Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 12 May 2022

The effect of perceived stress on cognition is mediated by personality and the underlying neural mechanism
- Ximei Zhu 1 na1 ,
- Wei Yan ORCID: orcid.org/0000-0002-5866-6230 1 na1 ,
- Xiao Lin 1 na1 ,
- Jianyu Que 1 ,
- Yuetong Huang 1 ,
- Haohao Zheng 1 ,
- Lin Liu 1 ,
- Jiahui Deng ORCID: orcid.org/0000-0002-2025-0336 1 ,
- Lin Lu ORCID: orcid.org/0000-0003-0742-9072 1 , 2 &
- Suhua Chang ORCID: orcid.org/0000-0001-7465-3985 1 , 2
Translational Psychiatry volume 12 , Article number: 199 ( 2022 ) Cite this article
3680 Accesses
10 Citations
5 Altmetric
Metrics details
- Human behaviour
- Neuroscience
- Pathogenesis
Perceived stress impairs cognitive function across the adult lifespan, but the extent to which cognition decline is variable across individuals. Individual differences in the stress response are described as personality traits. Substantial individual differences in the magnitude of cognitive impairment that is induced by short-term perceived stress are poorly understood. The present study tested the hypothesis that the relationship between short-term perceived stress and different aspects of cognition is mediated by personality traits. The study included 1066 participants with behavior and neuroimaging data from the Human Connectome Project after excluding individuals with missing variables. In the result, the parallel multiple mediation model demonstrated that the influence of perceived stress on the total and crystalized cognition is mainly mediated by neuroticism (indirect effect = −0.04, p < 0.05) and conscientiousness (indirect effect = 0.05, p < 0.05) in adults. Cortical thickness value ( n = 1066) of the right superior frontal gyrus (SFG) showed not only positive correlations with short-term perceived stress and neuroticism, but negative associations with cognition. The chain mediation model found that the right SFG and neuroticism play a small but significant chain mediating effect between stress and total cognition. The strength of the resting-state functional connectivity ( n = 968) between the left orbitofrontal cortex versus the left superior medial frontal cortex was positively correlated with crystallized cognition and negatively associated with conscientiousness. These results extend previous findings by the impacts of short-term perceived stress on cognitive function is mediated by neuroticism and the right SFG was the underlying neural mechanism.
Similar content being viewed by others
Stronger connectivity and higher extraversion protect against stress-related deterioration of cognitive functions
Jacek Rogala, Joanna Dreszer, … Andrzej Wróbel
Personality traits and brain health: a large prospective cohort study
Ya-Ru Zhang, Yue-Ting Deng, … Jin-Tai Yu

Covariation of preadult environmental exposures, adult brain imaging phenotypes, and adult personality traits
Kaizhong Xue, Bo Gao, … CHIMGEN Consortium
Introduction
Perceived stress is a feeling about how much strain individuals perceive when environmental demands exceed their adaptive capacity [ 1 ]. The prevalence of perceived stress in adults is 34.8–55%, which is higher than anxiety and depression [ 2 , 3 , 4 ]. Perceived stress influences the pathogenesis of physical disease by causing negative affective states, which in turn exert direct effects on biological processes or behavioral patterns that influence disease risk.
Perceived stress or challenge might enhance cognitive performance in some situations while limiting performance in other situations. Mild stress could help to enhance cognitive function, particularly in memory tasks or when the cognitive load is not excessive [ 5 , 6 ]. Exposure to high, acute stress impairs the complex cognitive function [ 7 , 8 ]. However, the extent to which cognition can be impaired is often variable across individuals [ 9 ]. The extent of perceived stress to cognition decline depends on the individual difference in stress response and brain function [ 10 , 11 ]. One widely cited explanation derives from the cognitive activation theory of stress agreed that the preference for individual coping styles in response to stress is a characteristic of personality [ 12 , 13 ]. Personality refers to individual differences in characteristic patterns of thinking, feeling, and behaving [ 14 ], which is frequently broken into several traits, including neuroticism, conscientiousness, openness, agreeableness, and extroversion. Evidence suggested that short-term perceived stress exposure affects stability and change of neuroticism [ 15 , 16 ]. Life events can lead to changes in personality traits and that those different life events may be differently related to specific trait domains [ 17 ]. Previous studies indicated that positive life events (enter a new relationship) in last month predict an increase in extraversion [ 18 ], and stressful life events in last 3 months predict an increase in neuroticism [ 19 ]. Neuroticism mediates the impact of menopause status on depressive symptoms among women [ 20 ]. Perceived stress significantly predicts neuroticism, which in turn cumulatively increases depression risk across the life span [ 21 , 22 ]. Personality trait changes are more strongly related to how individuals perceived in their lives rather than simply the occurrence of such stressful events [ 23 ]. Longitudinally, perceiving the event as a negative obstruction was associated with increases in neuroticism, whereas perceiving the event as a positive challenge was associated with increases in extraversion and conscientiousness.
Personality traits also influence the cognitive function across the adult lifespan and correlate to mild cognitive impairment in older adults [ 24 , 25 ]. Previous studies reported that lower neuroticism and extraversion are associated with better performance on the crystallized and fluid ability tasks [ 26 ], while higher openness is associated with higher cognitive performance [ 27 ]. Conscientiousness correlates negatively with abstract reasoning, verbal reasoning, as well as fluid cognition [ 28 , 29 ]. Furthermore, neuroticism and conscientiousness predict performance on the prospective memory tasks [ 30 , 31 , 32 ]. Interestingly, personality is more strongly related to crystallized intelligence than to fluid intelligence [ 33 ].
Prior imaging studies have observed that stress induces remodeling of the prefrontal cortex (PFC) and affects both the structure and function of PFC [ 34 , 35 ]. Chronic and acute stress induces cerebral blood flow changes, architectural changes, and dysfunction of the PFC [ 7 , 36 ]. Perceived stress in healthy adults induces hypoactivation of the working memory-related PFC and the reallocation of neural resources away from executive function networks [ 37 , 38 ]. Individuals with early life stress exhibit poorer processing speed and working memory, and have smaller volumes of the lateral orbitofrontal cortex and caudate nucleus [ 39 ]. According to previous studies, the regional brain structure especially the prefrontal cortex [ 40 , 41 ], and the functional connectivity have been proved to be related to cognition, as Zhu and colleagues have mentioned that the functional connectivity related to the superior frontal gyrus (SFG) played significant roles in higher-order cognitive functions [ 42 ]. A short-term study based on Human Connectome Project (HCP) database also found that personality is associated with the subcortical-medial prefrontal network and the dorsolateral PFC [ 43 ]. However, the relationship between stress, personality, and cognition is still unclear, especially in term of neuroimaging. Insights into the role of personality in the stress-cognition relationships are therefore important and may lead to new interventions to target vulnerability to stress-related cognitive decline rather than the manifestations of cognition impairment.
Based on previous studies, we hypothesized that the relationship between short-term perceived stress and different aspects of cognitive function (total, crystallized, and fluid cognition) is mediated by personality traits (neuroticism, conscientiousness, openness, agreeableness, and extroversion) in young adults. The data that were analyzed in the present study were derived from the HCP database, which included behavioral, magnetic resonance imaging (MRI), and genetic data that were collected from 1206 young adults. We sought to form a framework to explain how different personality traits mediate the effect of short-term perceived stress on different aspects of cognition decline and further explore the underlying neuroimaging mechanism by analyzing MRI data to probe the validity of our explanations and posited mechanisms.
Participants
The data in the present study were drawn from the HCP database (March 2017 public data release) from the Washington University-University of Minnesota (WU-Minn HCP) Consortium [ 44 , 45 ]. The research procedures and ethical guidelines were approved by Washington University’s institutional review board. The WU-Minn HCP Consortium obtained full informed consent from all participants.
Measurement and statistical analysis
The short-term perceived stress of the participants in the HCP was assessed by the perceived stress scale in the National Institutes of Health (NIH) toolbox [ 46 ], which measures the degree of stress a person feels. The scale includes 10 items about levels of experienced stress during the last month. Respondents rate the items on a 5-point Likert with higher scores reflecting higher perceived stress [ 47 ]. The total score of the 10 items was used to denote the perceived stress.
Personality was measured by the 60-item version of the Costa and McRae Neuroticism/Extroversion/Openness Five-Factor Inventory (NEO-FFI) [ 48 ]. The NEO-FFI is one of the most commonly used questionnaires to capture the major facets of human personality traits, including neuroticism, conscientiousness, openness, agreeableness, and extroversion.
Cognition was measured by a series of tests in the NIH Toolbox cognitive battery [ 49 ]. Composite cognition scores from the NIH Toolbox [ 50 ] included crystallized cognition composite score (i.e., the ability to solve problems based on prior knowledge and experience, including language decoding and language comprehension) [ 51 ], fluid cognition composite score (i.e., the ability to solve novel reasoning problems, which is correlated with executive function, episodic memory, working memory, and processing speed) [ 51 ] and the total cognitive function composite score (a combination of both crystallized and fluid scores). Compared with the gold standard composites of cognition (IQ scores of the Wechsler Adult Intelligence Scale), total cognitive function, crystallized cognition, and fluid cognition composite scores showed acceptable internal consistency, excellent test–retest reliability, strong convergent and discriminant validities [ 52 ]. The specific description of the NIH Toolbox cognition assessment scale is provided in the Supplementary Methods.
All of the analyses used the following variables as covariates: age, gender, race (categorized as white or other), handedness (Edinburgh Handedness Questionnaire scores), years of education, total household income, body mass index (BMI), adult self-report (ASR) anxious/depressed raw score, alcohol dependence diagnosis ( Diagnostic and Statistical Manual of Mental Disorders , 4th edition [DSM-IV], criteria for alcohol dependence), tobacco dependence diagnosis (DSM-IV criteria for tobacco dependence), marijuana dependence diagnosis (DSM-IV criteria for marijuana dependence), and positive tests for illicit drugs (including cocaine, tetrahydrocannabinol, opioids, amphetamines, methamphetamine, and oxycontin). The correlations among short-term perceived stress, personality traits, and cognitive function were calculated using partial correlation analyses after controlling the above covariates using SPSS 20 software. False discovery rate (FDR) was used to address the issue of multiple comparisons, and FDR-corrected p values ( pval_fdr ) < 0.05 were considered statistically significant.
To explore how personality traits contribute to the association between short-term perceived stress and cognitive function, we used model 4 and 6 in PROCESS [ 53 ] to construct simple, parallel multiple, and chain mediation models to analyze whether personality traits (mediation variable) mediate the association between short-term perceived stress (input variable) and cognitive function (output variable). The simple mediation models only explore the mediating effect of a single variable, while the parallel multiple mediation models explore the mediating effect of multiple variables which are parallel without causally influencing each other. If one variable influence another, then chain mediation models would be the appropriate choice. The bootstrap method is used to randomly sample 10,000 times from the original sample to estimate the indirect effect value. If the 95% corrected confidence intervals (CI) of the indirect effect value does not include zero, it suggests that the indirect effect is statistically significant at the 0.05 level [ 54 ].
Magnetic resonance imaging data acquisition and statistical analysis
High-resolution T1-weighted (T1w) and T2-weighted (T2w) structural images were acquired on a 3T Siemens Skyra scanner (Siemens AG, Erlanger, Germany) with a 32-channel head coil at a resolution of 0.7 mm isotropic (field of view [FOV] = 224 × 224, matrix = 320 × 320, 256 sagittal slices, repetition time [TR] of T1w = 2400 ms, TR of T2w = 3200 ms, echo time [TE] of T1w = 2.14 ms, TE of T2w = 565 ms). The structural (T1w and T2w MRI) data used in this study were from the HCP preprocessed pipeline and composed of FreeSurfer summary statistics data, including surface thickness, surface area, and subcortical segmentation volume [ 55 , 56 ]. Then brain structure data was processed by z transformation to improve normality. Association analysis with the whole-brain structure analysis is a fundamental tool in augmenting understanding of the brain structure related to phenotype data. Linear regression models were constructed to calculate the association between cortical thickness and area with cognition, stress, and personality after controlling the basic covariates above. pval_fdr < 0.05 was considered statistically significant. Linear regression models were constructed to calculate the association between cortical and subcortical volume with cognition, stress, and personality after controlling for total intracranial volume and the basic covariates above.
The resting-state functional MRI (rs-fMRI) images were acquired on a 3T Siemens Skyra scanner with a 32-channel head coil at a resolution of 2 mm isotropic (FOV = 208 × 180, matrix = 104 × 90, 72 slices, TR = 720 ms, TE = 33.1 ms). The rs-fMRI data has been preprocessed by the HCP with its uniform method [ 45 , 55 , 56 ]. Only participants with four rs-fMRI data (two scans and two directions for each scan) were used to construct the whole-brain functional network. After preprocessing, the gray matter of the whole brain was parcellated with Shen brain atlas as template [ 57 ]. Nodal signals were created by averaging the regional blood oxygen level-dependent signals of all voxels within each region of two directions of two scans. Pearson cross-correlations between all pairwise combinations of region signals were calculated for each participant, followed by z transformation to improve normality. Resting-state functional connectivity (FC) between brain areas, which reflects correlations of activity, has broad implications for the interpretation of the brain regions with altered connectivity in the process of cognition and behavior. The linear regression models were constructed between behavior data and the whole-brain functional connectivity (FC) network (250 × 250 regions with 31,125 edges), which reflected correlations between brain regions with altered connectivity and function after controlling the basic covariates [ 58 ]. FDR-corrected p values for each component using 5000 permutation testing were used to address the issue of multiple corrections, and the component with pval_fdr < 0.05 was considered statistically significant. Since the parcellation of the Shen brain atlas is based on objectively functional connectivity analysis [ 57 ], we used the Shen brain atlas as the main result. For comparison, we also tried to use the AAL brain atlas as a template, which included 116 brain regions, to repeat the analyses above.
Correlation and mediation model for short-term perceived stress, personality, and cognitive function
After deleting individuals with missing variables, a total of 1066 participants with behavior and brain structure data from HCP were used for the analysis. The analysis flowchart is shown in Supplementary Fig. S1 . The participants were young adults, 22–37 years old (mean = 28.82 years, standard deviation = 3.67 years), and 492 were male (Table 1 ). After controlling for covariates, short-term perceived stress negatively correlated with total cognition ( r = −0.08, pval_fdr < 0.05) and fluid cognition ( r = −0.07, pval_fdr < 0.05). Short-term perceived stress negatively correlated with agreeableness and conscientiousness (Table 2 ). Only neuroticism positively correlated with short-term perceived stress ( r = 0.41, pval_fdr < 0.01). The correlation between openness, extroversion, and short-term perceived stress was nonsignificant. Total cognition positively correlated with openness, and negatively correlated with conscientiousness and neuroticism (Table 2 ).
The simple mediation analysis found that the relationship between short-term perceived stress and total cognition was mediated by neuroticism, conscientiousness, and openness respectively (Supplementary Table S1 ). The relationship between short-term perceived stress and crystallized cognition was mediated by neuroticism, conscientiousness, and agreeableness respectively (Supplementary Table S1 ). No personality trait mediated the relationship between stress and fluid cognition. Personality traits that were significant in the simple mediation analysis were included simultaneously in the parallel multiple mediation model to compare the mediation effect and mediation ratio (i.e., the ratio of the specific indirect effect to the total effect). For total cognition, the mediation effect and ratio of neuroticism (indirect effect = −0.06, ratio = 0.43) was higher than the mediation effect and ratio of conscientiousness (indirect effect = −0.04, ratio = −0.30), whereas the mediation effect of openness was nonsignificant in the parallel multiple mediation model (Fig. 1a ). For crystallized cognition, the mediation effect and ratio of neuroticism (indirect effect = −0.04, ratio = 0.72) and conscientiousness (indirect effect = 0.05, ratio = −0.73) were higher than agreeableness (indirect effect = 0.01, ratio = 0.23; Fig. 1b ). The mediation effect of neuroticism, openness, and agreeableness had the same direction as the total effect of stress on cognition. In contrast, the mediation effect of conscientiousness was in the opposite direction of the total effect of stress and cognition (Fig. 1 ). Because of the different mediating directions of personality factors, the total indirect effect was statistically non-significant in the multiple mediation models. The total effect, which was a combination of the direct effect of stress and the indirect effect of personality, indicated that stress was negatively associated with cognition.
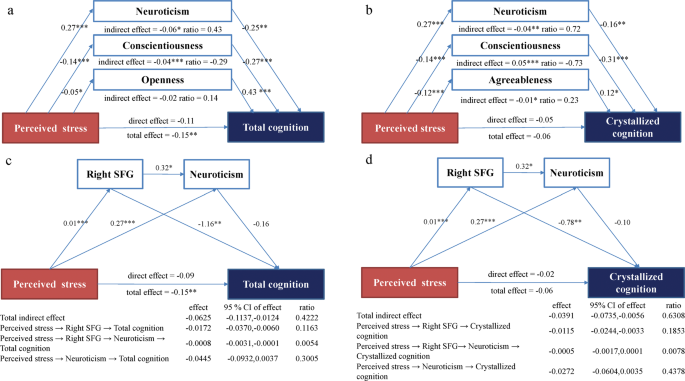
* p < 0.05, ** p < 0.01, *** p < 0.001. CI, confidence intervals. ratio, ratio of indirect effect to the total effect of each mediator. a The parallel multiple mediation model of neuroticism and conscientiousness mainly mediated the relationship between perceived stress and total cognition. Openness was nonsignificant in the mediation model. b The parallel multiple mediation model of neuroticism, conscientiousness, and agreeableness between perceived stress and crystallized cognition. c The chain mediation model of right SFG and neuroticism between perceived stress and total cognition. d The chain mediation model of right SFG and neuroticism between perceived stress and crystallized cognition.
Shared brain structural characteristics between perceived stress, personality, and cognitive function
For the whole-brain analysis after FDR correction, the linear regression models revealed that the cortical thickness of 10 brain regions was significantly associated with short-term perceived stress, including bilateral SFG, bilateral rostral middle frontal gyrus, bilateral caudal middle frontal gyrus, right pars opercularis gyrus, left pars orbitalis gyrus, left pars triangularis gyrus, and left precentral gyrus. Neuroticism was significantly associated with the cortical thickness of right SFG, whereas no significant brain region was associated with the other personality traits (Table 3 ). Total cognition was associated with cortical areas of 55 brain regions (Supplementary Table S2 ), and the cortical thickness of 7 brain regions which included bilateral SFG, right rostral middle frontal gyrus, right pars opercularis gyrus, left caudal middle frontal gyrus, left medial orbitofrontal gyrus, and left pars orbitalis gyrus (Table 3 ). Crystallized cognition was associated with cortical areas of 59 brain regions (Supplementary Table S2 ) and was associated with the cortical thickness of three brain regions which included right SFG, right rostral middle frontal gyrus, and left pars orbitalis gyrus (Table 3 ). And fluid cognition was associated with cortical areas of 24 brain regions (Supplementary Table S2 ). The cortical thickness of six brain regions was positively related to stress and negatively related to total cognition, which included bilateral SFG, right rostral middle frontal gyrus, left caudal middle frontal gyrus, right pars opercularis gyrus, left pars orbitalis gyrus. And the cortical thickness of the right SFG, right rostral middle frontal gyrus, left pars orbitalis gyrus were positively related to stress and negatively related to crystallized cognition (Table 3 ). Cortical thickness of the right SFG (the only shared brain region among stress, neuroticism, total and crystallized cognition) was positively associated with short-term perceived stress and neuroticism but negatively associated with total cognition and crystallized cognition.
To further analyze the role of right SFG, we constructed the chain mediation model of the right SFG and neuroticism between short-term perceived stress and total/crystallized cognition (Fig. 1c, d ). For total cognition, the sequential chain mediation effect of the right SFG and neuroticism between stress and total cognition was small but significant (effect = −0.0008, ratio = 0.0054), and the mediation effect of the right SFG between stress and total cognition was also small and significant (effect = −0.0172, ratio = 0.1163). For crystallized cognition, the sequential chain mediation effect of the right SFG and neuroticism between stress and crystallized cognition were insignificant, whereas the mediation effect of the right SFG between stress and crystallized cognition was significant (effect = −0.0115, ratio = 0.1853). The relationship between stress and total/crystallized cognition was mediated by the right SFG, and the relationship between stress and total cognition was slightly mediated by the sequential chain mediation effect of the right SFG and neuroticism.
Functional connectivity associated with conscientiousness and crystallized cognition
After excluding 98 participants without four fMRI scans, the rs-fMRI analysis included 968 participants. The linear regression models showed that all three measures had significant network FC after FDR correction. Further comparisons found overlapping FC in Shen brain atlas between conscientiousness and crystallized cognition, whereas no overlapping FC was found for perceived stress and other personality traits (Fig. S2 ). Conscientiousness was associated with 142 FCs and 100 nodes, including the bilateral frontal gyrus, bilateral orbitofrontal gyrus, right temporal gyrus, bilateral postcentral gyrus, left occipital gyrus, and bilateral precuneus gyrus (Fig. 2a ). Crystallized cognition was associated with 6 FCs and 12 nodes, including the bilateral SFG, right temporal gyrus, bilateral orbitofrontal cortex, right precentral gyrus, right cingulate gyrus, right lingual gyrus, and right fusiform gyrus (Fig. 2b ). Functional connectivity between the left lateral orbitofrontal cortex (OFC) and left superior medial frontal cortex was positively related to crystallized cognition and negatively related to conscientiousness after controlling for covariates (Fig. 2c, d ). However, comparisons of results did not find overlapping FC in the AAL atlas (Fig. S3 ).
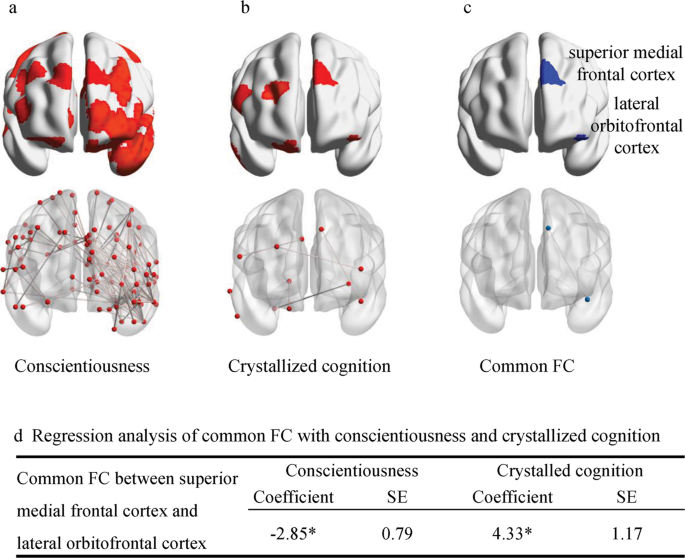
* pval_fdr < 0.05. SE, standard error. a Activation area and functional connectivity of conscientiousness included 142 edges and 100 nodes. b Activation area and functional connectivity of crystallized cognition included six edges and twelve nodes. c Functional connectivity of the left lateral orbitofrontal cortex and left superior medial frontal cortex was the only one shared FC among conscientiousness and crystallized cognition. d Linear regression models indicated that activation of FC was negatively related to conscientiousness but positively related to crystallized cognition after controlling for covariates.
In the present study, we examined the mediation effect of different personality traits on the relationship between short-term perceived stress and total/crystallized cognition and explored the potential mechanism based on neuroimaging data. The relationship between stress and cognition was mediated by neuroticism, conscientiousness, and agreeableness. The mediation effect of neuroticism and agreeableness modulated the adverse effect of short-term perceived stress on cognition, whereas conscientiousness mitigated this adverse effect. The total effect, which was a combination of the direct effect of stress and indirect effect of personality, indicated that stress was negatively associated with total/crystallized cognition. Further neuroimaging analysis showed that right SFG cortical thickness was not only positively associated with stress and neuroticism, but also was negatively associated with total and crystallized cognition. The chain mediation model found that the right SFG and neuroticism play a small chain mediating effect between stress and total cognition. Moreover, people with high conscientiousness had a worse cognitive function and exhibited the hypoactivation of FC between the left lateral OFC and left superior medial frontal cortex. Our study indicates that people with high neuroticism have a higher vulnerability to cognitive decline induced by stress, while people with low neuroticism have a lower vulnerability to cognitive decline induced by stress. These results broaden the understanding of the relationship between short-term perceived stress, cognition, and neuroticism, and indicate that the structural and functional changes of SFG may be underlying neural mechanisms.
Short-term perceived stress was negatively associated with total/crystallized cognitive function, and the relationship was mainly mediated by neuroticism and conscientiousness, and partially mediated by agreeableness. Acute stress exposure and chronic perceived stress across the life span increase the neuroticism level [ 15 , 59 ]. Individuals who score high on neuroticism are more likely than average to be moody and to experience negative feelings [ 60 ], respond worse to stressors, and are more likely to interpret ordinary situations as threatening and minor frustrations as hopelessly difficult. Continuous stress reduce conscientiousness which is associated with decreasing self-control and deliberation [ 16 , 61 ], and then lower conscientiousness improved cognitive flexibility [ 29 , 62 , 63 ], and language comprehension [ 64 ]. Thus, decreasing conscientiousness was not completely harmful, and may have a positive indirect effect, contrary to the direction of the total effect on total cognition and crystallized cognition. This opposite direction could be interpreted in such a way that the modulatory effect of conscientiousness partly relieves the effect of stress on cognition, whereas neuroticism and agreeableness transmit the effect of stress on cognition. In the present study, the mediated effect of personality traits on crystallized cognition and not fluid cognition, maybe because personality traits have a higher correlation with crystallized cognition rather than fluid cognition [ 33 , 65 ]. Support for the finding was provided by a study by Ashton and colleagues, which have reported openness is more strongly correlated with crystallized than with fluid abilities [ 66 ]. All in all, cognitive decline is caused by a combination of the direct effect of stress and the indirect effect of personality.
The modulatory effects of neuroticism and conscientiousness on this relationship were supported by the neuroimaging data. Firstly, the neuroimaging analysis found that right SFG cortical thickness was positively associated with perceived stress and neuroticism, and negatively associated with total/crystallized cognitive function. In addition, the chain mediation model further demonstrated that the cortical thickness of the right SFG and neuroticism play a small but significant chain mediating effect between perceived stress and total/crystallized cognition. The SFG, comprising one-third of the prefrontal cortex, contributes to higher cognitive functions and particularly to working memory and executive cognition [ 67 ]. The PFC is sensitive to the detrimental effects of stress exposure and the cortical thickness of PFC is associated with cognition impairement [ 34 , 68 , 69 , 70 ]. High levels of stress induced decreased blood flow and lower coherence regional homogeneity in the SFG [ 71 , 72 ]. A recent study based on HCP also found that the association between the SFG thickness and aggressive behavior is mediated by neuroticism, and the SFG thickness is positively associated with neuroticism, which supports our results [ 73 ]. People with high neuroticism who perceive more insecurity show greater activation in right SFG [ 74 ] which mediates the processing of subjective awareness and self-related information [ 75 ]. The findings showed that individuals with a higher right SFG cortical thickness had higher neuroticism and worse total/crystallized cognitive function than individuals with lower right SFG cortical thickness. Secondly, we also found that the FC between the left lateral OFC and left superior medial frontal cortex is negatively correlated with conscientiousness and positively correlated with crystallized cognition. The OFC in the ventral surface of the prefrontal lobe is involved in the cognitive process of sensory integration, emotion processing, and decision-making [ 76 , 77 , 78 ]. And the superior medial frontal cortex which is part of SFG is correlated with inhibitory control ability and other cognitive function [ 67 , 79 ]. The FC between left lateral OFC and left superior medial frontal cortex, the major component of the executive control network [ 80 ], is involved in cognitive control and decision-making [ 81 ]. Crystallized cognition refers to learned knowledge and experiences which enable people to categorize situations to make decisions [ 82 ], and people with high conscientiousness have poor decision-making capability due to the traits reflecting order and deliberation [ 63 ] which may explain why people with high conscientiousness have worse crystallized cognition than people with low conscientiousness.
The present study has several limitations. Firstly, the perceived stress in the study was measured by short-term perceived stress scales which assesses the degree to which situations are appraised as stressful in the last month and does not reflect the long-term stress level. Secondly, our study was based on whole-brain analysis without pre-defined regions of interest, and the further comparisons of results did not find overlapping FC in the AAL atlas. Therefore, the FC results are scattered and in-depth data analysis using another parcellation atlas is required to confirm the robustness of the current findings. Thirdly, the HCP data were cross-sectional without longitudinal follow-up, so it is difficult to prove a causal relationship between short-term perceived stress and cognitive function. Future studies should explore the influence of longitudinal changes in chronic stress and acute stress on cognitive function and the involvement of specific brain structure and neurocircuitry.
The present study tested the hypothesis that short-term perceived stress is negatively associated with total/crystallized cognition, mediated by neuroticism, conscientiousness, and agreeableness. Our findings might be useful for understanding the neuroimaging mechanisms that underlie stress-related cognition decline and preventing and controlling the adverse effects of perceived stress among individuals with susceptible personality traits.
Lazarus RS. Psychological stress and coping in adaptation and illness. Int J Psychiatry Med. 1974;5:321–33. https://doi.org/10.2190/t43t-84p3-qdur-7rtp .
Article CAS PubMed Google Scholar
Mirzaei M, Yasini Ardekani SM, Mirzaei M, Dehghani A. Prevalence of depression, anxiety and stress among adult population: Results of yazd health study. Iran J Psychiatry. 2019;14:137–46.
PubMed PubMed Central Google Scholar
Sherina MS, Rampal L, Kaneson N. Psychological stress among undergraduate medical students. Med J Malays. 2004;59:207–11.
CAS Google Scholar
Thomée S, Eklöf M, Gustafsson E, Nilsson R, Hagberg M. Prevalence of perceived stress, symptoms of depression and sleep disturbances in relation to information and communication technology (ICT) use among young adults—an explorative prospective study. Comput Hum Behav. 2007;23:1300–21. https://doi.org/10.1016/j.chb.2004.12.007 .
Article Google Scholar
Luethi, M, Meier, B, Sandi, C. Stress effects on working memory, explicit memory, and implicit memory for neutral and emotional stimuli in healthy men. Front Behav Neurosci. 2009. https://doi.org/10.3389/neuro.08.005.2008 .
Hidalgo V, Villada C, Almela M, Espín L, Gómez-Amor J, Salvador A. Enhancing effects of acute psychosocial stress on priming of non-declarative memory in healthy young adults. Stress. 2012;15:329–38. https://doi.org/10.3109/10253890.2011.624224 .
Article PubMed Google Scholar
Wang J, Rao H, Wetmore JS, Furlan PM, Korczykowski M, Dinges DF, et al. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proc Natl Acad Sci USA. 2005;102:17804–9. https://doi.org/10.1073/pnas.0503082102.
Article CAS PubMed PubMed Central Google Scholar
Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour, and cognition. Nat Rev Neurosci. 2009;10:434–45. https://doi.org/10.1038/nrn2639.
Sweis BM, Veverka KK, Dhillon ES, Urban JH, Lucas LR. Individual differences in the effects of chronic stress on memory: Behavioral and neurochemical correlates of resiliency. Neuroscience. 2013;246:142–59. https://doi.org/10.1016/j.neuroscience.2013.04.052 .
Tsai N, Eccles JS, Jaeggi SM. Stress and executive control: Mechanisms, moderators, and malleability. Brain Cogn. 2019;133:54–59. https://doi.org/10.1016/j.bandc.2018.10.004.
Kohn N, Hermans EJ, Fernández G. Cognitive benefit and cost of acute stress is differentially modulated by individual brain state. Soc Cogn Affect Neurosci. 2017;12:1179–87. https://doi.org/10.1093/scan/nsx043.
Article PubMed PubMed Central Google Scholar
Folkman, S, Lazarus, RS. Psychological and biological approaches to emotion. Lawrence Erlbaum Associates, Psychology Press 1990. 313–32.
Ursin H, Eriksen HR. The cognitive activation theory of stress. Psychoneuroendocrinology. 2004;29:567–92. https://doi.org/10.1016/S0306-4530(03)00091-X.
Matthews, G, Deary, IJ, Whiteman, MC Personality traits. Cambridge University Press; 2003.
