Roger Sperry’s Split Brain Experiments (1959–1968)
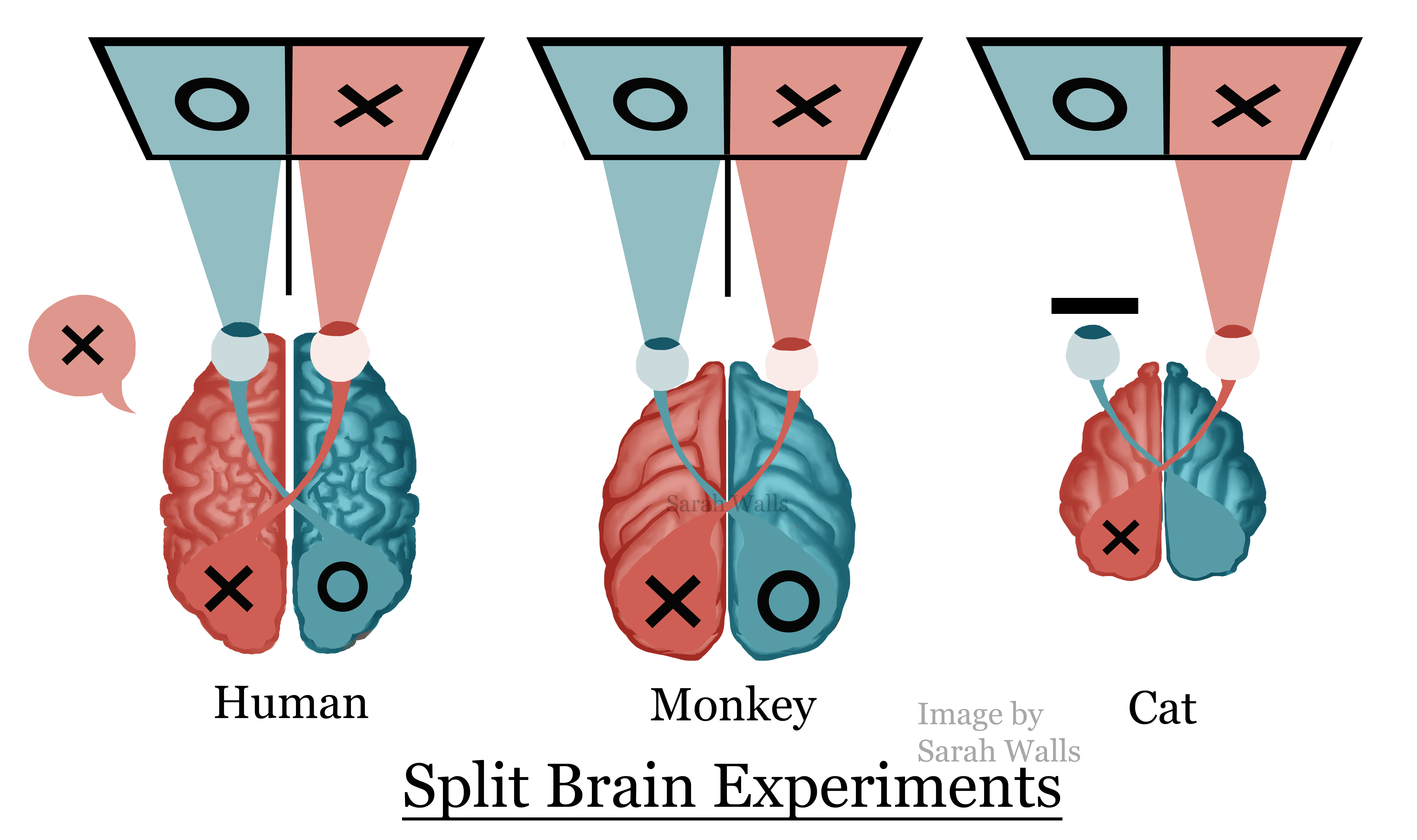
Editor's note: Sarah Walls created the above image for this article. You can find the full image and all relevant information here .
In the 1950s and 1960s, Roger Sperry performed experiments on cats, monkeys, and humans to study functional differences between the two hemispheres of the brain in the United States. To do so he studied the corpus callosum, which is a large bundle of neurons that connects the two hemispheres of the brain. Sperry severed the corpus callosum in cats and monkeys to study the function of each side of the brain. He found that if hemispheres were not connected, they functioned independently of one another, which he called a split-brain. The split-brain enabled animals to memorize double the information. Later, Sperry tested the same idea in humans with their corpus callosum severed as treatment for epilepsy, a seizure disorder. He found that the hemispheres in human brains had different functions. The left hemisphere interpreted language but not the right. Sperry shared the Nobel Prize in Physiology or Medicine in 1981for his split-brain research.
Sperry also studied other aspects of brain function and connections in mammals and humans, beyond split-brains, in 1940s and 1950s. In 1963, he developed the chemoaffinity hypothesis, which held that the axons, the long fiber-like process of brain cells, connected to their target organs with special chemical markers. This explained how complex nervous systems could develop from a set of individual nerves. Sperry then also studied brain patterns in frogs, cats, monkeys, and human volunteers. Sperry performed much of his research on the split-brain at California Institute of Technology, or Caltech, in Pasadena, California, where he moved in 1954.
Sperry began his research on split-brain in late 1950s to determine the function of the corpus callosum. He noted that humans with a severed corpus callosum did not show any significant difference in function from humans with intact corpus callosum, even though their hemispheres could not communicate due to the severing of the corpus callosum. Sperry postulated that there should be major consequences from cutting the brain structure, as the corpus callosum connected the two hemispheres of the brain, was large, and must have an important function. Sperry began designing experiments to document the effects of a severed corpus callosum. At the time, he knew that each hemisphere of the brain is responsible for movement and vision on the opposite side of the body, so the right hemisphere was responsible for the left eye and vice versa. Therefore, Sperry designed experiments in which he could carefully monitor what each eye saw and therefore what information is was going to each hemisphere.
Sperry experimented with cats, monkeys, and humans. His experiments started with split-brain cats. He closed one of their eyes and presented them with two different blocks, one of which had food under it. After that, he switched the eye patch to the other eye of the cat and put the food under the other block. The cat memorized those events separately and could not distinguish between the blocks with both eyes open. Next, Sperry performed a similar experiment in monkeys, but made them use both eyes at the same time, which was possible due to special projectors and light filters. The split-brain monkeys memorized two mutually exclusive scenarios in the same time a normal monkey memorized one. Sperry concluded that with a severed corpus callosum, the hemispheres cannot communicate and each one acts as the only brain.
Sperry moved on to human volunteers who had a severed corpus callosum. He showed a word to one of the eyes and found that split-brain people could only remember the word they saw with their right eye. Next, Sperry showed the participants two different objects, one to their left eye only and one to their right eye only and then asked them to draw what they saw. All participants drew what they saw with their left eye and described what they saw with their right eye. Sperry concluded that the left hemisphere of the brain could recognize and analyze speech, while the right hemisphere could not.
In the 1960s when Sperry conducted his split-brain research on humans, multiple scientists were studying brain lateralization, the idea that one hemisphere of the brain is better at performing some functions than the other hemisphere. However, researchers did not know which tasks each side of the brain was responsible for, or if each hemisphere acted independently from the other.
Sperry describes his research in cats in the article "Cerebral Organization and Behavior" published in 1961. To test how the cutting of the corpus callosum affected mammals, Sperry cut the corpus callosum of multiple cats and had them perform some tasks that involved their vision and response to a visual stimulus. After severing each cat´s corpus callosum, he covered one of the cat´s eyes to monitor with which eye the cat could see. Sperry could switch the eye patch from one eye to the other, depending on which visual field he wanted the cat to use. Next, Sperry showed the cats two wooden blocks with different designs, a cross and a circle. Sperry put food for the cat under one of the blocks. He taught the cats that when they saw the blocks with one eye, for instance, the right eye, the food was under the circle block, but when they saw it with the left eye, the food was under the block with a cross. Sperry taught the cats to differentiate between those two objects with their paws, pushing the correct wooden block away to get the food.
When Sperry removed the eye patch and the cats could see with both eyes, he performed the same experiment. When the cats could use both eyes, they hesitated and then chose both blocks almost equally. The right eye connects to the left hemisphere and the left eye connects to the right hemispheres. Sperry suspected that since he cut the corpus callosum in those cats, the hemispheres could not communicate. If the hemispheres could not communicate and the information from one eye only went to one hemisphere, then only that hemisphere would remember which block usually had food under it. From that, Sperry concluded that the cats remembered two different scenarios with two different hemispheres. He suspected that the cats technically had two different brains, as their hemispheres could not interact and acted as if the other one did not exist.
Sperry performed a similar experiment with monkeys, in which he also cut their corpus callosum. He wanted to test if both hemispheres could operate at the same time, even though they were not connected. That required separation of visual fields, or making sure that the right eye saw a circle, while the left eye saw a cross, like in the cat experiment, but without an eye patch and both eyes would see something at the same time instead of interchanging between the open eyes. Sperry solved that by using two projectors that were positioned side-by-side at an angle and showed mutually exclusive images. For example, the projector on the right showed a circle on the left and a cross on the right, while the projector on the left showed a cross on the left and a circle on the right. Sperry placed special light filters in front of each of the monkey´s eyes. The light filters made it so that each eye saw the images from only one of the projectors. That meant one of the eyes saw the circle on the right and the cross on the left, while the other eye saw the cross on the right and the circle on the left. From his experiments with cats, Sperry knew that there was no sharing of information from right and the left hemispheres, so he made the monkeys memorize two different scenarios at the same time.
The left eye saw a scenario where food would be dispersed when the monkey pressed the button corresponding to a cross, while the right eye saw a scenario where food would be dispersed when the monkey pressed a button corresponding to a circle. Ultimately, it was the same button, but the eyes saw it differently because of two projectors and special light filters. Sperry concluded that both hemispheres of the brain were learning two different, reversed, problems at the same time. He noted that the split-brain monkeys learned two problems in the time that it would take a normal monkey to learn one, which supported the assumption that the hemispheres were not communicating and each one was acting as the only brain. That seemed as a benefit of cutting corpus callosum, and Sperry questioned whether there were drawbacks to the procedure.
Sperry performed the next set of experiments on human volunteers, who had their corpus callosum severed previously due to outside factors, such as epilepsy. Sperry asked volunteers to perform multiple tests. From his previous experiments with cats and monkeys, Sperry knew that one, the opposite, hemisphere of the brain would only analyze information from one eye and the hemispheres would not be able to communicate to each other what they saw. He asked the participants to look at a white screen with a black dot in the middle. The black dot was the dividing point for the fields of view for a person, so the right hemisphere of the brain analyzed everything to the left of the dot and the left hemisphere of the brain analyzed everything that appeared to the right of the dot. Next, Sperry showed the participants a word on one side of the black dot for less than a second and asked them to tell him what they saw. When the participants saw the word with their right eye, the left hemisphere of the brain analyzed it and they were able to say what they saw. However, if the participants saw the word with their left eye, processed by right hemisphere, they could not remember what the word was. Sperry concluded that the left hemisphere could recognize and articulate language, while the right one could not.
Sperry then tested the function of the right hemisphere. He asked the participants of the same experiment that could not remember the word because it was in the left visual field to close their eyes and draw the object with their left hand, operated by the right hemisphere, to which he presented the word. Most people could draw the picture of the word they saw and recognize it. Sperry also noted that if he showed the word to the same visual field twice, then the person would recognize it as a word they saw, but if he showed it to the different visual fields, then the participants would not know that they saw the word before. Sperry concluded that the left hemisphere was responsible not only for articulating language, but also for understanding and remembering it, while the right hemisphere could only recognize words, but was not able to articulate them. That supported the previously known idea that the language center was in the left hemisphere.
Sperry performed another similar experiment in humans to further study the ability of the right hemisphere to recognize words. During that experiment, Sperry asked volunteers to place their left hand into a box with different tools that they could not see. After that, the participants saw a word that described one of the objects in the box in their left field of view only. Sperry noted that most participants then picked up the needed object from the box without seeing it, but if Sperry asked them for the name of the object, they could not say it and they did not know why they were holding that object. That led Sperry to conclude that the right hemisphere had some language recognition ability, but no speech articulation, which meant that the right hemisphere could recognize or read a word, but it could not pronounce that word, so the person would not be able to say it or know what it was.
In his last series of experiments in humans, Sperry showed one object to the right eye of the participants and another object to their left eye. Sperry asked the volunteers to draw what they saw with their left hand only, with closed eyes. All the participants drew the object that they saw with their left eye, controlled by the right hemisphere, and described the object that they saw with their right eye, controlled by the left hemisphere. That supported Sperry´s hypothesis that the hemispheres of brain functioned separately as two different brains and did not acknowledge the existence of the other hemisphere, as the description of the object did not match the drawing. Sperry concluded that even though there were no apparent signs of disability in people with a severed corpus callosum, the hemispheres did not communicate, so it compromised the full function of the brain.
Sperry received the 1981 Nobel Prize in Physiology or Medicine for his split-brain research. Sperry discovered that the left hemisphere of the brain was responsible for language understanding and articulation, while the right hemisphere could recognize a word, but could not articulate it. Many researchers repeated Sperry´sf experiments to study the split-brain patterns and lateralization of function.
- Sperry, Roger W. "Cerebral Organization and Behavior." Science 133 (1961): 1749–57. http://people.uncw.edu/puente/sperry/sperrypapers/60s/85-1961.pdf (Access December 8, 2017).
- Sperry, Roger W. "Hemisphere Deconnection and Unity in Conscious Awareness." American Psychologist 28 (1968): 723–33. http://people.uncw.edu/Puente/sperry/sperrypapers/60s/135-1968.pdf (Access December 8, 2017).
- Sperry, Roger W. "Split-brain Approach to Learning Problems." In The Neurosciences: A Study Program , eds. Gardner C. Quarton, Theodore Melnechuk, and Francis O. Schmitt, 714–22. New York: Rockefeller University Press, 1967. ttp://people.uncw.edu/puente/sperry/sperrypapers/60s/130-1967.pdf (Accessed November15, 2017).
- "The Split Brain Experiments." Nobelprize.org . https://www.nobelprize.org/educational/medicine/split-brain/background.html (Accessed May 3, 2017).

How to cite
Articles rights and graphics.
Copyright Arizona Board of Regents Licensed as Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported (CC BY-NC-SA 3.0)
Last modified
Share this page.
- Search Menu
- Sign in through your institution
- CNS Injury and Stroke
- Epilepsy and Sleep
- Movement Disorders
- Multiple Sclerosis/Neuroinflammation
- Neuro-oncology
- Neurodegeneration - Cellular & Molecular
- Neuromuscular Disease
- Neuropsychiatry
- Pain and Headache
- Advance articles
- Editor's Choice
- Author Guidelines
- Submission Site
- Why publish with this journal?
- Open Access
- About Brain
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Dispatch Dates
- Terms and Conditions
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, separated information processing in both hemispheres, lateralization of function, non-neural interhemispheric integration the concept of cross-cueing, the split-brain and concepts of neurological lesions, implications for understanding consciousness.
- < Previous
Interaction in isolation: 50 years of insights from split-brain research
- Article contents
- Figures & tables
- Supplementary Data
Lukas J. Volz, Michael S. Gazzaniga, Interaction in isolation: 50 years of insights from split-brain research, Brain , Volume 140, Issue 7, July 2017, Pages 2051–2060, https://doi.org/10.1093/brain/awx139
- Permissions Icon Permissions
Fifty years ago, one of the first studies that showed the neuropsychological consequences of sectioning the corpus callosum, that great bundle of fibres that connects the two cerebral hemispheres, was published in Brain ( Gazzaniga and Sperry, 1967 ). With the help of several patients who have undergone this procedure and generously given of their time as willing participants in research, a gold mine of information about the way brains function has been ferreted out. Research studies in the ensuing years have both confirmed and extended the findings, not only in the original patient group, but other groups as well. The insights gained from testing these so called ‘split-brain’ patients have contributed to the evolving field of cognitive neuroscience and have helped establish information processing models for how the brain governs behaviour and cognition.
The original ‘split-brain’ patients tested in California had undergone a complete transection of the corpus callosum and the anterior and hippocampal commissures (with some minor variance occurring between subjects) to alleviate intractable, severe epilepsy, which it did. Twenty years before, testing of another group of similar split-brain patients in Rochester, New York ( cf. Akelaitis, 1941 ) had not revealed any discernible differences between pre- and post-surgical behaviour, suggesting that not much would be learned from this new group. Using a behavioural testing device (which had not been used in New York) that allowed information to be fed to either hemisphere independent of the other, however, revealed that these patients were to provide a unique opportunity to investigate the separate functions of the two cerebral hemispheres ( Fig. 1 ).

Tachistoscope. Presenting visual stimuli with a tachistoscope allows selective presentation of visual information to one hemisphere at a time. Patients were asked to fix their gaze on the centre of the translucent screen, upon which the examiner projects visual stimuli for 0.1 s. Information projected onto the left half of the screen is subsequently processed by the right hemisphere, whereas stimuli presented in the right visual field are processed by the left hemisphere. The short presentation interval prevents visual information on one side of the screen from being processed by both hemispheres due to eye movements. Modified from Gazzaniga (2000) , with permission.
‘In general the post-surgical studies indicate a striking functional independence of the gnostic activities of the two hemispheres. Perceptual, cognitive, mnemonic, learned and volitional activities persist in each hemisphere, but can proceed separately in each case outside the realm of awareness of the other hemisphere.’
The goal of this article is to outline some of the challenges in interpreting the experience of interacting with split-brain patients. After briefly summarizing some elementary and uncontroversial findings derived from split-brain patients, we will focus on more controversial points that remain the topic of ongoing debate. In particular, we will review the concept of cross-cueing, which is a crucial and tangible reality when interpreting split-brain results. This may resonate with any reader who has had the experience of working with neurological patients.
