An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Springer Nature - PMC COVID-19 Collection


HIV/AIDS: Current Updates on the Disease, Treatment and Prevention
Praveen kumar gupta.
Department of Biotechnology, R.V College of Engineering, Bangalore, 560059 India
Apoorva Saxena
CCR5-delta 32 homozygous stem cell transplantation for HIV-infected individuals is being treated as a milestone in the global AIDS epidemic. Since 2008, when the second Berlin patient was cured from HIV after undergoing transplantation from a donor with delta-32 mutation, scientists are aiming for a long-term cure for the wider population. In 2019, a London patient became the second person to be free of HIV and came off the antiretroviral drugs completely. CCR5 gene is now being treated as a viable target for HIV treatment. It can be used in the treatment of HIV either through administration of drugs that bind to CCR5 and stop the receptor from working or through gene therapy to alter the CCR5 gene using CRISPR/Cas9 and prevent protein production. This review article aims to identify the obstacles and the need to overcome them in order to bridge the gap between current research and future potential cures for HIV.
Introduction
Human immunodeficiency virus or HIV is the cause of HIV infection that leads to the autoimmune disorder acquired immune deficiency syndrome (AIDS) [ 1 ] (Fig. 1 ). The major cause of spreading of HIV is through unprotected sex, during pregnancy from mother to foetus, through contaminated hypodermic needles and infected blood transfusions [ 1 ]. In the year 2016, an estimated 37 million people were living with HIV and 1 million deaths were reported. HIV/AIDS is a pandemic condition—an epidemic of diseases that spreads across large areas like multiple continents or even worldwide [ 1 ]. The first time AIDS was recognized was in the year 1981 by the United States Center for Disease Control and Prevention (CDC). Since the reported case of an individual who had successfully undergone a stem cell transplant from a person who showed a homozygous CCR5-delta 32 mutation, after receiving extensive high dose chemotherapy, there has been a greater interest in finding a potential cure.

Human Immunodeficiency Virus [ 5 ]
HIV is a type of retrovirus that adversely infects the immune system of a human, mainly targeting the CD4 + T-helper cells, accessory cells and the macrophages [ 2 ]. When it gains entry into the target cell, the viral genomic RNA undergoes a process of the reverse transcription with the help of reverse transcriptase enzyme and forms double stranded DNA (ds-DNA). This ds-DNA then gets integrated into the target cellular DNA with the help of enzyme integrase and other host co-factors [ 3 ]. The virus now can either become dormant or conceal itself and the target cell detection by the host immune system or it can get transcribed into new viral RNA and proteins that are released from the cell and begin the cycle again. HIV can be characterized into 2 major classes—HIV-1 and HIV-2. HIV-1, which is more virulent, infective and the major cause of HIV in humans, was discovered first and was initially referred to as HTLV-III or LAV [ 4 ] (Fig. 2 ). HIV-2 is less infective and far fewer people exposed to it are infected.

Structure of HIV-1 [ 8 ]
The crucial factor in gaining entry into target cell is through binding of HIV to the CD4 receptor present on the T-helper cells and to one of the chemokine receptors- either CCR5 or CXCR4 [ 6 , 7 ]. Binding to the co-receptor depends on the virus’s tropism which is the ability to bind to a specific receptor. Naturally, there are two types of tropic strains—R5 that bind to CCR5 and X4 which bind to CXCR4. Dual tropic strains are capable of binding to both. Of these two co-receptors, CCR5 is the prime receptor for virus’s entry into the target cell. R5-tropic strains prevail during early stages of infection, whereas the X4-tropic strains emerge later with disease progression. The envelope-like glycoprotein structure of HIV-1 is paramount in ensuring the viral entry into a target host cell [ 7 ]. This glycoprotein has 2 protein subunits: the gp41 (transmembrane) subunit and gp120 (external) subunit, which mimics a chemokine [ 6 , 7 ]. It does not manifest the unique structure of the chemokine but somehow manages to bind to both the co-receptors [ 6 ]. It forms a heterotrimeric complex wherein the gp120 subunit binds to the CD4 protein and specific co-receptor present on the target cell [ 6 ]. When this complex is formed, it triggers the release of a peptide which facilitates cell–cell fusion, that causes the viral membrane to fuse with the target cell membrane [ 6 ]. Binding to CD4 alone is not sufficient as it can result in gp120 shedding. So, it has to bind to the specific co-receptor for the fusion to proceed. The V1–V2 region of gp120 is recognized by the co-receptor, that influences which co-receptor will bind to the protein and is determined by degree of N-linked glycosylation and peptide composition. The highly variable V3 loop is the one that determines co-receptor specificity. The binding of gp120 glycoprotein to the CCR5 co-receptor is determined by two essential factors—the tyrosine-sulphated amino terminus of CCR5 receptor and following which there must be reciprocal action between the transmembrane domains of CCR5 and gp120 protein, i.e., inter-communication and synergy.
Antiretroviral Therapy
The usage of a combination of three or more antiretroviral drugs for suppression of the HIV infection is called antiretroviral therapy. Using multiple drugs in combination to increase the effectivity on various viral targets is called highly active antiretroviral therapy (HAART). It helps in maintaining the immune system to function, preventing HIV from developing resistance and other infections that potentially lead to death. The five classes of drugs used in combination to treat HIV infection are: entry inhibitors, nucleoside/nucleotide reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, integrase inhibitors and protease inhibitors.
Zidovudine/ZVD (also called azidothymidine) is an extensively used antiretroviral medication [ 9 ]. It is a thymidine analogue and is dosed twice daily in combination with other antiretrovirals. Its function is to particularly inhibit the reverse transcriptase enzyme which is necessary for the production of ds-DNA.
Cellular enzymes are used in converting AZT into the 5′-triphosphate form. Research studies suggest that the termination of forming ds-DNA chains is a crucial factor that leads to an inhibitory effect.
Studies have also shown that at very high dosage of this drug, its triphosphate form may inhibit the DNA polymerase enzyme which is used for cell division by the uninfected cells and mitochondria for replication. It may lead to toxic but reversible effects on certain skeletal and the cardiac muscles, causing the condition of myositis [ 10 ]. However, zidovudine also shows greater affinity for the reverse transcriptase enzyme, which is around 100-fold. This selectivity has been proven by the cell's ability to quickly repair its DNA strands if broken by AZT during its formation, whereas the HIV virus will lack this ability (Fig. 3 ).

Structure of zidovudine [ 11 ]
Zidovudine is commonly used in combination with nucleotide reverse transcriptase inhibitor, non-nucleoside reverse transcriptase inhibitor, HIV integrase strand transfer inhibitor and protease inhibitor [ 9 ]. The combination of lamivudine and zidovudine is not recommended for non-pregnant HIV-infected adults and adolescents due to greater toxicity but is used as an alternative, though not a preferred one, in antiretroviral-naive pregnant women as an initial treatment [ 9 ]. However, for paediatric patients (neonates, infants and children of age 12 or less), zidovudine with lamivudine/emtricitabine is a preferred option. For adolescents greater than the age of 12, it is an alternative [ 9 ].
Zidovudine Administration and Pharmacokinetics
Administration and dosage.
It is usually administered orally or by continuous IV infusion, although not rapid infusion and IM injection [ 9 ] (Tables (Tables1, 1 , ,2). 2 ). The dosage for paediatric patients and adult patients depends on their body weight (Tables (Tables3, 3 , ,4 4 ).
Oral administration [ 9 ]
IV administration [ 9 ]
Dosage for paediatric patients [ 9 ]
Dosage for adult patients [ 9 ]
Administration
Zidovudine: 1 mg/kg every 4 h [ 9 ].
Pharmacokinetics
Pharmacokinetics gives a detailed view of the fate of drugs in the human system. It includes various components like absorption, distribution, excretion or elimination and metabolism (Tables (Tables5, 5 , ,6, 6 , ,7). 7 ). The stability of such retroviral drugs should also be taken into account for both oral and parenteral dosage forms (Table (Table8 8 ).
Absorption [ 9 ]
Distribution [ 9 ]
Elimination process [ 9 ]
Stability of antiretrovirals [ 9 ]
Contraindications [ 9 ]
- Zidovudine has a history of life-threatening hypersensitivity reactions like Stevens–Johnson syndrome and anaphylaxis to the drug or maybe due to some ingredient in the formulation.
- Lamivudine/zidovudine: hypersensitivity history.
- Abacavir/zidovudine/lamivudine: history of hypersensitivity to abacavir, zidovudine or lamivudine; hepatic impairments may be mild or severe.
CCR5 Gene Structure
C–C chemokine receptor type 5 (also called CCR5 or CD195) is a receptor for chemokines present on the white blood cells. The CCR5 gene in humans is located on the short arm (p) at position 21 on chromosome number 3 (Fig. 4 ). It is mainly expressed cells like T-cells, macrophages, microglia, dendritic cells and eosinophils and is found within a cluster of genes coding for some other receptors like XCR1, CCBP2, etc. [ 12 , 13 ]. The gene has two promoters, three exons and two introns. Pu or PR2, the upstream promoter, has a 1.9 kb region, 57 bp in length and precedes the exon 1 [ 12 ]. Exon 1, which is the start of the coding region, is followed by the first intron, 501 bp in length. The second exon 2 is intron-less. It is found as exon 2a, 235 bp in length, and exon 2b, 54 bp in length. Pd or PR1, the second promoter, accommodates the intron 1 and exon 2 regions [ 12 ]. A 1.9 kb length intron is located between exon 2 and exon 3. Exon 3 is also intron-less and consists of the full ORF of the CCR5 gene, 11 bp of the 5′ untranslated regions and the complete 3′ untranslated regions [ 12 ].

Location of CCR5 gene on chromosome 21 [ 14 ]
These two promoters are devoid of the consensus TATA and CCAAT sequences, although the Pd promoter has a non-consensus TATA sequence and have an unusually high content of pyrimidine in them [ 12 ]. The upstream Pu promoter was found to be weaker than the downstream Pd promoter which had exhibited up to fivefold greater activity. But these results were established as erroneous [ 13 ]. With the help of RT-PCR technique, it was later identified that the Pu promoter was used in stimulated T-cells and the Pd promoter was used in unstimulated primary T-cells [ 13 ]. The error resulted due to the use of transformed T-cells affecting the overall expression of CCR5 protein via the Pu promoter [ 13 ]. Results also showed that transcription of the CCR5 gene when controlled by the Pu promoter containing exon 1 resulted in CCR5A or B and when controlled by the Pd promoter resulted in truncated isoforms [ 13 ].
CCR5 Gene Expression Regulation
The expression of CCR5 gene is regulated at three levels: 1. genetic factors, 2. factors involved in activation, signalling and trafficking of the receptor which includes desensitization, internalization and recycling and 3. environmental triggers [ 13 ].
CCR5 receptor is part of the G-protein coupled receptor family, which binds to its ligand and releases αi and βγ G-protein subunits. This results in a mediated effector response. Such responses stimulate the release of phospholipase Cβ and adenylyl cyclase. This in turn facilitates the release of intracellular calcium and form inositol triphosphate [ 13 ]. This leads to activation of phosphorylation of the CCR5 receptor which occurs at the serine and C-terminal residues via protein kinase C and G-protein coupled receptor kinases [ 13 ]. The regulatory proteins, β-arrestin 1 and 2, bind to the activated serine and the conserved DRY motif in the intracellular loop [ 13 ]. The β-arrestin proteins have functions like desensitizing the receptor to further stimulation and participating in endocytosis. The CCR5 expression level is controlled by the rates of recycling and endocytosis [ 13 ]. In the endocytosis process, β-arrestin protein facilitates the binding process between clathrin-coated pits and the phosphorylated receptor. Infection and entry of HIV into cells do not require CCR5 signalling, but the chemokine-induced endocytosis decreases the available receptor for HIV entry. This is the process of chemokine-mediated anti-HIV activity [ 13 ].
Environmental factors affecting CCR5 expression are infectious pathogenic agents like Mycobacterium tuberculosis , which increases the CCR5 expression. Studies have shown that CCR5 expression is considerably increased in all leukocyte subset cells during tuberculosis and dual infection with HIV [ 13 ]. However, the level of CCR5 expression on CD4 + T-cells was not increased. Conversely, it was also shown that HIV affects the level of expression of CCR5, due to a correlation with HIV disease progression. Individuals with end stage HIV were shown to have the highest percentages of CCR5 expressing CD4 + T-cells [ 13 ].
The regulation of CCR5 is complex. The introns as well as sequences in the 5′ UTR and 3′ UTR affect CCR5 gene regulation [ 13 ]. Therefore, mutations in these regions should be considered critical in the regulation process.
CCR5-Delta 32 Mutation
The discovery of CCR5-delta 32 mutation in the CCR5 gene in 1996 which exhibited some protection against HIV was a ground breaking one. Studies showed that the CD4 + T-cells when expressing this mutation prevented HIV envelope fusion [ 12 ] (Fig. 5 ). The mutant allele has a length of 215 in comparison to the wild type which contains 352 amino acid residues [ 13 ]. This mutation basically results due to the deletion of 32 base pairs from the position of nucleotides starting from 794 till 825, a frameshift mutation, and seven new amino acids are incorporated between amino acid 174 and stop codon at amino acid 182 [ 13 ] (Fig. 6 ). This mutation affects the region of second extracellular loop where the resultant protein lacked the last three transmembrane domains and also some regions necessary for G-protein interaction and signal transduction.

Comparison of HIV infecting cell with CCR5 and without CCR5 [ 15 ]

Difference between wild type CCR5 and CCR5-delta 32 [ 16 ]
This mutation is majorly restricted to people of European descent. The gene frequencies are found to be around 10% and shows a decline from north to south latitude. A 2–5% gene frequency in Europe, the Middle East and parts of the Indian subcontinent was observed in more than 3000 individuals. The highest frequency, at 20.93%, was discovered in the Ashkenazi Jewish population. The mutant allele is absent in Black populations excluding the African American group who may have acquired the mutation through genetic admixture [ 13 ].
The origin of the delta-32 mutant allele has been dated back to the year 275–1875, which increased over a period of time as a result of selective pressure, mainly the Black plague. However, historical data have shown that Black plague may not in fact be the cause [ 13 ]. The distribution of the delta-32 mutant allele in a north to south gradient does not correlate to the casualties of the plague and instead follows a south to the north gradient. The Black plague has shown the greatest casualties in areas like the Mediterranean region and China, with lowest allele frequencies of the mutation [ 13 ].
Studies suggested that delta-32 arose without a selective event. Tandem repeats found in the coding region of the CCR5 gene could cause unequal homologous recombination, which results in the delta-32 allele. The origins of the delta-32 mutation, however, remain a mystery [ 13 ].
The hype about the delta-32 mutation comes from its ability to protect homozygous individuals from HIV. The protective effect of the delta-32 mutation is a result of eliminating the expression of CCR5 protein on the cell surface, which prevents HIV’s entry into the cell. In the year 1997, however, studies showed that some of them having the homozygous delta-32 mutation were HIV-infected [ 13 ]. Further studies revealed the HIV virus was of the X4 type, which led to very rapid CD4 + T cell decline. Hence, this mutation is limited in its function and does not protect against viral strains which utilize other receptors or show dual-tropism [ 13 ].
In contrast, however, the delta-32 protein product which is localized to the endoplasmic reticulum is an important factor. It is shown to exert a trans-dominant negative effect on the wild-type CCR5 protein, which inhibits its transport to the cell surface. Further analysis in vitro showed the reduction of surface expression of wild type CCR5 and CXCR4 through dimerization by this mutant protein product [ 13 ]. This confers an inhibition to R5, X4 and R5X4 HIV infections [ 13 ]. Homozygous delta-32 individuals with this mutant protein were shown to have suppressed CXCR4 surface protein expression and decreased susceptibility to X4 infection. Experimental proofs also suggested that delta-32 heterozygous individuals with HIV infection do not stably express the mutant protein, are devoid of the molecular mechanism of complete protection and only maybe partially protected [ 13 ].
Stem Cell Transplantation
Stem cells are undifferentiated cells that can differentiate into specialized cells and can also undergo mitosis to produce more stem cells. There are mainly two classes—embryonic stem cells (ECS) and adult stem cells. Stem cells are also taken from the umbilical cord blood just after birth. These act as a repair mechanism for the body, such as skin, blood or intestinal tissues. Adult stem cells are majorly used in medical therapies like bone marrow transplantation. Bone marrow is the spongy tissue present inside the bones which serves as a rich source of adult stem cells. Long-term control of HIV is possible with CCR5-delta 32 stem cell transplantation [ 13 ].
Allogeneic transplantation of stem cells with this mutation in patients with HIV infection and malignancy has been considered as an option since the late 1990s (Fig. 7 ). Human leukocyte antigen (HLA) is a critical factor to be considered during the process of transplantation. The HLA should be a proper match; otherwise, it would lead to rejection by the recipient’s immune system. The limited availability of HLA-matched unrelated donors has made it even more difficult. Only about 1% of Caucasians possess this CCR5 null allele [ 13 ].

Allogeneic hematopoietic stem cell transplant [ 17 ]
Gene Therapy
Zinc finger nuclease technology is a popular tool which can be used for targeting specific DNA sequences in the genome. It falls in the class of restriction enzymes and is artificially made by fusing a zinc finger DNA-binding domain and DNA-cleavage domain. This technique is also engineered to eliminate the CCR5 expression over CD4 + T-cells, and the modified cells have shown to have a half-life of 48 weeks [ 13 ]. But it has its own issues. It is difficult to ensure that the desired repair mechanism is one which is used to repair the double stranded break (DBS) [ 13 ]. It is also challenging to scale it upwards and is an expensive technique.
A breakthrough technique, the CRISPR/Cas9 gene-editing system, is also used to eliminate the CCR5 receptor on the blood stem cells which can give rise to differentiated blood cells that are devoid of this receptor [ 18 ] (Fig. 8 ). These gene-edited stem cells can be established into an HIV-infected patient through bone marrow transplantation and give rise to an HIV-resistant immune system [ 18 ]. This technique, however, can also go sideways which leads to unwanted results that can cause ethical issues to rise. As seen in the highly controversial case of the Chinese scientist, He Jiankui, who with the help of this technology deleted the CCR5 gene in the twins, Lulu and Nana, introduced some unintended mutations in their genetic codes. There is still a lot of research needed to make this technology bioethically a safe tool.

CRISPR/Cas9 gene editing [ 19 ]
Researchers have also engineered a molecule called the chimeric antigen receptor (CAR) and introduced a gene for that molecule into blood-forming stem cells [ 18 ]. This molecule has two receptors that will recognize the antigen (HIV) and direct the immune cells to locate and kill the HIV-infected cells [ 18 ]. When transplanted into mice, which would have the CAR-carrying blood stem cells, it would result in reduced levels of HIV by inducing the immune cells to fight effectively against the virus [ 18 ]. An 80% to 95% drop in viral load was observed in the mice [ 18 ]. It was concluded that gene therapy could be a feasible option for treatment in HIV-positive humans.
Immunological Approaches
Studies have shown that vaccine can contribute effectively in viral clearance such as the Rhesus CMV vaccine vector [ 18 ]. A vaccine vector is a kind of vaccine which consists of chemically weakened viruses that are transported in the body to generate an immune response. The genes used in these vaccines are antigen coding surface proteins from that particular pathogen.
SAV001-H is the first and only preventive HIV vaccine which uses killed HIV-1 virus [ 18 ]. It is unique from other vaccines, as it uses genetically engineered whole virus genome, eliminating its pathogenicity and inactivating its virulence through irradiation and chemical treatments, finally approaching to the first “whole-killed virus”-based HIV vaccine [ 18 ]. The results of Phase 1 clinical trial, which were completed in the year 2013, were found to have serious and adverse effects in the 33 participants [ 18 ]. There was also a surprising boost in the antibody production against p24 and gp120. The HIV viral core is mostly made up of the structural protein, p24, which is called the capsid. A crucial factor in the diagnosis of primary HIV-infected individuals is the p24 antigen assay. High levels of p24 are found in the blood serum during the period between infection and seroconversion. The antibody production is found to increase as much as 64-fold [ 18 ]. The antibody production against gp120, which is a glycoprotein, necessary for attachment to a cell receptor and allow HIV entry, is found to increase up to eight-fold [ 18 ].
Another promising vaccine called the Kang's vaccine also uses the “whole-killed HIV-1,” which is similar to vaccines developed for rabies, polio and influenza [ 18 ]. However, HIV-1 is genetically engineered in such vaccines and raises questions about safety and possibility of large quantity production.
Researchers have also tested an immunogen called eOD-GT8 60mer, a protein nanoparticle, which is designed to mimic a crucial part of the HIV envelope protein which will bind to and activate the B cells to produce plasma cells that secrete antibodies needed to fight HIV [ 18 ]. This nanoparticle was developed in the Schief laboratory and tested in mouse models engineered by the Nemazee laboratory [ 18 ]. The researchers showed that immunization with eOD-GT8 60mer produced antibody progenitors with some of the characters crucial to recognize and block the HIV infection, proposing that it could be a promising first step in a series of immunizations against HIV [ 18 ]. The vaccine appears to work well in mouse models. The researchers are now investigating other immunogens that could work in coexistence with eOD-GT8 60mer [ 18 ].
Case Studies
The berlin patient [ 20 ].
The strongest proof available in favour of a HIV cure stems from the case of Timothy Brown who is popularly known as the Berlin patient (Fig. 9 ). He is considered the first person ever to be cured of HIV. The victory was predicated on doctors taking advantage of nature’s own experiment—the genetic mutation of CCR5 gene that produces a protein co-receptor present on the surface of CD4 + T-cells that HIV uses to gain entry. He was attending university in Berlin when was diagnosed HIV positive. His initial treatment include ART, and he was taking low doses of zidovudine and protease inhibitors. He continued to live a normal life for the next 10 years. But one day, he was again feeling extremely exhausted and the doctor had diagnosed it to be anaemia. He had received red blood cell transfusion for nearly a week and was then sent to an oncologist, Dr Huetter, when the previous doctor was unable to resolve the situation. The oncologist performed a painful bone marrow biopsy and after further diagnosis he was informed that he had acute myeloid leukaemia (AML).

Timothy Ray Brown a.k.a. “The Berlin patient” [ 21 ]
He then started receiving treatment at one of the Berlin University hospitals and had to receive four rounds of chemotherapy treatment. During the third round of chemotherapy, he had gotten a fatally dangerous infection and was immediately put into an induced coma. His blood sample was collected and sent to a stem cell donor bank with the German Red Cross to find matches in case he needed transplantation. Luckily, he had 267 matches which sparked an idea to locate donors with a homozygous CCR5 delta-32 mutation on CD4 + T-cells who are almost immune to HIV infection. A donor was found at the 61st attempt and had agreed to donate when necessary (Fig. 10 ).

Adam Castillejo a.k.a “The London patient” [ 23 ]
However, Timothy Brown had been reluctant and had said no to transplantation as the success rate was only 50–50. But at the end of 2006, leukaemia had rebounded and he desperately needed transplantation to survive. He received the stem cell transplant on February 6, 2007 and stopped taking his antiretroviral medication. Nearly 3 months after he underwent transplantation, HIV was no longer found in his body and he had thrived until the end of the year.
Unfortunately, life had other plans for him. After coming back from a trip to the USA, he was diagnosed with pneumonia and the leukaemia was back. The doctors decided to treat him with a second transplantation from the same donor in February 2008. The recovery was a tough one. He was almost paralyzed and went nearly blind. He had, however, eventually learnt to walk again and fully recovered 6 years later. He was continuously tested for HIV with extensive and precise tests. It was finally good news for him! Since 2010, when he decided to go public, he had interviewed for various magazines: POZ Magazine , New York Magazine and Science Magazine among others and decided to devote his life in supporting research for cures against HIV. In July 2012, he started the Timothy Ray Brown Foundation under World AIDS Institute and has worked with many scientists, organizations, research laboratories and universities to work on cures such as vaccination against HIV.
The London Patient [ 22 ]
The London patient may be the second person with HIV to no longer have the virus. In March 2019, in a report published in journal Nature , a group of investigators had announced the cure of a second HIV-positive patient. His success story depicts that CCR5 is a viable target for HIV research and treatment.
The London patient, who had chosen to remain anonymous, came out in public on March 9 th 2020. Adam Castillejo grew up in Caracas, Venezuela, and later shifted to London with his mother, as his parents were divorced. He was first diagnosed with HIV in 2003 and had started taking drugs to control the HIV infection in 2012. He had taken antiretroviral therapy for years before being diagnosed with an advanced form of blood cancer called Hodgkin’s lymphoma. Again, as in the case of the Berlin patient, the cancer was resistant to standard chemotherapy, so his doctors had advised more intensive chemotherapy along with bone marrow stem cell transplant. In 2016, he had agreed to transplantation and received it from a healthy donor who carried the CCR5 mutation. So, when his immune system regrew, it lacked the protein and was impervious to HIV. His virologist, Dr Ravindra Gupta, from the University of Cambridge, thinks it is a cure because a year had passed and they had carried out a few more tests for the viral load. In Adam Castillejo’s own words, “I don’t want people to think, “Oh, you’ve been chosen.” No, it just happened. I was in the right place, probably at the time right time, when it happened.” Adam Castillejo wants to be the “ambassador of hope” for people with this illness.
Although the scientists describe this case as a long-term remission, experts are calling it a potential cure. Such transplants are, however, dangerous and can be fatal. They are also an impractical approach to cure the millions already infected. These are highly risky procedures and can lead to serious complications. There still has to be a lot of research done to extend this type of treatment to a wider population infected with HIV.
A comparative study of the two patients reveals that their cases were in fact quite similar (Table (Table9 9 ).
Summary of the two cases—the Berlin patient and the London patient [ 24 ]
Lifestyle Practices to Prevent HIV Infection
Prevention is better than cure. And with HIV infections, one should practice prevention with utmost care and sincerity. An HIV diagnosis could turn one’s life upside down. So, it’s better to lead a healthy lifestyle by making the correct choices.
Measures for Protection Against HIV Infection
HIV is majorly spread through unprotected vaginal or anal sex. Choose less risky behaviour and be cautious. Not taking medicines to prevent or treat HIV is equally responsible for HIV infection. The number of sexual partners should be limited. One should get tested for sexually transmitted diseases and also know the sexual partner’s status. One can talk about pre-exposure prophylaxis to their respective healthcare provider. It is a preventive option for people who are not infected yet but are exposed to high risks of being HIV positive. HIV is also spread through intravenous injections and blood transfusions. Use of sterile equipment in such cases is a necessity.
Pre-exposure Prophylaxis
This is a preventive method of taking pills by people who are not HIV positive yet but who are at a high risk of getting infected and spreading it to others. A pill, named Truvada, contains two medicinal components, emtricitabine and tenofovir, that are used in combination with other drugs to treat HIV [ 25 ]. These medicines work on keeping the virus from creating a permanent infection.
Post-exposure Prophylaxis
Post-exposure prophylaxis (PEP) is a short course of HIV medicines taken soon after a possible exposure to HIV [ 25 ]. Every hour counts. For the treatment to be effective, the course should begin within 72 h after exposure to HIV; otherwise, it will not have any effect [ 25 ]. This treatment should be used only in cases of emergency. A person prescribed with PEP will need to take the medicines for 28 days at a stretch and then visit their respective healthcare provider for further tests [ 25 ]. Even if taken correctly, it may not be 100% effective. The sooner the medication is started, the better.
Healthy Practices to Follow When Living with HIV
A healthy, well-balanced and nutritious diet can help a person lead a better life by preventing health related issues like malnutrition and stopping the progression from HIV to AIDS. A well-balance diet is rich in whole grains, fresh fruits and vegetables, protein, low fat dairy products and multivitamins like zinc and B12. It also constitutes what should be cut down—fried foods, processed foods and sugary drinks. Smoking should be stopped when diagnosed with HIV. According to CDC, in the USA, the rate of adults with HIV, smoking is two to three times higher in adults infected with HIV than the nearly 18% of uninfected adults who smoke. Researchers at the Syracuse University analysed the data from 212 adults infected with HIV and found that the ones who smoked reported having more symptoms like dizziness and coughing.
Putting a stop to illegal drug use is equally necessary. People should seek treatment for addiction to illegal drugs like heroin, cocaine and methamphetamines. Sharing of needles for drugs can leave one exposed to other infections like hepatitis which might lead to a faster progression from HIV to AIDS. A recent study from the University of Pennsylvania School of Medicine showed a dramatic increase in the ability of HIV to attack healthy cells when methamphetamine is present in the bloodstream. This indicates that illegal drugs are also aiding in the HIV infection.
Being physically fit through a good work-out three to six times a week can help improve a person’s mood, perspective and overall quality of life. A good amount of moderate exercise can help fight HIV symptoms of nerve pain, loss of appetite and reduce the risks of other chronic diseases like heart disease, diabetes and osteoporosis. Taking the prescribed medication on time is known as adherence. This is vital to help reduce the risk of HIV becoming drug resistant and helps the immune system function for a longer time.
Nowadays, with the help of Internet of Things or IoT, patient’s health can be monitored 24/7. The quality of care provided can be increased many-folds with the help of monitoring devices enabled with current technology [ 26 ]. Concept of E-Health and M-Health is currently trending. E-Health makes use of electronic and communication processes with improved cyber security [ 26 ]. Some of the E-Health devices include GPS tracking, pedometer and electronic health records [ 26 ]. M-Health systems provide doctors with the complete medical history of the patient, so the treatment becomes easier and does not delay in case of emergencies. It makes use of mobile phones and other communication systems to help the patients with information about preventive health care services and collects data in real time as well [ 26 ]. The other important applications include chronic disease management, monitoring of diseases and tracking of epidemic outbreaks [ 26 ].
Genomic Diversity and Clinical Implications
Despite billions of dollars being invested, there is currently no HIV vaccine available that can either prevent the disease or treat those who suffer from it. An AIDS patient harbours 100 million genetically distinct variants of HIV [ 27 ]. This high diversity of HIV-1 is due to high replication rates, errors in reverse transcriptase and recombination events that mainly occur during the viral replication process. Reverse transcriptase enzyme has approximately a rate of 10 –4 nucleotide substitutions per replication cycle. Deletions, insertions and duplications are major contributing factors to the genetic variation of the virus [ 27 ]. Genetic recombination also plays an important role in creating genetic diversity. Template switches between two copies of RNA strands occur regularly during reverse transcription [ 27 ]. This generates a lot of mutations with the help of inter- and intra-molecular jumps. These mutations can either be drug resistant or inhibit the viral replication capacity.
HIV-1 can be classified into four main groups: M, N, O and the recently identified P. The M group is further identified into 4 subtypes (A to J). Studies have shown that there is a worldwide spread of non-B subtype viruses, and with the introduction of antiretroviral drugs, more research has to be conducted regarding the responsiveness of the drug resistance in non-B subtypes [ 27 ]. Different types of HIV-1 resistance are observed in different subtypes at varied levels. For example, subtypes B and G have shown to develop resistance against nelfinavir [ 27 ]. Research is also being done in the role of polymorphisms for development of drug resistance, to assess the genotypes before and after the therapy to be able to establish any association between the two [ 27 ].
Variation of Disease Progression Rate
There are 3 phases of the progression of HIV-1 infection- primary infection, chronic asymptomatic phase of infection and finally, AIDS. In the asymptomatic phase, neither signs nor symptoms of the disease are present, and this phase lasts an average of about 10 years. They can be divided as typical progressors, rapid progressors, slow progressors and long-term progressors. Rapid ones (10–20%) develop AIDS within 5 years of infection [ 28 ]. Slow progressors (5–15%) remain free of AIDS 15 years after infection [ 28 ]. Long-term progressors that constitute 1% show no signs and symptoms [ 28 ]. Factors like host genetic make-up, immune responses, co-infection and viral genetics and adaptation are attribute to this huge variation in disease progression [ 28 ]. But there is no solid evidence as such.
Some individuals known as elite controllers are able to manage the viral replication for longer durations, others are shown to rapidly lose CD4 + T-cells after seroconversion in the absence of cART (combination antiretroviral therapy). Scientists have conducted research studies that has led to the conclusion that rapid progression before administration of cART stops the recovery of CD4 + T-cells once the suppressive response to HIV-1 through cART is achieved. These findings have implications in public health policy making, clinical outcomes and science research. Ideally, cART should be initiated as soon the patient is diagnosed with HIV-1 irrespective of the CD4 + T-cell count. However, in clinical settings where cART is not widely available, these results would support strategies that may help in promoting frequent testing to reduce the proportion of patients initiating cART at low CD4 + T-cell counts. For those testing early, frequent CD4 + T-cell count should be monitored close to the time of HIV diagnoses to establish the rapid progressors phenotype in order to avoid unnecessary CD4 + T-cell count decay among rapid progressors. Finally, interpretation of the immunopathological basis of rapid progression can help improve individual clinical outcomes and limit its impact in the global HIV-1 pandemic.
Development of Drug Resistance as a Major Barrier to Treat HIV
HIV-1 has a high mutation rate. An estimated 10 10 virions per day can be produced in untreated patients that may result in variants called quasispecies. The complexity is also increased due to high recombination rate whenever more than one variant infects the same cell. All these are contributing factors that help in invading the host’s immune system and fostering drug resistance. Salvage therapy is also useful in cases when more than one regimen failed or a single regimen failed for a patient. It can be used to suppress the virus levels below the detection level and should have high genetic barrier to resistance to prevent rebound [ 29 ]. Clinicians need to focus on patient’s adherence as well as access to antiretrovirals (ARVs), drug interactions, tolerability, genotypic and phenotypic resistance testing, cross-resistance, genetic barrier and potency of ARVs [ 29 ].
Overcoming Obstacles and Future Prospects
At present, the reason for not being able to achieve a complete cure with the help of ART, in spite of achievement of undetectable viral load, is due to the presence of dormant virus or HIV latency. In a method call shock and kill, immune stimulants shock the latent virus from hidden reservoirs and then attempt to kill reactivated HIV [ 18 ]. An enzyme has been identified which is called histone deacetylase (HDAC) which is responsible for the sustained latency. Some studies show promise but are yet to be confirmed by clinical trials. Flushing these latent CD4 HIV-infected cells from their reservoirs with these HDAC-inhibitors into the blood circulation makes them susceptible to ART. Vorinostat and panobinostat are two such promising drugs [ 18 ].
Histone deacetylase inhibitors seem to have a broad spectrum of epigenetic activities. Vorinostat (also called Zolinza) is a U.S. Food and Drug Administration approved medicine, which has been used for the treatment of cutaneous T-cell lymphoma (CTCL) [ 18 ]. They help in flushing the virus from the reservoirs into the circulation. The dose is 400 mg. Other drugs on the pipeline are Protein kinase C agonist bryostatin-1 and GS-9620—TLR7 agonist [ 18 ].
Romidepsin (also called Istodax) is another HDAC inhibitor drug, which induces HIV-1 transcription to form plasma HIV-1 RNA that can be easily detected with standard assays [ 18 ]. This gives a possibility of reversing the HIV-1 latency in vivo without hindering T cell mediated immune response [ 18 ]. These findings will help the researchers with future clinical trials aiming to eliminate the HIV-1 reservoirs.
Research for curing HIV is at an infant stage but a promising one. Scientists are working on two broad types of HIV cures—a functional cure and a sterilising one.
The approach of the functional cure is to reduce the virus levels in the body to an undetectable stage, where the patient no longer needs to be on HIV medication or has no risk of progression to AIDS nor transferring the virus to others. Unlike the functional cure, however, a sterilising cure aims to get rid of HIV from the body completely by eliminating cells from latent reservoirs. It has proved to be an extremely challenging task for scientists, who believe it may be unachievable in the majority of them living with HIV. However, some findings by researchers at the University of Pittsburgh could lead to a foundation for an HIV vaccine. Clinical trials are in the works.
Abivax, a French company, is developing a drug that binds to some specific sequence of the viral RNA and inhibits its replication. During clinical trials, it has shown that this may have the potential to become a functional cure. The key is that it can target the reservoir of HIV viruses that hide inactive within our cells. It can target the reservoirs where HIV viruses act as inactive, within the infected cells. The result of phase IIa trial was quite promising. Fifteen patients were given the drug in combination with ART, and it was observed after 28 days of treatment that eight patients showed a 25% to almost 50% reduction of their HIV reservoirs compared to those only taking ART. The company is planning a phase IIb clinical trial to confirm the effects of the drug in the long term.
Research and development in HIV and its cure have come a long way since the disease was discovered in the 1980s. ART was a major milestone that has changed the lives of millions for good, but the next ambitious goal is to find an HIV cure before the year 2020. There are several approaches to an HIV cure ranging from shock and kill therapy, immunotherapy, vaccine development to gene editing using zinc finger nucleases and the CRISPR/Cas9 system, but finding the best possible solution is a challenge. One of the biggest challenges around any HIV treatment is the ability of the virus to rapidly mutate and develop resistance. Many of the new approaches do not provide any valuable insights as to whether the virus has the potential to become resistant. As of now, none of these functional cures have reached late-stage clinical trials, and the aim of finding an HIV cure until 2020 seems far-fetched. However, 2020 will likely be marked as an important milestone as the first late-stage trials will be executed. If successful, it could bring the approval of the first functional HIV cure in ten years.
There are two gene therapies undergoing human trials—one is to destroy the CCR5 receptor of the immune cells of people infected with HIV and the other therapy includes the CRISPR technology which is still under early trials. This mutation does not necessarily protect the person against all types of HIV. It was found that in one of the patients who had received the bone marrow treatment, it was found to have the CXCR4-tropic form. It uses a different type of receptor to enter and infect the cells. It was, however, not known whether this virus was acquired after the treatment or if some patients do contract a small amount of CXCR4-tropic virus that starts to multiply when other types are not present.
HIV research continues on many fronts that could provide the same results and only some of which rely on the CCR5 delta 32 mutation, which should be explored extensively. There are many strategies which are in the early stages of development. Scientific process can be slow but if done correctly, advances can be made to find a scalable, cost-effective cure for everyone.
Acknowledgments
The authors listed in this paper wish to express their appreciation to the RSST trust Bangalore for their continuous support and encouragement.
Authors Contribution
All authors have contributed equally with their valuable comments which made the manuscript to this form.
There was no funding provided for the above research and preparation of the manuscript.
Compliance with Ethical Standards
The authors declare that they have no conflict of interest.
All the authors listed hereby confirmed that in the above research, there were no human participants and/or animals involved in any kind of determination, evaluation or research studies.
There is also final confirmation given by all the listed authors for the submission of manuscript in its actual state. The authors listed above also confirm that the above-mentioned manuscript is in its original state and the manuscript is neither submitted anywhere nor in the submission process in any other journals. In addition, all the authors have solely contributed their original work in the preparation of this manuscript. If the copying or similarity have been found, then in all situations the listed authors are solely responsible.
Significance Statement
This article aims to increase awareness among the society about the current scenario of HIV/AIDS. The scientists are working on 2 types of cures—functional and sterilizing. The path to finding a cure is a promising one as late-phase trials begin in 2020.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Praveen Kumar Gupta, Email: ni.ude.ecvr@atpugkneevarp .
Apoorva Saxena, Email: [email protected] .
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Peer- and community-led responses to HIV: A scoping review
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliations MPact Global Action for Gay Men’s Health and Rights, Oakland, CA, United States of America, Alameda County Department of Public Health, Oakland, CA, United States of America
Roles Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Validation, Writing – review & editing
Affiliation Joint United Nations Programme on HIV/AIDS (UNAIDS), Geneva, Switzerland
Roles Conceptualization, Writing – review & editing
Affiliations Social, Health and Empowerment Feminist Collective of Transgender Women in Africa, East London, South Africa, Innovative Response Globally to Transgender Women and HIV (IRGT), Oakland, CA, United States of America
Roles Conceptualization, Validation, Writing – review & editing
Affiliation Global Network of Sex Work Projects, Edinburgh, Scotland
Affiliation International Network of People Who Use Drugs, London, United Kingdom
Roles Validation, Writing – review & editing
Affiliations MPact Global Action for Gay Men’s Health and Rights, Oakland, CA, United States of America, Arreola Research, San Francisco, CA, United States of America
Affiliation Graduate Institute, Geneva, Switzerland
Affiliation Rumah Cemara, Bandung, Indonesia
Affiliation The Global Fund to Fight AIDS, Tuberculosis, and Malaria, Geneva, Switzerland
Affiliation Gestos–HIV, Communication and Gender, Recife, Brazil
Affiliation International Community of Women Living with HIV Eastern Africa, Kampala, Uganda
Affiliation AIDS and Rights Alliance for Southern Africa, Windhoek, Namibia
- George Ayala,
- Laurel Sprague,
- L. Leigh-Ann van der Merwe,
- Ruth Morgan Thomas,
- Judy Chang,
- Sonya Arreola,
- Sara L. M. Davis,
- Aditia Taslim,
- Keith Mienies,

