- Open access
- Published: 14 October 2022

The effectiveness of case management for cancer patients: an umbrella review
- Nina Wang 1 , 2 ,
- Jia Chen 3 ,
- Wenjun Chen ORCID: orcid.org/0000-0001-5398-8508 4 , 5 ,
- Zhengkun Shi 1 ,
- Huaping Yang 1 ,
- Peng Liu 6 ,
- Xiao Wei 7 ,
- Xiangling Dong 6 ,
- Chen Wang 3 ,
- Ling Mao 8 &
- Xianhong Li 3
BMC Health Services Research volume 22 , Article number: 1247 ( 2022 ) Cite this article
2473 Accesses
24 Citations
1 Altmetric
Metrics details
Case management (CM) is widely utilized to improve health outcomes of cancer patients, enhance their experience of health care, and reduce the cost of care. While numbers of systematic reviews are available on the effectiveness of CM for cancer patients, they often arrive at discordant conclusions that may confuse or mislead the future case management development for cancer patients and relevant policy making. We aimed to summarize the existing systematic reviews on the effectiveness of CM in health-related outcomes and health care utilization outcomes for cancer patient care, and highlight the consistent and contradictory findings.
An umbrella review was conducted followed the Joanna Briggs Institute (JBI) Umbrella Review methodology. We searched MEDLINE (Ovid), EMBASE (Ovid), PsycINFO, CINAHL, and Scopus for reviews published up to July 8th, 2022. Quality of each review was appraised with the JBI Critical Appraisal Checklist for Systematic Reviews and Research Syntheses. A narrative synthesis was performed, the corrected covered area was calculated as a measure of overlap for the primary studies in each review. The results were reported followed the Preferred reporting items for overviews of systematic reviews checklist.
Eight systematic reviews were included. Average quality of the reviews was high. Overall, primary studies had a slight overlap across the eight reviews (corrected covered area = 4.5%). No universal tools were used to measure the effect of CM on each outcome. Summarized results revealed that CM were more likely to improve symptom management, cognitive function, hospital (re)admission, treatment received compliance, and provision of timely treatment for cancer patients. Overall equivocal effect was reported on cancer patients’ quality of life, self-efficacy, survivor status, and satisfaction. Rare significant effect was reported on cost and length of stay.
Conclusions
CM showed mixed effects in cancer patient care. Future research should use standard guidelines to clearly describe details of CM intervention and its implementation. More primary studies are needed using high-quality well-powered designs to provide solid evidence on the effectiveness of CM. Case managers should consider applying validated and reliable tools to evaluate effect of CM in multifaced outcomes of cancer patient care.
Peer Review reports
Cancer ranks as one of the leading causes of premature death among population around 30–69 years old across 134 countries [ 1 ], and the global incidence of cancer is about to reach 30.2 million new cases and 25.7 million deaths by 2040 [ 2 ]. Earlier detection and diagnosis, and development of diverse cancer treatments have increased the survival rate of cancer patients. According to Quaresma et al. [ 3 ], the cancer survival in the UK has doubled over the last 40 years alongside the advancement in cancer diagnosis and treatment. However, number of challenges exist in the current cancer care all over the world. Many cancer patients oftentimes receive a series of long-running and exhausting multi-modal treatments and experience descent in psychological, physical and social functioning, which have a significant negative impact on their quality of life (QoL) [ 4 , 5 ]. In addition, the significant healthcare spending and productivity losses of cancer patients lead to a heavy patient economic burden, which is another substantial issue with cancer care [ 6 ]. A systematic approach is needed to mobilize and deliver appropriate resources, provide accessible, safe, and well-coordinated care for cancer patients received stressful treatments and shouldered heavy economic burden [ 7 ].
Case management (CM) is defined by the Case Management Society of America (CMSA) as “a collaborative process of assessment, planning, facilitation, care coordination, evaluation, and advocacy for options and services to meet an individual’s and family’s comprehensive health needs through communication and available resources to promote quality, cost-effective outcomes” (P. 11) [ 8 ]. According to the definition, CM is designed to use resources effectively to improve the quality of treatments, patient care services, and QoL of patients while reducing the relevant healthcare costs.
With the worldwide utilization of CM in cancer patient care, studies examining the effect of CM in improving patient-related outcomes or healthcare service use outcomes have been skyrocketing. Numbers of systematic reviews and meta-analyses have been published to synthesis the effectiveness of CM in recent years and often arrive at discordant conclusions. For example, Joo et al. [ 9 ] retrieved and synthesised results from nine experimental studies and found that CM effectively improved patients’ QoL and symptom management. While Aubin et al. [ 10 ] reported equivocal effect on both QoL and symptom management. Chan et al. [ 11 ] reported that four of the five randomized controlled trials showed insignificant impact of CM on patients’ QoL. The inconsistent evidence on the impact of CM may confuse or mislead the future case management development and relevant policy making. Considering the exist of several systematic reviews and research synthesis available to inform the application of case management for cancer patient care improvement, umbrella review could now be undertaken to compare and contrast published reviews and to highlight the consistent or contradictory findings around the effect of CM on manifold aspects of cancer patient care [ 12 ]. Thus, the current review was conducted to 1) synthesis systematic reviews that assess the effects of CM on cancer patient outcomes (e.g., QoL, functioning status, symptom management, satisfaction, etc.) and health care utilization outcomes (e.g., cost, hospital admissions, length of stay, treatment received compliance, etc.), 2) summarize measurement used in evaluating patient outcomes and health care utilization outcomes.
This umbrella review followed the Joanna Briggs Institute (JBI) Umbrella Review (UR) methodology [ 12 ] and adhered to the Preferred Reporting Items for Overviews of systematic reviews (PRIO) checklist (see Additional file 1 ) [ 13 ]. This review has been registered with the Open Science Framework ( https://doi.org/10.17605/OSF.IO/7YQAP ).
Study searching methods
We performed literature search in five databases including MEDLINE (Ovid), EMBASE (Ovid), PsycINFO, CINAHL, and Scopus from inception to July 2022. Ethical approval and patient consent were not necessary since all analyses were based on previously published articles. The searching strategies in all five databases were developed with the help of a health science librarian. See Additional file 2 for the searching strategy and results in MEDLINE (Ovid). The studies were selected using the following inclusion and exclusion criteria.
Inclusion and exclusion criteria
Individuals diagnosed with any type of cancer at any cancer stages (early to advanced). Reviews targeted on people with no specified cancer diagnose were excluded.
Intervention
Case management interventions targeted on cancer patients. Case management is defined as a “collaborative process of assessment, planning, facilitation, care coordination, evaluation, and advocacy for options and services to meet an individual’s and family’s comprehensive health needs through communication and available resources to promote quality, cost-effective outcomes” [ 8 ]. Only reviews in which the effectiveness of CM as defined above was analyzed separately from other interventions were considered.
Individuals in comparison groups received “treatment as usual” (TAU). TAU may include various interventions called “standard of care,” “usual care,” or “standard treatment,” but generally refers to treatment as it is commonly provided. Only studies that compared case management with “TAU” were selected.
Patient outcomes (e.g., quality of life, symptom management, functioning status), health care utilization outcomes (e.g., cost, hospital admissions, length of stay), etc.
Acute care hospitals and primary care settings (e.g., long-term care, nursing homes, community care services). Hospital was defined as any department of internal medicine or surgery as well as unspecified hospital settings.
Study design
Systematic review/meta-analysis that only included quantitative studies. We excluded studies full-texts unavailable online.
Study selection
All retrieved studies were imported into Covidence systematic review software [ 14 ] and the duplicates were removed. Then, titles and abstracts were independently assessed by two researchers (XW and XD) according to the inclusion criteria. After that, the full texts of the selected abstracts were obtained and reviewed by the same two researchers (XW and XD) independently. The reference list of included studies was reviewed and searched for additional studies. Any disagreement between the two researchers were resolved through consultation with a senior researcher (PL).
Quality appraisal for included reviews
Two reviewers (NW and LM) independently assessed the methodological quality of the individual studies using the JBI Critical Appraisal Checklist for Systematic Reviews and Research Syntheses [ 15 ]. The tool aims to determine the extent to which the review has addressed the possibility of bias in its design, conduct and analysis [ 15 ]. It consists of 11 criteria scored as yes, no, unclear, or not applicable. We adopted a scoring system used in previously published systematic reviews [ 16 , 17 ]. For each article, a rating score was derived by taking the number obtained in the quality rating and dividing it by the total number of possible points allowed, giving each manuscript a total quality rating between 0 and 1. Studies were then classified as low (0–0.25), low-moderate (0.26–0.50), moderate (0.51–0.75), or high (0.76–1.0).
Data extraction
We developed the data extraction form based on the research questions, and extracted following information: characteristics of included reviews such as publication year range, whether conducted meta-analysis or not, type of cancer patients, age of population, type and number of primary studies included; intervention names, components, and duration; outcomes and evaluation tools used; author’s conclusions and interpretations. Two researchers (NW and LM) extracted data independently from all included articles into an Excel spreadsheet and another researcher (XL) verified it for accuracy.
Data synthesis
We were unable to statistically pool outcomes due to the heterogeneity of outcomes of the included reviews. Therefore, we conducted a narrative synthesis [ 18 ] of the numerical data of individual studies outcomes. The studies were summarized and synthesised by two reviewers (NW and ZS) independently and double checked by a third author (HY). Following the JBI UR methodology [ 12 ], we used a summary table to present clear, specific, and structured results from the selected reviews, and then synthesised these results to identify broad conclusions. To summarized information about the interventions we coded data into features, components and delivery strategies, and inductively developed themes within each domain as they emerged from the studies. As suggested by Li and colleagues [ 19 ], we grouped outcomes into: global QoL of patients, functional status (i.e. physical, cognitive, emotional, role, social), symptom management, cost, hospital (re)admission, length of stay, treatment received compliance, provision of timely treatment.
For clarity the term ‘primary studies’ refers to the articles found within the included reviews. As several primary studies are included in more than one review, the overall results and conclusions of an overview can be biased. To assess this bias, the degree of overlap between reviews was calculated with the Corrected Covered Area (CCA) method. The details of the CCA calculation have been described by Pieper and colleagues [ 20 ] elsewhere. A CCA score of less than 5% is regarded as a slight overlap, 5–9.9% as moderate overlap, 10–14.9% as high overlap and over 15% as a very high level of overlap. This measure has been validated in which the number of overlapped primary publications has a strong correlation with the CCA [ 21 ].
Search outcome
As shown in Fig. 1 , our search strategy generated 804 potentially relevant records. Upon removing the duplicates, 582 studies screened by title and abstract, 16 were identified for full text screening. We excluded eight of the 16 studies for the following reasons: no independent analysis on the effect of case management ( n = 6), or conference abstract ( n = 2). The eight remaining systematic reviews were selected and assessed for methodological quality. In total, all the eight reviews included 57 primary studies, among which 12 were duplicated included in two or three reviews. Forty-one of the 57 primary studies were randomized controlled trials (see Additional file 3 for included primary studies).
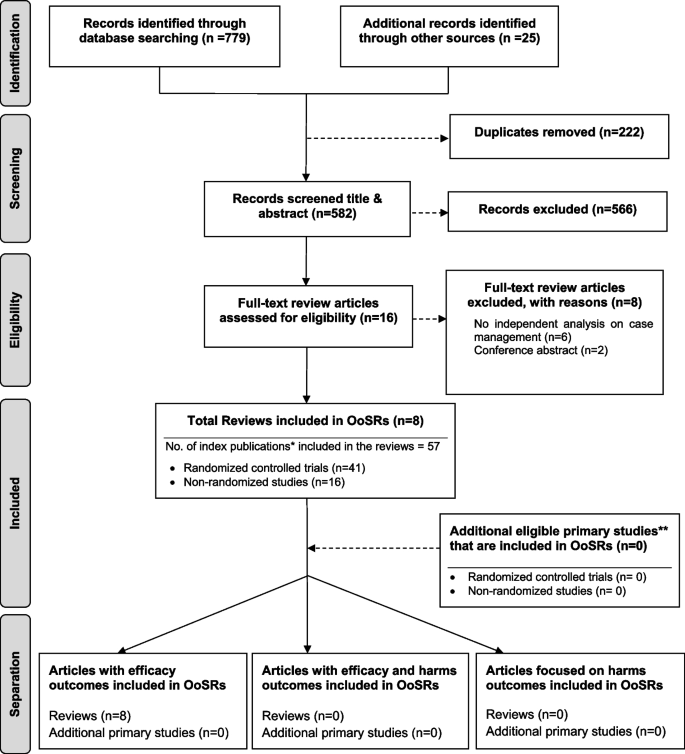
Flow chart for umbrella review. *Index publication is the first occurrence of a primary publication in the included reviews. **Additional eligible primary studies that had not been initially indentified by the search of the relevant reviews or obtained by updating the search of the included reviews
Methodological quality assessment
The quality assessment scores are presented in Table 1 . Only one review was rated as moderate because not clarify whether two or more reviewers independently assessed the quality of included primary studies, and did not report the methods to minimize errors in data extraction or publication bias. The other seven reviews were rated as high quality. Despite rated as strong, the seven reviews still companied with one or two issues on the assessment of heterogeneity, search strategy, and recommendations for policy and/or practice.
Characteristics of included studies
Table 2 presents a descriptive summary of characteristics of the eight systematic reviews [ 9 , 10 , 11 , 19 , 23 , 24 , 25 , 26 ]. The eight reviews aimed to identify evidence of the effectiveness of CM on cancer patients. Three of the studies were a systematic review with meta-analysis [ 10 , 25 , 26 ]. Five of the eight reviews adhered to the PRISMA statement [ 11 , 19 , 24 , 25 , 26 ], two adopted Cochrane systematic review methodology [ 9 , 10 ].
The eight reviews were published between 2008 and 2021, the primary studies in the reviews were published between 1983 and 2018. The number of primary studies regarding to CM included in each review ranged from three to 20. Five of the eight reviews included only randomized controlled trials (RCTs), the remaining reviews included a combination of study designs that involved RCTs, quasi-experimental and non-experimental studies (e.g., cohort study). The age of review participants ranged from 7 to 97 years and mean ages range from 48.63 to 66.31 years, which covers populations from children to elders. The total number of participants in each review ranged from 327 to 9601. Seven of the eight reviews included primary studies targeted on multiple types of cancer including breast, lung, colorectal, cervical, ovarian, prostate, gastric, hepatocellular, etc. Most of the primary studies included in the eight reviews were conducted in the United States, and there were also studies conducted in Canada, Australia, Europe (i.e., Germany, UK, Turkey, Switzerland, Denmark, Switzerland, Sweden, Norway, Netherlands) and East Asia (i.e., Hong Kong, Taiwan, South Korea, and Malaysia).
CM interventions
As shown in Table 2 , three studies reviewed trials of nurse-led CM interventions [ 9 , 25 , 26 ], two reviewed CM-like interventions that not termed as ‘CM’ while meet the CM definition by the CMSA [ 8 , 23 , 24 ]. Only one study reviewed CM focus solely on skill-training or symptom management [ 19 ]. All studies reviewed trials that facilitated the CM in a multidisciplinary collaboration approach. The duration of CM ranged from 4 days to 5 years. We presented the feature, components and delivery strategies of CM interventions for cancer patients in Fig. 2 by summarizing descriptions in each review. Congruent with the components defined by CMSA [ 8 ], all CM interventions included patient assessment, supportive services such as information and emotion support, care coordination by conducting education, consultation, and in-person, telephone or online coaching for regular follow-up. One critical component of CM interventions for cancer patients is the provision of palliative care. Control groups (CGs) of all studies reviewed in the reviews received usual treatment of care.
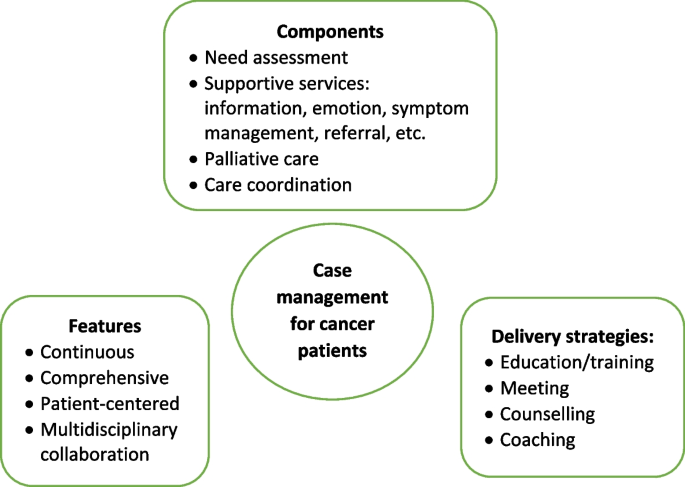
Features, components, and delivery strategies of case management for cancer patient care
Corrected Covered Area (CCA)
Table 3 presents the CCA for each outcome and as a whole. Overall, primary studies had a slight overlap across the eight reviews (CCA = 4.5%). In addition, no overlapping of primary studies was found for six of the 16 outcomes, including self-efficacy, psychological function, hospital (re)admissions, length of stay, and provision of timely treatment. Only one outcome (i.e., symptom management) showed slight overlap (0.7%). The CCA for other five outcomes (i.e., global QoL, physical function, role function, patient satisfaction, cost) evaluated by more than 2 reviews were between 5 to 9.9%, indicated a moderate overlap. The CCA for survivor status, cognitive function, emotional function, and treatment received compliance were over 10%.
Measurement used
Table 4 presents the quantitative measurement used in primary studies. As shown in Table 4 , studies investigated global QoL using different QoL-related scales, among which Functional Assessment of Cancer Therapy (FACT) (used in 15 primary studies) were most frequently applied, followed by the European Organisation for Research and Treatment of Cancer Core Quality of Life Questionnaire 30 (EORTC QLQ-C30) (used in 11 primary studies), and short form health survey (i.e., SF-8, SF-12, SF-36) (used in 10 primary studies). Different types of FACT tool were used according to the cancer types. For example, FACT-G was used for general cancer patients assessment, and FACT-B was used to evaluate breast cancer-related QoL. For the assessment of overall symptom management, SF-36 and Symptom Distress Scale (SDS) were used most frequently (used in four primary studies each). Different dimensions of SF-36 were also applied to evaluate other outcomes such as physical, emotional, and social function. Hospital Anxiety and Depression Scale (HADS) was the top employed tool in measuring the psychological function of patients. Patients’ sick leave days and the number of patients return to work were top employed metrics to evaluate the role function of patients. No unified tools were utilized to assess patient satisfaction towards the CM and majority of the primary studies used self-developed questionnaires.
Effect of CM on patient and health care utilization outcomes
The main outcomes from the seven systematic reviews are presented and summarized in Table 5 . Seven of the eight reviews reported the effects of case management on patients’ global QoL and showed mixed findings. Around half (49%, 19/39) of the primary studies included in the seven reviews reported significant positive impact of CM on global QoL. As for the functional status, there was a strong concordance among primary studies regarding the effectiveness of CM in improving cognitive function (e.g., uncertainty, health perceptions) (89%, 8/9); Equivocal effects were reported on psychological (e.g., patient anxiety, depression), physical (e.g., arm function), role function (e.g., sick leave days, patients returning to work), emotional (e.g., mood) and social function (e.g., social support) [ 9 , 11 , 26 ]. The findings regard to symptom management were more positive, with 75% (18/24) primary studies included in seven reviews revealed significant positive impact of CM on symptom severity and symptom distress decrease of pain, nausea, fatigue, discomfort, etc. Three of the four primary studies in two reviews [ 9 , 11 ] showed no significant influence of CM on patients’ self-efficacy. Wulff et al. [ 23 ] and Aubin et al. [ 10 ] reported mixed findings on the impact of CM on survivor status, with four of the six primary studies reported significant positive impact. The effect of CM on patient satisfaction was reported in five reviews and showed mixed results.
Of the eleven primary studies reported cost, only one controlled before-and-after study in Joo et al.’s [ 9 ] review reported significant impact on monthly cancer-related medical costs. The evidence concerning patients’ length of stay yielded no significant findings. Overall significant positive effect was reported on hospital (re)admission (e.g., inpatient and ICU admission rate), treatment received compliance (e.g., therapy acceptance or completion rate), and provision of timely treatment.
This umbrella review is the first to summarize the results of systematic reviews that synthesised the evidence on the effectiveness of CM on cancer patient outcomes and relevant health care utilization. Most reviews (7/8) showed a high methodological quality. Different tools were used to measure the effect of CM on the same outcome. The evidence regards to the effectiveness of CM is mixed. The summarized results revealed that CM was more likely to improve symptom management, cognitive function, hospital (re)admission, treatment received compliance, and provision of timely treatment for cancer patients. Overall equivocal effect was reported on cancer patients’ global QoL, psychological, physical, role, emotional and social function, self-efficacy, survivor status, and patient satisfaction.
No universal tools were used to measure improvement of each outcome in the CM group compared with the control group, making it challenging to conduct a meta-analysis of studies results [ 22 , 27 ]. This is a common issue faced the included reviews. Five of the eight reviews failed to conduct meta-analysis due to the heterogeneity [ 9 , 11 , 19 , 23 , 24 ]. Joo and Huber [ 22 ] conducted a review of reviews on the effect of CM on health care utilization outcome of chronic illness patients, they recognized the same problem and suggested using valid and standardized tools to minimize the differences in measurements. Despite various tools used, our review showed that FACT, EORTC QLQ-C30, and short form health survey (i.e., SF 36, SF 12, and SF 8) were most frequently applied to measure the effect of CM on the global QoL of cancer patients. These tools were also used in evaluating specific dimensions of QoL such as psychological, physical, emotional, and social function. This aligned with previous reviews [ 28 , 29 ] that found FACT and EORTC QLQ-C30 were the most common and well developed QoL instruments in cancer patients. FACT-G is considered appropriate for use with any types of cancer patients [ 30 ]. It is a 27-item tool that includes four primary QoL domains: physical well-being, social/family well-being, emotional well-being, and functional well-being [ 31 ]. Other versions of FACT (FACT-B [ 32 ], FACT-L [ 33 ] and FACT-E [ 34 ]) for specific type of cancer patients were developed by incorporating the four dimensions of FACT-G with additional cancer type-specific questions. EORTC QLQ-C30 was another type of QoL assessment tools for cancer patients specifically. It was developed by Aaronson et al. [ 35 ] and contains four domains: physical, emotional, cognitive and social functions, and a higher score indicates better QoL. The Short Form Health Survey is the most commonly used measure in evaluating QoL domains of patients suffering from a wide range of medical conditions [ 36 ]. Research found it provides reliable and valid indication of general health among cancer patients [ 37 , 38 ].
QoL is the most frequently evaluated outcome in our review with 39 primary studies in seven reviews reported the global QoL of cancer patients. Joo et al. [ 9 ] found that CM interventions improved QoL of cancer patients. Yin and colleagues [ 24 ] revealed that cancer patients achieved better physical and psychological condition through symptom management, needs assessment, direct referrals, and other services in CM. However, summarized results in our review show that the CM had equivocal effect on cancer patients’ global QoL and dimensions including psychological, physical, role, emotional and social function. Cognitive function is the only dimension showed positive change. Despite CM interventions share similar definitions and principles [ 8 ]. It is hard to foresee which aspect(s) of CM interventions contribute to certain effects due to their comprehensiveness [ 24 ]. Yin et al. [ 24 ] argued that the control group may receive a higher quality treatment than planned usual care since all the participants were not blinded and they have been informed about the aim of the study. Indicating a more rigorous design and evaluation is needed to avoid this information bias.
In the meantime, included reviews claimed that few primary studies reported enough details about CM interventions, including model used [ 10 , 11 ], dose and intensity [ 9 , 19 , 24 ], interventionist qualifications [ 11 ], protocol or manual used [ 9 , 23 ], and fidelity [ 23 ]. Particularly, the COVID-19 pandemic has considerable influence on the care delivery for cancer patients. For example, the more frequently utilization of remote patient monitoring technologies that incorporate community resources, primary care and allied health disciplines, as well as clinics to keep cancer patients away from acute care hospitals as much as possible [ 39 ]. Many of these changes have been integrated within routine case management for cancer care during the pandemic [ 39 ]. It is well-needed to report how those CM intervention were conducted follow standard reporting guidelines, in order to provide recommendation for future research.
Our review showed that CM is likely to improve the symptom management. Eighteen of the 24 included primary studies reported positive effect of CM on symptom management, including decrease symptom distress or severity of fatigue, pain, nausea, and vomiting. The same positive effect on symptom management was also revealed in other types of patients. Joo and colleagues [ 40 ] found that CM reduced substance use and significantly influenced abstinence rates among populations experienced substance disorders. Reviews by Stokes et al. [ 27 ] and Welch et al. [ 41 ] revealed positive effect on symptom release among people with long-term conditions and diabetes patients, respectively. The multidisciplinary collaboration approach adopted [ 10 ], and availability of professional support post-hospitalization [ 9 , 41 ] in CM might contribute to the improvement of symptom management. Specifically, multidisciplinary team involves physicians, nurses, and aligned healthcare professionals provides throughout and multifaced symptom assessment and management [ 10 ]. In addition, CM programs continuously follow up and advocate for patients’ concerns [ 8 ]. Specifically, case managers are available to patients 24 hours a day by phone call even after discharged, providing opportunity for immediate professional guidance on symptom management [ 9 ].
As for other patient outcomes, there is insufficient evidence of effect on self-efficacy and survivor status of cancer patients. Only three and four primary studies in total reported these two outcomes, respectively. Eleven primary studies in five reviews reported patient satisfaction and showed mixed results. Inconsistent results were found in a review of reviews by Buja et al. [ 7 ] which concluded strong evidence of CM improving satisfaction of patients with long term condition. In agreement with Joo and Huber’s [ 25 ] review, we found that CM favorably affect healthcare utilization outcomes such as treatment received compliance, hospital (re)admission, and provision of timely treatment. While the strength of the evidence was limited either by the high level of primary studies overlapping (CCA) (i.e., treatment received compliance, CCA = 13.3%) or the small number of studies reported certain outcomes (i.e., hospital admission, provision of timely treatment). Notably, the summarized results from included reviews conclude that despite theoretical benefits [ 8 ], in practice there is only slight evidence of benefits on reduction in the cost of care for cancer patients participated in CM interventions.
We provide some recommendations for future research based on the summarized results: 1) Future research should clearly describe details of CM intervention and its implementation, including theoretical underpinnings, dose and intensity, interventionist qualifications, protocol or manual used, fidelity, etc. In that way these details can be included in future systematic reviews, and effectiveness of individual elements of the intervention can be examined [ 27 ]. We recommend use standard guidelines to help organize the CM intervention reporting. For example, the Template for Intervention Description and Replication (TIDeiR) is one of the most popular guidelines that could be used to report the full breadth of CM interventions: from intervention rationale to assessments of treatment adherence and fidelity [ 42 ]. 2) More rigorous trials are needed to evaluate the effectiveness of CM. 3) Studies should also explore the barriers to and facilitators of CM implementation across various types of cancer patients at different stages, providing evidence for conducting successful CM implementation in the future.
Strengths and limitations
We conducted an umbrella review instead of a meta-analysis due to the heterogeneity of review outcomes. Although an umbrella review can only show the tendency or direction of the effect of CM rather than providing the magnitude or significance level of influence [ 12 ], the current evidence on the effect of CM in cancer patients was comprehensively summarized. There were some challenges when conducting the review. First, the quality of the umbrella reviews was greatly affected by the quality of the original reviews [ 12 ]. In this study, we confirmed that the quality of the original reviews were mostly high as assessed by the JBI Critical Appraisal Checklist [ 15 ]. Second, if the primary studies were included in several reviews, they may produce bias related to overlapping effects [ 20 ]. By calculating the CCA, we showed that 75% (12/16) of the individual outcomes had no to moderate overlapping of primary studies between included reviews, revealing that these results from each review were relatively independent. Cautious are needed on the summarized evidence regards to the effect of CM on survivor status, cognitive function, emotional function, and treatment received compliance because of the high overlapping (CCA > 10) between the reviews reported those outcomes.
There are limitations in our review. The first limitation concerns that the searching was limited to English-language articles and did not access unpublished papers. Second, as suggested by the JBI UR methodology [ 12 ], we did not assess the quality of evidence from included reviews, it increased the uncertainty of the review findings.
Effective CM aims to influence the health care delivery system in improving the health outcomes of cancer patients, enhancing their experience of health care, and reducing the cost of care. Our review found mixed effects of CM reported in cancer patient care. The summarized results revealed that CM was likely to improve symptom management for cancer patients. We also found CM has the tendency to enhance cancer patients’ experience of health care such as reducing hospital (re)admission rates, improving treatment received compliance and provision of timely treatment. Only slight evidence of benefits was reported on reducing the cost of care for cancer patients. Overall, more rigorous designed primary studies are needed to demonstrate the effects of CM on cancer patients and explore the elements of effective CM interventions.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
Corrected Covered Area
Control groups
- Case management
Case Management Society of America
European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire 30
Functional Assessment of Cancer Therapy - Breast Cancer
Functional Assessment of Cancer Therapy- Esophagus
Functional Assessment of Cancer Therapy- General
Functional Assessment of Cancer Therapy Scale-Lung
- Quality of life
Hospital Anxiety and Depression Scale
Joanna Briggs Institute
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Randomized controlled trials
Symptom Distress Scale
Medical Outcomes Study 8-item short form health survey
Medical Outcomes Study 12-item short form health survey
Medical Outcomes Study 36-item short form health survey
Treatment as usual
Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global cancer observatory: Cancer today: International Agency for Research on Cancer; 2020. https://gco.iarc.fr/today .
Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global Cancer observatory: Cancer tomorrow: International Agency for Research on Cancer; 2020. https://gco.iarc.fr/tomorrow .
Quaresma M, Coleman MP, Rachet B. 40-year trends in an index of survival for all cancers combined and survival adjusted for age and sex for each cancer in England and Wales, 1971-2011: a population-based study. Lancet. 2015;385:1206–18. https://doi.org/10.1016/S0140-6736(14)61396-9 .
Article PubMed Google Scholar
Yabroff KR, Dowling EC, Guy GP, Banegas MP, Davidoff A, Han X, et al. Financial hardship associated with cancer in the United States: findings from a population-based sample of adult cancer survivors. J Clin Oncol. 2016;34:259–67. https://doi.org/10.1200/JCO.2015.62.0468 .
Yan AF, Stevens P, Holt C, Walker A, Ng A, McManus P, et al. Culture, identity, strength and spirituality: a qualitative study to understand experiences of African American women breast cancer survivors and recommendations for intervention development. Eur J Cancer Care (Engl). 2019;28:e13013. https://doi.org/10.1111/ecc.13013 .
Article Google Scholar
Yabroff K, Mariotto A, Tangka F. Annual report to the nation on the status of Cancer, part II: patient economic burden associated with Cancer care. J Natl Cancer Inst. 2021;113:1670–82. https://doi.org/10.1093/jnci/djab192 .
Buja A, Francesconi P, Bellini I, Barletta V, Girardi G, Braga M, et al. Health and health service usage outcomes of case management for patients with long-term conditions: a review of reviews. Prim Heal Care Res Dev. 2020;21:1–21. https://doi.org/10.1017/S1463423620000080 .
Case Management Society of America. CMSA’s standards of practice for case management, Revised 2016. 2016. http://www.naylornetwork.com/cmsatoday/articles/index-v3.asp?aid=400028&issueID=53653 .
Google Scholar
Joo JY, Liu MF. Effectiveness of nurse-led case Management in Cancer Care: systematic review. Clin Nurs Res. 2019;28:968–91. https://doi.org/10.1177/1054773818773285 .
Aubin M, Giguère A, Martin M, Verreault R, Fitch MI, Kazanjian A, et al. Interventions to improve continuity of care in the follow-up of patients with cancer. Cochrane Database Syst Rev. 2012:1–193. https://doi.org/10.1002/14651858.CD007672.pub2 .
Chan RJ, Teleni L, McDonald S, Kelly J, Mahony J, Ernst K, et al. Breast cancer nursing interventions and clinical effectiveness: a systematic review. BMJ Support Palliat Care. 2020;10:276–86. https://doi.org/10.1136/bmjspcare-2019-002120 .
Aromataris E, Fernandez R, Godfrey C, Holly C, Khalil H, Tungpunkom P. JBI manual for evidence synthesis. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. JBI; 2020. https://doi.org/10.46658/JBIMES-20-11 .
Chapter Google Scholar
Bougioukas KI, Liakos A, Tsapas A, Ntzani E, Haidich AB. Preferred reporting items for overviews of systematic reviews including harms checklist: a pilot tool to be used for balanced reporting of benefits and harms. J Clin Epidemiol. 2018;93:9–24. https://doi.org/10.1016/j.jclinepi.2017.10.002 .
Veritas Health Innovation. Covidence systematic review sofware. Covidence. 2016. https://get.covidence.org/systematic-review-software?campaignid=15030045989&adgroupid=130408703002&gclid=EAIaIQobChMIvc7KuJDO-QIVh97ICh1BeQKnEAAYASAAEgI4APD_BwE .
Aromataris E, Fernandez R, Godfrey C, Holly C, Kahlil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Heal. 2015;13:132–40 http://joannabriggs.org/research/critical-appraisal-tools.html .
Gifford W, Rowan M, Dick P, Modanloo S, Benoit M, Al AZ, et al. Interventions to improve cancer survivorship among indigenous peoples and communities : a systematic review with a narrative synthesis. Support Care Cancer. 2021;29:7029–48. https://doi.org/10.1007/s00520-021-06216-7 .
Article PubMed PubMed Central Google Scholar
Gifford W, Squires J, Angus D, Ashley L, Brosseau L, Craik J, et al. Managerial leadership for research use in nursing and allied health care professions : a systematic review. Implement Sci. 2018;13:1–23. https://doi.org/10.1186/s13012-018-0817-7 .
Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M, et al. Guidance on the conduct of narrative synthesis in systematic reviews: a product from the ESRC methods Programme. Lancaster: Lancaster University; 2006. https://www.lancaster.ac.uk/media/lancaster-university/content-assets/documents/fhm/dhr/chir/NSsynthesisguidanceVersion1-April2006.pdf .
Li Q, Lin Y, Liu X, Xu Y. A systematic review on patient-reported outcomes in cancer survivors of randomised clinical trials: direction for future research. Psychooncology. 2014;23:721–30. https://doi.org/10.1002/pon.3504 .
Article CAS PubMed Google Scholar
Pieper D, Antoine SL, Mathes T, Neugebauer EAM, Eikermann M. Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol. 2014;67:368–75. https://doi.org/10.1016/j.jclinepi.2013.11.007 .
Choi J, Lee M, Lee JK, Kang D, Choi JY. Correlates associated with participation in physical activity among adults: a systematic review of reviews and update. BMC Public Health. 2017;17:1–13. https://doi.org/10.1186/s12889-017-4255-2 .
Joo JY, Huber DL. Case management effectiveness on health care utilization outcomes: a systematic review of reviews. West J Nurs Res. 2019;41:111–33. https://doi.org/10.1177/0193945918762135 .
Wulff CN, Thygesen M, Søndergaard J, Vedsted P. Case management used to optimize cancer care pathways: a systematic review. BMC Health Serv Res. 2008;8:1–7 http://www.biomedcentral.com/1472-6963/8/227 .
Yin YN, Wang Y, Jiang NJ, Long DR. Can case management improve cancer patients quality of life?: a systematic review following PRISMA. Medicine (Baltimore). 2020;99:1–7. https://doi.org/10.1097/MD.0000000000022448 .
Wu YL, Padmalatha KMS, Yu T, Lin YH, Ku HC, Tsai YT, et al. Is nurse-led case management effective in improving treatment outcomes for cancer patients? A systematic review and meta-analysis. J Adv Nurs. 2021;00:1–11. https://doi.org/10.1111/jan.14874 .
McQueen J, McFeely G. Case management for return to work for individuals living with cancer: a systematic review. Int J Ther Rehabil. 2017;24:203–10. https://doi.org/10.12968/ijtr.2017.24.5.203 .
Stokes J, Panagioti M, Alam R, Checkland K, Cheraghi-Sohi S, Bower P. Effectiveness of case management for “at risk” patients in primary care: a systematic review and meta-analysis. PLoS One. 2015;10:e0132340. https://doi.org/10.1371/journal.pone.0132340 .
Article CAS PubMed PubMed Central Google Scholar
Lemieux J, Goodwin PJ, Bordeleau LJ, Lauzier S, Théberge V. Quality-of-life measurement in randomized clinical trials in breast cancer: an updated systematic review (2001-2009). J Natl Cancer Inst. 2011;103:178–231. https://doi.org/10.1093/jnci/djq508 .
Montazeri A. Health-related quality of life in breast cancer patients: a bibliographic review of the literature from 1974 to 2007. J Exp Clin Cancer Res. 2008;27:1–31 https://jeccr.biomedcentral.com/articles/10.1186/1756-9966-27-32 .
Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–9. https://doi.org/10.1200/JCO.1993.11.3.570 .
Webster K, Cella D, Yost K. The F unctional a ssessment of C hronic I llness T herapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:79. https://doi.org/10.1186/1477-7525-1-79 .
Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, et al. Reliability and validity of the functional assessment of cancer therapy- breast quality-of-life instrument. J Clin Oncol. 1997;15:974–86. https://doi.org/10.1200/jco.1997.15.3.974 .
Cella DF, Bonomi AE, Lloyd SR, Tulsky DS, Kaplan E, Bonomi P. Reliability and validity of the functional assessment of cancer therapy-lung (FACT-L) quality of life instrument. Lung Cancer. 1995;12:199–220. https://doi.org/10.1016/0169-5002(95)00450-F .
Darling G, Eton DT, Sulman J, Casson AG, Cella D. Validation of the functional assessment of cancer therapy esophageal cancer subscale. Cancer. 2006;107:854–63. https://doi.org/10.1002/cncr.22055 .
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. https://doi.org/10.1093/jnci/85.5.365 .
Ware J, Kosinski M, Dewey J. How to score version two of the SF-36® health survey. Lincoln: QualityMetric Incorporated; 2000.
Treanor C, Donnelly M. A methodological review of the short form health survey 36 (SF-36) and its derivatives among breast cancer survivors. Qual Life Res. 2015;24:339–62. https://doi.org/10.1007/s11136-014-0785-6 .
Lins L, Carvalho FM. SF-36 total score as a single measure of health-related quality of life: scoping review. SAGE Open Med. 2016;4:1–12. https://doi.org/10.1177/2050312116671725 .
Chan RJ, Crawford-Williams F, Crichton M, Joseph R, Hart NH, Milley K, et al. Effectiveness and implementation of models of cancer survivorship care: an overview of systematic reviews. J Cancer Surviv. 2021:1–25. https://doi.org/10.1007/s11764-021-01128-1 .
Joo J, Huber D. Community-based case management effectiveness in populations that abuse substances. Int Nurs Rev. 2015;62:536–46. https://doi.org/10.1111/inr.12201 .
Welch G, Garb J, Zagarins S, Lendel I, Gabbay RA. Nurse diabetes case management interventions and blood glucose control: results of a meta-analysis. Diabetes Res Clin Pract. 2010;88:1–6. https://doi.org/10.1016/j.diabres.2009.12.026 .
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. https://doi.org/10.1136/bmj.g1687 .
Download references
Acknowledgements
Not applicable.
Dual (co-)authorship
We declared that no author has authored one or more of the included systematic reviews.
This study was supported by 1) Hunan Provincial Key Laboratory of Nursing (2017TP1004, PI: Jia Chen), Hunan Provincial Science and Technology Department, 2) Changsha Natural Science Foundation (kq2202365, PI: Nina Wang) Changsha Science and Technology Department, and 3) Management research foundation of Xiangya Hospital (2021GL12, PI: Nina Wang).
Author information
Authors and affiliations.
Department of Respiratory Medicine, Xiangya Hospital, Central South University, Changsha, China
Nina Wang, Zhengkun Shi & Huaping Yang
National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Changsha, China
Xiangya School of Nursing, Central South University, Changsha, China
Jia Chen, Chen Wang & Xianhong Li
School of Nursing, University of Ottawa, Ottawa, Canada
Wenjun Chen
Center for Research on Health and Nursing, University of Ottawa, Ottawa, Canada
Intensive Care Unit of Cardiovascular Surgery Department, Xiangya Hospital, Central South University, Changsha, China
Peng Liu & Xiangling Dong
The 956th Army Hospital, Linzhi, China
School of Nursing, Changsha Medical University, Changsha, China
You can also search for this author in PubMed Google Scholar
Contributions
All authors have contributed to the production of this review. NW and WC conceptualized and designed the study and are the guarantor of the paper. JC and ZS conducted the literature search. PL, XW and XD were involved in the study screening. NW, LM and XL participated in the quality appraisal and data extraction. NW, ZS and HY conducted the data analysis. NW drafted the manuscript. WC and XL revised the manuscript. All authors participated in the review of the manuscript and approved the final manuscript.
Corresponding author
Correspondence to Wenjun Chen .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1., additional file 2..
Searching strategies.
Additional file 3.
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Wang, N., Chen, J., Chen, W. et al. The effectiveness of case management for cancer patients: an umbrella review. BMC Health Serv Res 22 , 1247 (2022). https://doi.org/10.1186/s12913-022-08610-1
Download citation
Received : 04 April 2022
Accepted : 21 September 2022
Published : 14 October 2022
DOI : https://doi.org/10.1186/s12913-022-08610-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cancer patients
- Umbrella review
- Health care
- Outcome assessment
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]
Ohio State nav bar
The Ohio State University
- BuckeyeLink
- Find People
- Search Ohio State
Patient Case Presentation
Patient Mrs. B.C. is a 56 year old female who is presenting to her WHNP for her annual exam. She had to cancel her appointment two months ago and didn’t reschedule until now. Her last pap smear and mammogram were normal. Today, while performing her breast exam, her nurse practitioner notices dimpling in the left breast as the patient raises her arms over her head. When the NP mentions it to Mrs. B.C. she is surprised and denies noticing it before today. A firm, non-tender, immobile nodule is palpated in the upper quadrant of her breast . The NP then asks Mrs. B.C. how frequently she is performing breast self-exams, she admits to only doing them randomly when she remembers, which is about every few months. She reports no recent or abnormal drainage from her breast. Further examination reveals palpable axillary lymph nodes.
Mrs. B.C. is about 30 pounds overweight and walks her dog around her neighborhood every morning before work and every evening when she gets home. She reports drinking a glass of white wine before bed each night. She denies any history of tobacco use. She reports use of a combination birth control pill on and off for 25 years until she reached menopause. She is not currently taking any prescription medications.
Past Medical History
- Menarche (Age 10)
- Post-menopausal (Age 53)
- No other pertinent medical history
Family History:
- Father George- deceased from stroke (75 years old), history of hypertension, CAD, HLD
- Mother Maryanne alive- 76 years old, history of dementia, osteoporosis
- Brother Michael- alive, 57 years old, history of hypertension, CAD and cardiac stent placement (54 years old)
- Sister, Michelle- alive 53 years old, history of GERD, Asthma
- Brother- Jimmy- alive 50 years old, no past medical history
Social History:
Mrs. B.C. works Monday-Friday 8am-5pm at the local dentist’s office at the front desk as a schedule coordinator. She is planning to retire in a few years. In her spare time, she is involved in various community efforts to feed the homeless and helps to prepare dinners at her local church one night a week. She also enjoys cooking and baking at home, gardening, and nature photography.
Mrs. B.C. has two children. Her oldest son, Patrick, is 21 years old and is in his final year of pre-med. He is attending a public university about 2 hours away from home where he lives year-round. As an infant, Patrick was breastfed until 18 months when he self-weaned. Her daughter, Veronica, is 19 years old and lives at home while attending the local branch campus of a state university. She is in her second year of a business degree and then plans to transfer to the main campus next year. When Veronica was an infant she had difficulty latching onto the breast due to an undiagnosed tongue and lip ties resulting in Mrs. BC exclusively pumping and bottle feeding for six months. After six months, Mrs. B.C. was having a hard time keeping up while working and her found her supply diminished. Veronica had begun eating solid foods so Mrs. B.C. switched to supplemental formula, which was a big relief.
Mrs. B.C. was married to her now ex-husband Kent for 26 years. They divorced two years ago when Veronica was a senior in high school. They have remained friends and Kent lives 25 minutes away in a condo with his girlfriend. She also has two brothers who live nearby and a sister who lives out of state. Her 7 nieces and nephews range in age from 9 years old to 26 years old. Her father, George, passed away from a sudden stroke 4 years ago. Her mother, Maryanne, has dementia and is living in a nearby memory care facility. She also has many close friends.
Together we are beating cancer
About cancer
Cancer types
- Breast cancer
- Bowel cancer
- Lung cancer
- Prostate cancer
Cancers in general
- Clinical trials
Causes of cancer
Coping with cancer
- Managing symptoms and side effects
- Mental health and cancer
- Money and travel
- Death and dying
- Cancer Chat forum
Health Professionals
- Cancer Statistics
- Cancer Screening
- Learning and Support
- NICE suspected cancer referral guidelines
Get involved
- Make a donation
By cancer type
- Leave a legacy gift
- Donate in Memory
Find an event
- Race for Life
- Charity runs
- Charity walks
- Search events
- Relay For Life
- Volunteer in our shops
- Help at an event
- Help us raise money
- Campaign for us
Do your own fundraising
- Fundraising ideas
- Get a fundraising pack
- Return fundraising money
- Fundraise by cancer type
- Set up a Cancer Research UK Giving Page
- Find a shop or superstore
- Become a partner
- Cancer Research UK for Children & Young People
- Our We Are campaign
Our research
- Brain tumours
- Skin cancer
- All cancer types
By cancer topic
- New treatments
- Cancer biology
- Cancer drugs
- All cancer subjects
- All locations
By Researcher
- Professor Duncan Baird
- Professor Fran Balkwill
- Professor Andrew Biankin
- See all researchers
- Our achievements timeline
- Our research strategy
- Involving animals in research
Funding for researchers
Research opportunities
- For discovery researchers
- For clinical researchers
- For population researchers
- In drug discovery & development
- In early detection & diagnosis
- For students & postdocs
Our funding schemes
- Career Development Fellowship
- Discovery Programme Awards
- Clinical Trial Award
- Biology to Prevention Award
- View all schemes and deadlines
Applying for funding
- Start your application online
- How to make a successful application
- Funding committees
- Successful applicant case studies
How we deliver research
- Our research infrastructure
- Events and conferences
- Our research partnerships
- Facts & figures about our funding
- Develop your research career
- Recently funded awards
- Manage your research grant
- Notify us of new publications
Find a shop
- Volunteer in a shop
- Donate goods to a shop
- Our superstores
Shop online
- Wedding favours
- Cancer Care
- Flower Shop
Our eBay store
- Shoes and boots
- Bags and purses
- We beat cancer
- We fundraise
- We develop policy
- Our global role
Our organisation
- Our strategy
- Our Trustees
- CEO and Executive Board
- How we spend your money
- Early careers
Cancer news
- Cancer News
- For Researchers
- For Supporters
- Press office
- Publications
- Update your contact preferences
ABOUT CANCER
GET INVOLVED
NEWS & RESOURCES
FUNDING & RESEARCH
You are here

