- Open access
- Published: 28 June 2021

Impact of abortion law reforms on women’s health services and outcomes: a systematic review protocol
- Foluso Ishola ORCID: orcid.org/0000-0002-8644-0570 1 ,
- U. Vivian Ukah 1 &
- Arijit Nandi 1
Systematic Reviews volume 10 , Article number: 192 ( 2021 ) Cite this article
21k Accesses
2 Citations
3 Altmetric
Metrics details
A country’s abortion law is a key component in determining the enabling environment for safe abortion. While restrictive abortion laws still prevail in most low- and middle-income countries (LMICs), many countries have reformed their abortion laws, with the majority of them moving away from an absolute ban. However, the implications of these reforms on women’s access to and use of health services, as well as their health outcomes, is uncertain. First, there are methodological challenges to the evaluation of abortion laws, since these changes are not exogenous. Second, extant evaluations may be limited in terms of their generalizability, given variation in reforms across the abortion legality spectrum and differences in levels of implementation and enforcement cross-nationally. This systematic review aims to address this gap. Our aim is to systematically collect, evaluate, and synthesize empirical research evidence concerning the impact of abortion law reforms on women’s health services and outcomes in LMICs.
We will conduct a systematic review of the peer-reviewed literature on changes in abortion laws and women’s health services and outcomes in LMICs. We will search Medline, Embase, CINAHL, and Web of Science databases, as well as grey literature and reference lists of included studies for further relevant literature. As our goal is to draw inference on the impact of abortion law reforms, we will include quasi-experimental studies examining the impact of change in abortion laws on at least one of our outcomes of interest. We will assess the methodological quality of studies using the quasi-experimental study designs series checklist. Due to anticipated heterogeneity in policy changes, outcomes, and study designs, we will synthesize results through a narrative description.
This review will systematically appraise and synthesize the research evidence on the impact of abortion law reforms on women’s health services and outcomes in LMICs. We will examine the effect of legislative reforms and investigate the conditions that might contribute to heterogeneous effects, including whether specific groups of women are differentially affected by abortion law reforms. We will discuss gaps and future directions for research. Findings from this review could provide evidence on emerging strategies to influence policy reforms, implement abortion services and scale up accessibility.
Systematic review registration
PROSPERO CRD42019126927
Peer Review reports
An estimated 25·1 million unsafe abortions occur each year, with 97% of these in developing countries [ 1 , 2 , 3 ]. Despite its frequency, unsafe abortion remains a major global public health challenge [ 4 , 5 ]. According to the World health Organization (WHO), nearly 8% of maternal deaths were attributed to unsafe abortion, with the majority of these occurring in developing countries [ 5 , 6 ]. Approximately 7 million women are admitted to hospitals every year due to complications from unsafe abortion such as hemorrhage, infections, septic shock, uterine and intestinal perforation, and peritonitis [ 7 , 8 , 9 ]. These often result in long-term effects such as infertility and chronic reproductive tract infections. The annual cost of treating major complications from unsafe abortion is estimated at US$ 232 million each year in developing countries [ 10 , 11 ]. The negative consequences on children’s health, well-being, and development have also been documented. Unsafe abortion increases risk of poor birth outcomes, neonatal and infant mortality [ 12 , 13 ]. Additionally, women who lack access to safe and legal abortion are often forced to continue with unwanted pregnancies, and may not seek prenatal care [ 14 ], which might increase risks of child morbidity and mortality.
Access to safe abortion services is often limited due to a wide range of barriers. Collectively, these barriers contribute to the staggering number of deaths and disabilities seen annually as a result of unsafe abortion, which are disproportionately felt in developing countries [ 15 , 16 , 17 ]. A recent systematic review on the barriers to abortion access in low- and middle-income countries (LMICs) implicated the following factors: restrictive abortion laws, lack of knowledge about abortion law or locations that provide abortion, high cost of services, judgmental provider attitudes, scarcity of facilities and medical equipment, poor training and shortage of staff, stigma on social and religious grounds, and lack of decision making power [ 17 ].
An important factor regulating access to abortion is abortion law [ 17 , 18 , 19 ]. Although abortion is a medical procedure, its legal status in many countries has been incorporated in penal codes which specify grounds in which abortion is permitted. These include prohibition in all circumstances, to save the woman’s life, to preserve the woman’s health, in cases of rape, incest, fetal impairment, for economic or social reasons, and on request with no requirement for justification [ 18 , 19 , 20 ].
Although abortion laws in different countries are usually compared based on the grounds under which legal abortions are allowed, these comparisons rarely take into account components of the legal framework that may have strongly restrictive implications, such as regulation of facilities that are authorized to provide abortions, mandatory waiting periods, reporting requirements in cases of rape, limited choice in terms of the method of abortion, and requirements for third-party authorizations [ 19 , 21 , 22 ]. For example, the Zambian Termination of Pregnancy Act permits abortion on socio-economic grounds. It is considered liberal, as it permits legal abortions for more indications than most countries in Sub-Saharan Africa; however, abortions must only be provided in registered hospitals, and three medical doctors—one of whom must be a specialist—must provide signatures to allow the procedure to take place [ 22 ]. Given the critical shortage of doctors in Zambia [ 23 ], this is in fact a major restriction that is only captured by a thorough analysis of the conditions under which abortion services are provided.
Additionally, abortion laws may exist outside the penal codes in some countries, where they are supplemented by health legislation and regulations such as public health statutes, reproductive health acts, court decisions, medical ethic codes, practice guidelines, and general health acts [ 18 , 19 , 24 ]. The diversity of regulatory documents may lead to conflicting directives about the grounds under which abortion is lawful [ 19 ]. For example, in Kenya and Uganda, standards and guidelines on the reduction of morbidity and mortality due to unsafe abortion supported by the constitution was contradictory to the penal code, leaving room for an ambiguous interpretation of the legal environment [ 25 ].
Regulations restricting the range of abortion methods from which women can choose, including medication abortion in particular, may also affect abortion access [ 26 , 27 ]. A literature review contextualizing medication abortion in seven African countries reported that incidence of medication abortion is low despite being a safe, effective, and low-cost abortion method, likely due to legal restrictions on access to the medications [ 27 ].
Over the past two decades, many LMICs have reformed their abortion laws [ 3 , 28 ]. Most have expanded the grounds on which abortion may be performed legally, while very few have restricted access. Countries like Uruguay, South Africa, and Portugal have amended their laws to allow abortion on request in the first trimester of pregnancy [ 29 , 30 ]. Conversely, in Nicaragua, a law to ban all abortion without any exception was introduced in 2006 [ 31 ].
Progressive reforms are expected to lead to improvements in women’s access to safe abortion and health outcomes, including reductions in the death and disabilities that accompany unsafe abortion, and reductions in stigma over the longer term [ 17 , 29 , 32 ]. However, abortion law reforms may yield different outcomes even in countries that experience similar reforms, as the legislative processes that are associated with changing abortion laws take place in highly distinct political, economic, religious, and social contexts [ 28 , 33 ]. This variation may contribute to abortion law reforms having different effects with respect to the health services and outcomes that they are hypothesized to influence [ 17 , 29 ].
Extant empirical literature has examined changes in abortion-related morbidity and mortality, contraceptive usage, fertility, and other health-related outcomes following reforms to abortion laws [ 34 , 35 , 36 , 37 ]. For example, a study in Mexico reported that a policy that decriminalized and subsidized early-term elective abortion led to substantial reductions in maternal morbidity and that this was particularly strong among vulnerable populations such as young and socioeconomically disadvantaged women [ 38 ].
To the best of our knowledge, however, the growing literature on the impact of abortion law reforms on women’s health services and outcomes has not been systematically reviewed. A study by Benson et al. evaluated evidence on the impact of abortion policy reforms on maternal death in three countries, Romania, South Africa, and Bangladesh, where reforms were immediately followed by strategies to implement abortion services, scale up accessibility, and establish complementary reproductive and maternal health services [ 39 ]. The three countries highlighted in this paper provided unique insights into implementation and practical application following law reforms, in spite of limited resources. However, the review focused only on a selection of countries that have enacted similar reforms and it is unclear if its conclusions are more widely generalizable.
Accordingly, the primary objective of this review is to summarize studies that have estimated the causal effect of a change in abortion law on women’s health services and outcomes. Additionally, we aim to examine heterogeneity in the impacts of abortion reforms, including variation across specific population sub-groups and contexts (e.g., due to variations in the intensity of enforcement and service delivery). Through this review, we aim to offer a higher-level view of the impact of abortion law reforms in LMICs, beyond what can be gained from any individual study, and to thereby highlight patterns in the evidence across studies, gaps in current research, and to identify promising programs and strategies that could be adapted and applied more broadly to increase access to safe abortion services.
The review protocol has been reported using Preferred Reporting Items for Systematic review and Meta-Analysis Protocols (PRISMA-P) guidelines [ 40 ] (Additional file 1 ). It was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database CRD42019126927.
Eligibility criteria
Types of studies.
This review will consider quasi-experimental studies which aim to estimate the causal effect of a change in a specific law or reform and an outcome, but in which participants (in this case jurisdictions, whether countries, states/provinces, or smaller units) are not randomly assigned to treatment conditions [ 41 ]. Eligible designs include the following:
Pretest-posttest designs where the outcome is compared before and after the reform, as well as nonequivalent groups designs, such as pretest-posttest design that includes a comparison group, also known as a controlled before and after (CBA) designs.
Interrupted time series (ITS) designs where the trend of an outcome after an abortion law reform is compared to a counterfactual (i.e., trends in the outcome in the post-intervention period had the jurisdiction not enacted the reform) based on the pre-intervention trends and/or a control group [ 42 , 43 ].
Differences-in-differences (DD) designs, which compare the before vs. after change in an outcome in jurisdictions that experienced an abortion law reform to the corresponding change in the places that did not experience such a change, under the assumption of parallel trends [ 44 , 45 ].
Synthetic controls (SC) approaches, which use a weighted combination of control units that did not experience the intervention, selected to match the treated unit in its pre-intervention outcome trend, to proxy the counterfactual scenario [ 46 , 47 ].
Regression discontinuity (RD) designs, which in the case of eligibility for abortion services being determined by the value of a continuous random variable, such as age or income, would compare the distributions of post-intervention outcomes for those just above and below the threshold [ 48 ].
There is heterogeneity in the terminology and definitions used to describe quasi-experimental designs, but we will do our best to categorize studies into the above groups based on their designs, identification strategies, and assumptions.
Our focus is on quasi-experimental research because we are interested in studies evaluating the effect of population-level interventions (i.e., abortion law reform) with a design that permits inference regarding the causal effect of abortion legislation, which is not possible from other types of observational designs such as cross-sectional studies, cohort studies or case-control studies that lack an identification strategy for addressing sources of unmeasured confounding (e.g., secular trends in outcomes). We are not excluding randomized studies such as randomized controlled trials, cluster randomized trials, or stepped-wedge cluster-randomized trials; however, we do not expect to identify any relevant randomized studies given that abortion policy is unlikely to be randomly assigned. Since our objective is to provide a summary of empirical studies reporting primary research, reviews/meta-analyses, qualitative studies, editorials, letters, book reviews, correspondence, and case reports/studies will also be excluded.
Our population of interest includes women of reproductive age (15–49 years) residing in LMICs, as the policy exposure of interest applies primarily to women who have a demand for sexual and reproductive health services including abortion.
Intervention
The intervention in this study refers to a change in abortion law or policy, either from a restrictive policy to a non-restrictive or less restrictive one, or vice versa. This can, for example, include a change from abortion prohibition in all circumstances to abortion permissible in other circumstances, such as to save the woman’s life, to preserve the woman’s health, in cases of rape, incest, fetal impairment, for economic or social reasons, or on request with no requirement for justification. It can also include the abolition of existing abortion policies or the introduction of new policies including those occurring outside the penal code, which also have legal standing, such as:
National constitutions;
Supreme court decisions, as well as higher court decisions;
Customary or religious law, such as interpretations of Muslim law;
Medical ethical codes; and
Regulatory standards and guidelines governing the provision of abortion.
We will also consider national and sub-national reforms, although we anticipate that most reforms will operate at the national level.
The comparison group represents the counterfactual scenario, specifically the level and/or trend of a particular post-intervention outcome in the treated jurisdiction that experienced an abortion law reform had it, counter to the fact, not experienced this specific intervention. Comparison groups will vary depending on the type of quasi-experimental design. These may include outcome trends after abortion reform in the same country, as in the case of an interrupted time series design without a control group, or corresponding trends in countries that did not experience a change in abortion law, as in the case of the difference-in-differences design.
Outcome measures
Primary outcomes.
Access to abortion services: There is no consensus on how to measure access but we will use the following indicators, based on the relevant literature [ 49 ]: [ 1 ] the availability of trained staff to provide care, [ 2 ] facilities are geographically accessible such as distance to providers, [ 3 ] essential equipment, supplies and medications, [ 4 ] services provided regardless of woman’s ability to pay, [ 5 ] all aspects of abortion care are explained to women, [ 6 ] whether staff offer respectful care, [ 7 ] if staff work to ensure privacy, [ 8 ] if high-quality, supportive counseling is provided, [ 9 ] if services are offered in a timely manner, and [ 10 ] if women have the opportunity to express concerns, ask questions, and receive answers.
Use of abortion services refers to induced pregnancy termination, including medication abortion and number of women treated for abortion-related complications.
Secondary outcomes
Current use of any method of contraception refers to women of reproductive age currently using any method contraceptive method.
Future use of contraception refers to women of reproductive age who are not currently using contraception but intend to do so in the future.
Demand for family planning refers to women of reproductive age who are currently using, or whose sexual partner is currently using, at least one contraceptive method.
Unmet need for family planning refers to women of reproductive age who want to stop or delay childbearing but are not using any method of contraception.
Fertility rate refers to the average number of children born to women of childbearing age.
Neonatal morbidity and mortality refer to disability or death of newborn babies within the first 28 days of life.
Maternal morbidity and mortality refer to disability or death due to complications from pregnancy or childbirth.
There will be no language, date, or year restrictions on studies included in this systematic review.
Studies have to be conducted in a low- and middle-income country. We will use the country classification specified in the World Bank Data Catalogue to identify LMICs (Additional file 2 ).
Search methods
We will perform searches for eligible peer-reviewed studies in the following electronic databases.
Ovid MEDLINE(R) (from 1946 to present)
Embase Classic+Embase on OvidSP (from 1947 to present)
CINAHL (1973 to present); and
Web of Science (1900 to present)
The reference list of included studies will be hand searched for additional potentially relevant citations. Additionally, a grey literature search for reports or working papers will be done with the help of Google and Social Science Research Network (SSRN).
Search strategy
A search strategy, based on the eligibility criteria and combining subject indexing terms (i.e., MeSH) and free-text search terms in the title and abstract fields, will be developed for each electronic database. The search strategy will combine terms related to the interventions of interest (i.e., abortion law/policy), etiology (i.e., impact/effect), and context (i.e., LMICs) and will be developed with the help of a subject matter librarian. We opted not to specify outcomes in the search strategy in order to maximize the sensitivity of our search. See Additional file 3 for a draft of our search strategy.
Data collection and analysis
Data management.
Search results from all databases will be imported into Endnote reference manager software (Version X9, Clarivate Analytics) where duplicate records will be identified and excluded using a systematic, rigorous, and reproducible method that utilizes a sequential combination of fields including author, year, title, journal, and pages. Rayyan systematic review software will be used to manage records throughout the review [ 50 ].
Selection process
Two review authors will screen titles and abstracts and apply the eligibility criteria to select studies for full-text review. Reference lists of any relevant articles identified will be screened to ensure no primary research studies are missed. Studies in a language different from English will be translated by collaborators who are fluent in the particular language. If no such expertise is identified, we will use Google Translate [ 51 ]. Full text versions of potentially relevant articles will be retrieved and assessed for inclusion based on study eligibility criteria. Discrepancies will be resolved by consensus or will involve a third reviewer as an arbitrator. The selection of studies, as well as reasons for exclusions of potentially eligible studies, will be described using a PRISMA flow chart.
Data extraction
Data extraction will be independently undertaken by two authors. At the conclusion of data extraction, these two authors will meet with the third author to resolve any discrepancies. A piloted standardized extraction form will be used to extract the following information: authors, date of publication, country of study, aim of study, policy reform year, type of policy reform, data source (surveys, medical records), years compared (before and after the reform), comparators (over time or between groups), participant characteristics (age, socioeconomic status), primary and secondary outcomes, evaluation design, methods used for statistical analysis (regression), estimates reported (means, rates, proportion), information to assess risk of bias (sensitivity analyses), sources of funding, and any potential conflicts of interest.
Risk of bias and quality assessment
Two independent reviewers with content and methodological expertise in methods for policy evaluation will assess the methodological quality of included studies using the quasi-experimental study designs series risk of bias checklist [ 52 ]. This checklist provides a list of criteria for grading the quality of quasi-experimental studies that relate directly to the intrinsic strength of the studies in inferring causality. These include [ 1 ] relevant comparison, [ 2 ] number of times outcome assessments were available, [ 3 ] intervention effect estimated by changes over time for the same or different groups, [ 4 ] control of confounding, [ 5 ] how groups of individuals or clusters were formed (time or location differences), and [ 6 ] assessment of outcome variables. Each of the following domains will be assigned a “yes,” “no,” or “possibly” bias classification. Any discrepancies will be resolved by consensus or a third reviewer with expertise in review methodology if required.
Confidence in cumulative evidence
The strength of the body of evidence will be assessed using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) system [ 53 ].
Data synthesis
We anticipate that risk of bias and heterogeneity in the studies included may preclude the use of meta-analyses to describe pooled effects. This may necessitate the presentation of our main findings through a narrative description. We will synthesize the findings from the included articles according to the following key headings:
Information on the differential aspects of the abortion policy reforms.
Information on the types of study design used to assess the impact of policy reforms.
Information on main effects of abortion law reforms on primary and secondary outcomes of interest.
Information on heterogeneity in the results that might be due to differences in study designs, individual-level characteristics, and contextual factors.
Potential meta-analysis
If outcomes are reported consistently across studies, we will construct forest plots and synthesize effect estimates using meta-analysis. Statistical heterogeneity will be assessed using the I 2 test where I 2 values over 50% indicate moderate to high heterogeneity [ 54 ]. If studies are sufficiently homogenous, we will use fixed effects. However, if there is evidence of heterogeneity, a random effects model will be adopted. Summary measures, including risk ratios or differences or prevalence ratios or differences will be calculated, along with 95% confidence intervals (CI).
Analysis of subgroups
If there are sufficient numbers of included studies, we will perform sub-group analyses according to type of policy reform, geographical location and type of participant characteristics such as age groups, socioeconomic status, urban/rural status, education, or marital status to examine the evidence for heterogeneous effects of abortion laws.
Sensitivity analysis
Sensitivity analyses will be conducted if there are major differences in quality of the included articles to explore the influence of risk of bias on effect estimates.
Meta-biases
If available, studies will be compared to protocols and registers to identify potential reporting bias within studies. If appropriate and there are a sufficient number of studies included, funnel plots will be generated to determine potential publication bias.
This systematic review will synthesize current evidence on the impact of abortion law reforms on women’s health. It aims to identify which legislative reforms are effective, for which population sub-groups, and under which conditions.
Potential limitations may include the low quality of included studies as a result of suboptimal study design, invalid assumptions, lack of sensitivity analysis, imprecision of estimates, variability in results, missing data, and poor outcome measurements. Our review may also include a limited number of articles because we opted to focus on evidence from quasi-experimental study design due to the causal nature of the research question under review. Nonetheless, we will synthesize the literature, provide a critical evaluation of the quality of the evidence and discuss the potential effects of any limitations to our overall conclusions. Protocol amendments will be recorded and dated using the registration for this review on PROSPERO. We will also describe any amendments in our final manuscript.
Synthesizing available evidence on the impact of abortion law reforms represents an important step towards building our knowledge base regarding how abortion law reforms affect women’s health services and health outcomes; we will provide evidence on emerging strategies to influence policy reforms, implement abortion services, and scale up accessibility. This review will be of interest to service providers, policy makers and researchers seeking to improve women’s access to safe abortion around the world.
Abbreviations
Cumulative index to nursing and allied health literature
Excerpta medica database
Low- and middle-income countries
Preferred reporting items for systematic review and meta-analysis protocols
International prospective register of systematic reviews
Ganatra B, Gerdts C, Rossier C, Johnson BR, Tuncalp O, Assifi A, et al. Global, regional, and subregional classification of abortions by safety, 2010-14: estimates from a Bayesian hierarchical model. Lancet. 2017;390(10110):2372–81. https://doi.org/10.1016/S0140-6736(17)31794-4 .
Article PubMed PubMed Central Google Scholar
Guttmacher Institute. Induced Abortion Worldwide; Global Incidence and Trends 2018. https://www.guttmacher.org/fact-sheet/induced-abortion-worldwide . Accessed 15 Dec 2019.
Singh S, Remez L, Sedgh G, Kwok L, Onda T. Abortion worldwide 2017: uneven progress and unequal access. NewYork: Guttmacher Institute; 2018.
Book Google Scholar
Fusco CLB. Unsafe abortion: a serious public health issue in a poverty stricken population. Reprod Clim. 2013;2(8):2–9.
Google Scholar
Rehnstrom Loi U, Gemzell-Danielsson K, Faxelid E, Klingberg-Allvin M. Health care providers’ perceptions of and attitudes towards induced abortions in sub-Saharan Africa and Southeast Asia: a systematic literature review of qualitative and quantitative data. BMC Public Health. 2015;15(1):139. https://doi.org/10.1186/s12889-015-1502-2 .
Say L, Chou D, Gemmill A, Tuncalp O, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2(6):E323–E33. https://doi.org/10.1016/S2214-109X(14)70227-X .
Article PubMed Google Scholar
Benson J, Nicholson LA, Gaffikin L, Kinoti SN. Complications of unsafe abortion in sub-Saharan Africa: a review. Health Policy Plan. 1996;11(2):117–31. https://doi.org/10.1093/heapol/11.2.117 .
Abiodun OM, Balogun OR, Adeleke NA, Farinloye EO. Complications of unsafe abortion in South West Nigeria: a review of 96 cases. Afr J Med Med Sci. 2013;42(1):111–5.
CAS PubMed Google Scholar
Singh S, Maddow-Zimet I. Facility-based treatment for medical complications resulting from unsafe pregnancy termination in the developing world, 2012: a review of evidence from 26 countries. BJOG. 2016;123(9):1489–98. https://doi.org/10.1111/1471-0528.13552 .
Article CAS PubMed Google Scholar
Vlassoff M, Walker D, Shearer J, Newlands D, Singh S. Estimates of health care system costs of unsafe abortion in Africa and Latin America. Int Perspect Sex Reprod Health. 2009;35(3):114–21. https://doi.org/10.1363/3511409 .
Singh S, Darroch JE. Adding it up: costs and benefits of contraceptive services. Estimates for 2012. New York: Guttmacher Institute and United Nations Population Fund; 2012.
Auger N, Bilodeau-Bertrand M, Sauve R. Abortion and infant mortality on the first day of life. Neonatology. 2016;109(2):147–53. https://doi.org/10.1159/000442279 .
Krieger N, Gruskin S, Singh N, Kiang MV, Chen JT, Waterman PD, et al. Reproductive justice & preventable deaths: state funding, family planning, abortion, and infant mortality, US 1980-2010. SSM Popul Health. 2016;2:277–93. https://doi.org/10.1016/j.ssmph.2016.03.007 .
Banaem LM, Majlessi F. A comparative study of low 5-minute Apgar scores (< 8) in newborns of wanted versus unwanted pregnancies in southern Tehran, Iran (2006-2007). J Matern Fetal Neonatal Med. 2008;21(12):898–901. https://doi.org/10.1080/14767050802372390 .
Bhandari A. Barriers in access to safe abortion services: perspectives of potential clients from a hilly district of Nepal. Trop Med Int Health. 2007;12:87.
Seid A, Yeneneh H, Sende B, Belete S, Eshete H, Fantahun M, et al. Barriers to access safe abortion services in East Shoa and Arsi Zones of Oromia Regional State, Ethiopia. J Health Dev. 2015;29(1):13–21.
Arroyave FAB, Moreno PA. A systematic bibliographical review: barriers and facilitators for access to legal abortion in low and middle income countries. Open J Prev Med. 2018;8(5):147–68. https://doi.org/10.4236/ojpm.2018.85015 .
Article Google Scholar
Boland R, Katzive L. Developments in laws on induced abortion: 1998-2007. Int Fam Plan Perspect. 2008;34(3):110–20. https://doi.org/10.1363/3411008 .
Lavelanet AF, Schlitt S, Johnson BR Jr, Ganatra B. Global Abortion Policies Database: a descriptive analysis of the legal categories of lawful abortion. BMC Int Health Hum Rights. 2018;18(1):44. https://doi.org/10.1186/s12914-018-0183-1 .
United Nations Population Division. Abortion policies: A global review. Major dimensions of abortion policies. 2002 [Available from: https://www.un.org/en/development/desa/population/publications/abortion/abortion-policies-2002.asp .
Johnson BR, Lavelanet AF, Schlitt S. Global abortion policies database: a new approach to strengthening knowledge on laws, policies, and human rights standards. Bmc Int Health Hum Rights. 2018;18(1):35. https://doi.org/10.1186/s12914-018-0174-2 .
Haaland MES, Haukanes H, Zulu JM, Moland KM, Michelo C, Munakampe MN, et al. Shaping the abortion policy - competing discourses on the Zambian termination of pregnancy act. Int J Equity Health. 2019;18(1):20. https://doi.org/10.1186/s12939-018-0908-8 .
Schatz JJ. Zambia’s health-worker crisis. Lancet. 2008;371(9613):638–9. https://doi.org/10.1016/S0140-6736(08)60287-1 .
Erdman JN, Johnson BR. Access to knowledge and the Global Abortion Policies Database. Int J Gynecol Obstet. 2018;142(1):120–4. https://doi.org/10.1002/ijgo.12509 .
Cleeve A, Oguttu M, Ganatra B, Atuhairwe S, Larsson EC, Makenzius M, et al. Time to act-comprehensive abortion care in east Africa. Lancet Glob Health. 2016;4(9):E601–E2. https://doi.org/10.1016/S2214-109X(16)30136-X .
Berer M, Hoggart L. Medical abortion pills have the potential to change everything about abortion. Contraception. 2018;97(2):79–81. https://doi.org/10.1016/j.contraception.2017.12.006 .
Moseson H, Shaw J, Chandrasekaran S, Kimani E, Maina J, Malisau P, et al. Contextualizing medication abortion in seven African nations: A literature review. Health Care Women Int. 2019;40(7-9):950–80. https://doi.org/10.1080/07399332.2019.1608207 .
Blystad A, Moland KM. Comparative cases of abortion laws and access to safe abortion services in sub-Saharan Africa. Trop Med Int Health. 2017;22:351.
Berer M. Abortion law and policy around the world: in search of decriminalization. Health Hum Rights. 2017;19(1):13–27.
PubMed PubMed Central Google Scholar
Johnson BR, Mishra V, Lavelanet AF, Khosla R, Ganatra B. A global database of abortion laws, policies, health standards and guidelines. B World Health Organ. 2017;95(7):542–4. https://doi.org/10.2471/BLT.17.197442 .
Replogle J. Nicaragua tightens up abortion laws. Lancet. 2007;369(9555):15–6. https://doi.org/10.1016/S0140-6736(07)60011-7 .
Keogh LA, Newton D, Bayly C, McNamee K, Hardiman A, Webster A, et al. Intended and unintended consequences of abortion law reform: perspectives of abortion experts in Victoria, Australia. J Fam Plann Reprod Health Care. 2017;43(1):18–24. https://doi.org/10.1136/jfprhc-2016-101541 .
Levels M, Sluiter R, Need A. A review of abortion laws in Western-European countries. A cross-national comparison of legal developments between 1960 and 2010. Health Policy. 2014;118(1):95–104. https://doi.org/10.1016/j.healthpol.2014.06.008 .
Serbanescu F, Morris L, Stupp P, Stanescu A. The impact of recent policy changes on fertility, abortion, and contraceptive use in Romania. Stud Fam Plann. 1995;26(2):76–87. https://doi.org/10.2307/2137933 .
Henderson JT, Puri M, Blum M, Harper CC, Rana A, Gurung G, et al. Effects of Abortion Legalization in Nepal, 2001-2010. PLoS ONE. 2013;8(5):e64775. https://doi.org/10.1371/journal.pone.0064775 .
Goncalves-Pinho M, Santos JV, Costa A, Costa-Pereira A, Freitas A. The impact of a liberalisation law on legally induced abortion hospitalisations. Eur J Obstet Gynecol Reprod Biol. 2016;203:142–6. https://doi.org/10.1016/j.ejogrb.2016.05.037 .
Latt SM, Milner A, Kavanagh A. Abortion laws reform may reduce maternal mortality: an ecological study in 162 countries. BMC Women’s Health. 2019;19(1). https://doi.org/10.1186/s12905-018-0705-y .
Clarke D, Muhlrad H. Abortion laws and women’s health. IZA discussion papers 11890. Bonn: IZA Institute of Labor Economics; 2018.
Benson J, Andersen K, Samandari G. Reductions in abortion-related mortality following policy reform: evidence from Romania, South Africa and Bangladesh. Reprod Health. 2011;8(39). https://doi.org/10.1186/1742-4755-8-39 .
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Bmj-Brit Med J. 2015;349.
William R. Shadish, Thomas D. Cook, Donald T. Campbell. Experimental and quasi-experimental designs for generalized causal inference. Boston, New York; 2002.
Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46(1):348–55. https://doi.org/10.1093/ije/dyw098 .
Bernal JL, Cummins S, Gasparrini A. The use of controls in interrupted time series studies of public health interventions. Int J Epidemiol. 2018;47(6):2082–93. https://doi.org/10.1093/ije/dyy135 .
Meyer BD. Natural and quasi-experiments in economics. J Bus Econ Stat. 1995;13(2):151–61.
Strumpf EC, Harper S, Kaufman JS. Fixed effects and difference in differences. In: Methods in Social Epidemiology ed. San Francisco CA: Jossey-Bass; 2017.
Abadie A, Diamond A, Hainmueller J. Synthetic control methods for comparative case studies: estimating the effect of California’s Tobacco Control Program. J Am Stat Assoc. 2010;105(490):493–505. https://doi.org/10.1198/jasa.2009.ap08746 .
Article CAS Google Scholar
Abadie A, Diamond A, Hainmueller J. Comparative politics and the synthetic control method. Am J Polit Sci. 2015;59(2):495–510. https://doi.org/10.1111/ajps.12116 .
Moscoe E, Bor J, Barnighausen T. Regression discontinuity designs are underutilized in medicine, epidemiology, and public health: a review of current and best practice. Journal of Clinical Epidemiology. 2015;68(2):132–43. https://doi.org/10.1016/j.jclinepi.2014.06.021 .
Dennis A, Blanchard K, Bessenaar T. Identifying indicators for quality abortion care: a systematic literature review. J Fam Plan Reprod H. 2017;43(1):7–15. https://doi.org/10.1136/jfprhc-2015-101427 .
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. https://doi.org/10.1186/s13643-016-0384-4 .
Jackson JL, Kuriyama A, Anton A, Choi A, Fournier JP, Geier AK, et al. The accuracy of Google Translate for abstracting data from non-English-language trials for systematic reviews. Ann Intern Med. 2019.
Reeves BC, Wells GA, Waddington H. Quasi-experimental study designs series-paper 5: a checklist for classifying studies evaluating the effects on health interventions-a taxonomy without labels. J Clin Epidemiol. 2017;89:30–42. https://doi.org/10.1016/j.jclinepi.2017.02.016 .
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. https://doi.org/10.1136/bmj.39489.470347.AD .
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. https://doi.org/10.1002/sim.1186 .
Download references
Acknowledgements
We thank Genevieve Gore, Liaison Librarian at McGill University, for her assistance with refining the research question, keywords, and Mesh terms for the preliminary search strategy.
The authors acknowledge funding from the Fonds de recherche du Quebec – Santé (FRQS) PhD doctoral awards and Canadian Institutes of Health Research (CIHR) Operating Grant, “Examining the impact of social policies on health equity” (ROH-115209).
Author information
Authors and affiliations.
Department of Epidemiology, Biostatistics and Occupational Health, Faculty of Medicine, McGill University, Purvis Hall 1020 Pine Avenue West, Montreal, Quebec, H3A 1A2, Canada
Foluso Ishola, U. Vivian Ukah & Arijit Nandi
You can also search for this author in PubMed Google Scholar
Contributions
FI and AN conceived and designed the protocol. FI drafted the manuscript. FI, UVU, and AN revised the manuscript and approved its final version.
Corresponding author
Correspondence to Foluso Ishola .
Ethics declarations
Ethics approval and consent to participate.
Not applicable
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:.
PRISMA-P 2015 Checklist. This checklist has been adapted for use with systematic review protocol submissions to BioMed Central journals from Table 3 in Moher D et al: Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews 2015 4:1
Additional File 2:.
LMICs according to World Bank Data Catalogue. Country classification specified in the World Bank Data Catalogue to identify low- and middle-income countries
Additional File 3: Table 1
. Search strategy in Embase. Detailed search terms and filters applied to generate our search in Embase
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Ishola, F., Ukah, U.V. & Nandi, A. Impact of abortion law reforms on women’s health services and outcomes: a systematic review protocol. Syst Rev 10 , 192 (2021). https://doi.org/10.1186/s13643-021-01739-w
Download citation
Received : 02 January 2020
Accepted : 08 June 2021
Published : 28 June 2021
DOI : https://doi.org/10.1186/s13643-021-01739-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Abortion law/policies; Impact
- Unsafe abortion
- Contraception
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
A Defense of Abortion
Cite this chapter.