- Published: December 1, 2021
- https://doi.org/10.1371/journal.pone.0260555
- Reader Comments
Introduction
In June 2021, United Nations (UN) Member States committed to ambitious targets for scaling up community-led responses by 2025 toward meeting the goals of ending the AIDS epidemic by 2030. These targets build on UN Member States 2016 commitments to ensure that 30% of HIV testing and treatment programmes are community-led by 2030. At its current pace, the world is not likely to meet these nor other global HIV targets, as evidenced by current epidemiologic trends. The COVID-19 pandemic threatens to further slow momentum made to date. The purpose of this paper is to review available evidence on the comparative advantages of community-led HIV responses that can better inform policy making towards getting the world back on track.
We conducted a scoping review to gather available evidence on peer- and community-led HIV responses. Using UNAIDS’ definition of ‘community-led’ and following PRISMA guidelines, we searched peer-reviewed literature published from January 1982 through September 2020. We limited our search to articles reporting findings from randomized controlled trials as well as from quasi-experimental, prospective, pre/post-test evaluation, and cross-sectional study designs. The overall goals of this scoping review were to gather available evidence on community-led responses and their impact on HIV outcomes, and to identify key concepts that can be used to quickly inform policy, practice, and research.
Our initial search yielded 279 records. After screening for relevance and conducting cross-validation, 48 articles were selected. Most studies took place in the global south (n = 27) and a third (n = 17) involved youth. Sixty-five percent of articles (n = 31) described the comparative advantage of peer- and community-led direct services, e.g., prevention and education (n = 23) testing, care, and treatment programs (n = 8). We identified more than 40 beneficial outcomes linked to a range of peer- and community-led HIV activities. They include improved HIV-related knowledge, attitudes, intentions, self-efficacy, risk behaviours, risk appraisals, health literacy, adherence, and viral suppression. Ten studies reported improvements in HIV service access, quality, linkage, utilization, and retention resulting from peer- or community-led programs or initiatives. Three studies reported structural level changes, including positive influences on clinic wait times, treatment stockouts, service coverage, and exclusionary practices.
Conclusions and recommendations
Findings from our scoping review underscore the comparative advantage of peer- and community-led HIV responses. Specifically, the evidence from the published literature leads us to recommend, where possible, that prevention programs, especially those intended for people living with and disproportionately affected by HIV, be peer- and community-led. In addition, treatment services should strive to integrate specific peer- and community-led components informed by differentiated care models. Future research is needed and should focus on generating additional quantitative evidence on cost effectiveness and on the synergistic effects of bundling two or more peer- and community-led interventions.
Citation: Ayala G, Sprague L, van der Merwe LL-A, Thomas RM, Chang J, Arreola S, et al. (2021) Peer- and community-led responses to HIV: A scoping review. PLoS ONE 16(12): e0260555. https://doi.org/10.1371/journal.pone.0260555
Editor: Petros Isaakidis, Médecins Sans Frontières (MSF), SOUTH AFRICA
Received: August 19, 2021; Accepted: October 18, 2021; Published: December 1, 2021
Copyright: © 2021 Ayala et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its Supporting Information files.
Funding: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Communities affected by health emergencies have a long history of acting to promote and protect the wellness and rights of their members, a fact that has been generally accepted in the public health field [ 1 – 3 ]. Communities are recognized as a ‘critical catalyst’ to achieving the health-related targets in Sustainable Development Goal (SDG) 3 [ 4 ]. Stakeholders at all levels in the HIV sector are also increasingly recognizing, with some resolve, that communities living with and disproportionately affected by HIV must now play an even more prominent role in the global response [ 5 – 8 ]. This recognition comes with the realization that the world is off-track to meet global HIV targets [ 9 , 10 ] as evidenced by current epidemiologic trends in HIV incidence, prevalence, viral suppression, and AIDS deaths, especially among socially marginalized populations [ 11 , 12 ]. Underlying these trends are persistent inequities in access to and funding for HIV prevention, care, and treatment, which are experienced by people living with HIV, young women and girls (especially in Sub-Saharan Africa), gay and bisexual men, people who use drugs, prisoners, sex workers, and transgender people (key and vulnerable populations) [ 13 ]. Unabated stigmatization, discrimination, violence, and criminalization directed at key and vulnerable populations fuel inequities, undermining traction made towards achieving global targets [ 14 – 25 ]. Over 60% of all new HIV infections worldwide are among key populations, which reflect said inequalities [ 9 ].
The COVID-19 pandemic and its aftermath further threaten the gains made in a global HIV response that is already off-track [ 26 , 27 ]. People living with HIV are more likely than the general population to become severely ill with COVID-19 and more likely to die if hospitalized [ 28 ]. Investment in comprehensive HIV services, which is at present contracting [ 9 ], will likely shrink further as the world struggles to fund its response to the COVID-19 crisis. Moreover, key and vulnerable populations worldwide continue to be excluded from national social protection schemes, undermining critical and hard-fought gains in the fight against AIDS [ 29 ].
An international commitment to people-centred systems for health was enshrined in the United Nations (UN) 2021 Political Declaration on HIV and AIDS (“the Declaration”), building on strong commitments in the 2016 Political Declaration to ensure 30% of HIV service delivery would be community-led by 2030 [ 30 ]. In the 2021 Declaration, UN member States committed, as appropriate in the context of national programmes, to increase the proportion of HIV services delivered by community-led organizations to reach 30% of HIV testing and treatment services, 80% of HIV prevention services for high-risk populations, and 60% of programmes to achieve societally enabling environments by the year 2025 [ 31 – 33 ]
However, commitments made in 2016 have not yet translated into scaled-up coverage of community-led responses to HIV, despite donor recognition of the integral role communities can and do play [ 34 ]. The 2021 commitments are likely to see the same fate without concerted action. There are several reasons for this. First, the 2016 Declaration failed to clearly define what constitutes a ‘community-led’ programme, and until recently, the HIV sector had not come together to develop a shared definition of the term. As a result, activities led by people living with and disproportionately affected by HIV at the grassroots level have often been conflated with those led by national agencies or by international non-governmental organizations (INGOs), which may physically base themselves in communities, but that may not in fact have representatives from affected communities in senior management positions or on governance boards. This confusion over what legitimately constitutes a ‘community-led’ programme obfuscates responses at the local level, makes comparisons across studies challenging at best, and complicates monitoring, reporting, and analysis of progress towards commitments in the Declaration across regions.
Second, as previously mentioned, although there is recognition by governments, donors, and implementers of the need for community-led responses, the evidence to support them has lagged. This is because community-led organizations and networks, those with the greatest interest in documenting the effectiveness of their responses, seldom have the resources to conduct large-scale research [ 35 ]. Further, community-led studies might be critiqued as biased or conflicted or dismissed if experimental study designs, e.g., randomized control trials, which may be more appropriate for biomedical research, were not used to test for efficacy [ 36 – 39 ]. And because the HIV sector has been operating without a generally accepted definition, quantitatively measuring the comparative advantages of community-led responses is difficult to achieve.
Third, the global HIV response continues to operate with a “democratic deficit”. In other words, despite the expressed commitment to the Greater Involvement of People Living with AIDS (“the GIPA Principle”), a commitment which was explicitly mentioned for the first time in the 2021 Declaration, people living with and most affected by HIV are often not meaningfully and equitably engaged in decision-making, planning, financing, or implementing service delivery [ 32 , 40 – 43 ]. As a result, funding intended for community-led organizations has sometimes been captured by programmes that in practice fail to consult or meaningfully partner with the communities they serve. What some authors have called the biomedicalization of the HIV response has further complicated decision-making regarding the various roles communities can and should play, including in service delivery [ 5 ].
Clarifying terminology and examining the evidence for greater coverage of community-led responses are of urgent importance. This article presents a definition for community-led responses developed in 2019 during a 2-day expert consultation convened by the Joint UN Programme on HIV/AIDS (UNAIDS) and endorsed by a diverse group of government and civil society experts in late 2020. We then present the results of a scoping review that examined and synthesized research focused on community- and peer-led HIV responses published in the past 40 years. Our aim is to strengthen the case for expanded coverage of community-led HIV responses, supported by a clear definition, peer-reviewed evidence, and a set of recommendations for decision makers and funders.
Community experts’ meeting to define ‘community-led’
Recognizing the challenges in monitoring progress towards the commitments in the Declaration and the need for a clearer definition of “community-led”, the UNAIDS Secretariat (JAI, LS) convened a 2-day consultation with community experts in June 2019, to suggest an operational definition of community-led responses to HIV, at the request of its Programme Coordinating Board (PCB). A subsequent consultation was planned to define ‘woman-led’, building from the definitions developed during the 2019 Expert Consultation in Montreux, Switzerland. The meeting was postponed because of the COVID-19 pandemic.
Experts who participated in the June 2019 consultation included representatives from the leading global transnational networks of people living with HIV and key populations, who together represent hundreds of national and regional community-led organizations. They included: the International Community of Women Living with HIV (ICW), Global Network of People Living with HIV (GNP+), Global Network of Young People Living with HIV (Y+), International Treatment Preparedness Coalition (ITPC), Global Network of Sex Work Projects (NSWP), International Network of People Who Use Drugs (INPUD), Innovative Response Globally to Transgender Women and HIV (IRGT), MPact Global Action for Gay Men’s Health and Rights, TB People (the network of people living with tuberculosis), Gestos–HIV, Communication and Gender, representatives from the NGO delegation to the UNAIDS PCB, and members of the Communities Delegation to the Global Fund to Fight AIDS, Tuberculosis, and Malaria (“the Global Fund”). Staff members from the Global Fund and the U.S. Centres for Disease Control and Prevention were also in attendance. The meeting participants recognised the priorities of people living with HIV, including women and young people living with HIV, gay men and bisexual men, people who use drugs, sex workers, and transgender people as an integral part of their consensus-building deliberations.
Experts began their meeting with a review of findings from a global survey undertaken by UNAIDS just prior to the consultation. The survey, offered in four languages (English, French, Spanish, and Russian), was designed to canvass diverse definitions for ‘community’ and to identify core elements of ‘community-led’ and ‘key population-led’ in the context of the HIV/TB response. There were 475 completed surveys from respondents, representing 97 countries. Experts also studied policy documents and discussed ways to use the definition to monitor support and funding for critical community-led programmes. The meeting resulted in working definitions for the terms “community-led organizations”, “community-led responses”, “key population-led organizations”, and “key populations-led responses”. Meeting participants defined community-led responses as:
…actions and strategies that seek to improve the health and human rights of their constituencies, that are specifically informed and implemented by and for communities themselves and the organizations, groups, and networks that represent them . Community-led responses are determined by and respond to the needs and aspirations of their constituents. Community-led responses include advocacy, campaigning and holding decision-makers to account; monitoring of policies, practices, and service delivery; participatory research; education and information sharing; service delivery; capacity building, and funding of community-led organizations, groups, and networks. Community-led responses can take place at global, regional, national, subnational, and grassroots levels, and can be implemented virtually or in person . Not all responses that take place in communities are community-led .
Subsequent to this expert consultation, the proposed definitions of community-led responses and community-led organizations were vetted with two multistakeholder working groups for further input, resulting in minor changes to wording [ 44 , 45 ]. The careful distinctions made in the definitions, initially developed by community experts and further refined through the multistakeholder processes, are important and include a clear emphasis on the meaningful inclusion of people living with HIV, gay and bisexual men, people who use drugs, sex workers, and transgender people in designing, implementing, managing, and evaluating programmes. Similar distinctions have been made by other groups [ 46 ]. All four definitions are presented in Table 1 . The definition of community-led responses presented here informed the inclusion/exclusion criterion used in the scoping review, which focused on identifying evidence for the impact of community-led programmes on HIV outcomes.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0260555.t001
Scoping review
The scoping review began as a discussion between co-authors (GA, LS, JAI) and principal stakeholders involved in a technical consultation on social enablers as part of the UNAIDS-led 2025 Target Setting process. Building on this work, we conducted a literature search focused on research published between January 1982 (six months after the first cases of HIV/AIDS were published in the United States of America) and February 2021 [ 47 ]. The overall goals of this scoping study were to gather available evidence on community-led responses and their impact on HIV outcomes, and to identify key concepts that can be used to immediately inform policy, practice, and research [ 48 ]. We followed a five-step procedure that involved articulating a research question, identifying relevant studies, selecting studies, charting the data, and summarizing the findings [ 49 – 51 ]. Our study was guided by the question: What evidence is there about the comparative advantages of community-led HIV responses ?
Data sources and search strategy
The search was conducted on February 20, 21, and 22, 2021 by the lead author (GA) using PubMed/MEDLINE, Embase, and Web of Science. The search included articles published between January 1982-and February 2021. Due to resource limitations, we restricted the search to articles published in English and focused on HIV. We used a Boolean search strategy [ 52 ], which combined search terms as follows: “community led HIV” OR “peer led HIV” OR”community led AIDS” OR “peer led AIDS”.
Only titles were reviewed for the first level of screening. Second-level screening involved review of abstracts to exclude articles not relevant to the search and to remove duplicates. Studies were eligible for inclusion if they described community-led responses to HIV and their outcomes. Understanding that a common definition for community-led was absent when many studies were published, we included the search term ‘peer-led’ and evaluated each article against the criteria described in the definition developed at the Montreux consultation. Our search strategy included randomized controlled trials, quasi-experimental, prospective, pre/post-test evaluation, and cross-sectional study designs. We excluded study protocols, feasibility studies, case studies, case reports, editorials, behavioural surveillance studies, biomedical or pharmaceutical studies, and program reports. We also excluded articles that were not HIV-related, and/or that did not describe a program or intervention that was community- or peer-led.
After review and removal of non-relevant articles and duplicates, the two lead authors (GA, LS) cross-validated selected records, with inter-rater agreement reached for 86% of retrieved publications. Inclusion and exclusion discrepancies were discussed and resolved. Full text articles were then retrieved for review after consensus was reached. All co-authors were invited to identify additional peer-reviewed articles and grey literature, which were added if they appeared relevant to the review and conformed to the inclusion criteria. The study characteristics from full articles were extracted and compiled into a single spreadsheet for additional validation and coding. Authors communicated via email to resolve any additional outstanding questions or disagreements. Simple descriptive statistics were calculated to summarize the characteristics of research and data [ 47 ]. Other than what we describe in the methods section of this paper, no formal review protocol exists.
Search and selection of evidence
Our search yielded 279 potentially relevant records. After screening titles for relevance, 102 studies were excluded. After reviewing all abstracts remaining records for relevance, lead authors (GA, LS) then excluded another 56 articles. Sixty-two duplicate abstracts were identified and removed, leaving a total of 59 records. And after cross-validation and full text screening, 36 articles were selected. An additional 12 articles identified by co-authors and not captured by this scoping review were then added. The flow of articles in the selection process is presented in Fig 1 .
https://doi.org/10.1371/journal.pone.0260555.g001
Our search strategy yielded a total of 48 articles that met the inclusion criteria. Study methods and summary of findings are displayed in Table 2 .
https://doi.org/10.1371/journal.pone.0260555.t002
Study characteristics
While scarce, research on community-led responses and their outcomes appears to be gaining traction in recent years. Sixty-nine percent (n = 33) of articles included in this scoping review were published in the last 10 years between 2011 and early 2021. Fifty-six percent (n = 27) took place in the global south. South Africa (n = 4) and China (n = 3) were represented in the highest number of included studies from the global south [ 53 – 79 ]. The United States of America (n = 9) represented the highest number of studies that were implemented in the global north [ 80 – 94 ]. A diverse range of focus populations were represented in selected articles. Youth, gay and bisexual men, and people living with HIV were study populations in 27%, 23%, and 16% of articles included, respectively [ 56 , 67 , 69 , 72 , 75 , 76 , 78 , 81 , 84 , 86 , 88 , 89 , 94 – 99 ]. Research methods also varied. Twenty-seven percent of articles reported findings from quasi-experimental studies (n = 13) [ 54 , 57 , 58 , 64 , 67 , 70 , 72 , 74 , 77 , 80 , 81 , 84 , 86 ], 23% (n = 11) from systematic reviews [ 62 , 63 , 68 , 69 , 75 , 83 , 95 , 96 , 98 – 100 ], 19% (n = 9) from randomized control trials [ 55 , 59 , 61 , 73 , 76 , 87 – 90 ], and 15% (n = 7) from prospective or longitudinal studies [ 56 , 60 , 65 , 78 , 79 , 92 , 93 ].
A range of community-led approaches were described in the 48 articles we reviewed. Nearly half (48%, n = 23) described peer-led education or prevention interventions [ 54 , 55 , 57 – 62 , 64 – 66 , 70 , 74 , 79 , 80 , 82 , 83 , 85 – 88 , 91 , 96 ], of these more than half (n = 12) were focused on students or youth. Approaches in reviewed articles also include community-led testing, care, and treatment (n = 8) [ 56 , 63 , 67 , 69 , 73 , 94 , 97 , 98 ], community mobilization, advocacy, monitoring, and human rights programs (n = 5) [ 53 , 75 , 78 , 93 , 100 ], community support groups, clubs, and mentors (n = 4) [ 71 , 76 , 89 , 95 ], adherence programs (n = 4) [ 72 , 77 , 90 , 99 ], community empowerment (n = 3) [ 68 , 81 , 84 ], and drop-in centres (n = 1) [ 92 ]. General characteristics of studies included in this scoping study are presented in Fig 2 .
https://doi.org/10.1371/journal.pone.0260555.g002
Outcomes studied also varied. At the individual level, more than half of studies (58%, n = 28) reported improved prevention outcomes, i.e., condom use, sexual risk, self-efficacy, attitudes, and intentions [ 53 – 55 , 57 – 62 , 64 , 66 , 70 , 71 , 74 , 79 – 88 , 91 , 93 , 96 , 100 ]. There were 8 studies that reported improved HIV treatment adherence [ 89 , 90 , 95 , 99 ] and viral load or viral suppression [ 56 , 72 , 76 , 77 ]. Two studies report HIV incidence as an outcome [ 65 , 68 ]. Findings that community-led responses led to improvements in HIV incidence were associative. At the service level, improvements were reported in 10 studies, including in the areas of access, quality, demand, linkage to care, utilization, community-provider relationships, and coordination [ 63 , 67 , 69 , 73 , 75 , 78 , 92 , 94 , 97 , 98 ]. At the societal level, the beneficial effects of community-led HIV responses reported included: increases in community engagement, mobilization, social cohesion; and improvements in institutional norms and action planning [ 53 , 75 , 78 , 93 , 97 , 100 ]. Community empowerment was reported as critically important for engaging sex workers and gay and bisexual men, although its benefit was implied for other populations as well.
There were 3 studies reporting the beneficial effects of community-led HIV responses at the structural level, 2 of which are systematic reviews. Outcomes reported in this category included broadened recognition of gay men and other men who have sex with men as a priority population, secured positive influence on policy, reduced stock outs of HIV-related commodities, increased adoption of viral load testing to monitor clinical outcomes, improved access to legal aid, increased awareness of rights on the part of both rights holders and duty bearers, and improved community-government relations [ 75 , 78 , 100 ]. A recent systematic review that examined human rights-related interventions, found improvements in HIV-related health outcomes in addition to positive changes at the structural-level. The same review also found a small number of interventions that had no or negative influence. These failures appeared to be related to incomplete initiatives, limited dissemination, or limited enforcement of study protocols (100).
Nine studies in our scoping review reported mixed results or no differences in main outcomes measured between intervention and comparison arms [ 60 , 64 , 69 , 76 , 86 , 89 , 90 , 96 , 99 ]. Efficacy seemed to vary by study design, with no improvements reported more often when analyses were restricted to randomized control trials. Reasons given by investigators for mixed efficacy results included risk of bias, misalignment between intervention design and intervention objectives, and failure to adequately assess both the contexts in which risk behaviours occur and intervention preferences among populations for which the studied intervention was intended.
Finally, two studies, each a systematic review, reported that community-led responses were cost effective or cost saving (i.e., per patient costs associated with HIV testing and counselling, health-services, adherence clubs, and costs associated with accessing services like transportation, childcare, lost work time) [ 63 , 69 ]. Cost effectiveness is likely due to the adoption of community-led models with clinically stable patients, enabling communities to deliver care and treatment sustainably, cost-effectively, and equitably in resource-limited settings. Also contributing to cost effectiveness was the adoption of community-based or -led HIV testing and counselling approaches, which were found to be less expensive than facility-based strategies.
We found strong evidence to support expanded coverage of community-led HIV responses. Our scoping review revealed more than 40 beneficial outcomes linked to community-led HIV prevention, treatment, care, support, monitoring, and advocacy. More than half were prevention-related improvements. One prospective evaluation study of advocacy, conducted across 7 countries, documented, and verified 103 positive health and social inclusion outcomes over 24 months. Other investigators have similarly documented the critical importance of community engagement and the scale-up of peer-led prevention and treatment to fast-tracking the HIV response [ 2 , 101 – 103 ].
We found study designs varied, with only 9 randomized control trials reported in the last 40 years. This finding makes sense given that randomized control trials may be exceedingly difficult to design and implement, given the multi-faceted and complementary nature of community-led HIV responses and the challenges inherent with meaningfully engaging key and vulnerable populations [ 104 ]. The absence of a previously agreed to definition has added to the complexity of studying community-led HIV responses. Outcomes measured also varied greatly, making it exceedingly difficult to draw comparisons between community-led approaches.
Most studies in our review took place in the global south and focused on peer-led approaches for students or youth. Five of the 15 studies that took place in the global north focused on gay and bisexual men. Studies focused specifically on people who use drugs, and transgender women represented a very small proportion of studies we examined, despite the potential benefits of community-led responses for these groups. For example, using a differentiated service delivery approach to prevention, testing, care, and treatment, delivered by and designed in consultation with men who have sex with men and transgender women, in partnership with the public health sector, can improve service coverage, reach, utilization, and retention [ 105 ].
Sixty percent of studies (n = 29) described more than one beneficial outcome linked to community-led HIV responses. This finding suggests that comprehensive community-led responses, especially when combined with structural level interventions, may have synergistic and simultaneous effects at more than just the individual level. This could be because programs were designed to address more than one outcome, or because when programs are community-led, clients’ needs are addressed holistically [ 106 ]. However, beneficial structural-level outcomes, e.g., changes in repressive laws and social attitudes, were rarely reported and were of mixed effectiveness [ 100 ]. This is not surprising given that societal and structural or legal changes operate on a longer time horizon than do traditionally measured public health outcomes and have multiple inputs, making advocacy programs more difficult to evaluate [ 107 , 108 ].
Community-led HIV responses reported in the literature that we reviewed had several common characteristics that build on and reinforce the definition we used to conduct our scoping review. For example, some studies highlight the importance of empowerment and mobilization as effective strategies for engaging communities to lead HIV responses [ 41 , 68 , 93 ]. Relatedly, some studies underscore social cohesion as both an outcome and mediator of effective community-led HIV responses [ 93 , 109 ]. Social networks might be another engine driving success. For example, understanding community-led HIV responses through a social networking lens, may shed light on how criminalized or stigmatized groups build power to influence change at the local level. This may be linked with the experience of affiliation, support, feeling valued, and making meaningful contributions to one’s community, each fundamental to social action and well-being [ 110 – 112 ]. Other researchers point to the importance of understanding community-led HIV responses as an iterative process that feeds back onto itself, beginning with constituency engagement, followed by alignment of adopted approaches with needs, adaptation of adopted approaches, and application of evidence gathered from monitoring and evaluation activities to influence policy changes [ 113 ] Also key are the inclusion of community-led responses in national AIDS plans and their funded operationalization at the local level. Bringing accountability closer to the level of service provision through community-led monitoring can increase the uptake and quality of HIV and other health services [ 69 , 75 , 78 , 92 , 97 ]. Moreover, sustained community activism for improved and sustained political commitment is vital for meeting HIV-related targets at local, national, regional, and global levels [ 114 ].
Together, the 48 studies we reviewed suggest comparative advantages of community-led HIV responses over facility-based, standard-of-care. Quantitative studies with comparison arms reinforce the importance of community-led prevention (i.e., HIV testing and counselling, risk reduction education and other behaviour change programs). Likewise, community-led components in treatment programs (i.e., adherence support, decentralized medication delivery) yield better service utilization as well as clinical outcomes. Our review also suggests that communities living with and disproportionately affected by HIV can effectively deliver services and influence policy. The comparative advantage of community-led HIV responses is predicated on several factors, including credibility with community members, ability to adapt to changing contexts and policy priorities, maintaining influence both within the community and at the policy level, community ownership, and iterative interactions and alliances with authorities resulting in accountability gains [ 31 , 68 , 115 ]. Likewise, several studies reiterated the point that having interventions that are community-based is insufficient for producing improved outcomes–interventions must be peer-led, of high quality, and possess strengthened capacity through skills training to ensure stronger, community-endorsed outcomes [ 57 , 94 , 116 ]. Peer-led responses are not only feasible but are also effective in producing higher service-related yields [ 117 ].
Formidable structural barriers to enabling community-led HIV response were repeatedly named in studies we reviewed. They include regressive laws and policies, funding constraints, and intersecting social stigmatizations, discrimination, and violence [ 68 , 100 ]. Differentiated approaches to the delivery of HIV services might be a good bridge to enable expanded coverage of community-led HIV responses, especially in contexts that are hostile to key and vulnerable populations. This is because differentiated care flexibly tailors the provision of antiretroviral treatment for patients based on their acuity, greatly expanding the range of alternatives for how care occurs and who delivers it [ 72 , 118 – 121 ].
At a time when funding for HIV is becoming more difficult given COVID-19’s detrimental impact and other competing priorities, the global HIV response needs to become more strategic in the investments it makes. Although research focused on community-led structural interventions is rare, studies we reviewed suggest that targeting social determinants shown in research to be associated with improved HIV outcomes—such as the availability of syringe programmes and comprehensive sexuality education, or removing barriers to high quality HIV and health services—have long been recognized as effective [ 63 , 69 , 75 , 78 , 100 , 122 ]. Community empowerment and mobilization are also highly effective at engaging key and vulnerable populations, increasing service utilization and improving HIV-related health outcomes. They should become standard components of demand generation initiatives as well as testing, prevention, and violence mitigation programs [ 41 , 68 , 93 , 123 – 126 ]. Additionally, we can become more strategic in combining community-led biomedical, behavioural, and structural interventions, and in so doing, leverage their synergistic effects [ 41 ]. Based on our scoping review and corroborated by other researchers, we should pursue better coverage of community-led, differentiated prevention, care, support, and treatment, socio-economic impact mitigation and other non-HIV support services [ 72 , 118 , 121 ]. Community-led services can be optimized when conducted in tactical and supportive partnerships with healthcare providers and government officials across health sectors [ 127 – 130 ]. Concurrently, some investment in high impact ‘disruptive innovations’ like HIV self-testing, multi-dose ARV dispensing for both prevention and treatment, adherence clubs, and drop-in centres may also be warranted. Disruptive innovations are interventions and program approaches that are inexpensive, rapid, consumer-controlled, and can be easily delivered in and by communities [ 73 , 131 ].
Limitations and strengths
There are a few important limitations to note. We restricted our scoping review to articles and reports published in English. Research published in other languages may have added to and/or validated the findings reported in this paper or might have contradicted them. Also, we used only three search engines–PubMed, Embase, and Web of Science–to conduct the article search. Other search engines may have yielded studies not included here. Finally, the limited number of published works reviewed in this scoping study, as well as the heterogeneity of research designs and outcomes reported, make it difficult to draw conclusions in many areas where community-led HIV responses might be beneficial. There is a need for more research to strengthen the evidence base undergirding normative guidance on the expanded role communities can play towards more effective and cost-efficient HIV responses. There is also a need for more studies showing the impact of community-led advocacy strategies focused on different issues across diverse contexts. In addition, research tools and protocols should be developed and made available to support community-led research in these areas.
Limitations notwithstanding, our scoping review allowed us to examine a broad and diverse range of research designs and outcomes [ 132 ]. This was especially important given the scarcity of research focused on community-led HIV responses. Our scoping review uncovered 9 probability-based randomized control trials, which is also worth noting. Although this study design is considered the gold standard for generalizability, such studies are costly and may be unethical to implement, especially in contexts that criminalize or stigmatize key and vulnerable populations. Creative study designs that are fit-for-purpose and can be community-led are warranted [ 133 ]. Indeed, sampling experts have advocated for innovative nonprobability sampling methods that are useful and cost-efficient, such as Internet sampling, especially in research with marginalized communities [ 134 ].
Conclusions
Findings from this scoping review offer strong support for greater coverage of community-led HIV responses given their comparative advantages. To scale-up community-led HIV responses, we must first more meaningfully engage people living with HIV, key and vulnerable populations, and fund the organizations and networks they lead. In addition, we should:
- Promote broad adoption of the definition of community-led HIV response included here, which can be applied uniformly across research, practice, and policy spheres. A universally accepted definition would make it easier to track investments, monitor effectiveness, and report results.
- Implement prerequisite steps to establishing and supporting community-led HIV responses. They include strengthening technical and operational capacities of organizations led by people living with HIV, women, gay and bisexual men, people who use drugs, sex workers, transgender people, young people, and people with histories of incarceration. Special attention should be given to removing legal, policy, and funding barriers preventing community-led organizations from safely and efficiently operating [ 125 , 130 ]. In addition, funding community empowerment and other processes that promote peer support and social cohesion among key and vulnerable populations may optimize the impact community-led responses can have [ 114 , 135 ].
- Curate prevention portfolios that are predominantly community-led and include two or more of the following: outreach; HIV testing–including self-testing; STI testing and treatment; comprehensive sexuality education; condom and lubricants; pre- and post-exposure prophylaxis (PrEP and PEP); behavioural interventions; harm reduction, including needle and syringe programmes; peer support; risk reduction counselling; and drop-in centres [ 136 , 137 ]. Community-led prevention programs are especially important for driving down incidence curves among key and vulnerable populations [ 9 ].
- Design treatment programs that have two or more well-funded, community-led components. Essential components include linkage to and coordination of care [ 67 , 116 ]; decentralized dispensation of multi-dose ART that use differentiated care models to downstream treatment [ 72 , 118 , 121 ]; retention support [ 41 , 69 , 72 ]; adherence programs [ 56 , 69 , 72 , 76 , 95 , 118 ]; home health [ 56 , 138 ]; peer counselling and peer-led support groups [ 56 , 139 ]; and treatment education [ 56 ]. At present, 27% of all people living with HIV worldwide are without treatment [ 140 ]. Our scoping review revealed evidence on the beneficial outcomes from community-led treatment, care, and support programmes, which when implemented with differentiated care models, can help to bridge the treatment gap [ 120 ].
- Support community-led organizations that deliver services to empower and mobilize their clients/service recipients, monitor local HIV responses, advocate, expand access, mitigate and address violence, and generate demand for quality services [ 141 ]. Support for community-led monitoring and advocacy could also help ensure availability of medicines and diagnostics, while addressing service-related gaps and access barriers [ 75 ].
- Leverage the synergistic effects of multi-component community-led responses that can amplify beneficial changes at individual, service, societal, and structural levels.(13) Also, invest in interventions that target multiple outcomes that are proximally related to HIV [ 131 ].
- Conduct more research on community-led HIV responses, especially responses led by key and vulnerable populations. Research focused on programs led by people who use drugs and transgender people is especially needed. Studies are also needed on cost effectiveness of community-led HIV responses as well as on the long-term impact of structural-level interventions. Future research should adopt creative study designs and methods that are fit-for-purpose. For example, fractional factorial designs can identify independent and synergistic effects of intervention components and combination approaches [ 104 , 142 ]. Communities of people living with HIV, key and vulnerable populations should be supported to lead research, including policy and evaluation studies [ 143 – 146 ]. Finally, the use of a consistent set of outcome measures focused on HIV and stronger integration of metrics used by health ministries, researchers, and program implementers should be encouraged. The need for more research should not preclude scaling of community-led responses.
The leadership of people living with and disproportionately affected by HIV is central to the global response. We must act rapidly to scale-up coverage of peer- and community-led programs and advocacy initiatives if we are to achieve the 2030 targets.
Supporting information
S1 checklist. preferred reporting items for systematic reviews and meta-analyses extension for scoping reviews (prisma-scr) checklist..
https://doi.org/10.1371/journal.pone.0260555.s001
Acknowledgments
We would like to acknowledge the community experts convened by UNAIDS who worked tirelessly to develop the definitions for ‘community-led’ included in this paper. They are Timur Abdullaev, George Ayala, Victoria Bendaud, Judy Chang, Carlos Garcia de León, José Antonio Izazola-Licea, Renatta Langlais, Chad Martin, Dasha Ocheret Matyushina, Wame Mosime, Lillian Mworeko, Elani Nassif, Alessandra Nilo, Sharmeen Premjee, Gavin Reid, Laurel Sprague, Omar Syarif, Aditia Taslim, Ruth Morgan Thomas, and Leigh Ann van der Merwe. We also thank the civil society, academic, UN Member States, UNAIDS staff, and UN agency partners in the UNAIDS Multi-stakeholder Consultation on Social Enablers, June 2019, for their review and inputs to the definitions. Finally, we send appreciation to the 2020–2021 Multi-stakeholder Task Force on Community-led AIDS Responses, comprised equally of UN Member States and civil society members, which was co-convened by WHO, UNDP, and UNAIDS.
- 1. Declaration of Alma-Ata. International Conference on Primary Health Care; 1978; Geneva Switzerland: World Health Organization.
- View Article
- PubMed/NCBI
- Google Scholar
- 4. Towards a global action plan for healthy lives and well-being for all: uniting to accelerate progress towards the health-related SDGs. Geneva: World Health Organization; 2018. Contract No.: WHO/DCO/2018.3.
- 7. Rodriguez-García R, Bonnel R., Wilson D., N’Jie N. Investing in Communities Achieves Results: Findings from an Evaluation of Community Responses to HIV and AIDS. Washington D.C.: The World Bank; 2013.
- 9. UNAIDS. Seizing the moment: Tackling entrenched inequalities to end epidemics. Global AIDS Update. Geneva, Switzerland: UNAIDS; 2020.
- 13. Consolidated Guidelines on HIV Prevention, Diagnosis, Treatment and Care for Key Populations– 2016 Update. Geneva, Switzwerland: World Health Organization; 2016.
- 27. AIDS and Rights Alliance for Southern Africa HEaHARDotUoKN. Impact of the COVID-19 national measures on community-led HIV responses in the SADC region: Summary Brief. Windhoek, Namibia: ARASA and HEARD; 2020.
- 30. Political Declaration on HIV and AIDS: Ending Inequalities and Getting on Track to End AIDS by 2030. New York: United Nations; 2021. Contract No.: Agenda Item 10.
- 33. Political Declaration on HIV and AIDS: On the Fast-Track to Accelerate the Fight against HIV and to End the AIDS Epidemic by 2030. New York, New York: United Nations June 2016.
- 38. Befani B. Models of causality and causal inference. London: Department for International Development; 2012.
- 39. Bickman L, Reich S. M. Random controlled trials a gold standard with feet of clay. In: Donaldson CAC S.I., editor. What counts as credible evidence in applied research and evaluation practice. Thousand Oaks, California: Sage Publications; 2009. p. 51–72.
- 44. UNAIDS. 2025 AIDS Targets: Target-setting, impact and resource needs for the global AIDS response—technical consultation on social enablers. Geneva, Switzerland: UNAIDS; 2019 June 2019.
- 45. UNAIDS. Progress Report of the Multistakeholder Task Team on Community-led AIDS Responses. Geneva, Switzerland: UNAIDS; 2020.
- 95. CADTH Rapid Response Reports. Peer Support for Diabetes, Heart Disease and HIV/AIDS: A Review of the Clinical Effectiveness, Cost-effectiveness, and Guidelines. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2013.
- 105. De Lind Van Wijingaarden JW LD. Differentiated HIV-service delivery along the cascade for men who have sex with men and transgender women in Thailand: Lessons learned from the LINKAGES Project. Thai Red Cross AIDS Research Center, FHI360; 2020.
- 106. Solar O IA. A conceptual framework for action on the social determinants of health. World Health Organization; 2010. Report No. 2.
- 135. Jupp D, Ali SI. Measuring Empowerment? Ask Them—Quantifying Qualitative Outcomes from People’s Own Analysis. Swedish International Development Cooperation Agency, Sida; 2010 2010.
- 140. Fact Sheet 2021. Joint United Nations Programme on HIV/AIDS; 2021 2021.
- 146. Wallerstein N DB. Theoretical, historical and practice roots of community-based participatory research. In: Minkler M WN, editor. Community-based participatory research for health. San Francisco: Wiley; 2017.
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Research & training, advances in hiv/aids research.
For an update on what medical science is doing to fight the global HIV/AIDS pandemic, read a Parade article by NIH Director Francis S. Collins and NIAID Director Anthony S. Fauci, AIDS in 2010: How We're Living with HIV .
Over the past several decades, researchers have learned a lot about the human immunodeficiency virus (HIV) and the disease it causes, acquired immunodeficiency syndrome (AIDS). But still more research is needed to help the millions of people whose health continues to be threatened by the global HIV/AIDS pandemic.
At the National Institutes of Health, the HIV/AIDS research effort is led by the National Institute of Allergy and Infectious Diseases (NIAID). A vast network of NIAID-supported scientists, located on the NIH campus in Bethesda, Maryland, and at research centers around the globe, are exploring new ways to prevent and treat HIV infection, as well as to better understand the virus with the goal of finding a cure. For example, in recent months, NIAID and its partners made progress toward finding a vaccine to prevent HIV infection. Check out other promising areas of NIAID-funded research on HIV/AIDS at http://www.niaid.nih.gov/topics/hivaids/Pages/Default.aspx .
Other NIH institutes, including the Eunice Kennedy Shriver National Institute of Child Health and Human Development and National Institute on Alcohol Abuse and Alcoholism, also support research to better control and ultimately end the HIV/AIDS pandemic. Some of these researchers have found a simple, cost-effective way to cut HIV transmission from infected mothers to their breastfed infants. Others have developed an index to help measure the role of alcohol consumption in illness and death of people with HIV/AIDS.
Find out more about these discoveries and what they mean for improving the health of people in the United States and all around the globe.
This page last reviewed on August 20, 2015
Connect with Us
- More Social Media from NIH