Patient data use case studies

Questions about these case studies or how patient data is handled?
Contact the team
During our Review of Informed Choice for Cancer Registration, patients clearly told us they would like to know what their data are being used for. We agreed that as a charity, we would highlight where we are using patient-data for research and analysis. Relevant examples of work by the Cancer Intelligence team at Cancer Research UK will be available here, and we will add to this as we embark on new projects.
Lung cancer diagnostic pathways
Identifying common lung cancer diagnostic pathways using linked datasets.
Small proportions of patients in 2013-2015 meet the timings of the optimal pathway. Diagnostic pathway length varies by source of imaging request.
Variation in ovarian cancer treatment rates
Investigating variation in treatment for ovarian cancer patients.
We expect to establish if there is variation between Cancer Alliances/Trusts, identify the odds ratios/ regression co-efficients for predictor variables, and if it's the affect of healthcare system level factors.
Variation in lung cancer treatment rates
Investigating variation in treatment for non-small cell lung cancer patients.
We expect to establish if there is significant variation between Cancer Alliances/Trusts (geography still to TBC) and identify the odds ratios/regression co-effecients associated with each predictor variable.
Bowel cancer screening campaign evaluation
Evaluating the bowel cancer screening regional pilot campaign in the North West of England.
Analysis of interim results following the campaign are promising. We are currently working to finalise the analysis and results will follow in due course.
Colorectal cancer diagnostic pathways
Coming soon.
The impact of COVID-19 on radiotherapy in the UK
The aims of COVID RT are to understand why changes in radiotherapy treatment schedules were implemented during the pandemic and to then explore the impact of these changes on patient outcomes and the UK radiotherapy services. This project aims purely to understand the changes in patient's radiotherapy treatment from COVID-19.

Patient data – a vital tool that will help beat cancer
Cancer is the most complex health challenge that we face. There are 200 different types of cancer but even that is an oversimplification.
At a genetic level each individual cancer is as unique as the person with the disease. Despite this complexity our research has helped double cancer survival over the last 40 years. This extraordinary progress has been powered by relentlessly increasing our understanding of cancer’s intricacies.
But big questions about cancer remain, and our scientists are seeking the answers. Patient data has the potential to reveal some of these. Here we meet some outstanding scientists who are using patient data to grow our understanding even further, work that could underpin tomorrow’s cures.
Case study 1 – Prof. Crispin Miller
Professor_crispin_miller.jpg.

At the heart of the CRUK Scotland Institute is a team of brilliant scientists, led by Professor Crispin Miller, who specialise in using super computers to understand how cancer behaves.
This diverse and talented team - which includes mathematicians, physicists, biologists and software engineers - are using a range of techniques to dig deep into the huge amounts of data we have on the genetics and anatomy of tumours.
“Biology is exciting” says Crispin. “The questions of modern cancer research, like how the DNA in one cell can define an entire living person – to me that’s as exciting as understanding the first few microseconds of the Big Bang.”
Crispin’s goal is to identify patterns in a tumour’s DNA or its structure that might help us better understand why and how cancers develop. And ultimately this could inform how best to treat them.
But spotting those crucial patterns is difficult because tumours are so diverse. And so Crispin’s team turn to supercomputers. These powerful tools can sift through vast amounts of information to find patterns that would be impossible for a human to spot. Then the scientists can do what they do best – work out how the patterns are driving cancer progression or making a tumour more or less sensitive to an anticancer drug.
Professor Miller explains: “Data gives us insight into things you can’t see down a microscope. It has the potential to start developing truly targeted therapies. To find the right therapy for the right person at the right time that is the goal.”
His team are using a data approach on many different cancer-related questions. In one project, patients have kindly allowed their doctors to share anonymised scan images of tumours and the tumour genetic data. Crispin’s team are seeing whether they can build a sophisticated AI approach that has the power to use this data to predict how tumours are going to respond to treatment. This could help doctors pick the best treatment for each person.
What nearly all Professor Miller’s work has in common is that it is only possible due to the data that people with cancer have shared.
“Data is central to the research we are doing. The more tumours we study, the more we understand how they differ from each other and how we can use this information to improve outcomes for people with cancer. I am incredibly grateful to the patients who donate their data and tissue, I couldn’t do my work without it.”
Case study 2 – Dr Irene Lobon
“Data is everything” – how patient data is helping us understand how cancer spreads and resists treatment
For Dr Irene Lobon, everything is data and data is everything. From calculating nutrients in new recipes to studying tumour samples, analysing data is central to her life and work. While in her spare time Dr Lobon’s analytical skills help her to optimise her favourite recipes, in the laboratory, these skills could lead to lifesaving discoveries.
Dr Lobon is a biomedical researcher in the Cancer Dynamics Laboratory, led by Professor Samra Turajlic at The Francis Crick Institute. She works on a multidisciplinary team featuring scientists and clinicians, all working together to better understand cancer.
The team are studying advanced, spreading melanoma, called metastatic melanoma. This is a type of skin cancer that is particularly hard to treat and often becomes resistant to drugs. Professor Samra’s group want to know how melanoma changes over time, what happens inside the tumour as it spreads and what features lead to drug resistance. To answer these questions, the team relies on patient data. Specifically, samples from patients who have passed away from cancer.
To access these samples, the team makes use of the PEACE (Posthumous Evaluation of Advanced Cancer Environment) study, a huge collaborative project, set up to help researchers collect samples from patients who have died from cancer. In this study, researchers take samples from participants during their treatment journey and after they have died. This huge assembly of data has allowed clinicians and scientists to study cancer in a way that was not possible before.
Professor Turajlic’s team employed sophisticated genetic sequencing techniques to study nearly 600 separate samples from 14 different patients with metastatic melanoma from the PEACE study. “What we’ve observed is that there are many variables that contribute tiny bits to the cancer progression” said Dr Lobon.
Uncovering these small contributions allowed the team to piece together a complex timeline of how the cancer develops. “It’s like having a fossil. We can study cancers at different points in time.”
The team shared the results of this extensive study with the scientific community earlier this year. The incredible information uncovered has helped scientists to understand the complex ways that melanomas use to evade drugs. The researchers hope that it could also lead to the development of completely new drugs that could provide hope for those diagnosed with metastatic melanoma.
While Professor Samra’s team are using the PEACE study to study metastatic melanoma, there are many other scientists using the valuable data collected to investigate other cancer types. There are researchers investigating lung cancer, renal cancer and many more. What they have in common, is their reliance on patient data.
Dr Lobon emphasised that her work would be impossible without the generous people who agree to donate their samples after they pass. “It’s extremely important. There is no other way we could get such specific information without these samples.”
“Data is everything.”
Case study 3 – Prof Rebecca Fitzgerald
Professor_rebecca_fitzgerald.jpg.

Oesophageal cancer is one of the biggest challenges in cancer research today. The underlying biology of the disease is poorly understood and survival remains very low, with only 1 in 10 people surviving their disease for 10 years or more. It’s a big challenge – and one that researchers and clinicians can’t tackle alone.
For over 10 years, Professor Rebecca Fitzgerald at the University of Cambridge has been spearheading a huge project that brings together scientists, doctors and nurses from across the UK with the goal of better understanding oesophageal cancer.
Known in the research community as OCCAMS (Oesophageal Cancer Clinical and Molecular Stratification), this impressive programme of work has seen researchers collecting tumour and blood samples from people with oesophageal cancer and decoding the cancer’s genetic sequence so that they have a complete map of the cancer’s DNA.
These samples provide a treasure trove of information, allowing scientists to see how changes in DNA sequence affects progression of oesophageal cancer. And every person’s data provides a new piece of the puzzle.
“This really is a team effort,” says Rebecca. “By sharing information, we can see the patterns and the trends and that’s what allows the breakthroughs to happen. Right now we can’t predict how well someone will do just by how they look when we first see them in the clinic. Some people do well, and sadly some people do badly, and we need to know why.”
And there are a lot of questions that need answering. How do genetic changes in the cancer change the way it develops over time? Are there specific risk factors that could predict if oesophageal cancer is going to recur? Why do some people respond well to treatment and some people don’t? All these questions rely on matching up clinical data (data collected by doctors about how each person’s cancer progresses) with laboratory data (data that comes from samples that scientists take away to analyse in the lab).
“And we need a lot of data,” explains Rebecca. “Oesophageal cancer is really complicated. We can’t find the needle in the haystack by just using data from a few people. The current problem my team is working on – working out whether all oesophageal cancers start from the precursor condition Barrett’s oesophagus – is using data from 4,000 patients. And that is why we are so grateful to all the patients who allow us to do this work.”
In the end, this work is all driven by the desire to make the situation better for people with oesophageal cancer. Already the team have been able to describe for the first time some of the DNA mutations that they think cause cancer, and there are likely to be many more findings to come.
“We have seen some improvements over the 20 years I have been working on this,” reflects Rebecca, “but we’ve got a long way to go. Solving this problem is what gets me out of bed in the morning. And I want to get to the end of my career and see that it is really different. I can’t do what I do without patient data – and I am not done yet.”
Case study 4 – Dr Rajesh Jena
Personalising radiotherapy – how patient data is helping reduce the side effects of treatment
Radiotherapy is the gold standard of treatment for many types of cancers. In fact, more than 130,000 patients benefit from radiotherapy every year in the UK. Today, most people receive image-guided radiotherapy – that is, using imaging such as x-rays and MRIs to target the beam of radiation to the tumour site, to make it as accurate as possible.
“Many people think about imaging at the point of diagnosis to find a tumour, or to follow up on treatment to see if its working,” says Dr Rajesh Jena, a clinician scientist based in Cambridge who is finding ways to improve radiotherapy for people with tumours of the brain and spine. “But imaging can be so much more: it can be used to personalise and even predict response to treatment.”
With radiotherapy there can be side effects because the beam of radiation can also affect healthy tissue surrounding the tumour. Combining imaging while delivering radiotherapy can help minimise these side effects, and getting better at combining these approaches is where patient data can be so important.
By studying images taken during radiotherapy, researchers can determine which healthy tissues were affected by the radiation, and then use that information to help predict how a new patient might respond to treatment, potentially altering the treatment plan to make it as side-effect free as possible.
“The wealth of information an image holds comes with an incredible potential,” says Dr Jena. Such potential to inform patient care led Dr Jena and his team to develop the first continuously learning artificial intelligence (AI) medical device in his hospital. Using patient data to train the AI, they were able to develop a tool that speeds up analysis of images. The technology helps doctors by cutting the amount of time spent ‘sketching’ around healthy organs, as they create radiotherapy plans, a painstaking yet vital step in radiotherapy treatment to ensure healthy tissue is protected.
And this data offers value far beyond direct medical treatment. “It’s not only doctors that need access to imaging data like this,” Dr Jena adds. “Patients are so generous allowing us to use this data, so we try to maximise its value by working with mathematicians, physicists, biologists and many other experts.” Bringing together different specialisms like this gives the scientists the best chance of understanding a disease as complex as cancer.
“And we also look back at historical patient images – this is important to provide context, improve our understanding and build up a valuable database of information.”
Thanks to patient data, Dr Jena and his team have already been able to create a technology that frees up valuable time and ultimately enable patients to receive treatment sooner. Over the next year, Dr Jena and his team are working on improving this technology by providing the AI with even more examples of patient images to learn from, allowing it to become better than ever at identifying healthy tissue.
“We’re so grateful to have access to patient imaging data because without it we wouldn’t be able to develop technologies like this, which can make a real difference to people with cancer. We’re very careful with how we use patient data, we take care to ensure that all data is anonymised and is treated with respect.”
Case studies in detail
The earlier diagnosis of lung cancer will save lives but also puts additional pressure on diagnostic services. It is therefore important that lung cancer pathways are organised to be as effective and efficient as possible and to ensure patients are given their diagnosis as soon as possible.
We hoped to improve understanding of pre-diagnostic events, intervals and patterns for lung cancer patients on a national scale, and to benchmark to the timings in the newly adopted National Optimal Lung Cancer Pathway (NOLCP).
The data used included: lung cancer registrations (2013-2015) from National Cancer Registration Analysis Service (NCRAS), diagnostic imaging data (DID) and cancer waiting times (CWT) data from NHS England; and involved data from over 100,000 patients.
Following data linkage, time intervals between events were calculated, different scenarios of events were investigated and timings of events were compared with those from the NOLCP.
Many different diagnostic scenarios exist, from simple to complex, which varies by CCG. Time intervals from imaging to diagnosis differed by source of image referral, with those ordered by GP direct access imaging having longer pathways. Benchmarking to the NOLCP timings showed small proportions (less than 6%) of patients meeting timings, this also varied widely by CCG.
Partners: ACE Programme (funded by CRUK, Macmillan, NHS England), CRUK-NHS England Partnership, NCRAS
See also: ACE Programme website , More in-depth findings
Survival for ovarian cancer in the UK is lower than other comparable high-income countries and there is substantial regional variation within the UK. Access to and/or quality of treatment may be contributing factors.
This analysis will establish a detailed picture of ovarian cancer treatments and outcomes and is intended to provide initial insights into a planned national ovarian cancer audit benchmarking pilot, with the ultimate aim to act as a catalyst to reduce variation and drive improvements to clinical practice.
Data used will be: patients diagnosed with ovarian cancer (1st July 2014 to 31st March 2015) who were eligible for chemotherapy; information about their tumour, chemotherapy treatment received, and demographic information.
The linked data will be used to compare treatment access rates between Cancer Alliances and/ or Trust of Multi-Disciplinary Team/diagnosis, to determine the extent to which this can be explained by regional differences in patient demographics, such as age and socioeconomic status, and we will investigate the influence of healthcare system levels factors on access to treatment, such as provider and type, and consultant volume and specialisation.
Findings are due Winter 2018, but we expect to find how much geographical variation in access to treatment for ovarian cancer there is in England, and to what extent is this affected by patient demographics and healthcare system level factors.
Partners: NHS England
The purpose of this service evaluation is to establish; factors that affect access to lung cancer treatments for non-small cell lung cancer (NSCLC) patients, whether there is variation between Cancer Alliances/ hospital trusts in access to a range of treatment pathways, and the impact of patient, tumour and provider characteristics on access to treatments.
Data used will be: patients diagnosed with lung cancer (1st April 2014 to 31st March 2015) who were eligible for chemotherapy; information about their tumour, chemotherapy treatment received, and demographic information.
Understanding variation in accessing treatments will inform policy makers and commissioners regarding where efforts should be focused, to ensure equitable access to effective treatments for NSCLC, and improve patient outcomes.
The linked data will be used to calculate access rates for different treatments, and then statistical tests will measure the overall geographic variation and significance. Finally, logistic regression will identify which factors are predictive of whether people receive various treatments (e.g. age, deprivation, ethnicity).
Findings are due early 2019: we expect to establish the presence of variation at a geographic level, and the reaso
In England, less than 60% of eligible bowel cancer screening participants take part in the programme. Cancer Research UK, in partnership with Public Health England, carried out a regional Be Clear on Cancer pilot campaign in the North West of England to encourage participation.
The campaign ran in 33 CCGs and consisted of advertising (including TV) and direct mail. Advertising ran alone for 6 weeks and was later combined for 6 weeks with direct mail in 22 CCGs to first-timers and previous non-responders. The remaining 11 CCGs continued to receive advertising only. The campaign was aimed at people aged 55-74 in the lower socioeconomic groups (C2DE) and skewed towards men; this was based on pilots previously run by Cancer Research UK.
We evaluated whether the campaign advertising, or advertising paired with sending a CRUK-endorsement letter with the kit increased uptake. Differences have also been compared across different socioeconomic groups.
The data we used to do this were extracted from the national bowel cancer screening database. We used age, location, date of test kit received, and date kit sent back. For the direct mail element of the campaign, we obtained a set of data for bowel screening test kit recipients who received a CRUK-endorsement letter, and a separate set of data for bowel screening test kit recipients who did not. We did not receive any names, addresses, or NHS numbers, so no individuals could be identified.
We split participants into 3 groups for the evaluation:
- First timers – people who are invited for bowel screening for the first time
- Previously screened – those who were invited for screening previously and returned completed kits
- Previous non-responders – those who were invited previously but didn’t return completed kits
Results are promising. They showed an increase in uptake of the screening test kit during the live campaign for all groups, as well as a smaller sustained increase in the 3 months following the campaign.
There are upcoming changes to the bowel cancer screening programme which may impact on screening uptake. We recommend waiting for an appropriate time, once changes have been implemented, to consider another campaign.
See also: Definitions of socioeconomic groups , Previous pilots .
COVID RT – assessing the impact of COVID-19 on radiotherapy in the UK
The COVID-19 pandemic has had a significant impact on cancer patients and cancer services across the UK. During the peak of the pandemic, radiotherapy services across the UK continued to treat cancer patients in often challenging circumstances and implemented significant changes to standard practice to minimise the risks to patients of contracting COVID-19 and focus radiotherapy resources where they were most needed. The scale of these changes in radiotherapy practice, the clinical decision-making underpinning them and their impact on cancer patient outcomes is unknown. This study will give a national picture of the decision making by patients and clinical staff and the impact on patient's treatment. It also provides knowledge of how RT was used as a bridge to surgery when surgical services weren't viable due to the pandemic and therefore what are the further requirements for cohorts of patients.
Data have been collected by radiotherapy centres across England specifically for this study as part of patient's routine care. These data were submitted to NHS England (previously Public Health England) under regulation 2 of the Health Service Regulations 2002. NHS England (previously Public Health England) collected and stored these data. A pseudonymised extract of these data was created and transferred to our Cancer Research UK Secure Data Environment (SDE).
These data are being analysed within the CRUK SDE, by named analytical staff at Cancer Research UK and University of Leeds, alongside similar data for other UK nations. Summary results will be calculated and used as the first research project to understand the affect Covid had on radiotherapy treatment. These data will not be linked to other datasets.
Join us on X (previously known as Twitter)
Information on the patient-level data we hold
- Around the Practice
- Between the Lines
- Contemporary Concepts
- Readout 360
- Insights from Experts at Mayo Clinic on Translating Evidence to Clinical Practice
- Optimizing Outcomes in Patients with HER2+ Metastatic Breast Cancer

- Conferences
- Publications
- Career Center
Case 1: 48-Year-Old Patient With HER2+ Metastatic Breast Cancer
EP: 1 . Best Practices: HER2+ MBC With Brain Mets
EP: 2 . Frontline Standards of Care for HER2+ MBC
EP: 3 . Case 1: 48-Year-Old Patient With HER2+ Metastatic Breast Cancer
EP: 4 . Treatment Strategies for Relapsed/Refractory HER2+ MBC
EP: 5 . Case 2: 61-Year-Old Patient With R/R HER2+ MBC