- Judith Jarvis Thomson
1283 Accesses
14 Citations
34 Altmetric
Most opposition to abortion relies on the premise that the fetus is a human being, a person, from the moment of conception. The premise is argued for, but, as I think, not well. Take, for example, the most common argument. We are asked to notice that the development of a human being from conception through birth into childhood is continuous; then it is said that to draw a line, to choose a point in this development and say “before this point the thing is not a person, after this point it is a person” is to make an arbitrary choice, a choice for which in the nature of things no good reason can be given. It is concluded that the fetus is, or anyway that we had better say it is, a person from the moment of conception. But this conclusion does not follow. Similar things might be said about the development of an acorn into an oak tree, and it does not follow that acorns are oak trees, or that we had better say they are. Arguments of this form are sometimes called “slippery slope arguments” —the phrase is perhaps self-explanatory — and it is dismaying that opponents of abortion rely on them so heavily and uncritically.
I am very much indebted to James Thomson for discussion, criticism, and many helpful suggestions.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as EPUB and PDF
- Read on any device
- Instant download
- Own it forever
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Unable to display preview. Download preview PDF.
Similar content being viewed by others

If Abortion, then Infanticide

Anti-abortion Laws and the Ethics of Abortion
The moral significance of abortion inconsistency arguments.
You can also search for this author in PubMed Google Scholar
Editor information
Editors and affiliations.
Georgia State University, Atlanta, USA
James M. Humber & Robert F. Almeder &
Rights and permissions
Reprints and permissions
Copyright information
© 1976 Plenum Press, New York
About this chapter
Thomson, J.J. (1976). A Defense of Abortion. In: Humber, J.M., Almeder, R.F. (eds) Biomedical Ethics and the Law. Springer, Boston, MA. https://doi.org/10.1007/978-1-4684-2223-8_5
Download citation
DOI : https://doi.org/10.1007/978-1-4684-2223-8_5
Publisher Name : Springer, Boston, MA
Print ISBN : 978-1-4684-2225-2
Online ISBN : 978-1-4684-2223-8
eBook Packages : Springer Book Archive
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research

- DigitalGeorgetown Home
- Bioethics Research Library of the Kennedy Institute of Ethics
- Publications and Materials of the Bioethics Research Library
- BioethicsLine: 1972-1999

A Defense of Abortion
Bibliographic citation, permanent link, collections, related items.
Showing items related by title, author, creator and subject.
A Defense of Abortion

