A Survey of 3D Printing Technologies as Applied to Printed Electronics
Ieee account.
- Change Username/Password
- Update Address

Purchase Details
- Payment Options
- Order History
- View Purchased Documents
Profile Information
- Communications Preferences
- Profession and Education
- Technical Interests
- US & Canada: +1 800 678 4333
- Worldwide: +1 732 981 0060
- Contact & Support
- About IEEE Xplore
- Accessibility
- Terms of Use
- Nondiscrimination Policy
- Privacy & Opting Out of Cookies
A not-for-profit organization, IEEE is the world's largest technical professional organization dedicated to advancing technology for the benefit of humanity. © Copyright 2024 IEEE - All rights reserved. Use of this web site signifies your agreement to the terms and conditions.
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.
Early warning sign of extinction?

So much for summers of love

Are you a human? Select all that apply.
In this 3D printing process, the little dot of blue light triggers a chemical reaction that makes the resin harden into plastic.
Credit: Tracy H. Schloemer and Arynn O. Gallegos
Making 3D printing truly 3D
Juan Siliezar
Harvard Staff Writer
Researchers from Rowland Institute eliminate need for 2D layering
Don’t be fooled by the name. While 3D printers do print tangible objects (and quite well), how they do the job doesn’t actually happen in 3D, but rather in regular old 2D.
Working to change that is a group of former and current researchers from the Rowland Institute at Harvard.
First, here’s how 3D printing works: The printers lay down flat layers of resin, which will harden into plastic after being exposed to laser light, on top of each other, again and again from the bottom to the top. Eventually, the object, such as a skull , takes shape. But if a piece of the print overhangs, like a bridge or a wing of a plane, it requires some type of flat support structure to actually print, or the resin will fall apart.
The researchers present a method to help the printers live up to their names and deliver a “true” 3D form of printing. In a new paper in Nature, they describe a technique of volumetric 3D printing that goes beyond the bottom-up, layered approach. The process eliminates the need for support structures because the resin it creates is self-supporting.
“What we were wondering is, could we actually print entire volumes without needing to do all these complicated steps?” said Daniel N. Congreve, an assistant professor at Stanford and former fellow at the Rowland Institute, where the bulk of the research took place. “Our goal was to use simply a laser moving around to truly pattern in three dimensions and not be limited by this sort of layer-by-layer nature of things.”
The key component in their novel design is turning red light into blue light by adding what’s known as an upconversion process to the resin, the light reactive liquid used in 3D printers that hardens into plastic.
In 3D printing, resin hardens in a flat and straight line along the path of the light. Here, the researchers use nano capsules to add chemicals so that it only reacts to a certain kind of light — a blue light at the focal point of the laser that’s created by the upconversion process. This beam is scanned in three dimensions, so it prints that way without needing to be layered onto something. The resulting resin has a greater viscosity than in the traditional method, so it can stand support-free once it’s printed.
“We designed the resin, we designed the system so that the red light does nothing,” Congreve said. “But that little dot of blue light triggers a chemical reaction that makes the resin harden and turn into plastic. Basically, what that means is you have this laser passing all the way through the system and only at that little blue do you get the polymerization, [only there] do you get the printing happening. We just scan that blue dot around in three dimensions and anywhere that blue dot hits it polymerizes and you get your 3D printing.”
The researchers used their printer to produce a 3D Harvard logo, Stanford logo, and a small boat, a standard yet difficult test for 3D printers because of the boat’s small size and fine details like overhanging portholes and open cabin spaces.
The researchers, who included Christopher Stokes from the Rowland Institute, plan to continue developing the system for speed and to refine it to print even finer details. The potential of volumetric 3D printing is seen as a game changer, because it will eliminate the need for complex support structures and dramatically speed up the process when it reaches its full potential. Think of the “replicator” from “Star Trek” that materializes objects all at once.
But right now, the researchers know they have quite a ways to go.
“We’re really just starting to scratch the surface of what this new technique could do,” Congreve said.
Share this article
You might like.
Fossil record stretching millions of years shows tiny ocean creatures on the move before Earth heats up

Despite ‘hippie’ reputation, male bonobos fight three times as often as chimps, study finds

Philosopher Barba-Kay on CAPTCHA dilemma, Aristotle’s good life, and how the internet is changing us — not for the better
So what exactly makes Taylor Swift so great?
Experts weigh in on pop superstar's cultural and financial impact as her tours and albums continue to break records.
Exercise cuts heart disease risk in part by lowering stress, study finds
Benefits nearly double for people with depression
Do phones belong in schools?
Banning cellphones may help protect classroom focus, but school districts need to stay mindful of students’ sense of connection, experts say.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 18 August 2020
3D bioprinting of cells, tissues and organs
- Madhuri Dey ORCID: orcid.org/0000-0002-9523-8083 1 , 2 &
- Ibrahim T. Ozbolat 2 , 3 , 4 , 5
Scientific Reports volume 10 , Article number: 14023 ( 2020 ) Cite this article
54k Accesses
145 Citations
14 Altmetric
Metrics details
- Experimental models of disease
- Regeneration
- Tissue engineering
3D bioprinting has emerged as a promising new approach for fabricating complex biological constructs in the field of tissue engineering and regenerative medicine. It aims to alleviate the hurdles of conventional tissue engineering methods by precise and controlled layer-by-layer assembly of biomaterials in a desired 3D pattern. The 3D bioprinting of cells, tissues, and organs Collection at Scientific Reports brings together a myriad of studies portraying the capabilities of different bioprinting modalities. This Collection amalgamates research aimed at 3D bioprinting organs for fulfilling demands of organ shortage, cell patterning for better tissue fabrication, and building better disease models.
The discovery of a 3D printer dates back to early 1980s when Charles Hull, an American engineer, built the 1st 3D printer, capable of creating solid objects by following a computer-aided design (CAD). The printer deposited successive layers of an acrylic-based photopolymer which was then simultaneously crosslinked by UV light, thus creating a solid 3D object. This simple technology, called stereolithography (SLA), revolutionized the additive manufacturing industry. Gradually, by the late 1990s, 3D printing made its appearance in healthcare where surgeons began 3D printing dental implants, custom prosthetics, and kidney bladders. Subsequently the term ‘3D bioprinting’ emerged where the material being printed, called ‘bioink’ 1 , consisted of living cells, biomaterials, or active biomolecules. Analogous to additive manufacturing, 3D bioprinting involves layer-by-layer deposition of bioink to create 3D structures, such as tissues and organs 2 .
3D bioprinting can be broadly categorized as either extrusion 3 , droplet 4 , or laser-based bioprinting. Extrusion based bioprinting employs mechanical, pneumatic or solenoid dispenser systems to deposit bioinks in a continuous form of filaments, while droplet based bioprinting relies on the generation of bioink droplets by thermal, acoustic or electrical stimulation. Laser based bioprinting utilizes laser power to 3D print structures such as in SLA by a photopolymerization principle. It can also be used for precise positioning of cells such as in laser direct-write and Laser Induced Forward Transfer (LIFT). The selection of “bioinks” for each of these different bioprinting modalities usually varies based on the ink’s rheology, viscosity, crosslinking chemistry, and biocompatibility. Extrusion based bioprinting primarily requires shear thinning bioinks while droplet or inkjet bioprinting needs materials with low viscosity. Over the past few years, the design and synthesis of bioinks has evolved to meet the increasing needs of new bioprintable materials. Significant advancements have also been made to integrate secondary techniques accompanying the above-mentioned modalities of bioprinting. For example, creating 3D structures with low viscosity bioinks has always been a challenge. To overcome this issue, such bioinks can now be extruded in a granular support bath containing yield stress hydrogels which solidify around the extruded structure and prevent it from collapsing 5 . Apart from organ printing, bioprinting is also being used to fabricate in-vitro tissue models for drug screening, disease modelling, and several other in-vitro applications.
The 3D bioprinting of cells, tissues and organs Collection at Scientific Reports is dedicated to this field of research. This collection clearly portrays the diverse applications of different bioprinting modalities and how they could be utilized for improving various aspects of healthcare. Kim et al. 3D printed a novel two-layered polycaprolactone (PCL) -based tubular tracheal graft 6 . This tracheal graft, seeded with induced pluripotent stem cell (iPSC) -derived mesenchymal (MSCs) and chondrocyte stem cells supported the regeneration of tracheal mucosa and cartilage in a rabbit model of a segmental tracheal defect. Galarraga et al. used a norbornene-modified hyaluronic acid (NorHA) macromer as a representative bioink for cartilage tissue engineering 7 . Printed structures containing MSCs, on long term culture, not only led to an increase in compressive moduli, but also expressed biochemical content similar to native cartilage tissue. Vidal et al. used 3D printed customized calcium phosphate scaffolds with and without a vascular pedicle to treat large bone defects in sheep 8 . They used CT angioscan to scan the entire defect site and subsequently 3D print a personalized scaffold to anatomically fit the defect site. A bioink comprising decellularized matrix from mucosal and muscular layers of native esophageal tissues was used by Nam et al. to mimic the microenvironment of native esophagus 9 . Leucht et al. used gelatin based bioinks to study vasculogenesis in a bone-like microenvironment 10 . Kilian et al. used a calcium phosphate cement (CPC) and an alginate-methylcellulose based bioink containing primary chondrocytes to mimic the different layers of osteochondral tissue 11 .
This special issue also contains three notable research articles on the patterning of cells—two utilizing acoustics, and one, magnetism. Even though bioprinting enables the homogenous distribution of cells representing the macro-architectural properties, it lacks control of the tissue micro-architecture such as orientation of cells within the bioprinted constructs. Chansoria and Shirwaiker delved deep into the physics of ultrasound-assisted bioprinting (UAB) that utilizes the acoustophoresis principle to align MG63 cells within single and multi-layered extrusion-bioprinted alginate constructs 12 . Cells were aligned both orthogonally and in parallel to the printed filaments, thus mimicking cellular anisotropy in tissues such as ligaments, tendons, and cardiac muscle. Similarly, Sriphutkiat et al. used acoustic excitation to align skeletal myoblast cells (C2C12) and human umbilical vein endothelial cells (HUVECs) encapsulated in methacrylated gelatin (GelMA) bioink 13 . Goranov et al. magnetically labelled MSCs and HUVECs, and aligned them in a magnetic scaffold to mimic vascularization of bone constructs 14 .
It is important to note that the applications of 3D bioprinting are not limited to organ printing. It also holds great promise in less explored avenues, such as using scaffolds for drug delivery, studying disease mechanisms, or creating personalized medicines. In this Collection, Lee et al. 3D printed a rifampicin loaded PCL scaffold for possible treatment of osteomyelitis 15 . Xu and coworkers 3D printed paracetamol containing PVA tablets with three different geometries, each demonstrating different release profiles which could be tailored based on the patient's needs 16 . Further, Foresti et al. applied 5D additive manufacturing techniques to create personalized models of patients’ pathology 17 . Ding, Illsley and Chang 3D bioprinted GelMA-based models to investigate the trophoblast cell invasion phenomenon, enabling studies of key placental functions 18 .
Additionally, there are other notable articles in this Collection enumerating different aspects of bioprinting. Afghah et al. used a Pluronic-nanoclay based composite support bath to bioprint representative structures, for complex and hollow tissues, using cell laden alginate hydrogel 19 . Zhao et al. developed a 3D printed hanging drop dripper system for analyzing tumor spheroids in-situ 20 . Yumoto et al. performed RNA-seq analysis on inkjet-printed cells to analyze the effect of bioprinting on gene expression 21 . We would like to extend our utmost gratitude and thank all the authors and reviewers who devoted their time and effort towards this 3D bioprinting collection.
Even though 3D bioprinting is advancing at a commendable rate with researchers trying to develop new printing modalities as well as improve existing modalities, there still remains a multitude of challenges that need to be overcome. Currently, a limited number of bioinks exist which are both bioprintable and which accurately represent the tissue architecture needed to restore organ function post-printing. While bioinks made from naturally derived hydrogels are conducive to cell growth, synthetic hydrogels are mechanically robust. Thus, hybrid bioinks should be designed to amalgamate all these aspects. Moreover, the bioprinting process itself needs to be more cell-friendly. Shear stress applied to the cells during the printing process are detrimental to cell growth and might even alter the gene expression profiles. Stem cells, such as iPSCs, are sensitive to such physical forces and usually do not survive the printing process. As stem cell studies have mostly been performed on 2D environments, there exists a lot of unknowns for a 3D stem cell culture. Effective techniques need to be developed for high throughput generation and bioprinting of organoids 22 for personalized drug testing and predictive disease models. Additionally, vascularization of bioprinted constructs for proper nutrient exchange, as well as integration of printed vasculature with host vasculature post organ implantation, is another major obstacle. Overall, 3D bioprinting is a rapidly evolving field of research with immense challenges, but tremendous potential to revolutionize modern medicine and healthcare.
Hospodiuk, M., Dey, M., Sosnoski, D. & Ozbolat, I. T. The bioink: a comprehensive review on bioprintable materials. Biotechnol. Adv. 35 , 217–239 (2017).
PubMed CAS Google Scholar
Ozbolat, I. T. 3D Bioprinting: Fundamentals, Principles and Applications (Elsevier Inc., Amsterdam, 2016).
Google Scholar
Ozbolat, I. T. & Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 76 , 321–343 (2016).
Gudapati, H., Dey, M. & Ozbolat, I. A comprehensive review on droplet-based bioprinting: past, present and future. Biomaterials 102 , 20–42 (2016).
Heo, D. N. et al. 3D bioprinting of carbohydrazide-modified gelatin into microparticle-suspended oxidized alginate for the fabrication of complex-shaped tissue constructs. ACS Appl. Mater. Interfaces 12 , 20295–20306 (2020).
Kim, I. G. et al. Transplantation of a 3D-printed tracheal graft combined with iPS cell-derived MSCs and chondrocytes. Sci. Rep. 10 , 1–14 (2020).
Galarraga, J. H., Kwon, M. Y. & Burdick, J. A. 3D bioprinting via an in situ crosslinking technique towards engineering cartilage tissue. Sci. Rep. 9 , 1–12 (2019).
Vidal, L. et al. Regeneration of segmental defects in metatarsus of sheep with vascularized and customized 3D-printed calcium phosphate scaffolds. Sci. Rep. 10 , 1–11 (2020).
ADS Google Scholar
Nam, H. et al. Multi-layered free-form 3D cell-printed tubular construct with decellularized inner and outer esophageal tissue-derived bioinks. Sci. Rep. 10 , 1–14 (2020).
Leucht, A., Volz, A. C., Rogal, J., Borchers, K. & Kluger, P. J. Advanced gelatin-based vascularization bioinks for extrusion-based bioprinting of vascularized bone equivalents. Sci. Rep. 10 , 1–15 (2020).
Kilian, D. et al. 3D Bioprinting of osteochondral tissue substitutes-in vitro-chondrogenesis in multi-layered mineralized constructs. Sci. Rep. https://doi.org/10.1038/s41598-020-65050-9 (2020).
Article PubMed PubMed Central Google Scholar
Chansoria, P. & Shirwaiker, R. Characterizing the process physics of ultrasound-assisted bioprinting. Sci. Rep https://doi.org/10.1038/s41598-019-50449-w (2019).
Sriphutkiat, Y., Kasetsirikul, S., Ketpun, D. & Zhou, Y. Cell alignment and accumulation using acoustic nozzle for bioprinting. Sci. Rep. 9 , 1–12 (2019).
ADS CAS Google Scholar
Goranov, V. et al. 3D patterning of cells+in magnetic scaffolds for tissue engineering. Sci. Rep. https://doi.org/10.1038/s41598-020-58738-5 (2020).
Lee, J. H. et al. Development of a heat labile antibiotic eluting 3D printed scaffold for the treatment of osteomyelitis. Sci. Rep. 10 , 1–8 (2020).
Xu, X., Zhao, J., Wang, M., Wang, L. & Yang, J. 3D printed polyvinyl alcohol tablets with multiple release profiles. Sci. Rep. https://doi.org/10.1038/s41598-019-48921-8 (2019).
Foresti, R. et al. In-vivo vascular application via ultra-fast bioprinting for future 5D personalised nanomedicine. Sci. Rep. 10 , 3205 (2020).
ADS PubMed PubMed Central CAS Google Scholar
Ding, H., Illsley, N. P. & Chang, R. C. 3D bioprinted GelMA based models for the study of trophoblast cell invasion. Sci. Rep. 9 , 1–13 (2019).
Afghah, F., Altunbek, M., Dikyol, C. & Koc, B. Preparation and characterization of nanoclay-hydrogel composite support-bath for bioprinting of complex structures. Sci. Rep. 10 , 1–13 (2020).
Zhao, L. et al. A 3D printed hanging drop dripper for tumor spheroids analysis without recovery. Sci. Rep. 9 , 1–14 (2019).
Yumoto, M. et al. Evaluation of the effects of cell-dispensing using an inkjet-based bioprinter on cell integrity by RNA-seq analysis. Sci. Rep. 10 , 1–10 (2020).
Ayan, B. et al. Aspiration-assisted bioprinting for precise positioning of biologics. Sci. Adv. 6 , eaaw5111 (2020).
ADS PubMed PubMed Central Google Scholar
Download references
Author information
Authors and affiliations.
Department of Chemistry, Penn State University, University Park, PA, 16802, USA
Madhuri Dey
The Huck Institutes of the Life Sciences, Penn State University, University Park, PA, 16802, USA
Madhuri Dey & Ibrahim T. Ozbolat
Engineering Science and Mechanics Department, Penn State University, University Park, PA, 16802, USA
Ibrahim T. Ozbolat
Biomedical Engineering Department, Penn State University, University Park, PA, 16802, USA
Materials Research Institute, Penn State University, University Park, PA, 16802, USA
You can also search for this author in PubMed Google Scholar
Contributions
M.D. wrote the manuscript. I.T.O. reviewed and edited the manuscript.
Corresponding author
Correspondence to Ibrahim T. Ozbolat .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Dey, M., Ozbolat, I.T. 3D bioprinting of cells, tissues and organs. Sci Rep 10 , 14023 (2020). https://doi.org/10.1038/s41598-020-70086-y
Download citation
Published : 18 August 2020
DOI : https://doi.org/10.1038/s41598-020-70086-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Spheroid construction strategies and application in 3d bioprinting.
- Chunxiang Lu
- Yuanyuan Liu
Bio-Design and Manufacturing (2024)
3D Printing Technology in the Pharmaceutical and Biomedical Applications: A Critical Review
- Nahid Tyagi
- Vipul Bhardwaj
- Gaurav Sharma
Biomedical Materials & Devices (2024)
Infantile hemangioma models: is the needle in a haystack?
Journal of Translational Medicine (2023)
Fully 3D-printed organic electrochemical transistors
- Matteo Massetti
- Silan Zhang
- Simone Fabiano
npj Flexible Electronics (2023)
3D and 4D Bioprinting Technologies: A Game Changer for the Biomedical Sector?
- Reza Noroozi
- Zia Ullah Arif
- Xiongbiao Chen
Annals of Biomedical Engineering (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
- Reference Manager
- Simple TEXT file
People also looked at
Original research article, a 3d printing short course: a case study for applications in the geoscience teaching and communication for specialists and non-experts.

