

CERTIFICATE PROGRAM
Global clinical scholars research training.
Application Deadline: September 11, 2024 Program Begins: October 15, 2024 Standard Tuition : $15,900
- Who Should Apply
- Admissions & Tuition
Program Overview
Designed for clinicians and clinician-scientists in both the United States and abroad, our Global Clinical Scholars Research Training program provides advanced training in health care research and methods. Using a blended learning model that incorporates online tools, live virtual seminars, and dynamic workshops, the curriculum is focused on enhancing your ability at every stage of the research process – from writing grant proposals and launching new projects to analyzing data and presenting your results.
- Priority Enrollment Deposit Deadline: July 24, 2024
- Application Deadline: September 11, 2024
- Priority Enrollment Tuition: $14,900
- Standard Tuition: $15,900
View Admissions & Tuition for more information.
Program Format
This one-year blended program was designed with working professionals in mind and includes three in-person workshops with online seminars in between. Learners should expect to spend about 7-10 hours per week on coursework, viewing pre-recorded materials, attending live online lectures, reviewing sessions with faculty, and working on team assignments and your capstone.
In-Person Workshop Dates
Workshop 1:
- October 15-18, 2024 | Boston, MA
- November 4-7, 2024 | Lisbon, Portugal
Workshop 2:
- March 31 - April 3, 2025 | Boston, MA
- April 7-10, 2025 | Lisbon, Portugal
Workshop 3 & Graduation:
- October 14-18, 2025 | Boston, MA
Program Objectives
In this immersive program, you will explore the latest advancements and best practices in clinical research—improving your ability to drive health care innovation and achieve better outcomes. Throughout the program, you will learn alongside a talented group of peers from around the world, collaborating to create a deeper and more enduring understanding of the topics.
By attending Global Clinical Scholars Research Training, you will enhance your ability to:
- Design and perform observational and experimental clinical research
- Analyze, interpret, and present clinical research data
- Write and revise successful grant proposals
- Lead clinical teams across a variety of health care settings
The comprehensive program curriculum features five to six interactive webinars monthly, as well as more than 85 recorded online lectures. In addition to the core coursework, you can personalize your experience by choosing a concentration (Advanced Epidemiology or Clinical Trials) and an elective (Drug Development, Secondary Analysis of Clinical Trials or Survey Design) that both align with your interests. Throughout the program, scholars are expected to develop and submit a research proposal.
Upon fulfilling the Global Clinical Scholars Research Training program requirements, you will receive a Certificate of Completion. You also will become an Associate Member of the Harvard Medical School and Harvard University Alumni Associations and will be invited to attend Global Clinical Scholars Research Training program alumni events throughout the year.

Attend an In-Person Information Session
May 29 from 1 - 2 PM in Countway Library.

Building Clinical Research Skills to Advance Health in Saudi Arabia

A Bright Clinical Research Future: Clinician Completes Three HMS Programs and Becomes a Harvard Postdoc
Download the brochure, discover more information about the program and receive details as they're released by completing the form below:.

Program Director Ajay Singh shares an overview
Related Programs
Foundations of clinical research, master of medical sciences in clinical investigation.
© 2024 by the President and Fellows of Harvard College

Clinical Research Certification
Clinical research training.
Leaders in Online, Advanced Clinical Research Certification . Comprehensive Clinical Research Training in 1 to 4 weeks. See why 22,000+ students trust us and start or advance your clinical research career today.
Clinical research courses trusted by students at 1,200+ organizations, 6 government agencies, and 308 universities. Personalized clinical research job coaching with graduates placed at 1,600+ different organizations from large CROs to various trial sites.† Book advising session.
Advanced Clinical Research Coordinator Certification (ACRCC)
Course Info: Fee $300. 3,600+ enrollees. Requires 2 year degree. Salary $59k-80k. Finish in 8 days. Online, self-paced, instant Linkedin badge and online certificate after passing 70% final exam. Accredited by ACCRE, Transcelerate, Joint Accreditation (AMA, ANCC, ACPE for CME), Candidate MSA for federally-qualified post-graduate institute.
Graduates work at: AstraZeneca, Sloan Kettering Cancer Center, US VA, Cedars-Sinai, Mayo Clinic, Janssen, Thermo Fisher Scientific, NewYork-Presbyterian Hospital, University of Alabama, Stony Brook Medicine, NYU Langone Health, Nova Research Institute, Quest Diagnostics, Nestle, Impact H&R, and more†.
Graduate job roles post-course: Clinical Research Coordinator, Clinical Research Coordinator II, Lead Clinical Research Coordinator, Senior Clinical Research Coordinator, Oncology Research Coordinator, Clinical Study Coordinator, Clinical Research Data Coordinator, Clinical Research Nurse, Clinical Director/ Office Manager, Regulatory Contact/ Clinical Research Coordinator, Clinical Research/Regulatory Coordinator, Research Regulatory Specialist, Certified Clinical Research Coordinator, Clinical Research Specialist, Sr. Director of Clinical Operations, and more†. (2024 CCRPS graduate LinkedIn Survey)
Advanced Clinical Research Associate Certification (ACRAC)
Course Info: Fee $450 (payment plans available). 7,600 enrollees. Requires 4 year degree. Salary $49k-103k+. Finish in 10 days. Online, self-paced, instant Linkedin badge and online certificate after passing 70% final exam. Accredited by ACCRE, Transcelerate, Joint Accreditation (AMA, ANCC, ACPE for CME), Candidate MSA for federally-qualified post-graduate institute.
Graduates hired at: Moderna, Merck, IQVIA, ICON plc, Eli Lilly and Company, AstraZeneca, Deloitte, Procter & Gamble, St Jude Children's, Mount Sinai, U.S. DOH HHS, Mass General Hospital, Janssen Pharma, Memorial Sloan Kettering, Stanford University, and more.†
Graduate hired as: Clinical Research Associate, Clinical Trial Monitor II, Research Associate, CRA II, Senior Clinical Research Associate, Research Associate Immunology, Clinical Trial Monitor/CRA, Clinical Trials Project Manager, Clinical Director for R&D, Senior Clinical Research Associate, Clinical Research Professional, Clinical Trial Associate III, QA Associate II, IRB/SRC Analyst II, Clinical Trial Associate, Clinical Research Coordinator, Clinical Research Associate II, Clinical Operations Specialist, Advisor, Clinical Trial Management Associate, Quality Supervisor, and more.† (2024 CCRPS graduate LinkedIn Survey)
Advanced Pharmacovigilance and Drug Safety Certification (APVASC)
Course Info: Fee $300. 5,700+ enrollees. 4 year degree required. Salary $59k-140k. Finish in 6 days. Online, self-paced, instant Linkedin badge and online certificate after passing 70% final exam. Accredited by ACCRE, Transcelerate, Joint Accreditation (AMA, ANCC, ACPE for CME), Candidate MSA for federally-qualified post-graduate institute.
Graduates Hired At: Novo Nordisk, Moderna, Abbott, Accenture, CVS, Walgreens, FDA, VA, GoodRx, Merck, NIH, MOH Ontario, Thermo Fisher Scientific, Procter & Gamble, AbbVie, Parexel, Regeneron, Bristol Myers Squibb, IQVIA, CVS, Memorial Sloan Kettering, Johns Hopkins Medicine, MD Anderson, Emory Healthcare, Eli Lilly, and more.†
Graduate Hired As: Regulatory Affairs Associate, Clinical Trial Drug Safety Associate, Drug Safety Specialist, Patient Safety Senior Associate, Pharmacovigilance Scientist, Pharmacovigilance Manager, Senior Pharmacovigilance Associate, Pharmacovigilance Regional Head, Pharmacovigilance Analyst, Senior Director Quality, Pharmacovigilance Data Entry Manager, Principal Pharmacovigilance Scientist, QA & Medical Complaint Handling Associate, Senior Manager Medical Safety Officer, Medical Affairs Senior Scientist, Quality Manager, VP Medical Affairs, Pharmacovigilance Specialist, Regulatory Project Manager, Product Safety Manager, Manager Pharmacovigilance Operations, Regulatory Affairs Manager, Clinical Pharmacist Consultant, Product Vigilance Manager, Senior Manager Safety and Pharmacovigilance, Associate Director of Pharmacovigilance Department, Qualified Person Responsible for Pharmacovigilance, Pharmacovigilance Deputy, RA Manager, Cosmetovigilance, Drug Safety and Medical Information Specialist, Vice President Clinical Development, Operation Specialist, RA Supervisor & QPPV, Senior Director Clinical Operations, Regulatory Affairs Pharmacist, Vice President Operations, QA Executive, Health Advisor Pharmacist, and more.† (2024 CCRPS graduate LinkedIn Survey)
Advanced ICH GCP Certification (AGCPC)
Course Info: Fee $50. 3,100 enrollees. HS diploma required. Required every 2 years. Most comprehensive training available for ICH GCP. Finish in 2 days. Online, self-paced, instant Linkedin badge and online certificate after passing 70% final exam. Accredited by ACCRE, Transcelerate, Candidate MSA for federally-qualified post-graduate institute.
Graduates hired at: FDA, NHS, Novartis, Novo Nordisk, ICON PLC, PPD, IQVIA, Parexel, Johnson & Johnson, Medtronic, UNC Health, NYU Langone Health, MD Anderson, Colorado State University, Baylor, Kaiser, Cornell, Boston University, and more†.
Graduate hired as: Research Assistant, Lab Assistant, Research Coordinator, Research Scholar, Postdoctoral Researcher, Graduate Research Assistant, Research Assistant Intern, Outpatient Pharmacy Intern, Clinical Affairs Intern, Clinical Fellow, Clinical Nurse, Clinical Operations Manager, Clinical Research Assistant Professor, Lecturer, Graduate Teaching Assistant, Clinical Research Manager, Pharmacy Operations Manager, Associate Director of Clinical Development, Vice President of Clinical Development, Drug Safety Associate, Regulatory Specialist, Scientific Consultant, Medical Laboratory Scientist, Pharmacovigilance Associate, NHS Primary Care QI Facilitator, Government Healthcare Recruiter, Research Ethics Coordinator, and more†. (2024 CCRPS graduate LinkedIn Survey)
Advanced Clinical Research Project Manager Certification (ACRCC)
Course Info: Fee $350. 1,900 enrollees. Salary $65k-143k. Finish in 10 days. Prior PM or Research Experience required. Online, self-paced, instant Linkedin badge and online certificate after passing 70% final exam. Accredited by ACCRE, Transcelerate, Joint Accreditation (AMA, ANCC, ACPE for CME), Candidate MSA for federally-qualified post-graduate institute.
Graduates work at: Emory, ION Pharmaceuticals, Inc., Baim Institute for Clinical Research, Aya Healthcare, Dermavant Sciences, Inventprise, iSTAR Medical, Oregon Health & Science University, Flinders University, and more†.
Graduates hired as: Clinical Trial Project Manager, Research Nurse Manager, Clinical Research Coordinator-Data Manager, Clinical Research Associate, Transdisciplinary Research Project Manager, IT Project Manager in Clinical Research, Publicly Funded Research Project Manager, and more†. (2024 CCRPS graduate LinkedIn Survey)
Advanced Physician Medical Monitor Certification (APMMC)
Course Info: Fee $350. 1,500 enrollees. MBBS/IMG/MD preferred. Finish in 8 days. Salary $49k-110k. Online, self-paced, instant Linkedin badge and online certificate after passing 70% final exam. Accredited by ACCRE, Transcelerate, Joint Accreditation (AMA, ANCC, ACPE for CME), Candidate MSA for federally-qualified post-graduate institute.
Graduates hired at: US Army, Yale School of Medicine, Oxford University, Merck Healthcare, IQVIA Canada, CDC Foundation, MD Anderson Cancer and more†.
Graduates hired as: Clinical Research Medical Monitor, Medical Monitor, Principal Medical Monitor, Lead Medical Monitor, Clinical Trial Medical Monitor, Medical Oversight Director, Associate Medical Monitor, Senior Medical Monitor, Clinical Study Physician, Medical Advisor for Clinical Research, Clinical Research Physician, Medical Safety Monitor, Medical Director of Clinical Research, Drug Safety Medical Monitor, and more†. (2024 CCRPS graduate LinkedIn Survey)
Advanced Principal Investigator Physician Certification (APIPC)
Course Info: Fee $300. Active MD required. Salary range 42k-112k+ not including base physician salary. Finish in 6 days (optional modules). Online, self-paced, instant Linkedin badge and online certificate after passing 70% final exam. Accredited by ACCRE, Transcelerate, Joint Accreditation (AMA for CME), Candidate MSA for federally-qualified post-graduate institute.
Graduates Hired At: Accelemed Research, Zion Healthcare, CAP Research, Quotient Sciences, and more.†
Graduates Hired As: Principal Investigator in Clinical Research, Principal Research Investigator, Senior Principal Investigator, Clinical Trial Principal Investigator, Clinical Research Nurse Investigator, Oncology Principal Investigator, Radiation Therapy Principal Investigator, Academic Principal Investigator, Healthcare Settings Principal Investigator, and more.† (2024 CCRPS graduate LinkedIn Survey)
Advanced Clinical Trial Assistant Certification (ACTAC)
Course Info: Fee $150. 1,800+ enrollees. HS degree required. Finish in 6 days. Salary range $25k-70k+. Online, self-paced, instant Linkedin badge and online certificate after passing 70% final exam. Accredited by ACCRE, Transcelerate, Joint Accreditation (AMA, ANCC, ACPE for CME), Candidate MSA for federally-qualified post-graduate institute.
Graduates work at: Various universities, hospitals, clinics, scholarships, internships, and clinical research sites.†
Graduates hired as: Clinical research assistant, clinical trial assistant, clinical researcher professional, clinical research coordinator, trial assistant, research assistant, and more†. (2024 CCRPS graduate LinkedIn Survey).
CCRPS Reviews : View Recent Clinical Graduate Case Studies from April 2024
From Intern at a CRO to Regulatory Affairs Specialist at UPenn
From No Prior Experience to Clinical Researcher
IMG Used CCRPS to Advance as CRC, CRA, and now research project manager
Startup Associate to a Senior Clinical Research Startup Specialist
Senior Clinical Research Professional Uses CCRPS to Advance Her Career
From pharmacist to pharmacovigilance officer with CCRPS
Drug Safety Associate used CCRPS to Transition to American Job Market
From FMG to Clinical Research Coordinator
From Clinical Research Receptionist to Certified Study Coordinator
Transitioning from Physical Therapy to Clinical Research
From PV "student" to Lead Safety Associate with CCRPS
From Educational Research to Clinical Trials Project Management
Clinical Research Certification Courses
Clinical Research Associate Training
Requires bachelors of science . Monitor multiple clinical trial sites. Finish in 2-4 weeks.
Pharmacovigilance Clinical Research Training
Requires bachelors of science . Monitor drug safety. Finish in 2-3 weeks.
Clinical Research Coordinator Training
Requires 2 year degree . Support a clinical trial site. Finish in 1-3 weeks.
ICH GCP Clinical Research Training
Requires HS diploma. Required for all clinical trial professionals every 2 years .
Clinical Trial Assistant Training
Requires HS diploma . Assist in clinical trials. Finish in 1-2 weeks.
Clinical Research Management Certification Training
Requires clinical trial or project management experience . Finish in 2-4 weeks.
Principal Investigator Training
Requires active MD license or pending Sub-PI position . Conduct clinical trial at site. Finish in 1-3 weeks.
Medical Monitor Training
Requires MD or MBBS/IMG/FMG . Monitor clinical trials with medical knowledge. Finish in 2-4 weeks.
Get Clinical Research Certification with Comprehensive Clinical Trials Training Online
Get clinical research career training in 1 to 4 weeks with our online accredited clinical research courses. Trusted by organizations and experienced researchers.
Our clinical research courses are used by students at 1,200+ organizations, 6 government agencies, and 308 universities.† Graduates of our program work at 1,600+ different companies.
Demo clinical research courses:
Objectives: Provide an advanced and engaging review of International Conference on Harmonization Good Clinical Practice (ICH GCP) guidelines updated for 2024.
Students Enrolled: 3,166†
Students came from: Multiple universities, hospitals, research facilities, contract research organizations, medical practices, and biopharmaceutical companies at different stages
Requirements: HS Diploma or GED
Format: Advanced, online, self-paced.
Length: 16 Hours. Online, self paced, start anytime.
Accreditation: ACCRE, Transcelerate Biopharma
Certification: Clinical research certificate online. Exam score 70% or higher on 2 attempts.
Graduates work at: University research groups, hospitals, clinics, clinical trial sites, pharmaceutical companies, and government agencies
Graduate Job Roles after course: ICH GCP clinical research training is required every 2 years for all research roles thus our graduates work in a range of fields.
Research roles: Research Assistant, Lab Assistant, Research Coordinator, Research Scholar, Postdoctoral Researcher, Graduate Research Assistant, etc. Intern roles: Research Assistant Intern, Outpatient Pharmacy Intern, Clinical roles: Clinical Affairs Intern, Clinical Fellow, Clinical Nurse, Clinical Operations Manager, Clinical Research Professional Teaching roles: Assistant Professor, Lecturer, Graduate Teaching Assistant, etc. Management roles: Clinical Research Manager, Pharmacy Operations Manager, Associate Director of Clinical Development, Vice President of Clinical Development Specialized roles: Drug Safety Associate, Regulatory Specialist, Scientific Consultant, Medical Laboratory Scientist, Pharmacovigilance Associate Other roles: NHS Primary Care QI Facilitator, Government Healthcare Recruiter, Research Ethics Coordinator (based on review of new job positions after enrollment in course)
Pharmacovigilance and Drug Safety
Advanced Pharmacovigilance and Argus Safety Certification (APVASC)
Objectives: Gain advanced education in pharmacovigilance management and proficiency in international regulatory affairs and drug safety monitoring.
Students Enrolled: 5,708†
Students came from: CROs, pharmacies, pharmaceutical companies,
Requirements: Bachelors in Biology or Natural Science OR Pharmacist Degree. Many roles require prior clinical research experience which can be gained by other entry level positions through our CRC or ICH GCP clinical research training certification.
Length: 110 Hours. Online clinical research course, self paced, start anytime.
Accreditation: ACCRE, Joint Accreditation for CE with ACPE 17.5 CME for Pharmacists.
Certification: Online certificate. Exam score 70% or higher on 2 attempts.
Graduates work at: Pharmaceutical and Biotech Companies, Healthcare Service Providers, Regulatory Bodies and Research Institutes, Consulting and Services Companies, Consumer Goods Companies, Healthcare Information and Service.
Graduate Hired As: Regulatory Affairs Associate, Clinical Trial Drug Safety Associate, Drug Safety Specialist, Patient Safety Senior Associate, Pharmacovigilance Scientist, Pharmacovigilance Manager, Senior Pharmacovigilance Associate, Pharmacovigilance Regional Head, Pharmacovigilance Analyst, Senior Director Quality, Pharmacovigilance Data Entry Manager, Principal Pharmacovigilance Scientist, QA & Medical Complaint Handling Associate, Senior Manager Medical Safety Officer, Medical Affairs Senior Scientist, Quality Manager, VP Medical Affairs, Clinical Guidelines Coordinator, Clinical Data Monitor, Pharmacovigilance Specialist, Regulatory Project Manager, Product Safety Manager, Manager Pharmacovigilance Operations, Regulatory Affairs Manager, Clinical Pharmacist Consultant, Product Vigilance Manager, Epidemiologist, Senior Manager Safety and Pharmacovigilance, Associate Director of Pharmacovigilance Department, Qualified Person Responsible for Pharmacovigilance (QPPV), Pharmacovigilance Deputy, Regulatory Affairs Manager, Senior Scientist, Senior Medical Advisor, Cosmetovigilance, Drug Safety and Medical Information Specialist, Environmental Analyst, Vice President Clinical Development, Operation Specialist (Life Cycle Safety/Drug Safety/Medical Information), Regulatory Affairs Supervisor & QPPV, Senior Director Clinical Operations, Safety, and Customer Service Excellence, Regulatory Affairs - Pharmacist, Vice President Operations, QA Executive, Health Advisor Pharmacist, COVID Health Specialist - Case and Contact Manager, Senior Project Manager, Pharmacy Manager, R&D Post-Implementation Service, Pharmacy Manager, Investigations Associate, Lead Clinical Operations Safety/Quality Responsible, Pharmacist, Trial and Supply Management, etc. (based on review of new job positions post-enrollment)
Clinical Research Associate
Objective: Obtain a thorough understanding of clinical research to proficiently fulfill the duties of a Clinical Research Associate.
Students Enrolled: 7,536†
Students came from: Pharmaceuticals and Biotech Companies, Clinical Research and Consulting Services, Hospitals and Healthcare Providers, Universities and Academic Institutions
Requirements: Seeking candidates with a Bachelor's in Biology/Natural Science, Nursing Degree, or MBBS/IMG Degree for entry-level positions . Consider obtaining ACRP or SOCRA credentials after gaining 2 years of experience. Students with credentials and/or 2 years of experience (18% of cohort) still choose our course to refresh their knowledge because of our comprehensive review.
Length: 200 Hours. Online, self paced, start anytime.
Accreditation: ACCRE, Joint Accreditation for CE with ACCME, ANCC, ACPE, ICPE for 17.5 CME for Physicians, Nurses, Pharmacists, and Healthcare Professionals. Candidate for Federally Qualified Post-Graduate Institution with MSA-CESS.
Graduates work at: Pharmaceutical and Biotech Companies, Clinical Research and Consulting Services, Consulting and Professional Services, Consumer Goods Companies, Hospitals and Healthcare Providers, Government Health Departments, Universities and Academic Institutions, Healthcare IT and Services.
Graduate job roles post-course: Clinical Research Associate, Clinical Trial Monitor II, Research Associate, CRA II, Scientist, Quality Assurance Analyst, Senior Clinical Research Associate, Research Associate in Discovery Immunology, Clinical Trial Monitor/CRA, Clinical Trials Project Manager, Associate Director of Research Nursing, Clinical Trial Navigator, Clinical Director for R&D, Senior Clinical Research Associate, Clinical Research Professional, Medical Science Liaison, Clinical Trial Associate III, Quality Assurance Associate II, IRB/SRC Analyst II, Project Manager, Clinical Trial Associate, Clinical Research Coordinator, Public Health Advisor, Associate Scientist II, Strategy Analyst, Clinical Research Associate II, Clinical Operations Specialist, Advisor - Development Clinical Research Scientist, Neuroscience, Associate Clinical Engineer, Clinical Trial Management Associate, Quality Supervisor, Clinical Research Data Coordinator (based on review of new job positions post-enrollment)
Clinical Research Coordinator
Objective: Acquire comprehensive proficiency in clinical research coordinator training encompassing patient care, regulatory compliance, and trial oversight.
Students Enrolled: 3,653†
Students came from: Hospitals and Healthcare Providers:, Clinical Research and Consulting Services, Healthcare Services, Pharmaceutical and Biotech Companies, Clinical Research Centers
Requirements: HS Diploma or GED OR Nurses OR Professionals with patient experience.
Length: 150 Hours. Online, self paced, start anytime.
Accreditation: ACCRE, Joint Accreditation with ANCC for 17.5 CME for nurses.
Graduates work at: Pharmaceutical and Biotech Companies, Hospitals and Healthcare Providers, Universities and Academic Institutions, Clinical Research and Consulting Services, Diagnostic Services, Consumer Goods Companies, Healthcare Services, Cancer Treatment and Research Centers.
Graduate job roles post-course: Clinical Research Coordinator, Clinical Research Coordinator II, Lead Clinical Research Coordinator, Senior Clinical Research Coordinator, Oncology Research Coordinator, Clinical Study Coordinator, Clinical Research Data Coordinator, Clinical Research Nurse, Clinical Director/ Office Manager, Regulatory Contact/ Clinical Research Coordinator, Clinical Research/Regulatory Coordinator, Clinical Trials Specialist, Research Regulatory Specialist, Certified Clinical Research Coordinator, Clinical Research Specialist, Sr. Director of Clinical Operations (based on review of new job positions post-enrollment)
Clinical Research Assistant
Objective: Enhance skills required to support clinical trials, focusing on trial conduct, data collection, and administrative duties.
Students Enrolled: 1,800†
Students came from: Various universities, hospitals, clinics, and clinical research sites, etc.
Requirements: HS Diploma or GED. Current high-schoolers with evidence of active research internship can enroll.
Length: 50 Hours. Online, self paced, start anytime.
Accreditation: ACCRE, Transcelerate Biopharma.
Graduates work at: Various universities, hospitals, clinics, scholarships, internships, and clinical research sites.
Graduate job roles post-course: Clinical research assistant, clinical trial assistant, clinical researcher professional, clinical research coordinator, trial assistant, research assistant.
Clinical Research Project Manager
Objective: Prepare students for clinical research management certificate by teaching them how to effectively oversee large-scale clinical studies, ensuring adherence to protocols, budget, and timelines.
Students Enrolled: 1,190†
Students came from: Several CROs, universities, hospitals, clinics.
Requirements: Prior Project Management or Clinical Research Experience.
Accreditation: ACCRE, Joint Accreditation for CME
Certification: Online certificate. Exam score 70% or higher on 2 attempts.
Graduates work at: Pharmaceutical and Biotech Companies, Clinical Research and Consulting Services, Healthcare Staffing, Universities and Academic Institutions
Physician Medical Monitor
Prepare physicians for the specialized role of Medical Monitor, with an emphasis on patient safety, protocol adherence, and data interpretation.
Students Enrolled: 1,438†
Requirements: Medical Degree (MBBS, IMG, FMG).
Accreditation: ACCRE, Joint Accreditation with AMA for 17.5 CME.
Physician Principal Investigator
Equip licensed physicians in their country with the knowledge and skills to undertake the role of Principal Investigator in clinical research.
Students Enrolled: 391†
Requirements: Active Medical Degree. Nonactive medical doctors, PhDs, and PharmDs can work as Sub-I with this clinical research training certification.
Length: 100 Hours. Online, self paced, start anytime.
ICH GCP Training (required every 2 years)
Research Assistant Training (HS diploma)
Clinical Research Coordinator Training (2 year degree or LPN/RN)
Clinical Research Associate Training (4 year science degree or MBBS/IMG)
Pharmacovigilance & Regulatory Affairs Training (4 year science degree or PharmD)
Research Project Manager Training (prior Trials or PM experience)
Medical Monitor Training (nonclinical MD/MBBS/IMG/FMG)
Principal Investigator Training (active MD license)
Triple Accredited Clinical Research Certification Courses for 2024-2025
Transcelerate biopharma.
Recognizes CCRPS to be an accredited GCP trainer.
CCRPS is a candidate undergoing a 1 year intensive study for approval to be a federally recognized career and technical institution.
ACCRE accredits the professional program in Clinical Research leading to the Clinical Research Certification.
Institute for Credentialing Excellence
CCRPS maintains ICE organizational membership.
Upgrade your career or switch to a new path with our online clinical research training.
Joint accreditation.
CCRPS courses accredited by ACME, ICPE, and ANCC for doctors, pharmacists, and nurses for 17.5CME.
Postgraduate Institute for Medicine
CCRPS is audited by PIM for CME credit approval.
About CCRPS
Our online program for clinical research certification is trusted by thousands of students with our graduates finding careers at over 1,600 companies after taking our course per our 2024 survey. Ideal for career changers or those wanting to advance in roles like clinical research associate, coordinator, assistant, project manager, drug safety officer, principal investigator, or medical monitor.
Clinical research training courses by CCRPS are accredited by major organizations (ACCRE, Transcelerate Biopharma, AMA, ACPE, ANCC, ICPE for CME through JA) and recognized by small to large-size clinical research organizations.
Developed by senior clinical research training professionals to help students of all levels.
Training for a New Generation of Researchers
CCRPS provides affordable, industry-recognized clinical research training that will improve your job prospects and trial outcomes. We offer ICH GCP training, CRA certification, CRC certification, research assistant training, pharmacovigilance certification, PI training, medical monitor, and clinical research project manager training. We serve clinical professionals including nurses, physicians, pharmacists, PhDs, premeds, and science-field graduates who want to transition or accelerate their careers with CCRPS.
Do you want more information on our selection of clinical research training certification online programs? Read below.
Clinical Research Courses
The ICH-GCP certification is out for 2024 and offers hours worth of in depth clinical research certification training on all aspects about Good Clinical Practice as defined by the International Conference on Harmonization. The most advanced modules provide a complete overview no matter what your background with pharmaceutical research might be; this includes ethical practices that prioritize safety along side transparent decision making processes where there are none!
Requirements for ICH GCP Certification
The only diplomas needed to enroll in this program are high school or equivalent level education (such as GED). However, if you have more training than that and would like a head start on understanding the material being taught at our college then it is recommended that prior learning be taken into consideration when scheduling classes.
Is ICH GCP Certification right for you?
The ICH-GCP clinical research certification training is a great way for anyone who needs an introduction or refresh on ICH GCP guidelines in order to be an great CCRP. Candidates appearing before interviewers may find themselves unprepared when it comes down solely and exclusively them, but this course will give you all the basics that are needed!
Download the ICH GCP guidelines .
Why choose our ICH GCP training?
ICH-GCP clinical research certification training online confers multiple benefits not matched by any other GCP certification currently available, including being E6 (R2) compliant, having instant enrollment and flexible scheduling, being industry recognized, accredited by research authorities, and affordable with flexible payment options.
Institutions such as CROs that require employees to complete GCP certification can opt for a one-time annual fee payment which allows flexible scheduling for an unlimited number of trainees.
Research Assistant - Clinical Research Assistant
The research assistant certification provides you with the kick-start that will help gain better visibility for your application. The course is designed give thorough understanding of criteria needed in order conduct them effectively, what makes one organization more desirable than another when it comes time apply. The modules cover all aspects from planning through documentation, reporting & publication as well as safety practices necessary during participant recruitment/screening procedures
Requirements for Research Assistant Certification
The research assistant training is open to anyone, even without a high school diploma or equivalent.
High school students intending to work after graduation or interested in healthcare research may benefit from completing the clinical research assistant certification.
Premed students enrolled in an undergraduate degree program and majoring in one of the life sciences may also benefit from clinical research assistant training.
Why choose our clinical research assistant certification?
The research assistant certification is the leading choice for research assistant jobs because it is fully compliant with ICH-GCP and FDA CFR, covers all key concepts extensively, has flexible scheduling, is widely recognized and accepted, and is affordable.
Is research assistant training right for you?
The research assistant program provides a strong understanding of advanced Good Clinical Practice to have a successful career with room for growth. This program offers hands-on experience in subject-facing dimensions of clinical research trials, including training in: the proper protocol for obtaining informed consent, eliciting subject cooperation during trials, obtaining necessary background information for trial documentation.
Trainees learn about subject safety monitoring, which includes exposure to: Adverse Event (AE) identification, documentation and reporting, and trial protocol adherence.
The research assistant course materials contain real-life examples and case studies to help trainees develop insight into and build strategies for: increasing subject enrollment in new and ongoing studies, improving retention rates among subjects enrolled in an ongoing study.
This advanced clinical research coordinator training program is designed to provide in-depth coverage of all aspects, from basic pharmacovigilance and regulatory audits right up through planning for scientific integrity. The course teaches students everything they need know about how best handle each situation that may arise during their career as a Clinical Research Coordinator - no matter what field area interests them most!
Requirements for CRC Certification
The clinical research coordinator (CRC) is a senior member of the clinical research team with responsibilities in overseeing the smooth conduct of clinical research. Candidates must possess a minimum of an associates degree.
Is clinical research coordinator training right for you?
The objectives of candidates enrolling in the clinical research coordinator course are typically related to advancing their clinical research careers. Research professionals enroll in the program to build the relevant knowledge base and administrative skills needed to strengthen their applications for CRC positions. Use the clinical research coordinator course refresh or upgrade their skill-set and obtain clinical research certification in coordination.
Why choose our CRC Certification?
Our clinical research coordinator training has emerged as the clear industry preference when it comes to certifying candidates for on-site roles in clinical research, due to its updated compliance information, broad and deep content coverage, flexible scheduling, and industry-wide reputation for quality.
Our clinical research coordinator certification is accredited by the ACCRE, ACCME, ACPE and ANCC - the most widely recognized and accepted CRC programs across the industry - making it a sound investment for those looking to pursue a career in clinical research.
The course tuition is affordable and can be paid up-front or in easy monthly installments
The Clinical Research Associate Program is the perfect opportunity for you to have a career in research! This advanced program has over 200 hours of specialized clinical research training, which will teach students everything they need. You'll learn how to write reports and site visits with ease using our curriculum that covers all topics related directly or indirectly toward clinical trials work--and even teaches additional techniques for efficiency and workflow.
Requirement for CRA Certification
In order to enroll for the clinical research associate certification , one must have a bachelor’s degree in life science or a health-care science, or a graduate degree in medicine.
Is CRA training right for you?
Graduates with a bachelor's degree in science who are interested in exploring clinical research can benefit from taking this course. Aspirants to CRA jobs looking to boost their hire visibility can also benefit from taking the course. Health-care professionals (RNs, NPs, PAs and others) aiming to either transition to or advance a career in clinical research.
CRAs with less than 5 years of work experience wishing to fast-track. Also, CRAs, Senior CRAs and other clinical research personnel needing a refresher course.
Why choose our CRA Certification?
The clinical research associate course confers a number of advantages on the individual, whether they are entering the field of clinical research or working to advance their career.
CRAs certified through clinical research associate training have up-to-date knowledge of both ICH and FDA regulatory requirements for human subject safety in clinical research.
The program is flexible, allowing trainees to fit the training into a busy schedule. There is an emphasis on hands-on clinical research training using real-life clinical research examples and data sets.
Completing the clinical research associate course is recognized across the US as equivalent to 17.5 CME credits.
Qualifying candidates receive not only a widely accepted and recognized clinical research associate certificate.
Pharmacovigilance
The pharmacovigilance course is an advanced program that will prepare you for a career in PV, with the most comprehensive syllabus covering all aspects from pre-clinical phase to post market surveillance (Phase IV clinical trials).
Requirements for Pharmacovigilance Clinical Research Certification
The goal of pharmacovigilance is to ensure the safety of all drugs and medical devices. QPPVs are responsible for achieving this goal through and beyond clinical trials. To be a QPPV, one must have considerable medical knowledge, statistical skill, and analytical ability. Candidates for the pharmacovigilance and regulatory affairs certification must possess a minimum of:
A bachelor’s degree in life science OR a health-care science
Is clinical research drug safety certification right for you?
The pharmacovigilance certification is beneficial for those in the clinical research field who wish to upgrade their qualifications and expertise. CTAs/CRAs, SCRAs/CRCs, medical and nursing professionals, and QPPVs can all benefit from enrolling in the program.
Enrolling in the pharmacovigilance training gives aspirants an edge when applying for positions that require advanced knowledge of PV compliance, data analytics, software management skills, medical-legal awareness, etc.
Why choose our pharmacovigilance and drug safety training?
Our drug safety and regulatory affairs course is one of the leading pharmacovigilance clinical research certification programs by recruiters across the industry. The pharmacovigilance clinical research certification is compliant with FDA CFR and WHO-ISoP, providing trainees with up-to-date coaching on all relevant regulatory codes and standards. The focus areas of the pharmacovigilance course comprehensively cover all domains of knowledge and skill required for an effective QPPV. The pharmacovigilance course trains candidates in creating, managing and retrieving case report forms using Argus Safety software. CCRPS regulatory affairs clinical research certification offers on-demand, flexible scheduling to allow enrolled students to complete the program at their own pace. The pharmacovigilance and drug safety course tuition is payable either up front or in two easy monthly installments.Explore comprehensive clinical data management training and placement opportunities in the USA. Develop your skills and secure promising positions in this dynamic field. Unlock your potential for success today.
How to Become A Trial Project Manager
Requirements for clinical trial project manager training
Clinical research project managers must have a bachelor's degree in a scientific field. We require prior clinical trial experience in managerial roles or prior project manager experience though graduates seeking to grow in their current career can take the course. They must be able to manage and coordinate all aspects of clinical trials. They must be able to keep up with ever-changing regulations governing clinical trials
Why choose our medical monitor training?
Our clinical trial project manager training is the most comprehensive and up-to-date clinical research certificate program online. You will learn how to manage clinical trials from start to finish, including budgeting, scheduling, and communication.
Upon completion of the program, you will be a certified clinical trial project manager . Our tuition rates are very affordable compared to other programs in this field.
Is project manager clinical research certification right for you?
If you're a project manager or coordinator who is looking to enhance your skills and salary, then clinical research project manager training may be right for you. Earning clinical research project manager certification can help you stand out from other project managers and improve your career prospects.
You must also pass an exam that covers topics such as risk management, stakeholder relationships and data management.
If you meet these qualifications, then becoming certified can help you demonstrate your knowledge and expertise in the field of clinical research project management . Not only will this make you a more valuable asset to your current employer, but it can also open up doors to new opportunities down the road.
Certified clinical research professionals work in a booming industry and there’s no doubt that project managers are in high demand. If you want to make the jump into clinical trial project management, or if you’re already a project manager but want to specialize in pharmaceuticals, our course is exactly what you need.
How to Become a Medical Monitor
Requirements for medical monitor training
Medical monitors are senior members of the clinical research team who oversee the ethical, safe, and transparent conduct of clinical research.
To qualify as a medical monitor, trainees must have a degree in medicine (MD), a non-US degree in medicine (IMG/FMG), or a master’s degree in pharmacy (PharmD).
Physicians with one or more years of exposure to medical research may also qualify as medical monitors.
The medical monitor certification is a program that covers the full range of knowledge domains essential for an medical monitor role, from the philosophy behind GCP to present-day regulatory requirements for clinical research. The course curriculum reflects the most updated regulatory policies related to FDA’s CFR Title 21, as well as E6 (R2) ICH-GCP guidelines. Trainees have the option of on-demand scheduling to fit with their busy schedules.
Is Medical monitor certification right for you?
The medical monitor training offers a comprehensive overview of the principles of Good Clinical Practice, as well as compliance requirements for ethical and safe medical research.
This is the only program that provides in-depth training on all aspects of clinical research design and execution.
The medical monitor course also covers pharmacovigilance concepts crucial to a medical monitor’s role such as AE/SAE identification and tracking; probabilistic assessment of AEs/SAEs as ADRs; risk management in clinical trials.
Trainees gain working knowledge of financial regulatory compliance: disclosure documentation & updating; FDA audit protocols & strategies.
An added advantage is its focus on digitized elements such remote data monitoring tools (software & video), EDC capture & quality control
Principal Investigator
The principal investigator certification program is a great way for physicians involved in clinical research who want to transition into more senior roles, enhance their eligibility when applying or overseeing trials process. It provides PIs with the ideal means of upgrading career skills while also helping them become better fundraisers and managers!
Requirements for Principal Investigator Certification
To be a certified PI, you must be a practicing physician. You may also either be the PI or Co-PI of an ongoing clinical research study, or have been the Ex-PI or former Co-PI of a completed study.
Is Principal Investigator training right for you?
The principal investigator certification provides a thorough, yet quick refresher of the regulatory and compliance requirements for ethical, safe and transparent medical research. This is an in-depth review of all aspects of leading clinical research design and execution as a principal investigator, including: advanced trial design, randomization, blinding and unblinding; clinical site assessment, preparation active site monitoring and close-out; clinical trial protocol development and implementation, including trial monitoring tools and documentation; Investigational Product (IP) accountability storage and dispensing; Adverse Events (AEs), Serious Adverse Events (SAEs), Adverse Drug Reaction (ADR), Important Medical Event (IME) – identification tracking reporting; probabilistic assessment of AEs SAEs as ADRs – medical assessment statistical data analytics risk safety assessments in clinical trials.
Why choose our PI training course?
The principal investigator certification is the best choice for both physicians who want to get certified as a PI, and for industry experts who are looking for someone to fill a PI position. This is because the certification is very flexible and covers a lot of ground.
Additionally, those who become certified principal investigators will be up-to-date on the most recent regulatory policies related to FDA CFR Title 21 and the E6 (R2) ICH-GCP guidelines. This means that they will be qualified to manage compliance requirements in a clinical study.
The course curriculum includes all of the knowledge domains essential for clinical research principal investigator training , but busy professionals can review only the modules most relevant to them and their needs. This way, they can still update their knowledge and skills without having to spend a lot of time on it.
Clinical Research Staff Training
CCRPS works with pharmaceutical, biotech, medical device, and contract research organizations to efficiently train and certify their clinical research associates, coordinators, and assistants to meet ICH GCP and CFR compliance for their staff. We can provide outsourced clinical research staff training set up within 1-2 business days. We work with organization budgets and staff training size to provide comprehensive and transformational education for onboarding and updating staff compliance with ICH GCP and job training requirements. We have worked directly with organizations and groups ranging from 2 employees to 179 employees.
The Platform for Clinical Research Education
Ccrps case studies & reviews.
From IMG to Clinical Research Coordinator at Columbia University: " This course not only met but exceeded my expectations with its thorough curriculum and insightful modules." -Lisa-Pierre ( view full case study )
From IMG to Clinical Research Coordinator "The hands-on activities integrated throughout the course really helped solidify my understanding of complex concepts." -Umber Mahmood ( case study summary )
From Physical Therapist to Clinical Researcher: "The in-depth content and expert instructors provided me with invaluable insights into the field." - Celia Moon ( case study summary )
From International CRC to U.S. Lead CRC and CRA: "The flexible online format allowed me to balance my studies with my professional commitments seamlessly." - Aishwarya Sukumar ( view full case study )
Enjoyed Clinical Research Training through Examples "The real-world examples used throughout the course were incredibly useful for applying theory to practice." -Marta Marszalek ( view full case study )
Promoted to Senior Startup Specialist in Clinical Trials : "I appreciate how the course was structured—very interactive and engaging from start to finish." -Justin Scott Brathwaite ( transcript summary )
From Clinical Research Receptionist to Certified Study Coordinator with CCRPS: "I highly recommend this course for its comprehensive approach and practical applications." - Katie Decker ( view full case study )
Learning to Lead Safety Associate: "The course materials were clear, well-organized, and directly applicable to my work." - Renata Noronha ( view full case study )
From IMG to securing roles as a CRC, CRA, and now a project manager: "Joining this course was a pivotal step in my career advancement." - Dr. Vrushali Borawak ( view full case study )
From Physician to Confident Drug Safety Specialist: "The course provided a robust foundation in the field, which was critical for my professional development." - Rabiea Bilal ( view full case study )
From plant biologist to clinical recruitment administrative coordinator : "This program is a gateway to extensive knowledge and skills in a supportive learning environment." -Olajumoke Owati ( view full case study )
ICH GCP Expert: "Thanks to this course, I feel more competent and confident in my role." - Stephanie ( case study summary )
From International PV Roles To North American Market Success: "The detailed modules prepared me excellently for real-world applications." - John Vinil ( view full case study )
From Educational Research to Clinical Trials Project Management: "I was able to immediately apply what I learned in the course to my job. " - Rose Hyson ( view full case study )
From Coordinator to Clinical Research Grant Manager: "it really did a great job of the full scope of clinical research from start to finish." -Hannah Fischer
ICH GCP made her more confident in research: "this course just overall did a really good job going in depth, which I feel like wasn't just, it wasn't just covered just for the sake of covering content" - Aastha Shah (view full transcript)
From Clinical Research Intern to Regulatory Affairs Associate at UPenn: "I would say since then. I've completed this course. It's helped me get my job in regulatory affairs at a clinical research site." - Scott Boyle
From Masters in Health Safety to Clinical Researcher: " I will say quality of delivery, quality of the materials. - Ossai Opene ( view full case study )
- Technical Help
- CE/CME Help
- Billing Help
- Sales Inquiries
Clinical Research: An Introduction
Foundational course covering the core components of the clinical research enterprise.
About this Course
This course provides the basic concepts of what clinical research is, how it is carried out and by whom, and its underlying ethical and regulatory framework. It discusses the key principles of Good Clinical Practice such as data management and the protection of human subjects. It further explores specific issues in clinical research, including protocol design, critical regulations and oversight bodies, common types of clinical trials, regulatory compliance, and clinical research billing.
Note: The course provides an overview of the clinical research enterprise and its parts. It is meant to supplement (not replace) Human Subjects Research (HSR) and Good Clinical Practice (GCP) courses.
Course Preview:
Language Availability: English
Suggested Audiences: Clinical Billing Professionals, Clinical Data Managers, Clinical Research Coordinators (CRCs), Compliance Officers, Contract Research Organizations (CROs), Faculty and Post-Docs, IRB Administrators, IRB Members, Legal and Risk Management Staff, Research Administrators, Researchers, Sponsors, Students
Organizational Subscription Price: $675 per year/per site for government and non-profit organizations; $750 per year/per site for for-profit organizations Independent Learner Price: $99 per person
Course Content
- Understanding U.S. Clinical Research
This module explores the nature and purpose of clinical research, how it differs from clinical care, and the institutional and organizational conditions that shape the research enterprise. The module provides information on the pharmaceutical and medical device industries and regulatory oversight. It outlines the institutional roadblocks to an efficient research enterprise and also describes the role that clinical research plays for public health in general.
Recommended Use: Required ID (Language): 20463 (English) Author(s): Quincy Byrdsong, EdD, CIP, CCRP - Lipscomb University
- Common Types of Clinical Trials
This module walks the learner through different clinical trial types and study phases related to drug and medical device development. Learners explore common types of study designs, their various classifications, and how they address different research questions. The module also considers principles of quality by design and subject selection as they relate to the study design.
Recommended Use: Required ID (Language): 20464 (English) Author(s): Dawn N.L. Pittinger, MBA, CHRC, CRCP - Moffitt Cancer Center
- Critical Regulations and Oversight Bodies
This module summarizes ethical principles governing clinical research. It provides an overview of the FDA’s structure, jurisdiction, and regulatory functions and introduces the reader to IRBs. It discusses clinical investigations that generate the data to support FDA marketing applications and FDA enforcement of its requirements. Learners explore how investigations provide evidence that a new product is both safe and effective. The module touches on U.S. funding agencies’ requirements for clinical research and concludes with an overview of some major international GCP standards.
Recommended Use: Required ID (Language): 20465 (English) Author(s): Kris West, JD, MS - Council on Governmental Relations (COGR)
- Overview of the Clinical Research Enterprise
This module surveys the clinical research enterprise by focusing on the roles and responsibilities of different parties involved in clinical research administration, oversight, and operations. Learners examine the involvement of auxiliary offices as well as the use of different organizational structures to administer clinical research. The module concludes by contrasting sponsored research with non-sponsored research to identify common personnel and staffing practices.
Recommended Use: Required ID (Language): 20466 (English) Author(s): Quincy Byrdsong, EdD, CIP, CCRP - Lipscomb University
- Overview of a Protocol and Designing a Clinical Trial
This module describes protocol development and use in clinical research. It details how the protocol guides investigators, sponsors, monitors, and research stakeholders on how to conduct and oversee the trial. Learners will gain an appreciation of how a protocol helps regulators and ethics boards to understand study procedures and identify any risks for potential subjects. The module concludes with a discussion of study and investigator feasibility assessments.
Recommended Use: Required ID (Language): 20467 (English) Author(s): Melissa Byrn, MS, MBE - Polsky Center for Entrepreneurship and Innovation and University of Chicago
- Data Management in Clinical Research
This module introduces the sources of clinical data, how and where investigators collect data, and best practices for data management. It details how data is reported and the means of ensuring data quality, uniformity, and integrity across subject histories. Learners will explore methods for capturing and collecting data from paper sources, electronic health records, and other digital origins. The module concludes by identifying recent trends in best practices for data management.
Recommended Use: Supplemental ID (Language): 20468 (English) Author(s): Melissa Byrn, MS, MBE - Polsky Center for Entrepreneurship Innovation and University of Chicago
- Ensuring Compliance
This module defines compliance and outlines how it merges with ethics to encompass research integrity at an institution, site, or company. The module details how compliance with GCP serves core functions within clinical research. It reviews key areas of research compliance programs including policies and procedures, training and education, and risk assessments. Learners explore the application and limitations of privacy and confidentiality protections under HIPAA for research. The module concludes by outlining types of scientific misconduct and the means to prevent them.
Recommended Use: Supplemental ID (Language): 20469 (English) Author(s): Kelly Willenberg, DBA, RN, CHRC, CHC, CCRP - Kelly Willenberg & Associates
- Overview of Clinical Research Billing
This module highlights clinical trial budgeting and billing processes from a site perspective for industry sponsored clinical trials. It defines the components of a clinical research budget, discusses potential hidden costs for a research site, and identifies insurance billing requirements. The module concludes by detailing how good budgeting is important, how it relates to coverage analysis, and what guidelines and rules apply to research billing.
Recommended Use: Supplemental ID (Language): 20470 (English) Author(s): Marie Jackson, PhD, MBA - Methodist le Bonheur Healthcare
" role="button"> Computerized Systems in Clinical Research
This module discusses types of clinical research technologies used within clinical trial operations. It identifies how electronic systems enhance clinical trial compliance, improve site efficiencies, promote transparency in clinical trial conduct, and enhance safety and oversight of human research subjects. The module also explains the implications of the regulations at 21 CFR Part 11 on the implementation and use of computerized systems in clinical trials.
Recommended Use: Supplemental ID (Language): 20471 (English) Author(s): Candida Barlow, PhD, MSN, CRN-BC, RN - Oklahoma State University
Who should take the Clinical Research: An Introduction course?
The course is designed for individuals new to clinical research or looking to enter into a related field, including undergraduate and graduate students, university faculty and postdocs, research compliance officers, new clinical investigators, clinical research coordinators, research administrators, institutional officials, clinical data managers, and clinical billing professionals.
How does the Clinical Research: An Introduction course complement other CITI Program courses?
Clinical Research: An Introduction serves as a helpful precursor to taking CITI Program courses in the Good Clinical Practice (GCP) series, Human Subjects Research (HSR) series, and Responsible Conduct of Research (RCR) series. By providing a description of the conduct and context of clinical trials, this course provides leaners with the opportunity to better understand the regulatory and ethical dimensions of clinical research.
This course is not designed to replace other CITI Program courses (such as GCP , HSR , or RCR ).
Why should someone take the Clinical Research: An Introduction course?
Learners who wish to gain a foundational understanding of the clinical research enterprise should take this course to prepare for a career in clinical research or to gain necessary knowledge for those roles interfacing with clinical researchers.
This course can be used in onboarding for those new to research, or for those taking on new roles that involve interaction with research offices or include research responsibilities.
How long will the course take a learner to complete?
This course consists of eight modules. Each module contains detailed content and a quiz, as well as images, supplemental materials, and case studies.
Modules vary in length, and learners may require different amounts of time to complete them based on their familiarity and knowledge of the topic. As a rule of thumb, modules can take about 30 to 45 minutes to complete, which means it could take around four to six hours to complete all eight modules.
Is this course eligible for continuing medical education credits?
This course does not currently have CE/CME credits available.
What are the required and supplemental modules for learner groups?
This course is designed to be completed sequentially through its first five modules (we recommend they are set as “required”). The three following additional modules should be set for “supplemental.” These supplemental modules are recommended for individuals interested in those specific topics. The supplemental modules provide rich information relevant to clinical research but not essential for the learner to gain a foundational knowledge of clinical research.
Supplemental
What are the advantages of the Clinical Research: An Introduction course?
This course provides peer-reviewed training written by clinical research experts. Along with CITI Program's advantages, including our experience, customization options, cost effectiveness, and focus on organizational and learner needs, this makes it an excellent choice for clinical research training.
Related Content
GCP consists of basic and refresher courses that provide essential good clinical practice training for research teams involved in clinical trials.
Provides clinical research professionals with basic and advanced training tailored to the CRC’s critical role in the conduct of clinical trials.
This role-based course covers supervision, delegation, management, reports, and communication for investigators.
This course focuses on developing the knowledge and skills necessary to maintain compliance and best practices associated with clinical research billing.
An in-depth review of the development and execution of protocols.
This course provides an overview of research administration.
Privacy Overview
- Department of Health and Human Services
- National Institutes of Health