The Safety and Quality of Abortion Care in the United States (2018)
Chapter: 5 conclusions, 5 conclusions.
This report provides a comprehensive review of the state of the science on the safety and quality of abortion services in the United States. The committee was charged with answering eight specific research questions. This chapter presents the committee’s conclusions by responding individually to each question. The research findings that are the basis for these conclusions are presented in the previous chapters. The committee was also asked to offer recommendations regarding the eight questions. However, the committee decided that its conclusions regarding the safety and quality of U.S. abortion care responded comprehensively to the scope of this study. Therefore, the committee does not offer recommendations for specific actions to be taken by policy makers, health care providers, and others.
1. What types of legal abortion services are available in the United States? What is the evidence regarding which services are appropriate under different clinical circumstances (e.g., based on patient medical conditions such as previous cesarean section, obesity, gestational age)?
Four legal abortion methods—medication, 1 aspiration, dilation and evacuation (D&E), and induction—are used in the United States. Length of gestation—measured as the amount of time since the first day of the last
___________________
1 The terms “medication abortion” and “medical abortion” are used interchangeably in the literature. This report uses “medication abortion” to describe the U.S. Food and Drug Administration (FDA)-approved prescription drug regimen used up to 10 weeks’ gestation.
menstrual period—is the primary factor in deciding what abortion procedure is the most appropriate. Both medication and aspiration abortions are used up to 10 weeks’ gestation. Aspiration procedures may be used up to 14 to 16 weeks’ gestation.
Mifepristone, sold under the brand name Mifeprex, is the only medication specifically approved by the FDA for use in medication abortion. The drug’s distribution has been restricted under the requirements of the FDA Risk Evaluation and Mitigation Strategy program since 2011—it may be dispensed only to patients in clinics, hospitals, or medical offices under the supervision of a certified prescriber. To become a certified prescriber, eligible clinicians must register with the drug’s distributor, Danco Laboratories, and meet certain requirements. Retail pharmacies are prohibited from distributing the drug.
When abortion by aspiration is no longer feasible, D&E and induction methods are used. D&E is the superior method; in comparison, inductions are more painful for women, take significantly more time, and are more costly. However, D&Es are not always available to women. The procedure is illegal in Mississippi 2 and West Virginia 3 (both states allow exceptions in cases of life endangerment or severe physical health risk to the woman). Elsewhere, access to the procedure is limited because many obstetrician/gynecologists (OB/GYNs) and other physicians lack the requisite training to perform D&Es. Physicians’ access to D&E training is very limited or nonexistent in many areas of the country.
Few women are medically ineligible for abortion. There are, however, specific contraindications to using mifepristone for a medication abortion or induction. The drug should not be used for women with confirmed or suspected ectopic pregnancy or undiagnosed adnexal mass; an intrauterine device in place; chronic adrenal failure; concurrent long-term systemic corticosteroid therapy; hemorrhagic disorders or concurrent anticoagulant therapy; allergy to mifepristone, misoprostol, or other prostaglandins; or inherited porphyrias.
Obesity is not a risk factor for women who undergo medication or aspiration abortions (including with the use of moderate intravenous sedation). Research on the association between obesity and complications during a D&E abortion is less certain—particularly for women with Class III obesity (body mass index ≥40) after 14 weeks’ gestation.
A history of a prior cesarean delivery is not a risk factor for women undergoing medication or aspiration abortions, but it may be associated
2 Mississippi Unborn Child Protection from Dismemberment Abortion Act, Mississippi HB 519, Reg. Sess. 2015–2016 (2016).
3 Unborn Child Protection from Dismemberment Abortion Act, West Virginia SB 10, Reg. Sess. 2015–2016 (2016).
with an increased risk of complications during D&E abortions, particularly for women with multiple cesarean deliveries. Because induction abortions are so rare, it is difficult to determine definitively whether a prior cesarean delivery increases the risk of complications. The available research suggests no association.
2. What is the evidence on the physical and mental health risks of these different abortion interventions?
Abortion has been investigated for its potential long-term effects on future childbearing and pregnancy outcomes, risk of breast cancer, mental health disorders, and premature death. The committee found that much of the published literature on these topics does not meet scientific standards for rigorous, unbiased research. Reliable research uses documented records of a prior abortion, analyzes comparable study and control groups, and controls for confounding variables shown to affect the outcome of interest.
Physical health effects The committee identified high-quality research on numerous outcomes of interest and concludes that having an abortion does not increase a woman’s risk of secondary infertility, pregnancy-related hypertensive disorders, abnormal placentation (after a D&E abortion), preterm birth, or breast cancer. Although rare, the risk of very preterm birth (<28 weeks’ gestation) in a woman’s first birth was found to be associated with having two or more prior aspiration abortions compared with first births among women with no abortion history; the risk appears to be associated with the number of prior abortions. Preterm birth is associated with pregnancy spacing after an abortion: it is more likely if the interval between abortion and conception is less than 6 months (this is also true of pregnancy spacing in general). The committee did not find well-designed research on abortion’s association with future ectopic pregnancy, miscarriage or stillbirth, or long-term mortality. Findings on hemorrhage during a subsequent pregnancy are inconclusive.
Mental health effects The committee identified a wide array of research on whether abortion increases women’s risk of depression, anxiety, and/or posttraumatic stress disorder and concludes that having an abortion does not increase a woman’s risk of these mental health disorders.
3. What is the evidence on the safety and quality of medical and surgical abortion care?
Safety The clinical evidence clearly shows that legal abortions in the United States—whether by medication, aspiration, D&E, or induction—are
safe and effective. Serious complications are rare. But the risk of a serious complication increases with weeks’ gestation. As the number of weeks increases, the invasiveness of the required procedure and the need for deeper levels of sedation also increase.
Quality Health care quality is a multidimensional concept. Six attributes of health care quality—safety, effectiveness, patient-centeredness, timeliness, efficiency, and equity—were central to the committee’s review of the quality of abortion care. Table 5-1 details the committee’s conclusions regarding each of these quality attributes. Overall, the committee concludes that the quality of abortion care depends to a great extent on where women live. In many parts of the country, state regulations have created barriers to optimizing each dimension of quality care. The quality of care is optimal when the care is based on current evidence and when trained clinicians are available to provide abortion services.
4. What is the evidence on the minimum characteristics of clinical facilities necessary to effectively and safely provide the different types of abortion interventions?
Most abortions can be provided safely in office-based settings. No special equipment or emergency arrangements are required for medication abortions. For other abortion methods, the minimum facility characteristics depend on the level of sedation that is used. Aspiration abortions are performed safely in office and clinic settings. If moderate sedation is used, the facility should have emergency resuscitation equipment and an emergency transfer plan, as well as equipment to monitor oxygen saturation, heart rate, and blood pressure. For D&Es that involve deep sedation or general anesthesia, the facility should be similarly equipped and also have equipment to provide general anesthesia and monitor ventilation.
Women with severe systemic disease require special measures if they desire or need deep sedation or general anesthesia. These women require further clinical assessment and should have their abortion in an accredited ambulatory surgery center or hospital.
5. What is the evidence on what clinical skills are necessary for health care providers to safely perform the various components of abortion care, including pregnancy determination, counseling, gestational age assessment, medication dispensing, procedure performance, patient monitoring, and follow-up assessment and care?
Required skills All abortion procedures require competent providers skilled in patient preparation (education, counseling, and informed consent);
TABLE 5-1 Does Abortion Care in the United States Meet the Six Attributes of Quality Health Care?
a These attributes of quality health care were first proposed by the Institute of Medicine’s Committee on Quality of Health Care in America in the 2001 report Crossing the Quality Chasm: A New Health System for the 21st Century.
b Elsewhere in this report, effectiveness refers to the successful completion of the abortion without the need for a follow-up aspiration.
clinical assessment (confirming intrauterine pregnancy, determining gestation, taking a relevant medical history, and physical examination); pain management; identification and management of expected side effects and serious complications; and contraceptive counseling and provision. To provide medication abortions, the clinician should be skilled in all these areas. To provide aspiration abortions, the clinician should also be skilled in the technical aspects of an aspiration procedure. To provide D&E abortions, the clinician needs the relevant surgical expertise and sufficient caseload to maintain the requisite surgical skills. To provide induction abortions, the clinician requires the skills needed for managing labor and delivery.
Clinicians that have the necessary competencies Both trained physicians (OB/GYNs, family medicine physicians, and other physicians) and advanced practice clinicians (APCs) (physician assistants, certified nurse-midwives, and nurse practitioners) can provide medication and aspiration abortions safely and effectively. OB/GYNs, family medicine physicians, and other physicians with appropriate training and experience can perform D&E abortions. Induction abortions can be provided by clinicians (OB/GYNs,
family medicine physicians, and certified nurse-midwives) with training in managing labor and delivery.
The extensive body of research documenting the safety of abortion care in the United States reflects the outcomes of abortions provided by thousands of individual clinicians. The use of sedation and anesthesia may require special expertise. If moderate sedation is used, it is essential to have a nurse or other qualified clinical staff—in addition to the person performing the abortion—available to monitor the patient, as is the case for any other medical procedure. Deep sedation and general anesthesia require the expertise of an anesthesiologist or certified registered nurse anesthetist to ensure patient safety.
6. What safeguards are necessary to manage medical emergencies arising from abortion interventions?
The key safeguards—for abortions and all outpatient procedures—are whether the facility has the appropriate equipment, personnel, and emergency transfer plan to address any complications that might occur. No special equipment or emergency arrangements are required for medication abortions; however, clinics should provide a 24-hour clinician-staffed telephone line and have a plan to provide emergency care to patients after hours. If moderate sedation is used during an aspiration abortion, the facility should have emergency resuscitation equipment and an emergency transfer plan, as well as equipment to monitor oxygen saturation, heart rate, and blood pressure. D&Es that involve deep sedation or general anesthesia should be provided in similarly equipped facilities that also have equipment to monitor ventilation.
The committee found no evidence indicating that clinicians that perform abortions require hospital privileges to ensure a safe outcome for the patient. Providers should, however, be able to provide or arrange for patient access or transfer to medical facilities equipped to provide blood transfusions, surgical intervention, and resuscitation, if necessary.
7. What is the evidence on the safe provision of pain management for abortion care?
Nonsteroidal anti-inflammatory drugs (NSAIDs) are recommended to reduce the discomfort of pain and cramping during a medication abortion. Some women still report high levels of pain, and researchers are exploring new ways to provide prophylactic pain management for medication abortion. The pharmaceutical options for pain management during aspiration, D&E, and induction abortions range from local anesthesia, to minimal sedation/anxiolysis, to moderate sedation/analgesia, to deep sedation/
analgesia, to general anesthesia. Along this continuum, the physiological effects of sedation have increasing clinical implications and, depending on the depth of sedation, may require special equipment and personnel to ensure the patient’s safety. The greatest risk of using sedative agents is respiratory depression. The vast majority of abortion patients are healthy and medically eligible for all levels of sedation in office-based settings. As noted above (see Questions 4 and 6), if sedation is used, the facility should be appropriately equipped and staffed.
8. What are the research gaps associated with the provision of safe, high-quality care from pre- to postabortion?
The committee’s overarching task was to assess the safety and quality of abortion care in the United States. As noted in the introduction to this chapter, the committee decided that its findings and conclusions fully respond to this charge. The committee concludes that legal abortions are safe and effective. Safety and quality are optimized when the abortion is performed as early in pregnancy as possible. Quality requires that care be respectful of individual patient preferences, needs, and values so that patient values guide all clinical decisions.
The committee did not identify gaps in research that raise concerns about these conclusions and does not offer recommendations for specific actions to be taken by policy makers, health care providers, and others.
The following are the committee’s observations about questions that merit further investigation.
Limitation of Mifepristone distribution As noted above, mifepristone, sold under the brand name Mifeprex, is the only medication approved by the FDA for use in medication abortion. Extensive clinical research has demonstrated its safety and effectiveness using the FDA-recommended regimen. Furthermore, few women have contraindications to medication abortion. Nevertheless, as noted earlier, the FDA REMS restricts the distribution of mifepristone. Research is needed on how the limited distribution of mifepristone under the REMS process impacts dimensions of quality, including timeliness, patient-centeredness, and equity. In addition, little is known about pharmacist and patient perspectives on pharmacy dispensing of mifepristone and the potential for direct-to-patient models through telemedicine.
Pain management There is insufficient evidence to identify the optimal approach to minimizing the pain women experience during an aspiration procedure without sedation. Paracervical blocks are effective in decreasing procedural pain, but the administration of the block itself is painful, and
even with the block, women report experiencing moderate to significant pain. More research is needed to learn how best to reduce the pain women experience during abortion procedures.
Research on prophylactic pain management for women undergoing medication abortions is also needed. Although NSAIDs reduce the pain of cramping, women still report high levels of pain.
Availability of providers APCs can provide medication and aspiration abortions safely and effectively, but the committee did not find research assessing whether APCs can also be trained to perform D&Es.
Addressing the needs of women of lower income Women who have abortions are disproportionately poor and at risk for interpersonal and other types of violence. Yet little is known about the extent to which they receive needed social and psychological supports when seeking abortion care or how best to meet those needs. More research is needed to assess the need for support services and to define best clinical practice for providing those services.
Abortion is a legal medical procedure that has been provided to millions of American women. Since the Institute of Medicine first reviewed the health implications of national legalized abortion in 1975, there has been a plethora of related scientific research, including well-designed randomized clinical trials, systematic reviews, and epidemiological studies examining abortion care. This research has focused on examining the relative safety of abortion methods and the appropriateness of methods for different clinical circumstances. With this growing body of research, earlier abortion methods have been refined, discontinued, and new approaches have been developed.
The Safety and Quality of Abortion Care in the United States offers a comprehensive review of the current state of the science related to the provision of safe, high-quality abortion services in the United States. This report considers 8 research questions and presents conclusions, including gaps in research.
READ FREE ONLINE
Welcome to OpenBook!
You're looking at OpenBook, NAP.edu's online reading room since 1999. Based on feedback from you, our users, we've made some improvements that make it easier than ever to read thousands of publications on our website.
Do you want to take a quick tour of the OpenBook's features?
Show this book's table of contents , where you can jump to any chapter by name.
...or use these buttons to go back to the previous chapter or skip to the next one.
Jump up to the previous page or down to the next one. Also, you can type in a page number and press Enter to go directly to that page in the book.
Switch between the Original Pages , where you can read the report as it appeared in print, and Text Pages for the web version, where you can highlight and search the text.
To search the entire text of this book, type in your search term here and press Enter .
Share a link to this book page on your preferred social network or via email.
View our suggested citation for this chapter.
Ready to take your reading offline? Click here to buy this book in print or download it as a free PDF, if available.
Get Email Updates
Do you enjoy reading reports from the Academies online for free ? Sign up for email notifications and we'll let you know about new publications in your areas of interest when they're released.
- Share full article
Advertisement
Supported by
guest essay
Is There a Constitutional Right to Talk About Abortion?

