See highlights from GHTC's 2023 Innovating for Impact Awards
Sign up to receive news and updates from GHTC.
By submitting this form, you are agreeing to the data-use policies outlined in this privacy notice.

Why Research & Development

What is R&D?
Research and development (R&D) is the translation of an idea or discovery into a product that addresses a health need. The result should be a safe and effective product that is appropriate, affordable, acceptable, and accessible to those who need it most.

Why do we need R&D?
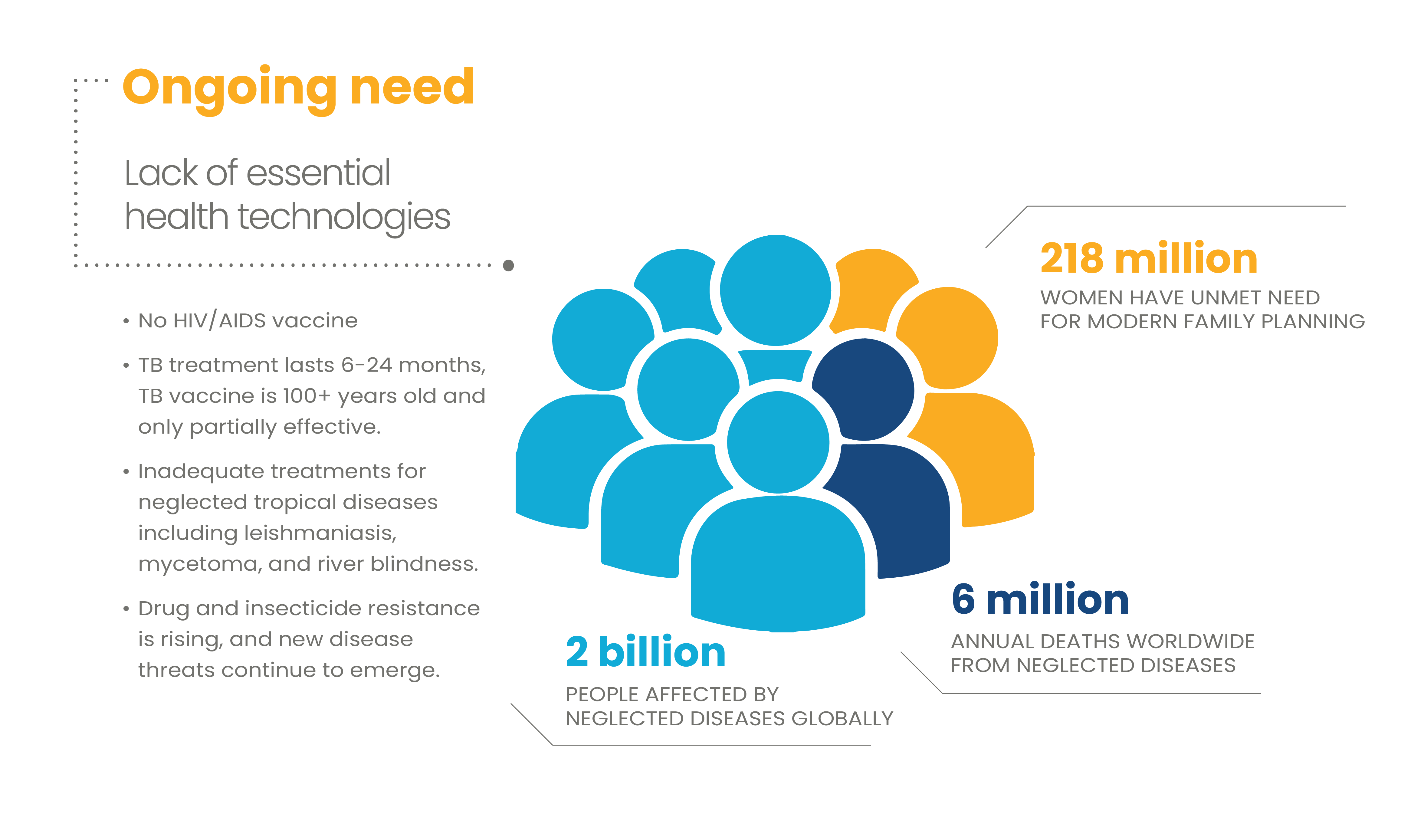
Despite tremendous progress in global health, millions of people still suffer or die each year because we don’t have the right technologies to combat many long-standing health challenges. And new health threats are emerging at a rapid pace. To overcome these challenges, new drugs, vaccines, diagnostics, and other tools are needed.
What has R&D achieved?
R&D has been the bedrock of progress in global health. Technologies developed through R&D have improved the life expectancy of those living with HIV/AIDS, driven remarkable declines in child mortality, and nearly eradicated polio.
within reach thanks to polio vaccines
lives saved annually by vaccines
AIDS-related deaths averted since 2001 due to antiretroviral therapies
Why is R&D a smart investment?
Global health R&D is an outcome multiplier. It not only saves and improves lives, but it also drives economic growth, saves costs, and improves health security.
A new child-friendly malaria medicine has saved an estimated 926,000 lives since its 2009 introduction.
A new treatment regimen for extensively drug-resistant TB had a cure rate of 90% in clinical trials, compared to prior treatments for which the rate was only 34%.
New formulations of the antiseptic chlorhexidine for umbilical cord care are projected to save more than 1 million babies by 2030.
$.86 of every dollar the US government invests in global health R&D goes to US-based researchers and €.80 of every euro of EU investment is spent within the EU.
Anti-malaria efforts are projected to provide $208.6 billion in net global economic gains by 2035.
US government investment in global health R&D from 2007-2022 generated an estimated 600,000 new US jobs and is project to yield $255 billion in long-term benefits to the American economy.
$26 million invested in polio vaccine R&D resulted in treatment cost savings of $180 billion since the 1950s.
Switching all patients with drug-resistant TB to regimens incorporating a new drug pretomanid could generate global cost savings of $740 million annually .
It cost $50 million to develop a low-cost meningitis A vaccine, which is estimated to have saved $9 billion in treatment and other costs between 2010 and 2020.
The 2014 Ebola outbreak claimed 11,000+ lives and cost the US $3 billion and cost the EU €1.2 billion to respond. Now, thanks to R&D efforts, we have vaccines and treatments.
COVID-19 vaccines, which built on decades of prior global health research, saved 14.4 million lives in the first year of the pandemic alone and contributed to an estimated $895 billion in healthcare cost savings from Dec. 2020-March 2022 .
By 2050, antimicrobial resistance could lead to 10 million deaths annually and cost the global economy $100 trillion .
What technologies are needed?
A diverse array of innovations is needed to prevent, diagnose, and treat the world’s most pressing global health challenges.
There are many types of devices that improve global health. Examples include injection tools that make vaccine delivery safer and easier; reproductive health technologies that ensure healthy timing and spacing of pregnancy and safe delivery; and bednets and other vector-control innovations that prevent mosquito-borne diseases. To reach patients in every corner of the globe, devices must be designed to be affordable, reliable, and easy to use, including in places where infrastructure is limited, electricity is unreliable, and trained health workers are scarce.
The first step in treating a disease or condition is identification. But in many places, health workers lack the tools to make an accurate diagnosis, either because the right tool does not exist or because the tool is too costly or challenging to use in resource-limited settings. Continued innovation is needed to create affordable, portable, easy-to-use, and quick diagnostics that deliver reliable and valid results, so every patient can get the right treatment at the right time, every time.
Every day, millions of people suffer or die because they do not have access to the right medicine. For many diseases of poverty, either effective treatments do not exist or are too cumbersome, toxic, or expensive. For diseases such as tuberculosis and malaria, emerging drug resistance is also of growing concern—underscoring the need for continued drug innovation.
Microbicides are biomedical products that block the transmission of HIV and other sexually transmitted infections (STIs). They come in many forms, including gels, tablets, films, vaginal rings, or as part of multipurpose prevention technologies designed to both prevent STIs and provide contraception. Given women are particularly vulnerable to HIV infection due to biology and gender inequalities, safe and effective microbicides are needed to fill a critical gap in HIV prevention.
Malnutrition and micronutrient deficiency can increase a person’s vulnerability to illness and impede intellectual development, robbing communities and nations of critical human capital and potential. An emerging field of nutrition science is studying new ways to improve nutrition through innovations such as biofortified crops with increased vitamin content, probiotic foods, and products that deliver nutritional supplements.
Vaccines are among the most effective public health interventions, not only saving lives but also saving significant costs for health systems. Vaccines have eliminated smallpox, put polio eradication within reach, and saved millions from COVID-19—but there are still many diseases of poverty and pandemic threats for which vaccines are poorly effective or have not yet been developed. Some are well known, such as HIV/AIDS, tuberculosis, and Zika, while others are less familiar but still devastating worldwide, including Chagas disease and Lassa fever.
What does the R&D process look like?
The path from promising discovery to lifesaving technology involves multiple steps:
Understanding needs and challenges of end-users to inform research
Uncovering insights into the biology of a disease and methods to address it
Translating discoveries into potential products and conducting lab testing
Testing products for safety and efficacy in humans
Confirming the safety, efficacy, and quality of new products
Launching and distributing products to meet global needs
Monitoring the ongoing safety, use, and effectiveness of products
Lives saved or improved / Health care costs reduced / Economic growth accelerated
Who are the players?
Many different sectors and stakeholders contribute to the development of global health technologies.
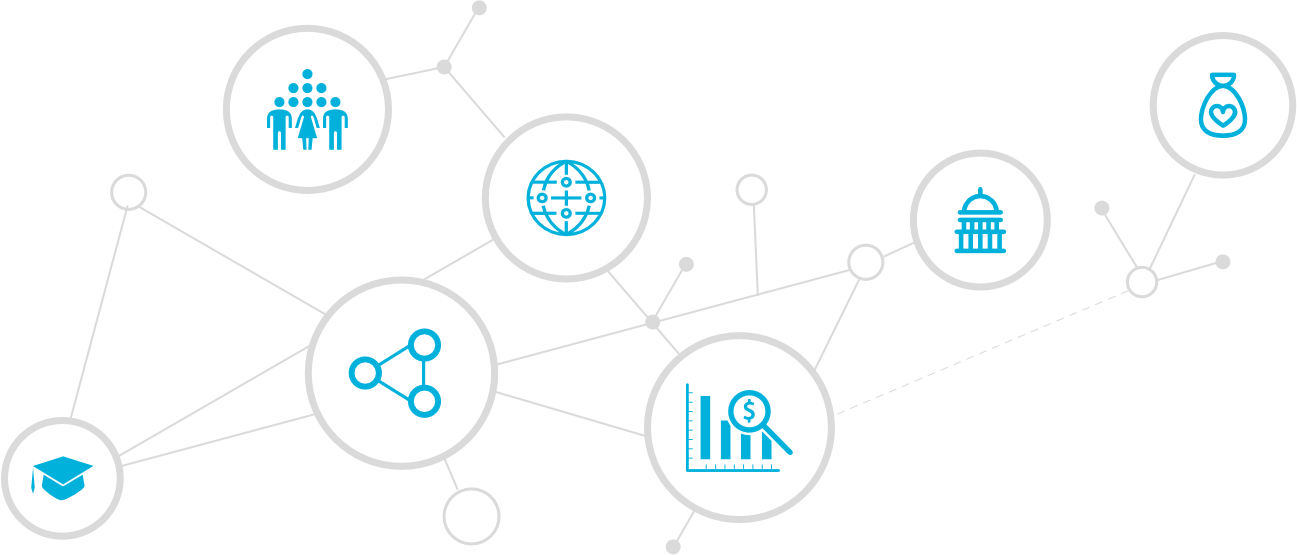
Conduct research to build foundational knowledge of diseases and conditions; advance product development, particularly at the early stage; and build an evidence base to inform R&D.