CDC provides national leadership for HIV prevention research, including the development and evaluation of HIV biomedical and behavioral interventions to prevent HIV transmission and reduce HIV disease progression in the United States and internationally. CDC’s research efforts also include identifying those scientifically proven, cost-effective, and scalable interventions and prevention strategies to be implemented as part of a high-impact prevention approach for maximal impact on the HIV epidemic.
The AIDS epidemic, although first recognized only 20 years ago, has had a profound impact in communities throughout the United States.
The Serostatus Approach to Fighting the HIV Epidemic: Prevention Strategies for Infected Individuals R. S. Janssen, D. R. Holtgrave, and K. M. De Cock led the writing of this commentary. R. O. Valdiserri, M. Shepherd, and H. D. Gayle contributed ideas and helped with writing and reviewing the manuscript.

CDC has provided funding to HIV partners to help implement programs that will help curb the increase of HIV infections. These programs facilitated with our partners and grantees are critical in the goal of eliminating HIV infection in the United States.

CDC has researched several HIV prevention interventions that have proven effective in helping to prevent HIV infection in certain populations and communities.

CDC has worked with key cities to create effective policies and programs to curb the tide of HIV infections in those cities. These cities have higher rates of HIV due to a number of factors therefore making them key locations for studies.

The Medical Monitoring Project (MMP) is a surveillance system designed to learn more about the experiences and needs of people who are living with HIV. It is supported by several government agencies and conducted by state and local health departments along with the Centers for Disease Control and Prevention.
- Assessment of 2010 CDC-funded Health Department HIV Testing Spending and Outcomes pdf icon [PDF – 359 KB]
- HIV Testing Trends in the United States, 2000-2011 pdf icon [PDF – 1 MB]
- HIV Testing at CDC-Funded Sites, United States, Puerto Rico, and the U.S. Virgin Islands, 2010 pdf icon [PDF – 691 KB]
- HIV Prevention Funding Allocations at CDC-Funded State and Local Health Departments, 2010 pdf icon [PDF – 792 KB]
Cost-effectiveness of HIV Prevention
- The cost-effectiveness of HIV prevention efforts has long been a criterion in setting program priorities. The basic principle is straightforward: choose those options that provide the greatest outcome for the least cost.
- The fact sheet Projecting Possible Future Courses of the HIV Epidemic in the United States pdf icon compares the cost-effectiveness of three different prevention investment scenarios.
The HIV/AIDS Prevention Research Synthesis (PRS) Project identifies evidence-based HIV behavioral interventions (EBIs) listed in the Compendium of Evidence-Based HIV Behavioral Interventions to help HIV prevention planners and providers in the United States choose the interventions most appropriate for their communities.
- On January 1, 2012, CDC began a new 5-year HIV prevention funding cycle with health departments, awarding $339 million annually.
- The STD/HIV National Network of Prevention Training Centers provides training for health departments and CBOs on the HIV prevention interventions.
- HIV by Group
- HIV Risk and Prevention
- HIV Nexus: Resources for Clinicians
- HIV Public Health Partners
- HIV Resource Library
- HIV Statistics Center
- About the Division of HIV Prevention
- VIH en Español
- @StopHIVTogether
- Get Email Updates
- Send Feedback
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
ORIGINAL RESEARCH article
Understanding hiv/aids dynamics: insights from cd4+t cells, antiretroviral treatment, and country-specific analysis.

