

COPD Case Study: Patient Diagnosis and Treatment (2024)
by John Landry, BS, RRT | Updated: Apr 4, 2024
Chronic obstructive pulmonary disease (COPD) is a progressive lung disease that affects millions of people around the world. It is primarily caused by smoking and is characterized by a persistent obstruction of airflow that worsens over time.
COPD can lead to a range of symptoms, including coughing, wheezing, shortness of breath, and chest tightness, which can significantly impact a person’s quality of life.
This case study will review the diagnosis and treatment of an adult patient who presented with signs and symptoms of this condition.
25+ RRT Cheat Sheets and Quizzes
Get access to 25+ premium quizzes, mini-courses, and downloadable cheat sheets for FREE.
COPD Clinical Scenario
A 56-year-old male patient is in the ER with increased work of breathing. He felt mildly short of breath after waking this morning but became extremely dyspneic after climbing a few flights of stairs. He is even too short of breath to finish full sentences. His wife is present in the room and revealed that the patient has a history of liver failure, is allergic to penicillin, and has a 15-pack-year smoking history. She also stated that he builds cabinets for a living and is constantly required to work around a lot of fine dust and debris.

Physical Findings
On physical examination, the patient showed the following signs and symptoms:
- His pupils are equal and reactive to light.
- He is alert and oriented.
- He is breathing through pursed lips.
- His trachea is positioned in the midline, and no jugular venous distention is present.
Vital Signs
- Heart rate: 92 beats/min
- Respiratory rate: 22 breaths/min
Chest Assessment
- He has a larger-than-normal anterior-posterior chest diameter.
- He demonstrates bilateral chest expansion.
- He demonstrates a prolonged expiratory phase and diminished breath sounds during auscultation.
- He is showing signs of subcostal retractions.
- Chest palpation reveals no tactile fremitus.
- Chest percussion reveals increased resonance.
- His abdomen is soft and tender.
- No distention is present.
Extremities
- His capillary refill time is two seconds.
- Digital clubbing is present in his fingertips.
- There are no signs of pedal edema.
- His skin appears to have a yellow tint.
Lab and Radiology Results
- ABG results: pH 7.35 mmHg, PaCO2 59 mmHg, HCO3 30 mEq/L, and PaO2 64 mmHg.
- Chest x-ray: Flat diaphragm, increased retrosternal space, dark lung fields, slight hypertrophy of the right ventricle, and a narrow heart.
- Blood work: RBC 6.5 mill/m3, Hb 19 g/100 mL, and Hct 57%.
Based on the information given, the patient likely has chronic obstructive pulmonary disease (COPD) .
The key findings that point to this diagnosis include:
- Barrel chest
- A long expiratory time
- Diminished breath sounds
- Use of accessory muscles while breathing
- Digital clubbing
- Pursed lip breathing
- History of smoking
- Exposure to dust from work
What Findings are Relevant to the Patient’s COPD Diagnosis?
The patient’s chest x-ray showed classic signs of chronic COPD, which include hyperexpansion, dark lung fields, and a narrow heart.
This patient does not have a history of cor pulmonale ; however, the findings revealed hypertrophy of the right ventricle. This is something that should be further investigated as right-sided heart failure is common in patients with COPD.
The lab values that suggest the patient has COPD include increased RBC, Hct, and Hb levels, which are signs of chronic hypoxemia.
Furthermore, the patient’s ABG results indicate COPD is present because the interpretation reveals compensated respiratory acidosis with mild hypoxemia. Compensated blood gases indicate an issue that has been present for an extended period of time.
What Tests Could Further Support This Diagnosis?
A series of pulmonary function tests (PFT) would be useful for assessing the patient’s lung volumes and capacities. This would help confirm the diagnosis of COPD and inform you of the severity.
Note: COPD patients typically have an FEV1/FVC ratio of < 70%, with an FEV1 that is < 80%.
The initial treatment for this patient should involve the administration of low-flow oxygen to treat or prevent hypoxemia .
It’s acceptable to start with a nasal cannula at 1-2 L/min. However, it’s often recommended to use an air-entrainment mask on COPD patients in order to provide an exact FiO2.
Either way, you should start with the lowest possible FiO2 that can maintain adequate oxygenation and titrate based on the patient’s response.
Example: Let’s say you start the patient with an FiO2 of 28% via air-entrainment mask but increase it to 32% due to no improvement. The SpO2 originally was 84% but now has decreased to 80%, and his retractions are worsening. This patient is sitting in the tripod position and continues to demonstrate pursed-lip breathing. Another blood gas was collected, and the results show a PaCO2 of 65 mmHg and a PaO2 of 59 mmHg.
What Do You Recommend?
The patient has an increased work of breathing, and their condition is clearly getting worse. The latest ABG results confirmed this with an increased PaCO2 and a PaO2 that is decreasing.
This indicates that the patient needs further assistance with both ventilation and oxygenation .
Note: In general, mechanical ventilation should be avoided in patients with COPD (if possible) because they are often difficult to wean from the machine.
Therefore, at this time, the most appropriate treatment method is noninvasive ventilation (e.g., BiPAP).
Initial BiPAP Settings
In general, the most commonly recommended initial BiPAP settings for an adult patient include this following:
- IPAP: 8–12 cmH2O
- EPAP: 5–8 cmH2O
- Rate: 10–12 breaths/min
- FiO2: Whatever they were previously on
For example, let’s say you initiate BiPAP with an IPAP of 10 cmH20, an EPAP of 5 cmH2O, a rate of 12, and an FiO2 of 32% (since that is what he was previously getting).
After 30 minutes on the machine, the physician requested another ABG to be drawn, which revealed acute respiratory acidosis with mild hypoxemia.
What Adjustments to BiPAP Settings Would You Recommend?
The latest ABG results indicate that two parameters must be corrected:
- Increased PaCO2
- Decreased PaO2
You can address the PaO2 by increasing either the FiO2 or EPAP setting. EPAP functions as PEEP, which is effective in increasing oxygenation.
The PaCO2 can be lowered by increasing the IPAP setting. By doing so, it helps to increase the patient’s tidal volume, which increased their expired CO2.
Note: In general, when making adjustments to a patient’s BiPAP settings, it’s acceptable to increase the pressure in increments of 2 cmH2O and the FiO2 setting in 5% increments.
Oxygenation
To improve the patient’s oxygenation , you can increase the EPAP setting to 7 cmH2O. This would decrease the pressure support by 2 cmH2O because it’s essentially the difference between the IPAP and EPAP.
Therefore, if you increase the EPAP, you must also increase the IPAP by the same amount to maintain the same pressure support level.
Ventilation
However, this patient also has an increased PaCO2 , which means that you must increase the IPAP setting to blow off more CO2. Therefore, you can adjust the pressure settings on the machine as follows:
- IPAP: 14 cmH2O
- EPAP: 7 cmH2O
After making these changes and performing an assessment , you can see that the patient’s condition is improving.
Two days later, the patient has been successfully weaned off the BiPAP machine and no longer needs oxygen support. He is now ready to be discharged.
The doctor wants you to recommend home therapy and treatment modalities that could benefit this patient.
What Home Therapy Would You Recommend?
You can recommend home oxygen therapy if the patient’s PaO2 drops below 55 mmHg or their SpO2 drops below 88% more than twice in a three-week period.
Remember: You must use a conservative approach when administering oxygen to a patient with COPD.
Pharmacology
You may also consider the following pharmacological agents:
- Short-acting bronchodilators (e.g., Albuterol)
- Long-acting bronchodilators (e.g., Formoterol)
- Anticholinergic agents (e.g., Ipratropium bromide)
- Inhaled corticosteroids (e.g., Budesonide)
- Methylxanthine agents (e.g., Theophylline)
In addition, education on smoking cessation is also important for patients who smoke. Nicotine replacement therapy may also be indicated.
In some cases, bronchial hygiene therapy should be recommended to help with secretion clearance (e.g., positive expiratory pressure (PEP) therapy).
It’s also important to instruct the patient to stay active, maintain a healthy diet, avoid infections, and get an annual flu vaccine. Lastly, some COPD patients may benefit from cardiopulmonary rehabilitation .
By taking all of these factors into consideration, you can better manage this patient’s COPD and improve their quality of life.
Final Thoughts
There are two key points to remember when treating a patient with COPD. First, you must always be mindful of the amount of oxygen being delivered to keep the FiO2 as low as possible.
Second, you should use noninvasive ventilation, if possible, before performing intubation and conventional mechanical ventilation . Too much oxygen can knock out the patient’s drive to breathe, and once intubated, these patients can be difficult to wean from the ventilator .
Furthermore, once the patient is ready to be discharged, you must ensure that you are sending them home with the proper medications and home treatments to avoid readmission.

Written by:
John Landry is a registered respiratory therapist from Memphis, TN, and has a bachelor's degree in kinesiology. He enjoys using evidence-based research to help others breathe easier and live a healthier life.
- Faarc, Kacmarek Robert PhD Rrt, et al. Egan’s Fundamentals of Respiratory Care. 12th ed., Mosby, 2020.
- Chang, David. Clinical Application of Mechanical Ventilation . 4th ed., Cengage Learning, 2013.
- Rrt, Cairo J. PhD. Pilbeam’s Mechanical Ventilation: Physiological and Clinical Applications. 7th ed., Mosby, 2019.
- Faarc, Gardenhire Douglas EdD Rrt-Nps. Rau’s Respiratory Care Pharmacology. 10th ed., Mosby, 2019.
- Faarc, Heuer Al PhD Mba Rrt Rpft. Wilkins’ Clinical Assessment in Respiratory Care. 8th ed., Mosby, 2017.
- Rrt, Des Terry Jardins MEd, and Burton George Md Facp Fccp Faarc. Clinical Manifestations and Assessment of Respiratory Disease. 8th ed., Mosby, 2019.
Recommended Reading
How to prepare for the clinical simulations exam (cse), faqs about the clinical simulation exam (cse), 7+ mistakes to avoid on the clinical simulation exam (cse), copd exacerbation: chronic obstructive pulmonary disease, epiglottitis scenario: clinical simulation exam (practice problem), guillain barré syndrome case study: clinical simulation scenario, drugs and medications to avoid if you have copd, the pros and cons of the zephyr valve procedure, the 50+ diseases to learn for the clinical sims exam (cse).
Chronic Obstructive Pulmonary Disease (COPD) Case Study (60 min)
Watch More! Unlock the full videos with a FREE trial
Included In This Lesson
Study tools.
Access More! View the full outline and transcript with a FREE trial
Mr. Whaley is a 65-year-old man with a history of COPD who presents to his primary care provider’s (PCP) office complaining of a productive cough off and on for 2 years and shortness of breath for the last 3 days. He reports that he has had several chest colds in the last few years, but this time it won’t go away. His wife says he has been feverish for a few days, but doesn’t have a specific temperature to report. He reports smoking a pack of cigarettes a day for 25 years plus the occasional cigar.
What nursing assessments should be performed at this time for Mr. Whaley?
- Full set of vital signs, including SpO2
- Heart and Lung sounds
- Gather any further details of illness or medical history, including allergies
Upon further assessment, Mr. Whaley has crackles throughout the lower lobes of his lungs, with occasional expiratory wheezes throughout the lung fields. His vital signs are as follows:
BP 142/86 mmHg HR 102 bpm
RR 32 bpm Temp 38.8°C
SpO 2 86% on room air
The nurse locates a portable oxygen tank and places the patient on 2 lpm oxygen via nasal cannula. Based on these findings, Mr. Whaley’s PCP decides to call an ambulance to send Mr. Whaley to the Emergency Department (ED). While waiting for the ambulance, the nurse repeats the SpO 2 and finds Mr. Whaley’s SpO 2 is only 89%. She increases his oxygen to 4 lpm, rechecks and notes an SpO 2 of 95%. The ambulance crew arrives, the nurse reports to them that the patient was short of breath and hypoxic, but sats are now 95% and he is resting. Per EMS, he is alert and oriented x 3.
What is going on with Mr. Whaley, physiologically?
- Mr. Whaley may have a lung infection, as evidenced by his fever and productive cough. This is causing a COPD exacerbation. COPD makes gas exchange difficult, which is why his SpO2 levels are low.
What would you have done differently? Why?
- Because of his COPD, Mr. Whaley should not have been placed on more than 2 lpm of supplemental O2 because it would decrease his respiratory drive and lead to CO2 toxicity.
- When Mr. Whaley’s sats didn’t improve, should have notified provider before adding more supplemental oxygen
Upon arrival to the ED, the RN finds Mr. Whaley is somnolent and difficult to arouse. He takes a set of vital signs and finds the following:
BP 138/78 mmHg HR 96 bpm
RR 16 bpm Temp 38.4°C
SpO 2 96% on 4 lpm nasal cannula
What is the possible cause of Mr. Whaley’s somnolence?
- His COPD makes gas exchange difficult, therefore he retains CO2. This means his respiratory drive to breathe is low O2 instead of high CO2. When the nurse gave too much supplemental oxygen, Mr. Whaley lost some of his respiratory drive. This is why his respiratory rate is so low.
- This can lead to CO2 toxicity, which presents as a decreased LOC and decreased respiratory rate, and can lead to the patient not protecting their airway and going into respiratory arrest
What orders do you expect from the ED provider?
- To remove the supplemental oxygen and only keep SpO2 between 88-92% to avoid over-oxygenating and CO2 toxicity
- Chest X-ray
- Blood Cultures, Sputum Cultures, CBC, BMP, ABG
- Bronchodilators, Corticosteroids, Breathing treatments from Respiratory Therapy
The provider writes the following orders:
Keep sats 88-92%
Labs: ABG, CBC, BMP
Insert peripheral IV
Albuterol nebulizer 2.5mg
Budesonide-formoterol 160/4.5 mcg
The nurse immediately removes the supplemental oxygen from Mr. Whaley and attempts to stimulate him awake. Mr. Whaley is still quite drowsy, but is able to awake long enough to state his full name. The nurse inserts a peripheral IV and draws the CBC and BMP, while the Respiratory Therapist (RT) draws an arterial blood gas (ABG). Blood gas results are as follows:
pCO 2 58 mmHg
HCO 3 – 30 mEq/L
pO 2 50 mmHg
Mr. Whaley’s chest x-ray shows consolidation in bilateral lower lobes.
Interpret the ABG. Explain.
- This is respiratory acidosis with partial compensation
- The ABG also shows hypoxemia
- Mr. Whaley retains CO2 chronically and his kidneys have tried to compensate (as evidenced by the HCO3- of 30 mEq/L). They weren’t able to fully compensate, though, so his pH is still acidic because of the high CO2
Which medication should be administered first? Why?
- Albuterol – because it is a bronchodilator and should always be administered before corticosteroids
Mr. Whaley’s condition improves after a bronchodilator and corticosteroid breathing treatment. His SpO 2 remains 90% on room air and his shortness of breath has significantly decreased. He is still running a fever of 38.3°C. The ED provider orders broad spectrum antibiotics for a likely pneumonia, which may have caused this COPD exacerbation. The provider also orders two inhalers for Mr. Whaley, one bronchodilator and one corticosteroid. Satisfied with his quick improvement, the provider decides it is safe for Mr. Whaley to recover at home with proper instructions for his medications and follow up from his PCP.
What are priority discharge teaching topics for Mr. Whaley?
- Mr. Whaley NEEDS to stop smoking!!!
- Proper use of inhalers, new medication instructions
- Reporting s/s respiratory infection to PCP sooner
- Pursed lip breathing and small, frequent meals to prevent shortness of breath
View the FULL Outline
When you start a FREE trial you gain access to the full outline as well as:
- SIMCLEX (NCLEX Simulator)
- 6,500+ Practice NCLEX Questions
- 2,000+ HD Videos
- 300+ Nursing Cheatsheets
“Would suggest to all nursing students . . . Guaranteed to ease the stress!”
Nursing Case Studies

This nursing case study course is designed to help nursing students build critical thinking. Each case study was written by experienced nurses with first hand knowledge of the “real-world” disease process. To help you increase your nursing clinical judgement (critical thinking), each unfolding nursing case study includes answers laid out by Blooms Taxonomy to help you see that you are progressing to clinical analysis.We encourage you to read the case study and really through the “critical thinking checks” as this is where the real learning occurs. If you get tripped up by a specific question, no worries, just dig into an associated lesson on the topic and reinforce your understanding. In the end, that is what nursing case studies are all about – growing in your clinical judgement.
Nursing Case Studies Introduction
Cardiac nursing case studies.
- 6 Questions
- 7 Questions
- 5 Questions
- 4 Questions
GI/GU Nursing Case Studies
- 2 Questions
- 8 Questions
Obstetrics Nursing Case Studies
Respiratory nursing case studies.
- 10 Questions
Pediatrics Nursing Case Studies
- 3 Questions
- 12 Questions
Neuro Nursing Case Studies
Mental health nursing case studies.
- 9 Questions
Metabolic/Endocrine Nursing Case Studies
Other nursing case studies.
- Login / Register

