder allows costs associated data submission in your grant budget. Some data repositories charge fees to cover curation and preservation costs. Even if you deposit your data to a repository that does not charge a fee, consider resources you may need for preparing the data for submission, submitting the data, and responding to questions and requests from the repository.
- Desirable Characteristics of Repositories for Federally Funded Research This guidance is provided by the National Science and Technology Council. It includes three main sections about organizational infrastructure, digital object management, and technology. There is a Table listing Additional Considerations for Repositories Storing Human Data.
- UVA Dataverse (LibraData) The LibraData homepage.
- Federally Funded Data Repository Characteristics Discusses alignment of LibraData with Federally Funded Data Repository Characteristics
Access and Reuse Restrictions
Data access and reuse may be restricted because of privacy protection requirements, data rights, or other reasons. Restrictions on data access or reuse should be addressed in your DMSP. Describe in your DMSP what you are going to share as well as what you are not sharing, and/or what you are sharing but only through proper de-identification and/or data use agreements.
If you have created new data from human subjects, you may need to redact your data to avoid re-identifying individuals. LibraData, the UVA data repository, requires that depositors remove any sensitive or confidential information from their data submissions. You may also determine that access to some or all your newly created data should be restricted because of data sensitivity or intellectual property claims. When submitting your data to a repository, you might have the option to recommend public access, which allows data sets to be downloaded by anyone, or restricted access, which requires researchers to request permission to access the data. You or the repository might also require other researchers might to sign a data use agreement.
If you are using existing data from a repository, a data use agreement may require that the data be shared only with certain members of your research team because of privacy requirements and then destroyed after a specified time period. If you are using existing data from a vendor through a UVA library subscription, the license probably will not allow reuse of the data for those not affiliated UVA.
Data redaction, data use agreements and vendor licenses are common examples of data access and reuse limitations. However, in some cases there may be other factors involved that restrict data access, such as informed consent language or federal, Tribal, or state laws, regulations, or policies. If you want a consultation or have questions, please contact email us at [email protected] .
For information about US human subjects and privacy laws and UVA Institutional Review Boards (IRBs) for compliance with federally mandated research guidelines, see Data Privacy and Human Subjects .
For information about intellectual property related to data and UVA ownership policies, see Data Rights and Policies .
- UVA IRB-SBS/Researcher Guide/Data The IRB-SBS Researcher Guide Data section covers topics, such as Protecting Privacy, Secondary Use of Existing Data, and Record Keeping – Retention of Research Records and Destruction of Data.
- What can be deposited in LibraData? See this FAQ for instructions to remove any confidential or sensitive information from data before submission.
Roles and Responsibilities
Indicate who is responsible responsible for overseeing data management and sharing activities and updating the DMSP. Include details about these activities (e.g., data collection, documentation, quality control, analysis, archiving and sharing) and identify who will be performing them. It may be useful to refer to this section of the DMSP when determining data and documentation access for team members.
- << Previous: About Research Data Management
- Next: Data Privacy and Human Subjects >>
- Last Updated: Mar 26, 2024 3:52 PM
- URL: https://guides.lib.virginia.edu/RDM
- 434-924-3021
- [email protected]
- Ask a Librarian
- UVA Shannon Library P.O. Box 400113 160 McCormick Road Charlottesville, VA 22904
About the Library
- Staff Directory
- Fellowships
Using the Library
- Library Use Policies
- Off-Grounds Access
- ITS Computing Accounts
- Accessibility Services
- Emergency Information
- UVA Privacy Policy
- Tracking Opt-out
Other Sites
- Cavalier Advantage
- Library Staff Site
Data Management Plan in Research: Characteristics and Development
- Conference paper
- First Online: 16 June 2020
- Cite this conference paper

- Paulo A. Cauchick-Miguel 16 ,
- Suzana R. Moro 16 ,
- Roberto Rivera 17 &
- Marlene Amorim 17
Part of the book series: Lecture Notes of the Institute for Computer Sciences, Social Informatics and Telecommunications Engineering ((LNICST,volume 319))
Included in the following conference series:
- International Conference on Data and Information in Online
579 Accesses
1 Citations
Data science is an interdisciplinary field that extracts value from data. One of the relevant areas is its application in research in order to define requirements of the data life cycle. Thus, data should be managed before, during, and after a research project completion. A robust data management plan (DMP) is a relevant and useful instrument to establish data-related requirements. In this context, this paper aims at highlighting some characteristics associated to research data management. To conduct this study peer-reviewed literature and secondary data are methodologically employed to fulfil the paper objective. The results discuss the development of DMP, provide some examples of documents and a check list related to data management, and present some recommendations for developing a suitable data management plan from the literature. The data management plan is one of the important instruments that should be considered with care when designing and applying it. Future work may consider providing a structure and guidance for research students in the field of industrial engineering as a valuable avenue to explore.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Michener, W.F.: Ten simple rules for creating a good data management plan. PLoS Comput. Biol. 11 (10), e1004525 (2015). https://doi.org/10.1371/journal.pcbi.1004525
Article Google Scholar
Davis, H.M., Cross, W.M.: Using a data management plan review service as a training ground for librarians. J. Librariansh. Sch. Commun. 3 (2), eP1243 (2015). https://doi.org/10.7710/2162-3309.1243
Antell, K., Foote, J.B., Turner, J., Shults, B.: Dealing with data: science librarians’ participation in data management at an association of research libraries institutions. Coll. Res. Libr. 75 (4), 557–574 (2014). https://doi.org/10.5860/crl.75.4.557
Surkis, A., Read, K.: Research data management. J. Med. Libr. Assoc. JMLA 103 (3), 154–156 (2015). https://doi.org/10.3163/1536-5050.103.3.011
Cox, A.M., Tam, W.W.T.: A critical analysis of lifecycle models of the research process and research data management. Aslib J. Inf. Manage. 70 (2), 142–157 (2018). https://doi.org/10.1108/AJIM-11-2017-0251
Vieira, R., Ferreira, F., Barateiro, J., Borbinha, J.: Data management with risk management in engineering and science projects. New Rev. Inf. Netw. 19 (2), 49–66 (2014). https://doi.org/10.1080/13614576.2014.918519
Anonymous: Nature editorial - making plans. Nature 555 (7696), 286 (2018)
Mannheimer, S.: Toward a better data management plan: the impact of DMPs on grant funded research practices. J. eSci. Librariansh. 7 (3), 5 (2018). https://doi.org/10.7191/jeslib.2018.1155
Bellgard, M.I.: ERDMAS: an exemplar-driven institutional research data management and analysis strategy. Int. J. Inf. Manage. 50 , 337–340 (2020). https://doi.org/10.1016/j.ijinfomgt.2019.08.009
Wright, A.: Electronic resources for developing data management skills and data management plans. J. Electron. Resour. Med. Libr. 13 (1), 43–48 (2016). https://doi.org/10.1080/15424065.2016.1146640
Holles, J.H., Schmidt, M.L.: Graduate research data management course content: teaching the Data Management Plan (DMP). In: 2018 ASEE Annual Conference and Exposition (2018)
Google Scholar
Reilly, M., Dryden, A.R.: Building an online data management plan tool. J. Librariansh. Sch. Commun. 1 (3), eP1066 (2013). https://doi.org/10.7710/2162-3309.1066
Van Loon, J.E., Akers, K.G., Hudson, C., Sarkozy, A.: Quality evaluation of data management plans at a research university. IFLA J. 43 (1), 98–104 (2017). https://doi.org/10.1177/0340035216682041
European Commission – European Union. https://ec.europa.eu/research/participants/docs/h2020-funding-guide/cross-cutting-issues/open-access-data-management/data-management_en.htm . Accessed 11 Jan 2020
Willaert, T., Cottyn, J., Kenens, U., Vandendriessche, T., Verbeke, D., Wyns, R.: Research data management and the evolutions of scholarship: policy, infrastructure and data literacy at KU Leuven. LIBER Q. 29 , 1–19 (2019). https://doi.org/10.18352/lq.20272
Elsevier. https://researcheracademy.elsevier.com/research-preparation/research-data-management . Accessed 11 Jan 2020
RDMLA – RDMLA. https://rdmla.github.io/about/ . Accessed 11 Jan 2020
Wilkinson, M.D., et al.: The FAIR guiding principles for scientific data management and stewardship. Sci. Data 3 , 160018 (2016). https://doi.org/10.1038/sdata.2016.18
Booth, A., Sutton, A., Papaioannou, D.: Systematic approaches to a successful literature review. Sage Publications Ltd., Thousand Oaks (2012)
European Commission. https://ec.europa.eu/research/participants/docs/h2020-funding-guide/cross-cutting-issues/open-access-data-management/data-management_en.htm . Accessed 9 Jan 2020
Digital Curation Centre –DCC. http://www.dcc.ac.uk/ . Accessed 8 Jan 2020
Digital Curation Centre –DCC. http://www.dcc.ac.uk/sites/default/files/documents/resource/DMP/DMP-checklist-flyer.pdf . Accessed 6 Jan 2020
University of Aveiro. https://www.ua.pt/pt/investigacao . Accessed 9 Jan 2020
European Commission. https://ec.europa.eu/programmes/erasmus-plus/about_en . Accessed 9 Jan 2020
Digital Curation Centre – DCC. http://www.dcc.ac.uk/sites/default/files/documents/resource/DMP/DMP_Checklist_2013.pdf . Accessed 6 Jan 2020
Download references
Author information
Authors and affiliations.
Universidade Federal de Santa Catarina, Florianópolis, SC, 88040-900, Brazil
Paulo A. Cauchick-Miguel & Suzana R. Moro
Universidade de Aveiro, 3810-193, Aveiro, Portugal
Roberto Rivera & Marlene Amorim
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Paulo A. Cauchick-Miguel .
Editor information
Editors and affiliations.
Universidade de São Paulo, São Paulo, Brazil
Rogério Mugnaini
Rights and permissions
Reprints and permissions
Copyright information
© 2020 ICST Institute for Computer Sciences, Social Informatics and Telecommunications Engineering
About this paper
Cite this paper.
Cauchick-Miguel, P.A., Moro, S.R., Rivera, R., Amorim, M. (2020). Data Management Plan in Research: Characteristics and Development. In: Mugnaini, R. (eds) Data and Information in Online Environments. DIONE 2020. Lecture Notes of the Institute for Computer Sciences, Social Informatics and Telecommunications Engineering, vol 319. Springer, Cham. https://doi.org/10.1007/978-3-030-50072-6_1
Download citation
DOI : https://doi.org/10.1007/978-3-030-50072-6_1
Published : 16 June 2020
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-50071-9
Online ISBN : 978-3-030-50072-6
eBook Packages : Computer Science Computer Science (R0)
Share this paper
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Data Management and Sharing
In this guide.
- Introduction
- Make a Plan
- Get Organized
- Save Your Data
- Document Your Process
- Share your Data
- Additional Resources
Planning for Data
Creating a plan that describes how data will be managed and shared throughout the course of a research project is an important step in ensuring that you, your collaborators, and potentially other researchers can find and use your data.
Many research funding agencies have begun to require data management plans (DMPs) , formal documents that specify how researchers plan to manage and share the data associated with a project, be submitted as part of grant proposals.
However, a DMP created as part of a grant application is really just the beginning. Your plan, as it is actually applied in the course of your research, can be more like a set of standard operating procedures that are put into practice by you and your collaborators. This page provides information about creating both DMPs for grant proposals and data-related plans to be shared with your research team.
Remember that, while it is important to create a plan, it is equally important that your plan is up-to-date and communicated to everyone involved in managing and sharing your data. The video below illustrates what can happen when a research team doesn't have a data management plan. Many data management issues can be handled easily or avoided entirely by planning ahead.
The Data Management Plan (DMP)
Funding agencies, including the National Science Foundation (NSF) and the Patient-Centered Outcomes Research Institute (PCORI) have laid out specific criteria for what should be included in a data management plan.
The table below outlines similar requirements for the National Institutes of Health , that are set to go into effect in 2023. If you would like assistance completing a data management plan for a grant proposal, please contact your liaison librarian to schedule a 1-1 consultation.
Because different funding agencies have different requirements for their data management plans, it can be helpful to use a tool like DMPTool . Created by a group of institutions led by the California Digital Library, DMPTool is designed to help researchers create high-quality DMPs that meet the requirements of their specific funding agency. Because we are an affiliated institution, Stanford researchers can sign into DMPtool using their SUNet ID.
The DMPTool can:
- Help you meet the funding requirements of your next grant.
- Collaborative plan creation and co-ownership with colleagues.
- Put you in touch with expert help at Lane Library and Stanford University Libraries
DMPTool2 Promotional Video from California Digital Library on Vimeo .
Data Management Planning
Even if you are not creating a DMP as part of a grant proposal, it is still helpful to maintain some kind of document that outlines how data should be managed over the course of a research project.
The table below outlines the elements to consider including in your data related plan (whether you call it a DMP or something else) as well as some questions to ask yourself when considering what information to include.
This document may be part of a broader set of standard operating procedures or it may stand alone. While it is important to create such a document, it is equally important to make sure its contents are updated and communicated to the relevant members of the research team.
- << Previous: Introduction
- Next: Get Organized >>
- Last Updated: Feb 14, 2024 1:21 PM
- URL: https://laneguides.stanford.edu/DataManagement
- Directories
- Research Data Management Workshop from UW Libraries
Data Management Plans
- Organization & Format
- Data Storage Comparison
- Selecting a Data Repository
- Resources for Publishing & Sharing Research Data
- Dryad Data Repository for UW Researchers
- NIH Data Management and Sharing Plan
- Frequently Asked Questions
- Washington State Data
- Undergraduate Guide to Using Data
- Digital Tools
- Reproducibility
- Scholarly Publishing and Open Access
- Start Your Research
- Research Guides
- University of Washington Libraries
- Library Guides
- UW Libraries
- Research Data Management
Research Data Management: Data Management Plans
What is a data management plan (dmp).
A data management plan is a document outlining how a researcher plans to manage data during and after a research project including how it will be organized, maintained, and shared.
Why do you need one?
Many funding agencies, including the National Science Foundation (NSF) and National Institutes for Health (NIH), are now requiring researchers to submit a formal DMP when applying for grants. Visit SPARC to view an up-to-date listing of data sharing policies by funding organization.
How do you create a DMP?
We recommend using the California Digital Library's DMPTool when creating a data management plan. This tool will walk you step-by-step through the requirements for several different funders and provide you with an exportable data management plan. UW is a participating institution; login using your NetID by clicking "Sign in" in the top right of the page and using Option 1 to select the University of Washington (UW). For more information see this guide on how to use the DMPTool .
Tools & Resources
- DMPTool An online tool to assist in writing data management plans for NSF, NIH, NEH, IMLS, or GBMF. Login using your NetID.
- SPARC SPARC provides an up-to-date listing of data sharing policies by funding organization.
Campus Services
If you have questions about data management planning or would like to request a data management plan consultation with a member of the Scholarly Communications and Publishing Team, please e-mail us at [email protected] .
- << Previous: Research Data Management Workshop from UW Libraries
- Next: Organization & Format >>
- Last Updated: May 2, 2024 3:09 PM
- URL: https://guides.lib.uw.edu/research/dmg
Research Data Management
- RDM Best Practices, 1 - 2 - 3
- 1.1 Data Management Plans (DMP)
- 1.2 Data Organization
- 1.3. Copyright & Intellectual Property
- 2.1 Data Documentation & Metadata
- 2.2 Ethical Issues - Sensitive Data
- 2.3 Data Storage & Backup
- 2.4 Data Security
- 3.1 Data Preservation
- 3.2 Data Sharing & Citation
- Education & Training
1.1 Data Management Plans - Page Contents