- Reservoir Geomechanics Research Group, Civil and Environmental Engineering Department, University of Alberta, Edmonton, AB, Canada
3D printing developed as a prototyping method in the early 1980s, yet it is considered as a 21st century technology for transforming digital models into tangible objects. 3D printing has recently become a critical tool in the geoscience research, education, and technical communication due to the expansion of the market for 3D printers and materials. 3D printing changes the perception of how we interact with our data and how we explain our science to non-experts, researchers, educators, and stakeholders. Hence, a one-day short course was designed and delivered to a group of professors, students, postdoctoral fellows, and technical staff to present the application of 3D printing in teaching and communication concepts in the geoscience. This case study was aimed at evaluating how a diverse group of participants with geoscience and engineering background and no prior experience with computer-aided modeling (CAD) or 3D printing could understand the principles of different 3D printing techniques and apply these methods in their respective disciplines. In addition, the course evaluation questionnaire allowed us to assess human perception of tangible and digital models and to demonstrate the effectiveness of 3D printing in data communication. The course involved five modules: 1) an introduction lecture on the 3D printing methods and materials; 2) an individual CAD modeling exercise; 3) a tour to 3D printing facilities with hands-on experience on model processing; 4) a tour to experimentation facilities where 3D-printed models were tested; and 5) group activities based on the examples of how to apply 3D printing in the current or future geoscience research and teaching. The participants had a unique opportunity to create a digital design at the beginning of the course using CAD software, analyze it and 3D print the final model at the end of the course. While this course helped the students understand how rendering algorithms could be used as a learning aid, educators gained experience in rapid preparation of visual aids for teaching, and researchers gained skills on the integration of the digital datasets with 3D-printed models to support societal and technical objectives.
Introduction
3D printing is a 21st century technology for transforming digital models into physical objects. This technology is rapidly evolving, with more access to 3D printing machines and materials ( Wohlers Report, 2019 ). This is an innovative tool in medical ( Baden et al., 2015 ) and biomedical sciences ( Hoy, 2013 ), engineering ( Meyers et al., 2016 ; Boyajian et al., 2020 ), and communication ( Baden et al., 2015 ; Malmström et al., 2020 ). 3D printing revolutionizes how we interact with our data and how we explain our science to non-experts ( Horowitz and Schultz, 2014 ). Creating repeatable, tangible models is emerging in the geoscience education and research as well as in the related industries, such as petroleum recovery, groundwater storage, and carbon dioxide sequestration ( Ishutov et al., 2018 ). One of the biggest advantages of 3D printing is that all the processes involved in the creation of a 3D object, from generating the design to obtaining the printed part, facilitate the learning of concepts and tools, which also develops creativity and communication skills. Earth science data are often modeled in 3D, and 3D printers can provide this 3D visualization and tangible aspect of digital data ( Figure 1 ).
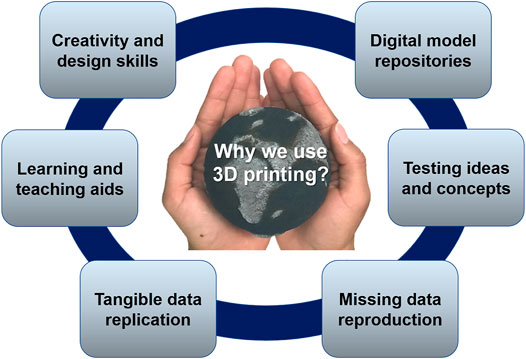
FIGURE 1 . Major benefits of using 3D printing in geosciences. It is useful for developing creativity and design skills through 3D modeling. 3D printing is a convenient tool for rapid manufacture of learning and teaching aids. Any 2D or 3D model can be replicated for a better communication, especially among non-specialists. Any digital data can be reproduced with 3D printing, even if the physical sample does not exist anymore. Research ideas and concepts can be repeatedly tested on the 3D-printed samples. All data can be retrieved or repeated from the digital repositories, which include files of 3D-printed models.
3D printing or so-called additive manufacturing of an object involves deposition of a material layer by layer ( Squelch, 2017 ). Therefore, this technology enables manufacturing models in various sizes and proportions (e.g., small objects can be printed large, so that more details are visible or large objects can be scaled down, so that one can hold the planet in the hand). Sustainable learning through a tangible approach is critical for understanding of complex geologic ideas, where learners can collect, gather and evaluate information about the exterior of the model and internal structures ( Szulżyk-Cieplak et al., 2014 ). Moreover, the same model can be used to communicate these ideas to others, including non-experts in a technical subject ( Dadi et al., 2014 ). 3D printing is essential for commination with impaired people, especially students who require special needs for education ( Kostakis et al., 2015 ; Jo et al., 2016 ; Pantazis and Priavolou, 2017 ; Koehler et al., 2018 ). In the Earth science curriculum, those students can learn common topics such as volcanoes or plate tectonics by using 3D-printed models in the classroom or at home. Buehler et al. (2016) demonstrates an example of a short course for students with intellectual disabilities in an inclusive context that results in enhancing digital literacy skills and reducing stigmas about these individuals at a community level.
Application of 3D printing in high-school education has already shown enhanced haptic perception of the learning material. Elrod (2016) emphasized that if 3D printing would be used in the K-12 environment, students could be better prepared for careers in emerging fields of technology [e.g., science, technology, engineering, and mathematics (STEM disciplines)]. Schelly et al. (2015) demonstrated that even a 3-day short course for middle- and high-school teachers from a variety of disciplines (sciences, engineering, and arts) gained a high interest in utilizing this technology in their classrooms. Chiu et al. (2015) presented a successful model for learning, self-learning, and mastery learning approaches for freshman students with different levels of technological literacy using 3D printers. Reggia et al. (2015) suggested that providing engineering students with an opportunity to perform a project-based design course using 3D printing was an essential curricular element in many engineering programs. Chien and Chu (2018) proposed that 3D printing could enable high-school students to improve their ability to transform from STEM to STEAM (science, technology, engineering, arts, and mathematics) using 3D printers and to create a bridging curriculum with respect to high-school and college students.
Roy and Brine (2017) developed a coursework model to build intellectual capital for the next generation who would vastly depend on 3D printing, because they would shape a smart community in both developing and developed economy context. Martin et al. (2014) explained an idea of “think globally, produce locally,” where 3D printing would become more affordable with the versatility of machines and the ability to engage students with many different STEM-based activities. Gatto et al. (2015) showed that engineering education is on the course of adapting to the social and industrial revolution brought by additive manufacturing, because the latter allowed for sharing digital data in repositories and repeatedly reproducing the data to test ideas and concepts ( Figure 1 ).
For the geoscience education, not many examples are found in the literature for using 3D printing in any full-time curriculum or short courses. Ford and Minshall (2019) demonstrate how teaching models of terrains, fossils, and mineral crystals can complement digital models for a better perception of 3D features. 3D printing is currently used in four geoscience areas, primarily for research and communication: paleontology, geomorphology, porous rocks, geomechanics ( Figure 2 ). These 3D-printed models help organizing a full description, classification, and preservation of geologic specimens. Resolution of 3D printers determines the accuracy of internal and external features of 3D-printed models and hence affects the repeatability of the digital design in different materials ( Figure 2 ). These characteristics are critical not only for creating teaching aids in the Earth Science curriculum, but also for conducting experimental research with 3D-pritned specimens ( Ishutov et al., 2018 ). 3D printing also has value for communication of geoscience to non-specialist audiences to convey technical information, to support legal arguments, and to provide general knowledge of the nature. Currently, there is no universal short course that can provide fast, but positive learning experience of digital modeling and 3D printing to understand and explain geologic concepts among both experts and generalists.
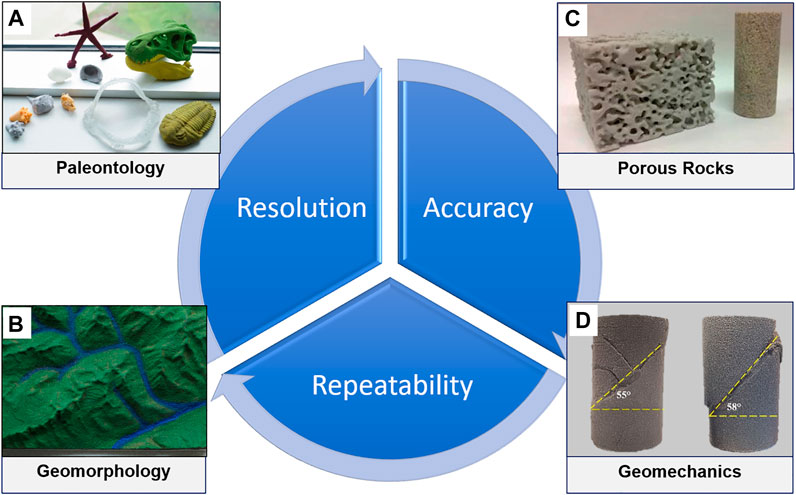
FIGURE 2 . Applications of 3D printing in the geoscience research areas: (A) paleontology, (B) geomorphology, (C) porous rocks, and (D) geomechanics. A blue chart indicates the characteristics of 3D-printed models that are critical for each of the geoscience areas. Materials used in a specific application have different physical and chemical properties, which affect the resolution of a 3D-printed model. 3D printer’s hardware and post-processing of 3D-printed models determine the accuracy of external and internal features. A combination of the three previous characteristics affects the repeatability of a digital design 3D-printed in multiple copies.
This course was developed to test how a group of participants from STEM disciplines, but with various academic backgrounds could perceive the fundamentals of available 3D printing techniques and materials and their relative merits. With little or no prior knowledge of CAD modeling and 3D printing, participants learnt about applications of 3D printing in studies of reservoir rocks ( Squelch 2017 ), fossils ( Rahman et al., 2012 ), geomechanics ( Hodder et al., 2018 ), geomorphology ( Hasiuk and Harding, 2016 ), and porous media ( Ishutov, 2019 ). This one-day short course was divided into five modules and involved students, postdoctoral fellows, technicians, and professors interested in current advances of 3D printing in research and teaching. In addition, participants explored the application of 3D printing in a technical communication. The objectives of the study included: 1) to evaluate if learners with versatile educational and cultural backgrounds could perceive the basic concepts of 3D printing techniques and material properties to provide an assessment of 3D-printed models for research in their respective discipline; 2) to test if fast learning of CAD modeling and 3D printing could help the participants utilize 3D-printed models to explain geologic concepts to generalist audiences; and 3) to prove that 3D-printed models were effective tools for the geoscience education.
Materials and Methods
The short course was designed for the participants without prior experience of CAD modeling or 3D printing. In addition, the course was open for students, professors, postdoctoral fellows, technicians, and research associates from the geoscience and engineering disciplines. The short course took place at the University of Alberta, Edmonton, Canada and involved 50 participants. The course learning outcomes were: 1) to understand capabilities and limitations of different 3D printing techniques; 2) to demonstrate how to digitally design 3D-printable models using CAD software, web platforms, and computed tomography data; 3) to provide the assessment of digital models and their relative replicas 3D-printed from real data; and 4) to characterize how 3D printing can increase the effectiveness of teaching and data communication.
Course Organization and Materials
The short course was organized in five modules: 1) an introduction lecture on the 3D printing methods and materials; 2) an individual CAD modeling exercise; 3) a tour to 3D printing facilities with hands-on experience on model processing; 4) a tour to experimentation facilities where 3D-printed models are tested; and 5) group activities based on the examples of how to apply 3D printing in current or future geoscience research and teaching ( Table 1 ). Each module was taught by one of the four instructors, and facility tours were led by four instructors, two instructors per facility. All instructions on how to complete each module were organized in a digital e-book (pdf).
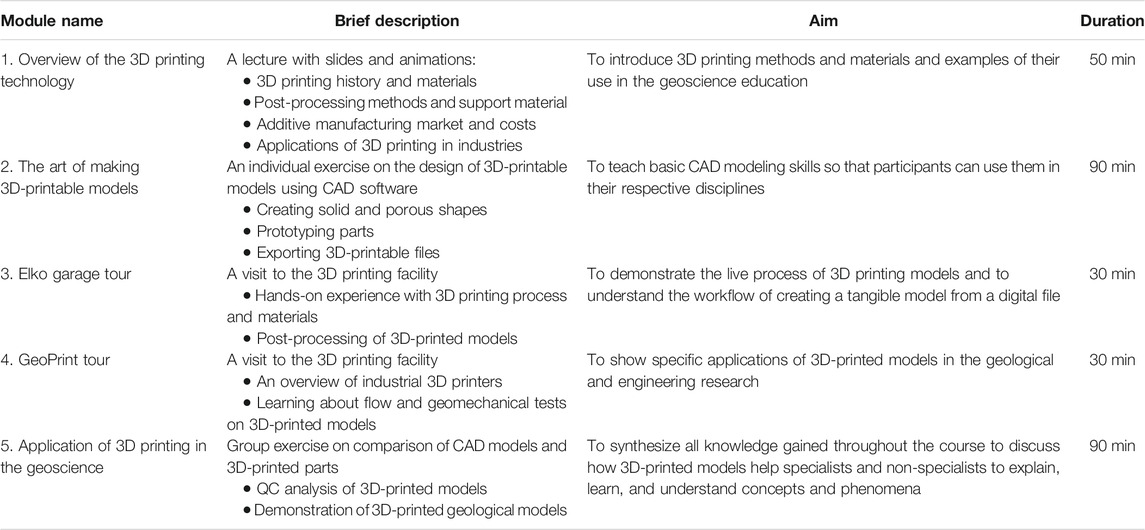
TABLE 1 . A brief description of topics covered in each module of the short course.
Module 1 included a lecture on the history of “rapid prototyping” and how 3D printing evolved as a tool for engineering industries. In addition, the workflow of creating a digital model and transferring it into a tangible object was covered. The model preparation for 3D printing was explained with examples of using printing specifications, such as the thickness of each layer, the vertical and horizontal dimensions, and the print speed. The lecture also contained post-processing methods, such as ultraviolet (UV) light curing or removal of support material that held the internal porous structure and external elements during printing to avoid deformation or damage of intricate designs. Instructors discussed 3D printing methods that differed by power source, resolution, precision, accuracy, build volume, materials, and price. The importance and applications of 3D-printed models were covered briefly for the areas of geoscience and engineering. At the end of the lecture, participants had a discussion session with instructors ( Figure 3A ).

FIGURE 3 . Photographs of the short course modules. (A) Module 1 “Overview of the 3D printing technology.” Course instructors presented a lecture on common additive manufacturing methods and materials and showed examples of 3D-printed models. (B) Module 2 “The art of making 3D-printable models.” Participants learned basic skills of CAD modeling using TinkerCAD. (C) Module 3 “Elko Garage Tour.” Live 3D printing process was shown to participants. (D) Module 4 “GeoPrint Tour.” Participants were shown industrial scale printing and experimental program performed with 3D-printed models. (E) Module 5 “Application of 3D printing in the geoscience.” Discussion of specific applications of geoscience models in edication and research.
Module 2 involved an individual CAD modeling exercise using an online platform on laptops or tablets ( Figure 3B ). The scale of 3D-printed models varied over the orders of magnitude: from nanometer-size features to the size of the 3D printer’s build volume. This activity was aimed at teaching the participants to create complex geological models (like rocks and fossils) using common shapes (e.g., cylinders, cubes) or multi-scale elements, which were then translated for 3D printing. At the end of this exercise, participants were able to export their model of choice for 3D printing and receive at the end of the course.
Module 3 represented a tour to the Elko Engineering Garage (University of Alberta, Edmonton, Canada) that introduced the participants to the activities associated with creating and 3D printing digital designs as well as post-processing of 3D-printed models ( Figure 3C ). Participants were exposed a variety of 3D printers and post-processing tools, as well as they had an opportunity to investigate a 3D laser scanner. Instructors made connections of the material covered in the lecture, such as material properties, 3D printing resolution, and model dimensions with the real applications in workspace. Participants were able to observe the 3D printing process of the digital models that they designed in module 2 and had a hands-on experience on post-processing their models to make give them a smooth, finished look.
Module 4 involved a visit to the GeoPRINT facility (University of Alberta, Edmonton, Canada), where an industrial-grade sand printer and a high-resolution stereolithography printer were located ( Figure 3D ). This tour introduced participants to two specific 3D printers used for geomechanical and flow research at Reservoir Geomechanics Research Group. Participants explored about the differences in material preparation, printing, and post-processing between these two technologies.
Module 5 included a group exercise on the comparison of CAD models for porous rocks, fossils and geomorphic features with their 3D-printed counterparts ( Figure 3E ). Participants assessed the differences in material finishes, accuracy of external and internal elements, and scales of 3D printing (using criteria in Figure 2 ). In addition, there was a discussion of potential application of 3D-printed models in the geoscience experiments to validate numerical simulations and complement existing laboratory tests. Instructors facilitated the discussion of 3D-printing techniques that participants have seen in modules 3 and 4 and how they could be applied to fundamental research in the areas of multi-phase fluid flow and reactive transport, discrete fracture networks, geomorphology, and paleontology ( Figure 3E ).
3D Printers and Software
Out of seven ASTM categories of 3D printing, four methods were shown in this short course: stereolithography, binder jetting, material extrusion, and material jetting. All 3D printers belonging to these categories were demonstrated in Modules 3 and 4. Materials used for demonstration of 3D printing techniques included polymers, plastics, sand, and resins.
The software used in module 2 for CAD modeling exercises was Autodesk TinkerCAD ( https://www.tinkercad.com ). It is a free online platform that requires only registration with email. The software used for processing of digital designs before 3D printing was Autodesk Meshmixer ( http://www.meshmixer.com ). It is a freeware that can be installed on most operating systems.
Post-Course Questionnaire
The course survey is proved to be one of the effective forms of analysis of the short course efficiency ( Chiu et al., 2015 ; Schelly et al., 2015 ; Meyers et al., 2016 ; Pantazis and Priavolou, 2017 ; Ford and Minshall, 2019 ; Assante et al., 2020 ). The surveys are usually conducted before and after the course to assess how learning objectives are fulfilled. In each module, the following criteria were used to build the course evaluation survey:
• fundamentals of 3D printing and its basic operating principles;
• advantages and disadvantages of 3D printing technologies;
• performance and functional constraints of 3D printing for specific applications.
• complete 3D-printing sequence of designing, fabricating, and measuring models;
• source of mismatch between digital and 3D-printed models.
• causes of errors and irregularities in 3D-printed models;
• hands-on experience of 3D printing in class for improved student understanding of subject matter.
• important 3D printing research challenges;
• resources to support experiments for teaching and classroom projects.
• understanding if humans learn better when using 3D-printed models;
• current and future 3D printing applications.
At the end of the course, instructors distributed an electronic evaluation form to all participants and asked them to complete it within 1 h. The questions in the survey were composed in a Google Docs form to allow for anonymous and individual response from each participant, who was required to indicate only their academic level. The post-course questionnaire was segmented into sections: 1) overall recommendation for the short course; 2) assessment of course materials (e-booklet, lecture slides, exercise instructions; 3) course content (cohesiveness of modules, ease of learning the material, laboratory tours, and visual aids); 4) time spent on each module; and 5) evaluation of instructors’ teaching abilities; 6) effectiveness of course learning outcomes. Section 1 responses were based on Yes/No scale. Responses in sections 2, 3, 5 were collected using the following scheme: strongly disagree, disagree, neutral, agree, and strongly agree. Responses in section 4 were registered using the following scheme: not enough, adequate, too much, no opinion. The last section was evaluated using Likert scale out of 5, where a higher value is a more positive response.
Results and Discussion
The short course involved 50 participants from geosciences and engineering ( Figure 4A ); it was expected to receive mixed comments about the course contents and organization of modules. Nonetheless, 97% of all participants responded that the course would be recommended to others ( Figure 4B ). In this case, others were referred to peer students, colleagues, and other academic staff. This outcome was positive to propose the course to various professional organizations as a customized workshop, e.g., for industry professionals interested in the use of 3D printing in research and technical communication. The instructors observed that despite the differences in age and academic background, the participants communicated with each other in a friendly manner. Based on the results of the post-course questionnaire, the short course outcomes were assessed for the adequacy and organization of the course materials, structure, and coherence of the course modules, and efficiency of the course instructors and learning objectives.
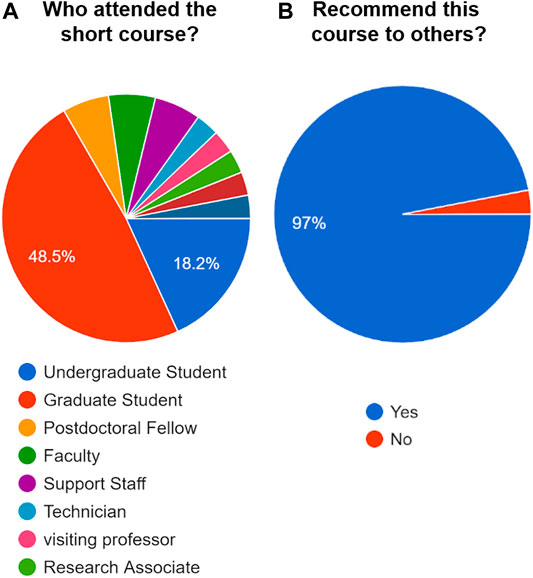
FIGURE 4 . Demographics of the short course participants. (A) Indication of the academic level and/or position. (B) Responses of participants from (A) to the question: “Will you recommend this short course to others?”
Course Materials
An e-book contained a set of short, descriptive instructions with images and figures about each module ( Figure 5 ) that was useful to most participants. Course objectives were clear, so that the short course agenda was understood by learners with different backgrounds (24 positive responses out of 32 responses in total). In addition, the survey showed that the e-book was a valuable component of the course as it helped navigating through activities and exercises (27 positive responses out of 33 responses in total). On the other hand, not all participants found the e-book visually appealing and suggested adding pseudo 3D cartoons that would visually simplify and outline different 3D printing processes (20 positive responses out of 33 responses in total; Figure 6 ). Other comments pointed out on the use of bolded text, underlining or different colors to highlight the key information in the e-book. Also, more than half of the class noted that activities were clearly defined by the instructors and suggested to include more details about the operation of software as numbered bullet points so there would be a step-by-step tutorial (21 positive responses out of 35 responses in total; Figure 6 ). A few additional notes were that the introductory lecture slides in module 1 were cohesive and well organized. For the next run of the course, instructors will prepare a short workflow with bullet points for each activity and exercise and will place them in the e-book as a support material. More images and snapshots will be added for each activity to allow the participants to navigate between the exercises.
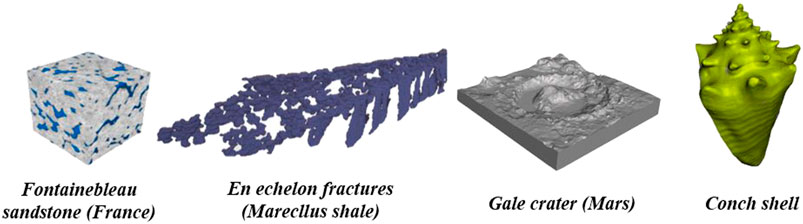
FIGURE 5 . An example of the module instructions from the course e-book. The full version of the e-book was available for participants a day before the course. Each module contained synopsis and a set of exercises.
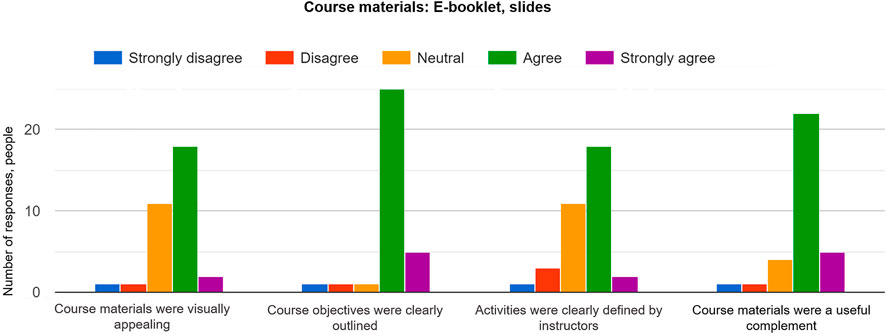
FIGURE 6 . Responses of participants for evaluation of the course materials, such as e-booklet and slides. All the course activities were described in the e-booklet provided on the short course day.