EP: 6 . Cancer Network Around the Practice: Relapsed/Refractory HER2+ Metastatic Breast Cancer
Adam M. Brufsky, MD, PhD: Let’s talk about this case. This is a 48-year-old woman who presented to her primary care physician a number of years ago with a lump in her breast. She had a 4.4-cm left breast mass and 3 palpable axillary lymph nodes. Her ultrasound and mammogram confirmed these physical findings.
She was referred to a medical oncologist and had a core needle biopsy that showed ER- [estrogen receptor-negative]/PR- [progesterone receptor-negative], HER2 [human epidermal growth factor receptor 2]-positive by IHC [immunohistochemistry score] that was 3+. A CT scan of the chest, abdomen, and pelvis showed 3 liver lesions, the largest being 3.1 cm. This is the de novo patient we always talk about. She had an MRI of the brain and it was negative for metastasis. She received 6 cycles of THP [docetaxel, trastuzumab, pertuzumab], followed by HP [trastuzumab, pertuzumab] for another 12 months. That’s 18 months of therapy.
She had a partial response in her breast mass, and her liver lesions fully responded. Later, she suddenly began to have rapid unexplained weight loss. The CT scan only showed 2 new liver lesions, so not quite the symptom I would imagine. She then got a brain MRI that showed about 30 widely scattered lesions, the largest being about 0.5 or 0.6 [cm]. They have all these little punctate ones; you’ve all seen those.
The question is: what treatment would you give this person? Let’s say the brain MRI shows 3 lesions, all in the frontal cortex, with the largest being 1.5 cm. That makes it a little bit of a different question because if there are widely scattered lesions, we’re not going to want to do SRS [stereotactic radiosurgery]. We are probably going to want to do whole brain radiation. Let’s say she’s asymptomatic with no edema. The polling question is: what treatment would you recommend? T-DM1 [trastuzumab emtansine], tucatinib/trastuzumab/capecitabine, SRS to the brain metastases, clinical trial, or other.
You guys could answer that question. Let me start with Sara. How would you approach this?
Sara A. Hurvitz, MD, FACP: They’re not totally mutually exclusive, right? You could do SRS and switch systemic therapy. She is progressing systemically in the liver, so I think switching systemic therapy makes sense. I like tucatinib because it does penetrate the blood-brain barrier, but I would still be tempted and would probably talk to my radiation oncology and neurosurgery colleagues. We’d probably end up doing both the SRS and tucatinib-based therapy.
Adam M. Brufsky, MD, PhD: That’s reasonable. VK, do you have any other comments on this?
VK Gadi, MD, PhD: Yes, I agree. The tolerability of the regimen is good. You might even give this lady an opportunity to fly without SRS and have that in your back pocket. If you’re not seeing control, you can go to SRS at a later time. I don’t think there’s a wrong answer here. You could probably do it both ways.
Adam M. Brufsky, MD, PhD: Neil, do you have something to add?
Neil M. Iyengar, MD: No. She fits perfectly into the HER2CLIMB population, so I agree with everything that has been said because there is demonstrated activity of the tucatinib-based regimen in terms of CNS [central nervous system] response. Coupling that with SRS is reasonable. This is the patient we were talking about earlier with whom we would discuss foregoing local therapy to the brain. That’s a reasonable discussion here. It’s a tricky poll question because my kneejerk response would be to put her on a clinical trial. We should all be trying to prioritize clinical trials, but in the absence of that clinical availability, tucatinib plus or minus radiation is a reasonable option.
Adam M. Brufsky, MD, PhD: There’s a clinical trial that’s great; it’s not scientifically spectacular, but clinically, it’s fabulous. I believe it’s called DESTINY. In fact, I put a patient on it today with trastuzumab deruxtecan and tucatinib together. That’s a great trial that’s going to accrue quickly. If we could put as many people as we can on that, we can answer the clinical question quickly. I would agree.
I have 1 last question before we go on to the last 25 minutes and the last segment. What do you tell people about [adverse] effects? Are you seeing a lot of [adverse] effects with tucatinib? Do you have to dose reduce it at all when you give it? These are questions people who haven’t had a lot of experience with it usually ask. I’ll start with Neil. Do you see a lot of diarrhea? Do you have to dose reduce with tucatinib?
Neil M. Iyengar, MD: In my experience, this regimen is quite tolerable. We all, as oncologists, have unfortunately become very comfortable with managing diarrhea, along with oncology nursing and so forth. What I have found with the tucatinib-based regimen is that with the initiation of antidiarrheal agents, the diarrhea usually resolves or improves pretty quickly. People have to know about it and be prepared to deal with it immediately. It does come on early, usually within the first cycle.
The other consideration to keep in mind with tucatinib is that many of the [adverse] effects are likely related to capecitabine. We’re all very comfortable with managing capecitabine-related toxicity and dose modifying capecitabine as needed. We see in the HER2CLIMB data that patients in the tucatinib arm stayed on study longer and were therefore exposed to capecitabine for longer than those in the placebo arm. I think a lot of the toxicities are familiar ones that are related to capecitabine and are quite manageable.
Adam M. Brufsky, MD, PhD: Great. VK and Sara, do you have any other comments about this toxicity? Do you see any toxicity at all with this, more than you’d expect?
Sara A. Hurvitz, MD, FACP: It’s well tolerated. About 13% had grade 3/4 diarrhea. Before getting on this call, I had to dose reduce a patient on this therapy. It’s hard to tell. On the clinical trial we enrolled patients, and I had a patient on who had severe colitis, hospitalization, etc, and I was sure she was getting tucatinib. When she was unblinded after the data came out, it turned out that she wasn’t on tucatinib. She was on placebo. I completely agree that these are [adverse] effects we’re used to with capecitabine. There’s not a whole lot of difference. Tucatinib is pretty well tolerated.
VK Gadi, MD, PhD: I agree. I think the capecitabine is the real culprit. The people on the trial were actually on it for so much longer that the toxicities from capecitabine emerged ongoing on the study. That has been my experience. Something important we don’t yet have is the PRO [patient-reported outcomes] data from these studies. A lot of my colleagues, especially those in communities where patients come in from a long way away, know that this is a tremendous pill burden with this regimen. Sometimes a parenteral regimen that you’re giving every 3 weeks is better for patients. I’m curious to see what those data look like when they come out. From our perspective as physicians, this is a slam dunk and it’s easy to give, but that’s not always the perspective that matters.
Adam M. Brufsky, MD, PhD: I agree.
Sara A. Hurvitz, MD, FACP: Yes, I think the quality of life PRO data were presented at the San Antonio [Breast Cancer Symposium]. I’m trying to pull it up. I don’t have it right at my fingertips, but my recollection was that it looked fairly good, that the quality of life was maintained.
Adam M. Brufsky, MD, PhD: Right, but they’re not going to tell you that they’re struggling to take all those pills. It’s a lot.
Sara A. Hurvitz, MD, FACP: That’s true.
Adam M. Brufsky, MD, PhD: It’s about 9 pills a day, which is a lot.
Neil M. Iyengar, MD: The quality of life data are always interesting because the end point of choice is time to deterioration and whether we are avoiding that. I think that’s a fairly low bar.
Adam M. Brufsky, MD, PhD: Exactly. Women are going to do anything they can.
Transcript edited for clarity.
- Biomarker-Driven Lung Cancer
- HER2-Positive Breast Cancer
- Chronic Lymphocytic Leukemia
- Small Cell Lung Cancer
- Renal Cell Carcinoma

- CONFERENCES
- PUBLICATIONS
Case 1: 72-Year-Old Woman With Small Cell Lung Cancer

EP: 1 . Case 1: 72-Year-Old Woman With Small Cell Lung Cancer
Ep: 2 . case 1: extensive-stage small cell lung cancer background, ep: 3 . case 1: impower133 trial in small cell lung cancer, ep: 4 . case 1: caspian trial in extensive-stage small cell lung cancer, ep: 5 . case 1: biomarkers in small cell lung cancer, ep: 6 . case 1: small cell lung cancer in the era of immunotherapy.

EP: 7 . Case 2: 67-Year-Old Woman With EGFR+ Non–Small Cell Lung Cancer
Ep: 8 . case 2: biomarker testing for non–small cell lung cancer, ep: 9 . case 2: egfr-positive non–small cell lung cancer, ep: 10 . case 2: flaura study for egfr+ metastatic nsclc, ep: 11 . case 2: egfr+ nsclc combination therapies.

EP: 12 . Case 2: Treatment After Progression of EGFR+ NSCLC

EP: 13 . Case 3: 63-Year-Old Man With Unresectable Stage IIIA NSCLC
Ep: 14 . case 3: molecular testing in stage iii nsclc, ep: 15 . case 3: chemoradiation for stage iii nsclc, ep: 16 . case 3: pacific trial in unresectable stage iii nsclc, ep: 17 . case 3: standard of care in unresectable stage iii nsclc, ep: 18 . case 3: management of immune-related toxicities in stage iii nsclc.
Mark Socinski, MD: Thank you for joining us for this Targeted Oncology ™ Virtual Tumor Board ® focused on advanced lung cancer. In today’s presentations my colleagues and I will review three clinical cases. We will discuss an individualized approach to treatment for each patient, and we’ll review key clinical trial data that impact our decisions. I’m Dr. Mark Socinski from the AdventHealth cancer institute in Orlando, Florida. Today I’m joined by Dr Ed Kim, a medical oncologist from the Levine Cancer Institute in Charlotte, North Carolina; Dr Brendon Stiles, who is a thoracic surgeon from the Weill Cornell Medical Center in New York ; and Dr Tim Kruser, radiation oncologist from Northwestern Medicine Feinberg School of Medicine in Chicago. Thank you all for joining me today. We’re going to move to the first case, which is a case of small cell lung cancer. I’m going to ask Dr Kim to do the presentation.
Edward Kim, MD: Thanks, Mark. It’s my pleasure to walk us through the first case, which is small cell lung cancer. This is a case with a 72-year-old woman who presents with shortness of breath, a productive cough, chest pain, some fatigue, anorexia, a recent 18-pound weight loss, and a history of hypertension. She is a schoolteacher and has a 45-pack-a-year smoking history; she is currently a smoker. She is married, has 2 kids, and has a grandchild on the way. On physical exam she had some dullness to percussion with some decreased-breath sounds, and the chest x-ray shows a left hilar mass and a 5.4-cm left upper-lobe mass. CT scan reveals a hilar mass with a bilateral mediastinal extension. Negative for distant metastatic disease. PET scan shows activity in the left upper-lobe mass with supraclavicular nodal areas and liver lesions, and there are no metastases in the brain on MRI. The interventional radiographic test biopsy for liver reveals small cell, and her PS is 1. Right now we do have a patient who has extensive-stage small cell lung cancer. Unfortunately, it’s what we found. It’s very common to see this with liver metastases.
Transcript edited for clarity.

FDA Approval Marks Amivantamab's Milestone in EGFR+ NSCLC
In this episode, Joshua K. Sabari, MD, discusses the FDA approval of amivantamab plus chemotherapy as a first-line treatment for patients with EGFR exon 20 insertion mutation-positive non-small cell lung cancer.

Treating Rare Driver Mutations in NSCLC: EGFR Exon 20 Insertions
During a Case-Based Roundtable® event, Misako Nagasaka, MD, discussed treatment for a patient with non–small cell lung cancer and an EGFR exon 20 insertion.

Lisberg Discusses Dato-DXd's Role in Advanced Lung Cancer Care
In this episode of Targeted Talks, Aaron Lisberg, MD, discusses results from the phase 3 TROPION-Lung01 study of datopotamab in advanced or metastatic non–small cell lung cancer.

Biomarker Testing Paves the Way for Better Targeted Therapies in NSCLC
At a live virtual event, Edward S. Kim, MD, MBA, discussed the evolving landscape of biomarker testing before making treatment decisions for patients with early-stage non–small cell lung cancer (NSCLC).

Dato-DXd Outperforms Docetaxel in Nonsquamous Lung Cancer
Aaron Lisberg, MD, discussed the phase 3 TROPION-Lung01 study which evaluated datopotamab deruxtecan for patients with advanced or metastatic non-small cell lung cancer.
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

- Research Note
- Open access
- Published: 28 April 2024
Higher peripheral blood mitochondrial DNA copy number and relative telomere length in under 48 years Indonesian breast cancer patients
- Prisca C. Limardi 1 , 2 , 3 ,
- Sonar Soni Panigoro 4 ,
- Nurjati Chairani Siregar 5 ,
- Noorwati Sutandyo 6 ,
- Fiastuti Witjaksono 7 ,
- Lidwina Priliani 2 , 3 ,
- Sukma Oktavianthi 2 , 3 &
- Safarina G. Malik 1 , 2 , 3
BMC Research Notes volume 17 , Article number: 120 ( 2024 ) Cite this article
189 Accesses
Metrics details
Breast cancer is the leading cause of cancer incidence and mortality among Indonesian women. A comprehensive investigation is required to enhance the early detection of this disease. Mitochondrial DNA copy number (mtDNA-CN) and relative telomere length (RTL) have been proposed as potential biomarkers for several cancer risks, as they are linked through oxidative stress mechanisms. We conducted a case–control study to examine peripheral blood mtDNA-CN and RTL patterns in Indonesian breast cancer patients (n = 175) and healthy individuals (n = 181). The relative ratios of mtDNA-CN and RTL were determined using quantitative real-time PCR (qPCR).
Median values of mtDNA-CN and RTL were 1.62 and 0.70 in healthy subjects and 1.79 and 0.73 in breast cancer patients, respectively. We found a positive association between peripheral blood mtDNA-CN and RTL ( p < 0.001). In under 48 years old breast cancer patients, higher peripheral blood mtDNA-CN (mtDNA-CN ≥ 1.73 (median), p = 0.009) and RTL (continuous variable, p = 0.010) were observed, compared to the corresponding healthy subjects. We also found a significantly higher ‘High-High’ pattern of mtDNA-CN and RTL in breast cancer patients under 48 years old ( p = 0.011). Our findings suggest that peripheral blood mtDNA-CN and RTL could serve as additional minimally invasive biomarkers for breast cancer risk evaluation.
Peer Review reports
Introduction
Breast cancer has been the leading cause of cancer incidence globally in 2020 and continues to be the primary cause of cancer-related deaths among women [ 1 , 2 ]. It is also the most common type of cancer in Indonesia, with a higher age-standardised death rate (15.3 per 100,000) [ 3 ] than the global mortality rate (13.6 per 100,000) in 2020 [ 4 ], indicating a relatively lower survival rate for breast cancer patient in Indonesia.
The search for non-invasive biomarkers for breast cancer screening remains a challenging process. One of these efforts involves exploring the potential utilisation of the peripheral blood mitochondrial DNA copy number (mtDNA-CN) and relative telomere length (RTL) [ 5 , 6 , 7 ].
The relationship between mitochondria and telomeres has been studied extensively, particularly in biological ageing. They are intertwined through the telomere-p53-PGC–1α-mitochondria axis and are intricately linked to oxidative stress [ 8 ]. Their functionality is commonly estimated by measuring the mtDNA-CN and RTL. Nevertheless, studies that simultaneously incorporating both biomarkers, particularly in association with breast cancer, remain limited. Independent studies with peripheral blood mtDNA-CN [ 6 , 9 , 10 , 11 , 12 , 13 ] and RTL [ 5 , 14 , 15 , 16 , 17 , 18 , 19 , 20 ] have also reported inconsistent findings. However, a prospective study by Campa et al. found a positive association between high peripheral blood mtDNA-CN and high RTL, along with an increased risk of breast cancer [ 7 ], suggesting that peripheral blood mtDNA-CN and RTL could be used as minimally invasive biomarkers for breast cancer risk evaluation.
Our study aimed to investigate the differences in peripheral blood mtDNA-CN and RTL between breast cancer patients and healthy subjects in Indonesia. To the best of our knowledge, such a study has not been conducted in Indonesia before. We hypothesise that there are significant differences in both biomarkers between breast cancer patients and healthy subjects, which can potentially be utilised as additional minimally invasive biomarkers for breast cancer risk evaluation in Indonesia.
Study design and participants
This retrospective case–control study was initially conducted between 2019 and 2020, following specific inclusion and exclusion criteria [ 21 ]. The case subjects are females diagnosed with breast cancer based on histopathology and immunohistochemistry assay, aged 19 years or older, who have not undergone any cancer therapies. The control subjects are healthy disease-free females, aged 19 years or older, without any history of cancer and chronic illnesses. Following the incorporation of incomplete data as additional exclusion criteria, this study further investigated 175 breast cancer patients and 181 healthy subjects from six public referral hospitals in Indonesia (Additional file 1 : Fig. S1). Approval from the Ethical Committee of Health Research at the Faculty of Medicine, Universitas Indonesia, Rumah Sakit Cipto Mangunkusumo, Jakarta, Indonesia, was obtained under reference number 450/UN2. F1/ETIK/2018.
Clinical samples and data measurements
We analysed 356 archived peripheral blood samples stored at −70 °C. Demographic data were obtained using a self-administered questionnaire, including age, menarche age, menopause, childbirth history, breastfeeding, hormonal contraceptive use, smoking status, and alcohol consumption. Body mass index (BMI) was calculated by dividing weight (kg) by height squared (m 2 ). Serum lipid concentrations were measured after 12 h of overnight fasting, including triglycerides (TG), high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), and total cholesterol (TC). We also calculated the triglyceride glucose (TyG) index using the following equation: Ln[fasting TG (mg/dL) x FPG (mg/dL)/2] [ 22 ].
Measurement of peripheral blood mtDNA-CN and RTL
Total DNA was extracted from archived peripheral blood samples using the salting-out methods. Blood samples with a total volume of 3 mL were extracted by employing Gentra® Puregene® Blood Kit (Qiagen, Hilden, Germany). Meanwhile, samples with a total volume of 1 mL were extracted using Geneius™ Micro gDNA Extraction Kit (Geneaid Biotech Ltd., New Taipei City, Taiwan), following both manufacturers’ instructions. Despite different extraction kits, no significant differences were found in mtDNA-CN and RTL measurement results (Additional file 1 : Table S1).
Each extracted DNA sample was diluted with nuclease-free water (Ambion, Texas, USA) to a concentration of 5 ng/μL and used as a quantitative real-time PCR (qPCR) template. The reactions were carried out using Power SYBR ™ Green PCR Master Mix (Applied Biosystems, California, USA) on a 7500 Real-Time PCR System (Applied Biosystems, California, USA). Each sample was evaluated in duplicate. The mtDNA-CN [ 23 ] and RTL [ 24 ] were measured by calculating their relative ratios to Beta-2-microglobulin ( B2M ), the single-copy nuclear-encoded reference gene. This calculation was based on the efficiency-corrected method implemented in the “qpcR” package [ 25 ]. A list of the primer pairs is provided in Additional file 1 : Table S2.
Statistical analyses
Data analysis was performed using R version 4.1.2 ( www.r-project.org ) with R Studio (version 2021.9.2.382; www.rstudio.com ). The normality of continuous variables was evaluated using the Shapiro–Wilk test. Medians (interquartile ranges) were reported for non-normally distributed variables (age, BMI, TG, HDL-C, LDL-C, TC, FPG, TyG index) and evaluated using the Wilcoxon-Mann Whitney U test. Categorical variables (alcohol consumption, smoking, menarche age, menopause, childbirth history, breastfeeding, hormonal contraceptive use) were evaluated using Pearson’s chi-square test and reported as the number of samples (percentage). Since the relative ratios of mtDNA-CN and RTL were not normally distributed even after being transformed into log e , their associations were estimated using rank-based linear regression models using the “Rfit” package [ 26 ]. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated using the likelihood ratio test, adjusted for potential confounders. Variable selections incorporated in the model were carried out using stepwise multiple regression implemented in the “MASS” package [ 27 ]. Confounders added to the models were age, BMI, HDL-C, LDL-C, TC, TyG index, alcohol consumption, and smoking status. Analyses were carried out on all subjects and sub-groups based on age (under 48 years and above 48 years subgroups). The cut-off age of 48 years was determined by the ‘SpEqualSe’ method in the “OptimalCutpoints” package, based on the equality of specificity and sensitivity values [ 28 ]. The effect size and power calculation were done using the “pwr” package [ 29 ]. Significance was indicated by a p -value of < 0.025, following Bonferroni correction.
Characteristics of study participants
The characteristics of the study participants are shown in Additional file 1 : Table S3. Compared to the healthy subjects, breast cancer patients had significantly higher median age (median value, healthy group vs. breast cancer group, 45 years vs. 48 years, p = 0.003) and lower BMI ( p = 0.003). Several clinical characteristics also showed significant differences (all p < 0.050). Breast cancer patients had a higher TG, lower HDL-C, higher FPG, and TyG index compared to healthy subjects. The prevalences of alcohol consumption and smoking were significantly higher in breast cancer patients. Those who developed breast cancer had a notably higher frequency of childbirth history, breastfeeding for less than 12 months, and hormonal contraceptive use.

