Artificial Intelligence-based System for Diagnosis of Cardiovascular Diseases
- Institutional Repository Home
- Jimma Institute of Technology
- Biomedical Engineering
Show full item record

Files in this item

This item appears in the following Collection(s)
- Biomedical Engineering [85]
- Communities & Collections
- By Issue Date
This Collection

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Heart-Healthy Living
- High Blood Pressure
- Sickle Cell Disease
- Sleep Apnea
- Information & Resources on COVID-19
- The Heart Truth®
- Learn More Breathe Better®
- Blood Diseases and Disorders Education Program
- Publications and Resources
- Blood Disorders and Blood Safety
- Sleep Science and Sleep Disorders
- Lung Diseases
- Health Disparities and Inequities
- Heart and Vascular Diseases
- Precision Medicine Activities
- Obesity, Nutrition, and Physical Activity
- Population and Epidemiology Studies
- Women’s Health
- Research Topics
- Clinical Trials
- All Science A-Z
- Grants and Training Home
- Policies and Guidelines
- Funding Opportunities and Contacts
- Training and Career Development
- Email Alerts
- NHLBI in the Press
- Research Features
- Past Events
- Upcoming Events
- Mission and Strategic Vision
- Divisions, Offices and Centers
- Advisory Committees
- Budget and Legislative Information
- Jobs and Working at the NHLBI
- Contact and FAQs
- NIH Sleep Research Plan
- < Back To Research Topics
Coronary Heart Disease Research
Language switcher.
For almost 75 years, the NHLBI has been at the forefront of improving the nation’s health and reducing the burden of heart and vascular diseases . Heart disease, including coronary heart disease, remains the leading cause of death in the United States. However, the rate of heart disease deaths has declined by 70% over the past 50 years, thanks in part to NHLBI-funded research. Many current studies funded by the NHLBI focus on discovering genetic associations and finding new ways to prevent and treat the onset of coronary heart disease and associated medical conditions.
NHLBI research that really made a difference
The NHLBI supports a wide range of long-term studies to understand the risk factors of coronary heart disease. These ongoing studies, among others, have led to many discoveries that have increased our understanding of the causes of cardiovascular disease among different populations, helping to shape evidence-based clinical practice guidelines.
- Risk factors that can be changed: The NHLBI Framingham Heart Study (FHS) revealed that cardiovascular disease is caused by modifiable risk factors such as smoking, high blood pressure , obesity , high cholesterol levels, and physical inactivity. It is why, in routine physicals, healthcare providers check for high blood pressure, high cholesterol, unhealthy eating patterns, smoking, physical inactivity, and unhealthy weight. The FHS found that cigarette smoking increases the risk of heart disease. Researchers also showed that cardiovascular disease can affect people differently depending on sex or race, underscoring the need to address health disparities.
- Risk factors for Hispanic/Latino adults: The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) found that heart disease risk factors are widespread among Hispanic/Latino adults in the United States , with 80% of men and 71% of women having at least one risk factor. Researchers also used HCHS/SOL genetic data to explore genes linked with central adiposity (the tendency to have excess body fat around the waist) in Hispanic/Latino adults. Before this study, genes linked with central adiposity, a risk factor for coronary heart disease, had been identified in people of European ancestry. These results showed that those genes also predict central adiposity for Hispanic/Latino communities. Some of the genes identified were more common among people with Mexican or Central/South American ancestry, while others were more common among people of Caribbean ancestry.
- Risk factors for African Americans: The Jackson Heart Study (JHS) began in 1997 and includes more than 5,300 African American men and women in Jackson, Mississippi. It has studied genetic and environmental factors that raise the risk of heart problems, especially high blood pressure, coronary heart disease, heart failure , stroke , and peripheral artery disease (PAD) . Researchers discovered a gene variant in African American individuals that doubles the risk of heart disease. They also found that even small spikes in blood pressure can lead to a higher risk of death. A community engagement component of the JHS is putting 20 years of the study’s findings into action by turning traditional gathering places, such as barbershops and churches, into health information hubs.
- Risk factors for American Indians: The NHLBI actively supports the Strong Heart Study , a long-term study that began in 1988 to examine cardiovascular disease and its risk factors among American Indian men and women. The Strong Heart Study is one of the largest epidemiological studies of American Indian people ever undertaken. It involves a partnership with 12 Tribal Nations and has followed more than 8,000 participants, many of whom live in low-income rural areas of Arizona, Oklahoma, and the Dakotas. Cardiovascular disease remains the leading cause of death for American Indian people. Yet the prevalence and severity of cardiovascular disease among American Indian people has been challenging to study because of the small sizes of the communities, as well as the relatively young age, cultural diversity, and wide geographic distribution of the population. In 2019, the NHLBI renewed its commitment to the Strong Heart Study with a new study phase that includes more funding for community-driven pilot projects and a continued emphasis on training and development. Read more about the goals and key findings of the Strong Heart Study.
Current research funded by the NHLBI
Within our Division of Cardiovascular Sciences , the Atherothrombosis and Coronary Artery Disease Branch of its Adult and Pediatric Cardiac Research Program and the Center for Translation Research and Implementation Science oversee much of our funded research on coronary heart disease.
Research funding
Find funding opportunities and program contacts for research on coronary heart disease.
Current research on preventing coronary heart disease
- Blood cholesterol and coronary heart disease: The NHLBI supports new research into lowering the risk of coronary heart disease by reducing levels of cholesterol in the blood. High levels of blood cholesterol, especially a type called low-density lipoprotein (LDL) cholesterol, raise the risk of coronary heart disease. However, even with medicine that lowers LDL cholesterol, there is still a risk of coronary heart disease due to other proteins, called triglyceride-rich ApoB-containing lipoproteins (ApoBCLs), that circulate in the blood. Researchers are working to find innovative ways to reduce the levels of ApoBCLs, which may help prevent coronary heart disease and other cardiovascular conditions.
- Pregnancy, preeclampsia, and coronary heart disease risk: NHLBI-supported researchers are investigating the link between developing preeclampsia during pregnancy and an increased risk for heart disease over the lifespan . This project uses “omics” data – such as genomics, proteomics, and other research areas – from three different cohorts of women to define and assess preeclampsia biomarkers associated with cardiovascular health outcomes. Researchers have determined that high blood pressure during pregnancy and low birth weight are predictors of atherosclerotic cardiovascular disease in women . Ultimately, these findings can inform new preventive strategies to lower the risk of coronary heart disease.
- Community-level efforts to lower heart disease risk among African American people: The NHLBI is funding initiatives to partner with churches in order to engage with African American communities and lower disparities in heart health . Studies have found that church-led interventions reduce risk factors for coronary heart disease and other cardiovascular conditions. NHLBI-supported researchers assessed data from more than 17,000 participants across multiple studies and determined that these community-based approaches are effective in lowering heart disease risk factors .
Find more NHLBI-funded studies on preventing coronary heart disease on the NIH RePORTER.

Learn about the impact of COVID-19 on your risk of coronary heart disease.
Current research on understanding the causes of coronary heart disease
- Pregnancy and long-term heart disease: NHLBI researchers are continuing the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-be (nuMoM2b) study to understand the relationship between pregnancy-related problems, such as gestational hypertension, and heart problems. The study also looks at how problems during pregnancy may increase risk factors for heart disease later in life. NuMoM2b launched in 2010, and long-term studies are ongoing, with the goal of collecting high-quality data and understanding how heart disease develops in women after pregnancy.
- How coronary artery disease affects heart attack risk: NHLBI-funded researchers are investigating why some people with coronary artery disease are more at risk for heart attacks than others. Researchers have found that people with coronary artery disease who have high-risk coronary plaques are more likely to have serious cardiac events, including heart attacks. However, we do not know why some people develop high-risk coronary plaques and others do not. Researchers hope that this study will help providers better identify which people are most at risk of heart attacks before they occur.
- Genetics of coronary heart disease: The NHLBI supports studies to identify genetic variants associated with coronary heart disease . Researchers are investigating how genes affect important molecular cascades involved in the development of coronary heart disease . This deeper understanding of the underlying causes for plaque buildup and damage to the blood vessels can inform prevention strategies and help healthcare providers develop personalized treatment for people with coronary heart disease caused by specific genetic mutations.
Find more NHLBI-funded studies on understanding the causes of coronary heart disease on the NIH RePORTER.

Recent findings suggest that cholesterol-lowering treatment can lower the risk of heart disease complications in people with HIV.
Current research on treatments for coronary heart disease
- Insight into new molecular targets for treatment: NHLBI-supported researchers are investigating the role of high-density lipoprotein (HDL) cholesterol in coronary heart disease and other medical conditions . Understanding how the molecular pathways of cholesterol affect the disease mechanism for atherosclerosis and plaque buildup in the blood vessels of the heart can lead to new therapeutic approaches for the treatment of coronary heart disease. Researchers have found evidence that treatments that boost HDL function can lower systemic inflammation and slow down plaque buildup . This mechanism could be targeted to develop a new treatment approach for coronary heart disease.
- Long-term studies of treatment effectiveness: The NHLBI is supporting the International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial EXTENDed Follow-up (EXTEND) , which compares the long-term outcomes of an initial invasive versus conservative strategy for more than 5,000 surviving participants of the original ISCHEMIA trial. Researchers have found no difference in mortality outcomes between invasive and conservative management strategies for patients with chronic coronary heart disease after more than 3 years. They will continue to follow up with participants for up to 10 years. Researchers are also assessing the impact of nonfatal events on long-term heart disease and mortality. A more accurate heart disease risk score will be constructed to help healthcare providers deliver more precise care for their patients.
- Evaluating a new therapy for protecting new mothers: The NHLBI is supporting the Randomized Evaluation of Bromocriptine In Myocardial Recovery Therapy for Peripartum Cardiomyopathy (REBIRTH) , for determining the role of bromocriptine as a treatment for peripartum cardiomyopathy (PPCM). Previous research suggests that prolactin, a hormone that stimulates the production of milk for breastfeeding, may contribute to the development of cardiomyopathy late in pregnancy or the first several months postpartum. Bromocriptine, once commonly used in the United States to stop milk production, has shown promising results in studies conducted in South Africa and Germany. Researchers will enroll approximately 200 women across North America who have been diagnosed with PPCM and assess their heart function after 6 months.
- Impact of mental health on response to treatment: NHLBI-supported researchers are investigating how mental health conditions can affect treatment effectiveness for people with coronary heart disease. Studies show that depression is linked to a higher risk for negative outcomes from coronary heart disease. Researchers found that having depression is associated with poor adherence to medical treatment for coronary heart disease . This means that people with depression are less likely to follow through with their heart disease treatment plans, possibly contributing to their chances of experiencing worse outcomes. Researchers are also studying new ways to treat depression in patients with coronary heart disease .
Find more NHLBI-funded studies on treating coronary heart disease on the NIH RePORTER.

Researchers have found no clear difference in patient survival or heart attack risk between managing heart disease through medication and lifestyle changes compared with invasive procedures.
Coronary heart disease research labs at the NHLBI
- Laboratory of Cardiac Physiology
- Laboratory of Cardiovascular Biology
- Minority Health and Health Disparities Population Laboratory
- Social Determinants of Obesity and Cardiovascular Risk Laboratory
- Laboratory for Cardiovascular Epidemiology and Genomics
- Laboratory for Hemostasis and Platelet Biology
Related coronary heart disease programs
- In 2002, the NHLBI launched The Heart Truth® , the first federally sponsored national health education program designed to raise awareness about heart disease as the leading cause of death in women. The NHLBI and The Heart Truth® supported the creation of the Red Dress® as the national symbol for awareness about women and heart disease, and also coordinate National Wear Red Day ® and American Heart Month each February.
- The Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) facilitates access to and maximizes the scientific value of NHLBI biospecimen and data collections. A main goal is to promote the use of these scientific resources by the broader research community. BioLINCC serves to coordinate searches across data and biospecimen collections and provide an electronic means for requesting additional information and submitting requests for collections. Researchers wanting to submit biospecimen collections to the NHLBI Biorepository to share with qualified investigators may also use the website to initiate the application process.
- Our Trans-Omics for Precision Medicine (TOPMed) Program studies the ways genetic information, along with information about health status, lifestyle, and the environment, can be used to predict the best ways to prevent and treat heart, lung, blood, and sleep disorders. TOPMed specifically supports NHLBI’s Precision Medicine Activities.
- NHLBI population and epidemiology studies in different groups of people, including the Atherosclerosis Risk in Communities (ARIC) Study , the Multi-Ethnic Study of Atherosclerosis (MESA) , and the Cardiovascular Health Study (CHS) , have made major contributions to understanding the causes and prevention of heart and vascular diseases, including coronary heart disease.
- The Cardiothoracic Surgical Trials Network (CTSN) is an international clinical research enterprise that studies heart valve disease , arrhythmias , heart failure, coronary heart disease, and surgical complications. The trials span all phases of development, from early translation to completion, and have more than 14,000 participants. The trials include six completed randomized clinical trials, three large observational studies, and many other smaller studies.

Learn how heart disease may be different for women than for men.
Explore more NHLBI research on coronary heart disease
The sections above provide you with the highlights of NHLBI-supported research on coronary heart disease. You can explore the full list of NHLBI-funded studies on the NIH RePORTER .
To find more studies:
- Type your search words into the Quick Search box and press enter.
- Check Active Projects if you want current research.
- Select the Agencies arrow, then the NIH arrow, then check NHLBI .
If you want to sort the projects by budget size — from the biggest to the smallest — click on the FY Total Cost by IC column heading.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 01 May 2024
Temporal dynamics of the multi-omic response to endurance exercise training
- MoTrPAC Study Group ,
- Lead Analysts &
MoTrPAC Study Group
Nature volume 629 , pages 174–183 ( 2024 ) Cite this article
41k Accesses
674 Altmetric
Metrics details
- Epigenetics
- Metabolomics
- Transcriptomics
Regular exercise promotes whole-body health and prevents disease, but the underlying molecular mechanisms are incompletely understood 1 , 2 , 3 . Here, the Molecular Transducers of Physical Activity Consortium 4 profiled the temporal transcriptome, proteome, metabolome, lipidome, phosphoproteome, acetylproteome, ubiquitylproteome, epigenome and immunome in whole blood, plasma and 18 solid tissues in male and female Rattus norvegicus over eight weeks of endurance exercise training. The resulting data compendium encompasses 9,466 assays across 19 tissues, 25 molecular platforms and 4 training time points. Thousands of shared and tissue-specific molecular alterations were identified, with sex differences found in multiple tissues. Temporal multi-omic and multi-tissue analyses revealed expansive biological insights into the adaptive responses to endurance training, including widespread regulation of immune, metabolic, stress response and mitochondrial pathways. Many changes were relevant to human health, including non-alcoholic fatty liver disease, inflammatory bowel disease, cardiovascular health and tissue injury and recovery. The data and analyses presented in this study will serve as valuable resources for understanding and exploring the multi-tissue molecular effects of endurance training and are provided in a public repository ( https://motrpac-data.org/ ).
Similar content being viewed by others

Multimodal cell atlas of the ageing human skeletal muscle

The impact of exercise on gene regulation in association with complex trait genetics