Engage in education or advocacy efforts to influence and advance R&D. Impacted communities help inform product design, development, and regulation and serve as critical partners in conducting clinical trials.
Directly fund R&D across all stages, particularly for diseases and conditions neglected by the private sector and those with epidemic potential; enact policies to incentivize private-sector R&D; support R&D infrastructure and capacity-building; and oversee regulatory standards and processes.
Convene and coordinate nations around shared health R&D objectives; build an evidence base and provide technical assistance to other stakeholders engaged in health R&D; produce guidelines for product use; and set product efficacy and safety standards for global procurement bodies that purchase and distribute products.
Conducts R&D directly in cases where a profitable market exists for a product or contributes as part of product development partnerships.
Bring together resources and talent from the public, private, and philanthropic sectors to develop critically needed health technologies for neglected diseases and conditions and emerging infectious diseases focusing on public health impact rather than profit.
Fund R&D for diseases and conditions neglected by the private sector, as well as other supportive efforts, including education, advocacy, and research to build an evidence base.
What are the challenges?
There are many obstacles throughout the process of developing new global health technologies.
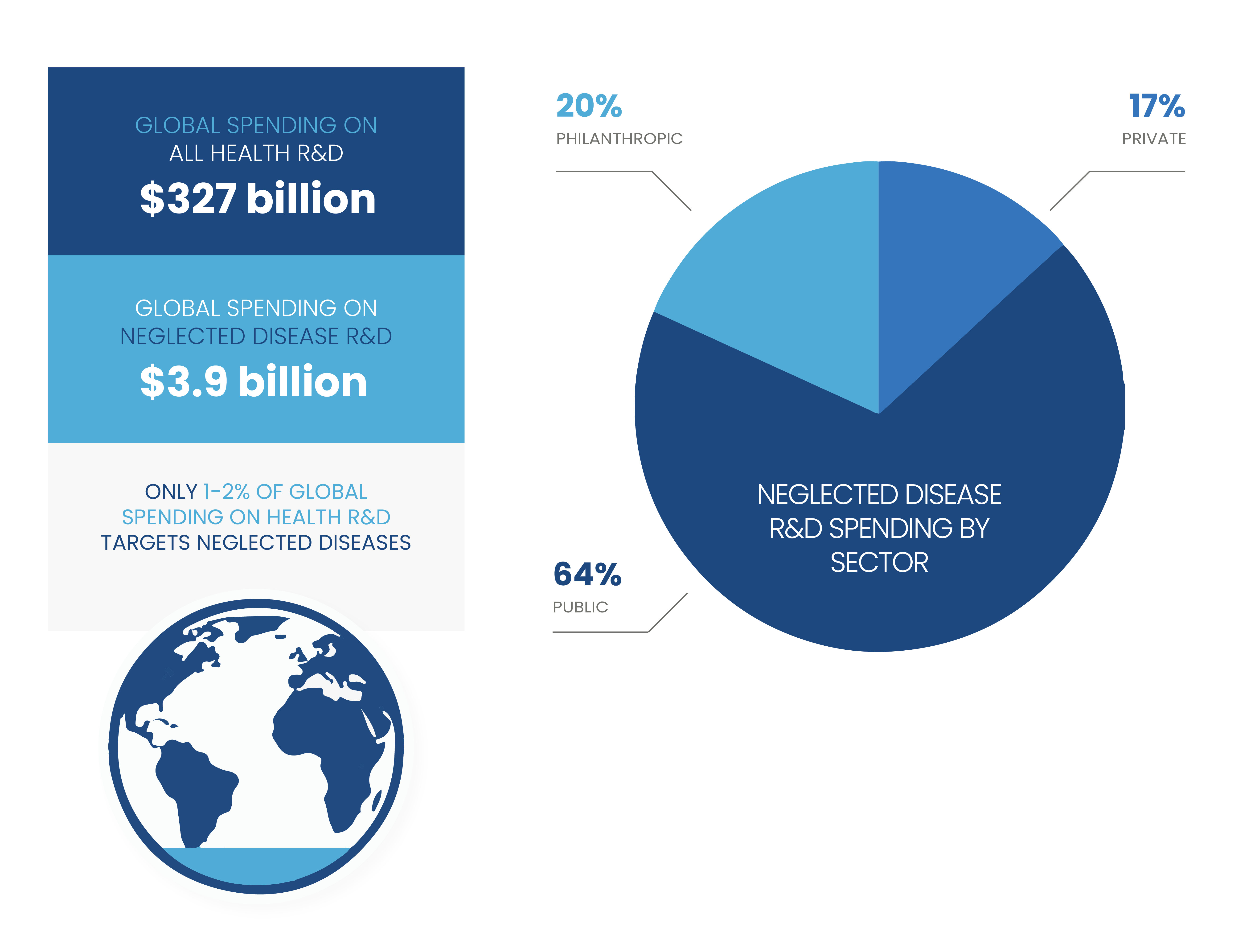
Most challenging is the lack of traditional market incentive. Emerging infectious diseases and diseases and conditions that disproportionately impact the world’s poorest places offer limited profit incentives to spur private-sector investments in R&D for new tools. Thus, sustained public-sector investment and philanthropic funding is critical to jump-start research and successfully move products through the R&D process.
Funding for neglected disease R&D
Designing for access
To reach people in need in every corner of the globe, products must be designed from the start to be fit-for-purpose for resource-limited settings. That means technologies should be affordable, easy to administer, and adapted for use in places without reliable electricity or infrastructure, and that early groundwork is laid to advance manufacturing and distribution approaches that will promote widespread availability.
Affordability
Will the product be available at a price low- and middle-income countries can afford?
Administration
How challenging is it to administer and what training is required of health workers?
Adaptability
Is it usable in settings without basic infrastructure, electricity, and equipment?
Availability
Can manufacturing and distribution mechanisms meet global need?
Capacity gaps
Under-resourced health systems in low- and middle-income countries (LMICs) can also increase the difficulty of conducting clinical trials. Additionally, limited staff and capacity at regulatory authorities, particularly in LMICs, can delay product review and approval, while different regulatory requirements across multiple countries can delay widespread product introduction. It can also be a challenge to secure local manufacturers that can produce products while meeting pricing, supply, and quality standards.
What is GHTC's role?
The challenges for global health R&D are large, but not insurmountable. GHTC works with academia, advocates, civil society, governments, multilateral organizations, the private sector, product development partnerships, and philanthropic donors to catalyze new investment and advance policy solutions to dismantle these obstacles and accelerate the development of critically needed health technologies.
Join our membership
Find out about GHTC’s membership options and the benefits of becoming a member.
Stay updated

An official website of the United States government
Here's how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. A lock ( ) or https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
Keyboard Navigation
- Agriculture and Food Security
- Anti-Corruption
- Conflict Prevention and Stabilization
- Democracy, Human Rights, and Governance
- Economic Growth and Trade
- Environment, Energy, and Infrastructure
- Gender Equality and Women's Empowerment
- Global Health
- Humanitarian Assistance
- Innovation, Technology, and Research
- Water and Sanitation
- Burkina Faso
- Central Africa Regional
- Central African Republic
- Côte d’Ivoire
- Democratic Republic of the Congo
- East Africa Regional
- Power Africa
- Republic of the Congo
- Sahel Regional
- Sierra Leone
- South Africa
- South Sudan
- Southern Africa Regional
- West Africa Regional
- Afghanistan
- Central Asia Regional
- Indo-Pacific
- Kyrgyz Republic
- Pacific Islands
- Philippines
- Regional Development Mission for Asia
- Timor-Leste
- Turkmenistan
- Bosnia and Herzegovina
- North Macedonia
- Central America and Mexico Regional Program
- Dominican Republic
- Eastern and Southern Caribbean
- El Salvador
- Middle East Regional Platform
- West Bank and Gaza
- Dollars to Results
- Data Resources
- Strategy & Planning
- Budget & Spending
- Performance and Financial Reporting
- FY 2023 Agency Financial Report
- Records and Reports
- Budget Justification
- Our Commitment to Transparency
- Policy and Strategy
- How to Work with USAID
- Find a Funding Opportunity
- Organizations That Work With USAID
- Resources for Partners
- Get involved
- Business Forecast
- Safeguarding and Compliance
- Diversity, Equity, Inclusion, and Accessibility
- Mission, Vision and Values
- News & Information
- Operational Policy (ADS)
- Organization
- Stay Connected
- USAID History
- Video Library
- Coordinators
- Nondiscrimination Notice
- Collective Bargaining Agreements
- Disabilities Employment Program
- Federal Employee Viewpoint Survey
- Reasonable Accommodations
- Urgent Hiring Needs
- Vacancy Announcements
- Search Search Search
Health Research and Development

USAID has played a critical role in promoting U.S. interests abroad by investing in research and development that has led to essential breakthroughs in prevention, diagnosis and treatment of global diseases.
USAID is committed to addressing some of the world’s most challenging health and development issues through research, introduction and scale-up of proven solutions. The Bureau for Global Health's investments in research and development have led to critical breakthroughs in prevention, diagnosis and treatment of deadly global diseases.
As a leader in the application of science and technology to achieve development objectives, USAID recognizes that a foundation in research is vital in creating game-changing innovations. Whether it's through product development or field implementation trials, we seek to broaden scientific progress and drive innovation that will empower host countries and improve lives. Through partnerships with our field missions, stakeholder countries and the private sector, the Bureau for Global Health's research and development agenda includes implementing high-impact solutions to prevent child and maternal deaths , control the HIV/AIDS epidemic and combat infectious diseases.

Learn how USAID's Health Research Program supports research and development, introduction, adoption and scale-up of products, technologies and systems to improve maternal, newborn, and child health and nutrition in low and middle income countries.

