An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Mol Sci


Burns: Classification, Pathophysiology, and Treatment: A Review
Wojciech Żwierełło.
1 Department of Medical Chemistry, Pomeranian Medical University, 70-204 Szczecin, Poland
Krzysztof Piorun
2 West Pomeranian Center for Treating Severe Burns and Plastic Surgery, 72-300 Gryfice, Poland
Marta Skórka-Majewicz
Agnieszka maruszewska.
3 Department of Physiology and Biochemistry, Institute of Biology, University of Szczecin, 71-412 Szczecin, Poland
Jacek Antoniewski
Izabela gutowska, associated data.
Not applicable.
Burns and their treatment are a significant medical problem. The loss of the physical barrier function of the skin opens the door to microbial invasion and can lead to infection. The repair process of the damage caused by the burn is impaired due to the enhanced loss of fluids and minerals through the burn wound, the onset of hypermetabolism with the concomitant disruption of nutrient supply, and derangements in the endocrine system. In addition, the initiated inflammatory and free radical processes drive the progression of oxidative stress, the inhibition of which largely depends on an adequate supply of antioxidants and minerals. Clinical experience and research provide more and more data to make the treatment of patients with thermal injury increasingly effective. The publication discusses disorders occurring in patients after thermal injury and the methods used at various stages of treatment.
1. Introduction
Skin is the human body’s largest organ, covering a surface area of about 2 sqm in an average adult. It consists of the epidermis and the dermis, deep within which are important skin appendage structures (including hair follicles, sweat glands and sebaceous glands). These deep structures are a source of proliferating epithelial cells (keratinocytes), which migrate into the clot and wound bed, playing an important role in the wound healing process. The loss of the physical barrier function of the skin opens the door to invasion by harmful microorganisms, which can lead to infection, and ultimately even to the development of sepsis. The repair process of burn injury, which begins as early as several hours after the traumatic event, may also be impaired by large fluid losses via the wound [ 1 ]. Any burn, even relatively minor, can have functional and aesthetic implications lasting throughout the patient’s lifetime.
Burns and their treatment have been regarded as an important medical problem since antiquity. The first formulations for concoctions to be used in burn care can be found already in prehistoric paintings, Egyptian papyri, and ancient Chinese art. The historical writings of Hippocrates, Celsus, and Galen describe increasingly elaborate methods for making ointments, dressings, and treatment regimens for different types of burns. In the mid-16th century, Ambrose Paré was one of the first to describe early burn wound excision. At the beginning of the 17th century, Guilhelmus Fabricius Hildanus ventured to discuss the pathophysiology of burns, making a unique contribution to the treatment of scar contractures, among other things. In 1797, Edward Kentish described the use of pressure dressings to alleviate the effects of burns and blistering, while in 1839, Dupuytren reviewed more than 50 cases of burns and presented a classification with six degrees of burn depth. His classification is still in use in many parts of the world. In the twentieth century, major developments in our knowledge of burn care occurred, particularly with regard to the problems of fluid loss and resuscitation, the hypermetabolic response to burns, infection control and the development of topical antimicrobials, early excision of burned tissue and wound closure with autologous or allogeneic skin grafts, keratinocyte culture, and, last but not least, the use of artificial skin substitutes. Efforts aimed at advancing our understanding of the problem of burns are gradually improving survival rates and the quality of life of burn patients. This does not change the fact that many aspects of the pathophysiology of this type of injury need further research, which will make it possible to develop a better, standardised, and generally accepted effective burn resuscitation regimen [ 2 , 3 ].
The use of appropriate treatment strategies in the shortest possible time from the occurrence of thermal injury can not only save the patient’s life, but also shorten their hospital stay and recovery time. Therefore, the aim of the study was to comprehensively discuss the disorders occurring in patients at different times after the occurrence of burns and the appropriate treatment methods.
A literature analysis was carried out on the PubMed database. The following keywords were used to search for available articles: “Burns”, “Burn”, “Burns pathophysiology”, “Burns treatment”, “Burn injury”, “Thermal injury”, “Burns treatment”. The time range of the searched articles was not established. We tried to use the latest reports on the pathophysiology and treatment of burns, but when discussing changes in the patient’s body after thermal injury, we did not want to eliminate older reports describing significant metabolic changes. Filters related to the type of articles (clinical trials, review, systematic review, book) were used. Repetitions were rejected from the found articles. The suitability for the inclusion of each study into the publication was thoroughly assessed. Eventually, 83 articles were included in the review.
2. Burn Injury
A burn injury results from skin contact with a heat source [ 4 ]. The factors that can cause burn injuries include high temperature, electricity, friction, radiation and chemicals [ 5 ]. Burn injuries vary, and an increase in the body surface area affected by the burn injury affects wound morbidity and patient mortality [ 6 ]. Other important factors directly impacting on the severity of injury include the location of the burn, temperature and time of exposure to the heat source, with a synergistic effect between them [ 7 ].
2.1. Classification of Burns
Burn injuries can be classified according to a number of factors, including their depth, aetiology and percentage of body surface area affected. The combination of the above classifications determines the degree of burn injury. Burns can be classified as “partial-thickness” and “full-thickness”. If the damage is limited to the epidermis and the outer part of the dermis (a superficial partial-thickness burn), with most of the appendage structures remaining intact, recovery will be rapid (10–14 days) and the risk of scarring low. If, on the other hand, the burn extends into the deeper layers of the dermis, with greater appendage damage, the epithelium will take longer to regenerate (3–6 weeks) and there will be a high probability of hypertrophic scarring. Full-thickness burns involve the destruction of all layers of the skin and usually require surgical intervention to ensure proper wound healing [ 1 , 8 ].
2.2. Aetiology
The origin of burn injuries can be thermal, electrical, chemical, radiation contact, etc.
2.2.1. Thermal Injuries
Thermal injuries account for about 90% of all burns, and the depth of injury depends on the temperature and duration of contact ( Figure 1 ). They can be divided into:
- - Injuries caused by hot liquids (scalds)—the most common type of burn injury, accounting for nearly 70% of burns in children, but also common in the elderly. Scalds usually cause partial-thickness burns that heal after a standard treatment regimen;
- - Dry heat injuries—usually caused by direct contact with a flame or radiant heat. Common in adults and often associated with complications due to smoke inhalation. They are usually deep (partial or full thickness) and generally require surgical intervention;
- - Contact injuries—result from direct contact with a hot object. Prolonged contact with a moderately hot object (e.g., a radiator) can also cause a thermal injury, which is commonly associated with loss of consciousness (e.g., in the elderly, patients with epilepsy, drug addicts and alcoholics). Contact burns are usually deep and require surgery [ 9 ].

A full-thickness 3rd degree thermal burn.
2.2.2. Electrical Injuries
Electrical injuries account for less than 5% of all burns. They are most common in children and male manual workers. The severity of injury is determined by the voltage and amperage, the type of current, duration of contact, and the pathway of the current through the body. Most tissues are good conductors, especially nerves and vessels. Skin and bones are poor conductors, although skin conductivity varies depending on its moisture content and temperature. The heat is generated by electricity around the tissues that are poor conductors, damaging the local surrounding tissues. Clinically, one often observes the so-called entrance and exit points, where the electrical current has passed through the body. An electrical voltage of <1000 V, typically found indoors, causes small, deep burns at the entrance and exit points. Alternating current can also interfere with heart function and lead to arrhythmias. High-voltage injuries (>1000 V) lead to extensive tissue damage, often with loss of limbs, asystole, cardiac arrhythmia, rhabdomyolysis (muscle breakdown) and renal failure. Fluid resuscitation is complex due to the invisible nature of the injuries. This type of injury is associated with a high mortality rate, and approximately 15% of victims have additional injuries from falls [ 10 ].
Burns can also be caused by the mere arc flash of a discharge between high-voltage sources. While current does not pass through the body, the heat from the arc can burn exposed parts of the body (hands and face). The resulting burns are usually partial-thickness, unless the arc causes clothing to ignite, leading to deeper injuries [ 11 ].
2.2.3. Chemical Injuries
Chemical injuries account for approx. 3% of burns. Incidents of this type mainly occur in domestic and industrial settings. This type of injury involves denaturation of proteins and the extent of injury depends on the concentration, amount, duration of contact, and mechanism of action of the given chemical, i.e., reduction and oxidation, corrosion, protoplasmic poison, vesication, and desiccation. While the clinical picture is similar for all groups of chemicals, the exact mechanisms of tissue damage may vary, hence chemicals have traditionally been classified into acids or alkalis [ 12 ].
Acid burns cause damage resulting in protein denaturation and necrosis, which is usually localised and short-lived. Alkaline burns, on the other hand, cause progressive liquefaction necrosis, with deeper tissue penetration and a prolonged effect. Cement causes alkaline burns, and when mixed with sweat it can induce an additional exothermic reaction. In addition, cement powder is highly hygroscopic and causes severe desiccation of the affected surface. Washing with copious amounts of water dilutes the chemical and helps reduce tissue damage [ 12 ]. Burns are most likely to be caused by acids (sulphuric, nitric, hydrofluoric, hydrochloric, acetic, formic, phosphoric, phenolic and chloroacetic acids), alkalis (sodium hydroxide, potassium hydroxide, calcium hydroxide and lithium hydroxide, sodium and calcium hypochlorite, ammonia, phosphate, silicate, sodium carbonate), oxidisers (bleaches such as chlorites used in the household, peroxides, chromates), or other chemicals (white phosphorus, hair colouring agents, mustard gas).
2.2.4. Radiation
Generally, harmful radiation is caused by alpha (α), beta (β) and gamma (γ) rays. Alpha particles are positively charged helium ions. They are heavy, can only travel a few centimetres in the air, and cannot penetrate the keratin layer of the skin. However, these are high energy particles with high Sv (sievert) value and can cause extensive tissue damage upon ingestion or inhalation. Beta particles are negatively charged electron beams that can travel several metres in the air and cause superficial sunburn-like injuries because of their limited ability to penetrate deep into tissue (1 cm) [ 13 ].
Gamma rays from X-rays and the natural decay of radioisotopes, such as 60 Co (cobalt) and 192 Ir (iryd), can travel several metres in the air and penetrate deep into tissues. Consequently, gamma rays can cause very deep damage involving vital structures such as the bone marrow and lungs. In addition to deep gamma burns on the skin, patients experience systemic symptoms described as Acute Radiation Syndrome (ARS) [ 13 ].
3. Pathophysiology of Burn Injuries
3.1. local effects of burn injuries.
Burn injuries cause coagulative necrosis of various layers of skin and underlying tissues. Because of its main function as a physiological barrier protecting underlying tissues, the skin usually limits the spread of damage to deeper layers, but the extent of damage is determined by the temperature, the energy transmitted by the causative agent, and the duration of exposure [ 14 ]. In principle, the site of a cutaneous burn injury can be divided into three zones:
- - Zone of coagulation—represents the area of necrosis with irreversible tissue damage incurred at the time of injury;
- - Zone of stasis—surrounds the coagulation zone and is moderately damaged with vascular transudate, elevated vasoconstricting factors, as well as local inflammatory reactions, resulting in impaired tissue perfusion. Depending on the wound environment, the zone may recover or progress to necrosis;
- - Zone of hyperaemia, with dilated vessels caused by inflammation. It is characterised by increased blood flow to healthy tissues without much risk of necrosis, unless there is severe sepsis or prolonged hypoperfusion [ 14 ].
3.2. Systemic Effects of Burn Injuries
Burns involving more than 30% of total body surface area (TBSA) result in considerable hypovolemia coupled with the formation and release of inflammatory mediators, leading to a subsequent systemic effect, namely a characteristic cardiovascular dysfunction known as burn shock. It is a complex process of circulatory and microcirculatory impairment, generating oedema in both burned and unaffected tissues. Even with prompt intervention and adequate fluid support, this pathophysiological state remains completely irreversible [ 15 ]. Plasma extravasation is another feature of burn injury, resulting in increased systemic vascular resistance (SVR) and reduced peripheral blood flow. This results in hemodynamic changes, which include a reduction in cardiac output due to the diminished plasma volume, as well as a decrease in urinary excretion [ 16 , 17 ].
Burn shock involves a state of inadequate tissue perfusion with resultant inadequate oxygen and nutrient delivery, as well as failure to remove metabolic waste from the tissues [ 15 ]. Despite proper fluid resuscitation and adequate preload, pulmonary and systemic vascular resistance increases and myocardial depression occurs. This in turn stimulates further exacerbation of the inflammatory response and contributes to the risk of multiple organ failure [ 15 ]. Importantly, elevated haemoglobin and haematocrit levels are also observed in burn injuries [ 16 , 17 ].
Another response of the body to a burn is oedema formation. Enema develops when the amount of fluid filtered out of microvessels is greater than the amount of fluid entering them [ 15 ]. The process of oedema formation is biphasic. The primary phase, initiated in the first hour after the burn injury, is caused by a rapid increase in the water content of the damaged tissues. The second phase, occurring 12–24 h after the burn injury, involves a slower, gradual increase in fluid flow in both the burned and intact skin and soft tissues [ 15 , 16 ].
In the development of post-burn oedema, an important role is played by the rate of increase in tissue water content, which is clearly influenced by the type and amount of fluid resuscitation administered to the patient. The tissue water content reaches double the original volume within the first hour, with 90% of the increase observed in the first few minutes. The use of fluid resuscitation contributes to further extravasation, influenced by increased blood flow and increased capillary pressure under the influence of the delivered fluids. On the other hand, oedema tends to be self-limiting when fluids are not administered [ 18 , 19 ].
In burn injuries exceeding 30% TBSA, thermal insults result in a decrease in the cellular transmembrane potentials in skeletal muscles not only at the site of injury, but also distant to the site of injury [ 20 ]. It has been shown that cell membranes in damaged and intact skeletal muscles demonstrate partial depolarisation of membrane potential from −90 mV to −80mV and −70 mV. As soon as there is a decline in membrane potential, the water and sodium content within cells increases. These alterations are also seen in haemorrhagic shock. Similar changes have been reported in cardiac, hepatic and endothelial cells [ 20 ]. Some scholars have linked membrane depolarisation to a decrease in adenosine triphosphate (ATP) and reduced ATPase activity in the respiratory chain. Others suggest that the mechanism depends on increased membrane permeability to sodium ions, associated with increased Na/K pump activity [ 21 ]. Research aimed at identifying the factors responsible for the cellular oedema seen in burn shock postulated the existence of unidentified and complex shock factor(s) [ 21 ]. This hypothesis was supported by Button et al., who demonstrated that burn-associated tissue oedema cannot be attributed solely to hypovolemia, and that burn shock should not be regarded as another form of haemorrhage [ 22 ].
The enormous energy demand, measured by resting energy expenditure, is a typical finding in burn patients, with the increase in metabolism (hypermetabolism) dependent on the size of burn. In patients with a TBSA of less than 10%, resting energy expenditure remains at physiological levels, but for TBSA in excess of 40%, this rate is twice as high during acute admission. Having reached the maximum value, the resting metabolic rate in severely burned patients gradually declines, amounting to 150%, 140%, 120% and 110% of baseline at the time of burn wound healing, 6, 9 and 12 months after thermal injury, respectively [ 23 ].
Underlying the hypermetabolic response following thermal injury are mechanisms of metabolic, hormonal and inflammatory dysregulation. This is a highly complex phenomenon, triggered by persistent increases in the secretion of catecholamine, cortisol, glucagon, and dopamine, and elevated concentrations of interleukin 1 (IL-1), interleukin 6 (IL-6), tissue necrosis factor (TNF), platelet-activating factor (PAF), complement cascades, as well as increased synthesis of reactive oxygen species (ROS) [ 24 ]. These metabolic regulations were found to occur in two phases: early (ebb) and late (flow). The “ebb” phase begins immediately after thermal injury and lasts approximately three days. It is characterised by hypodynamic circulation, reduced oxygen consumption and hyperglycaemia. These variables then begin to increase progressively until reaching the “flow” phase, which usually lasts up to a year since the burn injury [ 25 ].
The body’s hypermetabolic response has detrimental effects at the cellular and systemic level [ 26 ]. At the systemic level, the structure and function of major organs (heart, liver, skeletal muscle, skin), the immune system and the transmembrane transport system are compromised. Wound healing is impaired, which increases the risk of infection, hampers rehabilitation and delays the reintegration of patients back into society [ 26 , 27 ]. At the cellular level, hypermetabolic response increases thermogenesis [ 28 ] by uncoupling mitochondrial respiration from phosphorylation of ADP to ATP, resulting in heat generation [ 29 ]. Simultaneously, increased energy demand enhances oxygen consumption [ 28 ]. The adipose tissue of burn patients was reported to contain elevated levels of uncoupling protein 1 (UCP1), an important mediator of thermogenesis [ 30 , 31 ].
The endocrine disruption that occurs after a burn alters metabolic pathways. Catecholamines drive hypermetabolism, while an increase in the secretions of cortisol, adrenaline and glucagon (which are catabolic hormones), together with an increase in pro-inflammatory cytokines, inhibits protein and fat synthesis [ 26 , 32 ]. The observed negative nitrogen balance in burn patients [ 25 ] suggests that skeletal muscles are used as the main energy source [ 33 ]. Accelerated protein degradation leads to a significant loss of lean body mass (LBM) and muscle atrophy, resulting in reduced strength and compromised rehabilitation outcomes [ 26 , 34 ]. Depending on the magnitude of LBM loss, certain dysfunctions occur. While alterations in the immune system, increased rates of infection and delayed wound healing are correlated with a 20% loss of LBM, patients with a 30% loss of LBM present inhibited cough reflexes, prolonged requirements for mechanical ventilation, as well as an increased risk of pneumonia and pressure sores. With LBM loss reaching 40%, mortality among burn patients goes up to 50–100% [ 35 ].
Research has shown that impaired glucose metabolism can still be seen up to three years after thermal injury [ 24 ]. In severe burns, hypermetabolism and oxygen deprivation in the cells lead to anaerobic glycolysis, where glucose is converted to lactic acid [ 36 ]. In addition, patients with severe burns exhibit increased glucose production through activation of the gluconeogenesis pathway, with alanine as the main substrate (next to lactic acid). In this situation, amino acids become the main “fuel”, resulting in a deficit of amino acids for building proteins, as well as an increase in nitrogen excretion, mainly in the form of urea [ 26 ].
An increase in gluconeogenesis activity associated with an increase in gluconeogenic substrates, which include glycerol (derived from the breakdown of triacylglycerols), alanine (derived from the breakdown of proteins) and lactate (a product of anaerobic glycolysis), leads to hyperglycaemia in patients with severe burns. Research has shown that serum glucose levels are persistently elevated in these patients, reaching of up to 180 mg/dL. This condition is further compounded by an attenuation of the suppressive effect of insulin on hepatic glucose release and enhanced hepatic glycogenolysis [ 37 ]. Interestingly, determination of insulin levels in serum samples (showing a twofold increase) points to the development of insulin resistance in these patients [ 37 ].
Thermal injury also triggers changes in the circulatory system. Cardiac function is subject to several modifications starting already at the time of injury. Before detecting any reduction in plasma volume, receptors on thermally damaged skin trigger a neurogenic response, initiating a rapid decrease in cardiac output. This is associated with an initial reduction, followed by a significant increase in the cardiac index starting on the third day post-burn [ 25 ]. Other parameters, such as long-term increase in cardiac work, increased myocardial oxygen consumption, and heart rate acceleration, remain elevated during the recovery period [ 38 ]. Severe cardiac stress is accompanied by a persistent myocardial depression that can be attributed to hypovolemia, high SVR, low venous return and the effects of myocardial depressant substances. Fluid resuscitation usually fails to restore normal cardiac output.
Urinary dysfunction is a consequence of alterations in cardiovascular function and endocrine dysregulation (changes in angiotensin, vasopressin and aldosterone secretion). The development of hypovolemia, as well the diminished cardiac output following thermal injury bring down the glomerular filtration rate (GFR) as a result of reduced renal blood flow. These alterations usually manifest themselves in the form of oliguria, and if not managed promptly and appropriately it can lead to acute tubular necrosis (ATN), renal failure and even death [ 39 ].
Following thermal injury, it is critically important to provide for an adequate nutrient supply to meet the increased energy expenditure that occurs due to the hypermetabolic response. However, the digestive process is impaired in proportion to the magnitude of the burns. Due to the apoptosis of enterocytes (the cells making up the intestinal epithelium) and mucosal atrophy, absorptive capacity is reduced, particularly the uptake of glucose, amino acids and fatty acids, and the situation is further compounded by changes in the secretion and activity of digestive enzymes, including pancreatic lipase, involved in lipid digestion. In addition, with increased intestinal permeability, undesirable compounds can pass from the intestinal tract into the bloodstream [ 40 ].
Thermal trauma also disrupts liver function. Research has shown that thermal injury alters hepatic expression and serum concentrations of acute phase proteins. Serum complement C3 and α2-macroglobulin concentrations in burn patients initially fall, and then gradually rise. The redirection of substrates to synthesise these proteins, the increased use of muscle proteins for energy production due to the hypermetabolic response and the impaired absorption of nutrients (including amino acids) in burn patients are the likely factors suppressing the synthesis of constitutive hepatic proteins [ 25 ]. Lower production of the protein components for VLDL lipoproteins (transporters for triacylglycerols and fatty acids) reduces their release from the liver, which can lead to the fatty infiltration of this organ. This in turn increases the risk of sepsis. In addition, the use of TG as an energy substrate in extrahepatic tissues is reduced [ 25 ].
Endocrine response is one of the systemic reactions observed in severely burned patients and is characterised by significant functional alterations in the hypothalamic-pituitary axis. During the early post-burn phase, there is a marked upsurge in so-called stress hormones, which include catecholamine, glucagon, and cortisol [ 41 ]. They are thought to be the initiators of the body’s hypermetabolic, catabolic, and proteolytic response to the burn injury, with significant effects on cardiovascular function and resulting changes in water-electrolyte balance. Substantial changes are also observed in the production of thyroid hormones: TSH (thyroid-stimulating hormone), T3 (triiodothyronine), T4 (thyroxine) and parathyroid hormone (PTH), as well as testosterone and osteocalcin, whose serum concentrations were found to decrease in patients with thermal injuries. The initial stress-related hormonal response is followed by alterations at several points in the hypothalamic-pituitary-organ axes. Notably, severe burns cause specific modifications in the GH (growth hormone)—IGF-1 (insulin-like growth factor 1) axis. Significantly, the concentrations of IGF-1 and insulin-like growth factor binding protein-3 (IGFBP-3) were found to be more profoundly affected than GH [ 42 , 43 ].
Scholars have also demonstrated the effects of burns on the male reproductive system. Thermal injuries tend to affect the histology of the seminiferous epithelium with germ cell atrophy. A number of factors play a role in the aetiology of germ cell apoptosis and altered spermatogenesis: increased temperature in the scrotum, decreased hormone synthesis, systemic trauma, and oxidative stress subsequent to inadequate perfusion. Decreased blood testosterone levels are sometimes attributed to the presence of testicular toxicants. The harmful effects of these substances can be reversed by administering antioxidants, including ascorbic acid, which simultaneously reduces the body’s resuscitative fluid needs [ 43 , 44 ].
In the immunological context, thermal insults have a significant comprehensive impact on the immune system, particularly the cellular immune response. Immune deficiency in burn patients is thought to be caused by impaired expression of bone marrow granulocyte colony-stimulating factor ( G-CSF ) receptors. While the loss of skin and the mechanical barrier facilitates infection in patients with burn injuries, it has long been known that impaired immune mechanisms are key factors in bacterial, viral and fungal infections following burn injury [ 45 ].
4. Treatment of Patients after Thermal Injury
While none of the established therapeutic approaches to date have been able to completely reverse the complex reactions induced by burns, there is a number of pharmacological and non-pharmacological strategies which are effective in modulating burn-associated metabolism.
4.1. Cooling of Burned Areas
Research has shown that in the event of a burn, immediate removal of the cause and cooling of the injured area is beneficial to the burn victim. Reducing the elevated temperature of the burned tissue improves the physiological response. Importantly, it also provides palliative relief. The cooling agent should be applied as promptly as possible, but it must be at the right temperature. Extreme cold (e.g., ice) can cause further damage by reducing blood flow to the injured area (cold-induced vasoconstriction). Cooling of a large area of skin over a long period of time is likely to induce hypothermia. There is also a risk of frostbite on cooled surfaces. According to the available literature, the optimal temperature for cooling a burn injury is 10–20 °C [ 46 ].
4.2. Fluid Resuscitation
In the event of a severe burn, the first and most important therapeutic intervention is adequate resuscitation [ 47 ]. After a burn injury, fluid rapidly accumulates in damaged tissues and, to a lesser extent, in healthy tissues. Without resuscitation, burns greater than 15–20% TSBA can lead to hypovolemic shock, organ dysfunction and ultimately death of the victim. The 24-h fluid requirements of a burn victim are estimated using the Parkland formula for fluid resuscitation, which remains the most widely used protocol worldwide to date. Since its introduction by Baxter and Shires in 1968, it has become the gold standard for initial fluid resuscitation in burns [ 48 ]. The formula, based on the patient’s weight and the percentage of body surface area burned, is used in combination with regular measurements of physiological parameters and resuscitation endpoints, particularly the urine output. The Parkland formula estimates the total fluid requirement over 24 h at 4 mL/kg/% TBSA, with half of this volume to be administered in the first 8 h. In the past decade, concerns about the accuracy of this formula have led clinicians to re-evaluate the fluid resuscitation process, particularly in elderly patients. The concept of excessive resuscitation was addressed by in Pruitt in his 2000 report [ 49 ].
The phenomenon of excessive fluid loading usually results from a combination of several factors, i.e., inaccuracies in calculating fluid requirements, unnecessary fluid infusions, increased use of sedation and analgesic infusions, and excessive administration of crystalloid solutions [ 47 ]. In order to improve the accuracy of fluid resuscitation, attempts are being made to introduce adjunctive measures in the form of modern minimally invasive procedures, such as the insertion of a pulmonary artery catheter or translung thermodilution, allowing for continuous monitoring of venous oxygen saturation, intrathoracic blood volume, total blood volume index and extravascular lung water index, but irrespective of the above urine output remains the main indicator of adequate fluid resuscitation. Isotonic crystalloid resuscitation fluids (lactate or acetate Ringer’s solution) are recommended for fluid resuscitation. The simultaneous use of colloid and hypertonic lactated saline (HLS) is recommended as an option for fluid resuscitation [ 50 ].
4.3. Ventilation
Airway management and ventilator support are often required in cases of severe burns, particularly in thermal lung injuries. Ventilation strategies for respiratory failure in critically ill patients, including those with severe burns, are still being developed. The introduction of a lung-protective ventilation strategy has reduced the incidence of ventilator-associated lung damage. Overall technological advances in the field of ventilation have shown measurable improvements in outcomes for patients with severe burns and inhalation injuries [ 51 ].
4.4. Surgical Treatment
Early excision and closure of the burn wound is sometimes described as the greatest advance in the treatment of patients with severe thermal injuries. In fact, this strategy is crucial for reducing the incidence of complications associated with severe burns [ 52 ]. The metabolic rate in patients undergoing total excision and wound coverage with an autograft and/or deceased donor skin within the first 72 h following severe thermal injury (50% TBSA) is 40% lower than the metabolic rate in patients with similar burn severity who did not undergo excision within a week. Immediate excision also offers additional advantages, which include reduced protein loss, lower risk of infection and sepsis, and less pain compared to patients with delayed reconstruction [ 52 ].
Reconstructive burn surgery has greatly improved the quality of life for burn patients by restoring function and appearance to the affected areas. This type of surgery may involve skin grafts ( Figure 2 ), tissue expansion, and other techniques to repair damaged tissue and minimise scarring [ 53 , 54 ].

Intermediate-thickness skin grafts on a burn wound.
Eligibility for reconstructive burn surgery depends on several factors, including the extent and location of the burn, the patient’s overall health, and the presence of other medical conditions. In general, patients with burns that affect functional or cosmetically significant areas of the body, such as the face, hands, and feet, may be good candidates for reconstructive surgery. The timing of the surgery is also important and is usually carried out after the burn wounds have healed [ 54 ].
Assessment of burn depth poses a major challenge even to experienced surgeons, as there are no precise methods to do so that can be used at an early stage (up to several days after the injury). Physicians can take guidance from a few important clues, such as the mechanism of the burn injury, redness or sensory preservation in the tissues, but such an assessment is subject to considerable error. That is why Laser Doppler Imaging (LDI), an accurate diagnostic tool with high sensitivity and specificity, has proven to be an important adjunct to clinical assessment. It is used to measure the degree of disruption of dermal microvascular blood flow and makes it possible to assess total depth with a high degree of accuracy. The use of LDI has resulted in shorter hospital stays, lower rates of surgical interventions, shorter decision-making times for grafting procedures, and overall cost efficiency [ 55 ]. Another prospective assessment method may be active dynamic thermography, where the temperature of the burn wound is measured as an indicator of its depth [ 56 ].
In recent decades, many innovative techniques have been introduced to improve the surgical treatment of burn wounds. The use of various skin substitutes has been particularly important in the evolution of burn surgery, providing recovery options in injuries previously considered impossible to reconstruct. Skin substitutes are a diverse group of wound-covering materials. They are intended to help close the wound when autologous skin grafts are either unavailable, e.g., in extensive burns, or undesirable, e.g., in full-thickness burns with significant loss of dermis [ 57 ]. In addition to rapid wound closure, they help increase the dermal component of the treated wound, reduce or eliminate the factors that inhibit healing, reduce the inflammatory response, and, consequently, the risk of scar formation [ 58 ].
Skin substitutes have been categorised into temporary impervious dressing materials (Class I), single-layer durable epidermal or dermal substitutes (Class II), and composite skin substitutes (Class III). In comparison with autografts, biosynthetic skin substitutes and human cadaver skin showed comparable efficacy in early reconstructions. Nevertheless, there is currently no skin substitute that would have all the properties of human skin. With the use of substitutes, it is often possible to achieve tissue healing, but many skin functions (sensation, thermoregulation, secretion or UV protection) cannot be restored [ 59 ].
4.5. Sepsis
Given that sepsis plays a significant role in increasing mortality associated with burns and hypermetabolic response, every effort should be made to control its rate by taking appropriate measures. These will also help prevent infection in patients [ 60 ].
Prevention and early recognition of sepsis is a key element in the critical care of the burn patient [ 60 ]. Prevention strategies include topical antimicrobial dressings, early excision and grafting, as well as nutritional support. In patients with contaminated wounds or immunocompromised patients, such as diabetic patients, children, and perioperative patients, it is recommended to perform a bacterial culture of the wound and, as an option, introduce antibiotic prophylaxis [ 50 ]. However, preventive systemic administration of antibiotics is not (currently) recommended due to the absence of sufficient evidence to support its effectiveness. Nevertheless, burn infections should be proactively treated with a systemic antibiotic therapy and, if necessary, antifungal agents. The treatment is becoming increasingly difficult due to the drug resistance of many bacterial strains. Pro-calcitonin, white blood cell count and C-reactive protein (CRP) levels are, at present, the most commonly used markers of sepsis [ 61 ]. In patients with burns, procalcitonin was moderately sensitive (73%) and specific (75%) for sepsis, white blood cell count had poor sensitivity (47%) and moderate specificity (65%), and C-reactive protein was highly sensitive (86%) but poorly specific (54%). Other biomarkers such as stroke volume index, brain natriuretic peptide, TNF-alpha, and cell-free DNA measured on day 14 post injury showed the most promise [ 60 ].
4.6. Thermoregulation
Another conservative therapeutic measure that helps reduce resting energy expenditure in burn patients with more than 40% TBSA is to increase room temperature. This simple action raises the patient’s core body temperature, thus reducing body water loss. Severe physiological changes following severe thermal injury disrupt thermal homeostasis and render burn patients susceptible to hypothermia. Raising the ambient temperature in the operating room and intensive care unit can mitigate the loss of thermoregulation, prevent hypothermia and minimise the impact of hypermetabolism. However, there is still no conclusive scientific support for this recommendation [ 62 ].
4.7. Treatment of Contractures
Burn wound contracture is an inevitable consequence of burn injury, which, if not properly treated, remains with the patient for life. It can be prevented with early, progressive physical therapy using specific regimens aimed at improving body mass and muscle strength. There are many therapies designed to reduce contractures, including corticosteroid injection, antihistamine administration, hydrotherapy, dynamic or static splinting, laser therapy, compression therapy, as well as surgical excision and reconstruction [ 63 ].
4.8. Hormonal Regulation
Attempts to modulate burn-induced hormonal deregulation have led to the establishment of several pharmacological therapeutic strategies. They can be classified according to the use of anabolic agents or anti-catabolic agents. The former include GH, insulin, IGF-1, oxandrolone and testosterone. In turn, propranolol, an adrenergic antagonist, remains the most important anti-catabolic agent [ 26 , 64 ].
Recombinant human growth hormone (rhGH) is an effective modulator of the burn-initiated response with a broad spectrum of action. It suppresses the hepatic acute phase response by increasing the levels of constitutive hepatic proteins, reduces acute phase proteins and modulates cytokine expression, as well as reducing healing time of the skin graft site, improving muscle protein kinetics and maintaining anabolic muscular growth [ 26 ]. It also stimulates the production of many other proteins and attenuates nitrogen loss after injury. However, rhGH treatment has been linked to an increased risk of fatal complications in adult patients, which significantly restricts its administration [ 64 ].
In turn, recombinant human IGF-1 and IGFBP-3 have been shown to effectively improve muscle protein synthesis in burn patients with significantly fewer adverse effects compared to GH. In addition, these agents improve intestinal mucosal integrity in severe thermal trauma in children, attenuate muscle catabolism [ 26 ], and improve the parameters of hepatic acute phase, inflammatory response and immune response. Given that the clinical use of GH is severely limited, it appears that recombinant human insulin-like growth factor-1 (rhIGF-1) may be a better therapeutic agent to effectively attenuate adverse post-burn reactions [ 64 ].
