- Search by keyword
- Search by citation
Page 1 of 90

Curcumin-mediated enhancement of lung barrier function in rats with high-altitude-associated acute lung injury via inhibition of inflammatory response
Exposure to a hypobaric hypoxic environment at high altitudes can lead to lung injury. In this study, we aimed to determine whether curcumin (Cur) could improve lung barrier function and protect against high-a...
- View Full Text

Single-cell transcriptomics reveals e-cigarette vapor-induced airway epithelial remodeling and injury
In recent years, e-cigarettes have been used as alternatives among adult smokers. However, the impact of e-cigarette use on human bronchial epithelial (HBE) cells remains controversial.
Reliability of crackles in fibrotic interstitial lung disease: a prospective, longitudinal study
Although crackles on chest auscultation represent a fundamental component of the diagnostic suspect for fibrotic interstitial lung disease (ILD), their reliability has not been properly studied. We assessed th...
Localized delivery of therapeutics impact laryngeal mechanics, local inflammatory response, and respiratory microbiome following upper airway intubation injury in swine
Laryngeal injury associated with traumatic or prolonged intubation may lead to voice, swallow, and airway complications. The interplay between inflammation and microbial population shifts induced by intubation...
Extracorporeal membrane oxygenation as a bridge to lung transplantation: 5-year outcomes and bridge to decision in a large, older cohort
Extracorporeal membrane oxygenation (ECMO) as a bridge to lung transplantation (BTT) has expanded considerably, though evidence-based selection criteria and long-term outcome data are lacking. The purpose of t...
Differential proteins from EVs identification based on tandem mass tags analysis and effect of Treg-derived EVs on T-lymphocytes in COPD patients
Chronic obstructive pulmonary disease (COPD) is a widespread respiratory disease. This study examines extracellular vesicles (EVs) and proteins contained in EVs in COPD.
Chronic diesel exhaust exposure induced pulmonary vascular remodeling a potential trajectory for traffic related pulmonary hypertension
As one of the most common traffic-related pollutants, diesel exhaust (DE) confers high risk for cardiovascular and respiratory diseases. However, its impact on pulmonary vessels is still unclear.
Role of β-adrenergic signaling and the NLRP3 inflammasome in chronic intermittent hypoxia-induced murine lung cancer progression
Obstructive sleep apnea (OSA), characterized by chronic intermittent hypoxia (CIH), is a prevalent condition that has been associated with various forms of cancer. Although some clinical studies suggest a pote...
CXCL10 predicts autoimmune features and a favorable clinical course in patients with IIP: post hoc analysis of a prospective and multicenter cohort study
Interstitial pneumonia with autoimmune features (IPAF), which does not meet any of the criteria for connective tissue diseases (CTD), has been attracting an attention in patients with idiopathic interstitial p...
It takes two peroxisome proliferator-activated receptors (PPAR-β/δ and PPAR-γ) to tango idiopathic pulmonary fibrosis
Idiopathic pulmonary fibrosis (IPF) is characterized by aberrant lung epithelial phenotypes, fibroblast activation, and increased extracellular matrix deposition. Transforming growth factor-beta (TGF-β)1-induc...
Associations between vitamin D status and biomarkers linked with inflammation in patients with asthma: a systematic review and meta-analysis of interventional and observational studies
Numerous studies indicate an association between vitamin D status and inflammatory biomarkers in patients with asthma, but findings are inconsistent. This review aims to summarize the relationship between seru...
Metabolomic characterization of COVID-19 survivors in Jilin province
The COVID-19 pandemic has escalated into a severe global public health crisis, with persistent sequelae observed in some patients post-discharge. However, metabolomic characterization of the reconvalescent rem...
An algorithm for discontinuing mechanical ventilation in boys with x-linked myotubular myopathy after positive response to gene therapy: the ASPIRO experience
X-linked myotubular myopathy (XLMTM) is a rare, life-threatening congenital myopathy. Most (80%) children with XLMTM have profound muscle weakness and hypotonia at birth resulting in severe respiratory insuffi...
Serum tumor markers: potential indicators for occult lymph node metastasis in clinical T 1 − 2 N 0 M 0 small cell lung cancer patients
In their letter-to-the-editor entitled “Letter to the Editor: Incidence rate of occult lymph node metastasis in clinical T 1 − 2 N 0 M 0 small cell lung cancer patients and radiomic prediction based on contrast-enhanc...
The original article that this is related to.
Reference values for exhaled nitric oxide in healthy children aged 6–18 years in China: a cross-sectional, multicenter clinical study
The reference values of eNO have certain differences among people of different countries and races. We aimed to obtain the reference value of eNO in healthy children and adolescents (6–18 years old) in China a...
Effect of low climate impact vs. high climate impact inhalers for patients with asthma and COPD-a nationwide cohort analysis
Chronic obstructive pulmonary disease (COPD) and asthma can be treated with inhaled corticosteroids (ICS) delivered by low climate impact inhalers (dry powder inhalers) or high climate impact inhalers (pressur...
Tobacco and COPD: presenting the World Health Organization (WHO) Tobacco Knowledge Summary
The WHO recently published a Tobacco Knowledge Summary (TKS) synthesizing current evidence on tobacco and COPD, aiming to raise awareness among a broad audience of health care professionals. Furthermore, it ca...
Novel approach to exploring protease activity and targets in HIV-associated obstructive lung disease using combined proteomic-peptidomic analysis
Obstructive lung disease (OLD) is increasingly prevalent among persons living with HIV (PLWH). However, the role of proteases in HIV-associated OLD remains unclear.
Using machine learning for early detection of chronic obstructive pulmonary disease: a narrative review
Chronic obstructive pulmonary disease (COPD) is a prevalent respiratory disease and ranks third in global mortality rates, imposing a significant burden on patients and society. This review looks at recent res...
The association between cumulative exposure to PM 2.5 and DNA methylation measured using methyl-capture sequencing among COPD patients
Particulate matter with a diameter of < 2.5 μm (PM 2.5 ) influences gene regulation via DNA methylation; however, its precise mechanism of action remains unclear. Thus, this study aimed to examine the connection be...
Is YouTube a sufficient source of information on Sarcoidosis?
The internet is a common source of health information for patients and caregivers. To date, content and information quality of YouTube videos on sarcoidosis has not been studied. The aim of our study was to in...
Risk of incident chronic obstructive pulmonary disease during longitudinal follow-up in patients with nontuberculous mycobacterial pulmonary disease
The Global Initiative for Chronic Obstructive Lung Disease 2023 revision proposed that chronic obstructive pulmonary disease (COPD) has various etiologies including infections (COPD-I), such as tuberculosis an...
Characteristics of lung resistance and elastance associated with tracheal stenosis and intrapulmonary airway narrowing in ex vivo sheep lungs
Understanding the characteristics of pulmonary resistance and elastance in relation to the location of airway narrowing, e.g., tracheal stenosis vs. intrapulmonary airway obstruction, will help us understand l...
Mask side-effects are related to gender in long-term CPAP: results from the InterfaceVent real-life study
Over the past three decades, our understanding of sleep apnea in women has advanced, revealing disparities in pathophysiology, diagnosis, and treatment compared to men. However, no real-life study to date has ...
Recombinant thrombomodulin and recombinant antithrombin attenuate pulmonary endothelial glycocalyx degradation and neutrophil extracellular trap formation in ventilator-induced lung injury in the context of endotoxemia
Vascular endothelial damage is involved in the development and exacerbation of ventilator-induced lung injury (VILI). Pulmonary endothelial glycocalyx and neutrophil extracellular traps (NETs) are endothelial ...
CT-based whole lung radiomics nomogram for identification of PRISm from non-COPD subjects
Preserved Ratio Impaired Spirometry (PRISm) is considered to be a precursor of chronic obstructive pulmonary disease. Radiomics nomogram can effectively identify the PRISm subjects from non-COPD subjects, espe...
Unraveling the Mfn2-Warburg effect nexus: a therapeutic strategy to combat pulmonary arterial hypertension arising from catch-up growth after IUGR
The interplay between intrauterine and early postnatal environments has been associated with an increased risk of cardiovascular diseases in adulthood, including pulmonary arterial hypertension (PAH). While em...
Employing a synergistic bioinformatics and machine learning framework to elucidate biomarkers associating asthma with pyrimidine metabolism genes
Asthma, a prevalent chronic inflammatory disorder, is shaped by a multifaceted interplay between genetic susceptibilities and environmental exposures. Despite strides in deciphering its pathophysiological land...
Ubiquitination of angiotensin-converting enzyme 2 contributes to the development of pulmonary arterial hypertension mediated by neural precursor cell–expressed developmentally down-regulated gene 4-Like
In this study, we investigated whether neural precursor cell–expressed developmentally down-regulated gene 4-like (NEDD4L) is the E3 enzyme of angiotensin-converting enzyme 2 (ACE2) and whether NEDD4L degrades...
The burden of cough in idiopathic pulmonary fibrosis and other interstitial lung diseases: a systematic evidence synthesis
Cough remains a persistent symptom in patients with idiopathic pulmonary fibrosis (IPF) and other interstitial lung diseases (ILDs). To inform future research, treatment and care models, we conducted the first...
Cobalt exposure and pulmonary function reduction in chronic obstructive pulmonary disease patients: the mediating role of club cell secretory protein
Cobalt (Co) is a metal which is widely used in the industrial production. The previous studies found the toxic effects of environmental Co exposure on multiple organs. However, the correlation of blood Co conc...
Surgery versus intrapleural fibrinolysis for management of complicated pleural infections: a systematic review and meta-analysis
Complicated pleural infection comprises of complex effusions and empyema. When tube thoracostomy is ineffective, treatment options include surgical drainage, deloculation and decortication or intrapleural fibr...
Transcriptomic analysis reveals distinct effects of cigarette smoke on murine airspace and bone-marrow derived macrophages
Chronic obstructive pulmonary disease (COPD) is an inflammatory airway disease characterized by emphysema and chronic bronchitis and a leading cause of mortality worldwide. COPD is commonly associated with sev...
Vitamin D ameliorates particulate matter induced mitochondrial damages and calcium dyshomeostasis in BEAS-2B human bronchial epithelial cells
Mitochondria is prone to oxidative damage by endogenous and exogenous sources of free radicals, including particulate matter (PM). Given the role of mitochondria in inflammatory disorders, such as asthma and c...
Impact of radiomics features, pulmonary emphysema score and muscle mass on the rate of pneumothorax and chest tube insertion in CT-guided lung biopsies
Iatrogenic pneumothorax is a relevant complication of computed tomography (CT)-guided percutaneous lung biopsy. The aim of the present study was to analyze the prognostic significance of texture analysis, emph...
Artificial intelligence in COPD CT images: identification, staging, and quantitation
Chronic obstructive pulmonary disease (COPD) stands as a significant global health challenge, with its intricate pathophysiological manifestations often demanding advanced diagnostic strategies. The recent app...
Assessment and monitoring of lung disease in patients with severe alpha 1 antitrypsin deficiency: a european delphi consensus of the EARCO group
Currently, there is conflicting information and guidance on the effective management of Alpha 1 Antitrypsin Deficiency (AATD). Establishing a consensus of assessment and disease management specific to AATD is ...
Distinctive field effects of smoking and lung cancer case-control status on bronchial basal cell growth and signaling
Basal cells (BCs) are bronchial progenitor/stem cells that can regenerate injured airway that, in smokers, may undergo malignant transformation. As a model for early stages of lung carcinogenesis, we set out t...
Levosimendan mediates the BMP/Smad axis through upregulation of circUSP34-targeted miR-1298 to alleviate pulmonary hypertension
Pulmonary hypertension (PH) is a long-term disease that impacts approximately 1% of the world’s population. Currently, levosimendan (Lev) is proposed for PH treatment. However, the mechanism of Lev in the trea...
Expression of human Interferon Regulatory Factor 3 (IRF-3) in alveolar macrophages relates to clinical and functional traits in COPD.
Chronic obstructive pulmonary disease (COPD) is a frequent cause of morbidity and mortality. Dysregulated and enhanced immune-inflammatory responses have been described in COPD. Recent data showed impaired imm...
Pulmonary adaptation to repeated poly(I:C) exposure is impaired in asthmatic mice: an observational study
While asthma exacerbations remain a major challenge in patient management, few animal models exist to explore the underlying mechanisms. Here, we established an animal model of asthma that can be used to study...
The role and mechanism of thrombospondin-4 in pulmonary arterial hypertension associated with congenital heart disease
Due to a special hemodynamic feature, pulmonary vascular disease in pulmonary arterial hypertension associated with congenital heart disease (PAH-CHD) has two stages: reversible and irreversible. So far, the m...
Epidemiology, ventilation management and outcomes of COVID–19 ARDS patients versus patients with ARDS due to pneumonia in the Pre–COVID era
Ventilation management may differ between COVID–19 ARDS (COVID–ARDS) patients and patients with pre–COVID ARDS (CLASSIC–ARDS); it is uncertain whether associations of ventilation management with outcomes for C...
Inhaled tea polyphenol-loaded nanoparticles coated with platelet membranes largely attenuate asthmatic inflammation
Tea polyphenols (TPs), prominent constituents of green tea, possess remarkable antioxidant and anti-inflammatory properties. However, their therapeutic potential is limited due to low absorption and poor bioav...
PARK2 as a susceptibility factor for nontuberculous mycobacterial pulmonary disease
The genetic signatures associated with the susceptibility to nontuberculous mycobacterial pulmonary disease (NTM-PD) are still unknown. In this study, we performed RNA sequencing to explore gene expression pro...
Integrated omics characterization reveals reduced cancer indicators and elevated inflammatory factors after thermal ablation in non-small cell lung cancer patients
Thermal ablation is a minimally invasive treatment for non-small cell lung cancer (NSCLC). Aside from causing an immediate direct tumour cell injury, the effects of thermal ablation on the internal microenviro...
The clinical impacts of lung microbiome in bronchiectasis with fixed airflow obstruction: a prospective cohort study
Airflow obstruction is a hallmark of disease severity and prognosis in bronchiectasis. The relationship between lung microbiota, airway inflammation, and outcomes in bronchiectasis with fixed airflow obstructi...
Proportion of confluent B-Lines predicts respiratory support in term infants shortly after birth
To develop and evaluate the predictive value of a simplified lung ultrasound (LUS) method for forecasting respiratory support in term infants.
Small airway dysfunction links asthma exacerbations with asthma control and health-related quality of life
Small airway dysfunction not only affects asthma control, but also has adverse effects on the psychological and/or social activities of asthma patients. However, few long-term observational studies have explor...
Distinct enterotypes and dysbiosis: unraveling gut microbiota in pulmonary and critical care medicine inpatients
The gut-lung axis, pivotal for respiratory health, is inadequately explored in pulmonary and critical care medicine (PCCM) inpatients.
- Editorial Board
- Manuscript editing services
- Instructions for Editors
- Contact Support for Editors
- Sign up for article alerts and news from this journal
- Follow us on Twitter
Annual Journal Metrics
Citation Impact 2023 Journal Impact Factor: 4.7 5-year Journal Impact Factor: 5.3 Source Normalized Impact per Paper (SNIP): 1.192 SCImago Journal Rank (SJR): 1.498
Speed 2023 Submission to first editorial decision (median days): 3 Submission to acceptance (median days): 105
Usage 2023 Downloads: 2,949,876 Altmetric mentions: 2,397
- More about our metrics
Respiratory Research
ISSN: 1465-993X
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 20 April 2023
Interstitial lung disease diagnosis and prognosis using an AI system integrating longitudinal data
- Xueyan Mei ORCID: orcid.org/0000-0001-7224-7318 1 ,
- Zelong Liu ORCID: orcid.org/0000-0001-6968-6467 1 ,
- Ayushi Singh 2 ,
- Marcia Lange ORCID: orcid.org/0000-0003-2700-2279 3 ,
- Priyanka Boddu 3 ,
- Jingqi Q. X. Gong 4 ,
- Justine Lee 2 ,
- Cody DeMarco 2 ,
- Chendi Cao 1 ,
- Samantha Platt ORCID: orcid.org/0000-0002-4637-8370 3 ,
- Ganesh Sivakumar 3 ,
- Benjamin Gross 3 ,
- Mingqian Huang 2 ,
- Joy Masseaux 2 ,
- Sakshi Dua 5 ,
- Adam Bernheim 2 ,
- Michael Chung ORCID: orcid.org/0000-0002-0976-4088 2 ,
- Timothy Deyer 6 , 7 ,
- Adam Jacobi 2 ,
- Maria Padilla 5 ,
- Zahi A. Fayad ORCID: orcid.org/0000-0002-3439-7347 1 , 2 &
- Yang Yang ORCID: orcid.org/0000-0002-2841-4243 1 , 2 , 8
Nature Communications volume 14 , Article number: 2272 ( 2023 ) Cite this article
9067 Accesses
16 Citations
16 Altmetric
Metrics details
- Computed tomography
- Medical research
- Respiratory tract diseases
For accurate diagnosis of interstitial lung disease (ILD), a consensus of radiologic, pathological, and clinical findings is vital. Management of ILD also requires thorough follow-up with computed tomography (CT) studies and lung function tests to assess disease progression, severity, and response to treatment. However, accurate classification of ILD subtypes can be challenging, especially for those not accustomed to reading chest CTs regularly. Dynamic models to predict patient survival rates based on longitudinal data are challenging to create due to disease complexity, variation, and irregular visit intervals. Here, we utilize RadImageNet pretrained models to diagnose five types of ILD with multimodal data and a transformer model to determine a patient’s 3-year survival rate. When clinical history and associated CT scans are available, the proposed deep learning system can help clinicians diagnose and classify ILD patients and, importantly, dynamically predict disease progression and prognosis.
Similar content being viewed by others
Longitudinal lung cancer prediction convolutional neural network model improves the classification of indeterminate pulmonary nodules
Deep radiomics-based survival prediction in patients with chronic obstructive pulmonary disease
Classification of Interstitial Lung Abnormality Patterns with an Ensemble of Deep Convolutional Neural Networks
Introduction.
Interstitial lung disease (ILD) refers to a group of more than 200 pulmonary conditions which can exhibit varying degrees of lung parenchymal fibrosis 1 . Obtaining a specific diagnosis in cases of ILD is essential to guide patient management and treatment. High-resolution computed tomography (HRCT) plays a significant role in accurately classifying the various subtypes of ILD. According to the American Thoracic Society (ATS) guidelines, accurate diagnosis of ILD subtypes requires a multidisciplinary assessment reviewing clinical history, HRCT, and pathology 2 . In addition, longitudinal monitoring with CT can assess the progression of Serial CT that can reveal changes in the extent of parenchymal architectural distortion, reticulation, bronchiectasis and honeycombing, allowing for the identification of progressive fibrotic disease which correlates with poorer survival. Mortality is often not feasible as an end-point for diseases with chronic progressive fibrosis (such as IPF); change or lack of change in disease extent on HRCT represents a potential means of assessing treatment response 3 .
In some cases, diagnosis and classification of ILD types via CT are relatively straightforward. In other cases, the imaging findings can overlap multiple ILD patterns or may have no identifiable pattern, and is thus subject to substantial inter- and intra-observer variation among radiologists 4 . Interpretation of these difficult exams is challenging and can depend on the expertize of the radiologist.
Prior studies have shown that deep learning can be used to recognize different ILDs on CT images 5 , including detecting abnormal interstitial patterns 6 , automatic assessment of the extent of systemic sclerosis-related ILD 7 , and differentiation between nonspecific interstitial pneumonia (NSIP) and usual interstitial pneumonia (UIP) 8 . However, literature regarding accurate deep learning-aided diagnosis of multiple ILD subtypes as well as prediction of survival rate is limited at this time. The purpose of our study is to develop an AI system that (1) can classify 5 different types of ILD based on initial chest CT scans and relevant clinical history as well as (2) monitor a patient’s disease progression.
For our study, we collected clinical information retrospectively through a chart review of electronic medical records. Clinical information included age, sex, history of current/former smoking, history of rheumatic disease, home oxygen requirement, history of occupational exposures, pulmonary function test (PFT) values (FEV1/FVC ratio, FEV1 value, DLCO percentage), presence of pulmonary hypertension based on echocardiography or right heart catheterization, and history of lung biopsy. Clinical history was collected longitudinally for every CT scan available for the patient through the course of their treatment to account for changes in exposures or other variables. We collected CT scans and the corresponding clinical history obtained at every clinical encounter. To further predict a patient’s 3-year survival rate, we included medications and other therapeutic information to clinical history (Fig. 1 and Fig. 2 ).
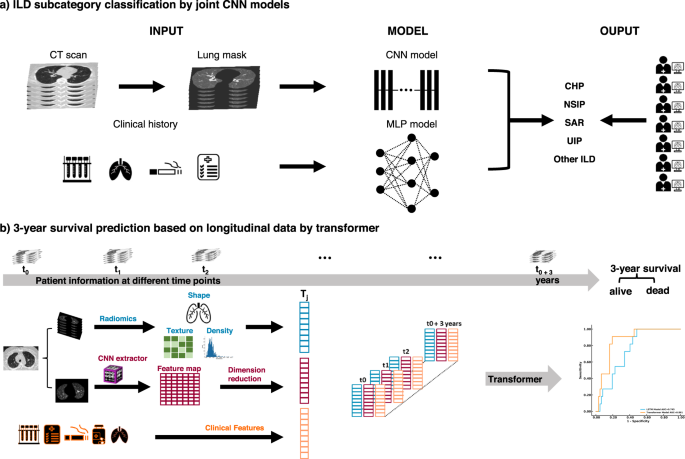
The ILD classification model was generated to predict the subtype of ILD for each patient based on CT scans of the chest and clinical information. A survival rate prediction AI model was generated based on the longitudinal data of each patient. a For the classification of ILD, we preprocessed CT scans to obtain the lung regions of each image. Then, we integrated the probability achieved by using a CNN model to study lung images and using an MLP model to study clinical information. Finally, we compared the ILD classification results from the joint AI model with human readers. b For the prediction of the 3-year survival rate, the patient information, including image features extracted via Radiomics and CNN model and clinical features, were collected during each visit and then used to generate a Transformer model to predict the risk of each patient.
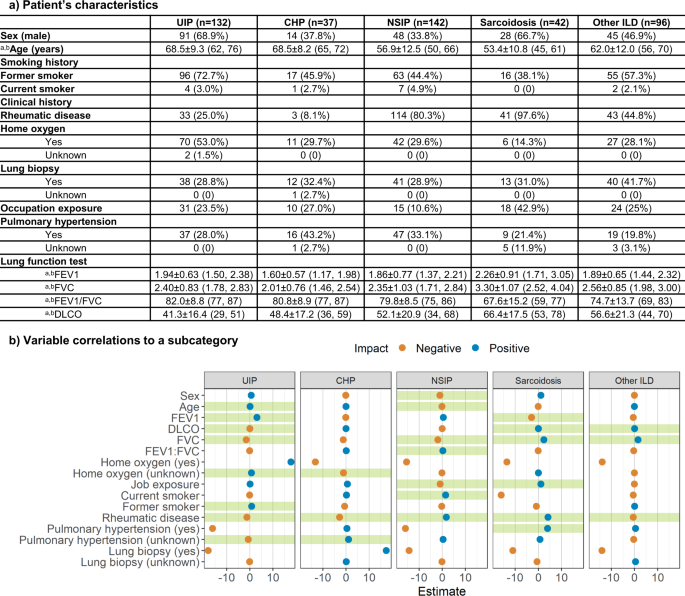
a Characteristics of patient’s clinical information for each ILD subtype. a Data in parentheses show interquartile range. b Indicates mean ± s.d. Data in parentheses shows the percentage of the population with the characteristic. b Correlations between clinical information and each ILD subcategory. The x-axis indicates the coefficient of each clinical variable evaluated by logistic regression. Green shades show a significant correlation.
For subtype classification, we first created a deep convolutional neural network (CNN) and a vision Transformer 9 (ViT) to learn image patterns of patients with ILD on the initial chest CT scan. We then used multilayer perceptron 10 (MLP), XGBoost 11 , and support vector machine 12 (SVM) classifiers to predict ILD subcategories based on clinical information. Finally, we developed a joint model integrating chest CT characteristics with associated clinical history to predict ILD subtypes. To predict a patient’s survival within 3 years from the initial visit, we created Transformer 13 and long-short-term memory 14 (LSTM) models to study longitudinal CT scans and longitudinal clinical information. The joint CNN model and the Transformer models showed the best scores on the validation set. Hereafter, the performance of the joint CNN and Transformer models is reported. The performances of other models can be found in Supplementary Figs. 2 – 6 .
The Mount Sinai Medical Center Research Registry for Interstitial Lung Disease (MSMC-ILD) was established in 2014. Patients enrolled in MSMC-ILD had a consensus diagnosis from radiology, pathology, and pulmonology. 449 patients with 1822 CT scans were collected between September 2014 and April 2021 from 230 centers in the United States. The patient population age ranged from 22–91 years (median 63, IQR 56-71), with 226 males and 223 females. All chest CT scans were obtained using a standard chest CT protocol and were reconstructed using multiple kernels and displayed with a lung window in axial view. A total of 132 patients (29.4%) were diagnosed with UIP, 37 patients (8.2%) with chronic hypersensitivity pneumonitis (CHP), 142 patients (31.6%) with NSIP, 42 patients (9.4%) with sarcoidosis and 96 patients (21.4%) with other various ILD. Of the 449 patients in the MSMC-ILD, 128 who had their initial scan and pulmonary function test performed at the Mount Sinai Hospital (MSH) were used as an external testing set. The remaining 321 patients were randomly split into a training set (80.4%, 258 cases with 78 UIP) and a validation set (19.6%, 63 cases with 20 UIP).
We performed a logistic regression with each ILD subcategory as the outcome and the clinical variables as the predictors to determine whether there existed a correlation between the type of ILD and the clinical history. Detailed descriptions and distributions of clinical history are reported in Fig. 2 . The logistic regression confirmed that age, FEV1, DLCO, FVC, home oxygen status, former smoking history, history of rheumatic disease, and history of pulmonary hypertension were strongly correlated to UIP ( p = 0.78). Home oxygen status, history of rheumatic disease, and history of pulmonary hypertension were significant features of CHP ( p = 0.91). Patient’s sex and age, FVC, FEV1/FVC ratio, occupational exposures, former smoking history, and history of rheumatic disease were strongly related to NSIP ( p = 0.096). Significant predictors of sarcoidosis were FEV1, DLCO, FVC, occupational exposures, history of rheumatic disease, and history of pulmonary hypertension ( p = 0.99). Finally, DLCO, FVC, and history of rheumatic disease were key features that correlated with other ILD ( p = 0.31).
We evaluated the AI models on the unseen external test set. The performance of the joint AI model was compared to seven readers who included a senior thoracic radiologist (STR) with 11 years of experience, two junior thoracic radiologists (JTR1 and JTR2) with 5 years of experience and 4 years of experience, respectively, a thoracic radiology fellow (TRF), two senior general radiologists (SGR1 and SGR2) with specialty in musculoskeletal and 10 years of experience and specialty in pediatric radiology and 15 years of experience respectively, and finally a senior pulmonologist (SP) with 10 years of experience. All readers were provided with the same deidentified lung CT scans and clinical information. The area under the receiver operating characteristic curve (AUROC), sensitivity, and specificity were calculated for each ILD category in our study. The performance and comparison of the AI model and human readers are reported in Fig. 3 . Comparisons between the joint model, STR, and SP were highlighted hereafter. Detailed performance of the other 5 human readers can be found in Supplementary Tables 1 – 3 .
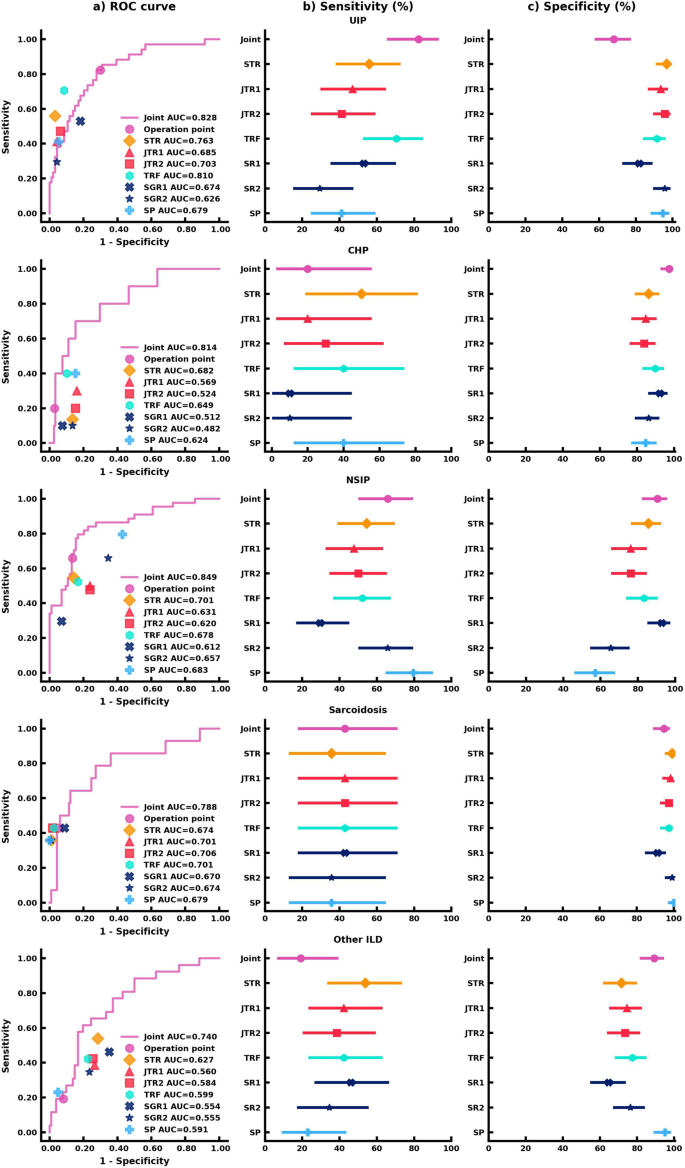
a AUC comparison between the joint AI model and human readers on the classification of each ILD subtype. The ( b ) Sensitivity analysis of the joint AI model and human readers’ results. The markers represent the sensitivity of the AI model and human readers on each ILD subtype, and the lines represent the confidence interval of sensitivity. c Specificity analysis of the joint AI model and human readers’ results. The markers represent the specificity of the AI model and human readers on each ILD subtype, and the lines represent the confidence interval of specificity. Each human reader was indicated with different markers. Sensitivity and specificity comparison were calculated via the exact Clopper-Pearson method to compute the 95% confidence interval (CI). In ( b , c ), data are presented as true sensitivity/specificity + /− 95% CI respectively.
For UIP classification, the joint model combining CT scans and clinical information had the highest sensitivity in comparison to all human readers, though the human readers had higher specificities. The joint model had a sensitivity of 82.4% (95% confidence interval (CI) 65.5%, 93.2%), an 68.1% specificity (95% CI 57.7%, 77.3%), and an AUROC of 0.828 (95% CI 0.748, 0.909). Importantly, the joint model outperformed the STR (55.9%; p < 0.05) and the SP (41.2%; p < 0.001) in sensitivity.
For CHP classification, the joint model displayed the highest specificity. The joint model achieved a 20.0% sensitivity (95% CI 2.5%, 55.6%), a 97.5% specificity (95% CI 92.8%, 99.5%), and an AUROC of 0.814 (95% CI 0.676, 0.951), which was equivalent to the STR and SP in sensitivity (50.0%, p = 0.38; 40.0%, p = 0.63) and significantly better in specificity as compared to both readers (86.4%, p < 0.01; 84.8%, p < 0.001).
The joint model was more sensitive and specific to classifying and diagnosing NSIP. The joint model achieved a 65.9% sensitivity (95% CI 50.1%, 79.5%), a 90.5% specificity (95% CI 82.1%, 95.8%), and an AUROC of 0.849 (95% CI 0.777, 0.922), which was comparable to the STR who had a 54.6% sensitivity (95% CI 38.9%, 69.6%; p = 0.33), an 85.7% specificity (95% CI 76.4%, 92.4%; p = 0.48), and an AUROC of 0.701 (95% CI 0.618, 0.785). The SP showed an equivalent 79.6% sensitivity (95% CI 64.7%, 90.2%; p = 0.11), but was outperformed in specificity (57.1%; 95% CI 45.9%, 67.9%; p < 0.001).
For the classification of sarcoidosis, the joint model and human readers had comparable sensitivities and specificities. The joint model achieved a 42.9% sensitivity (95% CI 17.7%, 71.1%), a 94.7% specificity (95% CI 88.9%, 98.0%), and an AUROC of 0.788 (95% CI 0.643, 0.933). The STR had a 35.7% sensitivity (95% CI 12.8%, 64.9%; p = 1), a 99.1% specificity (95% CI 95.2%, 100.0%; p = 0.13), and an AUROC of 0.674 (95% CI 0.544, 0.805). The SP had a 35.7% sensitivity (95% CI 12.8%, 64.9%; p = 1), a 100.0% specificity (95% CI 96.8%, 100.0%; p < 0.05), and an AUROC of 0.679 (95% CI 0.548, 0.809).
While human readers tended to be more sensitive than the joint model in classifying other ILD, the joint model was more specific. The joint model achieved a 19.2% sensitivity (95% CI 6.6%, 39.4%), an 89.2% specificity (95% CI 81.5%, 94.5%), and an AUROC of 0.740 (95% CI 0.636, 0.844). The STR had a 53.9% sensitivity (95% CI 33.4%, 73.4%; p < 0.05), a 71.6% specificity (95% CI 61.8%, 80.1%; p < 0.001), and an AUROC of 0.627 (95% CI 0.520, 0.734). The SP had a 23.1% sensitivity (95% CI 9.0%, 43.7%; p = 1), a 95.1% specificity (95% CI 88.9%, 98.4%; p = 0.11), and an AUROC of 0.591 (95% CI 0.506, 0.676).
The Transformer models using longitudinal radiomics and CT scan features and clinical information were used to predict a 3-year survival rate. We extracted 55,296 textual features based on volumetric CT studies. A pretrained CNN model containing underlying CT characteristics was used as an extractor to filter each CT image, and a total of 32 high-level CT features from each study were included. Medication history and other therapeutic information were added to clinical history, bringing the total to 18 clinical variables. A total of 165 features incorporating both imaging and clinical features were assessed longitudinally to create dynamic predictive models in a 3-year survival rate. Detailed descriptions of these 165 features and its correlations with the survival rate were reported in Supplementary Table 4 and details of medications and therapeutic classes were summarized in Supplementary Table 5 . Patients having 3-year follow-up information and known living status were included in the progression management study, resulting in 234 participants. Out of 599 visits from 234 patients in the time series analysis, 179 visits from 103 patients contain missing PFTs. We use the nearest visit PFT of each patient as the missing visit PFT. A total of 79 patients only had one visit in our system. The median number of visits within 3 years was 4, and the median time interval between each visit was 8 months. At the end of each year, the estimated mortality rate substantially increased from 2.1% then 6.4% then 9.4%.
We developed models at four endpoints starting from the initial visit to evaluate the patient’s response after treatment. Four Transformer models were developed using the patient’s initial visit information, and the data within 1 year, 2 years, and 3 years. False negatives were minimized. Only negative predictive value and sensitivity are reported hereafter. More details are demonstrated in Fig. 4 . The Transformer models tended to be more predictive with more follow-up data available, showing an uptrend AUROCs of 0.660 (95% CI 44.09%, 87.87%; p = 0.07981), 0.632 (95% CI 41.33%, 85.06%, p = 0.04951), 0.801 (95% CI 68.92%, 91.19%; p = 0.153), and 0.868 (95% CI 77.04%, 96.57%) evaluated at the initial visit, within 1 year, 2 years, and 3 years respectively. All models remained high, with negative predictive values ranging from 89.66 to 94.55%. The models became more sensitive when more follow up information was available, increasing in sensitivity from 54.55% (95% CI 23.38%, 83.25%) to 72.73% (95% CI 39.03%, 93.98%) at the end of year 1 and the end of year 3 respectively.
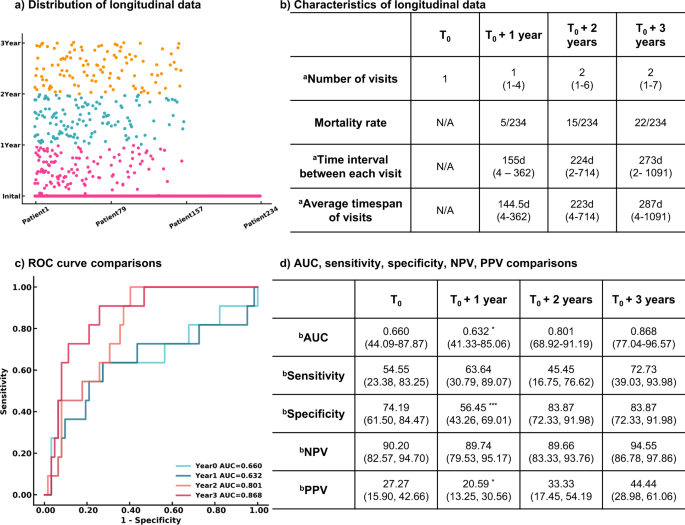
a Distribution of longitudinal visits from each patient. Each visit of 234 patients included in the survival rate study was presented. b Characteristics of longitudinal data. c ROC curves of 3-year survival rate prediction at different endpoints. d Performance and comparison of Transformer models developed at multiple endpoints. n = 234 for 3-year survival analysis. Two-sided P -values were calculated for all comparisons. a Data in parentheses indicate the range. b Data in parentheses indicate 95% CI. *** p < 0.001. ** p < 0.01. * p < 0.05.