Numbers at a Glance
Through Grand Challenges for Development, USAID and its partners have cultivated a pipeline of more than 150 innovations and supported their testing, development, and scale-up on their path to deliver health impact.
Reports to Congress
- USAID Global Health Research and Development Strategy 2017–2022 [PDF, 1.0MB]
- USAID Report to Congress on Health-Related Research and Development for FY 2017 [PDF, 313KB]
- Past Health-Related Research and Development Progress Reports
HEALTH RESEARCH 2018 WEBINAR SERIES
Webinar 1: USAID’s Support to Global Health R&D – Presentation [PDF, 4MB] and Adobe Connect
Webinar 3: USAID Global Health R&D and HIV/AIDS – Presentation [PDF, 5MB] and Adobe Connect .
Webinar 4: USAID Global Health Grand Health Challenges – Presentation [PDF, 7MB] and Adobe Connect .
Webinar 5: Health Systems and Maternal and Child Health – Presentation [PDF, 3MB] and Adobe Connect .
Health Research and Implementation Process

Part 1. Overview Information
National Institutes of Health ( NIH )
National Heart, Lung, and Blood Institute ( NHLBI )
National Institute on Aging ( NIA )
National Institute on Alcohol Abuse and Alcoholism ( NIAAA )
National Institute of Biomedical Imaging and Bioengineering ( NIBIB )
Eunice Kennedy Shriver National Institute of Child Health and Human Development ( NICHD )
National Institute on Deafness and Other Communication Disorders ( NIDCD )
National Institute of Diabetes and Digestive and Kidney Diseases ( NIDDK )
National Institute on Drug Abuse ( NIDA )
National Institute of Environmental Health Sciences ( NIEHS )
National Cancer Institute ( NCI )
All applications to this funding opportunity announcement should fall within the mission of the Institutes/Centers. The following NIH Offices may co-fund applications assigned to those Institutes/Centers.
Office of Research on Women's Health ( ORWH )
Office of Data Science Strategy ( ODSS )
Special Note: Not all NIH Institutes and Centers participate in Parent Announcements. Candidates should carefully note which ICs participate in this announcement and view their respective areas of research interest and requirements at the Table of IC-Specific Information, Requirements and Staff Contacts website. ICs that do not participate in this announcement will not consider applications for funding. Consultation with NIH staff before submitting an application is strongly encouraged.
- August 31, 2022 - Implementation Changes for Genomic Data Sharing Plans Included with Applications Due on or after January 25, 2023. See Notice NOT-OD-22-198 .
- August 5, 2022 - Implementation Details for the NIH Data Management and Sharing Policy. See Notice NOT-OD-22-189 .
See Section III. 3. Additional Information on Eligibility .
The purpose of the Mentored Quantitative Research Career Development Award (K25) is to attract to NIH-relevant research those investigators whose quantitative science and engineering research has thus far not been focused primarily on questions of health and disease. The K25 award will provide support and "protected time" for a period of supervised study and research for productive professionals with quantitative (e.g., mathematics, statistics, economics, computer science, imaging science, informatics, physics, chemistry) and engineering backgrounds to integrate their expertise with NIH-relevant research.
This Notice of Funding Opportunity (NOFO) is designed specifically for candidates proposing to serve as the lead investigator of an independent clinical trial, a clinical trial feasibility study, or a separate ancillary study to an existing trial, as part of their research and career development. Candidates not planning an independent clinical trial, or proposing to gain research experience in a clinical trial led by another investigator, must apply to companion NOFO ( PA-24-191 ).
Not Applicable
All applications are due by 5:00 PM local time of applicant organization.
Applicants are encouraged to apply early to allow adequate time to make any corrections to errors found in the application during the submission process by the due date.
It is critical that applicants follow the instructions in the Career Development (K) Instructions in the How to Apply - Application Guide except where instructed to do otherwise (in this NOFO or in a Notice from the NIH Guide for Grants and Contracts ). Conformance to all requirements (both in the How to Apply - Application Guide and the NOFO) is required and strictly enforced. Applicants must read and follow all application instructions in the How to Apply - Application Guide as well as any program-specific instructions noted in Section IV . When the program-specific instructions deviate from those in the How to Apply - Application Guide , follow the program-specific instructions. Applications that do not comply with these instructions may be delayed or not accepted for review.
There are several options available to submit your application through Grants.gov to NIH and Department of Health and Human Services partners. You must use one of these submission options to access the application forms for this opportunity.
- Use the NIH ASSIST system to prepare, submit and track your application online.
- Use an institutional system-to-system (S2S) solution to prepare and submit your application to Grants.gov and eRA Commons to track your application. Check with your institutional officials regarding availability.
- Use Grants.gov Workspace to prepare and submit your application and eRA Commons to track your application.
Part 2. Full Text of Announcement
Section i. funding opportunity description.
The overall goal of the NIH Research Career Development program is to help ensure that a diverse pool of highly trained scientists is available in appropriate scientific disciplines to address the Nation's biomedical, behavioral, and clinical research needs. NIH Institutes and Centers (ICs) support a variety of mentored and non-mentored career development award programs designed to foster the transition of new investigators to research independence and to support established investigators in achieving specific objectives. Candidates should review the different career development (K) award programs to determine the best program to support their goals. More information about Career programs may be found at the NIH Research Training and Career Development website.
The NIH Mentored Quantitative Research Career Development Award (K25) is designed to attract to NIH-relevant research those investigators whose quantitative science and engineering research has thus far not been focused primarily on questions of health and disease. Examples of quantitative scientific and technical backgrounds considered appropriate for this award include, but are not limited to: mathematics, statistics, economics, computer science, imaging science, informatics, physics, chemistry, and engineering. The K25 award is intended to attract talented individuals with highly-developed quantitative skills to the challenges of biomedical, behavioral, and clinical research. At the completion of the award, candidates will have the knowledge and skills necessary to compete for independent research support from NIH, or to participate as leading members of multidisciplinary research teams.
The specific objectives of the K25 award are to :
- Encourage research-oriented quantitative scientists and engineers with little or no experience in biomedicine, bioengineering, bioimaging, or behavioral research to gain fundamental knowledge in these areas, develop relevant research skills, and to gain experience in current concepts, advanced methods, and experimental approaches that will allow them to conduct basic or clinical biomedical, behavioral, bioimaging, or bioengineering research, and to become independent investigators or play leading roles in multi-disciplinary research teams.
- Increase the pool of quantitative researchers who can conduct biomedical, behavioral, or bioengineering studies, capitalizing on the quantitative backgrounds of these investigators to inform new directions in biomedical, behavioral, and bioengineering research.
- Provide a unique opportunity for candidates holding degrees in quantitative science or engineering to embark on three to five years of special study, including coursework, seminars, meetings, and mentored research, to achieve the career enhancement goals outlined above.
Because of the focus on a progression toward independence as a quantitative biomedical, behavioral, bioimaging, or bioengineering researcher, the prospective candidate for the Mentored Quantitative Research Career Development Award will require enhanced skills in the experimental, theoretical and conceptual approaches used in biomedicine, behavioral science, bioimaging or bioengineering. To satisfy this requirement, the candidate should propose a period of study and career development that is complementary to his or her previous research and experience. For example, a candidate with no or very limited experience in a given field of biomedical research may find a phased developmental program lasting for five years that includes a designated period of didactic training together with a closely supervised research experience the most efficient means of attaining independence. A candidate with, for example, more research experience in biomedicine may benefit from a program with greater emphasis on appropriate laboratory research with lower levels of supervision and direction. All programs should be carefully tailored to meet the individual needs of the candidate and must include (an) active mentor(s) who is (are) competent and willing to provide the appropriate research guidance. Candidates should strongly consider incorporating into their training plan formal courses in relevant areas of biomedicine, behavioral science, bioimaging, or bioengineering; this program offers a unique opportunity to devote protected time to this activity.
NIH defines a clinical trial as "A research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes." ( NOT-OD-15-015 ).
NIH not only supports trials of safety and efficacy, it also supports mechanistic exploratory studies that meet the definition of a clinical trial and are designed to explore or understand a biological or behavioral process, the pathophysiology of a disease, or the mechanism of action of an intervention. These studies may focus on basic and/or translational discovery research in healthy human subjects and in human subjects who are affected by the pathophysiology of diseases and disorders. By addressing basic questions and concepts in biology, behavior, and pathophysiology, these studies may provide insight into understanding human diseases and disorders along with potential treatments or preventive strategies. NIH also supports biomarker studies that meet the definition of a clinical trial and that may provide information about physiological function, target engagement of novel therapeutics, and/or the impact of therapeutics on treatment response. NIH thus supports studies that meet the definition of clinical trials (as noted above) but do not seek to establish safety, clinical efficacy, effectiveness, clinical management, and/or implementation of preventive, therapeutic, and services interventions.
Note: This Notice of Funding Opportunity (NOFO) is designed specifically for candidates proposing to serve as the lead investigator of an independent clinical trial, a clinical trial feasibility study, or a separate ancillary clinical trial, as part of their research and career development. Those not planning an independent clinical trial, or proposing to gain research experience in a clinical trial led by another investigator, must apply to companion NOFO ( PA-24-191 ).
Special Note: Because of the differences in individual Institute and Center (IC) program requirements for this NOFO, prospective applicants are strongly encouraged to consult the Table of IC-Specific Information, Requirements and Staff Contacts , to make sure that their application is appropriate for the requirements of one of the participating NIH ICs.
Investigators proposing NIH-defined clinical trials may refer to the Research Methods Resources website for information about developing statistical methods and study designs.
See Section VIII. Other Information for award authorities and regulations.
Section II. Award Information
Grant: A financial assistance mechanism providing money, property, or both to an eligible entity to carry out an approved project or activity.
The OER Glossary and the How to Apply - Application Guide provides details on these application types.
Required: Only accepting applications that propose an independent clinical trial(s).
The number of awards is contingent upon NIH appropriations and the submission of a sufficient number of meritorious applications.
Other Award Budget Information
The participating NIH Institutes and Centers will provide salary and fringe benefits for the award recipient (see Table of IC-Specific Information, Requirements and Staff Contacts ). Further guidance on budgeting for career development salaries is provided in the How to Apply - Application Guide .
In addition, the candidate may derive additional compensation for effort associated with other Federal sources or awards provided the total salary derived from all Federal sources does not exceed the maximum legislated salary rate (see http://grants.nih.gov/grants/policy/salcap_summary.html ) and the total percent effort does not exceed 100%. See also NOT-OD-17-094 .