Office of Clinical Research Training and Medical Education
For Students
- Summer Internships
- Medical Research Scholars Program
- Clinical Electives Program
For Recent Graduates
- Postbac Program
For Residents/Fellows
- Graduate Medical Education
- Elective Rotations
- Clinical Fellows Corner
- Loan Repayment Program
- FDA Rotation for NIH Fellows
Staff and Professionals Educational Opportunities
- Continuing Medical Education
- Responsible Conduct of Research Training
- Other NIH Educational Resources
NIH Clinical Center Department Fellowships and Opportunities
- Bioethics Fellowship Opportunities
- Critical Care Medicine - Fellowship Opportunities for Physicians
- Critical Care Medicine - Other Professional Opportunities
- Imaging Sciences Training Program
- Laboratory Medicine - Fellowship Programs
- Nursing - Professional Opportunities
- Nutrition - Dietetic Internship Program
- Pharmacy - Training Programs
- Social Work - Education and Training
- Transfusion Medicine - Education and Training
NIH-Wide Training Opportunities
- Courses in Clinical Research - NIH Office of Clinical Research
- NIH-Duke Master's Program in Clinical Research
- Research Training and Career Development - NIH Office of Extramural Research
- Training Opportunities - NIH Office of Intramural Training and Education
OCRTME News and Contacts
- Follow us @CCMedEd
NOTE: PDF documents require the free Adobe Reader .
This page last updated on 01/12/2024
You are now leaving the NIH Clinical Center website.
This external link is provided for your convenience to offer additional information. The NIH Clinical Center is not responsible for the availability, content or accuracy of this external site.
The NIH Clinical Center does not endorse, authorize or guarantee the sponsors, information, products or services described or offered at this external site. You will be subject to the destination site’s privacy policy if you follow this link.
More information about the NIH Clinical Center Privacy and Disclaimer policy is available at https://www.cc.nih.gov/disclaimers.html
- Department of Health and Human Services
- National Institutes of Health

Medical Research Scholars Program
Press Release : NIH Announces 2023-2024 Medical Research Scholars Program Class
The Medical Research Scholars Program is a year long research immersion program for future clinician-scientists that advances health by inspiring careers in biomedical research. By engaging students in basic, clinical, or translational research investigations, offering a curriculum rich in didactics and professional development, and featuring a robust mentorship and advising program, MRSP prepares its Scholars to become tomorrow's leaders in medicine and biomedical research.

Eligibility
The 10-12 month program is designed for students who are U.S. citizens or permanent residents, have a strong interest in conducting basic, translational, clinical or epidemiological research and are currently enrolled in their 2nd, 3rd, or 4th year at an accredited medical, dental, or veterinary program .
Dental and veterinary students: due to the integrated nature of the third and fourth (clinical) years, participation in the MRSP is recommended after you have completed your second or fourth year in school.
Acknowledgement Statement
The National Institutes of Health (NIH) Medical Research Scholars Program is a public-private partnership supported jointly by the NIH and contributions to the Foundation for NIH, alumni of student research programs, and other individual supporters via contributions to the Foundation for the National Institutes of Health.
NOTE: PDF documents require the free Adobe Reader .
This page last updated on 04/23/2024
You are now leaving the NIH Clinical Center website.
This external link is provided for your convenience to offer additional information. The NIH Clinical Center is not responsible for the availability, content or accuracy of this external site.
The NIH Clinical Center does not endorse, authorize or guarantee the sponsors, information, products or services described or offered at this external site. You will be subject to the destination site’s privacy policy if you follow this link.
More information about the NIH Clinical Center Privacy and Disclaimer policy is available at https://www.cc.nih.gov/disclaimers.html

- GRANTS & FUNDING HOME
- ABOUT GRANTS
- POLICY & COMPLIANCE
- NEWS & EVENTS
Division of Biomedical Research Workforce
- The Biomedical Research Workforce
- Reports on the Biomedical Research Workforce
- Extramural Diversity
- Undergraduate
- Graduate/Doctorate
- Postdoctoral/Residency
Early Career
Established investigator.
- Fellowships
- Career Development
- Other Training-Related
- Research Education
- Institute/Program Matrix
- Resources
NIH programs help to prepare the skilled, creative and diverse biomedical research workforce of tomorrow
Undergraduate and Postbaccalaureate
Engaging in research projects outside the classroom during undergraduate and postbaccalaureate years is important.
Graduate/Clinical Doctorate
The emphasis during these years is on acquiring the fundamental knowledge to master scientific research or clinical discipline. An experienced mentor is critical to successful training.
Postdoctorate/Residency
Research during this period is increasingly sophisticated and independent. The selection of a mentor during this period is very important for its impact on the researcher's future professional career.
The main goal for these early career researchers is to establish themselves and their teams as experts in their fields of research.

Established researchers are focused on their independent research, and use their broad knowledge and scientific expertise to impact public health and society at large.
Undergraduate and Postbaccalaureate Education
Predoctoral Training/Clinical Doctorate
Postdoctoral Training/Clinical Residency
Early Research Career Development
Investigator Development and Mentoring
Recent Funding Announcements
Nih research training and career development programs.
NIH programs help prepare individuals for careers in biomedical, behavioral, social, and clinical research.
- Learn more about how NIH Institutes and Centers may vary in research and training
- Contact NIH extramural research training representatives to discuss how specific programs fit your training and career goals.
- Explore this website for resources for training program leaders and individuals seeking research support at various career stages.
Research Career Pathways
Interactive guides describe NIH programs and links to support training and career development of biomedical scientists:
- Physician-Scientist Infographic
- Veterinarian-Scientist Infographic
- Dentist-Scientist Infographic
- Research-Scientist Infographic

Home About DBRW Career Path Programs Institute/Program Matrix Resources FAQ HHS Vulnerability Disclosure Disclaimer Contact Us
NIH Grants and Funding National Institutes of Health U.S. Department of Health and Human Services USA.gov – Government Made Easy
Older Versions of this Page
Global Clinical Scholars Research Training
This Harvard Medical School one-year, application-based certificate program provides advanced training in health care research and methods.

Associated Schools

Harvard Medical School
What you'll learn.
Design and perform observational and experimental clinical research
Analyze, interpret and present clinical research data
Write and revise successful grant proposals
Lead clinical teams across a variety of health care settings
Course description
Designed for clinicians and clinician-scientists in both the United States and abroad, our Global Clinical Scholars Research Training program aims to enable scholars to expand their knowledge and sharpen their skills in clinical research . Using a blended learning model that incorporates online tools, in-person seminars and dynamic workshops, the curriculum is focused on enhancing your ability at every stage of the research process–from writing grant proposals and launching new projects to analyzing data and presenting your results.
This program combines online learning with three intensive workshops that are designed to offer a mix of traditional and innovative approaches. Each month, you will participate in five to six interactive webinars – including review sessions by faculty and special seminars from leading experts–and also have access to more than 85 prerecorded online lectures. Both concentrations of Advanced Epidemiology or Clinical Trials require scholars to take the foundation courses and their choice of elective courses, in addition to a capstone project.
Participants will be eligible for Associate Alumni status upon successful completion of the program. Early tuition and need-based tuition reductions may be available.
Course Outline
Clinical Leadership in Medicine
Expand your leadership abilities and acquire the skills and framework you need to:
- Manage a health care team in diverse clinical settings
- Pilot successful collaborations within and outside your group
- Navigate the complexities of the institution
- Manage conflicts that arise in a high-stakes environment
Advanced Statistical Tools
Learn to utilize Stata statistical software and other advanced computing methods for:
- Analyzing longitudinal data
- Modeling regression and survival analysis
- Representing polynomial trends for time (e.g., linear or quadratic) and linear mixed-effects models
- Generating polished, manuscript-ready figures and tables
Research Ethics
Examine common challenges in the conduct and review of biomedical human subjects research, including:
- Evolution of ethical codes and regulations
- Responsibility of physicians as investigators
- Preparation of the research protocol application and informed consent documents
- Challenges of conducting research involving children and adolescents
Instructors

Ajay K. Singh

Jamie Robertson

Sagar Nigwekar
You may also like.

