Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 16 January 2023

CRISPR/Cas9 therapeutics: progress and prospects
- Tianxiang Li 1 na1 ,
- Yanyan Yang 2 na1 ,
- Hongzhao Qi 1 na1 ,
- Weigang Cui 3 ,
- Lin Zhang 4 ,
- Xiuxiu Fu 5 ,
- Xiangqin He 5 ,
- Meixin Liu 1 ,
- Pei-feng Li ORCID: orcid.org/0000-0002-0969-9407 1 &
- Tao Yu 1 , 5
Signal Transduction and Targeted Therapy volume 8 , Article number: 36 ( 2023 ) Cite this article
70k Accesses
94 Citations
27 Altmetric
Metrics details
- Gene delivery
- Molecular medicine
Clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) gene-editing technology is the ideal tool of the future for treating diseases by permanently correcting deleterious base mutations or disrupting disease-causing genes with great precision and efficiency. A variety of efficient Cas9 variants and derivatives have been developed to cope with the complex genomic changes that occur during diseases. However, strategies to effectively deliver the CRISPR system to diseased cells in vivo are currently lacking, and nonviral vectors with target recognition functions may be the focus of future research. Pathological and physiological changes resulting from disease onset are expected to serve as identifying factors for targeted delivery or targets for gene editing. Diseases are both varied and complex, and the choice of appropriate gene-editing methods and delivery vectors for different diseases is important. Meanwhile, there are still many potential challenges identified when targeting delivery of CRISPR/Cas9 technology for disease treatment. This paper reviews the current developments in three aspects, namely, gene-editing type, delivery vector, and disease characteristics. Additionally, this paper summarizes successful examples of clinical trials and finally describes possible problems associated with current CRISPR applications.
Similar content being viewed by others
Advances in CRISPR therapeutics
Spatiotemporal control of CRISPR/Cas9 gene editing
Applications of genome editing technology in the targeted therapy of human diseases: mechanisms, advances and prospects
Introduction.
Gene editing is a technology that precisely modifies the genome sequence to induce insertions, deletions, or base substitutions in the genome. 1 , 2 Many diseases are accompanied by changes in gene expression in vivo, particularly some genetic diseases caused by mutations in a single gene, and gene-editing technology is expected to control the occurrence of diseases at the genetic level. 3 To date, gene-editing technology has undergone three main generations of development: the first generation of gene-editing technology was zinc-finger nucleases (ZFNs); the second generation was transcription activator-like effector nucleases (TALENs); and the most widely used third generation gene-editing technology is clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas). 4 Unlike ZFNs and TALENs, which use proteins to target DNA strands, CRISPR technology directs Cas proteins to a specified location in the genome by changing the base sequence of a small segment of guide RNA, thus improving the efficiency of gene editing and expanding the applicability of gene-editing technology. 5
CRISPR/ Cas9 is a highly effective gene-editing tool that is widely used in the scientific community. 6 The CRISPR/Cas9 system evolved naturally in bacteria and archaea as a defense mechanism against phage infection and plasmid transfer. 7 , 8 Bacteria or archaea acquire a segment of their DNA sequence to insert into the CRISPR spacer region when first infiltrated by an exogenous phage or plasmid. If reinfected with homologous DNA, the bacterium will initiate transcription of the CRISPR region. After a series of processing and maturation processes to generate a single guide RNA (sgRNA), the sgRNA guides Cas9 to shear the DNA strand that disrupts the homologous spacer region. The recognition process of the sgRNA requires the involvement of protospacer-adjacent motifs (PAMs), a short guanine-enriched sequence. 9 The preferred PAM by Streptococcus pyogenes Cas9 (SpCas9) is NGG, which is common in the genomes of most organisms, thereby facilitating the use of CRISPR technology across the fields of plant and animal science, together with biomedicine. 10 , 11 , 12 , 13 , 14 By changing the nucleotide sequence of a small segment of guide RNA, CRISPR/Cas9 allows the accurate targeting of almost any desired genomic locus for the purpose of correcting disease-causing mutations or silencing genes associated with disease onset. 5 , 15 However, some highly chromatinized regions in the genome may not be accessible to CRISPR/Cas9. Promising applications for this technology include the treatment of cancers, cardiovascular diseases, sickle cell anemia, and neurodegenerative disease. 16 , 17 , 18 , 19
Wild-type Cas9 only cuts double-stranded DNA to form double-strand breaks (DSBs), which are repaired through DNA repair mechanisms, namely, homology-directed repair (HDR) and nonhomologous end joining (NHEJ). 20 , 21 , 22 The base sequence of the original gene is damaged, resulting in inactivation, but the inactivation of a single deleterious gene cannot address the complex processes of all disease events. 23 Therefore, researchers searched for possible ways to modify Cas9 by elucidating the physicochemical structure of Cas9, the mechanism of action by which Cas9 cleaves double chains, and other properties. They endowed Cas9 with new functions by mutating the structural domain of Cas9 and introducing effectors, including transcriptional regulatory tools such as dead Cas9 (dCas9) effectors and single-base substitution tools such as cytosine base editors (CBEs), adenine base editors (ABEs), and prime editors (PEs). Moreover, RNA recognition and cleavage functions can be performed by Cas13a isolated from Leptotrichia shahii. 24 , 25 , 26 , 27 , 28 These Cas9 variants and derivatives enrich the gene-editing paradigm and can be adapted to additional types of diseases.
Although several experiments have documented the use of gene-editing technology to modify cells in vitro for return to the body to treat some diseases, this approach is not applicable to most disease types. Achieving stable, efficient, and safe delivery in vivo is a challenge that must be overcome before CRISPR technology becomes a common treatment modality. CRISPR systems such as plasmid DNA (pDNA), mRNA and ribonucleoproteins (RNPs) are subject to degradation and immune clearance in vivo after direct delivery and therefore require the help of delivery vectors. 29 Adeno-associated virus (AAV) vectors are not suitable for application in most diseases because of the drawbacks of a limited loading capacity, a lack of specific targeting ability and inability to integrate into the host genome. 30 Nonviral vectors have been a hot topic of research in recent years, where lipid nanoparticles have been used in the clinic for the delivery of CRISPR gene drugs. 31 Polymeric nanoparticles, biomimetic nanomaterials, and exosomes have also shown potential for the delivery of CRISPR systems in animal experiments. 32 Further research and development are needed to apply nonviral vectors to a wide range of clinical applications.
Each disease has different characteristics, and our aim is not to develop a universal delivery vehicle but to develop multiple vehicles applicable to different types of diseases. Therefore, studying the pathogenesis of diseases and the pathological characteristics of disease cells and tissues and constructing environment-responsive and ligand-recognizing nanoparticles based on these characteristics will further enrich gene-targeting drugs in diseased tissues. 33 In addition, exosomes and cell membranes from immune cells or diseased organs can effectively avoid immune clearance, and the abundant membrane proteins on the surface enable gene-targeting drugs to be delivered to diseased cells.
In this review, we discuss the development of CRISPR technology and summarize the various types of gene-editing tools that have been developed in recent years. Delivery systems for CRISPR systems in the body are also summarized, with a focus on developing new systems more suitable for different diseases, and finally, the review addresses a collection of problems that may arise when applying CRISPR technology to treat diseases and the corresponding strategies. In conclusion, this approach has positive implications for providing the most effective gene therapy modalities for different diseases.
Discovery and development of CRISPR technology
CRISPR-related gene-editing technology is currently one of the hottest biological tools. Since 2013, explosive growth has been recorded in the study of CRISPR technology, with tens of thousands of CRISPR-related articles published. In October 2020, the Nobel Prize in Chemistry was awarded to French microbiologist Emmanuelle Charpentier and American biologist Jennifer Doudna for “developing a new approach to genome editing”. The method had been studied by scientists for nearly three decades before it received widespread attention (Fig. 1 ).
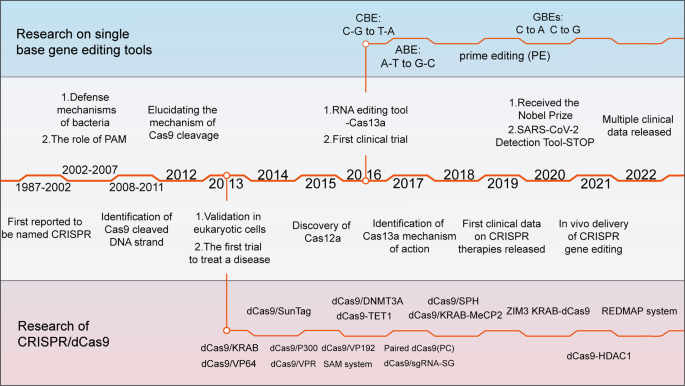
Timeline of major events in the development of CRISPR/Cas technology and representative Cas9 variants. In 1987, the CRISPR sequence was first reported. The mechanism by which Cas9 cuts DNA double strands was reported in 2012, and Cas9 was subsequently used for gene editing in mammalian cells. Since then, CRISPR technology has developed rapidly, and multiple Cas9 variants with specific functions have been identified. The representative variants are single-base substitution tools (e.g., CBE and PE) and transcriptional regulatory tools (e.g., dCas9-effector). Since 2016, CRISPR-based gene-editing technologies have been successively used in clinical treatment with great success. CRISPR clustered regularly interspaced short palindromic repeats, Cas CRISPR-associated, dCas9 dead Cas9, PAM protospacer-adjacent motifs, CBE cytosine base editors, ABE adenine base editors, GBE glycosylase base editors. (Figure was created with Adobe Illustrator)
Early detection using CRISPR technology
A special sequence of repeated intervals.
Like many great discoveries, the discovery of CRISPR technology was born out of an unexpected event. An unusual sequence identified in the 3′ end structural domain of the iap gene was first reported by Nakata et al. in 1987 while studying the iap gene of E. coli . The sequence consisted of five highly homologous sequences containing 29 nucleotides separated by 32 nucleotides. 34
Over the next decade, this particular repeat sequence was detected in a variety of bacteria and archaea. 35 , 36 , 37 , 38 , 39 In 2002, Janson et al. provided a generalized summary of the specific repeats that have been identified, naming these repeats as a family and using the acronym CRISPR for clustered regularly interspaced short palindromic repeats. 40 In addition, multiple CRISPR-associated proteins (Cas)-Cas1 to Cas4- have been revealed in previous studies.
Bacterial and archaeal defense weapons
In 2005, researchers discovered that the spacer sequences in CRISPR are not unique to each organism. 8 Mojica et al. found that most of the spacer sequences were derived from exogenous DNA, with only a small fraction unrelated to the outside world, and they found that viruses were more likely to infect cells without homologous spacer sequences. 8 They conjectured that CRISPR is involved in bacterial resistance to infection by external phages and in plasmid transfer. 11 , 41 The conjecture was confirmed 2 years later. 42 , 43 , 44
When first confronted with phage or plasmid infestation, bacteria containing CRISPR sequences acquire a segment of their DNA sequence, which serves as a spacer region between special repeat sequences. CRISPR RNA (crRNA) then undergoes a series of transcription and maturation processes to produce a single crRNA containing a protospacer sequence of 20 bases that binds to the invading DNA via complementary base pairing. 45 , 46 Recognition of the exogenous sequence by crRNA alone does not protect it from the phage; it also must be inactivated by disrupting the exogenous sequence through the cleavage activity of the Cas protein. 47 , 48
The CRISPR/Cas family of proteins is divided into two categories based on genomic and protein structure information, and the best-known protein Cas9 is among the Class II CRISPR/Cas systems. 49 , 50 Class I is characterized by a large Cas9 protein complex that shears the DNA strand, while Class II requires only a single shearing protein. Cas9 is characterized by the presence of two ribonuclease structural domains, a RuvC-like nuclease domain near the amino terminus and the HNH nuclease domain in the middle of the protein, both of which have the function of cleaving the DNA strand. 51 Notably, protospacer sequences are not randomly acquired from exogenous sequences but are always accompanied by a guanine-enriched sequence called protospacer-adjacent motifs (PAMs). 43 Subsequent studies have shown that PAM sequences play an important role in the acquisition of the spacer region, where Cas proteins perform cleavage. 5 , 15 , 52
Contributions of Charpentier and Doudna
The functional mechanism of CRISPR/Cas9 has been gradually revealed, and natural CRISPR/Cas9 has been rapidly applied to bacterial transformation. 44 , 53 In 2011, Siksnys et al. transferred the first CRISPR gene sequence from Streptococcus thermophilus to E. coli , and the E. coli that received the CRISPR gene sequence successfully resisted plasmid transformation, which was the first report that CRISPR/Cas9 functioned in a nonhost bacterium. 54 This finding suggested that CRISPR/Cas systems can be used as a defense mechanism against external infection and that their hosts are not necessary for the CRISPR system to function.
In 2012, Charpentier and Doudna purified Cas9 from S. thermophilus and Streptococcus pyogenes , enabling the cleavage of prokaryotic DNA in vitro. 47 , 55 They also elucidated the mechanism by which CRISPR/Cas9 works, noting that the cleavage site of Cas9 is controlled by a seed sequence in the crRNA and requires the involvement of PAM. Additionally, by altering the nucleotide sequence of a seed sequence, the system can function as a gene silencer in a variety of situations, providing gene targeting and gene editing by changing a nucleotide seed sequence.
The boom in CRISPR technology
Gene editing in mammalian cells.
Previous research on CRISPR/Cas9 has focused on prokaryotic cells, and CRISPR technology started to be used in medicine, agriculture, and other fields in a paper published by Zhang Feng et al. in 2013. 56 They used human-derived 293 T cells, into which they integrated trans-activating crRNA (tracrRNA), pre-crRNA, host factor ribonuclease (RNase) III, and Cas9 from S. pyogenes and added the respective promoters and two nuclear localization signals (NLSs) to ensure the entry of the structure into the nucleus. 47 , 48 , 54 , 57 This experiment targeted 30 base pairs located before the PAM at the human empty spiracle homeobox 1 (EMX1) locus, and the results showed that cleavage of EMX1 was achieved with the inclusion of at least spCas9, tracrRNA and pre-crRNA. Additionally, the function of Cas9 from S. thermophilus was verified by Zhang Feng et al. and produced consistent results.
In another paper published the same year, Church et al. constructed crRNA-tracrRNA fusion transcripts that became single guide RNAs (sgRNAs) and shrank crRNAs to 20 bp. 58 These studies had significant implications, both confirming that CRISPR motifs function in mammalian cells and simplifying the CRISPR gene-editing system, thereby providing more possibilities for the use of CRISPR.
The transcriptional regulatory tool dCas9
The DNA strand cleavage function of Cas9 was elucidated by designing a simple sgRNA segment to guide Cas9 to the target site, but many additional studies on genes have been performed to address functions other than DNA strand cleavage. Qi et al. mutated the RuvC1 and HNH nuclease domains (D10A and H841A) of the wild-type Cas9 mentioned above, causing Cas9 to lose its cleavage enzyme activity. 24 dCas9 showed efficient gene silencing when sgRNAs were designed for nontemplate DNA strands, while sgRNAs designed for template strands did not effectively silence gene expression. The relative positions of sgRNAs and target gene promoter sequences also had a significant effect on silencing efficiency. Importantly, for the sgRNA targeting promoter sequences, gene silencing occurs regardless of whether the target is the template or nontemplate strand. In July 2013, another study by Qi et al. revealed that dCas9 interacts with effectors related to transcriptional regulation, such as VP64 and KRAB, to coregulate gene expression, which is currently the most common use of dCas9. 59
First research using CRISPR technology for disease treatment
In the months after CRISPR/Cas9 was shown to function in mammalian cells, scientists rapidly achieved gene editing in animals such as mice, fruit flies, and rats and plants such as rice and wheat. 12 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 Nevertheless, treating disease was the greatest expectation of CRISPR technology, and in December 2013, Wu et al. published a study using CRISPR/Cas9 to treat cataracts in a mouse model with cataracts caused by base deletions. 68 , 69 They coinjected the mRNA encoding Cas9 with an sgRNA into fertilized eggs of mice that would have cataracts, and of the 22 mouse pups obtained, ten carried the mutant allele, including six NHEJ-mediated insertions and deletions and four HDR-mediated repairs. 20 , 21 , 22 All four mice with cataracts repaired by HDR induction were cured, and two of the NHEJ-induced mice were successfully cured. Based on these results, CRISPR/Cas9 can modify the genome to treat genetic diseases. In another study conducted during the same period, Schwank et al. isolated intestinal stem cells from two patients with cystic fibrosis transmembrane conductor receptor (CFTR) mutations and corrected the disease-causing mutation using CRISPR/Cas9 technology. 70 , 71 , 72 , 73 In addition, they proposed a protocol for the in vitro editing of genetically mutated stem cells and their subsequent introduction into the body to treat disease, which was successfully implemented for clinical use several years later. 74
Single-base gene-editing technology
Although CRISPR/Cas9 has successfully cured some diseases caused by point mutations by cleaving the double strand for re-repair, the inefficiency and uncertainty of this approach have limited its application. In 2016, Komor et al. argued that the treatment of genetic diseases should correct the mutated base rather than excising it to allow random recombination. 25 , 47 Cytidine deaminase catalyzes the deamidation of cytosine into uracil, which subsequently changes back to thymine through replicative division. Thus, they integrated CRISPR/dCas9 with rat-derived cytidine deaminase (APOBEC1) and successfully achieved C to U base conversion. 75 , 76 This base-editing technique was also improved to enhance the efficiency and precision of base substitution. The invention of single-base gene-editing technology not only provides a predictable method of base substitution but also designs a base substitution architecture that facilitates the subsequent invention of more base substitution methods, which is important for the treatment of genetic diseases caused by base mutations.
The RNA editing tool Cas13a
CRISPR/Cas13a (formerly known as C2c2) is a Class II Type VI CRISPR/Cas family protein extracted from the bacterium Leptotrichia shahii. 28 , 77 It is characterized by the inclusion of two higher eukaryotic and prokaryotic nucleotide-binding (HEPN) domains that efficiently degrade almost all single-stranded RNAs, and the recognition of the target RNA by Cas13a is mediated by an sgRNA. 78 Previously discovered Cas-related proteins act on the DNA strand, and the discovery of Cas13a provides a novel approach to the recognition and detection of RNA viruses, such as SARS-CoV-2. 79 , 80 Cas13a has also been shown to reduce the efficiency of gene expression in a manner similar to RNAi but with greater specificity. 81
First clinical trial of CRISPR/Cas9 technology
The first clinical trial of CRISPR/Cas9 technology was conducted by Lu and colleagues at West China Hospital in Sichuan, China. In October 2016, Lu et al. injected CRISPR/Cas9 gene-edited T cells back into patients, the world’s first human injection of gene-edited cells. 82 The T cells used for gene editing were derived from patients, and plasmids encoding Cas9 and sgRNA targeting the PD-1 gene were transfected into the cells by electroporation. The data showed a significant reduction in PD-1 expression in the gene-edited T cells. 83 , 84 Follow-up studies of patients who received T-cell injections showed that the patients did not experience significant adverse effects due to receiving gene-edited T cells, and two of them were in a stable condition. This study indicated the feasibility and safety of the clinical application of gene-editing technology, which is very important to promote the clinical application of gene-editing technology.
CRISPR-based gene-editing tools
CRISPR gene-editing technology facilitates gene editing in eukaryotic cells. Researchers have studied the mechanism of action of Cas9 and have obtained Cas9 variants with different functions and some other derivative gene-editing tools through special modifications and have discovered other Cas proteins in the Cas9 family, enriching the types of genes that can be edited using CRISPR technology. Researchers have developed some vectors to assist in transport and safely deliver the CRISPR system to the body.
Composition of CRISPR/Cas9
When invaded by exogenous phages or plasmids, bacteria and archaea containing CRISPR obtain a foreign DNA fragment inserted into the spacer region. 11 Re-entry of the foreign nucleic acid homologous to the spacer region into the bacteria activates transcription of the CRISPR array to produce pre-crRNA. Pre-crRNA contains sequences with complementary base pairing to tracrRNA, the repeat region of the CRISPR array. 47 , 85 TracrRNA first binds to the Cas9 protein after transcription; then, complementary base pairing between pre-crRNA and tracrRNA forms a double-stranded RNA, and the pre-crRNA binds to Cas9. After binding occurs, RNase III builds pre-crRNA in the primary process, and Cas9 cuts excess repetitive and spacer sequences in the secondary process. 46 , 86 After the two processes, the crRNA matures and gains the ability to target the DNA strand. The backbone RNA (tracrRNA) and crRNAs that target specific sequences together comprise the sgRNA. 14 , 47 , 58
Researchers constructed a crRNA-tracrRNA fusion transcript to simplify the aforementioned process and facilitate the application of the CRISPR/Cas9 system in eukaryotes, which greatly simplified the process of crRNA processing and maturation. 58 , 87 By designing a crRNA targeting sequence of only 20 bp of bases next to the PAM site, almost any position containing the PAM site can theoretically be targeted. The major difference between CRISPR-based gene-editing technology and ZFNs and TALENs is that CRISPR-based gene-editing technology relies on the RNA-mediated recognition of the target DNA. 4 , 88 The design of the sgRNA is the key to whether CRISPR gene editing is successful at the target site.
The sgRNA is responsible for guiding the gene-editing system to the target site, while the modification of the target DNA strand is performed by Cas9. Using SpCas9 as an example, the binding of SpCas9 to the target DNA depends on the recognition of the PAM sequence downstream of the target site, which triggers the separation of double-stranded DNA. 89 , 90 The 10 bases proximal to the PAM on crRNA are called the seed sequence, and the seed sequence first binds to the DNA strand through complementary base pairing to begin forming its R-loop structure. 91 The distal DNA of PAM interacts with the structural domains of REC2 and REC3 of Cas9 to accelerate the formation of the R-loop, and the formation of the intact R-loop promotes the activation of the structural domains of the HNH and RuvC nucleases that catalyze the cleavage of the double-stranded DNA. 90 , 92 , 93 , 94 , 95 , 96 , 97
When using wild-type Cas9 for gene editing, such as SpCas9 ( S. pyogenes Cas9) and SaCas9 ( Staphylococcus aureus Cas9), off-target effects, chromosomal translocations, large segment deletions, and other abnormalities often occur. 98 , 99 , 100 Due to the limitations of the PAM, the CRISPR/Cas9 gene-editing system often fails to target the proper sites. Therefore, the modification of Cas9 focuses on two goals: enhancing the security of Cas9 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 and freeing it from the limitations of PAM 111 , 112 , 113 , 114 , 115 , 116 , 117 (Tables 1 , 2 ).
Method for CRISPR delivery
Plasmid DNA (pDNA) is an ideal vector for loading the CRISPR system because it is not easily degradable, can be amplified in large quantities, and can be easily modified. 118 After entering the cell, the plasmid carrying CRISPR/Cas9 enters the nucleus with the assistance of NLS and transcribes the mRNA encoding Cas9 and sgRNA. 119 , 120 This process is very tedious, and loading CRISPR/Cas9 tools on mRNA may greatly simplify this process. However, mRNA is easily degraded and has low stability. In particular, gene-editing tools that deliver Cas9 to function in concert with effector proteins are difficult to apply because the number of bases in the mRNA encoding Cas9 and effector proteins is too large. 121 , 122
Cas9 RNPs, known as ribonucleoproteins (RNPs), are complexes formed by fusing purified Cas9 with sgRNA in vitro, and RNPs function immediately after entering cells. 123 , 124 However, RNPs are relatively difficult to deliver into cells due to their complex composition and charge properties, whereas proteins and nucleic acids are usually delivered using electroporation with the assistance of cell-penetrating peptides. 125 , 126 With continuous innovations in delivery vectors, scientists have identified exosomes as a promising approach to deliver Cas9 RNPs. 127 , 128
Functional categories of CRISPR tools
Dna strand cleavage tool.
CRISPR/Cas9 was initially studied for its powerful double-stranded DNA cleavage function. The sgRNA directs Cas9 to a designated site where DSBs form flat ends in the presence of HNH and RuvC nuclease structural domains. Subsequently, DNA repair mechanisms are activated, mainly NHEJ and HDR. 21 , 129 , 130 The repair of DSBs by NHEJ is imprecise and often leads to base mutations that result in targeted mutations. HDR repair is a complex and precise process that can repair broken DNA strands correctly. The perfectly repaired DNA strand is indistinguishable from the target DNA and will be cleaved by Cas9 again until the sgRNA becomes unrecognizable. Fortunately, the chance of HDR occurring in mature cells is much lower than that of NHEJ. 68
Cas9 efficiently cleaves double-stranded DNA, but in practice, the sgRNA often mismatches with double-stranded DNA, leading to off-target effects. 5 In addition, a more efficient method to mediate mutational inactivation of genes is needed to enhance the efficiency of gene knockdown and reduce unnecessary cleavage. Cas9 nickase (Cas9n), a Cas9 variant with mutations in the nuclease structural domain RuvC (D10A) of Cas9, only creates breaks in DNA strands complementary to the crRNA. 131 DNA single-strand breaks are repaired by a high-fidelity base excision repair (BER) pathway, and thus two adjacent sgRNA/Cas9n complexes are designed to shear a single site, which effectively prevents Cas9-mediated damage to nontarget DNA and greatly enhances the specificity of Cas9. 132 An offset of an appropriate distance between two Cas9ns facilitates the efficiency of gene editing. Zhang Feng and colleagues designed an online tool ( http://www.genome-engineering.org/ ) for the design of two Cas9n sgRNAs to facilitate follow-up research. 131
In 2015, Zhang Feng et al. extracted Cpf1 (CRISPR from Prevotella and Francisella ), now known as Cas12a. 133 Cas12a, belonging to the Class II Type V CRISPR‒Cas Cas12a, is a Class II Type V CRISPR‒Cas system with the same ability to cut DNA double strands as Cas9 but differs to a great extent from Cas9. In bacteria, crRNA maturation of Cas12a does not require the involvement of tracrRNA and RNase III, and when the CRISPR array is activated for transcription, the pre-crRNA is cleaved directly by Cas12a into a 43 bp nucleotide sequence serving as the sgRNA. SgRNA/Cas12a recognizes a T-rich PAM sequence, usually 5′-TTTV-3′, located upstream of the target site, followed by crRNA binding to the DNA strand. Cas12a has only one nuclease structural domain, RuvC, mediating the cleavage of double-stranded DNA. 134 Unlike the flat ends produced by Cas9 cutting the double strand, Cas12a generates a sticky end interface similar to the double sgRNA-guided Cas9n described above, producing a 4–5 base overhang. 135 This approach presents the advantage that if the first DNA strand repair creates insertions or deletions (indels), the target position could still be repaired the next time by HDR. 136 The resulting sticky end interface may exert a positive effect on gene insertion in NHEJ, which must be confirmed in subsequent studies. 137 , 138 In conclusion, the discovery of Cas12a enriches the gene-editing tools based on the CRISPR system, and it is the first PAM-less G-rich Cas protein that has been identified, which has important implications for some unknown genes in the genome (Fig. 2a–c ).
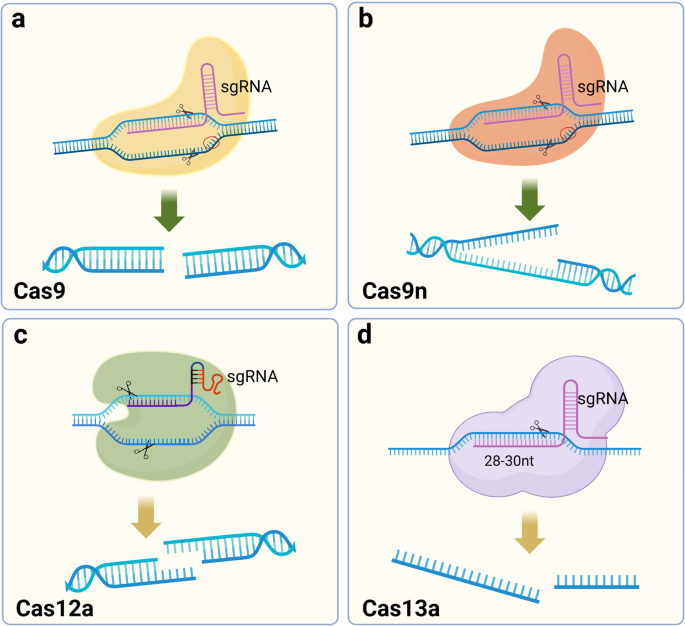
Schematic diagram of DNA strand cleavage tools. a Cas9 cleaves DNA double strands to form flat ends. b Cas9 nickase (Cas9n) cleaves the single DNA strand. c Cas12a cuts DNA double strands to form sticky ends. d Cas13a recognizes and cleaves RNA strands. (Figure was created with Biorender.com)
Regulation of gene expression and epigenetic modification tool
The ability of CRISPR/Cas9 to cleave double-stranded DNA depends on two nuclease structural domains, and mutating both nuclease structural domains results in dCas9 that loses enzyme-mediated cleavage activity. 24 These mutants are still able to bind to specific sites on the DNA strand under the guidance of RNA, which affects gene transcription, but more modestly, without severe off-target effects. Because Cas9 has been shown in other studies to load a number of proteins to reach a specific location in the genome and perform its function, designing a dCas9 incorporating transcription factors to regulate the expression of target genes is a potential research direction for realizing the application of the CRISPR/dCas9 system. 59 Many diseases are often accompanied by high expression of inflammatory factors or deleterious genes during the course of development, and measures to inhibit this activation or restore the expression of protective genes are important for targeting certain chronic diseases. 139 , 140 , 141 , 142 In addition, unlike CRISPR/Cas9 gene editing, because the genome has not been modified, CRISPR activation (CRISPRa) and CRISPR interference (CRISPRi) are reversible, which greatly reduces the unknown problems caused by off-target effects. 143 Since the base sequence of DNA is not directly changed, the efficiency of gene-editing limits the application of CRISPR/dCas9.
Gene regulation in eukaryotes is a complex process, and most genes are controlled by multiple regulatory elements interacting with each other. Epigenetic modifications also affect gene expression. Gilbert et al. fused dCas9 with multiple repressive chromatin modification domains and screened for a repressor domain KRAB (Krüppel-associated box) that significantly represses gene transcription. 59 CRISPR/dCas9 binding to activating structural domains also promotes gene expression; either VP64, composed of four copies of the transcriptional activator VP16, or the p65 activating structural domain enhance transcription. 59 A variety of activating or repressing structural domains have been developed to regulate gene expression. CRISPR/dCas9 is a universal transcriptional regulatory platform that can load activating or repressing structural domains to regulate gene expression. 144 , 145 , 146 , 147 , 148 In addition, epigenetic modifications may also be regulated by dCas9 loaded with epigenetic modification enzymes such as the DNA methyltransferase DNMT3A and acetyltransferase P300. 149 , 150 , 151 The risk of off-target effects of CRISPR/dCas9 is much lower than that of Cas9, and the effect is relatively efficient and mild, but the mechanism regulating gene expression is very complex. Thus, designing an sgRNA that targets one site may result in altered expression of multiple genes, and the risks must be further explored by performing more in-depth studies 152 (Fig. 3 ).
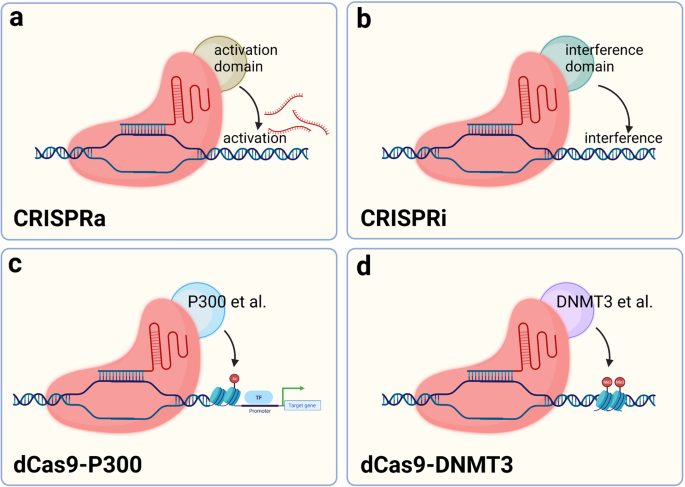
Schematic diagram of dCas9-based tools to regulate expression. a The dCas9 fusion VP64, VPR and other transcriptional activation effectors bind near the gene transcription start site to promote gene transcription. b dCas9 may be fused with KRAB or other transcriptional repressor effectors and bind to the gene transcription start site to silence gene transcription. c The complex formed by the fusion of dCas9 with P300 or other histone acetylases binds the gene transcription start site or enhancer region and promotes histone acetylation, which in turn enhances gene transcription. d dCas9 fused with DNMT3 and other DNA methyltransferases may bind the gene transcription start site to promote DNA methylation and thereby knock down gene transcription. (Figure was created with Biorender.com)
Base-editing technology
Many known genetic diseases are caused by a mutation in a base in a gene. The fundamental aim in treating these diseases is to restore the mutated base to the original base, not to cleave the DNA strand so that random repair mediated by HDR or NHEJ occurs, and the existing gene-editing tools are unable to achieve the desired function. 23 The five nucleotides are structurally similar to each other; for example, cytidine deaminase catalyzes the deamination of C into U. In the nucleus, U is replaced with T during cytokinesis, resulting in a C-G to T-A substitution, by agents later classified as cytosine base editors (CBEs). 153 , 154 , 155
Komor et al. selected APOBEC1 from rats as a first-generation base-editing tool (BE1) by comparing cytidine deaminase activity from humans, rats, and lamprey. 25 Catalytically inactive dCas9 was chosen as the target delivery vehicle to carry catalase, and 16-residue XTEN was also added as a stabilizer for this system. BE1 has good deamidation activity against nucleotides at the distal 4–8 positions of PAM, but in human genomic experiments, the conversion efficiency was only 0.8–7.7%. A possible explanation is that U is a base that does not belong in DNA and is easily repaired during DNA repair. In the second generation of base-editing tools (BE2), a stabilizer for U was added to prevent BER. 156 , 157 This improvement was successful, achieving a three-fold increase in the base substitution efficiency of BE2. Catalyzing a strand break complementary to the mutation site to replace G with A when BER occurs further improves the efficiency of base substitution. 24 , 131 Subsequent researchers have made some improvements to BE3 to enhance the efficiency of base editing, such as modifying and optimizing the nuclear localization signal, changing codons, and other methods, to improve the efficiency of BE3 base editing, reduce the formation of indels, and obtain more efficient gene-editing tools such as BE4max and BE4-Gam. 158 , 159 , 160 Kurt et al. successfully developed a gene-editing tool to induce a C-to-G substitution based on BE4max. 76 CBE frequently undergoes C-G mismutations during the process of achieving the C-to-T substitution, and the addition of two UGIs effectively stops this process. The human UNG (hUNG) enzyme with increased abasic site generation also has positive implications for base replacement between C and G. 160 Through a series of improvements, a novel base editor (BE4max (R33A) ΔUGI-hUNG complex (CGBE1)) was finally obtained. 76 This study improved the gene-editing tools for interbase substitution and facilitated the development of C-to-G base editors.
Achieving C-G to T-A and C-G to G-C substitutions is important for single-gene-editing efforts, but multiple types of base mutations cause disease, and achieving arbitrary substitutions between bases is an urgent task for applying CRISPR technology to disease treatment. 161 In 2017, Liu and his colleagues completed work to replace A-T base pairs with C-G base pairs. 26 Unlike the C-to-U substitution, which has been reported to occur only on free adenine, adenosine in RNA or adenosine in RNA‒DNA mismatches, no adenine deaminases are capable of deaminating A on double-stranded DNA. TadA is a tRNA adenine deaminase, and because of its homology to APOBECs, modifying TadA so that it can activate adenine deaminase activity on the DNA double strand is a promising approach. 162 , 163 When the antibiotic resistance gene in E. coli was mutated, E. coli survived only if they obtained the mutant site to achieve an A-to-I substitution. Using this method, researchers screened for TadA* capable of acting as a mutation on the DNA strand. During E. coli selection, the survival rate of E. coli in the presence of heterodimeric TadA-TadA* was higher, and the formation of heterodimers might significantly improve the editing efficiency of adenine bases. TadA-TadA*-Cas9n was finalized as ABE7.10 through several modifications. 152 Adenine is catalyzed by adenosine deaminase to become inosine, which eventually leads to the conversion of A-T to G-C. In subsequent studies of ABE, additional improvements were made to ABE7.10 to obtain a more efficient base-editing tool with fewer side effects. 159 , 164 , 165 , 166
The cytidine deaminase AID from a human source fused to the C-terminus of nCas9 efficiently achieves C-to-T editing. 167 However, in some strains, researchers have detected a high frequency of C-to-A mutations. CBEs added UGI to suppress the activity of the uracil-DNA glycosylase ( ung ) gene to increase the frequency of C-to-T mutations. 25 , 168 A high frequency of C-to-A mutations was observed in strains without suppressed ung activity, and this gene may be responsible for the C-to-A mutation. Finally, the Ung-nCas9-AID complex was constructed. This complex enables efficient C-to-A base substitution and fills a gap in single-base gene-editing technology. 75 Similarly, ung genes are involved in C-to-G base substitutions, and they construct the APOBEC-nCas9-Ung complex that allows efficient C-to-G substitutions. Researchers refer to this nCas9-cytidine deaminase- ung substitution as glycosylase base editors (GBEs).
Both ABE and CBE show efficient base substitution but do not achieve insertions, substitutions, and deletions between bases at will. Thus, a new single-base-editing technology may be needed. 169 The prime editor (PE) consists of two parts, a reverse transcriptase (RT) protein from Moloney murine leukemia virus (M-MLV) fused with Cas9n (H840A) and a 30 bp sgRNA (pegRNA), including a primer binding site (PBS) and an RT template. 27 , 170 , 171 , 172 , 173 After Cas9n reaches the designated position, it cuts the target DNA strand. PBS fixes the free 3′ DNA strand by complementary base pairing and reverse transcribes the new DNA strand with the RT template under the action of RT. Using this approach, arbitrary substitutions between bases are achieved, greatly increasing the applicability range of single-base gene editing, and base insertions and deletions can also be introduced. PE2 was obtained by optimizing M-MLV RT based on PE1, and the bases on the unedited strand must rely on DNA repair mechanisms to change. 27 BE3 in the system described above was modified by shearing the nonedited strand to obtain a much higher mutation efficiency than BE2. Therefore, in the improved PE2, another new sgRNA was added to cleave the nonedited strand to obtain PE3 and PE3b. 27 Although the editing efficiency of PE3/PE3b was increased by ~3-fold, Cas9 was unable to discriminate between these two different sgRNAs, introducing an unknown risk for this editing system (Fig. 4 ).
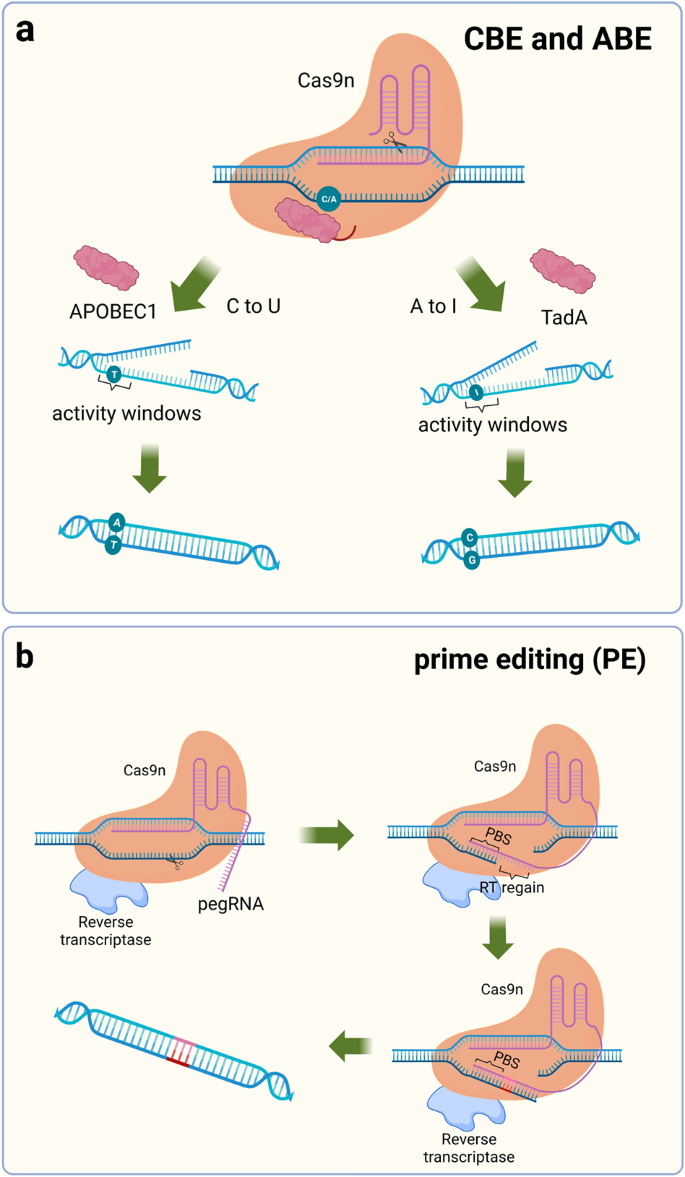
Schematic diagram of the single-base substitution tool. a Fusion of Cas9n with adenosine deaminase or cytidine deaminase enables the introduction of point mutations in the genome, APOBEC1 induces a C to U mutation, and TadA induces an A to I mutation. b PE contains a 30 bp segment of pegRNA, including the PBS sequence and RT region. PBS binds to the DNA strand and synthesizes the complementary strand of the RT region in the presence of reverse transcriptase. PBS primer binding site, RT reverse transcriptase, pegRNA prime editing guide RNA. (Figure was created with Biorender.com)
Tools for RNA strand identification and cleavage
CRISPR/Cas13a is an acquired immune defense mechanism for bacteria against RNA infestation. 28 Unlike Cas9, Cas13a recognizes the RNA strand and cleaves it using the HEPN nuclease structural domain. After cleaving the target RNA, the RNase activity is retained. The specificity is significantly reduced, leading to the cleavage of other nontarget RNAs, a phenomenon called collateral cleavage. RNA has an important role in the cell, and a relatively simple way to knockdown RNA has been developed based on a gene function screen. RNAi has good knockdown efficiency, but off-target effects are difficult to avoid. 174 , 175 CRISPR/Cas13a-based RNA gene-editing tools play a comparable role to RNAi but with much lower off-target efficiency. 81 Catalytically inactivated dCas13a, similar to dCas9, carries the corresponding effectors to regulate the function or translation of RNA, such as by regulating widespread m6A methylation on RNA, modifying the bases of RNA, and regulating protein translation 176 , 177 , 178 , 179 , 180 , 181 (Fig. 2d ).
CRISPR/Cas13a is also widely used to detect RNA. 78 In 2017, Zhang Feng and colleagues designed a nucleic acid detection tool called Specific High Sensitivity Enzymatic Reporter UnLOCKing (SHERLOCK) based on Cas13a. 182 They designed a reporter molecule that releases a fluorescent signal when the target single-stranded RNA (ssRNA) breaks, and they coincubated the constructed Cas13a, reporter molecule and crRNA with the target ssRNA and successfully observed the fluorescence; however, this approach is less sensitive. The amount of ssRNA detectable by Cas13a was increased by amplifying RNA using recombinase polymerase amplification (RPA) and T7 transcript binding to improve the sensitivity of Cas13a-based detection. 183 This method was improved for SARS-CoV-2 detection in 2020. 184
Carriers for delivering CRISPR technology
Plasmids or mRNAs loaded with CRISPR/Cas gene-editing systems are transfected into cells in vitro in the same manner as ordinary nucleic acids using transfection reagents, virus-mediated transfection, and other techniques. RNPs also enter cells through electroporation. However, most of these methods are less suitable in animals or humans. CRISPR tools undergo a long delivery process composed of three main phases to be effective in vivo: (1) the carrier must remain stable in the blood without degradation or immune clearance, (2) the carrier then accumulates in candidate tissues and triggers cell endocytosis, and (3) the CRISPR system escapes the lysosome into the cytoplasm to perform genome editing or regulate gene expression, particularly in the second phase of delivery, where enrichment in the target tissue is critical for successful delivery. The realization of this complex process requires the help of several delivery vehicles (Fig. 5 ).
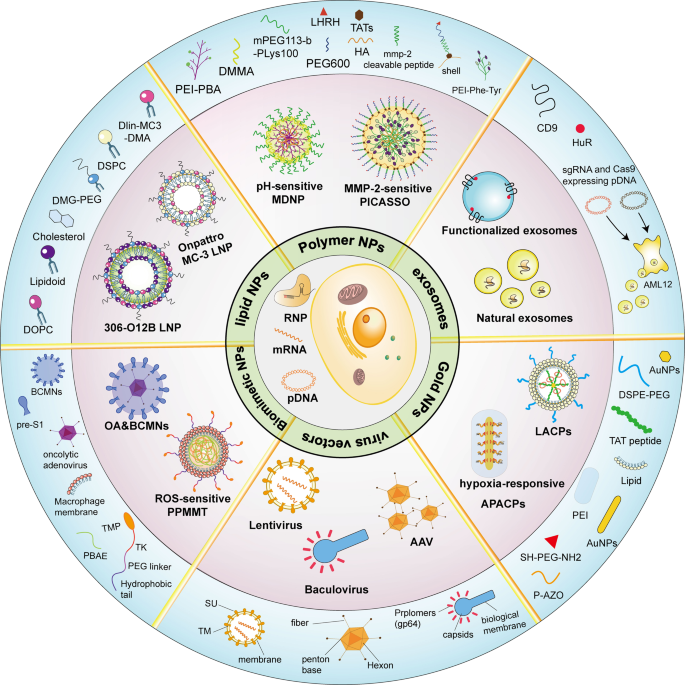
Schematic diagram showing multiple types of vectors for the in vivo delivery of CRISPR systems. The central region shows three forms of CRISPR action: pDNA, mRNA, and RNP. The middle circle section shows examples of delivery carriers, and the outermost area shows how the carriers are produced or the components. SU surface envelope protein, TM transmembrane envelope protein. (Figure was created with Adobe Illustrator and Biorender.com)
Virus vectors
In previous studies, viral vectors have been commonly used to deliver gene drugs. AAV is one of the most commonly used viral vectors for delivery, as it easily crosses the species barrier to infect cells and has very low immunogenicity, making it less likely to trigger an inflammatory response. 30 However, the CRISPR/Cas9 gene-editing system is very large compared to ordinary gene drugs, exceeding the maximum packaging capacity of AAV vectors by 4.7 kb. 185 In particular, when Cas9 carries effector proteins, special modifications are required for loading in AAV vectors, such as using the smaller SaCas9 or splitting the delivery system into two vectors. 186 , 187 , 188 Incorporating the coding sequence of the smaller Cas9 ortholog, SaCas9, into the regulatory cassette allows the coinclusion of effector-encoding sequences as epigenetic regulators to facilitate Cas9 regulatory activity while maintaining the plasmid size within the carrying capacity of AAV. For example, Himeda et al. established a CRISPRi system with dead SaCas9 (dSaCas9) and successfully inhibited the expression of full-length DUX4 mRNA (DUX-fl) in vitro, alleviating facioscapulohumeral muscular dystrophy (FSHD). 139 Double AAV vectors incorporate separately designed plasmids encoding a split Cas9 to accommodate the limited AAV carrying capacity, along with sgRNA. Upon cotransfection into a cell, the full Cas9 protein and sgRNA are produced to modulate gene expression; however, this method has a high risk of off-target effects.
Lentiviruses are retroviruses that infect dividing and nondividing cells and are therefore also often used as delivery vectors. 189 Due to the 10 kb loading capacity of lentiviruses, the entire CRISPR/Cas9 system can be loaded into it, but because lentiviruses integrate randomly into the host genome, they often trigger some immune responses and even cause cancer. 29 Baculovirus has also been used for CRISPR/Cas9 delivery. Baculovirus is a nonpathogenic insect virus with an extra-large loading capacity (~38 kb). 190 , 191 Moreover, as these viruses neither duplicate nor integrate into the genome, they have no heritability concerns. Nguyen et al. engineered baculovirus as a dCas9-VP64-p65-Rta (dCas9/VPR) delivery vehicle to significantly activate endogenous long noncoding RNA (lncRNA) differentiation-antagonizing nonprotein coding RNA (DANCR) in bone marrow-derived mesenchymal stem cells (BMSCs) and rat adipose-derived stem cells (rASCs). 192
Lipid-based nanocarriers
The first use of cationic liposomes for DNA transfection was reported in 1987 when Felgner et al. discovered the ability to use liposomes for gene delivery. 193 In the following decades, liposomes were frequently used as vectors for gene drug delivery, and liposome-based nanoparticles (LNPs) are considered promising tools for CRISPR/Cas9 transfer. 29 Unlike liposomes, LNPs do not have a continuous lipid bilayer and large inner aqueous pool, but they are mainly composed of lipid components such as natural phospholipids, cholesterol, and polyethylene glycol. 32 The simple synthesis of LNPs and their stable presence in serum have led to their frequent adaptation for the in vivo delivery of gene drugs. Unfortunately, since the liver is the dominant organ metabolizing lipids, lipid nanoparticles always show a high degree of enrichment in the liver. This targeting is very beneficial for the delivery of drugs for the treatment of liver diseases, but LNPs do not show high efficiency for diseases occurring in other organs. 194
Angiopoietin-like 3 (Angptl3) is an enzyme that regulates plasma lipoprotein levels. Loss of Angptl3 function reduces blood levels of triglycerides (TGs) and low-density lipoprotein cholesterol (LDL-C) without causing any clinical risk. Qiu et al. designed multiple LNPs for the delivery of Cas9 mRNA and an sgRNA targeting Angptl3. 195 , 196 A gold standard MC-3 LNP configured with cholesterol, DSPC and DMG-PEG was used as a control to screen for the most efficient 306-O12B LNP consisting of a leading tail-branched bioreducible lipidoid (306-O12B) and an optimized mixture of excipient lipid molecules. 197 The gene-editing efficiency of this LNP reached 38.5%, which is ~12 times that of MC-3 LNP. 195 The modification of LNPs to increase their enrichment in extrahepatic tissues might improve the scope of application of LNPs to deliver CRISPR/Cas9 for disease treatment. For example, Mohanna et al. constructed the novel LNP-based Incisive Delivery System (DS) that detected extensive genome editing in mouse corneas, 198 and Rosenblum et al. designed CRISPR-LNPs (sgPLK1-cLNPs) for tumor cells and observed ~80% gene-editing efficiency in tumor cells in vivo. 199
Polymer-based nanoparticles
Polymer nanoparticles have the advantages of low immunogenicity, good biocompatibility, and a high modification potential. 200 PLGA, chitosan, and other molecules, which are commonly used to construct polymer nanoparticle shells, improve the efficiency of polymer uptake by cells. The PEI of the core is often used as a transfection reagent for plasmid transfection, which is endocytosed by the cell and triggers the proton sponge effect into the cytoplasm. 201 In addition, polypeptides that recognize cell membrane surface receptors and polymers that are released by catabolism at specific pH, ATP, and hydrogen peroxide levels have be designed on polymer-based nanoparticle shells. 33 , 202 , 203 , 204 , 205
Liu et al. constructed a multistage delivery nanoparticle (MDNP) for delivering the CRISPR-dCas9 system. 206 They built the core-shell structure. The cationic polymer formed by PEI nanoparticles modified by phenylboronic acid (PBA) was used as the core. This core was then fused to the plasmid encoding dCas9 and sgRNA. The use of 2,3-dimethylmaleic anhydride (DMMA)-modified poly(ethylene glycol)-b-12 polylysine (mPEG113-b-PLys100/DMMA) as a shell wrapping the abovementioned cationic polymer allows the nanoparticles to exhibit different surface properties at different stages. The nanoparticles are injected into the bloodstream through the tail vein and stabilize in the bloodstream due to the negatively charged PEGylated surface of the shell. The tumor tissue has an acidic microenvironment (pH 6.5) in which the polymer shell rapidly dissociates and the core of the polymer becomes exposed due to a high level of surface sialylation on the surface of the cancer cells. The PBA moiety of the core binds to sialic acid, enhancing endocytosis by tumor cells. In cancer cells, PEI in the nucleus of the multimeric body escapes from lysosome via the proton sponge effect, causing water molecules and chloride ions from the lysosome to flow inward and plasmid DNA (pDNA) to successfully enter the cytoplasm of cancer cells. MDNPs overcome physiological barriers by changing the surface chemistry several times before finally entering tumor cells effectively. Changing the plasmids loaded with MDNP should allow it to become a novel technology for cancer treatment.
A dual-locking nanoparticle (DLNP) is another polymeric particle reported from the same team who developed MDNP. 207 DLNPs have a CRISPR/Cas13a core that targets PD-L1 in tumor cells. Cas13a enters tumor cells and is activated upon specific recognition of the PD-L1 mRNA. Activated Cas13a nonspecifically cleaves RNA and triggers the apoptosis of tumor cells. The tumor microenvironment has many typical features, and the slightly acidic environment may serve as a marker for polymer-based nanoparticles to discriminate tumors. In addition, reactive oxygen species (ROS) are also present at higher levels in the tumor environment than in normal tissues, and ROS also promote cellular DNA mutation and tumorigenesis. 208 The authors designed a responsive shell that disintegrates only under specific ROS and pH conditions to minimize irreversible damage to cells in other organs due to DLNP off-targeting. After DLNPs enter the body through the bloodstream, they are protected from immune clearance due to the presence of polyethylene glycol on their surface. When DLNPs reach the tumor through the blood, the microacidic environment and high ROS concentration in the tumor drive the disintegration of the DLNP shell, exposing the polymer core of the PEI/Cas13a complex. Eventually, the core is internalized into the tumor cells and released into the cytoplasm through the proton sponge effect.
The application of the CRISPR/dCas9 system in regenerative medicine is a hot topic. A layer-by-layer self-assembled peptide (SAP) coating was prepared on nanofibers and used to deliver the CRISPR-dCas9 system to promote the neurite growth of rat neurons. 209 Polycaprolactone (PCL) has several advantages that make it ideal for delivering the CRISPR-dCas9 system, including good stability, easy processing, good biocompatibility, and the ability to biodegrade. 210 However, experiments inspired by mussel adhesion chemistry showed that PCL does not readily adhere to cells. Zhang et al. developed a new method for PCL attachment using a layer of negatively charged amphiphilic SAP, and the pDNA encoding the CRISPRa system and SAP-RGD was absorbed through electrostatic interactions. 209 The RGD polymorphism supported cell adhesion and proliferation, effectively resolving the deficiency in PCL adhesion. SAP has a good affinity for many other biological peptides, and attaching SAP coatings to PCL is expected to be a routine strategy employed for in vivo targeted delivery of CRISPR/Cas9.
Natural and functionalized exosomes
Exosomes are membrane-bound vesicles that are 30–100 nm in diameter and originate from multivesicular bodies (MVBs) in organelles. 211 , 212 In living organisms, exosomes serve as a medium for intercellular transfer of proteins, lipids, nucleic acids, and other intracellular factors and carry virtually any biological component, including plasmids, with minimal side effects. 213 , 214 As exosomes also directly package sgRNAs and Cas9, thereby effectively decreasing the risk of off-target side effects during transport, they constitute a promising vehicle for CRISPR/dCas9 system delivery. Moreover, because exosomes retain proteins and lipids reflecting those of the parent cells, they preferentially interact and fuse with the parent cell type. 215 , 216