- 1 Department of Mathematics, Universitas Indonesia, Depok, Indonesia
- 2 Department of Mathematics, University of Raparin, Ranya, Iraq
- 3 Department of Mathematics, Universitas Tadulako, Palu, Indonesia
- 4 Department of Mathematics and Statistics, The University of Haripur, Haripur, KP, Pakistan
In this article, we present a mathematical model for human immunodeficiency virus (HIV)/Acquired immune deficiency syndrome (AIDS), taking into account the number of CD4+T cells and antiretroviral treatment. This model is developed based on the susceptible, infected, treated, AIDS (SITA) framework, wherein the infected and treated compartments are divided based on the number of CD4+T cells. Additionally, we consider the possibility of treatment failure, which can exacerbate the condition of the treated individual. Initially, we analyze a simplified HIV/AIDS model without differentiation between the infected and treated classes. Our findings reveal that the global stability of the HIV/AIDS-free equilibrium point is contingent upon the basic reproduction number being less than one. Furthermore, a bifurcation analysis demonstrates that our simplified model consistently exhibits a transcritical bifurcation at a reproduction number equal to one. In the complete model, we elucidate how the control reproduction number determines the stability of the HIV/AIDS-free equilibrium point. To align our model with the empirical data, we estimate its parameters using prevalence data from the top four countries affected by HIV/AIDS, namely, Eswatini, Lesotho, Botswana, and South Africa. We employ numerical simulations and conduct elasticity and sensitivity analyses to examine how our model parameters influence the control reproduction number and the dynamics of each model compartment. Our findings reveal that each country displays distinct sensitivities to the model parameters, implying the need for tailored strategies depending on the target country. Autonomous simulations highlight the potential of case detection and condom use in reducing HIV/AIDS prevalence. Furthermore, we identify that the quality of condoms plays a crucial role: with higher quality condoms, a smaller proportion of infected individuals need to use them for the potential eradication of HIV/AIDS from the population. In our optimal control simulations, we assess population behavior when control interventions are treated as time-dependent variables. Our analysis demonstrates that a combination of condom use and case detection, as time-dependent variables, can significantly curtail the spread of HIV while maintaining an optimal cost of intervention. Moreover, our cost-effectiveness analysis indicates that the condom use intervention alone emerges as the most cost-effective strategy, followed by a combination of case detection and condom use, and finally, case detection as a standalone strategy.
1 Introduction
Human immunodeficiency virus (HIV) is a virus that infects CD4+ T lymphocytes, leading to a weakened immune system in individuals. On the other hand, Acquired immune deficiency syndrome (AIDS) refers to the symptoms that occur as a result of a weakened immune system due to HIV infection ( 1 ). HIV can be transmitted through bodily fluids such as blood, semen, genital fluids, and breast milk. According to data from the WHO and the United Nations Programme on HIV/AIDS (UNAIDS), in 2016, there were 36.7 million people living with HIV (PLHIV)/AIDS worldwide. The majority of people living with HIV are in low and middle-income countries, such as Eswatini, where the prevalence is 25.9% among the adult population, Lesotho with a prevalence of 19.3%, South Africa with a prevalence of 17.8%, Botswana with a prevalence of 16.4%, and Mozambique with a prevalence of 11.6% ( 2 ). According to the same source, the top 10 countries with the highest HIV prevalence include 10 African nations, with South Africa having the highest number of people living with HIV, surpassing 7.6 million in 2020. Beyond Africa, the spread of HIV is also a significant concern, particularly in Indonesia, where the latest data report 519,158 individuals affected by HIV as of June 2022 ( 3 ). This places Indonesia as the third-highest country with people living with HIV in Asia, following India and Thailand.
The HIV illness may be divided into four phases. People living with HIV (PLHIV) and have entered stage one may experience mild symptoms such as flu-like symptoms, diarrhea, and fever. Those who have entered stage two may experience symptoms such as Tuberculosis (TB), swollen lymph nodes, and skin disorders. In stage three, individuals may experience symptoms in the mucous membranes, such as Tuberculosis (TB) in the lymph nodes. Finally, in stage four, individuals may experience systemic meningoencephalitis. Stage four is commonly referred to as AIDS. HIV attacks CD4+ T cells in the bodies of infected individuals. CD4+ T cells play a crucial role in the immune system and perform many functions in immune activation, coordination, modulation, and regulation ( 4 ). AIDS is defined as an outcome among those living with HIV if the CD4+ T cell count is < 200.
Antiretroviral therapy (ART) is available as a treatment to reduce the risk of HIV transmission and lower the amount of HIV in the blood. Highly active antiretroviral therapy (HAART) has been successful in reducing morbidity and mortality among individuals infected with HIV ( 5 ). According to the United Nations Programme on HIV/AIDS (UNAIDS), 1 there were 1.3 million people newly infected with HIV in 2022. Among 39 million people living with HIV in 2022, 630,000 people died of AIDS. According to the global HIV and AIDS epidemic, 2 only 86% of people with HIV globally knew their HIV status in 2022, the rest of them do not know that they were living with HIV. Therefore, antiretroviral therapy has the potential to reduce the number of individuals infected with HIV in sub-Saharan Africa and other countries.
The mathematical models have been used widely to model the spread of diseases, such as dengue ( 6 ), malaria ( 7 , 8 ), tuberculosis ( 9 – 11 ), HIV ( 12 ), COVID-19 ( 13 – 16 ), pneumonia ( 17 ). In the context of the HIV/AIDS infectious disease spread, the mathematical model can help researchers understand the impact of interventions and can be used to predict the potential outcome of scenarios in the field. An early mathematical model for HIV/AIDS can be found in Rahman et al.'s study ( 18 ). In 2009, Mukandavire et al. ( 19 ) presents a mathematical model for HIV/AIDS transmission dynamics, incorporating an explicit incubation period. It demonstrates that effective public health educational campaigns, when targeted at both sexually immature and sexually mature individuals, can significantly slow down the epidemic. The study also identifies the presence of backward bifurcation in the mathematical model, highlighting the complexity of disease dynamics and the potential impact of comprehensive intervention strategies. The presence of the backward bifurcation phenomenon in their model suggests that the extinction of HIV may not solely depend on the condition of the reproduction number being less than one. This is because another endemic possibility may emerge even when the reproduction number is already less than one. In lay terms, backward bifurcation in the model means that even if the conditions initially seem favorable for controlling and reducing HIV (for example, when the reproduction number is less than one), there is still a risk of a resurgence or a persistent presence of the infection. This phenomenon introduces a level of complexity, suggesting that the effectiveness of interventions and the possibility of HIV extinction are not solely determined by one factor (such as a low reproduction number). Instead, additional factors or conditions may influence the dynamics of the infection, making it more challenging to predict and control. Nyabadza and Mukandavire in ( 20 ) employ ordinary differential equations to investigate the dynamics of HIV/AIDS, particularly in the context of HIV testing and screening campaigns. The key findings include that having a basic reproduction number below one is necessary but not sufficient for disease control due to backward bifurcation phenomena. Additionally, the study fits the model to real data on HIV prevalence in South Africa, adding empirical validation to the model's insights that HIV counseling and testing itself has very little impact in reducing the prevalence of HIV unless the efficacy of the campaigns exceeds an evaluated threshold in the absence of backward bifurcation. Recently, Zhai et al. ( 21 ) develop a stochastic HIV/AIDS model that considers individuals with protection awareness. Their research revealed that HIV can become extinct when the R 0 s is <1. Furthermore, the study highlights that enhancing the protection efficiency of individuals with awareness and the implementation of continuous antiretroviral therapy (ART) both contribute to reducing the number of people living with HIV (PLHIV), offering potential strategies for disease control. In Jamil et al.'s study ( 22 ), a fractal fractional HIV/AIDS model is introduced, using fractional order differential equations. The study utilizes the first and second derivatives of the Lyapunov function to conduct a global analysis of the model. The research suggests that measures to reduce the effective contact rate between susceptible and infected individuals, coupled with improved treatment for those who are already infected, can enhance the effectiveness of interventions against HIV/AIDS. Recently, due to the COVID-19 pandemic, many mathematical models have been introduced to understand the impact of co-infection between HIV/AIDS with COVID-19. Xu et al. ( 23 ) focus on developing a mathematical model for HIV-TB co-infection dynamics and validate it using real incidence data from different regions. The article also delves into the comparison of numerical schemes to determine the most effective computational approach for simulating the model. Pinto et al. ( 24 ) explore models for HIV and TB coinfection dynamics, considering both fractional and integer order models. Their analysis encompasses treatment strategies for both diseases and includes considerations for the vertical transmission of HIV. Ringa et al. ( 25 ) conduct an analysis on sub-models (and co-infection model) related to HIV and COVID-19 co-infection. They apply an optimal control approach to control variables, finding that preventive measures can substantially reduce the burden of co-infections with COVID-19, and effective treatment of COVID-19 could, in turn, reduce co-infections with opportunistic infections such as HIV/AIDS. Please see Omami et al.'s study ( 26 ) for another model which incorporates a coinvection between HIV, dengue, and COVID-19. Another classical model was presented by Garba et al. ( 27 ), wherein they employed a mathematical framework to simulate the spread of HIV in Nigeria. Their model incorporates factors such as condom use and asymptomatic cases. The researchers utilized incidence data from Nigeria to calibrate the parameters of their model. Readers may refer to ( 28 – 34 ) for more HIV/AIDS related models.
This research is an extension of the study conducted by Rahman et al. ( 18 ) with modifications that include the addition of an AIDS compartment as a variable, taking into consideration the population engaged in unprotected sexual intercourse, and the intervention of antiretroviral therapy. This research aims to develop a comprehensive model for the spread of HIV, integrating the dynamics of CD4+T cells and considering key interventions such as condom use and treatment. By fitting model parameters based on data from four top countries with high HIV incidence rates, we seek to provide a nuanced understanding of how these factors influence the trajectory of the epidemic. Additionally, sensitivity analysis was conducted to assess the impact of various model parameters on the number of cases in each country, offering insights into the relative importance of different factors. Furthermore, optimal control techniques are employed to forecast potential optimal scenarios in the field, aiding in the design of effective strategies for HIV prevention and management.
In this study, a mathematical model for the spread of HIV/AIDS through unprotected sexual intercourse has been constructed based on the classification of the number of CD4+ T cells in the body, incorporating the intervention of antiretroviral therapy. The number of CD4+ T cells is crucial in constructing an HIV mathematical model because these cells play a central role in the immune system, and their depletion is a hallmark of HIV infection. CD4+ T cells are a type of white blood cell that helps coordinate the immune response to infections. HIV specifically targets and infects these cells, leading to a decline in their numbers over time. Incorporating the CD4+ T cell count into the mathematical model allows for a more realistic representation of the dynamics of HIV infection and disease progression. Applying some mathematical analysis tools, we conduct an analytical study on our model, including an analysis of existence, the stability analysis of equilibrium points, and the analysis of the basic reproduction number ℛ 0 . With this study, we can understand the long-term behavior of HIV transmission as it changes over time. The analysis of the basic reproduction number can quantify which factors play a significant role in efforts to control the spread of HIV. Following that, we conducted numerical simulations, which involved analyzing the elasticity and sensitivity of ℛ 0 , in addition to performing autonomous and optimal control simulations using the constructed model. The goal is to gain insights into the impact of antiretroviral therapy on the transmission of HIV/AIDS through unprotected sexual intercourse. This analysis is based on the classification of the number of CD4+ T cells in the body. By interpreting the outcomes from both the analytical study and numerical simulations, we aim to better understand the dynamics and effects of the treatment and condom use on the spread of the virus.
The article is structured as follows: Section 2 presents the construction and analysis of a simple case model where the number of CD4+T cells is not considered in the model. This section includes a global stability of equilibrium points and a sensitivity analysis of the basic reproduction number. Section 3 delves into the mathematical model analysis of the complete model, discussing equilibrium points, control reproduction number, data fitting, and model sensitivity analysis. Section 4 describes the modification of the complete model into a control optimal model. This section includes numerical experiments for different strategies, as well as a cost-effectiveness analysis. Finally, Section 5 provides conclusion.
2 A mathematical model of HIV/AIDS with antiretroviral treatment without considering the number of CD4+T cell number class
2.1 model construction.
In this section, we consider an antiretroviral treatment for an HIV-infected individual. First, let us consider the total human population (aged 15–49 years), denoted by N , to be be categorized into four different compartments based on their health statuses, namely, the susceptible individual ( S ), PLHIV with infection only ( I ), PLHIV receiving treatment ( T ), and PLHIV with AIDS illness ( A ). In this model, we assume that without any test, the HIV-infected individual cannot be detected. Hence, they cannot get a proper treatment. To construct the model, we use the transmission diagram as shown in Figure 1 .
Figure 1 . HIV transmission diagram with antiretroviral treatment.
The construction of the model is as follows. The recruitment rate of N is always considered as a susceptible person from a younger age class of <15 years, with a constant rate Λ. Infection only occurs due to successful contact between susceptible individuals with infected individuals I and T with a probability of β u and β t , respectively. The most effective way to prevent sexual transmission of HIV is through abstinence, that is, avoiding all vaginal, anal, and oral sex. In our model, we include the use of a male condom or a female condom that can avoid the transmission of HIV. The variables ϵ and κ are denoted as condom efficacy and the proportion of people who use condoms, then the transmission rate β u and β t can be reduced by a factor of 1 − ϵ κ . With this assumption, we have κ ∈ [0, 1] and ϵ ∈ [0, 1]. A bigger value of κ represents a better quality of a condom, and a bigger value of ϵ represents a condition where more people use condoms during their sexual activity. With the mass action infection process, then the infection force of infection is given by (1 − ϵ κ )( β u I + β t T ). In this model, we have a transition from I to T due to a medical test for HIV detection, which is denoted by τ . Furthermore, the transition from the HIV stage to AIDS is given by γ u and γ t for I and T individuals, respectively. Due to antiretroviral treatment, we assume that γ t < γ u . Each compartment has a natural death rate denoted by μ , except for A compartment which has an additional death rate due to AIDS, which is denoted by η. Based on this assumption, the complete model of HIV/AIDS transmission with the usage of condoms and the intervention of antiretroviral treatment is given by the following system of ordinary differential equations.
completed with the following initial condition
2.2 Preliminary analysis
In this section, we describe two theorems to guarantee the positiveness of the solution and also the positive invariant region of the system ( 1 ).
Theorem 1 . All solutions of the HIV/AIDS model in Equation (1) with a non-negative initial condition in ℝ + 4 remain positive for all time t > 0.
Proof : From the first equation on the ( 1 ), we have
The solution of S ( t ) is given by
Since S (0) > 0, then S ( t ) is always positive for all t > 0. The remaining variables I ( t ), T ( t ), and A ( t ) can be shown in a similar way. Hence the solution set { S, I, T, A } is always non-negative for all time t > 0. □
Theorem 2 . The region
is positively invariant and attracting with respect to the system ( 1 ) with a non-negative initial condition in ℝ + 4 .
Proof : Adding all equations in the system ( 1 ), we have
Solving this differential equation with respect to N ( t ), we have
Therefore, we can see that N ( t ) → Λ μ for t → ∞. If N ( 0 ) > Λ μ , then N ( t ) will monotonically decrease and tends to Λ μ . If N ( 0 ) < Λ μ , then N ( t ) will monotonically increase and tends to Λ μ . On the other hand, if N ( 0 ) = Λ μ , then N ( t ) will remain constant for all time t , where N ( t ) = Λ μ . Hence, according to Theorem 1 and previous calculation, we have 0 < S + I + T + A ≤ max { Λ μ , N ( 0 ) } . □
Based on Theorems 1 and 2, it is sufficient to consider the dynamics of the system ( 1 ) in the region D where the existence, uniqueness, and positiveness of the solution hold.
2.3 The HIV/AIDS-free equilibrium point and the basic reproduction number of the model ( 1 )
In this section, we analyze the existence and stability of the HIV/AIDS-free equilibrium points, and how they relate to the basic reproduction number of the system ( 1 ). The HIV/AIDS-free equilibrium point of the system ( 1 ) is given by
Before we calculate the local and global stability criteria of the HIV/AIDS-free equilibrium points, we first calculate the basic reproduction number of the system ( 1 ). First, we decompose the Jacobian matrix of an infected subsystem of the system ( 1 ) which is evaluated in E 0 in a transition Σ and transmission T matrix as follows:
Since the elements of the second row of T are all zero, then defining E = [10] t , the next-generation matrix is given by
The basic reproduction number of the system ( 1 ) is taken by the spectral radius of K , which is given by
The basic reproduction number in many epidemiological models play an important role in determining the local and global stability of the equilibrium points of their model. The basic reproduction number in our model represents the total number of secondary cases of HIV caused by one primary case of HIV in a completely virgin population during its infection period. The following theorem gives the local stability criteria of E 0 .
Theorem 3 . The HIV/AIDS-free equilibrium point E 1 is locally asymptotically stable if R 0 < 1 and unstable if R 0 > 1 .
Proof : The Jacobian matrix of the system ( 1 ) evaluated in E 0 is given by
which has two explicit eigenvalues, i.e, λ 1 = − μ and λ 2 = − ( μ + η), while the other two eigenvalues are taken by the solution of the following polynomial
where R 1 = Λ β u ( 1 - ϵ κ ) μ ( 2 μ + τ + γ u + γ t ) and R 0 = Λ ( β u ( γ t + μ ) + β t τ ) ( 1 - ϵ κ ) μ ( τ + γ u + μ ) ( γ t + μ ) . Since R 1 < R 0 , then the other two eigenvalues will have a negative real part if R 0 < 1 . Therefore, the HIV/AIDS-free equilibrium E 0 is locally asymptotically stable if R 0 < 1 . □
The following Theorem gives the global stability criteria of E 0 .
Theorem 4 . The HIV/AIDS-free equilibrium E 1 of the system ( 1 ) is globally stable if R 0 < 1 .
Proof : Using a same approach as authors in ( 35 ), let us define
where X = S ∈ ℝ + is the compartment of non-infected individuals, and Z = ( I , T , A ) ∈ ℝ + 3 is the infected compartments. Let X * = ( Λ μ , 0 ) .
From direct calculation, we have
Since I and T are always positive, then it is clear that Ĝ( X, Z ) ≥ 0. It is also clear that X * = (Λ/ μ ) is globally stable for F ( X , 0). Hence, E 0 is globally asymptotically stable. □
2.4 HIV/AIDS endemic equilibrium point of the system ( 1 )
The HIV/AIDS endemic equilibrium point of system ( 1 ) is given by
From the expression of E 2 , we have the following theorem.
Theorem 5 . The HIV/AIDS endemic equilibrium E 2 of the system ( 1 ) exist in ℝ + 4 if R 0 > 1 .
In the following theorem, we show the non-existence of backward-bifurcation phenomena of the system ( 1 ).
Theorem 6 . The system ( 1 ) always exhibits a forward bifurcation phenomenon at R 0 = 1 .
Proof : To analyze the bifurcation phenomena of system ( 1 ), we use the well-known Castillo-Song bifurcation theorem ( 35 ). Please see ( 36 – 38 ) for more examples of the implementation of this theorem in another epidemiological model. First, let us define S = x 1 , I = x 2 , T = x 3 , and A = x 4 and g i for i = 1, ..., 4 represent d S d t , d I d t , d T d t , and d A d t , respectively. Next, we choose β t as the bifurcation parameter. Solving R 0 = 1 with respect to β t , we have
Next, we calculate the Jacobian matrix of the system ( 1 ) and evaluate it at β t * and E 0 , we have
The eigenvalues of A are
We can see clearly that we have a simple zero eigenvalue, and λ 2 and λ 3 are negative. We have λ 4 < 0 if and only if Λ ( 1 - κ ϵ ) β u μ ( 2 μ + τ + γ t + γ u ) < 1 . Since
then we also have λ 4 < 0. Hence, we can proceed to the next step to analyze the bifurcation type of our model. The bifurcation type of our model can be determined with the following formula:
where v and w is the left and right eigenvectors of A with respect to the zero eigenvalue, respectively. The left eigenvalue of A with respect to the eigenvalue 0 is given by
On the other hand, the right eigenvector of A with respect to the eigenvalue 0 is given by
Hence, we have
Since a < 0 and b > 0, the system ( 1 ) always exhibits a forward bifurcation at R 0 = 1 . □
The following corollary is a direct implication from Theorem 6.
Corollary 1 . The HIV/AIDS endemic equilibrium E 2 of the system ( 1 ) is locally stable for R 0 > 1 but close to one.
In epidemiology, bifurcation refers to a qualitative change in the behavior of a dynamical system as a parameter is varied. Forward bifurcation specifically occurs when a stable endemic equilibrium coexists with an unstable disease-free equilibrium at parameter values where the basic reproduction number ( R 0 ) is larger than one. On the other hand, in cases where the reproduction number falls below one, a stable disease-free equilibrium exists without the existence of the endemic equilibrium. Consequently, the condition where the reproduction number equals one marks the bifurcation point. In the context of the HIV/AIDS model that we developed in Equation (1) , forward bifurcation has significant implications. It highlights the importance of not only reducing transmission rates but also addressing factors that contribute to the maintenance of stable disease-free equilibrium, such as the efficacy of condom use and treatment. By considering the implications of forward bifurcation, epidemiologists and policymakers can develop more nuanced and effective approaches to combating the HIV/AIDS epidemic.
2.5 Effect of R 0 to endemic size E 2
Here in this section, we will perform the elasticity index of the basic reproduction number R 0 and the endemic equilibrium size of the HIV/AIDS model in Equation (1) and also the parameter sensitivity to R 0 . We use parameter values for Lesotho (See table in Appendix 2 ), except for τ and κ , which is given as follows:
With this set of parameters, we have R 0 = 1 . 43 , which is larger than one. Hence, the HIV/AIDS endemic equilibrium E 2 exists and is given by
is locally stable. This confirms the results of Theorems 5 and 6.
Next, we calculate the elasticity index of R 0 and the endemic equilibrium size. To perform this simulation, we use the following formula ( 39 ):
where p is the model parameter, and Q is the quantity of the model output, such as R 0 or endemic equilibrium size E 2 . Using the above formula, we have
Substituting parameter values in Equation (2) yield E R 0 β u = + 0 . 969 , which means that increasing β u by 1% will increase R 0 by 0.969%. Therefore, if we increase β u from 1.4888 × 10 −7 to 1.503 × 10 −7 (increased by 1%), then R 0 increases from 1.43 to 1.44, which is an increase of 0.969%. With the same approach, we can calculate the elasticity of each parameter in the system ( 1 ) (except Λ that we ignored since the number of recruitment rates cannot be changed in the field) with respect to R 0 and E 2 . The results can be seen in Figure 2 .
Figure 2 . The elasticity of R 0 , S * , I * , T * , and A * with respect to (A) β u (blue), β t (cyan), γ u (green), and γ t (yellow), and with respect to (B) τ (blue), κ (cyan), and ϵ (yellow).
From Figure 2 , we can see that β u has a significant impact on R 0 and E 0 , more dominant than β t . Hence, infection from untreated individuals plays a significant role in determining the spread of HIV/AIDS. Increasing β u and β t will increase R 0 and all infected compartments in E 0 , but reduce S in E 0 . Furthermore, we can see that the progression to the A compartment, which is presented by parameters γ u and γ t , will reduce R 0 . We can also see that γ u is more significant in affecting R 0 or all compartments in E 0 compared to γ t . Another important result is that the case detection rate τ is promising in reducing R 0 and all infected compartments.
Since we can see that the use of condoms to reduce the infection rates β u and β t shows a promising potential to control HIV/AIDS, it is necessary to see the combination of ϵ and κ to reduce R 0 . By substituting all parameter values in Equation (2) , except ϵ and κ , into R 0 , we have R 0 ( ϵ , κ ) = 1 . 591 ( 1 - ϵ κ ) . Drawing this function in the ϵ − κ plane, we have the results in Figure 3 . It can be seen that if the efficacy of the condom or the proportion of people who use condoms is <0.37, then the basic reproduction number will always be larger than one (Area 1). Hence, the endemic situation will always appear in the population. On the other hand, if the two above mentioned parameters are >0.37 (Area 2), then there is a chance to eradicate HIV from the population (Area 2b).
Figure 3 . Dependency of R 0 on the condom efficacy and the proportion of people who use it.
3 A mathematical model of HIV/AIDS considering CD4+T cell number
3.1 model construction.
In this section, we modify our previous model in the system ( 1 ) by accommodating the number of CD4+T cells in the human body. To construct the model, we use the following assumptions:
1. We classify each of the I and T compartments in the system ( 1 ) into three compartments, which present the class of infected and treated individuals based on the number of CD4+T cells. We denote I 1 , I 2 , and I 3 as infected individuals who have not yet been detected and have different average numbers of CD4+T cells in their blood. Please see the descriptions of I i and T i for i = 1, 2, 3 in Table 1 .
2. We assume that since HIV infection itself is not very harmful, there is no additional death rate attributable directly to HIV individuals (compartments I i and T i ). However, additional deaths occur only among individuals with AIDS (compartment A ), and this occurs at a constant rate represented by the parameter η.
3. The case detection test can determine the level of CD4+T cells in the human body. Hence, we have a transition rate τ from I i to T i for i = 1, 2, 3 due to successful case detection.
4. Each treated compartment will get antiretroviral treatment which will delay the progression of HIV to AIDS. We assume that successful treatment will increase the number of CD4+T cells. With a duration of treatment is ρ , we have the probability of treatment being unsuccessful given by s, q , and r for individuals in T 1 , T 2 , and T 3 , respectively.
5. The infection rate of individuals in I i is given by β u while for individuals in T i is given by β t . As an effect of treatment, here we assume β t < β u .
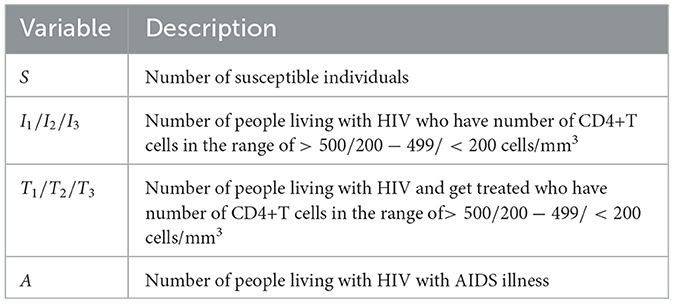
Table 1 . Description of variables of the HIV/AIDS model in the system ( 3 ).
Based on this model description and the transmission diagram in Figure 4 , the mathematical model of HIV/AIDS considering the level of CD4+T cells, antiretroviral treatment, and case detection is given by the following system of differential equations.
completed with a non-negative initial condition S (0) > 0, I 1 (0) ⩾ 0, I 2 (0) ⩾ 0, I 3 (0) ⩾ 0, T 1 (0) ⩾ 0, T 2 (0) ⩾ 0, T 3 (0) ⩾ 0, and A (0) ⩾ 0.
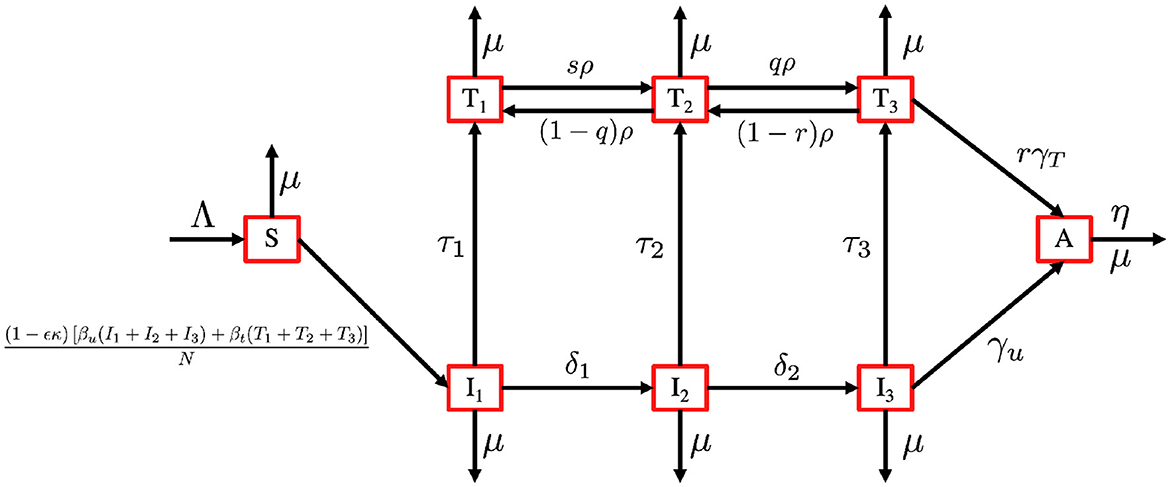
Figure 4 . Transmission diagram of the HIV/AIDS model in system ( 3 ).
3.2 Preliminary analysis
With a similar approach, as we have given for Theorems 1 and 2, the positivity and boundedness criteria of the system ( 3 ) is given in the following theorems.
Theorem 7 . All solutions of the HIV/AIDS model in Equation (3) with a non-negative initial condition in ℝ + 8 remain positive for all time t > 0.
Theorem 8 . The region
is positively invariant and attracting with respect to the system ( 1 ) with a non-negative initial condition in ℝ + 8 .
3.3 The HIV/AIDS-free equilibrium and the control reproduction number
The HIV/AIDS-free equilibrium of the system ( 3 ) is given by
Using the next-generation method ( 40 ), we determine the expression of the basic reproduction number of the system ( 3 ). Similar to the previous section, we first determine the transition (Σ) and transmission ( T ) matrices of the infected subcompartment of the system ( 3 ), which is given by
where a 1 = τ 1 + δ 1 + μ , a 2 = τ 2 + δ 2 + μ , , a 3 = τ 3 + γ u + μ , a 4 = ρ s + μ , a 5 = (1 − q ) ρ + q ρ + μ , and a 6 = (1 − r ) ρ + r γ t + μ . Since we have only the first row of T is non-zero, while the others are zero, we define
and calculate the control reproduction number using the formula of K = − E t T Σ −1 E . Hence, the control reproduction number of the system ( 3 ) is given by
Following ( 41 ) , the local stability criteria of ℰ 1 is given in the following theorem.
Theorem 9 . The HIV/AIDS-free equilibrium of the system ( 3 ) given by ℰ 1 is locally asymptotically stable if ℛ 0 < 1 and unstable if ℛ 0 > 1.
3.4 Data fitting
Here in this section, we fit the model ( 3 ) to the data of HIV prevalence (age 15–49 years) from Eswatini, Lesotho, Botswana, and South Africa, which represent the top four countries with the highest prevalence of HIV in the world in 2023. The prevalence data that is shown in Appendix 1 can be download from ( 42 ). Some parameters on the model in Equation (3) were estimated using the data-fitting process, while the other parameters were taken from the references. Here is the explanation of how we choose the value of these parameters.
1. The total population N, drawn from individuals aged between 15 and 64 years in each country, is sourced from The World Bank data in ( 43 ). According to this data, the populations of Eswatini, Lesotho, Botswana, and South Africa are 736,680, 1,425,560, 1,676,630, and 39,264,160, respectively.
2. The natural death rate is denoted by μ . Using data from the World Bank ( 44 ), we have μ Eswatini = 1 57 year - 1 , μ Lesotho = 1 53 year - 1 , μ Botswana = 1 61 year - 1 , and μ South Africa = 1 62 year - 1 .
3. Recruitment rate is denoted by (Λ). We assume that the total natural death is approximately equal to the total newborn, hence we have Λ = μ N .
4. Condom efficacy is denoted by ( ϵ ). Based on several references ( 45 ), the efficacy of the condom usage is between 90 and 95%. Hence, we assume that ϵ = 92.5%.
5. The progression rate due to the decreases in the number of CD4 + T Cells (δ 1 , δ 2 ). Based on ( 18 ), we choose δ 1 = 0.33 and δ 2 = 0.34.
6. Based on ( 27 ), we use γ u = 0.6. Since γ t < γ u due to treatment that has been followed by T individuals, then we assumed γ t = 0.3.
7. Since q, r , and s are proportions, we assume that ρ = 1.64, r = 0.5, q = 0.653, and s = 0.653.
8. Due to the treatment undertaken by individuals in T , then we assume that β t < β u .
9. We assume that individuals in T 3 are easier to be asked to follow the treatment process for HIV rather than individuals in T 1 and T 2 . Hence, we assume τ 3 > τ 2 > τ 1 > 0.
10. Since κ is the proportion of people who use condoms during sexual activity, we assume κ ∈ [0, 1].
Our aim is to estimate β u , β t , τ 1 , τ 2 , τ 3 , and ϵ which minimize the following error function
where P i is the HIV prevalence data at time step− i and N is the total population in 1990 for Eswatini, Lesotho, Botswana, and South Africa as described before. The initial conditions of I i (0), T i (0), and A (0), for i = 1, 2, 3 are also estimated here, with S ( 0 ) = N - ∑ i = 1 3 ( I i ( 0 ) + T i ( 0 ) + A ( 0 ) ) . The particle swarm optimization (PSO) is used to find the minimum of error function. By using the PSO algorithm as given in ( 46 ), with the number of particles given is 500, the maximum iteration is 1,000, and c 1 = c 2 = 1, we obtain the estimation of the parameters and the initial conditions as shown in Appendix 2 . Figure 5 displays the weekly prevalence calculations from the estimated results compared to the HIV prevalence data for Eswatini, Lesotho, Botswana, and South Africa. The model simulation in Figure 5 shows a better agreement with the actual data. The basic reproduction numbers for each country are given as follows: 1.095, 1.682, 1.732, and 1.65 for Eswatini, Lesotho, Botswana, and South Africa, respectively. A 95% confidence interval for the fitting results using bootstrap resampling residual approach is also given in Figure 5 . The lower and upper bounds of the confidence interval are computed by sorting 1,000 bootstrap samples and taking the 2.5 and 97.5% percentiles.
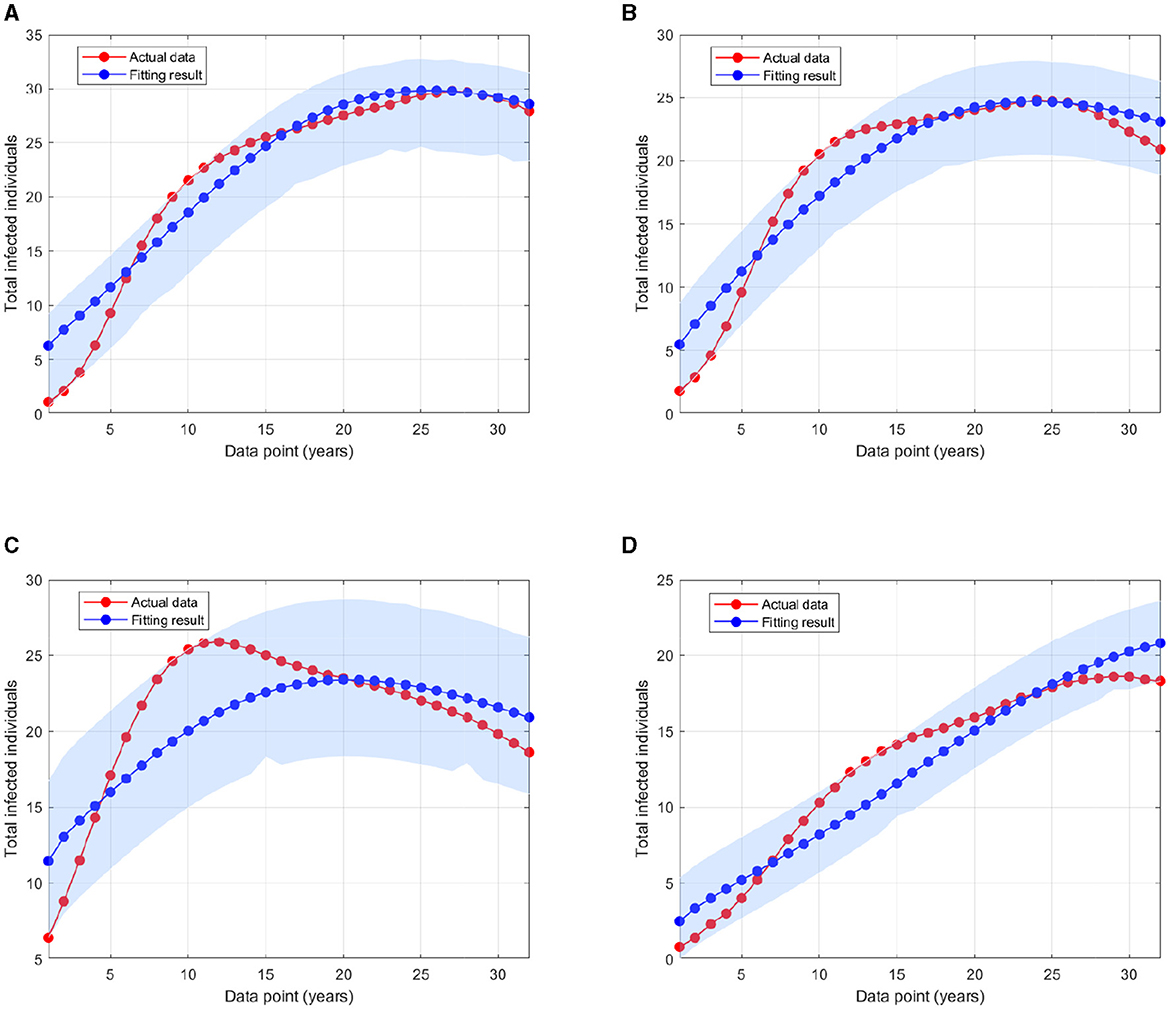
Figure 5 . A comparison of HIV prevalence based on actual data and the model obtained from the estimated parameters for (A) Eswatini; (B) Lesotho; (C) Botswana; and (D) South Africa.
3.5 Sensitivity analysis of the model and the control reproduction number
The sensitivity methods can be used on infectious disease models to determine which variable or parameter is sensitive to a specific condition. Identifying the key critical parameters is an effective way to study such models more widely and accurately. Recently, we have used the sensitivity methods to identify the critical parameters for some infectious disease models. Suppose that an infectious disease model has m compartments x i for i = 1, 2, ..., m and n parameters k j for j = 1, 2, ..., n , then, the local sensitivities can be calculated using three different techniques: non-normalizations, half-normalizations, and full-normalizations. First, the equation of non-normalization sensitivities is given by
where S k j x i is measured as a sensitivity coefficient of each x i with respect to each parameter k j . Then, the formula of half-normalization sensitivities is also defined below:
Finally, the equation of full-normalization sensitivities is defined by
We have used such estimated parameters and initial variables in computational simulations using MATLAB. The results given in this work provide an important step forward to understand the model dynamics more widely. This helps us to identify critical model parameters and how each model state is affected by the model parameters.
3.5.1 Model sensitivity analysis in Eswatini
In this computational simulation, results from Figure 6 are computed by using incidence data from Eswatini with the model initial populations S (0) = 854, 011, I 1 (0) = 9, 529, I 2 (0) = 11, I 3 (0) = 7, 970, T 1 (0) = 63, T 2 (0) = 3, 214, T 3 (0) = 8, 920, and A (0) = 1, 404, and the estimated model parameters are μ = 0.013, δ 1 = 0.33, δ 2 = 0.34, Λ = 11861.26, γ u = 0.1, γ t = 0.018, ρ 12 = 0.1462, ρ 21 = 0.57, ρ 23 = 0.1462, ρ 32 = 0.82, β u = 0.9529, β t = 0.001, τ = 0.7970, κ = 0.0063, and ϵ = 0.3214
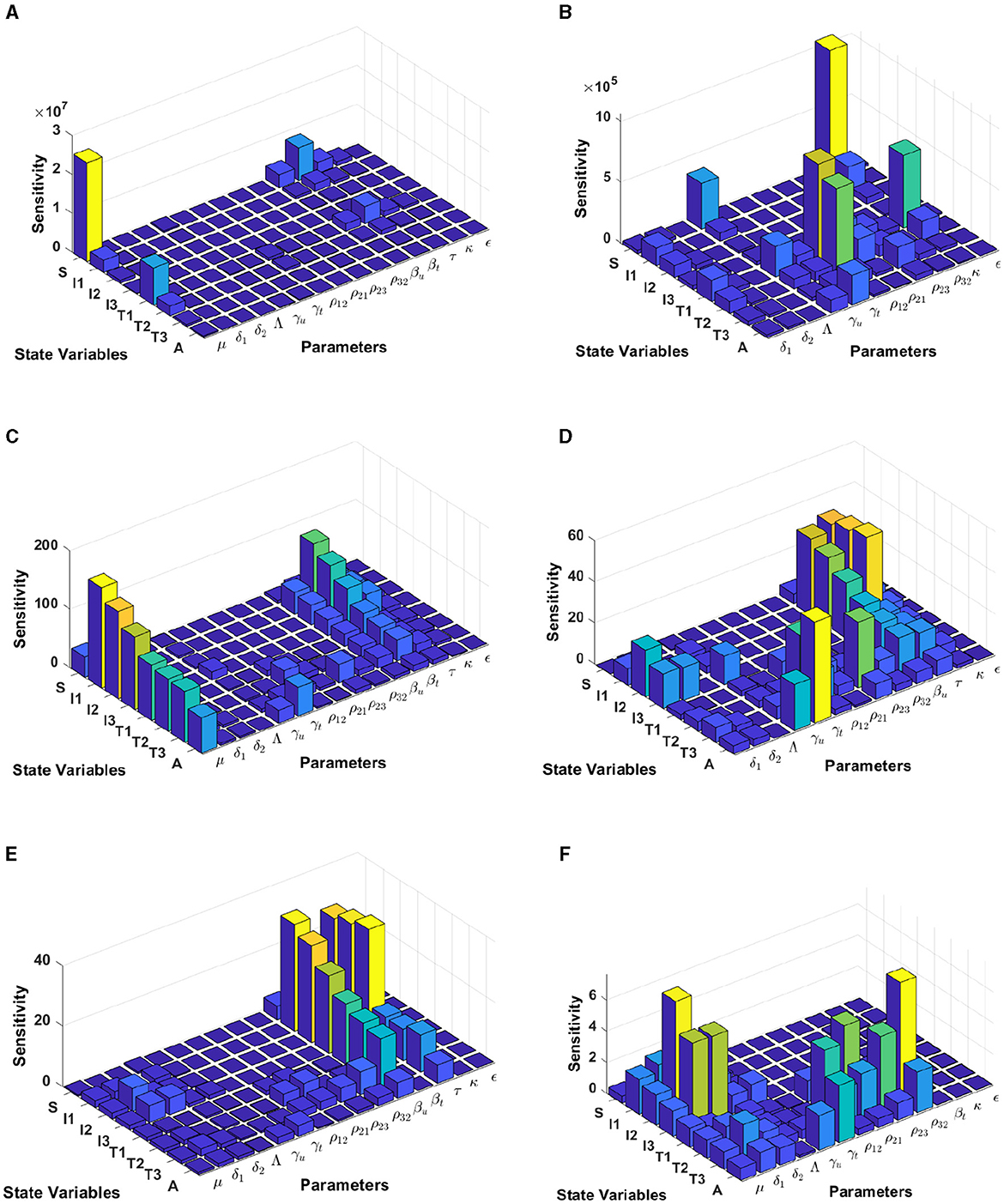
Figure 6 . Local sensitivity analysis with non-normalization (A, B) , half-normalization (C, D) , and full-normalization (E, F) techniques using the best-fit parameter for Eswatini.
When we use the non-normalization technique, it can be seen that the model states S and T 1 are very sensitive to μ , β t , β u , κ , and ρ 12 , while all model states are less sensitive to the model parameters δ 2 , Λ, ρ 32 , and ϵ ; see panels (A, B). Furthermore, using the half-normalization method shows that almost all model states are very sensitive to the model parameters μ , β t , β u , and τ , whereas they are less sensitive to the other model parameters; see panels (C, D). Interestingly, applying the full-normalization method shows that almost all model variables are sensitive to β u and τ , while the other variables have different sensitivities to the model parameters, this is clearly seen in panels (E, F).

