Getting Started in Clinical Research
Interested in a career in clinical research you’re in the right place, start by watching this brief video, then scroll down to continue your journey..

NEXT: INTRODUCTION TO CLINICAL TRIALS

This free online training program provides the foundational knowledge on which you can grow your competence as a clinical researcher. Learn how medical products are developed; how volunteer patients are protected; and who plays key roles in the development, research, review, and approval of medical products.
Create Your Free ACRP Account to Access >
Already have an account access course here >, next: explore career options.

Explore entry-level roles, their associated educational requirements, and sample career paths by downloading the ACRP Career Lattice. Reviewing this free resource will help you decide what might be the best role for you on the clinical research team.
Download ACRP Clinical Research Career Lattice™ >
Next: assess your education & background.
Now that you have reviewed the Clinical Research Career Lattice, the question is—Do you have the necessary education and background for an entry-level role?

Great. Now’s a good time to review competency guidelines for some of the roles you are interested in to get a better understanding of what the role involves.
View competency guidelines >.
No, I don’t.
That’s okay! Your next step should be to obtain the necessary educational background and/or explore obtaining a degree in clinical research.
Next: join acrp.
Join ACRP to connect with employers and others in the field, and gain complimentary access to resources that will help develop your skills and competencies.
Explore ACRP Member Benefits >
Next: finding an entry-level position.

Post your resume/CV in the ACRP Career Center so hiring employers can find you. Browse job and internship opportunities that seem like a good fit for you, and apply directly online.
Visit ACRP Career Center >
Tips & resources —be persistent. keep developing your competencies. network. and keep applying for new opportunities. in the meantime, get a glimpse into clinical research career journeys and more by reading these career-focused articles from clinical researcher ., next: congratulations on your first job.

Now that you’re a working clinical researcher, it’s time to start thinking about professional certification. You need 2 years’ experience to sit for an ACRP Certification Exam, but it’s never to early to start preparing to earn the flagship certification in clinical research and accelerating your career.
Learn About ACRP Certification >
Pro tip now that you’ve got your foot in the door, refer back to the acrp career lattice and competency guidelines periodically as you explore new roles and opportunities in your budding clinical research career..
- Program Overview
The SOCRA Certified Clinical Research Professional (CCRP) program is your gateway to excellence in clinical research. Elevate your career with our internationally recognized certification, tailored for professionals dedicated to upholding the highest standards in the field. Join a community committed to ethical practices, continuous learning, and advancing global health.
The Society of Clinical Research Associates (SOCRA) established the Certification Program for Clinical Research Professionals in order to create an internationally accepted standard of knowledge, education, and experience by which clinical research professionals will be recognized by the clinical research community. Those individuals so recognized may use the "Certified Clinical Research Professional" or "CCRP ® " designation.
Path to Certification
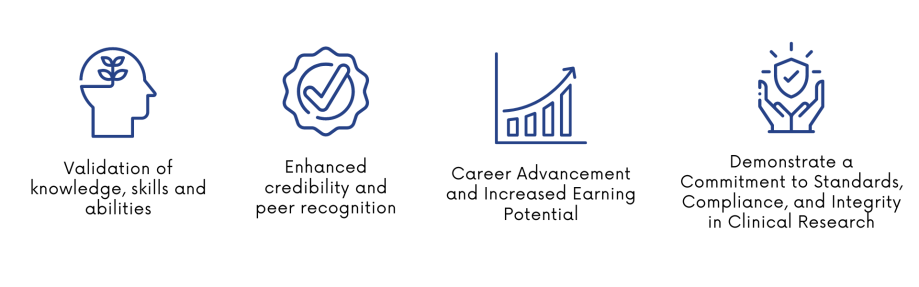
CCRP certification is awarded upon meeting two criteria: a successful written application and a passing CCRP examination score. The benefits of obtaining certification are numerous. It not only validates knowledge, skills, and abilities but also enhances credibility and peer recognition. Career advancement and increased earning potential become tangible outcomes, reflecting a commitment to standards, compliance, and integrity.
Scope and Standards of Practice
The standards upon which this certification program is based have been set forth by SOCRA to promote recognition and continuing excellence in the ethical conduct of clinical trials. It is the goal of SOCRA to encourage members, and assure the competency of certified members, in their knowledge, understanding, and application of the conduct of clinical investigations involving humans in accordance with the ICH Guidelines, the U.S. Code of Federal Regulations, and the ethical principles that guide clinical research. Members are expected to adhere to national, state, local and provincial regulations and to international guidelines published by the International Conference for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) and all applicable federal, state and local laws and policies.
Standards of Practice include an understanding of and application of basic concepts of Good Clinical (Research) Practice, including:
- The Nuremberg Code
- The Belmont Report
- The Declaration of Helsinki
- 21 U.S. Code of Federal Regulations – Parts 11, 50, 56, 312, 812
- 45 U.S. Code of Federal Regulations - Part 46
- ICH Harmonised Guideline for Good Clinical Practice E6(R2), and
- ICH Clinical Safety Data Management: Definitions and Standards for Expedited Reporting (E2A)
- 42 CFR Part 11 (ClinicalTrials.gov)
Certification Exam
The SOCRA Certification Examination is offered in two formats: paper and pencil (at SOCRA sponsored sites), and computer based (at Prometric testing centers or through Home Proctoring).
SOCRA Sponsored Sites: Paper and Pencil
- Hosted exams offered in various location throughout the US and Canada.
- Visit the paper and pencil exam schedule for dates and locations.
- A complete application must be received by the deadline date as stated on the examination schedule.
- Score reports mailed to you in 4-6 weeks after exam.
Computer Based Testing: Testing Centers and Remote Proctoring
- Offered at Prometric testing centers throughout the world or through Home Proctoring
- Click here for a list of test centers.
- Allow 2-4 weeks for application processing.
- Once application is approved, schedule exam at a testing center. Exam sessions are available at least 6 weeks in advance.
- Score reports received immediately upon completion of exam.
Candidate Handbook
For more information, please view the Candidate Handbook.
Certification
- CCRP Certification Quick Facts
- Definition of a Clinical Research Professional
- Certification Program Policies
- Removal of CCRP® Credential
- Verify Certification
- Exam Overview
- Candidate Eligibility
- Application and Fee
- Computer Based Testing Exams
- Paper and Pencil Exams
- Refunds, Rescheduling and Retesting
- SOCRA Sponsored Exam Schedule
- Preparing for the Exam
- Preparation Resources
- Examination Results
- Host an Exam at Your Site
- Apply Online
- Exam Schedule SOCRA Sponsored Sites
- Requirements for Maintaining Certification
- Continuing Education Requirements
- Descriptions of Acceptable CE
- CE Recordkeeping Requirements
- Request for SOCRA CE for Courses / Workshops
- Installment Plan Payment
- Renewal of Certification
- Recertification Audit
- Recertification Learning Module
- Accreditation
Summary of Certification Activities
11,145 CCRPs (as of 12/31/2022)
- 1,391 candidates took CCRP exam
- 73% passed CCRP exam
- 2,649 CCRPs recertified
- 946 candidates took CCRP exam
- 65% passed CCRP exam
- 2,783 CCRPs recertified
- 2,060 candidates took CCRP exam
- 70% passed CCRP exam
- 3,801 CCRPs recertified
- 1,980 candidates took CCRP exam
- 71% passed CCRP exam
- 3,188 CCRPs recertified
- 104 exam sites hosted
- 2,175 candidates took CCRP exam
- 2,491 CCRPs recertified
- 91 exam sites hosted
- 2,141 candidates took CCRP exam
- 2,421CCRPs recertified
Content types
- Infographics
- Case studies
- Press releases
- Career Advice
- Contracting/Freelancing
- Digital health
- Employee Engagement & Retention
- Employer Advice
- Employer Brand
- Life Science news
- Proclinical News
- Working in Recruitment
- Workplace Diversity
Latest jobs
Highly Competitive
City of London, England
Proclinical are seeking a dedicated Senior Statistical Programmer to join a dynamic team committed to advancing and improving patients' lives. This is a remote position based within the UK.
Frankfurt am Main (60329), Germany
Proclinical are seeking a Key Account Manager for a contract position based in Germany.
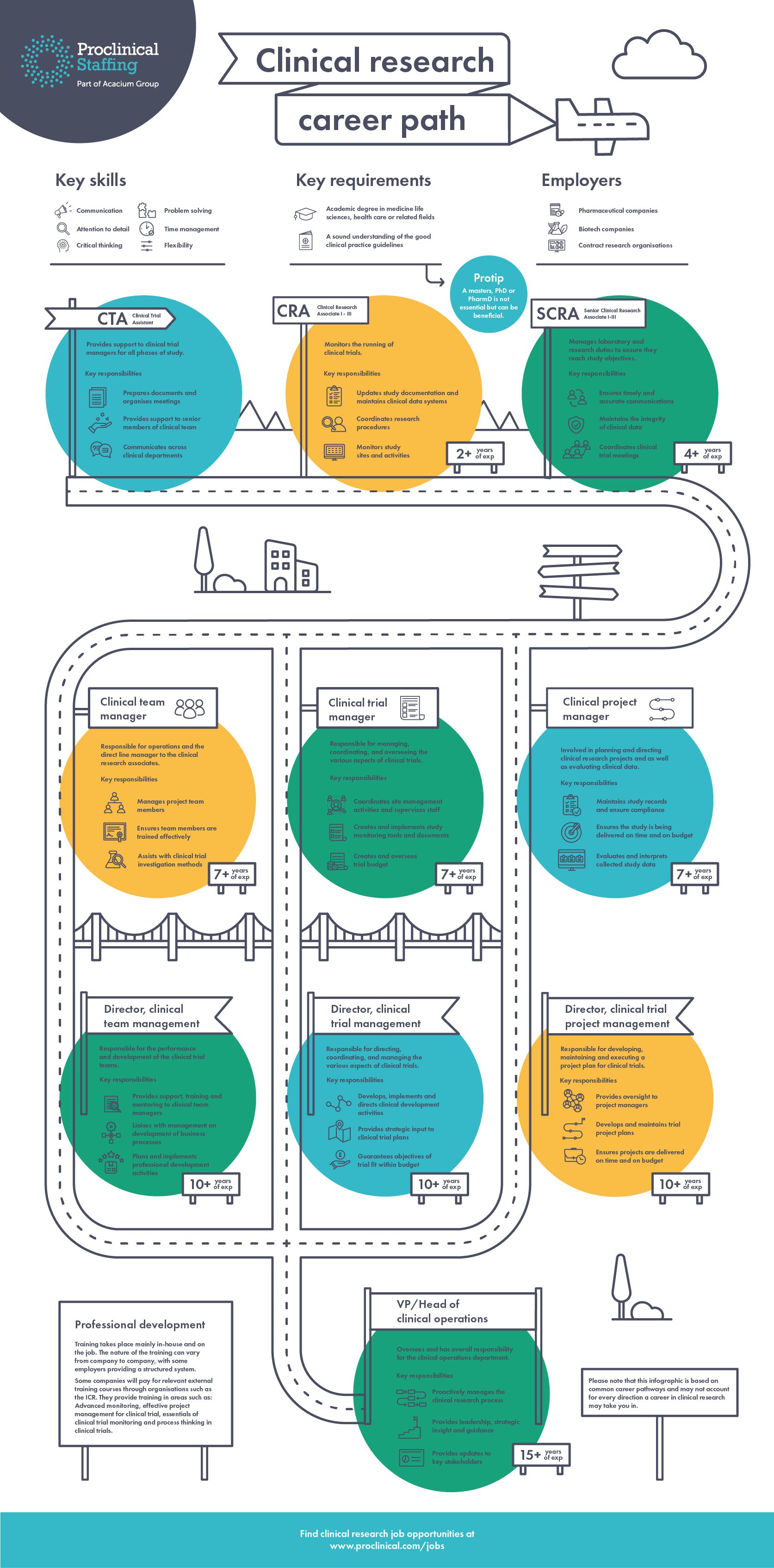
Infographic: Clinical research career paths
How to grow your career in clinical research
Clinical research is a competitive but growing field and provides rewarding career opportunities if you have qualifications or experience within life sciences . A career in clinical research involves playing a role in helping your employer conduct studies to ensure new treatments are safe and effective for patients. There are a variety of way to progress a career in the clinical research field with pharma, biotech, medical device companies and CROs all offering opportunities for professionals, such as the clinical research jobs listed on our website.
With the right experience, clinical professionals will have the potential to progress all the way to clinical director or even VP level. Once you have senior CRA level, there are typically three main routes a career in clinical research can take. You will either be a manager of the trial process, a manager of people or a project manager:
Clinical trial management:
Usually responsible for the managing, coordinating and overseeing various aspects of the trial. Typical duties will involve supervising staff, implementing study tools and documents, overseeing trial budgets and providing strategic input into trials.
Clinical team management:
Responsible for acting as the direct line manager to associates. Clinical team managers will manage and support team members, ensure staff are trained effectively, review the effectiveness of business processes and plan professional development activities.
Clinical project management:
Involved with the planning and directing of clinical trials and is also responsible for evaluating clinical data. Day-to-day tasks include, maintaining study records, ensuring activities are being delivered on time and on budget, interpreting study data and developing trial plans.
Here we discover what it takes to lead a fulfilling career in clinical research and the most common career pathways:
Looking to progress your career in clinical research?
We are hiring now for positions in clinical operations and clinical development within pharma and medical device companies and CROs. See our latest clinical research vacancies and apply today, or simply send us your CV to be considered for new positions as they become available.
Latest Posts
.png)
(VIDEO) Bridging the Gap: Candidates & Employers in Clinical Research

by Theodora Savlovschi - Wicks

Mid-sized biotech, consultant clinical data management solution

by Proclinical Staffing

Top 10 women in life sciences today

by Hannah Burke
.png)
Top 10 drugs with patents due to expire in the next five years

Leading remotely: How to retain remote workers

Proclinical broadens its network of offices by opening in Munich

Who are the top 10 medical device companies in the world in 2023?

by Dove Jociute

Proclinical honoured with International Recruitment Company of the Year award

Who are the top 10 pharmaceutical companies in the world? (2023)

Leadership strengths quiz

Highly Competitive Salary
New Haven, USA
Proclinical is seeking a dedicated and detail-oriented individual for the role of Supply Chain Planning Analyst. This is a remote contract position.

Cambridge, USA
Proclinical is actively seeking a Compensation Manager. This is a contract position located in Cambridge, MA.

Plainsboro, USA
Proclinical is seeking a dynamic and innovative Business Omni-Channel Marketing Lead. This is a contract position located in Plainsboro, NJ.
New York, USA
Proclinical is seeking a dynamic and strategic Business Development Manager/Director. This is a remote permanent position.

Boston, USA
Proclinical is seeking a Real World Evidence (RWE) Lead who will provide clinical leadership for evidence generation activities.
Paris-8e-Arrondissement, France
Proclinical is searching on behalf of one of our established clients for a Medical Science Liaison. This is a remote based role within France.

Springe (31832), Deutschland
Proclinical is seeking an IT Site Manager for a role based in Springe.

Zürich, Schweiz
Proclinical is seeking a JavaScript Developer for permanent position at a well established medical company.

Career advice
- Proclinical Staffing
- Proclinical Consulting
- Proclinical Executive
- Proclinical Engage
- Job Opportunities
- Our Services
- Submit vacancy
- Insights and Advice
- Meet the Team
Register a new account

- Modern slavery agreement
- Gender pay gap
- Privacy policy
- Carbon Reduction Plan PPN 06/21
- MTS Health Sciences How to Become a Clinical Research Associate
- Anesthesia Technician
- Audiologist & SLP
- Cardiovascular Technologist
- Dental Assistant
- Dental Hygienist
- Diagnostic Medical Sonographer
- Dialysis Technician
- EKG Technician
- EMT & Paramedic
- Kinesiologist
- Mammography Technologist
- Medical Assistant
- MRI Technologist
- Neurodiagnostic Technologist
- Nuclear Medicine Technologist
- Ophthalmic Technician
- Pharmacy Technician
- Phlebotomist
- Physical Therapist Assistant & Aide
- Psychiatric & Mental Health Technician
- Radiation Therapist
- Radiologic Technologist
- Respiratory Therapist
- Surgical Technologist
- Cytologist (Cytotechnologist)
- Dental Lab Technician
- Histotechnologist
- Medical Lab Assistant
- Medical Lab Technician
- Biological Sciences
- Biomedical Science
- Biotechnology
- Health Sciences
- Medical Laboratory Scientist
- Nutritionist & Dietitian
- Pathologists' Assistant (PathA)
- Pre-Vet (Veterinarian)
- Biomedical Equipment Technician
- Biomedical Informatics
- Health Informatics
- Health Information Management
- Health Information Technology
- Healthcare Administration
- Medical Billing & Coding
- Nursing Informatics
- Sterile Processing Technician
- Patient-Facing Technology Programs
- Laboratory Technology programs
- Natural & Clinical Lab Science
- Medical IT & Administrative
Certification Guides
Career guides, interviews & features, how to become a clinical research associate (cra), search for schools.
When you click on a sponsoring school or program advertised on our site, or fill out a form to request information from a sponsoring school, we may earn a commission. View our advertising disclosure for more details.
Scientists, researchers, and doctors make discoveries about drugs, surgical procedures, behavioral therapies, or medical devices through their work in laboratories and healthcare settings. This is only the beginning of the journey for pharmaceuticals, therapies, and devices, as bringing the findings from the lab to the street requires a vigorous scientific process known as a clinical trial. Clinical research associates (CRAs) are the professionals responsible for ensuring that clinical trials move forward following established guidelines and regulations for ethics, safety, and reporting.
Clinical research associates, also known as “monitors,” work on behalf of sponsors funding clinical trials for the new or existing drug, device, surgery, or behavioral intervention. Working directly for the sponsor or through a contract research organization, the main task of a CRA is to monitor the progress of an ongoing clinical trial.
Through in-person site visits or remote monitoring systems, a CRA serves as the central point of contact between a sponsor and testing sites; ensures that the trial is being administered per approved protocols; verifies that the clinical trial is being conducted ethically at all sites; and confirms the validity and accuracy of all data being collected and reported at test sites.
In addition to reading, interpreting, and understanding medical technology, clinical research associates must have excellent interpersonal and communication skills. The ability to understand best clinical practices, design protocols, and data standards requires CRAs to have outstanding attention to detail, analytical skills, and the capacity to deliver constructive feedback to participating research sites on their performance.
Although not a requirement, many CRAs travel between multiple research sites for study oversight, which may require a valid driver’s license, the physical capacity to travel, and/or willingness to fly or drive regularly.
This detailed guide explores the education and credentials required to become a clinical research associate (CRA).
Arizona State University
Johns hopkins university (aap), university of west florida, steps to become a clinical research associate (cra).
The pathways to becoming a clinical research associate are numerous and available to anyone with a high school diploma or higher. While formal education is not technically required to enter the field, having a bachelor’s degree or higher can make potential candidates much more competitive.
Certification in the field is also not required, but obtaining certification from the Society of Clinical Research Associates (SOCRA) or the Association of Clinical Research Professionals (ACRP) can result in more opportunities and even more competitive salaries.
Finally, all aspiring CRAs are advised to check out the International Conference on Harmonisation’s (ICH) guidelines for Good Clinical Practice (GCP) to get a feel for the professional expectations and responsibilities.
Here is how to become a CRA depending on one’s level of education. Please note that in the United States, there are two major certification bodies for CRAs: the Society of Clinical Research Associates (SOCRA) and the Association of Clinical Research Professionals (ACRP). Each pathway includes the eligibility requirements to pursue credentialing through either of these entities.
PATH 1: Earn a High School Diploma and Gain Experience
Perhaps the most strenuous route to this career is becoming a certified CRA with a high school diploma and between 3,000 and 3,500 hours of qualifying work experience (depending on the certification entity).
These candidates often start out in support positions assisting a more experienced or certified CRA with mundane tasks. An entry-level worker can earn increased responsibilities through a demonstrated capacity to learn the regulations, protocols, and ethical considerations. To qualify for the following CRA certification exams, high school graduates must:
SOCRA Category 1
- Complete two full-time years of CRA work within five years, or 3,500 hours of part-time work
ACRP CCRA (Certified Clinical Research Associate)
- Complete 3,000 hours performing essential duties
- Submit a resume documenting and demonstrating job performance
Please note that in some cases, additional education can be used to substitute for work experience hours. Please see credentialing websites for details.
PATH 2: Earn an Associate Degree and Gain Experience
Depending on the program, an associate’s degree of applied science (AAS) in clinical research can be a standalone degree or a stepping-stone to a bachelor’s or master’s. Licensed vocational or practical nurse (LVN or LPN) programs are designed specifically for practical, job-ready skills and may qualify aspiring CRAs for the ACRP certification.
Similar to the path taken by those with a high school diploma, having an associate degree, LPN, or LVN can open the door to some entry-level jobs in the industry. At this level, some prospective CRAs assist more experienced CRAs or some engage independently in entry-level tasks related to study monitoring. Those working as CRAs with an associate’s degree, LPN, or LVN can qualify for certification after working a certain number of hours in the field.
To qualify for the following CRA certification exams, associate degree graduates must:
SOCRA Category 2
- Hold a “clinical research” degree
- Complete one full-time year as a CRA or 1,750 hours part-time
ACRP Option 2 (Also for LVN, LPN)
- Hold a “clinical research degree” or complete 1,500 hours performing essential duties
PATH 3: Earn a Bachelor’s Degree and Gain Experience
Most entry-level clinical research associate positions require candidates to have a bachelor’s of science (BS) in a health-related field from an accredited four-year university. In some cases, programs are designed to add practical hours needed to qualify for certification tests.
Those interested in becoming a CRA can study nursing, health sciences, biological sciences, clinical research, clinical research administration, clinical research management, medical technology, or life sciences, among many other subjects. Because many entry-level positions are looking for those with previous work in the field, those earning a BS should seek internships, part-time work, and/or fellowships involving participation in research, if possible.
To qualify for the following CRA certification exams, bachelor’s degree graduates must:
SOCRA Category 3
- Hold a “clinical research” undergraduate degree
ACRP Options 1 & 2
- Complete 3,000 hours performing essential job duties or 1,500 hours of equivalent work experience requirements through ACRP certifications or approved clinical research degree programs accredited by the Council for Higher Education
PATH 4: Earn a Master’s Degree for Opportunities in Management
A master’s program in clinical research is generally designed for those already working as CRAs to expand their skills or to advance into management or supervisory roles within the field. However, for those with non-health science bachelor’s degrees who want to become CRAs, seeking a master’s of science in clinical research or a master’s of science in clinical research management could be a pathway to breaking into the field.
Because many of these programs are offered online, earning a degree is possible for even those students who need full flexibility of schedule to complete the degree. Although requirements for admission into master’s programs vary, those looking to gain admission into a master’s of science for clinical research commonly need the following:
- A bachelor’s degree
- Official transcripts demonstrating specific coursework in science
- A statement of purpose
- Letters of Recommendation or Reference
- A resume or CV
- An application fee
- TOEFL or IELTS scores (international students only)
Clinical Research Associate (CRA) Degree Programs
There is a range of formalized training programs that prepare professionals for this key role in ensuring the safe, and ethical development of medical technologies. Below you will find examples of programs at a range of educational levels available to those interested in a career as a CRA.
Durham Tech – AAS Program
Durham tech, located in Durham, North Carolina, offers a 71-credit hybrid on-campus and online clinical trials research associate (CTRA) associate of applied science (AAS) program. Durham’s CTRA AAS prepares graduates to work on any side of clinical research in an assistant’s role.
While most programs require the student to attend on-campus courses, there are several courses that are offered completely online. The program takes 20 to 21 months and includes coursework in research site management; clinical research management; research protocol design; an introduction to ethics; anatomy and physiology; an introduction to clinical data; pathophysiology; and clinical research terminology.
Graduates of the program may be eligible to sit for national certification examinations and will be prepared for opportunities at medical centers, pharmaceutical industries, hospitals, research facilities, clinics, physicians’ offices, and device companies.
- Location: Durham, NC
- Accreditation: Southern Association of Colleges and Schools Commission on Colleges (SACSCOC); Commission on Accreditation of Allied Health Education Programs (CAAHEP)
- Expected Time to Completion: 20 to 21 months
- Estimated Tuition: $5,396
Washington University in St.Louis University College – BS, MS, Certificates
Washington University in St. Louis, Missouri, has various degree options for CRAs at all stages of their career to work as monitors. Students can enhance their current skills and knowledge in clinical research management, as well as gain a deep mastery regarding how to best move clinical research forward in an ethical, compliant, and safe way.
Those with at least six units of transferable coursework qualify to apply to the 120-credit-hour bachelor of science in clinical research management to start their careers. Anyone with any educational background can pursue University College’s 21-credit undergraduate certificate in clinical research management to enhance career skills or make a resume more competitive.
Students who already have a BA or BS also have options at Washington University. Experienced professionals in the clinical research field who wish to seek formalized training can earn a 21-credit advanced certificate in clinical research management or a 30-credit master of science (MS) in clinical research management. Those with a non-healthcare bachelor’s degree who wish to become high-level CRAs can up their skills and knowledge by choosing the combined bachelor’s and master’s degree options.
Although the coursework in each program varies to suit the level of education, themes across all the programs include the fundamentals of clinical research management; research ethics and regulatory affairs; compliance, legal and regulatory issues; and data and information management in health sciences.
- Location: St. Louis, MO
- Accreditation: Higher Learning Commission (HLC)
- Expected Time to Completion: BS (up to 48 months); certificate (12 months); MS (24 months)
- Estimated Tuition: Undergraduate courses ($695 to 895 per credit); Graduate courses ($665 to 995 per credit)
Barnett International – Online Seminar
Designed for CRAs with two years of experience or less, this online clinical research associate onboarding program by Barnett International prepares entry-level employees to monitor clinical trials at high levels appropriate to industry standards.
Over ten weeks of synchronous online coursework lasting three hours per week, participants will learn topics including informed consent, investigational product accountability, safety definitions and reporting requirements, and regulatory compliance and quality assurance: audits and inspections. Participants receive 30 hours (3.0 CEUs) of continuing education credits.
- Location: Needham, MA
- Accreditation: Accreditation Council for Pharmacy Education
- Expected Time to Completion: Ten weeks
- Estimated Tuition: By Early Bird Deadline ($1,795); After Early Bird Deadline ($1,995); June 10 is the early bird deadline
Continuing Education for Clinical Research Associates (CRAs)
Both CRA certification bodies require continuing education to maintain active certification status.
SOCRA requires recertification every three years. It calls for 45 hours of CE to be completed over the course of the first three years beyond passing the initial test. Twenty-two CE units must be related to clinical research; the remainder can be in the professional or therapeutic area in which one works or specializes. In addition, those looking to maintain or renew certification must complete a “recertification continuing competence learning module.”
The ACRP expects certified CRAs to engage in continuing education (CE) and continuing involvement (CI) to maintain certifications. Continuing education should include coursework in research and healthcare, and continuing involvement requires candidates to engage in activities such as authorship, participating in investigator meetings, or working as a peer reviewer, among other opportunities. Notably, ACRP utilizes an ongoing point system for professionals to maintain their certifications.
CRA Career and Salary
Clinical trials and the objectivity they bring to advances in treatment are extremely important. In an increasingly globalized society, diseases spread across borders, and in an age of increased antibiotic resistance, new ways to fight bacteria will be needed. Furthermore, with an aging U.S. population comes increased rates of chronic conditions and the subsequent reliance on pharmaceuticals to improve people’s quality of life.
It’s not surprising that the Bureau of Labor Statistics (2022) predicted a 7 percent increase in openings for medical and clinical laboratory technicians between 2021 and 2031, much more than the average growth anticipated across all U.S. occupations during that same decade (5 percent). As far as the salaries are concerned, here are the salary percentiles for clinical laboratory technologists and technicians in the US ( BLS May 2022):
Lastly, while the BLS doesn’t track salaries for CRAs, PayScale.com (June 2023)—a site that relies on self-reported data—found that the median annual salary for a CRA was $72,393. Among the 1,391 CRAs reporting their annual salaries, Payscale found these percentiles:
- 10th percentile: $48,000
- 50th percentile (median): $72,393
- 90th percentile: $101,000
Specialized skills in CRA that increased salaries included medical devices (37 percent pay increase over average), team leadership (35 percent), and writing procedures & documentation (20 percent).
Years of experience, predictably, also have an impact on salary. Entry-level CRAs earn 15 percent below the average, while experienced CRAs (ten to 19 years) earn 16 percent above the average and late-career CRAs (20+ years) earn 25 percent above the average.
It is important to note that these figures also vary based on the data source. For illustration, Indeed.com (June 2023) found an average annual salary of $80,957 among United States clinical research associates.