Back to Pre-Research Stage
Data Management Plans
Facts About Data Management Plans:
A Data Management Plan (DMP) is a written living document that formally outlines what you will do with your research data during the course of your research project and afterwards. It is a living document because any time your research plans change, you should review your DMP in order to make sure that the plan still satisfies your essential data needs. It’s important to manage your data for many reasons. Firstly, it enhances the integrity of your research by virtue of increasing access and therefore, the reproducibility of your research data. Secondly, it safeguards and allows you to share your data for recognition and possibly to facilitate new scientific discoveries. Lastly, the number of funding agencies that require you to share and preserve your data is growing. Although a DMP can be designed throughout the research cycle (i.e. it’s never too late), it is best to plan for one early on in the research cycle in order to avoid many data management issues/headaches, which can be easily avoided by planning ahead.
Back to Page Contents
Funding Agency Requirements
Funding Agency Requirements:
Several funding agencies both federal and private require a DMP with every funding application.
- SPARC SPARC, which stands for Scholarly Publishing and Academic Resources Coalition, has assembled a great resource about data management and data sharing requirements from all of the federal funding agencies.
Examples of Federal Funding Agencies that Require Data Sharing or a DMP:
- 2011 - National Science Foundation An extension of the NSF Data Sharing Policy requires all applicants to submit a DMP with their funding request. Non-compliance could lead to award rejection.
- 2013 - National Institutes of Health's Public Access Policy Requires applicants to share their research findings and noncompliance can lead to award delays.

Back to Page Contents
DMP Examples
Examples of Data Management Plans:
- NIH Data Sharing Plans
- DataONE DMP Examples
- DMPTool This tool provides ample guidance on how to design a DMP for your specific type of research project and for your specific type of funding agency including ones from:
- The Gordon and Betty Moore Foundation
- National Institutes of Health (NIH)
- National Science Foundation (NSF)
The DMPTool will help cater your DMP to the needs/requirements of a specific funding agency. In general, it is important to consider these things when writing a DMP:
- Roles and Responsibilities
- Types of Data
- File Formats
- Organizing Files
- Metadata: Data Documentation
- Persistent Identifiers
- Security and Storage
- Sharing and Access
- Data Preservation and Archiving
- Citing Data and Data Redistribution
- Copyright & Privacy
Institutional DMPTool Partners:
- WSU WSU is a DMPTool institutional partner and WSU students and faculty need only to sign in with their WSU login and password.
- MIT MIT has great DMP questions that help guide the design of your respective DMP.
- UCLA UCLA has a great DMP template to assist you with your DMP planning and design.
- Harvard Harvard has a great best practice DMP template for you to use.