The participating NIH Institutes and Centers will provide research development support for the award recipient ( Table of IC-Specific Information, Requirements and Staff Contacts ). These funds may be used for the following expenses: (a) tuition and fees related to career development; (b) research-related expenses, such as supplies, equipment and technical personnel; c) travel to research meetings or training; and (d) statistical services including personnel and computer time.
Salary for mentors, secretarial and administrative assistants, etc. is not allowed.
NIH grants policies as described in the NIH Grants Policy Statement will apply to the applications submitted and awards made from this NOFO.
Section III. Eligibility Information
1. Eligible Applicants
Higher Education Institutions
- Public/State Controlled Institutions of Higher Education
- Private Institutions of Higher Education
The following types of Higher Education Institutions are always encouraged to apply for NIH support as Public or Private Institutions of Higher Education:
- Hispanic-serving Institutions
- Historically Black Colleges and Universities (HBCUs)
- Tribally Controlled Colleges and Universities (TCCUs)
- Alaska Native and Native Hawaiian Serving Institutions
- Asian American Native American Pacific Islander Serving Institutions (AANAPISIs)
Nonprofits Other Than Institutions of Higher Education
- Nonprofits with 501(c)(3) IRS Status (Other than Institutions of Higher Education)
- Nonprofits without 501(c)(3) IRS Status (Other than Institutions of Higher Education)
For-Profit Organizations
- Small Businesses
- For-Profit Organizations (Other than Small Businesses)
Local Governments
- State Governments
- County Governments
- City or Township Governments
- Special District Governments
- Indian/Native American Tribal Governments (Federally Recognized)
- Indian/Native American Tribal Governments (Other than Federally Recognized)
Federal Governments
- U.S. Territory or Possession
- Independent School Districts
- Public Housing Authorities/Indian Housing Authorities
- Native American Tribal Organizations (other than Federally recognized tribal governments)
- Faith-based or Community-based Organizations
- Regional Organizations
Non-domestic (non-U.S.) Entities (Foreign Organizations) are not eligible to apply.
Non-domestic (non-U.S.) components of U.S. Organizations are not eligible to apply.
Foreign components, as defined in the NIH Grants Policy Statement , are allowed.
Applicant Organizations
Applicant organizations must complete and maintain the following registrations as described in the How to Apply - Application Guide to be eligible to apply for or receive an award. All registrations must be completed prior to the application being submitted. Registration can take 6 weeks or more, so applicants should begin the registration process as soon as possible. Failure to complete registrations in advance of a due date is not a valid reason for a late submission, please reference NIH Grants Policy Statement 2.3.9.2 Electronically Submitted Applications for additional information.
- NATO Commercial and Government Entity (NCAGE) Code – Foreign organizations must obtain an NCAGE code (in lieu of a CAGE code) in order to register in SAM.
- Unique Entity Identifier (UEI) - A UEI is issued as part of the SAM.gov registration process. The same UEI must be used for all registrations, as well as on the grant application.
- eRA Commons - Once the unique organization identifier is established, organizations can register with eRA Commons in tandem with completing their Grants.gov registration; all registrations must be in place by time of submission. eRA Commons requires organizations to identify at least one Signing Official (SO) and at least one Program Director/Principal Investigator (PD/PI) account in order to submit an application.
- Grants.gov – Applicants must have an active SAM registration in order to complete the Grants.gov registration.
Program Directors/Principal Investigators (PD(s)/PI(s))
All PD(s)/PI(s) must have an eRA Commons account. PD(s)/PI(s) should work with their organizational officials to either create a new account or to affiliate their existing account with the applicant organization in eRA Commons. If the PD/PI is also the organizational Signing Official, they must have two distinct eRA Commons accounts, one for each role. Obtaining an eRA Commons account can take up to 2 weeks.
All PD(s)/PI(s) must be registered with ORCID . The personal profile associated with the PD(s)/PI(s) eRA Commons account must be linked to a valid ORCID ID. For more information on linking an ORCID ID to an eRA Commons personal profile see the ORCID topic in our eRA Commons online help .
Any candidate with the skills, knowledge, and resources necessary to carry out the proposed research as the Program Director/Principal Investigator (PD/PI) is invited to work with their mentor and organization to develop an application for support. Individuals from diverse backgrounds, including individuals from underrepresented racial and ethnic groups, individuals with disabilities, and women are always encouraged to apply for NIH support. See, Reminder: Notice of NIH's Encouragement of Applications Supporting Individuals from Underrepresented Ethnic and Racial Groups as well as Individuals with Disabilities , NOT-OD-22-019 . Multiple PDs/PIs are not allowed.
By the time of award, the individual must be a citizen or a non-citizen national of the United States or have been lawfully admitted for permanent residence (i.e., possess a currently valid Permanent Resident Card USCIS Form I-551, or other legal verification of such status).
Current and former PDs/PIs on NIH research project (R01), program project (P01), center grants (P50), Project Leads of program project (P01), or center grants (P50), other major individual career development awards (e.g., K01, K07, K08, K22, K23, K25, K76, K99/R00), or the equivalent are not eligible. Current and former PDs/PIs of an NIH Small Grant (R03), Exploratory/Developmental Grants (R21/R33), Planning Grant (R34/U34), Dissertation Award (R36), or SBIR/STTR (R41, R42, R43, R44) remain eligible, as do PD/PIs of Transition Scholar (K38) awards and individuals appointed to institutional K programs (K12, KL2). Candidates for the K25 award must have an advanced degree in a quantitative area of science or engineering (e.g., MSEE, PhD, DSc) and have demonstrated research interests in their primary quantitative discipline (including research outside of biomedicine, behavioral sciences, bioimaging, or bioengineering). The candidate should have demonstrated professional accomplishments consonant with his or her career stage. The K25 award is intended for research-oriented investigators at any level of experience, from the postdoctoral level to senior faculty level, who have shown clear evidence of productivity and research excellence in the field of their training, and who would like to expand their research capability, with the goal of making significant contributions to behavioral, biomedical (basic or clinical), bioimaging or bioengineering research that is relevant to the NIH mission.
2. Cost Sharing
This NOFO does not require cost sharing as defined in the NIH Grants Policy Statement Section 1.2 Definitions of Terms .
3. Additional Information on Eligibility
Applicant organizations may submit more than one application, provided that each application is scientifically distinct, and each is from a different candidate.
NIH will not accept duplicate or highly overlapping applications under review at the same time per NIH Grants Policy Statement Section 2.3.7.4 Submission of Resubmission Application . An individual may not have two or more competing NIH career development applications pending review concurrently. In addition, NIH will not accept:
- A new (A0) application that is submitted before issuance of the summary statement from the review of an overlapping new (A0) or resubmission (A1) application.
- A resubmission (A1) application that is submitted before issuance of the summary statement from the review of the previous new (A0) application.
- An application that has substantial overlap with another application pending appeal of initial peer review. (See NIH Grants Policy Statement 2.3.9.4 Similar, Essentially Identical, or Identical Applications ).
Candidates may submit research project grant (RPG) applications concurrently with the K application. However, any concurrent RPG application may not have substantial scientific and/or budgetary overlap with the career award application. K award recipients are encouraged to obtain funding from NIH or other Federal sources either as a PD/PI on a competing research grant award or cooperative agreement, or as project leader on a competing multi-project award as described in NOT-OD-18-157 .
At the time of award, the candidate must have a full-time appointment at the academic institution. Candidates are required to commit a minimum of 75% of full-time professional effort (i.e., a minimum of 9 person-months) to their program of career development. Candidates may engage in other duties as part of the remaining 25% of their full-time professional effort not covered by this award, as long as such duties do not interfere with or detract from the proposed career development program.
Candidates who have VA appointments may not consider part of the VA effort toward satisfying the full time requirement at the applicant institution. Candidates with VA appointments should contact the staff person in the relevant Institute or Center prior to preparing an application to discuss their eligibility.
After the receipt of the award, adjustments to the required level of effort may be made in certain circumstances. See NOT-OD-18-156 and NIH Grants Policy Statement , Section 12.3.6.4 Temporary Adjustments to the Percent Effort Requirement for more details.
Before submitting the application, the candidate must identify a mentor who will supervise the proposed career development and research experience. The mentor should be an active investigator in the area of the proposed research and be committed both to the career development of the candidate and to the direct supervision of the candidate’s research. The mentor must document the availability of sufficient research support and facilities. Candidates are encouraged to identify more than one mentor, i.e., a mentoring team, if this is deemed advantageous for providing expert advice in all aspects of the research career development program. In such cases, one individual must be identified as the primary mentor who will coordinate the candidate’s research. The candidate must work with the mentor(s) in preparing the application. The mentor, or a member of the mentoring team, should have a successful track record of mentoring individuals at the candidate’s career stage. The recruitment of women, individuals from underrepresented racial and ethnic groups, and individuals with disabilities as potential mentors is encouraged.
The mentor(s) or mentoring team must demonstrate appropriate expertise, experience, and ability to guide the applicant in the organization, management and implementation of the proposed research and clinical trial.
The applicant institution must have a strong, well-established record of research and career development activities and faculty qualified to serve as mentors in biomedical, behavioral, or clinical research.
Section IV. Application and Submission Information
1. Requesting an Application Package
Buttons to access the online ASSIST system or to download application forms are available in Part 1 of this NOFO. See your administrative office for instructions if you plan to use an institutional system-to-system solution.
2. Content and Form of Application Submission
It is critical that applicants follow the instructions in the Career Development (K) Instructions in the How to Apply - Application Guide except where instructed in this notice of funding opportunity to do otherwise. Conformance to the requirements in the How to Apply - Application Guide is required and strictly enforced. Applications that are out of compliance with these instructions may be delayed or not accepted for review.
For information on Application Submission and Receipt, visit Frequently Asked Questions – Application Guide, Electronic Submission of Grant Applications .
Page Limitations
All page limitations described in the How to Apply - Application Guide and the Table of Page Limits must be followed.
The following section supplements the instructions found in the How to Apply - Application Guide and should be used for preparing an application to this NOFO.
SF424(R&R) Cover
All instructions in the How to Apply - Application Guide must be followed.
SF424(R&R) Project/Performance Site Locations
Other Project Information
SF424(R&R) Senior/Key Person Profile Expanded
R&R Budget
PHS 398 Cover Page Supplement
PHS 398 Career Development Award Supplemental Form
The PHS 398 Career Development Award Supplemental Form is comprised of the following sections:
Candidate Research Plan Other Candidate Information Mentor, Co-Mentor, Consultant, CollaboratorsEnvironment & Institutional Commitment to the CandidateOther Research Plan Sections Appendix
Candidate Section
All instructions in the How to Apply - Application Guide must be followed, with the following additional instructions:
Candidate Information and Goals for Career Development
Candidate’s Background
- Describe prior training and research experience and how these relate to the objectives and long-term career plans of the candidate. Explain how the award will contribute to their attainment.