Riese H, Snieder H, Jeronimus BF, Korhonen T, Rose R, Kaprio J, et al. Timing of stressful life events affects stability and change of neuroticism. Eur J Pers 2014;28:193–200. https://doi.org/10.1002/per.1929.
Goldberg LR. The structure of phenotypic personality traits. Am Psychol. 1993;48:26–34. https://doi.org/10.1037//0003-066x.48.1.26.
Bleidorn W, Hopwood CJ, Lucas RE. Life events and personality trait change. J Pers. 2018;86:83–96. https://doi.org/10.1111/jopy.12286.
Lüdtke O, Roberts BW, Trautwein U, Nagy G. A random walk down university avenue: life paths, life events, and personality trait change at the transition to university life. J Pers Soc Psychol. 2011;101:620–37. https://doi.org/10.1037/a0023743.
Jeronimus, B, Riese, H, Ormel, J. Environmental influences on neuroticism in adulthood: A systematic review Ridderprint BV Press; 2015;75–131.
Lin MF, Ko HC, Wu JY, Chang FM. The impact of extroversion or menopause status on depressive symptoms among climacteric women in Taiwan: Neuroticism as moderator or mediator? Menopause. 2008;15:138–43. https://doi.org/10.1097/gme.0b013e31804b419d.
Vinkers CH, Joëls M, Milaneschi Y, Kahn RS, Penninx B, Boks M. Stress exposure across the life span cumulatively increases depression risk and is moderated by neuroticism. Depress Anxiety. 2014;31:737–45. https://doi.org/10.1002/da.22262.
Li D, Cui Y, Liu Y, Zheng Y, Zeng Y, Cheng A. A chain mediation model of perceived stress, neuroticism, and psychological inflexibility on depressive symptoms of chinese new fathers. Am J Mens Health. 2021;15:15579883211054351. https://doi.org/10.1177/15579883211054351.
Sutin AR, Costa PT, Wethington E, Eaton W. Turning points and lessons learned: Stressful life events and personality trait development across middle adulthood. Psychol Aging. 2010;25:524–33. https://doi.org/10.1037/a0018751.
Caselli RJ, Dueck AC, Locke DE, Henslin BR, Johnson TA, Woodruff BK, et al. Impact of personality on cognitive aging: a prospective cohort study. J Int Neuropsychol Soc. 2016;22:765–76. https://doi.org/10.1017/s1355617716000527.
Rouch I, Padovan C, Pongan E, Boublay N, Laurent B, Dorey JM, et al. Personality traits are related to selective cognitive impairment in early Alzheimer’s disease. J Alzheimers Dis. 2019;71:1153–62. https://doi.org/10.3233/jad-190459.
Wettstein M, Wahl HW, Siebert J, Schröder J. Still more to learn about late-life cognitive development: How personality and health predict 20-year cognitive trajectories. Psychol Aging. 2019;34:714–28. https://doi.org/10.1037/pag0000374.
Simon SS, Lee S, Stern Y. Personality-cognition associations across the adult life span and potential moderators: Results from two cohorts. J Pers. 2020;88:1025–39. https://doi.org/10.1111/jopy.12548.
Moutafi J, Furnham A, Crump J. What facets of openness and conscientiousness predict fluid intelligence score? Learn Individ Differ. 2006;16:31–42. https://doi.org/10.1016/j.lindif.2005.06.003.
Moutafi J, Furnham A, Paltiel L. Why is conscientiousness negatively correlated with intelligence? Pers Individ Dif. 2004;37:1013–22. https://doi.org/10.1016/j.paid.2003.11.010.
Arana JM, Meilan JJ, Perez E. The effect of personality variables in the prediction of the execution of different prospective memory tasks in the laboratory. Scand J Psychol. 2008;49:403–11. https://doi.org/10.1111/j.1467-9450.2008.00671.x.
Cuttler C, Graf P. Personality predicts prospective memory task performance: An adult lifespan study. Scand J Psychol. 2007;48:215–31. https://doi.org/10.1111/j.1467-9450.2007.00570.x.
Moutafi J, Furnham A, Paltiel L. Can personality factors predict intelligence? Pers Indiv Differ. 2005;38:1021–33. https://doi.org/10.1016/j.paid.2004.06.023.
Rammstedt, B, Lechner, CM, Danner, D. Relationships between personality and cognitive ability: A facet-level analysis. J Intell. 2018. https://doi.org/10.3390/jintelligence6020028 .
McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29. https://doi.org/10.1016/j.neuron.2013.06.028.
Hinwood M, Tynan RJ, Charnley JL, Beynon SB, Day TA, Walker FR. Chronic stress induced remodeling of the prefrontal cortex: Structural re-organization of microglia and the inhibitory effect of minocycline. Cereb Cortex. 2013;23:1784–97. https://doi.org/10.1093/cercor/bhs151.
Arnsten AF. Stress weakens prefrontal networks: Molecular insults to higher cognition. Nat Neurosci. 2015;18:1376–85. https://doi.org/10.1038/nn.4087.
Qin S, Hermans E, van Marle H, Luo J, Fernández G. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biol Psychiatry. 2009;66:25–32. https://doi.org/10.1016/j.biopsych.2009.03.006.
Luettgau L, Schlagenhauf F, Sjoerds Z. Acute and past subjective stress influence working memory and related neural substrates. Psychoneuroendocrinology. 2018;96:25–34. https://doi.org/10.1016/j.psyneuen.2018.05.036.
Saleh A, Potter GG, McQuoid DR, Boyd B, Turner R, MacFall JR, et al. Effects of early life stress on depression, cognitive performance and brain morphology. Psychol Med. 2017;47:171–81. https://doi.org/10.1017/S0033291716002403.
Schilling C, Kühn S, Paus T, Romanowski A, Banaschewski T, Barbot A, et al. Cortical thickness of superior frontal cortex predicts impulsiveness and perceptual reasoning in adolescence. Mol Psychiatry. 2013;18:624–30. https://doi.org/10.1038/mp.2012.56.
Parker N, Vidal-Pineiro D, French L, Shin J, Adams H, Brodaty H, et al. Corticosteroids and regional variations in thickness of the human cerebral cortex across the lifespan. Cereb Cortex. 2020;30:575–86. https://doi.org/10.1093/cercor/bhz108.
Zhu Y, Li X, Qiao Y, Shang R, Shi J, Shang Y. et al. Widespread plasticity of cognition-related brain networks in single-sided deafness revealed by randomized window-based dynamic functional connectivity. Med Image Anal. 2021;73:102163. https://doi.org/10.1016/j.media.2021.102163 .
Mulders P, Llera A, Tendolkar I, van Eijndhoven P, Beckmann C. Personality profiles are associated with functional brain networks related to cognition and emotion. Sci Rep. 2018;8:13874. https://doi.org/10.1038/s41598-018-32248-x.
Human Connectome Project. http://www.humanconnectomeproject.org/ (accessed Feb 27, 2022).
Van Essen DC, Smith SM, Barch DM, Behrens T, Yacoub E, Ugurbil K, et al. The WU-Minn Human Connectome Project: An overview. Neuroimage. 2013;80:62–79. https://doi.org/10.1016/j.neuroimage.2013.05.041.
Salsman J, Butt Z, Pilkonis PA, Cyranowski JM, Zill N, Hendrie HC, et al. Emotion assessment using the NIH Toolbox. Neurology. 2013;80:S76–86. https://doi.org/10.1212/WNL.0b013e3182872e11.
Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96.
Article CAS Google Scholar
Mccrae RR, Costa PT. A contemplated revision of the NEO Five-Factor Inventory. Pers Indiv Differ. 2004;36:587–96.
Akshoomoff N, Beaumont JL, Bauer PJ, Dikmen SS, Gershon RC, Mungas D, et al. VIII. NIH Toolbox Cognition Battery (CB): Composite scores of crystallized, fluid, and overall cognition. Monogr Soc Res Child Dev. 2013;78:119–32. https://doi.org/10.1111/mono.12038.
Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, et al. Cognition assessment using the NIH Toolbox. Neurology. 2013;80:S54–64. https://doi.org/10.1212/WNL.0b013e3182872ded.
Cattell, RB. Theory of fluid and crystallized intelligence: A critical experiment. J Educ Psychol. 1963;54:1–22.
Heaton RK, Akshoomoff N, Tulsky D, Mungas D, Weintraub S, Dikmen S, et al. Reliability and validity of composite scores from the NIH Toolbox Cognition Battery in adults. J Int Neuropsychol Soc. 2014;20:588–98. https://doi.org/10.1017/s1355617714000241.
Bolin JH, Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY: The Guilford Press. J Educ Meas. 2014;51:335–7. https://doi.org/10.1111/jedm.12050 .
Hayes, AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York: Guilford Publications; 2017.
Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013;80:105–24. https://doi.org/10.1016/j.neuroimage.2013.04.127.
Smith SM, Beckmann CF, Andersson J, Auerbach EJ, Bijsterbosch J, Douaud G, et al. Resting-state fMRI in the Human Connectome Project. Neuroimage. 2013;80:144–68. https://doi.org/10.1016/j.neuroimage.2013.05.039.
Shen X, Tokoglu F, Papademetris X, Constable RT. Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. Neuroimage. 2013;82:403–15. https://doi.org/10.1016/j.neuroimage.2013.05.081.
Zalesky A, Fornito A, Bullmore ET. Network-based statistic: Identifying differences in brain networks. Neuroimage. 2010;53:1197–207. https://doi.org/10.1016/j.neuroimage.2010.06.041.
Shields GS, Toussaint LL, Slavich GM. Stress-related changes in personality: A longitudinal study of perceived stress and trait pessimism. J Res Pers. 2016;64:61–68. https://doi.org/10.1016/j.jrp.2016.07.008.
Thompson ER. Development and validation of an international english big-five mini-markers. Pers Indiv Differ. 2008;45:542–8.
Muraven M, Baumeister R. Self-regulation and depletion of limited resources: Does self-control resemble a muscle? Psychol Bull. 2000;126:247–59. https://doi.org/10.1037/0033-2909.126.2.247.
Soubelet A. Age-cognition relations and the personality trait of conscientiousness. J Res Pers. 2011;45:529–34. https://doi.org/10.1016/j.jrp.2011.06.007.
Lepine JA, Colquitt JA, Erez A. Adaptability to changing task contexts: Effects of general cognitive ability, conscientiousness, and openness to experience. Pers Psychol. 2000;53:563–93. https://doi.org/10.1111/j.1744-6570.2000.tb00214.x .
Tõugu P, Tulviste T. Links between children’s language comprehension and expressive vocabulary and their personality traits: A longitudinal investigation. Learn Individ Differ. 2017;57:123–8. https://doi.org/10.1016/j.lindif.2017.03.015.
Brand CR. Open to experience—closed to intelligence: Why the ‘Big Five’ are really the ‘Comprehensive Six’. Eur J Pers. 1994;8:299–310. https://doi.org/10.1002/per.2410080407 .
Ashton MC, Lee K, Vernon PA, Jang KL. Fluid intelligence, crystallized intelligence, and the openness/intellect factor. J Res Pers. 2000;34:198–207. https://doi.org/10.1006/jrpe.1999.2276 .
du Boisgueheneuc F, Levy R, Volle E, Seassau M, Duffau H, Kinkingnehun S, et al. Functions of the left superior frontal gyrus in humans: A lesion study. Brain. 2006;129:3315–28. https://doi.org/10.1093/brain/awl244 .
Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–22. https://doi.org/10.1038/nrn2648.
Arnsten AFT, Raskind MA, Taylor FB, Connor DF. The effects of stress exposure on prefrontal cortex: Translating basic research into successful treatments for post-traumatic stress disorder. Neurobiol Stress. 2015;1:89–99. https://doi.org/10.1016/j.ynstr.2014.10.002.
Schnack HG, van Haren NEM, Brouwer RM, Evans A, Durston S, Boomsma DI, et al. Changes in thickness and surface area of the human cortex and their relationship with intelligence. Cereb Cortex. 2014;25:1608–17. https://doi.org/10.1093/cercor/bht357.
Soufer R, Bremner JD, Arrighi JA, Cohen I, Zaret BL, Burg MM, et al. Cerebral cortical hyperactivation in response to mental stress in patients with coronary artery disease. Proc Natl Acad Sci USA. 1998;95:6454–9. https://doi.org/10.1073/pnas.95.11.6454.
Chang J, Yu R. Acute social stress modulates coherence regional homogeneity. Brain Imaging Behav. 2019;13:762–70. https://doi.org/10.1007/s11682-018-9898-9.
Zhu X, Wang K, Cao A, Zhang Y, Qiu J. Personality traits and negative affect mediate the relationship between cortical thickness of superior frontal cortex and aggressive behavior. Neurosci Lett. 2020;718:134728 https://doi.org/10.1016/j.neulet.2019.134728.
Premkumar P, Ettinger U, Inchley-Mort S, Sumich A, Williams SCR, Kuipers E, et al. Neural processing of social rejection: the role of schizotypal personality traits. Hum Brain Mapp. 2012;33:695–706. https://doi.org/10.1002/hbm.21243.
Goldberg I, Harel M, Malach R. When the brain loses its self: Prefrontal inactivation during sensorimotor processing. Neuron. 2006;50:329–39. https://doi.org/10.1016/j.neuron.2006.03.015.
Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. https://doi.org/10.1038/nrn1747.
Bechara A, Damasio H, Damasio AR. Emotion, decision making, and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. https://doi.org/10.1093/cercor/10.3.295.
Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10:284–94. https://doi.org/10.1093/cercor/10.3.284.
Hsu T-Y, Tseng LY, Yu JX, Kuo WJ, Hung DL, Tzeng OJL, et al. Modulating inhibitory control with direct current stimulation of the superior medial frontal cortex. NeuroImage. 2011;56:2249–57. https://doi.org/10.1016/j.neuroimage.2011.03.059.
Liu L, Zeng LL, Li Y, Ma Q, Li B, Shen H, et al. Altered cerebellar functional connectivity with intrinsic connectivity networks in adults with major depressive disorder. PloS One. 2012;7:e39516 https://doi.org/10.1371/journal.pone.0039516.
Pessoa L. How do emotion and motivation direct executive control? Trends Cogn Sci. 2009;13:160–6. https://doi.org/10.1016/j.tics.2009.01.006.
Klein G. Naturalistic decision making. Hum Factors. 2008;50:456–60. https://doi.org/10.1518/001872008X288385.
Download references
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 81761128036, 81821092, 31871259, and 82171477), National Key Research and Development Program of China (no. 2019YFC0118601), and Beijing Brain Research Program (no. Z181100001518005).
Author information
These authors contributed equally: Ximei Zhu; Wei Yan; Xiao Lin.
Authors and Affiliations
Peking University Sixth Hospital, Peking University Institute of Mental Health, NHC Key Laboratory of Mental Health (Peking University), National Clinical Research Center for Mental Disorders (Peking University Sixth Hospital), Peking University, Beijing, 100191, China
Ximei Zhu, Wei Yan, Xiao Lin, Jianyu Que, Yuetong Huang, Haohao Zheng, Lin Liu, Jiahui Deng, Lin Lu & Suhua Chang
Research Unit of Diagnosis and Treatment of Mood Cognitive Disorder (2018RU006), Chinese Academy of Medical Sciences, Beijing, 100191, China
Lin Lu & Suhua Chang
You can also search for this author in PubMed Google Scholar
Contributions
XZ, XL, and SC analyzed the study data and wrote the initial draft. JQ, YH, HZ, LL, JD, LL, and SC revised the manuscript. WY, XL, LL, and SC participated in editing, interpretation, and revision. All authors contributed to and have approved the final manuscript.
Corresponding authors
Correspondence to Lin Lu or Suhua Chang .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary materials, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Zhu, X., Yan, W., Lin, X. et al. The effect of perceived stress on cognition is mediated by personality and the underlying neural mechanism. Transl Psychiatry 12 , 199 (2022). https://doi.org/10.1038/s41398-022-01929-7
Download citation
Received : 03 January 2021
Revised : 28 March 2022
Accepted : 04 April 2022
Published : 12 May 2022
DOI : https://doi.org/10.1038/s41398-022-01929-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
UTC Scholar
- UTC Scholar Home
- UTC Library
Preserving and Sharing UTC's Knowledge
- < Previous
Home > Student Research, Creative Works, and Publications > Masters Theses and Doctoral Dissertations > 512
Masters Theses and Doctoral Dissertations
The role of resilience, emotion regulation, and perceived stress on college academic performance.
Katherine A. Pendergast , University of Tennessee at Chattanooga Follow
Committee Chair
Ozbek, Irene Nichols, 1947-
Committee Member
Clark, Amanda J.; Rogers, Katherine H.
Dept. of Psychology
College of Arts and Sciences
University of Tennessee at Chattanooga
Place of Publication
Chattanooga (Tenn.)
Stress is a common problem for college students. The goal of this thesis was to examine the relationships between protective and risk factors to experiencing stress and how these factors may predict academic performance in college students. 125 college students were surveyed twice over the course of a semester on emotion regulation strategies, trait resilience, and perceived stress. The relationships between these variables and semester GPA were analyzed using correlational, multiple regression, and hierarchical regression analyses. It was determined that trait resilience scores do predict use of emotion regulation strategies but change in stress and trait resilience do not significantly predict variation in academic performance during the semester. Limitations and future directions are further discussed.
Acknowledgments
Thanks to my advisor, Dr. Ozbek, and committee members, Dr. Clark and Dr. Rogers, for invaluable feedback and support. Additional thanks to Dr. Jonathan Davidson, M.D., for his permission to use the CD-RISC to better understand resilience in the college population. Also, I would like to extend thanks to Linda Orth, Sandy Zitkus, and the entire records office staff of the University of Tennessee at Chattanooga for their willingness to collaborate and assist with this project. Lastly, I would like to thank the faculty and students of the Psychology Department for their overall support.
M. S.; A thesis submitted to the faculty of the University of Tennessee at Chattanooga in partial fulfillment of the requirements of the degree of Master of Science.
Stress (Psychology); Academic achievement -- Education (Higher)
Stress; Resilience; Emotion regulation; Academic performance
Document Type
Masters theses
xi, 72 leaves
https://rightsstatements.org/page/InC/1.0/?language=en
http://creativecommons.org/licenses/by-nc/3.0/
Recommended Citation
Pendergast, Katherine A., "The role of resilience, emotion regulation, and perceived stress on college academic performance" (2017). Masters Theses and Doctoral Dissertations. https://scholar.utc.edu/theses/512
Since April 24, 2017
Included in
Psychology Commons
Advanced Search
- Notify me via email or RSS
- Collections
- Disciplines
Author Corner
- Submission Guidelines
- Submit Research
- Graduate School Thesis and Dissertation Guidelines
Home | About | FAQ | My Account | Accessibility Statement
Privacy Copyright
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

More than a feeling: A unified view of stress measurement for population science
Elissa s. epel.
a Department of Psychiatry, University of California San Francisco, 401 Parnassus Avenue, San Francisco, CA, USA
Alexandra D. Crosswell
Stefanie e. mayer, aric a. prather, george m. slavich.
b Cousins Center for Psychoneuroimmunology and Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, CA, USA
Eli Puterman
c School of Kinesiology, University of British Columbia, Vancouver, BC, Canada
Wendy Berry Mendes
Stress can influence health throughout the lifespan, yet there is little agreement about what types and aspects of stress matter most for human health and disease. This is in part because “stress” is not a monolithic concept but rather, an emergent process that involves interactions between individual and environmental factors, historical and current events, allostatic states, and psychological and physiological reactivity. Many of these processes alone have been labeled as “stress.” Stress science would be further advanced if researchers adopted a common conceptual model that incorporates epidemiological, affective, and psychophysiological perspectives, with more precise language for describing stress measures. We articulate an integrative working model, highlighting how stressor exposures across the life course influence habitual responding and stress reactivity, and how health behaviors interact with stress. We offer a Stress Typology articulating timescales for stress measurement – acute, event-based, daily, and chronic – and more precise language for dimensions of stress measurement.
1. Introduction: Defining the problem
Today, most health researchers agree that stress is critical to human health and aging. Population-based studies that have measured perception of, or exposure to, stressors have documented its effects on health. Stress is tightly linked to psychological well-being, with stressful events acting as a precursor to many major psychiatric conditions ( Cohen et al., 2007 ; Hammen, 2005 ). The costs of morbidity associated with mental health conditions exceed that of any other diseases ( HALE Collaborators, 2016 ; Whiteford et al., 2013 ).
There is also now reliable evidence that stress is associated with greater risk of disease, including cardiovascular disease, hypertension, and infectious disease ( Cohen et al., 2007 ). A large but disjointed literature shows that stress affects slow-acting biological processes in the brain and body, accelerating diseases of aging. A deeper understanding of the mechanistic pathways by which psychosocial stress impacts physiology will lead to critical advances in both basic science and prevention; however, these advances cannot occur without better models and measures of stress. To better understand the effects of lifespan stress on health, ideally one would use prospective measures, starting during youth, and track people until after 50 years old when disease onset becomes more common. Fortunately, although diagnosable disease does not occur until later in life, there are several reliable indices of early damage to regulatory systems, allostatic load ( Hwang et al., 2014 ; Robertson et al., 2017 ; Seeman et al., 2001 ), and cellular aging such as inflammation, telomere length, and epigenetic clock ( Chen et al., 2016 ; Codd et al., 2013 ; Emerging Risk Factors Collaboration et al., 2010 ; Li et al., 2017 ; Zhang et al., 2017 ; Zhou et al., 2016 ) that can be measured during mid-life, and serve as markers, risk factors, and likely mechanistic precursors to disease or early mortality.
Despite widespread agreement that stress is important in the study of health and aging, there are critical barriers that prevent scientific progress. One major barrier, our focus here, is the lack of consistency and thoroughness in stress measurement. Measurement of stress is inherently complex because stress is experienced on multiple levels – social, psychological, and physiological. Therefore, there are few agreed upon ‘gold standard’ measures. Across studies, measurement is inconsistent and often superficial, and heterogeneous constructs are conflated. To improve stress measurement, we need to better articulate our measurement approaches using a common language of stress, as well as more complex and precise stress models that take into account the multi-level nature of stress. Here, we present a stress taxonomy as a step toward providing a common language for measurement, including dimensions of exposure, responses, and timescales ( Appendix 1 ). We also present a transdisciplinary model of stress that merges knowledge from both epidemiological and experimental approaches ( Fig. 1 ).

This figure presents a transdisciplinary model that describes “stress” as a set of interactive and emergent processes. The figure illustrates that stressors are experienced within the context of a person’s life, represented by the contextual factors in the blue triangle. These contextual factors include individual-level characteristics such as personality and demographic factors, the environment in which one lives, current and past stressor exposures, and protective factors; all of which combine to determine the baseline allostatic state of physiological regulation, and the lens through which stressors are perceived and assigned meaning. Contextual factors and habitual processes together influence psychological and physiological responses to acute and daily stressors. These responses, if dysregulated, are thought to lead to allostatic load and ultimately biological aging and early disease.
2. Toward more precise measurement and models of stress
2.1. what is stress, exactly.
There is an almost unbounded set of human experiences that can fall under the umbrella of ‘stress.’ The term stress is frequently used in both scientific circles and colloquially to refer to a number of different processes that are related but distinctly different. For example, “stress” is sometimes used to refer to actual life events or situations that happen to a person, such as losing a job or divorcing a spouse (hereafter “stressors” or “stressor exposures”). Stress is also used to refer to the cognitive, emotional, and biological reactions that such situations evoke (“stress responses”).
The definition of stress partly depends on whom you ask and their main discipline. For example, economists and social epidemiologists tend to define stressors in terms of social or economic contexts such as poverty or neighborhood deprivation. In contrast, psychologists tend to focus on individual level life events such as combat exposure, divorce, physical abuse, job loss, and daily hassles. Stress responses are assessed at many levels of analysis including self-reported perceptions and appraisals, affect ratings, patterns of neural activity, and physiological reactivity. All of these different measures of stressor exposures, perceptions of stress, and psychological and biological stress responses are at best loosely related ( Mauss et al., 2005 ). Furthermore, it is unclear which of these measures most accurately and consistently relate to health outcomes, and this often depends on the cohort sampled, which health outcomes are measured, and whether the outcomes are short or long term ( Cohen et al., 1997 ; Rehkopf et al., 2010 ). There is also lack of consensus about what the most health-damaging aspects of stress are, which has led to difficulty in moving the field beyond demonstrating associations between different measures of stress and specific health outcomes.
Because stress is so broadly defined, it can include antecedent, stimulus, or response. Hence, any measure tapping an exposure, perception, or biological or neural response can be labeled a measure of stress. This over-inclusiveness renders the construct of stress, as it is currently defined, of limited utility. This problem has led some researchers to suggest that we replace the term with detailed descriptions of stimuli meant to induce arousal ( Kagan, 2016 ). While this specificity could be a helpful way to operationalize laboratory tasks used to elicit stress responses, it would limit the ability for researchers to integrate findings. Too much descriptive specificity of stress processes would make it appear as if all studies are using and measuring heterogeneous tasks and processes, when in fact there are often core similarities. Identifying these similarities and describing them with common language allows for cross-study comparison and accumulated knowledge by looking across studies.
The traditional psychology definition of stress most adopted comes from stress and coping theory ( Lazarus and Folkman, 1984 ). In this definition, stress occurs when a person perceives the demands of an environmental stimuli to be greater than their ability to meet, mitigate, or alter those demands ( Lazarus et al., 1985 ). These perceptions of stress are not the same construct as trait measures of depressive symptoms or anxiety though there is some overlap. Trait-level measures of anxiety and depressive symptoms capture more diffuse individual differences in affective and behavioral experiences, whereas perceived stress refers to a response to specific conditions (though it can be chronic like anxiety and depressive symptoms). Perceived stress typically includes several psychological components of the stress response – feelings of overwhelm, or anxiety, as well as cognitions that demands outweigh resources, or not having control.
Perceived stress is also different from affect despite the important role negative affect plays in the conceptualization and measurement of perceived stress. Affective states are a large umbrella term for all emotional experiences, including emotional reactivity, longer-term mood states, and dispositional traits. Measures of perceived psychological stress capture a mix of affective states and cognitions in response to a situation. Researchers have tried to harmonize across the disparate literatures on acute stress and affective states like emotion and motivation in an attempt to understand how affective states shape our health and well-being (e.g., DeSteno et al., 2013 ). The overlap of these constructs are so widely accepted that the axiom in academia—stress is studied in medical schools; emotion is studied in psychology departments—underscores the similar phenomenology, biology, and consequences of stress and emotion while highlighting the different goals and approaches to studying these affective states. Stress research is more typically focused on mental and/or physical health outcomes, whereas emotion research focuses on antecedents of emotional states and short-term responses like neural activation, behavioral responses, and decision making.
Adding complexity to the differences between stress and affect, acute psychological stress responses are often measured by capturing specific emotional states. This is because negative emotional responses (fear, anxiety, sadness, anger) to an acute stressor are considered a core component of an acute stress response. Furthermore, emotions can be measured acutely and precisely as immediate responses to an eliciting event. Perceptions of stress can also be captured (e.g. by asking how overwhelmed one is by a task), but these do not capture the specific emotional experiences elicited by events (which motivate specific behavioral responses). Instead, they capture a vague construct of general distress that is less helpful in predicting behavioral and physiological outcomes. Another difference between perceived stress and negative emotions is the timescales in which they are typically experienced and measured. Emotions can be experienced and reconciled in milliseconds or seconds while perceptions of stress can be experienced over hours, weeks, or months. Lastly, emotions, traditionally, have a more specific eliciting agent. Snakes engender fear; gore engenders disgust; death brings on sadness. In contrast, acute stress tends to have eliciting situations that are more naturalistic, often require active responses, and are thus broad and diffuse in the emotions they may elicit. For example, the most common lab stressor used, delivering speeches in front of evaluators to elicit social stress, has elements of novelty, social and performance evaluation, uncontrollability, and negative feedback and engenders a wide range of emotional responses across individuals.
2.2. Considering multilevel assessment of stress
Another obstacle in stress research is that our typical model of how stress operates is not precise enough to generate highly predictive models. Stress is multilevel, emergent, and depends on context. By context, we mean the individual’s biographical context such as their age and genetic make-up, socio-cultural context such as socio-economic status and cultural norms, and their history of and current exposure to stress.
Predominant models of stress and health usually start with a potentially stressful event that typically has a beginning, an end, and is followed by a recovery period. In the typical model, stress is perceived by the brain, and feelings of distress and negative emotions trigger the body’s peripheral stress response to select the most appropriate behaviors to best adapt to the stressor ( Cohen et al., 2016 ). With repeated activation, as seen with recurrent stressor exposure, these brief physiological reactions are hypothesized to alter biological processes in the long-term, resulting in cumulative wear and tear on the body ( McEwen, 1998 ). This classic linear model of the relationship between stress and health can be put in a multilevel context that will be both more conceptually complete and predictive.
The linear model of stress where perceived stress and distress are central mediators of health impact is fitting for understanding immediate responses to an acute stressor. However, this model does not apply well to understanding the impact of major life events or chronic stressors that often have no clear end or recovery phase, such as poverty, and thus require ongoing adaptation. Rather, we need to know the additive and possibly interactive effects of historical and current stressors.
Decades of stress research have demonstrated that the timescales of stress are important to take into account. Global measures of perceived stress are helpful to capture recent perceptions (i.e., over the past month), but do not capture cumulative experiences and are not often reliably predictive of long term health outcomes. Similarly, specific affective responses to singular life events or daily stressors do not often have implications for long term health. However, these responses may be an indicator of how that person usually responds to stressors, thus providing insight into the person’s general response patterns. Indeed, affective responses to specific situations can have tremendous value in helping to elucidate a person’s traitlike vulnerability to the damaging effects of stressor exposure on health. For example, Almeida and colleagues have identified aspects of daily stress responses that predict health outcomes. Specifically, either high negative or low positive affect in response to minor daily stressors, measured each evening over at least a week (interpreted as poor recovery from daily stress) predicts worse long-term mental and physical health ( Mroczek et al., 2015 ; Piazza et al., 2013 ; Sin et al., 2015 ).
There are inherent limitations to using self-report measures. First, consciously perceived and self-reported ratings of distress and stress using standard scales explain a limited amount of variance in physiological stress reactivity and biological outcomes. This poses a problem given that the mechanistic pathway linking psychological stress to worse health is hypothesized to be through dysregulated stress reactivity profiles. The lack of association between self-reported ratings of stress and physiological indicators of stress arousal is likely due to many factors. First, events are not exclusively experienced through conscious perception as assumed in basic stress models. Emotional responses are constructed through iterative processes that incorporate the social world (this point is further described below).
Second, subjective reports of being “stressed” are potentially limited by individuals’ unwillingness or inability to report their veridical stress state. Unwillingness might be due to not wanting to appear weak or fragile. Inability to report might be due to lack of conscious perception. Additionally, there is a relative comparison process where one’s subjective understanding of “stress” is calibrated relative to other adverse aspects of their lives as well as to the lives of those in their community. Lastly, if an environment is physically dangerous, or basic resources are especially scarce, a subjective response to “how stressful is your life” might be met with less affirmation given that the lay understanding of stress does not necessarily capture physical danger or basic survival.
There are also cultural factors influencing the expression of feelings of distress, where they are experienced more in the body than in psychological terms, such as the somatization of depression in China ( Ryder and Chentsova-Dutton, 2012 ) or among immigrants with low social resources ( Lin et al., 1985 ). Thus, it is possible that in some cultural groups and circumstances, self-reports of somatic experiences that are outcomes of stressor exposure, such as pain or sleep disturbances, or somatic health symptoms such as headaches or stomachaches, may serve as better indicators of responses to stressor exposure than direct assessments of feelings or thoughts.
Finally, the association between self-reported stress and biological outcomes might be weaker due to psychometric differences in how the two concepts are measured – self-reports using limited Likert-type scaling that are interval responses and biological outcomes that are typically not linear. For example, there is usually a small correlation between self-reports of acute stress perception and cortisol reactivity. This should not be surprising given that cortisol is released in a pulsatile fashion with a pause-dump cycle that would limit the ability to see linear associations with a truncated ordinal self-report scale. Trait psychosocial tendencies can predict trait like allostatic responses to a small extent, such as accounting for variance (less than 5%) in the cortisol awaking response ( Boggero et al., 2017 ), serving as a piece of the foundation for understanding how stress becomes embedded in long term health.