Hepatic stellate cells (HSCs) secrete a large number of exosomes, and the exosomes secreted by these immortalized cells are less different from each other and more workable. 128 RNP is packaged in exosomes by electroporation to obtain the genome editing system exosome RNP . Wan et al. designed sgRNAs targeting p53 upregulated modulator of apoptosis (PUMA), Cyclin E1 (CcnE1), and K (lysine) acetyltransferase 5 (KAT5), which play important roles in liver disease development, in combination with Cas9 to construct the RNP. 217 , 218 , 219 A significant decrease in the expression of all three genes was detected. Exosome RNP is highly enriched in the liver and is an ideal vehicle for the targeted treatment of liver diseases such as cirrhosis and liver fibrosis. Nevertheless, artificial modifications are needed to enhance the exosome carrier targeting ability for certain cell types with low exosome secretion. Genome editing with designed extracellular vesicles (GEDEX) was developed for dCas9/VPR delivery to increase the delivery efficiency and precision. 220 Conversion of hepatic stellate cells (HSCs) to myofibroblasts (MFBs) is an important marker of liver fibrosis formation. 221 Upregulation of growth factor expression in endogenous hepatocytes effectively repairs liver injury in mice. GEDEX is similar to naturally occurring exosomes and thus can be modified to target a wide range of cells in vivo, highlighting its considerable potential for future clinical application.
Li et al. constructed a novel exosome by fusing the CD9 C-terminus with human antigen R (HuR) to improve the encapsulation ability of exosomes. 222 The length of the dCas9 mRNA increases the difficulty of encapsulating molecules in exosomes using methods such as electroporation, and the HuR recognition motif on this novel exosome facilitates dCas9 loading and thus shows significant promise for the targeted delivery of CRISPR/dCas9 systems to treat diseases.
Exosomes are endogenous delivery vehicles. They are less impeded due to their compositional similarities to cell membranes and are therefore less likely to be cleared by the immune system during cargo delivery than viral vectors, lipid nanocarriers, and polymorphic nanocarriers.
Gold nanoparticle delivery systems
Gold nanoparticles can be customized in size, and different sizes have different physical and chemical properties. One of the most typical features is that the surface electrons of Au nanoparticles (AuNPs) resonate at a frequency determined by the size of the NP, a phenomenon known in the scientific community as surface plasmon resonance. 223 , 224 Surface plasmon resonance is the most important application of AuNPs, which are used to prepare various low-cost sensors that can be observed with the naked eye. 225 , 226 Moreover, gold nanoparticles have good stability and biocompatibility, and surface modifications can easily be added, which makes them ideal carriers for delivering gene drugs. 227 In the treatment of diseases such as tumors, for example, the surface of AuNPs may be decorated with specific cancer cell ligands to enhance their recognition of cancerous tissue. In addition, gold nanoparticles themselves have anti-inflammatory and antibacterial properties, which are beneficial in the treatment of tumors.
Wang et al. combined lipid nanoparticles with good stability and a high drug loading rate with AuNPs that were released in vitro in a controlled manner. 228 A lipid-encapsulated AuNP/Cas9-sgPlk-1 plasmid (LACP) that was targeted for delivery to melanoma-bearing sites was constructed. First, the authors prepared AuNPs with a diameter of ~20 nm, which were attached to the TAT peptide, increasing the uptake of NPs by cells. 229 AuNPs form the core of the polymer through electrostatic interactions with negatively charged pDNA, and finally, the core is wrapped with cationic liposomes and then modified with PEG2000-DSPE to form LACP. The lipid shell stabilizes the structure of LACP and enhances cellular internalization. TAT directs nuclear targeting, and the AuNP core acts as both a carrier and a responder to photothermal conditions to release pDNA.
Biomimetic nanomaterials
The stable presence of nanomaterials in the circulation and their ability to undergo enriched accumulation at specific sites in the body can enhance the therapeutic efficacy of CRISPR gene-editing drugs. 230 However, carefully designed organic or inorganic carriers are inevitably partially cleared by the immune system in vivo. Researchers have expressed widespread interest in the use of a material from the organism itself, a biofilm that serves as a “pocket” for the contents of the cellular envelope, to prevent recognition by the immune system. 231 When a disease occurs, immune cells are usually triggered to enter the disease site and exert anti-inflammatory effects. The encapsulation of nanomaterials in the cell membranes of these cells not only prevents possible immune clearance but also enhances the enrichment of gene drugs at the disease site. 232
Yan et al. used the cationic polymer poly (β-amino ester) (PBAE) in complex with a plasmid encoding the CRISPR system as the core and covered the surface of the PBAE/pDNA complex with a macrophage membrane. Finally, the ROS response element (BAM-TK-TMP) was fused to the outer surface of the cell membrane. 233 In this bionanomaterial, the macrophage membrane targets inflammatory lesions, and TMP recognizes high ROS levels to promote the cellular internalization of nanoparticles. TMP may be tailored as an effector in response to multiple pathological or physiological conditions. 234 , 235 , 236
The occurrence of disease in vivo involves multiple genes, biochemical properties, and changes in the microenvironment. This complex mechanism poses great difficulties to drug delivery carriers, and LNPs, gold nanoparticles and bionanomaterials each have their own advantages and limitations. Moreover, various nanocarriers can be connected together, and the construction of composite nanoparticles can employ the different advantages of the carriers and enhance the delivery efficiency. 237 For example, Zhang et al. fused poly(ethylene glycol) methyl ether-block-poly(lactide-co-glycolide) (PEG-b-PLGA;PP)-based nanoparticles with PEI to obtain composite lipid and polymer nanoparticles: PP/PEI. 238 PP/PEI prevents the increased enrichment of lipid nanoparticles in the liver and allows efficient genome editing in the lung, heart, and blood vessels of adult mice after the administration of a single dose. In a follow-up study, the researchers also found that polyethylene glycolized nanoparticles have the ability to inhibit gene drug aggregation in individual organs and increase the duration of circulation in the body. In addition, the aforementioned delivery vehicle, LACP, is also a composite nanoparticle of liposomes and AuNPs. 228 In conclusion, the rational use of the advantages of various nanoparticles to design nanocarrier structures facilitates the combination of several excellent platforms for delivering gene drugs individually, which improves the efficiency of gene drug delivery and helps optimize the therapeutic effects of gene drugs (Table 3 ).
Application of gene-editing tools
In the preceding sections, we summarized a variety of genome-altering gene-editing approaches involving CRISPR systems and summarized the vectors available for delivering CRISPR tools in vivo or in vitro, including some brief descriptions of the characteristics, improvement options and applicability of these vectors. Next, we analyzed the alteration of the disease microenvironment or the salient features of diseased cells at the onset of some diseases from the disease perspective. We summarize the most promising CRISPR gene-editing tools with targeted delivery vectors for different types of diseases to facilitate subsequent studies.
Attempts to treat diseases
Cancer, a disease with high incidence and mortality rates, is standardly treated using surgical resection, radiotherapy, and chemotherapy; however, the latter two treatments engender serious side effects. 239 The process of cancer development is usually accompanied by abnormal expression of large numbers of genes, such as P53, Notch, and PD-L1. 240 , 241 , 242 , 243 In addition, the microenvironment in which tumorigenesis occurs also exhibits some abnormal changes. These characteristics are used to construct a nanoparticle that is released in a specific environment and efficiently deliver gene drugs to tumor cells. In tumor cells, aberrantly expressed genes may be silenced or overexpressed using CRISPR technology (Fig. 6b ).
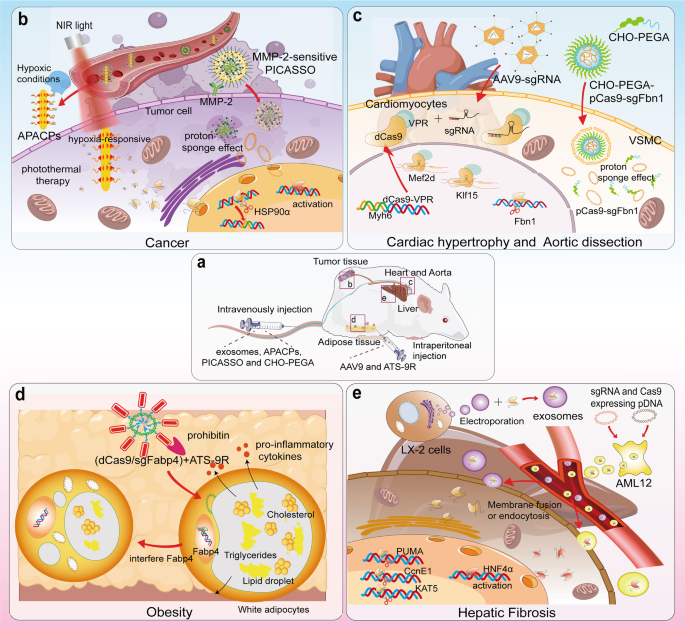
Delivering the CRISPR/Cas9 system to treat cancer, liver fibrosis, obesity, and cardiovascular diseases. a APACPs, exosomes, PICASSO and CHO-PEGA were intravenously injected into mice, and AAV9 was intraperitoneally injected. b APACPs are transported through the blood circulation to the tumor tissue, and hypoxic conditions promote the entry of APACPs into tumor cells. NPs release RNPs, silence the expression of HSP90α and reduce the hyperthermia tolerance of tumor cells. Externally applied NIR-induced photothermal therapy kills tumor cells. PICASSO responds to MMP-2 on the tumor cell membrane, and the shell disintegrates and the core enters the cell by endocytosis. The plasmid escapes from the lysosome into the cytoplasm through the proton sponge effect. c AAV9 delivered sgRNAs targeting Mef2d and Klf15 into dCas9-VPR transgenic mice. dCas9-VPR was synergistically transcribed with Myh6 and therefore specifically activated the expression of Mef2d and Klf15 in cardiomyocytes. Lipid nanoparticles CHO-PEGA deliver CRISPR/Cas9 to vascular smooth muscle cells in aortic coarctation to knockdown Fbn1. d Adipocyte targeting sequence to 9-mer arginine (ATS-9R) recognizes forbidden elements expressed at high levels in adipose tissue and delivers plasmids into white adipocytes, which contain huge lipid droplets and large amounts of triglycerides and cholesterol and release large amounts of inflammatory factors. After interfering with the expression of fatty acid binding protein 4 (Fabp4), the size of lipid droplets in white adipocytes decreases, and the release of inflammatory factors is inhibited for the purpose of treating obesity. e The exosomes secreted by LX-2 cells were extracted, and RNPs were loaded into the exosomes by electroporation. In studies targeting the knockdown of PUMA, CcnE1, and KAT5, exosomes were effective at alleviating liver diseases such as liver fibrosis. In vitro transfection of plasmids encoding sgRNA and dCas9/VP64 into mouse liver AML12 cells resulted in the secretion of AML12 exosomes carrying the CRISPR/dCas9 system. Delivery of these exosomes to HSCs elevated HNF4α expression and prompted cell differentiation into hepatocytes. (Figure was created with Adobe Illustrator and Biorender.com)
Rapid cell proliferation is a distinct feature of cancer development, a process that requires high oxygen consumption, leaving the tumor in a hypoxic microenvironment. 244 Photothermal therapy is defined as the delivery of infrared light-responsive nanomaterials to the body and the external application of infrared light to produce localized heat in the body and ablate tumors. 245 However, tumors can tolerate high temperatures up to 50 °C, which may lead to damage to paracancerous tissue. Therefore, reducing the temperature tolerance of tumor cells and using photothermal therapy at moderate to low temperatures may effectively hamper tumor development. The protein heat shock protein 90α (HSP90α), which is associated with cellular heat tolerance and is overexpressed in tumor cells, was obtained in a screen. Li et al. constructed a hypoxia-responsive nanoparticle based on gold nanorods that carried a CRISPR/Cas9 system to target HSP90α for knockdown. 244 After the vector entered the tumor cells, Cas9/sgRNA RNP was released into the cells, silencing HSP90α and causing the cells to lose their thermotolerance. Finally, infrared light activated the gold nanorods to ablate the tumor.
T lymphocytes play an important role in cancer immunotherapy. 246 The T-cell receptor (TCR) on the surface of the T-cell membrane recognizes peptides or antigens that bind to MHC molecules to identify and kill tumor cells. 247 For example, the surface of myeloma, melanoma and sarcoma cells contains NY-ESO-1 as an antigen that binds the MHC molecule. 248 , 249 , 250 However, mismatches between the therapeutic TCRα and β chains in T cells and endogenous TCR chains (TRAC and TRAB) reduce the expression of surface TCRs. 251 , 252 In addition, one study found that T cells from PD-1-deficient mice were more potent against cancer. 253 CRISPR was then used to knock out genes encoding TRAC, TRAB, and PD-1 (such as PDCD1) in vitro to improve the safety and efficacy of engineered T cells. This therapeutic strategy, called TCR-T therapy, significantly inhibited the growth of both hematologic and solid tumors. In addition, another study modified T cells to treat tumors, called CAR-T cells. 254 Tumor cells are killed by inserting a CAR gene-targeting CD19 into T cells, but the experiments have been indicated to be ineffective against solid tumors. Both TCR-T and CAR-T cells have been approved by the FDA and have been proven to be effective and safe in clinical trials. Other characteristics of tumor tissue or cells also have the potential to be applied for targeted drug recognition, such as membrane surface receptors, the tumor microenvironment, and proteins undergoing specific modifications. 255 , 256
Liver diseases
The liver is the main organ in the body that metabolizes lipids, and liposomes and lipid nanoparticles without special modifications are more likely to be enriched in the liver. 257 , 258 , 259 In addition, many cells in the liver secrete large amounts of exosomes, and these hepatocyte-derived exosomes with a homologous tissue targeting ability will be more easily enriched in the liver. 260 In contrast to synthetic lipid nanoparticles, these naturally occurring exosomes are originally carriers for the intercellular delivery of proteins, nucleic acids, and other molecules. Therefore, natural exosomes are very safe and are rarely cleared by the immune system (Fig. 6e ).
Various cell types in the liver produce or take up exosomes. 261 Thus, as exosomes from HSCs participate in establishing liver fibrosis, hepatocyte-derived exosomes may carry therapeutics for delivery into HSCs. Endogenous liver exosomes derived from the mouse liver AML12 cell line are thus safer and more effective as vectors. Encapsulation of the CRISPR-dCas9-VP64 system into AML12-derived exosomes successfully activated the expression of hepatocyte nuclear factor 4α (HNF4α), a transcriptional regulator of hepatocyte differentiation, in HSCs and a mouse model of liver fibrosis, thereby significantly attenuating liver fibrosis. In another experiment for the treatment of liver fibrosis, Luo et al. transfected plasmids expressing dCas/VP64 and sgRNA into the mouse hepatocyte line AML12. 261 The presence of dCas9 was detected in exosomes, suggesting that RNPs with transcriptional activity can be loaded in exosomes.
Phenylketonuria (PKU) is an autosomal recessive liver disease in which phenylalanine hydroxylase (PAH) enzyme deficiency results in decreased phenylalanine metabolism, causing hyperphenylalaninemia. 262 Repair of mutated bases using a single-base editor that converts C-G base pairs to T-A base pairs restores PAH expression and increases the reduced level of phenylalanine in blood. Villiger et al. used AAV carrying a single-base gene editor delivered to the Pah enu2 mouse model, and 63% of the mRNA had the corrected base sequence, a result that confirmed the effectiveness of this gene-editing system for the treatment of PKU.
Hepatitis B virus (HBV) is a serious threat to people’s health, and long-term treatment with drugs such as interferon may lead to a significant increase in viral resistance. 263 Moreover, these treatments do not eliminate HBV covalently closed circular DNA (cccDNA), and targeted destruction of cccDNA using Cas9 is an effective method for treating HBV. Wang et al. designed an infrared light-responsive bionanoparticle for delivery of the CRISPR/Cas9 system to HBV-infected cells. 264 This device effectively inactivated HBV cccDNA. CRISPR-based editing technology has shown significant efficacy in treating a variety of liver diseases and is an effective strategy for future treatment of these diseases.
Cardiovascular diseases
Cardiovascular disease is one of the major causes of death in humans. 265 , 266 Common cardiovascular diseases include atherosclerosis, myocardial hypertrophy, heart attack, and aortic dissection. 267 , 268 , 269 , 270 , 271 However, unlike tumors and liver diseases, blood flow in the heart and blood vessels is faster and blood pressure is greater, posing a challenge for nanoparticle enrichment at the lesion site 272 (Fig. 6c ).
During fetal development, the number of cardiomyocytes expands rapidly, whereas cardiomyocytes gradually lose their ability to proliferate with aging. 273 Although evidence of cardiomyocyte renewal has been obtained in many mammals, restoring the loss of cardiomyocytes caused by cardiomyopathy is not sufficient. Restoring the expression of genes associated with cardiomyocyte proliferation, such as myocyte enhancer factor 2 D (Mef2d) and Krüppel-like factor 15 (Klf15), in cardiomyocytes of adult animals may exert a positive effect on curing cardiomyocyte-related diseases. 274 , 275 Direct delivery of CRISPRa systems into cardiomyocytes is relatively difficult and may also lead to widespread off-target effects. Schoger et al. first constructed a dCas9/VPR transgenic mouse, and this sequence was inserted after the myosin heavy chain (Myh) 6 promotor and transcribed in concert with Myh6. 276 Since Myh6 is a cardiomyocyte-specific gene, expression of the dCas9/VPR system occurs only in cardiomyocytes. 277 The subsequent injection of AAV9 carrying sgRNA activates the transcription of cardiomyocyte proliferation-related genes in the cells. Using this approach, a cardiomyocyte-specific expression activation system was obtained, and the timing of transcriptional activation was controlled, providing an important reference for cardiovascular studies or studies of other organs or tissues that are difficult to access directly.
The development of aortic disease is usually accompanied by the inflammation of vascular endothelial cells and the phenotypic transformation of smooth muscle cells in the vascular mesoderm. 278 , 279 Zhang et al. constructed a hydroxyl-rich lipid nanoparticle capable of delivering CRISPR/Cas9 to vascular smooth muscle cells. 280 In another study, Zhao et al. combined lipid nanoparticles and polymer nanoparticles to construct a delivery vehicle for endothelial cells. 238 Although the CRISPR-based gene drug was successfully delivered to VSMCs and endothelial cells, the nanoparticles were still taken up by the liver in large quantities.
In addition, CRISPR/Cas9 technology is also widely used for bone regeneration and the treatment of CFTR, Alzheimer’s disease, obesity, and other diseases 72 , 73 , 192 , 281 , 282 , 283 , 284 , 285 (Fig. 6d ).
FDA-approved clinical treatments and diagnostics
Sars-cov-2 detection.
Coronaviruses can cause life-threatening respiratory infections in humans and have caused three epidemics in the 21st century: severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV), and still-unbeaten SARS-CoV-2. 286 , 287 , 288 All three highly pathogenic viruses belong to Betacoronavirus; however, high pathogenicity is not the greatest difficulty for humans to overcome. The susceptibility of humans to SARS-CoV-2, its ease of transmission, and its long incubation period make this virus difficult to eradicate. Therefore, the development of a test that rapidly detects SARS-CoV-2 in patients is more important than a treatment. RT‒PCR technology is a PCR method developed specifically for RNA detection that is convenient and reliable and is the current gold standard for detection. However, this assay requires a rigorous, high-level testing platform, which limits the number of people who can be tested. 289 , 290 Antigen antibody assays have also been used for SARS-CoV-2 detection but are costly and not suitable for large-scale application. Therefore, a new low-cost method that does not require an instrumental platform must be developed.
SHERLOCK is an RNA detection tool previously constructed by Zhang Feng et al. based on Cas13a. 182 However, SHERLOCK detection relies on multiple steps of RNA extraction and liquid handling, which may easily lead to cross-contamination or even infection of the assay personnel if not performed properly. The improved STOP (SHERLOCK testing in one pot) method based on SHERLOCK simplifies the detection method and increases the sensitivity of virus detection using isothermal amplification (LAMP). 184 , 291 LAMP operates at 55–70 °C, and thus the heat-tolerant Cas12b from Alicyclobacillus acidiphilus (AapCas12b) became the protein of choice for STOP. 292 Researchers then adopted the magnetic bead purification method to obtain RNA and concentrated the collected samples into the STOPCovid reaction mixture, which further shortened the detection time by directly using magnetic beads to adsorb RNA. Finally, STOPCovid.v2 was developed as the detection solution for SARS-CoV-2 based on CRISPR technology. In clinical assays, the method achieved a sensitivity of 93.1% and specificity of 98.5%, which were higher than the values of RT‒PCR. STOPCovid.v2 is a remarkable breakthrough that requires only a few simple instruments to perform the assay, and the results are easily distinguishable using test strips. It may become a “sharp sword” for humans to overcome SARS-CoV-2.
Sickle cell disease and β-thalassemia
Sickle cell disease (SCD) and transfusion-dependent β-thalassemia (TDT) are both caused by mutations in the hemoglobin β subunit gene and are among the most common single-gene genetic disorders worldwide. 293 , 294 Sickle cell disease is characterized by an imbalance in the hemoglobin chain and hemolytic anemia, which is usually treated by blood transfusion and iron-chelation therapy. Patients with β-thalassemia have sickle-shaped red blood cells that carry less oxygen and usually experience pain, and thus they are usually treated in the clinic with hydroxyurea, pain relievers, and blood transfusions. Bone marrow transplantation has also been used to treat both diseases, but matching is difficult. 74 When the pathogenesis of these two hematological diseases was studied at the genetic level, the transcription factor BCL11A was identified as a suppressor of fetal hemoglobin and γ-bead protein expression, and maintaining high levels of expression of these two proteins alleviated the symptoms of sickle cell disease and β-thalassemia. 295
In 2019, Wu et al. used CRISPR/Cas9 to cleave the BCL11A enhancer sequence in HSCs and successfully downregulated its expression without inducing significant side effects. 296 In December 2020, clinical data were released for a gene therapy called CTX001, a one-time therapy for SCD and TDT developed by CRISPR Therapeutics in association with Vertex Pharmaceuticals. 74 This clinical trial used Cas9 to cleave the BCL11A enhancer region in hematopoietic stem and progenitor cells (HSPCs), causing them to lose enhancer activity. This technique reduced the expression of BCL11A and restored the production of γ-hemoglobin and fetal hemoglobin. Subsequently, the researchers transplanted the edited HSPCs into two patients who had SCD and TDT. The follow-up study found that fetal hemoglobin levels exhibited a substantial elevation in both patients at 12 months postinjection. At the final follow-up visits after 18 and 15 months, both patients had achieved normal fetal hemoglobin levels. Subsequent treatment of eight patients yielded similar results to the first two patients, indicating the general applicability and efficiency of this strategy. However, this method is not absolutely harmless, as both of the initial patients experienced varying degrees of adverse effects, which were not life-threatening and resolved after treatment. In another clinical study for the treatment of SCD, an approach using RNAi to knock down BCL11A was used. 297 They constructed a lentiviral vector carrying short hairpin RNA (shRNA) and used this lentivirus to transduce CD34 + cells from SCD patients, and clinical success was also achieved.
Transthyretin amyloidosis
Transthyretin (TTR) amyloidosis is an autosomal dominant disorder mainly caused by the deposition of amyloid fibrils around cells that mainly threatens the human nervous system and heart. 298 , 299 , 300 In the normal state, TTR monomers are synthesized in the liver to form tetrameric complexes that are involved in the transport of thyroid hormones. Mutant TTRs do not stabilize the tetrameric structure and dissociate before reassembling into amyloid fibrils. However, patients with TTR amyloidosis do not show significant symptoms of thyroid hormone deficiency, suggesting that TTR may not be a major carrier of thyroid hormones and that reducing TTR expression may be a possible approach to treat this disease. 298 , 301 The main clinical treatment options are liver transplantation and stabilization of tetramers with the small molecule tafamidis; however, the latter is not a stable and effective approach. 31 The FDA has also approved the siRNA drug patisiran for the treatment of this disease. Patisiran blocks the translation of TTR to slow the disease process, but repeated injections are required throughout the patient’s life. 302 , 303
Since low TTR expression has no significant side effects, adopting a modality that permanently eliminates the mutated TTR gene may be able to eradicate TTR amyloidosis. In June 2021, Gillmore et al. reported the results of clinical trials for the in vivo delivery of a CRISPR-based gene-editing drug named NTLA-2001. 31 NTLA-2001 consists of a liver-targeting LNP encapsulating sgRNA against the TTR gene and mRNA for SpCas9. This LNP has been used several times to carry gene drugs for delivery to the liver. 304 , 305 In preliminary experiments in animal models, NTLA-2001 showed efficient permanent knockdown. Six patients were selected for treatment in this trial, and all patients received the drug injection without adverse effects during the treatment course. On the seventh day of receiving the drug, the patients’ blood indicators and liver function indicators were within normal limits. Three patients received a dose of 0.1 mg per kg, and the other three received a dose of 0.3 mg per kg to determine the efficacy of NTLA-2001. On day 28, 47%, 52%, and 56% reductions in blood TTR concentrations were detected in the three patients who received the low dose and 80%, 84%, and 96% reductions were detected in the three patients who received the high dose. This finding indicated that the efficacy of NTLA-2001 is dose-dependent and highly successful. A few months later, the method was granted orphan drug designation by the FDA, a recognition not only of NTLA-2001 but also of the in vivo delivery of CRISPR-based gene therapy.
In 2019, Maeder et al. developed a genome-edited therapy (EDIT-101) to treat Leber congenital amaurosis type 10 (LCA10). 306 They used an AAV5 vector loaded with saCas9 and sgRNA targeting the CEP290 mutant intron to deliver this gene-editing system into photoreceptor cells via a subretinal injection to delete or inactivate the mutated intron and restore normal expression of CEP290. The first clinical dosing of EDIT-101 was completed in March 2020. In September of the same year, clinical results for EDIT-101 showed that of the two groups receiving different doses, the mid-dose group experienced a more pronounced therapeutic effect, but the low-dose group experienced a poor therapeutic effect. Fortunately, none of the patients showed any serious adverse effects.
Human immunodeficiency virus (HIV) is a retrovirus that integrates into the host genome after infection and follows replication. 307 Antiretroviral therapy (ART) has shown good results in curbing HIV replication and improving immune function, but ART only controls the progression of HIV. 308 , 309 The HIV genome must be removed from the human genome to completely cure HIV infection. In 2020, Mancuso et al. reported the results of a study using AAV to deliver CRISPR/Cas9 in nonhuman primates for the treatment of HIV, and the results revealed that it is a viable strategy. 310 In September 2021, the FDA approved a CRISPR gene-editing technology-based therapy for the treatment of HIV infection (EBT-101).
In addition, in August 2022, the FDA approved a clinical application for CRISPR therapy CRD-TMH-001 for the treatment of Duchenne muscular dystrophy (DMD).
Together, these results suggest that the construction of an efficient, safe and stable targeting vector to deliver the CRISPR‒Cas9 system to the body is a promising new approach to treating diseases. Therefore, a number of delivery vehicles have been designed and manufactured for targeted delivery to disease sites according to the specific characteristics of a disease. These drugs show a good loading capacity and good prospects for clinical translation. Future research directions include improving vector targeting and designing more efficient CRISPR gene-editing systems according to the type of disease (Fig. 7 ).
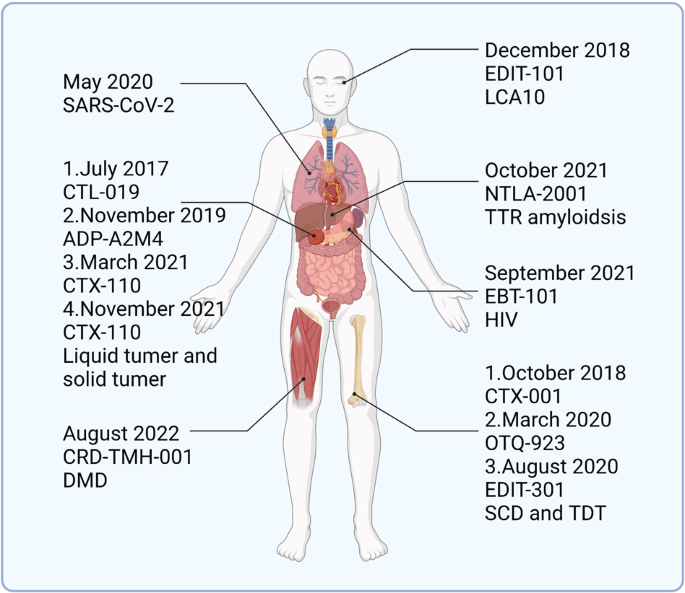
Summary chart of FDA-approved CRISPR therapies that can be used in clinical treatments. The text includes the date of FDA approval, the name of the therapy, and the type of applied diseases. DMD Duchenne muscular dystrophy, SCD sickle cell disease, TDT transfusion-dependent β-thalassemia, LCA10 Leber congenital amaurosis type 10, TTR transthyretin. (Figure was created with Biorender.com)
Limitations and challenges
The targeted delivery of CRISPR/Cas gene drugs to the body has the potential to treat diseases both in the laboratory and in the clinic. The advantages of a high specificity, effectiveness, and ease of handling make it one of the most sought-after technologies of the future. However, researchers have discovered some unexpected conditions when using CRISPR technology to edit genes.
Limitations of CRISPR/Cas9
Off-target effects.
Base mismatches between sgRNA and nontarget sequences may lead to off-target effects. The introduction of one or even multiple unknown mutations while repairing one error is clearly unacceptable. 311 , 312 , 313 When sgRNA binds to the DNA strand, the seed sequence at the proximal end of the PAM binds to the target strand strictly according to base complementary pairing. The distal three to five bases sometimes do not detach as imagined when mismatching occurs but form an unusual duplex conformation under a strong force. 314 , 315 This mechanism that allows mismatches may have arisen from the evolution of bacteria to counteract mutations in invading phages. Methods such as whole-genome sequencing and GUIDE-Seq have been developed to detect the occurrence of off-target effects. 316 , 317 The persistent expression of Cas9 in large numbers of cells increases the likelihood of off-target effects, and controlling Cas9 activation may reduce their occurrence (Table 2 ).
Improving the specificity of sgRNAs and detaching them from the DNA strand when mismatches occur is the key to solving this problem. The REC3 domain of Cas9 is critical for sensing mismatches arising at the distal end of the PAM, and researchers have mutated the REC3 domain to detect which variants might improve the accuracy of Cas9. 318 High-fidelity mutants such as HypaCas9 and Cas9-HF1 were rationally designed, and these mutants substantially improved the accuracy of Cas9. However, the interaction of mutated REC3 with the PAM-distal duplex is weakened, reducing the efficiency of Cas9. Bravo et al. found that the mismatch at bases 18–20 would form Ruv loop structure, which stabilized mismatch formation. They mutated all the residues involved in stabilization and obtained a Cas9 variant (SuperFi-Cas9) with a 500-fold reduction in the efficiency of DNA duplex cleavage at the 18th–20th base mismatch of sgRNA without affecting sgRNA-mediated double-strand cleavage with fully complementary bases. The addition a segment of a specialized structure to sgRNA to increase its specificity is also feasible. Kocak et al. designed a segment of hairpin structure at the 5′ end of sgRNA; this structure reduces the energy during mismatch and prevents the formation of an R-loop when mismatch occurs. 319 , 320 The R-loop is necessary for Cas9 activation, and thus this structure also prevents DNA duplex cleavage in the presence of mismatches. In conclusion, off-target effects are the greatest obstacle to the widespread application of CRISPR-based gene-editing technology, and modifying the sgRNA with Cas9 to make it more specific might prevent the occurrence of unknown mutations.
When CRISPR/dCas9 carrying activating or repressing structural domains is used to regulate gene expression, the upregulation or knockdown of the gene might not be sufficient to achieve a therapeutic effect. The CRISPRa system is divided into two parts: the sgRNA/Cas9 complex, which plays a targeting role, and the activating structural domain, which enhances transcription. 59 , 321 In general, when performing gene editing, only one sgRNA targeting the target site will be designed. Maeder et al. designed sgRNAs at four positions near the transcriptional start site of the target gene to obtain higher gene activation efficiency. 322 The transcriptional activation efficiency of multiple sgRNAs exerted a certain synergistic effect, and higher efficiency was obtained when more sgRNAs were present. Moreover, the transcriptional activation efficiency of sgRNAs at each position is not the same but is strongly linked to the cell and gene.
The initial transcriptional activation domain is the VP64 or p65 activation structural domain formed by the complex of four transcribed VP16, and the activation of this structure is not strong. Tanenbaum et al. constructed a synthetic system composed of a structure that contains a polypeptide chain that can recruit up to 24 copies of the protein to obtain higher activation efficiency. 148 The structure was used to recruit multiple copies of VP64 to form the dCas9-SunTag-VP64 transcriptional activation system. In a study of the activation of cell cycle suppressor cyclin-dependent kinase inhibitor 1B (CDKN1B), dCas9/VP64 did not affect cell cycle progression, whereas the same sgRNA carrying dCas9-SunTag-VP64 significantly inhibited cell cycle progression and reduced cell growth. VP64, p65 and Rta have been reported to have the ability to activate transcriptional. Chavez et al. used dCas9/VP64 as a backbone and added p65 and Rta to construct the transcriptional complex dCas9-VP64-p65-Rta (dCas9/VPR) as a transcriptional activation system. 145 With its simple structure and high efficiency, dCas9/VPR is one of the most frequently used activation systems for targeted delivery of CRISPRa. Other transcriptional activation systems, such as dCas9/SAM, dCas9/SPH and dCas9/VP192, are also able to substantially increase the efficiency of gene activation. 323 , 324 , 325 The activation or repression of a gene is related to many conditions, such as the location of the sgRNA, the selection of the effector structural domain, the type of cell and the targeted gene (Table 4 ).
Applicability
CRISPR/Cas9 can theoretically target any position in the genome but is limited to PAM sequences, which prevents Cas9 from reaching certain positions. 326 , 327 In particular, when using the base-editing tools CBEs or ABEs, the edited bases are located at specific relative positions of the PAM sites, and CBEs or ABEs may not be able to perform the base change function without a suitable PAM site. 26 Researchers have worked to modify Cas9 to ensure that it is not restricted to PAMs, and they have obtained multiple variants of Cas9 that are not restricted to recognizing NNG by mutating the Cas9 site or adding modified structural domains (Table 1 ).
Walton et al. mutated multiple amino acid sites of Cas9 to obtain a SpCas9 variant (SpRY) that is almost free from PAM restriction. 117 They first constructed a purine-rich PAM site, and SpRY was able to achieve partial gene editing at the site where the PAM is NRN (R is A or G) without lower editing efficiency than wild-type SpCas9. Next, they constructed pyrimidine-rich PAM loci, before which almost all Cas9 variants failed to recognize C- or T-rich loci, and SpRY exerted a gene-editing effect on 13 of the 31 loci constructed. Although SpRY still exhibits a stronger bias toward G-rich PAM sites, targeting pyrimidine-rich PAM sites using SpRY carrying a base-editing effector also promotes efficient base substitution. SpRY without the restriction of PAM is more prone to off-target effects. The previously reported Cas9-HF variant is effective at avoiding off-target effects, and after mutating the same site, SpRY-HF1 is able to eliminate almost all off-target effects. 108
Modifying Nme2Cas9, a Cas9 variant from Neisseria meningitidis , to recognize a wider variety of PAM sequences is a promising approach. 328 , 329 Compared to SpCas9, Nme2Cas9 is smaller and has greater potential for targeted delivery, and Nme2Cas9 also has strong gene-editing activity in mammalian cells. Researchers established a new selection platform to screen for Nme2Cas9 variants that recognize a single specified base. The final screen yielded four reliable variants: eNme2-C and eNme2-C.NR, eNme2-T.1 and eNme2-T.2. Compared with SpRY, these variants are not only PAM-independent but are also smaller in size. Except for eNme2-C.NR, the other three mutations exhibited stronger or similar gene-editing efficiency and fewer off-target effects. In conclusion, freeing Cas9 from the restriction of PAM sites may satisfy the need for single-base editing, the targeted cleavage of double strands, and other methods, which are important for the treatment of diseases caused by single-gene mutations.
Chromosomal disorganization
The safety of CRISPR-based gene-editing technology is a key topic of concern for researchers. The cleavage of double-stranded DNA by Cas9 usually triggers NHEJ repair, and these repaired DNA strands are usually missing a few base pairs or have a few added base pairs, which is the expected result. However, when verifying editing efficiency, researchers found that massive base deletions and chromosomal structural translocations sometimes occurred. 320 , 330 , 331 , 332 These errors may lead to positional diseases such as malignant tumors and are obviously not acceptable in clinical applications, although the probability of their occurrence is low. 110 , 333
The repeated cleavage of target genes by CRISPR/Cas9 is one of the important causes of chromosomal translocations and deletions. Yin et al. combined an exonuclease structural domain with Cas9 to reduce the occurrence of these mutations. 110 This structure performs end processing immediately upon the completion of cleavage, reducing the likelihood of producing intact ends. This approach effectively prevents perfect repair of the DNA strand and thus duplicate cleavage of the genome by Cas9. The authors fused spCas9 with optimized three-prime repair exonuclease 2 (TREX2) to generate a Cas9 exonuclease (Cas9TX). In experiments with engineered T cells and other cells, Cas9TX was clearly able to suppress chromosomal translocations relative to the high-fidelity SpCas9 variant.
Limitations of targeted delivery
Deviation from the desired position.
Viral and nonviral vectors are usually delivered to animals by systemic administration, and the vectors make CRISPR gene drugs immune to blood and tissue degradation. However, unmodified vectors are susceptible to capture by metabolic organs in the body. The CRISPR/Cas system does not lose activity when entering nontarget cells but genetically modifies healthy cells, which may lead to unpredictable consequences. Improved delivery vehicles are necessary to reduce the entry of gene drugs into nontarget cells. Approaches to improve delivery functionality include covering the carriers with a biofilm or adding peptides recognized by target cell receptors. 232 , 334 , 335 Designing environmentally responsive nanoparticles according to the target organ microenvironment enhances gene drug enrichment, such as variations in pH, reactive oxygen species (ROS), and adenosine triphosphate (ATP) levels. 33 The nanomaterial shell disintegrates in a specific environment, exposing the core, which then enters the cell through endocytosis. However, when the microenvironment in certain diseased tissues does not differ significantly from that of other tissues, constructing a nanoparticle that is induced by multiple conditions to release its contents is a feasible method for disease-specific targeting. In addition, light-, magnetic-, and ultrasound-responsive CRISPR/Cas9 delivery systems have been developed to support precision delivery. 33 When applied to the treatment of human diseases, the administration of drugs by in situ injection prevents them from being transported in the blood flow throughout the body. Regulating the expression of target genes may require a more modest CRISPRa or CRISPRi approach, and the changes imposed by CRISPR/dCas9-based transcriptional regulatory systems are reversible compared to altering genomic sequences to silence genes. 59 , 143
Biocompatibility
Suitable vectors must be constructed for candidate cells to reduce the possibility of adverse reactions caused by off-target CRISPR/Cas9 systems. The complex process from entry to function requires that the vector must be biocompatible, have a high encapsulation ability, and be able to traverse the cell membrane. 336 The immune response resulting from delivery of the material into the body must also be considered when designing the system. Commonly used Cas9 proteins derived from S. pyogenes and S. aureus have been reported to trigger an immune response in humans. As a method to overcome this challenge, a modified Cas9 lacking response-causing exons was delivered via AAV to effectively avoid humoral and cellular immune responses in juvenile and adult mice. 30 Moreover, even the modified Cas9 must also be transported in a vector designed to avoid triggering the host immune response. In vivo, viruses, lipids, and exosomes are effective at avoiding immune clearance, whereas synthetic chemical nanoparticles require a protective coating on the surface, such as modified PEG, which also stabilizes the polymer in the blood environment, or the inclusion of modified CD47 protein. 33 , 337 , 338 Furthermore, plant exosomes are more likely to escape detection by the immune system due to their natural origin. The use of plant exosomes for delivering CRISPR/dCas9 systems is also more acceptable for safety reasons due to the large differences between plant and mammalian pathogens. However, research on the delivery of gene drugs by plant exosomes is not yet mature, especially as many plants produce exosomes with different characteristics. 339 , 340 Overall, the development of additional delivery vehicles with low immunogenicity along with surface-modified proteins or polypeptides that effectively prevent the vehicle from being cleared by the immune system is necessary to facilitate the targeted delivery of gene drugs in the clinic, and the natural resistance of plant exosomes to immune clearance and their low pathogenicity highlighting their bright application prospects for this purpose.
Outlook and conclusions
After decades of development, CRISPR/Cas is no longer limited to cleaving DNA strands but has spawned a large family of single-base gene editing, transcriptional regulatory, and RNA strand cutting approaches. Thus, these systems may be applied to treat most human diseases, such as cancer, chronic diseases, and genetic diseases caused by a single gene. In cells, CRISPR/Cas9 and other systems have shown unparalleled gene-editing capabilities. However, the development of a safe, stable, and efficient strategy for delivering gene-editing tools to diseased cells in vivo is a major challenge to their use in clinical applications. CRISPR is usually delivered as plasmids, mRNA, or RNP, but all three forms are immunologically cleared both from the blood and from the digestive system.
AAV is one of the most commonly used vectors for delivering gene drugs, and even without considering loading capacity, the integration of AAV into the human genome may lead to disease and may be difficult to accept. Nonviral vectors with higher loading capacities and good safety profiles have also become ideal for delivery, especially LNPs, which have been used in clinical trials. Since vectors such as LNPs and AuNPs are not derived from organisms, they often trigger immunogenicity in vivo. These vectors are also susceptible to erroneous uptake by the digestive organs, although researchers have added modifications or peptides to prevent immune clearance. Exosomes from organisms or biofilms obtained as nonviral vector masks are very effective in avoiding immune clearance, and the proteins and peptides enriched in the biofilm may help the gene drug reach the target cells.
When a disease occurs, the microenvironment surrounding cells at the site of the disease is altered, which distinguishes it from normal tissue and creates favorable conditions for the development of delivery vectors. Disease onset is also accompanied by the overexpression of inflammatory factors in cells or other disease-associated membrane proteins. The addition of peptides that specifically recognize these membrane proteins allows nanomaterials to specifically recognize diseased cells and deliver CRISPR/Cas to the cells for gene-editing functions. In addition, the use of nanomaterials that respond to disease-specific conditions might also increase the enrichment of nanomaterials at the target site. Therefore, an in-depth study of the mechanism of disease onset, as well as the various pathways involved, the changes in the expression of various proteins, and the microenvironment in which the disease cells are located, may be very helpful in the construction of vectors for the targeted delivery of gene drugs.
In addition, the efficiency and safety of CRISPR/Cas9 itself are key facets to consider in clinical applications. Off-target CRISPR/Cas9 effects may lead to serious consequences, and these possible scenarios should be identified and improved before clinical application. Scientists have fully investigated the cleavage mechanism of Cas9 and developed several variants of Cas9 with a decreased likelihood of off-target effects and no reduction in efficiency. Off-target effects may be effectively avoided by modifying the sgRNA. In conclusion, in-depth studies of the mechanisms of disease occurrence, the development of more efficient and specific delivery vectors, and improvements in Cas9 variants with broader and safer adaptations are important. With these foundations, CRISPR/Cas technology will enter full clinical application to help treat human diseases.
Jiang, C., Meng, L., Yang, B. & Luo, X. Application of CRISPR/Cas9 gene editing technique in the study of cancer treatment. Clin. Genet. 97 , 73–88 (2020).
Article CAS Google Scholar
Manghwar, H., Lindsey, K., Zhang, X. & Jin, S. CRISPR/Cas system: recent advances and future prospects for genome editing. Trends Plant Sci. 24 , 1102–1125 (2019).
Roth, T. L. & Marson, A. Genetic disease and therapy. Annu. Rev. Pathol. 16 , 145–166 (2021).
Gaj, T., Gersbach, C. A. & Barbas, C. F. 3rd ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 31 , 397–405 (2013).
Hsu, P. D., Lander, E. S. & Zhang, F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 157 , 1262–1278 (2014).
Cox, D. B., Platt, R. J. & Zhang, F. Therapeutic genome editing: prospects and challenges. Nat. Med. 21 , 121–131 (2015).
Pickar-Oliver, A. & Gersbach, C. A. The next generation of CRISPR-Cas technologies and applications. Nat. Rev. Mol. Cell Biol. 20 , 490–507 (2019).
Mojica, F. J., Díez-Villaseñor, C., García-Martínez, J. & Soria, E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J. Mol. Evol. 60 , 174–182 (2005).
Marraffini, L. A. & Sontheimer, E. J. Self versus non-self discrimination during CRISPR RNA-directed immunity. Nature 463 , 568–571 (2010).
Li, J. et al. Genome editing mediated by SpCas9 variants with broad non-canonical PAM compatibility in plants. Mol. Plant. 14 , 352–360 (2021).
Bolotin, A., Quinquis, B., Sorokin, A. & Ehrlich, S. D. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology 151 , 2551–2561 (2005).
Chen, K. et al. CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu. Rev. Plant Biol. 70 , 667–697 (2019).
Platt, R. J. et al. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell 159 , 440–455 (2014).
Jiang, F. & Doudna, J. A. CRISPR-Cas9 structures and mechanisms. Annu. Rev. Biophys. 46 , 505–529 (2017).
Doudna, J. A. & Charpentier, E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346 , 1258096 (2014).
Article Google Scholar
Zhan, T. et al. CRISPR/Cas9 for cancer research and therapy. Semin. Cancer Biol. 55 , 106–119 (2019).
Vermersch, E., Jouve, C. & Hulot, J. S. CRISPR/Cas9 gene-editing strategies in cardiovascular cells. Cardiovasc. Res. 116 , 894–907 (2020).
Newby, G. A. et al. Base editing of haematopoietic stem cells rescues sickle cell disease in mice. Nature 595 , 295–302 (2021).
Heidenreich, M. & Zhang, F. Applications of CRISPR-Cas systems in neuroscience. Nat. Rev. Neurosci. 17 , 36–44 (2016).
Wu, Y. et al. Correction of a genetic disease by CRISPR-Cas9-mediated gene editing in mouse spermatogonial stem cells. Cell Res. 25 , 67–79 (2015).
Fu, Y. W. et al. Dynamics and competition of CRISPR-Cas9 ribonucleoproteins and AAV donor-mediated NHEJ, MMEJ and HDR editing. Nucleic Acids Res. 49 , 969–985 (2021).
Ran, F. A. et al. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8 , 2281–2308 (2013).
Landrum, M. J. et al. ClinVar: public archive of interpretations of clinically relevant variants. Nucleic Acids Res. 44 , D862–868 (2016).
Qi, L. S. et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152 , 1173–1183 (2013).
Komor, A. C. et al. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533 , 420–424 (2016).
Gaudelli, N. M. et al. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 551 , 464–471 (2017).
Anzalone, A. V. et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576 , 149–157 (2019).
Abudayyeh, O. O. et al. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science 353 , aaf5573 (2016).
Behr, M., Zhou, J., Xu, B. & Zhang, H. In vivo delivery of CRISPR-Cas9 therapeutics: progress and challenges. Acta Pharm. Sin. B. 11 , 2150–2171 (2021).
Wang, D., Zhang, F. & Gao, G. CRISPR-based therapeutic genome editing: strategies and in vivo delivery by AAV vectors. Cell 181 , 136–150 (2020).
Gillmore, J. D. et al. CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis. N. Engl. J. Med. 385 , 493–502 (2021).
Chen, F., Alphonse, M. & Liu, Q. Strategies for nonviral nanoparticle-based delivery of CRISPR/Cas9 therapeutics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 12 , e1609 (2020).
Cai, W., Luo, T., Mao, L. & Wang, M. Spatiotemporal delivery of CRISPR/Cas9 genome editing machinery using stimuli-responsive vehicles. Angew. Chem. Int. Ed. Engl. 60 , 8596–8606 (2021).
Ishino, Y. et al. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J. Bacteriol. 169 , 5429–5433 (1987).
Groenen, P. M., Bunschoten, A. E., van Soolingen, D. & van Embden, J. D. Nature of DNA polymorphism in the direct repeat cluster of Mycobacterium tuberculosis; application for strain differentiation by a novel typing method. Mol. Microbiol. 10 , 1057–1065 (1993).
Mojica, F. J., Ferrer, C., Juez, G. & Rodríguez-Valera, F. Long stretches of short tandem repeats are present in the largest replicons of the Archaea Haloferax mediterranei and Haloferax volcanii and could be involved in replicon partitioning. Mol. Microbiol. 17 , 85–93 (1995).
Hoe, N. et al. Rapid molecular genetic subtyping of serotype M1 group A Streptococcus strains. Emerg. Infect. Dis. 5 , 254–263 (1999).
Masepohl, B., Görlitz, K. & Böhme, H. Long tandemly repeated repetitive (LTRR) sequences in the filamentous cyanobacterium Anabaena sp. PCC 7120. Biochim. Biophys. Acta 1307 , 26–30 (1996).
Mojica, F. J., Díez-Villaseñor, C., Soria, E. & Juez, G. Biological significance of a family of regularly spaced repeats in the genomes of Archaea, Bacteria and mitochondria. Mol. Microbiol. 36 , 244–246 (2000).
Yang, Y., Xu, J., Ge, S. & Lai, L. CRISPR/Cas: advances, limitations, and applications for precision cancer research. Front. Med. 8 , 649896 (2021).
Pourcel, C., Salvignol, G. & Vergnaud, G. CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies. Microbiology 151 , 653–663 (2005).
Barrangou, R. et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315 , 1709–1712 (2007).
Deveau, H. et al. Phage response to CRISPR-encoded resistance in Streptococcus thermophilus. J. Bacteriol. 190 , 1390–1400 (2008).
Horvath, P. et al. Diversity, activity, and evolution of CRISPR loci in Streptococcus thermophilus. J. Bacteriol. 190 , 1401–1412 (2008).
Aliyari, R. & Ding, S. W. RNA-based viral immunity initiated by the Dicer family of host immune receptors. Immunol. Rev. 227 , 176–188 (2009).
Brouns, S. J. et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science 321 , 960–964 (2008).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337 , 816–821 (2012).
Gasiunas, G., Barrangou, R., Horvath, P. & Siksnys, V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc. Natl Acad. Sci. USA 109 , E2579–2586 (2012).
Haft, D. H., Selengut, J., Mongodin, E. F. & Nelson, K. E. A guild of 45 CRISPR-associated (Cas) protein families and multiple CRISPR/Cas subtypes exist in prokaryotic genomes. PLoS Comput. Biol. 1 , e60 (2005).
Makarova, K. S. et al. Evolution and classification of the CRISPR-Cas systems. Nat. Rev. Microbiol. 9 , 467–477 (2011).
Nishimasu, H. et al. Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell 156 , 935–949 (2014).
Wright, A. V., Nuñez, J. K. & Doudna, J. A. Biology and applications of CRISPR systems: harnessing nature’s toolbox for genome engineering. Cell 164 , 29–44 (2016).
Horvath, P. et al. Comparative analysis of CRISPR loci in lactic acid bacteria genomes. Int. J. Food Microbiol. 131 , 62–70 (2009).
Sapranauskas, R. et al. The Streptococcus thermophilus CRISPR/Cas system provides immunity in Escherichia coli. Nucleic Acids Res. 39 , 9275–9282 (2011).
Deltcheva, E. et al. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 471 , 602–607 (2011).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339 , 819–823 (2013).
Magadán, A. H., Dupuis, M., Villion, M. & Moineau, S. Cleavage of phage DNA by the Streptococcus thermophilus CRISPR3-Cas system. PLoS ONE 7 , e40913 (2012).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339 , 823–826 (2013).
Gilbert, L. A. et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 154 , 442–451 (2013).
Ren, X. et al. Optimized gene editing technology for Drosophila melanogaster using germ line-specific Cas9. Proc. Natl Acad. Sci. USA 110 , 19012–19017 (2013).
Kondo, S. & Ueda, R. Highly improved gene targeting by germline-specific Cas9 expression in Drosophila. Genetics 195 , 715–721 (2013).
Bassett, A. R., Tibbit, C., Ponting, C. P. & Liu, J. L. Highly efficient targeted mutagenesis of Drosophila with the CRISPR/Cas9 system. Cell Rep. 4 , 220–228 (2013).
Bassett, A. R. & Liu, J. L. CRISPR/Cas9 and genome editing in Drosophila. J. Genet. Genomics. 41 , 7–19 (2014).
Wang, H. et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153 , 910–918 (2013).
Ma, Y. et al. Generating rats with conditional alleles using CRISPR/Cas9. Cell Res. 24 , 122–125 (2014).
Jiang, W. et al. Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice. Nucleic Acids Res. 41 , e188 (2013).
Xie, K. & Yang, Y. RNA-guided genome editing in plants using a CRISPR-Cas system. Mol. Plant. 6 , 1975–1983 (2013).