‘Let’s hear it for the midwives and everything they do’
STEVE FORD, EDITOR
- You are here: COPD
Diagnosis and management of COPD: a case study
04 May, 2020
This case study explains the symptoms, causes, pathophysiology, diagnosis and management of chronic obstructive pulmonary disease
This article uses a case study to discuss the symptoms, causes and management of chronic obstructive pulmonary disease, describing the patient’s associated pathophysiology. Diagnosis involves spirometry testing to measure the volume of air that can be exhaled; it is often performed after administering a short-acting beta-agonist. Management of chronic obstructive pulmonary disease involves lifestyle interventions – vaccinations, smoking cessation and pulmonary rehabilitation – pharmacological interventions and self-management.
Citation: Price D, Williams N (2020) Diagnosis and management of COPD: a case study. Nursing Times [online]; 116: 6, 36-38.
Authors: Debbie Price is lead practice nurse, Llandrindod Wells Medical Practice; Nikki Williams is associate professor of respiratory and sleep physiology, Swansea University.
- This article has been double-blind peer reviewed
- Scroll down to read the article or download a print-friendly PDF here (if the PDF fails to fully download please try again using a different browser)
Introduction
The term chronic obstructive pulmonary disease (COPD) is used to describe a number of conditions, including chronic bronchitis and emphysema. Although common, preventable and treatable, COPD was projected to become the third leading cause of death globally by 2020 (Lozano et al, 2012). In the UK in 2012, approximately 30,000 people died of COPD – 5.3% of the total number of deaths. By 2016, information published by the World Health Organization indicated that Lozano et al (2012)’s projection had already come true.
People with COPD experience persistent respiratory symptoms and airflow limitation that can be due to airway or alveolar abnormalities, caused by significant exposure to noxious particles or gases, commonly from tobacco smoking. The projected level of disease burden poses a major public-health challenge and primary care nurses can be pivotal in the early identification, assessment and management of COPD (Hooper et al, 2012).
Grace Parker (the patient’s name has been changed) attends a nurse-led COPD clinic for routine reviews. A widowed, 60-year-old, retired post office clerk, her main complaint is breathlessness after moderate exertion. She scored 3 on the modified Medical Research Council (mMRC) scale (Fletcher et al, 1959), indicating she is unable to walk more than 100 yards without stopping due to breathlessness. Ms Parker also has a cough that produces yellow sputum (particularly in the mornings) and an intermittent wheeze. Her symptoms have worsened over the last six months. She feels anxious leaving the house alone because of her breathlessness and reduced exercise tolerance, and scored 26 on the COPD Assessment Test (CAT, catestonline.org), indicating a high level of impact.
Ms Parker smokes 10 cigarettes a day and has a pack-year score of 29. She has not experienced any haemoptysis (coughing up blood) or chest pain, and her weight is stable; a body mass index of 40kg/m 2 means she is classified as obese. She has had three exacerbations of COPD in the previous 12 months, each managed in the community with antibiotics, steroids and salbutamol.
Ms Parker was diagnosed with COPD five years ago. Using Epstein et al’s (2008) guidelines, a nurse took a history from her, which provided 80% of the information needed for a COPD diagnosis; it was then confirmed following spirometry testing as per National Institute for Health and Care Excellence (2018) guidance.
The nurse used the Calgary-Cambridge consultation model, as it combines the pathological description of COPD with the patient’s subjective experience of the illness (Silverman et al, 2013). Effective communication skills are essential in building a trusting therapeutic relationship, as the quality of the relationship between Ms Parker and the nurse will have a direct impact on the effectiveness of clinical outcomes (Fawcett and Rhynas, 2012).
In a national clinical audit report, Baxter et al (2016) identified inaccurate history taking and inadequately performed spirometry as important factors in the inaccurate diagnosis of COPD on general practice COPD registers; only 52.1% of patients included in the report had received quality-assured spirometry.
Pathophysiology of COPD
Knowing the pathophysiology of COPD allowed the nurse to recognise and understand the physical symptoms and provide effective care (Mitchell, 2015). Continued exposure to tobacco smoke is the likely cause of the damage to Ms Parker’s small airways, causing her cough and increased sputum production. She could also have chronic inflammation, resulting in airway smooth-muscle contraction, sluggish ciliary movement, hypertrophy and hyperplasia of mucus-secreting goblet cells, as well as release of inflammatory mediators (Mitchell, 2015).
Ms Parker may also have emphysema, which leads to damaged parenchyma (alveoli and structures involved in gas exchange) and loss of alveolar attachments (elastic connective fibres). This causes gas trapping, dynamic hyperinflation, decreased expiratory flow rates and airway collapse, particularly during expiration (Kaufman, 2013). Ms Parker also displayed pursed-lip breathing; this is a technique used to lengthen the expiratory time and improve gaseous exchange, and is a sign of dynamic hyperinflation (Douglas et al, 2013).
In a healthy lung, the destruction and repair of alveolar tissue depends on proteases and antiproteases, mainly released by neutrophils and macrophages. Inhaling cigarette smoke disrupts the usually delicately balanced activity of these enzymes, resulting in the parenchymal damage and small airways (with a lumen of <2mm in diameter) airways disease that is characteristic of emphysema. The severity of parenchymal damage or small airways disease varies, with no pattern related to disease progression (Global Initiative for Chronic Obstructive Lung Disease, 2018).
Ms Parker also had a wheeze, heard through a stethoscope as a continuous whistling sound, which arises from turbulent airflow through constricted airway smooth muscle, a process noted by Mitchell (2015). The wheeze, her 29 pack-year score, exertional breathlessness, cough, sputum production and tiredness, and the findings from her physical examination, were consistent with a diagnosis of COPD (GOLD, 2018; NICE, 2018).
Spirometry is a tool used to identify airflow obstruction but does not identify the cause. Commonly measured parameters are:
- Forced expiratory volume – the volume of air that can be exhaled – in one second (FEV1), starting from a maximal inspiration (in litres);
- Forced vital capacity (FVC) – the total volume of air that can be forcibly exhaled – at timed intervals, starting from a maximal inspiration (in litres).
Calculating the FEV1 as a percentage of the FVC gives the forced expiratory ratio (FEV1/FVC). This provides an index of airflow obstruction; the lower the ratio, the greater the degree of obstruction. In the absence of respiratory disease, FEV1 should be ≥70% of FVC. An FEV1/FVC of <70% is commonly used to denote airflow obstruction (Moore, 2012).
As they are time dependent, FEV1 and FEV1/FVC are reduced in diseases that cause airways to narrow and expiration to slow. FVC, however, is not time dependent: with enough expiratory time, a person can usually exhale to their full FVC. Lung function parameters vary depending on age, height, gender and ethnicity, so the degree of FEV1 and FVC impairment is calculated by comparing a person’s recorded values with predicted values. A recorded value of >80% of the predicted value has been considered ‘normal’ for spirometry parameters but the lower limit of normal – equal to the fifth percentile of a healthy, non-smoking population – based on more robust statistical models is increasingly being used (Cooper et al, 2017).
A reversibility test involves performing spirometry before and after administering a short-acting beta-agonist (SABA) such as salbutamol; the test is used to distinguish between reversible and fixed airflow obstruction. For symptomatic asthma, airflow obstruction due to airway smooth-muscle contraction is reversible: administering a SABA results in smooth-muscle relaxation and improved airflow (Lumb, 2016). However, COPD is associated with fixed airflow obstruction, resulting from neutrophil-driven inflammatory changes, excess mucus secretion and disrupted alveolar attachments, as opposed to airway smooth-muscle contraction.
Administering a SABA for COPD does not usually produce bronchodilation to the extent seen in someone with asthma: a person with asthma may demonstrate significant improvement in FEV1 (of >400ml) after having a SABA, but this may not change in someone with COPD (NICE, 2018). However, a negative response does not rule out therapeutic benefit from long-term SABA use (Marín et al, 2014).
NICE (2018) and GOLD (2018) guidelines advocate performing spirometry after administering a bronchodilator to diagnose COPD. Both suggest a FEV1/FVC of <70% in a person with respiratory symptoms supports a diagnosis of COPD, and both grade the severity of the condition using the predicted FEV1. Ms Parker’s spirometry results showed an FEV1/FVC of 56% and a predicted FEV1 of 57%, with no significant improvement in these values with a reversibility test.
GOLD (2018) guidance is widely accepted and used internationally. However, it was developed by medical practitioners with a medicalised approach, so there is potential for a bias towards pharmacological management of COPD. NICE (2018) guidance may be more useful for practice nurses, as it was developed by a multidisciplinary team using evidence from systematic reviews or meta-analyses of randomised controlled trials, providing a holistic approach. NICE guidance may be outdated on publication, but regular reviews are performed and published online.
NHS England (2016) holds a national register of all health professionals certified in spirometry. It was set up to raise spirometry standards across the country.
Assessment and management
The goals of assessing and managing Ms Parker’s COPD are to:
- Review and determine the level of airflow obstruction;
- Assess the disease’s impact on her life;
- Risk assess future disease progression and exacerbations;
- Recommend pharmacological and therapeutic management.
GOLD’s (2018) ABCD assessment tool (Fig 1) grades COPD severity using spirometry results, number of exacerbations, CAT score and mMRC score, and can be used to support evidence-based pharmacological management of COPD.

When Ms Parker was diagnosed, her predicted FEV1 of 57% categorised her as GOLD grade 2, and her mMRC score, CAT score and exacerbation history placed her in group D. The mMRC scale only measures breathlessness, but the CAT also assesses the impact COPD has on her life, meaning consecutive CAT scores can be compared, providing valuable information for follow-up and management (Zhao, et al, 2014).
After assessing the level of disease burden, Ms Parker was then provided with education for self-management and lifestyle interventions.
Lifestyle interventions
Smoking cessation.
Cessation of smoking alongside support and pharmacotherapy is the second-most cost-effective intervention for COPD, when compared with most other pharmacological interventions (BTS and PCRS UK, 2012). Smoking cessation:
- Slows the progression of COPD;
- Improves lung function;
- Improves survival rates;
- Reduces the risk of lung cancer;
- Reduces the risk of coronary heart disease risk (Qureshi et al, 2014).
Ms Parker accepted a referral to an All Wales Smoking Cessation Service adviser based at her GP surgery. The adviser used the internationally accepted ‘five As’ approach:
- Ask – record the number of cigarettes the individual smokes per day or week, and the year they started smoking;
- Advise – urge them to quit. Advice should be clear and personalised;
- Assess – determine their willingness and confidence to attempt to quit. Note the state of change;
- Assist – help them to quit. Provide behavioural support and recommend or prescribe pharmacological aids. If they are not ready to quit, promote motivation for a future attempt;
- Arrange – book a follow-up appointment within one week or, if appropriate, refer them to a specialist cessation service for intensive support. Document the intervention.
NICE (2013) guidance recommends that this be used at every opportunity. Stead et al (2016) suggested that a combination of counselling and pharmacotherapy have proven to be the most effective strategy.
Pulmonary rehabilitation
Ms Parker’s positive response to smoking cessation provided an ideal opportunity to offer her pulmonary rehabilitation (PR) – as indicated by Johnson et al (2014), changing one behaviour significantly increases a person’s chance of changing another.
PR – a supervised programme including exercise training, health education and breathing techniques – is an evidence-based, comprehensive, multidisciplinary intervention that:
- Improves exercise tolerance;
- Reduces dyspnoea;
- Promotes weight loss (Bolton et al, 2013).
These improvements often lead to an improved quality of life (Sciriha et al, 2015).
Most relevant for Ms Parker, PR has been shown to reduce anxiety and depression, which are linked to an increased risk of exacerbations and poorer health status (Miller and Davenport, 2015). People most at risk of future exacerbations are those who already experience them (Agusti et al, 2010), as in Ms Parker’s case. Patients who have frequent exacerbations have a lower quality of life, quicker progression of disease, reduced mobility and more-rapid decline in lung function than those who do not (Donaldson et al, 2002).
“COPD is a major public-health challenge; nurses can be pivotal in early identification, assessment and management”
Pharmacological interventions
Ms Parker has been prescribed inhaled salbutamol as required; this is a SABA that mediates the increase of cyclic adenosine monophosphate in airway smooth-muscle cells, leading to muscle relaxation and bronchodilation. SABAs facilitate lung emptying by dilatating the small airways, reversing dynamic hyperinflation of the lungs (Thomas et al, 2013). Ms Parker also uses a long-acting muscarinic antagonist (LAMA) inhaler, which works by blocking the bronchoconstrictor effects of acetylcholine on M3 muscarinic receptors in airway smooth muscle; release of acetylcholine by the parasympathetic nerves in the airways results in increased airway tone with reduced diameter.
At a routine review, Ms Parker admitted to only using the SABA and LAMA inhalers, despite also being prescribed a combined inhaled corticosteroid and long-acting beta 2 -agonist (ICS/LABA) inhaler. She was unaware that ICS/LABA inhalers are preferred over SABA inhalers, as they:
- Last for 12 hours;
- Improve the symptoms of breathlessness;
- Increase exercise tolerance;
- Can reduce the frequency of exacerbations (Agusti et al, 2010).
However, moderate-quality evidence shows that ICS/LABA combinations, particularly fluticasone, cause an increased risk of pneumonia (Suissa et al, 2013; Nannini et al, 2007). Inhaler choice should, therefore, be individualised, based on symptoms, delivery technique, patient education and compliance.
It is essential to teach and assess inhaler technique at every review (NICE, 2011). Ms Parker uses both a metered-dose inhaler and a dry-powder inhaler; an in-check device is used to assess her inspiratory effort, as different inhaler types require different inhalation speeds. Braido et al (2016) estimated that 50% of patients have poor inhaler technique, which may be due to health professionals lacking the confidence and capability to teach and assess their use.
Patients may also not have the dexterity, capacity to learn or vision required to use the inhaler. Online resources are available from, for example, RightBreathe (rightbreathe.com), British Lung Foundation (blf.org.uk). Ms Parker’s adherence could be improved through once-daily inhalers, as indicated by results from a study by Lipson et al (2017). Any change in her inhaler would be monitored as per local policy.
Vaccinations
Ms Parker keeps up to date with her seasonal influenza and pneumococcus vaccinations. This is in line with the low-cost, highest-benefit strategy identified by the British Thoracic Society and Primary Care Respiratory Society UK’s (2012) study, which was conducted to inform interventions for patients with COPD and their relative quality-adjusted life years. Influenza vaccinations have been shown to decrease the risk of lower respiratory tract infections and concurrent COPD exacerbations (Walters et al, 2017; Department of Health, 2011; Poole et al, 2006).
Self-management
Ms Parker was given a self-management plan that included:
- Information on how to monitor her symptoms;
- A rescue pack of antibiotics, steroids and salbutamol;
- A traffic-light system demonstrating when, and how, to commence treatment or seek medical help.
Self-management plans and rescue packs have been shown to reduce symptoms of an exacerbation (Baxter et al, 2016), allowing patients to be cared for in the community rather than in a hospital setting and increasing patient satisfaction (Fletcher and Dahl, 2013).
Improving Ms Parker’s adherence to once-daily inhalers and supporting her to self-manage and make the necessary lifestyle changes, should improve her symptoms and result in fewer exacerbations.
The earlier a diagnosis of COPD is made, the greater the chances of reducing lung damage through interventions such as smoking cessation, lifestyle modifications and treatment, if required (Price et al, 2011).
- Chronic obstructive pulmonary disease is a progressive respiratory condition, projected to become the third leading cause of death globally
- Diagnosis involves taking a patient history and performing spirometry testing
- Spirometry identifies airflow obstruction by measuring the volume of air that can be exhaled
- Chronic obstructive pulmonary disease is managed with lifestyle and pharmacological interventions, as well as self-management
Related files
200506 diagnosis and management of copd – a case study.
- Add to Bookmarks
Related articles
Have your say.
Sign in or Register a new account to join the discussion.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

2: Case Study #1- Chronic Obstructive Pulmonary Disease (COPD)
- Last updated
- Save as PDF
- Page ID 9896
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
- 2.1: Learning Objectives
- 2.2: Patient- Erin Johns
- 2.3: At Home
- 2.4: Emergency Room
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Perspective
- Open access
- Published: 07 May 2015
Four patients with a history of acute exacerbations of COPD: implementing the CHEST/Canadian Thoracic Society guidelines for preventing exacerbations
- Ioanna Tsiligianni 1 , 2 ,
- Donna Goodridge 3 ,
- Darcy Marciniuk 4 ,
- Sally Hull 5 &
- Jean Bourbeau 6
npj Primary Care Respiratory Medicine volume 25 , Article number: 15023 ( 2015 ) Cite this article
59k Accesses
12 Altmetric
Metrics details
- Respiratory tract diseases
The American College of Chest Physicians and Canadian Thoracic Society have jointly produced evidence-based guidelines for the prevention of exacerbations in chronic obstructive pulmonary disease (COPD). This educational article gives four perspectives on how these guidelines apply to the practical management of people with COPD. A current smoker with frequent exacerbations will benefit from support to quit, and from optimisation of his inhaled treatment. For a man with very severe COPD and multiple co-morbidities living in a remote community, tele-health care may enable provision of multidisciplinary care. A woman who is admitted for the third time in a year needs a structured assessment of her care with a view to stepping up pharmacological and non-pharmacological treatment as required. The overlap between asthma and COPD challenges both diagnostic and management strategies for a lady smoker with a history of asthma since childhood. Common threads in all these cases are the importance of advising on smoking cessation, offering (and encouraging people to attend) pulmonary rehabilitation, and the importance of self-management, including an action plan supported by multidisciplinary teams.
Similar content being viewed by others
Diagnostic spirometry in COPD is increasing, a comparison of two Swedish cohorts

COPD overdiagnosis in primary care: a UK observational study of consistency of airflow obstruction