- Describe the candidate’s research efforts and professional accomplishments consonant with career status, including any publications that demonstrate the candidate’s experience and interest in pursuing research (including research outside of biomedicine, behavior, bioimaging, or bioengineering).
- Provide a description of the candidate's commitment to a career in quantitative biomedical, bioimaging, behavioral, or bioengineering research that is relevant to the NIH mission.
Provide evidence of the candidate's potential to develop into a successful independent investigator. Usually this is evident from publications, prior research interests and experience, and reference letters.
- If applicable, describe the candidate's ability to organize, manage, and implement the proposed clinical trial, feasibility or ancillary clinical trial.
- If applicable, describe the candidate's prior efforts, interests and experience in clinical trials research.
Career Goals and Objectives
- Describe a systematic plan: (1) that shows a logical progression from prior research and training experiences to the research and career development experiences that will occur during the career award period and then to independent investigator status; and (2) that justifies the need for further career development to become an independent investigator.
- The candidate must demonstrate they have received training or will participate in courses such as: data management, epidemiology, study design (including statistics), hypothesis development, drug development, etc., as well as the legal and ethical issues associated with research on human subjects and clinical trials.
Candidate’s Plan for Career Development/Training Activities During Award Period
- Provide a description of the career development plan, incorporating consideration of the candidate's goals and prior experience. Propose a plan to obtain the necessary theoretical and conceptual background and research experience to launch an independent research career in quantitative biomedicine, bioengineering, bioimaging or behavioral research
- Include a list of the specific course of study in which the candidate will engage, including specific coursework which is essential to gaining the required theoretical and conceptual understanding of biomedicine, behavioral science, bioimaging, or bioengineering, important to the candidate's short- and long-term research interests and the manner of integration of these studies into the career development plan.
- The career development plan must be tailored to the needs of the individual candidate and the ultimate goal of achieving independence as a researcher in quantitative biomedicine, behavioral science, bioimaging, or bioengineering. Less experienced candidates may require a phased developmental period in which the first one to two year(s) of the award are largely didactic in nature that is followed by a period of intense, supervised research. Candidates with more experience at the time of application may need a shorter developmental period and may already have an adequate theoretical background.
- Describe the professional responsibilities/activities (including other research projects) beyond the minimum required 9 person months (75% full-time professional effort) commitment to the K25 award. Explain how these responsibilities/activities relate to the career development objectives of this award and will help ensure career progression to achieve independence as an investigator.
- The candidate and the mentor are jointly responsible for the preparation of the career development plan. A timeline is often helpful. The candidate or mentor may form a mentoring team or advisory committee to assist with the development of a program of study or to monitor the candidate's progress through the career development program.
Research Plan Section
All instructions in the How to Apply - Application Guide must be followed, with the following additional instructions:
Research Strategy
- Provide a sound quantitative biomedical, behavioral, or bioengineering research plan that is consistent with the candidate’s level of research development and objectives of their career development plan.
- The application must also describe the relationship between the mentor’s research and the candidate’s proposed research plan and the benefits of that relationship including how the candidate’s project will lead to an independent line of research. For research projects requiring team-based approaches, such as large epidemiological studies explain how the research will enhance the candidate’s expertise and prepare the candidate to have a major role in designing and leading future projects.
- Applicants proposing a clinical trial, ancillary or feasibility study should describe the planned analyses and statistical approach and how the expected analytical approach is suited to the available resources, proposed study design, scope of the project, and methods used to assign trial participants and deliver interventions.
- If proposing an ancillary clinical trial, provide a brief description of its relationship to the larger clinical trial.
- If proposing a feasibility study, to begin to address a clinical question, provide justification why this is warranted and how it will contribute the overall goals of the research project including planning and preliminary data for future, larger scale clinical trials.
- Describe the proposed timelines for the proposed clinical trial, feasibility study or ancillary clinical trial, including any potential challenges and solutions (e.g., enrollment shortfalls or inability to attribute causal inference to the results of an intervention when performing a small feasibility study).
- Describe how the proposed clinical trial or ancillary clinical trial will test the safety, efficacy or effectiveness of an intervention that could lead to a change in clinical practice, community behaviors or health care policy (This would not apply to a feasibility study).
Training in the Responsible Conduct of Research
- All applications must include a plan to fulfill NIH requirements for instruction in the Responsible Conduct of Research (RCR). See How to Apply - Application Guide for instructions.
Mentor, Co-Mentor, Consultant, Collaborators Section
Plans and Statements of Mentor and Co-mentor(s)
- The candidate must name a primary mentor who, together with the candidate, is responsible for the planning, directing, monitoring, and executing the proposed program. The candidate may also nominate co-mentors as appropriate to the goals of the program.
- The mentor should have sufficient independent research support to cover the costs of the proposed research project in excess of the allowable costs of this award.
- Include a statement that the candidate will commit at least 9 person months (75% of full-time professional effort) to the career development program and related career development activities.
- The application must include a statement from the mentor providing: 1) information on their research qualifications and previous experience as a research supervisor; 2) a plan that describes the nature of the supervision and mentoring that will occur during the proposed award period; 3) a plan for career progression for the candidate to move from the mentored stage of their career to independent research investigator status during the project period of the award; and 4) a plan for monitoring the candidate’s research, publications, and progression towards independence.
- Similar information must be provided by any co-mentor. If more than one co-mentor is proposed, the respective areas of expertise and responsibility of each should be described. Co-mentors should clearly describe how they will coordinate the mentoring of the candidate. If any co-mentor is not located at the sponsoring institution, a statement should be provided describing the mechanism(s) and frequency of communication with the candidate, including the frequency of face-to-face meetings.
- The mentor must agree to provide annual evaluations of the candidate’s progress as required in the annual progress report.
- The mentor or mentoring team must provide evidence of expertise, experience, and ability to guide the candidate in the organization, management and implementation of the proposed clinical trial, ancillary clinical trial or feasibility study and help him/her to meet timelines.
Letters of Support from Collaborators, Contributors and Consultants
- Signed statements must be provided by all collaborators and/or consultants confirming their participation in the project and describing their specific roles. Unless also listed as senior/key personnel, collaborators and consultants do not need to provide their biographical sketches. However, information should be provided clearly documenting the appropriate expertise in the proposed areas of consulting/collaboration.
- Advisory committee members (if applicable): Signed statements must be provided by each member of the proposed advisory committee. These statements should confirm their participation, describe their specific roles, and document the expertise they will contribute. Unless also listed as senior/key personnel, these individuals do not need to provide their biographical sketches.
Environmental and Institutional Commitment to the Candidate
Description of Institutional Environment
- The sponsoring institution must document a strong, well-established research and career development program related to the candidate's area of interest, including a high-quality research environment with key faculty members and other investigators capable of productive collaboration with the candidate.
- Describe how the institutional research environment is particularly suited for the development of the candidate's research career and the pursuit of the proposed research plan.
- Describe the resources and facilities that will be available to the candidate, including any clinical trial-related resources, such as specialized administrative, data coordinating, enrollment, and laboratory/testing support. If applicable, include a description of the resources and facilities available at international sites.
Institutional Commitment to the Candidate’s Research Career Development
- The sponsoring institution must provide a statement of commitment to the candidate's development into a productive, independent investigator and to meeting the requirements of this award. It should be clear that the institutional commitment to the candidate is not contingent upon receipt of this career award.
- Provide assurances that the candidate will be able to devote the required effort to activities under this award. The remaining effort should be devoted to activities related to the development of the candidate’s career as an independent scientist.
- Provide assurances that the candidate will have access to appropriate office and laboratory space, equipment, and other resources and facilities (including access to clinical and/or other research populations, as applicable) to carry out the proposed research plan.
- Provide assurance that appropriate time and support will be available for any proposed mentor(s) and/or other staff consistent with the career development plan.
Other Plan(s):
Note: Effective for due dates on or after January 25, 2023, the Data Management and Sharing Plan will be attached in the Other Plan(s) attachment in FORMS-H application forms packages.
- All candidates planning research (funded or conducted in whole or in part by NIH) that results in the generation of scientific data are required to comply with the instructions for the Data Management and Sharing Plan. All applications, regardless of the amount of direct costs requested for any one year, must address a Data Management and Sharing Plan.
Limited items are allowed in the Appendix. Follow all instructions for the Appendix as described in the How to Apply - Application Guide ; any instructions provided here are in addition to the How to Apply - Application Guide instructions.
PHS Human Subjects and Clinical Trials Information
When involving NIH-defined human subjects research, clinical research, and/or clinical trials (and when applicable, clinical trials research experience) follow all instructions for the PHS Human Subjects and Clinical Trials Information form in the How to Apply - Application Guide , with the following additional instructions:
If you answered “Yes” to the question “Are Human Subjects Involved?” on the R&R Other Project Information form, you must include at least one human subjects study record using the Study Record: PHS Human Subjects and Clinical Trials Information form or Delayed Onset Study record.
Study Record: PHS Human Subjects and Clinical Trials Information
Delayed Onset Study
Note: Delayed onset does NOT apply to a study that can be described but will not start immediately (i.e., delayed start).
All instructions in the SF424 (R&R) Application Guide must be followed.
PHS Assignment Request Form
Reference Letters
Candidates must carefully follow the How to Apply - Application Guide , including the time period for when reference letters will be accepted . Applications lacking the appropriate required reference letters will not be reviewed. This is a separate process from submitting an application electronically. Reference letters are submitted directly through the eRA Commons Submit Referee Information link and not through Grants.gov.
3. Unique Entity Identifier and System for Award Management (SAM)
See Part 2. Section III.1 for information regarding the requirement for obtaining a unique entity identifier and for completing and maintaining active registrations in System for Award Management (SAM), NATO Commercial and Government Entity (NCAGE) Code (if applicable), eRA Commons, and Grants.gov
4. Submission Dates and Times
Part I. contains information about Key Dates and Times. Applicants are encouraged to submit applications before the due date to ensure they have time to make any application corrections that might be necessary for successful submission. When a submission date falls on a weekend or Federal holiday , the application deadline is automatically extended to the next business day.
Organizations must submit applications to Grants.gov (the online portal to find and apply for grants across all Federal agencies) using ASSIST or other electronic submission systems. Applicants must then complete the submission process by tracking the status of the application in the eRA Commons , NIH’s electronic system for grants administration. NIH and Grants.gov systems check the application against many of the application instructions upon submission. Errors must be corrected and a changed/corrected application must be submitted to Grants.gov on or before the application due date and time. If a Changed/Corrected application is submitted after the deadline, the application will be considered late. Applications that miss the due date and time are subjected to the NIH Grants Policy Statement Section 2.3.9.2 Electronically Submitted Applications .
Applicants are responsible for viewing their application before the due date in the eRA Commons to ensure accurate and successful submission.