Becca Brewer is building a better future on a thriving earth by healing herself into wholeness, divesting from separation, and walking the path of the loving heart. Previously to her journey as an adventurer for a just, meaningful, and regenerative world, Becca was a formally trained sexuality educator with a master of education.
Related Articles
- Upskilling in the Allied Health Professions
- Single-use Plastics in Medicine Raise Concerns About Sustainability
- Clinical Research Certification - CCRA, CCRC, CPI
- Online Master’s in Clinical and Translational Research Programs
- Online Bachelor’s in Health Science Programs (BSHS Degree)
- Online Master's Programs in Clinical Management and Leadership
- Clinical Lab Science vs. Clinical Research
- BSHS (Health Sciences) vs BSHA (Health Administration) Programs
- Clinical vs Translational Research
- Guide to Health Science Careers
Related Programs
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US) Committee on Addressing Career Paths for Clinical Research; Kelley WN, Randolph MA, editors. Careers in Clinical Research: Obstacles and Opportunities. Washington (DC): National Academies Press (US); 1994.

Careers in Clinical Research: Obstacles and Opportunities.
- Hardcopy Version at National Academies Press
1 Introduction
Health care in the United States has improved markedly over the past five decades, in large measure because of the advances in health research that have been supported by a myriad of federal agencies, industry, the private nonprofit sector, and research institutions. Diverse teams of scientists composed of basic scientists, physicians, nurses, dentists, pharmacists, and other health professionals have been involved in research spanning a spectrum from fundamental biological discoveries about life processes, to behavioral and social research, to clinical and population-based studies, and to research on the organization, financing, and delivery of health care services. For example, imaging technology that allows investigators to peer into the human brain and observe the circuitry and biochemical reactions as humans construct thoughts and words is now available. Progress in genome mapping promises to provide monumental advances in understanding of genetic diseases and to aid investigators in finding biological therapies. Rational drug design is allowing researchers to custom design pharmacologic agents that can act on specific tissues, organs, or cell receptors and treat a broad spectrum of human maladies. Entirely new approaches to the treatment, cure, and prevention of human diseases are evolving with the availability of biological products and gene therapies. Research methods are being developed to permit investigators to evaluate the outcomes and effectiveness of health care practices. Research in these and other areas has formed a dynamic synergy that has positioned the United States at the forefront of innovation in medicine.
Despite all the advances, however, signs of stress are surfacing throughout the health care and health research systems. The soaring costs of health care and the escalating number of uninsured and underinsured people in the United States have thrown health issues into the policy arena at all levels of government. In medicine, highly subspecialized medical training, a declining interest by U.S. medical students in primary care training, and shortages of physicians willing to practice in rural or inner-city areas are all cited as symptoms of a worsening problem. The emergence of human immunodeficiency virus infection has demonstrated that new diseases can arise unexpectedly, and that a multifaceted approach spanning a variety of fields of research and a range of professional research scientists is needed to develop fundamental knowledge about a disease process, diagnosis, effective therapies, and prevention strategies and to assess the subsequent outcomes of health care practices. This can only be accomplished with highly talented and well-trained researchers in all areas of research, from basic to clinical research to outcomes and health services research.
Research is a highly social and political process of communication, interpersonal relationships, and scientific exchange that seeks to describe, explain, and modify biological and pathological processes. Researchers develop hypotheses and test them by collecting and analyzing data. The results add to existing knowledge. The unique feature of clinical research that distinguishes it from laboratory research is the direct involvement of human subjects. Although both laboratory and clinical research employ the same scientific principles for experimental procedures, the use of human subjects increases the complexities of scientific investigations. Whereas laboratory studies can more easily control for as many variables as possible to yield reproducible results, clinical research involves more heterogeneous populations, often is more expensive, takes longer to develop, requires long periods of time for data collection, and may be difficult to reproduce (Kimes et al., 1991). To advance medical care in patients, however, research must be performed in populations of patients with diseases.
Many research activities performed by a broad spectrum of professionals fall under the rubric of clinical research. Whereas many kinds of clinical research require similar skills and abilities, others may require different tools to achieve research objectives. Examples of how earlier investigations have influenced today's medical care are well known. Present research studies will improve tomorrow's medical practice, while future clinical research opportunities will affect care in the twenty-first century. What is the scope of clinical research, and what are the settings for conducting clinical research and the opportunities for future research?
- Scope Of Clinical Research
Clinical research is a relatively new discipline. Although the American Society for Clinical Investigation was formed in 1908, clinical advances prior to the 1950s were often based on imprecise observations by practicing clinicians (Cadman, 1994; Fox et al., 1992). In 1948 the British published the first randomized clinical trial, evaluating streptomycin in the treatment of malaria (Medical Research Council, 1948); the first clinical trial published in the United States was a study evaluating the effectiveness of penicillin for treating pneumoccocal pneumonia (Austrian et al., 1951). As methods for large-scale clinical studies became more refined, investigators gained an appreciation for new study designs, methodological advances, and the power of statistical analysis that permitted the validation of small differences between treatment regimens. Clinical research quickly became accepted as scholarly work and as an academic discipline (Fye, 1991; Ledley, 1991).
A major paradigm shift in clinical research was initiated in the 1970s when human cells were grown in vitro. As a result, some forms of clinical research could be performed on human cell lines grown in culture. This initiated a period some refer to as reductionism in which patients were no longer used as the primary focus of clinical research. This idea was extended by using the techniques of molecular biology, which permitted the study of human nucleic acid alterations in disease instead of requiring the study of the entire patient. Yet, in the final analysis, the application of these discoveries to improve medical care requires that these findings be used on the whole patient.
In parallel, during the last decade the discipline of clinical research has undergone a remarkable evolution in the scope, sophistication, and power of its methodologies. Changes have occurred in the approach to data collection, experimental design, and data analysis, and these changes provide a stronger basis for clinical research. In addition, understanding of the pathogenesis of diseases has provided more precise concepts of preclinical and subclinical disease states. The application of molecular epidemiology is a prime example of these changes in clinical research. Now the results of new biology are ripe for application to improve medical care, but many fear that a talented cohort of clinical investigators has not been prepared to translate these fundamental advances into improved medical care.
The revolution of fundamental research discovery is expected to accelerate in the future, driven by the explosion of science in biotechnology, molecular biology, computer technology, diagnostic systems, decision modeling, and clinical measurements technology. The sophisticated methods for clinical research require investigators with the requisite talents to design excellent clinical studies, recruit adequate numbers of research subjects, and analyze the large amounts of data collected. The need for cross-disciplinary teams to accomplish the objectives of multicenter, complex clinical trials is readily apparent. It is clear that training for a career in clinical research must be as rigorous as training for a career in the traditional basic sciences. Understanding of both the basic sciences and the evaluative sciences is essential to the success of clinical researchers. Moreover, novice clinical investigators require the same mentoring and nurturing in a supportive environment as those engaged in fundamental research disciplines if they are to develop into mature, independent scientists who remain competitive and productive over an extended period of time.
Numerous advances can be cited to describe opportunities in clinical research; the following allow one to comprehend their broad scope. One dramatic example of progress in fundamental research that has opened up immeasurable clinical research opportunities is the discovery in 1989 of the gene that is mutated in patients with cystic fibrosis. The gene was identified by using the advanced methodologies of positional gene mapping. Investigators delineated the nature of the mutation that leads to the production of a defective protein in the membranes of cells from patients with cystic fibrosis patients. Subsequent research demonstrated that this protein is associated with a membrane channel involved in the transport of chloride ions. This understanding has led to a number of chemical approaches to treat the disease. In addition, new efforts are under way to treat or cure the pulmonary manifestations of the disease by employing methods that are being developed in DNA transfer therapy. Research that is now being conducted in the laboratory will soon be carried over to use in patients with cystic fibrosis. Clinical investigators are crucial to the performance of this work and in bringing these novel therapies into common practice. Their participation will also be necessary to help determine how to deliver the technology efficiently, under what conditions and to which patients, and to assess the outcomes of these new therapies.
Hundreds, if not thousands, of other genetic disease are now being studied in the same fashion. As knowledge about the underlying genetic mechanisms for these diseases grows, new treatment approaches developed from basic laboratory techniques will be carried forward into clinical trials. In addition, genetic factors are being defined in diseases that have been regarded as multifactorial. For example, breast cancer scientists are on the threshold of discovering the genes that regulate its occurrence. Thus, approaches to modify the expression of these genes may be useful in the treatment of breast cancer. Genes that regulate the formation of atherogenic lesions in arteries, abnormalities that lead to coronary artery disease and heart disease have recently been identified. Blocking the activities of these genes using antisense gene therapy has been shown to block the progression of atherogenic lesions in arteries in animal models. On the basis of results of these promising studies, antisense gene therapies are being developed for use in humans. Novel therapies directed at blocking the genetic expression of the factors that determine at herogenesis as well as genetically directed products that can prevent or reverse these effects may be developed in the future and may lead to treatments or cures for ischemic heart disease and some forms of stroke. Clearly, clinical investigators will be critical for developing and testing these new therapies to determine their safety, efficacy, effectiveness, and cost-effectiveness in humans. Clinical investigators will also play a role in discussions regarding ethical considerations such as genetic testing and elucidating the behavioral or environmental factors influencing genetic diseases.
Although new therapies are being developed rapidly and require extensive clinical testing, old or current therapies should be rigorously evaluated as well. During the past few years several groups have initiated studies to examine the outcomes of current therapies for particular diseases or conditions (Eddy, 1984; Roper et al., 1984). For example, a broad-based research team has been investigating the treatment for benign prostate hypertrophy in a patient population in Maine (Wennberg et al., 1988). By taking into consideration the behavioral and social attributes of patients, the outcomes of the various treatments have been assessed. Not all treatment regimens are viewed favorably by patients, who have various needs and desired outcomes. Thus, the outcomes of particular therapies require sophisticated scientific methods to determine the effectiveness of therapy in patients with different expectations and needs. Other examples of opportunities for outcomes research can be cited by examining the topics under investigation by the Patient Outcomes Research Teams funded by the Agency for Health Care Policy and Research, such as low back pain, joint replacement, incontinence, and others. The research methods and tools used by those investigators are every bit as sophisticated as those needed to clone genes or isolate and characterize proteins. Similar studies in other fields of medical practice using these novel methodologies will be critical in the future.
The diversity of the preceding examples is a small sampling from a field rich in opportunity for improving medical care for millions of people in this country and around the world. An important interface in bringing these technologies to patients is the clinical investigator—the bridging scientist. The remarkable progress that has been evidenced in fundamental biology brings with it parallel opportunities for investigations in human populations. The realm of biomedical research can be viewed as a spectrum, with fundamental research occurring throughout the spectrum, some of which uses humans to answer crucial questions about human health and behavior. Thus, there is no discontinuity between fundamental biological science and clinical investigation. Indeed, it is progress throughout this research spectrum that frames the opportunities for progress in clinical research.
Increasing levels of sophistication and the assurance of an ample supply of excellent clinical investigators to carry technological advances to medical practice remain critical issues if the country is to continue to improve its health care system. There is growing evidence, however, of a discontinuity in the process of translating new research discoveries into improved health care; the process is further threatened by a potential lack of well-trained clinical investigators to provide the bridge to bring these discoveries into improved medical care (Kelley, 1988).
In the 20 years following World War II, bountiful resources were provided by the federal government to support research, primarily at the nation's research universities and medical schools (U.S. Department of Health, Education, and Welfare, Public Health Service, 1976). This paradigm of peer-reviewed, university-based research has been attributed to the wisdom and foresight of Vannevar Bush (Bush, 1945). Resources were not only plentiful for supporting research but numerous programs were also initiated to build the physical research infrastructure and train more highly talented scientists (Institute of Medicine, 1990). The biomedical research community responded, and the nation's health research capacity expanded significantly. During this period research that involved interactions with human subjects, possibly with the exception of psychological studies, was primarily the domain of physician-scientists. Many of these physician-scientists were motivated to pursue research careers because of the rapid advances in biomedicine and the potential to become critical players in medical discovery. Others may have pursued research to avoid military service in an unpopular war in Southeast Asia. Nonetheless, after completing their clinical training residencies, many physicians sought fellowships at the National Institutes of Health (NIH) and subsequently moved into academic and research positions around the country. Whatever their motivation, most of these scientists have contributed to the fount of knowledge that serves as the basis of modern health care.
In the late 1970s and early 1980s, many leaders in the medical research community expressed concern about a perceived decline in the participation rates of physicians engaged in all aspects of biomedical research (DiBona, 1979; Gill, 1984; Kelley, 1980 and 1985; Thier et al., 1980; Wyngaarden, 1979). This perception was supported by data demonstrating that the ratio of M.D.s to Ph.D.s successfully obtaining research grant awards from NIH was declining. More alarming was the notion that individuals who were highly trained in patient care and who were considered the technology transfer agents were not seeking rigorous scientific training, which widened the gap between basic research discoveries and application of these advances to improved health care (Glickman 1985; Healy, 1988). Furthermore, although some physicians were seeking training in the basic biological sciences, there was a perception that few were being trained to develop and test hypotheses in human subjects or populations (Forrest, 1980). Ironically, data show that the number of full-time faculty in medical schools has grown by more than 20,000 over the past decade, to nearly 65,000. (Data from the Association of American Medical Schools report that medical school faculty totaled 65,000 in 1990, whereas data collected for the Liaison Committee for Medical Education reports that faculty totals were nearly 80,000.) It has been hypothesized that this growth reflects a growing dependence on medical center profits to offset increasing constraints on research funds and shrinking subsidies for graduate medical education (Chin, 1985; Hughes et al., 1991). Although faculty members are required to perform scholarly activity, there appears to be an increasing demand on the clinical faculty to derive revenue through patient care. Furthermore, the growth in clinical faculty may have increased tensions between the faculty in basic science departments and those in the clinical departments. These tensions may arise because basic science faculty fear that their research funding base is being eroded by growing research activities in the clinical departments, and the growing number of clinical faculty bringing in patient care dollars positions the latter on a firmer financial footing. There is also a perception that some academic clinicians pursue research as a secondary interest and are not serious investigators. Many of these clinicians also feel that they cannot obtain tenure by performing human subject research, where the results may not be realized for many years and funding is believed to be extremely difficult to obtain. Moreover, those clinicians who focus on human research fear that they are perceived as second-rate scientists by their colleagues who perform fundamental research in both clinical and basic science departments.
A cause and effect has been difficult, if not impossible, to prove. Determining the size of the cohort of clinical investigators is fraught with error, because no database currently exists to track these investigators. Moreover, there has been no systematic way to collect and analyze data on the number of individuals who choose to perform clinical investigations, the availability of training pathways, or the outcomes of those few programs that do exist. Although many believe that quantitative factors such as debt and economic status directly influence decisions to pursue academic and research careers, there appear to be no measures for factoring in personal considerations such as the effects of mentors and role models, the desire to spend time with one's family, or having leisure time to pursue other personal interests. The growing base of fundamental science, the increasing complexity of medical care and understanding outcomes or effectiveness research, the difficulty (real or perceived) in obtaining research funding, and countless demands on an investigator's time all seem to weigh heavily against pursuing a career in research, particularly research that involves interactions with human subjects. The many employment sectors that require this expertise, such as federal agencies and industry, are also obstacles to conducting a thorough analysis.
Although most attention has been focused on the plight of physician-scientists, many other professional groups are experiencing similar difficulties in the area of human research. As in medicine, training for research careers in other professions is often fragmented, and the career pathways that young trainees should pursue are not clearly delineated. Although many of these other professions also provide outstanding training for delivering care, their programs may not be specifically structured for developing research capabilities. Thus, the Institute of Medicine ( IOM ) sought to undertake an analysis of the problems affecting the career paths leading to clinical research.
- Origins Of The Study
IOM has had an ongoing concern about the problems in the biomedical research arena, and particularly those problems confronting researchers who perform studies that require human subject participation. In 1988, IOM was commissioned by NIH to conduct a study to assess the availability of resources for performing research using patients. The committee was asked to consider a series of issues, including the effects of changes in the health care system on the environment for clinical research; how to improve the recruitment of medical students and residents into clinical research careers; identification of barriers to translating basic research advances into clinical practice; how to improve the relationships among clinical researchers, federal sponsors, and industry; the organization of clinical research; and how to stimulate interest in evaluative clinical sciences. Whereas that committee was asked to examine clinical research in the narrow sense of human subject research, the data from NIH that were available to the committee included all research on humans or human materials approved by institutional review boards, as indicated on Public Health Service grant application form number 398. This included research on all human material such as DNA, RNA, proteins, cells, or body fluids for in use in vitro studies, not necessarily material related to a patient's disease or involving the patient. Moreover, the committee was not able to glean any information from the private sector, either for-profit or nonprofit, to construct a complete picture of the resource base for patient-oriented clinical research.
Following the release of its report, Resources for Clinical Investigation (Institute of Medicine, 1988), the IOM Board on Health Sciences Policy convened a working group to reexamine issues related to clinical research. The working group recognized that the heterogeneous nature of the research training pathways for physician-scientists and the broad spectrum of research questions pursued by those investigators had complicated earlier analyses. The working group met twice to develop a strategy for exploring problems associated with the clinical research training pathways, particularly for physician-scientists. The working group sought to refine an approach that would isolate only the small portion of physician-scientist training that it felt was in a particularly vulnerable stage—patient-oriented clinical research—and did not attempt to address all the problems associated with physicians engaging in basic or health services research.
In December 1989, the National Research Council released the quadrennial report Biomedical and Behavioral Research Scientists: Their Training and Supply (National Research Council, 1989), which examined research training supported by the Public Health Service through National Research Service Awards (NRSAs). Although that report presented a detailed analysis of the doctoral biomedical and behavioral research workforce and recommended the numbers of NRSA trainees that should be supported, it paid scant attention to physician-scientists and largely ignored dentist- and nurse-scientists. The reasons for these omissions remain unclear, but they probably are the result of the inability to develop clearly defined populations of scientists in these professions. Whereas physicians, dentists, and nurses engage in a broad spectrum of fundamental research activities, they are critical players in clinical research. Although this group of scientists has often been referred to as clinical researchers because of their clinical degrees, they might be more appropriately referred to as clinician-researchers. Furthermore, the population of doctoral scientists engaged in human research also has remained undefined.
Following the release of the 1989 NRSA study, IOM 's Committee on Policies for Allocating Health Sciences Research Funds released a report in 1990, Funding Health Sciences Research: A Strategy to Restore Balance (Institute of Medicine, 1990). That committee also acknowledged that the limited understanding of the physician-scientist population and barriers to effective training of that population hampered the committee's attempts to recommend ways to overcome the barriers confronting those investigators. Thus, they recommended that a thorough analysis be performed on physician-scientists to clarify many of these issues.
- Charge To The Committee
The Committee on Addressing Career Paths for Clinical Research was formed in 1991 and was charged with identifying and evaluating issues in the education and training pathways for individuals pursuing careers in clinical investigation. In particular, the committee was asked to investigate ways to improve the quality of training for clinical investigators and to delineate pathways for individuals pursuing careers in clinical investigation in nursing, dentistry, medicine, and other related health professions engaged in human research. The committee was charged with the following: defining clinical research, how to stimulate individuals to pursue careers in clinical investigation, how to define appropriate curricula for training, how to identify mechanisms to bridge the gap between the basic and clinical sciences, how to address funding mechanisms for clinical investigation, how to establish measures of success in clinical research other than obtaining R01 grant support, how to encourage academic and industrial institutions to protect and reward these valuable investigators, and how to ensure adequate support mechanisms for retaining clinical researchers. For comparison, the committee also examined the pathways that lead physicians toward careers in basic research. The study focused on how existing structures and mechanisms in the federal government, universities, and industry might be used in new and innovative ways to foster careers for these groups of researchers.
The chair of the National Research Council appointed a 16-member committee to address the questions posed in the committee's charge. The committee was composed of active researchers and research administrators with expertise in nursing, dentistry, evaluative clinical sciences, surgery, epidemiology, and various medical subspecialties. The committee viewed several areas as deserving special attention, and these were addressed by task forces, including task forces in surgery, dentistry, nursing, and clinical psychology. The complete task force reports are included as appendixes to this report.
- Defining Clinical Research
The first item on the committee's agenda was to derive a working definition of clinical research. Various definitions have been used to describe or inventory research and development activities. Many lexicons classify research and development expenditures into the following three general categories: (1) basic research, (2) applied research, and (3) development. Although this classification scheme is useful for describing various research activities for budgetary purposes, it becomes less appropriate for describing cross-disciplinary clinical research, which may encompass portions of each of these categories.
Classification schemes often portray a linear progression of scientific knowledge from basic biological research, to applied research and development, and to improved diagnosis, treatment, and prevention of human disease. Many would argue, however, that a broad spectrum of research activities, from the most basic discoveries of nature to the application of knowledge in humans to understand and treat disease, would more accurately portray biomedical research ( Figure 1-1 ). Furthermore, research activity throughout the spectrum could be bidirectional or demonstrate circular feedback loops for generating new hypotheses. For example, many basic biomedical research questions arise from disease processes first observed in patients. Moreover, the boundaries between many of these subcategories are indistinct, with varying degrees of overlap and movement over time.
Diagram depicting the broad spectrum of clinical research, examples of training pathways, and possible sources of research funding. (Adapted from Kelley, 1988.)
Several clinical research classification methodologies have been attempted, each with its own limitations. Clinical research encompasses a vast range of research activities that are conducted by investigators in numerous disciplines. Ahrens has categorized the disparate activities encompassed under the rubric of clinical research into the following seven areas (Ahrens, 1992, pp. 40-48):
Studies on the mechanisms of human disease
- refinements in characterizations of disease processes
- explorations of unresolved questions in human biology
Studies on management of disease
- evaluations of new diagnostic and therapeutic techniques and devices
- drug trials (phases II, III, IV)
- studies of patient compliance and prevention measures
- searches for accurate prognostic markers
In vitro studies on materials of human origin
Animal models of human health or disease
Field surveys
Development of new technologies
Assessment of health care delivery.
All seven categories of research are essential to the progress of medical care and, ultimately, to the prevention of disease. Because the boundaries between these areas are indistinct, individuals can be working in more than one category at any given time.
The committee sought to derive a definition of clinical research that would cut across artificial boundaries to describe the universe of clinical research in terms of research activities or goals. Although there is a large amount of basic biological research that is not directly relevant to specific human diseases, such laboratory-based preclinical bench research may have direct links to understanding normal human function and disease. For example, control of human or retroviral gene expression as well as animal or cellular models of normal or diseased biological processes in humans is often clinically relevant, and under some classification schemes it is defined as clinical research.
At the other end of the spectrum is research on human subjects and populations that have direct application for understanding the prevention, diagnosis, and treatment of human disease by exploiting disciplines such as health services research, clinical epidemiology, and outcomes assessment. Undoubtedly, clinical research includes phase I-III human clinical trials to assess the effectiveness of new methods of intervention or patient management in defined populations. A body of research is also directed at understanding motivational factors for disease prevention and screening and the social and emotional impacts of disease and treatment by employing the disciplines of psychosocial, behavioral, and educational research, which can be considered clinical research. Thus, the committee agreed that there is a continuum of research spanning a wide range of activities that can be regarded as clinical research.
The committee emphasizes that clinical research is not simply that research performed by physicians or other professionals holding clinical degrees. Clearly, many scientists holding doctorates in the basic sciences are performing research that is very clinical in nature; many physicians are also outstanding basic scientists. Although it is very difficult to arrive at an unambiguous definition that will be agreeable to all parties, the committee believes that clinical research should be directed toward the elucidation of human biology and disease, and the control thereof.
The committee emphasized that a common definition should be as broad and inclusive as possible to accurately reflect the population of biomedical scientists generating knowledge about human ''disease." Furthermore, the committee acknowledges that clinical researchers may be performing research in more than one category; they may move back and forth along the spectrum as their line(s) of investigation matures or new research questions evolve. Thus, the committee proposes the following definition:
Clinical investigation , broadly defined, includes all studies intended to produce knowledge valuable to understanding the prevention, diagnosis, prognosis, treatment, or cure of human disease. This includes biomedical and health services research carried out in humans, usually by health care professionals, as well as research in organs, tissues, cells, subcellular elements, proteins, and genes derived from humans. It may also include the study of micro-organisms as well as studies of other members of the animal kingdom when this research is directed toward human disease.
Whereas this definition is suitably inclusive for all the researchers engaged in clinical research in the broadest sense, the committee identified specific areas that it believes need particular attention. The evolution of the new biology has begun to erode the perceived boundaries among the various medical disciplines as well as the boundaries between basic and clinical research. Moreover, the importance of basic research or other training experiences for teaching research methodology and study design to young clinical investigators cannot be overstated. Thus, the committee felt compelled to develop a broad definition for clinical research and then to focus on areas that it believes need immediate remediation to foster continued progress in clinical research. The special theme and focus of this study was patient-oriented clinical research, defined as that which requires "hands-on" participation with a human subject as opposed to the entire spectrum of clinical research. Interpreting its charge, the committee recognized that many professions are engaged in clinical research, including dentistry, nursing, pharmacy, osteopathic medicine, and the behavioral sciences, among others, and sought to include the perspectives of members of those professions as well. Nevertheless, the committee reinforced the common theme of the study and posed the following global questions about clinical research and the clinical research workforce:
- What can clinical research accomplish now and in the future to improve medical care?
- Is the current clinical research community poised and prepared to accomplish these goals?
- If the clinical research community is not prepared to accomplish these goals, what is the evidence that there is either inadequate clinical investigation or an inadequate number of well-trained clinical investigators to meet this need?
- What are the best approaches or best vehicles for change to improveclinical investigation and ensure a supply of highly competent clinicalinvestigators to meet these needs and accomplish the research goals?
- Limits On The Scope Of The Study
Although the committee developed a broad definition of clinical research, the major focus of the study was clinical research in which patients serve as the research subjects, often referred to as patient-oriented, patient-related , or preferably, human research . This category of clinical research includes research activities such as the characterization of healthy and diseased human function; evaluation of new diagnostic, therapeutic, and prognostic techniques, approaches, and devices; medical decision making; patient compliance and disease prevention research; health education research; drug trials; and the assessment of health care practices on patient populations. Thus, the committee's deliberations focused on the issues surrounding the preparation and training of clinical researchers who are engaged in research that requires the direct participation of human subjects. Lastly, although the committee frequently mentions areas of potential clinical research opportunities, it was not charged with developing a research agenda in clinical research and uses the examples only for reference.
- Conduct Of The Study
During the course of the study, the committee held four meetings to develop strategies and to analyze data. The committee used a variety of approaches to expand its expertise by involving as many avenues of input as possible to achieve its objectives, including four subcommittees, three task forces, a workshop, 11 commissioned papers, and information gained through solicitations of written input and interviews.
Subcommittees
First, the committee divided its members into the following four subcommittees to identify problems along the career pathways of clinical researchers: (1) undergraduate and precollege science education and research training, (2) research training during health professional school, (3) postdoctoral clinical research training, and (4) nurturing clinical research faculty. These subcommittees were convened separately to identify issues confronting their respective portions of the pathways and to develop approaches to collecting and analyzing data that could be used to draw conclusions.
Task Forces
Three task forces were convened in the spring of 1992 to address clinical research issues specific to (1) nursing and clinical psychology, (2) dentistry, and (3) surgery. Each of these task forces was chaired by a member of the committee and the membership was selected from those in the profession. They were charged with the following:
- Describe the clinical research performed by researchers in their respective professions and emphasize how it is different from that in other professions.
- Determine how many researchers in their profession are engaging in clinical research and estimate how many are needed.
- — Identify what needs to be enhanced or changed to encourage recruitment and the retention of clinical researchers in the profession.
- — Identify the funding sources for clinical research in their profession.
- — Explore the training backgrounds of the current cohort of clinical researchers in the profession.
- — Identify the education and training requirements for preparing clinical researchers in the profession.
- — Recommend changes necessary to address new clinical research questions for the profession in the future.
- — Describe how changes can be implemented or interwoven into existing organizational structures.
- — Identify the research training resources for individuals pursuing careers in clinical research for the profession.
- Recommend possible solutions to improving the career pathways leading to clinical research.
The complete task force reports can be found in Appendixes A , B , and C at the end of this report.
In June 1992 the committee sponsored a one-and-one-half-day workshop entitled "Clinical Research and Research Training: Spotlight on Funding." The overall goal of the workshop was to analyze training and research funding data and to explore innovative approaches to the training and support of clinical investigators. The first day of the workshop focused on the roles and responsibilities of research sponsors including the federal government, industry, the private nonprofit sector, third-party payers, and academic health centers and research institutions. The second day concentrated on the organizational barriers to clinical research training as well as the funding available for training. A transcript of the meeting was made for the use of the committee in preparing this report, but the committee chose not to publish a separate workshop proceedings.
Commissioned Papers
The committee commissioned 11 background papers to analyze topics of particular importance to the committee's deliberations. Although the findings of the papers are incorporated into this report, the committee felt that the papers were of such high-quality and made such significant contributions toward a better understanding of clinical research careers that they encouraged the authors to publish them separately. The following is a list of the paper titles and authors:
"Early Exposure to Research: Opportunities and Effects," by Marsha Lake Matyas of the American Association for the Advancement of Science.
"Advisers, Mentors, and Role Models in Graduate and Professional Education: Implications for the Recruitment, Training, and Retention of Physician-Investigators," by Judith P. Swazey of the Acadia Institute.
"The Effectiveness of Federally Supported Research Training in Preparing Clinical Investigators: Important Questions but Few Answers," by Georgine Pion of Vanderbilt University.
"Considerations of Educational Debt and the Selection of Clinical Research Careers," by Robert L. Beran of the Association of American Medical Colleges.
"Models of Postdoctoral Training for Clinical Research," by Thomas Lee and Lee Goldman of the Brigham and Women's Hospital.
"Models of Postdoctoral Training for Clinical Research," by David Atkins, Richard A. Deyo, Richard K. Albert, Donald J. Sherrard, and Thomas S. Inui of the University of Washington.
"Role of the GCRC in Establishing Career Paths in Clinical Research," by Charles Pak of the University of Texas Health Science Center.
"The Image of the Clinical Investigator," by Edwin Cadman of Yale University.
"University-Industry Relationships in Clinical Research: University Perspective," by David A. Blake of Johns Hopkins University.
"Roles and Responsibilities of Resident Review Committees and Certification Boards in Promoting Research Careers," by Linda Blank of the American Board of Internal Medicine.
"Clinical Research in Allied Health," by Leopold G. Selker of the University of Illinois.
Grants Analysis
The committee also undertook a detailed analysis of R01 grant awards that have been approved by institutional review boards to determine the fraction of awards that are truly patient-oriented, apart from those that use human materials or body fluids. Because the R01 pool represents about 55 percent of the total extramural funds awarded by NIH and because of the large time commitment required to read through grant files, the committee chose to limit the analysis to R01-type grant awards that were considered by initial review groups (study sections) in the Division of Research Grants ( DRG ). Of the more than 16,000 R01 grants active in fiscal year 1991, about 14,535 were reviewed by DRG study sections; of those, about 4,284 indicated the involvement of human subjects or materials. Of this 4,284, a random sample of 450 from 11 institutes was used for this analysis. The committee reviewed grants provided by the National Cancer Institute, National Heart, Lung, and Blood Institute, National Institute of Deafness and Communicative Disorders, National Institute of Arthritis, Musculoskeletal and Skin Diseases, National Institute of Child and Human Development, National Institute of Neurological Diseases and Stroke, National Institute of Allergy and Infectious Diseases, National Institute of Aging, National Institute of General Medical Sciences, National Institute of Diabetes, Digestive and Kidney Diseases, and National Eye Institute. Since the committee convened task forces on nursing and dentistry that evaluated grants in these disciplines, it did not include grants from the National Institute of Nursing Research (formerly the National Center for Nursing Research at the time the task force was convened) or the National Institute for Dental Research in the analysis. Furthermore, because National Institute of Mental Health, National Institute of Drug Abuse, and National Institute of Alcohol and Alcohol Abuse were not officially part of NIH at the start of this project and the transfer of these institutes was not assured in mid-June of 1992, grants from these institutes also were not included in the analysis. The results of this analysis are presented in Chapter 3 .
To supplement the information gleaned from each of the aforementioned mechanisms and to add breadth to the material available to the committee, IOM staff undertook several interviews of staff in various federal agencies, including NIH, the Food and Drug Administration, the Agency for Health Care Policy and Research, and Alcohol Drug Abuse and Mental Health Administration. Many of the data for the study came from NIH staff, to whom the committee is truly indebted. Because of the broad nature of the study, many sectors, public as well as private, contributed valuable information. Appendix E recognizes the many individuals who made important contributions to the report and are not cited elsewhere.
- Structure Of The Report
This report presents the findings from all the aforementioned methods of data collection and analysis. The following chapters elaborate on the issues the committee explored, presents its findings and conclusions, and offers its recommendations for improving clinical research career pathways. Chapter 2 examines the employment sectors and issues and obstacles confronting established clinical investigators, with an emphasis on academic clinical investigators. Chapter 3 discusses the available resources for funding clinical research. The obstacles and barriers to training pathways are presented in Chapter 4 . Chapter 5 explores the academic-industry relationships and the roles and responsibilities of investigators in these alliances.
- Cite this Page Institute of Medicine (US) Committee on Addressing Career Paths for Clinical Research; Kelley WN, Randolph MA, editors. Careers in Clinical Research: Obstacles and Opportunities. Washington (DC): National Academies Press (US); 1994. 1, Introduction.
- PDF version of this title (5.0M)
- Disable Glossary Links
In this Page
Other titles in this collection.
- The National Academies Collection: Reports funded by National Institutes of Health
Recent Activity
- Introduction - Careers in Clinical Research Introduction - Careers in Clinical Research
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