Phenotype and management of chronic obstructive pulmonary disease patients in general population in China: a nationally cross-sectional study
Case study 1: a 63-year-old man with moderate/severe copd and a chest infection.
A 63-year-old self-employed plumber makes a same-day appointment for another ‘chest infection’. He caught an upper respiratory tract infection from his grandchildren 10 days ago, and he now has a productive cough with green sputum, and his breathlessness and fatigue has forced him to take time off work.
He has visited his general practitioner with similar symptoms two or three times every year in the last decade. A diagnosis of COPD was confirmed 6 years ago, and he was started on a short-acting β 2 -agonist. This helped with his day-to-day symptoms, although recently the symptoms of breathlessness have been interfering with his work and he has to pace himself to get through the day. Recovering from exacerbations takes longer than it used to—it is often 2 weeks before he is able to get back to work—and he feels bad about letting down customers. He cannot afford to retire, but is thinking about reducing his workload.
He last attended a COPD review 6 months ago when his FEV 1 was 52% predicted. He was advised to stop smoking and given a prescription for varenicline, but he relapsed after a few days and did not return for the follow-up appointment. He attends each year for his ‘flu vaccination’. His only other medication is an ACE inhibitor for hypertension.
Managing the presenting problem. Is it a COPD exacerbation?
A COPD exacerbation is defined as ‘an acute event characterised by a worsening of the patient’s respiratory symptoms that is beyond normal day-to-day variation and leads to change in medications’. 1 , 2 The worsening symptoms are usually increased dyspnoea, increased sputum volume and increased sputum purulence. 1 , 2 All these symptoms are present in our patient who experiences an exacerbation triggered by a viral upper respiratory tract infection—the most common cause of COPD exacerbations. Apart from the management of the acute exacerbation that could include antibiotics, oral steroids and increased use of short-acting bronchodilators, special attention should be given to his on-going treatment to prevent future exacerbations. 2 Short-term use of systemic corticosteroids and a course of antibiotics can shorten recovery time, improve lung function (forced expiratory volume in one second (FEV 1 )) and arterial hypoxaemia and reduce the risk of early relapse, treatment failure and length of hospital stay. 1 , 2 Short-acting inhaled β 2 -agonists with or without short-acting anti-muscarinics are usually the preferred bronchodilators for the treatment of an acute exacerbation. 1
Reviewing his routine treatment
One of the concerns about this patient is that his COPD is inadequately treated. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) suggests that COPD management be based on a combined assessment of symptoms, GOLD classification of airflow limitation, and exacerbation rate. 1 The modified Medical Research Council (mMRC) dyspnoea score 3 or the COPD Assessment Tool (CAT) 4 could be used to evaluate the symptoms/health status. History suggests that his breathlessness has begun to interfere with his lifestyle, but this has not been formally asssessed since the diagnosis 6 years ago. Therefore, one would like to be certain that these elements are taken into consideration in future management by involving other members of the health care team. The fact that he had two to three exacerbations per year puts the patient into GOLD category C–D (see Figure 1 ) despite the moderate airflow limitation. 1 , 5 Our patient is only being treated with short-acting bronchodilators; however, this is only appropriate for patients who belong to category A. Treatment options for patients in category C or D should include long-acting muscarinic antagonists (LAMAs) or long-acting β 2 -agonists (LABAs), which will not only improve his symptoms but also help prevent future exacerbations. 2 Used in combination with LABA or LAMA, inhaled corticosteroids also contribute to preventing exacerbations. 2
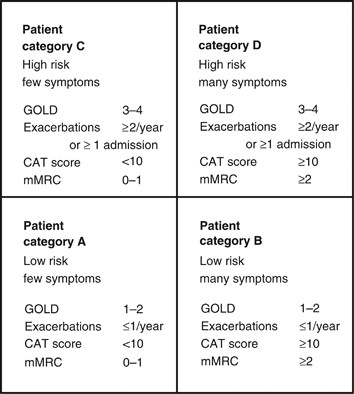
The four categories of COPD based on assessment of symptoms and future risk of exacerbations (adapted by Gruffydd-Jones, 5 from the Global Strategy for Diagnosis, Management and Prevention of COPD). 1 CAT, COPD Assessment Tool; COPD, chronic obstructive pulmonary disease; mMRC, modified Medical Research Council Dyspnoea Scale.

Prevention of future exacerbations
Exacerbations should be prevented as they have a negative impact on the quality of life; they adversely affect symptoms and lung function, increase economic cost, increase mortality and accelerate lung function decline. 1 , 2 Figure 2 summarises the recommendations and suggestions of the joint American College of Chest Physicians and Canadian Thoracic Society (CHEST/CTS) Guidelines for the prevention of exacerbations in COPD. 2 The grades of recommendation from the CHEST/CTS guidelines are explained in Table 1 .

