Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 23 November 2017
- Chronic kidney disease
- Paola Romagnani 1 ,
- Giuseppe Remuzzi 2 , 3 , 4 ,
- Richard Glassock 5 ,
- Adeera Levin 6 ,
- Kitty J. Jager 7 ,
- Marcello Tonelli 8 ,
- Ziad Massy 9 , 10 ,
- Christoph Wanner 11 &
- Hans-Joachim Anders 12
Nature Reviews Disease Primers volume 3 , Article number: 17088 ( 2017 ) Cite this article
28k Accesses
511 Citations
49 Altmetric
Metrics details
- Diabetes complications
- End-stage renal disease
- Public health
- Renal replacement therapy
Chronic kidney disease (CKD) is defined by persistent urine abnormalities, structural abnormalities or impaired excretory renal function suggestive of a loss of functional nephrons. The majority of patients with CKD are at risk of accelerated cardiovascular disease and death. For those who progress to end-stage renal disease, the limited accessibility to renal replacement therapy is a problem in many parts of the world. Risk factors for the development and progression of CKD include low nephron number at birth, nephron loss due to increasing age and acute or chronic kidney injuries caused by toxic exposures or diseases (for example, obesity and type 2 diabetes mellitus). The management of patients with CKD is focused on early detection or prevention, treatment of the underlying cause (if possible) to curb progression and attention to secondary processes that contribute to ongoing nephron loss. Blood pressure control, inhibition of the renin–angiotensin system and disease-specific interventions are the cornerstones of therapy. CKD complications such as anaemia, metabolic acidosis and secondary hyperparathyroidism affect cardiovascular health and quality of life, and require diagnosis and treatment.
This is a preview of subscription content, access via your institution

Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
92,52 € per year
only 92,52 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others

Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease
Chronic kidney disease in propionic acidemia
Nephrons, podocytes and chronic kidney disease: Strategic antihypertensive therapy for renoprotection
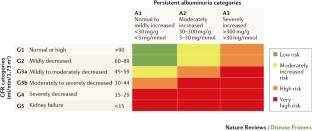
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 3 , 1–150 (2013). This study is the latest classification of CKD, now implementing albuminuria in a 2D matrix for stratification of the risk of CKD progression and complications.
Google Scholar
Zoccali, C. et al . The systemic nature of CKD. Nat. Rev. Nephrol. 13 , 344–358 (2017).
Hill, N. R. et al . Global prevalence of chronic kidney disease — a systematic review and meta-analysis. PLoS ONE 11 , e0158765 (2016).
Zhang, L. et al . Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 379 , 815–822 (2012).
Arora, P. et al . Prevalence estimates of chronic kidney disease in Canada: results of a nationally representative survey. CMAJ 185 , E417–E423 (2013).
White, S. L., Polkinghorne, K. R., Atkins, R. C. & Chadban, S. J. Comparison of the prevalence and mortality risk of CKD in Australia using the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) Study GFR estimating equations: the AusDiab (Australian Diabetes, Obesity and Lifestyle) Study. Am. J. Kidney Dis. 55 , 660–670 (2010).
Levey, A. S. & Coresh, J. Chronic kidney disease. Lancet 379 , 165–180 (2012).
Girndt, M., Trocchi, P., Scheidt-Nave, C., Markau, S. & Stang, A. The prevalence of renal failure. Results from the German Health Interview and Examination Survey for Adults, 2008–2011 (DEGS1). Dtsch. Arztebl. Int. 113 , 85–91 (2016).
Bruck, K. et al . CKD prevalence varies across the European general population. J. Am. Soc. Nephrol. 27 , 2135–2147 (2016).
Fraser, S. D. et al . Exploration of chronic kidney disease prevalence estimates using new measures of kidney function in the health survey for England. PLoS ONE 10 , e0118676 (2015).
Glassock, R. J., Warnock, D. G. & Delanaye, P. The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat. Rev. Nephrol. 13 , 104–114 (2017).
Stanifer, J. W., Muiru, A., Jafar, T. H. & Patel, U. D. Chronic kidney disease in low- and middle-income countries. Nephrol. Dial. Transplant. 31 , 868–874 (2016).
Stanifer, J. W. et al . The epidemiology of chronic kidney disease in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob. Health 2 , e174–e181 (2014).
Ene-Iordache, B. et al . Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study. Lancet Glob. Health 4 , e307–e319 (2016).
ESPN/ERA-EDTA Registry. Annual Report. ESPN/ERA-EDTA Registry www.espn-reg.org/index.jsp (2014).
Chesnaye, N. et al . Demographics of paediatric renal replacement therapy in Europe: a report of the ESPN/ERA-EDTA registry. Pediatr. Nephrol. 29 , 2403–2410 (2014).
Saran, R. et al . US Renal Data System 2016 Annual Data Report: epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 69 (Suppl. 1), A7–A8 (2017).
Harambat, J., van Stralen, K. J., Kim, J. J. & Tizard, E. J. Epidemiology of chronic kidney disease in children. Pediatr. Nephrol. 27 , 363–373 (2012).
Jha, V. et al . Chronic kidney disease: global dimension and perspectives. Lancet 382 , 260–272 (2013).
Stanifer, J. W. et al . Traditional medicines and kidney disease in low- and middle-income countries: opportunities and challenges. Semin. Nephrol. 37 , 245–259 (2017).
Charlton, J. R., Springsteen, C. H. & Carmody, J. B. Nephron number and its determinants in early life: a primer. Pediatr. Nephrol. 29 , 2299–2308 (2014).
Khalsa, D. D., Beydoun, H. A. & Carmody, J. B. Prevalence of chronic kidney disease risk factors among low birth weight adolescents. Pediatr. Nephrol. 31 , 1509–1516 (2016).
Komenda, P. et al . The prevalence of CKD in rural Canadian indigenous peoples: results from the First Nations Community Based Screening to Improve Kidney Health and Prevent Dialysis (FINISHED) screen, triage, and treat program. Am. J. Kidney Dis. 68 , 582–590 (2016).
Gifford, F. J., Gifford, R. M., Eddleston, M. & Dhaun, N. Endemic nephropathy around the world. Kidney Int. Rep. 2 , 282–292 (2017).
Glaser, J. et al . Climate change and the emergent epidemic of CKD from heat stress in rural communities: the case for heat stress nephropathy. Clin. J. Am. Soc. Nephrol. 11 , 1472–1483 (2016).
Jayasumana, C. et al . Chronic interstitial nephritis in agricultural communities: a worldwide epidemic with social, occupational and environmental determinants. Nephrol. Dial. Transplant. 32 , 234–241 (2017).
Levin, A. et al . Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet 390 , 1888–1917 (2017). This study presents a roadmap on how to close gaps in global kidney health.
ERA-EDTA Registry. ERA-EDTA Registry Annual Report 2014 (Department of Medical Informatics, Amsterdam, The Netherlands, 2016).
Li, P. K. et al . Changes in the worldwide epidemiology of peritoneal dialysis. Nat. Rev. Nephrol. 13 , 90–103 (2017).
Bello, A. K. et al . Assessment of global kidney health care status. JAMA 317 , 1864–1881 (2017). This study provides the latest overview about kidney health care in all regions of the world, revealing wide variation of access to nephrology specialists, quality of diagnostic workup and preferences for kidney replacement therapy.
Liyanage, T. et al . Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 385 , 1975–1982 (2015).
D. A. L. Ys, G. B. D. & Collaborators, H. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388 , 1603–1658 (2016).
Jager, K. J. & Fraser, S. D. S. The ascending rank of chronic kidney disease in the global burden of disease study. Nephrol. Dial. Transplant. 32 (Suppl. 2), ii121–ii128 (2017).
Thomas, B. et al . Global cardiovascular and renal outcomes of reduced GFR. J. Am. Soc. Nephrol. 28 , 2167–2179 (2017).
GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388 , 1459–1544 (2016).
Nitsch, D. et al . Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta-analysis. BMJ 346 , f324 (2013). This is a meta-analysis that provides the rationale for all-cause mortality risk prediction using eGFR and albuminuria levels as implemented in the KDIGO CKD stages.
van de Luijtgaarden, M. W. et al . Trends in dialysis modality choice and related patient survival in the ERA-EDTA Registry over a 20-year period. Nephrol. Dial. Transplant. 31 , 120–128 (2016).
Bertram, J. F., Douglas-Denton, R. N., Diouf, B., Hughson, M. D. & Hoy, W. E. Human nephron number: implications for health and disease. Pediatr. Nephrol. 26 , 1529–1533 (2011).
Brenner, B. M., Meyer, T. W. & Hostetter, T. H. Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N. Engl. J. Med. 307 , 652–659 (1982).
Hostetter, T. H., Olson, J. L., Rennke, H. G., Venkatachalam, M. A. & Brenner, B. M. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am. J. Physiol. 241 , F85–F93 (1981).
Benghanem Gharbi, M. et al . Chronic kidney disease, hypertension, diabetes, and obesity in the adult population of Morocco: how to avoid “over”- and “under”-diagnosis of CKD. Kidney Int. 89 , 1363–1371 (2016). This is a CKD population study performed in Morocco presenting percentiles for repeated estimates of GFR, which are extremely useful for patient care.
Ruggenenti, P., Cravedi, P. & Remuzzi, G. Mechanisms and treatment of CKD. J. Am. Soc. Nephrol. 23 , 1917–1928 (2012).
Laouari, D. et al . TGF-α mediates genetic susceptibility to chronic kidney disease. J. Am. Soc. Nephrol. 22 , 327–335 (2011). This is the first description of the transforming growth factor-α–epithelial growth factor receptor axis as a driver of compensatory growth of remnant nephrons. Targeting this pathway can limit the adaptive response from turning into a maladaptive mechanism of CKD progression.
Helal, I., Fick-Brosnahan, G. M., Reed-Gitomer, B. & Schrier, R. W. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat. Rev. Nephrol. 8 , 293–300 (2012).
Grams, M. E. et al . Kidney-failure risk projection for the living kidney-donor candidate. N. Engl. J. Med. 374 , 411–421 (2016).
Mueller, T. F. & Luyckx, V. A. The natural history of residual renal function in transplant donors. J. Am. Soc. Nephrol. 23 , 1462–1466 (2012).
D'Agati, V. D. et al . Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat. Rev. Nephrol. 12 , 453–471 (2016).
Tonneijck, L. et al . Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J. Am. Soc. Nephrol. 28 , 1023–1039 (2017).
Denic, A. et al . The substantial loss of nephrons in healthy human kidneys with aging. J. Am. Soc. Nephrol. 28 , 313–320 (2017).
Hodgin, J. B. et al . Glomerular aging and focal global glomerulosclerosis: a podometric perspective. J. Am. Soc. Nephrol. 26 , 3162–3178 (2015).
Kriz, W. & Lemley, K. V. The role of the podocyte in glomerulosclerosis. Curr. Opin. Nephrol. Hypertens. 8 , 489–497 (1999).
Kriz, W. & Lemley, K. V. A potential role for mechanical forces in the detachment of podocytes and the progression of CKD. J. Am. Soc. Nephrol. 26 , 258–269 (2015).
Benigni, A., Gagliardini, E. & Remuzzi, G. Changes in glomerular perm-selectivity induced by angiotensin II imply podocyte dysfunction and slit diaphragm protein rearrangement. Semin. Nephrol. 24 , 131–140 (2004).
Rizzo, P. et al . Nature and mediators of parietal epithelial cell activation in glomerulonephritides of human and rat. Am. J. Pathol. 183 , 1769–1778 (2013).
Clark, W. F. et al . Dipstick proteinuria as a screening strategy to identify rapid renal decline. J. Am. Soc. Nephrol. 22 , 1729–1736 (2011).
Abbate, M., Zoja, C. & Remuzzi, G. How does proteinuria cause progressive renal damage? J. Am. Soc. Nephrol. 17 , 2974–2984 (2006).
Schnaper, H. W. The tubulointerstitial pathophysiology of progressive kidney disease. Adv. Chron. Kidney Dis. 24 , 107–116 (2017).
Kaissling, B., Lehir, M. & Kriz, W. Renal epithelial injury and fibrosis. Biochim. Biophys. Acta 1832 , 931–939 (2013).
Peired, A. et al . Proteinuria impairs podocyte regeneration by sequestering retinoic acid. J. Am. Soc. Nephrol. 24 , 1756–1768 (2013). This study provides a description of how proteinuria suppresses podocyte regeneration from local podocyte precursors inside the glomerulus.
Brenner, B. M., Garcia, D. L. & Anderson, S. Glomeruli and blood pressure. Less of one, more the other? Am. J. Hypertens. 1 , 335–347 (1988).
Low Birth, W. & Nephron Number Working, G. The impact of kidney development on the life course: a consensus document for action. Nephron 136 , 3–49 (2017).
Hirano, D. et al . Association between low birth weight and childhood-onset chronic kidney disease in Japan: a combined analysis of a nationwide survey for paediatric chronic kidney disease and the National Vital Statistics Report. Nephrol. Dial. Transplant. 31 , 1895–1900 (2016).
Ruggajo, P. et al . Low birth weight and risk of progression to end stage renal disease in IgA nephropathy — a retrospective registry-based cohort study. PLoS ONE 11 , e0153819 (2016).
Becherucci, F., Roperto, R. M., Materassi, M. & Romagnani, P. Chronic kidney disease in children. Clin. Kidney J. 9 , 583–591 (2016).
Luyckx, V. A. et al . A developmental approach to the prevention of hypertension and kidney disease: a report from the Low Birth Weight and Nephron Number Working Group. Lancet 390 , 424–428 (2017).
Oliveira, B., Kleta, R., Bockenhauer, D. & Walsh, S. B. Genetic, pathophysiological, and clinical aspects of nephrocalcinosis. Am. J. Physiol. Renal Physiol. 311 , F1243–F1252 (2016).
Trautmann, A. et al . Spectrum of steroid-resistant and congenital nephrotic syndrome in children: the PodoNet registry cohort. Clin. J. Am. Soc. Nephrol. 10 , 592–600 (2015).
Eckardt, K. U. et al . Autosomal dominant tubulointerstitial kidney disease: diagnosis, classification, and management — A KDIGO consensus report. Kidney Int. 88 , 676–683 (2015).
Nicolaou, N., Renkema, K. Y., Bongers, E. M., Giles, R. H. & Knoers, N. V. Genetic, environmental, and epigenetic factors involved in CAKUT. Nat. Rev. Nephrol. 11 , 720–731 (2015).
Cain, J. E., Di Giovanni, V., Smeeton, J. & Rosenblum, N. D. Genetics of renal hypoplasia: insights into the mechanisms controlling nephron endowment. Pediatr. Res. 68 , 91–98 (2010).
Uy, N. & Reidy, K. Developmental genetics and congenital anomalies of the kidney and urinary tract. J. Pediatr. Genet. 5 , 51–60 (2016).
Vivante, A. & Hildebrandt, F. Exploring the genetic basis of early-onset chronic kidney disease. Nat. Rev. Nephrol. 12 , 133–146 (2016).
Kottgen, A. et al . Multiple loci associated with indices of renal function and chronic kidney disease. Nat. Genet. 41 , 712–717 (2009).
Dummer, P. D. et al . APOL1 kidney disease risk variants: an evolving landscape. Semin. Nephrol. 35 , 222–236 (2015).
Kruzel-Davila, E. et al . APOL1-mediated cell injury involves disruption of conserved trafficking processes. J. Am. Soc. Nephrol. 28 , 1117–1130 (2017).
Denic, A. et al . Single-nephron glomerular filtration rate in healthy adults. N. Engl. J. Med. 376 , 2349–2357 (2017).
Lu, J. L. et al . Association of age and BMI with kidney function and mortality: a cohort study. Lancet Diabetes Endocrinol. 3 , 704–714 (2015).
Kramer, H. et al . Waist circumference, body mass index, and ESRD in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study. Am. J. Kidney Dis. 67 , 62–69 (2016).
Chang, A. et al . Lifestyle-related factors, obesity, and incident microalbuminuria: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am. J. Kidney Dis. 62 , 267–275 (2013).
Foster, M. C. et al . Overweight, obesity, and the development of stage 3 CKD: the Framingham Heart Study. Am. J. Kidney Dis. 52 , 39–48 (2008).
Vivante, A. et al . Body mass index in 1.2 million adolescents and risk for end-stage renal disease. Arch. Intern. Med. 172 , 1644–1650 (2012).
Dunlop, W. Serial changes in renal haemodynamics during normal human pregnancy. Br. J. Obstet. Gynaecol. 88 , 1–9 (1981).
Nevis, I. F. et al . Pregnancy outcomes in women with chronic kidney disease: a systematic review. Clin. J. Am. Soc. Nephrol. 6 , 2587–2598 (2011).
Anders, H. J., Davis, J. M. & Thurau, K. Nephron protection in diabetic kidney disease. N. Engl. J. Med. 375 , 2096–2098 (2016).
Vallon, V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu. Rev. Med. 66 , 255–270 (2015). A comprehensive overview on the mechanism of action of SGLT2 inhibitors in diabetic kidney disease.
van Bommel, E. J. et al . SGLT2 inhibition in the diabetic kidney-from mechanisms to clinical outcome. Clin. J. Am. Soc. Nephrol. 12 , 700–710 (2017).
Anguiano Gomez, L., Lei, Y., Devarapu, S. K. & Anders, H. J. The diabetes pandemic suggests unmet needs for ‘CKD with diabetes’ in addition to ‘diabetic nephropathy’. Implications for pre-clinical research and drug testing. Nephrol. Dial. Transplant. http://dx.doi.org/10.1093/ndt/gfx219 (2017).
Wanner, C. et al . Empagliflozin and progression of kidney disease in type 2 diabetes. N. Engl. J. Med. 375 , 323–334 (2016). This is the first study to show profound nephroprotective effects of an SGLT2 inhibitor in patients with CKD and diabetes.
Bellomo, R., Kellum, J. A. & Ronco, C. Acute kidney injury. Lancet 380 , 756–766 (2012).
Venkatachalam, M. A., Weinberg, J. M., Kriz, W. & Bidani, A. K. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J. Am. Soc. Nephrol. 26 , 1765–1776 (2015).
Barton, A. L., Mallard, A. S. & Parry, R. G. One year's observational study of acute kidney injury incidence in primary care; frequency of follow-up serum creatinine and mortality risk. Nephron 130 , 175–181 (2015).
Eckardt, K. U. et al . Evolving importance of kidney disease: from subspecialty to global health burden. Lancet 382 , 158–169 (2013).
Portale, A. A. et al . Disordered FGF23 and mineral metabolism in children with CKD. Clin. J. Am. Soc. Nephrol. 9 , 344–353 (2014).
Denburg, M. R. et al . Fracture burden and risk factors in childhood CKD: results from the CKiD cohort study. J. Am. Soc. Nephrol. 27 , 543–550 (2016).
Eriksen, B. O. et al . Elevated blood pressure is not associated with accelerated glomerular filtration rate decline in the general non-diabetic middle-aged population. Kidney Int. 90 , 404–410 (2016).
Freedman, B. I. & Cohen, A. H. Hypertension-attributed nephropathy: what's in a name? Nat. Rev. Nephrol. 12 , 27–36 (2016).
Flynn, J. T. et al . Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension 52 , 631–637 (2008).
Vaziri, N. D. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am. J. Physiol. Renal Physiol. 290 , F262–F272 (2006).
Speer, T. et al . Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity 38 , 754–768 (2013).
Foley, R. N., Parfrey, P. S. & Sarnak, M. J. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am. J. Kidney Dis. 32 , S112–119 (1998).
Raschenberger, J. et al . Association of relative telomere length with cardiovascular disease in a large chronic kidney disease cohort: the GCKD study. Atherosclerosis 242 , 529–534 (2015).
Grabner, A. et al . Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab. 22 , 1020–1032 (2015).
de Jager, D. J. et al . Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 302 , 1782–1789 (2009).
De Cosmo, S., Menzaghi, C., Prudente, S. & Trischitta, V. Role of insulin resistance in kidney dysfunction: insights into the mechanism and epidemiological evidence. Nephrol. Dial. Transplant. 28 , 29–36 (2013).
Cozzolino, M., Ketteler, M. & Zehnder, D. The vitamin D system: a crosstalk between the heart and kidney. Eur. J. Heart Fail. 12 , 1031–1041 (2010).
Vervloet, M. & Cozzolino, M. Vascular calcification in chronic kidney disease: different bricks in the wall? Kidney Int. 91 , 808–817 (2017).
Carrero, J. J. et al . Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J. Ren. Nutr. 23 , 77–90 (2013).
Buchanan, C. et al . Intradialytic cardiac magnetic resonance imaging to assess cardiovascular responses in a short-term trial of hemodiafiltration and hemodialysis. J. Am. Soc. Nephrol. 28 , 1269–1277 (2017).
van der Heijden, B. J., van Dijk, P. C., Verrier-Jones, K., Jager, K. J. & Briggs, J. D. Renal replacement therapy in children: data from 12 registries in Europe. Pediatr. Nephrol. 19 , 213–221 (2004).
Tonshoff, B., Kiepe, D. & Ciarmatori, S. Growth hormone/insulin-like growth factor system in children with chronic renal failure. Pediatr. Nephrol. 20 , 279–289 (2005).
Rhee, C. M. et al . Thyroid functional disease: an under-recognized cardiovascular risk factor in kidney disease patients. Nephrol. Dial. Transplant. 30 , 724–737 (2015).
Andersen, K. et al . Intestinal dysbiosis, barrier dysfunction, and bacterial translocation account for CKD-related systemic inflammation. J. Am. Soc. Nephrol. 28 , 76–83 (2017).
Lau, W. L., Kalantar-Zadeh, K. & Vaziri, N. D. The gut as a source of inflammation in chronic kidney disease. Nephron 130 , 92–98 (2015).
Lu, R., Kiernan, M. C., Murray, A., Rosner, M. H. & Ronco, C. Kidney-brain crosstalk in the acute and chronic setting. Nat. Rev. Nephrol. 11 , 707–719 (2015).
Roumelioti, M. E. et al . Sleep and fatigue symptoms in children and adolescents with CKD: a cross-sectional analysis from the chronic kidney disease in children (CKiD) study. Am. J. Kidney Dis. 55 , 269–280 (2010).
De Broe, M. E., Gharbi, M. B., Zamd, M. & Elseviers, M. Why overestimate or underestimate chronic kidney disease when correct estimation is possible? Nephrol. Dial. Transplant. 32 (Suppl. 2), ii136–ii141 (2017).
Rule, A. D. et al . For estimating creatinine clearance measuring muscle mass gives better results than those based on demographics. Kidney Int. 75 , 1071–1078 (2009).
Rule, A. D. & Glassock, R. J. GFR estimating equations: getting closer to the truth? Clin. J. Am. Soc. Nephrol. 8 , 1414–1420 (2013).
Stevens, L. A. et al . Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 75 , 652–660 (2009).
Inker, L. A. et al . Performance of glomerular filtration rate estimating equations in a community-based sample of Blacks and Whites: the multiethnic study of atherosclerosis. Nephrol. Dial. Transplant. gfx042 (2017).
Praditpornsilpa, K. et al . The need for robust validation for MDRD-based glomerular filtration rate estimation in various CKD populations. Nephrol. Dial. Transplant. 26 , 2780–2785 (2011).
Inker, L. A. et al . Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 367 , 20–29 (2012).
Pottel, H. et al . An estimated glomerular filtration rate equation for the full age spectrum. Nephrol. Dial. Transplant. 31 , 798–806 (2016).
Delanaye, P. et al . Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: a review. Part 1: How to measure glomerular filtration rate with iohexol? Clin. Kidney J. 9 , 682–699 (2016).
Delanaye, P. et al . Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: a review. Part 2: Why to measure glomerular filtration rate with iohexol? Clin. Kidney J. 9 , 700–704 (2016).
Matsushita, K. et al . Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 3 , 514–525 (2015).
Fotheringham, J., Campbell, M. J., Fogarty, D. G., El Nahas, M. & Ellam, T. Estimated albumin excretion rate versus urine albumin-creatinine ratio for the estimation of measured albumin excretion rate: derivation and validation of an estimated albumin excretion rate equation. Am. J. Kidney Dis. 63 , 405–414 (2014).
Glassock, R. J. Evaluation of proteinuria redux. Kidney Int. 90 , 938–940 (2016).
Azurmendi, P. J. et al . Early renal and vascular changes in ADPKD patients with low-grade albumin excretion and normal renal function. Nephrol. Dial. Transplant. 24 , 2458–2463 (2009).
Glassock, R. J., Fervenza, F. C., Hebert, L. & Cameron, J. S. Nephrotic syndrome redux. Nephrol. Dial. Transplant. 30 , 12–17 (2015).
Atwell, T. D. et al . Incidence of bleeding after 15,181 percutaneous biopsies and the role of aspirin. AJR Am. J. Roentgenol. 194 , 784–789 (2010).
Lees, J. S. et al . Risk factors for bleeding complications after nephrologist-performed native renal biopsy. Clin. Kidney J. 10 , 573–577 (2017).
Xu, D. M., Chen, M., Zhou, F. D. & Zhao, M. H. Risk factors for severe bleeding complications in percutaneous renal biopsy. Am. J. Med. Sci. 353 , 230–235 (2017).
Glassock, R. J. Con: kidney biopsy: an irreplaceable tool for patient management in nephrology. Nephrol. Dial. Transplant. 30 , 528–531 (2015).
Li, J., An, C., Kang, L., Mitch, W. E. & Wang, Y. Recent advances in magnetic resonance imaging assessment of renal fibrosis. Adv. Chron. Kidney Dis. 24 , 150–153 (2017).
Becherucci, F. et al . Lessons from genetics: is it time to revise the therapeutic approach to children with steroid-resistant nephrotic syndrome? J. Nephrol. 29 , 543–550 (2016).