The use of insulin may be recommended in some burn injuries. It prevents muscle catabolism by activating anabolic processes in muscle [ 26 ] and preserves lean body mass without increasing hepatic triglyceride production. Apart from its ability to lower blood glucose levels by mediating peripheral glucose uptake into skeletal muscle and adipose tissue and suppressing hepatic gluconeogenesis, insulin is known to enhance DNA replication and protein synthesis by controlling amino acid uptake, increasing fatty acid synthesis and decreasing proteolysis. Insulin administration during hospitalisation has been shown to be very helpful in the treatment of hyperglycaemia in severely burned patients, by improving muscle protein synthesis, accelerating wound healing time and mitigating loss of lean body mass and acute phase response [ 64 , 65 ].
In turn, oxandrolone has found moderate clinical use in the prevention and treatment of the effects of burn injuries. As a synthetic analogue of testosterone, it restores serum testosterone levels, resulting in a sharp increase in gene expression of enzymes involved in muscle anabolism, as well as a decrease in protein catabolism. In addition to improving muscle protein synthesis, increasing lean body mass and bone mineral content, oxandrolone effectively counteracts the effects of hypermetabolism [ 26 , 64 ].
Due to the deleterious effects induced by elevated body catecholamine levels, anti-catabolic agents have been introduced into the burn injury management protocol. Propranolol has been shown to reduce compulsory thermogenesis, resting energy expenditure and tachycardia. It has also been found to play a role in increasing lean body mass, decreasing urinary nitrogen loss and urea production. It also reduces insulin resistance, peripheral lipolysis, hepatic acute phase protein response, fatty infiltration of the liver and skeletal muscle wasting [ 64 ].
4.9. Nutrition in Burn Patients
Implementation of well-balanced alternative nutrition is of utmost importance in the recovery process of burn patients ( Figure 3 ). Enteral nutrition has become the gold standard, in contrast to oral nutrition alone, as it usually succeeds in preserving total body weight and attenuates hypermetabolic response in burn patients [ 66 ]. It also helps preserve gastrointestinal motility and lowers the risk of sepsis [ 67 ]. In the presence of absolute contraindications for enteral feeding, such as prolonged ileus and intolerance to enteral feeding, or in cases where enteral nutrition alone fails to achieve the required calorie intake, parenteral feeding can be considered, but it is not universally recommended due to its possible side effects that include immunosuppression, impaired liver function and increased mortality. When it comes to the dietary profile best suited to the needs of burn patients, there are several factors to consider in order to maintain lean body mass. Given the high rates of amino acid oxidation in burn patients, it may be advisable to stimulate protein synthesis and maintain lean body mass with a high protein, high carbohydrate diet, which also increases endogenous insulin production [ 67 , 68 ].

Alternative nutrition in patients with burn injury.
4.9.1. Duration of Nutritional Support
Time to nutrition is an important factor affecting patient survival after severe burn injuries [ 69 ]. Significant damage to the intestinal mucosa and increased bacterial translocation that occur after a burn injury result in decreased nutrient absorption [ 67 , 69 ]. For this reason, nutritional support should preferably be started within 24 h of injury [ 70 ]. In animal models, early enteral feeding has been shown to significantly reduce the hypermetabolic response after severe burns [ 66 ]. Such a marked improvement in the hypermetabolic response has not been corroborated in human studies; however, early enteral feeding has been linked to a decrease in circulating catecholamines, cortisol and glucagon and preservation of intestinal mucosal integrity [ 69 , 71 , 72 ]. Early enteral feeding in humans also results in better muscle mass maintenance, accelerated wound healing, lower risk of Curling ulcer formation and shorter intensive care unit stays [ 68 ]. Nutrition, both enteral and parenteral, is generally administered in a continuous fashion. In the case of parenteral nutrition, this is carried out for logistical reasons, but with enteral nutrition, the reasons for continuous feeding are less clear. Initially, enteral feeding is administered continuously at a low volume, gradually working towards the target volume to ensure that the patient can tolerate the regimen. The continuous feeding schedule is usually continued even if the patient has no tolerance issues. Continuous enteral feeding is likely to be a remnant of parenteral schedules and to date, the superiority of either schedule has not been supported by data, albeit limited [ 73 ].
4.9.2. The Role of Micronutrients in the Nutrition of Burn Patients
Maintaining a healthy immune function and good wound healing is crucially important in patients following burn injury [ 69 ]. To this end, it is necessary to maintain normal metabolism of many vitamins and trace elements involved in these processes and to provide for sufficient nutrient intake to meet the increased energy requirements during this time [ 74 ]. Major burns trigger severe oxidative stress, which, combined with the substantial inflammatory response, contributes to the depletion of endogenous antioxidants, which in turn are highly dependent on adequate micronutrient concentrations [ 69 , 75 , 76 ].
In burns greater than 20% TBSA, it is very common to observe significant and fast progressing deficiencies of micro- and macroelements, which are associated with the production of large amounts of burn wound exudate. Significant amounts of Fe, Cu, Se and Zn have been found in exudative fluids [ 77 ]. One well-established strategy that has been observed to result in improved wound healing and fewer infectious complications is early intravenous supplementation, recommended by professional organisations in both Europe (European Society for Clinical Nutrition and Metabolism, European Burns Association) and America (American Burns Association). In this context, it is also vitally important to provide for weekly monitoring of serum levels of specific elements in such patients, or at the very least in those with burns exceeding 40% TBSA. It has been shown that in severe burns, such an approach makes it possible to detect pathologically low levels of these elements and intervene in a timely manner with intravenous supplementation [ 77 ].
Among the essential minerals is zinc, which plays a key role in wound healing, lymphocyte function, DNA replication and protein synthesis [ 74 , 78 ]. Iron acts as a cofactor for proteins involved in oxygen transport, while selenium improves cell-mediated immunity [ 75 ]. Copper is critical for the formation of mature, organised collagen, and its deficiency has been linked to cardiac arrhythmias, impaired immunity, and worse outcomes after burns [ 79 ].
Deficient levels of vitamins A, C, and D, as well as Fe, Cu, Se, and Zn, have been shown to adversely affect wound healing rates and skeletal muscle metabolism, as well as impair immune function [ 80 , 81 ]. Vitamin A accelerates wound healing by stimulating epithelial growth, while vitamin C promotes collagen maturation and cross-linking [ 82 ]. Vitamin D contributes to bone density and has been observed to be deficient after burns, but its exact role and optimal dose after severe burns remain unclear.
In paediatric burn patients, Ca and vitamin D homeostasis may be significantly dysfunctional for a number of reasons. Children with severe burns have increased bone resorption, osteoblast apoptosis, and urinary excretion of Ca. In addition, burned skin is unable to synthesise normal amounts of vitamin D3, leading to further deregulation in Ca and vitamin D levels. A study of children with burns showed that supplementation with a multivitamin containing 400 IU of vitamin D2 failed to correct vitamin D deficiency [ 83 ].
Funding Statement
This work was supported by statutory budget of Department of Medical Chemistry, Pomeranian Medical University in Szczecin, No. WFB-440-01/S/16/2022.
Author Contributions
Conceptualization, W.Ż. and I.G.; Funding acquisition, I.G.; Visualization, J.A.; Writing—original draft, W.Ż., K.P. and I.G.; Writing—review & editing, M.S.-M., I.G. and A.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Informed consent statement, data availability statement, conflicts of interest.
The authors declare they have no actual or potential competing financial interests.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
- Open access
- Published: 16 September 2019
Understanding acute burn injury as a chronic disease
- Lucy W. Barrett ORCID: orcid.org/0000-0001-9733-9841 1 , 2 ,
- Vanessa S. Fear 1 ,
- Jason C. Waithman 1 ,
- Fiona M. Wood 3 , 4 , 5 &
- Mark W. Fear 3 , 5
Burns & Trauma volume 7 , Article number: 23 ( 2019 ) Cite this article
21k Accesses
76 Citations
45 Altmetric
Metrics details
While treatment for burn injury has improved significantly over the past few decades, reducing mortality and improving patient outcomes, recent evidence has revealed that burn injury is associated with a number of secondary pathologies, many of which arise long after the initial injury has healed. Population studies have linked burn injury with increased risk of cancer, cardiovascular disease, nervous system disorders, diabetes, musculoskeletal disorders, gastrointestinal disease, infections, anxiety and depression. The wide range of secondary pathologies indicates that burn can cause sustained disruption of homeostasis, presenting new challenges for post-burn care. Understanding burn injury as a chronic disease will improve patient care, providing evidence for better long-term support and monitoring of patients. Through focused research into the mechanisms underpinning long-term dysfunction, a better understanding of burn injury pathology may help with the development of preventative treatments to improve long-term health outcomes. The review will outline evidence of long-term health effects, possible mechanisms linking burn injury to long-term health and current research into burns as a chronic disease.
Burn injury is a major public health issue, with an estimated 11 million incidences globally per year resulting in more than 300,000 deaths [ 1 ]. Burns are complex traumatic injuries, and much of the focus of research and clinical treatment has been on the acute trauma, appropriate surgical intervention and survival with reduced scarring. However, it is increasingly being acknowledged that burn injury can result in sustained and severe physiological and psychological problems. Some of these long-term effects have been well documented in the clinic, stemming from the prolonged healing period and the resulting physical scars. Other long-term health effects have been less well described. Recently, there has been increasing evidence of long-term health effects of a burn injury. Notably, the long-term effects have been observed after both severe and non-severe burns (< 20% total body surface area (TBSA)). This is significant, as the vast majority of burn patients, particularly in developed countries, suffer non-severe injuries. The review will outline evidence of long-term health effects, possible mechanisms linking burn injury to long-term health and current research into burns as a chronic disease.
Our initial literature search involved searching PubMed for articles containing the words “burn” AND “long-term”. This search returned 1274 references, 170 of which were identified as relevant to the topic of long-term health impacts of burn injury. Of these 170 references, 68 were about the long-term effects on mental health (the most well-known impact and therefore not a major focus of this review), 41 were discussing the long-term impacts of specific treatment regimens or specific types of burn and 30 were referring to what we consider to be acute stage (< 1 year post-burn). The remaining 31 references were all used in this review. The relatively small number of relevant publications returned by this search is indicative of the lack of research in this area, mainly due to the fact that many of the secondary pathologies discussed in this review were only linked to burn recently by long-term population studies. However, the data from these recently published studies will undoubtedly guide future research and lead to a better understanding of the overall impact of burn injury.
Long-term pathophysiology of burn injury
Metabolic changes, scarring and mental health disorders.
Compared to other traumatic injuries, burn patients face a prolonged healing process and are often left with physical and mental scars. Hypermetabolism is a well-characterised acute impact of burn [ 2 ]; however, recent evidence has shown that these changes persist in some manner years after the initial injury (reviewed in [ 3 ]). A study of 977 paediatric patients with severe burns analysed a variety of clinical markers and found that patients were still in a hypermetabolic state 3 years post-injury [ 4 ]. The persistence of the hypermetabolic state results in sustained loss of muscle mass and bone density [ 5 , 6 ]. An increase in muscle protein synthesis occurs in this hypermetabolic state, with a higher rate of protein degradation resulting in chronic amino acid loss that is sustained up to 1 year post-burn injury [ 7 ]. The respiratory capacity of muscle mitochondria also remains significantly reduced in burn patients 1 year post-injury [ 8 ], and muscle strength in patients with severe burns remains weaker at 1–5 years post-burn follow-up [ 9 ]. Loss of bone density as a result of inflammatory bone resorption and osteoblast apoptosis in paediatric patients with severe burns also persists long after the initial healing process [ 10 ].
While mortality rates for burn patients have significantly improved, hypertrophic scarring is a major long-term concern for survivors, especially for paediatric patients and patients suffering severe burns. Burn healing results in the deposition of excessive and disorganised extracellular matrix, reducing the pliability of scars. In hypertrophic scar, myofibroblasts persisting in the wound post-healing leads to continued contraction [ 11 ]. Treatments for scar include compression garments, massage, laser therapy, steroids and surgery [ 12 ], but there is a continued need for targeted therapies to reduce scar burden. Surgery may be required for hypertrophic scars that do not respond to other treatments, as depending on the location of the injury, scars can significantly impact movement and joint function.
Because of the context and severity of burn injuries, patients often suffer mental health problems during and long after the acute healing phase. Mental health disorders including post-traumatic stress disorder (PTSD) have been reported in burn patients more than a year after injury [ 13 ], and in one study of 90 burn patients 1–4 years postburn injury, 10% of patients suffered from major depression, 10% from anxiety and 7% from PTSD [ 14 , 15 ]. Patients with severe burns also frequently suffer from chronic persistent pain, which can have a significant impact on patient well-being in daily life. In a survey of 358 patients with severe burns, 52% of respondents reported suffering ongoing burn-related pain, despite their injuries occurring an average of 11 years prior [ 16 ]. The associated physical scars that remain after the burn has healed also contribute significantly to the pain and mental distress experienced by these patients [ 17 ].
Population studies identify long-term health impacts of burn injury
While clinical observations of hypermetabolism and the effects of burn injury on mental health and chronic pain have been reported for a number of years, other long-term impacts of burn injury have only recently been uncovered. The Western Australian (WA) Population-based Burn Injury Project is the most comprehensive long-term study of burn injury to date. This project undertaken by researchers from the Fiona Wood Foundation used linked hospital morbidity and death data from Western Australia from all patients hospitalised for a first burn injury from 1980 to 2012 ( n = 30,997) and a randomly selected, frequency matched uninjured comparison cohort ( n = 127,000). The burn injuries included minor (49% of patients) and severe burns (4%) (the severity of the remaining 47% were unspecified), with a range of depths. The scope of this data has allowed the investigation of the long-term impact of burn from many different angles. The major findings of these studies are summarised below and in Fig. 1 , and the potential cause(s) of these correlations will be discussed in more detail later.
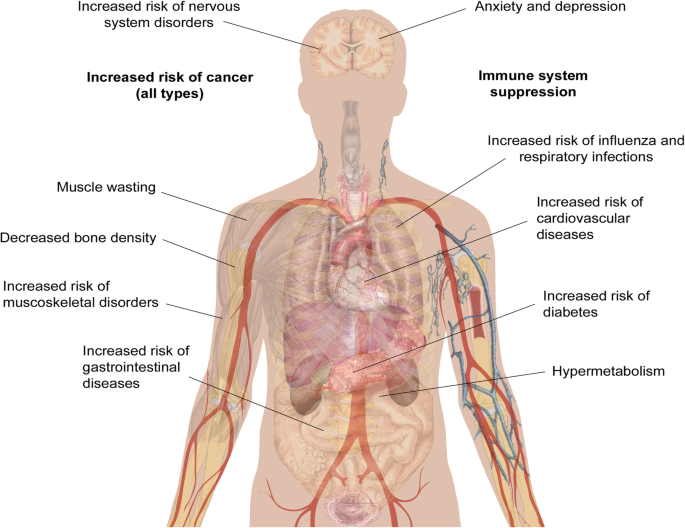
Long-term pathological effects of burn injury. Burn injury is associated with an increased risk of numerous secondary pathologies. The human body schematic is a copyright free image obtained from google images
Increased mortality
One of the first findings from the WA studies was that burn injury that requires hospitalisation results in higher long-term mortality rates for both children and adults. Paediatric burn patients had a 1.6 times (1.6×) higher age-adjusted mortality rate when compared to uninjured children over the 33-year study period, and this risk was increased in patients with severe burns compared to minor burns [ 18 ]. This increase in mortality was also seen in adolescents, young and middle-aged adults (aged 15–44 at the time of injury), who had a 1.8× higher mortality rate than observed in the uninjured cohort [ 19 ], and in older adults (45+), who had a 1.4× higher mortality rate [ 20 ]. Middle-aged and older adults who died during the follow-up period from the burn cohort were also statistically significantly younger than those in the uninjured cohort (43 vs 47 [ 19 ] and 76 vs 82 [ 20 ]). In support of these findings, another recent population study followed 1965 burn survivors and 8671 matched controls (mean age 44 years) for a median of 5 years. They found that the 5-year mortality was significantly increased among burn survivors, from 4% in controls to 11% in burn survivors [ 21 ].
Interestingly, comparing the effects of minor and severe burns in adults, minor burns were associated with a larger increase in mortality. This observation is supported by another hospital study that followed 365 critically ill adult burn patients who survived to hospital discharge found that patients with less severe burns had increased 5-year mortality compared to survivors with major burns [ 22 ]. A reason for this may be that individuals who survive major burns are strong physiologically, which provides a survival advantage post-hospital discharge. Another significant finding of these mortality studies is that in the adolescent, young and middle-aged adult cohort, females were found to have a higher increase in mortality compared to males [ 19 ]. The causes of death are varied and burn patients appear to be more at risk from deaths of all causes, including accidental and violent deaths [ 23 ].
Increased risk of disease
The population study revealed that burn patients frequently return to hospital for other conditions, indicating burn injury is associated with an increased risk of disease. These links are discussed below.
Cancer—all types
Duke et al. analyzed a sub-cohort of burn patients who were admitted to hospital between 1983 and 1987 (chosen as this group has the optimum follow-up time), which showed there was a 1.39× increase in cancer incidence in females compared to the matched uninjured cohort [ 24 ]. In this study, TBSA of the burn but not burn depth was associated with increased risk, with patients with severe burns found to have a 1.81× increased risk of cancer of all types. To strengthen this data, a second cohort of burn patients from Scotland was analysed. This cohort consisted of more than 38,000 patients admitted to hospital and followed up during the period from 1983 to 2008. This study showed a modest but significant increase in overall cancer risk for both genders and increase in cancer incidence in females, confirming the results from the WA study [ 25 ]. In this second paper, the types of cancer were also considered. Burn patients across all cohorts, genders and age groups had statistically significant increases in cancer of the buccal cavity, larynx, liver, respiratory tract and oesophagus. In addition, female burn survivors had higher incidences of breast and genital cancer.
Infectious disease
Burn injury increases susceptibility to infectious diseases, with higher rates of hospital admissions for infectious diseases found in both severe and minor burns, and the burn cohort was found to have a mortality rate 1.75× higher than the uninjured cohort [ 26 ]. Burn patients of all ages were found to have higher admission rates for influenza and viral pneumonia, bacterial pneumonia and other respiratory infections [ 27 ]. For these studies, patients with evidence of smoke inhalation of injury to the respiratory tract were removed. Admission rates for respiratory diseases were highest during the first 5 years post-burn; however, they remained elevated compared to the uninjured cohort for the duration of the 33-year study period.
Gastrointestinal disease
Boyd et al. and Stevenson et al. showed that both children and adults who experience a burn injury hospitalisation are at increased overall risk of developing gastrointestinal disease [ 28 , 29 ], which includes diseases of the oesophagus, stomach, duodenum and intestines, noninfective enteritis and colitis, and disorders of the gallbladder, biliary tract and pancreas. The paediatric burn cohort were found to have higher admission rates and spent longer in hospital than the uninjured cohort [ 28 ]. These data were similar in adults, who had more admissions and spent longer in hospital than the matched uninjured cohort [ 29 ]. This risk in adults was shown to decrease over time; however, rates of hospital admission did remain above the control group for the duration of the study period.
Negative impacts on the cardiovascular system
Paediatric burn patients had a higher rate of hospital admissions and days spent in hospital for circulatory diseases compared to the uninjured cohort [ 30 ]. Gender-specific analysis revealed this effect is more prominent in boys, with admissions remaining higher more than 20 years after the initial burn injury. A recent study in adolescent survivors of severe burns obtained during childhood found that burn injury is associated with myocardial fibrosis and reduced exercise tolerance [ 31 ]; however, more research is needed into non-severe burns and the mechanisms behind this increased risk in paediatric patients.
The increased risk of circulatory diseases was also seen in the adult cohort, with 1.46× more admissions and 2.9× more days spent in hospital [ 32 ]. More specifically, adult burn patients had a higher risk of ischaemic heart disease, heart failure and cerebrovascular disease, demonstrating burn injury has long-lasting systemic effects that impact on the heart and circulation [ 32 ]. These effects were also maintained in the sub-cohort of adult patients with non-severe burn injury [ 33 ].
Duke et al. found that the burn cohort had 2.21× more admissions for diabetes mellitus compared to the uninjured cohort. This increase was comparable amongst both genders and in both paediatric and adult patient cohorts and remained elevated for 5 years post-burn, after which there was no significant difference [ 34 ].
Musculoskeletal diseases
As discussed earlier, burn injury induces negative and sustained impacts on muscle and bone health. Randall et al. demonstrated that burn patients had nearly twice the hospital admission rate for musculoskeletal conditions compared to the uninjured cohort and spent longer in hospital, which included arthropathies, dorsopathies, osteopathies and soft tissue disorders [ 35 ]. Rates of fractures were also higher in the burn group, and this was significantly higher in females compared to males [ 36 ]. The admission rates for all musculoskeletal disorders remained high for the duration of the study and was elevated in all ages groups [ 37 ], demonstrating that both minor and severe burn injuries can affect muscle and bone integrity for at least 20 years post-injury. Holavanahalli et al. that used a self-report measure to investigate musculoskeletal impacts, patients who had sustained burn injuries an average of 17 years earlier reported joint pain and stiffness, problems walking and running and weak arms and hands [ 38 ]. The long-term impact of burn on musculoskeletal health has also been recently reviewed in depth [ 39 ].
Negative long-term impacts on the nervous system
Burn patients of all ages and genders included in the WA study were found to be at risk of nervous system conditions post-burn, with the burn cohort presenting at hospital more frequently than the uninjured cohort and spending 3.25 times the number of days in hospital [ 40 ]. Conditions with increased prevalence in burn patients include episodic and paroxysmal disorders such as epilepsy and migraine and nerve, nerve root and plexus disorders [ 41 ]. Hospital admissions for these conditions were significantly elevated during the first 5 years post-burn and were found to be sustained in paediatric patients for an extended period of 15 years post-burn.
Summary of population studies
The data from the WA population study revealed that burn injury has a wide range of significant long-lasting negative impacts on the overall health of patients and that these effects can also occur after a non-severe burn. This is an important finding and demonstrates the need for a greater understanding of the cellular and molecular effects of burn. The current knowledge regarding the effects of burn on long-term cellular function is discussed in detail in the next section.
Understanding the long-term impact on endocrine and immune system dysfunction in burn survivors
Burn injury has significant impacts on the endocrine and immune systems, and it is becoming evident that many of these changes are sustained long-term. To date, most long-term studies into these disruptions in burn patients have been done in severely burned paediatric patients. However, the results from the hospital data indicate that patients of all ages with non-severe burns also suffer from these dysfunctions [ 25 , 27 ]. Hormones are known to influence the immune system, and emerging evidence suggests that the numerous secondary pathologies associated with burn injury are the result of synergistic dysfunctions in these systems, with sustained changes in endocrine homeostasis contributing to long-term immune suppression that is characteristic of burn.
Endocrine changes
Following burn there is a rapid release of inflammatory cytokines, catecholamines and cortisol, initiating the hypermetabolic response and catabolic state. A recent study of severely burned children found that levels of urinary norepinephrine and cortisol remained significantly elevated 3 years post-burn [ 4 ]. These are stress hormones which inhibit lymphocyte proliferation as well as the activity of CD8+ T cells, natural killer (NK) cells and activated macrophages [ 42 ]. They also activate mast cells, leading to degranulation and the release of histamine, which stimulates the production of T helper type 2 (Th2) cytokine interleukin (IL)-10 and causes further vasodilation. Activation of the stress system suppresses the T helper type 1 (Th1) immune response (cellular immunity, generally pro-inflammatory) and favours a Th2 response (humoral immunity, generally anti-inflammatory). A healthy balance of Th1/Th2 responses is a hallmark of a normally functioning immune system and burn clearly disrupts this balance. Although the release of stress hormones is a normal response to trauma, a sustained increase in their expression as seen after burn can have detrimental effects and contribute to long-term immune suppression [ 43 ].
Other hormonal changes that occurred after burn in the paediatric study included a significant decrease of serum osteocalcin, parathyroid, insulin growth factor, insulin-like growth factor binding protein-3 and human growth hormone (GH) which were sustained at the 3-year time point and an increase in serum progesterone up to 2 years post-burn, indicative of long-term hormonal imbalance in these patients [ 4 ]. The more severe the burn, the greater the dysfunction; one study showed that children with burns > 80% TBSA had higher resting energy expenditure and urinary cortisol levels than patients with smaller burns [ 44 ]. Progesterone, which was shown to be increased in patients long after the initial healing process, exerts an immunosuppressive effect, reducing the activity of macrophages and NK cells and promoting a type 2 (Th2) immune response [ 45 ]. The Th2 shift may also be driven by the increase in catecholamines, which have been shown to inhibit Th1 and stimulate Th2 cytokine secretion [ 42 ]. GH, which is decreased after burn, also modulates the Th1/Th2 responses, with a mouse study looking at the effect of administering GH to burned mice showing that GH increases the production of Th1 cytokines interferon (IFN)-y and IL-2 [ 46 ]. It is evident that burn injury disrupts endocrine homeostasis and that this has long-term consequences for immune function.
Immune system changes
Compared to non-burn trauma, burn injury triggers a greater and more sustained inflammatory response [ 47 ]. Following an initial pro-inflammatory Th1 response where the release of cytokines such as tumor necrosis factor (TNF)-α and IL-6 activates the stress system [ 46 ], there is a rapid and sustained increase in IL-10 levels [ 42 ]. IL-10 is a Th2 cytokine that induces T regulatory cells and suppresses Th1 responses, leading to a deficient response to infection as a result of reduced cytotoxic T cell activity [ 48 , 49 ]. IL-10 has also been shown to stimulate the activation of mast cells, promote humoral immunity by differentiating B cells and inhibit macrophage activation and T cell proliferation [ 42 ]. In addition to IL-10, a more recent study showed that other Th2 cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF), TNF-α, IL-2 and IL-17 also remain elevated up to 3 years post-burn [ 4 ]. During the early immune response, there is also increased T regulatory cell activity [ 50 , 51 ], which is generally indicative of a suppressive immune phenotype.
While immune dysfunction has been recognised in the literature as a consequence of burn injury for more than 2 decades, the persistence of this dysfunction has only recently been investigated. A study investigating the effect of burn injury on immune function analysed cytokine release and immune cell populations in mouse models of burn and excision injuries at different time points [ 52 ]. Levels of inflammatory cytokines were measured in the serum of control, burn and excision groups taken on day 1, 3, 7 and 84 postburn injury, and whole blood was taken for analysis of immune cell populations. Comparison between the injury models confirmed that the response to a burn injury as opposed to an excision wound of the same size and depth is significantly different in both the innate and adaptive immune responses. In the acute phase response, the timing and profile of inflammatory cytokine production is significantly different between the two injury models. Increases in monocyte chemoattractant protein 1 (MCP1), MIP1α and MIP1β after burn injury lead to an increased number of monocytes at day 3 post-burn, demonstrating there are changes in immune cell populations early on. Changes in dendritic cell populations at day 28 are indicative of a reduced ability to prime T cells. At the long-term time point (day 84 postburn injury), burn-injured animals sustained a significant increase in IL-10 and decreased total numbers of white cells and lymphocytes in comparison to both control and excision wounded animals [ 52 ].
Studies in viral infection
Results from the population study highlighted a link between severe and non-severe burn injury and the subsequent development of respiratory infections. This included influenza and bacterial and viral pneumonia. To investigate this link, Fear et al. conducted a study in pre-clinical mouse models to examine the susceptibility to viral infection following a non-severe burn injury [ 27 ]. Mice exposed to the influenza virus 4 weeks postburn injury were shown to have increased viral titre in the bronchoalveolar lavage fluid and lung tissue. Analysis of the immune cell subsets showed that the CD8+ T cell proliferative response was diminished, and there were increased numbers of NK and natural killer T cells in the draining lymph nodes, indicating immune cell dysfunction [ 27 ]. In another recent murine study, it was found that burned mice were more susceptible to repeated infections which resulted in diminished innate immune cell function and increased anti-inflammatory environment [ 53 ].
Disruption of homeostasis and heart disease
Aside from the link with the development of infectious diseases, immune dysfunction in burns is likely to contribute to other secondary pathologies highlighted in the population studies. The excessive inflammatory response seen in the acute phase of burn healing could contribute to gastrointestinal damage, and changes in gut permeability after burns leads to increased risk of infection and endotoxin absorption [ 54 ]. Excessive hypermetabolism and immune changes after burn also have been shown to induce insulin resistance long-term, resulting in the heightened risk of diabetes associated with burn injury [ 34 , 55 ]. Inflammation, stress and hypermetabolism are likely to play a role in cardiac dysfunction after burn. Catecholamines, which are persistently elevated in burn, induce cardiac dysfunction by inducing Ca 2+ overload in cardiomyocytes and producing damaging oxidation products [ 56 ].
Data from the population studies demonstrated that burn patients have an increased risk of cancer [ 25 ]. The immune system plays an important role in cancer prevention, and therefore suppression of the immune system can lead to an increased risk of cancer [ 57 ]. Stress/hormone-induced immune suppression impairs the function of NK cells, which are critical to immune surveillance [ 58 ]. In addition, reduced activation of cytotoxic T cells reduces the chance of mutant cells being effectively removed following detection. In general, Th2 immunity is thought to enable tumour cells to evade immune surveillance more effectively [ 59 ]. Stress hormones also stimulate cell migration and invasion, suggesting a potential direct role in cancer growth and progression. For example, norepinephrine has been shown to increase the invasiveness of nasopharyngeal and ovarian cancer cells via the induction of matrix metalloproteinases which regulates angiogenesis [ 43 ]. High levels of histamine and mast cells have also been found in colorectal and breast cancer tissues [ 60 ].
Cancer risk and gender dimorphism postburn injury
Acute and long-term outcomes for burn patients are impacted by gender. In non-burn trauma, females generally have lower mortality and a lower risk of complications such as sepsis and organ failure as a result of more efficient innate and adaptive immune responses [ 61 ]. However, in burn, this is reversed, with males showing a lower risk of secondary complications and having an overall better prognosis [ 62 , 63 ]. As mentioned previously, female burn patients have a heightened risk of cancer; however, the WA population study found no difference in cancer incidence between male burn patients and uninjured controls [ 24 ]. This is a significant finding, especially considering males generally face a higher risk of cancer [ 64 ]. It is well known that the immune response is gender dimorphic, and sex hormones are likely to play an important role. Understanding this dimorphism and how it impacts outcomes after burn injury may provide vital clues to the mechanisms underlying the increase in cancer susceptibility in females.
A study in infected ovariectomised female mice found that they had a higher survival rate than control mice, indicating a role for oestrogen in immune function [ 65 ]. However, the effect of oestrogen on immune function is complex and not fully understood. Oestrogen receptors are found on numerous immune cells including B and T cells, NK cells, monocytes and macrophages [ 66 ]. In pregnancy, immune responses are altered to prevent foetal rejection, a process modulated by sex hormones including oestrogen and progesterone resulting in reduced activity of macrophages, NK cells and Th1 cells and a higher activity of T regulatory cells [ 67 ]. This is similar to the immune phenotype seen after burn injury. Pregnant women are also more susceptible to infectious diseases such as influenza [ 68 ]. Bird et al. have shown that physiological levels of oestrogen stimulate the immune response, while high levels of oestrogen such as those found in pregnancy have the opposite effect, causing immunosuppression [ 66 ]. Burn injury causes an increase in oestrogen levels in mice, and it has been hypothesised that this results in levels resembling pregnancy (immunosuppressive), while levels in male mice reach the levels of uninjured females (immunostimulatory) [ 69 ]. These results provide evidence for a likely role of oestrogen in gender dimorphism in burn injury.
Aside from oestrogen, other hormones may also play a role. Prostaglandin E2, which plays a role in mediating the cellular immune response by inhibiting T cell proliferation and macrophage antigen presentation, was shown to be increased in burn-injured female but not male mice 10 days post-injury [ 70 ]. Another factor that could play a role in burn injury gender dimorphism is mast cells. Mast cells are regarded as effector cells of allergic reactions, stimulating a Th2-type response. Mackey et al. showed that gene expression in mast cells is significantly different between males and females, with more than 8000 differentially expressed genes [ 71 ]. In mice, female mast cells were shown to possess an increased capacity for mediator synthesis and contained higher levels of histamine, tryptase, and chymase in their granules, which are released during times of stress and cause vasodilation, increased vascular permeability and increased production of reactive oxygen species [ 71 ]. The increased activation of mast cells in females following burn could contribute to poorer outcomes in both the short and long term.
In summary, burn injury is associated with a rapid influx of stress hormones and inflammatory factors resulting in a hyperactive acute innate response, followed by a switch to a Th2-type immune response and subsequent immune suppression that is sustained long-term (Fig. 2 ). We hypothesise that sustained immune suppression and disruption of homeostasis following burn injury underpins the development of numerous secondary pathologies. Stress arising from the burn and other factors such as pain may exacerbate this immune suppression [ 16 ], so better management of burn injury in the clinic could already be improving the impact of burn on immunity. However, more research needs to be done to fully understand the impact of burn on the immune system and the mechanisms that underpin the persistence of immune dysfunction, as well as whether there are specific patient groups at risk. Future studies will then enable the development of preventative treatments that could ideally be administered during the acute healing phase of burn care in order to reduce the risk of secondary complications. This will be beneficial to both the individual and the community by increasing the quality of life of burn survivors and reducing the burden on the health system and families of patients. Considering many burn patients are children and may face these complications relatively early in life, understanding burn injury as a chronic disease is an important step towards better burn care. In addition, the strong links between non-severe burn and secondary complications highlight the need for more in-depth studies on non-severe burns as opposed to severe burns, which to date have received more focus in the research community.
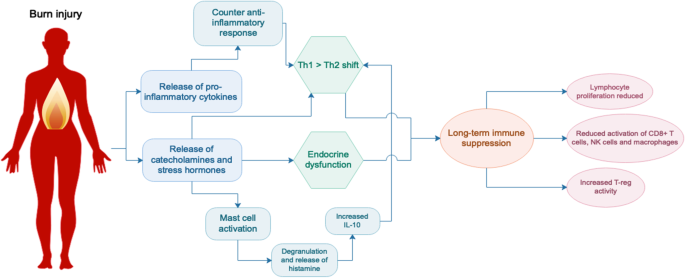
Endocrine and immune system changes following burn injury. Burn injury triggers the immediate release of pro-inflammatory cytokines, catecholamines and stress hormones, followed by a counter anti-inflammatory response and a shift towards a T helper type 2 (Th2) immune environment. Activation of mast cells contributes to this phenotype which is thought to be sustained, resulting in long-term suppression of the immune system. IL interleukin, NK natural killer
Conclusions
While acute clinical treatment for burns has improved significantly over the past few decades resulting in significantly higher rates of survival, there is increasing evidence of lifelong impacts of burn injury. Recent findings suggest burn injury can be considered a chronic disease, with secondary morbidity most likely linked to sustained changes to immune function. Future studies to understand the mechanisms involved will be critical to change clinical treatment pathways and reduce the long-term burden of burn injury for patients.