The positive association between peripheral blood mtDNA-CN and RTL
We found a consistent positive association between peripheral blood mtDNA-CN and RTL in all subjects and healthy and breast cancer groups. The association was found noteworthy ( p < 0.001; R 2 > 0.4) in each group, as shown in Fig. 1 .
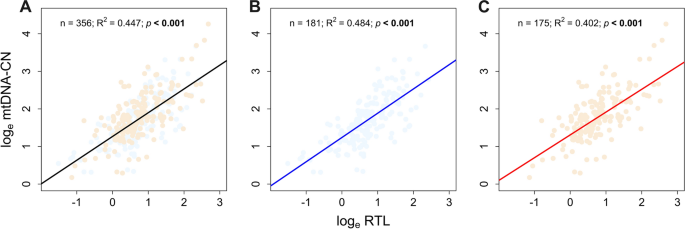
The positive association between peripheral blood mtDNA-CN and RTL. MtDNA-CN and RTL were positively associated with one another in A all samples, B healthy subjects, and C breast cancer patients
Comparison of peripheral blood mtDNA-CN and RTL between breast cancer patients and healthy subjects
The univariate comparison of peripheral blood mtDNA-CN and RTL between breast cancer patients and healthy subjects is presented in Additional file 1 : Fig. S2. The peripheral blood mtDNA-CN was significantly higher in the breast cancer patients compared to the healthy subjects (median value, healthy subjects vs. breast cancer patients, 1.62 vs. 1.79; p = 0.038). However, no significant differences in RTL were found between the two groups.
Additional analyses were performed on age subgroups, specifically those under and over 48 years old. Additional file 1 : Fig. S3 displays the results of a univariate comparison between mtDNA-CN and RTL levels in peripheral blood samples for healthy individuals and breast cancer patients in each age subgroup (under and above 48 years). Our findings indicated that mtDNA-CN ( p = 0.059) and RTL ( p = 0.008) tended to be higher in healthy subjects aged 48 years and above. In contrast, RTL levels ( p = 0.046) tended to be lower in breast cancer patients aged above 48 years.
We also conducted multivariate analyses in the age subgroups and presented the results as odds ratios. Breast cancer patients in the under 48 years subgroup had a significantly higher mtDNA-CN when analysed with classified median value (mtDNA-CN ≥ 1.73 (median), OR 2.81, 95% CI 1.31–6.22, p = 0.009, power = 1), and showed nominal significance when analysed as a continuous variable (OR 1.97, 95% CI 1.04–3.86, p = 0.040, power = 1) (Fig. 2 A).
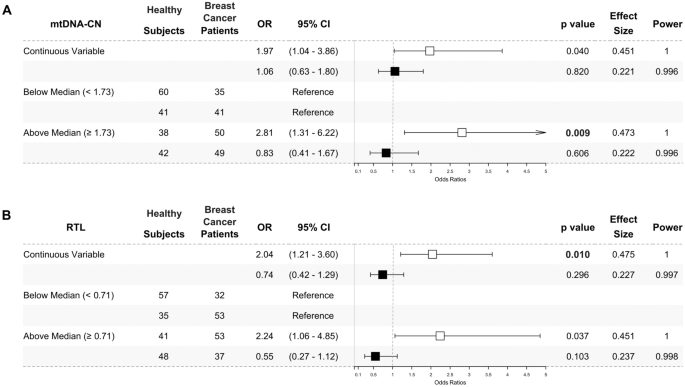
Higher peripheral blood mtDNA-CN ( A ) and RTL ( B ) in under 48 years breast cancer patients. The odds ratios of multivariate analysis for mtDNA-CN ( A ) and RTL ( B ) were presented as forest plots. The under 48 years subgroup was presented as white square (□). The above 48 years subgroup was presented as black square (■). The mtDNA-CN and RTL were analysed as a continuous variable and classified by the median value. Peripheral blood mtDNA-CN were significantly higher in under 48 years (□) breast cancer patients when analysed using the median cut-off (1.73) and nominally significant when analysed as a continuous variable. Meanwhile, peripheral blood RTL was significantly higher in under 48 years (□) breast cancer patients when being analysed as a continuous variable and nominally significant when being analysed using the median cut-off (0.71). No significant result was found in the above 48 years (■) subgroup. The likelihood ratio test was used to calculate odds ratios (ORs) with 95% confidence intervals (95% CIs) for association studies. Potential confounders added to the models were age, BMI, HDL-C, LDL-C, TC, TyG index, alcohol consumption, and smoking status. Significance was indicated by a p -value of < 0.025, following Bonferroni correction
RTL was also higher in under 48 years breast cancer patients when analysed as a continuous variable (OR 2.04, 95% CI 1.21–3.60, p = 0.010, power = 1) and showed a nominal significance when analysed with classified median value (RTL ≥ 0.71 (median), OR 2.24, 95% CI 1.06–4.85, p = 0.037, power = 1) (Fig. 2 B) as compared to the corresponding healthy subjects. Meanwhile, no significant odds ratios were found in the above 48-year subgroup.
We analysed the combination patterns of mtDNA-CN and RTL in peripheral blood using their median values. Values below the median were categorised as ‘Low’, while those above were considered 'High'. The median values for mtDNA-CN and RTL are 1.73 and 0.71, respectively. Consistent with individual analysis, we identified a significantly higher ‘High-High’ pattern of mtDNA-CN and RTL in breast cancer patients under 48 years old compared to healthy subjects of the same age group (OR 3.03, 95% CI 1.31–7.25, p = 0.011, power = 1). However, there were no significant differences in ‘Low–High’ and ‘High-Low’ patterns in both subgroups under and above 48 years old ( p > 0.050, power = 1), as depicted in Fig. 3 .
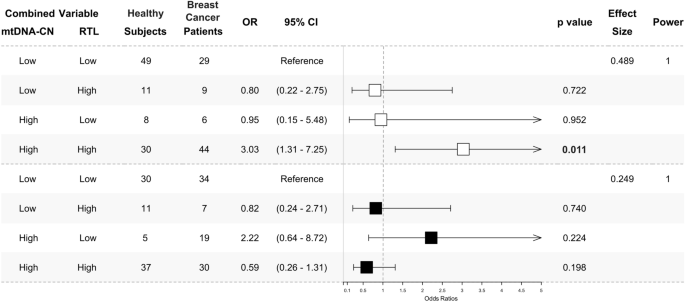
‘High-High’ pattern of peripheral blood mtDNA-CN and RTL in under 48 years breast cancer patients. The odds ratios of multivariate analysis for the mtDNA-CN and RTL combination pattern were presented as a forest plot. The under 48 years subgroup was presented as white square (□). The above 48 years subgroup was presented as black square (■). The combination was determined by the median value of each marker. The median cut-off is 1.73 for mtDNA-CN and 0.71 for RTL. A value below the median ( <) was classified as ‘Low’ and above the median ( ≥) as ‘High’. The ‘Low-Low’ combination was used as a reference. A ‘High-High’ combination was significantly found to be higher in under 48 years (□) breast cancer patients. No significant result was found in the above 48 years (■) subgroup. The multinomial regression test was used to calculate odds ratios (ORs) with 95% confidence intervals (95% CIs) for association studies. Potential confounders added to the models were age, BMI, HDL-C, LDL-C, TC, TyG index, alcohol consumption, and smoking status. Significance was indicated by a p -value of < 0.025, following Bonferroni correction
Breast cancer is a complex disease influenced by numerous factors. The development and presence of the disease contribute to systemic changes. These alterations can be observed in circulating blood, leading breast cancer to be recognised as a systemic disease [ 30 ]. Furthermore, in addition to its minimally invasive collection methods, blood circulates throughout the body and contains information regarding systemic status, e.g., the body’s response to malignancies [ 31 ]. This study observed distinct peripheral blood mtDNA-CN and RTL patterns between Indonesian breast cancer patients and healthy subjects, particularly in the under 48 years subgroup.
The mtDNA-CN and RTL are commonly associated with the ageing process, and numerous studies have highlighted an inverse relationship between these markers and age [ 32 , 33 ]. Both biomarkers exhibit dynamic regulation in response to environmental changes, mainly with antioxidant-oxidant imbalance. The lack of histone and less-adequate repair mechanism in mtDNA [ 34 ] and high-guanine residue in telomeres makes them susceptible to oxidative stress exposure [ 35 ]. Following previous studies in healthy adults [ 36 , 37 ], pregnant women [ 38 ], gastric cancer tissues [ 39 ], and breast cancer patients [ 40 ], our study also found a positive association between peripheral blood mtDNA-CN and RTL, indicating their significant interrelationship.
Our multivariate analysis revealed that breast cancer patients, particularly those under 48 years, exhibited a higher peripheral blood mtDNA-CN and RTL than healthy subjects. An increase in peripheral blood mtDNA-CN is potentially due to the activation of mitochondrial compensatory mechanism to maintain mitochondrial function in response to prolonged oxidative stress exposure during disease progression [ 41 , 42 , 43 , 44 ]. The dynamic regulation of blood telomere length occurs during hematopoiesis in the bone marrow. In the case of chronic diseases like breast cancer, prolonged exposure to oxidative stress may stimulate telomere lengthening as compensation for telomere loss in hematopoietic cells, which can later be observed in the peripheral blood cells [ 45 ]. Our finding also revealed that the ‘High-High’ combined pattern of both biomarkers was predominant in breast cancer patients under 48 years, which is consistent with previous research [ 7 ].
In separate and combined mtDNA-CN and RTL analyses, the subgroup of individuals under 48 years old consistently showed statistically significant results. This observation can be attributed to several factors. Firstly, it is worth noting that 48 years corresponds to the average age of menopause for Indonesian women [ 46 , 47 , 48 ]. Secondly, younger individuals may possess a better capacity for the compensatory mechanism of mitochondria and telomeres. The differences are more pronounced when comparing a relatively younger case–control subgroup [ 49 , 50 ].
The regulation of mtDNA-CN and RTL can be affected by the level and duration of exposure to oxidative stress [ 51 , 52 ]. Studies have shown that breast cancer patients typically exhibit higher levels of oxidative stress compared to healthy individuals [ 53 ]. Various risk factors for breast cancer can contribute to the development of oxidative stress during breast carcinogenesis. In this study, we identified several significant differences in specific clinical parameters (TG, HDL-C, FPG, TyG index) and lifestyle factors (alcohol consumption, smoking, breastfeeding duration, hormonal contraceptive use) between the breast cancer and healthy groups (Additional file 1 : Table S3). These variables are known to increase the risk of breast cancer. Alcohol consumption [ 54 ], smoking [ 55 ], and lifestyle changes that lead to alterations in serum lipid concentrations [ 56 , 57 ] have been linked to increased oxidative stress levels through various mechanisms.
On the other hand, cancer cells also produce ROS due to their high metabolic rate, accumulation of genetic alterations, relative hypoxia, and persistent inflammation [ 58 ]. Therefore, the altered level of oxidative stress in breast cancer patients might have resulted from either the presence of risk factors during carcinogenesis or the production of ROS by cancer cells. Nevertheless, determining the underlying mechanisms from a case–control study perspective can be pretty challenging.
Limitations
It is important to note that this study has some limitations. Firstly, the study cannot be generalised to other populations. Secondly, we did not measure any oxidative stress markers, which could provide valuable insights into the underlying mechanisms of mtDNA-CN and RTL regulations in breast cancer. Thirdly, the retrospective case–control study design cannot explain the direction of causation. Hence, future prospective studies enrolling other populations are warranted.
In summary, we have identified distinctive peripheral blood mtDNA-CN and RTL characteristics in under 48 years breast cancer patients. This pilot case–control study for Indonesian breast cancer patients has highlighted a potential utilisation of both biomarkers as additional minimally invasive tools for enhancing early breast cancer risk evaluation.
Availability of data and materials
The dataset supporting the conclusion of this article is available in the Mendeley Data repository, doi: https://dx.doi.org/ https://doi.org/10.17632/v7cz97s659.1
Abbreviations
Mitochondrial DNA copy number
Relative telomere length
Quantitative polymerase chain reaction
95% Confidence interval
Reactive oxygen species
Triglycerides
Total cholesterol
Fasting plasma glucose
Triglyceride and glucose index
High density lipoprotein-cholesterol
Low density lipoprotein-cholesterol
Peroxisome proliferator-activated receptor-gamma coactivator-1 alpha
Beta-2 microglobulin
Body mass index
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492 .
Article PubMed Google Scholar
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660 .
Article CAS PubMed Google Scholar
GLOBOCAN. Breast Fact Sheets. 2019. p. 2. https://gco.iarc.fr/today/data/factsheets/cancers/20-Breast-fact-sheet.pdf . Accessed 12 Sept 2020.
GLOBOCAN. Indonesia fact sheets. 2019. p. 2. https://gco.iarc.fr/today/data/factsheets/populations/360-indonesia-fact-sheets.pdf . Accessed 12 Sept 2020.
Pellatt AJ, Wolff RK, Torres-Mejia G, John EM, Herrick JS, Lundgreen A, et al. Telomere length, telomere-related genes, and breast cancer risk: the breast cancer health disparities study. Genes Chromosom Cancer. 2013;52(7):595–609.
Lemnrau A, Brook MN, Fletcher O, Coulson P, Tomczyk K, Jones M, et al. Mitochondrial DNA copy number in peripheral blood cells and risk of developing breast cancer. Cancer Res. 2015;75(14):2844–50.
Campa D, Barrdahl M, Santoro A, Severi G, Baglietto L, Omichessan H, et al. Mitochondrial DNA copy number variation, leukocyte telomere length, and breast cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Breast Cancer Res. 2018;20(1):29. https://doi.org/10.1186/s13058-018-0955-5 .
Article CAS PubMed PubMed Central Google Scholar
Sahin E, DePinho RA. Axis of ageing: telomeres, p53 and mitochondria. Nat Rev Mol Cell Biol. 2012;13(6):397.
Xia P, An HX, Dang CX, Radpour R, Kohler C, Fokas E, et al. Decreased mitochondrial DNA content in blood samples of patients with stage I breast cancer. BMC Cancer. 2009;9:454.
Article PubMed PubMed Central Google Scholar
Shen J, Platek M, Mahasneh A, Ambrosone CB, Zhao H. Mitochondrial copy number and risk of breast cancer: a pilot study. Mitochondrion. 2010;10(1):62–8.
Shen J, Wan J, Song R, Zhao H. Peripheral blood mitochondrial DNA copy number, length heteroplasmy and breast cancer risk: a replication study. Carcinogenesis. 2015;36(11):1307–13. https://doi.org/10.1093/carcin/bgv130 .
Thyagarajan B, Wang R, Nelson H, Barcelo H, Koh WP, Yuan JM. Mitochondrial DNA copy number is associated with breast cancer risk. PLoS ONE. 2013;8(6):65968.
Article Google Scholar
Zhao H, Chang D, Ye Y, Shen J, Chow WH, Wu X, et al. Associations of blood mitochondrial DNA copy number with social-demographics and cancer risk: results from the Mano-A-Mano Mexican American Cohort. Oncotarget. 2018;9(39):25491–502.
Svenson U, Ljungberg B, Roos G. Telomere length in peripheral blood predicts survival in clear cell renal cell carcinoma. Cancer Res. 2009;69(7):2896–901.
Gramatges MM, Telli ML, Balise R, Ford JM. Longer relative telomere length in blood from women with sporadic and familial breast cancer compared with healthy controls longer telomeres in breast cancer patients. Cancer Epidemiol Biomarkers Prev. 2010;19(2):605–13.
Samavat H, Xun X, Jin A, Wang R, Koh WP, Yuan JM. Association between prediagnostic leukocyte telomere length and breast cancer risk: the Singapore Chinese Health Study. Breast Cancer Res. 2019;21(1):50. https://doi.org/10.1186/s13058-019-1133-0 .
Shen J, Gammon MD, Terry MB, Wang Q, Bradshaw P, Teitelbaum SL, et al. Telomere length, oxidative damage, antioxidants and breast cancer risk. Int J Cancer. 2009;124(7):1637–43.
Pooley KA, Sandhu MS, Tyrer J, Shah M, Driver KE, Luben RN, et al. Telomere length in prospective and retrospective cancer case-control studies. Cancer Res. 2010;70(8):3170.
Ma H, Zhou Z, Wei S, Liu Z, Pooley KA, Dunning AM, et al. Shortened telomere length is associated with increased risk of cancer: a meta-analysis. PLoS ONE. 2011;6(6): e20466.
Duggan C, Risques R, Alfano C, Prunkard D, Imayama I, Holte S, et al. Change in peripheral blood leukocyte telomere length and mortality in breast cancer survivors. JNCI J Natl Cancer Inst. 2014;106(4): dju035.
Panigoro SS, Sutandyo N, Witjaksono F, Siregar NC, Ramli R, Hariani R, et al. The association between triglyceride-glucose index as a marker of insulin resistance and the risk of breast cancer. Front Endocrinol. 2021;11(12):1244.
Google Scholar
Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity: comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–51.
Venegas V, Wang J, Dimmock D, Wong LJ. Real-time quantitative PCR analysis of mitochondrial DNA content. Curr Protoc Hum Genet. 2011. https://doi.org/10.1002/0471142905.hg1907s68 .
O’Callaghan NJ, Dhillon VS, Thomas P, Fenech M. A quantitative real-time PCR method for absolute telomere length. Biotechniques. 2008;44(6):807–9.
Ritz C, Spiess AN. qpcR: an R package for sigmoidal model selection in quantitative real-time polymerase chain reaction analysis. Bioinformatics. 2008;24(13):1549–51.
Kloke JD, McKean JW. Rfit: Rank-based estimation for linear models. R J. 2012;4(2):57–64.
Ripley B, Venables B, Bates DM, Hornik K, Gebhardt A, Firth D. Support functions and datasets for venables and ripley’s MASS. 2022. https://cran.r-project.org/web/packages/MASS/MASS.pdf . Accessed 06 Apr 2023.
Lopez-Raton M, Xose Rodriguez-Alvarez M. Computing optimal cutpoints in diagnostic tests. 2021. https://cran.r-project.org/web/packages/OptimalCutpoints/OptimalCutpoints.pdf . Accessed 19 July 2023.
Champley S, Ekstrom C, Dalgaard P, Gill J, Weibelzahl S, Anandkumar A, et al. pwr: basic functions for power analysis. R Package version 13-0. 2017; p. 1–22. https://cran.r-project.org/web/packages/pwr/ . Accessed 24 June 2023
Kim R, Kin T. Reconsidering the meaning of curing primary breast cancer as a systemic disease. Front Oncol. 2021;18(11):696.
Mohr S, Liew CC. The peripheral-blood transcriptome: new insights into disease and risk assessment. Trends Mol Med. 2007;13(10):422–32.
Zhang R, Wang Y, Ye K, Picard M, Gu Z. Independent impacts of aging on mitochondrial DNA quantity and quality in humans. BMC Genomics. 2017;18(1):1–14. https://doi.org/10.1186/s12864-017-4287-0 .
Article CAS Google Scholar
Vyas CM, Ogata S, Reynolds CF, Mischoulon D, Chang G, Cook NR, et al. Lifestyle and behavioral factors and mitochondrial DNA copy number in a diverse cohort of mid-life and older adults. PLoS ONE. 2020;15(8): e0237235.
Salgado J, Honorato B, García-Foncillas J. Review: mitochondrial defects in breast cancer. Clin Med Oncol. 2008;2:199–207. https://doi.org/10.4137/CMO.S524 .
Coluzzi E, Colamartino M, Cozzi R, Leone S, Meneghini C, O’Callaghan N, et al. Oxidative stress induces persistent telomeric DNA damage responsible for nuclear morphology change in mammalian cells. PLoS ONE. 2014;9(10): e110963. https://doi.org/10.1371/journal.pone.0110963 .
Kim JH, Kim HK, Ko JH, Bang H, Lee DC. The relationship between leukocyte mitochondrial DNA copy number and telomere length in community-dwelling elderly women. PLoS ONE. 2013;8(6): e67227. https://doi.org/10.1371/journal.pone.0067227 .
Tyrka AR, Carpenter LL, Kao HT, Porton B, Philip NS, Ridout SJ, et al. Association of telomere length and mitochondrial DNA copy number in a community sample of healthy adults. Exp Gerontol. 2015;1(66):17–20.
Qiu C, Enquobahrie DA, Gelaye B, Hevner K, Williams MA. The association between leukocyte telomere length and mitochondrial DNA copy number in pregnant women: a pilot study. Clin Lab. 2015;61(3–4):363–9.
CAS PubMed Google Scholar
Jung SJ, Cho JH, Park WJ, Heo YR, Lee JH. Telomere length is correlated with mitochondrial DNA copy number in intestinal, but not diffuse, gastric cancer. Oncol Lett. 2017;14(1):925.
Park WJ, Lee JH. Positive correlation between telomere length and mitochondrial copy number in breast cancers. Ann Transl Med. 2019;7(8):23–23.
Lee HC, Yin PH, Lu CY, Chi CW, Wei YH. Increase of mitochondria and mitochondrial DNA in response to oxidative stress in human cells. Biochem J. 2000;348(Pt 2):425.
Liu CS, Tsai CS, Kuo CL, Chen HW, Lii CK, Ma YS, et al. Oxidative stress-related alteration of the copy number of mitochondrial DNA in human leukocytes. Free Radic Res. 2003;37(12):1307–17.
Shen M, Zhang L, Bonner MR, Liu CS, Li G, Vermeulen R, et al. Association between mitochondrial DNA copy number, blood cell counts, and occupational benzene exposure. Environ Mol Mutagen. 2008;49(6):453.
Zheng J, Ninghua C, Zhang S, Xuebin W, Ming L. Leukocyte mitochondrial DNA copy number and risk of thyroid cancer: a two-stage case-control study. Front Endocrinol. 2019;10:421.
Hu J, Hwang SS, Liesa M, Gan B, Sahin E, Jaskelioff M, et al. Antitelomerase therapy provokes ALT and mitochondrial adaptive mechanisms in cancer. Cell. 2012;148(4):651–63.
Yohanis M, Tiro E, Irianta T. Women in the rural areas experience more severe menopause symptoms. Indones J Obstet Gynecol. 2013;37(2):86–91.
Sepduwiana H. Usia Menopaouse pada Wanita di Wilayah Kerja Puskesmas Rambah Kabupaten Rokan Hulu. J Martenity Neonatal. 2017;4(1):145–53.
Dewi V. Association of socio-economic and demographic factors with Indonesian women’s premature menopause: analysis of the demographic and health surveys program (DHS) of 2017. Stud Ethno-Med. 2021;20:15.
Santos JM, Tewari S, Kowluru RA. A compensatory mechanism protects retinal mitochondria from initial insult in diabetic retinopathy. Free Radic Biol Med. 2012;53(9):1729.
Grandin N, Pereira B, Cohen C, Billard P, Dehais C, Carpentier C, et al. The level of activity of the alternative lengthening of telomeres correlates with patient age in IDH-mutant ATRX-loss-of-expression anaplastic astrocytomas. Acta Neuropathol Commun. 2019;7(1):1–3.
Solsona-Vilarrasa E, Fucho R, Torres S, Nuñez S, Nuño-Lámbarri N, Enrich C, et al. Cholesterol enrichment in liver mitochondria impairs oxidative phosphorylation and disrupts the assembly of respiratory supercomplexes. Redox Biol. 2019;24:101214.
Hou L, Joyce BT, Gao T, Liu L, Zheng Y, Penedo FJ, et al. Blood telomere length attrition and cancer development in the normative aging study cohort. EBioMedicine. 2015;2(6):591–6.
Bhattacharjee J, Jogdand S, Shinde RK, Goswami S. Assessment of oxidative stress in breast cancer patients: a hospital based study. Int J Basic Clin Pharmacol. 2018;7(5):966–70.
Nagamma T, Bhutia RD, Pokharel DR, Yadav S, Baxi J. Influence of alcohol consumption on oxidative stress and antioxidant status in cancer patients—case-control study from western Nepal. Asian Pacific J Cancer Prev. 2012;13(7):3513–7.
Nagamma T, Baxi J, Singh PP. Status of oxidative stress and antioxidant levels in smokers with breast cancer from western Nepal. Asian Pac J Cancer Prev. 2014;15(21):9467–70.
Yang RL, Shi YH, Hao G, Li W, Le GW. Increasing oxidative stress with progressive hyperlipidemia in human: relation between malondialdehyde and atherogenic index. J Clin Biochem Nutr. 2008;43(3):154.
Gupta RK, Patel AK, Kumari R, Chugh S, Shrivastav C, Mehra S, et al. Interactions between oxidative stress, lipid profile and antioxidants in breast cancer: a case control study. Asian Pac J Cancer Prev. 2012;13(12):6295–8.
Movahed ZG, Rastegari-Pouyani M, Hossein Mohammadi M, Mansouri K. Cancer cells change their glucose metabolism to overcome increased ROS: One step from cancer cell to cancer stem cell? Biomed Pharmacother. 2019;112:108690.
Download references
Acknowledgements
We would like to express our gratitude to all the patients who participated in the study. We also thank PT. Prodia Widyahusada Tbk. for their support of this study.
This study was supported by a block grant from the Government of Republic of Indonesia through the Ministry of Research and Technology for the Eijkman Institute for Molecular Biology.
Author information
Authors and affiliations.
Master’s Programme in Biomedical Sciences, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
Prisca C. Limardi & Safarina G. Malik
Genome Diversity and Diseases Laboratory, Eijkman Institute for Molecular Biology, Jakarta, Indonesia
Prisca C. Limardi, Lidwina Priliani, Sukma Oktavianthi & Safarina G. Malik
Genome Diversity and Diseases Division, Mochtar Riady Institute for Nanotechnology, Jl. Boulevard Jenderal Sudirman 1688, Lippo Karawaci, Tangerang, Banten, 15811, Indonesia
Department of Surgical Oncology, Dr. Cipto Mangunkusumo Hospital-Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
Sonar Soni Panigoro
Department of Anatomical Pathology, Dr. Cipto Mangunkusumo Hospital-Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
Nurjati Chairani Siregar
Department of Hematology and Medical Oncology, Dharmais Hospital National Cancer Center, Jakarta, Indonesia
Noorwati Sutandyo
Department of Nutrition, Dr. Cipto Mangunkusumo Hospital-Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
Fiastuti Witjaksono
You can also search for this author in PubMed Google Scholar
Contributions
SSP, NCS, NS, FW, and SGM conceived and designed the study. PCL performed the experiments. PCL, LP, SO, SGM performed the data analysis, wrote, and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Safarina G. Malik .
Ethics declarations
Ethics approval and consent to participate.
The study was conducted in 2019 to 2020 with written informed consent. Ethical approval was obtained from the Ethical Committee of Health Research of the Faculty of Medicine, Universitas Indonesia, Rumah Sakit Cipto Mangunkusumo, Jakarta, Indonesia (450/UN2. F1/ETIK/2018). All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
Figure S1. Flow diagram of the healthy subjects and breast cancer (BC) patients' enrolment Table S1. Comparison of mtDNA-CN and RTL between extraction methods Table S2. List of primer pairs Table S3. Characteristics of study participants Figure S2. Univariate comparison of peripheral blood mtDNA-CN and RTL between healthy subjects and breast cancer patients Figure S3. Univariate comparison of peripheral blood mtDNA-CN and RTL between under and above 48 years subgroup in healthy subjects and breast cancer patients.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Limardi, P.C., Panigoro, S.S., Siregar, N.C. et al. Higher peripheral blood mitochondrial DNA copy number and relative telomere length in under 48 years Indonesian breast cancer patients. BMC Res Notes 17 , 120 (2024). https://doi.org/10.1186/s13104-024-06783-y
Download citation
Received : 06 November 2023
Accepted : 18 April 2024
Published : 28 April 2024
DOI : https://doi.org/10.1186/s13104-024-06783-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast cancer
- Oxidative stress
BMC Research Notes
ISSN: 1756-0500
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Variants in structural cardiac genes in patients with cancer therapy-related cardiac dysfunction after anthracycline chemotherapy: a case control study
Affiliations.
- 1 Research Group Cardiovascular Diseases, GENCOR, University of Antwerp, Antwerp, Belgium. [email protected].
- 2 Department of Cardiology, Antwerp University Hospital, Antwerp, Belgium. [email protected].
- 3 Centrum of Medical Genetics, GENCOR, Antwerp University Hospital and University of Antwerp, Antwerp, Belgium.
- 4 Department of Cardiology, Antwerp University Hospital, Antwerp, Belgium.
- 5 Research Group Cardiovascular Diseases, GENCOR, University of Antwerp, Antwerp, Belgium.
- PMID: 38689299
- PMCID: PMC11059765
- DOI: 10.1186/s40959-024-00231-3
Background: Variants in cardiomyopathy genes have been identified in patients with cancer therapy-related cardiac dysfunction (CTRCD), suggesting a genetic predisposition for the development of CTRCD. The diagnostic yield of genetic testing in a CTRCD population compared to a cardiomyopathy patient cohort is not yet known and information on which genes should be assessed in this population is lacking.
Methods: We retrospectively included 46 cancer patients with a history of anthracycline induced CTRCD (defined as a decrease in left ventricular ejection fraction (LVEF) to < 50% and a ≥ 10% reduction from baseline by echocardiography). Genetic testing was performed for 59 established cardiomyopathy genes. Only variants of uncertain significance and (likely) pathogenic variants were included. Diagnostic yield of genetic testing was compared with a matched cohort of patients with dilated cardiomyopathy (DCM, n = 46) and a matched cohort of patients without cardiac disease (n = 111).
Results: Average LVEF at time of CTRCD diagnosis was 30.1 ± 11.0%. Patients were 52.9 ± 14.6 years old at time of diagnosis and 30 (65.2%) were female. Most patients were treated for breast cancer or lymphoma, with a median doxorubicin equivalent dose of 300 mg/m 2 [112.5-540.0]. A genetic variant, either pathogenic, likely pathogenic or of uncertain significance, was identified in 29/46 (63.0%) of patients with CTRCD, which is similar to the DCM cohort (34/46, 73.9%, p = 0.262), but significantly higher than in the negative control cohort (47/111, 39.6%, p = 0.018). Variants in TTN were the most prevalent in the CTRCD cohort (43% of all variants). All (likely) pathogenic variants identified in the CTRCD cohort were truncating variants in TTN. There were no significant differences in severity of CTRCD and in recovery rate in variant-harbouring individuals versus non-variant harbouring individuals.
Conclusions: In this case-control study, cancer patients with anthracycline-induced CTRCD have an increased burden of genetic variants in cardiomyopathy genes, similar to a DCM cohort. If validated in larger prospective studies, integration of genetic data in risk prediction models for CTRCD may guide cancer treatment. Moreover, genetic results have important clinical impact, both for the patient in the setting of precision medicine, as for the family members that will receive genetic counselling.
Keywords: Anthracyclines; Cancer therapy-related cardiac dysfunction; Cardio-oncology; Cardiogenetics; Risk stratification.
© 2024. The Author(s).
Grants and funding
- Genomia - ERC-COG-2017-771945/ERC_/European Research Council/International
- International edition
- Australia edition
- Europe edition