Siwy, J. et al . Noninvasive diagnosis of chronic kidney diseases using urinary proteome analysis. Nephrol. Dial. Transplant. gfw337 (2016).
Peralta, C. A. & Estrella, M. M. Preventive nephrology in the era of “I” evidence: should we screen for chronic kidney disease? Kidney Int. 92 , 19–21 (2017).
Taal, M. W. Screening for chronic kidney disease: preventing harm or harming the healthy? PLoS Med. 9 , e1001345 (2012).
Moyer, V. A. & Force, U. S. P. S. T. Screening for chronic kidney disease: U. S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 157 , 567–570 (2012).
Shardlow, A., McIntyre, N. J., Fluck, R. J., McIntyre, C. W. & Taal, M. W. Chronic kidney disease in primary care: outcomes after five years in a prospective cohort study. PLoS Med. 13 , e1002128 (2016).
Perkovic, V. et al . Management of patients with diabetes and CKD: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 90 , 1175–1183 (2016).
Hoerger, T. J. et al . A health policy model of CKD: 2. The cost-effectiveness of microalbuminuria screening. Am. J. Kidney Dis. 55 , 463–473 (2010).
Ozyilmaz, A. et al . Screening for albuminuria with subsequent screening for hypertension and hypercholesterolaemia identifies subjects in whom treatment is warranted to prevent cardiovascular events. Nephrol. Dial. Transplant. 28 , 2805–2815 (2013).
Tanaka, F. et al . Low-grade albuminuria and incidence of cardiovascular disease and all-cause mortality in nondiabetic and normotensive individuals. J. Hypertens. 34 , 506–512 (2016).
Grams, M. E. et al . Race, APOL1 risk, and eGFR decline in the general population. J. Am. Soc. Nephrol. 27 , 2842–2850 (2016).
Fink, H. A. et al . Screening for, monitoring, and treatment of chronic kidney disease stages 1 to 3: a systematic review for the U. S. Preventive Services Task Force American College of Physicians Clinical Practice Guideline. Ann. Intern. Med. 156 , 570–581 (2012). This study presents a critical discussion of the benefits and risks of CKD screening.
Imai, E. et al . Kidney disease screening program in Japan: history, outcome, and perspectives. Clin. J. Am. Soc. Nephrol. 2 , 1360–1366 (2007).
Caley, M., Chohan, P., Hooper, J. & Wright, N. The impact of NHS Health Checks on the prevalence of disease in general practices: a controlled study. Br. J. Gen. Pract. 64 , e516–e521 (2014).
Khwaja, A. & Throssell, D. A critique of the UK NICE guidance for the detection and management of individuals with chronic kidney disease. Nephron Clin. Pract. 113 , c207–c213 (2009).
Smith, J. M., Mott, S. A., Hoy, W. E. & International Federation of Kidney Foundations. Status of chronic kidney disease prevention programs: International Federation of Kidney Foundation Members 2005/2007. Kidney Int. 74 , 1516–1525 (2008).
Tonelli, M. et al . How to advocate for the inclusion of chronic kidney disease in a national noncommunicable chronic disease program. Kidney Int. 85 , 1269–1274 (2014).
Boulware, L. E., Jaar, B. G., Tarver-Carr, M. E., Brancati, F. L. & Powe, N. R. Screening for proteinuria in US adults: a cost-effectiveness analysis. JAMA 290 , 3101–3114 (2003).
Komenda, P. et al . Cost-effectiveness of primary screening for CKD: a systematic review. Am. J. Kidney Dis. 63 , 789–797 (2014).
Kovesdy, C. P., Furth, S. L., Zoccali, C. & World Kidney Day Steering Committee. Obesity and kidney disease: hidden consequences of the epidemic. Can. J. Kidney Health Dis. 4 , 2054358117698669 (2017).
Oellgaard, J. et al . Intensified multifactorial intervention in type 2 diabetics with microalbuminuria leads to long-term renal benefits. Kidney Int. 91 , 982–988 (2017).
Rebholz, C. M. et al . Dietary acid load and incident chronic kidney disease: results from the ARIC study. Am. J. Nephrol. 42 , 427–435 (2015).
Asghari, G. et al . Adherence to the Mediterranean diet is associated with reduced risk of incident chronic kidney diseases among Tehranian adults. Hypertens. Res. 40 , 96–102 (2017).
Dunkler, D. et al . Dietary risk factors for incidence or progression of chronic kidney disease in individuals with type 2 diabetes in the European Union. Nephrol. Dial. Transplant. 30 (Suppl. 4), iv76–iv85 (2015).
Liu, Y. et al . Dietary habits and risk of kidney function decline in an urban population. J. Ren. Nutr. 27 , 16–25 (2017).
Snelson, M., Clarke, R. E. & Coughlan, M. T. Stirring the pot: can dietary modification alleviate the burden of CKD? Nutrients 9 , 265 (2017).
Smyth, A. et al . Diet and major renal outcomes: a prospective cohort study. The NIH-AARP Diet and Health Study. J. Ren. Nutr. 26 , 288–298 (2016).
Rebholz, C. M. et al . DASH (Dietary Approaches to Stop Hypertension) diet and risk of subsequent kidney disease. Am. J. Kidney Dis. 68 , 853–861 (2016).
Dobre, M., Rahman, M. & Hostetter, T. H. Current status of bicarbonate in CKD. J. Am. Soc. Nephrol. 26 , 515–523 (2015).
Banerjee, T. et al . High dietary acid load predicts ESRD among adults with CKD. J. Am. Soc. Nephrol. 26 , 1693–1700 (2015).
Scialla, J. J. et al . Higher net acid excretion is associated with a lower risk of kidney disease progression in patients with diabetes. Kidney Int. 91 , 204–215 (2017).
Gross, O. et al . Early angiotensin-converting enzyme inhibition in Alport syndrome delays renal failure and improves life expectancy. Kidney Int. 81 , 494–501 (2012).
Schievink, B. et al . Early renin-angiotensin system intervention is more beneficial than late intervention in delaying end-stage renal disease in patients with type 2 diabetes. Diabetes Obes. Metab. 18 , 64–71 (2016).
Noris, M. & Remuzzi, G. Glomerular diseases dependent on complement activation, including atypical hemolytic uremic syndrome, membranoproliferative glomerulonephritis, and C3 glomerulopathy: core curriculum 2015. Am. J. Kidney Dis. 66 , 359–375 (2015).
Hildebrand, A. M., Huang, S. H. & Clark, W. F. Plasma exchange for kidney disease: what is the best evidence? Adv. Chron. Kidney Dis. 21 , 217–227 (2014).
Rauen, T. et al . Intensive supportive care plus immunosuppression in IgA nephropathy. N. Engl. J. Med. 373 , 2225–2236 (2015). This study shows that if conservative treatment is done well, it can be very potent in preventing CKD progression in IgA nephropathy.
Staplin, N. et al . Smoking and adverse outcomes in patients with CKD: the Study of Heart and Renal Protection (SHARP). Am. J. Kidney Dis. 68 , 371–380 (2016).
Cravedi, P., Ruggenenti, P. & Remuzzi, G. Intensified inhibition of renin-angiotensin system: a way to improve renal protection? Curr. Hypertens. Rep. 9 , 430–436 (2007).
Schrier, R. W. et al . Blood pressure in early autosomal dominant polycystic kidney disease. N. Engl. J. Med. 371 , 2255–2266 (2014).
Weir, M. R. et al . Effectiveness of patiromer in the treatment of hyperkalemia in chronic kidney disease patients with hypertension on diuretics. J. Hypertens. 35 (Suppl. 1), S57–S63 (2017).
Holtkamp, F. A. et al . An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 80 , 282–287 (2011).
Ruggenenti, P. et al . Role of remission clinics in the longitudinal treatment of CKD. J. Am. Soc. Nephrol. 19 , 1213–1224 (2008). This study shows that if conservative treatment is done well, it can be very potent in preventing CKD progression in many forms of kidney disease.
Daina, E. et al . A multidrug, antiproteinuric approach to alport syndrome: a ten-year cohort study. Nephron 130 , 13–20 (2015).
Cheung, A. K. et al . Effects of intensive BP control in CKD. J. Am. Soc. Nephrol. 28 , 2812–2823 (2017).
Li, K. et al . Effects of bariatric surgery on renal function in obese patients: a systematic review and meta analysis. PLoS ONE 11 , e0163907 (2016).
Guideline development group. Clinical Practice Guideline on management of patients with diabetes and chronic kidney disease stage 3b or higher (eGFR <45 mL/min). Nephrol. Dial. Transplant. 30 (Suppl. 2), ii1–ii142 (2015).
Neal, B. et al . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 377 , 644–657 (2017).
Zinman, B. et al . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 373 , 2117–2128 (2015).
Wanner, C., Amann, K. & Shoji, T. The heart and vascular system in dialysis. Lancet 388 , 276–284 (2016).
Rossignol, P. et al . Cardiovascular outcome trials in patients with chronic kidney disease: challenges associated with selection of patients and endpoints. Eur. Heart J. ehx209 (2017).
Xu, X. et al . Efficacy of folic acid therapy on the progression of chronic kidney disease: the renal substudy of the China stroke primary prevention trial. JAMA Intern. Med. 176 , 1443–1450 (2016).
Baigent, C. et al . The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 377 , 2181–2192 (2011).
Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. Suppl. 2 , 279–335 (2012).
Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. Suppl. 2 , 337–414 (2012).
Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int. Suppl. 7 , 1–59 (2017).
Sumida, K. & Kovesdy, C. P. Disease trajectories before ESRD: implications for clinical management. Semin. Nephrol. 37 , 132–143 (2017).
Tangri, N. et al . Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA 315 , 164–174 (2016).
Ricardo, A. C. et al . Influence of nephrologist care on management and outcomes in adults with chronic kidney disease. J. Gen. Intern. Med. 31 , 22–29 (2016).
Tordoir, J. et al . EBPG on vascular access. Nephrol. Dial. Transplant. 22 (Suppl. 2), ii88–ii117(2007).
Ravani, P. et al . Associations between hemodialysis access type and clinical outcomes: a systematic review. J. Am. Soc. Nephrol. 24 , 465–473 (2013).
Xue, H. et al . Hemodialysis access usage patterns in the incident dialysis year and associated catheter-related complications. Am. J. Kidney Dis. 61 , 123–130 (2013).
Alencar de Pinho, N. et al . Vascular access conversion and patient outcome after hemodialysis initiation with a nonfunctional arteriovenous access: a prospective registry-based study. BMC Nephrol. 18 , 74 (2017).
Wallace, E. L. et al . Catheter insertion and perioperative practices within the ISPD North American Research Consortium. Perit. Dial. Int. 36 , 382–386 (2016).
Leurs, P., Machowska, A. & Lindholm, B. Timing of dialysis initiation: when to start? Which treatment? J. Ren. Nutr. 25 , 238–241 (2015).
Abramowicz, D. et al . European Renal Best Practice Guideline on kidney donor and recipient evaluation and perioperative care. Nephrol. Dial. Transplant. 30 , 1790–1797 (2015).
Sebille, V. et al . Prospective, multicenter, controlled study of quality of life, psychological adjustment process and medical outcomes of patients receiving a preemptive kidney transplant compared to a similar population of recipients after a dialysis period of less than three years — the PreKit-QoL study protocol. BMC Nephrol. 17 , 11 (2016).
Chang, P. et al . Living donor age and kidney allograft half-life: implications for living donor paired exchange programs. Clin. J. Am. Soc. Nephrol. 7 , 835–841 (2012).
Allen, P. J. et al . Recurrent glomerulonephritis after kidney transplantation: risk factors and allograft outcomes. Kidney Int. 92 , 461–469 (2017).
Carson, R. C., Juszczak, M., Davenport, A. & Burns, A. Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin. J. Am. Soc. Nephrol. 4 , 1611–1619 (2009).
Morton, R. L. et al . Conservative management and end-of-life care in an Australian cohort with ESRD. Clin. J. Am. Soc. Nephrol. 11 , 2195–2203 (2016).
Verberne, W. R. et al . Comparative survival among older adults with advanced kidney disease managed conservatively versus with dialysis. Clin. J. Am. Soc. Nephrol. 11 , 633–640 (2016).
Crail, S. Walker, R., Brown, M. & Renal Supportive Care Working Group. Renal supportive and palliative care: position statement. Nephrology 18 , 393–400 (2013).
Birmele, B. et al . Death after withdrawal from dialysis: the most common cause of death in a French dialysis population. Nephrol. Dial. Transplant. 19 , 686–691 (2004).
Cox, K. J., Parshall, M. B., Hernandez, S. H., Parvez, S. Z. & Unruh, M. L. Symptoms among patients receiving in-center hemodialysis: a qualitative study. Hemodial. Int. 21 , 524–533 (2017).
Jesky, M. D. et al . Health-related quality of life impacts mortality but not progression to end-stage renal disease in pre-dialysis chronic kidney disease: a prospective observational study. PLoS ONE 11 , e0165675 (2016).
Rebollo Rubio, A. & Morales Asencio, J. M. & Eugenia Pons Raventos, M. Depression anxiety and health-related quality of life amongst patients who are starting dialysis treatment. J. Ren. Care 43 , 73–82 (2017). This is a study showing that improving quality of life starts with its proper assessment.
Davison, S. N., Jhangri, G. S. & Johnson, J. A. Cross-sectional validity of a modified Edmonton symptom assessment system in dialysis patients: a simple assessment of symptom burden. Kidney Int. 69 , 1621–1625 (2006).
Davison, S. N. Pain in hemodialysis patients: prevalence, cause, severity, and management. Am. J. Kidney Dis. 42 , 1239–1247 (2003).
Pereira, B. D. S. et al . Beyond quality of life: a cross sectional study on the mental health of patients with chronic kidney disease undergoing dialysis and their caregivers. Health Qual. Life Outcomes 15 , 74 (2017).
Tonelli, M. The roads less traveled? Diverging research and clinical priorities for dialysis patients and those with less severe CKD. J. Kidney Dis. 63 , 124–132 (2014).
Tinetti, M. E., Fried, T. R. & Boyd, C. M. Designing health care for the most common chronic condition — multimorbidity. JAMA 307 , 2493–2494 (2012).
Cabrera, V. J., Hansson, J., Kliger, A. S. & Finkelstein, F. O. Symptom management of the patient with CKD: the role of dialysis. Clin. J. Am. Soc. Nephrol. 12 , 687–693 (2017).
Manfredini, F. et al . Exercise in patients on dialysis: a multicenter, randomized clinical trial. J. Am. Soc. Nephrol. 28 , 1259–1268 (2017).
Cameron, J. I., Whiteside, C., Katz, J. & Devins, G. M. Differences in quality of life across renal replacement therapies: a meta-analytic comparison. Am. J. Kidney Dis. 35 , 629–637 (2000).
Iyasere, O. U. et al . Quality of life and physical function in older patients on dialysis: a comparison of assisted peritoneal dialysis with hemodialysis. Clin. J. Am. Soc. Nephrol. 11 , 423–430 (2016). This is a paper that evaluates alternative options to haemodialysis for older patients with ESRD.
Vanholder, R. et al . Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat. Rev. Nephrol. 13 , 393–409 (2017).
Dew, M. A. et al . Does transplantation produce quality of life benefits? A quantitative analysis of the literature. Transplantation 64 , 1261–1273 (1997).
Rhee, C. M., Brunelli, S. M., Subramanian, L. & Tentori, F. Measuring patient experience in dialysis: a new paradigm of quality assessment. J. Nephrol. http://dx.doi.org/10.1007/s40620-017-0401-2 (2017).
Wuttke, M. & Kottgen, A. Insights into kidney diseases from genome-wide association studies. Nat. Rev. Nephrol. 12 , 549–562 (2016).
Beeman, S. C. et al . MRI-based glomerular morphology and pathology in whole human kidneys. Am. J. Physiol. Renal Physiol. 306 , F1381–F1390 (2014).
Boor, P., Ostendorf, T. & Floege, J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat. Rev. Nephrol. 6 , 643–656 (2010).
Tampe, D. & Zeisberg, M. Potential approaches to reverse or repair renal fibrosis. Nat. Rev. Nephrol. 10 , 226–237 (2014).
Goicoechea, M. et al . Allopurinol and progression of CKD and cardiovascular events: long-term follow-up of a randomized clinical trial. Am. J. Kidney Dis. 65 , 543–549 (2015).
de Zeeuw, D. et al . Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Engl. J. Med. 369 , 2492–2503 (2013).
Pergola, P. E. et al . Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N. Engl. J. Med. 365 , 327–336 (2011).
Lazzeri, E., Romagnani, P. & Lasagni, L. Stem cell therapy for kidney disease. Expert Opin. Biol. Ther. 15 , 1455–1468 (2015).
Lasagni, L. et al . Podocyte regeneration driven by renal progenitors determines glomerular disease remission and can be pharmacologically enhanced. Stem Cell Rep. 5 , 248–263 (2015).
Mazzinghi, B., Romagnani, P. & Lazzeri, E. Biologic modulation in renal regeneration. Expert Opin. Biol. Ther. 16 , 1403–1415 (2016).
Pichaiwong, W. et al . Reversibility of structural and functional damage in a model of advanced diabetic nephropathy. J. Am. Soc. Nephrol. 24 , 1088–1102 (2013).
Cianciolo Cosentino, C. et al . Histone deacetylase inhibitor enhances recovery after AKI. J. Am. Soc. Nephrol. 24 , 943–953 (2013).
Klinkhammer, B. M., Goldschmeding, R., Floege, J. & Boor, P. Treatment of renal fibrosis-turning challenges into opportunities. Adv. Chron. Kidney Dis. 24 , 117–129 (2017).
Kramann, R. et al . Pharmacological GLI2 inhibition prevents myofibroblast cell-cycle progression and reduces kidney fibrosis. J. Clin. Invest. 125 , 2935–2951 (2015).
Peired, A. J., Sisti, A. & Romagnani, P. Mesenchymal stem cell-based therapy for kidney disease: a review of clinical evidence. Stem Cells Int. 2016 , 4798639 (2016).
Ninichuk, V. et al . Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen4A3-deficient mice. Kidney Int. 70 , 121–129 (2006).
Xinaris, C. et al . Functional human podocytes generated in organoids from amniotic fluid stem cells. J. Am. Soc. Nephrol. 27 , 1400–1411 (2016).
Takasato, M. et al . Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 536 , 238 (2016).
Takasato, M. & Little, M. H. Making a kidney organoid using the directed differentiation of human pluripotent stem cells. Methods Mol. Biol. 1597 , 195–206 (2017).
Xinaris, C., Brizi, V. & Remuzzi, G. Organoid models and applications in biomedical research. Nephron 130 , 191–199 (2015).
Anders, H. J., Jayne, D. R. & Rovin, B. H. Hurdles to the introduction of new therapies for immune-mediated kidney diseases. Nat. Rev. Nephrol. 12 , 205–216 (2016).
Holderied, A. & Anders, H. J. Animal models of kidney inflammation in translational medicine. Drug Discov. Today Dis. Models 11 , 19–27 (2014).
Levin, A., Lancashire, W. & Fassett, R. G. Targets, trends, excesses, and deficiencies: refocusing clinical investigation to improve patient outcomes. Kidney Int. 83 , 1001–1009 (2013).
Jayne, D. R. et al . Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J. Am. Soc. Nephrol. (2017).
Glassock, R., Delanaye, P. & El Nahas, M. An age-calibrated classification of chronic kidney disease. JAMA 314 , 559–560 (2015).
Levey, A. S., Inker, L. A. & Coresh, J. Chronic kidney disease in older people. JAMA 314 , 557–558 (2015).
Poggio, E. D. et al . Demographic and clinical characteristics associated with glomerular filtration rates in living kidney donors. Kidney Int. 75 , 1079–1087 (2009).
Pottel, H., Hoste, L., Yayo, E. & Delanaye, P. Glomerular filtration rate in healthy living potential kidney donors: a meta-analysis supporting the construction of the full age spectrum equation. Nephron 135 , 105–119 (2017).
Glassock, R. J. & Rule, A. D. The implications of anatomical and functional changes of the aging kidney: with an emphasis on the glomeruli. Kidney Int. 82 , 270–277 (2012).
Hallan, S. I. et al . Age and association of kidney measures with mortality and end-stage renal disease. JAMA 308 , 2349–2360 (2012).
Warnock, D. G., Delanaye, P. & Glassock, R. J. Risks for all-cause mortality: stratified by age, estimated glomerular filtration rate and albuminuria. Nephron 136 , 292–297 (2017).
Denic, A., Glassock, R. J. & Rule, A. D. Structural and functional changes with the aging kidney. Adv. Chron. Kidney Dis. 23 , 19–28 (2016).
Kidney Disease: Improving Global Outcomes (KDIGO) Lipid Work Group. KDIGO clinical practice guideline for lipid management in chronic kidney disease. Kidney Int. Suppl. 3 , 259–305 (2013).
Klessens, C. Q. et al . An autopsy study suggests that diabetic nephropathy is underdiagnosed. Kidney Int. 90 , 149–156 (2016).
Download references
Acknowledgements
P.R. is supported by the European Research Council under the Consolidator Grant RENOIR (ERC-2014-CoG), grant number 648274. G.R. and H.-J.A. have received support from the European Union's research and innovation programme (under grant agreement Horizon 2020, NEPHSTROM No. 634086). Z.M. has received research grants from the French government (the Investisssement d'Avenir programme). H.-J.A. has received support from the Deutsche Forschungsgemeinschaft (AN372/16-2, 23–1 and 24–1). The views expressed here are the responsibility of the authors only. The EU Commission takes no responsibility for any use made of the information set out.
Author information
Authors and affiliations.
Department of Experimental and Biomedical Sciences “Mario Serio” and Nephrology and Dialysis Unit, Meyer Children's University Hospital, Florence, Italy
Paola Romagnani
Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) - Istituto di Ricerche Farmacologiche Mario Negri, Bergamo, Italy
Giuseppe Remuzzi
Department of Medicine, Unit of Nephrology and Dialysis, Azienda Socio-Sanitaria Territoriale Papa Giovanni XXIII, Bergamo, Italy
Department of Biomedical and Clinical Science, L. Sacco, University of Milan, Milan, Italy
Department of Medicine, David Geffen School of Medicine at UCLA, Laguna Niguel, California, USA
Richard Glassock
Division of Nephrology, University of British Columbia, Vancouver, Canada
Adeera Levin
Department of Medical Informatics, European Renal Association–European Dialysis and Transplant Association (ERA–EDTA) Registry, Academic Medical Center, Amsterdam, The Netherlands
Kitty J. Jager
Division of Nephrology and Department of Community Health Sciences, Cumming School of Medicine, University of Calgary, Alberta, Canada
Marcello Tonelli
Division of Nephrology, Ambroise Paré University Hospital, Assistance Publique – Hôpitaux de Paris, University of Versailles-Saint-Quentin-en-Yvelines, Boulogne-Billancourt, France
INSERM U1018 Team5, Centre de Recherche en Épidémiologie et Santé des Populations (CESP), University of Versailles-Saint-Quentin-en-Yvelines, University Paris Saclay, Villejuif, France
Department of Medicine, Division of Nephrology, University Hospital of Würzburg, Würzburg, Germany
Christoph Wanner
Medizinische Klinik and Poliklinik IV, Klinikum der Ludwig Maximilians University (LMU) München – Innenstadt, Ziemssenstr. 1, München, 80336, Germany
Hans-Joachim Anders
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed equally to all sections of the Primer, with H.-J.A. coordinating the project.
Corresponding author
Correspondence to Hans-Joachim Anders .
Ethics declarations
Competing interests.
R.G. has received speaker honoraria from Genentech and consultancy honoraria from Bristol Myers Squibb; he has conducted compensated editorial tasks for the American Society of Nephrology and Karger and Wolters-Kluwer; and he owns stock in Reata. Z.M. has received grants for research from Amgen, Baxter, Dohme-Chibret, Fresenius Medical Care, GlaxoSmithKline, Lilly, Merck Sharp, Otsuka, and Sanofi-Genzyme; and has received personal fees and grants to charities from Amgen, Bayer and Sanofi-Genzyme. The other authors declare no competing interests.
PowerPoint slides
Powerpoint slide for fig. 1, powerpoint slide for fig. 2, powerpoint slide for fig. 3, powerpoint slide for fig. 4, powerpoint slide for fig. 5, powerpoint slide for fig. 6, powerpoint slide for fig. 7, powerpoint slide for fig. 8, powerpoint slide for fig. 9, rights and permissions.
Reprints and permissions
About this article
Cite this article.
Romagnani, P., Remuzzi, G., Glassock, R. et al. Chronic kidney disease. Nat Rev Dis Primers 3 , 17088 (2017). https://doi.org/10.1038/nrdp.2017.88
Download citation
Published : 23 November 2017
DOI : https://doi.org/10.1038/nrdp.2017.88
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Objectively measured daily steps as an outcome in a clinical trial of chronic kidney disease: a systematic review.
- Liuyan Huang
- Yifei Zhong
BMC Nephrology (2024)
Diffusion tensor imaging of brain changes in patients with chronic kidney disease before cognitive impairment with 3 T MRI device
- Sherif Abdel Fattah Moustafa
- Dina Abd Elnasser Rizk
- Wessam Mustafa
Egyptian Journal of Radiology and Nuclear Medicine (2024)
Uremic toxins mediate kidney diseases: the role of aryl hydrocarbon receptor
- Hongyan Xie
- Ninghao Yang
Cellular & Molecular Biology Letters (2024)
Realtime monitoring of thrombus formation in vivo using a self-reporting vascular access graft
- Daniel Hoare
- David Kingsmore
- John R. Mercer
Communications Medicine (2024)
Acute kidney injury during autologous stem cell transplantation in light chain amyloidosis with kidney involvement and their impact on prognosis
- Wencui Chen
- Xianghua Huang
Bone Marrow Transplantation (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Featured Clinical Reviews
- Screening for Atrial Fibrillation: US Preventive Services Task Force Recommendation Statement JAMA Recommendation Statement January 25, 2022
- Evaluating the Patient With a Pulmonary Nodule: A Review JAMA Review January 18, 2022
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
Chronic Kidney Disease Diagnosis and Management : A Review
- 1 Division of Nephrology, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland
- 2 Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins Medical Institutions, Baltimore, Maryland
Importance Chronic kidney disease (CKD) is the 16th leading cause of years of life lost worldwide. Appropriate screening, diagnosis, and management by primary care clinicians are necessary to prevent adverse CKD-associated outcomes, including cardiovascular disease, end-stage kidney disease, and death.