Information on the submission process and a definition of on-time submission are provided in the How to Apply - Application Guide .
5. Intergovernmental Review (E.O. 12372)
This initiative is not subject to intergovernmental review.
6. Funding Restrictions
All NIH awards are subject to the terms and conditions, cost principles, and other considerations described in the NIH Grants Policy Statement Section 7.9.1 Selected Items of Cost .
Pre-award costs are allowable only as described in the NIH Grants Policy Statement .
7. Other Submission Requirements and Information
Applications must be submitted electronically following the instructions described in the How to Apply - Application Guide . Paper applications will not be accepted.
Applicants must complete all required registrations before the application due date. Section III. Eligibility Information contains information about registration.
For assistance with your electronic application or for more information on the electronic submission process, visit How to Apply - Application Guide . If you encounter a system issue beyond your control that threatens your ability to complete the submission process on-time, you must follow the Dealing with System Issues guidance. For assistance with application submission, contact the Application Submission Contacts in Section VII.
Important reminders:
All PD(s)/PI(s) must include their eRA Commons ID in the Credential field of the Senior/Key Person Profile form . Failure to register in the Commons and to include a valid PD/PI Commons ID in the credential field will prevent the successful submission of an electronic application to NIH. See Section III of this NOFO for information on registration requirements.
The applicant organization must ensure that the unique entity identifier provided on the application is the same identifier used in the organization’s profile in the eRA Commons and for the System for Award Management. Additional information may be found in the How to Apply - Application Guide .
See more tips for avoiding common errors.
Upon receipt, applications will be evaluated for completeness and compliance with application instructions by the Center for Scientific Review, NIH. Applications that are incomplete or non-compliant will not be reviewed.
Post Submission Materials
Applicants are required to follow the instructions for post-submission materials, as described in the policy .
Any instructions provided here are in addition to the instructions in the policy.
Section V. Application Review Information
1. Criteria
Only the review criteria described below will be considered in the review process. Applications submitted to the NIH in support of the NIH mission are evaluated for scientific and technical merit through the NIH peer review system.
For this particular announcement, note the following : Reviewers should evaluate the candidate’s potential for developing an independent research program that will make important contributions to the field, taking into consideration the years of research experience and the likely value of the proposed research career development as a vehicle for developing a successful, independent research program.
Overall Impact
Reviewers should provide their assessment of the likelihood that the proposed career development and research plan will enhance the candidate’s potential for a productive, independent scientific research career in a health-related field, taking into consideration the criteria below in determining the overall impact score.
Reviewers will consider each of the review criteria below in the determination of scientific merit, and give a separate score for each. An application does not need to be strong in all categories to be judged likely to have major scientific impact.
The reviewers will consider that the clinical trial may include study design, methods, and intervention that are not by themselves innovative, but address important questions or unmet needs. Reviewers should also consider the scope of the clinical trial relative to the available resources, including the possibility that research support provided through career development awards may be sufficient to support only small feasibility studies.
Candidate
- Does the candidate have the potential to develop as an independent and productive researcher?
- Are the candidate's prior training and research experience appropriate for this award?
- Is the candidate’s academic, clinical (if relevant), and research record of high quality?
- Is there evidence of the candidate’s commitment to meeting the program objectives to become an independent investigator in research?
- Do the reference letters address the above review criteria, and do they provide evidence that the candidate has a high potential for becoming an independent investigator.
- Does the candidate have the potential to organize, manage, and implement the proposed clinical trial, feasibility or ancillary study?
- Does the candidate have training (or plans to receive training) in data management and statistics including those relevant to clinical trials?
Career Development Plan/Career Goals and Objectives
- What is the likelihood that the plan will contribute substantially to the scientific development of the candidate and lead to scientific independence?
- Are the candidate's prior training and research experience appropriate for this award?
- Are the content, scope, phasing, and duration of the career development plan appropriate when considered in the context of prior training/research experience and the stated training and research objectives for achieving research independence?
- Are there adequate plans for monitoring and evaluating the candidate’s research and career development progress?
Research Plan
- Is the prior research that serves as the key support for the proposed project rigorous?
- Has the candidate included plans to address weaknesses in the rigor of prior research that serves as the key support of the proposed project?
- Has the candidate presented strategies to ensure a robust and unbiased approach, as appropriate for the work proposed?
- Has the candidate presented adequate plans to address relevant biological variables, such as sex, for studies in vertebrate animals or human subjects?
- Is the research plan relevant to the candidate’s research career objectives?
- Is the research plan appropriate to the candidate's stage of research development and as a vehicle for developing the research skills described in the career development plan?
- Will the proposed research lead to an independent line of research for the candidate? If the proposed research discipline requires team-based approaches, will the candidate develop skills to play a major leadership role in the chosen research field?
- Are the scientific rationale and need for a clinical trial, ancillary clinical trial, or feasibility or ancillary study well supported by preliminary data, clinical and/or preclinical studies, or information in the literature or knowledge of biological mechanisms?
- If proposing a small feasibility study, is the study warranted and will it contribute to planning and preliminary data needed for design of future larger scale clinical trials?
- Is the clinical trial or ancillary clinical trial necessary for testing the safety, efficacy or effectiveness of an intervention, or in the case of a feasibility study necessary to establish feasibility of future clinical trial?
- Is the study design justified and relevant to the clinical, biological, and statistical hypothesis(es) being tested?
- Are the plans to standardize, assure quality of, and monitor adherence to, the protocol and data collection or distribution guidelines appropriate?
- Are planned analyses and statistical approach appropriate for the proposed study design and methods used to assign participants and deliver interventions, if interventions are delivered?
- For trials focusing on mechanistic, behavioral, physiological, biochemical, or other biomedical endpoints, is this trial needed to advance scientific understanding?
Mentor(s), Co-Mentor(s), Consultant(s), Collaborator(s)
- Are the qualifications of the mentor(s) in the area of the proposed research appropriate?
- Does the mentor(s) adequately address the candidate’s potential and his/her strengths and areas needing improvement?
- Is there adequate description of the quality and extent of the mentor’s proposed role in providing guidance and advice to the candidate?
- Is the mentor’s description of the elements of the research career development activities, including formal course work adequate?
- Is there evidence of the mentor s, consultant s, and/or collaborator’s previous experience in fostering the development of independent investigators?
- Is there evidence of the mentor's current research productivity and peer-reviewed support?
- Is active/pending support for the proposed research project appropriate and adequate?
- Are there adequate plans for monitoring and evaluating the career development awardee’s progress toward independence?
- Does the mentor or mentoring team have the expertise, experience, and ability to guide the applicant in the organization, management and implementation of the proposed clinical trial, ancillary clinical trial, or feasibility study and help him/her to meet timelines?
Environment & Institutional Commitment to the Candidate
- Is there clear commitment of the sponsoring institution to ensure that a minimum of 9 person-months (75% of the candidate’s full-time professional effort) will be devoted directly to the research and career development activities described in the application, with the remaining percent effort being devoted to an appropriate balance of research, teaching, administrative, and clinical responsibilities?
- Is the institutional commitment to the career development of the candidate appropriately strong?
- Are the research facilities, resources and training opportunities, including faculty capable of productive collaboration with the candidate adequate and appropriate?
- Is the environment for the candidate’s scientific and professional development of high quality?
- Is there assurance that the institution intends the candidate to be an integral part of its research program as an independent investigator?
- Are the administrative, data coordinating, enrollment and laboratory/testing centers, appropriate for the trial proposed?
- Does the application adequately address the capability and ability to conduct the trial, ancillary clinical trial, or feasibility study at the proposed site(s) or centers? If applicable, are there plans to add or drop enrollment centers, as needed, appropriate?
- If international site(s) is/are proposed, does the application adequately address the complexity of executing the clinical trial?
Study Timeline for Clinical Trials
Is the study timeline described in detail, taking into account start-up activities, the anticipated rate of enrollment, and planned follow-up assessment? Is the projected timeline feasible and well justified? Does the project incorporate efficiencies and utilize existing resources (e.g., CTSAs, practice-based research networks, electronic medical records, administrative database, or patient registries) to increase the efficiency of participant enrollment and data collection, as appropriate?
Are potential challenges and corresponding solutions discussed (e.g., strategies that can be implemented in the event of enrollment shortfalls)?
Protections for Human Subjects
For research that involves human subjects but does not involve one of the categories of research that are exempt under 45 CFR Part 46, the committee will evaluate the justification for involvement of human subjects and the proposed protections from research risk relating to their participation according to the following five review criteria: 1) risk to subjects, 2) adequacy of protection against risks, 3) potential benefits to the subjects and others, 4) importance of the knowledge to be gained, and 5) data and safety monitoring for clinical trials.
For research that involves human subjects and meets the criteria for one or more of the categories of research that are exempt under 45 CFR Part 46, the committee will evaluate: 1) the justification for the exemption, 2) human subjects involvement and characteristics, and 3) sources of materials. For additional information on review of the Human Subjects section, please refer to the Guidelines for the Review of Human Subjects .
Inclusion of Women, Minorities, and Individuals Across the Lifespan
When the proposed project involves human subjects and/or NIH-defined clinical research, the committee will evaluate the proposed plans for the inclusion (or exclusion) of individuals on the basis of sex/gender, race, and ethnicity, as well as the inclusion (or exclusion) of individuals of all ages (including children and older adults) to determine if it is justified in terms of the scientific goals and research strategy proposed. For additional information on review of the Inclusion section, please refer to the Guidelines for the Review of Inclusion in Clinical Research .
Vertebrate Animals
The committee will evaluate the involvement of live vertebrate animals as part of the scientific assessment according to the following three points: (1) a complete description of all proposed procedures including the species, strains, ages, sex, and total numbers of animals to be used; (2) justifications that the species is appropriate for the proposed research and why the research goals cannot be accomplished using an alternative non-animal model; and (3) interventions including analgesia, anesthesia, sedation, palliative care, and humane endpoints that will be used to limit any unavoidable discomfort, distress, pain and injury in the conduct of scientifically valuable research. Methods of euthanasia and justification for selected methods, if NOT consistent with the AVMA Guidelines for the Euthanasia of Animals, is also required but is found in a separate section of the application. For additional information on review of the Vertebrate Animals Section, please refer to the Worksheet for Review of the Vertebrate Animals Section.
Reviewers will assess whether materials or procedures proposed are potentially hazardous to research personnel and/or the environment, and if needed, determine whether adequate protection is proposed.
Resubmissions
For Resubmissions, the committee will evaluate the application as now presented, taking into consideration the responses to comments from the previous scientific review group and changes made to the project.