Associations of dietary patterns with brain health from behavioral, neuroimaging, biochemical and genetic analyses
Regular exercise provides wide-ranging health benefits, including reduced risks of all-cause mortality 1 , 5 , cardiometabolic and neurological diseases, cancer and other pathologies 2 , 6 , 7 . Exercise affects nearly all organ systems in either improving health or reducing disease risk 2 , 3 , 6 , 7 , with beneficial effects resulting from cellular and molecular adaptations within and across many tissues and organ systems 3 . Various ‘omic’ platforms (‘omes’) including transcriptomics, epigenomics, proteomics and metabolomics, have been used to study these events. However, work to date typically covers one or two omes at a single time point, is biased towards one sex, and often focuses on a single tissue, most often skeletal muscle, heart or blood 8 , 9 , 10 , 11 , 12 , with few studies considering other tissues 13 . Accordingly, a comprehensive, organism-wide, multi-omic map of the effects of exercise is needed to understand the molecular underpinnings of exercise training-induced adaptations. To address this need, the Molecular Transducers of Physical Activity Consortium (MoTrPAC) was established with the goal of building a molecular map of the exercise response across a broad range of tissues in animal models and in skeletal muscle, adipose and blood in humans 4 . Here we present the first whole-organism molecular map of the temporal effects of endurance exercise training in male and female rats and provide multiple insights enabled by this MoTrPAC multi-omic data resource.
Multi-omic analysis of exercise training
Six-month-old male and female Fischer 344 rats were subjected to progressive treadmill endurance exercise training (hereafter referred to as endurance training) for 1, 2, 4 or 8 weeks, with tissues collected 48 h after the last exercise bout (Fig. 1a ). Sex-matched sedentary, untrained rats were used as controls. Training resulted in robust phenotypic changes (Extended Data Fig. 1a–d ), including increased aerobic capacity (VO 2 max) by 18% and 16% at 8 weeks in males and females, respectively (Extended Data Fig. 1a ). The percentage of body fat decreased by 5% in males at 8 weeks (Extended Data Fig. 1b ), without a significant change in lean mass (Extended Data Fig. 1c ). In females, the body fat percentage did not change after 4 or 8 weeks of training, whereas it increased by 4% in sedentary controls (Extended Data Fig. 1b ). Body weight of females increased in all intervention groups, with no change for males (Extended Data Fig. 1d ).
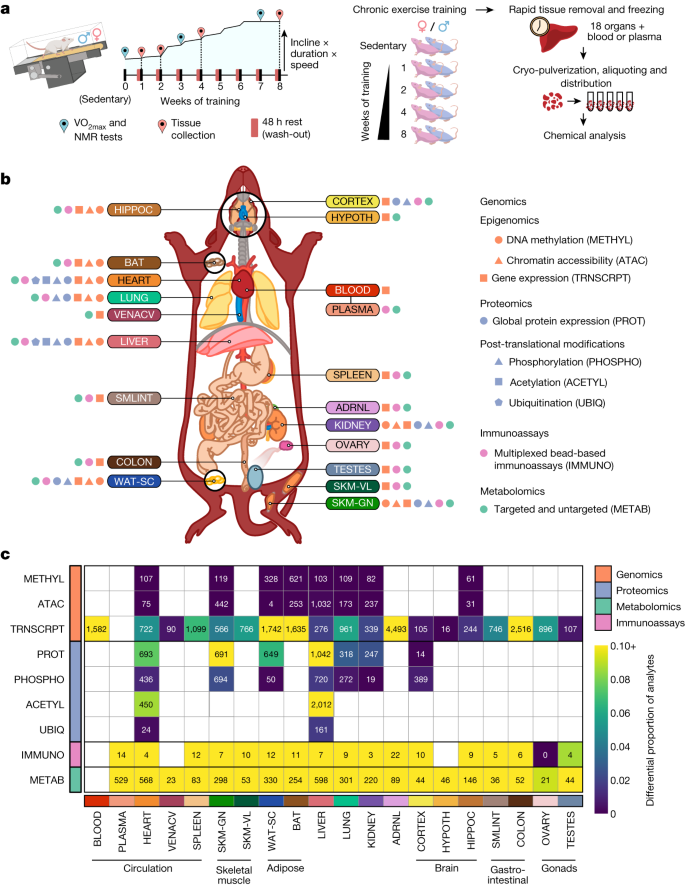
a , Experimental design and tissue sample processing. Inbred Fischer 344 rats were subjected to a progressive treadmill training protocol. Tissues were collected from male and female animals that remained sedentary or completed 1, 2, 4 or 8 weeks of endurance exercise training. For trained animals, samples were collected 48 h after their last exercise bout (red pins). b , Summary of molecular datasets included in this study. Up to nine data types (omes) were generated for blood, plasma, and 18 solid tissues, per animal: ACETYL: acetylproteomics; protein site acetylation; ATAC, chromatin accessibility, ATAC-seq data; IMMUNO, multiplexed immunoassays; METAB, metabolomics and lipidomics; METHYL, DNA methylation, RRBS data; PHOSPHO, phosphoproteomics; protein site phosphorylation; PROT, global proteomics; protein abundance; TRNSCRPT, transcriptomics, RNA-seq data; UBIQ, ubiquitylome, protein site ubiquitination. Tissue labels indicate the location, colour code, and abbreviation for each tissue used throughout this study: ADRNL, adrenal gland; BAT, brown adipose tissue; BLOOD, whole blood, blood RNA; COLON, colon; CORTEX, cerebral cortex; HEART, heart; HIPPOC, hippocampus; HYPOTH, hypothalamus; KIDNEY, kidney; LIVER, liver; LUNG, lung; OVARY, ovaries; PLASMA, plasma; SKM-GN, gastrocnemius (skeletal muscle); SKM-VL, vastus lateralis (skeletal muscle); SMLINT, small intestine; SPLEEN, spleen; TESTES, testes; VENACV, vena cava; WAT-SC, subcutaneous white adipose tissue. Icons next to each tissue label indicate the data types generated for that tissue. c , Number of training-regulated features at 5% FDR. Each cell represents results for a single tissue and data type. Colours indicate the proportion of measured features that are differential.
Whole blood, plasma and 18 solid tissues were analysed using genomics, proteomics, metabolomics and protein immunoassay technologies, with most assays performed in a subset of these tissues (Fig. 1b and Extended Data Fig. 1e,f ). Specific details for each omic analysis are provided in Extended Data Fig. 2 , Methods, Supplementary Discussion and Supplementary Table 1 . Molecular assays were prioritized on the basis of available tissue quantity and biological relevance, with the gastrocnemius, heart, liver and white adipose tissue having the most diverse set of molecular assays performed, followed by the kidney, lung, brown adipose tissue and hippocampus (Extended Data Fig. 1e ). Altogether, datasets were generated from 9,466 assays across 211 combinations of tissues and molecular platforms, resulting in 681,256 non-epigenetic and 14,334,496 epigenetic (reduced-representation bisulfite sequencing (RRBS) and assay for transposase-accessible chromatin using sequencing (ATAC-seq)) measurements, corresponding to 213,689 and 2,799,307 unique non-epigenetic and epigenetic features, respectively.
Differential analysis was used to characterize the molecular responses to endurance training (Methods). We computed the overall significance of the training response for each feature, denoted as the training P value, where 35,439 features at 5% false discovery rate (FDR) comprise the training-regulated differential features (Fig. 1c and Supplementary Table 2 ). Timewise summary statistics quantify the exercise training effects for each sex and time point. Training-regulated molecules were observed in the vast majority of tissues for all omes, including a relatively large proportion of transcriptomics, proteomics, metabolomics and immunoassay features (Fig. 1c ). The observed timewise effects were modest: 56% of the per-feature maximum fold changes were between 0.67 and 1.5. Permutation testing showed that permuting the group or sex labels resulted in a significant reduction in the number of selected analytes in most tissues (Extended Data Fig. 3a–d and Supplementary Discussion ). For transcriptomics, the hypothalamus, cortex, testes and vena cava had the smallest proportion of training-regulated genes, whereas the blood, brown and white adipose tissues, adrenal gland and colon showed more extensive effects (Fig. 1c ). For proteomics, the gastrocnemius, heart and liver showed substantial differential regulation in both protein abundance and post-translational modifications (PTMs), with more restricted results in white adipose tissue, lung and kidney protein abundance. For metabolomics, a large proportion of differential metabolites were consistently observed across all tissues, although the absolute numbers were related to the number of metabolomic platforms used (Extended Data Fig. 1e ). The vast number of differential features over the training time course across tissues and omes highlights the multi-faceted, organism-wide nature of molecular adaptations to endurance training.
Multi-tissue response to training
To identify tissue-specific and multi-tissue training-responsive gene expression, we considered the six tissues with the deepest molecular profiling: gastrocnemius, heart, liver, white adipose tissue, lung and kidney. In sum, 11,407 differential features from these datasets were mapped to their cognate gene, for a total of 7,115 unique genes across the tissues (Fig. 2a , Extended Data Fig. 4a and Supplementary Table 3 ). Most of the genes with at least one training-responsive feature were tissue-specific (67%), with the greatest number appearing in white adipose tissue (Fig. 2a ). We identified pathways enriched by these tissue-specific training-responsive genes (Extended Data Fig. 4b ) and tabulated a subset of highly specific genes to gain insight into tissue-specific training adaptation (Supplementary Table 4 ). Focusing on sexually conserved responses revealed tissue-dependent adaptations. These included changes related to immune cell recruitment and tissue remodelling in the lung, cofactor and cholesterol biosynthesis in the liver, ion flux in the heart, and metabolic processes and striated muscle contraction in the gastrocnemius ( Supplementary Discussion ). A detailed analysis of white adipose tissue adaptations to exercise training is provided elsewhere 14 . We also observed ‘ome’-specific responses, with unique transcript and protein responses at the gene and pathway levels (Extended Data Fig. 4c,d , Supplementary Discussion and Supplementary Tables 5 and 6 ).
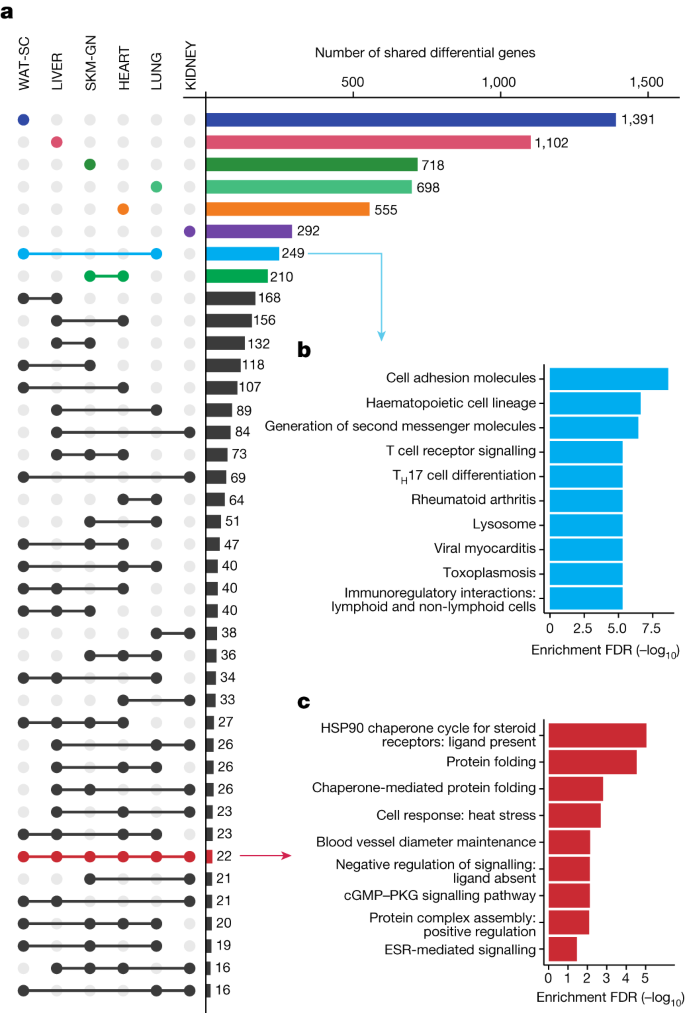
a , UpSet plot of the training-regulated gene sets associated with each tissue. Bars and dots indicating tissue-specific differential genes are coloured by tissue. Pathway enrichment analysis is shown for selected sets of genes in b , c as indicated by the arrows. b , c , Significantly enriched pathways (10% FDR) corresponding to genes that are differential in both LUNG and WAT-SC datasets ( b ) and the 22 genes that are training-regulated in all six tissues considered in a ( c ). Redundant pathways (those with an overlap of 80% or greater with an existing pathway) were removed. ESR, oestrogen receptor; T H 17, T helper 17.
2,359 genes had differential features in at least two tissues (Fig. 2a ). Lung and white adipose tissue had the largest set of uniquely shared genes ( n = 249), with predominantly immune-related pathway enrichments (Fig. 2b ); expression patterns suggested decreased inflammation in the lung and increased immune cell recruitment in white adipose tissue (Supplementary Tables 2 and 3 ). Heart and gastrocnemius had the second-largest group of uniquely shared genes, with enrichment of mitochondrial metabolism pathways including the mitochondria fusion genes Opa1 and Mfn1 (Supplementary Table 3 ).
Twenty-two genes were training-regulated in all six tissues, with particular enrichment in heat shock response pathways (Fig. 2c ). Exercise induces the expression of heat shock proteins (HSPs) in various rodent and human tissues 15 . A focused analysis of our transcriptomics and proteomics data revealed HSPs as prominent outliers (Extended Data Fig. 5a and Supplementary Discussion ). Specifically, there was a marked, proteomics-driven up-regulation in the abundance of HSPs, including the major HSPs HSPA1B and HSP90AA1 (Extended Data Fig. 5b,c ). Another ubiquitous endurance training response involved regulation of the kininogenases KNG1 and KNG2 (Supplementary Table 3 ). These enzymes are part of the kallikrein–kininogen system and have been implicated in the hypotensive and insulin-sensitizing effects of exercise 16 , 17 .
Transcription factors and phosphosignalling
We used proteomics and transcriptomics data to infer changes in transcription factor and phosphosignalling activities in response to endurance training through transcription factor and PTM enrichment analyses (Methods). We compared the most significantly enriched transcription factors across tissues (Fig. 3a , Extended Data Fig. 6a and Supplementary Table 7 ). In the blood, we observed enrichment of the haematopoietic-associated transcription factors GABPA, ETS1, KLF3 and ZNF143; haematopoietic progenitors are proposed to be transducers of the health benefits of exercise 18 . In the heart and skeletal muscle, we observed a cluster of enriched Mef2 family transcription factor motifs (Fig. 3a ). MEF2C is a muscle-associated transcription factor involved in skeletal, cardiac and smooth muscle cell differentiation and has been implicated in vascular development, formation of the cardiac loop and neuron differentiation 19 .
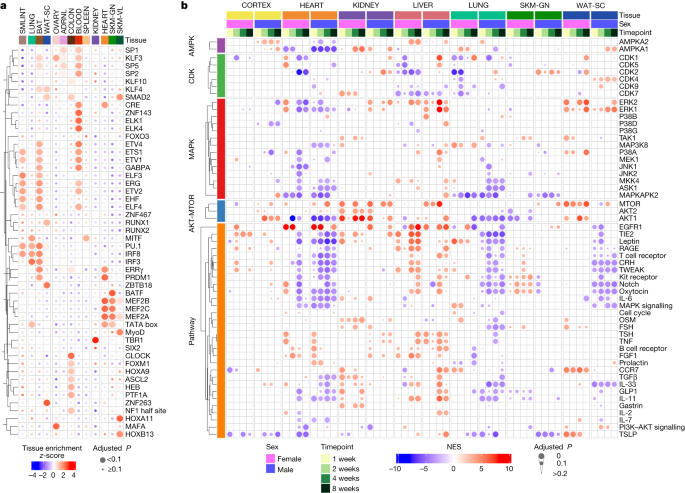
a , Transcription factor motif enrichment analysis of the training-regulated transcripts in each tissue. The heat map shows enrichment z -scores across the differential genes for the 13 tissues that had at least 300 genes after mapping transcript IDs to gene symbols. Transcription factors were hierarchically clustered by their enrichment across tissues. CRE, cAMP response element. b , Estimate of activity changes in selected kinases and signalling pathways using PTM signature enrichment analysis on phosphoproteomics data. Only kinases or pathways with a significant difference in at least one tissue, sex or time point ( q value < 0.05) are shown. The heat map shows normalized enrichment score (NES) as colour; tissue, sex and time point combinations as columns, and either kinases or pathways as rows. Kinases are grouped by family; rows are hierarchically clustered within each group. FSH, follicle-stimulating hormone; TSH, thyroid-stimulating hormone.
Phosphorylation signatures of key kinases were altered across many tissues (Fig. 3b and Supplementary Table 8 ). This included AKT1 across heart, kidney and lung, mTOR across heart, kidney and white adipose tissue, and MAPK across heart and kidney. The liver showed an increase in the phosphosignature related to regulators of hepatic regeneration, including EGFR1, IGF and HGF (Extended Data Fig. 6b , Supplementary Discussion ). Increased phosphorylation of STAT3 and PXN, HGF targets involved in cell proliferation, suggest a mechanism for liver regeneration in response to exercise (Extended Data Fig. 6c ). In the heart, kinases showed bidirectional changes in their predicted basal activity in response to endurance training (Extended Data Fig. 6d and Supplementary Discussion ). Several AGC protein kinases showed a decrease in predicted activity, including AKT1, whereas tyrosine kinases, including SRC and mTOR, were predicted to have increased activity. The known SRC target phosphorylation sites GJA1 pY265 and CDH2 pY820 showed significantly increased phosphorylation in response to training (Extended Data Fig. 6e ). Notably, phosphorylation of GJA1 Y265 has previously been shown to disrupt gap junctions, key transducers of cardiac electrical conductivity 20 . This suggests that SRC signalling may regulate extracellular structural remodelling of the heart to promote physiologically beneficial adaptations. In agreement with this hypothesis, gene set enrichment analysis (GSEA) of extracellular matrix proteins revealed a negative enrichment in response to endurance training, showing decreased abundance of proteins such as basement membrane proteins (Extended Data Fig. 6f–h and Supplementary Table 9 ).
Molecular hubs of exercise adaptation
To compare the dynamic multi-omic responses to endurance training across tissues, we clustered the 34,244 differential features with complete timewise summary statistics using an empirical Bayes graphical clustering approach (Methods). By integrating these results onto a graph, we summarize the dynamics of the molecular training response and identify groups of features with similar responses (Extended Data Fig. 7 and Supplementary Table 10 ). We performed pathway enrichment analysis for many graphically defined clusters to characterize putative underlying biology (Supplementary Table 11 ).
We examined biological processes associated with training using the pathway enrichment results for up-regulated features at 8 weeks of training (Extended Data Fig. 8 , Supplementary Table 12 and Supplementary Discussion ). Compared with other tissues, the liver showed substantial regulation of chromatin accessibility, including in the nuclear receptor signalling and cellular senescence pathways. In the gastrocnemius, terms related to peroxisome proliferator-activated receptors (PPAR) signalling and lipid synthesis and degradation were enriched at the protein level, driven by proteins including the lipid droplet features PLIN2, PLIN4 and PLIN5. At the metabolomic level, terms related to ether lipid and glycerophospholipid metabolism were enriched. Together, these enrichments highlight the well-known ability of endurance training to modulate skeletal muscle lipid composition, storage, synthesis and metabolism. The blood displayed pathway enrichments related to translation and organelle biogenesis and maintenance. Paired with the transcription factor analysis (Fig. 3a ), this suggests increased haematopoietic cellular mobilization in the blood. Less studied tissues in the context of exercise training, including the adrenal gland, spleen, cortex, hippocampus and colon, also showed regulation of diverse pathways ( Supplementary Discussion ).
To identify the main temporal or sex-associated responses in each tissue, we summarized the graphical cluster sizes by tissue and time (Extended Data Fig. 7a ). We observed that the small intestine and plasma had more changes at weeks 1 and 2 of training. Conversely, many up-regulated features in brown adipose tissue and down-regulated features in white adipose tissue were observed only at week 8. The largest proportion of opposite effects between males and females was observed at week 1 in the adrenal gland. Other tissues, including the blood, heart, lung, kidney and skeletal muscle (gastrocnemius and vastus lateralis), had relatively consistent numbers of up-regulated and down-regulated features.
We next focused on characterizing shared molecular responses in the three striated muscles (gastrocnemius, vastus lateralis and heart). The three largest graphical clustering paths of differential features in each muscle tissue converged to a sex-consistent response by week 8 (Fig. 4a ). Because of the large number of muscle features that were up-regulated in both sexes at week 8, we further examined the corresponding multi-omic set of analytes (Fig. 4b ). Pathway enrichment analysis of the genes associated with these differential features demonstrated a sex- and muscle-consistent endurance training response that reflected up-regulation of mitochondrial metabolism, biogenesis and translation, and cellular response to heat stress (Fig. 4c and Supplementary Table 11 ).
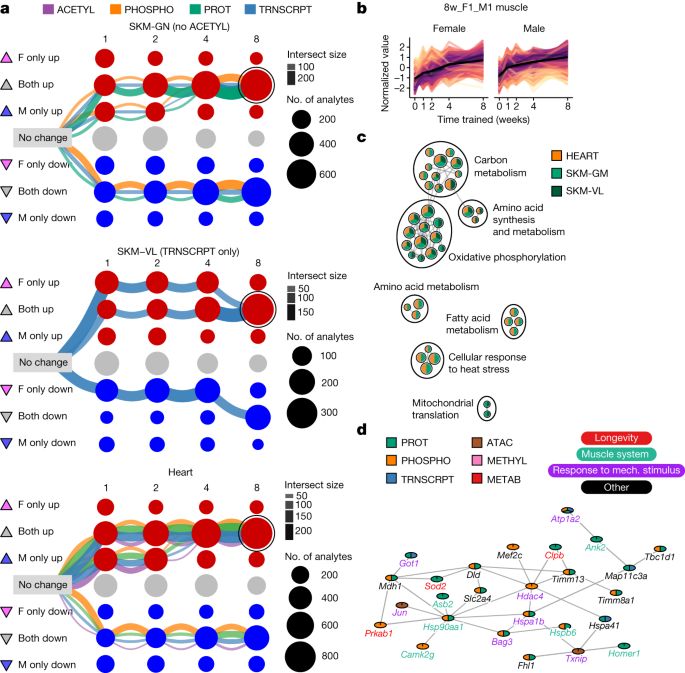
a , Graphical representation of training-differential features in the three muscle tissues: gastrocnemius (SKM-GN), vastus lateralis (SKM-VL) and heart. Each node represents one of nine possible states (rows) at each of the four training time points (columns). Triangles to the left of row labels map states to symbols used in Fig. 5a . Edges represent the path of differential features over the training time course (see Extended Data Fig. 7 for a detailed explanation). Each graph includes the three largest paths of differential features in that tissue, with edges split by data type. Both node and edge size are proportional to the number of features represented. The node corresponding to features that are up-regulated in both sexes at 8 weeks of training (8w_F1_M1) is circled in each graph. b , Line plots of standardized abundances of all 8w_F1_M1 muscle features. The black line represents the average value across all features. c , Network view of significant pathway enrichment results (10% FDR) corresponding to the features in b . Nodes represent pathways; edges represent functionally similar node pairs (set similarity ≥ 0.3). Nodes are included only if they are significantly enriched in at least two of the muscle tissues, as indicated by node colour. Node size is proportional to the number of differential feature sets (for example, gastrocnemius transcripts) for which the pathway is significantly enriched. High-level biological themes were defined using Louvain community detection of the nodes. d , A subnetwork of a larger cluster identified by network clustering 8w_F1_M1 features from SKM-GN. Mech., mechanical.
We used a network connectivity analysis to study up-regulated features in the gastrocnemius at week 8 (Extended Data Fig. 9a,b , Methods and Supplementary Discussion ). Mapping features to genes revealed overlaps between transcriptomic, chromatin accessibility, and proteomic assays, but no overlaps with methylation. Three molecular interaction networks were compared (Methods), and BioGRID 21 was used for further clustering analysis, which identified three clusters (Extended Data Fig. 9c and Supplementary Table 13 ). The largest cluster was significantly enriched for multiple muscle adaptation processes (Fig. 4d and Supplementary Table 14 ). This analysis illustrates the direct linkage among pathways and putative central regulators, emphasizing the importance of multi-omic data in identifying interconnected networks and understanding skeletal muscle remodelling.
Connection to human diseases and traits