Wu, Y. et al. Correction of a genetic disease in mouse via use of CRISPR-Cas9. Cell. Stem Cell. 13 , 659–662 (2013).
Zhao, L. et al. A 1-bp deletion in the gammaC-crystallin leads to dominant cataracts in mice. Mamm. Genome 21 , 361–369 (2010).
Schwank, G. et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell. Stem Cell. 13 , 653–658 (2013).
Shteinberg, M., Haq, I. J., Polineni, D. & Davies, J. C. Cystic fibrosis. Lancet 397 , 2195–2211 (2021).
Villamizar, O. et al. Targeted activation of cystic fibrosis transmembrane conductance regulator. Mol. Ther. 27 , 1737–1748 (2019).
Maule, G., Ensinck, M., Bulcaen, M. & Carlon, M. S. Rewriting CFTR to cure cystic fibrosis. Prog. Mol. Biol. Transl. Sci. 182 , 185–224 (2021).
Frangoul, H. et al. CRISPR-Cas9 gene editing for sickle cell disease and β-thalassemia. N. Engl. J. Med. 384 , 252–260 (2021).
Zhao, D. et al. Glycosylase base editors enable C-to-A and C-to-G base changes. Nat. Biotechnol. 39 , 35–40 (2021).
Kurt, I. C. et al. CRISPR C-to-G base editors for inducing targeted DNA transversions in human cells. Nat. Biotechnol. 39 , 41–46 (2021).
Ali, Z., Mahas, A. & Mahfouz, M. CRISPR/Cas13 as a tool for RNA interference. Trends Plant Sci. 23 , 374–378 (2018).
East-Seletsky, A. et al. Two distinct RNase activities of CRISPR-C2c2 enable guide-RNA processing and RNA detection. Nature 538 , 270–273 (2016).
V’Kovski, P. et al. Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol. 19 , 155–170 (2021).
Kim, D. et al. The architecture of SARS-CoV-2 transcriptome. Cell 181 , 914–921.e910 (2020).
Abudayyeh, O. O. et al. RNA targeting with CRISPR-Cas13. Nature 550 , 280–284 (2017).
Lu, Y. et al. Safety and feasibility of CRISPR-edited T cells in patients with refractory non-small-cell lung cancer. Nat. Med. 26 , 732–740 (2020).
Su, S. et al. CRISPR-Cas9-mediated disruption of PD-1 on human T cells for adoptive cellular therapies of EBV positive gastric cancer. Oncoimmunology 6 , e1249558 (2017).
Beane, J. D. et al. Clinical scale zinc finger nuclease-mediated gene editing of PD-1 in tumor infiltrating lymphocytes for the treatment of metastatic melanoma. Mol. Ther. 23 , 1380–1390 (2015).
Liao, C. & Beisel, C. L. The tracrRNA in CRISPR biology and technologies. Annu. Rev. Genet. 55 , 161–181 (2021).
Tang, H., Zhao, X. & Jiang, X. Synthetic multi-layer nanoparticles for CRISPR-Cas9 genome editing. Adv. Drug Deliv. Rev. 168 , 55–78 (2021).
Chuai, G. H., Wang, Q. L. & Liu, Q. In silico meets in vivo: towards computational CRISPR-based sgRNA design. Trends Biotechnol. 35 , 12–21 (2017).
Chandrasegaran, S. & Carroll, D. Origins of programmable nucleases for genome engineering. J. Mol. Biol. 428 , 963–989 (2016).
Mekler, V., Minakhin, L. & Severinov, K. Mechanism of duplex DNA destabilization by RNA-guided Cas9 nuclease during target interrogation. Proc. Natl Acad. Sci. USA 114 , 5443–5448 (2017).
Sternberg, S. H. et al. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature 507 , 62–67 (2014).
Szczelkun, M. D. et al. Direct observation of R-loop formation by single RNA-guided Cas9 and Cascade effector complexes. Proc. Natl Acad. Sci. USA 111 , 9798–9803 (2014).
Jiang, F. et al. STRUCTURAL BIOLOGY. A Cas9-guide RNA complex preorganized for target DNA recognition. Science 348 , 1477–1481 (2015).
Sternberg, S. H., LaFrance, B., Kaplan, M. & Doudna, J. A. Conformational control of DNA target cleavage by CRISPR-Cas9. Nature 527 , 110–113 (2015).
Pacesa, M. et al. R-loop formation and conformational activation mechanisms of Cas9. Nature 609 , 191–196 (2022).
Klum, S. M. et al. Helix-7 in Argonaute2 shapes the microRNA seed region for rapid target recognition. EMBO J. 37 , 75–88 (2018).
Mulepati, S., Héroux, A. & Bailey, S. Structural biology. Crystal structure of a CRISPR RNA-guided surveillance complex bound to a ssDNA target. Science 345 , 1479–1484 (2014).
Xiao, Y. et al. Structure basis for directional r-loop formation and substrate handover mechanisms in type I CRISPR-Cas system. Cell 170 , 48–60.e11 (2017).
Zhang, D., Zhang, Z., Unver, T. & Zhang, B. CRISPR/Cas: a powerful tool for gene function study and crop improvement. J. Adv. Res. 29 , 207–221 (2021).
Nussenzweig, A. & Nussenzweig, M. C. Origin of chromosomal translocations in lymphoid cancer. Cell 141 , 27–38 (2010).
Manghwar, H. et al. CRISPR/Cas systems in genome editing: methodologies and tools for sgRNA design, off-target evaluation, and strategies to mitigate off-target effects. Adv. Sci. 7 , 1902312 (2020).
Lee, J. K. et al. Directed evolution of CRISPR-Cas9 to increase its specificity. Nat. Commun. 9 , 3048 (2018).
Casini, A. et al. A highly specific SpCas9 variant is identified by in vivo screening in yeast. Nat. Biotechnol. 36 , 265–271 (2018).
Chen, J. S. et al. Enhanced proofreading governs CRISPR-Cas9 targeting accuracy. Nature 550 , 407–410 (2017).
Bravo, J. P. K. et al. Structural basis for mismatch surveillance by CRISPR-Cas9. Nature 603 , 343–347 (2022).
Zuo, Z. et al. Rational engineering of CRISPR-Cas9 nuclease to attenuate position-dependent off-target effects. CRISPR J. 5 , 329–340 (2022).
Xie, H. et al. High-fidelity SaCas9 identified by directional screening in human cells. PLoS Biol. 18 , e3000747 (2020).
Bratovič, M. et al. Bridge helix arginines play a critical role in Cas9 sensitivity to mismatches. Nat. Chem. Biol. 16 , 587–595 (2020).
Tan, Y. et al. Rationally engineered Staphylococcus aureus Cas9 nucleases with high genome-wide specificity. Proc. Natl Acad. Sci. USA 116 , 20969–20976 (2019).
Oakes, B. L. et al. CRISPR-Cas9 circular permutants as programmable scaffolds for genome modification. Cell 176 , 254–267.e216 (2019).
Yin, J. et al. Cas9 exo-endonuclease eliminates chromosomal translocations during genome editing. Nat. Commun. 13 , 1204 (2022).
Hirano, H. et al. Structure and engineering of Francisella novicida Cas9. Cell 164 , 950–961 (2016).
Kleinstiver, B. P. et al. Broadening the targeting range of Staphylococcus aureus CRISPR-Cas9 by modifying PAM recognition. Nat. Biotechnol. 33 , 1293–1298 (2015).
Huang, T. P. et al. High-throughput continuous evolution of compact Cas9 variants targeting single-nucleotide-pyrimidine PAMs. Nat. Biotechnol . https://doi.org/10.1038/s41587-022-01410-2 (2022).
Miller, S. M. et al. Continuous evolution of SpCas9 variants compatible with non-G PAMs. Nat. Biotechnol. 38 , 471–481 (2020).
Nishimasu, H. et al. Engineered CRISPR-Cas9 nuclease with expanded targeting space. Science 361 , 1259–1262 (2018).
Hu, J. H. et al. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity. Nature 556 , 57–63 (2018).
Walton, R. T., Christie, K. A., Whittaker, M. N. & Kleinstiver, B. P. Unconstrained genome targeting with near-PAMless engineered CRISPR-Cas9 variants. Science 368 , 290–296 (2020).
Lin, Y., Wagner, E. & Lächelt, U. Non-viral delivery of the CRISPR/Cas system: DNA versus RNA versus RNP. Biomater. Sci. 10 , 1166–1192 (2022).
Tachibana, R., Harashima, H., Shinohara, Y. & Kiwada, H. Quantitative studies on the nuclear transport of plasmid DNA and gene expression employing nonviral vectors. Adv. Drug Deliv. Rev. 52 , 219–226 (2001).
Liu, P. et al. Enhanced Cas12a editing in mammalian cells and zebrafish. Nucleic Acids Res. 47 , 4169–4180 (2019).
Luther, D. C. et al. Delivery approaches for CRISPR/Cas9 therapeutics in vivo: advances and challenges. Expert Opin. Drug Deliv. 15 , 905–913 (2018).
Youn, H. & Chung, J. K. Modified mRNA as an alternative to plasmid DNA (pDNA) for transcript replacement and vaccination therapy. Expert Opin. Biol. Ther. 15 , 1337–1348 (2015).
Seki, A. & Rutz, S. Optimized RNP transfection for highly efficient CRISPR/Cas9-mediated gene knockout in primary T cells. J. Exp. Med. 215 , 985–997 (2018).
Schumann, K. et al. Generation of knock-in primary human T cells using Cas9 ribonucleoproteins. Proc. Natl Acad. Sci. USA 112 , 10437–10442 (2015).
Kim, S. et al. Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 24 , 1012–1019 (2014).
Ramakrishna, S. et al. Gene disruption by cell-penetrating peptide-mediated delivery of Cas9 protein and guide RNA. Genome Res. 24 , 1020–1027 (2014).
Yao, X. et al. Engineered extracellular vesicles as versatile ribonucleoprotein delivery vehicles for efficient and safe CRISPR genome editing. J. Extracell. Vesicles. 10 , e12076 (2021).
Wan, T. et al. Exosome-mediated delivery of Cas9 ribonucleoprotein complexes for tissue-specific gene therapy of liver diseases. Sci. Adv. 8 , eabp9435 (2022).
Yang, H. et al. Methods favoring homology-directed repair choice in response to CRISPR/Cas9 induced-double strand breaks. Int. J. Mol. Sci. 21 , 6461 (2020).
Shen, S. et al. CRISPR as a strong gene editing tool. BMB Rep. 50 , 20–24 (2017).
Ran, F. A. et al. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154 , 1380–1389 (2013).
Dianov, G. L. & Hübscher, U. Mammalian base excision repair: the forgotten archangel. Nucleic Acids Res. 41 , 3483–3490 (2013).
Zetsche, B. et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 163 , 759–771 (2015).
Mao, Z. et al. CRISPR/Cas12a-based technology: a powerful tool for biosensing in food safety. Trends Food Sci. Technol. 122 , 211–222 (2022).
Stella, S., Alcón, P. & Montoya, G. Structure of the Cpf1 endonuclease R-loop complex after target DNA cleavage. Nature 546 , 559–563 (2017).
Paul, B. & Montoya, G. CRISPR-Cas12a: functional overview and applications. Biomed. J. 43 , 8–17 (2020).
Stella, S., Alcón, P. & Montoya, G. Class 2 CRISPR-Cas RNA-guided endonucleases: Swiss Army knives of genome editing. Nat. Struct. Mol. Biol. 24 , 882–892 (2017).
Fonfara, I. et al. The CRISPR-associated DNA-cleaving enzyme Cpf1 also processes precursor CRISPR RNA. Nature 532 , 517–521 (2016).
Himeda, C. L., Jones, T. I. & Jones, P. L. Targeted epigenetic repression by CRISPR/dSaCas9 suppresses pathogenic DUX4-fl expression in FSHD. Mol. Ther. Methods Clin. Dev. 20 , 298–311 (2021).
Di Maria, V. et al. Development and validation of CRISPR activator systems for overexpression of CB1 receptors in neurons. Front. Mol. Neurosci. 13 , 168 (2020).
Li, M. et al. miR-564: a potential regulator of vascular smooth muscle cells and therapeutic target for aortic dissection. J. Mol. Cell Cardiol. 170 , 100–114 (2022).
Yang, Y. et al. The lncRNA punisher regulates apoptosis and mitochondrial homeostasis of vascular smooth muscle cells via targeting miR-664a-5p and OPA1. Oxid. Med. Cell Longev. 2022 , 5477024 (2022).
Google Scholar
Dominguez, A. A., Lim, W. A. & Qi, L. S. Beyond editing: repurposing CRISPR-Cas9 for precision genome regulation and interrogation. Nat. Rev. Mol. Cell Biol. 17 , 5–15 (2016).
Balboa, D. et al. Conditionally stabilized dCas9 activator for controlling gene expression in human cell reprogramming and differentiation. Stem Cell Rep. 5 , 448–459 (2015).
Chavez, A. et al. Highly efficient Cas9-mediated transcriptional programming. Nat. Methods 12 , 326–328 (2015).
Konermann, S. et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature 517 , 583–588 (2015).
Larson, M. H. et al. CRISPR interference (CRISPRi) for sequence-specific control of gene expression. Nat. Protoc. 8 , 2180–2196 (2013).
Tanenbaum, M. E. et al. A protein-tagging system for signal amplification in gene expression and fluorescence imaging. Cell 159 , 635–646 (2014).
Hilton, I. B. et al. Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat. Biotechnol. 33 , 510–517 (2015).
Liu, X. S. et al. Editing DNA methylation in the mammalian genome. Cell 167 , 233–247.e217 (2016).
Vojta, A. et al. Repurposing the CRISPR-Cas9 system for targeted DNA methylation. Nucleic Acids Res. 44 , 5615–5628 (2016).
Hsu, M. N. et al. CRISPR technologies for stem cell engineering and regenerative medicine. Biotechnol. Adv. 37 , 107447 (2019).
Conticello, S. G. The AID/APOBEC family of nucleic acid mutators. Genome Biol. 9 , 229 (2008).
Rees, H. A. & Liu, D. R. Base editing: precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 19 , 770–788 (2018).
Jeong, Y. K., Song, B. & Bae, S. Current status and challenges of DNA base editing tools. Mol. Ther. 28 , 1938–1952 (2020).
Kunz, C., Saito, Y. & Schär, P. DNA Repair in mammalian cells: mismatched repair: variations on a theme. Cell Mol. Life Sci. 66 , 1021–1038 (2009).
Békési, A., Holub, E., Pálinkás, H. L. & Vértessy, B. G. Detection of genomic uracil patterns. Int. J. Mol. Sci. 22 , 3902 (2021).
Zafra, M. P. et al. Optimized base editors enable efficient editing in cells, organoids and mice. Nat. Biotechnol. 36 , 888–893 (2018).
Koblan, L. W. et al. Improving cytidine and adenine base editors by expression optimization and ancestral reconstruction. Nat. Biotechnol. 36 , 843–846 (2018).
Komor, A. C. et al. Improved base excision repair inhibition and bacteriophage Mu Gam protein yields C:G-to-T:A base editors with higher efficiency and product purity. Sci. Adv. 3 , eaao4774 (2017).
Lee, H. K. et al. Development of CRISPR technology for precise single-base genome editing: a brief review. BMB Rep. 54 , 98–105 (2021).
Shi, K. et al. Structural basis for targeted DNA cytosine deamination and mutagenesis by APOBEC3A and APOBEC3B. Nat. Struct. Mol. Biol. 24 , 131–139 (2017).
Kim, J. et al. Structural and kinetic characterization of Escherichia coli TadA, the wobble-specific tRNA deaminase. Biochemistry 45 , 6407–6416 (2006).
Richter, M. F. et al. Phage-assisted evolution of an adenine base editor with improved Cas domain compatibility and activity. Nat. Biotechnol. 38 , 883–891 (2020).
Lee, H. K. et al. Targeting fidelity of adenine and cytosine base editors in mouse embryos. Nat. Commun. 9 , 4804 (2018).
Huang, T. P. et al. Author Correction: Circularly permuted and PAM-modified Cas9 variants broaden the targeting scope of base editors. Nat. Biotechnol. 37 , 820 (2019).
Nishida, K. et al. Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. Science 353 , aaf8729 (2016).
Zheng, K. et al. Highly efficient base editing in bacteria using a Cas9-cytidine deaminase fusion. Commun. Biol. 1 , 32 (2018).
Lu, C. et al. Prime editing: an all-rounder for genome editing. Int. J. Mol. Sci. 23 , 9862 (2022).
Gerard, G. F. et al. The role of template-primer in protection of reverse transcriptase from thermal inactivation. Nucleic Acids Res. 30 , 3118–3129 (2002).
Gao, Z. et al. A truncated reverse transcriptase enhances prime editing by split AAV vectors. Mol. Ther. 30 , 2942–2951 (2022).
Anzalone, A. V., Koblan, L. W. & Liu, D. R. Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 38 , 824–844 (2020).
Chen, P. J. et al. Enhanced prime editing systems by manipulating cellular determinants of editing outcomes. Cell 184 , 5635–5652.e5629 (2021).
Setten, R. L., Rossi, J. J. & Han, S. P. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 18 , 421–446 (2019).
Schuster, A. et al. RNAi/CRISPR screens: from a pool to a valid hit. Trends Biotechnol. 37 , 38–55 (2019).
Wilson, C., Chen, P. J., Miao, Z. & Liu, D. R. Programmable m(6)A modification of cellular RNAs with a Cas13-directed methyltransferase. Nat. Biotechnol. 38 , 1431–1440 (2020).
Li, J. et al. Targeted mRNA demethylation using an engineered dCas13b-ALKBH5 fusion protein. Nucleic Acids Res. 48 , 5684–5694 (2020).
Zhao, J. et al. Photoactivatable RNA N(6) -Methyladenosine Editing with CRISPR-Cas13. Small 16 , e1907301 (2020).
Cox, D. B. T. et al. RNA editing with CRISPR-Cas13. Science 358 , 1019–1027 (2017).
Abudayyeh, O. O. et al. A cytosine deaminase for programmable single-base RNA editing. Science 365 , 382–386 (2019).
Zhao, X. et al. A CRISPR-Cas13a system for efficient and specific therapeutic targeting of mutant KRAS for pancreatic cancer treatment. Cancer Lett. 431 , 171–181 (2018).
Gootenberg, J. S. et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science 356 , 438–442 (2017).
Piepenburg, O., Williams, C. H., Stemple, D. L. & Armes, N. A. DNA detection using recombination proteins. PLoS Biol. 4 , e204 (2006).
Joung, J. et al. Detection of SARS-CoV-2 with SHERLOCK one-pot testing. N. Engl. J. Med. 383 , 1492–1494 (2020).
Sonntag, F., Schmidt, K. & Kleinschmidt, J. A. A viral assembly factor promotes AAV2 capsid formation in the nucleolus. Proc. Natl Acad. Sci. USA 107 , 10220–10225 (2010).
Backstrom, J. R. et al. Optimization of S. aureus dCas9 and CRISPRi elements for a single adeno-associated virus that targets an endogenous gene. Mol. Ther. Methods Clin. Dev. 19 , 139–148 (2020).
Chew, W. L. et al. A multifunctional AAV-CRISPR-Cas9 and its host response. Nat. Methods 13 , 868–874 (2016).
Böhm, S. et al. A gene therapy for inherited blindness using dCas9-VPR-mediated transcriptional activation. Sci. Adv. 6 , eaba5614 (2020).
Lin, S. C., Haga, K., Zeng, X. L. & Estes, M. K. Generation of CRISPR-Cas9-mediated genetic knockout human intestinal tissue-derived enteroid lines by lentivirus transduction and single-cell cloning. Nat. Protoc. 17 , 1004–1027 (2022).
Sung, L. Y. et al. Enhanced and prolonged baculovirus-mediated expression by incorporating recombinase system and in cis elements: a comparative study. Nucleic Acids Res. 41 , e139 (2013).
Lu, C. H. et al. Recent progresses in gene delivery-based bone tissue engineering. Biotechnol. Adv. 31 , 1695–1706 (2013).
Nguyen, N. T. K. et al. CRISPR activation of long non-coding RNA DANCR promotes bone regeneration. Biomaterials 275 , 120965 (2021).
Felgner, P. L. et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl Acad. Sci. USA 84 , 7413–7417 (1987).
Witzigmann, D. et al. Lipid nanoparticle technology for therapeutic gene regulation in the liver. Adv. Drug Deliv. Rev. 159 , 344–363 (2020).
Qiu, M. et al. Lipid nanoparticle-mediated codelivery of Cas9 mRNA and single-guide RNA achieves liver-specific in vivo genome editing of Angptl3. Proc. Natl Acad. Sci. USA 118 , e2020401118 (2021).
Romeo, S. et al. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J. Clin. Invest. 119 , 70–79 (2009).
CAS Google Scholar
Sedic, M. et al. Safety evaluation of lipid nanoparticle-formulated modified mRNA in the Sprague-Dawley rat and cynomolgus monkey. Vet. Pathol. 55 , 341–354 (2018).
Mirjalili Mohanna, S. Z. et al. LNP-mediated delivery of CRISPR RNP for wide-spread in vivo genome editing in mouse cornea. J. Control. Release 350 , 401–413 (2022).
Rosenblum, D. et al. CRISPR-Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci. Adv. 6 , eabc9450 (2020).
Mitchell, M. J. et al. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20 , 101–124 (2021).
Lee, Y. et al. Charge-conversion ternary polyplex with endosome disruption moiety: a technique for efficient and safe gene delivery. Angew. Chem. Int. Ed. Engl. 47 , 5163–5166 (2008).
Liu, C. et al. A programmable hierarchical-responsive nanoCRISPR elicits robust activation of endogenous target to treat cancer. Theranostics 11 , 9833–9846 (2021).
Sameiyan, E. et al. Aptamer-based ATP-responsive delivery systems for cancer diagnosis and treatment. Acta Biomater. 123 , 110–122 (2021).
Alsaiari, S. K. et al. Endosomal escape and delivery of CRISPR/Cas9 genome editing machinery enabled by nanoscale zeolitic imidazolate framework. J. Am. Chem. Soc. 140 , 143–146 (2018).
Jiang, C. et al. A non-viral CRISPR/Cas9 delivery system for therapeutically targeting HBV DNA and pcsk9 in vivo. Cell Res. 27 , 440–443 (2017).
Liu, Q. et al. Multistage delivery nanoparticle facilitates efficient CRISPR/dCas9 activation and tumor growth suppression in vivo. Adv. Sci. 6 , 1801423 (2019).
Zhang, Z. et al. Dual-locking nanoparticles disrupt the PD-1/PD-L1 pathway for efficient cancer immunotherapy. Adv. Mater. 31 , e1905751 (2019).
Cui, Q. et al. Modulating ROS to overcome multidrug resistance in cancer. Drug Resist. Updat. 41 , 1–25 (2018).
Zhang, K. et al. Localized delivery of CRISPR/dCas9 via layer-by-layer self-assembling peptide coating on nanofibers for neural tissue engineering. Biomaterials 256 , 120225 (2020).
Tamay, D. G. et al. 3D and 4D printing of polymers for tissue engineering applications. Front. Bioeng. Biotechnol. 7 , 164 (2019).
S, E. L. A., Mäger, I., Breakefield, X. O. & Wood, M. J. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 12 , 347–357 (2013).
Yang, B., Chen, Y. & Shi, J. Exosome biochemistry and advanced nanotechnology for next-generation theranostic platforms. Adv. Mater. 31 , e1802896 (2019).
Pegtel, D. M. & Gould, S. J. Exosomes. Annu. Rev. Biochem. 88 , 487–514 (2019).
Li, S. P., Lin, Z. X., Jiang, X. Y. & Yu, X. Y. Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools. Acta Pharmacol. Sin. 39 , 542–551 (2018).
Babuta, M. et al. Dysregulated autophagy and lysosome function are linked to exosome production by micro-RNA 155 in alcoholic liver disease. Hepatology 70 , 2123–2141 (2019).
Reshke, R. et al. Reduction of the therapeutic dose of silencing RNA by packaging it in extracellular vesicles via a pre-microRNA backbone. Nat. Biomed. Eng. 4 , 52–68 (2020).
Chen, D. et al. p53 up-regulated modulator of apoptosis induction mediates acetaminophen-induced necrosis and liver injury in mice. Hepatology 69 , 2164–2179 (2019).
Nevzorova, Y. A. et al. Cyclin E1 controls proliferation of hepatic stellate cells and is essential for liver fibrogenesis in mice. Hepatology 56 , 1140–1149 (2012).
Kwan, S. Y. et al. Depletion of TRRAP induces p53-independent senescence in liver cancer by down-regulating mitotic genes. Hepatology 71 , 275–290 (2020).
Lainšček, D. et al. Delivery of an artificial transcription regulator dCas9-VPR by extracellular vesicles for therapeutic gene activation. ACS Synth. Biol. 7 , 2715–2725 (2018).
DeRossi, C. et al. Mannose phosphate isomerase and mannose regulate hepatic stellate cell activation and fibrosis in zebrafish and humans. Hepatology 70 , 2107–2122 (2019).
Li, Z. et al. In vitro and in vivo RNA inhibition by CD9-HuR functionalized exosomes encapsulated with miRNA or CRISPR/dCas9. Nano Lett. 19 , 19–28 (2019).
Connor, D. M. & Broome, A. M. Gold nanoparticles for the delivery of cancer therapeutics. Adv. Cancer Res. 139 , 163–184 (2018).
Amina, S. J. & Guo, B. A review on the synthesis and functionalization of gold nanoparticles as a drug delivery vehicle. Int. J. Nanomed. 15 , 9823–9857 (2020).
Huang, C. B. et al. Highly sensitive strain sensors based on molecules-gold nanoparticles networks for high-resolution human pulse analysis. Small 17 , e2007593 (2021).
Lopes, L. C. et al. Gold nanoparticles capped with polysaccharides extracted from pineapple gum: Evaluation of their hemocompatibility and electrochemical sensing properties. Talanta 223 , 121634 (2021).
Peng, J. et al. Delivery of miR-320a-3p by gold nanoparticles combined with photothermal therapy for directly targeting Sp1 in lung cancer. Biomater. Sci. 9 , 6528–6541 (2021).
Wang, P. et al. Thermo-triggered release of CRISPR-Cas9 system by lipid-encapsulated gold nanoparticles for tumor therapy. Angew. Chem. Int. Ed. Engl. 57 , 1491–1496 (2018).
Wu, B. et al. Cell penetrating peptide TAT-functionalized liposomes for efficient ophthalmic delivery of flurbiprofen: penetration and its underlying mechanism, retention, anti-inflammation and biocompatibility. Int. J. Pharm. 598 , 120405 (2021).
Luk, B. T. & Zhang, L. Cell membrane-camouflaged nanoparticles for drug delivery. J. Control. Release 220 , 600–607 (2015).
Bose, R. J. et al. Cell membrane-coated nanocarriers: the emerging targeted delivery system for cancer theranostics. Drug Discov. Today 23 , 891–899 (2018).
Liu, J. et al. Macrophage-biomimetic anti-inflammatory liposomes for homing and treating of aortic dissection. J. Control. Release 337 , 224–235 (2021).
Yan, X. et al. Genome-editing prodrug: targeted delivery and conditional stabilization of CRISPR-Cas9 for precision therapy of inflammatory disease. Sci. Adv. 7 , eabj0624 (2021).
Yang, X. et al. Nanoscale ATP-responsive zeolitic imidazole framework-90 as a general platform for cytosolic protein delivery and genome editing. J. Am. Chem. Soc. 141 , 3782–3786 (2019).
Liu, J. et al. Fast and efficient CRISPR/Cas9 genome editing in vivo enabled by bioreducible lipid and messenger RNA nanoparticles. Adv. Mater. 31 , e1902575 (2019).
Wang, T. et al. MMP-responsive transformation nanomaterials with IAP antagonist to boost immune checkpoint therapy. J. Control. Release 343 , 765–776 (2022).
Li, X. et al. Multistage-responsive nanocomplexes attenuate ulcerative colitis by improving the accumulation and distribution of oral nucleic acid drugs in the colon. ACS Appl. Mater. Interfaces 14 , 2058–2070 (2022).
Zhang, X. et al. Robust genome editing in adult vascular endothelium by nanoparticle delivery of CRISPR-Cas9 plasmid DNA. Cell Rep. 38 , 110196 (2022).
Rupaimoole, R. & Slack, F. J. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 16 , 203–222 (2017).
Hu, J. et al. Targeting mutant p53 for cancer therapy: direct and indirect strategies. J. Hematol. Oncol. 14 , 157 (2021).
Majumder, S. et al. Targeting Notch in oncology: the path forward. Nat. Rev. Drug Discov. 20 , 125–144 (2021).
Cha, J. H. et al. Mechanisms controlling PD-L1 expression in cancer. Mol. Cell. 76 , 359–370 (2019).
Li, M. et al. Integrins as attractive targets for cancer therapeutics. Acta Pharm. Sin. B. 11 , 2726–2737 (2021).
Li, X. et al. Hypoxia-responsive gene editing to reduce tumor thermal tolerance for mild-photothermal therapy. Angew. Chem. Int. Ed. Engl. 60 , 21200–21204 (2021).
Zhi, D. et al. Photothermal therapy. J. Control. Release 325 , 52–71 (2020).
Thommen, D. S. & Schumacher, T. N. T cell dysfunction in cancer. Cancer Cell. 33 , 547–562 (2018).
Stadtmauer, E. A. et al. CRISPR-engineered T cells in patients with refractory cancer. Science 367 , eaba7365 (2020).
Robbins, P. F. et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. 29 , 917–924 (2011).
Rapoport, A. P. et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat. Med. 21 , 914–921 (2015).
Nowicki, T. S. et al. A pilot trial of the combination of transgenic NY-ESO-1-reactive adoptive cellular therapy with dendritic cell vaccination with or without ipilimumab. Clin. Cancer Res. 25 , 2096–2108 (2019).
Bendle, G. M. et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat. Med. 16 , 565–570 (2010).
Provasi, E. et al. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat. Med. 18 , 807–815 (2012).
Nishimura, H. et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11 , 141–151 (1999).
Larson, R. C. et al. CAR T cell killing requires the IFNγR pathway in solid but not liquid tumours. Nature 604 , 563–570 (2022).
Li, X. et al. Lactate metabolism in human health and disease. Signal Transduct. Target Ther. 7 , 305 (2022).
Qi, H. et al. Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes. Nanotechnol. Rev. 11 , 1511–1524 (2022).
Gong, J. et al. A versatile nonviral delivery system for multiplex gene-editing in the liver. Adv. Mater. 32 , e2003537 (2020).
Yang, T. et al. Therapeutic HNF4A mRNA attenuates liver fibrosis in a preclinical model. J. Hepatol. 75 , 1420–1433 (2021).
Bartneck, M. Lipid nanoparticle formulations for targeting leukocytes with therapeutic RNA in liver fibrosis. Adv. Drug Deliv. Rev. 173 , 70–88 (2021).
Wu, J. Y. et al. Exosome-Mimetic Nanovesicles from Hepatocytes promote hepatocyte proliferation in vitro and liver regeneration in vivo. Sci. Rep. 8 , 2471 (2018).
Luo, N. et al. Hepatic stellate cell reprogramming via exosome-mediated CRISPR/dCas9-VP64 delivery. Drug Deliv. 28 , 10–18 (2021).
Villiger, L. et al. Treatment of a metabolic liver disease by in vivo genome base editing in adult mice. Nat. Med. 24 , 1519–1525 (2018).
Zhang, Y. Y. & Hu, K. Q. Rethinking the pathogenesis of hepatitis B virus (HBV) infection. J. Med. Virol. 87 , 1989–1999 (2015).
Wang, D. et al. CRISPR/Cas9 delivery by NIR-responsive biomimetic nanoparticles for targeted HBV therapy. J. Nanobiotechnol. 20 , 27 (2022).
Zhao, D. et al. Epidemiology of cardiovascular disease in China: current features and implications. Nat. Rev. Cardiol. 16 , 203–212 (2019).
Li, X. et al. Recent advances in targeted delivery of non-coding RNA-based therapeutics for atherosclerosis. Mol. Ther. 30 , 3118–3132 (2022).
Hansson, G. K. & Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 12 , 204–212 (2011).
Oka, T., Akazawa, H., Naito, A. T. & Komuro, I. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ. Res. 114 , 565–571 (2014).
Mehta, L. S. et al. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation 133 , 916–947 (2016).
Zhu, Y. et al. Type A aortic dissection-experience over 5 decades: JACC historical breakthroughs in perspective. J. Am. Coll. Cardiol. 76 , 1703–1713 (2020).
Tadros, R. O. et al. Optimal treatment of uncomplicated type B aortic dissection: JACC review topic of the week. J. Am. Coll. Cardiol. 74 , 1494–1504 (2019).
Stevens, S. L. et al. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ 354 , i4098 (2016).
Derks, W. & Bergmann, O. Polyploidy in cardiomyocytes: roadblock to heart regeneration? Circ. Res. 126 , 552–565 (2020).
Gu, Z. et al. Genomic analyses identify recurrent MEF2D fusions in acute lymphoblastic leukaemia. Nat. Commun. 7 , 13331 (2016).
Noack, C. et al. KLF15-Wnt-dependent cardiac reprogramming up-regulates SHISA3 in the mammalian heart. J. Am. Coll. Cardiol. 74 , 1804–1819 (2019).
Schoger, E. et al. CRISPR-mediated activation of endogenous gene expression in the postnatal heart. Circ. Res. 126 , 6–24 (2020).
Madsen, A. et al. An important role for DNMT3A-mediated DNA methylation in cardiomyocyte metabolism and contractility. Circulation 142 , 1562–1578 (2020).
Li, X. et al. Targeting non-coding RNAs in unstable atherosclerotic plaques: mechanism, regulation, possibilities, and limitations. Int. J. Biol. Sci. 17 , 3413–3427 (2021).
Bai, B. et al. MicroRNA-302c-3p inhibits endothelial cell pyroptosis via directly targeting NOD-, LRR- and pyrin domain-containing protein 3 in atherosclerosis. J. Cell Mol. Med. 25 , 4373–4386 (2021).
Zhang, X. et al. CRISPR/Cas9 delivery mediated with hydroxyl-rich nanosystems for gene editing in aorta. Adv. Sci. 6 , 1900386 (2019).
Hsu, M. N. et al. Coactivation of endogenous Wnt10b and Foxc2 by CRISPR activation enhances BMSC osteogenesis and promotes calvarial bone regeneration. Mol. Ther. 28 , 441–451 (2020).
Lin, Y. T. et al. APOE4 causes widespread molecular and cellular alterations associated with Alzheimer’s disease phenotypes in human iPSC-derived brain cell types. Neuron 98 , 1141–1154.e1147 (2018).
Bhardwaj, S. et al. CRISPR/Cas9 gene editing: new hope for Alzheimer’s disease therapeutics. J. Adv. Res. 40 , 207–221 (2022).
Matharu, N. et al. CRISPR-mediated activation of a promoter or enhancer rescues obesity caused by haploinsufficiency. Science 363 , eaau0629 (2019).
Chung, J. Y. et al. Targeted delivery of CRISPR interference system against Fabp4 to white adipocytes ameliorates obesity, inflammation, hepatic steatosis, and insulin resistance. Genome Res. 29 , 1442–1452 (2019).
Fiolet, T. et al. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin. Microbiol. Infect. 28 , 202–221 (2022).
Harrison, A. G., Lin, T. & Wang, P. Mechanisms of SARS-CoV-2 transmission and pathogenesis. Trends Immunol. 41 , 1100–1115 (2020).
Tao, K. et al. The biological and clinical significance of emerging SARS-CoV-2 variants. Nat. Rev. Genet. 22 , 757–773 (2021).
Yüce, M., Filiztekin, E. & Özkaya, K. G. COVID-19 diagnosis—a review of current methods. Biosens. Bioelectron. 172 , 112752 (2021).
Safiabadi Tali, S. H. et al. Tools and techniques for severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2)/COVID-19 detection. Clin. Microbiol. Rev. 34 , e00228–20 (2021).
Soroka, M., Wasowicz, B. & Rymaszewska, A. Loop-mediated isothermal amplification (LAMP): the better sibling of PCR? Cells 10 , 1931 (2021).
Teng, F. et al. Repurposing CRISPR-Cas12b for mammalian genome engineering. Cell Discov. 4 , 63 (2018).
Taher, A. T., Weatherall, D. J. & Cappellini, M. D. Thalassaemia. Lancet 391 , 155–167 (2018).
Brandow, A. M. & Liem, R. I. Advances in the diagnosis and treatment of sickle cell disease. J. Hematol. Oncol. 15 , 20 (2022).
Sankaran, V. G. et al. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science 322 , 1839–1842 (2008).
Wu, Y. et al. Highly efficient therapeutic gene editing of human hematopoietic stem cells. Nat. Med. 25 , 776–783 (2019).
Esrick, E. B. et al. Post-transcriptional genetic silencing of BCL11A to treat sickle cell disease. N. Engl. J. Med. 384 , 205–215 (2021).
Wen, J. et al. Single AAV-mediated CRISPR-Nme2Cas9 efficiently reduces mutant hTTR expression in a transgenic mouse model of transthyretin amyloidosis. Mol. Ther. 30 , 164–174 (2022).
Rigopoulos, A. G. et al. Advances in the diagnosis and treatment of transthyretin amyloidosis with cardiac involvement. Heart Fail. Rev. 24 , 521–533 (2019).
Sousa, L., Coelho, T. & Taipa, R. CNS involvement in hereditary transthyretin amyloidosis. Neurology 97 , 1111–1119 (2021).
Sekijima, Y. Transthyretin (ATTR) amyloidosis: clinical spectrum, molecular pathogenesis and disease-modifying treatments. J. Neurol. Neurosurg. Psychiatry 86 , 1036–1043 (2015).
Adams, D. et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N. Engl. J. Med. 379 , 11–21 (2018).
Aimo, A. et al. RNA-targeting and gene editing therapies for transthyretin amyloidosis. Nat. Rev. Cardiol. 19 , 655–667 (2022).
Sabnis, S. et al. A novel amino lipid series for mRNA delivery: improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol. Ther. 26 , 1509–1519 (2018).
Akinc, A. et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 14 , 1084–1087 (2019).
Maeder, M. L. et al. Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10. Nat. Med. 25 , 229–233 (2019).
Burton, D. R. Advancing an HIV vaccine; advancing vaccinology. Nat. Rev. Immunol. 19 , 77–78 (2019).
Lorenzo-Redondo, R. et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature 530 , 51–56 (2016).
Noy, A. Optimizing treatment of HIV-associated lymphoma. Blood 134 , 1385–1394 (2019).
Mancuso, P. et al. CRISPR based editing of SIV proviral DNA in ART treated non-human primates. Nat. Commun. 11 , 6065 (2020).
Xu, C. F. et al. Rational designs of in vivo CRISPR-Cas delivery systems. Adv. Drug. Deliv. Rev. 168 , 3–29 (2021).
Zhang, S., Shen, J., Li, D. & Cheng, Y. Strategies in the delivery of Cas9 ribonucleoprotein for CRISPR/Cas9 genome editing. Theranostics 11 , 614–648 (2021).
Fu, Y. et al. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat. Biotechnol. 31 , 822–826 (2013).
Liu, M. S. et al. Engineered CRISPR/Cas9 enzymes improve discrimination by slowing DNA cleavage to allow release of off-target DNA. Nat. Commun. 11 , 3576 (2020).
Singh, D. et al. Mechanisms of improved specificity of engineered Cas9s revealed by single-molecule FRET analysis. Nat. Struct. Mol. Biol. 25 , 347–354 (2018).
Zischewski, J., Fischer, R. & Bortesi, L. Detection of on-target and off-target mutations generated by CRISPR/Cas9 and other sequence-specific nucleases. Biotechnol. Adv. 35 , 95–104 (2017).
Wienert, B. et al. Unbiased detection of CRISPR off-targets in vivo using DISCOVER-Seq. Science 364 , 286–289 (2019).
Zhu, X. et al. Cryo-EM structures reveal coordinated domain motions that govern DNA cleavage by Cas9. Nat. Struct. Mol. Biol. 26 , 679–685 (2019).
Kocak, D. D. et al. Increasing the specificity of CRISPR systems with engineered RNA secondary structures. Nat. Biotechnol. 37 , 657–666 (2019).
Nahmad, A. D. et al. Frequent aneuploidy in primary human T cells after CRISPR-Cas9 cleavage. Nat. Biotechnol. 40 , 1807–1813 (2022).
Becirovic, E. Maybe you can turn me on: CRISPRa-based strategies for therapeutic applications. Cell Mol. Life Sci. 79 , 130 (2022).
Maeder, M. L. et al. CRISPR RNA-guided activation of endogenous human genes. Nat. Methods 10 , 977–979 (2013).
Moradpour, M. & Abdulah, S. N. A. CRISPR/dCas9 platforms in plants: strategies and applications beyond genome editing. Plant Biotechnol. J. 18 , 32–44 (2020).
Zhou, H. et al. In vivo simultaneous transcriptional activation of multiple genes in the brain using CRISPR-dCas9-activator transgenic mice. Nat. Neurosci. 21 , 440–446 (2018).
Javaid, N., Pham, T. L. H. & Choi, S. Functional comparison between VP64-dCas9-VP64 and dCas9-VP192 CRISPR activators in human embryonic kidney cells. Int. J. Mol. Sci. 22 , 397 (2021).
Collias, D. & Beisel, C. L. CRISPR technologies and the search for the PAM-free nuclease. Nat. Commun. 12 , 555 (2021).
Anders, C., Niewoehner, O., Duerst, A. & Jinek, M. Structural basis of PAM-dependent target DNA recognition by the Cas9 endonuclease. Nature 513 , 569–573 (2014).
Edraki, A. et al. A compact, high-accuracy Cas9 with a dinucleotide PAM for in vivo genome editing. Mol. Cell. 73 , 714–726.e714 (2019).
Ibraheim, R. et al. Self-inactivating, all-in-one AAV vectors for precision Cas9 genome editing via homology-directed repair in vivo. Nat. Commun. 12 , 6267 (2021).
Tao, J. et al. Frequency and mechanisms of LINE-1 retrotransposon insertions at CRISPR/Cas9 sites. Nat. Commun. 13 , 3685 (2022).
Leibowitz, M. L. et al. Chromothripsis as an on-target consequence of CRISPR-Cas9 genome editing. Nat. Genet. 53 , 895–905 (2021).
Zuccaro, M. V. et al. Allele-specific chromosome removal after Cas9 cleavage in human embryos. Cell 183 , 1650–1664.e1615 (2020).
Adikusuma, F. et al. Large deletions induced by Cas9 cleavage. Nature 560 , E8–e9 (2018).
Jahromi, L. P. et al. Chemically engineered immune cell-derived microrobots and biomimetic nanoparticles: emerging biodiagnostic and therapeutic tools. Adv. Sci. 8 , 2002499 (2021).
Tang, D. et al. ROS-responsive biomimetic nanoparticles for potential application in targeted anti-atherosclerosis. Regen. Biomater. 8 , rbab033 (2021).
Xu, X. et al. Nanotechnology-based delivery of CRISPR/Cas9 for cancer treatment. Adv. Drug Deliv. Rev. 176 , 113891 (2021).
Gratton, S. E. et al. The effect of particle design on cellular internalization pathways. Proc. Natl Acad. Sci. USA 105 , 11613–11618 (2008).
Qie, Y. et al. Surface modification of nanoparticles enables selective evasion of phagocytic clearance by distinct macrophage phenotypes. Sci. Rep. 6 , 26269 (2016).
Dad, H. A. et al. Plant exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms. Mol. Ther. 29 , 13–31 (2021).
Cong, M. et al. Technology insight: plant-derived vesicles-How far from the clinical biotherapeutics and therapeutic drug carriers? Adv. Drug Deliv. Rev. 182 , 114108 (2022).
Kleinstiver, B. P. et al. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature 523 , 481–485 (2015).
Kleinstiver, B. P. et al. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature 529 , 490–495 (2016).
Slaymaker, I. M. et al. Rationally engineered Cas9 nucleases with improved specificity. Science 351 , 84–88 (2016).
Hsu, M. N. et al. CRISPR interference-mediated noggin knockdown promotes BMP2-induced osteogenesis and calvarial bone healing. Biomaterials 252 , 120094 (2020).
Fan, Y., Marioli, M. & Zhang, K. Analytical characterization of liposomes and other lipid nanoparticles for drug delivery. J. Pharm. Biomed. Anal. 192 , 113642 (2021).
Horodecka, K. & Düchler, M. CRISPR/Cas9: principle, applications, and delivery through extracellular vesicles. Int. J. Mol. Sci. 22 , 6072 (2021).
Fan, J., Cheng, Y. & Sun, M. Functionalized gold nanoparticles: synthesis, properties and biomedical applications. Chem. Rec. 20 , 1474–1504 (2020).
Chen, L. et al. Recent progress in targeted delivery vectors based on biomimetic nanoparticles. Signal Transduct. Target Ther. 6 , 225 (2021).
Wojnilowicz, M. et al. Super-resolution imaging of proton sponge-triggered rupture of endosomes and cytosolic release of small interfering RNA. ACS Nano. 13 , 187–202 (2019).
Perrin, A., Rousseau, J. & Tremblay, J. P. Increased expression of laminin subunit alpha 1 chain by dCas9-VP160. Mol. Ther. Nucleic Acids 6 , 68–79 (2017).
Download references
Acknowledgements
This work was supported by The National Natural Science Foundation of China (No. 81870331, China), Natural Science Foundation of Shandong Province (No. ZR2021MH280), and the Qingdao Municipal Science and Technology Bureau Project (No. 21-1-4-rkjk-12-nsh, China).
Author information
These authors contributed equally: Tianxiang Li, Yanyan Yang, Hongzhao Qi
Authors and Affiliations
Institute for Translational Medicine, The Affiliated Hospital of Qingdao University, No. 38 Dengzhou Road, 266021, Qingdao, People’s Republic of China
Tianxiang Li, Hongzhao Qi, Meixin Liu, Pei-feng Li & Tao Yu
Department of Immunology, School of Basic Medicine, Qingdao University, 266021, Qingdao, People’s Republic of China
Yanyan Yang
Department of Cardiology, People’s Hospital of Rizhao, No. 126 Taian Road, 276827, Rizhao, People’s Republic of China
Weigang Cui
Department of Microbiology, Linyi Center for Disease Control and Prevention, 276000, Linyi, People’s Republic of China
Department of Cardiac Ultrasound, The Affiliated Hospital of Qingdao University, 266000, Qingdao, People’s Republic of China
Xiuxiu Fu, Xiangqin He & Tao Yu
You can also search for this author in PubMed Google Scholar
Contributions
T.L. collected materials and wrote the manuscript. T.Y., H.Q., P.L., and Y.Y. provided the idea. L.Z. and T.L. are responsible for constructing the schematic diagrams presented within this article. T.Y., Y.Y., X.L., Z.W., P.L., L.Z., and X.F. helped with the final revision of the article. All authors reviewed the manuscript and approved the final manuscript.
Corresponding authors
Correspondence to Pei-feng Li or Tao Yu .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Li, T., Yang, Y., Qi, H. et al. CRISPR/Cas9 therapeutics: progress and prospects. Sig Transduct Target Ther 8 , 36 (2023). https://doi.org/10.1038/s41392-023-01309-7
Download citation
Received : 29 August 2022
Revised : 06 December 2022
Accepted : 27 December 2022
Published : 16 January 2023
DOI : https://doi.org/10.1038/s41392-023-01309-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Finely tuned ionizable lipid nanoparticles for crispr/cas9 ribonucleoprotein delivery and gene editing.
- Mincheol Jang
- Hyun Jung Chung
Journal of Nanobiotechnology (2024)
CRISPR/Cas9 targeting of passenger single nucleotide variants in haploinsufficient or essential genes expands cancer therapy prospects
- Hakhyun Kim
- Jang Hee Han
- Hyun Seok Kim
Scientific Reports (2024)
The consequences of viral infection on protists
- Victoria Fulgencio Queiroz
- Juliana Miranda Tatara
- Jonatas Santos Abrahao
Communications Biology (2024)
Gene targeting in adult organs using in vivo cleavable donor plasmids for CRISPR-Cas9 and CRISPR-Cas12a
- Riki Ishibashi
- Ritsuko Maki
- Fumiko Toyoshima
mRNA biotherapeutics landscape for rare genetic disorders
- V Rajesh Iyer
- Rakesh K Mishra
Journal of Biosciences (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
How CRISPR Is Changing Cancer Research and Treatment
July 27, 2020 , by NCI Staff
CRISPR is a highly precise gene editing tool that is changing cancer research and treatment.
Ever since scientists realized that changes in DNA cause cancer , they have been searching for an easy way to correct those changes by manipulating DNA . Although several methods of gene editing have been developed over the years, none has really fit the bill for a quick, easy, and cheap technology.
But a game-changer occurred in 2013, when several researchers showed that a gene-editing tool called CRISPR could alter the DNA of human cells like a very precise and easy-to-use pair of scissors.
The new tool has taken the research world by storm, markedly shifting the line between possible and impossible. As soon as CRISPR made its way onto the shelves and freezers of labs around the world, cancer researchers jumped at the chance to use it.
“CRISPR is becoming a mainstream methodology used in many cancer biology studies because of the convenience of the technique,” said Jerry Li, M.D., Ph.D., of NCI’s Division of Cancer Biology .
Now CRISPR is moving out of lab dishes and into trials of people with cancer. In a small study, for example, researchers tested a cancer treatment involving immune cells that were CRISPR-edited to better hunt down and attack cancer.
Despite all the excitement, scientists have been proceeding cautiously, feeling out the tool’s strengths and pitfalls, setting best practices, and debating the social and ethical consequences of gene editing in humans.
How Does CRISPR Work?
Like many other advances in science and medicine, CRISPR was inspired by nature. In this case, the idea was borrowed from a simple defense mechanism found in some microbes, such as bacteria.
To protect themselves against invaders like viruses, these microbes capture snippets of the intruder’s DNA and store them away as segments called CRISPRs, or clustered regularly interspersed short palindromic repeats. If the same germ tries to attack again, those DNA segments (turned into short pieces of RNA ) help an enzyme called Cas find and slice up the invader’s DNA.
After this defense system was discovered, scientists realized that it had the makings of a versatile gene-editing tool. Within a handful of years, multiple groups had successfully adapted the system to edit virtually any section of DNA, first in the cells of other microbes, and then eventually in human cells.
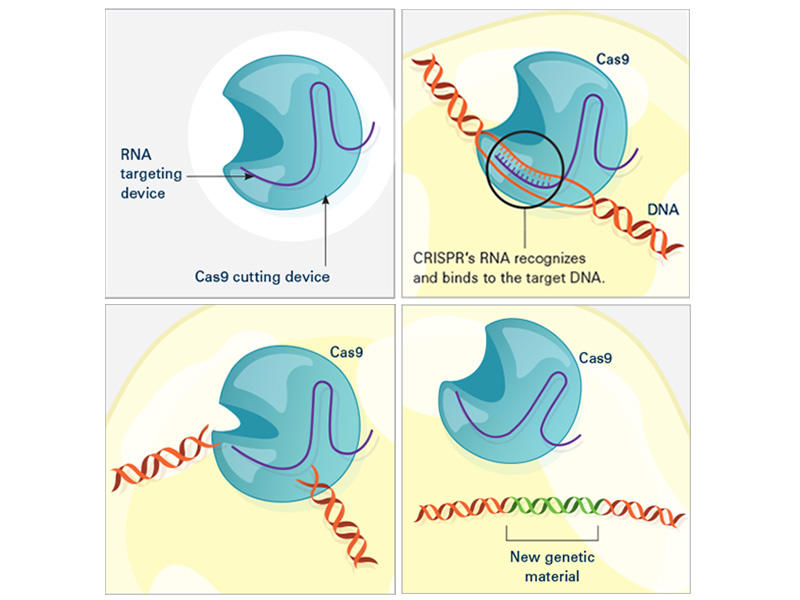
CRISPR consists of a guide RNA (RNA-targeting device, purple) and the Cas enzyme (blue). When the guide RNA matches up with the target DNA (orange), Cas cuts the DNA. A new segment of DNA (green) can then be added.
In the laboratory, the CRISPR tool consists of two main actors: a guide RNA and a DNA-cutting enzyme, most commonly one called Cas9. Scientists design the guide RNA to mirror the DNA of the gene to be edited (called the target). The guide RNA partners with Cas and—true to its name—leads Cas to the target. When the guide RNA matches up with the target gene's DNA, Cas cuts the DNA.
What happens next depends on the type of CRISPR tool that’s being used. In some cases, the target gene's DNA is scrambled while it's repaired, and the gene is inactivated . With other versions of CRISPR, scientists can manipulate genes in more precise ways such as adding a new segment of DNA or editing single DNA letters .
Scientists have also used CRISPR to detect specific targets, such as DNA from cancer-causing viruses and RNA from cancer cells . Most recently, CRISPR has been put to use as an experimental test to detect the novel coronavirus .
Why Is CRISPR a Big Deal?
Scientists consider CRISPR to be a game-changer for a number of reasons. Perhaps the biggest is that CRISPR is easy to use, especially compared with older gene-editing tools.
“Before, only a handful of labs in the world could make the proper tools [for gene editing]. Now, even a high school student can make a change in a complex genome ” using CRISPR, said Alejandro Chavez, M.D., Ph.D., an assistant professor at Columbia University who has developed several novel CRISPR tools.
CRISPR is also completely customizable. It can edit virtually any segment of DNA within the 3 billion letters of the human genome, and it’s more precise than other DNA-editing tools.
And gene editing with CRISPR is a lot faster. With older methods, “it usually [took] a year or two to generate a genetically engineered mouse model , if you’re lucky,” said Dr. Li. But now with CRISPR, a scientist can create a complex mouse model within a few months, he said.
Another plus is that CRISPR can be easily scaled up. Researchers can use hundreds of guide RNAs to manipulate and evaluate hundreds or thousands of genes at a time. Cancer researchers often use this type of experiment to pick out genes that might make good drug targets .
And as an added bonus, “it’s certainly cheaper than previous methods,” Dr. Chavez noted.
What Are CRISPR’s Limitations?
With all of its advantages over other gene-editing tools, CRISPR has become a go-to for scientists studying cancer. There’s also hope that it will have a place in treating cancer, too. But CRISPR isn’t perfect, and its downsides have made many scientists cautious about its use in people.
A major pitfall is that CRISPR sometimes cuts DNA outside of the target gene—what’s known as “off-target” editing. Scientists are worried that such unintended edits could be harmful and could even turn cells cancerous , as occurred in a 2002 study of a gene therapy .
“If [CRISPR] starts breaking random parts of the genome, the cell can start stitching things together in really weird ways, and there’s some concern about that becoming cancer,” Dr. Chavez explained. But by tweaking the structures of Cas and the guide RNA, scientists have improved CRISPR’s ability to cut only the intended target, he added.
Another potential roadblock is getting CRISPR components into cells. The most common way to do this is to co-opt a virus to do the job. Instead of ferrying genes that cause disease, the virus is modified to carry genes for the guide RNA and Cas.
Slipping CRISPR into lab-grown cells is one thing; but getting it into cells in a person's body is another story. Some viruses used to carry CRISPR can infect multiple types of cells, so, for instance, they may end up editing muscle cells when the goal was to edit liver cells.
Researchers are exploring different ways to fine-tune the delivery of CRISPR to specific organs or cells in the human body. Some are testing viruses that infect only one organ, like the liver or brain. Others have created tiny structures called nanocapsules that are designed to deliver CRISPR components to specific cells.
Because CRISPR is just beginning to be tested in humans, there are also concerns about how the body—in particular, the immune system —will react to viruses carrying CRISPR or to the CRISPR components themselves.
Some wonder whether the immune system could attack Cas (a bacterial enzyme that is foreign to human bodies) and destroy CRISPR-edited cells. Twenty years ago, a patient died after his immune system launched a massive attack against the viruses carrying a gene therapy he had received. However, newer CRISPR-based approaches rely on viruses that appear to be safer than those used for older gene therapies.
Another major concern is that editing cells inside the body could accidentally make changes to sperm or egg cells that can be passed on to future generations. But for almost all ongoing human studies involving CRISPR, patients’ cells are removed and edited outside of their bodies. This “ ex vivo ” approach is considered safer because it is more controlled than trying to edit cells inside the body, Dr. Chavez said.
However, one ongoing study is testing CRISPR gene editing directly in the eyes of people with a genetic disease that causes blindness, called Leber congenital amaurosis.
The First Clinical Trial of CRISPR for Cancer
The first trial in the United States to test a CRISPR-made cancer therapy was launched in 2019 at the University of Pennsylvania. The study, funded in part by NCI, is testing a type of immunotherapy in which patients’ own immune cells are genetically modified to better “see” and kill their cancer.
The therapy involves making four genetic modifications to T cells , immune cells that can kill cancer. First, the addition of a synthetic gene gives the T cells a claw-like protein (called a receptor ) that “sees” NY-ESO-1, a molecule on some cancer cells.
Then CRISPR is used to remove three genes: two that can interfere with the NY-ESO-1 receptor and another that limits the cells’ cancer-killing abilities. The finished product, dubbed NYCE T cells, were grown in large numbers and then infused into patients.
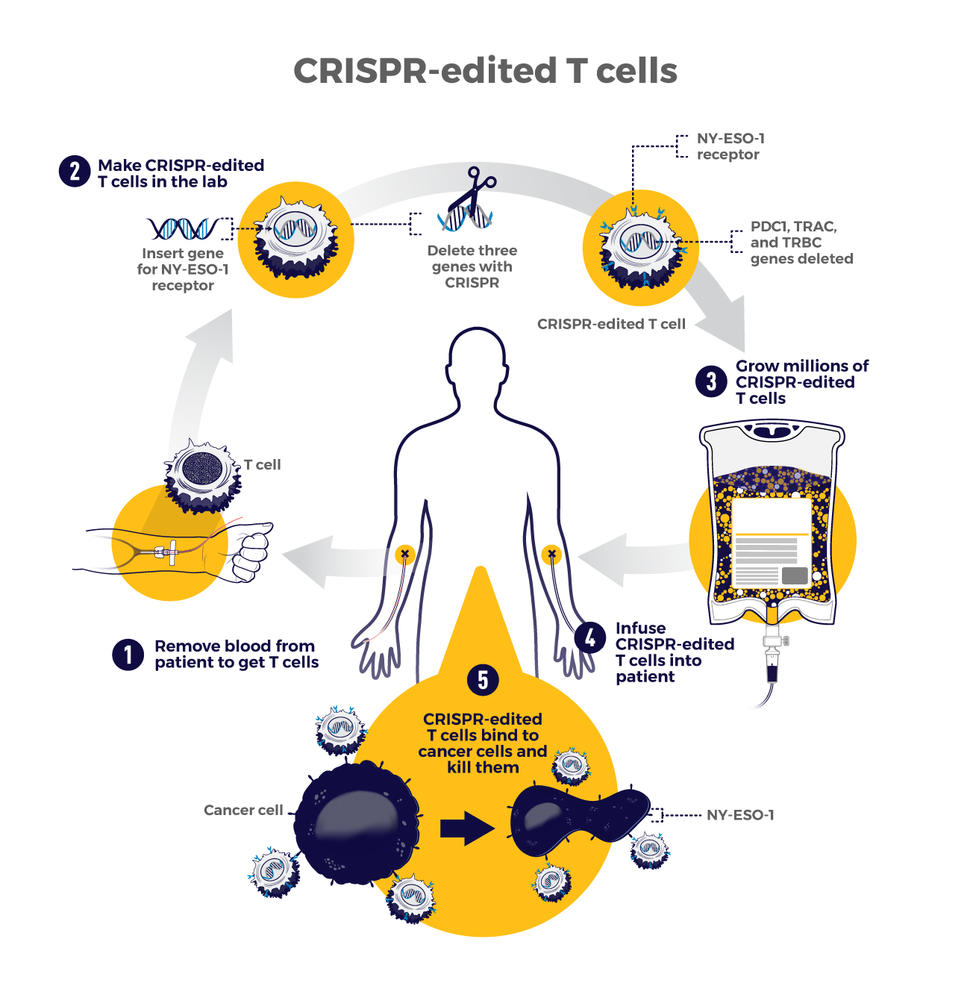
The first trial of CRISPR for patients with cancer tested T cells that were modified to better "see" and kill cancer. CRISPR was used to remove three genes: two that can interfere with the NY-ESO-1 receptor and another that limits the cells’ cancer-killing abilities.
“We had done a prior study of NY-ESO-1–directed T cells and saw some evidence of improved response and low toxicity ,” said the trial’s leader, Edward Stadtmauer, M.D., of the University of Pennsylvania. He and his colleagues wanted to see if removing the three genes with CRISPR would make the T cells work even better, he said.
The goal of this study was to first find out if the CRISPR-made treatment was safe. It was tested in two patients with advanced multiple myeloma and one with metastatic sarcoma . All three had tumors that contained NY-ESO-1, the target of the T-cell therapy.
Initial findings suggest that the treatment is safe . Some side effects did occur, but they were likely caused by the chemotherapy patients received before the infusion of NYCE cells, the researchers reported. There was no evidence of an immune reaction to the CRISPR-edited cells.
Only about 10% of the T cells used for the therapy had all four of the desired genetic edits. And off-target edits were found in the modified cells of all three patients. However, none of the cells with off-target edits grew in a way that suggested they had become cancer, Dr. Stadtmauer noted.
The treatment had a small effect on the patients’ cancers. The tumors of two patients (one with multiple myeloma and one with sarcoma) stopped growing for a while but resumed growing later. The treatment didn't work at all for the third patient.
It's exciting that the treatment initially worked for the sarcoma patient because “ solid tumors have been a much more difficult nut to crack with cellular therapy," Dr. Stadtmauer said. "Perhaps [CRISPR] techniques will enhance our ability to treat solid tumors with cell therapies.”
Although the trial shows that CRISPR-edited cell therapy is possible, the long-term effects still need to be monitored, Dr. Stadtmauer continued. The NYCE cells are “safe for as long as we’ve been watching [the study participants]. Our plan is to keep monitoring them for years, if not decades,” he said.
More Studies of CRISPR Treatments to Come
While the study of NYCE T cells marked the first trial of a CRISPR-based cancer treatment, there are likely more to come.
“This [trial] was really a proof-of-principle, feasibility, and safety thing that now opens up the whole world of CRISPR editing and other techniques of [gene] editing to hopefully make the next generation of therapies,” Dr. Stadtmauer said.
Other clinical studies of CRISPR-made cancer treatments are already underway. A few trials are testing CRISPR-engineered CAR T-cell therapies , another type of immunotherapy. For example, one company is testing CRISPR-engineered CAR T cells in people with B cell cancers and people with multiple myeloma .
There are still a lot of questions about all the ways that CRISPR might be put to use in cancer research and treatment. But one thing is for certain: The field is moving incredibly fast and new applications of the technology are constantly popping up.
“People are still improving CRISPR methods,” Dr. Li said. “It’s quite an active area of research and development. I’m sure that CRISPR will have even broader applications in the future.”
Featured Posts
March 27, 2024, by Edward Winstead
March 21, 2024, by Elia Ben-Ari
March 5, 2024, by Carmen Phillips
- Biology of Cancer
- Cancer Risk
- Childhood Cancer
- Clinical Trial Results
- Disparities
- FDA Approvals
- Global Health
- Leadership & Expert Views
- Screening & Early Detection
- Survivorship & Supportive Care
- February (6)
- January (6)
- December (7)
- November (6)
- October (7)
- September (7)
- February (7)
- November (7)
- October (5)
- September (6)
- November (4)
- September (9)
- February (5)
- October (8)
- January (7)
- December (6)
- September (8)
- February (9)
- December (9)
- November (9)
- October (9)
- September (11)
- February (11)
- January (10)