- << Previous: 1.0 Pre-Research Stage
- Next: 1.2 Data Organization >>
- Last Updated: Jan 4, 2024 11:01 AM
- URL: https://libguides.libraries.wsu.edu/rdmlibguide
Examples of data management plans
These examples of data management plans (DMPs) were provided by University of Minnesota researchers. They feature different elements. One is concise and the other is detailed. One utilizes secondary data, while the other collects primary data. Both have explicit plans for how the data is handled through the life cycle of the project.
School of Public Health featuring data use agreements and secondary data analysis
All data to be used in the proposed study will be obtained from XXXXXX; only completely de-identified data will be obtained. No new data collection is planned. The pre-analysis data obtained from the XXX should be requested from the XXX directly. Below is the contact information provided with the funding opportunity announcement (PAR_XXX).
Types of data : Appendix # contains the specific variable list that will be used in the proposed study. The data specification including the size, file format, number of files, data dictionary and codebook will be documented upon receipt of the data from the XXX. Any newly created variables from the process of data management and analyses will be updated to the data specification.
Data use for others : The post-analysis data may be useful for researchers who plan to conduct a study in WTC related injuries and personal economic status and quality of life change. The Injury Exposure Index that will be created from this project will also be useful for causal analysis between WTC exposure and injuries among WTC general responders.
Data limitations for secondary use : While the data involve human subjects, only completely de-identified data will be available and used in the proposed study. Secondary data use is not expected to be limited, given the permission obtained to use the data from the XXX, through the data use agreement (Appendix #).
Data preparation for transformations, preservation and sharing : The pre-analysis data will be delivered in Stata format. The post-analysis data will also be stored in Stata format. If requested, other data formats, including comma-separated-values (CSV), Excel, SAS, R, and SPSS can be transformed.
Metadata documentation : The Data Use Log will document all data-related activities. The proposed study investigators will have access to a highly secured network drive controlled by the University of Minnesota that requires logging of any data use. For specific data management activities, Stata “log” function will record all activities and store in relevant designated folders. Standard file naming convention will be used with a format: “WTCINJ_[six letter of data indication]_mmddyy_[initial of personnel]”.
Data sharing agreement : Data sharing will require two steps of permission. 1) data use agreement from the XXXXXX for pre-analysis data use, and 2) data use agreement from the Principal Investigator, Dr. XXX XXX ([email protected] and 612-xxx-xxxx) for post-analysis data use.
Data repository/sharing/archiving : A long-term data sharing and preservation plan will be used to store and make publicly accessible the data beyond the life of the project. The data will be deposited into the Data Repository for the University of Minnesota (DRUM), http://hdl.handle.net/11299/166578. This University Libraries’ hosted institutional data repository is an open access platform for dissemination and archiving of university research data. Date files in DRUM are written to an Isilon storage system with two copies, one local to each of the two geographically separated University of Minnesota Data Centers. The local Isilon cluster stores the data in such a way that the data can survive the loss of any two disks or any one node of the cluster. Within two hours of the initial write, data replication to the 2nd Isilon cluster commences. The 2nd cluster employs the same protections as the local cluster, and both verify with a checksum procedure that data has not altered on write. In addition, DRUM provides long-term preservation of digital data files for at least 10 years using services such as migration (limited format types), secure backup, bit-level checksums, and maintains a persistent DOIs for data sets, facilitating data citations. In accordance to DRUM policies, the de-identified data will be accompanied by the appropriate documentation, metadata, and code to facilitate reuse and provide the potential for interoperability with similar data sets.
Expected timeline : Preparation for data sharing will begin with completion of planned publications and anticipated data release date will be six months prior.
Back to top
College of Education and Human Development featuring quantitative and qualitative data
Types of data to be collected and shared The following quantitative and qualitative data (for which we have participant consent to share in de-identified form) will be collected as part of the project and will be available for sharing in raw or aggregate form. Specifically, any individual level data will be de-identified before sharing. Demographic data may only be shared at an aggregated level as needed to maintain confidentiality.
Student-level data including
- Pre- and posttest data from proximal and distal writing measures
- Demographic data (age, sex, race/ethnicity, free or reduced price lunch status, home language, special education and English language learning services status)
- Pre/post knowledge and skills data (collected via secure survey tools such as Qualtrics)
- Teacher efficacy data (collected via secure survey tools such as Qualtrics)
- Fidelity data (teachers’ accuracy of implementation of Data-Based Instruction; DBI)
- Teacher logs of time spent on DBI activities
- Demographic data (age, sex, race/ethnicity, degrees earned, teaching certification, years and nature of teaching experience)
- Qualitative field notes from classroom observations and transcribed teacher responses to semi-structured follow-up interview questions.
- Coded qualitative data
- Audio and video files from teacher observations and interviews (participants will sign a release form indicating that they understand that sharing of these files may reveal their identity)
Procedures for managing and for maintaining the confidentiality of the data to be shared
The following procedures will be used to maintain data confidentiality (for managing confidentiality of qualitative data, we will follow additional guidelines ).
- When participants give consent and are enrolled in the study, each will be assigned a unique (random) study identification number. This ID number will be associated with all participant data that are collected, entered, and analyzed for the study.
- All paper data will be stored in locked file cabinets in locked lab/storage space accessible only to research staff at the performance sites. Whenever possible, paper data will only be labeled with the participant’s study ID. Any direct identifiers will be redacted from paper data as soon as it is processed for data entry.
- All electronic data will be stripped of participant names and other identifiable information such as addresses, and emails.
- During the active project period (while data are being collected, coded, and analyzed), data from students and teachers will be entered remotely from the two performance sites into the University of Minnesota’s secure BOX storage (box.umn.edu), which is a highly secure online file-sharing system. Participants’ names and any other direct identifiers will not be entered into this system; rather, study ID numbers will be associated with the data entered into BOX.
- Data will be downloaded from BOX for analysis onto password protected computers and saved only on secure University servers. A log (saved in BOX) will be maintained to track when, at which site, and by whom data are entered as well as downloaded for analysis (including what data are downloaded and for what specific purpose).
Roles and responsibilities of project or institutional staff in the management and retention of research data
Key personnel on the project (PIs XXXXX and XXXXX; Co-Investigator XXXXX) will be the data stewards while the data are “active” (i.e., during data collection, coding, analysis, and publication phases of the project), and will be responsible for documenting and managing the data throughout this time. Additional project personnel (cost analyst, project coordinators, and graduate research assistants at each site) will receive human subjects and data management training at their institutions, and will also be responsible for adhering to the data management plan described above.
Project PIs will develop study-specific protocols and will train all project staff who handle data to follow these protocols. Protocols will include guidelines for managing confidentiality of data (described above), as well as protocols for naming, organizing, and sharing files and entering and downloading data. For example, we will establish file naming conventions and hierarchies for file and folder organization, as well as conventions for versioning files. We will also develop a directory that lists all types of data and where they are stored and entered. As described above, we will create a log to track data entry and downloads for analysis. We will designate one project staff member (e.g., UMN project coordinator) to ensure that these protocols are followed and documentation is maintained. This person will work closely with Co-Investigator XXXXX, who will oversee primary data analysis activities.
At the end of the grant and publication processes, the data will be archived and shared (see Access below) and the University of Minnesota Libraries will serve as the steward of the de-identified, archived dataset from that point forward.
Expected schedule for data access
The complete dataset is expected to be accessible after the study and all related publications are completed, and will remain accessible for at least 10 years after the data are made available publicly. The PIs and Co-Investigator acknowledge that each annual report must contain information about data accessibility, and that the timeframe of data accessibility will be reviewed as part of the annual progress reviews and revised as necessary for each publication.
Format of the final dataset
The format of the final dataset to be available for public access is as follows: De-identified raw paper data (e.g., student pre/posttest data) will be scanned into pdf files. Raw data collected electronically (e.g., via survey tools, field notes) will be available in MS Excel spreadsheets or pdf files. Raw data from audio/video files will be in .wav format. Audio/video materials and field notes from observations/interviews will also be transcribed and coded onto paper forms and scanned into pdf files. The final database will be in a .csv file that can be exported into MS Excel, SAS, SPSS, or ASCII files.
Dataset documentation to be provided
The final data file to be shared will include (a) raw item-level data (where applicable to recreate analyses) with appropriate variable and value labels, (b) all computed variables created during setup and scoring, and (c) all scale scores for the demographic, behavioral, and assessment data. These data will be the de-identified and individual- or aggregate-level data used for the final and published analyses.
Dataset documentation will consist of electronic codebooks documenting the following information: (a) a description of the research questions, methodology, and sample, (b) a description of each specific data source (e.g., measures, observation protocols), and (c) a description of the raw data and derived variables, including variable lists and definitions.
To aid in final dataset documentation, throughout the project, we will maintain a log of when, where, and how data were collected, decisions related to methods, coding, and analysis, statistical analyses, software and instruments used, where data and corresponding documentation are stored, and future research ideas and plans.
Method of data access
Final peer-reviewed publications resulting from the study/grant will be accompanied by the dataset used at the time of publication, during and after the grant period. A long-term data sharing and preservation plan will be used to store and make publicly accessible the data beyond the life of the project. The data will be deposited into the Data Repository for the University of Minnesota (DRUM), http://hdl.handle.net/11299/166578 . This University Libraries’ hosted institutional data repository is an open access platform for dissemination and archiving of university research data. Date files in DRUM are written to an Isilon storage system with two copies, one local to each of the two geographically separated University of Minnesota Data Centers. The local Isilon cluster stores the data in such a way that the data can survive the loss of any two disks or any one node of the cluster. Within two hours of the initial write, data replication to the 2nd Isilon cluster commences. The 2nd cluster employs the same protections as the local cluster, and both verify with a checksum procedure that data has not altered on write. In addition, DRUM provides long-term preservation of digital data files for at least 10 years using services such as migration (limited format types), secure backup, bit-level checksums, and maintains persistent DOIs for datasets, facilitating data citations. In accordance to DRUM policies, the de-identified data will be accompanied by the appropriate documentation, metadata, and code to facilitate reuse and provide the potential for interoperability with similar datasets.
The main benefit of DRUM is whatever is shared through this repository is public; however, a completely open system is not optimal if any of the data could be identifying (e.g., certain types of demographic data). We will work with the University of MN Library System to determine if DRUM is the best option. Another option available to the University of MN, ICPSR ( https://www.icpsr.umich.edu/icpsrweb/ ), would allow us to share data at different levels. Through ICPSR, data are available to researchers at member institutions of ICPSR rather than publicly. ICPSR allows for various mediated forms of sharing, where people interested in getting less de-identified individual level would sign data use agreements before receiving the data, or would need to use special software to access it directly from ICPSR rather than downloading it, for security proposes. ICPSR is a good option for sensitive or other kinds of data that are difficult to de-identify, but is not as open as DRUM. We expect that data for this project will be de-identifiable to a level that we can use DRUM, but will consider ICPSR as an option if needed.
Data agreement
No specific data sharing agreement will be needed if we use DRUM; however, DRUM does have a general end-user access policy ( conservancy.umn.edu/pages/drum/policies/#end-user-access-policy ). If we go with a less open access system such as ICPSR, we will work with ICPSR and the Un-funded Research Agreements (UFRA) coordinator at the University of Minnesota to develop necessary data sharing agreements.
Circumstances preventing data sharing
The data for this study fall under multiple statutes for confidentiality including multiple IRB requirements for confidentiality and FERPA. If it is not possible to meet all of the requirements of these agencies, data will not be shared.
For example, at the two sites where data will be collected, both universities (University of Minnesota and University of Missouri) and school districts have specific requirements for data confidentiality that will be described in consent forms. Participants will be informed of procedures used to maintain data confidentiality and that only de-identified data will be shared publicly. Some demographic data may not be sharable at the individual level and thus would only be provided in aggregate form.
When we collect audio/video data, participants will sign a release form that provides options to have data shared with project personnel only and/or for sharing purposes. We will not share audio/video data from people who do not consent to share it, and we will not publicly share any data that could identify an individual (these parameters will be specified in our IRB-approved informed consent forms). De-identifying is also required for FERPA data. The level of de-identification needed to meet these requirements is extensive, so it may not be possible to share all raw data exactly as collected in order to protect privacy of participants and maintain confidentiality of data.
Redirect Notice