Observations Defined as a persistent abnormality in kidney structure or function (eg, glomerular filtration rate [GFR] <60 mL/min/1.73 m 2 or albuminuria ≥30 mg per 24 hours) for more than 3 months, CKD affects 8% to 16% of the population worldwide. In developed countries, CKD is most commonly attributed to diabetes and hypertension. However, less than 5% of patients with early CKD report awareness of their disease. Among individuals diagnosed as having CKD, staging and new risk assessment tools that incorporate GFR and albuminuria can help guide treatment, monitoring, and referral strategies. Optimal management of CKD includes cardiovascular risk reduction (eg, statins and blood pressure management), treatment of albuminuria (eg, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers), avoidance of potential nephrotoxins (eg, nonsteroidal anti-inflammatory drugs), and adjustments to drug dosing (eg, many antibiotics and oral hypoglycemic agents). Patients also require monitoring for complications of CKD, such as hyperkalemia, metabolic acidosis, hyperphosphatemia, vitamin D deficiency, secondary hyperparathyroidism, and anemia. Those at high risk of CKD progression (eg, estimated GFR <30 mL/min/1.73 m 2 , albuminuria ≥300 mg per 24 hours, or rapid decline in estimated GFR) should be promptly referred to a nephrologist.
Conclusions and Relevance Diagnosis, staging, and appropriate referral of CKD by primary care clinicians are important in reducing the burden of CKD worldwide.
Read More About
Chen TK , Knicely DH , Grams ME. Chronic Kidney Disease Diagnosis and Management : A Review . JAMA. 2019;322(13):1294–1304. doi:10.1001/jama.2019.14745
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Case Report
- Open access
- Published: 22 April 2024
A loss-of-function AGTR1 variant in a critically-ill infant with renal tubular dysgenesis: case presentation and literature review
- Aljazi Al-Maraghi 1 ,
- Waleed Aamer 1 ,
- Mubarak Ziab 2 ,
- Elbay Aliyev 1 ,
- Najwa Elbashir 1 ,
- Sura Hussein 1 ,
- Sasirekha Palaniswamy 1 ,
- Dhullipala Anand 3 ,
- Donald R. Love 4 ,
- Adrian Charles 5 ,
- Ammira A.S.Akil 2 &
- Khalid A. Fakhro 1 , 2 , 6 , 7
BMC Nephrology volume 25 , Article number: 139 ( 2024 ) Cite this article
106 Accesses
Metrics details
Renal tubular dysgenesis (RTD) is a severe disorder with poor prognosis significantly impacting the proximal tubules of the kidney while maintaining an anatomically normal gross structure. The genetic origin of RTD, involving variants in the ACE, REN, AGT, and AGTR1 genes , affects various enzymes or receptors within the Renin angiotensin system (RAS). This condition manifests prenatally with oligohydramninos and postnatally with persistent anuria, severe refractory hypotension, and defects in skull ossification.
Case presentation
In this report, we describe a case of a female patient who, despite receiving multi vasopressor treatment, experienced persistent hypotension, ultimately resulting in early death at five days of age. While there was a history of parental consanguinity, no reported family history of renal disease existed. Blood samples from the parents and the remaining DNA sample of the patient underwent Whole Genome Sequencing (WGS). The genetic analysis revealed a rare homozygous loss of function variant (NM_000685.5; c.415C > T; p.Arg139*) in the Angiotensin II Receptor Type 1 ( AGTR1 ) gene.
This case highlights the consequence of loss-of-function variants in AGTR1 gene leading to RTD, which is characterized by high mortality rate at birth or during the neonatal period. Furthermore, we provide a comprehensive review of previously reported variants in the AGTR1 gene, which is the least encountered genetic cause of RTD, along with their associated clinical features.
Peer Review reports
Renal tubular dysgenesis (RTD) (MIM# 267,430) is a rare autosomal recessive disorder of renal tubular development that was first characterized in two stillborn siblings in 1983 [ 1 ]. The disease carries a poor prognosis and a high mortality rate due to the severity of the disease where patients may die in utero or soon after birth, despite the availability of high-quality clinical care. Although the exact prevalence of RTD is unknown, there are multiple reports of RTD cases [ 2 , 3 ].
The underlying pathophysiology of RTD involves reduced intrauterine renal perfusion leading to dysgenesis of proximal tubule formation in the kidneys, with preservation of grossly normal kidney structure [ 4 ].The clinical manifestations of RTD include persistent fetal anuria with subsequent oligohydramnios in pregnancy, pulmonary hypoplasia, and skull ossification defects of the bone due to persistent hypotension [ 5 ]. In addition, typical pathological changes seen on kidney sections taken from affected patients show the incomplete development of renal proximal tubules. These changes are attributed to the consequences of hypoperfusion and renal ischemia in the absence of Angiotensin II (ANG II) production or function, a defect responsible for the severe refractory hypotension observed at birth [ 6 ].
Previous studies have demonstrated the fundamental role of Renin Angiotensin System (RAS) during fetal development of the kidneys. Physiologically, the RAS pathway regulates extracellular fluid volume and maintains blood pressure levels in the body [ 7 ]. Several variants in four different genes encoding RAS signaling proteins ( AGT , REN , ACE , and AGTR1 ) have been described to cause RTD [ 8 ]. Variants in the AGTR1 gene constitute approximately 8% of the reported mutations causing RTD [ 9 ].
The Angiotensin II Receptor Type 1 ( AGTR1 ) gene encodes a receptor protein of the ligand angiotensin II, which is a potent vasopressor hormone in the RAS pathway [ 10 ]. The binding of ANG II to the Angiotensin II Type 1 receptor (AT1 receptor) promotes its activation, leading to vasoconstriction, sympathetic activity and aldosterone release from adrenals, ultimately increasing blood pressure [ 11 ]. Angiotensin II also regulates renal growth during fetal development [ 12 ].
Herein, we report a rare nonsense variant in the AGTR1 gene detected through whole genome sequencing (WGS) in a neonate exhibiting persistent anuria and resistant refractory pulmonary hypoplasia, ultimately resulting in early lethality.
The female patient, born to consanguineous parents (first degree cousins) with a family history of Oculocutaneous Albinism in the mother. This was the mother’s first pregnancy and antental ultrasound scans revealed oligohydramnios and Intra-Uterine Growth Retardation (IUGR). The patient was born prematurely at 36 weeks through an emergency cesarean section due to reduced fetal movement and failed induction. The baby was born weighing 2.0 kg with meconium stained liquor and Apgar scores were 6 and 9 at one and five minutes, respectively. The baby required minimal resuscitation and she was managed on continuous positive airway pressure (CPAP) in the first hour of life; however, within a few hours she deteriorated with bilateral pneumothoraces requiring chest drains, intubation, and ventilation. The patient was started on inhaled nitric oxide for hypoxic respiratory failure, and inotropes due to low blood pressure including dopamine, dobutamine, and epinephrine. The patient remained hypotensive with a mean blood pressure of 15–20 mmHg, which required the addition of hydrocortisone followed by vasopressin to improve her blood pressure. Her oxygen saturation measurements were 35%—45% in 100% FiO 2 . Supportive measures, including sedation, antibiotics, and fluids were administered. The patient didn’t have any urine output and she developed persistent hypoxia and hypotension, necessitating veno-arterial Extra Corporeal Membrane Oxygenation (ECMO) support on the second day of life, which led to an improvement in her oxygen saturation. However, the patients blood pressure remained low despite the ECMO and continuous inotropic support. While on ECMO, renal replacement therapy (CRRT) was initiated, effectively normalzing the creatinine levels, however the CRRT was discontinued due to the development of hypotension, resulting in progressive edema and fluid overload. Subsequently, the decision was made to decannulate and remove the ECMO support due to a substantial right-sided parenchymal hemorrhage and extra-axial hemorrhage observed on head ultrasound. The patient experienced coagulopathy, manifesting as oozing from the skin and chest tubes requiring multiple Fresh Frozen Plasma (FFP), cryoprecipitate, and red cell transfusions due to low hemoglobin, persistent thrombocytopenia and coagulopathy. On the fourth day, a multi-disciplinary team meeting, with the patient’s parents present, concluded to transition the patient from intensive care to comfort care with no further resuscitation. The patient was extubated the following day and passed away a few hours later.
Imaging studies that were done on the baby included: (1) Echocardiography, which showed a structurally normal heart but was associated with severe persistent pulmonary hypertension of newborn (PPHN) and complete right to left shunting across the ductus arteriosus; (2) Abdominal ultrasound, which showed non-specific bilateral echogenic kidneys; (3) Head ultrasound, which showed large left intra-parenchymal and extra-axial acute bleeding associated with mass effect.
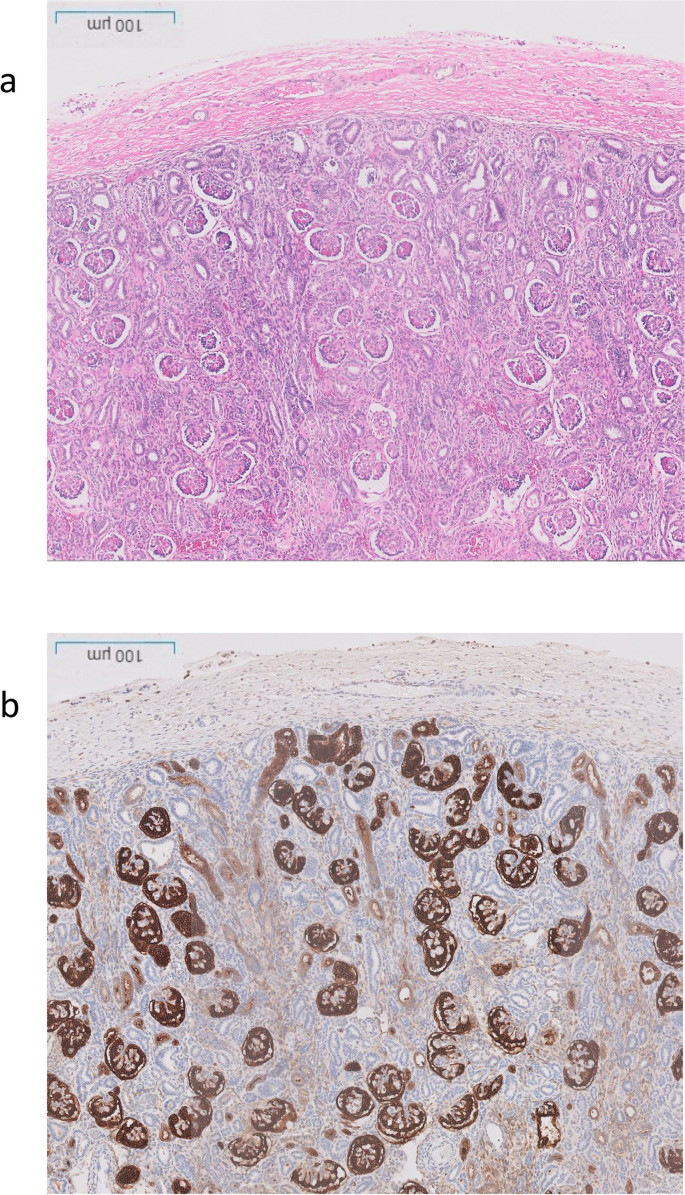
The post-mortem examination revealed mildly hypoplastic kidneys, moderate pulmonary hypoplasia, solid and poorly aerated lungs with diffuse alveolar damage, significantly reduced skull vault mineralization and bony development, indicative features of oligohydramnios sequence. Limbs exhibited some flexion changes, and there were characterestics findings of of Potters’ facies, marked edema, and a structurally normal heart. Histopathology showed changes of renal tubular dysgenesis with the renal cortex containing crowded glomeruli separated by small tubules with distal tubular morphology and absence of proximal tubules (Fig. 1 ). The proximal tubules should be as numerous as the glomeruli and have plump lining cells with abundant cytoplasm. The medulla appeared largely unremarkable. The family history of parental consanguinity and the severity of symptoms prompted enrolling the family in the Mendelian disease program at Sidra Medicine (Fig. 2 a) [ 13 ]. Genome sequencing was performed on all family members, and following our in-house analysis pipeline [ 14 ], the patient was, initially, found to carry six de novo and nine homozygous rare protein-altering variants, including two that were predicted to lead to loss-of-function (LoF) (Additional file 1 ). These two include a variant in OR1J4 (c.221C > G, p.Ser74*), an olfactory receptor gene not known to be associated with Mendelian disease, and a nonsense previously unreported variant (NM_000685.5; c.415C > T; p.Arg139*) was identified in the Angiotensin II Receptor Type 1 (AGTR1) gene (Table 1 ). Importantly, LoF variants in this gene have been associated with renal tubular dysgenesis (MIM# 267,430) [ 8 ]. Both parents were heterozygous carriers of the variant (Fig. 2 b) and in-silico pathogenicity scores predicted it to be highly damaging (CADD of 39 and GERP of 5.8).
Renal histological characteristics. a H&E renal cortex, showing crowding of the glomeruli, with intervening tubules mainly of distal tubule type, and lack of proximal tubules. b CD10 highlighting the glomeruli and the Bowmans capsule, but normal proximal tubules are not seen, only weak staining of the ureteric buds
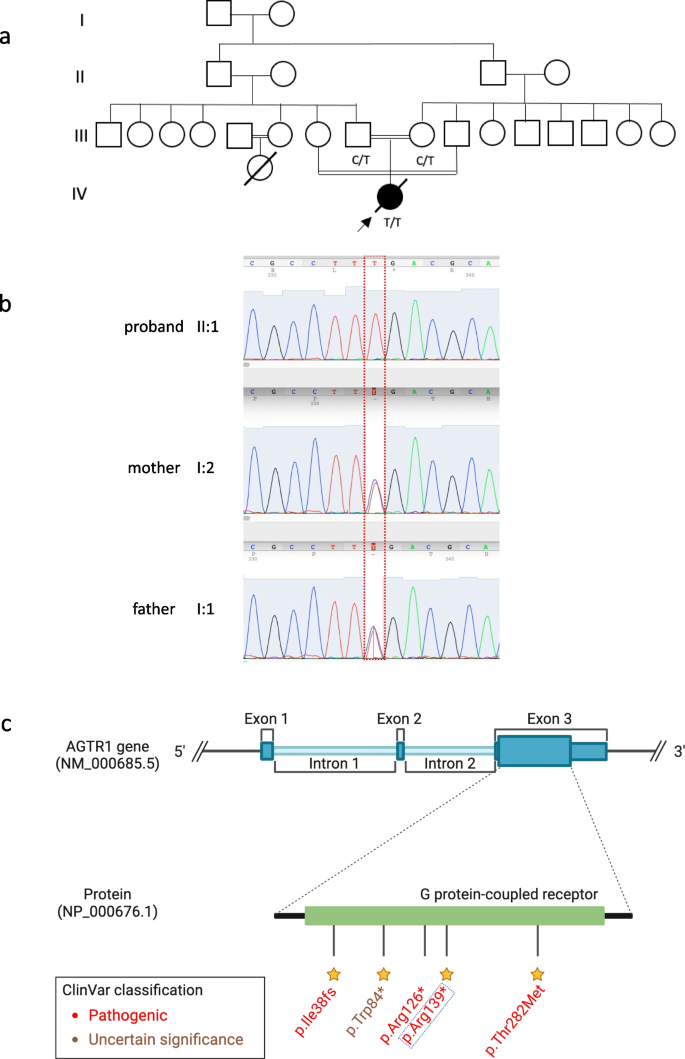
Patient characteristics and genetic findings. a Family pedigree of the patient along with genotypes of the nonesense AGTR1 variant (c.415C > T; p.Arg139*). b Chromatogram of Sanger sequencing showing the variant position and genotypes of the 3 family members. c Schematic of AGTR1 gene body with highlights of protein domains and reported ClinVar variants. The yellow stars refer to the staring system of ClinVar which indicate the review status of the variant
Discussion and conclusion
The molecular mechanisms underlying the genetic basis of RTD pathogenesis are still not fully elucidated; however, LoF/structural variants in genes encoding components of the RAS pathway are a major cause of the disease [ 8 ]. Disruption of the RAS leads to defects in the differentiation of proximal tubules during fetal development resulting in severe symptoms during pre- and postnatal periods including fetal anuria and oligohydramnios [ 15 ].
In this report, we present a case of a newborn female patient who suffered from congenital RTD and several severe complications, ultimately resulting in perinatal death at five days of life. Genetic analysis of the child and her parents identified a pathogenic nonsense variant in exon 3 of the AGTR1 gene. The predicted effects of this variant are protein truncation and possibly nonsense-mediated mRNA decay. To date, only eight RTD patients, including ours, have been reported with five different AGTR1 gene variants (Table 1 , Fig. 2 c), reflecting the rare nature of RTD and the significance of RAS signaling pathway in early development.
The genetic association of AGTR1 variants with an RTD phenotype is supported by the literature in which patients suffer severe symptoms during pre-and/or postnatal life [ 8 , 9 ]. In addition, recent evidence has pointed to the possibility of a milder form of the RTD depending on the variant position in the AGTR1 gene [ 16 ]. A male carrier of a homozygous LoF variant (p.Arg216*) in AGTR1 has been described who lived to 28 years of age under management with high doses of fludrocortisone which, along with vasopressin, have proved effective in managing RTD [ 16 ]. Overall, although the severity of symptoms in patients who carry AGTR1 mutations is consistent across all reported cases, it has been suggested that, similar to other genetic renal diseases, the phenotype is more severe when the affected protein is located more distally along the RAS pathway [ 17 ].
Reaching a final diagnosis of RTD prenatally has been challenging because all prenatal symptoms of oligohydramnios, and IUGR are not specific. This challenge leaves genetic testing as the only viable diagnostic option after none genetic causes have been excluded [ 18 ], particularly when offered in the context of prenatal diagnosis through chorionic villus sampling. Even when early symptoms began to emerge postnatally, the patient's instability did not indicate a specific diagnosis. The genetic finding complemented by the histopathology confirmed the diagnosis of RTD. Although the treatment remained supportive, providing prompt answers to healthcare providers and families is immensely valuable.
Given the severity of the condition, improved outcomes for RTD patients can be realized through early detection, facilitating clinical decision making and enhancing neonatal care, particularly in cases of severe congenital diseases with prenatal indications and symptoms. Genetic testing empowers carrier parents to make informed decisions regarding their future family plans. In the case of the newborn discussed here, the parents received appropriate counselling and were informed about the genetic results, and the disease risk in subsequent pregnancies. Early identification of recessive pathogenic variants, particularly in such highly consanguineous population, plays a pivotal role in the success of population screening programs and contributes to lowering the long-term burden of Mendelian diseases.
Availability of data and materials
The datasets analysed during the current study are available in the Genome Sequence Archive in Sidra Medicine, Qatar. Variant submitted in ClinVar under accession number VCV002430252.2.
Abbreviations
Angiotensin-converting enzyme
Angiotensin II Receptor Type 1
Continuous positive airway pressure
Renal replacement therapy
Extra Corporeal Membrane Oxygenation
Intra-uterine growth retardation
Persistent pulmonary hypertension of newborn
Renin angiotensin system
- Renal tubular dysgenesis
Allanson JE, Pantzar JT, Macleod PM. Possible new autosomal recessive syndrome with unusual renal histopathological changes. Am J Med Genet. 1983;16(1):57–60.
Article CAS PubMed Google Scholar
Tseng M-H, Huang S-M, Huang J-L, Fan W-L, Konrad M, Shaw SW, et al. Autosomal Recessive Renal Tubular Dysgenesis Caused by a Founder Mutation of Angiotensinogen. Kidney Int Rep. 2020;5(11):2042–51.
Article PubMed PubMed Central Google Scholar
Vincent KM, Alrajhi A, Lazier J, Bonin B, Lawrence S, Weiler G, et al. Expanding the clinical spectrum of autosomal-recessive renal tubular dysgenesis: two siblings with neonatal survival and review of the literature. Mol Genet Genomic Med. 2022;10(5):e1920.
Gubler M-C. Renal tubular dysgenesis. Pediatr Nephrol. 2014;29(1):51–9.
Article PubMed Google Scholar
Kumar D, Moss G, Primhak R, Coombs R. Congenital renal tubular dysplasia and skull ossification defects similar to teratogenic effects of angiotensin converting enzyme (ACE) inhibitors. J Med Genet. 1997;34(7):541–5.
Article CAS PubMed PubMed Central Google Scholar
Gubler MC, Antignac C. Renin–angiotensin system in kidney development: renal tubular dysgenesis. Kidney Int. 2010;77(5):400–6.
McDonough AA. Mechanisms of proximal tubule sodium transport regulation that link extracellular fluid volume and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2010;298(4):R851–61.
Gribouval O, Gonzales M, Neuhaus T, Aziza J, Bieth E, Laurent N, et al. Mutations in genes in the renin-angiotensin system are associated with autosomal recessive renal tubular dysgenesis. Nat Genet. 2005;37(9):964–8.
Gribouval O, Morinière V, Pawtowski A, Arrondel C, Sallinen S-L, Saloranta C, et al. Spectrum of mutations in the renin-angiotensin system genes in autosomal recessive renal tubular dysgenesis. Hum Mutat. 2012;33(2):316–26.
Higuchi S, Ohtsu H, Suzuki H, Shirai H, Frank GD, Eguchi S. Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology. Clin Sci (Lond). 2007;112(8):417–28.
Roks AJ, van Geel PP, Pinto YM, Buikema H, Henning RH, de Zeeuw D, et al. Angiotensin-(1–7) is a modulator of the human renin-angiotensin system. Hypertens (Dallas, Tex 1979). 1999;34(2):296–301.
Article CAS Google Scholar
Zhang S-L, Moini B, Ingelfinger JR. Angiotensin II Increases Pax-2 Expression in Fetal Kidney Cells Via the AT2 Receptor. J Am Soc Nephrol. 2004;15(6):1452–65.
Fakhro KA, Robay A, Rodrigues-Flores JL, Mezey JG, Al-Shakaki AA, Chidiac O, et al. Point of care exome sequencing reveals allelic and phenotypic heterogeneity underlying Mendelian disease in Qatar. Hum Mol Genet. 2019;28(23):3970–81.
CAS PubMed Google Scholar
Da’as SI, Aamer W, Hasan W, Al-Maraghi A, Al-Kurbi A, Kilani H, et al. PGAP3 Associated with hyperphosphatasia with mental retardation plays a novel role in brain morphogenesis and neuronal wiring at early development. Cells. 2020;9(8):1782.
Lacoste M, Cai Y, Guicharnaud L, Mounier F, Dumez Y, Bouvier R, et al. Renal tubular dysgenesis, a not uncommon autosomal recessive disorder leading to oligohydramnios: role of the renin-angiotensin system. J Am Soc Nephrol. 2006;17(8):2253–63.
Viering DHHM, Bech AP, de Baaij JHF, Steenbergen EJ, Danser AHJ, Wetzels JFM, et al. Functional tests to guide management in an adult with loss of function of type-1 angiotensin II receptor. Pediatr Nephrol. 2021;36(9):2731–7.
Demirgan EB, Saygili S, Canpolat N, Sever L, Kilicaslan I, Taylan D, et al. AGTR1-related renal tubular dysgeneses may not be fatal. Kidney Int reports. 2021;6(3):846–52.
Article Google Scholar
Banuelos R, Mallawaarachchi A, Doyle H, Mogra R. Oligohydramnios or anhydramnios and ultrasonically normal renal echotexture secondary to autosomal recessive renal tubular dysgenesis: an important consideration in the prenatal setting. Fetal Diagn Ther. 2023;50(1):17–21.
Download references
Acknowledgements
We are grateful to the families and individuals who participated in this study.
This work was supported by Qatar Foundation, National Priorities Research Program (NPRP11S-0110–180250).
Author information
Authors and affiliations.
Laboratory of Genomic Medicine, Sidra Medicine, P.O. Box 26999, Doha, Qatar
Aljazi Al-Maraghi, Waleed Aamer, Elbay Aliyev, Najwa Elbashir, Sura Hussein, Sasirekha Palaniswamy & Khalid A. Fakhro
Department of Human Genetics-Precision Medicine in Diabetes Prevention, Sidra Medicine, P.O. Box 26999, Doha, Qatar
Mubarak Ziab, Ammira A.S.Akil & Khalid A. Fakhro
Neonatology Division, Sidra Medicine, P.O. Box 26999, Doha, Qatar
Dhullipala Anand
Genetic Pathology, Sidra Medicine, P.O. Box 26999, Doha, Qatar
Donald R. Love
Anatomical Pathology, Sidra Medicine, P.O. Box 26999, Doha, Qatar
Adrian Charles
College of Health and Life Sciences, Hamad Bin Khalifa University, P.O. Box 34110, Doha, Qatar
Khalid A. Fakhro
Department of Genetic Medicine, Weill Cornell Medical College, P.O. Box 24144, Doha, Qatar
You can also search for this author in PubMed Google Scholar
Contributions
A. A. conducted genetic analysis and literature searches, taking the lead in writing the manuscript. W. A. played a key role in Whole Genome Sequence acquisition and manuscript drafting. A. D. contributed to clinical data collection and provided support to the patient. M. Z. and D. R. L. performed variant validation, while J. L. and S. H. were involved in patient enrollment and sample processing. E. A. performed structural variant analysis and reviewed the manuscript. D. R. L. offered clinical interpretation of genetic findings, and S. P. managed follow-up and reporting. A. C. provided images and description of renal histopathology. A. A. K. oversaw the original study, supervised patient recruitment, and handled manuscript review, editing, and regulatory requirements. K. A. F. secured funding, designed the study, coordinated the overall process, and conducted final proofreading and critical manuscript review. All authors participated in reading and approving the final manuscript.