- This is Broad Learn about our mission, history, and partner institutions.
- People Meet our members, staff scientists, fellows, leadership, and other Broadies.
- Join Broad Find out how to join the Broad as an employee or associate member.
- Contact us Find our contact information, directions to our buildings, and directory.
- Broad@20 Broad turns 20 this year! See how we’re celebrating our 20th anniversary.
- Cardiovascular disease
- Infectious disease and microbiome
- Kidney disease
- Psychiatric disorders
- Rare disease
- Chemical biology and therapeutics science
- Drug discovery
- Genome regulation, cellular circuitry, and epigenomics
- Medical and population genetics
- Data sciences
- Genetic perturbation
- Metabolomics
- Spatial technologies
- Patient-partnered research Patients partner with our scientists to accelerate the pace of discovery and find better treatments.
- Partnering and licensing We work closely with pharmaceutical, biotech, and technology partners to accelerate the translation of our discoveries.
- Publications A catalog of scientific papers published by our members and staff scientists.
- Resources, services, and tools Key scientific datasets and computational tools developed by our scientists and their collaborators.
- Collaborations and consortia We join with institutions and scientists the world over to address foundational challenges in science and health.
- Carlos Slim Center for Health Research The Slim Center aims to bring the benefits of genomics-driven medicine to Latin America, gleaning new insights into diseases with relevance to the region.
- Gerstner Center for Cancer Diagnostics The Gerstner Center is developing next-generation diagnostic technology for cancer detection and tracking disease progression.
- Klarman Cell Observatory The Klarman Cell Observatory is systematically defining mammalian cellular circuits, how they work together to create tissues and organs, and are perturbed to cause disease.
- Merkin Institute for Transformative Technologies in Healthcare The Merkin Institute is supporting early-stage ideas aimed at advancing powerful technological approaches for improving how we understand and treat disease.
- Novo Nordisk Foundation Center for Genomic Mechanisms of Disease This center is developing new paradigms and technologies to scale the discovery of biological mechanisms of common, complex diseases, by facilitating close collaborations between the Broad Institute and the Danish research community.
- Eric and Wendy Schmidt Center The EWSC is catalyzing a new field of interdisciplinary research at the intersection of data science and life science, aimed at improving human health.
- Stanley Center for Psychiatric Research The Stanley Center aims to reduce the burden of serious mental illness by contributing new insights into pathogenesis, identifying biomarkers, and paving the way toward new treatments.
- Art and science connection Explore the connection between art and science and how we bring together artists and Broad scientists through our artist-in-residence program, gallery exhibitions, and ongoing public conversations.
- Broad Discovery Center Visit our free public educational space that showcases how researchers at the Broad and their colleagues around the world seek to understand and treat human disease.
- Learning resources Access free classroom materials and more for STEM educators, parents, students, tutors, and others.
- Public programs Discover remarkable stories of scientific progress, and explore the intersections of science, medicine, and society.
- Student opportunities Learn about Broad Institute's mentored research offerings for high school students, college students, and recent college graduates.
- Visit Broad Come see what Broad is all about.
- Sign up for our newsletter Receive regular updates on Broad news, research and community.
- Research Highlights: CRISPR