Foundations of Clinical Research
This Harvard Medical School six-month, application-based certificate program provides the essential skill sets and fundamental knowledge required to begin or expand your clinical research career.

Clinical Drug Development
Learning about the process of clinical drug development has important implications for anyone working in health care and related sectors.

Gene Therapy
Explore recent advances in gene therapy and learn about the implications for patient care..
- NIH Employee Intranet
- Staff Directory
- En Español
OCRECO Home > Clinical Research Education > Introduction to the Principles and Practice of Clinical Research (IPPCR)
OFFICE OF CLINICAL RESEARCH EDUCATION AND COLLABORATION OUTREACH
- Clinical Research Education
- Funding Opportunities

Introduction to the Principles and Practice of Clinical Research (IPPCR)
- Course Information
- Description
- Registration
- Course Login
Description Important Dates General Information Course Objectives Individual (Non-Registered) Lecture Option Texbook Contact --> Welcome
The Introduction to the Principles and Practice of Clinical Research (IPPCR) course trains registrants on how to effectively and safely conduct clinical research. The course focuses on the spectrum of clinical research and the research process by highlighting biostatistical and epidemiologic methods, study design, protocol preparation, patient monitoring, quality assurance, ethical and legal issues, and much more.
Course Objectives
Provide an overview of basic biostatistical and epidemiologic methods involved in conducting clinical research.
Describe the principles involved in the ethical, legal, and regulatory issues in clinical human subjects research, including the role of Institutional Review Boards (IRBs).
Describe principles and issues involved in monitoring patient-oriented research.
Describe the infrastructure required in performing clinical research and the steps involved in developing and funding research studies.
Intended Audience
This course will be of interest to physicians, scientists, medical and dental students, nurses, public health professionals, and others conducting or planning a career in clinical research.
Course Directors
Social media links.
- Bookmark & Share
- E-mail Updates
Page Footer
- Visitor Information
- Privacy Notice
- Accessibility
- No Fear Act
- U.S. Department of Health and Human Services
- USA.gov – Government Made Easy
- HHS Vulnerability Disclosure
National Institutes of Health (NIH), 9000 Rockville Pike, Bethesda, Maryland 20892
NIH…Turning Discovery Into Health®
Research Training
Academic medical centers play a leading role in training the next generation of scientists. These researchers are trained across the continuum of research disciplines- to ensure future breakthroughs to improve health and transform health care. There are multiple training pathways for pursuing a career in medical research, each of which contribute to building a diverse research workforce. In addition to training the nation’s physicians, academic medical centers train the majority of biomedical scientists in the United States. For more information, check out these related pages:
Resources for students considering a career in medical research
Resources for deans, directors, and administrators of Ph.D., M.D.-Ph.D., and Postdoctoral Programs
Resources for research deans
Compact Between Biomedical Graduate Students and Their Research Advisors
This document offers a set of broad guidelines designed to initiate discussions at the local and national levels about the student-mentor relationship and to support the development of a positive mentoring relationship between the pre-doctoral student and their research advisor.
Compact Between Postdoctoral Appointees and Their Mentors
The Compact Between Postdoctoral Appointees and Their Mentors is intended to initiate discussions at the local and national levels about the postdoctoral appointee-mentor relationship and the commitments necessary for a high quality postdoctoral training experience. The Compact was drafted by the AAMC Group on Graduate Research, Education, and Training (GREAT) and its Postdoctorate Committee, and it is modeled on the AAMC Compact Between Resident Physicians and Their Teachers.
Compact Between Resident Physicians and Their Teachers
This compact is a declaration of the fundamental principles of graduate medical education and the major commitments of both residents and faculty to the educational process, to each other and to the patients they serve
- Research & Technology
- Medical Education
- Basic Science
ACRP Course Catalog
Refine Results:
Knowledge level:.
- Entry Level
- Intermediate
CATALOG GROUPS:
- In-Person Training
- Webinar Replays
- Recorded Conference
COMPETENCY:
- Clinical Trials Operations and GCPs
- Communication and Teamwork
- Data Management and Informatics
- Ethical and Participant Safety Considerations
- Leadership and Professionalism
- Medicines Development and Regulation
- Scientific Concepts and Research Design
- Study and Site Management
- Clinical Study Operations
PRIMARY ROLE:
- CTMS Administrator
- Clinical Research Nurse
- Business Development
- Clinical Research Scientist
- Clinical Data Coordinator
- Billing Compliance Officer
- Clinical Research Coordinator
- Data Manager
- Director of Pharmacovigilance
- Director of Scientific Affairs
- Director or Manager of Clinical Trial Operations
- Director or Manager of Regulatory Affairs
- Drug Safety Manager
- Drug Safety Physician
- Financial Analyst
- Investigator
- Medical Affairs
- Medical Director
- Medical Research Scientist
- Medical Safety Officer
- Medical Writer
- Monitor or Clinical Research Associate
- Patient Recruiter
- Project Manager
- Quality Control Specialist
- Regulatory Specialist
- Research Manager
- Research Technician or Assistant
- Site Selection and Start Up
- Statistician
- Sub-Investigator
108 results
For Individuals
For Business Partners
- Entry Level , Intermediate , Senior
The Future of Home Health Care in Clinical Trials
May 22, 2024—This live session will provide insights into the presenters experience with home health visits to-date and will prompt site and investigator attendees to understand what works and doesn’t work for sites when offered home health options in trial protocols.
Contact Hours
Merck Journeys: Career Conversations within Clinical Research
May 29, 2024—Discover the exciting world of clinical research at Merck during this informative event. Engage with professionals from Clinical Data Management, Clinical Science and Study Management, and Clinical Research Associate roles as they discuss their careers, the industry, and their experiences at Merck.
From Conflict to Collaboration: Enhancing Site and Sponsor/CRO Relationships
June 12, 2024—This live webinar delves into the perspectives and occasional conflicting interests of both parties, fostering mutual understanding. Representatives from each organization will share insights, real-life examples, and practical tools to overcome challenges, promote productive collaboration, and contribute to clinical trial success.
ACRP and the Academy Annual Membership Meeting
October 16, 2024—Join ACRP and the Academy leadership to explore highlights of ACRP’s year and what’s planned for 2025.
Employee Development and Succession Planning
This webinar targets site and organizational leaders with the kinds of details they need to pay attention to in order to develop the framework for an employee development program which will support succession planning.
Intro to In Vitro Diagnostics (IVDs): The Path to Working Together Globally and More Efficiently
This webinar addressed the pressing need for standardized regulatory practices in the field. With advancements in in-vitro diagnostics (IVD), an increasingly interconnected global healthcare landscape, and the growing demand for precise diagnostics, the need for harmonized regulations is paramount.
Clinical Trial Process History and Overview
This webinar covered phases of drug development, the history of regulatory development of human research protection, various types of research study designs, conducting a clinical trial, and generic name and trade name of the medicine.
Efficiency Unleashed: Optimizing Clinical Research Onboarding and Education
Clinical trial professionals with an interest or role in the onboarding and education of new staff can learn valuable best practices from the steps taken, barriers encountered, and resources available during one clinical trial site’s journey toward realizing its goal for optimal efficiency.
Building Your Team Through Transformational Leadership in Clinical Research
This webinar challenged attendees to self-reflect on their own leadership practices and how those might have been in need of transformation
ISO 14155 and FDA Requirements – Trials Conducted at the Research Site
This program explored medical devices and investigational medical devices.
Unlearn to Learn: The Evolution of Clinical Research/Trial Training
This session discussed the current way clinical trial training is implemented and ways that it could potentially be done more effectively and efficiently by industry stakeholders.
A Review of Medical Record Data Extraction and Adverse Event Reporting
This informative session explores some of the tools of the clinical research trade including data extraction and adverse event reporting.
Broadening Your Approach to Trial Diversity on a Global Scale
This live webinar explored diversity through a global lens by tapping into the perspectives of industry leaders who addressed how we can broaden access to a wide range of underrepresented groups, including those who possess attributes linked to troubling and challenging health disparities.
Merck Journeys: Elevating your Career within Clinical Research
This webinar offered a unique opportunity to learn about the rewarding career paths in CSSM. Experienced professionals from Merck shared insights into the innovative clinical research strategies, and discussed how your transferable skills can be leveraged in this field.
ACRP Annual Membership Meeting
ACRP and The Academy leadership joined together to explore highlights of the past year and see what was planned for 2024. Attendees heard how ACRP is working for them and the clinical research profession.
Investigator-Initiated Trial Tips and Tricks
This webinar highlighted best practices in various areas of clinical research management of investigator-initiated trials (IITs) and detailed how to navigate common challenges. The information included in this comprehensive review of IIT operations is experience-based and built upon good clinical research practices in alignment with NIH Regulations.
Good Clinical Practice (GCP) Simulation Renewal
The New Standard in GCP Training. This interactive simulation-powered training course helps ensure compliance with international standards for Good Clinical Practice in clinical trials (ICH E6).
Home Study: August 2023
Supplement more rigorous training, stay informed of the latest trends and developments in clinical research, and earn points for Maintenance of Certification with Home Study tests offered through ACRP’s flagship journal, Clinical Researcher.
- BCO, BD, CDC, CRC, CRN, CRS, CTMS Admin, DM, DoP, DoSA, DMCTO, DMRA, DSM, DSP, Executive, FA, Investigator, MA, MD, MRS, MSO, MW, MCRA, PR, Pharmacist, PMs, QCS, RS, RM, RTA, SSSU, Statistician, , Trainer
Introduction to Decentralized Clinical Trials (DCTs)
Gain the foundational knowledge clinical research professionals need to implement digital health components in clinical trials.
Investigational Drug Services Pharmacists: What They Wish You Knew
How the Investigational Drug Services (IDS) Pharmacy utilized by your clinical trials team works shouldn’t be a mystery, when it can instead be one of your biggest allies in research. This webinar provides “insider insights” from an IDS pharmacist on facts you should have at hand about IDS services when sponsors are considering your site for studies, or are sending monitors to keep track of ongoing projects.
How Do IRBs Review Virtual Trial Technology?
How will institutional review boards (IRBs) review study protocols involving the ever-increasing array of mobile health (mHealth) technologies and virtual trial apps on the market? This webinar helps clinical research professionals to understand the regulations and how the oversight of virtual trials might differ from what they have traditionally been used to.
Home Study: June 2023
Acrp 2023 full program – replay.
A replay package of 62 on-demand session recordings and presentation slides from the ACRP 2023 Conference, April 29 – May 1, Dallas, Texas. It includes content from the five educational tracks, the Signature Series sessions, Rapid-Fire session, and techXpo sessions, presented by a variety of clinical research service providers. Replay sessions will expire on May 31, 2026.
ACRP 2023 Workforce Development – Replay
This package is a replay of 8 on-demand session recordings and presentation slides from the Workforce Development Track at the ACRP 2023 Conference, April 29 – May 1, Dallas, Texas. Understand the workforce landscape and gain strategies to build smarter teams.
ACRP 2023 Technology & Future Trends – Replay
This package is a replay of 7 on-demand session recordings and presentation slides from the Technology & Future Trends Track at the ACRP 2023 Conference, April 29 – May 1, Dallas, Texas. Gain strategies to optimize technology and move your studies into the future.
ACRP 2023 Leadership & Career Growth – Replay
This package is a replay of 10 on-demand session recordings and presentation slides from the Leadership & Career Growth Track at the ACRP 2023 Conference, April 29 – May 1, Dallas, Texas. Get the insights you need to advance your career and grow as a leader.
ACRP 2023 Regulatory Trends & Compliance – Replay
This package is a replay of 10 on-demand session recordings and presentation slides from the Regulatory Trends & Compliance Track at the ACRP 2023 Conference, April 29 – May 1, Dallas, Texas. Hear tips and strategies to mitigate risk and keep your studies in compliance.
ACRP 2023 Study Management & Conduct – Replay
This package is a replay of 17 on-demand session recordings and presentation slides from the Study Management & Conduct Track at the ACRP 2023 Conference, April 29 – May 1, Dallas, Texas. Learn practical, proven ways to maximize study and site performance.
Home Study: April 2023
2022 site perceptions industry survey results: actionable insights for sites, sponsors, and cros.
Site leaders often feel their voices are not heard during the planning and conduct of sponsored clinical trials, while sponsors and contract research organization representatives feel at odds struggling to enhance relationships with those same sites. This webinar focuses on the results of an industry survey conducted by the Tufts Center for the Study of Drug Development on site perceptions, preparedness, and experiences with new clinical research execution solutions, and offers lessons learned on how you can improve site sustainability and lead more effective collaborations.
I Need a Mentor: Where Do I Start?
Career success shouldn’t be measured solely by how far one is able to climb the organizational ladder, especially when reaching career goals depends on so much more than mere effort. In an environment where achievement may rely less on “what you know” than on “who you know,” identifying the right individuals to be part of your knowledge bank and support squad can become crucial.
Building a More Equitable Future Together by Driving Inclusion in Clinical Trials
This webinar targets professionals across the clinical trial ecosystem with best practices learned about taking collective action to drive improvements focused on diversity, equity, and inclusion (DEI) issues. You will learn about building trust within historically underrepresented racial and ethnic minority communities, making clinical trials inclusive by design, and fostering partnership to address DEI-related issues in an integrated and sustainable manner.
Building Clinical Trial Diversity Action Plans for the Future, Now
Dive into the key elements of a diversity action plan for Phase III clinical trials, as explained in the latest recommendations and requirements from the U.S. Food and Drug Administration (FDA), including considerations of race, ethnicity, age, sex, gender, geographic location, and socioeconomic status. Learn how these diversity action plans contribute to the study site’s delivery of meaningful data on drug safety and efficacy from to sponsors.
Home Study: February 2023
Home study: december 2022, a review of ich e8 (r1) general considerations for clinical trials.
The ICH E8(R1) Guideline on General Considerations for Clinical Studies from the International Council for Harmonization guides the clinical development lifecycle for experimental therapies, including quality requirements for clinical study designs and data sources. Principles and practices for the conduct of clinical trials tied to this guideline, already in effect in such regions as the U.S., Canada, and Europe and forthcoming elsewhere, will be reviewed.
Achieving Global Health Equity Through More Diverse and Inclusive Trials
Ensuring a more equitable and accessible healthcare system starts with the healthcare professionals engaged for pre-drug launch and marketing, with the site investigators recruited by sponsors to run clinical trials, and with the location and availability of the study in terms of diverse and underrepresented populations of potential participants. Here’s a historic overview of what has been lacking in clinical trials when it comes to diversity and inclusion, how federal guidelines are beginning to turn the conversation within big pharma toward health equity, and more.
2022 ACRP/Academy Annual Membership Meeting
Join ACRP/Academy leadership to explore highlights of ACRP’s year and what’s planned for 2023 in the areas of membership, certification, and finances.
Home Study: October 2022
Self-leadership: winning strategies for fulfilling work and life (part 1): theoretical foundations and core competencies of self-leadership.
Learn how to use self-leadership strategies to survive and thrive in today’s volatile, uncertain, complex, and ambiguous world.
Ensuring Quality in Fast Enrolling Trials
Are new trials coming at you with break-neck speed? This webinar reviews a case study from a fast-enrolling trial and offers strategies for maintaining high quality standards and recognizing and mitigating the risks of accelerated enrollment.
Webinar—Self-Leadership: Winning Strategies for Fulfilling Work and Life (Part 2)
Learn even more about using self-leadership strategies to survive and thrive in today’s volatile, uncertain, complex, and ambiguous world, in part two of this webinar.
In the DCT Journey, Every Cloud Has a Silver Lining
This webinar focuses on some of the advantages and takeaways learned so far from the introduction, implementation, and adoption of decentralized clinical trials (DCTs). Panelists explore how they operationalized best practices in the areas of budgeting, contract language, remote monitoring practices, virtual visits, and more.
ICH E9: A Review and a Look Into the Addendum (R1)
Dive into an overview of both the ICH E9 Guideline on Statistical Principles for Clinical Trials from the International Council for Harmonization and the ICH E9(R1) Addendum, exploring how clinical trials are based on statistical principles and how treatment effects are measured.
Understanding the Role of DMCs and EACs in Research Oversight
Take a deep dive into the roles and functions of Data Monitoring Committees/Data Safety Monitoring Boards (DMCs/DSMBs) and Endpoint Adjudication Committees/Clinical Events Committees (EACs/CECs) in the lifecycle of clinical trials. These committees are increasingly tasked with providing independent, expert evaluations of clinical trial events and unbiased adjudications to determine if the definitions of certain clinical trial events have been met.
Everything You Need to Know About Holding an IND
This webinar provides a review of the purpose of and expectations set for holding an Investigational New Drug (IND) application through the U.S. Food and Drug Administration, explores common IND-related errors and how to avoid them, and outlines related requirements found in 21 CFR 312 of the Code of Federal Regulations for IND maintenance.
Deconstruct Your Clinical Trial to Plan a Positive Outcome: Effective Planning and Communication Strategies
This webinar examines the communication of intent and expectations to stakeholders in clinical trials to minimize risk and gain fluidity in operations, along with key elements behind planning a clinical trial and best practices for budgeting a study.
Streamline Your Clinical Research Organization’s Processes with End-to-End Promotions
Presenting a discussion on how consolidating the processes involved in the phases of drug discovery, pre-clinical research, clinical trials, and post-approval expectations on a single platform drives efficiencies, margin improvements, and real-time collaborations internally and externally. The result is better top- and bottom-line financial performance and consistency in process outcomes during trial execution.
Best of ACRP 2022
The “Best of ACRP 2022” package offers recordings from 25 of the most popular sessions from our most recent in-person event, along with seven techXpo sessions, presented by a variety of clinical research service providers.
Eliminating Barriers to Careers in Clinical Research
A perfect storm of negative trends is putting immense pressure on entry-level hiring, retention, and overall satisfaction and growth in the clinical research workforce. This webinar focuses on recognizing and overcoming the challenges that are unnecessary barriers to greater participation in the workforce by promising talent from a range of backgrounds and experiences.
The New Work Life Balance in Clinical Research
There’s no denying that “work as usual” has gone out the window in recent years–in the clinical research workforce as well as in most other settings. This webinar explores strategies for juggling a career in clinical research and your personal life in the “new normal,” including in the arenas of excelling at time management in the office and at home, reaching an ideal work/life balance, and acquiring tools to help make your goals a reality.
Virtual ACRP 2021 Full Program Replay
ACRP 2021 programming delivers practical strategies, best practices, and creative solutions needed to improve clinical trial quality.
Virtual ACRP 2021 Innovation in the Era of COVID Track Replay
Unlock your potential by learning innovative new ways clinical research is being conducted in response to COVID-19. This online, interactive program addresses telemedicine, remote monitoring, team management, study start-up and more in the era of COVID.
Virtual ACRP 2021 Regulatory Trends & Compliance Track Replay
Mitigate risk and improve regulatory compliance with insider advice from FDA officials and industry experts. This online, interactive program features FDA officials and industry experts addressing CDER BIMO compliance and enforcement, FDA inspections, ClinicalTrials.gov requirements, and more.
Investigative Site Diversity: Tufts CSDD Study on Staff Diversity at Clinical Research Sites
In this webinar, the Tufts Center for the Study of Drug Development team shares survey results on staff diversity at clinical research sites, with an emphasis on the impact diversity has on site performance and its practical implications, the major factors noted for diversity-related success and their associated barriers, and the relationship between site diversity and patient diversity.
The Push for Technology: A Discussion of Implementation Struggles, Strategies, and Lessons Learned
This webinar features a panel discussion on how the pandemic pushed the implementation of new technologies for clinical trials, with a site-centric focus on the struggles overcome and lessons learned, along with the impact on the workforce and how we all may want to prepare for the future.
Best Practices for Communicating Benefit, Risk, and Uncertainty in Medical Device Clinical Trials
This webinar focuses on how research participants cannot make an informed decision to participate in a clinical trial without clear communications from the research professionals supporting the trial. Evidence-based practices are covered to help you conduct patient education and informed consent tied to the benefits, risks, uncertainties, and patient preferences for using new medical technologies.
Using Agile Strategies to Solve Challenges in Research Operations
This webinar looks at how being agile in research settings is becoming a key performance indicator that partners and sponsors are seeking and expecting from sites, with a focus on techniques and strategies you can use to address and solve administrative and operational challenges at a faster rate via pragmatic and thoughtful “big swings of bold.”
Virtual ACRP 2021 Operational Efficiencies Track Replay
Boost efficiency in clinical trial management and execution with practical strategies for sites, sponsors, and CROs. This online, interactive program addresses collaboration, protocol feasibility, decentralized trials, remote monitoring, and more.
Clinical Research Staffing Reprioritizations and Resourcing Strategies
This webinar presents lessons learned from a survey on the impacts of the pandemic on staffing at research sites, and discusses implications for the future of clinical research workflows from the current shift to remote operations. When should you look for help or change your staffing approach?
The Future of DCTs: Are You Prepared?
This webinar is tailored for research professionals in sponsor, contract research organization, and study site settings who wonder how to embrace the challenges and benefits of decentralized clinical trials (DCTs), and includes a practical guide for what DCTs really are, how there is no going back, and how it is up to the research professional to adapt to change.
Research Ready: Leveraging Technology in the New Research Landscape
Join Advarra experts in a discussion on how to leverage technology in the new research landscape
Working with Site-Based Paperless Solutions and Ensuring Audit Readiness
Join RealTime experts for an overview of site-based electronic systems that the research industry is rapidly adopting.
Achieving Regulatory Compliance via Collaboration: Technology and Site Perspectives
Join experts from WCG Velos and the University of Kansas Medical Center to learn top practices and processes sites follow to meet compliance standards.
Communication Strategies for Conflict Resolution in Clinical Research Teams
This webinar examines group dynamics, team function, and how conflicts arise within the research team, and considers how specific communication behaviors can foster effective conflict resolution that facilitates team growth.
Career Paths in Clinical Research: Sharing the Journey and Providing Insight
Join a panel of clinical research professionals as they discuss their career paths into and through the clinical research industry, consider the future of their roles, and share advice with those wanting to join the industry. You will gain an understanding of the different research roles, areas that are ripe for career growth and development, and how you can prepare to thrive amidst future trends.
Let’s Talk Patient Recruitment: Strategies, Tools, Communication
This webinar reviews two participant recruitment strategies with guests who have helped increase efficiencies and increase recruitment into clinical trials. Be prepared to walk away with some new tips and tricks that you may be able to implement into your recruitment strategy as well.
Clinical Trial Diversity: Strategies to Support Patients, Sites, and Sponsors
This webinar focuses on ideas for educational outreach to promote the purposes and value of clinical research within organizations and communities, and considers what measures sponsors and sites can take to engage diverse patients in their clinical trials.
Monitoring Investigator-Initiated Trials
This webinar offers organizations valuable knowledge regarding how to create a process for internally monitoring the quality and safety of investigator-initiated trials. Templates, workflows, and other tools are shared so that learners may adapt the proposed program to their own institutional needs.
Improving Technology Proficiency in Clinical Research
This webinar considers how sites are shifting away from sponsor-provided systems into technology that meets their business needs. As the options for technology solutions continue to grow, research sites must develop a true technology strategy and develop proficiencies in selecting, implementing, maintaining, and connecting their technology.
How to Build Advanced Workflows with an Integrated Technology Environment
Join Florence Healthcare to learn how to harness the power of best-in-class software while avoiding digital fatigue, duplication, and wasted time.
Quality Improvements as a Result of Paperless Site-Based Systems
Join RealTime CTMS to examine how paperless sites and site networks are driving quality and improved timelines for the clinical research industry.
Exit the Feature Battle: How to Think Long Term and Prepare Your Site for the Future of Technology in Clinical Research
Join Veeva Systems to explore the key qualities and features sites need to look for in their technology partners to increase their connectivity with sponsors and patients.
Research and Expanded Access in Pandemic Times
This webinar provides an overview of the mechanisms through which, in pandemic conditions, experimental COVID-19 therapies and diagnostics were made available to patients and providers before clinical trials had established whether those products were safe and effective. Also considered are how this state of affairs affected researchers’ ability to generate substantial evidence of product effectiveness through high-quality trials and the U.S. Food and Drug Administration’s deliberations over potential COVID-19 vaccines.
Good Clinical Practice (GCP) Simulation
Esource: why this is the platform of the future.
Join experts from Clinical Research IO to explore how several independent trends in research will make eSource a platform technology for sites.
The Impact of Brexit and COVID-19 on Clinical Research Data Processing in the EU/UK
Join The DPO Centre for this exploration of data protection challenges relating to the handling of sensitive personal data of EU and UK trial participants and how COVID-19 has bought data protection to the forefront of people’s minds.
Transforming SOP Infrastructure for COVID Times (Session II)
This second of two webinars on best practices in crafting standard operating procedures (SOPs) for clinical trials applies the concept of emotional quotient-based design thinking to appropriately draft SOPs that include all potential stakeholders. Also considered will be common blind spots within basic compliance mechanisms and the keys for drafting more adaptive SOPs to serve as reference tools and training materials for preparing your organization for new ways of conducting clinical trials.
SARS-Cov-2, the Law, and You
In this webinar, the Association of Clinical Research Professionals and the Society for Clinical Research Sites offer a collaborative discussion on how new laws and regulations arising from the COVID-19 pandemic conditions affect small clinical research sites.
COVID-19 Technology Mad-Dash, What Worked and What Didn’t?
This webinar addresses the challenges of transforming clinical research operations with technology built during the upheavals research sites underwent in the midst of COVID-19 disruptions. You will gain a better understanding of the technology evolution experienced by the clinical research enterprise, how best to future-proof operations to avoid future disruptions such as this, and tips for operating your site with a view of both short- and long-term goals.
Innovative Approach of Using EHR Data to Improve the Speed, Efficiency, Quality and Costs of Conducting Clinical Trials
This webinar describes how a research site’s electronic health record (EHR) data can be used to precisely identify potential subjects from its patient population, thus reducing recruitment and screening efforts and accelerating time to completion of enrollment. Involved are the use of eConsent to enroll patients from their homes, the collection and use of relevant real-world data extracted from the EHR in ways that eliminate certain study visits and manual data entry, and the use of eSource for cutting the need for source data verification.
Practical Tips for Career Development
This webinar describes techniques for developing yourself as a better leader, as well as tools for developing a strong team that fosters open communication and respect of different personality styles. It provides practical tips for developing career ladders for individuals who wish to grow, hints on how to be flexible in terms of your team’s work environments and scheduling, and lessons learned on how to interact with external teams in order to facilitate strong inter-departmental communications and work flows.
Building Relationships With Healthcare Professionals and Community Groups to Increase Enrollment
This webinar focuses on best practices for building and maintaining relationships with healthcare professionals and community groups to increase study enrollment, particularly among diverse patient populations. You will learn how to manage productive physician and community networks that result in highly qualified patient referrals through real-world examples of successful site efforts, and from recommendations on practical adjustments sites can make to their existing strategies to maximize their efforts without overburdening their schedules.
Diversity of Research Personnel: Knowing Who We Are to Know Who We Serve
This webinar highlights how lack of diversity in the recruitment of study subjects impacts the generalizability of research findings, and how evaluating diversity among your research team members will inform study design and recruitment strategies. In turn, this will increase diversity among recruited subjects and make research findings more broadly applicable to wider populations.