3.5.2 Model sensitivity analysis in Lesotho
The results from Figure 7 are computed by using incidence data from Lesotho with the model initial populations S (0) = 1, 799, 000, I 1 (0) = 3, 676, I 2 (0) = 5, 018, I 3 (0) = 37, 272, T 1 (0) = 3, 420, T 2 (0) = 1, 701, T 3 (0) = 1, 982, and A (0) = 1, 163, and the estimated model parameters are μ = 0.013, δ 1 = 0.33, δ 2 = 0.34, Λ = 1, 799, 000/72, γ u = 0.1, γ t = 0.018, ρ 12 = 0.1462, ρ 21 = 0.57, ρ 23 = 0.1462, ρ 32 = 0.82, β u = 0.8999, β t = 0.0001, τ = 0.7909, κ = 0.0131, and ϵ = 0.2710
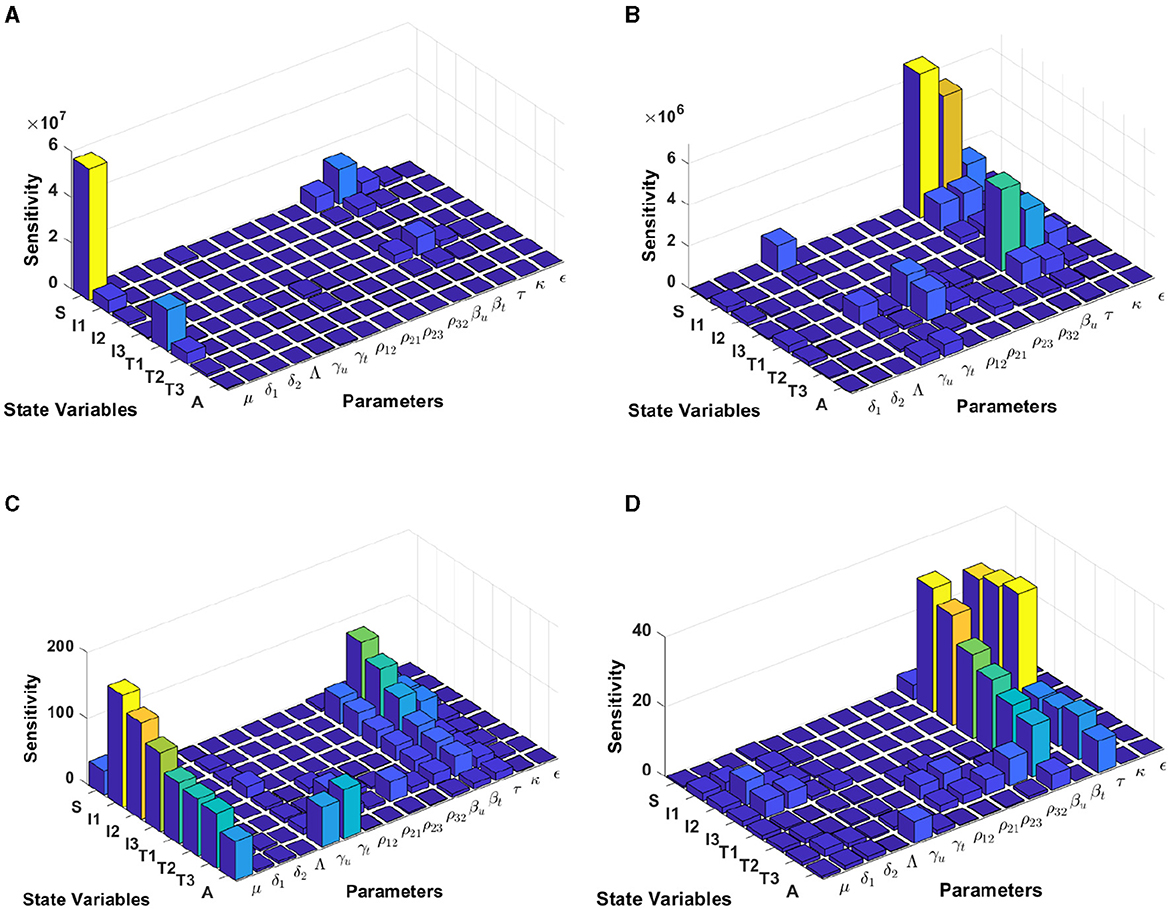
Figure 7 . Local sensitivity analysis with non-normalization (A, B) , half-normalization (C) , and full-normalization (D) techniques using the best-fit parameter for Lesotho.
By using the non-normalization technique, it shows that the model states S and T 1 are very sensitive to μ , β t , β u and τ , while the other model states are less sensitive to the model parameters; see panels (A, B). When we use the half-normalization method, it shows that almost all model states are very sensitive to the model parameters μ , β t , β u , and τ , whereas they are less sensitive to the other model parameters; see panel (C). Furthermore, by applying the full-normalization method shows that almost all model variables are sensitive to β u and τ , while we can also observe that there are different levels of sensitivities between model variables and parameters; see panel (D).
3.5.3 Model sensitivity analysis in Botswana
Using the model initial states and estimated model parameters in computational simulations, results from Figure 8 are computed by using incidence data from Botswana with the model initial populations S (0) = 1, 341, 000, I 1 (0) = 17, 426, I 2 (0) = 3, 379, I 3 (0) = 39, 459, T 1 (0) = 31, 384, T 2 (0) = 7, T 3 (0) = 14, 015, and A (0) = 1, 243, and the estimated model parameters are μ = 0.013, δ 1 = 0.33, δ 2 = 0.34, Λ = 1341000/72, γ u = 0.1, γ t = 0.018, ρ 12 = 0.1462, ρ 21 = 0.57, ρ 23 = 0.1462, ρ 32 = 0.82, β u = 0.8991, β t = 0.0001, τ = 0.8689, κ = 0.8974, and ϵ = 0.0067.
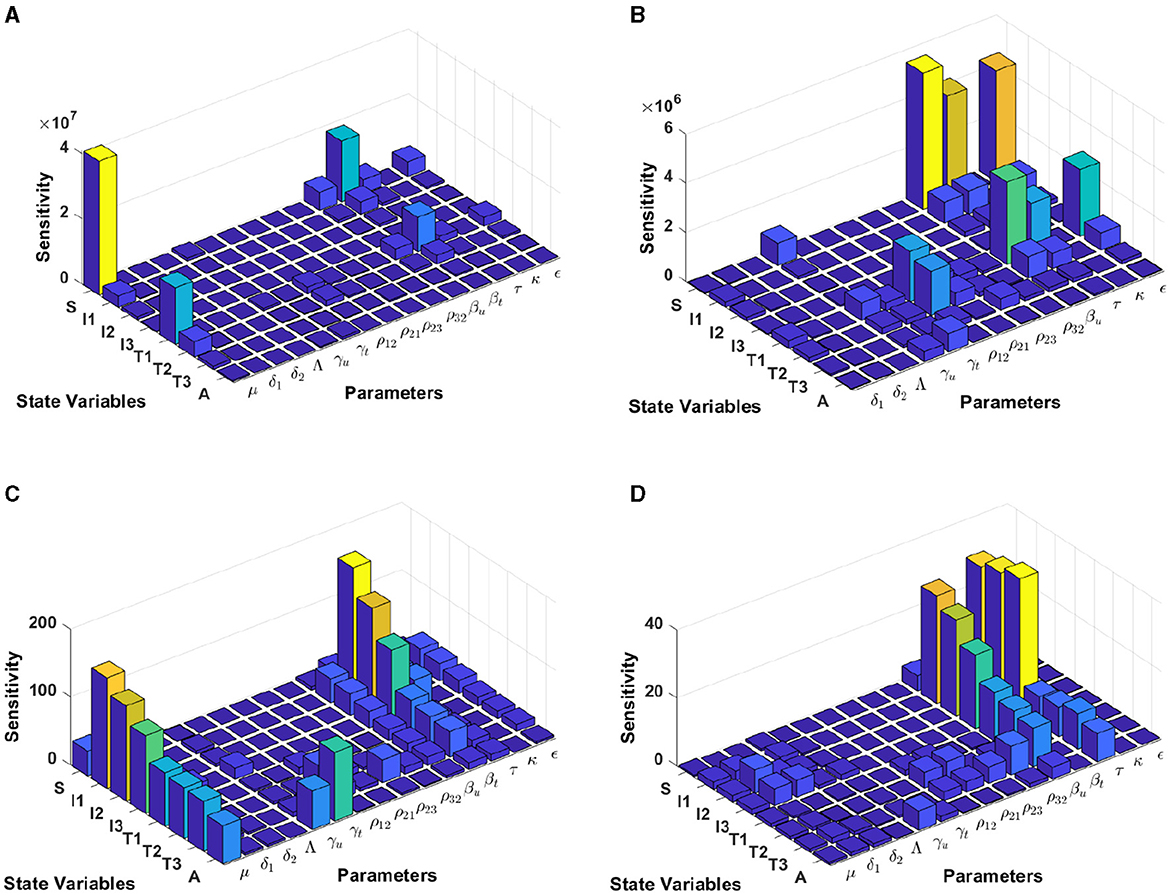
Figure 8 . Local sensitivity analysis with non-normalization (A, B) , half-normalization (C) , and full-normalization (D) techniques using the best-fit parameter for Botswana.
The results of the non-normalization technique shows that the model states S and T 1 are very sensitive to μ , β t , β u , τ , and ϵ , while there are different levels of sensitivities between other model states and parameters; see panels (A, B). Using the half-normalization method shows that almost all model states are very sensitive to the model parameters μ and β t , whereas there are sensitivities to the other model parameters; see panel (C). Furthermore, applying the full-normalization method shows that almost all model variables are sensitive to β u and τ , while we can also see that there are different levels of sensitivities between model variables and parameters; see Figure panel (D).
3.5.4 Model sensitivity analysis in South Africa
Computational results shown in Figure 9 are computed by using incidence data from South Africa with the model initial populations S (0) = 39, 880, 000, I 1 (0) = 127, 900, I 2 (0) = 145, 386, I 3 (0) = 101, 214, T 1 (0) = 104, T 2 (0) = 197, 709, T 3 (0) = 99, 926, and A (0) = 5, 033, and the estimated model parameters are μ = 0.013, δ 1 = 0.33, δ 2 = 0.34, Λ = 39, 880, 000/72, γ u = 0.1, γ t = 0.018, ρ 12 = 0.1462, ρ 21 = 0.57, ρ 23 = 0.1462, ρ 32 = 0.82, β u = 0.8999, β t = 0.0001, τ = 0.7945, κ = 0.6706, and ϵ = 0.0001.
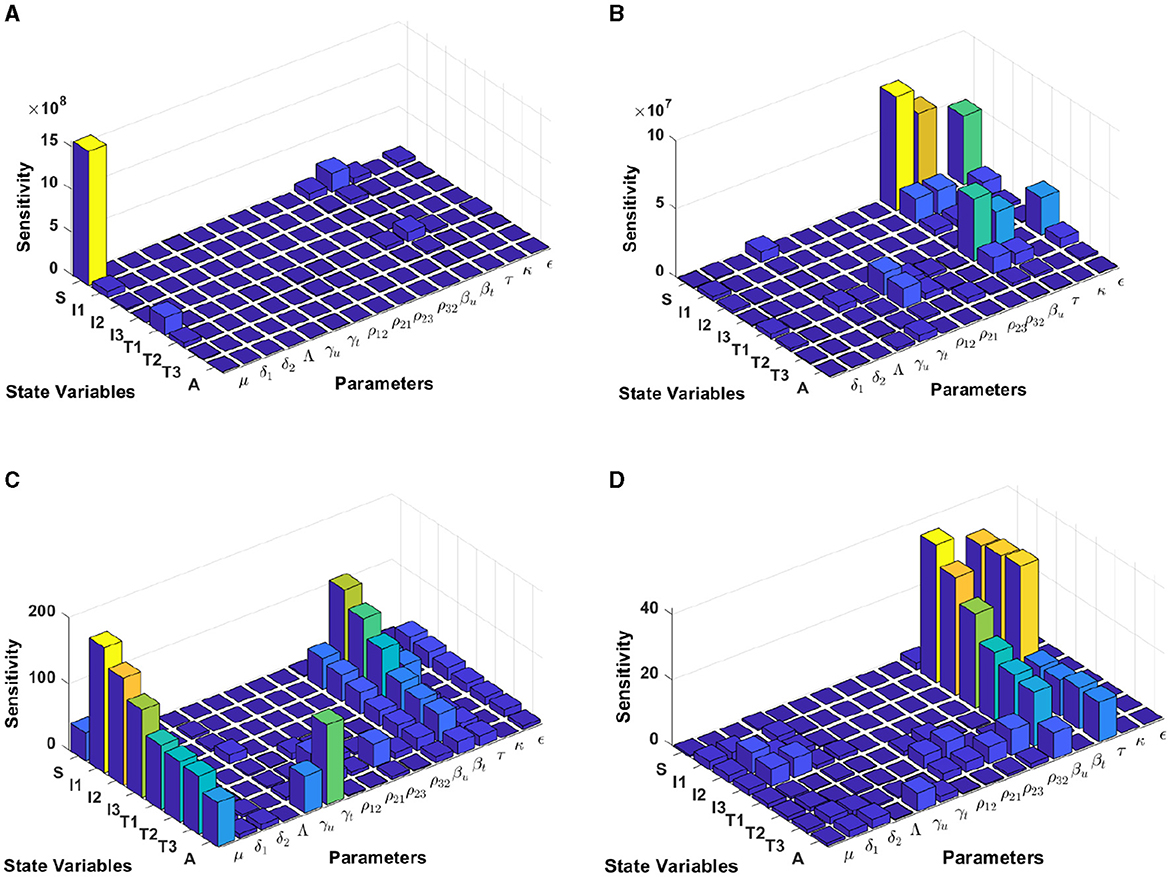
Figure 9 . Local sensitivity analysis with non-normalization (A, B) , half-normalization (C) , and full-normalization (D) techniques using the best-fit parameter for South Africa.
When we use the non-normalization technique, it can be seen that the model states S and T 1 are very sensitive to μ , β t , β u . κ , and ϵ , while the other states are less sensitive to the other model parameters; see panels (A, B). Furthermore, using the half-normalization method shows that almost all model states are very sensitive to the model parameters μ , β t , β u , and ϵ , whereas they are less sensitive to the other model parameters; see panel (C). Interestingly, applying the full-normalization method shows that almost all model variables are sensitive to β u and τ , while the other variables have different sensitivities to the model parameters, this is clearly seen in panel (D).
3.6 Autonomous simulation
In this simulation, we conduct the analysis using estimated parameters for Eswatini. We divide our simulation into three scenarios to understand the impact of the infection rate, the quality of prevention (condom use), and the effectiveness of massive detection. We employ bifurcation parameters and autonomous simulations at several sample points on the bifurcation diagram.
3.6.1 Effect of infection rate β u
To conduct the simulation, we set β u as the bifurcation parameter, while the other parameters are the best-fit parameter for Eswatini (see Section 3.5.1). The numerical results are presented in Figure 10 . It is clear to see that a larger value of β u will increase ℛ 0 and I 1 in endemic equilibrium. Based on this dataset, we determine that ℛ 0 = 1 when β u = 1 . 23 × 1 0 - 6 . At a sample point P 2 ( β u = 0 . 4 × 1 0 - 6 , ℛ 0 = 0.687), we observe that the HIV/AIDS-free equilibrium point is stable, with the following values:
On the other hand, at P 1 ( β u = 3 × 1 0 - 6 , we have ℛ 0 = 1.66), we observe that the HIV/AIDS-endemic equilibrium is stable, with the following values:
In panel (A) of Figure 10 , it is evident that for β u < 1 . 23 × 1 0 - 6 , ℛ 0 < 1, indicating the stability of the HIV/AIDS-free equilibrium. As β u increases, ℛ 0 also increases (as seen in the cyan curve). Upon reaching the branch point (BP), the HIV/AIDS-free equilibrium becomes unstable, leading to the stable HIV/AIDS-endemic equilibrium, which grows in significance as β u continues to increase. Panel (B) illustrates how trajectories from different initial conditions converge toward the same stable equilibrium point, namely, the HIV/AIDS-free equilibrium or the HIV/AIDS-endemic equilibrium. Figure 11 shows the dynamic of the system ( 3 ) with respect to various values of β u . It can be seen that larger β u will increase the number of infected individuals I i , T i , and A .
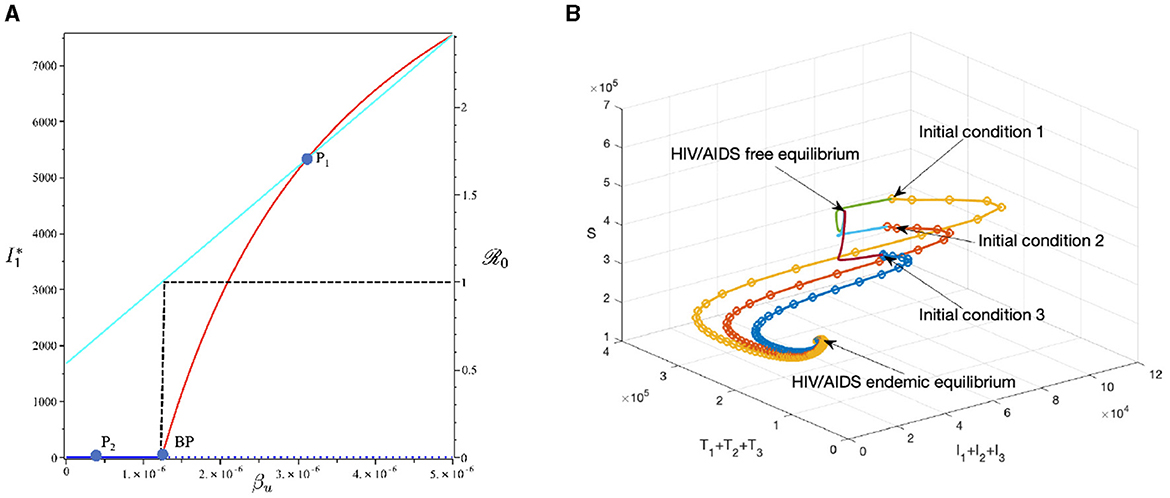
Figure 10 . The bifurcation diagram of the system ( 3 ) with respect to β u is presented in (A) . In this diagram, “BP" denotes the branch point occurring at ℛ 0 = 1. The cyan, red, and blue curves represent ℛ 0 , the endemic equilibrium, and the HIV/AIDS-free equilibrium, respectively. Solid and dotted curves indicate stable and unstable equilibria, respectively. (B) Depicts the trajectories of total infected, total treated, and susceptible individuals tending to HIV/AIDS free-equilibrium point for β u at P 1 and to HIV/AIDS endemic equilibrium for β u at P 2 .
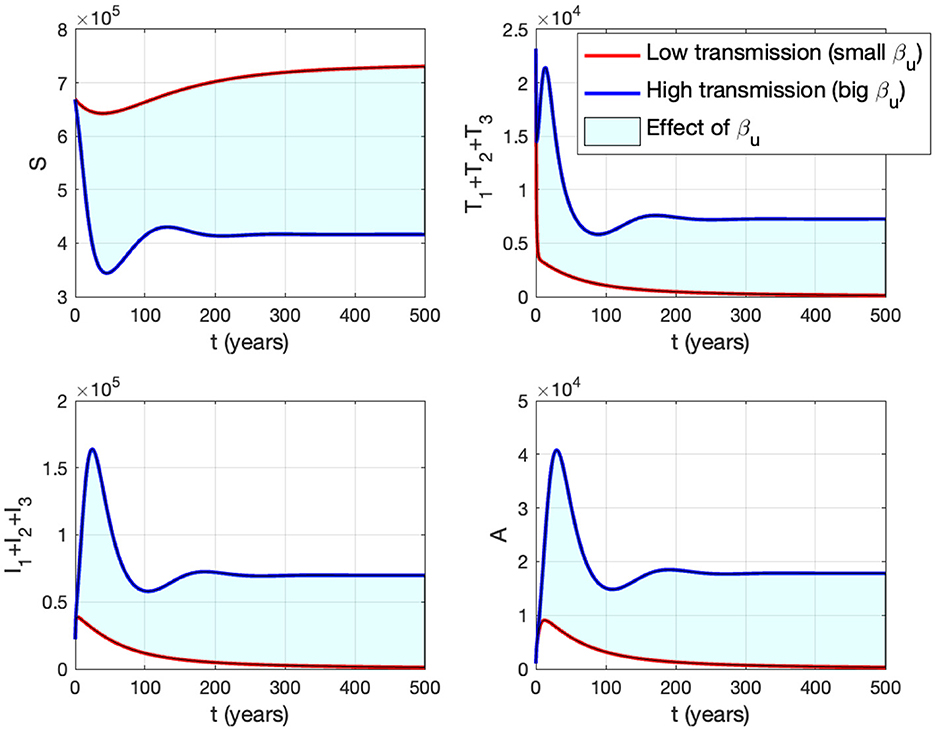
Figure 11 . The impact of β u on the dynamics of susceptible (up left), total infected (up right), total treated (below left), and AIDS (below right). The blue curve represents the dynamic for a low transmission rate β u (1.488 × 10 −7 ), while the red curve represents the dynamic for a high transmission rate β u (1.488 × 10 −6 ).
3.6.2 The effect of proportion of the condom use ( κ )
This section is dedicated to examining the impact of the proportion of people who use condoms during sexual contact ( κ ) on reducing the HIV/AIDS infection rate. For this simulation, we use the same parameter values as in Section 3.6.1, with the exception of κ , which is varying. The numerical results are presented in Figure 12 . With this data set, we determine that R 0 = 1 at ϵ = 0.691. As indicated by the cyan curve in panel (A), a higher quality of condoms (larger ϵ ) leads to a smaller ℛ 0 . At sample point P 1 , with ϵ = 0.2, we have ℛ 0 = 2.262, resulting in a stable HIV/AIDS-endemic equilibrium at
On the other hand, at sample point P 2 , when ϵ = 0.9, we have ℛ 0 = 0.464 which gives us a stable HIV/AIDS-free equilibrium point at
Panel (B) shows the trajectories of all solutions from different initial conditions tending toward their stable endemic equilibrium and disease-free equilibrium. Figure 13 shows the dynamic of the system ( 3 ) with respect to various values of κ . It can be seen that larger value of κ will reduce the number of infected individuals I i , T i , and A .
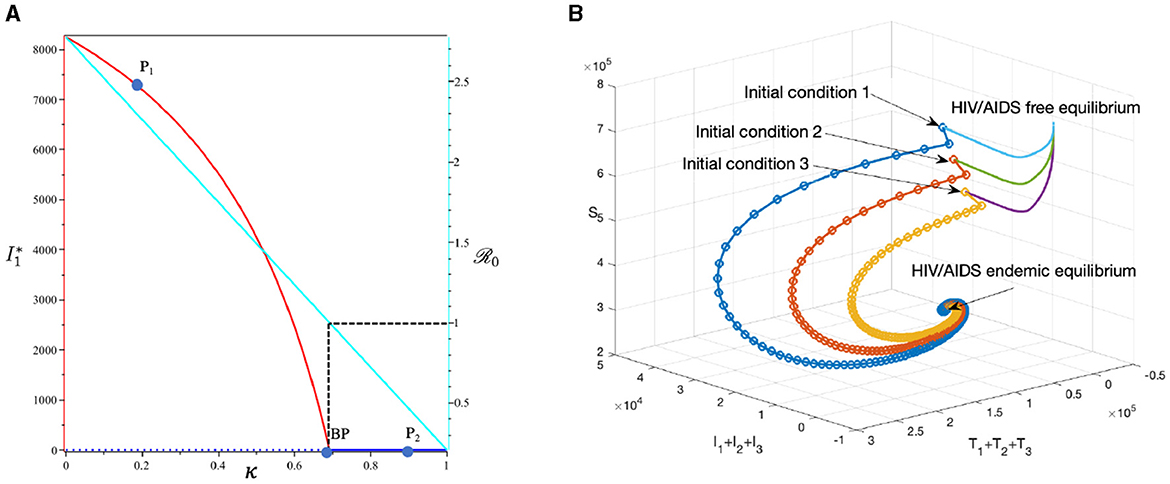
Figure 12 . The bifurcation diagram of the system ( 3 ) with respect to κ is presented in (A) . In this diagram, “BP” denotes the branch point occurring at ℛ 0 = 1. The cyan, red, and blue curves represent ℛ 0 , the endemic equilibrium, and the HIV/AIDS-free equilibrium, respectively. Solid and dotted curves indicate stable and unstable equilibria, respectively. (B) Depicts the trajectories of total infected, total treated, and susceptible individuals tending to HIV/AIDS-endemic equilibrium point for κ at P 1 and to HIV/AIDS-free equilibrium for κ at P 2 .
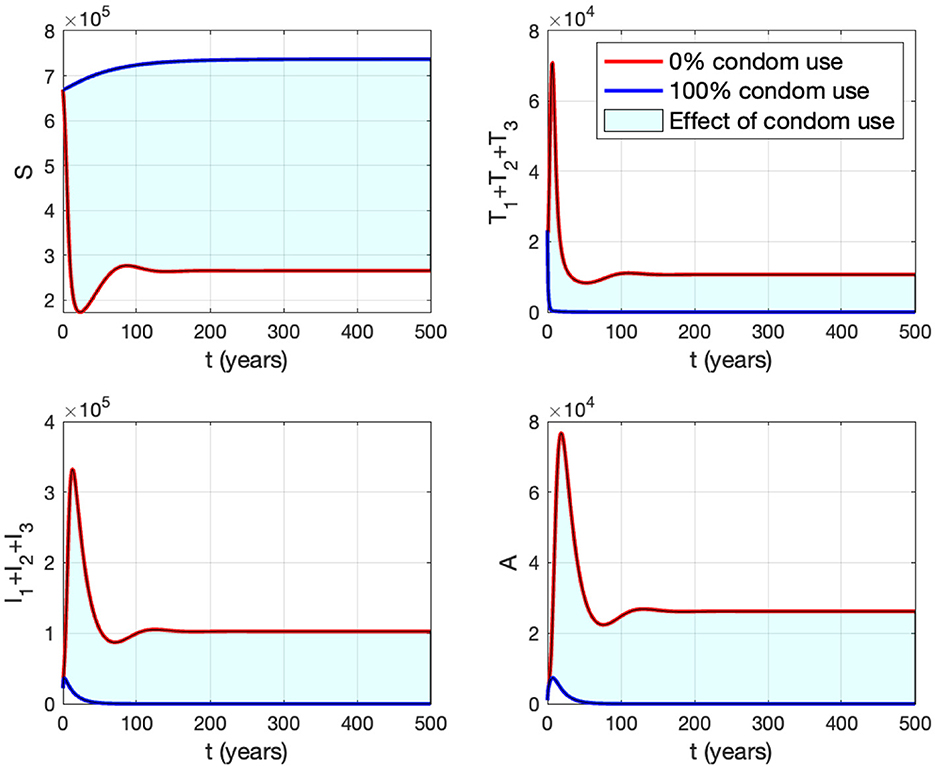
Figure 13 . The impact of κ to the dynamics of susceptible (up left), total infected (up right), total treated (below left), and AIDS (below right). The red curve represents the dynamic when no individuals use condom, while the blue curve represents the dynamic when all people use condoms during sexual contact.
3.6.3 The effect of massive case detection from I 1 to T 1 ( τ 1 )
This section is dedicated to exploring the impact of the rate of case detection ( τ 1 ) on the acceleration of treatment for individuals. In this simulation, we employ data for Eswatini, with the exception of τ 1 , which serves as a freely adjustable bifurcation parameter. The numerical findings are presented in Figure 14 . Within this dataset, we reveal the refined result that R 0 = 1 when τ 1 = 10.93. As described by the cyan curve in panel (A), a more intensive case detection (characterized by a larger τ 1 ) corresponds to a smaller ℛ 0 . At sample point P 1 , τ = 7 gives ℛ 0 = 1.04 and gives us the following endemic equilibrium.
On the other hand, at sample point P 2 , when τ = 15, we have ℛ 0 = 0.98, which gives us a stable HIV/AIDS-free equilibrium point at
Panel (B) shows the trajectory of all solutions from different initial conditions tends toward either how stable endemic equilibrium or disease-free equilibrium. Figure 15 shows the dynamic of the system ( 3 ) with respect to various values of τ 1 . It can be seen that a larger value of τ will reduce the number of infected individuals I i , T i , and A .
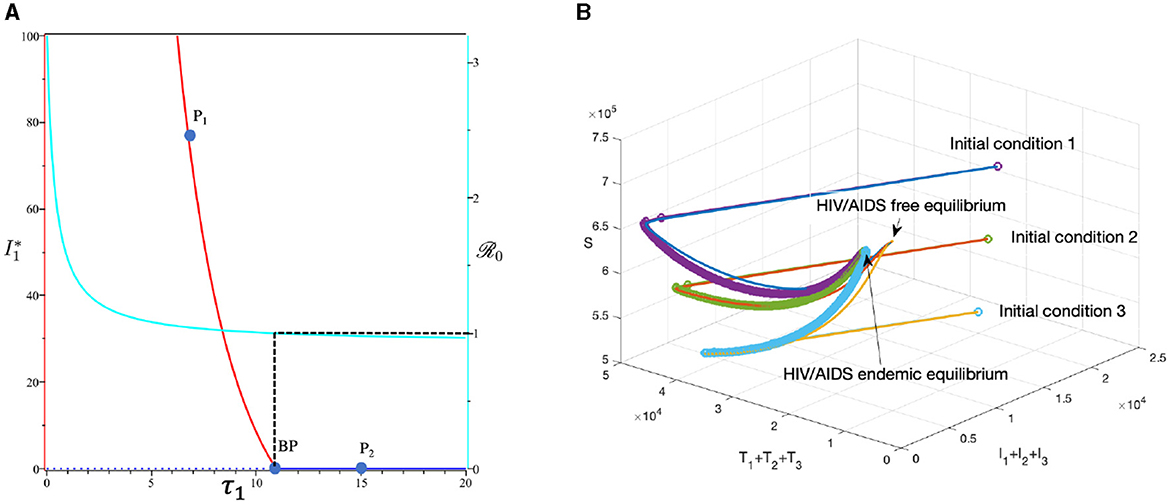
Figure 14 . The bifurcation diagram of the system ( 3 ) with respect to τ 1 is presented in (A) . In this diagram, “BP” denotes the branch point occurring at ℛ 0 = 1. The cyan, red, and blue curves represent ℛ 0 , the endemic equilibrium, and the HIV/AIDS-free equilibrium, respectively. Solid and dotted curves indicate stable and unstable equilibria, respectively. (B) Depicts the trajectories of total infected, total treated, and susceptible individuals tending to HIV/AIDS-endemic equilibrium point for τ 1 at P 1 and to HIV/AIDS-free equilibrium for τ at P 2 .
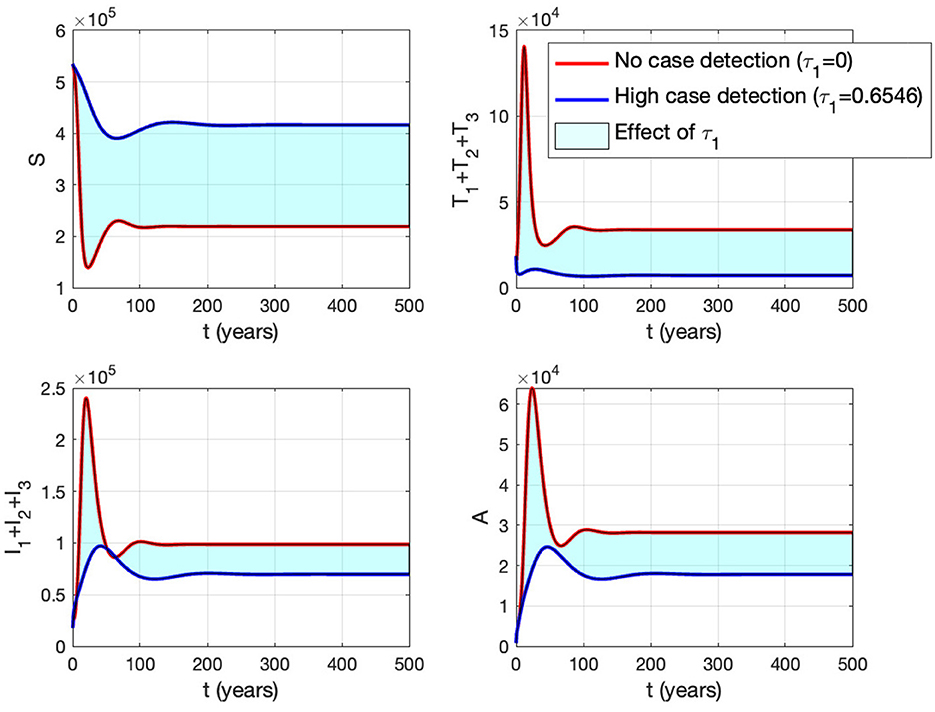
Figure 15 . The impact of τ 1 on the dynamics of susceptible (up left), total infected (up right), total treated (below left), and AIDS (below right). The red curve represents when no early case detection was implemented, while the blue curve represents when it was implemented.
3.7 Minimum proportion on the use of condoms to eliminate HIV/AIDS
In this section, we conduct numerical experiments to understand how the proportion of people using condoms impacts the spread of HIV/AIDS. We use four different datasets representing four countries: Eswatini, Lesotho, Botswana, and South Africa. All parameters are consistent with those in Appendix 2 , except for condom quality ( ϵ ). We calculate the minimum population proportion that needs to use condoms with a specific condom quality (between 70 and 100%) to achieve ℛ 0 < 1. The results are depicted in Figure 16 . It is clear that better condom quality requires a smaller proportion of the population to reduce ℛ 0 to less than one. Based on the analysis results, an efficacy of 70% for condoms indicates that Eswatini could eliminate HIV/AIDS if a minimum of 91.39% of the infected population consistently uses condoms during sexual activities. Simultaneously, with the same efficacy, it is evident that Lesotho would need to mandate 100% condom usage to achieve the same goal, while Botswana cannot solely rely on condom use to eliminate HIV/AIDS. If condom efficacy improves, for instance, reaching 90%, the proportion of the infected population required to use condoms during sexual activities decreases. It would be 71% for Eswatini and up to 81% for Botswana.
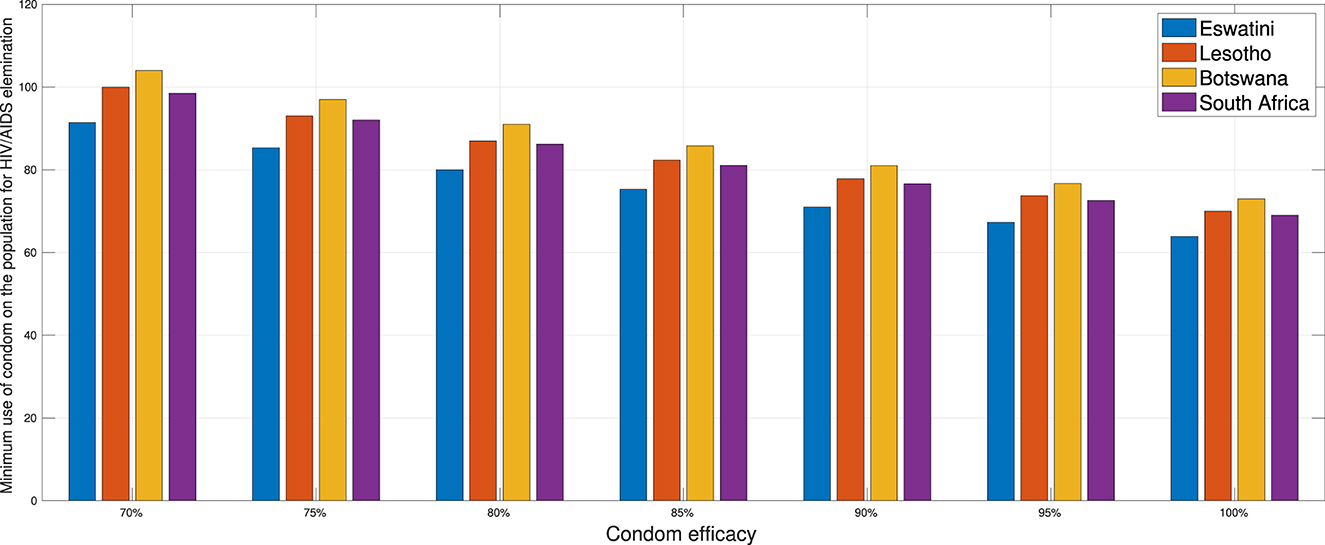
Figure 16 . A comparison of the minimum population percentage in each country required for condom usage, contingent upon the quality of the condom.
4 Extension of the HIV/AIDS model as an optimal control problem
4.1 optimal control model.
In this section, we expand our model from the system ( 3 ) to an optimal control problem by introducing time-dependent variables u 1 ( t ) to represent the proportion of condom use ( κ ) and the case detection rate ( τ 1 , τ 2 , τ 3 ), which is now denoted by u 2 ( t ), u 3 ( t ), u 4 ( t ), respectively. Consequently, our model now reads as follows:
The objective of this optimal control approach is to minimize the number of untreated infected individuals I 1 , I 2 , and I 3 , as well as A , by optimizing the intervention of the proportion of condom use ( u 1 ) and the case detection rate ( u 2 , u 3 , and u 4 ). Therefore, the cost function reads as follows:
where ω i for i = 1, 2, 3, and 4 and φ j for j = 1, 2, 3, and 4 are the positive weight parameter for each component on J .
4.2 Characterization of the problem
We define the Hamiltonian of our problem as follows:
First, by taking the partial derivative of H with respect to each variable, the adjoint system of our problem is given as follows:
completed with the transversality condition λ i ( t f ) = 0 for i = 1, 2, …8. The optimality condition is taken from ∂ H ∂ u i = 0 for i = 1, 2, 3, and 4. Hence, taking this into account with the lower and upper bounds for u i , we have the optimal control variables that should satisfy as follows:
To summarize our problem, we want to minimize the cost function given in Equation (5) subject to the state system in Equation (4) completed with its initial condition, the adjoint system in Equation (6) completed with its transversality condition, and the optimality condition in Equation (7) . We use a forward-backward iterative method to solve the problem. We begin by giving an intial guess for the control variables for all t and using it to solve the state system in Equation (4) forward in time. Then, we solve the adjoint system in Equation (6) backward in time with the given transversality condition. Hence, with these results, we can update the optimal control value using the formula in Equation (7) . We goback to the first step until the convergence criteria are achieved, which in our case is | J iteration-(i + 1) - J iteration-(i) | < 1 0 - 5 .
4.3 Numerical experiments
The simulation in this section was conducted using parameter values corresponding to the best-fit values for Eswatini. Please refer to Appendix 2 for details. Furthermore, we have set the value for the weight parameter on the cost function as follows:
and the initial condition given by
In the following, we set three scenarios for the implementation of controls.
• Scenario 1 : The implementation of condom use ( u 1 ) only, while case detection ( u 2 ) set to be zero. Hence u 1 ≠ 0 and u 2 = u 3 = u 4 = 0.
• Scenario 2 : The implementation of case detection ( u 2 , u 3 , u 4 ) only, while condom use ( u 1 ) set to be zero. Hence u 1 = 0, u 2 ≠ 0, u 3 ≠ 0, and u 4 ≠ 0.
• Scenario 3 : The combination of condom use ( u 1 ) and case detection ( u 2 , u 3 , and u 4 ). Hence u 1 ≠ 0, u 2 ≠ 0, u 3 ≠ 0, and u 4 ≠ 0.
The first numerical experiment involves the use of condoms as the sole strategy to prevent the spread of HIV/AIDS. The results are presented in Figure 17 where the dynamics of model output are given in panels (A–D) while the dynamics of control are shown in panels (E–H). It is clear to see that intervention in condom usage should be given almost maximal effort from the beginning of the simulation. Since condom usage can prevent new infections, we can observe an increase in the number of susceptible individuals [panel (A)] and significant decreases in the total number of PLHIV without treatment (total of I i ) and PLHIV with AIDS illness ( A ). Since the intervention of case detection was not used, we can see that the number of undetected case decreases and tends to zero [see panel (C)]. The total number of infections averted using this strategy is 7.59 × 10 6 at an optimal cost of 2.06 × 10 11 .
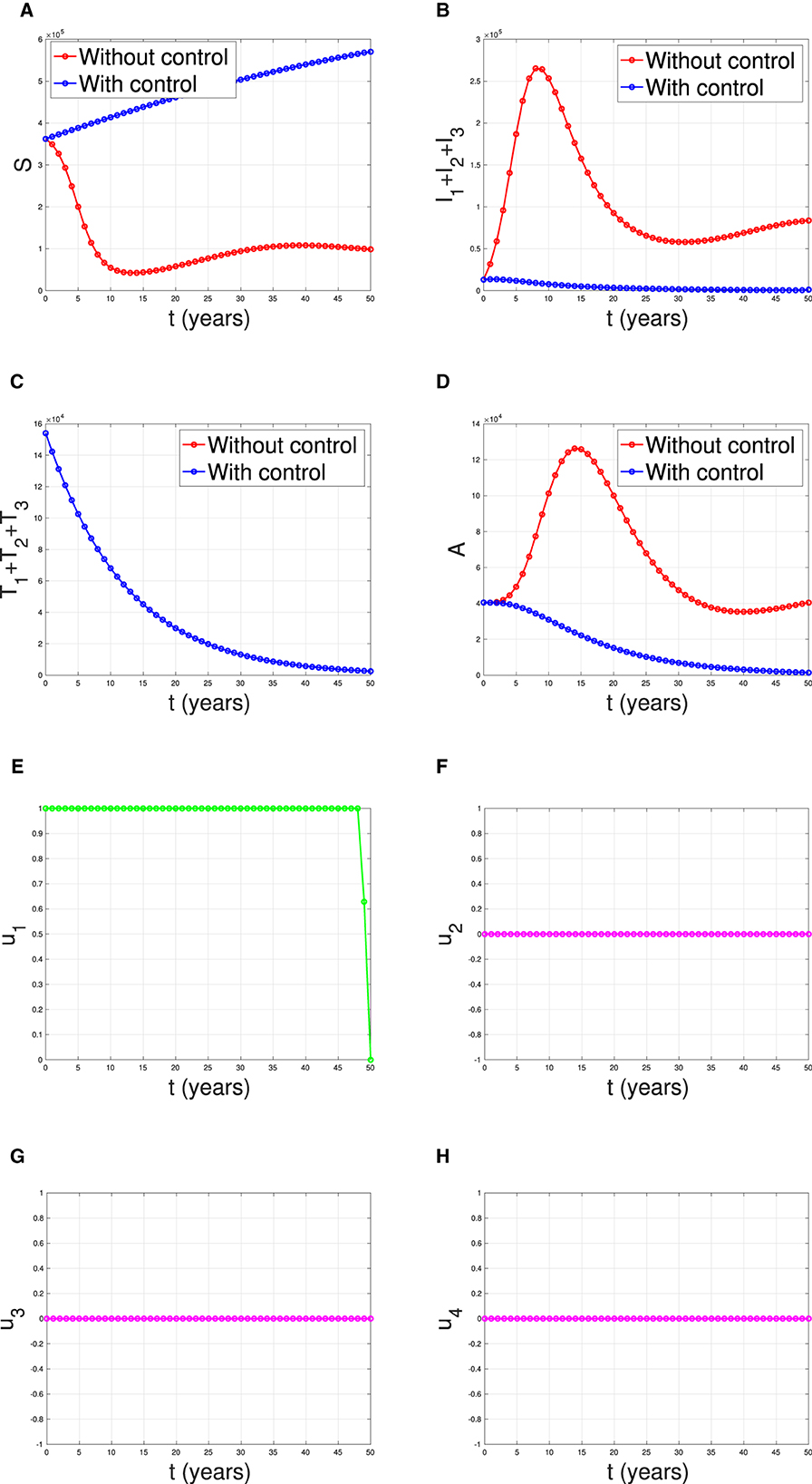
Figure 17 . The dynamic of model output for (A) susceptible, (B) total PLHIV untreated I , (C) total PLHIV receiving treatment T , (D) total PLHIV with AIDS ilness A , and (E–H) for control variables under the scenario when condom use used as a single intervention.
Figure 18 illustrates the situation when the government relied solely on the implementation of case detection as a single intervention ( u 2 , u 3 , u 4 only). The dynamics of control are depicted in panels (F–H). The intervention is applied with high intensity at the beginning of the simulation and then decreasing to an almost constant for a short period and decreases again when the final time approaches. With this strategy in place, we can observe a decrease in the number of PLHIV without and with treatment [panels (B, C)], resulting in a reduced number of individuals with AIDS [panel (D)]. The total number of infected individuals averted using this strategy is 3.74 × 10 4 at an optimal cost of 2.52 × 10 16 .
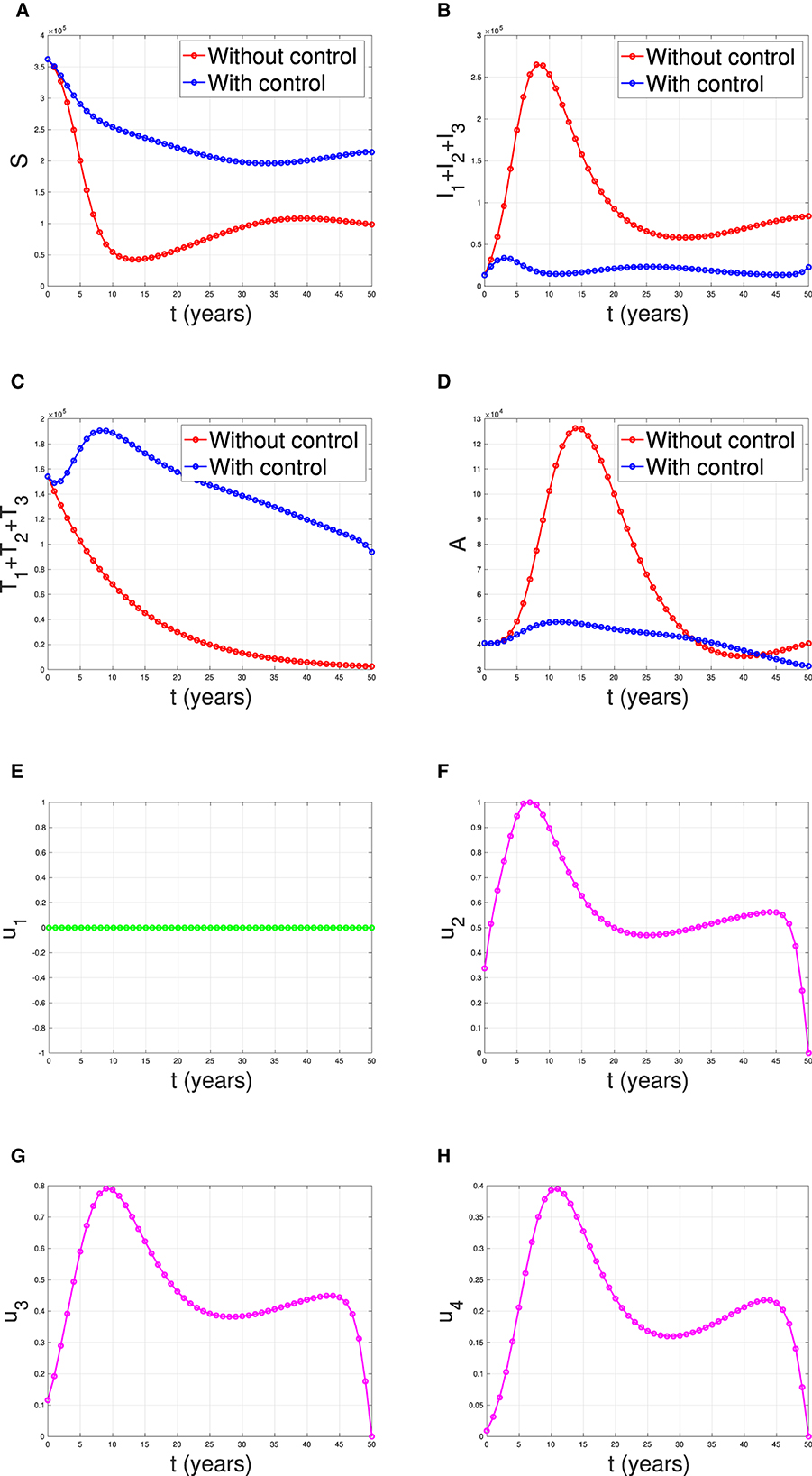
Figure 18 . The dynamic of model output for (A) susceptible, (B) total PLHIV untreated I , (C) total PLHIV receiving treatment T , (D) total PLHIV with AIDS illness A , and (E–H) for control variables under the scenario when case detection used as a single intervention.
The last simulation was conducted to assess the impact of combining condom usage and case detection in reducing the spread of HIV/AIDS in Eswatini. The results are presented in Figure 19 , with the dynamics of u 1 and u 2 shown in panels (E–H), respectively. We observe that the intervention involving condom use should be initiated at a high rate at the beginning of the simulation while the case detection followed the dynamic of infected individuals. As a result, we witness an initial increase in the number of susceptible individuals [panel (A)] and a significant decrease in the PLHIV without treatment and PLHIV with AIDS illness [panels (B, D), respectively]. On the other hand, since the number of infected PLHIV without treatment is already reduced due to condom use, then the number of treated PLHIV is not significantly different in the case of no control scenario. Finally, the combination of condom use and case detection leads to a significant reduction in the number of PLHIV. The total number of infections averted using this strategy is 7.55 × 10 6 at an optimal cost of 9.81 × 10 14 .
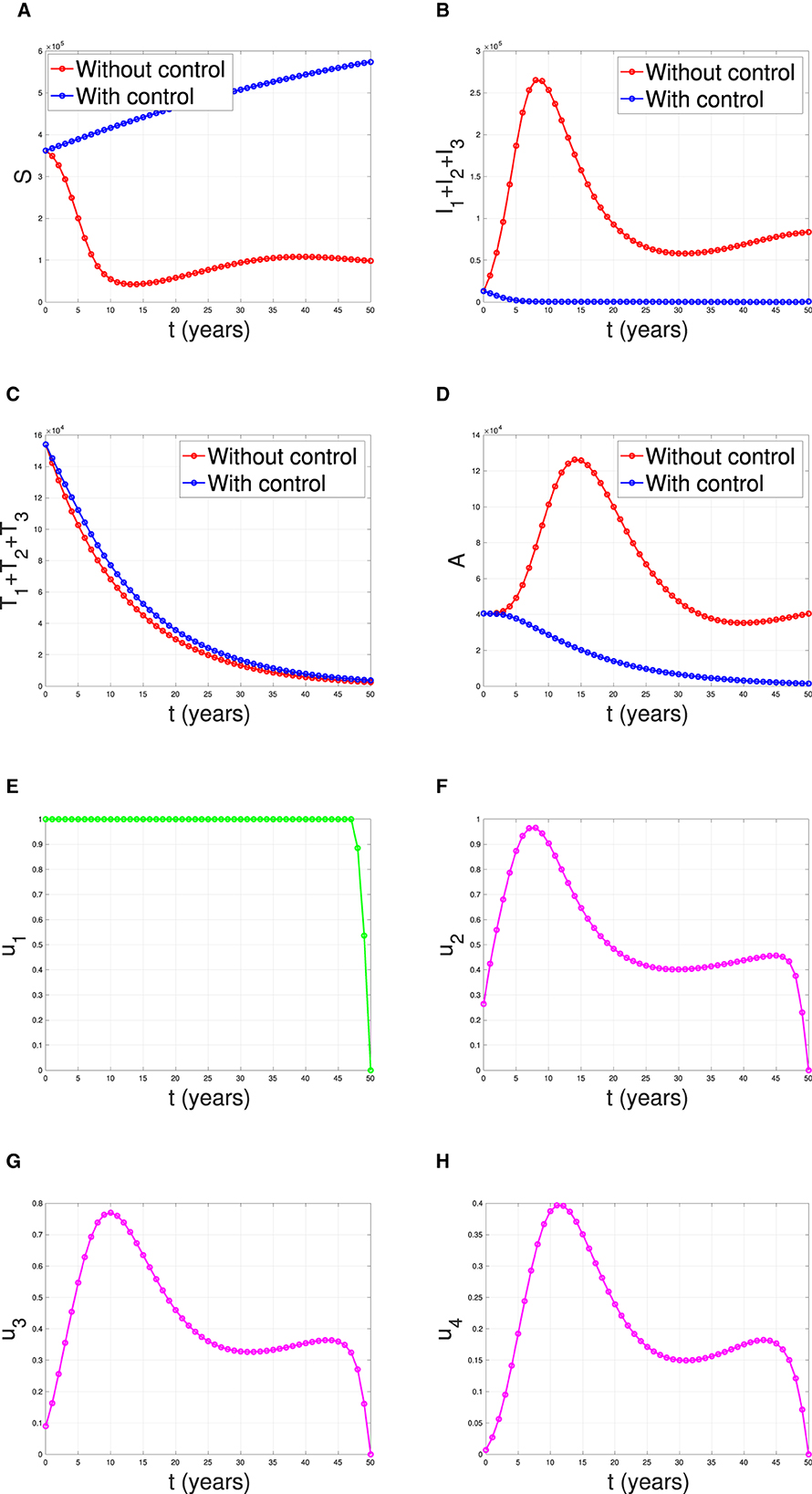
Figure 19 . The dynamic of model output for (A) Susceptible, (B) total PLHIV untreated I , (C) total PLHIV receiving treatment T , (D) total PLHIV with AIDS ilness A , and (E–H) for control variables under the scenario when condom use and case detection implemented together.
Cost-effectiveness analysis
In this section, we will calculate the most effective strategy between strategies 1,2, and 3 based on its average cost-effectiveness ratio (ACER) values. Several assumptions need to be elucidated in the cost-effectiveness calculations in this section. In computing the total cost ( TC ), it is assumed that the total expenses incurred as a consequence of heightened control interventions constitute the overall cost. On the other hand, total infections averted ( TIA ) are derived from the total number of individuals successfully prevented from infection as a consequence of control interventions. For further clarity, refer to Equations (8) and (9) below.
where symbol † and ‡ represent simulation results, without and with control, respectively. A smaller value of ACER represents the most effective strategy. The results of TC, TIA, and ACER are given in Table 2 .
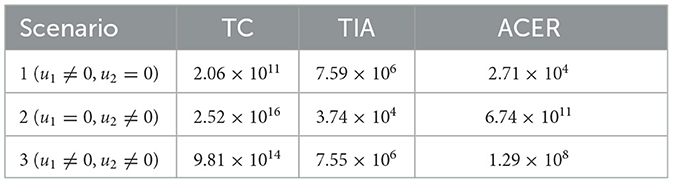
Table 2 . Simulation result for cost-effectiveness analysis.
Based on the calculations above, we can conclude that strategy 1, which focuses solely on condom use as the single intervention, is the most effective strategy. It is followed by strategy 3, which combines condom use and case detection. Strategy 2, which relies solely on case detection as the single intervention, is the least effective strategy. Figure 20 shows the impact of all possible scenarios on HIV prevalence in Eswatini. We can observe that the HIV prevalence between scenario 1 (condom use only) and scenario 3 (condom use and case detection) is only slightly different. This affirms that case detection is less effective in reducing HIV prevalence when the implementation of condom use is already taking place at maximum effort.
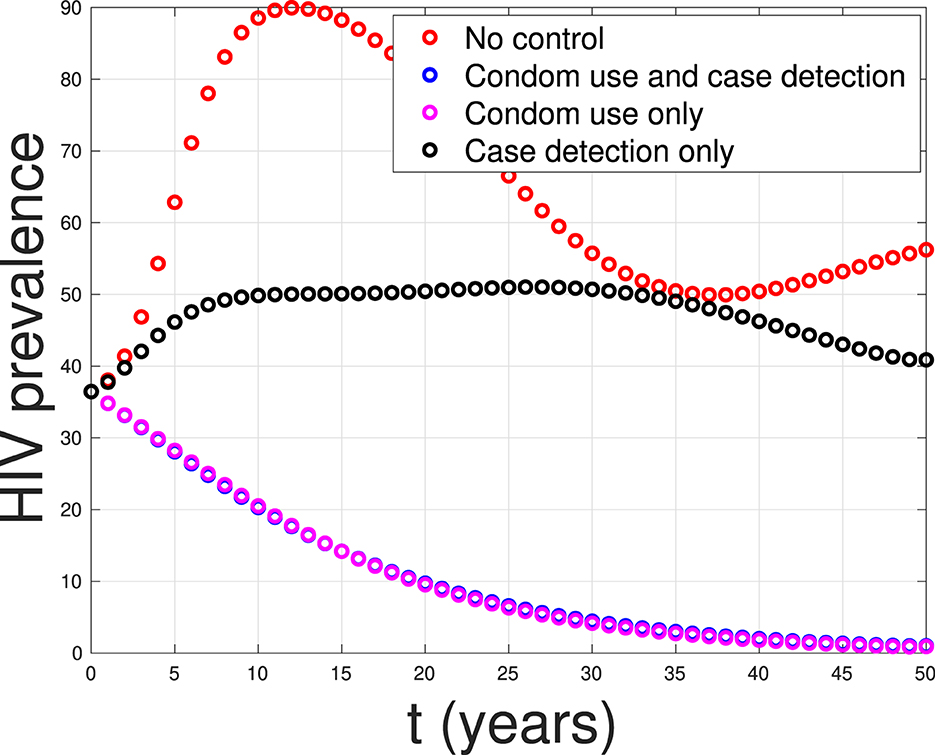
Figure 20 . A comparison between control scenarios on the HIV prevalence in Eswatini.
5 Conclusion