Corresponding author
Correspondence to Khalid A. Fakhro .
Ethics declarations
Ethics approval and consent to participate.
The institutional review board approval of this study was obtained from Sidra Medicine, Qatar (IRB 1636872). Furthermore, the family members signed informed consent and assent forms to participate in the proposed research study.
Consent for publication
We obtained written informed consent from the patient’s father to publish this potentially identifiable information from all of the family members included in this case report.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Al-Maraghi, A., Aamer, W., Ziab, M. et al. A loss-of-function AGTR1 variant in a critically-ill infant with renal tubular dysgenesis: case presentation and literature review. BMC Nephrol 25 , 139 (2024). https://doi.org/10.1186/s12882-024-03569-z
Download citation
Received : 28 November 2023
Accepted : 02 April 2024
Published : 22 April 2024
DOI : https://doi.org/10.1186/s12882-024-03569-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Middle East
- Rare Mendelian disease
- Whole genome sequencing
BMC Nephrology
ISSN: 1471-2369
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Open access
- Published: 29 April 2024
Efficacy and safety of direct oral anticoagulants in patients with atrial fibrillation combined with chronic kidney disease: a systematic review and meta-analysis
- Yaodi Li 1 na1 ,
- Shuyi Wu 1 na1 ,
- Jintuo Zhou 1 na1 &
- Jinhua Zhang ORCID: orcid.org/0000-0002-5629-0348 1
Thrombosis Journal volume 22 , Article number: 40 ( 2024 ) Cite this article
Metrics details
Currently published studies have not observed consistent results on the efficacy and safety of direct oral anticoagulants (DOACs) use in patients with chronic kidney disease (CKD) combined with atrial fibrillation (AF). Therefore, this study conducted a meta-analysis of the efficacy and safety of DOACs for patients with AF complicated with CKD.
Database literature was searched up to May 30, 2023, to include randomized controlled trials (RCT) involving patients with AF complicated with CKD DOACs and vitamin K antagonists (VKAs). Stroke, systemic embolism (SE), and all-cause mortality were used as effectiveness indicators, and major bleeding, intracranial hemorrhage (ICH), fatal bleeding, gastrointestinal bleeding (GIB), and clinically relevant non-major bleeding (CRNMB) were used as safety outcomes.
Nine RCT studies were included for analysis according to the inclusion criteria. Results of the efficacy analysis showed that compared with VKAs, DOACs reduced the incidence of stroke/SE (OR = 0.75, 95% CI 0.67–0.84) and all-cause deaths (OR = 0.84, 95% CI 0.75–0.93) in patients with AF who had comorbid CKD. Safety analyses showed that compared with VKAs, DOACs improved safety by reducing the risk of major bleeding (OR = 0.76, 95%CI 0.65–0.90), ICH (OR = 0.46, 95%CI 0.38–0.56), and fatal bleeding (OR = 0.75, 95%CI 0.65–0.87), but did not reduce the incidence of GIB and CRNMB.
Compared with VKAs, DOACs may increase efficacy and improve safety in AF patients with CKD (90 ml/min> Crcl≥15 ml/min), and shows at least similar efficacy and safety in AF patients with Kidney failure (Crcl<15 ml/min).
Introduction
Published studies have yielded inconsistent findings regarding the effectiveness and safety of DOACs in patients with AF and CKD. Patients with atrial fibrillation (AF) are susceptible to stroke or thromboembolic events due to increased heart rate, enlarged atria, and the special structure of the left atrium, which can lead to stagnation of blood flow [ 1 ]. To prevent thromboembolism, oral anticoagulants (OACs), including VKAs and direct oral anticoagulants (DOACs), are one of the preferred treatments for patients at risk of thromboembolism [ 2 ]. Compared with warfarin, DOACs is gradually being widely used in the clinic because of its fixed-dose, shorter half-life, and rapid elimination after discontinuation [ 3 ]. In addition, it has been shown that compared with warfarin, DOACs used in patients with atrial fibrillation with normal renal function can lead to a significant reduction in the risk of thrombosis and hemorrhage [ 4 , 5 ], and the efficacy and safety have been clinically proven to be superior to that of warfarin [ 6 , 7 ].
VKAs are metabolized by the liver and no dose adjustment is required in renal insufficiency. In contrast, DOACs has varying degrees of renal clearance, with approximately 80% of dabigatran, 50% of edoxaban, 35% of rivaroxaban, and 27% of apixaban excreted [ 8 ]. Owing to the exclusion of patients with advanced-stage CKD from phase 3 clinical trials of DOACs, the utilization of DOACs has lagged behind in this specific population. In dialysis patients, the utilization of VKAs poses heightened risks such as renal calcification and diminished platelet production due to renal failure, thus increasing the likelihood of bleeding within the typical INR range compared to the general population. Patients undergoing hemodialysis are subject to systemic heparin anticoagulant therapy during the treatment period. However, studies assessing bleeding complications in hemodialysis patients treated with AVKs or DOACs have not taken into consideration the extent of heparinization during hemodialysis treatment. Therefore, the efficacy and safety of DOACs in AF patients combined with CKD, particularly in cases involving kidney failurehas been controversial. The current literature review indicates that, in patients with moderate CKD, dabigatran and apixaban exhibit superior efficacy over VKAs in reducing the incidence of stroke and systemic embolism. However, no statistically significant differences emerge between apixaban, rivaroxaban, and VKAs in this regard. In terms of major bleeding risk reduction, edoxaban, apixaban, and VKAs demonstrate more favorable outcomes compared to VKAs alone. Conversely, no notable distinction is observed between rivaroxaban and dabigatran etexilate in this aspect [ 9 ]. A meta-analysis conducted by T. Ha et al. found insufficient evidence to determine the superiority of VKAs or DOACs in AF patients with advanced CKD [ 10 ]. Another study showed that DOACs were not associated with a reduced risk of thromboembolism in AF patients on long-term dialysis, whereas VKAs, dabigatran, and rivaroxaban were associated with a significantly higher risk of bleeding compared with apixaban and no anticoagulants [ 11 ]. However, one study showed that DOACs was significantly more effective and safer than VKAs in patients with CKD or ESRD combined with AF [ 12 ]. In addition, a meta-analysis of the use of DOACs and VKAs in end-stage dialysis patients showed at least similar efficacy and safety [ 13 ].
Therefore, to better investigate the efficacy and safety of DOACs use in patients with CKD combined with AF, the present study conducted a systematic review and meta-analysis of the available evidence from randomized controlled trials (RCTs) to inform clinical medication decisions.
We conducted a systematic review and meta-analysis of DOACs and VKAs for patients with AF comorbid CKD. CKD is defined as abnormalities of kidney structure or function, present for > 3 months, with implications for health. CKD is classified based on Cause, GFR category (G1–G5), and Albuminuria category (A1–A3) [ 14 ]. Renal insufficiency was defined as patients with CrCl < 95 ml/min, and patients with CrCl < 15 ml/min were defined as patients with kidney failure [ 15 ]. This study was conducted under the Preferred Reporting Initiative (PRISMA), registration number: CRD42023451323 [ 16 ].
Search strategies
The PubMed and Web of Science databases were searched and the time frame was from the creation of the database to May 31, 2023. To ensure a comprehensive literature search, we also identified additional studies by searching the reference lists of the literature. Search words: (“dabigatran” or “rivaroxaban” or “apixaban” or “edoxaban” or “NOAC” or “DOAC” or “non-vitamin K antagonist oral anticoagulantacting” or “novel oral anticoagulant” and “warfarin” or “coumadin” or “vitamin K antagonist”) and (“renal insufficient” or “kidney disease” or “chronic renal insufficiency” or “end-stage renal disease” or “renal dialysis or hemodialysis” and “atrial fibrillation” or “kidney failure”). Detailed search strategies for each database are provided in Table S 1 .
Study selection
Inclusion criteria: (1) RCT; (2) The study was conducted in AF patients with CKD; (3) DOACs, including comparative studies of apixaban, rivaroxaban, edoxaban, and dabigatran with VKAs. Both control and experimental groups reported at least one bleeding or thrombosis occurrence data; (4) Full text was available and relevant data could be extracted.
Exclusion criteria: (1) Patients with AF not comorbid with CKD; (2) Duplicate studies or incomplete experimental data.
Data extraction and study outcomes
Data extraction was done independently by two researchers (YL and SW). If there was a dispute, it will be discussed and resolved by a third researcher (JZ) to reach a consensus. Regarding missing data, we endeavor to communicate with the authors of the primary studies in an effort to obtain any unavailable data. If this proves unfeasible, we resort to the exclusion of studies with missing data. It is crucial to note, however, that this exclusionary approach may introduce selection bias if the missing data not missing completely at random. Furthermore, to ensure the reliability and robustness of our findings, we undertake a sensitivity analysis, scrutinizing the impact of varying scenarios on our results.
The following data were extracted from each study: study information (authors, year of publication), study characteristics (study population, sample size, duration of follow-up), intervention, and outcome indicators. Efficacy indicators were stroke, SE, and all-cause deaths. Safety indicators were major bleeding, intracranial hemorrhage (ICH), fatal bleeding, gastrointestinal bleeding (GIB), clinically relevant nonmajor bleeding (CRNMB), and minor bleeding.
Quality assessment
Using the Cochrane Collaboration Risk Assessment Tool [ 17 ], two authors independently evaluated each paper for bias in seven areas: generation of randomized sequences, allocation concealment, blinding of subjects and investigators, blinding of outcome evaluations, completeness of outcome data, selective reporting of outcomes, and other biases. The level of risk of bias was evaluated as “high”, “low”, and “unclear”. If there is a dispute, another researcher (JZ) will evaluate it and help to solve the problem.
Statistical analysis
Data on the incidence of all-cause death, stroke, SE, major bleeding, ICH, fatal bleeding, GIB, CRNMB, and minor bleeding were extracted for the inclusion of the experimental group and the control group. Forest plots were made using Review Manager 5.3 software. P -value, odds ratio (OR), and 95% confidence interval (Cl) were used as indicators of statistical differences in the comparison of the two groups. P -value < 0.05 and 95% Cl not containing 1 were considered as statistically significant differences. Statistical heterogeneity of the included studies was assessed using the Cochrane q-test p -value and I ² statistic, where a Cochrane q-test p -value < 0.1 or I ² value > 50% indicated significant heterogeneity. Fixed-effected model was used to calculate the pooled ORs and its 95% confidence interval (CI) if q-test p -value > 0.10 and I 2 < 50%. Otherwise, the random-effect model was applied.
Sensitivity analyses and subgroup analyses were also performed to look for sources of heterogeneity. In secondary analyses, data on dabigatran were excluded and the meta-analysis was re-run considering that the direct thrombin inhibitor dabigatran has the highest rate of renal excretion and renal function has a greater impact on it. In addition, to analyze whether DOACs and renal function levels affect the study indexes, subgroup analyses were performed according to the type of DOACs and the level of renal function of the patients. Since less than ten papers were included in this study, publication bias detection was not performed.
Literature search
According to the search strategy, a total of 2997 papers were included, 695 duplicates were removed, and 500 papers of special types (review, case, letter, guideline, comment, animal) were removed. After reading the titles and abstracts and removing uncontrolled studies, reviews, and literature that could not be accessed in full text, the remaining 55 could be downloaded in full text for reading. After removing 25 of the cohort studies, as well as 21 of the literature with incomplete data that could not be extracted as relevant, and 1 of the literature with data that could not be transformed [ 18 ], the remaining 9 randomized controlled studies were included in the meta-analysis [ 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 ] The flow chart for inclusion in the study is shown in Fig. 1 .
Flow chart for inclusion of studies
Baseline characteristics of included studies
The baseline characteristics of the included studies are shown in Table 1 . Nine randomized controlled studies with 15 subgroups were included in this study. The experimental group drugs DOACs included apixaban [ 19 , 25 , 26 , 27 ], rivaroxaban [ 20 , 22 , 24 ], dabigatran [ 21 ], and edoxaban [ 23 ]. The control drugs were dose-adjusted VKAs with INR values controlling between 2 and 3 except in literature where the control drug was warfarin (or matching placebo) [ 19 ] or phenprocoumon [ 27 ]. A total of 47,298 people were included, including 26,245 in the DOACs group and 21,053 in the VKAs group Table 2 .
Nine articles were at low risk for randomized sequence generation, completeness of outcome data, selective reporting of results, and other biases. Three articles were at high risk for allocation concealment and two were unclear. Three articles were at high risk for blinding of subjects and investigators, and one article was unclear about blinding of results (Fig. 2 ).
Risk of bias in each study. Green, low risk of bias; yellow, unclear risk of bias; and red, high risk of bias
Trial sequential analysis
Trial Sequential Analysis (TSA) was conducted in this study for various outcome indicators. A two-sided type I error probability (α) of 0.05 and type II error probability (β) of 0.20 were established, along with the definition of the Required Information Size (RIS). The control group’s incidence was computed using the data from the included studies. The TSA results showed the cumulative Z curves for stroke or SE, ICH and fetal bleeding crossed both the conventional and TSA boundaries and reached the the required information size (RIS), indicating that the meta-analysis results were stable and statistically significant (Fig. 3 C, F, J). The cumulative Z curves for all-cause death and major bleeding crossed the traditional threshold and TSA threshold, further confirming the credibility of the synthesized data (Fig. 3 D, E). The cumulative Z curves for stroke, minor bleeding, GIB crossed conventional test boundary; however, they did not cross Alpha-spending boundary, nor did it reach the required information size (Fig. 3 A, I, G). And the cumulative Z curves for SE and CRNMB did not cross trial sequential monitoring boundaries, and the sample size did not reach the RIS, suggesting no conclusive evidence to support a statistically significant difference in reducing SE and CRNMB, and larger randomized controlled trials are warranted to further investigate these outcomes (Fig. 3 B, H).
TSA results for various outcome measures in AF patients with CKD treated with DOACs versus VKAs. A stroke, B SE, C stroke or SE, D all-cause deaths, E major bleeding, F ICH, G GIB, H CRNMB, I minor bleeding, J fetal bleeding
Efficacy analysis
Comparison of efficacy metrics for stroke, SE, and all-cause death between DOACs and VKAs groups.
4 studies with a total of 6 data sets compared stoke, resulting in a 21% reduction in incidence with DOACs, although there was no statistically significant difference ( P = 0.008, OR = 0.79, 95% CI 0.67–0.94) and no significant heterogeneity between groups ( P = 0.35, I 2 = 10%) (Fig. 4 ).
Three studies with a total of 5 data sets compared SE, resulting in a 33% reduction in the incidence of DOACs, although there was no statistically significant difference in the results ( P = 0.25, OR = 0.67, 95%CI 0.34–1.32), and there was no significant heterogeneity between the groups ( P = 0.63, I 2 = 0%) (Fig. 5 ).
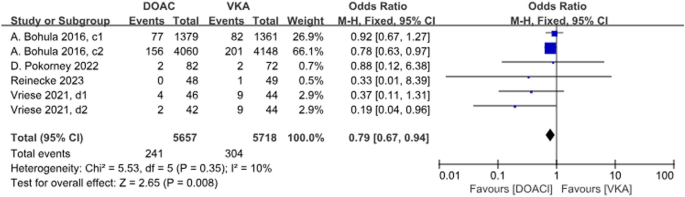
Forest plot for stroke in AF patients with CKD treated with DOACs versus VKAs. c1: CrCl 30-50 ml/min, edoxaban 30 mg daily; c2: CrCl 50-95 ml/min, edoxaban 60 mg daily; d1: CrCl<15 ml/min, rivaroxaban 10mg daily; d2: CrCl<15 ml/min, rivaroxaban and vitamin K2
There were 4 studies with a total of 8 data sets comparing stroke or SE. DOACs reduced the incidence by 25% compared with VKAs, a statistically different result ( P < 0.001, OR = 0.75, 95% CI 0.67–0.84), with no heterogeneity between groups ( P = 0.07, I 2 = 46%) (Fig. 6 ).
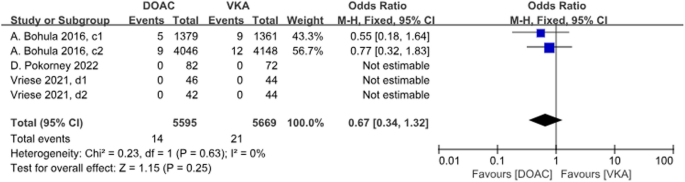
Forest plot for SE in AF patients with CKD treated with DOACs versus VKAs. c1: CrCl 30-50 ml/min, edoxaban 30 mg daily; c2: CrCl 50-95 ml/min, edoxaban 60 mg daily; d1: CrCl<15 ml/min, rivaroxaban 10mg daily; d2: CrCl<15ml/min, rivaroxaban and vitamin K2
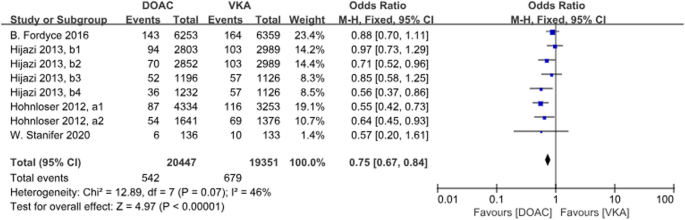
Forest plot for stroke or SE in AF patients with CKD treated with DOACs versus VKAs. a1: CrCl 51-80 ml/min, apixaban 5 mg twice daily or 2.5 mg twice daily; a2: CrCl≤50 ml/min, apixaban 5 mg twice daily or 2.5 mg twice daily; b1: CrCl 50-80 ml/min, dabigatran 110 mg twice daily; b2: CrCl 50-80 ml/min, dabigatran 150 mg twice daily; b3: CrCl 30-49 ml/min, dabigatran 110 mg twice daily; b4: CrCl 30-49 ml/min, dabigatran 150 mg twice daily
A total of 13 data sets from 7 studies compared all-cause deaths. The results were statistically significant when comparing the DOACs group to the VKAs group, with a 16% reduction in incidence with DOACs ( P = 0.0007, OR = 0.84, 95% CI 0.75–0.93), with heterogeneity between groups ( P = 0.01, I 2 = 53%) (Fig. 7 ).
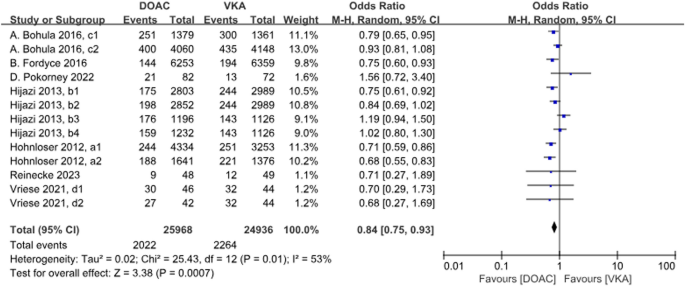
Forest plot for all-cause deaths in AF patients with CKD treated with DOACs versus VKAs. a1: CrCl 51–80 ml/min, apixaban 5 mg twice daily or 2.5 mg twice daily; a2: CrCl ≤ 50 ml/min, apixaban 5 mg twice daily or 2.5 mg twice daily; b1: CrCl 50–80 ml/min, dabigatran 110 mg twice daily; b2: CrCl 50–80 ml/min, dabigatran 150 mg twice daily; b3: CrCl 30–49 ml/min, dabigatran 110 mg twice daily; b4: CrCl 30–49 ml/min, dabigatran 150 mg twice daily; c1: CrCl 30–50 ml/min, edoxaban 30 mg daily; c2: CrCl 50–95 ml/min, edoxaban 60 mg daily; d1: CrCl<15 ml/min, rivaroxaban 10 mg daily; d2: CrCl<15 ml/min, rivaroxaban and vitamin K2
Safety analysis
Safety metrics such as major bleeding, ICH, and fatal bleeding were compared between the DOACs and VKAs groups.
A total of 9 studies with 15 data sets compared the incidence of major bleeding and the results were statistically different. DOACs compared with VKAs reduced the incidence by 24% ( P = 0.001, OR = 0.76, 95%CI 0.65–0.90), and there was heterogeneity between the groups ( P < 0.001, I 2 = 73%) (Fig. 8 ).
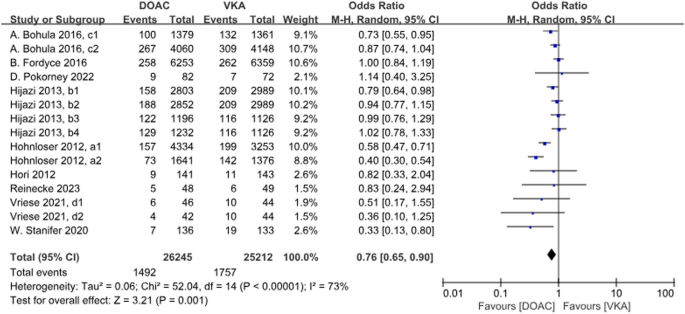
Forest plot for major bleeding in AF patients with CKD treated with DOACs versus VKAs. a1: CrCl 51–80 ml/min, apixaban 5 mg twice daily or 2.5 mg twice daily; a2: CrCl ≤ 50 ml/min, apixaban 5 mg twice daily or 2.5 mg twice daily; b1: CrCl 50–80 ml/min, dabigatran 110 mg twice daily. b2: CrCl 50–80 ml/min, dabigatran 150 mg twice daily. b3: CrCl 30–49 ml/min, dabigatran 110 mg twice daily; b4: CrCl 30–49 ml/min, dabigatran 150 mg twice daily; c1: CrCl 30–50 ml/min, edoxaban 30 mg daily; c2: CrCl 50–95 ml/min, edoxaban 60 mg daily; d1: CrCl<15 ml/min, rivaroxaban 10 mg daily; d2: CrCl<15 ml/min, rivaroxaban and Vitamin K2
A total of 6 studies with 10 data sets compared ICH incidence. There was a significant difference between the two groups, with DOACs reducing the incidence by 54% ( P <0.001, OR = 0.46, 95%CI 0.38–0.56) and no heterogeneity between groups ( P = 0.25, I 2 = 20%) (Fig. 9 ).
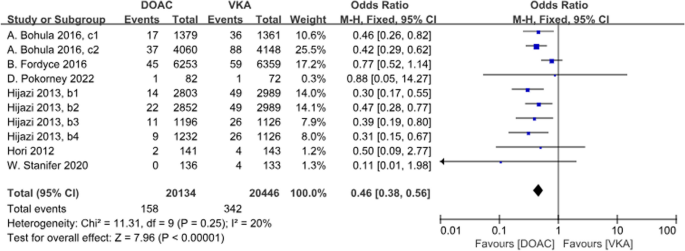
Forest plot for ICH in AF patients with CKD treated with DOACs versus VKAs. b1: CrCl 50–80 ml/min, dabigatran 110 mg twice daily. b2: CrCl 50–80 ml/min, dabigatran 150 mg twice daily. b3: CrCl 30–49 ml/min, dabigatran 110 mg twice daily; b4: CrCl 30–49 ml/min, dabigatran 150 mg twice daily; c1: CrCl 30–50 ml/min, edoxaban 30 mg daily; c2: CrCl 50–95 ml/min, edoxaban 60 mg daily
A total of 5 studies with 10 datasets compared the incidence of fatal bleeding and there was a significant difference between the two groups, with DOACs reducing the incidence by 25% ( P < 0.001, OR = 0.75, 95%CI 0.65–0.87). There was no heterogeneity between the groups ( P = 0.44, I 2 = 0%) (Fig. 10 ).
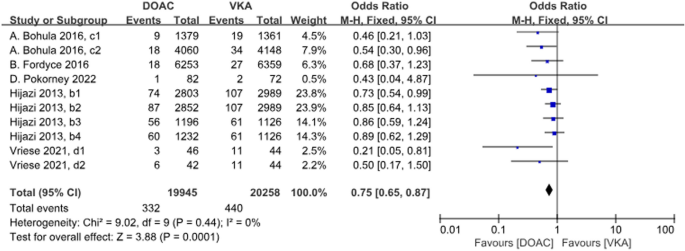
Forest plot for fetal bleeding in AF patients with CKD treated with DOACs versus VKAs. b1: CrCl 50–80 ml/min, dabigatran 110 mg twice daily. b2: CrCl 50–80 ml/min, dabigatran 150 mg twice daily. b3: CrCl 30–49 ml/min, dabigatran 110 mg twice daily; b4: CrCl 30–49 ml/min, dabigatran 150 mg twice daily; c1: CrCl 30–50 ml/min, edoxaban 30 mg daily; c2: CrCl 50–95 ml/min, edoxaban 60 mg daily; d1: CrCl<15 ml/min, rivaroxaban 10 mg daily; d2: CrCl<15 ml/min, rivaroxaban and Vitamin K2
A total of 4 studies with 6 data sets compared GIB, with no significant difference between the two groups ( P = 0.01, OR = 1.30, 95% CI 1.07–1.58) and no heterogeneity between the groups ( P = 0.35, I 2 = 10%) (Fig. 11 ).