Informed Consent Simulation
This interactive, simulation-powered training program helps ensure informed consent is obtained by the right subject, with the right forms, by the right people, through the right process, at the right time, and with the right documentation.
ACRP Clinical Research Knowledge Assessment™ (CRKA)
Whether you’re an emerging professional looking to demonstrate your foundational knowledge to prospective employers or an organizational leader looking for an efficient, effective way to vet new hires, ACRP’s new Entry-Level Knowledge Assessment (ELKA) is for you.
Certified Quality Manager Training
Increase your influence and expand your career opportunities by validating your commitment to quality. The Quality Management Institute and ACRP have partnered in a program of education for individual professionalism and the quality management of clinical research sites.
- BCO, CDC, CRC, CRN, CRS, CTMS Admin, DM, DoP, DoSA, DMCTO, DMRA, DSM, DSP, Executive, Investigator, MA, MD, MRS, MSO, MW, MCRA, PR, PMs, QCS, RS, RM, RTA, SSSU, Statistician, , Trainer
Clinical Trial Monitoring Basics
Become an expert on quality and risk-management strategies and procedures. This interactive eLearning course answers the fundamental questions: What is RBM and how is it different from the standard monitoring approach?
- Entry Level , Intermediate
- BCO, BD, CDC, CRC, CRN, CRS, CTMS Admin, DM, DoP, DoSA, DMCTO, DMRA, DSM, DSP, Executive, FA, Investigator, MA, MD, MRS, MSO, MW, MCRA, PR, Pharmacist, PMs, QCS, RS, RM, RTA, SSSU, , Trainer
Ethics and Human Subject Protection: A Refresher Course
When in need of a comprehensive refresher, this on-demand eLearning course provides a consolidated overview on the history and importance of ethical conduct in clinical trials involving human subjects.
- Investigator,
Investigator Responsibilities
Learn the various responsibilities of clinical investigators based on FDA Regulations, ICH Guidance Documents, and international standards.
Improving Recruitment, Accrual, and Retention in Clinical Trials
Explore best practices to improve recruitment and retention with a focus on operational efficiency, cultural competency, and patient centricity. Included tools will help you assess how to better communicate with potential participants and begin a critical reflection of your own skills and organizational practices.
Trial Feasibility and Selection: Their Impact on Accrual
Selecting the right trial for your clinical research site is key to the success of your accrual for the trial. This eLearning course will discuss how the menu or portfolio of studies offered at a site has an important impact on accrual and how research professionals can become part of the process.
- CRC, CRN, Investigator, PR, RM
Implementing a Patient-Centered Informed Consent Process
Improve your consent process by learning how to assess a participant’s reading level, health literacy, and overall understanding of clinical trial participation and address culture, learning styles, emotional states and language. This eLearning course will provide essential tools for those directly involved in informed consent discussions.
Using Metrics to Improve Subject Recruitment and Retention
Overcome challenges in subject recruitment and retention by learning how to leverage the metrics critical to success. This eLearning course will teach site personnel assess current site performance and readiness for action, and to implement appropriate metric measurement and monitoring.
- BCO, BD, CDC, CRC, CRN, CRS, CTMS Admin, DM, DoP, DoSA, DMCTO, DMRA, DSM, DSP, Executive, FA, Investigator, MA, MD, MRS, MSO, MW, MCRA, PR, Pharmacist, PMs, QCS, RS, RM, RTA, SSSU, Statistician, Trainer
Introduction to Clinical Trials
Fundamental education about the role clinical research plays in advancing medical knowledge and the work conducted by clinical research professionals. This free eLearning course details how medical products are developed, how volunteer patients are protected, and key roles in the development, research, review, and approval of medical products.
- Intermediate , Senior
Site Quality Management Tools: SOPs, Metrics and Training
Master quality-related processes and procedures required to ensure trial compliance. This eLearning course is a comprehensive training program that prepares you for the next step of setting up a quality management system– essential information for site personnel involved in quality management and improvement.
- BCO, CDC, CRC, CRN, CRS, DM, DoP, DoSA, DMCTO, DMRA, DSM, DSP, Executive, Investigator, MA, MD, MRS, MSO, MW, MCRA, PR, Pharmacist, PMs, QCS, RS, RM, RTA, SSSU, Statistician, Trainer
Inspection Readiness: Best Practices for Managing Clinical Trial Inspections
Reduce your anxiety by being prepared for clinical trial inspections. This eLearning course takes you through the full cycle of a regulatory authority GCP inspection and answers key questions including: Why, when and where are regulatory inspections performed? Who can be inspected? And more.
Mastering the Event Reporting Cycle: Understanding Your Impact on Patient Safety
Efficiently identify and report safety events in your clinical trials. This eLearning course, appropriate for all clinical research professionals, guides you through the complete event reporting cycle and critical timelines, as defined in ICH E2a and E6 Guidelines.
- BCO, BD, CDC, CRC, CRN, CRS, DM, Investigator, MA, MD, MRS, MW, MCRA, PR, Pharmacist, PMs, QCS, RS, RM, RTA, SSSU, Statistician, Trainer
Understanding Clinical Trial Protocols: Key Considerations for Effective Development and Feasibility Review
Expand your knowledge and skills in study protocol design and review. This e-Learning course covers the anatomy of a protocol, hypothesis development, study design, subject safety and data integrity risk considerations, protocol feasibility and protocol amendments.
Statistical Principles for Clinical Trials: Overview of ICH E9
Improve protocol interpretation and implementation by understanding trial scope, design, conduct, data analysis, and the evaluation and reporting of safety and tolerability data. This eLearning course explores the International Conference on Harmonization’s E9 guideline Statistical Principles for Clinical Trials.
- BCO, CRC, CRN, DMCTO, Executive, FA, Investigator, MCRA, PMs, RM, RTA, SSSU, Trainer
Mastering Budgeting at Your Site: Building and Negotiating Clinical Trial Budgets that Make Sense
An essential course for all clinical research professionals involved in the clinical trial agreement and/or budget process for industry-initiated trials.
Key Skills for Ensuring Quality Control through Risk-Based Decision Making
Gain confidence in making risk-based decisions and implementing actions related to quality control initiatives. This eLearning course covers key aspects related to risks in clinical trials, including: identification, analysis, planning, communication, change management and much more.
- CDC, CRC, CRN, CRS, CTMS Admin, DM, DoP, DoSA, DMCTO, DMRA, DSM, DSP, Investigator, MA, MD, MRS, MSO, MW, MCRA, PR, Pharmacist, PMs, QCS, RS, RM, RTA, SSSU, Statistician, Trainer
ICH Gap Analysis
Assess your and your team’s training needs quickly by conducting an ICH gap analysis. This eLearning course uses a game to challenge the learner’s knowledge and application of these important efficacy guidelines.
- Investigator, MCRA, PMs
Form FDA 1572: Get it Right the First Time
The Statement of Investigator (Form FDA 1572) doesn’t have to be complicated – let us help you get it right the first time. This eLearning course answers the questions of why, when and how to complete the FDA 1572 to make everyone’s jobs easier.
Ethics and Human Subject Protection: A Comprehensive Introduction
Explore the ethical considerations facing clinical research professionals and learn to put the rules into practice to ensure human subject safety and well-being. This eLearning course provides in-depth training on the importance of ethical conduct in clinical trials involving human subjects.
The Drug Development Process: ICH E8(R1) General Considerations for Clinical Trials
Develop a better understanding of the overall drug development process and how each study fits into the “big picture” of the development life cycle. This eLearning course puts into practice the ICH E8 guidance document General Considerations for Clinical Trials.
Building Quality Management Systems for Sites and Sponsors: Root Cause and CAPA
Implement a Quality Management System (QMS) and prevent findings related to patient safety, data quality and regulatory compliance. This eLearning course will teach you risk management strategies to avoid inaccurate trial data, regulatory non-compliance and more.
- BCO, CDC, CRC, CRN, CRS, CTMS Admin, DM, DoP, DoSA, DMCTO, DMRA, DSM, DSP, Executive, Investigator, MA, MD, MRS, MSO, MCRA, PR, Pharmacist, PMs, QCS, RS, RM, RTA, SSSU, Trainer
eResearch: Managing Clinical Trials in an Electronic Environment
Learn how to set up and manage electronic clinical research documents in compliance with U.S. and EU regulations, and current trends from both the site and sponsor perspectives. This eLearning course examines the challenges of working with electronic documents and how to overcome them.
- Online Courses
SOCRA has implemented an online component to the already robust array of educational opportunities. These online courses offer affordable, convenient access to quality education.
SOCRA's online courses are intended to provide access to training and continuing education that will promote quality clinical research, protect the welfare of research participants and improve global health.
Katrina A. Croghan, MS, CCRP
Anatoly Gorkun, MD, PhD, Chartered MCIPD and Hugh Devine, IMIS
Managing Data Management: Lessons from a Large CMS Demonstration Project
Erin O'Kelly Phillips, MPH, CCRP
Annette Oeser, BS, MLAS, CCRP
Training Game Plan: Ensuring Successful Project Team Member Transitions Through Training
Carolyn Rugloski, MSc, CCRP
Amber Ashley Parker, BA, CCRP
Rachel Kingsford, MS, CCRP
Resiliency in the Workplace
Barbara van der Schalie, MS
Prevention = Safety: No Tears Approach to Research Compliance
Angie Price, MSN, CCRC
Laura Holtz, MS, CCRP
Patricia Beers Block, MDEd, BS, BS, CCRP
Amy Jo Jenkins, MS, CCRP
SOCRA Recertification Module
JoAnn Mick, PhD, RN, NEA-BC
Mtonya Hunter, MBA, CCRP
- Program Information
- Register Online
- Program information
- Complete Learning Module
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español

Join Us for Healthy Vision Month 2024!
NEI is shining a light on vision loss and mental health — and sharing steps people with a visual impairment can take to thrive in their daily lives.
Learn more »

RECOVER Initiative launches clinical trials evaluating treatments for exercise intolerance, post-exertional malaise, and sleep disturbances.

May is National Speech-Language-Hearing Month
Learn about developmental language disorder—a disorder affecting 1 in 14 kindergarteners.

Celebrating at graduation this year?
Talk with your high school grads about celebrating safely.

STEM Teaching Resources
Innovative NIH-funded content to engage K-12 students in health science.
In the News

High-Risk Prostate Cancer
New urine test can identify aggressive cancers that need treatment

Parents Lost to Overdose
From 2011-21, more than 321,000 children lost a parent to a drug overdose.

Alzheimer’s Disease
People with two copies the APOE4 gene are at much higher risk.

Fentanyl Pill Seizures
Law enforcement sees massive jump in confiscated amount of the drug.
NIH at a Glance
Virtual-tour-screenshot-square.jpg.

Take the Virtual Tour
Explore the Bethesda campus and how NIH turns discovery into health.
monica-bertagnolli-thumbnail.jpg

The NIH Director
Monica M. Bertagnolli, M.D., is the NIH Director and provides leadership for the 27 Institutes and Centers that make up NIH.
nih-at-a-glance-funding.jpg
Funding for Research
NIH is the largest source of funding for medical research in the world, creating hundreds of thousands of high-quality jobs.
nih-at-a-glance-labs.jpg

Labs at NIH
Scientists conduct research on NIH campuses across the U.S., as part of our Intramural Research Program.
improving-health-collage.jpg

Impact of NIH Research
NIH-supported research has had a major positive impact on nearly all of our lives.
researcher-holding-petri-dish.jpg

Jobs at NIH
The central recruitment point of access to all NIH jobs and training opportunities
A public-private partnership to develop a coordinated research strategy to speed the most promising COVID-19 vaccines and treatments.
Rapid Acceleration of Diagnostics (RADx)
An initiative to speed innovation in the development, commercialization, and implementation of technologies for COVID-19 testing.
A new research initiative to understand, prevent, and treat the long-term effects of COVID-19.
COVID-19 Treatment Guidelines
Guidelines from NIH for the diagnosis, treatment, and control of COVID-19.
Research information from NIH
NIH supports research in COVID-19 testing, treatments, and vaccines. También disponible en español.
NIH COVID-19 Safety Plan
Guidance to NIH staff, including employees, contractors, trainees, and volunteers, related to COVID-19.
Featured Resources & Initiatives
A new science agency proposed by President Joseph Biden as part of NIH to drive biomedical breakthroughs and provide transformative solutions for all patients.
Anti-Sexual Harassment
NIH does not tolerate pervasive or severe harassment of any kind, including sexual harassment.
Ending Structural Racism
Learn more about NIH’s efforts to end structural racism in biomedical research through the UNITE initiative.
All of Us Research Program
A research effort to revolutionize how we improve health and treat disease.
NIH HEAL Initiative
Trans-agency effort to speed scientific solutions to stem the national opioid crisis.
Clinical Trials
Learn about participating in clinical trials and where to find them.
Accelerating Medicines Partnership
A bold venture to help identify new treatments and cures for diseases.
Medical Research Initiatives
Important initiatives aimed at improving medical research.
Training at NIH
NIH provides training opportunities internally, as well as at universities and other institutions across the U.S.
Connect with Us
- More Social Media from NIH

Virtual Tour
Experience University of Idaho with a virtual tour. Explore now
- Discover a Career
- Find a Major
- Experience U of I Life
More Resources
- Admitted Students
- International Students
Take Action
- Find Financial Aid
- View Deadlines
- Find Your Rep

Helping to ensure U of I is a safe and engaging place for students to learn and be successful. Read about Title IX.
Get Involved
- Clubs & Volunteer Opportunities
- Recreation and Wellbeing
- Student Government
- Student Sustainability Cooperative
- Academic Assistance
- Safety & Security
- Career Services
- Health & Wellness Services
- Register for Classes
- Dates & Deadlines
- Financial Aid
- Sustainable Solutions
- U of I Library

- Upcoming Events
Review the events calendar.
Stay Connected
- Vandal Family Newsletter
- Here We Have Idaho Magazine
- Living on Campus
- Campus Safety
- About Moscow

The largest Vandal Family reunion of the year. Check dates.
Benefits and Services
- Vandal Voyagers Program
- Vandal License Plate
- Submit Class Notes
- Make a Gift
- View Events
- Alumni Chapters
- University Magazine
- Alumni Newsletter

U of I's web-based retention and advising tool provides an efficient way to guide and support students on their road to graduation. Login to VandalStar.
Common Tools
- Administrative Procedures Manual (APM)
- Class Schedule
- OIT Tech Support
- Academic Dates & Deadlines
- U of I Retirees Association
- Faculty Senate
- Staff Council
Idaho WWAMI
- View the Curriculum
- Learn About WWAMI
WWAMI Idaho Offices
Foundations at the university of idaho.
Idaho WWAMI 1st & 2nd Year
Physical Address: 121 W. Sweet Avenue Moscow, ID 83844-4061
Anatomy Lab 803 S. Main Street Moscow, ID 83843
Jeff Seegmiller, Ed.D., Director [email protected]
Christine DePriest, Administrative Specialist [email protected]
Phone: 208-885-6696
Fax: 208-885-7910
Email: [email protected]
Web: WWAMI Medical Education Program
Clinical Phase – Boise
Idaho WWAMI Clinical & Explore and Focus Phase
Idaho WWAMI Medical Education Program 322 E. Front Street, Suite 462 Boise, ID 83702
Phone: 208-364-4544 Fax: 208-334-2344 Email: [email protected] Web: Idaho WWAMI Clinical Office
Mary Barinaga, M.D. Assistant Clinical Dean of Regional Affairs Idaho TRUST Co-Director [email protected] 208-364-4548
Frank Batcha, M.D. Assistant Clinical Dean of Regional Affairs Idaho TRUST Co-Director [email protected] 208-364-4546
Sarah Keshian Program Operations Administrator [email protected] 208-364-4546
Eden J Roberts Program Operations Specialist [email protected] 208-364-4544
Lydia Carbis Medical Student Service Coordinator [email protected] [email protected] 208-422-1000 Ext 7642 208-332-4414

TRUST Scholars
Preparing students for future practice in underserved rural areas.

Project ECHO Idaho
A telementoring program for Idaho’s healthcare community.

North Idaho AHEC
Uniting academic programs and regional health needs.

Help Support Idaho WWAMI Students

Rural Emergency Training
Students Excel in Rural Healthcare Simulation

Healthcare Exploration Workshop
Diverse Careers Unveiled in Idaho Healthcare Event
Idaho WWAMI is a partnership between the nationally-ranked University of Washington School of Medicine and Washington, Wyoming, Alaska, Montana and Idaho (WWAMI). Since 1972, the University of Idaho has partnered with the UW School of Medicine to help educate and provide training for Idaho’s future physicians.
Idaho WWAMI students - all considered Idaho residents - begin their training at the University of Idaho, where they will complete the first 18 months of curriculum, also known as the Foundations Phase. Students then move into the Clinical Phase, where they participate in required clinical experiences. These experiences can be based primarily out of Idaho, are offered in urban and/or rural settings, and/or across the WWAMI region, depending on the student's interests.
The curriculum taught at each WWAMI site, is a joint product of the UW School of Medicine. While each site has its own instructors and may offering specific electives, (such as wilderness medicine offered to Idaho students), all WWAMI students learn the same core curriculum at the same time.
Idaho WWAMI has five primary goals for the State of Idaho:
- Provide publicly supported medical education
- Increase the number of primary care physicians
- Provide community-based medical education
- Expand graduate medical education (residency training) and continuing medical education
- Provide all of this in a cost-effective manner
Idaho WWAMI has an excellent rate of return: 51% of our graduates choose to practice in Idaho — well above the national average of 39%.
- Learn about WWAMI
- How WWAMI Helps Your Community
- UWSOM Admissions Response to COVID-19
- U of I Immunization Policy
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Trans Am Clin Climatol Assoc
- v.125; 2014
Teaching Evidence-Based Medicine in The Former Soviet Union: Lessons Learned
Between 2009 and 2012, I taught principles of evidence-based medicine and clinical research in Russia, Tatarstan, Moldova, and Kazakhstan. The Soviet Union left a medical legacy characterized by balkanization of top tier medicine in highly specialized centers, so there was little capability for multidiscipinary care. In addition, the authoritarian government led to a persistently top-down tradition of medical education and practice, which one of my Russian colleagues aptly named “eminence-based medicine.” After the fall of the Soviet Union, funding for science and medical research was drastically cut, leading to a struggle for resources and politicization of resource decisions. At present, prejudices and beliefs about disease and treatment persist untested, limited English language competency impedes acquisition of new knowledge, and restriction of resources cripples innovation. Yet none of these conditions are unknown to us in the United States. Physicians may resist evidence that challenges long-held beliefs, and patients want us to make decisions based on their individual case, not evidence arising from studying other people. As physicians, we need to understand how to communicate with and frame our arguments so that they can be understood and received favorably. Can we draw lessons from trying to teach evidence-based medicine in the former Soviet Union?
INTRODUCTION
Between 2009 and 2012, I had the opportunity to teach principles of evidence-based medicine and clinical research in four very divergent venues in the former Soviet Union (FSU). These experiences both illuminated for me the obstacles to the practice of good medicine in many parts of the world and also taught me some lessons I think are applicable to how we talk to our colleagues and patients here in the United States.
SOVIET MEDICINE
In the Soviet Union, all health care and its personnel were owned and employed by the state. Healthcare was considered a right of all citizens. In addition, the population of the Soviet Union became relatively well educated, with literacy rates (for those 15 years old) of 99.7% for males and 99.2% for females by the 1990s. However, both medical education and health care were quite substandard, despite the accomplishments of the Soviet Union in other areas of science.
Primary care was largely provided by minimally trained and overworked physicians in environments with minimal resources. Local hospitals outside the major cities often lacked basic laboratories and sometimes even sufficient plumbing to maintain adequate hygiene. Student physicians graduated from enormous undergraduate medical schools, and most received little practical medical training comparable to US residency programs. Once working, physicians were mostly poorly compensated, often earning no more than laborers. They were unmotivated to continually expand and update their knowledge, as they anticipated being unable to use it as well as remaining uncompensated for their additional efforts. Physicians working in clinics were mandated to see eight patients per hour. Giving physicians bribes to ensure that a family member received at least adequate medical treatment became commonplace in the Soviet Union, so that such bribes were often a physician's main source of income. In addition, both linguistic and political barriers kept Soviet physicians out of step with medical progress elsewhere in the world.
Secondary and tertiary hospitals were organized by specialty, with a great deal of compartmentalization of resources and specialties. Typically, a cardiovascular hospital would not have neurologists, neurosurgeons, or trauma doctors, whereas neurology hospitals would not have physicians representing most internal medicine specialties, such as infectious diseases, endocrinology, etc. Use of clinical laboratories was sparse, so that patients undergoing major surgery, such as colectomy for cancer, were discharged after a relatively long hospital stay that involved at most only two or three laboratory measurements of blood counts or electrolytes.
Pharmaceuticals during Soviet days were of poor quality if made domestically, and prohibitively expensive if imported. While health care was considered a right, medicines had to be purchased by the patients, putting many medicines out of reach of most citizens. In addition, nutritional support in most hospitals was so inadequate that patients were obligated to have family members bring meals.
Medicine was traditionally conducted in an authoritarian manner, in which patients were obligated to accept the treatments ordered by their doctors. If a patient asked a nurse what medicine the nurse was administering, he would likely receive as answer “I'm giving you what the doctor ordered,” without further explanation. Choices were essentially never offered, even when feasible. Thus, while patients did not trust their doctors, they had little choice in their healthcare. Nevertheless, in the waning days of the USSR, life expectancy was slightly more than 70 years.
MEDICINE AND HEALTH IN THE RUSSIAN FEDERATION
After the break-up of the FSU, the new Russian Federation's constitution provided all citizens the right to free healthcare under a mandatory health insurance mechanism. Although in place since 1996, this resulted in per capita annual health expenditures of only US$158, compared to $4187 in the US in 2000. In 2008, 621,000 doctors and 1.3 million nurses were employed in the Russian healthcare system. The number of doctors per 10,000 people was 43.8, but only 12.1 doctors per 10,000 people served in rural areas. In contrast, there are about 24 physicians per 10,000 people in the US (data.worldbank.org). Furthermore, the number of general practitioners as a share of the total number of doctors was only 1.26%.
The large shifts in both the Russian economy as well as in healthcare resulted in a sharp decrease in life expectancy, which has only recently been improving ( Figure 1 ). Mortality appears largely due to cardiovascular disease (55%) and cancer (15.2%). Russia is also, however, the world leader in smoking (43.9 million adults, 31% of the total population) and a leader in the consumption of alcohol on a per capita basis.

Life expectancy in Russia. By LokiiT [CC-BY-3.0 http://creativecommons.org/licenses/by/3.0 )], via Wikimedia Commons, data derived from Rosstat.
Moreover, reforms have in many respects made the medical system worse. Medical education remains largely un-reformed: A medical degree is a 6-year bachelor's degree, and a majority of those who obtain such a degree never practice medicine. In fact, a degree in medicine is considered a suitable education for a housewife, much like a degree in home economics was two generations ago in the US. Education in basic sciences is generally substandard and rarely addresses the experimental evidence underlying concepts of physiology and pathophysiological mechanisms of disease. Post-graduate education/training is usually 1–2 years, even for specialists. Academicians do a 3-year “research” training ending with award of a PhD. Understanding the English language is not required in many schools, and therefore students and post-graduate trainees often still learn in isolation from the world's medical literature.
Private insurance, although now legal and available, has not resulted in market competition outside of a few large cities. Most Russians receive health insurance through their workplace. These insurance organizations dictate what doctors they will see and which hospitals they will go to. Medicines must still often be purchased by the patient without benefit of insurance. Insurers provide house-call physicians (usually minimally trained general practitioners) for acute illnesses; if the physician feels the patient needs medical care or diagnostic testing beyond what can be done at home, the patient is sent to a designated hospital, depending on what the physician thinks is going on. Outpatient clinics are generally available only for well-patient visits and some specialty visits.
In general, hospitals are under-resourced, and allocation of resources remains in the hands of the centralized government agencies and their regional sub-agencies, and thus highly political. Accordingly, budgetary decisions are often made to please political constituencies rather than to meet clinical needs.
TEACHING EVIDENCE-BASED MEDICINE IN THE FSU
Medical education in moscow, russian federation.
As a Fulbright scholar in Moscow for 3 months in 2010, my main job was to lecture senior students and residents at the Russian State Medical University based in Moscow ( Figure 2 ). The student population is quite international, drawing from the FSU as well as a large number of developing countries in Africa and Asia. This university provides bachelor degree-level education to approximately 1900 students in medicine, pharmacology, dentistry, and other allied health disciplines ( Figure 2 ).

The Russian State Medical University. The main hall connecting the two primary buildings of the university is prominently decorated with a Soviet-style mural extolling achievements of Russian medicine ( www.rsmu.ru ).
Foreign citizens must provide evidence of completion of a secondary school education and then must satisfactorily complete a 1-year course in the “preparatory department,” during which they study the Russian language, biology, physics, mathematics, and chemistry. After completion of medical studies, students may enter into a Candidate of Science degree, which ordinarily takes about 3 years and requires performance of research and presentation of a dissertation; this is ordinarily considered equivalent to our PhD, but it does not require extensive didactic training. There is also a further Doctor of Science degree, which many budding academicians pursue. One year of post-graduate clinical training is needed to actually practice medicine, even as a specialist.
My lectures were presented in the late afternoons and early evenings, to enable both students and residents posted in various city hospitals to attend. In addition, I presented several lectures in the city hospitals themselves to reach more of the residents and faculty there. Among the medical students, most attendees were Asian and African, as they tended to have better English skills, and my lectures were given in English with bilingual English/Russian slides. Those Russian students who chose to attend likewise had good English skills, and many sought advice about pursuing clinical training or education in the US. Faculty members both at the medical school and various teaching hospitals generally did not have the ability to converse in English, although there were a few exceptions, largely among those who had done some training outside of Russia.

During their clinical experiences, students had little role in provision of patient care in the hospital. They did not participate in work rounds and made no entries into the medical record. They had no direct patient care responsibilities, and their work day was generally 9 am to 2 or 3 pm . They were not expected to read beyond their textbooks and, occasionally, a review article, despite the fact that the medical university had online library subscriptions to a great deal of Western medical journals (all of which, however, required English skills). When told how American medical students participate in patient care, several students reacted by saying that they thought such roles were abusive of patients. However, at the same time, their professors rarely saw fit to explain their decision making to their students or patients. Most often, clinic visits ended when a nurse handed a patient instructions or a prescription with the simple comment that “this is what the doctor recommends.” Thus, it was an uphill battle to convince students and residents of the value of such basics as controls and blinding in clinical trials. In general, what we call the scientific method did not seem to these students to be applicable to the practice of clinical medicine.
Compartmentalization of Care
Another phenomenon I discovered in Russia was their traditional institutionalized compartmentalization of care. During Soviet days, Stalin sought to disperse the means of production so that if cotton was grown in Georgia, textile manufacture would be accomplished elsewhere, so no one region could wield control over the whole process, thus ensuring that the federal government had overall power. Although I have been unable to discover a similar reasoning as the source of the organization of medical care and research in the FSU, the facts on the ground are similar. Care has traditionally been delivered either by hospitals that provide general care only or those that provide specialty care only. Therefore, a patient with major trauma must be taken either to the neurosurgical hospital to have his head wound dealt with, or to the general hospital, where there is a cardiologist or cardiothoracic surgeon who can deal with his chest trauma. No hospital has both. Likewise, research institutes have specialized but limited facilities: one might have excellent animal facilities but be unable to do biochemical analyses, whereas another has excellent chemistry laboratories but no way to house or work with animals.
One of the newest hospitals in Moscow is the Scientific and Practical Center for Pediatric Craniofacial Surgery and Neuropathology. This brand new hospital is a tertiary care center that does head and neck surgery, including craniofacial surgery, neurosurgery for brain tumors, and for other neurovascular conditions. It also has an extensive seizure disorder program and an intensive care unit that cares for newborns with intracranial bleeds as well as head trauma. The physical facilities are impressively up to date and architecturally impressive ( Figure 3 ).

Scientific and Practical Center for Pediatric Craniofacial Surgery and Neuropathology.
However, there are no cardiologists, orthopedists, or other medical or pediatric specialists available for consultation. Among the advanced technologies available at this center are up-to-date MRI and CT scanners and digital video EEG. In contrast, there is no laboratory that does basic coagulation testing; they cannot measure PT, aPTT, fibrinogen, or clotting factor levels. It is not that the physicians do not understand the utility of such testing. It is that donors and politicians would rather be responsible for budgets that make headlines. While I was in Moscow, this hospital received a second MRI machine, and the Patriarch of the Russian Orthodox Church appeared on the front page, above the centerfold, to present this MRI machine; a simple coagulation analyzer, although cheaper, does not make headlines extolling the advances of the Russian medical system.
Clinical Research at Teaching Hospitals
One general hospital I visited was Moscow Clinical Hospital #15. This hospital's website states it serves nearly 35,000 patients per year, trains residents, and performs research.
The hospital does not have specialized hematology or oncology services, but it is a general hospital with active trauma, obstetric, and cardiac surgery services. Posters on the walls illustrate the research its faculty conducts. One example was entitled “Clinical Research for Individual Simulator Inhaler,” by the Moscow State University of Medicine and Dentistry, Department of Exercise Therapy, Sport Medicine and Physiotherapy, City Clinical Hospital #15. A summary of this research essentially stated the following:
- There were 37 subjects, of which 21 were females and 16 males.
- Different forms of respiratory diseases were treated, including acute and chronic bronchitis, bronchial asthma, pneumonia, and “dystonia of hypo- and hypertonic types.”
- The treatment consisted of a 2-week course of “health restoration through application of the Individual Simulator Inhaler,” which appears to be a device similar to an incentive spirometer.
- The investigators' conclusion was that “all the tested persons showed improvement.”
- The investigators further stated that “as the result of our research we revealed that the health restoring effect [of the device] occurs due to its influence on the main factors of lung diseases.”
Nowhere in the report was there mention of controls of any sort, any attempt to control for the type of pulmonary process being treated, or any quantitative measurements (such as might have been made by formal pulmonary function testing). No mechanism of action was hypothesized. Although such “studies” appear to be acceptable as research in Russian medicine, they may also explain why physicians and students do not see clinical research as providing valuable evidence on which to base practice. Furthermore, I saw that learning medicine by memorization was stifling logical reasoning. For example, one student asked: “If you treat a patient with high cholesterol with a statin, and the cholesterol goes down, can't you stop the statin?” Only when asked whether the statin would have permanently addressed the cause of the hypercholesterolemia did the student admit that perhaps an analogy could not be drawn to how antibiotics are used and that the process leading to hypercholesterolemia would not have been reversed permanently.
Innovation in Kazan, Tatarstan
Tatarstan and its capital Kazan are far from the corridors of power in Moscow. In fact, most Russians, including those in the federal government, do not much care what happens in Tatarstan as long as it remains peaceful and does not threaten the rest of the country. Tatarstan is a semi-autonomous region with a large Tatar Muslim population (52%), as well as a large ethnic Russian Orthodox population (43%); several other minority groups are also present. It has a medical school (Kazan State Medical University) created on the Soviet model but poorly supported by the government. However, without political support (or resources), the head of the Therapy Department (their nomenclature for a Department of Medicine) is bringing his medical school and hospital into the 21st century. English is emphasized, and residents and faculty attend European medical conferences to hear about current clinical research methodology and results. Medical students are encouraged to conduct research projects, often providing useful information, such as surveying culture results and use of empiric antibiotics to help guide local practice. The faculty is proud of being up-to-date regarding evidence supporting current therapeutic approaches. And despite limited resources, the Hematology Department faculty has improved their acute myelogenous leukemia discharge-in-remission rate to 50% without flow cytometry, molecular diagnosis, or platelet transfusions! In fact, the hematology ward has five patients per room, with less than a yard between some patient beds, making isolation impossible.
Morning report in Kazan would be familiar to anyone at a US medical school ( Figure 4 ). All residents and medical students on the Therapy wards are in attendance as a resident presents a case. The chair of the department and the chief resident sit at the front of the room listening to the case presentation and then asking questions. In one case I heard presented, the house staff had missed the diagnosis of pulmonary embolism while they kept looking for laboratory and ECG signs of myocardial infarction. The department chair firmly but kindly used the Socratic method to lead the presenting resident through an uncomfortable series of questions designed to let her learn from her own mistakes.