Diagnosing, treating, and managing interstitial lung disease and its subtypes remains a complex clinical challenge, often requiring the expertize of highly specialized physicians, such as thoracic fellowship-trained radiologists, and the synthesis of an array of clinical information. Lack of human resources and limited access to clinicians with specialized expertize in ILD is a worldwide barrier in ILD management 15 . Furthermore, quantifying a patient’s response to treatment and disease progression is a second barrier to clinical care 16 . Walsh 17 et al. developed a deep learning model of 1157 high resolution CT scans to classify UIP and non-UIP. It achieved accuracy 79% in classifying 29 UIP cases. Choe 18 et al. created a content-based image retrieval method to classify four subtypes of ILD, UIP, NSIP, COP and CHP based on CT scans of 288 patients and showed that their proposed framework can improve radiologists’ ILD classification accuracy from 52.4% to 72.8%. Both studies only used CT images for algorithm development, while in our study we combine CT images with clinical information together to develop a joint model in order to develop a more comprehensive algorithm to study ILD subtype classification. In addition, we conducted a 3 year survival analysis using longitudinal data of patients to monitor patient’s disease progression. Our present study created a joint CNN model by integrating CT images with clinical information. This model accurately predicted five ILD subtypes and outperformed a senior thoracic radiologist and a senior pulmonologist in diagnosing true cases of UIP ( p < 0.05; p < 0.001). Our joint CNN model also performed as well as all human readers in sensitivity when diagnosing CHP, sarcoidosis, NSIP, and other ILD ( p > 0.05). In addition to the diagnostic joint CNN model, we created a Transformer model that can predict a patient’s 3-year survival rate after a visit with high sensitivity and negative predictive value while remaining a reasonably high specificity and positive predictive value.
The joint CNN model showed superior performance in the classification of ILD subtypes. Pretrained weights from the RadImageNet models 19 were used as starting points for CNN. The RadImageNet pre-trained model contained CT features such as pulmonary infiltrates. These features shared high-level similarity to our target ILD data, which further improved the CNN performance on CT images. While ViT showed great potential on large natural image datasets 9 , the ViT model was outperformed by the CNN model using transfer learning due to the small sample size of images from our ILD dataset (Supplementary Fig. 4 ). After synthesizing CT images and clinical history and using weights pre-trained from similar studies, the joint CNN was more sensitive to diagnosing UIP that outperformed the STR ( p < 0.05), JTRs ( p < 0.001), SGRs ( p < 0.001), and SP ( p < 0.001).
For the diagnosis of CHP, the joint model achieved equivalent performance in sensitivity ( p > 0.05) to all human readers and outperformed the STR ( p < 0.01), JTRs ( p < 0.001), TRF ( p < 0.05), SGR2 ( p < 0.01), and SP ( p < 0.001) in specificity. Regarding NSIP prediction, the joint model performed equally well in sensitivity as compared to six readers ( p > 0.05) and outperformed SGR1 ( p < 0.01). Similarly, it demonstrated higher specificity compared to the JTRs ( p < 0.001), SP ( p < 0.001), and SGR2 ( p < 0.001) and performed comparably to the STR ( p = 0.48). Both the joint model and human readers performed equally well in reading sarcoidosis ( p > 0.05) in sensitivity and specificity, except that the joint model was outperformed by the SP ( p < 0.05) in specificity. For the diagnosis of other ILD, the joint model demonstrated similar performance to six human readers ( p > 0.05) and was only outperformed by the STR ( p < 0.05); the model was significantly more specific than six human readers ( p < 0.01) except for the SP ( p = 0.11).
To analyze the 3-year survival rate, we developed two-time series models, the LSTM and Transformer; both models consisted of multiple factors including quantitative CT information, clinical history, and medication history within 3 years. The average of the Transformer models achieved 7.5% better performance than the average of LSTM models, and the ensemble Transformer model achieved 15.8% better performance than the ensemble LSTM model. Thus, the Transformer algorithm was applied to train patients’ data within 1 year, 2 years, and 3 years. The confidence of 3-year survival prediction via Transformer was increased with more follow-up information. The AUROC was dramatically improved by 31.5% between the evaluation at the initial visit and the end of year 3. There was no difference between the evaluation at the end of year 2 and year 3 ( p = 0.153). This shows that response to treatment may require more than one year. After 2 years of treatment, there is high confidence (95%) in predicting the patient’s survival.
Our study sought to address two major barriers in interstitial lung disease management. Firstly, the diagnosis of ILD subtypes often requires thoracic fellowship-trained radiologists, and specialists with such expertize are scarce. This potentially limits the timely diagnosis and treatment of persons living with ILD. Thus, with widespread implementation of our deep learning system, we hope to alleviate the burden on these highly specialized clinicians while enhancing patient care. The implementation of our deep learning system could provide a useful diagnostic tool for the general radiologists in the community who infrequently encounter interstitial lung disease. Instead of simply reporting these diverse disease processes using broad terms such as “pulmonary fibrosis”, the general radiologist could use this deep learning system to reach a specific diagnosis. Our diagnostic joint model, for example, showed superior sensitivity in identifying UIP ( p < 0.05) and significant improvement in specificity for the diagnosis of CHP ( p < 0.05), NSIP ( p < 0.05), and other ILD ( p < 0.05) as compared to human readers. The second barrier in interstitial lung disease management is disease prognosis and progression. It is important to evaluate treatment efficacy and patient prognosis at each visit so that patients may be counseled about their condition and what to expect. Accurately predicting patient response and prognosis is extremely challenging but has great value by ultimately improving patient outcomes. Our Transformer model can evaluate the 3-year survival rate at each visit by integrating information from each visit. Moreover, the Transformer model demonstrated significant advancement in predicting a 3-year survival rate when current follow-up information was integrated. Our deep learning system has the potential to be integrated into the daily workflow of pulmonologists, rheumatologists, pathologists, and radiologists, where it could serve as a second opinion for a diagnosis of ILD subtypes and dynamically provide personalized insights regarding current and future treatment efficacy using its 3-year survival prediction feature. Installation of the deep learning models would require cloud computing with the integration of PACS and Epic or other clinical databases, which is relatively easy to achieve in most modern healthcare systems.
Our proposed deep learning system has limitations. One major limitation is that a patient’s initial visit in the registry may not be the patient’s first evaluation for ILD since our patients come from multiple areas. For the unknown values in the categorical variables, we made an additional class within each variable to indicate them. In the ILD classification part, our lung segmentation algorithm uses a high threshold, so some opacity in the image might be missed. In the time series study, one limitation is a small sample size since 79 of 234 patients had only one visit. Because we split the training, validation, and test dataset based on different hospital resources, the prevalence of death differs between the test dataset and training/validation datasets which have around 10% death in the whole sample, making it more difficult for the model to study the characteristics from the deceased patients. In addition, deaths were determined from the chart declaration. The causes of death might not be only associated with ILD. Lastly, during preprocessing clinical variables for the time series study, missing PFTs were filled with the same data from the nearest visit.
In future studies, more clinical history and additional clinical data, including symptoms after treatment and long-term survival rate, can be analyzed when further follow-up information is gathered. We also aim to collect pathology slides and genetic data to comprehensively diagnose ILD subtypes and improve treatment and outcomes. Deploying the models in a cloud setting could help clinicians access the results faster. The reproducibility of the models needs further evaluation at multiple medical centers.
In conclusion, the proposed deep learning system demonstrates high potential in accurately diagnosing five subtypes of ILD. This could help clinicians without access to specialized thoracic training fellow, to diagnose and make dynamic predictions regarding patient prognosis and disease progression. We believe the proposed models, which integrate CT images with clinical history, demonstrate equivalent performance to a senior thoracic radiologist and a senior pulmonologist and also evaluate survival rate at each follow-up visit, which could be a useful tool to distinguish ILD subcategories and manage the long-term progression of patients.
Ethics oversight
The study was approved by the Institutional Review Board (IRB) of the Mount Sinai School of Medicine, in accordance with Mount Sinai’s Federal Wide Assurances to the Department of Health and Human Services (ID# STUDY-14-00584-CR001). Written informed consent has been obtained from patients enrolled in this research registry. A Data and Safety Monitoring Board (DSMB) from Mount Sinai IRB had oversight of the study.
Study population
We collected chest CT scans and clinical information from 458 patients enrolled in the MSMC-ILD between September 2014 and April 2021. Individuals for participation in Mount Sinai Medical Center Research Registry for Interstitial Lung Disease (MSMC-ILD) included all adult (age > 18 years old) patients who were receiving or seeking medical care for the treatment of interstitial lung disease at Mount Sinai Medical Center, St Luke’s and Beth Israel Medical Centers. Patients with lung fibrosis or other interstitial lung disease were enrolled in the MSMC-ILD and assessing the extent of the disease. MSMC-ILD was established in 2014. The diagnosis of an ILD subtype followed the ATS2018 guidelines. All registry patients had a consensus diagnosis from radiology, pathology, and pulmonology. In this study, occupational exposure or other environmental exposure is included as a clinical feature. It is likely that the patient cohort at MSMC might be different from other patient cohorts. For example, patients at MSMC might be influenced by World Trade Center exposure. There were nine patients excluded due to low image quality resulting in a total of 449 patients with both clinical information and CT images that were included in our ILD diagnosis study. The patient population age ranged from 22 to 91 years (median 63, IQR 56-71), with 226 males and 223 females. A total of 132 patients (29.4%) were diagnosed with usual interstitial pneumonia (UIP), 37 patients (8.2%) with chronic hypersensitivity pneumonitis (CHP), 142 patients (31.6%) with nonspecific interstitial pneumonia (NSIP), 42 patients (9.4%) with sarcoidosis and 96 patients (21.4%) with other various ILD. 234 patients were selected for the 3-year survival analysis (see Supplementary Fig. 1 for inclusion and exclusion criteria). Sex information was used in the diagnosis of ILD subtypes as well as the prediction of 3 year survival analysis. Study participants did not receive compensation.
Clinical information
Clinical information was retrospectively collected by medical students, radiology residents, and thoracic radiology fellows through chart review via electronic medical records. The following data were collected within 6 months of the study date of each patient’s CT scan: age, sex, history of current or former smoking, history of rheumatic disease, home oxygen requirement, history of occupational or other exposures (including pets), World Trade Center exposure, pulmonary function test (PFT) values (FEV1/FVC ratio, FEV1 value, DLCO percentage), presence of pulmonary hypertension based on echocardiography or right heart catheterization, and history/results of lung biopsy. Clinical information was collected from pulmonology visit notes in the Electronic Medical Record and PFT flowcharts. If data was not available within the 6-month time frame, the data entry for that variable was left blank. Incomplete clinical variables were later filled with values from the nearest visit.
We also recorded the medications being used at or about the time of the CT to treat the ILD. There were eight types of medicine used for patients, including azathioprine (immunosuppressant), bosentan (cardiovascular), cyclophosphamide (antineoplastics), mycophenolate (immunosuppressant), nintedanib (unclassified), pirfenidone (unclassified), prednisone (hormone), rituximab (unclassified).
The dataset was split by patient ID and hospital. For ILD subtype classification, 128 (28.5%) patients with their initial CT scan and clinical information collected at the Mount Sinai Hospital were used as the external test set. The rest of the 321 (71.5%) patients whose initial data were collected at outside hospitals were used for model development, with 258 (57.5%) patients within the training set and 63 (14.0%) patients into the validation set. For the analysis of the 3-year survival rate, a subset of 234 patients meeting the criteria in Supplementary Fig. 1 was utilized. These 234 patients were split into a training dataset containing 123 patients (6 dead), a validation dataset containing 38 patients (5 dead), and a test dataset containing 73 patients (11 dead).
Human reader studies
The predictions of the joint CNN AI model were compared to seven human readers on the test set. Six board-certified and fellowship-trained radiologists and a pulmonologist, as well as one thoracic radiology fellow, were provided with the same initial CT scan and associated clinical information that were used to develop the AI models. A senior thoracic radiologist (A.J.) with 10 years of post graduate experience, two junior thoracic radiologists (M.C. and A.B.) with 5-years post graduate experience, a thoracic radiology fellow (A.S.), two senior radiologists (M.H. and J.M.) with 10 years of experience in non thoracic radiology specialties(musculoskeletal radiology and pediatric radiology respectively), and a senior pulmonologist (S.D.) with 10 years experience each reviewed the 128 studies and associated clinical information from the test set. Their predictions were compared to the predictions of the joint deep learning model and the consensus diagnosis.
The consensus diagnosis of UIP, CHP, NSIP, sarcoidosis, and other various ILD was used as the gold standard to develop the AI models in ILD subcategory classification. We created five models using image data and clinical information. First, a CNN model (model 1) using pre-trained weights from the RadImageNet 19 and ViT model (model 2) based on CT images were developed. Second, machine learning models (model 3), including MLP, SVM, and XGBoost, were generated based on the clinical information. Finally, a joint CNN model (model 4) and a joint ViT model (model 5) were developed which integrated both the imaging and clinical data.
ILD subtype classification model training
We used the same optimization strategies for all classification AI models by employing the Adam optimizer with a learning rate of 0.001 and weight decay of 0.0001, except model 4 used a learning rate of 0.0001. Each model was trained with 40 epochs. We used categorical cross-entropy as the objective function.
To predict patients’ 3-year survival rate longitudinal radiological and clinical data were used to create time series models based on each time point (initial visit, year 1, year 2, year 3) with LSTM 14 and Transformer 13 , respectively.
Three-year survival rate model training
We used the same optimization strategies for all longitudinal AI models by employing the Adam optimizer of learning rate of 0.0001 and weight decay of 0.0001. Both LSTM and the Transformer were developed in two different parameter settings. For each parameter setting, we repeated the simulation 30 times. Each simulation was trained with 100 epochs with a batch size of 64. We used categorical cross-entropy as the objective function. The top two models from each parameter setting with the best performance on the validation dataset were selected for the ensemble model. A total of four models through averaging probability for each patient were then calculated for their performance on the test dataset. The details of parameter settings were reported in later sections.
Data preprocessing
Clinical information and ct data collection.
The following clinical data were collected: patients’ sex, age, lung function lab test results (FEV1, DLCO, FVC, FEV1/FVC), smoking history, occupational exposure, rheumatic disease, hypertension, lung biopsy, and the use of home oxygen. CT imaging data were collected from the study DICOM header. For missing data, we added an unknown class to each categorical variable. The LabelEncoder function in the scikit-learn package was utilized to encode these categorical data into numerical variables. The StandardScaler function in the scikit-learn package was used to normalize each feature to unit variance with the mean set as 0.
Image preprocessing
First, all CT scans were resampled to an isotropic voxel. Next, we generated lung regions for each image in each study. This was achieved by applying a threshold of -400HU to each CT slice to effectively convert the CT image into a binary image consisting of two densities—air and not air. The “not air” periphery of the binary image was removed, and the two largest “air” regions were kept. The binary mask was then used on the input raw CT image to separate the lung regions. After lung segmentation, a standard lung window (width = 1500HU and level = -400HU) was used to normalize pixel intensities between 0 and 255 for each segmented lung CT slice. GE Centricity Universal Viewer 6.0 was used to review the CT studies. Preprocessed images were used to develop CT-based models in Tensorflow (2.4.0).
CT-based convolutional neural network model (model 1)
We designed a CT-based convolutional neural network model to diagnose ILD using the CT images. This CT-based CNN model was built via transfer learning using pre-trained weights from a RadImageNet convolutional neural network Inception-ResNet-V2 (IRV2) 19 , 20 , 21 . We froze all layers from the pre-trained model and only trained the top10 layers that incorporated high-level features. An average pooling layer and the last dense classifier layers were followed by the last convolutional layer. Using a RadImageNet pre-trained model provided a better starting point than an ImageNet model as the RadImageNet database contains CT lung images and therefore shares higher similarity with the target data.
CT-based vision Transformer model (model 2)
We trained a CT-based vision Transformer model. ViT model was developed to transfer the success of the self-attention mechanism on NLP tasks into imaging applications 9 . Our ViT model first split the input image into 10 patches and encoded each embedded patch into a self-attention based deep neural network. Then, two fully connected layers with 2048 and 1024 nodes and the final prediction layer were followed by the encoded embedding layers.
Machine learning model (model 3)
To classify ILD subtypes based on clinical information, we applied MLP, SVM, and XGBoost classifiers to build machine learning models. We evaluated the performance of these three classifiers on the validation dataset (Supplementary Fig 5 ). We fine-tuned the model’s hyperparameters on the training and validation dataset and evaluated the best model on the test dataset. For the SVM classifier, we assessed the ‘C’ and kernel type. For the XGBoost classifier, the learning rate and several iterations were tuned. For MLP, we assessed the number of hidden nodes in each layer, the learning rate, activation method, and solver for weight optimization. After the hyperparameter optimization, the two-layer MLP model with 64 and 32 nodes was selected because it achieved the highest AUC score on the validation dataset.
Joint CNN and MLP model (model 4)
A joint model combining CT images and clinical information was developed. The inception-res-net-v2 architecture using pre-trained weights derived from the RadImageNet database 19 was used to learn features from imaging data. Given the pre-trained weights included CT imaging patterns relevant to our targeted CT images we froze the base layers that stored fundamental information from CT features and only trained the top10 layers that incorporated high-level features. An average pooling layer and three full layers with 1024, 512, and 32 nodes were followed by the last convolutional layer. CT images were finally presented in a vector with 32 features. 16 clinical variables were learned by the MLP model that had two fully connected layers with 64 and 32 nodes, respectively. The last MLP layer was combined with the vector containing CT features. The joint vector was then fed into a fully connected layer having 512-dimensional features before the output layer.
Joint ViT and MLP model (model 5)
A joint ViT and MLP model was also developed to study the combined information of CT images and clinical data. Because the location of lung regions might vary in CT images from different centers, we chose to split the input image into 32 patches. Then, patches were processed via the Transformer encoder, which contained four independent self-attention heads to repeat the computation in parallel. The image features extracted from the Transformer encoder were then connected with three fully connected layers with 1024, 512, and 32 nodes. All CT images were presented in a vector with 32 features. Similar to model 4, a total of 16 clinical variables were learned by the MLP model that had two fully connected layers with 64 and 32 nodes, respectively. The last layer of the MLP model was combined with the vector containing CT features. The joint vector was then fed into a fully connected layer having 512-dimensional features before the output layer.
Radiomics was used to extract textual features of normal lung regions from CT images 22 . We first converted our segmented lung CT images into binary images as the masked images to indicate the region of interest for Radiomics. Then, we applied the PyRadiomics tool to combine CT images and masked CT images to obtain textual features based on volumetric data. The features extracted from PyRadiomics contain information about the size, shape, spatial relationship, and image intensity of medical images 23 . A total of 116 radiomics features were obtained for further model development in predicting a 3-year survival rate.
CNN extractor and Uniform Manifold Approximation and Projection (UMAP)
We used a pre-trained RIN-generic IRV2 CNN model developed on the RadImageNet database as the extractor to obtain high-level CT features. The last convolutional layer conv_7b having 1536 kernel maps in 6 by 6 matrix size, was used to screen each CT image. Each CT image was presented as a vector of 55,296 features. We then averaged the CT slices from each study. After CNN feature extraction, we used UMAP 24 to reduce the dimension of features while preserving the global structure allowing the 55, 296 features to be reduced to 32.
Time Series data preprocessing
The time-series data included clinical information, medication information, and imaging features for each patient visit were extracted from Radiomics and CNN. The MinMaxScaler function was used to normalize all features. The maximum visit number from our dataset was seven, so patients who had less than seven visits were given data values of zero for the “missing” visits as the sign for our model to skip the data during processing. Sklearn (0.24.1) was used to preprocess and develop the models.
Transformer time series model
We developed a Transformer time series model to study the temporal information from the time series data of patients’ clinical information and CT image features. The Transformer time series model was developed by stacking 16 Transformer encoders together to evaluate data at each time point. The time-series data were processed via the Transformer encoders and then followed by an average pooling layer and a fully connected layer with 128 nodes. We fine-tuned the hyperparameters of the Transformer model on the training and validation dataset and evaluated the best model on the test dataset. We assessed the number of heads in the Transformer encoder.
LSTM time series model
LSTM is an improved form of a Recurrent Neural Network, designed to solve the problem of vanishing long-term gradients 14 . The LSTM time series model was developed to predict living status based on patients’ clinical information and CT image features over time. The time-series input was first passed through two layers of LSTM, which computes the corresponding sequence of input data at different time states and then outputs a sequence of hidden state vectors in forward and reverse directions. Then, the features extracted from LSTM were followed by three fully connected layers and one final classifier layer. We fine-tuned the hyperparameters of the LSTM model on the training and validation dataset and evaluated the best model on the test dataset. We assessed the number of LSTM layers.
Statistical analysis
Comparisons of AUROCs were performed by bootstrap in the pROC package (version 1.18.0) 25 in R. A total of 2000 bootstrap permutations were simulated to calculate 95% CIs and p -values. The 95%CIs of sensitivity and specificity for AI models and human readers were calculated by the exact Clopper-Pearson method 26 . McNemar’s test 27 was used to compare sensitivity and specificity. Generalized score statistic test 28 was used to calculate p values for negative predictive values and positive predictive values. Two-sided p values were assessed for all statistical analyses, and p -value < 0.05 was defined as statistical significance. We performed logistic regression to evaluate the correlations between clinical variables and each ILD subcategory. The Hosmer–Lemeshow test 29 confirmed the goodness of logistic regression. McNemar’s and the generalized score statistic tests were performed in the DTComPair 30 package (version 1.0.3) in R 4.1.3.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The in-house datasets generated and/or analysed during the current study are not publicly available due to HIPAA compliance and were used with Mount Sinai institutional permission for the purposes of this project. All requests for access to in-house data will be addressed to the corresponding authors, Dr. Xueyan Mei ([email protected]), Dr. Yang Yang ([email protected]) or Dr. Zahi Fayad ([email protected]), and will be processed in accordance with Mount Sinai institutional guidelines. Mount Sinai Innovation Partners (MSIP) will assess all requests based on the purpose of data request, and it may take up to one month to process the request. A material-transfer or data-usage agreement will be required between Mount Sinai and the receiving organization. The requesting organization must provide comprehensive details, including the name and full contact information of the individual and institution making the request, along with specific identification of the data being requested. Additionally, the requesting organization must clearly state the intended purpose of the data transfer and provide assurances that the transferred data will only be used for non-commercial academic and educational purposes in compliance with Mount Sinai institutional guidelines. The pretrained models used in this paper are available at https://doi.org/10.1148/ryai.210315 . Source data are provided as a Source Data file. Source data are provided with this paper.
Code availability
All the codes we used to train the models have been posted in this github repository https://github.com/lzl199704/ILD .
Soffer, S. et al. Artificial intelligence for interstitial lung disease analysis on chest computed tomography: a systematic review. Acad. Radiol. 29 , S226–S235 (2022).
Article PubMed Google Scholar
Raghu, G. et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 183 , 788–824 (2011).
Article PubMed PubMed Central Google Scholar
Walsh, S. L. F. et al. Role of imaging in progressive-fibrosing interstitial lung diseases. Eur. Respir. Rev. 27 , 180073 (2018).
Grenier, P. et al. Chronic diffuse interstitial lung disease: diagnostic value of chest radiography and high-resolution CT. Radiology 179 , 123–132 (1991).
Article CAS PubMed Google Scholar
Trusculescu, A. A., Manolescu, D., Tudorache, E. & Oancea, C. Deep learning in interstitial lung disease-how long until daily practice. Eur. Radiol. 30 , 6285–6292 (2020).
Bermejo-Peláez, D., Ash, S. Y., Washko, G. R., San José Estépar, R. & Ledesma-Carbayo, M. J. Classification of interstitial lung abnormality patterns with an ensemble of deep convolutional neural networks. Sci. Rep. 10 , 338 (2020).
Article PubMed PubMed Central ADS Google Scholar
Chassagnon, G. et al. Deep learning–based approach for automated assessment of interstitial lung disease in systemic sclerosis on CT images. Radiology: Artif. Intell. 2 , e190006 (2020).
Google Scholar
Christe, A. et al. Computer-aided diagnosis of pulmonary fibrosis using deep learning and CT images. Invest. Radiol. 54 , 627–632 (2019).
Dosovitskiy, A. et al. An image is worth 16x16 words: transformers for image recognition at scale. arXiv https://doi.org/10.48550/arxiv.2010.11929 (2020).
Gardner, M. W. & Dorling, S. R. Artificial neural networks (the multilayer perceptron)—a review of applications in the atmospheric sciences. Atmos. Environ. 32 , 2627–2636 (1998).
Article CAS ADS Google Scholar
Chen, T. & Guestrin, C. XGBoost: A scalable tree boosting system. in Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining—KDD ’16 785–794 (ACM Press, 2016).
Hearst, M. A., Dumais, S. T., Osuna, E., Platt, J. & Scholkopf, B. Support vector machines. IEEE Intell. Syst. Their Appl. 13 , 18–28 (1998).
Article Google Scholar
Vaswani, A. et al. Attention is all you need. Adv. Neural Inf. Process. Syst. https://doi.org/10.48550/arXiv.1706.03762 (2017).
Hochreiter, S. & Schmidhuber, J. Long short-term memory. Neural Comput . 9 , 1735–1780 (1997).
Cosgrove, G. P., Bianchi, P., Danese, S. & Lederer, D. J. Barriers to timely diagnosis of interstitial lung disease in the real world: the INTENSITY survey. BMC Pulm. Med. 18 , 9 (2018).
Wong, A. W., Ryerson, C. J. & Guler, S. A. Progression of fibrosing interstitial lung disease. Respir. Res. 21 , 32 (2020).
Walsh, S. L. F., Calandriello, L., Silva, M. & Sverzellati, N. Deep learning for classifying fibrotic lung disease on high-resolution computed tomography: a case-cohort study. Lancet Respir. Med 6 , 837–845 (2018).
Choe, J. et al. Content-based image retrieval by using deep learning for interstitial lung disease diagnosis with chest CT. Radiology 302 , 187–197 (2022).
Mei, X. et al. RadImageNet: an open radiologic deep learning research dataset for effective transfer learning. Radiology: Artif. Intell. 4 , e210315 (2022).
Xueyan, M. Cats to CATs with RadImageNet: A Transformative Platform for Medical Imaging AI Research (Icahn School of Medicine at Mount Sinai, 2022).
Szegedy, C., Ioffe, S., Vanhoucke, V. & Alemi, A. A. Thirty-first AAAI Conference on Artificial Intelligence (ACM, 2017).
van Griethuysen, J. J. M. et al. Computational radiomics system to decode the radiographic phenotype. Cancer Res 77 , e104–e107 (2017).
Zhang, X. et al. Deep learning with radiomics for disease diagnosis and treatment: challenges and potential. Front. Oncol. 12 , 773840 (2022).
McInnes, L., Healy, J. & Melville, J. UMAP: Uniform manifold approximation and projection for dimension reduction. arXiv https://doi.org/10.48550/arxiv.1802.03426 (2018).
Robin, X. et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinforma. 12 , 77 (2011).
Clopper, C. J. & Pearson, E. S. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26 , 404 (1934).
Article MATH Google Scholar
McNemar, Q. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika 12 , 153–157 (1947).
Leisenring, W., Alonzo, T. & Pepe, M. S. Comparisons of predictive values of binary medical diagnostic tests for paired designs. Biometrics 56 , 345–351 (2000).
Article CAS PubMed MATH Google Scholar
Hosmer, D. W., Lemeshow, S. & Sturdivant, R. X. Applied Logistic Regression (Wiley, 2013).
Stock, C. & Hielscher, T. DTComPair: Comparison of Binary Diagnostic Tests in a Paired Study Design . https://rdrr.io/cran/DTComPair/ (2014).
Download references
Acknowledgements
X.M. was supported by the National Center for Advancing Translational Sciences (NCATS) TL1TR004420 NRSA TL1 Training Core in Transdisciplinary Clinical and Translational Science (CTSA).
Author information
Authors and affiliations.
BioMedical Engineering and Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Xueyan Mei, Zelong Liu, Chendi Cao, Zahi A. Fayad & Yang Yang
Department of Diagnostic, Molecular, and Interventional Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Ayushi Singh, Justine Lee, Cody DeMarco, Mingqian Huang, Joy Masseaux, Adam Bernheim, Michael Chung, Adam Jacobi, Zahi A. Fayad & Yang Yang
Icahn School of Medicine at Mount Sinai, New York, NY, USA
Marcia Lange, Priyanka Boddu, Samantha Platt, Ganesh Sivakumar & Benjamin Gross
Department of Pharmaceutical Sciences, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Jingqi Q. X. Gong
Department of Medicine, Pulmonary, Critical Care and Sleep Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Sakshi Dua & Maria Padilla
Department of Radiology, Cornell Medicine, New York, NY, USA
Timothy Deyer
Department of Radiology, East River Medical Imaging, New York, NY, USA
Department of Radiology and Biomedical Imaging, University of California, San Francisco, CA, USA
You can also search for this author in PubMed Google Scholar
Contributions
X.M. and Z.L. developed the modeling. X.M., Z.L. and J.Q.X.G. created the figures. X.M., Z.L., A.S., M.L., P.B., C.D., C.C, T.D., M.P., A.J., Z.A.F. and Y.Y. wrote the manuscript. X.M., M.P., M.C., A.B., A.J., Z.A.F. and Y.Y. designed the experiments. A.S., M.C., A.B., A.J., S.D., J.M., and M.H. evaluated and read the test set cases. M.L, A.S., P.B., J.L., C.D., S.P., G.S., and B.G. collected the dataset. X.M. and Z.L. performed the statistical analysis. X.M, Z.A.F. and Y.Y. supervised the work.