Decision tree for prevention of acute exacerbations of COPD (reproduced with permission from the CHEST/CTS Guidelines for the prevention of exacerbations in COPD). 2 This decision tree for prevention of acute exacerbations of COPD is arranged according to three key clinical questions using the PICO format: non-pharmacologic therapies, inhaled therapies and oral therapies. The wording used is ‘Recommended or Not recommended’ when the evidence was strong (Level 1) or ‘Suggested or Not suggested’ when the evidence was weak (Level 2). CHEST/CTS, American College of Chest Physicians and Canadian Thoracic Society; COPD, chronic obstructive pulmonary disease; FEV 1 , forced expiratory volume in one second; FVC, forced vital capacity; LABA, long-acting β-agonist; LAMA, long-acting muscarinic antagonist; ICS, inhaled corticosteroids; SAMA, short-acting muscarinic antagonist; SABA, short-acting β-agonist; SM, self-management.
Pharmacological approach
In patients with moderate-to-severe COPD, the use of LABA or LAMA compared with placebo or short-acting bronchodilators is recommended to prevent acute exacerbations (Grades 1B and 1A, respectively). 2 , 6 , 7 LAMAs are associated with a lower rate of exacerbations compared with LABAs (Grade 1C). 2 , 6 The inhaler technique needs to be checked and a suitable device selected. If our patient does not respond to optimizing inhaled medication and continues to have two to three exacerbations per year, there are additional options that offer pulmonary rehabilitation and other forms of pharmacological therapy, such as a macrolide, theophylline, phosphodieseterase (PDE4) inhibitor or N -acetylocysteine/carbocysteine, 2 although there is no information about their relative effectiveness and the order in which they should be prescribed. The choice of prescription should be guided by the risk/benefit for a given individual, and drug availability and/or cost within the health care system.
Non-pharmacological approach
A comprehensive patient-centred approach based on the chronic care model could be of great value. 2 , 8
This should include the following elements
Vaccinations: the 23-valent pneumococcal vaccine and annual influenza vaccine are suggested as part of the overall medical management in patients with COPD. 2 Although there is no clear COPD-specific evidence for the pneumococcal vaccine and the evidence is modest for influenza, the CHEST/CTS Guidelines concur with advice of the World Health Organization (WHO) 9 and national advisory bodies, 10 – 12 and supports their use in COPD patients who are at risk for serious infections. 2
Smoking cessation (including counselling and treatment) has low evidence for preventing exacerbations (Grade 2C). 2 However, the benefits from smoking cessation are outstanding as it improves COPD prognosis, slows lung function decline and improves the quality of life and symptoms. 1 , 2 , 13 , 14 Our patient has struggled to quit in the past; assessing current readiness to quit, and encouraging and supporting a future attempt is a priority in his care.
Pulmonary rehabilitation (based on exercise training, education and behaviour change) in people with moderate-to-very-severe COPD, provided within 4 weeks of an exacerbation, can prevent acute exacerbations (Grade 1C). 2 Pulmonary rehabilitation is also an effective strategy to improve symptoms, the quality of life and exercise tolerance, 15 , 16 and our patient should be encouraged to attend a course.
Self-management education with a written action plan and supported by case management providing regular direct access to a health care specialist reduces hospitalisations and prevents severe acute exacerbations (Grade 2C). 2 Some patients with good professional support can have an emergency course of steroids and antibiotics to start at the onset of an exacerbation in accordance with their plan.
Finally, close follow-up is needed for our patient as he was inadequately treated, relapsed from smoking cessation after a few days despite varenicline, and missed his follow-up appointment. A more alert health care team may have been able to identify these issues, avoid his relapse and take a timely approach to introducing additional measures to prevent his recurrent acute exacerbations.
Case study 2: A 74-year-old man with very severe COPD living alone in a remote community
A 74-year-old man has a routine telephone consultation with the respiratory team. He has very severe COPD (his FEV 1 2 years ago was 24% of predicted) and he copes with the help of his daughter who lives in the same remote community. He quit smoking the previous year after an admission to the hospital 50 miles away, which he found very stressful. He and his family managed another four exacerbations at home with courses of steroids and antibiotics, which he commenced in accordance with a self-management plan provided by the respiratory team.
His usual therapy consists of regular long-acting β 2 -agonist/inhaled steroid combination and a long-acting anti-muscarinic. He has a number of other health problems, including coronary heart disease and osteoarthritis and, in recent times, his daughter has become concerned that he is becoming forgetful. He manages at home by himself, steadfastly refusing social help and adamant that he does not want to move from the home he has lived in for 55 years.
This is a common clinical scenario, and a number of important issues require attention, with a view to optimising the management of this 74-year-old man suffering from COPD. He has very severe obstruction, is experiencing frequent acute flare-ups, is dependent and isolated and has a number of co-morbidities. To work towards preventing future exacerbations in this patient, a comprehensive plan addressing key medical and self-care issues needs to be developed that accounts for his particular context.
Optimising medical management
According to the CHEST/CTS Guidelines for prevention of acute exacerbations of COPD, 2 this patient should receive an annual influenza vaccination and may benefit from a 23-valent pneumococcal vaccine (Grades 1B and 2C, respectively). Influenza infection is associated with greater risk of mortality in COPD, as well as increased risk of hospitalisation and disease progression. 1 A diagnosis of COPD also increases the risk for pneumococcal disease and related complications, with hospitalisation rates for patients with COPD being higher than that in the general population. 10 , 17 Although existing evidence does not support the use of this vaccine specifically to prevent exacerbations of COPD, 1 administration of the 23-valent pneumococcal vaccine is recommended as a component of overall medical management. 9 – 12
Long-term oxygen therapy has been demonstrated to improve survival in people with chronic hypoxaemia; 18 it would be helpful to obtain oxygen saturation levels and consider whether long-term oxygen therapy would be of benefit to this patient.
Even though this patient is on effective medications, further optimisation of pharmacologic therapy should be undertaken, including reviewing administration technique for the different inhaler devices. 19 Maintenance PDE4 inhibitors, such as roflumilast or theophyllines, long-term macrolides (i.e., azithromycin) or oral N -acetylcysteine are potential considerations. Each of these therapeutic options has demonstrated efficacy in preventing future acute exacerbations, although they should be used with caution in this frail elderly man. 2 This patient would benefit from a review of co-morbidities, including a chest X-ray, electrocardiogram, memory assessment and blood tests including haemoglobin, glucose, thyroid and renal function assessments.
Pulmonary rehabilitation, supported self-management and tele-health care
Pulmonary rehabilitation for patients who have recently experienced an exacerbation of COPD (initiated <4 weeks following the exacerbation) has been demonstrated to prevent subsequent exacerbations (Grade 1C). 2 Existing evidence suggests that pulmonary rehabilitation does not reduce future exacerbations when the index exacerbation has occurred more than 4 weeks earlier; 2 however, its usefulness is evident in other important patient-centred outcomes such as improved activity, walking distance and quality of life, as well as by reduced shortness of breath. It would be appropriate to discuss this and enable our patient to enrol in pulmonary rehabilitation.
The patient’s access to pulmonary rehabilitation in his remote location, however, is likely to be limited. Several reports have noted that only one to two percent of people with COPD are able to access pulmonary rehabilitation programmes within Canada, 20 the United States 21 and the United Kingdom. 22 Alternatives to hospital-based pulmonary rehabilitation programmes, such as home-based programmes or programmes offered via tele-health, may be options for this patient. 23 Home-based pulmonary rehabilitation programmes have been found to improve exercise tolerance, symptom burden and quality of life. 24 – 27 Outcomes of a pulmonary rehabilitation programme offered via tele-health have also been found to be comparable to those of a hospital-based programme, 28 and may be worth exploring.
Written self-management (action) plans, together with education and case management, are suggested in the CHEST/CTS guidelines as a strategy to reduce hospitalisation and emergency department visits attributable to exacerbations of COPD (Grade 2B). 2 Our patient has an existing action plan, which has enabled him and his family to manage some exacerbations at home. Although the patient has likely had some education on COPD and its management in the past, on-going reinforcement of key principles may be helpful in preventing future exacerbations.
The self-management plan should be reviewed regularly to ensure the advice remains current. The patient’s ability to use the self-management plan safely also needs to be assessed, given his daughter’s recent observation of forgetfulness and his living alone. Cognitive impairment is being increasingly recognised as a significant co-morbidity of COPD. 29 , 30 Patients who were awaiting discharge from hospital following an exacerbation of COPD were found to perform significantly worse on a range of cognitive functional measures than a matched group with stable COPD, a finding that persisted 3 months later. 29 Cognitive impairment may contribute independently to the risk for future exacerbations by increasing the likelihood of incorrect inhaler device use and failure to adhere to recommended treatments. 29
Given that this patient resides in a remote location, access to case management services that assist in preventing future exacerbations may be difficult or impossible to arrange. Although there is currently insufficient evidence that in general the use of telemonitoring contributes to the prevention of exacerbations of COPD, 2 tele-health care for this remotely located patient has potential to allow for case management at a distance, with minimal risk to the patient. Further study is needed to address this potential benefit.
Assessing for and managing frailty
Recognising this patient’s co-morbid diagnoses of coronary heart disease and osteoarthritis, careful assessment of functional and self-care abilities would be appropriate. Almost 60% of older adults with COPD meet the criteria for frailty. 31 Frailty is defined as a dynamic state associated with decline of physiologic reserves in multiple systems and inability to respond to stressful insults. 32 Frailty is associated with an increased risk for institutionalisation and mortality. 33 , 34 Given the complex needs of those who are frail, screening this patient for frailty would constitute patient-centred and cost-effective care. Frailty assessment tools, such as the seven-point Clinical Frailty Index, 35 may provide structure to this assessment.
Admission to a hospital 50 miles away from our patient’s home last year for an exacerbation was stressful. Since his hospitalisation, this patient has experienced four additional exacerbations that have been managed at home in his remote community. It would be appropriate to explore the patient’s treatment wishes and determine whether the patient has chosen to refuse further hospitalisations. Our patient’s risk of dying is significant, with risk factors increasing the risk of short-term mortality following an exacerbation of COPD (GOLD Stage 4, age, male sex, confusion). 36 Mortality rates between 22 and 36% have been documented in the first and second years, respectively, following an exacerbation, 37 , 38 which also increase with the frequency and severity of hospitalisations. 39
Our patient has refused social help and does not want to be relocated from his home. Ageing in their own home is a key goal of many older adults. 40 , 41 Efforts to ensure that adequate resources to support the patient are available (and to support the daughter who is currently providing a lot of his care) will form an important part of the plan of care.
Case study 3: A 62-year-old woman with severe COPD admitted with an exacerbation
A 62-year-old lady is admitted for the third time this year with an acute exacerbation of her severe COPD. Her FEV 1 was 35% predicted at the recent outpatient visit. She retired from her job as a shop assistant 5 years ago because of her breathlessness and now devotes her time to her grandchildren who ‘exhaust her’ but give her a lot of pleasure.
She quit smoking 5 years ago. Over the years, her medication has increased, as nothing seemed to relieve her uncomfortable breathlessness, and, in addition to inhaled long-acting β 2 -agonist/ inhaled steroid combination and a long-acting anti-muscarinic, she is taking theophylline and carbocysteine, although she is not convinced of their beneficial effect. Oral steroid courses help her dyspnoea and she has taken at least six courses this year: she has an action plan and keeps an emergency supply of medication at home.
A secondary care perspective on the management strategy for this woman
Acute exacerbations of COPD have serious negative consequences for health care systems and patients. The risk of future events and complications, such as hospital admission and poor patient outcomes (disability and reduced health status), can be improved through a combination of non-pharmacological and pharmacological therapies. 2
Evaluation of the patient, risk assessment and adherence to medication
The essential first step in the management of this lady (as for any patient) includes a detailed medical evaluation. Our patient has a well-established diagnosis of COPD with severe airflow obstruction (GOLD grade 3), significant breathlessness that resulted in her retiring from her job, and recurrent exacerbations. She does not have significant co-morbidity, although this requires to be confirmed. Further to the medical evaluation, it is important to assess her actual disease management (medication and proper use) as well as making sure she has adopted a healthy lifestyle (smoking cessation, physical activities and exercise). Does she live in a smoke-free environment? Effect of and evidence for smoking cessation in the prevention of acute exacerbations of COPD is low, but evidence exists for a reduction in cough and phlegm after smoking cessation and less lung function decline upon sustained cessation. With respect to the medication, never assume that it is taken as prescribed. When asking the patient, use open questions such as ‘I would like to hear how you take your medication on a typical day?’ instead of ‘Did you take the medication as prescribed’. Open questions tend to elicit more useful and pertinent information, and invite collaboration. Asking the patient to demonstrate her inhalation technique shows you the way she uses her different inhalation devices.
Optimising the pharmacological therapy
The second step is to assess whether the patient is on optimal treatment to prevent exacerbations. In other words, can we do better helping the patient manage her disease and improving her well-being. As in the previous cases, vaccination, in particular, annual administration of the influenza vaccine, should be prescribed for this lady. We should evaluate other alternatives of pharmacological therapy that could improve symptoms, prevent exacerbations and reduce the use of repeated systemic corticosteroids with their important adverse effects (such as osteoporosis, cataracts, diabetes). Prescribing a PDE4 inhibitor (Grade 2A) or a long-term macrolide (Grade 2A) once a day would be a consideration for this lady. 2 As there is no superiority trial comparing these two medications, our preference will be based on potential side effects, as well as cost and access to treatment. For PDE4 inhibitors, there are limited data on supplemental effectiveness in patients with COPD and chronic bronchitis concurrently using inhaled therapies, and they potentially have side effects such as diarrhoea, nausea, headache and weight loss. The side effects tend to diminish over time, but some patients may have to discontinue the therapy. Long-term macrolides have been studied in COPD patients already treated with inhaled therapies and shown to be effective, although clinicians need to consider in their individual patients the potential for harm, such as prolongation of the QT interval, hearing loss and bacterial resistance. Furthermore, the duration (beyond 1 year) and exact dosage of macrolide therapy (for example, once daily versus three times per week) are unknown.
Making non-pharmacological therapy an essential part of the management
The third step, often neglected in the management of COPD patients, is non-pharmacological therapy. For this lady, we suggest self-management education with a written action plan and case management to improve how she deals with exacerbations (Grade 2B). 2 The expectation will not be to reduce exacerbations but to prevent emergency department visits and hospital admissions. However, despite general evidence of efficacy, 42 not all self-management interventions have been shown to be effective or to benefit all COPD patients 43 , 44 (some have been shown to be potentially harmful 44 ). The effectiveness of any complex intervention such as self-management in COPD critically depends on the health care professionals who deliver the intervention, as well on the patient and the health care system. The patient may not have the motivation or desire to change or to commit to an intensive programme. The individual patient’s needs, preferences and personal goals should inform the design of any intervention with a behavioural component. For this lady, it is essential to apply integrated disease management and to refer the patient to a pulmonary rehabilitation programme. Pulmonary rehabilitation has high value, including reducing the risk for hospitalisation in COPD patients with recent exacerbations (Grade 1C). 2 The most important benefits our patient can expect from participating in structured supervised exercise within pulmonary rehabilitation are improved health status, exercise tolerance and a reduction in dyspnoea (Grade 1A). 2 , 15 Pulmonary rehabilitation programmes provide clinicians with an opportunity to deliver education and self-management skills to patients with COPD, and are well established as a means of enhancing standard therapy to control and alleviate symptoms, optimise functional capacity and improve health-related quality of life.
Case study 4: A 52-year-old lady with moderate COPD—and possibly asthma
A 52-year-old lady attends to discuss her COPD and specifically the problem she is having with exacerbations and time ‘off sick’. She is a heavy smoker, and her progressively deteriorating lung function suggests that she has moderate COPD, although she also has a history of childhood asthma, and had allergic rhinitis as a teenager. Recent spirometry showed a typical COPD flow-volume loop, although she had some reversibility (250 ml and 20%) with a post-bronchodilator FEV 1 of 60% predicted.
She has a sedentary office job and, although she is breathless on exertion, this generally does not interfere with her lifestyle. The relatively frequent exacerbations are more troublesome. They are usually triggered by an upper respiratory infection and can take a couple of weeks to recover. She has had three exacerbations this winter, and as a result her employer is not happy with her sickness absence record and has asked her to seek advice from her general practitioner.
She has a short-acting β 2 -agonist, although she rarely uses it except during exacerbations. In the past, she has used an inhaled steroid, but stopped that some time ago as she was not convinced it was helping.
It is a welcome opportunity when a patient comes to discuss her COPD with a particular issue to address. With a history of childhood asthma, and serial COPD lung function tests, she has probably been offered many components of good primary care for COPD, but has not yet fully engaged with her management. We know that ~40% of people with COPD continue to smoke, and many are intermittent users of inhaled medications. 45 It is easy to ignore breathlessness when both job and lifestyle are sedentary.
Understanding her diagnosis and setting goals
Her readiness to engage can be supported by a move to structured collaborative care, enabling the patient to have the knowledge, resources and support to make the necessary changes. Much of this can be done by the primary care COPD team, including the pharmacist. Regular recall to maintain engagement is essential.
The combination of childhood asthma, rhinitis and a long history of smoking requires diagnostic review. This might include serial peak flows over 2 weeks to look for variability, and a chest X-ray, if not done recently, to rule out lung cancer as a reason for recent exacerbations. Her spirometry suggests moderate COPD, 1 , 46 but she also has some reversibility, not enough to place her in the asthma camp but, combined with her past medical history, being enough to explore an asthma COPD overlap syndrome. This is important to consider as it may guide decisions on inhaled medication, and there is evidence that lung function deteriorates faster in this group. 47 It is estimated that up to 20% of patients have overlap diagnoses, although the exact prevalence depends on the definition. 48
Reducing the frequency of exacerbations
Exacerbations in COPD are debilitating, often trigger hospital admission and hasten a progressive decline in pulmonary function. 2 Written information on interventions that can slow down the course of COPD and reduce the frequency and impact of exacerbations will help to support progressive changes in management.
Smoking cessation
Few people are unaware that cessation of smoking is the key intervention for COPD. Reducing further decline in lung function will slow down the severity of exacerbations. Finding a smoking cessation programme that suits her working life, exploring previous attempts at cessation, offering pharmacotherapy and a non-judgemental approach to further attempts at stopping are crucial.
Immunisations
Many, but not all, exacerbations of COPD are triggered by viral upper respiratory tract infections. Annual flu immunisation is a part of regular COPD care and reduces exacerbations and hospitalisation when flu is circulating (Grade 1B). Pneumococcal immunisation should be offered, although evidence for reducing exacerbations is weak; those with COPD will be at greater risk for pneumococcal infection. 2
Pulmonary rehabilitation
Pulmonary rehabilitation improves symptoms, quality of life and reduces hospital admission. 49 It is most efficacious in patients who are symptomatic (MRC dyspnoea scale 3 and above) and in terms of reducing exacerbations is most effective when delivered early after an exacerbation (Grade 1C). 2 The major hurdle is encouraging patients to attend, with most programmes showing an attrition rate of 30% before the first appointment, and high rates of non-completion. 45 , 50 Effective programmes that maintain the gains from aerobic exercise are more cost-effective than some of the inhaled medications in use (see Figure 3 ). 50
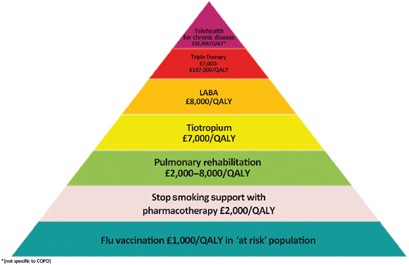
The COPD value pyramid (developed by the London Respiratory Network with The London School of Economics and reproduced with permission from the London Respiratory Team report 2013). 48 This 'value' pyramid reflects what we currently know about the cost per QALY of some of the commonest interventions in COPD. It was devised as a tool for health care organisations to use to promote audit and to ensure adequate commissioning of non-pharmacological interventions. COPD, chronic obstructive pulmonary disease; LABA, long-acting β-agonist; QALY, quality-adjusted life-year.
Inhaled medication is likely to improve our patient’s breathlessness and contribute to a reduction in exacerbation frequency. Currently, she uses only a short-acting β 2 -agonist. One wonders if she has a spacer? How much of the medicine is reaching her lungs? Repeated observation and training in inhaler use is essential if patients are to benefit from expensive medications.
With her history of asthma and evidence of some reversibility, the best choice of regular medication may be a combination of inhaled corticosteroid and a LABA. Guidelines suggest the asthma component in asthma COPD overlap syndrome should be the initial treatment target, 48 and a LABA alone should be avoided. Warn about oral thrush, and the increased risk for pneumonia. 46 If she chooses not to use an inhaled steroid, then a trial of a LAMA is indicated. Both drugs reduce exacerbation rates. 2 , 51
Finally, ensuring early treatment of exacerbations speeds up recovery. 52 Prescribe rescue medication (a 5–7-day course of oral steroids and antibiotic) to be started when symptomatic, and encourage attendance at a post-exacerbation review.
Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD. Updated 2014. http://www.goldcopd.org. Accessed January 2015.
Criner GJ, Bourbeau J, Diekemper RL, Ouellette DR, Goodridge D, Hernandez P et al. Prevention of acute exacerbations of chronic obstructive pulmonary disease: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest 2015; 147 : 894–942.
Article Google Scholar
Fletcher CM . Standardised questionnaire on respiratory symptoms: a statement prepared and approved by the MRC Committee on the Aetiology of Chronic Bronchitis (MRC breathlessness score). BMJ 1960; 2 : 1665.
Google Scholar
Jones PW, Harding G, Berry P, Wiklund I, Chen W-H, Leidy NK . Development and first validation of the COPD Assessment Test. Eur Respir J 2009; 34 : 648–654.
Article CAS Google Scholar
Gruffydd-Jones K . GOLD guidelines 2011: what are the implications for primary care? Prim Care Respir J 2012; 21 : 437–441.
Chong J, Karner C, Poole P . Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012; 9 : CD009157.
Cheyne L, Irvin-Sellers MJ, White J . Tiotropium versus ipratropium bromide for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2013; 9 : CD009552.
Bodenheimer T, Wagner EH, Grumbach K . Improving primary care for patients with chronic illness. JAMA 2002; 288 : 1775–1779.
World Health Organization. 23-valent pneumococcal polysaccharide vaccine. WHO position paper. Wkly Epidemiol Rec 2008; 83 : 373–384.
Center for Disease Control and Prevention Pneumococcal vaccination. http://www.cdc.gov/vaccines/vpd-vac/pneumo/ . Accessed January 2015.
Public Health England (2014a) Immunisation against infectious disease - "The Green Book". Chapter 19 - Influenza. https://www.gov.uk/government/collections/immunisation-against-infectious-disease-the-green-book . Accessed January 2015.
Public Health Agency of Canada. Canadian immunization guide: Pneumococcal vaccine, 2014. http://www.phac-aspc.gc.ca/publicat/cig-gci/p04-pneu-eng.php . Accessed January 2015.
Fletcher C, Peto R . The natural history of chronic airflow obstruction. Br Med J 1977; 1 : 1645–1648.
Hersh CP, DeMeo DL, Al-Ansari E, Carey VJ, Reilly JJ, Ginns LC et al. Predictors of survival in severe, early onset COPD. Chest 2004; 126 : 1443–1451.
Lacasse Y, Goldstein R, Lasserson TJ, Martin S . Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006; (4): CD003793.
Lacasse Y, Wong E, Guyatt GH, King D, Cook DJ, Goldstein RS . Meta-analysis of respiratory rehabilitation in chronic obstructive pulmonary disease. Lancet 1996; 348 : 1115–1119.
Lee T, Weaver F, Weiss K . Impact of pneumococcal vaccination on pneumonia rates in patients with COPD and asthma. J Gen Intern Med 2007; 22 : 62–67.
Crockett AJ, Cranston JM, Moss JR, Allpers JH . A review of long-term oxygen therapy for chronic obstructive pulmonary disease. Respir Med 2001; 95 : 437–443.
Newman SP . Inhaler treatment options in COPD. Eur Respir Rev 2005; 96 : 102–108.
Brooks D, Sottana R, Bell B, Hanna M, Laframboise L, Selvanayagarajah S et al. Characterization of pulmonary rehabilitation programs in Canada in 2005. Can Respir J 2007; 14 : 87–92.
Bickford LS, Hodgkin JE, McInturff SL . National pulmonary rehabilitation survey. Update. J Cardiopulm Rehabil 1995; 15 : 406–411.
Yohannes AM, Connolly MJ . Pulmonary rehabilitation programmes in the UK: a national representative survey. Clin Rehabil 2004; 18 : 444–449.
Marciniuk DD, Brooks D, Butcher S, Debigare R, Dechman G, Ford G et al. Optimizing pulmonary rehabilitation in chronic obstructive pulmonary disease – practical issues: a Canadian Thoracic Society Clinical Practice Guideline. Can Respir J 2010; 17 : 159–168.
Guell M, De-Lucas P, Galdiz J, Montemayor T, Rodriguez Gonzalez-Moro J, Gorostiza A . Home vs hospital-based pulmonary rehabilitation for patients with chronic obstructive pulmonary disease: disease: a Spanish multicentre trial. Arch Bronconeumol 2008; 44 : 512–518.
Maltais F, Bourbeau J, Shapiro S, Lacasse Y, Perrault H, Baltzan M . Effects of home-based pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2008; 149 : 869–878.
Puente-Maestu L, Sanz M, Sanz P, Cubillo J, Mayol J, Casaburi R . Comparison of effects of supervised versus self-monitored training programmes in patients with chronic obstructive pulmonary disease. Eur Respir J 2000; 15 : 517–525.
Strijbos J, Postma D, Van-Altena R, Gimeno F, Koeter G . A comparison between an outpatient hospital-based pulmonary rehabilitation program and a home-care pulmonary rehabilitation program in patients with COPD. A follow-up of 18 months. Chest 1996; 109 : 366–372.
Stickland M, Jourdain T, Wong EY, Rodgers WM, Jendzjowsky NG, MacDonald GF . Using Telehealth technology to deliver pulmonary rehabilitation in chronic obstructive pulmonary disease patients. Can Respir J 2011; 18 : 216–220.
Dodd JW, Getov SV, Jones PW . Cognitive function in COPD. Eur Respir J 2010; 35 : 913–922.
Cleutjens FA, Spruit MA, Ponds RW, Dijkstra JB, Franssen FM, Wouters EF et al. Cognitive functioning in obstructive lung disease: results from the United Kingdom biobank. J Am Med Dir Assoc 2014; 15 : 214–219.
Park SK, Richardson CR, Holleman RG, Larson JL . Frailty in people with COPD, using the National Health and Nutrition Evaluation Survey dataset (2003-2006). Heart Lung 2013; 42 : 163–170.
Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G . Untangling the concepts of disability, frailty and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 2004; 59 : 255–263.
Lahousse L, Maes B, Ziere G, Loth DW, Verlinden VJ, Zillikens MC et al. Adverse outcome of frailty in the elderly: the Rotterdam Study. Eur J Epidemiol 2014; 29 : 419–427.
Rockwood K, Howlett SE, MacKnight C, Beattie BL, Bergman H, Hebert R et al. Prevalence, attributes and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian study of health and aging. J Gerontol A Biol Sci Med Sci 2004; 59 : 1310–1317.
Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173 : 489–495.
Singanayagam A, Schembri S, Chalmers JD . Predictors or mortality in hospitalized adults with acute exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc 2013; 10 : 81–89.
Almagro P, Calbo E, Ochoa de Echaguen A, Barreiro B, Quintana S, Heredia JL et al. Mortality after hospitalization for COPD. Chest 2002; 121 : 1441–1448.
Groenewegen KH, Schols AM, Wouters EF . Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest 2003; 124 : 459–467.
Soler-Cataluna JJ, Martinez-Garcia MA, Sanchez PR, Salcedo E, Navarro M, Ochando R . Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005; 60 : 925–931.
Ball MM, Perkins MM, Whittington FJ, Connell BR, Hollingsworth C, King SV et al. Managing decline in assisted living: the key to aging in place. J Gerontol B Psychol Sci Soc Sci 2004; 59 : S202–S212.
Pynoos J, Caraviello R, Cicero C . Lifelong housing: the anchor in aging-friendly communities. Generations 2009; 33 : 26–32.
Zwerink M, Brusse-Keizer M, van der Valk PD, Zielhuis GA, Monninkhof EM, van der Palen J et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014; 3 : CD002990.
Bucknall CE, Miller G, Lloyd SM, Cleland J, McCluskey S, Cotton M et al. Glasgow supported self-management trial (GSuST) for patients with moderate to severe COPD: randomised controlled trial. BMJ 2012; 344 : e1060.
Fan VS, Gaziano JM, Lew R, Bourbeau J, Adams SG, Leatherman S et al. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: a randomized, controlled trial. Ann Intern Med 2012; 156 : 673–683.
Hull S, Mathur R, Lloyd-Owen S, Round T, Robson J . Improving outcomes for people with COPD by developing networks of general practices: evaluation of a quality improvement project in east London. NPJ Prim Care Respir Med 2014; 24 : 14082.
National Institute of Health and Clinical Excellence. Management of chronic obstructive pulmonary disease in adults in primary and secondary care. Updated 2010. NICE CG101. http://www.nice.org.uk/ . Accessed January 2015.
Gibson PG, Simpson JL . The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax 2009; 64 : 728–735.
Global INitiative for Asthma. Asthma, COPD and asthma-COPD overlap syndromes (ACOS). GINA 2014. http://www.ginasthma.org/documents/14 . Accessed January 2015.
Ries AL, Bauldoff GS, Carlin BW, Casaburi R, Emery CF, Mahler DA et al. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest 2007; 131 (5 Suppl): 4S–42S.
London Respiratory Team. Take a breath in, keep it in and now try to breathe again. NHS England, 2013. http://www.networks.nhs.uk/nhs-networks/london-respiratory-network . Accessed January 2015.
Guyatt G, Gutterman D, Baumann MH, Addrizzo-Harris D, Hylek EM, Phillips B et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians task force. Chest 2006; 129 : 174–181.
Wilkinson TM, Donaldson GC, Hurst JR, Seemungal TA, Wedzicha JA . Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004; 169 : 1298–1303.
Download references
Acknowledgements
The authors declare that no funding was received.
Author information
Authors and affiliations.
Agia Barbara Health Care Center, Heraklion, Crete, Greece
Ioanna Tsiligianni
Department of Thoracic Medicine, Clinic of Social and Family Medicine, University of Crete, Heraklion, Crete, Greece
Department of Medicine, College of Medicine, University of Saskatchewan, Saskatoon, SK, Canada
Donna Goodridge
Division of Respirology, Critical Care and Sleep Medicine, University of Saskatchewan, Saskatoon, SK, Canada
Darcy Marciniuk
Centre for Primary Care and Public Health, Blizard Institute, Queen Mary University of London, London, UK
Respiratory Epidemiology and Clinical Research Unit, Montreal Chest Institute, McGill University Health Centre, Montréal, QC, Canada
Jean Bourbeau
You can also search for this author in PubMed Google Scholar
Contributions
IT, DG and DM, JB, SH wrote the perspectives on case studies 1, 2, 3 and 4, respectively. The handling editor (Hilary Pinnock) collated and edited the individual sections.
Corresponding author
Correspondence to Jean Bourbeau .
Ethics declarations
Competing interests.
JB declares peer reviewed government grants (for conducting research in COPD self-management 'Living Well with COPD' and the longitudinal population-based Canadian Cohort Obstructive Lung Disease (CanCOLD) study) from Canadian Institute of Health Research Rx&D collaborative programme (Astra Zeneca, Boehringer- Ingelheim, GlaxoSmithKline, Merck, Nycomed, Novartis), Canadian Respiratory Research Network (CRRN), Respiratory Health Network of the FRQS and Research Institute of the MUHC. The remaining authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Reprints and permissions
About this article
Cite this article.
Tsiligianni, I., Goodridge, D., Marciniuk, D. et al. Four patients with a history of acute exacerbations of COPD: implementing the CHEST/Canadian Thoracic Society guidelines for preventing exacerbations. npj Prim Care Resp Med 25 , 15023 (2015). https://doi.org/10.1038/npjpcrm.2015.23
Download citation
Received : 22 February 2015
Accepted : 24 February 2015
Published : 07 May 2015
DOI : https://doi.org/10.1038/npjpcrm.2015.23
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Case study: 33-year-old female presents with chronic sob and cough.
Sandeep Sharma ; Muhammad F. Hashmi ; Deepa Rawat .
Affiliations
Last Update: February 20, 2023 .
- Case Presentation
History of Present Illness: A 33-year-old white female presents after admission to the general medical/surgical hospital ward with a chief complaint of shortness of breath on exertion. She reports that she was seen for similar symptoms previously at her primary care physician’s office six months ago. At that time, she was diagnosed with acute bronchitis and treated with bronchodilators, empiric antibiotics, and a short course oral steroid taper. This management did not improve her symptoms, and she has gradually worsened over six months. She reports a 20-pound (9 kg) intentional weight loss over the past year. She denies camping, spelunking, or hunting activities. She denies any sick contacts. A brief review of systems is negative for fever, night sweats, palpitations, chest pain, nausea, vomiting, diarrhea, constipation, abdominal pain, neural sensation changes, muscular changes, and increased bruising or bleeding. She admits a cough, shortness of breath, and shortness of breath on exertion.
Social History: Her tobacco use is 33 pack-years; however, she quit smoking shortly prior to the onset of symptoms, six months ago. She denies alcohol and illicit drug use. She is in a married, monogamous relationship and has three children aged 15 months to 5 years. She is employed in a cookie bakery. She has two pet doves. She traveled to Mexico for a one-week vacation one year ago.
Allergies: No known medicine, food, or environmental allergies.
Past Medical History: Hypertension
Past Surgical History: Cholecystectomy
Medications: Lisinopril 10 mg by mouth every day
Physical Exam:
Vitals: Temperature, 97.8 F; heart rate 88; respiratory rate, 22; blood pressure 130/86; body mass index, 28
General: She is well appearing but anxious, a pleasant female lying on a hospital stretcher. She is conversing freely, with respiratory distress causing her to stop mid-sentence.
Respiratory: She has diffuse rales and mild wheezing; tachypneic.
Cardiovascular: She has a regular rate and rhythm with no murmurs, rubs, or gallops.
Gastrointestinal: Bowel sounds X4. No bruits or pulsatile mass.
- Initial Evaluation
Laboratory Studies: Initial work-up from the emergency department revealed pancytopenia with a platelet count of 74,000 per mm3; hemoglobin, 8.3 g per and mild transaminase elevation, AST 90 and ALT 112. Blood cultures were drawn and currently negative for bacterial growth or Gram staining.
Chest X-ray
Impression: Mild interstitial pneumonitis
- Differential Diagnosis
- Aspiration pneumonitis and pneumonia
- Bacterial pneumonia
- Immunodeficiency state and Pneumocystis jiroveci pneumonia
- Carcinoid lung tumors
- Tuberculosis
- Viral pneumonia
- Chlamydial pneumonia
- Coccidioidomycosis and valley fever
- Recurrent Legionella pneumonia
- Mediastinal cysts
- Mediastinal lymphoma
- Recurrent mycoplasma infection
- Pancoast syndrome
- Pneumococcal infection
- Sarcoidosis
- Small cell lung cancer
- Aspergillosis
- Blastomycosis
- Histoplasmosis
- Actinomycosis
- Confirmatory Evaluation
CT of the chest was performed to further the pulmonary diagnosis; it showed a diffuse centrilobular micronodular pattern without focal consolidation.
On finding pulmonary consolidation on the CT of the chest, a pulmonary consultation was obtained. Further history was taken, which revealed that she has two pet doves. As this was her third day of broad-spectrum antibiotics for a bacterial infection and she was not getting better, it was decided to perform diagnostic bronchoscopy of the lungs with bronchoalveolar lavage to look for any atypical or rare infections and to rule out malignancy (Image 1).
Bronchoalveolar lavage returned with a fluid that was cloudy and muddy in appearance. There was no bleeding. Cytology showed Histoplasma capsulatum .
Based on the bronchoscopic findings, a diagnosis of acute pulmonary histoplasmosis in an immunocompetent patient was made.
Pulmonary histoplasmosis in asymptomatic patients is self-resolving and requires no treatment. However, once symptoms develop, such as in our above patient, a decision to treat needs to be made. In mild, tolerable cases, no treatment other than close monitoring is necessary. However, once symptoms progress to moderate or severe, or if they are prolonged for greater than four weeks, treatment with itraconazole is indicated. The anticipated duration is 6 to 12 weeks total. The response should be monitored with a chest x-ray. Furthermore, observation for recurrence is necessary for several years following the diagnosis. If the illness is determined to be severe or does not respond to itraconazole, amphotericin B should be initiated for a minimum of 2 weeks, but up to 1 year. Cotreatment with methylprednisolone is indicated to improve pulmonary compliance and reduce inflammation, thus improving work of respiration. [1] [2] [3]
Histoplasmosis, also known as Darling disease, Ohio valley disease, reticuloendotheliosis, caver's disease, and spelunker's lung, is a disease caused by the dimorphic fungi Histoplasma capsulatum native to the Ohio, Missouri, and Mississippi River valleys of the United States. The two phases of Histoplasma are the mycelial phase and the yeast phase.
Etiology/Pathophysiology
Histoplasmosis is caused by inhaling the microconidia of Histoplasma spp. fungus into the lungs. The mycelial phase is present at ambient temperature in the environment, and upon exposure to 37 C, such as in a host’s lungs, it changes into budding yeast cells. This transition is an important determinant in the establishment of infection. Inhalation from soil is a major route of transmission leading to infection. Human-to-human transmission has not been reported. Infected individuals may harbor many yeast-forming colonies chronically, which remain viable for years after initial inoculation. The finding that individuals who have moved or traveled from endemic to non-endemic areas may exhibit a reactivated infection after many months to years supports this long-term viability. However, the precise mechanism of reactivation in chronic carriers remains unknown.
Infection ranges from an asymptomatic illness to a life-threatening disease, depending on the host’s immunological status, fungal inoculum size, and other factors. Histoplasma spp. have grown particularly well in organic matter enriched with bird or bat excrement, leading to the association that spelunking in bat-feces-rich caves increases the risk of infection. Likewise, ownership of pet birds increases the rate of inoculation. In our case, the patient did travel outside of Nebraska within the last year and owned two birds; these are her primary increased risk factors. [4]
Non-immunocompromised patients present with a self-limited respiratory infection. However, the infection in immunocompromised hosts disseminated histoplasmosis progresses very aggressively. Within a few days, histoplasmosis can reach a fatality rate of 100% if not treated aggressively and appropriately. Pulmonary histoplasmosis may progress to a systemic infection. Like its pulmonary counterpart, the disseminated infection is related to exposure to soil containing infectious yeast. The disseminated disease progresses more slowly in immunocompetent hosts compared to immunocompromised hosts. However, if the infection is not treated, fatality rates are similar. The pathophysiology for disseminated disease is that once inhaled, Histoplasma yeast are ingested by macrophages. The macrophages travel into the lymphatic system where the disease, if not contained, spreads to different organs in a linear fashion following the lymphatic system and ultimately into the systemic circulation. Once this occurs, a full spectrum of disease is possible. Inside the macrophage, this fungus is contained in a phagosome. It requires thiamine for continued development and growth and will consume systemic thiamine. In immunocompetent hosts, strong cellular immunity, including macrophages, epithelial, and lymphocytes, surround the yeast buds to keep infection localized. Eventually, it will become calcified as granulomatous tissue. In immunocompromised hosts, the organisms disseminate to the reticuloendothelial system, leading to progressive disseminated histoplasmosis. [5] [6]
Symptoms of infection typically begin to show within three to17 days. Immunocompetent individuals often have clinically silent manifestations with no apparent ill effects. The acute phase of infection presents as nonspecific respiratory symptoms, including cough and flu. A chest x-ray is read as normal in 40% to 70% of cases. Chronic infection can resemble tuberculosis with granulomatous changes or cavitation. The disseminated illness can lead to hepatosplenomegaly, adrenal enlargement, and lymphadenopathy. The infected sites usually calcify as they heal. Histoplasmosis is one of the most common causes of mediastinitis. Presentation of the disease may vary as any other organ in the body may be affected by the disseminated infection. [7]
The clinical presentation of the disease has a wide-spectrum presentation which makes diagnosis difficult. The mild pulmonary illness may appear as a flu-like illness. The severe form includes chronic pulmonary manifestation, which may occur in the presence of underlying lung disease. The disseminated form is characterized by the spread of the organism to extrapulmonary sites with proportional findings on imaging or laboratory studies. The Gold standard for establishing the diagnosis of histoplasmosis is through culturing the organism. However, diagnosis can be established by histological analysis of samples containing the organism taken from infected organs. It can be diagnosed by antigen detection in blood or urine, PCR, or enzyme-linked immunosorbent assay. The diagnosis also can be made by testing for antibodies again the fungus. [8]
Pulmonary histoplasmosis in asymptomatic patients is self-resolving and requires no treatment. However, once symptoms develop, such as in our above patient, a decision to treat needs to be made. In mild, tolerable cases, no treatment other than close monitoring is necessary. However, once symptoms progress to moderate or severe or if they are prolonged for greater than four weeks, treatment with itraconazole is indicated. The anticipated duration is 6 to 12 weeks. The patient's response should be monitored with a chest x-ray. Furthermore, observation for recurrence is necessary for several years following the diagnosis. If the illness is determined to be severe or does not respond to itraconazole, amphotericin B should be initiated for a minimum of 2 weeks, but up to 1 year. Cotreatment with methylprednisolone is indicated to improve pulmonary compliance and reduce inflammation, thus improving the work of respiration.
The disseminated disease requires similar systemic antifungal therapy to pulmonary infection. Additionally, procedural intervention may be necessary, depending on the site of dissemination, to include thoracentesis, pericardiocentesis, or abdominocentesis. Ocular involvement requires steroid treatment additions and necessitates ophthalmology consultation. In pericarditis patients, antifungals are contraindicated because the subsequent inflammatory reaction from therapy would worsen pericarditis.
Patients may necessitate intensive care unit placement dependent on their respiratory status, as they may pose a risk for rapid decompensation. Should this occur, respiratory support is necessary, including non-invasive BiPAP or invasive mechanical intubation. Surgical interventions are rarely warranted; however, bronchoscopy is useful as both a diagnostic measure to collect sputum samples from the lung and therapeutic to clear excess secretions from the alveoli. Patients are at risk for developing a coexistent bacterial infection, and appropriate antibiotics should be considered after 2 to 4 months of known infection if symptoms are still present. [9]
Prognosis
If not treated appropriately and in a timely fashion, the disease can be fatal, and complications will arise, such as recurrent pneumonia leading to respiratory failure, superior vena cava syndrome, fibrosing mediastinitis, pulmonary vessel obstruction leading to pulmonary hypertension and right-sided heart failure, and progressive fibrosis of lymph nodes. Acute pulmonary histoplasmosis usually has a good outcome on symptomatic therapy alone, with 90% of patients being asymptomatic. Disseminated histoplasmosis, if untreated, results in death within 2 to 24 months. Overall, there is a relapse rate of 50% in acute disseminated histoplasmosis. In chronic treatment, however, this relapse rate decreases to 10% to 20%. Death is imminent without treatment.
- Pearls of Wisdom
While illnesses such as pneumonia are more prevalent, it is important to keep in mind that more rare diseases are always possible. Keeping in mind that every infiltrates on a chest X-ray or chest CT is not guaranteed to be simple pneumonia. Key information to remember is that if the patient is not improving under optimal therapy for a condition, the working diagnosis is either wrong or the treatment modality chosen by the physician is wrong and should be adjusted. When this occurs, it is essential to collect a more detailed history and refer the patient for appropriate consultation with a pulmonologist or infectious disease specialist. Doing so, in this case, yielded workup with bronchoalveolar lavage and microscopic evaluation. Microscopy is invaluable for definitively diagnosing a pulmonary consolidation as exemplified here where the results showed small, budding, intracellular yeast in tissue sized 2 to 5 microns that were readily apparent on hematoxylin and eosin staining and minimal, normal flora bacterial growth.
- Enhancing Healthcare Team Outcomes
This case demonstrates how all interprofessional healthcare team members need to be involved in arriving at a correct diagnosis. Clinicians, specialists, nurses, pharmacists, laboratory technicians all bear responsibility for carrying out the duties pertaining to their particular discipline and sharing any findings with all team members. An incorrect diagnosis will almost inevitably lead to incorrect treatment, so coordinated activity, open communication, and empowerment to voice concerns are all part of the dynamic that needs to drive such cases so patients will attain the best possible outcomes.
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Histoplasma Contributed by Sandeep Sharma, MD
Disclosure: Sandeep Sharma declares no relevant financial relationships with ineligible companies.
Disclosure: Muhammad Hashmi declares no relevant financial relationships with ineligible companies.
Disclosure: Deepa Rawat declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Sharma S, Hashmi MF, Rawat D. Case Study: 33-Year-Old Female Presents with Chronic SOB and Cough. [Updated 2023 Feb 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Review Palliative Chemotherapy: Does It Only Provide False Hope? The Role of Palliative Care in a Young Patient With Newly Diagnosed Metastatic Adenocarcinoma. [J Adv Pract Oncol. 2017] Review Palliative Chemotherapy: Does It Only Provide False Hope? The Role of Palliative Care in a Young Patient With Newly Diagnosed Metastatic Adenocarcinoma. Doverspike L, Kurtz S, Selvaggi K. J Adv Pract Oncol. 2017 May-Jun; 8(4):382-386. Epub 2017 May 1.
- Review Breathlessness with pulmonary metastases: a multimodal approach. [J Adv Pract Oncol. 2013] Review Breathlessness with pulmonary metastases: a multimodal approach. Brant JM. J Adv Pract Oncol. 2013 Nov; 4(6):415-22.
- A 50-Year Old Woman With Recurrent Right-Sided Chest Pain. [Chest. 2022] A 50-Year Old Woman With Recurrent Right-Sided Chest Pain. Saha BK, Bonnier A, Chong WH, Chenna P. Chest. 2022 Feb; 161(2):e85-e89.
- Suicidal Ideation. [StatPearls. 2024] Suicidal Ideation. Harmer B, Lee S, Duong TVH, Saadabadi A. StatPearls. 2024 Jan
- Review Domperidone. [Drugs and Lactation Database (...] Review Domperidone. . Drugs and Lactation Database (LactMed®). 2006
Recent Activity
- Case Study: 33-Year-Old Female Presents with Chronic SOB and Cough - StatPearls Case Study: 33-Year-Old Female Presents with Chronic SOB and Cough - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
It seems you are using an outdated browser. Please upgrade to a modern browser to improve your experience on this website.