By Linda Greenhouse
Ms. Greenhouse, the recipient of a 1998 Pulitzer Prize, reported on the Supreme Court for The Times from 1978 to 2008 and was a contributing Opinion writer from 2009 to 2021.
There has hardly ever been as fierce a defender of free speech as the current Supreme Court.
Since John Roberts became chief justice almost 19 years ago, the court has expanded the protective net of the First Amendment to cover such activities as selling videos depicting animal torture, spending unlimited amounts of money in support of political candidates and refusing to pay dues (or a dues-like fee) to a public employee union.
This last decision, Janus v. American Federation of State, County and Municipal Employees, Council 31, overturned a 41-year-old precedent and led a dissenting justice, Elena Kagan, to accuse the majority of “weaponizing the First Amendment.” In the 303 Creative case last year, the court gave a Christian web designer the First Amendment right not to do business with would-be customers whose same-sex wedding websites would violate her views about marriage.
The court’s version of free speech has become a powerful tool against government regulation. Six years ago, effectively striking down a California law, the court gave so-called crisis pregnancy centers — offices that try to imitate abortion clinics but strive to persuade women to continue their pregnancies — a First Amendment right not to provide information on where a woman could actually get an abortion. The state said the notice was needed to help women who came to such centers under the false impression that they provided abortions. In his majority opinion, Justice Clarence Thomas said the “unduly burdensome” requirement amounted to unconstitutionally compelled speech.
Now the question is whether the court’s solicitude toward those who would rather not talk about abortion extends in the other direction. What about state laws that prohibit rather than require offering information about where to get an abortion?
While there is not yet such a case on the Supreme Court’s docket, lower courts have been tightening a First Amendment noose around efforts by anti-abortion states to curb the flow of information about how to obtain legal abortion care across state lines. Federal District Courts in Indiana and Alabama both ruled this month that while states in the wake of Roe v. Wade’s demise can ban abortion, they cannot make it illegal to give abortion-related advice, including advice to minors seeking abortions without parental consent.
A federal magistrate judge issued a similar ruling last November on Idaho’s abortion law, one of the most extreme in the country, which makes it a crime to assist a minor in obtaining an abortion in any state without a parent’s consent. Idaho could criminalize abortion, the judge, Debora Grasham, wrote. “What the state cannot do,” she went on, “is craft a statute muzzling the speech and expressive activities of a particular viewpoint with which the state disagrees under the guise of parental rights.” The United States Court of Appeals for the Ninth Circuit heard Idaho’s appeal on May 7.
With the Supreme Court extremely unlikely to revisit its decision 23 months ago in Dobbs v. Jackson Women’s Health Organization that eradicated the constitutional right to abortion, the question of how far states can go to prevent their citizens from finding alternative ways to terminate a pregnancy will become increasingly urgent. In his concurring opinion in the Dobbs case, Justice Brett Kavanaugh raised the question of whether a state could now “bar a resident of that state from traveling to another state to obtain an abortion.” The answer was “no,” he continued, “based on the constitutional right to interstate travel.” It is worth noting that Justice Kavanaugh wrote only for himself; none of the other conservatives who made up the Dobbs majority joined him. “Other abortion-related legal questions may emerge in the future,” Justice Kavanaugh offered noncommittally.
The future arrived quickly enough in the form of the two abortion-related cases awaiting decision before the court’s current term, which concludes at the end of June or in early July. Both are anomalous in that they involve questions of federal rather than state authority.
One, Food and Drug Administration v. Alliance for Hippocratic Medicine , concerns the government’s approval of the expanded use of the medication that first received F.D.A. approval 24 years ago. Medication abortion now accounts for more than half of abortions in the United States. The case contains an off-ramp for the court that, based on the argument in March, the justices appear likely to take: Because the anti-abortion doctors, dentists and medical groups who challenged the F.D.A. suffered no harm from the availability of the medication, and are unlikely to suffer harm in the future, they never had standing to bring the case in the first place.
The other, Moyle v. United States, results from a clash between the federal government and Idaho over whether federal law requires the state to provide emergency abortion care in its hospitals. The outcome largely depends on whether the court accepts the Biden administration’s view that there is no abortion exception to the law at issue, which prohibits hospitals from turning away people who need emergency care.
In the abortion cases in Indiana, Idaho and Alabama that may yet find their way to the Supreme Court, the justices would face the acute dilemma of reconciling their fealty to the First Amendment with the profound anti-abortion sentiment the Dobbs majority opinion displayed.
In defending their laws, the states argue that what they are prohibiting is not actually speech but conduct, namely inducing criminal activity. Rejecting this argument in the Indiana case, Judge Sarah Evans Barker of Federal District Court wrote that the Planned Parenthood affiliate that challenged the law simply “seeks to provide truthful information to clients regarding out-of-state options and medical referrals to out-of-state providers for abortion services that are legal in those states.” A prohibition on providing such information, the judge said, “does not further any interest Indiana may have in investigating criminal conduct within its borders.” In the Alabama case, another Federal District Court judge, Myron Thompson, observed that “unable to proscribe out-of-state abortions, the attorney general interprets state law as punishing the speech necessary to obtain them.”
From the cases they are in the process of deciding this term, the justices are well aware that their effort to wash their hands of the nettlesome business of abortion has failed. One or more of the First Amendment cases is likely to reach the court during its next term. I wonder if the justices have a clue about how much pain lies ahead when they have to decide whether the right to speak inevitably encompasses the right to choose.
Linda Greenhouse, the recipient of a 1998 Pulitzer Prize, reported on the Supreme Court for The Times from 1978 to 2008 and was a contributing Opinion writer from 2009 to 2021.
The Times is committed to publishing a diversity of letters to the editor. We’d like to hear what you think about this or any of our articles. Here are some tips . And here’s our email: [email protected] .
Follow the New York Times Opinion section on Facebook , Instagram , TikTok , WhatsApp , X and Threads .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 18 May 2024
Vasectomy incidence in the military health system after the reversal of Roe v. Wade
- Benjamin C. Pierson ORCID: orcid.org/0009-0004-8642-293X 1 ,
- Amanda Banaag 1 , 2 ,
- Miranda Lynn Janvrin ORCID: orcid.org/0000-0002-3083-5581 1 , 2 &
- Tracey Pérez Koehlmoos ORCID: orcid.org/0000-0003-1377-8615 1
International Journal of Impotence Research ( 2024 ) Cite this article
Metrics details
- Health care
- Risk factors
Dobbs v. Jackson Women’s Health Organization (Dobbs decision) has already had profound impact on reproductive health care in the United States. Some studies have reported increased incidence of vasectomy after the Dobbs decision. The Military Health System (MHS) provides a unique opportunity to evaluate this relationship in a universally insured, geographically representative population. We conducted a retrospective cross-sectional study of vasectomies among all male beneficiaries in the MHS, ages 18 to 64, from 2018 to 2022. Beneficiaries receiving a vasectomy were identified via billing data extraction from the MHS Data Repository (MDR). Descriptive statistics of demographic factors of all those receiving a vasectomy in the study period were evaluated. Crude and multivariate logistic regression models were used to evaluate for differences in demographic variables in those receiving a vasectomy pre-Dobb’s decision as compared to after the Dobb’s decision. The total number of men receiving a vasectomy each month over the study period was analyzed, as were the numbers in a state immediately implementing abortion access restrictions (Texas), and one without any restrictions on abortion access (Virginia). Our analysis found that men receiving a vasectomy post-Dobbs decision were more likely to be younger, unmarried, and of junior military rank than prior to the Dobbs decision. In the months following the Dobbs decision in 2022 (June-December), there was a 22.1% increase in vasectomy utilization as compared to the averages of those months in 2018–2021. Further, it was found that the relative increase in vasectomy after the Dobbs decision was greater in Texas (29.3%) compared to Virginia (10.6%). Our findings highlight the impact of the Dobbs decision on reproductive health care utilization outside of abortion.
Introduction
The Supreme Court ruling in the Dobbs decision delivered on June 24, 2022, has already had a profound impact on the delivery of reproductive health care in the United States [ 1 ]. The most visible of these has been the restriction on access to abortion and other reproductive health care services implemented by several states with the overturning of Roe v. Wade [ 2 ]. There have also been several important downstream effects from this decision on health systems and the delivery of contraceptive services including the utilization of vasectomy procedures throughout the United States [ 3 ].
Vasectomy is a safe, effective, reversible, relatively inexpensive contraceptive service that can be performed in an outpatient setting and requires minimal recovery time [ 4 , 5 ]. It is associated with significantly fewer complications and shorter recovery times than female tubal ligation [ 6 ]. However, the prevalence of vasectomy has traditionally lagged that of tubal ligation [ 7 ]. Historically, motivations for men to request vasectomy have included previous unwanted pregnancy, completed family size, or dislike of other contraceptive options [ 8 ]. Other studies have shown vasectomy rates to be associated with multiple individual factors including knowledge of procedure, cultural and religious norms, insurance coverage status, and other socioeconomic indicators [ 9 , 10 ]. In 2009, the reported prevalence of vasectomy in men aged 30–45 years was 11.4% [ 10 ]. In 2002, the reported incidence of vasectomy in the United States was 10.2 per 1 000 men aged 25–49 [ 11 ]. During the early 21st century, studies found a decline in the rates of vasectomy in the United States [ 12 , 13 ]. However, more recently vasectomy rates increased in the United States in data from 2014 to 2021 [ 14 ].
In the wake of the Dobbs decision, however, studies have shown up to a 29% increase in vasectomy rates throughout the United States [ 15 , 16 , 17 ]. Larger increases have been seen in states with ‘trigger bans’ on abortion access as compared to those that did not immediately implement access restrictions to abortion services [ 15 ]. These more recent studies have also shown shifts in the demographics of men receiving vasectomies in the United States, with shifts towards younger and unmarried men increasingly utilizing this method of contraception [ 14 , 16 , 17 ]. The factors and motivations for these shifts in vasectomy demographics have not been rigorously studied, however stated reasons for seeking vasectomy amongst individuals motivated by the Dobbs decision included the high success rate of the procedure, concern that vasectomy may be outlawed next, or as an act of solidarity with women [ 15 ].
The Military Health System (MHS) provides health care to approximately 9.6 million beneficiaries comprising service members, retirees, and their families. The system is composed of 49 inpatient hospitals and medical centers and 465 ambulatory care clinics throughout the United States and internationally as well as over 500 000 network providers. Analysis of the MHS allows for a unique opportunity to examine the effects of health policies in a universally insured, geographically representative population [ 18 ]. Given the age and gender distribution in the military, the population provides a diverse sample of individuals of male reproductive capacity in which to study utilization of vasectomy services. Historically, the military has lagged the general population in vasectomy utilization, with an estimated incidence of 7.1 vasectomies per 1 000 male service members per year from 2000 to 2009 and 8.6 vasectomies per 1 000 male service members per year from 2000 to 2017 [ 13 , 19 ]. Additionally, with the dispersion of the MHS throughout the United States, the system provides additional opportunity to study vasectomy rates between states implementing more onerous restrictions on abortion access post Dobbs decision (Texas) as compared to those not implementing restrictions on abortion access (Virginia). Aside from the differences in abortion policies between the two states, these states were chosen for further analysis due to having the second and third highest number of stationed active duty military servicemembers, and having sizable, representative populations from all military services present. The objective of this study was to retrospectively assess the utilization of vasectomy within the MHS with evaluation of the impact of the Dobbs decision, and differences amongst states with differing levels of restriction on abortion access.
Materials/subjects and methods
Data source and study population.
We retrospectively evaluated administrative health care claims data extracted from the MHS Data Repository (MDR). The MDR holds administrative and healthcare claims for all service members and retirees of the United States Army, Air Force, Navy, Marine Corps and Coast Guard and their dependents. It includes data on care provided directly through Military Treatment Facilities (MTFs) as well as private sector care through the Department of Defense’s TRICARE benefit. It does not include care delivered in a deployed setting, or through the Veterans Health Administration.
Study population
Our study population included all male beneficiaries, ages 18 to 64, of the MHS during the time period of 2018 through 2022. Through query of the MDR we are able to achieve complete ascertainment of vasectomies covered by the MHS. We excluded service members (and their dependents) who were members of the National Guard or Military Reserves due to their inconsistent access to the MHS which may have resulted in bias of mis ascertainment of the outcome of interest by not fully capturing men receiving a vasectomy. There was minimal (< 2%) variation in total number of Active Duty men in the study population year to year, with a low of 1 261 768 in 2022 and a high of 1 285 952 in 2019. The consistency of the denominator indicates that the count of men receiving a vasectomy each year will provide an appropriate approximation of the vasectomy rate year over year.
Data extraction
We queried all encounter data for International Classification of Diseases 10 (ICD-10) codes Z30.2 and Z98.52 and Current Procedural Terminology (CPT) codes 55450 and 55250 to identify vasectomies performed within the MHS. For each unique individual identified, their first visit with one of these codes was counted as a vasectomy. Each unique individual could only be counted for one vasectomy during the study period.
Data analysis
We conducted a descriptive analysis on patient demographics and time period for individuals receiving a vasectomy. Descriptive analysis included patient demographics (age, race/ethnicity, military service branch, and rank), the month and year of their first vasectomy visit, and the state in which the visit occurred. Age was evaluated as a categorical variable by decade (ages 18 and 19 combined with 20 s, all over 60 combined). Rank has been utilized in a variety of military studies both as an indicator of socioeconomic status as well as educational attainment. We performed logistic regression analysis on the effect of these demographic covariates to determine if certain groups were more likely to receive a vasectomy after the Dobbs decision as compared to before the decision. We examined crude logistic regression models on each covariate, as well as a full logistic regression model adjusting for all covariates. We further evaluated numbers of individuals receiving a vasectomy in each month of each year under study to evaluate for general trends, and any potential deviation from these trends in 2022 following the release of the Dobbs decision in June of 2022. Additionally, we assessed the relative change in numbers of vasectomies in the months following the release of the Dobbs decision in 2022 as compared to those month’s average from 2018 through 2021 between a state immediately imposing restrictions on abortion access (Texas) and one that had no such immediate increase in abortion access restrictions (Virginia). All analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC). This study was considered exempt by the Institutional Review Board of the Uniformed Services University of the Health Sciences. This manuscript adheres to the STROBE reporting guidelines.
A total of 96 617 men aged 18–64 received a vasectomy covered by the MHS from January 2018 to December 2022. The demographic breakdown of these men is presented in Table 1 . The groups in which the largest number of vasectomies were performed was in those aged 30–39 (53.97%), White (73.82%), active duty service members (78.24%), in the Army (36.58%), of senior enlisted rank (64.11%), and married (85.37%). We evaluated these demographics in a logistic regression model to determine factors associated with higher odds of receiving a vasectomy after the Dobbs decision (Table 2 ). The results of our adjusted logistic regression model show that the odds of receiving a vasectomy post-Dobbs decision as compared to pre-Dobbs decision were the highest in MHS beneficiaries in ages 18–29 (aOR 1.11, 95% CI 1.07–1.15) as compared to the reference group of those aged 30–39, and in unmarried beneficiaries (aOR 1.06, 95% CI: 1.02–1.10). Dependent beneficiaries were more likely to receive a vasectomy (aOR 1.42, 95% CI: 1.27–1.58) as compared to active duty service members. Senior officers were the least likely of the formal military ranks to receive a vasectomy. No significant differences in odds of vasectomy by race were noted.
In the evaluation of the month during which men received vasectomies from January 2018 through December 2022 (Fig. 1 ) we observed fairly consistent levels between 1 500 and 2000 vasectomies per month during our study, with notable exceptions of large decreases seen during 2020, and a short increase seen immediately following the Dobbs decision in 2022. Overall, the year 2022 had the highest cumulative number of vasectomies performed among all calendar years (22 248) representing a 19.7% increase as compared to the average of 2018–2021. There was also a significant decrease in vasectomies performed in 2020, with only 14,642 men receiving a vasectomy. When discounting this significant decrease in 2020, we still observed an 11.8% increase in vasectomies in 2022 as compared to the average of 2018, 2019, and 2021. In the months following the Dobbs decision release, we observed a noticeable uptick in numbers of vasectomies performed, with a peak of 2236 in August 2022. In analysis of the average of the months June to December in each year, vasectomy incidence increased substantially after the release of the Dobbs decision in June of 2022, with a relative increase from June through December 2022 of 22.1% as compared to the average of the same months from 2018 to 2021. Analysis by state (Fig. 2 ) showed that the relative increase in vasectomy after the Dobbs decision from June through December 2022 as compared to the average of the same period in 2018–2021 was greater in a state immediately implementing a restriction on abortion access (Texas, 29.3%) as compared to a state with no such restriction (Virginia, 10.6%).
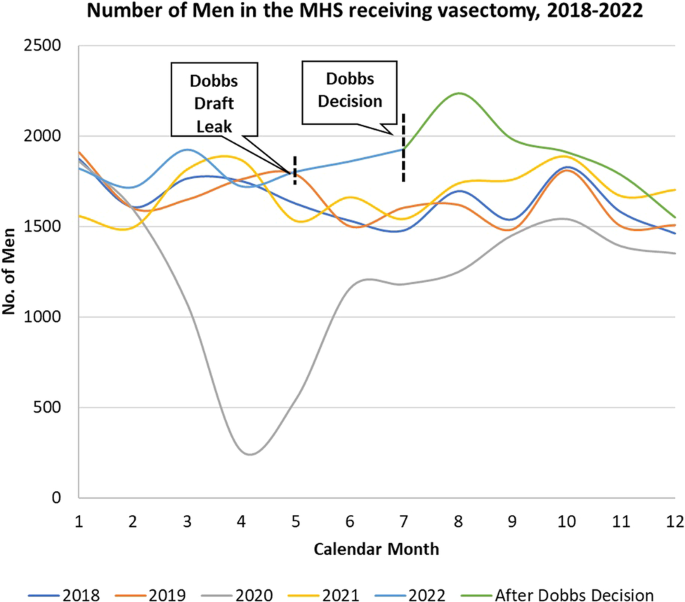
Number of men in the MHS receiving vasectomy, 2018–2022. Source: Authors analysis of MDR Data.
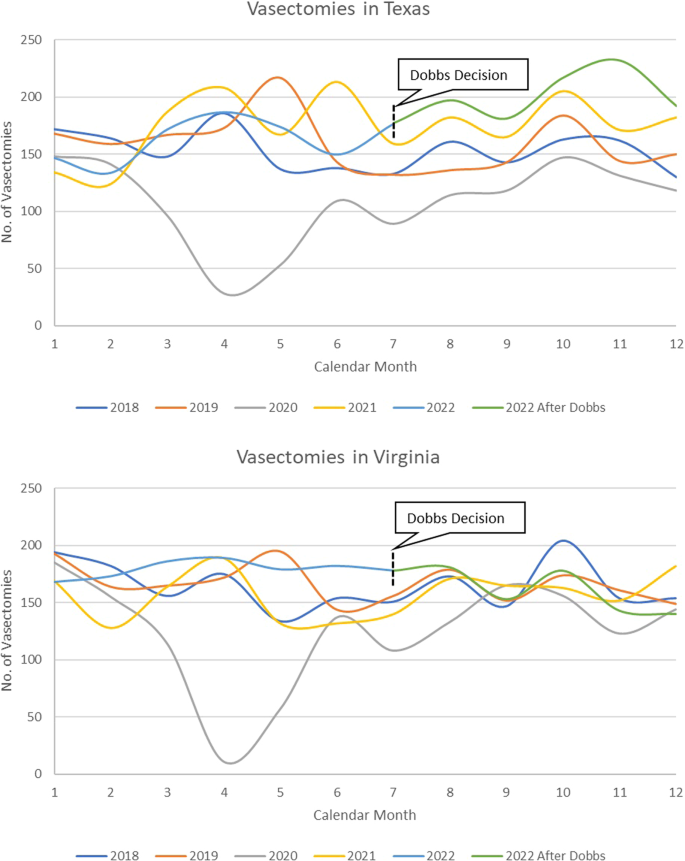
Number of men in the MHS receiving vasectomies in Texas and Virginia, 2018–2022. Source: Authors analysis of MDR Data.
Our findings demonstrate the striking changes in the incidence of vasectomy following the Dobbs decision in the MHS. Not only did 2022 have the highest number of men receiving a vasectomy, but monthly analysis showed a significant increase in vasectomy incidence in the months following the Dobbs decision release in 2022. Additionally, there was a notable difference in vasectomy incidence in Texas, which implemented a trigger restriction on abortion access after the reversal of Roe v. Wade , compared to Virginia, which had no such immediate restriction on abortion access. Texas had a substantially larger increase in vasectomy incidence from June to December 2022 as compared to expected rates from previous years. Further, our study provided interesting insight into the demographics of men receiving a vasectomy within the MHS, with unmarried and younger men having higher odds of receiving a vasectomy. Our analysis also indicated that there was a precipitous decrease in vasectomy incidence in 2020, during the height of the COVID-19 pandemic.
Increased incidence of vasectomy in the wake of the Dobbs decision has been noted in several other studies evaluating vasectomy incidence regional health centers and by insurance claims data [ 15 , 16 ]. Our study shows that similar increases in vasectomy utilization were seen after the release of the Dobbs decision within the MHS as compared to the populations included in previous studies. However, in analysis of the monthly utilization of vasectomy within the MHS, there was a noticeable surge in vasectomies immediately following the Dobb’s decision with incidence largely returning to normal levels several months after the decision. This suggests that it may be possible that the release of the Dobbs decision acted as a ‘call to action’ in the short-term leading those already considering a vasectomy to receive one, but that the effect may be short lived, leading to vasectomy incidence returning to normal levels over time. Further study of the rate of incidence of vasectomy over the next several years could help to determine if this trend persists and vasectomy becomes a more mainstream and utilized form of contraceptive care.
Our findings highlight the potential importance of state-by-state level abortion access restrictions on the utilization of vasectomy, which has notable implications for the MHS. The legalities around providing access to abortion care within the MHS are muddied due to the Hyde Amendment of 1976, which restricts the use of federal funds to be used for abortion care. Typically, for MHS beneficiaries, these services were utilized as needed on an out-of-pocket basis. In some circumstances, beneficiaries were eligible for reimbursement for travel for the procedures due to the care not being available in network [ 20 , 21 , 22 ]. Our findings show that in our two comparator states (Texas and Virginia), the immediate implementation of abortion access restrictions was associated with higher rates of vasectomy utilization post Dobbs decision. This finding has been replicated in at least one other study; however no direct analysis on the effect of restrictions by differing gestational age, or other factors on the rate of vasectomy post Dobbs has been published to date [ 15 ]. Further research into the persistence of these effects, as well as analysis including differing levels of abortion access restrictions to better understand the effects these State level policies have on driving vasectomy utilization is important to better understand the issue and in the allocation of assets and resources for reproductive health care services.
Our demographic analysis indicated that younger (<30) and unmarried men had increased odds of utilizing vasectomy services post Dobbs decision as compared to pre-Dobbs. These findings suggest a significant shift in the demographics of vasectomy utilization, with previous studies typically showing older men at time of family completion were the most likely to utilize the service [ 10 ]. Other more recent studies have demonstrated these trends towards increased utilization of vasectomy services by younger men after the Dobbs decision, with Bole and colleagues showing a significant decline in median age of vasectomy utilization, and larger proportions of unmarried and childless men receiving vasectomy post Dobbs [ 16 ]. These findings suggest a shift in the landscape of perceptions and beliefs regarding vasectomy in younger generations, and further qualitative study of younger men receiving vasectomy to better characterize their attitudes regarding reproductive care could help provide greater insight into this trend. Additionally, longitudinal follow up of these younger and unmarried men receiving vasectomies may be indicated to assess if they are more likely to utilize procedures to reverse their vasectomy in the future. Substantially increased utilization of the more complex reversal procedures could drive significant requirements for resources and training for reproductive urologists and may further highlight the importance of evidence-based pre-vasectomy counseling [ 23 , 24 ].
The impact of the COVID-19 pandemic on vasectomy utilization was an additional significant finding, with 2020 having the lowest number of vasectomies in our study. This finding continues to highlight the myriad impacts that the pandemic had on health care access and utilization, which may drive untold further healthcare needs in the future. Multiple studies have shown varying degrees of decreases in medical care access and utilization from cancer screenings to routine immunizations, and other reproductive services were all significantly impacted by the pandemic [ 25 , 26 , 27 ]. Further work on the downstream effects of these impacts could help to develop campaigns to ‘catch up’ on the immense amount of delayed care.
Our study had several key limitations. First, we were only able to capture vasectomies paid for (directly or indirectly) by the MHS, therefore any beneficiary receiving this service outside of the MHS would not be included. Additionally, we only utilized data back to 2018, which was primarily done to present data comparable to other studies that have been published on this issue [ 15 , 16 ]. Further, as we only allowed for each individual to be counted for one vasectomy, it is possible that individuals received a vasectomy, had it reversed, and then received another vasectomy, in which case their second vasectomy would not be counted; however, we believe this to be rare, with only approximately 5% of men seeking an initial vasectomy reversal, and an even smaller proportion seeking re-vasectomy after a reversal [ 28 ].
Our analysis supports the hypothesis that the Dobbs decision had an immediate effect on vasectomy utilization within the MHS. We assess that the reversal of Roe v. Wade was a significant driver of a sizable increase in vasectomy incidence within the MHS, specifically amongst younger and unmarried men. Additionally, state level restrictions on abortion access may have mediated this effect; with more stringent restrictions resulting in higher rates of vasectomy utilization. These findings demonstrate the robustness of changes in the landscape of vasectomy utilization in a universally insured, geographically representative population. The MHS must be cognizant of the profound effect that the Dobbs decision has had on the state of reproductive health care access in America. It must be agile in appropriately allocating reproductive care assets and resources to those areas greatest effected by the reversal of Roe v. Wade to best support the needs of service members and their families.
The contents of this publication are the sole responsibility of the authors and do not necessarily reflect the views, assertions, opinions or policies of the Uniformed Services University of the Health Sciences (USUHS), the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. (HJF), the Department of Defense (DoD), or the Departments of the Army, Navy, or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the United States Government.
Data availability
The data that support the findings of this study are available from the United States Defense Health Agency. Restrictions apply to the availability of these data, which were used under federal Data User Agreements for the current study, and so are not publicly available.
Stein TB, Vasan A. Public health’s role in a Post-Dobbs World - The New York City abortion access hub. N Engl J Med. 2023;388:1926–9.
Article PubMed Google Scholar
Kulczycki A. Dobbs: navigating the new quagmire and its impacts on abortion and reproductive health care. Health Educ Behav Off Publ Soc Public Health Educ. 2022;49:924–8.
Google Scholar
Paltrow LM, Harris LH, Marshall MF. Beyond abortion: the consequences of overturning roe. Am J Bioeth AJOB. 2022;22:3–15.
Jamieson DJ, Costello C, Trussell J, Hillis SD, Marchbanks PA, Peterson HB, et al. The risk of pregnancy after vasectomy. Obstet Gynecol. 2004;103:848–50.
Schwingl PJ, Guess HA. Safety and effectiveness of vasectomy. Fertil Steril. 2000;73:923–36.
Article CAS PubMed Google Scholar
Bartz D, Greenberg JA. Sterilization in the United States. Rev Obstet Gynecol. 2008;1:23–32.
PubMed PubMed Central Google Scholar
Mosher WD, Martinez GM, Chandra A, Abma JC, Willson SJ. Use of contraception and use of family planning services in the United States: 1982–2002. Adv Data. 2004:1–36.
Howard G. Motivation for vasectomy. Lancet Lond Engl. 1978;1:546–8.
Article CAS Google Scholar
Dejene Wolde Y, Ali M, Gebremeskel F, Ukke GG, Gebreselassie R, Demelash M, et al. Knowledge, attitude and associated factors towards vasectomy among married men in Arba Minch Town, Southern Ethiopia, 2021; A Cross-Sectional Study. Open Access J Contracept. 2023;14:1–13.
Article PubMed PubMed Central Google Scholar
Eisenberg ML, Henderson JT, Amory JK, Smith JF, Walsh TJ. Racial differences in vasectomy utilization in the United States: data from the national survey of family growth. Urology. 2009;74:1020–4.
Barone MA, Hutchinson PL, Johnson CH, Hsia J, Wheeler J. Vasectomy in the United States, 2002. J Urol. 2006;176:232–6.
Punjani N, Goldstein M. Vasectomy: is the apparent decline real or not? Nat Rev Urol. 2022;19:69–70.
Zhang X, Eisenberg ML. Vasectomy utilization in men aged 18–45 declined between 2002 and 2017: Results from the United States National Survey for Family Growth data. Andrology. 2022;10:137–42.
Huang Z, Hyman MJ, Raheem OA. Trends in the vasectomy rate among privately insured men aged 18-64 in the United States Between 2014 and 2021. Urology. 2023;179:80–6.
Vasectomies rose by 29% in the three months after the end of Roe. Econ Lond. 2023.
Bole R, Lundy SD, Pei E, Bajic P, Parekh N, Vij SC. Rising vasectomy volume following reversal of federal protections for abortion rights in the United States. Int J Impot Res. [Internet]. 2023. Available from: https://doi.org/10.1038/s41443-023-00672-x
Zhang TR, Able C, Ramasamy R, Kohn TP. United States vasectomy incidence rises after the reversal of Roe v. Wade in a national clinical and claims database. Fertil Steril. 2023;120:196–7.
Tanielian T, Farmer C. The US military health system: promoting readiness and providing health care. Health Aff Proj Hope. 2019;38:1259–67.
Article Google Scholar
Santomauro M, Masterson J, Marguet C, Crain D. Demographics of Men Receiving Vasectomies in the US Military 2000-2009. Curr Urol. 2012;6:15–20.
Rosoff JI. The Hyde Amendment and the future. Fam Plann Perspect. 1980;12:172.
Fix L, Seymour JW, Grossman D, Johnson DM, Aiken ARA, Gomperts R, et al. Abortion Need among U.S. Servicewomen: Evidence from an Internet Service. Womens Health Issues Off Publ Jacobs Inst Womens Health. 2020;30:161–6.
Grindlay K, Seymour JW, Fix L, Reiger S, Keefe-Oates B, Grossman D. Abortion knowledge and experiences among U.S. Servicewomen: a qualitative study. Perspect Sex Reprod Health. 2017;49:245–52.
Fantus RJ, Halpern JA. Vasovasostomy and vasoepididymostomy: indications, operative technique, and outcomes. Fertil Steril. 2021;115:1384–92.
Hyman MJ, Huang Z, Raheem OA The percentage of men counseled by urologists who received a vasectomy mildly increased after the publication of the AUA vasectomy guideline. Int J Impot Res. 2024. https://doi.org/10.1038/s41443-024-00829-2 .
Becker NV, Moniz MH, Tipirneni R, Dalton VK, Ayanian JZ. Utilization of women’s preventive health services during the COVID-19 pandemic. JAMA Health Forum. 2021;2:e211408.
Mafi JN, Craff M, Vangala S, Pu T, Skinner D, Tabatabai-Yazdi C, et al. Trends in US ambulatory care patterns during the COVID-19 pandemic, 2019–2021. JAMA. 2022;327:237–47.
Article CAS PubMed PubMed Central Google Scholar
Steenland MW, Geiger CK, Chen L, Rokicki S, Gourevitch RA, Sinaiko AD, et al. Declines in contraceptive visits in the United States during the COVID-19 pandemic. Contraception. 2021;104:593–9.
Ramasamy R, Schlegel PN. Vasectomy and vasectomy reversal: an update. Indian J Urol IJU J Urol Soc India. 2011;27:92–7.
Download references
Acknowledgements
We acknowledge critical advice from the Center for Health Services Research and faculty of the Uniformed Services University Department of Preventive Medicine and Biostatistics. This study was funded by the Department of Defense, Defense Health Agency: Grant #HU0001–20–20035.
Author information
Authors and affiliations.
Uniformed Services University of the Health Sciences, Bethesda, MD, USA
Benjamin C. Pierson, Amanda Banaag, Miranda Lynn Janvrin & Tracey Pérez Koehlmoos
The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, MD, USA
Amanda Banaag & Miranda Lynn Janvrin
You can also search for this author in PubMed Google Scholar
Contributions
Pierson – Conceived and designed work that led to submission, drafted and revised manuscript, approved final version of manuscript and is accountable for all aspects of the work. Banaag – Designed analysis plan, acquired and interpreted data, revised manuscript, approved final version and is accountable for all aspects of the work. Janvrin – Played an important role in interpreting results, revised manuscript, approved final version and is accountable for all aspects of the work. Koehlmoos – Conceived and designed work that lead to submission, revised manuscript, approved final version of manuscript and is accountable for all aspects of the work.
Corresponding author
Correspondence to Benjamin C. Pierson .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Ethical approval
Due to the secondary analysis of existing, de-identified data, this study was deemed exempt from human subjects review by the Institutional Review Board of the Uniformed Services University of the Health Sciences. Permission and conditions for use of these data were granted by the United States Defense Health Agency.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Pierson, B.C., Banaag, A., Janvrin, M.L. et al. Vasectomy incidence in the military health system after the reversal of Roe v. Wade. Int J Impot Res (2024). https://doi.org/10.1038/s41443-024-00905-7
Download citation
Received : 15 February 2024
Revised : 26 April 2024
Accepted : 08 May 2024
Published : 18 May 2024
DOI : https://doi.org/10.1038/s41443-024-00905-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cell Rep Med
- v.4(1); 2023 Jan 17