Furthermore, growing research shows that stressor exposure alone has different effects depending on one’s neurobiological predisposition to be vulnerable to stress. High vulnerability does not just confer risk but also confers benefits and thriving in response to supportive environments (differential susceptibility). The interaction between a person’s biological and historical context and acute stress responses are pivotal for uncovering how stressor exposures influence long-term health ( Boyce, 2016 ). There have also been rapid advances in the basic science of stress processes, which have helped to unpack the cognitive and affective response components, and links to neural and peripheral physiological responses. For example, we know that within a day, there are emergent influences that shape acute stress processes – phenomena such as stress contagion that are not mediated through conscious perception ( Palumbo et al., 2017 ; Thorson et al., 2017 ). Stress models can be improved by taking into account the reciprocal relationship between individual-level factors such as age and personality, the context of the person’s life (including socio-economic status and historical stressor exposures), habitual responses such as baseline allostatic physiological states and mental filters, acute stress processes, and the neural and peripheral responses to stressors, as shown in Fig. 1 and described below.
2.3. Toward an integrative model of lifespan stress and health
We present a model of stress and health that suggests how individual and environmental contextual factors and stress processes interact over the lifespan to shape biological aging and disease, shown in Fig. 1 . First, there are contextual factors that shape an individual’s vulnerability or resilience to stress. These include individual-level factors such as genetic and developmental contexts, and environmental factors such as the socio-economic and cultural contexts. One’s cultural and socio-economic context provides the framework from which experiences are interpreted and assigned meaning ( Worthman, 2010 ), and thus influence the extent to which an event will be appraised as threatening or as challenging ( Folkman et al., 1986 ). The person also experiences stressors within the context of their own life experiences, including past exposure to stressful events and past or current experience of chronic stress. An important factor missing in many models of stress is cumulative stress exposure, which includes historical factors and current stress experiences. Having a severe history of stressor exposures (traumatic stress in particular, especially if experienced in childhood) or being under current chronic stress greatly impacts the likelihood of being exposed to more frequent stressors (life events and daily hassles), and of developing maladaptive acute stress responses. Contextual factors and cumulative stress, together with protective factors, shape how people habitually view events and respond to stressors affectively and physiologically.
Protective factors – typically malleable social, psychological, and behavioral traits – influence one’s resilience to stress. For example, protective factors include supportive family structures and maintenance of a physically active lifestyle that allows one to withstand or bounce back from stress ( Cacioppo et al., 2002 ). In the next part of the figure, we introduce habitual processes . Habitual processes include mental filters and allostatic states. Mental filters are the lens from which we see the world. Cognitive biases (e.g., pessimistic expectations of the future) and allostatic states are the basal level of functioning of regulatory systems. The context of one’s life greatly influences these habitual processes. In cases of excessive historical exposure to stressful life events, one’s mental filter is prone to habitually amplify cognitive and emotional responses to stressful stimuli leading to exaggerated threat appraisals, and prolonged, blunted, or otherwise dysregulated physiological reactivity.
Modern views of development of emotional and stress responses view the brain as a ‘prediction machine’ where appraisals of events are shaped in part by one’s personal memory bank of what to expect as well as from the current stimuli ( Barrett and Simmons, 2015 ). For illustration, early childhood adversity is associated with alterations in social, cognitive and behavioral processes in daily life including greater threat appraisal, difficulty regulating emotions, and ineffective social behaviors ( Repetti et al., 2002 ) all of which are associated with alterations in stress physiology ( Woody and Szechtman, 2011 ).
There are several examples of altered basal states. These trait-like responses, response stereotypy, include both habitual arousal patterns that stems from various sources (genetic, historical exposure to stress, or engagement in health maintaining behaviors) or altered trait like reactivity. There can be either low or high basal cortisol, as found in depression, trauma exposure, or chronically high social strain ( Friedman et al., 2012 ; Bernard et al., 2017 ; McEwen, 1998 ). Some people tend to be high cortisol reactors over time even when exposed to the same stressors, without habituating ( Kirschbaum et al., 1995 ; Schommer et al., 2003 ). Some individuals do not have the usual dips in blood pressure at night, and in some studies these nondippers have greater exposure to trauma or low social support ( Mellman et al., 2009 ; Stepnowsky et al., 2004 ; Ulmer et al., 2013 ).
When there is a history of chronic or traumatic stress, there are changes to the neural brain architecture for stress responding – typically amygdala volume enlarges, hippocampal volume decreases, and there is greater connectivity promoting exaggerated responses with poor recovery ( Fig. 1 , Path A). There are also changes to systemic and cellular markers of allostatic load, as detailed in Part 7. These neural pathways for stress become more primed and prepared for future stress, in turn leading to one’s resting allostasis geared toward higher maladaptive patterns of reactivity, defined below as patterns that cause more damage than protection ( Fig. 1 , Path B) promoting a feed forward cycle.
We can take a close-up lens to examine the acute stress processes that unfold momentarily in response to a stressor. Within a day, we respond to internal stressors (such as anticipation and rumination about potential or past stressors), as well as unfolding events. Acute stress processes including cognitive, affective, and biological responses evoked by an acute stressor is, theoretically, a critical nexus in characterizing and explaining lifespan stress and effects on aging.
Psychological responses include cognitive processes of appraisal, and perseverative cognitions (anticipation and rumination) that are associated with allostatic states and reactivity. Affective responses include emotional responses, motivational states, and then efforts to manage the affective and physiological arousal –emotion regulation strategies and coping efforts. Post stressor rumination appears to be a particularly important stress process that may prolong cortisol activation during stress recovery, altering allostatic states ( Gianferante et al., 2014 ; Ottaviani et al., 2016 ; Verkuil et al., 2009 ). These responses are further described in Part 5.
The acute stress response involves multisystem physiological responses , which are interdependent responses between neural pathways and the autonomic, neuroendocrine, and immune systems ( McEwen, 2007 ). While most aspects of the acute response are protective, there are certain profiles of response that are more prone to damage than protection, likely because the response does not adequately match the demands of the situation. We call these response profiles maladaptive stress responses. The dominant theory of how stress exposure ‘gets under the skin’ to impact long term health is that repeated physiological reactivity coupled with maladaptive responses to those repeated hits lead to allostatic load over time. Maladaptive response profiles can take multiple forms: heightened anticipation prior to an event, heightened reactivity to an event, prolonged recovery after the event is over, and lack of habituation which can be defined as both lack of effective adaptation to the event as it is occurring or lack of effective adaption to the same event after repeatedly experiencing the same event on different occasions.
The basic premise underlying the identification of these maladaptive profiles is that an acute response that activates and then shuts off quickly does not harm the body. These systems are dynamic, responding quickly to the environment in order to meet our needs, and at the most basic level – to keep us safe physically and psychologically. However, these same systems and the associated responses can be harmful if the responses are sustained or slow to return to baseline. This is because it requires extra physiological effort, causing wear and tear over time, or alternatively because it represents a physiological system that cannot respond as quickly or nimbly as is needed to effectively cope with environmental demands. Prospective evidence linking maladaptive acute stress response profiles to disease risk remains limited. The most well studied association between stress reactivity and disease outcomes has been cardiovascular reactivity and future cardiovascular disease. A meta-analysis of studies examining cardiovascular reactivity to acute mental stress and future cardiovascular disease found that greater reactivity and poorer recovery from stress were associated with worse cardiovascular health at subsequent study visits, though the effect sizes were small (e.g. probability of incident hypertension increased by ~23%, hazard ratio: 1.23, for individuals with greater compared to weaker stress reactivity; Chida and Steptoe, 2010 ). Past models and research on acute reactivity and health have been overly simplified, assuming that ‘high reactors’ are the individuals more likely to get disease. The relationship between stress reactivity and disease risk is likely more complex and influenced by factors researchers have not consistently or thoroughly examined. In the following section we describe what will likely be a highly promising focus in psychological science stress research – the invisible emergent influences on stress reactivity that ultimately determine if acute stress responses will be maladaptive and lead to disease.
Allostatic load is defined as the perturbation of several physiological systems toward consistently high or low or non-adaptive states even when stressors remit, whose combined perturbations lead to wear and tear on the body. Altered stress reactivity appears to play an important role in the development of psychiatric disorders ( Ehlert et al., 2001 ) as well as cardiovascular disease ( Phillips et al., 2013 ; Lovallo, 2011 ; Treiber et al., 2003 ). However, reactivity alone is a weak predictor of outcomes. This may be because of a lack of focus on defining the specifically malignant aspects of a stress response. Mounting a stress response is in itself a healthy and adaptive physiological response, and more attention must be paid to differentiating between the adaptive function of a “stress” response (such as energizing when energy is needed to act), and responses that are damaging.
These maladaptive stress processes can be applied to both physiological states and affective responses, and the two of these interact and synergize eachother. There is prolonged anticipation of future events , elevation of affective states such as anxiety and worry, or physiological states of vigilant preparedness, reflected in autonomic nervous system and neuroendocrine arousal. There is also exaggerated, or underactive peak reactivity , that reflect a mismatch to the demands of the situation – potentially in combination with delayed stress recovery Over time, there is overreacting repeatedly to the same minor stressor – lack of habituation .
Health behaviors play a role in each part of the model. Health behaviors have a direct role contributing to allostatic states and load, and also interact with stress exposure and stress responses. As proposed elsewhere ( Umberson et al., 2008 ; Mezuk et al., 2013 ), the experience of lifespan stressors may shape the selection and engagement of poor health behaviors. These behaviors, in the short term, dampen the psychological experience of stress and the physiological stress responses while, in the long-term, may potentially damage physiological functioning. For example, consuming high sugar beverages ( Tryon et al., 2015 ), smoking ( Wardle et al., 2011 ) and alcohol intake ( Stephens and Wand, 2012 ) can dampen the physiological stress response in the short run, but are clearly damaging to health if maintained over the lifespan. Healthy behaviors, on the other hand, dampen the negative consequences of the repeated acute psychological and physiological stress response ( Puterman and Epel, 2012 ). For example, while rumination prolongs the hypothalamic pituitary adrenal (HPA) axis response to an acute stressor in sedentary adults, it has no such effect on those with a healthy lifestyle ( Puterman et al., 2011 ). A bout of exercise on any day can dampen the affective response to stressors ( Puterman et al., 2017 ), perhaps protecting from stress related depression and disease ( Piazza et al., 2013 ; Charles et al., 2013 ). For a fuller picture, daily health behaviors must be included in studies that aim to understand the effects of stress.
2.4. Invisible emergent influences on stress reactivity
Reactivity is often thought of as an invariant or stable response over time. Indeed, the reason reactivity is interesting in stress-health research is that it can be predictive of future health outcomes. However, these predictive studies are infrequent. Part of the reason that cortisol reactivity, for example, may not be predictive of long term outcomes is that reactivity is highly influenced by many contextual factors relevant to the current situation and to a person’s psychological state at that time – thus, it is influenced by both conscious and unconscious influences. Therefore, we briefly review the non-conscious influences on reactivity, so that researchers using reactivity paradigms can be aware of these influences to minimize them or capitalize on them. A more nuanced understanding of the influences on emotional and physiological acute stress reactivity may lead to better interventions and prediction of health and disease spans.
Reported stress perceptions (e.g., cognitive appraisals, emotional responses) result from a highly constructed emergent process. Although they are critical to measure, we also now know that these states cannot be reported with high accuracy, as they are difficult to identify. Reported stress is associated with autonomic reactivity with small relationships around r = .20 ( Mendes et al., 2008 ). There are individual differences in over or under perception, and, as described below, personality and processes such as goals, affective style, interoception and aging may play a role. Emotional states are constructed not as a linear function of the level of objective stress present, but also with input from one’s bodily state (e.g., if they have had caffeine, bodily posture and positions), and external stimuli.
Internal influences include signals emanating from the body that shape stress responses and include but are not limited to bottom-up processes such as afferent information signaled through proprioceptive cues, like body position, microbiome imbalances, interoceptive sensitivity and accuracy, and individual and developmental differences in response stereotypy ( Cowan et al., 2017 ). Although much (if not most) information regarding stress context is assessed via explicit or implicit perception, there is also evidence that bottom up influences can shape affective experiences. In one notable study, for example, manipulated supine versus upright body position affected prefrontal cortical asymmetry measured through electroencephalogram ( Harmon-Jones and Peterson, 2009 ). When individuals are in an approach-motivated state (similar to challenge) they tend to have more left frontal cortical activation ( Koslov et al., 2011 ). Harmon-Jones and Peterson randomly assigned participants to be fully supine or upright while being insulted by a confederate. As anger is primarily an approach-oriented state, the upright participants showed greater shifts in left frontal cortical activation from a resting state, but supine participants did not show left shifting asymmetry. Presumably the supine body position blunted the approach oriented response providing intriguing evidence that bottom up influences, like body positions, can subtly influence the downstream neural and physiologic responses of affective states, which are a central component of the stress response.
External influences are features in the environment that shape stress responses. Some situational features that shape stress responses are obvious – information that an environment is unsafe or unpredictable would be perceived as more stressful or threatening – but some features are less obvious. For example, social environments can signal safety or danger via subtle information communicated by conspecifics. Emotion research has studied the concept of emotion contagion for decades with the idea being that a person’s emotional state can emanate from them via voice, facial expression, posture, and behavior and influence those around them ( Hatfield et al., 1994 ). Similarly, acute stress can be “caught” without explicit information and manifested in a physiologic change. In one study, mothers of 12–14 month infants were randomly assigned to experience a standardized laboratory stressor (TSST) or a non-stressful control condition in a separate room from their infant. When the mother and infant were reunited and the infants were placed directly on the mothers’ laps, infants of stressed mothers showed an immediate increase in sympathetic nervous system activation compared to a resting baseline, whereas infants of non-stressed mothers did not show an increase ( Waters et al., 2014 ). Furthermore, mothers and infants from the stress condition showed greater physiologic covariation – a mirroring of the rise and fall of the sympathetic nervous system for the rest of the experiment – compared to mothers and infants from the non-stress condition. This study was then replicated and extended to test the role of touch in stress contagion and in addition to replicating the initial effect, it was also observed that only infants who were placed on the mothers’ laps showed stress contagion. When the infants were placed in a high chair right next to the mothers the infants did not show any evidence of stress contagion ( Waters et al., 2017 ). These studies underscore how effortlessly stress can be transmitted from one person to another.
3. Dimensions of stressor exposure and response for transdisciplinary stress science
While any lifespan model of stress and health will necessarily be complex, including some unmeasurable components, there are several easily measureable aspects of stressor exposures and responses. These include historical exposures, current exposures and responses across different time scales. In Appendix 1 , we present a taxonomy of terms (the ‘Stress Typology’) as a first step toward providing a common language, including descriptive dimensions of exposure and responses to stress. The purpose of this tool is to highlight the important conceptual dimensions of stress relevant to the study of health and well-being. Researchers describing any type of psychological stress should use this as a reference guide for how to describe the stressor exposure and response, as well as a tool during study development to make sure key aspects of the stressor of interest are being captured. Using consistent language when describing the aspects of stress and its measurement – and using a theoretical lens to do so – is important for building a cumulative science of stress and harmonizing around critical theoretical dimensions. For example, the seminal meta-analysis by Dickerson and Kemeny (2004) evaluated more than 200 studies and identified that social-evaluative threat was the key ingredient for situations that elicit cortisol increases. Few other conceptual dimensions of stress have been identified as being essential in understanding and explaining the impact of stress on health and well-being.
The most important distinction identified in the Typology is between stressor exposure (“stressors”) and psychological responses to the stressor. Often these two concepts are confused or conceptualized as overlapping. It is essential for researchers to state whether the form of stress being referred to is the exposure to the stressful event or stimulus, or the response to it, which we define as the person’s subjective cognitive appraisal, emotional response, and behavioral response to the event or stimulus (physiological reactivity, while technically part of a stress response, is not included in the typology). In the following sections we describe characteristics of stressor exposure and stress response that are important to consider conceptually and methodologically. These are the components included in the Stress Typology.
3.1. Stressor exposure characteristics
3.1.1. timescale.
One of the most defining characteristics of a stressor is duration. Here we describe four timescales—acute stressors, daily events, life events, and chronic stressors. Acute stressors are intense short-term exposures. These are typically examined under standardized laboratory conditions but can be examined as naturally occurring events, with lower granularity due to limitations of measurement in the field. Examining the response trajectory of one acute stressor using a magnifying glass in real time over minutes allows us to examine an individual’s stress response kinetics, anticipation, peak reactivity, habituation, recovery, and regulation processes. A subset of people have stereotyped maladaptive ‘stress signatures’ (habitual patterns of over-responding to acute stressors) that may lead to allostatic load and early disease over time ( McEwen, 1998 ).
There is some stability in daily emotional stress responses, at least in midlife, and these may weaken with age ( Sliwinski et al., 2009 ). The variance in people’s ‘stress signatures’ is in part embedded in a person’s historical and current context ( Fig. 1 ). There are many factors that influence daily reactivity. For example, EMA studies have shown that anticipation of stressors leads to more negative affect ( Neubauer et al., 2017 ) as well as greater cortisol reactivity both on the morning of anticipation and during the stressor ( Wetherell et al., 2015 ). Rumination also prolongs reactivity. Rumination predicts greater cortisol reactivity in response to an acute stressor, and higher cortisol that evening ( Zoccola and Dickerson, 2015 ; Puterman et al., 2010 ). Inducing rumination after a stressor leads to greater vasoconstriction and prolonged blood pressure recovery ( Ottaviani et al., 2017 ). In contrast, mindful acceptance training can lead to less exaggerated cortisol and blood pressure reactivity responses to a standardized stressor ( Lindsay et al., 2018 ). Acute stressors are described in detail in Part 5.
Daily events , sometimes called “daily hassles,” are the more minor hassles that happen frequently such as rushing, arguments, deadlines, and child care strains. When someone faces the same daily stressors frequently, whether the actual event or just threat of the event, this can be considered a type of chronic stressor. In turn, to understand how chronic stress emerges at a daily level, we can examine the daily lives and daily stressful events of those under chronic stress.
Life events are time-limited and episodic in nature, such as getting into an accident, being laid off, being broken up with, or receiving a life-threatening diagnosis. Life events can be events that seem positive on the surface but are in fact quite demanding such as getting promoted at work or getting married. These circumstances occur in a specific moment in time, with an identifiable onset. Although the actual event can be relatively brief, events can have varying long-term consequences, depending on the nature of the event and its sequelae, especially in relation to initiating chronic stressors. Traumatic events are life events that are particularly severe in that they clearly threaten the physical and/or psychological safety of the individual or those close to them. Examples are witnessing or experiencing violence, the death of a loved one, experiencing abuse, or experiencing a natural disaster. A greater number of traumatic events across the lifespan is associated with worse self-reported health, greater health care utilization, functional disability, arthritis, greater number of acute and chronic illnesses, and mortality ( Gawronski et al., 2014 ; Keyes et al., 2013 ; Krause et al., 2004 ; Rosengren et al., 2004 ). Experiencing trauma in childhood is particularly deleterious for health; there is strong evidence that early childhood adversity is associated with higher rates of illness in adulthood including cancer, depression, cognitive decline, and premature mortality ( Brown et al., 1995 ; Kelly-Irving et al., 2013 ; Barnes et al., 2012 ; Montez and Hayward, 2014 ).
Chronic stressors , sometimes called “chronic difficulties,” are stressors that are present for longer periods of time and include circumstances such as caregiving, being unemployed, living in a dangerous neighborhood, financial strain, or being in a conflictual relationship. Specific life events like losing a job can initiate chronic stressors, such as persistent financial difficulties, although this is not always the case. Likewise, specific chronic stressors, such as living in an unsafe neighborhood, can give rise to different life events, such as being a victim of crime, but again, this is not always the case ( Brown and Harris, 1978 ). The criteria for the duration of a situation for a chronic stressor vary greatly. The Life Events and Difficulties Scale (LEDS; Brown and Harris, 1978 ) uses only 4 weeks as a cut off for a chronic difficulty. We suggest that a situation should be ongoing for at least six months to be considered chronic, although longer periods (one year or more) will lead to a more stringent criteria for whether a chronic stressor might have long term health effects.
Chronic stress can be defined in other ways besides quantifying duration. For example, Pearlin (1989) defines chronic stressors as “relatively enduring problems, conflicts, and threats that many people face in their daily lives” (p. 245). Hobfoll (2001) proposes a model of stress where frequent stressors deplete one’s resources more quickly. When a daily stressor stems from the same ongoing situation, this is conceptualized as a form of chronic stress. Empirical evidence shows that chronic stress in this form is associated with high negative and low positive daily affect ( Koffer et al., 2016 ).
The different timescales are nested within each other as depicted in Fig. 2 . Although chronic stress, life events, and daily stressors can occur randomly, in general, people’s stress levels at each of these timescales is greatly influenced by the social context of their lives. In other words, if you are already experiencing a chronic stressor (such as financial strain, job insecurity, or being a caregiver), you are more likely to report a greater number of daily stressors and greater general perceived stress at any timepoint (as demonstrated below with a caregiver sample). Furthermore, on a monthly basis, those under chronic stress will also report more frequent or severe major life events, and greater peaks of perceived stress responses during those times, greater variability, and greater mean perceived stress over time. In sum, being under chronic stress puts you at greater risk for experiencing a greater number and more severe life events and daily hassles, and reporting greater perceived stress at any given moment.

This figure describes how different time scales of stressor exposure – months, daily, momentary – are nested within each other. In short, chronic stressor exposure shapes how an individual perceives daily or acute stressors.
3.1.2. Developmental life stages: In utero, childhood, adulthood, and cumulative stressors
A second characteristic of stressor exposures is the life period in which the stressor exposure occurred. Stressors occur at any time of life, beginning with in utero, a critical period. The impact on mental and physical health depends partly on the developmental phase ( Andersen and Teicher, 2008 ; Masten and Narayan, 2010 ). Stress during fetal development has imprinting effects on adult mental and physical health ( Barker, 2004 ; Van den Bergh et al., 2017 ). Early childhood is another critical period ( Lupien et al., 2009 ). Severe stress (chronic or traumatic stressors) in childhood is associated with vulnerability to psychological and physical illness in adulthood, including depression, lung disease, heart disease, diabetes, cancer, and premature mortality ( Anda et al., 2009 ; Danese and Baldwin, 2017 ; Danese et al., 2007 ; Felitti et al., 1998 ; Jacobs and Bovasso, 2000 ; Kelly-Irving et al., 2013 ; Koupil et al., 2009 ; Rich-Edwards et al., 2010 ; Wegman and Stetler, 2009 ). Most of the research in this area has focused on the long-term impact of severe forms of early adversity, such as physical or sexual abuse, though less severe and more common forms of early adversity such as disrupted parent-child relationships have also been associated with worse health in adulthood ( Russek and Schwartz, 1997 ). The pathway from early life stress to disease development may be mediated by early changes in neural pathways regulating stress, such as amygdala connectivity (e.g., Tottenham et al., 2010 ), biological pathways such as chronic inflammation in adulthood ( Slopen et al., 2010 ; Surtees et al., 2003 ; Danese et al., 2007 ) and telomere shortening ( Hanssen et al., 2017 ; Ridout et al., 2017 ), cognitive and affective processes ( Pechtel and Pizzagalli, 2011 ), and behavioral proclivities ( Miller et al., 2011 ).
The majority of research thus far has focused on differentiating childhood from adulthood experiences of stressful life events and chronic stressors, though researchers are now focused on identifying smaller units of time that are important in a developmental context (e.g., puberty) during which the body may be more susceptible to stressors ( Heim and Binder, 2012 ). Late adulthood stressors are also important in that people have lower homeostatic capacity, physiological resilience, as well as less coupling between their internal and external stimuli ( Mendes, 2010 described in Section 6.3 ).
Summing up exposures from across the lifetime is theoretically important and allows us to test the importance of a linear dose response model. It may also be that those with early adversity are more impacted by later life events, a double hit model, although there is little research so far testing this. Most studies examining links between lifespan stressors and health have relied on trauma checklists which often do not capture major stressors that are not considered traumatic, such as moving, the breakup of a relationship, or getting fired from a job, and it is not clear how important these stressful but normative life events are.
A life course perspective can help us understand both cumulative stressors as well as whether one event will have a severe impact. The life course perspective takes into account the examination of individuals over time, their social roles, historical and cultural contexts, and biologically sensitive critical periods. It can help us understand the typically greater adverse events in those of low socio-economic status, starting from childhood. Social disadvantage leads to a cumulative disadvantage and helps explain certain events such as unplanned teen pregnancy, which is a result of many differences in opportunities and decisions over time, rather than being a singular event ( Elder, 1998 ). In turn that event makes other events more likely, such as dropping out of school and economic hardship.
Social role stress becomes particularly important in mid to older life, when people are engaged in work, parenting, and multiple social roles, before aging related diseases or disabilities take their toll, and these work roles can have positive effects on health. While role strain can occur with too many social roles, in general, having many work and social roles predicts better mental and physical health ( Nordenmark, 2004 ). Retirement can have positive or negative effects on health, depending in part on whether it is forced or voluntary, and the subjective meaning of retirement for the person ( Moen, 1996 ).
When considering how much impact stressful life events have on health, events have different effects at different developmental periods of life. There are sensitive periods when people are biologically more vulnerable to stressors. There are also socially sensitive developmental periods. The impact of stressful events may also depend on the timing of the event in one’s life course, and whether or not it is normative at that time in life, vs. a violation of normative experiences. For example, retirement and caregiving in later years do not violate expectations of aging and thus may be easier to adjust to, whereas caregiving for a special needs child as a young or midlife parent often violates expectations. Midlife is a time characterized by high career and social demands with which chronic caregiving responsibilities conflict. For similar reasons, loss of a spouse and natural disasters have bigger impacts on people in midlife than older age ( Bonanno and Kaltman, 1999 ).
3.1.3. Stress assessment window
The third characteristic of stressor exposure is the assessment window, meaning the timeframe of the tool being used (e.g., retrospective reporting on past two weeks, or a current momentary assessment) and the proximity of the assessment to the stressor exposure (e.g., number of minutes from stimuli, or number of years since traumatic event).
3.2. Stress response characteristics
The second category of the Stress Typology is the psychological and behavioral responses to the stressor. Stress responses include global stress appraisals about one’s life and not specifically about a stressor (often measured with the Perceived Stress Scale; Cohen et al., 1983 ), subjective psychological stress within a specific life domain (such as work stress), and responses to specific stimuli or events (i.e. motivational states, emotional responses, cognitive appraisals, behavioral coping strategies, emotion regulation, and perseverative cognitions). These responses may be the most proximal determinants for engaging in healthy or unhealthy behaviors to seek relief from a stressor.
Stress is not a singular construct wherein stressors of different types have similar effects on health. Rather, stress research has shown that some types of stressors are particularly deleterious. Additionally, different situations can evoke distinct social and psychological responses. These include, for example, feelings of interpersonal loss, physical threat, a threat to one’s social standing, social humiliation, entrapment, change in one’s social roles, or blocked opportunities. Early research focused on identifying stressors that cause substantial upheaval or change in one’s life ( Holmes and Rahe, 1967 ). Other work has focused on the degree of controllability that different stressors possess ( Maier and Watkins, 2005 ). A third tradition has focused on the extent to which different stressors lead to upheaval or disruption of a person’s goals, plans, and aspirations for the future ( Brown and Harris, 1978 ). A fourth tradition has focused on the extent to which life stressors, typically traumatic events, violate a person’s worldview—such as that the world is benevolent, predictable, and meaningful ( Janoff-Bulman, 1992 ; Silver and Updegraff, 2013 ).
Stress responses can be closely examined using standardized lab stressors. Lab stressors, while not naturalistic, are invaluable tools because they allow us to manipulate and understand contextual effects as well as physiological, cognitive, and affective responses as they unfold during a stressor. They also reveal whether an individual has an embedded exaggerated or blunted stress response. Catching the onset of a stressor in the field is difficult. Technological innovations that utilize ecological momentary assessment (EMA) and bio-sensing tools will allow the capture of both psychological and physiological states, and passive contextual data throughout the day. Importantly, however, these measures will have limited ability to capture stress trajectories until they can capture the onset, peak, and recovery parameters of responses to daily events. A further description of acute stressor tasks and responses is presented in Section 6 .
3.3. Example: From chronic stressor to momentary stress responses
Earlier we presented and defined several ‘types’ of stressors (e.g., chronic and acute stressors); these different types of stressors interact, and it is likely the interaction that explains the pathway from stress to health detriments. We offer an example of the interaction of stressor type by looking at a sample that is defined by caregiver status. Caregiving for an ill or disabled family member is a model often used in chronic stress research. Caregiving is stressful because it requires daily, and sometimes moment-by-moment, intense caring for someone else who may have difficulty managing physical, behavioral, and cognitive needs, which at the same time limits time for self-care and paid work. If one has few financial resources, common stressful events may have a bigger impact. Caregivers tend to have high rates of anxiety and depression and poor physical health. We have studied the life events, daily stressors and perceived stress responses of caregiving mothers of children with an autism spectrum disorder (a chronic stressor) and mothers of neurotypical children over 3 weeks, each week separated by nine months. This included roughly 180 women, described elsewhere ( Catalino et al., 2017 ; Felder et al., 2017 ).
First of all, the caregivers reported much higher global perceived life stress ( Cohen et al., 1983 ) over the prior month at study baseline, [mean=21.9 for caregivers (SD=4.7) vs. 15.7 for controls (SD=4.4), p < .0001, a large effect d=1.36] and this magnitude of discrepancy persisted over the years they were studied. Caregivers also reported a greater number of stressful life events in the past year compared to controls. A greater number of stressful events in the previous year was associated with greater levels of perceived stress over the past month (r=0.39, p < .0001).
When shifting to examining daily stress, we can assess whether a well-defined chronic stressor (caregiving) predicted more daily stressful events. Daily stressors were coded as absent or as a very minor hassle (“non-stressor day”) or moderate/major daily event (“stressor-day”) each day for the 21 days. Caregivers had greater frequency of moderate daily stressors (51% vs. 42%, T=2.74, p < .007). Extrapolating to over a year, this would lead to 35 more days with a moderately stressful event per year, roughly an extra month of moderately or highly stressful days, compared to controls. Both controls and caregivers reported higher nightly perceived stress on the days they had a significant stressor, with caregivers showing higher levels than controls (Caregivers: 1.92, SD=.83; Controls: 1.21, SD=.62, T=6.55, p < .0001, a large effect where d=.98). Caregivers also had larger increases from their baseline perceived stress levels on non-stressor days even though they had a relatively high baseline (baseline levels: Caregivers: 1.42 SD=.68; Controls 0.88, SD=.51; T=5.96 p < .0001). As shown, the mean perceived stress level on a high stressor day for controls was similar to the mean nightly stress level on a low stressor day for caregivers. Relatedly, in a separate study of acute stress responses to a laboratory social evaluative stressor, we found that caregivers had greater appraisals of threat than controls ( O’Donovan et al., 2012b ). This suggests that exposure to the context of chronic stress of caregiving may shift one’s mental filter to appraise even non-caregiving situations as carrying greater threat (requiring more demands and having fewer resources).
This example demonstrates that the context of the person’s life – in this case being exposed to a chronic stressor – shapes the number of significant daily stressors, as well as the emotional reactivity and recovery from those stressors. This is not just significant to the psychological wellbeing of the caregivers, because such an increase in number of daily stressors may help explain the greater systemic inflammation in caregivers compared to controls ( Gouin et al., 2012 ). In sum, chronic exposures, daily events, and perceived stress responses are interrelated, and together they give us a view of one’s stress landscape, from macro to micro scale.
4. Chronic psychological stress and cumulative life stressors
Chronic stressors have an important place in stress science. There is a strong relationship between experiencing chronic stress and health-related outcomes, including biomarkers of disease, early disease conditions, and in some cases, mortality. The chronicity of a stressor – the length of time the event and its aftermath continue for – is a major factor determining the extent of its effects on health. Furthermore, chronic stress acts as a background or contextual layer for which individuals encounter other types of stressors (daily hassles, acute events), increasing the likelihood that they will not have the resources to cope as efficiently with the additional stressors (see Fig. 2 ). For example, individuals who live in neighborhoods in which they feel unsafe have to be ‘on guard’ all the time in order to stay alert and aware of their environment.