Writing a Data Management & Sharing Plan
Learn what NIH expects Data Management & Sharing Plans to address, as well as how to submit your Plan.
- Applications for Receipt Dates BEFORE Jan 25 2023
- Applications for Receipt Dates ON/AFTER Jan 25 2023
Writing a Data Sharing Plan
Under its 2003 data sharing policy , NIH expects investigators to submit a data sharing plan with requests for funding or grants, cooperative agreements, intramural research, contracts, or other funding agreements of $500,000 or more per year.
Data sharing plans should describe how an applicant will share their final research data. The specifics of the plan will vary on a case-by-case basis, depending on the type of data to be shared and how the investigator plans to share the data.
Examples of information to cover in a data sharing plan include:
- The expected schedule for data sharing
- The format of the dataset
- The documentation to be provided with the dataset
- Whether any analytic tools also will be provided
- A brief description of such an agreement
- Criteria for deciding who can receive the data
- Whether or not any conditions will be placed on their use
- Investigators choosing to handle their own data sharing may wish to enter into a data-sharing agreement.
Generating large-scale genomic data? NIH’s Genomic Data Sharing (GDS) policy may also apply to your research. See our GDS Policy Overview to learn more.
Examples of Data Sharing Plans
The exact content and level of detail to be included in a data sharing plan depends on the specifics of the project, such as how the investigator is planning to share data, or the size and complexity of the dataset. The examples below give a sense of what a data sharing plan can look like.
Example 1 This application requests support to collect public-use data from a survey of more than 22,000 Americans over the age of 50 every 2 years. Data products from this study will be made available without cost to researchers and analysts. User registration is required in order to access or download files. As part of the registration process, users must agree to the conditions of use governing access to the public release data, including restrictions against attempting to identify study participants, destruction of the data after analyses are completed, reporting responsibilities, restrictions on redistribution of the data to third parties, and proper acknowledgment of the data resource. Registered users will receive user support, as well as information related to errors in the data, future releases, workshops, and publication lists. The information provided to users will not be used for commercial purposes, and will not be redistributed to third parties.
Example 2 The proposed research will include data from approximately 500 subjects being screened for three bacterial sexually transmitted diseases (STDs) at an inner city STD clinic. The final dataset will include self-reported demographic and behavioral data from interviews with the subjects and laboratory data from urine specimens provided. Because the STDs being studied are reportable diseases, we will be collecting identifying information. Even though the final dataset will be stripped of identifiers prior to release for sharing, we believe that there remains the possibility of deductive disclosure of subjects with unusual characteristics. Thus, we will make the data and associated documentation available to users only under a data-sharing agreement that provides for: (1) a commitment to using the data only for research purposes and not to identify any individual participant; (2) a commitment to securing the data using appropriate computer technology; and (3) a commitment to destroying or returning the data after analyses are completed.
Example 3 The proposed research will involve a small sample (less than 20 participants) recruited from clinical facilities in the New York City area with Williams syndrome. This rare craniofacial disorder is associated with distinguishing facial features. Even with the removal of all identifiers, we believe that it would be difficult if not impossible to protect the identities of subjects given the physical characteristics of subjects, the type of clinical data (including imaging) that we will be collecting, and the relatively restricted area from which we are recruiting subjects. Therefore, we are not planning to share the data.
What data that will be shared:
I will share phenotypic data associated with the collected samples by depositing these data at ________________ which is an NIH-funded repository. Genotype data will be shared by depositing these data at ________________. Additional data documentation and de-identified data will be deposited for sharing along with phenotypic data, which includes demographics, family history of XXXXXX disease, and diagnosis, consistent with applicable laws and regulations. I will comply with the NIH GWAS Policy and the funding IC’s existing policies on sharing data on XXXXXX disease genetics to include secondary analysis of data resulting from a genome wide association study through the repository. Meta-analysis data and associated phenotypic data, along with data content, format, and organization, will be available at ____________. Submitted data will confirm with relevant data and terminology standards.
Who will have access to the data:
I agree that data will be deposited and made available through ________________ which is an NIH-funded repository, and that these data will be shared with investigators working under an institution with a Federal Wide Assurance (FWA) and could be used for secondary study purposes such as finding genes that contribute to process of XXXXXX. I agree that the names and Institutions of persons either given or denied access to the data, and the bases for such decisions, will be summarized in the annual progress report. Meta-analysis data and associated phenotypic data, along with data content, format, and organization, will be made available to investigators through ____________.
Where will the data be available:
I agree to deposit and maintain the phenotypic data, and secondary analysis of data (if any) at ________________, which is an NIH-funded repository and that the repository has data access policies and procedures consistent with NIH data sharing policies.
When will the data be shared:
I agree to deposit genetic outcome data into ________________ repository as soon as possible but no later than within one year of the completion of the funded project period for the parent award or upon acceptance of the data for publication, or public disclosure of a submitted patent application, whichever is earlier.
How will researchers locate and access the data:
I agree that I will identify where the data will be available and how to access the data in any publications and presentations that I author or co-author about these data, as well as acknowledge the repository and funding source in any publications and presentations. As I will be using ________________, which is an NIH-funded repository, this repository has policies and procedures in place that will provide data access to qualified researchers, fully consistent with NIH data sharing policies and applicable laws and regulations.
How to Submit Data Sharing Plans
The plan should be included in the Resource Sharing section of the application. See the How to Apply – Application Guide for form instructions.
Writing a Data Management and Sharing Plan
Under the 2023 Data Management and Sharing (DMS) Policy , NIH expects researchers to maximize the appropriate sharing of scientific data, taking into account factors such as legal, ethical, or technical issues that may limit the extent of data sharing and preservation.
NIH requires all applicants planning to generate scientific data to prepare a DMS Plan that describes how the scientific data will be managed and shared. For more on what constitutes scientific data, see Research Covered Under the Data Management & Sharing Policy .
Applications subject to NIH’s Genomic Data Sharing (GDS) Policy should also address GDS-specific considerations within the elements of a DMS Plan (see NOT-OD-22-189 and details below).
Submitting Data Management and Sharing Plans
The DMS Plan should be submitted as follows:
- DMS Plans should be included within the “Other Plan(s)” field on the PHS 398 Research Plan or PHS 398 Career Development Award Supplemental Form as indicated in the Application Instructions . See below for details on developing and formatting Plans.
- A brief summary and associated costs should be submitted as part of the budget and budget justification (see Budgeting for Data Management and Sharing and the Application Instructions for details).
- Extramural (contracts) : as part of the technical evaluation
- Intramural : determined by the Intramural Research Program
- Other funding agreements : prior to the release of funds
Data Management and Sharing Plan Format
DMS Plans are recommended to be two pages or less in length.
NIH has developed an optional DMS Plan format page that aligns with the recommended elements of a DMS Plan.
Important: Do not include hypertext (e.g., hyperlinks and URLs) in the DMS Plan attachment.

Elements to Include in a Data Management and Sharing Plan
As outlined in NIH Guide Notice Supplemental Policy Information: Elements of an NIH Data Management and Sharing Plan , DMS Plans should address the following recommended elements and are recommended to be two pages or less in length. As described in the Application Guide, the DMS Plan should be attached to the application as a PDF file. See NIH’s Format Attachments page.
1. Data Type
Briefly describe the scientific data to be managed and shared:
- Summarize the types (for example, 256-channel EEG data and fMRI images) and amount (for example, from 50 research participants) of scientific data to be generated and/or used in the research. Descriptions may include the data modality (e.g., imaging, genomic, mobile, survey), level of aggregation (e.g., individual, aggregated, summarized), and/or the degree of data processing.
- Describe which scientific data from the project will be preserved and shared. NIH does not anticipate that researchers will preserve and share all scientific data generated in a study. Researchers should decide which scientific data to preserve and share based on ethical, legal, and technical factors. The plan should provide the reasoning for these decisions.
A brief listing of the metadata, other relevant data, and any associated documentation (e.g., study protocols and data collection instruments) that will be made accessible to facilitate interpretation of the scientific data
For data subject to the GDS Policy: Data types expected to be shared under the GDS Policy should be described in this element. Note that the GDS Policy expects certain types of data to be shared that may not be covered by the DMS Policy’s definition of “scientific data”. For more information on the data types to be shared under the GDS Policy, consult Data Submission and Release Expectations .
2. Related Tools, Software and/or Code
Indicate whether specialized tools are needed to access or manipulate shared scientific data to support replication or reuse, and name(s) of the needed tool(s) and software. If applicable, specify how needed tools can be accessed.
3. Standards
Describe what standards, if any, will be applied to the scientific data and associated metadata (i.e., data formats, data dictionaries, data identifiers, definitions, unique identifiers, and other data documentation).
4. Data Preservation, Access, and Associated Timelines
Give plans and timelines for data preservation and access, including:
- The name of the repository(ies) where scientific data and metadata arising from the project will be archived. See Selecting a Data Repository for information on selecting an appropriate repository.
- How the scientific data will be findable and identifiable, i.e., via a persistent unique identifier or other standard indexing tools.
When the scientific data will be made available to other users and for how long. Identify any differences in timelines for different subsets of scientific data to be shared.
- Note that NIH encourages scientific data to be shared as soon as possible, and no later than the time of an associated publication or end of the performance period, whichever comes first. NIH also encourages researchers to make scientific data available for as long as they anticipate it being useful for the larger research community, institutions, and/or the broader public.
For data subject to the GDS Policy: For human genomic data: Investigators are expected to submit data to a repository acceptable under the Genomic Data Sharing Policy. See Where to Submit Genomic Data . Human genomic data is expected to be shared according to NIH’s Data Submission and Release Expectations , but no later than the end of the performance period, whichever comes first. For Non-human genomic data: Investigators may submit data to any widely used repository. Non-human genomic data is expected to be shared as soon as possible, but no later than the time of an associated publication, or end of the performance period, whichever is first.
5. Access, Distribution, or Reuse Considerations
Describe any applicable factors affecting subsequent access, distribution, or reuse of scientific data related to:
- Informed consent
- Privacy and confidentiality protections consistent with applicable federal, Tribal, state, and local laws, regulations, and policies
- Whether access to scientific data derived from humans will be controlled
- Any restrictions imposed by federal, Tribal, or state laws, regulations, or policies, or existing or anticipated agreements
Any other considerations that may limit the extent of data sharing. Any potential limitations on subsequent data use should be communicated to the individuals or entities (for example, data repository managers) that will preserve and share the scientific data. The NIH ICO will assess whether an applicant’s DMS plan appropriately considers and describes these factors. For more examples, see Frequently Asked Questions for examples of justifiable reasons for limiting sharing of data.
Expectations for human genomic data subject to the GDS Policy: Informed Consent Expectations: For research involving the generation of large-scale human genomic data from cell lines or clinical specimens that were created or collected AFTER the effective date of the GDS Policy (January 25, 2015): NIH expects that informed consent for future research use and broad data sharing will have been obtained. This expectation applies to de-identified cell lines or clinical specimens regardless of whether the data meet technical and/or legal definitions of de-identified (i.e. the research does not meet the definition of “human subjects research” under the Common Rule). For research involving the generation of large-scale human genomic data from cell lines or clinical specimens that were created or collected BEFORE the effective date of the GDS Policy: There may or may not have been consent for research use and broad data sharing. NIH will accept data derived from de-identified cell lines or clinical specimens lacking consent for research use that were created or collected before the effective date of this Policy. Institutional Certifications and Data Sharing Limitation Expectations: DMS Plans should address limitations on sharing by anticipating sharing according to the criteria of the Institutional Certification . In cases where it is anticipated that Institutional Certification criteria cannot be met (i.e., data cannot be shared as expected by the GDS Policy), investigators should state the institutional Certification criteria in their DMS Plan, explaining why the element cannot be met, and indicating what data, if any, can be shared and how to enable sharing to the maximal extent possible (for example, sharing data in a summary format). In some instances, the funding NIH ICO may need to determine whether to grant an exception to the data submission expectation under the GDS Policy. Genomic Summary Results: Investigators conducting research subject to the GDS Policy should indicate in their DMS Plan if a study should be designated as “sensitive” for the purposes of access to Genomic Summary Results (GSR), as described in NOT-OD-19-023 .
6. Oversight of Data Management and Sharing
Indicate how compliance with the DMS Plan will be monitored and managed, the frequency of oversight, and by whom (e.g., title, roles). This element refers to oversight by the funded institution, rather than by NIH. The DMS Policy does not create any expectations about who will be responsible for Plan oversight at the institution.
Sample Plans
NIH has provided sample DMS Plans as examples of how a DMS Plan could be completed in different contexts, conforming to the elements described above. These sample DMS Plans are provided for educational purposes to assist applicants with developing Plans but are not intended to be used as templates and their use does not guarantee approval by NIH.
Note that the sample DMS Plans provided below may reflect additional expectations established by NIH or specific NIH Institutes, Centers, or Offices that go beyond the DMS Policy. Applicants will need to ensure that their Plan reflects any additional, applicable expectations (including from NIH policies and any ICO- or program-specific expectations as stated in the FOA).
Assessment of Data Management and Sharing Plans
Program staff at the proposed NIH Institute or Center (IC) will assess DMS Plans to ensure the elements of a DMS Plan have been adequately addressed and to assess the reasonableness of those responses. Applications selected for funding will only be funded if the DMS Plan is complete and acceptable.
During peer review, reviewers will not be asked to comment on the DMS Plan nor will they factor the DMS Plan into the Overall Impact score, unless sharing data is integral to the project design and specified in the funding opportunity (see NOT-OD-22-189 ).
If data sharing is integral to the project and tied to a scored review criterion in the funding opportunity, program staff will assess the adequacy of the DMS Plan per standard procedure, but peer reviewers will also be able to view the DMS Plan attachment and may factor that information into scores as outlined in the evaluation criteria.
For information about budget assessment by peer reviewers, see Budgeting for Data Management and Sharing .
Revising Data Management and Sharing Plans
Pre-Award Plan Revisions: If the DMS Plan provided in the application cannot be approved based on the information provided, applicants will be notified that additional information is needed. This will occur through the Just-in-Time (JIT) process. Applicants will be expected to communicate with their Program Officer and/or Grants Management Specialist to resolve any issues that prevent the funding IC from approving the DMS Plan. If needed, applicants should submit a revised DMS Plan. Refer to NIH Grants Policy Statement Section 2.5.1 Just-in-Time Procedures for additional guidance.
Post-Award Plan Revisions: Although investigators submit plans before research begins, plans may need to be updated or revised over the course of a project for a variety of reasons for example, if the type(s) of data generated change(s), a more appropriate data repository becomes available, or if the sharing timeline shifts. If any changes occur during the award or support period that affects how data is managed or shared, investigators should update the Plan to reflect the changes. It may be helpful to discuss potential changes with the Program Officer. In addition, the funding NIH ICO will need to approve the updated Plan. NIH staff will monitor compliance with approved DMS Plans during the annual RPPR process as well. For more details, please refer to NOT-OD-23-185: Prior Approval Requests for Revisions to an Approved Data Management and Sharing (DMS) Plan Must be Submitted Using the Prior Approval Module .
Additional Considerations
Note that funding opportunities or ICs may have specific expectations (for example: scientific data to share, relevant standards, repository selection). View a list of NIH Institute or Center data sharing policies . Investigators are encouraged to reach out to program officers with questions about specific ICO requirements.
Please note that a Plan is part of an application, and, as such, an institution takes responsibility for the Plan and the rest of the application's contents when submitting an application. Although part of the official submission, when not considered during peer review the attachment is maintained as a separate “Data Management and Sharing (DMS) Plan” document in the grant folder viewable via the Status Information screen in eRA Commons. This document is viewable by authorized users and is not part of the assembled e-Application.
New Data Management & Sharing Policy Effective January 25, 2023!
Related resources.
Selecting a Data Repository
Budgeting for Data Management & Sharing
Data Management
NIH Institute or Center Data Sharing Policies