Abortion bans and their impacts: A view from the United States
Laura j. frye.
1 Gynuity Health Projects, New York, NY, USA
Beverly Winikoff
A retrospective study of abortion facilities in and around Texas by White et al. 1 and a spatial analysis by Rader et al. 2 are combined to illustrate the detrimental effects of abortion bans enacted in the United States.
Abortion restrictions have been introduced in various forms across many states for years, but since June 2022, when the right to abortion was no longer federally protected, we have seen a rapid increase in these restrictions. We are just starting to quantify and qualify their effects. Two recent studies published in JAMA offer early indications of the effects of draconian bans.
In “Association of Texas’ 2021 Ban on Abortion in Early Pregnancy with the Number of Facility-Based Abortion in Texas and Surrounding States,” White et al. used a large dataset containing information before and after the passage of SB8 in September 2021. 1 This bill banned most abortions after 6 weeks in the state of Texas. The data presented in this article allow for a careful examination of the law’s effects, and the authors paint a picture of how rapidly destabilizing such bans can be. The study clearly shows that, in the immediate aftermath of SB8’s implementation, there was both an absolute drop in documented abortions and a shift in the location of abortions as Texans went to neighboring states for medical care.
The paper explicitly examines abortions after 12 weeks as an important indicator of change, not because of the small decrease in safety and efficacy with increasing gestational durations, but rather because of the major increase in burdens to affected individuals (cost, time, travel) and to clinics (resources, scheduling) with gestations beyond this point.
A clearer and more detailed sense of how these patient travel dynamics play out can be found in the “Estimated Travel Time and Spatial Access to Abortion Facilities in the US Before and After the Dobbs v Jackson Women’s Health Decision” by Rader et al., which uses simulation and spatial analysis to measure changes in surface travel time to the closest abortion facility before and after the June 2022 Dobbs decision. 2
The average travel time to reach the nearest abortion facility significantly increased in the simulated post-Dobbs world, and, while the median change from 11 to 17 min is not jaw dropping, the spread of the data and the extremes of the curve are where the biggest problems lie. The authors show a doubling of the number of individuals who must travel more than 60 min to access abortion care. Then, through sensitivity analyses on geographic heterogeneity, they illustrate some of the extreme increases in travel time for people in the South, as in Texas, with a mean increase of over 7 h.
While the White paper notes that their data did not include individual-level demographic information (and thus was not able to explore the disparate effects of the ban on various subpopulations), the Raden paper is able to shed some light on the disproportionate impacts of abortion restrictions by use of census data. The latter paper shows that longer travel times occur more frequently in populations without insurance, with lower incomes, and who are racial and ethnic minorities. Documentation of these effects is important for advocacy, policy change, and resource allocation.
The White et al. paper wisely uses care in describing the data they have as “documented facility-based abortions,” acknowledging the now-frequent practice of non-facility-based self-managed abortion with pills. Similarly, Rader et al. note that their data are predicated on the idea of traveling to a physical facility and do not account for the mailing of pills to a person’s home. The TelAbortion study from 2016 to 2021 provided evidence on the safety and efficacy of direct-to-patient telemedicine abortion with mailing of pills, 3 , 4 and the FDA now allows for this method of abortion pill provision. We also know that self-managed abortion can be a safe and effective option 5 and is currently common in the United States. 6 , 7 There is increasing interest in determining its role in the care landscape. 8 , 9 , 10 Moving forward, it would be beneficial to see more information on how remote provision of care and self-management play into the dynamics illustrated in these articles.
These two papers, used together, can help prepare clinics in protective states for the influx of affected individuals as additional oppressive laws are passed in other states. The lessons documented only grow in relevance as the map of the United States darkens with more and more states passing restrictive abortion laws. We can use these data both to decry the negative and disproportionate effect of these bans and to call for action to prepare receiving clinics in protective states as they take on the care of more people who are denied medical services in their home states.
Declaration of interests
The authors declare no competing interests.