Morning report in Kazan.
With this approach, it appeared that the faculty, residents, and students acquire excellent clinical skills and are familiar with current medical literature and treatment guidelines. And although they cannot obtain such supportive items such as platelet transfusions for patients with leukemia in most cases, they have a fairly good armamentarium of diagnostic tests and can obtain new and even expensive medications for inpatient use. Perhaps most tellingly, in walking the halls, one sees posters announcing the latest activities and meetings of the medical student “Anticorruption Club”! Thus, Kazan is an example of how a visionary leader can affect medical education, as well as medical practice and clinical outcomes.
Chisenau, Moldova
In another part of the FSU, I also taught is Chisenau, the capital of Moldova. Moldova is the poorest country in Eastern Europe. Its gross national product per capita was estimated to be $2037 US/y in 2012 and has actually decreased in recent years. Over one third of the gross domestic product is supplied by remittances from Moldovans working abroad (mostly in Europe). Moldova's total health expenditures per capita are approximately $386 US ( 2 ), and Soviet era practices persist throughout the country. In fact, although Moldova has two official languages (Romanian and Russian), medical doctors largely come from the Russian-speaking population.
Medical care is largely delivered through low-tech delivery systems. Medical records outside of inpatient records are kept by the patients themselves, who hand-carry them to clinics and doctor appointments. Examination rooms tend to be extremely simply furnished, with low exam tables that have no clean paper or other removable covering. There are no patient gowns and often no exam gloves. Equipment, when present, is often falling apart. Otoscopes and ophthalmoscopes are rarely available and even more rarely used. Shortages of equipment and supplies are constants. Basic tests not done due to their cost include such things as thyroid panels, cholesterol screening, and HgbA1C ( Figure 5 ).

A “Reanimation Unit” (Intensive Care Unit) at the Oncology Center Hospital in Chisinau.
Again, as in the Russian Federation, many doctors who graduate from medical school do not practice. In Chisenau, medical doctors earned $35/month in 2010, whereas store clerks earned three to four times more. Physicians who do work at public hospitals and clinics often moonlight elsewhere, including at a growing number of private clinics catering to the small but slowly increasing population of more well-to-do citizens or those who receive support from relatives abroad when they need medical care.
During my first trip there, I was part of a team sponsored by the Ministry of Health of Moldova, which was trying to modernize transfusion and hematology laboratories. My role was to educate doctors about standard practices in transfusion medicine and laboratory hematology. I lectured for 6 hours a day about the use of laboratory information, decision-making about blood product use, and interpretation of red cell serology. Physicians at several of the largest hospitals in Chisenau were required to attend my lectures, and my topics were pre-approved by the Ministry of Health. Mandatory attendance at lectures being held during a heat wave in un−air-conditioned classrooms understandably left these physicians less than enthusiastic! It soon became clear that I would need to do more than just give straightforward lectures to get them to entertain new approaches to doing their jobs. My strategy for improving communication became to first briefly present my personal “bio,” both to establish credibility and to build a relationship in which we had things in common. Thus, after introducing myself and what I did at work in the US, I shared cases in order to develop rapport and participation in problem solving. Finally, I gave my lectures and solicited give and take. Often this meant holding my tongue while listing to their practices, which certainly went against what we in the US teach as good medicine. Nevertheless, by encouraging their participation in case-centered problem solving and allowing for back and forth discussion about various points, they transformed from a passive to an active audience, thus allowing discussion about some of their, from the US perspective, unusual medical practices.
I also visited and lectured at the main National Transfusion Center and lectured physicians and advanced technologists there. For the most part, my audience at the Transfusion Center was enthusiastic and eager to bring their practices up to date. But in addition to knowing little about advanced red cell serological techniques, they also knew little about good laboratory practices in general. Blood tubes were left open on carts in hallways. Instruments were calibrated once annually. Glove use was optional, and gloves were often re-used. The physical environment was clean but marginal in other ways; our translator fainted from the heat in the room where blood shipments to hospitals were being prepared! And while there were many staff members, there was relatively little activity. We visited the apheresis unit, where they proudly showed off their six modern apheresis machines; but only one was at work that day.
At the other end of the economic spectrum extant in the FSU, Kazakhstan is an oil-rich country whose leader, Nursultan Nazarbayev, is determined to compete with the West. Kazakhstan is a huge but relatively sparsely populated country, larger than Western Europe and four times the size of Texas, but with only approximately 17 million inhabitants (approximately 10 million less than Texas). As a former Soviet Republic, it shares a 4000-mile border with Russia and a 1400-mile frontier with China.
One of Nazarbayev's initiatives is to build a new, post-graduate, English-language curriculum medical school and hospital in their newly created capital of Astana ( Figure 6 ).

New construction in Astana, Kazakhstan.
Many US universities have scrambled to get a piece of this “knowledge economy” pie, and Duke also has contributed in this sphere. Our crew of three physicians and three administrators were there to advise the administration about how to bring together five separate but closely situated hospitals, each with its own specialty area consistent with the Soviet model, and their faculties in order to create the clinical teaching facility of the new medical school. We then participated in a national medical conference during which I discussed the use of EBM as an important part of formulating national health policy. The inherited structure of the hospitals was clearly creating rivalries and conflicts, as they were not used to considering themselves allied in any way whatsoever. Politically and strategically, therefore, gradual unification and coordination of their clinical services and faculties has proven to be quite challenging. And, although many physicians and administrators clearly perceived the value of EBM as a guide to multiple areas of health policy, they still found acquisition of state-of-the-art MRI machines and establishment of bone marrow transplant programs more attractive than addressing the public health needs of their population.
PRESENTING EBM RECOMMENDATIONS TO OUR PATIENTS
If we have trouble convincing our colleagues coming from other traditions in medical education about the value of evidence-based medicine, how can we hope to use EBM to help communicate about medical decision-making to our patients? Will it help them understand why their doctor may no longer recommend (or their insurance companies pay for) annual mammograms or PSA tests? While we believe that how we should treat our patients can be tested in a scientifically valid manner, we also know that we cannot find good evidence to solve all our medical dilemmas. We often give our patients major and complicated choices. Do they want surgery or radiation therapy? Do they want to “watch and wait” or undergo an invasive biopsy? Yet when we try to explain the “evidence” for our recommendations, we often need to explain how the clinical trials were conducted and whether the patient we are treating is like or different from the trial subjects. We also need to explain what questions and controversies about the treatment remain, and why. Can we express these conundrums in a way that helps our patients make the choices we impose on them?
For myself, I believe that trying to teach principles of EBM to physicians with different educational backgrounds and different cultures has made me understand a little more about how to communicate with my patients. Nevertheless, in the long run, I also believe that our own country has to make sure that all secondary educational institutions teach the principles of the scientific method, logic, and human biology in a competent manner if we are going to succeed in providing our patients with the opportunity to make good choices about their health care. Our patients, and especially our future patients, need to have the tools to understand EBM when we explain it. Only then will we be able to determine if understanding what EBM is will make it easier for patients to choose the treatment they prefer and make it more likely that they will adhere to the choice they make. Or will they still ask: “What would you do if I were your mother / father / brother…?”
Potential Conflicts of Interest: None disclosed.
Ausiello, Boston: I think there is an incredibly important lesson in what you're doing. And I'll take it from my own experiences in helping the Russian government build a new university called Skoltech, which you may be familiar with. The strong, strong science that has evolved in Russia in physics, mathematics, computation, never was part of the cultural heritage in the development of medicine. Medicine was low on the food chain, and it was never appreciated to be a scientific enterprise. And they're now trying to build a multidisciplined approach to science. In activity just north of Moscow, it is evident that bringing together “scientists” in physics, chemistry, and mathematics are largely going to be the tool kits and skill sets that develop a biological repertoire that ultimately will come back to medicine. And so I would posit that the biggest skill and tool that we bring to our patients is that we do feel that we are well-grounded in a scientific enterprise, and that we should continue to emphasize that to all of our patients no matter whether there is evidence or not for an existing treatment.
Telen, Durham: Thank you.
Weinblatt, Boston: I'd like your perspective on therapeutic trials that are now being done in Russia. As major pharmaceutical and biotech companies have noted difficulty in recruitment from North America and Western Europe, there has been a significant shift to studies being done now in the FSU. Some of us have concerns about the validity of the data and the high placebo, as well as active drug effects and low adverse event rates that are being reported. What is your experience in Russia? Tell us about these studies, this trend towards increasing recruitment from some other developing countries, and the impact that has upon the pharmaceutical industry.
Telen, Durham: So I think that you raise some really important questions. Certainly the environment that patients are being recruited from in Russia is different in many respects. Although healthcare is guaranteed, it is certainly not always up to what we would consider a standard acceptable to us. Not only that, healthcare's guaranteed; drugs are not. And so, often the patients have to buy their own drugs even if their physician's care or their hospital care is guaranteed. And so, participating in clinical trials is often seen, I think, by Russian patients as possibly, you know, a big advantage, because it helps them get the basic care that they need. So that may be a lot of the placebo effect. There is a very different style of communication between physicians and patients in Russia. And so I am absolutely sure that for most patients, unless you really question people hard about adverse events, you'll find out very little. So I wouldn't be surprised if most adverse events, unless they're serious adverse events, do not get reported. And so, the other thing is that I think the motivations for physicians are sometimes very different; why they are participating in trials. A lot of these hospitals are strapped. I mean, they are strapped for really basic equipment. So if a pharmaceutical company is going to come in—and even if it's just going to buy them a couple of new ECG machines—this is very attractive to a lot of these hospitals. And so it's not that we in the United States who do clinical research do not do it for the money, because we do. But we do it for other reasons too. But we also do it for the money! But there is a different driver here. And the last thing that someone pointed out to me, actually before I left—when I started trying to read up on all of this stuff—was that there can be a difference in impacts of drugs in different places because of the underlying care. So there was a study of activated protein-C, I think in sepsis, that was published a number of years ago. And when they looked at where the study was done, it was international. So it was really all over the place. In the countries that had what we would consider as maybe poorer quality ICUs, the activated protein-C seemed to improve things. In the United States, it had no effect. So in total, you might get an answer one way or the other, but it was really dependent on what the infrastructure of care was. And I think that may also be a problem in at least some of these pharmaceutical studies.
- Partner with us
- Apply Online

Lomonosov Moscow State University
The university.
- Recognitions
- Eligibility
- Fees Structure
Originally founded in 1755 as Moscow State University in the city of Moscow, Lomonosov Moscow State University is one of the oldest and well-recognized medical universities in Russia.The University offers a range of medical programs, most popular of which is the General Medicine Program called MBBS. Presently, the University has about 47,000 students studying on its campus and an academic staff of about 5,000 members. The scientific library of Lomonosov Moscow State University has a collection of about 10 million copies of books and medical periodicals and facilitates more than 57,000 readers. To facilitate advanced research and international clinical training, Lomonosov Moscow State University has collaboration with more than 15 research institutes and clinics and more than six medical schools and universities. For facilitating clinical rotations of the students, the university has more than 24 clinical bases across the city. Lomonosov Moscow State University is approved by the Medical Council of India (MCI) and offers a 6-Year Program for MBBS in Russia. Students in India, who have qualified NEET, can apply for direct admission to the MBBS Program of Lomonosov Moscow State University.

Ministry of Science and Higher Education, Russia

World Directory of Medical Schools (WDOMS)

Medical Council of India (MCI)

Educational Commission for Foreign Medical Graduates (ECFMG)

Medical Council of Canada (MCC)

Foundation for Advancement of International Medical Education and Research (FAIMER)
To get admission to the MBBS Program of Lomonosov Moscow State University, the student must qualify NEET-UG (National Eligibility cum Entrance Test-Undergraduate).
Besides NEET-UG, there is no requirement to go through any additional entrance examination.
RUS EDUCATION SUPPORT

INDIAN FOOD
MODERN CLASSROOMS
Medical Laboratories
Clinical Training
Recreational Facilities
Ensured Safety
FMGE (Foreign Medical Graduates Examination) Preparation

USMLE (United States Medical Licensing Examination) Preparation

- Lomonosov Moscow State University was established in 1755 in the city of Moscow.
- Presently, Lomonosov Moscow State University has about 47,000 students and an academic staff of about 5,000 members.
- Library of Lomonosov Moscow State University has a collection of about 10 million books and medical periodicals.
- For clinical rotations, the Lomonosov Moscow State University has more than 24 clinical bases across Moscow city.
University Address
Mbbs program, admission & support, examination support, student life.

Originally founded in 1755 as Moscow State University in the city of Moscow, Lomonosov Moscow State University is one of the oldest and well-recognized medical universities in Russia.
Counted among the leaders in the list of institutions for medical education in Russia, Lomonosov Moscow State University was started to spread higher medical education in Russia. Now, the University offers a range of medical programs, most popular of which is the General Medicine Program called MBBS.
Presently, the University has about 47,000 students studying on its campus and an academic staff of about 5,000 members.
The scientific library of Lomonosov Moscow State University has a collection of about 10 million copies of books and medical periodicals and facilitates more than 57,000 readers to get the required study material for their medical studies and research activities. For research projects, students can work in scientific laboratories ad share their research in the form of articles in leading Russian and international medical journals and research papers.
To facilitate advanced research and international clinical training, Lomonosov Moscow State University has collaboration with more than 15 research institutes and clinics and more than six medical schools and universities. Besides, the University also operates a Medical Research and Education Center, where students are involved in medical research to solve real-life health issues and provide world-class industrial training.
To keep its students up-to-date with academic updates and the latest research in the field of medical science, the University publishes its own newspaper as well.
To encourage students to perform to their best abilities and explore their potential various inter-university competitions are organized on the campus where students get the opportunity to showcase their work and expertise to their peers and leading professions from the medical industry.
For personalized learning experience, the University maintains a low student-teacher ratio, which allows students and faculty members to develop an individual rapport with each other and discuss student matters in an open environment.
To facilitate clinical rotations of the students, the university has more than 24 clinical bases across the city and provides hands-on training under the guidance and supervision of expert healthcare professionals. Depending on their area of interest, students can choose their clinical discipline and get specialized training. To put an extra focus on the quality of education and training provided to medical aspirants, the University also formed the Educational and Training Commission.
The University has preserved its rich history of more than 26 decades in its museums located on the campus which are kept open for visits by all students and visitors. One of the most visited museums is The Museum of Natural History which provides knowledge of earth sciences.
Lomonosov Moscow State University is listed in the World Directory of Medical Schools (WDOMS) and certified by the Educational Commission for Foreign Medical Graduates (ECFMG), United States of America. Lomonosov Moscow State University is also approved by the Medical Council of Canada (MCC) and the Medical Council of India (MCI). The University offers a 6-Year Program for MBBS in Russia for local as well as international medical aspirants. Students in India, who have qualified NEET, can apply for direct admission to the MBBS Program of Lomonosov Moscow State University.

Lomonosov Moscow State University Lomonosov Ave., 27 Moscow, 119192 Russian Federation

Lomonosov Moscow State University offers a 6-Year MBBS Program in the Russian language. For international students, classes for initial years may be organized in English medium.
The Program for MBBS in Russia is focused on building a strong academic base with a pragmatic approach to education and medical research. To provide hands-on clinical experience, the students studying MBBS in Russia are involved in clinical training from the second year of MBBS. While education in classrooms and laboratories helps the students develop academic skills and sound theoretical understanding, clinical training in University-affiliated hospitals help them apply their knowledge into practice.

To get admission to the MBBS Program of Lomonosov Moscow State University, you can apply online at Rus Education website.
Rus Education is duly authorized by the Russian Centre for Science and Culture (Cultural Department of The Embassy of the Russian Federation in India) to promote Russian Education among Indian Citizens. Rus Education is also an authorized associate of Lomonosov Moscow State University. We facilitate one-window admission to the MBBS Program of Lomonosov Moscow State University with no requirement of any donation or capitation and without any entrance examination.

The campus of Lomonosov Moscow State University offers excellent living and recreational facilities to help students live a comfortable, fulfilling, and fruitful student life.
To provide students with safe and comfortable accommodation, the University maintains a fully-managed hostel facility with the help of its Dormitory Management Unit.
The University promotes a healthy lifestyle for all its students and members and has well-maintained sports sections where students can play football, volleyball, basketball, or any other sports of their choice. For sports competitions, students can join sports clubs including Mountaineering Club, Diving Club, Yacht Club. For girl students, the University also has separate sports facilities and teams including a Women Football Team.
To involve students in extracurricular activities, the University organizes various cultural activities, concerts, excursion trips, and community and welfare programs. The purpose of these activities is to improve the personal and moral qualities of the students and to ensure their all-round development.
To help students capture precious memories from their campus life, the University has a dedicated students’ media center, which helps students with photo and video shootings as well as print media.
On the campus, the University encourages a culture of cooperation and support where students are taught to respect the culture and traditions of each other and maintain a respectful attitude towards fellow human beings.
To protect the rights and interest of the students and for better peer support, the University has also established a Student Council which offers moral support and help to solve all kinds of student problems.
Students are also allowed to organize extracurricular events in coordination with Student Committee and get full University support preferably for initiatives of social importance like medical outreach programs, blood donation drives, health awareness camps, etc.
For a soothing break, Students can visit the centuries-old Botanical Garden of the University and calm down in the natural environment of the garden.
Students who love to travel and explore new places can join outdoor trips organized to visit nearby interesting places in Moscow, the biggest city in Russia. In Moscow, students can explore its cultural heritage, museums, historic buildings, the world-famous Alexander Garden, and much more. For traveling in Moscow, students don’t face any problems, thanks to its convenient and cheap transportation system, especially the Moscow Metro.
TOP MEDICAL UNIVERSITIES IN RUSSIA
.jpg)
Perm State Medical University
.jpg)
Tver State Medical University
.jpg)
Orenburg State Medical University

Mari State University
.jpg)
Siberian State Medical University
A php error was encountered.
Severity: Notice
Message: Undefined variable: countries
Filename: includes/footer.php
Line Number: 26
File: /home/mbbsinrussia/public_html/application/views/includes/footer.php Line: 26 Function: _error_handler
File: /home/mbbsinrussia/public_html/application/controllers/University.php Line: 46 Function: view
File: /home/mbbsinrussia/public_html/index.php Line: 315 Function: require_once
Severity: Warning
Message: Invalid argument supplied for foreach()
Message: Undefined variable: state
Line Number: 44
File: /home/mbbsinrussia/public_html/application/views/includes/footer.php Line: 44 Function: _error_handler
©2024-25 Rus Education.
- Open access
- Published: 14 May 2024
Protocol for a scoping review study on learning plan use in undergraduate medical education
- Anna Romanova ORCID: orcid.org/0000-0003-1118-1604 1 ,
- Claire Touchie 1 ,
- Sydney Ruller 2 ,
- Victoria Cole 3 &
- Susan Humphrey-Murto 4
Systematic Reviews volume 13 , Article number: 131 ( 2024 ) Cite this article
79 Accesses
Metrics details
The current paradigm of competency-based medical education and learner-centredness requires learners to take an active role in their training. However, deliberate and planned continual assessment and performance improvement is hindered by the fragmented nature of many medical training programs. Attempts to bridge this continuity gap between supervision and feedback through learner handover have been controversial. Learning plans are an alternate educational tool that helps trainees identify their learning needs and facilitate longitudinal assessment by providing supervisors with a roadmap of their goals. Informed by self-regulated learning theory, learning plans may be the answer to track trainees’ progress along their learning trajectory. The purpose of this study is to summarise the literature regarding learning plan use specifically in undergraduate medical education and explore the student’s role in all stages of learning plan development and implementation.
Following Arksey and O’Malley’s framework, a scoping review will be conducted to explore the use of learning plans in undergraduate medical education. Literature searches will be conducted using multiple databases by a librarian with expertise in scoping reviews. Through an iterative process, inclusion and exclusion criteria will be developed and a data extraction form refined. Data will be analysed using quantitative and qualitative content analyses.
By summarising the literature on learning plan use in undergraduate medical education, this study aims to better understand how to support self-regulated learning in undergraduate medical education. The results from this project will inform future scholarly work in competency-based medical education at the undergraduate level and have implications for improving feedback and supporting learners at all levels of competence.
Scoping review registration:
Open Science Framework osf.io/wvzbx.
Peer Review reports
Competency-based medical education (CBME) has transformed the approach to medical education to focus on demonstration of acquired competencies rather than time-based completion of rotations [ 1 ]. As a result, undergraduate and graduate medical training programs worldwide have adopted outcomes-based assessments in the form of entrustable professional activities (EPAs) comprised of competencies to be met [ 2 ]. These assessments are completed longitudinally by multiple different evaluators to generate an overall impression of a learner’s competency.
In CBME, trainees will progress along their learning trajectory at individual speeds and some may excel while others struggle to achieve the required knowledge, skills or attitudes. Therefore, deliberate and planned continual assessment and performance improvement is required. However, due to the fragmented nature of many medical training programs where learners rotate through different rotations and work with many supervisors, longitudinal observation is similarly fragmented. This makes it difficult to determine where trainees are on their learning trajectories and can affect the quality of feedback provided to them, which is a known major influencer of academic achievement [ 3 ]. As a result, struggling learners may not be identified until late in their training and the growth of high-performing learners may be stifled [ 4 , 5 , 6 ].
Bridging this continuity gap between supervision and feedback through some form of learner handover or forward feeding has been debated since the 1970s and continues to this day [ 5 , 7 , 8 , 9 , 10 , 11 ]. The goal of learner handover is to improve trainee assessment and feedback by sharing their performance and learning needs between supervisors or across rotations. However, several concerns have been raised about this approach including that it could inappropriately bias subsequent assessments of the learner’s abilities [ 9 , 11 , 12 ]. A different approach to keeping track of trainees’ learning goals and progress along their learning trajectories is required. Learning plans (LPs) informed by self-regulated learning (SRL) theory may be the answer.
SRL has been defined as a cyclical process where learners actively control their thoughts, actions and motivation to achieve their goals [ 13 ]. Several models of SRL exist but all entail that the trainee is responsible for setting, planning, executing, monitoring and reflecting on their learning goals [ 13 ]. According to Zimmerman’s SRL model, this process occurs in three stages: forethought phase before an activity, performance phase during an activity and self-reflection phase after an activity [ 13 ]. Since each trainee leads their own learning process and has an individual trajectory towards competence, this theory relates well to the CBME paradigm which is grounded in learner-centredness [ 1 ]. However, we know that medical students and residents have difficulty identifying their own learning goals and therefore need guidance to effectively partake in SRL [ 14 , 15 , 16 , 17 ]. Motivation has also emerged as a key component of SRL, and numerous studies have explored factors that influence student engagement in learning [ 18 , 19 ]. In addition to meeting their basic psychological needs of autonomy, relatedness and competence, perceived learning relevance through meaningful learning activities has been shown to increase trainee engagement in their learning [ 19 ].
LPs are a well-known tool across many educational fields including CBME that can provide trainees with meaningful learning activities since they help them direct their own learning goals in a guided fashion [ 20 ]. Also known as personal learning plans, learning contracts, personal action plans, personal development plans, and learning goals, LPs are documents that outline the learner’s roadmap to achieve their learning goals. They require the learner to self-identify what they need to learn and why, how they are going to do it, how they will know when they are finished, define the timeframe for goal achievement and assess the impact of their learning [ 20 ]. In so doing, LPs give more autonomy to the learner and facilitate objective and targeted feedback from supervisors. This approach has been described as “most congruent with the assumptions we make about adults as learners” [ 21 ].
LP use has been explored across various clinical settings and at all levels of medical education; however, most of the experience lies in postgraduate medical education [ 22 ]. Medical students are a unique learner population with learning needs that appear to be very well suited for using LPs for two main reasons. First, their education is often divided between classroom and clinical settings. During clinical training, students need to be more independent in setting learning goals to meet desired competencies as their education is no longer outlined for them in a detailed fashion by the medical school curriculum [ 23 ]. SRL in the workplace is also different than in the classroom due to additional complexities of clinical care that can impact students’ ability to self-regulate their learning [ 24 ]. Second, although most medical trainees have difficulty with goal setting, medical students in particular need more guidance compared to residents due to their relative lack of experience upon which they can build within the SRL framework [ 25 ]. LPs can therefore provide much-needed structure to their learning but should be guided by an experienced tutor to be effective [ 15 , 24 ].
LPs fit well within the learner-centred educational framework of CBME by helping trainees identify their learning needs and facilitating longitudinal assessment by providing supervisors with a roadmap of their goals. In so doing, they can address current issues with learner handover and identification as well as remediation of struggling learners. Moreover, they have the potential to help trainees develop lifelong skills with respect to continuing professional development after graduation which is required by many medical licensing bodies.
An initial search of the JBI Database, Cochrane Database, MEDLINE (PubMed) and Google Scholar conducted in July–August 2022 revealed a paucity of research on LP use in undergraduate medical education (UGME). A related systematic review by van Houten–Schat et al. [ 24 ] on SRL in the clinical setting identified three interventions used by medical students and residents in SRL—coaching, LPs and supportive tools. However, only a couple of the included studies looked specifically at medical students’ use of LPs, so this remains an area in need of more exploration. A scoping review would provide an excellent starting point to map the body of literature on this topic.
The objective of this scoping review will therefore be to explore LP use in UGME. In doing so, it will address a gap in knowledge and help determine additional areas for research.
This study will follow Arksey and O’Malley’s [ 26 ] five-step framework for scoping review methodology. It will not include the optional sixth step which entails stakeholder consultation as relevant stakeholders will be intentionally included in the research team (a member of UGME leadership, a medical student and a first-year resident).
Step 1—Identifying the research question
The overarching purpose of this study is to “explore the use of LPs in UGME”. More specifically we seek to achieve the following:
Summarise the literature regarding the use of LPs in UGME (including context, students targeted, frameworks used)
Explore the role of the student in all stages of the LP development and implementation
Determine existing research gaps
Step 2—Identifying relevant studies
An experienced health sciences librarian (VC) will conduct all searches and develop the initial search strategy. The preliminary search strategy is shown in Appendix A (see Additional file 2). Articles will be included if they meet the following criteria [ 27 ]:
Participants
Medical students enrolled at a medical school at the undergraduate level.
Any use of LPs by medical students. LPs are defined as a document, usually presented in a table format, that outlines the learner’s roadmap to achieve their learning goals [ 20 ].
Any stage of UGME in any geographic setting.
Types of evidence sources
We will search existing published and unpublished (grey) literature. This may include research studies, reviews, or expert opinion pieces.
Search strategy
With the assistance of an experienced librarian (VC), a pilot search will be conducted to inform the final search strategy. A search will be conducted in the following electronic databases: MEDLINE, Embase, Education Source, APA PsycInfo and Web of Science. The search terms will be developed in consultation with the research team and librarian. The search strategy will proceed according to the JBI Manual for Evidence Synthesis three-step search strategy for reviews [ 27 ]. First, we will conduct a limited search in two appropriate online databases and analyse text words from the title, abstracts and index terms of relevant papers. Next, we will conduct a second search using all identified key words in all databases. Third, we will review reference lists of all included studies to identify further relevant studies to include in the review. We will also contact the authors of relevant papers for further information if required. This will be an iterative process as the research team becomes more familiar with the literature and will be guided by the librarian. Any modifications to the search strategy as it evolves will be described in the scoping review report. As a measure of rigour, the search strategy will be peer-reviewed by another librarian using the PRESS checklist [ 28 ]. No language or date limits will be applied.
Step 3—Study selection
The screening process will consist of a two-step approach: screening titles/abstracts and, if they meet inclusion criteria, this will be followed by a full-text review. All screening will be done by two members of the research team and any disagreements will be resolved by an independent third member of the team. Based on preliminary inclusion criteria, the whole research team will first pilot the screening process by reviewing a random sample of 25 titles/abstracts. The search strategy, eligibility criteria and study objectives will be refined in an iterative process. We anticipate several meetings as the topic is not well described in the literature. A flowchart of the review process will be generated. Any modifications to the study selection process will be described in the scoping review report. The papers will be excluded if a full text is not available. The search results will be managed using Covidence software.
Step 4—Charting the data
A preliminary data extraction tool is shown in Appendix B (see Additional file 3 ). Data will be extracted into Excel and will include demographic information and specific details about the population, concept, context, study methods and outcomes as they relate to the scoping review objectives. The whole research team will pilot the data extraction tool on ten articles selected for full-text review. Through an iterative process, the final data extraction form will be refined. Subsequently, two members of the team will independently extract data from all articles included for full-text review using this tool. Charting disagreements will be resolved by the principal and senior investigators. Google Translate will be used for any included articles that are not in the English language.
Step 5—Collating, summarising and reporting the results
Quantitative and qualitative analyses will be used to summarise the results. Quantitative analysis will capture descriptive statistics with details about the population, concept, context, study methods and outcomes being examined in this scoping review. Qualitative content analysis will enable interpretation of text data through the systematic classification process of coding and identifying themes and patterns [ 29 ]. Several team meetings will be held to review potential themes to ensure an accurate representation of the data. The PRISMA Extension for Scoping Reviews (PRISMA-ScR) will be used to guide the reporting of review findings [ 30 ]. Data will be presented in tables and/or diagrams as applicable. A descriptive summary will explain the presented results and how they relate to the scoping review objectives.
By summarising the literature on LP use in UGME, this study will contribute to a better understanding of how to support SRL amongst medical students. The results from this project will also inform future scholarly work in CBME at the undergraduate level and have implications for improving feedback as well as supporting learners at all levels of competence. In doing so, this study may have practical applications by informing learning plan incorporation into CBME-based curricula.
We do not anticipate any practical or operational issues at this time. We assembled a team with the necessary expertise and tools to complete this project.
Availability of data and materials
All data generated or analysed during this study will be included in the published scoping review article.
Abbreviations
- Competency-based medical education
Entrustable professional activity
- Learning plan
- Self-regulated learning
- Undergraduate medical education
Frank JR, Snell LS, Cate OT, et al. Competency-based medical education: theory to practice. Med Teach. 2010;32(8):638–45.
Article PubMed Google Scholar
Shorey S, Lau TC, Lau ST, Ang E. Entrustable professional activities in health care education: a scoping review. Med Educ. 2019;53(8):766–77.
Hattie J, Timperley H. The power of feedback. Rev Educ Res. 2007;77(1):81–112.
Article Google Scholar
Dudek NL, Marks MB, Regehr G. Failure to fail: the perspectives of clinical supervisors. Acad Med. 2005;80(10 Suppl):S84–7.
Warm EJ, Englander R, Pereira A, Barach P. Improving learner handovers in medical education. Acad Med. 2017;92(7):927–31.
Spooner M, Duane C, Uygur J, et al. Self-regulatory learning theory as a lens on how undergraduate and postgraduate learners respond to feedback: a BEME scoping review : BEME Guide No. 66. Med Teach. 2022;44(1):3–18.
Frellsen SL, Baker EA, Papp KK, Durning SJ. Medical school policies regarding struggling medical students during the internal medicine clerkships: results of a National Survey. Acad Med. 2008;83(9):876–81.
Humphrey-Murto S, LeBlanc A, Touchie C, et al. The influence of prior performance information on ratings of current performance and implications for learner handover: a scoping review. Acad Med. 2019;94(7):1050–7.
Morgan HK, Mejicano GC, Skochelak S, et al. A responsible educational handover: improving communication to improve learning. Acad Med. 2020;95(2):194–9.
Dory V, Danoff D, Plotnick LH, et al. Does educational handover influence subsequent assessment? Acad Med. 2021;96(1):118–25.
Humphrey-Murto S, Lingard L, Varpio L, et al. Learner handover: who is it really for? Acad Med. 2021;96(4):592–8.
Shaw T, Wood TJ, Touchie T, Pugh D, Humphrey-Murto S. How biased are you? The effect of prior performance information on attending physician ratings and implications for learner handover. Adv Health Sci Educ Theory Pract. 2021;26(1):199–214.
Artino AR, Brydges R, Gruppen LD. Chapter 14: Self-regulated learning in health professional education: theoretical perspectives and research methods. In: Cleland J, Duning SJ, editors. Researching Medical Education. 1st ed. John Wiley & Sons; 2015. p. 155–66.
Chapter Google Scholar
Cleland J, Arnold R, Chesser A. Failing finals is often a surprise for the student but not the teacher: identifying difficulties and supporting students with academic difficulties. Med Teach. 2005;27(6):504–8.
Reed S, Lockspeiser TM, Burke A, et al. Practical suggestions for the creation and use of meaningful learning goals in graduate medical education. Acad Pediatr. 2016;16(1):20–4.
Wolff M, Stojan J, Cranford J, et al. The impact of informed self-assessment on the development of medical students’ learning goals. Med Teach. 2018;40(3):296–301.
Sawatsky AP, Halvorsen AJ, Daniels PR, et al. Characteristics and quality of rotation-specific resident learning goals: a prospective study. Med Educ Online. 2020;25(1):1714198.
Article PubMed PubMed Central Google Scholar
Pintrich PR. Chapter 14: The role of goal orientation in self-regulated learning. In: Boekaerts M, Pintrich PR, Zeidner M, editors. Handbook of self-regulation. 1st ed. Academic Press; 2000. p. 451–502.
Kassab SE, El-Sayed W, Hamdy H. Student engagement in undergraduate medical education: a scoping review. Med Educ. 2022;56(7):703–15.
Challis M. AMEE medical education guide No. 19: Personal learning plans. Med Teach. 2000;22(3):225–36.
Knowles MS. Using learning contracts. 1 st ed. San Francisco: Jossey Bass; 1986.
Parsell G, Bligh J. Contract learning, clinical learning and clinicians. Postgrad Med J. 1996;72(847):284–9.
Article CAS PubMed PubMed Central Google Scholar
Teunissen PW, Scheele F, Scherpbier AJJA, et al. How residents learn: qualitative evidence for the pivotal role of clinical activities. Med Educ. 2007;41(8):763–70.
Article CAS PubMed Google Scholar
van Houten-Schat MA, Berkhout JJ, van Dijk N, Endedijk MD, Jaarsma ADC, Diemers AD. Self-regulated learning in the clinical context: a systematic review. Med Educ. 2018;52(10):1008–15.
Taylor DCM, Hamdy H. Adult learning theories: Implications for learning and teaching in medical education: AMEE Guide No. 83. Med Teach. 2013;35(11):e1561–72.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32.
Peters MDJ, Godfrey C, McInerney P, Munn Z, Tricco AC, Khalol H. Chapter 11: Scoping reviews. In: Aromataris E, Munn Z, eds. JBI Manual for Evidence Synthesis. JBI; 2020. https://synthesismanual.jbi.global. . Accessed 30 Aug 2022.
McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J Clin Epidemiol. 2016;75:40–6.
Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–88.
Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
Venables M, Larocque A, Sikora L, Archibald D, Grudniewicz A. Understanding indigenous health education and exploring indigenous anti-racism approaches in undergraduate medical education: a scoping review protocol. OSF; 2022. https://osf.io/umwgr/ . Accessed 26 Oct 2022.
Download references
Acknowledgements
Not applicable.
This study will be supported through grants from the Department of Medicine at the Ottawa Hospital and the University of Ottawa. The funding bodies had no role in the study design and will not have any role in the collection, analysis and interpretation of data or writing of the manuscript.
Author information
Authors and affiliations.
The Ottawa Hospital – General Campus, 501 Smyth Rd, PO Box 209, Ottawa, ON, K1H 8L6, Canada
Anna Romanova & Claire Touchie
The Ottawa Hospital Research Institute, Ottawa, Canada
Sydney Ruller
The University of Ottawa, Ottawa, Canada
Victoria Cole
The Ottawa Hospital – Riverside Campus, Ottawa, Canada
Susan Humphrey-Murto
You can also search for this author in PubMed Google Scholar
Contributions
AR designed and drafted the protocol. CT and SH contributed to the refinement of the research question, study methods and editing of the manuscript. VC designed the initial search strategy. All authors reviewed the manuscript for final approval. The review guarantors are CT and SH. The corresponding author is AR.
Authors’ information
AR is a clinician teacher and Assistant Professor with the Division of General Internal Medicine at the University of Ottawa. She is also the Associate Director for the internal medicine clerkship rotation at the General campus of the Ottawa Hospital.
CT is a Professor of Medicine with the Divisions of General Internal Medicine and Infectious Diseases at the University of Ottawa. She is also a member of the UGME Competence Committee at the University of Ottawa and an advisor for the development of a new school of medicine at Toronto Metropolitan University.
SH is an Associate Professor with the Department of Medicine at the University of Ottawa and holds a Tier 2 Research Chair in Medical Education. She is also the Interim Director for the Research Support Unit within the Department of Innovation in Medical Education at the University of Ottawa.
CT and SH have extensive experience with medical education research and have numerous publications in this field.
SR is a Research Assistant with the Division of General Internal Medicine at the Ottawa Hospital Research Institute.
VC is a Health Sciences Research Librarian at the University of Ottawa.
SR and VC have extensive experience in systematic and scoping reviews.
Corresponding author
Correspondence to Anna Romanova .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. prisma-p 2015 checklist., 13643_2024_2553_moesm2_esm.docx.
Additional file 2: Appendix A. Preliminary search strategy [ 31 ].
Additional file 3: Appendix B. Preliminary data extraction tool.
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Romanova, A., Touchie, C., Ruller, S. et al. Protocol for a scoping review study on learning plan use in undergraduate medical education. Syst Rev 13 , 131 (2024). https://doi.org/10.1186/s13643-024-02553-w
Download citation
Received : 29 November 2022
Accepted : 03 May 2024
Published : 14 May 2024
DOI : https://doi.org/10.1186/s13643-024-02553-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Skip to main content
- Keyboard shortcuts for audio player
- Your Health
- Treatments & Tests
- Health Inc.
- Public Health
Medical residents are starting to avoid states with abortion bans, data shows
Julie Rovner
Rachana Pradhan