Deprivation linked to higher second cancer risk among England breast cancer survivors
Cambridge study finds those from poorest areas have 35% higher risk of second non-breast cancer
Female survivors of breast cancer living in the most deprived areas have a 35% higher risk of developing second, unrelated cancers, compared with those from the most affluent areas, research shows.
Breast cancer is the most commonly diagnosed cancer in the UK, with about 56,000 people being told they have it each year. Improved diagnosis and treatments mean that five-year survival rates are now 86% in England.
People who survive breast cancer have a greater likelihood of second primary (unrelated) cancer, but until now the exact risk has not been clear.
A team of researchers led by the University of Cambridge analysed NHS data from almost 600,000 patients in England and found, compared with the general female population, women who had survived breast cancer had an increased risk of developing 12 other primary cancers.
They had double the risk of developing cancer in the unaffected (contralateral) breast, an 87% higher risk of endometrial cancer, a 58% increased likelihood of myeloid leukaemia and a 25% higher risk of ovarian cancer.
The study, published in Lancet Regional Health – Europe, found that the risk of second primary cancers was higher in people living in areas of greater socioeconomic deprivation.
Compared with the most affluent, the least well-off female survivors of breast cancer had a 166% greater chance of developing lung cancer, a 78% higher risk of stomach cancer, more than 50% increased risk of bladder and oesophagus cancers, 48% higher risk of head and neck cancer and 43% increased risk of kidney cancer.
Overall, those from the most deprived areas had a 35% higher risk of a second non-breast cancer.
This may be because risk factors such as smoking, obesity and alcohol consumption are more common among more deprived groups. A 2023 study found that deprivation causes 33,000 extra cancer cases in the UK each year .
The first author, Isaac Allen, from the department of public health and primary care at the University of Cambridge, said: “This is the biggest study ever to examine second cancers after breast cancer and the first to show that women diagnosed with breast cancer in deprived regions are more likely to get second cancers. Many cancers are caused by deprivation, but more research is clearly needed to identify the specific factors driving the higher risks and how best to reduce these inequalities.”
In addition to data from more than 580,000 women, the authors examined the risk of second primary cancers for more than 3,500 male breast cancer survivors diagnosed between 1995 and 2019 using the national cancer registration dataset.
Male breast cancer survivors were 55 times more likely than the general male population to develop contralateral breast cancer, 58% more likely than the general male population to develop prostate cancer and had four times the risk of thyroid cancer, although the actual numbers of these cancers were low.
Responding to the findings, Prof Pat Price, a leading oncologist and co-founder of the Catch Up With Cancer campaign , said: “This highlights yet another instance of alarming inequalities within cancer, underscoring the urgent need for a dedicated cancer plan. Where one comes from or their socioeconomic status should not determine the chances of developing or surviving cancer.”
Dr Simon Vincent, the director of research, support and influencing at Breast Cancer Now, said while the higher risk of secondary cancer may occur due to genetic factors or the effects of initial breast cancer treatment, more research was needed into the causes of second primary cancers and how to follow up patients completing primary breast cancer treatment.
- Breast cancer
- Women's health
- Social exclusion
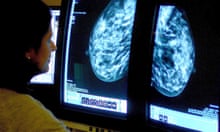
Medics design AI tool to predict side-effects in breast cancer patients