Cultural Relativity and Acceptance of Embryonic Stem Cell Research
Article sidebar.
Main Article Content
There is a debate about the ethical implications of using human embryos in stem cell research, which can be influenced by cultural, moral, and social values. This paper argues for an adaptable framework to accommodate diverse cultural and religious perspectives. By using an adaptive ethics model, research protections can reflect various populations and foster growth in stem cell research possibilities.
INTRODUCTION
Stem cell research combines biology, medicine, and technology, promising to alter health care and the understanding of human development. Yet, ethical contention exists because of individuals’ perceptions of using human embryos based on their various cultural, moral, and social values. While these disagreements concerning policy, use, and general acceptance have prompted the development of an international ethics policy, such a uniform approach can overlook the nuanced ethical landscapes between cultures. With diverse viewpoints in public health, a single global policy, especially one reflecting Western ethics or the ethics prevalent in high-income countries, is impractical. This paper argues for a culturally sensitive, adaptable framework for the use of embryonic stem cells. Stem cell policy should accommodate varying ethical viewpoints and promote an effective global dialogue. With an extension of an ethics model that can adapt to various cultures, we recommend localized guidelines that reflect the moral views of the people those guidelines serve.
Stem cells, characterized by their unique ability to differentiate into various cell types, enable the repair or replacement of damaged tissues. Two primary types of stem cells are somatic stem cells (adult stem cells) and embryonic stem cells. Adult stem cells exist in developed tissues and maintain the body’s repair processes. [1] Embryonic stem cells (ESC) are remarkably pluripotent or versatile, making them valuable in research. [2] However, the use of ESCs has sparked ethics debates. Considering the potential of embryonic stem cells, research guidelines are essential. The International Society for Stem Cell Research (ISSCR) provides international stem cell research guidelines. They call for “public conversations touching on the scientific significance as well as the societal and ethical issues raised by ESC research.” [3] The ISSCR also publishes updates about culturing human embryos 14 days post fertilization, suggesting local policies and regulations should continue to evolve as ESC research develops. [4] Like the ISSCR, which calls for local law and policy to adapt to developing stem cell research given cultural acceptance, this paper highlights the importance of local social factors such as religion and culture.
I. Global Cultural Perspective of Embryonic Stem Cells
Views on ESCs vary throughout the world. Some countries readily embrace stem cell research and therapies, while others have stricter regulations due to ethical concerns surrounding embryonic stem cells and when an embryo becomes entitled to moral consideration. The philosophical issue of when the “someone” begins to be a human after fertilization, in the morally relevant sense, [5] impacts when an embryo becomes not just worthy of protection but morally entitled to it. The process of creating embryonic stem cell lines involves the destruction of the embryos for research. [6] Consequently, global engagement in ESC research depends on social-cultural acceptability.
a. US and Rights-Based Cultures
In the United States, attitudes toward stem cell therapies are diverse. The ethics and social approaches, which value individualism, [7] trigger debates regarding the destruction of human embryos, creating a complex regulatory environment. For example, the 1996 Dickey-Wicker Amendment prohibited federal funding for the creation of embryos for research and the destruction of embryos for “more than allowed for research on fetuses in utero.” [8] Following suit, in 2001, the Bush Administration heavily restricted stem cell lines for research. However, the Stem Cell Research Enhancement Act of 2005 was proposed to help develop ESC research but was ultimately vetoed. [9] Under the Obama administration, in 2009, an executive order lifted restrictions allowing for more development in this field. [10] The flux of research capacity and funding parallels the different cultural perceptions of human dignity of the embryo and how it is socially presented within the country’s research culture. [11]
b. Ubuntu and Collective Cultures
African bioethics differs from Western individualism because of the different traditions and values. African traditions, as described by individuals from South Africa and supported by some studies in other African countries, including Ghana and Kenya, follow the African moral philosophies of Ubuntu or Botho and Ukama , which “advocates for a form of wholeness that comes through one’s relationship and connectedness with other people in the society,” [12] making autonomy a socially collective concept. In this context, for the community to act autonomously, individuals would come together to decide what is best for the collective. Thus, stem cell research would require examining the value of the research to society as a whole and the use of the embryos as a collective societal resource. If society views the source as part of the collective whole, and opposes using stem cells, compromising the cultural values to pursue research may cause social detachment and stunt research growth. [13] Based on local culture and moral philosophy, the permissibility of stem cell research depends on how embryo, stem cell, and cell line therapies relate to the community as a whole. Ubuntu is the expression of humanness, with the person’s identity drawn from the “’I am because we are’” value. [14] The decision in a collectivistic culture becomes one born of cultural context, and individual decisions give deference to others in the society.
Consent differs in cultures where thought and moral philosophy are based on a collective paradigm. So, applying Western bioethical concepts is unrealistic. For one, Africa is a diverse continent with many countries with different belief systems, access to health care, and reliance on traditional or Western medicines. Where traditional medicine is the primary treatment, the “’restrictive focus on biomedically-related bioethics’” [is] problematic in African contexts because it neglects bioethical issues raised by traditional systems.” [15] No single approach applies in all areas or contexts. Rather than evaluating the permissibility of ESC research according to Western concepts such as the four principles approach, different ethics approaches should prevail.
Another consideration is the socio-economic standing of countries. In parts of South Africa, researchers have not focused heavily on contributing to the stem cell discourse, either because it is not considered health care or a health science priority or because resources are unavailable. [16] Each country’s priorities differ given different social, political, and economic factors. In South Africa, for instance, areas such as maternal mortality, non-communicable diseases, telemedicine, and the strength of health systems need improvement and require more focus. [17] Stem cell research could benefit the population, but it also could divert resources from basic medical care. Researchers in South Africa adhere to the National Health Act and Medicines Control Act in South Africa and international guidelines; however, the Act is not strictly enforced, and there is no clear legislation for research conduct or ethical guidelines. [18]
Some parts of Africa condemn stem cell research. For example, 98.2 percent of the Tunisian population is Muslim. [19] Tunisia does not permit stem cell research because of moral conflict with a Fatwa. Religion heavily saturates the regulation and direction of research. [20] Stem cell use became permissible for reproductive purposes only recently, with tight restrictions preventing cells from being used in any research other than procedures concerning ART/IVF. Their use is conditioned on consent, and available only to married couples. [21] The community's receptiveness to stem cell research depends on including communitarian African ethics.
c. Asia
Some Asian countries also have a collective model of ethics and decision making. [22] In China, the ethics model promotes a sincere respect for life or human dignity, [23] based on protective medicine. This model, influenced by Traditional Chinese Medicine (TCM), [24] recognizes Qi as the vital energy delivered via the meridians of the body; it connects illness to body systems, the body’s entire constitution, and the universe for a holistic bond of nature, health, and quality of life. [25] Following a protective ethics model, and traditional customs of wholeness, investment in stem cell research is heavily desired for its applications in regenerative therapies, disease modeling, and protective medicines. In a survey of medical students and healthcare practitioners, 30.8 percent considered stem cell research morally unacceptable while 63.5 percent accepted medical research using human embryonic stem cells. Of these individuals, 89.9 percent supported increased funding for stem cell research. [26] The scientific community might not reflect the overall population. From 1997 to 2019, China spent a total of $576 million (USD) on stem cell research at 8,050 stem cell programs, increased published presence from 0.6 percent to 14.01 percent of total global stem cell publications as of 2014, and made significant strides in cell-based therapies for various medical conditions. [27] However, while China has made substantial investments in stem cell research and achieved notable progress in clinical applications, concerns linger regarding ethical oversight and transparency. [28] For example, the China Biosecurity Law, promoted by the National Health Commission and China Hospital Association, attempted to mitigate risks by introducing an institutional review board (IRB) in the regulatory bodies. 5800 IRBs registered with the Chinese Clinical Trial Registry since 2021. [29] However, issues still need to be addressed in implementing effective IRB review and approval procedures.
The substantial government funding and focus on scientific advancement have sometimes overshadowed considerations of regional cultures, ethnic minorities, and individual perspectives, particularly evident during the one-child policy era. As government policy adapts to promote public stability, such as the change from the one-child to the two-child policy, [30] research ethics should also adapt to ensure respect for the values of its represented peoples.
Japan is also relatively supportive of stem cell research and therapies. Japan has a more transparent regulatory framework, allowing for faster approval of regenerative medicine products, which has led to several advanced clinical trials and therapies. [31] South Korea is also actively engaged in stem cell research and has a history of breakthroughs in cloning and embryonic stem cells. [32] However, the field is controversial, and there are issues of scientific integrity. For example, the Korean FDA fast-tracked products for approval, [33] and in another instance, the oocyte source was unclear and possibly violated ethical standards. [34] Trust is important in research, as it builds collaborative foundations between colleagues, trial participant comfort, open-mindedness for complicated and sensitive discussions, and supports regulatory procedures for stakeholders. There is a need to respect the culture’s interest, engagement, and for research and clinical trials to be transparent and have ethical oversight to promote global research discourse and trust.
d. Middle East
Countries in the Middle East have varying degrees of acceptance of or restrictions to policies related to using embryonic stem cells due to cultural and religious influences. Saudi Arabia has made significant contributions to stem cell research, and conducts research based on international guidelines for ethical conduct and under strict adherence to guidelines in accordance with Islamic principles. Specifically, the Saudi government and people require ESC research to adhere to Sharia law. In addition to umbilical and placental stem cells, [35] Saudi Arabia permits the use of embryonic stem cells as long as they come from miscarriages, therapeutic abortions permissible by Sharia law, or are left over from in vitro fertilization and donated to research. [36] Laws and ethical guidelines for stem cell research allow the development of research institutions such as the King Abdullah International Medical Research Center, which has a cord blood bank and a stem cell registry with nearly 10,000 donors. [37] Such volume and acceptance are due to the ethical ‘permissibility’ of the donor sources, which do not conflict with religious pillars. However, some researchers err on the side of caution, choosing not to use embryos or fetal tissue as they feel it is unethical to do so. [38]
Jordan has a positive research ethics culture. [39] However, there is a significant issue of lack of trust in researchers, with 45.23 percent (38.66 percent agreeing and 6.57 percent strongly agreeing) of Jordanians holding a low level of trust in researchers, compared to 81.34 percent of Jordanians agreeing that they feel safe to participate in a research trial. [40] Safety testifies to the feeling of confidence that adequate measures are in place to protect participants from harm, whereas trust in researchers could represent the confidence in researchers to act in the participants’ best interests, adhere to ethical guidelines, provide accurate information, and respect participants’ rights and dignity. One method to improve trust would be to address communication issues relevant to ESC. Legislation surrounding stem cell research has adopted specific language, especially concerning clarification “between ‘stem cells’ and ‘embryonic stem cells’” in translation. [41] Furthermore, legislation “mandates the creation of a national committee… laying out specific regulations for stem-cell banking in accordance with international standards.” [42] This broad regulation opens the door for future global engagement and maintains transparency. However, these regulations may also constrain the influence of research direction, pace, and accessibility of research outcomes.
e. Europe
In the European Union (EU), ethics is also principle-based, but the principles of autonomy, dignity, integrity, and vulnerability are interconnected. [43] As such, the opportunity for cohesion and concessions between individuals’ thoughts and ideals allows for a more adaptable ethics model due to the flexible principles that relate to the human experience The EU has put forth a framework in its Convention for the Protection of Human Rights and Dignity of the Human Being allowing member states to take different approaches. Each European state applies these principles to its specific conventions, leading to or reflecting different acceptance levels of stem cell research. [44]
For example, in Germany, Lebenzusammenhang , or the coherence of life, references integrity in the unity of human culture. Namely, the personal sphere “should not be subject to external intervention.” [45] Stem cell interventions could affect this concept of bodily completeness, leading to heavy restrictions. Under the Grundgesetz, human dignity and the right to life with physical integrity are paramount. [46] The Embryo Protection Act of 1991 made producing cell lines illegal. Cell lines can be imported if approved by the Central Ethics Commission for Stem Cell Research only if they were derived before May 2007. [47] Stem cell research respects the integrity of life for the embryo with heavy specifications and intense oversight. This is vastly different in Finland, where the regulatory bodies find research more permissible in IVF excess, but only up to 14 days after fertilization. [48] Spain’s approach differs still, with a comprehensive regulatory framework. [49] Thus, research regulation can be culture-specific due to variations in applied principles. Diverse cultures call for various approaches to ethical permissibility. [50] Only an adaptive-deliberative model can address the cultural constructions of self and achieve positive, culturally sensitive stem cell research practices. [51]
II. Religious Perspectives on ESC
Embryonic stem cell sources are the main consideration within religious contexts. While individuals may not regard their own religious texts as authoritative or factual, religion can shape their foundations or perspectives.
The Qur'an states:
“And indeed We created man from a quintessence of clay. Then We placed within him a small quantity of nutfa (sperm to fertilize) in a safe place. Then We have fashioned the nutfa into an ‘alaqa (clinging clot or cell cluster), then We developed the ‘alaqa into mudgha (a lump of flesh), and We made mudgha into bones, and clothed the bones with flesh, then We brought it into being as a new creation. So Blessed is Allah, the Best of Creators.” [52]
Many scholars of Islam estimate the time of soul installment, marked by the angel breathing in the soul to bring the individual into creation, as 120 days from conception. [53] Personhood begins at this point, and the value of life would prohibit research or experimentation that could harm the individual. If the fetus is more than 120 days old, the time ensoulment is interpreted to occur according to Islamic law, abortion is no longer permissible. [54] There are a few opposing opinions about early embryos in Islamic traditions. According to some Islamic theologians, there is no ensoulment of the early embryo, which is the source of stem cells for ESC research. [55]
In Buddhism, the stance on stem cell research is not settled. The main tenets, the prohibition against harming or destroying others (ahimsa) and the pursuit of knowledge (prajña) and compassion (karuna), leave Buddhist scholars and communities divided. [56] Some scholars argue stem cell research is in accordance with the Buddhist tenet of seeking knowledge and ending human suffering. Others feel it violates the principle of not harming others. Finding the balance between these two points relies on the karmic burden of Buddhist morality. In trying to prevent ahimsa towards the embryo, Buddhist scholars suggest that to comply with Buddhist tenets, research cannot be done as the embryo has personhood at the moment of conception and would reincarnate immediately, harming the individual's ability to build their karmic burden. [57] On the other hand, the Bodhisattvas, those considered to be on the path to enlightenment or Nirvana, have given organs and flesh to others to help alleviate grieving and to benefit all. [58] Acceptance varies on applied beliefs and interpretations.
Catholicism does not support embryonic stem cell research, as it entails creation or destruction of human embryos. This destruction conflicts with the belief in the sanctity of life. For example, in the Old Testament, Genesis describes humanity as being created in God’s image and multiplying on the Earth, referencing the sacred rights to human conception and the purpose of development and life. In the Ten Commandments, the tenet that one should not kill has numerous interpretations where killing could mean murder or shedding of the sanctity of life, demonstrating the high value of human personhood. In other books, the theological conception of when life begins is interpreted as in utero, [59] highlighting the inviolability of life and its formation in vivo to make a religious point for accepting such research as relatively limited, if at all. [60] The Vatican has released ethical directives to help apply a theological basis to modern-day conflicts. The Magisterium of the Church states that “unless there is a moral certainty of not causing harm,” experimentation on fetuses, fertilized cells, stem cells, or embryos constitutes a crime. [61] Such procedures would not respect the human person who exists at these stages, according to Catholicism. Damages to the embryo are considered gravely immoral and illicit. [62] Although the Catholic Church officially opposes abortion, surveys demonstrate that many Catholic people hold pro-choice views, whether due to the context of conception, stage of pregnancy, threat to the mother’s life, or for other reasons, demonstrating that practicing members can also accept some but not all tenets. [63]
Some major Jewish denominations, such as the Reform, Conservative, and Reconstructionist movements, are open to supporting ESC use or research as long as it is for saving a life. [64] Within Judaism, the Talmud, or study, gives personhood to the child at birth and emphasizes that life does not begin at conception: [65]
“If she is found pregnant, until the fortieth day it is mere fluid,” [66]
Whereas most religions prioritize the status of human embryos, the Halakah (Jewish religious law) states that to save one life, most other religious laws can be ignored because it is in pursuit of preservation. [67] Stem cell research is accepted due to application of these religious laws.
We recognize that all religions contain subsets and sects. The variety of environmental and cultural differences within religious groups requires further analysis to respect the flexibility of religious thoughts and practices. We make no presumptions that all cultures require notions of autonomy or morality as under the common morality theory , which asserts a set of universal moral norms that all individuals share provides moral reasoning and guides ethical decisions. [68] We only wish to show that the interaction with morality varies between cultures and countries.
III. A Flexible Ethical Approach
The plurality of different moral approaches described above demonstrates that there can be no universally acceptable uniform law for ESC on a global scale. Instead of developing one standard, flexible ethical applications must be continued. We recommend local guidelines that incorporate important cultural and ethical priorities.
While the Declaration of Helsinki is more relevant to people in clinical trials receiving ESC products, in keeping with the tradition of protections for research subjects, consent of the donor is an ethical requirement for ESC donation in many jurisdictions including the US, Canada, and Europe. [69] The Declaration of Helsinki provides a reference point for regulatory standards and could potentially be used as a universal baseline for obtaining consent prior to gamete or embryo donation.
For instance, in Columbia University’s egg donor program for stem cell research, donors followed standard screening protocols and “underwent counseling sessions that included information as to the purpose of oocyte donation for research, what the oocytes would be used for, the risks and benefits of donation, and process of oocyte stimulation” to ensure transparency for consent. [70] The program helped advance stem cell research and provided clear and safe research methods with paid participants. Though paid participation or covering costs of incidental expenses may not be socially acceptable in every culture or context, [71] and creating embryos for ESC research is illegal in many jurisdictions, Columbia’s program was effective because of the clear and honest communications with donors, IRBs, and related stakeholders. This example demonstrates that cultural acceptance of scientific research and of the idea that an egg or embryo does not have personhood is likely behind societal acceptance of donating eggs for ESC research. As noted, many countries do not permit the creation of embryos for research.
Proper communication and education regarding the process and purpose of stem cell research may bolster comprehension and garner more acceptance. “Given the sensitive subject material, a complete consent process can support voluntary participation through trust, understanding, and ethical norms from the cultures and morals participants value. This can be hard for researchers entering countries of different socioeconomic stability, with different languages and different societal values. [72]
An adequate moral foundation in medical ethics is derived from the cultural and religious basis that informs knowledge and actions. [73] Understanding local cultural and religious values and their impact on research could help researchers develop humility and promote inclusion.
IV. Concerns
Some may argue that if researchers all adhere to one ethics standard, protection will be satisfied across all borders, and the global public will trust researchers. However, defining what needs to be protected and how to define such research standards is very specific to the people to which standards are applied. We suggest that applying one uniform guide cannot accurately protect each individual because we all possess our own perceptions and interpretations of social values. [74] Therefore, the issue of not adjusting to the moral pluralism between peoples in applying one standard of ethics can be resolved by building out ethics models that can be adapted to different cultures and religions.
Other concerns include medical tourism, which may promote health inequities. [75] Some countries may develop and approve products derived from ESC research before others, compromising research ethics or drug approval processes. There are also concerns about the sale of unauthorized stem cell treatments, for example, those without FDA approval in the United States. Countries with robust research infrastructures may be tempted to attract medical tourists, and some customers will have false hopes based on aggressive publicity of unproven treatments. [76]
For example, in China, stem cell clinics can market to foreign clients who are not protected under the regulatory regimes. Companies employ a marketing strategy of “ethically friendly” therapies. Specifically, in the case of Beike, China’s leading stem cell tourism company and sprouting network, ethical oversight of administrators or health bureaus at one site has “the unintended consequence of shifting questionable activities to another node in Beike's diffuse network.” [77] In contrast, Jordan is aware of stem cell research’s potential abuse and its own status as a “health-care hub.” Jordan’s expanded regulations include preserving the interests of individuals in clinical trials and banning private companies from ESC research to preserve transparency and the integrity of research practices. [78]
The social priorities of the community are also a concern. The ISSCR explicitly states that guidelines “should be periodically revised to accommodate scientific advances, new challenges, and evolving social priorities.” [79] The adaptable ethics model extends this consideration further by addressing whether research is warranted given the varying degrees of socioeconomic conditions, political stability, and healthcare accessibilities and limitations. An ethical approach would require discussion about resource allocation and appropriate distribution of funds. [80]
While some religions emphasize the sanctity of life from conception, which may lead to public opposition to ESC research, others encourage ESC research due to its potential for healing and alleviating human pain. Many countries have special regulations that balance local views on embryonic personhood, the benefits of research as individual or societal goods, and the protection of human research subjects. To foster understanding and constructive dialogue, global policy frameworks should prioritize the protection of universal human rights, transparency, and informed consent. In addition to these foundational global policies, we recommend tailoring local guidelines to reflect the diverse cultural and religious perspectives of the populations they govern. Ethics models should be adapted to local populations to effectively establish research protections, growth, and possibilities of stem cell research.
For example, in countries with strong beliefs in the moral sanctity of embryos or heavy religious restrictions, an adaptive model can allow for discussion instead of immediate rejection. In countries with limited individual rights and voice in science policy, an adaptive model ensures cultural, moral, and religious views are taken into consideration, thereby building social inclusion. While this ethical consideration by the government may not give a complete voice to every individual, it will help balance policies and maintain the diverse perspectives of those it affects. Embracing an adaptive ethics model of ESC research promotes open-minded dialogue and respect for the importance of human belief and tradition. By actively engaging with cultural and religious values, researchers can better handle disagreements and promote ethical research practices that benefit each society.
This brief exploration of the religious and cultural differences that impact ESC research reveals the nuances of relative ethics and highlights a need for local policymakers to apply a more intense adaptive model.
[1] Poliwoda, S., Noor, N., Downs, E., Schaaf, A., Cantwell, A., Ganti, L., Kaye, A. D., Mosel, L. I., Carroll, C. B., Viswanath, O., & Urits, I. (2022). Stem cells: a comprehensive review of origins and emerging clinical roles in medical practice. Orthopedic reviews , 14 (3), 37498. https://doi.org/10.52965/001c.37498