A COPD Case Study: Susan M.

This post was written by Jane Martin, BA, LRT, CRT, Assistant Director of Education at the COPD Foundation .
Meet Susan M! Share your impressions in our latest COPD case study.
Summary of in-patient admission: Susan M. is being discharged today following a 6-day ICU and step-down admission for acute exacerbation of COPD with bacterial pneumonia requiring intubation and mechanical ventilation for a period of 32 hours. Subsequent to her extubation and transfer to the step down unit she was treated with oral antibiotics and Albuterol and Ipratropium nebulizer q 4 hrs. and prn at noc.
Past utilization: Susan was admitted to the hospital for eight days last winter for acute exacerbation of COPD with bacterial pneumonia requiring 48-hour intubation and mechanical ventilation. Since then she has been seen in the ER x 2 for extreme shortness of breath with anxiety with no evidence of infection. On both occasions her shortness of breath subsided with nebulizer treatments, low flow O2, and coaching in relaxation techniques. On one of those visits Susan reported that her shortness of breath “ramped up” when she was unable to contact her daughter who, at the time, was driving alone, long-distance. “I worry a lot. I try to tell myself not to worry, but I just can’t help it.”
Medical history: COPD, systemic hypertension, hip replacement 5 years ago. FEV1 is 50% normal predicted. 35-pack year cigarette history, quit at age 50. Bone density: T score: -2 (low bone density possibly leading to osteoporosis).
Family history: Father died of stroke at age 80, mother died of injuries due to a MVA at age 75. Has three adult children with no known medical problems.
CXR at discharge: Mild hyperinflation, no pneumonia.
Pulse oximetry: Room air 95%.
Height: 65” Weight: 130 lbs. Susan has lost 5 lbs. within the last year with no intention of losing weight.
Psych/Social: Widowed. Lives alone. Husband died of internal injuries following a MVA 2 years ago. Spends meal times alone. “I used to make big meals when everybody was here but now, why make a big deal out of cooking when I’m the only one?” Our youngest son and his family live 15 miles away. “They’re so busy, I hate to bother them.” Susan drives her car only during the day and when “absolutely necessary,” sometime not leaving the house for up to 6 days at a time.
Here are a few questions for your consideration.
- What are your impressions?
- What are your post-acute recommendations for this patient?
- What follow up would you conduct with this patient and within what time frame?
- What education would you ensure this patient has at discharge?
- Would you recommend any consults in addition to nutrition and behavioral health?
Share your thoughts in the comments below!
This page was reviewed on March 3, 2020 by the COPD Foundation Content Review and Evaluation Committee
12 Comments
Join Us on COPD360social
Join the Conversation
Already a Member?
- Open access
- Published: 13 December 2023
The patient journey in Chronic Obstructive Pulmonary Disease (COPD): a human factors qualitative international study to understand the needs of people living with COPD
- Nicola Scichilone 1 ,
- Andrew Whittamore 2 ,
- Chris White 3 ,
- Elena Nudo 4 ,
- Massimo Savella 4 &
- Marta Lombardini 4
BMC Pulmonary Medicine volume 23 , Article number: 506 ( 2023 ) Cite this article
2372 Accesses
1 Altmetric
Metrics details
Chronic obstructive pulmonary disease (COPD) is a common condition that causes irreversible airway obstruction. Fatigue and exertional dyspnoea, for example, have a detrimental impact on the patient’s daily life. Current research has revealed the need to empower the patient, which can result in not only educated and effective decision-making, but also a considerable improvement in patient satisfaction and treatment compliance.
The current study aimed to investigate the perspectives and requirements of people living with COPD to possibly explore new ways to manage their disease.
Adults with COPD from 8 European countries were interviewed by human factor experts to evaluate their disease journey through the gathering of information on the age, performance, length, and impact of diagnosis, symptoms progression, and family and friends' reactions. The assessment of present symptoms, services, and challenges was performed through a 90-min semi-structured interview. To identify possible unmet needs of participants, a generic thematic method was used to explore patterns, themes, linkages, and sequences within the data collected. Flow charts and diagrams were created to communicate the primary findings. Following analysis, the data was consolidated into cohesive insights and conversation themes relevant to determining the patient's unmet needs.
The 62, who voluntarily accepted to be interviewed, were patients (61% females, aged 32–70 years) with a COPD diagnosis for at least 6 months with stable symptoms of different severity. The main challenges expressed by the patients were the impact on their lifestyle, reduced physical activity, and issues with their mobility. About one-fourth had challenges with their symptoms or medication including difficulty in breathing. Beyond finding a cure for COPD was the primary goal for patients, their main needs were to receive adequate information on the disease and treatments, and to have adequate support to improve physical activity and mobility, helpful both for patients and their families.
Conclusions
These results could aid in the creation of new ideas and concepts to improve our patient’s quality of life, encouraging a holistic approach to people living with COPD and reinforcing the commitment to understanding their needs.
Peer Review reports
Introduction
Chronic obstructive pulmonary disease (COPD) is defined by irreversible airway obstruction linked with comorbidities or systemic effects [ 1 ]. COPD is a worldwide epidemic that contributes significantly to healthcare expenses due to high morbidity and mortality rates [ 2 , 3 ]. The clinical assessment of fixed airflow limitation and symptoms such as coughing and wheezing determine a COPD diagnosis; nevertheless, COPD symptoms negatively impact the patient's daily activities and lifestyle [ 4 ]. Patients may encounter a variety of debilitating physical symptoms, resulting in functional loss and high degrees of psychosocial anguish [ 5 , 6 , 7 ].
Integrated approaches to disease assessment and management are required to better understand and address the burden of COPD symptoms from a patient's perspective [ 8 ].
According to a recent observational study, regardless of disease severity, more than half of COPD patients experienced symptoms during the whole 24-h day, and almost 80% of patients reported experiencing symptoms at least twice a day. Symptoms are linked to poor health, depression, anxiety, and poor sleep quality [ 9 , 10 ].
Patients with COPD and comorbidities remain particularly challenging to manage because in Europe there is, generally, no guidance at the national level except in the UK, Slovenia, and Germany [ 11 , 12 , 13 ]. In Nordic countries and France, the management of patients with COPD is mainly performed by general practitioners with an inadequate level of assistance [ 14 , 15 , 16 ]. In other countries, patient management is performed at the discretion of the local structures, and the need for a comprehensive, holistic approach is looked forward [ 17 , 18 , 19 , 20 ]. Other chronic conditions increase symptom load, impair functional performance, and negatively impact health status; thus, management strategies must be adjusted accordingly [ 10 ].
Care plans, within the healthcare system, emphasize the importance of addressing these patients' particular physical, psychological, social, and spiritual needs through holistic supportive input offered as person-centered care [ 21 ]. Understanding the patient's perspective on their support requirements (those areas of living with COPD for which they require assistance, such as help controlling symptoms or accessing financial benefits) is critical to facilitating this approach. A recent systematic literature review has identified a whole range of support needs for COPD patients, based on the perspectives of the patients themselves [ 7 ].
Our human factor study aims to explore how COPD has affected the patients’ daily lives and the lives of those around them, through the assessment of symptoms, treatment, and service availability, identifying what challenges the patient faces in living with COPD, and which are the unmet needs in the different stages of the journey of care.
This human factors COPD patient needs study was conducted in November 2022 by an ISO 13485 certified specialist human factors consultant (Rebus Medical Ltd), both in-person or remotely, via video call using the Zoom platform. Remote interviews were needed to enable more severe patients to attend the sessions and to ensure that the intended study sample was achieved. As for other qualitative analyses, a minimum of 48 participants were planned to be interviewed.
Interviews were conducted on a 1–1 basis, with patients who voluntarily accepted to be interviewed from 8 countries: Denmark, France, Germany, Italy, Slovenia, Spain, Sweden, and the UK. Each interview was 90 min long and followed a semi-structured approach allowing for unscripted discussion when the participants’ responses raised new questions. For interviews that took place outside of the UK, a native-speaking moderator conducted the interview, whilst an interpreter translated the conversation live to a data analyst (Fig. 1 ).
Summary of the study methods. Countries involved in the study are indicated in grey
Participants included in the study, aged 18 years or older, with a current COPD diagnosis, were screened for COPD severity according to GOLD criteria-2020-document [ 22 ] and voluntarily provided their informed consent.
Because the objectives were connected to identifying unmet requirements through video conference, the formative interviews were deemed low to minimal risk to participants and, thus, no formal approval to an Ethical Committee was required.
For interviews conducted in a language other than English, a simultaneous translator was recruited to enable a member of Rebus Medical staff to watch the interview listen to the translation, and record notes. Digital video recordings were collected to accurately account for each test session. Notes were verified at the end of each interview, while participant faces recorded on the videos were blurred to anonymize the footage. When all interviews were complete, the raw notes from each interview were collated and verified using the recorded videos in a master data capture spreadsheet.
The interviews were conducted to evaluate the journey of care through the collection of information on the gender, age, performance, length, and impact of diagnosis, symptoms progression, and family and friends’ reactions through questions that were designed on purpose to identify the unmet need and main challenges of each step of the patient’s journey. The evaluation of the current symptoms (fluctuations, flare-ups, alleviations, effect on sleep and daily activities including the use of electronic devices), services (health care providers support, insurance, available information on COPD), and challenges (in lifestyle, daily activities, treatments, symptoms management, emotional and environmental) was included in the semi-structured interview (Table 1 ).
As this was an exploratory insight interview, protocol deviations like alterations to the interviewer’s script to reformulate questions, ad hoc addition of questions and probes to the interviewer’s script to focus on points of interest specific to each participant, and changes to the interviewer’s script as the study progresses to allow for study learnings were permitted and expected.
A generic thematic approach was employed to uncover patterns, themes, links, and sequences within the data collected to identify probable unmet needs of participants through the patient journey of people living with COPD.
To communicate the major findings, flow charts, and diagrams were constructed. Following analysis, the data were synthesized and refined into cohesive insights and discussion themes pertinent to identifying the patient's unmet needs along the different stages of the patient journey.
A total of 62 patients (38—61% females) with COPD aged between 32 and 70 years ( N = 1 aged 25–40 years, N = 42 aged 41–65 years, N = 19 aged > 65 years) were interviewed. Most of the patients (35—56%) had severe COPD (Table 2 ).
Current- or past smokers were 49 (80%) of the 61 respondents. A larger proportion of patients with severe COPD (9/35, 26%) had never smoked compared to the moderate COPD patient group (3/27, 11%); in fact, 26 (74%) severe patients and 24 (89%) moderate were smokers or had smoked in the past (Fig. 2 ).
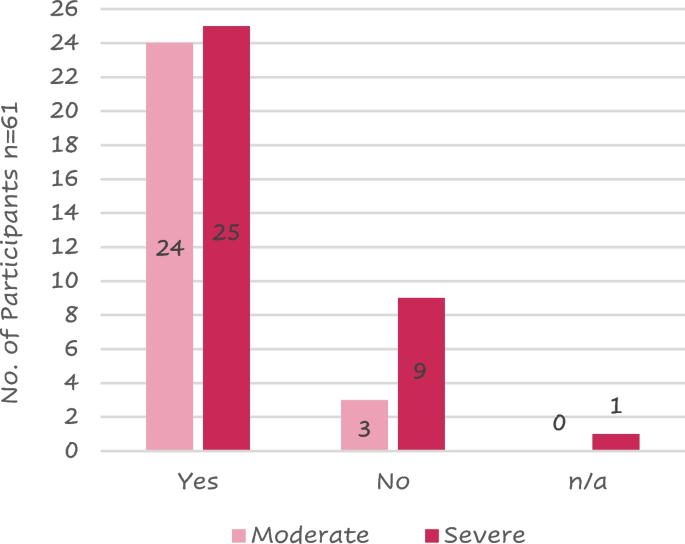
Distribution of patients that have ever been a smoker against COPD severity
Legend: n/a = not available
Patient journey
A total of 113 symptoms of COPD were recorded because most patients reported more than 1 symptom at the onset of the disease; 78 (69%) of these symptoms were related to dyspnoea. The highest reported symptoms were difficulty breathing and coughing (Fig. 3 ).
Patient’s reported signs and symptoms leading to COPD diagnosis
Note—Other includes chest tightness, hereditary respiratory issues, persistent flare ups, unable to walk upstairs, difficulty talking, difficulty walking, difficulty swallowing, bronchitis as a child and headaches
Fourteen (30%) of the 46 respondents referred to being diagnosed with COPD more than 1 year after initial symptoms, while 6 (13%) were diagnosed from 7 to 12 months from the onset of symptoms. Ten (64%) of the 14 requiring > 1 year for their diagnosis had severe COPD.
Most of the 56 patients who answered (41 – 73%) were diagnosed by a lung specialist mainly using spirometry (Fig. 4 ).
Tests performed at the visit of diagnosis
Legend: FR = France, GE = Germany, IT = Italy, SL = Slovenia, SP = Spain, NO = Northern (Sweden Denmark), UK = United Kingdom. “Other” includes: MRI, pressure cabin test, swabs collected, endoscope to check lungs, chamber, PET scan, Blood taken from the ear, blood gas test, oxygen saturation, walking/ running tests, echocardiogram, pulse oximeter/O 2 saturation, sleep test
About half of the responders (23 of 45 – 51%) felt their symptoms stable from the diagnosis (Fig. 5 ).
Symptom progression
Legend: FR = France, GE = Germany, IT = Italy, SL = Slovenia, SP = Spain, NO = Northern (Sweden Denmark), UK = United Kingdom, n/a = not available
Thirteen (29%) of those interviewed stated that their family and friends were supportive at the time of COPD diagnosis while 8 (18%) were worried about the diagnosis. Seven of them received no reaction from their family or friends and a further 7 did not tell anyone about their diagnosis. ‘Other’ reactions that were received from family and friends included: acceptance, anger, fear, shock, anguish, and expected, while some patients “prefer not to speak about it”.
The COPD diagnosis hurt 26 (58%) of the responders who described a negative impact of their COPD diagnosis, mainly because of their inability to be active, while 13 of them (29%) felt a positive impact mainly because they stopped or reduced smoking (Table 3 ).
Six (19%) of the 31 patients who provided details on the reason for quitting smoking reported they received more information about how to give up smoking and the risks associated with smoking, 3 patients mentioned some form of medication to support smoking cessation may have helped them give up, and 2 patients reported that they would give up for a family member but would struggle to have the motivation to do it themselves. Three patients reported that nothing would have helped them stop smoking while 8 patients reported that, despite knowing the impact smoking has, they still chose to smoke. Other suggestions to stop smoking reported by participants included: the threat of death, vaping if the smoking affected their fitness, cigarettes stopped being sold, stopping because of asthma and its diagnosis, quitting when they were in the hospital for a week giving it up after then, or because the smell was horrible.
A total of 59 patients answered about their changes in symptoms throughout the day; seventeen (29%) felt no changes while 13 (22%) worsened in the morning, 11 (19%) worsened at night, and 6 (10%) worsened both in the morning and at night.
Twenty-five (41%) of the 61 responders were hospitalized due to a COPD flare-up at least once after their COPD diagnosis; most of them had severe disease (Fig. 6 ).
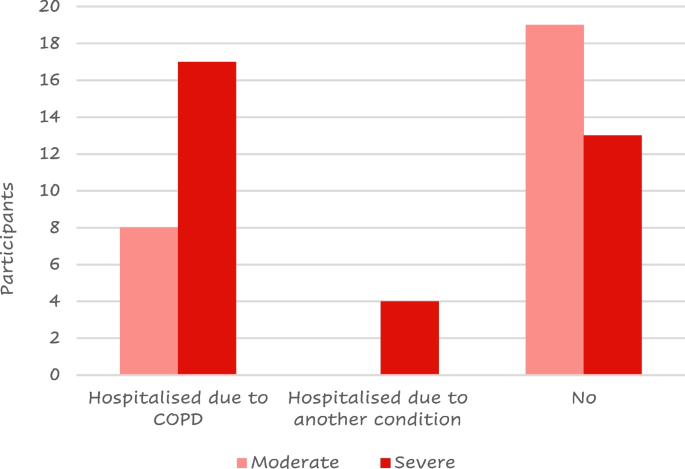
Number of patients that have experienced a COPD flare-up by COPD severity
Seven (30%) of the 23 patients who took any action to alleviate their symptoms, before seeing a doctor and getting a diagnosis, reduced their physical exercise to not trigger symptoms. While others were more vigilant with their health, received help from family and friends, or used inhalers, a rescue pack, or menthol sweets.
Thirty seven out of 58 participants reported sleep disruption. Of these, 12 (32%), reported disruption due to COPD while 10 (17%) had sleep negatively affected by another condition. Other causes for patients’ sleep disruption included coughing, the need to change sleeping positions, and cold weather.
Patients reported needing more support including more information about their condition, financial support for transportation, improved treatment options, accessibility badges, and help in carrying out chores in the house such as cooking, cleaning, and general housekeeping. Some patients also indicated a wish for personal training. Some patients were unaware of what type of support they may require or what type of support could be available to them while others were looking for a different inhaler or treatment to alleviate their cough or a device that assists deep breathing, transplant, a dog or a sport requiring a limited physical effort that would help them be more active, and/or meeting a COPD support group.
About half of the respondents (26/56 – 46%) used electronic devices to monitor their health status including a finger pulse oximeter ( n = 9), smartwatch ( n = 8), or a blood pressure cuff ( n = 5).
A total of 64 responses were collected from the 58 patients who shared their opinion on the treatment they were utilizing; 33 (52%) of the feedback was positive (Table 4 ).
While 20 (31%) of the respondents felt neutral about their current prescribed treatment, 11 (17%) reported either that their medicine had "no therapeutic impact", that they faced "psychological restraint" with their prescribed regime, or that they had issues with treatment compliance.
Six (12%) of the 52 respondents confirmed using digital or analogic reminders to take their dose. Three patients were currently using a dose counter on their device to remind them if their doses had not been taken, and two patients were using a timer on their mobile phones to remind them when their next dose was due. One participant used digital/analogic support but did not indicate which.
The main strategies used to remind them to take their medication include:
leaving the medication in a specific location to prompt them to take their dose at the correct time,
relying on habit or routine to prompt them to take their medication,
taking the COPD medication at the same time as other medications,
feeling unwell to prompt themselves to take their medication.
A total of 32 (56%) of the 57 respondents reported missing a medicine dose; eight of them cited a change in their schedule or routine as its cause. Other reasons for missing a dose reported by patients included: not taking the medication seriously, forgetting to take their dose in the evening, forgetting to bring their medication with them when leaving the house, a change in their environment, a missed medication delivery, and “not taking regular doses”.
The primary reasons why patients appreciate their present treatments were the drug's functionality ( n = 18), the device design ( n = 10), the convenience of use ( n = 8), and the medication's quick and uncomplicated administration ( n = 5). Other patients expressed liking for current medication including feeling comfortable with their present treatment, feeling in charge, and independence.
On the other hand, the device design ( n = 14), the necessity to take their medication ( n = 8), and the side effects of the drug ( n = 5) were the most reported characteristics that patients disliked therapy. Other reported reasons included uncertainty about what the treatment is supposed to do, a sense of guilt when their medication is forgotten, the fact that they are still limited in their activity, and the sensation or taste inside their mouth. Three patients stated that they did not enjoy their current prescribed treatment. "You have to accept what is available," one patient said. Other patients referred detest having to take their medications daily.
About two-thirds ( n = 34 – 67%) of those polled ( n = 51) claimed no involvement with the selection of their present treatment option.
Most of the patients ( n = 42 – 69% of the 61 respondents) reported receiving training for the use of their current treatment. The remaining 31% of the patients did not receive any training, reporting that they “would have liked more formal training, the current device is more complex”, or believed it “could have been useful to receive training and would have loved the explanation, demo training”. Three patients also stated that they did not need training, whether they received it or not.
Twenty-two (52%) of the 42 patients that received training, thought that it was effective and only 5 (12%) did not believe their training to be effective. Fifteen (36%) of patients who received training did not provide feedback on the efficacy of the training they received.
Eight Italian patients reported receiving instruction mostly from a lung specialist, while the majority of British ( n = 5) and Nordic ( n = 4) patients reported receiving training primarily from a nurse (Fig. 7 ); this is probably due to the different structures of the national health systems.
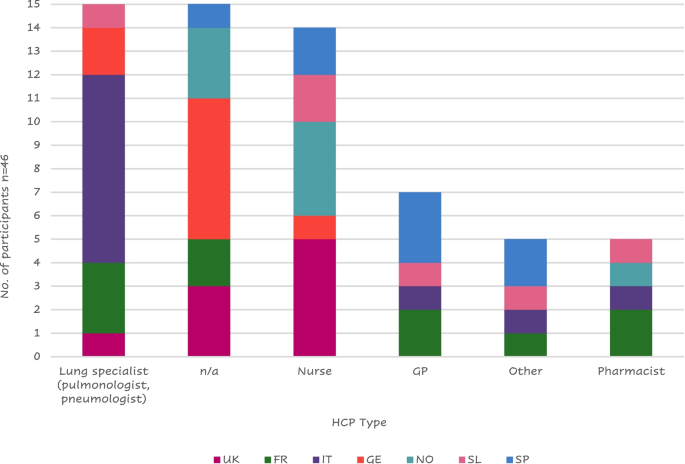
Health care provider (HCP) that administered training to patients by country
Legend: FR = France, GE = Germany, GP = General Practitioner; IT = Italy, SL = Slovenia, SP = Spain, n/a = not applicable; NO = Northern (Sweden Denmark), UK = United Kingdom
One Italian patient stated he received no specific training but was told by his pneumologist to look inside the package and read the instructions; a Frenchman mentioned that his wife was a doctor, so she just showed him how to use the device. Other participants’ training was received at meetings of a lung association from the pharmacists or at a live course organized by the doctor or during rehabilitation.
Six (18% of the 34 respondents) received help from their family or friends to find training materials or treatment information. Most patients received help to find further information and one participant mentioned that he was able to speak to a relative with COPD.