Availability of data and materials
Abbreviations.
Growth hormone
Granulocyte-macrophage colony-stimulating factor
Monocyte chemoattractant protein 1
Macrophage inflammatory protein
Natural killer
Natural killer T
Post-traumatic stress disorder
Total body surface area
Tumour necrosis factor alpha
Western Australia
WHO, Burns fact sheet. 2018.
Google Scholar
Porter C, Tompkins RG, Finnerty CC, Sidossis LS, Suman OE, Herndon DN. The metabolic stress response to burn trauma: current understanding and therapies. Lancet. 2016;388(10052):1417–26.
Article PubMed PubMed Central Google Scholar
Clark A, Imran J, Madni T, Wolf SE. Nutrition and metabolism in burn patients. Burns Trauma. 2017;5:11.
Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One. 2011;6(7):e21245.
Article CAS PubMed PubMed Central Google Scholar
Pereira C, Murphy K, Jeschke M, Herndon DN. Post burn muscle wasting and the effects of treatments. Int J Biochem Cell Biol. 2005;37(10):1948–61.
Article CAS PubMed Google Scholar
Klein GL, Herndon DN, Langman CB, Rutan TC, Young WE, Pembleton G, et al. Long-term reduction in bone mass after severe burn injury in children. J Pediatr. 1995;126(2):252–6.
Chao T, Herndon DN, Porter C, Chondronikola M, Chaidemenou A, Abdelrahman DR, et al. Skeletal muscle protein breakdown remains elevated in pediatric burn survivors up to one-year post-injurY. Shock. 2015;44(5):397–401.
Porter C, Herndon DN, Borsheim E, Bhattarai N, Chao T, Reidy PT, et al. Long-term skeletal muscle mitochondrial dysfunction is associated with hypermetabolism in severely burned children. J Burn Care Res. 2016;37(1):53–63.
St-Pierre DM, Choiniere M, Forget R, Garrel DR. Muscle strength in individuals with healed burns. Arch Phys Med Rehabil. 1998;79(2):155–61.
Klein GL. Disruption of bone and skeletal muscle in severe burns. Bone Res. 2015;3:15002.
Ehrlich HP, Desmouliere A, Diegelmann RF, Cohen IK, Compton CC, Garner WL, et al. Morphological and immunochemical differences between keloid and hypertrophic scar. Am J Pathol. 1994;145(1):105–13.
CAS PubMed PubMed Central Google Scholar
Finnerty CC, Jeschke MG, Branski LK, Barret JP, Dziewulski P, Herndon DN. Hypertrophic scarring: the greatest unmet challenge after burn injury. Lancet. 2016;388(10052):1427–36.
Ehde DM, Patterson DR, Wiechman SA, Wilson LG. Post-traumatic stress symptoms and distress 1 year after burn injury. J Burn Care Rehabil. 2000;21(2):105–11.
Ter Smitten MH, de Graaf R, Van Loey NE. Prevalence and co-morbidity of psychiatric disorders 1-4 years after burn. Burns. 2011;37(5):753–61.
Article PubMed Google Scholar
Duke JM, Randall SM, Boyd JH, Wood FM, Fear MW, Rea S. A population-based retrospective cohort study to assess the mental health of patients after a non-intentional burn compared with uninjured people. Burns. 2018;44(6):1417–26.
Dauber A, Osgood PF, Breslau AJ, Vernon HL, Carr DB. Chronic persistent pain after severe burns: a survey of 358 burn survivors. Pain Med. 2002;3(1):6–17.
Dalal PK, Saha R, Agarwal M. Psychiatric aspects of burn. Indian J Plast Surg. 2010;43(Suppl):S136–42.
Duke JM, Rea S, Boyd JH, Randall SM, Wood FM. Mortality after burn injury in children: a 33-year population-based study. Pediatrics. 2015;135(4):e903–10.
Duke JM, Boyd JH, Randall SM, Wood FM. Long term mortality in a population-based cohort of adolescents, and young and middle-aged adults with burn injury in Western Australia: A 33-year study. Accid Anal Prev. 2015;85:118–24.
Duke JM, Boyd JH, Rea S, Randall SM, Wood FM. Long-term mortality among older adults with burn injury: a population-based study in Australia. Bull World Health Organ. 2015;93(6):400–6.
Mason SA, Nathens AB, Byrne JP, Diong C, Fowler RA, Karanicolas PJ, et al. Increased rate of long-term mortality among burn survivors: a population-based matched cohort study. Ann Surg. 2018.
Nitzschke S, Offodile AC 2nd, Cauley RP, Frankel JE, Beam A, Elias KM, et al. Long term mortality in critically ill burn survivors. Burns. 2017;43(6):1155–62.
Onarheim H, Vindenes HA. High risk for accidental death in previously burn-injured adults. Burns. 2005;31(3):297–301.
Duke J, Rea S, Semmens J, Edgar DW, Wood F. Burn and cancer risk: a state-wide longitudinal analysis. Burns. 2012;38(3):340–7.
Duke JM, Bauer J, Fear MW, Rea S, Wood FM, Boyd J. Burn injury, gender and cancer risk: population-based cohort study using data from Scotland and Western Australia. BMJ Open. 2014;4(1):e003845.
Duke JM, Randall SM, Wood FM, Boyd JH, Fear MW. burns and long-term infectious disease morbidity: a population-based study. Burns. 2017;43(2):273–81.
Fear VS, Boyd JH, Rea S, Wood FM, Duke JM, Fear MW. Burn injury leads to increased long-term susceptibility to respiratory infection in both mouse models and population studies. PLoS One. 2017;12(1):e0169302.
Boyd JH, Wood FM, Randall SM, Fear MW, Rea S, Duke JM. Effects of pediatric burns on gastrointestinal diseases: a population-based study. J Burn Care Res. 2017;38(2):125–33.
Stevenson AW, Randall SM, Boyd JH, Wood FM, Fear MW, Duke JM. Burn leads to long-term elevated admissions to hospital for gastrointestinal disease in a West Australian population based study. Burns. 2017;43(3):665–73.
Duke JM, Randall SM, Fear MW, Boyd JH, Rea S, Wood FM. Long-term effects of pediatric burns on the circulatory system. Pediatrics. 2015;136(5):e1323–30.
Hundeshagen G, Herndon DN, Clayton RP, Wurzer P, McQuitty A, Jennings K, et al. Long-term effect of critical illness after severe paediatric burn injury on cardiac function in adolescent survivors: an observational study. Lancet Child Adolesc Health. 2017;1(4):293–301.
Duke JM, Randall SM, Fear MW, Boyd JH, Rea S, Wood FM. Understanding the long-term impacts of burn on the cardiovascular system. Burns. 2016;42(2):366–74.
O'Halloran E, Shah A, Dembo L, Hool L, Viola H, Grey C, et al. The impact of non-severe burn injury on cardiac function and long-term cardiovascular pathology. Sci Rep. 2016;6:34650.
Duke JM, Randall SM, Fear MW, Boyd JH, O'Halloran E, Rea S, et al. Increased admissions for diabetes mellitus after burn. Burns. 2016;42(8):1734–9.
Randall SM, Fear MW, Wood FM, Rea S, Boyd JH, Duke JM. Long-term musculoskeletal morbidity after adult burn injury: a population-based cohort study. BMJ Open. 2015;5(9):e009395.
Duke JM, Randall SM, Fear MW, Boyd JH, Wood FM. Fracture admissions after burns: a retrospective longitudinal study. Burns. 2017;43(6):1175–82.
Duke JM, Randall SM, Fear MW, Boyd JH, Rea S, Wood FM. Increased admissions for musculoskeletal diseases after burns sustained during childhood and adolescence. Burns. 2015;41(8):1674–82.
Holavanahalli RK, Helm PA, Kowalske KJ. Long-term outcomes in patients surviving large burns: the musculoskeletal system. J Burn Care Res. 2016;37(4):243–54.
Polychronopoulou E, Herndon DN, Porter C. The long-term impact of severe burn trauma on musculoskeletal health. J Burn Care Res. 2018;39(6):869–80.
Vetrichevvel TP, Randall SM, Fear MW, Wood FM, Boyd JH, Duke JM. Burn injury and long-term nervous system morbidity: a population-based cohort study. BMJ Open. 2016;6(9):e012668.
Duke JM, Randall SM, Fear MW, Boyd JH, Rea S, Wood FM. Burn induced nervous system morbidity among burn and non-burn trauma patients compared with non-injured people. Burns. 2019.
Elenkov IJ, Chrousos GP. Stress hormones, Th1/Th2 patterns, pro/anti-inflammatory cytokines and susceptibility to disease. Trends Endocrinol Metab. 1999;10(9):359–68.
Webster Marketon JI, Glaser R. Stress hormones and immune function. Cell Immunol. 2008;252(1-2):16–26.
Jeschke MG, Mlcak RP, Finnerty CC, Norbury WB, Gauglitz GG, Kulp GA, et al. Burn size determines the inflammatory and hypermetabolic response. Crit Care. 2007;11(4):R90.
Al-Tarrah K, Moiemen N, Lord JM. The influence of sex steroid hormones on the response to trauma and burn injury. Burns Trauma. 2017;5:29.
Takagi K, Suzuki F, Barrow RE, Wolf SE, Herndon DN. Recombinant human growth hormone modulates Th1 and Th2 cytokine response in burned mice. Ann Surg. 1998;228(1):106–11.
Mace JE, Park MS, Mora AG, Chung KK, Martini W, White CE, et al. Differential expression of the immunoinflammatory response in trauma patients: burn vs. non-burn. Burns. 2012;38(4):599–606.
O'Sullivan ST, O'Connor TP. Immunosuppression following thermal injury: the pathogenesis of immunodysfunction. Br J Plast Surg. 1997;50(8):615–23.
Hunt JP, Hunter CT, Brownstein MR, Giannopoulos A, Hultman CS, deSerres S, et al. The effector component of the cytotoxic T-lymphocyte response has a biphasic pattern after burn injury. J Surg Res. 1998;80(2):243–51.
MacConmara MP, Maung AA, Fujimi S, McKenna AM, Delisle A, Lapchak PH, et al. Increased CD4+ CD25+ T regulatory cell activity in trauma patients depresses protective Th1 immunity. Ann Surg. 2006;244(4):514–23.
PubMed PubMed Central Google Scholar
Hanschen M, Tajima G, O'Leary F, Ikeda K, Lederer JA. Injury induces early activation of T-cell receptor signaling pathways in CD4+ regulatory T cells. Shock. 2011;35(3):252–7.
Valvis SM, Waithman J, Wood FM, Fear MW, Fear VS. The immune response to skin trauma is dependent on the etiology of injury in a mouse model of burn and excision. J Invest Dermatol. 2015;135(8):2119–28.
Kartchner LB, Gode CJ, Dunn JLM, Glenn LI, Duncan DN, Wolfgang MC, et al. One-hit wonder: late after burn injury, granulocytes can clear one bacterial infection but cannot control a subsequent infection. Burns. 2019;45(3):627–40.
Ziegler TR, Smith RJ, O'Dwyer ST, Demling RH, Wilmore DW. Increased intestinal permeability associated with infection in burn patients. Arch Surg. 1988;123(11):1313–9.
Gauglitz GG, Herndon DN, Kulp GA, Meyer WJ 3rd, Jeschke MG. Abnormal insulin sensitivity persists up to three years in pediatric patients post-burn. J Clin Endocrinol Metab. 2009;94(5):1656–64.
Adameova A, Abdellatif Y, Dhalla NS. Role of the excessive amounts of circulating catecholamines and glucocorticoids in stress-induced heart disease. Can J Physiol Pharmacol. 2009;87(7):493–514.
Finn OJ. Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol. 2012;23(Suppl 8):viii6–9.
Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5(10):617–25.
Moreno-Smith M, Lutgendorf SK, Sood AK. Impact of stress on cancer metastasis. Future Oncol. 2010;6(12):1863–81.
Elenkov IJ, Webster E, Papanicolaou DA, Fleisher TA, Chrousos GP, Wilder RL. Histamine potently suppresses human IL-12 and stimulates IL-10 production via H2 receptors. J Immunol. 1998;161(5):2586–93.
CAS PubMed Google Scholar
Haider AH, Crompton JG, Oyetunji T, Stevens KA, Efron DT, Kieninger AN, et al. Females have fewer complications and lower mortality following trauma than similarly injured males: a risk adjusted analysis of adults in the National Trauma Data Bank. Surgery. 2009;146(2):308–15.
Karimi K, Faraklas I, Lewis G, Ha D, Walker B, Zhai Y, et al. Increased mortality in women: sex differences in burn outcomes. Burns Trauma. 2017;5:18.
Wasiak J, Lee SJ, Paul E, Shen A, Tan H, Cleland H, et al. Female patients display poorer burn-specific quality of life 12 months after a burn injury. Injury. 2017;48(1):87–93.
Dorak MT, Karpuzoglu E. Gender differences in cancer susceptibility: an inadequately addressed issue. Front Genet. 2012;3:268.
Plackett TP, Deburghraeve CR, Palmer JL, Gamelli RL, Kovacs EJ. Effects of estrogen on bacterial clearance and neutrophil response after combined burn injury and wound infection. J Burn Care Res. 2016;37(5):328–33.
Bird MD, Karavitis J, Kovacs EJ. Sex differences and estrogen modulation of the cellular immune response after injury. Cell Immunol. 2008;252(1-2):57–67.
Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav. 2012;62(3):263–71.
Kourtis AP, Read JS, Jamieson DJ. Pregnancy and infection. N Engl J Med. 2014;370(23):2211–8.
Gregory MS, Duffner LA, Faunce DE, Kovacs EJ. Estrogen mediates the sex difference in post-burn immunosuppression. J Endocrinol. 2000;164(2):129–38.
Gregory MS, Duffner LA, Hahn EL, Tai HH, Faunce DE, Kovacs EJ. Differential production of prostaglandin E(2) in male and female mice subjected to thermal injury contributes to the gender difference in immune function: possible role for 15-hydroxyprostaglandin dehydrogenase. Cell Immunol. 2000;205(2):94–102.
Mackey E, Ayyadurai S, Pohl CS, D’ Costa S, Li Y, Moeser AJ. Sexual dimorphism in the mast cell transcriptome and the pathophysiological responses to immunological and psychological stress. Biol Sex Differ. 2016;7:60.
Download references
Acknowledgements
Supported by the Fiona Wood Foundation
Author information
Authors and affiliations.
Telethon Kids Institute, University of Western Australia, Northern Entrance, Perth Children’s Hospital, 15 Hospital Ave, Nedlands, WA, 6009, Australia
Lucy W. Barrett, Vanessa S. Fear & Jason C. Waithman
Institute for Respiratory Health, Ground Floor, E Block Sir Charles Gairdner Hospital, Hospital Avenue, Nedlands, WA, 6009, Australia
Lucy W. Barrett
Fiona Wood Foundation, Fiona Stanley Hospital, MNH (B) Main Hospital, CD 15, Level 4, Burns Unit, 102-118 Murdoch Drive, Murdoch, WA, 6150, Australia
Fiona M. Wood & Mark W. Fear
Burns Service of Western Australia, WA Department of Health, Nedlands, WA, 6009, Australia
Fiona M. Wood
Burn injury research unit, School of Biomedical Sciences, University of Western Australia, Crawley, WA, 6009, Australia
You can also search for this author in PubMed Google Scholar
Contributions
LB drafted the manuscript with input from VF, JW, FW and MF in terms of the overall concept and scope of the review. VF and MF revised the manuscript in preparation for submission. All authors read and approved the final manuscript for submission.
Corresponding author
Correspondence to Lucy W. Barrett .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Barrett, L.W., Fear, V.S., Waithman, J.C. et al. Understanding acute burn injury as a chronic disease. Burn Trauma 7 , 23 (2019). https://doi.org/10.1186/s41038-019-0163-2
Download citation
Received : 11 April 2019
Accepted : 18 June 2019
Published : 16 September 2019
DOI : https://doi.org/10.1186/s41038-019-0163-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Immune system
- Endocrine system
- Homeostasis
- Patient care
- Chronic disease
Burns & Trauma
ISSN: 2321-3876
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 30 April 2021
Development of a framework for managing severe burns through a 17-year retrospective analysis of burn epidemiology and outcomes
- Ling Chen 1 na1 ,
- Xiaochong He 2 na1 ,
- Jishu Xian 3 ,
- Jianmei Liao 3 ,
- Xuanji Chen 4 ,
- Yue Luo 5 ,
- Zonghua Wang 2 na1 &
- Ning Li 5 na1
Scientific Reports volume 11 , Article number: 9374 ( 2021 ) Cite this article
1451 Accesses
12 Citations
1 Altmetric
Metrics details
- Disease prevention
Burns are one of the most common injuries in daily life for all ages of population. This study was to investigate the epidemiology and outcomes among burn patients in one of the largest burn centers in the southwest of China. The study was performed at the Institute of Burn Research in the first affiliated with the Army Medical University (AMU). A total of 17,939 burn patients were included in this retrospective study. Information regarding burn epidemiology and outcomes in 17 years were collected, calculated and compared. The age ranged from 257 days to 95 years old. Scalding and flame were the two most common causes to burn injuries, comprising of 91.96% in total. Limbs, head/face/neck, and trunk were the most frequently occurred burn sites, with the number and the percent of 12,324 (68.70%), 7989 (44.53%), and 7771 (43.32%), respectively. The average total body surface area (TBSA) was 13.64 ± 16.83% (median 8%) with a range of 0.1–100%. A total of 874 (4.9%) patients had TBSA > 50%. The presence of a burn with an inhalation injury was confirmed in 543 patients (3.03%). The average LOS was 32.11 ± 65.72 days (median: 17 days). Eventually, the retrospective analysis resulted in the development of a burn management continuum used for developing strategies to prevent and manage severe burns. The annual number of burn injuries has kept decreasing, which was partially attributed to the increased awareness and education of burn prevention and the improved burn-preventative circumstances. However, the burn severity and the economic burden were still in a high level. And the gender difference and age difference should be considered when making individualized interventions and rehabilitative treatments.
Similar content being viewed by others
Cohort analysis of 50% lethal area (LA50) and associating factors in burn patients based on quality improvements and health policies
Reza Shahriarirad, Ramin Shekouhi, … Abdolkhalegh Keshavarzi

Epidemiology and mortality in patients hospitalized for burns in Catalonia, Spain
L. Abarca, P. Guilabert, … Maria J. Colomina

Burn injury
Marc G. Jeschke, Margriet E. van Baar, … Sarvesh Logsetty
Introduction
Burns are one of the most common injuries primarily resulted from heat, but can also be due to radiation, electricity, and chemicals 1 . Burns can be minor or life-threatening medical problems. In the European Union, burns are one of the three most common “unintentional fatal injuries”, together with poisoning and drowning (34.1%) 2 . According to the American Burn Association, it is reported that one in 1442 Americans died from injuries exposure to fire, flames, or smoke 3 .
In addition to enormous death of more than 250,000 annually, severe burn injuries can lead to unavoidable complications including scarring, disfigurement, physical disabilities, and require long-term rehabilitation, reconstruction and anti-scar therapy 4 . These physical problems are great challenges to maintain psychological health for patients and also cause major economic burden on family and society. Previous studies have revealed that burn patients experienced burn-associated abuse, stigma and rejection, resulting in high occurrence of mental health disorders including post-traumatic stress disorder (30%), anxiety (13–47%) and depression (23–61%) 5 . Moreover, burns are also among the most expensive traumatic injuries, because of long hospitalization and rehabilitation, and costly wound and scar treatment 2 .
The South-East Asian region including China is one of the three WHO regions with the greatest burden of injuries, accounting for almost half of the worldwide burn deaths 6 , 7 , 8 . Therefore, more studies are needed to investigate the epidemiology, etiology and outcomes of burn populations in this region in order to further improve the effects of preventive measures to mortality and deformities. As Peck et al. mentioned 9 , a detailed understanding of epidemiology is essential in order to take steps to prevent injuries. However, most of the previous studies on burn epidemiology and outcomes are limited to vulnerable age groups such as children 10 , 11 , 12 , 13 , 14 and elderly population or a specific type of burn such as thermal burns or chemical burns 15 , 16 . The adults between the age of 18 and 60 years old have not been fully studied although they occupied the largest number of burn patients. Besides, their post-burn recovery and rehabilitation becomes especially important considering the primary responsibilities they have taken to society and family. Most of them are backbone of the family regarding physical, emotional and economic support. This retrospective study has therefore been performed to investigate the burn epidemiology and outcomes in the southwest of China over a long-time span of 17 years (2002–2019), which in particular analyzed the epidemiology and outcomes among young and middle-aged adults between 18 and 40 years old.
Materials and methods
Study setting.
The data were collected in the Institute of Burn Research (IBR), located in the first affiliated with the Army Medical University (AMU). This burn specialized center is one of the earliest department for burns in China and is also one of the largest burn centers across the world with 150 inpatient beds (including 18 ICU beds). An estimated of 1300 burn patients are admitted to the center annually. The year of 2018 was the 60th anniversary of this center; and a lot of achievements on burn treatment has been reached including the Chinese Rule of Nine, fluid resuscitation protocol, experience in inhalation injury, wound treatment strategies, prevention and treatment of burn infections 17 .
Another significant achievement of this center was to develop the first and the biggest burn database in the mainland of China. This database could directly access to the hospital information system (HIS), the laboratory information management system (LIS) and the electronic medical record (EMR). This database was established to provide a comprehensive and convenient access to the information of burn inpatients and outpatients to clinical professionals and researchers (with access permission) for the purpose of case review and therefore promoting clinical practice, research and burn prevention.
Data extraction
We reviewed all 18,138 cases with the diagnosis of burn admitted to the Institute of Burn Research between January 2002 and December 2019. Fifty-one duplicates were removed according to name, gender and birth date; and another 148 records were excluded due to data missing. Eventually, a total of 17,939 cases were included for final analysis. Demographic and clinical information of burn patients were collected including age, gender, burn size and depth, burn sites, inhalation injury and the outcomes (including length of stay, length of stay in BICU, total cost and count of death).
All methods were performed in accordance the Declaration of Helsinki. This study was a retrospective analysis, and no individual information that may identify the patients were not reported; therefore, informed consent from the patients were not required. The need for informed consent was waived and the study protocol was approved by the medical ethics committee in southwest hospital affiliated to the Third Military Medical University (Army Medical University) (approval number: KY201904).
Statistical analysis
The data were primarily entered and processed using Microsoft Excel 2010 (Microsoft Corporation). The data analysis was performed and the figures were drawn using the GraphPad Prism 5 (GraphPad Software Inc, San Diego, CA). The software of Statistical Package for the Social Sciences 21 (SPSS Inc, Chicago, IL) was adopted to analyse the descriptive statistics and headcounts. Pearson chi-square test or Fisher’s exact test was used to compare the patient numbers in different groups. T-tests or one-way ANOVA were used to compare two or more quantitative variables (e.g., LOS, LOS in ICU, cost, ABSI and BI score), and Scheffe’s test was performed as a post-hoc test in the comparison of two groups. The Abbreviated Burn Severity Index (ABSI) 12 and the index of burn severity (Burn Index, BI) were calculated as follows: ABSI = Gender (female = 1, male = 0) + Age (0–20 = 1, 21–40 = 2, 41–60 = 3, 61–80 = 4, 80–100 = 5) + Inhalation injury (yes = 1, no = 0) + Full-thickness burns (yes = 1, no = 0) + Total body surface area (TBSA) (1–10% = 1, 11–20% = 2, 21–30% = 3, 31–0% = 4, 41–50% = 5, 51–60% = 6, 61–70% = 7, 71–80% = 8, 81–90% = 9, 91–100% = 10); BI = TBSA of the full-thickness burn% + 1/2 TBSA of the deep partial-thickness burn% 12 .
Multiple linear regression of approach (entry: P = 0.05; removal: P = 0.10) was used to examine the factors that interpreting the medical cost. Multiple logistic regression (entry: P = 0.05; removal: P = 0.10) was used to screen the factors contributing to mortality. P values < 0.05 were considered significant.
Ethics approval and consent to participate
Our study has received ethics approval from southwest hospital affiliated to the Third Military Medical Universitiy (Army Medical University) (approval number: KY201904).
Demographic characteristics
Table 1 and Figure 1 illustrated the general characteristics of the burn patients investigated in this study. The age of the 17,939 burn patients ranged from 257 days to 95 years old. The patients under the age of 18 years old accounted for the highest proportion of patients with a total number of 7192 (40.1%) and followed by the number of young and middle-aged adults between 18 and 40 years old accounting for nearly a third of all the patients (5383, 30.0%).
Patient demographic distribution. ( A ) Distribution of different age groups of patients by months. ( B ) Distribution of different age groups of patients by years. ( C ) Distribution of the number of the total patients by years. ( D ) The distribution of the ratio of male to female by years.
Distribution of burns
Seasonality.
Figure 1 A showed a change of burn incidence over month in different age groups. For the elderly patients over the age of 61 years old, the incidence of burns kept at an average level in every month. For adult patients under the age of 60 years old, the burns that most frequently occurred were during summer season from June to September while the occurrence stayed in a low level during autumn season from October to December; and the number increased to the peak in July and reduced to the nadir in February. For pediatric patients, burns occurred most frequently during Chinese spring festival of January and February while in November it showed the lowest occurrence of burns.
A similar change trend was indicated in the Fig. 1 B for the age groups of pediatric patients and adult patients under the age of 60 years old, increasing first to the peak in 2011 and then kept dropping year by year. However, for the elderly burn patients, the number has kept the trend of increase as the year went on. As the Fig. 1 C shown, the total number of burn patients kept increasing year by year until the year of 2013 and reached at a high level in the year of 2011 (n = 1496), 2012 (n = 1226), 2013 (n = 1423), and 2014 (n = 1323). Since then, the number began to drop, and the decrease kept going on, and reached at the nadir in 2017 (n = 651).
The ratios of males to females presented a significant decline across the seventeen years and kept dropping from 2.59:1 in 2002 to 1.68:1 in 2017, with a slight increase after that in 2018 and 2019 (Fig. 1 D). Moreover, among the adult patients between the age of 18 and 40 years old, the number of male patients was far larger than that of female patients with an estimated ratio of 3.22:1 (Table 1 ).
Age, gender and season difference on burn causes
Scalding was the leading cause of burns, accounting for nearly a half of all cases (48.0%), and the second most common cause was flame, accounting for 44.0% of all patients. Electrical, chemical, and other types of burns accounted for 6.8%, 0.2%, and 1.1% of all the patients, respectively (Table 1 ).
Scalding and flame were indicated as the two most common causes of burns in all groups (Fig. 2 A). However, the leading cause of burns was significantly different in pediatric patients and adult patients. The cause of scalding was in the first place among pediatric patients, with the number accounting for almost three fourth of all the patients (5393, 75.0%) (Figs. 2 B, 3 B); In comparison, flame was the most common cause of burns in adult patients under the age of 60 years old (5751, 59.61%) (Figs. 2 C, 3 B).
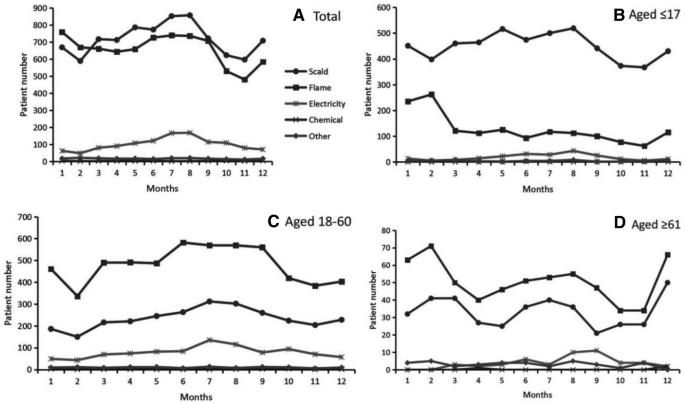
Distribution of burn causes by months in different age groups. ( A ) In total patients. ( B ) In patients under the age of 18 years old. ( C ) In patients aged between 18 and 60 years old. ( D ) In patients above the age of 60 years old.
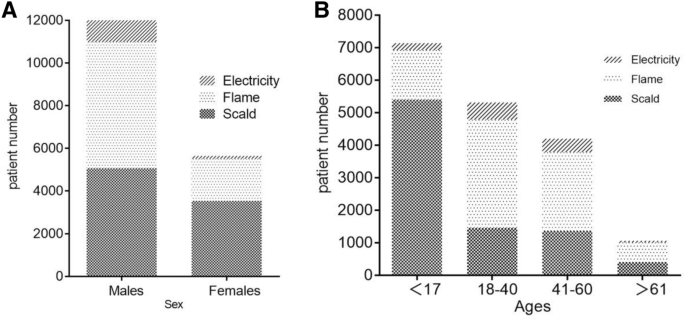
Etiology analysis. ( A ) Distribution of etiology by ages. ( B ) Distribution of etiology by gender.
It was also showed the gender difference on the causes of burns. The incidence of burns caused by flames was significantly larger than the burns caused by scalding among male patients (48.24% versus 41.08%, P < 0.001); while the burn caused by scalding was significantly larger than that caused by flames among female patients (61.04% versus 33.41%, P < 0.001) (Fig. 3 A).
As shown in the Fig. 2 B, flame burns in pediatric patients occurred most frequently in January and February. The incidence of burns caused by scalding stayed in a high level across spring and summer seasons from March to August compared to a low level in the autumn season from October to November. Different from the pediatric patients, the number of burns caused by flame and scalding in adult patients was both reaching at the nadir in February (Fig. 2 C). And the number of scalding burns reached at peak in July while the flame burns stayed in a high level across spring and summer seasons from March to September. Like the pediatric patients, the incidence of flame burns in the elderly patients aged over 60 years old reached at the peak in February and reached at the nadir in October (Fig. 2 D). Regarding the scalding burns, there were three periods with high level of incidence: from February to March, from June to August, and the month of December.
As shown in Table 1 , Limbs, head/face/neck, and trunk were the most frequently occurred burn sites, with the number and the percent of 12,324 (68.7%), 7989 (44.5%), and 7771 (43.3%), respectively. The top three burn sites in all adult patients aged 18 years old and above were in sequence of limbs (7554, 70.3%), head/face/neck (5104, 47.5%) and truck (4183, 38.9%); the top three burn sites in pediatric patients were in sequence of limbs (4770, 66.3%), trunk (3588, 49.9%) and head/face/neck (2885, 40.1%).
Burn severity and cure rates
The average total body surface area (TBSA) was 13.64 ± 16.83% (median 8%) with a range of 0.1–100%. Patients with TBSA of 0–10% and 11–20% accounted for 11296 (62.97%) and 3496 (19.49%) of all cases, respectively. A total of 874 (4.87%) patients had TBSA > 50%. With the improvement of burn treatment, the cure rates of burn patients have continuously increased in the last 17 years. As shown in Table 2 , the results indicated great improvements in treatment outcomes in our burn center. The cure rates among patients with ≤ 90% TBSA remained a high level from 78.30% to 99.59%. For severe burns with ≥ 91% TBSA, the cure rates have dramatically increased from 23.08% in 2002–2005 to 76.47% in 2016–2020.
Table 3 presented the burn severity of ABSI index in different age groups. The results suggested that the elderly patients were most likely to suffer severe burns compared to other age groups. The majority of the pediatric patients (4890, 68.0%) had a very low ABSI burn score; while the majority of the adult patients under the age of 60 years old had a moderate to severe ABSI burn score (7576, 78.5%). The elderly patients had a moderately severe to serious ABSI burn severity.
Health outcomes
The presence of a burn with an inhalation injury was confirmed in 543 patients (3.02%) (Table 4 ), with the lowest occurrence rate in pediatric patients (0.8%). The average LOS was 32.11 ± 65.72 days (median: 17 days). The adult patients aged between 18 and 60 years old confirmed the longest length of stay in hospital with more than 40 days. The length of staying in BICU was significantly higher in burn patients between the age of 40 and 60 years old. A significantly longer stay in hospitals and BICU were revealed among male patients compared to female ones, with an average of 9 days longer in hospital and 0.5 day longer in BICU. Patients injured by flame and electrical burns spent significantly longer time in hospitals and BICU, compared to those patients caused by scald, chemical and other reasons, with an average of more than 20 days longer.
Table 5 showed the multiple linear regression of factors contributed to the total medical cost. The demographic information of age and gender, the cause of burns, BI score, the thickness of burns, and the length of stay in hospital were included as contributors. Compared to the reference item (Ref), the higher of the absolute value of the β score, the higher contribution of the item to the medical cost. The results suggested that electrical burns, the gender of male, and the middle age between 41 and 60 years old showed significant association with higher medical cost.
In total, there were 145 deaths among the 17939 patients, for a mortality of 0.81%. Our results showed in Table 6 that older age with more than 41 years old had the greatest influence on mortality (OR 3.38, P < 0.001), followed by full-thickness burns (OR = 3.09, P < 0.05) and older age with more than 61 years old (OR 2.88, P < 0.05). Moreover, a shorter length of stay in hospitals (OR 0.97, P < 0.001) and female gender (OR 0.57, P < 0.05) were protective factors for mortality.
Framework of severe burn management continuum
Figure 4 described the model of burn management continuum used for developing strategies to prevent and manage severe burns. This model was designed to decrease burn injuries to populations, and promote recovery and rehabilitation to burn patients. It was worthy to note that this model presented a continuous process of burn management from the stage of burn prevention to burn treatment and finally to burn rehabilitation and reconstruction.