News & information
Keep up with the latest from r&d partners, 10 clinical research career paths and progression opportunities, felicia rodriguez.
- October 30, 2023
Clinical research careers contribute to the development of safe and effective treatments and therapies for patients. The responsibilities may vary based on the organization, therapeutic area, and specific study requirements, but they all share the common goal of advancing medical science and improving healthcare outcomes.
Progressing in clinical research jobs involves a combination of experience, education, certifications, and networking. All of these are fairly essential for career growth, although the specific path and opportunities may vary depending on your interests, the organization you work for, and the area you specialize in.
In this guide, we explore some of the many careers in clinical research, from entering the profession to potential progression opportunities.
Clinical research career paths
Clinical research careers can follow a range of routes. Here are ten clinical research jobs you can go into, along with their responsibilities.
1. Clinical research coordinator (CRC) An entry-level role, CRCs assist with patient recruitment, obtaining informed consent, data collection, and ensuring protocol adherence. They coordinate study visits, maintain documentation, and communicate with investigators.
2. Clinical research associate (CRA) Entry-level clinical research associate jobs involve monitoring clinical trial sites, verifying data, ensuring regulatory compliance and study protocols, and assessing the safety and wellbeing of study subjects.
3. Clinical trial manager Clinical trial managers oversee all operations of a clinical trial, from study initiation to close-out. They manage budgets, timelines, and teams of CRAs, ensuring that trials are executed successfully.
4. Clinical project manager Clinical project managers manage and oversee multiple trials within a program. They collaborate with cross-functional teams, manage resources, and ensure that each phase of a project aligns with organizational goals.
5. Regulatory affairs specialist Regulatory affairs specialists are largely responsible for the administrative side of compliance. They compile, submit, and maintain regulatory documents for approval. You’ll have to stay informed on changing regulations and liaise with regulatory agencies, ensuring that all policies are adhered to.
6. Data manager Data managers manage clinical trial data, overseeing data collection, cleaning, and database management. They ensure data quality and see to it that all standards are followed, working closely with biostatisticians.
7. Clinical research scientist Clinical research scientists design study protocols, collect and analyze data, and interpret results. They also write study reports and publish findings in scientific journals.
8. Medical monitor Medical monitors oversee patient and subject safety during the trial, review adverse events, and make recommendations for study adjustments or halts based on medical knowledge.
9. Clinical quality assurance auditor Auditors conduct regular inspections and audits to adhere to regulations and quality standards. They identify non-compliance issues and recommend corrective actions.
10. Clinical research consultant Consultants provide expert guidance on various aspects of clinical research, including study design, regulatory strategies, and data analysis. They work independently or with organizations to solve complex problems.
Clinical research progression opportunities
To advance in clinical research careers, you can further your education by pursuing advanced degrees. These might include a Master’s in Clinical Research or an MBA. You could also obtain certifications relevant to your role, like the Clinical Research Professional (CCRP) or Project Management Professional (PMP).
Networking can also help you to get ahead by building rewarding relationships, connecting with peers and mentors in the industry and learning from more senior professionals. This could involve attending conferences, joining professional organizations, and asking to shadow leaders.
Here’s more detail on clinical research progression, and areas the above ten roles can move into.
1. Senior CRC or Clinical Research Associate (CRA) Clinical research coordinators (CRCs) can progress to a senior CRC or clinical research associate (CRA). Responsibilities include more independent study management, training junior coordinators, and handling complex trials.
2. Senior CRA or clinical trial manager Clinical research associates (CRAs) can progress to a senior CRA or clinical trial manager position. The duties of senior clinical research associate jobs are focused on starting to mentor junior CRAs. Clinical trial managers take on even more responsibility by overseeing an entire team of CRAs.
3. Clinical project manager or senior clinical trial manager Clinical trial managers can progress to clinical project manager roles, which involve managing the entire project portfolio. Senior clinical trial managers then demonstrate their capabilities by handling significantly more complex trials.
4. Director of clinical operations Clinical project managers can look to become directors of clinical operations. Directors play a pivotal role in overseeing the management and execution of clinical research programs within an organization, including teams of project managers and CRAs.
5. Regulatory affairs manager or director Over time, regulatory affairs specialists might be able to take up a manager position, which would involve handling larger portfolios of products. Eventually, you could become a director, overseeing an entire regulatory department.
6. Senior data manager or clinical data scientist Data managers often move into senior roles, which means managing larger datasets. You could then set your sights on becoming a data scientist, specializing in data analysis.
7. Senior research scientist or director of clinical research With experience, clinical research scientists can aim to become senior scientists. You would take on more significant research projects, possibly with the goal of becoming a director. This would mean running an entire research department.
8. Chief medical officer (CMO) Medical monitors can progress to chief medical officers (CMOs), who are responsible for all medical aspects of clinical research within an organization.
9. Senior auditor or quality assurance manager Later in their careers, clinical quality assurance auditors might become senior auditors, overseeing an audit team. You could then take up a quality assurance manager position, managing the entire quality assurance program.
10. Clinical research consultant As a consultant, your options for progression are slightly different. Rather than looking to move into a different role, the goal is usually to build a larger client base and gain expertise in specific therapeutic areas. Your earnings and reputation can then grow as you become an expert in the field.
Progressing your career with R&D Partners clinical research staffing agency
R&D Partners are dedicated to helping you excel in the rewarding field of clinical research. As experienced clinical research job recruiters, we understand that a rewarding career in this industry can shape the future of healthcare, making a positive impact on people’s lives.
Whether you’re looking for entry-level clinical research associate jobs or senior leadership roles, our goal is to provide you with insights, strategies, and guidance to chart your path to success in this ever-evolving industry. As partners to many leading life science organizations on the east and west coast, we can bring you exclusive career opportunities not available anywhere else.
Contact our friendly team to discuss your career options, or browse our current opportunities in clinical research careers.
Our global FSP & strategic staffing firm specializes in Scientific, Clinical Research, and Engineering.
Explore our top job opportunities.
We offer life sciences niche functional service provider & strategic staffing solutions to help you reach your goals.