To systematically evaluate the translational value of our data, we integrated our results with extant exercise studies and disease ontology (DO) annotations (Methods). First, we compared our vastus lateralis transcriptomics results to a meta-analysis of long-term training gene-expression changes in human skeletal muscle tissue 8 , demonstrating a significant and direction-consistent overlap (Extended Data Fig. 9d–g and Supplementary Discussion ). We also identified a significant overlap between differential transcripts in the gastrocnemius of female rats trained for 8 weeks and differentially expressed genes identified in the soleus in a study of sedentary and exercise-trained female rats selectively bred for high or low exercise capacity 22 (Extended Data Fig. 9h ). Similarly, adaptations from high-intensity interval training in humans 23 significantly overlapped with the proteomics response in rats (Extended Data Fig. 9i ), particularly for female rats trained for 8 weeks (Extended Data Fig. 9j ). Finally, we performed DO enrichment analysis using the DOSE R package 24 (Supplementary Table 15 and Methods). Down-regulated genes from white adipose tissue, kidney and liver were enriched for several disease terms, suggesting a link between the exercise response and type 2 diabetes, cardiovascular disease, obesity and kidney disease (5% FDR; Extended Data Fig. 9k and Supplementary Discussion ), which are all epidemiologically related co-occurring diseases 25 . Overall, these results support a high concordance of our data from rats with human studies and their relevance to human disease.
Sex-specific responses to exercise
Many tissues showed sex differences in their training responses (Extended Data Fig. 10 ), with 58% of the 8-week training-regulated features demonstrating sex-differentiated responses. Opposite responses between the sexes were observed in adrenal gland transcripts, lung phosphosites and chromatin accessibility features, white adipose tissue transcripts and liver acetylsites. In addition, proinflammatory cytokines exhibited sex-associated changes across tissues (Extended Data Fig. 11a,b and Supplementary Table 16 ). Most female-specific cytokines were differentially regulated between weeks 1 and 2 of training, whereas most male-specific cytokines were differentially regulated between weeks 4 and 8 (Extended Data Fig. 11c ).
We observed extensive transcriptional remodelling of the adrenal gland, with more than 4,000 differential genes. Notably, the largest graphical path of training-regulated features was negatively correlated between males and females, with sustained down-regulation in females and transient up-regulation at 1 week in males (Extended Data Fig. 11d ). The genes in this path were also associated with steroid hormone synthesis pathways and metabolism, particularly those pertaining to mitochondrial function (Supplementary Table 11 ). Further, transcription factor motif enrichment analysis of the transcripts in this path showed enrichment of 14 transcription factors (5% FDR; Supplementary Table 17 ), including the metabolism-regulating factors PPARγ, PPARα and oestrogen-related receptor gamma (ERRγ). The gene-expression levels of several significantly enriched transcription factors themselves followed the same trajectory as this path (Extended Data Fig. 11e ).
In the rat lung, we observed decreased phosphosignalling activity with training primarily in males (Fig. 3b ). Among these, the PRKACA phosphorylation signature showed the largest sex difference at 1 and 2 weeks (Extended Data Fig. 11f–h and Supplementary Table 8 ). PRKACA is a kinase that is involved in signalling within multiple cellular pathways. However, four PRKACA substrates followed this pattern and were associated with cellular structures (such as cytoskeleton and cell–cell junctions): DSP, MYLK, STMN1 and SYNE1 (Extended Data Fig. 11i ). The phosphorylation of these proteins suggests a sex-dependent role of PRKACA in mediating changes in lung structure or mechanical function with training. This is supported as DSP and MYLK have essential roles in alveolar and epithelial cell remodelling in the lung 26 , 27 .
Immune pathway enrichment analysis of training-regulated transcripts at 8 weeks showed limited enrichment in muscle (heart, gastrocnemius and vastus lateralis) and brain (cortex, hippocampus, hypothalamus), down-regulation in the lung and small intestine, and strong up-regulation in brown and white adipose tissue in males only (Fig. 5a , Extended Data Fig. 12a and Supplementary Table 11 ). Many of the same immune pathways (Supplementary Table 18 ) and immune-related transcription factors (Supplementary Table 19 ) were enriched in both adipose tissues in males. Furthermore, correlation between the transcript expression profiles of male-specific up-regulated features in the adipose tissues and immune cell markers from external cell-typing assays revealed a strong positive correlation for many immune cell types, including B, T and natural killer cells, and low correlation with platelets, erythrocytes and lymphatic tissue (Fig. 5b,c , Methods and Supplementary Table 20 ). These patterns suggest recruitment of peripheral immune cells or proliferation of tissue-resident immune cells as opposed to non-biological variation in blood or lymph content. Correlations at the protein level were not as marked (Extended Data Fig. 12b,c ). Complementary analyses using CIBERTSORTx produced similar results (Extended Data Fig. 12d,e ). In summary, our data suggest an important role of immune cell activity in the adaptation of male adipose tissue to endurance training.
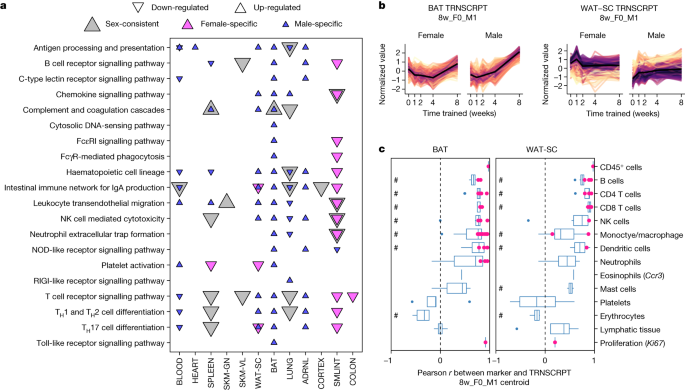
a , Enrichment analysis results of the training-differential transcripts at 8 weeks in Kyoto Encyclopedia of Genes and Genomes (KEGG) immune system pathways (10% FDR). NK, natural killer. b , Line plots of standardized abundances of selected training-differential transcripts. Brown and white adipose tissue show male-specific up-regulation at week 8 (8w_F0_M1). The small intestine (SMLINT) shows down-regulation in females and partial down-regulation in males at week 8 (8w_F-1_M0 or 8w_F-1_M-1). c , Box plots of the sample-level Pearson correlation between markers of immune cell types, lymphatic tissue or cell proliferation and the average value of features in b at the transcript level. A pink dot indicates that the marker is also one of the differential features plotted in b . A pound sign indicates that the distribution of Pearson correlations for a set of at least two markers is significantly different from 0 (two-sided one-sample t -test, 5% FDR). When only one marker is used to define a category on the y axis, the gene name is provided in parentheses. In box plots, the centre line represents median, box bounds represent 25th and 75th percentiles, whiskers represent minimum and maximum excluding outliers and blue dots represent outliers.
The small intestine was among the tissues with the highest enrichment in immune-related pathways (Extended Data Fig. 12a ), with down-regulation of transcripts at 8 weeks, and a more robust response in females (Fig. 5b ). This transcript set was significantly enriched with pathways related to gut inflammation (Supplementary Table 11 ). We observed positive associations between these transcripts and markers of several immune cell types, including B, T, natural killer and dendritic cells, suggesting decreased abundance (Fig. 5c and Supplementary Discussion ). Endurance training also decreased the expression of transcripts with genetic risk loci for inflammatory bowel disease (IBD), including major histocompatability complex class II 28 , a finding that also emerged through the DO enrichment analysis (Supplementary Table 15 ). Endurance training is suggested to reduce systemic inflammation, in part by increasing gut microbial diversity and gut barrier integrity 29 . In accordance, we observed decreases in Cxcr3 and Il1a with training (Extended Data Fig. 12f ), both of which are implicated in the pathogenesis of IBD 30 , 31 . Together, these data suggest that endurance training improves gut homeostasis, potentially conferring systemic anti-inflammatory effects.
Multi-tissue changes in mitochondria and lipids
We summarized the organism-wide metabolic changes for metabolomic datasets using RefMet metabolite classes (Fig. 6a and Supplementary Table 21 ) and for non-metabolomics datasets using metabolic subcategories of KEGG pathways (10% FDR; Extended Data Fig. 13a and Supplementary Table 11 ). The liver showed the greatest number of significantly enriched metabolite classes, followed by the heart, lung and hippocampus (Fig. 6a and Supplementary Discussion ). Inspection of individual metabolites and acylcarnitine groups revealed changes associated with functional alterations in response to training (Extended Data Fig. 13b–d and Supplementary Discussion ). Of particular interest, trimethylamine- N -oxide has been associated with cardiovascular disease 32 . We observed up-regulation of 1-methylhistidine, a marker of muscle protein turnover, in the kidney at 1, 2 and 4 weeks, which may indicate muscle breakdown and clearance through the kidney during early training time points. Cortisol levels were increased as expected from the physiological stress of training, and we observed a substantial increase in the kidney, again probably owing to renal clearance 33 . The liver showed up-regulation of 1-methylnicotinamide, which may have a role in inflammation 34 , at 8 weeks.
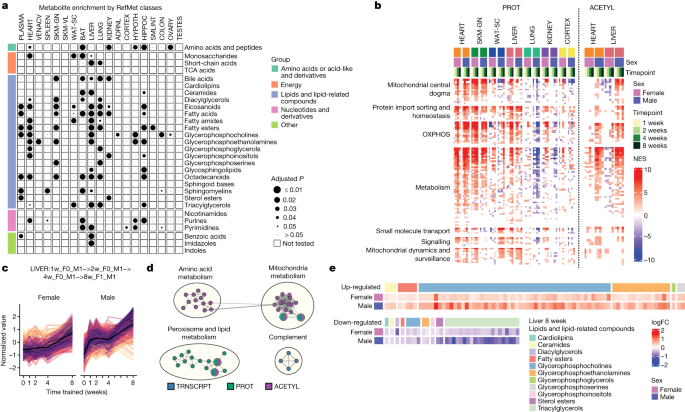
a , RefMet metabolite class enrichment calculated using GSEA with the −log 10 training P value. Significant chemical class enrichments (5% FDR) are shown as black circles with size is proportional to FDR. Small grey circles are chemical class enrichments that were not significant, and blank cells were not tested owing to low numbers of detected metabolites. TCA, tricarboxylic acid cycle. b , GSEA results using the MitoCarta MitoPathways gene set database and proteomics (PROT) or acetylome (ACETYL) timewise summary statistics for training. NESs are shown for significant pathways (10% FDR). Mitochondrial pathways shown as rows are grouped using the parental group in the MitoPathways hierarchy. OXPHOS, oxidative phosphorylation. c , Line plots of standardized abundances of liver training-differential features across all data types that are up-regulated in both sexes, with a later response in females (LIVER: 1w_F0_M1 − >2w_F0_M1 − >4w_F0_M1 − >8w_F1_M1). The black line represents the average value across all features. d , Network view of pathway enrichment results corresponding to features in c . Nodes indicate significantly enriched pathways (10% FDR); edges connect nodes if there is a similarity score of at least 0.375 between the gene sets driving each pathway enrichment. Node colours indicate omes in which the enrichment was observed. e , log 2 fold changes (logFC) relative to sedentary controls for metabolites within the ‘Lipids and lipid related compounds’ category in the 8-week liver. Heat map colour represents fold change (red, positive; blue, negative). Compounds are grouped into columns based on category (coloured bars).
The heart showed enrichment of various carbohydrate metabolism subcategories across many omes (Extended Data Fig. 13a ), and remarkably, all enzymes within the glycolysis–gluconeogenesis pathway showed a consistent increase in abundance, except for GPI, FBP2 and DLAT (Extended Data Fig. 13e ). Oxidative phosphorylation was enriched in most tissues and is consistent with the joint analyses of the muscle tissues (Fig. 4c ), suggesting potential changes in mitochondria biogenesis. We estimated proportional mitochondrial changes to endurance training using mitochondrial RNA-sequencing (RNA-seq) reads (Extended Data Fig. 14a–c ) and changes of mitochondrial functions through GSEA using gene expression, protein abundance and protein PTMs (Fig. 6b , Extended Data Fig. 14d and Supplementary Tables 22 – 25 ). Increased mitochondrial biogenesis was observed in skeletal muscle, heart and liver across these analyses. Moreover, sex-specific mitochondrial changes were observed in the adrenal gland, as described above, and in the colon, lung and kidney. These results highlight a highly adaptive and pervasive mitochondrial response to endurance training; a more in-depth analysis of this response is provided elsewhere 35 .
In the liver, we observed substantial regulation of metabolic pathways across the proteome, acetylome and lipidome (Fig. 6a,b and Extended Data Fig. 13a ). For example, there was significant enrichment in 12 metabolite classes belonging to ‘lipids and lipid-related compounds’ (Fig. 6a and Supplementary Table 26 ). We therefore focused on the large group of features that increased in abundance over time for both sexes (Fig. 6c ). Most of these liver features corresponded to protein abundance and protein acetylation changes in the mitochondrial, amino acid and lipid metabolic pathways (Fig. 6d and Supplementary Table 27 ). We also observed an increase in phosphatidylcholines and a concomitant decrease in triacylglycerols (Fig. 6e ). Finally, there was increased abundance and acetylation of proteins from the peroxisome, an organelle with key functions in lipid metabolism (Extended Data Fig. 14e ). To our knowledge, these extensive changes in protein acetylation in response to endurance training have not been described previously. Together, these molecular adaptations may constitute part of the mechanisms underlying exercise-mediated improvements in liver health, particularly protection against excessive intrahepatic lipid storage and steatosis 36 .
Mapping the molecular exercise responses across a whole organism is critical for understanding the beneficial effects of exercise. Previous studies are limited to a few tissues, a narrow temporal range, or a single sex. Substantially expanding on the current work in the field, we used 25 distinct molecular platforms in as many as 19 tissues to study the temporal changes to endurance exercise training in male and female rats. Accordingly, we identified thousands of training-induced changes within and across tissues, including temporal and sex-biased responses, in mRNA transcripts, proteins, post-translational modifications and metabolites. Each omic dataset provides unique insights into exercise adaptation, where a holistic understanding requires multi-omic analysis. This work illustrates how mining our data resource can both recapitulate expected mechanisms and provide novel biological insights.
This work can be leveraged to deepen our understanding of exercise-related improvement of health and disease management. The global heat shock response to exercise may confer cytoprotective effects, including in pathologies related to tissue damage and injury recovery 37 . Increased acetylation of liver mitochondrial enzymes and regulation of lipid metabolism may link exercise to protection against non-alcoholic fatty liver disease and steatohepatitis 36 . Similarly, exercise-mediated modulation of cytokines, receptors and transcripts linked to intestinal inflammation or IBD may be associated with improved gut health. These examples highlight unique training responses illuminated by a multi-omics approach that can be leveraged for future hypothesis-driven research on how exercise improves whole-body and tissue-specific health.
We note limitations in our experimental design, datasets and analyses ( Supplementary Discussion ). In short, samples were collected 48 h after the last exercise bout to capture sustained alterations, thereby excluding acute responses. Our assays were performed on bulk tissue and do not cover single-cell platforms. Our resource has limited omic characterization for certain tissues, and additional platforms with emerging biological relevance were not utilized, including microbiome profiling. Moreover, our results are hypothesis-generating and require biological validation; supporting this, we have established a publicly accessible tissue bank from this study.
This MoTrPAC resource provides future opportunities to enhance and refine the molecular map of the endurance training response. We expect that this dataset will remain an ongoing platform to translate tissue- and sex-specific molecular changes in rats to humans. MoTrPAC has made extensive efforts to facilitate access, exploration and interpretation of this resource. We developed the MoTrPAC Data Hub to easily explore and download data ( https://motrpac-data.org/ ), software packages to provide reproducible source code and facilitate data retrieval and analysis in R (MotrpacRatTraining6mo and MotrpacRatTraining6moData 38 , 39 ), and visualization tools for data exploration ( https://data-viz.motrpac-data.org ). Altogether, this multi-omic resource serves as a broadly useful reference for studying the milieu of molecular changes in endurance training adaptation and provides new opportunities to understand the effects of exercise on health and disease.
All methods are included in the Supplementary Information .
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
MoTrPAC data are publicly available via http://motrpac-data.org/data-access . Data access inquiries should be sent to [email protected]. Additional resources can be found at http://motrpac.org and https://motrpac-data.org/ . Interactive data visualizations are provided through a website ( https://data-viz.motrpac-data.org ) and HTML reports summarizing the multi-omic graphical analysis results in each tissue 40 . Processed data and analysis results are additionally available in the MotrpacRatTraining6moData R package 39 ( https://github.com/MoTrPAC/MotrpacRatTraining6moData ). Raw and processed data for were deposited in the appropriate public repositories as follows. RNA-seq, ATAC-seq and RRBS data were deposited at the Sequence Read Archive under accession PRJNA908279 and at the Gene Expression Omnibus under accession GSE242358 ; multiplexed immunoassays were deposited at IMMPORT under accession SDY2193 ; metabolomics data were deposited at Metabolomics Workbench under project ID PR001020 ; and proteomics data were deposited at MassIVE under accessions MSV000092911 , MSV000092922 , MSV000092923 , MSV000092924 , MSV000092925 and MSV000092931 . We used the following external datasets: release 96 of the Ensembl R. norvegicus (rn6) genome ( https://ftp.ensembl.org/pub/release-96/fasta/rattus_norvegicus/dna/ ) and gene annotation ( https://ftp.ensembl.org/pub/release-96/gtf/rattus_norvegicus/Rattus_norvegicus.Rnor_6.0.96.gtf.gz ); RefSeq protein database ( https://ftp.ncbi.nlm.nih.gov/refseq/R_norvegicus/ , downloaded 11/2018); the NCBI gene2refseq mapping files ( https://ftp.ncbi.nlm.nih.gov/gene/DATA/gene2refseq.gz , accessed 18 December 2020); RGD rat gene annotation ( https://download.rgd.mcw.edu/data_release/RAT/GENES_RAT.txt , accessed 12 November 2021); BioGRID v4.2.193 ( https://downloads.thebiogrid.org/File/BioGRID/Release-Archive/BIOGRID-4.2.193/BIOGRID-ORGANISM-4.2.193.tab3.zip ); STRING v11.5 ( https://stringdb-downloads.org/download/protein.physical.links.v11.5/10116.protein.physical.links.v11.5.txt.gz ); GENCODE release 39 metadata and annotation files ( https://ftp.ebi.ac.uk/pub/databases/gencode/Gencode_human/release_39/ , accessed 20 January 2022); MatrisomeDB ( https://doi.org/10.1093/nar/gkac1009 ); MitoPathways database available through MitoCarta ( https://personal.broadinstitute.org/scalvo/MitoCarta3.0/ ); PTMSigDB v1.9.0 PTM set database ( https://doi.org/10.1074/mcp.TIR118.000943 ); UniProt human proteome FASTA for canonical protein sequences (UniProtKB query “reviewed:true AND proteome:up000005640”, download date 3 March 2021); the CIBERSORT LM22 leukocyte gene signature matrix ( https://doi.org/10.1007/978-1-4939-7493-1_12 ); published results from Amar et al. 8 , Bye et al. 22 and Hostrup et al. 23 ; and GTEx v8 gene-expression data (dbGaP Accession phs000424.v8.p2). Details are provided in the Supplementary Information , Methods.
Code availability
Code for reproducing the main analyses is provided in the MotrpacRatTraining6mo R package 38 ( https://motrpac.github.io/MotrpacRatTraining6mo/ ). MoTrPAC data processing pipelines for RNA-seq, ATAC-seq, RRBS and proteomics are available in the following Github repositories: https://github.com/MoTrPAC/motrpac-rna-seq-pipeline 41 , https://github.com/MoTrPAC/motrpac-atac-seq-pipeline 42 , https://github.com/MoTrPAC/motrpac-rrbs-pipeline 43 and https://github.com/MoTrPAC/motrpac-proteomics-pipeline 44 . Normalization and quality control scripts are available at https://github.com/MoTrPAC/MotrpacRatTraining6moQCRep 45 .
Blair, S. N. et al. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 262 , 2395–2401 (1989).
Article CAS PubMed Google Scholar
Booth, F. W., Roberts, C. K. & Laye, M. J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2 , 1143–1211 (2012).
Article PubMed PubMed Central Google Scholar