The starting point for many split-brain experiments is to provide information to one hemisphere at a time ( Fig. 1 ). This is most easily accomplished through the visual system, thanks to its tidy anatomy ( Fig. 2 ). If you stare straight ahead at a spot, information on the right side of space perceived by both eyes will end up in the left hemisphere and information on the left side of space will end up in the right hemisphere. This is true for all of us, including our split-brain patients. Since our hemispheres are connected, it is natural for our brains to stitch the two sides together and create a unified visual world ( Gazzaniga et al. , 1965 ). Yet, for the split-brain patient with no such connection, each hemisphere sees only the opposite half of the space.

Neuroanatomical basis for processing of visual information. When fixating the centre of the screen (cross), visual information presented on the left half of the screen (blue square) is processed by neurons located in the nasal half of the retina in the left eye and lateral half of the retina in the right eye. While the latter directly project into the right hemisphere, axons of retinal neurons in the nasal half of the left eye (blue) cross from the left to the right hemisphere in the optic chiasm. As a result, visual stimuli presented to the left visual field are processed by the right hemisphere, while stimuli presented to the right visual field (red circle) are processed by the left hemisphere.
This neat separation of visual input makes it possible to provide visual information to one hemisphere of split-brain patients without the knowledge of the other hemisphere. For example, when an object is shown in the right visual field, the visual information travels to the left hemisphere and the patient is effortlessly able to name it ( Fig. 3 A). When shown to the left visual field, however, the information travels to the right hemisphere, and when asked, the patient will typically answer that no object was seen ( Fig. 3 B). This phenomenon is easily explained by the fact that most people’s speech centre is located in their left hemisphere. When the hemispheres are separated, the left will be capable of naming an object, while the right hemisphere stays mute. Moreover, the left hemisphere will also eagerly answer the question intended for the right hemisphere. When it hears the question directed to the right hemisphere asking what the object was, the left hemisphere correctly and honestly reports that it did not see anything at all.

Separated information processing. ( A ) When two different letters are presented in each visual field, the patient will report the letter projected onto the right half of the screen (‘R’, processed by the verbally dominant left hemisphere). The letter presented on the left half of the screen (‘B’, processed by the right hemisphere) is not verbally reported, but can be identified via tactile information using the left hand (controlled by the right hemisphere). ( B ) If visual stimuli are exclusively presented in the left visual field (processed by the right hemisphere), they can again be identified by the patient via tactile information from the left hand (also processed by the right hemisphere). Intriguingly, the patient will verbally report that he did not see any stimulus, due to the lack of information in the verbal left hemisphere. Modified from Sperry et al. (1969) , with permission.
Now picture yourself listening to the completely normal looking person sitting in front of you saying that he did not see the object. He sounds absolutely sure about this. One might jump to the conclusion that the right hemisphere did not perceive the stimulus. Yet this interpretation drastically changes when the right hemisphere is asked to communicate non-verbally. For example, when instructed to point out the object from a group of objects with the left hand, patients reliably identify the object that had been presented to the right hemisphere. Not just better than chance. Every time.
From an anatomical perspective, this hardly seems surprising: the right hemisphere perceives and processes the visual input and then uses its loyal henchman, the left hand, to point it out. The left hand does this because it receives its neuronal input from corticospinal fibres that originate from the right hemisphere. Phenomenologically for the onlooker, however, the observation is far more challenging: the left hand is now confidently pointing out the object that the person just categorically and confidently denied seeing. This is where things get really interesting. Ask the person why he is pointing to that object. Since the left hemisphere and its speech centre do not know what the right hemisphere saw and do not know why the left hand is pointing to a particular object, one might think that the person would once again answer correctly and honestly by admitting ignorance with a simple ‘I don’t know’. This never happens. The left hemisphere always comes up with a story about why the left hand is doing what it is doing, ‘It is pointing to the apple because I like red’. The results of this very simple experiment led to numerous questions and more testing of the split-brain patients, resulting in more intriguing answers and inferences which are well summarized by the notion of the ‘left hemisphere interpreter’ ( Fig. 4 ; for a detailed account see Gazzaniga and LeDoux, 1978 ; Gazzaniga, 2000 ).

Example of the left hemisphere interpreter. In a classic test, a chicken claw was shown to the (speaking) left hemisphere and a snow scene was shown to the (silent) right hemisphere. Patient P.S. easily picked out related pictures from a set of eight options. His left hand chose a snow shovel and his right hand chose a chicken. When asked why he had picked those particular pictures, P.S. said, ‘Oh, that’s simple. The chicken claw goes with the chicken, and you need a shovel to clean out the chicken shed’. Modified from Gazzaniga (2000) , with permission.
On the one hand, the strict separation of information processing seems to be a logical consequence of well understood basic neuroanatomy. At the same time, however, interpreting the consequences of two independent information processing systems housed in the same body challenges our intuitive understanding of fundamental aspects of psychology, such as conscious awareness of perception (when one hemisphere reports, ‘I didn’t see anything’) or agency (yet the other chooses the correct object) and causation (‘because I like red’), which ultimately led to the question how these independent systems can coexist and coordinate a single physical body despite the lack of direct, neural interaction. And there was the other nagging notion: can a flick of a knife really produce two separate-consciousness autonomous brains? If so, what exactly does that mean for, say, personal identity?
The fact that the left hemisphere jumps in to offer an explanation whenever asked, even if it does not know what its counterpart to the right is up to, may suggest that the right hemisphere is unable to process language at all. While, indeed, the right hemisphere is typically, at first, not capable of speech production, it does, however, understand both spoken and written language. Since auditory stimuli are typically processed bilaterally, the experimental design had to be adjusted to test lateralization of phoneme processing. For example, after verbally presenting a target word (perceived by both hemispheres) such as ‘chair’, a series of words was visually presented to the right hemisphere only. The left hand then successfully indicated that it recognized the target word by pointing to it ( Gazzaniga and Sperry, 1967 ). To accomplish this, the spoken word had to be interpreted by the right hemisphere in order to produce the correct response from the left hand, since only the right hemisphere could see the list of words from which to choose. In a similar fashion, the right hemisphere can also process the semantic meaning of short sentences. For example, changing the initial verbal target from a single word to a description (‘Used to tell the time’), also leads to a correct response with the left hand pointing to ‘clock’ from a list of words.
Despite the obvious dominance of the left hemisphere, various follow-up experiments have established and further characterized that both hemispheres possess the ability to process language independently. In a complementary fashion, the right hemisphere shows superior specialization for visuospatial processing, as observed in tasks involving part-whole relations, spatial relationships, apparent motion detection, mental rotation, spatial matching and mirror image discrimination (for further details see Gazzaniga, 2005 ).
More recent findings suggest that in the split-brain, the right hemisphere may be specialized to infer causality from physical interactions, whereas the left hemisphere may be involved in more abstract inference of causality ( Roser et al. , 2005 ). The right hemisphere is also better at recognizing familiar faces and human faces. The clinical observation that prosopagnosia typically occurs after lesions to the right hemisphere converges with results from split-brain research ( Turk et al. , 2002 ), as well as neuroimaging findings in both healthy subjects and neurological patients alike ( Rossion et al. , 2011 ). It also appears that the right hemisphere plays a major role in our ability to determine what the intentions of another person might be ( Young and Saxe, 2009 ). Even more startling the right hemisphere can develop speech following callosal section ( Gazzaniga et al. , 1979 , 1984 ; Baynes et al. , 1995 ).
The fact that the split-brain separately processes information in each hemisphere has been replicated numerous times for various domains and, by itself, constitutes an uncontroversial and accepted concept. The degree of hemispheric separation, however, is a topic of ongoing debate. Does surgically disconnecting (most) cortical interhemispheric fibres result in two distinct conscious systems? Are the two hemispheres each perceiving the world and processing information in a slightly different fashion, leading to two independent minds constructing and following their own respective goals?
A first objection might be that two completely separated neural systems should have trouble coordinating one body, given that each of these systems governs the motor function of half of the body. Indeed, some split-brain patients transiently experienced symptoms of an alien hand syndrome, where typically the left hand is perceived to be moving as if following its own goals with a reduced experience of agency over those movements ( Gazzaniga, 2015 ). Moreover, for some patients an intermanual conflict was observed. For example, when trying to arrange a set of blocks with both hands, one hand often undoes what the other has just arranged rather than cooperating to optimize task performance ( Gazzaniga, 2015 ). It is no surprise that the right hemisphere, with its specialized skills for visuospatial reasoning, runs circles around the left hemisphere outperforming it ‘hands down’ in this task. Yet very quickly after surgery, patients are able to walk and run while avoiding obstacles ( Holtzman et al. , 1981 ), even swim ( Gazzaniga, 2015 ), dance and play the piano ( Akelaitis, 1941 ).
Such behaviours critically rely on the coordinated interactions between the hemispheres and the movements they control. It seems almost impossible that two separated hemispheres should be able to swim or play piano, naturally leading to the question of whether the split-brain uses some alternative mysterious non-callosal pathway to transfer information. Could visual information from both hemi-fields be transferred via non-callosal fibres and used to adjust motor controls to avoid bumping into objects while walking or running? While in monkeys, visual information can indeed be exchanged between hemispheres via the anterior commissure, a similar mechanism has been ruled out in humans ( Gazzaniga, 2005 ).
A more likely explanation lies in behavioural ‘cross-cueing’ between hemispheres. A popular analogy illustrating the concept of cross-cueing lies in the coordinated behaviour displayed by conjoined twins. If two unquestionably independent brains control one body, as is the case if the conjunction is sufficiently high, we see a wonderful example of two distinct neural systems integrating information without direct pathways linking the two. Abby and Brittany Hensel are such a pair, each with different desires, likes and dislikes, and personalities. They are conjoined at the chest and torso with a single pair of arms and legs. Even though Abby controls one arm and leg and Brittany the other, they are athletically coordinated. By picking up on behavioural cues, for example when Brittany perceives a movement initiated by Abby (and vice versa), they are able to unconsciously and effortlessly coordinate their movements to a degree that allows them to do such things as play softball.
Split-brain patients might be in a related situation—in some instances only one hemisphere may have access to crucial information needed to perform a certain task. With the abundant amount of constant practice starting right after the surgery, it seems logical that split-brain patients quickly develop nuanced ways to integrate such crucial pieces of information, even in the absence of fibre bundles carrying it from one hemispheres to the other. Since patients are used to constantly relying on cross-cueing, these subtle behavioural cues, which allow them to accomplish complex behaviour, can turn into a profound problem for an experimenter who is trying to test the hemispheres in isolation.
In a manner similar to a patient with early dementia, who creatively dodges questions that would reveal his inability to recall recent events, a split-brain patient will use cueing mechanisms when faced with a task that requires integration of information between hemispheres. Neither of these patients, however, intend to trick the examiner. Their intent, like anyone’s, is simply to perform as well as they can when faced with a challenge. Over the decades, various findings seemed to support the notion of information integration across hemispheres in split-brain patients at first glance. Yet this support dissolved when meticulous re-examination prevented any possibility of cross-cueing ( Gazzaniga and Hillyard, 1971 ). Depending on the experimental design, this can be highly challenging or even impossible ( Seymour et al. , 1994 ).
Recently, Pinto et al. (2017) investigated the degree to which processing of visual information is segregated between hemispheres in two split-brain patients. In line with the canonical interpretation of independent visual processing, they observed that visual stimuli could not be compared across visual half-fields. The authors, however, also observed that some features, such as the presence or location of visual stimuli, were correctly reported throughout the entire visual field for responses obtained verbally or with either hand ( Pinto et al. , 2017 ). This seems at odds with two separated perceptual streams of information. For example, how can the patients verbally report or indicate with their right hand (both controlled by the left hemisphere) whether a visual stimulus was presented to the left visual half-field (i.e. the right hemisphere)? The authors conclude that a certain degree of information exchange has to occur between hemispheres through non-callosal fibres. They suggest that although the information is not sufficient to inform the other hemisphere about its details, there is enough to let it know if and where a stimulus was presented.
These findings can easily be explained by cross-cueing, even though the authors quickly discarded this explanation in their discussion. By characterizing cross-cueing as ‘behavioural tricks, such as touching the left hand with the right hand’ the authors reveal that they underestimate the potential range and subtlety of cueing behaviour, which has been flushed out over decades. In fact, their data and observations fall nicely in line with previous observations of non-neural communication occurring via cross-cueing.
As noted by the authors, the amount of information transferred from one hemisphere to the other by cross-cueing is limited. Accordingly, the patients answered the simple question of whether a visual stimulus was presented or not (almost) perfectly. With the more difficult question of the stimulus’s localization, the answers were not so perfect: though reported above chance level, there was a higher error rate (see Figure 2 in Pinto et al. , 2017 ). Thus, cueing binary information (stimulus/no stimulus) is easy for two separated hemispheres, even without a highly obvious manoeuvre such as touching hands. Informing the other hemisphere about the location of the stimulus is more difficult, however, as readily reflected in the increased error rates. The fact that patients localized stimuli above chance level, even in the crossed case (e.g. stimulus presented to the left hemisphere and response with left hand), can be explained by the experimental design: while an eye-tracking device made sure that a patient fixated on the centre of the screen during the presentation of the visual stimulus, the patients did not have to focus their gaze on the centre of the screen while consecutively indicating the stimulus location. Because split-brain patients have the capacity to cross-cue the location of visual stimuli by eye movements (a glance to the upper-left or right would be cue enough), this allowed them to cue the opposite hemisphere ( Gazzaniga, 1969 ).
Even without the cue of eye movements, intriguing previous data suggest that attentional capacities can be controlled by either hemisphere in split-brain patients, hence giving yet another alternative explanation for the above chance localization of visual stimuli ( Fig. 5 ; Holtzman et al. , 1981 ). For example, after a visual stimulus was exclusively perceived by the right hemisphere, it can direct the attention of the left hemisphere to the given spot in the consecutive relocation condition, by using eye movements or neural connections via collicular-cortical projections or the intact anterior commissure ( Holtzman et al. , 1981 ). In summary, cross-cueing directing hemispheric attention may well explain the findings, rendering the concluded direct inter-hemispheric transfer of visual information unnecessary. This explanation is also in perfect agreement with the observation that two stimuli simultaneously presented in different visual half-fields, could not be compared by the patients (in line with the canonical view of two independent processing systems).

Interhemispheric transfer of spatial location. In this experiment, patients were instructed to locate target stimuli by fixating them with their right eye, while the left eye was occluded. In the first condition, the target stimulus location was highlighted ( A and B ). Unsurprisingly, subjects correctly moved their right eye to the target location when the target was presented in the left visual field, processed by the right hemisphere (within-field trial). In the second between-field condition ( B ), the subject was required to move the eyes to the relative point in the right visual field (not processed by the right hemisphere). Split-brain subjects were able to do this, suggesting cross-integration of spatial information between hemispheres. In the second part of the experiment, information on the identity of the target was presented, either within the left visual field (processed by the right hemisphere, C ) or in the right visual field (not processed by the right hemisphere, D ). While patients had no problems correctly identifying the indicated target stimulus in within-field trials ( C ), they had to guess the target-identity in between-field trials ( D ), as reflected by chance-level accuracy. Hence, while crude information on the spatial localization of a stimulus can be cross-integrated between hemispheres ( B ), more complex information such as stimulus identity ( D ) is not integrated in split-brain patients. Modified from Gazzaniga (1995) , with permission.
Cross-cueing mechanism and mirror neurons
If cross-cueing indeed plays a prominent role in integrating information between hemispheres lacking direct neural connections, how does one hemisphere express content in a way that allows the other hemisphere to understand it? As mentioned above, an obvious possibility lies in initiating a motor action that is perceived by the other hemisphere, for example touching the right hand with the left or tapping a finger. But many more subtle possibilities exist. For example, some of the facial musculature is innervated bilaterally. Thus, a contraction instigated by one hemisphere can attract the other hemisphere’s attention. As discussed above, eye movements and direction of attention via subcortical pathways may be particularly suitable ways to convey the location of a stimulus.
The success of cross-cueing critically relies on the capacity of the recipient hemisphere to decipher the meaning of a given cue. This leads to the question of whether specific mechanisms are involved in the perception and interpretation of cues. Does each hemisphere possess neural circuitry that specializes in picking up, deciphering and potentially even anticipating actions initiated by the other hemisphere? A suitable candidate for this job may be mirror neurons, a set of neurons in the cortical motor system that are active each time an individual performs an action or observes another individual performing the same action ( Rizzolatti et al. , 1996 ). While the initial studies described the mirror mechanism for hand movements with neuronal representations in the ventral premotor cortex, similar neurons have been reported throughout a parieto-frontal network, reacting to a range of different actions, including movements of the mouth and face ( Rizzolatti and Sinigaglia, 2010 ). Could these specialized neurons also be activated in one hemisphere of a split-brain when it detects an action initiated by the other hemisphere? Indeed, when healthy subjects imitate actions, mirror neurons in the hemisphere not controlling the motor output show stronger activation than in the contralateral hemisphere’s network that performs the actual movement ( Aziz-zadeh et al. , 2006 ). Moreover, mirror neurons in the parietal cortex have been characterized as encoding the goal of a perceived action ( Rizzolatti and Sinigaglia, 2010 ), thus making them prominent candidates to decode action cues.
The sports’ world illuminates just how specialized the prediction of movements can be. For example, standing at bat, a skilled baseball player, unconsciously predicting a fastball’s trajectory from the pitcher’s movement, initiates his swing before the ball even leaves the pitcher’s hands. Similarly, the split-brain may rely on the mirror neuron network to become more and more efficient at interpreting and, in the case of sequences of cues, even anticipating such cues thrown to it by the other hemisphere. While this hypothesis remains pure speculation, it may explain how split-brain patients become more adept at using cross-cueing over time and some have even gained the capacity to produce simple speech, such as one-word utterances, from the formerly mute right hemisphere ( cf. Gazzaniga, 2000 ).