Clinical Research Certification
Clinical research training.
Get Certification in Clinical Research with Comprehensive Clinical Trials Training Online
Get clinical research career training in 1 to 4 weeks with our online accredited clinical research courses. Trusted by organizations and experienced researchers.
Our clinical research courses are used by students at 1,200+ organizations, 6 government agencies, and 308 universities.† Graduates of our program work at 1,600+ different companies.‡
Schedule Career and Course Advising with Liz or Hazel
View 18 Graduate Case Studies and Reviews from April 2024
Advanced ICH GCP Certification (AGCPC)
Objectives: Provide an advanced and engaging review of International Conference on Harmonization Good Clinical Practice (ICH GCP) guidelines updated for 2024.
Students Enrolled: 3,166†
Students came from: Multiple universities, hospitals, research facilities, contract research organizations, medical practices, and biopharmaceutical companies at different stages
Requirements: HS Diploma or GED
Format: Advanced, online, self-paced.
Length: 16 Hours. Online, self paced, start anytime.
Accreditation: ACCRE, Transcelerate Biopharma
Certification: Online certificate. Exam score 70% or higher on 2 attempts.
Graduates work at: University research groups, hospitals, clinics, clinical trial sites, pharmaceutical companies, and government agencies
Graduate Job Roles after course: ICH GCP training is required every 2 years for all research roles thus our graduates work in a range of fields.
Research roles: Research Assistant, Lab Assistant, Research Coordinator, Research Scholar, Postdoctoral Researcher, Graduate Research Assistant, etc. Intern roles: Research Assistant Intern, Outpatient Pharmacy Intern, Clinical roles: Clinical Affairs Intern, Clinical Fellow, Clinical Nurse, Clinical Operations Manager, Clinical Research Professional Teaching roles: Assistant Professor, Lecturer, Graduate Teaching Assistant, etc. Management roles: Clinical Research Manager, Pharmacy Operations Manager, Associate Director of Clinical Development, Vice President of Clinical Development Specialized roles: Drug Safety Associate, Regulatory Specialist, Scientific Consultant, Medical Laboratory Scientist, Pharmacovigilance Associate Other roles: NHS Primary Care QI Facilitator, Government Healthcare Recruiter, Research Ethics Coordinator (based on review of new job positions after enrollment in course)
Pharmacovigilance and Drug Safety
Advanced pharmacovigilance and argus safety certification (apvasc).
Objectives: Gain advanced education in pharmacovigilance management and proficiency in international regulatory affairs and drug safety monitoring.
Students Enrolled: 5,708†
Students came from: CROs, pharmacies, pharmaceutical companies,
Requirements: Bachelors in Biology or Natural Science OR Pharmacist Degree. Many roles require prior clinical research experience which can be gained by other entry level positions through our CRC or ICH GCP training.
Length: 110 Hours. Online, self paced, start anytime.
Accreditation: ACCRE, Joint Accreditation for CE with ACPE 17.5 CME for Pharmacists.
Graduates work at: Pharmaceutical and Biotech Companies, Healthcare Service Providers, Regulatory Bodies and Research Institutes, Consulting and Services Companies, Consumer Goods Companies, Healthcare Information and Service.
Graduate Hired As: Regulatory Affairs Associate, Clinical Trial Drug Safety Associate, Drug Safety Specialist, Patient Safety Senior Associate, Pharmacovigilance Scientist, Pharmacovigilance Manager, Senior Pharmacovigilance Associate, Pharmacovigilance Regional Head, Pharmacovigilance Analyst, Senior Director Quality, Pharmacovigilance Data Entry Manager, Principal Pharmacovigilance Scientist, QA & Medical Complaint Handling Associate, Senior Manager Medical Safety Officer, Medical Affairs Senior Scientist, Quality Manager, VP Medical Affairs, Clinical Guidelines Coordinator, Clinical Data Monitor, Pharmacovigilance Specialist, Regulatory Project Manager, Product Safety Manager, Manager Pharmacovigilance Operations, Regulatory Affairs Manager, Clinical Pharmacist Consultant, Product Vigilance Manager, Epidemiologist, Senior Manager Safety and Pharmacovigilance, Associate Director of Pharmacovigilance Department, Qualified Person Responsible for Pharmacovigilance (QPPV), Pharmacovigilance Deputy, Regulatory Affairs Manager, Senior Scientist, Senior Medical Advisor, Cosmetovigilance, Drug Safety and Medical Information Specialist, Environmental Analyst, Vice President Clinical Development, Operation Specialist (Life Cycle Safety/Drug Safety/Medical Information), Regulatory Affairs Supervisor & QPPV, Senior Director Clinical Operations, Safety, and Customer Service Excellence, Regulatory Affairs - Pharmacist, Vice President Operations, QA Executive, Health Advisor Pharmacist, COVID Health Specialist - Case and Contact Manager, Senior Project Manager, Pharmacy Manager, R&D Post-Implementation Service, Pharmacy Manager, Investigations Associate, Lead Clinical Operations Safety/Quality Responsible, Pharmacist, Trial and Supply Management, etc. (based on review of new job positions post-enrollment)
Clinical Research Associate
Advanced clinical research associate certification (acrac).
Objective: Obtain a thorough understanding of clinical research to proficiently fulfill the duties of a Clinical Research Associate.
Students Enrolled: 7,536†
Students came from: Pharmaceuticals and Biotech Companies, Clinical Research and Consulting Services, Hospitals and Healthcare Providers, Universities and Academic Institutions
Requirements: Seeking candidates with a Bachelor's in Biology/Natural Science, Nursing Degree, or MBBS/IMG Degree for entry-level positions . Consider obtaining ACRP or SOCRA credentials after gaining 2 years of experience. Students with credentials and/or 2 years of experience (18% of cohort) still choose our course to refresh their knowledge because of our comprehensive review.
Length: 200 Hours. Online, self paced, start anytime.
Accreditation: ACCRE, Joint Accreditation for CE with ACCME, ANCC, ACPE, ICPE for 17.5 CME for Physicians, Nurses, Pharmacists, and Healthcare Professionals. Candidate for Federally Qualified Post-Graduate Institution with MSA-CESS.
Graduates work at: Pharmaceutical and Biotech Companies, Clinical Research and Consulting Services, Consulting and Professional Services, Consumer Goods Companies, Hospitals and Healthcare Providers, Government Health Departments, Universities and Academic Institutions, Healthcare IT and Services.
Graduate job roles post-course: Clinical Research Associate, Clinical Trial Monitor II, Research Associate, CRA II, Scientist, Quality Assurance Analyst, Senior Clinical Research Associate, Research Associate in Discovery Immunology, Clinical Trial Monitor/CRA, Clinical Trials Project Manager, Associate Director of Research Nursing, Clinical Trial Navigator, Clinical Director for R&D, Senior Clinical Research Associate, Clinical Research Professional, Medical Science Liaison, Clinical Trial Associate III, Quality Assurance Associate II, IRB/SRC Analyst II, Project Manager, Clinical Trial Associate, Clinical Research Coordinator, Public Health Advisor, Associate Scientist II, Strategy Analyst, Clinical Research Associate II, Clinical Operations Specialist, Advisor - Development Clinical Research Scientist, Neuroscience, Associate Clinical Engineer, Clinical Trial Management Associate, Quality Supervisor, Clinical Research Data Coordinator (based on review of new job positions post-enrollment)
Clinical Research Coordinator
Advanced clinical research coordinator certification (acrcc).
Objective: Acquire comprehensive proficiency in clinical research coordinator training encompassing patient care, regulatory compliance, and trial oversight.
Students Enrolled: 3,653†
Students came from: Hospitals and Healthcare Providers:, Clinical Research and Consulting Services, Healthcare Services, Pharmaceutical and Biotech Companies, Clinical Research Centers
Requirements: HS Diploma or GED OR Nurses OR Professionals with patient experience.
Length: 150 Hours. Online, self paced, start anytime.
Accreditation: ACCRE, Joint Accreditation with ANCC for 17.5 CME for nurses.
Graduates work at: Pharmaceutical and Biotech Companies, Hospitals and Healthcare Providers, Universities and Academic Institutions, Clinical Research and Consulting Services, Diagnostic Services, Consumer Goods Companies, Healthcare Services, Cancer Treatment and Research Centers.
Graduate job roles post-course: Clinical Research Coordinator, Clinical Research Coordinator II, Lead Clinical Research Coordinator, Senior Clinical Research Coordinator, Oncology Research Coordinator, Clinical Study Coordinator, Clinical Research Data Coordinator, Clinical Research Nurse, Clinical Director/ Office Manager, Regulatory Contact/ Clinical Research Coordinator, Clinical Research/Regulatory Coordinator, Clinical Trials Specialist, Research Regulatory Specialist, Certified Clinical Research Coordinator, Clinical Research Specialist, Sr. Director of Clinical Operations (based on review of new job positions post-enrollment)
Clinical Research Assistant
Advanced clinical trial assistant certification (actac).
Objective: Enhance skills required to support clinical trials, focusing on trial conduct, data collection, and administrative duties.
Students Enrolled: 1,800†
Students came from: Various universities, hospitals, clinics, and clinical research sites, etc.
Requirements: HS Diploma or GED. Current high-schoolers with evidence of active research internship can enroll.
Length: 50 Hours. Online, self paced, start anytime.
Accreditation: ACCRE, Transcelerate Biopharma.
Graduates work at: Various universities, hospitals, clinics, scholarships, internships, and clinical research sites.
Graduate job roles post-course: Clinical research assistant, clinical trial assistant, clinical researcher professional, clinical research coordinator, trial assistant, research assistant.
Clinical Research Project Manager
Advanced Clinical Research Project Manager Certification (ACRCC)
Objective: Prepare students for clinical trial management certification by teaching them how to effectively oversee large-scale clinical studies, ensuring adherence to protocols, budget, and timelines.
Students Enrolled: 1,190†
Students came from: Several CROs, universities, hospitals, clinics.
Requirements: Prior Project Management or Clinical Research Experience.
Accreditation: ACCRE, Joint Accreditation for CME
Certification: Online certificate. Exam score 70% or higher on 2 attempts.
Graduates work at: Pharmaceutical and Biotech Companies, Clinical Research and Consulting Services, Healthcare Staffing, Universities and Academic Institutions
Physician Medical Monitor
Advanced physician medical monitor certification (apmmc).
Prepare physicians for the specialized role of Medical Monitor, with an emphasis on patient safety, protocol adherence, and data interpretation.
Students Enrolled: 1,438†
Requirements: Medical Degree (MBBS, IMG, FMG).
Accreditation: ACCRE, Joint Accreditation with AMA for 17.5 CME.
Physician Principal Investigator
Advanced principal investigator physician certification (apipc).
Equip licensed physicians in their country with the knowledge and skills to undertake the role of Principal Investigator in clinical research.
Students Enrolled: 391†
Requirements: Active Medical Degree. Nonactive medical doctors, PhDs, and PharmDs can work as Sub-I with this training.
Length: 100 Hours. Online, self paced, start anytime.
Clinical Research Courses
Clinical research associate training.
Requires bachelors of science . Monitor multiple clinical trial sites. Finish in 2-4 weeks.
Pharmacovigilance Training
Requires bachelors of science . Monitor drug safety. Finish in 2-3 weeks.
Clinical Research Coordinator Training
Requires 2 year degree . Support a clinical trial site. Finish in 1-3 weeks.
ICH GCP Training
Requires HS diploma. Required for all clinical trial professionals every 2 years .
Clinical Trial Assistant Training
Requires HS diploma . Assist in clinical trials. Finish in 1-2 weeks.
Clinical Research Project Manager Training
Requires clinical trial or project management experience . Finish in 2-4 weeks.
Principal Investigator Training
Requires active MD license or pending Sub-PI position . Conduct clinical trial at site. Finish in 1-3 weeks.
Medical Monitor Training
Requires MD or MBBS/IMG/FMG . Monitor clinical trials with medical knowledge. Finish in 2-4 weeks.
CCRPS Reviews : View 18 Recent Graduate Case Studies from April 2024 including Video and Transcript Highlights d Transcript
Triple accredited clinical research courses for 2024-2025, transcelerate biopharma.
Recognizes CCRPS to be an accredited GCP trainer.
CCRPS is a candidate undergoing a 1 year intensive study for approval to be a federally recognized career and technical institution.
ACCRE accredits the professional program in Clinical Research leading to the Certificate of Clinical Research.
Institute for Credentialing Excellence
CCRPS maintains ICE organizational membership.
Upgrade your career or switch to a new path with our online clinical research training.
Joint accreditation.
CCRPS courses accredited by ACME, ICPE, and ANCC for doctors, pharmacists, and nurses for 17.5CME.
Postgraduate Institute for Medicine
CCRPS is audited by PIM for CME credit approval.
About CCRPS
Our online program for clinical research certification is trusted by thousands of students with our graduates finding careers at over 1,600 companies after taking our course per our 2024 survey. Ideal for career changers or those wanting to advance in roles like clinical research associate, coordinator, assistant, project manager, drug safety officer, principal investigator, or medical monitor.
Clinical research courses by CCRPS are accredited by major organizations (ACCRE, Transcelerate Biopharma, AMA, ACPE, ANCC, ICPE for CME through JA) and recognized by small to large-size clinical research organizations.
Developed by senior clinical research professionals to help students of all levels.
Training for a New Generation of Researchers
CCRPS provides affordable, industry-recognized research training that will improve your job prospects and trial outcomes. We offer ICH GCP training, CRA certification, CRC certification, research assistant training, pharmacovigilance certification, PI training, medical monitor, and clinical research project manager training. We serve clinical professionals including nurses, physicians, pharmacists, PhDs, premeds, and science-field graduates who want to transition or accelerate their careers with CCRPS.
Do you want more information on our selection of clinical research online training programs? Read below.
The ICH-GCP certification is out for 2024 and offers hours worth of in depth training on all aspects about Good Clinical Practice as defined by the International Conference on Harmonization. The most advanced modules provide a complete overview no matter what your background with pharmaceutical research might be; this includes ethical practices that prioritize safety along side transparent decision making processes where there are none!
Requirements for ICH GCP Certification
The only diplomas needed to enroll in this program are high school or equivalent level education (such as GED). However, if you have more training than that and would like a head start on understanding the material being taught at our college then it is recommended that prior learning be taken into consideration when scheduling classes.
Is ICH GCP Certification right for you?
The ICH-GCP training is a great way for anyone who needs an introduction or refresh on ICH GCP guidelines in order to be an great CCRP. Candidates appearing before interviewers may find themselves unprepared when it comes down solely and exclusively them, but this course will give you all the basics that are needed!
Download the ICH GCP guidelines .
Why choose our ICH GCP training?
Certification through the online ICH-GCP training confers multiple benefits not matched by any other GCP certification currently available, including being E6 (R2) compliant, having instant enrollment and flexible scheduling, being industry recognized, accredited by research authorities, and affordable with flexible payment options.
Institutions such as CROs that require employees to complete GCP certification can opt for a one-time annual fee payment which allows flexible scheduling for an unlimited number of trainees.
Research Assistant - Clinical Research Assistant
The research assistant certification provides you with the kick-start that will help gain better visibility for your application. The course is designed give thorough understanding of criteria needed in order conduct them effectively, what makes one organization more desirable than another when it comes time apply. The modules cover all aspects from planning through documentation, reporting & publication as well as safety practices necessary during participant recruitment/screening procedures
Requirements for Research Assistant Certification
The research assistant training is open to anyone, even without a high school diploma or equivalent.
High school students intending to work after graduation or interested in healthcare research may benefit from completing the clinical research assistant certification.
Premed students enrolled in an undergraduate degree program and majoring in one of the life sciences may also benefit from clinical research assistant training.
Why choose our clinical research assistant certification?
The research assistant certification is the leading choice for research assistant jobs because it is fully compliant with ICH-GCP and FDA CFR, covers all key concepts extensively, has flexible scheduling, is widely recognized and accepted, and is affordable.
Is research assistant training right for you?
The research assistant program provides a strong understanding of advanced Good Clinical Practice to have a successful career with room for growth. This program offers hands-on experience in subject-facing dimensions of clinical research trials, including training in: the proper protocol for obtaining informed consent, eliciting subject cooperation during trials, obtaining necessary background information for trial documentation.
Trainees learn about subject safety monitoring, which includes exposure to: Adverse Event (AE) identification, documentation and reporting, and trial protocol adherence.
The research assistant course materials contain real-life examples and case studies to help trainees develop insight into and build strategies for: increasing subject enrollment in new and ongoing studies, improving retention rates among subjects enrolled in an ongoing study.
This advanced clinical research coordinator training program is designed to provide in-depth coverage of all aspects, from basic pharmacovigilance and regulatory audits right up through planning for scientific integrity. The course teaches students everything they need know about how best handle each situation that may arise during their career as a Clinical Research Coordinator - no matter what field area interests them most!
Requirements for CRC Certification
The clinical research coordinator (CRC) is a senior member of the clinical research team with responsibilities in overseeing the smooth conduct of clinical research. Candidates must possess a minimum of an associates degree.
Is clinical research coordinator training right for you?
The objectives of candidates enrolling in the clinical research coordinator course are typically related to advancing their clinical research careers. Research professionals enroll in the program to build the relevant knowledge base and administrative skills needed to strengthen their applications for CRC positions. Use the clinical research coordinator course refresh or upgrade their skill-set and obtain certification in research coordination.
Why choose our CRC Certification?
Our clinical research coordinator training has emerged as the clear industry preference when it comes to certifying candidates for on-site roles in clinical research, due to its updated compliance information, broad and deep content coverage, flexible scheduling, and industry-wide reputation for quality.
Our clinical research coordinator certification is accredited by the ACCRE, ACCME, ACPE and ANCC - the most widely recognized and accepted CRC programs across the industry - making it a sound investment for those looking to pursue a career in clinical research.
The course tuition is affordable and can be paid up-front or in easy monthly installments
The Clinical Research Associate Program is the perfect opportunity for you to have a career in research! This advanced program has over 200 hours of specialized training, which will teach students everything they need. You'll learn how to write reports and site visits with ease using our curriculum that covers all topics related directly or indirectly toward clinical trials work--and even teaches additional techniques for efficiency and workflow.
Requirement for CRA Certification
In order to enroll for the clinical research associate certification , one must have a bachelor’s degree in life science or a health-care science, or a graduate degree in medicine.
Is CRA training right for you?
Graduates with a bachelor's degree in science who are interested in exploring clinical research can benefit from taking this course. Aspirants to CRA jobs looking to boost their hire visibility can also benefit from taking the course. Health-care professionals (RNs, NPs, PAs and others) aiming to either transition to or advance a career in clinical research.
CRAs with less than 5 years of work experience wishing to fast-track. Also, CRAs, Senior CRAs and other clinical research personnel needing a refresher course.
Why choose our CRA Certification?
The clinical research associate course confers a number of advantages on the individual, whether they are entering the field of clinical research or working to advance their career.
CRAs certified through clinical research associate training have up-to-date knowledge of both ICH and FDA regulatory requirements for human subject safety in clinical research.
The program is flexible, allowing trainees to fit the training into a busy schedule. There is an emphasis on hands-on training using real-life clinical research examples and data sets.
Completing the clinical research associate course is recognized across the US as equivalent to 17.5 CME credits.
Qualifying candidates receive not only a widely accepted and recognized clinical research associate certificate.
Pharmacovigilance
The pharmacovigilance course is an advanced program that will prepare you for a career in PV, with the most comprehensive syllabus covering all aspects from pre-clinical phase to post market surveillance (Phase IV clinical trials).
Requirements for Pharmacovigilance Certification
The goal of pharmacovigilance is to ensure the safety of all drugs and medical devices. QPPVs are responsible for achieving this goal through and beyond clinical trials. To be a QPPV, one must have considerable medical knowledge, statistical skill, and analytical ability. Candidates for the pharmacovigilance and regulatory affairs certification must possess a minimum of:
A bachelor’s degree in life science OR a health-care science
Is clinical research drug safety certification right for you?
The pharmacovigilance certification is beneficial for those in the clinical research field who wish to upgrade their qualifications and expertise. CTAs/CRAs, SCRAs/CRCs, medical and nursing professionals, and QPPVs can all benefit from enrolling in the program.
Enrolling in the pharmacovigilance training gives aspirants an edge when applying for positions that require advanced knowledge of PV compliance, data analytics, software management skills, medical-legal awareness, etc.
Why choose our pharmacovigilance and drug safety training?
Our drug safety and regulatory affairs course is one of the leading pharmacovigilance certification program by recruiters across the industry. The pharmacovigilance certification is compliant with FDA CFR and WHO-ISoP, providing trainees with up-to-date coaching on all relevant regulatory codes and standards. The focus areas of the pharmacovigilance course comprehensively cover all domains of knowledge and skill required for an effective QPPV. The pharmacovigilance course trains candidates in creating, managing and retrieving case report forms using Argus Safety software. CCRPS regulatory affairs certification offers on-demand, flexible scheduling to allow enrolled students to complete the program at their own pace. The pharmacovigilance and drug safety course tuition is payable either up front or in two easy monthly installments.Explore comprehensive clinical data management training and placement opportunities in the USA. Develop your skills and secure promising positions in this dynamic field. Unlock your potential for success today.
How to Become A Trial Project Manager
Requirements for clinical trial project manager training
Clinical research project managers must have a bachelor's degree in a scientific field. We require prior clinical trial experience in managerial roles or prior project manager experience though graduates seeking to grow in their current career can take the course. They must be able to manage and coordinate all aspects of clinical trials. They must be able to keep up with ever-changing regulations governing clinical trials
Why choose our medical monitor training?
Our clinical trial project manager training is the most comprehensive and up-to-date program available You will learn how to manage clinical trials from start to finish, including budgeting, scheduling, and communication.
Upon completion of the program, you will be a certified clinical trial project manager . Our tuition rates are very affordable compared to other programs in this field.
Is project manager certification right for you?
If you're a project manager or coordinator who is looking to enhance your skills and salary, then clinical research project manager training may be right for you. Earning clinical research project manager certification can help you stand out from other project managers and improve your career prospects.
You must also pass an exam that covers topics such as risk management, stakeholder relationships and data management.
If you meet these qualifications, then becoming certified can help you demonstrate your knowledge and expertise in the field of clinical research project management . Not only will this make you a more valuable asset to your current employer, but it can also open up doors to new opportunities down the road.
Certified clinical research professionals work in a booming industry and there’s no doubt that project managers are in high demand. If you want to make the jump into clinical trial project management, or if you’re already a project manager but want to specialize in pharmaceuticals, our course is exactly what you need.
How to Become a Medical Monitor
Requirements for medical monitor training
Medical monitors are senior members of the clinical research team who oversee the ethical, safe, and transparent conduct of clinical research.
To qualify as a medical monitor, trainees must have a degree in medicine (MD), a non-US degree in medicine (IMG/FMG), or a master’s degree in pharmacy (PharmD).
Physicians with one or more years of exposure to medical research may also qualify as medical monitors.
The medical monitor certification is a program that covers the full range of knowledge domains essential for an medical monitor role, from the philosophy behind GCP to present-day regulatory requirements for clinical research. The course curriculum reflects the most updated regulatory policies related to FDA’s CFR Title 21, as well as E6 (R2) ICH-GCP guidelines. Trainees have the option of on-demand scheduling to fit with their busy schedules.
Is Medical monitor certification right for you?
The medical monitor training offers a comprehensive overview of the principles of Good Clinical Practice, as well as compliance requirements for ethical and safe medical research.
This is the only program that provides in-depth training on all aspects of clinical research design and execution.
The medical monitor course also covers pharmacovigilance concepts crucial to a medical monitor’s role such as AE/SAE identification and tracking; probabilistic assessment of AEs/SAEs as ADRs; risk management in clinical trials.
Trainees gain working knowledge of financial regulatory compliance: disclosure documentation & updating; FDA audit protocols & strategies.
An added advantage is its focus on digitized elements such remote data monitoring tools (software & video), EDC capture & quality control
Principal Investigator
The principal investigator certification program is a great way for physicians involved in clinical research who want to transition into more senior roles, enhance their eligibility when applying or overseeing trials process. It provides PIs with the ideal means of upgrading career skills while also helping them become better fundraisers and managers!
Requirements for Principal Investigator Certification
To be a certified PI, you must be a practicing physician. You may also either be the PI or Co-PI of an ongoing clinical research study, or have been the Ex-PI or former Co-PI of a completed study.
Is Principal Investigator training right for you?
The principal investigator certification provides a thorough, yet quick refresher of the regulatory and compliance requirements for ethical, safe and transparent medical research. This is an in-depth review of all aspects of leading clinical research design and execution as a principal investigator, including: advanced trial design, randomization, blinding and unblinding; clinical site assessment, preparation active site monitoring and close-out; clinical trial protocol development and implementation, including trial monitoring tools and documentation; Investigational Product (IP) accountability storage and dispensing; Adverse Events (AEs), Serious Adverse Events (SAEs), Adverse Drug Reaction (ADR), Important Medical Event (IME) – identification tracking reporting; probabilistic assessment of AEs SAEs as ADRs – medical assessment statistical data analytics risk safety assessments in clinical trials.
Why choose our PI training course?
The principal investigator certification is the best choice for both physicians who want to get certified as a PI, and for industry experts who are looking for someone to fill a PI position. This is because the certification is very flexible and covers a lot of ground.
Additionally, those who become certified principal investigators will be up-to-date on the most recent regulatory policies related to FDA CFR Title 21 and the E6 (R2) ICH-GCP guidelines. This means that they will be qualified to manage compliance requirements in a clinical study.
The course curriculum includes all of the knowledge domains essential for clinical research principal investigator training , but busy professionals can review only the modules most relevant to them and their needs. This way, they can still update their knowledge and skills without having to spend a lot of time on it.
Clinical Research Staff Training
CCRPS works with pharmaceutical, biotech, medical device, and contract research organizations to efficiently train and certify their clinical research associates, coordinators, and assistants to meet ICH GCP and CFR compliance for their staff. We can provide outsourced clinical research staff training set up within 1-2 business days. We work with organization budgets and staff training size to provide comprehensive and transformational education for onboarding and updating staff compliance with ICH GCP and job training requirements. We have worked directly with organizations and groups ranging from 2 employees to 179 employees.
The Platform for Clinical Research Education
Ccrps case studies & reviews.
From IMG to Clinical Research Coordinator at Columbia University: " This course not only met but exceeded my expectations with its thorough curriculum and insightful modules." -Lisa-Pierre ( view full case study )
From IMG to Clinical Research Coordinator "The hands-on activities integrated throughout the course really helped solidify my understanding of complex concepts." -Umber Mahmood ( case study summary )
From Physical Therapist to Clinical Researcher: "The in-depth content and expert instructors provided me with invaluable insights into the field." - Celia Moon ( case study summary )
From International CRC to U.S. Lead CRC and CRA: "The flexible online format allowed me to balance my studies with my professional commitments seamlessly." - Aishwarya Sukumar ( view full case study )
Enjoyed Clinical Research Training through Examples "The real-world examples used throughout the course were incredibly useful for applying theory to practice." -Marta Marszalek ( view full case study )
Promoted to Senior Startup Specialist in Clinical Trials : "I appreciate how the course was structured—very interactive and engaging from start to finish." -Justin Scott Brathwaite ( transcript summary )
From Clinical Research Receptionist to Certified Study Coordinator with CCRPS: "I highly recommend this course for its comprehensive approach and practical applications." - Katie Decker ( view full case study )
Learning to Lead Safety Associate: "The course materials were clear, well-organized, and directly applicable to my work." - Renata Noronha ( view full case study )
From IMG to securing roles as a CRC, CRA, and now a project manager: "Joining this course was a pivotal step in my career advancement." - Dr. Vrushali Borawak ( view full case study )
From Physician to Confident Drug Safety Specialist: "The course provided a robust foundation in the field, which was critical for my professional development." - Rabiea Bilal ( view full case study )
From plant biologist to clinical recruitment administrative coordinator : "This program is a gateway to extensive knowledge and skills in a supportive learning environment." -Olajumoke Owati ( view full case study )
ICH GCP Expert: "Thanks to this course, I feel more competent and confident in my role." - Stephanie ( case study summary )
From International PV Roles To North American Market Success: "The detailed modules prepared me excellently for real-world applications." - John Vinil ( view full case study )
From Educational Research to Clinical Trials Project Management: "I was able to immediately apply what I learned in the course to my job. " - Rose Hyson ( view full case study )
From Coordinator to Clinical Research Grant Manager: "it really did a great job of the full scope of clinical research from start to finish." -Hannah Fischer
ICH GCP made her more confident in research: "this course just overall did a really good job going in depth, which I feel like wasn't just, it wasn't just covered just for the sake of covering content" - Aastha Shah (view full transcript)
From Clinical Research Intern to Regulatory Affairs Associate at UPenn: "I would say since then. I've completed this course. It's helped me get my job in regulatory affairs at a clinical research site." - Scott Boyle
From Masters in Health Safety to Clinical Researcher: " I will say quality of delivery, quality of the materials. - Ossai Opene ( view full case study )
How to Become a Research Nurse
What is a research nurse.
- Career Outlook

Research Nurses, also referred to as Clinical Nurse Researchers or Nurse Researchers, develop and implement studies to investigate and provide information on new medications, vaccinations, and medical procedures. They assist in providing evidence-based research that is essential to safe and quality nursing care. This guide will explain what a Research Nurse does, how much they make, how to become one, and more!
Research nurses play a pivotal role in developing new and potentially life-saving medical treatments. Typically, clinical research nurses have advanced degrees, assist in the development of studies regarding medications, vaccines, and medical procedures, and also the care of research participants.
Nurses that know they want to be a clinical research nurse will often work as a research assistant, a clinical data collector, and/or clinical research monitor. It is essential to gain some bedside experience, but not as important as other nursing specialties.
Clinical research nurses have advanced degrees such as an MSN or Ph.D. This is vital to those that want to conduct independent research. For that reason, most clinical research nurses do not work in this field until they are in their 40s-50s.
Find Nursing Programs
What does a research nurse do.
Research Nurses primarily conduct evidence-based research through these two types of research methods:
- Quantitative: Meaning it’s researched that can be measured via statistical, mathematical, or computational techniques.
- Phenomenology
- Grounded Theory
- Ethnography
- Narrative Inquiry
Clinical research nurses perform a variety of tasks, all centered around research. These specific job responsibilities include:
- Collaborating with industry sponsors and other investigators from multi-institutional studies
- Educating and training of new research staff
- Overseeing the running of clinical trials
- Administering questionnaires to clinical trial participants
- Writing articles and research reports in nursing or medical professional journals or other publications
- Monitoring research participants to ensure adherence to study rules
- Adhering to research regulatory standards
- Writing grant applications to secure funding for studies
- Reporting findings of research, which may include presenting findings at industry conferences, meetings and other speaking engagements
- Adhering to ethical standards
- Maintaining detailed records of studies as per FDA guidelines, including things such as drug dispensation
- Participating in subject recruitment efforts
- Ensuring the necessary supplies and equipment for a study are in stock and in working order
- Engaging with subjects and understanding their concerns
- Providing patients with thorough explanation of trial prior to obtaining Informed Consent, in collaboration with treating physician and provides patient education on an ongoing basis throughout the patient’s course of trial.
>> Show Me Online MSN Programs
Research Nurse Salary
Glassdoor.com states an annual median salary of $95,396 for Research Nurses and Payscale reports that Clinical Research Nurses earn an average annual salary of $75,217 or $36.86/hr .
Research Nurse Salary by Years of Experience
Research Nurses can earn a higher annual salary with increased years of experience.
- Less than 1 year of experience earn an average salary of $68,000
- 1-4 years of experience earn an average salary of $73,000
- 5-9 years of experience earns an average salary of $73,000
- 10-19 years of experience earns an average salary of $80,000
- 20 years or more of experience earns an average salary of $78,000
Via Payscale
To become a Research Nurse, you’ll need to complete the following steps:
Step 1: Attend Nursing School
You’ll need to earn either an ADN or a BSN from an accredited nursing program in order to take the first steps to become a registered nurse.
Step 2: Pass the NCLEX-RN
Become a Registered Nurse by passing the NCLEX examination.
Step 3: Gain Experience at the Bedside
Though not as important as in some other nursing careers, gaining experience is still a vital step for those wanting to become Nurse Researchers.
Step 4: Earn an MSN and/or Ph.D
Research Nurses typically need an advanced degree, so ADN-prepared nurses will need to complete an additional step of either completing their BSN degree or entering into an accelerated RN to MSN program which will let them earn their BSN and MSN at the same time.
Step 5: Earn Your Certification
There are currently two certifications available for Clinical Research Nurses. They are both offered by the Association of Clinical Research Professionals.
- Clinical Research Association (CCRA)
- Clinical Research Coordinator (CCRC)
These certifications are not specific to nurses but rather those that work in the research field.
CCRA Certification
In order to be deemed eligible for the CCRA Certification exam, applicants must attest to having earned 3,000 hours of professional experience performing the knowledge and tasks located in the six content areas of the CRA Detailed Content Outline. Any experience older than ten years will not be considered.