Neufer, P. D. et al. Understanding the cellular and molecular mechanisms of physical activity-induced health benefits. Cell Metab. 22 , 4–11 (2015).
Sanford, J. A. et al. Molecular Transducers of Physical Activity Consortium (MoTrPAC): mapping the dynamic responses to exercise. Cell 181 , 1464–1474 (2020).
Article CAS PubMed PubMed Central Google Scholar
Nocon, M. et al. Association of physical activity with all-cause and cardiovascular mortality: a systematic review and meta-analysis. Eur. J. Cardiovasc. Prev. Rehabil. 15 , 239–246 (2008).
Article PubMed Google Scholar
Moore, S. C. et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA . Intern. Med. 176 , 816–825 (2016).
Google Scholar
Pedersen, B. K. & Saltin, B. Exercise as medicine — evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 25 , 1–72 (2015).
Amar, D. et al. Time trajectories in the transcriptomic response to exercise - a meta-analysis. Nat. Commun. 12 , 3471 (2021).
Article ADS CAS PubMed PubMed Central Google Scholar
Gibb, A. A. et al. Exercise-induced changes in glucose metabolism promote physiological cardiac growth. Circulation 136 , 2144–2157 (2017).
Lindholm, M. E. et al. An integrative analysis reveals coordinated reprogramming of the epigenome and the transcriptome in human skeletal muscle after training. Epigenetics 9 , 1557–1569 (2014).
Overmyer, K. A. et al. Maximal oxidative capacity during exercise is associated with skeletal muscle fuel selection and dynamic changes in mitochondrial protein acetylation. Cell Metab. 21 , 468–478 (2015).
Pillon, N. J. et al. Transcriptomic profiling of skeletal muscle adaptations to exercise and inactivity. Nat. Commun. 11 , 470 (2020).
Sato, S. et al. Atlas of exercise metabolism reveals time-dependent signatures of metabolic homeostasis. Cell Metab. 34 , 329–345.e8 (2022).
Many, G. M. Sexual dimorphism and the multi-omic response to exercise training in rat subcutaneous white adipose tissue. Nat. Metab. https://doi.org/10.1038/s42255-023-00959-9 (2024).
Henstridge, D. C., Febbraio, M. A. & Hargreaves, M. Heat shock proteins and exercise adaptations. Our knowledge thus far and the road still ahead. J. Appl. Physiol. 120 , 683–691 (2016).
Dumke, C. L., Kim, J., Arias, E. B. & Cartee, G. D. Role of kallikrein–kininogen system in insulin-stimulated glucose transport after muscle contractions. J. Appl. Physiol. 92 , 657–664 (2002).
Vettor, R. et al. Effect of exercise on plasma kallikrein and muscular phospholipase A2 activity in rats. Mol. Cell. Endocrinol. 45 , 65–70 (1986).
De Lisio, M. & Parise, G. Exercise and hematopoietic stem and progenitor cells: protection, quantity, and function. Exerc. Sport Sci. Rev. 41 , 116–122 (2013).
Cho, E.-G. et al. MEF2C enhances dopaminergic neuron differentiation of human embryonic stem cells in a parkinsonian rat model. PLoS ONE 6 , e24027 (2011).
Lin, R., Warn-Cramer, B. J., Kurata, W. E. & Lau, A. F. v-Src phosphorylation of connexin 43 on Tyr247 and Tyr265 disrupts gap junctional communication. J. Cell Biol. 154 , 815–827 (2001).
Oughtred, R. et al. The BioGRID database: a comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Sci. 30 , 187–200 (2021).
Bye, A. et al. Gene expression profiling of skeletal muscle in exercise-trained and sedentary rats with inborn high and low VO 2max . Physiol. Genomics 35 , 213–221 (2008).
Hostrup, M. et al. High-intensity interval training remodels the proteome and acetylome of human skeletal muscle. eLife 11 , e69802 (2022).
Yu, G., Wang, L.-G., Yan, G.-R. & He, Q.-Y. DOSE: an R/Bioconductor package for disease ontology semantic and enrichment analysis. Bioinformatics 31 , 608–609 (2015).
Aguilar, D. Heart failure, diabetes mellitus, and chronic kidney disease: a clinical conundrum. Circ. Heart Fail. 9 , e003316 (2016).
van Moorsel, C. H. M. Desmoplakin: an important player in aging lung disease. Am. J. Respir. Crit. Care Med. 202 , 1201–1202 (2020).
Wang, T. et al. Myosin light chain kinase (MYLK) coding polymorphisms modulate human lung endothelial cell barrier responses via altered tyrosine phosphorylation, spatial localization, and lamellipodial protrusions. Pulm. Circ. 8 , 2045894018764171 (2018).
Jostins, L. et al. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491 , 119–124 (2012).
Clarke, S. F. et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 63 , 1913–1920 (2014).
Lammers, K. M. et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology 135 , 194–204.e3 (2008).
Scarpa, M. et al. The epithelial danger signal IL-1α is a potent activator of fibroblasts and reactivator of intestinal inflammation. Am. J. Pathol. 185 , 1624–1637 (2015).
Wang, Z. et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472 , 57–63 (2011).
Daly, W., Seegers, C., Timmerman, S. & Hackney, A. C. Peak cortisol response to exhausting exercise: effect of blood sampling schedule. Med. Sportiva 8 , 17–20 (2004).
CAS Google Scholar
Zhang, W. et al. Nicotinamide N- methyltransferase ameliorates renal fibrosis by its metabolite 1-methylnicotinamide inhibiting the TGF-β1/Smad3 pathway. FASEB J. 36 , e22084 (2022).
CAS PubMed Google Scholar
Amar, D. et al. The mitochondrial multi-omic response to exercise training across tissues. Prepint at BioRxiv https://doi.org/10.1101/2023.01.13.523698 (2023).
Thyfault, J. P. & Rector, R. S. Exercise combats hepatic steatosis: potential mechanisms and clinical implications. Diabetes 69 , 517–524 (2020).
Dornbos, D. et al. Preischemic exercise reduces brain damage by ameliorating metabolic disorder in ischemia/reperfusion injury. J. Neurosci. Res. 91 , 818–827 (2013).
Gay, N. R., Amar, D., Beltran, P. M. J. & MoTrPAC Study Group. MotrpacRatTraining6mo: Analysis of the MoTrPAC endurance exercise training study in 6-month-old. R package version 1.6.3 https://motrpac.github.io/MotrpacRatTraining6mo/ (2023).
Gay, N. R. & MoTrPAC Study Group. MotrpacRatTraining6moData: Data for analysis of the MoTrPAC endurance exercise training study in 6-month-old rats. R package version 1.9.0 https://motrpac.github.io/MotrpacRatTraining6moData/ (2023).
Gay, N. R., Amar, D. & MoTrPAC Study Group. Visualization of graphical analysis results: Temporal dynamics of the multi-omic response to endurance exercise training across tissues. Zenodo https://doi.org/10.5281/zenodo.7703294 (2023).
Raja, A. et al. MoTrPAC/motrpac-rna-seq-pipeline. GitHub https://github.com/MoTrPAC/motrpac-rna-seq-pipeline (2023).
Gay, N. R., Raja, A. & MoTrPAC Study Group. MoTrPAC/motrpac-atac-seq-pipeline. GitHub https://github.com/MoTrPAC/motrpac-atac-seq-pipeline (2023).
Akre, S., Raja, A., Samdarshi, M. & MoTrPAC Study Group. MoTrPAC/motrpac-rrbs-pipeline. GitHub https://github.com/MoTrPAC/motrpac-rrbs-pipeline (2023).
Jimenez-Morales, D., Samdarshi, M., Hershman, S. & MoTrPAC Study Group. MoTrPAC/motrpac-proteomics-pipeline. GitHub https://github.com/MoTrPAC/motrpac-proteomics-pipeline (2023).
Amar, D., Samdarshi, M., Raja, A. & Gay, N. R. MoTrPAC/MotrpacRatTraining6moQCRep. GitHub https://github.com/MoTrPAC/MotrpacRatTraining6moQCRep (2023).
McCarron, A. et al. Phenotypic characterization and comparison of cystic fibrosis rat models generated using CRISPR/Cas9 gene editing. Am. J. Pathol. 190 , 977–993 (2020).
Download references
Acknowledgements
Funding: The MoTrPAC Study is supported by NIH grants U24OD026629 (Bioinformatics Center), U24DK112349, U24DK112342, U24DK112340, U24DK112341, U24DK112326, U24DK112331, U24DK112348 (Chemical Analysis Sites), U01AR071133, U01AR071130, U01AR071124, U01AR071128, U01AR071150, U01AR071160, U01AR071158 (Clinical Centers), U24AR071113 (Consortium Coordinating Center), U01AG055133, U01AG055137 and U01AG055135 (PASS/Animal Sites). This work was also supported by other funding sources: NHGRI Institutional Training Grant in Genome Science 5T32HG000044 (N.R.G.), National Science Foundation Graduate Research Fellowship Grant No. NSF 1445197 (N.R.G.), National Heart, Lung, and Blood Institute of the National Institute of Health F32 postdoctoral fellowship award F32HL154711 (P.M.J.B.), the Knut and Alice Wallenberg Foundation (M.E.L.), National Science Foundation Major Research Instrumentation (MRI) CHE-1726528 (F.M.F.), National Institute on Aging P30AG044271 and P30AG003319 (N.M.), and NORC at the University of Chicago grant no. P30DK07247 (E.R.). Parts of this work were performed in the Environmental Molecular Science Laboratory, a US Department of Energy national scientific user facility at Pacific Northwest National Laboratory in Richland, WA. The views expressed are those of the authors and do not necessarily reflect those of the NIH or the US Department of Health and Human Services. Some figures were created using Biorender.com. Fig. 1b was modified with permission from ref. 46 .
Author information
These authors contributed equally: David Amar, Nicole R. Gay, Pierre M. Jean-Beltran
These authors jointly supervised this work: Sue C. Bodine, Steven A. Carr, Karyn A. Esser, Stephen B. Montgomery, Simon Schenk, Michael P. Snyder, Matthew T. Wheeler
Authors and Affiliations
Department of Medicine, Stanford University, Stanford, CA, USA
David Amar, David Jimenez-Morales, Malene E. Lindholm, Shruti Marwaha, Archana Natarajan Raja, Jimmy Zhen, Euan Ashley, Matthew T. Wheeler, Karen P. Dalton, Steven G. Hershman, Mihir Samdarshi & Christopher Teng
Department of Genetics, Stanford University, Stanford, CA, USA
Nicole R. Gay, Bingqing Zhao, Jose J. Almagro Armenteros, Nasim Bararpour, Si Wu, Stephen B. Montgomery, Michael P. Snyder, Clarisa Chavez, Roxanne Chiu, Krista M. Hennig, Chia-Jui Hung, Christopher A. Jin & Navid Zebarjadi
Proteomics Platform, Broad Institute of MIT and Harvard, Cambridge, MA, USA
Pierre M. Jean-Beltran, Hasmik Keshishian, Natalie M. Clark, Steven A. Carr, D. R. Mani, Charles C. Mundorff & Cadence Pearce
Department of Internal Medicine, University of Iowa, Iowa City, IA, USA
Dam Bae, Ana C. Lira, Sue C. Bodine, Michael Cicha, Luis Gustavo Oliveira De Sousa, Bailey E. Jackson, Kyle S. Kramer, Andrea G. Marshall & Collyn Z-T. Richards
Department of Quantitative Health Sciences, Mayo Clinic, Rochester, MN, USA
Surendra Dasari
Metabolomics Platform, Broad Institute of MIT and Harvard, Cambridge, MA, USA
Courtney Dennis, Julian Avila-Pacheco & Clary B. Clish
Department of Internal Medicine, University of Michigan, Ann Arbor, MI, USA
Charles R. Evans & Charles F. Burant
School of Chemistry and Biochemistry, Georgia Institute of Technology, Atlanta, GA, USA
David A. Gaul, Evan M. Savage & Facundo M. Fernández
Department of Medicine, Duke University, Durham, NC, USA
Olga Ilkayeva, William E. Kraus & Kim M. Huffman
Duke Molecular Physiology Institute, Duke University, Durham, NC, USA
Olga Ilkayeva, Michael J. Muehlbauer, William E. Kraus, Christopher Newgard, Kim M. Huffman & Megan E. Ramaker
Emory Integrated Metabolomics and Lipidomics Core, Emory University, Atlanta, GA, USA
Anna A. Ivanova, Xueyun Liu & Kristal M. Maner-Smith
BRCF Metabolomics Core, University of Michigan, Ann Arbor, MI, USA
Maureen T. Kachman, Alexander (Sasha) Raskind & Tanu Soni
Division of Endocrinology, Nutrition, and Metabolism, Mayo Clinic, Rochester, MN, USA
Ian R. Lanza
Department of Neurology, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Venugopalan D. Nair, Gregory R. Smith, Yongchao Ge, Stuart C. Sealfon, Mary Anne S. Amper, Kristy Guevara, Nada Marjanovic, German Nudelman, Hanna Pincas, Irene Ramos, Stas Rirak, Aliza B. Rubenstein, Frederique Ruf-Zamojski, Nitish Seenarine, Sindhu Vangeti, Mital Vasoya, Alexandria Vornholt, Xuechen Yu & Elena Zaslavsky
Environmental Molecular Sciences Division, Pacific Northwest National Laboratory, Richland, WA, USA
Paul D. Piehowski
Department of Pathology and Laboratory Medicine, University of Vermont, Burlington, VT, USA
Jessica L. Rooney, Russell Tracy, Elaine Cornell, Nicole Gagne & Sandy May
Department of Pathology, Stanford University, Stanford, CA, USA
Kevin S. Smith, Nikolai G. Vetr, Stephen B. Montgomery & Daniel Nachun
Department of Biostatistics and Data Science, Wake Forest University School of Medicine, Winston-Salem, NC, USA
Cynthia L. Stowe, Fang-Chi Hsu, Scott Rushing & Michael P. Walkup
Biological Sciences Division, Pacific Northwest National Laboratory, Richland, WA, USA
Gina M. Many, James A. Sanford, Joshua N. Adkins, Wei-Jun Qian, Marina A. Gritsenko, Joshua R. Hansen, Chelsea Hutchinson-Bunch, Matthew E. Monroe, Ronald J. Moore, Michael D. Nestor, Vladislav A. Petyuk & Tyler J. Sagendorf
Department of Biochemistry, Emory University, Atlanta, GA, USA
Tiantian Zhang, Zhenxin Hou & Eric A. Ortlund
Section on Integrative Physiology and Metabolism, Joslin Diabetes Center, Boston, MA, USA
David M. Presby, Laurie J. Goodyear, Brent G. Albertson, Tiziana Caputo, Michael F. Hirshman, Nathan S. Makarewicz, Pasquale Nigro & Krithika Ramachandran
Department of Human Genetics, University of Michigan, Ann Arbor, MI, USA
Alec Steep & Jun Z. Li
Department of Pharmacological Sciences, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Yifei Sun & Martin J. Walsh
Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Aging and Metabolism Research Program, Oklahoma Medical Research Foundation, Oklahoma City, OK, USA
- Sue C. Bodine
Department of Physiology and Aging, University of Florida, Gainesville, FL, USA
Karyn A. Esser & Marco Pahor
Department of Orthopaedic Surgery, School of Medicine, University of California, San Diego, La Jolla, CA, USA
Simon Schenk
Department of Biomedical Data Science, Stanford University, Stanford, CA, USA
Stephen B. Montgomery
Department of Biostatistics, University of Alabama at Birmingham, Birmingham, AL, USA
Gary Cutter
Division of Cardiovascular Medicine, Beth Israel Deaconess Medical Center, Boston, MA, USA
Robert E. Gerszten & Jeremy M. Robbins
Division of Public Health Sciences, Wake Forest University School of Medicine, Winston-Salem, NC, USA
Michael E. Miller
Department of Medicine, Mayo Clinic, Rochester, MN, USA
K. Sreekumaran Nair
Department of Statistics, Stanford University, Stanford, CA, USA
Trevor Hastie & Rob Tibshirani
Department of Biomedical Data Sciences, Stanford University, Stanford, CA, USA
Rob Tibshirani
Department of Aging and Geriatric Research, University of Florida, Gainesville, FL, USA
Brian Bouverat, Christiaan Leeuwenburgh & Ching-ju Lu
Section on Gerontology and Geriatric Medicine, Wake Forest University School of Medicine, Winston-Salem, NC, USA
- Barbara Nicklas
Department of Health and Exercise Science, Wake Forest University School of Medicine, Winston-Salem, NC, USA
W. Jack Rejeski
National Institute on Aging, National Institutes of Health, Bethesda, MD, USA
- John P. Williams
National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, USA
Applied Physiology and Kinesiology, University of Florida, Gainesville, FL, USA
Elisabeth R. Barton
Department of Biomedical Sciences, University of Missouri, Columbia, MO, USA
Frank W. Booth
Department of Medical Pharmacology and Physiology, University of Missouri, Columbia, MO, USA
Department of Nutrition and Exercise Physiology, University of Missouri, Columbia, MO, USA
Frank W. Booth & R. Scott Rector
Dalton Cardiovascular Research Center, University of Missouri, Columbia, MO, USA
Department of Kinesiology and Health Education, University of Texas, Austin, TX, USA
Roger Farrar
Department of Medicine, Division of Endocrinology and Diabetes, University of California, Los Angeles, CA, USA
Andrea L. Hevener
Center for Public Health Genomics, University of Virginia School of Medicine, Charlottesville, VA, USA
Benjamin G. Ke & Chongzhi Zang
Section on Clinical, Behavioral, and Outcomes Research, Joslin Diabetes Center, Boston, MA, USA
Sarah J. Lessard
Department of Molecular Physiology and Biophysics, Vanderbilt University, Nashville, TN, USA
Andrea G. Marshall
Department of Health Sciences, Stetson University, Deland, FL, USA
Scott Powers
Department of Medicine, University of Missouri, Columbia, MO, USA
R. Scott Rector
NextGen Precision Health, University of Missouri, Columbia, MO, USA
Cell Biology and Physiology, Internal Medicine, University of Kansas Medical Center, Kansas City, KS, USA
John Thyfault
Center for Skeletal Muscle Research at Robert M. Berne Cardiovascular Research Center, University of Virginia School of Medicine, Charlottesville, VA, USA
Department of Medicine, University of Virginia School of Medicine, Charlottesville, VA, USA
Department of Pharmacology, University of Virginia School of Medicine, Charlottesville, VA, USA
Department of Molecular Physiology and Biological Physics, University of Virginia School of Medicine, Charlottesville, VA, USA
Fralin Biomedical Research Institute, Center for Exercise Medicine Research at Virginia Tech Carilion, Roanoke, VA, USA
Department of Human Nutrition, Foods, and Exercise, College of Agriculture and Life Sciences, Virginia Tech, Blacksburg, VA, USA
Department of Computational and Systems Biology, University of Pittsburgh, Pittsburgh, PA, USA
Ali Tugrul Balci & Maria Chikina
Petit Institute of Bioengineering and Biosciences, Georgia Institute of Technology, Atlanta, GA, USA
Samuel G. Moore
Department of Medicine, Emory University, Atlanta, GA, USA
Karan Uppal
Department of Cell, Developmental, and Integrative Biology, University of Alabama at Birmingham, Birmingham, AL, USA
Marcas Bamman & Anna Thalacker-Mercer
Department of Medicine, University of Colorado Anschutz Medical Campus, Aurora, CO, USA
Bryan C. Bergman, Daniel H. Bessesen, Wendy M. Kohrt, Edward L. Melanson, Kerrie L. Moreau, Irene E. Schauer & Robert S. Schwartz
Department of Medicine, University of Alabama at Birmingham, Birmingham, AL, USA
Thomas W. Buford
Human Performance Laboratory, Ball State University, Muncie, IN, USA
Toby L. Chambers, Bridget Lester, Scott Trappe & Todd A. Trappe
Translational Research Institute, AdventHealth, Orlando, FL, USA
Paul M. Coen, Bret H. Goodpaster & Lauren M. Sparks
Department of Pediatrics, University of California, Irvine, CA, USA
Dan Cooper, Fadia Haddad & Shlomit Radom-Aizik
Pennington Biomedical Research Center, Baton Rouge, LA, USA
Kishore Gadde, Melissa Harris, Neil M. Johannsen, Tuomo Rankinen & Eric Ravussin
College of Nursing, University of Colorado Anschutz Medical Campus, Aurora, CO, USA
Catherine M. Jankowski
Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA
Nicolas Musi
Population and Public Health, Pennington Biomedical Research Center, Baton Rouge, LA, USA
Robert L. Newton Jr
Biochemistry and Structural Biology, Center for Metabolic Health, Barshop Institute for Longevity and Aging Studies, University of Texas Health Science Center, San Antonio, TX, USA
Blake B. Rasmussen
Barshop Institute for Longevity and Aging Studies, University of Texas Health Science Center, San Antonio, TX, USA
Elena Volpi
MoTrPAC Study Group
- Primary authors
Lead Analysts
- , Nicole R. Gay
- , Pierre M. Jean-Beltran
Lead Data Generators
- , Surendra Dasari
- , Courtney Dennis
- , Charles R. Evans
- , David A. Gaul
- , Olga Ilkayeva
- , Anna A. Ivanova
- , Maureen T. Kachman
- , Hasmik Keshishian
- , Ian R. Lanza
- , Ana C. Lira
- , Michael J. Muehlbauer
- , Venugopalan D. Nair
- , Paul D. Piehowski
- , Jessica L. Rooney
- , Kevin S. Smith
- , Cynthia L. Stowe
- & Bingqing Zhao
- Natalie M. Clark
- , David Jimenez-Morales
- , Malene E. Lindholm
- , Gina M. Many
- , James A. Sanford
- , Gregory R. Smith
- , Nikolai G. Vetr
- , Tiantian Zhang
- , Bingqing Zhao
- , Jose J. Almagro Armenteros
- , Julian Avila-Pacheco
- , Nasim Bararpour
- , Yongchao Ge
- , Zhenxin Hou
- , Shruti Marwaha
- , David M. Presby
- , Archana Natarajan Raja
- , Evan M. Savage
- , Alec Steep
- , Yifei Sun
- , Si Wu
- & Jimmy Zhen
Animal Study Leadership
- , Karyn A. Esser
- , Laurie J. Goodyear
- & Simon Schenk
Manuscript Writing Group Leads
- Nicole R. Gay
- & David Amar
Manuscript Writing Group
- Malene E. Lindholm
- , Simon Schenk
- , Stephen B. Montgomery
- , Sue C. Bodine
- , Facundo M. Fernández
- , Stuart C. Sealfon
- , Michael P. Snyder
- & Tiantian Zhang
Senior Leadership
- Joshua N. Adkins
- , Euan Ashley
- , Charles F. Burant
- , Steven A. Carr
- , Clary B. Clish
- , Gary Cutter
- , Robert E. Gerszten
- , William E. Kraus
- , Jun Z. Li
- , Michael E. Miller
- , K. Sreekumaran Nair
- , Christopher Newgard
- , Eric A. Ortlund
- , Wei-Jun Qian
- , Russell Tracy
- , Martin J. Walsh
- & Matthew T. Wheeler
Co-corresponding Authors
Bioinformatics center.
- , Karen P. Dalton
- , Trevor Hastie
- , Steven G. Hershman
- , Mihir Samdarshi
- , Christopher Teng
- , Rob Tibshirani
- , Matthew T. Wheeler
Biospecimens Repository
- Elaine Cornell
- , Nicole Gagne
- , Sandy May
- & Russell Tracy
Administrative Coordinating Center
- Brian Bouverat
- , Christiaan Leeuwenburgh
- , Ching-ju Lu
- & Marco Pahor
Data Management, Analysis, and Quality Control Center
- Fang-Chi Hsu
- , Scott Rushing
- & Michael P. Walkup
Exercise Intervention Core
- & W. Jack Rejeski
- & Ashley Xia