How could that formerly mute right hemisphere possibly learn to speak? This skill can emerge years after surgery in some patients and may partially rely on neural plasticity in the right hemisphere. As discussed above, the right hemisphere understands words and hence readily represents their semantic meaning. What could be holding back the right hemisphere’s verbal floodgates may be that it lacks the capacity to coordinate muscle activation in order to produce intelligible speech. Over those intervening years, every time a split-brain patient uses the left hemisphere to speak, the right hemisphere will perceive both intonation-related movements in the thorax, neck and face, and the auditory result. Using the capacity of the mirror neuron system, the right hemisphere might be able to emulate movements to produce speech-related motor output itself. Support for this hypothesis stems from the observation that some ‘audiovisual’ mirror neurons discharge both when seeing or hearing an action, such as when ripping paper or snapping a stick in two ( Kohler et al. , 2002 ). Such neurons may help to evolve the skill to generate motor commands that result in production of simple speech. How difficult it must be to accomplish this complex task is clear to anyone who has tried to speak a foreign language with a perfect accent, a major challenge even with both hemispheres on the job.
Beyond the insights into the functional specialization of the hemispheres and how much hemispheric integration is necessary to produce behaviour, the split-brain also offers a unique perspective on our understanding of brain lesions. In 1965, Norman Geschwind published his seminal paper entitled ‘ Disconnexion syndromes in animals and man ’ ( Geschwind, 1965 ), which reinvigorated the much older idea that the disconnection of communication pathways may lead to specific patterns of functional impairment, introduced by Karl Wernicke (1874). The prototypical example for a disconnection syndrome is conduction aphasia, where a person understands what they hear, can speak fluently, but may use the wrong words or parts of words and has difficulty or is unable to repeat spoken phrases. This condition is produced by lesions to the bundle of neural fibres connecting Broca’s area, which is responsible for the motor component of language and Wernicke’s area, responsible for the sensory component of language. Thus, the clinical observation linking lesions in communication pathways to specific deficits presented neuroscience a path worth pursuing, paving the way for the concept of distributed functional networks, a hot topic in contemporary neuroscience (for a review see Catani and ffytche, 2005 ).
While the split-brain is clearly an example of a disconnexion syndrome, it provides an opportunity that other examples of disconnection syndromes do not. This is the opportunity to study the presence of mental capacities, not the absence of mental capacity caused by lesions ( Gazzaniga, 2015 ). For example, in some patients, the corpus callosum was surgically sectioned in stages over a period of months, in the hope that the patient’s seizures could be controlled without sectioning the entire structure. Testing patients throughout this process revealed the functional organization of the corpus callosum: the more posterior regions transfer basic sensory information that relates to vision, audition and somatosensory information, while anterior regions are involved in the transfer of attentional resources and higher cognitive information ( cf. Gazzaniga, 2005 ). Moreover, split-brain research led to the development of several methodological advances that derived from questions specifically occurring in split-brain patients. One such question lies in accurately assessing the surgical result of the sectioning, that is, the actual extent of the corpus callosum sectioning. This led to the development of a specific neuroimaging approach that allows one to assess the extent of callosal disconnection in split-brain patients ( Gazzaniga et al. , 1985 ; Corballis et al. , 2001 ) and callosal lesions due to all kinds of pathologies ( Fig. 6 ).
![split brain research conclusion Imaging the corpus callosum. The necessity to determine the extent of the callosotomy in split-brain patients motivated the advancement of neuroimaging methodology to investigate if the corpus callosum was entirely resected or if residual fibres allow information transfer between hemispheres. The first assessment of a split-brain patient via MRI in 1985 suggested two remaining interhemispheric connections in the anterior and posterior end of the corpus callosum (bright spots in white boxes). Reassessment of the same patient with advanced imaging technology (higher spatial resolution and 3D acquisition) in 2001 confirmed the remaining anterior connection, while showing that the posterior fibres were clearly severed. Modern imaging techniques allow reconstruction of callosal fibres from diffusion imaging data [diffusion spectrum imaging (DSI)] and hence a more direct assessment of corpus callosum integrity. Modified from Corballis et al. (2001), with permission.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/brain/140/7/10.1093_brain_awx139/2/m_awx139f6.jpeg?Expires=1733245689&Signature=l2wyBxfq0WgyghCsJEJgtzd~0qYFPWyTWqh3WY1gDoQIt9mFvhqz0-hHbOziT3ZphUB31f~fgPtnNShbRQxDcG11Hr~GupHAT~lxnVP7~ATcC4CVIgi9VtW74-rIKnYecvp8p7bEQjcM-skWMFOy4bfa5mWDBl~aP15MCSMOrTs87R~oCmOu4UpQ5nxCZN0B5D-thTt2sBk6x3ksgk9xrrIZ6z4UFotfuN3jpLcF6XinzyMpYJTVwcoZXTFBINGf6UieLYv4a9cvjEZGIW~6m1Qpx7ODKgsMdVVD56f-XwkuEyOXIDV8rzXFmQWp8aU~gE9r~GNKMnSQgvvgUOlW2g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Imaging the corpus callosum. The necessity to determine the extent of the callosotomy in split-brain patients motivated the advancement of neuroimaging methodology to investigate if the corpus callosum was entirely resected or if residual fibres allow information transfer between hemispheres. The first assessment of a split-brain patient via MRI in 1985 suggested two remaining interhemispheric connections in the anterior and posterior end of the corpus callosum (bright spots in white boxes). Reassessment of the same patient with advanced imaging technology (higher spatial resolution and 3D acquisition) in 2001 confirmed the remaining anterior connection, while showing that the posterior fibres were clearly severed. Modern imaging techniques allow reconstruction of callosal fibres from diffusion imaging data [diffusion spectrum imaging (DSI)] and hence a more direct assessment of corpus callosum integrity. Modified from Corballis et al. (2001) , with permission.
Besides the various insights on aspects of functional specialization of the hemispheres or the functional anatomy of the corpus callosum that were obtained from split-brain work, these extraordinary cases of separated hemispheres raise an even more general question: how much integration of information between specialized brain modules is necessary to give rise to our skilled behaviour and to create our unique experience of the world around us? It seems puzzling that the verbal IQ and problem solving capacities of split-brain patients are typically unaffected by the surgery. Moreover, patients do not report any difference in the nature of their personal experience—despite the fact that their hemispheres are separated, they report that they experience a single consciousness ( cf. Gazzaniga, 2000 ). Not surprisingly, theoretical frameworks of consciousness often include the split-brain as a test-case for their respective theory. Yet claims of support are made regardless of whether conscious experience is interpreted to result from the integration of regional resources, as in the Global Workspace Theory ( cf. Baars, 1997 ) or the Information Integration Theory ( cf. Tononi and Koch, 2015 ) or, in contrast, is hypothesized to stem from focal activity, as suggested by the local recurrent processing theory of consciousness for example ( cf. Lamme, 2006 ).
A set of observations from split-brain experiments may be particularly suitable to inform such theoretical frameworks of consciousness. In several domains of problem-solving, the left hemisphere shows fundamentally different strategic tendencies compared to the right hemisphere. For example, the right hemisphere adheres to factual knowledge when asked to identify previously presented stimuli and thus outperforms the left hemisphere, which falsely recognizes similar yet unseen objects ( Phelps and Gazzaniga, 1992 ). This observation is in line with the notion that the left hemisphere ‘gets the gist’ and tends to integrate information into theories, which can help to predict future events and offer a coherent interpretative framework. Interpretive qualities unique to the left hemisphere were also observed in a probability-guessing paradigm ( Wolford et al. , 2000 ) where it tries to find patterns, i.e. a ‘theory’ in random events. The left hemisphere is not shy to interpret the behaviour of or physiological responses evoked by emotional stimuli presented to the right hemisphere, even when it is bound to fail to come up with a veridical story due to the lack of critical information exclusively present in the right hemisphere. Why would the left hemisphere interpreter bother to do so? By constantly offering explanations for what it perceives, the left hemisphere interpreter may generate a feeling in all of us that we are integrated and unified ( Gazzaniga, 2000 ). Hence, the interpretive function that strings events together to form our seemingly coherent autobiographies is hosted by the left hemisphere.
Of course, the distinct interpretive capacities of both hemispheres are but a small piece in the puzzle of deciphering the neurobiological foundations that give rise to our conscious experience of the world. These findings also intriguingly illustrate the vast scope of impactful insights that can be gained from the persistent study of a unique group of neurological patients.
L.J.V. and M.S.G. thankfully acknowledge funding by the SAGE Center for the Study of the Mind, University of California.
Akelaitis AJ . Psychobiological studies following section of the corpus callosum. A preliminary report . Am J Psychiatry 1941 ; 97 : 1147 – 57 .
Google Scholar
Aziz-zadeh L , Koski L , Zaidel E , Mazziotta J , Iacoboni M . Lateralization of the human mirror neuron system . J Neurosci 2006 ; 26 : 2964 – 70 .
Baars BJ . In the Theater of Consciousness: Global Workspace Theory, A Rigorous Scientific Theory of Consciousness . Journal of Consciousness Studies 1997 ; 4 : 292 – 309 .
Baynes K , Wessinger CM , Fendrich R , Gazzaniga MS . The emergence of the capacity to name left visual field stimuli in a callosotomy patient: implications for functional plasticity . Neuropsychologia 1995 ; 33 : 1225 – 42 .
Catani M , ffytche DH . The rises and falls of disconnection syndromes . Brain 2005 ; 128 : 2224 – 39 .
Corballis PM , Inati S , Funnell MG , Grafton ST , Gazzaniga MS . MRI assessment of spared fibers following callosotomy: a second look . Neurology 2001 ; 57 : 1345 – 6 .
Gazzaniga MS . Cross-cuing mechanisms control and ipsilateral monkeys eye-hand in split-brain monkeys . Exp Neurol 1969 ; 23 : 11 – 17 .
Gazzaniga MS . Principles of human brain organization derived from split-brain studies . Neuron 1995 ; 14 : 217 – 28 .
Gazzaniga MS . Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain 2000 ; 123 : 1293 – 326 .
Gazzaniga MS . Forty-five years of split-brain research and still going strong . Nat Rev Neurosci 2005 ; 6 : 653 – 9 .
Gazzaniga MS . Tales from both sides of the brain: a life in neuroscience . New York : Ecco/HarperCollins ; 2015 .
Google Preview
Gazzaniga MS , Bogen JE , Sperry RW . Observations on visual perception after disconnexion of the cerebral hemispheres in man . Brain 1965 ; 88 : 221 – 36 .
Gazzaniga MS , Hillyard SA . Language and speech capacity of the right hemisphere . Neuropsychologia 1971 ; 9 : 273 – 80 .
Gazzaniga MS , Holtzman JD , Deck MD , Lee BC . MRI assessment of human callosal surgery with neuropsychological correlates . Neurology 1985 ; 35 : 1763 – 6 .
Gazzaniga MS , LeDoux JE . The integrated mind . New York : Plenum Press ; 1978 .
Gazzaniga MS , Smylie CS , Baynes K , Hirst W , McCleary C . Profiles of right hemisphere language and speech following brain bisection . Brain Lang 1984 ; 22 : 206 – 20 .
Gazzaniga MS , Sperry RW . Language after section of the cortical commissures . Brain 1967 ; 90 : 131 – 48 .
Gazzaniga MS , Volpe BT , Smylie CS , Wilson DH , Le Doux JE . Plasticity in speech organization following commissurotomy . Brain 1979 ; 102 : 805 – 15 .
Geschwind N . Disconnection syndromes in animals and man . Brain 1965 ; 88 : 237 – 644 .
Holtzman JD , Sidtis JJ , Volpe BT , Wilson DH , Gazzaniga MS . Dissociation of spatial information for stimulus localization and the control of attention . Brain 1981 ; 104 : 861 – 72 .
Kohler E , Keysers C , Umiltà MA , Fogassi L , Gallese V , Rizzolatti G . Hearing sounds, understanding actions: action representation in mirror neurons . Science 2002 ; 297 : 846 – 8 .
Lamme VAF . Towards a true neural stance on consciousness . Trends Cogn Sci 2006 ; 10 : 494 – 501 .
Phelps EA , Gazzaniga MS . Hemispheric differences in mnemonic processing: the effects of left hemisphere interpretation . Neuropsychologia 1992 ; 30 : 293 – 7 .
Pinto Y , Neville DA , Otten M , Corballis PM , Lamme VAF , de Haan EHF et al. Split brain: divided perception but undivided consciousness . Brain 2017 : pii : aww358 .
Rizzolatti G , Fadiga L , Gallese V , Fogassi L . Premotor cortex and the recognition of motor actions . Cogn Brain Res 1996 ; 3 : 131 – 41 .
Rizzolatti G , Sinigaglia C . The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations . Nat Rev Neurosci 2010 ; 11 : 264 – 74 .
Roser ME , Fugelsang JA , Dunbar KN , Corballis PM , Gazzaniga MS . Dissociating processes supporting causal perception and causal inference in the brain . Neuropsychology 2005 ; 19 : 591 – 602 .
Rossion B , Dricot L , Goebel R , Busigny T . Holistic face categorization in higher order visual areas of the normal and prosopagnosic brain: toward a non-hierarchical view of face perception . Front Hum Neurosci 2011 ; 4 : 225 .
Seymour SE , Reuter-lorenz PA , Gazzaniga MS . The disconnection syndrome: Basic findings reaffirmed . Brain 1994 ; 117 : 105 – 15 .
Sperry RW , Gazzaniga MS , Bogen JE . Interhemispheric relationships: the neocortical commissures; syndromes of hemisphere disconnection . Handb Clin Neurol 1969 ; 4 : 273 – 90 .
Tononi G , Koch C . Consciousness: here, there and everywhere? Phil Trans R Soc B 2015 ; 370 : 20140167 .
Turk DJ , Heatherton TF , Kelley WM , Funnell MG , Gazzaniga MS , Macrae CN . Mike or me? Self-recognition in a split-brain patient . Nat Neurosci 2002 ; 5 : 841 – 2 .
Wernicke C . The aphasia symptom-complex. 1874. Breslau, Cohn and Weigert . Translated in: Eling P, editor. Reader in the history of aphasia. Vol. 4. Amsterdam: John Benjamins; 1994. p. 69–89 .
Wolford G , Miller MB , Gazzaniga MS . The left hemisphere’s role in hypothesis formation . J Neurosci 2000 ; 20 : RC64 .
Young L , Saxe R . Innocent intentions: a correlation between forgiveness for accidental harm and neural activity . Neuropsychologia 2009 ; 47 : 2065 – 72 .
Email alerts
Citing articles via, looking for your next opportunity.
- Contact the editorial office
- X (formerly Twitter)
- Guarantors of Brain
- Recommend to your Library
Affiliations
- Online ISSN 1460-2156
- Print ISSN 0006-8950
- Copyright © 2024 Guarantors of Brain
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
One Brain. Two Minds? Many Questions
Victoria rosen.
- Author information
- Article notes
- Copyright and License information
Address correspondence to: Victoria Rosen, School of Psychology and Neuroscience, St Mary’s Quad, South Street, St Andrews, Fife, KY16 9JP. Email: [email protected]
Corresponding author.
Received 2018 Mar 9; Revised 2018 May 23; Accepted 2018 May 31; Collection date 2018 Spring.
For several decades, split-brain research has provided valuable insight into the fields of psychology and neuroscience. These studies have progressed our knowledge of hemispheric specialization, language processing, the role of the corpus callosum, cognition, and even human consciousness. Following a recent empirical paper by Pinto et al. (2017a) and review by Volz and Gazzaniga (2017) , a debate has ensued about the nature of conscious perception of visual stimuli in split-brain patients. This exchange is an ideal platform for generating discussion about both the implications of recent findings and the interpretation of results from split-brain studies in general.
Keywords: hemispheric specialization, split-brain, cross-cueing, corpus callosum, cognition, consciousness
From its beginnings fifty years ago, split-brain research has continually proved to be a vital field within the greater scope of psychology and neuroscience. Split-brain research refers to research and insights garnered from studying patients who have had their corpus callosum, a bundle of fibers connecting the two hemispheres of the brain, severed, in most cases to treat severe epilepsy. This unique condition, combined with a novel technique of presenting information to each hemisphere independently, led to a field that has been prominent for five decades, and still continues to produce new and exciting revelations in neuroscience. However, the field also continues to spark debate and controversy. This is best demonstrated by a recent exchange in journal Brain .
In a 2017 empirical paper, Pinto and colleagues offer evidence against a dominant view in split-brain research: that after severing the corpus callosum visual information cannot be transferred through other fibers ( Pinto et al., 2017a ). Going even further, they interpret results indicative of conscious reporting across hemispheres as suggesting the two hemispheres are not separately conscious following the surgery. In their recent review, Volz and Gazzaniga (2017) , argue against these interpretations by Pinto et al. Together, these papers triggered a debate within the field leading to further responses in the form of letters to the editor from Pinto et al. (2017b) , Volz et al. (2018) , and Corballis et al. (2018) . Here, I summarize each component of the current debate, and also argue why the exchange as a whole can serve as a valuable teaching tool.
I will start by summarizing sections of the review by Volz and Gazzaniga (2017) that give context to both this exchange and the field as a whole. A group of patients in Rochester, New York in 1939 were the first to undergo surgery designed to treat severe epilepsy by severing the corpus callosum, but these first patients were not actually the first group of split-brain patients that we think of today. That is because though they were studied extensively, these patients appeared not to be significantly different after the surgery compared to before ( Akelaitis, 1941 ). This conclusion was accepted by many for two decades, until a novel experimental design was able to present information to each hemisphere in isolation, which for the first time gave experimenters the ability to observe the two hemispheres individually ( Gazzaniga and Sperry, 1967 ; Volz & Gazzaniga, 2017 ). I am including this not just as an interesting anecdote, but also because it is a great example of how difficult it can be to design an experiment in split-brain research. In this line of research, it is of the utmost importance that each hemisphere receives information independently. Because of the nature of the condition and the way patients learn to adapt to their new circumstance after surgery, this is not trivial, and therefore relevant for the debate at hand.