There are many reasons someone may be under chronic stress. Decades of stress research have examined the impact of a wide range of difficult and demanding experiences, referring to these experiences with different terminology such as chronic strain, ongoing stressors, and chronic stress. We use the term chronic stress to mean any experience that is demanding and distressing nearly every day for 6 months or more. We reviewed the literature linking chronic stress to health outcomes and identified types of chronic stressors that have extensive evidence linking them to disease and mortality. These types are: neighborhood environment, financial strain, interpersonal stress (i.e., loneliness, social isolation, relationship conflict, & discrimination), work stress, and caregiving. In Appendix 2 , we list exemplar studies linking these experiences of chronic stress to physical health outcomes, pulling from epidemiological studies and meta-analytic evidence when possible. We present only positive findings in the table and have selected representative studies rather than presenting a complete review of all relevant research.
A particularly important type of chronic stressor is one that is interpersonal in nature. There is an abundance of work suggesting that social interpersonal stressors are particularly harmful for health, given the fundamental motivation for humans to form and maintain close social bonds ( Baumeister and Leary, 1995 ; Gilbert, 1992 ; Slavich et al., 2010a ). Interpersonal stressors are amongst the strongest predictors of emotional distress, systemic inflammation, poor health, and survival (e.g., Brown et al., 1995 ; House et al., 1988 ; Kendler et al., 2003 ; Miller et al., 2009 ; Sheets and Craighead, 2014 ). Interestingly, social stressors that involve an element of rejection (feeling excluded from a social group) have a notably large impact on future depression ( Slavich et al., 2014 ; Slavich et al., 2009 ) and inflammatory biology ( Murphy et al., 2015 , 2013 ). The primary social stressors found to be associated with worse health are social isolation, loneliness, relationship conflict, and discrimination. Links between these chronic stressors and health are outlined in Appendix 2 .
Financial strain is another important chronic stressor as level of financial resources are known to predict health, across the socio-economic (SES) gradient. Results from the nationally representative Health and Retirement Study (HRS) show that lower SES during both childhood and adulthood predict worse self-rated health, greater chronic conditions in later life ( Luo and Waite, 2005 ), and increased mortality ( Galobardes et al., 2004 ; Nandi et al., 2014 ). In addition to measuring objective resources by SES indices, it is important to measure perceived financial strain, which is a relative measure and can exist at any level of SES. In HRS, perceived financial strain predicted earlier disability ( Matthews et al., 2005 ) and increased mortality independent of education and income ( Szanton et al., 2008 ).
Exposure to these chronic stressors is important but equally important is the psychological response to the stressor. One example of this basic principle has been demonstrated by research on caregivers. Caring for someone who is disabled or chronically ill is difficult, and can have detrimental effects on the caregiver’s mental and physical health. Informal caregivers are at increased risk for health problems compared to age-matched non-caregivers ( Pinquart and Sörensen, 2003 ; Vitaliano et al., 2003 ). Mere exposure to caregiving is predictive of some negative outcomes, particularly because it tends to be ongoing for many years, but the distress associated with caregiving is often more predictive. For example, in a classic study on caregiving stress, older adults were followed over five years. Those caring for a partner with a condition did not have higher mortality than control non-caregivers, but of caregivers, those who reported high distress from caregiving had 63% higher mortality ( Schulz and Beach, 1999 ). In another study of younger maternal caregivers, when compared to age-matched controls, the two groups had similar telomere length and oxidative stress. However, greater perceptions of global perceived stress among the caregivers was associated with a worse biomarker profile ( Epel et al., 2004 ). To measure chronic stress well, characteristics of the stressor (e.g. duration, severity, controllability) and how someone responds to the situation (e.g. affective and cognitive responses) need to be measured. These measurement components are outlined further in the Appendix 1 .
Most of the chronic stress measures included in epidemiological studies capture current experiences within selected life domains and therefore miss past experiences and cumulative experiences across the lifespan. Cumulative lifetime stressor exposure is important as several theoretical models suggest that the impact of stress accumulates over time, with greater exposure leading to more health problems. However, cumulative life stress is time intensive and burdensome to measure and thus has not been captured in most epidemiological studies. Furthermore, the validity of retrospective measures is inherently an issue, and childhood trauma questionnaires have been examined in this regard. Studies directly comparing retrospective and prospective reports of adverse childhood experiences have found slight to fair agreement rates (reviewed in Newbury et al., 2018 , Reuben et al., 2016 ).
Self-reports of lifetime stressors, including childhood traumas, are typically assessed many years after their occurrence, introducing biases such as forgetting or infantile amnesia, inaccuracy regarding the timing of events, or mood-congruent memory that result in underreporting of childhood adversity estimates (reviewed in Hardt and Rutter, 2004 ).
On the other hand, prospective measures of childhood adversity may be limited due to under-reporting of respondents/caregivers or under-detection by agencies ( Hardt and Rutter, 2004 ). Overall, prospective measures show better psychometric properties and prospective cohort studies are clearly of advantage. Despite the limitations of retrospective measures of historical life stress exposure, studies relying on retrospective reports of cumulative life stress have been illuminating in showing that lifetime stressor exposure negatively affects health-related processes and outcomes, including autonomic nervous system activity ( Lampert et al., 2016 ), systemic inflammatory activity ( O’Donovan et al., 2012a ), brain structure ( Ansell et al., 2012 ) and function ( Seo et al., 2014 ), rates of alcohol use ( Lloyd and Turner, 2008 ), and physical and mental health status ( Stults-Kolehmainen and Sinha, 2014 ; Turner and Lloyd, 1995 ). In the Health and Retirement study, a retrospective measure of life events across the lifespan has been examined and childhood versus later life stressors have been compared. When broken down by life period, childhood adversity had a significant effect on proinflammatory gene expression ( Levine et al., 2015 ), as well as telomere length ( Puterman et al., 2016 ), both potential mechanisms of disease development.
To address the need to capture a stressor exposure across the lifespan without lengthy in-person interviews, an automated, computer-based interview method was developed. The Stress and Adversity Inventory (STRAIN) is an online system that measures a person’s lifetime exposure to 55 types of acute and chronic stressors that may affect health ( Slavich and Shields, 2018 ). The stressors that are assessed cover all major life domains (e.g., health, relationships, education, work, and finances) and focus on experiences that have a moderate baserate in adolescent and adult populations. Cumulative life stress measured with the STRAIN has so far been associated with worse health, including poor self-reported physical health ( Toussaint et al., 2016 ), impaired cognitive functioning ( Goldfarb et al., 2017 ; Shields et al., 2017 ), and greater cancer-related depression and fatigue ( Bower et al., 2014 ; Dooley et al., 2017 ).

5. What makes stress stressful? The role of stress appraisals
There is great variability in individual responses to stressors. One key component of both psychological and physiological responses to potentially stressful events or stimuli is cognitive appraisals (see Fig. 3 ). Appraisals are people’s evaluative judgment of the situation or event that is influenced by individual-level and environmental factors. There are several types of stress appraisals that have been identified to influence acute stress reactivity, and fewer that have been identified to shape responses to chronic stress. Classic dimensions from animal and human research that shape acute stress reactivity include evaluations of the situation as novel, unpredictable, and uncontrollable ( Mason, 1968 ). Other stress appraisals that offer potential insight into links between stressor exposure, reactivity, and aging include appraisals of the stressor as a threat vs. a challenge, and threats to one’s physical safety or to one’s ego/social sense of self.

This figure shows that stressor context, cognitive factors, developmental stage, and individual differences including historical stress influence one’s physiological response to an acute stressor.
The idea that some types of stress responses might be beneficial or adaptive has a long history in stress research. Lazarus and Folkman’s (1984) theory of stress and coping provided the critical extension to this idea by offering that there were not simply types of situations that were associated with benign or maladaptive stress, but rather the perception, or appraisal, that determines the positive or negative impact of the stressor. Psychological states of challenge compared to threat have been conceptualized as a relative ratio between the demands of a situation – the psychological or physical danger present, the uncertainty or novelty of a situation, and the required effort – and the personal resources to cope—individual and dispositional styles, social support, and knowledge and abilities ( Blascovich and Mendes, 2000 ). A situation can be demanding because the stakes are high, it is novel, and requires effort to do well, but to the extent that someone has experience (more familiarity), feels supported, has well-honed abilities, or is knowledgeable about the topic, they can off-set the demands. As a heuristic, to the extent that demands exceed resources, a threat state is expected, whereas when resources meet or exceed demands a challenge state is anticipated. Importantly, these psychological states have been linked to distinct affective, motivational, neuroendocrine, autonomic, and behavioral responses – with threat appraisals being associated with more maladaptive/harmful reactivity patterns ( Blascovich and Mendes, 2000 ; Jamieson et al., 2017 ). Furthermore, threat appraisals in the context of an acute laboratory stressor have been associated with indices of cellular aging, including shorter telomere length ( O’Donovan et al., 2012b ). This distinction between threat and challenge offers a way for researchers to interpret how the same stressor might be harmful for some but not all people, and that some stressors might in fact be beneficial ( Sapolsky, 2015 ; Seery et al., 2010 ). Additionally, threat and challenge appraisals are an important and powerful area of stress research given that appraisals are modifiable ( Jamieson et al., 2012 ) and thus provide a potential target for intervention.
Another cognitive appraisal that has been identified as ‘toxic’ across a variety of stressor types is feeling unsafe (both physically and psychologically). Threats to physical survival and safety have been shown to dysregulate the HPA axis and other physiological systems in animals and humans ( Sapolsky et al., 2000 ). Threats in the daily environment likely influence biological functioning. Evidence to support this point include studies showing that subjective appraisals of neighborhood safety and disorder (e.g., vandalism, theft) are associated with flatter daily diurnal cortisol slopes ( Do et al., 2011 ; Karb et al., 2012 ) and accelerated cellular aging, even after adjusting for demographic and socioeconomic characteristics ( Park et al., 2015 ). Similarly, threats to the social self, including negative social evaluation and threats to one’s social status, elicit strong emotional, physiological, and behavioral responses. Negative social evaluation (social-evaluative threat) in the context of an uncontrollable performance acute stress task is a particularly powerful HPA axis activator ( Dickerson and Kemeny, 2004 ). Negative social evaluation also elicits autonomic nervous system activation, proinflammatory cytokine increases, and self-reported negative affect ( Akinola and Mendes, 2008 ; Bosch et al., 2009 ; Dickerson et al., 2009 ). Similarly, perceived threats to one’s social status, including lower subjective standing in the general community and perceived inequality in important life domains (in respect to work, home and family life) have been associated with biological dysregulation ( Seeman et al., 2014 ).
In sum, appraisal processes critically shape an individual’s response to acute stress. Appraisals of threat to an individual’s physical and social integrity may be particularly salient in shaping physiological responses to acute stress, and likely play a prominent role in aging-related processes and diseases. The importance of appraisals in responses to chronic stressors has received significantly less attention. Importantly, there are also stress appraisals that have beneficial effects on health, or on the ability to endure the stressor, such as seeing a stressor as beneficial (benefit finding) or meaningful ( Moskowitz et al., 2007 ).
6. Acute stress: Specificity of conditions, responses, and reactivity profiles
Chronic stress is often characterized as an oppressive, unremitting long-term aversive state that can accumulate and lead to poor psychological and physical health. In contrast, acute stress occurs over a shorter period of time and typically has a relatively clear start and end point. In general, chronic stress occurs on a time scale of weeks, months and potentially years, whereas acute stress occurs over minutes and maybe hours. Although repeated acute stressors experienced over a long period of time (days, weeks, months) might become chronic stressors – for example a discriminatory work environment where every day a superior is disparaging toward an employee – acute stressors differ from chronic stressors in many critical aspects. Here we review how acute stress is conceptualized, the affective, cognitive, and developmental factors that modulate acute stress, the biology underlying acute stress responses, the short and long term consequences, and how acute and chronic stress influence one another.
6.1. Characteristics of the acute stressor
Acute stress is characterized by the occurrence of a specific eliciting event. This event can be an identifiable, punctate situation, like a job interview or public talk, or something more diffuse like a first date. The responses to the event flow through conscious processes, like appraisals, and also through unconscious processes that exert influences on the brain and body without explicit awareness ( Bechara et al., 1997 ; Blascovich and Mendes, 2000 ; Critchley, 2005 ). The stressor type, how the stressor is coped with or regulated, and contextual factors, determine the immediate physiologic response, as well as the behavioral and long-term consequences.
One feature that is important is whether the event is active or passive ( Blascovich and Tomaka, 1996 ; Lawler et al., 1976 ). Active events are defined as evocative situations that require an instrumental response. In a laboratory setting, active stressors include spontaneous speeches, reaction time tasks, or evaluated mental arithmetic. In real world settings, active stressors include events such as job interviews, public speaking, test taking, work and relationship-related discussions. Passive stressors are evocative situations that are experienced without any instrumental response requirement. In a laboratory setting passive stressors include watching disturbing films or experiencing an unavoidable shock. In real world settings, passive stressors include events like watching a loved one experience something difficult, having dental work performed, or waiting for test results. The active/passive distinction is important for interpreting the physiologic responses stemming from the event, and for interpreting the coping strategies engaged. In active stressors mobilizing metabolic energy to devote to the task at hand could be viewed as functional, whereas in passive tasks recruitment of metabolic energy is less likely to serve a functional purpose and ultimately could be more health damaging. For example, Obrist (1981) argued that attempts to cope with passive stressors, such as inevitable pain, could exacerbate painful experiences by creating greater muscle tension. Iwata and LeDoux (1988) found that when rats were conditioned to expect an electric shock, their physiological responses changed based on whether they were unrestrained (and escape was presumably possible) versus restrained (with no possibility of escape). Unrestrained compared to restrained rats showed greater sympathetic arousal and lower blood pressure reactivity prior to an electric shock, consistent with the idea that greater SNS activation provides more oxygenated blood to the periphery to enhance flight, which would be adaptive in the unrestrained condition. Thus, for human stress measurement interpretation, it is critical to understand the context of the stressor, and the possible functions of the response. It cannot be assumed that physiologic arousal or activation in response to an acute stressor is health damaging.
Controllability is also an important feature of the stressor and can alter appraisals of the event and responses to it. In a meta-analysis of cortisol reactivity, tasks coded as motivated performance situations (i.e., active tasks) were associated with a significant cortisol increase only when they were also coded as uncontrollable and not when the task was coded as controllable ( Byron et al., 2010 ). These authors defined uncontrollability as situations when “behavioral responses could not appreciably affect outcomes.” Thus, situations where people receive false feedback (negative feedback that is untethered to performance) or experiences of discrimination in which one is rejected for some characteristic of themselves for which they have no control (e.g. sex, race/ethnicity), are expected to engender more negative acute stress responses than when stressors are controllable.
The metabolic demand required during a stressor needs to be considered to interpret the physiologic consequences. Some acute stress tasks inherently require metabolic demand that by itself activates physiological responses. Therefore, the changes in physiologic responses might be a combination of the metabolic demands and the psychological state induced by the stressor. In some situations the metabolic demand is obvious. For example, standing versus sitting during a speech delivery task differentially affects blood pressure given that sitting blood pressure is lower than standing blood pressure. Further, mentally preparing to give a speech versus delivering a speech differentially affects respiration and heart rate variability.
Some metabolic demands of the task are less obvious yet can influence physiology and obscure the extent to which the psychological state of stress is affecting physiologic changes. For example, greater cognitive effort increases blood pressure and heart rate responses in a linear, monotonic manner ( Wright and Kirby, 2001 ). To the extent that a stressor is more cognitively demanding than other tasks, or difficult for some people and not others, it might differentially increase blood pressure and heart rate responses not because of the experience of stress, but because the task is more cognitively difficult. Cortisol levels also increase during more cognitively demanding tasks ( Lovallo and Thomas, 2000 ). Thus, all changes in “stress physiology” cannot be interpreted as due to the psychological state of stress, they might be due to the more mundane or distinct features of the task like body position, physical movement, effort, motivation, or cognitive processing.
6.2. Varieties of acute stress responses
Acute stress is often measured by examining short term changes in physiology with the general principle being that events or psychological states that increase arousal can be characterized as “stress.” This is an over-simplification given that changes in psychological and physiologic functioning support behavior, and in situations in which the stressor is active, recruitment of metabolic resources are often necessary to meet the situational demands. Indeed, what we consider the two primary physiological “stress systems” – the sympathetic adrenal medullary and the hypothalamic pituitary adrenal cortical axes – are associated with many psychological states including positive and negative emotion, cognitive effort, and approach and avoidant motivational states. Thus, examining acute psychological or physiologic responses and assuming that changes from a baseline or resting state indicate that a person is feeling “stressed” is problematic. Instead, considering the task demands, examining the profile of the responses, temporal aspects related to response, and habituation and recovery, provides a more comprehensive portrait of the acute stress response.
6.2.1. Profiles of responses: Challenge and threat
How the body and mind respond to a stressor can have short and long-term consequences on behavior and health. One way to begin to differentiate stress types and stress responses physiologically is to consider the profile of responses across systems. Several theories have attempted to differentiate acute stress responses into broad categories of beneficial and harmful responses (e.g. Dienstbier, 1989 ; Frankenhaeuser, 1986 ; Henry, 1986 ). One theory that integrates Dienstbier’s “physiological toughness” theory and Lazarus and Folkman’s stress appraisal theory in the context of acute stressful situations, is the biopsychosocial model of challenge and threat (for a review see Blascovich and Mendes, 2010 ). In this model, both challenge and threat states occur during acute “stressful” situations; however, the states differ in their antecedent appraisal processes and subsequent downstream cardiovascular reactivity. For example, as reviewed earlier, challenge states occur when individuals appraise their resources as exceeding the demands of the task, whereas threat states occur when situational demands are perceived to exceed resources (as described in Part 5 above). These responses are considered profiles because there are multiple physiologic responses—one single physiologic response is insufficient—the theory makes predictions regarding both the direction and the relative intensity of the response, and there are specific expectations of the temporal nature of the responses. Specifically, even though both states are characterized by sympathetic nervous system activation, challenge is characterized by increases from a resting state in cardiac output (CO, the total volume of oxygenated blood the heart pumps in a minute) and decreases in total peripheral resistance (TPR)—vasodilation. Threat is characterized by little or no increase in CO and increases in TPR—vasoconstriction. Challenge states have quick SNS responses and habituate during a task, whereas threat states have slower rise in SNS upon exposure to the task and SNS tends to stay elevated for a longer portion of the task. Furthermore, in some contexts, SNS activation is greater in challenge than threat states, consistent with Dienstbier’s idea of physiological toughness, which suggests that larger increases in SNS activation to novel situations is related to effective coping and better performance (see also Jamieson et al., 2010 ).
Research has identified benefits of challenge states compared to threat states in cognitive performance, emotional responses, and health. For example, challenge, relative to threat, states have been associated with better decision-making ( Kassam et al., 2009 ), higher perceived social standing ( Scheepers et al., 2012 ), more approach-oriented behavior, and increased positive affect ( Mendes et al., 2008 ). Larger increases in sympathetic activation (commonly measured using changes in ventricle contractility, a relatively pure measure of sympathetic activation, or changes in catecholamine levels such as epinephrine) also tend to produce better performance in physical and cognitive tasks. For example, students preparing to take the GRE were assigned to either a stress reappraisal manipulation, which encouraged participants to interpret their physiological arousal during test-taking as a beneficial response that would enhance cognitive performance or a no-instruction control condition. Reappraisal participants exhibited a larger increase in sympathetic activation (measured with salivary alpha amylase) immediately before taking a practice GRE and performed better at the math exam than those who were assigned to the control condition. Indeed, consistent with the challenge and threat framework, the greater the SNS increase the better the math performance (see also Dienstbier, 1989 , for additional examples). Furthermore, the reappraisal participants, compared to control, earned higher GRE exam scores when they took the actual test in the following months ( Jamieson et al., 2010 ). Indeed, Dientsbier in reviewing decades of arousal and cognitive performance (test-taking) data concluded “despite the high difficulty level…these data indicate no curvilinear relations; naturally evoked peripheral catecholamines never seem to be too high for optimal performance” (page 86). These relationships are consistent with the physical activity literature where higher activation is associated with better performance with little evidence of a U-shaped relation.
Although the mechanisms through which long term health benefits of challenge have not yet been specified, there is cross sectional and longitudinal data suggesting that benign challenge responses may accumulate over time to produce more positive health outcomes. In the Framingham Heart Study sample of more than 1500 participants ( Jefferson et al., 2010 ), higher levels of cardiac output, one of the primary cardiovascular determinants of challenge states, was associated with increased cognitive processing speed in older adulthood. These researchers speculated that increased oxygenated blood produced by the heart may have long-term protective effects in the brain.
In our own data on response to a standardized stressor in healthy women, we have observed moderate correlations between cardiovascular reactivity during an acute stressful laboratory task and telomerase – an enzyme that adds telomeric DNA to shortened telomeres – among female caregivers ( N =58) (study described elsewhere ( Epel et al., 2006 ). Higher levels of basal telomerase were associated with greater increases in CO during speech and math tasks (Δ CO: speech β = .37, t =2.23, p < .05; math β = .42, t =2.88, p < .01) and lower TPR (Δ TPR: speech β = −.38, t =−2.48, p < .05; math β = −.36, t =−2.30, p < .05). These data point to the intriguing, albeit preliminary, suggestion that repeated threat reactivity during acute stress might speed biological aging.
Lastly, psychological and physiological profiles of threat and challenge may have implications for behavior change. Acute stress reactivity profiles may lead to latent behavioral tendencies. For example, motivational states of engagement should facilitate proactive behaviors (like exercise), whereas motivational states of inhibition may lead to more passive coping such as eating or substance abuse to modulate negative emotions.
6.2.2. Temporal trajectories of acute physiologic responses
In addition to considering the magnitude of a specific measure of physiologic activation to acute stress, it is also important to consider that marker’s temporal trajectory during the stressor ( Fig. 4 ). We build on a very similar model proposed by McEwen (1998) which focused on responses to repeated events and, implicitly, on HPA axis activation. Here we focus on autonomic nervous system arousal within the context of a single event, though the current figure and McEwen’s seminal figure have much overlap.

Acute reactivity profiles associated with vulnerability.
The acute reactivity approach to understanding health outcomes makes assumptions regarding how affective experiences bring about acute changes (reactivity) in biological systems, which might accumulate over time to induce excessive wear and tear on biological health via allostatic load ( McEwen, 1998 ). Scholars who use this approach typically expose participants to standardized tasks like watching videos, giving evaluated speeches, and engaging in social interactions that activate physiologic changes, and interpret the profile of the resulting activation as maladaptive or harmful to health. In the simplest case, the “reactivity hypothesis” examines physiologic changes from a resting state to an activated state with the assumption that the greater the activation, the more harmful the physiological response would be if experienced repeatedly. For example, in a study in which women described being unjustly accused of shoplifting, African American women who reported experiencing past discrimination had greater diastolic blood pressure than African American women who reported little experiences of prior discrimination in their lives ( Guyll et al., 2001 ). The authors of this work interpreted the findings as showing that “discrimination may act as a stressor that adversely affects cardiovascular health and that the effect may be mediated by pathogenic events associated with physiologic reactivity” (p. 322). Although the general reactivity hypothesis is intriguing, it likely cannot yield the full story on how acute reactivity affects health. For example, in this same paper European American women completing the same “discrimination” task showed larger blood pressure reactivity than African American women. Thus, reactivity might be part of the pathway from affective experiences to health outcomes, but the simple interpretation that greater reactivity in the lab provides a snapshot of typical reactivity in daily life is not sufficient.
Intensity of reactivity provides an initial picture of how individuals respond, but examining a more dynamic profile over time may provide a more comprehensive understanding. Fig. 4 presents four trajectory profiles of reactivity (c.f. McEwen, 1998 ). A maladaptive (or unhealthy) response in anticipatory reactions would be characterized by a heightened response prior to the onset of an event (top left graph). As depicted in Fig. 4 , anticipatory responses might create more wear and tear on the system because of the lengthened reactivity that precedes an event. This could be a function of having negative expectations for a social interaction, test, or job interview, which might be reflected in increased vigilance or anxiety. These negative expectations might be especially harmful when transitioning to new environments.
Unhealthy psychological and physiologic responses can also be characterized by the lack of recovery once a stressor is over. As depicted in the upper right graph, whereas “healthy” reactivity is characterized by a return to baseline levels once a stressor is over, an unhealthy response would show a continued elevation in reactivity once the stressor has ended. Rumination, in particular, has been implicated in poor post-stress recovery ( Brosschot et al., 2005 ).
When responding to a novel event, a typical psychological and physiologic response would include an initial strong activation that coordinates metabolic systems to contend with the task at hand, and also fairly quick habituation (represented by the blue line), which has been labeled a “physiologically tough” response ( Dienstbier, 1989 ). In contrast, a lack of habituation during a stressor (bottom left graph) might reflect an inflexibility of the system to quickly adapt, which may also ultimately create excessive wear and tear. To the extent that some individuals are hyper-vigilant during a task either due to stigmatized status or individual differences like fear of evaluation or rejection-sensitivity, they might show less habituation to a stress task. Also, profiles don’t occur in isolation and individuals can have any combination of the profiles such as lack of habituation and recovery (bottom right graph) with the assumption that the combination of unhealthy trajectories may be additively pathologic, but we are unaware of any data on this specific question.
Finally, although we have focused primarily on trajectory of physiologic responses to acute stress this approach could be easily adapted to encompass psychological reactions (e.g., affective responses). Anticipation, for example, can be characterized psychologically as worry, whereas blunted recovery indicates rumination or preservative thinking. Lastly, strong activation physiologically could index alertness or engagement, whereas weaker or blunted activation might be construed as disengagement or loss of motivation.
6.3. Sex and aging effects on acute stress physiology
There are important sex differences in acute stress responses. First, there are reliable sex differences in resting cardiovascular system functioning. For example, women exhibit higher basal heart rate at rest while men exhibit higher blood pressure at rest. Differences in resting cardiovascular functioning can influence the interpretation of the reactivity of these parameters. Additionally, there tends to be activation differences between men and women with men showing greater overall changes in peripheral physiological responses ( Blascovich and Tomaka, 1996 ). However, many main effects of sex are reduced, if not eliminated, once BMI is controlled. Further, men and women do not reliably show differences in patterning of physiologic responses during acute stress, so there is little evidence that there are sex differences in general approach versus avoidant (challenge versus threat) physiologic patterns ( Mendes et al., 2008 ; Kassam et al., 2009 ; Jamieson et al., 2012 ).
Associations between psychological states (mind) and physiologic responses (body) are not static across the life course. Some of the changes across the life span are due directly to developmental and aging processes. In early life, afferent and efferent projections are still developing compared to the other end of the life stage when neuropathy, decay, and loss of flexibility affects mind-body relations. In addition to these direct physical changes, old age (typically 65 and older) can be associated with a loss of interoception—the ability to detect internal changes—and proprioception—ability to detect static and dynamic body positioning. The ability to quickly and accurately detect bodily states decreases as we age, and may result in a type of dissociation between top-down and bottom-up processing affecting both awareness of physiologic activation as well as the ability to effectively down-regulate responses. This mind-body dissociation has been noted for decades and led Jung to conclude that “emotions become more cognitive in older age.”
Much of modern stress research assumes there are reliable mindbody connections—that acute stress responses influence bodily responses, and vice versa, that the biological milieu can shape psychological stress reactions. Aging has dramatic effects on our brains and bodies. Though there is a great deal of individual variation, cognitive declines such as deterioration in short term memory, reaction times, and attention can occur even in the absence of neurological diseases (e.g., Levy, 1994 ). In the body, loss of muscle mass, deficiencies of growth hormones, hardening of the vasculature, and blunted activation reduces the flexibility of responding to different environmental demands (e.g., Epel et al., 2007 ; Matthews, 2005 ). Age related changes may also influence the relations between perception and physiological response. Proprioception and interoception both decline with old age. Using a heart beat detection paradigm with participants ranging in age from 22 to 63, older subjects showed poorer detection of their heart beats than younger and middle aged adults, and the overall bivariate correlation between age and accurate heart beat detection was r =−.49 and r =−.45 at two time points ( Khalsa et al., 2009 ). Proprioception declines are well documented ( Goble et al., 2009 ).
Declines in interoception and proprioception are not the only dramatic physiological change that occurs in aging that are relevant for acute stress. As people age there is tremendous blunting of key physiological systems, like SNS responses. For example, Levenson and colleagues observed lower heart rate responses for anger, fear, and sadness in older adults compared to younger adults during a directed facial action task ( Levenson et al., 1991 ). In some cases younger adults have twice as large SNS increases as older adults (a finding that mirrors physical exercise).
Importantly, in research with older adults, anger manipulations did not engender increases in peripheral (finger) skin temperature as has been observed in younger adults ( Levenson et al., 1991 ). One possibility underlying this lack of change could be that the flexibility of the vasculature—is affected by neuropathy that occurs with aging and the extremities (arms, hands, legs, feet) tend to be affected first. However, it is important to note that the flexibility of the vasculature is compromised in an asymmetrical form with age—vessels can still constrict easily but are harder to dilate. A psychological interpretation of this finding is that older adults are better able to modulate responses to negative stimuli, especially short term ones. These enhanced regulatory processes are related to better emotional well-being ( Charles, 2010 ). Importantly, though, this theoretical perspective also underscores that older adults have greater difficulties recovering from adverse experiences and these homeostatic regulatory processes could lead to more health damaging responses to sustained or especially intense negative affective experiences. Therefore, stress states associated with more approach-orientation may be compromised earlier than states of threat and withdrawal, which might become the default response in older age.
Evidence of declines in sensory perception of the body as we age, and how this decline can interrupt the mind-body connection, has been used as evidence of maturational dualism , a phenomenon that suggests that the bodily changes that co-occur with the aging process can influence the experience of affective states ( Mendes, 2010 ). For older adults, acute stressors may be experienced in the mind (and brain) but not be embodied in the same way as in younger adults. The weakening of the mind-body connection in older adulthood is primarily due to a loss of peripheral perception and blunted physiological reactivity, and may blunt the ability to use internal states to guide decisions and behavior.
There are intriguing clues in the literature regarding how the loss of mind-body connections in older age may influence acute stress responses. For example, one study examined the somatic marker hypothesis in older adults ( Denburg et al., 2005 ). In previous papers, Damasio and his colleagues (e.g., Bechara et al., 1997 ) described the somatic marker hypothesis, which suggested that bodily states outside of conscious awareness could influence behavior. To examine this hypothesis, participants (brain damaged and control) were presented with four decks of cards with various gains and losses associated with the cards. Two of the decks resulted in overall losses—large gains, but large losses as well—whereas the other two decks resulted in smaller gains, but smaller losses. They found that as participants turned over cards from the various decks, changes in skin conductance (activity in the eccrine gland, indicating sympathetic activation innervated by acetylcholine) co-occurred with choices from the riskier decks. Importantly, these bodily changes preceded conscious reporting of which decks were risky by approximately 40 trials. Thus, the somatic marker hypothesis claims that bodily changes can indicate psychological or mental states prior to conscious reporting. In this original article, normal participants were compared to patients with ventral medial lesions. While normal participants consciously reported which decks were risky by about the 40th trial, lesion patients were not able to learn this pattern.
In the extension of this earlier study with older adults, similar to the studies on patients with VM lesions, they did not show preferences for the advantageous decks across five trials (or 100 cards; Denburg et al., 2005 ). When examining individual responses, the authors reported that among the younger group, 37 out of 40 participants eventually picked from the advantaged deck; among the older group, only 15 out of 40 showed this same “unimpaired” pattern. The remaining older participants either showed more preference for the disadvantaged deck or no preference. There are at least two possible interpretations of these data in light of the ideas presented here: (1) similar to Levenson’s data, older participants had blunted physiological responses during the task, which limited the ability to sense internal states vis-á-vis the somatic marker hypothesis, or (2) the SNS response was intact and as strong as that experienced by younger participants, but the ability to sense the bodily changes—interoceptive awareness—was diminished ( Khalsa et al., 2009 ). Of course another possibility is that the lack of choice of the advantageous decks was due to a combination of blunted reactivity and loss of interoception.