Preclinical Animal Study Sites
- Brent G. Albertson
- , Dam Bae
- , Elisabeth R. Barton
- , Frank W. Booth
- , Tiziana Caputo
- , Michael Cicha
- , Luis Gustavo Oliveira De Sousa
- , Roger Farrar
- , Andrea L. Hevener
- , Michael F. Hirshman
- , Bailey E. Jackson
- , Benjamin G. Ke
- , Kyle S. Kramer
- , Sarah J. Lessard
- , Nathan S. Makarewicz
- , Andrea G. Marshall
- , Pasquale Nigro
- , Scott Powers
- , Krithika Ramachandran
- , R. Scott Rector
- , Collyn Z-T. Richards
- , John Thyfault
- , Zhen Yan
- & Chongzhi Zang
Chemical Analysis Sites
- , Mary Anne S. Amper
- , Ali Tugrul Balci
- , Clarisa Chavez
- , Maria Chikina
- , Roxanne Chiu
- , Natalie M. Clark
- , Marina A. Gritsenko
- , Kristy Guevara
- , Joshua R. Hansen
- , Krista M. Hennig
- , Chia-Jui Hung
- , Chelsea Hutchinson-Bunch
- , Christopher A. Jin
- , Xueyun Liu
- , Kristal M. Maner-Smith
- , D. R. Mani
- , Nada Marjanovic
- , Matthew E. Monroe
- , Ronald J. Moore
- , Samuel G. Moore
- , Charles C. Mundorff
- , Daniel Nachun
- , Michael D. Nestor
- , German Nudelman
- , Cadence Pearce
- , Vladislav A. Petyuk
- , Hanna Pincas
- , Irene Ramos
- , Alexander (Sasha) Raskind
- , Stas Rirak
- , Jeremy M. Robbins
- , Aliza B. Rubenstein
- , Frederique Ruf-Zamojski
- , Tyler J. Sagendorf
- , Nitish Seenarine
- , Tanu Soni
- , Karan Uppal
- , Sindhu Vangeti
- , Mital Vasoya
- , Alexandria Vornholt
- , Xuechen Yu
- , Elena Zaslavsky
- , Navid Zebarjadi
Clinical Sites
- Marcas Bamman
- , Bryan C. Bergman
- , Daniel H. Bessesen
- , Thomas W. Buford
- , Toby L. Chambers
- , Paul M. Coen
- , Dan Cooper
- , Fadia Haddad
- , Kishore Gadde
- , Bret H. Goodpaster
- , Melissa Harris
- , Kim M. Huffman
- , Catherine M. Jankowski
- , Neil M. Johannsen
- , Wendy M. Kohrt
- , Bridget Lester
- , Edward L. Melanson
- , Kerrie L. Moreau
- , Nicolas Musi
- , Robert L. Newton Jr
- , Shlomit Radom-Aizik
- , Megan E. Ramaker
- , Tuomo Rankinen
- , Blake B. Rasmussen
- , Eric Ravussin
- , Irene E. Schauer
- , Robert S. Schwartz
- , Lauren M. Sparks
- , Anna Thalacker-Mercer
- , Scott Trappe
- , Todd A. Trappe
- & Elena Volpi
Contributions
All authors reviewed and revised the manuscript. Detailed author contributions are provided in the Supplementary Information .
Corresponding authors
Correspondence to Sue C. Bodine , Karyn A. Esser , Simon Schenk , Stephen B. Montgomery , Michael P. Snyder , Steven A. Carr or Matthew T. Wheeler .
Ethics declarations
Competing interests.
S.C.B. has equity in Emmyon, Inc. G.R.C. sits on data and safety monitoring boards for AI Therapeutics, AMO Pharma, Astra-Zeneca, Avexis Pharmaceuticals, Biolinerx, Brainstorm Cell Therapeutics, Bristol Meyers Squibb/Celgene, CSL Behring, Galmed Pharmaceuticals, Green Valley Pharma, Horizon Pharmaceuticals, Immunic, Mapi Pharmaceuticals, Merck, Mitsubishi Tanabe Pharma Holdings, Opko Biologics, Prothena Biosciences, Novartis, Regeneron, Sanofi-Aventis, Reata Pharmaceuticals, NHLBI (protocol review committee), University of Texas Southwestern, University of Pennsylvania, Visioneering Technologies, Inc.; serves on consulting or advisory boards for Alexion, Antisense Therapeutics, Biogen, Clinical Trial Solutions LLC, Genzyme, Genentech, GW Pharmaceuticals, Immunic, Klein-Buendel Incorporated, Merck/Serono, Novartis, Osmotica Pharmaceuticals, Perception Neurosciences, Protalix Biotherapeutics, Recursion/Cerexis Pharmaceuticals, Regeneron, Roche, SAB Biotherapeutics; and is the president of Pythagoras Inc., a private consulting company. S.A.C. is a member of the scientific advisory boards of Kymera, PrognomiQ, PTM BioLabs, and Seer. M.P.S. is a cofounder and scientific advisor to Personalis, Qbio, January AI, Filtricine, SensOmics, Protos, Fodsel, Rthm, Marble and scientific advisor to Genapsys, Swaz, Jupiter. S.B.M. is a consultant for BioMarin, MyOme and Tenaya Therapeutics. D.A. is currently employed at Insitro, South San Francisco, CA. N.R.G. is currently employed at 23andMe, Sunnyvale, CA. P.M.J.B. is currently employed at Pfizer, Cambridge, MA. Insitro, 23andMe and Pfizer had no involvement in the work presented here.
Peer review
Peer review information.
Nature thanks Atul Deshmukh, Jorge Ruas and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer review reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended data fig. 1 animal phenotyping and data availability..
a-d) Clinical measurements before and after the training intervention in untrained control rats (SED), 4-week trained rats (4w), and 8-week trained rats (8w). Data are displayed pre and post for each individual rat (connected by a line), with males in blue and females in pink. Filled symbols (n = 5 per sex and time point) represent rats used for all omics analyses, whereas the rat utilized for proteomics only (n = 1 per sex and time point) is represented by a non-filled symbol. Significant results by ANOVA of the overall group effect (#, p < 0.05; ##, p < 0.01) and interaction between group and time (§, p < 0.05; §§ p < 0.01) are indicated. Significant within-group differential responses from a Bonferroni post hoc test are indicated (*, q-value < 0.05; **, q-value < 0.01). a) Aerobic capacity through a VO 2 max test until exhaustion. Data are reported in ml/(kg.min) for all individual rats and time points. b) Body fat percentage. c) Percent lean mass. ( b-c ) were assessed through nuclear magnetic resonance spectroscopy. d) Body weight (in grams). e) Description of available datasets. Colored cells indicate that data are available for that tissue and assay. Individual panels and platforms are shown for metabolomics and the multiplexed immunoassays. f) Detailed availability of sample-level data across assays. Each column represents an individual animal, ordered by training group and colored by sex. Gray cells indicate that data were generated for that animal and assay; black cells indicate that data were not generated. Rows are ordered by ome and colored by assay and tissue.
Extended Data Fig. 2 Quality control metrics for omics data.
a) Proteomics multiplexing design using TMT11 reagents for isobaric tagging and a pooled reference sample. The diagram describes processing of a single tissue. Following multiplexing, peptides were used for protein abundance analysis, serial PTM enriched for phosphosite and optional acetylsite quantification, or ubiquitylsite quantification through enrichment of lysine-diglycine ubiquitin remnants. b) Total number of fully quantified proteins per plex in each global proteome dataset. c-e) The total number of fully quantified phosphosites (c) , acetylsites (d) , and ubiquitylsites (e) per plex in each dataset. f) Distributions of coefficients of variation (CVs) calculated from metabolomics features identified in pooled samples and analyzed periodically throughout liquid chromatography-mass spectrometry runs. CVs were aggregated and plotted separately for named and unnamed metabolites. g) Transcription start site (TSS) enrichment (top) and fraction of reads in peaks (FRiP, bottom) across ATAC-seq samples per tissue. h) Distributions of RNA integrity numbers (RIN, top) and median 5′ to 3′ bias (bottom) across samples in each tissue in the RNA-Seq data. i) Percent methylation of CpG, CHG and CHH sites in the RRBS data. For boxplots in (h,i) : center line represents median; box bounds represent 25th and 75th percentiles; whiskers represent minimum and maximum excluding outliers; filled dots represent outliers. j) Number of wells across multiplexed immunoassays with fewer than 20 beads. Measurements from these 182 wells were excluded from downstream analysis. k) 2D density plot of targeted analytes’ mean fluorescence intensity (MFI) versus corresponding CHEX4 MFI from the same well for each multiplexed immunoassay measurement, where CHEX4 is a measure of non-specific binding.
Extended Data Fig. 3 Permutation tests.
a-b) Permutation tests of groups within males (a) and females (b) . For each sex, the original group labels were shuffled to minimize the number of animal pairs that remain in the same group. Only the group labels were shuffled and all other covariates remained as in the original data. For each permuted dataset, the differential abundance pipeline was rerun and the number of transcripts that were selected at 5% FDR adjustment were re-counted. c-d) Permutation tests of sex within groups. For each group and each sex, half of the animals were selected randomly and their sex was swapped. Only the sex labels were shuffled and all other covariates remained as in the original data. For each permutation the differential analysis pipeline was rerun and the timewise summary statistics were extracted. A gene was considered sexually dimorphic if for at least one time point the z-score (absolute) difference between males and females was greater than 3. c) Counts of sexually dimorphic genes among the IHW-selected genes of the original data. d) Counts of sexually dimorphic genes among the 5% FDR selected genes within each permuted dataset. Each boxplot in (a-d) represents the differential abundance analysis results over 100 permutations of the transcriptomics data in a specific tissue. Center line represents median; box bounds represent 25th and 75th percentiles; whiskers represent minimum and maximum excluding outliers; open circles represent outliers. Added points represent the results of the true data labels, and their shape corresponds to the empirical p-value ( ● : p > 0.05; ×: 0.01 < p < 0.05; *: p ≤ 0.01).
Extended Data Fig. 4 Correlations between proteins and transcripts throughout endurance training.
a) Number of tissues in which each gene, including features mapped to genes from all omes, is training-regulated. Only differential features from the subset of tissues with deep molecular profiling (lung, gastrocnemius, subcutaneous white adipose, kidney, liver, and heart) and the subset of omes that were profiled in all six of these tissues (DNA methylation, chromatin accessibility, transcriptomics, global proteomics, phosphoproteomics, multiplexed immunoassays) were considered. Numbers above each bar indicate the number of genes that are differential in exactly the number of tissues indicated on the x-axis. b) Pathways significantly enriched by tissue-specific training-regulated genes represented in Fig. 2a (q-value < 0.1). KEGG and Reactome pathways were queried, and redundant pathways were removed (i.e., those with an overlap of 80% or greater with an existing pathway). c) Heatmaps showing the Pearson correlation between the TRNSCRPT and PROT timewise summary statistics (z- and t-scores, respectively) (top, gene-level) and pathway-level enrichment results (Gene Set Enrichment Analysis normalized enrichment scores) (bottom, pathway-level). d) Scatter plots of pathway GSEA NES of the TRNSCRPT and PROT datasets in the seven tissues for which these data were acquired. Pathways showing high discordance or agreement across TRNSCRPT and PROT and with functional relevance or general interest were highlighted.
Extended Data Fig. 5 Heat shock response.
a) Scatter plots of the protein t-scores (PROT) versus the transcript z-scores (TRNSCRPT) by gene at 8 weeks of training (8 W) relative to sedentary controls. Data are shown for the seven tissues for which both proteomics and transcriptomics was acquired. Red points indicate genes associated with the heat shock response, and the labeled points indicate those with a large differential response at the protein level. b-c) Line plots showing protein b) and transcript (c) log 2 fold-changes relative to the untrained controls for a subset of heat shock proteins with increased abundance during exercise training. Each line represents a protein in a single tissue.
Extended Data Fig. 6 Regulatory signaling pathways modulated by endurance training.
a) Heatmap of differences in TF motif enrichment in training-regulated genes across tissues. Each value reflects the average difference in motif enrichment for shared transcription factors. Tissues are clustered with complete linkage hierarchical clustering. b) (left) Filtered PTM-SEA results for the liver showing kinases and signaling pathways with increased activity. (right) Heatmap showing t-scores for phosphosites within the HGF signaling pathway. c) Hypothetical model of HGF signaling effects during exercise training. Phosphorylation of STAT3 and PXN is known to modulate cell growth and cell migration, respectively. Error bars=SEM. d) Filtered PTM-SEA results for the heart showing selected kinases with significant enrichments in at least one time point. Heatmap shows the NES as color and enrichment p-value as dot size. Kinases are grouped by kinase family and sorted by hierarchical clustering. e) (top) Log 2 fold-change of GJA1 and CDH2 protein abundance in the heart. No significant response to exercise training was observed for these proteins (F-test; q-value > 0.05). (bottom) Log 2 fold-changes for selected Src kinase phosphosite targets, GJA1 pY265 and CDH2 pY820, in the heart. These phosphosites show a significant response to exercise training (F-test, 5% FDR). Error bars=SEM. f) Gene Set Enrichment Analysis (GSEA) results from the heart global proteome dataset using the matrisome gene set database. Heatmap shows NES as color and enrichment p-value as dot size. Rows are clustered using hierarchical clustering. g) Log 2 fold-change for basement membrane proteins in heart. Proteins showing a significant response to exercise training are highlighted in orange (F-test; 5% FDR). Error bars=SEM. h) Log 2 protein fold-change of NTN1 protein abundance in heart. A significant response to exercise training was observed for these proteins (F-test; 5% FDR). Error bars=SEM.
Extended Data Fig. 7 Graphical representation of differential results.
a) Number of training-regulated features assigned to groups of graphical states across tissues and time. Red points indicate features that are up-regulated in at least one sex (e.g., only in males: F0_M1; only in females: F1_M0; in both sexes: F1_M1), and blue points indicate features down-regulated in at least one sex (only in males: F0_M-1; only in females: F-1_M0; in both sexes: F-1_M-1). Green points indicate features that are up-regulated in males and down-regulated in females or vice versa (F-1_M1 and F1_M-1, respectively). Point size is proportional to the number of features. Point opacity is proportional to the within-tissue fraction of features represented by that point. Features can be represented in multiple points. The number of omes profiled in each tissue is provided in parentheses next to the tissue abbreviation. b) A schematic example of the graphical representation of the differential analysis results. Top: the z-scores of four features. A positive score corresponds to up-regulation (red), and a negative score corresponds to down regulation (blue). Bottom: the assignment of features to node sets and full path sets (edge sets are not shown for conciseness but can be easily inferred from the full paths). Node labels follow the [time]_F[x]_M[y] format where [time] shows the animal sacrifice week and can take one of (1w, 2w, 4w, or 8w), and [x] and [y] are one of (−1,0,1), corresponding to down-regulation, no effect, and up-regulation, respectively. c) Graphical representation of the feature sets. Columns are training time points, and rows are the differential abundance states. Node and edge sizes are proportional to the number of features that are assigned to each set.
Extended Data Fig. 8 Key pathway enrichments per tissue.
Key pathway enrichments for features that are up-regulated in both sexes at 8 weeks of training in each tissue. For display purposes, enrichment q-values were floored to 1e-10 (Enrichment FDR (−log10) = 10). Bars are colored by the number of omes for which the pathway was significantly enriched (q-value < 0.01) (lighter gray: 1 ome; darker gray: 2 omes; black: 3 omes). Pathways were selected from Supplementary Table 10 .
Extended Data Fig. 9 Associations with signatures of human health and complex traits.
a) Jaccard coefficients between gene sets identified by different omes in 8-week gastrocnemius up-regulated features (“X” marks overlap p > 0.05). b) Network connectivity p-values (Pathways, Biogrid, and string) among the gastrocnemius week-8 multi-omic genes and with the single-omic genes. c) Proportion of features from each ome represented in the gastrocnemius response clusters, identified by the network clustering analysis. d-g) Overlap between our rat vastus lateralis differential expression results and the meta-analysis of human long-term exercise studies by Amar et al. d-e) Spearman correlation (d) and its significance (e) between the meta-analysis fold-changes and the log 2 fold-changes foreach sex and time point. f) GSEA results. Genes were ranked by meta-analysis (−log 10 p-value*log 2 fold-change) and the rat training-differential, sex-consistent gene sets were tested for enrichment at the bottom of the ranking (negative scores) or the top (positive scores). g) Overlap between the rat gene sets from (f) and the high-heterogeneity human meta-analysis genes (I 2 > 75%). h) -log 10 overlap p-values (Fisher’s exact test), comparing rat female gastrocnemius and vastus lateralis week-8 differential transcripts from this study (p < 0.01) and the differential genes from the rat female soleus data of Bye et al. (p < 0.01). HCR: high capacity runners, LCR: low capacity runners. i) A comparison of rat gastrocnemius differential proteins from this study (p < 0.01) and the human endurance training proteomics results of Hostrup et al. (p < 0.01) using Fisher’s exact test. Left: -log 10 overlap p-values. Right: -log 10 sex concordance p-values. j) Statistics of the overlapping proteins from ( i ), week-8 female comparison (y: rat z-scores, x: human t-scores). k) DOSE disease enrichment results of the white adipose, kidney, and liver gene sets. DOSE was applied only on diseases that are relevant for each tissue. The network shows the results for the sex-consistent down-regulated features at week-8.
Extended Data Fig. 10 Characterization of the extent of sex difference in the endurance training response.
The extent of sex differences in the training response were characterized in two ways: first, by correlating log 2 fold-changes between males and females for each training-differential feature; second, by calculating the difference between the area under the log 2 fold-change curve for each training-differential feature, including a (0,0) point (Δ AUC , males - females). The first approach characterizes differences in direction of effect while the second approach characterizes differences in magnitude. Left plot for each tissue: density line plots of correlations from the first approach. Densities or correlations corresponding to features in each ome are plotted separately, with a label that provides the ome and the number of differential features represented. Right plot for each tissue: 2D density plot of Δ AUC against the correlation between the male and female log 2 fold-changes for each training-differential feature used to simultaneously evaluate sex differences in the direction and magnitude of the training response. Points at the top-center of these 2D density plots represent features with high similarity between males and females in terms of both direction and magnitude; features on the right and left sides of the plots represent features with greater magnitudes of response in males and females, respectively.
Extended Data Fig. 11 Sex differences in the endurance training response.
a) Heatmap of the training response of immunoassay analytes across tissues. Gray indicates no data. Bars indicate the number of training-regulated analytes in each tissue (top) and the number of tissues in which the analyte is training-regulated (right, 5% FDR). b) Training-differential cytokines across tissues. 5, 24, and 9 cytokines were annotated as anti-, pro-, and pro/anti- inflammatory, respectively. Bars indicate the number of annotated cytokines in each category that are differential (5% FDR). c) Counts of early vs. (1- or 2-week) vs. late (4- or 8-week) differential cytokines, according to states assigned by the graphical analysis, including all tissues. Cytokines with both early and late responses in the same tissue were excluded. d) Line plots of standardized abundances of training-differential features that follow the largest graphical path in the adrenal gland (i.e., 1w_F-1_M1 − >2w_F-1_M0 − >4w_F-1_M0 − >8w_F-1_M0 according to our graphical analysis notation). The black line represents the average value across all features. The closer a colored line is to this average, the darker it is (distance calculated using sum of squares). e) Line plots of transcript-level log 2 fold-changes corresponding to six transcription factors (TFs) whose motifs are significantly enriched by transcripts in (d) . TF motif enrichment q-values are provided in the legend (error bars = SEM). f) Male versus female NES from PTM-SEA in the lung. Anticorrelated points corresponding to PRKACA NES are in dark red. g) Line plots of standardized abundances of training-differential phosphosites that follow the largest graphical edges of phosphosites in the lung (1w_F1_M-1 − >2w_F1_M-1 − >4w_F0_M-1). h) Top ten kinases with the greatest over-representation of substrates (proteins) corresponding to training-differential phosphosites in (g) . MeanRank scores by library are shown, as reported by KEA3. i) Line plots showing phosphosite-level log 2 fold-changes of PRKACA phosphosite substrates identified in the lung as differential with disparate sex responses (error bars = SEM).
Extended Data Fig. 12 Assessment of immune responses to endurance training.
a) Heatmap of the number and percent of KEGG and Reactome immune pathways significantly enriched by training-regulated features at 8 weeks. b) Line plots of standardized abundances of training-differential proteins in white adipose tissue up-regulated only in males at 8 weeks. Black line shows average across all features. c) Boxplots of the sample-level Pearson correlation between markers of immune cell types, lymphatic tissue, or cell proliferation and the average value of features in (b) at the protein level. Center line represents median; box bounds represent 25th and 75th percentiles; whiskers represent minimum and maximum excluding outliers; filled dots represent outliers. A pink point indicates that the marker is also one of the differential features plotted in (b) . # indicates when the distribution of Pearson correlations for a set of at least two markers is significantly different from 0 (two-sided one-sample t-test, 5% BY FDR). When only one marker is used to define a category on the y-axis, the gene name is provided in parentheses. d) Trajectories of mean absolute signal of various immune cell types in BAT or WAT-SC following deconvolution of bulk RNA-Seq with CIBERSORTx (error bars = SEM). e) Immune cell type enrichment analysis results of training-differentially expressed transcripts. Points represent significant enrichments (5% FDR, one-sided Mann-Whitney U test). f) Line plots showing the log 2 fold-changes for Cxcr3 and Il1a transcripts in the small intestine (error bars = SEM).
Extended Data Fig. 13 Metabolic effects of endurance training.
a) Significant enrichments for relevant categories of KEGG metabolism pathways from features that are up- or down- regulated in both sexes at 8 weeks (8w_F1_M1 and 8w_F-1_M-1 nodes, respectively). Triangles point in the direction of the response (up or down). Points are colored by ome. b) Log 2 fold-change of metabolites regulated across many tissues (F-Test, 5% FDR, error bars=SEM). c) Log 2 fold-change of training-regulated metabolites: 1-methylhistidine in the kidney, cortisol in the kidney, and 1-methylnicotinamide in the liver (F-Test, 5% FDR, error bars = SEM). d) Volcano plots showing abundance changes (log 2 fold-changes; logFC) and significance (-log 10 nominal p-values) for acyl-carnitines. Features are colored based on the carnitine chain length. e) Protein abundance changes in the glycolysis and gluconeogenesis pathway in the heart tissue after 8 weeks of training. Line plots show the log 2 fold-changes over the training time course (error bars = SEM). Red and blue boxes indicate a statistically significant (F-test, 5% FDR) increase and decrease in abundance, respectively, for both males and females at 8 weeks.
Extended Data Fig. 14 Mitochondria and peroxisome adaptations to endurance training.
a) Boxplots showing the percent of mitochondrial genome reads across samples in each tissue that map to the mitochondrial genome (% MT reads). b) Comparison of % MT reads between untrained controls and animals trained for 8 weeks. Plot shows tissues with a statistically significant change after 8 weeks in at least one sex (red asterisk, two-sided Dunnett’s test, 10% FDR). For boxplots in (b,c) : center line represents median; box bounds represent 25th and 75th percentiles; whiskers represent minimum and maximum excluding outliers; filled dots represent outliers. c) Boxplots showing the percent of mitochondrial genome reads across tissue, sex, and time points. Center line represents median; box bounds represent 25th and 75th percentiles; whiskers represent minimum and maximum excluding outliers; open circles represent outliers. Red asterisks indicate a significant change throughout the training time course (F-test, 5% FDR). Center line represents median; box bounds represent 25th and 75th percentiles; whiskers represent minimum and maximum excluding outliers; blue dots represent outliers. d) GSEA using the MitoCarta MitoPathways gene set database and transcriptome (TRNSCRPT) or phosphoproteome (PHOSPHO) differential analysis results. NES are shown for significant pathways (10% FDR) for all tissues, sexes, and time points within the heatmap. Mitochondria pathways (rows) are grouped using the parental group in the MitoPathways hierarchy. e) Protein abundance and protein acetylation level changes in the peroxisome KEGG pathway in the liver tissue after 8 weeks of training. Red boxes indicate an increase in abundance for both males and females, while red circles indicate an increase in at least one acetylsite within the protein (8w_F1_M1 cluster).
Supplementary information
Supplementary information.
Supplementary Methods, Tables, discussion, author contributions and references.
Reporting Summary
Supplementary tables.
Supplementary Tables 1–27. See ‘README’ tab for details.
Peer Review File
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
MoTrPAC Study Group., Lead Analysts. & MoTrPAC Study Group. Temporal dynamics of the multi-omic response to endurance exercise training. Nature 629 , 174–183 (2024). https://doi.org/10.1038/s41586-023-06877-w
Download citation
Received : 21 September 2022
Accepted : 16 November 2023
Published : 01 May 2024
Issue Date : 02 May 2024
DOI : https://doi.org/10.1038/s41586-023-06877-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.