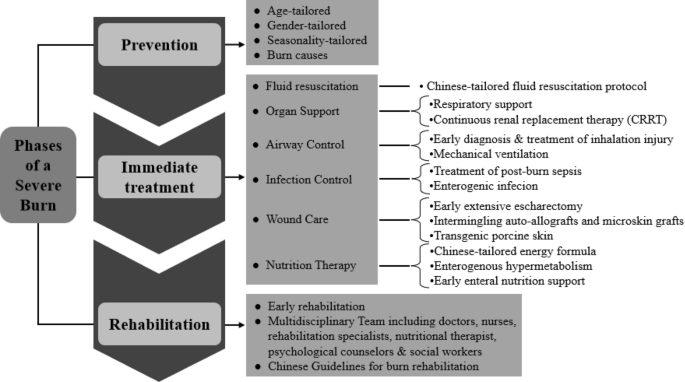
Burn prevention and management continuum.
Prevention was the process designed to prevent or minimize the risks related to burn injuries. As our data showed, with the improvement of burn prevention strategies, the number of burn injuries has continuously declined since the year of 2011 (Fig. 1 C). Notably, the effect of gender, age and seasonality on occurrence of burn injuries was significant. Another factor should be considered was the top two causes of burn injuries: scalding and flame. All these results suggested a prevention strategy should be tailored according to the population’s characteristics. For example, the public education should be different between teenagers and the elderly.
The phase of in-hospital treatment encompassed the immediate actions taken in the face of a severe burn. The six aspects and related strategies in the framework were developed on the basis of our accumulated and extensive experience through treatment of large number of cases. Most importantly, these aspects have drawn from clinical and basic research and from the experience of translating the basic findings into clinical practice. Some strategies such as estimation of burn area, fluid resuscitation protocol and energy formula were developed and tailored according to the characteristics among Chinese populations.
The phase of rehabilitation was important for severe burn patients to recover from the impact of burns and return to society. Once immediate needs were met, the rehabilitation phase could begin. Our institute was one of the pioneers of implementing early rehabilitation for burn patients in Mainland China. The burn rehabilitation have gained increase attention since 1995 in our center, and a whole floor of special burn rehabilitation center was established in 2011, comprising of multidisciplinary team including doctors, nurses, rehabilitation specialists, nutritional therapist, psychological counselors & social workers.
Burn patients accounted for a large proportion of hospitalized injury patients worldwide. They suffered from great physical and psychological burden because of the associated morbidity, rehabilitation, mortality and requirement of high cost medical services. Therefore, investigations on epidemiological parameters related to burns and treatment outcomes could assist with the adoption of effective interventions and individualized prevention approaches in southwest China. In particular, this study focused on the clinical characteristics and treatment outcomes of different age groups of burn patients. The primary result we found was that the annual number of burn injuries has kept decreasing since the year of 2014, which was consistent with the global trends 18 , 19 . This change might be partially attributed to the increased awareness and education of burn prevention and the improved burn-preventative environment. Another notable finding was that the gender difference and age difference should be considered when making individualized interventions and rehabilitative treatments. The etiology, the frequently burn sites, the occurrence rate of inhalation injury, the LOS, the cost and the mortality rates were significantly different between male patients and female patients, and among patients of different age groups. However, the burn severity showed by the indicators of TBSA, ABSI and BI, and the economic burden indicated by the medical cost were still in a high level, which suggested that the current prevention and care of burns remained inadequate, demonstrating that more effective interventions should be introduced in the future.
Same as the results from other studies 20 , 21 , 22 , scalding and flame were identified as the two most common causes of burning in the southwest of China. Furthermore, the age difference and gender difference on etiology should be noticed. Firstly, considering the age difference, the scalding was in the first cause to burn injuries among pediatric patients, comprising of 74.24% of all cases under the age of 18 years old. Scald burns were primarily caused by hot steam, hot water, hot soup and hot oil. This suggested that hot steam and hot fluid should be cautioned among children population. In contrast, flame burns were the leading etiology in adult patients. The patients between the age of 18 and 40 years suffered most from flame burning, accounting for 62.07% of all the cases in this age group. Flame injuries were mainly generated by gas and bomb explosion, short circuit of electricity and fireworks. Secondly, considering the gender difference, the incidence of burns caused by flames was significantly larger than those caused by scalding among male patients; while the burns caused by scalding was significantly larger than those caused by flames among female patients. Electricity was the third most common cause of burns in the pediatric population and the adult patients under the age of 60 years old; while chemical burns was the third cause in the elderly population above the age of 60 years old. It was worthwhile to note that although the flame and scald were the first two etiologies of burn injuries, the electrical burn was the one that costed the highest medical expense (Table 5 ). According to the previous studies, this was likely because that 10–68% 23 of the electrical burns resulted in amputation, which increased the medical cost. The above findings indicated that burn education was necessary for the population of all ages, and particularly preventive strategies should be individualized by age, gender, and burn causes. For example, children were curious about their surroundings but they were too young to be aware of burn-related dangers. Therefore, it was their parents or guardians as the main target of education about providing safe environment and eliminating possible burn dangers to their children, such as putting the hot water or oil away from children and protecting the electrical plugs in case of electricity burns.
Our findings demonstrated that the adult patients aged between 18 and 60 years old were the main victims of burn injuries, accounting for 53.8% of the total cases. The first cause to burns in this population was the flame, followed by scald, and then the electricity. This may relate to the potential burn-related hazards in work place 16 . In particular, the number of male patients at this age was significantly larger than that of female patients, with the ratio of three to one (Table 1 ). Moreover, almost half of those patients were self-funded on the medical expense; and the male patients spent significantly more money on treatment compared to the cost among female patients. Furthermore, the sites of head/face/neck, trunk and limbs were the most significantly parts of body that burn occurred. During the process of post-burn recovery and rehabilitation, the scar was a notable problem. The scar on head/face/neck will influence the facial appearance, and the scar on limbs will affect the physical functions. All of these results suggested that the adult patients especially the male patients should become the major prevention target in the future considering their responsibilities to the family and society. Additionally, the early rehabilitation on physical abilities should be emphasized.
When great awareness and focus has been placed on the elderly and young children, the population of females should be also considered as a vulnerable group of burn injuries and worthy of increased attention. It was interesting to find out that the percentage of female burn patients increased annually despite of the decreased number of the overall adult patients (Fig. 1 D). It suggested that the risk for Chinese females to suffer from burn injuries kept increasing. After further analysis of the etiology, we found that scalding was the priority cause to burns in females, accounting for over 60 percent of the total female patients (Table 1 ). Surprisingly, in another developing country India, similar finding has been reported before that Indian population of young females between the ages of 16 and 35 years were high-risk of burning. The reason lied in that females cooked over open flames at floor level, often with faulty equipment and loose clothing susceptible to catching fire. Based on years of clinical experience on burn center, our research team has discussed the reasons for the high risk of burning in Chinese females. Considering the priority etiology of scalding, we assumed the first reason was that women still took the main responsibility of cooking for the family, so they were more likely exposure to hot water/oil and cooking flames compared to men. Secondly, more and more women were involved in the workforce. The results showed that the labor force participation rate in Chinese females reached 70% 24 . And the chance of exposure to burn risks was increasing if the precautions were not sufficient in the workplace. The third reason we considered may be due to the culture factor of gender inequality in developing countries. Women in those countries such as India and China were mainly work in the areas with relatively high risk of burn injuries such as housemaid and catering services.
Building on the previous findings 19 of those the burn size determining major complications and survival rates, this study also identified that the burn size and depth contributed to the total cost of burn patients according to burn index (BI). BI was an indicator of burn severity calculated on the basis of TBSA and burn depth. In Dr. Jeschke and Prof. Herndon’s study of an sample of 952 severely burned pediatric patients, they confirmed that burn size of 62% TBSA was a crucial threshold for post-burn morbidity and mortality 25 ; and the cost of burn treatment was increasing accordingly. Besides the TBSA, ABSI, and BI, there were other indicators developed to predict burn outcomes such as the modified Baux score 26 and the Pediatric Risk of Mortality (PRISM) score 27 . Most of these indices were formula developed by calculating risk factors for post-burn morbidity and mortality. For example, the Baux score was calculated in consideration of age, burn size and the presence of inhalation injury. Further analysis and comparisons were needed to figure out which of the above indicators would be more accurate and effective in predicting mortality and hospital length of stay among patients with burns.
Some limitations should be noted when interpreting these findings. First, due to system default, we could not access to the data of other poor outcomes such as infection and sepsis. Therefore, we failed to find out the risk factors contributed to these poor outcomes. However, our research team is now conducting a longitudinal study trying to investigate risk factors for these poor outcomes in Chinese burn patients. Another limitation was that our data only partially reflected the epidemiology of burn injuries in southwest of China, and the patients in this study mainly originated from Chongqing, Sichuan, Yunnan and Guizhou Province. Due to unequal economic development between the south cities and the east cities in China, the findings cannot represent the status in the eastern cities of China. Therefore, more studies with large sample sizes and multiple centers are still needed. Thirdly, since our center is in a tertiary hospital, so some of the severe burn patients have received treatments from other hospitals before they were transferred into our center. Therefore, the burn severity observed in our study might be higher than average.
This 17-year retrospective study examined the epidemiology and outcomes of burn patients in southwest of China. The annual number of burn injuries has kept decreasing, which was partially attributed to the increased awareness and education of burn prevention and the improved burn-preventative circumstances. However, the burn severity and the economic burden were still in a high level. And the gender difference and age difference should be considered when making individualized interventions and rehabilitative treatments.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Army Medical University
Institute of Burn Research
Hospital Information System
Laboratory Information Management System
Electronic Medical Record
Abbreviated Burn Severity Index
Total Body Surface Area
Length of Stay
Li, H. et al. Epidemiology and outcome analysis of 6325 burn patients: A five-year retrospective study in a major burn center in Southwest China. Sci. Rep. 7 , 46066. https://doi.org/10.1038/srep46066 (2017).
Article ADS CAS PubMed PubMed Central Google Scholar
Brusselaers, N., Monstrey, S., Vogelaers, D., Hoste, E. & Blot, S. Severe burn injury in Europe: A systematic review of the incidence, etiology, morbidity, and mortality. Crit. Care 14 , R188. https://doi.org/10.1186/cc9300 (2010).
Article PubMed PubMed Central Google Scholar
American Burn Association. Burn Incident Fact Sheet (2016). http://ameriburn.org/who-we-are/media/burn-incidence-fact-sheet/ . Accessed 19 April 2021.
Smolle, C. et al. Recent trends in burn epidemiology worldwide: A systematic review. Burns 43 , 249–257. https://doi.org/10.1016/j.burns.2016.08.013 (2017).
Article PubMed Google Scholar
Wiechman, S. A. & Patterson, D. R. ABC of burns. Psychosocial aspects of burn injuries. BMJ 329 , 391–393. https://doi.org/10.1136/bmj.329.7462.391 (2004).
Tripathee, S. & Basnet, S. J. Epidemiology of burn injuries in Nepal: A systemic review. Burns Trauma 5 , 10. https://doi.org/10.1186/s41038-017-0075-y (2017).
Boerma, T. & Mathers, C. D. The World Health Organization and global health estimates: Improving collaboration and capacity. BMC Med. 13 , 50. https://doi.org/10.1186/s12916-015-0286-7 (2015).
Golshan, A., Patel, C. & Hyder, A. A. A systematic review of the epidemiology of unintentional burn injuries in South Asia. J. Public Health (Oxf.) 35 , 384–396. https://doi.org/10.1093/pubmed/fds102 (2013).
Article Google Scholar
Peck, M. D. et al. Burns and injuries from non-electric-appliance fires in low- and middle-income countries. Part II. A strategy for intervention using the Haddon matrix. Burns 34 , 312–319. https://doi.org/10.1016/j.burns.2007.08.009 (2008).
Kai-Yang, L. et al. Epidemiology of pediatric burns requiring hospitalization in China: A literature review of retrospective studies. Pediatrics 122 , 132–142. https://doi.org/10.1542/peds.2007-1567 (2008).
Wang, S. et al. Epidemiology of burns in pediatric patients of Beijing City. BMC Pediatr. 16 , 166. https://doi.org/10.1186/s12887-016-0686-7 (2016).
Li, H. et al. Epidemiology of pediatric burns in southwest China from 2011 to 2015. Burns 43 , 1306–1317. https://doi.org/10.1016/j.burns.2017.03.004 (2017).
Tan, A. et al. Smoke inhalation increases intensive care requirements and morbidity in paediatric burns. Burns 42 , 1111–1115. https://doi.org/10.1016/j.burns.2016.02.010 (2016).
Williams, F. N. et al. The leading causes of death after burn injury in a single pediatric burn center. Crit. Care 13 , R183. https://doi.org/10.1186/cc8170 (2009).
Ye, C. et al. Ten-year epidemiology of chemical burns in western Zhejiang Province, China. Burns 42 , 668–674. https://doi.org/10.1016/j.burns.2015.12.004 (2016).
Li, H. et al. Wound management and outcome of 595 electrical burns in a major burn center. J. Surg. Res. 214 , 182–189. https://doi.org/10.1016/j.jss.2017.02.032 (2017).
Li, H. et al. The progress of Chinese burn medicine from the Third Military Medical University-in memory of its pioneer, Professor Li Ao. Burns Trauma 5 , 16. https://doi.org/10.1186/s41038-017-0082-z (2017).
Peleg, K., Goldman, S. & Sikron, F. Burn prevention programs for children: Do they reduce burn-related hospitalizations? Burns 31 , 347–350. https://doi.org/10.1016/j.burns.2004.10.028 (2005).
Seo, D. K. et al. Epidemiological trends and risk factors in major burns patients in South Korea: A 10-year experience. Burns 41 , 181–187. https://doi.org/10.1016/j.burns.2014.05.004 (2015).
Ciftci, I., Arslan, K., Altunbas, Z., Kara, F. & Yilmaz, H. Epidemiologic evaluation of patients with major burns and recommendations for burn prevention. Ulus Travma Acil Cerrahi Derg 18 , 105–110 (2012).
Iqbal, T., Saaiq, M. & Ali, Z. Epidemiology and outcome of burns: Early experience at the country’s first national burns centre. Burns 39 , 358–362. https://doi.org/10.1016/j.burns.2012.07.011 (2013).
Yates, J., McKay, M. & Nicholson, A. J. Patterns of scald injuries in children–has anything changed? Iran. Med. J. 104 , 263–265 (2011).
CAS Google Scholar
Arnoldo, B., Klein, M. & Gibran, N. S. Practice guidelines for the management of electrical injuries. J. Burn Care Res. 27 , 439–447. https://doi.org/10.1097/01.BCR.0000226250.26567.4C (2006).
Chen, Y. Y., Chen, M., Lui, C. S. M. & Yip, P. S. F. Female labour force participation and suicide rates in the world. Soc. Sci. Med. 195 , 61–67. https://doi.org/10.1016/j.socscimed.2017.11.014 (2017).
Kraft, R. et al. Burn size and survival probability in paediatric patients in modern burn care: A prospective observational cohort study. Lancet 379 , 1013–1021. https://doi.org/10.1016/S0140-6736(11)61345-7 (2012).
Osler, T., Glance, L. G. & Hosmer, D. W. Simplified estimates of the probability of death after burn injuries: Extending and updating the baux score. J. Trauma 68 , 690–697. https://doi.org/10.1097/TA.0b013e3181c453b3 (2010).
Berndtson, A. E., Sen, S., Greenhalgh, D. G. & Palmieri, T. L. Estimating severity of burn in children: Pediatric risk of mortality (PRISM) score versus Abbreviated Burn Severity Index (ABSI). Burns 39 , 1048–1053. https://doi.org/10.1016/j.burns.2013.05.001 (2013).
Download references
This study was supported by Army Medical Science and Technology Programme [Grant Number 20QNPY005], by Talent Project of Army Medical University [Grant Number 410301060194] and by Talent Project of Army Medical University [Grant Number 410301060196]. The sponsor or funding organization had no role in the design or conduct of this research, data analysis or manuscript preparation.
Author information
These authors contributed equally: Ling Chen and Xiaochong He. These authors jointly supervised this work: Zonghua Wang and Ning Li.
Authors and Affiliations
Department of Emergency, The 958th Hospital of PLA, The Affiliated Hospital of Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing, 400020, People’s Republic of China
School of Nursing, Third Military Medical University (Army Medical University), Chongqing, 400038, People’s Republic of China
Xiaochong He & Zonghua Wang
Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing, 400038, People’s Republic of China
Jishu Xian & Jianmei Liao
School of Public Health and Management, Chongqing Medical University, Chongqing, 400016, People’s Republic of China
Xuanji Chen
Institute of Burn Research, Southwest Hospital, Third Military Medical University (Army Medical University), Gaotanyan Street No.30, Shapingba District, Chongqing, 400038, People’s Republic of China
Yue Luo & Ning Li
You can also search for this author in PubMed Google Scholar
Contributions
C.L., H.X., W.Z. and L.N. designed of the study. L.N., X.J. and L.J. acquired the data. C.L., W.Z., H.X. and C.X. analyzed and interpreted data. This manuscript was originally prepared and written by C.L. and W.Z., with edits and revisions from L.N., H.X. and L.Y. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Zonghua Wang or Ning Li .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Chen, L., He, X., Xian, J. et al. Development of a framework for managing severe burns through a 17-year retrospective analysis of burn epidemiology and outcomes. Sci Rep 11 , 9374 (2021). https://doi.org/10.1038/s41598-021-88507-x
Download citation
Received : 12 January 2021
Accepted : 07 April 2021
Published : 30 April 2021
DOI : https://doi.org/10.1038/s41598-021-88507-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
- P. Guilabert
- Maria J. Colomina
Scientific Reports (2023)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Published: 01 December 2015

Burn wound healing and treatment: review and advancements
- Matthew P. Rowan 1 ,
- Leopoldo C. Cancio 1 ,
- Eric A. Elster 2 ,
- David M. Burmeister 1 ,
- Lloyd F. Rose 1 ,
- Shanmugasundaram Natesan 1 ,
- Rodney K. Chan 1 , 3 ,
- Robert J. Christy 1 &
- Kevin K. Chung 1 , 2
Critical Care volume 19 , Article number: 243 ( 2015 ) Cite this article
66k Accesses
519 Citations
23 Altmetric
Metrics details
A Letter to this article was published on 20 January 2016
Burns are a prevalent and burdensome critical care problem. The priorities of specialized facilities focus on stabilizing the patient, preventing infection, and optimizing functional recovery. Research on burns has generated sustained interest over the past few decades, and several important advancements have resulted in more effective patient stabilization and decreased mortality, especially among young patients and those with burns of intermediate extent. However, for the intensivist, challenges often exist that complicate patient support and stabilization. Furthermore, burn wounds are complex and can present unique difficulties that require late intervention or life-long rehabilitation. In addition to improvements in patient stabilization and care, research in burn wound care has yielded advancements that will continue to improve functional recovery. This article reviews recent advancements in the care of burn patients with a focus on the pathophysiology and treatment of burn wounds.
Introduction
Acute thermal injuries requiring medical treatment affect nearly half a million Americans each year, with approximately 40,000 hospitalizations and 3,400 deaths annually [ 1 ]. The survival rate for admitted burn patients has improved consistently over the past four decades [ 2 ] and is currently a favorable 97 % for patients admitted to burn centers [ 3 ]. This can be largely attributed to national decreases in burn size, improvements in burn critical care, and advancements in burn wound care and treatment that have been driven by research, as reflected in the dramatic increase in burn publications over the last several decades [ 4 , 5 ]. Since the first International Congress on Research in Burns over 50 years ago, progress has been made in a host of areas, and vital improvements in early resuscitation, infection management, wound excision and coverage, and fluid management have helped in the fight against burn mortality [ 6 , 7 ]. This review presents an update on the care of burn patients, with special emphasis on the mechanisms underlying burn wound healing and recent advancements in burn wound care.
Pathophysiology of burn wounds
Thermal burns from dry sources (fire or flame) and wet sources (scalds) account for approximately 80 % of all reported burns [ 8 ] and can be classified based on the depth of burn [ 9 , 10 ]. In addition to local injury at the site of burn, severe thermal injury over a large area of the skin, roughly 20 % total body surface area (TBSA) or greater, results in acute systemic responses collectively known as burn shock [ 11 ]. Burn shock is characterized by increased capillary permeability, increased hydrostatic pressure across the microvasculature, protein and fluid movement from the intravascular space into the interstitial space, increased systemic vascular resistance, reduced cardiac output, and hypovolemia requiring fluid resuscitation [ 12 ]. The edema that forms in the interstitial space forms rapidly in the first 8 h following burn injury, and continues to form more slowly for at least 18 h [ 13 ]. Volume requirements for resuscitation can be estimated by the total burn size and the patient’s weight (or body surface area). Additional factors influencing these needs include the presence or absence of inhalation injury, the extent of full-thickness burns, and the time since injury [ 12 ]. The actual fluid infusion rate is then titrated hourly, based on the adequacy of physiological responses, such as the urine output [ 14 ].
Following successful resuscitation, patients with larger burns then enter a more prolonged period of hypermetabolism, chronic inflammation, and lean body mass wasting, all of which may impair wound healing [ 15 ]. Additionally, an increased susceptibility to infection due to altered immune status may lead to sepsis, further exacerbating systemic inflammation [ 16 ]. Sustained hypermetabolism and inflammation impair wound healing through delayed re-epithelialization [ 17 , 18 ]. The extent of inflammation and hypermetabolism is related to the extent [ 19 ] and depth of burn, as deeper burns show higher levels of circulating cytokines [ 20 ] and a greater hypermetabolic response [ 21 ]. Similarly, the extent of burn is an efficient predictor of hospital length of stay [ 19 , 22 ] and mortality [ 19 , 23 ].
According to one model, the burn wound can be divided into three zones based on the severity of tissue destruction and alterations in blood flow [ 10 , 24 – 26 ]. The central part of the wound, known as the zone of coagulation, is exposed to the greatest amount of heat and suffers the most damage. Proteins denature above 41 °C (106 °F), so excessive heat at the site of injury results in extensive protein denaturation, degradation, and coagulation, leading to tissue necrosis. Around the central zone of coagulation is the zone of stasis, or zone of ischemia, which is characterized by decreased perfusion and potentially salvageable tissue [ 10 ]. In this zone, hypoxia and ischemia can lead to tissue necrosis within 48 h of injury in the absence of intervention [ 27 ]. The mechanisms underlying apoptosis and necrosis in the ischemic zone remain poorly understood, but appear to involve immediate autophagy within the first 24 h following injury and delayed-onset apoptosis around 24 to 48 h postburn [ 27 ]. Other studies have shown apoptosis to be active as early as 30 min postburn [ 28 ] depending on the intensity of the burn injury [ 29 ]. Oxidative stress may play a role in the development of necrosis, as preclinical studies have demonstrated promising reductions in necrosis with systemic antioxidant administration [ 30 ]. At the outermost regions of the burn wound is the zone of hyperemia that receives increased blood flow via inflammatory vasodilation and will likely recover, barring infection or other injury [ 25 ].
Although burns are different from other wounds in some respects, such as the degree of systemic inflammation [ 31 ], healing of all wounds is a dynamic process with overlapping phases [ 32 ] (Table 1 ). The initial inflammatory phase brings neutrophils and monocytes to the site of injury via localized vasodilation and fluid extravasation, thereby initiating an immune response that is later sustained by the recruitment of macrophages by chemokines [ 31 ]. The inflammatory phase serves not only to prevent infection during healing, but also to degrade necrotic tissue and activate signals required for wound repair [ 33 ]. Following, and overlapping with the inflammatory response, the proliferative phase is characterized by keratinocyte and fibroblast activation by cytokines and growth factors [ 34 ]. In this phase, keratinocytes migrate over the wound to assist in closure and restoration of a vascular network, which is a vital step in the wound healing process [ 35 ]. This network of communication between stromal, endothelial, and immune cells determines the course of healing, including closure and revascularization.
Overlapping with the proliferative phase, the final phase of healing involves remodeling the wound [ 36 ]. During the remodeling phase, the wound scar matures [ 31 ] as collagen and elastin are deposited and continuously reformed as fibroblasts become myofibroblasts [ 37 ]. Myofibroblasts adopt a contractile phenotype, and thus are involved in wound contracture [ 38 ]. The conversion from fibroblasts to myofibroblasts controls a delicate balance between contraction and re-epithelialization that, in part, determines the pliability of the repaired wound [ 39 ]. In addition to fibroblast conversion, apoptosis of keratinocytes and inflammatory cells are key steps in the termination of wound healing and the overall final appearance of the wound [ 40 ].
Optimization of burn wound healing
Inflammation.
Inflammation is vital to successful burn wound healing, and inflammatory mediators (cytokines, kinins, lipids, and so forth) provide immune signals to recruit leukocytes and macrophages that initiate the proliferative phase [ 37 ]. Wound re-epithelialization, or closure, in the proliferative phase via keratinocyte and fibroblast activation, or migration from dedifferentiated hair follicles and other epidermal analogs [ 41 , 42 ], is mediated by cytokines recruited in the inflammatory phase. While this indicates that inflammation is essential for wound healing, aberrant inflammatory pathways have also been linked to hypertrophic scarring, and anti-inflammatory treatments could potentially aggravate symptoms and delay wound healing [ 40 , 43 , 44 ].
Significant edema that is initiated by several factors including vasodilation, extravascular osmotic activity, and increased microvascular permeability often accompanies inflammation [ 45 ]. Excessive or prolonged edema and inflammation exacerbate pain and impair wound healing [ 17 , 18 ]. Interestingly, studies suggest that in the absence of infection, inflammation might not be required for tissue repair [ 46 ]. Since inflammation can have both beneficial and detrimental effects on burn wound healing, the clinical challenge becomes management, applying therapeutic intervention only when inflammation and edema become excessive.
Treatment of inflammation in large burns is difficult, as recently discussed in detail elsewhere [ 16 ]. Traditional anti-inflammatory treatments that focus on the inhibition of prostaglandin synthesis, such as nonsteroidal anti-inflammatory drugs or glucocorticoids, impair wound healing [ 47 ]. However, steroid administration has been shown to reduce inflammation, pain, and length of hospital stay in burn patients in several small studies [ 48 , 49 ]. Early excision and grafting has become the gold standard for treatment of full and deep partial thickness burns [ 50 , 51 ], in part because early excision helps reduce the risk of infection and scarring [ 52 – 54 ]. The timing of debridement coincides with the inflammatory phase of healing, as the burn eschar removed during excision is an inflammatory nidus and a rich pabulum for bacterial proliferation.
Nontraditional anti-inflammatory treatments, such as opioids, have gained considerable attention but have yet to translate promising preclinical results into clinical practice for wound healing. While the majority of animal studies have demonstrated consistent anti-inflammatory effects of opioids on peripheral neurons [ 55 ], clinical studies have shown little to no effect on inflammation [ 56 ]. Furthermore, topical morphine delayed the early inflammatory phase and accelerated the later proliferative phase [ 57 , 58 ], which is supported by in vitro studies showing opioid stimulation of keratinocyte migration [ 59 ]. Large-scale clinical trials evaluating opioid efficacy on wound healing have not yet been conducted [ 60 ].
The skin functions as a barrier to the external environment to maintain fluid homeostasis and body temperature, while providing sensory information along with metabolic and immunological support. Damage to this barrier following a burn disrupts the innate immune system and increases susceptibility to bacterial infection [ 61 ]. Burn wound infection was defined in a rat model with Pseudomonas aeruginosa [ 62 , 63 ], in which the following progression was observed: burn wound colonization; invasion into subjacent tissue within 5 days; destruction of granulation tissue; visceral hematogenous lesions; and leukopenia, hypothermia, and death. Burn patients are at high risk for infection [ 64 ], especially drug-resistant infection [ 65 ], which often results in significantly longer hospital stays, delayed wound healing, higher costs, and higher mortality [ 66 ]. Infection can lead to the development of a pronounced immune response, accompanied by sepsis or septic shock, which results in hypotension and impaired perfusion of end organs, including the skin – all processes that delay wound healing. Furthermore, the leading causes of death following a severe burn are sepsis and multiorgan failure [ 67 – 69 ], so prevention and management of infection is a primary concern in the treatment of burn patients. Early and accurate diagnosis of infection is difficult: C-reactive protein and the white blood cell count are most often used, since the diagnostic power of procalcitonin is questionable in burns [ 70 ]. Consensus definitions of sepsis and infection have recently been proposed that are more relevant to the burn population and are often used clinically but still require validation [ 71 ].
The management of burn wound infections has been extensively reviewed elsewhere [ 61 , 64 – 66 , 72 – 77 ]. Since the adoption of topical antibiotics, such as mafenide in the 1960s and silver sulfadiazine in the 1970s, and of early excision and grafting in the 1970s and thereafter, systemic infections and mortality have consistently decreased [ 68 , 72 , 78 ]. However, Gram-positive and Gram-negative bacterial infections still remain one of the most common causes of mortality following burn injury [ 73 ]. Bacterial cultures can aid in the selection of an appropriate antibiotic, especially in cases of bacterial drug resistance, but altered pharmacokinetic parameters in burn patients must be considered and dosing should be adjusted accordingly to maximize antibiotic efficacy [ 79 ]. Importantly, effective topical antimicrobials do not exist for invasive fungal infections, and fungal wound infections are associated with greater mortality rates in large burns (>30 % TBSA) [ 80 ]. Owing to high lethality, suspicion of an invasive burn wound infection mandates rapid diagnosis, often by histopathology, and excision or re-excision of the wound.
Sustained hypermetabolism, hormone elevations, and muscle wasting following severe burn injury all contribute to the clinical outcome, with magnitude and duration that are unique to burns [ 81 , 82 ]. Accordingly, reducing the impact of a hypermetabolic state and providing adequate nutrition are key factors that affect burn wound healing and recovery [ 83 ], as has been reviewed elsewhere [ 84 ]. There is a difficult balance between the additional caloric needs to meet the demand from hypermetabolism and the consequences of nutrient overconsumption. Nutritional support following a burn injury is a complex issue. For example, early excision and aggressive feeding in children does not diminish energy expenditure but is associated with decreased muscle protein catabolism, a decreased rate of burn sepsis, and significantly lower bacterial counts from excised tissue [ 85 ]. In adults, early nutritional support is correlated with shorter stays, accelerated wound healing, and decreased risk of infection [ 86 ].
Several nutritional factors must be considered. For example, excess carbohydrate consumption may lead to hyperglycemia [ 87 ] that can exacerbate systemic inflammation and muscle degradation [ 88 , 89 ]. Furthermore, excess fat consumption may exaggerate the immunosuppressed state [ 90 ]; and since major burn injuries may also result in immunosuppression [ 91 ], this exaggeration may increase the risk for infection and sepsis. Carbohydrate and fat intake must therefore be closely monitored in burn patients. Guidelines for nutritional support of burn patients vary, but consensus recommendations have been given by the American Burn Association and the American Society for Parenteral and Enteral Nutrition for carbohydrates, proteins, and fats [ 84 ].
In addition to support with amino acids and vitamins [ 84 ], administration of insulin has been shown to decrease healing time by reducing protein catabolism and increasing skeletal muscle protein synthesis [ 92 – 96 ]. More research is needed to optimize insulin delivery, as many recombinant growth factors, such as epidermal growth factor and transforming growth factor, are often cost prohibitive [ 93 ]. Other anabolic agents, such as oxandrolone, have been shown to increase lean body mass recovery, decrease length of stay, and improve overall outcomes, including wound healing [ 97 – 100 ]. Additionally, while conventional theory suggests that hemoglobin levels must be maintained above 10 g/dl to promote wound healing [ 101 ], preliminary evidence suggests that mild to moderate anemia has no effect on graft success if perfusion is maintained with proper circulatory volume [ 102 ]. The results of a multicenter, randomized, controlled trial (ClinicalTrials.gov NCT01079247) comparing blood transfusion with lower volumes (target hemoglobin of 7 to 8 g/dl) and conventional volumes (target hemoglobin >10 g/dl) for a large cohort of patients are expected soon and will allow for more definitive clinical guidelines on blood transfusion volumes.
Resuscitation
Severe thermal injuries over a large area of the skin (>20 % TBSA) require fluid resuscitation for stabilization. Although volume guidelines and fluid compositions vary widely between centers, the goal of fluid resuscitation is to maintain organ perfusion with the least amount of fluid necessary [ 12 ]. Common traditional resuscitation formulas, such as the modified Brooke, and Parkland formulas, employ crystalloids such as lactated Ringer’s that contain sodium, chloride, calcium, potassium, and lactate. During large-volume resuscitations, the addition of colloids (for example, albumin, fresh frozen plasma) as adjuncts has been successful in reducing the total volume [ 12 ]. Despite extensive research into resuscitation fluid compositions and volumes, little is known about the effect of resuscitation on wound healing. A recent meta-analysis showed a positive association between the number of grafting procedures and hypernatremia, suggesting that high serum sodium levels may inhibit graft take [ 103 ]. Additionally, we have recently shown that the rate of wound closure (healing rate) is significantly faster in patients who received lower 24-h fluid resuscitation volumes [ 104 ]. More work is needed to evaluate the effect of resuscitation on wound healing trajectories before clinical recommendations for preferred fluid compositions and volumes can be made.
Wound coverage and grafting
Early excision and grafting has been the standard of care for decades. Most studies have shown that excision within 24 to 48 h after injury is associated with decreased blood loss, infection, length of hospital stay and mortality, and increased graft take [ 105 – 108 ], although mortality reductions may only occur in patients without inhalation injury [ 109 ]. Since one of the main challenges in treating acute thermal injuries is preventing infection, excising the eschar and covering the wound as early as possible are critical. The standard for rapid and permanent closure of full-thickness burns is a split-thickness skin graft from an uninjured donor site on the same patient (autograft). Such grafting provides sufficient coverage without risk of rejection, although meta-analyses have yet to determine the failure rate of split-thickness skin grafts in burn patients. Split-thickness skin grafts can be meshed with variable expansion ratios to increase the coverage area, but concerns remain over the effect that meshing has on range of motion [ 110 ] and the graft site healing rate. On the other hand, donor sites are painful and impose their own wound-healing burden on the patient [ 111 ]. Various dressings have been used to cover donor sites during healing, with variable results [ 112 ].