[2] Poliwoda, S., Noor, N., Downs, E., Schaaf, A., Cantwell, A., Ganti, L., Kaye, A. D., Mosel, L. I., Carroll, C. B., Viswanath, O., & Urits, I. (2022). Stem cells: a comprehensive review of origins and emerging clinical roles in medical practice. Orthopedic reviews , 14 (3), 37498. https://doi.org/10.52965/001c.37498
[3] International Society for Stem Cell Research. (2023). Laboratory-based human embryonic stem cell research, embryo research, and related research activities . International Society for Stem Cell Research. https://www.isscr.org/guidelines/blog-post-title-one-ed2td-6fcdk ; Kimmelman, J., Hyun, I., Benvenisty, N. et al. Policy: Global standards for stem-cell research. Nature 533 , 311–313 (2016). https://doi.org/10.1038/533311a
[4] International Society for Stem Cell Research. (2023). Laboratory-based human embryonic stem cell research, embryo research, and related research activities . International Society for Stem Cell Research. https://www.isscr.org/guidelines/blog-post-title-one-ed2td-6fcdk
[5] Concerning the moral philosophies of stem cell research, our paper does not posit a personal moral stance nor delve into the “when” of human life begins. To read further about the philosophical debate, consider the following sources:
Sandel M. J. (2004). Embryo ethics--the moral logic of stem-cell research. The New England journal of medicine , 351 (3), 207–209. https://doi.org/10.1056/NEJMp048145 ; George, R. P., & Lee, P. (2020, September 26). Acorns and Embryos . The New Atlantis. https://www.thenewatlantis.com/publications/acorns-and-embryos ; Sagan, A., & Singer, P. (2007). The moral status of stem cells. Metaphilosophy , 38 (2/3), 264–284. http://www.jstor.org/stable/24439776 ; McHugh P. R. (2004). Zygote and "clonote"--the ethical use of embryonic stem cells. The New England journal of medicine , 351 (3), 209–211. https://doi.org/10.1056/NEJMp048147 ; Kurjak, A., & Tripalo, A. (2004). The facts and doubts about beginning of the human life and personality. Bosnian journal of basic medical sciences , 4 (1), 5–14. https://doi.org/10.17305/bjbms.2004.3453
[6] Vazin, T., & Freed, W. J. (2010). Human embryonic stem cells: derivation, culture, and differentiation: a review. Restorative neurology and neuroscience , 28 (4), 589–603. https://doi.org/10.3233/RNN-2010-0543
[7] Socially, at its core, the Western approach to ethics is widely principle-based, autonomy being one of the key factors to ensure a fundamental respect for persons within research. For information regarding autonomy in research, see: Department of Health, Education, and Welfare, & National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research (1978). The Belmont Report. Ethical principles and guidelines for the protection of human subjects of research.; For a more in-depth review of autonomy within the US, see: Beauchamp, T. L., & Childress, J. F. (1994). Principles of Biomedical Ethics . Oxford University Press.
[8] Sherley v. Sebelius , 644 F.3d 388 (D.C. Cir. 2011), citing 45 C.F.R. 46.204(b) and [42 U.S.C. § 289g(b)]. https://www.cadc.uscourts.gov/internet/opinions.nsf/6c690438a9b43dd685257a64004ebf99/$file/11-5241-1391178.pdf
[9] Stem Cell Research Enhancement Act of 2005, H. R. 810, 109 th Cong. (2001). https://www.govtrack.us/congress/bills/109/hr810/text ; Bush, G. W. (2006, July 19). Message to the House of Representatives . National Archives and Records Administration. https://georgewbush-whitehouse.archives.gov/news/releases/2006/07/20060719-5.html
[10] National Archives and Records Administration. (2009, March 9). Executive order 13505 -- removing barriers to responsible scientific research involving human stem cells . National Archives and Records Administration. https://obamawhitehouse.archives.gov/the-press-office/removing-barriers-responsible-scientific-research-involving-human-stem-cells
[11] Hurlbut, W. B. (2006). Science, Religion, and the Politics of Stem Cells. Social Research , 73 (3), 819–834. http://www.jstor.org/stable/40971854
[12] Akpa-Inyang, Francis & Chima, Sylvester. (2021). South African traditional values and beliefs regarding informed consent and limitations of the principle of respect for autonomy in African communities: a cross-cultural qualitative study. BMC Medical Ethics . 22. 10.1186/s12910-021-00678-4.
[13] Source for further reading: Tangwa G. B. (2007). Moral status of embryonic stem cells: perspective of an African villager. Bioethics , 21(8), 449–457. https://doi.org/10.1111/j.1467-8519.2007.00582.x , see also Mnisi, F. M. (2020). An African analysis based on ethics of Ubuntu - are human embryonic stem cell patents morally justifiable? African Insight , 49 (4).
[14] Jecker, N. S., & Atuire, C. (2021). Bioethics in Africa: A contextually enlightened analysis of three cases. Developing World Bioethics , 22 (2), 112–122. https://doi.org/10.1111/dewb.12324
[15] Jecker, N. S., & Atuire, C. (2021). Bioethics in Africa: A contextually enlightened analysis of three cases. Developing World Bioethics, 22(2), 112–122. https://doi.org/10.1111/dewb.12324
[16] Jackson, C.S., Pepper, M.S. Opportunities and barriers to establishing a cell therapy programme in South Africa. Stem Cell Res Ther 4 , 54 (2013). https://doi.org/10.1186/scrt204 ; Pew Research Center. (2014, May 1). Public health a major priority in African nations . Pew Research Center’s Global Attitudes Project. https://www.pewresearch.org/global/2014/05/01/public-health-a-major-priority-in-african-nations/
[17] Department of Health Republic of South Africa. (2021). Health Research Priorities (revised) for South Africa 2021-2024 . National Health Research Strategy. https://www.health.gov.za/wp-content/uploads/2022/05/National-Health-Research-Priorities-2021-2024.pdf
[18] Oosthuizen, H. (2013). Legal and Ethical Issues in Stem Cell Research in South Africa. In: Beran, R. (eds) Legal and Forensic Medicine. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-32338-6_80 , see also: Gaobotse G (2018) Stem Cell Research in Africa: Legislation and Challenges. J Regen Med 7:1. doi: 10.4172/2325-9620.1000142
[19] United States Bureau of Citizenship and Immigration Services. (1998). Tunisia: Information on the status of Christian conversions in Tunisia . UNHCR Web Archive. https://webarchive.archive.unhcr.org/20230522142618/https://www.refworld.org/docid/3df0be9a2.html
[20] Gaobotse, G. (2018) Stem Cell Research in Africa: Legislation and Challenges. J Regen Med 7:1. doi: 10.4172/2325-9620.1000142
[21] Kooli, C. Review of assisted reproduction techniques, laws, and regulations in Muslim countries. Middle East Fertil Soc J 24 , 8 (2020). https://doi.org/10.1186/s43043-019-0011-0 ; Gaobotse, G. (2018) Stem Cell Research in Africa: Legislation and Challenges. J Regen Med 7:1. doi: 10.4172/2325-9620.1000142
[22] Pang M. C. (1999). Protective truthfulness: the Chinese way of safeguarding patients in informed treatment decisions. Journal of medical ethics , 25(3), 247–253. https://doi.org/10.1136/jme.25.3.247
[23] Wang, L., Wang, F., & Zhang, W. (2021). Bioethics in China’s biosecurity law: Forms, effects, and unsettled issues. Journal of law and the biosciences , 8(1). https://doi.org/10.1093/jlb/lsab019 https://academic.oup.com/jlb/article/8/1/lsab019/6299199
[24] Wang, Y., Xue, Y., & Guo, H. D. (2022). Intervention effects of traditional Chinese medicine on stem cell therapy of myocardial infarction. Frontiers in pharmacology , 13 , 1013740. https://doi.org/10.3389/fphar.2022.1013740
[25] Li, X.-T., & Zhao, J. (2012). Chapter 4: An Approach to the Nature of Qi in TCM- Qi and Bioenergy. In Recent Advances in Theories and Practice of Chinese Medicine (p. 79). InTech.
[26] Luo, D., Xu, Z., Wang, Z., & Ran, W. (2021). China's Stem Cell Research and Knowledge Levels of Medical Practitioners and Students. Stem cells international , 2021 , 6667743. https://doi.org/10.1155/2021/6667743
[27] Luo, D., Xu, Z., Wang, Z., & Ran, W. (2021). China's Stem Cell Research and Knowledge Levels of Medical Practitioners and Students. Stem cells international , 2021 , 6667743. https://doi.org/10.1155/2021/6667743
[28] Zhang, J. Y. (2017). Lost in translation? accountability and governance of Clinical Stem Cell Research in China. Regenerative Medicine , 12 (6), 647–656. https://doi.org/10.2217/rme-2017-0035
[29] Wang, L., Wang, F., & Zhang, W. (2021). Bioethics in China’s biosecurity law: Forms, effects, and unsettled issues. Journal of law and the biosciences , 8(1). https://doi.org/10.1093/jlb/lsab019 https://academic.oup.com/jlb/article/8/1/lsab019/6299199
[30] Chen, H., Wei, T., Wang, H. et al. Association of China’s two-child policy with changes in number of births and birth defects rate, 2008–2017. BMC Public Health 22 , 434 (2022). https://doi.org/10.1186/s12889-022-12839-0
[31] Azuma, K. Regulatory Landscape of Regenerative Medicine in Japan. Curr Stem Cell Rep 1 , 118–128 (2015). https://doi.org/10.1007/s40778-015-0012-6
[32] Harris, R. (2005, May 19). Researchers Report Advance in Stem Cell Production . NPR. https://www.npr.org/2005/05/19/4658967/researchers-report-advance-in-stem-cell-production
[33] Park, S. (2012). South Korea steps up stem-cell work. Nature . https://doi.org/10.1038/nature.2012.10565
[34] Resnik, D. B., Shamoo, A. E., & Krimsky, S. (2006). Fraudulent human embryonic stem cell research in South Korea: lessons learned. Accountability in research , 13 (1), 101–109. https://doi.org/10.1080/08989620600634193 .
[35] Alahmad, G., Aljohani, S., & Najjar, M. F. (2020). Ethical challenges regarding the use of stem cells: interviews with researchers from Saudi Arabia. BMC medical ethics, 21(1), 35. https://doi.org/10.1186/s12910-020-00482-6
[36] Association for the Advancement of Blood and Biotherapies. https://www.aabb.org/regulatory-and-advocacy/regulatory-affairs/regulatory-for-cellular-therapies/international-competent-authorities/saudi-arabia
[37] Alahmad, G., Aljohani, S., & Najjar, M. F. (2020). Ethical challenges regarding the use of stem cells: Interviews with researchers from Saudi Arabia. BMC medical ethics , 21 (1), 35. https://doi.org/10.1186/s12910-020-00482-6
[38] Alahmad, G., Aljohani, S., & Najjar, M. F. (2020). Ethical challenges regarding the use of stem cells: Interviews with researchers from Saudi Arabia. BMC medical ethics , 21(1), 35. https://doi.org/10.1186/s12910-020-00482-6
Culturally, autonomy practices follow a relational autonomy approach based on a paternalistic deontological health care model. The adherence to strict international research policies and religious pillars within the regulatory environment is a great foundation for research ethics. However, there is a need to develop locally targeted ethics approaches for research (as called for in Alahmad, G., Aljohani, S., & Najjar, M. F. (2020). Ethical challenges regarding the use of stem cells: interviews with researchers from Saudi Arabia. BMC medical ethics, 21(1), 35. https://doi.org/10.1186/s12910-020-00482-6), this decision-making approach may help advise a research decision model. For more on the clinical cultural autonomy approaches, see: Alabdullah, Y. Y., Alzaid, E., Alsaad, S., Alamri, T., Alolayan, S. W., Bah, S., & Aljoudi, A. S. (2022). Autonomy and paternalism in Shared decision‐making in a Saudi Arabian tertiary hospital: A cross‐sectional study. Developing World Bioethics , 23 (3), 260–268. https://doi.org/10.1111/dewb.12355 ; Bukhari, A. A. (2017). Universal Principles of Bioethics and Patient Rights in Saudi Arabia (Doctoral dissertation, Duquesne University). https://dsc.duq.edu/etd/124; Ladha, S., Nakshawani, S. A., Alzaidy, A., & Tarab, B. (2023, October 26). Islam and Bioethics: What We All Need to Know . Columbia University School of Professional Studies. https://sps.columbia.edu/events/islam-and-bioethics-what-we-all-need-know
[39] Ababneh, M. A., Al-Azzam, S. I., Alzoubi, K., Rababa’h, A., & Al Demour, S. (2021). Understanding and attitudes of the Jordanian public about clinical research ethics. Research Ethics , 17 (2), 228-241. https://doi.org/10.1177/1747016120966779
[40] Ababneh, M. A., Al-Azzam, S. I., Alzoubi, K., Rababa’h, A., & Al Demour, S. (2021). Understanding and attitudes of the Jordanian public about clinical research ethics. Research Ethics , 17 (2), 228-241. https://doi.org/10.1177/1747016120966779
[41] Dajani, R. (2014). Jordan’s stem-cell law can guide the Middle East. Nature 510, 189. https://doi.org/10.1038/510189a
[42] Dajani, R. (2014). Jordan’s stem-cell law can guide the Middle East. Nature 510, 189. https://doi.org/10.1038/510189a
[43] The EU’s definition of autonomy relates to the capacity for creating ideas, moral insight, decisions, and actions without constraint, personal responsibility, and informed consent. However, the EU views autonomy as not completely able to protect individuals and depends on other principles, such as dignity, which “expresses the intrinsic worth and fundamental equality of all human beings.” Rendtorff, J.D., Kemp, P. (2019). Four Ethical Principles in European Bioethics and Biolaw: Autonomy, Dignity, Integrity and Vulnerability. In: Valdés, E., Lecaros, J. (eds) Biolaw and Policy in the Twenty-First Century. International Library of Ethics, Law, and the New Medicine, vol 78. Springer, Cham. https://doi.org/10.1007/978-3-030-05903-3_3
[44] Council of Europe. Convention for the protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine: Convention on Human Rights and Biomedicine (ETS No. 164) https://www.coe.int/en/web/conventions/full-list?module=treaty-detail&treatynum=164 (forbidding the creation of embryos for research purposes only, and suggests embryos in vitro have protections.); Also see Drabiak-Syed B. K. (2013). New President, New Human Embryonic Stem Cell Research Policy: Comparative International Perspectives and Embryonic Stem Cell Research Laws in France. Biotechnology Law Report , 32 (6), 349–356. https://doi.org/10.1089/blr.2013.9865
[45] Rendtorff, J.D., Kemp, P. (2019). Four Ethical Principles in European Bioethics and Biolaw: Autonomy, Dignity, Integrity and Vulnerability. In: Valdés, E., Lecaros, J. (eds) Biolaw and Policy in the Twenty-First Century. International Library of Ethics, Law, and the New Medicine, vol 78. Springer, Cham. https://doi.org/10.1007/978-3-030-05903-3_3
[46] Tomuschat, C., Currie, D. P., Kommers, D. P., & Kerr, R. (Trans.). (1949, May 23). Basic law for the Federal Republic of Germany. https://www.btg-bestellservice.de/pdf/80201000.pdf
[47] Regulation of Stem Cell Research in Germany . Eurostemcell. (2017, April 26). https://www.eurostemcell.org/regulation-stem-cell-research-germany
[48] Regulation of Stem Cell Research in Finland . Eurostemcell. (2017, April 26). https://www.eurostemcell.org/regulation-stem-cell-research-finland
[49] Regulation of Stem Cell Research in Spain . Eurostemcell. (2017, April 26). https://www.eurostemcell.org/regulation-stem-cell-research-spain
[50] Some sources to consider regarding ethics models or regulatory oversights of other cultures not covered:
Kara MA. Applicability of the principle of respect for autonomy: the perspective of Turkey. J Med Ethics. 2007 Nov;33(11):627-30. doi: 10.1136/jme.2006.017400. PMID: 17971462; PMCID: PMC2598110.
Ugarte, O. N., & Acioly, M. A. (2014). The principle of autonomy in Brazil: one needs to discuss it ... Revista do Colegio Brasileiro de Cirurgioes , 41 (5), 374–377. https://doi.org/10.1590/0100-69912014005013
Bharadwaj, A., & Glasner, P. E. (2012). Local cells, global science: The rise of embryonic stem cell research in India . Routledge.
For further research on specific European countries regarding ethical and regulatory framework, we recommend this database: Regulation of Stem Cell Research in Europe . Eurostemcell. (2017, April 26). https://www.eurostemcell.org/regulation-stem-cell-research-europe
[51] Klitzman, R. (2006). Complications of culture in obtaining informed consent. The American Journal of Bioethics, 6(1), 20–21. https://doi.org/10.1080/15265160500394671 see also: Ekmekci, P. E., & Arda, B. (2017). Interculturalism and Informed Consent: Respecting Cultural Differences without Breaching Human Rights. Cultura (Iasi, Romania) , 14 (2), 159–172.; For why trust is important in research, see also: Gray, B., Hilder, J., Macdonald, L., Tester, R., Dowell, A., & Stubbe, M. (2017). Are research ethics guidelines culturally competent? Research Ethics , 13 (1), 23-41. https://doi.org/10.1177/1747016116650235
[52] The Qur'an (M. Khattab, Trans.). (1965). Al-Mu’minun, 23: 12-14. https://quran.com/23
[53] Lenfest, Y. (2017, December 8). Islam and the beginning of human life . Bill of Health. https://blog.petrieflom.law.harvard.edu/2017/12/08/islam-and-the-beginning-of-human-life/
[54] Aksoy, S. (2005). Making regulations and drawing up legislation in Islamic countries under conditions of uncertainty, with special reference to embryonic stem cell research. Journal of Medical Ethics , 31: 399-403.; see also: Mahmoud, Azza. "Islamic Bioethics: National Regulations and Guidelines of Human Stem Cell Research in the Muslim World." Master's thesis, Chapman University, 2022. https://doi.org/10.36837/ chapman.000386
[55] Rashid, R. (2022). When does Ensoulment occur in the Human Foetus. Journal of the British Islamic Medical Association , 12 (4). ISSN 2634 8071. https://www.jbima.com/wp-content/uploads/2023/01/2-Ethics-3_-Ensoulment_Rafaqat.pdf.
[56] Sivaraman, M. & Noor, S. (2017). Ethics of embryonic stem cell research according to Buddhist, Hindu, Catholic, and Islamic religions: perspective from Malaysia. Asian Biomedicine,8(1) 43-52. https://doi.org/10.5372/1905-7415.0801.260
[57] Jafari, M., Elahi, F., Ozyurt, S. & Wrigley, T. (2007). 4. Religious Perspectives on Embryonic Stem Cell Research. In K. Monroe, R. Miller & J. Tobis (Ed.), Fundamentals of the Stem Cell Debate: The Scientific, Religious, Ethical, and Political Issues (pp. 79-94). Berkeley: University of California Press. https://escholarship.org/content/qt9rj0k7s3/qt9rj0k7s3_noSplash_f9aca2e02c3777c7fb76ea768ba458f0.pdf https://doi.org/10.1525/9780520940994-005
[58] Lecso, P. A. (1991). The Bodhisattva Ideal and Organ Transplantation. Journal of Religion and Health , 30 (1), 35–41. http://www.jstor.org/stable/27510629 ; Bodhisattva, S. (n.d.). The Key of Becoming a Bodhisattva . A Guide to the Bodhisattva Way of Life. http://www.buddhism.org/Sutras/2/BodhisattvaWay.htm
[59] There is no explicit religious reference to when life begins or how to conduct research that interacts with the concept of life. However, these are relevant verses pertaining to how the fetus is viewed. (( King James Bible . (1999). Oxford University Press. (original work published 1769))
Jerimiah 1: 5 “Before I formed thee in the belly I knew thee; and before thou camest forth out of the womb I sanctified thee…”
In prophet Jerimiah’s insight, God set him apart as a person known before childbirth, a theme carried within the Psalm of David.
Psalm 139: 13-14 “…Thou hast covered me in my mother's womb. I will praise thee; for I am fearfully and wonderfully made…”
These verses demonstrate David’s respect for God as an entity that would know of all man’s thoughts and doings even before birth.
[60] It should be noted that abortion is not supported as well.
[61] The Vatican. (1987, February 22). Instruction on Respect for Human Life in Its Origin and on the Dignity of Procreation Replies to Certain Questions of the Day . Congregation For the Doctrine of the Faith. https://www.vatican.va/roman_curia/congregations/cfaith/documents/rc_con_cfaith_doc_19870222_respect-for-human-life_en.html
[62] The Vatican. (2000, August 25). Declaration On the Production and the Scientific and Therapeutic Use of Human Embryonic Stem Cells . Pontifical Academy for Life. https://www.vatican.va/roman_curia/pontifical_academies/acdlife/documents/rc_pa_acdlife_doc_20000824_cellule-staminali_en.html ; Ohara, N. (2003). Ethical Consideration of Experimentation Using Living Human Embryos: The Catholic Church’s Position on Human Embryonic Stem Cell Research and Human Cloning. Department of Obstetrics and Gynecology . Retrieved from https://article.imrpress.com/journal/CEOG/30/2-3/pii/2003018/77-81.pdf.
[63] Smith, G. A. (2022, May 23). Like Americans overall, Catholics vary in their abortion views, with regular mass attenders most opposed . Pew Research Center. https://www.pewresearch.org/short-reads/2022/05/23/like-americans-overall-catholics-vary-in-their-abortion-views-with-regular-mass-attenders-most-opposed/
[64] Rosner, F., & Reichman, E. (2002). Embryonic stem cell research in Jewish law. Journal of halacha and contemporary society , (43), 49–68.; Jafari, M., Elahi, F., Ozyurt, S. & Wrigley, T. (2007). 4. Religious Perspectives on Embryonic Stem Cell Research. In K. Monroe, R. Miller & J. Tobis (Ed.), Fundamentals of the Stem Cell Debate: The Scientific, Religious, Ethical, and Political Issues (pp. 79-94). Berkeley: University of California Press. https://escholarship.org/content/qt9rj0k7s3/qt9rj0k7s3_noSplash_f9aca2e02c3777c7fb76ea768ba458f0.pdf https://doi.org/10.1525/9780520940994-005
[65] Schenker J. G. (2008). The beginning of human life: status of embryo. Perspectives in Halakha (Jewish Religious Law). Journal of assisted reproduction and genetics , 25 (6), 271–276. https://doi.org/10.1007/s10815-008-9221-6
[66] Ruttenberg, D. (2020, May 5). The Torah of Abortion Justice (annotated source sheet) . Sefaria. https://www.sefaria.org/sheets/234926.7?lang=bi&with=all&lang2=en
[67] Jafari, M., Elahi, F., Ozyurt, S. & Wrigley, T. (2007). 4. Religious Perspectives on Embryonic Stem Cell Research. In K. Monroe, R. Miller & J. Tobis (Ed.), Fundamentals of the Stem Cell Debate: The Scientific, Religious, Ethical, and Political Issues (pp. 79-94). Berkeley: University of California Press. https://escholarship.org/content/qt9rj0k7s3/qt9rj0k7s3_noSplash_f9aca2e02c3777c7fb76ea768ba458f0.pdf https://doi.org/10.1525/9780520940994-005
[68] Gert, B. (2007). Common morality: Deciding what to do . Oxford Univ. Press.
[69] World Medical Association (2013). World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA , 310(20), 2191–2194. https://doi.org/10.1001/jama.2013.281053 Declaration of Helsinki – WMA – The World Medical Association .; see also: National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. (1979). The Belmont report: Ethical principles and guidelines for the protection of human subjects of research . U.S. Department of Health and Human Services. https://www.hhs.gov/ohrp/regulations-and-policy/belmont-report/read-the-belmont-report/index.html
[70] Zakarin Safier, L., Gumer, A., Kline, M., Egli, D., & Sauer, M. V. (2018). Compensating human subjects providing oocytes for stem cell research: 9-year experience and outcomes. Journal of assisted reproduction and genetics , 35 (7), 1219–1225. https://doi.org/10.1007/s10815-018-1171-z https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6063839/ see also: Riordan, N. H., & Paz Rodríguez, J. (2021). Addressing concerns regarding associated costs, transparency, and integrity of research in recent stem cell trial. Stem Cells Translational Medicine , 10 (12), 1715–1716. https://doi.org/10.1002/sctm.21-0234
[71] Klitzman, R., & Sauer, M. V. (2009). Payment of egg donors in stem cell research in the USA. Reproductive biomedicine online , 18 (5), 603–608. https://doi.org/10.1016/s1472-6483(10)60002-8
[72] Krosin, M. T., Klitzman, R., Levin, B., Cheng, J., & Ranney, M. L. (2006). Problems in comprehension of informed consent in rural and peri-urban Mali, West Africa. Clinical trials (London, England) , 3 (3), 306–313. https://doi.org/10.1191/1740774506cn150oa
[73] Veatch, Robert M. Hippocratic, Religious, and Secular Medical Ethics: The Points of Conflict . Georgetown University Press, 2012.
[74] Msoroka, M. S., & Amundsen, D. (2018). One size fits not quite all: Universal research ethics with diversity. Research Ethics , 14 (3), 1-17. https://doi.org/10.1177/1747016117739939
[75] Pirzada, N. (2022). The Expansion of Turkey’s Medical Tourism Industry. Voices in Bioethics , 8 . https://doi.org/10.52214/vib.v8i.9894
[76] Stem Cell Tourism: False Hope for Real Money . Harvard Stem Cell Institute (HSCI). (2023). https://hsci.harvard.edu/stem-cell-tourism , See also: Bissassar, M. (2017). Transnational Stem Cell Tourism: An ethical analysis. Voices in Bioethics , 3 . https://doi.org/10.7916/vib.v3i.6027
[77] Song, P. (2011) The proliferation of stem cell therapies in post-Mao China: problematizing ethical regulation, New Genetics and Society , 30:2, 141-153, DOI: 10.1080/14636778.2011.574375
[78] Dajani, R. (2014). Jordan’s stem-cell law can guide the Middle East. Nature 510, 189. https://doi.org/10.1038/510189a
[79] International Society for Stem Cell Research. (2024). Standards in stem cell research . International Society for Stem Cell Research. https://www.isscr.org/guidelines/5-standards-in-stem-cell-research
[80] Benjamin, R. (2013). People’s science bodies and rights on the Stem Cell Frontier . Stanford University Press.
Mifrah Hayath
SM Candidate Harvard Medical School, MS Biotechnology Johns Hopkins University
Olivia Bowers
MS Bioethics Columbia University (Disclosure: affiliated with Voices in Bioethics)
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License .
- Nation & World Politics
- Abortion rights initiatives make the ballot in South Dakota and Colorado
Voters in Colorado and South Dakota will have a say on abortion rights this fall after enough signatures were collected to put measures on the ballots.
South Dakota voters will get a chance at direct democracy on the contentious issue in a conservative state where a trigger law banning nearly all abortions went into effect after Roe v. Wade was overturned.
Colorado’s measure, which made the ballot Friday, would enshrine abortion protections into the state constitution. Colorado already has broad protections for abortion, becoming a haven for those in states that have restricted abortion, particularly Texas.
South Dakota’s top election official announced Thursday that about 85% of the more than 55,000 signatures submitted in support of the ballot initiative are valid, exceeding the required 35,017 signatures.
Voters will vote up or down on prohibiting the state from regulating abortion before the end of the first trimester and allowing the state to regulate abortion after the second trimester, except when necessary to preserve the life or physical or emotional health of a pregnant woman.
More on abortion, law and politics
- Judge says South Carolina can enforce 6-week abortion ban amid dispute over when a heartbeat begins
- Anti-abortion activist who led a clinic occupation is sentenced to nearly 5 years in prison
- Survey finds 8,000 women a month got abortion pills despite their states’ bans or restrictions
- Texas man seeking info on partner’s reported out-of-state abortion
- What’s EMTALA, the patient protection law at the center of Supreme Court abortion arguments?
Dakotans for Health, which sponsored the amendment, said in a statement Thursday that the signatures’ validation “certified that the people of South Dakota, not the politicians in Pierre, will be the ones to decide whether to restore Roe v. Wade as the law of South Dakota.”
Abortion rights are also on the ballot in Florida and Maryland, and advocates are still working toward that goal in states including Arizona, Montana and Nebraska in the aftermath of the U.S. Supreme Court’s 2022 reversal of Roe.
Voters of seven other states have already approved abortion access in ballot measures, including four that wrote abortion rights into their constitution.
Supporters for Colorado’s ballot measure said they turned in over 225,000 signatures, nearly double the required number of just over 124,000. Amending the state constitution will require the support of 55% of voters.
“In this time of uncertainty, we need to secure abortion rights and access in the Colorado Constitution, beyond the reach of politics and politicians,” Karen Middleton, president of Cobalt Abortion Fund based in Colorado, said in a statement Friday.
South Dakota outlaws all abortions, except to save the life of the mother.
Despite securing language on the ballot, abortion rights advocates in South Dakota face an uphill battle to success in November. Republican lawmakers strongly oppose the measure, and a major abortion rights advocate has said it doesn’t support it.
The American Civil Liberties Union of South Dakota warned when the signatures were submitted that the language as written doesn’t convey the strongest legal standard for courts to evaluate abortion laws and could risk being symbolic only.
Life Defense Fund, a group organized against the initiative, said they will continue to research the signatures.
Opponents still have 30 days — until June 17 — to file a challenge with the secretary of state’s office.
“We are grateful to the many dedicated volunteers who have put in countless hours, and we are resolute in our mission to defend unborn babies,” co-chairs Leslee Unruh and state Rep. Jon Hansen said in a statement.
This story has been edited to remove New York from the list of states where abortion rights are on the ballot this fall. Earlier this month, a judge removed the amendment from the ballot, finding lawmakers missed a procedural step when they put it there.
Dura reported from Bismarck, North Dakota. Associated Press writer Hannah Fingerhut contributed from Oakland, New Jersey.
Most Read Local Stories
- Funder of proposed play area at Seattle nude beach revealed, city plan draws ire
- WA trooper shoots, kills man on I-5 in Everett
- UW encampment to come down after a deal with administrators
- Residents of Seattle low-income high-rise go a week without elevators
- Interim CEO of King County homeless agency withdraws candidacy