Generative A.I. Arrives in the Gene Editing World of CRISPR
Much as ChatGPT generates poetry, a new A.I. system devises blueprints for microscopic mechanisms that can edit your DNA.
The physical structure of OpenCRISPR-1, a gene editor created by A.I. technology from Profluent. Credit... Video by Profluent Bio
Supported by
- Share full article

By Cade Metz
Has reported on the intersection of A.I. and health care for a decade.
- April 22, 2024
Generative A.I. technologies can write poetry and computer programs or create images of teddy bears and videos of cartoon characters that look like something from a Hollywood movie.
Now, new A.I. technology is generating blueprints for microscopic biological mechanisms that can edit your DNA, pointing to a future when scientists can battle illness and diseases with even greater precision and speed than they can today.
Described in a research paper published on Monday by a Berkeley, Calif., startup called Profluent, the technology is based on the same methods that drive ChatGPT, the online chatbot that launched the A.I. boom after its release in 2022 . The company is expected to present the paper next month at the annual meeting of the American Society of Gene and Cell Therapy.
Much as ChatGPT learns to generate language by analyzing Wikipedia articles, books and chat logs, Profluent’s technology creates new gene editors after analyzing enormous amounts of biological data, including microscopic mechanisms that scientists already use to edit human DNA.
These gene editors are based on Nobel Prize-winning methods involving biological mechanisms called CRISPR. Technology based on CRISPR is already changing how scientists study and fight illness and disease , providing a way of altering genes that cause hereditary conditions, such as sickle cell anemia and blindness.