Corresponding authors
Correspondence to Xueyan Mei , Zahi A. Fayad or Yang Yang .
Ethics declarations
Competing interests.
T.D. is managing partner of RadImageNet LLC.
Peer review
Peer review information.
Nature Communications thanks the other anonymous reviewer(s) for their contribution to the peer review of this work. Peer review reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, reporting summary, source data, source data, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Mei, X., Liu, Z., Singh, A. et al. Interstitial lung disease diagnosis and prognosis using an AI system integrating longitudinal data. Nat Commun 14 , 2272 (2023). https://doi.org/10.1038/s41467-023-37720-5
Download citation
Received : 16 June 2022
Accepted : 29 March 2023
Published : 20 April 2023
DOI : https://doi.org/10.1038/s41467-023-37720-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Use of artificial intelligence algorithms to analyse systemic sclerosis-interstitial lung disease imaging features.
Rheumatology International (2024)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Journal Proposal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu
- Subscribe SciFeed
- Recommended Articles
- PubMed/Medline
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
A survey of deep learning for lung disease detection on medical images: state-of-the-art, taxonomy, issues and future directions.

1. Introduction
2. methodology, 3. the basic process to apply deep learning for lung disease detection, 3.1. image acquisition phase, 3.2. preprocessing phase, 3.3. training phase, 3.4. classification phase, 4. the taxonomy of state-of-the-art work on lung disease detection using deep learning, 4.1. image type, 4.1.1. chest x-rays, 4.1.2. ct scans, 4.1.3. sputum smear microscopy images, 4.1.4. histopathology images, 4.2. features, 4.3. data augmentation, 4.4. types of deep learning algorithm, 4.5. transfer learning, 4.6. ensemble of classifiers, 4.7. type of disease, 4.7.1. tuberculosis, 4.7.2. pneumonia, 4.7.3. lung cancer, 4.7.4. covid-19, 4.8. dataset, 5. analysis of trend, issues and future directions of lung disease detection using deep learning, 5.1. an analysis of the trend of lung disease detection in recent years, 5.1.1. trend analysis of the image type used, 5.1.2. trend analysis of the features used, 5.1.3. trend analysis of the usage of data augmentation, 5.1.4. trend analysis of the types of deep learning algorithm used, 5.1.5. trend analysis of the usage of transfer learning, 5.1.6. trend analysis of the usage of ensemble, 5.1.7. trend analysis of the type of lung disease detected using deep learning, 5.2. issues and future direction of lung disease detection using deep learning, 5.2.1. issues, 5.2.2. potential future works, 6. limitation of the survey, 7. conclusions, author contributions, conflicts of interest.
- Bousquet, J. Global Surveillance, Prevention and Control of Chronic Respiratory Diseases ; World Health Organization: Geneva, Switzerland, 2007; pp. 12–36. [ Google Scholar ]
- Forum of International Respiratory Societies. The Global Impact of Respiratory Disease , 2nd ed.; European Respiratory Society: Sheffield, UK, 2017; pp. 5–42. [ Google Scholar ]
- World Health Organization. Coronavirus Disease 2019 (COVID-19) Situation Report ; Technical Report March; World Health Organization: Geneva, Switzerland, 2020. [ Google Scholar ]
- Rahaman, M.M.; Li, C.; Yao, Y.; Kulwa, F.; Rahman, M.A.; Wang, Q.; Qi, S.; Kong, F.; Zhu, X.; Zhao, X. Identification of COVID-19 samples from chest X-Ray images using deep learning: A comparison of transfer learning approaches. J. X-Ray Sci. Technol. 2020 , 28 , 821–839. [ Google Scholar ] [ CrossRef ]
- Yahiaoui, A.; Er, O.; Yumusak, N. A new method of automatic recognition for tuberculosis disease diagnosis using support vector machines. Biomed. Res. 2017 , 28 , 4208–4212. [ Google Scholar ]
- Hu, Z.; Tang, J.; Wang, Z.; Zhang, K.; Zhang, L.; Sun, Q. Deep learning for image-based cancer detection and diagnosis-A survey. Pattern Recognit. 2018 , 83 , 134–149. [ Google Scholar ] [ CrossRef ]
- American Thoracic Society. Diagnostic Standards and Classification of Tuberculosis in Adults and Children. Am. J. Respir. Crit. Care Med. 2000 , 161 , 1376–1395. [ Google Scholar ] [ CrossRef ]
- Setio, A.A.A.; Traverso, A.; de Bel, T.; Berens, M.S.; van den Bogaard, C.; Cerello, P.; Chen, H.; Dou, Q.; Fantacci, M.E.; Geurts, B.; et al. Validation, comparison, and combination of algorithms for automatic detection of pulmonary nodules in computed tomography images: The LUNA16 challenge. Med. Image Anal. 2017 , 42 , 1–13. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Shen, D.; Wu, G.; Suk, H.I. Deep Learning in Medical Image Analysis. Annu. Rev. Biomed. Eng. 2017 , 19 , 221–248. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Wu, C.; Luo, C.; Xiong, N.; Zhang, W.; Kim, T.H. A Greedy Deep Learning Method for Medical Disease Analysis. IEEE Access 2018 , 6 , 20021–20030. [ Google Scholar ] [ CrossRef ]
- Ma, J.; Song, Y.; Tian, X.; Hua, Y.; Zhang, R.; Wu, J. Survey on deep learning for pulmonary medical imaging. Front. Med. 2019 , 14 , 450–469. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Rajaraman, S.; Candemir, S.; Xue, Z.; Alderson, P.O.; Kohli, M.; Abuya, J.; Thoma, G.R.; Antani, S.; Member, S. A novel stacked generalization of models for improved TB detection in chest radiographs. In Proceedings of the 2018 40th Annual International Conference the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 17–21 July 2018; pp. 718–721. [ Google Scholar ] [ CrossRef ]
- Ardila, D.; Kiraly, A.P.; Bharadwaj, S.; Choi, B.; Reicher, J.J.; Peng, L.; Tse, D.; Etemadi, M.; Ye, W.; Corrado, G.; et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat. Med. 2019 , 25 , 954–961. [ Google Scholar ] [ CrossRef ]
- Gordienko, Y.; Gang, P.; Hui, J.; Zeng, W.; Kochura, Y.; Alienin, O.; Rokovyi, O.; Stirenko, S. Deep Learning with Lung Segmentation and Bone Shadow Exclusion Techniques for Chest X-Ray Analysis of Lung Cancer. Adv. Intell. Syst. Comput. 2019 , 638–647. [ Google Scholar ] [ CrossRef ]
- Kieu, S.T.H.; Hijazi, M.H.A.; Bade, A.; Yaakob, R.; Jeffree, S. Ensemble deep learning for tuberculosis detection using chest X-Ray and canny edge detected images. IAES Int. J. Artif. Intell. 2019 , 8 , 429–435. [ Google Scholar ] [ CrossRef ]
- Dietterich, T.G. Ensemble Methods in Machine Learning. Int. Workshop Mult. Classif. Syst. 2000 , 1–15. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Webb, A. Introduction To Biomedical Imaging ; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [ Google Scholar ] [ CrossRef ]
- Kwan-Hoong, N.; Madan M, R. X ray imaging goes digital. Br. Med J. 2006 , 333 , 765–766. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Lopes, U.K.; Valiati, J.F. Pre-trained convolutional neural networks as feature extractors for tuberculosis detection. Comput. Biol. Med. 2017 , 89 , 135–143. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ayan, E.; Ünver, H.M. Diagnosis of Pneumonia from Chest X-Ray Images using Deep Learning. Sci. Meet. Electr.-Electron. Biomed. Eng. Comput. Sci 2019 , 1–5. [ Google Scholar ] [ CrossRef ]
- Salman, F.M.; Abu-naser, S.S.; Alajrami, E.; Abu-nasser, B.S.; Ashqar, B.A.M. COVID-19 Detection using Artificial Intelligence. Int. J. Acad. Eng. Res. 2020 , 4 , 18–25. [ Google Scholar ]
- Herman, G.T. Fundamentals of Computerized Tomography ; Springer: London, UK, 2009; Volume 224. [ Google Scholar ] [ CrossRef ]
- Song, Q.Z.; Zhao, L.; Luo, X.K.; Dou, X.C. Using Deep Learning for Classification of Lung Nodules on Computed Tomography Images. J. Healthc. Eng. 2017 , 2017 . [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Gao, X.W.; James-reynolds, C.; Currie, E. Analysis of tuberculosis severity levels from CT pulmonary images based on enhanced residual deep learning architecture. Neurocomputing 2019 , 392 , 233–244. [ Google Scholar ] [ CrossRef ]
- Rao, P.; Pereira, N.A.; Srinivasan, R. Convolutional neural networks for lung cancer screening in computed tomography (CT) scans. In Proceedings of the 2016 2nd International Conference on Contemporary Computing and Informatics, IC3I 2016, Noida, India, 14–17 December 2016; pp. 489–493. [ Google Scholar ] [ CrossRef ]
- Gozes, O.; Frid, M.; Greenspan, H.; Patrick, D. Rapid AI Development Cycle for the Coronavirus (COVID-19) Pandemic: Initial Results for Automated Detection & Patient Monitoring using Deep Learning CT Image Analysis Article. arXiv 2020 , arXiv:2003.05037. [ Google Scholar ]
- Shah, M.I.; Mishra, S.; Yadav, V.K.; Chauhan, A.; Sarkar, M.; Sharma, S.K.; Rout, C. Ziehl–Neelsen sputum smear microscopy image database: A resource to facilitate automated bacilli detection for tuberculosis diagnosis. J. Med. Imaging 2017 , 4 , 027503. [ Google Scholar ] [ CrossRef ]
- López, Y.P.; Filho, C.F.F.C.; Aguilera, L.M.R.; Costa, M.G.F. Automatic classification of light field smear microscopy patches using Convolutional Neural Networks for identifying Mycobacterium Tuberculosis. In Proceedings of the 2017 CHILEAN Conference on Electrical, Electronics Engineering, Information and Communication Technologies (CHILECON), Pucon, Chile, 18–20 October 2017. [ Google Scholar ]
- Kant, S.; Srivastava, M.M. Towards Automated Tuberculosis detection using Deep Learning. In Proceedings of the 2018 IEEE Symposium Series on Computational Intelligence (SSCI), Bengaluru, India, 18–21 November 2018; pp. 1250–1253. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Oomman, R.; Kalmady, K.S.; Rajan, J.; Sabu, M.K. Automatic detection of tuberculosis bacilli from microscopic sputum smear images using deep learning methods. Integr. Med. Res. 2018 , 38 , 691–699. [ Google Scholar ] [ CrossRef ]
- Mithra, K.S.; Emmanuel, W.R.S. Automated identification of mycobacterium bacillus from sputum images for tuberculosis diagnosis. Signal Image Video Process. 2019 . [ Google Scholar ] [ CrossRef ]
- Samuel, R.D.J.; Kanna, B.R. Tuberculosis ( TB ) detection system using deep neural networks. Neural Comput. Appl. 2019 , 31 , 1533–1545. [ Google Scholar ] [ CrossRef ]
- Gurcan, M.N.; Boucheron, L.E.; Can, A.; Madabhushi, A.; Rajpoot, N.M.; Yener, B. Histopathological Image Analysis: A Review. IEEE Rev. Biomed. Eng. 2009 , 2 , 147–171. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Coudray, N.; Ocampo, P.S.; Sakellaropoulos, T.; Narula, N.; Snuderl, M.; Fenyö, D.; Moreira, A.L.; Razavian, N.; Tsirigos, A. Classification and mutation prediction from non–small cell lung cancer histopathology images using deep learning. Nat. Med. 2018 , 24 , 1559–1567. [ Google Scholar ] [ CrossRef ]
- O’Mahony, N.; Campbell, S.; Carvalho, A.; Harapanahalli, S.; Hernandez, G.V.; Krpalkova, L.; Riordan, D.; Walsh, J. Deep Learning vs. Traditional Computer Vision. Adv. Intell. Syst. Comput. 2020 , 128–144. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Vajda, S.; Karargyris, A.; Jaeger, S.; Santosh, K.C.; Candemir, S.; Xue, Z.; Antani, S.; Thoma, G. Feature Selection for Automatic Tuberculosis Screening in Frontal Chest Radiographs. J. Med Syst. 2018 , 42 . [ Google Scholar ] [ CrossRef ]
- Jaeger, S.; Karargyris, A.; Candemir, S.; Folio, L.; Siegelman, J.; Callaghan, F.; Xue, Z.; Palaniappan, K.; Singh, R.K.; Antani, S.; et al. Automatic tuberculosis screening using chest radiographs. IEEE Trans. Med. Imaging 2014 , 33 , 233–245. [ Google Scholar ] [ CrossRef ]
- Antony, B.; Nizar Banu, P.K. Lung tuberculosis detection using x-ray images. Int. J. Appl. Eng. Res. 2017 , 12 , 15196–15201. [ Google Scholar ]
- Chauhan, A.; Chauhan, D.; Rout, C. Role of gist and PHOG features in computer-aided diagnosis of tuberculosis without segmentation. PLoS ONE 2014 , 9 , e112980. [ Google Scholar ] [ CrossRef ]
- Al-Ajlan, A.; Allali, A.E. CNN—MGP: Convolutional Neural Networks for Metagenomics Gene Prediction. Interdiscip. Sci. Comput. Life Sci. 2019 , 11 , 628–635. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Domingos, P. A Few Useful Things to Know About Machine Learning. Commun. ACM 2012 , 55 , 78–87. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Mikołajczyk, A.; Grochowski, M. Data augmentation for improving deep learning in image classification problem. In Proceedings of the 2018 International Interdisciplinary PhD Workshop, Swinoujscie, Poland, 9–12 May 2018; pp. 117–122. [ Google Scholar ] [ CrossRef ]
- Shorten, C.; Khoshgoftaar, T.M. A survey on Image Data Augmentation for Deep Learning. J. Big Data 2019 , 6 . [ Google Scholar ] [ CrossRef ]
- O’Shea, K.; Nash, R. An Introduction to Convolutional Neural Networks. arXiv 2015 , arXiv:1511.08458v2. [ Google Scholar ]
- Ker, J.; Wang, L. Deep Learning Applications in Medical Image Analysis. IEEE Access 2018 , 6 , 9375–9389. [ Google Scholar ] [ CrossRef ]
- Pan, S.J.; Yang, Q. A Survey on Transfer Learning. IEEE Trans. Knowl. Data Eng. 2010 , 22 , 1345–1359. [ Google Scholar ] [ CrossRef ]
- Lanbouri, Z.; Achchab, S. A hybrid Deep belief network approach for Financial distress prediction. In Proceedings of the 2015 10th International Conference on Intelligent Systems: Theories and Applications (SITA), Rabat, Morocco, 20–21 October 2015; pp. 1–6. [ Google Scholar ] [ CrossRef ]
- Hinton, G.E.; Osindero, S. A fast learning algorithm for deep belief nets. Neural Comput. 2006 , 18 , 1527–1554. [ Google Scholar ] [ CrossRef ]
- Cao, X.; Wipf, D.; Wen, F.; Duan, G.; Sun, J. A practical transfer learning algorithm for face verification. In Proceedings of the IEEE International Conference on Computer Vision, Sydney, Australia, 1–8 December 2013; pp. 3208–3215. [ Google Scholar ] [ CrossRef ]
- Wang, C.; Chen, D.; Hao, L.; Liu, X.; Zeng, Y.; Chen, J.; Zhang, G. Pulmonary Image Classification Based on Inception-v3 Transfer Learning Model. IEEE Access 2019 , 7 , 146533–146541. [ Google Scholar ] [ CrossRef ]
- Krizhevsky, A.; Sutskeve, I.; Hinton, G.E. ImageNet Classification with Deep Convolutional Neural Networks. Adv. Neural Inf. Process. Syst. 2012 . [ Google Scholar ] [ CrossRef ]
- Tajbakhsh, N.; Shin, J.Y.; Gurudu, S.R.; Hurst, R.T.; Kendall, C.B.; Gotway, M.B.; Liang, J. Convolutional Neural Networks for Medical Image Analysis: Full Training or Fine Tuning? IEEE Trans. Med. Imaging 2016 , 35 , 1299–1312. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Nogueira, K.; Penatti, O.A.; dos Santos, J.A. Towards better exploiting convolutional neural networks for remote sensing scene classification. Pattern Recognit. 2017 , 61 , 539–556. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Kabari, L.G.; Onwuka, U. Comparison of Bagging and Voting Ensemble Machine Learning Algorithm as a Classifier. Int. J. Adv. Res. Comput. Sci. Softw. Eng. 2019 , 9 , 1–6. [ Google Scholar ]
- Chouhan, V.; Singh, S.K.; Khamparia, A.; Gupta, D.; Albuquerque, V.H.C.D. A Novel Transfer Learning Based Approach for Pneumonia Detection in Chest X-ray Images. Appl. Sci. 2020 , 10 , 559. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Lincoln, W.P.; Skrzypekt, J. Synergy of Clustering Multiple Back Propagation Networks. Adv. Neural Inf. Process. Syst. 1990 , 2 , 650–659. [ Google Scholar ]
- Lakhani, P.; Sundaram, B. Deep Learning at Chest Radiography: Automated Classification of Pulmonary Tuberculosis by Using Convolutional Neural Networks. Radiology 2017 , 284 , 574–582. [ Google Scholar ] [ CrossRef ]
- Divina, F.; Gilson, A.; Goméz-Vela, F.; Torres, M.G.; Torres, J.F. Stacking Ensemble Learning for Short-Term Electricity Consumption Forecasting. Energies 2018 , 11 , 949. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- World Health Organisation. Global Health TB Report ; World Health Organisation: Geneva, Switzerland, 2018; p. 277. [ Google Scholar ]
- Murphy, K.; Habib, S.S.; Zaidi, S.M.A.; Khowaja, S.; Khan, A.; Melendez, J.; Scholten, E.T.; Amad, F.; Schalekamp, S.; Verhagen, M.; et al. Computer aided detection of tuberculosis on chest radiographs: An evaluation of the CAD4TB v6 system. Sci. Rep. 2019 , 10 , 1–11. [ Google Scholar ] [ CrossRef ]
- Melendez, J.; Sánchez, C.I.; Philipsen, R.H.; Maduskar, P.; Dawson, R.; Theron, G.; Dheda, K.; Van Ginneken, B. An automated tuberculosis screening strategy combining X-ray-based computer-aided detection and clinical information. Sci. Rep. 2016 , 6 , 1–8. [ Google Scholar ] [ CrossRef ]
- Heo, S.J.; Kim, Y.; Yun, S.; Lim, S.S.; Kim, J.; Nam, C.M.; Park, E.C.; Jung, I.; Yoon, J.H. Deep Learning Algorithms with Demographic Information Help to Detect Tuberculosis in Chest Radiographs in Annual Workers’ Health Examination Data. Int. J. Environ. Res. Public Health 2019 , 16 , 250. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Pasa, F.; Golkov, V.; Pfeiffer, F.; Cremers, D.; Pfeiffer, D. Efficient Deep Network Architectures for Fast Chest X-Ray Tuberculosis Screening and Visualization. Sci. Rep. 2019 , 9 , 2–10. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Cao, Y.; Liu, C.; Liu, B.; Brunette, M.J.; Zhang, N.; Sun, T.; Zhang, P.; Peinado, J.; Garavito, E.S.; Garcia, L.L.; et al. Improving Tuberculosis Diagnostics Using Deep Learning and Mobile Health Technologies among Resource-Poor and Marginalized Communities. In Proceedings of the 2016 IEEE 1st International Conference on Connected Health: Applications, Systems and Engineering Technologies, CHASE, Washington, DC, USA, 27–29 June 2016; pp. 274–281. [ Google Scholar ] [ CrossRef ]
- Liu, J.; Liu, Y.; Wang, C.; Li, A.; Meng, B. An Original Neural Network for Pulmonary Tuberculosis Diagnosis in Radiographs. In Lecture Notes in Computer Science, Proceedings of the International Conference on Artificial Neural Networks, Rhodes, Greece, 4–7 October 2018 ; Springer: Berlin/Heidelberg, Germany, 2018; pp. 158–166. [ Google Scholar ] [ CrossRef ]
- Stirenko, S.; Kochura, Y.; Alienin, O. Chest X-Ray Analysis of Tuberculosis by Deep Learning with Segmentation and Augmentation. In Proceedings of the 2018 IEEE 38th International Conference on Electronics andNanotechnology (ELNANO), Kiev, Ukraine, 24–26 April 2018; pp. 422–428. [ Google Scholar ]
- Andika, L.A.; Pratiwi, H.; Sulistijowati Handajani, S. Convolutional neural network modeling for classification of pulmonary tuberculosis disease. J. Phys. Conf. Ser. 2020 , 1490 . [ Google Scholar ] [ CrossRef ]
- Ul Abideen, Z.; Ghafoor, M.; Munir, K.; Saqib, M.; Ullah, A.; Zia, T.; Tariq, S.A.; Ahmed, G.; Zahra, A. Uncertainty assisted robust tuberculosis identification with bayesian convolutional neural networks. IEEE Access 2020 , 8 , 22812–22825. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hwang, E.J.; Park, S.; Jin, K.N.; Kim, J.I.; Choi, S.Y.; Lee, J.H.; Goo, J.M.; Aum, J.; Yim, J.J.; Park, C.M. Development and Validation of a Deep Learning—based Automatic Detection Algorithm for Active Pulmonary Tuberculosis on Chest Radiographs. Clin. Infect. Dis. 2019 , 69 , 739–747. [ Google Scholar ] [ CrossRef ]
- Hwang, S.; Kim, H.E.; Jeong, J.; Kim, H.J. A Novel Approach for Tuberculosis Screening Based on Deep Convolutional Neural Networks. Med. Imaging 2016 , 9785 , 1–8. [ Google Scholar ] [ CrossRef ]
- Islam, M.T.; Aowal, M.A.; Minhaz, A.T.; Ashraf, K. Abnormality Detection and Localization in Chest X-Rays using Deep Convolutional Neural Networks. arXiv 2017 , arXiv:1705.09850v3. [ Google Scholar ]
- Nguyen, Q.H.; Nguyen, B.P.; Dao, S.D.; Unnikrishnan, B.; Dhingra, R.; Ravichandran, S.R.; Satpathy, S.; Raja, P.N.; Chua, M.C.H. Deep Learning Models for Tuberculosis Detection from Chest X-ray Images. In Proceedings of the 2019 26th International Conference on Telecommunications (ICT), Hanoi, Vietnam, 8–10 April 2019; pp. 381–385. [ Google Scholar ] [ CrossRef ]
- Kieu, T.; Ho, K.; Gwak, J.; Prakash, O. Utilizing Pretrained Deep Learning Models for Automated Pulmonary Tuberculosis Detection Using Chest Radiography. Intell. Inf. Database Syst. 2019 , 4 , 395–403. [ Google Scholar ] [ CrossRef ]
- Abbas, A.; Abdelsamea, M.M. Learning Transformations for Automated Classification of Manifestation of Tuberculosis using Convolutional Neural Network. In Proceedings of the 2018 13th International Conference on Computer Engineering andSystems (ICCES), Cairo, Egypt, 18–19 December 2018; IEEE: New York, NY, USA, 2018; pp. 122–126. [ Google Scholar ]
- Karnkawinpong, T.; Limpiyakorn, Y. Classification of pulmonary tuberculosis lesion with convolutional neural networks. J. Phys. Conf. Ser. 2018 , 1195 . [ Google Scholar ] [ CrossRef ]
- Liu, C.; Cao, Y.; Alcantara, M.; Liu, B.; Brunette, M.; Peinado, J.; Curioso, W. TX-CNN: Detecting Tuberculosis in Chest X-Ray Images Using Convolutional Neural Network. In Proceedings of the 2017 IEEE International Conference on Image Processing (ICIP), Beijing, China, 17–20 September 2017. [ Google Scholar ]
- Yadav, O.; Passi, K.; Jain, C.K. Using Deep Learning to Classify X-ray Images of Potential Tuberculosis Patients. In Proceedings of the 2018 IEEE International Conference on Bioinformatics and Biomedicine(BIBM), Madrid, Spain, 3–6 December 2018; IEEE: New York, NY, USA, 2018; pp. 2368–2375. [ Google Scholar ]
- Sahlol, A.T.; Elaziz, M.A.; Jamal, A.T.; Damaševičius, R.; Hassan, O.F. A novel method for detection of tuberculosis in chest radiographs using artificial ecosystem-based optimisation of deep neural network features. Symmetry 2020 , 12 , 1146. [ Google Scholar ] [ CrossRef ]
- Hooda, R.; Mittal, A.; Sofat, S. Automated TB classification using ensemble of deep architectures. Multimed. Tools Appl. 2019 , 78 , 31515–31532. [ Google Scholar ] [ CrossRef ]
- Rashid, R.; Khawaja, S.G.; Akram, M.U.; Khan, A.M. Hybrid RID Network for Efficient Diagnosis of Tuberculosis from Chest X-rays. In Proceedings of the 2018 9th Cairo International Biomedical Engineering Conference(CIBEC), Cairo, Egypt, 20–22 December 2018; IEEE: New York, NY, USA, 2018; pp. 167–170. [ Google Scholar ]
- Kieu, S.T.H.; Hijazi, M.H.A.; Bade, A.; Saffree Jeffree, M. Tuberculosis detection using deep learning and contrast-enhanced canny edge detected x-ray images. IAES Int. J. Artif. Intell. 2020 , 9 . [ Google Scholar ] [ CrossRef ]
- Rajaraman, S.; Antani, S.K. Modality-Specific Deep Learning Model Ensembles Toward Improving TB Detection in Chest Radiographs. IEEE Access 2020 , 8 , 27318–27326. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Melendez, J.; Ginneken, B.V.; Maduskar, P.; Philipsen, R.H.H.M.; Reither, K.; Breuninger, M.; Adetifa, I.M.O.; Maane, R.; Ayles, H.; Sánchez, C.I. A Novel Multiple-Instance Learning-Based Approach to Computer-Aided Detection of Tuberculosis on Chest X-Rays. IEEE Trans. Med. Imaging 2014 , 34 , 179–192. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Becker, A.S.; Bluthgen, C.; van Phi, V.D.; Sekaggya-Wiltshire, C.; Castelnuovo, B.; Kambugu, A.; Fehr, J.; Frauenfelder, T. Detection of tuberculosis patterns in digital photographs of chest X-ray images using Deep Learning: Feasibility study. Int. J. Tuberc. Lung Dis. 2018 , 22 , 328–335. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Li, L.; Huang, H.; Jin, X. AE-CNN Classification of Pulmonary Tuberculosis Based on CT images. In Proceedings of the 2018 9th International Conference on Information Technology inMedicine and Education (ITME), Hangzhou, China, 19–21 October 2018; IEEE: New York, NY, USA, 2018; pp. 39–42. [ Google Scholar ] [ CrossRef ]
- Pattnaik, A.; Kanodia, S.; Chowdhury, R.; Mohanty, S. Predicting Tuberculosis Related Lung Deformities from CT Scan Images Using 3D CNN ; CEUR-WS: Lugano, Switzerland, 2019; pp. 9–12. [ Google Scholar ]
- Zunair, H.; Rahman, A.; Mohammed, N. Estimating Severity from CT Scans of Tuberculosis Patients using 3D Convolutional Nets and Slice Selection ; CEUR-WS: Lugano, Switzerland, 2019; pp. 9–12. [ Google Scholar ]
- Llopis, F.; Fuster-Guillo, A.; Azorin-Lopez, J.; Llopis, I. Using improved optical flow model to detect Tuberculosis ; CEUR-WS: Lugano, Switzerland, 2019; pp. 9–12. [ Google Scholar ]
- Wardlaw, T.; Johansson, E.W.; Hodge, M. Pneumonia: The Forgotten Killer of Children ; United Nations Children’s Fund (UNICEF): New York, NY, USA, 2006; p. 44. [ Google Scholar ]
- Wunderink, R.G.; Waterer, G. Advances in the causes and management of community acquired pneumonia in adults. BMJ 2017 , 1–13. [ Google Scholar ] [ CrossRef ]
- Tobias, R.R.; De Jesus, L.C.M.; Mital, M.E.G.; Lauguico, S.C.; Guillermo, M.A.; Sybingco, E.; Bandala, A.A.; Dadios, E.P. CNN-based Deep Learning Model for Chest X-ray Health Classification Using TensorFlow. In Proceedings of the 2020 RIVF International Conference on Computing and Communication Technologies, RIVF 2020, Ho Chi Minh, Vietnam, 14–15 October 2020. [ Google Scholar ]
- Stephen, O.; Sain, M.; Maduh, U.J.; Jeong, D.U. An Efficient Deep Learning Approach to Pneumonia Classification in Healthcare. J. Healthc. Eng. 2019 , 2019 . [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Kermany, D.S.; Goldbaum, M.; Cai, W.; Lewis, M.A. Identifying Medical Diagnoses and Treatable Diseases by Image-Based Deep Learning. Cell 2018 , 172 , 1122–1131.e9. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Young, J.C.; Suryadibrata, A. Applicability of Various Pre-Trained Deep Convolutional Neural Networks for Pneumonia Classification based on X-Ray Images. Int. J. Adv. Trends Comput. Sci. Eng. 2020 , 9 , 2649–2654. [ Google Scholar ] [ CrossRef ]
- Moujahid, H.; Cherradi, B.; Gannour, O.E.; Bahatti, L.; Terrada, O.; Hamida, S. Convolutional Neural Network Based Classification of Patients with Pneumonia using X-ray Lung Images. Adv. Sci. Technol. Eng. Syst. 2020 , 5 , 167–175. [ Google Scholar ] [ CrossRef ]
- Rajpurkar, P.; Irvin, J.; Zhu, K.; Yang, B.; Mehta, H.; Duan, T.; Ding, D.; Bagul, A.; Ball, R.L.; Langlotz, C.; et al. CheXNet: Radiologist-Level Pneumonia Detection on Chest X-Rays with Deep Learning. arXiv 2017 , arXiv:1711.05225v3. [ Google Scholar ]
- Rahman, T.; Chowdhury, M.E.H.; Khandakar, A.; Islam, K.R.; Islam, K.F.; Mahbub, Z.B.; Kadir, M.A.; Kashem, S. Transfer Learning with Deep Convolutional Neural Network (CNN) for Pneumonia Detection Using Chest X-ray. Appl. Sci. 2020 , 10 , 3233. [ Google Scholar ] [ CrossRef ]
- Hashmi, M.; Katiyar, S.; Keskar, A.; Bokde, N.; Geem, Z. Efficient Pneumonia Detection in Chest Xray Images Using Deep Transfer Learning. Diagnostics 2020 , 10 , 417. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Acharya, A.K.; Satapathy, R. A Deep Learning Based Approach towards the Automatic Diagnosis of Pneumonia from Chest Radio-Graphs. Biomed. Pharmacol. J. 2020 , 13 , 449–455. [ Google Scholar ] [ CrossRef ]
- Elshennawy, N.M.; Ibrahim, D.M. Deep-Pneumonia Framework Using Deep Learning Models Based on Chest X-Ray Images. Diagnostics 2020 , 10 , 649. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Emhamed, R.; Mamlook, A.; Chen, S. Investigation of the performance of Machine Learning Classifiers for Pneumonia Detection in Chest X-ray Images. In Proceedings of the 2020 IEEE International Conference on Electro Information Technology (EIT), Chicago, IL, USA, 31 July–1 August 2020; pp. 98–104. [ Google Scholar ]
- Kumar, A.; Tiwari, P.; Kumar, S.; Gupta, D.; Khanna, A. Identifying pneumonia in chest X-rays: A deep learning approach. Measurement 2019 , 145 , 511–518. [ Google Scholar ] [ CrossRef ]
- Hurt, B.; Yen, A.; Kligerman, S.; Hsiao, A. Augmenting Interpretation of Chest Radiographs with Deep Learning Probability Maps. J. Thorac. Imaging 2020 , 35 , 285–293. [ Google Scholar ] [ CrossRef ]
- Borczuk, A.C. Benign tumors and tumorlike conditions of the lung. Arch. Pathol. Lab. Med. 2008 , 132 , 1133–1148. [ Google Scholar ] [ CrossRef ]
- Hua, K.L.; Hsu, C.H.; Hidayati, S.C.; Cheng, W.H.; Chen, Y.J. Computer-aided classification of lung nodules on computed tomography images via deep learning technique. OncoTargets Ther. 2015 , 8 , 2015–2022. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Kurniawan, E.; Prajitno, P.; Soejoko, D.S. Computer-Aided Detection of Mediastinal Lymph Nodes using Simple Architectural Convolutional Neural Network. J. Phys. Conf. Ser. 2020 , 1505 . [ Google Scholar ] [ CrossRef ]
- Ciompi, F.; Chung, K.; Van Riel, S.J.; Setio, A.A.A.; Gerke, P.K.; Jacobs, C.; Th Scholten, E.; Schaefer-Prokop, C.; Wille, M.M.; Marchianò, A.; et al. Towards automatic pulmonary nodule management in lung cancer screening with deep learning. Sci. Rep. 2017 , 7 , 1–11. [ Google Scholar ] [ CrossRef ]
- Shakeel, P.M.; Burhanuddin, M.A.; Desa, M.I. Lung cancer detection from CT image using improved profuse clustering and deep learning instantaneously trained neural networks. Meas. J. Int. Meas. Confed. 2019 , 145 , 702–712. [ Google Scholar ] [ CrossRef ]