Course Content
The course content was developed using several approaches: lecture slides, individual exercises, group exercises, and facility tours. The majority of the class responded that modules were cohesive (29 positive responses out of 33 responses in total; Figure 7 ). Participants were mostly engaged during the visits to the Elko Garage and GeoPrint facilities (modules 3 and 4), because these tours improved their understanding of the 3D printing process (30 positive responses out of 32 responses in total). Observing the printing methods and interaction with 3D-printed models provided a motivation for the learners to incorporate this technology in their research, teaching, or other activities (29 positive responses out of 34 responses in total; Figure 7 ). In addition, the majority of participants could understand all aspects of digital design, processing, and post-processing of 3D-printed models via the CAD modeling exercise (module 2) (31 positive responses out of 34 responses it total). Instructors observed that even those participants who did not have any experience with digital modeling of simple shapes could learn it fast, because at the end of the exercise everyone was on the same level.
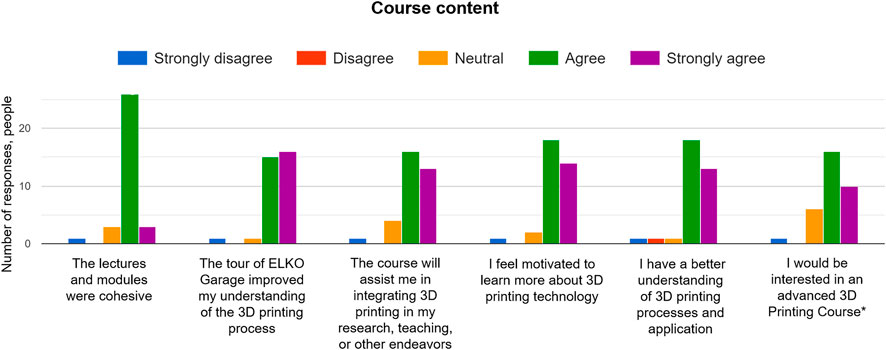
FIGURE 7 . Responses of participants for evaluation of the course content. Participants assessed each activity at the end of the short course. *A question about the advanced 3D printing course is whether participants would like to have a short course on the applications of 3D printing in their respective discipline (not geoscience).
The group exercise involving comparison of digital models with their 3D-printed counterparts and the discussion of applications in the geosciences (Module 5) was expected to be challenging, because the participants were divided into mixed groups of 10 people to avoid accumulating representatives of the same department and academic level in one group. E.g., one group might have consisted of two undergraduate students from civil engineering and geology, three professors from electrical engineering, computer engineering and geophysics, three postdoctoral fellows from mechanical engineering, and petroleum engineering, and two research associates from atmospheric science and computer science, respectively. Most of the class responded positively to such combination of groups, because it allowed them to share a broader spectrum of ideas given the versatility of backgrounds (32 positive responses out of 35 responses in total; Figure 7 ). Some participants responded that they would prefer to classify the groups by the department, so that they would share the same interest in 3D printing and might make the group work more cohesive. This model could be another option for the group activity, where the groups could be formed by the department only, but the course contents would need to be more general, rather than focusing on the geoscience and engineering applications.
Participants would also asked to have more group activities to share the knowledge learnt, which confirmed that this intentional split into mixed groups worked well for leaning the unknown concepts. A few people were not interested in the geoscience applications and would have liked to participate in the content related to their discipline only or in a more generic content. This was a viable comment, and more than half of the class responded that they would like to have an advanced 3D printing course to explore the applications in their relative subjects of interest (26 positive responses out of 30 responses in total; Figure 7 ). Perhaps a separate short course covering specific applications of 3D printing in STEM disciplines might be developed to satisfy this interest. The most expected comment was that participants were thinking of getting their own 3D printer to manufacture models for research, teaching, and communication.
Each module had a different time period for completion, because it depended on the speed of the instructor’s delivery and the pace of participants ( Figure 8 ). It was designed to spend more time on individual and group exercises (Modules 2 and 5), so that the pace between the participants could be averaged as some people needed more time to learn new tools. In general, almost all learners (29 out of 33) agreed that the 50-min lecture in module 1 was sufficient to grasp the main concepts. Some participants (12 out of 33) noted that they would need more time to go through the functionalities of the software in Module 2 to complete the CAD exercises. In future, this module could be timed in a different way, where the participants would have an extensive, detailed introduction into the software and then they would be given a set of exercises to complete. Also, for those who could complete a mandatory set of exercises faster, additional activities would be provided. For the group exercises (module 5), about half of the class completed their assignments on time, while a quarter of the class felt that the time could be reduced ( Figure 8 ). To adjust this module, more exercises would be provided, specifically a small section discussing case studies in the geoscience.
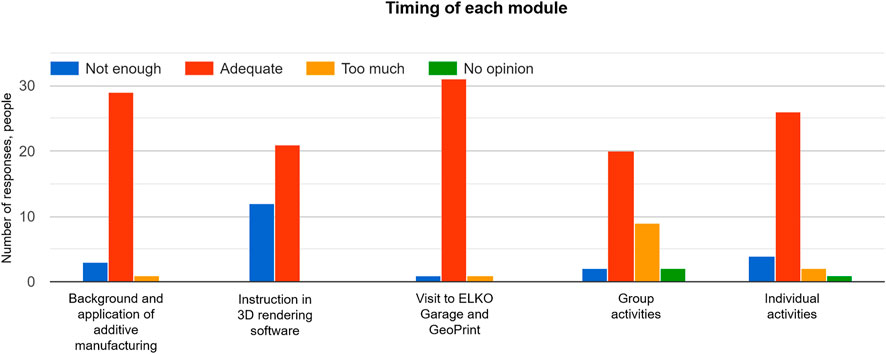
FIGURE 8 . Responses of participants for evaluation of the time spent on each module of the short course.
Efficiency of Instructors
The next set of questions in the survey was aimed at revealing any flaws in the style and structure of the instruction. It was found that the majority of the class was satisfied with the teaching style and delivery of the modules by instructors (28 positive responses out of 33 responses in total; Figure 9 ). One participant noted that it would be useful to have solutions for each exercise, mainly for the ones related to the group activity. The answers could not be compiled for each activity as they varied by the group and the amount of material covered in each case. A few participants would like to have more one-to-one communications with instructors, but it might not always possible, given the size of the class and time allocated for each activity. It is foreseen that the class size will be reduced to have more time assisting each participant in all activities, even though the majority of participants (31 out of 33; Figure 9 ) felt supported during the course.
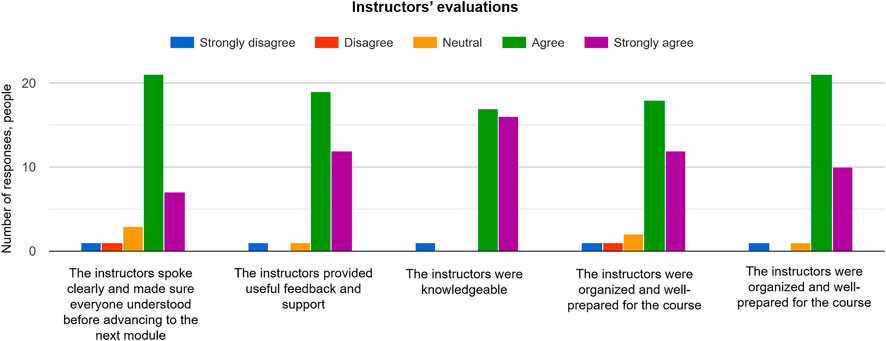
FIGURE 9 . Responses of participants for evaluation of the instructors’ delivery of the short course.
The survey showed that instructors were knowledgeable (32 positive responses out of 33 responses in total) and well-prepared (30 positive responses out of 34 responses in total) for the course, which fulfilled the course objective of sustainable learning and communication through tangible models. It is confirmed that 3D printing promoted the curiosity among the learners and facilitated an interest in creation of a model simultaneously with the instructor. Developing creative potential entailed improving a problem-based approach to demonstrate theoretical concepts that could be accessible by different groups of participants. This short course demonstrated that diverse groups were able to assimilate, apply, and describe new knowledge more effectively, including collaborative and individual learning. There is a need in studying how these methods can complement traditional instruction in terms of retention of material and motivating learners to study and develop their communication and problem-solving skills.
Efficiency of Learning Objectives
The course learning objectives were evaluated during interactive exercises of the course as well as post-course questionnaire. After completion of each module, participants were asked to complete the same set of three questions based on the course objectives. Their responses were averaged using Likert scale, where more positive responses were approaching 5 and less positive responses were approaching 1 ( Table 2 ). Participants were scoring how each of the three objectives was fulfilled when they completed modules subsequently. It was evident that more confidence was gained toward the end of the short course when all three course objectives were assessed (increasing scores from Module 1 to Module 5 in Table 2 ). While not all participants had geoscience background, collaborative learning is proven to be effective in enhancing creativity and hence enabling a large class to adopt the new technology. Post-course questionaries demonstrated that faculty, students, research fellows, and technicians could effectively work in teams to understand basic concepts of 3D printing techniques and material properties. They used this information to provide an assessment of 3D-printed models and to generate ideas for research in their respective disciplines.