Previously, CRISPR methods used mechanisms found in nature — biological material gleaned from bacteria that allows these microscopic organisms to fight off germs.
“They have never existed on Earth,” said James Fraser, a professor and chair of the department of bioengineering and therapeutic sciences at the University of California, San Francisco, who has read Profluent’s research paper. “The system has learned from nature to create them, but they are new.”
The hope is that the technology will eventually produce gene editors that are more nimble and more powerful than those that have been honed over billions of years of evolution.
On Monday, Profluent also said that it had used one of these A.I.-generated gene editors to edit human DNA and that it was “open sourcing” this editor, called OpenCRISPR-1. That means it is allowing individuals, academic labs and companies to experiment with the technology for free.
A.I. researchers often open source the underlying software that drives their A.I. systems , because it allows others to build on their work and accelerate the development of new technologies. But it is less common for biological labs and pharmaceutical companies to open source inventions like OpenCRISPR-1.
Though Profluent is open sourcing the gene editors generated by its A.I. technology, it is not open sourcing the A.I. technology itself.

The project is part of a wider effort to build A.I. technologies that can improve medical care. Scientists at the University of Washington, for instance, are using the methods behind chatbots like OpenAI’s ChatGPT and image generators like Midjourney to create entirely new proteins — the microscopic molecules that drive all human life — as they work to accelerate the development of new vaccines and medicines.
(The New York Times has sued OpenAI and its partner, Microsoft, on claims of copyright infringement involving artificial intelligence systems that generate text.)
Generative A.I. technologies are driven by what scientists call a neural network , a mathematical system that learns skills by analyzing vast amounts of data. The image creator Midjourney, for example, is underpinned by a neural network that has analyzed millions of digital images and the captions that describe each of those images. The system learned to recognize the links between the images and the words. So when you ask it for an image of a rhinoceros leaping off the Golden Gate Bridge, it knows what to do.
Profluent’s technology is driven by a similar A.I. model that learns from sequences of amino acids and nucleic acids — the chemical compounds that define the microscopic biological mechanisms that scientists use to edit genes. Essentially, it analyzes the behavior of CRISPR gene editors pulled from nature and learns how to generate entirely new gene editors.
“These A.I. models learn from sequences — whether those are sequences of characters or words or computer code or amino acids,” said Profluent’s chief executive, Ali Madani, a researcher who previously worked in the A.I. lab at the software giant Salesforce.
Profluent has not yet put these synthetic gene editors through clinical trials, so it is not clear if they can match or exceed the performance of CRISPR. But this proof of concept shows that A.I. models can produce something capable of editing the human genome.
Still, it is unlikely to affect health care in the short term. Fyodor Urnov, a gene editing pioneer and scientific director at the Innovative Genomics Institute at the University of California, Berkeley, said scientists had no shortage of naturally occurring gene editors that they could use to fight illness and disease. The bottleneck, he said, is the cost of pushing these editors through preclinical studies, such as safety, manufacturing and regulatory reviews, before they can be used on patients.
But generative A.I. systems often hold enormous potential because they tend to improve quickly as they learn from increasingly large amounts of data. If technology like Profluent’s continues to improve, it could eventually allow scientists to edit genes in far more precise ways. The hope, Dr. Urnov said, is that this could, in the long term, lead to a world where medicines and treatments are quickly tailored to individual people even faster than we can do today.
“I dream of a world where we have CRISPR on demand within weeks,” he said.
Scientists have long cautioned against using CRISPR for human enhancement because it is a relatively new technology that could potentially have undesired side effects, such as triggering cancer, and have warned against unethical uses, such as genetically modifying human embryos.
This is also a concern with synthetic gene editors. But scientists already have access to everything they need to edit embryos.
“A bad actor, someone who is unethical, is not worried about whether they use an A.I.-created editor or not,” Dr. Fraser said. “They are just going to go ahead and use what’s available.”
Cade Metz writes about artificial intelligence, driverless cars, robotics, virtual reality and other emerging areas of technology. More about Cade Metz
Explore Our Coverage of Artificial Intelligence
News and Analysis
Eight daily newspapers owned by Alden Global Capital sued OpenAI and Microsoft , accusing the tech companies of illegally using news articles to power their A.I. chatbots.
The spending that the tech industry’s giants expect A.I. to require, for the chips and data centers , is starting to come into focus — and it is jarringly large.
The table stakes for A.I. start-ups to compete with the likes of Microsoft and Google are in the billions of dollars. And even that may not be enough .
The Age of A.I.
A new category of apps promises to relieve parents of drudgery, with an assist from A.I . But a family’s grunt work is more human, and valuable, than it seems.
Despite Mark Zuckerberg’s hope for Meta’s A.I. assistant to be the smartest , it struggles with facts, numbers and web search.
Much as ChatGPT generates poetry, a new A.I. system devises blueprints for microscopic mechanisms that can edit your DNA.
Could A.I. change India’s elections? Avatars are addressing voters by name, in whichever of India’s many languages they speak. Experts see potential for misuse in a country already rife with disinformation.
Which A.I. system writes the best computer code or generates the most realistic image? Right now, there’s no easy way to answer those questions, our technology columnist writes .
Advertisement
- International edition
- Australia edition
- Europe edition

‘You a scam artist’: the most brutal moments in Kendrick Lamar’s Drake diss track
The long-awaited rebuttal to Drake has arrived: Euphoria, a track in which Lamar dismantles Drake’s street credibility. But why use misogyny and homophobia to do it?
Haymakers were thrown yesterday by Kendrick Lamar towards Drake as rap’s top bout enters another round, following duels involving undercards such as J Cole, Rick Ross, Future and A$AP Rocky.
Lamar initially rang the bell a month ago with Like That, slagging off Drake as a mere pop star. Drake came back with Push Ups, featuring the allegation that Lamar’s wife was cheating on him (among various other barbs). Before Lamar could respond, Drake released Taylor Made Freestyle, which used AI to cheekily have 2Pac and Snoop Dogg – two of Lamar’s west coast forebears – chide Lamar for not following up soon enough.
Follow up he now has, with Euphoria, which many are putting straight into the diss track hall of fame thanks to a sustained barrage of blows against Drake’s Blackness, street credibility, artistic depth, financial agreements, romantic relationships and more.
The central thesis
The thrust of Lamar’s track is that Drake certainly isn’t street, and isn’t really Black – or at the very least, isn’t fully embraced by Black culture. He instructs him not to say the N-word, says he’s “faking for likes and digital hugs” and raps: “You’re not a rap artist, you a scam artist with the hopes of being accepted / Tommy Hilfiger stood out, but Fubu never had been your collection.” The suggestion is that Drake wears Tommy Hilfiger, a designer regarded distrustfully in hip-hop following a rumour that he was hostile to Black consumers (which he denied), but not Fubu, the Black-owned brand For Us, By Us.
As if that wasn’t clear enough, later there’s this: “How many more fairytale stories ’bout your life till we had enough? / How many more Black features till you finally feel that you’re Black enough?” And “you got shit twisted / What is it? The braids?” is a funny line that takes aim at Drake’s braided hair, seen as an attempt to appear more conventionally Black.
Euphoria at times echoes what was previously the most high-profile and successful diss track against Drake: The Story of Adidon, by Pusha T. While the revelation of Drake having a secret child with a porn star was that track’s biggest gotcha, other lines, alleging that Drake paraded his Black father to assert his Blackness, were equally stinging. Pusha also isolated his hair: “Confused, always felt you weren’t Black enough / Afraid to grow it ’cause your ’fro wouldn’t nap enough.”
The threats
Another major echo of The Story of Adidon comes in how Lamar chides Drake for not, in his eyes, properly raising his son Adonis, in a sustained series of unadorned lines: “Giving him tools to walk through life like day-by-day, know nothing ’bout that / Teaching him morals, integrity, discipline, listen, man, you don’t know nothing ’bout that.” Lamar’s hackles are clearly up after Drake’s allegation about his wife, and escalates the family drama: “Talk about me and my family, crodie? / Someone gon’ bleed in your family, crodie” is the track’s most blatant threat.
The wordplay
There’s another threat in another line about family: “Dementia must run in his family, but let it get shaky / I’ll park his son” – “park” being shorthand for shutting someone up with force, and a play on Parkinson’s. Who the son and father are is open to interpretation – it’s not quite the “motherfucking quintuple entendre” that Drake asked for on Taylor Made Freestyle, but there’s a lot to unpack, even if the pun veers on being laboured and clever-clever. More straightforwardly fun is stuff like “let your core audience stomach that, then tell ’em where you get your abs from”, a reference to the longstanding rumour that Drake had cosmetic surgery on his abdominals.
The hidden meanings
“I hate the way that you walk, the way that you talk, I hate the way that you dress” works well as a bracingly straightforward statement – remarkable in an age when dissing tends to be folded into layers of subliminal meaning – but it’s actually a callback to how DMX once described Drake. Another choice Easter egg is at the very beginning, with a reversed recording of Richard Pryor in The Wiz, saying: “Everything they say about me is true” – the next line in the film being: “I’m a phoney.”
The low points
There can be something hectoring about Lamar at times in his discography, less the Mr Morale of his last album and more Mr Moralising – and this turns ugly with some outright misogyny on Euphoria. He refers to gossiping as “ho shit” even as he delivers a gossipy diss track, and “we hate the bitches you fuck, ’cause they confuse themselves with real women” is gross even if you take it as a subliminal reference to Drake’s oft-remarked-upon friendships with teenage girls and women. There are frequent lines comparing Drake to a woman, which Lamar tries to legitimise by amping women up – “I believe you don’t like women, it’s real competition” – but it’s patronising, and there’s a whiff of homophobia in framing Drake as being less than a real man for having feminine qualities. That also comes through in his loaded praise of Drake’s danceable music – “Keep making me dance, waving my hand, and it won’t be no threat” – and in saying Drake “pops ass” while dancing with women.
The homophobia becomes horribly explicit on a line that calls Drake’s OVO labelmates “dick riders”, using gay sex as a diss. It was repellent when Nas was doing it on his own hall-of-fame diss track Ether, and that was back in 2001; Lamar, whose artistry rests on his self-presentation as flawed but enlightened, demeans himself here. Also, the three-part beat is blah even if Lamar does ride it with skill and animation. And no one apart from accountants cares about percentage splits in label deals, subject matter which results in some very dull lines.
The next steps
There will probably be much social media discussion around mixed-race identity, colourism and what constitutes “real” Blackness – though Lamar isn’t really talking about mixed-race people generally, just one in particular. And that man-to-man enmity will no doubt continue. “If you take it there, I’m taking it further”: Lamar promises that if Drake retaliates, he’ll retaliate again. For all that endless war is an integral part of American life, that would probably be more than enough from this chapter. Drake has already shot back, posting a scene of Julia Stiles in 10 Things I Hate About You saying lines similar to Lamar’s “I hate the way that you dress” screed – a neat puncturing of Lamar’s occasional pomposity. Humour rather than anger will be Drake’s best recourse now, and we’ll certainly need some good jokes to keep the combat worth watching.
- Kendrick Lamar
Most viewed
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Coronary artery disease.
Rai Dilawar Shahjehan ; Beenish S. Bhutta .
Affiliations
Last Update: August 17, 2023 .
- Continuing Education Activity
Coronary artery disease is a common heart condition that involves atherosclerotic plaque formation in the vessel lumen. This leads to impairment in blood flow and thus oxygen delivery to the myocardium. It is a cause of major morbidity and mortality in the US and worldwide. To avoid the high morbidity and mortality associated with this condition, it must be promptly diagnosed and treated. This activity illustrates the evaluation, diagnosis, and management of coronary artery disease and highlights the role of the healthcare team in evaluating and treating patients with this condition.
- Identify the etiology of coronary artery disease.
- Outline the evaluation of coronary artery disease.
- Discuss the management options available for coronary artery disease.
- Introduction
Coronary artery disease is a condition in which there is an inadequate supply of blood and oxygen to the myocardium. It results from occlusion of the coronary arteries and results in a demand-supply mismatch of oxygen. It typically involves the formation of plaques in the lumen of coronary arteries that impede blood flow. It is the major cause of death in the US and worldwide. At the beginning of the 20th century, it was an uncommon cause of death. Deaths due to CAD peaked in the mid-1960s and then decreased however, it still is the leading cause of death worldwide. [1]
Coronary artery disease is a multifactorial phenomenon. Etiologic factors can be broadly categorized into non-modifiable and modifiable factors. Non-modifiable factors include gender, age, family history, and genetics. Modifiable risk factors include smoking, obesity, lipid levels, and psychosocial variables. In the Western world, a faster-paced lifestyle has led people to eat more fast foods and unhealthy meals which has led to an increased prevalence of ischemic heart diseases. In the US, better primary care in the middle and higher socioeconomic groups has pushed the incidence towards the later part of life. Smoking remains the number one cause of cardiovascular diseases. In 2016, the prevalence of smoking among the United States among adults was found to be at 15.5 %. [2]
The male gender is more predisposed than the female gender. Hypercholesterolemia remains an important modifiable risk factor for CAD. Increased low-density lipoproteins (LDL) increased the risk for CAD and elevated high-density lipoproteins (HDL) decrease the incidence of CAD. An individual's 10-year risk of atherosclerotic cardiovascular disease can be calculated using the ASCVD equation available online on the American Heart Association portal. Markers of inflammation are also strong risk factors for coronary artery disease. High sensitivity CRP (hsCRP) is thought to be the best predictor of coronary artery disease in some studies although uses for it in a practical setting are controversial. [3]
- Epidemiology
Coronary artery disease is very common in both developed and developing worlds. In one study, it was estimated that CAD represented 2.2% of the overall global burden of disease and 32.7% of cardiovascular diseases. It costs over 200 billion dollars annually to the health care system in the United States. It is estimated that 7.6% of men and 5.0% of women in the US lived with coronary artery disease from 2009 to 2012 based on the national health survey done by the American Heart Association (AHA). This amount to 15.5 million Americans afflicted with the disease during this time. [4] [5]
The incidence of CAD is observed to rise with age, regardless of gender. In the ONACI registry in France, the incidence of CAD was about 1% in the 45 to 65 age group, which increased to about 4% as the age group reached 75 to 84 years. [6]
- Pathophysiology
The hallmark of the pathophysiology of CAD is the development of atherosclerotic plaque. Plaque is a build-up of fatty material that narrows the vessel lumen and impedes the blood flow. The first step in the process is the formation of a "fatty streak." Fatty streak is formed by subendothelial deposition of lipid-laden macrophages, also called foam cells. When a vascular insult occurs, the intima layer breaks, and monocytes migrate into the subendothelial space where they become macrophages. These macrophages take up oxidized low-density lipoprotein (LDL) particles, and foam cells are formed. T cells get activated, which releases cytokines only to aid in the pathologic process. Growth factors released activate smooth muscles, which also take up oxidized LDL particles and collagen and deposit along with activated macrophages and increase the population of foam cells. This process leads to the formation of subendothelial plaque.
Over time, this plaque could grow in size or become stable if no further insult occurs to the endothelium. If it becomes stable, a fibrous cap will form, and the lesion will become calcified over time. As time passes, the lesion can become hemodynamically significant enough that not enough blood would reach the myocardial tissue at the time of increased demands, and angina symptoms would occur. However, symptoms would abate at rest as the oxygen requirement comes down. For a lesion to cause angina at rest, it must be at least 90% stenosed. Some plaques can rupture and lead to exposure of tissue factor, which culminates in thrombosis. This thrombosis could cause subtotal or total occlusion of the lumen and could result in the development of acute coronary syndrome (ACS) in the form of unstable angina, NSTEMI, or STEMI, depending on the level of insult. [7]
Classification of coronary artery disease is typically done as under:
- Stable ischemic heart disease (SIHD)
- ST-elevation MI (STEMI)
- Non-ST elevation MI (NSTEMI)
- Unstable angina
- History and Physical
It is very important to take a detailed history and physical examination before proceeding towards further workup. Coronary artery disease could manifest as stable ischemic heart disease (SIHD) or acute coronary syndrome (ACS). It can further progress into congestive heart failure (CHF) if not controlled. Patients should be asked about chest pain, its relation to physical activity, and radiation of the pain into the jaw, neck, left arm, or into the back. Dyspnea should be evaluated for rest and also on activity. The patient should also be asked about syncope, palpitations, tachypnea, lower extremity edema, orthopnea, and exercise capacity. A family history of ischemic heart diseases should be obtained along with dietary, smoking, and lifestyle habits.
Physical examination should include inspection, palpation, and auscultation. One should inspect for any acute distress, jugular venous distention, and peripheral edema. In palpation, one should palpate for fluid thrill and heave. The extent of peripheral edema if present should be evaluated. The distension of the jugular vein should be measured. In auscultation, the heart should be auscultated in all four locations and lungs should also be auscultated with a special focus on the lower zones.
There are several modalities to evaluate for coronary artery disease including EKG, Echo, CXR, Stress test, cardiac catheterization, and blood work to name the main ones. These tests are done depending on the context in which patients are presenting. The following are details on different diagnostic modalities we have available for the evaluation of coronary artery disease:
Electrocardiogram (EKG)
EKG is a very basic yet enormously helpful test in the evaluation of coronary artery disease. It measures electrical activity in the cardiac conduction system and is measured by 10 leads attached to the skin at standardized locations. It provides information about both the physiology and anatomy of the heart. It typically has 12 leads on the paper that is printed once the test is performed and each lead correlates with the specific location of the heart. Important information to notice on an EKG is a heart's rate, rhythm, and axis. After that, information regarding acute and chronic pathologic processes can be obtained. In acute coronary syndrome, one can see ST-segment changes and T wave changes. If an ACS has degenerated into arrhythmias, that can also be seen. In chronic settings, EKG can show information like axis deviation, bundle branch blocks, and ventricular hypertrophy. EKG is also a cost-effective and readily available testing modality that is not user-dependent.
Echocardiography
Echocardiography is an ultrasound of the heart. It is a useful and non-invasive mode of testing that is performed in both acute and chronic and inpatient and outpatient settings. In acute settings, it could tell about wall motion, valvular regurgitation and stenosis, infective or autoimmune lesions, and chamber sizes. It also is useful in the diagnosis of acute pulmonary pathologies like pulmonary embolism. It also evaluates the pericardial cavity. In chronic settings, it can be done to see the same information mentioned above and also a response to the therapy. It also is used in an outpatient setting as part of stress testing. In addition to diagnostics, it also has a role in therapeutics for example, pericardiocentesis could be performed with the needle-guided by echocardiography. This test is user-dependent and could be costly compared to EKG. [8]
Stress Test
The stress test is a relatively non-invasive test to evaluate for coronary artery disease. It is used in the setting of suspected angina or angina equivalent and is helpful in ruling in or out coronary pathology when interpreted in an appropriate setting. During the test, the heart is artificially exposed to stress and if the patient gets certain abnormal EKG changes in ST segments or gets symptoms of angina, the test is aborted at that point and coronary artery disease is diagnosed. EKGs are obtained before, during, and after the procedure, and the patient is continuously monitored for any symptoms. There are mainly two types of stress tests; exercise stress test and pharmacologic stress test. In exercise stress tests, the patient has to run on a treadmill until he achieves 85% of the age-predicted maximal heart rate. If a patient develops exertional hypotension, hypertension (>200/110 mmHg), ST-segment elevations or depression, or ventricular or supraventricular arrhythmias. [9]
Chest X-ray
Chest X-ray is an important component of the initial evaluation of cardiac disease. The standard imaging films include standing posteroanterior (PA) and left lateral decubitus. Sometimes, anteroposterior (AP) projection is obtained especially in inpatient settings with the patient lying down, however, this interpretation of AP films is significantly limited. Proper analysis of PA and AP views provides useful and cost-effective information about the heart, lungs, and vasculature. Interpretation should be done in a stepwise pattern so that important information is not overlooked.
Blood work aids in establishing the diagnosis and assessing therapeutic responses. In acute settings, cardiac enzymes and B-type natriuretic peptides are often done along with complete blood counts and metabolic panels. BNP provides information about volume overload of cardiogenic origin however it has its limitations. It can be falsely elevated in kidney diseases and falsely low in obesity. Cardiac enzymes like CK and troponin provide information about an acute ischemic event. In chronic settings, lipid panel provides important prognostic information. C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) aid in assessing disease like acute pericarditis. Liver function tests (LFT) can be done to evaluate for an infiltrative process that can affect the liver and heart simultaneously like hemochromatosis. Liver tests are also done to assess increased right heart pressures, especially in chronic settings.
Cardiac Catheterization
Cardiac catheterization is the gold standard and most accurate modality to evaluate ischemic coronary heart disease. It is however an invasive procedure with associated complications. Not everyone is a candidate for the procedure. In non ACS settings, patients with intermediate pretest probability for CAD are usually the right candidates for it. In the ACS setting, all STEMI patients and selected NSTEMI patients get an emergent cardiac catheterization. This procedure is done in a cardiac catheterization lab, is expertise dependent, and is done under moderate sedation. There is contrast exposure in the procedure which could cause serious allergic reactions and kidney injury.
- Treatment / Management
Coronary artery disease could present either as stable ischemic heart disease (SIHD) or acute coronary syndrome (ACS). The former present in a chronic setting while the latter presents more in an acute setting. The management depends on the particular disease type. We will discuss the management of each subtype separately:
Stable Ischemic Heart Disease
Stable ischemic heart disease presents as stable angina. Stable angina typically presents as substernal chest pain or pressure that worsens with exertion or emotional stress and gets relieved with rest or nitroglycerin and is of 2 months duration. It is important to know that classic anginal symptoms could be absent and it could present differently with atypical symptoms and exertional dyspnea instead in certain demographic groups including women, elderly age, and diabetics. Management of SIHD includes both non-pharmacologic and pharmacologic interventions. Lifestyle modifications include smoking cessation, regular exercise, weight loss, good control of diabetes and hypertension, and a healthy diet. Pharmacologic interventions include cardioprotective and antianginal medications.
Every patient should get guideline-directed medical therapy (GDMT) which includes low dose aspirin, beta-blocker, as-needed nitroglycerin, and moderate to high-intensity statin. If symptoms are not controlled with this, beta-blocker therapy should be titrated up to heart rates 55-60, and the addition of calcium channel blocker and long-acting nitrates should be considered. [10] Ranolazine could also be added to relieve refractory anginal symptoms. If maximal GDMT has failed to relive angina, cardiac catheterization should be done to visualize the coronary anatomy and a decision should be made for percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) based on the patient profile. [11]
Acute Coronary Syndrome
The acute coronary syndrome presents as sudden onset substernal chest pain or pressure typically radiating to the neck and left arm and may be accompanied by dyspnea, palpitations, dizziness, syncope, cardiac arrest, or new-onset congestive heart failure. Prompt EKG is necessary for all patients with ACS to assess for STEMI and typically is done pre-hospital by an emergency medical services crew. STEMI is recognized by the presence of ST elevation in contiguous leads of 1 mm in limb leads or precordial leads excepting V2 and V3. In V2 and V3, men need to have 2 mm elevations and women 1.5 mm to qualify for STEMI diagnosis. New-onset left bundle branch block (LBBB) is also considered a STEMI equivalent. If STEMI is present, emergency PCI is warranted in a PCI capable facility or if a PCI facility is available within 2 hours distance. If the PCI capable facility is more than 2 hours away, intravenous thrombolytic therapy is indicated after making sure there are no contraindications to it.
It is important to differentiate a true STEMI from other conditions that mimic STEMI on EKG like acute pericarditis, Brugada syndrome, early repolarization changes, and LVH associated changes. All patients should get a full dose of sublingual aspirin (324 mg) upon presentation. Nitrates should be given for pain relief after making sure there are no contraindications to nitrates like hypotension, RV failure, and consumption of phosphodiesterase inhibitors in the past 24-48 hours. High-dose statin therapy and beta-blockers should also be initiated early. P2Y12 inhibitors (prasugrel, ticagrelor, or prasugrel) should be started based on the patient profile. Patients who have NSTE ACS should get anticoagulation, typically heparin or enoxaparin are used. For NSTEMI, early invasive therapy within 24 hours is advised for patients with intermediate to high TIMI scores (>2). [12] [13]
Regular visits with cardiologists and family physicians are key to good long term management of coronary artery disease. Medication adherence and lifestyle modification are important.
- Differential Diagnosis
Coronary artery disease has a wide range of differential diagnoses because of the proximity of the heart with adjacent organs, including the lungs, stomach, big vessels, and musculoskeletal organs. Acute anginal chest pain could mimic acute pericarditis, myocarditis, prinzmetal angina, pericardial effusion, acute bronchitis, pneumonia, pleuritis, pleural effusion, aortic dissection, GERD, peptic ulcer disease, esophageal motility disorders, and costochondritis. Stable ischemic heart disease could also mimic GERD, Peptic ulcer disease, costochondritis, and pleuritis. History, physical examination, and diagnostic studies should be carefully carried out to narrow down the differential diagnosis and reach an accurate diagnosis.
- Toxicity and Adverse Effect Management
Both medical and surgical management for ischemic heart disease is associated with their side effects and complications. These undesirable effects could be mitigated by careful selection, physician expertise, and patient education. Aspirin therapy is associated with bleeding, idiosyncratic, and allergic drug reactions. [14] Statin therapy can cause myalgias, diarrhea, and arthralgias among side effects. [15]
Beta-blockers could cause bradycardia and hypotension. ACEIs could result in hypotension, dizziness, creatinine elevation, cough, and allergic reactions including angioedema. [16] PCI can possibly cause coronary artery perforation, stent thrombosis in an acute setting, and in-stent restenosis on chronic basis. [17] CABG can have its own complications including but not limited to arrhythmias, cardiac tamponade, post-op bleeding, infection, renal impairment, and phrenic nerve injury.
The prognosis of the disease depends on multiple factors some of which could be modified while others are non-modifiable. Patient's age, gender, family history and genetics, ethnicity, dietary and smoking habits, medication compliance, availability of healthcare and financial status, and the number of arteries involved are some of the factors. Comorbid conditions including diabetes mellitus, hypertension, dyslipidemia, and chronic kidney disease also have a role in the overall outcome. [18]
- Complications
Arrhythmias, acute coronary syndrome, congestive heart failure, mitral regurgitation, ventricular free wall rupture, pericarditis, aneurysm formation, and mural thrombi are the main complication associated with coronary artery disease. [19] [20] [10]
- Deterrence and Patient Education
Coronary artery disease is caused by a combination of modifiable and nonmodifiable factors. Primary care providers should focus on the modifiable risk factor modification on each routine visit. Tight control of diabetes, hypertension and lipid levels in addition to smoking cessation, weight loss and exercise can make a huge difference. Since it is a global public health concern, in school curriculums and different avenues of media, more awareness needs to be created.
- Pearls and Other Issues
Several landmark trials were done over the past few decades which have totally changed the way we care for coronary artery disease patients. It is beyond the scope of this article to discuss individual trial results, however, the following are the names of some important studies. ISIS-2, CURE, CLARITY-TIMI 28, TIRTON-TIMI 38, PLATO, and CURRENT-OASIS 7 trials were done regarding guidelines for antiplatelet medications. [21] SYNERGY, ACUITY, ExTRACT-TIMI 25, OASIS-5, and ATLAS ACS/ TIMI 52 were done about the use of anticoagulation. ADMIRAL, ACUITY, ISAR-REACT 3, and HORIZONS-AMI are famous trials regarding GpIIb/IIIa use, COMMIT for beta-blockers while SHOCK, DANAMI-2, BASKET-LATE, TIMACS, and BASKET-PROVE are about PCI and CABG. MIRACL trial was done about statin use.
- Enhancing Healthcare Team Outcomes
Evaluation of ischemic heart disease can frequently present a diagnostic dilemma. Such patients can present non-specific symptoms like chest pain or shortness of breath. The cause of chest pain or shortness of breath could be due to a myriad of diseases including gastrointestinal, cardiac, musculoskeletal, psychological, and pulmonary causes. While a cardiologist is often involved as a central player, it is important to take other team members on board as indicated including a gastroenterologist, pulmonologist, and psychiatrist. Radiologists also are an important resource in the whole process. Nurses are a very important part of the diagnostic and therapeutic workup as well as they provide key bedside information not witnessed by the physicians in their short encounters.
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Classification of CAD. Image created by the author Rai Dilawar Shahjehan, MD.
Disclosure: Rai Dilawar Shahjehan declares no relevant financial relationships with ineligible companies.
Disclosure: Beenish Bhutta declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Shahjehan RD, Bhutta BS. Coronary Artery Disease. [Updated 2023 Aug 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Instantaneous Wave-Free Ratio. [StatPearls. 2024] Instantaneous Wave-Free Ratio. Soos MP, Gonzalez-Morales D, McComb D. StatPearls. 2024 Jan
- Review The epidemic of the 20(th) century: coronary heart disease. [Am J Med. 2014] Review The epidemic of the 20(th) century: coronary heart disease. Dalen JE, Alpert JS, Goldberg RJ, Weinstein RS. Am J Med. 2014 Sep; 127(9):807-12. Epub 2014 May 5.
- Myocardial oxygen supply:demand ratio as reference for coronary vasodilatory drug effects in humans. [Heart. 1997] Myocardial oxygen supply:demand ratio as reference for coronary vasodilatory drug effects in humans. Vergroesen I, Kal JE, Spaan JA, Van Wezel HB. Heart. 1997 Aug; 78(2):117-26.
- Multidetector computed tomography for coronary artery disease screening in asymptomatic populations: evidence-based analysis. [Ont Health Technol Assess Ser....] Multidetector computed tomography for coronary artery disease screening in asymptomatic populations: evidence-based analysis. Medical Advisory Secretariat. Ont Health Technol Assess Ser. 2007; 7(3):1-56. Epub 2007 May 1.
- Review Biomarkers to monitor the prognosis, disease severity, and treatment efficacy in coronary artery disease. [Expert Rev Cardiovasc Ther. 2023] Review Biomarkers to monitor the prognosis, disease severity, and treatment efficacy in coronary artery disease. Yazdani AN, Pletsch M, Chorbajian A, Zitser D, Rai V, Agrawal DK. Expert Rev Cardiovasc Ther. 2023 Jul-Dec; 21(10):675-692. Epub 2023 Oct 26.
Recent Activity
- Coronary Artery Disease - StatPearls Coronary Artery Disease - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