The Match Day ceremony at the University of California, Irvine, on March 15. Match Day is the day when medical students seeking residency and fellowship training positions find out their options. Increasingly, medical students are choosing to go to states that don't restrict abortion. Jeff Gritchen/MediaNews Group via Getty Images hide caption
The Match Day ceremony at the University of California, Irvine, on March 15. Match Day is the day when medical students seeking residency and fellowship training positions find out their options. Increasingly, medical students are choosing to go to states that don't restrict abortion.
Isabella Rosario Blum was wrapping up medical school and considering residency programs to become a family practice physician when she got some frank advice: If she wanted to be trained to provide abortions, she shouldn't stay in Arizona.
Blum turned to programs mostly in states where abortion access — and, by extension, abortion training — is likely to remain protected, like California, Colorado and New Mexico. Arizona has enacted a law banning most abortions after 15 weeks.
"I would really like to have all the training possible," she said, "so of course that would have still been a limitation."
In June, she will start her residency at Swedish Cherry Hill hospital in Seattle.
According to new statistics from the Association of American Medical Colleges (AAMC), for the second year in a row, students graduating from U.S. medical schools this year were less likely to apply for residency positions in states with abortion bans and other significant abortion restrictions.
Since the Supreme Court in 2022 overturned the constitutional right to an abortion, state fights over abortion access have created plenty of uncertainty for pregnant patients and their doctors. But that uncertainty has also bled into the world of medical education, forcing some new doctors to factor state abortion laws into their decisions about where to begin their careers.