Because of the straightforward nature of the visual system when compared with our knowledge of how the other senses are processed, it is commonly used to deliver stimuli in split-brain experiments ( Volz and Gazzaniga, 2017 ). To explain briefly how this works, when an image is shown in right visual field, it is ‘seen’ and processed by the left hemisphere and vice versa. Meaning, if a split-brain patient were to see information only in one half of their visual space, it would be processed only by the contralateral hemisphere ( Volz and Gazzaniga, 2017 ). Interestingly though, when an object is shown in the right visual field and the patient is asked what was seen they can and do answer correctly, but when shown an object in left visual field and asked the same question, the patient will often answer that nothing was seen ( Volz and Gazzaniga, 2017 ). This is because the left hemisphere houses most language capabilities. So, when something is presented in the right visual field (to the left hemisphere) patients are able to respond verbally; however, when an image is presented in the left visual field, though the patient may not be able to respond verbally, they are able to non-verbally. For example, participants can use their left hands (controlled by the right hemisphere) to point out what was seen from a group of objects ( Volz and Gazzaniga, 2017 ).
In their 2017 empirical paper, Pinto et al. (2017a) nicely summarize this phenomenon postulating that the left hemisphere can only perceive the right side of visual space with expression through verbal language and the right hand, while the right hemisphere can only perceive the left side of visual space with expression through the left hand. However, following this summary, Pinto et al. (2017a) also mention that though this is widely taught and believed, there are no quantitative data supporting the idea, only clinical observations.
Now I will outline the empirical findings by Pinto et al. (2017a) that have sparked the current controversy. The researchers studied two split-brain patients, and though some of their results replicate past findings, others appear to challenge the status quo in the field. While two patients may seem like a small number, Pinto et al. justify this by explaining that there are very few split-brain patients remaining today. It is also worth noting that both patients were tested at least a decade after surgery. In their first experiment, Pinto and colleagues (2017a) examined if the patients could detect a stimulus and indicate its location when presented in only one visual half field. They asked patients to respond with their left hands, right hands, and verbally. Researchers observed near perfect accuracy for detection of the stimulus, regardless of response type (left hand, right hand, verbal), and well above chance accuracy for indicating location ( Pinto et al., 2017a ). Even more interesting, however, is that there was no observed interaction between response type and stimulus location (left visual field, right visual field).
This led to further testing to determine if the results above could be due to transfer of visual information across the two hemispheres. In follow-up experiments only one of the patients was asked to compare stimuli across and within visual half fields, as well as name and match pictures within visual half fields. The patient could not compare stimuli across half fields but was able to within half fields. Additionally, the same patient showed better performance when labeling objects presented to the right visual field, and matching objects presented to the left ( Pinto et al., 2017a ). These findings, consistent with previous research, suggest that visual processing is indeed independent for each hemisphere in split-brain patients. However, the authors note there was still no interaction between response type and visual field. This leaves the question of how patients were able to correctly report what was processed regardless of which side did the processing. To test if this phenomenon was due to conscious or unconscious processes, the experimenters asked the patient to complete similar testing, but this time with confidence ratings. Based on confidence ratings being higher for correct responses, the researchers concluded that the patient was indeed consciously aware of his reporting. Again, there was no interaction between response type and stimulus location ( Pinto et al., 2017a ).
The authors entertain several interpretations of their data, but ultimately, they take the stance that that visual perception remains divided in split-brain patients, but that in reporting what was perceived, consciousness is undivided. They refer to this as “‘split phenomenality’ combined with ‘unity of consciousness’” ( Pinto et al., 2017a ). This interpretation lies in direct contrast with both previous theories of processing in split-brain patients and dominant theories of consciousness.
Pinto and colleagues (2017a) go into a lengthy explanation as to why cross-cueing should be ruled out. First, they define cross-cueing as “one hemisphere informing the other hemisphere with behavioral ticks, such as touching the left hand with the right hand” and that it can only transfer “one bit of information” ( Pinto et al., 2017a ). Using this definition, they claim cross-cueing is not likely responsible for their results. They reason that: 1) cross-cueing could not transfer the amount of information needed for correct responses, 2) there were significant differences in performance on visual tasks between hemifields (this refers to the experiment in which the patient was better at matching objects shown in the left visual field but better at labeling objects shown in the right visual field), 3) the experiment was set up to prevent hands from touching each other, 4) in an experiment of reaction times with a colored circle appearing in either the left or right visual field there were no significant time differences between ipsilateral and contralateral responses, which would be expected if cross-cueing were to take place as it should slow down ipsilateral responses. After this lengthy discussion on cross-cueing, the authors conclude with one final possibility that because testing began several years after the operation and both patients were operated on as young adults, it could be that over time patients develop new structural connections to transfer information across hemispheres ( Pinto et al., 2017a ).
Switching back to the review by Volz and Gazzaniga (2017) , after summarizing basics in the field, the authors take the time to discuss recent findings focusing primarily on the empirical paper by Pinto et al. (2017a) . Volz and Gazzaniga (2017) describe cross-cueing as one hemisphere using knowledge gained by perceiving behavioral cues from the other to overcome a challenge or complete a task that would require information to be shared between hemispheres. The authors also note that this is not done actively or consciously and the cues can often be exceptionally subtle. This emphasis on subtle cues marks a difference in definition of cross-cueing between the two sets of authors, which is noted in the review. Volz and Gazzaniga (2017) critique Pinto et al.’s (2017a) willingness to write-off cross-cueing far too quickly. Although Pinto et al. (2017a) used eye tracking technology to ensure the patient was fixating (maintaining visual gaze on a specific location) during stimulus presentation, fixation was not monitored while the patient was responding. According to Volz and Gazzaniga (2017) this meant that cross-cueing could occur in the form of an eye movement when asked to indicate the location of the stimulus.
Pinto and colleagues (2017b) subsequently responded to Volz and Gazzaniga’s review in a letter to the editor of Brain . In this letter they once again assert why they believe cross-cueing is an unlikely explanation, responding more specifically to points brought up in the review. They contend that even cross-cueing cannot explain the lack of an interaction between response type and location. Though they do give way that an alternative explanation broached by Volz and Gazzaniga (2017) (transfer through subcortical routes) could be more likely, they assert that there is a larger problem in the whole interpretation framework, namely that the term cross-cueing is not clearly defined ( Pinto et al. 2017b ). In a subsequent reply to Pinto et al. (2017b) , Volz and colleagues (2018) concede that the lack of a formal definition of cross-cueing is a significant issue, but still reassert their stance. They emphasize that due to the passing of time between the patients’ surgery and testing, they could have learned much more subtle and efficient ways to transfer information through behavioral cues. In a final response in the form of a letter to the editor, a third party weighs in. Corballis and colleagues (2018) cite the ongoing debate and argue that it is a mistake to focus so heavily on cross-cueing. Instead the authors assert that both groups should return to the idea of subcortical routes. The authors provide anatomical evidence citing a ‘second visual system’ pathway involving midbrain structures. This pathway is believed to go through the superior colliculi, the pulvinar nuclei, and subsequently to the parietal lobes with a subcortical interhemispheric connection at the collicular commissure ( Trevarthen and Sperry, 1973 ; Corballis et al., 2018 ). In addition to the anatomical evidence, Corballis et al (2018) summarize results from previous behavioral experiments involving split-brain patients that support this possibility. Overall the authors make a strong case for subcortical connections as a possible explanation for Pinto and colleagues’ (2017a) observations.
The above exchange serves as an example of a lively and provocative conversation in neuroscience emerging from competing interpretations of published data. The value of this exchange as a teaching tool comes not from which interpretation (if any) the reader chooses to accept, but rather from understanding why these different interpretations exist, and how each group of authors was able to use scientific evidence to support their ideas. In a classroom setting, research is often presented as producing facts, but it is important to remember that different scientists can draw different conclusions from the same data. This means that our interpretations of scientific work are just as much a part of science as the actual evidence. Though this may seem obvious to researchers, it is something that is often overlooked by students.
The current debate in split-brain research brings the audience’s attention to critical components of scientific research in general, including experimental design and interpretation, as well as communication within the field. Though the separate sets of authors may disagree, they communicate effectively and publicly, and in doing so demonstrate that there can be wide variation in interpretation of scientific evidence which can largely affect the implications of a study as well as guide future research.
In addition to being a great teaching tool for the aspects mentioned above, this exchange is also useful in that it can introduce students to a variety of publication types. The inclusion of an empirical paper, a review, and responses in the form of letters to the editor, teaches students that scientific research is not done in isolation, and shows how and when to use different forms of publication.
I believe there is a place for this set of papers in almost any introductory psychology or neuroscience class, as well as cognitive neuroscience classes. Additionally, this exchange could be especially useful in upper level psychology and neuroscience classes with a focus on evaluating scientific literature, interpretation, or experimental design. The authors’ emphasis on critical thinking and interpretation creates a springboard for classroom discussion and ideas for future directions in the field.
If I were to teach this exchange in a classroom, I would have students read these manuscripts in the order I have presented them here: starting with the review by Volz and Gazzaniga which contains relevant background of the field, followed by the empirical by paper by Pinto et al. (2017a) . I would then ask the students to discuss if they believe the criticism in the review was fair and why (or why not). Afterwards, I would follow up the discussion with the three letters to the editor and ask the students to decide which interpretation they side with and why, or to come up with their own interpretation supported by empirical evidence.
Regardless of how this set of papers is taught, it has the potential to stimulate thought and discussion. It will be exciting to see how this debate continues to develop over time.
The author would like to thank all involved in the University of St. Andrews MRes in Neuroscience program, especially Dr. Stefan Pulver.
- Akelaitis AJ. Psychobiological studies following section of the corpus callosum. A preliminary report. Am J Psychiatry. 1941;97:1147–1157. [ Google Scholar ]
- Corballis MC, Corballis PM, Berlucchi G, Marzi CM. Perceptual unity in the split brain: the role of subcortical connections. Brain. 2018;141(6):e46. doi: 10.1093/brain/awy085. [ DOI ] [ PubMed ] [ Google Scholar ]
- Gazzaniga MS, Sperry RW. Language after section of the cerebral commissures. Brain. 1967;90:131–148. doi: 10.1093/brain/90.1.131. [ DOI ] [ PubMed ] [ Google Scholar ]
- Pinto Y, Lamme VAF, de Haan EHF. Cross-cueing cannot explain unified control in split-brain patients. Brain. 2017b;140:e68. doi: 10.1093/brain/awx235. [ DOI ] [ PubMed ] [ Google Scholar ]
- Pinto Y, Neville DA, Otten M, Corballis PM, Lamme VAF, de Haan EHF, Foschi N, Fabri M. Split brain: divided perception but undivided consciousness. Brain. 2017a;140:1231–1237. doi: 10.1093/brain/aww358. [ DOI ] [ PubMed ] [ Google Scholar ]
- Trevarthen C, Sperry RW. Perceptual unity of the ambient visual field in human commissurotomy patients. Brain. 1973;96:547–570. doi: 10.1093/brain/96.3.547. [ DOI ] [ PubMed ] [ Google Scholar ]
- Volz LJ, Gazzaniga MS. Interaction in isolation: 50 years of insights from split-brain research. Brain. 2017;140:2051–2060. doi: 10.1093/brain/awx139. [ DOI ] [ PubMed ] [ Google Scholar ]
- Volz LJ, Hillyard SA, Miller MB, Gazzaniga MS. Unifying control over the body: consciousness and cross-cueing in split-brain patients. Brain. 2018;141(3):e15. doi: 10.1093/brain/awx359. [ DOI ] [ PubMed ] [ Google Scholar ]
- PDF (97.3 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 01 August 2005
Forty-five years of split-brain research and still going strong
- Michael S. Gazzaniga 1
Nature Reviews Neuroscience volume 6 , pages 653–659 ( 2005 ) Cite this article
11k Accesses
274 Citations
31 Altmetric
Metrics details
Forty-five years ago, Roger Sperry, Joseph Bogen and I embarked on what are now known as the modern split-brain studies. These experiments opened up new frontiers in brain research and gave rise to much of what we know about hemispheric specialization and integration. The latest developments in split-brain research build on the groundwork laid by those early studies. Split-brain methodology, on its own and in conjunction with neuroimaging, has yielded insights into the remarkable regional specificity of the corpus callosum as well as into the integrative role of the callosum in the perception of causality and in our perception of an integrated sense of self.
This is a preview of subscription content, access via your institution
Relevant articles
Open Access articles citing this article.
A review of combined functional neuroimaging and motion capture for motor rehabilitation
- Emanuel A. Lorenz
- , Xiaomeng Su
- & Nina Skjæret-Maroni
Journal of NeuroEngineering and Rehabilitation Open Access 03 January 2024
A frontal transcallosal inhibition loop mediates interhemispheric balance in visuospatial processing
- Yanjie Wang
- , Zhaonan Chen
- … Siyu Zhang
Nature Communications Open Access 25 August 2023
Mapping lesion, structural disconnection, and functional disconnection to symptoms in semantic aphasia
- Nicholas E. Souter
- , Xiuyi Wang
- … Elizabeth Jefferies
Brain Structure and Function Open Access 04 July 2022
Access options
Subscribe to this journal
Receive 12 print issues and online access
176,64 € per year
only 14,72 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
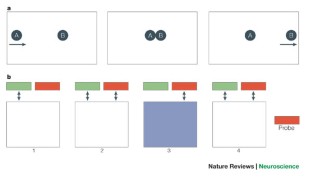
Lashley, K. S. In search of the engram. Symp. Soc. Exp. Biol. 4 , 454–482 (1950).
Google Scholar
Hebb, D. O. The Organization of Behavior: a Neuropsychological Theory (Wiley, New York, USA, 1949).
Sperry, R. W. Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc. Natl Acad. Sci. USA 50 , 703–710 (1963).
CAS PubMed Google Scholar
Weiss, P. A. In vitro experiments on the factors determining the course of the outgrowing nerve fiber. J. Exp. Zool. 68 , 393–448 (1934).
Van Wagenen, W. P & Herren, R. Y. Surgical division of commissural pathways in the corpus callosum: relation to spread of an epileptic attack. Arch. Neurol. Psychiatry 44 , 740–759 (1940).
Bogen, J. E. & Vogel, P. J. Cerebral commissurotomy in man. Bull. Los Angel. Neuro. Soc. 27 , 169–172 (1962).
Akelaitis, A. J. A study of gnosis, praxis and language following section of the corpus callosum and anterior commissure. J. Neurosurg. 1 , 94–102 (1944).
Myers, R. E. Function of the corpus callosum in interocular transfer. Brain 79 , 358–363 (1956).
Myers, R. E. & Sperry, R. W. Interhemispheric communication through the corpus callosum: mnemonic carry-over between the hemispheres. Arch. Neurol. Psychiatry 80 , 298–303 (1958).
CAS Google Scholar
Gazzaniga, M. S. Split brain research: a personal history. Cornell Univ. Alumni Q. 45 , 2–12 (1982).
Lettvin, J. Y. 1981 Nobel prize for physiology or medicine. Science 214 , 517–520 (1981).
Gazzaniga, M. S. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain 123 , 1293–1326 (2000).
PubMed Google Scholar
Zaidel, E. in Handbook of Neuropsychology Vol. 4 (eds Boller, F. & Grafman, J.) 115–150 (Elsevier, Amsterdam, 1991).
Funnell, M. G., Corballis, P. M. & Gazzaniga, M. S. Handbook of Neuropsychology 2nd Edn Vol. 1 (eds Boller, F. & Grafman, J.) 103–120 (Elsevier, Amsterdam, 2000).
Milner, B. in Interhemispheric Relations and Cerebral Dominance (ed. Mountcastle, V. B.) 177–198 (Johns Hopkins Press, Baltimore, Maryland, 1962).
Zaidel, E. & Peters, A. M. Phonological encoding and ideographic reading by the disconnected right hemisphere: two case studies. Brain and Language 14 , 205–234 (1981).
Zaidel, E. in The Dual Brain (eds Benson, D. F. & Zaidel, E.) 205–231 (Guildford, New York, 1985).
Baynes, K., Eliassen, J. C., Lutsep, H. L & Gazzaniga, M. S. Modular organization of cognitive systems masked by interhemispheric integration. Science 280 , 902–905 (1998).
Nebes, R. Superiority of the minor hemisphere in commissurotomized man on a test of figural unification. Brain 95 , 633–638 (1972).
Nebes, R. Perception of spatial relationships by the right and left hemispheres of a commissurotomized man. Neuropsychologia 11 , 285–289 (1973).
Forster, B. A., Corballis, P. M. & Corballis, M. C. Effect of luminance on successiveness discrimination in the absence of the corpus callosum. Neuropsychologia 38 , 441–450 (2000).
Corballis, M. C. & Sergent, J. Imagery in a commissurotomized patient. Neuropsychologia 26 , 13–26 (1988).
Corballis, P. M., Funnell, M. G. & Gazzaniga, M. S. A dissociation between spatial and identity matching in callosotomy patients. Neuroreport 10 , 2183–2187 (1999).
Funnell, M. G., Corballis, P. M. & Gazzaniga, M. S. A deficit in perceptual matching in the left hemisphere of a callosotomy patient. Neuropsychologia 37 , 1143–1154 (1999).
Corballis, P. M., Fendrich, R., Shapley, R. & Gazzaniga, M. S. Illusory contours and amodal completion: evidence for a functional dissociation in callosotomy patients. J. Cogn. Neurosci. 11 , 459–466 (1999).