Six (15%) of the 41 respondents had gone online for help with their equipment (looking for tutorials online on forums and finding animated videos on how to use their inhalers). The main reasons for not using the internet for support were a lack of trust in online information ("would rather trust a doctor than go online"), an unwillingness to read more about their condition due to a fear of "reading too much" and becoming "depressed" if they investigated their disease. Other patients did not feel the need for additional support from the internet because their devices were "easy to use" or they wouldn't need further support due to their disease. One patient stated that he looked online and "found it strange that the messages were exclusively for persons with moderate to severe COPD, with only a few messages from people with mild COPD".
Lung specialists were the health care providers (HCPs) who most frequently provided support to patients with COPD ( n = 24/60—40%) followed by general practitioners (23 – 38%) (Fig. 8 ); only 3 patients reported not having received any support.
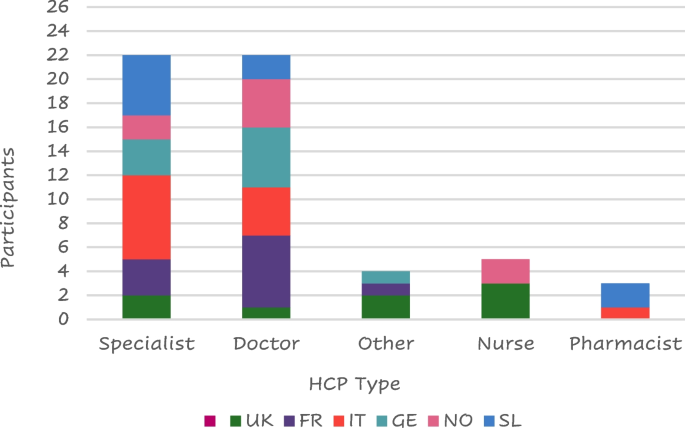
Type of HCP support by country
Legend: FR = France, GE = Germany, IT = Italy, SL = Slovenia, SP = Spain, NO = Northern (Sweden Denmark), UK = United Kingdom
The most frequent answers to the question “If you had a magic wand what would you wish for to improve your life with COPD?” were to find a cure ( n = 18), followed by more regular visits from their doctor/specialist ( n = 11), stop smoking ( n = 5), more information ( n = 4), HCP contact number and COPD support group ( n = 3), and digital monitoring ( n = 2) (Fig. 9 ).
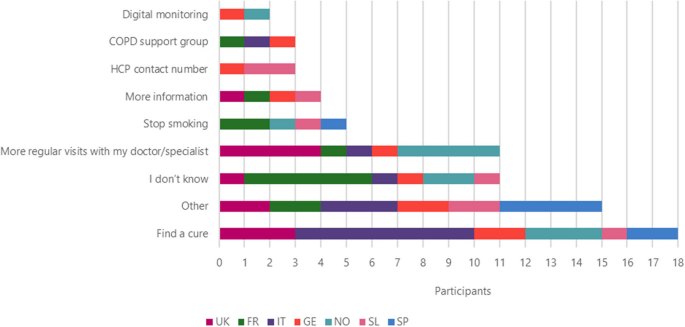
Improvements that patients wish to be made to improve their life by country
Other improvements that patients wish for include: access to new drugs, information about COPD, current and new drugs, reduced side effects, holding COPD workshops, investment in more research, provide cheaper treatment options, new lungs, something to help be more active, to be told that they would not need to take medication anymore, a new type of drug delivery that wouldn’t need to be taken with patient everywhere (like a nicotine patch), instant relief and doctors and nurses to be more humane.
Other services they felt were useful for them included physiotherapy ( n = 12), the use of support groups ( n = 8), exercise classes and psychological assistance ( n = 6), nutrition ( n = 4) while 1 patient from the UK suggested lifestyle (Fig. 10 ).
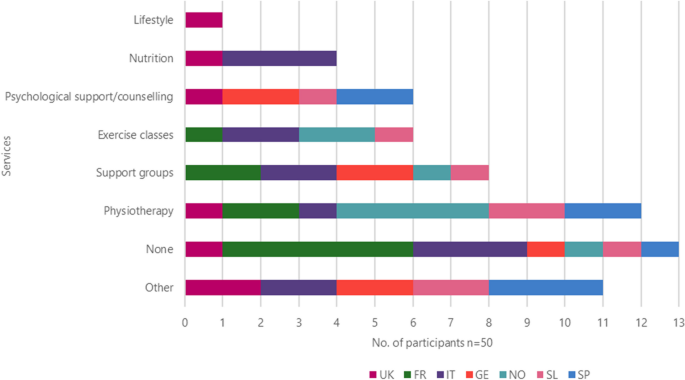
Other services the patient would like to use by country
Other services that patients would like to use included easier access to their HCP, paid, private physiotherapy sessions, smoking cessation support, disability card, training (videos and tutorials) including emergencies, lung transplants, more information about new drugs and the benefits of medication, hear more from doctors and pharmacists, and workshops for families and friends to help them understand what patients are going through.
Even if 3 patients reported having insurance covering additional services, they were generally unaware of the support they could receive through medical insurance. Many had concerns that such services would cost more money.
All the patients included in the study provided a total of 122 daily challenges they must face. 53 (43%) of the responses were related to their lifestyle. Reduced physical activity was referred by more than half ( n = 32) of them and difficulty in mobility was reported by 16; 28 (23%) reported challenges with their symptoms or medication (mainly difficult breathing, n = 15) (Fig. 11 ) while 13 (11%) reported emotional challenges including anxiety, depression, embarrassment due to symptoms or treatment, fear of the conditioning worsening, people recognizing they have a condition, acceptance of the condition and dependence on the medication.
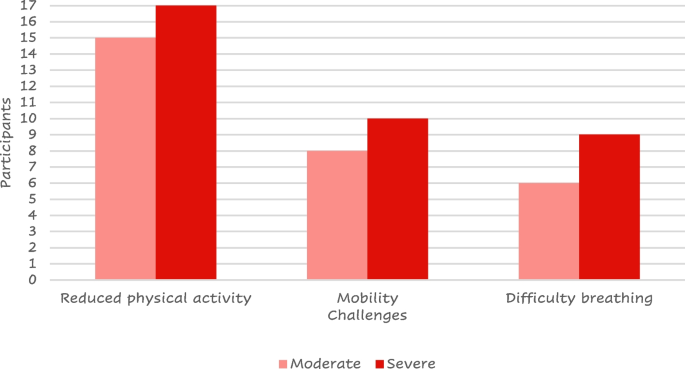
Most reported challenges by COPD severity
The objective of this human factors research was to identify the unmet needs along the different stages of people living with COPD through a one-to-one, semi-structured interview exploring the patient’s feelings and attitudes toward their journeys with the disease.
Differently from other studies exploring similar aspects of the impact of the disease on patient’s daily life where the data belong to medical databases, [ 4 , 6 , 7 , 9 , 10 , 23 ] the current approach is unique, in that it systematically investigates the patient’s feelings in a structured fashion, thus allowing us to better understand the patient’s emotions, which is becoming a relevant aspect of COPD management [ 7 , 24 ]. Furthermore, because of the consistent and wide heterogeneity between the different countries, patients included in this study could have been considered representative of the entire population of European patients with COPD.
The patient reported feelings highlighted that reduced physical activity, mobility challenges, and difficulty breathing resulted as the main challenges in daily life. According to the current international guidelines on COPD management, [ 22 , 25 , 26 ] physical activity is encouraged and monitored to evaluate the prognosis or looked forward to as a target for the evaluation of the treatment efficacy. [ 25 ] Our results confirm that patients perceive COPD as the cause of their reduced physical activity, [ 27 ] having a strong impact on their self-perception. Differently from other studies where increased physical activity was observed independently from patients’ counseling, [ 28 ] general psychological support and accepting their mobility challenges were described as important aims by the patients. Our patients felt reduced mobility as one of their main challenges; aids to improve mobility were described in the available literature as crucial to maintaining the patient’s independence [ 7 ] and have been included in the 2023 GOLD guidelines [ 29 ].
The HCP approach is mainly focused on improving the patient’s breathlessness and exercise intolerance [ 22 , 25 ]; the feeling depicted by the interviewed patients confirms the lack of information about how to manage breathlessness. [ 30 ] The only positive aspect of the COPD diagnosis, reported by 6 of the interviewees, was smoke quitting. Patients frequently feel angry and depressed when they think about the difficulties they have described. Participants discussed a variety of coping mechanisms to deal with these difficulties, including cutting back on physical activity, making sure they stayed active (as much as possible), and utilizing their rescue inhaler as a preventative step.
About one-fourth of the patients did not report having performed spirometry at diagnosis; as spirometry is the landmark of diagnosis; any other method is not gold standard and subjected to criticism [ 22 ]. Because of the qualitative nature of this study, we cannot exclude that this issue was linked to the patient’s reduced memory at the time of diagnosis.
As observed in other studies, [ 31 ] negative behavior has a strong influence on the patient’s quality of life. Patients in the current study generally felt negative emotions before receiving their diagnosis; however, a supportive role of relatives and caregivers was referred by interviewed subjects at the time of diagnosis. About forty percent of patients complained of having waited long before the diagnosis. When asked about the impact of their current treatment, participants gave primarily positive feedback and commonly described their current therapy as “good” and doing its job. Even if most of the patients included in our study felt stable symptoms, some were still looking for a “miraculous” cure. The need for support beyond just pharmacological treatments, such as psychological support and physiotherapy, became clear through the in-depth discussions with patients, confirming the requirement for an integrated and patient-tailored interview to identify the profile of each patient [ 27 , 32 ] to share the most appropriate interventions in the periodic visits, without the need of the patient’s hospitalizations to allow the introduction of new therapies suggested by other research [ 33 ].
As expected, our results show that the information about COPD and the training on both the disease and treatment were provided by different HCPs in various European countries. However, patients often felt that they were not provided with enough information at the point of diagnosis regarding the condition itself or the range of treatment options available. Some felt they did not receive adequate training on how to take their medication correctly, whereas others highlighted that the public should be made more aware of the condition, in general, to help them feel accepted and understood by their family and friends. When asked about the current support they were receiving for their disease, patients reported wanting more information about their clinical condition or treatment options, more regular visits with their HCP, smoking cessation assistance, and support in their day-to-day lives such as housework and improved accessibility, confirming the need of self-management education and skills training highlighted by other authors [ 22 , 25 , 26 ]. However, many patients were unsure or unaware of what support/services were available to them or did not feel they needed any additional support.
This study had a qualitative approach and was, thus, not designed to provide any definitive answer to a study hypothesis. Differently from other studies on general populations of patients with COPD where males and elderly are the most frequent patients [ 34 , 35 ], those who agreed to participate in this study were mostly women and aged between 42 and 65 years. Due to the inclusion of patients that could not be fully representative of the global patients with COPD and the study approach, the outcomes have to be properly generalized. Furthermore, the nature of the study required interviews to be carried out in the participant’s local language with the use of translators to support analysis leading to a potential loss of nuance in meaning.
In conclusion, the current findings show that an apparent discrepancy exists between the traditional lung functional and pharmacological approaches in diagnosing and managing COPD and patient’s needs and challenges in daily activities. In this respect, human factor studies play a relevant role in intercepting gaps in the care of people suffering from COPD, encouraging a novel holistic approach when designing clinical research or shepherding patients along their COPD daily journey.
Availability of data and materials
The data that support the findings of this study are available from Chris White (Rebus Medical), but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are not available without permission of Chiesi Farmaceutici.
Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012 [cited 2023 May 29];379(9823):1341–51. Available from: https://pubmed.ncbi.nlm.nih.gov/22314182/ .
Roggeri A, Micheletto C, Roggeri DP. Outcomes and costs of treating chronic obstructive pulmonary disease with inhaled fixed combinations: the Italian perspective of the PATHOS study. Int J Chron Obstruct Pulmon Dis. 2014 Jun 5 [cited 2023 May 29];9:569–76. Available from: https://pubmed.ncbi.nlm.nih.gov/24940053/ .
Blasi F, Cesana G, Conti S, Chiodini V, Aliberti S, Fornari C, et al. The clinical and economic impact of exacerbations of chronic obstructive pulmonary disease: a cohort of hospitalized patients. PLoS One. 2014 Jun 27 [cited 2023 May 29];9(6). Available from: https://pubmed.ncbi.nlm.nih.gov/24971791/ .
Rennard S, Decramer M, Calverley PMA, Pride NB, Soriano JB, Vermeire PA, et al. Impact of COPD in North America and Europe in 2000: subjects’ perspective of Confronting COPD International Survey. Eur Respir J. 2002 Oct 1 [cited 2023 May 29];20(4):799–805. Available from: https://pubmed.ncbi.nlm.nih.gov/12412667/ .
Sundh J, Ekström M. Persistent disabling breathlessness in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2016 Nov 9 [cited 2023 May 29];11(1):2805–12. Available from: https://pubmed.ncbi.nlm.nih.gov/27877034/ .
Ouellette DR, Lavoie K. Recognition, diagnosis, and treatment of cognitive and psychiatric disorders in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2017 Feb [cited 2023 May 29];12:639–50. Available from: https://pubmed.ncbi.nlm.nih.gov/28243081/ .
Gardener AC, Ewing G, Kuhn I, Farquhar M. Support needs of patients with COPD: a systematic literature search and narrative review. Int J Chron Obstruct Pulmon Dis. 2018 Mar 26 [cited 2023 May 29];13:1021–35. Available from: https://pubmed.ncbi.nlm.nih.gov/29628760/ .
Spruit MA, Singh SJ, Garvey C, Zu Wallack R, Nici L, Rochester C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013 Oct 15 [cited 2023 May 29];188(8). Available from: https://pubmed.ncbi.nlm.nih.gov/24127811/ .
Miravitlles M, Worth H, Soler Cataluña JJ, Price D, De Benedetto F, Roche N, et al. Observational study to characterise 24-hour COPD symptoms and their relationship with patient-reported outcomes: results from the ASSESS study. Respir Res. 2014 Oct 21 [cited 2023 May 29];15(1). Available from: https://pubmed.ncbi.nlm.nih.gov/25331383/ .
Jones PW, Watz H, Wouters FM, Cazzola M, COPD: the patient perspective. cited 2023 May 29. Available from: 2016. https://doi.org/10.2147/COPD.S85977 .
Article PubMed Central Google Scholar
Škrgat S, Triller N, Košnik M, Susič TP, Petek D, Jamšek VV, et al. Priporočila za obravnavo bolnika s kronično obstruktivno pljučno boleznijo na primarni in specialistični pulmološki ravni v Sloveniji. Zdravniski Vestnik. 2017;86(1–2):1–12.
Google Scholar
Mehring M, Donnachie E, Fexer J, Hofmann F, Schneider A. Disease management programs for patients with COPD in Germany: a longitudinal evaluation of routinely collected patient records. Respir Care. 2014 [cited 2023 Nov 15];59(7):1123–32. Available from: https://pubmed.ncbi.nlm.nih.gov/24222706/ .
Overview | Chronic obstructive pulmonary disease in over 16s: diagnosis and management | Guidance | NICE. [cited 2023 Nov 15]. Available from: https://www.nice.org.uk/guidance/ng115 .
Molin KR, Søndergaard J, Lange P, Egerod I, Langberg H, Lykkegaard J. Danish general practitioners’ management of patients with COPD: a nationwide survey. Scand J Prim Health Care. 2020 [cited 2023 Nov 15];38(4):391–8. Available from: https://pubmed.ncbi.nlm.nih.gov/33164618/ .
Sandelowsky H, Natalishvili N, Krakau I, Modin S, Ställberg B, Nager A. COPD management by Swedish general practitioners – baseline results of the PRIMAIR study. Scand J Prim Health Care. 2018 Jan 2 [cited 2023 Nov 15];36(1):5. Available from: /pmc/articles/PMC5901441/.
Meeraus W, Wood R, Jakubanis R, Holbrook T, Bizouard G, Despres J, et al. COPD treatment pathways in France: a retrospective analysis of electronic medical record data from general practitioners. Int J Chron Obstruct Pulmon Dis. 2018 Dec 18 [cited 2023 Nov 15];14:51–63. Available from: https://www.dovepress.com/copd-treatment-pathways-in-france-a-retrospective-analysis-of-electron-peer-reviewed-fulltext-article-COPD .
Come migliorare la qualità di vita dei pazienti colpiti da bronco-pneumopatia cronica e dei loro caregiver? | Azienda Ospedaliera Nazionale SS. Antonio e Biagio e Cesare Arrigo Alessandria. [cited 2023 Nov 15]. Available from: https://www.ospedale.al.it/it/comunicazione/notizie/come-migliorare-qualita-vita-pazienti-colpiti-bronco-pneumopatia-cronica-loro-caregiver .
Aiello F, Alunni A, Berardi M, Bordoni F, Calzolari M, Coviello AP, et al. Medicina Pratica L’associazione LABA-LAMA nella gestione del paziente con BPCO-Il punto di vista della Medicina Generale. Rivista Società Italiana di Medicina Generale n 3 • [Internet]. [cited 2023 Nov 15];29:2022. Available from: https://goldcopd.org/ .
ALLEGATOA alla Dgr n. 206 del 24 febbraio 2015.
Miravitlles M, Soler-Cataluña JJ, Calle M, Molina J, Almagro P, Quintano JA, et al. Spanish Guidelines for Management of Chronic Obstructive Pulmonary Disease (GesEPOC) 2017. Pharmacological Treatment of Stable Phase. Arch Bronconeumol. 2017 Jun 1 [cited 2023 Nov 15];53(6):324–35. Available from: https://pubmed.ncbi.nlm.nih.gov/28477954/ .
NHS England » Ambitions for Palliative and End of Life Care: A national framework for local action 2021–2026. [cited 2023 May 29]. Available from: https://www.england.nhs.uk/publication/ambitions-for-palliative-and-end-of-life-care-a-national-framework-for-local-action-2021-2026/ .
POCKET GUIDE TO COPD DIAGNOSIS, MANAGEMENT, AND PREVENTION A Guide for Health Care Professionals. 2020 [cited 2023 May 29]; Available from: www.goldcopd.org .
Rayner J, Khan T, Chan C, Wu C. Illustrating the patient journey through the care continuum: leveraging structured primary care electronic medical record (EMR) data in Ontario, Canada using chronic obstructive pulmonary disease as a case study. Int J Med Inform. 2020 Aug 1 [cited 2023 Jun 3];140. Available from: https://pubmed.ncbi.nlm.nih.gov/32473567/ .
Walker S, Andrew S, Hodson M, Roberts CM. Stage 1 development of a patient-reported experience measure (PREM) for chronic obstructive pulmonary disease (COPD). NPJ Prim Care Respir Med. 2017 Dec 1 [cited 2023 Jun 3];27(1). Available from: https://pubmed.ncbi.nlm.nih.gov/28740181/ .
Agustí A, Celli BR, Criner GJ, Halpin D, Anzueto A, Barnes P, et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Eur Respir J. 2023 Apr 1 [cited 2023 May 29];61(4):2300239. Available from: https://erj.ersjournals.com/content/61/4/2300239 .
Reddel HK, Bacharier LB, Bateman ED, Brightling CE, Brusselle GG, Buhl R, et al. Global Initiative for Asthma Strategy 2021 Executive Summary and Rationale for Key Changes. Am J Respir Crit Care Med [Internet]. 2022 Jan 1 [cited 2023 Jun 3];205(1):17–35. Available from: https://pubmed.ncbi.nlm.nih.gov/34658302/ .
Jones PW, Watz H, Wouters EFM, Cazzola M. COPD: the patient perspective. Int J Chron Obstruct Pulmon Dis. 2016 [cited 2023 Jun 3];11 Spec Iss(Spec Iss):13–20. Available from: https://pubmed.ncbi.nlm.nih.gov/26937186/ .
Aggarwal AN, Gupta D, Jindal SK. The relationship between FEV1 and peak expiratory flow in patients with airways obstruction is poor. Chest. 2006 [cited 2023 Jun 3];130(5):1454–61. Available from: https://pubmed.ncbi.nlm.nih.gov/17099024/ .
2023 GOLD guidelines for chronic obstructive pulmonary disease. [cited 2023 Jun 26]. Available from: https://www.jwatch.org/na56004/2023/04/20/2023-gold-guidelines-chronic-obstructive-pulmonary-disease .
Oliver SM. Living with failing lungs: the doctor–patient relationship. Fam Pract. 2001 Aug 1 [cited 2023 Jun 3];18(4):430–9. Available from: https://academic.oup.com/fampra/article/18/4/430/620192 .
JIACI · J Invest Allergol Clin Immunol. [cited 2023 Jun 3]. Available from: https://www.jiaci.org/summary/vol16-issue4-num90 .
Martínez-Guiu J, Arroyo-Fernández I, Rubio R. Impact of patients’ attitudes and dynamics in needs and life experiences during their journey in COPD: an ethnographic study. Expert Rev Respir Med. 2022 [cited 2023 Jun 3];16(1):121–32. Available from: https://pubmed.ncbi.nlm.nih.gov/34238094/ .
Lainscak M, Gosker HR, Schols AMWJ. Chronic obstructive pulmonary disease patient journey: hospitalizations as window of opportunity for extra-pulmonary intervention. Curr Opin Clin Nutr Metab Care. 2013 May [cited 2023 Jun 3];16(3):278–83. Available from: https://pubmed.ncbi.nlm.nih.gov/23507875/ .
Kim-Dorner SJ, Schmidt T, Kuhlmann A, Graf von der Schulenburg JM, Welte T, Lingner H. Age- and gender-based comorbidity categories in general practitioner and pulmonology patients with COPD. NPJ Prim Care Respir Med. 2022 Dec 1 [cited 2023 Nov 15];32(1). Available from: /pmc/articles/PMC9061861/.
Maestri R, Vitacca M, Paneroni M, Zampogna E, Ambrosino N. Gender and age as determinants of success of pulmonary rehabilitation in individuals with chronic obstructive pulmonary disease. Arch Bronconeumol. 2023 Mar 1 [cited 2023 Nov 15];59(3):174–7. Available from: https://www.archbronconeumol.org/en-gender-age-as-determinants-success-articulo-S0300289622005683 .
Download references
Acknowledgements
Rebus Medical (St Nicholas House, 31-34 High St, Bristol BS1 2AW, United Kingdom) was responsible for contacting the patients, data collection, and statistical analyses. The authors thank Andrea Rossi for the medical writing support and the Chiesi and Rebus study team for the management of the Human factors study (Marta Lombardini, Ilaria Milesi, Lorenzo Ventura, Veronica Giminiani, Massimo Savella, Elena Zeni, Elena Nudo, Lisa Forde, Shivani Bhalsod, Elsie Barker, and Chris White).
The authors thank the patients, the interviewers, and the translators who made this study possible.
All the activities were funded by Chiesi Farmaceutici S.p.A. (Parma, Italy).
Author information
Authors and affiliations.
Division of Respiratory Medicine, Department PROMISE, “Giaccone” University Hospital, University of Palermo, Palermo, Italy
Nicola Scichilone
GP, Portsdown Group Practice, Portsmouth, UK
Andrew Whittamore
Rebus Medical LTD, Bristol, UK
Chris White
Chiesi Farmaceutici S.P.A, Via Paradigna 131/A – 43122, Parma, Italy
Elena Nudo, Massimo Savella & Marta Lombardini
You can also search for this author in PubMed Google Scholar
Contributions
NS contributed to the design of the study and critically revised the outcomes according to the clinical needs from a specialistic point of view. AW contributed to the design of the study and critically revised the outcomes according to the clinical needs from a general practitioner’s point of view. CW designed the study, managed and coordinated the study activities. EN, MS, and ML contributed to the design of the study and critically revised the outcomes from a treatment producer’s point of view. All authors critically revised the drafted article and read and approved the final manuscript.
Corresponding author
Correspondence to Nicola Scichilone .
Ethics declarations
Ethics approval and consent to participate.
The DL 20 marzo 2008 specifies that interviews to the patients without any clinical intervention (as the present study) are not considered observational studies and, thus, don't need to be submitted to the revision and approval of an Ethical committee.
All patients provided their informed consent to participate in this study. The informed consent included statements that required participants to agree to maintain confidentiality regarding the information shared during the study session, as well as described the conditions for the collection, use, processing, retention, and transfer of their personal data (including personally identifiable information and personal health information).
Consent for publication
Not applicable.
Competing interests
NS declares no competing interests.
AW declares no competing interests.
CW is a full-time employee of Rebus Medical.
EN, MS, and ML are full-time employees of Chiesi Farmaceutici.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Scichilone, N., Whittamore, A., White, C. et al. The patient journey in Chronic Obstructive Pulmonary Disease (COPD): a human factors qualitative international study to understand the needs of people living with COPD. BMC Pulm Med 23 , 506 (2023). https://doi.org/10.1186/s12890-023-02796-8
Download citation
Received : 18 September 2023
Accepted : 29 November 2023
Published : 13 December 2023
DOI : https://doi.org/10.1186/s12890-023-02796-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Chronic obstructive pulmonary disease
- Assessment of healthcare needs
- Qualitative research
- Patient-centered care
- Human-centered design
- Human factors science
- Pharmacologic therapy
- Qualitative evaluation
- Determination of healthcare needs
- Multinational perspective
- Quality of life
- Quality of healthcare
BMC Pulmonary Medicine
ISSN: 1471-2466
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Discounts and promotions
- Delivery and payment
Cart is empty!
Case study definition