Shots - Health News
How florida and arizona supreme court rulings change the abortion access map.
Fourteen states, primarily in the Midwest and South, have banned nearly all abortions. The new analysis by the AAMC — exclusively reviewed by KFF Health News before its public release — found that the number of applicants to residency programs in states with near-total abortion bans declined by 4.2% between 2024 and 2023, compared with a 0.6% drop in states where abortion remains legal.
Notably, the AAMC's findings illuminate the broader problems that abortion bans can create for a state's medical community, particularly in an era of provider shortages: The organization tracked a larger decrease in interest in residencies in states with abortion restrictions not only among those in specialties most likely to treat pregnant patients, like OB-GYNs and emergency room doctors, but also among aspiring doctors in other specialties.
"It should be concerning for states with severe restrictions on reproductive rights that so many new physicians — across specialties — are choosing to apply to other states for training instead," wrote Atul Grover, executive director of the AAMC's Research and Action Institute.
The AAMC analysis found that the number of applicants to OB-GYN residency programs in abortion-ban states dropped by 6.7%, compared with a 0.4% increase in states where abortion remains legal. For internal medicine, the drop observed in abortion-ban states was over five times as much as in states where abortion is legal.
'Geographic misalignment'
In its analysis, the AAMC said that an ongoing decline in interest in abortion-ban states among new doctors ultimately "may negatively affect access to care in those states."
Dr. Jack Resneck Jr., immediate past president of the American Medical Association, said the data demonstrates yet another consequence of the post- Roe v. Wade era.
The AAMC analysis notes that even in states with abortion bans, residency programs are filling their positions — mostly because there are more graduating medical students in the U.S. and abroad than there are residency slots.
Still, Resneck said, "we're extraordinarily worried." For example, physicians without adequate abortion training may not be able to manage miscarriages, ectopic pregnancies or potential complications, such as infection or hemorrhaging, that could stem from pregnancy loss.
Those who work with students and residents say their observations support the AAMC's findings. "People don't want to go to a place where evidence-based practice and human rights in general are curtailed," said Beverly Gray, an associate professor of obstetrics and gynecology at Duke University School of Medicine.
Abortion in North Carolina is banned in nearly all cases after 12 weeks. Women who experience unexpected complications or discover their baby has potentially fatal birth anomalies later in pregnancy may not be able to receive care there.
Gray said she worries that even though Duke is a highly sought training destination for medical residents, the abortion ban "impacts whether we have the best and brightest coming to North Carolina."
Rohini Kousalya Siva will start her obstetrics and gynecology residency at MedStar Washington Hospital Center in Washington, D.C., this year. She said she did not consider programs in states that have banned or severely restricted abortion, applying instead to programs in Maryland, New Hampshire, New York and Washington, D.C.
"We're physicians," said Kousalya Siva, who attended medical school in Virginia and was previously president of the American Medical Student Association. "We're supposed to be giving the best evidence-based care to our patients, and we can't do that if we haven't been given abortion training."
Another consideration: Most graduating medical students are in their 20s, "the age when people are starting to think about putting down roots and starting families," said Gray, who added that she is noticing many more students ask about politics during their residency interviews.
And because most young doctors make their careers in the state where they do their residencies, "people don't feel safe potentially having their own pregnancies [while] living in those states" with severe restrictions, said Debra Stulberg, chair of the Department of Family Medicine at the University of Chicago.
Stulberg and others worry that this self-selection away from states with abortion restrictions will exacerbate the shortages of physicians in rural and underserved areas.
"The geographic misalignment between where the needs are and where people are choosing to go is really problematic," she said. "We don't need people further concentrating in urban areas where there's already good access."
From Tennessee to California
After attending medical school in Tennessee, which has adopted one of the most sweeping abortion bans in the U.S., Hannah Light-Olson will start her OB-GYN residency at the University of California San Francisco this summer.
It was not an easy decision, she said. "I feel some guilt and sadness leaving a situation where I feel like I could be of some help," she said. "I feel deeply indebted to the program that trained me and to the patients of Tennessee."
Light-Olson said some of her fellow students applied to programs in abortion-ban states "because they think we need pro-choice providers in restrictive states now more than ever." In fact, she said, she also applied to programs in abortion-ban states when she was confident the program had a way to provide abortion training.
"I felt like there was no perfect 100% guarantee. We've seen how fast things can change," she said. "I don't feel particularly confident that California and New York aren't going to be under threat too."
As a condition of a scholarship she received for medical school, Blum said, she will have to return to Arizona to practice, and it is unclear what abortion access will look like then. But she is worried about long-term impacts.
"Residents, if they can't get the training in the state, then they're probably less likely to settle down and work in the state as well," she said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling and journalism.
- rural health
- medical residency
- abortion bans
- medical provider shortage
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- For authors
- New editors
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Online First
- Research in specialist sport and exercise medicine training
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Bruce Hamilton 1 , 2 ,
- http://orcid.org/0000-0002-8413-2814 Larissa Trease 3 , 4 ,
- Corey Cunningham 4 , 5
- 1 Sports Medicine , High Performance Sport New Zealand AUT Millennium Institute of Sport and Health , Auckland , New Zealand
- 2 SPRINZ , Auckland University of Technology , Auckland , New Zealand
- 3 La Trobe Sport and Exercise Medicine Research Centre (LASEM) , La Trobe University , Bundoora , Victoria , Australia
- 4 Australasian College of Sport and Exercise Physicians , Melbourne , Victoria , Australia
- 5 New South Wales Institute of Sport , Sydney Olympic Park , New South Wales , Australia
- Correspondence to Dr Bruce Hamilton, Sports Medicine, High Performance Sport New Zealand AUT Millennium Institute of Sport and Health, Auckland, New Zealand; bruce.hamilton{at}hpsnz.org.nz
https://doi.org/10.1136/bjsports-2024-108554
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
- Sports medicine
Over 20 years ago, Thomas Best and Domhnall MacAuley rhetorically posited that evidence-based sports medicine was potentially a ‘contradiction in terms’. 1 In 2010, Evert Verhagen and Willem van Mechelen stated that ‘most individuals involved in sports medicine are not thoroughly trained in epidemiological and methodological rigour’. 2 Despite these somewhat disparaging views, research has long been recognised as an important component of specialist training in sport and exercise medicine, 3 4 at least in part as a result of academic medical centres demonstrating better patient outcomes. 5 Indeed, the Australasian College of Sport and Exercise Physicians (ACSEP) has centralised the role of research in sports medicine training since its inception in 1985, incorporating a requirement to complete original research as part of fellowship training. 6 Until 2023, in order to graduate from the training programme, registrars were required to complete a series of mandatory research modules and undertake ‘an original research project, and [be] published as first author in an international refereed journal’. 7
As part of an internal 2022 review of the college’s research requirements, several limitations with this research approach were identified including:
A focus on publication in a high-level journal as a binary outcome, rather than the process of research.
Inconsistency with the research requirements with other specialist training programmes in Australia and New Zealand. 8
A reliance on the nuances and publication imperatives of academic journals to determine registrar research outcomes, with resultant delays, difficulties in publishing and an inability to complete the fellowship requirements.
A lack of focus on identifying and developing research competencies.
A lack of access for registrars to research environments, resources and technical capability.
Registrar dissatisfaction, frustration and disengagement with research activities.
The review highlighted a conflict between the desirability of incorporating research requirements into specialist sports medicine training, and the unavoidable challenges of performing quality research. Reflecting this, the Medical Council of New Zealand specifically highlights the importance of ‘enquiry, intellectual curiosity and evidence-based practice’ in specialist training, but also acknowledges that ‘not all trainees will have the inclination, opportunity or aptitude for an extended period of research activity’. 9
Following the 2022 review, the ACSEP ‘doubled down’ on its desire to develop specialists who were competent in critically interpreting, applying and undertaking sports medicine research. While recognising that trainee approaches to research engagement may vary, all trainees are required to contribute to or lead a research study. 8 In essence, the college recognised that while not all specialist sport and exercise registrars were destined to be researchers, all specialists must be able to engage positively in research activity. One size does not fit all. Subsequently, the college overhauled the training programme approach to research with the goal of achieving greater research engagement from both registrars and fellows.
In 2023, the ACSEP formally evolved its training requirements to a competency-based assessment with the removal of the singular publication outcome requirement and providing a range of means by which registrars could complete their individualised ‘research-based activity (RBA)’. While the participation in original research remained a requirement, evidence of developing research competencies such as the formulation of research questions and hypotheses, literature reviews and the development of a research methodology allowed registrars to establish a broad research portfolio in order to complete the training requirements. Furthermore, evidence of ongoing involvement in research and the demonstration of the translation of novel research in sporting or clinical environments can contribute to a registrar’s RBA portfolio.
In recognition of the constraints many registrars face in linking with effective research environments and supervisors, the ACSEP has recruited a technical advisor to support registrars in developing appropriate projects and to guide them towards national and international research support networks. Finally, the college has committed to promoting research from its registrars and fellows with a view to ensuring the ongoing involvement in research is seen as a viable and rewarding professional pathway for sport and exercise physicians.
For the specialty of sport and exercise medicine to thrive requires highly skilled and informed clinicians who are able to interpret and use a broad range of 21st century research techniques. In modernising its research curriculum, the ACSEP hopes to be at the forefront of clinical and evidence-based sports medicine in the decades to come. Evidence-based sports medicine should not be a contradiction.
Ethics statements
Patient consent for publication.
Not applicable.
Ethics approval
- MacAuley D ,
- Verhagen E ,
- van Mechelen W
- Humphries D ,
- Dijkstra HP , et al
- Khullar D ,
- Orav EJ , et al
- Brukner PD ,
- Crichton KJ ,
- Stehlik P ,
- Brandenburg C , et al
X @DrLarissaTrease
Contributors All authors contributed to the development of this editorial.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests CC is the president of the Australasian College of Sport and Exercise Physicians (ACSEP) and BH is the chair of the Research Committee of ACSEP.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:
- Open access
- Published: 17 May 2024
Behavioral skills training for teaching safety skills to mental health service providers compared to training-as-usual: a pragmatic randomized control trial
- Elizabeth Lin 1 ,
- Mais Malhas 1 ,
- Emmanuel Bratsalis 1 ,
- Kendra Thomson 1 , 2 ,
- Fabienne Hargreaves 1 ,
- Kayle Donner 1 ,
- Heba Baig 1 ,
- Rhonda Boateng 1 ,
- Rajlaxmi Swain 1 ,
- Mary Benisha Benadict 1 &
- Louis Busch 1
BMC Health Services Research volume 24 , Article number: 639 ( 2024 ) Cite this article
40 Accesses
Metrics details
Violence in the healthcare workplace has been a global concern for over two decades, with a high prevalence of violence towards healthcare workers reported. Workplace violence has become a healthcare quality indicator and embedded in quality improvement initiatives of many healthcare organizations. The Centre for Addiction and Mental Health (CAMH), Canada’s largest mental health hospital, provides all clinical staff with mandated staff safety training for self-protection and team-control skills. These skills are to be used as a last resort when a patient is at imminent risk of harm to self or others. The purpose of this study is to compare the effectiveness of two training methods of this mandated staff safety training for workplace violence in a large psychiatric hospital setting.
Using a pragmatic randomized control trial design, this study compares two approaches to teaching safety skills CAMH’s training-as-usual (TAU) using the 3D approach (description, demonstration and doing) and behavioural skills training (BST), from the field of applied behaviour analysis, using instruction, modeling, practice and feedback loop. Staff were assessed on three outcome measures (competency, mastery and confidence), across three time points: before training (baseline), immediately after training (post-training) and one month later (follow-up). This study was registered with the ISRCTN registry on 06/09/2023 (ISRCTN18133140).
With a sample size of 99 new staff, results indicate that BST was significantly better than TAU in improving observed performance of self-protection and team-control skills. Both methods were associated with improved skills and confidence. However, there was a decrease in skill performance levels at the one-month follow-up for both methods, with BST remaining higher than TAU scores across all three time points. The impact of training improved staff confidence in both training methods and remained high across all three time points.
Conclusions
The study findings suggest that BST is more effective than TAU in improving safety skills among healthcare workers. However, the retention of skills over time remains a concern, and therefore a single training session without on-the-job-feedback or booster sessions based on objective assessments of skill may not be sufficient. Further research is needed to confirm and expand upon these findings in different settings.
Peer Review reports
Introduction
Violence in the healthcare workplace has been a global concern for over two decades. In 2002, a joint task force of the International Labour Office (ILO), World Health Organization, Public Services International, and the International Council of Nurses created an initiative to address this issue [ 1 ]. One result was the documentation of a high international prevalence of violence towards healthcare workers showing that as many as half or more experienced physical or psychological violence in the previous year [ 2 , 3 ]. Since then, workplace violence has become a healthcare quality indicator and been embedded in the quality improvement initiatives of many healthcare organizations (for example, Health Quality Ontario [ 4 ]). Conceptually, it is also reflected in the expansion of the Triple Aim framework to the Quintuple Aim to include staff work-life experience [ 5 ].
Despite these efforts, the high prevalence of workplace violence in healthcare persists [ 6 ]. Two meta-analyses, representing 393,344 healthcare workers, found a 19.3% pooled prevalence of workplace violence in the past year among which 24.4% and 42.5% reported physical and psychological violence experiences, respectively [ 7 , 8 ]. The literature also highlighted that workers in mental health settings were at particular risk [ 8 , 9 ]. A systematic review of violence in U.S. psychiatric hospitals found between 25 to 85 percent of staff encountering physical aggression in the past year [ 10 ]. Partial explanations for this wide range include methodological, population, and setting differences. For example, Gerberich and colleagues [ 11 ] surveyed nearly 4,000 Minnesota nurses and found 13 percent reporting physical assault and 38 percent reporting verbal or other non-physical violence in the previous year. Further analyses showed that nurses on psychiatric or behavioral units were twice as likely as those on medical/surgical units to experience physical violence and nearly three times as likely to experience non-physical violence. Ridenour, et al., [ 12 ] in a hospital-record study of acute locked psychiatric wards in U.S. Veteran’s Hospitals found that 85 percent of nurses had experienced aggression in a 30-day period (85 percent verbal; 81 percent physical). And, in a prospective study of a Canadian psychiatric hospital, Cooper and Mendonca [ 13 ] found over 200 physical assaults on nurses within 27 months. While they do not indicate what percentage of nurses were assaulted, their results are consistent with a frequency of between 1 and 2 assaults per week.
Workplace violence has been associated with negative psychological, physical, emotional, financial, and social consequences which impact staff’s ability to provide care and function at work [ 14 , 15 , 16 ]. A 7-year, population-based, follow-up study in Denmark highlighted the long-term impact of physical and psychological health issues owing to physical workplace violence [ 17 ]. Two studies, one in Italy [ 18 ] and one in Pakistan [ 19 ], have linked workplace violence to demoralization and declining quality of healthcare delivery and job satisfaction among healthcare workers.
Building on these efforts, the ILO published a 2020 report recommending the need for national and organizational work environment policies and workplace training “…on the identified hazards and risks of violence and harassment and the associated prevention and protection measures….” ([ 20 ], p. 55). Consequently, many countries [ 21 , 22 , 23 ] have committed to creating a safe work environment. In Ontario, Canada, the government has provided guidelines for preventing workplace violence in healthcare [ 4 , 24 ], and our institution, the Centre for Addiction and Mental Health, launched a major initiative in 2018 to address the physical and psychological safety of patients and staff [ 25 ]. A priority component of this initiative is mandatory training for all new clinical staff on trauma-informed crisis prevention, de-escalation skills, and, in particular, safe physical intervention skills [ 26 , 27 ].
However, the effects of such training, especially for managing aggressive behaviour, are only partially understood. A 2015 systematic review on training for mental health staff [ 28 ] and a more recent Cochrane review on training for healthcare staff [ 29 ] reported remarkably similar findings. Both noted the inconsistent evidence (due to methodological issues, small numbers of studies, heterogenous results) which made definitive conclusions about the merits and efficacy of training difficult. The more consistent impacts found by Price and colleagues [ 28 ] were improved knowledge and staff confidence in their ability to manage aggression. There was some evidence of improved de-escalation skills including the ability to deal with physical aggression [ 30 , 31 ] and verbal abuse [ 32 ]. However, these studies were limited because they used unvalidated scales or simulated, rather than real-world, scenarios. For outcomes such as assault rates, injuries, the incidence of aggressive events, and the use of physical restraints, the findings were mixed or difficult to generalize due to the inconsistent evidence.
Similarly, Geoffrion and colleagues [ 29 ] found some positive effect of skills-training on knowledge and attitudes, at least short-term, but noted that support for longer-term effects was less sure. The evidence for impacts on skills or the incidence of aggressive behaviour was even more uncertain. They also noted that the literature was limited because it focused largely on nurses. They concluded, “education combined with training may not have an effect on workplace aggression directed toward healthcare workers, even though education and training may increase personal knowledge and positive attitudes” ([ 29 ], p. 2). Among their recommendations were the need to evaluate training in higher-risk settings such as mental healthcare, include other healthcare professionals who also have direct patient contact in addition to nurses, and use more robust study designs. In addition, the literature evaluating training procedures focussed on self-reported rather than objective measures of performance.
Given the concerns with demonstrating effectiveness, the violence prevention literature has tended to focus on training modalities and immediate post-training assessment rather than on skill retention over time. In a systematic review of prevention interventions in the emergency room, Wirth et al. [ 21 ] found only five out of 15 included studies that noted any kind of evaluation in the period after training (generally two to nine months post-training) while Geoffrion, et al. [ 29 ] identified only two among the nine studies in their meta-analysis that had follow-up skills assessments. However, for both of these reviews, the studies doing follow-up evaluations focused on subjective, self-reported outcomes (empathy, confidence, self-reported knowledge) with no objective behavioral skills measures. Both Wirth et al. [ 21 ] and Leach et al. [ 33 ] cite studies noting a loss of effectiveness of prevention skills (between three to six months post-training), but specific percentages of retention were not provided.
The present study sought to address these gaps by comparing two approaches to teaching safety skills for managing aggressive patient/client behaviour. The setting was a large psychiatric teaching hospital; the sample was drawn from all new clinical staff attending their mandated on-boarding training; and we used a pragmatic randomized control trial design. In addition, we added a 1-month post-training assessment to evaluate skill retention. Our control intervention was the current training-as-usual (TAU) in which trainers “describe” and “demonstrate”, and trainees “do” by practicing the demonstrated skill but without objective checklist-guided performance assessment by the trainer. Our test intervention was behavioural skills training (BST) [ 34 , 35 ] drawn from the field of applied behaviour analysis [ 36 ]. BST is a performance- and competency-based training model that uses an instructional, modeling, practice, and feedback loop to teach targeted skills to a predetermined performance level. Checklists guide the instructional sequence and the determination of whether or not the predetermined performance threshold has been reached. Considerable evidence indicates that BST can yield significant improvement in skills post-training, over time, and across different settings [ 37 , 38 , 39 ]. It has been used to train a wide range of participants, including behavior analysts, parents, and educators, to build safety-related skills and manage aggressive behavior [ 37 , 40 , 41 ].
As previously described [ 42 ], our objective was to compare the effectiveness of TAU against BST. Our hypotheses, stated in null form, were that these methods would not differ significantly in:
Observer assessment of self-protection and team-control physical skills.
Self-assessed confidence in using those skills.
Study participants were recruited from all newly-hired clinical staff attending a mandatory two-week orientation. Staff were required to register beforehand for a half-day, in-person, physical safety skills session. They were randomized to a session at the time of registration, and the sessions were then randomized to TAU or BST. All randomization was performed by RB using GraphPad software [ 43 ].
The physical skills training was scheduled for a 3.5 h session on one day of the mandatory onboarding. At the end of the previous day, attendees were introduced to the study (including the fact that it was a randomized study) and asked for consent to email them a copy of the informed consent. On the morning of the physical skills training, a research team member met with attendees to answer questions and then meet privately with each individual to ascertain if they wished to participate and sign the informed consent. The trainers and session attendees were thus unaware of who was or was not in the study. Recruitment began January 2021, after ethics approval, and continued until September 2021 when the target of at least 40 study participants completing all assessments for each training condition was reached. The target sample size was chosen to allow 80-percent power to detect a medium to large effect size [ 44 ].
Both methods taught the same 11 target skills for safely responding to patients/clients that may exhibit harm to self or others (e.g., aggressive behaviour) during their hospital admission. These skills, defined by the hospital as mandatory for all newly hired staff, included six self-protection and five team-control (physical restraint) skills (see Appendix A ). Each target skill had defined components and a specific sequence in which they were taught as outlined on performance checklists (see Appendix B for a checklist example).
The two methods differed in how these sequences were administered. For BST, the trainers used the performance checklists to guide the training sequence (instruction, modeling, rehearsal, and feedback) and to indicate when the trainee was ready to move on to the next skill [ 34 ] (see Appendix C for BST sequence). In BST, common practice is to define successful performance criteria a priori (e.g., up to three correct, consecutive executions at 100% [ 45 ]). However, because the physical skills training session in our study had to be completed within the scheduled 3.5 h, the criterion was lowered for practical reasons to one correct performance (defined as 80% of the components comprising that skill) with the added goal of aiming for up to 5 times in a row if time allowed before moving on to the next skill. In contrast, while TAU included elements of modeling, practice, and feedback, it did not systematically assess skill acquisition nor impose any specific level of success before proceeding to the next skill.
There were three outcome measures, two observer-based assessments of skill acquisition (competence and mastery) and one self-reported confidence measure. Competence was defined as the percentage of components comprising an individual skill that were correctly executed (e.g., if a skill had 10 components and only six were executed properly, the competence score for that skill would be 60%). Mastery was the threshold defining when a competence score was felt to indicate successful achievement of a skill and to indicate some degree of the durability of the skill acquisition [ 46 ]. For our study, we expanded mastery to apply to the two categories of self-protection and team-control (rather than to each individual skill) using the average competence scores for the skills within each category. Mastery was pre-defined as 80-percent, a commonly used threshold [ 28 , 47 ].
The outcome measures were assessed at three time points: immediately before training (baseline), immediately after training (post-training), and one month later (follow-up). The hospital provided limited descriptive information (professional role, department) for all registrants for administrative purposes but for confidentiality reasons did not provide personal information such as age or gender/sex. The research team elected not to collect personal information for two reasons. First, the primary study concern was to evaluate the main effect of training method rather than developing predictive models, and the expected result of the randomization process was that potential covariates would not be systematically biased in the two study groups. Second, we would not be able to use this information to compare participants with non-participants to identify biases in who consented to be in the study. We were able to compare them on department role and profession by subtracting the aggregated study-participant information from the aggregated hospital-provided information – the only form of the hospital-provided information available to the research team (see Table 1 below). In addition, since degree of patient contact was an important factor in the likelihood of needing to exercise safety skills, the research team also created an algorithm estimating which combinations of professional role and department were likely to have direct, less direct, or rare/low patient contact.
Participants were also asked at baseline and follow-up how many events they encountered in the previous month that required the use of these skills. This information was collected because of our interest in testing a post-hoc hypothesis that those with actual experience would score higher than those who did not.
All assessments were carried out following a standardized protocol. To ensure that registrants remained blinded to which colleagues were in the study, each registrant’s skill acquisition was assessed privately by a research team member at baseline and post-training using the performance checklists. Only assessments for those consenting to participate were videotaped. Study participants were then asked to return one month later for a follow-up assessment which was also videotaped. For the purposes of post-hoc analyses, participants completing all three assessments were defined as ‘completers’ while those completing baseline and post-training assessments but not the one-month follow-up were ‘non-completers.’
The same performance checklists used by the BST trainers were then used by trained observers blinded to the participant’s training method to assess the videotapes. As described previously [ 42 ], interobserver agreement (IOA) was routinely evaluated throughout the study with the final value being 96% across the 33% of the performance assessment videos scored for the IOA calculation.
Skill acquisition outcomes were calculated using the checklist-based observer assessments of the videotapes. The percentage of correctly executed components for each target skill was established. Then, these percentages were averaged across the six self-protection target skills and across the five team-control target skills to create competence scores. Finally, the predefined threshold of 80% was applied to the competence scores to determine which participants met the mastery threshold [ 47 , 48 ].
Self-reported confidence was assessed on a 10-point Likert scale (‘not at all’ to ‘extremely’ confident) using a version of our institution’s standard assessment questions adapted for this study (See Appendix D ).
Statistical analysis
R software was used to generate descriptive statistics (frequencies, percentages) and test our hypotheses [ 49 ]. Generalized linear mixed models (GLMM) were used to test nested main and interaction effects using likelihood-ratio chi-square statistics for the post-training and follow-up results as there were no baseline differences. GLMM was also used to evaluate BST-TAU differences at the three study time points [ 50 , 51 ]. For the BST-TAU comparisons, we used Cohen’s d as a guide for evaluating the practical significance of the differences for the continuous measures (competence, confidence). We used Cohen’s suggested thresholds [ 52 ] of 0.2, 0.5, and 0.8 for small, medium, and large effect sizes conservatively by applying them to both the point estimates and 95% confidence intervals. Thus, for example, a Cohen’s d where the confidence interval went below 0.2 would be interpreted as non-meaningful. For the categorical measure of mastery, we used BST-TAU risk ratios. Confidence intervals for all effect size measures were obtained using bootstrapping. Independent-samples t -tests were used for the post-hoc analyses and, along with chi-square tests, to compare the completers and non-completers.
One hundred ninety-nine staff consented to participate in the study out of a total of 360 session attendees (55%). Of these, 108 (54%) had been randomly assigned to a BST session and 91 (46%) to a TAU session. Half ( n = 99) completed assessments at all three time points (44% TAU; 55% BST). These 99 (hereafter ‘study completers’) constituted 28 percent of all session attendees.
Among the non-completers, 53 had been assigned to BST and 47 to TAU. Eight were classified as incomplete because of technical software issues when video-recording one of their assessments and one (the first participant) because the IOA process prompted substantive changes to the assessment checklist. The primary reason for the remaining non-completers was missing the follow-up assessment (91 individuals: 50/53 BST, 41/47 TAU) largely due to difficulties scheduling a non-mandatory event during the pandemic (e.g., units restricting staff from leaving because of clinical staff shortages or patient outbreaks, staff illness).
Descriptive information for the expected degree of patient contact and for hospital department is shown in Table 1 for study participants (completers, non-completers), non-participants, and the total group of session attendees. No significant differences were found when comparing participants versus non-participants or study completers versus non-completers in terms of expected patient contact ( χ 2 (2) = 0.36, n.s.; χ 2 (2) = 2.22, n.s.; respectively) or department type ( χ 2 (3) = 4.40; ( χ 2 (3) = 1.00, n.s.; respectively).
Figure 1 depicts the self-protection and team-control competence scores for the study completers (left and right sides, respectively). The hypothesis-testing results showed a significant difference by training Method (self-protection: χ 2 (1) = 34.46, p < 0.001; team-control: χ 2 (1) = 50.42, p < 0.001). There was also a significant decline between post-training and follow-up (Time) for both skill categories independent of Method (self-protection: χ 2 (1) = 81.29, p < 0.001; team-control: χ 2 (1) = 56.51, p < 0.001), and a significant Method-by-Time interaction independent of Method and Time for team-control skills ( χ 2 (1) = 17.41, p < 0.001). BST-TAU comparisons showed no difference at baseline for either type of skill (not shown). However, BST was significantly better than TAU at both post-training (self-protection: Cohen’s d = 1.45 [1.02, 1.87], large effect size; team-control: Cohen’s d = 2.55 [2.08, 3.02]; large effect size) and follow-up (respectively – Cohen’s d = 0.82 [0.40, 1.23]; Cohen’s d = 0.62 [0.21, 1.03], both small effect sizes). For both methods, competence scores dropped between post-training and follow-up although not to the original baseline levels.
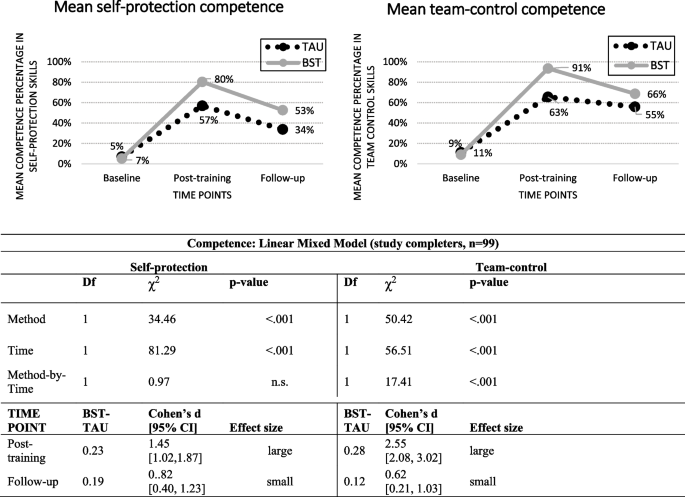
Observer-rated self-protection and team-control competence skills in TAU and BST across time-points
The skill mastery results for the study completers are shown in Fig. 2 . The mastery patterns paralleled the competence patterns in that BST was significantly better than TAU (self protection: χ 2 (1) = 28.82, p < 0.001; team-control: χ 2 (1) = 72.87, p < 0.001). There was also a significant Time effect independent of Method (self-protection: χ 2 (1) = 27.54, p < 0.001; team-control: χ 2 (1) = 33.03, p < 0.001). There were no significant interactions for either type of skill once the effects of Method and Time were accounted for. BST-TAU comparisons showed no difference in percent achieving Mastery at baseline (not shown) but large risk ratios at both post-training (self-protection: 13.43 [4.01, > 1000]; team-control: 31.24 [8.45, > 1000] and follow-up [self-protection: 12.30 [1.58, > 1000]; team-control: 30.60 [6.75. > 1000]).
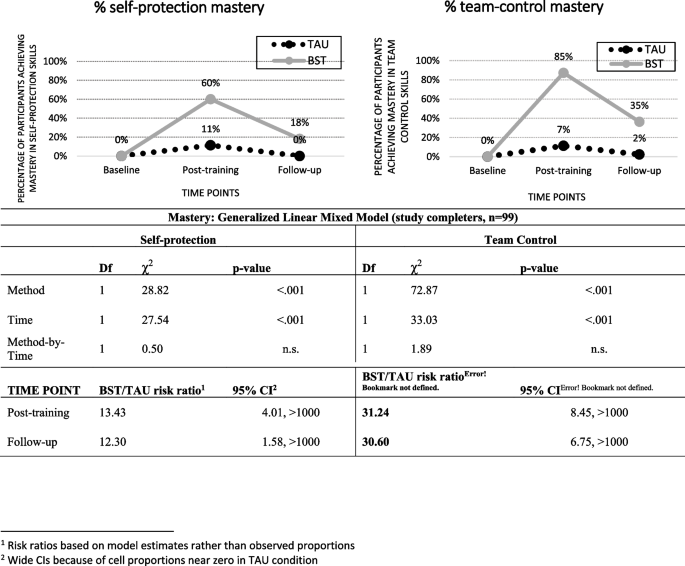
Observer-rated self-protection and team-control mastery (Predefined as 80% or better competence) by TAU and BST across time-points
Confidence scores for the study completers are shown in Fig. 3 . The only significant main effect was for Time (self-protection: χ 2 (1) = 36.87, p < 0.001; team-control: χ 2 (1) = 21.08, p < 0.001). For both skill categories, the scores increased between baseline and post-training and then dropped at follow-up but not to the original baseline levels.
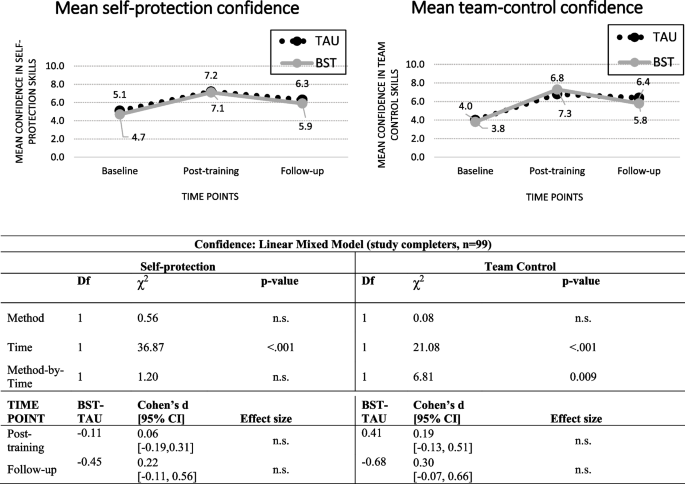
Self-rated self-protection and team-control confidence in TAU and BST across time-points
To assess what impact the high no-show rate for the one-month follow-up could have had, we compared the completers and the non-completers on the six post-training outcomes (competence, mastery, and confidence for self-protection and for team-control). Non-completers had slightly lower scores than completers except for the two confidence measures where their self-assessments were higher (not shown). However, the only significant difference between the two groups was for self-protection competence means (0.70 vs 0.63, completers vs non- completers, t (195) = 2.40, p = 0.017).
In terms of past-month experience, few study completers reported events requiring self-protection (19 at baseline, 9 at follow-up) or team-control skills (14 at baseline, 14 at follow-up). Consequently, we only examined the presence or absence of experience without breaking it down by training method. We found non-significant results for both competence and mastery (not shown) but a potential impact on confidence for self-protection skills at follow-up and for team-control skills at baseline and post-training (Fig. 4 ).
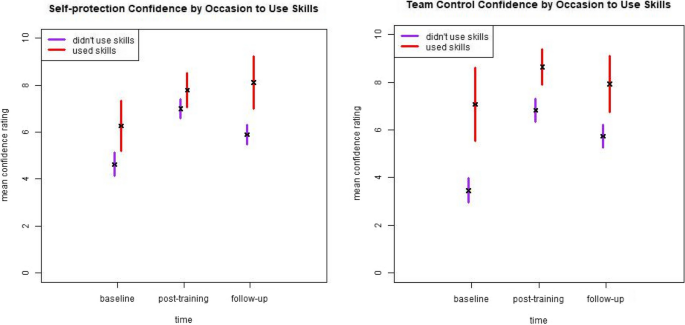
Self-rated self-protection and team-control confidence by occasion to use skills in the past month across time-points
4. Summary and discussion.
Our strongest finding was that BST was significantly better than TAU in improving the observed performance of self-protection and team-control skills. While follow-up scores decreased for both methods, BST scores remained higher than TAU scores. The impact of training on staff confidence differs from these patterns in that confidence scores improved noticeably at post-training and remained relatively high at follow-up. Further, our post-hoc analyses suggested that recent experience using safety skills might have a greater impact on confidence than on observed skill performance. We also found that training, regardless of method, was independently associated with improved observer-scored skills and self-reported confidence.
The better performance of BST is consistent with the fact that it incorporates training elements that are supported both by current educational and learning theories and evidence of effectiveness [ 46 , 53 , 54 , 55 ]. While both BST and TAU can be considered ‘outcomes based’ [ 54 ], the key difference is the BST’s use of the checklist. Based directly on the desired behavioral outcomes, this tool simultaneously creates a common understanding because it is shared with the trainees, ensures consistent and systematic training across all BST trainees, pinpoints where immediate and personalized feedback is needed to either correct or reinforce performance, and tracks the number of correct repetitions required to meet mastery criteria as well as support retention [ 46 , 56 , 57 ]. By contrast, TAU does not use a checklist and the kind and amount of feedback or practice repetitions is left to the trainer’s discretion.
However, there are at least two questions regarding whether BST produced the expected results. The BST framework requires continued rehearsal and feedback until a specified performance criterion is reached [ 34 ]. However, our mandatory safety training had practical, unmodifiable constraints. The institution required the safety-training sessions be completed in 3.5 h which meant that BST trainers were limited in their ability to use the more stringent performance criteria described in the literature. For example, it was not practical to set the performance criterion at higher than 80 percent. In addition, all BST completers were able to demonstrate 80-percent correct performance for each skill at least once, but not all were able to demonstrate five consecutive, correct executions within the allotted time. If the requirement of five in a row at 80% or higher had been implemented, then the post-training scores (and potentially the 1-month follow-up scores) for the BST completers could have been higher.
A second question is what level of skill retention should be expected at follow-up. The BST scores at one-month follow-up constituted 66% and 73% of the competence scores at post-training (self-protection and team-control, respectively) and 30% and 41% of the mastery percentages at post-training (self-protection and team-control, respectively). Although BST and elements of performance feedback models have been found to be effective in staff training with successful retention over time [ 58 , 59 , 60 , 61 , 62 ], finding appropriate comparators for our study was challenging because there are no studies where BST has been used for training such a large and diverse group of staff. Further, as noted above, the body of workplace violence prevention literature has not consistently focussed on retention. However, the broader training and education literature does suggest that our results are consistent with or somewhat lower than those from other studies. Offiah et al. [ 63 ] found that 45 percent of medical students retained the full set of clinical skills 18 months after completing simulation training, and Bruno and colleagues [ 64 ] found published retention rates ranging between 75 and 85 percent across time periods between four to 24 months and across diverse disciplinary fields. Regardless of the comparators, the loss in skill performance after one-month post-training is a concern.
Our interpretation is that reliance on a single session, even with highly structured and competency-based methods, is not adequate particularly in the context of managing distressing events. Efforts should be made to allow for flexibility with respect to setting higher thresholds for success despite organizational restraints for staff training. Furthermore, settings that require these skills to be performed more reliably for both patient and staff safety (e.g., emergency departments, acute care settings, security services) should consider on-the-job feedback or booster sessions based on objective assessments of skill rather than on pre-set amounts of time (e.g. annual refresher). This would be more consistent with the BST literature, as on-the-job training should occur based on an evidence-based approach.
Our finding of a differential impact of training on confidence versus demonstrable skills is consistent with a long-standing, substantial body of research examining the relationship between self-assessment and objective measures of learning [ 28 , 65 , 66 ]. The pattern of non-existent, weak, or even inverse relationships between the two has been shown for a variety of medical staff trainee and education learner groups [ 28 , 29 , 67 , 68 , 69 , 70 , 71 , 72 ]. Consequently, many researchers recommend either not using self-assessments at all or at least ensuring that objective measures are also collected (e.g.,[ 64 , 65 ]).
The literature does offer some hypotheses for why this discrepancy occurs and, further, why self-assessment continues to be used in medical education and training despite the robust evidence that it does not accurately reflect learning. Katowa-Mukwato and Banda [ 70 ] in a study of Zambian medical students suggest that fear of revealing their weaknesses led to a negative correlation between self- and objective-ratings. Persky, et al. [ 69 ] reference the theory of ‘metacognition’ – defined as ‘thinking about thinking’ (p. 993, [ 69 ] – and the ‘Dunning-Kruger’ effect that the ability to recognize competence (i.e., accurate metacognition) is unevenly distributed. There is also discussion as to why these measures continue to be used and suggestions of how best to use them. Yates et al. [ 65 ] suggest that ease of collecting this information is a factor. More complex and nuanced explanations are offered by Lu, et al. [ 66 ] and Tavares, et al. [ 73 ] who note that self-assessment is an important component in theories of learning and evaluation and that self-perception and self-reflection (particularly when objective findings are shared) are critical ingredients for supporting medical and continuing profession education in a self-regulating profession.
Because the goal of our study was to assess the effectiveness of two training methods, we did not collect information or have the opportunity to explore any of these potential reasons for why self-reported and objective measures are discrepant or to evaluate the best use of that discrepancy. The modest contributions that our study adds are that selecting a higher-risk setting, including non-nursing healthcare professionals, using a more rigorous study design (as recommended by Geoffrion, et al. [ 29 ]), and attempting to account for recent experience do not appear to alter this pattern.
The major strength of our study is its design. Currently, we have identified only one other study evaluating the impact of BST training for clinical staff using a randomized control trial design [ 41 ]. Other strengths are our inclusion of a large percentage of non-nursing, direct-care staff, our use of both self-reported and observer-assessed outcome measures, and our findings regarding retention. These strengths allow us to add to the evidence base already established in the literature.
However, interpretation of our results should consider several limitations. Conducting a research study on full-time clinical staff during a pandemic meant that a high percentage of those consenting to be in the study did not complete their 1-month follow-up assessment. The reported reasons for missing the third assessment (unit restrictions or short staffing because of the pandemic) are consistent with the demographic differences between completers and non-completers in that they were more likely to be nurses or working on inpatient units. Our comparison of the post-training scores of the completers and non-completers suggested that the no-shows had slightly lower post-training observed skill performance (but slightly better confidence ratings). If we had managed to assess the non-completers at follow-up, our reported findings may have been diluted although it is unlikely that this would have completely negated the large effect sizes.
The time constraints on the mandatory training meant that we were unable to fully apply either the BST mastery criteria commonly reported in the literature (i.e., three correct, consecutive executions [ 28 , 47 ] or the one we would have preferred (i.e., five correct executions). While this type of limitation is consistent with the pragmatic nature of our design, it likely had an impact on our findings in terms of potentially lowering the post-training BST competency and mastery scores and, perhaps more importantly, contributing to the lower retention rates at 1-month follow-up [ 56 ].
The 45-percent refusal rate by the training registrants is another concerning issue. Anecdotal reports from the training team were that the response rate was very low at the start of the study because many of the new hires were nervous about being videotaped (a specific comment reported was that it reminded some of the new graduates of ‘nursing school.’) and were unsure of the purpose of the study. The team then changed to a more informal, conversational introduction describing the need for the study as well as reassuring attendees that it was the training, not the participants, that was being evaluated. The team’s impression was that this improved the participation rate. The participants and non-participants were not statistically different in terms of their expected patient contact and department role. However, we cannot preclude that there may have been systematic biases for other unmeasured characteristics.
Another limitation, as identified by Price, et al. [ 28 ], is that we used artificial training scenarios, though this may be unavoidable given the low frequency of aggressive events and the ethics of deliberately exposing staff to these events. Also, we only measured the skills directly related to handling client/patient events. We were not able to access information on event frequency or severity, staff distress and complaints, or institutional-level measures such as lost workdays due to sick leave, staff turnover, or expenditures [ 29 , 33 ]. A further gap, which is important but difficult to assess, is whether there is any impact of staff safety training on the clients or patients who are involved.
Given these strengths and limitations, we see our study as adding one piece of evidence that needs to be a) confirmed or disconfirmed by other researchers in both the same and different settings and b) understood as part of a complex mix of ingredients. Specific areas for further research arising directly out of our findings include evaluating whether less constrained training time would improve attainment of skill mastery, exploration and evaluation of methods to increase skill retention over time, and, most importantly but also more difficult to assess, the impact on patients and clients of staff safety skills training. More evidence on these fronts will hopefully contribute to maintaining and improving workplace safety.
Availability of data and materials
The dataset generated and analysed during the current study is not publicly available due to the fact that it is part of a larger internal administrative data collection but is available from the corresponding author on reasonable request.
International Labour Organization. Joint Programme Launches New Initiative Against Workplace Violence in the Health Sector. 2002. Available from: https://www.ilo.org/global/about-the-ilo/newsroom/news/WCMS_007817/lang--en/index.htm . Cited 2022 Aug 5.
Di Martino V. Workplace violence in the health sector. Country case studies Brazil, Bulgaria, Lebanon, Portugal, South Africa, Thailand and an Additional Australian Study. Geneva: ILO/ICN/WHO/PSI Joint Programme on Workplace Violence in the Health Sector; 2002.
Needham I, Kingma M, O’Brien-Pallas L, McKenna K, Tucker R, Oud N, editors. Workplace Violence in the Health Sector. In: Proceedings of the First International Conference on Workplace Violence in the Health Sector - Together, Creating a Safe Work Environment. The Netherlands: Kavanah; 2008. p. 383.
Health Quality Ontario. Quality Improvement Plan Guidance: Workplace Violence Prevention. 2019.
Google Scholar
Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12(6):573–6.
Article PubMed PubMed Central Google Scholar
Somani R, Muntaner C, Hillan E, Velonis AJ, Smith P. A Systematic review: effectiveness of interventions to de-escalate workplace violence against nurses in healthcare settings. Saf Health Work. 2021;12(3):289–95. https://doi.org/10.1016/j.shaw.2021.04.004 .
Li Y, Li RQ, Qiu D, Xiao SY. Prevalence of workplace physical violence against health care professionals by patients and visitors: a systematic review and meta-analysis. Int J Environ Res Public Health. 2020;17(1):299.
Liu J, Gan Y, Jiang H, Li L, Dwyer R, Lu K, et al. Prevalence of workplace violence against healthcare workers: a systematic review and meta-analysis. Occup Environ Med. 2019;76(12):927–37.
Article PubMed Google Scholar
O’Rourke M, Wrigley C, Hammond S. Violence within mental health services: how to enhance risk management. Risk Manag Healthc Policy. 2018;11:159–67.
Odes R, Chapman S, Harrison R, Ackerman S, Hong OS. Frequency of violence towards healthcare workers in the United States’ inpatient psychiatric hospitals: a systematic review of literature. Int J Ment Health Nurs. 2021;30(1):27–46.
Gerberich SG, Church TR, McGovern PM, Hansen HE, Nachreiner NM, Geisser MS, et al. An epidemiological study of the magnitude and consequences of work related violence: the minnesota nurses’ study. Occup Environ Med. 2004;61(6):495–503.
Article CAS PubMed PubMed Central Google Scholar
Ridenour M, Lanza M, Hendricks S, Hartley D, Rierdan J, Zeiss R, et al. Incidence and risk factors of workplace violence on psychiatric staff. Work. 2015;51(1):19–28.
Cooper AJ, Mendonca JD. A prospective study of patient assaults on nurses in a provincial psychiatric hospital in Canada. Acta Psychiatr Scand. 1991;84(2):163–6.
Article CAS PubMed Google Scholar
Hiebert BJ, Care WD, Udod SA, Waddell CM. Psychiatric nurses’ lived experiences of workplace violence in acute care psychiatric Units in Western Canada. Issues Ment Health Nurs. 2022;43(2):146–53.
Lim M, Jeffree M, Saupin S, Giloi N, Lukman K. Workplace violence in healthcare settings: the risk factors, implications and collaborative preventive measures. Ann Med Surg. 2022;78:103727.
Article Google Scholar
Lanctôt N, Guay S. The aftermath of workplace violence among healthcare workers: a systematic literature review of the consequences. Aggress Violent Behav. 2014;19(5):492–501.
Friis K, Pihl-Thingvad J, Larsen FB, Christiansen J, Lasgaard M. Long-term adverse health outcomes of physical workplace violence: a 7-year population-based follow-up study. Eur J Work Organ Psychol. 2019;28(1):101–9.
Berlanda S, Pedrazza M, Fraizzoli M, de Cordova F. Addressing risks of violence against healthcare staff in emergency departments: the effects of job satisfaction and attachment style. Biomed Res Int. 2019;28(5430870):1–12.
Baig LA, Ali SK, Shaikh S, Polkowski MM. Multiple dimensions of violence against healthcare providers in Karachi: results from a multicenter study from Karachi. J Pakistani Med Assoc. 2018;68(8):1157–65.
International Labour Organization. Safe and healthy working environments free from violence and harassment. International Labour Organization. Geneva: International Labour Organization; 2020. Available from: https://www.ilo.org/global/topics/safety-and-health-at-work/resources-library/publications/WCMS_751832/lang--en/index.htm .
Wirth T, Peters C, Nienhaus A, Schablon A. Interventions for workplace violence prevention in emergency departments: a systematic review. Int J Environ Res Public Health. 2021;18(16):8459.
U.S. Congress. Workplace Violence Prevention for Health Care and Social Service Workers Act. U.S.A.; 2020. Available from: https://www.congress.gov/bill/116th-congress/house-bill/1309 .
Lu A, Ren S, Xu Y, Lai J, Hu J, Lu J, et al. China legislates against violence to medical workers. TneLancet Psychiatry. 2020;7(3):E9.
PubMed Google Scholar
Government of Ontario Ministry of Labour T and SD. Preventing workplace violence in the health care sector | ontario.ca. 2020. Available from: https://www.ontario.ca/page/preventing-workplace-violence-health-care-sector . Cited 2020 Jul 28.
Safe and Well Committee. Safe & Well Newsletter. 2018. Available from: http://insite.camh.net/files/SafeWell_Newsletter_October2018_107833.pdf .
Patel MX, Sethi FN, Barnes TR, Dix R, Dratcu L, Fox B, et al. Joint BAP NAPICU evidence-based consensus guidelines for the clinical management of acute disturbance: de-escalation and rapid tranquillisation. J Psychiatr Intensive Care. 2018;14(2):89–132.
Heckemann B, Zeller A, Hahn S, Dassen T, Schols JMGA, Halfens RJG. The effect of aggression management training programmes for nursing staff and students working in an acute hospital setting. A narrative review of current literature. Nurse Educ Today. 2015;35(1):212–9.
Price O, Baker J, Bee P, Lovell K. Learning and performance outcomes of mental health staff training in de-escalation techniques for the management of violence and aggression. Br J Psychiatry. 2015;206:447–55.
Geoffrion S, Hills DJ, Ross HM, Pich J, Hill AT, Dalsbø TK, et al. Education and training for preventing and minimizing workplace aggression directed toward healthcare workers. Cochrane Database Syst Rev. 2020;9(Art. No.: CD011860). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8094156/ . Cited 2021 Dec 3.
Paterson B, Turnbull J, Aitken I. An evaluation of a training course in the short-term management of violence. Nurse Educ Today. 1992;12(5):368–75.
Rice ME, Helzel MF, Varney GW, Quinsey VL. Crisis prevention and intervention training for psychiatric hospital staff. Am J Community Psychol. 1985;13(3):289–304.
Wondrak R, Dolan B. Dealing with verbal abuse: evaluation of the efficacy of a workshop for student nurses. Nurse Educ Today. 1992;12(2):108–15.
Leach B, Gloinson ER, Sutherland A, Whitmore M. Reviewing the evidence base for de-escalation training: a rapid evidence assessment. RAND research reports. RAND Corporation; 2019. Available from: https://www.rand.org/pubs/research_reports/RR3148.html . Cited 2021 Nov 26.
Parsons MB, Rollyson JH, Reid DH. Evidence-based staff training: a guide for practitioners. Behav Anal Pract. 2012;5(2):2–11.
Miltenberger RG, Flessner C, Gatheridge B, Johnson B, Satterlund M, Egemo K. Evaluation of behavioral skills training to prevent gun play in children. J Appl Bahavior Anal. 2004;37:513–6.
Baer DM, Wolf MM, Risley TR. Some still-current dimensions of applied behavior analysis. J Appl Behav Anal. 1987;20(4):313–27.
Dillenburger K. Staff training. Handbook of treatments for autism spectrum disorder. In: Matson JL, editor. Handbook of treatments for autism spectrum disorder. Switzerland: Springer Nature; 2017. p. 95–107.
Kirkpatrick M, Akers J, Rivera G. Use of behavioral skills training with teachers: a systematic review. J Behav Educ. 2019;28(3):344–61.
Sun X. Behavior skills training for family caregivers of people with intellectual or developmental disabilities: a systematic review of literature. Int J Dev Disabil. 2020:68(3):247-73.
Davis S, Thomson K, Magnacca C. Evaluation of a caregiver training program to teach help-seeking behavior to children with autism spectrum disorder. Int J Dev Disailities. 2020;66(5):348–57.
Gormley L, Healy O, O’Sullivan B, O’Regan D, Grey I, Bracken M. The impact of behavioural skills training on the knowledge, skills and well-being of front line staff in the intellectual disability sector: a clustered randomised control trial. J Intellect Disabil Res. 2019;63(11):1291–304.
Lin E, Malhas M, Bratsalis E, Thomson K, Boateng R, Hargreaves F, et al. Behavioural skills training for teaching safety skills to mental health clinicians: A protocol for a pragmatic randomized control trial. JMIR Res Protoc. 2022;11(12):e39672.
GraphPad. Randomly assign subjects to treatment groups. Available from: https://www.graphpad.com/quickcalcs/randomize1.cfm . Cited 2023 June 6.
Van Voorhis CRW, Morgan BL. Understanding power and rules of thumb for determining sample sizes. Tutor Quant Methods Psychol. 2007;3(2):43–50.
Erath TG, DiGennaro Reed FD, Sundermeyer HW, Brand D, Novak MD, Harbison MJ, et al. Enhancing the training integrity of human service staff using pyramidal behavioral skills training. J Appl Behav Anal. 2020;53(1):449–64.
Wong KK, Fienup DM, Richling SM, Keen A, Mackay K. Systematic review of acquisition mastery criteria and statistical analysis of associations with response maintenance and generalization. Behav Interv. 2022;37(4):993–1012.
Richling SM, Williams WL, Carr JE. The effects of different mastery criteria on the skill maintenance of children with developmental disabilities. J Appl Behav Anal. 2019;52(3):701–17 (Available from: https://pubmed.ncbi.nlm.nih.gov/31155708/ ). Cited 2022 Oct 14.
Pitts L, Hoerger ML. Mastery criteria and the maintenance of skills in children with developmental disabilities. Behav Interv. 2021;36(2):522–31. Available from: https://doi.org/10.1002/bin.1778 . Cited 2022 Oct 17.
R Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2021.
Pinheiro J, Bates D. Mixed-effects models in S and S-PLUS. New York: Springer Science+Business Media; 2006.
Bosker R, Snijders TA. Multilevel analysis: An introduction to basic and advanced multilevel modeling. In: Analysis M, editor. London. UK: Sage Publishers; 2012. p. 1–368.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988.
Taylor DCM, Hamdy H. Adult learning theories: Implications for learningand teaching in medical education: AMEEGuide No. 83. Med Teach. 2013;35(11):e1561-72.
Nodine TR. How did we get here? A brief history of competency-based higher education in the United States. Competency Based Educ. 2016;1:5–11.
Novak MD, Reed FDD, Erath TG, Blackman AL, Ruby SA, Pellegrino AJ. Evidence-Based Performance Management: Applying behavioral science to support practitioners. Perspect Behav Sci. 2019;42:955–72.
Fienup DM, Broadsky J. Effects of mastery criterion on the emergence of derived equivalence relations. J Appl Behav Anal. 2017;40:843–8.
Fuller JL, Fienup DM. A preliminary analysis of mastery criterion level: effects on response maintenance. Behav Anal Pract. 2018;11(1):1–8.
Alavosius MP, Sulzer-Azaroff B. The effects of performance feedback on the safety of client lifting and transfer. J Appl Behav Anal. 1986;19:261–7.
Hogan A, Knez N, Kahng S. Evaluating the use of behavioral skills training to improve school staffs’ implementation of behavior intervention plans. J Behav Educ. 2015;24(2):242–54.
Nabeyama B, Sturmey P. Using behavioral skills training to promote safe and correct staff guarding and ambulation distance of students with multiple physical disabilities. J Appl Behav Anal. 2010;43(2):341–5.
Parsons MB, Rollyson JH, Reid DH. Teaching practitioners to conduct behavioral skills training: a pyramidal approach for training multiple human service staff. Behav Anal Pract. 2013;6(2):4–16.
Sarakoff RA, Sturmey P. The effects of behavioral skills training on staff implementation of discrete-trial teaching. J Appl Behav Anal. 2004;37(4):535–8.
Offiah G, Ekpotu LP, Murphy S, Kane D, Gordon A, O’Sullivan M, et al. Evaluation of medical student retention of clinical skills following simulation training. BMC Med Educ. 2019;19:263.
Bruno P, Ongaro A, Fraser I. Long-term retention of material taught and examined in chiropractic curricula: its relevance to education and clinical practice. J Can Chiropr Assoc. 2007;51(1):14–8.
PubMed PubMed Central Google Scholar
Yates N, Gough S, Brazil V. Self-assessment: With all its limitations, why are we still measuring and teaching it? Lessons from a scoping review. Med Teach. 2022;44(11):1296–302. https://doi.org/10.1080/0142159X.2022.2093704 .
Lu FI, Takahashi SG, Kerr C. Myth or reality: self-assessment is central to effective curriculum in anatomical pathology graduate medical education. Acad Pathol. 2021;8:23742895211013530.
Magnacca C, Thomson K, Marcinkiewicz A, Davis S, Steel L, Lunsky Y, et al. A telecommunication model to teach facilitators to deliver acceptance and commitment training. Behav Anal Pract. 2022;15(3):752–752.
Naughton CA, Friesner DL. Comparison of pharmacy students’ perceived and actual knowledge using the pharmacy curricular outcomes assessment. Am J Pharm Educ. 2012;76(4):63.
Persky AM, Ee E, Schlesselman LS. Perception of learning versus performance as outcome measures of educational research. Am J Pharm. 2020;84(7):993–1000.
Katowa-Mukwato P, Sekelani SB. Self-perceived versus objectively measured competence in performing clinical pratical procedures by final medical students. Int J Med Educ. 2016;7:122–9.
Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Residents’ procedural experience does not ensure competence: a research synthesis. J Grad Med Educ. 2017;9(2):201–8.
Choudhry NK, Fletcher RH, Soumerai SB. Systematic review: the relationship between clinical experience and quality of health care. Ann Intern Med. 2005;142(4):260–73.
Tavares W, Sockalingam S, Valanci S, Giuliani M, Davis D, Campbell C, et al. Performance Data Advocacy for Continuing Professional Development in Health Professions. Acad Med. 2024;99(2):153-8. https://doi.org/10.1097/ACM.0000000000005490 .
Download references
Acknowledgements
We thank Sanjeev Sockalingam, Asha Maharaj, Katie Hodgson, Erin Ledrew, Sophie Soklaridis, and Stephanie Sliekers for their guidance and for dedicating the human and financial resources needed to support this study. We also want to express our sincere gratitude to the following individuals for facilitating physical skills sessions and for volunteering as actors in the physical skills demonstrations: Kate Van den Borre, Steven Hughes, Paul Martin Demers, Ross Violo, Genevieve Poulin, Stacy de Souza, Narendra Deonauth, Joanna Zygmunt, Tessa Donnelly, Lawren Taylor, and Bobby Bonner. Finally, we are grateful to Marcos Sanchez for statistical consultation and Quincy Vaz for research support.
This research was funded internally by the Centre for Addiction and Mental Health.
Author information
Authors and affiliations.
Department of Education, Centre for Addiction and Mental Health, Toronto, ON, Canada
Elizabeth Lin, Mais Malhas, Emmanuel Bratsalis, Kendra Thomson, Fabienne Hargreaves, Kayle Donner, Heba Baig, Rhonda Boateng, Rajlaxmi Swain, Mary Benisha Benadict & Louis Busch
Department of Applied Disability Studies, Brock University, St. Catharines, ON, Canada
Kendra Thomson
You can also search for this author in PubMed Google Scholar
Contributions
All authors were involved in the study design, monitoring and implementing the study, and review of manuscript drafts. EL was responsible for the original study design and drafting of the full manuscript. MM, EB, and FH led the implementation of the training sessions. EB, FH, HB, KT, and LB were involved in the reliability assessments (IOA). KD and HB were primarily responsible for data analysis. HB and RB monitored the data collection and the ongoing study procedures. RS and MBB assisted in the literature review.
Corresponding author
Correspondence to Elizabeth Lin .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the Research Ethics Board of the Centre for Addiction and Mental Health (#101/2020). Informed consent was obtained from all subjects participating in the study. All interventions were performed in accordance with the Declaration of Helsinki. This study was registered with the ISRCTN registry on 06/01/2023 (ISRCTN18133140).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1., additional file 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Lin, E., Malhas, M., Bratsalis, E. et al. Behavioral skills training for teaching safety skills to mental health service providers compared to training-as-usual: a pragmatic randomized control trial. BMC Health Serv Res 24 , 639 (2024). https://doi.org/10.1186/s12913-024-10994-1
Download citation
Received : 06 September 2023
Accepted : 16 April 2024
Published : 17 May 2024
DOI : https://doi.org/10.1186/s12913-024-10994-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Workplace violence
- Violence prevention
- Behavioural skills training
- Performance and competency-based staff training
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]
COLUMBIA UNIVERSITY IN THE CITY OF NEW YORK