In this study, we developed a mathematical model to assess the effectiveness and cost-effectiveness of various strategies aimed at controlling the spread of HIV/AIDS. We considered the impact of case detection, treatment, and condom use in our model. One key aspect of our model is its acknowledgment of the fact that treatment outcomes may not always be successful, making it a more realistic representation of the disease's dynamics. We begin our analysis for a special case model, where we do not consider the number of CD4+T cells. Our mathematical investigation on the expression of the equilibrium points and the reproduction number reveals the potential of case detection to reduce the reproduction number of the virus.
Our comprehensive analysis is extended to the complete model, where we assessed the stability of the HIV/AIDS-free equilibrium point. We found that this equilibrium is stable when the control reproduction number is less than one, indicating the feasibility of disease containment.
To validate our model, we estimate our parameter values using data from four different countries: Eswatini, Lesotho, Botswana, and South Africa. Parameter estimation was performed using incidence data from these regions, and we found that the reproduction of each country is larger than one, which indicates the endemicity of HIV/AIDS in those countries. Furthermore, sensitivity analysis sheds light on the impact of condom use and case detection on HIV spread dynamics. It allowed us to identify the most influential factors in disease control.
Finally, our study delved into optimal control strategies, considering the dynamics of infected individuals when all control variables are time-dependent. From a cost-effectiveness perspective, we found that employing condom use as a sole intervention is the most effective strategy in terms of the average cost for each averted infected individual. It is worth noting that condom use not only serves as a cost-effective approach but is also a crucial tool in preventing the transmission of HIV/AIDS. While the simulation results for case detection in this research indicate its lesser effectiveness in reducing the number of HIV prevalence, it still holds importance. Case detection, despite its limitations, plays a crucial role in assisting the government in mitigating the broad social impact of HIV in the population. In light of the complexities surrounding HIV/AIDS, a comprehensive strategy that combines both condom use and case detection could offer a more robust approach to tackling the multifaceted challenges posed by the HIV epidemic. In conclusion, our research provides valuable insights into the control of HIV/AIDS, offering a comprehensive assessment of strategies and emphasizing the importance of case detection as a highly efficient and cost-effective means of disease containment.
Beyond its potential in reducing HIV spreads, there are several issues about the implementation of condom use and case detection. In societies where discussions about sex and sexual health are taboo or stigmatized, condom use campaigns may face challenges in gaining acceptance. Cultural norms and religious beliefs can influence attitudes toward condom use. Accessibility to condoms may be limited in certain cultural contexts due to factors such as affordability, availability, and distribution channels. Addressing these barriers is crucial for the success of condom use campaigns. On the other hand, HIV-related stigma and discrimination can impede case detection efforts. Fear of being ostracized or discriminated against may discourage individuals from seeking HIV testing and treatment. Privacy concerns are paramount in HIV testing and case detection. Trust in healthcare systems is essential for successful case detection. In some communities, historical distrust or negative experiences with healthcare providers may affect the willingness to engage with HIV testing and treatment services. In summary, the effectiveness of condom use campaigns and case detection for HIV depends on their alignment with social and cultural contexts. Interventions need to be culturally sensitive, addressing barriers related to stigma, discrimination, and accessibility to effectively prevent the spread of HIV and promote early detection and treatment. In diverse social and cultural settings, collaborating with local communities, leaders, and organizations can enhance the relevance and acceptance of these interventions.
Although the research results in this article show in-depth insights into the potential use of condoms and early detection in reducing HIV prevalence in case studies across four countries, there are still some aspects that can be further developed to achieve more satisfying outcomes. One of these aspects is the fact that condom use is not only for suppressing or preventing the spread of HIV but also for other sexually transmitted infections (STIs) such as syphilis, chlamydia, herpes, and human papillomavirus (HPV). Therefore, condom use as an intervention can be applied in models that consider co-infection between two or more STIs. Only few researchers have discussed coinfection models involving HIV and other STIs, as seen in ( 47 – 51 ). The use of condoms as an intervention that can prevent both diseases simultaneously would be intriguing to explore in future research.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary material , further inquiries can be directed to the corresponding author.
Author contributions
DA: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. RD: Formal analysis, Investigation, Writing – original draft. SK: Methodology, Software, Validation, Visualization, Writing – review & editing. JW: Data curation, Investigation, Software, Validation, Visualization, Writing – review & editing. PK: Formal analysis, Funding acquisition, Investigation, Supervision, Validation, Writing – review & editing. MS: Investigation, Software, Visualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by Universitas Indonesia through the PUTI-Q1 research grant scheme (ID:NKB-485/UN2.RST/HKP.05.00/2023).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1324858/full#supplementary-material
1. ^ AIDS by the Numbers (2023). Available online at: https://www.unaids.org/en .
2. ^ The Global HIV and AIDS Epidemic (2023). Available online at: https://www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics/#::text=HIV .
1. Kementrian Kesehatan (Kemkes). Ayo Cari Tahu Apa Itu HIV . (2022). Available online at: https://yankes.kemkes.go.id/view_artikel/754/ayo-cari-tahu-apa-itu-hiv (accessed July 20, 2023).
Google Scholar
2. World Population Review. HIV Rates by Country 2024 . (2024). Available online at: https://worldpopulationreview.com/country-rankings/hiv-rates-by-country (accessed July 20, 2023).
3. Universitas Negeri Surabaya (UNESA). Hari AIDS Sedunia 2022 . (2022). Available online at: https://www.unesa.ac.id/hari-aids-sedunia-2022-angka-penderita-tinggi-begini-catatan-dosen-unesa (accessed July 20, 2023).
4. Raphael I, Joern RR, Forsthuber TG. Memory CD4 + T cells in immunity and autoimmune diseases. Cells . (2020) 9:531. doi: 10.3390/cells9030531
PubMed Abstract | Crossref Full Text | Google Scholar
5. Oyovwevotu SO. Mathematical modelling for assessing the impact of intervention strategies on HIV/AIDS high risk group population dynamics. Heliyon . (2021) 7:e07991. doi: 10.1016/j.heliyon.2021.e07991
6. Li Y, Liu L. The Impact of Wolbachia on Dengue Transmission Dynamics in an SEI-SIS model . Nonlinear Analysis: Real World Applications. Elsevier BV. (2021). 103363 p.
7. Oke SI, Ojo MM, Adeniyi MO, Matadi MB. Mathematical modeling of malaria disease with control strategy. Commun Math Biol Neurosci . (2020) 2020:43. doi: 10.28919/cmbn/4513
Crossref Full Text | Google Scholar
8. Handari BD, Aldila D, Tamalia E, Khoshnaw SHA, Shahzad M. Assessing the impact of medical treatment and fumigation on the superinfection of malaria: a study of sensitivity analysis. Commun Biomath Sci . (2023) 6:51–73. doi: 10.5614/cbms.2023.6.1.5
9. Das K, Murthy B, Samad SA, Biswas MHA. Mathematical transmission analysis of SEIR tuberculosis disease model. Sens Int . (2021) 2:100120. doi: 10.1016/j.sintl.2021.100120
10. Aldila D, Latifa SL, Dumbela PA. Dynamical analysis of mathematical model for Bovine Tuberculosis among human and cattle population. Commun Biomath Sci . (2019) 2:55–64. doi: 10.5614/cbms.2019.2.1.6
11. Aldila D, Chavez JP, Wijaya KP, Ganegoda NC, Simorangkir GM, Tasman H, et al. A tuberculosis epidemic model as a proxy for the assessment of the novel M 72/ AS 01 E vaccine. Commun Nonlinear Sci Numer Simul . (2023) 20:107162. doi: 10.1016/j.cnsns.2023.107162
12. Maimunah, D A. Mathematical model for HIV spreads control program with art treatment. J Phys . (2018) 974:012035. doi: 10.1088/1742-6596/974/1/012035
13. Paul JN, Mbalawata IS, Mirau SS, Masandawa L. Mathematical modeling of vaccination as a control measure of stress to fight COVID-19 infections. Chaos Solit Fract . (2023) 166:30. doi: 10.1016/j.chaos.2022.112920
14. Balya MA, Dewi BO, Lestari FI, Ratu G, Rosuliyana H, Windyhani T, et al. Investigating the impact of social awareness and rapid test on A COVID-19 transmission model. Commun Biomath Sci . (2021) 4:46–64. doi: 10.5614/cbms.2021.4.1.5
15. Bentout S, Chekroun A, Kuniya T. Parameter estimation and prediction for coronavirus disease outbreak 2019 (COVID-19) in Algeria. AIMS Public Health . (2020) 7:306–18. doi: 10.3934/publichealth.2020026
16. Bentout S, Tridane A, Djilali S. Age-structured modeling of COVID-19 epidemic in the USA, UAE and Algeria. Alexandria Eng J . (2021) 60:401–11. doi: 10.1016/j.aej.2020.08.053
17. Aldila D, Nadya AF, Herdicho FF, Ndii MZ, Chukwu CW. Optimal control of pneumonia transmission model with seasonal factor: learning from Jakarta incidence data. Heliyon . (2023) 9:e18096. doi: 10.1016/j.heliyon.2023.e18096
18. Rahman SA, Vaidya NK, Zou X. Impact of early treatment programs on HIV epidemics: an immunity-based mathematical model. Math Biosci . (2016) 280:38–49. doi: 10.1016/j.mbs.2016.07.009
19. Mukandavire Z, Garira W, Tchuenche JM. Modelling effects of public health educational campaigns on HIV/AIDS transmission dynamics. Appl Math Model . (2009) 33:2084–95. doi: 10.1016/j.apm.2008.05.017
20. Nyabadza F, Mukandavire Z. Modelling HIV/AIDS in the presence of an HIV testing and screening campaign. J Theor Biol . (2011) 280:167–79. doi: 10.1016/j.jtbi.2011.04.021
21. Zhai X, Li W, Wei F, Mao X. Dynamics of an HIV/AIDS transmission model with protection awareness and fluctuations. Chaos Solit Fract . (2023) 169:113224. doi: 10.1016/j.chaos.2023.113224
22. Jamil S, Farman M, Akgul A. Qualitative and quantitative analysis of a fractal fractional HIV/AIDS model. Alexand Eng J . (2023) 76:167–77. doi: 10.1016/j.aej.2023.06.021
23. Xu C, Liu Z, Pang Y, Akgul A, Baleanu D. Dynamics of HIV-TB coinfection model using classical and Caputo piecewise operator: a dynamic approach with real data from South-East Asia, European and American regions. Chaos Solit Fract . (2022) 165:112879. doi: 10.1016/j.chaos.2022.112879
24. Pinto CMA, Carvalho ARM. New findings on the dynamics of HIV and TB coinfection models. Appl Math Comput . (2014) 242:36–46. doi: 10.1016/j.amc.2014.05.061
25. Ringa N, Diagne ML, Rwezaura H, Omame A, Tchoumi SY, Tchuenche JM, et al. and COVID-19 co-infection: a mathematical model and optimal control. Inf Med Unlocked . (2022) 31:100978. doi: 10.1016/j.imu.2022.100978
26. Omame A, Raezah AA, Diala UH, Onuoha C. The optimal strategies to be adopted in controlling the co-circulation of COVID-19, dengue and HIV: insight from a mathematical model. Axioms . (2023) 12:773. doi: 10.3390/axioms12080773
27. Garba S, Gumel A. Mathematical recipe for HIV elimination in Nigeria. J Niger Math Soc . (2010) 29:51–112.
28. Greenhalgh D, Hay G. Mathematical modelling of the spread of HIV/AIDS amongst injecting drug users. Math Med Biol . (1997) 14:11–38. doi: 10.1093/imammb/14.1.11
29. Gumel AB, McCluskey CC, Van Den Driessche P. Mathematical study of a staged-progression HIV model with imperfect vaccine. Bull Math Biol . (2006) 68:2105–28. doi: 10.1007/s11538-006-9095-7
30. Punyacharoensin N, Edmunds WJ, De Angelis D, White RG. Mathematical models for the study of HIV spread and control amongst men who have sex with men. Eur J Epidemiol . (2011) 26:695–709. doi: 10.1007/s10654-011-9614-1
31. Ofosuhene OA, Prince PO, Noor AI, Aline C. Analysing the impact of migration on HIV/AIDS cases using epidemiological modelling to guide policy makers. Infect Math Model . (2022) 7:252–61. doi: 10.1016/j.idm.2022.01.002
32. Fatmawati, Khan MA, Odinsyah HP. Fractional model of HIV transmission with awareness effect. Chaos Solit Fract . (2020) 138:109967. doi: 10.1016/j.chaos.2020.109967
33. Yusuf A, Mustapha UT, Sulaiman TA, Hincal E, Bayram M. Modeling the effect of horizontal and vertical transmissions of HIV infection with Caputo fractional derivative. Chaos Solit Fract . (2021) 145:110794. doi: 10.1016/j.chaos.2021.110794
34. Tabassum MF, Saeed M, Akgul A, Farman M, Chaudhry NA. Treatment of HIV/AIDS epidemic model with vertical transmission by using evolutionary Padé-approximation. Chaos Solit Fract . (2020) 2020:1099686. doi: 10.1016/j.chaos.2020.109686
35. Castillo-Chavez C, Feng Z, Huang W. On the computation of R 0 and its role on global stability. Math Approach Emerg Reemerg Infect Dis . (2002) 1:229–50. doi: 10.1007/978-1-4757-3667-0_13
36. Aldila D, Angelina M. Optimal control problem and backward bifurcation on malaria transmission with vector bias. Heliyon . (2021) 7:e06824. doi: 10.1016/j.heliyon.2021.e06824
37. Aldila D. Optimal control for dengue eradication program under the media awareness effect. Int J Nonlinear Sci Numer Simul . (2023) 24:95–122. doi: 10.1515/ijnsns-2020-0142
38. Aldila D, Puspadani CA, Rusin R. Mathematical analysis of the impact of community ignorance on the population dynamics of dengue. Front Appl Math Stat . (2023) 9:1094971. doi: 10.3389/fams.2023.1094971
39. Chitnis N, Hyman JM, Cushing JM. Determining important parameters in the spread of malaria through the sensitivity analysis of a mathematical model. Bull Math Biol . (2008) 70:1272. doi: 10.1007/s11538-008-9299-0
40. Diekmann O, Heesterbeek J, Roberts MG. The construction of next-generation matrices for compartmental epidemic models. J R Soc Interface . (2010) 7:873–85. doi: 10.1098/rsif.2009.0386
41. Van den Driessche P, Watmough J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci . (2002) 180:29–48. doi: 10.1016/S0025-5564(02)00108-6
42. The World Bank. Prevalence of HIV . (2023). Available online at: https://data.worldbank.org/indicator/SH.DYN.AIDS.ZS (accessed July 20, 2023).
43. The World Bank. Population Ages 15-64, Total . (2022). Available online at: https://data.worldbank.org/indicator/SP.POP.1564.TO (accessed January 13, 2024).
44. The World Bank. Life Expectancy at Birth, Total (Years) . (2022). Available online at: https://data.worldbank.org/indicator/SP.DYN.LE00.IN (accessed January 13, 2024).
45. Pinkerton SD, Abramson PR. Effectiveness of condoms in preventing HIV transmission. Soc Sci Med . (1997) 44:1303–12. doi: 10.1016/S0277-9536(96)00258-4
46. Puspita JW, Fakhruddin M, Fahlena H, Rohim F, Sutimin. On the reproduction ratio of dengue incidence in Semarang, Indonesia 2015-2018. Commun Biomath Sci . (2019) 2:118–26. doi: 10.5614/cbms.2019.2.2.5
47. Mahiane SG, Nguema EP, Pretorius C, Auvert B. Mathematical models for coinfection by two sexually transmitted agents: the human immunodeficiency virus and herpes simplex virus type 2 case. J R Stat Soc Ser C . (2010) 59:547–72. doi: 10.1111/j.1467-9876.2010.00719.x
48. Nwankwo A, Okuonghae D. Mathematical analysis of the transmission dynamics of HIV syphilis co-infection in the presence of treatment for syphilis. Bull Math Biol . (2018) 80:437–92. doi: 10.1007/s11538-017-0384-0
49. David JF, Lima VD, Zhu J, Brauer F. A co-interaction model of HIV and syphilis infection among gay, bisexual and other men who have sex with men. Infect Dis Model . (2020) 5:855–70. doi: 10.1016/j.idm.2020.10.008
50. Teklu SW, Terefe BB. COVID-19 and syphilis co-dynamic analysis using mathematical modeling approach. Front Appl Math Stat (2023) 8:1–13. doi: 10.3389/fams.2022.1101029
51. Wang C, Gao S, Li X, Martcheva M. Modeling syphilis and HIV coinfection: a case study in the USA. Bull Math Biol . (2023) 85:20–32. doi: 10.1007/s11538-023-01123-w
Keywords: HIV/AIDS, antiretroviral treatment, condom, global stability, sensitivity analysis, optimal control, cost-effectiveness
Citation: Aldila D, Dhanendra RP, Khoshnaw SHA, Wijayanti Puspita J, Kamalia PZ and Shahzad M (2024) Understanding HIV/AIDS dynamics: insights from CD4+T cells, antiretroviral treatment, and country-specific analysis. Front. Public Health 12:1324858. doi: 10.3389/fpubh.2024.1324858
Received: 20 October 2023; Accepted: 14 March 2024; Published: 11 April 2024.
Reviewed by:
Copyright © 2024 Aldila, Dhanendra, Khoshnaw, Wijayanti Puspita, Kamalia and Shahzad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dipo Aldila, aldiladipo@sci.ui.ac.id
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Case Report
- Open access
- Published: 05 April 2024
Missed opportunities for HIV testing and sexual health-related challenges in an individual with intellectual disability: a case report
- Lina Martina Würfel 1 , 2 ,
- Anja Potthoff 1 , 2 ,
- Sandeep Nambiar 1 &
- Adriane Skaletz-Rorowski 1 , 2
AIDS Research and Therapy volume 21 , Article number: 20 ( 2024 ) Cite this article
143 Accesses
1 Altmetric
Metrics details
HIV testing remains an important tool in identifying people living with HIV/AIDS (PLWHA). An early diagnosis of HIV can lead to a prolonged life expectancy if treatment is initiated promptly. Indicator conditions can be the first sign of an HIV infection and should therefore be recognised and consequently a HIV test should be carried out. Testing should occur in all individuals as sexuality can be experienced by everyone, and stigma can lead to the exclusion of vulnerable groups, leading to a gap in diagnosis and treatment [ 1 , 2 ].
Case presentation
A 63-year-old man, who identifies as bisexual and has had an intellectual disability since birth, presented at our health care centre for HIV testing. A decade ago, the patient was diagnosed with Stage III Diffuse Large B-cell Non-Hodgkin Lymphoma, an AIDS defining cancer. The patient presented at a Haematology and Oncology department 3 months prior, due to a weight loss of 10 kg over the past 5 months. Oral thrush, an HIV-indicator condition, had been diagnosed by the otolaryngologists shortly before. During this medical evaluation, pancytopenia was identified. Despite the presence of indicator conditions, the patient was never tested for HIV in the past. Staff members from the care facility for intellectually disabled suggested conducting a HIV test in our clinic through the public health department, where HIV positivity was revealed. The AIDS-defining diagnosis, along with a CD4 + cell count of 41/µl, suggests a prolonged period of HIV positivity.
Due to the presence of existing indicator conditions, an earlier HIV diagnosis was possible. We contend that most of the recent illnesses could have been prevented if earlier testing had been carried out. Therefore, patients presenting with AIDS indicator conditions, including those with mental disabilities, should be given the opportunity to be tested for HIV. HIV/AIDS trainings should be made available to health care professionals as well as to personnel interacting with vulnerable groups.
HIV testing continues to be a crucial method for identifying people living with HIV/AIDS (PLWHA) [ 3 ]. An early detection of HIV, followed by prompt initiation of treatment, can contribute to an extended life expectancy. Recognizing indicator conditions as potential early signs of HIV infection is essential, underscoring the importance of promptly conducting an HIV test. HIV-indicator conditions are those associated with or as a result of immunodeficiency and include AIDS-defining conditions [ 2 , 4 ]. Testing should be inclusive, as everyone, regardless of their sexual orientation and their mental capacity, can be vulnerable to HIV.
A 63-year-old bisexual man, with an intellectual disability since birth, presented at our center for HIV testing. Ten years prior, he had been diagnosed with Diffuse Large B-cell Non-Hodgkin Lymphoma in Stage III, an AIDS-defining cancer that requires an HIV Test [ 5 ]. Subsequently, he underwent therapy with Rituximab (8x) and CHOP (cyclophosphamide, doxorubicin hydrochloride (hydroxydaunomycin), vincristine sulfate (Oncovin) and prednisone) (6x). Three months before attending our center, he presented at a department of Hematology, Oncology, and Palliative Medicine for further investigation due to pancytopenia and a weight loss of 10 kg over the last 5 months (BMI: 17.6 kg/m2). At that time, the patient also reported experiencing heartburn. The patient denied having fever, chills, and night sweats. Additionally, there were recurrent middle ear infections, with the most recent one resulting in a perforated eardrum. Furthermore, there was an increase in episodes of panic attacks and the possibility of epilepsy was evaluated. The otolaryngologists had diagnosed oral thrush, a HIV-indicator condition, and the patient had already been receiving treatment with Amphotericin B suspension.