- Chen, S.; Han, Y.; Lin, J.; Zhao, X.; Kong, P. Pulmonary nodule detection on chest radiographs using balanced convolutional neural network and classic candidate detection. Artif. Intell. Med. 2020 , 107 , 101881. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hosny, A.; Parmar, C.; Coroller, T.P.; Grossmann, P.; Zeleznik, R.; Kumar, A.; Bussink, J.; Gillies, R.J.; Mak, R.H.; Aerts, H.J. Deep learning for lung cancer prognostication: A retrospective multi-cohort radiomics study. PLoS Med. 2018 , 15 , 1–25. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Xu, Y.; Hosny, A.; Zeleznik, R.; Parmar, C.; Coroller, T.; Franco, I.; Mak, R.H.; Aerts, H.J. Deep learning predicts lung cancer treatment response from serial medical imaging. Clin. Cancer Res. 2019 , 25 , 3266–3275. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Kuan, K.; Ravaut, M.; Manek, G.; Chen, H.; Lin, J.; Nazir, B.; Chen, C.; Howe, T.C.; Zeng, Z.; Chandrasekhar, V. Deep Learning for Lung Cancer Detection: Tackling the Kaggle Data Science Bowl 2017 Challenge. arXiv 2017 , arXiv:1705.09435. [ Google Scholar ]
- Ausawalaithong, W.; Thirach, A.; Marukatat, S.; Wilaiprasitporn, T. Automatic Lung Cancer Prediction from Chest X-ray Images Using the Deep Learning Approach. In Proceedings of the 2018 11th Biomedical Engineering International Conference (BMEiCON), Chiang Mai, Thailand, 21–24 November 2018. [ Google Scholar ]
- Xu, S.; Guo, J.; Zhang, G.; Bie, R. Automated detection of multiple lesions on chest X-ray images: Classification using a neural network technique with association-specific contexts. Appl. Sci. 2020 , 10 , 1742. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020 , 395 , 497–506. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Velavan, T.P.; Meyer, C.G. The COVID-19 epidemic. Trop. Med. Int. Health 2020 , 25 , 278–280. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Shibly, K.H.; Dey, S.K.; Islam, M.T.U.; Rahman, M.M. COVID faster R–CNN: A novel framework to Diagnose Novel Coronavirus Disease (COVID-19) in X-Ray images. Inform. Med. Unlocked 2020 , 20 , 100405. [ Google Scholar ] [ CrossRef ]
- Alsharman, N.; Jawarneh, I. GoogleNet CNN neural network towards chest CT-coronavirus medical image classification. J. Comput. Sci. 2020 , 16 , 620–625. [ Google Scholar ] [ CrossRef ]
- Zhu, J.; Shen, B.; Abbasi, A.; Hoshmand-Kochi, M.; Li, H.; Duong, T.Q. Deep transfer learning artificial intelligence accurately stages COVID-19 lung disease severity on portable chest radiographs. PLoS ONE 2020 , 15 , e0236621. [ Google Scholar ] [ CrossRef ]
- Sethi, R.; Mehrotra, M.; Sethi, D. Deep Learning based Diagnosis Recommendation for COVID-19 using Chest X-Rays Images. In Proceedings of the 2020 Second International Conference on Inventive Research in Computing Applications (ICIRCA), Coimbatore, India, 15–17 July 2020. [ Google Scholar ]
- Das, D.; Santosh, K.C.; Pal, U. Truncated inception net: COVID-19 outbreak screening using chest X-rays. Phys. Eng. Sci. Med. 2020 , 43 , 915–925. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Panwar, H.; Gupta, P.K.; Siddiqui, M.K.; Morales-Menendez, R.; Singh, V. Application of deep learning for fast detection of COVID-19 in X-Rays using nCOVnet. Chaos Solitons Fractals 2020 , 138 , 109944. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Narin, A.; Kaya, C.; Pamuk, Z. Automatic Detection of Coronavirus Disease (COVID-19) Using X-ray Images and Deep Convolutional Neural Networks. arXiv 2020 , arXiv:2003.10849. [ Google Scholar ]
- Apostolopoulos, I.D.; Mpesiana, T.A. Covid—19: Automatic detection from X-ray images utilizing transfer learning with convolutional neural networks. Phys. Eng. Sci. Med. 2020 , 1–6. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Chowdhury, M.E.H.; Rahman, T.; Khandakar, A.; Mazhar, R.; Kadir, M.A.; Reaz, M.B.I.; Mahbub, Z.B.; Islam, K.R.; Salman, M.; Iqbal, A.; et al. Can AI help in screening Viral and COVID-19 pneumonia? arXiv 2020 , arXiv:2003.13145. [ Google Scholar ] [ CrossRef ]
- Wang, L.; Lin, Z.Q.; Wong, A. COVID-Net: A Tailored Deep Convolutional Neural Network Design for Detection of COVID-19 Cases from Chest X-Ray Images. Sci. Rep. 2020 , 10 , 1–12. [ Google Scholar ] [ CrossRef ]
- Sethy, P.K.; Behera, S.K.; Ratha, P.K.; Biswas, P. Detection of coronavirus disease (COVID-19) based on deep features and support vector machine. Int. J. Math. Eng. Manag. Sci. 2020 , 5 , 643–651. [ Google Scholar ] [ CrossRef ]
- Islam, M.Z.; Islam, M.M.; Asraf, A. A combined deep CNN-LSTM network for the detection of novel coronavirus (COVID-19) using X-ray images. Inform. Med. Unlocked 2020 , 20 , 100412. [ Google Scholar ] [ CrossRef ]
- Shorfuzzaman, M.; Masud, M. On the detection of covid-19 from chest x-ray images using cnn-based transfer learning. Comput. Mater. Contin. 2020 , 64 , 1359–1381. [ Google Scholar ] [ CrossRef ]
- Li, L.; Qin, L.; Xu, Z.; Yin, Y.; Wang, X.; Kong, B.; Bai, J.; Lu, Y.; Fang, Z.; Song, Q.; et al. Using Artificial Intelligence to Detect COVID-19 and Community-acquired Pneumonia Based on Pulmonary CT: Evaluation of the Diagnostic Accuracy. Radiology 2020 , 296 , 65–71. [ Google Scholar ] [ CrossRef ]
- Alazab, M.; Awajan, A.; Mesleh, A.; Abraham, A.; Jatana, V.; Alhyari, S. COVID-19 prediction and detection using deep learning. Int. J. Comput. Inf. Syst. Ind. Manag. Appl. 2020 , 12 , 168–181. [ Google Scholar ]
- Waheed, A.; Goyal, M.; Gupta, D.; Khanna, A.; Al-Turjman, F.; Pinheiro, P.R. CovidGAN: Data Augmentation Using Auxiliary Classifier GAN for Improved Covid-19 Detection. IEEE Access 2020 , 8 , 91916–91923. [ Google Scholar ] [ CrossRef ]
- Ouyang, X.; Huo, J.; Xia, L.; Shan, F.; Liu, J.; Mo, Z.; Yan, F.; Ding, Z.; Yang, Q.; Song, B.; et al. Dual-Sampling Attention Network for Diagnosis of COVID-19 from Community Acquired Pneumonia. IEEE Trans. Med. Imaging 2020 , 39 , 2595–2605. [ Google Scholar ] [ CrossRef ]
- Mahmud, T.; Rahman, M.A.; Fattah, S.A. CovXNet: A multi-dilation convolutional neural network for automatic COVID-19 and other pneumonia detection from chest X-ray images with transferable multi-receptive feature optimization. Comput. Biol. Med. 2020 , 122 , 103869. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Shi, F.; Xia, L.; Shan, F.; Wu, D.; Wei, Y.; Yuan, H.; Jiang, H. Large-Scale Screening of COVID-19 from Community Acquired Pneumonia using Infection Size-Aware Classification. arXiv 2020 , arXiv:2003.09860. [ Google Scholar ]
- Breiman, L. Random forests. Mach. Learn. 2001 , 45 , 5–32. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Xu, X.; Jiang, X.; Ma, C.; Du, P.; Li, X.; Ly, S.; Yu, L.; Chen, Y.; Su, J.; Lang, G.; et al. A Deep Learning System to Screen Novel Coronavirus Disease 2019 Pneumonia. Engineering 2020 . [ Google Scholar ] [ CrossRef ]
- Singh, D.; Kumar, V.; Kaur, M. Classification of COVID-19 patients from chest CT images using multi-objective differential evolution–based convolutional neural networks. Eur. J. Clin. Microbiol. Infect. Dis. 2020 , 39 , 1379–1389. [ Google Scholar ] [ CrossRef ]
- Sedik, A.; Iliyasu, A.M.; El-Rahiem, B.A.; Abdel Samea, M.E.; Abdel-Raheem, A.; Hammad, M.; Peng, J.; Abd El-Samie, F.E.; Abd El-Latif, A.A. Deploying machine and deep learning models for efficient data-augmented detection of COVID-19 infections. Viruses 2020 , 12 , 769. [ Google Scholar ] [ CrossRef ]
- Ahsan, M.M.; Alam, T.E.; Trafalis, T.; Huebner, P. Deep MLP-CNN model using mixed-data to distinguish between COVID-19 and Non-COVID-19 patients. Symmetry 2020 , 12 , 1526. [ Google Scholar ] [ CrossRef ]
- Afshar, P.; Heidarian, S.; Naderkhani, F.; Oikonomou, A.; Plataniotis, K.N.; Mohammadi, A. COVID-CAPS: A capsule network-based framework for identification of COVID-19 cases from X-ray images. Pattern Recognit. Lett. 2020 , 138 , 638–643. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rosenthal, A.; Gabrielian, A.; Engle, E.; Hurt, D.E.; Alexandru, S.; Crudu, V.; Sergueev, E.; Kirichenko, V.; Lapitskii, V.; Snezhko, E.; et al. The TB Portals: An Open-Access, Web- Based Platform for Global Drug-Resistant- Tuberculosis Data Sharing and Analysis. J. Clin. Microbiol. 2017 , 55 , 3267–3282. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Cid, Y.D.; Liauchuk, V.; Klimuk, D.; Tarasau, A. Overview of ImageCLEFtuberculosis 2019—Automatic CT—Based Report Generation and Tuberculosis Severity Assessment ; CEUR-WS: Lugano, Switzerland, 2019; pp. 9–12. [ Google Scholar ]
- Demner-Fushman, D.; Kohli, M.D.; Rosenman, M.B.; Shooshan, S.E.; Rodriguez, L.; Antani, S.; Thoma, G.R.; McDonald, C.J. Preparing a collection of radiology examinations for distribution and retrieval. J. Am. Med. Inform. Assoc. 2016 , 23 , 304–310. [ Google Scholar ] [ CrossRef ]
- Shiraishi, J.; Katsuragawa, S.; Ikezoe, J.; Matsumoto, T.; Kobayashi, T.; Komatsu, K.I.; Matsui, M.; Fujita, H.; Kodera, Y.; Doi, K. Development of a digital image database for chest radiographs with and without a lung nodule: Receiver operating characteristic analysis of radiologists’ detection of pulmonary nodules. Am. J. Roentgenol. 2000 , 174 , 71–74. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jaeger, S.; Candemir, S.; Antani, S.; Wáng, Y.x.J.; Lu, P.x.; Thoma, G. Two public chest X-ray datasets for computer-aided screening of pulmonary diseases. Quant. Imaging Med. Surg. 2014 , 4 , 475–477. [ Google Scholar ] [ CrossRef ]
- Xiaosong, W.; Yifan, P.; Le, L.; Lu, Z.; Mohammadhadi, B.; Summers, R.M. ChestX-ray8: Hospital-scale chest X-ray database and benchmarks on weakly-supervised classification and localization of common thorax diseases. In Proceedings of the IEEE conference on computer vision and pattern recognition, Honolulu, HI, USA, 21–26 July 2017; pp. 3462–3471. [ Google Scholar ]
- Costa, M.G.; Filho, C.F.; Kimura, A.; Levy, P.C.; Xavier, C.M.; Fujimoto, L.B. A sputum smear microscopy image database for automatic bacilli detection in conventional microscopy. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology ociety, EMBC, Chicago, IL, USA, 26–30 August 2014; pp. 2841–2844. [ Google Scholar ] [ CrossRef ]
- Armato, S.G.; McLennan, G.; Bidaut, L.; McNitt-Gray, M.F.; Meyer, C.R.; Reeves, A.P.; Zhao, B.; Aberle, D.R.; Henschke, C.I.; Hoffman, E.A.; et al. The Lung Image Database Consortium (LIDC) and Image Database Resource Initiative (IDRI): A completed reference database of lung nodules on CT scans. Med. Phys. 2011 , 38 , 915–931. [ Google Scholar ] [ CrossRef ]
- Grossman, R.L.; Allison, P.; Ferrentti, V.; Varmus, H.E.; Lowy, D.R.; Kibbe, W.A.; Staudt, L.M. Toward a Shared Vision for Cancer Genomic Data. N. Engl. J. Med. 2016 , 375 , 1109–1112. [ Google Scholar ] [ CrossRef ]
- Cohen, J.P.; Morrison, P.; Dao, L.; Roth, K.; Duong, T.Q.; Ghassemi, M. COVID-19 Image Data Collection: Prospective Predictions Are the Future. arXiv 2020 , arXiv:2006.11988. [ Google Scholar ]
Click here to enlarge figure
| Authors | Deep Learning Technique | Features | Dataset |
|---|---|---|---|
| [ ] | CNN with transfer learning and data augmentation | Features extracted from CNN | Montgomery |
| [ ] | K-nearest neighbour, Simple Linear Regression and Sequential Minimal Optimisation (SMO) Classification | Area, major axis, minor axis, eccentricity, mean, kurtosis, skewness and entropy | Shenzhen |
| [ ] | ViDi | Features extracted from CNN | Unspecified |
| [ ] | CNN | Gabor, LBP, SIFT, PHOG and Features extracted from CNN | Private dataset |
| [ ] | CNN | Features extracted from CNN | ImageCLEF 2018 dataset |
| [ ] | CNN with transfer learning, with demographic information | Features extracted from CNN + demographic information | Private dataset |
| [ ] | CNN with data augmentation, and ensemble by weighted averages of probability scores | Features extracted from CNN | Montgomery, Shenzhen, Belarus, JSRT |
| [ ] | CNN with transfer learning and data augmentation | Features extracted from CNN | Private dataset, Montgomery, Shenzhen |
| [ ] | CNN | Features extracted from CNN | Private datasets, Montgomery, Shenzhen |
| [ ] | CNN with transfer learning and ensemble by simple linear probabilities averaging | Features extracted from CNN + rule-based features | Indiana, JSRT, Shenzhen |
| [ ] | CNN | HoG features | ZiehlNeelsen Sputum smear Microscopy image DataBase |
| [ ] | CNN and shuffle sampling | Features extracted from CNN | Private datasets |
| [ ] | CNN with transfer learning and ensemble by averaging | CNN extracted features from edge images | Montgomery, Shenzhen |
| [ ] | CNN with transfer learning, data augmentation and ensemble by weighted probability scores average | Features extracted from CNN | Private dataset, Montgomery, Shenzhen, Belarus |
| [ ] | AutoEncoder-CNN | Features extracted from CNN | Private dataset |
| [ ] | CNN with transfer learning and shuffle sampling | Features extracted from CNN | Private dataset |
| [ ] | End-to-end CNN | Features extracted from CNN | Montgomery, Shenzhen |
| [ ] | Optical flow model | Activity Description Vector on optical flow of video sequences | ImageCLEF 2019 dataset |
| [ ] | CNN | Colours | TBimages dataset |
| [ ] | Modified maximum pattern margin support vector machine (modified miSVM) | First four moments of the intensity distributions | Private datasets |
| [ ] | CAD4TB with clinical information | Features extracted from CNN + clinical features | Private dataset |
| [ ] | DBN | LoH + SURF features | ZiehlNeelsen Sputum smear Microscopy image DataBase |
| [ ] | CAD4TB | Features extracted from CNN | Private dataset |
| [ ] | CNN with transfer learning and data augmentation | Features extracted from CNN | Montgomery, Shenzhen, NIH-14 dataset |
| [ ] | CNN | Features extracted from CNN | TBimages dataset |
| [ ] | CNN from scratch and data augmentation | Features extracted from CNN | Montgomery, Shenzhen, Belarus |
| [ ] | 3D CNN | Features extracted from CNN + lung volume + patient attribute metadata | ImageCLEF 2019 dataset |
| [ ] | CNN with transfer learning and ensemble by stacking | local and global feature descriptors + features extracted from CNN | Private dataset, Montgomery, Shenzhen, India |
| [ ] | CNN with transfer learning and feature level ensemble | Features extracted from CNN | Shenzhen |
| [ ] | CNN with transfer learning and ensemble by averaging | CNN extracted features from edge images | Montgomery, Shenzhen |
| [ ] | CNN with transfer learning | Features extracted from CNN | ZiehlNeelsen Sputum smear Microscopy image DataBase |
| [ ] | CNN with data augmentation | Features extracted from CNN | Shenzhen |
| [ ] | CNN with transfer learning and data augmentation | Features extracted from CNN | NIH-14, Montgomery, Shenzhen |
| [ ] | CNN with transfer learning, Bag of CNN Features and ensemble by a simple soft-voting scheme | Features extracted from CNN + BOW | Private dataset, Montgomery, Shenzhen |
| [ ] | Neural network | Shape, curvature descriptor histograms, eigenvalues of Hessian matrix | Montgomery, Shenzhen |
| [ ] | CNN with transfer learning and data augmentation | Features extracted from CNN | Montgomery, Shenzhen, NIH-14 |
| [ ] | 3D CNN | Features extracted from CNN | ImageCLEF 2019 dataset |
| [ ] | CNN and Artificial Ecosystem-based Optimisation algorithm | Features extracted from CNN | Shenzhen |
| [ ] | CNN | Features extracted from CNN | Shenzhen |
| [ ] | Bayesian based CNN | Features extracted from CNN | Montgomery, Shenzhen |
| [ ] | CNN with transfer learning, and ensemble by majority voting, simple averaging, weighted averaging, and stacking | Features extracted from CNN | Montgomery, Shenzhen, LDOCTCXR, 2018 RSNA pneumonia challenge dataset, Indiana dataset |
| Reference | Deep Learning Technique | Features | Dataset |
|---|---|---|---|
| [ ] | Deep Siamese based neural network | CNN extracted features from the left half and right half of the lungs | Unspecified Kaggle dataset |
| [ ] | CNN with transfer learning and data augmentation | Features extracted from CNN | LDOCTCXR |
| [ ] | CNN with transfer learning, data augmentation and ensemble by majority voting. | Features extracted from CNN | LDOCTCXR |
| [ ] | CNN with transfer learning | Features extracted from CNN | LDOCTCXR |
| [ ] | CNN with transfer learning, data augmentation and ensemble by combining confidence scores and bounding boxes. | Features extracted from CNN | Radiological Society of North America (RSNA) pneumonia dataset |
| [ ] | CNN with transfer learning and data augmentation | Features extracted from CNN | NIH Chest X-ray Dataset |
| [ ] | CNN from scratch and data augmentation | Features extracted from CNN | LDOCTCXR |
| [ ] | CNN with transfer learning | Features extracted from CNN | LDOCTCXR |
| [ ] | CNN | Features extracted from CNN | Mooney’s Kaggle dataset |
| [ ] | CNN and LSTM-CNN, with transfer learning and data augmentation | Features extracted from CNN | Mooney’s Kaggle dataset |
| [ ] | CNN with probabilistic map of pneumonia | Features extracted from CNN | 2018 RSNA pneumonia challenge dataset |
| [ ] | Decision Tree, Random Forest, K-nearest neighbour, AdaBoost, Gradient Boost, XGBboost, CNN | Multiple features | Mooney’s Kaggle dataset |
| [ ] | CNN with transfer learning, data augmentation and ensemble by weighted averaging | Features extracted from CNN | LDOCTCXR |
| [ ] | CNN with transfer learning and data augmentation | Features extracted from CNN | Mooney’s Kaggle dataset |
| [ ] | CNN with transfer learning | Features extracted from CNN | Private dataset |
| Reference | Deep Learning Technique | Features | Dataset |
|---|---|---|---|
| [ ] | CNN | Features extracted from CNN | LUNA, LIDC, NLST |
| [ ] | CNN with transfer learning | Features extracted from CNN | JSRT Dataset, NIH-14 dataset |
| [ ] | Multi-stream multi-scale convolutional networks | Features extracted from CNN | MILD dataset DLCST dataset |
| [ ] | CNN with transfer learning | Features extracted from CNN | NCI Genomic Data Commons |
| [ ] | CNN with transfer learning and data augmentation | Features extracted from CNN | NSCLC-Radiomics, NSCLC-Radiomics-Genomics, RIDER Collections and several private datasets |
| [ ] | CNN and DBN | Features extracted from CNN and DBN | LIDC-IDRI |
| [ ] | CNN with transfer learning | Features extracted from CNN | Kaggle Data Science Bowl 2017 dataset, Lung Nodule Analysis 2016 (LUNA16) dataset |
| [ ] | CNN | Features extracted from CNN | LIDC-IDRI |
| [ ] | CNN | Features extracted from CNN | LIDC-IDRI |
| [ ] | CNN with data augmentation | Features extracted from CNN | LIDC-IDRI database |
| [ ] | CNN with transfer learning and data augmentation | Features extracted from CNN | Private dataset |
| [ ] | Bone elimination and lung segmentation before training with CNN | Features extracted using CNN from bone eliminated lung images and segmented lung images | JSRT dataset |
| [ ] | CNN-long short-term memory network | Features extracted from CNN | NIH-14 dataset |
| [ ] | CNN with transfer learning and data augmentation | Features extracted from CNN | JSRT database |
| [ ] | CNN with data augmentation | Features extracted from CNN | Cancer Imaging Archive |
| Authors | Deep Learning Technique | Features | Dataset |
|---|---|---|---|
| [ ] | CNN with transfer learning and location-attention classification mechanism | Features extracted from CNN | Private dataset |
| [ ] | CNN with transfer learning and data augmentation | Features extracted from CNN | SIRM database, Cohen’s Github dataset, Chowdhury’s Kaggle dataset |
| [ ] | RADLogics Inc., CNN with transfer learning and data augmentation | Features extracted from RADLogics Inc and CNN | Chainz Dataset, A dataset from a hospital in Wenzhou, China, Dataset from El-Camino Hospital (CA) and Lung image database consortium (LIDC) |
| [ ] | CNN with transfer learning | Features extracted from CNN | Cohen’s Github dataset and LDOCTCXR |
| [ ] | CNN with transfer learning and data augmentation | Features extracted from CNN | Cohen’s Github dataset and unspecified Kaggle dataset |
| [ ] | VB-Net and modified random decision forests method | 96 handcrafted image features | Dataset obtained from Tongji Hospital of Huazhong University of Science and Technology, Shanghai Public Health Clinical Center of Fudan University, and China-Japan Union Hospital of Jilin University. |
| [ ] | CNN from scratch and data augmentation | Features extracted from CNN | COVIDx Dataset |
| [ ] | CNN with transfer learning | Features extracted from CNN | Cohen’s Github dataset, Andrew’s Kaggle dataset, LDOCTCXR |
| [ ] | CNN with transfer learning | Features extracted from CNN | Cohen’s Github dataset, RSNA pneumonia dataset, COVIDx |
| [ ] | CNN with transfer learning and data augmentation | Features extracted from CNN | Sajid’s Kaggle dataset |
| [ ] | CNN with transfer learning and data augmentation | Features extracted from CNN | Cohen’s Github dataset, Mooney’s Kaggle dataset |
| [ ] | CNN with transfer learning | Features extracted from CNN | COVID-CT-Dataset |
| [ ] | CNN as feature extractor and long short-term memory (LSTM) network as classifier | Features extracted from CNN | GitHub, Radiopaedia, The Cancer Imaging Archive, SIRM, Kaggle repository, NIH dataset, Mendeley dataset |
| [ ] | CNN with transfer learning and synthetic data generation and augmentation | Features extracted from CNN | Cohen’s Github, Chowdhury’s Kaggle dataset, COVID-19 Chest X-ray Dataset, Initiative |
| [ ] | CNN with transfer learning, data augmentation and ensemble by majority voting | Features extracted from CNN | Cohen’s Github, LDOCTCXR |
| [ ] | CNN with transfer learning and stacking ensemble | Features extracted from CNN | Private dataset, LDOCTCXR |
| [ ] | CNN | Features extracted from CNN | Private dataset |
| [ ] | Multi-objective differential evolution-based CNN | Features extracted from CNN | Unspecified |
| [ ] | CNN with transfer learning | Features extracted from CNN | Cohen’s Github |
| [ ] | CNN and ConvLSTM with data augmentation | Features extracted from CNN | Cohen’s Github, COVID-CT-Dataset |
| [ ] | CNN with transfer learning | Features extracted from CNN | Cohen’s Github |
| [ ] | CNN with ensemble by weighted averaging | Features extracted from CNN | Private hospital datasets |
| [ ] | CNN with transfer learning | Features extracted from CNN | Cohen’s Github, Mooney’s Kaggle dataset, Shenzhen and Montgomery datasets |
| [ ] | MLP-CNN based model | Features extracted from CNN | Cohen’s Github |
| [ ] | CNN with transfer learning | Features extracted from CNN | Cohen’s Github, unspecified Kaggle dataset |
| [ ] | Capsule Network-based framework with transfer learning | Features extracted from CNN | Cohen’s Github, Mooney’s Kaggle dataset |
| Name | Disease | Image Type | Reference | Number of Images | Link |
|---|---|---|---|---|---|
| Belarus dataset | Tuberculosis | X-ray and CT | [ ] | 1299 | |
| ImageCLEF 2018 dataset | Tuberculosis | CT | 2287 | ||
| ImageCLEF 2019 dataset | Tuberculosis | CT | [ ] | 335 | |
| India | Tuberculosis | X-ray | [ ] | 78 tuberculosis and 78 normal | |
| Indiana Dataset | Multiple diseases with annotations | X-ray | [ ] | 7284 | |
| JSRT dataset | Lung nodules and normal | X-ray and CT | [ ] | 154 nodule and 93 non-nodule | |
| Montgomery and Shenzhen datasets | Tuberculosis and normal | X-ray | [ ] | 394 tuberculosis and 384 normal | |
| NIH-14 dataset | Pneumonia and 13 other diseases | X-ray | [ ] | 112120 | |
| TBimages dataset | Tuberculosis | Sputum smear microscopy image | [ ] | 1320 | |
| ZiehlNeelsen Sputum smear Microscopy image DataBase | Tuberculosis | Sputum smear microscopy image | [ ] | 620 tuberculosis and 622 normal | |
| Large Dataset of Labeled Optical Coherence Tomography (OCT) and Chest X-Ray Images (LDOCTCXR) | Pneumonia and normal | X-ray | [ ] | 3883 pneumonia and 1349 normal | |
| Radiological Society of North America (RSNA) pneumonia dataset | Pneumonia and normal | X-ray | 5528 |
| Name | Disease | Image Type | Reference | Number of Images | Link |
|---|---|---|---|---|---|
| LDOCTCXR | X-ray | [ ] | 3883 pneumonia and 1349 normal | ||
| NIH Chest X-ray Dataset | Pneumonia and 13 other diseases | X-ray | [ ] | 112,120 | |
| Radiological Society of North America (RSNA) pneumonia dataset | Pneumonia and normal | X-ray | 5528 | ||
| Mooney’s Kaggle dataset | Pneumonia and normal | X-ray | 5863 |
| Name | Disease | Image Type | Reference | Number of Images | Link |
|---|---|---|---|---|---|
| JSRT dataset | Lung nodules and normal lungs | X-ray and CT | [ ] | 154 nodule and 93 non-nodule | |
| Kaggle Data Science Bowl 2017 dataset | Lung Cancer | CT scans | 601 | ||
| LIDC-IDRI | Lung Cancer | CT | [ ] | 1018 | |
| Lung Nodule Analysis 2016 (LUNA16) dataset | Location and size of lung nodules | CT scans | [ ] | 888 | |
| NCI Genomic Data Commons | Lung Cancer | histopa- thology images | [ ] | More than 575,000 | |
| NIH-14 dataset | 14 lung diseases | X-ray | [ ] | 112,120 | |
| NLST | Lung Cancer | CT | Approximately 200,000 | ||
| NSCLC-Radiomics | Lung Cancer | CT | 422 | ||
| NSCLC- Radiomics -Genomics | Lung Cancer | CT | 89 | ||
| RIDER Collections | Lung Cancer | CT | Approximately 280,000 |
| Name | Disease | Image Type | Reference | Number of Images | Link |
|---|---|---|---|---|---|
| Andrew’s Kaggle dataset | COVID-19 | X-ray and CT | 79 | ||
| Chainz Dataset | COVID-19 and normal | CT | 50 COVID-19, 51 normal | ||
| Chowdhury’s Kaggle dataset | COVID-19, normal and pneumonia | X-ray | [ ] | 219 COVID-19, 1341 normal and 1345 pneumonia | |
| Cohen’s Github dataset | COVID-19 | X-ray and CT | [ ] | 123 | |
| COVIDx Dataset | COVID-19, normal and pneumonia | X-ray | [ ] | 573 COVID-19, 8066 normal and 5559 pneumonia | |
| Italian Society Of Medical And Interventional Radiology (SIRM) COVID-19 Database | COVID-19 | X-ray and CT | 68 | ||
| LDOCTCXR | Pneumonia and normal | X-ray | [ ] | 3883 pneumonia and 1349 normal | |
| Lung image database consortium (LIDC) | Lung Cancer | CT | [ ] | 1018 | |
| Sajid’s Kaggle dataset | COVID-19 and normal | X-ray | 28 normal, 70 COVID-19 | ||
| Mooney’s Kaggle dataset | Pneumonia and normal | X-ray | 5863 | ||
| COVID-CT Dataset | COVID-19 and normal | CT | 349 COVID-19 and 463 non-COVID-19 | ||
| Mendeley Augmented COVID-19 X-ray Images Dataset | COVID-19 and normal | X-ray | 912 | ||
| COVID-19 Chest X-Ray Dataset Initiative | COVID-19 | X-ray | 55 |
| Attributes | Subattributes | References |
|---|---|---|
| Image types | X-Ray | [ , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , ] |
| CT Scans | [ , , , , , , , , , , , , , , , , , , , , ] | |
| Sputum Smear Microscopy Images | [ , , , , ] | |
| Histopathology images | [ ] | |
| Features | Extracted from CNN | [ , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , ] |
| Others | [ , , , , , , , , , , , , , , , , , ] | |
| Data augmentation | Yes | [ , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , ] |
| Types of deep learning algorithm | CNN | [ , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , ] |
| Non-CNN | [ , , , , , , , , ] | |
| Transfer learning | Fixed feature extractor | [ , , , , , , , , , , , , , , , , , , ] |
| Fine-tuning CNN | [ , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , ] | |
| Ensemble | Majority voting | [ , , , ] |
| Probability score averaging | [ , , , , , , , , ] | |
| Stacking | [ , , ] | |
| Other | [ ] | |
| Disease types | Tuberculosis | [ , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , ] |
| Pneumonia | [ , , , , , , , , , , , , , , ] | |
| Lung cancer | [ , , , , , , , , , , , , , , ] | |
| COVID-19 | [ , , , , , , , , , , , , , , , , , , , , , , , , , , ] |
| MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
Share and Cite
Kieu, S.T.H.; Bade, A.; Hijazi, M.H.A.; Kolivand, H. A Survey of Deep Learning for Lung Disease Detection on Medical Images: State-of-the-Art, Taxonomy, Issues and Future Directions. J. Imaging 2020 , 6 , 131. https://doi.org/10.3390/jimaging6120131
Kieu STH, Bade A, Hijazi MHA, Kolivand H. A Survey of Deep Learning for Lung Disease Detection on Medical Images: State-of-the-Art, Taxonomy, Issues and Future Directions. Journal of Imaging . 2020; 6(12):131. https://doi.org/10.3390/jimaging6120131
Kieu, Stefanus Tao Hwa, Abdullah Bade, Mohd Hanafi Ahmad Hijazi, and Hoshang Kolivand. 2020. "A Survey of Deep Learning for Lung Disease Detection on Medical Images: State-of-the-Art, Taxonomy, Issues and Future Directions" Journal of Imaging 6, no. 12: 131. https://doi.org/10.3390/jimaging6120131
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
Advertisement
Air pollution exposure—the (in)visible risk factor for respiratory diseases
- Review Article
- Open access
- Published: 04 March 2021
- Volume 28 , pages 19615–19628, ( 2021 )
Cite this article
You have full access to this open access article

