- Open access
- Published: 10 March 2023

Recent advances in CRISPR-based genome editing technology and its applications in cardiovascular research
- Zhen-Hua Li 1 na1 ,
- Jun Wang 1 na1 ,
- Jing-Ping Xu 1 , 2 ,
- Jian Wang 1 &
- Xiao Yang 1
Military Medical Research volume 10 , Article number: 12 ( 2023 ) Cite this article
17k Accesses
6 Citations
17 Altmetric
Metrics details
The rapid development of genome editing technology has brought major breakthroughs in the fields of life science and medicine. In recent years, the clustered regularly interspaced short palindromic repeats (CRISPR)-based genome editing toolbox has been greatly expanded, not only with emerging CRISPR-associated protein (Cas) nucleases, but also novel applications through combination with diverse effectors. Recently, transposon-associated programmable RNA-guided genome editing systems have been uncovered, adding myriads of potential new tools to the genome editing toolbox. CRISPR-based genome editing technology has also revolutionized cardiovascular research. Here we first summarize the advances involving newly identified Cas orthologs, engineered variants and novel genome editing systems, and then discuss the applications of the CRISPR-Cas systems in precise genome editing, such as base editing and prime editing. We also highlight recent progress in cardiovascular research using CRISPR-based genome editing technologies, including the generation of genetically modified in vitro and animal models of cardiovascular diseases (CVD) as well as the applications in treating different types of CVD. Finally, the current limitations and future prospects of genome editing technologies are discussed.
Genome editing technology refers to a series of technologies capable of manipulating cellular DNA sequences at desired genomic sites by generating altered DNA sequences through nuclease-mediated site-specific DNA breaks that are resolved through DNA repair pathways [ 1 , 2 , 3 ]. Among genome editing-associated nucleases, clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein (Cas) nucleases are convenient, efficient, and precise, and are currently the most widely used [ 4 , 5 , 6 , 7 , 8 ]. After the CRISPR-Cas9 system was characterized and programmed to perform RNA-guided DNA cleavage at specific sites in prokaryotes, it was immediately proven to be an efficient tool for editing eukaryotic genomes [ 9 ]. Since then, CRISPR-based genome editing technology has drawn a worldwide attention and initiated extensive development. Emerging CRISPR-based tools with broadened targeting ranges, improved editing specificity and efficiency, and other distinct capabilities have facilitated eukaryotic genome editing by selecting optimal CRISPR-Cas tools. In addition to expanding the CRISPR-Cas nuclease arsenal, this system has also been applied to transcriptional regulation, epigenetic modification, and live-cell imaging by incorporation with other effector proteins [ 7 , 10 , 11 ].
The exponential development of genome editing technology has dramatically changed the landscape of biological and medical research, heralding a new era of precision medicine based on genome editing [ 12 ]. CRISPR-based nucleases are able to cut target DNA and generate double-strand breaks (DSBs), followed by the introduction of random mutations mediated by non-homologous end-joining (NHEJ), or make precise editing through homology-directed repair (HDR) [ 13 ]. The therapeutic potential of CRISPR-based tools has been investigated using the mouse models of various human diseases [ 7 , 14 ]. However, precise gene correction for in vivo therapeutic utility remains challenging, which is partially due to the low efficiency of HDR-mediated DNA replacement. This strategy is usually not applicable to post-mitotic cells, as HDR occurs mainly in the S/G 2 phase during cell division [ 15 ]. Precise genome editing tools have been developed and continuously optimized by fusing activity-impaired Cas nucleases with deaminases, called base editors, or with reverse transcriptases, called prime editors (PEs) [ 16 , 17 , 18 ]. Despite not achieving the goal of arbitrarily introducing any genetic substitutions at any targeted genomic site, we are now closer to this aspiration than ever.
Cardiovascular disease (CVD) is a group of disorders of the heart and blood vessels that has consistently been ranked as the leading threat to human health worldwide. Many gene mutations have been linked to CVD, and the number is still increasing [ 19 , 20 ]. Loss-of-function studies in animals are required to address the causal relationship between these mutations and cardiovascular pathologies. With the help of the CRISPR-based toolbox, creating animal models of human diseases has become much easier, faster, and more flexible than ever before; these models will greatly advance our understanding of cardiovascular pathogenesis and the development of therapeutic strategies [ 14 ]. Furthermore, CRISPR-based genome editing technology holds promise for treating inherited CVD caused by rare mutations.