TABLE 2 . Comparison of student responses on fulfilling the course learning objectives.
Individual CAD modeling exercise (module 2) helped the participants understand how geological and engineering models could be designed and utilized to explain ideas and concepts to generalist audiences. In module 5, instructors provided an example of 3D-printed porous rock created from a digital model ( Figure 10 ). All participants were asked to use this workflow to characterize how the rock porosity could have been formed and to explain why the rock grains had angular or rounded geometry and how they were transported to form a larger formation. Participants with a geoscience background were assessing responses of participants that did not have any background in the geoscience. It was noted that comparison of images, 3D digital models, and 3D-printed samples altogether provided better understanding of the rock properties rather than each model separately. Also. participants with good technical background in CAD within the team could help teaching other teammates, providing additional peer learning element in the process.
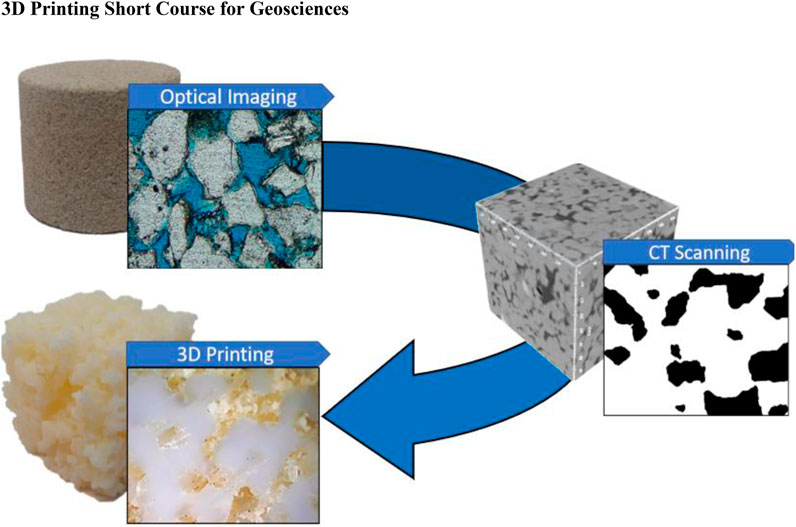
FIGURE 10 . Workflow for generation of 3D-printed samples from digital models. Source data are either optical or CT images of natural rocks (e.g., Berea sandstone). Images are segmented into pores and grains; the grain volume is transferred to 3D printing software as a CAD model. Selected 3D printer creates a tangible model layer-by-layer (polymer in this example). Pore space is filled with support material (soft polymer) that is removed by post-processing.
Module 5 was very useful for synthesizing previous modules and providing exercises linking CAD modeling from module 2 with 3D printing methods presented in module 1 and materials observed in modules 3 and 4. Participants were asked to choose one model for which both CAD and 3D-printed models were available ( Figure 11 ). Their task was to prepare a 1-min presentation of the model intended for general audience. The exercise was aimed at evaluating if 3D-printed models could improve geoscience learning for non-specialists. This collaborative learning approach demonstrated that expertise from students with different backgrounds could contribute to the cognitive process. Instead of learning under the instructions of tutors, participants collaboratively worked and learnt together. Participants noted that those teammates without geoscience background provided more intuitive and comprehensive description of selected models. It might be due to the fact that specialists could not often formulate higher-level explanation of concepts and phenomena.
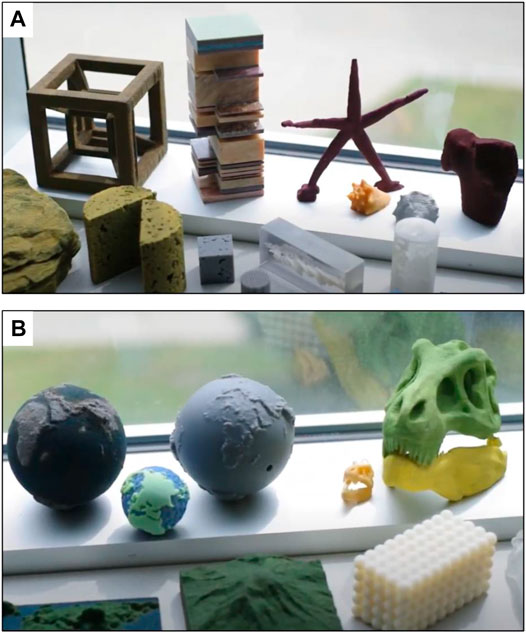
FIGURE 11 . Examples of 3D-printed models used in course exercises. (A) Fossil and rock specimens. (B) Geomorphology and porous models.
Post-course questionnaire showed that 3D printing was an efficient tool in teaching and communication geological data and hypotheses to many types of diverse audiences. This study proved that non-specialists could learn, understand, and explain scientific concepts without prior knowledge about them. This finding is important because 3D printing can be used in many university curricula where students with any background can learn sciences in any environment. In particular, tangible aspect of 3D-printed models is vital for the geoscience education where most of the data are in a 3D format. Future development of the short course will involve several examples of non-geoscience data (e.g., engineering, medicine) to challenge participants in interpretation of concepts that are far beyond their expertise. This approach will help identifying if 3D-printed models are useful in communicating more complex phenomena to non-specialist audience.
3D printing is an emerging technology in the geoscience that provides additional teaching support, enhances technical communication using visual aids, and enables repeatable experimentation in research. While the process of incorporating this technology into the regular curriculum in academic institutions may take years, short courses can help this process by improving student and faculty engagement and by developing skills for a more qualitative knowledge acquisition. The short course presented in this study was useful for a diverse group of participants including professors, students, postdoctoral fellows, and technicians from the geoscience and engineering disciplines, because it allowed them to communicate geological concepts using digital models and their tangible counterparts. Participants demonstrated that this technology allowed them having the capacity for modification and sharing digital data and supporting educators who wanted to produce teaching models without prior expertise and in a rapid manner.
While this one-day short course had five modules, participants acknowledged that the time spent on each module was adequate as the modules contained the right amount of instructions and activities. It was designed in a way that participants would create their digital model, learn about different 3D printing techniques, observe how these techniques worked live and how 3D-printed models were experimented with in the laboratory, and finally 3D print their own model and discuss its properties. It was noted by the participants that course materials, such as e-booklet and slides with instructions, helped them digesting technical information in a cohesive way.
The main objectives of the short course was fulfilled, because the majority of participants responded that they would start using 3D printing for their research, teaching, or communication. Moreover, many participants had an interest in taking an advanced short course on the applications of this technology in their respective disciplines and to recommend this short course to others. Each module can certainly be modified and adjusted according to the background of the audience. This short course can be a primer for educators willing to introduce creative modeling in their teaching schedule and prepare students for problem-solving skills using tangible models. Making testable analogs of natural phenomena for the geoscience researchers is critical and can be achieved through acquiring CAD modeling skills in this course. Besides creating visual and teaching aids, this technology is a powerful tool in communication, as shown in the short course, because the participants with diverse academic backgrounds could discuss ideas and concepts without prior knowledge about them, only using 3D-printed models.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Written informed consent was obtained from the relevant individuals for the publication of any potentially identifiable images or data included in this article.
Author Contributions
SI was the primary designer of the short course contents and the paper outline. He presented a poster at 2019 American Geophysical Union Conference on that study. SI developed exercises for the short course and prepared introduction and methods sections. KH developed presentation slides for the short course and wrote sections on results and discussion. RC was responsible for the introduction and conclusions. Figures were collected and analyzed by all authors. GZ-N was responsible for the lab tours.
The course was partially funded by MIP-CONACYT-280097 Grant, Mexico and NSERC 549236, Natural Sciences and Engineering Research Council of Canada. The funds covered the costs of 3D-printed models for participants of the short course.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the University of Alberta and Faculty of Engineering for the opportunity to host this short course on campus. Our special gratitude is to the Elko Engineering Garage for providing a demonstration tour and 3D printing the short course models. We are grateful to the Reservoir Geomechanics Research Group [RG] 2 for support in preparation of this course. We also thank NSERC for support in continuous running of GeoPRINT GeoInnovation Environment at the Department of Civil and Environmental Engineering.
Assante, D., Cennamo, G. M., and Placidi, L. (2020). “3D Printing in Education: an European Prospective,” in Proceedings from the IEEE Global Engineering Education Conference (EDUCON) . Porto: Portugal , 1133–1138.
Google Scholar
Baden, T., Chagas, A. M., Gage, G., Marzullo, T., Prieto-Godino, L. L., and Euler, T. (2015). Open Labware: 3-D Printing Your Own Lab Equipment. Plos Biol. 13, e1002086. doi:10.1371/journal.pbio.1002086
PubMed Abstract | CrossRef Full Text | Google Scholar
Boyajian, M. K., Lubner, R. J., Roussel, L. O., Crozier, J. W., Ryder, B. A., and Woo, A. S. (2020). A 3D Printed Suturing Trainer for Medical Students. Clin. Teach. 17, 1–5. doi:10.1111/tct.13176
CrossRef Full Text | Google Scholar
Buehler, E., Comrie, N., Hofmann, M., McDonald, S., and Hurst, A. (2016). Investigating the Implications of 3D Printing in Special Education. ACM Trans. Access. Comput. 8, 1–28. doi:10.1145/2870640
Chien, Y.-H., and Chu, P.-Y. (2018). The Different Learning Outcomes of High School and College Students on a 3D-Printing STEAM Engineering Design Curriculum. Int. J. Sci. Math. Educ. 16, 1047–1064. doi:10.1007/s10763-017-9832-4
Chiu, P. H. P., Chiu Lai, K. W., Fan, T. K. F., and Cheng, S. H. (2015). “A Pedagogical Model for Introducing 3D Printing Technology in a Freshman Level Course Based on a Classic Instructional Design Theory,” in Proceedings From the IEEE Frontiers In Education Conference (FIE) . El Paso, TX, USA , 1–6. doi:10.1109/FIE.2015.7344287
Dadi, G. B., Goodrum, P. M., Taylor, T. R., and Maloney, W. F. (2014). Effectiveness of Communication of Spatial Engineering Information through 3D CAD and 3D Printed Models. Vis. Eng. 2, 114. doi:10.1186/s40327-014-0009-8
Elrod, R. E. (2016). Classroom Innovation through 3D Printing. Libr. Hi Tech. News , 33 (3), 5–7. doi:10.1108/LHTN-12-2015-0085
Ford, S., and Minshall, T. (2019). Invited Review Article: Where and How 3D Printing Is Used in Teaching and Education. Addit. Manuf. 25, 131–150. doi:10.1016/j.addma.2018.10.028
Gatto, A., Bassoli, E., Denti, L., Iuliano, L., and Minetola, P. (2015). Multi-disciplinary Approach in Engineering Education: Learning with Additive Manufacturing and Reverse Engineering. Rapid Prototyping J. 21, 598–603. doi:10.1108/RPJ-09-2014-0134
Hasiuk, F., and Harding, C. (2016). Touchable Topography: 3D Printing Elevation Data and Structural Models to Overcome the Issue of Scale. Geology. Today. 32, 16–20. doi:10.1111/gto.12125
Hodder, K. J., Nychka, J. A., and Chalaturnyk, R. J. (2018). Process Limitations of 3D Printing Model Rock. Prog. Additive Manufacturing 3, 172–182. doi:10.1007/s40964-018-0042-6
Horowitz, S. S., and Schultz, P. H. (2014). Printing Space: Using 3D Printing of Digital Terrain Models in Geosciences Education and Research. J. Geosci. Education. 62 (1), 138–145. doi:10.5408/13-031.1
Hoy, M. B. (2013). 3D Printing: Making Things at the Library. Med. Ref. Serv. Q. 32 (1), 93–99. doi:10.1080/02763869.2013.749139
Ishutov, S. (2019). Establishing Framework for 3D Printing Porous Rock Models in Curable Resins. Transp Porous Med. 129, 431–448. doi:10.1007/s11242-019-01297-9
Ishutov, S., Jobe, T. D., Zhang, S., Gonzalez, M., Agar, S. M., Hasiuk, F. J., et al. (2018). Three-dimensional Printing for Geoscience: Fundamental Research, Education, and Applications for the Petroleum Industry. Bulletin. 102, 1–26. doi:10.1306/0329171621117056
Jo, W., Hee, I., J., Harianto, R. A., So, J. H., Lee, H., Ju Lee, H., et al. (2016). Instead of Seeing and Hearing, Students Can Use Their Sense of Touch to Recognize the 3D Tactile Aids, Which Might Improve Their Learning and Memory Processes. J. Vis. Impairment Blindness. 110, 115–121. doi:10.1159/000390709
Koehler, K. E., Wild, T. A., and Tikkun, S. (2018). Implications of 3-D Printing for Teaching Geoscience Concepts to Students with Visual Impairments. J. Sci. Educ. Stud. Disabil. 2, 49–81. doi:10.14448/jsesd.10.0004
Kostakis, V., Niaros, V., and Giotitsas, C. (2015). Open Source 3D Printing as a Means of Learning: An Educational experiment in Two High Schools in Greece. Telematics Inform. 32, 118–128. doi:10.1016/j.tele.2014.05.001
Malmström, H., Enger, J., Karlsteen, M., and Weidow, J. (2020). Integrating CAD, 3D-Printing Technology and Oral Communication to Enhance Students' Physics Understanding and Disciplinary Literacy. Eur. J. Phys. 41, 065708. doi:10.1088/1361-6404/aba6bd
Martin, R. K., Bowden, N. S., and Merril, C. (2014). 3D Printing in Technology and Engineering Education. Technol. Eng. Teach. 73, 30–35. doi:10.1163/9789004415133_006
Meyers, K. L., Morgan, A. S., and Conner, B. P. (2016). 3D Printing to Introduce Design in a Cornerstone Project. Glob. J. Eng. Educ. 18, 22–29. doi:10.1007/978-1-4842-0946-2_2
Pantazis, A., and Priavolou, C. (2017). 3D Printing as a Means of Learning and Communication: The 3 Ducation Project Revisited. Telematics Inform. 34, 1465–1476. doi:10.1016/j.tele.2017.06.010
Rahman, I. A., Adcock, K., and Garwood, R. J. (2012). Virtual Fossils: a New Resource for Science Communication in Paleontology. Evo Edu Outreach 5, 635–641. doi:10.1007/s12052-012-0458-2
Reggia, E., Calabro, K., and Albrecht, J. (2015). “A Scalable Instructional Method to Introduce First-Year Engineering Students to Design and Manufacturing Processes by Coupling 3D Printing with CAD Assignments,” in Proceedings of the ASEE Annual Conference & Exposition . Seattle, WA . doi:10.18260/p.23447
Roy, D., and Brine, J. (2017). “3D Printing for Multidisciplinary Education: a Technology with Diverse Potential,” in Proceedings Of the INTED2017 Conference . Valencia, Spain . doi:10.21125/inted.2017.0039
Schelly, C., Anzalone, G., Wijnen, B., and Pearce, J. M. (2015). Open-source 3-D Printing Technologies for Education: Bringing Additive Manufacturing to the Classroom. J. Vis. Languages Comput. 28, 226–237. doi:10.1016/j.jvlc.2015.01.004
Squelch, A. (2017). 3D Printing Rocks for Geo-Educational, Technical, and Hobbyist Pursuits. Geosphere 14, 360–366. doi:10.1130/GES01364.1
Szulżyk-Cieplak, J., Duda, A., and Sidor, B. (2014). 3D Printers—New Possibilities in Education. Adv. Sci. Techn. Res. J. 8, 96–101. doi:10.12913/22998624/57510.12913/22998624/568
Wohlers Report (2019). 3D Printing and Additive Manufacturing. ISBN 978-0-9913332-5-7.
Keywords: 3D printing, learning aid, visualization, reservoir, porous rock, geomodeling, fossils, geomorphology
Citation: Ishutov S, Hodder K, Chalaturnyk R and Zambrano-Narvaez G (2021) A 3D printing Short Course: A Case Study for Applications in the Geoscience Teaching and Communication for Specialists and Non-experts. Front. Earth Sci. 9:601530. doi: 10.3389/feart.2021.601530
Received: 01 September 2020; Accepted: 13 May 2021; Published: 28 May 2021.
Reviewed by:
Copyright © 2021 Ishutov, Hodder, Chalaturnyk and Zambrano-Narvaez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sergey Ishutov, [email protected]
This article is part of the Research Topic
3D Printing in Geology and Geophysics: A New World of Opportunities in Research, Outreach, and Education
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Adv Sci (Weinh)
- v.8(10); 2021 May
3D Tissue and Organ Printing—Hope and Reality
Assaf shapira.
1 Shmunis School of Biomedicine and Cancer Research, Faculty of Life Sciences, Tel Aviv University, Tel Aviv 6997801 Israel
2 Department of Materials Science and Engineering, Faculty of Engineering, Tel Aviv University, Tel Aviv 6997801 Israel
3 The Center for Nanoscience and Nanotechnology, Tel Aviv University, Tel Aviv 6997801 Israel
4 Sagol Center for Regenerative Biotechnology, Tel Aviv University, Tel Aviv 6997801 Israel
Three‐dimensional (3D) bioprinting is an emerging, groundbreaking strategy in tissue engineering, allowing the fabrication of living constructs with an unprecedented degree of complexity and accuracy. While this technique greatly facilitates the structuring of native tissue‐like architectures, many challenges still remain to be faced. In this review, the fruits of recent research that demonstrate how advanced bioprinting technologies, together with inspiring creativity, can be used to address these challenges are presented and discussed. Next, the future of the field is discussed, in terms of expected developments, as well as possible directions toward the realization of the vision of fully functional, engineered tissues, and organs. Last, a few hypothetical scenarios for the role 3D bioprinting may play in future tissue engineering are depicted, with an emphasis on its impact on tomorrow's regenerative medicine.
3D bioprinting is an emerging strategy in tissue engineering. While it greatly facilitates the structuring of tissue‐like architectures, many challenges still remain. Here, the fruits of the latest research that address these challenges are presented. The future of the field is then discussed and hypothetical scenarios for the role of 3D bioprinting in future tissue engineering and regenerative medicine are depicted.

1. Introduction
Since ancient times, humans have been fascinated by the unimaginable complexity of living creatures. The orchestrated function of multiple structures with incredible geometries ignited the imagination of our ancestors, making them raise existential questions. The invention of the microscope further enhanced this enthusiasm, revealing the existence of a new, concealed world of sophisticated, functional, tiny bio‐architectures. For medical experts and clinicians, however, these observations were accepted with an ambivalent feeling. On the one hand, they shed light on the mechanisms that support life with far‐reaching implications on medical care. On the other hand, they stressed the difficulties one may face while trying to regenerate such complicated, delicate systems. Nevertheless, the idea to artificially construct living tissues, or even whole organs, has never been abandoned, setting up the base for the rising field of tissue engineering (TE). The concept of TE is generally focused on the construction of acellular or cellularized patches that can be implanted alongside or instead of a damaged tissue, leading to regeneration of its hampered or lost function. To achieve an optimal therapeutic effect, the engineered patch is usually designed to mimic the native tissue in terms of the cellular, biochemical, mechanical, and structural features. [ 1 , 2 ] While numerous studies have demonstrated the feasibility of this concept, in the vast majority of these cases the structure of the engineered tissues is still considerably different from that of their native counterparts. This can be largely attributed to the fact that traditional fabrication methods do not provide an adequate capacity to precisely control the spatial positioning of the building materials. Moreover, while some forms of basic biostructures can be generated by spontaneous cellular organization processes, these are very difficult to control and manipulate. Given the high compositional and structural complexity of living tissues, a fabrication method capable of precisely depositing different materials and cells in pre‐defined locations in the 3D space is highly desirable. This capacity was introduced with the development of techniques for additive manufacturing (AM), commonly known as “3D printing.” [ 3 , 4 ]
The “classic” AM/3D printing process can be described as a procedure in which a 3D physical object is built, layer‐by‐layer, on the basis of data from a computer‐aided design (CAD). [ 4 , 5 , 6 ] Traditionally used for the rapid prototyping of objects made of industrial‐grade plastic, glass, metal, ceramics, etc., the technique has recently been adopted and modified for the fabrication of both acellular and cell‐containing tissue‐like structures made of biocompatible, cell‐friendly materials. This technology, termed “3D bioprinting,” has revolutionized the field of TE by taking it a step forward toward a new era in which the fabrication of complex, composite bio‐architectures is within reach. [ 7 ] Indeed, the last years have been characterized by a massive burst of intriguing research and fascinating developments in this field. 3D bioprinting techniques have been fine‐tuned and refined so that they can now be used to deposit a growing diversity of meticulously formulated biomaterials with unprecedented accuracy, without compromising on the viability of encapsulated cells. [ 8 , 9 , 10 , 11 , 12 ] The fact that 3D biofabricated tissue‐like structures share more and more features with their natural equivalents indicates the enormous potential of the technology to bring us closer to the desired goal of manufacturing functional replacement body parts. Nevertheless, there are still many challenges to overcome, some of which relate to the printing technology itself, some to the structural and supporting biomaterials, and some derive from the quality of the biostructures to be printed. In this article, we briefly discuss several of the prominent, recently published works in which innovative approaches and advanced technologies were harnessed to face some of these challenges. An emphasis is given to extrusion and photopolymerization‐based fabrication strategies that allow structuring with an exceptional degree of complexity and accuracy. [ 12 , 13 ] After a short review of the state of the art, we bring our own insights and vision for the near‐ and far‐future of 3D bioprinting and its foreseen impact on research and clinical practice.
2. Facing the Challenges
3D bioprinting techniques are based on similar principles to conventional AM approaches, for example, extrusion, inkjet, and light‐based printing (which includes stereolithography (SLA), two‐photon polymerization (2PP), and laser‐assisted printing (LAP)). These techniques, however, have undergone modifications and adaptations dictated by the nature of the building materials, incorporated cells, and working environment. [ 9 ] That is to say that the processes should be gentle enough so as not to involve any steps that expose delicate printing materials and loaded biofactors to conditions that may adversely affect their quality. Things get much more complicated, though, when living cells are present in the formulation (referred to as a “bioink” [ 14 ] ). In these cases, the process becomes even less forgiving, forcing the user to work in a very narrow range of conditions. Last, to all of these restraints is joined the challenge of performing the process under sterile conditions. While bioprinting processes are, by far, less permissive than the more common, conventional AM techniques, they are not less capable of endowing the user with extraordinary creative liberty. To realize this power and bring it into practice, however, one should take advantage of the unique capabilities of the specific working platform, while at the same time confronting its challenges. By integrating biology with excellent engineering, leading research groups have creatively used advanced, customized 3D bioprinting techniques to define the cutting edge of engineered tissues and biostructures. We have categorized these recent works according to the way they addressed three main challenges in the field: the complexity of the fabricated structure, the accuracy of the printing, and the speed of the process.
2.1. Making It Complex
Most of the work published during the earliest years of TE was based on the fabrication of homogenous, porous scaffolds with simple geometries. These scaffolds were either acellular or contained unpatterned cells. [ 15 ] While this was acceptable at the time as a proof of concept, the design of modern engineered bio‐constructs has evolved to better reflect the complex composition and architecture of native tissues. [ 16 ] A special emphasis was given to the multiplicity of biomolecules and cell types, the spatial arrangement of which is crucial for proper physiological function. An intuitive example in this regard is the human skin, where the proper function depends on a particular arrangement of distinct layers, each dominated by a specific type of cells. [ 17 , 18 , 19 ] Recent advances in mechanical and material engineering have led to the accelerated development of extrusion‐based 3D bioprinters. These can be loaded with a wide variety of materials and cells, which, when forced out through a printhead nozzle, form a continuous strand. [ 13 , 20 ] When precisely deposited in pre‐defined positions according to a meticulously planned digital design, heterogeneous, composite, tissue‐like structures can be fabricated. [ 8 ] An example of a unique method for fabricating such structures has been presented by Liu et al. [ 21 ] In this study, the authors developed a 3D bioprinter capable of fabricating structures with high compositional complexity using a single printhead. The printer, which consisted of a bundle of seven thin capillaries individually connected to unique bioink reservoirs, enabled the extrusion of multiple bioinks in a fast and continuous manner. In an impressive eye‐catching demonstration, cellular and acellular, sophisticated, planar, and 3D patterns were printed using both individual and simultaneous bioink injection modes ( Figure 1 A – G ). Importantly, the constructs were fabricated at a speed that is up to 15 times faster than that which is achieved when printing using existing nozzle‐based platforms without compromising either accuracy or cell viability. Shape fidelity was degraded to some extent, though, as a result of partial collapse of large multi‐layered structures. With printing resolution of 100–200 µm and the ability to generate gradient structures that mimic those occurring in natural tissues, this bioprinting strategy is definitely an interesting choice for complex, multimaterial 3D structuring. [ 21 ]