- Gabriel-Petrică Bălă ORCID: orcid.org/0000-0002-1877-1327 1 ,
- Ruxandra-Mioara Râjnoveanu 2 ,
- Emanuela Tudorache 1 ,
- Radu Motișan 3 &
- Cristian Oancea ORCID: orcid.org/0000-0003-2083-0581 1
19k Accesses
117 Citations
87 Altmetric
12 Mentions
Explore all metrics
There is increasing interest in understanding the role of air pollution as one of the greatest threats to human health worldwide. Nine of 10 individuals breathe air with polluted compounds that have a great impact on lung tissue. The nature of the relationship is complex, and new or updated data are constantly being reported in the literature. The goal of our review was to summarize the most important air pollutants and their impact on the main respiratory diseases (chronic obstructive pulmonary disease, asthma, lung cancer, idiopathic pulmonary fibrosis, respiratory infections, bronchiectasis, tuberculosis) to reduce both short- and the long-term exposure consequences. We considered the most important air pollutants, including sulfur dioxide, nitrogen dioxide, carbon monoxide, volatile organic compounds, ozone, particulate matter and biomass smoke, and observed their impact on pulmonary pathologies. We focused on respiratory pathologies, because air pollution potentiates the increase in respiratory diseases, and the evidence that air pollutants have a detrimental effect is growing. It is imperative to constantly improve policy initiatives on air quality in both high- and low-income countries.
Similar content being viewed by others