In this review, we highlight the recent advances in CRISPR-based genome editing technology, mainly in the past three years, and discuss the tremendous innovation this epoch-making technology has brought to the field of cardiovascular research.
Novel Cas orthologs and engineered variants
Natural CRISPR-Cas systems are originally identified as adaptive immune systems in bacteria and archaea, and can be divided into two classes based on their composition and mechanisms. These systems are further divided into six types (I–VI) and dozens of subtypes based on the characteristics and accessory genes flanking the CRISPR array [ 21 ]. The most widely used class 2 CRISPRs are characterized by their single effector proteins, including type II Cas9 and type V-A Cas12a. Although class 2 natural Cas nucleases have long been used for efficient genome editing, their applications are limited because of the requirement of specific protospacer adjacent motif (PAM) sequences, off-target DNA cleavage, and occasionally, large sizes. Class 1 CRISPR systems possess multiple effector molecules that have unique features, such as distinct PAM preferences, higher on-target specificity through longer target recognition, and production of long-range genomic deletion [ 22 , 23 , 24 , 25 ]. However, the requirement of multiple effectors and the relatively low editing efficiency must be improved before their widespread application. Continuous efforts have been made to characterize novel Cas orthologs and engineered Cas variants to improve genome editing efficiency and broaden compatibility (Fig. 1 ).
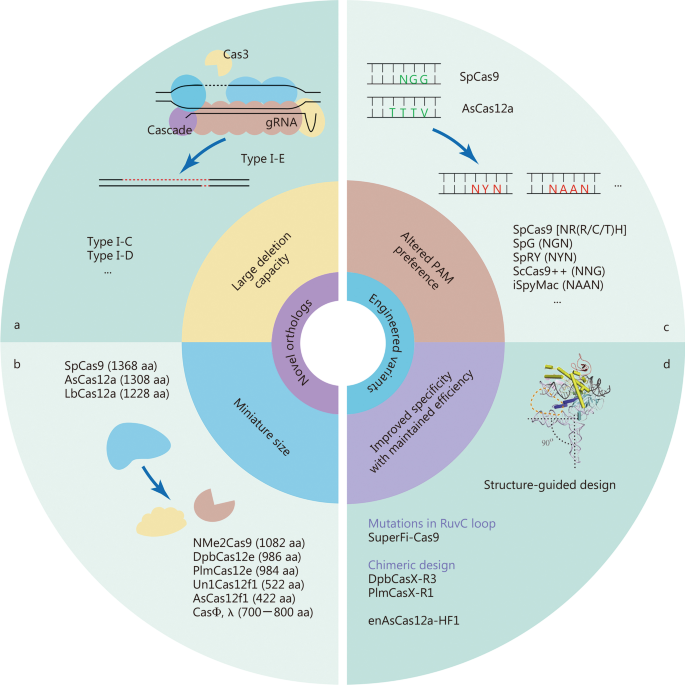
Characteristics of novel Cas orthologs and engineered variants. a Representative type I Cas orthologs capable of large-range deletions. b Representative Cas orthologs of miniature sizes. c Engineered Cas variants with diverse protospacer adjacent motif recognition capabilities. d Structure-guided strategies for improving DNA specificity without affecting the on-target cleavage efficiency
Characterizing novel Cas orthologs with distinctive features
The class 1 type I CRISPR system is the most prevalent CRISPR system, in which the multi-subunit CRISPR-associated complex for antiviral defense (Cascade) identifies DNA targets, and the helicase-nuclease enzyme Cas3 degrades DNA [ 26 ] (Fig. 1 a). Several type I CRISPR systems have been characterized and applied to mammalian genome editing. Type I-E and type I-D systems have been used to induce unidirectional and bidirectional long-range deletions in human cells [ 22 , 23 , 24 ]. Recently, supplying Cas11 was shown to enable divergent I-C, I-D, and I-B CRISPR-Cas3 editors for eukaryotic applications, and efficiently produced large unidirectional deletions [ 25 ]. Therefore, type I CRISPR systems can greatly expand the genome editing toolbox owing to their unique mechanisms and advantages in deleting full-length genes, gene clusters, and non-coding sequences.