What’s on the Exam?
- Scientific Concepts and Research Design
- Ethical and Participant Safety Considerations
- Product Development and Regulation
- Clinical Trial Operations (GCPs)
- Study and Site Management
- Data Management and Informatics
Exam Information
- Exam Fee: $435 Member; $485 Nonmember
- Exam Fee: $460 Member; $600 Nonmember
- Multiple choice examination with 125 questions (25 pretest non-graded questions)
CCRC Certification
In order to be deemed eligible for the CCRC Certification exam, applicants must attest to having earned 3,000 hours of professional experience performing the knowledge and tasks located in the six content areas of the CCRC Detailed Content Outline. Any experience older than ten years will not be considered.
Where Do Research Nurses Work?
Clinical Research nurses can work in a variety of locations, including:
- Government Agencies
- Teaching Hospitals
- Medical Clinics
- International Review Board
- Medicine manufacturing
- Pharmaceutical companies
- Medical research organizations
- Research Organizations
- International Health Organizations
- Private practice
- Private and public foundations
What is the Career Outlook for a Research Nurse?
According to the BLS , from 2022 to 2032, there is an expected growth of 6% for registered nurses. With the aging population and nursing shortage, this number is expected to be even higher.
The BLS does identify medical scientists, which includes clinical research nurses, as having a growth potential of 10% between 2022-2032.
What are the Continuing Education Requirements for a Research Nurse?
Generally, in order for an individual to renew their RN license, they will need to fill out an application, complete a specific number of CEU hours, and pay a nominal fee. Each state has specific requirements and it is important to check with the board of nursing prior to applying for license renewal.
If the RN license is part of a compact nursing license, the CEU requirement will be for the state of permanent residence. Furthermore, some states require CEUs related to child abuse, narcotics, and/or pain management.
A detailed look at Continuing Nurse Education hours can be found here .
Where Can I Learn More About Becoming a Research Nurse?
- American Nurses Association (ANA)
- Nurse Researcher Magazine
- National Institute of Nursing Research
- International Association of Clinical Research Nurses
- Association of Clinical Research Professionals
- Society of Clinical Research Associates
- American Association of Colleges of Nursing
Research Nurse FAQs
What is the role of a research nurse.
- Research nursing is a nursing practice with a specialty focus on the care of research participants.
What makes a good Research Nurse?
- Research Nurses should be excellent communicators, have strong attention to detail, be self-assured, have strong clinical abilities, be flexible, autonomous, organized, and eager to learn new information.
How much does a Research Nurse make?
- Research nurses earn an average salary of $95,396 according to Glassdoor.com.
What is it like being a Research Nurse?
- Research Nurses provide and coordinate clinical care. Research Nurses have a central role in ensuring participant safety, maintaining informed consent, the integrity of protocol implementation, and the accuracy of data collection and data recording.

Kathleen Gaines (nee Colduvell) is a nationally published writer turned Pediatric ICU nurse from Philadelphia with over 13 years of ICU experience. She has an extensive ICU background having formerly worked in the CICU and NICU at several major hospitals in the Philadelphia region. After earning her MSN in Education from Loyola University of New Orleans, she currently also teaches for several prominent Universities making sure the next generation is ready for the bedside. As a certified breastfeeding counselor and trauma certified nurse, she is always ready for the next nursing challenge.