Air Pollution and Lung Diseases

Effects on health of air pollution: a narrative review
External Environmental Pollution as a Risk Factor for Asthma
Explore related subjects.
- Environmental Chemistry
Avoid common mistakes on your manuscript.
Introduction
Air pollution represents one of the biggest risk factors for human health. It is an invisible killer that hides around us, influencing both young and old generations. According to the World Health Organization (WHO), each year, 7 million people die due to air pollution. The most affected pathologies are chronic obstructive pulmonary disease, lung cancer, and respiratory infections, including pneumonia, stroke, and heart disease. Nine out of 10 individuals breathe air with polluted compounds, which penetrate deep into the lung tissue, and furthermore in the cardiovascular system (Ghebreyesus 2018 ) (World Health Organization 2018 ) (Tiotiu et al. 2020 ).
The most exposed individuals are elderly persons, infants, pregnant women, and persons with comorbidities (Mannucci et al. 2015 ). An estimated 43% of lung diseases and 24% of strokes are attributed to air pollution.
We performed an electronic search on PubMed for literature published in the last 5 years, with the last search date on February 15, 2020. The following terms were used: “air pollution,” “particulate matter,” “biomass,” “smoke,” “sulfur dioxide,” “nitrogen dioxide,” “carbon monoxide,” “ozone,” “chronic obstructive pulmonary disease,” “asthma,” “lung cancer,” “idiopathic pulmonary fibrosis,” “respiratory infections,” “bronchiectasis,” and “tuberculosis.” The terms were also searched in combination, such as “particulate matter and lung cancer” and “air pollution and chronic obstructive pulmonary disease.” The results were restricted to full-text studies of humans and mechanisms via animal experiments. Systematic reviews, meta-analyses, reviews, and publications from the WHO were included in the search. We additionally included older references if they had an important impact on the subject area, according to our knowledge.
General air pollution
Air pollutants are classified into two main categories: primary air pollutants (pollutants emitted directly into the atmosphere) and secondary air pollutants (pollutants that are formed within the atmosphere itself) (World Health Organization 2005 ) (Mannucci et al. 2015 ).
Primary air pollutants are those released from a direct source, such as exhaust pipes from a mobile vehicle, or from a stationary source, such as factory chimneys. At the same time, contaminated dust can also be distributed by the wind to uncontaminated areas. These pollutants can be calculated by measuring the amounts emitted by the source itself. Primary air pollutants are represented by oxides of nitrogen, carbon monoxide (CO), sulfur dioxide (SO 2 ), volatile organic compounds (VOCs), and carbonaceous and non-carbonaceous primary particles. The International Agency for Research on Cancer (IARC) has classified emissions from burning coal in an indoor environment as potentially carcinogenic to humans. These were observed with sufficient evidence in both animals and humans (Barone-Adesi et al. 2012 ).
There are many sources of primary air pollutants, but the most significant are road traffic and power plants. Additionally, industrial and residential heating based on wood, coal. or oil contributes to increasing the degree of air pollution (World Health Organization 2005 ) (Guarnieri and Balmes 2014 ) (Kravchenko and Lyerly 2018 ) (Minichilli et al. 2019 ).
Secondary air pollutants are formed through chemical reactions in the atmosphere, with natural components such as water and oxygen. Secondary air pollutants include ozone (O 3 ), oxides of nitrogen, and particulate matter (PM) (World Health Organization 2005 ) (Guarnieri and Balmes 2014 ) (Mannucci et al. 2015 )
The chemical composition of air pollutants is diverse and depends on the source. Additionally, a seasonal pattern is observed, with higher average daily concentration levels of nitrogen dioxide (NO 2 ), CO, PM10, and fine particulate matter (PM2.5) during the cold season, while O 3 concentration levels tended to be higher during the warm season (Bernardini et al. 2019 )
Air pollutants released by coal-fired power plants have raised concerns about their impact on public health. PM2.5 can have both short-term and long-term consequences on human health. In a study conducted by Cheng-Kuan Lin et al., a strong association was observed between the increase in coal capacity per person and an increase in the relative risk for lung cancer, both in men and women. These were observed by a factor of 85% among women and 59% among men. Based on these data, it is predicted that in 2025, a total of 1.37 million cases of lung cancer will be correlated with coal-fired power plants (Lin et al. 2019 ).
Outdoor (ambient) air pollutants
Sulfur dioxide.
Sulfur dioxide (SO 2 ) and PM come from the process of burning fossil fuels and represent the essential components of air pollution. Sulfurous and sulfuric acids are formed as a result of the oxidation process of SO 2 . Natural sources include volcanoes, but significant concerns have been encountered in large metropolitan regions where coal is being used for domestic heating or for poorly controlled combustion for industrial installations (World Health Organization 2005 ).
Exacerbation of respiratory symptoms has been shown to be related to exposure to SO 2 emitted by coal-burning power plants, and lower concentrations were associated with respiratory deaths. The major anthropogenic sources of SO 2 are found in developing countries and come from burning fossil fuels that contain sulfur. The reason for burning fossil fuels is due to heating homes, use in power plants, and powering vehicles (Kravchenko and Lyerly 2018 ). Sulfur dioxide concentrations are lower since indoor concentrations are absorbed by walls, furniture, and inhalation systems (World Health Organization 2005 ).
Nitrogen dioxide
There are many species of nitrogen oxides, but the one with the most important effect on human health is NO 2 . NO 2 is a gas with a brown color, having a distinctive powerful scent. Nitric oxide spontaneously produces dioxide when it is exposed to air. It is a powerful oxidant that produces nitric acid and nitric oxide by reacting with water, and it is an important trace gas affecting human health. It absorbs solar radiation, contributing to low visibility in the atmosphere and plays a direct role in global climate change.
NO 2 undergoes further transformations, and after the photochemical reaction sequence is initiated by its solar radiation-induced activation, newly generated pollutants are created, containing organic, nitrate, and sulfate particles, all measured at PM2.5 and PM10. Among natural sources, the represented sources include lightning, inclusion of stratospheric nitrogen oxides, and bacterial and volcanic actions. The major anthropogenic sources are mobile sources (combustion engines) and stationary combustion sources (power generation sources) (World Health Organization 2005 ) (Kravchenko and Lyerly 2018 )
Patients with asthma and chronic obstructive pulmonary disease (COPD) have been associated with an increased risk of respiratory hospitalization after exposure to NO 2 (Kravchenko and Lyerly 2018 ). In addition, exposure to air pollution due to traffic vehicles increases the risk of developing bronchiolitis obliterans post-lung transplant syndrome (Johannson et al. 2015 ).
In China, a systematic review and meta-analysis by Sun et al. identified a positive correlation between short-term ambient exposure to NO 2 and pulmonary diseases. A 10-μg/m 3 increase in NO 2 concentration was associated with an increase of 1.4% in mortality due to respiratory disease and 1.0% in hospital admission. Elderly individuals had an even higher susceptibility (Sun et al. 2017 ).
Carbon monoxide
The most important source of environmental CO is incomplete combustion of traffic-related fossil fuels, leading to >50% of emissions in urban areas, other sources (such as manufacturing and natural processes, etc.) being less prominent (World Health Organization 2005 ).
Carbon monoxide is considered to be a “silent killer” due to its toxicity arising from its ability to bind hemoglobin more strongly than oxygen, increasing the risk of asphyxia-related deaths at high levels of exposure or hypoxic tissue damage at low levels of exposure (World Health Organization 2005 ).
Asthma, bronchiectasis, and pneumonia have been associated with ambient short-term exposure to CO (Zhao et al. 2019 ). The study of Zhao et al. conducted over 4 and 1/2 years, with a daily mean ambient CO of 0.88 mg/m 3 , varying from 0.40 to 3.13 mg/m 3 , reported an increased risk for daily outpatient visits for respiratory disease, with a higher effect on women and elderly patients (Zhao et al. 2019 ).
In different studies, a positive association between daily exposure to PM2.5, SO 2 , and CO and an increased risk of mortality from respiratory diseases and lung cancer was reported. For a 1-mg/m 3 increase in CO and a 10-μg/m 3 increase in PM10, there has been a 1.9% and 4.8% increase in total deaths, respectively (Xue et al. 2018 ) (Table 1 ).
Volatile organic compounds
Volatile organic compounds (VOCs) are compounds with a high vapor pressure of one or more carbon atoms, which will lead to their release in the atmosphere (Ciganek and Neca 2008 ). Compounds from the atmosphere, in a state of a vapor phase, such as oxygenates, hydrocarbons, halogenates, and other carbon compounds, are the main components of VOCs (World Health Organization 2005 ).
There are different sources of VOCs. They can arise from natural causes, such as forest fires, vegetation, and animals, but also from artificial causes, such as vehicles. Natural sources of VOCs represent a higher percentage, but anthropogenic sources contribute significantly to reducing air quality.
The most important sources of VOCs are released by industrial and agricultural sources. At the same time, handling solvents or solvent-based products contributes significantly to VOC concentrations. Samples that were collected from road dust or soil were based on volatile organic compounds, such as benzene, toluene, styrene, ethylbenzene, and xylene, as well as aliphatic hydrocarbons (mostly n -alkanes), dichloromethane, and disulfide carbon (Ciganek and Neca 2008 ).
Oxidative stress and decreased lung function are related to exposure to low levels of VOCs. Additionally, airway inflammation could be related to exposure to increased levels of VOCs in everyday life (Kwon et al. 2018 ).
In a national cross-sectional representative survey that was conducted by the Indoor Air Quality Observatory, N -undecane and 1,2,4-trimethylbenzene were correlated with asthma in 8.6% of cases, while trichloroethylene, ethylbenzene, and m/p- and o-xylene were associated with rhinitis (Billionnet et al. 2011 ).
In a French cross-sectional study on farmers, indoor mean VOC concentrations were smaller in workplaces than in dwellings. Working in a rural environment involves a degree of exposure to various risk factors such as agricultural machinery and fires, exposure to capricious weather, agricultural land working, and exposure to various organic compounds. Following this study, individuals mentioned that respiratory symptoms, such as dyspnea, cough, sneezing, and wheezing, were the most common. They were present in 44% of people when manipulating plants that had been harvested. Asthma and early airway obstruction were linked with exposure to VOCs and PM and in farmers (Maesano et al. 2019 ).
Ozone (O 3 ) is a chemical compound that is not directly emitted into the air but is formed through a series of complex reactions. Atomic oxygen and nitric oxide are formed after NO 2 splits. Atomic oxygen later combines with oxygen-forming ozone. Ozone is disintegrated by reacting with nitric oxide, resulting in NO 2 and oxygen. Ambient concentrations depend on several factors: the concentration of NO 2 and VOCs, sunshine intensity, atmospheric convection, and the proportion of VOCs to nitrogen oxides (World Health Organization 2005 ) (Guarnieri and Balmes 2014 ).
Daily concentrations of air pollutants are higher in the cool seasons than in the warm seasons, except for O 3 , which is higher during warm seasons (Wang et al. 2019d ). Ground-level ozone (O 3 ) is considered one of the most dangerous air pollutants in the USA and the European Union, being a strong oxidizing compound. In recent years, O 3 levels have remained high without showing any decline and will remain a constant public health problem, especially with the progression of global warming (Guarnieri and Balmes 2014 ) (Wang et al. 2019c ).
Concentrations of O 3 can increase during late spring and summer months due to photochemical reactions, along with its precursors, such as VOCs. High concentrations of O 3 can be associated with various local and long-range transports of anthropogenic emissions. In winter, lower photochemical processes result in a smaller contribution of this factor to PM2.5 mass (18%) (Bari and Kindzierski 2017 ). Human exposure to ozone is correlated with a high risk of respiratory disorders, such as asthma exacerbation and lung inflammation, loss of lung function, and cystic fibrosis (Johannson et al. 2014 ). Additionally, it has been shown to interact with cerebral blood vessels by modulating the expression of genes involved in brain vasoreactivity, irritating mucous membranes, altering the levels of serotonin, and affecting the immune system (Bernardini et al. 2019 ).
- Particulate matter
According to the World Health Organization (WHO), the standard for daily PM2.5 concentration is 25 μg/m 3 , while the annual average is 10 μg/m 3 . Approximately 92% of the world’s population lives in locations where the mean PM2.5 mass concentration surpasses this amount. Approximately 3 million persons die from outdoor air pollution each year (Wang et al. 2019b ).
Particulate matter (PM) is represented by a complex mixture containing components with diverse physical and chemical characteristics. The potential for these particles to cause injury varies due to their chemical composition and source. Additionally, their size and physical characteristics represent major concerns for public health (World Health Organization 2005 ).
Particles are classified in general by their aerodynamic diameter. PM can generally be classified into three major fractions: coarse particles, exceeding 2.5 μm in aerodynamic diameter; fine particles, which are smaller than 2.5 μm; and ultrafine particles, which are smaller than 0.1 μm (100 nm). PM10 contains PM2.5 and thoracic coarse mass (the distinction between PM10 and PM2.5 is generally presented as coarse mass). In general, PM10 mass contains 40–90% of the PM2.5, the rest being considered coarse PM (World Health Organization 2005 ) (Guarnieri and Balmes 2014 ) (Mannucci et al. 2015 ).
PM with a 2.5–10-μm aerodynamic diameter, also known as coarse PM, is stored particularly in the head and in the upper respiratory tract. PM2.5 is usually stored in the deep respiratory airways, primarily in the small airways and alveoli, while ultrafine PM (<0.1μm) is stored in the alveoli (World Health Organization 2005 ) (Guarnieri and Balmes 2014 ) (Liang et al. 2019 ).
PM2.5 are primarily formed from gases. These particles usually emerge as ultrafine particles created by the formation of very small particles (nuclei) by condensation-nucleation of low vapor pressure substances generated by chemical reaction into the atmosphere or by high-temperature vaporization (World Health Organization 2005 ) (Mannucci et al. 2015 ) (Kravchenko and Lyerly 2018 ). The principal precursor gases are represented by nitrogen oxides, ammonia, SO 2 , and VOCs. At the same time, fluctuations in the concentrations of these compounds may alter ambient PM concentrations. On the days when PM10 concentration exceeds 50 μg/m 3 PM, nitrate becomes the main compound of PM10 and PM2.5 (World Health Organization 2005 )(Kravchenko and Lyerly 2018 ).
There are numerous sources of both human activity and natural source-related particles. Specific sources impact different regions of the world, but more than two-thirds of PM 2.5 is due to industrialization in developed areas.
The origin of PM2.5 is variable. It can come from several sources, such as vehicle traffic, followed by dust generation, aerosols from regional transport, agricultural activities, and the burning of biomass for cooking or heating. It is difficult to appreciate the contribution of each source and to further recognize the PM formation mechanism (Zhang and Cao 2015 ).
Photochemical conversion of secondary pollutants (SO 4 2− , NO 3 − , and NH4+) represents 3.7% of PM2.5 and 2.4% of PM10 (Lee et al. 2018 ). Concentrations of NH 4 + , NO 3 − , and SO 4 2− on days with higher pollution can be two to four times higher than on unpolluted days ( p < 0.01), according to the study of Pan et al, where PM2.5 samples were gathered from two metropolitan areas (Beijing and Shanghai) on polluted and unpolluted days in the fall of 2017 (Pan et al. 2019 ). Additionally, seasonal variations in PM2.5 have been described in different regions of the world.
In a Canadian study conducted by Md. Aynul Bari from May 2009 to December 2015, the overall mean and median concentrations of PM2.5 were comparatively higher in winter than in summer (Bari and Kindzierski 2017 ). The same observation was reported in the Chinese study of Yan-Lin Zhang et al., with remarkable seasonal variability in PM2.5, which was highest during winter and lowest during summer. On the other hand, increased levels of PM2.5 are also found in the spring and autumn due to the contribution of dust particles and the start of burning biomass. In addition, the lowest and highest PM2.5 concentrations frequently occur in the afternoon and evening hours (Johannson et al. 2014 ).
An investigation of the origin of wintertime high PM2.5 pollution days revealed that in addition to traffic emissions, another significant source that helped increase PM2.5 in winter was a mixed factor represented by local industry and agriculture (deduced as gas emission sources and upstream oil) (Bari and Kindzierski 2017 ).
Indoor air pollutants
Biomass smoke.
Biomass smoke is a major public health problem (Balcan et al. 2016 ). Coal and biomass fuels are used by almost 3 billion people worldwide. A large part of the world’s population still depends on solid fuel for cooking, firewood, and charcoal (Nsoh et al. 2019 ). Individuals generally use different types of fuels for heating and cooking, such as “smoky coal” (bituminous), “smokeless coal” (anthracite), and wood (Barone-Adesi et al. 2012 ).
Compounds emitted from smoky coal combustion are present in abundance, such as polycyclic aromatic hydrocarbons (PAHs), methylated PAHs, and heterocyclic aromatic compounds containing nitrogen. Following an incomplete combustion process, solid fuel releases a significant amount of toxic particles. These will then be inhaled and cause multiple respiratory symptoms. Symptoms may include upper respiratory tract conditions, such as cough, nasal obstruction, vocal dysfunction, rhinorrhea, laryngeal spasm, and lower respiratory tract symptoms, such as dyspnea, wheezing, and cough (Nsoh et al. 2019 ).
Approximately half of the world’s population cooks and heats using unprocessed biomass fuels and coal. Several diseases are associated with exposure to solid fuel smoke, including lung cancer, chronic obstructive pulmonary disease, and respiratory infections (Barone-Adesi et al. 2012 ). In the study by Barone-Ades et al., it was observed that mortality due to lung cancer was higher in people who used smoked charcoal than in those who used no-smoke charcoal throughout their lives. In the study, 9962 people used smokeless coal, and 27,310 used smoked coal. The absolute risk of death from lung cancer in individuals who used smoked charcoal was higher for women (20%) than men (18%). As a comparison, the percentage of people who used smokeless charcoal and developed lung cancer was only 0.5%. These values are similar to those observed in heavy smokers in Western countries, with a value between 20 and 26% (Barone-Adesi et al. 2012 ).
Lung function begins to deteriorate after exposure to smoke for more than 15 years. The chance of having modified pulmonary function increases as the duration of exposure increases. In rural areas, women are generally more exposed due to their conventional lifestyle. In a case-control study, from a total of 115 women exposed to biomass smoke, 23.8% had small airway disease, 19.1% had obstruction, and 17.3% had a restriction pattern on pulmonary function tests (Balcan et al. 2016 ).
Public health focus on respiratory disease
- Chronic obstructive pulmonary disease
COPD is a multifactorial condition characterized by chronic airway obstruction that is incompletely reversible, progressive, and associated with an abnormal inflammatory response of the lung to harmful particles or gases (Singh et al. 2019 ).
Ambient air pollution is associated with COPD morbidity and mortality. From systemic analyses, it was observed that morbidity from COPD is correlated with a short-term increase in air pollution (Adar et al. 2014 )(Zhang et al. 2016 ) (Tian et al. 2018 ).
The body’s response may differ from person to person. The ability of each person to react to air pollution may differ in the Chinese population compared with the North American or European population due to differences in air pollution concentration and the composition of the polluted air. At the same time, the pre-existing pathology of one population may be different from another. In a study conducted in Beijing, China, a reduction in the average concentration of PM2.5 up to 58 μg/m 3 was observed in 2017 compared with 2013 when the average concentration was 87 μg/m 3 . Although a significant reduction was observed, the value was still high, given that the reference value was 10 μg/m 3 according to the WHO. However, it was observed that there were 161,613 hospitalizations for exacerbations of COPD (most patients were men over 65 years of age). Short-term exposure to air pollutants was correlated with hospital visits in the COPD emergency sections, resulting in subsequent hospitalizations and mortality (Liang et al. 2019 ).
In a population-based study involving 3941 nonsmoking Taiwanese adults, 791 had COPD. Exposure to PM2.5 at concentrations higher than 38.98 μg/m 3 was associated with increased predisposition to COPD among nonsmokers in Taiwan. However, exposures to concentrations of 32.07–38.98 μg/m 3 and 29.38–32.07 μg/m 3 were not significant (Huang et al. 2019 ).
From the multitude of studies that have shown a link between COPD and air pollution, we selected this study.
At high concentrations, air pollutants have a direct inflammatory effect on airway neuroreceptors and the epithelium. In addition, oxidative stress has been associated with pollutant exposure (O 3 , NO 2 , PM2.5) (Johannson et al. 2014 ). Airway inflammation can be induced by specific pollutants (O 3 , NO 2 , PM2.5), while airway hyperresponsiveness can be induced by O 3 and NO 2 (Johannson et al. 2014 ) (Kravchenko and Lyerly 2018 ).
The EGEA study conducted on 204 adult asthmatic patients revealed important data about the role of oxidative stress in the association between air pollution and asthma. The levels of fluorescent oxidation products (FlOPs), an oxidative stress-related biomarker, increased with PM10 and O 3 , and the risk of persistent asthma increased with plasma FlOP levels (Havet et al. 2019 ).
Air pollution represents one of the most important factors aggravating asthma in children, with higher incidences in European and Caribbean regions (Cadelis et al. 2014 )(Akpinar-Elci et al. 2015 ). One of the contributing factors is Saharan dust (Gyan et al. 2005 ). Saharan particles are composed of mineral origins. They are composed of a multitude of particles, such as clay, quartz, silicon oxide, and carbonates. They are lined with organic matter represented by bacteria and spores or pollen grains. Saharan dust contains PM10 and PM2.5–10, which can further predispose to an increase in visits to the emergency service for patients aged 5–15 years (Cadelis et al. 2014 ).
In a retrospective study of 5 years conducted by Muge Akpinar-Elci, the relationship between Saharan dust and exposure, climatic variables, and asthma was analyzed. There were 4411 recorded asthma-related emergency visits, and variation in asthma was correlated with dust concentration (Akpinar-Elci et al. 2015 ). Additionally, in a study conducted by Cadelis et al., there were 836 visits for asthma, with 514 boys and 322 girls (Cadelis et al. 2014 ).
In a study that took place in 10 European cities, the incidence of asthma among children was 14%, and after exposure to air with polluting compounds from road traffic, children with exacerbated asthma constituted 15% of the cohort. Asthma symptoms have been correlated with short-term exposure to ambient PM2.5 and PM10 in prospective cohorts, particularly in children with allergic sensitivity (Guarnieri and Balmes 2014 ). In a cohort study conducted by Bowatte et al., exposure to traffic-related air pollution (TRAP) was associated with both persistent and new-onset asthma in adults. Living < 200 m from a major road was correlated with greater odds of new asthma for middle-aged persons who never had asthma by 45 years. Asthmatic participants at 45 years had an increased risk of persistent asthma up to 53 years if they were living < 200 m from a major road compared with asthmatic participants living > 200 m from a major road (Bowatte et al. 2018 ).
- Lung cancer
Lung cancer represents one of the most common types of cancer and has a poor prognosis. The most important risk factor incriminated in developing lung cancer is active smoking, but exposure to environmental occupational carcinogens, residential radon, and passive smoke is also recognized as risk factors (Raaschou-Nielsen et al. 2013 ).
Although the association between lung cancer and long-term exposure to air pollution has been clarified, the link between lung cancer mortality and short-term exposure to air pollution remains unknown. The number of lung cancer cases is expected to increase due to continuous exposure to air pollution in regard to massive industrialization, an aging population and constant high smoking prevalence (Wang et al. 2019d ). PM2.5, PM10, and O 3 contribute to oxidative stress within the respiratory system and therefore potentially facilitate pulmonary inflammation and could initiate or promote the mechanisms of carcinogenesis (Xing et al. 2019 ). PM2.5 is considered the most relevant pollutant (Hamra et al. 2014 ).
In 2010, cancers of the trachea, bronchial tree, or lungs attributable to exposure to PM2.5 accounted for approximately 7% of total mortality. The mechanisms that have been incriminated in the association between PM2.5 and lung cancer include DNA deterioration and cell cycle changes (Longhin et al. 2013 ). PM2.5 was also related to increased production of reactive oxidative species.
A correlation was observed between the aerodynamic diameter of the fine particles in the medium ≤ 2.5 (PM2.5) and the incidence and mortality from lung cancer. Based on a meta-analysis of 18 studies, the correlation between PM2.5 and PM10 and the incidence and mortality of lung cancer were studied. Following the analysis, it was observed that the meta-relative risk was 1.09 for lung cancer related to PM2.5 and 1.09 for PM10. Additionally, the risk of adenocarcinoma associated with PM 10 was 1.29, while for PM2.5, it was 1.4. These results can help us better analyze the pathology of bronchopulmonary cancer in connection with air pollution (Hamra et al. 2014 ).
In the Ahsmong-2 study, it was shown that for each 10 μg/m 3 increase in ambient PM2.5, the incidence of lung cancer increased, although the individuals from the study were exposed to low levels of ambient PM2.5 and had never smoked. The percentage was higher for individuals who had a longer period of residence and who had spent more than 1 h/day outside. The predominant type of cancer was adenocarcinomas, with a percentage of 66.4% (Gharibvand et al. 2017 ).
Wang et al. suggested that the carcinogenic effects of PM2.5 vary by gender as well as by the environment in which individuals live, i.e., rural or urban. It has also been observed that younger people have a lower sensitivity than elderly people. For individuals in rural areas, it was observed that with a growth level of the average concentration of PM2.5 by 10 μg/m 3 , the incidence and mortality from lung cancer were 15% and 23% among men, compared with 22% and 24% among women, respectively. Thus, following this study, the results showed that women have a meaningful risk of developing lung cancer in correlation to PM2.5 exposure (Wang et al. 2019a ).
Idiopathic pulmonary fibrosis
Idiopathic pulmonary fibrosis (IPF) is defined as a specific form of chronic, progressive fibrosing interstitial pneumonia of unknown cause, occurring primarily in older adults, and limited to the lungs. It is a progressive lung disease with a complex etiology (Johannson et al. 2018 ) characterized by progressive worsening of dyspnea and lung function and is associated with poor prognosis (Raghu et al. 2011 ).
There are not enough studies to certify the effects of air pollution on interstitial lung disease. However, in a study conducted by Johansson, it was shown that acute exacerbations of IPF were associated with an increase in the mean level, the maximum level, and the number of exceedances above accepted standards of O 3 and NO 2 (Johannson et al. 2014 ). Of the six criteria regulated by the US Environmental Protection Agency, particulate matter (PM), ground level (O 3 ), and NO 2 were strongly related to adverse respiratory effects (Johannson et al. 2015 ).
One potential mechanism by which ambient air pollution may cause interstitial lung disease is oxidative stress through the generation of excess reactive oxygen species (ROS), such as radical hydroxide and superoxide anion. IPF patients exhibit evidence of reduced antioxidant capacity, suggesting that they may have an increased vulnerability to excess ROS (Johannson et al. 2015 ). Another explanation for the progressive evolution of the disease was highlighted by the study of Winterbottom et al. on 135 subjects evaluated between 2007 and 2013. The results showed a strong association between PM10 levels and a decrease in forced vital capacity (FVC). With each μg/m 3 increase in PM10, there was an additional 46 cc/year decline in FVC. The significant relationship observed between the exposure to coarse (PM10) and the decline rate of FVC was not reported for PM2.5, showing an inverse relationship between the diameter size of the particle and penetration into the airways. Each 5 μg/m 3 increase in ambient PM2.5 concentration at residences corresponded with an additional 1.15 L/year increase in oxygen use on the 6-min walking test (6MWT) (Winterbottom et al. 2018 ). The results are equivocal, because the study conducted by Kerri A. Johannson et al. showed that PM10, PM2.5, and NO2 were associated with reduced lung function, but the changes were independent of air pollution exposure (Johannson et al. 2018 ).
There were no significant relationships between mean weekly change in air pollutant levels and concurrent weekly changes in forced vital capacity (FVC), forced expiratory volume during the first second (FEV1), University of California San Diego Shortness of Breath Questionnaire (UCSD-SOBQ), or visual analog scale (VAS) scores. Nevertheless, regarding the duration or interval of assessment periods, there was no significant association between the mean level of air pollutants and subsequent changes in lung function. Additionally, neither higher cumulative mean exposures nor maximal exposures to air pollution were associated with a more rapid decline in FVC or FEV1 over the study period. However, in patients with IPF, average exposures to NO 2 , PM2.5, and PM10 were associated with lower FVC, indicating that air pollution may influence the severity of disease in some individuals (Johannson et al. 2018 ).
In a study with 436 patients performed by Johannson et al., 75 of them had at least one acute exacerbation, and a subgroup of 13 patients had more than one exacerbation. During the exposure period, acute exacerbation of IPF was correlated significantly with increased mean rates, maximum levels, and amounts of O 3 and NO 2 exceedances. Increased exposure to O 3 and NO 2 over the preceding 6 weeks was associated with a high risk of acute exacerbation of IPF. This suggests that air pollution could be correlated with the development of this clinically significant event. At the same time, there were no consistent relationships between PM10, SO 2 , or CO and acute exacerbation of IPF compared with NO 2 , O 3 , and PM2.5 (Johannson et al. 2014 ).
Respiratory infections
In the cross-sectional study of Nsoh et al., 1849 patients diagnosed from January 2013 to April 2016 with acute respiratory infections (ARIs) were registered. Of the selected patients, more than 70% used at least one form of solid fuel for cooking. In poorly ventilated homes, the impact of this exposure was irritation of the respiratory tract and eyes and an increased risk of cancers related to long-term inhalation of this poor-quality air. The probability of developing ARI was 3.62 times higher for people who were exposed to cooking indoors than for those who were not exposed. Additionally, the chances of developing ARI were 1.91 times higher for those exposed to open fire than for those who were not exposed. Thus, PM2.5 values were 13.2 times higher than what the WHO recommends. Dry weather and dust also increase the risk of developing ARI (Nsoh et al. 2019 ).
A study conducted in China found that with increasing concentrations of PM2.5 and PM2.5–10 compounds, the number of hospital visits for upper airway infections and pneumonia meaningfully increased. The increase in the average concentration, which accumulated over 6 days, was 10 μg/m 3 (Z. Zhang et al. 2019 ).
Zheng P et al. constructed a seasonal model of cases of respiratory infections, revealing a higher preponderance in the period with lower temperatures. While children aged 5–14 years had a higher chance of developing acute respiratory infections (55.1%), those under 5 years had a higher chance (60.5%) of developing lower acute respiratory infections. The concentrations of air pollutants PM10, NO 2 , and SO 2 exhibited lower values in the warmer period. Young children have a higher degree of susceptibility than older individuals due to their less developed immune system, tighter airways, higher frequency of respiration, and higher long-term exposure to air pollutants of the lower respiratory tract. Due to the excessive use of coal for heating during the colder season, winds also contribute to increased concentrations of air pollutants (P. Zheng et al. 2017 ).
Bronchiectasis
Bronchiectasis is defined as inappropriate and permanent dilatation of the bronchi. It is a chronic respiratory disease, with many patients having frequent exacerbations. Due to their exacerbations, lung function will subsequently decrease, furthermore, increasing mortality (Garcia-Olivé et al. 2018 ).
Infectious pathogens are often incriminated in the majority of bronchiectasis exacerbations, but frequently, no pathogen can be identified. In a study conducted by C. Pieter Goeminne et al. on 432 patients diagnosed through high-resolution computed tomography (HRCT) and clinically confirmed bronchiectasis, for a 10 μg/m 3 increase in PM10 and NO 2 , the chance of developing an exacerbation in that same day increased by 4.5% and 3.2%. In total, 11.2% for PM10 and 4.7% for NO 2 were the risk of having an exacerbation for a 10-μg/m 3 increase in the concentration of air pollutants. Subanalysis showed considerably higher relative risks through spring and summer due to increased expected outdoor air pollution exposure (Goeminne et al. 2018 ).
Additionally, in a retrospective observational study conducted in Badalona, SO 2 was considerably related to an increase in the hospitalization number (Garcia-Olivé et al. 2018 ). Through our search of the literature, we noticed that there are few studies on the connection between air pollution and bronchiectasis.
Tuberculosis
According to the WHO, in the 2019 Global Tuberculosis Report, approximately 10 million people worldwide fell ill with tuberculosis in 2018, and it is the leading cause of a single infectious agent. Worldwide, tuberculosis is considered to be the 10th leading cause of death (WHO-Global Tuberculosis Report 2019 n.d. ) Tuberculosis (TB) is a disease whose prevalence has been associated with socioeconomic risk factors that has a stronger association with urban settings, where there is greater exposure to air pollution (Jassal et al. 2012 ).
There is a direct correlation between air quality and tuberculosis incidence. Precipitation, atmospheric pressure, and relative humidity affect the incidence of tuberculosis by indirectly reducing the quantity of inhalable PM and SO 2 concentrations. On the other hand, wind plays a major role by increasing the incidence of tuberculosis by spreading pathogens (Zhang and Zhang 2019 ). Fine particulate matter and traffic-related air pollution might be associated with an increased risk of developing tuberculosis. This association is not due to direct exposure but rather to the impairment of the individual’s immunity (Lai et al. 2016 ). Popovic et al. showed in a systematic review that the pollutant most frequently associated with tuberculosis is PM2.5 (Popovic et al. 2019 ).
The Chengdu study also documented that exposure to ambient PM10, NO 2 , and SO 2 was linked to increased tuberculosis morbidity in China, but the lag time was 28 days for PM10 and 5 days for SO 2 and NO 2 , which can only be attributed to short-term effects (Zhu et al. 2018 ). Another study conducted by Lai et al. highlighted that an increased risk of active tuberculosis is related to exposure to fine particulate matter PM2.5. Furthermore, traffic-related air pollution, including nitrogen dioxide, nitrogen, and carbon monoxide, was associated with tuberculosis risk. On the other hand, PM10 was not linked with active tuberculosis, and O 3 was inversely associated with the risk of TB (Lai et al. 2016 ).
Similar to the last results, O 3 levels could not be significantly correlated with acid fast bacilli (AFB)-positive smears in the retrospective study of Jamal et al. Medical records of 196 individuals diagnosed with TB positivity at Los Angeles County and University of Southern California Medical Center Hospital were analyzed. A total of 111 had smear positivity, while 85 had smear negativity. There was a significant correlation in single pollutant models analyzing PM2.5 levels and smear-positive TB (Jassal et al. 2012 ).
The link between PM2.5 concentration, notably a 10 μg/m 3 increase in PM2.5 levels, and active TB was also noted in a study conducted from 2014 to 2017 in Lianyungang. For the single-pollutant model, the association between a 10-μg/m 3 increase in PM10 concentration and SO 2 concentration and active TB was significant. Additionally, a potential correlation between relatively long-term outdoor exposure to PM2.5, PM10, SO 2 , and NO 2 and active TB was observed in the time-series study conducted in the northeastern region of Jiangsu Province, China. In the multipollutant models, ambient PM10 and NO 2 remained significant, and the association was not altered in subgroups of different genders or ages (Li et al. 2019 ).
In addition, exposure to pollution over different periods of time may be associated with drug resistance. Exposure to PM2.5, PM10, O 3 , and CO has been associated with drug-resistant TB, including first-line monodrug resistance, polydrug resistance, and multidrug resistance (MDR), in both single- and multiregression models. In the retrospective study of Yao et al., conducted in Jinan city, China, from January 1, 2014 to December 31, 2015, 752 new culture-confirmed TB cases reported in TB prevention and control institutions of Jinan were included. The results showed significant monodrug resistance, and polydrug resistance increased the risk for ambient PM2.5, PM10, O 3 , and CO exposure. The most significant association for PM2.5 was noticed at 540 days of exposure, for O 3 was noticed at 180 days of exposure, and for PM10 and CO, it was noted from 90 to 540 days of exposure. Of the 752 cases, 18.8% were first-line drug-resistant cases with streptomycin having the highest rate of resistance (15.3%), 13% were second-line resistant, fluoroquinolones having the highest rate of resistance (11.3%), 12.3% were resistant to more than 1 drug but not MDR, and 3.3% were MDR-TB (Yao et al. 2019 ).
NO 2 nitrogen dioxide, SO 2 sulfur dioxide, PM2.5 particulate matter with diameter < 2.5μm in diameter, PM10 particulate matter with diameter < 10μm in diameter, O 3 ozone, CO carbon monoxide, COPD chronic obstructive pulmonary disease, IPF idiopathic pulmonary fibrosis
Animal experiments
Animal experiments have opened up new perspectives on air pollution (Edwards et al. 2020 ). Air pollution contributes to increased inflammation. When polluted air is inhaled, its first stop is the lungs. This is where oxidation-reduction first occurs (Gangwar et al. 2020 ). Oxidative stress arises from altering the balance between oxidants and antioxidants. Altering this balance will increase oxidative stress and cause the increase of lung pathologies through promoting inflammation of the airways. PM consists of a number of components capable of generating ROS, which subsequently increase inflammatory mediators in the lungs (Valavanidis et al. 2013 ).
In a study conducted by Edward et al., it was observed that rats exposed to TRAP, compared with those not exposed, exhibited increased gene expression changes related to oxidative stress, inflammation, aging, and fibrosis in the heart (Edwards et al. 2020 ). While Zheng et al. showed that following exposure to tracheal diesel particles, mice presented an increase in transient oxidative stress in the lungs, Sun et al. showed that PM2.5 accentuates the degree of atherosclerosis, degrades vasomotor tone, and determines vascular inflammation in mice that have been chronically exposed to low concentrations of PM2.5 (Q. Sun et al. 2015 ) (X. Zheng et al. 2019 ) (Gangwar et al. 2020 ). Rats that were exposed to ozone showed 8-hydroxy-2′-deoxyguanosine (8-OHdG) and heme oxygenase-1 (HO-1) in macrophages, developing rigid lungs with reduced function (Sunil et al. 2013 ) (Valavanidis et al. 2013 ).
Fibrosis and reversible cardiac dysfunction were observed in mice after intratracheal exposure to PM2.5 (Gangwar et al. 2020 ). Oxidative stress in the myocardium is increased in those exposed to ultrafine particles (Cozzi et al. 2021 ). Qin and all demonstrated that after intratracheal exposure to PM2.5, the most sensitive were the extremes of age compared with adult animals, developing heart dysfunction and reversible fibrosis (Qin et al. 2018 ) (Gangwar et al. 2020 ). Cozzi et al. showed that myocardial damage in mice exposed to ultrafine particles is double that in those not exposed (Cozzi et al. 2021 ).
General information
When the level of pollution is high, informing the population should be a priority. This information should be free and easy to access so that outdoor activity is reduced during periods with higher air pollution (Tiotiu et al. 2020 ).
Air quality alerts are beneficial to the population. The population is notified when air quality alerts occur (Wen et al. 2009 ). In a study conducted by Graff and Neidell, it was found that when the population was alerted by smog alerts, outdoor physical activity on visits to the Griffith Park Observatory and Los Angeles Zoo decreased by 8% and 15%, respectively. However, when alerts were repeated on days 2 and 3, people did not take into account the smog alerts, with values of 0% and 5%, respectively (Graff and Neidell 2009 ). These warnings should be repeated as often as possible and should be of particular interest for patients with cardiopulmonary pathology, as well as healthy patients who may subsequently develop chronic diseases (Wen et al. 2009 ).
From a real estate point of view, both large cities and those with fewer inhabitants should be channeled on development so that the degree of pollution does not affect the quality of life of the population. This should be done from the beginning and not developed later, after the urbanization plan has been made. This would help reduce air pollution from the start. Public institutions, as well as the community, must contribute to reducing the degree of pollution. However, although institutions should play a key role in reducing pollution, it can also be reduced by individual freewill (Carlsten et al. 2020 ).
As medical staff inform asthmatics to avoid aeroallergens, patients with chronic cardiopulmonary disease should also be informed by the degree of air pollution and how it may affect their health. Otherwise, they may develop new symptoms or experience worsening of pre-existing symptoms. The air quality index (AQI) should be consulted frequently by patients to cancel outdoor activities when air quality is poor (Shofer et al. 2007 ) (Wen et al. 2009 ).
The use of masks helps reduce the degree of inhalation of noxious substances. However, not all masks are equally effective, and this depends on both the type of mask and the filter it has (Carlsten et al. 2020 ). In a study conducted by Shakya et al., masks made from material were beneficial to a low degree in protecting particles with a diameter of 2.5 μm, while surgical masks were more effective. The most efficient in eliminating most tested particles was N95 masks. The material masks have a higher comfort but are much weaker than N95 masks (Shakya et al. 2016 ).
Conclusions
Today, although we know the impact of pollution on the respiratory system, we have tried to describe up-to-date information on how pollution affects the respiratory system and the pathologies associated with it (Fig. 1 ). This depends on the type of pollutant, its concentration in the environment, and its size. Air pollution potentiates the increase in respiratory pathology. It is important to constantly measure the quality of the air, both in developed and less-developed countries to ensure continued improvement.
NO 2− nitrogen dioxide, SO 2 sulfur dioxide, VOCs volatile organic compounds, CO carbon monoxide, PM2.5 particulate matter with diameter < 2.5 μm in diameter, PM10 particulate matter with diameter < 10 μm in diameter
Strength of this review
The characteristics of this review refer in particular to the lung diseases caused by air pollutants. The lung is one of the main human organs that have direct contact with the air and is able to filter inhalable pollutants. Lung damage by any other pathology corroborated with inhalable pollutants can later affect other organs and the whole body. For this reason, we considered it of major importance to classify the air pollutants and to present how each pollutant influences lung pathologies and can later affect the whole body.
Abbreviations
World Health Organization
sulfur dioxide
nitrogen dioxide
carbon monoxide
volatile organic compounds
International Agency for Research on Cancer
particulate matter
particulate matter with diameter < 2.5 μm in diameter
particulate matter with diameter < 10 μm in diameter
chronic obstructive pulmonary disease
fluorescent oxidation products
traffic-related air pollution
idiopathic pulmonary fibrosis
reactive oxygen species
forced vital capacity
6-min walking test
forced expiratory volume during the first second
University of California San Diego Shortness of Breath Questionnaire
visual analog scale
acute respiratory infections
high-resolution computed tomography
tuberculosis
multiresistance drug resistance
Adar SD, Filigrana PA, Clements N, Peel JL (2014) Ambient coarse particulate matter and human health: a systematic review and meta-analysis. Current Environmental Health Reports 1(3):258–274. https://doi.org/10.1007/s40572-014-0022-z
Article Google Scholar
Akpinar-Elci M, Martin FE, Behr JG, Diaz R (2015) Saharan dust, climate variability, and asthma in Grenada, the Caribbean. International Journal of Biometeorology 59(11):1667–1671. https://doi.org/10.1007/s00484-015-0973-2
Balcan B, Aakan S, Ugurlu AO, Hhandemir BO, Ceyhan BB, Ozkaya S (2016) Effects of biomass smoke on pulmonary functions: a case control study. International Journal of COPD 11(1):1615–1622. https://doi.org/10.2147/COPD.S109056
Bari MA, Kindzierski WB (2017) Characteristics of air quality and sources affecting fine particulate matter (PM2.5) levels in the City of Red Deer, Canada. Environmental Pollution 221:367–376. https://doi.org/10.1016/j.envpol.2016.11.087
Article CAS Google Scholar
Barone-Adesi F, Chapman RS, Silverman DT, He X, Hu W, Vermeulen R, Ning B, Fraumeni JF, Rothman N, Lan Q (2012) Risk of lung cancer associated with domestic use of coal in Xuanwei, China: Retrospective Cohort Study. BMJ (Online) 345(7874):1–10. https://doi.org/10.1136/bmj.e5414
Bernardini F, Attademo L, Trezzi R, Gobbicchi C, Balducci PM, Del Bello V, Menculini G et al (2019) Air pollutants and daily number of admissions to psychiatric emergency services: evidence for detrimental mental health effects of ozone. Epidemiology and Psychiatric Sciences 29:1–7. https://doi.org/10.1017/S2045796019000623
Billionnet C, Gay E, Kirchner S, Leynaert B, Annesi-Maesano I (2011) Quantitative assessments of indoor air pollution and respiratory health in a population-based sample of French dwellings. Environmental Research 111(3):425–434. https://doi.org/10.1016/j.envres.2011.02.008
Bowatte G, Lodge CJ, Knibbs LD, Erbas B, Perret JL, Jalaludin B, Morgan GG et al (2018) Traffic related air pollution and development and persistence of asthma and low lung function. Environment International 113(January):170–176. https://doi.org/10.1016/j.envint.2018.01.028
Cadelis G, Tourres R, Molinie J (2014) Short-term effects of the particulate pollutants contained in Saharan dust on the visits of children to the emergency department due to asthmatic conditions in Guadeloupe (French Archipelago of the Caribbean). PLoS ONE 9(3):e91136. https://doi.org/10.1371/journal.pone.0091136
Carlsten C, Salvi S, Wong GWK, Chung KF (2020) Personal strategies to minimise effects of air pollution on respiratory health : advice for providers , Patients and the Public. https://doi.org/10.1183/13993003.02056-2019
Ciganek M, Neca J (2008) Chemical characterization of volatile organic compounds on animal farms. Veterinarni Medicina 53(12):641–651. https://doi.org/10.17221/1969-VETMED
Cozzi E, Hazarika S, Stallings Iii HW, Cascio WE, Devlin RB, Lust RM, Wingard CJ et al (2021) Ultrafine particulate matter exposure augments ischemia-reperfusion injury in mice. 27834:894–903. https://doi.org/10.1152/ajpheart.01362.2005
Edwards S, Zhao G, Tran J, Patten KT, Valenzuela A, Wallis C, Bein KJ, Wexler AS, Lein PJ, Rao X (2020) Pathological cardiopulmonary evaluation of rats chronically exposed to traffic- related air pollution 128 (December): 1–14.
Gangwar RS, Bevan GH, Palanivel R, Das L (2020) Redox biology oxidative stress pathways of air pollution mediated toxicity : recent insights. Redox Biology 34(March):101545. https://doi.org/10.1016/j.redox.2020.101545
Garcia-Olivé I, Stojanovic Z, Radua J, Rodriguez-Pons L, Martinez-Rivera C, Manzano JR (2018) Effect of air pollution on exacerbations of bronchiectasis in Badalona, Spain, 2008-2016. Respiration 96(2):111–116. https://doi.org/10.1159/000488646
Gharibvand L, Shavlik D, Mark G, Lawrence Beeson W, Soret S, Knutsen R (2017) The association between ambient fine particulate air pollution and lung cancer incidence : results from the. AHSMOG-2 Study 378(3):378–384
Google Scholar
Ghebreyesus TA (2018) 9 out of 10 people worldwide breathe polluted air , but more countries are taking actioN 39 (May): 641–43.
Goeminne PC, Cox B, Finch S, Loebinger MR, Bedi P, Hill AT, Fardon TC, de Hoogh K, Nawrot TS, Chalmers JD (2018) The impact of acute air pollution fluctuations on bronchiectasis pulmonary exacerbation: a case-crossover analysis. European Respiratory Journal 52(1):1702557. https://doi.org/10.1183/13993003.02557-2017
Graff J, Neidell M (2009) Days of haze : environmental information disclosure and intertemporal avoidance behavior. Journal of Environmental Economics & Management 58(2):119–128. https://doi.org/10.1016/j.jeem.2009.03.001
Guarnieri M, Balmes JR (2014) Outdoor air pollution and asthma. The Lancet 383(9928):1581–1592. https://doi.org/10.1016/S0140-6736(14)60617-6
Gyan K, Henry W, Lacaille S, Laloo A, Lamsee-Ebanks C, McKay S, Antoine RM, Monteil MA (2005) African dust clouds are associated with increased paediatric asthma accident and emergency admissions on the Caribbean Island of Trinidad. International Journal of Biometeorology 49(6):371–376. https://doi.org/10.1007/s00484-005-0257-3
Hamra GB, Guha N, Cohen A, Laden F, Raaschou-Nielsen O, Samet JM, Vineis P, Forastiere F, Saldiva P, Yorifuji T, Loomis D (2014) Outdoor particulate matter exposure and lung cancer: a systematic review and meta-analysis. Environmental Health Perspectives 122(9):906–911. https://doi.org/10.1289/ehp.1408092
Havet A, Li Z, Zerimech F, Sanchez M, Siroux V, Le Moual N, Brunekreef B et al (2019) Does the oxidative stress play a role in the associations between outdoor air pollution and persistent asthma in adults? Findings from the EGEA study. Environmental Health: A Global Access Science Source 18(1):1–9. https://doi.org/10.1186/s12940-019-0532-0
Huang HC, Lin FCF, Wu MF, Nfor ON, Hsu SY, Lung CC, Ho CC, Chen CY, Liaw YP (2019) Association between chronic obstructive pulmonary disease and PM2.5 in Taiwanese nonsmokers. International Journal of Hygiene and Environmental Health 222(5):884–888. https://doi.org/10.1016/j.ijheh.2019.03.009
Jassal MS, Bakman I, Jones B (2012) Correlation of ambient pollution levels and heavily-trafficked roadway proximity on the prevalence of smear-positive tuberculosis. Public Health 127(3):268–274. https://doi.org/10.1016/j.puhe.2012.12.030
Johannson KA, Balmes JR, Collard HR (2015) Air pollution exposure: a novel environmental risk factor for interstitial lung disease? Chest 147(4):1161–1167. https://doi.org/10.1378/chest.14-1299
Johannson KA, Vittinghoff E, Lee K, Balmes JR, Ji W, Kaplan GG, Kim DS, Collard HR (2014) Acute exacerbation of idiopathic pulmonary fibrosis associated with air pollution exposure. European Respiratory Journal 43(4):1124–1131. https://doi.org/10.1183/09031936.00122213
Johannson KA, Vittinghoff E, Morisset J, Wolters PJ, Noth EM, Balmes JR, Collard HR (2018) Air pollution exposure is associated with lower lung function, but not changes in lung function, in patients with idiopathic pulmonary fibrosis. Chest 154(1):119–125. https://doi.org/10.1016/j.chest.2018.01.015
Kravchenko J, Lyerly HK (2018) The impact of coal-powered electrical plants and coal ash impoundments on the health of residential communities. North Carolina Medical Journal 79(5):289–300. https://doi.org/10.18043/ncm.79.5.289
Kwon JW, Park HW, Kim WJ, Kim MG, Lee SJ (2018) Exposure to volatile organic compounds and airway inflammation. Environmental Health: A Global Access Science Source 17(1):1–8. https://doi.org/10.1186/s12940-018-0410-1
Lai TC, Chiang CY, Wu CF, Yang SL, Liu DP, Chan CC, Lin HH (2016) Ambient air pollution and risk of tuberculosis: a cohort study. Occupational and Environmental Medicine 73(1):56–61. https://doi.org/10.1136/oemed-2015-102995
Lee Y, Lee YC, Kim T, Choi JS, Park D (2018) Sources and characteristics of particulate matter in subway tunnels in Seoul, Korea. International Journal of Environmental Research and Public Health 15(11):1–17. https://doi.org/10.3390/ijerph15112534
Li Z, Mao X, Liu Q, Song H, Ji Y, Xu D, Qiu B, Tian D, Wang J (2019) Long-term effect of exposure to ambient air pollution on the risk of active tuberculosis. International Journal of Infectious Diseases 87:177–184. https://doi.org/10.1016/j.ijid.2019.07.027
Liang L, Cai Y, Barratt B, Lyu B, Chan Q, Hansell AL, Xie W, Zhang D, Kelly FJ, Tong Z (2019) Associations between daily air quality and hospitalisations for acute exacerbation of chronic obstructive pulmonary disease in Beijing, 2013–17: an ecological analysis. The Lancet Planetary Health 3(6):e270–e279. https://doi.org/10.1016/S2542-5196(19)30085-3
Lin CK, Lin RT, Chen T, Zigler C, Wei Y, Christiani DC (2019) A global perspective on coal-fired power plants and burden of lung cancer. Environmental Health: A Global Access Science Source 18(1):1–11. https://doi.org/10.1186/s12940-019-0448-8
Longhin E, Holme JA, Gutzkow KB, Arlt VM, Kucab JE, Camatini M, Gualtieri M (2013) Cell cycle alterations induced by urban PM2.5 in bronchial epithelial cells: characterization of the process and possible mechanisms involved. Particle and Fibre Toxicology 10(1):1–19. https://doi.org/10.1186/1743-8977-10-63
Maesano CN, Caillaud D, Youssouf H, Banerjee S, Homme JP, Audi C, Horo K, Toloba Y, Ramousse O, and Annesi-maesano I (2019) Indoor exposure to particulate matter and volatile organic compounds in dwellings and workplaces and respiratory health in French farmers 3: 1–12.
Mannucci PM, Harari S, Martinelli I, Franchini M (2015) Effects on health of air pollution: a narrative review. Internal and Emergency Medicine 10(6):657–662. https://doi.org/10.1007/s11739-015-1276-7
Minichilli F, Gorini F, Bustaffa E, Cori L, Bianchi F (2019) Mortality and hospitalization associated to emissions of a coal power plant: a population-based cohort study. Science of the Total Environment 694:133757. https://doi.org/10.1016/j.scitotenv.2019.133757
Nsoh M, Olga B, Mankollo Y, Ebongue M, Cyprien KN, Louise J, Likeng N et al (2019) Acute respiratory infection related to air pollution in Bamenda, North West Region of Cameroon. 8688:1–8. https://doi.org/10.11604/pamj.2019.32.99.15228
Pan Y, Pan X, Xiao H, Xiao H (2019) Structural Characteristics and functional implications of PM2.5 bacterial communities during fall in Beijing and Shanghai, China. Frontiers in Microbiology 10(October):1–11. https://doi.org/10.3389/fmicb.2019.02369
Popovic I, Soares RJ, Magalhaes EG, Marks GB, Dong GH, Wei X, Knibbs LD (2019) A systematic literature review and critical appraisal of epidemiological studies on outdoor air pollution and tuberculosis outcomes. Environmental Research 170(November 2018):33–45. https://doi.org/10.1016/j.envres.2018.12.011
Qin G, Jin X, Zhang Y, Guo L, Chen R, Sang N (2018) Ambient fine particulate matter exposure induces reversible cardiac dysfunction and fibrosis in juvenile and older female mice, 1–14.
Raaschou-Nielsen O, Andersen ZJ, Beelen R, Samoli E, Stafoggia M, Weinmayr G, Hoffmann B, Fischer P, Nieuwenhuijsen MJ, Brunekreef B, Xun WW, Katsouyanni K, Dimakopoulou K, Sommar J, Forsberg B, Modig L, Oudin A, Oftedal B, Schwarze PE, Nafstad P, de Faire U, Pedersen NL, Östenson C-G, Fratiglioni L, Penell J, Korek M, Pershagen G, Eriksen KT, Sørensen M, Tjønneland A, Ellermann T, Eeftens M, Peeters PH, Meliefste K, Wang M, Bueno-de-Mesquita B, Key TJ, de Hoogh K, Concin H, Nagel G, Vilier A, Grioni S, Krogh V, Tsai M-Y, Ricceri F, Sacerdote C, Galassi C, Migliore E, Ranzi A, Cesaroni G, Badaloni C, Forastiere F, Tamayo I, Amiano P, Dorronsoro M, Trichopoulou A, Bamia C, Vineis P, Hoek G (2013) Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). The Lancet Oncology 14(9):813–822. https://doi.org/10.1016/S1470-2045(13)70279-1
Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV et al (2011) An Official ATS/ERS/JRS/ALAT Statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. American Journal of Respiratory and Critical Care Medicine 183(6):788–824. https://doi.org/10.1164/rccm.2009-040GL
Shakya KM, Noyes A, Kallin R, Peltier RE (2016) Evaluating the ef fi cacy of cloth facemasks in reducing particulate matter exposure. 27(3):352–357. https://doi.org/10.1038/jes.2016.42
Shofer S, Chen T-m, Gokhale J, Kuschner WG (2007) Outdoor air pollution : counseling and exposure risk reduction. The Medical Science 333(4):257–260. https://doi.org/10.1097/MAJ.0b013e31803b913c
Singh D, Agusti A, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Criner GJ et al (2019) Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: The GOLD Science Committee Report 2019. The European Respiratory Journal 53(5):1900164. https://doi.org/10.1183/13993003.00164-2019
Wen X-j, Balluz L, Mokdad A (2009) Association between media alerts of air quality index and change of outdoor activity among adult asthma in six states, BRFSS, 2005. J Community Health 34(1):40–46. https://doi.org/10.1007/s10900-008-9126-4
Sun J, Barnes AJ, He D, Wang M, Wang J (2017) Systematic review and meta-analysis of the association between ambient nitrogen dioxide and respiratory disease in China. International Journal of Environmental Research and Public Health 14(6). https://doi.org/10.3390/ijerph14060646
Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD (2015) Acceleration of atherosclerosis and vascular inflammation in an animal model 294 (23): 3003–10.
Sunil VR, Vayas KN, Massa CB, Gow AJ, Laskin JD, Laskin DL (2013) Ozone-induced injury and oxidative stress in bronchiolar epithelium are associated with altered pulmonary mechanics. 133(2):309–319. https://doi.org/10.1093/toxsci/kft071
Tian Y, Xiang X, Juan J, Song J, Cao Y, Huang C, Li M, Yonghua H (2018) Short-term effects of ambient fine particulate matter pollution on hospital visits for chronic obstructive pulmonary disease in Beijing, China. Environmental Health: A Global Access Science Source 17(1):1–8. https://doi.org/10.1186/s12940-018-0369-y
Tiotiu AI, Novakova P, Nedeva D, Chong-neto HJ, Novakova S (2020) Paschalis Steiropoulos, and Krzysztof Kowal. Impact of air pollution on asthma outcomes, 1–29.
Valavanidis A, Vlachogianni T, Fiotakis K, Loridas S (2013) Pulmonary oxidative stress , inflammation and cancer : respirable particulate matter , fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms:3886–3907. https://doi.org/10.3390/ijerph10093886
Wang H, Gao Z, Ren J, Liu Y, Chang L T-c, Cheung K, Feng Y, Li Y (2019a) Chemosphere an urban-rural and sex differences in cancer incidence and mortality and the relationship with PM 2 . 5 exposure: an ecological study in the southeastern side of Hu Line. Chemosphere 216:766–773. https://doi.org/10.1016/j.chemosphere.2018.10.183
Wang L, Xiong Q, Wu G, Gautam A, Jiang J, Liu S, Zhao W, Guan H (2019b) Spatio-temporal variation characteristics of PM2.5 in the Beijing–Tianjin–Hebei Region, China, from 2013 to 2018. International Journal of Environmental Research and Public Health 16(21). https://doi.org/10.3390/ijerph16214276
Wang M, Sampson PD, Sheppard LE, Stein JH, Vedal S, Kaufman JD (2019c) Long-term exposure to ambient ozone and progression of subclinical arterial disease : the multi-ethnic study of atherosclerosis and air pollution 127 (May): 1–8.
Wang N, Mengersen K, Tong S, Kimlin M, Zhou M, Wang L, Yin P et al (2019d) Short-term association between ambient air pollution and lung cancer mortality. Environmental Research 179(June):108748. https://doi.org/10.1016/j.envres.2019.108748
WHO (n.d.) Global Tuberculosis Report 2019
Winterbottom CJ, Shah RJ, Patterson KC, Kreider ME, Panettieri RA, Rivera-Lebron B, Miller WT et al (2018) Exposure to ambient particulate matter is associated with accelerated functional decline in idiopathic pulmonary fibrosis. Chest 153(5):1221–1228. https://doi.org/10.1016/j.chest.2017.07.034
World Health Organization, WHO (2005) WHO air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide: global update 2005, 1–21. 10.1016/0004-6981(88)90109-6.
World Health Organization, WHO (2018) 9 out of 10 people worldwide breathe polluted air, but more countries are taking action. WHO.INT. 2018. https://www.who.int/news/item/02-05-2018-9-out-of-10-people-worldwide-breathe-polluted-air-but-more-countries-are-taking-action .
Xing DF, Xu CD, Liao XY, Xing TY, Cheng SP, Hu MG, Wang JX (2019) Spatial association between outdoor air pollution and lung cancer incidence in China. BMC Public Health 19(1):1–11. https://doi.org/10.1186/s12889-019-7740-y
Xue X, Chen J, Sun B, Zhou B, Li X. (2018) Temporal trends in respiratory mortality and short-term effects of air pollutants in Shenyang , China, 11468–79.
Yao L, Cui LL, Liu JY, Song WM, Su L, Li YF, Li HC (2019) Ambient air pollution exposures and risk of drug-resistant tuberculosis. Environment International 124(January):161–169. https://doi.org/10.1016/j.envint.2019.01.013
Zhang CY, Zhang A (2019) Climate and air pollution alter incidence of tuberculosis in Beijing, China. Annals of Epidemiology 37(2):71–76. https://doi.org/10.1016/j.annepidem.2019.07.003
Zhang S, Li G, Lin T, Guo Q, Pan X (2016) Short-term exposure to air pollution and morbidity of COPD and asthma in East Asian area: a systematic review and meta-analysis. Environmental Research 148:15–23. https://doi.org/10.1016/j.envres.2016.03.008
Zhang YL, Cao F (2015) Fine particulate matter (PM 2.5) in China at a city level. Scientific Reports 5(2014):1–12. https://doi.org/10.1038/srep14884
Zhang Z, Chai P, Wang J, Ye Z, Shen P, Lu H, Jin M, et al. (2019) Association of particulate matter air pollution and hospital visits for respiratory diseases : a time-series study from China.”
Zhao Y, Hu J, Tan Z, Liu T, Zeng W, Li X, Huang C, Wang S, Huang Z, Ma W (2019) Ambient carbon monoxide and increased risk of daily hospital outpatient visits for respiratory diseases in Dongguan, China. Science of the Total Environment 668:254–260. https://doi.org/10.1016/j.scitotenv.2019.02.333
Zheng P-w, Wang J-b, Zhang Z-y, Shen P, Chai P-f, Li D, Jin M-j, Tang M-L, Lu H-c, Lin H-b, Chen K (2017) Air pollution and hospital visits for acute upper and lower respiratory infections among children in Ningbo, China: A Time-Series Analysis. 24:18860–18869. https://doi.org/10.1007/s11356-017-9279-8
Zheng X, Wang G, Bin P, Meng T, Niu Y, Yang M, Zhang L (2019) Time-course e ff ects of antioxidants and phase II enzymes on diesel exhaust particles-induced oxidative damage in the mouse lung. Toxicology and Applied Pharmacology 366(January):25–34. https://doi.org/10.1016/j.taap.2019.01.010
Zhu S, Xia L, Wu J, Chen S, Chen F, Zeng F, Chen X, Chen C, Xia Y, Zhao X, Zhang J (2018) Ambient air pollutants are associated with newly diagnosed tuberculosis: a time-series study in Chengdu, China. Science of the Total Environment 631–632(17):47–55. https://doi.org/10.1016/j.scitotenv.2018.03.017
Download references
Availability of data and materials
The authors confirm that all data underlying the findings are fully available without restriction. Data can be obtained after submitting a request to correspondence author.
Author information
Authors and affiliations.
Department of Pulmonology, University of Medicine and Pharmacy “Victor Babeș”, P-ța Eftimie Murgu nr.2, Timișoara, 300041, Timiș, Romania
Gabriel-Petrică Bălă, Emanuela Tudorache & Cristian Oancea
Department of Pulmonology, University of Medicine and Pharmacy “Iuliu Hațieganu”, Cluj-Napoca, Romania
Ruxandra-Mioara Râjnoveanu
Magnasci SRL, Timișoara, Romania
Radu Motișan
You can also search for this author in PubMed Google Scholar
Contributions
GPB selected the articles included in the review and wrote the draft. RMR reviewed and edited the final draft and provided supervision of the activity. ET and RM analyzed the selected articles and performed the analysis of the collected data. CO managed and coordinated the research activity and provided validation of the final results. All the authors read and approved the manuscript.
Corresponding author
Correspondence to Ruxandra-Mioara Râjnoveanu .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflicts of interest.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Bălă, GP., Râjnoveanu, RM., Tudorache, E. et al. Air pollution exposure—the (in)visible risk factor for respiratory diseases. Environ Sci Pollut Res 28 , 19615–19628 (2021). https://doi.org/10.1007/s11356-021-13208-x
Download citation
Received : 10 June 2020
Accepted : 24 February 2021
Published : 04 March 2021
Issue Date : April 2021
DOI : https://doi.org/10.1007/s11356-021-13208-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Air pollution
- Respiratory diseases
- Find a journal
- Publish with us
- Track your research
- Internal Medicine
- Respiratory tract diseases
- Lung Diseases
Lung Disease Detection Using Deep Learning
- Affiliation: BMS Institute of Technology