Research Administrator I
- P&S Vice Dean For Research
- Columbia University Medical Center
- Opening on: May 16 2024
- Job Type: Officer of Administration
- Bargaining Unit:
- Regular/Temporary: Regular
- End Date if Temporary:
- Hours Per Week: 35
- Standard Work Schedule:
- Salary Range: $72,000 - $85,000
Position Summary
The mission of the Vagelos College of Physicians and Surgeons (VP&S) Office for Research is to facilitate the highest caliber of biomedical research, from basic to translational to clinical, among the VP&S faculty, students, and staff at Columbia University Irving Medical Center (CUIMC).
As a member of the Pre-Award team, the Research Administrator I contributes to CUIMC’s research mission by supporting grant-related pre-award administrative activities for the basic sciences and other participating departments in the Vagelos College of Physicians and Surgeons.
In this role, the Research Administrator I will leverage their pre-award grant administration knowledge to assist faculty in submission and pre-award administration of grants and contracts. Key areas of responsibility will include assisting PIs in the development, review, and submission of grants; preparing grant budgets; drafting supporting materials; and assisting with annual progress reports. Through this role, the Administrator I can expect to broaden their knowledge of academic grant-funded environments, including complex grants and the landscape of funded research at CUIMC, and contribute to the development of peers on the Pre-Award team.
To be successful in this role, the Research Administrator I should be able to apply their full breadth of pre-award grant administration knowledge while demonstrating strong interpersonal and communication skills, attention to detail, and an ability to work collaboratively and independently in a deadline-driven environment. The Administrator I should be enthusiastic about building relationships and working closely with principal investigators to contribute to the advancement of research at CUIMC.
Responsibilities
Pre-Award Grant Administration
Assists PIs in the development, review, and submission of all Federal, State, and foundation grants and contracts applications, with a primary focus on individual investigator grants and multi-component grants. Manages timelines and the overall progression of submission to ensure the on-time completion of high quality, responsive and compliant proposal applications.
Trains under the direction of senior staff to gain knowledge and expertise in complex grants, such as center grants, and training grants. Assists senior staff on pre-award administration of said grants.
Prepares grant budgets and budget justifications based on the aims provided and RFA technical content requirements in full compliance with sponsor/donor and CU policies, as well as engages outside collaborators and establishes subcontracts.
Prepares and compiles all components of each grant submission, ensures that the proposal is formatted, packaged, and submitted in accordance with granting agency requirements.
Drafts supporting documents for grants including letters of intent, letters of support, cover letters, facilities, and resources.
Maintains detailed, up-to-date records of PI’s other support, biosketches, and grant submission documents.
Responds to RFAs and summary statements and addresses issues prior to submission.
In coordination with the Post-Award Team and the principal investigators, promotes accurate preparation and timely submission of progress reports as required by sponsoring program.
Tracks the status of pending applications, follows up with investigator regarding review/funding status of application.
Supports investigators in responding to Just-In-Time requests.
Ensures files are complete for awarded applications, secures missing information from principal investigators and/or administrators.
Maintains standards: budget templates, standardized checklists for core staff and PIs tailored to the sponsor/mechanism, standardized filing system using shared drives, updated resources and facilities.
Performs all other duties as assigned within scope of practice and/or training.
People and Stakeholder Management
Works collaboratively and cooperatively with faculty, grant writers, P&S proposal development and Pre-Award team on all aspects of grant administration.
Manages and maintains positive relationships with internal and external stakeholders, such as faculty, internal offices, funding agencies, and collaborating organizations.
Contributes to team learning culture by providing support to colleagues, demonstrating self-development, and keeping current on relevant professional development topics.
Models professionalism and accountability in interactions with internal clients through clear, respectful, and timely communication and responsive follow-up.
Strategy and Continuous Improvement
Maintains sound knowledge of federal rules and regulations that govern research grants as well as stays informed about sponsor terms and conditions for submitting and administering grant awards.
Participates in and/or supports team projects and initiatives. Uses tools and reporting mechanisms to track progress and ensure timely communication of issues and status.
Keeps current on all organizational policies, goals, and initiatives.
Successfully completes all required university and department trainings.
- Perform other related duties and responsibilities as assigned/requested.
Minimum Qualifications
Bachelor's Degree or combination of education and experience.
A minimum of 3 years of related experience, including prior experience in an academic grant funded environment.
Knowledge of and experience with full scope of pre-award grant management, including mastery of terminology, standards, and tools for grant management; Ability to serve as a resource to peers.
Strong customer service orientation, with the ability to interact and collaborate positively, constructively and effectively with multiple constituencies. Ability to model strong service to others.
Project management skills, including ability to efficiently lead and execute technical project activities, attention to detail, effectively coordinate and communicate with stakeholders.
Interpersonal and emotional intelligence skills focused on establishing and maintaining productive relationships with faculty, peers, leadership, and other stakeholders.
Capacity to work with a high degree of independence and successfully within a deadline driven, multi-tasking operating environment. Ability to handle multiple projects at increasing levels of complexity, demonstrate sound judgment in managing time, prioritizing, and decision-making.
Excellent analytical, organizational, interpersonal, oral and written communication skills.
Self-motivated and demonstrates initiative, patience, and resourcefulness in adapting to changes.
Proficiency in problem assessment and collaborative problem solving in interdisciplinary settings. Ability to communicate and escalate complex issues to management and research offices.
Proficiency in Microsoft Office (especially Excel) or similar software is required and an ability and willingness to learn new systems and programs.
Ability to work with a variety of individuals and groups in a constructive and respectful manner while appreciating the unique contributions of an inclusive workforce that brings together the talents of people across multiple identities.
Strong commitment to fostering diversity and equity.
Must successfully complete systems training requirements.
Preferred Qualifications
Bachelor's degree in biological science or business/administration degree such as finance, accounting, business administration, business, public health, public administration, or a directly related field.
Prior experience with NIH submissions and systems (era COMMONS, ASSIST, RePORTER)
Equal Opportunity Employer / Disability / Veteran
Columbia University is committed to the hiring of qualified local residents.
Commitment to Diversity
Columbia university is dedicated to increasing diversity in its workforce, its student body, and its educational programs. achieving continued academic excellence and creating a vibrant university community require nothing less. in fulfilling its mission to advance diversity at the university, columbia seeks to hire, retain, and promote exceptionally talented individuals from diverse backgrounds. , share this job.
Thank you - we'll send an email shortly.
Other Recently Posted Jobs
Non-Student Short-Term Casual
Refer someone to this job

- ©2022 Columbia University
- Accessibility
- Administrator Log in
Wait! Before you go, are you interested in a career at Columbia University? Sign up here!
Thank you, for sharing your information. A member of our team will reach out to you soon!

This website uses cookies as well as similar tools and technologies to understand visitors' experiences. By continuing to use this website, you consent to Columbia University's usage of cookies and similar technologies, in accordance with the Columbia University Website Cookie Notice .
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
May 17, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
New data outline positive effects of endurance exercise training
by American Physiological Society
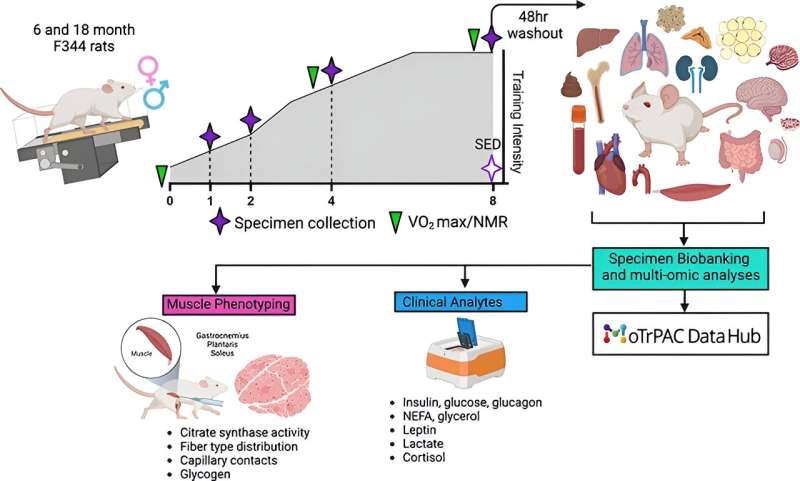
Treadmill training, a form of endurance exercise, was found to be highly effective "with robust improvements in skeletal muscle citrate synthase activity in as little as [one to two] weeks, and improvements in maximum run speed and maximal uptake by [four to eight] weeks." The full effect of endurance exercise training was previously unexplained until this study.
Researchers sought to develop and implement a standardized endurance exercise protocol in more than 340 rats participating in progressive treadmill training five days a week for one, two, four or eight weeks.
The researchers collected and measured 18 samples of tissues, blood and plasma to determine the effectiveness of endurance exercise. Improving skeletal muscle citrate synthase activity—a marker of mitochondrial density—in rats that exercised is significant because it feeds more energy to working muscles so they can function longer and faster.
The article , "Physiological adaptations to progressive endurance exercise training in adult and aged rats: insights from the molecular transducers of physical activity consortium (MoTrPAC)," is published ahead of print in the journal Function .
"This work in mature, treadmill-trained rats represents the most comprehensive and unprecedented resource for studying temporal, sex- and age-specific responses to endurance exercise training in a pre-clinical rat model," the researchers wrote.
Explore further
Feedback to editors
New analysis estimates the effects of race-neutral lung function testing on patients, hospitals, and beyond
6 hours ago

World-first trial shows benefits of finding and treating undiagnosed asthma and COPD
9 hours ago

Acetaminophen shows promise in warding off acute respiratory distress syndrome, organ injury in patients with sepsis
13 hours ago

Modular communicative leadless ICD found to be safe and exceeds performance expectations
May 18, 2024

Creativity and humor shown to promote well-being in older adults via similar mechanisms

Sweet taste receptor affects how glucose is handled metabolically by humans

Better medical record-keeping needed to fight antibiotic overuse, studies suggest

Repeat COVID-19 vaccinations elicit antibodies that neutralize variants, other viruses

A long-term ketogenic diet accumulates aged cells in normal tissues, new study shows

Gut bacteria enhance cancer immunotherapy in mouse study
Related stories.

Study in rats helps scientists work out the effects of exercise at the cellular level
May 1, 2024
Body-wide molecular map explains why exercise is so good for you
May 14, 2024
First steps toward a whole-body map of molecular responses to exercise
May 5, 2024

Novel insights into how muscles change during endurance training
Sep 12, 2023
Muscle health may be informed by activity level rather than aging process
Mar 21, 2024

High-intensity interval training improves spatial memory in rats
May 17, 2021
Recommended for you
Consistent exercise changes how saturated fat is used by the body, study finds
May 16, 2024

Discovery of a master neuron that controls movement in worms has implications for human disease
Study investigates cardiac cell regeneration in search of novel therapeutics
Study finds astrocytic pH regulator can repair blood-brain barrier, reverse brain damage caused by ischemic stroke
May 15, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

IMAGES
VIDEO
COMMENTS
Clinical research training not only creates potential independent investigators, but also enables clinicians to advance their careers through a greater understanding of research evidence. Designed to provide learners with the foundational knowledge and skill sets required to produce high-quality clinical research, our program will lay the ...
By attending Global Clinical Scholars Research Training, you will enhance your ability to: Design and perform observational and experimental clinical research. Analyze, interpret, and present clinical research data. Write and revise successful grant proposals. Lead clinical teams across a variety of health care settings.
The best free clinical research courses available are Clinical Data Management, Clinical Research, Research Methods, Researcher Management and Leadership Training, and Medical Research. All of these courses are free and offer a great introduction to the world of clinical research.
CCRPS provides online, accredited clinical research training in 1 to 4 weeks utilized by over 22,000 researchers to get hired or promoted within the field. Our clinical research courses are used by students at 1,200+ organizations, 6 government agencies, and 308 universities. Graduates of our progra
This course provides peer-reviewed training written by clinical research experts. Along with CITI Program's advantages, including our experience, customization options, cost effectiveness, and focus on organizational and learner needs, this makes it an excellent choice for clinical research training.
The NIH Clinical Center offers training to help prepare the next generation of translational and clinician scientists. The innovative programs include summer internships, postbaccalaureate fellowships, clinical elective rotations, medical student research programs, graduate medical education, and continuing medical education. An interactive ...
Learn Clinical Research, earn certificates with paid and free online courses from Harvard, Stanford, University of Michigan, Johns Hopkins and other top universities around the world. Read reviews to decide if a class is right for you. Follow 6.3k. Share 101 courses ...
Global Clinical Scholars Research Training. This Harvard Medical School one-year, application-based certificate program provides advanced training in health care research and methods. $14,900 - $15,900. Register by Sep 11.
The 10-12 month program is designed for students who are U.S. citizens or permanent residents, have a strong interest in conducting basic, translational, clinical or epidemiological research and are currently enrolled in their 2nd, 3rd, or 4th year at an accredited medical, dental, or veterinary program. Dental and veterinary students: due to ...
NIH Research Training and Career Development Programs. NIH programs help prepare individuals for careers in biomedical, behavioral, social, and clinical research. Learn more about how NIH Institutes and Centers may vary in research and training. Contact NIH extramural research training representatives to discuss how specific programs fit your ...
The Office of Clinical Research Education and Collaboration Outreach has the responsibility for courses which provide clinical research training for the spectrum of investigators and others involved in clinical research. Official website of the National Institutes of Health (NIH). NIH is one of the world's foremost medical research centers.
Designed for clinicians and clinician-scientists in both the United States and abroad, our Global Clinical Scholars Research Training program aims to enable scholars to expand their knowledge and sharpen their skills in clinical research.Using a blended learning model that incorporates online tools, in-person seminars and dynamic workshops, the curriculum is focused on enhancing your ability ...
Official website of the National Institutes of Health (NIH). NIH is one of the world's foremost medical research centers. An agency of the U.S. Department of Health and Human Services, the NIH is the Federal focal point for health and medical research. The NIH website offers health information for the public, scientists, researchers, medical professionals, patients, educators, and students.
Program Overview. The SOCRA Certified Clinical Research Professional (CCRP) program is your gateway to excellence in clinical research. Elevate your career with our internationally recognized certification, tailored for professionals dedicated to upholding the highest standards in the field. Join a community committed to ethical practices ...
Research Training. Academic medical centers play a leading role in training the next generation of scientists. These researchers are trained across the continuum of research disciplines- to ensure future breakthroughs to improve health and transform health care. There are multiple training pathways for pursuing a career in medical research ...
ACRP 2023 Study Management & Conduct - Replay. This package is a replay of 17 on-demand session recordings and presentation slides from the Study Management & Conduct Track at the ACRP 2023 Conference, April 29 - May 1, Dallas, Texas. Learn practical, proven ways to maximize study and site performance.
These online courses offer affordable, convenient access to quality education. SOCRA's online courses are intended to provide access to training and continuing education that will promote quality clinical research, protect the welfare of research participants and improve global health. Source Documentation.
Research & Training. precision-medicine-silhouettes.jpg. Medical Research Initiatives. Precision Medicine Initiative, The BRAIN Initiative, Accelerating Medicines Partnership, Rigor and Reproducibility, Data Science at NIH, and more. 20150330-white-blood-cells.jpg. Science Highlights.
Official website of the National Institutes of Health (NIH). NIH is one of the world's foremost medical research centers. An agency of the U.S. Department of Health and Human Services, the NIH is the Federal focal point for health and medical research. The NIH website offers health information for the public, scientists, researchers, medical professionals, patients, educators,
Idaho WWAMI has five primary goals for the State of Idaho: Provide publicly supported medical education. Increase the number of primary care physicians. Provide community-based medical education. Expand graduate medical education (residency training) and continuing medical education. Provide all of this in a cost-effective manner.
Abstract. Between 2009 and 2012, I taught principles of evidence-based medicine and clinical research in Russia, Tatarstan, Moldova, and Kazakhstan. The Soviet Union left a medical legacy characterized by balkanization of top tier medicine in highly specialized centers, so there was little capability for multidiscipinary care.
For research projects, students can work in scientific laboratories ad share their research in the form of articles in leading Russian and international medical journals and research papers. To facilitate advanced research and international clinical training, Lomonosov Moscow State University has collaboration with more than 15 research ...
The current paradigm of competency-based medical education and learner-centredness requires learners to take an active role in their training. However, deliberate and planned continual assessment and performance improvement is hindered by the fragmented nature of many medical training programs. Attempts to bridge this continuity gap between supervision and feedback through learner handover ...
Building details. University entrance. Pirogov Russian National Research Medical University (formerly known as Russian State Medical University or RSMU) is a medical higher education institution in Moscow, Russia founded in 1906. It is fully accredited and recognized by Russia's Ministry of Education and Science and is under the authority of ...
The Match Day ceremony at the University of California, Irvine, on March 15. Match Day is the day when medical students seeking residency and fellowship training positions find out their options.
Training; Research; Sports medicine; Over 20 years ago, Thomas Best and Domhnall MacAuley rhetorically posited that evidence-based sports medicine was potentially a 'contradiction in terms'. 1 In 2010, Evert Verhagen and Willem van Mechelen stated that 'most individuals involved in sports medicine are not thoroughly trained in epidemiological and methodological rigour'. 2 Despite these ...
The lack of menopause education in medical training programs and limited intergenerational discourse contributes to the knowledge gap. ... increasing clinical research, and encouraging open ...
Offiah et al. found that 45 percent of medical students retained the full set of clinical skills 18 months after completing simulation training, and Bruno and colleagues found published retention rates ranging between 75 and 85 percent across time periods between four to 24 months and across diverse disciplinary fields. Regardless of the ...
In this role, the Research Administrator I will leverage their pre-award grant administration knowledge to assist faculty in submission and pre-award administration of grants and contracts. Key areas of responsibility will include assisting PIs in the development, review, and submission of grants; preparing grant budgets; drafting supporting ...
DOI: 10.1093/function/zqae014. Provided by American Physiological Society. Treadmill training, a form of endurance exercise, was found to be highly effective "with robust improvements in skeletal ...