For Revisions, the committee will consider the appropriateness of the proposed expansion of the scope of the project. If the Revision application relates to a specific line of investigation presented in the original application that was not recommended for approval by the committee, then the committee will consider whether the responses to comments from the previous scientific review group are adequate and whether substantial changes are clearly evident.
As applicable for the project proposed, reviewers will consider each of the following items, but will not give scores for these items, and should not consider them in providing an overall impact score.
Resource Sharing Plans
Reviewers will comment on whether the Resource Sharing Plan(s) (i.e., Sharing Model Organisms ) or the rationale for not sharing the resources, is reasonable.
All applications for support under this NOFO must include a plan to fulfill NIH requirements for instruction in the Responsible Conduct of Research (RCR). Taking into account the level of experience of the candidate, including any prior instruction or participation in RCR as appropriate for the candidate’s career stage, the reviewers will evaluate the adequacy of the proposed RCR training in relation to the following five required components: 1) Format - the required format of instruction, i.e., face-to-face lectures, coursework, and/or real-time discussion groups (a plan with only on-line instruction is not acceptable); 2) Subject Matter - the breadth of subject matter, e.g., conflict of interest, authorship, data management, human subjects and animal use, laboratory safety, research misconduct, research ethics; 3) Faculty Participation - the role of the mentor(s) and other faculty involvement in the fellow’s instruction; 4) Duration of Instruction - the number of contact hours of instruction (at least eight contact hours are required); and 5) Frequency of Instruction – instruction must occur during each career stage and at least once every four years. Plans and past record will be rated as ACCEPTABLE or UNACCEPTABLE , and the summary statement will provide the consensus of the review committee. See also: NOT-OD-10-019 .
Select Agent Research
Reviewers will assess the information provided in this section of the application, including 1) the Select Agent(s) to be used in the proposed research, 2) the registration status of all entities where Select Agent(s) will be used, 3) the procedures that will be used to monitor possession use and transfer of Select Agent(s), and 4) plans for appropriate biosafety, biocontainment, and security of the Select Agent(s).
Authentication of Key Biological and/or Chemical Resources
For projects involving key biological and/or chemical resources, reviewers will comment on the brief plans proposed for identifying and ensuring the validity of those resources.
Budget and Period of Support
Reviewers will consider whether the budget and the requested period of support are fully justified and reasonable in relation to the proposed research.
2. Review and Selection Process
Applications will be evaluated for scientific and technical merit by (an) appropriate Scientific Review Group(s), in accordance with NIH peer review policies and practices , using the stated review criteria. Assignment to a Scientific Review Group will be shown in the eRA Commons.
As part of the scientific peer review, all applications:
- May undergo a selection process in which only those applications deemed to have the highest scientific and technical merit (generally the top half of applications under review) will be discussed and assigned an overall impact score.
- Will receive a written critique.
Applications will be assigned on the basis of established PHS referral guidelines to the appropriate NIH Institute or Center. Applications will compete for available funds with all other recommended applications. Following initial peer review, recommended applications will receive a second level of review by the appropriate national Advisory Council or Board.
- Scientific and technical merit of the proposed project as determined by scientific peer review.
- Availability of funds.
- Relevance of the proposed project to program priorities
3. Anticipated Announcement and Award Dates
After the peer review of the application is completed, the PD/PI will be able to access his or her Summary Statement (written critique) via the eRA Commons . Refer to Part 1 for dates for peer review, advisory council review, and earliest start date.
Information regarding the disposition of applications is available in the NIH Grants Policy Statement Section 2.4.4 Disposition of Applications .
Section VI. Award Administration Information
1. Award Notices
If the application is under consideration for funding, NIH will request "just-in-time" information from the applicant as described in the NIH Grants Policy Statement . This request is not a Notice of Award nor should it be construed to be an indicator of possible funding.
A formal notification in the form of a Notice of Award (NoA) will be provided to the applicant organization for successful applications. The NoA signed by the grants management officer is the authorizing document and will be sent via email to the recipient’s business official.
Recipients must comply with any funding restrictions described in Section IV.6. Funding Restrictions . Selection of an application for award is not an authorization to begin performance. Any costs incurred before receipt of the NoA are at the recipient's risk. These costs may be reimbursed only to the extent considered allowable pre-award costs.
Any application awarded in response to this NOFO will be subject to terms and conditions found on the Award Conditions and Information for NIH Grants website. This includes any recent legislation and policy applicable to awards that is highlighted on this website.
Specific to applications proposing clinical trials, ancillary or feasibility studies
Additionally, ICs may specify any special reporting requirements for the proposed clinical trial to be included under IC-specific terms and conditions in the NoA.
For example: If the proposed clinical trial has elevated risks, ICs may require closer programmatic monitoring and it may be necessary to require the awardee to provide more frequent information and data as a term of the award (e.g., to clarify issues, address and evaluate concerns, provide documentation). All additional communications and information related to programmatic monitoring must be documented and incorporated into the official project file.
Individual awards are based on the application submitted to, and as approved by, the NIH and are subject to the IC-specific terms and conditions identified in the NoA.
ClinicalTrials.gov: If an award provides for one or more clinical trials. By law (Title VIII, Section 801 of Public Law 110-85), the "responsible party" must register and submit results information for certain “applicable clinical trials” on the ClinicalTrials.gov Protocol Registration and Results System Information Website ( https://register.clinicaltrials.gov ). NIH expects registration and results reporting of all trials whether required under the law or not. For more information, see https://grants.nih.gov/policy/clinical-trials/reporting/index.htm
Institutional Review Board or Independent Ethics Committee Approval: Recipient institutions must ensure that all protocols are reviewed by their IRB or IEC. To help ensure the safety of participants enrolled in NIH-funded studies, the recipient must provide NIH copies of documents related to all major changes in the status of ongoing protocols.
Data and Safety Monitoring Requirements: The NIH policy for data and safety monitoring requires oversight and monitoring of all NIH-conducted or -supported human biomedical and behavioral intervention studies (clinical trials) to ensure the safety of participants and the validity and integrity of the data. Further information concerning these requirements is found at http://grants.nih.gov/grants/policy/hs/data_safety.htm and in the application instructions (SF424 (R&R) and PHS 398).
Investigational New Drug or Investigational Device Exemption Requirements: Consistent with federal regulations, clinical research projects involving the use of investigational therapeutics, vaccines, or other medical interventions (including licensed products and devices for a purpose other than that for which they were licensed) in humans under a research protocol must be performed under a Food and Drug Administration (FDA) investigational new drug (IND) or investigational device exemption (IDE).
2. Administrative and National Policy Requirements
All NIH grant and cooperative agreement awards include the NIH Grants Policy Statement as part of the NoA. For these terms of award, see the NIH Grants Policy Statement Part II: Terms and Conditions of NIH Grant Awards, Subpart A: General and Part II: Terms and Conditions of NIH Grant Awards, Subpart B: Terms and Conditions for Specific Types of Grants, Recipients, and Activities , including of note, but not limited to:
- Federal-wide Standard Terms and Conditions for Research Grants
- Prohibition on Certain Telecommunications and Video Surveillance Services or Equipment
- Acknowledgment of Federal Funding
If a recipient is successful and receives a Notice of Award, in accepting the award, the recipient agrees that any activities under the award are subject to all provisions currently in effect or implemented during the period of the award, other Department regulations and policies in effect at the time of the award, and applicable statutory provisions.
If a recipient receives an award, the recipient must follow all applicable nondiscrimination laws. The recipient agrees to this when registering in SAM.gov. The recipient must also submit an Assurance of Compliance ( HHS-690 ). To learn more, see Laws and Regulations Enforced by the HHS Office for Civil Rights website .
HHS recognizes that NIH research projects are often limited in scope for many reasons that are nondiscriminatory, such as the principal investigator’s scientific interest, funding limitations, recruitment requirements, and other considerations. Thus, criteria in research protocols that target or exclude certain populations are warranted where nondiscriminatory justifications establish that such criteria are appropriate with respect to the health or safety of the subjects, the scientific study design, or the purpose of the research. For additional guidance regarding how the provisions apply to NIH grant programs, please contact the Scientific/Research Contact that is identified in Section VII under Agency Contacts of this NOFO.
In accordance with the statutory provisions contained in Section 872 of the Duncan Hunter National Defense Authorization Act of Fiscal Year 2009 (Public Law 110-417), NIH awards will be subject to System for Award Management (SAM.gov) requirements. SAM.gov requires Federal agencies to review and consider information about an applicant in the designated integrity and performance system (currently SAM.gov) prior to making an award. An applicant can review and comment on any information in the responsibility/qualification records available in SAM.gov. NIH will consider any comments by the applicant, in addition to the information available in the responsibility/qualification records in SAM.gov, in making a judgement about the applicant’s integrity, business ethics, and record of performance under Federal awards when completing the review of risk posed by applicants as described in 2 CFR Part 200.206 “Federal awarding agency review of risk posed by applicants.” This provision will apply to all NIH grants and cooperative agreements except fellowships.
3. Data Management and Sharing
Consistent with the 2023 NIH Policy for Data Management and Sharing, when data management and sharing is applicable to the award, recipients will be required to adhere to the Data Management and Sharing requirements as outlined in the NIH Grants Policy Statement . Upon the approval of a Data Management and Sharing Plan, it is required for recipients to implement the plan as described.
4. Reporting
When multiple years are involved, recipients will be required to submit the Research Performance Progress Report (RPPR) annually and financial statements as required in the NIH Grants Policy Statement . The Supplemental Instructions for Individual Career Development (K) RPPRs must be followed. For mentored awards, the Mentor’s Report must include an annual evaluation statement of the candidate’s progress.
A final RPPR, invention statement, and the expenditure data portion of the Federal Financial Report are required for closeout of an award, as described in the NIH Grants Policy Statement . NIH NOFOs outline intended research goals and objectives. Post award, NIH will review and measure performance based on the details and outcomes that are shared within the RPPR, as described at 2 CFR 200.301.
The Federal Funding Accountability and Transparency Act of 2006 as amended (FFATA), includes a requirement for recipients of Federal grants to report information about first-tier subawards and executive compensation under Federal assistance awards issued in FY2011 or later. All recipients of applicable NIH grants and cooperative agreements are required to report to the Federal Subaward Reporting System (FSRS) available at www.fsrs.gov on all subawards over the threshold. See the NIH Grants Policy Statement for additional information on this reporting requirement.