Luck, S. J., Hillyard, S. A., Mangun, G. R. & Gazzaniga, M. S. Independent hemispheric attentional systems mediate visual search in split-brain patients. Nature 342 , 543–545 (1989).
Lambert, A. J. Interhemispheric interaction in the split-brain. Neuropsychologia 29 , 941–948 (1991).
Corballis, M. C. Split decisions: problems in the interpretation of results from commissurotomized subjects. Behav. Brain Res. 64 , 163–172 (1994).
Levy, J. & Trevarthen, C. Metacontrol of hemispheric function in human split-brain patients. J. Exp. Psychol. Hum. Percept. Perform. 2 , 299–312 (1976).
Holtzman, J. D. & Gazzaniga, M. S. Dual task interactions due exclusively to limits in processing resources. Science 218 , 1325–1327 (1982).
Weissman, D. H. & Banich, M. T. The cerebral hemispheres cooperate to perform complex but not simple tasks. Neuropsychology 14 , 41–59 (2000).
Belger, A. & Banich, M. T. Costs and benefits of integrating information between the cerebral hemispheres: a computational perspective. Neuropsychology 12 , 380–398 (1998).
Banich, M. T. & Belger, A. Interhemispheric interaction: how do the hemispheres divide and conquer a task? Cortex 26 , 77–94 (1990).
Gordon, H. W., Bogen, J. E. & Sperry, R. W. Absence of deconnexion syndrome in two patients with partial section of the neocommissures. Brain 94 , 327–336 (1971).
Gazzaniga, M. S. & Freedman, H. Observations on visual processes after posterior callosal section. Neurology 23 , 1126–1130 (1973).
Risse, G. L., Gates, J., Lund, G., Maxwell, R. & Rubens, A. Interhemispheric transfer in patients with incomplete section of the corpus-callosum. Anatomic verification with magnetic resonance imaging. Arch. Neurol. 46 , 437–443 (1989).
Gazzaniga, M. S. The split brain in man. Sci. Am. 217 , 24–29 (1967).
Corballis, M. C. Visual integration in the split brain. Neuropsychologia 33 , 937–959 (1995).
Baynes, K. Language and reading in the right hemisphere: highways or byways of the brain? J. Cogn. Neurosci. 2 , 159–179 (1990).
Gazzaniga, M. S. Interhemispheric communication of visual learning. Neuropsychologia 4 , 183–189 (1966).
Seymour, S. A., Reuter-Lorenz, P. A. & Gazzaniga, M. S. The disconnection syndrome: basic findings reaffirmed. Brain 117 , 105–115 (1994).
Gazzaniga, M. S., Bogen, J. E. & Sperry, R. W. Observations on visual perception after disconnexion of the cerebral hemispheres in man. Brain 88 , 221–236 (1965).
Funnell, M. G., Corballis, P. M. & Gazzaniga, M. S. Cortical and subcortical interhemispheric interactions following partial and complete callosotomy. Arch. Neurol. 57 , 185–189 (2000).
Funnell, M. G., Corballis, P. M. & Gazzaniga, M. S. Insights into functional specificity of the human corpus callosum. Brain 123 , 920–926 (2000).
Fabri, M. et al. Posterior corpus callosum and interhemispheric transfer of somatosensory information: an fMRI and neuropsychological study of a partially callosotomized patient. J. Cogn. Neurosci. 13 , 1071–1079 (2001).
Ihori, N., Kawamura, M., Fukuzawa, K. & Kamaki, M. Somesthetic disconnection syndromes in patients with callosal lesions. Eur. Neurol. 44 , 65–71 (2000).
Arguin, M. et al. Divided visuo-spatial attention systems with total and anterior callosotomy. Neuropsychologia 15 , 295–302 (2000).
Basser, P. J. & Jones, D. K. Diffusion-tensor MRI: theory, experimental design and data analysis - a technical review. NMR Biomed. 15 , 456–467 (2002).
Basser, P. J., Mattiello, J. & LeBihan, D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J. Magn. Reson. B 103 , 247–254 (1994).
Basser, P. J., Mattiello, J. & LeBihan, D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 66 , 259–267 (1994).
CAS PubMed PubMed Central Google Scholar
Le Bihan, D. Looking into the functional architecture of the brain with diffusion MRI. Nature Rev. Neurosci. 4 , 469–480 (2003).
Sundgren, P. C. et al. Diffusion tensor imaging of the brain: review of clinical applications. Neuroradiology 46 , 339–350 (2004).
Chepuri, N. B. et al. Diffusion anisotropy in the corpus callosum. Am. J. Neuroradiol. 23 , 803–808 (2002).
Baird, A. A., Colvin, M. K., Van Horn, J. D., Inati, S. & Gazzaniga, M. S. Functional connectivity: integrating behavioral, DTI and fMRI data sets. J. Cogn. Neurosci. 17 , 687–693 (2005).
Warrington, E. K. & Taylor, A. M. The contribution of the right parietal lobe to object recognition. Cortex 9 , 152–164 (1973).
Humphreys, G. W., Price, C. J. & Riddoch, M. J. From objects to names: a cognitive neuroscience approach. Psychol. Res. 62 , 118–130 (1999).
Colvin, M. K., Funnell, M. G., Hahn, B. & Gazzaniga, M. S. Identifying functional channels in the corpus callosum: correlating interhemispheric transfer time with white matter organization. Poster presented at the annual meeting of the Society for Neuroscience, San Diego, California, 2004. J. Cogn. Neurosci. 139 (suppl. 5), (2005).
Aboitiz, F. & Montiel, J. One hundred million years of interhemispheric communication: the history of the corpus callosum. Braz. J. Med. Biol. Res. 36 , 409–420 (2003).
LaMantia, A. S. & Rakic, P. Cytological and quantitative characteristics of four cerebral commissures in the rhesus monkey. J. Comp. Neurol. 291 , 520–537 (1990).
Banich, M. T. The missing link: the role of interhemispheric interaction in attentional processing. Brain Cogn. 36 , 128–157 (1998).
Cabeza, R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol. Aging 17 , 85–100 (2002).
Colvin, M. K., Wig, G. S., Kelley, W. M., Grafton, S. T. & Gazzaniga, M. S. Callosal organization predicts the level and effect of right frontal activity during verbal encoding on subsequent memory in healthy young adults. Soc. Neurosci. Abstr. 204.4 (2005).
Colvin, M. K. Individual differences in callosal organization: relationship to interhemispheric communication and hemispheric asymmetries. Diss. Abstr. (in the press).
Leslie, A. M. & Keeble, S. Do six-month-old infants perceive causality? Cognition 25 , 265–288 (1987).
Michotte, A. The Perception of Causality (Basic Books, New York, USA, 1963) (Translated from original, published 1946).
Roser, M. E., Fugelsang, J. A., Dunbar, K. N., Corballis, P. M. & Gazzaniga, M. S. Dissociating causal perception and causal inference in the brain. Neuropsychology (in the press).
Fugelsang, J. A., Roser, M. E., Corballis, P. M., Gazzaniga, M. S. & Dunbar, K. N. Brain mechanisms underlying perceptual causality. Cogn. Brain Res. (in the press).
Turk, D. J., Heatherton, T. F., Macrae, C. N., Kelley, W. M. & Gazzaniga, M. S. Out of contact, out of mind: the distributed nature of self. Ann. NY Acad. Sci. 1001 , 65–78 (2003).
Gazzaniga, M. S. One brain — two minds? Am. Sci. 60 , 311–317 (1972).
Gazzaniga, M. S. & Smylie, C. S. Facial recognition and brain asymmetries: clues to underlying mechanisms. Ann. Neurol. 13 , 536–540 (1983).
DeRenzi, E. Prosopagnosia in two patients with CT scan evidence of damage confined to the right-hemisphere. Neuropsychologia 24 , 385–389 (1986).
Landis, T., Cummings, J. L., Christen, L., Bogen, J. E. & Imhof, H. G. Are unilateral right posterior cerebral lesions sufficient to cause prosopagnosia? Clinical and radiological findings in six additional patients. Cortex 22 , 243–252 (1986).
Michel, F., Poncet, M. & Signoret, J. L. Les lesions responsables de la prosopagnosie sont-elles toujours bilateral. Rev. Neurol. (Paris) 145 , 764–770 (1989) (in French).
Wada, Y. & Yamamoto, T. Selective impairment of facial recognition due to a haematoma restricted to the right fusiform and lateral occipital region. J. Neurol. Neurosurg. Psychiatry 71 , 254–257 (2001).
Whiteley, A. M. & Warrington, E. K. Prosopagnosia: a clinical, psychological, and anatomical study of three patients. J. Neurol. Neurosurg. Psychiatry 40 , 395–403 (1977).
Keenan, J. P., Nelson, A., O'Connor, M. & Pascual-Leone, A. Neurology: self-recognition and the right hemisphere. Nature 409 , 305 (2001).
Keenan, J. P. et al. Left hand advantage in a self-face recognition task. Neuropsychologia 37 , 1421–1425 (1999).
Keenan, J. P., Ganis, G, Freund, S. & Pascual-Leone, A. Self-face identification is increased with left hand responses. Laterality 5 , 259–268 (2000).
Conway, M. A. et al. A positron emission tomography (PET) study of autobiographical memory retrieval. Memory 7 , 679–702 (1999).
Conway, M. A. & Pleydell-Pearch, C. W. The construction of autobiographical memories in the self-memory system. Psychol. Rev. 107 , 261–288 (2000).
Kircher, T. T. et al. The neural correlates of intentional and incidental self processing. Neuropsychologia 40 , 683–692 (2002).
Maguire, E. A. & Mummery, C. J. Differential modulation of a common memory retrieval network revealed by positron emission tomography. Hippocampus 9 , 54–61 (1999).
Turk, D. J. Mike or me? Self-recognition in a split-brain patient. Nature Neurosci. 5 , 841–842 (2002).
Cooney, J. W. & Gazzaniga, M. S. Neurologic disorders and the structure of human consciousness. Trends Cogn. Sci. 7 , 161–164 (2003).
Erikson, T. C. Spread of epileptic discharge. Arch. Neurol. Psychiatry 43 , 429–452 (1940).
Gazzaniga, M. S., Bogen, J. E. & Sperry, R. W. Some functional effects of sectioning the cerebral commissures in man. Proc. Natl Acad. Sci. USA 48 , 1765–1769 (1962).
Gazzaniga, M. S. Effects of commissurotomy on a preoperatively learned visual discrimination. Exp. Neurol. 8 , 14–19 (1963).
Gazzaniga, M. S., Bogen, J. E. & Sperry, R. W. Dyspraxia following division of cerebral commissures. Arch. Neurol. 16 , 606–612 (1967).
Bogen, J. E. & Gazzaniga, M. S. Cerebral commissurotomy in man — minor hemisphere dominance for certain visuospatial functions. J. Neurosurg. 23 , 394–399 (1965).
Gazzaniga, M. S. & LeDoux, J. The Integrated Mind (Plenum, New York, USA, 1978).
Corballis, P. M. Visuospatial processing and the right-hemisphere interpreter. Brain Cogn. 53 , 171–176 (2003).
Download references
Acknowledgements
This research was supported by National Institutes of Health grants to the author. It was also supported by a graduate reseach fellowship from the National Science Foundation to M. Colvin. I would like to thank my collaborators, M. Colvin, M. Funnell, M. Roser and D. Turk, for their scientific input as well as their assistance in reviewing this paper. I would also like to thank R. Townsend for her editorial assistance.
Author information
Authors and affiliations.
the Center for Cognitive Neuroscience, 6162 Moore Hall, Dartmouth College, Hanover, 03755-3547, New Hampshire, USA
Michael S. Gazzaniga
You can also search for this author in PubMed Google Scholar
Ethics declarations
Competing interests.
The author declares no competing financial interests.
Related links
Further information.
Center for Cognitive Neuroscience
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Gazzaniga, M. Forty-five years of split-brain research and still going strong. Nat Rev Neurosci 6 , 653–659 (2005). https://doi.org/10.1038/nrn1723
Download citation
Issue Date : 01 August 2005
DOI : https://doi.org/10.1038/nrn1723

Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
- Xiaomeng Su
- Nina Skjæret-Maroni
Journal of NeuroEngineering and Rehabilitation (2024)
- Zhaonan Chen
Nature Communications (2023)
Impact of corpus callosum integrity on functional interhemispheric connectivity and cognition in healthy subjects
- Michele Porcu
- Luigi Cocco
Brain Imaging and Behavior (2023)
Impaired neurovascular coupling and cognitive deficits in anti-N-methyl-D-aspartate receptor encephalitis
- Yuanyuan Guo
- Yanghua Tian
Brain Imaging and Behavior (2022)
- Elizabeth Jefferies
Brain Structure and Function (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Split-Brain: What We Know Now and Why This is Important for Understanding Consciousness
- Open access
- Published: 12 May 2020
- Volume 30 , pages 224–233, ( 2020 )
Cite this article
You have full access to this open access article

- Edward H. F. de Haan 1 ,
- Paul M. Corballis 2 ,
- Steven A. Hillyard 3 ,
- Carlo A. Marzi 4 ,
- Anil Seth 5 ,
- Victor A. F. Lamme 1 ,
- Lukas Volz 6 ,
- Mara Fabri 7 ,
- Elizabeth Schechter 8 ,
- Tim Bayne 9 ,
- Michael Corballis 2 &
- Yair Pinto 1
53k Accesses
44 Citations
76 Altmetric
Explore all metrics
Recently, the discussion regarding the consequences of cutting the corpus callosum (“split-brain”) has regained momentum (Corballis, Corballis, Berlucchi, & Marzi, Brain , 141 (6), e46, 2018 ; Pinto et al., Brain, 140 (5), 1231–1237, 2017a ; Pinto, Lamme, & de Haan, Brain, 140 (11), e68, 2017 ; Volz & Gazzaniga, Brain , 140 (7), 2051–2060, 2017 ; Volz, Hillyard, Miller, & Gazzaniga, Brain , 141 (3), e15, 2018 ). This collective review paper aims to summarize the empirical common ground, to delineate the different interpretations, and to identify the remaining questions. In short, callosotomy leads to a broad breakdown of functional integration ranging from perception to attention. However, the breakdown is not absolute as several processes, such as action control, seem to remain unified. Disagreement exists about the responsible mechanisms for this remaining unity. The main issue concerns the first-person perspective of a split-brain patient. Does a split-brain harbor a split consciousness or is consciousness unified? The current consensus is that the body of evidence is insufficient to answer this question, and different suggestions are made with respect to how future studies might address this paucity. In addition, it is suggested that the answers might not be a simple yes or no but that intermediate conceptualizations need to be considered.
Similar content being viewed by others
Split-brain syndrome and extended perceptual consciousness.

Split-Brain Human Subjects

What Is the Unconscious? A Novel Taxonomy of Psychoanalytic, Psychological, Neuroscientific, and Philosophical Concepts
Avoid common mistakes on your manuscript.
Introduction
The term “split-brain” refers to patients in whom the corpus callosum has been cut for the alleviation of medically intractable epilepsy. Since the earliest reports by van Wagenen and Herren ( 1940 ) and Akelaitis ( 1941 , 1943 ) on the repercussions of a split-brain, two narratives have emerged. First and foremost is the functional description, pioneered by Gazzaniga, Sperry and colleagues (Gazzaniga, Bogen, & Sperry, 1963 ; Gazzaniga, Bogen, & Sperry, 1962 ; Sperry, 1968 ), in which the intricacies, the exceptions, the effects of different testing conditions, and the experimental confounds have been delineated by decades of extensive research with a relatively small group of patients (Berlucchi, Aglioti, Marzi, & Tassinari, 1995 ; Corballis, 1994 ; Corballis et al., 2010 ; Corballis, 2003 ; Luck, Hillyard, Mangun, & Gazzaniga, 1989 ; Pinto, Lamme, & de Haan, 2017b ; Volz, Hillyard, Miller, & Gazzaniga, 2018 ). It is important to note that even in this small group there are differences. In some patients all commissures were severed (“commissurotomy”), in others only the corpus callosum was cut (“callosotomy”) and some patients fall somewhere in between these two boundaries. Now, the search term “split-brain” results in a total of 2848 publications in the database of the Web-of-Science and 29,300 hits on Google Scholar, indicating a wealth of detailed information. The other depiction of split-brain patients entails the first-person perspective of the split-brain. In other words, “what is it like” to be a split-brain patient? It is especially this perspective that has captured the attention of the general press, popular science books and basic textbooks. By its nature, this second narrative lacks the detail of the functional description of the phenomenon, but it captures the intriguing question of how unity of consciousness is related to brain processes. Dominant in this description is the idea that in a split-brain each hemisphere houses an independent conscious agent. This notion, and particularly the concept of an isolated but conscious right hemisphere that is unable to express its feelings, desires or thinking due a lack of language, has captured the imagination (Gazzaniga, 2014 ).
It is important to clarify what we mean by unified consciousness. Here, we use the term in the sense of “subject unity” as defined by Bayne (Bayne & Chalmers, 2003 ; Bayne, 2008 ; Bayne, 2010 ). Subject unity is present if all the experiences generated in a system belong to one subject. In other words, if a system contains a first person perspective, then subject unity is preserved if that system only contains one such perspective, but subject unity is absent if the system contains multiple first person perspectives. Thus, in our definition of conscious unity, consciousness in a split-brain is split if each cortical hemisphere houses an independent conscious agent.
The view that consciousness is split in a split-brain has significantly impacted cognitive neuroscience at large. For instance, currently dominant theories about conscious awareness - the Integrated Information Theory (Tononi, 2005 ; Tononi, 2004 ) and the Global Neuronal Workspace Theory (Dehaene & Naccache, 2001 ; Dehaene, Kerszberg, & Changeux, 1998 ) - may be critically dependent on the validity of this view. Both theories imply that without massive communication between different subsystems, for instance cortical hemispheres, independent conscious agents arise. Thus, if the split consciousness view is invalid, these theories may be critically challenged.