- BMS Institute of Technology

Abstract and Figures

Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations

- Vishnu Sharma

- Akram Bennour

- Sameer Algburi

- Baidaa Mutasher Rashed
- Nirvana Popescu
- Dipali Chandre
- Namrata Bhosale
- Radhika Darode
- Devyani S. Jadhav
- J. Lakshmi Narayana
- Manyam Thaile

- Sachin Kumar

- Olga Vorfolomeyeva

- Devesh Pratap Singh

- Hanan Aljuaid
- Raida Alotaibi
- Hilah Almatrafi
- Leen Alfuraih
- Sara Sabour
- Nicholas Frosst
- Geoffrey E. Hinton
- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Heart-Healthy Living
- High Blood Pressure
- Sickle Cell Disease
- Sleep Apnea
- Information & Resources on COVID-19
- The Heart Truth®
- Learn More Breathe Better®
- Blood Diseases & Disorders Education Program
- Publications and Resources
- Clinical Trials
- Blood Disorders and Blood Safety
- Sleep Science and Sleep Disorders
- Lung Diseases
- Health Disparities and Inequities
- Heart and Vascular Diseases
- Precision Medicine Activities
- Obesity, Nutrition, and Physical Activity
- Population and Epidemiology Studies
- Women’s Health
- Research Topics
- All Science A-Z
- Grants and Training Home
- Policies and Guidelines
- Funding Opportunities and Contacts
- Training and Career Development
- Email Alerts
- NHLBI in the Press
- Research Features
- Ask a Scientist
- Past Events
- Upcoming Events
- Mission and Strategic Vision
- Divisions, Offices and Centers
- Advisory Committees
- Budget and Legislative Information
- Jobs and Working at the NHLBI
- Contact and FAQs
- NIH Sleep Research Plan
- < Back To Research Topics
Interstitial Lung Diseases Research
Language switcher.
As part of its broader commitment to research on lung diseases, the NHLBI leads and supports research and programs on interstitial lung diseases (ILDs). The NHLBI has funded several studies and programs to help develop new treatments for long-term lung diseases. Current studies aim to understand the causes of ILDs and develop new ways to diagnose and treat these lung diseases.
NHLBI research that really made a difference
- An NHLBI-funded study discovered new mutations, or changes, in two genes associated with familial pulmonary fibrosis . This is a type of ILD that runs in families, and it causes life-threatening lung scarring in older adults. These findings helped advance our understanding of the causes of ILDs to help prevent lung scarring and improve quality of life.
- The NHLBI supports the Centers for Advanced Diagnostics and Experimental Therapeutics in Lung Diseases (CADET) program. This program stimulated the development of new treatments for lung diseases and sleep disorders. The first stage, CADET I, supported research on how lung diseases develop. The second stage, CADET II, funded research on new drugs. To learn more about new therapies for ILDs, visit NHLBI Supports CADET Researchers to Produce New Pulmonary Disease Drugs .
- NHLBI-funded researchers are helping find a new treatment for scleroderma-associated ILD. A medicine called oral cyclophosphamide (CYC) helps improve lung function in scleroderma-related ILD. However, patients can take this medicine for only a short term — up to a year. The researchers have tested another medicine, mycophenolate mofetil (MMF), and found that MMF is just as effective and easier for patients to tolerate without feeling sick. Also, research partially supported by the NHLBI has found that the medicine metformin, which is already approved to treat type 2 diabetes, has the potential to help treat lung fibrosis that gets worse over time.
Current research funded by the NHLBI
Our Division of Lung Diseases and its Lung Biology and Disease Branch oversee much of the research on ILDs we fund.
Find funding opportunities and program contacts for ILD research.
Current research on lung biology and the causes of ILDs
- Older adults have a higher risk of some types of ILDs. The NHLBI supports research to find out how normal changes in our immune system due to aging can raise the risk of lung scarring. The researchers are also working to determine how age-related changes in the lungs can lead to ILDs.
- NHLBI-funded researchers are studying the role of the immune system and molecules called oxidants in lung scarring. The researchers are using animal models and donated lung tissue from people who have ILDs and asthma to find out how a protein called S-glutathionylation contributes to lung scarring. This research may lead to the development of specific antioxidants to prevent and treat ILDs.
- The NHLBI funds research to identify how specific cell types in the lungs are involved in lung scarring. The researchers are studying how the injury and death of cells in the lungs can trigger lung scarring and cause ILDs.
Find more NHLBI-funded studies on the causes of ILDs at the NIH RePORTER.
Current research on genetics and ILDs
- Some ILDs run in families. These conditions are caused by mutations, or changes in genes that control how our lungs develop or how our lungs respond to damage. The NHLBI supports research to identify genes that cause or raise the risk of a type of ILD called familial interstitial pneumonia. The researchers also aim to find out how mutations in these genes lead to lung damage.
- Idiopathic pulmonary fibrosis (IPF) is the most common type of ILD. NIH-funded researchers are working with the Pulmonary Fibrosis Foundation to identify genes that are linked to IPF and to understand the various types of IPF. The researchers are also working to find out whether a person’s genes determine whether a medicine called N-acetylcysteine is effective in treating IPF.
Find more NHLBI-funded studies on genetics and ILDs at the NIH RePORTER.
Current research on diagnosing and monitoring ILDs
- The NHLBI funds research to develop new methods to diagnose and monitor a type of ILD called hypersensitivity pneumonitis . NHLBI-supported researchers are focused on developing blood tests to monitor people who have hypersensitivity pneumonitis to identify which patients will develop serious complications from this type of ILD.
- To help better diagnose ILDs, NHLBI-funded researchers are using a new technique called high attenuation areas to improve the quality of images from computed tomography (CT) scans. The researchers will use this method to develop ways to predict which people who have mild lung scarring will go on to develop ILDs. This research will involve participants from the NHLBI-funded Multi-Ethnic Study of Atherosclerosis (MESA) . The researchers will also establish a new study called the Families-At-risk for ILD (FAR-ILD) study.
- NHLBI-funded researchers are developing new methods to use magnetic resonance imaging (MRI) technology to measure and monitor lung function in people who have IPF. This will help doctors and researchers find out whether new treatments for ILDs are effective in slowing down or stopping lung scarring. This research will involve both animal models and patients who have IPF.
Find more NHLBI-funded studies on diagnosing ILDs at the NIH RePORTER.
Current research on treating ILDs
- Childhood interstitial lung disease (chILD) can be caused by mutations, or changes, in the genes that control how the body makes a substance called surfactant. Surfactant coats the inside of the lungs and helps the lungs work better. NHLBI-supported researchers are focused on using CRISPR-Cas9 gene editing technology to correct gene mutations that cause problems with surfactant production. This method of gene therapy could help treat life-threatening chILD.
- The NHLBI supports research to find new treatments to slow down or stop lung scarring in people who have ILDs. The researchers are working to identify specific proteins in the body that cause scar tissue to form in the lungs. They will then develop medicines to block these proteins. The researchers will also find out whether removing lung cells that usually form scar tissue can help prevent lung scarring from getting worse in people who have ILDs.
Find more NHLBI-funded studies on treating ILDs at the NIH RePORTER.

Learn more about NHLBI-supported research on treating ILDs: Researchers engineer cells to help reverse pulmonary fibrosis .
ILD research labs at the NHLBI
Researchers from the Laboratory of Applied Precision Omics , within the Pulmonary Branch of our Division of Intramural Research , are developing new methods to monitor and treat lung transplant rejection. Researchers from the Laboratory of Translational Research , also within the Pulmonary Branch, are focused on understanding the cause of an ILD called lymphangioleiomyomatosis (LAM).
Read more about these projects and ongoing clinical trials.
Read about how NHLBI researchers helped develop new imaging methods to monitor lung health: NIH researchers develop high-performance low-field MRI for cardiac and lung imaging .
Related ILD programs
- The Molecular Atlas of Lung Development Program (LungMAP) is building a molecular map of the developing lungs in humans and mice. The program is helping advance lung research, in part through its web-based data resource, called BREATH, that allows users to access LungMAP data and findings. Learn more about LungMAP: NHLBI project breathes life into first in-depth atlas of the human lung .
- The Lung Tissue Research Consortium (LTRC) provides human lung tissues to qualified investigators for use in their research. The program enrolls patients who are planning to have lung surgery, collects blood and other clinical data from these donors, and stores donated tissue that otherwise would be discarded after the lung surgery. The LTRC provides tissue samples and data at no cost to approved investigators.
- The Pulmonary Trials Cooperative brings together patients, researchers, and healthcare professionals from more than 50 institutions, with a common goal of developing new treatments and testing current clinical care practices for ILDs and other lung diseases.
- The Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) centralizes and integrates biospecimens and clinical data that were once stored in separate repositories. Researchers can find and request available resources on BioLINCC’s secure website, which maximizes the value of these resources and advances heart, lung, blood, and sleep research.
- Our Trans-Omics for Precision Medicine (TOPMed) program includes participants who have ILDs, which may help us understand how genes contribute to differences in disease severity and how patients respond to treatment.
- Defining and Promoting Pediatric Pulmonary Health (DAP3H)
- NHLBI Workshop on Intersection between Aging Biology and Pathobiology of Lung Diseases
Explore more NHLBI research on ILDs
The sections above provide you with the highlights of NHLBI-supported research on ILDs. You can explore the full list of NHLBI-funded studies on the NIH RePORTER .
To find more studies:
- Type your search words into the Quick Search box and press enter.
- Check Active Projects if you want current research.
- Select the Agencies arrow, then the NIH arrow, then check NHLBI .
If you want to sort the projects by budget size — from the biggest to the smallest — click on the FY Total Cost by IC column heading.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Angiol
- v.16(3); Autumn 2007