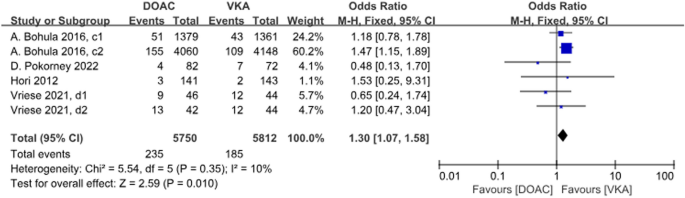
Forest plot for GIB AF patients with CKD treated with DOACs versus VKAs. c1: CrCl 30–50 ml/min, edoxaban 30 mg daily; c2: CrCl 50–95 ml/min, edoxaban 60 mg daily; d1: CrCl<15 ml/min, rivaroxaban 10 mg daily; d2: CrCl<15 ml/min, rivaroxaban and Vitamin K2
A total of 3 studies with 3 data sets compared CRNMB. There was no significant difference between the two groups ( P = 0.83, OR = 0.99, 95% CI 0.88–1.11) and no heterogeneity between groups ( P = 0.80, I 2 = 0%). Forest plot results (Fig. 12 ).
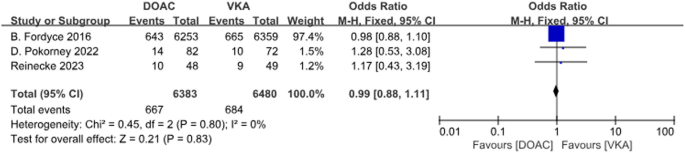
Forest plot for CRNMB in AF patients with CKD treated with DOACs versus VKAs
A total of 2 studies with 4 sets of data compared minor bleeding. DOACs reduced the incidence by 13%, statistically different between the two groups ( P = 0.03, OR = 0.87, 95% CI 0.77–0.99), with no heterogeneity between the groups ( P = 0.39, I 2 = 0%) (Fig. 13 ).
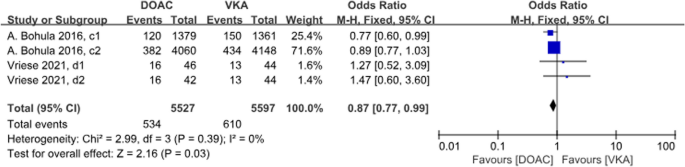
Forest plot for minor bleeding in AF patients with CKD treated with DOACs versus VKAs. c1: CrCl 30-50 ml/min, edoxaban 30 mg daily; c2: CrCl 50-95 ml/min, edoxaban 60 mg daily; d1: CrCl<15 ml/min, rivaroxaban 10mg daily; d2: CrCl<15 ml/min, rivaroxaban and Vitamin K2
Sensitivity analysis
There was heterogeneity in all-cause deaths. Further sensitivity analyses showed a decrease in I 2 from 53 to 32% after the removal of the Hijazi et al. 2014c group [ 21 ], indicating that the CrCl 30–50 ml/min dabigatran 110 mg group was a possible source of heterogeneity in the all-cause deaths.
There was heterogeneity in the incidence of major bleeding. Further sensitivity analyses showed a decrease in I 2 from 73 to 55% after removal in the Hohnloser et al. 2012, a1 group [ 19 ], from 73 to 68% after removal in the Hohnloser et al. 2012, a2 group [ 19 ], and from 73 to 19% after simultaneous removal in both groups, which suggests that there is a possible source of heterogeneity in major bleeding in the apixaban group.

Subgroup analysis
For AF patients with CKD, subgroup analyses performed differently for DOACs showed that rivaroxaban and apixaban were superior in reducing all-cause deaths compared with VKAs, with rivaroxaban reducing the incidence by 25% ( P = 0.01, OR = 0.75, 95% CI 0.60–0.93) and apixaban reducing the incidence by 29% ( P < 0.001, OR = 0.71, 95% CI 0.62–0.81). There was no statistical difference between edoxaban and dabigatran ( P = 0.09; P = 0.44). There was heterogeneity between groups ( P = 0.1, I 2 = 51.7%), suggesting that different DOACs may be a source of heterogeneity (Figure S 1 ).
Subgroup analyses of AF patients with kidney failure differing for DOACs showed that neither rivaroxaban nor apixaban reduced the incidence of all-cause deaths compared with VKAs (Figure S 2 ).
Subgroup analysis showed that DOACs reduced all-cause deaths in patients with CrCl 50–95 ml/min with heterogeneity between groups ( P <0.001, OR = 0.82, 95% CI 0.75–0.90, I 2 = 51%), and did not reduce all-cause deaths in patients with CrCl < 30 ml/min. There was no heterogeneity between groups ( P = 0.69, I 2 = 0%) (Figure S 3 ).
For AF patients with CKD, subgroup analyses differing according to DOACs showed that apixaban significantly reduced the incidence of major bleeding compared with VKAs ( P < 0.001, OR = 0.47, 95% CI 0.34–0.64); edoxaban reduced the incidence of major bleeding by 18% ( P = 0.02, OR = 0.82, 95% CI 0.70–0.97), and no significant difference between rivaroxaban and dabigatran ( P = 0.95; P = 0.14). Heterogeneity existed between the different DOACs study groups ( P = 0.0004, I 2 = 83.7%), suggesting that the different DOACs may be the source of the heterogeneity in major bleeding (Figure S 4 ).
Subgroup analysis of AF patients with Kidney failure with different DOACs showed that neither rivaroxaban nor apixaban reduced the incidence of major bleeding compared with VKAs (Figure S 5 ).
Subgroup analyses based on renal function levels showed that, in terms of major bleeding, compared with VKAs, DOACs reduced the incidence by 21% in patients with CrCl 50–95 ml/min ( P = 0.02, OR = 0.79, 95% CI 0.64–0.96); by 67% in patients with CrCl 15–29 ml/min (OR 0.33, 95% CI 0.13–0.80). There was no significant difference in patients with CrCl 30–49 ml/min and CrCl < 15 ml/min ( P = 0.28; P = 0.18). There was no heterogeneity between groups for different renal function subgroups ( P = 0.20, I 2 = 34.8%) (Figure S 6 ).
The current study shows that in patients with AF combined with CKD, DOACs may be able to reduce the incidence of stroke and SE as well as all-cause deaths compared with VKAs in terms of efficacy. In terms of safety, it may reduce the incidence of major bleeding, ICH, fatal bleeding, and minor bleeding, and may not reduce the incidence of GIB and CRNMB. Subgroup analyses showed that [ 1 ] in AF patients with comorbid CKD (90 ml/min> Crcl≥15 ml/min), apixaban and rivaroxaban reduced all-cause deaths, apixaban and dabigatran reduced the incidence of stroke or SE, apixaban and edoxaban reduced the incidence of major bleeding, and edoxaban and dabigatran reduced ICH and fatal bleeding, compared to VKAs [ 2 ]. In AF patients with comorbid kidney failure, there were no significant differences in the efficacy and safety of rivaroxaban compared with VKAs, except for an advantage in reducing stroke and fatal bleeding, while there were no significant differences in the efficacy and safety of apixaban. Upon excluding direct thrombin inhibitors, specifically dabigatran, the analysis revealed that inhibitors targeting thrombin and factor X can further diminish the occurrence of stroke or systemic embolism, all-cause mortality, massive bleeding, and fatal bleeding in patients with CKD.
In this study, we found that apixaban and dabigatran were superior to VKAs in reducing stroke and SE in patients with CKD and that there was no significant difference between rivaroxaban and edoxaban compared with VKAs. Apixaban and edoxaban reduced the risk of major bleeding significantly compared to VKAs, which is similar to the findings of Feldberg et al. [ 9 ], but when compared to VKAs, edoxaban and dabigatran had a significant advantage in reducing ICH and fatal bleeding, and rivaroxaban and apixaban had a significant advantage in reducing all-cause deaths, which was not reported by Feldberg et al. The study conducted by Feldberg et al. included only six RCTs, evaluated only stroke, SE, and hemorrhage, and included aspirin in addition to VKAs in the control group. In contrast, this paper uses 9 RCTs, all of which were conducted in patients with AF, and all of which had VKAs including warfarin as the control drug, making the results more comparable. Comparison with the analysis of Kuno et al. [ 11 ] in terms of efficacy. In terms of safety, Kuno et al. concluded that the use of anticoagulants increased the risk of bleeding in dialysis patients, whereas our study showed a similar risk of bleeding. The reason for this may be that Kuno et al. included 16 observational studies, which considered dialysis patients excluded from RCT trials, only 2 studies out of 16 combined AF, and there was a high degree of heterogeneity in the studies. In contrast, 3 RTC trials were included in our study of dialysis patients. RCT trials have strict nadir criteria and the level of evidence for their results is higher than that of observational studies. A study by Chen et al. showed that DOACs was significantly more effective and safer than warfarin in patients with CKD combined with AF [ 12 ]. The study by Chen et al. included 6 RCTs and 19 observational studies; there were no RCTs for Kidney failure, and 2 of these studies reported on venous thromboembolism populations non-AF populations. Whereas our article used all RCT studies with a high level of evidence, including 3 RCTs with a CrCl < 15 ml/min. Secondly, Chen et al. analyzed stroke, SE, and VTE together as the same efficacy outcome, which may introduce bias due to the differences in pathomechanisms of SE and VTE. Chen et al. did not conduct further subgroup analysis based on renal function staging and failed to analyze the effectiveness and safety of kidney failure combined with AF patients. The preceding meta-analysis, concentrating on end-stage patients, aligns with our study’s findings [ 13 ]. Li et al.‘s investigation encompassed one randomized controlled trial and five observational studies. Despite our inclusion of three randomized controlled trials, it is noteworthy that due to recruitment challenges in some of these trials, the level of evidence in our study did not surpass that of Li et al.‘s. Both investigations illustrated that the use of DOACs, namely rivaroxaban or apixaban, in patients with kidney failure combined with AF yielded comparable efficacy and safety outcomes to those observed with VKAs. A meta-analysis concerning AF patients with kidney failure, comparing DOACs and warfarin, utilized three RCTs [ 28 ]; however, one study had missing data [ 24 ]. Their outcome measures focused solely on major bleeding, systemic embolism, and cardiovascular death, lacking the depth of analysis found in our article. Additionally, our study included a subgroup analysis of patients with CrCl between 15 ml/min and 90 ml/min, augmenting overall comprehensiveness and persuasiveness. Another meta-analysis on DOACs and warfarin in AF patients with CKD did not specifically explore kidney failure patients with distinct physiological and functional changes [ 29 ]. This analysis relied on five pre-2016 RCTs and 14 observational studies. In contrast, a network meta-analysis concluded that DOACs out performed warfarin in preventing thromboembolic events and reducing bleeding risk in AF patients with mild to moderate kidney disease [ 30 ]. However, the study acknowledged a limitation in the strength of evidence, precluding a definitive preference for a particular DOACs. Conversely, our study suggests an elevated risk of bleeding without significant benefits from OACs in dialysis patients with AF. In comparison to previous meta-analyses on the efficacy and safety of DOACs and VKAs in AF patients with CKD. This article incorporates nine RCTs with robust evidence levels, encompassing all DOACs. Among these, three RCTs were specifically scrutinized concerning patients with kidney failure, with two employing apixaban and one utilizing rivaroxaban. The use of warfarin in dialysis patients is associated with an increased risk of bleeding, even when maintaining an INR within the normal range, posing challenges to its practical application. Hemodialysis patients typically undergo systemic heparin anticoagulation during their sessions, a variable inconsistently considered in studies examining bleeding complications related to both VKAs and DOACs in this population. As a result, research in this domain has consistently lagged behind. This investigation disclosed no significant disparity in efficacy and safety between VKAs and DOACs for patients with kidney failure and concurrent AF. This finding introduces a novel and more convenient option for anticoagulant therapy in this patient demographic.Our study showed that increased DOACs efficacy and reduced side effects were associated with renal clearance of the drug. Dabigatran has the greatest dependence on renal function due to renal excretion as a prototype [ 31 ], apixaban has the lowest renal clearance renal impairment has less effect on its excretion [ 32 ], and renal function is moderately affected by rivaroxaban [ 33 ]. Compared with warfarin, DOACs has a protective effect on renal function [ 34 ]. Our study shows that apixaban may be the best choice when compared to VKAs in the case of an adjusted degree of renal impairment.
Fordyce et al.’s study revealed that the incidence of major bleeding attributed to rivaroxaban at doses of 10 mg daily and 15–20 mg daily in patients with a Crcl of 30–49 ml/min was 6.38% and 4.12%, respectively, with no statistically significant difference observed. In individuals with kidney failure, the incidence of major bleeding increased to 13.04% for those receiving rivaroxaban at a daily dose of 10 mg, and when combined with vitamin K2, the incidence was 9.52%. This underscores the heightened bleeding risk associated with kidney failure. Among patients with a Crcl of 25–30 ml/min receiving apixaban, the incidence of major bleeding for doses of 2.5 mg bid compared to 5 mg bid was 3.42% and 4.39%, respectively, while the incidence of major or clinically relevant non-major bleeding was 4.28% and 7.35%, respectively. The study conducted by A. Mavrakanas et al. concluded that a dosage of apixaban at 2.5 mg bid is the appropriate choice for patients with kidney failure. This suggests a correlation between reduced renal function levels and the necessity for dose reduction.
VKAs is metabolized in the liver by cytochrome P450s and excreted as a metabolite via the kidneys, whereas all DOACs have varying degrees of prototypic drug excretion via the kidneys, and moderate-to-severe renal insufficiency has a significant effect on their pharmacokinetics. Therefore, dose adjustment is required for use in patients with CKD. However VKAs leads to the risk of calcification of the renal arteries, calcification of the aortic valve, and decreased bone calcium [ 35 ]. Calcification of small arteries may lead to an increased incidence of ischemic stroke [ 36 ]. There is no specific treatment for the defense of calcification due to warfarin anticoagulation, which has a poor prognosis and high morbidity and mortality once it occurs. Warfarin anticoagulation therapy is a risk factor for the development of calcification defense [ 37 ]. The US guidelines recommend anticoagulation with warfarin in kidney failure patients with CrCl < 15 ml/min [ 38 ]. The nephrology guidelines also recommend warfarin as the drug of choice for anticoagulation in kidney failure patients [ 39 ]. However, European guidelines do not recommend DOACs anticoagulation in kidney failure patients with CrCl < 15 ml/min [ 40 ]. Previous meta-studies also showed that warfarin for AF patients undergoing dialysis did not show significant benefits or harms [ 13 , 41 ]. In contrast, our study showed at least similar efficacy and safety when comparing DOACs and warfarin in patients with CrCl < 15 ml/min.
For AF patients with comorbid CKD, since thrombopoietin mRNA can be expressed in the kidneys, decreased renal function can lead to impaired platelet production and an increased risk of bleeding [ 42 ]. Inadequate anticoagulation therapy exists for this group of patients, especially dialysis patients, due to the concern that anticoagulant use may lead to an increased incidence of bleeding [ 43 ].
This study demonstrates that the use of DOACs improves safety and reduces the incidence of major bleeding, fatal bleeding, and ICH in patients with CKD and concurrent AF, as compared to VKAs therapy. For patients with kidney failure combined with AF, rivaroxaban and apixaban showed at least similar effectiveness and safety when compared to warfarin. This may inform drug selection for oral anticoagulation in patients with CKD combined with AF. Moreover, this article employed nine randomized controlled trials (RCTs), providing a higher level of evidence compared to alternative meta-analyses. Notably, three RCT studies were specifically dedicated to patients with kidney failure, thereby addressing a gap in prior research.
This study also has some limitations: (1) The method of CrCl calculation was not consistent across studies, with 6 studies using the Cockcroft-Gault formula and two using Hemodialysis. (2) Lack of data on the DOACs dose-adjustment regimen, in addition to the level of renal function, the dose of drug use may be affected by factors such as body weight, age, etc., so further studies are needed to investigate the pharmacokinetics of DOACs use in this population. (3) Failure to perform subgroup analysis based on DOACs high and low dose groups.
Compared with VKAs, DOACs improves the efficacy and safety of anticoagulation in patients with CKD combined with AF. In patients with atrial fibrillation combined with Kidney failure DOACs has at least similar effectiveness and safety when compared with VKAs.
Availability of data and materials
All data relevant to the study are included in the article or uploaded as supplementary information.
Soliman EZ, Prineas RJ, Go AS, et al. Chronic kidney disease and prevalent atrial fibrillation: the chronic renal insufficiency cohort (CRIC). Am Heart J. 2010;159:1102–7.
Article PubMed PubMed Central Google Scholar
Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–962.
Article PubMed Google Scholar
Levy JH, Spyropoulos AC, Samama CM, et al. Direct oral anticoagulants: new drugs and new concepts. JACC Cardiovasc Interv. 2014;7(12):1333–51.
Article CAS PubMed Google Scholar
Noll G, Noll S, Huerlimann D. Direct oral anticoagulants in atrial fibrillation. Semin Hematol. 2014;51(2):139–46.
Wetmore JB, Roetker NS, Yan H, et al. Direct-acting oral anticoagulants versus warfarin in medicare patients with chronic kidney disease and atrial fibrillation. Stroke. 2020;51(8):2364–73.
Paravattil B, Elewa H. Approaches to direct oral anticoagulant selection in practice. J Cardiovasc Pharmacol Therap. 2019;24(2):95–102.
Article CAS Google Scholar
Rafflenbeul E, Mueller-Ehmsen J. Vitamin-K-antagonists: is their prescription really a art defect. Today? Internist. 2017;58(1):90–9.
Park H, Yu HT, Kim TH, et al. Oral anticoagulation therapy in atrial fibrillation patients with advanced chronic kidney disease: CODE-AF registry. Yonsei Med J. 2023;64(1):18–24.
Feldberg J, Patel P, Farrell A, et al. A systematic review of direct oral anticoagulant use in chronic kidney disease and dialysis patients with atrial fibrillation. Nephrol Dial Transpl. 2019;34(2):265–77.
Ha JT, Neuen BL, Cheng LP, et al. Benefits and harms of oral anticoagulant therapy in chronicn kidney disease a systematic review and meta-analysis. Ann Intern Med. 2019;171(3):181–9.
Kuno T, Takagi H, Ando T, et al. Oral anticoagulation for patients with atrial fibrillation on long-term hemodialysis. J Am Coll Cardiol. 2020;75(3):273–85.
Chen HY, Ou SH, Huang CW, et al. Efficacy and safety of direct oral anticoagulants vs warfarin in patients with chronic kidney disease and dialysis patients: a systematic review and meta-analysis. Clin Drug Investig. 2021;41(4):341–51.
Li W, Zhou Y, Chen S, et al. Use of non-vitamin K antagonists oral anticoagulants in atrial fibrillation patients on dialysis. Front Cardiovasc Med. 2022;9:9.
Google Scholar
KDIGO. Clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2022;2022(102):S1-s127.
Hahn K, Lamparter M. Prescription of DOACs in patients with atrial fibrillation at different stages of renal insufficiency. Adv Ther. 2023;40(10):4264–81.
Article CAS PubMed PubMed Central Google Scholar
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339: b2700.
Higgins JPT, Page MJ, Sterne J. RoB 2.0: a revised Cochrane risk-of-bias tool for randomized trials. Oxford: The Cochrane Collaboration; 2016.
Fox KA, Piccini JP, Wojdyla D, et al. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J. 2011;32(19):2387–94.
Hohnloser SH, Hijazi Z, Thomas L, Hanna M, et al. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J. 2012;33(22):2821–30.
Hori M, Matsumoto M, Tanahashi N, et al. Safety and Efficacy of Adjusted Dose of Rivaroxaban in Japanese patients with non-valvular atrial fibrillation: Subanalysis of J-ROCKET AF for patients with moderate renal impairment. Circ J. 2013;77(3):632–8.
Hijazi Z, Hohnloser SH, Oldgren J, et al. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: a RE-LY (Randomized evaluation of long-term anticoagulation therapy) trial analysis. Circulation. 2014;129(9):961–70.
Christopher B, Hellkamp AS, Lokhnygina Y, et al. On-Treatment outcomes in patients with worsening renal function with Rivaroxaban compared with warfarin: insights from ROCKET AF. Circulation. 2016;134(1):37–47.
Article Google Scholar
Bohula EA, Giugliano RP, Ruff CT, et al. Impact of renal function on outcomes with Edoxaban in the ENGAGE AF-TIMI 48. Trial Circulation. 2016;134(1):24–36.
Vriese AS, Caluwé R, Van Der Meersch H, et al. Safety and efficacy of vitamin K antagonists versus rivaroxaban in hemodialysis patients with atrial fibrillation: a multicenter randomized controlled trial. J Am Soc Nephrology. 2021;32(6):1474–83.
John W, Stanife, Stanifer JW, Pokorney SD, et al. Apixaban versus warfarin in patients with atrial fibrillation and advanced chronic kidney disease. Circulation. 2020;141(17):1384–92.
Sean D, Pokorney, Pokorney SD, Chertow GM, et al. Apixaban for patients with atrial fibrillation on hemodialysis: a multicenter randomized controlled trial. Circulation. 2022;146(23):1735–45.
Reinecke H, Engelbertz C, Bauersachs R, et al. A randomized controlled trial comparing apixaban with the vitamin K antagonist phenprocoumon in patients on chronic hemodialysis: the AXADIA-AFNET 8 study. Circulation. 2023;147(4):296–309.
Mapili JA, Lim LC, Velando BM, et al. The safety and efficacy of direct oral anticoagulants among chronic kidney disease patients on dialysis with non-valvular atrial fibrillation: a meta-analysis. Front Cardiovasc Med. 2023;10:1261183.
Rhee TM, Lee SR, Choi EK. Efficacy and safety of oral anticoagulants for atrial fibrillation patients with chronic kidney disease: a systematic review and meta-analysis. Front Cardiovasc Med. 2022;9:885548.
Xiaole Su B, Wang YL, et al. Oral anticoagulant agents in patients with atrial fibrillation and CKD: a systematic review and pairwise network meta-analysis. Am J Kidney Dis. 2021;78(5):678–89.
Stangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet. 2008;47(5):285–95.
Chang M, Yu Z, Shenker A, et al. Effect of renal impairment on the pharmacokinetics, pharmacodynamics, and safety of apixaban. J Clin Pharmacol. 2016;56(5):637–45.
Grandone E, Aucella F, Barcellona D, et al. Position paper on the safety/efficacy profile of direct oral anticoagulants in patients with chronic kidney disease. Consensus document from the SIN, FCSA and SISET. Blood Transfus. 2020;18(6):478–85.
PubMed PubMed Central Google Scholar
Lullo LD, Mariani MV, Ronco C, et al. Atrial fibrillation and anticoagulant treatment in end-stage renal disease patients: where do we stand? Cardiorenal Med. 2022;12(4):131–40.
Krüger T, Westenfeld R, Schurgers LJ, et al. Coagulation meets calcification: the vitamin K system. Int J Artif Organs. 2009;32:67–74.
Shah M, Avgil Tsadok M, Jackevicius CA, et al. Warfarin use and the risk for stroke and bleeding in patients with atrial fibrillation undergoing. dialysis Circulation. 2014;129:1196–203.
Guzmán UV, De La Cueva P, Verde E, et al. Calciphylaxis: fatal complication of cardiometabolic syndrome in patients with end stage kidney disease. Nefrologia. 2008;28(1):32–6.
January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of cardiology/american heart association task force on practice guidelines and the heart rhythm society. J Am Coll Cardiol. 2014;64:2246–80.
Wanner C, Herzog CA, Turakhia MP. Chronic kidney disease and arrhythmias: highlights from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 2018;94:231–4.
Steffel J, Verhamme P, Potpara TS, et al. The (2018). European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39:1330–93.
Harel Z, Chertow GM, Shah PS, et al. Warfarin and the risk of stroke and bleeding in patients with atrial fibrillation receiving dialysis. A systematic review and meta-analysis. Can J Cardiol. 2017;33:737–46.
Lambert MP. Platelets in liver and renal disease. Hematology Am Soc Hematol Educ Program. 2016;2016(1):251–5.
Piccini JP, Hernandez AF, Zhao X, et al. Quality of care for atrial fibrillation among patients hospitalized for heart failure. J Am Coll Cardiol. 2009;54(14):1280–9.
Download references
Code availability
Not applicable.
No specific funding was necessary for the conduct of this study.
Author information
Yaodi Li, Shuyi Wu and Jintuo Zhou contributed equally to this work.
Authors and Affiliations
Department of Pharmacy, Fujian Maternity and Child Health Hospital College of Clinical Medicine for Obstetrics & Gynecology and Pediatrics, Fujian Medical University, #18 Daoshan Road, Fuzhou, 350001, China
Yaodi Li, Shuyi Wu, Jintuo Zhou & Jinhua Zhang
You can also search for this author in PubMed Google Scholar
Contributions
JHZ initiated the study. YDL and SYW performed data extraction and analyses. YDL drafted the first version of the manuscript. YDL, JTZ and SYW critically reviewed the manuscript and revised it. YDL, JTZ and SYW contributed to the analysis of data and provided critical revisions. JHZ, YDL, and SYW contributed to the conception and design, and they provided critical revisions of the paper for crucial intellectual content. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Jinhua Zhang .
Ethics declarations
Ethics approval and consent to participate.
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Li, Y., Wu, S., Zhou, J. et al. Efficacy and safety of direct oral anticoagulants in patients with atrial fibrillation combined with chronic kidney disease: a systematic review and meta-analysis. Thrombosis J 22 , 40 (2024). https://doi.org/10.1186/s12959-024-00608-5
Download citation
Received : 25 October 2023
Accepted : 15 April 2024
Published : 29 April 2024
DOI : https://doi.org/10.1186/s12959-024-00608-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Direct oral anticoagulants
- Chronic kidney disease
- Kidney failure
- Atrial fibrillation
- meta-analysis
Thrombosis Journal
ISSN: 1477-9560
- Submission enquiries: [email protected]
Diet and Kidney Function: a Literature Review
- Nutrition and Hypertension (T Mori, Section Editor)
- Open access
- Published: 03 February 2020
- Volume 22 , article number 14 , ( 2020 )
Cite this article
You have full access to this open access article

- A. C. van Westing 1 ,
- L. K. Küpers 1 &
- J. M. Geleijnse 1
13k Accesses
40 Citations
103 Altmetric
31 Mentions
Explore all metrics
Purpose of Review
The burden of chronic kidney disease (CKD) is increasing worldwide. For CKD prevention, it is important to gain insight in commonly consumed foods and beverages in relation to kidney function.