Research Data Management: Data Management Plan
- What is Research Data Management
- What is Research Data
- Research Data Cycle
- Funder Requirements
- RDM Policy & Legislation
- Library Support for Research Data Management
- RDM Training
- Data Sharing & Publishing
- Data Citing
- Research Data & Research Data Management
- Data Storage
About Data Management Plans
What is Data Management Plan (DMP)
A data management plan (DMP) is a written document that describes the data you expect to acquire or generate during the course of a research project, how you will manage, describe, analyze, and store those data, and what mechanisms you will use at the end of your project to share and preserve your data.
You may have already considered some or all of these issues with regard to your research project, but writing them down helps you formalize the process, identify weaknesses in your plan, and provide you with a record of what you intend(ed) to do. Data management is best addressed in the early stages of a research project, but it is never too late to develop a data management plan.
Creating a Data Management Plan
Research is all about discovery, and the process of doing research sometimes requires you to shift gears and revise your intended path. Your DMP is a living document that you may need to alter as the course of your research changes. Remember that any time your research plans change, you should review your DMP to make sure that it still meets your needs.
What should be covered in the Data Plan
The framework below, adapted from one developed by the Inter-University Consortium for Political and Social Research (ICPSR) , shows one approach to the elements of a data management plan.
DMPTool @NWU
Online tool for creating a dmp @nwu.
North-West University Libraries provides access to the online Data Management Planning (DMP) Tool . The DMPTool includes data management plan templates, along with a wealth of information and assistance to guide you through the process of creating a ready-to-use DMP for your specific research project and funding agency.

We can review your data management plan and make suggestions. We are also happy to verify whether your intended use of the Dayta Ya Rona Digital Repository as described in your plan matches up with the Dayta Ya Rona services we provide.
DMP submission
Once your data management plan is complete, you will include it with the rest of your proposal to the funding agency. North-West University Research's Office has further information on proposal development and submission.
- << Previous: RDM Policy & Legislation
- Next: Library Support for Research Data Management >>
- Last Updated: Feb 9, 2024 4:29 PM
- URL: https://libguides.nwu.ac.za/research-data-management
- AUT Library
- Library Guides
- Research Support
Managing your research data
- Writing a data management plan
- Research data & data management
Research data management plan
Aut dmp tool, other guides and checklists.
- Collecting data
- Managing data
- Sharing data
A data management plan ( DMP ) is a formal document that outlines how you will handle your data both during your research, and after the project is completed. This ensures that data are well-managed in the present, and prepared for preservation in the future. A DMP is often required in grant proposals.
A research data management plan is a living document and should be reviewed and updated regularly.
WSG format for a data management plan mentioned in the video.
Your DMP should include the a brief description about your project and how data will be managed:
- Roles and responsibilities
- Ethics and policies/guidelines compliance
- Types of data, data format and documentation
- Data storage, file backup and security
- Access, sharing and archiving
AUT guidelines and policies
- Principles, policies and codes
- Data management guidelines - AUT ethical guidelines and confidentiality requirements
- AUT guide for drafting a data management plan
The AUT Data Management Planning Tool makes use of a platform developed and hosted by University of California Digital Library. By using this tool you will create a data management plan based on current AUT data management guidance.
Plans can be drafted on DMPTool and once complete are downloadable in PDF form for your own records. Settings in the tool allow you to control whether your plan is private, institutionally viewable or open to public view.
The questions and structure of the DMPTool have been customised for AUT researchers as part of a joint project between AUT Library and the University Research Office. If you would like to give constructive feedback on the tool please contact: [email protected]
Sign in to AUT DMPTool:
- Enter your AUT email to the sign in box
- On the next screen, click Sign in with Institution (SSO)
Important: To access the AUT Template, you must select 'No funder associated with this plan or my funder is not listed' on the Create Plan page.
- Video - Creating a Data Management Plan (DMP) - Curtin University
- ANDS guide for Data management plans By Australian National Data Service.
- Example DMPs Examples on the Digital Curation Centre website (UK).
- Data management costing tool and checklist - UK Data Service
- DDC guidance - The Digital Curation Centre (DCC) UK
- Digital Creation Centre (DCC), Edinburgh
- UK Data Archive
- << Previous: Research data & data management
- Next: Collecting data >>
- Last Updated: Nov 21, 2023 11:28 AM
- URL: https://aut.ac.nz.libguides.com/RDM
A data management plan (DMP) is a document which defines how data handled throughout the lifecycle of a project—that is, from its acquisition to archival.
While these documents are typically used for research projects to meet funder requirements, they can be leveraged within a corporate environment as well to create structure and alignment between stakeholders.
Since DMPs highlight the types of data that will be used within the project and addresses the management of it throughout the data lifecycle , stakeholders, such as governance teams, can provide clear feedback on the storage and dissemination of sensitive data, such as personally identifiable information (PII), at the onset of a project. These documents allow teams to avoid compliance and regulatory pitfalls, and they can serve as templates on how to approach and manage data for future projects.
Learn the building blocks and best practices to help your teams accelerate responsible AI.
Register for the white paper on AI governance
A data management plan typically has five components:
1. A statement of purpose 2. Data definitions 3. Data collection and access 4. Frequently asked questions (FAQs) 5. Research data limitations
Each of these focus areas enables research agencies and research funders (or perhaps your data management team) to assess the amount of risk associated with a given project. The data management plan also addresses how to manage that risk. For example, if sensitive data is used within a project, is it appropriate to re-use that data for future projects? Depending on the sensitivity of that data, it may not be appropriate, or it may require additional user consent.
Each component of a data management plan focuses on a particular piece of information, we’ll delve more into each one.
1. Statement of purpose: This explains why the team needs to acquire specific types of data over the course of the project. It should clearly outline the question that the team is attempting to answer with this dataset.
2. Data definitions: Data descriptions help end users and their audiences understand naming conventions and their correspondence with specific datasets. Some of this information may also be held within the metadata, typically labeling data by its data sources and file formats. Creating and abiding by pre-defined metadata standards throughout the data acquisition process will also ensure a more consistent collection and smoother integration process.
3. Data collection and access: This section of a DMP highlights how data will be collected, stored, and accessed from a data repository. It will likely address the data source of any existing data or the approach that will be taken to create new data, such as an experiment. It should also contain information around the timing of data—i.e. how often it will be updated and over what period of time. The type and timing of the data will generally inform its storage and access to third-parties. For example, unstructured data will require a non-relational system versus a relational one, and larger datasets will require more compute power compared to smaller ones. There also may be restrictions around data sharing due to privacy or intellectual property rights. Since project stakeholders will expect that sensitive data, such as personally identifiable information (PII), is treated with the upmost care and security, it’s important for data owners to be clear about their data management practices, particularly in this area. This will include answers to questions around the data’s long-term preservation, such as data archiving or data re-use. For data that is not sensitive in nature, there will be an expectation to provide a pathway for third parties to access raw data and research results.
4. Frequently Asked Questions: This section can be considered a “catch-all” for other popular questions within data management projects, such as sharing plans, citation preferences, and data backup methods. Researchers or data owners may to highlight any digital object identifiers (DOI) for owners of adjacent or related projects. Additionally, if project owners are archiving data, they’ll also need to address the length of the archive’s existence. Will it live for one year, five years, or perhaps indefinitely?
5. Research data limitations: This section addresses upfront limitations with the dataset, which will limit its ability to generalize more broadly to populations. For example, data may be focused on a specific demographic, such as a geography, gender, race, age group, et cetera.
Data management plans are predominantly used in more academic settings, particularly for federal government funded programs, such as the National Institutes of Health (NIH) and National Science Foundation (NSF), but corporations can also leverage them in either their research or data governance functions. While academics and researchers need to comply with funder requirements in grant applications, many research institutions create a DMP tool to provide participants with the relevant template for their research project. Data governance teams within organizations can set up similar protocols to ingest data requests from stakeholders advocating for new data initiatives.
Grant applications
Researchers in both private and public sectors look to different funding agencies to sponsor research and innovation initiatives. DMPs mitigate risk for both parties, ensuring that data owners have assessed the value as well as their own personal responsibility (i.e. security and disaster recovery measures) to research data management.
Data governance initiatives Data management plans are also incredibly helpful for new data initiatives in business settings, assisting all stakeholders in understanding the importance of new data sources and how it can tie to business outcomes. As developments within hybrid cloud , artificial intelligence , the internet of things (IoT), and edge computing continue to spur the growth of big data, enterprises will need to find ways to manage the complexity of it within their data systems.
Read the free report to learn how data management on a unified platform for data, analytics and AI can accelerate time to insights.
Learn the best practices to ensure data quality, accessibility, and security as a foundation to an AI-centric data architecture.
Scale AI workloads for all your data, anywhere, with IBM watsonx.data, a fit-for-purpose data store built on an open data lakehouse architecture.
Research Data Management
- Getting Started
Data Management Plans
Roles & responsibilities, tools & resources, agencies that require a dmp, funder policies.
- Managing Your Data
- Documentation & Metadata
- Sharing & Preservation
- Reproducibility & Replicability
- Tools & Software
- Citing Data
A Data Management Plan (DMP) serves two main purposes:
- As a requirement for most publicly funded research grants.
- As a guide for you and your lab as you collect and analyze data.
General components of a data management plan:
- Managing your data
- Standards for your metadata (data describing your data)
- Policies for storing & sharing your data (including privacy, confidentiality, and copyright issues)
- Policies for re-use
- Plans for long-term storage
- Roles & Responsibilities for all parties throughout the life of the data
Best practices for dealing with data on a daily basis:
- Define roles and assign responsibilities for data management.
- For each task identified in your data management plan, identify the skills needed to perform the task.
- Match skills needed to available staff and identify gaps.
- Develop training plans for continuity.
- Assign responsible parties and monitor results.
- Talk to your librarian about best practices for managing lab notebooks. We can help.
- Data Management Plan Tool The DMPTool will walk you through the process of writing your data management plan for a variety of funding agencies.
- DART Rubric for NSF DMPs The DART Rubric for NSF DMPs provides explanations and guideance for different sections of the DMP required by the NSF.
- DART Guidance for NSF Directorates Additional guideance for the DART Rubric for NSF DMPs. In addition to providing some additional help with the sections in the rubric, this guidance also details requirements for particular NSF Directorates.
- McMaster University Data Management Plan Database Database of example DMPs from the Digital Research Alliance of Canada, National Institutes of Health (NIH), Qualitative DMP Competition, DataOne, Digital Curation Centre, Liber, the Working Group on NIH DMSP Guidance, and UC San Diego Research Data Curation compiled into one searchable, open-access platform.
- DMPTool Example DMPs Example DMPs that were made public by users of the DMPTool.
Most public funding agencies require a DMP of some sort in the grant application process.
- SPARC (the Scholarly Publishing and Academic Resources Coalition) - Search for the data policies of various funders. - Find your funder and look for the Data Management Planning section to see what kind of DMP is required
NIH and NSF policies
- NSF Data Management and Sharing Policies (including by Directorate and Division)
- NIH Data Sharing Policy Important Note: A new policy took effect on January 25, 2023.
Supplemental information for new NIH Data Sharing Policy
- Elements of an NIH Data Management and Sharing Plan
- Allowable Costs for Data Management and Sharing
- Selecting a Repository for Data Resulting from NIH-Supported Research
- Additional NIH Data Sharing Policies by Institute and Center
- Browse Data Sharing Requirements by Federal Agency Beautiful & easy to use site for finding out what your funder expects from you in sharing your data. From SPARC
- << Previous: Getting Started
- Next: Managing Your Data >>
- Last Updated: May 10, 2024 10:35 AM
- URL: https://courseguides.trincoll.edu/data-management
Creating a good research data management plan