The idea of split consciousness in a split-brain had its origin in the early split-brain studies (Gazzaniga, 1967 ; Gazzaniga, 1975 ; Gazzaniga et al., 1962 ; Sperry, 1968 ). These studies tested patients primarily in the two perceptual domains where processing is largely restricted to the contralateral hemisphere, that is vision and touch. In these early studies, stimuli, for instance objects, that were presented to the left hemisphere either physically in the right hand or as an image in the right visual half-field, could be readily named (as the left hemisphere is dominant for language) or pointed out with the right hand (which is controlled by the left hemisphere). The patient’s behavior became intriguing when the stimuli were presented in the left visual field or in the left hand. Now the patient, or at least the verbal left hemisphere, appeared oblivious to the fact that there had been a stimulus at all but was nevertheless able to select the correct object from an array of alternatives presented to the left hand or the left visual half-field (see Fig. 1 ). In a particularly dramatic recorded demonstration, the famous patient “Joe” was able to draw a cowboy hat with his left hand in response to the word “Texas” presented in his left visual half field. His commentary (produced by the verbal left hemisphere) showed a complete absence of insight into why his left hand had drawn this cowboy hat. Another astonishing example involved the same patient. MacKay and MacKay ( 1982 ) flashed a digit in the left visual field and trained the patient to play a version of ‘20 questions’ across hemispheres. The left hemisphere guessed the answer vocally, and the right hemisphere provided responses by pointing ‘up’ (meaning ‘guess a higher number’) or ‘down’ with the left hand. In this way the patient managed to vocalize the right answer. This suggests two independent conscious agents communicating with each other (one steering the left hand, the other agent controlling vocal expressions). However, note that an alternative interpretation is possible. Perhaps the patient knows the answer but finds it hard to vocalize. The ‘20 questions’ then simply help him in finding the correct vocalization.
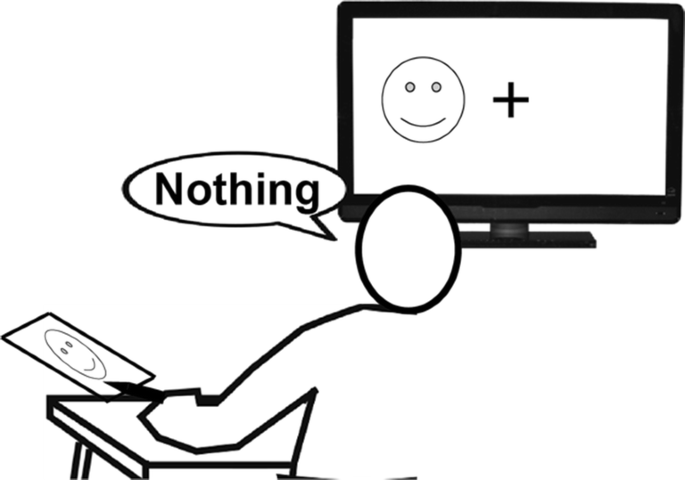
One of the most well-known split-brain findings is that the patient claims verbally not to have seen the stimulus in the left visual field, yet indicates the identity of it with their left hand. This suggests that the left hemisphere (controlling verbal output) is blind to the left visual field, while the right hemisphere (controlling the left hand) does perceive it
Thus, these early observations suggested that there is no meaningful communication between the two hemispheres in split-brain patients. This led to the hypothesis that there might be two separate conscious agents, a left hemisphere that is able to talk to us and can explain what it sees and feels, and a mute right hemisphere that cannot communicate in language but that can nevertheless show that it has perceived and recognized objects and words. However, over time this view has eroded somewhat due to several anomalies (even right from the start) that may challenge this view.
Common Ground
An early observation, suggesting some remaining unification concerned what Joe Bogen called the “social ordinariness” of split-brain patients. Apart from a number of anecdotal incidents in the subacute phase following the surgery, these patients seem to behave in a socially ordinary manner and they report feeling unchanged after the operation (Bogen, Fisher, & Vogel, 1965 ; Pinto et al., 2017a ; R. W. Sperry, 1968 ; R. Sperry, 1984 ), although their extra-experiment behavior has not been systematically observed in great detail (Schechter, 2018 ). While the right hemisphere appears to be better at recognizing familiarity from faces, self-face recognition, that is the ability to realise immediately that a presented photograph represents you, appears to be available equally to both hemispheres in a split-brain patient (Uddin et al., 2008 ; Uddin, 2011 ). Thus, it seems unlikely that a mute but conscious right hemisphere would not have made itself known one way or the other. Thus, right from the start a paradox arose. The controlled lab tests suggested that consciousness is split in split-brain patients. Yet, everyday experiences of the patient and their close ones suggests that only one person exists in a split-brain. Additionally, it has been suggested that the two separate consciousnesses-hypothesis presumes that in the intact brain (before surgery) both hemispheres were conscious but connected via the corpus callosum, and they only became dissociable due to the operation. This casts doubt on the viability of the two consciousness view.
Crucially, the lab tests themselves were not always supportive of the split-consciousness view. Multiple experimental results showed that capacity for communication between the hemispheres varies both across patients and across tasks. For instance, a central observation in split-brain patients concerns the inability to compare visual stimuli across the midline. In other words, when one stimulus is presented to the left visual hemifield and the other to the right hemifield, the patient cannot accurately indicate whether both stimuli are the same or not, although they can do so when both stimuli are presented within one visual field. This is consistent with the notion that each hemisphere independently perceives the contralateral visual field, and that an intact corpus callosum is necessary for integration. Although there are indeed many examples of split-brain patients who are incapable of comparing stimuli across the midline, prominent examples can also be found of patients who can compare stimuli across the midline (Johnson, 1984 ; but see Seymour, Reuter-Lorenz, & Gazzaniga, 1994 ). This points to an important problem in the field, namely, individual differences. One aspect that may be important for individual differences is handedness differences. Variations in handedness may lead to differences in language capabilities in the right hemisphere, and could even underly differences in inter-hemispheric integration.
Moreover, under certain circumstances nearly all tested split-brain patients seem able to compare, or integrate, particular types of stimuli across the two visual half-fields (see Fig. 2 ). An early demonstration of across hemifields integration is the study by Eviatar and Zaidel ( 1994 ). They showed that split-brain patients could accurately indicate the identity and shape of upper- and lower-case letters in either hemifield, regardless of with which hand they responded, for instance accurately identifying the letter A in the left visual field with the right hand. Yet these patients were mostly unable to compare these same stimuli across visual fields. In another experiment, two tilted lines were presented with a gap between them. The lines were positioned in such a way that extending them across the gap would either cause the lines to coincide or to run in parallel. When split-brain patients indicate whether the lines are parallel or coincident, they are highly accurate, even when both line segments are located in different half-fields (Corballis, 1995 ; Pinto, de Haan, Lamme, & Fabri, n.d. ; Sergent, 1987 ; Trevarthen & Sperry, 1973 ). Another example of visual integration across the midline involves apparent motion. When two dots are presented in succession at a short distance (2 to 14 visual degrees), split-brain patients are able to accurately indicate whether the dots create apparent motion, or that they were presented simultaneously or with delays too long to provoke apparent motion. Critically, they are able to do so even when one dot appears in the left visual field, and the other in the right visual field (Knapen et al., 2012 ; Naikar & Corballis, 1996 ; Ramachandran, Cronin-Golomb, & Myers, 1986 ). Clearly, under specific conditions, there is meaningful communication between the two hemispheres in the absence of the corpus callosum.
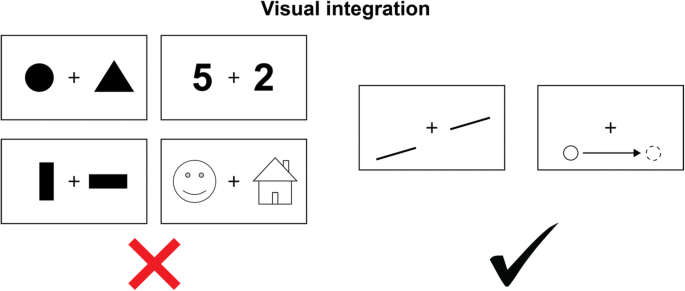
Although most split-brain patients cannot compare visual features such as shape and object identity across the midline, other features, such as good continuation of lines, and apparent motion, are integrated without a corpus callosum
Another observation that suggests some form of unity across the two visual half fields concerns detection and localization of stimulation, for instance, a brief flash (see, for example, an early study on the response times to light flashes with the ipsi- or contralateral hand: Clarke & Zaidel, 1989 ). Several investigations (Corballis, Corballis, Fabri, Paggi, & Manzoni, 2005 ; Pinto et al., 2017a ; Trevarthen & Sperry, 1973 ) have demonstrated convincingly that split-brain patients can accurately report the presence and location of stimuli for any position in the whole visual field, with either hand, and even verbally. Accurate detection and localization appears to be possible for all patients and all stimuli (different shapes, figures, equiluminant stimuli) tested so far. Thus, when patients in earlier studies said that they saw “nothing” when a stimulus was presented in the left visual half-field, they may have meant that they could see it but that they could not identify or retrieve the name of the object.
Other findings point to a crucial difference between the degree of lateralization of visual-perceptual processing and producing overt responses. Perception appears to be more split, while responding remains largely unified. Whether a stimulus appears in the left or the right visual hemifield strongly impacts performance of split-brain patients. However, response type (left hand, right hand or verbally) seems to have a much smaller, or no effect at all. For instance, Pinto et al., 2017a ) found that the split-brain patient was much better at matching pictures to sample stimuli in the left visual field. Yet, for the exact same stimuli matching pictures to verbal labels was vastly superior when the stimuli appeared in the right visual field. Crucially, response type did not play any role. The patient was better in matching pictures to sample for stimuli in the left visual field, even if they responded verbally or with the right hand. Similarly, Levy, Trevarthen, and Sperry ( 1972 ) presented split-brain patients with chimeric or composite faces, that is, one half-face in each visual field. Subsequently the patient either matched the chimeric face to sample, or attached a verbal label to it. Verbal matching was mostly based on the half-face in the right visual field, while matching to sample was mainly driven by the half-face in the left visual field. But crucially, the latter was the case, independent of whether the patient responded with the left or the right hand.
Thus, it seems that in split-brain patients perceptual processing is largely split, yet response selection and action control appear to be unified under certain conditions. This, by itself, does not prove whether a split-brain houses one or two conscious agents. One explanation could be that the split-brain houses two agents, each having their own experiences, who synchronize their behavioral output through various means. Another possible explanation is that a split-brain houses one agent who experiences an unintegrated stream of information who controls the entire body, comparable to watching a movie where sight and sound are out-of-sync. At any rate, these findings challenge the previously mentioned classic split-brain description, which is still found in reviews and text books (Gray, 2002 ; Wolman, 2012 ). In this classic characterization the patient indicates that they saw nothing when a stimulus appeared in the left visual field. Yet, to their own verbal surprise, the left hand correctly draws the stimulus. The aforementioned examples of unity in action control suggests that these effects may depend on the type and complexity of the response that is required.
Interpretations
There are three, not-mutually exclusive, hypotheses concerning the mechanisms involved in, seemingly, preserved unity in the split-brain. The first notion is that information is transferred subcortically. The second idea is that ipsilateral motor control underlies unity in action control. The third idea claims that information transfer is based on varies forms of inter-hemispheric collaboration, including subtle behavioral cues. The first proposal (Corballis Corballis, Berlucchi, & Marzi, 2018 ; de Haan et al., 2019 ; Pinto, Lamme, & de Haan, 2017b ; Pinto et al., 2017a ; Savazzi et al., 2007 ; Mancuso, Uddin, Nani, Costa, & Cauda, 2019 ) suggests that the multitude of subcortical connections that are spared during surgery are responsible for the transfer of information. As was initially pointed out by Trevarthen ( 1968 ) and Trevarthen and Sperry ( 1973 ) and recently stressed by Pinto, de Haan, and Lamme ( 2017a ) and Corballis et al. ( 2018 ), there are many commissures (white matter tracts that connect homologous structures on both sides of the central nervous system) and decussations (bundles that connect different structures on both sides) that link nuclei that are known to be involved in perceptual processing. The importance of these commisural connections for transferring visual information in split-brain patients has been highlighted by Trevarthen and Sperry ( 1973 ). Moreover, the role of these connections in a split-brain has recently been demonstrated by bilateral fMRI activations in the first somatosensory cortex, after unilateral stimulation of trunk midline touch receptors (Fabri et al., 2006 ) and in the second somatic sensory area after unilateral stimulation of hand pain receptors (Fabri, Polonara, Quattrini, & Salvolini, 2002 ). Uddin and colleagues used low-frequency BOLD fMRI resting state imaging to investigate functional connectivity between the two hemispheres in a patient in whom all major cerebral commissures had been cut (Uddin et al., 2008 ). Compared to control subjects, the patient’s interhemispheric correlation scores fell within the normal range for at least two symmetrical regions. In addition, Nomi and colleagues suggested that split-brain patients might rely particularly on dorsal and ventral pontine decussations of the cortico-cerebellar interhemispheric pathways as evidenced by increased fractional anisotropy (FA) on diffusion weighted imaging (Nomi, Marshall, Zaidel, Biswal, Castellanos, Dick, Uddin & Mooshagian, 2019). Interhemispheric exchange of information also seems to occur in the domain of taste sensitivity, activation of primary gustatory cortex in the fronto-parietal operculum was reported in both hemispheres after unilateral gustatory stimulation of the tongue receptors (Mascioli, Berlucchi, Pierpaoli, Salvolini, Barbaresi, Fabri, & Polonara, 2015 ). Note that patients may differ with respect to how many of these connections have been cut, and this might also explain some of the individual variance among patients. Moreover, in all patients subcortical structures remain intact. For instance, the superior colliculus is known to integrate visual information from both hemispheres and project information to both hemispheres (Meredith & Stein, 1986 ; Comoli et al., 2003 ). Such structures may support attentional networks, and may enable the right hemisphere to attend to the entire visual field. In turn, attentional unity could help in unifying cognitive and motor control, which may subserve ipsilateral motor control.
The second point concerns the ipsilateral innervation of the arms. Manual action is not strictly lateralized, and the proximal (but not the distal) parts of the arm are controlled bilaterally, although the ipsilateral contribution remains undetermined. This could explain why split-brain patients may respond equally well with both hands in certain experimental conditions (Corballis, 1995 ; Gazzaniga, Bogen, & Sperry, 1967 ; Pinto, de Haan, & Lamme, 2017a ). First, there is substantial evidence that bilateral cortical activations can be observed during unilateral limb movements in healthy subjects. In addition, ipsilesional motor problems in arm control have been observed in patients with unilateral cortical injuries, and finally there is evidence from electrocorticography with implanted electrodes for localization of epileptic foci showing similar spatial and spectral encoding of contralateral and ipsilateral limb kinematics (Bundy, Szrama, Pahwa, & Leuthardt, 2018 ). While these observations argue convincingly for a role in action control by the ipsilateral hemisphere, they do not prove that a hemisphere on it’s own can purposefully control the movements of the ipsilateral hand. Thus, the role of ipsilateral arm-hand control in explaining split-brain findings is currently not settled.
The third hypothesis argues that in addition to whatever direct neural communication may exist between the hemispheres, they may inform one another via strategic cross-cueing processes (Volz & Gazzaniga, 2017 ; Volz et al., 2018 ). The split-brain patients underwent surgery many years prior to testing, and the separated perceptual systems have had ample time to learn how to compensate for the lack of commissural connections. For example, subtle cues may be given by minimal movements of the eyes or facial muscles, which might not even be visible to an external observer but are capable of encoding, for example, the location of a stimulus for the hemisphere that did not “see” it. A cross-cueing mechanism might also allow one hemisphere to convey to the other which one of a limited set of known items had been shown (Gazzaniga & Hillyard, 1971 ; Gazzaniga, 2013 ).
Finally, it is possible to entertain combinations of the different explanations. For instance, it is conceivable that in the subacute phase following split-brain surgery the hemispheres are ineffective in communicating with each other. During this initial phase, phenomena such as an “alien hand” - that is a hand moving outside conscious control of the (verbal) person - may be present. In the ensuing period, the patients may have learned to utilize the information that is exchanged via subcortical connections, ipsilateral motor control or cross-cueing to coordinate the processing of the two hemispheres. In such a way, the patient may counteract some of the effects of losing the corpus callosum.
What do We Need to Know?
This paper aims to contribute to the agenda for the next decade of split-brain research. Full split-brain surgery is rare these days, and it is important that we try to answer the central questions while these patients are still available for study. In order to examine the variations between patients it would be useful to test as many of the available patients as possible with the same tests.
One important goal is to map out precisely how much functionality and information is still integrated across hemispheres in the split-brain, and what the underlying principles are. For instance, in some cases the two hemispheres seem to carry out sensory-motor tasks, such as visual search, independently from one another (Arguin et al., 2000 ; Franz, Eliassen, Ivry, & Gazzaniga, 1996 ; Hazeltine, Weinstein, & Ivry, 2008 ; Luck, Hillyard, Mangun, & Gazzaniga, 1994 ; Luck et al., 1989 ), while in other cases functions such as attentional blink, or attentional cueing, seem to be integrated across hemispheres (Giesbrecht & Kingstone, 2004 ; Holtzman, Volpe, & Gazzaniga, 1984 ; Holtzman, Sidtis, Volpe, Wilson, & Gazzaniga, 1981 ; Pashler et al., 1994 ; Ptito, Brisson, Dell’Acqua, Lassonde, & Jolicœur, 2009 ). An important challenge is to unveil why some cognitive functions can be carried out independently in the separated hemispheres while other functions engage both hemispheres. Furthermore, it is now clear that accurate detection and localization is possible across the whole visual field, and there is some evidence that even more information concerning visual images can be transferred between hemispheres. Although we have some understanding of what types of information can be transferred in the visual domain, our knowledge base in the somatosensory domain is much more limited. This is probably due to a bias throughout cognitive neuroscience and psychology, leading to a strong focus on vision in split-brain research. It is important to collect converging evidence by investigating the somatosensory system which is also strongly lateralized. Note that in somatosensory processing transfer between hemispheres (about 80% correct for the bimanual conditions) has been observed for basic same-different matching of real objects (Fabri, Del Pesce et al., 2005 ).