Plus, get exclusive access to discounts for nurses, stay informed on the latest nurse news, and learn how to take the next steps in your career.
By clicking “Join Now”, you agree to receive email newsletters and special offers from Nurse.org. We will not sell or distribute your email address to any third party, and you may unsubscribe at any time by using the unsubscribe link, found at the bottom of every email.
Part 1. Overview Information
National Institutes of Health ( NIH )
National Eye Institute ( NEI )
National Heart, Lung, and Blood Institute ( NHLBI )
National Human Genome Research Institute ( NHGRI )
National Institute on Aging ( NIA )
National Institute on Alcohol Abuse and Alcoholism ( NIAAA )
National Institute of Allergy and Infectious Diseases ( NIAID )
National Institute of Arthritis and Musculoskeletal and Skin Diseases ( NIAMS )
National Institute of Biomedical Imaging and Bioengineering ( NIBIB )
Eunice Kennedy Shriver National Institute of Child Health and Human Development ( NICHD )
National Institute on Deafness and Other Communication Disorders ( NIDCD )
National Institute of Dental and Craniofacial Research ( NIDCR )
National Institute of Diabetes and Digestive and Kidney Diseases ( NIDDK )
National Institute on Drug Abuse ( NIDA )
National Institute of Environmental Health Sciences ( NIEHS )
National Institute of General Medical Sciences ( NIGMS )
National Institute of Mental Health ( NIMH )
National Institute of Neurological Disorders and Stroke ( NINDS )
National Institute on Minority Health and Health Disparities ( NIMHD )
National Library of Medicine ( NLM )
National Center for Complementary and Integrative Health ( NCCIH )
National Cancer Institute ( NCI )
All applications to this funding opportunity announcement should fall within the mission of the Institutes/Centers. The following NIH Offices may co-fund applications assigned to those Institutes/Centers.
Office of Research on Women's Health ( ORWH )
Office of Data Science Strategy ( ODSS )
Special Note: Not all NIH Institutes and Centers participate in Parent Announcements. Candidates should carefully note which ICs participate in this announcement and view their respective areas of research interest and requirements at the Table of IC-Specific Information, Requirements and Staff Contacts website. ICs that do not participate in this announcement will not consider applications for funding. Consultation with NIH staff before submitting an application is strongly encouraged.
- August 31, 2022 - Implementation Changes for Genomic Data Sharing Plans Included with Applications Due on or after January 25, 2023. See Notice NOT-OD-22-198 .
- August 5, 2022 - Implementation Details for the NIH Data Management and Sharing Policy. See Notice NOT-OD-22-189 .
See Section III. 3. Additional Information on Eligibility .
The primary purpose of the NIH Mentored Clinical Scientist Research Career Development Awards (K08) program is to prepare qualified individuals for careers that have a significant impact on the health-related research needs of the Nation. This program represents the continuation of a long-standing NIH program that provides support and "protected time" to individuals with a clinical doctoral degree for an intensive, supervised research career development experience in the fields of biomedical and behavioral research, including translational research.
This Notice of Funding Opportunity (NOFO) is designed specifically for applicants proposing research that does not involve leading an independent clinical trial, a clinical trial feasibility study, or an ancillary clinical trial . Under this NOFO applicants are permitted to propose a research experience in a clinical trial led by a mentor or co-mentor. Those proposing a clinical trial or an ancillary clinical trial as lead investigator, should apply to the companion NOFO ( PA-24-181 ).
Not Applicable
All applications are due by 5:00 PM local time of applicant organization.
Applicants are encouraged to apply early to allow adequate time to make any corrections to errors found in the application during the submission process by the due date.
It is critical that applicants follow the instructions in the Career Development (K) Instructions in the How to Apply - Application Guide except where instructed to do otherwise (in this NOFO or in a Notice from the NIH Guide for Grants and Contracts ). Conformance to all requirements (both in the How to Apply - Application Guide and the NOFO) is required and strictly enforced. Applicants must read and follow all application instructions in the How to Apply - Application Guide as well as any program-specific instructions noted in Section IV . When the program-specific instructions deviate from those in the How to Apply - Application Guide , follow the program-specific instructions. Applications that do not comply with these instructions may be delayed or not accepted for review.
There are several options available to submit your application through Grants.gov to NIH and Department of Health and Human Services partners. You must use one of these submission options to access the application forms for this opportunity.
- Use the NIH ASSIST system to prepare, submit and track your application online.
- Use an institutional system-to-system (S2S) solution to prepare and submit your application to Grants.gov and eRA Commons to track your application. Check with your institutional officials regarding availability.
- Use Grants.gov Workspace to prepare and submit your application and eRA Commons to track your application.
Part 2. Full Text of Announcement
Section i. funding opportunity description.
The overall goal of the NIH Research Career Development program is to help ensure that a diverse pool of highly trained scientists is available in appropriate scientific disciplines to address the Nation's biomedical, behavioral, and clinical research needs. NIH Institutes and Centers (ICs) support a variety of mentored and non-mentored career development award programs designed to foster the transition of new investigators to research independence and to support established investigators in achieving specific objectives. Candidates should review the different career development (K) award programs to determine the best program to support their goals. More information about Career programs may be found at the NIH Research Training and Career Development website.
The objective of the NIH Mentored Clinical Scientist Research Career Development Award (K08) is to provide salary and research support for a sustained period of “protected time” (3-5 years) to support didactic study and/or mentored research for individuals with clinical doctoral degrees (e.g., MD, DDS, DMD, DO, DC, OD, ND, DVM, PharmD, or PhD in clinical disciplines). The K08 provides support for an intensive, mentored research career development experience in biomedical or behavioral research, including translational research. For the purpose of this award, translational research is defined as the application of basic research discoveries toward the diagnosis, management, and prevention of human disease. Individuals with a clinical doctoral degree interested in pursuing a career in patient-oriented research should refer to the NIH Mentored Patient Oriented Research Career Development Award (K23).
The K08 award may be used by candidates with different levels of prior research training and at different stages in their mentored career development. For example, a candidate with limited experience in a given field of research may use an award to support a career development experience that includes a designated period of didactic training followed by a period of closely supervised research experience. A candidate with previous research experience and training may not require extensive additional didactic preparation, and may use an award to support a career development experience that focuses on an intensive, supervised research experience.
Note: This Notice of Funding Opportunity (NOFO) is designed specifically for proposing research that does not involve leading an independent clinical trial, a clinical trial feasibility study, or an ancillary clinical trial. Under this NOFO are permitted to propose a research experience in a clinical trial led by a mentor or co-mentor. Those proposing a clinical trial or an ancillary clinical trial as lead investigator, should apply to the companion NOFO ( PA-24-181 ).
Special Note: Because of the differences in individual Institute and Center (IC) program requirements for this NOFO, prospective applicants are strongly encouraged to consult the Table of IC-Specific Information, Requirements and Staff Contacts , to make sure that their application is appropriate for the requirements of one of the participating NIH ICs.
See Section VIII. Other Information for award authorities and regulations.
Section II. Award Information
Grant: A financial assistance mechanism providing money, property, or both to an eligible entity to carry out an approved project or activity.
The OER Glossary and the How to Apply - Application Guide provides details on these application types.
Not Allowed: Only accepting applications that do not propose clinical trials.
Note: Applicants may propose to gain experience in a clinical trial led by a mentor/co-mentor as part of their research career development.
The number of awards is contingent upon NIH appropriations and the submission of a sufficient number of meritorious applications.
Other Award Budget Information
The participating NIH Institutes and Centers will provide salary and fringe benefits for the award recipient (see Table of IC-Specific Information, Requirements and Staff Contacts ). Further guidance on budgeting for career development salaries is provided in the How to Apply - Application Guide .
In addition, the candidate may derive additional compensation for effort associated with other Federal sources or awards provided the total salary derived from all Federal sources does not exceed the maximum legislated salary rate (see http://grants.nih.gov/grants/policy/salcap_summary.html ) and the total percent effort does not exceed 100%. See also NOT-OD-17-094 .
These funds may be used for the following expenses: (a) tuition and fees related to career development; (b) research-related expenses, such as supplies, equipment and technical personnel; c) travel to research meetings or training; and (d) statistical services including personnel and computer time.
Salary for mentors, secretarial and administrative assistants, etc. is not allowed.
NIH grants policies as described in the NIH Grants Policy Statement will apply to the applications submitted and awards made from this NOFO.
Section III. Eligibility Information
1. Eligible Applicants
Higher Education Institutions
- Public/State Controlled Institutions of Higher Education
- Private Institutions of Higher Education
The following types of Higher Education Institutions are always encouraged to apply for NIH support as Public or Private Institutions of Higher Education:
- Hispanic-serving Institutions
- Historically Black Colleges and Universities (HBCUs)
- Tribally Controlled Colleges and Universities (TCCUs)
- Alaska Native and Native Hawaiian Serving Institutions
- Asian American Native American Pacific Islander Serving Institutions (AANAPISIs)
Nonprofits Other Than Institutions of Higher Education
- Nonprofits with 501(c)(3) IRS Status (Other than Institutions of Higher Education)
- Nonprofits without 501(c)(3) IRS Status (Other than Institutions of Higher Education)
For-Profit Organizations
- Small Businesses
- For-Profit Organizations (Other than Small Businesses)
Local Governments
- State Governments
- County Governments
- City or Township Governments
- Special District Governments
- Indian/Native American Tribal Governments (Federally Recognized)
- Indian/Native American Tribal Governments (Other than Federally Recognized)
Federal Governments
- U.S. Territory or Possession
- Independent School Districts
- Public Housing Authorities/Indian Housing Authorities
- Native American Tribal Organizations (other than Federally recognized tribal governments)
- Faith-based or Community-based Organizations
- Regional Organizations
Non-domestic (non-U.S.) Entities (Foreign Organizations) are not eligible to apply.
Non-domestic (non-U.S.) components of U.S. Organizations are not eligible to apply.
Foreign components, as defined in the NIH Grants Policy Statement , are allowed.
Applicant Organizations
Applicant organizations must complete and maintain the following registrations as described in the How to Apply - Application Guide to be eligible to apply for or receive an award. All registrations must be completed prior to the application being submitted. Registration can take 6 weeks or more, so applicants should begin the registration process as soon as possible. Failure to complete registrations in advance of a due date is not a valid reason for a late submission, please reference NIH Grants Policy Statement 2.3.9.2 Electronically Submitted Applications for additional information.
- NATO Commercial and Government Entity (NCAGE) Code – Foreign organizations must obtain an NCAGE code (in lieu of a CAGE code) in order to register in SAM.
- Unique Entity Identifier (UEI) - A UEI is issued as part of the SAM.gov registration process. The same UEI must be used for all registrations, as well as on the grant application.
- eRA Commons - Once the unique organization identifier is established, organizations can register with eRA Commons in tandem with completing their Grants.gov registration; all registrations must be in place by time of submission. eRA Commons requires organizations to identify at least one Signing Official (SO) and at least one Program Director/Principal Investigator (PD/PI) account in order to submit an application.
- Grants.gov – Applicants must have an active SAM registration in order to complete the Grants.gov registration.
Program Directors/Principal Investigators (PD(s)/PI(s))
All PD(s)/PI(s) must have an eRA Commons account. PD(s)/PI(s) should work with their organizational officials to either create a new account or to affiliate their existing account with the applicant organization in eRA Commons. If the PD/PI is also the organizational Signing Official, they must have two distinct eRA Commons accounts, one for each role. Obtaining an eRA Commons account can take up to 2 weeks.
All PD(s)/PI(s) must be registered with ORCID . The personal profile associated with the PD(s)/PI(s) eRA Commons account must be linked to a valid ORCID ID. For more information on linking an ORCID ID to an eRA Commons personal profile see the ORCID topic in our eRA Commons online help .
Any candidate with the skills, knowledge, and resources necessary to carry out the proposed research as the Program Director/Principal Investigator (PD/PI) is invited to work with their mentor and organization to develop an application for support. Individuals from diverse backgrounds, including individuals from underrepresented racial and ethnic groups, individuals with disabilities, and women are always encouraged to apply for NIH support. See, Reminder: Notice of NIH's Encouragement of Applications Supporting Individuals from Underrepresented Ethnic and Racial Groups as well as Individuals with Disabilities , NOT-OD-22-019 . Multiple PDs/PIs are not allowed.
By the time of award, the individual must be a citizen or a non-citizen national of the United States or have been lawfully admitted for permanent residence (i.e., possess a currently valid Permanent Resident Card USCIS Form I-551, or other legal verification of such status).
Current and former PDs/PIs on NIH research project (R01), program project (P01), center grants (P50), or Project Leads of program project (P01), or center grants (P50), other major individual career development awards (e.g., DP5, K01, K07, K08, K22, K23, K25, K76, K99/R00), or Project Leads of program project (P01) or center grants (P50) or the equivalent are not eligible. Current and former PDs/PIs of an NIH Small Grant (R03), Exploratory/Developmental Grants (R21/R33), Planning Grant (R34/U34), Dissertation Award (R36), or SBIR/STTR (R41, R42, R43, R44) remain eligible, as do PD/PIs of Transition Scholar (K38) awards and individuals appointed to institutional K programs (K12, KL2). Candidates for the K08 award must have a clinical doctoral degree. Such degrees include, but are not limited to, the MD, DO, DDS, DMD, OD, DC, PharmD, ND (Doctor of Naturopathy), and DVM. Individuals with the PhD or other doctoral degree in clinical disciplines such as clinical psychology, nursing, clinical genetics, speech-language pathology, audiology or rehabilitation are also eligible. Individuals holding the PhD in a non-clinical discipline who are certified to perform clinical duties should contact the appropriate Institute concerning their eligibility for a K08 award.
2. Cost Sharing
This NOFO does not require cost sharing as defined in the NIH Grants Policy Statement Section 1.2 Definitions of Terms .
3. Additional Information on Eligibility
Applicant organizations may submit more than one application, provided that each application is scientifically distinct, and each is from a different candidate.
NIH will not accept duplicate or highly overlapping applications under review at the same time per NIH Grants Policy Statement Section 2.3.7.4 Submission of Resubmission Application . An individual may not have two or more competing NIH career development applications pending review concurrently. In addition, NIH will not accept:
- A new (A0) application that is submitted before issuance of the summary statement from the review of an overlapping new (A0) or resubmission (A1) application.
- A resubmission (A1) application that is submitted before issuance of the summary statement from the review of the previous new (A0) application.
- An application that has substantial overlap with another application pending appeal of initial peer review. (See NIH Grants Policy Statement 2.3.9.4 Similar, Essentially Identical, or Identical Applications ).
Candidates may submit research project grant (RPG) applications concurrently with the K application. However, any concurrent RPG application may not have substantial scientific and/or budgetary overlap with the career award application. K award recipients are encouraged to obtain funding from NIH or other Federal sources either as a PD/PI on a competing research grant award or cooperative agreement, or as project leader on a competing multi-project award as described in NOT-OD-18-157.
At the time of award, the candidate must have a “full-time” appointment at the academic institution. Candidates are required to commit a minimum of 75% of full-time professional effort (i.e., a minimum of 9 person-months) to their program of career development. Candidates may engage in other duties as part of the remaining 25% of their full-time professional effort not covered by this award, as long as such duties do not interfere with or detract from the proposed career development program.
Candidates who have VA appointments may not consider part of the VA effort toward satisfying the full time requirement at the applicant institution. Candidates with VA appointments should contact the staff person in the relevant Institute or Center prior to preparing an application to discuss their eligibility.
After the receipt of the award, adjustments to the required level of effort may be made in certain circumstances. See NOT-OD-18-156 and NIH Grants Policy Statement , Section 12.3.6.4 Temporary Adjustments to the Percent Effort Requirement for more details.
Before submitting the application, the candidate must identify a mentor who will supervise the proposed career development and research experience. The mentor should be an active investigator in the area of the proposed research and be committed both to the career development of the candidate and to the direct supervision of the candidate’s research. The mentor must document the availability of sufficient research support and facilities. Candidates are encouraged to identify more than one mentor, i.e., a mentoring team, if this is deemed advantageous for providing expert advice in all aspects of the research career development program. In such cases, one individual must be identified as the primary mentor who will coordinate the candidate’s research. The candidate must work with the mentor(s) in preparing the application. The mentor, or a member of the mentoring team, should have a successful track record of mentoring individuals at the candidate’s career stage. The recruitment of women, individuals from underrepresented racial and ethnic groups, and individuals with disabilities as potential mentors is encouraged.
The mentor(s) or mentoring team must demonstrate appropriate expertise, experience, and ability to guide the candidate in the organization, management and implementation of the proposed research and/or clinical trial.
The applicant institution must have a strong, well-established record of research and career development activities and faculty qualified to serve as mentors in biomedical, behavioral, or clinical research.
Section IV. Application and Submission Information
1. Requesting an Application Package
Buttons to access the online ASSIST system or to download application forms are available in Part 1 of this NOFO. See your administrative office for instructions if you plan to use an institutional system-to-system solution.
2. Content and Form of Application Submission
It is critical that applicants follow the instructions in the Career Development (K) Instructions in the How to Apply - Application Guide except where instructed in this notice of funding opportunity to do otherwise. Conformance to the requirements in the How to Apply - Application Guide is required and strictly enforced. Applications that are out of compliance with these instructions may be delayed or not accepted for review.
For information on Application Submission and Receipt, visit Frequently Asked Questions – Application Guide, Electronic Submission of Grant Applications .
Page Limitations
All page limitations described in the How to Apply - Application Guide and the Table of Page Limits must be followed.
The following section supplements the instructions found in the How to Apply - Application Guide and should be used for preparing an application to this NOFO.
SF424(R&R) Cover
All instructions in the How to Apply - Application Guide must be followed.
SF424(R&R) Project/Performance Site Locations
Other Project Information
SF424(R&R) Senior/Key Person Profile Expanded
R&R Budget
PHS 398 Cover Page Supplement
PHS 398 Career Development Award Supplemental Form
The PHS 398 Career Development Award Supplemental Form is comprised of the following sections:
Candidate Research Plan Other Candidate Information Mentor, Co-Mentor, Consultant, Collaborators Environment & Institutional Commitment to the Candidate Other Research Plan Sections Appendix
Candidate Section
All instructions in the How to Apply - Application Guide must be followed, with the following additional instructions:
Candidate Information and Goals for Career Development
Candidate’s Background
- Describe the candidate's commitment to a health-related research career. Describe all the candidate's professional responsibilities in the recipient institution and elsewhere and describe their relationship to the proposed activities on the career award.
- Describe prior training and how it relates to the objectives and long-term career plans of the candidate.
- Describe the candidate's research efforts to this point in their research career, including any publications, prior research interests and experience.
- Provide evidence of the candidate's potential to develop into an independent investigator.
Career Goals and Objectives
- Describe a systematic plan: (1) that shows a logical progression from prior research and training experiences to the research and career development experiences that will occur during the career award period and then to independent investigator status; and (2) that justifies the need for further career development to become an independent investigator.
Candidate’s Plan for Career Development/Training Activities During Award Period
- The candidate and the mentor(s) are jointly responsible for the preparation of the career development plan. A career development timeline is often helpful.
- The didactic (if any) and the research aspects of the plan must be designed to develop the necessary knowledge and research skills in scientific areas relevant to the candidate's career goals.
- Describe the professional responsibilities/activities including other research projects beyond the minimum required 9 person months (75% full-time professional effort) commitment to the career award. Explain how these responsibilities/activities will help ensure career progression to achieve independence as an investigator.
Research Plan Section
All instructions in the How to Apply - Application Guide must be followed, with the following additional instructions:
Research Strategy
- A sound research project that is consistent with the candidate’s level of research development and objectives of their career development plan must be provided. The research description should demonstrate the quality of the candidate’s research thus far and also the novelty, significance, creativity and approach, as well as the ability of the candidate to carry out the research.
- The application must also describe the relationship between the mentor’s research and the candidate’s proposed research plan.
- If the applicant is proposing to gain experience in a clinical trial, ancillary clinical trial or a clinical trial feasibility study as part of his or her research career development, describe the relationship of the proposed research project to the clinical trial.
Training in the Responsible Conduct of Research
- All applications must include a plan to fulfill NIH requirements for instruction in the Responsible Conduct of Research (RCR). See How to Apply - Application Guide for instructions.
Mentor, Co-Mentor, Consultant, Collaborators Section
Plans and Statements of Mentor and Co-mentor(s)
- The candidate must name a primary mentor who, together with the candidate, is responsible for the planning, directing, monitoring, and executing the proposed program. The candidate may also nominate co-mentors as appropriate to the goals of the program.
- The mentor should have sufficient independent research support to cover the costs of the proposed research project in excess of the allowable costs of this award.
- Include a statement that the candidate will commit at least 9 person months (75% of full-time professional effort) to the career development program and related career development activities.
- The application must include a statement from the mentor providing: 1) information on his/her research qualifications and previous experience as a research supervisor; 2) a plan that describes the nature of the supervision and mentoring that will occur during the proposed award period; 3) a plan for career progression for the candidate to move from the mentored stage of his/her career to independent research investigator status during the project period of the award; and 4) a plan for monitoring the candidate’s research, publications, and progression towards independence.
- Similar information must be provided by any co-mentor. If more than one co-mentor is proposed, the respective areas of expertise and responsibility of each should be described. Co-mentors should clearly describe how they will coordinate the mentoring of the candidate. If any co-mentor is not located at the sponsoring institution, a statement should be provided describing the mechanism(s) and frequency of communication with the candidate, including the frequency of face-to-face meetings.
- The mentor must agree to provide annual evaluations of the candidate’s progress as required in the annual progress report.
- If the candidate is proposing to gain experience in a clinical trial as part of his or her research career development, the mentor or co-mentor of the mentoring team must include a statement to document leadership of the clinical trial, and appropriate expertise to guide the applicant in any proposed clinical trials research experience.
Letters of Support from Collaborators, Contributors and Consultants
- Signed statements must be provided by all collaborators and/or consultants confirming their participation in the project and describing their specific roles. Unless also listed as senior/key personnel, collaborators and consultants do not need to provide their biographical sketches. However, information should be provided clearly documenting the appropriate expertise in the proposed areas of consulting/collaboration.
- Advisory committee members (if applicable): Signed statements must be provided by each member of the proposed advisory committee. These statements should confirm their participation, describe their specific roles, and document the expertise they will contribute. Unless also listed as senior/key personnel, these individuals do not need to provide their biographical sketches.
Environmental and Institutional Commitment to the Candidate
Description of Institutional Environment
- The sponsoring institution must document a strong, well-established research and career development program related to the candidate's area of interest, including a high-quality research environment with key faculty members and other investigators capable of productive collaboration with the candidate.
- Describe how the institutional research environment is particularly suited for the development of the candidate's research career and the pursuit of the proposed research plan.
- Describe the resources and facilities that will be available to the candidate.
Institutional Commitment to the Candidate’s Research Career Development
- The sponsoring institution must provide a statement of commitment to the candidate's development into a productive, independent investigator and to meeting the requirements of this award. It should be clear that the institutional commitment to the candidate is not contingent upon receipt of this career award.
- Provide assurances that the candidate will be able to devote the required effort to activities under this award. The remaining effort should be devoted to activities related to the development of the candidate’s career as an independent scientist.
- Provide assurances that the candidate will have access to appropriate office and laboratory space, equipment, and other resources and facilities (including access to clinical and/or other research populations, as applicable) to carry out the proposed research plan.
- Provide assurance that appropriate time and support will be available for any proposed mentor(s) and/or other staff consistent with the career development plan.
Other Plan(s):
Note: Effective for due dates on or after January 25, 2023, the Data Management and Sharing Plan will be attached in the Other Plan(s) attachment in FORMS-H application forms packages.
- All applicants planning research (funded or conducted in whole or in part by NIH) that results in the generation of scientific data are required to comply with the instructions for the Data Management and Sharing Plan. All applications, regardless of the amount of direct costs requested for any one year, must address a Data Management and Sharing Plan.
Limited items are allowed in the Appendix. Follow all instructions for the Appendix as described in the How to Apply - Application Guide ; any instructions provided here are in addition to the How to Apply - Application Guide instructions.
PHS Human Subjects and Clinical Trials Information
When involving NIH-defined human subjects research, clinical research, and/or clinical trials (and when applicable, clinical trials research experience) follow all instructions for the PHS Human Subjects and Clinical Trials Information form in the How to Apply - Application Guide , with the following additional instructions:
If you answered “Yes” to the question “Are Human Subjects Involved?” on the R&R Other Project Information form, you must include at least one human subjects study record using the Study Record: PHS Human Subjects and Clinical Trials Information form or Delayed Onset Study record.
Study Record: PHS Human Subjects and Clinical Trials Information
- For NOFOs that do not allow independent clinical trials, do not complete Section 4 – Protocol Synopsis information or Section 5 - Other Clinical Trial-related Attachments.
Delayed Onset Study
Note: Delayed onset does NOT apply to a study that can be described but will not start immediately (i.e., delayed start).
All instructions in the SF424 (R&R) Application Guide must be followed.
PHS Assignment Request Form
Reference Letters
Candidates must carefully follow the How to Apply - Application Guide , including the time period for when reference letters will be accepted . Applications lacking the appropriate required reference letters will not be reviewed. This is a separate process from submitting an application electronically. Reference letters are submitted directly through the eRA Commons Submit Referee Information link and not through Grants.gov.
3. Unique Entity Identifier and System for Award Management (SAM)
See Part 2. Section III.1 for information regarding the requirement for obtaining a unique entity identifier and for completing and maintaining active registrations in System for Award Management (SAM), NATO Commercial and Government Entity (NCAGE) Code (if applicable), eRA Commons, and Grants.gov
4. Submission Dates and Times
Part I. contains information about Key Dates and Times. Applicants are encouraged to submit applications before the due date to ensure they have time to make any application corrections that might be necessary for successful submission. When a submission date falls on a weekend or Federal holiday , the application deadline is automatically extended to the next business day.
Organizations must submit applications to Grants.gov (the online portal to find and apply for grants across all Federal agencies) using ASSIST or other electronic submission systems. Applicants must then complete the submission process by tracking the status of the application in the eRA Commons , NIH’s electronic system for grants administration. NIH and Grants.gov systems check the application against many of the application instructions upon submission. Errors must be corrected and a changed/corrected application must be submitted to Grants.gov on or before the application due date and time. If a Changed/Corrected application is submitted after the deadline, the application will be considered late. Applications that miss the due date and time are subjected to the NIH Grants Policy Statement Section 2.3.9.2 Electronically Submitted Applications .
Applicants are responsible for viewing their application before the due date in the eRA Commons to ensure accurate and successful submission.
Information on the submission process and a definition of on-time submission are provided in the How to Apply - Application Guide .
5. Intergovernmental Review (E.O. 12372)
This initiative is not subject to intergovernmental review.
6. Funding Restrictions
All NIH awards are subject to the terms and conditions, cost principles, and other considerations described in the NIH Grants Policy Statement Section 7.9.1 Selected Items of Cost .
Pre-award costs are allowable only as described in the NIH Grants Policy Statement .
7. Other Submission Requirements and Information
Applications must be submitted electronically following the instructions described in the How to Apply - Application Guide . Paper applications will not be accepted.
Applicants must complete all required registrations before the application due date. Section III. Eligibility Information contains information about registration.
For assistance with your electronic application or for more information on the electronic submission process, visit How to Apply - Application Guide . If you encounter a system issue beyond your control that threatens your ability to complete the submission process on-time, you must follow the Dealing with System Issues guidance. For assistance with application submission, contact the Application Submission Contacts in Section VII.
Important reminders:
All PD(s)/PI(s) must include their eRA Commons ID in the Credential field of the Senior/Key Person Profile form . Failure to register in the Commons and to include a valid PD/PI Commons ID in the credential field will prevent the successful submission of an electronic application to NIH. See Section III of this NOFO for information on registration requirements.
The applicant organization must ensure that the unique entity identifier provided on the application is the same identifier used in the organization’s profile in the eRA Commons and for the System for Award Management. Additional information may be found in the How to Apply - Application Guide .
See more tips for avoiding common errors.
Upon receipt, applications will be evaluated for completeness and compliance with application instructions by the Center for Scientific Review, NIH. Applications that are incomplete or non-compliant will not be reviewed.
Post Submission Materials
Applicants are required to follow the instructions for post-submission materials, as described in the policy .
Any instructions provided here are in addition to the instructions in the policy.
Section V. Application Review Information
1. Criteria
Only the review criteria described below will be considered in the review process. Applications submitted to the NIH in support of the NIH mission are evaluated for scientific and technical merit through the NIH peer review system.
For this particular announcement, note the following : Reviewers should evaluate the candidate’s potential for developing an independent research program that will make important contributions to the field, taking into consideration the years of research experience and the likely value of the proposed research career development as a vehicle for developing a successful, independent research program.
Overall Impact
Reviewers should provide their assessment of the likelihood that the proposed career development and research plan will enhance the candidate’s potential for a productive, independent scientific research career in a health-related field, taking into consideration the criteria below in determining the overall impact score.
Reviewers will consider each of the review criteria below in the determination of scientific merit, and give a separate score for each. An application does not need to be strong in all categories to be judged likely to have major scientific impact.
Candidate
- Does the candidate have the potential to develop as an independent and productive researcher?
- Are the candidate's prior training and research experience appropriate for this award?
- Is the candidate’s academic, clinical (if relevant), and research record of high quality?
- Is there evidence of the candidate’s commitment to meeting the program objectives to become an independent investigator in research?
- Do the reference letters address the above review criteria, and do they provide evidence that the candidate has a high potential for becoming an independent investigator?
Career Development Plan/Career Goals and Objectives
- What is the likelihood that the plan will contribute substantially to the scientific development of the candidate and lead to scientific independence?
- Are the candidate's prior training and research experience appropriate for this award?
- Are the content, scope, phasing, and duration of the career development plan appropriate when considered in the context of prior training/research experience and the stated training and research objectives for achieving research independence?
- Are there adequate plans for monitoring and evaluating the candidate’s research and career development progress?
- If proposed, will the clinical trial experience contribute to the applicant's research career development?
Research Plan
- Are the proposed research questions, design, and methodology of significant scientific and technical merit?
- Is the prior research that serves as the key support for the proposed project rigorous?
- Has the candidate included plans to address weaknesses in the rigor of prior research that serves as the key support for the proposed project?
- Has the candidate presented strategies to ensure a robust and unbiased approach, as appropriate for the work proposed?
- Has the candidate presented adequate plans to address relevant biological variables, such as sex, for studies in vertebrate animals or human subjects?
- Is the research plan relevant to the candidate's research career objectives?
- Is the research plan appropriate to the candidate's stage of research development and as a vehicle for developing the research skills described in the career development plan?
- If proposed, will the clinical trial experience contribute to the research project?
Mentor(s), Co-Mentor(s), Consultant(s), Collaborator(s)
- Are the qualifications of the mentor(s) in the area of the proposed research appropriate?
- Does the mentor(s) adequately address the candidate’s potential and their strengths and areas needing improvement?
- Is there adequate description of the quality and extent of the mentor’s proposed role in providing guidance and advice to the candidate?
- Is the mentor’s description of the elements of the research career development activities, including formal course work adequate?
- Is there evidence of the mentor’s, consultant’s, and/or collaborator’s previous experience in fostering the development of independent investigators?
- Is there evidence of the mentor's current research productivity and peer-reviewed support?
- Is active/pending support for the proposed research project appropriate and adequate?
- Are there adequate plans for monitoring and evaluating the career development recipient’s progress toward independence?
- Are the mentor’s research qualifications, scientific stature, experience, and mentoring track record appropriate for the candidate's research career development needs?
- Are the nature and extent of the proposed mentorship adequate and appropriate, and is the commitment of the mentor(s) to the candidate's advanced research career development appropriate?
- Does the mentor(s) have a history of research productivity and support?
- If the applicant is proposing to gain experience in a clinical trial as part of his or her research career development, is there evidence of the appropriate expertise, experience, and ability on the part of the mentor(s) to guide the applicant during participation in the clinical trial?
Environment & Institutional Commitment to the Candidate
- Is there clear commitment of the sponsoring institution to ensure that the required minimum of the candidate’s effort will be devoted directly to the research described in the application, with the remaining percent effort being devoted to an appropriate balance of research, teaching, administrative, and clinical responsibilities?
- Is the institutional commitment to the career development of the candidate appropriately strong?
- Are the research facilities, resources and training opportunities, including faculty capable of productive collaboration with the candidate adequate and appropriate?
- Is the environment for the candidate’s scientific and professional development of high quality?
- Is there assurance that the institution intends the candidate to be an integral part of its research program as an independent investigator?
Protections for Human Subjects
For research that involves human subjects but does not involve one of the categories of research that are exempt under 45 CFR Part 46, the committee will evaluate the justification for involvement of human subjects and the proposed protections from research risk relating to their participation according to the following five review criteria: 1) risk to subjects, 2) adequacy of protection against risks, 3) potential benefits to the subjects and others, 4) importance of the knowledge to be gained, and 5) data and safety monitoring for clinical trials.
For research that involves human subjects and meets the criteria for one or more of the categories of research that are exempt under 45 CFR Part 46, the committee will evaluate: 1) the justification for the exemption, 2) human subjects involvement and characteristics, and 3) sources of materials. For additional information on review of the Human Subjects section, please refer to the Guidelines for the Review of Human Subjects .
Inclusion of Women, Minorities, and Individuals Across the Lifespan
When the proposed project involves human subjects and/or NIH-defined clinical research, the committee will evaluate the proposed plans for the inclusion (or exclusion) of individuals on the basis of sex/gender, race, and ethnicity, as well as the inclusion (or exclusion) of individuals of all ages (including children and older adults) to determine if it is justified in terms of the scientific goals and research strategy proposed. For additional information on review of the Inclusion section, please refer to the Guidelines for the Review of Inclusion in Clinical Research .
Vertebrate Animals
The committee will evaluate the involvement of live vertebrate animals as part of the scientific assessment according to the following three points: (1) a complete description of all proposed procedures including the species, strains, ages, sex, and total numbers of animals to be used; (2) justifications that the species is appropriate for the proposed research and why the research goals cannot be accomplished using an alternative non-animal model; and (3) interventions including analgesia, anesthesia, sedation, palliative care, and humane endpoints that will be used to limit any unavoidable discomfort, distress, pain and injury in the conduct of scientifically valuable research. Methods of euthanasia and justification for selected methods, if NOT consistent with the AVMA Guidelines for the Euthanasia of Animals, is also required but is found in a separate section of the application. For additional information on review of the Vertebrate Animals Section, please refer to the Worksheet for Review of the Vertebrate Animals Section.
Reviewers will assess whether materials or procedures proposed are potentially hazardous to research personnel and/or the environment, and if needed, determine whether adequate protection is proposed.
Resubmissions
For Resubmissions, the committee will evaluate the application as now presented, taking into consideration the responses to comments from the previous scientific review group and changes made to the project.
For Revisions, the committee will consider the appropriateness of the proposed expansion of the scope of the project. If the Revision application relates to a specific line of investigation presented in the original application that was not recommended for approval by the committee, then the committee will consider whether the responses to comments from the previous scientific review group are adequate and whether substantial changes are clearly evident.
As applicable for the project proposed, reviewers will consider each of the following items, but will not give scores for these items, and should not consider them in providing an overall impact score.
Resource Sharing Plans
Reviewers will comment on whether the Resource Sharing Plan(s) (i.e., Sharing Model Organisms ) or the rationale for not sharing the resources, is reasonable.
All applications for support under this NOFO must include a plan to fulfill NIH requirements for instruction in the Responsible Conduct of Research (RCR). Taking into account the level of experience of the candidate, including any prior instruction or participation in RCR as appropriate for the candidate’s career stage, the reviewers will evaluate the adequacy of the proposed RCR training in relation to the following five required components: 1) Format - the required format of instruction, i.e., face-to-face lectures, coursework, and/or real-time discussion groups (a plan with only on-line instruction is not acceptable); 2) Subject Matter - the breadth of subject matter, e.g., conflict of interest, authorship, data management, human subjects and animal use, laboratory safety, research misconduct, research ethics; 3) Faculty Participation - the role of the mentor(s) and other faculty involvement in the fellow’s instruction; 4) Duration of Instruction - the number of contact hours of instruction (at least eight contact hours are required); and 5) Frequency of Instruction – instruction must occur during each career stage and at least once every four years. Plans and past record will be rated as ACCEPTABLE or UNACCEPTABLE , and the summary statement will provide the consensus of the review committee. See also: NOT-OD-10-019 .
Select Agent Research
Reviewers will assess the information provided in this section of the application, including 1) the Select Agent(s) to be used in the proposed research, 2) the registration status of all entities where Select Agent(s) will be used, 3) the procedures that will be used to monitor possession use and transfer of Select Agent(s), and 4) plans for appropriate biosafety, biocontainment, and security of the Select Agent(s).
Authentication of Key Biological and/or Chemical Resources
For projects involving key biological and/or chemical resources, reviewers will comment on the brief plans proposed for identifying and ensuring the validity of those resources.
Budget and Period of Support
Reviewers will consider whether the budget and the requested period of support are fully justified and reasonable in relation to the proposed research.
2. Review and Selection Process
Applications will be evaluated for scientific and technical merit by (an) appropriate Scientific Review Group(s), in accordance with NIH peer review policies and practices , using the stated review criteria. Assignment to a Scientific Review Group will be shown in the eRA Commons.
As part of the scientific peer review, all applications:
- May undergo a selection process in which only those applications deemed to have the highest scientific and technical merit (generally the top half of applications under review) will be discussed and assigned an overall impact score.
- Will receive a written critique.
Applications will be assigned on the basis of established PHS referral guidelines to the appropriate NIH Institute or Center. Applications will compete for available funds with all other recommended applications. Following initial peer review, recommended applications will receive a second level of review by the appropriate national Advisory Council or Board.
- Scientific and technical merit of the proposed project as determined by scientific peer review.
- Availability of funds.
- Relevance of the proposed project to program priorities
3. Anticipated Announcement and Award Dates
After the peer review of the application is completed, the PD/PI will be able to access his or her Summary Statement (written critique) via the eRA Commons . Refer to Part 1 for dates for peer review, advisory council review, and earliest start date.
Information regarding the disposition of applications is available in the NIH Grants Policy Statement Section 2.4.4 Disposition of Applications .
Section VI. Award Administration Information
1. Award Notices
If the application is under consideration for funding, NIH will request "just-in-time" information from the applicant as described in the NIH Grants Policy Statement . This request is not a Notice of Award nor should it be construed to be an indicator of possible funding.
A formal notification in the form of a Notice of Award (NoA) will be provided to the applicant organization for successful applications. The NoA signed by the grants management officer is the authorizing document and will be sent via email to the recipient’s business official.
Recipients must comply with any funding restrictions described in Section IV.6. Funding Restrictions . Selection of an application for award is not an authorization to begin performance. Any costs incurred before receipt of the NoA are at the recipient's risk. These costs may be reimbursed only to the extent considered allowable pre-award costs.
Any application awarded in response to this NOFO will be subject to terms and conditions found on the Award Conditions and Information for NIH Grants website. This includes any recent legislation and policy applicable to awards that is highlighted on this website.
Institutional Review Board or Independent Ethics Committee Approval: Grantee institutions must ensure that all protocols are reviewed by their IRB or IEC. To help ensure the safety of participants enrolled in NIH-funded studies, the recipient must provide NIH copies of documents related to all major changes in the status of ongoing protocols.
2. Administrative and National Policy Requirements
All NIH grant and cooperative agreement awards include the NIH Grants Policy Statement as part of the NoA. For these terms of award, see the NIH Grants Policy Statement Part II: Terms and Conditions of NIH Grant Awards, Subpart A: General and Part II: Terms and Conditions of NIH Grant Awards, Subpart B: Terms and Conditions for Specific Types of Grants, Recipients, and Activities , including of note, but not limited to:
- Federal-wide Standard Terms and Conditions for Research Grants
- Prohibition on Certain Telecommunications and Video Surveillance Services or Equipment
- Acknowledgment of Federal Funding
If a recipient is successful and receives a Notice of Award, in accepting the award, the recipient agrees that any activities under the award are subject to all provisions currently in effect or implemented during the period of the award, other Department regulations and policies in effect at the time of the award, and applicable statutory provisions.
If a recipient receives an award, the recipient must follow all applicable nondiscrimination laws. The recipient agrees to this when registering in SAM.gov. The recipient must also submit an Assurance of Compliance ( HHS-690 ). To learn more, see Laws and Regulations Enforced by the HHS Office for Civil Rights website .
HHS recognizes that NIH research projects are often limited in scope for many reasons that are nondiscriminatory, such as the principal investigator’s scientific interest, funding limitations, recruitment requirements, and other considerations. Thus, criteria in research protocols that target or exclude certain populations are warranted where nondiscriminatory justifications establish that such criteria are appropriate with respect to the health or safety of the subjects, the scientific study design, or the purpose of the research. For additional guidance regarding how the provisions apply to NIH grant programs, please contact the Scientific/Research Contact that is identified in Section VII under Agency Contacts of this NOFO.
In accordance with the statutory provisions contained in Section 872 of the Duncan Hunter National Defense Authorization Act of Fiscal Year 2009 (Public Law 110-417), NIH awards will be subject to System for Award Management (SAM.gov) requirements. SAM.gov requires Federal agencies to review and consider information about an applicant in the designated integrity and performance system (currently SAM.gov) prior to making an award. An applicant can review and comment on any information in the responsibility/qualification records available in SAM.gov. NIH will consider any comments by the applicant, in addition to the information available in the responsibility/qualification records in SAM.gov, in making a judgement about the applicant’s integrity, business ethics, and record of performance under Federal awards when completing the review of risk posed by applicants as described in 2 CFR Part 200.206 “Federal awarding agency review of risk posed by applicants.” This provision will apply to all NIH grants and cooperative agreements except fellowships.
3. Data Management and Sharing
Consistent with the 2023 NIH Policy for Data Management and Sharing, when data management and sharing is applicable to the award, recipients will be required to adhere to the Data Management and Sharing requirements as outlined in the NIH Grants Policy Statement . Upon the approval of a Data Management and Sharing Plan, it is required for recipients to implement the plan as described.
4. Reporting
When multiple years are involved, recipients will be required to submit the Research Performance Progress Report (RPPR) annually and financial statements as required in the NIH Grants Policy Statement . The Supplemental Instructions for Individual Career Development (K) RPPRs must be followed. For mentored awards, the Mentor’s Report must include an annual evaluation statement of the candidate’s progress.
A final RPPR, invention statement, and the expenditure data portion of the Federal Financial Report are required for closeout of an award, as described in the NIH Grants Policy Statement . NIH NOFOs outline intended research goals and objectives. Post award, NIH will review and measure performance based on the details and outcomes that are shared within the RPPR, as described at 2 CFR 200.301.
The Federal Funding Accountability and Transparency Act of 2006 as amended (FFATA), includes a requirement for recipients of Federal grants to report information about first-tier subawards and executive compensation under Federal assistance awards issued in FY2011 or later. All recipients of applicable NIH grants and cooperative agreements are required to report to the Federal Subaward Reporting System (FSRS) available at www.fsrs.gov on all subawards over the threshold. See the NIH Grants Policy Statement for additional information on this reporting requirement.
In accordance with the regulatory requirements provided at 2 CFR Part 200.113 and Appendix XII to 2 CFR Part 200, recipients that have currently active Federal grants, cooperative agreements, and procurement contracts from all Federal awarding agencies with a cumulative total value greater than $10,000,000 for any period of time during the period of performance of a Federal award, must report and maintain the currency of information reported in the System for Award Management (SAM) about civil, criminal, and administrative proceedings in connection with the award or performance of a Federal award that reached final disposition within the most recent five-year period. The recipient must also make semiannual disclosures regarding such proceedings. Proceedings information will be made publicly available in the designated integrity and performance system (Responsibility/Qualification in SAM.gov, formerly FAPIIS). This is a statutory requirement under section 872 of Public Law 110-417, as amended (41 U.S.C. 2313). As required by section 3010 of Public Law 111-212, all information posted in the designated integrity and performance system on or after April 15, 2011, except past performance reviews required for Federal procurement contracts, will be publicly available. Full reporting requirements and procedures are found in Appendix XII to 2 CFR Part 200 – Award Term and Condition for Recipient Integrity and Performance Matters.
5. Evaluation
In carrying out its stewardship of human resource-related programs, NIH may request information essential to an assessment of the effectiveness of this program from databases and from participants themselves. Participants may be contacted after the completion of this award for periodic updates on various aspects of their employment history, publications, support from research grants or contracts, honors and awards, professional activities, and other information helpful in evaluating the impact of the program.
Section VII. Agency Contacts
We encourage inquiries concerning this funding opportunity and welcome the opportunity to answer questions from potential applicants.
Because of the difference in individual Institute and Center (IC) program requirements for this NOFO, prospective applications MUST consult the Table of IC-Specific Information, Requirements, and Staff Contacts , to make sure that their application is responsive to the requirements of one of the participating NIH ICs. Prior consultation with NIH staff is strongly encouraged.
eRA Service Desk (Questions regarding ASSIST, eRA Commons, application errors and warnings, documenting system problems that threaten on-time submission, and post-submission issues)
Finding Help Online: https://www.era.nih.gov/need-help (preferred method of contact) Telephone: 301-402-7469 or 866-504-9552 (Toll Free)
General Grants Information (Questions regarding application processes and NIH grant resources) Email: [email protected] (preferred method of contact) Telephone: 301-637-3015
Grants.gov Customer Support (Questions regarding Grants.gov registration and Workspace) Contact Center Telephone: 800-518-4726 Email: [email protected]
See Table of IC-Specific Information, Requirements and Staff Contacts .
Examine your eRA Commons account for review assignment and contact information (information appears two weeks after the submission due date).
Section VIII. Other Information
Recently issued trans-NIH policy notices may affect your application submission. A full list of policy notices published by NIH is provided in the NIH Guide for Grants and Contracts . All awards are subject to the terms and conditions, cost principles, and other considerations described in the NIH Grants Policy Statement .
Please note that the NIH Loan Repayment Programs (LRPs) are a set of programs to attract and retain promising early-stage investigators in research careers by helping them to repay their student loans. Recipients of career development awards are encouraged to consider applying for an extramural LRP award.
Awards are made under the authorization of Sections 301 and 405 of the Public Health Service Act as amended (42 USC 241 and 284) and under Federal Regulations 42 CFR Part 52 and 2 CFR Part 75.