About this video
Increasingly, funding bodies require researchers to submit a data management plan alongside their grant applications. These plans describe how data will be acquired, treated and preserved, both during and after the research project.
Good research data management has a role to play beyond funding. As well as saving researchers time and effort when running experiments, it benefits the wider scientific community, as well-organized data can be used by other researchers in their work.
In this webinar recording, produced in collaboration with Dr. Rob Hooft, Technical Coordinator of the Dutch TechCentre for Life Sciences , you will learn what a data management plan is and when you might need one. He explains why research data management is important, whatever your career stage, and helps you understand how to apply the FAIR principles (findable, accessible, interoperable, and reusable).
You’ll come away with the knowledge required to build your own research data management plan and get the most out of your data.
About the presenter

Manager ELIXIR NL, Dutch Techcentre for Life Sciences
Rob Hooft was educated as a structural chemist at Utrecht University and did his PhD studies in the same lab on the subject of structure-activity relationships of sweet tasting compounds. After spending four years at the EMBL in Heidelberg where he developed tools to verify the quality of protein structures, he worked for a variety of companies developing software then managing a team. When the final firm he worked for, Bruker AXS, stopped their R&D in the Netherlands, Rob moved back to the academic world and joined the Netherlands Center for Bioinformatics, NBIC, as CTO for the service-directed program. Following a two-year excursion to the Netherlands eScience Center - where he ran the data program of the Dutch Techcenter for Life Sciences (DTL) - he joined DTL managing the Dutch tasks in the European ELIXIR infrastructure for Life Sciences data.

How researchers benefit from citing data

Data Repositories to store your data

Make your data findable- It's Not FAIR! Improving Data Publishing Practices in Research

Make your data accessible -It's Not FAIR! Improving Data Publishing Practices in Research
Make your data interoperable - It's Not FAIR! Improving Data Publishing Practices in Research
Data management plans, how to write a good data in brief article.
Mendeley Data for Journals
Research data management
Data in Brief
Research Data Management Librarian Academy course
Mendeley Data
Research Data Management
- Literature/Guides for RDM Best Practices
- Data Information Literacy
- Publishing and Sharing Your Data
- Funding Agency Requirements
- Ownership of Data
- Scholarly Communication Libguide This link opens in a new window
- Research Data Management Working Group
- Manage Your Researcher Profile with ORCID This link opens in a new window
Rowan University Office of Sponsored Programs
- Research forms for grant proposals Information on preparing Data Management Plans from the Rowan University Office of Sponsored Programs.
Data Management Plans
Before beginning a research project, it is critical to have a clearly written data management plan (DMP). Most funding agencies require grant proposals to include a DMP that clearly outlines how the researcher will collect, manage, organize, preserve and distribute the research data that they will be generating during the study. Each funding agency has its own set of requirements for DMPs, so identify all elements that need to be addressed for a DMP for that specific agency before writing the plan. You may use free, web-based tools such as DMPTool that helps you construct data management plans using templates that address specific funder requirements.
Core elements of a Data Management Plan will include answers to the following questions:
- What’s the purpose of the research?
- What is the data? How and in what format will the data be collected? Is it numerical data, image data, text sequences, or modeling data?
- How much data will be generated for this research?
- How long will the data be collected and how often will it change?
- Are you using data that someone else produced? If so, where is it from?
- Who is responsible for managing the data? Who will ensure that the data management plan is carried out?
- What documentation will you be creating in order to make the data understandable by other researchers?
- Are you using metadata that is standard to your field? How will the metadata be managed and stored?
- What file formats will be used? Do these formats conform to an open standard and/or are they proprietary?
- Are you using a file format that is standard to your field? If not, how will you document the alternative you are using?
- What directory and file naming convention will be used?
- What are your local storage and backup procedures? Will this data require secure storage?
- What tools or software are required to read or view the data?
- Who has the right to manage this data? Is it the responsibility of the PI, student, lab, MIT, or funding agency?
- What data will be shared, when, and how?
- Does sharing the data raise privacy, ethical, or confidentiality concerns? Do you have a plan to protect or anonymize data, if needed?
- Who holds intellectual property rights for the data and other information created by the project? Will any copyrighted or licensed material be used? Do you have permission to use/disseminate this material?
- Are there any patent- or technology-licensing-related restrictions on data sharing associated with this grant?
- Will this research be published in a journal that requires the underlying data to accompany articles?
- Will there be any embargoes on the data?
- Will you permit re-use, redistribution, or the creation of new tools, services, data sets, or products (derivatives)? Will commercial use be allowed?
- How will you be archiving the data? Will you be storing it in an archive or repository for long-term access? If not, how will you preserve access to the data?
- Is a discipline-specific repository available? If not, you could consider depositing your data into Rowan Digital Works (RDW). Email the Library's Institutional Repository team at [email protected] for more information.
- How will you prepare data for preservation or data sharing? Will the data need to be anonymized or converted to more stable file formats?
- Are software or tools needed to use the data? Will these be archived?
- How long should the data be retained? 3-5 years, 10 years, or forever?
[Credit: Text for questions for DMP from MIT Libraries]
Sample Data Management Plans
- Public DMPs (from DMPTool website)
- ICPSR sample plans (for deposit with ICPSR)
- Natural Science examples (from ICPSR website)
- Guidelines for Effective Data Management Plans (ICPSR)
- Managing and Sharing Data: Best Practice for Researchers (UK Data Archive)
- Australian National University Data Management Manual
- NIH examples of data sharing plans
- UK Data Archive Managing and Sharing Data Guide
DMP Resources and Guides
- JHU Grant Reviews Guide Guide to evaluating DMPs in grant proposals prepared by Johns Hopkins University. more... less... Knowing what the reviewers are looking for should be helpful in creating your own.
- Digital Curation Centre DMP Checklist Checklist for creating data management plans provided by the UK's Digital Curation Centre.
- << Previous: Home
- Next: DMPTool >>
- Last Updated: Jan 3, 2024 12:06 PM
- URL: https://libguides.rowan.edu/researchdatamanagement