Printing of complex structures. Continuous multimaterial extrusion bioprinter. A) Schematic illustration of the mutimaterial printhead and a photograph of a printed microfiber. B) Human organ‐like structures bioprinted using multiple bioinks. Lower panel: C) A macroscopic image of a multicomponent heart‐like structure loaded with fluorescent microbeads and D–G) microscopic images of junction regions showing coexistence of differently pre‐labeled embedded cells. Adapted with permission. [ 21 ] Copyright 2016, Wiley‐VCH. Sacrificial writing into functional tissue (SWIFT). H) Process illustration. I) viability staining showing improved cell survival in channeled, perfused tissue (right) versus non‐channeled tissue (left). Scale bars: 500 µm. J) The left anterior descending (LAD) artery together with diagonal and septal branches were printed into septal‐anterior wall wedge of cardiac tissue matrix (right), with structural data derived from a 3D CAD model downloaded from the NIH 3D Print Exchange (left). Adapted with permission. [ 29 ] Copyright 2019, AAAS. A 3D printed vascularized proximal tubule model. K) Model design. L) Printing of several model architectures with an increasing degree of complexity (Scale bar: 10 mm). M,N) Immunofluorescence staining of a cellularized printed tissue stained for Na+/K+ ATPase (Green, in proximal tubule lined with epithelial cells), CD31 (Red, in vascular channel lined with endothelial cells) and nuclei (Blue). Scale bars: 1 mm in (M), 100 µm in inset, and in (N). Reproduced with permission. [ 31 ] Copyright 2019, National Academy of Sciences. Biofabrication of mechanically stable, human‐scale tissue constructs using integrated tissue‐organ printer (ITOP). O) Illustration of the ITOP system designed to deliver multiple cell‐laden hydrogels, supporting PCL and sacrificial Pluronic‐F127 and P) the basic patterning of a printed 3D architecture. Q) A representative 3D bioprinting process from the data acquisition stage to a fabricated, engineered tissue product. Reproduced with permission. [ 32 ] Copyright 2016, Springer Nature.
In addition to material and cell heterogeneity, another basic feature of higher organisms is the presence of a vascular system that ensures a constant supply of oxygen and nutrients and removal of waste from each and every cell in the body. As a requirement for the survival of cells in 3D structures, where the rate of diffusive transport into the core of the bulk is insufficient, vascularization has become a major aim for tissue engineers. [ 22 ] Endothelial cells, seeded in engineered tissues, can spontaneously organize into vessel‐like structures that are able to anastomize with the host. Nevertheless, this process is relatively slow and cannot keep pace with the metabolic requirements of newly implanted tissue. [ 23 ] For this reason, the strategy of generating pre‐vascularized engineered tissues that can be rapidly perfused upon completion of the fabrication process has gained popularity. The last decade has been characterized by an abundance of publications in which different concepts were applied to accomplish this goal. [ 22 , 24 , 25 , 26 ] One common fabrication strategy is to use fugitive/sacrificial materials, such as Pluronic F127, gelatin, and carbohydrates, that temporarily define and support the structure of the printed vessel network within the engineered, surrounding parenchyma. Upon completion of the fabrication process, the structure is cured while the sacrificial material is discarded. This process generates voids that can be perfused with oxygen and nutrient‐rich cell‐media throughout the whole volume of the construct. [ 27 , 28 ] A distinguished work that elegantly demonstrated such a strategy was recently published by Lewis and co‐workers. [ 29 ] In this work, the authors developed a biomanufacturing method referred to as “SWIFT” (sacrificial writing into functional tissue). At the core of this strategy, induced pluripotent stem cell (iPSC)‐derived organoids are grown and harvested to generate organ‐specific building blocks. These are then mixed with extracellular matrix (ECM) solution and compacted to yield a densely cellular, granular matrix. Next, a gelatin‐based sacrificial ink is deposited into the matrix, which embraces and stabilizes the printed pattern by virtue of its self‐healing, viscoplastic properties. Curing the matrix by incubating at 37 °C and removing the liquefied, embedded, fugitive ink then yields a channel system within the living construct. The resulting channels can then be perfused with endothelial cells that cover the inner part and form a monolayer on the lumen, recapitulating blood vessel endothelium. The researchers showed that SWIFT‐printed perfused vascularized structures resulted in a significant improvement in cell viability compared to non‐vascularized controls. As expected, the most dramatic effect was observed at the core of the constructs. The SWIFT method was then used to demonstrate the fabrication of a perfusable, engineered cardiac tissue that remained viable and beat synchronously over a 7‐day period [ 29 ] (Figure 1H – J ). A second publication from this group gave yet another example of mimicking the complex architecture of native tissue. This time, the researchers focused on modeling the proximal tubule (PT) of the kidney. By using Pluronic F127 as a fugitive ink, a PT model was fabricated, consisting of an ECM‐embedded, open lumen circumscribed by PT epithelial cells (PTECs). A perfusable tissue chip was used to house the model, providing it with physiological shear stresses. As demonstrated, the resulting 3D PTs promoted the formation of a renal tubular‐like epithelium. This cell monolayer exhibited several morphological features and functional markers akin to native PTECs, including the presence of cilia, albumin uptake, and the expression of Na + /K + ATPase, Aquaporin 1, and K cadherin. [ 30 ] In a follow‐up study, the researchers enhanced the model to also contain a second, adjacent, endothelialized open lumen that recapitulated a peritubular capillary (Figure 1K – N ). The dually perfused construct enabled the investigation of selective reabsorption of solutes via tubular–vascular exchange, akin to the native kidney tissue. This physiological‐like behavior indicates the capacity of the platform to serve as a model to study kidney function under both homeostasis and disease conditions. [ 31 ] It should be noted, however, that in the three aforementioned works, the printed fugitive ink is embedded in casted media that eventually becomes an integral part of the final construct. This may limit the construct's design, as the printer is unable to control either the composition of this component, or its geometry, which is dictated by the shape of the cast mold. In addition, a second step, post‐printing perfusion, needs to be introduced into the fabrication scheme in order to obtain cell‐lined channels.
Another layer of complexity that characterizes the tissues and organs of higher organisms is their geometry and macro‐structure. This constitutes a significant hurdle, especially for the printing of large, volumetric structures, as many materials commonly used in bioprinting are soft. The weak mechanical properties of these materials are incapable of providing adequate self‐support, at least until the constructs are fully cured. This typically results in a distorted geometry of multi‐layered constructs that may eventually collapse under their own weight. A similar problem also exists when the geometry of the structure dictates the printing of bridges (when a material is deposited on “thin air” without an underlying material layer) and/or overhangs (when an underlying material layer provides only partial support). To address this problem, several strategies have been implemented, most of which are based on the integration of some sort of permanent or temporal support for the printed structures. [ 28 ] A comprehensive work performed by Kang et al. provided an excellent example of such a strategy. [ 32 ] In this work, Pluronic F127 and poly( ε ‐caprolactone) (PCL) were used as temporal and permanent printing materials, respectively, to support the fabrication of cellular, human‐scale, tissue constructs. These materials were loaded, alongside cell‐laden composite hydrogels, into a multifunctional system denoted as an “integrated tissue‐organ printer” (ITOP). The device, equipped with multiple extrusion‐based cartridges, was used to fabricate porous, volumetric biostructures on the basis of digital data acquired by medical imaging modalities (Figure 1O – Q ). Externally supported by the fugitive Pluronic F127 and internally by PCL, structurally stable constructs of a mandible and a calvarial bone, as well as ear cartilage and skeletal muscle, were fabricated. The viability of cells inside these constructs was maintained with a constant increase in cell number over a 15‐day period. Importantly, in vivo structural robustness, host integration and tissue formation were well evident in animal‐implantation experiments. [ 32 ] Another approach to support the biofabrication of volumetric structures composed of soft materials has been proposed by Bhattacharjee et al. and Hinton et al. [ 33 , 34 ] In two innovative works, the authors demonstrated a technique in which free‐form 3D printing is performed inside non‐thixotropic, particulate gel. This is achieved by virtue of the capacity of the granular material to fluidize around the traversing writing needle and at the point of injection, while rapidly solidifying to embed the extruded material behind the moving tip ( Figure 2 A ). The transparent, granular support medium that was developed by Bhattacharjee et al. was composed of jammed, hydrogel micro‐particles made of Carbopol, a cross‐linked polyacrylic acid copolymer. Extrusion of a wide variety of soft materials into this medium enabled the fabrication of complex, hierarchical structures with features ≈100 µm in diameter (Figure 2B – D ). Moreover, living cells could be deposited and grown inside the particulate support material when prepared using growth medium as a solvent. The printed construct, which was embraced and stabilized by the support medium throughout the whole fabrication process, could be cured during or after the writing. As Carbopol cannot be liquefied or degraded by gentle, cell‐friendly treatments, extraction of the printout was performed by washing. [ 33 ] It should be taken into account, however, that this mechanical extraction step may jeopardize the integrity of delicate structures. Moreover, removal of the support from narrow or internal voids could be very challenging.

Printing of complex structures (continued). Writing inside Carbopol microgel support bath. A) Schematic representation of the principle behind printing inside a granular support medium. B) Printing of complex structures by extrusion of fluorescent microsphere suspension inside a microgel support bath. C) A continuous network of hollow vessels made of photo‐crosslinkable PVA before and D) after crosslinking and extraction from the support. Adapted with permission. [ 33 ] Copyright 2015, Published by AAAS. 3D bioprinting using freeform reversible embedding of suspended hydrogels (FRESH). E) Time‐laps sequence of printing using FRESH. F) Perfused 3D vascular network, G) tri‐leaflet heart valve and H) neonatal‐scale human heart printed from acidified collagen. The underlying digital models are shown above the pictures of the actual printed constructs. Adapted with permission. [ 35 ] Copyright 2019, AAAS. I) 3D bioprinting using pepsinized ECM‐based bioinks in particulate, alginate‐xanthan gum hybrid support media. The main panel shows an in‐process image of a printed, small‐scale cellularized human heart with major blood vessels fabricated using two bioinks. Reproduced under the terms of the CC‐BY license. [ 38 ] Copyright 2019, the Authors, Published by Wiley‐VCH. Inset: A printed, acellular coronal cross‐section of the miniaturized heart. The structures were supplemented with colored microbeads for visualization. Scale bar: 1 mm. Adapted with permission. [ 37 ] Copyright 2020, IOP.
Circumventing this difficulty, Hinton and colleagues introduced a process termed “freeform reversible embedding of suspended hydrogels” or “FRESH.” In this technique, a semi‐transparent support medium, composed of gelatin microparticle slurry, embraces the extruded material and preserves the geometry of the plotted shape. The printed construct, which undergoes curing concurrently with and/or after the completion of the writing process, can then be easily extracted by melting the granular gelatin support at a cell‐friendly temperature of 37 °C. Using the FRESH method for printing natural biopolymers, the researchers demonstrated the fabrication of complex acellular bio‐architectures such as a femur, a coronary arterial tree, a heart, and a brain. [ 34 ] In a follow‐up study, the group proved the capability of the system to support the printing of acellular heart components, ranging in scale from capillaries to a tri‐leaflet valve and finally to a full organ (Figure 2E – H ). This was performed using acid‐solubilized, high‐concentration collagen ink that cured while undergoing rapid neutralization upon extrusion into the granular gelatin support. This rapid equilibration to physiological pH was also found to allow for cells to be safely deposited, in a second step, in close proximity to the collagen component. Using a dual‐material printing process with collagen ink as the structural component and a high cell‐density bio‐ink, a contracting, cellular model of a heart's left ventricle was fabricated. [ 35 ] The FRESH technique also served as the means for the fabrication of a synchronously contracting human chambered muscle pump. This time, a photo‐crosslinkable ECM formulation containing human iPSCs (hiPSCs) was used as a bioink to print two‐chambered structures with a vessel inlet and outlet. The cells then expanded and differentiated into cardiomyocytes (CM) within the photo‐cured structure. This in situ proliferation and differentiation strategy resulted in enhanced cell density and tissue connectivity, manifested as contiguous electrical function and pump dynamics. Nevertheless, it should be noted that this approach does not allow for the generation of constructs containing more than a single cell type since all the cells in the printout are inevitably treated with the same differentiation protocol. [ 36 ]
It is worth noting that, while the ease of printout extraction is a major strength of the FRESH method, the mechanism behind it concurrently limits its application. That is to say, the heat‐sensitivity of the gelatin particles restricts the use of printing materials that require prolonged curing at elevated temperatures, such as the commonly used pepsin‐treated ECM‐derived collagen preparations. In contrast to acid‐solubilized collagen, pepsin‐treated ECM‐derived collagen remains soluble at cell‐friendly pH (and thus can be used to encapsulate living cells) and gradually undergoes physical crosslinking at body temperature.
Recently, our group presented a modified version of a support medium, specifically developed for the printing of cell‐containing, pepsin‐treated, neutral, ECM‐based bioinks. This transparent, hybrid formulation, comprised of calcium‐alginate nanoparticles and xanthan gum, is thermally stable. Thus, it allows the thermal curing of collagenous bioinks upon extended incubation at 37 °C. Extraction from the support, in this case, is performed by using a delicate treatment with alginate‐degrading enzymes, or, alternatively, by calcium chelation. [ 37 ] It should be noted, however, that these extraction procedures require the addition of external reagents. In addition, they usually take longer to accomplish than the above‐mentioned FRESH technique. Taking advantage of the high printing accuracy that can be achieved using this hybrid support medium, we were able to fabricate complex multimaterial geometries and cellular anatomical‐like structures. As a demonstration, we fabricated miniaturized cellular human hearts containing the major blood vessels (Figure 2I ). Importantly, in this study, both the cells and the ECM‐based component of the bioinks were derived from a single human omentum tissue. [ 38 ] It should be stressed that these organ‐like structures lacked internal branched vascular networks and were not tested for electromechanical function. Nevertheless, the presented capability, although still far from realization in the clinic, represents a significant step toward the 3D printing of fully personalized tissues and organs.
Overall, the described studies demonstrate the potential of innovative extrusion‐based bioprinting strategies to fabricate constructs with an exceptional degree of complexity. This potential can be attributed to the ability of these methods to accurately deliver a diversity of materials and cells to pre‐determined spatial positions, whether on top of a substrate or within a surrounding medium. However, this versatile scheme has some points of weakness stemming from the mechanism of dispensing materials through a nozzle. The first is the limited resolution that can be achieved. As a rule, a higher resolution requires the use of a finer dispensing nozzle. Unfortunately, narrowing the nozzle through which the materials pass results in the application of increased shear forces that may eventually rupture the encapsulated cells. This restricts the extrusion of bioinks to nozzles with an inner diameter of ≈150 µm, thus limiting the printing resolution of cellular constructs to approximately this value. [ 13 , 39 ] The second limitation relates to the process throughput, the effect of which is most pronounced when fabricating large objects. This limitation results from the localized nature of the material deposition mechanism, which relies on movements of the printhead and/or printer's stage for plotting the pattern of each layer. [ 13 ]
The weaknesses of this printing strategy, as well as of other fabrication schemes that will be outlined below, may be addressed by creative means and new concepts. These are discussed in the “Future Perspectives” section below.
2.2. Making It Accurate
Sophisticated geometries and micro/nano‐scale features are basic properties of biological structures. Obviously, as recapitulation of the native tissue architecture is fundamental for regenerative medicine, vast efforts have been invested in the development of accurate, ultra‐high‐resolution fabrication techniques. When it comes to sketching with high accuracy, a creator will tend to pick the finest writing implement that comes to hand. In the case of 3D printing, light is definitely the sharpest pencil in the box. SLA is a light‐assisted 3D printing method based on photopolymerization. In this method, a photo‐sensitive resin is successively cured, layer‐by‐layer, by either a point‐scanning laser beam (referred to as “direct/laser write” or “scanning SLA”) or selective exposure to a projected image plane (referred to as “projection‐based stereolithography,” PSL). [ 12 , 40 , 41 , 42 ] An inspiring demonstration of using SLA to create bio‐mimicking structures was provided by Chen and colleagues. In their research, the group generated a 3D hepatic model containing hiPSC‐derived hepatic progenitor cells cultured with supporting endothelial cells and adipose‐derived stem‐cells. [ 43 ] To recapitulate the native liver module architecture, the researchers encapsulated the cells in photopolymerizable gelatin methacrylate (GelMA) and glycidal methacrylate‐hyaluronic acid (GMHA) hydrogels. These were then used as printing substances in a rapid, two‐step fabrication process, in which complementary shapes were generated by exposure to patterned UV light. The procedure resulted in constructs that consisted of microscale hexagonal lobule units of liver cells and supporting cells ( Figure 3 A – C ) that showed improved morphological organization and higher liver‐specific gene expression in comparison to two‐dimensional (2D) or hepatic progenitor cells‐only models. Moreover, the engineered tissues exhibited enhanced metabolic product secretion and induction of cytochrome P450, a family of key enzymes in liver drug metabolism. [ 43 ] In a follow‐up study, the researchers used a similar printing technique to fabricate biomimetically patterned cellular heart and liver tissue constructs. [ 44 ] In this work, the hydrogels used for cell encapsulation were based on photo‐crosslinkable decellularized‐ECM incorporating tissue‐specific, native biochemical constituents. These materials were shown to provide the encapsulated hiPSC‐derived cells with a highly supportive environment for maturation and organization. Importantly, this was done without compromising on design complexity and printing resolution, thus allowing the fabrication of structures with 30 µm features. [ 44 ] Overall, these meticulously engineered tissues are definitely a step forward toward the development of improved, physiologically relevant in vitro models for disease studies, personalized medicine, and drug screening. It should be noted, though, that the above‐mentioned cellular constructs were not designed as thick, multilayered structures. Rather, they were built as low‐profile microarchitectures with a width and length of 3 mm and a thickness of only 250 µm. In other words, while the cells indeed experienced a true 3D environment, the macrostructure was more like that of a thin sheet.