Recently, the IS200/605 transposon family encoded RNA-guided nucleases have been identified as ancestors of CRISPR-Cas nucleases [ 27 , 28 ]. Cas9 endonucleases could likely have evolved from ancestral IscB proteins, whereas Cas12 endonucleases descended from TnpB proteins [ 27 ]. These transposon-encoded nucleases, together with the IsrB proteins, which are shorter IscB homologs also encoded in IS200/605 superfamily transposons, are called the obligate mobile element-guided activity (OMEGA) system [ 27 ]. IscB and TnpB are guided by non-coding RNAs called ωRNAs, which are derived from the left- or right-end elements of a transposon and combine the functions of CRISPR RNA (crRNA) and trans-activating CRISPR RNA (tracrRNA) [ 27 , 28 , 29 ]. Being only two-fifths the size of Cas9, IscB and TnpB can mediate double-strand DNA cleavage at the target sites with a 3′ or 5′ transposon-associated motif (TAM), and both have been adopted for genome editing in human cells [ 27 , 28 ].
The in vivo application of most CRISPR systems is challenging because of their large size, especially when delivered by the widely used adeno-associated virus (AAV). As a result, the exploitation of miniature Cas proteins with high efficiency is in sustained demand (Fig. 1 b). For instance, SaCas9 (1053 aa), CjCas9 (984 aa), and Nme1Cas9 (1082 aa) have been validated as mammalian genomic editors [ 30 , 31 , 32 ]. Recently, a compact Nme2Cas9 (1082 aa) recognizing an N 4 CC PAM was described with an identical target density as SpCas9 and few off-target effects [ 33 ]. In addition to the Cas9 orthologs mentioned above, several Cas12 nucleases with smaller sizes, such as Cas12e (or CasX, 986 aa), and Cas12f (400–700 aa), have also been identified as genome editing tools in mammalian cells [ 34 , 35 , 36 , 37 ]. The Un1Cas12f1 (522 aa) system was optimized to enable efficient genome editing in human cells [ 35 ]. Notably, with only a size of 422 aa, AsCas12f1 is currently the smallest RNA-guided Cas nuclease, and has been shown to be an effective programmed genome editing tool in both bacterial and human cells [ 36 ]. With approximately equal to or less than half the size of the widely used SpCas9, these miniature CRISPR tools facilitate the AAV-mediated all-in-one delivery of CRISPR components or catalytically inactive Cas variants fused with other functional proteins.
Very recently, CRISPR-Cas systems were found to be widely encoded in the genomes of diverse bacteriophages, where they are involved in competition with other viruses [ 38 , 39 ]. The bacteriophage-encoded Cas proteins contain all known types of CRISPR-Cas systems, but have phage-specific properties. These Cas proteins, such as CasΦ [ 38 ] and Casλ [ 39 ], tend to have remarkably small sizes due to the compact viral genome. These hypercompact systems have been shown to edit the genomes of human and plant cells, indicating that viral Cas nucleases could serve as a new source of genome editing tools.
Expanding the range of genomic targets
Genomic targeting by Cas nucleases requires a PAM sequence near the site where the Cas nuclease binds DNA sequences complementary to the single guide RNA (sgRNA). The requirement of PAM is the gatekeeper for CRISPR-Cas mediated genome targeting, as whether a genomic sequence possesses a PAM for a certain Cas nuclease determines whether the site can be targeted and edited by CRISPR. Efforts have been made to develop Cas nucleases with broader PAM compatibility to pursue true PAM-free nucleases. However, PAM-free nucleases might have potential drawbacks, such as self-targeting of gRNA-expressing DNA constructs and reduced efficiency as more time is required for interrogating the whole genome. Therefore, it is better to develop an arsenal of divergent PAM-dependent Cas nucleases that collectively cover all genomic sequences [ 40 ] (Fig. 1 c).