Patients with more extensive burns often require temporary coverage with an allograft, xenograft, skin substitute, or dermal analog due to insufficient or unavailable donor sites. Allografts, or tissue taken from a living or deceased human donor, and xenografts, taken from a different species, promote re-epithelialization and prepare the wound bed for autograft, increasing the healing rate when compared with traditional dressings [ 113 ]. A recent meta-analysis suggested that since allografts and xenografts appear to be equally effective, xenografts may be a superior choice for their increased safety and reduced price [ 114 ]. However, caution should be exercised in drawing broad conclusions from this meta-analysis because the cited studies lack standardization and critical details such as depth and size of burn, and many studies cited were merely anecdotal. A cadaver allograft is thus widely considered the best material for temporary closure of excised wounds in patients with extensive, life-threatening burns and inadequate donor sites. The cadaver allograft is also the preferred material for protection of widely meshed autografts (3:1 or higher meshing ratios) during healing. In the latter setting, the allograft is applied over the meshed autograft in the manner of a sandwich.
A variety of different skin substitutes and dermal analogs exist [ 115 – 119 ] (Table 2 ) that can be broadly divided into those which replace the epidermis or replace the dermis [ 120 , 121 ]. Epidermal substitutes are normally only a few cell layers thick and lack normal dermal components [ 122 , 123 ]. Commercially available dermal substitutes include acellular matrices, commonly from human – for example, Alloderm (LifeCell, Bridgewater, NJ, USA) or GraftJacket (KCI, San Antonio, TX, USA) – or other sources (for example, Integra; Integra LifeSciences, Plainsboro, NJ, USA). Biobrane (Smith & Nephew, London, UK) is a semisynthetic, bilaminar material consisting of a nylon-mesh dermal analog (bonded with porcine collagen) and a silicone epidermal analog. Biobrane is used for temporary closure of superficial burns and donor sites [ 124 , 125 ]. Products currently under development integrate the concept of dermal scaffolds that actively promote revascularization by incorporating stem cells and growth factors to recreate a favorable cellular microenvironment [ 126 , 127 ].
Numerous options exist for dressings [ 128 , 129 ]. The selection of an appropriate dressing depends on several factors, including depth of burn, condition of the wound bed, wound location, desired moisture retention and drainage, required frequency of dressing changes, and cost. While many factors must be considered in dressing selection, the goals in selecting the most appropriate dressing should include providing protection from contamination (bacterial or otherwise) and from physical damage, allowing gas exchange and moisture retention, and providing comfort to enhance functional recovery. The traditional approach to burn wound care developed at the US Army Burn Center includes alternation of mafenide acetate cream in the morning and silver sulfadiazine cream in the evening, with gauze dressings used over the creams. More recently, silver-impregnated and other dressings have been introduced. Major classes of dressings include: alginate, for example Aquacel (ConvaTec, Bridgewater, NJ, USA), Comfeel (Coloplast, Minneapolis, MN, USA), or Sorbsan (Mylan, Morgantown, WV, USA); antimicrobial, for example Acticoat (Smith & Nephew, London, UK) or Silverlon (Argentum, Geneva, IL, USA); collagen, for example Fibracol (Johnson & Johnson, New Brunswick, NJ) or Puracol (Medline, Mundelein, IL, USA); hydrocolloid, for example Duoderm (ConvaTec, Bridgewater, NJ, USA), Granuflex (ConvaTec, Bridgewater, NJ, USA), or Tegaderm (3M, Maplewood, MN, USA); hydrogel, for example Dermagel (Maximilian Zenho & Co, Brussels, Belgium), SilvaSorb (Medline, Mundelein, IL, USA), or Skintegrity (Medline, Mundelein, IL, USA); and polyurethane foam, for example Allevyn (Smith & Nephew, London, UK) or Lyofoa (Molnycke, Gothenburg, Sweden). Notably, many of these dressings exhibit antimicrobial properties through silver impregnation, but recent studies suggest silver may delay wound healing and should not be routinely used on uninfected donor skin [ 130 , 131 ] even though silver dressings may reduce wound pain [ 132 ]. In patients with extensive or deep burns, antimicrobial efficacy should be the first priority in burn wound care.
Alternatively, cell-based techniques for more permanent coverage have made progress. Research on cultured epithelial cells has made advancements, especially with respect to culture time. Culture-based options, such as Epicel (Genzyme, Cambridge, MA, USA), use a small biopsy of the patient’s skin to provide keratinocytes, which are expanded over 2 to 3 weeks (for Epicel, in the presence of proliferation-arrested murine fibroblasts) into a confluent epidermal autograft. Other options, such as ReCell (Avita, Northridge, CA, USA), take a small biopsy of the patient’s skin and prepare a mixture of keratinocytes, melanocytes, and stem cells in a liquid formulation for spraying onto the excised burn wound during the same operation [ 133 – 135 ]. These techniques may reduce the amount of donor skin needed for treatment of large burns, significantly reducing the healing time of both the donor and the burn sites, and increasing overall graft success and scar quality [ 136 ]. More work is needed on cell-based coverage options before widespread implementation can be recommended.
Keratinocytes and stem cells
As mentioned previously, keratinocytes play a vital role in wound closure. Cytokine activation causes keratinocyte migration in the proliferative phase, leading to closure and restoration of a vascular network [ 35 ]. Keratinocytes can also be activated by mu opioid receptor agonists [ 59 ] but the role of these agonists on inflammation and wound closure remains unclear [ 57 , 58 ]. Despite positive studies with EpiDex (Modex, Lausanne, Switzerland) – an engineered, fully differentiated autologous skin substitute derived from keratinocytes showing efficacy comparable with split-thickness skin grafts in wound closure and healing [ 137 ] – results have yet to translate into clinically viable options. Studies evaluating expansion of keratinocytes on human fibroblasts following trypsin extraction [ 138 ], and using engineered skin with keratinocytes on a fibrin matrix [ 139 ], have demonstrated improvements in wound healing. Retrospective analyses on autologous keratinocytes showed that cultured allogeneic or autologous keratinocytes may accelerate wound healing [ 140 , 141 ]. Taken together, the future impact of keratinocyte-mediated cell coverage options is promising, but more research is needed [ 134 ]. Additionally, keratinocyte-based treatments should be pursued carefully, as overactivation of keratinocytes can contribute to the development of hypertrophic scarring [ 43 , 142 ].
The use of adult stem cells, including bone marrow stem cells, hair follicle stem cells, and adipose stem cells, in acute burn care is an exciting topic [ 143 ]. Addition of bone marrow stem cells to nonhealing chronic wounds leads to engraftment of cells and enhanced wound healing [ 144 , 145 ]. Moreover, studies have reported that bone marrow stem cells can transdifferentiate towards multiple skin cell types [ 146 ]. Mechanisms of action of bone marrow stem cells in burns are not fully elucidated, but modulation of inflammation has occurred after radiation burns in humans [ 147 ]. Similarly, adipose stem cells accelerate re-epithelialization by paracrine activation of host cells via growth factor secretion [ 148 , 149 ]. Also, hair follicle stem cells are capable of generating a stratified epidermis on human burn wounds [ 150 ]. Additionally, the possibility of generating a cellular skin equivalent is being explored. Hair follicle stem cells have been incorporated into products, such as Integra, to investigate wound healing [ 151 ]. A cultured skin substitute using adipose stem cells and keratinocytes has been developed that produces epidermal, dermal, and hypodermal stratification [ 152 ]. Moreover, human adipose stem cells that would normally be discarded have recently been isolated from debrided burn eschar tissue [ 153 ] and used to generate a tri-layered, vascularized construct [ 154 ]. Promising data with nonembryonic stems cells such as these have stimulated interest into future applications and development, and undoubtedly further investigations will produce exciting results.
Other considerations and future directions
Monitoring and predicting wound healing.
No new skin-based technology can substitute for careful attention by the burn team to the progress (or lack thereof) of wound healing. The WoundFlow computer software program was developed as an enhancement over the traditional paper Lund–Browder diagram to more accurately quantify and track burn injuries over time [ 104 , 155 ]. WoundFlow is an electronic mapping program that calculates burn size and tracks wound healing [ 104 , 155 ]. The ability to accurately track burn wound healing over time will support both clinical care and future studies that compare healing rates and outcomes following different treatments. Notably, this study demonstrated that delayed wound healing was associated with a significantly higher risk of mortality [ 104 , 155 ].
The ability to predict whether a burn wound will spontaneously heal or not would greatly improve patient care. Furthermore, the ability to uniquely tailor treatment to each individual patient would improve patient outcomes and decrease the time to functional recovery, reducing the overall cost of care. Biomarkers may provide a means to allow for tailored treatments and to give insight into wound healing mechanisms [ 156 – 161 ]. Significant efforts in the search for predictive biomarkers for wound failure have determined that serum cytokines, such as interleukin-3 and 12p70, and serum procalcitonin are independently associated with wound failure [ 161 ]. Additional candidates have been identified [ 158 – 160 ] but further work is needed to model complex, temporal serum cytokine profiles into an effective predictor for wound healing. In addition to evaluating serum cytokine profiles, candidate biomarkers have been identified in wound effluent [ 161 ], which may be a better medium for predicting local wound healing than cytokines in the circulation [ 162 ]. Wound exudate has been shown to contain elevated levels of immunosuppressive and proinflammatory cytokines, such as interleukin-1β, interleukin-2, interleukin-6, and tumor necrosis factor alpha [ 163 ]. In fact, dipeptidyl peptidase IV and aminopeptidase have been identified in burn wound exudate with a significantly different ratio from that found in plasma [ 164 ]. Other work on local wound biomarkers using biopsies has shown that a host of proteins are upregulated during wound healing [ 165 ]. More work is needed to establish a biomarker profile that can accurately predict wound healing and to identify potential novel areas for therapeutic intervention.
In addition to examining burn wounds directly, and the wound exudate, another potential method for examining the ability of burn wounds to heal is non-invasive imaging [ 166 ]. To this end, a number of non-invasive imaging techniques have been investigated for their use in determining burn depth. Such techniques include terahertz imaging, spatial-frequency-domain imaging, near-infrared spectroscopic imaging, and reflectance-mode confocal microscopy, among others [ 167 – 172 ]. While many of these techniques have not yet been refined sufficiently for clinical application, the most successful research efforts into imaging techniques for burn wounds examine blood flow, such as laser Doppler imaging and indocyanine green angiography [ 173 ]. Laser Doppler imaging provides the most evidence for accurately assessing burn severity [ 174 ], but it has been shown that laser Doppler imaging is only superior to visual assessment 48 h after thermal injury [ 175 ]. Additional studies are needed to fully explore the potential for incorporation of non-invasive imaging modalities into the routine treatment of burn wounds.
Obese patients
As the obese population continues to grow [ 176 ], new treatment approaches will be required. Obese burn patients present with a variety of unique characteristics that include: increased rates of diabetes, hypertension, cardiac disease, and pulmonary disease; altered pharmacokinetics and pharmacodynamics; and altered immune responses [ 177 ]. Even the commonly used Lund–Browder chart for estimation of TBSA is problematic for obese patients because it fails to account for altered body-mass distribution in these patients [ 178 ]. Hence, analysis of group differences and controlled clinical studies in unique patient populations are needed [ 179 ].
Older patients
Census predictions suggest that the older population will double in the next 20 years. Since older people are at increased risk for burn injury, an increasing number of burn injuries among the older population should be expected. A recent review delineated the unique burn pathophysiology, comorbidities, and treatment strategies for the older population [ 180 ]. Detailing all of the unique considerations for the older burn population is outside the scope of this review, but several key points are noteworthy. Most burns among older people occur at home, especially in the kitchen and bathroom, due to diminished alertness, slower reaction time, and reduced mobility [ 181 ]. Reductions in metabolic rate and skin thickness with age result in more severe burns, and more extensive full-thickness burns are associated with increased mortality [ 182 ]. Comorbidities such as diabetes and cardiovascular disease complicate treatment, and may exacerbate the postburn hypermetabolic response [ 183 ]. Several formulas for predicting the survival of older patients, such as the Baux score [ 184 ], have received wide acceptance and can help guide clinicians in patient treatment. Unique treatment considerations for older patients should include attentive resuscitation to reduce the risk of volume overload, judicious ventilator support, careful analgesic administration, prudently timed excision and grafting, and extended rehabilitation for functional recovery [ 180 ]. The older population presents a unique challenge to the burn clinician, and the treatment of patients must be carefully considered on a case-by-case basis.
Future directions
Adult burn patients with increased markers of inflammatory stress exhibit reduced serum levels of vitamin A despite normal markers of oxidative stress [ 185 – 187 ]. Additionally, limited preclinical studies show that polyprenoic acid and retinol can facilitate wound healing [ 188 ], and that retinoids are efficacious on a variety of other skin conditions [ 189 ]. Moreover, early clinical studies have shown that retinoid treatment effectively increases scar elasticity [ 190 , 191 ]. Taken together, these data highlight the need for studies evaluating retinoids on burn wound healing outcomes.
Pirfenidone was originally developed as an antihelminthic and antipyretic agent, but recent work has demonstrated that it also has anti-inflammatory, antioxidative, and antiproliferative effects [ 192 ]. In particular, the antifibrotic properties of pirfenidone attenuate fibroblast proliferation and collagen deposition in vitro and in preclinical models [ 192 ]. Pirfenidone is approved for the treatment of idiopathic pulmonary fibrosis in Europe, Japan, and the USA. The antifibrotic actions of pirfenidone and other data suggest that pirfenidone could modulate the tissue response to injury at multiple stages of wound repair to improve scarring and function as an adjuvant for abnormal wound healing processes. Preclinical investigations are currently underway in rabbits [ 193 , 194 ] and rats [ 195 ], but controlled clinical studies are needed to evaluate the safety and efficacy of pirfenidone on abnormal wound healing.
The treatment of burn wounds with hyperbaric oxygen was first investigated in the mid-1960s and garnered some attention in the decades following, but controversy remains over potential risks and costs [ 196 , 197 ]. Recent work in rat models has shown that hyperbaric oxygen reduces healing time and improves scar appearance of burn injuries [ 198 ]. Advancements in hyperbaric chambers have reduced the overall cost associated with treatment, and controlled clinical trials in humans are beginning to produce data supporting the conclusion that hyperbaric oxygen is safe and effective for improving burn wound healing [ 199 – 201 ]. However, more data are needed before broad conclusions can be made about the overall utility of hyperbaric oxygen for treating burns.
Future research on burn patient care will focus on a variety of areas [ 202 ]. Considering a current survival rate of over 97 % for burn patients [ 3 ], major advancements from the past several decades have improved patient care such that significant future improvements in patient survival rate will be more difficult. However, improvements are still needed in individualized care, namely prediction of patient outcomes and the ability to tailor treatment to optimize functional recovery. Improvements are also needed to accelerate wound closure and healing and to improve psychological care to promote successful reintegration. Research in inflammation, infection, stem cells, grafting, biomarkers, inflammation control, and rehabilitation will continue to improve individualized care and create new treatment options.
The various clinical challenges in treating acute thermal injuries include balancing the many factors that affect wound healing to reduce the length of stay (and associated cost of treatment), the risk of infection, the time to wound closure, and the overall time to functional recovery. The treatment of burn wounds has evolved over several decades through clinical and preclinical research. Significant advancements have been made in patient care, including tracking wound healing, developing novel graft and coverage options, controlling inflammation, optimizing dietary needs, and testing unique pharmacological interventions. As a result of these efforts, patient survival has improved along with a concomitant decrease in the length of stay, which in turn results in a decreased cost to the patient and the medical providers. A summary of selected clinical recommendations is provided (Table 3 ) to aid the intensivist, but it is important to remember that burn patients present unique challenges based on multiple variables (for example, age, TBSA, comorbidities) and treatment decisions must be tailored to each patient’s needs. Current and future research will continue to identify novel targets and treatment paradigms to further improve burn wound care.
Abbreviations
Total body surface area
Gibran NS, Wiechman S, Meyer W, Edelman L, Fauerbach J, Gibbons L, et al. American Burn Association consensus statements. J Burn Care Res. 2013;34:361–5.
Article PubMed Google Scholar
Mann R, Heimbach D. Prognosis and treatment of burns. West J Med. 1996;165:215–20.
CAS PubMed PubMed Central Google Scholar
American Burn Association. Burn incidence and treatment in the United States: 2013 fact sheet. 2013. http://www.ameriburn.org/resources_factsheet.php . Accessed 12 May 2015.
Sen S, Palmieri T, Greenhalgh D. Review of burn research for the year 2013. J Burn Care Res. 2014;35:362–8.
Wolf SE, Arnoldo BD. The year in burns 2011. Burns. 2012;38:1096–108.
Burd A. Research in burns – present and future. Indian J Plast Surg. 2010;43:S11–4.
Article PubMed PubMed Central Google Scholar
Thomas SJ, Kramer GC, Herndon DN. Burns: military options and tactical solutions. J Trauma. 2003;54:S207–18.
PubMed Google Scholar
American Burn Association. National Burn Repository 2014. 2014. http://www.ameriburn.org/2014NBRAnnualReport.pdf . Accessed 12 May 2015.
Kagan RJ, Peck MD, Ahrenholz DH, Hickerson WL, Holmes J, Korentager R, et al. Surgical management of the burn wound and use of skin substitutes: an expert panel white paper. J Burn Care Res. 2013;34:e60–79.
Nisanci M, Eski M, Sahin I, Ilgan S, Isik S. Saving the zone of stasis in burns with activated protein C: an experimental study in rats. Burns. 2010;36:397–402.
Robins EV. Burn shock. Crit Care Nurs Clin North Am. 1990;2:299–307.
Article CAS PubMed Google Scholar
Pham TN, Cancio LC, Gibran NS, American Burn Association. American Burn Association practice guidelines burn shock resuscitation. J Burn Care Res. 2008;29:257–66.
Shirani KZ, Vaughan GM, Mason Jr AD, Pruitt Jr BA. Update on current therapeutic approaches in burns. Shock. 1996;5:4–16.
Dries DJ. Management of burn injuries – recent developments in resuscitation, infection control and outcomes research. Scand J Trauma Resusc Emerg Med. 2009;17:14.
Porter C, Hurren NM, Herndon DN, Borsheim E. Whole body and skeletal muscle protein turnover in recovery from burns. Int J Burns Trauma. 2013;3:9–17.
PubMed PubMed Central Google Scholar
Farina Jr JA, Rosique MJ, Rosique RG. Curbing inflammation in burn patients. Int J Inflamm. 2013;2013:715645.
Article CAS Google Scholar
Edgar DW, Fish JS, Gomez M, Wood FM. Local and systemic treatments for acute edema after burn injury: a systematic review of the literature. J Burn Care Res. 2011;32:334–47.
Sommer K, Sander AL, Albig M, Weber R, Henrich D, Frank J, et al. Delayed wound repair in sepsis is associated with reduced local pro-inflammatory cytokine expression. PLoS One. 2013;8, e73992.
Article CAS PubMed PubMed Central Google Scholar
Wilmore DW, Long JM, Mason Jr AD, Skreen RW, Pruitt Jr BA. Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg. 1974;180:653–69.
Sakallioglu AE, Basaran O, Karakayali H, Ozdemir BH, Yucel M, Arat Z, et al. Interactions of systemic immune response and local wound healing in different burn depths: an experimental study on rats. J Burn Care Res. 2006;27:357–66.
Pereira CT, Herndon DN. The pharmacologic modulation of the hypermetabolic response to burns. Adv Surg. 2005;39:245–61.
Hussain A, Dunn KW. Predicting length of stay in thermal burns: a systematic review of prognostic factors. Burns. 2013;39:1331–40.
Colohan SM. Predicting prognosis in thermal burns with associated inhalational injury: a systematic review of prognostic factors in adult burn victims. J Burn Care Res. 2010;31:529–39.
Jackson DM. The diagnosis of the depth of burning. Br J Surg. 1953;40:588–96.
Hettiaratchy S, Dziewulski P. ABC of burns: pathophysiology and types of burns. BMJ. 2004;328:1427–9.
Kowalske KJ. Burn wound care. Phys Med Rehab Clin North Am. 2011;22:213–27.
Article Google Scholar
Tan JQ, Zhang HH, Lei ZJ, Ren P, Deng C, Li XY, et al. The roles of autophagy and apoptosis in burn wound progression in rats. Burns. 2013;39:1551–6.
Singer AJ, McClain SA, Taira BR, Guerriero JL, Zong W. Apoptosis and necrosis in the ischemic zone adjacent to third degree burns. Acad Emerg Med. 2008;15:549–54.
Matylevitch NP, Schuschereba ST, Mata JR, Gilligan GR, Lawlor DF, Goodwin CW, et al. Apoptosis and accidental cell death in cultured human keratinocytes after thermal injury. Am J Pathol. 1998;153:567–77.
Deniz M, Borman H, Seyhan T, Haberal M. An effective antioxidant drug on prevention of the necrosis of zone of stasis: N-acetylcysteine. Burns. 2013;39:320–5.
Tiwari VK. Burn wound: how it differs from other wounds? Indian J Plast Surg. 2012;45:364–73.
Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–21.
Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012;49:35–43.
Werner S, Krieg T, Smola H. Keratinocyte–fibroblast interactions in wound healing. J Invest Dermatol. 2007;127:998–1008.
Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, Sawaya A, et al. Epithelialization in wound healing: a comprehensive review. Adv Wound Care. 2014;3:445–64.
Widgerow AD. Cellular/extracellular matrix cross-talk in scar evolution and control. Wound Repair Regen. 2011;19:117–33.
Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–46.
Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–37.
Snowden JM. Wound closure: an analysis of the relative contributions of contraction and epithelialization. J Surg Res. 1984;37:453–63.
Shih B, Garside E, McGrouther DA, Bayat A. Molecular dissection of abnormal wound healing processes resulting in keloid disease. Wound Repair Regen. 2010;18:139–53.
Claudinot S, Nicolas M, Oshima H, Rochat A, Barrandon Y. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc Natl Acad Sci U S A. 2005;102:14677–82.
Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–4.
Curran TA, Ghahary A. Evidence of a role for fibrocyte and keratinocyte-like cells in the formation of hypertrophic scars. J Burn Care Res. 2013;34:227–31.
Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166–72.
Arturson G. Forty years in burns research – the postburn inflammatory response. Burns. 2000;26:599–604.
Szpaderska AM, DiPietro LA. Inflammation in surgical wound healing: friend or foe? Surgery. 2005;137:571–3.
Franz MG, Steed DL, Robson MC. Optimizing healing of the acute wound by minimizing complications. Curr Probl Surg. 2007;44:691–763.
Stubhaug A, Romundstad L, Kaasa T, Breivik H. Methylprednisolone and ketorolac rapidly reduce hyperalgesia around a skin burn injury and increase pressure pain thresholds. Acta Anaesthesiol Scand. 2007;51:1138–46.
CAS PubMed Google Scholar
Huang G, Liang B, Liu G, Liu K, Ding Z. Low dose of glucocorticoid decreases the incidence of complications in severely burned patients by attenuating systemic inflammation. J Crit Care. 2015;30:e7–11.
Janzekovic Z. A new concept in the early excision and immediate grafting of burns. J Trauma. 1970;10:1103–8.
Orgill DP. Excision and skin grafting of thermal burns. N Engl J Med. 2009;360:893–901.
Barret JP, Herndon DN. Effects of burn wound excision on bacterial colonization and invasion. Plast Reconstruct Surg. 2003;111:744–50. discussion 751–2.
Cramer LM, McCormack CR, Carroll DB. Progressive partial excision and early graftin in lethal burns. Plast Reconstruct Surg Transplant Bull. 1962;30:595–9.
Engrav LH, Heimbach DM, Reus JL, Harnar TJ, Marvin JA. Early excision and grafting vs. nonoperative treatment of burns of indeterminant depth: a randomized prospective study. J Trauma. 1983;23:1001–4.
Stein C, Kuchler S. Non-analgesic effects of opioids: peripheral opioid effects on inflammation and wound healing. Curr Pharm Des. 2012;18:6053–69.
Brack A, Rittner HL, Stein C. Immunosuppressive effects of opioids – clinical relevance. J Neuroimmune Pharmacol. 2011;6:490–502.
Rook JM, Hasan W, McCarson KE. Morphine-induced early delays in wound closure: involvement of sensory neuropeptides and modification of neurokinin receptor expression. Biochem Pharmacol. 2009;77:1747–55.
Rook JM, McCarson KE. Delay of cutaneous wound closure by morphine via local blockade of peripheral tachykinin release. Biochem Pharmacol. 2007;74:752–7.
Bigliardi PL, Buchner S, Rufli T, Bigliardi-Qi M. Specific stimulation of migration of human keratinocytes by mu-opiate receptor agonists. J Recept Signal Transduct Res. 2002;22:191–9.
Stein C, Kuchler S. Targeting inflammation and wound healing by opioids. Trends Pharmacol Sci. 2013;34:303–12.
Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006;19:403–34.
Teplitz C, Davis D, Walker HL, Raulston GL, Mason Jr AD, Moncrief JA. Pseudomonas burn wound sepsis. II Hematogenous infection at the junction of the burn wound and the unburned hypodermis. J Surg Res. 1964;4:217–22.
Teplitz C, Davis D, Mason Jr AD, Moncrief JA. Pseudomonas burn wound sepsis. I Pathogenesis of experimental pseudomonas burn wound sepsis. J Surg Res. 1964;4:200–16.
Coban YK. Infection control in severely burned patients. World J Crit Care Med. 2012;1:94–101.
Branski LK, Al-Mousawi A, Rivero H, Jeschke MG, Sanford AP, Herndon DN. Emerging infections in burns. Surg Infect. 2009;10:389–97.
Shupp JW, Pavlovich AR, Jeng JC, Pezzullo JC, Oetgen WJ, Jaskille AD, et al. Epidemiology of bloodstream infections in burn-injured patients: a review of the national burn repository. J Burn Care Res. 2010;31:521–8.
Bloemsma GC, Dokter J, Boxma H, Oen IM. Mortality and causes of death in a burn centre. Burns. 2008;34:1103–7.
Chipp E, Milner CS, Blackburn AV. Sepsis in burns: a review of current practice and future therapies. Ann Plastic Surg. 2010;65:228–36.
Williams FN, Herndon DN, Hawkins HK, Lee JO, Cox RA, Kulp GA, et al. The leading causes of death after burn injury in a single pediatric burn center. Crit Care. 2009;13:R183.
Mann EA, Wood GL, Wade CE. Use of procalcitonin for the detection of sepsis in the critically ill burn patient: a systematic review of the literature. Burns. 2011;37:549–58.
Greenhalgh DG, Saffle JR, Holmes JH, Gamelli RL, Palmieri TL, Horton JW, et al. American Burn Association consensus conference to define sepsis and infection in burns. J Burn Care Res. 2007;28:776–90.
D'Avignon LC, Chung KK, Saffle JR, Renz EM, Cancio LC. Prevention of Combat-Related Infections Guidelines Panel. Prevention of infections associated with combat-related burn injuries. J Trauma. 2011;71:S282–9.
D'Avignon LC, Hogan BK, Murray CK, Loo FL, Hospenthal DR, Cancio LC, et al. Contribution of bacterial and viral infections to attributable mortality in patients with severe burns: an autopsy series. Burns. 2010;36:773–9.
Hospenthal DR, Murray CK, Andersen RC, Bell RB, Calhoun JH, Cancio LC, et al. Guidelines for the prevention of infections associated with combat-related injuries: 2011 update: endorsed by the Infectious Diseases Society of America and the Surgical Infection Society. J Trauma. 2011;71:S210–34.
Hospenthal DR, Murray CK, Andersen RC, Blice JP, Calhoun JH, Cancio LC, et al. Guidelines for the prevention of infection after combat-related injuries. J Trauma. 2008;64:S211–20.
Rafla K, Tredget EE. Infection control in the burn unit. Burns. 2011;37:5–15.
Rowley-Conwy G. Infection prevention and treatment in patients with major burn injuries. Nurs Stand. 2010;25:51–2. 54, 56–8 passim.
Brown TP, Cancio LC, McManus AT, Mason Jr AD. Survival benefit conferred by topical antimicrobial preparations in burn patients: a historical perspective. J Trauma. 2004;56:863–6.
Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis. 2014;14:498–509.
Horvath EE, Murray CK, Vaughan GM, Chung KK, Hospenthal DR, Wade CE, et al. Fungal wound infection (not colonization) is independently associated with mortality in burn patients. Ann Surg. 2007;245:978–85.
Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363:1895–902.
Williams FN, Herndon DN, Jeschke MG. The hypermetabolic response to burn injury and interventions to modify this response. Clin Plast Surg. 2009;36:583–96.
Andel H, Kamolz LP, Horauf K, Zimpfer M. Nutrition and anabolic agents in burned patients. Burns. 2003;29:592–5.
Abdullahi A, Jeschke MG. Nutrition and anabolic pharmacotherapies in the care of burn patients. Nutr Clin Pract. 2014;29:621–30.
Hart DW, Wolf SE, Chinkes DL, Beauford RB, Mlcak RP, Heggers JP, et al. Effects of early excision and aggressive enteral feeding on hypermetabolism, catabolism, and sepsis after severe burn. J Trauma. 2003;54:755–61. discussion 761–4.
Mosier MJ, Pham TN, Klein MB, Gibran NS, Arnoldo BD, Gamelli RL, et al. Early enteral nutrition in burns: compliance with guidelines and associated outcomes in a multicenter study. J Burn Care Res. 2011;32:104–9.
Mecott GA, Al-Mousawi AM, Gauglitz GG, Herndon DN, Jeschke MG. The role of hyperglycemia in burned patients: evidence-based studies. Shock. 2010;33:5–13.
Gore DC, Chinkes DL, Hart DW, Wolf SE, Herndon DN, Sanford AP. Hyperglycemia exacerbates muscle protein catabolism in burn-injured patients. Crit Care Med. 2002;30:2438–42.
Kulp GA, Tilton RG, Herndon DN, Jeschke MG. Hyperglycemia exacerbates burn-induced liver inflammation via noncanonical nuclear factor-kappaB pathway activation. Mol Med. 2012;18:948–56.
Cunningham-Rundles S, McNeeley DF, Moon A. Mechanisms of nutrient modulation of the immune response. J Allergy Clin Immunol. 2005;115:1119–28. quiz 1129.
Schwacha MG, Chaudry IH. The cellular basis of post-burn immunosuppression: macrophages and mediators. Int J Mol Med. 2002;10:239–43.
Ferrando AA, Chinkes DL, Wolf SE, Matin S, Herndon DN, Wolfe RR. A submaximal dose of insulin promotes net skeletal muscle protein synthesis in patients with severe burns. Ann Surg. 1999;229:11–8.
Hrynyk M, Neufeld RJ. Insulin and wound healing. Burns. 2014;40:1433–46.
Pidcoke HF, Baer LA, Wu X, Wolf SE, Aden JK, Wade CE. Insulin effects on glucose tolerance, hypermetabolic response, and circadian-metabolic protein expression in a rat burn and disuse model. Am J Physiol Regul Integr Comp Physiol. 2014;307:R1–10.
Pidcoke HF, Wade CE, Wolf SE. Insulin and the burned patient. Crit Care Med. 2007;35:S524–30.
Sakurai Y, Aarsland A, Herndon DN, Chinkes DL, Pierre E, Nguyen TT, et al. Stimulation of muscle protein synthesis by long-term insulin infusion in severely burned patients. Ann Surg. 1995;222:283–94.
Hart DW, Wolf SE, Ramzy PI, Chinkes DL, Beauford RB, Ferrando AA, et al. Anabolic effects of oxandrolone after severe burn. Ann Surg. 2001;233:556–64.
Tuvdendorj D, Chinkes DL, Zhang XJ, Suman OE, Aarsland A, Ferrando A, et al. Long-term oxandrolone treatment increases muscle protein net deposition via improving amino acid utilization in pediatric patients 6 months after burn injury. Surgery. 2011;149:645–53.
Wolf SE, Edelman LS, Kemalyan N, Donison L, Cross J, Underwood M, et al. Effects of oxandrolone on outcome measures in the severely burned: a multicenter prospective randomized double-blind trial. J Burn Care Res. 2006;27:131–9. discussion 140–1.
Wolf SE, Thomas SJ, Dasu MR, Ferrando AA, Chinkes DL, Wolfe RR, et al. Improved net protein balance, lean mass, and gene expression changes with oxandrolone treatment in the severely burned. Ann Surg. 2003;237:801–10. discussion 810–1.
Bains JW, Crawford DT, Ketcham AS. Effect of chronic anemia on wound tensile strength: correlation with blood volume, total red blood cell volume and proteins. Ann Surg. 1966;164:243–6.
Agarwal P, Prajapati B, Sharma D. Evaluation of skin graft take following post-burn raw area in normovolaemic anaemia. Indian J Plast Surg. 2009;42:195–8.
Namdar T, Stollwerck PL, Stang FH, Eisenbeiss W, Siemers F, Mailander P, et al. Impact of hypernatremia on burn wound healing: results of an exploratory, retrospective study. Ostomy Wound Manage. 2011;57:30–4.
Nitzschke SL, Aden JK, Serio-Melvin ML, Shingleton SK, Chung KK, Waters JA, et al. Wound healing trajectories in burn patients and their impact on mortality. J Burn Care Res. 2014;35:474–9.
Desai MH, Herndon DN, Broemeling L, Barrow RE, Nichols Jr RJ, Rutan RL. Early burn wound excision significantly reduces blood loss. Ann Surg. 1990;211:753–9. discussion 759–62.
Herndon DN, Barrow RE, Rutan RL, Rutan TC, Desai MH, Abston S. A comparison of conservative versus early excision. Therapies in severely burned patients. Ann Surg. 1989;209:547–52. discussion 552–3.
Saaiq M, Zaib S, Ahmad S. Early excision and grafting versus delayed excision and grafting of deep thermal burns up to 40 % total body surface area: a comparison of outcome. Ann Burns Fire Disasters. 2012;25:143–7.
Vinita P, Khare NA, Chandramouli M, Nilesh S, Sumit B. Comparative analysis of early excision and grafting vs delayed grafting in burn patients in a developing country. J Burn Care Res. 2014; doi:10.1097/BCR.0b013e31827e4ed6.
Ong YS, Samuel M, Song C. Meta-analysis of early excision of burns. Burns. 2006;32:145–50.
Schwanholt C, Greenhalgh DG, Warden GD. A comparison of full-thickness versus split-thickness autografts for the coverage of deep palm burns in the very young pediatric patient. J Burn Care Rehab. 1993;14:29–33.