The ability to precisely edit the genome of a living cell holds enormous potential to accelerate life science research, improve biotechnology, and even treat human disease.
Methods for genome editing — primarily zinc finger nucleases and Transcription Activator-Like Effector (TALE) Nucleases — have existed for several years, but in 2013 these were quickly eclipsed by the efficiency, effectiveness and precision of the engineered CRISPR-Cas9 system that was first harnessed for mammalian genome editing by Feng Zhang of the Broad Institute and MIT.
The CRISPR system
Like zinc fingers and TALEs, CRISPR systems are natural products. However, CRISPR-Cas differs from zinc fingers and TALEs in one crucial aspect that makes it superior for genome editing applications: whereas zinc fingers and TALEs bind to DNA through a direct protein-DNA interaction, requiring the protein to be redesigned for each new target DNA site, CRISPR-Cas achieves target specificity through a small RNA that can easily be swapped for other RNAs targeting new sites.
In nature, CRISPR-Cas systems help bacteria defend against attacking viruses (known as bacteriophage or just phage). They consist of two components, the CRISPR (clustered, regularly interspaced palindromic repeats) array and Cas (CRISPR-associated) proteins. CRISPR sequences bookend short stretches of DNA that bacteria have copied from invading phages, preserving a memory of the viruses that have attacked them in the past. These sequences are then transcribed into short RNAs that guide Cas proteins to matching viral sequences. The Cas proteins destroy the matching viral DNA by cutting it. There are a number of different types of CRISPR-Cas systems in nature, which vary in their components; the CRISPR-Cas9 system uses just a single protein, Cas9, to find and destroy target DNA. In 2015, Zhang and colleagues successfully harnessed a second system, called CRISPR-Cpf1 , which has the potential for even simpler and more precise genome engineering.
Engineering the CRISPR toolbox
In early 2011, Feng Zhang was just starting his own research group at the Broad Institute and MIT, where he is an investigator at the McGovern Institute for Brain Research and a faculty member in the departments of Brain and Cognitive Sciences and Biological Engineering. After learning about existing CRISPR research at a scientific meeting at the Broad, he quickly realized that the system, with a single RNA-guided protein, could be a game changer in genome editing technology. He was already working on DNA targeting methods, having helped to develop the TALE system as a Junior Fellow at Harvard. This system could target and activate genes in mammalian genomes.
Zhang and his team focused on harnessing CRISPR-Cas9 for use in human cells. In January 2013, he reported the first successful demonstration of Cas9-based genome editing in human cells in what has become the most-cited CRISPR paper (Cong et al., Science, 2013). Researchers from George Church’s lab at Harvard University reported similar findings in the same issue of Science (Mali et al., Science, 2013). The Zhang and Church papers showed that Cas9 could be targeted to a specific location in the human genome and cut the DNA there. The cut DNA was then repaired by inserting a new stretch of DNA, supplied by the researchers, essentially achieving “find and replace” functionality in the human genome.
In September, 2015, Zhang and partners described a different system, Cpf1, which appears to have significant implications for research and therapeutics. The Cpf1 system is simpler in that it requires only a single RNA. The Cpf1 enzyme is also smaller than the standard SpCas9, making it easier to deliver into cells and tissues.
Refining and sharing CRISPR tools

At the Broad Institute, the system has also been used for genome-wide screens to identify genes involved in resistance to cancer drugs and dissect immune regulatory networks. CRISPR has been used to rapidly create mouse models of cancer that arise from multiple gene alterations (Platt et al., Cell, 2014). In 2015, Zhang and his team reported success with Cas9 derived from a different bacterium , Staphylococcus aureus (SaCas9). SaCas9 is smaller than the original Cas9, which has advantages for gene therapy (Ran et al., Nature, 2015).
The Zhang lab has trained thousands of researchers in the use of CRISPR-Cas9 genome editing technology through direct education and by sharing more than 40,000 CRISPR-Cas9 components with academic laboratories around the world to help accelerate global research that will benefit human health. In September 2015, the Zhang lab also began to share Cpf1 components.
Users can obtain guide sequences for knock-outs and transcriptional activation as well as information about genome-wide libraries for CRISPR-based screening. The Zhang lab plasmids can be obtained via Addgene , a non-profit plasmid repository dedicated to improving life science research. Additional information about materials can be found on the Zhang laboratory website.
What is CRISPR
- Questions and Answers about CRISPR
- CRISPR Timeline
- CRISPR Research News
- Feng Zhang Bio
- David Liu Bio
- Genetic Perturbation Platform
Patents & Licensing
- For journalists: Updates on the patent process
- Information about licensing of CRISPR tools
- Information about CRISPR-Cpf1 systems
For Researchers
Broad Institute Media Relations 617-714-8600 [email protected]
Latest news

A Review on the Mechanism and Applications of CRISPR/Cas9/Cas12/Cas13/Cas14 Proteins Utilized for Genome Engineering
- Review Paper
- Published: 27 September 2022
- Volume 65 , pages 311–325, ( 2023 )
Cite this article

- V. Edwin Hillary ORCID: orcid.org/0000-0002-6208-5332 1 &
- S. Antony Ceasar ORCID: orcid.org/0000-0003-4106-1531 1
21k Accesses
48 Citations
24 Altmetric
Explore all metrics
The clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated protein (CRISPR/Cas) system has altered life science research offering enormous options in manipulating, detecting, imaging, and annotating specific DNA or RNA sequences of diverse organisms. This system incorporates fragments of foreign DNA (spacers) into CRISPR cassettes, which are further transcribed into the CRISPR arrays and then processed to make guide RNA (gRNA). The CRISPR arrays are genes that encode Cas proteins. Cas proteins provide the enzymatic machinery required for acquiring new spacers targeting invading elements. Due to programmable sequence specificity, numerous Cas proteins such as Cas9, Cas12, Cas13, and Cas14 have been exploited to develop new tools for genome engineering. Cas variants stimulated genetic research and propelled the CRISPR/Cas tool for manipulating and editing nucleic acid sequences of living cells of diverse organisms. This review aims to provide detail on two classes (class 1 and 2) of the CRISPR/Cas system, and the mechanisms of all Cas proteins, including Cas12, Cas13, and Cas14 discovered so far. In addition, we also discuss the pros and cons and recent applications of various Cas proteins in diverse fields, including those used to detect viruses like severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). This review enables the researcher to gain knowledge on various Cas proteins and their applications, which have the potential to be used in next-generation precise genome engineering.
Similar content being viewed by others

Non-coding RNAs: Classification, Biology and Functioning

Unraveling the mechanisms of PAMless DNA interrogation by SpRY-Cas9
Advances in miniature CRISPR-Cas proteins and their applications in gene editing
Avoid common mistakes on your manuscript.
Introduction
Genome editing or gene editing is a popular technology used in medicines, therapeutic drugs, infectious studies, and agricultural biotechnology. The genome-editing tools have been employed to study the precise function of a gene by cutting and altering at a programmed locus through insertion, deletion, or replacement of targeted bases. In the beginning, conventional gene-editing techniques like homologous recombination (HR) were utilized for gene inactivation, but the effectiveness of HR was extremely low with the labor-intensive process [ 1 ]. Later, targeted gene knock-down utilizing RNA interference (RNAi) technique has provided researchers with rapid and low-cost technology to silence the gene of interest to study its functions. However, it also could not completely knock-down the targeted sequence, which faced unpredictable off-target effects and delivered only temporary or partial inhibition of gene function [ 2 ].
The genome-editing technique needs programmable sequence-specific endonucleases to produce the site-specific single-stranded breaks (SSBs) or double-stranded breaks (DSBs) at the targeted site that allow the endogenous repair mechanisms to fill the breaks [ 3 ]. These breaks are fixed by either of the two major repair mechanisms, (1) homology-directed repair (HDR) and (2) non-homologous end-joining repair (NHEJ) [ 4 ]. To facilitate specific DNA breaks, various genome-editing tools are developed previously, such as meganucleases or homing endonucleases [ 5 ], zinc-finger nucleases (ZFNs) [ 6 ], and transcription activator-like effector nucleases (TALENs) [ 7 ]. But these tools demand laborious efforts for cloning and protein construction to make DSBs, which hinders these tools from routine applications of genome editing. In 2012, the researchers developed clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated proteins (Cas) system-based genome editing tools [ 8 ]. CRISPR/Cas system mediates diverse adaptive immune systems against phages or plasmids. Due to unique features of simplicity in design, cost-effectiveness, and labor intensity, the research community has immediately adopted the CRISPR/Cas system as a user-friendly and robust RNA-guided DNA targeting tool for genome editing in various species [ 8 , 9 , 10 , 11 ]. Based on the mechanism, the CRISPR system has been divided into two main classes (1 and 2) and six types (I-VI) [ 12 ]. In these, types I to III were extensively studied, whereas types IV and VI were recently discovered. Types I, II, and V cut DNA, type VI cleaves RNA, type III cleaves both DNA and RNA and the cleavage activity of type IV has not yet been identified [ 13 ].
Cas9 protein has been utilized for diverse applications such as fluorescent imaging, base-editing, and transcriptional activation, apart from targeted cleavage of dsDNA. Like Cas9, the Cas10 protein is also involved in various applications such as fluorescent imaging, base-editing, and RNA tracking. As an alternative to Cas9 and Cas10, the Cas12 protein enhanced genome-editing efficiency by targeting only T-rich motifs without utilizing tracrRNA. So Cas12 system has expanded editing applications such as base-editing and detecting transcriptional variations. Cas13 protein is also used for diverse applications such as imaging, base-editing, and detection of transcriptional variations. Recently, the Cas14 protein has advanced genome-editing efficiency without needing an adjacent protospacer motif (PAM) and performs transcriptional regression and base-editing. This review describes the Cas variants of two classes of CRISPR systems used for genome editing.
History of CRISPR/Cas System
A group of researchers detected CRISPRs by analyzing the alkaline phosphatase gene, which is liable for the isozyme conversion of alkaline phosphatase ( iap ) in the E. coli K-12 strain [ 14 ]. They identified a genomic region that contains a series of 32 nucleotides of distinctive sequences flanked by invariable palindromic repeats on the 3′ end of the iap gene [ 14 ]. Later, distinctive parallel sequences were found in the other E. coli strains and Enterobacteria ( Shigella dysenteriae and Salmonella enterica ) [ 15 ]. Similarly, during the study of Mycobacterium tuberculosis strains, researchers identified 36 bp repeats interspaced with unique spacers of 35–41 bp [ 16 ].
In subsequent works, the CRISPR array was found in archaea ( Haloferax mediterranei and Streptococcus thermopile ), and the same was identified in 90% bacterial and 40% archaeal genomes [ 17 ]. However, this odd genomic sequence first turned out to be the outline of the CRISPR array. Still, due to the absence of necessary genome sequence data, the biological function of CRISPR remained elusive. Jansen et al. in 2002 described the CRISPR-associated genes [ 18 ]. Following this, several CRISPR/Cas genes were identified. In 2005, spacer sequence was identified in many genomes, and unique spacer regions were found within the CRISPR array [ 19 ]. These results showed that CRISPR is an adaptive immune system to defend prokaryotic cells against phage infection through the RNA-guided process.
Classification of CRISPR/Cas System
The CRISPR system has been classified into two major classes. In the Class 1 system, the RNA-guided target cleavage needs several effector proteins, but the Class 2 system requires only one RNA-guided endonuclease to cleave the DNA sequences [ 12 , 20 ]. The class 1 system of CRISPR is divided into three types I, III, and IV, and the Class 2 system is divided into types II, V, and VI [ 21 , 22 ]. In the type I system, the CRISPR/Cas locus contains the Cas3 signature gene that encodes a large protein with a helicase to unwind DNA-DNA and RNA–DNA duplexes [ 23 ]. The type II locus encodes multidomain protein to target and cleave the dsDNA [ 8 ]. The type III CRISPR/Cas possesses the Cas10 signature gene, encodes a multidomain protein with palm domain to target, and cleaves ssDNA [ 24 ]. Type IV system contains CRISPR-associated splicing factor 1 (Csf1), which encodes a ribonucleic protein, but the detailed function of this system is yet to be identified [ 21 ]. Type V locus possesses Cas12 signature gene (known as CRISPR from Prevotella and Francisella 1 (Cpf1), C2c1 or C2c3 protein) encodes RuvC (an E. coli protein involved in DNA repair) domain, which cleaves both dsDNA or ssDNA [ 25 ]. Type VI contains Cas13 (C2c2) that encodes higher eukaryotes and prokaryotes' nucleotide-binding domain (HEPN), which cleaves ssRNA [ 26 ] (Table 1 ).
The CRISPR/Cas system uses three stages to defend against viruses or foreign genetic materials [ 27 ] (Fig. 1 ). In the first stage, protospacers are incorporated into the host CRISPR locus as spacers between crRNA repeats [ 28 ]; next, Cas proteins are expressed, a spacer is transcribed into pre-crRNA, and the pre-crRNA is cleaved by Cas proteins and becomes functional as a mature crRNA [ 28 ]. In the third stage, Cas protein recognizes the target with the help of crRNA and generates cleavage of the genome [ 28 ]. Many CRISPR systems act based on the presence of a sequence-specific PAM, which is adjacent to the crRNA-specific site within the target genome.
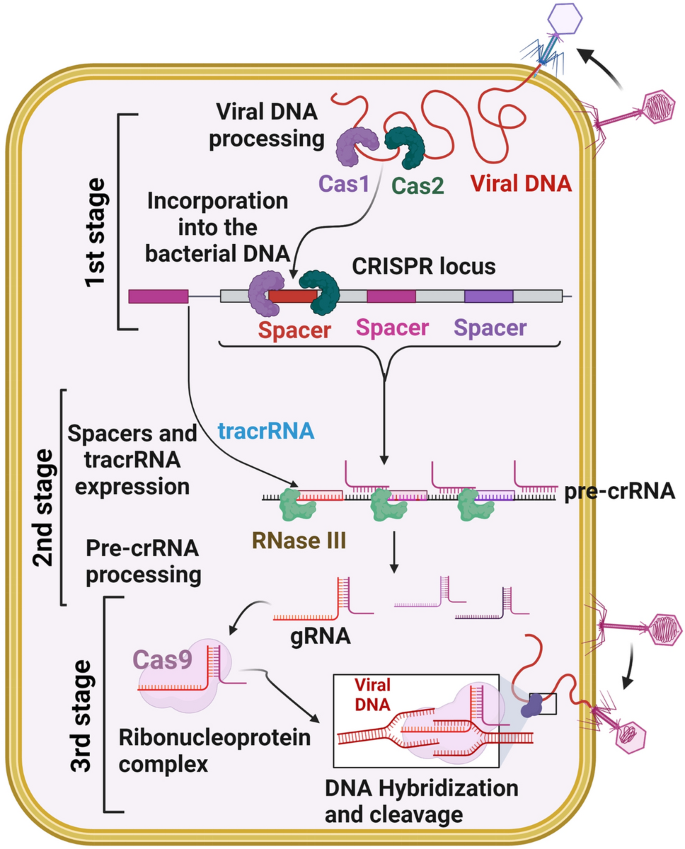
CRISPR/Cas adaptive immunity system. The three stages such as CRISPR adaptation (stage 1), CRISPR RNA biogenesis (stage 2), and CRISPR interference (stage 3) are schematically illustrated. During the adaption stage, the injection of genetic material (virus) into bacterial cells triggers the Cas1 and Cas2 adaption module proteins, which cleaves the invading sequences (spacers) and are then incorporated into the CRISPR array. During CRISPR/RNA biogenesis stage, the CRISPR array is transcribed into a precursor to crRNA molecules (pre-crRNA), which are then cleaved into mature crRNAs. These mature crRNAs form effector complexes with Cas proteins. When foreign genetic material sequences match a CRISPR spacer, the matching crRNA binds to the invading strand and cleaves the invading strand with the help of Cas nuclease (CRISPR interference stage)
CRISPR/Cas System
Scientists have discovered a novel microbial defense system that defends itself from viral and mobile genetic elements. One of the defense mechanisms found in bacterium and archaea is referred to as CRISPR/Cas system. By integrating DNA sequences into their genome identical to previous invaders, bacteria and archaea generate a cellular memory of them [ 29 ]. These acquired sequences permit them to spot viruses or mobile genetic invaders resulting in the degradation of the invading sequences and work as an adaptive immune system [ 29 , 30 ].
CRISPR immunity is denoted by distinct phases. In the adaptation phase, bacterium and archaea gain cellular memory of the invading phages [ 30 ]. The phage genome sequences are integrated into the CRISPR locus of the bacterial or archaeal genomes, composed of 24 to 47 base pair (bp) repeats and separated by spacers. The unique loci of the CRISPR system were first discovered in 1987 [ 14 ]. Then Bolotin et al. in 2005 identified CRISPR’s function (enzymes encoding Cas genes generate DNA fragmentation) involved in protecting the bacteria and archaea from foreign invaders [ 31 ]. Later, scientists revealed that the CRISPR/Cas system works as an adaptive immune system [ 32 ] that was eventually adopted as a versatile system for RNA programmable genome editing [ 8 ].
Cas Proteins of the CRISPR System
Cas proteins have gained popularity among the research community for broader genome engineering applications and are currently used in diverse fields, including biotechnology, agriculture, and medical research. Recently discovered programmable Cas proteins like Cas 12, Cas 13, and Cas 14 have improved the precision of the CRISPR/Cas-mediated genome editing (Table 2 ). The mechanism of these different Cas proteins, pros and cons, and their applications are summarized in detail below.
Cas1 and Cas2 Proteins
Cas1 and Cas2 are generally conserved proteins in the prokaryotic adaptive immune system [ 28 ]. CRISPR/Cas system consists of a CRISPR array containing the repeats (∼30 to 40 bp) separated by spacers, an adjacent module comprising Cas1 and Cas2, and the distinctive signature proteins [ 41 ].
Mechanism of Cas1 and Cas2 Proteins
Cas1 and Cas2 belong to the Type II CRISPR system found in E. coli [ 42 ]. E. coli Cas1-Cas2 complex mediates spacer acquisition in vivo; however, the molecular mechanism behind these proteins throughout immunity is unclear [ 43 ]. Cas1 and Cas2 proteins formed an integrase complex consisting of two distal Cas1 dimers bridged by a Cas2 dimer [ 44 ]. The Cas1 and Cas2 proteins bind to the pre-spacer like twin-folded DNA. The pre-spacer integrated the proximal leader region of the CRISPR array guided by the leader sequence with a pair of inverted repeats inside the CRISPR repeat [ 45 ].
Applications of Cas1 and Cas2 Proteins
Cas1 and Cas2 are the conserved proteins among all CRISPR/Cas systems. The molecular mechanism behind the Cas1 and Cas2 proteins is still unclear. Hence, researchers have not applied these proteins for genome-editing in various fields.