Case study, a term which some of you may know from the "Case Study of Vanitas" anime and manga, is a thorough examination of a particular subject, such as a person, group, location, occasion, establishment, phenomena, etc. They are most frequently utilized in research of business, medicine, education and social behaviour. There are a different types of case studies that researchers might use:
• Collective case studies
• Descriptive case studies
• Explanatory case studies
• Exploratory case studies
• Instrumental case studies
• Intrinsic case studies
Case studies are usually much more sophisticated and professional than regular essays and courseworks, as they require a lot of verified data, are research-oriented and not necessarily designed to be read by the general public.
How to write a case study?
It very much depends on the topic of your case study, as a medical case study and a coffee business case study have completely different sources, outlines, target demographics, etc. But just for this example, let's outline a coffee roaster case study. Firstly, it's likely going to be a problem-solving case study, like most in the business and economics field are. Here are some tips for these types of case studies:
• Your case scenario should be precisely defined in terms of your unique assessment criteria.
• Determine the primary issues by analyzing the scenario. Think about how they connect to the main ideas and theories in your piece.
• Find and investigate any theories or methods that might be relevant to your case.
• Keep your audience in mind. Exactly who are your stakeholder(s)? If writing a case study on coffee roasters, it's probably gonna be suppliers, landlords, investors, customers, etc.
• Indicate the best solution(s) and how they should be implemented. Make sure your suggestions are grounded in pertinent theories and useful resources, as well as being realistic, practical, and attainable.
• Carefully proofread your case study. Keep in mind these four principles when editing: clarity, honesty, reality and relevance.
Are there any online services that could write a case study for me?
Luckily, there are!
We completely understand and have been ourselves in a position, where we couldn't wrap our head around how to write an effective and useful case study, but don't fear - our service is here.
We are a group that specializes in writing all kinds of case studies and other projects for academic customers and business clients who require assistance with its creation. We require our writers to have a degree in your topic and carefully interview them before they can join our team, as we try to ensure quality above all. We cover a great range of topics, offer perfect quality work, always deliver on time and aim to leave our customers completely satisfied with what they ordered.
The ordering process is fully online, and it goes as follows:
• Select the topic and the deadline of your case study.
• Provide us with any details, requirements, statements that should be emphasized or particular parts of the writing process you struggle with.
• Leave the email address, where your completed order will be sent to.
• Select your payment type, sit back and relax!
With lots of experience on the market, professionally degreed writers, online 24/7 customer support and incredibly low prices, you won't find a service offering a better deal than ours.