Akan M, Yildirim S, Misirlioglu A, Ulusoy G, Akoz T, Avci G. An alternative method to minimize pain in the split-thickness skin graft donor site. Plast Reconstruct Surg. 2003;111:2243–9.
Voineskos SH, Ayeni OA, McKnight L, Thoma A. Systematic review of skin graft donor-site dressings. Plast Reconstruct Surg. 2009;124:298–306.
Hermans MH. Preservation methods of allografts and their (lack of) influence on clinical results in partial thickness burns. Burns. 2011;37:873–81.
Hermans MH. Porcine xenografts vs. (cryopreserved) allografts in the management of partial thickness burns: is there a clinical difference? Burns. 2014;40:408–15.
Ehrenreich M, Ruszczak Z. Tissue-engineered temporary wound coverings. Important options for the clinician. Acta Dermatovenerol Alp Pannonica Adriat. 2006;15:5–13.
Ehrenreich M, Ruszczak Z. Update on tissue-engineered biological dressings. Tissue Eng. 2006;12:2407–24.
Groeber F, Holeiter M, Hampel M, Hinderer S, Schenke-Layland K. Skin tissue engineering – in vivo and in vitro applications. Clin Plast Surg. 2012;39:33–58.
Mansbridge J. Skin tissue engineering. J Biomater Sci Polym Ed. 2008;19:955–68.
Mansbridge JN. Tissue-engineered skin substitutes in regenerative medicine. Curr Opin Biotechnol. 2009;20:563–7.
Catalano E, Cochis A, Varoni E, Rimondini L, Azzimonti B. Tissue-engineered skin substitutes: an overview. J Artif. 2013;16:397–403.
Shevchenko RV, James SL, James SE. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J R Soc Interface. 2010;7:229–58.
Atiyeh BS, Costagliola M. Cultured epithelial autograft (CEA) in burn treatment: three decades later. Burns. 2007;33:405–13.
Fang T, Lineaweaver WC, Sailes FC, Kisner C, Zhang F. Clinical application of cultured epithelial autografts on acellular dermal matrices in the treatment of extended burn injuries. Ann Plast Surg. 2014;73:509–15.
Jeschke MG, Finnerty CC, Shahrokhi S, Branski LK, Dibildox M, Organization ABA, et al. Wound coverage technologies in burn care: novel techniques. J Burn Care Res. 2013;34:612–20.
Supp DM, Boyce ST. Engineered skin substitutes: practices and potentials. Clin Dermatol. 2005;23:403–12.
Kampmann A, Lindhorst D, Schumann P, Zimmerer R, Kokemuller H, Rucker M, et al. Additive effect of mesenchymal stem cells and VEGF to vascularization of PLGA scaffolds. Microvasc Res. 2013;90:71–9.
Park KM, Gerecht S. Harnessing developmental processes for vascular engineering and regeneration. Development. 2014;141:2760–9.
Broussard KC, Powers JG. Wound dressings: selecting the most appropriate type. Am J Clin Dermatol. 2013;14:449–59.
Wasiak J, Cleland H, Campbell F, Spinks A. Dressings for superficial and partial thickness burns. Cochrane Database Syst Rev. 2013;3, CD002106.
Google Scholar
Aziz Z, Abu SF, Chong NJ. A systematic review of silver-containing dressings and topical silver agents (used with dressings) for burn wounds. Burns. 2012;38:307–18.
Nikkhah D, Gilbert P, Booth S, Dheansa B. Should we be using silver based compounds for donor site dressing in thermal burns? Burns. 2013;39:1324–5.
Abboud EC, Legare TB, Settle JC, Boubekri AM, Barillo DJ, Marcet JE, et al. Do silver-based wound dressings reduce pain? A prospective study and review of the literature. Burns. 2014;40:S40–7.
Navarro FA, Stoner ML, Park CS, Huertas JC, Lee HB, Wood FM, et al. Sprayed keratinocyte suspensions accelerate epidermal coverage in a porcine microwound model. J Burn Care Rehab. 2000;21:513–8.
Wood FM, Kolybaba ML, Allen P. The use of cultured epithelial autograft in the treatment of major burn wounds: eleven years of clinical experience. Burns. 2006;32:538–44.
Wood FM, Kolybaba ML, Allen P. The use of cultured epithelial autograft in the treatment of major burn injuries: a critical review of the literature. Burns. 2006;32:395–401.
Tenenhaus M, Rennekampff HO. Surgical advances in burn and reconstructive plastic surgery: new and emerging technologies. Clin Plast Surg. 2012;39:435–43.
Tausche AK, Skaria M, Bohlen L, Liebold K, Hafner J, Friedlein H, et al. An autologous epidermal equivalent tissue-engineered from follicular outer root sheath keratinocytes is as effective as split-thickness skin autograft in recalcitrant vascular leg ulcers. Wound Repair Regen. 2003;11:248–52.
Bisson F, Rochefort E, Lavoie A, Larouche D, Zaniolo K, Simard-Bisson C, et al. Irradiated human dermal fibroblasts are as efficient as mouse fibroblasts as a feeder layer to improve human epidermal cell culture lifespan. Int J Mol Sci. 2013;14:4684–704.
Idrus RB, Rameli MA, Low KC, Law JX, Chua KH, Latiff MB, et al. Full-thickness skin wound healing using autologous keratinocytes and dermal fibroblasts with fibrin: bilayered versus single-layered substitute. Adv Skin Wound Care. 2014;27:171–80.
Auxenfans C, Menet V, Catherine Z, Shipkov H, Lacroix P, Bertin-Maghit M, et al. Cultured autologous keratinocytes in the treatment of large and deep burns: a retrospective study over 15 years. Burns. 2015;41:71–9.
Auxenfans C, Shipkov H, Bach C, Catherine Z, Lacroix P, Bertin-Maghit M, et al. Cultured allogenic keratinocytes for extensive burns: a retrospective study over 15 years. Burns. 2014;40:82–8.
van der Veer WM, Bloemen MC, Ulrich MM, Molema G, van Zuijlen PP, Middelkoop E, et al. Potential cellular and molecular causes of hypertrophic scar formation. Burns. 2009;35:15–29.
Lewis CJ. Stem cell application in acute burn care and reconstruction. J Wound Care. 2013;22:7–8. 10, 12–6.
Badiavas EV. The potential of bone marrow cells to orchestrate homeostasis and healing in skin. Blood Cells Mol Dis. 2004;32:21–3.
Badiavas EV, Abedi M, Butmarc J, Falanga V, Quesenberry P. Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol. 2003;196:245–50.
Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180:2581–7.
Bey E, Prat M, Duhamel P, Benderitter M, Brachet M, Trompier F, et al. Emerging therapy for improving wound repair of severe radiation burns using local bone marrow-derived stem cell administrations. Wound Repair Regen. 2010;18:50–8.
Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ, et al. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48:15–24.
Nakagami H, Maeda K, Morishita R, Iguchi S, Nishikawa T, Takami Y, et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 2005;25:2542–7.
Kurata S, Itami S, Terashi H, Takayasu S. Successful transplantation of cultured human outer root sheath cells as epithelium. Ann Plast Surg. 1994;33:290–4.
Navsaria HA, Ojeh NO, Moiemen N, Griffiths MA, Frame JD. Reepithelialization of a full-thickness burn from stem cells of hair follicles micrografted into a tissue-engineered dermal template (Integra). Plast Reconstruct Surg. 2004;113:978–81.
Trottier V, Marceau-Fortier G, Germain L, Vincent C, Fradette J. IFATS collection: using human adipose-derived stem/stromal cells for the production of new skin substitutes. Stem Cells. 2008;26:2713–23.
Natesan S, Wrice NL, Baer DG, Christy RJ. Debrided skin as a source of autologous stem cells for wound repair. Stem Cells. 2011;29:1219–30.
Chan RK, Zamora DO, Wrice NL, Baer DG, Renz EM, Christy RJ, et al. Development of a vascularized skin construct using adipose-derived stem cells from debrided burned skin. Stem Cells Int. 2012;2012:841203.
Article PubMed PubMed Central CAS Google Scholar
Williams JF, King BT, Aden JK, Serio-Melvin M, Chung KK, Fenrich CA, et al. Comparison of traditional burn wound mapping with a computerized program. J Burn Care Res. 2013;34:e29–35.
Brown TS, Safford S, Caramanica J, Elster EA. Biomarker use in tailored combat casualty care. Biomark Med. 2010;4:465–73.
Hawksworth JS, Stojadinovic A, Gage FA, Tadaki DK, Perdue PW, Forsberg J, et al. Inflammatory biomarkers in combat wound healing. Ann Surg. 2009;250:1002–7.
Hahm G, Glaser JJ, Elster EA. Biomarkers to predict wound healing: the future of complex war wound management. Plast Reconstruct Surg. 2011;127:21S–6S.
Chromy BA, Eldridge A, Forsberg JA, Brown TS, Kirkup BC, Elster E, et al. Proteomic sample preparation for blast wound characterization. Proteome Sci. 2014;12:10.
Chromy BA, Eldridge A, Forsberg JA, Brown TS, Kirkup BC, Jaing C, et al. Wound outcome in combat injuries is associated with a unique set of protein biomarkers. J Transl Med. 2013;11:281.
Forsberg JA, Potter BK, Polfer EM, Safford SD, Elster EA. Do inflammatory markers portend heterotopic ossification and wound failure in combat wounds? Clin Orthop Relat Res. 2014;472:2845–54.
Mikhal'chik EV, Piterskaya JA, Budkevich LY, Pen'kov LY, Facchiano A, De Luca C, et al. Comparative study of cytokine content in the plasma and wound exudate from children with severe burns. Bull Exp Biol Med. 2009;148:771–5.
Widgerow AD, King K, Tussardi IT, Banyard DA, Chiang R, Awad A, et al. The burn wound exudate – an under-utilized resource. Burns. 2015;41:11–7.
Prager MD, Sabeh F, Baxter CR, Atiles L, Hartline B. Dipeptidyl peptidase IV and aminopeptidase in burn wound exudates: implications for wound healing. J Trauma. 1994;36:629–33.
Mauskar NA, Sood S, Travis TE, Matt SE, Mino MJ, Burnett MS, et al. Donor site healing dynamics: molecular, histological, and noninvasive imaging assessment in a porcine model. J Burn Care Res. 2013;34:549–62.
Kaiser M, Yafi A, Cinat M, Choi B, Durkin AJ. Noninvasive assessment of burn wound severity using optical technology: a review of current and future modalities. Burns. 2011;37:377–86.
Arbab MH, Dickey TC, Winebrenner DP, Chen A, Klein MB, Mourad PD. Terahertz reflectometry of burn wounds in a rat model. Biomed Opt Express. 2011;2:2339–47.
Cross KM, Leonardi L, Gomez M, Freisen JR, Levasseur MA, Schattka BJ, et al. Noninvasive measurement of edema in partial thickness burn wounds. J Burn Care Res. 2009;30:807–17.
Cross KM, Leonardi L, Payette JR, Gomez M, Levasseur MA, Schattka BJ, et al. Clinical utilization of near-infrared spectroscopy devices for burn depth assessment. Wound Repair Regen. 2007;15:332–40.
Nguyen JQ, Crouzet C, Mai T, Riola K, Uchitel D, Liaw LH, et al. Spatial frequency domain imaging of burn wounds in a preclinical model of graded burn severity. J Biomed Opt. 2013;18:66010.
Sowa MG, Leonardi L, Payette JR, Cross KM, Gomez M, Fish JS. Classification of burn injuries using near-infrared spectroscopy. J Biomed Opt. 2006;11:054002.
Sowa MG, Leonardi L, Payette JR, Fish JS, Mantsch HH. Near infrared spectroscopic assessment of hemodynamic changes in the early post-burn period. Burns. 2001;27:241–9.
Devgan L, Bhat S, Aylward S, Spence RJ. Modalities for the assessment of burn wound depth. J Burns Wounds. 2006;5, e2.
Pape SA, Skouras CA, Byrne PO. An audit of the use of laser Doppler imaging (LDI) in the assessment of burns of intermediate depth. Burns. 2001;27:233–9.
Hoeksema H, Van de Sijpe K, Tondu T, Hamdi M, Van Landuyt K, Blondeel P, et al. Accuracy of early burn depth assessment by laser Doppler imaging on different days post burn. Burns. 2009;35:36–45.
Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–14.
Goutos I, Sadideen H, Pandya AA, Ghosh SJ. Obesity and burns. J Burn Care Res. 2012;33:471–82.
Neaman KC, Andres LA, McClure AM, Burton ME, Kemmeter PR, Ford RD. A new method for estimation of involved BSAs for obese and normal-weight patients with burn injury. J Burn Care Res. 2011;32:421–8.
Liodaki E, Senyaman O, Stollwerck PL, Mollmeier D, Mauss KL, Mailander P, et al. Obese patients in a burn care unit: a major challenge. Burns. 2014;40:1738–42.
Keck M, Lumenta DB, Andel H, Kamolz LP, Frey M. Burn treatment in the elderly. Burns. 2009;35:1071–9.
Lewandowski R, Pegg S, Fortier K, Skimmings A. Burn injuries in the elderly. Burns. 1993;19:513–5.
Hunt JL, Purdue GF. The elderly burn patient. Am J Surg. 1992;164:472–6.
Williams GJ, Herndon DN. Modulating the hypermetabolic response to burn injuries. J Wound Care. 2002;11:87–9.
Roberts G, Lloyd M, Parker M, Martin R, Philp B, Shelley O, et al. The Baux score is dead. Long live the Baux score: a 27-year retrospective cohort study of mortality at a regional burns service. J Trauma Acute Care Surg. 2012;72:251–6.
Nordlund MJ, Pham TN, Gibran NS. Micronutrients after burn injury: a review. J Burn Care Res. 2014;35:121–33.
Pintaudi AM, Tesoriere L, D'Arpa N, D'Amelio L, D'Arpa D, Bongiorno A, et al. Oxidative stress after moderate to extensive burning in humans. Free Radic Res. 2000;33:139–46.
Vinha PP, Martinez EZ, Vannucchi H, Marchini JS, Farina Jr JA, Jordao Jr AA, et al. Effect of acute thermal injury in status of serum vitamins, inflammatory markers, and oxidative stress markers: preliminary data. J Burn Care Res. 2013;34:e87–91.
Aida T, Murata J, Asano G, Kanda Y, Yoshino Y. Effects of polyprenoic acid on thermal injury. Br J Exp Pathol. 1987;68:351–8.
Nickle SB, Peterson N, Peterson M. Updated physician's guide to the off-label uses of oral isotretinoin. J Clin Aesthet Dermatol. 2014;7:22–34.
Dematte MF, Gemperli R, Salles AG, Dolhnikoff M, Lancas T, Saldiva PH, et al. Mechanical evaluation of the resistance and elastance of post-burn scars after topical treatment with tretinoin. Clinics. 2011;66:1949–54.
Salles AG, Gemperli R, Toledo PN, Ferreira MC. Combined tretinoin and glycolic acid treatment improves mouth opening for postburn patients. Aesthet Plast Surg. 2006;30:356–62.
Macias-Barragan J, Sandoval-Rodriguez A, Navarro-Partida J, Armendariz-Borunda J. The multifaceted role of pirfenidone and its novel targets. Fibrogenesis Tissue Repair. 2010;3:16.
Jung KI, Choi JS, Kim HK, Shin SY. Effects of an anti-transforming growth factor-beta agent (pirfenidone) on strabismus surgery in rabbits. Curr Eye Res. 2012;37:770–6.
Zhong H, Sun G, Lin X, Wu K, Yu M. Evaluation of pirfenidone as a new postoperative antiscarring agent in experimental glaucoma surgery. Invest Ophthalmol Vis Sci. 2011;52:3136–42.
Chowdhury S, Guha R, Trivedi R, Kompella UB, Konar A, Hazra S. Pirfenidone nanoparticles improve corneal wound healing and prevent scarring following alkali burn. PLoS One. 2013;8, e70528.
Cianci P, Sato R. Adjunctive hyperbaric oxygen therapy in the treatment of thermal burns: a review. Burns. 1994;20:5–14.
Cianci P, Williams C, Lueders H, Lee H, Shapiro R, Sexton J, et al. Adjunctive hyperbaric oxygen in the treatment of thermal burns. An economic analysis. J Burn Care Rehab. 1990;11:140–3.
Selcuk CT, Ozalp B, Durgun M, Tekin A, Akkoc MF, Alabalik U, et al. The effect of hyperbaric oxygen treatment on the healing of burn wounds in nicotinized and nonnicotinized rats. J Burn Care Res. 2013;34:e237–43.
Cianci P, Slade Jr JB, Sato RM, Faulkner J. Adjunctive hyperbaric oxygen therapy in the treatment of thermal burns. Undersea Hyperb Med. 2013;40:89–108.
Eskes A, Vermeulen H, Lucas C, Ubbink DT. Hyperbaric oxygen therapy for treating acute surgical and traumatic wounds. Cochrane Database Syst Rev. 2013;12, CD008059.
Eskes AM, Ubbink DT, Lubbers MJ, Lucas C, Vermeulen H. Hyperbaric oxygen therapy: solution for difficult to heal acute wounds? Systematic review. World J Surg. 2011;35:535–42.
Wolf SE, Tompkins RG, Herndon DN. On the horizon: research priorities in burns for the next decade. Surg Clin North Am. 2014;94:917–30.
Download references
Acknowledgements
The authors would like to thank the staff of the Clinical Trials task area at the US Army Institute of Surgical Research for administrative support. The authors would also like to thank Dr Harold Klemcke for critical review of this manuscript. This work was supported in part by an appointment (MPR) to the Postgraduate Research Participation Program and an appointment (LCC) to the Knowledge Preservation Program at the US Army Institute of Surgical Research administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and US Army Medical Research and Materiel Command.
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
Author information
Authors and affiliations.
United States Army Institute for Surgical Research, 3698 Chambers Pass, Fort Sam Houston, TX, 78234, USA
Matthew P. Rowan, Leopoldo C. Cancio, David M. Burmeister, Lloyd F. Rose, Shanmugasundaram Natesan, Rodney K. Chan, Robert J. Christy & Kevin K. Chung
Uniformed Services University of the Health Sciences, 4301 Jones Bridge Rd, Bethesda, MD, 20814, USA
Eric A. Elster & Kevin K. Chung
Brooke Army Medical Center, 3551 Roger Brook Dr, Fort Sam Houston, TX, 78234, USA
Rodney K. Chan
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Matthew P. Rowan .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors’ contributions
MPR and KKC outlined the paper. MPR wrote all drafts of the manuscript, with primary editing and revision support from LCC. All authors contributed information for the manuscript, participated in its revision, and approved the final version for publication.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Rowan, M.P., Cancio, L.C., Elster, E.A. et al. Burn wound healing and treatment: review and advancements. Crit Care 19 , 243 (2015). https://doi.org/10.1186/s13054-015-0961-2
Download citation
Published : 01 December 2015
DOI : https://doi.org/10.1186/s13054-015-0961-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Wound Healing
- Idiopathic Pulmonary Fibrosis
- Bone Marrow Stem Cell
- Pirfenidone
- Total Body Surface Area
View archived comments (1)
Critical Care
ISSN: 1364-8535
- Submission enquiries: [email protected]
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Publish with us
- About the journal
- Meet the editors
- Specialist reviews
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 1, Issue 1
- Improving the comparability and quality of burn research
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0001-5238-4796 Sarah E Bache and
- David Barnes
- Adult and Paediatric Burns Centre , St Andrew's Centre of Plastic Surgery and Burns, Broomfield Hospital , Chelmsford , Essex , UK
- Correspondence to Sarah E Bache, c/o Burns Centre, St Andrew's Centre for Plastic Surgery and Burns, Broomfield Hospital, Chelmsford CM1 7ET, Essex, UK; sarahbache{at}doctors.org.uk
https://doi.org/10.1136/bmjmed-2022-000273
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Determining pertinent outcomes in burn care to be reported in all future trials
Burn injuries contribute considerably to the global healthcare burden, with an estimated 11 million people annually affected worldwide. 1 The impact of burn trauma can be destructive, lifelong, and indiscriminate. People of all ages, ethnic origins, and backgrounds are at risk. But it is the most vulnerable in society who are disproportionately affected: children, elderly people, individuals with poor mental or physical health, and those of low socioeconomic status. However, the past 50 years have seen substantial reductions in burn mortality, largely due to early excision and grafting, improved burn resuscitation, intensive care treatment, and better management of sepsis and wound care. Survival is now expected for the vast majority of people, even after severe burn injuries. 2 The focus of the next 50 years will be on improving outcomes for survivors. Scarring, functionality, cosmesis, psychological, and long term physical impact are just a few examples of areas of research and focus for the worldwide burn community.
The evaluation and comparison of burn treatments presents a difficult challenge because they are a heterogenous group of injuries. Burn size, depth, anatomical location, cause, and patient factors are inconsistent. Additionally, the outcomes measured are also heterogenous, reliant on the preferences of researchers and not necessarily those most important to patients. Decreasing incidence of major burn in high income countries limits recruitment to trials, so evidence increasingly relies either on multicentre collaboration or systematic reviews. In the past, authors of systematic reviews have been prohibited from drawing firm conclusions by a lack of comparable outcomes in burn studies. The linked article by Young et al 3 (doi:10.1136/bmjmed-2022-000183) is the first step towards reporting consistency through the development of a core outcome set for burn care research (COSB-i). The ultimate aim is for future burn research papers to include (but not be limited to) this common set of pertinent outcomes.
But what outcomes are considered most important—not only to those delivering burn care, but also to the patients and carers receiving it? Previous papers identified a framework of outcomes following the agreement of multidisciplinary burn teams. 4 However, Young and colleagues have built on this work by producing a core outcome set through established methods outlined by the COMET (core outcome measures in effectiveness trials) initiative. 5 6 Core outcome sets are scientifically identified by stakeholders (including clinical staff, commissioners and, most importantly, patients) as those most crucial in determining the effects of an intervention. They have the potential to prevent wasted time and resources by directing researchers towards only the most relevant outcomes when embarking on a trial. In addition, core outcome sets can reduce selective reporting of favourable findings, and ensure that study outcomes are meaningful and relevant to stakeholders. If widely used, they will facilitate comparison between trials, evidence synthesis, and better quality systematic reviews and will therefore have a considerable impact on the quality of future burn research.
The authors’ methodology is clearly described, following their published protocol and using the core outcome set standards for development (COS-STAD). 7 8 Three key stages are described. Firstly, a comprehensive long list of 1021 unique outcomes was identified through a systematic literature review of randomised controlled trials on burn injury, patient reported outcomes, and semi-structured interviews of 15 patients and 10 clinicians. 9–11 Secondly, a Delphi survey comprising two rounds of questionnaires completed by 668 worldwide health professionals and 126 UK patients or carers enabled the creation of a shortlist of 31 outcomes. Thirdly, a stakeholder meeting of 28 UK and 19 international clinical staff was held to decide by vote the final seven core outcomes.
The final seven outcomes were: death; specified complications (eg, sepsis or wound infection); ability to do daily tasks; time to wound healing; long term neuropathic pain and itch; psychological wellbeing; and return to school or work. Several of these outcomes emphasise long term function, rather than the short term physiological markers that are often used by clinicians. This list reflects the co-production and participation of a wide group of stakeholders, and is a strength of the work. The inclusion of these outcomes in any study of burn research will be a marker of the standard of research and the importance of findings to the burn community.
Young and colleagues have demonstrated an admirable commitment to co-production in their strategy, through the involvement of clinicians and patients. The process was overseen by a steering group of members of the burn multidisciplinary team and UK patients. Patients were also involved in the study design, both rounds of questionnaires, and the consensus meeting. The authors were unable to recruit international patients, owing to the financial and time implications of translating and distributing the survey to patients worldwide. This limitation could restrict the relevance of the outcomes globally. The study remains, however, a comprehensive attempt to garner international opinion on burn outcomes, with 77 countries represented by professionals, 18% of whom were from low and low middle income countries.
Research into burn and scar management is on the cusp of further advances in the coming years. The creation of the COSB-i is therefore a timely and important first step in improving the quality and comparability of burn research. The resulting seven outcomes are broad and undefined in nature and scope. While the COSB-i identifies what should be reported in future burn trials, the next step is to determine how these outcomes should be defined and measured.
- Jackson PC ,
- Hardwicke J ,
- Bamford A , et al
- Edgar D , et al
- Williamson PR ,
- Altman DG ,
- Bagley H , et al
- Blazeby JM , et al
- Brookes S ,
- Rumsey N , et al
- Kirkham JJ ,
- Altman DG , et al
- Bland S , et al
- Griffiths C ,
- Armstrong-James L ,
- White P , et al
Contributors Both authors have substantially contributed to the editorial, and agree on the final document.
Competing interests We have read and understood the BMJ policy on declaration of interests and declare the following interests: DB has been an expert witness for the UK Crown Prosecution Service and UK family court; and has received commercial research funding from Smart Matrix and Smith and Nephew for wound care and dressings research, and travel and conference fees from Mediwound, a manufacturer and supplier of debridement agents.
Provenance and peer review Commissioned; not externally peer reviewed.
Linked Articles
- Research Establishment of a core outcome set for burn care research: development and international consensus Amber Young Anna Davies Carmen Tsang Jamie Kirkham Tom Potokar Nicole Gibran Zephanie Tyack Jill Meirte Teruichi Harada Baljit Dheansa Jo Dumville Chris Metcalfe Rajeev Ahuja Fiona Wood Sarah Gaskell Sara Brookes Sarah Smailes Marc Jeschke Murat Ali Cinar Nukhba Zia Amr Moghazy Jonathan Mathers Sian Falder Dale Edgar Jane Mary Blazeby BMJ Medicine 2022; 1 - Published Online First: 08 Jul 2022. doi: 10.1136/bmjmed-2022-000183
Read the full text or download the PDF:
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Initial assessment and...
Initial assessment and management of burns
- Related content
- Peer review
- Philip McCaughey , fourth year medical student 1 ,
- Sandra McAllister , academic clinical lecturer 2
- 1 Queen’s University Belfast, Northern Ireland
- 2 Centre for Experimental Medicine, Queen’s University Belfast, Northern Ireland
How to examine, treat, and refer severe burns injuries
Burns represent a substantial healthcare burden, accounting for more than 300 000 global deaths annually. 1 In the United Kingdom, an estimated 250 000 patients present to primary care with a burns injury every year and 175 000 attend emergency departments, 2 3 so medical students are highly likely to encounter such patients during rotations. Most burns occur at home, and children are more likely than adults to be scalded. 4
Many burns can be adequately managed with appropriate first aid treatment at home or in primary care. In more severe cases, however, prompt recognition, assessment, and appropriate management can be life saving.
The initial management has an important effect on the patient’s long term outcome, so your interventions can have a positive impact. Initial management consists of following a structured plan and frequently reassessing the patient’s condition and response to what you are doing. This article aims to provide you with a basic understanding of the pathology of burns, a safe and effective system for assessing patients with burns, and guidance on appropriate initial management strategies.
What is a burn?
According to the Oxford English Dictionary , a burn is “an injury caused by exposure to heat or flame.” Most burns occur as a result of thermal injury—the largest proportion of these are scalds, especially in the paediatric population. 2 Burns can also be caused by several other mechanisms including electricity, chemicals, or radiation (box 1). Burns are often considered as injuries of the skin only, but this is not the case—any tissue can be burnt, including cornea and lung. This is important to remember—inhalation injuries must never be overlooked.
Box 1: Types of burn injuries and their causes
Thermal burns.
Flame—Accelerants such as petroleum, ignition of clothing by candles, or cigarettes
Scald—Boiling water from bath, kettle, or hot drink. These are the most common causes of thermal injury (60% of paediatric burns)
Contact—Radiators, irons, hobs, and hair straighteners
Flash—Ignition of a volatile substance, often after using accelerants when burning rubbish
Electrical burns
Low voltage—Domestic electrical supplies <240 V. Electrocardiography is needed to rule out arrhythmias. May cause cardiac arrest
High voltage—Power cables >1000 V, industrial accidents, lightning strikes. Injury can also occur through a high tension “flash” burn, in which the current arc does not pass through the patient but can cause clothing to catch fire and can cause deep burns
Lightning—Not a common mechanism in the UK (2-5 people a year) but important worldwide, with 10 000 deaths annually as a result of lightning strikes
Chemical burns
Acids—Common agents are acetic, hydrochloric, sulphuric, and hydrofluoric acid. Note that with hydrofluoric acid severe hypocalcaemia can occur, combined with hypomagnesaemia, leading to fatal cardiac arrhythmias. Small burns (2% total body surface area) caused by hydrofluoric acid can be fatal. Consult burns unit promptly. Do not contaminate yourself while washing the patient
Alkali—Household cleaning agents such as bleach. Contact burns from wet cement
Organic compounds—Bitumen/tarmac or petroleum contact burns
Radiation burns
Ultraviolet light—Sun, tanning booths. Varies greatly with skin type
Ionising radiation—Radiation therapy, x rays, radioactive fallout. Severity is related to the volume of exposure
Treatment of a burn begins at the scene of the incident. As with any trauma patient, a primary survey using an ABCDE (Airway, Breathing, Circulation, Disability, Exposure) approach should be adopted so that life threatening abnormalities can be recognised promptly and corrected. Courses such as advanced trauma life support (ATLS) teach a systematic approach to treatment and links are provided in the further reading section to refresh your knowledge of this approach. This article assumes knowledge of this and will focus on burns management.
If you are first on the scene do not rush in immediately. Adopt a SAFE approach (Shout for help; Assess the scene; ensure it is Free from danger; Evaluate the casualty) as you would for any pre-hospital emergency and undertake an initial primary survey. When dealing with burns patients and assessing ABCDE it is important to consider inhalation injuries, which might not be immediately apparent but can cause rapid airway obstruction, as well as trauma, carbon monoxide exposure, and inhalation of hydrogen cyanide gas.
The importance of assessing for inhalation burns cannot be overstated because such injuries can be rapidly fatal. Look for facial, mouth, nose, and pharynx burns; singeing of nasal hairs and eyebrows; soot in sputum; or signs of respiratory distress. Inhalation injury often presents with increasing oedema of the airways, progressing to obstruction over hours, so it is essential to reassess patients often. Definitive airway management may be needed in patients with an inhalation injury or those with a decreased level of consciousness who might not be able to maintain a patent airway. Involve the anaesthetic team promptly, because early intubation to protect the airway is better than trying to intubate a patient whose airway has become occluded.
Appropriate first aid has a measurable effect on outcomes, 5 preventing further tissue damage and reducing associated morbidity. Stop the burning process by removing the patient from the source of burning. Remove clothing and jewellery unless they are melted or adherent to the wound, in which case they should be left in place.
Management of a burn
Management of the burn wound itself is best remembered by the three Cs: Cool, Call, and Cover 6 :
Cool by irrigating with cool running tap water (around 15ºC) for 20 minutes. Cooling is beneficial for up to three hours after injury. Do not apply butter or oils. It is important to keep the patient, especially children, as warm as possible while cooling the burn wound to prevent hypothermia—“cool the burn, but warm the patient.” Keep unburned areas wrapped up (warming blankets) while running water over burned areas
Call for an ambulance
Cover the cooled burn loosely with clingfilm, omitting the face. If clingfilm is not available, cover with a clean cloth or non-adherent dressing. Facial burns can be covered with wet gauze or hydrogel dressings for transfer. Burn gel wraps can be used for their analgesic properties, but only after the burn has been sufficiently cooled. Do not wrap limbs too tightly. Swelling can occur rapidly after burns injury, and the dressing can then act as a tourniquet, restricting blood flow. Remember that wet or gel dressings will cool the patient, so wrap the patient in blankets to prevent hypothermia.
How to examine a burn
The severity of a burns injury is related to the proportion of the body surface area that has been burnt and the depth (thickness) of the burn. Accurate estimation of the size of the burn, given as a percentage of total body surface area (% TBSA) is the main factor in deciding whether patients need active resuscitation. As part of this assessment, do not count areas of erythema—reddening of the skin without blistering or loss of the epidermis. These areas will heal spontaneously and are not included in the estimated % TBSA of the burn.
A straightforward assessment tool for estimating % TBSA is Wallace’s “rule of nines,” in which the head and arms are each calculated as occupying 9% TBSA, the anterior and posterior surfaces of the lower limbs are each 9% (18% in total for each lower limb), the chest and back are 18% each, and the perineum is 1% (fig 1 ⇓ ). ⇓ This approach cannot be used for patients under 16 years and slightly overestimates body surface area. 7

Fig 1 Wallace’s rule of nines 8
- Download figure
- Open in new tab
- Download powerpoint
Alternatively, Lund and Browder charts (fig 2 ⇓ ) are often available in emergency departments and account for age related differences in body surface area, making them a more appropriate tool for evaluating TBSA in the paediatric population. Children have relatively large heads in proportion to their body size, so it is important to use an appropriate paediatric chart for your calculations.

Fig 2 Lund and Browder chart 9
Another simple yet subjective method is to equate the area of the patient’s hand, inclusive of palm and fingers, to 1% of TBSA. Although each method has its advantages, all are subject to varying degrees of inter-rater variability, and several studies have highlighted the need for more reliable methods of estimating TBSA. 10 11
Burns are classified according to depth and may be described as:
Superficial dermal
Deep dermal
Full thickness.
These would previously have been described as first, second, or third degree burns (superficial partial and deep partial would formerly have been classified as second degree burns). You are quite likely to hear this terminology used, but it has been superseded by depth, rather than degree. The table ⇓ describes the features of these burns.
Clinical determination of burn depth 4
- View inline
The skin loses its elasticity as the depth of the burn increases. Deep dermal and full thickness burns, particularly those that extend all the way around a limb (known as circumferential) can act as a tourniquet when swelling inevitably develops. This can lead to complete ischaemia of the limbs or respiratory compromise if the chest wall is involved. In both scenarios, rapid recognition of the potential for problems is essential. Burnt skin may have to be incised, in a procedure known as escharotomy, to allow lung ventilation or to restore or maintain limb circulation.
When assessing a burn you should:
Follow a systematic ABCDE approach, be alert to the possibility of inhalation injury, and call for help early
Estimate the area that has been burnt because this indicates the need for fluid resuscitation and is important for deciding whether the patient needs to be referred to the burns team
Recognise areas of erythema and exclude them from your calculations
Try to gauge the burn depth—it will usually be a mixture, such as 25% partial thickness and 20% full thickness. Check for circumferential burns
Consider the possibility of carbon monoxide or cyanide poisoning.