Another important goal is to obtain a more detailed description of the perceptual, cognitive and linguistic capabilities of the disconnected right hemisphere. For understanding unity of mind, two capabilities specifically are crucial. First, experiments investigating aspects of the conscious mind often go beyond simple visual processing, and future studies will thus critically depend on testing high-level cognitive abilities of both hemispheres. Specifically, language abilities, crucial for understanding questions and instructions, will likely play a pivotal role. Thus, the first question is to what extent the right hemisphere is capable of language processing. Note that complicated instructions (Gazzaniga, Smylie, Baynes, Hirst, & McCleary, 1984 ; Pinto et al., 2017a ; Zaidel, 1983 ), for instance relating to mental imagery (Johnson, Corballis, & Gazzaniga, 2001 ; Kosslyn, Holtzman, Farah, & Gazzaniga, 1985 ; Sergent & Corballis, 1990 ), seem to be well within the reach of the right hemisphere. Moreover, right hemisphere language capabilities seem to improve over time (Gazzaniga, Volpe, Smylie, Wilson, & LeDoux, 1979 ; Gazzaniga et al., 1996 ). Longitudinal language tests (for instance with a Token test: De Renzi & Vignolo, 1962 ) would further illuminate the extent of right hemisphere language processing.
Second, unveiling to what extent each hemisphere is capable of subserving consciousness at all seems relevant for unity of mind as well. If the disconnected right hemisphere can produce full-blown consciousness, then questions regarding unity of mind are clearly more pertinent then if the right hemisphere only produces minimal amounts of consciousness. Right hemisphere consciousness can be studied through novel neural paradigms (Bekinschtein et al., 2009 ; Casali et al., 2013 ; Pitts, Metzler, & Hillyard, 2014 ; Shafto & Pitts, 2015 ). For instance, Bekinschtein et al. employed EEG to measure if the brain detected irregularities (as indicated by an event-related potential [ERP] signal called the P3) in different states of consciousness. They found that when consciousness was reduced, local irregularities were still detected - for instance after three high auditory tones a low tone evoked a P3. However, global irregularities - several times a low tone followed three high tones, then on the critical trial three high tones were followed by another high tone - did not evoke a P3 when consciousness was reduced. Crucially, when consciousness was unimpaired both local and global irregularities evoked a P3 response. Right hemisphere consciousness may also be studied in other patient groups where interhemispheric communication is hampered. One particularly interesting group are post-hemispherotomy patients (Lew, 2014 ). These patients have been surgically treated to disconnect an entire hemisphere (usually for intractable epilepsy), but unlike hemispherectomy patients the disconnected hemisphere remains in place in the cranium and remains vascularized.
Clearly, the central question, whether each hemisphere supports an independent conscious agent, is not settled yet. Novel paradigms in this respect could lead to progress. For instance, a pivotal question is whether each hemisphere makes its own decisions independent of the other hemisphere. If each hemisphere produces its own autonomous conscious agent then this should be the case. That is, if two agents are asked to freely choose a random number, then the odds that they consistently pick the same number are small. And vice versa, if each hemisphere makes its own conscious decisions, independent of the other hemisphere, then this seems to rule out unity of mind. Note that each hemisphere making its own decisions is different from information processing occurring independently per hemisphere. Unconscious information processing is almost certainly split across hemispheres in a split-brain. However, this does not prove that consciousness is split or unified. Even in a healthy brain, where consciousness is unified, many unconscious processes run independently, and in parallel.
One way to tackle the central question is by having the hemispheres respond to questions in parallel. Overt behavior most likely does not allow for this, due to bilateral motor control processes sketched earlier. However, perhaps parallel responding is possible if the hemispheres produce covert responses. For instance, the patient could be asked to pick one of four options and indicate their choice by carrying out certain content-specific mental imagery tasks. This imagery can then be decoded in parallel from each hemisphere using neuroimaging techniques (see Owen et al., 2006 for a similar approach with vegetative state patients). If each hemisphere harbors an autonomous conscious agent, then it is highly unlikely that the two hemispheres will consistently make the same choices. Thus, if the choices are uncorrelated across hemispheres, then this may critically challenge the unified mind view.
Another way to tackle the question of unified consciousness in the split-brain is to employ ERPs as markers of concurrent conscious processing in the left and right hemispheres. For instance, in one study (Kutas, Hillyard, Volpe, & Gazzaniga, 1990 ) visual targets were presented either separately to the left or right visual field or to both visual fields simultaneously. It was found that the P300 - a signal possibly reflecting conscious processing of a visual target (Dehaene & Changeux, 2011 ; Dehaene, Charles, King, & Marti, 2014 ; Salti, Bar-Haim, & Lamy, 2012 ) - was reduced for bilateral targets. This suggests some type of integration of conscious processing. Studies employing ERPs may indicate whether conscious processing is unified, while unconscious processing is split, which would be suggestive of unified consciousness.
Conclusions
In summary, the pivotal issue in split-brain research is whether dividing the brain divides consciousness. That is, do we find evidence for the existence of one, or two conscious agents in a split-brain? Note that intermediate results may be found. Perhaps some measures indicate unified consciousness while others do not. This would then provoke further interesting questions on the unity of consciousness. What are the crucial measures for unity of consciousness? If intermediate results are found, more unconventional possibilities should be entertained as well. For instance, although difficult to fathom, some philosophers have suggested that a split-brain does not contain one or two observers, but a non-whole number of conscious agents (Nagel, 1971 ; Perry, 2009 ), for instance one and a half first-person perspectives. If evidence for this position is found, then its implications would stretch beyond split-brain patients. It would suggest that our intuitions on the indivisibility of the experiential self may be mistaken. One way to think of this is as with the difference between conscious and unconscious processing. Perhaps this is not a dichotomous distinction, but a continuum between more or less conscious. Similarly, perhaps the existence of a first-person perspective is not dichotomous, but gradual as well. Another possibility is that a split-brain does contain a whole number of conscious agents, but that consciousness across these agents is only partially unified. That is, the agents share some conscious experiences and decisions, but not all (Lockwood, 1989 ; Schechter, 2014 ; Schechter, 2018 ; Schechter, 2013 ). Finally, another way to look at this is in terms of ‘dissociation’, as in depersonalization (Phillips et al., 2001 ; Sierra et al., 2002 ). Perhaps the number of agents is not altered, but the agent feels depersonalized in some situations, and therefore no longer feels that they control the actions, or even experience the information, that has just occurred in their brain.
New findings on the unity of consciousness in a split-brain could fundamentally impact currently dominant consciousness theories. Global Neuronal Workspace Theory (Dehaene & Naccache, 2001 ; Dehaene et al., 1998 ) asserts that consciousness arises when information that is processed in unconscious (or preconscious) modules is broadcast to a central ‘workspace’, primarily residing in frontal regions of the brain. Although not very explicit on the unity of consciousness in split-brain patients, Global Neuronal Workspace Theory seems to endorse the split consciousness idea, given that each hemisphere has its own prefrontal hub, enabling broadcasting of whatever information is processed in that hemisphere.
Integrated Information Theory (Tononi, 2005 ; Tononi, 2004 ) has specifically addressed the issue of split brain (for instance in Tononi, 2004 ). Integrated Information Theory asserts that ‘phi’, a measure of how integrated information is, determines the level of consciousness. The higher phi, the more conscious a system is. Moreover, local maxima in phi correspond to conscious agents. If in a system all subsystems are highly interconnected, then phi is highest for the system as a whole, and local maxima are absent. Thus, such a system produces only one conscious agent. However, if subsystems only exchange minimal amounts of information, then phi per subsystem is higher than phi for the system as whole. In such a case each subsystem creates its own conscious agent. In a split-brain, connectedness, that is integration of information, is much higher within than across hemispheres. Therefore, according to Integrated Information Theory consciousness should be split in a split-brain.
Recurrent Processing theory (Lamme & Roelfsema, 2000 ; Lamme, 2004 ; Lamme, 2010 ) argues for the independence of consciousness from attention, access, or report. This theory has addressed the issue of report specifically, making the case that consciousness and reportability, whether verbal or manual, should be viewed as entirely independent (Lamme, 2006 ; Tsuchiya, Wilke, Frässle, & Lamme, 2015 ). Crucially, this theory states that even in the normal mind, ‘islands’ of unattended yet conscious information reside (Lamme, 2006 ). In these cases, all the information, although functionally unintegrated, is nonetheless experienced by the same mind. Support for this view comes from findings in multiple object tracking (Pinto, Howe, Cohen, & Horowitz, 2010 ; Pinto, Scholte, & Lamme, 2012 ; Pylyshyn & Storm, 1988 ). Here, evidence indicates that when moving objects in two visual hemifields are tracked, attention is split (Howe, Cohen, Pinto, & Horowitz, 2010 ) and each hemisphere processes the relevant information in the contralateral visual field independently of the other (Alvarez & Cavanagh, 2005 ; Cavanagh & Alvarez, 2005 ; Drew, Mance, Horowitz, Wolfe, & Vogel, 2014 ). That is, the left hemisphere only tracks the right visual field and vice versa. Yet, although the visual information is not integrated across hemispheres, from a first person perspective, it seems clear that the subject experiences all moving objects across the entire visual field. Another example of the dissociation between consciousness and reportability is the so-called partial report paradigm (Pinto et al., 2017b ; Pinto, Sligte, Shapiro, & Lamme, 2013 ; Sligte, Scholte, & Lamme, 2008 ; Sperling, 1960 ). In these paradigms subjects seem to remember more than they can report. Thus, reportability and consciousness are dissociated. Perhaps in split-brain patients this dissociation is simply more pronounced. That is, consciousness remains unified, but reportability has become more dissociated, thereby inducing the appearance of two independent agents. In sum, according to the Recurrent Processing theory, integration of information is not needed for a unified mind, implying that the mind may remain unified when the brain is split. Thus, different theories of consciousness have different predictions on the unity of mind in split-brain patients, and await the results of further investigation into this intriguing phenomenon.
Akelaitis, A. J. (1941). Studies on the corpus callosum: II. The higher visual functions in each homonymous field following complete section of the corpus callosum. Archives of Neurology and Psychiatry , 45 (5), 788–796.
Google Scholar
Akelaitis, A. J. (1943). Studies on the corpus callosum: VII. Study of language functions (tactile and visual lexia and graphia) unilaterally following section of the corpus callosum. Journal of Neuropathology & Experimental Neurology , 2 (3), 226–262.
Alvarez, G. A., & Cavanagh, P. (2005). Independent resources for attentional tracking in the left and right visual hemifields. Psychological Science , 16 (8), 637–643.
PubMed Google Scholar
Arguin, M., Lassonde, M., Quattrini, A., Del Pesce, M., Foschi, N., & Papo, I. (2000). Divided visuo-spatial attention systems with total and anterior callosotomy. Neuropsychologia , 38 (3), 283–291.
CAS PubMed Google Scholar
Bayne, T. (2008). The unity of consciousness and the split-brain syndrome. The Journal of Philosophy , 105 (6), 277–300.
Bayne, T. (2010). The unity of consciousness. Oxford: Oxford University Press.
Bayne, T., & Chalmers, D. J. (2003). What is the unity of consciousness? In A. Cleeremans (Ed.), The unity of consciousness: Binding, integration and dissociation (pp. 23–58). Oxford, England: Oxford University press.
Bekinschtein, T. A., Dehaene, S., Rohaut, B., Tadel, F., Cohen, L., & Naccache, L. (2009). Neural signature of the conscious processing of auditory regularities. Proceedings of the National Academy of Sciences of the United States of America , 106 (5), 1672–1677.
CAS PubMed PubMed Central Google Scholar
Berlucchi, G., Aglioti, S., Marzi, C., & Tassinari, G. (1995). Corpus callosum and simple visuomotor integration. Neuropsychologia , 33 (8), 923–936.
Bogen, J. E., Fisher, E., & Vogel, P. (1965). Cerebral commissurotomy: A second case report. Jama , 194 (12), 1328–1329.
Bundy, D. T., Szrama, N., Pahwa, M., & Leuthardt, E. C. (2018). Unilateral, 3D arm movement kinematics are encoded in ipsilateral human cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience , 38 (47), 10042–10056.
CAS Google Scholar
Casali, A. G., Gosseries, O., Rosanova, M., Boly, M., Sarasso, S., Casali, K. R., et al. (2013). A theoretically based index of consciousness independent of sensory processing and behavior. Science Translational Medicine , 5 (198), 198ra105.
Cavanagh, P., & Alvarez, G. A. (2005). Tracking multiple targets with multifocal attention. Trends in Cognitive Sciences , 9 (7), 349–354.
Clarke, J. M., & Zaidel, E. (1989). Simple reaction times to lateralized light flashes. Varieties of interhemispheric communication routes. Brain , 112 , 849–870.
Comoli, E., Coizet, V., Boyes, J., Bolam, J. P., Canteras, N. S., Quirk, R. H., … Redgrave, P. (2003). A direct projection from superior colliculus to substantia nigra for detecting salient visual events. Nature Neuroscience , 6 (9), 974–980.
Corballis, M. C. (1994). Can commissurotomized subjects compare digits between the visual fields? Neuropsychologia , 32 (12), 1475–1486.
Corballis, M. C. (1995). Visual integration in the split brain. Neuropsychologia , 33 (8), 937–959.
Corballis, M. C., Birse, K., Paggi, A., Manzoni, T., Pierpaoli, C., & Fabri, M. (2010). Mirror-image discrimination and reversal in the disconnected hemispheres. Neuropsychologia , 48 (6), 1664–1669.
Corballis, M. C., Corballis, P. M., Berlucchi, G., & Marzi, C. A. (2018). Perceptual unity in the split brain: The role of subcortical connections. Brain , 141 (6), e46.
Corballis, M. C., Corballis, P. M., Fabri, M., Paggi, A., & Manzoni, T. (2005). Now you see it, now you don't: Variable hemineglect in a commissurotomized man. Cognitive Brain Research , 25 (2), 521–530.
Corballis, P. M. (2003). Visuospatial processing and the right-hemisphere interpreter. Brain and Cognition , 53 (2), 171–176.
de Haan, E. H., Fabri, M., Dijkerman, H. C., Foschi, N., Lattanzi, S., & Pinto, Y. (2019). Unified tactile detection and localisation in split-brain patients. Cortex , 124 , 217–223.
De Renzi, A., & Vignolo, L. A. (1962). Token test: A sensitive test to detect receptive disturbances in aphasics. Brain: A Journal of Neurology, 85 , 665–678. https://doi.org/10.1093/brain/85.4.665
Dehaene, S., & Changeux, J. (2011). Experimental and theoretical approaches to conscious processing. Neuron , 70 (2), 200–227.
Dehaene, S., Charles, L., King, J., & Marti, S. (2014). Toward a computational theory of conscious processing. Current Opinion in Neurobiology , 25 , 76–84.
Dehaene, S., Kerszberg, M., & Changeux, J. P. (1998). A neuronal model of a global workspace in effortful cognitive tasks. Proceedings of the National Academy of Sciences of the United States of America , 95 (24), 14529–14534.
Dehaene, S., & Naccache, L. (2001). Towards a cognitive neuroscience of consciousness: Basic evidence and a workspace framework. Cognition , 79 (1), 1–37.
Drew, T., Mance, I., Horowitz, T. S., Wolfe, J. M., & Vogel, E. K. (2014). A soft handoff of attention between cerebral hemispheres. Current Biology , 24 (10), 1133–1137.
Eviatar, Z., & Zaidel, E. (1994). Letter matching within and between the disconnected hemispheres. Brain and Cognition , 25 (1), 128–137.
Fabri, M., Del Pesce, M., Paggi, A., Polonara, G., Bartolini, M., Salvolini, U., et al. (2005). Contribution of posterior corpus callosum to the interhemispheric transfer of tactile information. Cognitive Brain Research , 24 (1), 73–80.
Fabri, M., Polonara, G., Mascioli, G., Paggi, A., Salvolini, U., & Manzoni, T. (2006). Contribution of the corpus callosum to bilateral representation of the trunk midline in the human brain: An fMRI study of callosotomized patients. European Journal of Neuroscience , 23 (11), 3139–3148.
Mascioli, G., Berlucchi, G., Pierpaoli, C., Salvolini, U., Barbaresi, P., Fabri, M. and Polonara, G. (2015) Functional MRI cortical activations from unilateral tactile-taste stimulations of the tongue. Physiology and Behavior, 151 , 221–229. https://doi.org/10.1016/j.physbeh.2015.07.031 .
Fabri, M., Polonara, G., Quattrini, A., & Salvolini, U. (2002). Mechanical noxious stimuli cause bilateral activation of parietal operculum in callosotomized subjects. Cerebral Cortex , 12 (4), 446–451.
Franz, E. A., Eliassen, J. C., Ivry, R. B., & Gazzaniga, M. S. (1996). Dissociation of spatial and temporal coupling in the bimanual movements of callosotomy patients. Psychological Science , 7 (5), 306–310.
Gazzaniga, M., Bogen, J., & Sperry, R. (1967). Dyspraxia following division of the cerebral commissures. Archives of Neurology , 16 (6), 606–612.