Labour MP Foy says she has breast cancer and urges others to get checked

Cost of breast cancer to UK economy could reach £3.6bn by 2034, study shows

Consumer genetic test results ‘causing unnecessary breast cancer alarm’
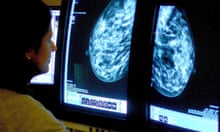
Most early-stage breast cancer patients will be long-term survivors – study
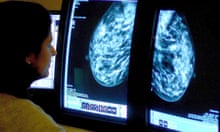
Breast cancer drug cuts risk of most common form returning by 25%
Should I worry about the cancer risk from hormonal contraceptives?
Advising older patients against breast cancer surgery is ‘age bias’, UK study finds
Drug may help more women survive hereditary breast cancer
Most viewed.
- Today's news
- Reviews and deals
- Climate change
- 2024 election
- Fall allergies
- Health news
- Mental health
- Sexual health
- Family health
- So mini ways
- Unapologetically
- Buying guides
Entertainment
- How to Watch
- My Portfolio
- Latest News
- Stock Market
- Premium News
- Biden Economy
- EV Deep Dive
- Stocks: Most Actives
- Stocks: Gainers
- Stocks: Losers
- Trending Tickers
- World Indices
- US Treasury Bonds
- Top Mutual Funds
- Highest Open Interest
- Highest Implied Volatility
- Stock Comparison
- Advanced Charts
- Currency Converter
- Basic Materials
- Communication Services
- Consumer Cyclical
- Consumer Defensive
- Financial Services
- Industrials
- Real Estate
- Mutual Funds
- Credit cards
- Balance Transfer Cards
- Cash-back Cards
- Rewards Cards
- Travel Cards
- Personal Loans
- Student Loans
- Car Insurance
- Morning Brief
- Market Domination
- Market Domination Overtime
- Opening Bid
- Stocks in Translation
- Lead This Way
- Good Buy or Goodbye?
- Fantasy football
- Pro Pick 'Em
- College Pick 'Em
- Fantasy baseball
- Fantasy hockey
- Fantasy basketball
- Download the app
- Daily fantasy
- Scores and schedules
- GameChannel
- World Baseball Classic
- Premier League
- CONCACAF League
- Champions League
- Motorsports
- Horse racing
- Newsletters
New on Yahoo
- Privacy Dashboard
Yahoo Finance
New case study highlights community oncology practices as preferred site of service over hospitals and academic centers.
FORT MYERS, Fla. , May 1, 2024 /PRNewswire/ -- In a case study released today by Florida Cancer Specialists & Research Institute, LLC , community oncology practices emerge as the preferred site of service for cancer treatment, challenging the traditional dominance of hospitals and academic centers. The summarized findings within this case study shed light on the significant advantages offered by community oncology practices in delivering high-quality care to cancer patients.
"The collective insights we reveal in this case study are poised to spark meaningful discussions among policymakers, health care stakeholders, and the general public about the future of cancer care delivery," says FCS Chief Executive Officer Nathan H. Walcker . "By amplifying the voice of community oncology practices, the study advocates for a more inclusive, patient-centric healthcare system that celebrates diversity and innovation.
The study, titled " Evaluating the Site of Service and Oncology Care Costs," delves into key factors influencing patient outcomes, cost-effectiveness, and overall satisfaction with care delivery models. Contrary to common perceptions, the findings reveal that community oncology practices outperform hospitals and academic centers in several critical areas: personalized care, convenience and accessibility, cost-effectiveness and a collaborative approach.
Community oncology practices excel in providing personalized treatment plans tailored to individual patient needs. With a patient-centric approach, these practices prioritize empathy, communication, and accessibility, fostering strong patient-provider relationships crucial for navigating the complexities of cancer care.
Patients receiving care in a community oncology setting, often located close to their homes, experience reduced travel times and related expenses and find it easier to access care. Patient proximity to care is a large contributor to improved patient adherence to treatment regimens, which directly impacts patient outcomes.
The study also underscores the cost-effectiveness of care delivery in community oncology settings. By avoiding the overhead expenses associated with large hospital systems and through close relationships with insurers, community practices offer competitive pricing without compromising the quality of care, thereby alleviating financial burdens on patients and payers alike.
Contrary to misconceptions, community oncology practices foster collaboration with academic institutions and hospitals, leveraging shared expertise and resources to optimize patient outcomes. This collaborative spirit promotes innovation, knowledge exchange, and continuous improvement in cancer care delivery.
Recognizing the fundamental role played by community oncology practices in the healthcare landscape, FCS President & Managing Physician Lucio N. Gordan , MD says, "The findings summarized in this case study challenge the conventional wisdom surrounding cancer care delivery. Community oncology practices represent a cornerstone of comprehensive cancer care, offering a holistic approach that prioritizes patient well-being and clinical excellence."
Read the case study here.
About Florida Cancer Specialists & Research Institute, LLC: (FLCancer.com)
Florida Cancer Specialists & Research Institute (FCS) offers patients access to more clinical trials than any private oncology practice in Florida . The majority of new cancer drugs recently approved for use in the U.S. were studied in clinical trials with FCS participation.* Recognized for our research, FCS is a recipient of the national Clinical Trials Participation Award presented by the American Society of Clinical Oncology (ASCO). FCS physicians, trained in prestigious medical schools and research institutes, are consistently ranked nationally as Top Doctors by U.S. News & World Report.
Celebrating its 40 th year in 2024, FCS has built a national reputation for excellence that is reflected in exceptional and compassionate patient care, driven by innovative clinical research, cutting-edge technologies and advanced treatments, including targeted therapies genomic-based treatment and immunotherapy. Our highest values are embodied by our outstanding team of highly trained and dedicated physicians, clinicians and staff.
*Prior to approval
View original content to download multimedia: https://www.prnewswire.com/news-releases/new-case-study-highlights-community-oncology-practices-as-preferred-site-of-service-over-hospitals-and-academic-centers-302133211.html
SOURCE Florida Cancer Specialists & Research Institute
- Open access
- Published: 03 May 2024
Does depressurization of the portal vein before liver transplantation affect the recurrence of HCC? A nested case-control study
- Guo Wei 1 ,
- Yong Zhao 1 ,
- Shifeng Feng 1 ,
- Jingsheng Yuan 3 ,
- Gang Xu 3 ,
- Jian Yang 2 ,
- Lingxiang Kong 2 , 3 &
- Jiayin Yang 2
BMC Cancer volume 24 , Article number: 558 ( 2024 ) Cite this article
Metrics details
Portal hypertension (PHT) has been proven to be closely related to the development of hepatocellular carcinoma (HCC). Whether PHT before liver transplantation (LT) will affect the recurrence of HCC is not clear.
110 patients with depressurization of the portal vein (DPV) operations (Transjugular Intrahepatic Portosystemic Shunt—TIPS, surgical portosystemic shunt or/and splenectomy) before LT from a HCC LT cohort, matched with 330 preoperative non-DPV patients; this constituted a nested case-control study. Subgroup analysis was based on the order of DPV before or after the occurrence of HCC.
The incidence of acute kidney injury and intra-abdominal bleeding after LT in the DPV group was significantly higher than that in non-DPV group. The 5-year survival rates in the DPV and non-DPV group were 83.4% and 82.7% respectively ( P = 0.930). In subgroup analysis, patients in the DPV prior to HCC subgroup may have a lower recurrence rate (4.7% vs.16.8%, P = 0.045) and a higher tumor free survival rate (88.9% vs.74.4%, P = 0.044) after LT under the up-to-date TNMI–II stage, while in TNM III stage, there was no difference for DPV prior to HCC subgroup compared with the DPV after HCC subgroup or the non-DPV group.
Compared with DPV after HCC, DPV treatment before HCC can reduce the recurrence rate of HCC after early transplantation (TNM I-II). DPV before LT can reduce the recurrence of early HCC.
Peer Review reports
Introduction
At present, liver transplantation (LT) is still the best choice for hepatocellular carcinoma (HCC) patients with liver cirrhosis with a better long-term survival rate and lower recurrence rate of HCC [ 1 , 2 ]. Gastrointestinal bleeding and refractory ascites caused by portal hypertension (PHT) are the most common complications that occur during the transplantation waiting period [ 3 , 4 , 5 ]. Once gastrointestinal hemorrhage occurs, the mortality in Child-Pugh C patients is as high as 40% within the first 6 weeks of bleeding [ 6 ]. Depressurization of the portal vein (DPV), not only controls gastrointestinal bleeding, but also reduces ascites relieving abdominal discomfort, and significantly reduces hepatorenal syndrome caused by replenishing insufficient volume [ 3 , 7 , 8 , 9 ]. DPV practices and preferences vary throughout the world, the main operative methods for DPV include splenectomy and portosystemic shunt and particular radiological DPV. DPV has become a frequently-used bridging therapy for LT [ 10 , 11 , 12 , 13 , 14 ].
Cirrhosis is the most significant independent risk factor for HCC [ 15 , 16 ]. Although PHT is mainly caused by cirrhosis, it has also been shown to be a risk factor of HCC independent of cirrhosis [ 17 ]. In addition, HCC recurrence may be related to PHT in HCC radiofrequency ablation treatment [ 18 ]. According to the theory of soil and seeds [ 19 ], LT is different from other surgical treatments that remove seeds (HCC). LT completely removes the soil (sick liver) and seeds together, in theory, eliminating the possibility of recurrence. However, 5-year recurrence rates are still 7.8%, and can even reach up to 40% in HCC LT beyond the Milan standard [ 20 ]. A 2018 retrospective analysis by Mazzaferro et al. found that the 5-year cumulative mortality rate related to the recurrence of HCC after LT was 8.1%, accounting for 1/3 of the total death of the LT recipients [ 21 ]. Therefore, HCC recurrence following LT is still an important research area. Based on the evidence that PHT is closely related to HCC; the purpose of this study was to observe whether reducing the portal pressure limits the recurrence of HCC after LT.
This work has been reported in line with the STROCSS criteria [ 22 ]. The unique identifying number of this retrospective research is ChiCTR2000032141(date of first registration 20/04/2020, http://www.chictr.org.cn/showproj.aspx?proj=52598 ). Sichuan University West China Hospital and the Public Health Clinical Center of Chengdu Hospital are cooperative hospitals. The Public Health Clinical Center of Chengdu provides initial outpatient and follow-up services for some liver disease patients, but all surgeries are completed by West China Hospital. The two closely cooperate to form a medical whole, so patients are not distinguished.
Inclusion and exclusion criteria
The inclusion criteria of the open cohort were as follows: HCC patients 18 years or older without invasion of the main branch of the portal vein or the hepatic vein; HCC that did not directly invade the adjacent organs (except the gallbladder) or penetrate the peritoneum; There was no extrahepatic metastasis (TNM ≤ III A). The patients all had liver cirrhosis and portal hypertension. The diameter of portal vein was measured by ultrasound to determine whether the diameter was increased and to detect liver cirrhosis. Exclusion criteria included retransplantation, multiple organ transplantation, domino LT, double donor LT, or treatment with mTOR inhibitors (e.g., Everolimus/Rapamune) after transplantation. LT grafts were donated by patients who suffered cardiac death ( n = 494) or brain death ( n = 298). All the HCC were examined by pathology after undergoing a DPV operation. All included tumors were simple HCC, other tumor types such as cholangiocarcinoma or other special types of liver tumors were excluded. Patients with portal hypertension related complications, such as severe gastrointestinal varices, were informed of the risk of gastrointestinal bleeding and voluntarily decided to undergo DPV treatment.
Diagnostic criteria and follow up for HCC LT
TNM classification of HCC was conducted following the American Joint Committee of Cancer (AJCC) AJCC Cancer Staging Manual 8th edition in 2017 ( [ 23 ]). Acute renal injury (AKI) was assessed using the 2015 edition of the International Club of Ascites: the increase of sCr in 48 h was ≥ 0.3 mg/dl (26.5 μmol/L); or the increase of sCr in 48 h was ≥ 1.5 times of baseline value; or the urine volume lasted for 6 h < 0.5 ml·kg − 1 ·H− 1 [ 24 , 25 , 26 ]. Follow-up in the outpatient clinic was conducted routinely. Measurements of alpha fetoprotein and hepatitis B virus deoxyribonucleic acid (DNA), and abdominal ultrasonography were done every 3 months, and a CT scan was performed every 6 months. All hepatitis B virus DNA-positive patients were treated with anti-viral therapy before and after surgery. When intrahepatic recurrence was difficult to ascertain, MRI or contrast-enhanced ultrasonography was performed. Tumor recurrence was determined mainly based on radiographic evidence and/or AFP level. Patients who showed tumor recurrence after surgery were treated with the following options: resection, radio frequency ablation, re-LT, transcatheter arterial chemoembolization, or sorafenib. Patients were monitored until October 2019 or until their death, and their medical records were retrospectively reviewed. Our center requires standard treatment guidelines be followed for HCC patients with elevated HCV RNA. Patients are treated with direct-acting antiviral drugs (DAAS) while waiting for transplantation. If HCV recurrence occurs after transplantation, DAAS should be performed as soon as possible. Additionally, the use of glucocorticoids should be withdrawn as soon as possible after LT, and calcineurin inhibitor maintenance therapy (e.g., tacrolimus) should be minimized. No patients included in this study received any downstage therapy prior to LT. Downstaging refers to methods of treating HCC, such as radiation therapy, chemotherapy, and molecular targeted therapy. Patients with PHT are diagnosed through a comprehensive assessment, which includes a medical history review of liver disease, radiological findings indicative of liver fibrosis or sclerosis, endoscopic identification of gastric varices, and portal vein color Doppler ultrasound revealing portal vein dilatation or collateral circulation formation.
Case-control study composition
A total of 792 HCC patients underwent their first LT between September 2007 and January 2022. From these patients, we conducted a propensity score matching (PSM) analysis of 114 patients who underwent DPV (TIPS, surgical portosystemic shunt or/and splenectomy) before LT (32 Transjugular Intrahepatic Portosystemic Shunt-TIPS, 82 surgical portosystemic shunt or/and splenectomy), and 342 matched non-DPV control patients based on their PSM score on the day of LT but prior to surgery (Fig. S1 ).
Demographic and disease characteristics
Baseline characteristics of the DPV and non-DPV groups, matched prior to surgery on the day of LT, are shown in Table 1 . There was no significant difference between the DPV and non-DPV groups with respect to disease or population characteristics before transplantation.
Intraoperative and postoperative outcomes between the DPV and non-DPV groups
The postoperative complication data for the DPV and non-DPV groups are shown in Fig. 1 A. Intra-abdominal bleeding, incidence of postoperative AKI, and Clavien–Dindo Grade III–V complications were significantly higher in the DPV group than those in the non-DPV group. There was no significant difference in cumulative survival rate between the DPV and non-DPV groups within the different TNM stages (Fig. 2 ).
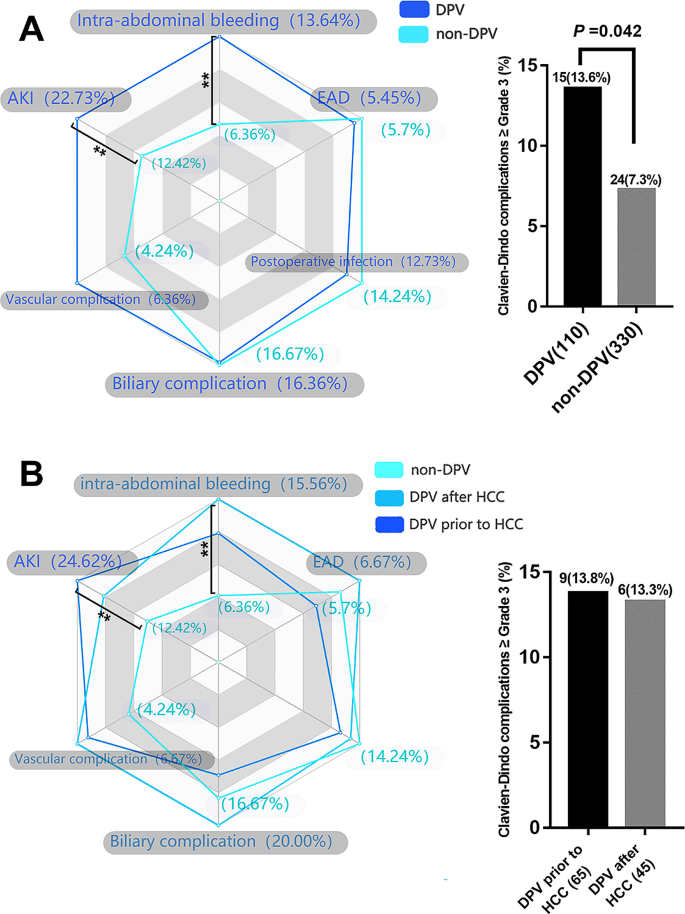
Analysis of main postoperative complications by radar chart and histogram. ** P < 0.05 ( A ) The incidence of AKI (22.7% vs. 12.4, P = 0.011) and intra-abdominal bleeding (13.6% vs. 6.4%, P = 0.012) in DPV group was significantly higher than that in non-DPV group, while the incidence of Clavien–Dindo III-V complication in DPV group was higher than that in non-DPV group. ( B ) In DPV subgroup comparison (DPV prior to HCC and DPV after HCC), there was no significant difference between the two subgroups in the incidence of specific major complications and the incidence of Clavien–Dindo III-V overall complications
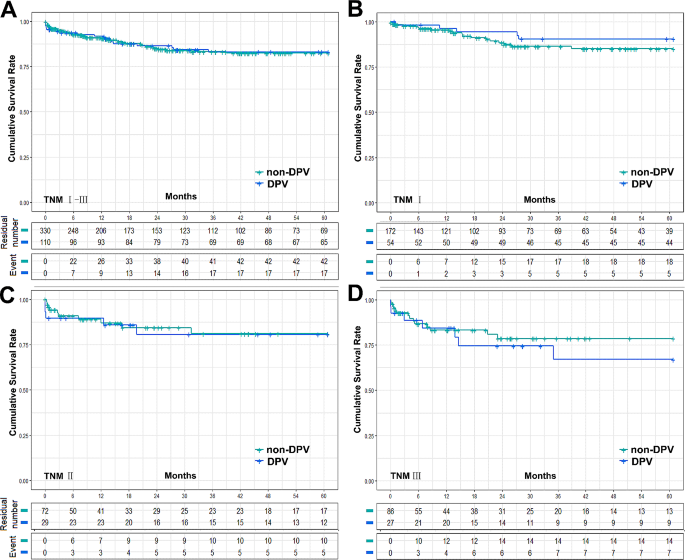
Cumulative survival rate between DPV and non-DPV group. ( A ) The 5-year survival rates of DPV ( n = 110) and non-DPV ( n = 330) group were 83.0% and 82.4% respectively, log-rank P = 0.934, and the 5-year median survival times were 52.52 ± 1.87 (95% Cl 48.85 to 56.19) and 52.30 ± 1.21 (95% Cl 49.93 to 54.68), respectively. ( B ) In HCC TNMIpatients, the 5-year survival rates of DPV ( n = 54) and non-DPV ( n = 172) were 90.4% and 85.1% respectively, log-rank P = 0.342, and the 5-year median survival times were 56.55 ± 1.87 (95% Cl 52.89 to 60.22) and 54.18 ± 1.47 (95% Cl 51.31 to 57.05), respectively. ( C ) In HCC TNMIIpatients, The 5-year survival rates of DPV ( n = 29) and non DPV ( n = 72) group were 80.7% and 81.0% respectively, log-rank P = 0.862, and the 5-year median survival times were 50.59 ± 4.15 (95% Cl 42.45 to 58.73) and 51.28 ± 2.77 (95% Cl 45.85 to 56.72), respectively. ( D ) In HCC TNM III patients, The 5-year survival rates of DPV ( n = 27) and non DPV ( n = 86) group were 67.0% and 78.5% respectively, log-rank P = 0.483, and the 5-year median survival times were 45.17 ± 5.00 (95% Cl 35.38 to 54.97) and 49.37 ± 2.77 (95% Cl 43.95 to 54.79), respectively
HCC and DPV subgroups
DPV patients were divided into two subgroups according to the sequence of their DPV treatment: DPV prior to HCC ( n = 65) and DPV after HCC ( n = 45). Subgroup analysis found no significant differences between these groups in postoperative complications (Fig. 1 B). However, patients with TNM I–II HCC had a significantly better cumulative recurrence rate and tumor-free survival rate when undergoing DPV prior to HCC compared to the non-DPV group. However, there was no significant difference between the subgroup that received DPV after HCC and the non-DPV group. In TNM III HCC patients, there was no significant difference in tumor recurrence rate or tumor-free survival rate between the non-DPV group and either DPV subgroup (Fig. 3 ).
Comparison of cumulative recurrence rate and tumor free survival rate between two DPV subgroups (DPV prior to HCC ( n = 65) or DPV after HCC ( n = 45)) and non-DPV ( n = 330) group after LT. (A1-A2) The 5-year cumulative recurrence rate and tumor free survival rate of DPV after HCC subgroup and non-DPV group were 16.4% vs. 18.7%, log-rank P = 0.928, 75.3% vs. 73.0% log-rank P = 0.979, respectively. The 5-year cumulative recurrence rate and tumor free survival rate of DPV prior to HCC subgroup and non-DPV group were 9.0% vs. 18.7%, log-rank P = 0.098, 82.5% vs. 73.0%, log-rank P = 0.134. (B1-B2) In HCC TNM I-II patients, DPV prior to HCC subgroup were significantly better than non-DPV group in 5-year recurrence rate and tumor free survival rate (4.7% vs. 17.3%, log-rank P = 0.039 and 88.9% vs. 73.8%, log-rank P = 0.037). There was no significant difference between DPV after HCC subgroup and non-DPV group (13.2% vs. 17.3%, log-rank P = 0.619, 79.1% vs. 73.8%, log-rank P = 0.671). (C1-C2) The 5-year cumulative recurrence rate and tumor free survival rate of DPV prior to HCC, DPV after HCC and non-DPV were 25.0%, 27.3%, 22.8%, and 58.0%, 66.7%, 71.6%, respectively. There was no significant difference between groups (log-rank P > 0.05)
At present, HCC recurrence remains the main problem affecting the LT prognosis ( [ 27 ]). Although PHT has been shown to be closely associated with the development of HCC ( [ 28 ]) and to significantly affect prognosis following other non-LT radical treatments for HCC ( [ 18 , 29 , 30 ]), whether preoperative DPV can affect the prognosis of LT for HCC has not been reported. Our main finding is that patients who underwent DPV prior to HCC had a lower cumulative recurrence rate and higher tumor-free survival rate after LT in the AJCC TNM I–II stages, while in TNM III stage, DPV prior to HCC resulted in no difference between DPV after HCC or non-DPV patients.
The AJCC levels of evidence were established by the AJCC 8th Edition cancer staging system. Most of the confirmed risk factors related to the prognosis of LT for HCC were controlled in the latest TNM stage ( [ 31 , 32 ]). It is possible that there are relevant HCC risk factors that have not yet been reported due to the uncontrolled nature of even multiple-center studies and the difficulty of performing epidemiological studies on HCC ( [ 33 ]). One of the potential limitations of this study is the fact that DPV consists of three different procedures. Although there is no theoretical and clinical evidence suggesting that surgical portosystemic shunt and/or splenectomy will affect HCC recurrence after LT or other radical treatment, TIPS may increase the risk of metastasis caused by intrahepatic shunt through HCC ( [ 34 , 35 ]). If TIPS after HCC could lead to HCC extrahepatic metastasis, there would be a selection bias in our study. Since LT is not recommended for patients with extrahepatic metastasis, patients who developed extrahepatic metastasis due to TIPS after HCC will be excluded from LT. Wallace et al. reported that 2 of 9 patients developed lung metastases from TIPS crossing through a hepatic malignancy ( [ 35 ]). However, Bettinger et al. ( [ 36 ]). reported no metastasis was observed in 40 patients with centrally located tumors in the liver (segment VIII, V, and IV). A recent case-control study observing 217 patients found that TIPS is safe for PHT in patients with HCC ( [ 37 ]). Although we were unable to confirm that DPV after HCC does not increase the risk of HCC metastasis after transplantation, our results showed there is no increase in HCC recurrence or metastasis in patients who met the criteria for LT and underwent DPV after HCC. Therefore, early LT (before metastasis) for patients undergoing DPV after HCC may prevent this possibility of postoperative potential HCC metastasis.
As early as 1985, Bjørneboe et al. demonstrated that portal-systemic shunt may increase the risk of primary HCC in cirrhosis of the liver based on an observational cohort study of 201 people ( [ 38 ]). Banares et al. observed that TIPS may lead to an increase in 5-year cumulative incidence of HCC to 34%, significantly higher than the 25% observed in the control group ( [ 33 ]). However, this view has not been unanimously accepted ( [ 39 ]). A series of related studies shows that DPV may cause nodular regenerative hyperplasia, but it does not increase the incidence of HCC ( [ 40 , 41 , 42 ]) and a recent meta-analysis also found that the incidence of HCC was not increased ( [ 43 ]). Moreover, in contrast with other radical treatments, multiple HCC in the liver is not an absolute contraindication for LT. At present, there is still a lack of relevant research reports on this topic. In our study we found no difference between the DPV prior to HCC subgroup and the non-DPV group in the recurrence rate of HCC.
Although we did not observe a difference between patients who received DPV prior to HCC and non-DPV patients in the overall cohort, subgroup analysis highlighted differences between the different tumor TNM stages. Our results found that patients who met the criteria for AJCC TNMI–II stage who underwent DPV prior to HCC had a lower recurrence rate after LT, while patients in TNM III stage, showed no difference between DPV after HCC and non-DPV patients. Relatively low portal pressure may have a protective effect in early HCC patients, but when HCC develops past a certain point this may no longer be effective and this threshold is likely to be between TNM II and III stages. According to the theory by Tanaka et al., portal vein pressure is positively correlated with the pressure of both liver tissue and HCC, and the greater the pressure difference between the tumor and the adjacent tissue, the more likely the tumor is to invade tissue with relatively low pressure ( [ 44 ]). Therefore, it is possible that reducing the pressure of the portal vein simultaneously reduces the pressure of the liver tissue. HCC occurs in the liver tissue with relatively normal pressure compared to PHT, which makes the pressure in HCC also relatively low. Therefore, HCC with lower internal pressure is less likely spread into the adjacent tissue. However, with the development of HCC, and an increase in HCC pressure, the pressure difference between the tumor and surrounding tissue begins to be significant. In this scenario, the relatively low pressure of the liver tissue would no longer play a protective role but instead may allow HCC to reach the hepatic vein and enter the circulation easily, eventually leading to an increased risk of recurrence. However, it is worth noting that this interpretation is only a reasonable deduction based on our results and the theory suggested by Tanaka et al. and there is still a lack of direct evidence to support this.