Fluid resuscitation
Burns cause enormous systemic insult, with huge volumes of fluid shifting into the injured area in response to direct damage to the microcirculation and the production of inflammatory mediators at the site of the burn. Fluid resuscitation aims to deal with the systemic insult promptly. When the burn is greater than 20-30% TBSA, overwhelming production of inflammatory mediators can trigger an increase in vascular permeability, leading to generalised oedema. 4 When combined with evaporative loss from the surface of the burns, this can cause hypovolaemia, which can cause failure of other organs, especially the kidney, if left untreated. Burns cause a central area of tissue destruction, with a surrounding area of stasis (critically reduced blood flow). Persistent hypotension increases the likelihood of injury to this zone, so the restoration of circulation volume with prompt fluid resuscitation can help minimise progression of the burn injury.
Fluid resuscitation should be started in all burns estimated at greater than 10% TBSA in children and greater than 15% TBSA in adults. 4 Use the modified Parkland formula to calculate fluid requirements (box 2).
Box 2: Modified Parkland formula
All patients.
Give 3-4 mL Hartmann’s solution per kg body weight per % TBSA over 24 hours:
Give half calculated volume over first 8 hours
Give second half over next 16 hours
Also give maintenance fluid—for example, 0.45% saline + 5% dextrose—according to weight and local policy
For a 70 kg adult with 15% full thickness burns:
4 mL×70 kg × 15% TBSA=4200 mL in total
Give 2100 mL during the first 8 hours after the burn and then 2100 mL during the next 16 hours
The resuscitation clock begins at the time of the burn, not the time when the patient arrives in your department.
Assessing the adequacy of resuscitation
The most sensitive way to assess the adequacy of resuscitation is to monitor the patient’s urine output. This can be done by inserting a urinary catheter and taking hourly readings, aiming for a urinary output of 0.5 mL per kg body weight per hour in adults and 1 mL per kg body weight per hour in children.
Under-resuscitation may occur if the patient’s arrival at hospital has been delayed or if you have underestimated the extent of the burn (or missed additional injuries or inhalation burns). Remember that you cannot see the extent of an inhalation burn and that these burns also lead to fluid losses. If under-resuscitation has occurred, increase the infusion rate and reassess.
If the urinary output is much higher than expected—for example, 2-3 mL per kg per hour—the patient may be over-resuscitated and you should consider reducing the infusion volumes. This can also occur if you have overestimated the extent of the burn. As at all stages in the process reassess the patient and adjust infusion rates accordingly.
Monitor vital signs and check serum electrolytes regularly. Dilutional hyponatraemia is common and hyperkalaemia is often seen in patients with extensive muscle damage—for example, after electrocution or escharotomy.
Dark (coffee coloured) urine can be caused by myoglobin that is released from necrotic muscle and excreted by the kidneys. This can be seen when external compression from full thickness burns has resulted in muscle ischaemia, when electrocution has caused rhabdomyolysis, or when the patient has been lying in one position for a prolonged period. Myoglobin will rapidly block the renal filtration system, leading to acute tubular necrosis. The first line of management is to increase fluid resuscitation to achieve twice the suggested hourly output of urine—1 mg per kg body weight per hour in adults, and 2 mg per kg body weight per hour in children. Discuss the patient’s condition with the burns team at an early stage.
Additional considerations
Patients are often in pain and emotional distress. Give analgesia intravenously because when it is given intramuscularly absorption will vary according to the systemic insult. Give aliquots of morphine (0.05-0.1 mg/kg), titrated to effect
Perform imaging as indicated by the primary survey
Gastroparesis often occurs in patients with a large burn: consider inserting a nasogastric tube
Consider the possibility of non-accidental injury, especially in vulnerable adults or children. It may be a case of deliberate injury, such as the child who is plunged into a hot bath, or burned with an iron. Injury may also occur as a consequence of inadequate supervision—the older person who has fallen out of bed in a care facility or the child who has gained access to caustic household chemicals. Ask yourself if the pattern of injury fits the explanation. If you are worried, seek senior advice then follow your hospital’s policy for involving social services. The diagnosis of non-accidental injury is not one to make in haste, but it is better to have a fairly low threshold for referring patients according to your clinical suspicion, rather than to miss the opportunity to intervene on behalf of a vulnerable person
Ensure a secondary survey, including a full history and head to toe examination, is undertaken after the patient has been stabilised. Use the AMPLE acronym when taking a history (Allergies, Medications, Past medical history, Last ate (time), Events, and Environment relating to injury).
Dress burns with a non-adherent dressing, cover with gauze, and bandage—not tightly because the area will swell. Consider whether the patient needs to be transferred to a specialist unit. If so, clingfilm may be used to cover burns.
Referral and transfer
Knowing when to refer is an important part of your assessment. Multidisciplinary, definitive care is essential for the patient with a burns injury. Burns teams include plastic surgeons, anaesthetists, nursing staff, occupational therapists, physiotherapists, speech and language therapists, dietitians, psychologists, and social workers who are all experienced in burns care.
The National Network for Burn Care has produced referral guidelines that are endorsed by the British Burn Association. If a specialist burns team is within one hour’s journey, patients should be transported there in the first instance. The exception to this advice is where immediate intervention is needed to preserve life, such as endotracheal intubation for inhalation injuries.
Patients who have been assessed and stabilised at a hospital without specialist burns services may need to be transferred to the regional burns service. If patients have additional injuries, the local trauma team should reach an agreement with the burns team about the severity of the injuries. Patients may need to be treated in the trauma unit, with advice from the burns team, until they are fit for transfer.
All patients who meet the criteria in box 3 should be referred. If you are unsure, it is better to discuss the patient with your nearest burns team. Similarly, if there are concerns regarding healing of the burn, infection, or suspected toxic shock syndrome, the burns team should be consulted for advice.
Box 3: Criteria for referral to a specialist burns team 12
Refer all patients in the following groups to a specialist burns team:
Total body surface area—≥2% in children, ≥3% in adults
Depth—All full thickness burns
Distribution—All circumferential burns
Duration—Any burn that has not healed within two weeks
Non-accidental injury—Refer any patient in whom non-accidental injury is suspected within 24 hours
Patients with the following features should be discussed with a burns consultant, and referral should be considered:
Location—All burns to hands, feet, face, perineum, or genitalia
Any chemical, friction, or electrical burn; any cold injury
Other considerations—Unwell or febrile child with a burn, any comorbid conditions or concerns regarding burn injuries that may affect management or healing of the burn.
Outcomes for patients with burns injuries
You may have been taught that the sum of the patient’s age and the % TBSA give an indication of the mortality rate for that patient (Baux score). However, as survival rates after burn injury continue to improve, 13 this score now tends to overestimate mortality rates. Twenty five years ago, a young adult with a 50% total body surface area burn had a 50% mortality rate; this has now been reduced to 10%. 14 Improvements in our understanding of resuscitation, nutritional support, and the prevention and management of infection, as well as developments in surgical techniques, have all combined to improve the outlook for patients.
The care provided by the multidisciplinary burns team is essential for patients with severe burns. The initial aim is to save life and allow people to get back to their home. Rehabilitation can be prolonged and patients may be left with restricting or visible scarring. Scar revision surgery is possible over time, and it is helpful for patients to feel that they are supported by the burns team, acutely and in the longer term.
This 40 year old woman has a history of epilepsy. She was sitting down to drink a cup of freshly made tea, when she had a seizure. She spilt tea over her right thigh and perineum. This happened about 30 minutes ago, and a relative brought the patient straight to hospital. ⇓

Fig 3 Burns on patient’s right thigh
Describe what you see in the photograph of the patient’s right thigh.
The patient has burnt about 1.5% TBSA on the anteromedial aspect of her right thigh. The area has been blistered, but these have burst, showing an area of erythema, which is painful and has brisk capillary refill—consistent with an area of superficial dermal burn.
The patient has an additional area of similar burn (1% TBSA) on the perineal area. There are no other injuries. How will you manage this patient?
This patient has 2.5% TBSA superficial dermal burns, and therefore does not require resuscitation fluids. The area has not been cooled, and the burn was less than three hours ago, and so the area should be cooled for 20 minutes with tepid running water. Showering the area can be a convenient way to cool the burn. As the burn involves the perineum, it is appropriate to discuss the injury with a specialist burns unit. The burn can be covered with clingfilm until a decision has been made about further care.
As the area was very painful, and the perineum was involved, the patient was transferred to the regional burns unit for analgesia and wound care. A urinary catheter was inserted, and the area was dressed with simple, non-adherent dressings. The burn healed after around two weeks of conservative care, and the patient was discharged home.
You receive a telephone call from the emergency department in your hospital. A 49 year old woman has just been brought in by ambulance. She was cleaning a portable bio-oil heater, and was relighting it to burn off some residue, when it exploded. She has burns to her right arm and both legs.
What do you ask next?
Be systematic—remember ABCDEF. Ask about the details of the burn—was the patient inside or outside? Does she have any symptoms or signs suggesting an inhalation injury? Did her clothing catch fire? Has the burn been cooled? Has she any other injuries?
The burn happened in the garden, and she has no obvious signs of inhalation injury. The patient’s clothing caught fire, and she rolled on the ground and then got into a cool shower, before being brought to hospital. There are no other injuries. You go to the emergency department, and examine the patient. ⇓ ⇓

Fig 4 shows the patient’s right leg, with mixed depth burns (mid and deep dermal)

Fig 5 Anterior aspect of the right arm
Figure 5 shows the anterior aspect of the right arm. There is a mixed depth burnt area, most of which is insensate with delayed capillary refill time, in keeping with deep dermal or full thickness burn. The burn is circumferential. There is a small (0.5% TBSA) deep dermal burn on the left knee. You estimate a total of 10.5% TBSA burn, and so the patient does not require resuscitation fluids.
What specific examination would you now perform?
As there is a circumferential burn of the right arm, the next step is to assess the circulation in the limb. On examination, the limb feels swollen and firm, and the capillary refill time in nailbeds of the fingers on the right hand is greater than 4 seconds. You can palpate a radial pulse.
What do you do now?
The presence of a palpable pulse does not mean that perfusion of the limb is adequate—and remember that the limb will swell over the next 24-72 hours. You should prepare to perform escharotomies of the limb, to release the pressure on the limb.
Escharotomies were performed (fig 6), and the capillary refill time returned to normal. Note the midaxial incision, avoiding the ulnar nerve at the medial epicondyle; you will also see the gap between the skin edges after release of the constricting eschar. The patient was managed conservatively for one week, until the mixed depth areas had fully delineated. She then required excision of 5% TBSA burn from the right arm, and 2% TBSA from the right leg. The excised areas were reconstructed with split skin grafts. These healed well, and the patient has been discharged home. ⇓

Fig 6 Escharotomies perfomed on patient’s right arm
It is natural to feel overwhelmed when faced with a patient with a burn injury. Taking a calm, systematic approach to assessment and initial management will give the patient the best possible chance of a good outcome. Once you have completed your initial management, consider whether the patient needs to be referred for specialist treatment. If you are not sure, discuss the case with the regional burns service. You might also consider attending an emergency management of severe burns course—this one day, multidisciplinary course covers the immediate management of burns in more detail than this article.
Further reading
Emergency Management of Severe Burns: In the UK, this course is arranged by the British Burn Association— www.britishburnassociation.org/emsb
Resuscitation Council (UK). A systematic approach to the acutely ill patient, ABCDE— www.resus.org.uk/resuscitation-guidelines/a-systematic-approach-to-the-acutely-ill-patient-abcde
Royal College of Surgeons. Advanced Trauma Life Support (ATLS) Provider Programme— www.rcseng.ac.uk/courses/course-search/atls.html
Originally published as: Student BMJ 2016;24:h5583
SMA initiated the article, revised the draft article, and is guarantor. PMC performed the literature search, wrote the draft article, and produced the final version.
Competing interests: None declared.
Patient consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
- ↵ Mock C, Peck M, Peden M, Krug E, eds. A WHO plan for burn prevention and care. 2008. http://apps.who.int/iris/bitstream/10665/97852/1/9789241596299_eng.pdf .
- ↵ National Burn Care Review. National burn injury referral guidelines. In: Standards and strategy for burn care. NBCR, 2001:68-9.
- ↵ Wilkinson E. The epidemiology of burns in secondary care, in a population of 2.6 million people. Burns 1998 ; 24 : 139 -43. OpenUrl CrossRef PubMed Web of Science
- ↵ British Burn Association. Emergency management of severe burns (EMSB) course manual. 15th ed. 2012. www.britishburnassociation.org/emsb .
- ↵ Skinner A, Peat B. Burns treatment for children and adults: a study of initial burns first aid and hospital care. N Z Med J 2002 ; 115 : 1 -9. OpenUrl PubMed Web of Science
- ↵ British Burn Association. First aid position statement, 2014. www.britishburnassociation.org/downloads/BBA_First_Aid_Position_Statement_-_8.10.14.pdf .
- ↵ Wachtel TL, Berry CC, Wachtel EE, et al. The inter-rater reliability of estimating the size of burns from various burn area chart drawings. Burns 2000 ; 26 : 156 -70. OpenUrl CrossRef PubMed Web of Science
- ↵ Van Hasselt EJ. 2008. Burns manual. 2nd ed. Nederlandse Brandwonden Stichting.
- ↵ Lund C, Browder N. The estimation of areas of burns. Surg Gynecol Obstet 1944 ; 79 : 352 -8. OpenUrl
- ↵ Nichter LS, Williams J, Bryant CA, et al. Improving the accuracy of burn-surface estimation. Plast Reconstr Surg 1985 ; 76 : 428 -33. OpenUrl CrossRef PubMed
- ↵ Miller SF, Finley RK, Waltman M, et al. Burn size estimate reliability: a study. J Burn Care Rehabil 1991 ; 12 : 546 -5. OpenUrl CrossRef PubMed
- ↵ National Network for Burn Care. Burn care referral guidance. Version 1. 2012. www.britishburnassociation.org/downloads/National_Burn_Care_Referral_Guidance_-_5.2.12.pdf .
- ↵ Jackson PC, Hardwicke J, Bamford A, et al. Revised estimates of mortality from the Birmingham Burn Centre, 2001-2010. Ann Surg 2014 ; 259 : 979 -84. OpenUrl
- ↵ Richards A, Dafydd H. Key notes on plastic surgery . 2nd ed. Wiley Blackwell, 2015 .
- Open access
- Published: 14 December 2021
The psychological impact of paediatric burn injuries: a systematic review
- Alix Woolard 1 , 2 ,
- Nicole T. M. Hill 1 ,
- Matthew McQueen 1 ,
- Lisa Martin 3 ,
- Helen Milroy 2 ,
- Fiona M. Wood 4 ,
- Indijah Bullman 1 &
- Ashleigh Lin 1 , 2
BMC Public Health volume 21 , Article number: 2281 ( 2021 ) Cite this article
7567 Accesses
22 Citations
Metrics details
To review and synthesise qualitative literature regarding the psychological outcomes following paediatric burn injuries, and to determine if children and adolescents who experience a burn injury have elevated risk of psychopathology following the injury.
Systematic review of quantitative and qualitative studies.
Data sources
Informit health, Medline, Embase, and PsycINFO were searched from January 2010 to December 2020.
Data extraction and synthesis
Two reviewers screened articles, and one reviewer extracted data (with cross-checking from another reviewer) from the included studies and assessed quality using an established tool. Narrative synthesis was used to synthesise the findings from the quantitative studies, and thematic synthesis was used to synthesise the findings of included qualitative studies.
Searches yielded 1240 unique titles, with 130 retained for full-text screening. Forty-five studies from 17 countries were included. The psychological outcomes included in the studies were mental health diagnoses, medication for mental illness, depression, anxiety, stress, fear, post-traumatic stress, post-traumatic growth, emotional issues, self-harm, self-esteem, self-concept, stigmatisation, quality of life, level of disability, resilience, coping, and suicidality.
Conclusions
Our findings highlight paediatric burn patients as a particularly vulnerable population following a burn injury. Studies suggest elevated anxiety and traumatic stress symptoms, and higher rates of psychopathology in the long-term. Further research is recommended to determine the psychological outcomes in the other mental health domains highlighted in this review, as findings were mixed.
Clinical care teams responsible for the aftercare of burn patients should involve psychological support for the children and families to improve outcomes.
Peer Review reports
Introduction
Burns are one of the most severe injuries that a child can experience and are a common cause of emergency presentations. The most recent Australian annual report showed 5430 cases of hospitalisation for burns, the majority of which were young children (aged 0–4) [ 1 ]. Paediatric burn management and medical aftercare has made significant advances in recent decades (for a review, see; [ 2 ]), such that current survival rates are high for even very serious burns [ 3 , 4 ]. Historically, burn aftercare has focused on physical healing, in recent years, however, research has uncovered the poor psychological outcomes in children who experience burns, including a minor burn injury [ 5 ]. This is because burn injuries do not only have a serious physical impact on a child, they also seriously impact the psychological and emotional wellbeing of the child and their family.
Burn injuries are painful, both physically and mentally. This is especially the case for young children who may not understand that procedural pain (e.g., dressing changes) is a necessary component of recovery [ 6 ]. In fact, early burn studies provided some of the founding research for the original clinical outline of posttraumatic stress disorder (PTSD) in the Diagnostic and Statistical Manual of Mental Disorders-III [ 7 , 8 ]. The event in which the injury occurred is often traumatising [ 9 ], and hospitalisation can be scary for children and often involves separation from family or peers, which is traumatic in itself [ 10 ]. Further, the immediate and subsequent wound care following a burn injury is not only physically painful, but also invasive and pervasive [ 11 ]. Scarring is also common for a burn wound, despite medical advances, which requires long-term medical care and can also contribute to poor psychological outcomes [ 9 ].
Given the severity and prevalence of paediatric burn injuries, as well as the accompanying trauma during and after a burn, there is a need to address the psychological after-care of paediatric burns. Symptoms of PTSD are often reported in children who have experienced a burn injury [ 12 , 13 , 14 ]. Other studies have also found that post-burn, individuals can report high rates of acute stress disorder [ 15 ], anxiety, and depression [ 9 ].
It is important when attempting to address clinical outcomes, such as the psychological after-care of paediatric burns, to first review the available evidence on the subject and make evidence-based evaluations. The existing reviews of paediatric burn outcomes reveal that the majority of children who have experienced paediatric burn injuries often go on to experience long-term psychopathology [ 16 , 17 , 18 ]. There are studies that contradict these findings, with reports of children adjusting well after this injury [ 19 ]. However, the most recent review on psychopathology following paediatric burns, conducted 8 years ago, found that the overwhelming majority of studies report short- and long-term psychopathology in both children and their parents after a burn injury [ 10 ]. This review highlighted that the majority of children who have experienced a burn injury go on to display some sort of distressed behaviour, either internalising (such as anxiety and withdrawal) or externalising (such as aggression and opposition) behaviours [ 10 ]. The findings highlight the importance of psychological aftercare and how advances in mental health care following a burn injury had greatly improved between 2000 and 2012, giving clinicians a better understanding of the broader needs of those who have experienced paediatric burns [ 10 ].
There is a need for an updated review of the work by Bakker et al. [ 10 ] of the current research on the psychological impact and outcomes of paediatric burns. Clinicians need to be aware of the current research on the psychological outcome of paediatric burns survivors to be able to target areas of need – which extend beyond treating the physical burn. The aim of this current review was to search, review, and evaluate the current research that has been conducted on the psychological impact on children’s mental health following paediatric burn injury. The primary objective of this study was to examine the research (conducted in the last 10 years) that exists on the psychological impact on children’s mental health following a paediatric burn injury. We hypothesised that children or adolescents who sustained a burn injury would be more likely to go on to experience psychopathology than those who had not experienced a burn injury.
We conducted this review following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines [ 20 ]. Our review methodology was registered with the PROSPERO international prospective register of systematic reviews (CRD42020215553, National Institute for Health Research, n.d.).
Search strategy
Electronic databases Informit health, Medline, Embase, and PsycINFO were searched on the 19th of October 2020, for English-language empirical peer-reviewed articles published in the last 10 years (2010–2021). Our search terms included: ‘children’, ‘chil*’, ‘paediatric’, ‘pediatric’, ‘youth’, ‘young’, ‘adolesc*’, AND ‘burn*’, ‘thermal*’ AND ‘psycholog*’, ‘psychopathology’, ‘mental health’, ‘depression’, ‘anxiety’. These terms were derived from our research question, similar published reviews, and through consultation with experts in the field. Additional articles were identified through comprehensive hand searches of the reference lists of included articles and Google Scholar searches. Two authors (AW, NTMH) conducted the initial screening of abstract and titles, and the full-text screen of potential articles. Any discrepancies were resolved by two authors (AW, AL).
Inclusion and exclusion criteria
Articles were included if they involved: 1) data on the psychological impact or psychological outcome of paediatric burn injury, 2) mean age of children in the study was under 18 years of age at the time of the burn injury, 3) the injury involved a burn that required hospitalisation (including emergency presentation). Psychological outcomes were reported from parent-report or caregiver-report questionnaires, child self-report questionnaires, or from observational assessment. Cross-sectional or cohort studies were eligible if the psychological outcomes were assessed after the burn injury. Randomised control trials (RCTs) were eligible if psychological measures were assessed at baseline (pre-intervention). Eligible control groups included child or adolescent populations that did not experience a burn injury.
Articles were excluded if they; 1) only included physiological or functional outcomes of the child only, 2) articles with adult populations, 3) papers not peer-reviewed (e.g. grey literature and book chapters), 4) intervention studies that focussed on a drug or behavioural treatment of the burn injury and did not measure psychological variables at baseline, 5) psychological outcomes related to parents of the child only, 6) burn injury arose from self-harm, potentially indicating prior psychopathology, and 7) burn injury was reported as the result of intentional harm (such as maltreatment by a caregiver). Non-English language studies were included and translated using Google translate. The first author contacted authors for further details (e.g., unpublished data, further information from abstracts) on grey literature to avoid publication bias.
Data collection, risk of bias and quality assessment
Data were extracted using the software Covidence [ 21 ], which has a data extraction template. Results were reported according to the PRISMA statement (Liberati et al., 2009). Study quality was assessed using the National Heart, Lung and Blood Institute quality assessment tool for observational, cohort and cross-sectional studies, with the addition of the following criteria relevant to this review: (1) the study involved a comparison groups: a paediatric burn group and a control non-burn group; (2) reliable and validated psychological outcome measures were used; (3) a statistical power analysis was conducted to determine optimal sample size to find differences between groups; (4) appropriate statistical analyses were conducted to determine differences between groups; and (5) the study used a prospective design rather than a cross-sectional design [ 22 ]. The quality assessment tool provides a rating for each study based on 14 criteria relating to the study population selection, blinding, confounding, outcome measures, and missing data or attrition. One reviewer (AW) rated each study using this tool, another author cross-checked (NTMH), and any discrepancies were sent to a third independent reviewer (AL) for deliberation.
Study selection and characteristics
Forty-five studies we included in the review. Study selection was summarized using the PRISMA chart in Fig. 1 . Study characteristics are outlined in Table 1 . The studies comprised 32 mixed method, 10 interventions, and four qualitative. The age ranges of the children at the time of the burn injury varied in each study and ranged from 0 to 18 years of age. The settings of the studies included the United States ( n = 17), Australia ( n = 12), Sweden ( n = 3), Canada ( n = 2), the United Kingdom ( n = 1), South Africa (n = 1), Nicaragua (n = 1), Mexico (n = 1), Iran (n = 1), Poland (n = 1), Switzerland (n = 2), Finland (n = 1), Brazil (n = 1), Spain (n = 1), the Netherlands ( N = 1) and Turkey (n = 1). The psychological outcomes included in the studies were mental health diagnoses, medication for mental illness, depression, anxiety, stress, fear, post-traumatic stress, post-traumatic growth, emotional issues, self-harm, self-esteem, self-concept, stigmatisation, quality of life, level of disability, resilience, coping, and suicidality. Eighty-eight per cent of studies used validated and standardised assessments.
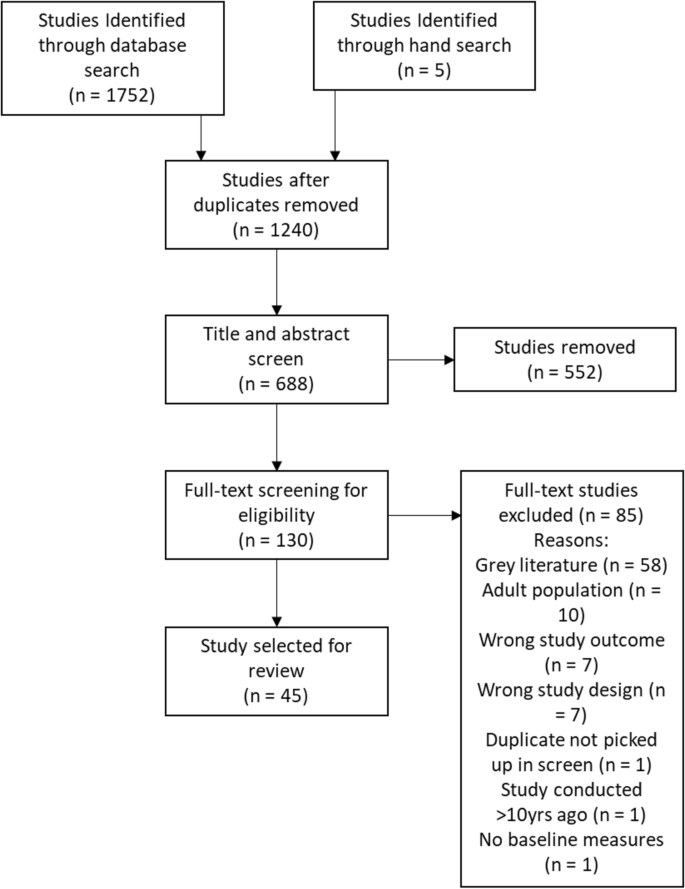
PRISMA flowchart of study selection
Results of quantitative studies
The most commonly reported psychological outcome in this review was anxiety in children and adolescents following a burn injury [ 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 ]. The six cohort studies investigating the presence of anxiety following a pediatric burn examined children with burns versus normative data on uninjured children, or how anxiety related to differing individual factors in the child or adolescent population, such as age or gender. One study compared the anxiety of children or adolescents who had been hospitalised for burns for at least 24-h to normative data and found that state (i.e. situational) and not trait (i.e. related to personality) anxiety was higher in children and adolescents who had experienced a burn injury [ 31 ]. Another study that explored anxiety and pain found that pain was significantly associated with anxiety in children or adolescents who had been discharged from hospital for a burn 6–12 months prior [ 30 ]. One study investigating age of the child at injury found that age was significantly related to anxiety, with pre-school children exhibiting more anxiety around wound dressings than older children [ 25 ]. Rimmer et al. [ 34 , 35 ] conducted two studies investigating anxiety in burn-injured children and adolescents; one study examined concordance of parent and child ratings of anxiety, and one study investigating anxiety levels generally. Children self-reported their anxiety levels as significantly higher than proxy-reports by their parents [ 35 ]. A high proportion of children and adolescents screened positively for anxiety symptoms (39%), with 28% of the population scoring above the cut-off to indicate an anxiety disorder. This is compared to 6.9% of Australian children in the general population [ 34 , 38 ]. This study also observed that girls with a burn injury scored higher on anxiety measures than boys [ 35 ]. Riobueno-Naylor et al. [ 36 ] found that children who reported concerns with their appearance after burn were more likely to have social anxiety compared to those who had no appearance concerns, regardless of burn size, bodily location of burn or gender of the child.
There were nine RCTs that investigated anxiety and pediatric burns [ 23 , 24 , 26 , 27 , 28 , 29 , 32 , 33 , 37 ]. Three of the studies used the Visual Analog Scale – Anxiety (VAS-A), which has not been validated in a control group for children, however baseline anxiety was comparable for all children with burn injuries in all three studies (mean = 2.6–2.76 [ 23 ]; mean = 2.7–2.9 [ 24 ]; mean = 2.0 [ 27 ]). One of the studies used a measure of anxiety that was created specifically for the intervention (Yoga Evaluation Questionnaire), and thus is not able to be compared to normative data [ 26 ]. Khadra et al. [ 29 ] found that children’s anxiety (indexed on the Procedural Behaviour Checklist [ 39 ]) was low at baseline, but was strongly positively related to procedural pain and fear. Jeffs et al. [ 28 ] reported that the children in their study at baseline scored an average of 31.5 (state) and 34 (trait) on the State-Trait Anxiety Inventory [ 40 ], which is just below the clinical cut-off for an anxiety disorder [ 39 , 41 ]. Conversely, Parlak-Gurol et al. [ 32 ] reported much higher baseline scores (range = 46.71–45.96) for state anxiety using the same measure, placing their cohort in the clinical range. One study found that children recovering from a burn injury had a baseline score of 32 on the Beck Anxiety Inventory [ 42 ], which indicates severe anxiety [ 37 ]. Finally, another RCT reported that the children in their study scored at baseline much higher on the Spence Children’s Anxiety Scale [ 43 ] than what is reported in normative studies (77.4–90.4, compared to 21.72, respectively; [ 33 ]). In summary, anxiety was consistently identified as a common outcome of pediatric burns. This relationship was replicated across multiple studies of differing methodological quality, with age, gender, perceived body-image, procedural pain, and fear noted as influential factors.
Traumatic stress
Another common psychological outcome investigated in the literature was traumatic stress or post-traumatic stress symptoms [ 24 , 30 , 44 , 45 , 46 , 47 , 48 , 49 , 50 ]. One study found that 8% of children required treatment for Acute Stress Disorder after their burn injury [ 49 ]. Another study examining PTSD symptoms found that 11.7% of children met full criteria for PTSD, and a further 15–66.7% met subclinical thresholds for at least one cluster of symptoms (i.e. re-experiencing the event, avoidance, emotional numbing or increased arousal) [ 48 ]. Similarly, Graf et al. [ 47 ] found that 13.2% of toddlers in their study (aged 9–48 months) met full criteria for PTSD, and between 19.7–73.7% met criteria for at least one cluster of PTSD symptoms. This study also found that the burn severity and quality of family relations were all associated with toddler PTSD symptoms [ 47 ]. Several of the studies also found that maternal or parental distress and PTSD symptoms were associated with child PTSD or traumatic stress [ 45 , 46 , 47 , 48 ]. Brown et al. [ 44 ] showed that the stress the child experienced during wound dressings were associated with higher PTSD symptoms 3 months post-injury. De Young et al. [ 45 ] found that parental trauma history, child premorbid problems, and burn severity and size were related to child PTSD symptoms 6 months after the injury. In contrast, Nelson et al. [ 30 ] found no significant associations between pain and intensity of the burn and PTSD symptoms. Finally, Stoddard et al. [ 50 ] found that PTSD symptoms decreased gradually over time for children who had experienced a burn.
Depression/mood disturbances
Eight studies investigated depression symptoms in children after a burn injury [ 30 , 33 , 37 , 50 , 51 , 52 , 53 ]. Boles et al. [ 51 ] found that 32% of their sample of children who had experienced a frostbite burn had symptoms of depression at the time of the injury. Nodoushani et al. [ 52 ] found similarly high rates of depressive symptoms in their study, with around 45% of participants indicating they felt depressed at some point in the 36 months after their injury, although these results were found using a study-specific questionnaire that has not been validated. Three studies used the Child Depression Inventory [ 54 ] to assess depressive symptoms in their samples. The first found scores to exceed the clinical cut-off for depression immediately after hospitalisation [ 33 ], with similar high scores in a the same sample 5 years post-burn injury [ 37 ]. In contrast, the third study showcased scores that were below the clinical cut-off during hospitalisation [ 50 ]. Another study by Nicolosi et al. [ 55 ] found that mean depression scores in their sample indicated that most participants were not depressed or displayed only a few symptoms of depression post-burn injury, regardless of where the burn was situated on the body. In contrast, Rosenberg et al. [ 53 ] found that 19% of patients with electrical burns had a diagnosis of depression 3 years post-injury. Finally, Nelson et al. [ 30 ] found that the pain and intensity of a burn was associated with depression 6- and 12-months post-burn injury.
Emotional issues
Eight studies investigated emotional issues, mostly measured via the Child Behaviour Checklist (CBCL) [ 31 , 47 , 53 , 56 ], the Strengths and Difficulties Questionnaire [ 57 , 58 , 59 ], and the Burn Outcomes Questionnaire (BOQ) [ 60 , 61 ]. Of the studies using the CBCL, one study found that the children displayed a higher proportion of emotional problems than a normative sample [ 31 ], while another found their sample displayed fewer emotional problems than a normative one [ 47 ]. A third study using the CBCL reported that 7–9% of participants displayed emotional and behavioural problems [ 53 ], however, their results were not compared with normative data. Of the two studies that used the Strengths and Difficulties Questionnaire, one reported comparable results to normative data [ 59 ] whereas another study found their sample displayed more emotional problems than normative data [ 58 ]. Both studies reporting on the BOQ found significant associations between burns and emotional health [ 60 , 61 ]. Sveen et al. [ 60 ]) identified that pain, itch, and parental concern were associated with fear-avoidance and emotional health, and Warner et al. [ 61 ] found that larger burn sizes and facial burns decelerated the pace of emotional recovery [ 60 ]. Russell et al. [ 62 ] investigated the relationship between self-concept (perception of themselves and subsequent self-esteem) and emotional issues, and found that poorer self-concept was associated with emotional problems.
Self-esteem
Findings were mixed with regards to self-esteem in children and adolescents who had experienced a burn injury. Three studies reported that their sample had healthy levels of self-esteem that were comparable to normative or control data of non-burned children [ 37 , 55 , 58 ], whereas two studies reported low self-esteem in their sample [ 36 , 62 ]. Russell et al. [ 62 ] reported that children with an anxiety disorder also had lower self-concept. Riobueno-Naylor et al. [ 36 ] found that appearance concerns were associated with lower self-worth, and that the majority of adolescents receiving follow-up care after a burn were concerned with their appearance, although this wasn’t related to burn size, location or gender of the adolescent.