Using phage-assisted non-continuous and continuous evolution strategies, three new SpCas9 variants (SpCas9-NRRH, SpCas9-NRCH, and SpCas9-NRTH) were characterized to recognize most NR PAM sequences, together with SpCas9-NG (N = A/T/C/G, R = A/G, H = A/C/T) [ 41 ]. To relax the PAM preference of SpCas9, two SpCas9 variants, SpG (targeting NGN) and SpRY (targeting NYN), have been generated by structure-guided substitutions in several residues, making most of the genome targetable (Y = C/T) [ 42 ]. Structure-motivated engineering has also been used to expand targeting range of LbCas12a and AsCas12a [ 43 , 44 ]. Chimeric Cas proteins created by exchanging PAM-interacting domains between naturally occurring Cas orthologs have also been applied to expand PAM recognition. Substituting the loop sequence of Cas9 from Streptococcus anginosus , together with the T1227K mutation, into the open reading frame (ORF) of ScCas9 generates ScCas9++ with NNG PAM compatibility [ 45 ]. A similar strategy was used to generate a variant iSpyMac by grafting the PAM-interacting domain of SmacCas9 into SpyCas9, which recognizes all adenine dinucleotide PAM (NAAN PAM) sequences [ 46 ]. The chimera generation approach has also been applied to replace PAM-interacting domains in SaCas9 [ 47 ] and Cas12a [ 48 ].
Improving the DNA specificity without affecting on-target cleavage efficiency
Off-target activity is a major challenge when using CRISPR tools for disease-related gene therapy. Over the years, continuous efforts have been made to construct high-fidelity Cas9 variants including eSpCas9(1.1) [ 49 ], SpCas9-HF1 [ 50 ], HypaCas9 [ 51 ], evoCas9 [ 52 ], HiFi Cas9 [ 53 ] and Sniper-Cas9 [ 54 ]. In addition, high-fidelity SaCas9s have been identified either by rational engineering [ 55 ] or directional screening [ 56 ]. The achievement of enhanced discrimination between on-target and off-target binding of these variants relies mainly on the energetic destabilization of the Cas9:sgRNA:DNA complex at off-target sites [ 57 ]. However, the improvement of these high-fidelity Cas9 variants seems to occur at the cost of decreased on-target efficiency [ 58 , 59 ]. Recently, kinetics-guided cryo-electron microscopy was used to show that mismatches distal to PAM can be stabilized by a loop in the RuvC domain, allowing Cas9 activation [ 60 ]. Based on this observation, they designed a high-fidelity variant with mutations in the RuvC domain, named SuperFi-Cas9, which displayed significantly improved mismatch discrimination without compromising on-target DNA cleavage efficiency [ 60 ].
DpbCas12e has been validated as a naturally occurring high-fidelity Cas nuclease with striking avoidance of off-target activity [ 61 ]. In a recent cryo-EM-based structural engineering study, unique nucleotide-binding loops within Cas12e were found to be important for DNA cleavage efficacy. Based on this finding, newly designed chimeric Cas12e proteins (DpbCasX-R3 and PlmCasX-R1) and sgRNA (sgRNAv2) exhibited substantially improved DNA editing efficiency in mammalian cells [ 62 ]. Structure-guided protein engineering has also been used to improve the performance of AsCas12a [ 43 ]. E174R/S542R/K548R substitutions were introduced into AsCas12a to construct a variant called enAsCas12a that possesses an expanded targeting range and increased cleavage activity. A high-fidelity version of enAsCas12a (enAsCas12a-HF1) with an additional N282A substitution was also engineered to reduce off-target effects [ 43 ] (Fig. 1 d).
Advances in precise genome editing
Precise genome editing is essential for preclinical research and clinical gene therapy, and HDR-mediated gene editing has long been the only option. Efforts have been made to improve HDR efficiency, such as the use of rationally designed single-stranded oligodeoxynucleotide (ssODN) templates instead of double-stranded DNA [ 63 ] or the addition of NHEJ chemical inhibitors [ 64 ]. The delivery of Cas9 and HDR templates by AAVs has accomplished precise genome editing in post-mitotic hippocampal neurons and cardiomyocytes in mice [ 65 , 66 , 67 ]. However, the efficiency of HDR-mediated editing is still relatively low compared to that of the predominant NHEJ repair pathway, and DSBs made by this conventional method may introduce undesired damage to the genome [ 68 , 69 , 70 ]. Its clinical application is hampered by the need for additional DNA templates and HDR-promoting chemical agents with potential cytotoxicity, such as SCR7 [ 64 ], azidothymidine, trifluridine [ 71 ], NU7026, and NU7441 [ 72 ]. Motivated by these problems, novel precise genome editing tools that do not require DSBs or exogenous DNA templates have been developed (Fig. 2 ).