Cigarette smoke and adverse health effects: An overview of research trends and future needs
Sibu p saha.
1 Gill Heart Institute, University of Kentucky, Lexington, Kentucky
Deepak K Bhalla
2 Department of Pharmaceutical Sciences, Wayne State University, Detroit, Michigan
Thomas F Whayne, Jr
3 Graduate Center for Toxicology, University of Kentucky, Lexington, Kentucky, USA
A large volume of data has accumulated on the issues of tobacco and health worldwide. The relationship between tobacco use and health stems initially from clinical observations about lung cancer, the first disease definitively linked to tobacco use. Almost 35 years ago, the Office of the Surgeon General of the United States Health Service reviewed over 7000 research papers on the topic of smoking and health, and publicly recognized the role of smoking in various diseases, including lung cancer. Since then, numerous studies have been published that substantiate the strong association of tobacco use with a variety of adverse human health effects, most prominently with cancer and cardiovascular diseases. Cigarette smoking is regarded as a major risk factor in the development of lung cancer, which is the main cause of cancer deaths in men and women in the United States and the world. Major advances have been made by applying modern genetic technologies to examine the relationship between exposure to tobacco smoke and the development of diseases in human populations. The present review summarizes the major research areas of the past decade, important advances, future research needs and federal funding trends.
A repository for the collection, analysis, validation and dissemination of all smoking and health-related data was established by the World Health Organization. The data received from various member countries were compiled into a book entitled Tobacco or Health: A Global Status Report, 1997 ( 1 ). This report showed smoking prevalence and other tobacco use-related data from various countries and presented an analysis. It is estimated that there are approximately 1.1 billion smokers worldwide, of which 900 million are men and 200 million are women. The sex ratio of men to women is 2:1 for developed nations and 7:1 for developing nations. Smoking prevalence in men and women averages 42% and 24%, respectively, for developed countries, and 48% and 7%, respectively, for less developed countries. In comparison, approximately 47 million people smoke cigarettes in the United States ( 2 ), and smoking prevalence in the United States is estimated at 28% and 23% for men and women, respectively. The Surgeon General’s report in 2004 concluded that in the United States, cigarette smoking has caused 12 million deaths since 1964, at a cost to the nation of approximately US$157.7 billion each year ( 3 ). There has been a significant decline in the consumption of cigarettes in the United States since 1964. The production of cigarettes continues at a steady pace mainly to meet export demands, which continue to rise due to increasing tobacco use in the rest of the world, especially in far eastern and southeastern Asia. On the basis of consumption and disease incidence trends, it is predicted that there will be an epidemic of tobacco-related diseases in various countries of the world in the next 20 to 30 years.
EPIDEMIOLOGY OF TOBACCO-RELATED DISEASE
As part of the Global Burden of Disease Study carried out by the Harvard University School of Public Health in 1997 ( 4 ), it was projected that mortality and morbidity from tobacco use will increase by almost threefold worldwide in 20 to 25 years. Similar predictions have been made by the Oxford University Center headed by Sir Richard Doll, who was one of the first researchers to link cigarette smoking with lung cancer in the 1950s ( 5 , 6 ). Cancer, cardiovascular diseases and chronic obstructive pulmonary disease continue to be the main health problems associated with cigarette smoking. An extensive database has accumulated, which has consistently documented a relationship between smoking and these specific diseases. The strength of the association is further demonstrated by measuring the RR and the presence of a dose-response relationship (ie, direct relationship between the intensity of exposure to cigarette smoke and the risk of disease). According to a 2004 Centers for Disease Control and Prevention report ( 3 ), approximately 2600 people die of cardiovascular disease in the United States every day, which translates into one death every 33 s. Furthermore, the likelihood of dying from heart disease increases fourfold as a result of smoking. The cost of heart disease and stroke in terms of health care expenses and lost productivity was estimated at US$351 billion in the United States alone in 2003.
An analysis by European health experts ( 7 ) determined that in developed countries as a whole, tobacco is responsible for 24% of all male deaths and 7% of all female deaths; these figures rise to over 40% in men in some countries of central and eastern Europe and to 17% in women in the United States. The average decreased life span of smokers is approximately eight years. Among United Kingdom doctors followed for 40 years, overall death rates in middle age were approximately three times higher among physicians who smoked cigarettes than in nonsmokers. In those United Kingdom physicians who stopped smoking, even in middle age, a substantial improvement in life expectancy was noticed. These same experts found that worldwide, smoking kills three million people each year and this figure is increasing. They predict that in most countries, the worst is yet to come, because by the time the young smokers of today reach middle or old age, there will be approximately 10 million deaths per year from tobacco use. Approximately 500 million individuals alive today can expect to be killed by tobacco and 250 million of these deaths will occur in the middle age group. Tobacco is already the biggest cause of adult death in developed countries. Over the next few decades tobacco is expected to become the biggest cause of adult death in the world. For men in developed countries, the full effects of smoking can already be seen. Tobacco causes one-third of all male deaths in the middle age group (plus one-fifth in the old age group) and is the cause of approximately one-half of all male cancer deaths in the middle age group (plus one-third in the old age group). Of those who start smoking in their teenage years and continue smoking, approximately one-half will be killed by tobacco. One-half of these deaths will be in middle-aged individuals (35 to 69 years of age) and each will lose an average of 20 to 25 years of nonsmoker life expectancy. In contrast, the total mortality is decreasing rapidly and cancer mortality is decreasing slowly in nonsmokers in many countries. Throughout Europe in the 1990s, tobacco smoking caused three-quarters of a million deaths in the middle age group. In the Member States of the European Union in the 1990s, there were over one-quarter of a million deaths in the middle age group directly caused by tobacco smoking, which included 219,700 deaths in men and 31,900 in women. There were many more deaths caused by tobacco at older ages. In countries of central and eastern Europe, including the former Union of Soviet Socialist Republics, there were 441,200 deaths in middle-aged men and 42,100 deaths in women. Several epidemiological studies examining the factors responsible for the interindividual differences in the susceptibility to tobacco-related cancers and cardiovascular diseases are being performed in the United States, Europe and Japan. Although still not common practice, many of the newer studies are employing molecular genetic assays in conjunction with epidemiology to identify genotypes susceptible to disease development and select suitable biomarkers of tobacco smoke exposure.
The frequency of investigations in the area of cigarette smoke composition and chemistry decreased during the last decade. Nonetheless, there are ample data to suggest that cigarette smoke is a highly complex mixture that contains approximately 4800 different compounds ( 8 ). Approximately 100 of these compounds are known carcinogens, cocarcinogens and/or mutagens. The complex mixture also contains gases such as ozone, formaldehyde, ammonia, carbon monoxide, toluene and benzene, and about 10 10 particles of different sizes in each mL of mainstream smoke. In addition, a number of other toxic, mutagenic, tumour promoter and/or cocarcinogenic substances have been identified in both mainstream and sidestream cigarette smoke over the years. Many chemical and biological assays of smoke condensates have also documented the presence of potent inhibitors of carcinogenesis in smoke. Such a complex chemical composition of smoke has made it difficult to determine the active constituent(s) responsible for the tobacco-related health risks of smoking and has led to studies of individual constituents of smoke such as polycyclic aromatic hydrocarbons (PAH), nitrosamines and nicotine. Thus, over the years, various individual groups of smoke constituents have been the focus of research at different times. For example, studies of PAH were in vogue during the 1970s and 1980s, followed by nitrosamines in the 1990s. Tobacco alkaloids have long been studied because of their pharmacological activity and have attracted increased attention because of their suspected role in addiction, smoking behaviour and cessation. However, it is also being realized now that the health effects of this complex mixture are likely to result from a combined effect of these chemicals through multiple mechanisms rather than as result of the effects of a single smoke constituent. The mixture contains compounds belonging to almost every class of chemicals that are toxic and protective, agonist and antagonist, carcinogenic and anticarcinogenic, and exists in the gaseous as well as the particulate phase. Extensive studies on the chemical constituents of tobacco smoke and their relationship to disease were published by Hoffmann and Hoffmann of the American Health Foundation ( 8 ). Newer studies have largely focused on the comparative chemistry of mainstream and sidestream smoke. Interest in the free radical chemistry of smoke has resurfaced due to the realization that smoke-induced oxidative injury may play an important role in the etiology of a variety of tobacco-related diseases. Pioneering studies on the free radical chemistry of tobacco smoke, performed in the laboratory of William Pryor at the Louisiana State University ( 9 ), identified short- and long-lived radicals in mainstream and sidestream cigarette smoke, and implicated them in various smoking-associated disease etiologies.
TOBACCO-RELATED CARDIOVASCULAR DISEASE
Cardiovascular diseases, and atherosclerosis in particular, are the leading causes of death in industrial societies. The predominant underlying cause of coronary artery disease (CAD) is atherogenesis, which also causes atherosclerotic aortic and peripheral vascular diseases. Cigarette smoking, independently and synergistically with other risk factors such as hypertension and hypercholesterolemia, contributes to the development and promotion of the atherosclerotic process. Various studies have shown that the risk of developing CAD increases with the number of cigarettes smoked per day, total number of smoking years and the age of initiation, thus indicating a dose-related response. In contrast, cessation of smoking is reported to reduce mortality and morbidity from atherosclerotic vascular disease.
The mechanisms through which smoking influences the development and progression of atherosclerosis are poorly understood at present, but recent studies point to an adverse effect of smoking on endothelial and smooth muscle cell functions as well as thrombotic disturbances produced by tobacco smoke ( 10 , 11 ). With the use of modern ultrasonographic techniques, three independent studies performed in the United States, Europe and Australia have demonstrated that both active and passive smokers exhibit impaired endothelium-dependent vasoregulation ( 12 – 14 ). Some degree of recovery of endothelial function in ex-passive smokers who have stayed away from smoke-contaminated environments further supported a secondary role of smoke in endothelial dysfunction ( 15 ).
Evidence has been presented that tobacco-related impairment of endothelial function may be related to its adverse effects on endothelial nitric oxide (NO) synthase ( 16 , 17 ). An association between a genetic polymorphism of the endothelial NO synthase gene and the predisposition of smokers to CAD was reported ( 18 , 19 ). Additionally, studies report that smoke interferes with L-arginine and NO metabolism, resulting in reduced NO formation ( 20 ). Upregulation of the expression of endothelial cell adhesion molecules (CAMs) such as vascular CAM-1 and intercellular CAM-1 by smoke condensates, and stimulation of leukocyte and endothelium attachment by exposure to cigarette smoke was demonstrated ( 21 ). Cigarette smoke extract has been shown to induce expression of CAMs ( 22 ). However, the expression of a specific adhesion molecule is determined in vivo and the relationship between various events is poorly understood.
Exposure to tobacco smoke is known to increase oxidative stress in the body by various mechanisms, including depletion of plasma antioxidants such as vitamin C. At least two studies have been performed to determine the role of oxidative stress in increasing leukocyte-endothelial interactions that precede the development of atherosclerosis in smokers. One study showed that a high intake of vitamin C by smokers significantly reduced the adhesiveness of their monocytes to endothelial cells ( 23 ). However, in a second study, sera from young smokers was collected before and after a single oral supplementation with vitamin C and L-arginine (a substrate for NO production). The sera were tested for promotion of the adherence of human monocytes to human umbilical vein endothelial cell monolayers. It was shown that while oral L-arginine caused reduction in such leukocyte adherence, no reduction was seen with vitamin C supplementation ( 24 ). This suggested that the NO levels may be important in smoking-induced leukocyte-endothelial interactions, at least during the early stages. Neither NO nor any other markers of oxidative stress were measured in either of these studies.
The levels of 8-hydroxydeoxyguanosine, an oxidized DNA product, and F2-isoprostane, an oxidative arachidonic acid product, were found to be elevated in passive smokers ( 25 , 26 ). Oxidation of low-density lipoprotein (LDL), which is a gold standard risk factor of the atherosclerotic process, was also found to be elevated in smokers, as determined by the presence of increased levels of autoantibodies against oxidized LDL. It was further demonstrated that dietary supplementation with a lipid-soluble antioxidant, α-tocopherol, significantly reduced plasma levels of oxidized LDL autoantibodies ( 27 ). Similarly, intake of a mixture of antioxidants was found to increase the resistance of smoker LDL to oxidative modification ( 28 ) and reduce the plasma levels of 8-hydroxydeoxyguanosine in passive smokers ( 25 ). These studies have thus identified newer, more specific markers of oxidative stress that can be used as biomarkers of oxidant injury and used for the development of dietary and/or pharmacological interventions against disease development.
Relatively few studies related to cardiovascular effects of cigarette smoke have been performed in rodent models. Such animal studies are, however, needed to delineate the role of different mechanisms in promoting atherosclerotic disease and for developing appropriate interventions.
TOBACCO-RELATED CANCERS
Tobacco carcinogenesis has remained a focus of research during the past 10 years, and various epidemiological and experimental studies have not only confirmed the major role of tobacco smoke exposure in lung and bladder cancers, but have also reported on its association with cancers of various other sites, such as the oral cavity, esophagus, colon, pancreas, breast, larynx and kidney. It is also associated with leukemia, especially acute myeloid leukemia.
In addition to the highly recognized role of cigarette smoking in lung cancer, it has been implicated in many other chronic diseases, including chronic bronchitis and pulmonary emphysema. In the United States, the reduction in smoking has resulted in a decline in death due to lung cancer in men since the mid 1980s. However, the incidence of lung cancer in women has surpassed that of breast cancer and continues to rise; it will likely be the focus of future studies ( 29 , 30 ). Both active and passive smoking are implicated in this increase, and several studies of smoking behaviour and disease incidence in women suggest greater susceptibility of women to tobacco carcinogens ( 31 ). It is believed that 80% to 90% of all respiratory cancers are related to active smoking.
Because of the antiestrogenic protective effects of smoking, the role of smoking in breast cancer is controversial. However, recent studies suggest that both active and passive smoking may have a role in the occurrence of breast cancer. One example is a study that found an OR of 4.5 for breast cancer among women who were exposed to passive smoke before 12 years of age and an OR of 7.5 for active smokers. Women who were first exposed to passive smoke after 12 years of age had a lower, although still elevated, OR ( 32 ).
In both men and women, cancers of the head and neck are also on the rise, and this has been attributed to increased use of smokeless tobacco products. Also, a synergistic interaction between cigarette smoking and radon exposure was confirmed in a large study that showed that lung cancer incidence due to an interaction between smoking and radon exposure exceeded incidence accounted for by additive effects and, therefore, indicated multiplicative effects ( 33 ).
Comparative toxicity studies have shown that in comparison with standard cigarettes, the new experimental cigarettes that heat tobacco have a relatively low toxicity ( 34 ). In comparing lung cancer risk in smokers of different types of cigarettes, Lee ( 35 ) determined in 2001 that the risk was 36% lower in individuals smoking filtered cigarettes than in those smoking unfiltered cigarettes, and the risk was 23% lower for smokers of low-tar cigarettes than smokers of high-tar cigarettes. The risk increased by 42% in hand-rolled cigarette smokers and by 75% in smokers using black tobacco.
One interesting observation relates to the nature of lung cancer, which has changed over the years with respect to the location and the types of lung tumours observed in smokers. In the past, the primary tumours observed among smokers were the centrally located squamous cell carcinomas of the airways. Now, the predominant lung tumours in smokers are peripheral adenocarcinomas and other non-small-cell lung cancers. This shift in tumour types has been attributed to changes in the composition of cigarettes and its effect on the smoking patterns of tobacco users over the past 30 years ( 8 , 36 ). Significant reductions in cigarette tar and nicotine and increased levels of nitrates in cigarettes have markedly altered the manner in which cigarettes are smoked. The number and volume of puffs taken by smokers have increased from a single 35 mL puff/min with 1950s cigarettes to two to four 50 mL puffs/min of low-tar or low-nicotine cigarettes; the depth of inhalation has also increased. These changes in smoking patterns have promoted greater deposition of smoke constituents into the peripheral lungs, where adenocarcinomas develop.
Major advances are being made in the area of molecular epidemiology of tobacco-related cancers in human populations. Many recent epidemiological studies have focused on the differential susceptibility to tobacco-related cancers; they have employed polymerase chain reaction-based molecular assays that permit genotypic analysis of small human samples and supplement the information generated by enzymatic and immunological assays. These assays are increasingly being used in human and experimental studies to examine various gene-gene and gene-environment interactions. One area that has received considerable attention in recent years is the role of polymorphic enzymes in the development of diseases. It is now well recognized that genetic polymorphism strongly influences cancer susceptibility and incidence. The frequencies of mutated alleles of proto-oncogenes, tumour suppressor genes and xenobiotic bio-transformation genes vary significantly among different populations and impact substantially on their susceptibility to cancer. Nearly every enzyme in the carcinogen metabolism pathways has been found to exist in multiple forms, many of which vary in binding affinity and/or turnover efficiency. Some are even entirely absent in individuals, thereby influencing their susceptibility to disease development.
The chemical complexity of tobacco smoke and the metabolic activation requirements for many of its carcinogenic constituents have drawn particular attention to genetic polymorphisms of biotransformation enzymes that metabolize tobacco smoke carcinogens. Thus, genes for various activating enzymes such as cytochrome P450 (CYP) proteins, and deactivating enzymes such as glutathione S-transferase (GST), N-acetyl transferase (NAT) and uridine diphosphate-glucose transferase have been the main target of many recent studies in the context of tobacco carcinogenesis. Also, pre-existing inherited mutations and/or mutation susceptibility of tumour suppressor genes such as p53 , which are known to play a major role in determining cancer susceptibility, have been a subject of investigations in tobacco-related carcinogenesis ( 37 , 38 ).
Several human studies have suggested a strong interplay of various polymorphic CYP1A1, CYP1A2, CYP2E1, NAT1, NAT2, GSTM1 and GSTT1 enzymes in modulating the formation of DNA adducts, induction of mutations and chromosomal damage, and/or the incidence of cancers of various sites in different populations ( 39 – 47 ).
The CYP1A1 gene has been extensively studied in Japanese populations. Two polymorphic variants that interact with smoking to modify lung cancer risk have been identified ( 47 , 48 ). Thus, a homozygous minor allele combined with smoking was found to increase lung cancer risk. Studies of the same gene in Western populations have, however, yielded negative or conflicting results ( 49 ), although an interaction of CYP1A1 variants with the GST null genotype has been reported to significantly increase lung cancer risks in non-Japanese populations ( 50 , 51 ).
NATs are polymorphic conjugation enzymes (produced by the NAT1 and NAT2 genes) involved in the detoxification of aromatic amines by N-acetylation. Depending on the presence or absence of a particular variant, individuals can be categorized as slow or fast acetylators, which in turn can influence the incidence of bladder cancer. It was shown that slow acetylator NAT2 is an important modifier of the amount of aromatic amine-DNA adduct formation even at a low dose of tobacco smoke exposure ( 52 ). Slow acetylator NAT2 genotype was also a significant risk factor for bladder cancer in moderate and heavy smokers, but had no effect in nonsmokers ( 53 ).
GSTs are another group of metabolic detoxification enzymes that have attracted a great deal of interest in recent years because of their association with risks for different types of cancers. Based on their sequences, these enzymes are divided into five classes. Three of these classes – GSTM1, GSTT1 and GSTPi – are important in the context of tobacco-related cancers. Extensive studies on the relationship of these genes to cancer risks have shown that most populations studied have very high frequencies (20% to 50%) of homozygous GSTM1 and GSTT1 deletion carriers. GSTM1 and GSTT1 may be involved in the etiology of cancer at more than one site. Furthermore, the risk to individuals who carry homozygous deletions is generally small but increases significantly on interaction with cigarette smoking ( 54 ). Among all metabolic cancer susceptibility genes, the association of GSTM1 deficiency with cancer risk is the most consistent and unidirectional. Various experimental and epidemiological observations support the role of this gene in tobacco-related cancers. For example, it has been observed that the excretion of urinary mutagens and the number of lung tissue DNA adducts in GSTM1-deficient smokers is significantly greater than those carrying the wild-type allele ( 55 – 57 ). Various epidemiological studies also support the premise that deficiency of this enzyme predisposes for lung and bladder cancers ( 58 ). Furthermore, low activity alleles of GSTPi have been often found in association with different types of human cancers ( 59 , 60 ).
In addition to anomalies of biotransformation enzyme genes, inactivation of tumour suppressor genes such as p53 , and activation of the proto-oncogene K-ras are also involved in tobacco-related cancers. Various mutated forms of tumour suppressor gene p53 have been commonly detected in lung tumours and it has been found that these mutations are predominantly located in exons 5 to 8. The nature of point mutations in this gene has been extensively investigated and studies show that the most common mutant allele of the p53 gene possesses a G:C to A:C transversion ( 61 ), which is associated with tobacco use ( 62 , 63 ).
The above studies show that several genetically controlled polymorphic enzymes and enzyme systems are linked to tobacco carcinogen activation and deactivation. Some of these genes have been identified and characterized, but others remain undiscovered. Not only the independent effects of single gene polymorphisms, but an interplay of multiple gene interactions appear to be involved. The complexity of epidemiological studies, which have many uncontrollable variables, makes it difficult to study such interactions and their control in human studies. Additionally, many of the enzymes involved in tobacco carcinogen metabolism are also induced by other environmental factors such as alcohol use, dietary constituents, pesticide and xenobiotic exposure, hormonal status, etc, further complicating the interpretation of data. The interaction of many of these genes with each other and the effect of environmental factors are just beginning to be examined. Experimental studies in specifically constructed transgenic and knock-out animals will be important for a systematic evaluation of the contribution of specific cancer genes and/or cancer susceptibility genes to the tobacco carcinogenic process, and to help identify the mechanisms through which environmental agents, such as cigarette smoke, influence these processes.
SECONDHAND SMOKE
The adverse effects of cigarette smoke on human health are widely recognized. It is the main etiological agent in chronic obstructive pulmonary disease and lung cancer, and is a known human carcinogen. While the risks to human health from active smoking are accepted, evidence supporting the risk of involuntary exposure to environmental tobacco smoke (ETS) has accumulated in recent years. It is the main source of toxicant exposure by inhalation in nonsmokers. Despite recent regulations, smoking in public enterprises is not uncommon. However, despite an occasional report on the effect of secondhand smoke in nonsmokers, little attention was given to this aspect of smoking until about 1970. ETS is now regarded as a risk factor for development of lung cancer, cardiovascular disease and altered lung functions in passive smokers ( 64 ). In general, children exposed to ETS show deterioration of lung function, more days of restricted activity, more pulmonary infections, more days in bed, more absences from school and more hospitalization than children living in nonsmoking homes ( 65 ).
Passive smoking is also implicated in increasing atherosclerosis in individuals 15 to 65 years of age. Children exposed to ETS are at higher risk of developing cardiovascular disorders. Quantitative risk estimates were obtained by measuring the intimal-medial thickness of the carotid artery in a large longitudinal atherosclerosis risk study of 10,914 individuals. Increases of 50%, 25% and 20% were shown over nonsmokers in current, ex-and passive smokers, respectively, thus suggesting a role of all types of tobacco smoke exposure in the progression of atherosclerosis ( 66 ). A recent meta-analysis ( 67 ) of 18 epidemiological studies (10 cohort and eight case-control) further showed an increased RR of CAD in ETS-exposed individuals. These investigators also identified a significant dose-response relationship between the intensity of smoke exposure and risk of CAD in passive smokers. Cardiovascular health risks of smoke-exposed women are of particular concern. Although the exposure to ETS is a current topic of debate in tobacco-related cancers and other lung diseases, the limited research at the basic experimental level provides a strong argument for launching experimental studies to support human data and explore disease mechanisms.
Follow-up of news stories, and local and state ordinances, leads to the conclusion that more communities and states are restricting exposure to secondhand smoke.
NATIONAL INSTITUTES OF HEALTH RESEARCH FUNDING FOR STUDIES OF HEALTH EFFECTS OF CIGARETTE SMOKE
To determine the extent of federal support for experimental studies in the area of health effects of cigarette smoke, the National Institutes of Health (NIH) database of all R01 research grant awards was searched for titles and abstracts containing the words ‘cigarette smoke’ from 1985 to 1998. The results are summarized below. A total of 127 hits were obtained and a careful review of the abstracts provided the following distribution:
- Grants involving experimental animal studies = 12 (9.4%)
- Grants involving experimental animal studies in which whole tobacco smoke was used = 3 (2.3%)
- Grants involving experimental animal studies using smoke components (nicotine, PAH, cadmium and quinones) = 8 (6.2%)
- One grant involved aging
A similar search of the NIH database from 1999 to 2006 revealed 907 grants in all award categories. The grant distribution by category was as follows:
- Total number of R01s = 383
- Grants involving experimental animal studies = 77 (20.1%)
- Grants involving experimental animal studies in which whole tobacco smoke was used = 29 (7.6%)
- Grants involving experimental animal studies using smoke components (nicotine, PAH, cadmium and quinones) = 29 (7.6%)
All the remaining grants generally supported behavioural and epidemiological studies in humans or other systems. Although the number of grants supporting animal studies increased between 1999 and 2006 compared with 1985 to 1998, a significant portion of NIH funding still went to research projects in the area of tobacco use and smoking behaviour, tobacco use among youth and interventions, nicotine addiction and neurobiology of nicotine (areas not covered in this review), presumably in agreement with the NIH’s recent goal of finding effective smoking cessation programs to reduce tobacco usage in the general population. Thus, it is clear that the need for basic experimental research in the field of smoking-associated diseases and the mechanisms through which tobacco smoke causes various diseases remain as important as they ever were. The escalation of health care costs makes it even more necessary to find ways to protect the health of smokers and smoke-exposed individuals with any dietary or therapeutic interventions that hold promise.
DIRECTIONS FOR FUTURE RESEARCH
The most benefit is likely to result from detailed epidemiological studies complemented by specific molecular genotyping of various populations. Ideally, studies of this type will re-evaluate the prevalence of smoking and tobacco use and determine the exact nature of tobacco-related disease incidence, the role of contributory factors such as dietary habits, exposure to other substances and the genetic composition of subpopulations most at risk. Various biochemical and molecular assays will need to be applied to screen nonsmoker and smoker populations for a variety of health risks. Analysis of the results from such studies will help identify the main interacting factors for various health risks and define relationships among various epidemiological parameters. It would appear necessary to assemble teams of multidisciplinary investigators to perform these coordinated human studies in the field and in the laboratory. By nature, such studies are expensive and will involve commitment of resources, time and substantial amounts of funds to obtain meaningful results. Given the limited resources and competing priorities for research funding, it is not easy to undertake such human studies. Hence, the experimental studies in animal models using inhalation exposure to whole smoke, and not individual constituents of smoke, is probably the next best approach for smoking and health programs.
The human epidemiological studies described in the present review have identified a number of genes that appear to have a distinct role in various tobacco-related diseases, and cancers in particular. Inability to control all the different variables in human studies has made it difficult to clearly define the contribution of various suspect genes in tobacco carcinogenesis. With the recent commercial availability of a variety of transgenic and knock-out animals for research, it would be most desirable, as a first step, to use these animals to establish experimental models of various tobacco-related diseases which can then be used for determining the contribution of different genes to disease processes and for elucidation of the mechanism(s) of disease development. Furthermore, these animal models can be used to identify various agents possessing protective and therapeutic potential.
Research efforts in the area of smoking and health would benefit by focusing on studies of the in vivo effects of inhaled whole cigarette smoke in animal models of known specific genetic composition. Selection of the genetic composition would also require a thorough consideration of the information available from human molecular epidemiological studies. As indicated earlier, there are a number of genes that clearly influence the development of smoke-related diseases. In this context, many relevant transgenic and knock-out animals that can be effectively used for the study of tobacco-related diseases are now becoming available.
Tobacco abuse is a major public health problem and includes secondhand smoke exposure. Continued efforts to control and eliminate this abuse are a medical necessity.
- Help & Support
- Research & Reports
About Our Research
More than a century of progress.
For over a century, the American Lung Association has funded thousands of critical lung disease research projects. These projects include examining the cause and prevention of lung diseases like tuberculosis, asthma, COPD and lung cancer, as well as how to manage and eradicate lung disease. Advancements in medical and scientific research to move us closer to a world free of lung disease, through basic, translational and clinical research .
More than 4 in 10 Americans live with unhealthy air, and more than 33 million live with chronic lung diseases like asthma, COPD and lung cancer. In fact, lung disease is the third leading cause of death in the U.S. But research can—and doe— save lives.
For over a century, the American Lung Association has advanced medical and scientific research to move us closer to a world free of lung disease, through basic, translational and clinical research.
To put it simply, basic research is where all research begins to find new ideas, principles and theories that form the basis of scientific development that leads to exciting breakthroughs.
Translational research, also known as “bench – to- bedside” moves theory into medical practice and health outcomes.
Clinical research is then conducted to ensure that a new treatment, device, product or procedure is safe and successful.
It takes all of the pieces of this puzzle, working together to save lives through research. We’ve seen many successes, like our funded researcher Dr. Mary Ellen Avery who made a discovery in the lungs of babies with respiratory distress syndrome that saved an estimated 800,000 children over 50 years following her discovery and led her to win the national medal of science.
And when our Airways Clinical Research Centers discovered that the flu vaccine was safe for people with asthma, the Centers for Disease Control and Prevention changed their recommendations so that all individuals receive the flu vaccine. This life-changing discovery is estimated to avoid 104,000 hospitalizations every year if everyone with asthma got the flu shot.
It takes all of us to work to save lives through research. Together we can watch as the number of lives saved continues to grow and we get one step closer to a world without lung disease.
Medical breakthroughs pioneered by American Lung Association researchers and their colleagues worldwide have reduced the burden of lung disease on patients and their loved ones, allowing them to live healthier, more active lives.
Since 1915, our researchers have made significant contributions to the fight against lung disease by revolutionizing treatment and unlocking secrets of the body's immune system. We have funded breakthroughs in the fight against tuberculosis, identified genes that cause the development of lung cancer and cystic fibrosis, and developed new ways to treat respiratory distress syndrome.
Building Blocks of Scientific Research
Basic research, translational research, clinical research, meet our current researchers.
Our mission is sustained by the innovative lung disease research projects we fund each year. See the scientists poised to make breakthroughs that could improve prevention, detection and treatment of lung disease.
Research News
Research milestones, president's research report, research partnerships, advancing research.
Our annual publication illustrates how lung disease research is helping improve lives and making a difference for all those who have been touched by lung disease.
Important Research Findings
The results of clinical trials from our Airways Clinical Research Centers Network are changing the nature of asthma and COPD patient care across the United States. See important findings from our studies that impact you.
View all research videos on YouTube
Funding & Impact
"The way in which we have the healthcare of tomorrow is to do research today. We need to tackle those major problems that seem intractable, but will be mitigated by discoveries that we make today. There’s no way to do this research without funding, and the vision that our American Lung Association supporters have to bring together a workforce to tackle these major problems is crucial. There can be no more important work."
—Steven Dubinett, MD, Associate Vice Chancellor for Research, and Professor of Medicine, UC.
Page last updated: August 7, 2024
A Breath of Fresh Air In Your Inbox
Join over 700,000 people who receive the latest news about lung health, including research, lung disease, air quality, quitting tobacco, inspiring stories and more!
Thanks for submitting your email.
Make a Donation
Your tax-deductible donation funds lung disease and lung cancer research, new treatments, lung health education, and more.
Become a Lung Health Insider
Thank you! You will now receive email updates from the American Lung Association.
Select Your Location
Select your location to view local American Lung Association events and news near you.
Change Language
Lung helpline.
Talk to our lung health experts at the American Lung Association. Our service is free and we are here to help you.
1-800-LUNG-USA
(1-800-586-4872)

IMAGES
VIDEO
COMMENTS
1. Introduction. The burden of chronic respiratory diseases (CRDs) that affect both adults and children is constantly increasing globally. The mortality and morbidity cause of respiratory diseases is unclear; however, recent statistics published by WHO and other agencies found an estimate of around 400 million people around the globe are suffering with mild to moderate conditions of Asthma and ...
Chronic respiratory diseases are among the most common non-communicable diseases worldwide, largely due to the ubiquity of noxious environmental, occupational, and behavioural inhalational exposures.1 In addition to chronic obstructive pulmonary disease (COPD) and asthma, chronic respiratory diseases include interstitial lung disease, pulmonary sarcoidosis, and pneumoconioses, such as ...
The gut-lung axis, pivotal for respiratory health, is inadequately explored in pulmonary and critical care medicine (PCCM) inpatients. Naijian Li, Guiyan Tan, Zhiling Xie, Weixin Chen, Zhaowei Yang, Zhang Wang, Sha Liu and Mengzhang He. Respiratory Research 2024 25:304. Research Published on: 10 August 2024.
Abstract. COPD and idiopathic pulmonary fibrosis (IPF) together represent a considerable unmet medical need, and advances in their treatment lag well behind those of other chronic conditions. Both diseases involve maladaptive repair mechanisms leading to progressive and irreversible damage. However, our understanding of the complex underlying ...
Long-Term Oxygen Therapy for 24 or 15 Hours per Day in Severe Hypoxemia. M. Ekström and OthersN Engl J Med 2024;391:977-988. Oxygen therapy prolongs survival in patients with severe hypoxemia but ...
Background: In the past two decades, many advances have been made to our understanding of interstitial lung disease (ILD) and the way we approach its treatment. Despite this, many questions remain unanswered, particularly those related to how the disease and its therapies impact outcomes that are most important to patients.
The third edition of the Global Impact of Respiratory Disease 1 begins with the statement "We take our breathing and our respiratory health for granted, but our lungs enable us to live, laugh, love, and enjoy activities." At no time in our recent history has that truth become more evident and more relevant. The COVID-19 pandemic, in a short period of 24 months, has claimed the lives of > 5 ...
Respiratory tract diseases are illnesses that affect components of the respiratory system, including the nasal passages, the bronchi and the lungs. They range from acute infections, such as ...
Chronic obstructive pulmonary disease (COPD) is a disease of the respiratory tract that is characterized narrowing of the small airways and destruction of lung tissue. This leads to poor airflow ...
The Mount Sinai Medical Center Research Registry for Interstitial Lung Disease (MSMC-ILD) was established in 2014. Patients enrolled in MSMC-ILD had a consensus diagnosis from radiology, pathology ...
Over 200 interstitial lung diseases, from ultra rare to relatively common, are recognised. Most interstitial lung diseases are characterised by inflammation or fibrosis within the interstitial space, the primary consequence of which is impaired gas exchange, resulting in breathlessness, diminished exercise tolerance, and decreased quality of life. Outcomes vary considerably for each of the ...
The recent developments of deep learning support the identification and classification of lung diseases in medical images. Hence, numerous work on the detection of lung disease using deep learning can be found in the literature. This paper presents a survey of deep learning for lung disease detection in medical images. There has only been one survey paper published in the last five years ...
Since the arrival of the novel Covid-19, several types of researches have been initiated for its accurate prediction across the world. The earlier lung disease pneumonia is closely related to Covid-19, as several patients died due to high chest congestion (pneumonic condition). It is challenging to differentiate Covid-19 and pneumonia lung diseases for medical experts. The chest X-ray imaging ...
There is increasing interest in understanding the role of air pollution as one of the greatest threats to human health worldwide. Nine of 10 individuals breathe air with polluted compounds that have a great impact on lung tissue. The nature of the relationship is complex, and new or updated data are constantly being reported in the literature. The goal of our review was to summarize the most ...
According to a research study done by the Forum of International Respiratory Societies called "The Global Impact of Respiratory Disease," 10.4 million people suffered mild or severe symptoms of tuberculosis, and 1.4 million of those affected died as per the survey reported . Lung cancer kills an astounding number of people every year.
Coming research initiatives can be summarized in 10 main areas. (1) From epidemiology the impact of new forms of electronic cigarettes on prevalence and mortality of COPD will be sought. (2) The study of the disease endotypes and its relationship phenotypes will have to be unraveled in the next decade. (3) Diagnosis of COPD faces several ...
Interstitial lung disease (ILD) is a collective term representing a diverse group of lung conditions characterised by the presence of non-infective infiltrates, most commonly in the pulmonary interstitium and alveoli, which in certain cases manifest as architectural distortion and irreversible fibrosis. ... Due to the high volume of papers, we ...
Learning about Lung Diseases and their characterization is one of the most interesting research topics in recent years. With the various uses of medical images in hospitals, pathologies, and ...
Become a Lung Health Insider. Join over 700,000 people who receive the latest news about lung health, including research, lung disease, air quality, quitting tobacco, inspiring stories and more! The Lung Association is helping to improve the quality of life for lung disease patients and their families through our groundbreaking research.
Analysis of trend, on the other hand, provides an overview of the research direction of the topic of interest identified from the previous work. In this paper, a taxonomy of deep learning applications on lung diseases and a trend analysis on the topic are presented. ... which are the final contributions of this paper. The state-of-the-art lung ...
The American Lung Association Epidemiology and Statistics Unit monitors trends in lung disease and behavioral risk factors. We analyze raw data from government surveys available through the National Center for Health Statistics and other agencies to develop reports on lung disease mortality, prevalence, hospitalization, economic costs and risk factors.
As part of its broader commitment to research on lung diseases, the NHLBI leads and supports research and programs on interstitial lung diseases (ILDs). The NHLBI has funded several studies and programs to help develop new treatments for long-term lung diseases. Current studies aim to understand the causes of ILDs and develop new ways to ...
Almost 35 years ago, the Office of the Surgeon General of the United States Health Service reviewed over 7000 research papers on the topic of smoking and health, and publicly recognized the role of smoking in various diseases, including lung cancer.
For over a century, the American Lung Association has funded thousands of critical lung disease research projects. These projects include examining the cause and prevention of lung diseases like tuberculosis, asthma, COPD and lung cancer, as well as how to manage and eradicate lung disease. Advancements in medical and scientific research to ...