IMAGES
VIDEO
COMMENTS
Cardiovascular diseases (CVDs), principally ischemic heart disease (IHD) and stroke, are the leading cause of. global mortality and a major contributor to disability. This paper reviews the ...
3) Machine Learning algorithms allowed us to analyze clinical data, draw. relationships between diagnostic variables, design the predictive model, and. tests it against the new case. The predictive model achieved an accuracy of 89.4. percent using RandomForest Classifier's default setting to predict heart diseases.
This paper proposes heart disease prediction using different machine-learning algorithms like logistic regression, naïve bayes, support vector machine, k nearest neighbor (KNN), random forest ...
This paper aims to provide a comprehensive overview of recent research on heart disease diagnosis by reviewing articles published by reputable sources between 2014 and 2022.
Introduction and background. Atherosclerosis is the process of formation of plaque composed of cholesterol, fat, calcium, and other substances in the wall of large and medium-sized arteries causing diminished blood flow to an area of the body [1,2].Moreover, atherosclerotic vascular disease can be classified into two arenas: a cardiovascular disease affecting the heart and peripheral blood ...
Cardiovascular diseases (CVDs), principally ischemic heart disease (IHD) and stroke, are the leading cause of global mortality and a major contributor to disability. This paper reviews the magnitude of total CVD burden, including 13 underlying causes of cardiovascular death and 9 related risk factors, using estimates from the Global Burden of ...
Most importantly, pooled analyses indicate that, in general, ML algorithms are accurate (AUC 0.8-0.9 s) in overall cardiovascular disease prediction. In subgroup analyses of each ML algorithms ...
Overview of thesis papers (Background and context) ... regurgitation is the most frequent valvular heart disease in patients over the age of 65 years. Elderly patients account for about 40% of all patients with mitral regurgitation and 4.5% are over 80 years of age. Mitral valve diseases are increasing in prevalence and require valve
Coronary artery disease (CAD) continues to pose a health challenge, impacting numerous individuals and placing strain on healthcare systems worldwide. The narrowing and blockage of arteries due to CAD can lead to complications like heart attacks and heart failure. Over time, dedicated efforts from researchers, professionals, and governments ...
Globally, cardiovascular disease (CVDs) is the primary cause of morbidity and mortality, accounting for more than 70% of all fatalities. According to the 2017 Global Burden of Disease research, cardiovascular disease is responsible for about 43% of all fatalities [1,2].Common risk factors for heart disease in high-income nations include lousy diet, cigarette use, excessive sugar consumption ...
This thesis studies the possible determinants for imaging markers of cardiovascular disease risk, including carotid intima media thickness and pulse wave arterial stiffness index and further investigates putative circulating miRNAs as novel biomarkers for cardiovascular diseases. The overarching aim is to provide insight
Introduction. Thought of broadly, the management of patients with stable coronary artery disease (CAD) involves significant complexity including the diagnosis of CAD, assessment of associated risk, treatment, and monitoring of symptoms during follow up. 1 To provide sufficient focus in this month's topic review in Circulation: Cardiovascular Quality and Outcomes, we have concentrated on the ...
Heart disease and stroke statistics-2011 update: a report from the American Heart Association. Circulation. 2011; 123:e18-e209. Link Google Scholar; 5. Dhruva SS, Bero LA, Redberg RF. Gender bias in studies for Food and Drug Administration premarket approval of cardiovascular devices. Circ Cardiovasc Qual Outcomes. 2011; 4:165-171. Link ...
Heart disease is a fatal human disease, rapidly increases globally in both developed and undeveloped countries and consequently, causes death. Normally, in this disease, the heart fails to supply ...
The causes and prevention of heart disease have been studied for years, and new information is emerging. For the last several decades, saturated fat and cholesterol have been thought to be major contributors to coronary artery disease, and therefore people are typically advised to strictly limit these in their diet.
In this paper, an AI-based system has been proposed for the diagnosis of cardiovascular diseases using clinical data, patient information, and electrocardiogram (ECG) signal. The proposed system includes an ECG processor part that allows cardiologists to process and analyze the different waveforms, a machine learning-based heart disease ...
Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2012; 33:2451-2496. Crossref Medline Google Scholar; 60.
Cardiovascular disease (CVD) is a collective term designating all types of affliction affecting the blood circulatory system, including the heart and vasculature, which, respectively, displaces and conveys the blood. This multifactorial disorder encompasses numerous congenital and acquired maladies. CVD represents the leading noncommunicable cause of death in Europe (∼50% of all deaths; ∼ ...
This paper analyzes few parameters and predicts heart disease, suggesting a prediction system based entirely on data mining approaches. a) Quantitative comparison with different algorithms using ...
For almost 75 years, the NHLBI has been at the forefront of improving the nation's health and reducing the burden of heart and vascular diseases. Heart disease, including coronary heart disease, remains the leading cause of death in the United States. However, the rate of heart disease deaths has declined by 70% over the past 50 years, thanks ...
Fig. 1: Summary of the study design and multi-omics dataset. To identify the main temporal or sex-associated responses in each tissue, we summarized the graphical cluster sizes by tissue and time ...
Our findings demonstrate that atherosclerosis is an SMC-driven tumor-like disease, advancing our understanding of its pathogenesis and opening prospects for innovative precision molecular strategies aimed at preventing and treating atherosclerotic cardiovascular disease.
Introduction. Cardiovascular diseases (CVDs) are the foremost reason of disease burden and mortality all over the world. Approximately 30% of total deaths (17.9 million) occurred due to CVDs globally in 2016. 1 The situation is critically serious in low- and middle-income countries like India. During the past three decades, the number of deaths due to CVDs has increased significantly from 15.2 ...
Described in a research paper published on Monday by a Berkeley, Calif., startup called Profluent, the technology is based on the same methods that drive ChatGPT, the online chatbot that launched ...
The central thesis. The thrust of Lamar's track is that Drake certainly isn't street, and isn't really Black - or at the very least, isn't fully embraced by Black culture.
Coronary artery disease is a condition in which there is an inadequate supply of blood and oxygen to the myocardium. It results from occlusion of the coronary arteries and results in a demand-supply mismatch of oxygen. It typically involves the formation of plaques in the lumen of coronary arteries that impede blood flow. It is the major cause of death in the US and worldwide. At the beginning ...