Pros and Cons of Cas1 and Cas2 Proteins
So far, no genome editing studies have been demonstrated via Cas1 and Cas2 proteins; therefore, significant merits and demerits of Cas1 and Cas2 proteins should be evaluated before applying these in diverse fields.
Cas9 Protein
Cas9 is a protein associated with the CRISPR adaptive immune systems of S. pyogenes and is referred to as Spy Cas9 protein. The Spy Cas9 protein had a large multifunctional domain of 1368 amino acids and acts as a DNA endonuclease in natural and artificial CRISPR/Cas systems.
Mechanism of Cas9 Protein
The mechanism of Cas9 protein has been studied extensively. Cas9 protein has six domains (1) Recognition lobe (REC I), (2) REC II, (3) Arginine-rich bridge helix, (4) PAM Interacting, (5) HNH, and (6) RuvC. REC I is the major domain responsible for binding with the gRNA; the REC II function is not studied [ 46 ]. The arginine-rich bridge helix initiates cleavage activity upon binding to targeted sequences. The interaction with PAM confers PAM specificity, which is responsible for binding with the target sequence. The HNH and RuvC are nuclease domains to chop the target sequence [ 46 , 47 ] (Fig. 2 ).
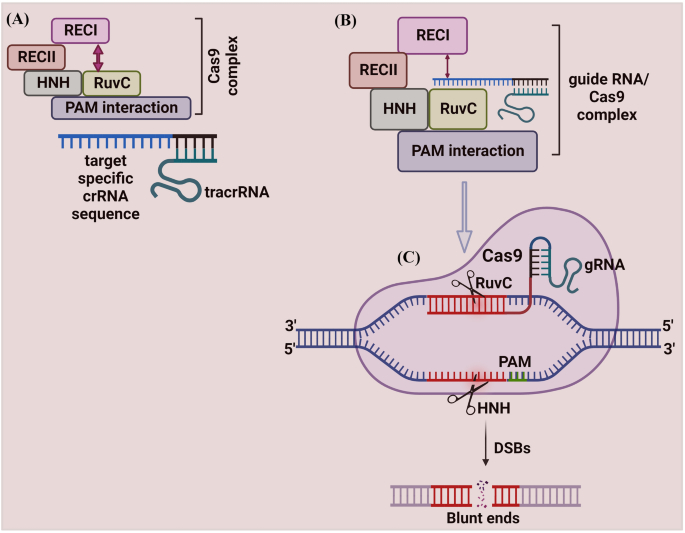
Schematic illustration of CRISPR/Cas9 mechanism. A The Cas9 protein complex contains six domains (Recognition lobe (REC I), REC II, Arginine-rich bridge helix, PAM Interacting, HNH, and RuvC). REC I is the major domain responsible for binding with the gRNA, the REC II function is not studied. The arginine-rich bridge helix initiates cleavage activity upon binding to targeted sequences. The interaction with PAM confers PAM specificity, which is responsible for binding with the target sequence. The HNH and RuvC are nuclease domains to chop the target sequence. The Cas9 protein remains inactive due to the absence of gRNA. B The programmed gRNA binds to the Cas9 and generates changes in the protein, which leads the inactive Cas9 protein into its active form. Once triggered, it searches the target sequence by binding with a sequence that matches the PAM sequence (5′-NGG-3′). Then Cas9 generates DSBs at 3 bp upstream of the PAM using its HNH and RuvC domains
The Cas9 protein remains inactive due to the absence of gRNA. The engineered gRNA forms a T-shape involved in 1 tetra-loop and 3 stem-loops [ 8 , 46 ]. The gRNA is programmed to own a 5′ end, which is complementary to the target sequence. The programmed gRNA binds to the Cas9 and generates changes in the protein, which leads the inactive Cas9 protein into its active form. Once triggered, it searches the target sequence by binding with a sequence that matches the PAM sequence (5′-NGG-3′). Then Cas9 cuts dsDNA at 3 bp upstream of the PAM using its HNH and RuvC domains. The HNH domain cleaves the DNA strand that is complementary to the 20-nucleotide sequence (gRNA) of crRNA (target strand)) and the RuvC domain cleaves the DNA strand opposite to the complementary strand (non-target DNA strand). The spy Cas9 system for cleaving target DNA recognizes a short ‘seed’ sequence with the 5'-NGG-3' di-nucleotide containing PAM [ 8 ]. The twin tracrRNA: crRNA was fused into a single guide RNA (sgRNA), making the CRISPR/Cas9 system to cut the targeted dsDNA or ssDNA sequences [ 8 ].
Application of Cas9 Protein
Cas9 protein holds great promise for efficient and targeted genome engineering applications in research, medicine, and biotechnology. The ease of genome modification in many species by simply designing a gRNA sequence enables large-scale genome editing experiments to probe genome function more than traditional gene editing techniques like TALEN and ZFN. Further, the Cas9 protein is also converted into an RNA-guided homing device (dCas9) by inactivating the nuclease domains to slow down the transcription. Using effector fusion (Cas9 proteins or sgRNA) to alter transcription states of specific genomic loci, or rearrange the genome, can significantly expand the genome engineering modification utilizing the CRISPR/Cas9 system. CRISPR/Cas9 system has been widely adopted by researchers and applied in diverse fields, including microbes [ 48 ], plants [ 49 , 50 , 51 ], animals [ 52 ], insects [ 53 , 54 ], and human cell lines [ 55 ] (briefly explained in Fig. 3 ).
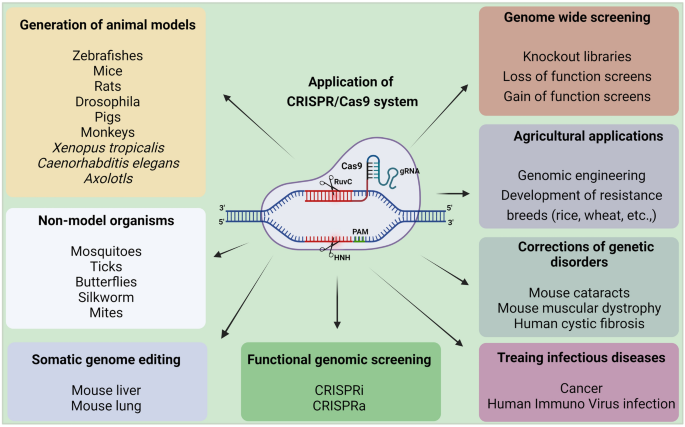
Applications of CRISPR/Cas9 system. CRISPR/Cas9 system has revolutionized genome engineering: its accuracy, rapidity, and affordability permit its use in a nearly limitless range of applications. Since its discovery, researchers have been using the CRISPR/Cas9 system to cure diseases, discover new treatments, and for precision medicine. It does not stop there; beyond treating human diseases, CRISPR/Cas9 is also being utilized for studying the model and non-model insects’ biology, somatic genome editing, manufacturing biofuels, and engineering better crops (rice, wheat, etc.), etc. These advances made possible by the invention of the CRISPR/Cas9 system will change the lives of people globally
Pros and Cons of Cas9 Protein
The advantage of the CRISPR/Cas9 system is the design simplicity with more efficiency than existing ZFN and TALEN systems [ 8 ]. Multiplexed genome editing is another significant advantage of Cas9, which can be achieved by designing multiple sequence-specific gRNAs simultaneously [ 8 ].
Despite numerous advances, CRISPR/Cas9 system faced several disadvantages and also opened numerous queries about risks associated with editing. One example is the gRNA, which guides the Cas9 to cleave dsDNA or ssDNA showed higher on- and off-target mutations in the targeted organisms [ 9 , 55 ]. Even the finest accessible CRISPR/Cas system that uses HDR also induces undesirable mutations. However, these off-target effects can be reduced by utilizing the modified Cas9 version called null Cas9 (nCas9), which generates a nick in only one strand than the DSBs [ 55 ]. However, 100% off-targets cannot be reduced using nCas9, which requires upgraded Cas9 versions in the future.
Cas12 Protein
Cas12 is a versatile protein with more dynamic applications, including epigenome editing. The Cas12 protein belongs to the type V CRISPR system [ 56 ]. The Cas12 protein had recently emerged as an effector RNA-guided DNA endonuclease that becomes an alternative to the Cas9 protein for genome editing [ 25 ]. The Cas12 protein was isolated from the Acidaminococcus species ( As Cas12a) and Lachnospiraceae bacterium (LbCas12a) that fight against invading viruses. Cas9 protein discriminates more accurately against mismatches within the initial ~ 10 bp of the RNA–DNA helix proximal to the PAM sequence [ 25 ]. Moreover, Cas12 isn’t a bit like the Cas9 protein; as a result, this protein can process the precursor crRNA by itself, which doesn’t require tracrRNA or RNase III. This process enhanced researchers to use Cas12 protein for multiplex genome editing [ 25 ].
Mechanism of Cas12 Protein
The Cas12 protein requires only the crRNAs to create an efficient cut at ssDNA and dsDNA. Cas12 protein contains the RuvC and nuclease lobe (NUC) domains for cleavage activity [ 56 ]. Like Cas9, Cas12 encounters a potential target site beside a PAM sequence. Once Cas12 starts encountering, it initiates R-loop, which forms base-pair hybridization between the crRNA and the target DNA strand. During this step, Cas12 matches the < 17 bp of the target sequence and leads to an R-loop formation [ 47 ]. Once R-loop is formed, the Cas12 protein uses its active RuvC domain to cleave the non-target strand with the help of the PAM sequence [ 56 ] (Fig. 4 ). However, the function of the RuvC domain of Cas12 protein in cleaving the targeted DNA strand is not yet clearly studied.
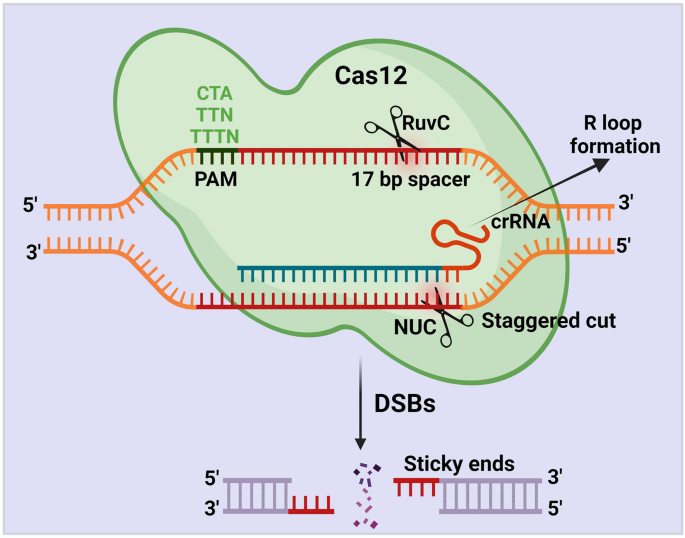
Schematic illustration of CRISPR/Cas12 mechanism. The Cas12 protein requires only the crRNAs to generate DSBs. Cas12 protein cleaves the target region beside a PAM sequence (CTA, TTN, TTTN) with the help of the RuvC and nuclease lobe (NUC) domains. Once Cas12 starts encountering, it initiates R-loop, which forms base-pair hybridization between the crRNA and the target DNA strand. During this step, Cas12 matches the < 17 bp of the target sequence and leads to an R-loop formation. Once R-loop is formed, the Cas12 protein uses its active RuvC domain and generates a staggering cut in the non-target strand with the help of the PAM sequence
Application of Cas12 Protein
A more concise system, CRISPR/Cas12 cleaves, dsDNA or ssDNA via the RuvC domain and does not require tracrRNA. Recently, Doudna’s group introduced a novel CRISPR/Cas diagnostic tool termed DNA endonuclease-targeted CRISPR trans-reporter (DETECTR) based on Cas12 protein (Fig. 5 b) [ 57 ]. This DETECTR technique uses type V enzyme to cleave ssDNA sequence in a three-stage process: (1) Cas12a protein and target crRNA are complemented to the DNA reporter probes; once crRNA recognizes its target sequence through Cas12a protein, the Cas12a protein turns on collateral action and cleaves the target ssDNA or dsDNA. (2) Target DNA probes bind with fluorophores and a quencher molecule. (3) Degradation of the DNA probes releases fluorophore and quencher, producing a robust fluorescent signal for detecting targeted ssDNA or dsDNA cleavage [ 58 ]. In addition, this system detected even a single molecule of viral particles (Fig. 5 b). For instance, Chen et al. (2018) used the DETECTR technique and detected Human Papilloma Viruses (HPV) [ 57 ]. In brief, they combined the non-specific ssDNA of Cas12 with DETECTR and differentiated several HPV16 and HPV18 strains from crude DNA extracts of clinical samples within 1 h. Furthermore, researchers detected African Swine Fever Virus (ASFV) by employing the CRISPR/Cas12 system [ 59 ]. They combined a fluorescent-based point of care (POC) system with CRISPR/Cas12 and detected ASFV within 2 h. From this result, they reported that the CRISPR/Cas12 is very specific and can detect even up to a single nucleotide of a targeted virus [ 59 ]. Recently, DETECTR is also utilized for detecting novel severe acute respiratory syndrome coronavirus-2 (SARS-Cov-2). Mammoth Biosciences, Inc. targeted two Nucleocapsid ( N ) and Envelope (E) genes of SARS-Cov-2 and observed faster virus detection within 1 h [ 60 ]. In brief, they generated Cas12-gRNAs to specifically target the SARS-CoV-2 and optimized the DETECTR assay for E and N genes. They utilized this upgraded DETECTR assay and detected the SARS-CoV-2 on a lateral flow strip within 1 h. Similar to this, Argentina and CASPR Biotech used a quick and portable SARS-CoV-2 diagnostic method based on CRISPR/Cas12 system [ 61 ]. They gathered saliva samples from COVID-19 patients and reported that the naturally occurring proteins in saliva had no inhibitory effects on the CRISPR/Cas12-based paper strip experiment [ 61 ]. Additionally, a Chinese institution used the CRISPR/Cas12-based DETECTR system to confirm a clear detection of SARS-CoV-2 [ 62 ]. They engineered gRNA that targeted the orf1a , orf1b , N , and E genes of the SARS (Wuhan-Hu-1 strain) and related viruses, in which they detected single nucleotide polymorphisms (SNPs). From these results, researchers stated that CRISPR/Cas12-based detection could be employed for the efficient and rapid diagnosis of COVID-19. Jiang et al. (2021) recently developed a magnetic-pull-down-assisted colorimetric (M-CDC) technique coupled to the CRISPR/Cas12 system to detect SARS-CoV-2. They used gold nanoparticle (AuNP) probes for this technique to detect SARS-CoV-2. Additionally, they screened 41 viral samples and reported that M-CDC is a useful technique for screening SARS-CoV-2 variants without requiring advanced instruments [ 63 ]. These applications of Cas12 nuclease enhanced scientists to increase the scope of genome-editing in various fields, including in diagnosing novel viruses [ 64 , 65 , 66 ].
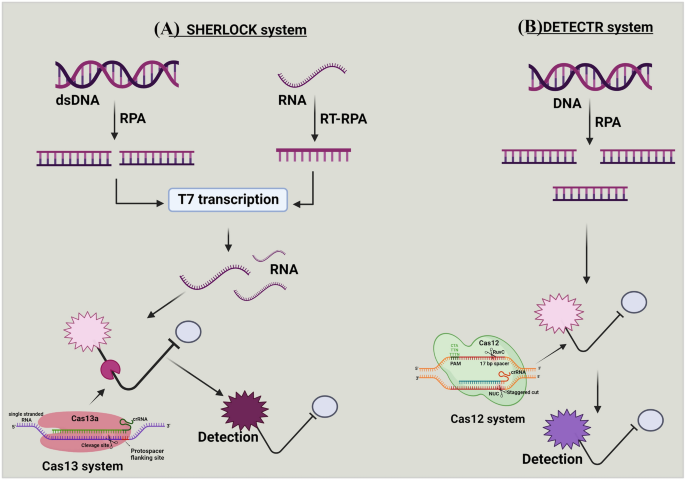
a and b Mechanism of SHERLOCK and DETECTR systems. (A) Targeted double-stranded DNA (dsDNA) or RNA is amplified with recombinase polymerase amplification (RPA) or reverse transcription (RT)-RPA. The RPA is coupled with T7 transcription to covert targeted RNA for detection by Cas13 system. This amplification step with the combination of reporter probe, enable specific high-sensitivity enzymatic reporter unlocking (SHERLOCK) to detect the targeted sequence, (B) In DNA endonuclease-targeted CRISPR trans reporter (DETECTR), DNA is amplified with RPA. The Cas12 system pairs with the single-stranded DNA (ssDNA) of interest, and the DNase activity of Cas12 system is initiated. This amplification step, combined with the reporter probe, enables DETECR to detect the targeted sequence
Pros and Cons of Cas12 Protein
Like Cas9, Cas12 protein was also considered as a sole member of the CRISPR family for genome editing. But in most circumstances, Cas12 was deemed superior to Cas9 protein because Cas12 protein generates staggered DSBs and promotes HDR repair mechanism instead of both NHEJ & HDR [ 67 ]. Cas12 system also overcomes disadvantages associated with diagnostic strategies. For example, diagnosing SARS-COV-2 utilizing quantitative qRT-PCR demands a longer time to get the results [ 66 ]. But the CRISPR/Cas12-based DETECTR detects the SARS-CoV-2 within 1 h [ 66 ]. These results proved the advantage of the CRISPR/Cas12 system, which can also be utilized in detecting newly emerging viruses in the future.
The rapid advancement of the CRISPR-Cas12 system for genome editing has proved revolutionary for the life sciences. Despite this technology's wide application areas, the CRISPR/Cas12 system faces several significant flaws. For example, the CRISPR/Cas12 system is dependent on host cell DNA repair machinery with or without the presence of a template [ 68 ]. Although this system has been successfully used to acquire accurate DNA insertion into the desired genomic loci, its effectiveness varies depending on the cell type. DNA repair through HDR is also associated with active cell division, making these tools ineffective in cell division (for example, neurons) [ 69 ]. However, significant continuing research aims to tailor the Cas12 system further to ensure accurate DNA insertion into the targeted genome. Apart from this drawback, this system has a wide range of applicability, and ongoing endeavors are striving to generate enhanced CRISPR/Cas12 for robust genome engineering.
Cas13 Protein
The most recently discovered Cas protein is Cas13. CRISPR/Cas13 system functions as an ‘adaptive’ immune system in archaea and bacteria to defend against the invading RNA elements [ 70 ]. The Cas13 protein family contains two subtypes: (1) Cas13a protein from Leptotrichia shahii bacterium ( Lsh Cas13a), which is formally known as C2c2 and belongs to type VI, and (2) Cas13b from Prevotella sp. ( Psp Cas13b) belongs to the type III CRISPR/Cas system. This system targets and cleaves only the ssRNA, not the ssDNA or dsDNA [ 71 ].
Mechanism of Cas13a Protein
Cas13a protein is activated through a single crRNA, like Cas12 protein from pre-crRNA processing. Cas13a protein comprises crRNA, NUC lobes, and two nucleotide-binding (HEPN) RNase domains for targeting RNA (Fig. 6 ). The Lsh Cas13a cleaves ssRNA, upon recognizing the target sequence (22–28 nt) complementary to the crRNA spacer [ 72 , 73 ]. The target sequence is flanked by a protospacer-flanking site (PFS) at the 3′-end, which has a bias to adenosine (A), uracil (U), and cytosine(C). Lsh Cas13a and crRNA bind together and cleave the target region of ssRNA without the tracrRNA.
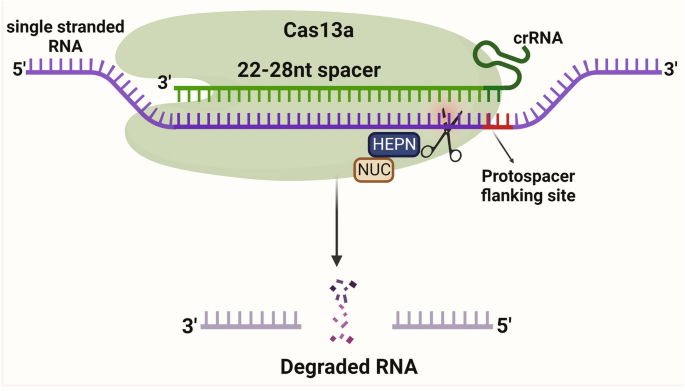
Schematic illustration of CRISPR/Cas13a mechanism. Cas13a protein is activated through a single crRNA. Cas13a protein comprises crRNA, NUC lobes, and two nucleotide-binding (HEPN) RNase domains for targeting RNA. The Cas13a cleaves ssRNA, upon recognizing the target sequence (22–28 nt) complementary to the crRNA spacer. The target sequence is flanked by a protospacer-flanking site (PFS) at the 3′-end and crRNA binds together and cleaves the target region of ssRNA without the tracrRNA
Mechanism of Cas13b Protein
The Cas13b protein is more precise than the Cas13a protein since a PFS flanks RNA targeting with A, U, or G at the 5′ end and PAM (NAN/NNA) at the 3′ end [ 74 ]. Cas13b protein is associated with the mature crRNA. ÇRISPR/Cas13b complex searches for the target ssRNA and induces conformational changes at the ssRNA target, resulting in nonspecific RNA cleavage [ 75 ]. However, the mechanism behind the Cas13b protein is not fully revealed, but scientists tested the ability of the cas13b protein for RNA editing (Fig. 7 ).
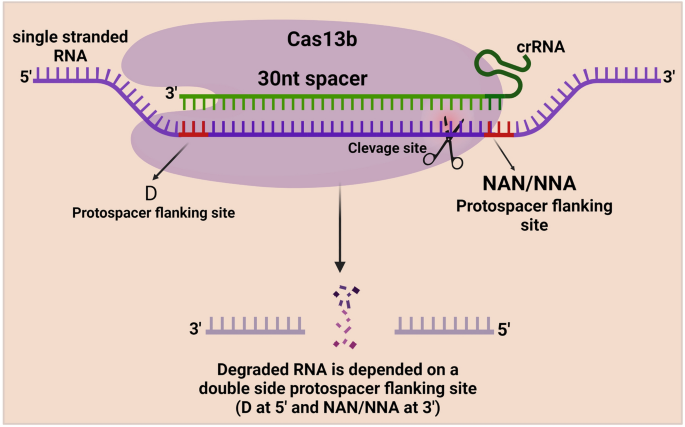
Schematic illustration of CRISPR/Cas13b mechanism. Cas13b protein is associated with the mature crRNA. This ÇRISPR/Cas13b complex searches for the target ssRNA and induces precise conformational changes at the ssRNA target with the help of the Protospacer flanking site (PFS), which flanks RNA targeting at the 5′ end and PAM sequence (NAN/NNA) at the 3′ end, resulting in nonspecific RNA cleavage
Application of Cas13 Protein
Cas13 proteins had wider application in various genome-editing and diagnostic fields. Beyond other applications like base editing, the Cas13a is also utilized for single-nucleotide detection at any site on the target sequence [ 25 ]. Like the DETECTR system, which depends on the activity of Cas12 protein, the specific high-sensitivity enzymatic reporter unlocking (SHERLOCK) technique depends on Cas13 protein (Fig. 5 a) [ 76 , 77 ]. The SHERLOCK system works by integrating the targeted RNA fragments with Cas13 crRNA and fluorescent RNA probes. If the corresponding target sequence exists in the sample, Cas13 recognizes it via crRNA and cleaves fluorescent DNA probes, influencing fluorophore and quencher. This results in detecting the target via a fluorescent signal [ 73 ]. In addition, while SHERLOCK combined with reverse transcription-recombinase polymerase amplification (RT-RPA) or isothermal-RPA, the crRNA-Cas13a complex binds and cleaves a specific target sequence with high specificity [ 72 ]. SHERLOCK is used for various functions, including RNA detection, sensitive detection of nucleic acid contamination, and general RNA/DNA quantitation of specific qPCR assays [ 77 ].
Additionally, Cas13 nuclease could potentially track the allele-specific expression of transcripts or disease-associated mutations in cells. These attractive features opened new avenues to discriminate single nucleotide changes with high sensitivity in many organisms, including viruses (Fig. 5 a). For instance, Gootenberg et al. (2018) diagnosed E. coli and Pseudomonas aeruginosa with the DNA isolated from these strains using the SHERLOCK system. They have also distinguished the isolates of Klebsiella pneumonia with two types of resistance genes, such as K. pneumoniae carbapenemase (KPC) and New Delhi metallo-β-lactamase 1 [ 70 ]. Furthermore, SHERLOCK differentiated African and American strains of the zika virus, the dengue virus (DENV) serotype, and cancer-related DNA targets. In addition, SHERLOCK detected Zika virus and DENV with higher sensitivity within 30 min. Researchers also developed a unique technique for detecting viral infection by combining SHERLOCK with heating unextracted diagnostic samples to obliterate nucleases (HUDSON) [ 75 ]. Within 2 hours, updated SHERLOCK identified DENV in a patient's saliva, blood, and serum. From these existing results, SHERLOCK Biosciences, Inc. and Mammoth Biosciences, Inc. used the SHERLOCK technique for the detection of SARS-CoV-2. They targeted S and Orf1ab genes and detected the SARS-Cov-2 RNA sequence within an hour. Metsky et al. (2020) also detected the SARS-CoV-2 RNA using the Cas13a proteins. They combined synthetic RNA targets with fluorescent visualizers and detected SARS-CoV-2-RNA [ 76 ]. To test the effectiveness of detecting SARS-CoV-2, Rauch et al. (2020) recently utilized the Cas13 based-Ragged-Equitable-Scalable Testing (CREST) method [ 77 ]. This technology uses portable and lightweight LED visualizers built with plastic filters. Researchers also used lateral flow immunochromatography strips to detect target RNAs, but these strips are expensive. Therefore, they created a P51 cardboard fluorescent visualizer, which was less expensive and could detect 10 copies of target RNA per millilitre. Additionally, they utilised the smartphone camera to capture data, which they then uploaded to the cloud to enable POC testing [ 77 ]. It improves nucleic acid detection based on CRISPR and demonstrates its power to change diagnostic and surveillance efforts by direct screening even on a vast population. Later, the CRISPR/Cas13a test based on single-molecule RNA diagnosis was developed by the Tian et al. (2020) group, which eliminated reverse transcriptase and nucleic acid extension procedures [ 78 ]. They stated that the SARS-CoV-2 would be easily detectable using the CRISPR/Cas13a test [ 78 , 79 ]. These advantages of Cas13 protein enabled researchers to target any non-targetable sequences in diverse organisms [ 80 , 81 , 82 ].
Pros and Cons of Cas13 Protein
Like Cas9 and Cas12, CRISPR/Cas13 is also a robust, precise, and versatile RNA-targeting system, which opens novel research horizons in diverse fields. Compared with earlier RNA manipulation systems, CRISPR/Cas13 offers numerous advantages. For example, its modular construction, which consists of a single protein effector module and an RNA guide module, allows for significant scalability by enabling the production of whole libraries of various guide RNAs in addition to easy and quick design [ 83 ]. The recently discovered Cas13 mutant versions (dCas13, Cas13x), which function as programmable RNA-binding proteins, efficiently target different effectors to specific RNAs to induce specific mutations [ 83 ]. Due to the inherent crRNA biogenesis, multiple RNAs can be targeted using Cas13 precisely. Compared with RNAi, CRISPR/Cas13 mediated genome modifications are not limited to targeting cytoplasmic transcripts. In addition, Cas13 enables faster downregulation of gene expression by directly knocking out the cytoplasmic mRNA transcripts [ 83 ]. Recently, CRISPR/Cas13-based SHERLOCK and SHERLOCKv2 also played a vital role in developing novel molecular diagnostic tools to detect viruses, including SARS-CoV-2. Apart from these major advantages, CRISPR/Cas13 faced off-target mutations [ 55 ], which is the major drawback of this system. However, future research would help overcome this obstacle and aid in developing novel RNA knockdown approaches with more specificity and efficiency.
Cas14 Protein
Doudna’s group investigated other and similar types (Cas9, Cas12, and Cas13) of Cas systems that were available in nature [ 84 ]. They explored by creating a metagenomic database of the bacterial genome to search for uncharacterized Cas genes [ 84 ]. Surprisingly, they found Cas14 protein, which codes for smaller Cas protein with MW 40–70 kd. This Cas14 protein is extremely smaller (400–700 amino acids) than the other characterized Cas proteins [ 84 ]. Due to their small size, Doudna’s lab reported that Cas14 protein can target ssDNA without a PAM [ 84 ].
Mechanism of Cas14 Protein
This Cas14 cleaves ssDNA and confers immunity against viruses with ssDNA genomes or mobile genetic elements (MGEs) [ 84 ]. Cas14 protein recognizes the ssDNA, mediates seed sequence interaction with the target ssDNA, and cleaves the ssDNA, not dsDNA or ssRNA. Like Cas9, the Cas14 protein requires both tracrRNA and crRNA to target the ssDNA (Fig. 8 ). The cleavage efficiency of the Cas14 protein is more specific than Cas9, Cas12, and Cas 13 proteins without the presence of the PAM region [ 40 ]. Thus, this system meets all the criteria for high-fidelity genome editing.
Schematic illustration of CRISPR/Cas14 mechanism. The Cas14 protein comprises both tracrRNA and crRNA to target ssDNA. Cas14 protein recognizes the ssDNA with the help of tracrRNA and crRNA, mediates seed sequence interaction with the target ssDNA, and cleaves the ssDNA, not dsDNA or ssRNA. The cleavage efficiency of the Cas14 protein is more specific than Cas9, Cas12, and Cas 13 proteins without the presence of the PAM region
Application of Cas14 Protein
CRISPR/Cas14 system is now harnessed as superior to the Cas13 system. Researchers combined the CRISPR/Cas14 system with the DETECTR as a diagnostic approach for high-fidelity detection of ssDNA. Harrington et al. (2018) first used CRISPR/Cas14 system with the DETECTR technique. They designed gRNA targeting the HECT and RLD Domain Containing E3 Ubiquitin Protein Ligase 2 ( HERC2 ) gene of human saliva samples from blue-eyed single nucleotide polymorphisms (SNP) individuals. They exhibited strong activation of recognition of the blue-eyed SNP by Cas14, while the Cas12 system failed to detect the blue-eyed SNP [ 40 ]. This result represents a cost-effective method for screening pathogenic mutations and provides a great opportunity for mapping the candidate genes associated with different pathogens. Another group proposes the Cas14 for viral diagnostic in combination with the simplified nucleic acid extraction, which does not involve complicated sample extraction like heating unextracted diagnostic samples to obliterate nucleases (HUDSON). The results reported that Cas14 could provide an advanced platform in the future for viral screening [ 72 , 79 ]. This novel CRISPR/Cas14 system crosses criteria with user-friendly, sensitive, and more specific applications for future genome engineering. It can be harnessed for other purposes, including diagnostic applications like phylogenetic association (new viruses), epidemiology association with other pathogens, and taxonomic analysis [ 85 ].
Pros and Cons of Cas14 Protein
The Cas14 protein, which edits dsDNA, ssDNA, and RNA, holds various advantages over traditional Cas9 protein. For example, the Cas14 protein that has around 500 amino acid is very small, so this protein could be easily delivered to target any tissue than Cas9 protein [ 86 ]. Additionally, the Cas14 protein's selectivity improves the fidelity of single nucleotide polymorphism (SNP) [ 83 ]. Finally, the Cas14 protein has less limiting PAM requirements (uses only T-rich sequences) [ 83 ], allowing it to edit more targeted genomic sequences than the Cas9 protein. However, only a few studies have been demonstrated, which requires extensive studies to assist Cas14 in several fields of research in the future.
Conclusion and Future Perspectives
The exploitation of CRISPR/Cas tools made a breakthrough in genome engineering in the last few years. The discovery of Cas proteins has revolutionized natural science research and enabled advances in basic research, therapeutics, and diagnostics. The ease to use, the requirement of very little equipment, and the low cost have made many laboratories utilize these CRISPR/Cas-based systems to study the function of genes in various organisms. The initial discovery of the Cas9 protein, which shows its spotlight in the CRISPR system, has enhanced researchers to find different Cas variants like Cas12, Cas13, and Cas14 proteins. This research improvement in Cas proteins has provided exciting platforms in genome engineering, including detecting RNA viruses from plants, animals, and humans and treating infections. Several studies are currently underway to find novel Cas variants from nature. However, numerous aspects of the mechanism of Cas proteins still lack sufficient understanding. Therefore, understanding the molecular mechanism behind the Cas proteins, identification of PAM-less Cas proteins, and accurate targeting specificity to minimize off-target effects will increase the potential of utilizing Cas proteins for precise applications of genome engineering. In addition, identifying the uncharacterized advance Cas proteins that may still exist in many bacteria or archaea will revolutionize multiple fields, including diagnosing novel viruses, therapeutics, agriculture, breeding, etc. If these proteins are fully addressed with improved genome-editing abilities, it might kick-start new CRISPR “fever” offering influential and groundbreaking CRISPR technologies into mainstream use in the near future.
Capecchi, M. R. (2005). Gene targeting in mice: Functional analysis of the mammalian genome for the twenty-first century. Nature Reviews Genetics, 6 (6), 507–512.
Article CAS PubMed Google Scholar
McManus, M. T., & Sharp, P. A. (2002). Gene silencing in mammals by small interfering RNAs. Nature Reviews Genetics, 3 (10), 737–747.
Rudin, N., Sugarman, E., & Haber, J. E. (1989). Genetic and physical analysis of double-strand break repair and recombination in Saccharomyces cerevisiae . Genetics, 122 (3), 519–534.
Article CAS PubMed PubMed Central Google Scholar
Kass, E. M., & Jasin, M. (2010). Collaboration and competition between DNA double-strand break repair pathways. FEBS Letters, 584 (17), 3703–3708.
Stoddard, B. L. (2005). Homing endonuclease structure and function. Quarterly Reviews of Biophysics, 38 (1), 49.
Sander, J. D., Dahlborg, E. J., Goodwin, M. J., Cade, L., Zhang, F., Cifuentes, D., & Qi, Y. (2011). Selection-free zinc-finger-nuclease engineering by context-dependent assembly (CoDA). Nature Methods, 8 (1), 67–69.
Reyon, D., Tsai, S. Q., Khayter, C., Foden, J. A., Sander, J. D., & Joung, J. K. (2012). FLASH assembly of TALENs for high-throughput genome editing. Nature Biotechnology, 30 (5), 460.
Jinek, M., Chylinski, K., Fonfara, I., Hauer, M., Doudna, J. A., & Charpentier, E. (2012). A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science, 337 (6096), 816–821.
Ceasar, S. A., Rajan, V., Prykhozhij, S. V., Berman, J. N., & Ignacimuthu, S. (2016). Insert, remove or replace: A highly advanced genome editing system using CRISPR/Cas9. Biochimica et Biophysica Acta - Molecular Cell Research, 1863 (9), 2333–2344.
Article CAS Google Scholar
Hille, F., Richter, H., Wong, S. P., Bratovič, M., Ressel, S., & Charpentier, E. (2018). The biology of CRISPR-Cas: Backward and forward. Cell, 172 (6), 1239–1259.
Xu, Y., & Li, Z. (2020). CRISPR-Cas systems: Overview, innovations and applications in human disease research and gene therapy. Computational and Structural Biotechnology Journal, 18 , 2401–2415.
Makarova, K. S., Haft, D. H., Barrangou, R., Brouns, S. J. J., Charpentier, E., Horvath, P., & Yakunin, A. F. (2011). Evolution and classification of the CRISPR–Cas systems. Nature Reviews Microbiology, 9 (6), 467–477.
Wang, F., Wang, L., Zou, X., Duan, S., Li, Z., Deng, Z., & Chen, S. (2019). Advances in CRISPR-Cas systems for RNA targeting, tracking and editing. Biotechnology Advances, 37 (5), 708–729.
Ishino, Y., Shinagawa, H., Makino, K., Amemura, M., & Nakata, A. (1987). Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli , and identification of the gene product. Journal of Bacteriology, 169 (12), 5429–5433.
Nakata, A., Amemura, M., & Makino, K. (1989). Unusual nucleotide arrangement with repeated sequences in the Escherichia coli K-12 chromosome. Journal of Bacteriology, 171 (6), 3553–3556.
Hermans, P. W., Van Soolingen, D., Bik, E. M., De Haas, P. E., Dale, J. W., & Van Embden, J. D. (1991). Insertion element IS987 from Mycobacterium bovis BCG is located in a hot-spot integration region for insertion elements in Mycobacterium tuberculosis complex strains. Infection and Immunity, 59 (8), 2695–2705.
Mojica, F. J. M., Díez-Villaseñor, C., Soria, E., & Juez, G. (2000). Biological significance of a family of regularly spaced repeats in the genomes of archaea, bacteria and mitochondria. Molecular Microbiology, 36 (1), 244–246.
Jansen, R., van Embden, J. D. A., Gaastra, W., & Schouls, L. M. (2002). Identification of genes that are associated with DNA repeats in prokaryotes. Molecular Microbiology, 43 (6), 1565–1575.
Haft, D. H., Selengut, J., Mongodin, E. F., & Nelson, K. E. (2005). A guild of 45 CRISPR-associated (Cas) protein families and multiple CRISPR/Cas subtypes exist in prokaryotic genomes. PLoS Computational Biology, 1 (6), e60.
Article PubMed PubMed Central Google Scholar
Koonin, E. V., Makarova, K. S., & Zhang, F. (2017). Diversity, classification and evolution of CRISPR-Cas systems. Current Opinion in Microbiology, 37 , 67–78.
Koonin, E. V., & Makarova, K. S. (2017). Mobile genetic elements and evolution of CRISPR-Cas systems: All the way there and back. Genome Biology and Evolution, 9 (10), 2812–2825.
Shabalina, S. A., & Koonin, E. V. (2008). Origins and evolution of eukaryotic RNA interference. Trends in Ecology & Evolution, 23 (10), 578–587.
Article Google Scholar
Brouns, S. J. J., Jore, M. M., Lundgren, M., Westra, E. R., Slijkhuis, R. J. H., Snijders, A. P. L., & Van Der Oost, J. (2008). Small CRISPR RNAs guide antiviral defense in prokaryotes. Science, 321 (5891), 960–964.
Marraffini, L. A., & Sontheimer, E. J. (2008). CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science, 322 (5909), 1843–1845.
Zetsche, B., Gootenberg, J. S., Abudayyeh, O. O., Slaymaker, I. M., Makarova, K. S., Essletzbichler, P., & Regev, A. (2015). Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell, 163 (3), 759–771.
Abudayyeh, O. O., Gootenberg, J. S., Essletzbichler, P., Han, S., Joung, J., Belanto, J. J., & Regev, A. (2017). RNA targeting with CRISPR–Cas13. Nature, 550 (7675), 280–284.
Deltcheva, E., Chylinski, K., Sharma, C. M., Gonzales, K., Chao, Y., Pirzada, Z. A., & Charpentier, E. (2011). CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature, 471 (7340), 602–607.
Barrangou, R., Fremaux, C., Deveau, H., Richards, M., Boyaval, P., Moineau, S., & Horvath, P. (2007). CRISPR provides acquired resistance against viruses in prokaryotes. Science, 315 (5819), 1709–1712.
Horvath, P., & Barrangou, R. (2010). CRISPR/Cas, the immune system of bacteria and archaea. Science, 327 (5962), 167–170.
Semenova, E., Jore, M. M., Datsenko, K. A., Semenova, A., Westra, E. R., Wanner, B., & Severinov, K. (2011). Interference by clustered regularly interspaced short palindromic repeat (CRISPR) RNA is governed by a seed sequence. Proceedings of the National Academy of Sciences, 108 (25), 10098–10103.
Bolotin, A., Quinquis, B., Sorokin, A., & Ehrlich, S. D. (2005). Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology, 151 (8), 2551–2561.
Koonin, E. V., & Wolf, Y. I. (2009). Is evolution Darwinian or/and Lamarckian? Biology Direct, 4 (1), 1–14.
Mojica, F. J. M., Díez-Villaseñor, C., García-Martínez, J., & Soria, E. (2005). Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. Journal of Molecular Evolution, 60 (2). 174–182. https://doi.org/10.1007/s00239-004-0046-3
Cong, L., Ran, F. A., Cox, D., Lin, S., Barretto, R., Habib, N., Hsu, P. D., Wu, X., Jiang, W., Marraffini, L. A., & Zhang, F. (2013). Multiplex genome engineering using CRISPR/Cas systems. Science, 339 (6121). 819–823. https://doi.org/10.1126/science.1231143
Esvelt, K. M., Mali, P., Braff, J. L., Moosburner, M., Yaung, S. J., & Church, G. M. (2013). Orthogonal Cas9 proteins for RNA-guided gene regulation and editing. Nature Methods, 10 (11), 1116–1121. https://doi.org/10.1038/nmeth.2681
Horvath, P., Romero, D. A., Coûté-Monvoisin, A. C., Richards, M., Deveau, H., Moineau, S., Boyaval, P., Fremaux, C., & Barrangou, R. (2008). Diversity, activity, and evolution of CRISPR loci in Streptococcus thermophilus. Journal of Bacteriology, 190 (4), 1401–1412. https://doi.org/10.1128/JB.01415-07
Kim, E., Koo, T., Park, S. W., Kim, D., Kim, K., Cho, H.-Y., Song, D. W., Lee, K. J., Jung, M. H., Kim, S., Kim, J. H., Kim, J. H., & Kim, J.-S. (2017). In vivo genome editing with a small Cas9 orthologue derived from Campylobacter jejuni . Nature Communications, 8 (1), 14500. https://doi.org/10.1038/ncomms14500
Yang, H., Gao, P., Rajashankar, K. R., & Patel, D. J. (2016). PAM-dependent target DNA recognition and cleavage by C2c1 CRISPR-Cas endonuclease. Cell, 167 (7), 1814–1828.e12. https://doi.org/10.1016/j.cell.2016.11.053
Yamano, T., Nishimasu, H., Zetsche, B., Hirano, H., Slaymaker, I. M., Li, Y., Fedorova, I., Nakane, T., Makarova, K. S., Koonin, E. V., Ishitani, R., Zhang, F., & Nureki, O. (2016). Crystal structure of Cpf1 in complex with guide RNA and target DNA. Cell, 165 (4), 949–962. https://doi.org/10.1016/j.cell.2016.04.003
Harrington, L. B., Burstein, D., Chen, J. S., Paez-Espino, D., Ma, E., Witte, I. P., & Doudna, J. A. (2018). Programmed DNA destruction by miniature CRISPR-Cas14 enzymes. Science, 362 (6416), 839–842.
Wiedenheft, B., Zhou, K., Jinek, M., Coyle, S. M., Ma, W., & Doudna, J. A. (2009). Structural basis for DNase activity of a conserved protein implicated in CRISPR-mediated genome defense. Structure, 17 (6), 904–912.
Datsenko, K. A., Pougach, K., Tikhonov, A., Wanner, B. L., Severinov, K., & Semenova, E. (2012). Molecular memory of prior infections activates the CRISPR/Cas adaptive bacterial immunity system. Nature Communications, 3 (1), 1–7.
Babu, M., Beloglazova, N., Flick, R., Graham, C., Skarina, T., Nocek, B., & Binkowski, A. (2011). A dual function of the CRISPR–Cas system in bacterial antivirus immunity and DNA repair. Molecular Microbiology, 79 (2), 484–502.
Yosef, I., Goren, M. G., & Qimron, U. (2012). Proteins and DNA elements essential for the CRISPR adaptation process in Escherichia coli . Nucleic Acids Research, 40 (12), 5569–5576.
Wright, A. V., & Doudna, J. A. (2016). Protecting genome integrity during CRISPR immune adaptation. Nature Structural & Molecular Biology, 23 (10), 876.
Nishimasu, H., Ran, F. A., Hsu, P. D., Konermann, S., Shehata, S. I., Dohmae, N., & Nureki, O. (2014). Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell, 156 (5), 935–949.
Anders, C., Niewoehner, O., Duerst, A., & Jinek, M. (2014). Structural basis of PAM-dependent target DNA recognition by the Cas9 endonuclease. Nature, 513 (7519), 569–573.
Liu, R., Chen, L., Jiang, Y., Zhou, Z., & Zou, G. (2015). Efficient genome editing in filamentous fungus Trichoderma reesei using the CRISPR/Cas9 system. Cell Discovery, 1 (1), 1–11.
Zhang, Y., Massel, K., Godwin, I. D., & Gao, C. (2018). Applications and potential of genome editing in crop improvement. Genome Biology, 19 (1), 1–11.
Hillary, V. E., & Ceasar, S. A. (2019). Application of CRISPR/Cas9 genome editing system in cereal crops. The Open Biotechnology Journal, 13 (1), 173–179.
Ceasar, S. A., Maharajan, T., Hillary, E., & Krishna, T. P. A. (2022). Insights to improve the plant nutrient transport by CRISPR/Cas system. Biotechnology Advances, 59 , 107963.
Lee, H., Yoon, D. E., & Kim, K. (2020). Genome editing methods in animal models. Animal Cells and Systems, 24 (1), 8–16.
Hillary, V. E., Ceasar, S. A., & Ignacimuthu, S. (2020). Genome engineering in insects: focus on the CRISPR/Cas9 system. Genome engineering via CRISPR-Cas9 system (pp. 219–249). Elsevier.
Chapter Google Scholar
Hillary, V. E., & Ceasar, S. A. (2021). Genome engineering in insects for the control of vector borne diseases. Progress in Molecular Biology and Translational Science, 179 , 197–223.
Rodríguez-Rodríguez, D. R., Ramírez-Solís, R., Garza-Elizondo, M. A., Garza-Rodríguez, M. D. L., & Barrera-Saldaña, H. A. (2019). Genome editing: A perspective on the application of CRISPR/Cas9 to study human diseases. International Journal of Molecular Medicine, 43 (4), 1559–1574.
PubMed PubMed Central Google Scholar
Fonfara, I., Richter, H., Bratovič, M., Le Rhun, A., & Charpentier, E. (2016). The CRISPR-associated DNA-cleaving enzyme Cpf1 also processes precursor CRISPR RNA. Nature, 532 (7600), 517–521.
Chen, J. S., Ma, E., Harrington, L. B., Da Costa, M., Tian, X., Palefsky, J. M., & Doudna, J. A. (2018). CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science, 360 (6387), 436–439.
Makarova, K. S., Wolf, Y. I., Iranzo, J., Shmakov, S. A., Alkhnbashi, O. S., Brouns, S. J. J., & Horvath, P. (2020). Evolutionary classification of CRISPR–Cas systems: A burst of class 2 and derived variants. Nature Reviews Microbiology, 18 (2), 67–83.
He, Q., Yu, D., Bao, M., Korensky, G., Chen, J., Shin, M., & Du, K. (2020). High-throughput and all-solution phase African Swine Fever Virus (ASFV) detection using CRISPR-Cas12a and fluorescence based point-of-care system. Biosensors and Bioelectronics, 154 , 112068.
Broughton, J. P., Deng, X., Yu, G., Fasching, C. L., Servellita, V., Singh, J., & Sotomayor-Gonzalez, A. (2020). CRISPR–Cas12-based detection of SARS-CoV-2. Nature Biotechnology, 38 (7), 870–874.
Satyanarayana, M. (2020). A COVID-19 diagnostic that uses CRISPR gets a nod from the FDA chemical & engineering news.
Feng, M., & Li, X. (2020). Land cover mapping toward finer scales. Science Bulletin, 65 (19), 1604–1606.
Article PubMed Google Scholar
Jiang, Y., Hu, M., Liu, A.-A., Lin, Y., Liu, L., Yu, B., & Pang, D. W. (2021). Detection of SARS-CoV-2 by CRISPR/Cas12a-enhanced colorimetry. ACS Sensors, 6 (3), 1086–1093.
Bandyopadhyay, A., Kancharla, N., Javalkote, V. S., Dasgupta, S., & Brutnell, T. P. (2020). CRISPR-Cas12a (Cpf1): A versatile tool in the plant genome editing tool box for agricultural advancement. Frontiers in Plant Science, 11 , 1589.
Tsukamoto, T., Sakai, E., Iizuka, S., Taracena-Gándara, M., Sakurai, F., & Mizuguchi, H. (2018). Generation of the adenovirus vector-mediated CRISPR/Cpf1 system and the application for primary human hepatocytes prepared from humanized mice with chimeric liver. Biological and Pharmaceutical Bulletin, 41 (7), 1089–1095.
Teng, F., Li, J., Cui, T., Xu, K., Guo, L., Gao, Q., & Zhou, Q. (2019). Enhanced mammalian genome editing by new Cas12a orthologs with optimized crRNA scaffolds. Genome Biology, 20 (1), 1–6.
Hillary, V. E., & Ceasar, S. A. (2022). Prime editing in plants and mammalian cells: Mechanism, achievements, limitations, and future prospects. BioEssays, 44 , 2200032.
Hillary, V. E., Ignacimuthu, S., & Ceasar, S. A. (2021). Potential of CRISPR/Cas system in the diagnosis of COVID-19 infection. Expert Review of Molecular Diagnostics, 21 (11), 1179–1189.
Al-Shayeb, B., Sachdeva, R., Chen, L. X., Ward, F., Munk, P., Devoto, A., & Amano, Y. (2020). Clades of huge phages from across Earth’s ecosystems. Nature, 578 (7795), 425–431.
East-Seletsky, A., O’Connell, M. R., Knight, S. C., Burstein, D., Cate, J. H. D., Tjian, R., & Doudna, J. A. (2016). Two distinct RNase activities of CRISPR-C2c2 enable guide-RNA processing and RNA detection. Nature, 538 (7624), 270–273.
Liu, L., Li, X., Wang, J., Wang, M., Chen, P., Yin, M., & Wang, Y. (2017). Two distant catalytic sites are responsible for C2c2 RNase activities. Cell, 168 (1–2), 121–134.
Gootenberg, J. S., Abudayyeh, O. O., Lee, J. W., Essletzbichler, P., Dy, A. J., Joung, J., & Freije, C. A. (2017). Nucleic acid detection with CRISPR-Cas13a/C2c2. Science, 356 (6336), 438–442.
Gootenberg, J. S., Abudayyeh, O. O., Kellner, M. J., Joung, J., Collins, J. J., & Zhang, F. (2018). Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science, 360 (6387), 439–444.
Konermann, S., Lotfy, P., Brideau, N. J., Oki, J., Shokhirev, M. N., & Hsu, P. D. (2018). Transcriptome engineering with RNA-targeting type VI-D CRISPR effectors. Cell, 173 (3), 665–676.
Smargon, A. A., Cox, D. B. T., Pyzocha, N. K., Zheng, K., Slaymaker, I. M., Gootenberg, J. S., & Makarova, K. S. (2017). Cas13b is a type VI-B CRISPR-associated RNA-guided RNase differentially regulated by accessory proteins Csx27 and Csx28. Molecular Cell, 65 (4), 618–630.
Kellner, M. J., Koob, J. G., Gootenberg, J. S., Abudayyeh, O. O., & Zhang, F. (2020). SHERLOCK: Nucleic acid detection with CRISPR nucleases. Nature Protocols, 15 (3), 1311.
Kellner, M. J., Koob, J. G., Gootenberg, J. S., Abudayyeh, O. O., & Zhang, F. (2019). SHERLOCK: Nucleic acid detection with CRISPR nucleases. Nature Protocols, 14 (10), 2986–3012.
Tian, T., Shu, B., Jiang, Y., Ye, M., Liu, L., Guo, Z., & Zhou, X. (2020). An ultralocalized Cas13a assay enables universal and nucleic acid amplification-free single-molecule RNA diagnostics. ACS Nano., 15 , 1167.
Myhrvold, C., Freije, C. A., Gootenberg, J. S., Abudayyeh, O. O., Metsky, H. C., Durbin, A. F., & Parham, L. A. (2018). Field-deployable viral diagnostics using CRISPR-Cas13. Science, 360 (6387), 444–448.
Metsky, H. C., Freije, C. A., Kosoko-Thoroddsen, T. S. F., Sabeti, P. C., & Myhrvold, C. (2020). CRISPR-based surveillance for COVID-19 using genomically-comprehensive machine learning design. BioRxiv . https://doi.org/10.1101/2020.02.26.967026
Rauch, S., Roth, N., Schwendt, K., Fotin-Mleczek, M., Mueller, S. O., & Petsch, B. (2020). mRNA based SARS-CoV-2 vaccine candidate CVnCoV induces high levels of virus neutralizing antibodies and mediates protection in rodents. bioRxiv., 11 , 12.
Google Scholar
Palaz, F., Kalkan, A. K., Can, O., Demir, A. N., Tozluyurt, A., Özcan, A., & Ozsoz, M. (2021). CRISPR-Cas13 system as a promising and versatile tool for cancer diagnosis, therapy, and research. ACS Synthetic Biology, 10 (6), 1245–1267.
Lotfi, M., & Rezaei, N. (2020). CRISPR/Cas13: A potential therapeutic option of COVID-19. Biomedicine & Pharmacotherapy, 131 , 110738.
Burstein, D., Harrington, L. B., Strutt, S. C., Probst, A. J., Anantharaman, K., Thomas, B. C., & Banfield, J. F. (2017). New CRISPR–Cas systems from uncultivated microbes. Nature, 542 (7640), 237–241.
Nayak, S., Blumenfeld, N. R., Laksanasopin, T., & Sia, S. K. (2017). Point-of-care diagnostics: Recent developments in a connected age. Analytical Chemistry, 89 (1), 102–123.
Mahas, A., Stewart, C. N., Jr., & Mahfouz, M. M. (2018). Harnessing CRISPR/Cas systems for programmable transcriptional and post-transcriptional regulation. Biotechnology Advances, 36 (1), 295–310.
Download references
No funding was received for this article.
Author information
Authors and affiliations.
Department of Biosciences, Rajagiri College of Social Sciences, 683 104, Cochin, Kerala, India
V. Edwin Hillary & S. Antony Ceasar
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the study's conception and design. EHV and SAC wrote the article. EHV and SAC drew the images.
Corresponding author
Correspondence to S. Antony Ceasar .
Ethics declarations
Competing interest.
The authors declare that there are no competing interests associated with the manuscript.
Ethical Approval
No ethics approval is needed for this article.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Hillary, V.E., Ceasar, S.A. A Review on the Mechanism and Applications of CRISPR/Cas9/Cas12/Cas13/Cas14 Proteins Utilized for Genome Engineering. Mol Biotechnol 65 , 311–325 (2023). https://doi.org/10.1007/s12033-022-00567-0
Download citation
Received : 16 May 2022
Accepted : 15 September 2022
Published : 27 September 2022
Issue Date : March 2023
DOI : https://doi.org/10.1007/s12033-022-00567-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Genome engineering
- Find a journal
- Publish with us
- Track your research
CRISPR/CAS9: A promising approach for the research and treatment of cardiovascular diseases
Affiliations.
- 1 Department of Cardiology, The Second Hospital of Jilin University, 218 Ziqiang Road, Changchun 130041, China.
- 2 Department of Neurology, The Liaoning Province People's Hospital, 33 Wenyi Road, ShenYang 110016, China.
- 3 Department of Cardiology, The Second Hospital of Jilin University, 218 Ziqiang Road, Changchun 130041, China. Electronic address: [email protected].
- 4 Department of Cardiology, The Second Hospital of Jilin University, 218 Ziqiang Road, Changchun 130041, China. Electronic address: [email protected].
- PMID: 36191879
- DOI: 10.1016/j.phrs.2022.106480
The development of gene-editing technology has been one of the biggest advances in biomedicine over the past two decades. Not only can it be used as a research tool to build a variety of disease models for the exploration of disease pathogenesis at the genetic level, it can also be used for prevention and treatment. This is done by intervening with the expression of target genes and carrying out precise molecular targeted therapy for diseases. The simple and flexible clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 gene-editing technology overcomes the limitations of zinc finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs). For this reason, it has rapidly become a preferred method for gene editing. As a new gene intervention method, CRISPR/Cas9 has been widely used in the clinical treatment of tumours and rare diseases; however, its application in the field of cardiovascular diseases is currently limited. This article reviews the application of the CRISPR/Cas9 editing technology in cardiovascular disease research and treatment, and discusses the limitations and prospects of this technology.
Keywords: CRISPR/Cas9; Cardiovascular disease; Gene editing.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Publication types
- Research Support, Non-U.S. Gov't
- CRISPR-Cas Systems*
- Cardiovascular Diseases* / genetics
- Cardiovascular Diseases* / therapy
- Gene Editing / methods
- Genetic Engineering / methods

- May 14, 2024 | Atomic Ballet: Scientists Make Surprising Discovery in Battery Technology
- May 14, 2024 | Chilling Discoveries: Princeton Physicists Unlock Secrets of Kinetic Magnetism
- May 14, 2024 | Years After His Death, a Late Scientist’s Work Has Opened the Door for Life-Saving New Cancer Treatments
- May 14, 2024 | Gene Linked to Learning Difficulties Has Direct Impact on Learning and Memory
- May 14, 2024 | XRISM Spacecraft Detects Iron Signatures in Nearby Active Galaxy
Gene Editing Breakthrough: CRISPR Improves Vision in Clinical Trial
By Massachusetts Eye And Ear May 13, 2024

In a recent clinical trial, CRISPR gene editing was applied to 14 patients suffering from a form of inherited blindness. The treatment proved safe and resulted in measurable vision improvements in 11 of the participants. The trial, named BRILLIANCE, signifies a significant step forward in gene therapy for ocular diseases. Credit: SciTechDaily.com
Mass Eye and Ear-led phase 1/2 trial, which included 14 participants, found that the first-of-its-kind experimental treatment was safe and efficacious.
- BRILLIANCE trial results showed 11 out of 14 treated participants experienced some improvements in vision and quality of life measures.
- CRISPR-based therapy was found safe with no dose-limiting toxicities reported.
- Mass Eye and Ear researchers say their findings support continued research and clinical trials of CRISPR therapies for inherited retinal disorders.
Results from a groundbreaking clinical trial of CRISPR gene editing in 14 individuals with a form of inherited blindness show that the treatment is safe and led to measurable improvements in 11 of the participants treated. The phase 1/2 trial called BRILLIANCE, was led by principal investigator Eric Pierce, MD, PhD, of Mass Eye and Ear, a member of the Mass General Brigham healthcare system, and sponsored by Editas Medicine, Inc. Findings are reported on May 6th in The New England Journal of Medicine .
“This research demonstrates that CRISPR gene therapy for inherited vision loss is worth continued pursuit in research and clinical trials,” said Pierce, director of Ocular Genomics Institute and Berman-Gund Laboratory for the Study of Retinal Degenerations at Mass Eye and Ear and Harvard Medical School. “While more research is needed to determine who may benefit most, we consider the early results promising. To hear from several participants how thrilled they were that they could finally see the food on their plates –that is a big deal. These were individuals who could not read any lines on an eye chart and who had no treatment options, which is the unfortunate reality for most people with inherited retinal disorders.”
Jason Comander, MD, PhD, performs the procedure to deliver the CRISPR-based medicine as part of the BRILLIANCE trial in September 2020 at Mass Eye and Ear. Credit: Mass Eye and Ear
Participant Demographics and Trial Procedures
All 14 trial participants, including 12 adults (ages 17 to 63) and two children (ages 10 and 14), were born with a form of Leber Congenital Amaurosis (LCA) caused by mutations in the centrosomal protein 290 ( CEP290 ) gene. They underwent a single injection of a CRISPR/Cas9 genome editing medicine, EDIT-101 in one eye via a specialized surgical procedure. This trial, which included the first patient to ever receive a CRISPR-based investigational medicine directly inside the body, focused primarily on safety with a secondary analysis for efficacy.
No serious treatment or procedure-related adverse events were reported, nor were there any dose-limiting toxicities. For efficacy, the researchers looked at four measures: best-corrected visual acuity (BCVA); dark-adapted full-field stimulus testing (FST), visual function navigation (VNC, as measured by a maze participants completed), and vision-related quality of life.
Eleven participants demonstrated improvements in at least one of those outcomes, while six demonstrated improvement in two or more. Four participants had clinically meaningful improvement in BCVA. Six participants experienced meaningful improvements in cone-mediated vision as indicated by FSTs, five of whom had improvements in at least one of the three other outcomes. Cone photoreceptors are used for daytime and central vision.

Infographic explaining the phase 1/2 results of the BRILLIANCE trial. Credit: Mass General Brigham
CRISPR’s Potential and Early Successes
“The results from the BRILLIANCE trial provide proof of concept and important learnings for the development of new and innovative medicines for inherited retinal diseases. We’ve demonstrated that we can safely deliver a CRISPR-based gene editing therapeutic to the retina and have clinically meaningful outcomes,” said Baisong Mei, MD, PhD, Chief Medical Officer, Editas Medicine.
Studies like this one show the promise of gene therapy for treating incurable conditions. Mass General Brigham’s Gene and Cell Therapy Institute is helping to translate scientific discoveries made by researchers into first-in-human clinical trials and, ultimately, life-changing treatments for patients.
Mutations in the CEP290 gene are the leading cause of inherited blindness taking place during the first decade of life. The mutations cause rod and cone photoceptors in the eye’s retina to function improperly, which after some time will lead to irreversible vision loss. Pierce compares it to a small part of an engine breaking down, which eventually leads the entire engine to falter.
CRISPR-Cas9 is a gene editing toolkit that acts as a GPS -guided scissor to cut a portion of the mutated genome to leave a functional gene. For inherited blindness, the goal was to inject CRISPR to reach the eye’s retina to restore the ability to produce the gene and protein responsible for light-sensing cells.
The CEP290 gene is larger than what traditional adeno-associated virus (AAV) vector gene therapies, including one FDA-approved for a different type of inherited vision loss, can accommodate. The genome editing company Editas Medicine began exploring how to tackle the CEP290 mutation in 2014, conducting preclinical studies to determine whether a gene editing approach like CRISPR-Cas9 might be feasible to target these large gene mutations. This work led to the BRILLIANCE trial, which began in mid-2019.
Jason Comander, MD, PhD, director of the Inherited Retinal Disorders Service at Mass Eye and Ear, examines the CRISPR-based medicine prior to performing a surgery of the novel treatment in September 2020, at Mass Eye and Ear in Boston. Credit: Mass Eye and Ear
Trial Outcomes and Future Directions
The first patient to receive a CRISPR treatment inside the body ( in vivo ) took place at the Casey Eye Institute at Oregon Health & Science University (OHSU), under the leadership of Mark Pennesi, MD, PhD.
“This trial shows CRISPR gene editing has exciting potential to treat inherited retinal degeneration,” Pennesi said. “There is nothing more rewarding to a physician than hearing a patient describe how their vision has improved after a treatment. One of our trial participants has shared several examples, including being able to find their phone after misplacing it and knowing that their coffee machine is working by seeing its small lights. While these types of tasks might seem trivial to those who are normally sighted, such improvements can have a huge impact on quality of life for those with low vision.”
The second patient was treated at Mass Eye and Ear in September 2020, following delays caused by the COVID-19 pandemic. Additional participants were treated across three other trial sites: Bascom Palmer Eye Institute, W.K. Kellogg Eye Center, and Scheie Eye Institute at the Children’s Hospital of Philadelphia (CHOP) and the Hospital of the University of Pennsylvania. Two adults received low-dose therapy, five received mid-dose, and another five received a high-dose treatment. Two children, treated at CHOP under the leadership of Tomas S. Aleman, MD, received a mid-dose treatment.