An implication of aging effects on physiology is that older individuals may have to rely more on the external environment to determine their internal states. This is consistent with Carstensen’s socioemotional selectivity theory ( Carstensen, 2006 ), which describes a positivity effect in older adults including a shifting away from negative stimuli toward more positive stimuli, and favoring positive and avoiding negative emotions. Although speculative, an implication of this theory is that older participants would be more susceptible to suggestions of an affective state since they might have to rely more on their external world to provide information about their internal states. Although theory and evidence suggest that environmental cues can strongly influence affective states and meaning ( Barrett, 2009 ), the loss of exquisite ability to detect internal states may make older participants more sensitive to environmental cues in determining their stress responses.
There are also psychological and social aspects of aging that influence how we perceive and respond to stress exposures. One of the drivers of these changes is that as we age, our motivations, goals, and behaviors adapt to match the new circumstances of our life. For example, the Selection, Optimization, and Compensation model proposed by Baltes and Baltes (1990) describes that in order to cope with changes during aging such as loss of resources and physical function, people tend to maximize their strengths and compensate for losses. They select new realistic goals that add purpose to life, and there is a shift in goals from growth, in young adulthood, to maintenance or prevention in older adulthood. This shift is related to better well-being ( Ebner et al., 2006 ). Another adaptation of aging is to shift one’s environment to match needs, weeding out negative situations and people, which leads to more positive affect ( Charles and Carstensen, 2010 ). Furthermore, our social networks decrease in size, because we reduce the number of superficial social connections. This leads to an overall more positive emotional tone from close personal relationships ( English and Carstensen, 2014 ). Stressor exposures are also interpreted differently at different points in our life course since one’s developmental stage changes the meaning and expectations of life events ( Pearlin, 1989 ). For example, being forced to retire because of problems with physical functioning is likely to have a less negative impact at age 80 (when this event is an expected life course transition) than being forced to leave the workforce because of a physical decline at age 40.
6.4. Life stressors shape acute stress reactivity
Stress reactivity profiles alone are not deterministic as they are largely influenced by recent and situational factors related to the stressor. However, certain reactivity profiles may reflect an embedded history of stress, serving as a phenotypic signature of exposure. This can be determined by measuring both history and current reactivity. Integrating historical stress and acute stress processes should help us to better understand how stress impacts aging-related processes and chronic diseases of aging. There is a disparate body of studies showing that types of chronic stressors or current life events can shape acute reactivity profiles. For example, a quantitative review of 30 years of research showed that general life stress was associated with worse cardiovascular (heart rate and blood pressure) recovery from acute stress ( Chida and Hamer, 2008 ). Meta-analytic and descriptive reviews show that chronic stress is associated with elevated long-term cortisol secretion ( Stalder et al., 2017 ; Staufenbiel et al., 2013 ), with stressor duration and recency since stressor onset being important factors in shaping basal cortisol levels ( Miller et al., 2007 ). However, greater levels of chronic stress conversely can dampen cardiovascular and neuroendocrine responses to acute stress ( Matthews et al., 2001 ). Furthermore, in response to acute stress tasks, a history of childhood adversity has been associated with a blunted cortisol response ( Carpenter et al., 2011 ), autonomic responses indicative of threat appraisal ( McLaughlin et al., 2014a ), and greater inflammatory reactivity (reviewed in Fagundes et al., 2013 ).
These findings underscore the need for a more comprehensive model of stress that takes into account historical and current stress, as well as current reactivity across different regulatory systems, to understand one’s risk profile for disease and interpret reactivity patterns. There is a robust dynamic interplay between different time scales of stress (early life stress, chronic stressors, life events, and acute stressors) and thus we should predict and interpret acute stress reactivity profiles in the context of these important historical and contextual factors. Information across the different time scales will provide us with a more nuanced understanding of what differentiates a healthy acute stress response from a burnt-out or disengaged one.
7. Moderators of resilience and vulnerability
There is growing acceptance that stressors confer risk for disease outcomes ( Cohen et al., 2007 ), but there is tremendous individual variability in how vulnerable or resilient one is to stress. An unexpected story is that most people are resilient in the face of major trauma – possibly up to 80% returning to previous levels of psychological functioning ( Bonanno et al., 2011 ; Donoho et al., 2017 ) although the exact percentage is debated ( Infurna and Luthar, 2016 ). Moderators are critical in tracing how and for whom chronic stress confers biological risk, and may explain variability in aging trajectories ( Bherer et al., 2013 ). Here we briefly highlight known moderators of the stress—health relationship because a careful characterization of vulnerability and resilience processes is beyond the scope of this article, covered elsewhere ( Bonanno and Diminich, 2013 ; Southwick et al., 2005 ).
There are known resilience factors for an adaptive acute stress response. Personality traits and individual differences align in expected directions with experiencing threat or challenge during acute stress episodes. People who believe more strongly in a just world, individuals with high, stable self-esteem ( Seery et al., 2004 ), and socially connected individuals ( Cacioppo et al., 2002 ) typically exhibit cardiovascular reactivity consistent with challenge states during acute stressful tasks more than individuals who score lower on these constructs. Individual differences in resting neurological activity have also been linked to challenge and threat states. Specifically, individuals with higher left, relative to right, frontal cortical activity (a neurological pattern previously linked to positive affect and well-being) were more likely to respond to acute stress with challenge appraisals and challenge reactivity (higher CO and lower TPR; Koslov et al., 2011 ), suggesting a direct relationship between resting neurological activity and cardiovascular reactivity during acute stress. Additionally, there is emerging evidence that physically active adults have healthier acute stress responses than those who are sedentary. For example, physically inactive adults who ruminated after an acute laboratory stressor were found to have prolonged cortisol responses with delayed recovery whereas those who were physically active recovered as efficiently as low ruminating adults ( Puterman et al., 2011 ).
Stress effects may have little impact on people who have high levels of resources, and high levels of social support in particular ( Southwick et al., 2016 ). Social support has long been identified as a critical buffer to the deleterious effects of sustained stress ( Cohen and Willis, 1985 ). The positive impact of perceived social support (the perception that support from others is available and satisfying; Gottlieb and Bergen, 2010 ) on health has received considerable empirical evidence. Social support is suggested to be associated with a healthier and more resilient ‘biological profile’ (Uchino, 2006), which may help the person mount an adaptive psychological and physiological response to stressors. Constructs related to social support such as loneliness and social isolation have been linked to stress-related physiological systems including neural, cardiovascular, immune, and neuroendocrine functioning ( Cohen, 2004 ; Taylor et al., 2006 ). For example, loneliness predicts earlier mortality ( Holt-Lunstad et al., 2015 ), and is associated with less salutary profiles of cortisol and inflammatory responses to acute stress ( Cacioppo et al., 2015 ; Eisenberger et al., 2017 ; Hawkley and Cacioppo, 2010 ). There are likely many other positive dispositional factors that modulate the experience of stress, such as mindfulness, that need to be further explicated.
8. Biological impact of chronic stress on the brain, periphery, and future experiences of stress
Repeated acute stressors, life events, chronic stressors, and cumulative life stressors contribute to disease through complex pathways, where the brain serves as the central mediator of the stress response. The brain is a ‘prediction machine’ that bases its emerging appraisals of stressors on both external stimuli as well as on one’s personal memory bank of what to expect ( Barrett and Simmons, 2015 ). A history of feeling or being threatened might shift the appraisal of a current stimulus to more of a threat than challenge appraisal. The brain then plays a fundamental role in regulating the psychological, physiological, and behavioral responses to the stimuli. Further, it dynamically responds to internal stimuli that facilitates adaptation. The brain is more than a mediator, however, since accumulated stress directly and meaningfully impacts neural functioning and structure. Exposure to chronic stress and major life events, especially during sensitive developmental periods, can result in alterations in neural function and structure, thus shaping future affective and physiological stress responses.
Where does stress live in the brain? The neural stress response is not localized in any particular area but rather reflected in intrinsic neural networks that change and adapt to demands, both external and internal. An example of this is seen with autonomic nervous system responses to stress. Human neuroimaging studies demonstrate that the distribution of neural networks in brain areas involved in visceral control – such as the medial and orbital prefrontal cortex, anterior cingulate cortex (ACC), insula, amygdala, thalamus, and hippocampus among others – regulate autonomic nervous system functioning to coordinate hemodynamics and immune system response to external stimuli including stress ( Critchley et al., 2011 ; Gianaros and Wager, 2015 ). For example, research from Gianaros and colleagues demonstrates that stress-evoked increases in blood pressure are associated with a number of functional changes in neural activation, including increased medial prefrontal cortex and periaqueductal grey activity, the latter of which is a critical subcortical region responsible for autonomic regulation (reviewed in Gianaros and Wager, 2015 ). This finding is consistent with animal research showing that the mPFC plays a causal role in stress-related cardiovascular reactivity ( Resstel and Corrêa, 2006 ). In addition, neural responses to social rejection in the dACC and anterior insula predict individuals’ inflammatory reactivity to social stress ( Slavich et al., 2010b ). Given the longstanding evidence that sustained and exaggerated cardiovascular and inflammatory stress responses confer risk for cardiovascular disease processes ( Chida and Steptoe, 2010 ), advances in mapping the neural regions activated by stress may lead to more precise predictions about who is vulnerable to stress-related disease.
Exposure to traumatic stressors, particularly when they occur early in life (e.g., prenatal, childhood), appears to affect the brain in important ways ( Lupien et al., 2009 ; McLaughlin et al., 2014b ), including how the brain perceives and reacts to future threats and stressors. There is consistent evidence that early trauma exposure is associated with smaller hippocampal volume in adulthood ( Teicher et al., 2012 ) and alterations in amygdala-dependent emotional processing, marked by increased threat sensitivity ( McCrory et al., 2011 ). Additionally, early life trauma is linked to less cortical thickness of the prefrontal cortex ( Hanson et al., 2010 ), and in a sample of adolescents exposed to child abuse, a reduction in resting state connectivity between the amygdala and ventral medial PFC ( Herringa et al., 2013 ). In this sample of adolescents, reduced connectivity mediated the link between child abuse exposure and internalizing symptoms, including depression and anxiety. Altered neural development caused by early adversity may also lead to worse physical health in adulthood through pathways that lead to elevated peripheral inflammation ( Chiang et al., 2015 ).
While historical stressor exposure, such as early life trauma, can leave an indelible mark on the brain, chronic stress and cumulative stressor exposure during adulthood also produce changes to brain function and structures. Higher cumulative adversity has been associated with reduced gray matter in the several areas within the prefrontal cortex, including the mPFC, anterior cingulate, and insula (Ansell et al., 2012). Higher scores on global perceived stress have been associated with reduced white matter in the PFC ( Moreno et al., 2017 ). In a prospective cohort study of postmenopausal women, higher scores on perceived stress, averaged over nearly two decades, predicted smaller hippocampal grey matter ( Gianaros et al., 2007 ). This latter finding is important because the hippocampus is replete with glucocorticoid receptors and thus renders the hippocampus at risk for atrophy when exposed to the high doses of glucocorticoids observed in response to prolonged stress in animal models ( McEwen, 1999 ; Sapolsky, 1999 ), as well as excitatory amino acid neurotransmitters and other endogenous mediators ( McEwen and Gianaros, 2011 ). Inflammatory processes, which are consistently upregulated during periods of acute and prolonged stress ( Marsland et al., 2017 ; Segerstrom and Miller, 2004 ) are also implicated in signaling the brain in ways relevant to the processing of stressors. For example, endotoxin administration, a well-known inflammatory challenge, resulted in an exaggerated amygdala response to social stress when compared to those who received the placebo ( Muscatell et al., 2009 ).
The neural responses to stress do not appear to be uniform between men and women, though there are few fMRI stress studies that study sex differences. In a small study employing an acute laboratory stressor, men showed increased activation in the prefrontal cortex while women displayed elevated activity in limbic regions, including the ventral striatum and cingulate ( Wang et al., 2007 ). In terms of structural changes due to stress, childhood trauma may impact the hippocampal volume of males more than females (reviewed in Tiwari and Gonzalez, 2018 ) and may enlarge amygdala and decrease connectivity of salience hubs such as the dACC in females more than males ( Helpman et al., 2017 ). Animal studies have delineated some of the mechanisms of sex differences. For example, in rats, chronic restraint stress leads to dendritic atrophy of the hippocampus more so in male rats than female rats ( Galea et al., 1997 ). Sex steroids, which have a myriad of effects on neurons and glial cells, and various stress response systems, help explain these differential effects ( McEwen and Milner, 2017 ). Sex differences in acute stress responses and stress-related psychiatric disorders are well established, require further study, and should be included in the development of prevention and treatment strategies ( Gobinath et al., 2017 ).
Together these findings suggest that high levels of cumulative life stress, especially experienced early in life, shape stress related neural pathways and brain architecture ( Fig. 1 , Path A), and these alter how people perceive and respond to potentially stressful stimuli ( Fig. 1 , Path B). Sustained and exaggerated stress responses likely result in feed forward mechanisms, with important implications for allostatic load and disease risk.
9. Conclusion
In order to advance our understanding of how stress influences trajectories of aging and health, stress must be measured in context . Context includes individual and environmental factors, personal histories of stressor exposure (stress in childhood in particular but also cumulative life stress), current chronic stressors, and existing protective factors. Examining the impact of a single stressor exposure without measuring the contextual factors in which a person is experiencing the stressor limits the predictive ability: The historical context influences the habitual responses to stress that ultimately determine whether it will have cumulative effects, contributing to allostatic load and early disease. Traditional models of stimulus-response framework are useful for seeing and studying individual components of the stress process, but these tend to be linear and limited to measures of conscious explicit recall. To advance health research, we need to examine and describe context along with stressor exposures and stress responses, taking into account (both analytically and theoretically) the recursive and multilevel processes that link stress to health as described in Fig. 1 . It is difficult to understand a person’s risk for stress-related disease with only life history self-report, or only a reactivity measure. However, looking at these in tandem may help us uncover who is at highest risk for stress related disease. Relying only on retrospective measures for landmark events will also lead to a limited and possibly biased view of stress effects and stress resilience. We need longitudinal studies, and ideally transgenerational studies, that collect social, individual, and physiological indices of well-being over time, as well as health behaviors.
Careful measurement of stress processes is essential to propelling stress science forward. This begins with choosing a stress measure from a theoretical, or at least conceptual lens, and that appropriately fits the research question. For example, asking someone if they help a family member with activities of daily living may mean you are able to group them as a caregiver or not, but it does not mean you can make assumptions that they are ‘chronically stressed’ since you have not asked them about their levels of subjective distress from caregiving. The Stress Typology should be used to guide decisions and descriptions of stress measures. Importantly, the Typology provides a list of the psychosocial features that characterize stressor exposures, highlighting the need to identify and describe these (i.e. physical threat/danger, social status threat, humiliation, and role change/disruption). Identifying these features of the stressor should lead to insights into mechanisms by which the stressor impacts psychological responses and physiology and enhance the ability to harmonize across multiple stressors that might not be identical, but share common features (e.g., acute political instability and unsafe neighborhoods both share a common core of physical threat/danger). We do not yet know all the key attributes that make a stressor toxic. It is likely the Typology is missing key components that must be measured, and that some subjective components may not in fact be important. Much work is to be done in refining existing, and developing new stress measurement tools. For example, a recent study showed that metrics of language (particularly lower output and words than self reported measures of stress, suggesting that unconscious/behavioral measures have a potentially large unexplored role in tapping individual differences related to poor health ( Mehl et al., 2017 ).
Future research will also need to focus on cultural validation of existing stress measures. Furthermore, better measurement of profiles of acute stress reactivity (e.g., threat vs. challenge, recovery) may lead to interventions more targeted toward stress resilience and promoting healthy behaviors. The next generation of research will embrace a systems perspective – one that incorporates a person’s life history and context into hypotheses about how stressor exposures influence health, as well as protective and damaging health behaviors – which directly impact allostatic load and brain health. We now have the benefit of rich measures of daily stress through ecological momentary assessments using technology to obtain both psychological and physiological responses and detailed profiles of stressor exposures and social/physical contexts. Future research will also have an even more granular lens, with the ability to measure stress responses as they unfold in the moment. With these new measurement techniques we can better assess stressor exposures and responses, including recovery speed, at multiple levels of analysis. We can build models that encompass better measurement of lifespan exposures and responses, as well as short term or acute stress responses. In addition, with more specific measures and predictive models, we can lay the foundation for individual and social interventions, and policies, that are geared toward promoting the wellbeing and healthspan of our aging society.
Acknowledgement
The Stress Measurement Network has the goal to promote better theory and measurement of stress to deepen our understanding of stress processes and healthy aging. This paper is based partly on past meetings of the Network and we are grateful to our many colleagues who contributed to these discussions, and especially grateful to Lisbeth Nielsen, Ph.D., Division of Behavioral and Social Research, NIA, who has spearheaded this effort, both within NIA and across NIH. We are grateful to Lis Nielsen and Bruce McEwen for their impactful insights and comments on this manuscript. We are also grateful to Tom Kamarck, Ph.D., who helped develop an earlier version of the Stress Typology. This work was supported by the National Institutes of Health [NIA R24 AG048024, “Advancing Psychosocial & Biobehavioral Stress Measurement to Understanding Aging”].
Appendix 1. Stress typology for stress measurement
A. stressor exposure characteristics.
Stressor exposure characteristics include the timescale of the stressor, the life period in which it occurs, and the assessment window of the measurement tool used. These can be either objectively recorded or self-reported by the participant.
A.1. Timescale
- Acute Stressors: These are short term exposures under either naturalistic or standardized laboratory conditions.
- Daily Events/Hassles: These are the moreminor hassles that happen frequently, such as rushing, arguments, deadlines, and child caregiving strains.
- Life Events: These are stressful events that are event-based – meaning they are episodic in nature, and have an identifiable onset – such as getting into an accident, being laid off, being broken up with, or receiving a life-threatening diagnosis. Traumatic events are a special category of life events in which physical and/or psychological safety is threatened.
- Chronic Stressors: Stressors that are identified by participants, interviewer, or external raters as being demanding, distressing, and ongoing (e.g., 6 months or more).
A.2. Life period
- In utero: Exposure to maternal stress and associated hormones that traverse the umbilical cord and modify the resting allostatic state and response signatures prenatally.
- Childhood: Childhood is typically defined up to age 18 years old, though some measures focus on early childhood such as before age 5.
- Adulthood: Exposures that occur at 18 years or older.
- Lifespan/Cumulative: Measures that ask about exposures during childhood and adulthood. This can be measured cross-sectionally with retrospective measures, or this can be calculated from prospective studies that take measures over time.
A.3. Assessment window
- Current rating (usually captures in-the-moment reports, can also be reporting on the past 10 min, past hour, etc.)
- Daily ratings (typically given at the end of day, reporting on the day)
- Retrospective periods of more than a day (week, month, year, lifetime)
- Proximity of assessment to stressor exposure. This more typically applies to objective assessments since subjective assessments are harder to recall retrospectively. The proximity of the assessment to the event can be current or retrospective. It can be assessed as a continuous variable, such as the number of minutes or years between when the exposure occurred and when it was assessed.
A.4. Stressor attributes
- Duration: Captured in measurement units such as minutes, days, months, or years.
- Severity: Measured on a continuous scale, from low-to-high severity, that can be rated by others or self-rated.
- Controllability: Measured on a continuous scale by others or self-rated. Tasks can also be defined as controllable (giving a speech) or uncontrollable (cold-pressor test) by task design.
- Life domain: Life domains are specific areas of life that stress can exist within such as Education, Work, Reproductive Health, Housing, Money, Crime, Legal, Health, Intimate Relationships, Friend Relationships, Children, Death, Possessions. Stressors can cross and affect multiple domains.
- Target of stressor: This identifies who the stressor targets and can include the self, close-others such as family and friends, or the participants’ community.
- Potential of the stressor to elicit potentially harmful emotional responses: There are qualities inherent to some stressors that lead to feelings of social threat or shame that are associated with worse adjustment outcomes. These stressor qualities include interpersonal loss, physical threat/danger, social status threat, humiliation, entrapment, and role change/disruption.
B. Psychological and behavioral responses to specific stimuli or events
Responses to stressful stimuli or a an acute event include appraisals and perceptions of the situation, as well as affective, emotional, and cognitive responses to it. Measures of trait affect are not included as these are not context-specific and thus we do not consider them stress responses, however, momentary emotional responses are considered a component of the stress response.
- Global subjective stress: This is often measured with the Perceived Stress Scale, and thought of when we colloquially say we are “feeling stressed.”
- Subjective stress within a life domain: These measures typically include both extent of exposures (existence of stressor, to frequency of experience) as well as subjective ratings of how much distress the situation causes. Examples of common domains include neighborhood environment, financial resources and strain, work strain, unemployment, and social/interpersonal stress such as caregiver, loneliness, isolation, relationship strain, and discrimination.
- Motivational states (e.g. approach and avoidance, which can be measured with subjective and behavioral indicators)
- Emotional responses (negative and positive affect ratings and specific emotions in response to stimuli)
- Cognitive appraisals (threat vs. challenge appraisals, loss appraisals, threats to safety, lack of controllability)
- Behavioral coping (e.g. behaviors such as smoking, overeating, and strategies such as seeking support)
- Emotion regulation (e.g. cognitive re-appraisal, situation modification, response modulation, emotion focused coping)
- Perseverative cognition (e.g. worry, rumination)
Typology Legend: An essential distinction in studying stress is whether the form of stress being referred to is the exposure to the stressful event or stimulus (A), or the response to the it (B), which we define as the person’s subjective psychological appraisal, emotional, and cognitive response to the event or stimulus. Below are dimensions of stressors and stress responses to promote unified descriptions across studies and fields.
Appendix 2.
Chronic stressors and physical health outcomes
Declarations of interest
- Ahola K, Hakanen J, 2007. Job strain, burnout, and depressive symptoms: a prospective study among dentists . J. Affect. Disord 104 ( 1 ), 103–110. [ PubMed ] [ Google Scholar ]
- Akinola M, Mendes WB, 2008. The dark side of creativity: biological vulnerability and negative emotions lead to greater artistic creativity . Pers. Soc. Psychol. Bull 34 ( 12 ), 1677–1686. 10.1177/0146167208323933. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Anda RF, Dong M, Brown DW, Felitti VJ, Giles WH, Perry GS, et al., 2009. The relationship of adverse childhood experiences to a history of premature death of family members . BMC Publ. Health 9 , 106. 10.1186/1471-2458-9-106. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Andersen SL, Teicher MH, 2008. Stress, sensitive periods and maturational events in adolescent depression . Trends Neurosci 31 ( 4 ), 183–191. 10.1016/j.tins.2008.01.004. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R, 2012a. Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions . Biol. Psychiat 72 ( 1 ), 57–64. 10.1016/j.biopsych.2011.11.022. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baltes PB, Baltes MM, 1990. Psychological perspectives on successful aging: the model of selective optimization with compensation. In: Baltes PB, Baltes MM (Eds.), Successful Aging: Perspectives from the Behavioral Sciences . Cambridge University Press, Cambridge, U.K., pp. 1–34. [ Google Scholar ]
- Barker DJP, 2004. The developmental origins of chronic adult disease . Acta Paediatr. (Oslo, Norway: 1992) 93 ( Supplement 446 ), 26–33. [ PubMed ] [ Google Scholar ]
- Barnes LL, de Leon CFM, Lewis TT, Bienias JL, Wilson RS, Evans DA, 2008. Perceived discrimination and mortality in a population-based study of older adults . Am. J. Publ. Health 98 ( 7 ), 1241–1247. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Barnes LL, Wilson RS, Everson-Rose SA, Hayward MD, Evans DA, de Leon CFM, 2012. Effects of early-life adversity on cognitive decline in older African Americans and whites . Neurology 79 ( 24 ), 2321–2327. 10.1212/WNL.0b013e318278b607. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Barrett LF, 2009. Variety is the spice of life: a psychological construction approach to understanding variability in emotion . Cogn. Emot 23 ( 7 ), 1284–1306. 10.1016/j.biopsych.2011.11.022. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Barrett LF, Simmons WK, 2015. Interoceptive predictions in the brain . Nat. Rev. Neurosci 16 ( 7 ), 419–429. 10.1038/nrn3950. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baumeister RF, Leary MR, 1995. The need to belong: desire for interpersonal attachments as a fundamental human motivation . Psychol. Bull 117 ( 3 ), 497–529. [ PubMed ] [ Google Scholar ]
- Beatty DL, Matthews KA, Bromberger JT, Brown C, 2014. Everyday discrimination prospectively predicts inflammation across 7-years in racially diverse midlife women: Study of women’s health across the nation . J. Soc. Issues 70 ( 2 ), 298–314. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bechara A, Damasio H, Tranel D, Damasio AR, 1997. Deciding advantageously before knowing the advantageous strategy . Science 275 ( 5304 ), 1293–1295. [ PubMed ] [ Google Scholar ]
- Bernard K, Frost A, Bennett CB, Lindhiem O, 2017. Maltreatment and diurnal cortisol regulation: a meta-analysis . Psychoneuroendocrinology 78 , 57–67. 10.1016/j.psyneuen.2017.01.005. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bherer L, Erickson KI, Liu-Ambrose T, 2013. A review of the effects of physical activity and exercise on cognitive and brain functions in older adults . J. Aging Res 657508. 10.1155/2013/657508. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Blascovich J, Mendes WB, 2000. Challenge and threat appraisals: the role of affective cues. In: Forgas J (Ed.), Feeling and Thinking: The Role of Affect in Social Cognition . Cambridge University Press, New York, NY, pp. 59–82. [ Google Scholar ]
- Blascovich J, Mendes WB, 2010. Social psychophysiology and embodiment. In: Fiske ST, Gilbert DT, Lindzey G (Eds.), The Handbook of Social Psychology , fifth ed. John Wiley & Sons Inc, New York, NY, pp. 194–227. [ Google Scholar ]
- Blascovich J, Tomaka J, 1996. The biopsychosocial model of arousal regulation . In: Advances in Experimental Social Psychology , vol. 28 , pp. 1–51. [ Google Scholar ]
- Boggero IA, Hostinar CE, Haak EA, Murphy MLM, Segerstrom SC, 2017. Psychosocial functioning and the cortisol awakening response: meta-analysis, P-curve analysis, and evaluation of the evidential value in existing studies . Biol. Psychol 129 , 207–230. 10.1016/j.biopsycho.2017.08.058. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bonanno GA, Diminich ED, 2013. Annual Research Review: positive adjustment to adversity–trajectories of minimal-impact resilience and emergent resilience . J. Child Psychol. Psychiat 54 ( 4 ), 378–401. 10.1111/jcpp.12021. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bonanno GA, Westphal M, Mancini AD, 2011. Resilience to loss and potential trauma . Annu. Rev. Clin. Psychol 7 , 511–535. 10.1146/annurevclinpsy-032210-104526. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bonanno G, Kaltman S, 1999. Toward an Integrative Perspective on Bereavement , vol. 125 . < 10.1037//0033-2909.125.6.760 >. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bosch JA, de Geus EJC, Carroll D, Goedhart AD, Anane LA, van Zanten JJV, et al., 2009. A general enhancement of autonomic and cortisol responses during social evaluative threat . Psychosom. Med 71 ( 8 ), 877–885. 10.1097/PSY.0b013e3181baef05. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bower JE, Crosswell AD, Slavich GM, 2014. Childhood adversity and cumulative life stress: risk factors for cancer-related fatigue . Clin. Psychol. Sci 2 ( 1 ), 108–115. 10.1177/2167702613496243. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Boyce WT, 2016 . Differential susceptibility of the developing brain to contextual adversity and stress . Neuropsychopharmacology 41 ( 1 ), 142–162. 10.1038/npp.2015.294. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brosschot JF, Pieper S, Thayer JF, 2005. Expanding stress theory: prolonged activation and perseverative cognition . Psychoneuroendocrinology 30 ( 10 ), 1043–1049. [ PubMed ] [ Google Scholar ]
- Brown GW, Harris TO, 1978. Social Origins of Depression: A Study of Psychiatric Disorder in Women . The Free Press, New York, NY. [ Google Scholar ]
- Brown GW, Harris TO, Hepworth C, 1995. Loss, humiliation and entrapment among women developing depression: a patient and non-patient comparison . Psychol. Med 25 ( 1 ), 7–21. [ PubMed ] [ Google Scholar ]
- Byron K, Khazanchi S, Nazarian D, 2010. The relationship between stressors and creativity: a meta-analysis examining competing theoretical models . J. Appl. Psychol 95 ( 1 ), 201. [ PubMed ] [ Google Scholar ]
- Cacioppo JT, Cacioppo S, Capitanio JP, Cole SW, 2015. The neuroendocrinology of social isolation . Annu. Rev. Psychol . 66 , 733–767. 10.1146/annurev-psych-010814-015240. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cacioppo JT, Hawkley LC, Crawford LE, Ernst JM, Burleson MH, Kowalewski RB, et al., 2002. Loneliness and health: potential mechanisms . Psychosom. Med 64 ( 3 ), 407–417. [ PubMed ] [ Google Scholar ]