High‐accuracy printing. 3D bioprinted hepatic construct. A) Illustration of the two‐step, projection‐based stereolithography approach in which B) sequential exposure to two complementary shapes of patterned UV light resulted in C) liver lobule‐like structures containing hepatic cells (green) and supporting cells (red). Scale bars: 500 µm. Reproduced with permission. [ 43 ] Copyright 2016, PNAS. Fabrication of complex, vascular architectures in biocompatible hydrogels. D) Schematic representation of a 3D printing process based on projection stereolithography. E) Perfused, entangled vascular networks printed within hydrogels. Scale bars: 3 mm. F) A scheme of a distal lung subunit (left), an actual printed structure during red‐blood cells (RBCs) perfusion and tidal ventilation (center), and a graph showing the RBC sensitivity to ventilation gas (right). Scale bar: 1 mm. Adapted with permission. [ 45 ] Copyright 2019, AAAS. G) The two‐photon polymerization (2PP) fabrication method. A focused infrared or near‐infrared light is emitted from a femtosecond laser into a volume of photo‐crosslinkable substance to induce polymerization only at the focal point. Adapted with permission. [ 46 ] Copyright 2018, Royal Society of Chemistry. 2PP‐fabricated retinal cell grafts. H) A scanning electron microscope image showing three scaffolds surrounded by a retaining wall. Each scaffold presents a different vertical pore size (25, 20, or 15 µm) and a horizontal pore size of 7 µm. I) A fluorescence image of a scaffold containing 25 µm vertical pores loaded with retinal progenitor cells (red). The bottom panel provides a side view, showing that the cells formed neuronal processes that extended into and aligned with the vertical pores. Adapted with permission. [ 48 ] Copyright 2017, Elsevier. Generation of 3D cell networks using 2PP‐fabricated microcage‐containing scaffolds. J) The concept of micro‐scaffolds for confined cell growth. Blocks of complementary, half‐cell cages in the shape of truncated octahedrons are designed and printed. Cells are then seeded and grown inside the hemispherical containers, followed by stacking the cellular structures one on top of the other. K,L) Scanning electron microscopy image of a tri‐layer stack, with neurites projecting from the cages (red arrows) to establish connections between neighboring confined PC12 cells. Adapted with permission. [ 50 ] Copyright 2019, The Royal Society of Chemistry.
A different approach for harnessing the power of SLA to accurately fabricate sophisticated geometries was presented by Grigoryan et al. [ 45 ] In a colorful article, the researchers developed a modified PSL scheme capable of printing at a high resolution of 50 µm. The fabrication technique was initially utilized to produce poly(ethylene glycol) diacrylate (PEGDA) hydrogels containing intricate vascular architectures with functional internal topologies such as mixers and valves. Next, it served to explore the oxygenation and flow of human red blood cells (RBCs) during tidal ventilation. To this end, the authors developed a bioinspired alveolar model, in which RBCs were perfused through ensheathing vasculature that closely tracks the curvature of 3D airway topography. Tidal ventilation with oxygen caused a distention of the airway upon inflation, leading to the compression of adjacent blood vessels and the redirection of fluid streams to neighboring vessel segments. Furthermore, the perfused RBCs were found to respond to the ventilation. Expectedly, they presented significantly higher oxygen partial pressure and saturation relative to deoxygenated RBCs that were loaded at the inlet, or ventilated with nitrogen gas (Figure 3D – F ). Next, the authors used their customized printing scheme to fabricate cellular structures made of PEGDA:GelMA‐based bioinks. To this end, lung‐mimetic architectures were populated with human lung fibroblasts in the bulk of the interstitial space, and human epithelial‐like cells were attached to the airway lumen. In another demonstration, human mesenchymal stem cells within fabricated hydrogels were found to maintain high viability for 24 h. The cells also showed osteogenic differentiation as a function of soluble factor delivery via vascular perfusion. Last, implantation experiments were performed in mice, demonstrating the in vivo survival and activity of engineered cellular hepatic tissues with an incorporated perfusable vasculature. [ 45 ] The unprecedented degree of geometrical intricacy achieved by this rapid, precise, and cell‐friendly process, constitutes a significant milestone in the production of functional, vascularized, bio‐mimicking constructs. This advance may constitute the basis for the development of more accurate and physiologically relevant tissue models, accelerating progress in biomedical and pharmacological research. Limited compositional complexity, however, is still a major downside of this printing scheme, as will be elaborated further on.
While SLA is a preferred technique for printing accurate constructs at microscale resolution, it is by far the only strategy that is commonly used for the precise fabrication of sub‐micrometer features. This can be optimally achieved by virtue of a distinct type of laser‐based direct writing system: the highly precise two‐photon polymerization (TPP/2PP) method. In this method, characterized by a spatial resolution of down to 100 nm, a focused infrared or near‐infrared light is emitted from a femtosecond laser to induce polymerization inside a volume of photo‐crosslinkable substance. As the photon density required for polymerization is reached only at the focal point, a defined 3D structure can be patterned by moving the beam focus and/or the photo‐reactive material in the X , Y , and Z axes [ 46 , 47 ] (Figure 3G ). Worthington et al. described a photoreceptor cell replacement concept for the treatment of retinal degenerative blindness using 2PP‐fabricated retinal cell grafts. [ 48 ] The group used 2PP to recapitulate the fine natural structure of the outer retina, in which photoreceptor cells are tightly packed and aligned parallel to the light path. Using this precise fabrication method, non‐degradable 3D scaffolds with closely packed vertical pores 25 µm in diameter were fabricated. Interconnected, 7 µm horizontal pores were introduced to these 1 mm‐wide and 120 µm‐high structures in order to facilitate the diffusion of nutrients and oxygen. hiPSC‐derived retinal progenitor cells were then loaded into the scaffolds, forming neuronal processes that extended into and aligned with the vertical pores. Cell bodies were also found to populate the structure's columns, with the latter providing them with a proper vertical guidance [ 48 ] (Figure 3H , ,I). I ). The design of these constructs constituted the basis for a follow‐up study in which degradable, biocompatible, two‐photon polymerized PCL‐based scaffolds were fabricated. No inflammation, pyrogenicity, or other local or systemic toxicities were observed following sub‐retinal implantation of cell‐free scaffolds, indicating their future potential in the treatment of retinal degenerative diseases. [ 49 ]
The ultra‐high‐resolution capacity of 2PP has also been utilized for structuring stackable micro‐scaffolds comprised of synthetic photoresist. These scaffolds were engineered to allow confined cell growth in a specific, pre‐determined spatial organization. In these constructs, developed by Larramendy et al., blocks of complementary, half‐cell cages in the shape of truncated octahedrons were designed as stackable structural layers. [ 50 ] Neuron‐like PC12 cells were then seeded and grown inside the hemispherical containers, followed by stacking the cellular structures one on top of the other. As the 50 µm‐diameter containers were designed as cages that restrain the cell bodies, cell‐to‐cell connections could only be realized between neurites. Indeed, neurites were found to project from the hexagonal openings of the cages and interact with those of neighboring cells, a first step toward the establishment of a 3D neuronal network. Such a technique thus holds potential for applications in which the formation of controlled, 3D cellular networks is desirable [ 50 ] (Figure 3J – L ).
While the exceptional capabilities of SLA in terms of accuracy and resolution are unquestioned, this printing strategy suffers from several weaknesses that deserve attention. In extrusion and inkjet‐based printing, for example, the materials of choice are selectively deposited at specific spatial locations. In contrast, SLA is traditionally based on the selective curing of a single, homogenous photoreactive material. This significantly limits the applicability of this method to the fabrication of structures with low‐compositional complexity, that is, a single bioink. As demonstrated, multicomponent structures can be fabricated by manually exchanging the photoreactive material between projections. [ 43 ] However, such non‐continuous fabrication processes can be tedious, slow, and inaccurate. Another consideration that needs to be accounted for, especially in the context of 2PP, is the process throughput. The highly confined region of polymerization, which endows this fabrication method with its phenomenal accuracy, also imposes an extended process duration. This limits 2PP‐fabricated structures to the millimeter range, and even in this scale, fabrication can require days to accomplish. [ 51 , 52 , 53 ] Recent works, however, indicate a trend toward the development of faster 2PP printing platforms, as discussed below.
2.3. Making It Fast
As mentioned above, the presence of living cells constitutes a limiting factor in bioprinting, profoundly narrowing the range of compatible materials and fabrication conditions. Moreover, as a rule, the conditions required to support long‐term cell viability cannot be optimally maintained during printing. For this reason, the cells need to be transferred as fast as possible to an environment that supports their metabolic demands, with a replenishing supply of oxygen and nutrients. Printing time, which is derived from the printing resolution, printout composition, object size, and fabrication technique, is thus a critical parameter that may directly impact the fate of the incorporated cells. The significance of printing duration is especially prominent in the generation of large, volumetric constructs composed of numerous thin layers. Projection‐based stereolithography presents a huge advantage over direct‐write SLA and extrusion‐based printing, as it enables fabrication in a layer‐at‐once fashion instead of tracing a set of coordinates for each layer. [ 12 , 40 , 54 ] While using this strategy spares a considerable amount of processing time, it is still based on the conventional approach of consecutive material layering.
Recently, a new paradigm in photopolymer‐based additive fabrication has been proposed by Spadaccini and colleagues, enabling the fabrication of 3D geometries on a time scale of seconds. [ 55 ] This incredible processing speed is achieved by the superposition of patterned optical fields from multiple beams, projected at orthogonal directions into a photo‐sensitive resin. The region in which the beams intersect defines the object's geometry, where the energy of the absorbed light overcomes a curing threshold. Using this unique holographic patterning system, a variety of 3D shapes made of PEGDA have been fabricated by a single light exposure of up to 10 s ( Figure 4 A – F ). These structures, however, were limited in their geometry due to the prismatic nature of the overlapping beams. [ 55 ] To overcome these limitations, another novel approach denoted as “computed axial lithography” (CAL), has been developed. This technique, pioneered by Taylor and co‐workers, enables ultra‐fast printing of large and geometrically complex objects within a matter of seconds. [ 56 ] This was achieved by using an innovative volumetric printing approach inspired by the image reconstruction procedures of computed tomography. The method is based on the concurrent printing of all points within a given 3D geometry by projecting a set of 2D images through a rotating tank containing a photo‐sensitive resin. The superposition of exposures from multiple rotational angles eventually reaches an energy dose that is sufficient for curing the geometry of choice. The non‐crosslinked photo‐sensitive resin is then washed away, leaving behind a solid 3D printout (Figure 4G , ,H). H ). In addition to speed, this unique “volume‐at‐once” type of fabrication also offers several advantages over layer‐based printing. First, the fabricated objects present a smooth surface and are devoid of anisotrophic mechanical performance that may result from material layering. Second, the technique can be applied on high‐viscosity fluids and even on solids, such that the cured structure remains embedded in the surrounding material with minimal relative motion between the two. This eliminates the need to support the structure during the fabrication process, enabling the printing of bridges, overhanging elements, and disconnected parts. Third, the technique allows printing around preexisting objects, enabling the incorporation of external elements into the fabricated construct. Using this technique, the group demonstrated the fabrication of a large array of geometries and centimeter‐scale objects made of acrylate polymers and GelMA. The structures, containing features as small as 300 µm, were fabricated in an extremely short time frame of 30–300 s. [ 56 ] A later publication by Loterie et al. demonstrated tomographic volumetric printing of acrylic and silicone parts with improved geometric fidelity. This was achieved by using an optimized projection source with an integrated feedback system, allowing high‐resolution fabrication of centimeter‐scale objects with features as thin as 80 µm in less than 30 s. [ 57 ] In an intriguing study, Bernal et al. demonstrated the use of such volumetric printing techniques to biofabricate structures that are difficult to reproduce through conventional AM processes [ 58 ] (Figure 4I – K ). Using GelMA as a photocurable resin, the group printed a fluidic ball‐cage valve with free‐floating elements and a human auricle model, both of which were fabricated in less than 23 s. To prove the biocompatibility of the system, an anatomical trabecular bone model loaded with mesenchymal stromal cells was generated. This living construct, which contained an interconnected porous network, was reproduced with the smallest resolved feature measuring ≈145 µm. Printing speed was extremely high, with less than 13 s being required to complete the fabrication of an 8.5 × 9.3 mm structure. High cell‐viability of more than 85% was maintained throughout a 7‐day period, during which the culture was primed with osteogenic medium so as to mimic the osteoblast population within native bone. Vascular endothelial cells were then introduced into the pore network of the structure, leading to the formation of early angiogenic sprouts that began to invade the bone compartment of the construct. Finally, to assess the capacity of printed cells to synthesize new tissue matrix, the researchers fabricated a meniscus‐shaped implant in which articular chondroprogenitor cells were encapsulated at a density of 10 7 cells mL −1 . The cells, which exhibited high long‐term viability and metabolic activity, were found to synthesize neo‐ECM. This newly synthesized matrix increased the compressive modulus of the graft from ≈15 to ≈265 kPa, comparable to native human fibrocartilage. [ 58 ]

High‐speed volumetric printing. Holographic 3D fabrication. A) Prism mirrors direct beams at orthogonal directions into a photo‐sensitive resin that B) is consequently cured at the region of intersection. This results in generation of 3D shapes C–F) by a single short exposure of up to 10 s. Scale bars: 2 mm. Adapted with permission. [ 55 ] Copyright 2017, AAAS. Computed axial lithography (CAL). G) Graphical illustration of the CAL approach. A set of 2D images is projected through a rotating tank filled with photo‐sensitive material. The superposition of exposures from multiple rotational angles eventually reaches an energy dose that is sufficient for curing the geometry of choice. H) The printed object, generated in less than 1 min, after extraction from the uncured material. A sequential view of the process is presented at the bottom. Scale bars: 10 mm. Adapted with permission. [ 56 ] Copyright 2019, AAAS. Tomographic volumetric bioprinting. I) A cell‐laden biocompatible resin in a rotating tank is J) projected by 2D light patterns from multiple rotational angles. K) The resin then solidifies in selected areas where the accumulative absorbed dose overcomes a gelation threshold (Main: structure rendering. Inset: the actual printed structure). Scale bar: 2 mm. Reproduced with permission. [ 58 ] Copyright 2019, Wiley‐VCH.
Altogether, these innovative volumetric printing schemes, which allow the fabrication of large, geometrically complex structures at unimaginably high speeds, are nothing less than game changers. Importantly, the ability to generate such constructs with densely packed, viable cells is an important milestone and a significant breakthrough in TE. Without a doubt, this technology is expected to play a central role in modern biofabrication, with far‐reaching implications on future developments and applications. It shares, however, a major drawback with the other above‐mentioned photopolymerization‐based printing techniques. Namely, as volumetric printing is based on the selective curing of a single type, homogenous, pre‐casted material, the printed construct inevitably presents low compositional complexity.
3. Future Perspectives
TE has taken enormous steps forward in recent years, with the latest advances in biofabrication techniques being a major driving force. The progress that has been made and the innovations described above address important aspects and bottlenecks in the field, speeding up its evolution. They also, however, reveal new complications to be overcome and further raise the bar for future developments. In the sections below we discuss potential directions for progress in the 3D bioprinting domain. An outlook on the impact of this emerging discipline on next‐generation research and medicine is also brought and discussed.
3.1. What Is in the Pipeline?
Obviously, current biofabrication protocols are far from providing the capacity to generate transplantable, functional, complex tissues and organs. From a technical point of view, this may result, in part, from the fact that each fabrication method is characterized by an inherent set of strengths and weaknesses. That is to say, a technique that excels in fabricating specific types of materials and structures will probably give sub‐optimal results for different kinds of compositions and geometries. As discussed, tissues and organs are generally composed of an assortment of cells, materials, and architectures. Thus, low efficiency and/or reduced performance and building quality are to be expected during the fabrication of some elements of the final printout. With this in mind, it is reasonable to expect future 3D bioprinting developments in which attempts will be made to broaden the applicability of existing fabrication protocols. Indeed, scientists have already begun to develop modified printing schemes that compensate, to some extent, for the inherent shortcomings that characterize their underlying working principles. For example, stereolithographic bioprinting can give excellent results in terms of accuracy. However, as mentioned, it usually yields constructs that are made of a single bioink. To address this limitation, the printing device may be re‐configured to enable easy and rapid in‐process exchange of the photocurable resin. Such a configuration has been proposed by Khademhosseini and colleagues, who developed a stereolithographic bioprinting platform with an integrated microfluidics device. The novel system enables projection‐based printing with the option to quickly and efficiently switch between different bioinks during the process. Using this automated system, multimaterial and multicellular microstructures and biomimetic heterogeneous tissue constructs were continuously fabricated, at high‐resolution, within a time‐frame of seconds [ 59 ] ( Figure 5 A – D ). In a later work, Mayer et al. demonstrated the use of a microfluidics system integrated into a 2PP‐based laser lithography apparatus. Using this setup, the authors printed multimaterial, fluorescent, 3D security features based on four emission colors. While this research did not assess the functionality of the system for working with biomaterials and cells, it elegantly proved that integration with microfluidic systems can also greatly increase the complexity of 2PP‐printed structures. [ 60 ]