Principal investigator of the BRILLIANCE trial, Eric Pierce, MD, PhD, director of Ocular Genomics Institute and Berman-Gund Laboratory for the Study of Retinal Degenerations at Mass Eye and Ear and Harvard Medical School. Credit: Mass Eye and Ear
“Our patients are the first congenitally blind children to be treated with gene-editing, which significantly improved their daytime vision. Our hope is that the study will pave the road for treatments of younger children with similar conditions and further improvements in vision,” said Aleman , the Irene Heinz-Given and John LaPorte Research Professor in Ophthalmology at Penn Medicine with the Scheie Eye Institute and a pediatric ophthalmologist at CHOP who served as a site principal investigator and study co-author. “This trial represents a landmark in the treatment of genetic diseases, in specific, genetic blindness, by offering an important alternative treatment, when traditional forms of gene therapy, such as gene augmentation, are not an option.”
Participants were monitored every three months for one year, and then followed less frequently for two additional years. At visits, they would undergo a series of serum and vision tests to examine safety and efficacy outcome measures.
In November 2022, Editas paused enrollment on the BRILLIANCE trial. Pierce and colleagues are exploring working with other commercial partners to conduct additional trials, in collaboration with Editas. The researchers hope future studies can examine ideal dosing, whether a treatment effect is more pronounced in certain age groups such as younger patients, and include refined endpoints to measure the effects of improved cone function on activities of daily living.
For more on this research, see Pioneering CRISPR Gene Editing Trial: 79% of Participants See Improvement .
Reference: “Gene-editing for CEP290-associated Retinal Degeneration” by Eric A. Pierce, Tomas S. Aleman, Kanishka T. Jayasundera, Bright S. Ashimatey, Keunpyo Kim, Alia Rashid, Michael Jaskolka, Rene L. Myers, Bryon L. Lam, Steven T. Bailey, Jason I. Commander, Andreas K. Lauer, Albert M. Maguire and Mark E. Pennesi, 6 May 2024, New England Journal of Medicine . DOI: 10.1056/NEJMoa2309915
The senior corresponding author of this study was Eric A. Pierce, MD, PhD (Mass Eye and Ear), and Tomas S. Aleman, MD (CHOP) and Mark E. Pennesi, MD, PhD (OHSU) were co-corresponding authors. Additional co-authors include Kanishka T. Jayasundera, MD (Kellogg), Bright S. Ashimatey, OD, PhD (Editas), Keunpyo Kim, PhD (Editas), Alia Rashid, MD (Editas), Michael C. Jaskolka, PhD (Editas), Rene L. Myers, PhD (Editas), Byron L. Lam, MD (Bascom Palmer), Steven T. Bailey, MD (OHSU), Jason I. Comander, MD, PhD (Mass Eye and Ear), Andreas K. Lauer, MD (OHSU), Albert M. Maguire, MD (CHOP).
This research was funded by Editas medicine. This research was also supported by the National Institute of Health P30 EY014104 core grant to Mass Eye and Ear, P30 EY010572 core grant, the Malcolm M. Marquis MD Endowed Fund for Innovation, and an unrestricted grants from Research to Prevent Blindness to Casey Eye Institute and the Scheie Eye Institute. Additional support was provided by the Irene Heinz Given and John La Porte Given Endowment, and Hope for Vision.
More on SciTechDaily

AI Transforms Drug Discovery With Faster, Safer Cancer Treatments

Astronomers Reveal Previously Unknown Details About The Milky Way
New Approach to Gene Therapy: Prime Editing System Inserts Entire Genes in Human Cells

Revolutionizing Water Decontamination With Plasma Technology

Brain-Eating Amoeba Meets Its Match: Unusual Giant Virus Discovered in Austria

Unleashing Photonic Snake States: A New Era of Light Perception

MIT Scientists Find Clues to Why Fake News Snowballs on Social Media

Webb Space Telescope Captures Nearby Planetary System in Breathtaking Detail
Be the first to comment on "gene editing breakthrough: crispr improves vision in clinical trial", leave a comment cancel reply.
Email address is optional. If provided, your email will not be published or shared.
Save my name, email, and website in this browser for the next time I comment.
Welcome to the IDT family!
Your product is now available from Integrated DNA Technologies.
Many of the Swift products you have grown to love are now part of our new complete portfolio, xGen™ NGS. Through this new partnership we are pleased to offer you comprehensive next generation sequencing solutions.
xGen NGS—made for you.
Unsure of what products are available? Or, perhaps you’d like guidance on which products are compatible? If so, try our xGen NGS Solutions Builder Tool today.
Find Archer now at IDT!
All Archer information is now available on IDT’s website. You can view Archer assays alongside IDT’s xGen™ NGS portfolio to find the best next generation sequencing solution for your lab.
Confidently detect more with Archer NGS assay solutions for your solid tumor, blood cancer, immune profiling, and genetic disease research.
Explore how our NEW cGMP gRNA manufacturing service can accelerate your CRISPR therapeutics project

- Order history

- 1-800-328-2661
- How to order and request a quote
- Videos & webinars
Choose your region, country/territory, and preferred language
Crispr-cas nucleases: understanding pam requirements and cas diversification strategies, mastering nucleases to unlock crispr’s potential.

PAM sequences are a requirement for CRIPSR-Cas experiments and should be considered carefully during experimental design. This DECODED takes a look at what PAM requirements are and how researchers have genetically engineered Cas nucleases to unlock the full potential of CRISPR gene editing.
What are PAM requirements for CRISPR?
In CRISPR experiments, protospacer adjacent motifs (PAM) are short sequences of genomic DNA located directly next to the targeted modification site. PAM sequences are an important requirement for any CRISPR experiment as they are the parts of DNA that the Cas nuclease recognizes. PAM sequences act as a signal for Cas nucleases to let them know they have found the correct modification site.
The Streptococcus pyogenes Cas9 ( sp Cas9) nuclease recognizes an NGG PAM (Figure 1), other nucleases recognize different PAMs. For example, the Cas12 nuclease from Acidaminococcus sp . ( as Cas12a or Cpf1 ) relies on a TTTV sequence (Figure 2). Cas nucleases won’t cut the PAM site itself but modify the DNA upstream or downstream of the PAM site. More specifically, Cas nuclease is thought to destabilize the adjacent sequence, allowing interrogation of the sequence by the crRNA, and resulting in RNA-DNA pairing when a matching sequence is present [ 1,2 ].
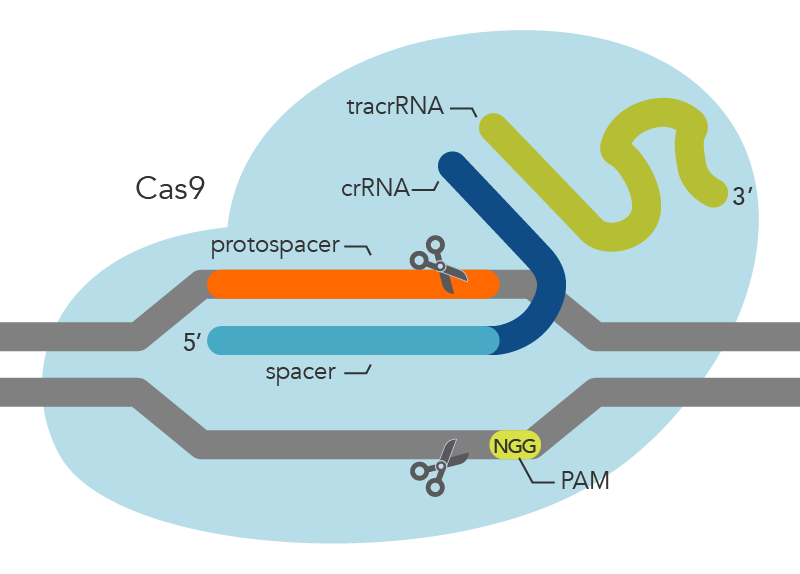
Engineering Cas nucleases to recognize novel PAMs
One example of Cas nucleases modified to recognize different PAMs are the Alt-R™ CRISPR-Cas12a nucleases, which come in two formats:
- Alt-R Cas12a V3 which recognizes a TTTV PAM sequence
- Alt-R Cas12a Ultra which works with a TTTN (N = any nucleotide) PAM site
These Cas nucleases are recombinant Cas12a enzymes from Acidminococcus sp. (V3 Cas) and Lachnospiraceae ( Ultra Cas). The Alt-R Cas12a Ultra nucleases have higher on-target potency than their wild-type Cas12a nuclease counterparts. These mutants recognize TTTT PAM sites in addition to TTTV motifs, which increases their target range for genome editing studies. Additionally, the Alt-R Cas12a Ultra mutant has an increased temperature tolerance, providing more flexibility for gene editing in systems that require lower culture temperatures.
Other Cas nuclease variants from different bacterial species that recognize different PAM sequences are also exist (Table 1)[ 3,4 ].
Table 1. Overview of diverse Cas nucleases and their associated PAM sequence.
Continuing efforts by researchers to isolate novel Cas nucleases as well as modify already identified Cas enzymes have greatly increased the expanded ways that CRISPR can be applied to gene editing applications.
Learn more about CRISPR
CRISPR has proven to be a powerful tool for gene editing and for advancing genomic medicine. CRISPR-Cas9 is one of many options available for gene editing, meaning it is important to fully understand this approach and others when developing an experiment.
- Anders C, Niewoehner O, Duerst A, et al . Structural basis of PAM-dependent target DNA recognition by the Cas9 endonuclease . Nature . 2014;513(7519):569-573.
- Sternberg SH, Redding S, Jinek M, et al . DNA interrogation by the CRISPR RNA-guided endonuclease Cas9 . Nature . 2014;507(7490):62-67.
- Kleinstiver BP, Prew MS, Tsai SQ, et al . Engineered CRISPR-Cas9 nucleases with altered PAM specificities . Nature . 2015;523(7561):481-485.
- Xin C, Yin J, Yuan S, et al . Comprehensive assessment of miniature CRISPR-Cas12f nucleases for gene disruption . Nat Commun . 2022;13(1):5623.
RUO24-2780_001
Courtney Thomas, PhD, Scientific Writer, IDT
Published May 14, 2024
DECODED online newsletter

Additional resources
- Importance of the PAM sequences in CRISPR-Cas9 gene editing »
- CRISPR-Cas9: What are the pros and cons? »
- What is CRISPR screening? »
Review other DECODED Online newsletter articles on CRISPR genome editing applications.
Product focus
Alt-r™ crispr-cas12a (cpf1).
Cas12a-based genome editing with TTTV PAM sequence. Provides expanded target site selection.
Uses modified crRNA and RNP delivery for higher on-target potency. No tracrRNA needed.
Alt-R™ Genome Editing Detection Kit
T7 endonuclease I (T7EI) mismatch cleavage assay detects on-target or known off-target genome editing in cultured cells.
Technically simple, providing easy-to-read electrophoretic results. For single samples or high throughput.
Alt-R™ CRISPR-Cas9 System
Cas9 nucleases, guide RNAs, enhancers, and controls for successful genome editing.
Use for knock-out (non-homologous end joining [NHEJ]) or knock-in (homology-directed repair [HDR]) experiments.
- Today's news
- Reviews and deals
- Climate change
- 2024 election
- Fall allergies
- Health news
- Mental health
- Sexual health
- Family health
- So mini ways
- Unapologetically
- Buying guides
Entertainment
- How to Watch
- My Portfolio
- Latest News
- Stock Market
- Premium News
- Biden Economy
- EV Deep Dive
- Stocks: Most Actives
- Stocks: Gainers
- Stocks: Losers
- Trending Tickers
- World Indices
- US Treasury Bonds
- Top Mutual Funds
- Highest Open Interest
- Highest Implied Volatility
- Stock Comparison
- Advanced Charts
- Currency Converter
- Basic Materials
- Communication Services
- Consumer Cyclical
- Consumer Defensive
- Financial Services
- Industrials
- Real Estate
- Mutual Funds
- Credit cards
- Balance Transfer Cards
- Cash-back Cards
- Rewards Cards
- Travel Cards
- Personal Loans
- Student Loans
- Car Insurance
- Morning Brief
- Market Domination
- Market Domination Overtime
- Opening Bid
- Stocks in Translation
- Lead This Way
- Good Buy or Goodbye?
- Fantasy football
- Pro Pick 'Em
- College Pick 'Em
- Fantasy baseball
- Fantasy hockey
- Fantasy basketball
- Download the app
- Daily fantasy
- Scores and schedules
- GameChannel
- World Baseball Classic
- Premier League
- CONCACAF League
- Champions League
- Motorsports
- Horse racing
- Newsletters
New on Yahoo
- Privacy Dashboard
Yahoo Finance
Crispr therapeutics provides business update and reports first quarter 2024 financial results.
-More than 25 authorized treatment centers (ATCs) activated globally for CASGEVY™ and multiple patients have already had cells collected-
-Clinical trials ongoing for next generation CAR T product candidates, CTX112™ and CTX131™ targeting CD19 and CD70 respectively, across multiple indications-
-Clinical trials ongoing for in vivo gene editing product candidates, CTX310™ and CTX320™ targeting ANGPTL3 and Lp(a), respectively -
-Expands pipeline with new preclinical programs utilizing lipid nanoparticle (LNP) mediated delivery to the liver for refractory hypertension targeting angiotensinogen (AGT) and acute hepatic porphyria (AHP) targeting 5’-aminolevulinate synthase 1 (ALAS1)-
-Clinical trial ongoing for CTX211™ , an allogeneic, hypoimmune, gene-edited, stem cell derived product candidate for the treatment of Type 1 Diabetes (T1D)-
-Strong balance sheet with approximately $2.1 billion in cash, cash equivalents, and marketable securities as of March 31, 2024-
ZUG, Switzerland and BOSTON, May 08, 2024 (GLOBE NEWSWIRE) -- CRISPR Therapeutics (Nasdaq: CRSP), a biopharmaceutical company focused on creating transformative gene-based medicines for serious diseases, today reported financial results for the first quarter ended March 31, 2024.
“This quarter, in addition the robust launch of CASGEVY, we are pleased to have nominated additional in vivo programs targeting both rare and common diseases to our portfolio based on promising preclinical data,” said Samarth Kulkarni, Ph.D., Chief Executive Officer and Chairman of CRISPR Therapeutics. “Additionally, we continue to advance our portfolio of clinical trials across oncology, autoimmune, diabetes and cardiovascular indications in a capital efficient manner. With multiple data read-outs in the next 12-18 months, we are poised to broaden the number of patients that could potentially benefit from transformative gene-editing based therapies.”
Recent Highlights and Outlook
Hemoglobinopathies and CASGEVY™ (exagamglogene autotemcel [exa-cel])
CASGEVY is approved in the U.S., Great Britain, the European Union (EU), the Kingdom of Saudi Arabia (KSA), and the Kingdom of Bahrain (Bahrain) for the treatment of both sickle cell disease (SCD) and transfusion-dependent beta thalassemia (TDT). Regulatory submissions for CASGEVY have been completed in both SCD and TDT in Switzerland and Canada; the submission in Canada was granted priority review. CASGEVY is the first therapy to emerge from a strategic partnership between CRISPR Therapeutics and Vertex Pharmaceuticals established in 2015. As part of an amendment to the collaboration agreement in 2021, Vertex now leads global development, manufacturing, regulatory and commercialization of CASGEVY with support from CRISPR Therapeutics.
As of mid-April, more than 25 authorized treatment centers (ATCs) have been activated globally, including centers in all regions where CASGEVY is approved, and multiple patients have already had cells collected.
Vertex has signed multiple agreements with both commercial and government health insurance providers in the U.S. to provide access to CASGEVY. Vertex has also secured reimbursed access for eligible people with SCD or TDT in KSA and Bahrain, as well as for people with TDT in France through an early access program.
CRISPR Therapeutics has two next-generation approaches with the potential to significantly expand the addressable population with SCD and TDT. CRISPR Therapeutics continues to advance its internally developed targeted conditioning program, an anti-CD117 (c-Kit) antibody-drug conjugate (ADC), through preclinical studies. Additionally, the Company has ongoing research efforts to enable in vivo editing of hematopoietic stem cells. This work could obviate the need for conditioning altogether, expand geographic reach, and enable the treatment of multiple additional other diseases beyond SCD and TDT.
Immuno-Oncology and Autoimmune Diseases
CRISPR Therapeutics’ next-generation allogeneic CAR T candidates reflect the Company’s mission of innovating continuously to bring potentially transformative medicines to patients as quickly as possible. Clinical trials are ongoing for the Company’s next-generation CAR T product candidates, CTX112™ and CTX131™, targeting CD19 and CD70, respectively, across multiple indications. CTX112 and CTX131 both contain novel potency edits which can lead to significantly higher CAR T cell expansion and cytotoxicity, potentially representing best-in-class allogeneic CAR T products for these targets.
CTX112 is being developed for both oncology and autoimmune indications. In oncology settings, CTX112 is in a Phase 1/2 trial for CD19 positive relapsed or refractory B-cell malignancies, and the Company expects to report preliminary clinical data this year.
The Company remains on track to initiate a clinical trial for CTX112 in systemic lupus erythematosus (SLE) in the first half of this year, with the potential to expand into additional autoimmune indications in the future. Early clinical studies have shown that CD19-directed autologous CAR T therapy can produce long-lasting remissions in multiple autoimmune indications by deeply depleting B cells. The Company’s first generation allogeneic CD19-directed CAR T program has demonstrated effective depletion of B cells in oncology settings, which supports the potential for CTX112 in autoimmune diseases.
CTX131, CRISPR Therapeutics’ next generation CAR T targeting CD70, is currently in an ongoing clinical trial in solid tumors. The Company remains on track to initiate a clinical trial for CTX131 in hematologic malignancies in the first half of this year.
CRISPR Therapeutics has established a proprietary lipid nanoparticle (LNP) platform for the delivery of CRISPR/Cas9 to the liver. The first two in vivo programs utilizing this proprietary platform, CTX310™ and CTX320™, are directed towards validated therapeutic targets associated with cardiovascular disease, and are in ongoing clinical trials. Earlier today, the Company announced the addition of two additional preclinical programs, CTX340™ and CTX450™, utilizing this LNP delivery system, demonstrating the modularity and scalability of the platform.
Refractory hypertension is a serious unmet medical need affecting approximately 1.5 million patients in the U.S. alone. CTX340 is designed to inhibit production of hepatic angiotensinogen (AGT), a validated target to modulate the renin-angiotensin-aldosterone system (RAAS) and normalize blood pressure durably with a one-time treatment. In preclinical studies, CTX340 showed ~60% liver editing and ~90% AGT protein reduction, resulting in sustained ~30 mmHg blood pressure (BP) reduction out to 3 months in the spontaneously hypertensive rat (SHR) model.
Acute hepatic porphyria (AHP) is a group of rare genetic diseases of heme biosynthesis. Symptomatic patients have acute attacks, characterized by debilitating neurovascular symptoms, as well as multiple chronic symptoms, such as pain. There are approximately 5,000 patients diagnosed with AHP in the U.S., although the disease remains underdiagnosed. CTX450 is specifically designed to inhibit production of ALAS1 in the liver, preventing accumulation of neurotoxic aminolevulinic acid (ALA) and porphobilinogen (PBG). In preclinical studies, CTX450 showed ~70% liver editing and ~97% ALAS1 protein reduction, resulting in reduction of ALA and PBG disease biomarkers to normal levels in an AHP mouse model.
CRISPR Therapeutics has initiated IND/CTA-enabling studies for CTX340 and CTX450 and expects to initiate both clinical trials in the second half of 2025.
In addition to the pipeline updates expanding the liver-targeted in vivo pipeline, CRISPR Therapeutics reported initial data at the American Society of Gene and Cell Therapy Annual Meeting demonstrating its proprietary capabilities to deliver to and edit genes in the eye, opening a potential new focus area.
Regenerative Medicine
CRISPR Therapeutics continues to advance a Phase 1 clinical trial for CTX211™ for the treatment of Type 1 Diabetes (T1D). CRISPR Therapeutics remains committed to its goal of developing a beta-cell replacement product that does not require chronic immunosuppression.
Vertex has non-exclusive rights to certain CRISPR Therapeutics’ CRISPR/Cas9 technology to accelerate development of potentially curative cell therapies for T1D. CRISPR Therapeutics remains eligible for development milestones and would receive royalties on any future products resulting from this agreement.
Other Corporate Matters
In March, CRISPR Therapeutics announced its proposal to elect Christian Rommel, Ph.D., to its Board of Directors at the Company’s annual general meeting to be held this year. Dr Rommel brings in-depth experience in successfully accelerating innovation and advancing drug candidates across a breadth of modalities and disease areas.
In February, CRISPR Therapeutics announced that it had entered into an investment agreement for the sale of approximately $280 million of its common shares to a select group of institutional investors in a registered direct offering.
First Quarter 2024 Financial Results
Cash Position: Cash, cash equivalents, and marketable securities were $2,108.1 million as of March 31, 2024, compared to $1,695.7 million as of December 31, 2023. The increase in cash was primarily driven by proceeds from the February 2024 registered direct offering, a $200.0 million milestone payment received from Vertex in connection with the approval of CASGEVY, proceeds from employee option exercises as well as interest income, offset by operating expenses.
R&D Expenses: R&D expenses were $76.2 million for the first quarter of 2024, compared to $99.9 million for the first quarter of 2023. The decrease in R&D expense was primarily driven by reduced variable external research and manufacturing costs.
G&A Expenses: General and administrative expenses were $18.0 million for the first quarter of 2024, compared to $22.4 million for the first quarter of 2023. The decrease in G&A expense was primarily driven by a decrease in employee related and stock-based compensation expense.
Collaboration Expense: Collaboration expense, net, was $47.0 million for the first quarter of 2024, compared to $42.2 million for the first quarter of 2023. The increase in collaboration expense, net, was primarily attributable to commercial and manufacturing costs.
Net Loss: Net loss was $116.6 million for the first quarter of 2024, compared to a net loss of $53.1 million for the first quarter of 2023.
About CASGEVY™ (exagamglogene autotemcel [exa-cel])
CASGEVY™ is a non-viral, ex vivo CRISPR/Cas9 gene-edited cell therapy for eligible patients with SCD or TDT, in which a patient’s own hematopoietic stem and progenitor cells are edited at the erythroid specific enhancer region of the BCL11A gene. This edit results in the production of high levels of fetal hemoglobin (HbF; hemoglobin F) in red blood cells. HbF is the form of the oxygen-carrying hemoglobin that is naturally present during fetal development, which then switches to the adult form of hemoglobin after birth. CASGEVY has been shown to reduce or eliminate VOCs for patients with SCD and transfusion requirements for patients with TDT.
CASGEVY is approved for certain indications in multiple jurisdictions for eligible patients.
About the CRISPR Therapeutics-Vertex Collaboration CRISPR Therapeutics and Vertex entered into a strategic research collaboration in 2015 focused on the use of CRISPR/Cas9 to discover and develop potential new treatments aimed at the underlying genetic causes of human disease. CASGEVY (exa-cel) represents the first potential treatment to emerge from the joint research program. Under an amended collaboration agreement, Vertex now leads global development, manufacturing, and commercialization of CASGEVY and splits program costs and profits worldwide 60/40 with CRISPR Therapeutics. Vertex is the manufacturer and exclusive license holder of CASGEVY™.
About CTX112 CTX112 is a next-generation, wholly-owned, allogeneic CAR T product candidate targeting Cluster of Differentiation 19, or CD19, which incorporates additional edits designed to enhance CAR T potency and reduce CAR T exhaustion. CTX112 is being investigated in an ongoing clinical trial designed to assess safety and efficacy of the product candidate in adult patients with relapsed or refractory CD19-positive B-cell malignancies who have received at least two prior lines of therapy.
About CTX131 CTX131 is a next-generation, wholly-owned, allogeneic CAR T product candidate targeting Cluster of Differentiation 70, or CD70, an antigen expressed on various solid tumors and hematologic malignancies. CTX131 incorporates additional edits designed to enhance CAR T potency and reduce CAR T exhaustion. CTX131 is being investigated in a clinical trial designed to assess the safety and efficacy of the product candidate in adult patients with relapsed or refractory solid tumors.
About In Vivo Programs CRISPR Therapeutics has established a proprietary LNP platform for the delivery of CRISPR/Cas9 to the liver. The Company’s in vivo portfolio includes its lead investigational in vivo programs, CTX310 (directed towards angiopoietin-related protein 3 (ANGPTL3)) and CTX320 (directed towards lipoprotein(a) (Lp(a)), two validated therapeutic targets for cardiovascular disease, are in ongoing clinical trials. In addition, the Company’s research and preclinical development candidates include CTX340 and CTX450, targeting angiotensinogen (AGT) for refractory hypertension and 5’-aminolevulinate synthase 1 (ALAS1) for acute hepatic porphyria (AHP), respectively.
About CTX211 CTX211 is an allogeneic, gene-edited, stem cell-derived investigational therapy for the treatment of T1D, which incorporates gene edits that aim to make cells hypoimmune and enhance cell fitness. This immune-evasive cell replacement therapy is designed to enable patients to produce their own insulin in response to glucose.
About CRISPR Therapeutics Since its inception over a decade ago, CRISPR Therapeutics has transformed from a research-stage company advancing programs in the field of gene editing, to a company that recently celebrated the historic approval of the first-ever CRISPR-based therapy and has a diverse portfolio of product candidates across a broad range of disease areas including hemoglobinopathies, oncology, regenerative medicine, cardiovascular, autoimmune, and rare diseases. CRISPR Therapeutics advanced the first-ever CRISPR/Cas9 gene-edited therapy into the clinic in 2018 to investigate the treatment of sickle cell disease or transfusion-dependent beta thalassemia, and beginning in late 2023, CASGEVY™ (exagamglogene autotemcel) was approved in some countries to treat eligible patients with either of those conditions. The Nobel Prize-winning CRISPR science has revolutionized biomedical research and represents a powerful, clinically validated approach with the potential to create a new class of potentially transformative medicines. To accelerate and expand its efforts, CRISPR Therapeutics has established strategic partnerships with leading companies including Bayer and Vertex Pharmaceuticals. CRISPR Therapeutics AG is headquartered in Zug, Switzerland, with its wholly-owned U.S. subsidiary, CRISPR Therapeutics, Inc., and R&D operations based in Boston, Massachusetts and San Francisco, California, and business offices in London, United Kingdom. To learn more, visit www.crisprtx.com .
CRISPR THERAPEUTICS® standard character mark and design logo, CTX112™, CTX131™, CTX211™, CTX310™, CTX320™, CTX340™ and CTX450™ are trademarks and registered trademarks of CRISPR Therapeutics AG. The CASGEVY™ word mark and design are trademarks of Vertex Pharmaceuticals Incorporated. All other trademarks and registered trademarks are the property of their respective owners.
CRISPR Therapeutics Forward-Looking Statement
This press release may contain a number of “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including statements made by Dr. Kulkarni in this press release, as well as statements regarding CRISPR Therapeutics’ expectations about any or all of the following: (i) its plans for and its preclinical studies, clinical trials and pipeline products and programs, including, without limitation, manufacturing capabilities, status of such studies and trials, potential expansion into new indications and expectations regarding data generally; (ii) the data that will be generated by ongoing and planned clinical trials, and the ability to use that data for the design and initiation of further clinical trials; (iii) plans and expectations for the commercialization of, and anticipated benefits of, CASGEVY, including the anticipated patient populations eligible for CASGEVY in jurisdictions where it has been or may be approved; (iv) the sufficiency of its cash resources; (v) the expected benefits of its collaborations; and (vi) the therapeutic value, development, and commercial potential of CRISPR/Cas9 gene editing technologies and therapies. Without limiting the foregoing, the words “believes,” “anticipates,” “plans,” “expects” and similar expressions are intended to identify forward-looking statements. You are cautioned that forward-looking statements are inherently uncertain. Although CRISPR Therapeutics believes that such statements are based on reasonable assumptions within the bounds of its knowledge of its business and operations, forward-looking statements are neither promises nor guarantees and they are necessarily subject to a high degree of uncertainty and risk. Actual performance and results may differ materially from those projected or suggested in the forward-looking statements due to various risks and uncertainties. These risks and uncertainties include, among others: the efficacy and safety results from ongoing clinical trials will not continue or be repeated in ongoing or planned clinical trials or may not support regulatory submissions; regulatory authorities may not approve exa-cel on a timely basis or at all; adequate pricing or reimbursement may not be secured to support continued development or commercialization of exa-cel following regulatory approval; clinical trial results may not be favorable; one or more of its product candidate programs will not proceed as planned for technical, scientific or commercial reasons; future competitive or other market factors may adversely affect the commercial potential for its product candidates; initiation and completion of preclinical studies for its product candidates is uncertain and results from such studies may not be predictive of future results of future studies or clinical trials; regulatory approvals to conduct trials or to market products are uncertain; uncertainties inherent in the operation of a manufacturing facility; it may not realize the potential benefits of its collaborations; uncertainties regarding the intellectual property protection for its technology and intellectual property belonging to third parties, and the outcome of proceedings (such as an interference, an opposition or a similar proceeding) involving all or any portion of such intellectual property; and those risks and uncertainties described under the heading "Risk Factors" in CRISPR Therapeutics’ most recent annual report on Form 10-K, quarterly report on Form 10-Q and in any other subsequent filings made by CRISPR Therapeutics with the U.S. Securities and Exchange Commission, which are available on the SEC's website at www.sec.gov. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date they are made. CRISPR Therapeutics disclaims any obligation or undertaking to update or revise any forward-looking statements contained in this press release, other than to the extent required by law.
Investor Contact: Susan Kim +1-617-307-7503 [email protected]
Media Contact: Rachel Eides +1-617-315-4493 [email protected]
CRISPR Therapeutics AG Condensed Consolidated Statements of Operations (Unaudited, In thousands except share data and per share data)
CRISPR Therapeutics AG Condensed Consolidated Balance Sheets Data (Unaudited, in thousands)
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Oncol
CRISPR Gene Therapy: Applications, Limitations, and Implications for the Future
Fathema uddin.
1 Department of Medicine, Thoracic Oncology Service, Memorial Sloan Kettering Cancer Center, New York, NY, United States
Charles M. Rudin
2 Weill Cornell Medicine, Cornell University, New York, NY, United States
Triparna Sen
A series of recent discoveries harnessing the adaptive immune system of prokaryotes to perform targeted genome editing is having a transformative influence across the biological sciences. The discovery of Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and CRISPR-associated (Cas) proteins has expanded the applications of genetic research in thousands of laboratories across the globe and is redefining our approach to gene therapy. Traditional gene therapy has raised some concerns, as its reliance on viral vector delivery of therapeutic transgenes can cause both insertional oncogenesis and immunogenic toxicity. While viral vectors remain a key delivery vehicle, CRISPR technology provides a relatively simple and efficient alternative for site-specific gene editing, obliviating some concerns raised by traditional gene therapy. Although it has apparent advantages, CRISPR/Cas9 brings its own set of limitations which must be addressed for safe and efficient clinical translation. This review focuses on the evolution of gene therapy and the role of CRISPR in shifting the gene therapy paradigm. We review the emerging data of recent gene therapy trials and consider the best strategy to move forward with this powerful but still relatively new technology.
Introduction
Gene therapy as a strategy to provide therapeutic benefit includes modifying genes via disruption, correction, or replacement ( 1 ). Gene therapy has witnessed both early successes and tragic failures in a clinical setting. The discovery and development of the CRISPR/Cas9 system has provided a second opportunity for gene therapy to recover from its stigma and prove to be valuable therapeutic strategy. The recent advent of CRISPR technology in clinical trials has paved way for the new era of CRISPR gene therapy to emerge. However, there are several technical and ethical considerations that need addressing when considering its use for patient care. This review aims to (1) provide a brief history of gene therapy prior to CRISPR and discuss its ethical dilemmas, (2) describe the mechanisms by which CRISPR/Cas9 induces gene edits, (3) discuss the current limitations and advancements made for CRISPR technology for therapeutic translation, and (4) highlight a few recent clinical trials utilizing CRISPR gene therapy while opening a discussion for the ethical barriers that these and future trials may hinge upon.
Gene Therapy Prior to Crispr—History, Hurdles, and its Future
Origins of gene therapy.
The introduction of gene therapy into the clinic provided hope for thousands of patients with genetic diseases and limited treatment options. Initially, gene therapy utilized viral vector delivery of therapeutic transgenes for cancer treatment ( 2 ) or monogenic disease ( 3 ). One of these pioneering clinical trials involved ex vivo retroviral delivery of a selective neomycin-resistance marker to tumor infiltrating leukocytes (TILs) extracted from advanced melanoma patients ( 4 ). Although the neomycin tagging of TILs did not have a direct therapeutic intent and was used for tracking purposes, this study was the first to provide evidence for both the feasibility and safety of viral-mediated gene therapy. Soon after, the first clinical trial that used gene therapy for therapeutic intent was approved in 1990 for the monogenic disease adenosine deaminase-severe combined immunodeficiency (ADA-SCID). Two young girls with ADA-SCID were treated with retroviruses for ex vivo delivery of a wildtype adenosine deaminase gene to autologous T-lymphocytes, which were then infused back into the patients ( 5 , 6 ). While one patient showed moderate improvement, the other did not ( 5 , 6 ) Although initial results were suboptimal, the early evidence of feasibility prompted multiple subsequent gene therapy trials using viral-mediated gene edition. However, this was followed by some major setbacks.
Tragic Setbacks for Gene Therapy
Jesse Gelsinger, an 18-year-old with a mild form of the genetic disease ornithine transcarbamylase (OTC) deficiency, participated in a clinical trial which delivered a non-mutated OTC gene to the liver through a hepatic artery injection of the recombinant adenoviral vector housing the therapeutic gene. Unfortunately, Jesse passed away 4 days after treatment ( 7 ). The adenovirus vector triggered a much stronger immune response in Jesse than it had in other patients, causing a chain of multiple organ failures that ultimately led to his death ( 8 ). At the time of the trial, adenoviral vectors were considered reasonably safe. In preclinical development, however, two of the rhesus monkeys treated with the therapy developed a similar pattern of fatal hepatocellular necrosis ( 9 ). Shortly after, another gene therapy trial led to the development of leukemia in several young children induced by insertional oncogenesis from the therapy ( 10 ). These trials opened for two forms of SCID (SCID-X1 or common ɤ chain deficiency) and adenosine deaminase deficiency (ADA). The therapy used ɤ-retroviral vectors for ex vivo delivery of therapeutic transgenes to autologous CD34+ hematopoietic stem cells, which were reintroduced to the patients ( 10 ). Five patients developed secondary therapy-related leukemia, one of whom died from the disease ( 11 ). Further investigation revealed integration of the therapeutic gene into the LMO2 proto-oncogene locus, presumably resulting in the development of leukemia ( 12 ). Subsequent analyses have suggested a higher frequency of insertional mutagenesis events with ɤ-retroviral vectors relative to other vectors ( 13 ). Together, these tragic events prompted substantial post-hoc concerns regarding the nature of appropriate informed consent and the stringency of safety and eligibility parameters for gene therapy experimentation in humans ( 14 ).
Shifting the Gene Therapy Paradigm
Almost two decades after these cases, gene therapy returned in clinical trials with reengineered viruses designed with safety in mind. Current clinical approaches are being scrutinized for evidence of insertional mutagenesis and adverse immunogenic reactions ( 15 – 18 ). Non-viral vectors have been used as an alternative method for gene delivery, which have reduced immunogenicity compared to their viral counterparts and therefore greater tolerance for repeated administration. A concern is whether these methods can be optimized to provide equivalent efficiency of gene delivery to that provided by viruses ( 19 ).
While viral vectors continue to be essential for current gene therapy, the concerns and limitations of viral-mediated gene edition has broadened the diversity of gene-editing approaches being considered. Rather than introducing the therapeutic gene into a novel (and potentially problematic) locus, a more attractive strategy would be to directly correct the existing genetic aberrations in situ . This alternative would allow the pathological mutation to be repaired while averting the risk of insertional oncogenesis. The discovery and repurposing of nucleases for programmable gene editing made this possible, beginning with the development of zinc finger nucleases (ZFN) ( 20 , 21 ), followed by transcription activator-like effector nucleases (TALENs), meganucleases, and most recently, the CRISPR/Cas system ( 22 ). While the other gene-editing tools can induce genome editing at targeted sites under controlled conditions, the CRISPR/Cas system has largely supplanted these earlier advances due to its relatively low price, ease of use, and efficient and precise performance. However, this technology is often delivered with adeno-associated virus (AAV) vectors, and thus does not completely avert risks associated with viruses. Other delivery options are available to circumvent this issue, each with their own advantages and challenges (see Delivery of CRISPR Gene Therapy section). Of the CRISPR/Cas systems, CRISPR/Cas9 is the most developed and widely used tool for current genome editing.
CRISPR/Cas9 Mediated Gene Editing
Pioneering discoveries in crispr/cas9 technology.
The bacterial CRISPR locus was first described by Francisco Mojica ( 23 ) and later identified as a key element in the adaptive immune system in prokaryotes ( 24 ). The locus consists of snippets of viral or plasmid DNA that previously infected the microbe (later termed “spacers”), which were found between an array of short palindromic repeat sequences. Later, Alexander Bolotin discovered the Cas9 protein in Streptococcus thermophilus , which unlike other known Cas genes, Cas9 was a large gene that encoded for a single-effector protein with nuclease activity ( 25 ). They further noted a common sequence in the target DNA adjacent to the spacer, later known as the protospacer adjacent motif (PAM)—the sequence needed for Cas9 to recognize and bind its target DNA ( 25 ). Later studies reported that spacers were transcribed to CRISPR RNAs (crRNAs) that guide the Cas proteins to the target site of DNA ( 26 ). Following studies discovered the trans-activating CRISPR RNA (tracrRNA), which forms a duplex with crRNA that together guide Cas9 to its target DNA ( 27 ). The potential use of this system was simplified by introducing a synthetic combined crRNA and tracrRNA construct called a single-guide RNA (sgRNA) ( 28 ). This was followed by studies demonstrating successful genome editing by CRISPR/Cas9 in mammalian cells, thereby opening the possibility of implementing CRISPR/Cas9 in gene therapy ( 29 ) ( Figure 1 ).

Hallmarks of CRISPR Gene Therapy. Timeline highlighting major events of traditional gene therapy, CRISPR development, and CRISPR gene therapy. The text in red denotes gene therapy events which have raised significant ethical concerns.
Mechanistic Overview of CRISPR/Cas9-Mediated Genome Editing
CRISPR/Cas9 is a simple two-component system used for effective targeted gene editing. The first component is the single-effector Cas9 protein, which contains the endonuclease domains RuvC and HNH. RuvC cleaves the DNA strand non-complementary to the spacer sequence and HNH cleaves the complementary strand. Together, these domains generate double-stranded breaks (DSBs) in the target DNA. The second component of effective targeted gene editing is a single guide RNA (sgRNA) carrying a scaffold sequence which enables its anchoring to Cas9 and a 20 base pair spacer sequence complementary to the target gene and adjacent to the PAM sequence. This sgRNA guides the CRISPR/Cas9 complex to its intended genomic location. The editing system then relies on either of two endogenous DNA repair pathways: non-homologous end-joining (NHEJ) or homology-directed repair (HDR) ( Figure 2 ). NHEJ occurs much more frequently in most cell types and involves random insertion and deletion of base pairs, or indels, at the cut site. This error-prone mechanism usually results in frameshift mutations, often creating a premature stop codon and/or a non-functional polypeptide. This pathway has been particularly useful in genetic knock-out experiments and functional genomic CRISPR screens, but it can also be useful in the clinic in the context where gene disruption provides a therapeutic opportunity. The other pathway, which is especially appealing to exploit for clinical purposes, is the error-free HDR pathway. This pathway involves using the homologous region of the unedited DNA strand as a template to correct the damaged DNA, resulting in error-free repair. Experimentally, this pathway can be exploited by providing an exogenous donor template with the CRISPR/Cas9 machinery to facilitate the desired edit into the genome ( 30 ).