- Capistrant BD, Berkman LF, Glymour MM, 2014. Does duration of spousal caregiving affect risk of depression onset? Evidence from the Health and Retirement Study . Am. J. Geriat. Psychiat 22 ( 8 ), 766–770. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Carstensen LL, 2006. The influence of a sense of time on human development . Science 312 ( 5782 ), 1913–1915. 10.1126/science.1127488. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, Price LH, 2011. Effect of childhood physical abuse on cortisol stress response . Psychopharmacology 214 ( 1 ), 367–375. 10.1007/s00213-010-2007-4. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Catalino LI, Arenander J, Epel E, Puterman E, 2017. Trait acceptance predicts fewer daily negative emotions through less stressor-related rumination . Emotion 17 ( 8 ), 1181–1186. 10.1037/emo0000279. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Charles ST, 2010. Strength and vulnerability integration: a model of emotional well-being across adulthood . Psychol. Bull 136 ( 6 ), 1068–1091. 10.1037/a0021232. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Charles ST, Carstensen LL, 2010. Social and emotional aging . Annu. Rev. Psychol 61 , 383–409. 10.1146/annurev.psych.093008.100448. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Charles ST, Piazza JR, Mogle J, Sliwinski MJ, Almeida DM, 2013. The wear-andtear of daily stressors on mental health . Psychol. Sci 24 ( 5 ), 733–741. 10.1177/0956797612462222. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chen BH, Marioni RE, Colicino E, Peters MJ, Ward-Caviness CK, Tsai P-C, et al., 2016. DNA methylation-based measures of biological age: meta-analysis predicting time to death . Aging 8 ( 9 ), 1844–1865. 10.18632/aging.101020. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chiang JJ, Taylor SE, Bower JE, 2015. Early adversity, neural development, and inflammation . Dev. Psychobiol 57 ( 8 ), 887–907. 10.1002/dev.21329. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chida Y, Hamer M, 2008. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: a quantitative review of 30 years of investigations . Psychol. Bull 134 ( 6 ), 829–885. 10.1037/a0013342. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chida Y, Steptoe A, 2010. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence . Hypertension 55 ( 4 ), 1026–1032. 10.1161/HYPERTENSIONAHA.109.146621. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chandola T, Brunner E, Marmot M, 2006. Chronic stress at work and the metabolic syndrome: prospective study . BMJ 332 ( 7540 ), 521–525. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Codd V, Nelson CP, Albrecht E, Mangino M, Deelen J, Buxton JL, et al., 2013. Identification of seven loci affecting mean telomere length and their association with disease . Nat. Genet 45 ( 4 ), 422–427. 10.1038/ng.2528.427-2. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cohen S, 2004. Social relationships and health . Am. Psychol 59 ( 8 ), 676–684. 10.1037/0003-066X.59.8.676. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cohen S, Gianaros PJ, Manuck SB, 2016. A stage model of stress and disease . Perspect. Psychol. Sci 11 ( 4 ), 456–463. 10.1177/1745691616646305. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cohen S, Janicki-Deverts D, Miller GE, 2007. Psychological stress and disease . JAMA 298 ( 14 ), 1685–1687. 10.1001/jama.298.14.1685. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cohen S, Kamarck T, Mermelstein R, 1983. A global measure of perceived stress . J. Health Soc. Behav 24 ( 4 ), 385–396. [ PubMed ] [ Google Scholar ]
- Cohen S, Kessler R, Underwood G, Gordon L, 1997. Measuring Stress: A Guide for Health and Social Scientists . Oxford University Press, New York, NY. [ Google Scholar ]
- Cohen S, Wills TA, 1985. Stress, social support, and the buffering hypothesis . Psychol. Bull 98 ( 2 ), 310–357. 10.1037/0033-2909.98.2.310. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cowan CSM, Hoban AE, Ventura-Silva AP, Dinan TG, Clarke G, Cryan JF, 2017. Gutsy moves: the amygdala as a critical node in microbiota to brain signaling . BioEssays: News Rev. Mol., Cell. Dev. Biol 10.1002/bies.201700172. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Critchley HD, 2005. Neural mechanisms of autonomic, affective, and cognitive integration . J. Comp. Neurol 493 ( 1 ), 154–166. 10.1002/cne.20749. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Critchley HD, Nagai Y, Gray MA, Mathias CJ, 2011. Dissecting axes of autonomic control in humans: insights from neuroimaging . Auton. Neurosci.: Basic Clin 161 ( 1 ), 34–42. [ PubMed ] [ Google Scholar ]
- Danese A, Baldwin JR, 2017. Hidden wounds? Inflammatory links between childhood trauma and psychopathology . Annu. Rev. Psychol 68 ( 1 ), 517–544. 10.1146/annurev-psych-010416-044208. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R, 2007. Childhood maltreatment predicts adult inflammation in a life-course study . PNAS 104 ( 4 ), 1319–1324. 10.1073/pnas.0610362104. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Denburg NL, Tranel D, Bechara A, 2005. The ability to decide advantageously declines prematurely in some normal older persons . Neuropsychologia 43 ( 7 ), 1099–1106. 10.1016/j.neuropsychologia.2004.09.012. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- DeSteno D, Gross JJ, Kubzansky L, 2013. Affective science and health: the importance of emotion and emotion regulation . Health Psychol . 32 ( 5 ), 474–486. 10.1037/a0030259. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dickerson SS, Gable SL, Irwin MR, Aziz N, Kemeny ME, 2009 . Social-evaluative threat and proinflammatory cytokine regulation: an experimental laboratory investigation . Psychol. Sci 20 ( 10 ), 1237–1244. 10.1111/j.1467-9280.2009.02437.x. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dickerson SS, Kemeny ME, 2004. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research . Psychol. Bull 130 ( 3 ), 355–391. 10.1037/0033-2909.130.3.355. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dienstbier RA, 1989. Arousal and physiological toughness: implications for mental and physical health . Psychol. Rev 96 ( 1 ), 84–100. [ PubMed ] [ Google Scholar ]
- Do DP, Diez Roux AV, Hajat A, Auchincloss AH, Merkin SS, Ranjit N, et al., 2011 . Circadian rhythm of cortisol and neighborhood characteristics in a populationbased sample: the multi-ethnic study of atherosclerosis . Health Place 17 ( 2 ), 625–632. 10.1016/j.healthplace.2010.12.019. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Donoho CJ, Bonanno GA, Porter B, Kearney L, Powell TM, 2017. A decade of war: prospective trajectories of post-traumatic stress disorder symptoms among deployed US military personnel and the influence of combat exposure . Am. J. Epidemiol 10.1093/aje/kwx318. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dooley LN, Slavich GM, Moreno PI, Bower JE, 2017. Strength through adversity: moderate lifetime stress exposure is associated with psychological resilience in breast cancer survivors . Stress Health 33 ( 5 ), 549–557. 10.1002/smi.2739. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ebner NC, Freund AM, Baltes PB, 2006. Developmental changes in personal goal orientation from young to late adulthood: from striving for gains to maintenance and prevention of losses . Psychol. Aging 21 ( 4 ), 664–678. 10.1037/0882-7974.21.4.664. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ehlert U, Gaab J, Heinrichs M, 2001. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: the role of the hypothalamus-pituitary-adrenal axis . Biol. Psychol 57 ( 1–3 ), 141–152. [ PubMed ] [ Google Scholar ]
- Eisenberger NI, Moieni M, Inagaki TK, Muscatell KA, Irwin MR, 2017. In sickness and in health: the co-regulation of inflammation and social behavior . Neuropsychopharmacology 42 ( 1 ), 242–253. 10.1038/npp.2016.141. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Elder G, 1998. The life course as developmental theory . Child Dev . 69 ( 1 ), 1–12. [ PubMed ] [ Google Scholar ]
- Emerging Risk Factors Collaboration,Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, et al., 2010. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis . Lancet (London, England) 375 ( 9709 ), 132–140. 10.1016/S0140-6736(09)61717-7. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- English T, Carstensen LL, 2014. Selective narrowing of social networks across adulthood is associated with improved emotional experience in daily life . Int. J. Behav. Dev 38 ( 2 ), 195–202. 10.1177/0165025413515404. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Epel E, Burke H, Wolkowitz OM, 2007. The psychoneuroendocrinology of aging: anabolic and catabolic hormones. In: Aldwin CM, Park CL, Spiro A (Eds.), Handbook of Health Psychology and Aging . Guilford Press, New York, NY, pp. 119–141. [ Google Scholar ]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM, 2004. Accelerated telomere shortening in response to life stress . PNAS 101 ( 49 ), 17312–17315. 10.1073/pnas.0407162101. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE, et al., 2006 . Cell aging in relation to stress arousal and cardiovascular disease risk factors . Psychoneuroendocrinology 31 ( 3 ), 277–287. 10.1016/j.psyneuen.2005.08.011. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fagundes CP, Glaser R, Kiecolt-Glaser JK, 2013. Stressful early life experiences and immune dysregulation across the lifespan . Brain Behav. Immun 27 ( 1 ), 8–12. 10.1016/j.bbi.2012.06.014. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Felder JN, Epel ES, Coccia M, Puterman E, Prather AA, 2017. Effects of daily maladaptive coping on nightly sleep in mothers . Psychol. Health 1–14. 10.1080/08870446.2017.1310863. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al., 1998. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the Adverse Childhood Experiences (ACE) study . Am. J. Prev. Med 14 ( 4 ), 245–258. [ PubMed ] [ Google Scholar ]
- Folkman S, Lazarus RS, Gruen RJ, DeLongis A, 1986. Appraisal, coping, health status, and psychological symptoms . J. Pers. Soc. Psychol 50 ( 3 ), 571–579. [ PubMed ] [ Google Scholar ]
- Fonareva I, Oken BS, 2014. Physiological and functional consequences of caregiving for relatives with dementia . Int. Psychogeriatr 26 ( 5 ), 725–747. 10.1017/S1041610214000039. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Frankenhaeuser M, 1986. A psychobiological framework for research on human stress and coping. In: Dynamics of Stress . Springer, Boston, MA, pp. 101–116. [ Google Scholar ]
- Friedman EM, Karlamangla AS, Almeida DM, Seeman TE, 2012. Social strain and cortisol regulation in midlife in the US . Soc. Sci. Med. (1982) 74 ( 4 ), 607–615. 10.1016/j.socscimed.2011.11.003. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Galea LAM, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS, 1997. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress . Neuroscience 81 ( 3 ), 689–697. 10.1016/S0306-4522(97)00233-9. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Galobardes B, Lynch JW, Davey Smith G, 2004. Childhood socioeconomic circumstances and cause-specific mortality in adulthood: systematic review and interpretation . Epidemiol. Rev 26 , 7–21. 10.1093/epirev/mxh008. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gawronski KA, Kim ES, Miller LE, 2014. Potentially traumatic events and serious life stressors are prospectively associated with frequency of doctor visits and overnight hospital visits . J. Psychosom. Res 77 ( 2 ), 90–96. [ PubMed ] [ Google Scholar ]
- Gianaros PJ, Jennings JR, Sheu LK, Greer PJ, Kuller LH, Matthews KA, 2007. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus . NeuroImage 35 ( 2 ), 795–803. 10.1016/j.neuroimage.2006.10.045. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gianaros PJ, Wager TD, 2015. Brain-body pathways linking psychological stress and physical health . Curr. Dir. Psychol. Sci 24 ( 4 ), 313–321. 10.1177/0963721415581476. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gianferante D, Thoma MV, Hanlin L, Chen X, Breines JG, Zoccola PM, Rohleder N, 2014. Post-stress rumination predicts HPA axis responses to repeated acute stress . Psychoneuroendocrinology 49 , 244–252. 10.1016/j.psyneuen.2014.07.021. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gilbert P, 1992. Depression: The Evolution of Powerlessness . The Guilford Press, New York, NY. [ Google Scholar ]
- Gobinath AR, Choleris E, Galea LAM, 2017. Sex, hormones, and genotype interact to influence psychiatric disease, treatment, and behavioral research . J. Neurosci. Res 95 ( 1–2 ), 50–64. 10.1002/jnr.23872. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Goble DJ, Coxon JP, Wenderoth N, Van Impe A, Swinnen SP, 2009. Proprioceptive sensibility in the elderly: degeneration, functional consequences and plastic-adaptive processes . Neurosci. Biobehav. Rev 33 ( 3 ), 271–278. 10.1016/j.neubiorev.2008.08.012. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Goldfarb EV, Shields GS, Daw ND, Slavich GM, Phelps EA, 2017. Low lifetime stress exposure is associated with reduced stimulus-response memory . Learn. Memory 24 , 162–168. 10.1101/lm.045179.117. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gottlieb BH, Bergen AE, 2010. Social support concepts and measures . J. Psychosom. Res 69 ( 5 ), 511–520. 10.1016/j.jpsychores.2009.10.001. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gouin J-P, Glaser R, Malarkey WB, Beversdorf D, Kiecolt-Glaser J, 2012. Chronic stress, daily stressors, and circulating inflammatory markers . Health Psychol . 31 ( 2 ), 264–268. 10.1037/a0025536. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Guyll M, Matthews KA, Bromberger JT, 2001. Discrimination and unfair treatment: relationship to cardiovascular reactivity among African American and European American women . Health Psychol . 20 ( 5 ), 315–325. [ PubMed ] [ Google Scholar ]
- Hakulinen C, Pulkki-Råback L, Jokela M, Ferrie JE, Aalto A-M, Virtanen M, et al., 2016. Structural and functional aspects of social support as predictors of mental and physical health trajectories: whitehall II cohort study . J. Epidemiol. Commun. Health 70 ( 7 ), 710–715. [ PubMed ] [ Google Scholar ]
- HALE Collaborators, 2016. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015 . Lancet 388 ( 10053 ), 1603–1658. 10.1016/S0140-6736(16)31460-X. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hammen C, 2005. Stress and depression . Annu. Rev. Clin. Psychol 1 , 293–319. 10.1146/annurev.clinpsy.1.102803.143938. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, Pollak SD, 2010. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk . J. Neurosci 30 ( 22 ), 7466–7472. 10.1523/JNEUROSCI.0859-10.2010. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hanssen LM, Schutte NS, Malouff JM, Epel ES, 2017. The relationship between childhood psychosocial stressor level and telomere length: a meta-analysis . Health Psychol. Res 5 ( 1 ), 6378. 10.4081/hpr.2017.6378. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Harmon-Jones E, Peterson CK, 2009. Supine body position reduces neural response to anger evocation . Psychol. Sci 20 ( 10 ), 1209–1210. 10.1111/j.1467-9280.2009.02416.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hardt J, Rutter M, 2004. Validity of adult retrospective reports of adverse childhood experiences: review of the evidence . J. Child Psychol. Psychiat 45 ( 2 ), 260–273. [ PubMed ] [ Google Scholar ]
- Hatfield E, Cacioppo JT, Rapson RL, 1994. Emotional Contagion . Cambridge University Press. [ Google Scholar ]
- Hawkley LC, Cacioppo JT, 2010. Loneliness matters: a theoretical and empirical review of consequences and mechanisms . Ann. Behav. Med 40 ( 2 ), 218–227. 10.1007/s12160-010-9210-8. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Heikkilä K, Nyberg ST, Theorell T, Fransson EI, Alfredsson L, Bjorner JB, et al., 2013. Work stress and risk of cancer: meta-analysis of 5700 incident cancer events in 116000 European men and women . Br. Med. J 346 , f165. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Heim C, Binder EB, 2012 . Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics . Exp. Neurol 233 ( 1 ), 102–111. 10.1016/j.expneurol.2011.10.032. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Helpman L, Zhu X, Suarez-Jimenez B, Lazarov A, Monk C, Neria Y, 2017. Sex differences in trauma-related psychopathology: a critical review of neuroimaging literature (2014–2017) . Curr. Psychiat. Rep 19 ( 12 ), 104. 10.1007/s11920-017-0854-y. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Henry JP, 1986. Neuroendocrine patterns of emotional response . In: Biological Foundations of Emotion , pp. 37–60. [ Google Scholar ]
- Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, Essex MJ, 2013. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence . PNAS 110 ( 47 ), 19119–19124. 10.1073/pnas.1310766110. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hobfoll SE, 2001. The influence of culture, community, and the nested-self in the stress process: advancing conservation of resources theory . Appl. Psychol.: Int. Rev 50 ( 3 ), 337–421. 10.1111/1464-0597.00062. [ CrossRef ] [ Google Scholar ]
- Holmes TH, Rahe RH, 1967. The social readjustment rating scale . J. Psychosom. Res 11 ( 2 ), 213–218. [ PubMed ] [ Google Scholar ]
- Holt-Lunstad J, Smith TB, Baker M, Harris T, Stephenson D, 2015. Loneliness and social isolation as risk factors for mortality: a meta-analytic review . Perspect. Psychol. Sci 10 ( 2 ), 227–237. 10.1177/1745691614568352. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- House JS, Landis KR, Umberson D, 1988. Social relationships and health . Science 241 ( 4865 ), 540–545. [ PubMed ] [ Google Scholar ]
- Hwang A-C, Peng L-N, Wen Y-W, Tsai Y-W, Chang L-C, Chiou S-T, Chen L-K, 2014. Predicting all-cause and cause-specific mortality by static and dynamic measurements of allostatic load: a 10-year population-based cohort study in Taiwan . J. Am. Med. Directors Assoc 15 ( 7 ), 490–496. 10.1016/j.jamda.2014.02.001. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Infurna FJ, Luthar SS, 2016. Resilience to major life stressors is not as common as thought . Perspect. Psychol. Sci 11 ( 2 ), 175–194. 10.1177/1745691615621271. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Iwata J, LeDoux JE, 1988. Dissociation of associative and nonassociative concomitants of classical fear conditioning in the freely behaving rat . Behav. Neurosci 102 ( 1 ), 66–76. [ PubMed ] [ Google Scholar ]
- Jacobs JR, Bovasso GB, 2000. Early and chronic stress and their relation to breast cancer . Psychol. Med 30 ( 3 ), 669–678. [ PubMed ] [ Google Scholar ]
- Jamieson J, Mendes WB, Blackstock E, Schmader T, 2010. Turning the knots in your stomach into bows: reappraising arousal improves performance on the GRE . J. Exp. Soc. Psychol 46 , 208–212. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jamieson JP, Hangen EJ, Lee HY, Yeager DS, 2017. Capitalizing on appraisal processes to improve affective responses to social stress . Emotion Rev 10.1177/1754073917693085. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jamieson JP, Nock MK, Mendes WB, 2012. Mind over matter: reappraising arousal improves cardiovascular and cognitive responses to stress . J. Exp. Psychol. Gen 141 , 417–422. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lindsay EK, Young S, Smyth JM, Brown KW, Creswell JD, 2018. Acceptance lowers stress reactivity: dismantling mindfulness training in a randomized controlled trial . Psychoneuroendocrinology 87 , 63–73. 10.1016/j.psyneuen.2017.09.015. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Janoff-Bulman R, 1992. Shattered Assumptions: Towards a New Psychology of Trauma . Free Press, New York. [ Google Scholar ]
- Jefferson AL, Himali JJ, Beiser AS, Au R, Massaro JM, Seshadri S, et al., 2010. Cardiac index is associated with brain aging: the Framingham Heart Study . Circulation 122 ( 7 ), 690–697. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kagan J, 2016. An overly permissive extension . Perspect. Psychol. Sci 11 ( 4 ), 442–450. 10.1177/1745691616635593. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Karb RA, Elliott MR, Dowd JB, Morenoff JD, 2012. Neighborhood-level stressors, social support, and diurnal patterns of cortisol: the Chicago Community Adult Health Study . Soc. Sci. Med 75 ( 6 ), 1038–1047. 10.1016/j.socscimed.2012.03.031. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kassam K, Koslov K, Mendes WB, 2009. Decisions under distress: stress profiles influence anchoring and adjustment . Psychol. Sci 20 , 1394–1399. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kelly-Irving M, Lepage B, Dedieu D, Lacey R, Cable N, Bartley M, et al., 2013. Childhood adversity as a risk for cancer: findings from the 1958 British birth cohort study . BMC Publ. Health 13 , 767. 10.1186/1471-2458-13-767. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kendler KS, Hettema JM, Butera F, Gardner CO, Prescott CA, 2003. Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety . Arch. Gen. Psychiat 60 ( 8 ), 789–796. [ PubMed ] [ Google Scholar ]
- Keyes KM, McLaughlin KA, Demmer RT, Cerdá M, Koenen KC, Uddin M, Galea S, 2013. Potentially traumatic events and the risk of six physical health conditions in a population-based sample . Depress. Anx 30 ( 5 ), 451–460. 10.1002/da.22090. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Khalsa SS, Rudrauf D, Tranel D, 2009. Interoceptive awareness declines with age . Psychophysiology 46 ( 6 ), 1130–1136. 10.1111/j.1469-8986.2009.00859.x. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kirschbaum C, Prüssner JC, Stone AA, Federenko I, Gaab J, Lintz D, et al., 1995. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men . Psychosom. Med 57 ( 5 ), 468–474. [ PubMed ] [ Google Scholar ]
- Koffer RE, Ram N, Conroy DE, Pincus AL, Almeida DM, 2016. Stressor diversity: introduction and empirical integration into the daily stress model . Psychol. Aging 31 ( 4 ), 301–320. 10.1037/pag0000095. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Koslov K, Mendes WB, Pajtas PE, Pizzagalli DA, 2011. Asymmetry in resting intracortical activity as a buffer to social threat . Psychol. Sci 22 ( 5 ), 641–649. 10.1177/0956797611403156. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Koupil I, Plavinskaja S, Parfenova N, Shestov DB, Danziger PD, Vågerö D, 2009. Cancer mortality in women and men who survived the siege of Leningrad (1941–1944) . Int. J. Cancer 124 ( 6 ), 1416–1421. 10.1002/ijc.24093. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Krause N, Shaw BA, Cairney J, 2004. A descriptive epidemiology of lifetime trauma and the physical health status of older adults . Psychol. Aging 19 ( 4 ), 637–648. 10.1037/0882-7974.19.4.637. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lampert R, Tuit K, Hong K-I, Donovan T, Lee F, Sinha R, 2016. Cumulative stress and autonomic dysregulation in a community sample . Stress 19 ( 3 ), 269–279. 10.1080/10253890.2016.1174847. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lawler KA, Obrist PA, Lawler JE, 1976. Cardiac and somatic response patterns during a reaction time task in children and adults . Psychophysiology 13 ( 5 ), 448–455. 10.1111/j.1469-8986.1976.tb00859.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lazarus RS, Folkman S, 1984. Stress, Appraisal & Coping . Springer, New York. [ Google Scholar ]
- Lazarus RS, DeLongis A, Folkman S, Gruen R, 1985. Stress and adaptational outcomes. The problem of confounded measures . Am. Psychol 40 ( 7 ), 770–785. [ PubMed ] [ Google Scholar ]
- Levenson RW, Carstensen LL, Friesen WV, Ekman P, 1991. Emotion, physiology, and expression in old age . Psychol. Aging 6 ( 1 ), 28–35. [ PubMed ] [ Google Scholar ]
- Levine ME, Cole SW, Weir DR, Crimmins EM, 2015. Childhood and later life stressors and increased inflammatory gene expression at older ages . Soc. Sci. Med . 1982 ( 130 ), 16–22. 10.1016/j.socscimed.2015.01.030. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Levy R, 1994. Aging-associated cognitive decline. Working Party of the International Psychogeriatric Association in collaboration with the World Health Organization . Int. Psychogeriatr 6 ( 1 ), 63–68. [ PubMed ] [ Google Scholar ]
- Li Y, Zhong X, Cheng G, Zhao C, Zhang L, Hong Y, et al., 2017. Hs-CRP and all-cause, cardiovascular, and cancer mortality risk: a meta-analysis . Atherosclerosis 259 , 75–82. 10.1016/j.atherosclerosis.2017.02.003. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lin EH, Carter WB, Kleinman AM, 1985. An exploration of somatization among Asian refugees and immigrants in primary care . Am. J. Publ. Health 75 ( 9 ), 1080–1084. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lloyd DA, Turner RJ, 2008. Cumulative lifetime adversities and alcohol dependence in adolescence and young adulthood . Drug Alcohol Depend . 93 ( 3 ), 217–226. 10.1016/j.drugalcdep.2007.09.012. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lovallo WR, 2011. Do low levels of stress reactivity signal poor states of health? Biol. Psychol 86 ( 2 ), 121–128. 10.1016/j.biopsycho.2010.01.006. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lovallo WR, Thomas TL, 2000. Stress hormones in psychophysiological research: emotional, behavioral and cognitive implications. In: Cacioppo JT, Tassinary LG, Berntson GG (Eds.), Handbook of Psychophysiology . Cambridge University Press, Cambridge, U.K., pp. 342–367. [ Google Scholar ]
- Luo Y, Hawkley LC, Waite LJ, Cacioppo JT, 2012. Loneliness, health, and mortality in old age: a national longitudinal study . Soc. Sci. Med 74 ( 6 ), 907–914. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Luo Y, Waite LJ, 2005. The impact of childhood and adult SES on physical, mental, and cognitive well-being in later life . J. Gerontol. Ser. B, Psychol. Sci. Soc. Sci 60 ( 2 ), S93–S101. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C, 2009. Effects of stress throughout the lifespan on the brain, behaviour and cognition . Nat. Rev. Neurosci 10 ( 6 ), 434–445. 10.1038/nrn2639. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Maier SF, Watkins LR, 2005. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor . Neurosci. Biobehav. Rev 29 ( 4–5 ), 829–841. 10.1016/j.neubiorev.2005.03.021. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Marsland AL, Walsh C, Lockwood K, John-Henderson NA, 2017. The effects of acute psychological stress on circulating and stimulated inflammatory markers: a systematic review and meta-analysis . Brain Behav. Immun 64 , 208–219. 10.1016/j.bbi.2017.01.011. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mason JW, 1968. A review of psychoendocrine research on the pituitary-adrenal cortical system . Psychosom. Med 30 ( 5 Suppl ), 576–607. [ PubMed ] [ Google Scholar ]
- Matthews KA, 2005. Psychological perspectives on the development of coronary heart disease . Am. Psychol 60 ( 8 ), 783–796. 10.1037/0003-066X.60.8.783. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Matthews KA, Gump BB, Owens JF, 2001. Chronic stress influences cardiovascular and neuroendocrine responses during acute stress and recovery, especially in men . Health Psychol 20 ( 6 ), 403–410. [ PubMed ] [ Google Scholar ]
- Matthews RJ, Smith LK, Hancock RM, Jagger C, Spiers NA, 2005. Socioeconomic factors associated with the onset of disability in older age: a longitudinal study of people aged 75 years and over . Soc. Sci. Med. (1982) 61 ( 7 ), 1567–1575. 10.1016/j.socscimed.2005.02.007. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ, 2005. The tie that binds? Coherence among emotion experience, behavior, and physiology . Emotion 5 ( 2 ), 175–190. 10.1037/1528-3542.5.2.175. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McCrory EJ, De Brito SA, Sebastian CL, Mechelli A, Bird G, Kelly PA, Viding E, 2011. Heightened neural reactivity to threat in child victims of family violence . Curr. Biol 21 ( 23 ), R947–948. 10.1016/j.cub.2011.10.015. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McEwen BS, 1998. Stress, adaptation, and disease. Allostasis and allostatic load . Ann. N. Y. Acad. Sci 840 , 33–44. [ PubMed ] [ Google Scholar ]
- McEwen BS, 1999. Stress and hippocampal plasticity . Annu. Rev. Neurosci 22 , 105–122. 10.1146/annurev.neuro.22.1.105. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McEwen BS, 2007. Physiology and neurobiology of stress and adaptation: central role of the brain . Physiol. Rev 87 ( 3 ), 873–904. 10.1152/physrev.00041.2006. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McEwen BS, Gianaros PJ, 2011. Stress- and allostasis-induced brain plasticity . Annu. Rev. Med 62 , 431–445. 10.1146/annurev-med-052209-100430. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McEwen BS, Milner TA, 2017. Understanding the broad influence of sex hormones and sex differences in the brain . J. Neurosci. Res 95 ( 1–2 ), 24–39. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- McLaughlin KA, Sheridan MA, Alves S, Mendes WB, 2014a. Child maltreatment and autonomic nervous system reactivity: identifying dysregulated stress reactivity patterns by using the biopsychosocial model of challenge and threat . Psychosom. Med 76 ( 7 ), 538–546. 10.1097/PSY.0000000000000098. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McLaughlin KA, Sheridan MA, Lambert HK, 2014b. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience . Neurosci. Biobehav. Rev 47 ( Supplement C ), 578–591. 10.1016/j.neubiorev.2014.10.012. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mehl MR, Raison CL, Pace TWW, Arevalo JMG, Cole SW, 2017. Natural language indicators of differential gene regulation in the human immune system . PNAS 114 ( 47 ), 12554–12559. 10.1073/pnas.1707373114. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Melamed S, Shirom A, Toker S, Shapira I, 2006. Burnout and risk of type 2 diabetes: a prospective study of apparently healthy employed persons . Psychosom. Med 86 ( 6 ), 863–869. [ PubMed ] [ Google Scholar ]
- Mellman TA, Brown DD, Jenifer ES, Hipolito MMS, Randall OS, 2009. Posttraumatic stress disorder and nocturnal blood pressure dipping in young adult African Americans . Psychosom. Med 71 ( 6 ), 627–630. 10.1097/PSY.0b013e3181a54341. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mendes WB, 2010. Weakened links between mind and body in older age: the case for maturational dualism in the experience of emotion . Emotion Rev . 2 , 240–244. [ Google Scholar ]
- Mendes WB, McCoy S, Major B, Blascovich J, 2008. How attributional ambiguity shapes physiological and emotional responses to social rejection and acceptance . J. Pers. Soc. Psychol 94 ( 2 ), 278–291. 10.1037/0022-3514.94.2.278. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mezuk B, Kershaw KN, Hudson D, Lim KA, Ratliff S, 2011. Job strain, workplace discrimination, and hypertension among older workers: the health and retirement study . Race Soc. Probl 3 ( 1 ), 38–50. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mezuk B, Abdou CM, Hudson D, Kershaw KN, Rafferty JA, Lee H, Jackson JS, 2013. “White Box” epidemiology and the social neuroscience of health behaviors: the environmental affordances model . Soc. Ment. Health 3 , 79–95. 10.1177/2156869313480892. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Miller GE, Chen E, Parker KJ, 2011. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms . Psychol. Bull 137 ( 6 ), 959. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Miller GE, Chen E, Zhou ES, 2007. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans . Psychol. Bull 133 ( 1 ), 25–45. 10.1037/0033-2909.133.1.25. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Miller GE, Rohleder N, Cole SW, 2009. Chronic interpersonal stress predicts activation of pro- and anti-inflammatory signaling pathways 6 months later . Psychosom. Med 71 ( 1 ), 57–62. 10.1097/PSY.0b013e318190d7de. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Moen P, 1996. A life course perspective on retirement, gender, and well-being . J. Occup. Health Psychol 1 ( 2 ), 131–144. [ PubMed ] [ Google Scholar ]
- Mohren DC, Swaen GW, Kant IJ, van Amelsvoort LG, Borm PJ, Galama JM, 2003. Common infections and the role of burnout in a Dutch working population . J. Psychosom. Res 55 ( 3 ), 201–208. [ PubMed ] [ Google Scholar ]
- Montez JK, Hayward MD, 2014. Cumulative childhood adversity, educational attainment, and active life expectancy among US adults . Demography 51 ( 2 ), 413–435. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Moreno GL, Bruss J, Denburg NL, 2017. Increased perceived stress is related to decreased prefrontal cortex volumes among older adults . J. Clin. Exp. Neuropsychol 39 ( 4 ), 313–325. 10.1080/13803395.2016.1225006. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Moskowitz JT, Butensky E, Harmatz P, Vichinsky E, Heyman MB, Acree M, et al., 2007. Caregiving time in sickle cell disease: psychological effects in maternal caregivers . Pediatr. Blood Cancer 48 ( 1 ), 64–71. 10.1002/pbc.20792. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Moody DLB, Chang YF, Brown C, Bromberger JT, Matthews KA, 2018. Everyday discrimination and metabolic syndrome incidence in a racially/ethnically diverse sample: study of Women’s Health Across the Nation (SWAN) . Psychosom. Med 80 ( 1 ), 114–121. 10.1097/PSY.0000000000000516. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mroczek DK, Stawski RS, Turiano NA, Chan W, Almeida DM, Neupert SD, Spiro A, 2015. Emotional reactivity and mortality: longitudinal findings from the VA normative aging study . J. Gerontol. Ser. B, Psychol. Sci. Soc. Sci 70 ( 3 ), 398–406. 10.1093/geronb/gbt107. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Murayama H, Fujiwara Y, Kawachi I, 2012. Social capital and health: a review of prospective multilevel studies . J. Epidemiol 22 ( 3 ), 179–187. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Murphy MLM, Slavich GM, Chen E, Miller GE, 2015. Targeted rejection predicts decreased anti-inflammatory gene expression and increased symptom severity in youth with asthma . Psychol. Sci 26 ( 2 ), 111–121. 10.1177/0956797614556320. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Murphy MLM, Slavich GM, Rohleder N, Miller GE, 2013. Targeted rejection triggers differential pro- and anti-inflammatory gene expression in adolescents as a function of social status . Clin. Psychol. Sci 1 ( 1 ), 30–40. 10.1177/2167702612455743. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Muscatell KA, Slavich GM, Monroe SM, Gotlib IH, 2009. Stressful life events, chronic difficulties, and the symptoms of clinical depression . J. Nerv. Ment. Dis 197 ( 3 ), 154–160. 10.1097/NMD.0b013e318199f77b. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nandi A, Glymour MM, Subramanian SV, 2014. Association among socioeconomic status, health behaviors, and all-cause mortality in the United States . Epidemiology 25 ( 2 ), 170–177. 10.1097/EDE.0000000000000038. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Neubauer AB, Smyth JM, Sliwinski MJ, 2017. When you see it coming: stressor anticipation modulates stress effects on negative affect . Emotion (Washington, D.C.) 10.1037/emo0000381. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Newbury JB, Arseneault L, Moffitt TE, Caspi A, Danese A, Baldwin JR, Fisher HL, 2018. Measuring childhood maltreatment to predict early-adult psychopathology: comparison of prospective informant-reports and retrospective self-reports . J. Psychiatr. Res 96 , 57–64. 10.1016/j.jpsychires.2017.09.020. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nordenmark M, 2004. Multiple social roles and well-being: a longitudinal test of the role stress theory and the role expansion theory . Acta Sociol . 47 ( 2 ), 115–126. [ Google Scholar ]
- Norton MC, Smith KR, Østbye T, Tschanz JT, Corcoran C, Schwartz S, et al., 2010. Increased risk of dementia when spouse has dementia? The cache county study . J. Am. Geriatr. Soc . 58 ( 5 ), 895–900. 10.1111/j.1532-5415.2010.02806.x. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Obrist PA, 1981. Cardiovascular Psychophysiology: A Perspective . Plenum Press, New York. [ Google Scholar ]