Note: For help accessing PDF, RTF, MS Word, Excel, PowerPoint, Audio or Video files, see Help Downloading Files .
opens in a new window

Physician Assistant Career Paths: Clinical Practice vs. Clinical Research
Whether you’re already a practicing physician assistant (PA) or are still studying to become a PA, you’re likely aware that there is more than one exciting career pathway available to you. While 93.7 percent of physician assistants choose clinical practice after completing their studies and certification, others may decide to pursue a career in clinical research. Some PAs even do both, participating in clinical research trials within a practice setting in conjunction with caring for a panel of patients.
What to Expect as a PA in Clinical Research
Clinical research trials are critical to the advancement of medicine. Not only are they used to develop and test potentially lifesaving treatments, but they can also be designed to further the understanding of disease processes and short- and long-term outcomes.
Physician assistants employed in clinical research participate in a variety of ways—from the recruiting, screening, and retaining of participants to implementing trial protocols. They often serve as clinical research coordinators, and may even advance to co-investigator and principal investigator positions.
Principal—or primary—investigators (also known as PIs) are responsible for running clinical trials according to study protocols, keeping accurate records, and reporting adverse effects. Co-investigators (also known as sub-investigators) work under the PI and complete many of the same duties. They also supervise the clinical research coordinators who are responsible for monitoring trial-related activities and the clinical research associates who are part of the research team.
Caring for patients who are enrolled in clinical trials is an increasingly common role for physician assistants as well. These PAs basically provide patient care to the study participants in the same way they would do so within clinical practice.
How to Become a Clinical Research PA
Many physician assistants who work in clinical research learn through on-the-job training. Often, their entrance into the field is through a position working with a physician or on a medical team that’s involved in clinical trials. This may happen directly after certification or after years in clinical practice. Either way, knowledge of the clinical research lifecycle, an understanding of biostatics, or a strong desire to learn more in these areas will be helpful.
If you think you may want to advance to the level of co-investigator or primary investigator, earning a doctorate degree may give you an advantage over others seeking promotion to these leadership positions. Look for PA doctorate programs with a focus on clinical research.
It can also be helpful to connect with other physician assistants who are already involved in research. These PAs can give you a firsthand account of their experience and may be willing to refer you for available positions and serve as mentors.
Other ways to find your first opportunity include searching for PA jobs that combine clinical practice with clinical trials. And, if you’re still a PA student, you can pursue opportunities within your school’s academic research lab or at local offices of research attached to your academic center.
Pros and Cons of Choosing Both Clinical Research and Clinical Practice as a PA
Because clinical research trials often test developing therapies and explore the forefront of medical physiology, physician assistants who choose to work in both clinical research and clinical practice have a serious advantage over their counterparts who focus on clinical practice alone.
These PAs gain a direct understanding of how treatment is evolving, and they can incorporate that knowledge into their clinical practice. Rather than waiting for a medical body (like the American Medical Association, for example) to put together new treatment guidelines years down the line, they’re able to treat their patients according to the latest discoveries now.
Of course, there are also potential drawbacks when working in both research and practice. These include the possibility that your research work may not be factored into your clinical salary (resulting in more work for the same pay), and the need to cover unbudgeted travel costs for attending conferences if you’re asked to present your research.

Related Articles

How to Launch Your Physician Assistant Career

A Day In the Life of a Cardiology PA

The Pros and Cons of Doctoral Degrees for PAs
The IU School of Medicine Department of Obstetrics and Gynecology is proud to offer the Clinical Research and Academic Success in Obstetrics and Gynecology course . The course will take place in Stowe, Vermont, at the Topnotch Resort, from Aug. 26–29, 2024. Vermont was selected because it welcomes, affirms, and respects reproductive rights. We look forward to seeing you in Vermont! Questions should be directed to the course director, Dr. Jeffery Peipert, MD, PhD, Clarence E. Ehrlich Professor and Department of Obstetrics and Gynecology Chair at IU School of Medicine.
Who the program is for
What learners will gain.
- List the key attributes of an appropriate research question
- Describe the advantages and disadvantages of longitudinal cohort studies, case-control studies and randomized clinical trials
- Use the basic functions of a statistical software package
- Understand the basic principles of article publication and manuscript review processes
- Critically read research papers by understanding the main sources of bias and confounding
- List the attributes of a good mentor and the key principles of a successful mentor-mentee relationship
- Describe the process for promotion from Assistant to Full Professor and the “currency” of academic faculty for career advancement
Submit an application
To apply, please email Dr. Peipert the following:
- A brief statement (one page or less) indicating your reason for applying
- Potential clinical research interests
- A letter of commitment for funding from chair or division director.
Participants and their departments will be responsible for travel, accommodations and tuition ($4,000).
Applications are due by May 15, 2024.
Email your application now
Course faculty

Kavita Nanda, MD, MHS
Director of Medical Research at FHI360
Kavita Nanda, MD, MS, is an internationally recognized expert in contraception. She is the director of medical research at FHI360, where she has worked as an obstetrician gynecologist, epidemiologist and scientist for almost 25 years.
For the past 18 years, she has worked as a temporary advisor to WHO, working to update the WHO Medical Eligibility Criteria and Selected Practice Recommendations for Contraceptive Use. She is also a member of the CDC guidelines development group for contraception.
Nanda received her medical degree from Albany Medical College, completed her residency at Thomas Jefferson University, and did a Women’s Health Research Fellowship and Master of Health Sciences (Clinical Research) at Duke University.

Christina M. Scifres, MD
Associate Professor of Obstetrics & Gynecology
Christina Scifres, MD, is a maternal-fetal medicine specialist at Indiana University School of Medicine. She joined the Department of Obstetrics and Gynecology faculty as an associate professor in 2018 and was named the maternal-fetal medicine division director in 2020.
Her clinical and research interests include gestational diabetes, ovarian cysts, and improving outcomes in pregnancies complicated by diabetes and obesity. She was a co-investigator for an NIH-funded randomized clinical trial comparing two screening strategies for gestational diabetes.
Scifres graduated from the University of Oklahoma College of Medicine and completed residency and a fellowship at Washington University School of Medicine.
Read Bio Christina M. Scifres, MD

Jeffrey F. Peipert, MD, PhD
Chair, Department of Obstetrics & Gynecology
Jeffrey F. Peipert, MD, PhD, is the Clarence E. Ehrlich Professor and chair of the Department of Obstetrics and Gynecology at Indiana University School of Medicine. He is board-certified in obstetrics and gynecology and has a doctorate in epidemiology.
He has conducted numerous studies including: NICHD-funded randomized trial of a computer-based intervention to encourage dual method contraceptive use to prevent unplanned pregnancy and STIs; randomized trial of therapy for pelvic inflammatory disease (PEACH Study). He was also the principal investigator of a large prospective study, the Contraceptive CHOICE Project, which recruited 9,256 women and successfully followed them for two to three years for contraceptive effectiveness, satisfaction, and continuation rates.
Read Bio Jeffrey F. Peipert, MD, PhD
Clinical Research Supervisor - 128461
Job description, #128461 clinical research supervisor.
UCSD Layoff from Career Appointment : Apply by 03/08/2024 for consideration with preference for rehire. All layoff applicants should contact their Employment Advisor.
Special Selection Applicants : Apply by 03/20/2024. Eligible Special Selection clients should contact their Disability Counselor for assistance.
DESCRIPTION
The Hamilton Glaucoma Center (HGC) clinical research group of the Department of Ophthalmology and the Shiley Eye Institute (SEI) at UCSD is involved in research to better understand the pathophysiology of glaucoma and to identify effective approaches to early diagnosis of the disease. HGC is the coordinating center for several multi-center studies including the African Descent and Glaucoma Evaluation Study (ADAGES) and the Diagnostic Innovations in Glaucoma Study (DIGS). It also participates in numerous sponsor studies initiated by companies researching glaucoma treatments and devices. The Department of Ophthalmology currently receives over $6 million annually from federal funding programs of the National Institutes of Health, National Eye Institute and other sources.
Supervise the glaucoma clinic research team under the direction of principal investigators at the Hamilton Glaucoma Center. Supervise study staff, conduct performance evaluations and hiring. Act as a resource for other Glaucoma Center personnel. Act as a resource for the research studies' regulatory activities including, but not limited to, interpreting, preparation, coordination, implementation and compliance with the Institutional Review Board (IRB) guidelines, FDA requirements, and Good Clinical Practice procedures. Interpret research protocols, coordinate and supervise subject testing, data collection and design data analysis protocols to ensure compliance and completion for a variety of studies simultaneously. Supervise and train personnel in the operation of clinical and research instruments, ensuring quality and efficient assessment of visual function, physiology and anatomy in normal, glaucoma suspect and glaucoma patients. Participate in the design, management, and maintenance of database program in both IBM and Macintosh environments.
Supervises staff involved in routine research study coordination. Receives predetermined work assignments that are subject to a moderate level of control and review. Oversees staff in execution of assignments; trains and mentors staff to improve quality and quantity of work.
MINIMUM QUALIFICATIONS
Seven years of related experience, education/training, OR an Bachelor’s degree in related area plus three years of related experience/training.
Working knowledge of clinical or laboratory research, clinical trial recruitment, eligibility, protocol adherence, quality data submission and adverse event reporting.
Ability to effectively manage multiple priorities, prioritize projects and meet the demands of a fast-paced and dynamic work environment. Adaptable to quickly changing priorities.
Demonstrated skills in employee supervision and HR administration. Demonstrated experience in training others, particularly in the field of research.
Critical thinking skills to evaluate issues and identify a potential solution.
Clear and concise communicator; good verbal and written communication skills; both.
Good interpersonal skills, including but not limited to: problem-solving, teamwork development, leadership, mentorship. Interpersonal skills to effectively motivate others. Works well with others to achieve common goals. Ability to cultivate relationships with multiple stakeholders at various levels of administration.
Ability to perform all commonly applicable functions in word processing and spreadsheet software. Effectively uses campus' clinical information and documentation application programs. Proven ability utilizing clinical trial management systems.
Ability to collect and analyze patient data in a clinical ophthalmology setting and report findings in a written form.
Advanced knowledge of medical terminology in the field of Ophthalmology.
Demonstrated excellent ability to work with a variety of individuals including elderly patients, faculty, clinicians, staff and students in a medical setting.
Demonstrated strong ability to maintain confidentiality involving medical records, personnel and work related materials.
BART training & certification within 6 months of hire date.
Demonstrated knowledge of academic institutional review board clinical trials submission process and regulations.
Demonstrated experience in interpreting medical charts and extracting accurate data from medical records.
Demonstrated ability to understand and interpret complex research protocols in order to screen patients for eligibility, initiate study plan, collect data, evaluated for adverse events and protocol deviations.
Demonstrated experience working with FDA regulations, policies and procedures, and good Clinical Practice guidelines for the conduct of clinical research.
PREFERRED QUALIFICATIONS
Advanced knowledge of quality control algorithms and relational database design and administration.
Experience as an ophthalmic technician and/or coordinator in the field of ophthalmology. Demonstrated strong ability to instruct patients regarding ophthalmic services such as explaining and reinforcing physician instructions.
Knowledge and ability to perform diagnostic imaging equipment such as Optical Coherence Topography (OCT) instruments, Fundus photography, and other clinical equipment including Standard & Color Visual Fields, Snellen Visual Acuity, ETDRS, Contrast Sensitivity Acuity, Noncontact & Applanation Tonometry, Pachymetry, Keratometry, IOL Master, Lensometer, Autorefractor, Refraction with Trial Lenses & Phoropter, Various Color Visit Tests, Anthropometric Measurements, Lens opacity measurements, and other anatomical/physiological tests, techniques & instruments.
SPECIAL CONDITIONS
Must be able to work various hours and locations based on business needs.
Must be willing to travel to occasional investigator meetings and training sessions in the U.S.
Employment is subject to a criminal background check and pre-employment physical.
Pay Transparency Act
Annual Full Pay Range: $82,500 - $151,500 (will be prorated if the appointment percentage is less than 100%)
Hourly Equivalent: $39.51 - $72.56
Factors in determining the appropriate compensation for a role include experience, skills, knowledge, abilities, education, licensure and certifications, and other business and organizational needs. The Hiring Pay Scale referenced in the job posting is the budgeted salary or hourly range that the University reasonably expects to pay for this position. The Annual Full Pay Range may be broader than what the University anticipates to pay for this position, based on internal equity, budget, and collective bargaining agreements (when applicable).
If employed by the University of California, you will be required to comply with our Policy on Vaccination Programs, which may be amended or revised from time to time. Federal, state, or local public health directives may impose additional requirements. If applicable, life-support certifications (BLS, NRP, ACLS, etc.) must include hands-on practice and in-person skills assessment; online-only certification is not acceptable.
UC San Diego Health Sciences is comprised of our School of Medicine, Skaggs School of Pharmacy and Pharmaceutical Sciences, The Herbert Wertheim School of Public Health and Human Longevity Science, and our Student Health and Well-Being Department. We have long been at the forefront of translational - or "bench-to-bedside" - research, transforming patient care through discovery and innovation leading to new drugs and technologies. Translational research is carried out every day in the hundreds of clinical trials of promising new therapies offered through UC San Diego Health, and in the drive of our researchers and clinician-scientists who are committed to having a significant impact on patient care. We invite you to join our team!
Applications/Resumes are accepted for current job openings only. For full consideration on any job, applications must be received prior to the initial closing date. If a job has an extended deadline, applications/resumes will be considered during the extension period; however, a job may be filled before the extended date is reached.
To foster the best possible working and learning environment, UC San Diego strives to cultivate a rich and diverse environment, inclusive and supportive of all students, faculty, staff and visitors. For more information, please visit UC San Diego Principles of Community .
UC San Diego is an Equal Opportunity/Affirmative Action Employer. All qualified applicants will receive consideration for employment without regard to race, color, religion, sex, sexual orientation, gender identity, national origin, disability, age or protected veteran status.
For the University of California’s Affirmative Action Policy please visit: https://policy.ucop.edu/doc/4010393/PPSM-20 For the University of California’s Anti-Discrimination Policy, please visit: https://policy.ucop.edu/doc/1001004/Anti-Discrimination
UC San Diego is a smoke and tobacco free environment. Please visit smokefree.ucsd.edu for more information.
UC San Diego Health maintains a marijuana and drug free environment. Employees may be subject to drug screening.
Application Instructions
Please click on the link below to apply for this position. A new window will open and direct you to apply at our corporate careers page. We look forward to hearing from you!
Share This Page
Posted : 4/25/2024
Job Reference # : 128461
JOIN OUR TALENT COMMUNITY
Interested in working at UC San Diego and UC San Diego Health but can't find a position that's right for you? Submit your resume to our Talent Community to be considered for future opportunities that may align with your expertise. Please note, by joining our Talent Community, you are not applying for a position with UC San Diego Campus and Health. Rather, this is an additional way for our Talent Acquisition team to find candidates with specific credentials, if an opportunity arises. You are still encouraged to regularly check back on our career site or sign up for Job Alerts to apply for openings that are a match for your background.
- Career Sites by Recruiting.com
What Can You Do With a Medical Degree?
If you don't want to be a doctor who treats patients, options range from research and writing to consulting and counseling.
Different jobs with med degree

Getty Images
Doctors in the pharmaceutical research field may work for pharmaceutical companies, research organizations or regulatory agencies in medicine discovery, development and licensing.
Key Takeaways
- There are options aside from being a doctor who treats patients.
- An alternative career may require additional training or education.
- Medical schools prefer to admit students who will practice medicine.
After graduating with a medical degree, most doctors complete a full three-year residency training program. While it's possible for medical school graduates to earn a general medical license after completing a single postgraduate year and passing all three steps of the United States Medical Licensing Examination, clinical opportunities are extremely limited for those who don't complete residency and become board-certified.
Most doctors go on to become traditional clinicians, examining, diagnosing and treating patients.
For those who want to treat patients, the long list of medical and surgical specialties includes pediatrics, internal medicine, radiology, psychiatry, oncology, emergency medicine, anesthesiology, general surgery, plastic and maxillofacial surgery, ophthalmology and orthopedics.
Because a medical degree represents enormous financial and personal sacrifice, and pursuing a clinical career means years of rigorous training beyond medical school, most physicians expect to stay in clinical practice for decades. And while almost two-thirds of doctors say they’d still choose the profession if they had their career to do over, according to a 2023 survey by The Physicians Foundation, life as a clinician is not for everyone.
Dr. Steve Liggett, vice dean for research at the Morsani College of Medicine at the University of South Florida and associate vice president for research at USF Health, is a doctor who isn't in clinical practice. As a professor of internal medicine and of molecular pharmacology and physiology, he focuses on research and leads the USF Health Office of Research.
“To succeed in the nonclinical space is going to take additional training,” he says. “It's not really feasible, for example, to be a molecular biologist – which is what I am – and not having had any molecular biology training.”
Here are some careers you can pursue as a doctor, including some that don't require completing residency or additional training.
Pharmaceutical Research
Liggett says he sees doctors who have completed their clinical training move on, after just a few years of practice, to work in early clinical trials and drug development in the pharmaceutical industry.
"They really love it. They don't see as many patients, but they're really involved at the cutting edge of what's going to be the next generation of drugs that are going to come out, often in their specialty,” he says, “and so that is one of the more common paths that I've seen.”
Doctors in this field may work for pharmaceutical companies, research organizations or regulatory agencies in medicine discovery, development and licensing. Potential roles include medical adviser or medical science liaison officer, medical reviewer, clinical research physician, pharmacovigilance (drug safety) practitioner and medical affairs specialist.
Health care consulting is a common path for medical school graduates who move into a nonclinical career, says Dr. Daniel Clinchot, vice dean for education at the Ohio State University College of Medicine and a professor at Ohio State's Wexner Medical Center .
They may consult for corporations or insurance companies, he says, or use their knowledge of clinical care to help doctors and clinical health care facilities improve their business and management practices.
Other consulting roles include being an adviser to a medical startup company, working with a market research company, educating physicians on equipment or technology, or giving input on the operations of a hospital or health care system.
Teaching and Clinical Education
Medical schools rely on doctors who can teach for clinical rotations, where third- and fourth-year students learn from preceptors – and experts say schools across the U.S. are struggling to find them. Clinchot says most med schools prefer to hire teachers who have some clinical experience in addition to a medical degree.
Liggett says doctors who choose teaching typically provide didactic instruction for first- and second-year medical students, or bedside teaching with small groups.
"Medical schools are taking that very seriously and want full-time teachers," Liggett says, "and an M.D. could certainly do that.”
Public Health
Public health deals with population-level health problems, including causes, prevention and intervention.
“Some people get a master's in public health " after graduating from medical school, Liggett says, "and then they're able to be more of an epidemiologist and look at national trends, analyze data across states or across zip codes to try to understand environmental basis of disease or the way communicable diseases might be passed, for example.”
But there are public health roles for doctors beyond epidemiology. According to PublicHealth.org, physicians who transition to work in public health "may still provide individual clinical care, but they also devote more of their time to developing public health programs and initiatives. Their credentials as medical doctors uniquely qualify them to advise and author public health initiatives and provide community-wide medical advice and education."
Medical Writing
Medical writers communicate complex scientific and clinical data to diverse audiences. The field includes scientific writers, technical writers , regulatory writers, promotional writers, health care marketers and health care journalists, according to the American Medical Writers Association.
Per AMWA, there's growing demand for medical writers to produce continuing medical education materials, health care policy documents, scientific and medical journal articles , abstracts for medical conferences, magazine and newspaper articles, medical books, advertising materials, regulatory documents including U.S. Food and Drug Administration submissions, white papers and decision aids for patients.
Other Careers for Doctors
- Clinical informatics specialist
- Genetic counselor
- Forensic specialist
- Policy adviser
- Grant writer
- Health care benefits adviser
Choosing a Path
While there are many alternate careers for doctors who want to move away from clinical care, Clinchot says medical schools aim to admit students who will ultimately practice medicine – in part because the U.S. faces a serious doctor shortage that's expected to worsen as medical students and doctors struggle with debilitating stress, burnout and disruptive changes in health care practice.
The Association of American Medical Colleges estimates the U.S. could face a shortage of up to 86,000 doctors by 2036, and according to a June 2023 survey by Merritt Hawkins for The Physicians Foundation, 28% of doctors who responded said they would like to retire within the next year – up from 21% in 2022.
“Our entire curriculum and our career counseling is all geared towards clinical careers, so we don't even have a track within the M.D. program for nonclinical-related careers,” Clinchot says. “We have several tracks (including a track) for research that's a clinician scientist, so you're doing both clinical work and research. We don't have a track for students that just all they want to do is nonclinical work.”
Clinchot says most students – even those who choose not to complete a three-year residency – don’t stop at earning their medical degree.
“Most will go on for at least one year," he says, "because the one year of additional training after your M.D. degree gives you an ability to get a license to practice medicine."
That extra year is worth it for most, Clinchot says, since they've already devoted several years to medical school.
Should You Become a Doctor?

Join the Conversation
Tags: medical school , doctors , graduate schools , education , careers , students
America 2024

Health News Bulletin
Stay informed on the latest news on health and COVID-19 from the editors at U.S. News & World Report.
Sign in to manage your newsletters »
Sign up to receive the latest updates from U.S News & World Report and our trusted partners and sponsors. By clicking submit, you are agreeing to our Terms and Conditions & Privacy Policy .
You May Also Like
The 10 worst presidents.
U.S. News Staff Feb. 23, 2024

Cartoons on President Donald Trump
Feb. 1, 2017, at 1:24 p.m.