Research Data Management (RDM): Data Management Plan (DMP)
- What is Research Data
- What is Research Data Management
- FAIR Principles
- Open Datasets
- Data Management Policies
- External Funder Requirements
- Journals and Publishers Policies
- Research Data Lifecycle
- Data Classification
- Data Storage
- Data Security
- Sensitive data
- RDM and Primary Materials Checklist
Data Management Plan (DMP)
- Existing Data
- Data Retention
- Publishing Research Data

Click the image above to start building your Research Data Management Plan
A Data Management Plan is a document that specifies how research data will be handled both during and after a research project.
It identifies key actions and strategies to ensure that research data are of high quality, secure, sustainable, and – to the extent possible – accessible and reusable.
Many public research funders and ethics committees require a data management plan to be submitted as part of their application.
What to include in DMP
A good DMP takes into account the applicable regulations and data policies and considers the whole research data lifecycle. It typically addresses the following topics:
- What data will be collected, and how.
- How data will be documented.
- How any ethical and legal issues will be dealt with.
- How data will be stored and backed up during research.
- Any plans for preserving (some of the) data beyond the project's end.
- Any plans for sharing or providing access to (some of the) data.
- Who will be responsible for data management, and what additional resources may be required.
- What to cover in your DMP may also depend on your funder, who may provide their own DMP template.
Charles Darwin University recommends completing the RDM and Primary Materials Checklist as part of the DMP and provides a full DMP template
- << Previous: RDM and Primary Materials Checklist
- Next: Existing Data >>
- Last Updated: May 13, 2024 12:06 PM
- URL: https://libguides.cdu.edu.au/RDM
Create a research data management plan
All researchers at UWE Bristol must complete a research data management plan before collecting any data as part of research, or using any data for research.
A research data management plan is a document drawn up at the start of the research process. It outlines how all research data will be generated or collected, managed, stored and preserved, shared or disposed of. All researchers at UWE, including doctoral researchers, must complete a research data management plan before collecting any data as part of research, or using any data for research.
UWE Bristol research data management plan template
There is a specific research data management plan template for use by UWE Bristol researchers. Each question has accompanying guidance notes which can be accessed via hyperlinks from within the template itself, or can be viewed below.
An online training module on research data management to help you to complete your research data management plan is available on the My Learning platform for staff, and on the UWE Bristol Blackboard for students. Please note, the online module is mandatory for staff who conduct or supervise research. Please see the research governance pages on research data management for the most up-to-date information.
Access the UWE Bristol Research data management plan template (DOC) .
Note 1: What data will you collect, create or use?
Outline the types of research data that are expected to be created or collected.
Research data can be in any form, for example, electronic or hard copy, video, audio, artefacts, machine readouts. It can include survey/questionnaire data, consent forms, laboratory log books, videos of artistic performances, audio records of interviews and their transcripts, physical samples (including biological samples of animal or human origin), photographic images, environmental or habitat data, observational data (of humans or animals).
Please include:
- The source of the data - is the data coming from primary sources including data subjects, for example, survey participants, video recordings), being generated, or from a third party (such as secondary data)?
- The volume of data do you expect to generate.
- Who are the data subjects (for example survey participants or interviewees)? Are there, or might there be, any vulnerable subjects? These could include, but are not limited to, children, older people, patients, prisoners, asylum seekers, those who are physically or mentally unwell.
- How many data subjects will there be?
- What is the purpose of the data that is being collected?
- Are you creating or collecting personal, sensitive or special category data such as health, religion, race, sexual orientation, genetics and biometrics, relating to a living person or persons?
Note 2: How will you collect, create or access the data?
For primary data, briefly describe how the data will be collected or created.
- Will you be using recorded interviews, observation, physical measurement, taking samples, computer/instrument generated, survey, film etc.
- Outline any professional or community standards that will be adhered to.
- If you are generating digital survey data, what system will you use? Please note: Qualtrics is currently the only survey tool authorised by UWE Bristol.
If you are collaborating with any third party organisation or individual
- Outline any data handling agreements that are in place - such as data processing agreements, data sharing agreements or collaboration agreements which include data sharing/handling arrangements.
- If data is to be held by a third party describe when, where, and how the data will be held by the third party. Please note: personal data should not be moved outside of the European Economic area without safeguards being in place (please email The Data Protection Team at [email protected] for advice).
If you are using third party data (such as a secondary data source), say how this will be obtained
- Outline any restrictions on third party data use, or any access or use agreements that you/the University will need to sign. Please note: you should never sign anything without first consulting with Megan Wiggins in The Contracts Team at [email protected] , as you do not have authority to do so.
- Are you collecting or accessing data under restricted access conditions, for example within the NHS , ONS , or Criminal Justice System?
UWE Bristol Ethics process for human participants - Consent
Ethical approval MUST be granted by UWE Bristol's College or University Research Ethics Committee in respect of research and/or evaluations involving human participant data. Both legal and ethical dimensions of research data must be considered (please see note 7 in relation to Data Protection). In relation to personal data, the combined effect of ‘law’ and ‘ethics’ is generally that you may only do with data what you have told research participants you will do, and which you have gained consent for.
Please explain here briefly how you will:
- Please ensure that you consult the guidance on participant consent forms (DOC) . Will this consent enable future sharing and re-use? If this is not appropriate, and you do not propose to share and re-use, say so here.
- If you will not be gaining consent please set out your justification. Where professional standards may exist, for example in covert research, or in filming crowds, please set out any professional standards you will be adhering to.
- provide participant information Please see the guidance on participant information sheets (DOC) and the recommended Participant Privacy Notice (DOC) .
- handle personal, sensitive or other special category data.
- protect the interests and identity of any research participants - for example data anonymisation.
Note 3: Classify your data as public, restricted or confidential
See the Information Handling Policy (PDF) for definitions of data classifications.
Note 4: How will the data be stored and backed up at all stages of its lifecycle?
Please be clear how data will be stored at each stage of the research. You must comply with the Information Security Policy (PDF) and take account of the Information Security Toolkit .
You should take a "life course" approach to your data, being clear about the "data journey". For example, if you are interviewing human participants, taking samples, or collecting measurements or other data in the field, how will the data be stored at all stages of the research, how will it be securely transported, backed up, anonymised or pseudonymised, archived, securely disposed of?
Outline where data will be stored during the research
It is usually a requirement that research data is stored on a UWE Bristol networked drive (S or H), or on UWE Bristol OneDrive. If this is not possible, for example in the case of very large datasets, or situations where you will not have network access, you should consult the IT Service Desk for advice.
Please note: the only Cloud provision which is acceptable for use is UWE Bristol OneDrive, not Dropbox or any other provision.
How will data be backed up?
- Who is responsible for conducting data back up, and version control?
- How frequently will data be backed up?
- Is sufficient storage available via UWE Bristol systems, or do you need to consider additional storage needs and associated costs?
See UWE Bristol's data storage pages (intranet only) for information.
Note 5: How will the data be documented, described and maintained?
How will you manage file versioning during the course of the research.
Is there a standard that will be used?
Note what file format(s) you intend to use to store data
Where possible it is better to use non-proprietary file formats that are in widespread use to ensure on-going readability of data.
The UK Data Archive has a list of file formats which are in common use.
Outline how you will record information about your data
This is so that you, and others where appropriate, can access and use the data. This might be during the course of the research, or afterwards. Say where this information will be held, for example within the data itself, in a readme.txt file, in a database, or using another system.
As a minimum, you should record date, name and purpose of the data, the creator, details of how the data was created/analysed, explanations of any codes or abbreviations used.
For further information, see the Research Data Management guidance on documenting data .
Note 6: How will your data be processed?
Please describe here what you will actually do with the data, throughout its life course.
Set out the data security measures that you will take in relation to the way you will use the data. Data security measures should be appropriate to the data involved.
UK Data Protection legislation requires that personal data will be processed in a manner that ensures appropriate security including protection against unlawful processing, accidental loss, destruction or damage (also see Note 7 ). However, it is not just personal data; other kinds of data will also need careful handling, for example security sensitive research, commercially sensitive data, data subject to export controls, or environmental information (for example, in relation to rare or endangered populations of animals).
- Will any third party processing of data take place? If so, by whom and for what purpose? (for example, use of a UWE Bristol approved transcription service)
- How will you ensure only those who have a legitimate right to access the data can do so?
- By what mechanisms will data be shared between research partners?
- If you are generating data in the field, how will you ensure its safe transfer to UWE Bristol secure systems?
Note 7: Does the Data Protection Act (2018) apply to your research?
Collecting personal data and complying with data protection.
The General Data Protection Regulation (GDPR), implemented in the UK by the Data Protection Act (2018), states that there must be lawfulness, fairness and transparency in relation to any data subjects. Use this section to consider how you will ensure that this data principle is upheld. Please refer to the Data Protection Research Standard (intranet only) for information.
If you are working with human participants
- Outline how you will ensure you comply with the principles of accuracy and minimisation of data collected.
- Outline how you will ensure that data is kept in a form which permits identification for no longer than is necessary (storage limitation under GDPR ). Where data needs to be kept in this form for a longer period of time (for example, clinical trials data) please justify that here. Consult the Data Protection Research Standard (intranet only).
- Please set out here whether you have conducted a Data Protection Impact Assessment (DPIA) (staff intranet), and any necessary subsequent action. If you do not need to conduct a DPIA , please justify this here.
Note 8: Export controls and other legislation and regulation
- Please see Export Controls Regulations Guidance .
- If your research data may have a “dual use” or potentially be used in weapons of mass destruction (such as certain materials or algorithms) then you must set out here how you will ensure that you comply with the relevant regulations. Please note: the consequences of a breach of Export Controls can be extremely serious, so if you are uncertain, seek guidance from your College Management.
- In relation to other legislation and regulations, are there any specific implications for your research data? If so, please set them out here.
Note 9: What Intellectual Property will be collected or used in this research?
Who owns the ipr of any data collected or created.
- In the case of partnership working, is this covered by a collaboration agreement?
- In the case of derived products (including derived datasets), who owns, controls and has permission to use these?
- Are the measures you have set in place in relation to the research data appropriate to protect IP and not infringe third party IP?
Refer to The UWE Bristol Policy on Intellectual Property (PDF) for information.
Note 10: What are your plans for long-term preservation and data sharing, where appropriate, and data disposal?
Researchers are encouraged to think about preserving their data and, where appropriate, making it available for sharing and re-use. Some funders and publishers have specific requirements relating to data retention.
Please explain here:
- How you will decide which datasets to keep beyond the end of the research. Include the reasons for your decisions and be clear how this relates to participant consent if you are working with human participants.
- Are you using a schedule from a funder/publisher?
- Outline if there is any data which must be securely destroyed during, or at the end of, the research and how this will be achieved.
- Where any data to be preserved will be deposited. For example, in an established discipline specific, or other, national data repository, or in UWE Bristol's data repository.
- If you do not plan to use a data repository, explain how your data will curated and preserved beyond the end of the research, or why and how it will be securely and safely disposed of.
- If and how you intend to share your research data for re-use. If appropriate, note how participant consent allows for this.
- Note any access limits to the data. For example, does your data contain personal information about research participants that requires access controls, or are you working with an external collaborator?
- Will there be an embargo period?
- Do you have a funder that has a specific data release schedule?
If it is not appropriate to share your data, state why here.
For further information, see guidance on Preserving, Sharing and Disposing of Research Data .
Outline the proposed arrangements if any member of the research team leaves the University
Please note: no student or staff member is permitted to remove data from the University without the permission of the data owner. This is the Pro-Vice Chancellor Research and Business Engagement, delegated to College Deans. This permission will only be granted where it is consistent with the University’s interests and legal and ethical obligations.
Note 11: Who is responsible for enacting the different elements of the research data management plan?
The UWE Bristol project manager has overall responsibility for enacting the research data management plan, but others such as other UWE Bristol research team members, external collaborators, or other third parties will have a role to play. Please set this out here:
- What are the different roles within your research team - at UWE Bristol and/or external collaborators, in relation to the data?
- Do others have a role in relation to research data - for example, data gatekeepers, archivists, personnel in other organisations who are not part of the research team.
- Have formal agreements been set in place by the Contracts team - as appropriate, with research funders, collaborators or other partners?
- What are the different roles within your research team in relation to the data?
- How these roles, including the UWE project manager, will be fulfilled in the case of a significant period of unanticipated absence (such as sickness leave, or a member of staff leaving at short notice).
Note 12: What resources are needed to deliver the plan, and are these available?
Outline any resources you will need to fulfil your plan.
Consider, for example:
- equipment needs - such as an encrypted UWE Bristol laptop or recording device
- long-term storage provision - including resource to pay for it
- transcription costs
- staff time - to ensure data is properly handled
- encrypted additional hard drive or other additional storage
- training or additional staff expertise
- preservation costs - including time for preparation for archiving
- costs associated with any third party data.
See the UK Data Service guidance on costing data management for further guidance.
Funder Requirements
Many research funders also have requirements regarding the submission of a research data management plan. If you are externally funded, find out about your funder's requirements .
The University of Bristol has shared examples of data plans submitted to a range of funders. The University of Leeds has shared two examples of examples of ESRC data plans .
UK Data Archive's managing and sharing data guide provides information to assist researchers in sharing data.
Getting help with submitting a grant application
For help with submitting a data management plan as part of a research funding bid, please contact the Research and Knowledge Exchange Team .