In accordance with the regulatory requirements provided at 2 CFR Part 200.113 and Appendix XII to 2 CFR Part 200, recipients that have currently active Federal grants, cooperative agreements, and procurement contracts from all Federal awarding agencies with a cumulative total value greater than $10,000,000 for any period of time during the period of performance of a Federal award, must report and maintain the currency of information reported in the System for Award Management (SAM) about civil, criminal, and administrative proceedings in connection with the award or performance of a Federal award that reached final disposition within the most recent five-year period. The recipient must also make semiannual disclosures regarding such proceedings. Proceedings information will be made publicly available in the designated integrity and performance system (Responsibility/Qualification in SAM.gov, formerly FAPIIS). This is a statutory requirement under section 872 of Public Law 110-417, as amended (41 U.S.C. 2313). As required by section 3010 of Public Law 111-212, all information posted in the designated integrity and performance system on or after April 15, 2011, except past performance reviews required for Federal procurement contracts, will be publicly available. Full reporting requirements and procedures are found in Appendix XII to 2 CFR Part 200 – Award Term and Condition for Recipient Integrity and Performance Matters.
5. Evaluation
In carrying out its stewardship of human resource-related programs, NIH may request information essential to an assessment of the effectiveness of this program from databases and from participants themselves. Participants may be contacted after the completion of this award for periodic updates on various aspects of their employment history, publications, support from research grants or contracts, honors and awards, professional activities, and other information helpful in evaluating the impact of the program.
Section VII. Agency Contacts
We encourage inquiries concerning this funding opportunity and welcome the opportunity to answer questions from potential applicants.
Because of the difference in individual Institute and Center (IC) program requirements for this NOFO, prospective applications MUST consult the Table of IC-Specific Information, Requirements, and Staff Contacts , to make sure that their application is responsive to the requirements of one of the participating NIH ICs. Prior consultation with NIH staff is strongly encouraged.
eRA Service Desk (Questions regarding ASSIST, eRA Commons, application errors and warnings, documenting system problems that threaten on-time submission, and post-submission issues)
Finding Help Online: https://www.era.nih.gov/need-help (preferred method of contact) Telephone: 301-402-7469 or 866-504-9552 (Toll Free)
General Grants Information (Questions regarding application processes and NIH grant resources) Email: [email protected] (preferred method of contact) Telephone: 301-637-3015
Grants.gov Customer Support (Questions regarding Grants.gov registration and Workspace) Contact Center Telephone: 800-518-4726 Email: [email protected]
See Table of IC-Specific Information, Requirements and Staff Contacts .
Examine your eRA Commons account for review assignment and contact information (information appears two weeks after the submission due date).
Section VIII. Other Information
Recently issued trans-NIH policy notices may affect your application submission. A full list of policy notices published by NIH is provided in the NIH Guide for Grants and Contracts . All awards are subject to the terms and conditions, cost principles, and other considerations described in the NIH Grants Policy Statement .
Please note that the NIH Loan Repayment Programs (LRPs) are a set of programs to attract and retain promising early-stage investigators in research careers by helping them to repay their student loans. Recipients of career development awards are encouraged to consider applying for an extramural LRP award.
Awards are made under the authorization of Sections 301 and 405 of the Public Health Service Act as amended (42 USC 241 and 284) and under Federal Regulations 42 CFR Part 52 and 2 CFR Part 75.

Note: For help accessing PDF, RTF, MS Word, Excel, PowerPoint, Audio or Video files, see Help Downloading Files .

IMAGES
VIDEO
COMMENTS
The Global Observatory on Health R&D is a comprehensive source of information and analyses on global health R&D for human diseases. It builds on existing data/reports from a range of sources, and gathers new information to help monitor health R&D and decision-making related to: health R&D gaps. priorities for new investments in health R&D.
Primary Health Care Research & Development is a fully open access journal aimed specifically at both researchers and practitioners in primary health care, bridging the gap between primary health care research policy and practice.It provides a forum for the publication of international, interdisciplinary research and development in primary health care.
Health research entails systematic collection or analysis of data with the intent to develop generalizable knowledge to understand health challenges and mount an improved response to them. The full spectrum of health research spans five generic areas of activity: measuring the health problem; understanding its cause(s); elaborating solutions; translating the solutions or evidence into policy ...
Innovations are the source of all human development and improvement of quality of life. At the same time, they challenge existing standards, solutions and societal patterns. ... Despite the abundance and increasing need for innovations in health care, theoretical scientific research in this area is still very limited . At the same time, the ...
Applied research and development involves seeking just enough information, through studies with just enough methodologic rigor, to improve practice within a short time. Such research can identify p...
Research for Health: our role in the global public health research community. WHO's technical units are just one part of a global web of research for health, encompassing academia, national and regional research bodies, product development partnerships and the private sector. WHO helps to provide global guidance for research priority setting.
USAID's Global Health Research and Development (R&D) Strategy (2023 - 2028) outlines the Agency's approach to ensuring research is translated into timely action to improve health, well-being, and resilience of people around the world. To achieve this vision, USAID focuses on: the development of new technologies, tools, and approaches; and implementation science, knowledge management, and ...
Find out about GHTC's membership options and the benefits of becoming a member. Learn More. Find out why research and development for new health technologies is an essential part of the solution to the world's greatest global health challenges and one of the biggest drivers of health improvements worldwide.
1. Introduction 'High quality healthcare' is one of the seven pillars forming the foundation of the Kuwait Vision 2035 Development Plan, which is the driving force behind the development efforts set forth by the Kuwait government in 2017 [].The state of Kuwait has experienced rapid and extensive economic, socio-demographic, and epidemiological transformations over the past five decades ...
The rapid growth of medical research and technology development has vastly improved the health of Americans. Nonetheless, a significant knowledge gap affects their care, and it continues to expand: the gap in knowledge about what approaches work best, under what circumstances, and for whom. The dynamic nature of product innovation and the increased emphasis on treatments tailored to the ...
Overall U.S. health spending (including spending on R&D) was estimated to be $4.1 trillionin 2020, a 25.0% increase since 2016. Medical and health R&D investment accounts for 5.9%of overall health spending in the U.S., or just under 6 cents of each health care dollar. Reaching $161.8 billion, industry accounts for nearly two- thirds (66%) of U ...
USAID is committed to addressing some of the world's most challenging health and development issues through research, introduction and scale-up of proven solutions. The Bureau for Global Health's investments in research and development have led to critical breakthroughs in prevention, diagnosis and treatment of deadly global diseases.
What research and development is. It is the way new medicines become available for use: Working with health care professionals is essential to studying how medicines work, including designing and learning more about medicines and treatment approaches through clinical trials both before and after a medicine is approved for use by patients. Research is an ongoing process: Our work isn't done ...
Ever since Merck was founded 350 years ago, we have gained unmatched experience in the discovery, development and manufacturing of medicines. On top of leveraging our experience and expertise, we also believe that a healthier future deserves a sizable investment. In 2018 our Healthcare business devoted roughly 20% of total sales to R&D ...
This special Research in Context feature explores the development of more effective ways to treat depression, including personalized treatment approaches and both old and new drugs. Choat / Adobe Stock. Everyone has a bad day sometimes. People experience various types of stress in the course of everyday life. These stressors can cause sadness ...
The World Health Organization's Global Observatory on Health Research and Development (R&D) has released its latest findings, revealing stark disparities in global health research funding, resource allocation, and capacity between high-income and low- and middle-income countries. The commentary "Tracking global resources and capacity for health research: time to reassess strategies and ...
R&E drives the discovery of new capabilities to improve medical care through management of the Defense Health Program (DHP) Research, Development, Test and Evaluation (RDT&E) appropriations that support the health and medical mission of the MHS. R&E shepherds innovative medical materiel products and clinical practice knowledge solutions from ...
Research Evaluation and Commercialization Hubs (REACH) support academic innovators to convert promising scientific discoveries into medical products while training a diverse biomedical workforce that is globally competitive in technology development and entrepreneurship. Early consideration of the healthcare impact and commercial viability of ...
Collectively, these forms of health research have led to significant discoveries, the development of new therapies, and a remarkable improvement in health care and public health. 4 Economists have found that medical research can have an enormous impact on human health and longevity, and that the resulting increased productivity of the ...
April 17, 2024. The following is attributed to Jeff Shuren, M.D., J.D., director of the FDA's Center for Devices and Radiological Health (CDRH) Today, CDRH is issuing two companion reports that ...
Reviewers should provide their assessment of the likelihood that the proposed career development and research plan will enhance the candidate's potential for a productive, independent scientific research career in a health-related field, taking into consideration the criteria below in determining the overall impact score.
An Open Comprative Study of the Prophylactic Efficacy and a Non-comparative Study of the Immunogenicity and Safety of the Inactivated Whole-virion Concentrated Purified Coronavirus Vaccine (CoviVac) Produced by FSBSI "Chumakov Federal Scientific Center for Research and Development of Immune-and-Biological Products" for Adults Aged 60 Years and ...
Recruitment of volunteers will be competitive. A maximum of 450 children aged 12 to 17 years inclusive will be screened in the study, of which it is planned to include and randomize 300 children who meet the criteria for inclusion in the study and do not have non-inclusion criteria, data on which will be used for subsequent safety and immunogenicity analysis.
Key Takeaways. Healthcare-related spaces performed well in the first quarter of 2024, however availability remains in short supply. Despite the delivery of 32,622 square feet, vacancy remained at 3.6% as 32,204 square feet were absorbed. Overall lease rates increased to $28.95 per square foot with 160,922 square feet under construction.
The research trajectory appeared to be unexplored in healthcare. Interestingly, a comprehensive framework involving micro-meso and macro perspective to evaluate change actions and the importance of change outcome was found to be emerging trends only in the general literature on organisational change.
Health research entails systematic collection or analysis of data with the intent to develop generalizable knowledge to understand health challenges and mount an improved response to them. The full spectrum of health research spans five generic areas of activity: measuring the health problem; understanding its cause(s); elaborating solutions; translating the solutions or evidence into policy ...
The life tests started after successful completion of hydraulic tests (hydraulic filling) of the mock-up with the aim to determine RK3+ hydraulic resistance. Life tests are carried out on a full-scale research hot run-in test bench V-440 and will last for full 1500 hours. The aim of tests is to study mechanical stability of RK3+ components ...
Elektrostal has a long history of industrial development, contributing to the growth and progress of the region. Read also: 49 Facts About Nellore . ... Elektrostal serves as an important hub for scientific research, particularly in the fields of metallurgy, materials science, and engineering. ... Provides a high standard of healthcare.
The Center for Public Health and Environmental Assessment (CPHEA) within U.S. EPA's Office of Research and Development is announcing a workshop entitled "Workshop to Discuss Policy-Relevant Science to Inform EPA's Integrated Plan for the Review of the Ozone National Ambient Air Quality Standards". This workshop is being organized by CPHEA ...
To have a robust clinical antifungal pipeline it's essential to invest and monitor its upstream development. In November 2022 WHO released the WHO fungal priority pathogens list (FPPL), a catalogue of the 19 fungi that represent the greatest threat to public health. The list is the first global effort to systematically prioritize fungal pathogens, considering the unmet research and development ...