Photos: Obama Behind the Scenes
April 8, 2022

Photos: Who Supports Joe Biden?
March 11, 2020

Nationwide Campus Protests Escalate
Laura Mannweiler April 30, 2024

What to Know: Trump Gag Order Violations
Lauren Camera April 30, 2024

Motion To Oust Johnson Dead On Arrival
Aneeta Mathur-Ashton April 30, 2024

Consumers Losing Confidence in Economy
Tim Smart April 30, 2024

Home Prices Rise, Defying Mortgage Rates

The Week in Cartoons April 29 - May 3
April 30, 2024, at 12:49 p.m.


Clinical Research Nurse
- Madison, Wisconsin
- SCHOOL OF MEDICINE AND PUBLIC HEALTH/CARBONE COMP CANCER CENTER
- Health and Wellness Services
- Partially Remote
- Staff-Full Time
- Staff-Part Time
- Opening at: Apr 26 2024 at 14:25 CDT
- Closing at: May 10 2024 at 23:55 CDT
Job Summary:
The Clinical Research Nurse will join the Clinical Research Central Office (CRCO) at the University of Wisconsin Carbone Cancer Center (UWCCC) to coordinate cancer clinical research within one or more Disease-Oriented Teams. The primary duties of this job involve the management of subjects enrolled in clinical research studies at the UW Carbone Cancer Center. This position will report to the Clinical Team Manager and work under the general direction of the Principal Investigator of each research study. This position has the ability to work remotely 1 day/week and does not include any nights or weekends. Prefer full time FTE, but will consider down to 0.8 FTE for the right candidate. The Clinical Research Nurse must have a high degree of clinical expertise with a specific focus on the treatment of patients with anticancer agents and a specialized nursing competence in the field of Oncology Research.
Responsibilities:
- 10% Secures and schedules logistics for clinical research projects according to the research plan
- 10% Assists in the recruitment and screening of subjects for clinical studies by conducting physical health assessments
- 10% Provides professional nursing care to patients according to established protocols
- 15% Provides appropriate treatment plan direction and information to study participants
- 20% Serves as main point of contact and liaison to project participants, investigators, research sponsors, and the research team delivering study information in accordance with established research project standards and protocols
- 10% Collects, verifies, and enters data into database and analyzes clinical information data
- 15% Serves a primary point of contact for emergent study participant situations related to adverse effects or complications of the study
- 10% May provide expertise, training, and guidance to the community, peers, and/or students
Institutional Statement on Diversity:
Diversity is a source of strength, creativity, and innovation for UW-Madison. We value the contributions of each person and respect the profound ways their identity, culture, background, experience, status, abilities, and opinion enrich the university community. We commit ourselves to the pursuit of excellence in teaching, research, outreach, and diversity as inextricably linked goals. The University of Wisconsin-Madison fulfills its public mission by creating a welcoming and inclusive community for people from every background - people who as students, faculty, and staff serve Wisconsin and the world. For more information on diversity and inclusion on campus, please visit: Diversity and Inclusion
Preferred Bachelor's Degree Nursing
Qualifications:
Minimum one year nursing experience required. Candidates should have exceptional clinical nursing skills and expertise coupled with a strong interest in clinical research. Prior experience working with Oncology patients is preferred. Prior clinical research experience preferred. License/Certification should also include BLS certification required
License/Certification:
Required RN - Registered Nurse - State Licensure And/Or Compact State Licensure Required BCLS - Basic Life Support
Full or Part Time: 80% - 100% This position may require some work to be performed in-person, onsite, at a designated campus work location. Some work may be performed remotely, at an offsite, non-campus work location.
Appointment Type, Duration:
Ongoing/Renewable
Minimum $68,000 ANNUAL (12 months) Depending on Qualifications
Additional Information:
- Work experience should demonstrate dependability, flexibility, and maturity. Candidates must be effective at building interpersonal relationships with constructive interactions, be clear and effective communicators, promote and create collegial environments that value accountability. Employees will also be expected to uphold UWCCC core values as defined below: - Respect: Demonstrate respect for self and others -- behave professionally. - Integrity: Act with integrity and honesty. - Teamwork: Commit to and demonstrate teamwork. - Excellence: Ensure excellence, quality, and high ethical standards in conduct and performance. -TB testing and a Caregiver Background Check will be required at the time of employment. This position has been identified as a position of trust with access to vulnerable populations. The selected candidate will be required to pass an initial Caregiver Check to be eligible for employment under the Wisconsin Caregiver Law and then every four years. - The successful applicant will be responsible for ensuring eligibility for employment in the United States on or before the effective date of the appointment.
How to Apply:
To apply for this position, please click on the "Apply Now" button. You will be asked to upload a resume and cover letter, and provide three professional/supervisor references as a part of the application process. Please ensure that the resume and cover letter address how you meet the minimum/preferred qualifications for the position.
Jennifer Wilkie [email protected] 608-262-8025 Relay Access (WTRS): 7-1-1. See RELAY_SERVICE for further information.
Official Title:
Research Nurse(HS042)
Department(s):
A53-MEDICAL SCHOOL/CARBONE CANC CTR/CANC CTR
Employment Class:
Academic Staff-Renewable
Job Number:
The university of wisconsin-madison is an equal opportunity and affirmative action employer..
You will be redirected to the application to launch your career momentarily. Thank you!
Frequently Asked Questions
Applicant Tutorial
Disability Accommodations
Pay Transparency Policy Statement
Refer a Friend
You've sent this job to a friend!
Website feedback, questions or accessibility issues: [email protected] .
Learn more about accessibility at UW–Madison .
© 2016–2024 Board of Regents of the University of Wisconsin System • Privacy Statement
- Current Employees
- Duke & Durham
- Human Resources
- Connect With Us
- External Applicants
- Current Duke Employees
- Duke Health Careers
Clinical Research Coordinator - Obstetrics and Gynecology
Durham, NC, US, 27710
School of Medicine
Established in 1930, Duke University School of Medicine is the youngest of the nation's top medical schools. Ranked sixth among medical schools in the nation, the School takes pride in being an inclusive community of outstanding learners, investigators, clinicians, and staff where interdisciplinary collaboration is embraced and great ideas accelerate translation of fundamental scientific discoveries to improve human health locally and around the globe. Composed of more than 2,600 faculty physicians and researchers, nearly 2,000 students, and more than 6,200 staff, the Duke University School of Medicine along with the Duke University School of Nursing, and Duke University Health System comprise Duke Health, a world-class academic medical center. The Health System encompasses Duke University Hospital, Duke Regional Hospital, Duke Raleigh Hospital, Duke Health Integrated Practice, Duke Primary Care, Duke Home Care and Hospice, Duke Health and Wellness, and multiple affiliations.
Duke University’s Department of Obstetrics and Gynecology has an immediate opening for a Clinical Research Coordinator. This position will involve clinical research activities with the Urogynecology research team.
Urogynecology is a medical discipline focused on pelvic floor issues including vaginal prolapse, bladder control issues, bowel control issues, and recurrent urinary tract infections. Given the breadth of our research program, we are looking to add an additional research coordinator to our experienced team. We are specifically looking for a highly motivated, team-oriented individual to integrate into several NIH-funded and industry-funded trials. This is an in-person position which will require rotating days between our two clinical offices (one in Durham and one in Raleigh).
Operations: Knowledgeable in regulatory and institutional policies and processes; applies these appropriately in study documentation, protocol submissions, and SOPs. Recruits research participants according to study protocol. Screens participants for simple and complex studies (e.g., procedural and interventional studies). Maintains participant level documentation for all studies, including those that are complex in nature (e.g., procedural and interventional studies) and/or require access to the Duke EHR. Follows SOPs. Independently employs simple procedures for collecting, preparing, processing, shipping, and maintaining inventory of specimens. Assists with establishing and maintaining study level documentation. Conducts activities for study visits in compliance with the protocol. Participates in study team meetings. Employ strategies to maintain participant retention in clinical studies. May train or oversee others in all of the above activities. Under supervision, assists with managing investigational products including arrival, storage, and handling (requisitions, inventory, and reordering). Under supervision, prepares for study monitoring and audit visits. Ethics: Knowledgeable in data security, safeguarding participant privacy, and ethical conduct of research. Identifies all adverse events, and determines whether or not they are reportable. Collaborates with the Principal Investigator to determine adverse event attributes, including relatedness to study. May train or oversee others. Conducts and documents consent for participants for all types of studies, including those that are complex in nature and/or require any orders in Maestro Care. May train or oversee others. Assists with the development of consent plans and documents for participants. Under supervision, for non-complex studies (e.g., survey studies and registries), develops and submits documentation and information for IRB review. Prepares and submits documents needed for regulatory and safety reporting to sponsors and other agencies. May train or oversee others. Data: Enters and collects basic data for research studies. May score scripted or validated tests and measures. Independently corrects and documents incomplete, inaccurate or missing data for non-complex studies. Follows SOPs for quality assurance. Runs summaries and reports on existing data. Follows required processes, policies, and systems to ensure data security and provenance. In addition, recognizes and reports security of physical and electronic data vulnerabilities. Learns and uses new technology when required. Assists in updating reports on study progress for the Principal Investigator and other study team members and collaborators. Science: Under guidance, develops elements of research protocols for simple studies (e.g., registries, survey studies). Demonstrates a basic understanding of the elements of research study designs. Study and Site Management: Prepares for, coordinates, and actively participates in site visits. Communicates effectively with sponsors and/or CROs. Uses clinical research management system and its reports to manage research participants' activities, calendars, tracking/marking financial milestones, and all aspects of study visits. Uses required EMR functionalities to manage participants and study visits. May train others. Records basic protocol information in clinical research management system. For studies with supplies or equipment, ensures that there are ample supplies and that equipment is in good working order. May forecast effort needs. May train or oversee others. Ensure that studies are conducted in compliance with institutional requirements and other policies. Follows protocol-specific systems and process flows. As directed, assists in preparing studies for closeout, (e.g., packing files, documenting files for storage, shipping extra supplies back to sponsor). Leadership: Proactively seeks opportunities to add relevant skills and certifications to own portfolio. Keeps current with research updates by attending key external offerings (i.e. Research Wednesday, RPN, events outside of Duke, etc.) and applies the learned material to the job. May disseminate information to others. Serves on committees and workgroups internal to Duke or externally in therapeutic area of research. Demonstrates interpersonal skills to get work done efficiently. Recognizes and escalates organizational issues that could be optimized to improve research process. Demonstrates resilience and is adaptive to change. Uses advanced subject matter expertise in the therapeutic area or clinical research to solve problems. Communicates effectively with others, regardless of reporting relationship, to accomplish shared work objectives. Education/Training
Completion of an Associate's degree
Work requires a minimum of two years of relevant research experience. A Bachelor's degree may substitute for 2 years required experience.
Minimum Qualifications
Duke is an Affirmative Action/Equal Opportunity Employer committed to providing employment opportunity without regard to an individual's age, color, disability, gender, gender expression, gender identity, genetic information, national origin, race, religion, sex, sexual orientation, or veteran status.
Duke aspires to create a community built on collaboration, innovation, creativity, and belonging. Our collective success depends on the robust exchange of ideas—an exchange that is best when the rich diversity of our perspectives, backgrounds, and experiences flourishes. To achieve this exchange, it is essential that all members of the community feel secure and welcome, that the contributions of all individuals are respected, and that all voices are heard. All members of our community have a responsibility to uphold these values.
Essential Physical Job Functions: Certain jobsat Duke University and Duke University Health System may include essential job functions that require specific physical and/or mental abilities. Additional information and provision for requests for reasonable accommodation will be provided by each hiring department.
Nearest Major Market: Durham Nearest Secondary Market: Raleigh
Duke is an Affirmative Action / Equal Opportunity Employer committed to providing employment opportunity without regard to an individual’s age, color, disability, gender, gender expression, gender identity, genetic information, national origin, race, religion, sex, sexual orientation, or veteran status. Read more about Duke’s commitment to affirmative action and nondiscrimination at hr.duke.edu/eeo.
Clinical Research Coord Inter
The Division of Gastroenterology and Hepatology at the University of Michigan is recruiting a full-time Clinical Research Coordinator to join its thriving team. One specific focus for this position will be to help manage projects related to the complications of cirrhosis with a focus on liver cancer development. The primary responsibility for this position will be managing a portfolio of studies around liver cancer screening and development. The responsibilities include consenting patients, interacting with sponsors, and other sites, and maintaining regulatory approval. If desired candidates may also develop an academic resume in the position, with opportunities for writing and publication. All studies are industry or federally funded.
This clinical research coordinator (CRC) position may provide study coordination for multiple clinical research studies of any complexity. Coordinator experience and mastery of all job duties from the CRC-Associate position on the Michigan Medicine CRC Career Ladder is required. This position should begin to serve on various clinical research committees at the University level. This position demonstrates advanced skills and knowledge along with the ability to support, guide, train, and demonstrate the implementation of study related activities. This position applies critical thinking and creative problem-solving skills across a wide variety of clinical studies. This position contributes to the development of new processes, procedures, and tools to enhance clinical research activities across the competency domains and conducts quality assurance/quality control checks on their work. This level of CRC continues to build on their competency foundation by making greater investments in their ongoing continuing education and professional development. Key behavioral competency descriptors include: Design, demonstrate, develop, guide, and support.
Mission Statement
Michigan Medicine improves the health of patients, populations and communities through excellence in education, patient care, community service, research and technology development, and through leadership activities in Michigan, nationally and internationally. Our mission is guided by our Strategic Principles and has three critical components; patient care, education and research that together enhance our contribution to society.
Why Join Michigan Medicine?
Michigan Medicine is one of the largest health care complexes in the world and has been the site of many groundbreaking medical and technological advancements since the opening of the U-M Medical School in 1850. Michigan Medicine is comprised of over 30,000 employees and our vision is to attract, inspire, and develop outstanding people in medicine, sciences, and healthcare to become one of the world’s most distinguished academic health systems. In some way, great or small, every person here helps to advance this world-class institution. Work at Michigan Medicine and become a victor for the greater good.
What Benefits can you Look Forward to?
- Excellent medical, dental and vision coverage effective on your very first day
- 2:1 Match on retirement savings
Responsibilities*
- Verifying patient eligibility for studies via medical chart reviews
- Recruiting patients for study participation and obtaining informed consent
- Coordinating study visits with patients and hepatology providers
- Performing data collection (face-to-face surveys, chart reviews) and data quality assurance checks
- Performing sample collection and initial processing
- Monitoring study inventory and purchasing supplies
- Maintaining study data using REDCap (Research Electronic Data Capture) or other programs.
- Preparing study reports, annual reviews, and Institutional Review Board documentation
- Monitoring and evaluating protocol compliance
- Assisting with data analysis and preparation of manuscripts and conference presentations
Characteristic Duties and Responsibilities: Expert level knowledge, skills, and abilities within all 8 competency domains is expected:
- Scientific Concepts and Research Design
- Ethical Participant Safety Considerations
- Investigational Products Development and Regulation
- Clinical Study Operations (GCPs)
- Study and Site Management
- Data Management and Informatics
- Leadership and Professionalism
- Communication and Teamwork
Supervision Received: This position reports directly to a faculty PI
Supervision Exercised: Could provide Functional supervision (likely in limited capacity such as training) of staff in titles within the CRC Career Ladder.
Required Qualifications*
- Bachelor's degree in Health Science or an equivalent combination of related education and experience is necessary.
- Certification is required through Association of Clinical Research Professionals (ACRP) as a Certified Clinical Research Coordinator (CCRC) or Society of Clinical Research Association (SOCRA) as a Certified Clinical Research Professionals (CCRP) or equivalent. Candidates must be eligible to register or take the exam at date of hire and the certification must be completed or passed etc. within six months of date of hire. (Please review eligibility criteria from SoCRA or ACRP )
- Minimum 3 years of directly related experience in clinical research and clinical trials is necessary. Please review SoCRA's Definition of a Clinical Research Professional qualifying experience prior to applying.
Desired Qualifications*
- 6+ years of direct related experience
Work Schedule
Work typically occurs during the business hours of the week however some flexibility is possible.
Additional Information
Michigan Medicine is firmly committed to advancing inclusion, diversity, equity, accessibility, and belonging, which are core to the culture and values of the Medical School Office of Research. Our community supports recruiting and cultivating a diverse workforce as a reflection of our commitment to serve the diverse people of Michigan and the world. We strive to create a work culture where each team member feels respected, valued, and safe.
Background Screening
Michigan Medicine conducts background screening and pre-employment drug testing on job candidates upon acceptance of a contingent job offer and may use a third party administrator to conduct background screenings. Background screenings are performed in compliance with the Fair Credit Report Act. Pre-employment drug testing applies to all selected candidates, including new or additional faculty and staff appointments, as well as transfers from other U-M campuses.
Application Deadline
Job openings are posted for a minimum of seven calendar days. The review and selection process may begin as early as the eighth day after posting. This opening may be removed from posting boards and filled anytime after the minimum posting period has ended.
U-M EEO/AA Statement
The University of Michigan is an equal opportunity/affirmative action employer.
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
- Current Funding Opportunities
Mentored Research Scientist Development Award (Parent K01 - Independent Clinical Trial Required)
The purpose of the NIH Mentored Research Scientist Development Award (K01) is to provide support and protected time (three to five years) for an intensive, supervised career development experience in the biomedical, behavioral, or clinical sciences leading to research independence. Although all of the participating NIH Institutes and Centers (ICs) use this support mechanism to support career development experiences that lead to research independence, some ICs use the K01 award for individuals who propose to train in a new field or for individuals who have had a hiatus in their research career because of illness or pressing family circumstances. Other ICs offer separate K01 NOFOs intended to increase research workforce diversity.
Funding Opportunity Details
Full Announcement: PA-24-175 Related Notices or Announcements: None
Program Contacts: Tracy L. Rankin, Ph.D.; Voula Osganian, M.D., Sc.D., M.P.H.
Open Date: 5/12/2024
Letter of Intent Due Date: Not Applicable

IMAGES
VIDEO
COMMENTS
Here are four steps you can take to become a researcher: 1. Take relevant classes. Clinical researchers typically pursue an undergraduate degree in biology, chemistry, medicine, psychology or a related field. Many also earn a master's, especially if they hope to work at a university or pharmaceutical company.
Recent research has revealed that academic medical centers, which employ a large number of clinical research professionals, are suffering from high staff turnover rates in this arena, with issues such as uncertainty of the job, dissatisfaction with training, and unclear professional development and role progression pathways being reported as ...
5—Write and speak clearly. Aside from strong technical skills for many jobs, you may also need to demonstrate above-average written and verbal skills. This is important because clinical research is a cross-functional, team-oriented field. For most roles, you'll be working in a team environment. When the job description states, "candidate ...
Now that you're a working clinical researcher, it's time to start thinking about professional certification. You need 2 years' experience to sit for an ACRP Certification Exam, but it's never to early to start preparing to earn the flagship certification in clinical research and accelerating your career. Learn About ACRP Certification >.
The SOCRA Certified Clinical Research Professional (CCRP) program is your gateway to excellence in clinical research. Elevate your career with our internationally recognized certification, tailored for professionals dedicated to upholding the highest standards in the field. Join a community committed to ethical practices, continuous learning ...
The ACRP offers the Certified Clinical Research Associate credential. To earn this certification, you must have one of the following: A bachelor's degree and at least 3,000 hours of experience as a CRA. A current CCRC, CPI or ACRP-CP certification and be able to substitute 1,500 hours of work experience.
How to grow your career in clinical research . Clinical research is a competitive but growing field and provides rewarding career opportunities if you have qualifications or experience within life sciences.A career in clinical research involves playing a role in helping your employer conduct studies to ensure new treatments are safe and effective for patients.
Hold a "clinical research degree" or complete 1,500 hours performing essential duties. Submit a resume documenting and demonstrating job performance. Please note that in some cases, additional education can be used to substitute for work experience hours. Please see credentialing websites for details.
A career as a clinical research associate can be rewarding for individuals who are excited by the prospect of a dynamic role overseeing many different kinds of clinical trials. ... You can start applying for jobs when you have the necessary qualifications to become a CRA. Visit job sites such as Indeed or LinkedIn and type in "clinical ...
A clinical research coordinator is an integral part of the research team for medical studies. They conduct and manage clinical trials, providing outcomes that shape medical advances in preventative care, curing diseases, and immunizations, among other areas. With employment options available in hospitals, pharmaceutical companies, and private ...
It is clear that training for a career in clinical research must be as rigorous as training for a career in the traditional basic sciences. Understanding of both the basic sciences and the evaluative sciences is essential to the success of clinical researchers. Moreover, novice clinical investigators require the same mentoring and nurturing in ...
Clinical research careers contribute to the development of safe and effective treatments and therapies for patients. The responsibilities may vary based on the organization, therapeutic area, and specific study requirements, but they all share the common goal of advancing medical science and improving healthcare outcomes.
Get clinical research career training in 1 to 4 weeks with our online accredited clinical research courses. Trusted by organizations and experienced researchers. Our clinical research courses are used by students at 1,200+ organizations, 6 government agencies, and 308 universities.† Graduates of our program work at 1,600+ different companies.‡
Required Qualifications* Bachelor's degree in Health Science or an equivalent combination of related education and experience. Certification is required through Association of Clinical Research Professionals (ACRP) as a Certified Clinical Research Coordinator (CCRC) or Society of Clinical Research Association (SOCRA) as a Certified Clinical Research Professionals (CCRP) or equivalent.
Glassdoor.com states an annual median salary of $95,396 for Research Nurses and Payscale reports that Clinical Research Nurses earn an average annual salary of $75,217 or $36.86/hr.. Research Nurse Salary by Years of Experience. Research Nurses can earn a higher annual salary with increased years of experience. Less than 1 year of experience earn an average salary of $68,000
Required Qualifications* Bachelor's degree in Health Science or an equivalent combination of related education and experience. Certification is required through Association of Clinical Research Professionals (ACRP) as a Certified Clinical Research Coordinator (CCRC) or Society of Clinical Research Association (SOCRA) as a Certified Clinical Research Professionals (CCRP) or equivalent.
More information about Career programs may be found at the NIH Research Training and Career Development website. The objective of the NIH Mentored Clinical Scientist Research Career Development Award (K08) is to provide salary and research support for a sustained period of "protected time" (3-5 years) to support didactic study and/or ...
While 93.7 percent of physician assistants choose clinical practice after completing their studies and certification, others may decide to pursue a career in clinical research. Some PAs even do both, participating in clinical research trials within a practice setting in conjunction with caring for a panel of patients.
The course is intended for OBGYN fellows and junior faculty interested in advancing their skills in clinical and medical education research. The course is also designed to help junior faculty with their academic advancement and career progression. What learners will gain Participants will gain knowledge and skills crucial to professional ...
Seven years of related experience, education/training, OR an Bachelor's degree in related area plus three years of related experience/training. Working knowledge of clinical or laboratory research, clinical trial recruitment, eligibility, protocol adherence, quality data submission and adverse event reporting.
Keeps current with research updates by attending key external departmental meetings (i.e. Research Wednesday, RPN, additional training, etc.). Navigates processes and people involved in Duke clinical research, demonstrates the organizational awareness, and has the interpersonal skills necessary to get work done efficiently.
There are options aside from being a doctor who treats patients. An alternative career may require additional training or education. Medical schools prefer to admit students who will practice ...
Job Summary: The Clinical Research Nurse will join the Clinical Research Central Office (CRCO) at the University of Wisconsin Carbone Cancer Center (UWCCC) to coordinate cancer clinical research within one or more Disease-Oriented Teams. The primary duties of this job involve the management of subjects enrolled in clinical research studies at the UW Carbone Cancer Center.
Records basic protocol information in clinical research management system. For studies with supplies or equipment, ensures that there are ample supplies and that equipment is in good working order. May forecast effort needs. May train or oversee others. Ensure that studies are conducted in compliance with institutional requirements and other ...
Job Type: Officer of Administration Regular/Temporary: Regular Hours Per Week: 35 Salary Range: $62,400 - $65,000 The salary of the finalist selected for this role will be set based on a variety of factors, including but not limited to departmental budgets, qualifications, experience, education, licenses, specialty, and training. The above hiring range represents the University's good faith ...
Required Qualifications* Bachelor's degree in Health Science or an equivalent combination of related education and experience is necessary. Certification is required through Association of Clinical Research Professionals (ACRP) as a Certified Clinical Research Coordinator (CCRC) or Society of Clinical Research Association (SOCRA) as a Certified Clinical Research Professionals (CCRP) or ...
Mentored Research Scientist Development Award (Parent K01 - Independent Clinical Trial Required) The purpose of the NIH Mentored Research Scientist Development Award (K01) is to provide support and protected time (three to five years) for an intensive, supervised career development experience in the biomedical, behavioral, or clinical sciences leading to research independence.