Quality of life
Four studies examined Quality of Life (QoL) [ 58 , 63 , 64 , 65 ]. Laitakari et al. [ 63 ] found that QoL was comparable for children who had experienced a burn injury when compared to control groups of non-burned children. Maskell et al. [ 58 ] and Weedon and Potterton [ 65 ] both found that QoL was lower for children or adolescents who had experienced a burn injury, with the latter study showing that children who were burned with hot water scored the poorest in terms of QoL. Murphy et al. [ 64 ] did not compare QoL to normative data, however did note that QoL 2.5–12.5 years after the injury was poorer for children who sustained the injury after school entry (i.e. they were older).
One study investigated the incidence of self-harm following a burn injury and found that 10–26 years after the injury, 2.7% of individuals who had experienced a burn injury had been admitted to hospital for self-harm, which is more than double the number of admissions from the non-burned control cohort [ 66 ].
Suicidality
Goodhew et al. [ 67 ] found that in their sample of adults who had sustained a burn injury as children, 11% had reported a suicide attempt in their lifetime. This study indicates individuals who sustained a burn in childhood have higher rates of suicide attempts compared to the general population, which is estimated to be between 3.2–4.5% [ 68 , 69 ].
Mental health diagnoses
Five studies reported on the incidence of mental health diagnoses following a pediatric burn injury [ 53 , 66 , 67 , 70 , 71 , 72 ]. Bushroe et al. [ 70 ] reported that young children (0–4 years) were most likely to receive a mental health diagnosis (8.56 risk-ratio) compared to older children (10–14 years; 1.02 risk-ratio). Duke et al. [ 66 ] found that burn-injured pediatric patients were 2.6 times more likely to be admitted to hospital for psychiatric conditions 10–26 years after the injury, regardless of burn size or severity. This study also found that age was a factor, with older children (10–15 years) being five times more likely to be admitted than younger children. Goodhew et al. [ 67 ] reported that 42% of their cohort received a mental health diagnosis in their lifetime, with female gender and burn visibility increasing this risk ratio. Thomas et al. [ 72 ] reported that 49% of their sample met criteria for one or more Cluster A (25.5%), Cluster B (31.6%), Cluster C (21.5%), or Other (35.7%; Personality Not Otherwise Specified, Passive Aggressive, or Depressive) personality disorders post a severe paediatric burn injury. Rosenberg et al. [ 53 ] investigated diagnosis based on burn type (electrical injury versus other burns) and found comparable incidences of mental health diagnoses at the acute stage post-injury for electrical injury compared to other burns. Specifically, they found that at acute presentation, 45–52% of the sample were experiencing anxiety, 31–51% were experiencing PTSD or Acute Stress Disorder, and 12–19% were experiencing depression. Two-years post-injury these rates dropped to 10–14% for anxiety, 5–7% for PTSD, and 13–19% for depression. Finally, De Young et al. [ 72 ] found that the children or adolescents with diagnosed PTSD after they had experienced a burn injury were more likely to also have Major Depressive Disorder, Oppositional Defiance Disorder, Seasonal Affective Disorder and Specific Phobia 1 month post-injury (73% of cases). Six months post-injury, the children with PTSD were more likely to have an additional diagnosis of Attention Deficit Hyperactivity Disorder, Oppositional Defiance Disorder and Seasonal Affective Disorder (85% of cases).
Post-traumatic growth, resilience and coping
Two studies examined post-traumatic growth (i.e. positive psychological change), resilience or coping in children and adolescents who had experienced a burn injury [ 30 , 73 ]. Nelson et al. [ 30 ] reported that pain from burns did not interrupt post-traumatic growth in children. Quezada et al. [ 73 ] showed that the children in their study had generally high levels of resilience following a burn injury, and that age was significantly associated with resilience, with younger children showing more resilience (as measured via the Resilience Questionnaire for children and adolescents) than older children.
Results of qualitative studies
Two of the three qualitative studies investigated the experiences of children or adolescents who experienced a burn, and the third investigated parent perception of their child or adolescent following a burn (see Table 2 ; [ 72 , 73 , 74 ]). Themes that arose in all studies were that the child experienced significant anxiety, stress, or “worry” after the burn incident.
Risk of bias within studies
Based on our criteria (see Table 3 ), we found that six independent studies met a high level of reliability and quality (58,63,64,66,70,77). Only those six studies used a non-burn comparison group with which to compare psychological outcomes. Fourteen studies reported on longitudinal outcomes (36, 37, 51–53, 55, 60–64, 66, 67, 73), whereas the others reported on outcomes during the first 18 months following the injury.
Due to the heterogeneity of study designs, we were unable to conduct a meta-analysis on whether children and adolescents who experienced a burn injury went on to experience elevated rates or risk of psychopathology later in life.
General discussion
This study aimed to examine the research conducted on the psychological impact on children’s mental health following paediatric burn injury from the years 2010–2020. In line with our hypothesis, we found that most studies reported elevated levels of psychopathology and psychological symptoms following paediatric burn injuries. However, findings were mixed for most mental health concerns and kinds of symptoms, except for increased anxiety symptoms and traumatic stress post burn injury.
Regarding psychological symptoms, studies reported that children generally experience more anxiety following a burn injury, that the anxiety they experience is more likely to be state-based (than trait-based), and the anxiety following a burn is associated with pain, the age at which the burn occurred (older children exhibit more difficulties), and the gender of the child (with girls more likely to report experiencing more anxiety than boys) [ 28 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 ]. Qualitatively, we found that parents and children/adolescents both report the child or adolescent experiencing heightened anxiety after a burn compared to prior [ 75 , 76 , 78 ]. The majority of studies that investigated traumatic stress following a burn injury also reported children experiencing higher PTSD and acute stress symptoms, and that these symptoms could also be related to parental distress and the child’s premorbid psychological problems [ 45 , 47 , 48 , 49 , 71 ].
In terms of mental health diagnoses, the six studies which reported these had different designs, making it difficult to generalise their results [ 53 , 66 , 67 , 70 , 71 , 72 ]. There does appear to be evidence, however, of elevated risk for mental health diagnoses and hospitalisation following paediatric burn injury, and in particular diagnoses such as Anxiety Disorders, PTSD, Acute Stress Disorder, Depression, and Personality Disorders were reported by the studies to be higher for this population.
Findings were mixed regarding depressive symptoms, emotional issues, self-esteem and QoL [ 30 , 47 , 51 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 77 ], and thus we cannot conclude on the experiences of children following a burn in these mental health concerns. Further, there were few studies investigating self-harm, resilience and suicidality following paediatric burn injury [ 66 , 67 , 73 ], and further evidence is required for conclusions to be drawn in these domains.
Limitations and future considerations
There were methodological strengths and issues that arose from the studies in this review. Impressively, we found that 88% of the studies in our review used standardised measures, an improvement in comparison to the review by Bakker et al. [ 10 ], who reported that just 75% of included studies used standardised measures. This increase in the use of standardised measures allows for more reliable and generalisable findings. One of the main limitations we found was the issue of heterogeneity meaning we were unable to conduct a meta-analysis. Some studies reported lifetime diagnoses, and did not differentiate whether the diagnosis was pre- or post-burn injury [ 67 ], and other studies did not report on factors that would likely influence outcomes (such as whether the children received psychological support after the injury) [ 53 ]. Standardised reporting of mental health outcomes, for instance routinely collecting comorbid mental health diagnoses at admission or collecting standardised measures of depression and anxiety for all burns patients, would help address the issue of heterogeneity across studies. Further, more studies are needed in terms of long-term diagnostic outcomes of paediatric burns patients. The paper that reported the strongest evidence for long-term elevated rates of psychopathology was that of Bushroe et al. [ 70 ] who examined risk ratios for mental health diagnoses longitudinally and provided clear indication of the long-term impact of a paediatric burn injury. Most studies in our review reported on short-term (acute-18 months) psychological outcomes following a burn injury, and it is difficult to determine causality in such studies. Despite this, we believe that the findings are still useful and able to inform clinical care and practice.
Given that most of the participants in the studies experienced increased anxiety, and that many experienced other psychological symptoms following a burn injury, it is clear that children and adolescents are particularly vulnerable following this type of traumatic injury. The longitudinal studies included in this review demonstrate that risk for psychopathology following a paediatric burn injury is much higher and ongoing than in the general population, which suggests that psychological recovery for these children and adolescents needs to be an area of focus in the future. The clinical care teams involved in a child’s recovery from a burn need to support psychological recovery for the child and the family to promote optimal outcomes. Ideally, the clinical care team should include consultation from mental health professionals. Our findings also demonstrate that factors in a child’s life (such as parental distress, age, and gender) may influence their psychological recovery and should be taken into consideration by the clinical care team.
Availability of data and materials
Data was available to authors via University databases. The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
Post-traumatic Stress Disorder
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Randomised Controlled Trial
Visual Analog Scale – Anxiety
Child Behaviour Checklist
Burns Outcomes Questionnaire
Quality of Life
Australian Institute of Health and Welfare. Hospitalised burn injuries Australia [Internet]. Australian Institute of Health and Welfare. 2013 [cited 2020 Oct 19]. Available from: https://www.aihw.gov.au/reports/injury/hospitalised-burn-injuries-australia-2013-14/contents/summary
Toon MH, Maybauer DM, Arceneaux LL, Fraser JF, Meyer W, Runge A, et al. Children with burn injuries-assessment of trauma, neglect, violence and abuse. J Inj Violence Res. 2011;3(2):98–110.
Article PubMed PubMed Central Google Scholar
Chong HP, Quinn L, Cooksey R, Molony D, Jeeves A, Lodge M, et al. Mortality in paediatric burns at the Women’s and Children’s hospital (WCH), Adelaide, South Australia: 1960–2017. Burns. 2020;46(1):207–12.
Article PubMed Google Scholar
Yarbrough DR. Improving survival in the burned patient. J S C Med Assoc 1975. 1990 Jun;86(6):347–9.
De Sousa A. Psychological aspects of Paediatric burns (a clinical review). Ann Burns Fire Disasters. 2010;23(3):155–9.
PubMed PubMed Central Google Scholar
Martin-Herz SP, Thurber CA, Patterson DR. Psychological principles of burn wound pain in children II: treatment applications. J Burn Care Rehabil. 2000;21(5):458–72.
Article CAS PubMed Google Scholar
Andreasen NJ, Noyes R, Hartford CE. Factors influencing adjustment of burn patients during hospitalization. Psychosom Med. 1972;34(6):517–25.
Andreasen NJ, Hartford CE, Knott JR, Canter DA. EEG changes associated with burn delirium. Dis Nerv Syst. 1977;38(1):27–31.
CAS PubMed Google Scholar
Van Loey NEE, Van Son MJM. Psychopathology and psychological problems in patients with burn scars: epidemiology and management. Am J Clin Dermatol. 2003;4(4):245–72.
Bakker A, Maertens KJP, Van Son MJM, Van Loey NEE. Psychological consequences of pediatric burns from a child and family perspective: a review of the empirical literature. Clin Psychol Rev. 2013;33(3):361–71.
Dise-Lewis JE. A Developmental Perspective on Psychological Principles of Burn Care: J Burn Care Rehabil. 2001;22(3):255–60.
Landolt MA, Buehlmann C, Maag T, Schiestl C. Brief report: quality of life is impaired in pediatric burn survivors with posttraumatic stress disorder. J Pediatr Psychol. 2020;34(1):14–21.
Article Google Scholar
Saxe GN, Stoddard FJ, Hall E, Chawla N, Lopez C, Sheridan R, et al. Pathways to PTSD, part I: children with burns. Am J Psychiatry. 2005;6.
Young ACD, Kenardy JA, Cobham VE, Kimble R. Prevalence, comorbidity and course of trauma reactions in young burn-injured children. J Child Psychol Psychiatry. 2011;8.
Stoddard FJ, Ronfeldt H, Kagan J, Drake JE, Snidman N, Murphy JM, et al. Young burned children: the course of acute stress and physiological and behavioral responses. Am J Psychiatry. 2006;7.
Meyer WJ, Robert R, Murphy L, Blakeney PE. Evaluating the psychosocial adjustment of 2- and 3-year-old pediatric burn survivors. J Burn Care Rehabil. 2000;21(2):179–84.
Piazza-Waggoner C, Dotson C, Adams CD, Joseph K, Goldfarb IW, Slater H. Preinjury Behavioral and Emotional Problems Among Pediatric Burn Patients. J Burn Care Rehabil. 2005;26(4):371–8.
Stoddard FJ, Norman DK, Murphy MJ, Beardslee WR. Psychiatric outcome of burned children and adolescents. J Am Acad Child Adolesc Psychiatry. 1989;28(4):589–95.
Landolt MA, Grubenmann S, Meuli M. Family Impact Greatest: Predictors of Quality of Life and Psychological Adjustment in Pediatric Burn Survivors. J Trauma Inj Infect Crit Care. 2002;53(6):1146–51.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34.
Veritas health innovation. Covidence systematic review software [Internet]. Covidence. [cited 2020 Oct 22]. Available from: https://app.covidence.org/
National Heart Lung and Blood Institute. Study Quality Assessment Tools: Study quality assessment tool for observational cohort and cross-sectional studies [Internet]. : https://www.nhlbi.nih.gov/health-topics/study-qualityassessment-tools . 38. Abrutyn S, Mueller AS. 2020 [cited 2020 Oct 22]. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
Brown NJ, Kimble RM, Rodger S, Ware RS, Cuttle L. Play and heal: randomized controlled trial of ditto™ intervention efficacy on improving re-epithelialization in pediatric burns. Burns. 2014;40(2):204–13.
Chester SJ, Tyack Z, De Young A, Kipping B, Griffin B, Stockton K, et al. Efficacy of hypnosis on pain, wound-healing, anxiety, and stress in children with acute burn injuries: a randomized controlled trial. Pain. 2018;159(9):1790–801.
Chrapusta A, Pąchalska M. Evaluation of differences in health-related quality of life during the treatment of post-burn scars in pre-school and school children. Ann Agric Environ Med. 2014;21(4):5.
Conn AS, Hall MS, Quinn K, Wiggins B, Memmott C, Brusseau TA. An examination of a yoga intervention with pediatric burn survivors. J Burn Care Res. 2017;38(1):e337–42.
Hyland EJ, D’Cruz R, Harvey JG, Moir J, Parkinson C, Holland AJA. An assessment of early child life therapy pain and anxiety management: a prospective randomised controlled trial. Burns. 2015;41(8):1642–52.
Jeffs D, Dorman D, Brown S, Files A, Graves T, Kirk E, et al. Effect of Virtual Reality on Adolescent Pain During Burn Wound Care: J Burn Care Res. 2014;35(5):395–408.
PubMed Google Scholar
Khadra C, Ballard A, Déry J, Paquin D, Fortin J-S, Perreault I, et al. Projector-based virtual reality dome environment for procedural pain and anxiety in young children with burn injuries: a pilot study. J Pain Res. 2018;11:343–53.
Nelson S, Uhl K, Wright LA, Logan D. Pain is associated with increased physical and psychosocial impairment in youth with a history of burn injuries. J Pain. 2020;21(3–4):355–63.
Pardo GD, García IM, Gómez-Cía T. Psychological Effects Observed in Child Burn Patients During the Acute Phase of Hospitalization and Comparison With Pediatric Patients Awaiting Surgery. J Burn Care Res. 2010;31(4):569–78.
Parlak Gürol A, Polat S, Nuran AM. Itching, Pain, and Anxiety Levels Are Reduced With Massage Therapy in Burned Adolescents. J Burn Care Res. 2010;31(3):429–32.
Rezazadeh H, Froutan R, Ahmad Abadi A, Mazloum SR, Moghaddam K. Effects of art therapy program on anxiety and depression among 6-12-year-old burned children. Open Access Maced J Med Sci. 2020;8(B):126–32.
Rimmer RB, Bay RC, Alam NB, Sadler IJ, Hansen L, Foster KN, et al. Burn-Injured Youth May Be at Increased Risk for Long-Term Anxiety Disorders: J Burn Care Res 2014;35(2):154–161.
Rimmer RB, Bay RC, Sadler IJ, Alam NB, Foster KN, Caruso DM. Parent vs burn-injured child self-report: contributions to a better understanding of anxiety levels. J Burn Care Res. 2014;35(4):296–302.
Riobueno-Naylor A, Williamson H, Canenguez K, Kogosov A, Drexler A, Sadeq F, et al. Appearance Concerns, Psychosocial Outcomes, and the Feasibility of Implementing an Online Intervention for Adolescents Receiving Outpatient Burn Care. J Burn Care Res. 2020 Jun 28;iraa108.
Tropez-Arceneaux LL, Castillo Alaniz AT, Lucia Icaza I, Alejandra Murillo E. The Psychological Impact of First Burn Camp in Nicaragua: J Burn Care Res 2017;38(1):e1–e7.
AIHW. Australia’s children, Children with mental illness [Internet]. Australian Institute of Health and Welfare. 2020 [cited 2021 Feb 3]. Available from: https://www.aihw.gov.au/reports/children-youth/australias-children/contents/health/children-with-mental-illness
Elliott CH, Jay SM, Woody P. An observation scale for measuring children’s distress during medical procedures. J Pediatr Psychol. 1987;12(4):543–51.
Knight RG, Waal-Manning HJ, Spears GF. Some norms and reliability data for the state--trait anxiety inventory and the Zung self-rating depression scale. Br J Clin Psychol. 1983;22(Pt 4):245–9.
Julian LJ. Measures of Anxiety. Arthritis Care Res [Internet]. 2011 Nov [cited 2021 Feb 3];63(0 11). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3879951/
Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–7.
Spence MJ, Moore DS. Categorization of infant-directed speech: development from 4 to 6 months. Dev Psychobiol. 2003;42(1):97–109.
Brown NJ, Kimble RM, Rodger S, Ware RS, McWhinney BC, Ungerer JPJ, et al. Biological markers of stress in pediatric acute burn injury. Burns. 2014;40(5):887–95.
De Young AC, Hendrikz J. Kenardy J.a. JA, Cobham VE, Kimble RM. prospective evaluation of parent distress following pediatric burns and identification of risk factors for young child and parent posttraumatic stress disorder. J Child Adolesc Psychopharmacol. 2014;24(1):9–17.
Enlow PT, Brown Kirschman KJ, Mentrikoski J, Szabo MM, Butz C, Aballay AM, et al. The role of youth coping strategies and caregiver psychopathology in predicting posttraumatic stress symptoms in pediatric burn survivors. J Burn Care Res. 2019;40(5):620–6.
Graf A, Schiestl C, Landolt MA. Posttraumatic stress and behavior problems in infants and toddlers with burns. J Pediatr Psychol. 2011;36(8):923–31.
Haag A-C, Landolt MA. Young Children’s acute stress after a burn injury: disentangling the role of injury severity and parental acute stress. J Pediatr Psychol. 2017;42(8):861–70.
Sharp S, Thomas C, Rosenberg L, Rosenberg M, Meyer W. Propranolol Does Not Reduce Risk for Acute Stress Disorder in Pediatric Burn Trauma. J Trauma Inj Infect Crit Care. 2010;68(1):193–7.
Article CAS Google Scholar
Stoddard FJ. Therapeutic play in the diagnosis and treatment of hospitalized children recovering from acute burns. J Am Acad Child Adolesc Psychiatry. 2017;56(10):S118.
Boles R, Gawaziuk JP, Cristall N, Logsetty S. Pediatric frostbite: a 10-year single-center retrospective study. Burns. 2018;44(7):1844–50.
Nodoushani A.Y., Murphy J.M., Wang S.L., Stoddard FJ, Kazis L., Lydon M., et al. Prevalence of depressive symptoms over time in pediatric burn survivors. J Burn Care Res. 2018;39(Supplement 1):S102.
Rosenberg M, Mehta N, Rosenberg L, Ramirez M, Meyer WJ, Herndon DN, et al. Immediate and long-term psychological problems for survivors of severe pediatric electrical injury. Burns. 2015 Dec;41(8):1823–30.
Kovacs M. Children’s depression inventory. MHS; 2011.
Google Scholar
Nicolosi JT, De Carvalho VF, Sabates AL. A quantitative, cross-sectional study of depression and self-esteem in teenage and young adult burn victims in rehabilitation. Ostomy Wound Manag. 2013;59(9):22–9.
Achenbach TM, Rescorla LA. Manual for the ASEBA School-age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth and Families; 2013.
Goodman R. Psychometric properties of the strengths and difficulties questionnaire. J Am Acad Child Adolesc Psychiatry. 2001;40(11):1337–45.
Maskell J, Newcombe P, Martin G, Kimble R. Psychosocial Functioning Differences in Pediatric Burn Survivors Compared With Healthy Norms: J Burn Care Res 2013;34(4):465–476.
Willebrand M, Sveen J, Ramklint M, Bergquist M, Huss F, Sjöberg F. Psychological problems in children with burns—parents’ reports on the strengths and difficulties questionnaire. Burns. 2011 Dec;37(8):1309–16.
Sveen J, Huss F, Sjoberg F, Willebrand M. Psychometric properties of the swedish version of the burn outcomes questionnaire for children aged 5 to 18 years. J Burn Care Res. 2012;33(6):e286–94.
Warner P, Stubbs TK, Kagan RJ, Herndon DN, Palmieri TL, Kazis LE, et al. The effects of facial burns on health outcomes in children aged 5 to 18 years: J trauma acute care Surg. Sep. 2012;73:S189–96.
Russell W, Robert RS, Thomas CR, Holzer CE, Blakeney P, Meyer WJ. Self-Perceptions of Young Adults Who Survived Severe Childhood Burn Injury: J Burn Care Res. 2013;34(4):394–402.
Laitakari E, Koljonen V, Pyorala S, Rintala R, Roine RP, Sintonen H. The long-term health-related quality of life in children treated for burns as infants 5-9 years earlier. Burns. 2015;41(6):1186–92.
Murphy ME, Holzer CE, Richardson LM, Epperson K, Ojeda S, Martinez EM, et al. Quality of life of young adult survivors of pediatric burns using world health organization disability assessment scale II and burn specific health scale-brief: a comparison. J Burn Care Res. 2015;36(5):521–33.
Weedon M, Potterton J. Socio-economic and clinical factors predictive of paediatric quality of life post burn. Burns. 2011;37(4):572–9.
Duke JM, Randall SM, Vetrichevvel TP, McGarry S, Boyd JH, Rea S, et al. Long-term mental health outcomes after unintentional burns sustained during childhood: a retrospective cohort study. Burns Trauma. 2018 Dec 1;6:s41038–018-0134-z.
Goodhew F, Van Hooff M, Sparnon A, Roberts R, Baur J, Saccone EJ, et al. Psychiatric outcomes amongst adult survivors of childhood burns. Burns. 2014;40(6):1079–88.
Johnston AK, Pirkis JE, Burgess PM. Suicidal thoughts and Behaviours among Australian adults: findings from the 2007 National Survey of mental health and wellbeing. Aust N Z J Psychiatry. 2009 Jul 1;43(7):635–43.
De Leo D, Cerin E, Spathonis K, Burgis S. Lifetime risk of suicide ideation and attempts in an Australian community: prevalence, suicidal process, and help-seeking behaviour. J Affect Disord. 2005 Jun;86(2–3):215–24.
Bushroe KM, Hade EM, McCarthy TA, Bridge JA, Leonard JC. Mental Health after Unintentional Injury in a Pediatric Managed-Medicaid Population. J Pediatr. 2018 Aug;199:29–34.e16.
De Young AC, Kenardy JA, Cobham VE, Kimble R. Prevalence, comorbidity and course of trauma reactions in young burn-injured children. J Child Psychol Psychiatry. 2012;53(1):56–63.
Thomas CR, Russell W, Robert RS, Holzer CE, Blakeney P, Meyer WJ. Personality disorders in Young adult survivors of pediatric burn injury. J Personal Disord. 2012;26(2):255–66.
Quezada L, González MT, Mecott GA. Explanatory Model of Resilience in Pediatric Burn Survivors: J Burn Care Res. 2016;37(4):216–25.
Coombes J, Hunter K, Mackean T, Ivers R. The journey of aftercare for Australia’s first nations families whose child had sustained a burn injury: a qualitative study. BMC Health Serv Res. 2020;20(1):536.
Horridge G, Cohen K, Gaskell S. BurnEd: parental, psychological and social factors influencing a burn-injured child’s return to education. Burns. 2010;36(5):630–8.
McGarry S, Elliott C, McDonald A, Valentine J, Wood F, Girdler S. Paediatric burns: from the voice of the child. Burns. 2014;40(4):606–15.
Maskell J, Newcombe P, Martin G, Kimble R. Psychological and psychosocial functioning of children with burn scarring using cosmetic camouflage: a multi-Centre prospective randomised controlled trial. Burns. 2014;40(1):135–49.
Egberts MR, van de Schoot R, Geenen R, Van Loey NEE. Parents’ posttraumatic stress after burns in their school-aged child: a prospective study. Health Psychol. 2017;36(5):419–28.
Download references
Acknowledgements
AL is supported by an NHMRC Career Development Fellowship (#1148793).
This work was supported by a Channel 7 Telethon Trust grant.
Author information
Authors and affiliations.
Telethon Kids Institute, Perth Children’s Hospital, 15 Hospital Avenue, Nedlands, Australia
Alix Woolard, Nicole T. M. Hill, Matthew McQueen, Indijah Bullman & Ashleigh Lin
The University of Western Australia, Perth, Australia
Alix Woolard, Helen Milroy & Ashleigh Lin
Fiona Wood Foundation, Perth, Australia; Child and Adolescent Health Service, Perth Children’s Hospital, University of Western Australia, Perth, Western Australia, Australia
Lisa Martin
Fiona Wood Foundation, Perth, Australia
Fiona M. Wood
You can also search for this author in PubMed Google Scholar
Contributions
AW, FMW and HM conceived of the study. AW conducted the searches. Screening was completed by AW and NTMH. AL acted as a consensus reviewer. AW extracted data and assessed quality of the included papers, NTMH and MM double checked this process. AW was responsible for the data analysis, with input from HM, AL, IB, MM and LM. The initial draft of the manuscript was prepared by AW, then circulated among all authors for critical revision. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Alix Woolard .
Ethics declarations
Ethics approval and consent for publication.
Ethical approval was not required, as no primary data were collected as part of this study. Consent was therefore not required for publication.
Competing interests
AL is supported by an NHMRC Career Development Fellowship (#1148793). NTMH is supported by a Forrest Fellowship. AW, MM, LM, HM, FMW and IB declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Woolard, A., Hill, N.T.M., McQueen, M. et al. The psychological impact of paediatric burn injuries: a systematic review. BMC Public Health 21 , 2281 (2021). https://doi.org/10.1186/s12889-021-12296-1
Download citation
Received : 23 August 2021
Accepted : 23 November 2021
Published : 14 December 2021
DOI : https://doi.org/10.1186/s12889-021-12296-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Psychological outcomes
- Psychopathology
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Search Menu
- Advance Articles
- Supplements
- Virtual Collections
- Author Guidelines
- Submission Site
- Open Access
- Self-Archiving Policy
- Benefits of Publishing with JBCR
- About Journal of Burn Care and Research
- About the American Burn Association
- Editorial Board
- Advertising & Corporate Services
- Journals Career Network
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Timing of nanocrystalline silver-based dressing application – a retrospective single-center pediatric cohort stud y.
- Article contents
- Figures & tables
- Supplementary Data
Marshall Thibedeau, Joel Fish, Charis Kelly, Julia Wenskus, Jennifer Zuccaro, Eduardo Gus, Timing of nanocrystalline silver-based dressing application – A retrospective single-center pediatric cohort stud y , Journal of Burn Care & Research , 2024;, irae056, https://doi.org/10.1093/jbcr/irae056
- Permissions Icon Permissions
Recent evidence has demonstrated that silver has anti-inflammatory properties that are independent from the known antimicrobial ones. In our current model of care, non-adherent, non-silver dressings are applied for acute presentations of pediatric partial thickness burn injuries. The wounds are re-assessed after the progression phase (48-72 hours after injury) and silver dressings are applied. However, when logistical obstacles prevent re-assessment within the 48–72-hour window, nanocrystalline silver-based dressings are applied on presentation. The objective of this study was to test our model of care. We hypothesized that immediate application (< 24 hours after injury) of nanocrystalline silver-based dressings would reduce surgical interventions. This was a retrospective single-center cohort study. All patients <18 years old treated at a pediatric burn center for acute partial thickness burn injuries, between January 1, 2020, and December 31, 2021 were included. Multivariable logistic regression was used to compare surgical treatment rates between patients with different timing of nanocrystalline silver-based dressing application. Four hundred seventy-six patients were included for analysis. One hundred four (21.8%) had nanocrystalline silver-based dressings and 372 (78.2%) had non-silver non-adherent dressings applied within 24 hours of injury. Multivariable logistic regression identified three statistically significant variables as predictors for surgical treatment: age (OR = 1.14, 95% CI [1.06-1.23]), total body surface area (OR = 1.15, 95% CI [1.06-1.25]), and burns to buttocks/lower extremity (OR = 2.39, 95% CI [1.26-4.53]). Immediate (< 24 hours after injury) application of nanocrystalline silver-based dressings does not affect surgical treatment rate in pediatric patients with partial thickness burns.
Email alerts
Citing articles via, looking for your next opportunity.
- Recommend to Your Librarian
- Advertising and Corporate Services
Affiliations
- Online ISSN 1559-0488
- Print ISSN 1559-047X
- Copyright © 2024 American Burn Association
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

COMMENTS
Research aimed at identifying the factors responsible for the cellular oedema seen in burn shock postulated the existence of unidentified and complex shock factor(s) . ... A study of children with burns showed that supplementation with a multivitamin containing 400 IU of vitamin D2 failed to correct vitamin D deficiency .
A recent study indicated that one third of hospitalized patients with burns were extubated within 1 day after admission. 8 Patients who require intubation tend to have larger and deeper burns ...
Burn injuries are a significant problem with more than 500,000 people seeking medical treatment, 40,000 resultant hospitalizations, and 4000 deaths per year in the United States. 1 The annual cost of treating these burns is estimated to be in excess of U.S. $ 1 billion, not including the indirect costs of disability and rehabilitation. 1 These statistics have driven a multitude of studies that ...
While treatment for burn injury has improved significantly over the past few decades, reducing mortality and improving patient outcomes, recent evidence has revealed that burn injury is associated with a number of secondary pathologies, many of which arise long after the initial injury has healed. Population studies have linked burn injury with increased risk of cancer, cardiovascular disease ...
The WHO estimates that 11 million burn injuries of all types occur annually worldwide, 180,000 of which are fatal 13. There is a wide variability in the incidence of burn injury 23. For example ...
Incidence of burns. Globally, a total of 8,955,228 new cases (95% UI 6,820,977-11157666cases) of burns were identified in 2019, which is almost evenly split between men and women, and most of the new cases were concentrated in the 10-19-year age group (Table 1, Fig. 1A). From 1990 to 2015, the number of incident cases fluctuates within a certain degree, but the number of incident cases ...
This is the first systematic review of qualitative research studies in burns to focus on outcome domains relevant to patient-centred assessment in burns research. It is based upon a large body of qualitative evidence gathered across various settings and adult burn patient populations. It has utilized a review process incorporating well ...
A multitude of epidemiological studies published in Europe over the past 10 years have reported a decrease in incidence, mortality, and severity of burns (a major burn is defined as > 20% TBSA 5 ...
The study was performed at the Institute of Burn Research in the first affiliated with the Army Medical University (AMU). A total of 17,939 burn patients were included in this retrospective study.
Previous research has indicated that mast cell over-activation post-burn is linked to the fibrotic processes of scarring and hypertrophy of scars [30, 33], suggesting that the ongoing systemic inflammatory challenge brought about by upregulated inflammatory markers and free radical production in the body after non- and severe burn injuries is ...
Burns is a major global problem that affects the mental, social, and physical conditions of patients. 65 People who suffer from burns have a change in their life satisfaction after a burn. 66 The results of this study showed that patients with burns are slightly satisfied with their lives. On the other hand, differences in life satisfaction can ...
A study in mice [ 427, 428] showed that burn injury caused a greater reduction in the antinociceptive response to opioids (morphine, oxycodone and hydrocodone) in the contralateral limb than in the burned limb. It is likely that the effects of opioids are antagonized by inflammatory signals in the damaged tissue.
Burns are a prevalent and burdensome critical care problem. The priorities of specialized facilities focus on stabilizing the patient, preventing infection, and optimizing functional recovery. Research on burns has generated sustained interest over the past few decades, and several important advancements have resulted in more effective patient stabilization and decreased mortality, especially ...
The inclusion of these outcomes in any study of burn research will be a marker of the standard of research and the importance of findings to the burn community. Young and colleagues have demonstrated an admirable commitment to co-production in their strategy, through the involvement of clinicians and patients.
How to examine, treat, and refer severe burns injuries Burns represent a substantial healthcare burden, accounting for more than 300 000 global deaths annually.1 In the United Kingdom, an estimated 250 000 patients present to primary care with a burns injury every year and 175 000 attend emergency departments,23 so medical students are highly likely to encounter such patients during rotations ...
4. Discussion. Survey research related to burn care is commonly published, and the rate of publication appears to have increased over the past two decades. The results of this study suggest that the quality of survey research in burn care is generally poor and inconsistent.
To review and synthesise qualitative literature regarding the psychological outcomes following paediatric burn injuries, and to determine if children and adolescents who experience a burn injury have elevated risk of psychopathology following the injury. Systematic review of quantitative and qualitative studies. Informit health, Medline, Embase, and PsycINFO were searched from January 2010 to ...
Background: Implementing innovations emerging from clinical research can be challenging. Thermal imagers provide an accessible diagnostic tool to increase the accuracy of burn wound depth assessment. This mixed-methods implementation study aimed to assess the barriers and facilitators, design implementation strategies, and guide the implementation process of thermal imaging in the outpatient ...
This was a retrospective single-center cohort study. All patients <18 years old treated at a pediatric burn center for acute partial thickness burn injuries, between January 1, 2020, and December 31, 2021 were included.
The research has continued for 75 years and now includes second and third generations of participants. The MRIs were conducted between 1999 and 2019 with FHS participants born during the 1930s through the 1970s. The brain study consisted of 3,226 participants (53% female, 47% male) with an average age of about 57 at the time of the MRI.