Library Services
- Using the Library
- Finding Resources
- Learning & Teaching Support
- Open Science & Research Support
- Collections

New UCL Data Management Plan template
24 April 2024
Need to write a data management plan? Have you considered using the UCL Data Management Plan template?

Writing a data management plan can be challenging, but here is a simple way to structure your plan and follow best practice in data management and sharing.
What is a Data Management Plan?
A Data Management Plan (DMP) describes your planned and/or actioned data management and sharing activities. It is generally 1-3 pages in length and should cover the four phases of the research data lifecycle. It is generally written at the start of a research project and should be revisited at different stages of the project and updated where necessary. DMPs may be published in the UCL Research Data Repository and assigned a persistant, uniqure link in the form of a Digital Object Identifier (DOI).
Download our Data Management Plan template (MS Word)
Research Data news
Funnelback feed: https://cms-feed.ucl.ac.uk/s/search.json?collection=drupal-professional-... Double click the feed URL above to edit

IMAGES
VIDEO
COMMENTS
A data management plan, or DMP, is a formal document that outlines how data will be handled during and after a research project. Many funding agencies, especially government sources, require a DMP as part of their application processes. Even if you are not seeking funding for your research, documenting a plan for your research data is a best ...
Much of research data management is simply good research practice so you will already be some way down the line. Data plans are just a way of ensuring (and/or showing) that you have thought about how to create, store, backup, share and preserve your data. The Digital Curation Centre ( DCC) has produced an interactive online tool to help ...
A data management plan (DMP) will help you manage your data, meet funder requirements, and help others use your data if shared. The DMPTool is a web-based tool that helps you construct data management plans using templates that address specific funder requirements. From within this tool, you can save your plans, access MIT-specific information & resources, and request a review of your DMP by a ...
A data-management plan explains how researchers will handle their data during and after a project, and encompasses creating, sharing and preserving research data of any type, including text ...
Data management plans (DMPs) are documents that outline how data will be collected, stored, secured, analyzed, disseminated, and preserved over the lifecycle of a research project. They are typically created in the early stages of a project, and they are typically short documents that may evolve over time.
For this reason, your plan should describe data management and sharing during your research and, importantly, after your research is complete. Many funding agencies and sponsors require a data management plan with each proposal, but any researcher or team will benefit from developing a data management plan at the beginning of a project.
Research papers and data products are key outcomes of the science enterprise. Governmental, nongovernmental, and private foundation sponsors of research are increasingly recognizing the value of research data. As a result, most funders now require that sufficiently detailed data management plans be submitted as part of a research proposal.
DMPTool. The DMPTool is web-based and provides basic templates to help you construct a Data Management Plan. Using DMPTool, researchers can access a template, example answers, and guiding resources to successfully write a data management plan for any research project or grant.
Research data could be observational, experimental, simulated, or derived. Some examples include tables of numbers, transcripts of interviews, survey results, images, video or audio recordings, genomic data, or code, among others. A Data Management Plan (DMP) is an outline of what you will do with your data during and after a research project ...
A Data Management Plan outlines how data will be collected, organized, stored, secured, shared, and preserved in a research project. It covers data collection methods, organization, storage, sharing, preservation, ethics, and researcher responsibilities. Data Management Plans promote transparency and maximize research impact by ensuring your ...
Introduction. A Data Management & Sharing Plan (DMSP), also referred to as a Data Management Plan (DMP), is a formal document that outlines what you will do with your data during the active phase of the research project and after the project ends. This document may also be called a Data Management Plan (DMP) depending on the funding agency.
A data management plan is a written description document detailing how a researcher plans to collect, store, describe, preserve, and make data available [ 10 ]. The DMP goes through peer review and can be used in part to evaluate a research project merit [ 1 ].
A DMP (or DMSP, Data Management and Sharing Plan) describes what data will be acquired or generated as part of a research project, how the data will be managed, described, analyzed, and stored, and what mechanisms will be used to at the end of your project to share and preserve the data. One of the key advantages to writing a DMP is that it ...
Creating a plan that describes how data will be managed and shared throughout the course of a research project is an important step in ensuring that you, your collaborators, and potentially other researchers can find and use your data. Many research funding agencies have begun to require data management plans (DMPs), formal documents that ...
What is a data management plan (DMP)? A data management plan is a document outlining how a researcher plans to manage data during and after a research project including how it will be organized, maintained, and shared.. Why do you need one? Many funding agencies, including the National Science Foundation (NSF) and National Institutes for Health (NIH), are now requiring researchers to submit a ...
A Data Management Plan (DMP) is a written living document that formally outlines what you will do with your research data during the course of your research project and afterwards. It is a living document because any time your research plans change, you should review your DMP in order to make sure that the plan still satisfies your essential ...
These examples of data management plans (DMPs) were provided by University of Minnesota researchers. They feature different elements. One is concise and the other is detailed. One utilizes secondary data, while the other collects primary data. Both have explicit plans for how the data is handled through the life cycle of the project.
Writing a Data Sharing Plan. Under its 2003 data sharing policy, NIH expects investigators to submit a data sharing plan with requests for funding or grants, cooperative agreements, intramural research, contracts, or other funding agreements of $500,000 or more per year.. Data sharing plans should describe how an applicant will share their final research data.
A data management plan (DMP) is a written document that describes the data you expect to acquire or generate during the course of a research project, how you will manage, describe, analyze, and store those data, and what mechanisms you will use at the end of your project to share and preserve your data. You may have already considered some or ...
A data management plan (DMP) is a formal document that outlines how you will handle your data both during your research, and after the project is completed.This ensures that data are well-managed in the present, and prepared for preservation in the future. A DMP is often required in grant proposals.. A research data management plan is a living document and should be reviewed and updated regularly.
A data management plan (DMP) is a document which defines how data handled throughout the lifecycle of a project—that is, from its acquisition to archival. While these documents are typically used for research projects to meet funder requirements, they can be leveraged within a corporate environment as well to create structure and alignment ...
A Data Management Plan (DMP) describes your planned and/or actioned data management and sharing activities. It is generally 1-3 pages in length and should cover the four phases of the research data lifecycle. It is generally written at the start of a research project and should be revisted at different stages of the project and updated where ...
A Data Management Plan (DMP) serves two main purposes: As a requirement for most publicly funded research grants. As a guide for you and your lab as you collect and analyze data. General components of a data management plan: Managing your data; Standards for your metadata (data describing your data)
management plans, data management toolkits, and template agreements to support effective data management and access. Delserone (2008) discusses the University of Minnesota Libraries' research data ...
Increasingly, funding bodies require researchers to submit a data management plan alongside their grant applications. These plans describe how data will be acquired, treated and preserved, both during and after the research project. Good research data management has a role to play beyond funding. As well as saving researchers time and effort when running experiments, it benefits the wider ...
Data Management Plans. Before beginning a research project, it is critical to have a clearly written data management plan (DMP). Most funding agencies require grant proposals to include a DMP that clearly outlines how the researcher will collect, manage, organize, preserve and distribute the research data that they will be generating during the ...
A Data Management Plan is a document that specifies how research data will be handled both during and after a research project. It identifies key actions and strategies to ensure that research data are of high quality, secure, sustainable, and - to the extent possible - accessible and reusable.
A good data management plan will ensure the availability and accessibility of your research results after your project is complete and you have published the results, increasing the value of your research and possible reuse by other researchers. (University of Arizona). It is important to note that a Data Management Plan is usually required by ...
A research data management plan is a document drawn up at the start of the research process. It outlines how all research data will be generated or collected, managed, stored and preserved, shared or disposed of. All researchers at UWE, including doctoral researchers, must complete a research data management plan before collecting any data as ...
A Data Management Plan (DMP) describes your planned and/or actioned data management and sharing activities. It is generally 1-3 pages in length and should cover the four phases of the research data lifecycle. It is generally written at the start of a research project and should be revisited at different stages of the project and updated where ...