Recent Findings
We included 21 papers of prospective cohort studies with 3–24 years of follow-up. We focused on meat, fish, dairy, vegetables, fruit, coffee, tea, soft drinks, and dietary patterns. There was convincing evidence that a healthy dietary pattern may lower CKD risk. Plant-based foods, coffee, and dairy may be beneficial. Unhealthy diets and their components, such as red (processed) meat and sugar-sweetened beverages, may promote kidney function loss. For other foods and beverages, associations with CKD were neutral and/or the number of studies was too limited to draw conclusions.
Healthy dietary patterns are associated with a lower risk of CKD. More research is needed into the effects of specific food groups and beverages on kidney function.
Similar content being viewed by others

Dietary patterns and chronic kidney disease risk: a systematic review and updated meta-analysis of observational studies
The western diet and chronic kidney disease.

Food Patterns are Associated with Likelihood of CKD in US Adults
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is a major public health burden [ 1 , 2 ], with a global prevalence of ~ 11% in the general adult population [ 1 ]. If left untreated, CKD slowly progresses to end-stage renal disease, which requires dialysis or kidney transplant [ 2 , 3 ]. CKD is bidirectionally associated with cardiovascular diseases (CVD) [ 4 , 5 ]. Hypertension [ 6 ] and type 2 diabetes mellitus (T2DM) [ 7 , 8 ] are independent risk factors for CKD [ 6 , 7 ], and their global prevalences are increasing [ 9 , 10 ], which will likely impact CKD. Worldwide, a 31.7% increase of CKD mortality was observed over the last decade [ 11 ].
Lifestyle factors, including smoking [ 12 ], alcohol use [ 13 ], and physical inactivity [ 14 ], could promote CKD. Apart from that, there is increasing scientific interest in the potential role of diet [ 15 , 16 ]. High salt intake is an established risk factor for kidney function decline [ 17 , 18 ], mainly through its adverse effect on blood pressure and vascular health [ 19 , 20 , 21 ]. Less is known about other dietary factors. Therefore, we reviewed the current evidence on foods, beverages, and overall dietary quality in relation to the risk of incident CKD using data from prospective cohort studies.
We performed a comprehensive search in PubMed of papers published until August 2019 describing prospective cohort studies, supplemented by manual searches of reference lists from appropriate studies. The review is based on prospective cohort studies with at least 3 years of follow-up that reported on the relation between food groups, beverages, and dietary patterns and kidney function in populations free from CKD (defined as mean estimated glomerular filtration rate (eGFR) > 60 ml/min/1.73 m 2 ).
Foods of interest were red (processed) meat, poultry, fish, dairy, vegetables, legumes, nuts, and fruits. Beverages included coffee, tea, sugar-sweetened beverages (SSBs), and diet beverages. Dietary patterns included adherence to the Dietary Approach to Stop Hypertension (DASH) diet, Mediterranean diet, and other healthy dietary patterns. Unhealthy diets were high fat, high sugar diets, and diets with a high acid load.
Concerning kidney function, we selected studies with data on the eGFR, derived from the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [ 22 , 23 ] and Modification of Diet in Renal Disease (MDRD) [ 24 ].
Reasons for exclusion of articles were studies with (1) follow-up less than 3 years, (2) study design other than prospective cohort study, (3) study population with T2DM and analgesic use, (4) no full-text available, and (5) focus on end-stage renal disease. The selection process is shown in eFig. 1 .
From selected papers, we extracted data on population characteristics, study design, intakes of foods and/or beverages, kidney function outcomes, risk estimates for diet-kidney function associations, and potential confounders.
The primary outcome for this review was “incident CKD” based on eGFR cutoff criteria, described in eTable 1 . Associations between foods, beverages, and incident CKD in different studies were expressed as odds ratios (OR), obtained from logistic regression analysis, or hazard ratios (HR), obtained from Cox proportional hazard analysis with corresponding 95% confidence intervals (CI). In this review, OR and HR are both denoted as relative risks (RRs). Continuous associations between food groups, beverages, and change in eGFR are expressed as beta regression coefficients, obtained from multivariable linear regression.
RRs and betas from fully adjusted models are reported in tables with potential confounders. When these models included possible intermediates (i.e., factors could play a role in the biological pathway), risk estimates from less adjusted models are given. Two-sided P values < 0.05 for risk estimates were considered statistically significant.
An overview of studies of foods, beverages, and dietary patterns and their associations with incident CKD is presented in eTable 1 . Studies that focused on eGFR change, albuminuria, or hyperuricemia are described in eTable 2 and eTable 3 . Graphical displays of the point estimates with 95% CI related to incident CKD using forest plots are presented in Figs. 1 , 2 , and 3 .

Forest plot for associations between commonly consumed foods and incident chronic kidney disease
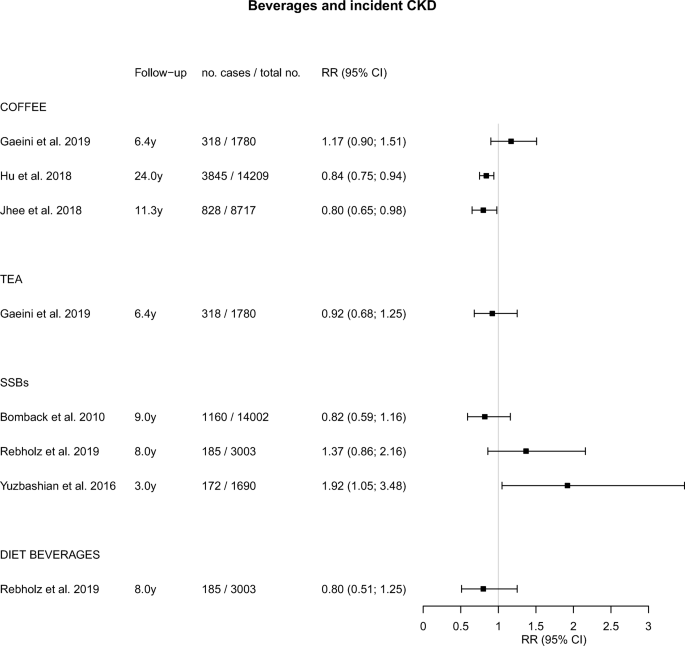
Forest plot for associations between commonly consumed beverages and incident chronic kidney disease. SSB, sugar-sweetened beverages
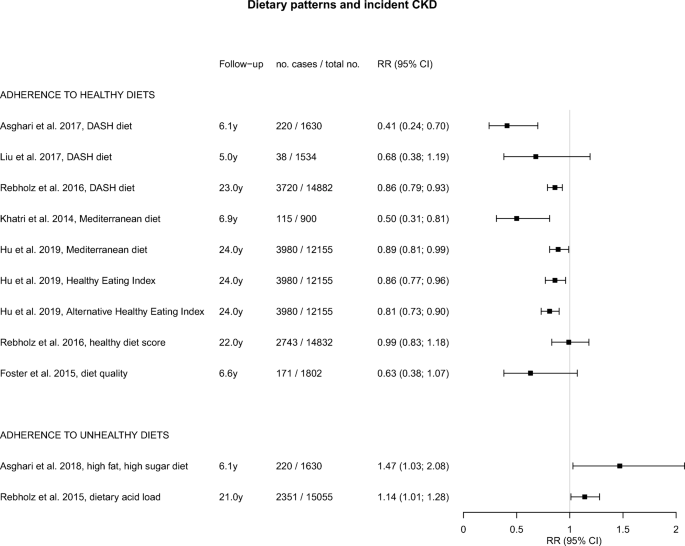
Forest plot for associations between dietary patterns and incident chronic kidney disease. DASH, Dietary Approach to Stop Hypertension
Two studies evaluated the consumption of red (processed) meat and poultry in relation to incident CKD (Fig. 1 ) [ 25 ••, 26 ••]. Red meat intake in these studies varied between 0.17 to 0.34 servings per day (low intake) and 1.15 to 2.52 servings per day (high intake). In the Atherosclerosis Risk in Communities (ARIC) study of ~ 12,000 US participants with 23 years of follow-up, a total of 2632 participants developed CKD [ 25 ••]. In this population, the HR for high versus low intake of red meat and CKD risk was 1.19 (95% CI, 1.03; 1.36; Fig. 1 ) [ 25 ••]. In a study of 4881 Iranian participants followed for 3 years, 613 participants developed CKD with an OR of 1.73 (95% CI, 1.33; 2.24) for high versus low red meat intake (Fig. 1 ) [ 26 ••]. Findings for processed meat were similar to those for red meat in both studies, and no significant associations with kidney function were found for poultry (Fig. 1 ) [ 25 ••].
Two studies evaluated the association between fish consumption and incident CKD (Fig. 1 ) [ 25 ••, 27 ]. The Strong Heart Study among American Indians followed 2261 participants for 5.4 years of whom 4% developed CKD. Fish intake was analyzed in four categories ranging from 0 to > 15 g per day [ 27 ]. No significant associations were found with an OR of 1.46 (95% CI, 0.65; 3.26) for high versus zero fish intake [ 27 ]. In the ARIC study [ 25 ••], fish intake was analyzed in quintiles ranging from 0.07 to 0.64 servings per day. A borderline significant HR of 0.89 (95% CI, 0.78; 1.01) was found in the upper versus lower quintile of intake (Fig. 1 ) [ 25 ••].
Dairy consumption and incident CKD were examined in the ARIC study among US individuals (Fig. 1 ) [ 25 ••]. Intake of low-fat dairy ranged from 0.00 to 2.04 servings per day and intake of high-fat dairy from 0.13 to 1.61 servings per day [ 25 ••]. A significantly lower risk of CKD was found for low-fat dairy intake, with a HR of 0.75 (95% CI, 0.65; 0.85) for high versus low intake. High-fat dairy intake was also inversely associated with CKD, albeit non-significant (Fig. 1 ) [ 25 ••].
We found 3 studies of vegetable intake and CKD risk (Fig. 1 ) [ 28 •, 29 ••, 30 ]. In a study of 1780 Iranians from the Tehran Lipid Glucose Study (TLGS), followed for 6 years, 319 participants developed CKD [ 28 •]. Allium vegetable intake was analyzed in tertiles ranging from 1 to 39 g per week [ 28 •]. A significant inverse association with CKD risk was found, with a HR of 0.68 (95% CI, 0.48; 0.98) in the upper versus lower tertiles of intake [ 28 •]. In 9229 participants from the Korean Genome and Epidemiology Study, 1741 incident CKD cases were reported during 8.2 years [ 29 ••]. Intake of non-fermented vegetables ranged from 49 to 222 g per day, and intake of fermented vegetables from 164 to 227 g per day [ 29 ••]. Non-fermented vegetables were inversely related to CKD risk, with a HR of 0.86 (95% CI, 0.76; 0.98) for high versus low intake (Fig. 1 ) [ 29 ••]. For fermented vegetables, an inverse but non-significant association was found (Fig. 1 ) [ 29 ••]. In the abovementioned TLGS, nitrate-containing vegetable intake ranged from 146 to 428 g per day [ 30 ]. No significant association with CKD risk was found after 3 years of follow-up (Fig. 1 ) [ 30 ].
Legumes and Nuts
In the ARIC study with 23 years of follow-up, legume intake ranged from 0.07 to 0.68 servings per day and nut intake ranged from 0.03 to 0.86 servings per day [ 25 ••]. Both legumes and nuts were significantly associated with lower risks of CKD, with HRs of 0.83 (95% CI, 0.72; 0.95) and 0.81 (95% CI, 0.72; 0.92) for high versus low intakes, respectively (Fig. 1 ) [ 25 ••].
One study in 9229 South Koreans, followed for 8.2 years, reported on fruit consumption and incident CKD [ 29 ••]. Fruit intake ranged from 143 to 345 g per day and showed no association with incident CKD (HR of 1.00) (Fig. 1 ) [ 29 ••].
Three studies examined coffee consumption and incident CKD (Fig. 2 ) [ 31 , 32 , 33 ]. The Iranian TLGS compared coffee drinkers (median intake 8.3 ml per day) to non-drinkers [ 31 ]. In the ARIC study in the USA [ 32 ] and the Korean Genome and Epidemiology Study in South Korea [ 33 ], those drinking at least 3 cups [ 32 ] or at least 2 cups [ 33 ] were compared with non-coffee drinkers. In the Iranian study, a non-significant direct association between coffee and CKD was found [ 31 ], whereas in the US and Korean studies, significant inverse associations were observed in those with higher coffee intakes, with HR of 0.84 [ 32 ] and 0.80 [ 33 ], respectively (Fig. 2 ).
The Iranian TLGS also reported on tea consumption, ranging from < 250 ml (low intake) to > 750 ml per day (high intake) (Fig. 2 ) [ 31 ]. Unfortunately, data on the type of tea and its preparation method was not collected [ 31 ]. However, a previous study reported that in Iran, black tea is often consumed [ 34 ] with added sweets and sugar, including a variety of additives [ 31 ]. No significant association with incident CKD was found (Fig. 2 ) [ 31 ].
Soft Drinks
Three studies reported on SSBs and incident CKD (Fig. 2 ) [ 35 , 36 , 37 ], of which one American study also reported on diet beverages (Fig. 2 ) [ 36 ]. In the ARIC study with 9 years of follow-up, consumption of SSB (cutoff 1 drink per day) was not significantly associated with CKD risk [ 35 ]. In the Jackson Heart Study (3003 participants, 185 CKD cases) with 8 years of follow-up, a direct, non-significant association of SSB with CKD risk was found [ 36 ]. In the Iranian TLGS, SSB consumption ranged from < 0.5 to > 4 servings per week [ 37 ]. A significantly elevated risk of CKD was found when comparing high with low intakes, with an OR (95% CI) of 1.92 (1.05; 3.48) (Fig. 2 ) [ 37 ]. Diet beverages were studied in the Jackson Heart Study and showed no significant association with CKD risk (Fig. 2 ) [ 36 ].
Dietary Patterns
Healthy diets.
A number of studies examined healthy dietary patterns and incident CKD [ 38 , 39 , 40 •, 41 , 42 ••, 43 , 44 ], including the DASH diet [ 39 , 40 •, 41 ], Mediterranean diet [ 38 , 42 ••], and other healthy dietary patterns [ 42 ••, 43 , 44 ], for which findings are shown in Fig. 3 . The DASH diet was examined in the Healthy Aging in Neighborhoods of Diversity across the Life Span cohort with 5 years of follow-up [ 40 •], in the ARIC study with 23 years of follow-up [ 41 ] and in the Iranian TLGS with 6.1 years of follow-up [ 39 ]. All studies suggested a beneficial effect of the DASH diet, with RRs between 0.41 and 0.86 for high versus low adherence (Fig. 3 ). The association was statistically significant for 2 studies [ 39 , 41 ].
Mediterranean diet scores were examined in the Northern Manhattan Study [ 38 ] and ARIC study [ 42 ••], with 6.9 years [ 38 ] and 24 years [ 42 ••] of follow-up, respectively. Reduced RRs of 0.50 [ 38 ] and 0.89 [ 42 ••] were found for high versus low adherence, which were significant for both studies (Fig. 3 ).
The ARIC study also examined [ 42 ••] adherence to healthy dietary patterns assessed using the Healthy Eating Index-2015 (HEI-2015) and the alternative HEI-2010 [ 42 ••]. The HEI-2015 was designed to assess adherence to US Dietary Guidelines for Americans [ 45 ], while the alternative HEI-2010 was designed to identify key components associated with chronic diseases [ 46 ]. For both diet quality scores, significantly lower risks of CKD were found for higher adherence, with RRs of 0.86 and 0.81, respectively (Fig. 3 ) [ 42 ••].
In the ARIC study with 22 years of follow-up, the Healthy Diet Score based on American Heart Association’s Life’s Simple 7 was studied, which appeared not to be associated with incident CKD [ 43 ]. In the Framingham Offspring cohort followed for 6.6 years (1802 participants, 171 CKD cases), the Dietary Guidelines Adherence Index was borderline significantly inversely associated with CKD risk [ 44 ].
Healthy dietary patterns were also beneficially associated with other renal function outcomes, such as rapid eGFR decline [ 40 •, 44 ] and ≥ 25% eGFR decline [ 40 •] (Supplementary material; eTable 1 ).
Unhealthy Diets
Two studies reported on unhealthy dietary patterns and incident CKD (Fig. 3 ) [ 47 , 48 ]. In the TLGS, a high-fat, high-sugar diet was related to a significantly higher risk of CKD, with OR of 1.46 [ 47 ]. In participants of the ARIC study, an increased HR of 1.13 was found for a diet with a high acid load (12.2 to 100.7 mEq per day), which is characterized by high levels of salt, animal protein, and phosphorus, compared with a low acid load (− 119.1 to − 3.2 mEq per day).
This review of 21 prospective cohort studies among individuals with (relatively) normal kidney function shows a consistently lower risk of CKD in those adhering to a healthy dietary pattern [ 38 , 39 , 40 •, 41 , 42 ••, 43 , 44 ]. For individual food groups and beverages, the observed associations were more variable and weaker. We found adverse associations for red (processed) meat and SSBs in some studies and beneficial associations for dairy, vegetables, legumes, and nuts.
Two recent reviews have indicated that healthy dietary patterns may prevent incident CKD [ 15 , 16 ]. Ajjarapu et al. included 26 prospective cohort studies and found that adherence to a DASH or Mediterranean diet may be useful to prevent CKD [ 16 ]. Similar results were found in a meta-analysis of 15 prospective and retrospective cohort studies performed by Bach et al. [ 15 ]. A low animal/vegetable protein ratio is often considered an indicator of a healthy dietary pattern. In this regard, the ARIC study [ 25 ••] showed that after 23 years of follow-up, high (> 22.8 g per day) versus low (< 12.1 g per day) intake of vegetable protein was significantly associated with lower risk of CKD, whereas no association was found for high (> 69.6 g per day) versus low (< 36.4 g per day) intake of animal protein [ 25 ••]. Similar results on animal protein intake were found in 1135 participants with normal renal function (defined as eGFR > 80 ml/min/1.73 m 2 ) from the Nurses’ Health Study [ 49 ].
A lower risk of incident CKD for those consuming more vegetables and legumes may partly be attributable to fiber, as shown in a study among Iranian TLGS participants, with 6.1 years of follow-up [ 50 ]. Consumption of whole grains has also been linked to less kidney function decline in the Doetinchem Study in The Netherlands, with 15 years of follow-up [ 51 ]. In a study of vegetables and fruit intake in relation to kidney function decline, assessed by the annual change in eGFR, inverse associations were found [ 51 ] (Supplementary material; eTable 2 ), which strengthens our findings on healthy dietary patterns.
We found no association of CKD with fish intake, which is often considered part of a healthy diet. This was confirmed in another study among American Indians with 5.4 years of follow-up, where fish intake was not related to change in kidney function [ 27 ] (Supplementary material; eTable 2 ). For poultry, we could only include one study, and more research is needed.
Our results for coffee, indicating a potentially protective effect, are also in line with the results from a study on kidney function change [ 52 ] (Supplementary material; eTable 2 ). In this study, the coffee was mainly caffeinated [ 52 ] and likely to be filtered. The Iranian study suggested an increased, albeit non-significant, risk of CKD, which could be attributable to the regularly consumed unfiltered type of coffee in this country [ 31 ]. However, more information regarding the type of coffee and its preparation methods is needed, including amounts of added sugar and other condiments, before results can be correctly interpreted. We found no beneficial associations for tea and incident CKD, which was in line with the results from a Dutch study on kidney function decline [ 52 ] (Supplementary material; eTable 2 ). However, our review included only one study on incident CKD from Iran [ 31 ]. More information about the types of tea in relation to kidney function, including amounts of added sugar, is needed before drawing conclusions.
For low-fat dairy products and incident CKD, we found some evidence for a potentially protective effect on kidney function, though based on only one study [ 25 ••]. This is in line with a study in Dutch participants in which less kidney function loss was found during 15 years of follow-up who consumed more milk and low-fat dairy [ 53 ] (Supplementary material; eTable 2 ).
With regard to other kidney function outcomes (Supplementary material; eTable 3 ), studies on the risk of albuminuria [ 27 , 35 , 54 , 55 ] and hyperuricemia [ 35 ] were in accordance with those for CKD. A higher, albeit non-significant risk of hyperuricemia was found for high versus low SSB consumption [ 35 ]. Also, a good versus poor diet quality, based on eight fundamental DASH diet components, was associated with a lower risk of incident microalbuminuria [ 55 ], and fruit intake was related to a lower risk of albuminuria [ 54 ]. Fish intake was not associated with albuminuria [ 27 , 56 ].
To summarize, this review shows that a healthy dietary pattern may help prevent kidney function decline and lower the risk of CKD. The number of studies of individual foods and beverages in this field, however, is limited and most of the evidence comes from a limited number of cohorts. More research on the components of healthy (and unhealthy) diets and indicators of kidney health in different populations is needed to fill these knowledge gaps.
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hill NR, Fatoba ST, Oke JL, Hirst JA, Callaghan AO, Lasserson DS, et al. Global prevalence of chronic kidney disease – a systematic review and meta-analysis. PLoS One. 2016;11:1–18.
Google Scholar
Thomas R, Kanso A, Sedor JR. Chronic kidney disease and its complications. Prim Care. 2008;35:329–44.
Article PubMed PubMed Central Google Scholar
Centers for disease control and prevention chronic kidney disease basics. https://www.cdc.gov/kidneydisease/basics.html . Accessed 23 Oct 2019.
Sun X, He J, Ji XL, Zhao YM, Lou HY, Song XX, et al. Association of chronic kidney disease with coronary heart disease and stroke risks in patients with type 2 diabetes mellitus: an observational cross-sectional study in Hangzhou, China. Chin Med J. 2017;130:57–63.
Hoogeveen EK, Geleijnse JM, Giltay EJ, Soedamah-Muthu SS, De Goede J, Oude Griep LM, et al. Kidney function and specific mortality in 60-80 years old post-myocardial infarction patients: a 10-year follow-up study. PLoS One. 2017;12:1–17.
Tedla FM, Brar A, Browne R, Brown C. Hypertension in chronic kidney disease: navigating the evidence. Int J Hypertens. 2011. https://doi.org/10.4061/2011/132405 .
Shen Y, Cai R, Sun J, Dong X, Huang R, Tian S, et al. Diabetes mellitus as a risk factor for incident chronic kidney disease and end-stage renal disease in women compared with men: a systematic review and meta-analysis. Endocrine. 2017;55:66–76.
Article CAS PubMed Google Scholar
National Kidney Foundation Diabetes - a major risk factor for kidney disease. https://www.kidney.org/atoz/content/diabetes . Accessed 23 Oct 2019.
Mills K, Bundy J, Kelly T, Reed J, Kearney P, Reynolds K, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134:441–50.
IDF Diabetes Atlas (2019) International Diabetes Federation. http://www.diabetesatlas.org . Accessed 26 Nov 2019.
Wang H, Naghavi M, Allen C, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–544.
Article Google Scholar
Xia J, Wang L, Ma Z, Zhong L, Wang Y, Gao Y, et al. Cigarette smoking and chronic kidney disease in the general population: a systematic review and meta-analysis of prospective cohort studies. Nephrol Dial Transplant. 2017;32:475–87.
White SL, Polkinghorne KR, Cass A, Shaw JE, Atkins RC, Chadban SJ. Alcohol consumption and 5-year onset of chronic kidney disease: the AusDiab study. Nephrol Dial Transplant. 2009;24:2464–72.
Article PubMed Google Scholar
Zelle D, Klaassen G, van Adrichem E, Bakker S, Corpeleijn E, Navis G. Physical inactivity: a risk factor and target for intervention in renal care. Nat Rev Nephrol. 2017;13:152–68.
Bach KE, Kelly JT, Palmer SC, Khalesi S, Strippoli GFM, Campbell KL. Healthy dietary patterns and incidence of CKD: a meta-analysis of cohort studies. Clin J Am Soc Nephrol. 2019;14:1441–9.
Ajjarapu AS, Hinkle SN, Li M, Francis EC, Zhang C. Dietary patterns and renal health outcomes in the general population: a review focusing on prospective studies. Nutrients. 2019;11:1877.
Article CAS PubMed Central Google Scholar
Sugiura T, Takase H, Ohte N, Dohi Y. Dietary salt intake is a significant determinant of impaired kidney function in the general population. Kidney Blood Press Res. 2018;43:1245–54.
Malta D, Petersen KS, Johnson C, Trieu K, Rae S, Jefferson K, et al. High sodium intake increases blood pressure and risk of kidney disease. From the science of salt: a regularly updated systematic review of salt and health outcomes (August 2016 to March 2017). J Clin Hypertens. 2018;20:1654–65.
Article CAS Google Scholar
Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension. 2006;47:296–308.
Rust P, Ekmekcioglu C. Impact of salt intake on the pathogenesis and treatment of hypertension. In: Islam MS, editor. Hypertens. from basic Res. to Clin. Pract. Cham: Springer International Publishing AG; 2017. p. 61–84.
Ohta Y, Tsuchihashi T, Kiyohara K, Oniki H. High salt intake promotes a decline in renal function in hypertensive patients: a 10-year observational study. Hypertens Res. 2013;36:172–6.