Amongst the diagnostics that were carried out to further investigate the symptoms the patient was presenting were, a CT scan, an esophagogastroduodenoscopy, and a bone marrow biopsy. The CT scan of the neck and thorax revealed a persistently stable lymphadenopathy, with some additional regression; a recurrence of Diffuse Large B-cell Non-Hodgkin Lymphoma could therefore be excluded. The CT scan showed an incidental finding of a hepatosplenomegaly and 4 small nodules up to 8 mm in the right lung. The esophagogastroduodenoscopy only revealed scars and transverse furrows of unclear etiology, with no evidence of a sustained fungal infection. Amphotericin B suspension was subsequently discontinued. A bone marrow biopsy was also performed but yielded no significant findings. The patient was subsequently released from the hospital. An HIV test was not carried out.
Following a training session on HIV, employees from the care facility for disabled individuals suggested carrying out an HIV test in our patient. Testing was carried out in our clinic in collaboration with the public health department, which led to the identification of HIV positivity.
The highest viral load was 73,763 copies/ml with a CD4 + helper cell count of 41/µl. The reduced CD4 + helper cell count, and the history of AIDS-defining and HIV-associated diseases imply that the diagnosis of HIV had been delayed for an extended period.
At the time of presentation at our medical center, there were no indications for other sexually transmitted infections.
Upon conversation with the patient, it emerged that he has resided in a residential facility for individuals with intellectual disabilities since 1996, with his legal caregiver being his brother. In terms of his sexual history, he was in a heterosexual relationship for eight years in the past. In 2011, the patient established a stable relationship with a homosexual man. Following this, he engaged in regular sexual encounters with different partners both inside and outside the facility. There is no record of drug use, and condom usage was infrequent.
Given the patient’s HIV-indicator conditions among the medical history such as oral thrush, pancytopenia, and wasting syndrome, as well as Diffuse Large B-cell Non-Hodgkin Lymphoma, an AIDS-defining cancer diagnosed in 2013, the question now arises as to when the HIV infection may have occurred and whether an earlier diagnosis would have been possible if HIV testing had been carried out, since indicator conditions were present.
Possible barriers hindering a timely diagnosis may have been: Stigma among physicians, which could entail erroneous assumptions regarding the sexuality of individuals with mental disabilities, failure to identify indicator conditions and test for HIV, lack of awareness among affected individuals with mental disabilities, resulting in a limited understanding of HIV and potential omission of crucial information during medical consultations, the failure to acknowledge sexuality and inadequate collection of sexual history, and insufficient inclusivity in HIV testing for all individuals.
The estimated median time for seroconversion to a CD4 + cell count below 200 cells/mm3 lies at 7,93 years [ 6 ], although not definitive, there is a strong likelihood that HIV would have been detected if HIV testing had been conducted at the onset of an indicator condition, a decade earlier. Furthermore, early HIV treatment initiation would likely have mitigated a significant part, if not all, of the more recent illness and probable HIV-related complications. This highlights the significance of HIV testing.
Assumptions and stereotyping may lead a physician to wrongly believe that a mentally disabled person is incapable of engaging in sexual relations [ 7 ].
Consequently, this stereotyping could undermine the patient’s diagnosis, impede treatment, and hinder the attainment of positive health outcomes [ 8 ]. It is crucial to diagnose HIV early in order to initiate treatment promptly, as PLWHA who start highly active antiretroviral therapy (HAART) at a later stage, with a lower CD4 + cell count, seem to exhibit a higher propensity for AIDS-related complications at advanced ages, in contrast to those who initiated treatment earlier [ 9 ].
Provider-initiated testing for indicator conditions may hold particular significance for individuals with intellectual disabilities, as it could hinder their comprehension of HIV, their ability to disclose risky behavior, and/or their capacity to seek testing independently.
On the other hand, individuals at risk of acquiring HIV and PLWHA frequently experience elevated rates of mental health issues in comparison to the general population. Therefore, it is of great importance to integrate diagnostic methods such as HIV tests among the routine checkups to reduce the impact of stigma. HIV Testing should be inclusive for everyone, regardless of the social status, disabilities, and living conditions, sexuality should be addressed openly and assumptions should be avoided [ 10 , 11 ].
Providing HIV/AIDS training through workshops and trainings for healthcare workers and related personnel who interact with vulnerable groups has proven to be highly significant, as demonstrated in this instance. If the center for disabled individuals had not proposed an HIV test for our patient, the diagnosis might not have been uncovered [ 12 ].
Additionally, sexuality needs to be acknowledged and addressed in individuals with disabilities, including those with learning disabilities, to provide education on safer sex practices and to facilitate HIV testing.
Overall, medical teams failed to recommend HIV testing in this patient multiple times, initially during the 2013 lymphoma diagnosis and at subsequent presentations with symptoms suggestive of AIDS. However, care facility staff deserve credit for recognizing the necessity of HIV testing after training and ensuring its arrangement.
Conclusions
Patients presenting with indicator conditions, including those with mental disabilities, should be tested for HIV to ensure an early diagnosis, and all patients should be asked about their sexuality as everyone can be vulnerable to HIV. Furthermore, more trainings should be made available to health care professionals and related personnel regarding sexual health.
Data availability
No datasets were generated or analysed during the current study.
Jordans CCE, Vasylyev M, Rae C, Jakobsen ML, Vassilenko A, Dauby N et al. National medical specialty guidelines of HIV indicator conditions in Europe lack adequate HIV testing recommendations: a systematic guideline review. Euro Surveill. 2022;27(48).
UN Targets [Available from. https://www.unaids.org/en/topics/2025_target_setting .
Vaz-Pinto I, Gorgulho A, Esteves C, Guimarães M, Castro V, Carrodeguas A, Medina D. Increasing HIV early diagnosis by implementing an automated screening strategy in emergency departments. HIV Med. 2022;23(11):1153–62.
Article PubMed PubMed Central Google Scholar
Bull L, Rayment M. HIV-indicator-condition-driven HIV testing: clinically effective but still rarely implemented. Clin Med (Lond). 2016;16(2):175–9.
Article PubMed Google Scholar
Lodi S, Phillips A, Touloumi G, Geskus R, Meyer L, Thiébaut R, et al. Time from human immunodeficiency virus seroconversion to reaching CD4 + cell count thresholds < 200, <350, and < 500 Cells/mm³: assessment of need following changes in treatment guidelines. Clin Infect Dis. 2011;53(8):817–25.
Article CAS PubMed Google Scholar
Deffew A, Coughlan B, Burke T, Rogers E. Staff member’s views and attitudes to supporting people with an intellectual disability: a multi-method investigation of intimate relationships and sexuality. J Appl Res Intellect Disabil. 2022;35(4):1049–58.
Nyblade L, Stockton MA, Giger K, Bond V, Ekstrand ML, Lean RM, et al. Stigma in health facilities: why it matters and how we can change it. BMC Med. 2019;17(1):25.
Wada N, Jacobson LP, Cohen M, French A, Phair J, Muñoz A. Cause-specific mortality among HIV-infected individuals, by CD4(+) cell count at HAART initiation, compared with HIV-uninfected individuals. Aids. 2014;28(2):257–65.
Remien RH, Stirratt MJ, Nguyen N, Robbins RN, Pala AN, Mellins CA. Mental health and HIV/AIDS: the need for an integrated response. Aids. 2019;33(9):1411–20.
Deblonde J, De Koker P, Hamers FF, Fontaine J, Luchters S, Temmerman M. Barriers to HIV testing in Europe: a systematic review. Eur J Public Health. 2010;20(4):422–32.
Mangurian C, Cournos F, Schillinger D, Vittinghoff E, Creasman JM, Lee B, et al. Low rates of HIV Testing among adults with severe Mental illness receiving care in Community Mental Health settings. Psychiatr Serv. 2017;68(5):443–8.
Download references
No external funding was obtained for the research and preparation of this manuscript. All aspects of this work were conducted using resources available within the authors’ affiliated institutions.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and affiliations.
WIR-Walk In Ruhr – Center for Sexual Health and Medicine, Große Beckstraße 12, 44787, Bochum, Germany
Lina Martina Würfel, Anja Potthoff, Sandeep Nambiar & Adriane Skaletz-Rorowski
Interdisciplinary Immunological Outpatient Clinic, Center for Sexual Health and Medicine, Department of Dermatology, Venereology and Allergology, Ruhr-Universität Bochum, Bochum, Germany
Lina Martina Würfel, Anja Potthoff & Adriane Skaletz-Rorowski
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization, A.P. and A.S.R.; writing—original draft preparation, L.M.W.; writing—review and editing, L.M.W., A.P., A.S.R., S.N.; supervision, A.P.; project administration, A.P.; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Correspondence to Adriane Skaletz-Rorowski .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Informed consent Statement
Written informed consent has been obtained from the legal caregiver of the patient.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Würfel, L.M., Potthoff, A., Nambiar, S. et al. Missed opportunities for HIV testing and sexual health-related challenges in an individual with intellectual disability: a case report. AIDS Res Ther 21 , 20 (2024). https://doi.org/10.1186/s12981-024-00606-7
Download citation
Received : 26 February 2024
Accepted : 19 March 2024
Published : 05 April 2024
DOI : https://doi.org/10.1186/s12981-024-00606-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Intellectual disability
- HIV testing
- Public health
AIDS Research and Therapy
ISSN: 1742-6405
- Submission enquiries: [email protected]
- What Are HIV and AIDS?
- How Is HIV Transmitted?
- Who Is at Risk for HIV?
- Symptoms of HIV
- U.S. Statistics
- Impact on Racial and Ethnic Minorities
- Global Statistics
- HIV and AIDS Timeline
- In Memoriam
- Supporting Someone Living with HIV
- Standing Up to Stigma
- Getting Involved
- HIV Treatment as Prevention
- Pre-exposure Prophylaxis (PrEP)
- Post-exposure Prophylaxis (PEP)
- Preventing Sexual Transmission of HIV
- Alcohol and HIV Risk
- Substance Use and HIV Risk
- Preventing Perinatal Transmission of HIV
- HIV Vaccines
- Long-acting HIV Prevention Tools
- Microbicides
- Who Should Get Tested?
- HIV Testing Locations
- HIV Testing Overview
- Understanding Your HIV Test Results
- Living with HIV
- Talking About Your HIV Status
- Locate an HIV Care Provider
- Types of Providers
- Take Charge of Your Care
- What to Expect at Your First HIV Care Visit
- Making Care Work for You
- Seeing Your Health Care Provider
- HIV Lab Tests and Results
- Returning to Care
- HIV Treatment Overview
- Viral Suppression and Undetectable Viral Load
- Taking Your HIV Medicine as Prescribed
- Tips on Taking Your HIV Medication Every Day
- Paying for HIV Care and Treatment
- Other Health Issues of Special Concern for People Living with HIV
- Alcohol and Drug Use
- Coronavirus (COVID-19) and People with HIV
- Hepatitis B & C
- Vaccines and People with HIV
- Flu and People with HIV
- Mental Health
- Mpox and People with HIV
- Opportunistic Infections
- Sexually Transmitted Infections
- Syphilis and People with HIV
- HIV and Women's Health Issues
- Aging with HIV
- Emergencies and Disasters and HIV
- Employment and Health
- Exercise and Physical Activity
- Food Safety and Nutrition
- Housing and Health
- Traveling Outside the U.S.
- Civil Rights
- Workplace Rights
- Limits on Confidentiality
- National HIV/AIDS Strategy (2022-2025)
- Implementing the National HIV/AIDS Strategy
- Prior National HIV/AIDS Strategies (2010-2021)
- Key Strategies
- Priority Jurisdictions
- HHS Agencies Involved
- Learn More About EHE
- Ready, Set, PrEP
- Ready, Set, PrEP Pharmacies
- Ready, Set, PrEP Resources
- AHEAD: America’s HIV Epidemic Analysis Dashboard
- HIV Prevention Activities
- HIV Testing Activities
- HIV Care and Treatment Activities
- HIV Research Activities
- Activities Combating HIV Stigma and Discrimination
- The Affordable Care Act and HIV/AIDS
- HIV Care Continuum
- Syringe Services Programs
- Finding Federal Funding for HIV Programs
- Fund Activities
- The Fund in Action
- About PACHA
- Members & Staff
- Subcommittees
- Prior PACHA Meetings and Recommendations
- I Am a Work of Art Campaign
- Awareness Campaigns
- Global HIV/AIDS Overview
- U.S. Government Global HIV/AIDS Activities
- U.S. Government Global-Domestic Bidirectional HIV Work
- Global HIV/AIDS Organizations
- National Black HIV/AIDS Awareness Day February 7
- HIV Is Not A Crime Awareness Day February 28
- National Women and Girls HIV/AIDS Awareness Day March 10
- National Native HIV/AIDS Awareness Day March 20
- National Youth HIV & AIDS Awareness Day April 10
- HIV Vaccine Awareness Day May 18
- National Asian & Pacific Islander HIV/AIDS Awareness Day May 19
- HIV Long-Term Survivors Awareness Day June 5
- National HIV Testing Day June 27
- Zero HIV Stigma July 21
- Southern HIV/AIDS Awareness Day August 20
- National Faith HIV/AIDS Awareness Day August 27
- National African Immigrants and Refugee HIV/AIDS and Hepatitis Awareness Day September 9
- National HIV/AIDS and Aging Awareness Day September 18
- National Gay Men's HIV/AIDS Awareness Day September 27
- National Latinx AIDS Awareness Day October 15
- World AIDS Day December 1
- Event Planning Guide
- U.S. Conference on HIV/AIDS (USCHA)
- National Ryan White Conference on HIV Care & Treatment
- AIDS 2020 (23rd International AIDS Conference Virtual)
Want to stay abreast of changes in prevention, care, treatment or research or other public health arenas that affect our collective response to the HIV epidemic? Or are you new to this field?
HIV.gov curates learning opportunities for you, and the people you serve and collaborate with.
Stay up to date with the webinars, Twitter chats, conferences and more in this section.
PACHA Members Discuss HIV Research Highlights from CROI 2024 By HIV.gov
- Share on Facebook
- Share on Twitter
- Share on LinkedIn
- Share on Email
Each year, the Conference on Retroviruses and Opportunistic Infections (CROI) features an array of exciting new developments in HIV research that can help support the health and well-being of people across the globe. Before the start of the 80 th full council meeting of the Presidential Advisory Council on HIV/AIDS (PACHA) in Houston, TX, HIV.gov had the opportunity to speak with PACHA members Patrick Sullivan, DrPH, MPH, Professor of Epidemiology at Emory University’s Rollins School of Public Health, and Jeff Taylor, Executive Director of the HIV and Aging Research Project, who both attended CROI 2024, about what they presented and what stood out to them at this year’s conference. Watch their conversation Exit Disclaimer :
Mentorship and HIV and Aging Research
Mr. Taylor noted that this year was the first time a formal mentorship program for advocates was launched at the conference, with seasoned mentors paired with new Community Educator Scholars to support and engage them to ensure they got the most out of CROI. He also reflected on the range of research presented at CROI related to HIV and aging. He noted that there continue to be findings presented via abstracts and presentations from the NIH-supported REPRIEVE trial , a global study that demonstrated that a statin, a cholesterol-lowering medication, may offset the high risk of cardiovascular disease in people with HIV by more than a third, potentially preventing one in five major cardiovascular events (e.g., heart attacks, strokes, or surgery to open a blocked artery) or premature deaths in this population. As he noted, new clinical guidelines were recently published based on those findings, helping clinicians better support the health of those ages 40-75. ( View HIV.gov’s CROI 2024 conversation with Dr. Carl Dieffenbach about new REPRIEVE trial findings .)
The Further Promise of PrEP
Dr. Sullivan discussed new evidence from a study he and his colleagues at Emory University conducted Exit Disclaimer showing that, over the past decade, U.S. states with high PrEP coverage among those who need it experienced steeper declines in new HIV diagnoses rates than states with low PrEP coverage. Their analysis showed that from 2012 to 2021, states with the lowest levels of PrEP coverage saw an annual increase in new HIV diagnoses, while all other states saw an annual decrease in HIV diagnoses, with the largest decreases among states with the highest levels of PrEP coverage. In other words, he emphasized, while we’ve known for decades that PrEP works to prevent HIV at the individual level, we now know that when we remove barriers to PrEP access and take PrEP to scale, we can see an impact on the population level as well. He further noted that other studies presented at CROI 2024 about all stages in the PrEP cascade—awareness, access, uptake, and adherence—show that we have the tools to get us to that high level of PrEP coverage and better knowledge of how to deploy them.
Catch Up on Other CROI HIV Research Updates
Mr. Taylor also shared an important observation about the opening session with the HIV.gov team as we were working on this blog. He noted, “There was ongoing discussion at CROI regarding stigma as an obstacle to ending the epidemic. Ugandan activist Frank Mugisha, Sexual Minorities Uganda (SMUG), highlighted the chilling impact of new laws criminalizing LGBTQ individuals during the conference's opening plenary. He added that these laws hinder access to HIV care and threaten progress against HIV and that discriminatory policies can cripple the fight against HIV.”
HIV.gov has shared other interviews from CROI 2024 with federal HIV leaders, participating researchers, and community members. You can find all of them on HIV.gov’s social media channels and with recaps here on the blog available by using the CROI topic tag .
More than 3,600 HIV and infectious disease researchers from 73 countries gathered in Denver and virtually from March 3-6 this year for CROI, an annual scientific meeting on the latest research that can help accelerate global progress in the response to HIV and other infectious diseases, including STIs and viral hepatitis. Over 1,000 summaries of original research were presented. Visit the conference website Exit Disclaimer for more information. Session webcasts and more information will be published there for public access in 30 days.
Related HIV.gov Blogs
- Aging Aging and HIV
- CROI Conference on Retroviruses & Opportunistic Infections
- PACHA Presidential Advisory Council on HIV/AIDS
- PrEP Pre-Exposure Prophylaxis
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 24 November 2021
A study of awareness on HIV/AIDS among adolescents: A Longitudinal Study on UDAYA data
- Shobhit Srivastava ORCID: orcid.org/0000-0002-7138-4916 1 ,
- Shekhar Chauhan ORCID: orcid.org/0000-0002-6926-7649 2 ,
- Ratna Patel ORCID: orcid.org/0000-0002-5371-7369 3 &
- Pradeep Kumar ORCID: orcid.org/0000-0003-4259-820X 1
Scientific Reports volume 11 , Article number: 22841 ( 2021 ) Cite this article
9891 Accesses
6 Citations
11 Altmetric
Metrics details
- Health care
- Health services
- Public health
Acquired Immunodeficiency Syndrome caused by Human Immunodeficiency Virus (HIV) poses a severe challenge to healthcare and is a significant public health issue worldwide. This study intends to examine the change in the awareness level of HIV among adolescents. Furthermore, this study examined the factors associated with the change in awareness level on HIV-related information among adolescents over the period. Data used for this study were drawn from Understanding the lives of adolescents and young adults, a longitudinal survey on adolescents aged 10–19 in Bihar and Uttar Pradesh. The present study utilized a sample of 4421 and 7587 unmarried adolescent boys and girls, respectively aged 10–19 years in wave-1 and wave-2. Descriptive analysis and t-test and proportion test were done to observe changes in certain selected variables from wave-1 (2015–2016) to wave-2 (2018–2019). Moreover, random effect regression analysis was used to estimate the association of change in HIV awareness among unmarried adolescents with household and individual factors. The percentage of adolescent boys who had awareness regarding HIV increased from 38.6% in wave-1 to 59.9% in wave-2. Among adolescent girls, the percentage increased from 30.2 to 39.1% between wave-1 & wave-2. With the increase in age and years of schooling, the HIV awareness increased among adolescent boys ([Coef: 0.05; p < 0.01] and [Coef: 0.04; p < 0.01]) and girls ([Coef: 0.03; p < 0.01] and [Coef: 0.04; p < 0.01]), respectively. The adolescent boys [Coef: 0.06; p < 0.05] and girls [Coef: 0.03; p < 0.05] who had any mass media exposure were more likely to have an awareness of HIV. Adolescent boys' paid work status was inversely associated with HIV awareness [Coef: − 0.01; p < 0.10]. Use of internet among adolescent boys [Coef: 0.18; p < 0.01] and girls [Coef: 0.14; p < 0.01] was positively associated with HIV awareness with reference to their counterparts. There is a need to intensify efforts in ensuring that information regarding HIV should reach vulnerable sub-groups, as outlined in this study. It is important to mobilize the available resources to target the less educated and poor adolescents, focusing on rural adolescents.
Similar content being viewed by others