Gazzaniga, M. S. (1967). The split brain in man. Scientific American , 217 (2), 24–29.
Gazzaniga, M. S. (1975). Review of the split brain. Journal of Neurology , 209 (2), 75–79.
Gazzaniga, M. S. (2013). Shifting gears: Seeking new approaches for mind/brain mechanisms. Annual Review of Psychology , 64 (1), 1–20.
Gazzaniga, M. S. (2014). The split-brain: Rooting consciousness in biology. Proceedings of the National Academy of Sciences of the United States of America , 111 (51), 18093–18094.
Gazzaniga, M. S., Bogen, J. E., & Sperry, R. W. (1962). Some functional effects of sectioning the cerebral commissures in man. Proceedings of the National Academy of Sciences of the United States of America , 48 , 1765–1769.
Gazzaniga, M. S., Bogen, J. E., & Sperry, R. W. (1963). Laterality effects in somesthesis following cerebral commissurotomy in man. Neuropsychologia , 1 (3), 209–215.
Gazzaniga, M. S., Eliassen, J. C., Nisenson, L., Wessinger, C. M., Fendrich, R., & Baynes, K. (1996). Collaboration between the hemispheres of a callosotomy patient: Emerging right hemisphere speech and the left hemisphere interpreter. Brain , 119 (4), 1255–1262.
Gazzaniga, M. S., & Hillyard, S. A. (1971). Language and speech capacity of the right hemisphere. Neuropsychologia , 9 (3), 273–280.
Gazzaniga, M. S., Smylie, C. S., Baynes, K., Hirst, W., & McCleary, C. (1984). Profiles of right hemisphere language and speech following brain bisection. Brain and Language , 22 (2), 206–220.
Gazzaniga, M. S., Volpe, B., Smylie, C. S., Wilson, D. H., & LeDoux, J. E. (1979). Plasticity in speech organization following commissurotomy. Brain , 102 (4), 805–815.
Giesbrecht, B., & Kingstone, A. (2004). Right hemisphere involvement in the attentional blink: Evidence from a split-brain patient. Brain and Cognition , 55 (2), 303–306.
Gray, P. (2002). The nervous system. Psychology (pp. 160-161) worth publishers, New York, NY, USA.
Hazeltine, E., Weinstein, A., & Ivry, R. B. (2008). Parallel response selection after callosotomy. Journal of Cognitive Neuroscience , 20 (3), 526–540.
Holtzman, J. D., Sidtis, J. J., Volpe, B. T., Wilson, D. H., & Gazzaniga, M. S. (1981). Dissociation of spatial information for stimulus localization and the control of attention. Brain : A Journal of Neurology , 104 (Pt 4), 861–872.
Holtzman, J. D., Volpe, B. T., & Gazzaniga, M. S. (1984). Spatial orientation following commisural section. In R. Parasuram & D. R. Davies (Eds.), Varieties of attention (pp. 375–394). Orlando (FL): Academic press.
Howe, P. D., Cohen, M. A., Pinto, Y., & Horowitz, T. S. (2010). Distinguishing between parallel and serial accounts of multiple object tracking. Journal of Vision , 10 (8), 11–11.
Johnson, L. E. (1984). Bilateral visual cross-integration by human forebrain commissurotomy subjects. Neuropsychologia , 22 (2), 167–175.
Johnson, S. H., Corballis, P. M., & Gazzaniga, M. S. (2001). Within grasp but out of reach: Evidence for a double dissociation between imagined hand and arm movements in the left cerebral hemisphere. Neuropsychologia , 39 (1), 36–50.
Knapen, T., Pinto, Y., Scholte, H. S., Lamme, V., Foschi, N., & Fabri, M. (2012). Perception of apparent motion in a split-brain observer. Journal of Vision , 12 (9), 548–548.
Kosslyn, S. M., Holtzman, J. D., Farah, M. J., & Gazzaniga, M. S. (1985). A computational analysis of mental image generation: Evidence from functional dissociations in split-brain patients. Journal of Experimental Psychology: General , 114 (3), 311–341.
Kutas, M., Hillyard, S. A., Volpe, B. T., & Gazzaniga, M. S. (1990). Late positive event-related potentials after commissural section in humans. Journal of Cognitive Neuroscience , 2 (3), 258–271.
Lamme, V. A. (2004). Separate neural definitions of visual consciousness and visual attention; a case for phenomenal awareness. Neural Networks , 17 (5), 861–872.
Lamme, V. A. (2006). Towards a true neural stance on consciousness. Trends in Cognitive Sciences , 10 (11), 494–501.
Lamme, V. A. (2010). How neuroscience will change our view on consciousness. Cognitive Neuroscience , 1 (3), 204–220.
Lamme, V. A., & Roelfsema, P. R. (2000). The distinct modes of vision offered by feedforward and recurrent processing. Trends in Neurosciences , 23 (11), 571–579.
Levy, J., Trevarthen, C., & Sperry, R. W. (1972). Perception of bilateral chimeric figures following hemispheric deconnexion. Brain : A Journal of Neurology , 95 (1), 61–78.
Lew, S. M. (2014). Hemispherectomy in the treatment of seizures: A review. Translational Pediatrics , 3 (3), 208–217.
PubMed PubMed Central Google Scholar
Lockwood, M. (1989). Mind, brain and the quantum: The compound 'I' . Oxford: Basil Blackwell.
Luck, S. J., Hillyard, S. A., Mangun, G. R., & Gazzaniga, M. S. (1989). Independent hemispheric attentional systems mediate visual-search in split-brain patients. Nature , 342 (6249), 543–545.
Luck, S. J., Hillyard, S. A., Mangun, G. R., & Gazzaniga, M. S. (1994). Independent attentional scanning in the separated hemispheres of split-brain patients. Journal of Cognitive Neuroscience , 6 (1), 84–91.
MacKay, D. M., & MacKay, V. (1982). Explicit dialogue between left and right half-systems of split brains. Nature , 295 (5851), 690–691.
Mancuso, L., Uddin, L. Q., Nani, A., Costa, T., & Cauda, F. (2019). Brain functional connectivity in individuals with callosotomy and agenesis of the corpus callosum: A systematic review. Neuroscience & Biobehavioral Reviews , 105 , 231–248.
Meredith, M. A., & Stein, B. E. (1986). Visual, auditory, and somatosensory convergence on cells in superior colliculus results in multisensory integration. Journal of Neurophysiology , 56 (3), 640–662.
Nagel, T. (1971). Brain bisection and the unity of consciousness. Synthese , 22 (3–4), 396–413.
Naikar, N., & Corballis, M. C. (1996). Perception of apparent motion across the retinal midline following commissurotomy. Neuropsychologia , 34 (4), 297–309.
Owen, A. M., Coleman, M. R., Boly, M., Davis, M. H., Laureys, S., & Pickard, J. D. (2006). Detecting awareness in the vegetative state. Science (New York, N.Y.) , 313 (5792), 1402.
Pashler, H., Luck, S. J., Hillyard, S. A., Mangun, G. R., OʼBrien, S., & Gazzaniga, M. S. (1994). Sequential operation of disconnected cerebral hemispheres in split-brain patients. Neuroreport, 5 , 2381–2384.
Perry, J. (2009). Diminished and fractured selves. Personal Identity and Fractured Selves , 129–162.
Phillips, M. L., Medford, N., Senior, C., Bullmore, E. T., Suckling, J., Brammer, M. J., … David, A. S. (2001). Depersonalization disorder: Thinking without feeling. Psychiatry Research: Neuroimaging , 108 (3), 145–160.
Pinto, Y., de Haan, E. H. F., & Lamme, V. A. F. (2017a). The split-brain phenomenon revisited: A single conscious agent with split perception. Trends in Cognitive Sciences , 21 (11), 835–851.
Pinto, Y., de Haan, E. H. F., Lamme, V. A. F., & Fabri, M. (n.d.). Intact gestalt and gist processing in split-brain patients.
Pinto, Y., Howe, P. D., Cohen, M. A., & Horowitz, T. S. (2010). The more often you see an object, the easier it becomes to track it. Journal of Vision , 10 (10), 4–4.
Pinto, Y., Scholte, H. S., & Lamme, V. (2012). Tracking moving identities: After attending the right location, the identity does not come for free. PLoS One , 7 (8), e42929.
Pinto, Y., Sligte, I. G., Shapiro, K. L., & Lamme, V. A. F. (2013). Fragile visual short-term memory is an object-based and location-specific store. Psychonomic Bulletin & Review , 20 (4), 732–739.
Pinto, Y., Lamme, V. A., & de Haan, E. H. (2017). Cross-cueing cannot explain unified control in split-brain patients. Brain , 140 (11), e68.
Pinto, Y., Neville, D. A., Otten, M., Corballis, P. M., Lamme, V. A., de Haan, E. H., et al. (2017a). Split brain: Divided perception but undivided consciousness. Brain , 140 (5), 1231–1237.
Pinto, Y., Vandenbroucke, A. R., Otten, M., Sligte, I. G., Seth, A. K., & Lamme, V. A. F. (2017b). Conscious visual memory with minimal attention. Journal of Experimental Psychology: General , 146 (2), 214–226.
Pitts, M. A., Metzler, S., & Hillyard, S. A. (2014). Isolating neural correlates of conscious perception from neural correlates of reporting one's perception. Frontiers in Psychology , 5 , 1078.
Ptito, A., Brisson, B., Dell’Acqua, R., Lassonde, M., & Jolicœur, P. (2009). The attentional blink within and across the hemispheres: Evidence from a patient with a complete section of the corpus callosum. Biological Psychology , 82 (1), 64–69.
Pylyshyn, Z. W., & Storm, R. W. (1988). Tracking multiple independent targets: Evidence for a parallel tracking mechanism. Spatial Vision , 3 (3), 179–197.
Ramachandran, V., Cronin-Golomb, A., & Myers, J. (1986). Perception of apparent motion by commissurotomy patients. Nature , 320 (6060), 358–359.
Salti, M., Bar-Haim, Y., & Lamy, D. (2012). The P3 component of the ERP reflects conscious perception, not confidence. Consciousness and Cognition , 21 (2), 961–968.
Savazzi, S., Fabri, M., Rubboli, G., Paggi, A., Tassinari, C. A., & Marzi, C. A. (2007). Interhemispheric transfer following callosotomy in humans: Role of the superior colliculus. Neuropsychologia , 45 (11), 2417–2427.
Schechter, E. (2013). The unity of consciousness: Subjects and objectivity. Philosophical Studies , 165 (2), 671–692.
Schechter, E. (2014). Partial unity of consciousness. In D. J. Bennett & C. S. Hill (Eds.), Sensory integration and the unity of consciousness (pp. 347–374). Cambridge, MA: MIT press.
Schechter, E. (2018). Self-consciousness and "split" brains: The minds' I . Oxford, UK: Oxford University Press.
Sergent, J. (1987). A new look at the human split brain. Brain , 110 (5), 1375–1392.
Sergent, J., & Corballis, M. C. (1990). Generation of multipart images in the disconnected cerebral hemispheres. Bulletin of the Psychonomic Society , 28 (4), 309–311.
Seymour, S. E., Reuter-Lorenz, P. A., & Gazzaniga, M. S. (1994). The disconnection syndrome - basic findings reaffirmed. Brain , 117 (1), 105–115.
Shafto, J. P., & Pitts, M. A. (2015). Neural signatures of conscious face perception in an inattentional blindness paradigm. Journal of Neuroscience , 35 (31), 10940–10948.
Sierra, M., Senior, C., Dalton, J., McDonough, M., Bond, A., Phillips, M. L., … David, A. S. (2002). Autonomic response in depersonalization disorder. Archives of General Psychiatry , 59 (9), 833–838.
Sligte, I. G., Scholte, H. S., & Lamme, V. A. F. (2008). Are there multiple visual short-term memory stores? PLoS One , 3 (2), e1699.
Sperling, G. (1960). The information available in brief visual presentations. Psychological Monographs: General and Applied , 74 (11), 1–29.
Sperry, R. (1984). Consciousness, personal identity and the divided brain. Neuropsychologia , 22 (6), 661–673.
Sperry, R. W. (1968). Hemisphere deconnection and unity in conscious awareness. American Psychologist , 23 (10), 723–733.
Tononi, G. (2004). An information integration theory of consciousness. BMC Neuroscience , 5 (1), 42.
Tononi, G. (2005). Consciousness, information integration, and the brain. Progress in Brain Research , 150 , 109–126.
Trevarthen, C., & Sperry, R. W. (1973). Perceptual unity of the ambient visual system in human commissurotomy patients. Brain , 96 (3), 547–570.
Trevarthen, C. B. (1968). Two mechanisms of vision in primates. Psychologische Forschung , 31 (4), 299–337.
Tsuchiya, N., Wilke, M., Frässle, S., & Lamme, V. A. (2015). No-report paradigms: Extracting the true neural correlates of consciousness. Trends in Cognitive Sciences , 19 (12), 757–770.
Uddin, L. Q. (2011). Brain connectivity and the self: The case of cerebral disconnection. Consciousness and Cognition , 20 (1), 94–98.
Uddin, L. Q., Mooshagian, E., Zaidel, E., Scheres, A., Margulies, D. S., Kelly, A. M. C., … Milham, M. P. (2008). Residual functional connectivity in the split-brain revealed with resting-state fMRI. Neuroreport , 19 (7), 703–709.
Van Wagenen, W. P., & Herren, R. Y. (1940). Surgical division of commissural pathways in the corpus callosum: Relation to spread of an epileptic attack. Archives of Neurology and Psychiatry , 44 (4), 740.
Volz, L. J., & Gazzaniga, M. S. (2017). Interaction in isolation: 50 years of insights from split-brain research. Brain , 140 (7), 2051–2060.
Volz, L. J., Hillyard, S. A., Miller, M. B., & Gazzaniga, M. S. (2018). Unifying control over the body: Consciousness and cross-cueing in split-brain patients. Brain , 141 (3), e15.
Wolman, D. (2012). A tale of two halves. Nature , 483 (7389), 260–263.
Zaidel, E. (1983). A response to Gazzaniga: Language in the right hemisphere, convergent perspectives. American Psychologist, 38 (5), 542–546. https://doi.org/10.1037/0003-066X.38.5.542
Download references
Acknowledgements
This work was supported by an Advanced Investigator Grant by the European Research Council (ERC grant FAB4V (#339374) to EdH and a Templeton grant (ID# 61382, "Towards understanding a unified mind") to YP and VL.
Author information
Authors and affiliations.
Department of Psychology, University of Amsterdam, Amsterdam, the Netherlands
Edward H. F. de Haan, Victor A. F. Lamme & Yair Pinto
School of Psychology, University of Auckland, Auckland, New Zealand
Paul M. Corballis & Michael Corballis
School of Health Sciences, University of California Dan Diego, La Jolla, CA, USA
Steven A. Hillyard
School of Medicine and Surgery, University of Verona, Verona, Italy
Carlo A. Marzi
Sackler Centre for Consciousness Science, Sussex University, Brighton, UK
Klinik für Neurologie, Universitätsklinikum Köln, Kerpener Str, 62, Köln, Germany
Dipartimento di Medicina Sperimentale e Clinica, Via Tronto 10/A, 60020, Ancona, Italy
Department of Philosophy, Washington University, St. Louis, MO, USA
Elizabeth Schechter
Department of Philosophy, Monash University, Melbourne, Australia
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Edward H. F. de Haan .
Ethics declarations
Competing interests.
The authors report no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
de Haan, E.H.F., Corballis, P.M., Hillyard, S.A. et al. Split-Brain: What We Know Now and Why This is Important for Understanding Consciousness. Neuropsychol Rev 30 , 224–233 (2020). https://doi.org/10.1007/s11065-020-09439-3
Download citation
Received : 05 November 2019
Accepted : 16 April 2020
Published : 12 May 2020
Issue Date : June 2020
DOI : https://doi.org/10.1007/s11065-020-09439-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Split-brain
- Lateralization
- Visual perception
- Consciousness agents
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
Through studies of this group, neuroscientists now know that the healthy brain can look like two markedly different machines, cabled together and exchanging a torrent of data. But when the...
Sperry severed the corpus callosum in cats and monkeys to study the function of each side of the brain. He found that if hemispheres were not connected, they functioned independently of one another, which he called a split-brain. The split-brain enabled animals to memorize double the information.
In our current studies we reproduced the classic finding that split-brain patients are unable to integrate visual information across the two visual half-fields. However, we also investigated systematically to what extent performance depends on where a stimulus appears.
Beyond the insights into the functional specialization of the hemispheres and how much hemispheric integration is necessary to produce behaviour, the split-brain also offers a unique perspective on our understanding of brain lesions.
Split-brain research refers to research and insights garnered from studying patients who have had their corpus callosum, a bundle of fibers connecting the two hemispheres of the brain, severed, in most cases to treat severe epilepsy.
In the intervening decades, split-brain research has continued to illuminate many areas of neuroscience. Not only have we and others learned even more about how the hemispheres differ, but we also have been able to understand how they communicate once they have been separated. Split-brain studies have shed light on
This new evidence from neuroimaging is, therefore, causing us to rethink earlier conclusions about interhemispheric interaction and recruitment that were drawn from split-brain testing alone.
The book presents a review of Gazzaniga’s work on the human split brain—the theory that the right and left hemispheres of the brain can act independently, have diff erent strengths, and separate agendas.
Does a split-brain harbor a split consciousness or is consciousness unified? The current consensus is that the body of evidence is insufficient to answer this question, and different suggestions are made with respect to how future studies might address this paucity.
split-brain studies. These experiments opened up new frontiers in brain research and gave rise to much of what we know about hemispheric specialization and integration. The latest developments in split-brain research build on the groundwork laid by those early studies. Split-brain methodology, on its own and in conjunction with neuroimaging, has