Inker L, Schmid C, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–9.
Article CAS PubMed PubMed Central Google Scholar
National Kidney Foundation CKD-EPI creatinine-cystatin equation (2012). https://www.kidney.org/content/ckd-epi-creatinine-cystatin-equation-2012 . Accessed 23 Oct 2019.
Botev R, Mallié JP, Couchoud C, Schück O, Fauvel JP, Wetzels JFM, et al. Estimating glomerular filtration rate: Cockcroft-gault and modification of diet in renal disease formulas compared to renal inulin clearance. Clin J Am Soc Nephrol. 2009;4:899–906.
•• Haring B, Selvin E, Liang M, Coresh J, Grams M, Petruski-Ivleva N, et al. Dietary protein sources and risk for incident chronic kidney disease: results from the Atherosclerosis Risk in Communities (ARIC) study. J Ren Nutr. 2017;27:233–42 This study investigated different sources of protein and concluded that red and processed meat were adversely associated with risk of CKD and nuts, and low-fat dairy and legumes were associated with lower risk of CKD.
•• Mirmiran P, Yuzbashian E, Aghayan M, Mahdavi M, Asghari G, Azizi F. A prospective study of dietary meat intake and risk of incident chronic kidney disease. J Ren Nutr. 2019:1–8 A study on meat consumption among Iranian participants that found a significant higher risk of CKD for highest versus lowest group of meat consumption.
Lee C, Howard B, Mete M, Wang H, Jolly S, Adler A. Association between fish consumption and nephropathy in American Indians--the Strong Heart Study. J Ren Nutr. 2012;22:221–7.
• Bahadoran Z, Mirmiran P, Momenan AA, Azizi F. Allium vegetable intakes and the incidence of cardiovascular disease, hypertension, chronic kidney disease, and type 2 diabetes in adults: a longitudinal follow-up study. J Hypertens. 2017;35:1909–16 A study on allium vegetable consumption among Iranian participants, which suggested a potential protective association for highest versus lowest intake of allium vegetables on risk of CKD.
•• Jhee JH, Kee YK, Park JT, Chang TI, Kang EW, Yoo TH, et al. A diet rich in vegetables and fruit and incident CKD: a community-based prospective cohort study. Am J Kidney Dis. 2019;XX:1–10 A study that observed a significantly lower risk of CKD for those consuming more non-fermented vegetables.
Mirmiran P, Bahadoran Z, Golzarand M, Asghari G, Azizi F. Consumption of nitrate containing vegetables and the risk of chronic kidney disease: Tehran Lipid and Glucose Study. Ren Fail. 2016;38:937–44.
Gaeini Z, Bahadoran Z, Mirmiran P, Azizi F. Tea, coffee, caffeine intake and the risk of cardio-metabolic outcomes: findings from a population with low coffee and high tea consumption. Nutr Metab. 2019;16:1–10.
Hu EA, Selvin E, Grams ME, Steffen LM, Coresh J, Rebholz CM. Coffee consumption and incident kidney disease: results from the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis. 2018;72:214–22.
Jhee JH, Nam KH, An SY, et al. Effects of coffee intake on incident chronic kidney disease: a community-based prospective cohort study. Am J Med. 2018;131:1482–1490.e3.
Islami F, Pourshams A, Nasrollahzadeh D, et al. Tea drinking habits and oesophageal cancer in a high risk area in northern Iran: population based case-control study. Br Med J. 2009. https://doi.org/10.1136/bmj.b929 .
Bomback AS, Derebail VK, Shoham DA, Anderson CA, Steffen LM, Rosamond WD, et al. Sugar-sweetened soda consumption, hyperuricemia, and kidney disease. Kidney Int. 2010;77:609–16.
Rebholz CM, Young BA, Katz R, Tucker KL, Carithers TC, Norwood AF, et al. Patterns of beverages consumed and risk of incident kidney disease. Clin J Am Soc Nephrol. 2019;14:49–56.
Yuzbashian E, Asghari G, Mirmiran P, Zadeh-Vakili A, Azizi F. Sugar-sweetened beverage consumption and risk of incident chronic kidney disease: Tehran Lipid and Glucose Study. Nephrology. 2016;21:608–16.
Khatri M, Moon YP, Scarmeas N, Gu Y, Gardener H, Cheung K, et al. The association between a mediterranean-style diet and kidney function in the northern Manhattan study cohort. Clin J Am Soc Nephrol. 2014;9:1868–75.
Asghari G, Yuzbashian E, Mirmiran P, Azizi F. The association between Dietary Approaches to Stop Hypertension and incidence of chronic kidney disease in adults: the Tehran Lipid and Glucose Study. Nephrol Dial Transplant. 2017;32:ii224–30.
• Liu Y, Kuczmarski MF, Miller ER, Nava MB, Zonderman AB, Evans MK, et al. Dietary habits and risk of kidney function decline in an urban population. J Ren Nutr. 2017;27:16–25 This study observed a lower, albeit non-significant, risk of CKD for those with greater adherence to DASH diet.
Rebholz C, Crews D, Grams M, Steffen L, Levey A, Miller E 3rd, et al. DASH (Dietary Approaches to Stop Hypertension) diet and risk of subsequent kidney disease. Am J Kidney Dis. 2016;68:853–61.
•• Hu EA, Steffen LM, Grams ME, Crews DC, Coresh J, Appel LJ, et al. Dietary patterns and risk of incident chronic kidney disease: the Atherosclerosis Risk in Communities study. Am J Clin Nutr. 2019;110:713–21 Dietary quality was assessed according to three different adherence measures: Healthy Eating Index, alternative Healthy Eating Index and Mediterranean diet score. A lower risk of CKD was found for the group with the highest dietary quality, based on three adherence measures.
Rebholz CM, Anderson CAM, Grams ME, Bazzano LA, Crews DC, Chang AR, et al. Relationship of the American Heart Association’s impact goals (Life’s Simple 7) with risk of chronic kidney disease: results from the Atherosclerosis Risk in Communities (ARIC) cohort study. J Am Heart Assoc. 2016;5:1–10.
Foster M, Hwang S, Massaro J, Jacques P, Fox C, Chu A. Lifestyle factors and indices of kidney function in the Framingham Heart Study. Am J Nephrol. 2015;41:267–74.
Krebs-Smith S, Pannucci T, Subar A, Kirkpatrick S, Lerman J, Tooze J, et al. Update of the Healthy Eating Index: HEI-2015. J Acad Nutr Diet. 2018;118:1591–602.
Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–18.
Asghari G, Momenan M, Yuzbashian E, Mirmiran P, Azizi F. Dietary pattern and incidence of chronic kidney disease among adults: a population-based study. Nutr Metab. 2018;15:1–11.
Rebholz C, Coresh J, Grams M, Steffen L, Anderson C, Appel L, et al. Dietary acid load and incident chronic kidney disease: results from the ARIC study. Am J Nephrol. 2015;42:427–35.
Knight EL, Stampfer MJ, Hankinson SE, Spiegelman D, Curhan GC. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med. 2003. https://doi.org/10.7326/0003-4819-138-6-200303180-00009 .
Mirmiran P, Yuzbashian E, Asghari G, Sarverzadeh S, Azizi F. Dietary fibre intake in relation to the risk of incident chronic kidney disease. Br J Nutr. 2018;119:479–85.
Herber-Gast GCM, Boersma M, Verschuren WMM, Stehouwer CDA, Gansevoort RT, Bakker SJL, et al. Consumption of whole grains, fruit and vegetables is not associated with indices of renal function in the population-based longitudinal Doetinchem study. Br J Nutr. 2017;118:375–82.
Herber-Gast GCM, Van Essen H, Verschuren WMM, Stehouwer CDA, Gansevoort RT, Bakker SJL, et al. Coffee and tea consumption in relation to estimated glomerular filtration rate: results from the population-based longitudinal Doetinchem Cohort Study. Am J Clin Nutr. 2016;103:1370–7.
Herber-Gast GCM, Biesbroek S, Verschuren WMM, Stehouwer CDA, Gansevoort RT, Bakker SJL, et al. Association of dietary protein and dairy intakes and change in renal function: results from the population-based longitudinal Doetinchem cohort study1,2. Am J Clin Nutr. 2016;104:1712–9.
Wen J, Hao J, Zhang Y, et al. Fresh fruit consumption and risk of incident albuminuria among rural Chinese adults: a village-based prospective cohort study. PLoS One. 2018;13:1–3.
CAS Google Scholar
Chang A, Van Horn L, Jacobs DJ, et al. Lifestyle-related factors, obesity, and incident microalbuminuria: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Kidney Dis. 2013;62:267–75.
Park I, Xun P, Tsinovoi CL, Klemmer P, Liu K, He K. Intakes of long-chain omega-3 polyunsaturated fatty acids and non-fried fish in relation to incidence of chronic kidney disease in young adults: a 25-year follow-up. Eur J Nutr. 2019:1–9. https://doi.org/10.1007/s00394-019-02022-4 .
Download references
Author information
Authors and affiliations.
Division of Human Nutrition and Health, Wageningen University, Stippeneng 4, 6708 WE, Wageningen, The Netherlands
A. C. van Westing, L. K. Küpers & J. M. Geleijnse
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to A. C. van Westing .
Ethics declarations
Conflict of interest.
The research presented in this paper has been funded by the Jaap Schouten Foundation (JSF_SU_10_2018).
Human and Animal Rights and Informed Consent
All reported studies/experiments with human subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki Declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Nutrition and Hypertension
Electronic Supplementary Material
(DOCX 28.9 kb)
(DOCX 66.7 kb)
(DOCX 19.3 kb)
(DOCX 19.5 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and permissions
About this article
van Westing, A.C., Küpers, L.K. & Geleijnse, J.M. Diet and Kidney Function: a Literature Review. Curr Hypertens Rep 22 , 14 (2020). https://doi.org/10.1007/s11906-020-1020-1
Download citation
Published : 03 February 2020
DOI : https://doi.org/10.1007/s11906-020-1020-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Dietary patterns
- Kidney function
- Chronic kidney disease
- Prospective cohort studies
- Glomerular filtration rate
Advertisement
- Find a journal
- Publish with us
- Track your research
Case report
- Open access
- Published: 23 April 2024
Surgical management of renal cell carcinoma with subhepatic inferior vena cava tumor thrombus: a case report and review of the literature
- Bekim Ademi 1 ,
- Luan Jaha 1 ,
- Isa Haxhiu 2 ,
- Xhevdet Çuni 2 ,
- Afrim Tahiri 3 ,
- Jetmir Gashi 1 ,
- Adhurim Koshi 1 &
- Art Jaha 1
Journal of Medical Case Reports volume 18 , Article number: 201 ( 2024 ) Cite this article
155 Accesses
1 Altmetric
Metrics details
Renal cell carcinomas are the most common form of kidney cancer in adults. In addition to metastasizing in lungs, soft tissues, bones, and the liver, it also spreads locally. In 2–10% of patients, it causes a thrombus in the renal or inferior vena cava vein; in 1% of patients thrombus reaches the right atrium. Surgery is the only curative option, particularly for locally advanced disease. Despite the advancements in laparoscopic, robotic and endovascular techniques, for this group of patients, open surgery continues to be among the best options.
Here we present a case of successful tumor thrombectomy from the infrahepatic inferior vena cava combined with renal vein amputation and nephrectomy. Our patient, a 58 year old Albanian woman presented to the doctors office with flank pain, weight loss, fever, high blood pressure, night sweats, and malaise. After a comprehensive assessment, which included urine analysis, complete blood count, electrolytes, renal and hepatic function tests, as well as ultrasonography and computed tomography, she was diagnosed with left kidney renal cell carcinoma involving the left renal vein and subhepatic inferior vena cava. After obtaining informed consent from the patient we scheduled her for surgery, which went well and without complications. She was discharged one week after to continue treatment with radiotherapy, chemotherapy, and immunotherapy.
Open surgery is a safe and efficient way to treat renal cell carcinoma involving the renal vein and inferior vena cava. It is superior to other therapeutic modalities. When properly done it provides acceptable long time survival and good quality of life to patients.
Peer Review reports
Renal cell carcinomas (RCCs) in adults account for around 85% of kidney neoplasms [ 1 , 2 ] and may be linked to various risk factors, such as genetics, smoking, obesity, and exposure to certain chemicals. Other potential risk factors include hypertension, exposure during work to trichloroethylene, benzene or herbicides, the use of nonsteroidal anti-inflammatory drugs, dialysis, hepatitis C infection, and kidney stones [ 3 , 4 ].
RCC can remain clinically undetected throughout much of its progression. In around 90% of cases, RCC does not present with the hallmark symptoms of flank mass, hematuria, and flank pain until the disease has progressed significantly. Other signs and symptoms are weight loss, malaise, fever, night sweats, hypercalcemia, and hypertension. Male patients may also experience varicoceles on their left side as a result of obstruction of the testicular vein. However, almost one third of patients show no symptoms and end up discovering the carcinoma incidentally [ 5 ].
Around one third of RCC patients develop metastatic disease, with metastases being present either at the time of diagnosis or, in up to half of cases, later after a nephrectomy. The most common sites for metastatic disease are the lungs, soft tissues, bones, and liver, although the skin and central nervous system are also frequently affected [ 6 ].
A comprehensive diagnostic approach typically involves urinalysis, blood tests, renal and hepatic profiles, and imaging techniques, such as PET scans and angiotomography. Percutaneous core biopsy may also be performed to determine malignancy status.
RCC is staged using tumor, node, metastasis (TNM) classification and the American Joint Committee on Cancer (AJCC) staging system. Higher grade tumors are associated with poorer prognosis; inferior cava vein involvement is classified as stage III within these staging systems [ 6 ].
Surgical intervention is currently the only effective treatment for localized RCC, although it may also be utilized to relieve symptoms in cases of metastatic disease. The specific surgical approach depends on the location of the tumor thrombus. Several surgical staging systems have been proposed, including the Neves, Novick, and Hinman systems. In the Novick system, which we have used in this case report, a tumor thrombus found in the renal vein that extends less than 2 cm within the inferior vena cava (IVC) is classified as a level I thrombus. An infrahepatic thrombus is classified as a level II thrombus. A level III classification is given to an intrahepatic IVC thrombus below the diaphragm, while a level IV classification is reserved for an IVC tumor thrombus that extends above the diaphragm, as illustrated in Fig. 1 [ 7 , 8 , 9 ].
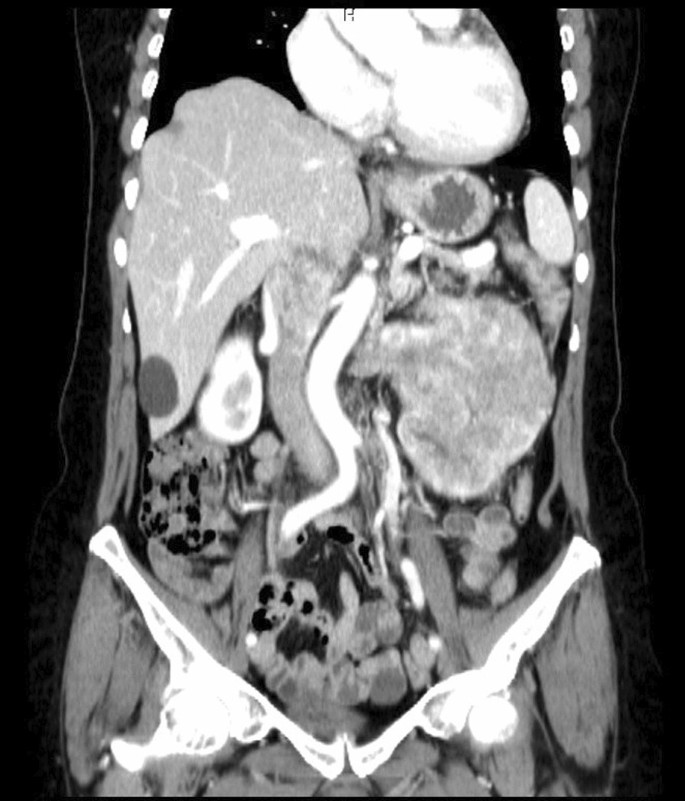
Preoperative computed tomography angiography, coronary view
We are pleased to present a successful tumor thrombectomy from the infrahepatic inferior vena cava (class II) in patients with renal cell carcinoma. Our patient was a 58-year-old Albanian housewife who presented at the doctors office with a range of symptoms, including flank pain, hematuria, weight loss, fever, hypertension, night sweats, and malaise.
Despite these symptoms, she remained well-oriented to person, place, and time. Her vital signs were slightly altered, with a blood pressure of 150/95 mmHg, heart rate of 90 beats per minute, respiratory rate of 20 breaths per minute, and temperature of 38 °C. The skin appeared reddened, warm, and moist, without any lesions or rashes observed. The head was normocephalic and the neck was supple with no masses or lymphadenopathy. No visual deficits, ptosis, or facial asymmetry indicating cranial nerve pathology were noted. Muscle strength was 5/5 bilaterally, and sensations were intact to light touch and pinprick throughout. Reflexes were 2 and symmetric in all extremities, with no pathological reflexes present. Gait was steady and coordinated, without any observed abnormalities. Palpation over the left flank elicited tenderness, pain, and guarding, as did percussion. No abnormalities were heard on auscultation of the abdomen.
After a comprehensive assessment, which included urine analysis, complete blood count, electrolytes, renal and hepatic function tests, as well as ultrasonography and computed tomography, she was diagnosed with left kidney renal cell carcinoma involving the left renal vein and subhepatic inferior vena cava (Figs. 1 , 2 ). All tests came back normal, except for microhematuria and slight hypoalbuminemia. A minor increase in C-reactive protein was also noted (Table 1 ).
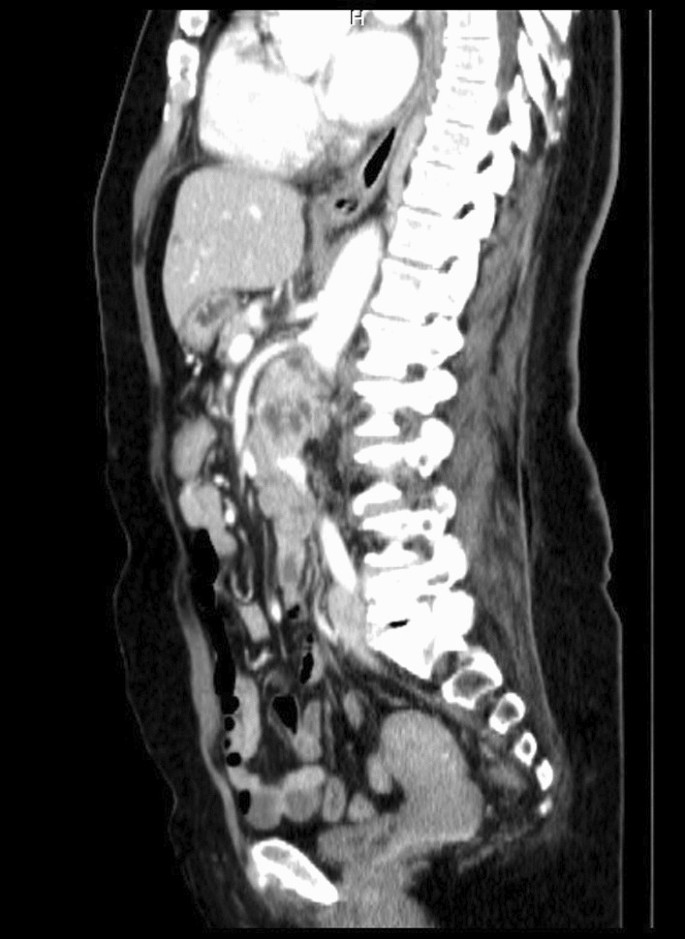
Preoperative computed tomography angiography, sagittal view
Apart from for a beta blocker (carvedilol 6.25 mg, twice a day orally) that she was taking for hypertension, she was on no other medications. The patient was a nonsmoker and did not consume alcohol. There was no history of kidney or other malignancy in the family.
The cancer extension into the left renal vein and subhepatic inferior vena cava corresponded to Neves II stage disease. After careful consideration, we decided to perform a nephrectomy, renal vein amputation and thrombectomy of the subhepatic vena cava.
A team composed of vascular surgeons, urologists, a hepatobiliary surgeon, and an anesthesiologist came together to form a multidisciplinary team. Adequate amounts of blood products, such as packed red blood cells, platelets, cryoprecipitate, fresh frozen plasma, and clotting factors, were made available for surgery. The surgical procedure was carried out under general endotracheal anesthesia. To monitor and resuscitate the patient during surgery, a large bore central venous catheter, a 15 F catheter in the right internal jugular vein, and a right radial arterial catheter were inserted.
A midline incision was made to approach the abdomen as it was thought to provide optimal exposure of the inferior vena cava and contralateral kidney, enable thorough metastatic evaluation, and minimize postoperative pain. Since we decided to remove the kidney first and cancer thrombus after, we mobilized the colon medially, brought the kidney outside of Gerota’s fascia, and tied the ureter. After tying the renal artery and leaving the kidney attached only by the renal vein, the kidney was removed (Fig. 3 ).
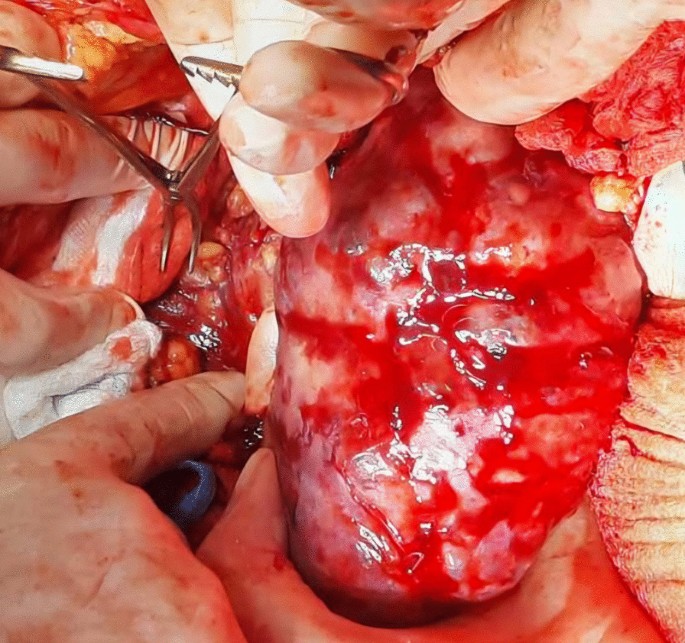
Nephrectomy
Afterwards, liver was separated from its connections and tourniquets were positioned around the suprahepatic IVC, which was then mobilized together with the contralateral renal vein. To manage and identify lumbar veins, vascular clamps were placed above and below the tumor thrombus (Fig. 4 ). Patient response was monitored with a 1 minute test clamp, during which no hemodynamic changes were observed, allowing the clamps to remain in place as we performed cavotomy. A precise incision was created around the ostium of the renal vein into the IVC, followed by placement of a Fogarty catheter to facilitate thrombus removal (Fig. 5 ). The tumor itself was successfully removed without any issues and the cavotomy incision was closed using a 3–0 polypropylene suture (Figs. 6 , 7 ). After ensuring meticulous hemostasis, the abdominal cavity was closed using multiple layers.
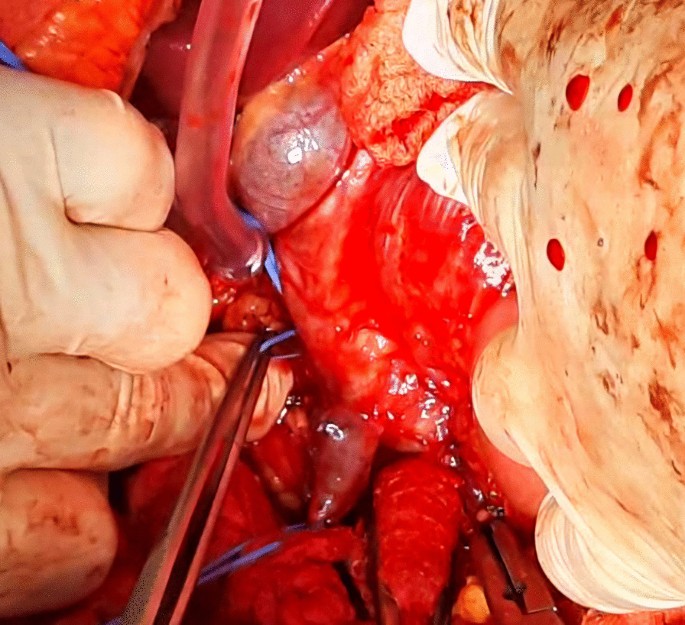
Vascular control of the inferior vena cava, right renal vein, and ovarian vein
Tumor thrombus removal
Suturing of the inferior vena cava
Overall, the patient received 10 units of packed red blood cells, 10 units of fresh frozen plasma, 10 units of platelets, and 10 units of cryoprecipitate. The surgery proceeded without complications. The patient was then referred for further treatment in the ICU, where she stayed for a day for postoperative care. She received an additional two units of red blood cells and one unit of plasma to achieve a hematocrit value of 38% and hemoglobin level of 115 g/L. Blood urea nitrogen (BUN) and creatinine levels were within the normal range, as were the electrolyte levels. Liver enzyme levels were also normal. Hemodynamically, she remained stable, and her oxygen saturation consistently stayed above 98%. At 6 hours later, she was successfully extubated. On the second day after surgery, she was transferred to the ward, and 1 week later, she was discharged to continue treatment with radiotherapy, chemotherapy, and immunotherapy. During her ward stay, she received intravenous antibiotics (1 g of ceftriaxone twice daily and metronidazole 500 mg three times daily), subcutaneous low molecular weight heparin (enoxaparin 4000 IU/day), intravenous proton pump inhibitor (pantoprazole 40 mg once daily), and analgesics (diclofenac 50 mg intravenously twice daily for the first two days and as needed for pain thereafter). No additional blood transfusions were required (Fig. 8 ).