PHT results from elevated intrahepatic vascular resistance due to sinusoidal alterations and liver fibrosis. This condition is compounded by the opening of portosystemic collateral vessels and the formation of new vessels, driven by vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) ( [ 45 ]). HCC, a common complication of cirrhosis, shares similar underlying factors with PHT, increasing the complexity of liver pathology. Inhibition of VEGF receptor 2 signaling has shown potential in reducing collateral vessel formation in experimental models ( [ 46 ]). VEGF plays a crucial role in neovascularization and fibrosis within the liver parenchyma, involving hepatocytes and hepatic stellate cells (HSCs). Activated HSCs, stimulated by VEGF, contribute to fibrosis, while portal myofibroblasts aid angiogenesis through collagen production. Liver stiffness influences sinusoidal angiogenesis and fibrosis progression. The resultant intrahepatic neovessels Increased hypoxia, inflammatory cytokines (IL-6, IL-1α), growth factors (epidermal growth factor, transforming growth factor-α and -β, fibroblast growth factor, PDGF) ( [ 46 , 47 ]). Anti-VEGF therapies like Sorafenib offer promise in mitigating PHT ( [ 48 ]). Chronic inflammation and angiogenesis triggered by PHT play a significant role in hepatocarcinogenesis, implying that early intervention targeting angiogenesis in liver disease could hold therapeutic promise.
Since DPV prior to HCC followed by LT can significantly reduce the recurrence rate in the early stage of the tumor, we wondered whether DPV might be applicable to all patients with PHT. However, we are still unsure if early DPV will increase the incidence of HCC and the postoperative complications of DPV are still worthy of consideration. Portal vein thrombosis (PVT) induced by DPV can progress to cavernous transformation, escalating the challenges of LT and amplifying the likelihood of intraoperative and short-term postoperative complications ( [ 49 ]). Vigilance is paramount when employing Tips, ensuring they do not penetrate the inferior vena cava excessively, particularly into the right atrium. Misplaced Tips can precipitate severe bleeding and jeopardize patient survival post-LT. Nevertheless, conventional pre-transplant TIPS placement primarily correlates with heightened portal flow gradient and does not significantly impede subsequent LT outcomes ( [ 50 ]). . Our study shows that DPV patients had a higher incidence of complications including AKI and postoperative blood loss in HCC stages III–V than non-DPV patients in all TNM stages, but there was no significant difference in the long-term survival rate. At present, there have been few reports about the complications of DPV after LT. Tripathi et al. found that patients who underwent LT after TIPS had higher dialysis rates and the long post-operative hospital stages, but that the overall survival rate is not affected ( [ 51 ]). Other associated reports did not focus on postoperative complications, but they all reported that there was still no significant difference in survival between their DPV and non DPV group ( [ 52 ]). Therefore, DPV is still a safe and feasible bridging treatment for LT, but it may be accompanied by relatively high complications after LT. It can also be reasonably inferred that the corresponding treatment costs will be increased. Altogether, we believe that it is still necessary to be cautious before deeming DPV applicable to all patients with PHT, especially for those without gastrointestinal hemorrhage or refractory ascites caused by PHT.
The retrospective nature of our work should be acknowledged as a key limitation, even when using a case-control study design based on PSM. However, PSM may lead to other biases due to unmeasured patient characteristics which may have influenced outcomes. Furthermore, because it is difficult to measure, we were unable to directly measure the portal vein pressure. We could only infer a reduction in portal vein pressure through demonstrated methods such as TIPS and obvious postoperative improvement in symptoms. However, we do not know the relationship between the degree of reduction and our results. At present, noninvasive methods of detecting portal vein pressure are indirect and cannot directly reflect the accurate pressure value. Therefore, a technical breakthrough that is able to provide a more in-depth demonstration of the relationship between portal pressure and tumor recurrence is necessary. In addition, our data source was a single center which limits the study’s scope. In HCC TNM III patients, there were only 14 cases of DPV prior to HCC and 12 cases of DPV after HCC. Further multi-center studies are required to verify these findings.
Based on our results, we propose that non-HCC patients with current DPV indications (such as patients with severe gastrointestinal bleeding and irreversible ascites) should be treated more actively with DPV. If HCC occurred in such patients and LT performed early (TNM I–II), such patients may have relatively lower recurrence rates than patients in non-DPV or DPV after HCC patients. DPV before LT can reduce the recurrence of early HCC.
Data availability
All related data of our center are stored in the Chinese Liver Transplant Registry, a platform for unified management of LT centers in mainland China (CLTR: http://cltr.cotr.cn ). The data that support the findings of this study are available from the Chinese Liver Transplant Registry (CLTR: http://cltr.cotr.cn ), but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. But all related data in this study are available from the corresponding author on reasonable request.
Mehta N, Bhangui P, Yao FY, Mazzaferro V, Toso C, Akamatsu N, et al. Liver transplantation for hepatocellular carcinoma. Working Group Report from the ILTS Transplant Oncology Consensus Conference. Transplantation. 2020;104(6):1136–42.
Article PubMed Google Scholar
de’Angelis N, Landi F, Carra MC, Azoulay D. Managements of recurrent hepatocellular carcinoma after liver transplantation: a systematic review. World J Gastroenterol. 2015;21(39):11185–98.
Article PubMed PubMed Central Google Scholar
Lee EW, Shahrouki P, Alanis L, Ding P, Kee ST. Management options for gastric variceal hemorrhage. JAMA Surg. 2019;154(6):540–8.
Rajwani K, Fortune BE, Brown RS, Jr. Critical care management of gastrointestinal bleeding and ascites in liver failure. Semin Respir Crit Care Med. 2018;39(5):566–77.
Yoshiji H, Nagoshi S, Akahane T, Asaoka Y, Ueno Y, Ogawa K, et al. Evidence-based clinical practice guidelines for liver cirrhosis 2020. J Gastroenterol. 2021;56(7):593–619.
Cremers I, Ribeiro S. Management of variceal and nonvariceal upper gastrointestinal bleeding in patients with cirrhosis. Th Adv Gastroenterol. 2014;7(5):206–16.
Article Google Scholar
Wong F, Sniderman K, Liu P, Blendis L. The mechanism of the initial natriuresis after transjugular intrahepatic portosystemic shunt. Gastroenterology. 1997;112(3):899–907.
Article CAS PubMed Google Scholar
Stanley AJ, Laine L. Management of acute upper gastrointestinal bleeding. BMJ. 2019;364:l536.
Lesmana CRA, Raharjo M, Gani RA. Managing liver cirrhotic complications: overview of esophageal and gastric varices. Clin Mol Hepatol. 2020;26(4):444–60.
Sellers CM, Nezami N, Schilsky ML, Kim HS. Transjugular intrahepatic portosystemic shunt as a bridge to liver transplant: current state and future directions. Transplantation Reviews (Orlando Fla). 2019;33(2):64–71.
Kong L, Li M, Li L, Jiang L, Yang J, Yan L. Splenectomy before adult liver transplantation: a retrospective study. BMC Surg. 2017;17(1):44.
de Ville J, D’Ambrosio G, Grimaldi C. Surgical management of portal hypertension in children. Semin Pediatr Surg. 2012;21(3):219–32.
Bai D-S, Zhou B-H, Qian J-J, Zhang C, Jin S-J, Jiang G-Q. Effects of laparoscopic splenectomy and azygoportal disconnection on liver synthesis function and cirrhosis: a 2-year prospective study. Surg Endosc. 2019. https://doi.org/10.1007/s00464-019-7307-7
Yoshizumi T, Itoh S, Shimokawa M, Inokuchi S, Harada N, Takeishi K, et al. Simultaneous splenectomy improves outcomes after adult living donor liver transplantation. J Hepatol. 2021;74(2):372–9.
Singal AG, Zhang E, Narasimman M, Rich NE, Waljee AK, Hoshida Y, et al. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: a meta-analysis. J Hepatol. 2022;77(1):128–39.
Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(5 Suppl 1):S35–50.
Ripoll C, Groszmann RJ, Garcia-Tsao G, Bosch J, Grace N, Burroughs A, et al. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J Hepatol. 2009;50(5):923–8.
Kim R, Jeong WK, Kang TW, Song KD, Lee MW, Ahn SH et al. Intrahepatic distant recurrence after radiofrequency ablation of hepatocellular carcinoma: relationship with portal hypertension. Acta radiologica (Stockholm, Sweden: 1987). 2019;60(12):1609-18.
Mancuso A, Perricone G. Hepatocellular carcinoma and liver transplantation: state of the art. J Clin Translational Hepatol. 2014;2(3):176–81.
Google Scholar
Gundlach JP, Ellrichmann M, van Rosmalen M, Vogelaar S, Eimer C, Rheinbay C, et al. Liver transplantation for HCC in cirrhosis: are Milan criteria outdated? Z Gastroenterol. 2024;62(1):43–9.
Article CAS PubMed PubMed Central Google Scholar
Mazzaferro V, Sposito C, Zhou J, Pinna AD, De Carlis L, Fan J, et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology. 2018;154(1):128–39.
Agha R, Abdall-Razak A, Crossley E, Dowlut N, Iosifidis C, Mathew G. STROCSS 2019 guideline: strengthening the reporting of cohort studies in surgery. Int J Surg. 2019;72:156–65.
Amin MBGD. AJCC cancer staging manual. 8th ed. New York, NY: Springer; 2017.
Angeli P, Gines P, Wong F, Bernardi M, Boyer TD, Gerbes A, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the international club of ascites. J Hepatol. 2015;62(4):968–74.
Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31.
Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1). Crit Care. 2013;17(1):204.
Tabrizian P, Holzner ML, Mehta N, Halazun K, Agopian VG, Yao F, et al. Ten-year outcomes of liver transplant and downstaging for hepatocellular carcinoma. JAMA Surg. 2022;157(9):779–88.
PubMed PubMed Central Google Scholar
Thabut D, Kudo M. Treatment of portal hypertension in patients with HCC in the era of Baveno VII. J Hepatol. 2023;78(3):658–62.
Jung KS, Kim JH, Kim SU, Song K, Kim BK, Park JY, et al. Liver stiffness value-based risk estimation of late recurrence after curative resection of hepatocellular carcinoma: development and validation of a predictive model. PLoS ONE. 2014;9(6):e99167–e.
Berzigotti A, Reig M, Abraldes JG, Bosch J, Bruix J. Portal hypertension and the outcome of surgery for hepatocellular carcinoma in compensated cirrhosis: a systematic review and meta-analysis. Hepatology (Baltimore MD). 2015;61(2):526–36.
Liu Z, Liu Y, Zhang W, Hong Y, Meng J, Wang J, et al. Deep learning for prediction of hepatocellular carcinoma recurrence after resection or liver transplantation: a discovery and validation study. Hepatol Int. 2022;16(3):577–89.
Vauthey J-N, Ribero D, Abdalla EK, Jonas S, Bharat A, Schumacher G, et al. Outcomes of liver transplantation in 490 patients with hepatocellular carcinoma: validation of a uniform staging after surgical treatment. J Am Coll Surg. 2007;204(5):1016–28.
Bañares R, Núñez O, Escudero M, Fernández C, Vaquero J, Beceiro I, et al. Patients with cirrhosis and bare-stent TIPS may have increased risk of hepatocellular carcinoma. Hepatology (Baltimore MD). 2005;41(3):566–71.
Fichtl A, Seufferlein T, Zizer E. Risks and benefits of TIPS in HCC and other liver malignancies: a literature review. BMC Gastroenterol. 2023;23(1):403.
Wallace M, Swaim M. Transjugular intrahepatic portosystemic shunts through hepatic neoplasms. J Vascular Interventional Radiology: JVIR. 2003;14(4):501–7.
Bettinger D, Knüppel E, Euringer W, Spangenberg HC, Rössle M, Thimme R, et al. Efficacy and safety of transjugular intrahepatic portosystemic shunt (TIPSS) in 40 patients with hepatocellular carcinoma. Aliment Pharmacol Ther. 2015;41(1):126–36.
Luo S-H, Chu J-G, Huang H, Yao K-C. Safety and efficacy of transjugular intrahepatic portosystemic shunt combined with palliative treatment in patients with hepatocellular carcinoma. World J Clin Cases. 2019;7(13):1599–610.
Bjørneboe M, Andersen JR, Christensen U, Skinhøj P, Jensen OM. Does a portal-systemic shunt increase the risk of primary hepatic carcinoma in cirrhosis of the liver? Scand J Gastroenterol. 1985;20(1):59–64.
Libbrecht L, Maleux G, Verslype C, Nevens F, Roskams T. Influence of TIPS on development of hepatocellular carcinoma in cirrhosis. Hepatology (Baltimore MD). 2005;42(1):236–7.
Cazals-Hatem D, Vilgrain V, Genin P, Denninger M-H, Durand F, Belghiti J, et al. Arterial and portal circulation and parenchymal changes in Budd-Chiari syndrome: a study in 17 explanted livers. Hepatology (Baltimore MD). 2003;37(3):510–9.
De Santis A, Iegri C, Kondili L, Riggio O, Salvatori FM, Catalano C, et al. Hepatocellular carcinoma in cirrhotic patients with transjugular intrahepatic portosystemic shunt: a retrospective case-control study. Dig Liver Disease: Official J Italian Soc Gastroenterol Italian Association Study Liver. 2014;46(8):726–30.
Borentain P, Garcia S, Gregoire E, Vidal V, Ananian P, Ressiot E, et al. Transjugular intrahepatic porto-systemic shunt is a risk factor for liver dysplasia but not hepatocellular carcinoma: a retrospective study of explanted livers. Dig Liver Disease: Official J Italian Soc Gastroenterol Italian Association Study Liver. 2015;47(1):57–61.
Chen B, Pang L, Chen H-B, Wu D-B, Wang Y-H, Chen E-Q. TIPS is not associated with a higher risk of developing HCC in Cirrhotic patients: a systematic review and meta-analysis. J Clin Translational Hepatol. 2019;7(3):232–7.
Tanaka T, Yamanaka N, Oriyama T, Furukawa K, Okamoto E. Factors regulating tumor pressure in hepatocellular carcinoma and implications for tumor spread. Hepatology. 1997;26(2):283–7.
Fernandez M. Molecular pathophysiology of portal hypertension. Hepatology. 2015;61(4):1406–15.
Allaire M, Rudler M, Thabut D. Portal hypertension and hepatocellular carcinoma: Des liaisons dangereuses?. Liver Int. 2021;41(8):1734–43.
Kong L, Zhou Y, Bu H, Lv T, Shi Y, Yang J. Deletion of interleukin-6 in monocytes/macrophages suppresses the initiation of hepatocellular carcinoma in mice. J Exp Clin Cancer Res. 2016;35(1):131.
Ma R, Chen J, Liang Y, Lin S, Zhu L, Liang X, et al. Sorafenib: a potential therapeutic drug for hepatic fibrosis and its outcomes. Biomed Pharmacother. 2017;88:459–68.
Kurata N, Ogura Y, Ogiso S, Onishi Y, Kamei H, Kodera Y. Splenectomy in living donor liver transplantation and risk factors of portal vein thrombosis. Hepatobiliary Pancreat Dis Int. 2019;18(4):337–42.
Matsushima H, Fujiki M, Sasaki K, Cywinski JB, D’Amico G, Uso TD, et al. Can pretransplant TIPS be harmful in liver transplantation? A propensity score matching analysis. Surgery. 2020;168(1):33–9.
Tripathi D, Therapondos G, Redhead DN, Madhavan KK, Hayes PC. Transjugular intrahepatic portosystemic stent-shunt and its effects on orthotopic liver transplantation. Eur J Gastroenterol Hepatol. 2002;14(8):827–32.
Barbier L, Hardwigsen J, Borentain P, Biance N, Daghfous A, Louis G, et al. Impact of transjugular intrahepatic portosystemic shunting on liver transplantation: 12-year single-center experience. Clin Res Hepatol Gastroenterol. 2014;38(2):155–63.
Huang J, Millis JM, Mao Y, Millis MA, Sang X, Zhong S. A pilot programme of organ donation after cardiac death in China. Lancet. 2012;379(9818):862–5.
Download references
Acknowledgements
Not Applicable.
This study was supported by grants from Chengdu Science and Technology Projects (2022-YF05-01253-SN); Sichuan Natural Science Foundation (2022NSFSC0805); China Postdoctoral Science Foundation (2022M720102); Sichuan Medical Association (2021HR58); Research Project of Chengdu Health Commission (2023179).
Author information
Authors and affiliations.
Department of General Surgery, Public health clinical center of chengdu, Chengdu, Sichuan Province, China
Guo Wei, Yong Zhao & Shifeng Feng
Department of Liver transplantation center, West China Hospital of Sichuan University, Chengdu, Sichuan Province, China
Tao Lv, Jian Yang, Lingxiang Kong & Jiayin Yang
Department of Liver transplantation Laboratory, West China Hospital of Sichuan University, Chengdu, Sichuan Province, China
Jingsheng Yuan, Gang Xu & Lingxiang Kong
You can also search for this author in PubMed Google Scholar
Contributions
LK, WG and Jiayin Y designed the study; WG, GX, Jiayin Y, YZ, SF, Jian Y, TL, LK performed the research and collected the data; LK, SF, YZ analyzed and interpreted the data; WG, LK wrote the first draft of the manuscript; All authors edited the manuscript and approved the final draft; The acquisition of funding is from Jiayin Y.
Corresponding authors
Correspondence to Lingxiang Kong or Jiayin Yang .
Ethics declarations
Ethical approval and source of grafts.
In our study, the liver grafts utilized for LT since 2007 were obtained from deceased citizens in accordance with a new organ procurement and allocation policy implemented in China after 2012 ([ 53 ]). No prisoners were included as donors. The protocol was approved by the Ethics Committee of the West China Hospital of Sichuan University West China Hospital. Written informed consent was obtained from all the recipients prior to their surgery, and all of donations were voluntary and altruistic in all cases, and were in accordance with the ethical guidelines of the Declaration of Helsinki. The written informed consents from the next of kin of the dead citizen are kept at the Red Cross Society of Sichuan Province, China. ( www.scredcross.org.cn ).
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Wei, G., Zhao, Y., Feng, S. et al. Does depressurization of the portal vein before liver transplantation affect the recurrence of HCC? A nested case-control study. BMC Cancer 24 , 558 (2024). https://doi.org/10.1186/s12885-024-12322-6
Download citation
Received : 25 April 2023
Accepted : 30 April 2024
Published : 03 May 2024
DOI : https://doi.org/10.1186/s12885-024-12322-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Hepatocellular carcinoma (HCC)
- Liver transplantation (LT)
- Tumor recurrence
- Portal hypertension (PHT)
- Portosystemic Shunt (PSS)
- Splenectomy
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]
New study offers hope for a rare and devastating eye cancer
After more than a decade studying a rare eye cancer that produces some of the hardest-to-fight tumors, researchers from University of Pittsburgh Medical Center have found a treatment that works on some patients and, more importantly, a tool that can predict when it is likely to succeed.
The work, published in Nature Communications, is being validated in a clinical trial involving at least 30 patients. It could pave the way for similar methods designed to overcome one of the enduring frustrations of cancer care.
Because tumors differ, not only between patients but even inside the same patient, a treatment that works on one mass may fail on another, even when both are of the same cancer type.
The researchers in Pittsburgh tackled this problem in uveal melanoma, an eye cancer that afflicts only 5 people in a million, but that half the time spreads to other parts of the body, often the liver. The median survival once uveal melanoma has spread has been less than seven months, according to a 2018 study in the journal JAMA Ophthalmology.
“We chose this because it was one of the only cancers that 10 years ago when we started, there was nothing approved for it,” said Udai Kammula, who led the study and directs the Solid Tumor Cell Therapy Program at UPMC Hillman Cancer Center in Pittsburgh.
Scientists had long speculated that the reason uveal melanoma is so tough to fight is that something helps the tumor keep out T cells, a key part of the body’s immune system that develops in bone marrow. However, previous studies by Kammula and his colleagues showed that uveal melanoma tumors actually have T cells inside, and they are turned on.
The problem? The cells lie dormant instead of multiplying and reaching numbers large enough to overwhelm the tumor.
The culprit appears to reside somewhere inside the tumor’s ecosystem of cells, molecules and blood vessels, known formally as the tumor’s “microenvironment.” Kammula compares this ecosystem to the infrastructure that supports a city. Something in that infrastructure helps protect uveal melanoma tumors by preventing the critical T cells from multiplying.
“Ultimately, if we’re going to get rid of cancer, we have to get rid of this infrastructure,” Kammula said.
A tool for predicting success
He and his colleagues have had some success using a treatment known as adoptive cell therapy, which was developed in the 1980s by Steven Rosenberg at the National Institutes of Health.
The treatment involves removing the T cells from the tumor, where they have been unable to proliferate. Scientists then take those T cells and grow them outside the body in a lab dish. They treat patients with chemotherapy to kill off the last of their old immune systems. Finally, they reinfuse the lab-grown T cells into the patient’s blood stream and the cells, now in much greater numbers, go on to attack the tumor.
In this treatment, the T cells are often referred to as tumor-infiltrating leukocytes, or TILs.
Kammula said his team has found that tumors shrink partially or completely in about 35% of patients who receive the treatment. But they wanted to know why it doesn’t work in the majority of cases, and whether there might be some way to predict beforehand when it will succeed.
To find out, the researchers analyzed samples from 100 different uveal melanoma tumors that had spread to different parts of the body in 84 patients, seeking to examine all of the tumors’ genetic material.
“We basically put the tumor biopsy in a blender that had the stroma (supportive tissue), the blood vessels, the immune cells, the tumor cells. It had everything,” Kammula said, explaining that they then analyzed all of the tumor’s genetic material.
They found 2,394 genes that could have helped make the tumor susceptible to treatment, some of them genes that experts would regard as “the usual suspects” and others that were unexpected. Using this long list of genes, the scientists searched for characteristics that they shared.
The genes were predominantly involved in helping the body defend itself against viruses, bacteria and other foreign invaders by removing the invaders and helping tissue heal. Kammula and the study’s lead author, Shravan Leonard-Murali, a postdoctoral fellow in the lab, used the different activity levels of these genes to develop a clinical tool.
The tool, known as a biomarker, assigns a score to a uveal melanoma tumor based on the likelihood that it will respond well to the treatment – removing T cells, growing them outside the body, then reinfusing them.
So far, Kammula said, the biomarker has been “extremely good,” in predicting when the treatment will be effective, though he added, “these findings will need confirmation in the current ongoing clinical trial.”
“I thought it was somewhat of a tour de force, honestly,” said Eric Tran, an associate member of the Earle A. Chiles Research Institute, a division of Providence Cancer Institute in Portland, Ore. Tran did not participate in the study.
He said that while it will be important to validate these results, “I was certainly encouraged by their studies. And from my perspective, I wonder if that sort of strategy can be deployed in other cancers.”
Ryan J. Sullivan, an oncologist at Massachusetts General Hospital and associate professor at Harvard Medical School who was not involved in the study, called the team’s work “timely” and said “it is even more significant that they appear to have a [tool] that appears to predict which patients will benefit.”
The team at UPMC is already investigating possible wider application of the treatment and the biomarker in a second clinical trial that involves a dozen different cancers.
Bridging the digital divide in Spokane County
There is a major challenge in cities all across Washington state, big and small.
- Weird But True
- Sex & Relationships
- Viral Trends
- Human Interest
- Fashion & Beauty
- Food & Drink
- Health Care
- Men’s Health
- Women’s Health
- Mental Health
- Health & Wellness Products
- Personal Care Products
trending now in Lifestyle