- O’Donovan A, Neylan TC, Metzler T, Cohen BE, 2012a. Lifetime exposure to traumatic psychological stress is associated with elevated inflammation in the Heart and Soul Study . Brain Behav. Immun 26 ( 4 ), 642–649. 10.1016/j.bbi.2012.02.003. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- O’Donovan A, Tomiyama AJ, Lin J, Puterman E, Adler NE, Kemeny M, et al., 2012b. Stress appraisals and cellular aging: a key role for anticipatory threat in the relationship between psychological stress and telomere length . Brain Behav. Immun 26 ( 4 ), 573–579. 10.1016/j.bbi.2012.01.007. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Oken BS, Fonareva I, Wahbeh H, 2011. Stress-related cognitive dysfunction in dementia caregivers . J. Geriatr. Psychiat. Neurol 24 ( 4 ), 191–198. 10.1177/0891988711422524. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ottaviani C, Thayer JF, Verkuil B, Lonigro A, Medea B, Couyoumdjian A, Brosschot JF, 2016. Physiological concomitants of perseverative cognition: a systematic review and meta-analysis . Psychol. Bull 142 ( 3 ), 231–259. 10.1037/bul0000036. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ottaviani C, Brosschot JF, Lonigro A, Medea B, Van Diest I, Thayer JF, 2017. Hemodynamic profiles of functional and dysfunctional forms of repetitive thinking. Ann. Behav. Med. : Publ. Soc. Behav. Med 51 ( 2 ), 261–271. 10.1007/s12160-016-9851-3. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Palumbo R, Marraccini M, Weyandt L, Wilder-Smith O, McGee H, Liu S, Goodwin M, 2017. Interpersonal autonomic physiology: a systematic review of the literature . Person. Soc. Psychol. Rev . 21 , 99–141. [ PubMed ] [ Google Scholar ]
- Park M, Verhoeven JE, Cuijpers P, Reynolds Iii CF, Penninx BWJH, 2015. Where you live may make you old: the association between perceived poor neighborhood quality and leukocyte telomere length . PloS One 10 ( 6 ), e0128460. 10.1371/journal.pone.0128460. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pearlin LI, 1989. The sociological study of stress . J. Health Soc. Behav 30 ( 3 ), 241–256. 10.2307/2136956. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pechtel P, Pizzagalli DA, 2011. Effects of early life stress on cognitive and affective function: an integrated review of human literature . Psychopharmacology 214 ( 1 ), 55–70. 10.1007/s00213-010-2009-2. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Phillips AC, Robertson T, Carroll D, Der G, Shiels PG, McGlynn L, Benzeval M, 2013. Do symptoms of depression predict telomere length? Evidence from the west of Scotland twenty-07 study . Psychosom. Med . 75 ( 3 ), 288–296. 10.1097/PSY.0b013e318289e6b5. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Piazza JR, Charles ST, Sliwinski MJ, Mogle J, Almeida DM, 2013. Affective reactivity to daily stressors and long-term risk of reporting a chronic physical health condition . Ann. Behav. Med 45 ( 1 ), 110–120. 10.1007/s12160-012-9423-0. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pinquart M, Sörensen S, 2003. Differences between caregivers and noncaregivers in psychological health and physical health: a meta-analysis . Psychol. Aging 18 ( 2 ), 250–267. [ PubMed ] [ Google Scholar ]
- Puterman E, Epel E, 2012. An intricate dance: life experience, multisystem resiliency, and rate of telomere decline throughout the lifespan . Soc. Person. Psychol. Compass 6 ( 11 ), 807–825. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Puterman E, Gemmill A, Karasek D, Weir D, Adler NE, Prather AA, Epel ES, 2016. Lifespan adversity and later adulthood telomere length in the nationally representative US Health and Retirement Study . PNAS 113 ( 42 ), E6335–E6342. 10.1073/pnas.1525602113. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Puterman E, Lin J, Blackburn E, O’Donovan A, Adler N, Epel E, 2010. The power of exercise: buffering the effect of chronic stress on telomere length . PloS One 5 ( 5 ), e10837. 10.1371/journal.pone.0010837. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Puterman E, O’Donovan A, Adler NE, Tomiyama AJ, Kemeny M, Wolkowitz OM, Epel E, 2011. Physical activity moderates effects of stressor-induced rumination on cortisol reactivity . Psychosom. Med 73 ( 7 ), 604–611. 10.1097/PSY.0b013e318229e1e0. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Puterman E, Weiss J, Beauchamp MR, Mogle J, Almeida DM, 2017. Physical activity and negative affective reactivity in daily life . Health Psychol . 36 ( 12 ), 1186–1194. 10.1037/hea0000532. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rehkopf DH, Kuper H, Marmot MG, 2010. Discrepancy between objective and subjective measures of job stress and sickness absence . Scand. J. Work Environ. Health 36 ( 6 ), 449–457. [ PubMed ] [ Google Scholar ]
- Repetti RL, Taylor SE, Seeman TE, 2002. Risky families: family social environments and the mental and physical health of offspring . Psych. Bull . 128 ( 2 ), 330. [ PubMed ] [ Google Scholar ]
- Resstel LBM, Corrêa FMA, 2006. Involvement of the medial prefrontal cortex in central cardiovascular modulation in the rat . Auton. Neurosci.: Basic Clin 126 – 127 , 130–138. 10.1016/j.autneu.2006.02.022. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Reuben A, Moffitt TE, Caspi A, Belsky DW, Harrington H, Schroeder F, et al., 2016. Lest we forget: comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health . J. Child Psychol. Psychiat 57 ( 10 ), 1103–1112. 10.1111/jcpp.12621. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rich-Edwards JW, Spiegelman D, Lividoti Hibert EN, Jun H-J, Todd TJ, Kawachi I, Wright RJ, 2010. Abuse in childhood and adolescence as a predictor of type 2 diabetes in adult women . Am. J. Prev. Med 39 ( 6 ), 529–536. 10.1016/j.amepre.2010.09.007. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ridout KK, Levandowski M, Ridout SJ, Gantz L, Goonan K, Palermo D, et al., 2017. Early life adversity and telomere length: a meta-analysis . Mol. Psychiat 10.1038/mp.2017.26. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Robertson T, Beveridge G, Bromley C, 2017. Allostatic load as a predictor of all-cause and cause-specific mortality in the general population: evidence from the Scottish Health Survey . PloS One 12 ( 8 ), e0183297. 10.1371/journal.pone.0183297. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Robinette JW, Charles ST, Almeida DM, Gruenewald TL, 2016. Neighborhood features and physiological risk: an examination of allostatic load . Health Place 41 , 110–118. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Robinette JW, Charles ST, Mogle JA, Almeida DM, 2013. Neighborhood cohesion and daily well-being: results from a diary study . Soc. Sci. Med 96 , 174–182. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rosengren A, Wilhelmsen L, Orth-Gomér K, 2004. Coronary disease in relation to social support and social class in Swedish menA 15 year follow-up in the study of men born in 1933 . Eur. Heart J 25 ( 1 ), 56–63. 10.1016/j.ehj.2003.10.005. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Russek LG, Schwartz GE, 1997. Feeling of parental caring predict health status in midlife: a 35-year follow-up of the Harvard Mastery of Stress Study . J. Behav. Med 20 ( 1 ), 1–13. 10.1023/A:1025525428213. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ryder AG, Chentsova-Dutton YE, 2012. Depression in cultural context: “Chinese somatization”, revisited . Psychiatr. Clin. North Am 35 ( 1 ), 15–36. 10.1016/j.psc.2011.11.006. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Masten A, Narayan A, 2010. Child development in the context of disaster, war, and terrorism : pathways of risk and resilience 63 , 227–257. < 10.1146/annurev-psych-120710-100356 >. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sapolsky RM, 1999. Glucocorticoids, stress, and their adverse neurological effects: relevance to aging . Exp. Gerontol 34 ( 6 ), 721–732. [ PubMed ] [ Google Scholar ]
- Sapolsky RM, 2015. Stress and the brain: Individual variability and the inverted-U . Nat. Neurosci 18 ( 10 ), 1344–1346. [ PubMed ] [ Google Scholar ]
- Sapolsky RM, Romero LM, Munck AU, 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions . Endocr. Rev 21 ( 1 ), 55–89. 10.1210/edrv.21.1.0389. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Scheepers D, de Wit F, Ellemers N, Sassenberg K, 2012. Social power makes the heart work more efficiently: evidence from cardiovascular markers of challenge and threat . J. Exp. Soc. Psychol 48 ( 1 ), 371–374. [ Google Scholar ]
- Schommer NC, Hellhammer DH, Kirschbaum C, 2003. Dissociation between reactivity of the hypothalamus-pituitary-adrenal axis and the sympathetic-adrenalmedullary system to repeated psychosocial stress . Psychosom. Med 65 ( 3 ), 450–460. [ PubMed ] [ Google Scholar ]
- Schulz R, Beach SR, 1999. Caregiving as a risk factor for mortality: the caregiver health effects study . JAMA 282 ( 23 ), 2215–2219. 10.1001/jama.282.23.2215. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Seeman M, Stein Merkin S, Karlamangla A, Koretz B, Seeman T, 2014. Social status and biological dysregulation: the “status syndrome” and allostatic load . Soc. Sci. Med 1982 ( 118 ), 143–151. 10.1016/j.socscimed.2014.08.002. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Seeman TE, McEwen BS, Rowe JW, Singer BH, 2001. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging . PNAS 98 ( 8 ), 4770–4775. 10.1073/pnas.081072698. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Seery MD, Blascovich J, Weisbuch M, Vick SB, 2004. The relationship between self-esteem level, self-esteem stability, and cardiovascular reactions to performance feedback . J. Person. Soc. Psychol 87 ( 1 ), 133. [ PubMed ] [ Google Scholar ]
- Seery MD, Holman EA, Silver RC, 2010. Whatever does not kill us: cumulative lifetime adversity, vulnerability, and resilience . J. Person. Soc. Psychol 99 ( 6 ), 1025. [ PubMed ] [ Google Scholar ]
- Segerstrom SC, Miller GE, 2004. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry . Psychol. Bull 130 ( 4 ), 601–630. 10.1037/0033-2909.130.4.601. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Seo D, Tsou KA, Ansell EB, Potenza MN, Sinha R, 2014. Cumulative adversity sensitizes neural response to acute stress: association with health symptoms . Neuropsychopharmacology 39 ( 3 ), 670–680. 10.1038/npp.2013.250. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shankar A, McMunn A, Demakakos P, Hamer M, Steptoe A, 2017. Social isolation and loneliness: prospective associations with functional status in older adults . Health Psychol . 36 ( 2 ), 179–187. 10.1037/hea0000437. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sheets ES, Craighead WE, 2014. Comparing chronic interpersonal and non-interpersonal stress domains as predictors of depression recurrence in emerging adults . Behav. Res. Ther 63 , 36–42. 10.1016/j.brat.2014.09.001. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shields GS, Doty D, Shields RH, Gower G, Slavich GM, Yonelinas AP, 2017. Recent life stress exposure is associated with poorer long-term memory, working memory, and self-reported memory . Stress 20 ( 6 ), 598–607. 10.1080/10253890.2017.1380620. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Silver RC, Updegraff JA, 2013. Searching for and finding meaning following personal and collective traumas. In: Markman KD, Proulx T, Lindberg MJ (Eds.), The Psychology of Meaning . American Psychological Association, Washington, D.C.. [ Google Scholar ]
- Sin NL, Graham-Engeland JE, Ong AD, Almeida DM, 2015. Affective reactivity to daily stressors is associated with elevated inflammation . Health Psychol . 34 ( 12 ), 1154–1165. 10.1037/hea0000240. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Slavich GM, O’Donovan A, Epel ES, Kemeny ME, 2010a. Black sheep get the blues: a psychobiological model of social rejection and depression . Neurosci. Biobehav. Rev 35 ( 1 ), 39–45. 10.1016/j.neubiorev.2010.01.003. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Slavich GM, Shields GS, 2018. Assessing lifetime stress exposure using the Stress and Adversity Inventory for Adults (Adult STRAIN): an overview and initial validation . Psychosom. Med 80 , 17–27. 10.1097/PSY.0000000000000534. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Slavich GM, Tartter MA, Brennan PA, Hammen C, 2014. Endogenous opioid system influences depressive reactions to socially painful targeted rejection life events . Psychoneuroendocrinology 49 , 141–149. 10.1016/j.psyneuen.2014.07.009. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Slavich GM, Thornton T, Torres LD, Monroe SM, Gotlib IH, 2009. Targeted rejection predicts hastened onset of major depression . J. Soc. Clin. Psychol 28 ( 2 ), 223–243. 10.1521/jscp.2009.28.2.223. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Slavich GM, Way BM, Eisenberger NI, Taylor SE, 2010b. Neural sensitivity to social rejection is associated with inflammatory responses to social stress . PNAS 107 , 14817–14822. 10.1073/pnas.1009164107. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sliwinski MJ, Almeida DM, Smyth J, Stawski RS, 2009. Intraindividual change and variability in daily stress processes: findings from two measurement-burst diary studies . Psychol. Aging 24 ( 4 ), 828–840. 10.1037/a0017925. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Slopen N, Lewis TT, Gruenewald TL, Mujahid MS, Ryff CD, Albert MA, Williams DR, 2010. Early life adversity and inflammation in African Americans and whites in the midlife in the United States survey . Psychosom. Med 72 ( 7 ), 694–701. 10.1097/PSY.0b013e3181e9c16f. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Smith SG, Jackson SE, Kobayashi LC, Steptoe A, 2018. Social isolation, health literacy, and mortality risk: findings from the English Longitudinal Study of Ageing . Health Psychol 37 , 160–169. 10.1037/hea0000541. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Southwick SM, Vythilingam M, Charney DS, 2005. The psychobiology of depression and resilience to stress: implications for prevention and treatment . Annu. Rev. Clin. Psychol 1 , 255–291. 10.1146/annurev.clinpsy.1.102803.143948. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Southwick SM, Sippel L, Krystal J, Charney D, Mayes L, Pietrzak R, 2016. Why are some individuals more resilient than others: the role of social support . World Psychiat . 15 ( 1 ), 77–79. 10.1002/wps.20282. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stalder T, Steudte-Schmiedgen S, Alexander N, Klucken T, Vater A, Wichmann S, et al., 2017. Stress-related and basic determinants of hair cortisol in humans: a metaanalysis . Psychoneuroendocrinology 77 , 261–274. 10.1016/j.psyneuen.2016.12.017. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Staufenbiel SM, Penninx BWJH, Spijker AT, Elzinga BM, van Rossum EFC, 2013. Hair cortisol, stress exposure, and mental health in humans: a systematic review . Psychoneuroendocrinology 38 ( 8 ), 1220–1235. 10.1016/j.psyneuen.2012.11.015. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stephens MA, Wand G, 2012. Stress and the HPA axis: role of glucocorticoids in alcohol dependence . Alcohol Res . 34 , 468–483. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stepnowsky CJ, Nelesen RA, DeJardin D, Dimsdale JE, 2004. Socioeconomic status is associated with nocturnal blood pressure dipping . Psychosom. Med 66 ( 5 ), 651–655. 10.1097/01.psy.0000138124.58216.6c. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stults-Kolehmainen MA, Sinha R, 2014. The effects of stress on physical activity and exercise . Sports Med 44 ( 1 ), 81–121. 10.1007/s40279-013-0090-5. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Surtees P, Wainwright N, Day N, Brayne C, Luben R, Khaw K-T, 2003. Adverse experience in childhood as a developmental risk factor for altered immune status in adulthood . Int. J. Behav. Med 10 ( 3 ), 251–268. [ PubMed ] [ Google Scholar ]
- Szanton SL, Allen JK, Thorpe RJ, Seeman T, Bandeen-Roche K, Fried LP, 2008. Effect of financial strain on mortality in community-dwelling older women . J. Gerontol. Ser. B, Psychol. Sci. Soc. Sci 63 ( 6 ), S369–374. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Taylor SE, Lehman BJ, Kiefe CI, Seeman TE, 2006. Relationship of early life stress and psychological functioning to adult c-reactive protein in the Coronary Artery Risk Development in Young Adults Study . Biol. Psychiat 60 ( 8 ), 819–824. 10.1016/j.biopsych.2006.03.016. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Teicher MH, Anderson CM, Polcari A, 2012. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum . PNAS 109 ( 9 ), E563–572. 10.1073/pnas.1115396109. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Thorson K, West T, Mendes WB, 2017. Measuring physiological influence in dyads: a guide to designing, implementing, and analyzing dyadic physiological studies . Psych. Methods 10.1037/met0000166. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tiwari A, Gonzalez A, 2018. Biological alterations affecting risk of adult psychopathology following childhood trauma: a review of sex differences . Clin. Psychol. Rev 10.1016/j.cpr.2018.01.006. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, et al., 2010. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation . Dev. Sci 13 ( 1 ), 46–61. 10.1111/j.1467-7687.2009.00852.x. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Toussaint L, Shields GS, Dorn G, Slavich GM, 2016. Effects of lifetime stress exposure on mental and physical health in young adulthood: how stress degrades and forgiveness protects health . J. Health Psychol 21 ( 6 ), 1004–1014. 10.1177/1359105314544132. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T, 2003. Cardiovascular reactivity and development of preclinical and clinical disease states . Psychosom. Med 65 ( 1 ), 46–62. [ PubMed ] [ Google Scholar ]
- Tryon MS, Stanhope KL, Epel ES, Mason AE, Brown R, Medici V, et al., 2015. Excessive sugar consumption may be a difficult habit to break: a view from the brain and body . J. Clin. Endocrinol. Metab 100 ( 6 ), 2239–2247. 10.1210/jc.2014-4353. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Turner RJ, Lloyd DA, 1995. Lifetime traumas and mental health: the significance of cumulative adversity . J. Health Soc. Behav 36 ( 4 ), 360–376. [ PubMed ] [ Google Scholar ]
- Ulmer CS, Calhoun PS, Bosworth HB, Dennis MF, Beckham JC, 2013. Nocturnal blood pressure non-dipping, posttraumatic stress disorder, and sleep quality in women . Behav. Med. (Washington, D.C.) 39 ( 4 ), 111–121. 10.1080/08964289.2013.813434. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Umberson D, Liu H, Reczek C, 2008. Stress and health behaviors. In: Turner H, Schiemann S (Eds.), Advances in Life Course Research: Stress Processes Across the Life Course . Elsevier, Oxford, England, pp. 19–44. [ Google Scholar ]
- Van den Bergh BRH, van den Heuvel MI, Lahti M, Braeken M, de Rooij SR, Entringer S, et al., 2017. Prenatal developmental origins of behavior and mental health: the influence of maternal stress in pregnancy . Neurosci. Biobehav. Rev 10.1016/j.neubiorev.2017.07.003. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Verkuil B, Brosschot JF, de Beurs DP, Thayer JF, 2009. Effects of explicit and implicit perseverative cognition on cardiac recovery after cognitive stress . Int. J. Psychophysiol.: Off. J. Int. Org. Psychophysiol . 74 ( 3 ), 220–228. 10.1016/j.ijpsycho.2009.09.003. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vitaliano PP, Zhang J, Scanlan JM, 2003. Is caregiving hazardous to one’s physical health? A meta-analysis . Psychol. Bull 129 ( 6 ), 946–972. 10.1037/0033-2909.129.6.946. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wang J, Korczykowski M, Rao H, Fan Y, Pluta J, Gur RC, et al., 2007. Gender difference in neural response to psychological stress . Soc. Cogn. Affect. Neurosci 2 ( 3 ), 227–239. 10.1093/scan/nsm018. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wardle MC, Munafo MR, de Wit H, 2011. Effect of social stress during acute nicotine abstinence . Psychopharmacology 218 , 39–48. 10.1007/s00213-010-2150-y. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Waters SF, West TV, Karnilowicz HR, Mendes WB, 2017. Affect contagion between mothers and infants: examining valence and touch . J. Exp. Psychol. Gen 146 ( 7 ), 1043–1051. 10.1037/xge0000322. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Waters SF, West TV, Mendes WB, 2014. Stress contagion: physiological covariation between mothers and infants . Psychol. Sci 25 ( 4 ), 934–942. 10.1177/0956797613518352. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wegman HL, Stetler C, 2009. A meta-analytic review of the effects of childhood abuse on medical outcomes in adulthood . Psychosom. Med 71 ( 8 ), 805–812. 10.1097/PSY.0b013e3181bb2b46. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wetherell MA, Lovell B, Smith MA, 2015. The effects of an anticipated challenge on diurnal cortisol secretion . Stress (Amsterdam, Netherlands) 18 ( 1 ), 42–48. 10.3109/10253890.2014.993967. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al., 2013. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010 . Lancet 382 ( 9904 ), 1575–1586. 10.1016/S0140-6736(13)61611-6. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Woody EZ, Szechtman H, 2011. Adaptation to potential threat: the evolution, neurobiology, and psychopathology of the security motivation system . Neurosci. Biobehav. Rev 35 ( 4 ), 1019–1033. 10.1016/j.neubiorev.2010.08.003. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Worthman C, 2010. The ecology of human development: evolving models for cultural psychology . J. Cross Cult. Psychol 41 , 546–562. [ Google Scholar ]
- Wright RA, Kirby LD, 2001. Effort determination of cardiovascular response: an integrative analysis with applications in social psychology 33 , 255–307. [ Google Scholar ]
- Zhang X, Zhao Q, Zhu W, Liu T, Xie S-H, Zhong L-X, et al., 2017. The association of telomere length in peripheral blood cells with cancer risk: a systematic review and meta-analysis of prospective studies . Cancer Epidemiol. Biomark. Prev 26 ( 9 ), 1381–1390. 10.1158/1055-9965.EPI-16-0968. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhou Y, Han W, Gong D, Man C, Fan Y, 2016. Hs-CRP in stroke: a meta-analysis . Clin. Chim. Acta; Int. J. Clin. Chem 453 , 21–27. 10.1016/j.cca.2015.11.027. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zoccola PM, Dickerson SS, 2015. Extending the recovery window: effects of trait rumination on subsequent evening cortisol following a laboratory performance stressor . Psychoneuroendocrinology 58 , 67–78. 10.1016/j.psyneuen.2015.04.014. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Open access
- Published: 04 August 2021
Perceived stress among university students in Oman during COVID-19-induced e-learning
- Mustafa Malik ORCID: orcid.org/0000-0002-0450-6629 1 &
- Sarfaraz Javed 2
Middle East Current Psychiatry volume 28 , Article number: 49 ( 2021 ) Cite this article
55k Accesses
28 Citations
Metrics details
Higher education institutions across the world moved to e-learning in response to the disruptions caused by the pandemic. While e-learning has an advantage for students that they can attend to their courses from anywhere at their convenience, yet the sudden disruptive shift to e-learning during the pandemic saw students facing many challenges, which had strong ability to induce mental health issues among the students. This study aimed at examining the impact of COVID-19-induced e-learning on the university students’ stress perceptions in Oman.
A total of 966 usable responses were received. The results showed that 96.9% (moderate stress = 82.5% and high stress = 14.4%) reported having experienced stress through e-learning during COVID-19. The results further indicated that there was a significant negative correlation between perceived stress and the students’ academic performance. The family support and institutional support were observed to have a significant effect on students’ stress perception.
Conclusions
Students are away from the physical campuses over a year now, continuing their courses and programs online. The numerous challenges they are facing through e-learning, together with the prevailing uncertainty, have intensified stress among them. The continued stress over time will not only affect their academic performance, but their mental and physical health as well, as the stress has proven to be one of the major causes of various physical and mental disorders. While e-learning seems to emerge as a new normal, the students require proper attention, help, and support from their families and institutions. The institutions should revisit their online courses and program delivery mechanisms, methods, and practices to ensure that students are not over stressed.
The spread of novel coronavirus (COVID-19) disrupted all economic and social activities around the world. Higher education has been one of the worst hit sectors. Being intensively interactive in nature, this sector saw a comparatively early and complete shutdown across the globe. In Oman, the government announced the closure of all educational institutions by mid of March 2020. Responding to the pandemic and the subsequent advisories by regulating bodies to suspend on-campus academic activities, higher education institutions (HEIs) switched to online mode to deliver academic programs. Like elsewhere, most of the HEIs in Oman had no prior experience of delivering programs completely online and therefore, applied varied approaches depending on their size, governance models, and disciplinary differences [ 1 ]. Over more than a year in pandemic now, HEIs in Oman are continuing their programs online. With many improvements and innovations in online teaching and learning process over the past 1 year, students have started adapting to this new normal.
However, weakness of online teaching infrastructure, inexperience of teachers regarding new technologies, information gap, and complex home environment still exist [ 2 ]. Students, through COVID-19-induced online education are facing numerous challenges, such as instructional [ 3 ] technological, and technical [ 3 , 4 , 5 ]; social and family atmosphere [ 5 ]; home confinement [ 6 , 7 ]; emotional and psychological [ 3 , 8 ]. Furthermore, many students do not find a suitable space in their homes for effective learning and do not have access to sufficient hardware or internet which constrains their home learning [ 9 ].
Though online education for HEIs is not anything new, as many HEIs have been offering full courses and/or full programs online, yet students enrolled in full online instruction-based programs, who were not familiar with such experiences faced a system shock [ 10 ].
All these challenges faced by the students in online education during COVID-19 have a strong ability to induce mental health issues among the students. One commonly experienced mental health issue by university students is the academic stress, which is mostly due to the students’ apprehension of loss of grades and fear of failure [ 11 ]. Fear of lower performance and delay in completion of studies are also the reasons to induce stress among students during COVID-19. Many studies conducted during last 1 year focusing on university students’ mental health during COVID-19, such as [ 12 , 13 , 14 , 15 , 16 ] concluded that COVID-19 had a negative impact on the mental health and wellbeing of the university students. Besides earlier studies such as [ 17 , 18 , 19 ] have also shown that uncertainties due to public health emergencies, such as H1N1 influenza, Ebola, SARS, and MERS, caused negative psychological effects on university and college students. Furthermore, the negative psychological impacts are aggravated if the public emergencies accompany with home confinement [ 6 , 7 ], which is what happened across the globe, during COVID-19.
In the context of Oman, there are very few studies conducted regarding the impact of COVID-19 on the mental health of people and these are mostly related to the youth in general [ 20 ], general population [ 21 ], and health workers [ 21 ]. Regarding the mental health of university students during COVID-19 in the context of Oman, we did not find any study except for one by Alqassabi et al. [ 22 ], published in Arabic language. The available literature indicates that there is little being published on impact of COVID-19-induced e-learning on the stress perceptions of university students in Oman. While there is considerable literature on this subject available in the Western and Eastern contexts, these cannot necessarily be generalized to Arab world considering that its culture, social structure, and social norms vary greatly from the Western and Eastern cultures.
Thus, the present study aimed at examining the impact of COVID-19-induced e-learning on the university students’ stress perceptions in Oman, thereby filling the gap in the literature that exists in the Oman context on the topic under study. The results of this study provide insights to the higher education educators, administrators, and policy makers to enhance e-learning implementation in a way that ensures mental and psychological wellbeing of the students.
This study was undertaken at the University of Nizwa, Oman. It is the second largest and the only non-profit private university in the country. The university comprises of four colleges namely College of Arts and Sciences (CAS), College Economics, Management and Information Systems (CEMIS), College of Pharmacy and Nursing (CPN), and the College of Engineering and Architecture (CEA). A total of 5269 students were registered across all undergraduate programs in the university for Fall 2020 [CAS = 2556 (48.5%); CEMIS = 1569 (29.8%); CPN = 552 (10.5%); and CEA = 592 (11.2%)] ( data received from Center for Information Systems of the University by email on the request of the corresponding author ). A self-administered questionnaire was sent online to all the registered students. A total of 966 [CAS = 573 (53.5%); CEMIS = 273 (28.3%); CPN = 74 (7.7%); and CEA = 102 (10.6%)] completed responses were received. The figures show that the percentage of respondents from each college is fairly proportionate to the percentage of total students in each college, thus the sample is fairly representative of each college of the university under study.
Perceived Stress Scale (PSS-10)
To assess the stress as perceived by the students, the Cohen’s Perceived Stress Scale (PSS-10 [ 23 ];) was used. It is a self-reported questionnaire that measures the degree to which an individual appraises situation in his or her life as stressful. The scale consists of 10 items including 6 positively phrased and 4 negatively phrased items. Each item is rated by respondents on a 5-point scale ranging from ‘ never (0)’ to ‘ very often (4)’. The scores for negatively phrased items are reversed and then the scores for all 10 items are added for each individual. The individual scores on PSS can range from 0 to 40 with higher scores indicating higher perceived stress (‘ low stress = 0–13’, ‘ moderate stress = 14–26’, and ‘ high stress = 27–40’). The scale has been used in numerous studies and has shown good reliability. Lee [ 24 ] in his review paper on the use of PSS-10 reported that Cronbach’s alpha was evaluated at > 0.70 in all 12 studies his research referred to. For the present study the Cronbach’s alpha for PSS-10 scale was 0.76.
Potential factors leading to stress among the university students were derived from the literature [ 25 , 26 , 27 , 28 ] and after informal discussion with the students. The students’ inputs were very beneficial as it helped the authors to identify factors that were more relevant to online education and in the present pandemic situation. A total of 14 potential source of stress were identified. These were grouped into three categories as academic stressors (7 items), psychological stressors (4 items), and social stressors (3 items). For each potential stressor, the respondents were asked to show their agreement or disagreement on a 5-point Likert scale ranged between ‘ highly agree as 5’ and ‘ highly disagree as 1’. The 14 stressors used in this study showed an overall Cronbach’s alpha of 0.78.
The survey for the present research was conducted online using Google forms, with the undergraduate students registered for Fall Semester 2020 at the University of Nizwa in Oman. A bilingual (Arabic and English) questionnaire was sent to the students through their university email and was also posted on the University’s Moodle home page with the help of Center for Information Systems of the University to ensure wider participation. The survey was open between 6 December 2020 and 31 December 2020 which corresponded to week 13 to week 16 of the academic semester (last 4 weeks before the final examination). During this period, three email reminders were sent to all the students for completing the survey. In the introductory part of the questionnaire, the purpose of this research was briefly explained. Students were informed that the participation in the survey was completely voluntary and were asked to express their consent before proceeding to respond to the questionnaire. To conduct this study, we received an ethical approval from the Human Ethics Committee in the Office of the Vice Chancellor for Graduate Studies, Research and International Relations, University of Nizwa through its letter (EC Ref. No.: HREC-12-2020) on 17 November 2020.
Statistical analysis
The statistical analysis was performed using the Statistical Package for Social Science (SPSS-20). Apart from descriptive statistics, means, standard deviations, and Pearson correlation was used to analyze and interpret the data and write the results.
Descriptive statistics
As presented in Table 1 , a total of 966 student-participants responded to the survey. By gender, the respondents comprised of 84.4% females ( n = 815) and 15.6% males ( n = 151). The female:male ratio of respondents was proportionate to the female:male ratio of the total student population ( n = 5269) registered for Fall 2020 in the university (females = 4603, 87.3%) and males = 666, 12.6%).
Perceived stress among respondents
As presented in Table 2 , the results of this study showed that the mental health of students is negatively affected during the pandemic. Of 966 students surveyed, 936 (96.9%) reported having experienced stress (moderate stress = 82.5% and high stress = 14.4%). There were only 30 (3.1%) students who reported having experienced low stress.
When comparing mean stress scores between groups, the stress score varied between 21.0 and 22.6 among all categories of respondents, thereby showing that on an average all the respondents experienced a high-moderate stress. Similarly, female students ( M = 22.2, SD = 4.2) showed higher symptoms of stress compared to male students ( M = 21.0, SD = 4.3), and the mean stress score observed for married students ( M = 22.6, SD = 4.3) was higher as compared to unmarried students ( M = 21.3, SD = 4.2). Similarly, students from urban areas were comparatively more stressed than those living in rural areas, students living in joint families were comparatively more stressed than those living in nuclear families, students enrolled in bachelor’s degree programs were comparatively more stressed than their counterparts in diploma degree programs. While comparing mean stress score of students based on the year of study, the students studying in Year 2 and Year 4 showed comparatively higher stress than those studying in Year 1 and Year 3. Based on the CGPA of the students, the students falling at the middle of the CGPA continuum (2.50–3.50) showed comparatively higher stress than those with lower (≤ 2.0–2.5) and higher (3.5–4.0) CGPA. When comparing the students’ mean stress scores based on the college they belonged to, the engineering students showed a comparatively higher stress (CEA, M = 22.5, SD = 4.5) than students of other three colleges (CEMIS, M = 21.9, SD = 3.6; CAS, M = 2.19, SD = 4.5 and CPN, M = 21.9, SD = 3.6).