Postoperative computed tomography
The ICV is free of thrombus, but there are already developed regional and liver metastases.
On the follow-up computed tomography (CT) scan performed one month after surgery, the inferior vena cava was free of tumor, but unfortunately, the tumor had already spread to the liver and retroperitoneum (Fig. 7 ). The patient’s condition deteriorated 3 months after surgery and despite aggressive chemotherapy, she passed away 6 months later. Owing to religious reasons, no autopsy was performed.
In the midst of the ongoing debate concerning the role of open surgery in the treatment of renal cell carcinoma involving the renal vein and inferior vena cava, especially when compared with less invasive surgical approaches, our case presentation aims to align with those who consider open surgical thrombectomy from the infrahepatic inferior vena cava combined with renal vein amputation and the removal of the affected kidney, a viable treatment option.
However, it requires extensive preoperative management, a multidisciplinary team, precise technical expertise during the procedure, exceptional anesthesia management, and diligent postoperative care. The surgical team must be well-prepared for any unexpected situations that may arise during the procedure while also having a clear understanding of the procedure at hand. When dealing with cases above the liver, the team should include vascular, urology, hepatobiliary, and cardiac surgeons. Additionally, strategies, such as kidney immobilization and tyrosine inhibitors, are recommended to decrease the size of the tumor before surgery.
The first routine preoperative arterial embolization of the kidney to decrease the tumor size and facilitated the surgical procedure was advocated by Pouliot and coworkers [ 10 ], but was found to have no significant advantage over no embolization. Preoperative arterial embolization of the kidney has not shown significant advantages and is only recommended in specific situations, as it is associated with complications, such as angioinfarction syndrome and inflammation around the kidney and surgical field [ 11 ]. The usefulness of tyrosine kinase inhibitors is still debated and remains to be confirmed through randomized trials despite its effectiveness in treating metastatic RCC [ 12 , 13 , 14 ].
There have been reports of surgeons placing IVC Greenfield Filters during surgery to prevent pulmonary embolism, but studies have found this practice to be ineffective and potentially dangerous. The filter may become clogged with thrombus, requiring complete removal and IVC reconstruction [ 15 ].
When dealing with a patient with RCC and IVC tumor thrombus, the surgical approach must be personalized on the basis of the level of the thrombus and characteristics of the tumor. Precise preoperative imaging plays a critical role in effective planning [ 16 ]. It is imperative to ascertain the location of the tumor thrombus—whether it is infrahepatic, intrahepatic, or suprahepatic—as this will dictate the appropriate surgical approach, methodology for controlling the inferior vena cava, and potential requirement for vascular bypass [ 6 ].
In patients with RCC and IVC tumor thrombus, general endotracheal anesthesia is favored over regional epidural anesthesia owing to the risk of significant blood loss and coagulopathy, which increases the potential for epidural hematomas.
Various modes of surgical treatment have been described for patients with RCC involving renal vein and IVC, including laparoscopic, robotic, and endovascular surgery [ 17 , 18 , 19 , 20 ]. In certain exceptional situations of renal cell carcinoma (RCC) and tumor thrombus in level I of the inferior vena cava (IVC), a laparoscopic method might be feasible. However, recorded cases have indicated an elevated possibility of intraoperative complications with minimal proof regarding its cancer-fighting efficacy and safety. Moreover, robotic radical nephrectomy has proven to be effective in certain instances of low-level thrombi, but thorough research remains inadequate, and these minimally invasive procedures are not commonly recommended [ 6 ].
Open surgery, specifically nephrectomy and IVC thrombectomy, remains the preferred option and has shown usefulness even in metastatic disease, provided the patient is a candidate for surgery and has local symptoms [ 21 ]. In patients with metastatic RCC, the most reliable indicators of their prognosis are their response to systemic therapy and the burden of their metastatic disease [ 22 , 23 , 24 ].
The factors that could affect the survival of patients in this cohort are related to pathological TNM stage, nuclear grade, histological tumor subtype, regional lymph node status, and perinephritic fat invasion. It is worth noting, however, that there is little or no correlation between the level of IVC tumor thrombus and overall survival (OS). According to a recent study, the recurrence rate might be influenced by the extent of IVC tumor thrombus, however, there does not seem to be any correlation with OS [ 25 ]. Additionally, research on the results of surgery in patients with RCC who underwent nephrectomy and IVC thrombectomy revealed that the existence of fragile venous thrombus could augment the probability of synchronous nodal or distant metastases [41]. According to reports, the 5-year OS rate following surgical approach ranges from 32 to 69% in patients with IVC tumor thrombus wall invasion [ 25 , 26 , 27 , 28 ].
As for the procedure itself, radical nephrectomy with concomitant IVC thrombectomy has a reported perioperative mortality rate of 5–10%. However, it has been associated with significant morbidity, resulting in an overall complication rate of 38% [50]. As such complex surgeries require extensive vascular, hepatobiliary, cardiothoracic, and anesthesia support, it is recommended that only centers of excellence with the necessary resources undertake these procedures. Specifically, they should be reserved for patients with more advanced level III and IV IVC tumor thrombi [ 29 , 30 , 31 , 32 ].
The effective management of renal cell carcinoma and inferior vena cava tumor thrombus relies heavily on a skilled multidisciplinary surgical team. Adequate preoperative imaging is crucial for the planning and facilitation of surgical procedures for such cases. The surgical strategy should be customized to suit the specific characteristics and extent of the tumor thrombus situated in the inferior vena cava. While minimally invasive surgical techniques (including robotics) may have a role in this matter, their use should be limited to specific cases, particularly level I IVC tumor thrombus cases with favorable anatomic and tumor characteristics. For smaller lesions in patients who are not candidates for surgery, thermal ablation may provide a viable alternative. Metastatic disease in RCC can be treated with targeted therapy and immunotherapy, whereas chemotherapy and hormonal therapy have generally been unsuccessful.
Availability of data and materials
The data are available under consideration of the corresponding author upon reasonable request.
Abbreviations
- Renal cell carcinoma
Inferior vena cava
American Joint Committee on Cancer
Kidney Cancer: Introduction. Cancer.net. Available at https://www.cancer.net/cancer-types/kidney-cancer/introduction . August 2019; Accessed 18 Feb 2021.
National Cancer Institute. Cancer stat facts: kidney and renal pelvis cancer. Surveillance, Epidemiology, and End Results (SEER) Program. Available at: http://seer.cancer.gov/statfacts/html/kidrp.html . Accessed 26 Mar 2019.
DeVita VT Jr, Lawrence TS, Rosenberg SA. DeVita, Hellman, and Rosenberg’s cancer principles and practice of oncology. Philadelphia: Wolters Kluwer Health; 2015.
Google Scholar
Cheungpasitporn W, Thongprayoon C, O’Corragain OA, Edmonds PJ, Ungprasert P, Kittanamongkolchai W, et al . The risk of kidney cancer in patients with kidney stones: a systematic review and meta-analysis. QJM. 2015;108(3):205–12.
Article CAS PubMed Google Scholar
Sachdeva K, Curti B, Jana BRP. Renal cell carcinoma. Medscape Drugs & Diseases from WebMD. Updated February 21, 2019. Available at http://emedicine.medscape.com/article/281340-overview . Accessed 26 Mar 2019.
Sachdeva K, Makhoul I, Javeed M, Curti B, Renal cell carcinoma. 2022. eMedicine Guideline] Renal Mass and Localized Renal Cancer: AUA Guideline. American Urological Association. Available at https://www.auanet.org/guidelines-and-quality/guidelines/renal-mass-and-localized-renal-cancer-evaluation-management-and-follow-up . 2021.
Neves RJ, Zincke H. Surgical treatment of renal cancer with vena cava extension. Br J Urol. 1987;59:90–5.
Article Google Scholar
Novick AC. Stewart’s operative urology. 2nd ed. Baltimore: Williams & Wilkins; 1989.
Hinman F. Atlas of urologic surgery. Philadelphia: W.B. Saunders Co; 1998.
Pouliot F, Shuch B, Larochelle JC, Pantuck A, Belldegrun AS. Contemporary management of renal tumors with venous tumor thrombus. J Urol. 2010;184:833–41.
Article PubMed Google Scholar
Subramanian VS, Stephenson AJ, Goldfarb DA, Fergany AF, Novick AC, Krishnamurthi V. Utility of preoperative renal artery embolization for management of renal tumors with inferior vena caval thrombi. Urology. 2009;74:154–9.
Motzer RJ, Hutson TE, Tomczak P, et al . Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–90.
Article CAS PubMed PubMed Central Google Scholar
Di Silverio F, Sciarra A, Parente U, et al . Neoadjuvant therapy with sorafenib in advanced renal cell carcinoma with vena cava extension submitted to radical nephrectomy. Urol Int. 2008;80:451–3.
Cost NG, Delacroix SE Jr, Sleeper JP, et al . The impact of targeted molecular therapies on the level of renal cell carcinoma vena caval tumor thrombus. Eur Urol. 2011;59:912–8.
Blute ML, Boorjian SA, Leibovich BC, Lohse CM, Frank I, Karnes RJ. Results of inferior vena caval interruption by greenfield filter, ligation or resection during radical nephrectomy and tumor thrombectomy. J Urol. 2007;178:440–5.
Woodruff DY, Van Veldhuizen P, Muehlebach G, Johnson P, Williamson T, Holzbeierlein JM. The perioperative management of an inferior vena caval tumor thrombus in patients with renal cell carcinoma. Urol Oncol. 2009;122:2299–302.
Ayati M, Nikfallah A, Jabalameli P, Najjaran Tousi V, Noroozi M, Jamshidian H. Extensive surgical management for renal tumors with inferior vena cava thrombus. Urol J. 2006;3:212–5.
PubMed Google Scholar
Hattori R, Osamu K, Yoshino Y, et al . Laparoscopic radical nephrectomy for large renal-cell carcinomas. J Endourol. 2009;23:1523–6.
Tian X, Hong P, Liu Z, Huang Y, Wang G, Hou X, Zhang S, Ma L. En bloc retroperitoneal laparoscopic radical nephrectomy with inferior vena cava thrombectomy for renal cell carcinoma with level 0 to II venous tumor thrombus: a single-center experience. Cancer. 2020;126:2073–8.
Abaza R. Robotic surgery and minimally invasive management of renal tumors with vena caval extension. Curr Opin Urol. 2011;21:104–9.
González J, Gorin MA, Garcia-Roig M, Ciancio G. Inferior vena cava resection and reconstruction: technical considerations in the surgical management of renal cell carcinoma with tumor thrombus. Urol Oncol Semin Orig Investig. 2014;32(1):34-e19.
Kakoti S, Jena R, Sureka SK, Srivastava A, Mandhani A, Singh UP. Experience with management of renal cell carcinoma with inferior vena cava/right atrial tumor thrombus. Indian J Urol IJU J Urolog Soc India. 2021;37(3):234.
Whitson JM, Reese AC, Meng MV. Population based analysis of survival in patients with renal cell carcinoma and venous tumor thrombus. Urol Oncol. 2011. https://doi.org/10.1016/j.urolonc.2010.11.017 .
Lambert EH, Pierorazio PM, Shabsigh A, Olsson CA, Benson MC, McKiernan JM. Prognostic risk stratification and clinical outcomes in patients undergoing surgical treatment for renal cell carcinoma with vascular tumor thrombus. Urology. 2007;69:1054–8.
Al Otaibi M, Abou Youssif T, Alkhaldi A, et al . Renal cell carcinoma with inferior vena caval extention: impact of tumor extent on surgical outcome. BJU Int. 2009;104:1467–70.
Bertini R, Roscigno M, Freschi M, et al . Impact of venous tumor thrombus consistency (solid vs friable) on cancer-specific survival in patients with renal cell carcinoma. Eur Urol. 2011;60:358–65.
Klatte T, Pantuck AJ, Riggs SB, et al . Prognostic factors for renal cell carcinoma with tumor thrombus extension. J Urol. 2007;178:1189–95.
Kaag MG, Toyen C, Russo P, et al . Radical nephrectomy with vena caval thrombectomy: a contemporary experience. B JU Int. 2011;1(07):1386–93.
Yuan SM. Surgical treatment of renal cell carcinoma with inferior vena cava tumor thrombus. Surgery Today. 2022;52:1125–33.
Topaktaş R, Ürkmez A, Tokuç E, Kayar R, Kanberoğlu H, Öztürk Mİ. Surgical management of renal cell carcinoma with associated tumor thrombus extending into the inferior vena cava: a 10-year single-center experience. Turk J Urol. 2019;45(5):345.
Article PubMed PubMed Central Google Scholar
Lawindy SM, Kurian T, Kim T, Mangar D, Armstrong PA, Alsina AE, Sheffield C, Sexton WJ, Spiess PE. Important surgical considerations in the management of renal cell carcinoma (RCC) with inferior vena cava (IVC) tumor thrombus. BJU Int. 2012;110(7):926–39.
Ralla B, Adams L, Maxeiner A, Mang J, Krimphove M, Dushe S, Makowski M, Miller K, Fuller F, Busch J. Perioperative and oncologic outcome in patients treated for renal cell carcinoma with an extended inferior vena cava tumor thrombus level II–IV. Aktuelle Urol. 2019;53:431–8.
Download references
Acknowledgements
Not applicable.
Author information
Authors and affiliations.
Department of Vascular Surgery, University Clinical Center of Kosovo, Prishtina, Kosovo
Bekim Ademi, Luan Jaha, Jetmir Gashi, Adhurim Koshi & Art Jaha
Department of Urology, University Clinical Center of Kosovo, Prishtina, Kosovo
Isa Haxhiu & Xhevdet Çuni
Department of Abdominal Surgery, University Clinical Center of Kosovo, Prishtina, Kosovo
Afrim Tahiri
You can also search for this author in PubMed Google Scholar
Contributions
The surgical procedure was carried out by BA, LJ, IH, XC, AK, and AT. LJ and AJ conducted extensive research on medical literature, gathered relevant data, and were the main contributors to writing the manuscript. All authors read and approved final manuscript.
Corresponding author
Correspondence to Luan Jaha .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the ethical committee of the Kosovo Chamber of Physicians.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Ademi, B., Jaha, L., Haxhiu, I. et al. Surgical management of renal cell carcinoma with subhepatic inferior vena cava tumor thrombus: a case report and review of the literature. J Med Case Reports 18 , 201 (2024). https://doi.org/10.1186/s13256-024-04517-z
Download citation
Received : 05 September 2023
Accepted : 20 March 2024
Published : 23 April 2024
DOI : https://doi.org/10.1186/s13256-024-04517-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Inferior vena cava involvement
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Single-Center Experience of Pediatric Cystic Kidney Disease and Literature Review
Affiliations.
- 1 Department of Pediatrics, School of Medicine, University of Zagreb, 10000 Zagreb, Croatia.
- 2 Department of Pediatrics, University Hospital Center Zagreb, 10000 Zagreb, Croatia.
- 3 Department of Nephrology, Arterial Hypertension, Dialysis and Transplantation, University Hospital Center Zagreb, 10000 Zagreb, Croatia.
- 4 Department of Internal Medicine, School of Medicine, University of Zagreb, 10000 Zagreb, Croatia.
- PMID: 38671609
- PMCID: PMC11048964
- DOI: 10.3390/children11040392
Introduction: Pediatric cystic kidney disease (CyKD) includes conditions characterized by renal cysts. Despite extensive research in this field, there are no reliable genetics or other biomarkers to estimate the phenotypic consequences. Therefore, CyKD in children heavily relies on clinical and diagnostic testing to predict the long-term outcomes.
Aim: A retrospective study aimed to provide a concise overview of this condition and analyze real-life data from a single-center pediatric CyKD cohort followed during a 12-year period.
Methods and materials: Medical records were reviewed for extensive clinical, laboratory, and radiological data, treatment approaches, and long-term outcomes.
Results: During the study period, 112 patients received a diagnosis of pediatric CyKD. Male patients were more involved than female (1:0.93). Fifty-six patients had a multicystic dysplastic kidney; twenty-one of them had an autosomal dominant disorder; fifteen had an isolated renal cyst; ten had been diagnosed with autosomal recessive polycystic kidney disease; three had the tuberous sclerosis complex; two patients each had Bardet-Biedl, Joubert syndrome, and nephronophthisis; and one had been diagnosed with the trisomy 13 condition. Genetic testing was performed in 17.9% of the patients, revealing disease-causing mutations in three-quarters (75.0%) of the tested patients. The most commonly presenting symptoms were abdominal distension (21.4%), abdominal pain (15.2%), and oligohydramnios (12.5%). Recurrent urinary tract infections (UTI) were documented in one-quarter of the patients, while 20.5% of them developed hypertension during the long-term follow-up. Antibiotic prophylaxis and antihypertensive treatment were the most employed therapeutic modalities. Seventeen patients progressed to chronic kidney disease (CKD), with thirteen of them eventually reaching end-stage renal disease (ESRD). The time from the initial detection of cysts on an ultrasound (US) to the onset of CKD across the entire cohort was 59.0 (7.0-31124.0) months, whereas the duration from the detection of cysts on an US to the onset of ESRD across the whole cohort was 127.0 (33.0-141.0) months. The median follow-up duration in the cohort was 3.0 (1.0-7.0) years. The patients who progressed to ESRD had clinical symptoms at the time of initial clinical presentation.
Conclusion: This study is the first large cohort of patients reported from Croatia. The most common CyKD was the multicystic dysplastic kidney disease. The most common clinical presentation was abdominal distention, abdominal pain, and oliguria. The most common long-term complications were recurrent UTIs, hypertension, CKD, and ESRD.
Keywords: ADPKD; ARPKD; Bardet–Biedl syndrome; Joubert syndrome; cystic kidney disease; multicystic dysplastic kidney; nephronophthisis complex; tuberous sclerosis complex.
Grants and funding

IMAGES
VIDEO
COMMENTS
Introduction. Chronic kidney disease (CKD) is a major public health burden [1, 2], with a global prevalence of ~ 11% in the general adult population [].If left untreated, CKD slowly progresses to end-stage renal disease, which requires dialysis or kidney transplant [2, 3].CKD is bidirectionally associated with cardiovascular diseases (CVD) [4, 5]. ...
Introduction. An estimated 840 million people worldwide have chronic kidney disease (CKD) [], which was responsible for 1.2 million deaths and 35.8 million disability-adjusted life years in 2017 [].However, only 12% of sufferers are aware of their condition [].CKD is diagnosed when the estimated glomerular filtration rate (eGFR) declines below 60 mL/min/1.73 m 2 or the urinary albumin-to ...
Darlington O, Dickerson C, Evans M, et al. Costs and healthcare resource use associated with risk of cardiovascular morbidity in patients with chronic kidney Disease: evidence from a systematic literature review. Adv Ther. 2021;38(2):994-1010. [PMC free article]
Chronic kidney disease (CKD) is a syndrome defined as persistent alterations in kidney structure, function or both with implications for the health of the individual 1, 2. Examples of structural ...
This narrative review discusses the diagnosis, evaluation, staging, and management of chronic kidney disease (CKD), including discussion of new calculators for determining risk of CKD progression, treatment of complications, and considerations for referral to a nephrologist and dialysis initiation.
Chronic kidney disease is a progressive disease with no cure and high morbidity and mortality that occurs commonly in the general adult population, especially in people with diabetes and hypertension. Preservation of kidney function can improve outcomes and can be achieved through non-pharmacological strategies (eg, dietary and lifestyle adjustments) and chronic kidney disease-targeted and ...
Delaying disease progression and reducing the risk of mortality are key goals in the treatment of chronic kidney disease (CKD). New drug classes to augment renin-angiotensin-aldosterone system (RAAS) inhibitors as the standard of care have scarcely met their primary endpoints until recently. This systematic literature review explored treatments evaluated in patients with CKD since 1990 to ...
Chronic kidney disease is a progressive disease with no cure and high morbidity and mortality that occurs commonly in the general adult population, especially in people with diabetes and hypertension. Preservation of kidney function can improve outcomes and can be achieved through non-pharmacological strategies (eg, dietary and lifestyle ...
Abstract. Importance: Chronic kidney disease (CKD) is the 16th leading cause of years of life lost worldwide. Appropriate screening, diagnosis, and management by primary care clinicians are necessary to prevent adverse CKD-associated outcomes, including cardiovascular disease, end-stage kidney disease, and death.
Chronic kidney disease (CKD) is a complex disease which affects approximately 13% of the world's population. Over time, CKD can cause renal dysfunction and progression to end-stage kidney disease and cardiovascular disease. ... A Narrative Review of Chronic Kidney Disease in Clinical Practice: Current Challenges and Future Perspectives Adv Ther ...
A review of the medical literature from 2000 found 23 different designations for kidney disease. 3 In 2002, the National Kidney Foundation's (NKF) Kidney Disease Outcomes Quality Initiative (KDOQI) published the first guideline, which offered both a definition of CKD and standards of care for patients with the disease. 4
Chronic kidney disease (CKD) has emerged as one of the most prominent causes of death and suffering in the 21 st century. Due in part to the rise in risk factors, such as obesity and diabetes mellitus, the number of patients affected by CKD has also been increasing, affecting an estimated 843.6 million individuals worldwide in 2017. 1 Although mortality has declined in patients with end-stage ...
Abstract and Figures. Chronic kidney disease is a progressive, irreversible decline in renal function in which the body's ability to maintain metabolic and fluid and electrolyte balance fails ...
Delaying disease progression and reducing the risk of mortality are key goals in the treatment of chronic kidney disease (CKD). New drug classes to augment renin-angiotensin-aldosterone system (RAAS) inhibitors as the standard of care have scarcely met their primary endpoints until recently. This sy …
This systematic literature review explored the treatments evaluated in patients with CKD since 1990 to allow an assessment of contem-porary data relative to the overall treatment landscape. METHODS This systematic literature review was conducted according to the recommendations of Cochrane [18], the Centre for Reviews and Dissemination
Cystatin C (Cys C) is a cationic non-glycosylated low-molecular-weight (13,389 kDa) cystine proteinase. This compound is stably produced by all nucleated cells in constitutive fashion and meets the criteria for a GFR marker. Use of serum Cys C value or its reciprocal (1/Cys C) as a measure of GFR was proposed in 1985.
Background Renal tubular dysgenesis (RTD) is a severe disorder with poor prognosis significantly impacting the proximal tubules of the kidney while maintaining an anatomically normal gross structure. The genetic origin of RTD, involving variants in the ACE, REN, AGT, and AGTR1 genes, affects various enzymes or receptors within the Renin angiotensin system (RAS). This condition manifests ...
Background Currently published studies have not observed consistent results on the efficacy and safety of direct oral anticoagulants (DOACs) use in patients with chronic kidney disease (CKD) combined with atrial fibrillation (AF). Therefore, this study conducted a meta-analysis of the efficacy and safety of DOACs for patients with AF complicated with CKD. Methods Database literature was ...
Abstract. Purpose of review: The burden of chronic kidney disease (CKD) is increasing worldwide. For CKD prevention, it is important to gain insight in commonly consumed foods and beverages in relation to kidney function. Recent findings: We included 21 papers of prospective cohort studies with 3-24 years of follow-up.
Chronic kidney disease (CKD) is a complex disease which affects approximately 13% of the world's population. Over time, CKD can cause renal dysfunction and progression to end-stage kidney disease and cardiovascular disease. Complications associated with CKD may contribute to the acceleration of disease progression and the risk of cardiovascular-related morbidities. Early CKD is asymptomatic ...
Delaying disease progression and reducing the risk of mortality are key goals in the treatment of chronic kidney disease (CKD). New drug classes to augment renin-angiotensin-aldosterone system ...
Abstract. Chronic kidney disease (CKD) is a prevalent and progressive condition affecting millions worldwide. It is a long-term condition characterized by gradual loss of kidney function over time. The management of CKD is complex and requires a multidisciplinary approach. This review aims to outline the current management guidelines for CKD.
Purpose of Review The burden of chronic kidney disease (CKD) is increasing worldwide. For CKD prevention, it is important to gain insight in commonly consumed foods and beverages in relation to kidney function. Recent Findings We included 21 papers of prospective cohort studies with 3-24 years of follow-up. We focused on meat, fish, dairy, vegetables, fruit, coffee, tea, soft drinks, and ...
Chronic kidney disease (CKD) is a serious comorbidity affecting liver transplant recipients (LTRs). Calcineurin inhibitor dosing minimization protocols and everolimus use purportedly increased from 2010, potentially impacting CKD development. This systematic literature review was designed to identify CKD incidence in adult LTRs, focusing on ...
Renal cell carcinomas are the most common form of kidney cancer in adults. In addition to metastasizing in lungs, soft tissues, bones, and the liver, it also spreads locally. In 2-10% of patients, it causes a thrombus in the renal or inferior vena cava vein; in 1% of patients thrombus reaches the right atrium. Surgery is the only curative option, particularly for locally advanced disease.
Background. Chronic kidney disease (CKD) is one of the main comorbidities affecting liver transplant recipients [].Kidney disease is diagnosed in 20-25% of patients with liver disease [] and can be due to pathological conditions affecting both the kidney and the liver (eg, sarcoidosis or diabetes), or a complication of certain liver diseases, such as hepatitis B, hepatitis C, or alcoholic ...
The most common CyKD was the multicystic dysplastic kidney disease. The most common clinical presentation was abdominal distention, abdominal pain, and oliguria. ... Single-Center Experience of Pediatric Cystic Kidney Disease and Literature Review Children (Basel). 2024 Mar 25;11(4):392. doi: 10.3390/children11040392. Authors Sara ...