IMAGES
VIDEO
COMMENTS
Chronic obstructive pulmonary disease (COPD) is a progressive lung disease that affects millions of people around the world. It is primarily caused by smoking and is characterized by a persistent obstruction of airflow that worsens over time. COPD can lead to a range of symptoms, including coughing, wheezing, shortness of breath, and chest ...
Case Presentation. The patient is a 60-year-old white female presenting to the emergency department with acute onset shortness of breath. Symptoms began approximately 2 days before and had progressively worsened with no associated, aggravating, or relieving factors noted. She had similar symptoms approximately 1 year ago with an acute, chronic ...
COPD: a case study Authors Debbie Price is lead practice nurse, Llandrindod Wells Medical Practice; Nikki Williams is associate professor of respiratory and sleep physiology, Swansea University. Abstract This article uses a case study to discuss the symptoms, causes and management of chronic obstructive pulmonary disease, describing the patient's
The nurse locates a portable oxygen tank and places the patient on 2 lpm oxygen via nasal cannula. Based on these findings, Mr. Whaley's PCP decides to call an ambulance to send Mr. Whaley to the Emergency Department (ED). While waiting for the ambulance, the nurse repeats the SpO2 and finds Mr. Whaley's SpO2 is only 89%.
Click the card to flip 👆. Chronic obstructive pulmonary disease (COPD) is a group of common chronic respiratory disorders, such as chronic bronchitis, emphysema, and chronic asthma, which are characterized by progressive tissue degeneration and obstruction in the airways of the lungs. Emphysema occurs when the air sacs (alveoli) at the end ...
Nurse Seema works on a medical-surgical unit and is caring for Richard, a 75-year-old male with a history of smoking, who was admitted for an acute exacerbation of chronic obstructive pulmonary disease, or COPD. After settling Richard in his room, Nurse Seema goes through the steps of the Clinical Judgment Measurement Model to make clinical ...
COPD case study answers. Which of the clinical manifestations are abnormal and why? What would normal findings be? Click the card to flip 👆. • Dyspnea at rest, cough, fever; T=101.1, BP=162/90, HR=108, O2 sats 89%; labored respirations, coarse crackles, accessory muscle use.
On examination, the temperature was 36.4°C, the heart rate 103 beats per minute, the blood pressure 79/51 mm Hg, the respiratory rate 30 breaths per minute, and the oxygen saturation 99% while ...
Darrell Johnson is a 62-year-old male who comes to the Emergency Department (ED) with a 4-day history of increased sputum production, a change in the character of sputum, increased shortness of breath, and a fever of 101° F. He has a smoking history of 2 packs a day for the past 20 years, and he smoked 1 pack a day prior to that, beginning at ...
This article uses a case study to discuss the symptoms, causes and management of chronic obstructive pulmonary disease, describing the patient's associated pathophysiology. Diagnosis involves spirometry testing to measure the volume of air that can be exhaled; it is often performed after administering a short-acting beta-agonist.
The elegance of a parsimonious diagnosis — one answer to explain all of a patient's ... mechanical intervention. 20 In this case, ... disease 2019: retrospective study. BMJ 2020;368:m1091 ...
This page titled 2: Case Study #1- Chronic Obstructive Pulmonary Disease (COPD) is shared under a CC BY-SA 4.0 license and was authored, remixed, and/or curated by Glynda Rees, Rob Kruger, and Janet Morrison via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request.
This clinical case report highlights the usefulness of FEF 25-75 evaluation in early COPD diagnosis and monitoring and confirms the efficacy of LAMA-LABA association for small airways obstruction treatment. Keywords: COPD, LAMA, LABA, FEF25-75, Treatment. Abbreviations: COPD, chronic obstructive pulmonary disease; LAMA, long acting muscarinic ...
This case study incorporates a common presentation seen by the nurse in clinical practice: community acquired pneumonia with a history of COPD causing an acute exacerbation. Principles of spiritual care are also naturally situated in this scenario to provide rich discussion of "how to" practically incorporate this into the nurse's practice.
Case study 1: A 63-year-old man with moderate/severe COPD and a chest infection A 63-year-old self-employed plumber makes a same-day appointment for another 'chest infection'.
Case Presentation. History of Present Illness: A 33-year-old white female presents after admission to the general medical/surgical hospital ward with a chief complaint of shortness of breath on exertion. She reports that she was seen for similar symptoms previously at her primary care physician's office six months ago.
practice 8-10 repetitions of purse lip breathing 3-4 times a day. examples of pursed lip breathing. inhale for 3 seconds (silently count 1-2-3) and then exhale for 9 seconds (silently count 1-2-3-4-5-6-7-8-9) inhale for 2 seconds count THEN exhale for a 6 second count. diaphragmatic breathing education.
Case Study 29 COPD Exacerbation. Difficulty: Advanced Setting: Hospital Index Words: chronic obstructive pulmonary disease (COPD), medications, ... Class Activity-1460C Module 6 Review Quiz-Answer Key; Case Study 76 Diabetes Mellitus Type 2-A; Related documents. Case Study 81 Diabetes Mellitus Type 1-Answers;
Meet Susan M! Share your impressions in our latest COPD case study. Summary of in-patient admission: Susan M. is being discharged today following a 6-day ICU and step-down admission for acute exacerbation of COPD with bacterial pneumonia requiring intubation and mechanical ventilation for a period of 32 hours. Subsequent to her extubation and transfer to the step down unit she was treated with ...
Chronic obstructive pulmonary disease (COPD) is a common condition that causes irreversible airway obstruction. Fatigue and exertional dyspnoea, for example, have a detrimental impact on the patient's daily life. Current research has revealed the need to empower the patient, which can result in not only educated and effective decision-making, but also a considerable improvement in patient ...
1) CASE SUMMARY. Mr TLT is a 58 year old taxi driver who was admitted to Hospital Batu Pahat due to newly diagnosed chronic obstructive pulmonary disease. He has had hypertension for the past one year and is taking T Amlodipine 5mg od. He is also a chronic smoker for the past 40 years who smokes about 20 sticks of cigarettes a day.
The ordering process is fully online, and it goes as follows: • Select the topic and the deadline of your case study. • Provide us with any details, requirements, statements that should be emphasized or particular parts of the writing process you struggle with. • Leave the email address, where your completed order will be sent to.