CRISPR/Cas9 mediated gene editing. Cas9 in complex with the sgRNA targets the respective gene and creates DSBs near the PAM region. DNA damage repair proceeds either through the NHEJ pathway or HDR. In the NHEJ pathway, random insertions and deletions (indels) are introduced at the cut side and ligated resulting in error-prone repair. In the HDR pathway, the homologous chromosomal DNA serves as a template for the damaged DNA during repair, resulting in error-free repair.
Limitations and Advancements of CRISPR/Cas9
Off-target effects.
A major concern for implementing CRISPR/Cas9 for gene therapy is the relatively high frequency of off-target effects (OTEs), which have been observed at a frequency of ≥50% ( 31 ). Current attempts at addressing this concern include engineered Cas9 variants that exhibit reduced OTE and optimizing guide designs. One strategy that minimizes OTEs utilizes Cas9 nickase (Cas9n), a variant that induces single-stranded breaks (SSBs), in combination with an sgRNA pair targeting both strands of the DNA at the intended location to produce the DSB ( 32 ). Researchers have also developed Cas9 variants that are specifically engineered to reduce OTEs while maintaining editing efficacy ( Table 1 ). SpCas9-HF1 is one of these high-fidelity variants that exploits the “excess-energy” model which proposes that there is an excess affinity between Cas9 and target DNA which may be enabling OTEs. By introducing mutations to 4 residues involved in direct hydrogen bonding between Cas9 and the phosphate backbone of the target DNA, SpCas9-HF1 has been shown to possess no detectable off-target activity in comparison to wildtype SpCas9 ( 35 ). Other Cas9 variants that have been developed include evoCas9 and HiFiCas9, both of which contain altered amino acid residues in the Rec3 domain which is involved in nucleotide recognition. Desensitizing the Rec3 domain increases the dependence on specificity for the DNA:RNA heteroduplex to induce DSBs, thereby reducing OTEs while maintaining editing efficacy ( 38 , 39 ). One of the more recent developments is the Cas9_R63A/Q768A variant, in which the R63A mutation destabilizes R-loop formation in the presence of mismatches and Q768A mutation increases sensitivity to PAM-distal mismatches ( 49 ). Despite the different strategies, the rational for generating many Cas9 variants with reduced OTEs has been to ultimately reduce general Cas9 and DNA interactions and give a stronger role for the DNA:RNA heteroduplex in facilitating the edits.
Cas9 variants.
Optimizing guide designs can also reduce the frequency of OTEs ( 31 ). Many features in an sgRNA determine specificity including the seed sequence (a 10–12 bp region proximal to PAM on 3′ of spacer sequence) ( 29 , 53 ), GC content ( 54 , 55 ), and modifications such as 5′ truncation of the sgRNA ( 56 ). Several platforms have also been designed to provide optimized guide sequences against target genes, including E-Crisp ( 31 , 57 ), CRISPR-design, CasOFFinder, and others ( 31 ). However, many of these tools are designed based on computational algorithms with varying parameters or rely on phenotypic screens that may be specific to cell types and genomes, generating appreciable noise and lack of generalizability across different experimental setups ( 58 , 59 ). Recently, an additional guide design tool named sgDesigner was developed that addressed these limitations by employing a novel plasmid library in silico that contained both the sgRNA and the target site within the same construct. This allowed collecting Cas9 editing efficiency data in an intrinsic manner and establish a new training dataset that avoids the biases introduced through other models. Furthermore, a comparative performance evaluation to predict sgRNA efficiency of sgDesigner with 3 other commonly used tools (Doench Rule Set 2, Sequence Scan for CRISPR and DeepCRISPR) revealed that sgDesigner outperformed all 3 designer tools in 6 independent datasets, suggesting that sgDesigner may be a more robust and generalizable platform ( 60 ).
Protospacer Adjacent Motif Requirement
An additional limitation of the technology is the requirement for a PAM near the target site. Cas9 from the bacteria Streptococcus pyogenes (SpCas9) is one of the most extensively used Cas9s with a relatively short canonical PAM recognition site: 5′NGG3′, where N is any nucleotide. However, SpCas9 is relatively large and difficult to package into AAV vectors ( 61 , 62 ), the most common delivery vehicle for gene therapy. Staphylococcus aureus Cas9 (SaCas9) is a smaller ortholog that can be packaged more easily in AAV vectors but has a longer PAM sequence: 5′NNGRRT3′ or 5′NNGRR(N)3′, where R is any purine, which further narrows the window of therapeutic targeting sites. Engineered SaCas9 variants have been made, such as KKH SaCas9, which recognizes a 5′NNNRRT3′ PAM, broadening the human targeting sites by 2- to 4-fold. OTEs, however, are observed with frequencies similar to wildtype SaCas9 and need to be considered in designing any therapeutic application ( 33 ). Several other variants of SpCas9 have also been engineered for broadening the gene target window including SpCas9-NG, which recognizes a minimal NG PAM ( 44 ) and xCas9, which recognizes a broad range of PAM including NG, GAA, and GAT ( 43 ). A side by side comparison of both variants revealed that while SpCas9-NG had a broader PAM recognition, xCas9 had the lowest OTE in human cells ( 63 ). Another Cas9 ortholog from the bacteria Streptococcus canis , ScCas9, has been recently characterized with a minimal PAM specificity of 5′NNG3′ and an 89.2% sequence homology to SpCas9 and comparable editing efficiency to SpCas9 in both bacterial and human cells ( 52 ). The most recent development is a variant of SpCas9 named SpRY that has been engineered to be nearly PAMless, recognizing minimal NRN > NYN PAMs. This new variant can potentially edit any gene independent of a PAM requirement, and hence can be used therapeutically against several genetic diseases ( 47 ).
Alternatively, RNA-targeting Cas9 variants have been developed which also broaden the gene targeting spectrum by mitigating PAM requirement restrictions. S. pyogenese Cas9 (SpyCas9) can be manipulated to target RNA by providing a short oligonucleotide with a PAM sequence, known as a PAMmer ( 64 , 65 ), and thus eliminates the need for a PAM site within the target region. Other subsets of Cas enzymes have also been discovered that naturally target RNA independent of a PAM, such as Cas13d. Upon further engineering of this effector, CasRx was developed for efficient RNA-guided RNA targeting in human cells ( 66 , 67 ). Although RNA-targeting CRISPR advances provide a therapeutic opportunity without the risk of DNA-damage toxicity, they exclude the potential for editing a permanent correction into the genome.
DNA-Damage Toxicity
CRISPR-induced DSBs often trigger apoptosis rather than the intended gene edit ( 68 ). Further safety concerns were revealed when using this tool in human pluripotent stem cells (hPSCs) which demonstrated that p53 activation in response to the toxic DSBs introduced by CRISPR often triggers subsequent apoptosis ( 69 ). Thus, successful CRISPR edits are more likely to occur in p53 suppressed cells, resulting in a bias toward selection for oncogenic cell survival ( 70 ). In addition, large deletions spanning kilobases and complex rearrangements as unintended consequences of on-target activity have been reported in several instances ( 71 , 72 ), highlighting a major safety issue for clinical applications of DSB-inducing CRISPR therapy. Other variations of Cas9, such as catalytically inactive endonuclease dead Cas9 (dCas9) in which the nuclease domains are deactivated, may provide therapeutic utility while mitigating the risks of DSBs ( 73 ). dCas9 can transiently manipulate expression of specific genes without introducing DSBs through fusion of transcriptional activating or repressing domains or proteins to the DNA-binding effector ( 74 ). Other variants such as Cas9n can also be considered, which induces SSBs rather than DSBs. Further modifications of these Cas9 variants has led to the development of base editors and prime editors, a key innovation for safe therapeutic application of CRISPR technology (see Precision Gene Editing With CRISPR section).
Immunotoxicity
In addition to technical limitations, CRISPR/Cas9, like traditional gene therapy, still raises concerns for immunogenic toxicity. Charlesworth et al. showed that more than half of the human subjects in their study possessed preexisting anti-Cas9 antibodies against the most commonly used bacterial orthologs, SaCas9 and SpCas9 ( 75 ). Furthermore, AAV vectors are also widely used to deliver CRISPR components for gene therapy. To this end, several Cas9 orthologs and AAV serotypes were tested based on sequence similarities and predicted binding strength to MHC class I and class II to screen for immune orthologs that can be used for safe repeated administration of AAV-CRISPR gene therapy. Although no two AAV serotypes were found to completely circumvent immune recognition, the study verified 3 Cas9 orthologs [SpCas9, SaCas9, and Campylobacter jejuni Cas9 (CjCas9)] which showed robust editing efficiency and tolerated repeated administration due to reduced immunogenic toxicity in mice immunized against AAV and Cas9 ( 76 ). A major caveat is pre-existing immunity in humans against 2 of these orthologs—SpCas9 and SaCas9, leaving CjCas9 as the only current option for this cohort of patients. However, this ortholog has not been well-studied in comparison to the other 2 orthologs and will need further investigation to provide evidence for its safety and efficacy for clinical use. Future studies may also identify other Cas9 immune-orthogonal orthologs for safe repeated gene therapy.
Precision Gene Editing With CRISPR
Precise-genome editing is essential for prospects of CRISPR gene therapy. Although HDR pathways can facilitate a desired edit, its low efficiency renders its utility for precise gene editing for clinical intervention highly limiting, with NHEJ as the default pathway human cells take for repair. Enhancement of HDR efficiency has been achieved via suppression of the NHEJ pathway through chemical inhibition of key NHEJ modulating enzymes such as Ku ( 77 ), DNA ligase IV ( 78 ), and DNA-dependent protein kinases (DNA-PKcs) ( 79 ). Other strategies that improve HDR efficiency include using single-stranded oligodeoxynucleotide (ssODN) template, which contains the homology arms to facilitate recombination and the desired edit sequence, instead of double-stranded DNA (dsDNA). Rationally designed ssODN templates with optimized length complementarity have been shown to increase HDR rates up to 60% in human cells for single nucleotide substitution ( 80 ). Furthermore, cell cycle stage plays a key role in determining the DNA-damage repair pathway a cell may take. HDR events are generally restricted to late S and G2 phases of the cell cycle, given the availability of the sister chromatid to serve as a template at these stages, whereas NHEJ predominates the G1, S, and G2 phases ( 81 ). Pharmacological arrest at the S phase with aphidicolin increased HDR frequency in HEK293T with Cas9-guide ribonucleoprotein (RNP) delivery. Interestingly, cell arrest in the M phase using nocodazole with low concentrations of the Cas9-guide RNP complex yielded higher frequencies of HDR events in these cells, reaching a maximum frequency of up to 31% ( 82 ). Although HDR is considered to be restricted to mitotic cells, a recent study revealed that the CRISPR/Cas9 editing can achieve HDR in mature postmitotic neurons. Nishiyama et al. successfully edited the CaMKIIα locus through HDR in postmitotic hippocampal neurons of adult mice in vitro using an AAV delivered Cas9, guide RNA, and donor template in the CaMKIIα locus, which achieved successful HDR-mediated edits in ~30% of infected cells. Although HDR efficiency was dose-dependent on AAV delivered HDR machinery and off-target activity was not monitored, this study demonstrated CRISPR's potential utility for translational neuroscience after further developments ( 83 ). To further exploit cell-cycle stage control as a means to favor templated repair, Cas9 conjugation to a part of Geminin, a substrate for G1 proteosome degradation, can limit Cas9 expression to S, G2, and M stages. This strategy was shown to facilitate HDR events while mitigating undesired NHEJ edits in human immortalized and stem cells ( 84 , 85 ). A more recent strategy combined a chemically modified Cas9 to the ssODN donor or a DNA adaptor that recruits the donor template, either of which improved HDR efficiency by localizing the donor template near the cleavage site ( 86 ). Despite these advancements, HDR is still achieved at a relatively low efficiency in eukaryotic cells and use of relatively harmful agents in cells such as NHEJ chemical inhibitors may not be ideal in a clinical setting.
A recent advancement that allows precision gene editing independent of exploiting DNA damage response mechanisms is the CRISPR base editing (BE) system. In this system, a catalytically inactive dead Cas9 (dCas9) is conjugated to deaminase, which can catalyze the conversion of nucleotides via deamination. For increased editing efficiency, Cas9 nickase (Cas9n) fused with deaminase is recently being more utilized over dCas9 for base editing, as the nicks created in a single strand of DNA induce higher editing efficiency. Currently, the two types of CRISPR base editors are cytidine base editors (CBEs) and adenosine base editors (ABEs). CBEs catalyze the conversion of cytidine to uridine, which becomes thymine after DNA replication. ABEs catalyze the conversion of adenosine to inosine which becomes guanine after DNA replication ( 87 ). Base editors provide a means to edit single nucleotides without running the risk of causing DSB-induced toxicity. However, base editors are limited to “A to T” and “C to G” conversions, narrowing its scope for single-base gene edition to only these bases. In addition, base editors still face some of the same challenges as the previously described CRISPR systems, including OTEs, more so with CBEs than ABEs ( 88 , 89 ) and packaging constraints, namely in AAV vectors due to the large size of base editors ( 90 ). Furthermore, the editing window for base editors are limited to a narrow range of a few bases upstream of the PAM ( 90 ). More recently, prime editing has been developed as a strategy to edit the genome to insert a desired stretch of edits without inducing DSBs ( 91 ). This technology combines fusion of Cas9n with a reverse transcriptase and a prime editing guide RNA (pegRNA), which contains sgRNA sequence, primer binding site (PBS), and an RNA template encoding the desired edit on the 3′ end. Prime editors use Cas9n to nick one strand of the DNA and insert the desired edit via reverse transcription of the RNA template. The synthesized edit is incorporated into the genome and the unedited strand is cleaved and repaired to match the inserted edit. With an optimized delivery system in place, base editors and primer editors can open the door for precision gene editing to correct and potentially cure a multitude of genetic diseases ( Figure 3 ).

Precise Gene Editing. (A) CRISPR/Cas9-HDR. Cas9 induces a DSB. The exogenous ssODN carrying the sequence for the desired edit and homology arms is used as a template for HDR-mediated gene modification. (B) Base Editor. dCas9 or Cas9n is tethered to the catalytic portion of a deaminase. Cytosine deaminase catalyzes the formation of uridine from cytosine. DNA mismatch repair mechanisms or DNA replication yield an C:G to T:A single nucleotide base edit. Adenosine deaminase catalyzes the formation of inosine from adenosine. DNA mismatch repair mechanisms or DNA replication yield an A:T to G:C single nucleotide base edit. (C) Prime Editor. Cas9n is tethered to the catalytic portion of reverse transcriptase. The prime editor system uses pegRNA, which contains the guide spacer sequence, reverse transcriptase primer, which includes the sequence for the desired edit and a primer binding site (PBS). PBS hybridizes with the complementary region of the DNA and reverse transcriptase transcribes new DNA carrying the desired edit. After cleavage of the resultant 5′ flap and ligation, DNA repair mechanisms correct the unedited strand to match the edited strand. HDR, homology directed repair. DSB, double stranded break; SSB, single-stranded break; ssODN, single-stranded oligodeoxynucleotide.
Delivery of CRISPR Gene Therapy
The delivery modality of CRISPR tools greatly influences its safety and therapeutic efficacy. While traditional gene therapy utilizing viruses have been scrutinized for the risk of immunotoxicity and insertional oncogenesis, AAV vectors remain a key delivery vehicle for CRISPR gene therapy and continues to be extensively used for its high efficiency of delivery ( 92 ). The CRISPR toolkit can be packaged as plasmid DNA encoding its components, including Cas9 and gRNA, or can be delivered as mRNA of Cas9 and gRNA. Nucleic acids of CRISPR can be packaged in AAV vectors for delivery or introduced to target cells via electroporation/nucleofection or microinjection, with the latter methods averting virus-associated risks. However, microinjection can be technically challenging and is only suited for ex vivo delivery. Electroporation is also largely used for ex vivo but can be used in vivo for certain target tissues ( 93 ). However, high-voltage shock needed to permeabilize cell membranes via electroporation can be toxic and can lead to permanent permeabilization of treated cells ( 94 ). In addition to viral toxicity, AAV delivery of CRISPR components yields longevity of expression, leading to greater incidence of OTEs. Alternatively, delivery of the Cas9 protein and gRNA as RNP complexes has reduced OTEs while maintained editing efficacy, owing to its transient expression and rapid clearance in the cell ( 95 ).
Once the delivery modality is selected, CRISPR/Cas9 edits can be facilitated either ex vivo where cells are genetically modified outside of the patient and reintroduced back, or in vivo with delivery of the CRISPR components directly into the patient where cells are edited ( Figure 4 ). Both systems pose their own set of advantages and challenges. Advantages for ex vivo delivery include greater safety since patients are not exposed to the gene altering tool, technical feasibility, and tighter quality control of the edited cells. However, challenges to this method include survival and retention of in vivo function of cells outside the patient after genetic manipulation and extensive culture in vitro . Also, an adequate supply of cells is needed for efficient re-engraftment. These conditions limit this method to certain cell types that can survive and be expanded in culture, such as hematopoietic stem and progenitor cells (HSPCs) ( 96 ) and T cells ( 97 ).

Delivery of CRISPR Therapy. Nucleic acids encoding CRISPR/Cas9 or its RNP complex can be packaged into delivery vehicles. Once packaged, edits can be facilitated either ex vivo or in vivo . Ex vivo editing involves extraction of target cells from the patient, cell culture, and expansion in vitro , delivery of the CRISPR components to yield the desired edits, selection, and expansion of edited cells, and finally reintroduction of therapeutic edited cells into the patient. In vivo editing can be systemically delivered via intravenous infusions to the patient, where the CRISPR cargo travels through the bloodstream via arteries leading to the target tissue, or locally delivered with injections directly to target tissue. Once delivered, the edits are facilitated in vivo to provide therapeutic benefit.
While ex vivo gene therapy has provided therapeutic benefit for hematological disorders and cancer immunotherapy, many tissue types are not suited for this method, severely limiting its therapeutic utility for other genetic diseases. in vivo manipulation is thus needed to expand CRISPR's utility to treat a broader range of genetic diseases, such as Duchenne muscular dystrophy (DMD) ( 98 ) and hereditary tyrosinemia ( 99 ). CRISPR components can be delivered in vivo systemically through intravenous injections or can be locally injected to specific tissues ( Figure 4 ). With systemic delivery, the CRISPR components and its vehicle are introduced into the circulatory system where expression of the gene editing toolkit can be controlled to target specific organs via tissue-specific promoters ( 100 ). However, challenges of in vivo delivery include degradation by circulating proteases or nucleases, opsonization by opsonins, or clearance by the mononuclear phagocyte system (MPS). Furthermore, the cargo must reach the target tissue and bypass the vascular endothelium, which are often tightly connected by cell-cell junctions ( 101 ), preventing accessibility to larger delivery vehicles (>1 nm diameter). Additionally, once the cargo has reached the target cells, they must be internalized, which is generally facilitated through endocytosis where they can be transported and degraded by lysosomal enzymes ( 102 ). In addition, localization of the editing machinery near the point of injection can result in uneven distribution of the edited cell repertoire within the tissue, which may result in suboptimal therapeutic outcomes ( 102 ). While advancements are continuing to refine delivery techniques, the current systems have allowed CRISPR gene therapy to be used in the clinic.
Biological Intervention of CRISPR/Cas9 in Clinical Trials
Cancer immunotherapy.
The first CRISPR Phase 1 clinical trial in the US opened in 2018 with the intent to use CRISPR/Cas9 to edit autologous T cells for cancer immunotherapy against several cancers with relapsed tumors and no further curative treatment options. These include multiple myeloma, melanoma, synovial sarcoma and myxoid/round cell liposarcoma. This trial was approved by the United States Food and Drug Administration (FDA) after careful consideration of the risk to benefit ratios of this first application of CRISPR gene therapy into the clinic. During this trial, T lymphocytes were collected from the patients' blood and ex vivo engineered with CRISPR/Cas9 to knockout the α and β chains of the endogenous T cell receptor (TCR), which recognizes a specific antigen to mediate an immune response, and the programmed cell death-1 (PD-1) protein, which attenuates immune response. The cells were then transduced with lentivirus to deliver a gene encoding a TCR specific for a NY-ESO-1 antigen, which has been shown to be highly upregulated in the relapsed tumors and thus can serve as a therapeutic target. Since then, many trials have opened for CRISPR-mediated cancer immunotherapy and is currently the most employed strategy for CRISPR gene therapy ( Table 2 ). A trial implementing this strategy using other tools had already been conducted in both pre-clinical and clinical settings, but this was the first time CRISPR/Cas9 was used to generate the genetically modified T cells ( 97 ). The moderate transition of switching only the tool used for an already approved therapeutic strategy may have been key to paving the road for using CRISPR's novel abilities for gene manipulation, such as targeted gene disruption.
Biological intervention of CRISPR gene therapy in clinical trials.
Gene Disruption
The first clinical trial in the US using CRISPR to catalyze gene disruption for therapeutic benefit were for patients with sickle-cell anemia (SCD) and later β-thalassemia, by Vertex Pharmaceuticals and CRISPR Therapeutics. This therapy, named CTX001, increases fetal hemoglobin (HbF) levels, which can occupy one or two of four hemoglobin binding pockets on erythrocytes and thereby provides clinical benefit for major β-hemoglobin diseases such as SCD and β-thalassemia ( 103 ). The trial involved collecting autologous hematopoietic stem and progenitor cells from peripheral blood and using CRISPR/Cas9 to disrupt the intronic erythroid-specific enhancer for the BCL11A gene ( {"type":"clinical-trial","attrs":{"text":"NCT03745287","term_id":"NCT03745287"}} NCT03745287 ) as disruption of this gene increases HbF expression ( 104 – 106 ). Genetically modified hematopoietic stem cells with BCL11A disruption are delivered by IV infusion after myeloablative conditioning with busulfan to destroy unedited hematopoietic stem cells in the bone marrow. Preliminary findings from two patients receiving this treatment seem promising. One SCD patient was reported to have 46.6% HbF and 94.7% erythrocytes expressing HbF after 4 months of CTX001 transfusions and one β-thalassemia patient is expressing 10.1 g/dL HbF out of 11.9 g/dL total hemoglobin, and 99.8% erythrocytes expressing HbF after 9 months of the therapy. Results from the clinical trial that has opened for this therapy ( {"type":"clinical-trial","attrs":{"text":"NCT04208529","term_id":"NCT04208529"}} NCT04208529 ) to assess the long-term risks and benefits of CTX001 will dictate whether this approach can provide a novel therapeutic opportunity for a disease that otherwise has limited treatment options.
In vivo CRISPR Gene Therapy
While the aforementioned trials rely on ex vivo editing and subsequent therapy with modified cells, in vivo approaches have been less extensively employed. An exciting step forward with CRISPR gene therapy has been recently launched with a clinical trial using in vivo delivery of CRISPR/Cas9 for the first time in patients. While in vivo editing has been largely limited by inadequate accessibility to the target tissue, a few organs, such as the eye, are accessible. Leber congenital amaurosis (LCA) is a debilitating monogenic disease that results in childhood blindness caused by a bi-allelic loss-of-function mutation in the CEP290 gene, with no treatment options. This therapy, named EDIT-101, delivers CRISPR/Cas9 directly into the retina of LCA patients specifically with the intronic IVS26 mutation, which drives aberrant splicing resulting in a non-functional protein. The therapy uses an AAV5 vector to deliver nucleic acid instructions for Staphylococcus aureus Cas9 and two guides targeting the ends of the CEP290 locus containing the IVS26 mutation. The DSB induced by Cas9 and both guides result in either a deletion or inversion of the IVS26 intronic region, thus preventing the aberrant splicing caused by the genetic mutation and enabling subsequent translation of the functional protein ( 107 ). Potential immunotoxicity or OTEs arising from nucleic acid viral delivery will have to be closely monitored. Nonetheless, a possibly curative medicine for genetic blindness using an in vivo approach marks an important advancement for CRISPR gene therapy.
CRISPR Editing in Human Embryos and Ethical Considerations
While somatic editing for CRISPR therapy has been permitted after careful consideration, human germline editing for therapeutic intent remains highly controversial. With somatic edition, any potential risk would be contained within the individual after informed consent to partake in the therapy. Embryonic editing not only removes autonomy in the decision-making process of the later born individuals, but also allows unforeseen and permanent side effects to pass down through generations. This very power warrants proceeding with caution to prevent major setbacks as witnessed by traditional gene therapy. However, a controversial CRISPR trial in human embryos led by Jiankui He may have already breached the ethical standards set in place for such trials. This pilot study involved genetic engineering of the C-C chemokine receptor type 5 ( CCR5 ) gene in human embryos, with the intention of conferring HIV-resistance, as seen by a naturally occurring CCR5 Δ 32 mutation in a few individuals ( 108 ). However, based on the limited evidence, CRISPR/Cas9 was likely used to target this gene, but rather than replicate the naturally observed and beneficial 32-base deletion, the edits merely induced DSBs at one end of the deletion, allowing NHEJ to repair the damaged DNA while introducing random, uncharacterized mutations. Thus, it is unknown whether the resultant protein will function similarly to the naturally occurring CCR5 Δ 32 protein and confer HIV resistance. In addition, only one of the two embryos, termed with the pseudonym Nana, had successful edits in both copies of the CCR5 gene, whereas the other embryo, with pseudonym Lulu, had successful editing in only one copy. Despite these findings, both embryos were implanted back into their mother, knowing that the HIV-resistance will be questionable in Nana and non-existent in Lulu ( 109 , 110 ).
Furthermore, recent studies have shown that the mechanism for infection of some variants of the highly mutable HIV virus may heavily rely on the C-X-C chemokine receptor type 4 ( CXCR4 ) co-receptor ( 108 , 111 ). With no attempts at editing CXCR4 , this adds yet another layer of skepticism toward achieving HIV resistance by this strategy. In addition, OTEs, particularly over the lifetime of an individual, remain a major concern for applying this technology in humans. The recent advances in the editing tool to limit OTEs, such as using high fidelity Cas9 variants, has not been exploited. Furthermore, the rationale for selecting HIV prevention for the first use of CRISPR in implanted human embryos contributes to the poor risk to benefit ratio of this study, considering HIV patients can live long, healthy lives on a drug regimen. A more appropriate first attempt would have been to employ this technology for a more severe disease. For example, correction of the MYBPC3 gene is arguably a better target for embryonic gene editing, as mutations in MYBPC3 can cause hypertrophic cardiomyopathy (HCM), a heart condition responsible for most sudden cardiac deaths in people under the age of 30. Gene correction for this pathological mutation was achieved recently for the first time in the US in viable human embryos using the HDR-mediated CRISPR/Cas9 system. However, these embryos were edited for basic research purposes and not intended for implantation. In this study, sperm carrying the pathogenic MYBPC3 mutation and the CRISPR/Cas9 machinery as an RNP complex were microinjected into healthy donor oocytes arrested at MII, achieving 72.4% homozygous wildtype embryos as opposed to 47.4% in untreated embryos. The HDR-mediated gene correction was observed at considerably high frequencies with no detectable OTEs in selected blastomeres, likely owing to the direct microinjection delivery of the RNP complex in the early zygote. Interestingly, the maternal wildtype DNA was used preferentially for templated repair over the provided exogenous ssODN template ( 112 ). While evidence for gene correction was promising, NHEJ mediated DNA repair was still observed in many embryos, highlighting the need to improve HDR efficiency before clinical application can be considered. Although strategies have been developed to improve HDR, such as chemical inhibitors of NHEJ ( 77 – 79 ), such techniques may have varying outcomes in embryonic cells and side effects that may arise from treatment needs to be investigated. Germline gene editing will remain to be ethically unfavorable at its current state and its discussions may not be considered until sufficient long-term studies of the ongoing somatic CRISPR therapy clinical trials are evaluated.
Potential for CRISPR Therapeutics During COVID-19 Pandemic
The rapidly advancing CRISPR technology may provide aid during our rapidly evolving times. The recent outbreak of a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to a global pandemic ( 113 ). These pressing times call for an urgent response to develop quick and efficient testing tools and treatment options for coronavirus disease 2019 (COVID-19) patients. Currently available methods for testing are relatively time consuming with suboptimal accuracy and sensitivity ( 114 ). The two predominant testing methods are molecular testing or serological testing. The US Centers for Disease Control and Prevention (CDC) has developed a real-time RT-PCR assay for molecular testing for the presence of viral RNA to detect COVID-19 ( 115 ). However, this assay has a roughly ~30% false negative rate ( 116 , 117 ) with the turnaround time of several hours to >24 h. Serological testing methods are much more rapid but lack the ability to detect acute respiratory infection since antibodies used to detect infection can take several days or weeks to develop.
Recently, a CRISPR Cas12-based assay named SARS-CoV-2 DETECTR has been developed for detection of COVID-19 with a short turnaround time of about 40 min and a 95% reported accuracy. The assay involves RNA extraction followed by reverse transcription and simultaneous isothermal amplification using the RT-LAMP method. Cas12 and a guide RNA against regions of the N (nucleoprotein) gene and E (envelope) gene of SARS-CoV-2 are then targeted, which can be visualized by cleavage of a fluorescent reporter molecule. The assay also includes a laminar flow strip for a visual readout, where a single band close to where the sample was applied indicates a negative test and 2 higher bands or a single higher band would indicate cleavage of the fluorescent probe and hence positive for SARS-CoV-2 ( 118 ).
In addition to CRISPR's diagnostic utility, CRISPR may provide therapeutic options for COVID-19 patients. The recently discovered Cas13 is an RNA-guided RNA-targeting endonuclease may serve as a potential therapeutic tool against COVID-19. PAC-MAN (Prophylactic Antiviral CRISPR in huMAN cells) has been developed, which utilizes the Ruminococcus flavefaciens derived VI-D CRISPR-Cas13d variant, selected for its small size facilitating easier packaging in viral vehicles, high specificity, and strong catalytic activity in human cells. This technique was developed to simultaneously target multiple regions for RNA degradation, opening the door for a much-needed pan-coronavirus targeting strategy, given the evidence suggesting relatively high mutation and recombination rates of SARS-CoV-2 ( 119 ). With these advances, the CRISPR/Cas machinery may again be implemented to serve its original purpose as a virus-battling system to provide aid during this pandemic.
The birth of gene therapy as a therapeutic avenue began with the repurposing of viruses for transgene delivery to patients with genetic diseases. Gene therapy enjoyed an initial phase of excitement, until the recognition of immediate and delayed adverse effects resulted in death and caused a major setback. More recently, the discovery and development of CRISPR/Cas9 has re-opened a door for gene therapy and changed the way scientists can approach a genetic aberration—by fixing a non-functional gene rather than replacing it entirely, or by disrupting an aberrant pathogenic gene. CRISPR/Cas9 provides extensive opportunities for programmable gene editing and can become a powerful asset for modern medicine. However, lessons learned from traditional gene therapy should prompt greater caution in moving forward with CRISPR systems to avoid adverse events and setbacks to the development of what may be a unique clinically beneficial technology. A failure to take these lessons into account may provoke further backlash against CRISPR/Cas9 development and slow down progression toward attaining potentially curative gene editing technologies.
Although CRISPR editing in humans remains a highly debated and controversial topic, a few Regulatory Affairs Certification (RAC)-reviewed and FDA-approved CRISPR gene therapy trials have opened after thorough consideration of the risk to benefit ratios. These first few approved trials, currently in Phase I/II, are only for patients with severe diseases, such as cancers or debilitating monogenic diseases. The outcomes of these trials will dictate how rapidly we consider using this system to treat less severe diseases, as the risks of the technology are better understood. A concern remains whether normalizing CRISPR/Cas9 editing for less debilitating diseases may act as a gateway for human genome editing for non-medical purposes, such as altering genes in embryos to create offspring with certain aesthetic traits. This fear of unnatural selection for unethical reasons has likely become more tangible in the public's view with the strong media attention of the edited “CRISPR babies.” The lasting effects of that trial and outcomes of the approved clinical trials will greatly influence CRISPR's future in gene therapy and begin to answer the key questions we must consider as we further explore this technology. These key questions include how to avoid the mistakes of the past, who should decide CRISPR's therapeutic future, and how the ethical boundaries of its applications should best be drawn.
Author Contributions
FU researched and drafted the article. TS and CR supervised the content. All authors wrote, reviewed, and edited the manuscript before submission.
Conflict of Interest
CR has consulted regarding oncology drug development with AbbVie, Amgen, Ascentage, Astra Zeneca, Celgene, Daiichi Sankyo, Genentech/Roche, Ipsen, Loxo, and Pharmar, and is on the scientific advisory boards of Harpoon Therapeutics and Bridge Medicines. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Ms. Emily Costa, Dr. Alvaro Quintanal Villalonga, and Dr. Rebecca Caesar for their excellent assistance with editing the review.
Funding. This work was supported by grants from the US National Institutes of Health, including U24CA213274 and R01CA197936 (CR); Parker Institute of Cancer Immunotherapy grant (TS).

CRISPR HIV Gene Therapy Disappoints in Early Study
© 2024 Smart + Strong . All Rights Reserved. Terms of use and Your privacy . Smart + Strong ® is a registered trademark of CDM Publishing, LLC.
EBT-101 recipients who stopped antiretrovirals experienced HIV viral rebound, but similar therapies may hold more promise for herpes and hepatitis B.
May 13, 2024 • By Liz Highleyman
EBT-101, a CRISPR-based gene-editing therapy from Excision BioTherapeutics, was safe and well tolerated in a Phase I/II study, but it did not prevent viral rebound in the first three participants who stopped antiretroviral treatment, according to a presentation last week at the American Society of Gene & Cell Therapy (ASGCT) annual meeting .
Excision put a positive spin on the findings, noting that favorable safety data is a necessary step on the path to developing therapies for latent viral infections. The company also touted promising early results from studies of CRISPR-based therapies for herpes simplex virus and hepatitis B. But the HIV rebound news is disappointing, and it underscores the importance of remaining wary of exaggerated claims from industry and the mainstream press about the state of HIV cure research.
“Initial data from the EBT-101-001 trial provides important clinical evidence that a gene editing treatment modality can be safely delivered for targeting the HIV DNA reservoirs in human cells,” study investigator Rachel Presti, MD, PhD, of Washington University St. Louis School of Medicine, said in a news release . “This study provides researchers with invaluable insights for how CRISPR technology can be applied for addressing infectious disease and was an important first step towards additional programs designed to optimize this treatment modality for treating the millions of individuals who are impacted by HIV and other infectious disease.”
Antiretroviral therapy can keep HIV replication suppressed indefinitely, but the virus inserts its genetic blueprints into the DNA of human cells and establishes a long-lasting reservoir that the drugs can’t reach. This integrated HIV DNA lies dormant in resting T cells during treatment, but it can start churning out new virus when antiretrovirals are stopped, making a cure nearly impossible. The only way to tell whether an intervention leads to long-term remission is to discontinue antiretroviral therapy with careful monitoring, known as an analytic treatment interruption.
Kamel Khalili, PhD, of Temple University, and colleagues have been studying gene therapy to cure HIV for more than a decade. Their work employs CRISPR-Cas9, a technology that combines guide RNAs that home in on specific segments of DNA and a nuclease enzyme that cuts the genetic material at the desired site. In 2014, they reported that a CRISPR-Cas9 tool could cut out a segment of integrated HIV DNA necessary for viral replication in a laboratory study. Another study published in 2019 showed that this approach could remove integrated HIV genes and clear latent viral reservoirs in mice.
This led to the development of EBT-101, a CRISPR-based therapy delivered by an adeno-associated virus that uses dual guide RNAs to target three sites on the integrated HIV genome. Making cuts at these locations prevents the production of intact virus. Last August, researchers reported that a single dose of a simian version of the therapy safely and effectively removed an HIV-like virus from viral reservoirs in monkeys on antiretroviral therapy, but this study did not include a treatment interruption.
The first human clinical trial of EBT-101 ( NCT05144386 ) started in 2022 , enrolling people on antiretroviral therapy with a stable undetectable viral load. The study protocol called for participants who maintained viral suppression at 12 weeks after receiving the gene therapy to undergo an analytic treatment interruption.
At the European Society of Gene & Cell Therapy annual meeting last October, Presti reported that EBT-101 was detectable in the blood of the first three treated participants after a single IV infusion at the initial dose level. EBT-101 was well tolerated with only mild temporary side effects. She did not present treatment interruption outcomes , but that didn’t stop the Daily Mail from proclaiming that a cure for HIV “ could be months away .”
Presti gave an update last week, and the news generally wasn’t good. Of the five participants who have so far received the initial dose of EBT-101, three stopped antiretroviral therapy. Unfortunately, all three experienced viral rebound and had to restart their antiretrovirals. This likely occurred because the gene therapy did not reach all cells harboring latent HIV, and even a very small number of cells containing residual HIV DNA is enough to reignite viral replication.
But the news was not all bad. One EBT-101 recipient was able to maintain viral suppression for four months after treatment discontinuation—considerably longer than it typically takes for the virus to rebound after stopping antiretrovirals. This suggests that EBT-101 or similar CRISPR therapies might play a role in a combination functional cure strategy.
“We know that many people were hopeful that a first trial could provide evidence of a possible cure for HIV because the field has been waiting over 20 years for a cure,” Excision senior vice president William Kennedy, MD, said in a news release . “However, it was essential that this clinical trial establish safety for EBT-101 as a gene therapy product as well as safety related to the use of CRISPR for the field.”
Excision is now testing a higher dose of EBT-101 in a second cohort and is exploring new CRISPR delivery methods that might be more efficient than the adeno-associated virus vector. One possibility is lipid nanoparticles like the ones used to deliver messenger RNA (mRNA) in COVID-19 vaccines.
The company is also exploring CRISPR-based approaches for other latent viral infections. Herpes simplex virus (HSV) persists in nerve cells, and it can reactivate to cause cold sores, genital herpes or eye inflammation (keratitis). Hepatitis B virus (HBV) establishes chronic infection in the liver, where it can potentially lead to cirrhosis and liver cancer. Unlike HIV and other retroviruses, however, HSV and HBV do not integrate their genetic blueprints into the chromosomes of human cells, so they may be easier to remove. In other presentations at the ASGCT meeting, researchers reported preclinical results for another experimental CRISPR therapy dubbed EBT-104, showing that a single dose reduced HSV DNA by more than 99% in laboratory cell cultures. What’s more, it eliminated viral shedding in 11 of 12 rabbits with herpes keratitis, according to the news release.
In other preclinical research, a single dose of EBT-107—a CRISPR compound delivered by lipid nanoparticles—reduced HBV DNA, hepatitis B surface antigen and hepatitis B e antigen by 98%, 97% and 92%, respectively, in a mouse model of hepatitis B. Unlike CRISPR delivered by viral vectors, EBT-107 in nanoparticles and could potentially be given as multiple doses to reach more latent virus.
Click here for more news about HIV cure research .
Read More About:
- #functional cure
- #gene therapy
- #hepatitis B
- #viral persistence
- #viral rebound
RELATED articles

A Week of Action Against Homophobia and Transphobia 2024

Many Older People With HIV Have Unmet Needs

My Secret Life No More

HIV as the Common Denominator
Stay logged in.
You have been inactive for 60 minutes and will be logged out in . Any updates not saved will be lost.
You Have Been Logged Out
Click here to log back in.
POZ uses cookies to provide necessary website functionality, improve your experience, analyze our traffic and personalize ads. Our Privacy Policy
POZ uses cookies to provide necessary website functionality, improve your experience, analyze our traffic and personalize ads. By remaining on our website, you indicate your consent to our Privacy Policy and our Cookie Usage .

IMAGES
VIDEO
COMMENTS
Previous research on CRISPR/Cas9 has focused on prokaryotic cells, and CRISPR technology started to be used in medicine, agriculture, and other fields in a paper published by Zhang Feng et al. in ...
A HISTORY OF THE DISCOVERY OF THE MAIN COMPONENTS OF THE CRISPR-Cas9 SYSTEM. CRISPR - clustered regularly interspaced short palindromic repeats - were first discovered in the sequences of DNA from Escherichia coli bacteria and described in 1987 by Ishino et al. [] from Osaka University (Japan).At that time sequencing of these difficult-to-study DNA fragments took several months, but ...
The mechanism of CRISPR/Cas-9 genome editing can be generally divided into three steps: recognition, cleavage, and repair. 13 The designed sgRNA directs Cas-9 and recognizes the target sequence in the gene of interest through its 5ʹcrRNA complementary base pair component. The Cas-9 protein remains inactive in the absence of sgRNA.
Introduction. Clustered regularly interspaced palindromic repeats (CRISPR)/Cas9 is a gene-editing technology causing a major upheaval in biomedical research. It makes it possible to correct errors in the genome and turn on or off genes in cells and organisms quickly, cheaply and with relative ease. It has a number of laboratory applications ...
In the laboratory, the CRISPR tool consists of two main actors: a guide RNA and a DNA-cutting enzyme, most commonly one called Cas9. Scientists design the guide RNA to mirror the DNA of the gene to be edited (called the target). The guide RNA partners with Cas and—true to its name—leads Cas to the target.
CRISPR-Cas9 applications in plants and fungi also promise to change the pace and course of agricultural research. Future research directions to improve the technology will include engineering or identifying smaller Cas9 variants with distinct specificity that may be more amenable to delivery in human cells.
The historical foundation of CRISPR-Cas9 research can be traced to its identification as an adaptive immune defense mechanism in bacterial cells, serving as a robust defense against foreign DNA. Scientists have adeptly utilized CRISPR-Cas9 for precise genome editing across diverse organisms, resulting in substantial advancements and a refined ...
A decade of CRISPR. In the decade since the publication of CRISPR-Cas9 as a genome-editing technology, the CRISPR toolbox and its applications have profoundly changed basic and applied biological research. Wang and Doudna now review the origins and utility of CRISPR-based genome editing, the successes and current limitations of the technology ...
The Zhang lab has trained thousands of researchers in the use of CRISPR-Cas9 genome editing technology through direct education and by sharing more than 40,000 CRISPR-Cas9 components with academic laboratories around the world to help accelerate global research that will benefit human health.
Jiang et al. induced site-specific chromosome translocation in mouse embryonic stem cells by CRISPR-Cas9, in order to establish a cell and animal model for subsequent research on congenital genetic diseases, infertility, and cancer related to chromosomal translocation .
Presently, the combined application of the CRISPR/Cas9 technology and human-induced pluripotent stem cells (hiPSCs) can accurately and rapidly establish various in vitro cell models for targeted gene modification. Edited cell lines can not only be used in disease research, but also to gradually replace diseased structures by inducing their differentiation in target tissues or organs.
Like Cas9 and Cas12, CRISPR/Cas13 is also a robust, precise, and versatile RNA-targeting system, which opens novel research horizons in diverse fields. Compared with earlier RNA manipulation systems, CRISPR/Cas13 offers numerous advantages.
CRISPR-Cas9. CRISPR gene editing (pronounced / ˈ k r ɪ s p ə r / "crisper") standing for "Clustered Regularly Interspaced Short Palindromic Repeats" is a genetic engineering technique in molecular biology by which the genomes of living organisms may be modified. It is based on a simplified version of the bacterial CRISPR-Cas9 antiviral defense system. By delivering the Cas9 nuclease ...
As a new gene intervention method, CRISPR/Cas9 has been widely used in the clinical treatment of tumours and rare diseases; however, its application in the field of cardiovascular diseases is currently limited. This article reviews the application of the CRISPR/Cas9 editing technology in cardiovascular disease research and treatment, and ...
"This research demonstrates that CRISPR gene therapy for inherited vision loss is worth continued pursuit in research and clinical trials," said Pierce, director of Ocular Genomics Institute and Berman-Gund Laboratory for the Study of Retinal Degenerations at Mass Eye and Ear and Harvard Medical School. ... CRISPR-Cas9 is a gene editing ...
Figure 1. Components of a CRISPR-Cas9 system for directing Cas9 endonuclease to genomic targets. The crRNA:tracrRNA complex uses crRNA and tracrRNA sequences that hybridize and then form a complex with Cas9 endonuclease to guide targeted cleavage of genomic DNA. The cleavage site is specified by the spacer element of the crRNA (light blue bar).
Thereafter, CRISPR research continued to bloom year after year in thousands of laboratories across the world; the success stories of this novel technology are schematically depicted in few articles. ... The CRISPR-Cas9 system is utilized in the type II system and provides its function via a single effector complex comprising a Cas9 protein ...
Scientists have said they used a human gene editing tool, CRISPR-Cas9, to restore vision in people with a rare form of inherited or congenital blindness.. The researchers said 11 out of the 14 ...
Global Change Research Institute of the Czech Academy of Sciences, Brno, Czech Republic. These authors share first authorship. Search for more papers by this author. ... Thirdly, we generated CRISPR-Cas9 knockout mutants (KO) of the key CK biosynthetic genes coding for LOG (PH145 and PH147) and IPT (PH219, PH220, PH221, and PH222). Our findings ...
These enzymes, which include Cas12a and Cas9, are guided to their DNA targets by CRISPR RNA. Cas12a exhibits superior accuracy, displaying fewer off-target effects compared to Cas9, as well as the ability to edit multiple genome locations simultaneously.
Although Cas9 has already been widely used as a research tool, a particularly exciting future direction is the development of Cas9 as a therapeutic technology for treating genetic disorders. For a monogenic recessive disorder due to loss-of-function mutations (such as cystic fibrosis, sickle-cell anemia, or Duchenne muscular dystrophy), Cas9 ...
CRISPR Therapeutics and Vertex entered into a strategic research collaboration in 2015 focused on the use of CRISPR/Cas9 to discover and develop potential new treatments aimed at the underlying ...
CRISPR/Cas9 is a simple two-component system used for effective targeted gene editing. The first component is the single-effector Cas9 protein, which contains the endonuclease domains RuvC and HNH. RuvC cleaves the DNA strand non-complementary to the spacer sequence and HNH cleaves the complementary strand.
In 2014, they reported that a CRISPR-Cas9 tool could cut out a segment of integrated HIV DNA necessary for viral replication in a laboratory study. ... In other preclinical research, a single dose of EBT-107—a CRISPR compound delivered by lipid nanoparticles—reduced HBV DNA, hepatitis B surface antigen and hepatitis B e antigen by 98%, 97% ...