Predictors of never testing for HIV among sexually active individuals aged 15–56 years in Rwanda
Hosee Niyompano, Emmanuel Biracyaza, … Aline Umubyeyi
Awareness and use of HIV pre-exposure prophylaxis and factors associated with awareness among MSM in Beijing, China
Yanming Sun, Hongyan Lu, … Guiying Li

Identification of adolescent girls and young women for targeted HIV prevention: a new risk scoring tool in KwaZulu Natal, South Africa
Sarah Gabrielle Ayton, Martina Pavlicova & Quarraisha Abdool Karim
Introduction
Acquired Immunodeficiency Syndrome (AIDS) caused by Human Immunodeficiency Virus (HIV) poses a severe challenge to healthcare and is a significant public health issue worldwide. So far, HIV has claimed almost 33 million lives; however, off lately, increasing access to HIV prevention, diagnosis, treatment, and care has enabled people living with HIV to lead a long and healthy life 1 . By the end of 2019, an estimated 38 million people were living with HIV 1 . More so, new infections fell by 39 percent, and HIV-related deaths fell by almost 51 percent between 2000 and 2019 1 . Despite all the positive news related to HIV, the success story is not the same everywhere; HIV varies between region, country, and population, where not everyone is able to access HIV testing and treatment and care 1 . HIV/AIDS holds back economic growth by destroying human capital by predominantly affecting adolescents and young adults 2 .
There are nearly 1.2 billion adolescents (10–19 years) worldwide, which constitute 18 percent of the world’s population, and in some countries, adolescents make up as much as one-fourth of the population 3 . In India, adolescents comprise more than one-fifth (21.8%) of the total population 4 . Despite a decline projection for the adolescent population in India 5 , there is a critical need to hold adolescents as adolescence is characterized as a period when peer victimization/pressure on psychosocial development is noteworthy 6 . Peer victimization/pressure is further linked to risky sexual behaviours among adolescents 7 , 8 . A higher proportion of low literacy in the Indian population leads to a low level of awareness of HIV/AIDS 9 . Furthermore, the awareness of HIV among adolescents is quite alarming 10 , 11 , 12 .
Unfortunately, there is a shortage of evidence on what predicts awareness of HIV among adolescents. Almost all the research in India is based on beliefs, attitudes, and awareness of HIV among adolescents 2 , 12 . However, few other studies worldwide have examined mass media as a strong predictor of HIV awareness among adolescents 13 . Mass media is an effective channel to increase an individuals’ knowledge about sexual health and improve understanding of facilities related to HIV prevention 14 , 15 . Various studies have outlined other factors associated with the increasing awareness of HIV among adolescents, including; age 16 , 17 , 18 , occupation 18 , education 16 , 17 , 18 , 19 , sex 16 , place of residence 16 , marital status 16 , and household wealth index 16 .
Several community-based studies have examined awareness of HIV among Indian adolescents 2 , 10 , 12 , 20 , 21 , 22 . However, studies investigating awareness of HIV among adolescents in a larger sample size remained elusive to date, courtesy of the unavailability of relevant data. Furthermore, no study in India had ever examined awareness of HIV among adolescents utilizing information on longitudinal data. To the author’s best knowledge, this is the first study in the Indian context with a large sample size that examines awareness of HIV among adolescents and combines information from a longitudinal survey. Therefore, this study intends to examine the change in the awareness level of HIV among adolescents. Furthermore, this study examined the factors associated with a change in awareness level on HIV-related information among adolescents over the period.
Data and methods
Data used for this study were drawn from Understanding the lives of adolescents and young adults (UDAYA), a longitudinal survey on adolescents aged 10–19 in Bihar and Uttar Pradesh 23 . The first wave was conducted in 2015–2016, and the follow-up survey was conducted after three years in 2018–2019 23 . The survey provides the estimates for state and the sample of unmarried boys and girls aged 10–19 and married girls aged 15–19. The study adopted a systematic, multi-stage stratified sampling design to draw sample areas independently for rural and urban areas. 150 primary sampling units (PSUs)—villages in rural areas and census wards in urban areas—were selected in each state, using the 2011 census list of villages and wards as the sampling frame. In each primary sampling unit (PSU), households to be interviewed were selected by systematic sampling. More details about the study design and sampling procedure have been published elsewhere 23 . Written consent was obtained from the respondents in both waves. In wave 1 (2015–2016), 20,594 adolescents were interviewed using the structured questionnaire with a response rate of 92%.
Moreover, in wave 2 (2018–2019), the study interviewed the participants who were successfully interviewed in 2015–2016 and who consented to be re-interviewed 23 . Of the 20,594 eligible for the re-interview, the survey re-interviewed 4567 boys and 12,251 girls (married and unmarried). After excluding the respondents who gave an inconsistent response to age and education at the follow-up survey (3%), the final follow-up sample covered 4428 boys and 11,864 girls with the follow-up rate of 74% for boys and 81% for girls. The effective sample size for the present study was 4421 unmarried adolescent boys aged 10–19 years in wave-1 and wave-2. Additionally, 7587 unmarried adolescent girls aged 10–19 years were interviewed in wave-1 and wave-2 23 . The cases whose follow-up was lost were excluded from the sample to strongly balance the dataset and set it for longitudinal analysis using xtset command in STATA 15. The survey questionnaire is available at https://dataverse.harvard.edu/file.xhtml?fileId=4163718&version=2.0 & https://dataverse.harvard.edu/file.xhtml?fileId=4163720&version=2.0 .
Outcome variable
HIV awareness was the outcome variable for this study, which is dichotomous. The question was asked to the adolescents ‘Have you heard of HIV/AIDS?’ The response was recorded as yes and no.
Exposure variables
The predictors for this study were selected based on previous literature. These were age (10–19 years at wave 1, continuous variable), schooling (continuous), any mass media exposure (no and yes), paid work in the last 12 months (no and yes), internet use (no and yes), wealth index (poorest, poorer, middle, richer, and richest), religion (Hindu and Non-Hindu), caste (Scheduled Caste/Scheduled Tribe, Other Backward Class, and others), place of residence (urban and rural), and states (Uttar Pradesh and Bihar).
Exposure to mass media (how often they read newspapers, listened to the radio, and watched television; responses on the frequencies were: almost every day, at least once a week, at least once a month, rarely or not at all; adolescents were considered to have any exposure to mass media if they had exposure to any of these sources and as having no exposure if they responded with ‘not at all’ for all three sources of media) 24 . Household wealth index based on ownership of selected durable goods and amenities with possible scores ranging from 0 to 57; households were then divided into quintiles, with the first quintile representing households of the poorest wealth status and the fifth quintile representing households with the wealthiest status 25 .
Statistical analysis
Descriptive analysis was done to observe the characteristics of unmarried adolescent boys and girls at wave-1 (2015–2016). In addition, the changes in certain selected variables were observed from wave-1 (2015–2016) to wave-2 (2018–2019), and the significance was tested using t-test and proportion test 26 , 27 . Moreover, random effect regression analysis 28 , 29 was used to estimate the association of change in HIV awareness among unmarried adolescents with household factors and individual factors. The random effect model has a specific benefit for the present paper's analysis: its ability to estimate the effect of any variable that does not vary within clusters, which holds for household variables, e.g., wealth status, which is assumed to be constant for wave-1 and wave-2 30 .
Table 1 represents the socio-economic profile of adolescent boys and girls. The estimates are from the baseline dataset, and it was assumed that none of the household characteristics changed over time among adolescent boys and girls.
Figure 1 represents the change in HIV awareness among adolescent boys and girls. The percentage of adolescent boys who had awareness regarding HIV increased from 38.6% in wave-1 to 59.9% in wave-2. Among adolescent girls, the percentage increased from 30.2% in wave-1 to 39.1% in wave-2.
The percenate of HIV awareness among adolescent boys and girls, wave-1 (2015–2016) and wave-2 (2018–2019).
Table 2 represents the summary statistics for explanatory variables used in the analysis of UDAYA wave-1 and wave-2. The exposure to mass media is almost universal for adolescent boys, while for adolescent girls, it increases to 93% in wave-2 from 89.8% in wave-1. About 35.3% of adolescent boys were engaged in paid work during wave-1, whereas in wave-II, the share dropped to 33.5%, while in the case of adolescent girls, the estimates are almost unchanged. In wave-1, about 27.8% of adolescent boys were using the internet, while in wave-2, there is a steep increase of nearly 46.2%. Similarly, in adolescent girls, the use of the internet increased from 7.6% in wave-1 to 39.3% in wave-2.
Table 3 represents the estimates from random effects for awareness of HIV among adolescent boys and girls. It was found that with the increases in age and years of schooling the HIV awareness increased among adolescent boys ([Coef: 0.05; p < 0.01] and [Coef: 0.04; p < 0.01]) and girls ([Coef: 0.03; p < 0.01] and [Coef: 0.04; p < 0.01]), respectively. The adolescent boys [Coef: 0.06; p < 0.05] and girls [Coef: 0.03; p < 0.05] who had any mass media exposure were more likely to have an awareness of HIV in comparison to those who had no exposure to mass media. Adolescent boys' paid work status was inversely associated with HIV awareness about adolescent boys who did not do paid work [Coef: − 0.01; p < 0.10]. Use of the internet among adolescent boys [Coef: 0.18; p < 0.01] and girls [Coef: 0.14; p < 0.01] was positively associated with HIV awareness in reference to their counterparts.
The awareness regarding HIV increases with the increase in household wealth index among both adolescent boys and girls. The adolescent girls from the non-Hindu household had a lower likelihood to be aware of HIV in reference to adolescent girls from Hindu households [Coef: − 0.09; p < 0.01]. Adolescent girls from non-SC/ST households had a higher likelihood of being aware of HIV in reference to adolescent girls from other caste households [Coef: 0.04; p < 0.01]. Adolescent boys [Coef: − 0.03; p < 0.01] and girls [Coef: − 0.09; p < 0.01] from a rural place of residence had a lower likelihood to be aware about HIV in reference to those from the urban place of residence. Adolescent boys [Coef: 0.04; p < 0.01] and girls [Coef: 0.02; p < 0.01] from Bihar had a higher likelihood to be aware about HIV in reference to those from Uttar Pradesh.
This is the first study of its kind to address awareness of HIV among adolescents utilizing longitudinal data in two indian states. Our study demonstrated that the awareness of HIV has increased over the period; however, it was more prominent among adolescent boys than in adolescent girls. Overall, the knowledge on HIV was relatively low, even during wave-II. Almost three-fifths (59.9%) of the boys and two-fifths (39.1%) of the girls were aware of HIV. The prevalence of awareness on HIV among adolescents in this study was lower than almost all of the community-based studies conducted in India 10 , 11 , 22 . A study conducted in slums in Delhi has found almost similar prevalence (40% compared to 39.1% during wave-II in this study) of awareness of HIV among adolescent girls 31 . The difference in prevalence could be attributed to the difference in methodology, study population, and study area.
The study found that the awareness of HIV among adolescent boys has increased from 38.6 percent in wave-I to 59.9 percent in wave-II; similarly, only 30.2 percent of the girls had an awareness of HIV during wave-I, which had increased to 39.1 percent. Several previous studies corroborated the finding and noticed a higher prevalence of awareness on HIV among adolescent boys than in adolescent girls 16 , 32 , 33 , 34 . However, a study conducted in a different setting noticed a higher awareness among girls than in boys 35 . Also, a study in the Indian context failed to notice any statistical differences in HIV knowledge between boys and girls 18 . Gender seems to be one of the significant determinants of comprehensive knowledge of HIV among adolescents. There is a wide gap in educational attainment among male and female adolescents, which could be attributed to lower awareness of HIV among girls in this study. Higher peer victimization among adolescent boys could be another reason for higher awareness of HIV among them 36 . Also, cultural double standards placed on males and females that encourage males to discuss HIV/AIDS and related sexual matters more openly and discourage or even restrict females from discussing sexual-related issues could be another pertinent factor of higher awareness among male adolescents 33 . Behavioural interventions among girls could be an effective way to improving knowledge HIV related information, as seen in previous study 37 . Furthermore, strengthening school-community accountability for girls' education would augment school retention among girls and deliver HIV awareness to girls 38 .
Similar to other studies 2 , 10 , 17 , 18 , 39 , 40 , 41 , age was another significant determinant observed in this study. Increasing age could be attributed to higher education which could explain better awareness with increasing age. As in other studies 18 , 39 , 41 , 42 , 43 , 44 , 45 , 46 , education was noted as a significant driver of awareness of HIV among adolescents in this study. Higher education might be associated with increased probability of mass media and internet exposure leading to higher awareness of HIV among adolescents. A study noted that school is one of the important factors in raising the awareness of HIV among adolescents, which could be linked to higher awareness among those with higher education 47 , 48 . Also, schooling provides adolescents an opportunity to improve their social capital, leading to increased awareness of HIV.
Following previous studies 18 , 40 , 46 , the current study also outlines a higher awareness among urban adolescents than their rural counterparts. One plausible reason for lower awareness among adolescents in rural areas could be limited access to HIV prevention information 16 . Moreover, rural–urban differences in awareness of HIV could also be due to differences in schooling, exposure to mass media, and wealth 44 , 45 . The household's wealth status was also noted as a significant predictor of awareness of HIV among adolescents. Corroborating with previous findings 16 , 33 , 42 , 49 , this study reported a higher awareness among adolescents from richer households than their counterparts from poor households. This could be because wealthier families can afford mass-media items like televisions and radios for their children, which, in turn, improves awareness of HIV among adolescents 33 .
Exposure to mass media and internet access were also significant predictors of higher awareness of HIV among adolescents. This finding agrees with several previous research, and almost all the research found a positive relationship between mass-media exposure and awareness of HIV among adolescents 10 . Mass media addresses such topics more openly and in a way that could attract adolescents’ attention is the plausible reason for higher awareness of HIV among those having access to mass media and the internet 33 . Improving mass media and internet usage, specifically among rural and uneducated masses, would bring required changes. Integrating sexual education into school curricula would be an important means of imparting awareness on HIV among adolescents; however, this is debatable as to which standard to include the required sexual education in the Indian schooling system. Glick (2009) thinks that the syllabus on sexual education might be included during secondary schooling 44 . Another study in the Indian context confirms the need for sex education for adolescents 50 , 51 .
Limitations and strengths of the study
The study has several limitations. At first, the awareness of HIV was measured with one question only. Given that no study has examined awareness of HIV among adolescents using longitudinal data, this limitation is not a concern. Second, the study findings cannot be generalized to the whole Indian population as the study was conducted in only two states of India. However, the two states selected in this study (Uttar Pradesh and Bihar) constitute almost one-fourth of India’s total population. Thirdly, the estimates were provided separately for boys and girls and could not be presented combined. However, the data is designed to provide estimates separately for girls and boys. The data had information on unmarried boys and girls and married girls; however, data did not collect information on married boys. Fourthly, the study estimates might have been affected by the recall bias. Since HIV is a sensitive topic, the possibility of respondents modifying their responses could not be ruled out. Hawthorne effect, respondents, modifying aspect of their behaviour in response, has a role to play in HIV related study 52 . Despite several limitations, the study has specific strengths too. This is the first study examining awareness of HIV among adolescent boys and girls utilizing longitudinal data. The study was conducted with a large sample size as several previous studies were conducted in a community setting with a minimal sample size 10 , 12 , 18 , 20 , 53 .
The study noted a higher awareness among adolescent boys than in adolescent girls. Specific predictors of high awareness were also noted in the study, including; higher age, higher education, exposure to mass media, internet use, household wealth, and urban residence. Based on the study findings, this study has specific suggestions to improve awareness of HIV among adolescents. There is a need to intensify efforts in ensuring that information regarding HIV should reach vulnerable sub-groups as outlined in this study. It is important to mobilize the available resources to target the less educated and poor adolescents, focusing on rural adolescents. Investment in education will help, but it would be a long-term solution; therefore, public information campaigns could be more useful in the short term.
WHO. HIV/AIDS . https://www.who.int/news-room/fact-sheets/detail/hiv-aids (2020).
Singh, A. & Jain, S. Awareness of HIV/AIDS among school adolescents in Banaskantha district of Gujarat. Health Popul.: Perspect. Issues 32 , 59–65 (2009).
Google Scholar
WHO & UNICEF. adolescents Health: The Missing Population in Universal Health Coverage . 1–31 https://www.google.com/search?q=adolescents+population+WHO+report&rlz=1C1CHBF_enIN904IN904&oq=adolescents+population+WHO+report&aqs=chrome..69i57.7888j1j7&sourceid=chrome&ie=UTF-8 (2018).
Chauhan, S. & Arokiasamy, P. India’s demographic dividend: state-wise perspective. J. Soc. Econ. Dev. 20 , 1–23 (2018).
Tiwari, A. K., Singh, B. P. & Patel, V. Population projection of India: an application of dynamic demographic projection model. JCR 7 , 547–555 (2020).
Troop-Gordon, W. Peer victimization in adolescence: the nature, progression, and consequences of being bullied within a developmental context. J. Adolesc. 55 , 116–128 (2017).
PubMed Google Scholar
Dermody, S. S., Friedman, M., Chisolm, D. J., Burton, C. M. & Marshal, M. P. Elevated risky sexual behaviors among sexual minority girls: indirect risk pathways through peer victimization and heavy drinking. J. Interpers. Violence 35 , 2236–2253 (2020).
Lee, R. L. T., Loke, A. Y., Hung, T. T. M. & Sobel, H. A systematic review on identifying risk factors associated with early sexual debut and coerced sex among adolescents and young people in communities. J. Clin. Nurs. 27 , 478–501 (2018).
Gurram, S. & Bollampalli, B. A study on awareness of human immunodeficiency virus among adolescent girls in urban and rural field practice areas of Osmania Medical College, Hyderabad, Telangana, India. (2020).
Jain, R., Anand, P., Dhyani, A. & Bansal, D. Knowledge and awareness regarding menstruation and HIV/AIDS among schoolgoing adolescent girls. J. Fam. Med. Prim Care 6 , 47–51 (2017).
Kawale, S., Sharma, V., Thaware, P. & Mohankar, A. A study to assess awareness about HIV/AIDS among rural population of central India. Int. J. Commun. Med. Public Health 5 , 373–376 (2017).
Lal, P., Nath, A., Badhan, S. & Ingle, G. K. A study of awareness about HIV/AIDS among senior secondary school Children of Delhi. Indian J. Commun. Med. 33 , 190–192 (2008).
CAS Google Scholar
Bago, J.-L. & Lompo, M. L. Exploring the linkage between exposure to mass media and HIV awareness among adolescents in Uganda. Sex. Reprod. Healthcare 21 , 1–8 (2019).
McCombie, S., Hornik, R. C. & Anarfi, J. K. Effects of a mass media campaign to prevent AIDS among young people in Ghana . Public Health Commun. 163–178 (Routledge, 2002). https://doi.org/10.4324/9781410603029-17 .
Sano, Y. et al. Exploring the linkage between exposure to mass media and HIV testing among married women and men in Ghana. AIDS Care 28 , 684–688 (2016).
Oginni, A. B., Adebajo, S. B. & Ahonsi, B. A. Trends and determinants of comprehensive knowledge of HIV among adolescents and young adults in Nigeria: 2003–2013. Afr. J. Reprod. Health 21 , 26–34 (2017).
Rahman, M. M., Kabir, M. & Shahidullah, M. Adolescent knowledge and awareness about AIDS/HIV and factors affecting them in Bangladesh. J. Ayub. Med. Coll. Abbottabad 21 , 3–6 (2009).
Yadav, S. B., Makwana, N. R., Vadera, B. N., Dhaduk, K. M. & Gandha, K. M. Awareness of HIV/AIDS among rural youth in India: a community based cross-sectional study. J. Infect. Dev. Countries 5 , 711–716 (2011).
Alhasawi, A. et al. Assessing HIV/AIDS knowledge, awareness, and attitudes among senior high school students in Kuwait. MPP 28 , 470–476 (2019).
Chakrovarty, A. et al. A study of awareness on HIV/AIDS among higher secondary school students in Central Kolkata. Indian J. Commun. Med. 32 , 228 (2007).
Katoch, D. K. S. & Kumar, A. HIV/AIDS awareness among students of tribal and non-tribal area of Himachal Pradesh. J. Educ. 5 , 1–9 (2017).
Shinde, M., Trivedi, A., Shinde, A. & Mishra, S. A study of awareness regarding HIV/AIDS among secondary school students. Int. J. Commun. Med. Public Health https://doi.org/10.18203/2394-6040.ijcmph20161611 (2016).
Article Google Scholar
Council, P. UDAYA, adolescent survey, Bihar and Uttar Pradesh, 2015–16. Harvard Dataverse https://doi.org/10.7910/DVN/RRXQNT (2018).
Kumar, P. & Dhillon, P. Household- and community-level determinants of low-risk Caesarean deliveries among women in India. J. Biosoc. Sci. 53 , 55–70 (2021).
Patel, S. K., Santhya, K. G. & Haberland, N. What shapes gender attitudes among adolescent girls and boys? Evidence from the UDAYA Longitudinal Study in India. PLoS ONE 16 , e0248766 (2021).
CAS PubMed PubMed Central Google Scholar
Fan, C., Wang, L. & Wei, L. Comparing two tests for two rates. Am. Stat. 71 , 275–281 (2017).
MathSciNet Google Scholar
Kim, T. K. T test as a parametric statistic. Korean J. Anesthesiol. 68 , 540–546 (2015).
PubMed PubMed Central Google Scholar
Bell, A., Fairbrother, M. & Jones, K. Fixed and random effects models: making an informed choice. Qual. Quant. 53 , 1051–1074 (2019).
Jarrett, R., Farewell, V. & Herzberg, A. Random effects models for complex designs. Stat. Methods Med. Res. 29 , 3695–3706 (2020).
MathSciNet CAS PubMed PubMed Central Google Scholar
Neuhaus, J. M. & Kalbfleisch, J. D. Between- and within-cluster covariate effects in the analysis of clustered data. Biometrics 54 , 638–645 (1998).
CAS PubMed MATH Google Scholar
Kaur, G. Factors influencing HIV awareness amongst adolescent women: a study of slums in Delhi. Demogr. India 47 , 100–111 (2018).
Alene, G. D., Wheeler, J. G. & Grosskurth, H. Adolescent reproductive health and awareness of HIV among rural high school students, North Western Ethiopia. AIDS Care 16 , 57–68 (2004).
CAS PubMed Google Scholar
Oljira, L., Berhane, Y. & Worku, A. Assessment of comprehensive HIV/AIDS knowledge level among in-school adolescents in eastern Ethiopia. J. Int. AIDS Soc. 16 , 17349 (2013).
Samkange-Zeeb, F. N., Spallek, L. & Zeeb, H. Awareness and knowledge of sexually transmitted diseases (STDs) among school-going adolescents in Europe: a systematic review of published literature. BMC Public Health 11 , 727 (2011).
Laguna, E. P. Knowledge of HIV/AIDS and unsafe sex practices among Filipino youth. in 15 (PAA, 2004).
Teitelman, A. M. et al. Partner violence, power, and gender differences in South African adolescents’ HIV/sexually transmitted infections risk behaviors. Health Psychol. 35 , 751–760 (2016).
Oberth, G. et al. Effectiveness of the Sista2Sista programme in improving HIV and other sexual and reproductive health outcomes among vulnerable adolescent girls and young women in Zimbabwe. Afr. J. AIDS Res. 20 , 158–164 (2021).
DeSoto, J. et al. Using school-based early warning systems as a social and behavioral approach for HIV prevention among adolescent girls. Preventing HIV Among Young People in Southern and Eastern Africa 280 (2020).
Ochako, R., Ulwodi, D., Njagi, P., Kimetu, S. & Onyango, A. Trends and determinants of Comprehensive HIV and AIDS knowledge among urban young women in Kenya. AIDS Res. Ther. 8 , 11 (2011).
Peltzer, K. & Supa, P. HIV/AIDS knowledge and sexual behavior among junior secondary school students in South Africa. J. Soc. Sci. 1 , 1–8 (2005).
Shweta, C., Mundkur, S. & Chaitanya, V. Knowledge and beliefs about HIV/AIDS among adolescents. WebMed Cent. 2 , 1–9 (2011).
Anwar, M., Sulaiman, S. A. S., Ahmadi, K. & Khan, T. M. Awareness of school students on sexually transmitted infections (STIs) and their sexual behavior: a cross-sectional study conducted in Pulau Pinang, Malaysia. BMC Public Health 10 , 47 (2010).
Cicciò, L. & Sera, D. Assessing the knowledge and behavior towards HIV/AIDS among youth in Northern Uganda: a cross-sectional survey. Giornale Italiano di Medicina Tropicale 15 , 29–34 (2010).
Glick, P., Randriamamonjy, J. & Sahn, D. E. Determinants of HIV knowledge and condom use among women in madagascar: an analysis using matched household and community data. Afr. Dev. Rev. 21 , 147–179 (2009).
Rahman, M. Determinants of knowledge and awareness about AIDS: Urban-rural differentials in Bangladesh. JPHE 1 , 014–021 (2009).
Siziya, S., Muula, A. S. & Rudatsikira, E. HIV and AIDS-related knowledge among women in Iraq. BMC. Res. Notes 1 , 123 (2008).
Gao, X. et al. Effectiveness of school-based education on HIV/AIDS knowledge, attitude, and behavior among secondary school students in Wuhan, China. PLoS ONE 7 , e44881 (2012).
ADS CAS PubMed PubMed Central Google Scholar
Kotecha, P. V. et al. Reproductive health awareness among urban school going adolescents in Vadodara city. Indian J. Psychiatry 54 , 344–348 (2012).
Dimbuene, T. Z. & Defo, K. B. Fostering accurate HIV/AIDS knowledge among unmarried youths in Cameroon: do family environment and peers matter?. BMC Public Health 11 , 348 (2011).
Kumar, R. et al. Knowledge attitude and perception of sex education among school going adolescents in Ambala District, Haryana, India: a cross-sectional study. J. Clin. Diagn. Res. 11 , LC01–LC04 (2017).
ADS PubMed PubMed Central Google Scholar
Tripathi, N. & Sekher, T. V. Youth in India ready for sex education? Emerging evidence from national surveys. PLoS ONE 8 , e71584 (2013).
Rosenberg, M. et al. Evidence for sample selection effect and Hawthorne effect in behavioural HIV prevention trial among young women in a rural South African community. BMJ Open 8 , e019167 (2018).
Gupta, P., Anjum, F., Bhardwaj, P., Srivastav, J. & Zaidi, Z. H. Knowledge about HIV/AIDS among secondary school students. N. Am. J. Med. Sci. 5 , 119–123 (2013).
Download references
This paper was written using data collected as part of Population Council’s UDAYA study, which is funded by the Bill and Melinda Gates Foundation and the David and Lucile Packard Foundation. No additional funds were received for the preparation of the paper.
Author information
Authors and affiliations.
Ph.D. Research Scholar, Department of Survey Research & Data Analytics, International Institute for Population Sciences, Mumbai, India
Shobhit Srivastava & Pradeep Kumar
Ph.D. Research Scholar, Department of Family and Generations, International Institute for Population Sciences, Mumbai, India
Shekhar Chauhan
Ph.D. Research Scholar, Department of Public Health and Mortality Studies, International Institute for Population Sciences, Mumbai, India
Ratna Patel
You can also search for this author in PubMed Google Scholar
Contributions
Conception and design of the study: S.S. and P.K.; analysis and/or interpretation of data: P.K. and S.S.; drafting the manuscript: S.C., and R.P.; reading and approving the manuscript: S.S., P.K., S.C. and R.P.
Corresponding author
Correspondence to Pradeep Kumar .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Srivastava, S., Chauhan, S., Patel, R. et al. A study of awareness on HIV/AIDS among adolescents: A Longitudinal Study on UDAYA data. Sci Rep 11 , 22841 (2021). https://doi.org/10.1038/s41598-021-02090-9
Download citation
Received : 05 June 2021
Accepted : 29 September 2021
Published : 24 November 2021
DOI : https://doi.org/10.1038/s41598-021-02090-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
Approximately 1.2 million people in the United States are currently living with HIV, and an estimated 14% are infected, yet unaware of their status (Office of Infectious Disease and HIV/AIDS Policy, 2020).HIV and AIDS continue to have a disproportionate impact on certain populations, including youth—gay, bisexual, and other men who have sex with men (MSM)—racial and ethnic minorities ...
R.T. Gandhi and OthersN Engl J Med 2023;389:2468-2476. A 70-year-old woman with advanced HIV infection was evaluated because of cough, shortness of breath, and malaise. Eleven months earlier, she ...
Interview with Dr. Anthony Fauci on progress made during the past four decades of the HIV/AIDS pandemic and ongoing efforts to end this threat. 18m 44s Download. The dramatic saga of the acquired ...
HIV testing remains an important tool in identifying people living with HIV/AIDS (PLWHA). An early diagnosis of HIV can lead to a prolonged life expectancy if treatment is initiated promptly. Indicator conditi... Lina Martina Würfel, Anja Potthoff, Sandeep Nambiar and Adriane Skaletz-Rorowski. AIDS Research and Therapy 2024 21 :20.
An effective and scalable cure strategy is a top priority for the HIV research field; this Review discusses recent advances, knowledge gaps, and priority research areas for the next 5 years.
Since its inception, PEPFAR, which one of us (J. N.) now leads, has ensured crucial access to life-saving treatment for more than 20 million people in at least 50 countries. Twenty years after the ...
In this Review, Landovitz, Scott and Deeks explore the current state of the art for HIV prevention and treatment, including unmet needs and emerging tools. They describe how researchers are ...
HIV research continues on many fronts that could provide the same results and only some of which rely on the CCR5 delta 32 mutation, which should be explored extensively. There are many strategies which are in the early stages of development. ... This article aims to increase awareness among the society about the current scenario of HIV/AIDS ...
Introduction In June 2021, United Nations (UN) Member States committed to ambitious targets for scaling up community-led responses by 2025 toward meeting the goals of ending the AIDS epidemic by 2030. These targets build on UN Member States 2016 commitments to ensure that 30% of HIV testing and treatment programmes are community-led by 2030. At its current pace, the world is not likely to meet ...
While still elusive, cure remains the ultimate long-term goal for Gilead's HIV research and development efforts. ... Aiken C. Current Opinion in HIV and AIDS. 2018; 13(4): 359-365.
Human immunodeficiency virus (HIV) is an infection that attacks the body's immune system. Acquired immunodeficiency syndrome (AIDS) is the most advanced stage of the disease. HIV targets the body's white blood cells, weakening the immune system. This makes it easier to get sick with diseases like tuberculosis, infections and some cancers.
Advances in HIV/AIDS Research. HIV virions budding and releasing from an infected cell. NIAID, NIH. For an update on what medical science is doing to fight the global HIV/AIDS pandemic, read a Parade article by NIH Director Francis S. Collins and NIAID Director Anthony S. Fauci, AIDS in 2010: How We're Living with HIV.
Research. CDC provides national leadership for HIV prevention research, including the development and evaluation of HIV biomedical and behavioral interventions to prevent HIV transmission and reduce HIV disease progression in the United States and internationally. CDC's research efforts also include identifying those scientifically proven ...
Progress in scientific research rarely follows a straight path. Generally, it entails many unexpected meanderings, with a mix of good and bad ideas, good and bad luck. The discovery of the human im...
Readers may refer to (28-34) for more HIV/AIDS related models. This research is an extension of the study conducted by Rahman et al. with modifications that include the addition of an AIDS compartment as a variable, taking into consideration the population engaged in unprotected sexual intercourse, and the intervention of antiretroviral ...
Background HIV testing remains an important tool in identifying people living with HIV/AIDS (PLWHA). An early diagnosis of HIV can lead to a prolonged life expectancy if treatment is initiated promptly. Indicator conditions can be the first sign of an HIV infection and should therefore be recognised and consequently a HIV test should be carried out. Testing should occur in all individuals as ...
Blockbuster obesity drug leads to better health in people with HIV. Semaglutide reduces weight and fat accumulation associated with the antiretroviral regimen that keeps HIV at bay. Mariana ...
More than 3,600 HIV and infectious disease researchers from 73 countries gathered in Denver and virtually from March 3-6 this year for CROI, an annual scientific meeting on the latest research that can help accelerate global progress in the response to HIV and other infectious diseases, including STIs and viral hepatitis.
From the identification of HIV as the agent that causes AIDS, to the development of effective antiretroviral drugs, the scientific achievements in HIV research in the past 20 years have been ...
Chronic HIV-1 infection can cause neurological illness, also known as HIV-associated neurocognitive disorders (HAND). The elevated level of pro-inflammatory cytokines and chemokines, such as C-C Chemokine Ligand 5 (CCL5/RANTES), is one of the ways of causing HIV-1-mediated neuroinflammation. C-C Chemokine Receptor 5 (CCR5) is the main coreceptor for viral entry into host cells and for ...
Almost all the research in India is based on beliefs, attitudes, and awareness of HIV among adolescents 2,12. However, few other studies worldwide have examined mass media as a strong predictor of ...