Emerging concepts. A stereolithographic 3D bioprinting platform with an integrated microfluidics device designed for fabrication of multimaterial and multicellular microstructures. A) Illustration of the setup. B) Operation of the microfluidics device that enables quick switching between different bioinks with intermediate washing steps. C) Schematics of the cyclic, 4‐steps bioprinting process inside the microfluidics chip. D) A single component and a three‐component structure made of PEGDA. Adapted with permission. [ 59 ] 2018, Wiley‐VCH. Multimaterial, multinozzle 3D printing of voxelated matter. E) Four‐material printheads with a single nozzle, F) four nozzles at a 1 × 4 1D setup, and G) 16 nozzles at a 4 × 4 2D setup. H) Voxalated matter is extruded from a four‐material, 2D printhead with 4 × 4 nozzle setup. Inset: Operation of a two‐material nozzle that produces a continuous voxelated filament at different material switching frequencies. Adapted with permission. [ 62 ] Copyright 2019, Springer Nature. 4D bioprinting of shape‐transforming structures. I) Layers of printed acellular or cell‐containing shape‐morphing hydrogels J) undergo photo‐crosslinking and mild drying and K,L) instantly fold into tubes upon immersion in aqueous media. Reproduced with permission. [ 66 ] Copyright 2017, Wiley‐VCH. Bioprinting‐assisted tissue emergence (BATE). M) Illustration of the BATE concept. The fabrication process is based on deposition of high‐density cell suspensions into liquid precursors of ECM hydrogels that facilitate effective cellular self‐organization into macrostructures. N) Tube evolution of BATE‐printed intestinal tissue with lumen and budding structures formed at day 6 and crypts at day 9. Scale bars: 200 µm. Adapted with permission. [ 70 ] Copyright 2020, Springer Nature. Endoscopic additive manufacturing. O,P) Illustration of the intracorporeal TE concept in which 3D printing is performed on the patient's internal organs by minimally invasive procedures using miniaturized printing platforms. Adapted with permission. [ 74 ] Copyright 2020, IOP. Q–S) A microbioprinting platform can be installed on an endoscope to treat gastric wall injuries. Scale bar: 1 cm. Adapted with permission. [ 75 ] Copyright 2020, IOP. T–W) Printed stackable microcage modules for manual assembly. Printed rigid stackable microcage scaffolds with 1 × 1, 2 × 2, and 4 × 4 designs can be manually assembled and scaled to adopt a desired geometry. Additionally, each microcage can be loaded with a cargo of choice, such as cells and/or therapeutics (demonstrated in (W) using fluorescent microgels). Scale bars: 1.5 mm. Adapted with permission. [ 79 ] Copyright 2020, Wiley‐VCH.
As with compositional complexity, improvements in printing speed can also dramatically broaden the applicability of fabrication methods that do not excel in terms of throughput. For instance, the production rate of the accurate (yet slow) 2PP method can be greatly enhanced if polymerization is executed in a layer‐by‐layer, instead of point‐by‐point, fashion. This concept was realized in a work conducted by Saha et al. [ 61 ] In this study, the performance of a novel parallel process, based on femtosecond projection, was compared to the commonly implemented point‐by‐point writing scheme. Using layer‐by‐layer projection of digital masks, the group succeeded in increasing the throughput up to three orders of magnitude compared to that achieved by existing serial techniques. Importantly, the improved printing rate, reaching 8.7 mm 3 h −1 , was attained without compromising the characteristic 2PP sub‐micrometer resolution. [ 61 ]
In addition to 2PP printing techniques, extrusion‐based fabrication procedures would benefit from improved process throughput, especially when applied to the construction of large objects. This can be achieved, for example, by parallelizing several multimaterial deposition processes. An intriguing approach in this direction was presented in a recent study by Lewis and colleagues. [ 62 ] The group developed a unique setup in which a single printhead is capable of depositing up to eight different materials (modeled in this work by silicone, wax, epoxy, and gelatin‐based inks). The different materials flow through independent channels that eventually merge into a single ink flow, immediately before the nozzle outlet. High‐frequency switching between the printing materials allows extrusion of filaments composed of distinct volume elements (voxels) along their length. When adjacently deposited, in a layer‐by‐layer manner, a multimaterial 3D structure is formed, with a voxel volume approaching that of the nozzle diameter cubed. The printing heads can also be designed to contain multiple nozzles as a 1D array (e.g., 4 nozzles in a 1 × 4 setup) or 2D array (e.g., 16 nozzles in a 4 × 4 setup) (Figure 5E – H ). This multimaterial, multinozzle design thus considerably boosts printing throughput, not only by avoiding the need for an individual printhead for each material, but also by parallelizing the fabrication process. To demonstrate the performance of this setup, a soft robotic walker equipped with sixteen 12 mm x 12 mm x 17 mm actuators was printed within 17 min using stiff and flexible silicone inks. [ 62 ]
Another strategy for speeding up extrusion‐based fabrication processes may be based on our vision of an “inside‐out” printing scheme. In this hypothetical mechanism, the object is simultaneously fabricated by multiple three‐axis controllable dispensing tips that follow distinct, non‐intersecting paths. In contrast to the canonical printing scheme, the fabrication begins from the core of the object and continues, in a layer‐by‐layer fashion, toward its periphery. This process is theoretically feasible due to the presence of a support medium that envelops the extruded material and holds it in place, simulating printing in a zero‐gravity environment. By printing inside a support bath that is considerably larger than the printout, each dispensing needle can approach the object from a different angle, including from the bottom. In this way, the fabrication time of large, volumetric structures could be considerably reduced as a function of the number of simultaneously operated dispensing tips.
While boosting the processing speed is highly advantageous, the major limitation of extrusion‐based 3D fabrication is the printing resolution. As discussed above, the intuitive strategy of decreasing the diameter of the dispensing tip is limited due to the increasing shear stress, to which the cells will eventually succumb. Thus, in this case, alternative, out‐of‐the‐box thinking is highly desired. An interesting approach would be to use “smart materials” as inks for the fabrication of structures that can transform their shape in response to stimuli. Such a technique, denoted “4D printing,” could be utilized for the fabrication of structures with an attainable resolution using a standard extrusion‐based printer. Upon stimulation, however, the printout would undergo a structural transformation to attain dimensions that are beyond the building capability of the underlying fabrication method. [ 6 , 63 , 64 , 65 ] A proof for the feasibility of this approach was provided by Kirillova et al., who used photo‐crosslinkable methacrylated alginate and hyaluronic acid as shape‐morphing hydrogels. [ 66 ] The materials were loaded with cells and used as bioinks for the extrusion‐based printing of 2D, rectangular shapes. Following photo‐crosslinking at 530 nm, mild drying, and immersion in aqueous media, the printed layers instantly folded into tubes with an internal diameter of as low as 20 µm (Figure 5I – L ). This value is on the scale of the internal diameters of the smallest blood vessels, the geometries of which are extremely challenging to reproduce using existing extrusion‐based printing techniques. Notably, neither the printing process nor the post‐printing treatment adversely affected the cells that survived for at least 7 days without any decrease in their viability. [ 66 ]
Another strategy for overcoming the limitations of using a particular fabrication technique is to synergistically combine several complimentary printing schemes into a single platform, whereby the strengths of one cover for the weaknesses of the other. An intriguing example of the implementation of such a strategy has been presented by Shanjani et al. [ 67 ] In this work, PSL and extrusion‐based printing techniques were combined for the fabrication of complex, multimaterial cellular constructs. The structures were composed of extruded, thermoplastic PCL that formed a porous, rigid scaffold, combined with soft, photo‐crosslinkable PEGDA hydrogel that contained living endothelial cells and mesenchymal stem cells. The fabrication was based on a repeating process in which strands of molten PCL were deposited on the build platform, followed by immersion into the pre‐polymer solution and photo‐curing of the regions that needed to be gelled. Using this scheme, various complex designs were generated, including cellular scaffolds with integrated perfusable conduits. [ 67 ] For more information and insights on such multi‐technological, hybrid fabrication methods, we recommend the readers to peruse these two recently published articles. [ 68 , 69 ]
Aside from improving established printing methods, or combining them into integrated platforms, the future of the field also depends on the development of new 3D biofabrication techniques. While not in the scope of this review, it is worth mentioning that the last several years have been characterized by the emergence of a variety of innovative printing schemes and concepts. These include, among others, procedures that involve magnetic and acoustic‐based printing, electrohydrodynamic processing, and new methods for the 3D patterning of spheroids/organoids. Most of these techniques are still in their infancy and require further development and tuning. Nevertheless, a taste of their performance can already be obtained from recently published works. [ 9 , 68 , 69 ] An intriguing example of such a technique was recently presented by Lotolf and colleagues. [ 70 ] In their work, organoid‐forming stem cells were used as building blocks that can spatially self‐arrange according to a pre‐defined geometry. The process was based on the deposition of high‐density cell suspensions into liquid precursors of ECM hydrogels that facilitated effective cellular self‐organization. Using this approach, termed bioprinting‐assisted tissue emergence, centimeter‐scale epithelial, connective, and vascular tissues were fabricated. Importantly, the printed biostructures were characterized by native‐like features such as lumens, crypts, and branches and responded to chemical stimuli, indicating their high physiological relevance [ 70 ] (Figure 5M , ,N N ).
Also worth mentioning is the evolving approach of patient‐specific in situ 3D printing, in which constructs are printed, in vivo, directly at the target site. [ 71 , 72 , 73 ] A subset of this approach, the newly emerged concept of intracorporeal 3D printing, or endoscopic AM, is performed by minimally invasive procedures using miniaturized printing platforms [ 74 , 75 ] (Figure 5O – S ). Either way, as the constructs are fabricated on or inside the patient's body, which serves as a living bioreactor, there is no need for an in vitro maturation phase. Another approach that targets a clinical need is the production of “off‐the‐shelf” tissue substitutes. At the heart of this concept is the ambition to provide clinicians with a pool of available, readily transplantable, pre‐prepared, engineered body parts. The advantage of this approach is clear: malfunctioning tissue might be repaired or replaced without going through tedious preliminary design and manufacturing processes. One of the major obstacles in this concept, however, is the limited capacity to personalize the pre‐prepared tissue so that it matches the patient, both structurally and immunologically. Currently, resolving the problem of immune rejection of cell‐containing implants requires complicated procedures (i.e., cellularization of the implant with patient‐derived cells, [ 76 ] Human Leukocyte Antigens (HLA) matched [ 77 ] or engineered, “universal,” hypoimmunogenic cells [ 78 ] ). The process of structural matching, on the other hand, could be significantly simplified. This could be done, for example, by enabling the clinician to produce patient‐specific geometries from pre‐printed building blocks without the need for special equipment or long training. Such an approach was elegantly demonstrated by Subbiah et al. [ 79 ] The group used lithography‐based 3D printing to construct a microcage scaffold assembly system for regeneration of hard tissues. The rigid, miniaturized, stackable microcage modules could be manually assembled and scaled by the user to generate the required geometry. Moreover, as each module is amenable to loading with a cargo of choice, cells and therapeutic agents could be patterned in 3D within the composed construct [ 79 ] (Figure 5T – W ).
Finally, it should be pointed out that the described progress and future advances should go hand in hand with the continuous improvement of printing materials, design tools, process algorithms, and post‐printing culturing and maturation techniques. While not thoroughly discussed in this review, it must be remembered that these elements are inseparable from the printing process. Information on the latest advances in these important disciplines can be found in recent reviews. [ 8 , 80 , 81 , 82 , 83 , 84 ]
A summary table that presents some of the key features of the printing methods covered in this review can be found below ( Table 1 ).
Key features of the printing methods covered in this review
3.2. The Future of Printed Tissues and Organs—At the Crossroad of Reality
So, what should we expect to see in the near and far future? What will be the impact of the evolving 3D bioprinting field on modern healthcare, biotechnology, and academic research? In this section, we try to depict three hypothetical scenarios. Reality will most probably navigate its way somewhere in between.
The first is an ideal scenario for tissue engineers and is governed by technology and know‐how. That is to say, progress in the 3D biofabrication field will be dictated mainly by our capacity to build more advanced printing machines, formulate improved bioinks, and efficiently expand cells and culture the printed structures. In this scenario, the basic assumption is that biology will not pose an obstacle that cannot eventually be overcome on the journey toward engineered functional tissues and organs. Contrariwise, given a precise spatial positioning of the proper cells in meticulously formulated materials and under specific controlled conditions, the printed living components will organize and mature to form the desired structures. This does not mean that the cellular component of the engineered tissues will not require special preparation, guidance, and care. Rather, the biological knowledge that will be gained as the field evolves will suffice to fuel the progress. Under these hypothetical conditions, it is not too ambitious to assume that our ability to 3D fabricate basic, physiologically functional biostructures will mature in the foreseen future. Such a capacity will enable the production of the core constituents of animal and human tissues to a level at which most, or almost all, of the functionality of the native components is mimicked by the printed counterparts. Obviously, the progress must be accompanied by the development of advanced bioreactors and supporting accessories that enable controlled, long‐term cultivation of the living constructs. Such achievements will boost biological research, facilitating a much deeper investigation of the molecular, developmental, and physiological processes that are at the heart of life. They are also expected to revolutionize the fields of pharmacology and drug screening that currently rely on less reliable models such as 2D cell cultures, organ‐on‐a‐chip models, 3D non‐vascularized cellular constructs, and animals. Successful fabrication of 3D hierarchical tissue structures containing heterogeneous cell populations and supportive vasculature will gradually trigger attempts to use them for regenerative purposes. Animal models will first be used to prove the capacity of engineered tissues seeded with autologous cells to integrate into the host and to maintain long‐term activity. Follow‐up experiments will then be conducted to test whether a printed implant can regain the functionality of a defective tissue, or at least compensate, to some extent, for the loss of its activity. An array of integrated microsensors and actuators may be used to provide these crucial data, together with an indication of the tissue's activity and physiological state during maturation and post‐implantation. Such an integrated electronic system will work in a bi‐directional way, also allowing on‐demand intervention by electrical excitation or release of active compounds into the implant's surroundings. [ 85 ] After confirming a therapeutic benefit in animal models, a race toward the development of clinical applications will start. First, cooperation will be established between, on one side, academia and the biotechnological industry and, on the other side, healthcare providers and hospitals. The latter will then set up their own bioprinting centers in which the whole process will take place. A typical procedure might begin with the harvest of cells and/or biomaterials from the patient, followed by their being processed into bioinks. Alternative sources of immune‐compatible cells, such as iPSC banks or “universal” iPSC lines, can also be used. [ 76 , 77 , 78 ] In the next step, the engineered tissue will be fabricated on the basis of data extracted from 3D imaging of the patient's own anatomy, or from a generic model that will undergo personalized adaptations. When the integral sensory system and additional complementary assays indicate the maturation of the tissue, transplantation will be performed. The patient will then be continuously monitored by the healthcare provider with the assistance of wireless communication between the integrated electronics and an extracorporeal receiver. From this stage, the next significant step will be toward a higher level in the hierarchy, which is the level of the organ. Since constructs' volumes will greatly increase in the 3D bioprinting of full‐size human organs, the integration of ultra‐fast fabrication techniques may be required. Nevertheless, as speed will probably still come at the expense of printing resolution and complexity, such methods should be used in combination with other complementary, more accurate fabrication procedures. A representative scheme may be based on a hybrid platform in which an organ's parenchyma is fabricated at high speed around accurately pre‐printed organ‐specific microstructures and branched vascular system. After printing, the engineered organs will be connected to computer‐guided bioreactors that will constantly monitor their culturing environment and physiological status. The recorded data will be processed to generate a feedback loop that ensures a proper supply of oxygen, nutrients, essential biofactors, and external stimuli to the living organ. When the organ is functional and fully mature, it will be transplanted instead of, or in parallel to, its faulty natural counterpart, to regenerate function. Optionally, as discussed above, the engineered organs may be designed to maintain reciprocal communication with a medical specialist by virtue of integrated arrays of sensors and actuators. The integrated electronics may also be controlled by an internal feedback loop that can automatically intervene in the transplant's activity in cases of rapidly emerging, life‐threatening complications.
While the scenario depicts an optimal outcome, it presumably will not be realized in the near future. This is due to the long list of associated biological and technological challenges that will probably require prolonged research and development. An example of such a challenge is the current absence of efficient cell expansion techniques. The human adult heart, for instance, contains ≈4 billion muscle cells (CM). Hence, a huge number of these cells first needs to be attained in order to print a full size, transplantable, cellular organ. As adult human CM exhibit a very limited self‐renewal capacity, an enormous population of patient‐specific stem cells must first be established and differentiate accordingly. This requires execution of complicated procedures for attaining a highly pure CM culture with the proper phenotype. Unfortunately, these procedures, in their current form, are particularly costly and very demanding for scaling up. [ 86 , 87 , 88 , 89 , 90 ] Another challenge that has largely stayed out of focus, is the innervation of engineered tissues and organs. While not essential for tissue organization and survival, its role in organ development, functionality, and regeneration is increasingly being recognized. Addressing this issue adds another layer of complexity that may require expanding both knowledge and laboratory practice. [ 91 ] A wide perspective on the challenges presented by whole organ bioprinting and future directions for the field can be found in a recent comprehensive review. [ 92 ]
In the next hypothetical scenario, biology is much less cooperative. Referred to here as the “glass ceiling” scenario, it depicts a situation in which most of the complex engineered cellular constructs will not reach an adequate level of functional resemblance to the native tissue. In other words, although fabricated to precisely mimic the composition, architecture, and hierarchy of the native tissue, and albeit treated with the most updated differentiation and culturing protocols, the vast majority of printed tissues will display only limited functionality. Thus, while still being able to provide substantial benefits for research and biotechnological applications like basic drug screening, cultured meat, bioproduct production, etc., the non‐ideal performance of printed biostructures will prevent their clinical use. That being the case, what could be the reason that the engineered tissue does not organize and perform like a native one? If we precisely recapitulate the composition and spatial position of the tissue's elements, introduce the cells into a supportive environment and provide them with appropriate cues, what else is required for the formation of a native‐like, functional tissue? Two possible options are time and the sequence of events. The reason we choose to focus on these specific parameters is that they are prominent during natural development, but are not reflected, or taken into consideration, in current 3D bioprinting protocols. During the natural development of higher organisms, complex biological structures are progressively generated in time frames that are significantly longer than the course of an average 3D bioprinting session. These processes are also characterized by an orchestrated sequence of events with a defined hierarchy in terms of onset times. Moreover, cells that initially reside in one location may migrate to another, and the whole process may include additional spatiotemporal events of cell differentiation, proliferation, and death. In contrast, the common 3D bioprinting schemes are based on rapid patterning processes in which materials and cells are positioned at their final, desired location. Although post‐printing cell differentiation, proliferation, and even migration can be induced and manipulated to some extent, the native time frame and order of events will probably not be recapitulated. The nature of these parameters, in terms of their effect on the end result of tissue formation processes, still needs to be investigated. It is clear, however, that if the course of the process, by itself, plays a substantial role in the functionality of the tissue, it will be challenging to use 3D bioprinting for regenerative medicine purposes. In any case, it is reasonable to assume that there are variables in developmental biology that are either well concealed or too complicated to be recapitulated or managed by current technology. Obviously, there is also no guarantee that the required know‐how will be attained in the foreseeable future. Considering the complexity of living systems, with their interwoven signal routes and numerous feedback loops, it may not be unrealistic to consider a situation in which biology will eventually put a glass ceiling above our heads. While this may considerably hinder progress toward clinical application, it should be remembered that 3D bioprinting is a means, not an end. That is to say that if regenerative medicine is an ultimate goal, maybe fabrication of functional substitutions for malfunctioning tissues and organs will eventually be realized via alternative technologies.
The third scenario depicts a situation in which technologies other than 3D bioprinting will eventually dominate TE, or at least some of its derived clinical developments and applications. For instance, let us assume that highly functional, 3D bioprinted complex tissues and even organs can be fabricated, but only by a process that requires an enormous amount of resources, making them inaccessible to healthcare providers. For example, we mentioned the huge number of cells required for the construction of engineered human organs. While reaching these numbers may not be a completely uncrossable barrier, it may require an exceptionally prolonged and costly process in the absence of much improved culturing technologies. Another example in this regard is the recapitulation of the fine architectures that characterize living tissues. As discussed, the rapid advances in fabrication techniques endow researchers with the capacity to generate complicated geometries at very high resolution. These techniques, however, suffer from a low throughput and compositional complexity. Thus, scientists largely rely on spontaneous cell‐organization processes to create, for instance, the finest capillary networks in small, engineered cellular constructs. Indeed, such processes may take place when providing cells with a rough spatial guidance and proper biochemical cues. It is also known that such processes rapidly and efficiently occur as part of the natural response to tissue damage. [ 25 ] We cannot be sure, however, that these processes will suffice to establish a proper blood vessel infrastructure that is capable of supporting full‐size, engineered, functional organs. And, in case they do not, ultra‐high resolution printing procedures, which will probably be adapted in the future for higher compositional complexity, may be the only available solution. [ 24 ] Nevertheless, the cost of massive use of these techniques, required for generating full‐scale organs for transplantation, may make the process practically unattainable for most patients.
Thus, if top‐notch, state‐of‐the art 3D bioprinting technology does not yield affordable, transplantation‐ready engineered body parts, what solution will modern medicine offer to patients with failing tissues and organs? If artificial means for mimicking or bypassing developmental processes are not the answer, natural developmental processes may be harnessed for this purpose. While still immature and ethically controversial, somatic cell nuclear transfer techniques enable the generation of a genetic clone of an adult animal. [ 93 , 94 ] It may be possible that in the future, this technology will allow scientists to initiate developmental processes that yield functional organs without the necessity of generating a conscious, living, whole organism. Another intriguing option is to use animals as a source of transplantable tissues and organs (xenotransplantation), with recent interesting research performed on genetically modified pigs. [ 95 ] An entirely different direction may be the construction of artificial, synthetic organs. [ 96 , 97 , 98 ] Although currently not sufficiently developed to provide fully functional implantable or wearable replacements for malfunctioning organs, the technology may reach that point in the future.
With that being said, we believe that 3D‐bioprinting of functional tissues and organs will continue to develop, even in the case where it is not the method of choice for manufacturing body part substitutes. This is because research may substantially benefit from the ability to control the structure and composition of these native‐like structures. For instance, 3D bioprinting may enable the incorporation of genetically modified cells expressing a reporter gene at specific locations in an engineered organ. It may also allow the integration of actuators and sensors that will shed light on hard‐to‐detect physiological processes and activities. This freedom of design, not offered by the described alternatives, may rise above the cost of production, maintaining the demand for these functional printed bioconstructs.
4. Conclusions
3D printing is an ingenious concept and a groundbreaking technology that impacts a wide range of disciplines such as architecture, industrial design, manufacturing, and art. Owing to its power to grant users the exceptional capability to quickly and accurately convert a digital design into a 3D physical object, 3D printing gradually caught the attention of tissue engineers. Today, 3D bioprinting is one of the most desirable tools in TE, offering an advanced means (and in many cases the only means) for the construction of complex biostructures. Major efforts are now being made to refine the process, aiming to improve printing accuracy and speed as well as the complexity of the resulting printout. Indeed, the latest works overviewed in this article prove that motivation and creativity can be combined with knowledge and talent to achieve these goals. Aided by knowledge in cell biology and the expected advances in our understanding of developmental processes, 3D bioprinting may be the spearhead in the future of TE, taking it to new and higher levels. Obviously, any major advances in this field will open new gates, expedite developments, and accelerate progress in applicative regenerative medicine. Will 3D bioprinting be the technology of choice for generating transplantation‐ready, complex engineered tissues and organs? Or, should we humbly ask, will any technology bring us to this point in the foreseeable future? We believe that biology holds the key. It may or may not comply with attempts to control and manipulate it according to our needs and desires. But while we wonder if and when the transplantation of complex biofabricated constructs will become a routine clinical procedure, it seems that 3D bioprinting technology is rapidly evolving toward the realization of this vision.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
The authors acknowledge the support from the European Research Council (ERC) Starting Grant No. 637943 and the Slezak Foundation. The authors would like to thank Mr. Eric Silberman for his editorial assistance.
Biographies
Assaf Shapira is a research associate in the Shmunis School of Biomedicine and Cancer Research at the Faculty of Life Science at Tel Aviv University, Israel. His current research focuses on the development of advanced biomaterials and biofabrication techniques, with an emphasis on 3D bioprinting.
Tal Dvir is a professor in the Shmunis School of Biomedicine and Cancer Research at the Faculty of Life Science and in the Department of Materials Science and Engineering at the Faculty of Engineering at Tel Aviv University, Israel. He is also the director of the Tel Aviv University Center for Nanoscience and Nanotechnology and the founding director of the Sagol Center for Regenerative Biotechnology. His laboratory designs and develops smart bio and nanomaterials and technologies for engineering complex tissues and organs, such as the heart, brain, spinal cord, intestine, eyes, and more.
Shapira A., Dvir T., 3D Tissue and Organ Printing—Hope and Reality . Adv. Sci. 2021, 8 , 2003751. 10.1002/advs.202003751 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
A Novel 3D Printing Technology for Synthetic Hard Rock and the Fabrication of Jinping Marble
- Original Paper
- Published: 13 September 2022
- Volume 55 , pages 7695–7714, ( 2022 )
Cite this article