TikTokker reveals little-known detail in the 7-Eleven logo: 'How...

My daughter heard 'monsters' in the wall — what we discovered...

NYC is having a thrilling steakhouse boom — and these are the...

Forget 10,000 steps a day — here's what to focus on instead

Hairstylist reveals the top 10 'icks' people do when they get...

I'm 35 and love sex, but I've been celibate for 8 years — this...

I was terrified I was a pedophile —here's what was actually...

Sad state of Prince Harry and Kate Middleton’s relationship...
Mom went to hospital for blindness — discovered she had lung cancer.
- View Author Archive
- Follow on Twitter
- Get author RSS feed
Thanks for contacting us. We've received your submission.

Don’t turn a blind eye to the possibility of lung cancer in patients with vision problems, one doctor warned recently.
A 32-year-old woman from India who never smoked went to the doctor after going blind in one eye. Her lungs were filled with cancerous tumors, despite reporting no pulmonary disturbances, according to a case study published in the Elsevier journal Radiology Case Reports.

“Despite widespread malignant involvement, the patient was completely asymptomatic and active except for visual disturbances,” Dr. Alok Pratap Singh, of the Sanjay Gandhi Postgraduate Institute of Medical Sciences, writes in the study.
“This case further emphasized the necessity of prompt and priority-based evaluation of patients for lung carcinoma whenever doubtful intraocular lesions are noted. It seems that these cases represent a distinct subset of lung malignancy,” he adds.
The woman sought attention at a hospital after she lost her vision in her right eye and saw flashing lights, a visual disturbance called photopsia, in her left eye.

Tests revealed that she had lung cancer along with choroidal metastasis in both of her eyes. Choroidal metastasis is a cancer that starts elsewhere in the body but moves into the eyes via the blood, according to the New York Eye Cancer Center.
The doctor said that visual disturbances should be taken seriously as a potential cancer symptom.

“It should be stressed that visual disturbances owing to choroidal secondaries obligated these patients to seek medical assistance. Since visual input is the most sensitive sensory input, patients are able to notice even slightest of disturbance in vision,” Singh writes in the case study.
“We do not know for how long [the cancer] would have been clinically silent if it [wasn’t] for symptomatic choroidal metastasis,” he adds.
Although it is uncommon to experience blurred vision due to lung cancer, it is more common that blurred vision can be a sign of a brain tumor. Either way — it’s best to see a doctor for such symptoms immediately.
Share this article:

Advertisement

IMAGES
VIDEO
COMMENTS
In one study, 69.8% of the patients had a partial or complete response after receiving an ... Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of cancer patients with COVID-19 in a New York ...
Cancer is a risk factor for venous thromboembolism, and this patient presents with chest pain, dyspnea, and unexplained tachycardia. In the context of cancer, an elevated d -dimer level can ...
Methods. A case study approach was used because it allowed us to examine the complexity of cancer management from the perspective of one person's case as interpreted by multiple people, retaining its holistic and meaningful characteristics while being studied 17 answering how and why questions. 18 Interviews from four participants presented multiple perspectives of the same interested topic ...
Thenew england journal ofmedicine. Presentation of Case. Dr. Mathew S. Lopes:A 65-year-old woman was transferred to this hospital because of chest pain. Six months before the current presentation ...
This is a qualitative study in which 21 women with breast cancer or survivors were interviewed. Participants were recruited at 9 large hospitals in Spain and intentional sampling methods were applied. Data were collected using a semi-structured interview that was elaborated with the help of medical oncologists, nurses, and psycho-oncologists.
Background. Worldwide, male breast cancer is extremely rare, accounting for <1% of all breast tumors and <1% of all malignancies in men [1-3].Recently, the incidence of male breast cancer has increased from 1.0 per 100,000 men in the late 1970s to 1.2 per 100,000 men from 2000 to 2004 [4-7].The American Cancer Society reported a similar trend in the incidence of breast cancer in men from ...
This patient has a stomach cancer that appears to be locoregionally advanced on imaging studies. The first decision regarding management is whether to resect it immediately or to administer preoperative chemotherapy, with or without preoperative radiation therapy. Treatment of Locoregionally Advanced Gastric Cancer
example, in a series of 8422 patients enrolled on International Breast Cancer Study Group trials between 1978 and 1999, the rate of node-negativity for medial compared to lateral/central tumors was 44 versus 33 percent, respectively. The most likely explanation for this difference is preferential drainage of some medial tumors to the IM nodes.
Case management (CM) is widely utilized to improve health outcomes of cancer patients, enhance their experience of health care, and reduce the cost of care. While numbers of systematic reviews are available on the effectiveness of CM for cancer patients, they often arrive at discordant conclusions that may confuse or mislead the future case management development for cancer patients and ...
Patient Case Presentation. Patient Mrs. B.C. is a 56 year old female who is presenting to her WHNP for her annual exam. She had to cancel her appointment two months ago and didn't reschedule until now. Her last pap smear and mammogram were normal. Today, while performing her breast exam, her nurse practitioner notices dimpling in the left ...
Lessons for the future. "This case is unusual, not only because of the multimodal approach but also the age of the patient," explains Dr. Kamath. "It underscores the unfortunate rise of young-onset colorectal cancer. Thirty years ago, the median age at onset of colorectal cancer was 72 years. It has fallen to 66 years, and we are seeing ...
Hepin* had been diagnosed with triple negative breast cancer late in 2014, before going on to have surgery. Her treatment was initially successful, and for a number of years she led an active lifestyle. But in May 2018 she started to notice a change. 'I was feeling more tired than usual - yawning and flagging easily,' she explains.
And this person certainly did. This patient had 2 years from completion of her platinum-based chemotherapy before she had evidence of recurrence. These bigger platinum-free intervals are also associated with better likelihood to respond to a platinum rechallenge. Case Overview: A 57-Year-Old Woman With Ovarian Cancer. Initial Presentation
Cancer Patients Have Increased Risk of Death From COVID-19, Studies Show. Encorafenib Alone or With Binimetinib Provides Long-Term Benefits in Melanoma.
Presentation of Case. Dr. Christopher T. Chen: A 34-year-old woman was evaluated in the oncology clinic of this hospital for the management of relapsed, metastatic intrahepatic cholangiocarcinoma ...
Case study 4 - Dr Rajesh Jena. Personalising radiotherapy - how patient data is helping reduce the side effects of treatment. Radiotherapy is the gold standard of treatment for many types of cancers. In fact, more than 130,000 patients benefit from radiotherapy every year in the UK.
The aim of the study was to report a rare case of BRCA 1-mutated breast cancer in a young patient with multiple affected relatives. Breast cancer is due to a genetic predisposition with BRCA1 and BRCA2 representing a significant proportion of families with a very high risk of developing the disease over a lifetime of up to 50-80%.
Application error: a client-side exception has occurred (see the browser console for more information) . Home to the journal Oncology, Cancer Network provides research and opinion on the screening, early detection, diagnosis, treatment, and prevention of cancers.
The patient was diagnosed with stage IV colorectal cancer, and the ECOG PS [performance status] of the patient was 1. At that time, the patient was started on treatment with bevacizumab and FOLFOX [5-fluorouracil, leucovorin, oxaliplatin]. The patient received about 4 months of this treatment with scans at 2- and 4-months showing response.
During a Case-Based Roundtable® event, Yasir Y. Elamin, MD, discussed the intracranial efficacy of brigatinib from the ALTA-1L trial for patients with ALK+ non-small cell lung cancer in the first article of a 2-part series.
Study design and participants. This retrospective case-control study was initially conducted between 2019 and 2020, following specific inclusion and exclusion criteria [].The case subjects are females diagnosed with breast cancer based on histopathology and immunohistochemistry assay, aged 19 years or older, who have not undergone any cancer therapies.
In the United States, the American Cancer Society reported that the demographics of cancer patients are increasingly shifting from older individuals to middle-aged people. While adults older than ...
Conclusions: In this case-control study, cancer patients with anthracycline-induced CTRCD have an increased burden of genetic variants in cardiomyopathy genes, similar to a DCM cohort. If validated in larger prospective studies, integration of genetic data in risk prediction models for CTRCD may guide cancer treatment. Moreover, genetic results ...
Introduction. Cervical cancer is one of the major causes of cancer-related death in women worldwide [].It is the fourth most prevalent cause of malignancy in women after breast, colorectal, and lung cancer [].]. The incidence of cervical cancer and mortality rate has declined by 70% in the United States since the 1950s because of age-appropriate screening [].
Most early-stage breast cancer patients will be long-term survivors - study. 13 Jun 2023. ... Advising older patients against breast cancer surgery is 'age bias', UK study finds.
FORT MYERS, Fla., May 1, 2024 /PRNewswire/ -- In a case study released today by Florida Cancer Specialists & Research Institute, LLC, community oncology practices emerge as the preferred site of ...
Case-control study composition. A total of 792 HCC patients underwent their first LT between September 2007 and January 2022. From these patients, we conducted a propensity score matching (PSM) analysis of 114 patients who underwent DPV (TIPS, surgical portosystemic shunt or/and splenectomy) before LT (32 Transjugular Intrahepatic Portosystemic Shunt-TIPS, 82 surgical portosystemic shunt or ...
Abstract. This paper presents an atypical case of a patient with brain tumor of the glioblastoma multiforme (GBM) type who achieved a 5-year survival. Some general information is provided including epidemiology, diagnostic and treatment procedures (surgery and radio-chemo-therapy), and prognosis of survival related to GBM.
News; Health; New study offers hope for a rare and devastating eye cancer Thu., April 25, 2024 Udai Kammula of University of Pittsburgh Medical Center stands with a patient who was treated with ...
A woman who went blind in one eye discovered she had lung cancer. utah51 - stock.adobe.com Don't turn a blind eye to the possibility of lung cancer in patients with vision problems, one doctor ...