DOD Issues Open Announcement for Research Projects Under Defense Industrial Base Consortium OTA; Laura Taylor-Kale Quoted

The Department of Defense has issued an open announcement to solicit white papers on proposed research ideas that could be developed into prototypes as part of a push to accelerate the delivery of critical capabilities to warfighters.
DOD said Tuesday the open announcement released through the Defense Industrial Base Consortium’s other transaction authority is open to companies from the U.S., Canada, Australia, and the U.K.
The white papers should fall within one or more of the critical sectors and areas of interest and will be evaluated to be considered for Defense Production Act Title III and Industrial Base Analysis and Sustainment funding.
The critical sectors listed in the announcement are kinetic capabilities, energy storage and batteries, castings and forgings, strategic and critical materials, microelectronics and workforce development.
Additional areas of interest are small unmanned aerial systems, submarine industrial base, space industrial base and emerging manufacturing technology.
“This Open Announcement will solicit new ideas for research or prototype project solutions that will benefit our industrial base,” said Laura Taylor-Kale , assistant secretary of defense for industrial base policy at DOD.
“Expanding sources of supply is a key element of the vision articulated in the National Defense Industrial Strategy to help build resilient supply chains,” added Taylor-Kale.
White papers are due Sept. 30.

ADVERTISEMENT
Abortion-rights measure validated for South Dakota ballot, but challenge expected
Opponents have 30 days to challenge the validity of the petition..

A state office said Thursday that a petition seeking to reinstate abortion rights has enough valid signatures to make the Nov. 5 ballot, but opponents have already promised a legal challenge.
Based on a random sample of signatures, the South Dakota Secretary of State’s Office estimated that 85% percent of the nearly 55,000 signatures on the petition are from South Dakota registered voters, which means the estimated number of valid signatures is 46,098. The petition needed 35,017 to qualify for the election.
The proposed state constitutional amendment would legalize abortions in the first trimester of pregnancy but allow the state to impose limited regulations in the second trimester and a ban in the third trimester, with exceptions for the life and health of the mother. Abortions are currently banned in the state, except to “preserve the life of the pregnant female.”
Opponents have 30 days to challenge the validity of the petition. Leslee Unruh, co-chair of the Life Defense Fund, said earlier this month that the group “can’t wait to get to court.” The Life Defense Fund is a ballot question committee organized to oppose the ballot measure.
Thursday, Unruh and Life Defense Fund co-chair Rep. John Hansen, R-Dell Rapids, issued a joint statement: “This fight is about saving the lives of countless unborn children in our state. We are grateful to the many dedicated volunteers who have put in countless hours, and we are resolute in our mission to defend unborn babies. We will continue to research these signatures and announce a challenge at the appropriate time.”
Opponents of the ballot measure have alleged some petition signers were duped into believing they were signing a petition to repeal the state sales tax on groceries, when they were actually signing the abortion-rights petition. The Dakotans for Health ballot question committee circulated both measures and denies any wrongdoing.
Hansen recently formed the South Dakota Petition Integrity political action committee. People associated with the committee have been calling hundreds of petition signers from the sample list, as part of an effort to gather evidence for a court challenge and tell signers about a new state law passed in March allowing them to withdraw their signature.
Complaints about the calls led to a press release from the Secretary of State’s Office earlier this week labeling the calls a “scam,” due to callers allegedly giving the impression that they’re officially affiliated with the Secretary of State’s Office.
Rick Weiland, of Dakotans for Health, said earlier this week that the phone campaign was a sign of “desperation.”
“They are desperate to keep abortion off the ballot this fall and let the people decide,” Weiland said.
The abortion measure could be one of many on the ballot in November. Two measures already on the ballot were placed there by the Legislature: a proposal to change male-specific officeholder references in the state constitution to neutral language, and a proposal that would allow the state to impose work requirements on some Medicaid expansion enrollees.
Validated but within the 30-day challenge window are the abortion measure and an initiated measure prohibiting state sales taxes on items sold for human consumption, specifically targeting state sales taxes on groceries.
Validation is pending for citizen-led petitions that would create open primary elections and legalize adult recreational marijuana use .
Meanwhile, a citizen-led group is trying to refer a new pipeline law to the ballot. The Legislature passed the law last winter to implement new protections for landowners affected by a proposed carbon dioxide pipeline, while still allowing a regulatory path forward for the project
This story was originally published on SouthDakotaSearchlight.com.


IMAGES
COMMENTS
Abortion services are a vital component of reproductive health care. Since the Supreme Court's 2022 ruling in Dobbs v.Jackson Women's Health Organization, access to abortion services has been increasingly restricted in the United States. Jung and colleagues review current practice and evidence on medication abortion, procedural abortion, and associated reproductive health care, as well as ...
Abstract. This article presents a research study on abortion from a theoretical and empirical point of view. The theoretical part is based on the method of social documents analysis, and presents a complex perspective on abortion, highlighting items of medical, ethical, moral, religious, social, economic and legal elements.
Abortion is a common medical or surgical intervention used to terminate pregnancy. Although a controversial and widely debated topic, approximately 73 million induced abortions occur worldwide each year, with 29% of all pregnancies and over 60% of unintended pregnancies ending in abortion. Abortions are considered safe if they are carried out using a method recommended by WHO, appropriate to ...
Introduction. Globally, access to safe abortion is limited. As a result, an estimated 25 million unsafe abortion occur each year, and at least 22 800 women die from resulting complications, almost all in low- and middle-income countries. 1 This is often due to restrictive laws which prohibit abortion; but even in contexts where abortion is legal, other barriers, such as cost, distance and ...
Abortion access is a component of economic justice because parenthood is expensive. In the USA, 49% of abortion patients have incomes below the poverty line and an additional 26% have low incomes; 73% of abortion patients list "can't afford a baby now" as one of their reasons, and 23% list it as "the most important reason".
On December 1, 2021, the Supreme Court heard oral arguments in Dobbs v. Jackson Women's Health Organization. At the center of the case is a Mississippi law banning nearly all abortions after 15 ...
abortion and diminish the number of quali fied medical pro-fessionals who can provide abortion care. In 32 states, abor-tions can be performed only by a licensed physician, 18 a requirement that limits the practice of advanced-practice clinicians. Research has shown no differences in outcomes of medication and aspiration abortion in relation ...
Wade and Planned Parenthood v. Casey, we present a special collection of political science research drawn from APSA and Cambridge journals addressing the politics of abortion and abortion rights. The decision has already changed abortion policy dramatically in several US states and has opened a new era of contentious politics over both abortion ...
The legal landscape surrounding abortion in the United States has shifted dramatically since the Supreme Court's June 2022 decision in Dobbs v. Jackson Women's Health Organization eliminated a nationwide right to abortion ( 1 ). In the year since, roughly half of US states have expanded abortion restrictions.
This review will systematically appraise and synthesize the research evidence on the impact of abortion law reforms on women's health services and outcomes in LMICs. ... Waddington H. Quasi-experimental study designs series-paper 5: a checklist for classifying studies evaluating the effects on health interventions-a taxonomy without labels. J ...
Yes, science can weigh in on abortion law. Why, as a scientist, I signed an amicus brief for the US Supreme Court's case on abortion. The world is moving towards greater reproductive rights for ...
This paper interprets this as the claim that inconsistency arguments are morally irrelevant for any (widely held) OA view. ... even if OA choose (i) and conclude they ought to do more to prevent spontaneous abortion (education, research, increased access to healthcare (Simkulet 2017, ... A defense of abortion. New York, NY: Cambridge University ...
JUDITH JARVIS THOMSON A Defense of Abortion'. Most opposition to abortion relies on the premise that the fetus is a human being, a person, from the moment of conception. The premise is argued for, but, as I think, not well. Take, for example, the most common argument. We are asked to notice that the development of a human being from conception ...
©2009—2024 Bioethics Research Library Box 571212 Washington DC 20057-1212 202.687.3885
A defense of abortion. D. Boonin. Published 1 November 2002. Philosophy, Law. David Boonin has written the most thorough and detailed case for the moral permissibility of abortion yet published. Critically examining a wide range of arguments that attempt to prove that every human fetus has a right to life, he shows that each of these arguments ...
Abstract. Most opposition to abortion relies on the premise that the fetus is a human being, a person, from the moment of conception. The premise is argued for, but, as I think, not well. Take, for example, the most common argument. We are asked to notice that the development of a human being from conception through birth into childhood is ...
In an overview of the contributors' papers, the editor, Dr Gregory Wilmoth, concluded, ... and other defense mechanisms, clearly works. Research has shown that the subset of women who anticipate the most difficulty dealing well with their abortions ... A major problem with abortion research and reviews is a failure to address all of the ...
Pavlischek, Keith J. 1998. "Abortion Logic and Paternal Responsibility: One More Look at Judith Thomson's Argument and a Critique of David Boonin-Vail's Defense of It" (revised version of Pavlischek 1993). In Louis P. Pojman and Francis J. Beckwith, eds. The Abortion Controversy: 25 Years after Roe v.
1: The (typical) human fetus is a person. 2: Every person has a right to life. C 1: The (typical) human fetus has a right to life. P 3: If the (typical) human fetus has a right to life, then abortion (at least in typical circumstances) is morally impermissible.
"A Defense of Abortion" is a moral philosophy essay by Judith Jarvis Thomson first published in Philosophy & Public Affairs in 1971. Granting for the sake of argument that the fetus has a right to life, Thomson uses thought experiments to argue that the right to life does not include, entail, or imply the right to use someone else's body to survive and that induced abortion is therefore ...
A Defense of Abortion. Creator. Thomson, Judith Jarvis. Bibliographic Citation. ... ©2009—2024 Bioethics Research Library Box 571212 Washington DC 20057-1212 202.687.3885 . Toggle navigation. Login; Toggle navigation. View Item DigitalGeorgetown Home; Bioethics Research Library of the Kennedy Institute of Ethics ...
With this growing body of research, earlier abortion methods have been refined, discontinued, and new approaches have been developed. The Safety and Quality of Abortion Care in the United States offers a comprehensive review of the current state of the science related to the provision of safe, high-quality abortion services in the United States ...
Federal District Courts in Indiana and Alabama both ruled this month that while states in the wake of Roe v. Wade's demise can ban abortion, they cannot make it illegal to give abortion-related ...
The legalities around providing access to abortion care within the MHS are muddied due to the Hyde Amendment of 1976, which restricts the use of federal funds to be used for abortion care.
In "Association of Texas' 2021 Ban on Abortion in Early Pregnancy with the Number of Facility-Based Abortion in Texas and Surrounding States," White et al. used a large dataset containing information before and after the passage of SB8 in September 2021. 1 This bill banned most abortions after 6 weeks in the state of Texas.
Voices in Bioethics is currently seeking submissions on philosophical and practical topics, both current and timeless. Papers addressing access to healthcare, the bioethical implications of recent Supreme Court rulings, environmental ethics, data privacy, cybersecurity, law and bioethics, economics and bioethics, reproductive ethics, research ethics, and pediatric bioethics are sought.
Life Defense Fund, a group organized against the initiative, said they will continue to research the signatures. Opponents still have 30 days — until June 17 — to file a challenge with the ...
Life Defense Fund, a group organized against the initiative, said they will continue to research the signatures. Opponents still have 30 days — until June 17 — to file a challenge with the ...
The Department of Defense has issued an open announcement to solicit white papers on proposed research ideas that could be developed into prototypes as part of a push to accelerate the delivery of ...
People associated with the Life Defense Fund protest outside a Sioux Falls library on May 1, 2024, as an abortion-rights group conducts a press conference inside.