- Shiming Mei 1 ,
- Xia-Ting Feng 1 ,
- Zhengwei Li 1 ,
- Chengxiang Yang 1 &
- Jikai Gao 1
842 Accesses
4 Citations
Explore all metrics
This paper presents a novel 3D printing technology for synthetic hard rock based on self-developed 3D printing equipment and a systematic 3D printing method. The 3D printing equipment adopts a wet-material extrusion deposition moulding (WEDM) process and consists of a frame structure, feeding subsystem and control module. Aiming at the characteristics of wet similar materials of rock, the feeding subsystem was designed based on pneumatic actuation-spiral control, which could realize the printing of multicomponent and multiparticle materials. A systematic 3D printing method considering similarity theory was established to achieve the printing of synthetic hard rock with different physical and mechanical properties. Using the aforementioned 3D printing equipment and method, the 3D printing of synthetic hard rock was successfully performed. The results show that the printability of similar materials of rock might be controlled to achieve a reasonable initial setting time, extrudability and certain self-supporting ability by adding a set retarder and water-retaining agent. The mechanical performance and failure patterns of the printed samples were conveniently controlled by adjusting the proportion of the components in the similar materials. The quantitative similarity evaluation of mechanical parameters between the printed samples and Jinping marble was performed based on similarity theory, and the optimal mix proportion for simulating Jinping marble was obtained. Compared with other 3D printing technologies for rock mechanics with WEDM, the surface microstructure and mechanical characteristics of the printed samples are more similar to those of natural rock. These research results can be used to expand the application of 3D printing technology in rock mechanics.
Sample-scale 3D printing equipment was independently developed based on WEDM process.
A systematic 3D printing method for synthetic hard rock was established.
The 3D printing of synthetic hard rock is successfully completed.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
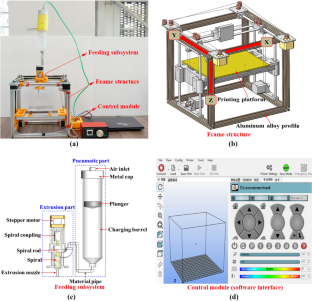
Similar content being viewed by others

A response surface methodology study on 4D printing for layered PLA/TPU structures
Influence of 3D-printing deposition parameters on crystallinity and morphing properties of PLA-based materials
Fused deposition modeling: process, materials, parameters, properties, and applications
Bos F, Wolfs R, Ahmed Z, Salet T (2016) Additive manufacturing of concrete in construction: potentials and challenges of 3D concrete printing. Virtual Phys Prototyp 11(3):209–225. https://doi.org/10.1080/17452759.2016.1209867
Article Google Scholar
Chen MX, Li LB, Zheng Y, Zhao PQ, Lu LC, Cheng X (2018) Rheological and mechanical properties of admixtures modified 3D printing sulphoaluminate cementitious materials. Constr Build Mater 189(20):601–611. https://doi.org/10.1016/j.conbuildmat.2018.09.037
Cheon DS, Jeon S, Park C, Song WK, Park ES (2011) Characterization of brittle failure using physical model experiments under polyaxial stress conditions. Int J Rock Mech Min 48(1):152–160. https://doi.org/10.1016/j.ijrmms.2010.10.001
Chia HN, Wu BM (2015) Recent advances in 3D printing of biomaterials. J Biol Eng 9(4):4. https://doi.org/10.1186/s13036-015-0001-4
Feng XT, Chen BR, Zhang CQ, Li SJ, Wu SY (2013) Mechanism, warning and dynamic control of rock-burst development process. Science Press, Beijing ( in Chinese )
Google Scholar
Feng XT, Gong YH, Zhou YY, Li ZW, Liu XF (2019) The 3D-printing technology of geological models using rock-like materials. Rock Mech Rock Eng 52(7):2261–2277. https://doi.org/10.1007/s00603-018-1703-y
Fereshtenejad S, Song JJ (2016) Fundamental study on applicability of powder-based 3D printer for physical modeling in rock mechanics. Rock Mech Rock Eng 49(6):2065–2074. https://doi.org/10.1007/s00603-015-0904-x
Gell EM, Walley SM, Braithwaite CH (2019) Review of the validity of the use of artificial specimens characterizing the mechanical properties of rocks. Rock Mech Rock Eng 52:2949–2961. https://doi.org/10.1007/s00603-019-01787-8
He BG, Zelig R, Hatzor YH, Feng XT (2014) Rockburst generation in discontinuous rock masses. Rock Mech Rock Eng 49:4103–4124. https://doi.org/10.1007/s00603-015-0906-8
Hu L, Feng XT, Xiao YX, Feng GL, Li SJ, Pan PZ, Yao ZB (2019) Characteristics of the microseismicity resulting from the construction of a deeply-buried shaft. Tunn under Sp Tech 85(8):114–127. https://doi.org/10.1016/j.tust.2018.12.016
Jiang C, Zhao GF (2015) A preliminary study of 3D printing on rock mechanics. Rock Mech Rock Eng 48(3):1041–1050. https://doi.org/10.1007/s00603-014-0612-y
Jiang C, Zhao GF, Zhu JB, Zhao YX, Shen LM (2016a) Investigation of dynamic crack coalescence using a gypsum-like 3D printing material. Rock Mech Rock Eng 49(10):3983–3998. https://doi.org/10.1007/s00603-016-0967-3
Jiang Q, Feng XT, Song LB, Gong YH, Zheng H, Cui J (2016b) Modeling rock specimens through 3D printing: tentative experiments and prospects. Acta Mech Sin 32(1):101–111. https://doi.org/10.1007/s10409-015-0524-4
Ju Y, Xie HP, Zheng Z, Lu J, Mao L, Gao F, Peng R (2014) Visualization of the complex structure and stress field inside rock by means of 3D printing technology. Chin Sci Bull 59(36):5354–5365. https://doi.org/10.1007/s11434-014-0579-9
Ju Y, Wang L, Xie HP, Ma GW, Mao LT, Zheng ZM, Lu JB (2017a) Visualization of the three-dimensional structure and stress field of aggregated concrete materials through 3D printing and frozen-stress techniques. Constr Build Mater 143:121–137. https://doi.org/10.1016/j.conbuildmat.2017.03.102
Ju Y, Wang L, Xie HP, Ma GW, Zheng ZM, Mao LT (2017b) Visualization and transparentization of the structure and stress field of aggregated geomaterials through 3D printing and photoelastic techniques. Rock Mech Rock Eng 50(6):1383–1407. https://doi.org/10.1007/s00603-017-1171-9
Khoshnevis B (2004) Automated construction by contour crafting-related robotics and information technologies. Automat Constr 13(1):5–19. https://doi.org/10.1016/j.autcon.2003.08.012
Khoshnevis B, Bukkapatnam S, Kwon H, Saito J (2001) Experimental investigation of contour crafting using ceramics materials. Rapid Prototyp J 7(1):32–42. https://doi.org/10.1108/13552540110365144
Kong LY, Ostadhassan M, Li CX, Tamimi N (2018) Can 3-D printed gypsum samples replicate natural rocks? An experimental study. Rock Mech Rock Eng 51(10):3061–3074. https://doi.org/10.1007/s00603-018-1520-3
Li ZJ, Ma GW, Wang F, Wang L, Sanjayan J (2022) Expansive cementitious materials to improve micro-cable reinforcement bond in 3D concrete printing. Cem Concrete Comp 125:104304. https://doi.org/10.1016/j.cemconcomp.2021.104304
Liang F, Liang YH (2014) Study on the status quo and problems of 3D printed buildings in China. Glob J Hum Soc Sci 14(5):7–10
Wu ZJ, Zhang B, Weng L, Liu QS, Wong LNY (2020) A new way to replicate the highly stressed soft rock: 3D printing exploration. Rock Mech Rock Eng 53(1):467–476. https://doi.org/10.1007/s00603-019-01926-1
Lim S, Le T, Webster J, Buswell R, Austin S, Gibb A, Thorpe T (2009) Fabricating construction components using layer manufacturing technology. Proceedings of International Conference on Global Innovation in Construction, Loughborough, UK, 512-520
Liu YR, Guan FH, Yang Q, Yang RQ, Zhou WY (2013) Geomechanical model test for stability analysis of high arch dam based on small blocks masonry technique. Int J Rock Mech Min 61:231–243 https://doi.org/10.1016/j.ijrmms.2013.03.003
Ma GW, Wang L, Ju Y (2018) State-of-the-art of 3D printing technology of cementitious material-An emerging technique for construction. Sci China Technol Sc 61(4):475–495. https://doi.org/10.1007/s11431-016-9077-7
Michael E (2013) 3D printing for children: What to build next? Int J Child Comput Interact 1(1):7–13. https://doi.org/10.1016/j.ijcci.2012.08.004
Mohan MK, Rahul AV, Tittelboom KV, Schutter GD (2020) Extrusion-based concrete 3D printing from a material perspective: a state-of-the-art. Cem Concr Compos 115:103855. https://doi.org/10.1016/j.ijcci.2012.08.004
NASA (2014) Space station 3-D printer builds ratchet wrench to complete first phase of operations. http://www.nasa.gov/mission_pages/station/research/news/3Dratchet_wrench . Accessed 26 Apr 2015.
Sanjayan JG, Nematollahi B, Xia M, Marchment T (2018) Effect of surface moisture on inter-layer strength of 3D printed concrete. Constr Build Mater 172:468–475. https://doi.org/10.1016/j.conbuildmat.2018.03.232
Sharafisafa M, Shen LM, Xu QF (2018) Characterisation of mechanical behaviour of 3D printed rock-like material with digital image correlation. Int J Rock Mech Min 112:122–138. https://doi.org/10.1016/j.ijrmms.2018.10.012
Song LB, Jiang Q, Shi YE, Feng XT, Li YH, Su FS, Liu C (2018) Feasibility investigation of 3D printing technology for geotechnical physical models: study of tunnels. Rock Mech Rock Eng 52:2949–2961. https://doi.org/10.1007/s00603-018-1504-3 ( 51:2617-2637 )
Tay YWD, Panda B, Paul SC, Mohamed NAN, Tan MJ, Leong KF (2017) 3D printing trends in building and construction industry: a review. Virtual Phys Prototyp 12(3):261–276. https://doi.org/10.1080/17452759.2017.1326724
Tian W, Han N (2017) Mechanical properties of rock specimens containing pre-existing flaws with 3D printed materials. Strain 53(6):1–13. https://doi.org/10.1111/str.12240
Vogler D, Walsh SDC, Dombrovski E, Perras MA (2017) A comparison of tensile failure in 3D-printed and natural sandstone. Eng Geol 226:221–235. https://doi.org/10.1016/j.enggeo.2017.06.011
Wang L, Ma GW, Liu TH, Buswell R, Li ZJ (2021) Interlayer reinforcement of 3D printed concrete by the in-process deposition of U-nails. Cem Concr Res 148:106535. https://doi.org/10.1016/j.cemconres.2021.106535
Wolfs RJM, Bos FP, Salet TAM (2018) Early age mechanical behaviour of 3D printed concrete: Numerical modelling and experimental testing. Cem Concr Res 106:103–116. https://doi.org/10.1016/j.cemconres.2018.02.001
Xia YJ, Zhang CQ, Zhou H, Hou J, Su GS, Gao Y, Liu N, Singh HK (2020) Mechanical behavior of structurally reconstructed irregular columnar jointed rock mass using 3D printing. Eng Geol 268(4):105509. https://doi.org/10.1016/j.enggeo.2020.105509
Zhao XG, Cai M, Wang J, Li PF (2015) Strength comparison between cylindrical and prism specimens ofBeishan granite under uniaxial compression. Int J Rock Mech Min 76:10–17. https://doi.org/10.1016/j.ijrmms.2015.02.009
Zhou T, Zhu JB (2018) Identification of a suitable 3D printing material for mimicking brittle and hard rocks and Its brittleness enhancements. Rock Mech Rock Eng 51(3):765–777. https://doi.org/10.1007/s00603-017-1335-7
Zhu JB, Zhou T, Liao ZY, Sun L, Li XB, Chen R (2018) Replication of internal defects and investigation of mechanical and fracture behaviour of rock using 3D printing and 3D numerical methods in combination with X-ray computerized tomography. Int J Rock Mech Min 106:198–212. https://doi.org/10.1016/j.ijrmms.2018.04.022
Zhu GQ, Feng XT, Zhou YY, Li ZW, Fu LJ, Xiong YR (2019a) Physical model experimental study on spalling failure around a tunnel in synthetic marble. Rock Mech Rock Eng 53(2):909–926. https://doi.org/10.1007/s00603-019-01952-z
Zhu GQ, Feng XT, Zhou YY, Li ZW, Yang CX, Gao YH (2019b) Experimental study to design an analog material for Jinping marble with high strength, high brittleness and high unit weight and ductility. Rock Mech Rock Eng 52(7):2279–2292. https://doi.org/10.1007/s00603-018-1710-z
Download references
Acknowledgements
The authors sincerely acknowledge the financial support from the National Natural Science Foundation of China under Grant No. 51839003, the 111 Project under Grant No. B17009 and the Liao Ning Revitalization Talents Program under Grant No. XLYCYSZX1902. The authors are grateful to Dr. Yanhua Gong, Dr. Meizhu Zhang, Dr. Guoqiang Zhu, and Mr. Qiang Han and for their valuable assistance. The authors would also like to thank the journal editors and anonymous reviewers for their valuable suggestions.
Author information
Authors and affiliations.
Key Laboratory of Ministry of Education on Safe Mining of Deep Metal Mines, Northeastern University, Shenyang, 110819, Liaoning, China
Shiming Mei, Xia-Ting Feng, Zhengwei Li, Chengxiang Yang & Jikai Gao
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Xia-Ting Feng .
Ethics declarations
Conflict of interest.
The authors have no conflicts of interest to declare.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Mei, S., Feng, XT., Li, Z. et al. A Novel 3D Printing Technology for Synthetic Hard Rock and the Fabrication of Jinping Marble. Rock Mech Rock Eng 55 , 7695–7714 (2022). https://doi.org/10.1007/s00603-022-03054-9
Download citation
Received : 20 September 2021
Accepted : 25 August 2022
Published : 13 September 2022
Issue Date : December 2022
DOI : https://doi.org/10.1007/s00603-022-03054-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- 3D printing technology
- Wet-material extrusion deposition moulding
- Synthetic hard rock
- Similarity theory
- Jinping marble
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
The more information about 3D printing technology will help the company and government to upgrade and improve the infrastructure of 3D printing technology. Thus, this paper is to overview the types of 3D printing technologies, materials used for 3D printing technology in manufacturing industry and lastly, the applications of 3D printing technology.
The paper discusses numerous 3D printing processes, their advantages and disadvantages. A comprehensive description of different materials compatible for each type of 3D printing process is presented. The paper also presents the various application areas of each type of process. A dedicated section on industry 4.0 has also been included.
Learn how scientists are improving 3D printing techniques and materials to create larger, faster and stronger products. Find out how these innovations are changing the image of a once-niche technology and attracting commercial interest.
In addition, this paper discussed the main processing challenges with void formation, anisotropic behaviour, the limitation of computer design and layer-by-layer appearance. Overall, this paper gives an overview of 3D printing, including a survey on its benefits and drawbacks as a benchmark for future research and development.
3D Printing Technology: A Future Perspective. May 2021. Conference: (ICRISET-2020) International Conference on Research and Innovations in Science, Engineering & Technology, 4th-5th Sept. 2020. At ...
This is an analytical research paper in which the reader is introduced to the 3D printing technology, its definition, history, basic components, and operation theory. So what is this technology?
Jawaharlal Nehru Technological University Hyderabad, India. Abstract: 3D printing also known as Additive manufacturing technology has been dubbed the next big thing and be as equally wide. spread ...
Numerous 3D printing methods, such as material jetting, extrusion, polymerization, fusion, and sintering, have been researched to form 3D structures of various material groups, including insulators, semiconductors, and conductors, to provide 3D-printed electronics. As a result, remarkable research progress has been achieved in the integration ...
According to current advances in 3D Printing and Design for Sustainability of the research framework before described, this paper aims to identify new and promising open research topics to be taken into account in the near future, to rethink their impacts, to suggest potential combinations and to imagine new roles and future sustainable applications for 3D Printing.
This paper reviews the electromagnetic aspects of 3D printing technologies (3DP) applied to printed electronics, a fast-growing market. It covers the materials, processes, structures and applications of 3DP in the EM domain, as well as the challenges and opportunities for future research.
3D printing has revolutionized various industries by enabling the production of complex designs and shapes. Recently, the potential of new materials in 3D printing has led to an exponential increase in the technology's applications. However, despite these advancements, the technology still faces significant challenges, including high costs, low printing speeds, limited part sizes, and strength.
The researchers present a method to help the printers live up to their names and deliver a "true" 3D form of printing. In a new paper in Nature, they describe a technique of volumetric 3D printing that goes beyond the bottom-up, layered approach. The process eliminates the need for support structures because the resin it creates is self ...
3D printing is a constantly expanding technology that represents one of the most exciting and disruptive production possibilities available today. This technology has gained global recognition and garnered considerable attention in recent years. However, technological breakthroughs, particularly in the field of material science, continue to be the focus of research, particularly in terms of ...
The discovery of a 3D printer dates back to early 1980s when Charles Hull, an American engineer, built the 1st 3D printer, capable of creating solid objects by following a computer-aided design (CAD).
3D printing processes are well established to produce 3D objects composed of polymers and polymer composites. Some 3D printing techniques are well developed, such as material extrusion (ME) (we mainly focus on FFF in this review and for direct ink writing we refer to a previous review paper 28), VP (or stereolithography [SLA]), material jetting (MJ), binder jetting (BJ), and PBF, but many ...
3D printing has evolved as a disruptive technology for fabrication of industrial components, however due to the intrinsic nature of the process, the mechanical strength of the parts developed by 3D printing is a subject of research.
Abstract This paper provides a critical review of the related literature on 3D printing in construction. The paper discusses and evaluates the different 3D printing techniques in construction. The paper also discusses and categorizes the benefits, challenges, and risks of 3D printing in construction. The use of 3D printing technology offers several advantages over traditional methods. However ...
The papers were sorted according to the scope of the study, focusing on the research topic and 3D printing as a method for recycling polymers. A total of 144 articles were excluded as they focused on issues other than 3D printing as a method for recycling plastics.
3D printing developed as a prototyping method in the early 1980s, yet it is considered as a 21st century technology for transforming digital models into tangible objects. 3D printing has recently become a critical tool in the geoscience research, education, and technical communication due to the expansion of the market for 3D printers and materials. 3D printing changes the perception of how we ...
Digital fabrication technology, known as 3D printing, develops physical objects from a geometrical representation by adding materials (Shahrubudin et al., 2019). With the support of computer-aided ...
1. Introduction. Bioprinting is a subcategory of additive manufacturing (AM), also known as three-dimensional (3D) printing. It is defined as the printing of structures using viable cells, biomaterials and biological molecules [1,2].Bioprinting must produce scaffolds with a suitable microarchitecture to provide mechanical stability and promote cell ingrowth whilst also considering the impact ...
First, the authors conducted the database search using a pre-defined search string that consisted of seven 3D printing terms, seven construction and engineering terms, and 28 viability-related terms, as shown in Table 1.Conference papers and journal articles that matched at least one 3D printing term, one engineering and construction term, and one viability term in their title, keywords, or ...
Abstract. Three‐dimensional (3D) bioprinting is an emerging, groundbreaking strategy in tissue engineering, allowing the fabrication of living constructs with an unprecedented degree of complexity and accuracy. While this technique greatly facilitates the structuring of native tissue‐like architectures, many challenges still remain to be faced.
This paper presents a novel 3D printing technology for synthetic hard rock based on self-developed 3D printing equipment and a systematic 3D printing method. The 3D printing equipment adopts a wet-material extrusion deposition moulding (WEDM) process and consists of a frame structure, feeding subsystem and control module. Aiming at the characteristics of wet similar materials of rock, the ...
To solve this problem, the anodic oxidation behavior of 3D printing AlSi10Mg alloy was systematically studied in this paper, focusing on the mechanism of the effect of oxidation voltage on the film structure characteristics, corrosion resistance and wear resistance.
1. Introduction. 3D printing construction technology was an amazing breakthrough in the past decade and potentially can revolutionize the construction industry, resolve residential crises, serve the green house, and brings in sustainability benefits, such as increased resource efficiency, improved construction productivity, compensates for shortages of skilled labors, and building of complex ...