Perceived institutional and family support
Organizational (institutional) support [ 29 ] and social (including family) support [ 30 ] is generally perceived to have positive influence on peoples’ health and wellbeing. In academic settings, the institutional support may refer to the academic and non-academic support and services provided by the institutions to its students. In the context of e-learning during COVID-19, it may refer to the online academic support available to the students from their mentors, advisors, and teachers as well as from the institution’s academic support services such as online learning support, technical and technological support, communication with students, and response to students’ academic concerns. On the other hand, family support during COVID-19 may refer to the support provided by a student’s family in terms of time and space and family responsibilities etc. The respondents (students) were asked to evaluate the institutional and family support during COVID-19-induced e-learning as poor , moderate , or good . The results of this study, as presented in Table 3 , showed that 95.2% (good = 66.9% and moderate = 28.3%) students perceived family support as favorable compared to 72.5% (good = 21.7 and moderate = 50.8%) perceiving institutional support as favorable. A considerable percentage of students (27.4%) perceived the institutional support as poor.
Self-reported academic performance
One of the major factors leading to stress among the students and widely reported in the literature is the fear of losing grades and poor academic performance. In this study, the students when asked to compare their academic performance with that of pre-COVID-19 period, 39% reported poor academic performance compared to 27.6% reporting their academic performance has improved, and the rest 33.4% said that they have not observed any change in academic performance (Table 3 ).
Stress factors
Various factors contributed to the stress experienced by students in online education during the pandemic. These factors were categorized as (a) academic factors, (b) psychological factors, and (c) social factors. Table 4 presents ranking of perceived stress factors based on their mean. Among the academic factors, while all factors have been perceived as contributing to the students’ stress with the mean varying between 3.63 (lowest) and 4.12 (highest), increased number of exams during online distance learning was perceived as the strongest factor leading to stress among students (mean = 4.12, SD = 0.98) and sufficiency of learning materials provided by instructors was ranked at lowest (mean = 3.89, SD = 1.01). Among the psychological factors, again all the factors were perceived as contributing to the stress among the students with the mean score varying between 4.33 (highest) and 3.50 (lowest). Constant fear of losing grades (mean = 4.33, SD = 0.93), and worry about performance (mean = 4.27, SD = 0.96) were ranked as top two psychological stress factors. Among the social factors, concern for family sufferings, my family is suffering due to my over engagement in academic work (mean = 3.91, SD = 1.06) was ranked at highest.
Correlation among variables
This study showed that the academic performance was negatively correlated with the overall perceived stress ( r = − .349, p < 0.01), negatively correlated with perceived academic stressors ( r = − .511, p < 0.01), negatively correlated with perceived psychological stressors ( r = − .383, p < 0.01), and also negative correlated with perceived social stressors ( r = − .100, p < 0.01) (Table 5 ). Furthermore, when comparing the correlation of academic performance with the perceived stressors, it was observed that perceived academic stressors had a significantly higher negative correlation with academic performance ( r = − .511, p < 0.01) compared to perceived psychological stressors ( r = − .383, p < 0.01) and perceived social stressors ( r = − .100, p < 0.01).
The results of this study showed that COVID-19-induced online learning had a negative impact on the mental health of university students in terms of perceived stress, which was reported as moderate to high by 96.9% of the respondents. These results are consistent with most of the studies conducted during last 1 year such as 12], [ 13 , 14 , 15 , 16 ]. Higher stress among university students has been, most often, related to lower academic performance [ 31 , 32 , 33 ]. Through this study, it was observed that the students’ perceived academic performance had a negative relationship with their overall perceived stress on PSS10 ( r = − .349, p < 0.01).
Furthermore, the results showed that perceived family support has a negative relationship with perceived stress ( r = − .203, p < 0.01) and positive relationship with the academic performance ( r = .262, p < 0.01). Deihl et al. [ 34 ] observed that people who perceived lower support from family and friends were emotionally less positive and hence more stressed. Moreover, Solberg [ 35 ] opined that social support moderates the relationship between stress and distress, which means that people who perceive higher social (family) support report lower distress. It is important to mention here that Cohen et al. [[ 30 ], p. 4] defined social support as ‘a process through which the social relationships promote health and wellbeing’.
While many studies have studied impact of social support on students’ stress, the impact of institutional support on students’ perceived stress has received little focus, especially in the studies those have been conducted during the pandemic. Since during the pandemic there was an abrupt shift from the physical classroom to online platforms, the institutional support to the students, in terms of advising systems, instructions, program progress tracking, career planning, and technology support, had a considerable impact on how comfortable or not they were with the online learning. This study showed that the students’ perception of institutional support has significant negative relation with students’ perception of stress ( r = − .296, p < 0.01). This demonstrates that higher education institutions’ student support systems have a potential to mediate the stress, especially the academic stress, which in this study is significantly negatively correlated to perceived institutional support ( r = − .401, p < 0.01) and academic performance ( r = − .511, p < 0.01). Pascoe et al. [ 36 ] argue that enhancing student support in the education setting may improve the mental health of young people.
When comparing mean stress scores between groups, the stress score varied between 21.0 and 22.6 among all categories of respondents, thereby showing that on an average all the respondents experienced a high-moderate stress. These results are similar to other studies conducted during the pandemic such as [ 3 , 5 , 6 , 7 , 8 , 9 ].
When comparing the mean stress scores (see Table 1 ), female students ( M = 22.2, SD = 4.2) showed higher symptoms of stress compared to male students ( M = 21.0, SD = 4.3). The mean stress score observed for married students ( M = 22.6, SD = 4.3) was higher as compared to unmarried students ( M = 21.3, SD = 4.2). Similarly, students from urban areas were comparatively more stressed than those living in rural areas, students living in joint families were comparatively more stressed than those living in nuclear families, and students enrolled in bachelor’s degree programs were comparatively more stressed than their counterparts in diploma degree programs. While comparing mean stress score of students based on the year of study, the students studying in Year 2 and Year 4 showed comparatively higher stress than those studying in Year 1 and Year 3. Based on the CGPA of the students, the students falling of the middle of the CGPA continuum (2.50–3.50) showed comparatively higher stress than those with lower (≤ 2.0–2.5) and higher (3.5–4.0) CGPA. When comparing the students’ mean stress scores based on the college they belonged to, the engineering students showed a comparatively higher stress (CEA, M = 22.5, SD = 4.5) than students of other three colleges (CEMIS, M = 21.9, SD = 3.6; CAS, M = 2.19, SD = 4.5 and CPN, M = 21.9, SD = 3.6).
The spread of COVID-19 affected every aspect of human life. Its impact has been unprecedented. The closure of universities for on-campus face-to-face teaching and learning forced higher education institution across the globe to deliver programs online. Initially thought to be a temporary arrangement to deal with the crises, online education is emerging as a new normal. In Oman, as of now, higher education institutions are closed for on-campus teaching and learning for over a year, and courses and programs are delivered either completely online or through blended learning mode. The recent resurge in COVID-19-infected cases in the country has once again triggered a sense of uncertainty on the opening of universities for on-campus activities. Sahu [ 14 ] and Tayefi [ 37 ] observed that consistent closure of universities for on-campus classes triggered a sense of uncertainty among the students about their academic and professional career and intensified among them persistent mental health challenges. Given this context, the present study was conducted to investigate the impact of the COVID-19-induced online learning on university students’ psychological health in Oman. The study further investigated the relationship between the students’ perceived level of stress and their perceived family support and institutional support, as well as their academic performance.
The study showed the during the online teaching and learning induced by the pandemic, students in general have experienced a moderate to higher stress. The students’ mental health is significantly affected by the support they perceive from their family and the institution, as these were observed to be negatively correlated to the perceived stress by students. Although, academic, social and psychological stressors were observed to have negative impact on students’ mental health (perceived stress), yet academic stressors were observed to have comparatively higher negative correlation with students’ perceived stress as compared to psychological and social stressors, during COVID-19-induced online learning.
Various studies have shown that mental health of populations is significantly affected when faced with public health emergencies, and university students are no exception to this fact. The COVID-19 pandemic has been more catastrophic than any other public health emergency witnessed in the recent past. University students have been out of the physical campuses over a year now, and courses and programs are, more or less, completely delivered online, and there seems to be no end to it, at least not for the next six months to 1 year.
If the students are perceiving continued stress over time, it will not only affect their mental health but physical health as well, as the stress has proven to be one of the major causes of various physical and mental disorders such as hypertension, depression, diabetes, asthma, obesity, and cardiovascular diseases [ 38 , 39 , 40 , 41 ]. Hence, the students require proper attention, help, and support from their families and institutions [ 12 ]. The institutions should revisit their online courses and program delivery mechanisms, methods, and practices to ensure that students are not over stressed, particularly in terms of number of assessments, academic workload, and technical difficulties they face.
Limitations
This study focuses on stress perceived by university students in Oman during COVID-19-induced online learning. Although the results of this study provide significant insights on the students’ perceived stress during online learning, as not many studies are available on the subject in the context of Oman, yet the findings of this study cannot be generalized for the entire country as the study was conducted on the students in a specific university. Further studies could be conducted involving a wider cross-sectional sample across higher education institutions in Oman to generalize the findings.
Availability of data and materials
All the data and materials relevant to this study are available with the corresponding author and can be made available on a reasonable request.
Abbreviations
Coronavirus disease 2019
Higher education institutions
Middle East respiratory syndrome
Perceived stress scale-10
Severe acute respiratory syndrome
Statistical package for social science
International Association of Universities, IAU (2020) Regional/National Perspectives on the Impact of COVID-19 on Higher Education. https://www.iau-aiu.net/IMG/pdf/iau_covid-19_regional_perspectives_on_the_impact_of_covid-19_on_he_july_2020_.pdf . Accessed 08 Feb 2021
Ali W (2020) Online and remote learning in higher education institutes: a necessity in light of Covid-19 pandemic. High Educ 10(3):16–25
Google Scholar
Martin, A. (2020) How to optimize online learning in the age of coronavirus (COVID-19): A 5-point guide for educators. https://www.researchgate.net/publication/339944395_How_to_Optimize_Online_Learning_in_the_Age_of_Coronavirus_COVID-19_A_5-Point_Guide_for_Educators/link/5e71637ca6fdcc37caf31289/download . Accessed 8 Feb 2021
Almaiah MA, Al-Khasawneh A, Althunibat A (2020) Exploring the critical challenges and factors influencing the e-learning system usage during COVID-19 pandemic. Educ Inf Technol 25(6):5261–5280. https://doi.org/10.1007/s10639-020-10219-y
Article Google Scholar
Zhong, R. (2020) The coronavirus exposes education’s digital divide. The New York Times https://www.nytimes.com/2020/03/17/technology/china-schools-coronavirus.html . Accessed 17 Jan 2020
Wang C, Pan R, Wan X, Tan Y, Xu L, McIntyre RS et al (2020a) A longitudinal study on the mental health of general population during the COVID-19 epidemic in China. Brain Behav Immunity 87:40–48. https://doi.org/10.1016/j.bbi.2020.04.028
Article CAS Google Scholar
Wang C, Pan R, Wan X, Tan Y, Xu L, Ho CS (2020b) Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. Int J Environ Res Public Health 17:1729
Daniel SJ (2020) Education and the COVID-19 pandemic. Prospects 49(1-2):91–96. https://doi.org/10.1007/s11125-020-09464-3
Zhang W, Wang Y, Yang L, Wang C (2020) Suspending classes without stopping learning: China’s education emergency management policy in the Covid-19 outbreak. J Risk Financ Manage 13(3):55
Ngampornchai A, Adams J (2016) Students’ acceptance and readiness for E-learning in Northeastern Thailand. Int J Educ Technol in Higher Education 13(1):1–13
Goff AM (2011) Stressors, academic performance, and learned resourcefulness in baccalaureate nursing students. Int J Nurs Educ Scholarsh 8(1):1–20. https://doi.org/10.2202/1548-923X.2114
Cao W, Fang Z, Hou G, Han M, Xu X, Dong J, Zheng J (2020) The psychological impact of the COVID-19 epidemic on college students in China. Psychiatry Res 287:112934. https://doi.org/10.1016/j.psychres.2020.112934
Article CAS PubMed PubMed Central Google Scholar
Marelli S, Castelnuovo A, Somma A, Castronovo V, Mombelli S, Bottoni D, Ferini-Strambi L (2020) Impact of COVID-19 lockdown on sleep quality in university students and administration staff. J Neurol 268:1–8
Sahu P (2020) Closure of universities due to Coronavirus Disease 2019 (COVID-19): impact on education and mental health of students and academic staff. Cureus 12(4):1–6
Son C, Hegde S, Smith A, Wang X, Sasangohar F (2020) Effects of COVID-19 on college students’ mental health in the United States: interview survey study. J Med Internet Res 22(9):e21279. https://doi.org/10.2196/21279
Article PubMed PubMed Central Google Scholar
Zhai Y, Du X (2020) Mental health care for international Chinese students affected by the COVID-19 outbreak. Lancet Psychiatry 7(4):e22. https://doi.org/10.1016/S2215-0366(20)30089-4
Sprang G, Silman M (2013) Posttraumatic stress disorder in parents and youth after health related disasters. Disaster Med Public health Prep 7(1):105–110. https://doi.org/10.1017/dmp.2013.22
Article PubMed Google Scholar
Wu P, Fang Y, Guan Z, Fan B, Kong J, Yao Z, Liu X, Fuller CJ, Susser E, Lu J, Hoven CW (2009) The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry 54(5):302–311. https://doi.org/10.1177/070674370905400504
Bai Y, Lin CC, Lin CY, Chen JY, Chue CM, Chou P (2004) Survey of stress reactions among health care workers involved with the SARS outbreak. Psychiatry Serv. 55(9):1055–1057. https://doi.org/10.1176/appi.ps.55.9.1055
Al Omari O, Al Sabei S, Al Rawajfah O, Abu Sharour L, Aljohani K, Alomari K, Alhalaiqa F (2020) Prevalence and predictors of depression, anxiety, and stress among youth at the time of COVID-19: An online cross-sectional multicountry study. Depress Res Treat 2020:1–9. https://doi.org/10.1155/2020/8887727
Sinawi HA, Al Balushi N, Al-Mahrouqi T, Al Ghailani A, McCall RK, Sultan A, Al-Alawi M (2021) Predictors of psychological distress among the public in Oman amid coronavirus disease 2019 pandemic: a cross-sectional analytical study. Psychol Health Med 26(1):131–144. https://doi.org/10.1080/13548506.2020.1842473
Alqassabi K, Althehli R, Alkhawaja A (2021) The predictive capacity of Coronavirus impacts with psychological adjustment among university students in Oman. J Educ Psychol Res 18(68):312–336
Cohen S, Kamarck T, Mermelstein R (1983) Perceived Stress Scale. J Health Soc Behav 24(4):385–396. https://doi.org/10.2307/2136404
Lee EH (2012) Review of the psychometric evidence of the perceived stress scale. Asian Nurs Res 6(4):121–127. https://doi.org/10.1016/j.anr.2012.08.004
Sreeramareddy CT, Shankar PR, Binu VS, Mukhopadhyay C, Ray B, Menezes RG (2007) Psychological morbidity, sources of stress and coping strategies among undergraduate medical students of Nepal. BMC Med Educ 7(1):1–8
Hamaideh SH (2011) Stressors and reactions to stressors among university students. Int J Soc Psychiatry 57 (1):69–80. https://doi.org/10.1177/0020764009348442
El Ansari W, Khalil K, Stock C (2014) Symptoms and health complaints and their association with perceived stressors among students at nine Libyan universities. Int J Environ Res Public Health 11(12):12088–12107. https://doi.org/10.3390/ijerph111212088
Pluut H, Curşeu PL, Ilies R (2015) Social and study related stressors and resources among university entrants: Effects on well-being and academic performance. Learn Individ Differences 37:262–268. https://doi.org/10.1016/j.lindif.2014.11.018
Eisenberger R, Armeli S, Rexwinkel B, Lynch PD, Rhoades L (2001) Reciprocation of perceived organizational support. J Appl Psychol 86(1):42–51. https://doi.org/10.1037/0021-9010.86.1.42
Article CAS PubMed Google Scholar
Cohen S, Gottlieb B, Underwood L (2000) Social relationships and health. In: Cohen S, Underwood L, Gottlieb B (eds) Measuring and intervening in social support. Oxford University Press, New York. https://doi.org/10.1093/med:psych/9780195126709.003.0001
Chapter Google Scholar
Alotaibi AD, Alosaimi FM, Alajlan AA, Abdulrahman KAB (2020) The relationship between sleep quality, stress, and academic performance among medical students. J Fam Community Med 27(1):23–28. https://doi.org/10.4103/jfcm.JFCM_132_19
Lin XJ, Zhang CY, Yang S, Hsu ML, Cheng H, Chen J, Yu H (2020) Stress and its association with academic performance among dental undergraduate students in Fujian, China: a cross-sectional online questionnaire survey. BMC Med Educ 20:1–9
Meyer S, Larson M (2018) Physical activity, stress, and academic performance in college: does exposure to stress reduction information make a difference? Coll Stud J 52(4):452–457
Deihl LM, Vicary JR, Deike RC (1997) Longitudinal trajectories of self-esteem from early to middle adolescence and related psychosocial variables among rural adolescents. J Res on Adolescents 7(4):393–411. https://doi.org/10.1207/s15327795jra0704_3
Solberg VS (1997) Examination of self-efficacy, social support, and stress as predictors of psychological and physical distress among Hispanic college students. Hisp J Behav Sci 19(2):182–201. https://doi.org/10.1177/07399863970192006
Pascoe MC, Hetrick SE, Parker AG (2020) The impact of stress on students in secondary school and higher education. Int J Adolesc Youth 25(1):104–112. https://doi.org/10.1080/02673843.2019.1596823
Tayefi B, Eftekhar M, Tayefi M, Darroudi S, Khalili N, Mottaghi A (2020) Prevalence and socio-demographic correlates of mental health problems among Iranian health sciences students. Acad Psychiatry 44(1):73–77. https://doi.org/10.1007/s40596-019-01121-y
McEwen BS (1998) Protective and damaging effects of stress mediators. N Engl J Med 338(3):171–179. https://doi.org/10.1056/NEJM199801153380307
Hwang A, Peng L, Wen Y, Tsai Y, Chang L, Chiou S, Chen L (2014) Predicting all-cause and cause specific mortality by static and dynamic measurements of allostatic load: a 10-year population-based cohort study in Taiwan. J Am Med Dir Assoc 15(7):490–496. https://doi.org/10.1016/j.jamda.2014.02.001
Stauder A, Cserháti Z, Thege BK (2018) Decreasing the negative effects of work-related stress in unchanged working environments. Eur J Ment Health 13(2):163–183. https://doi.org/10.5708/EJMH.13.2018.2.4
Li M, Shu Q, Huang H, Bo W, Wang L, Wu H (2020) Associations of occupational stress, workplace violence, and organizational support on chronic fatigue syndrome among nurses. J Adv Nurs 76(5):1151–1161. https://doi.org/10.1111/jan.14312
Download references
Acknowledgements
The lead author acknowledges the support of the Center for Information Systems at his university (University of Nizwa) for disseminating the survey questionnaire for this study to students and providing the data related to the number of students registered in the University during Fall 2020.
This research did not receive any funding from any source.
Author information
Authors and affiliations.
Department of Management, University of Nizwa, Nizwa, Oman
Mustafa Malik
Department of Commerce, Aligarh Muslim University, Aligarh, India
Sarfaraz Javed
You can also search for this author in PubMed Google Scholar
Contributions
MM conceived and designed this research. He designed the questionnaire, collected data, performed the data analysis, and wrote the manuscript. SJ contributed in literature review and the proofreading of the manuscript. All the authors have read and approved the manuscript.
Corresponding author
Correspondence to Mustafa Malik .
Ethics declarations
Ethics approval and consent to participate.
This study received the ethical approval from the Human Ethics Committee in the Office of the Vice Chancellor for Graduate Studies, Research and International Relations, University of Nizwa through its letter (EC Ref. No.: HREC-12-2020) on 17 November 2020. The online survey questionnaire used for this study contained an introduction which explained the purpose and objectives of the study and asked the respondents to complete the survey voluntarily. Since the survey was conducted online, the respondents’ consent was sought through a declaration made at the end of the introduction to the survey on the first page of the questionnaire. Respondents could proceed to the next page of the questionnaire only if they have accepted to participate in the survey.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Malik, M., Javed, S. Perceived stress among university students in Oman during COVID-19-induced e-learning. Middle East Curr Psychiatry 28 , 49 (2021). https://doi.org/10.1186/s43045-021-00131-7
Download citation
Received : 21 May 2021
Accepted : 25 July 2021
Published : 04 August 2021
DOI : https://doi.org/10.1186/s43045-021-00131-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Higher education
- Share full article
Advertisement
Supported by
What Doctors Want You to Know About Beta Blockers for Anxiety
Start-ups are making it easier to get the pills online, but experts warn they should be used with caution.

By Christina Caron
Anxious ahead of a big job interview? Worried about giving a speech? First date nerves?
The solution, some digital start-ups suggest, is a beta blocker, a type of medication that can slow heart rate and lower blood pressure — masking some of the physical symptoms of anxiety.
Typically a trip to the doctor’s office would be necessary to get a prescription, but a number of companies are now connecting patients with doctors for quick virtual visits and shipping the medication to people’s homes.
“No more ‘Shaky and Sweaty,’” one online ad promised. “Easy fast 15 minute intake.”
That worries Dr. Yvette I. Sheline, a professor of psychiatry at the University of Pennsylvania Perelman School of Medicine.
“The first question is: What is going on with this person?” Dr. Sheline said. Are they depressed in addition to anxious? Do they have chronic anxiety or is it just a temporary case of stage fright? “You don’t want to end up prescribing the wrong thing,” she added.
In addition, although beta blockers are generally considered safe, experts say they can carry unpleasant side effects and should be used with caution.
What are beta blockers?
Beta blockers such as propranolol hydrochloride have been approved by the Food and Drug Administration for chest pain, migraine prevention, involuntary tremors, abnormal heart rhythms and other uses.
Some are still prescribed for hypertension, although they’re no longer considered the preferred treatment , mainly because other medications are more effective in preventing stroke and death.
Beta blockers can ease the physical symptoms of the “fight or flight” response to stress, such as tremors, sweaty palms or a racing heart, but they are not F.D.A.-approved to treat anxiety disorders.
For decades, doctors have prescribed them for issues other than their approved uses, including for problems like stage fright. In recent years, celebrities like Robert Downey Jr. and Khloé Kardashian have said the medications helped them overcome performance anxiety.
How do they work?
When we start feeling anxious or stressed, our bodies produce adrenaline, which prepares us to respond to perceived danger. The hormone signals our heart to beat faster and narrows our blood vessels to redirect blood to important organs like the heart and lungs. Breathing quickens, and we start to sweat.
Beta blockers work by “blocking” the effects of adrenaline. They cause the heart to beat more slowly and with less force, which helps lower blood pressure.
But if you’re feeling especially anxious, “your mind is still going to race, you’re still going to ruminate and worry,” said Regine Galanti, a psychologist in Cedarhurst, N.Y., who treats people with anxiety disorders.
In other words, beta blockers are not going to address the root of your fears. “If it becomes like a weekly, ‘Oh, I’m just having a hard time in this course. I’ll just pop a beta blocker every single time.’ I would say, ‘What’s the long-term goal here?’” she added.
Patients are typically only prescribed a few pills for specific situations where they might experience performance anxiety, said Dr. Joseph Bienvenu, a professor of psychiatry at Johns Hopkins University School of Medicine. But some online companies dole out as many as 48 at a time.
Are there any side effects?
Yes. Beta blockers can make people feel dizzy. Other potential side effects include fatigue, cold hands or feet, trouble sleeping and nightmares. They can also cause stomach problems like nausea or diarrhea and, less often, difficulty breathing.
This is why some doctors tell their patients to avoid taking them for the first time on the day of a big event.
Dr. Bienvenu advises patients to initially try the medication on the weekend, or “when you don’t have anything else to do.”
“I just want people to know how it’s going to affect them,” he said.
Is it OK to take them for a potentially scary task, like a big presentation?
Possibly. But experts suggested visiting your general practitioner first.
Beta blockers may not be advised for some people with diabetes, low blood pressure or bradycardia, which is a slow heart beat — or people with asthma or another lung disease. And certain drugs, including some cholesterol and cardiovascular medications, can interact with them.
Online doctors do not have your full medical history and have not examined you in person, said Arthur Caplan, a professor of bioethics at the N.Y.U. Grossman School of Medicine.
Without a physical exam, some patients might not know that they have an underlying issue like an irregular heartbeat, he added. And they may not know who to call if they have questions after getting a prescription.
“You need to be managed on these kinds of drugs,” he said.
For those who often face anxiety-provoking tasks like public speaking, the experts said, it might be most beneficial to try breathing techniques or exposure therapy , which involves directly confronting what makes us anxious to break a pattern of fear and avoidance.
“Masking your anxiety symptoms is not going to teach you how to manage your anxiety symptoms,” Dr. Galanti said.
Christina Caron is a Times reporter covering mental health. More about Christina Caron
Managing Anxiety and Stress
Stay balanced in the face of stress and anxiety with our collection of tools and advice..
How are you, really? This self-guided check-in will help you take stock of your emotional well-being — and learn how to make changes .
These simple and proven strategies will help you manage stress , support your mental health and find meaning in the new year.
First, bring calm and clarity into your life with these 10 tips . Next, identify what you are dealing with: Is it worry, anxiety or stress ?
Persistent depressive disorder is underdiagnosed, and many who suffer from it have never heard of it. Here is what to know .
If you notice drastic shifts in your mood during certain times of the year, you could have seasonal affective disorder. Here are answers to your top questions about the condition .
How much anxiety is too much? Here is how to establish whether you should see a professional about it .

IMAGES
VIDEO
COMMENTS
levels of perceived stress, although only weakly (r = -0.15 to -0.25). Regression analysis revealed that of the three experiences, the amount of pleasure students experienced explained the largest amount of the variance in perceived stress; but, it was a weak predictor, explaining only 3% of the variance independently.
An undergraduate honors thesis submitted in partial fulfillment of the requirements for the degree of Bachelor of Science in University Honors and Public Health Studies: Pre-Clinical Health Science ... Perceived stress of the study population was higher than normative college student values. Greater use of adaptive coping mechanisms than ...
The current study explores the relationships between reappraisal, suppression, social support, resilience, perceived stress and. academic performance while controlling for other factors that may affect academic performance. The goal of this research was to establish if these predictors can uniquely predict real world.
THESIS PERCEIVED STRESS, CAREGIVER BURDEN, AND EMOTIONAL DISTRESS IN CAREGIVERS OF HEAD AND NECK CANCER AND LUNG CANCER Submitted by Marissa C. Alliegro Department of Psychology In partial fulfillment of the requirements For the Degree of Master of Science Colorado State University Fort Collins, Colorado Fall 2019 Master's Committee:
Stress among college students has been well documented. Pierceal and Keim (2007) reported that 75% to 80% of college students are moderately stressed, and 10% to 12% are. severely stressed. Brougham et al. (2009) found that an average of 52% of college students. experience high levels of stress in a typical semester.
This study will contribute to the limited literature about university students' perceived stress, resilience, optimism, and psychological well-being, especially in relation to Pakistani culture.
For a perceived stress prevalence of 50% and a sample size of 100, the 95% confidence interval (CI) is 48-52% —a reasonable 95% CI estimate for perceived stress prevalence measure. Studies with missing information for any of the domains were classified as having an unclear risk of bias for that specific domain. ... Thesis. University of ...
thesis director, Dr. William Milberg, for his insight and his refusal to accept anything less . than my best quality work. Finally, I owe a great deal of gratitude to Dr. Adrienne . Tierney for her guidance and helpful direction during this thesis process. ... Perceived Stress Scale (Cohen et al., 1983) ...
Perceived stress is a feeling about how much strain individuals perceive when environmental demands exceed their adaptive capacity [].The prevalence of perceived stress in adults is 34.8-55% ...
AN ASSESSMENT OF PERCEIVED STRESS, STRESSORS, COPING STRATEGIES, AND STRESS MINDSETS AMONG LA CROSSE COUNTY, WISCONSIN EMPLOYEES A Chapter Style Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Public Health in Community Health Education Janessa L. VandenBerge College of Science and Health ...
Scoring: First, reverse the scoring for questions 4, 5, 7, and 8. On these 4 questions, change the scores so that 0 = 4, 1 = 3, 2 = 2, 3 = 1, and 4 = 0. Then, add up your scores for each item to get a total. Scores on this measure can range from 0-40 with higher scores representing higher perceived stress.
Students with higher perceived stress indicated missing two or more days of class and had higher avoidant coping strategies (Thomas & Borrayo, 2016). Digital stress adds to perceived stress in a person's life (Weinstein & Selman, 2014). Psychological health effects can result from the use of products such as smartphones and laptops.
The overall prevalence of academic stress among participants was 92%, with a mean score (24.99 ± 5.159), and the levels of academic stress were ranged from low (4.3%), moderate (73.2%), to high (22.5%). Approximately 80% of the percipients reported academic stress during all exam times with a mean score (25.33 ± 4.976).
Abstract. Stress is a common problem for college students. The goal of this thesis was to examine the relationships between protective and risk factors to experiencing stress and how these factors may predict academic performance in college students. 125 college students were surveyed twice over the course of a semester on emotion regulation strategies, trait resilience, and perceived stress.
Given that perceived stress is commonly considered a personality style rather than a response to environmental stressors (Cohen et al., 1983;Cohen, Tyrrell, & Smith, 1993; Phillips, 2012), there ...
academic stress and perceived stress either through correlations or differences— demographics, academic classification, ma jor or field of study, athletic scholarship status, and season of sport (in-season/out of season) . To assess this relationship, an online questionnaire, including two perceived stress scales, was collected at the
The authors report that substance use was associated with a greater desire for academic achievement, higher perceived stress, less effective coping strategies, and less closeness with parents (Leonard et al., Citation 2015). In 7th and 8th Grade students from the United States, self-perceived stress has similarly been reported to be related to ...
INTRODUCTION. Stress is an inevitable life experience that develops when an individual fails to cope with the external physiological and cognitive distress of daily life [1, 2].Perceived stress is defined as how an individual understands the amount of stress he or she is exposed to in a period of time [].It is related to a feeling of uncertainty and instability about life and depends on the ...
Perceived stress, which reflects the tendency to appraise one's life situations as stressful and overwhelming, has emerged as a stable predictor for depressive symptoms. However, the neurobiological bases of perceived stress and how perceived stress influences depressive symptoms in the healthy brain remain largely unknown. Here, we ...
Perceived stress is also different from affect despite the important role negative affect plays in the conceptualization and measurement of perceived stress. Affective states are a large umbrella term for all emotional experiences, including emotional reactivity, longer-term mood states, and dispositional traits. ...
PERCEIVED STRESS, CAFFEINE CONSUMPTION, AND GPA OF UNDERGRADUATE STUDENTS AT A LARGE PUBLIC UNIVERSITY Emma Simpson University of Kentucky, [email protected] ... This Master's Thesis is brought to you for free and open access by the Dietetics and Human Nutrition at UKnowledge. It has been accepted for inclusion in Theses and Dissertations ...
The study further investigated the relationship between the students' perceived level of stress and their perceived family support and institutional support, as well as their academic performance. The study showed the during the online teaching and learning induced by the pandemic, students in general have experienced a moderate to higher stress.
Beta blockers work by "blocking" the effects of adrenaline. They cause the heart to beat more slowly and with less force, which helps lower blood pressure. But if you're feeling especially ...
Both perceived job stress and job-related strain may affect physical and mental health. House's framework also includes conditioning variables, such as social support, which may modify the relationships between the environment, perceived job stress, and job-related strain. Conceptual Framework "Social and psychological factors have been