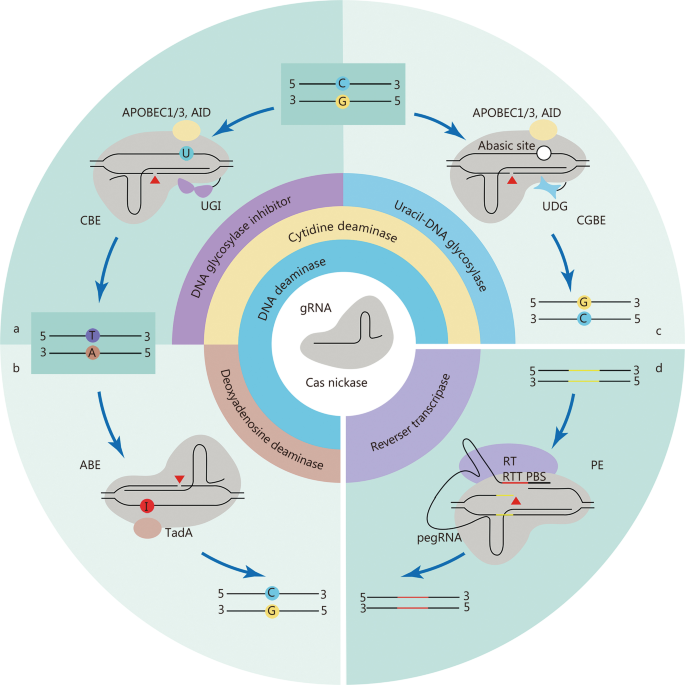
CRISPR-Cas-based DNA base editing tools. a-c Schematic diagrams of CBE ( a ), ABE ( b ), and CGBE ( c ). d Schematic of PE. UGI uracil-DNA glycosylase inhibitor, AID activation-induced cytidine deaminase, UNG uracil-DNA glycosylase, TadA deoxyadenosine deaminases, RT reverse transcriptase, PBS primer binding site, RTT RT template, CBE cytosine base editor, ABE adenine base editor, CGBE C-to-G base editor, PE Prime editor
Base editors
Base editors can make precise base substitutions without requiring DSBs or donor DNA templates, and are independent of HDR, providing a promising therapeutic tool for human genetic diseases in which the most relevant variants are single nucleotide mutations [ 73 , 74 ]. Current base editors are constructed by fusing DNA deaminase enzymes to catalytically impaired Cas nucleases, which can precisely change a single base in a targeted sequence [ 75 ]. Base editing jointly harnesses the genome-targeting function of a Cas protein and the DNA base modification role of a deaminase, and sometimes additional regulatory elements are also required to achieve the desired performance. To date, base editors can be used not only for precise genome editing at specific single loci, but also for large-scale functional screening of genetic variants or key amino acid residues [ 76 , 77 , 78 ].
The cytosine base editor (CBE) and adenine base editor (ABE) are widely used base editors that enable the editing of all four types of base transitions (C-to-T, A-to-G, T-to-C, and G-to-A) [ 16 , 17 ] (Fig. 2 a, b). Since these two editors were developed in 2016 and 2017, subsequent efforts have significantly expanded their genome-targeting range and improved their efficiency and product purity. The use of Cas nickase, fusion of a second uracil-DNA glycosylase inhibitor (UGI) domain, the addition of a nuclear localization sequence, and linker and codon optimization have greatly increased the editing efficiencies of base editors [ 79 , 80 , 81 ]. To maximize the editing scope of base editors, diverse base editors with natural, engineered, or evolved Cas variants that recognize alternative PAMs and various deaminases have been created [ 82 , 83 , 84 , 85 , 86 , 87 , 88 ].
Programmable C-to-G base editors (CGBEs) that can achieve targeted C-to-G and G-to-C base transversions have recently been developed [ 89 , 90 ]. CGBEs originate from CBE by replacing the UGI with a uracil-DNA glycosylase (UNG), which excises the U base generated by cytosine deaminase, resulting in an abasic site followed by the preferential installation of a G base through the DNA repair mechanism (Fig. 2 c). Although CGBEs can provide efficient C-to-G and G-to-C editing, very few sites are suitable for CGBE editing. Several studies have used machine learning to optimize CGBEs to improve editing efficiency and product purity by changing the species origin, modifying the relative positions of UNG and deaminase, and optimizing codons [ 91 , 92 ].
The therapeutic applications of base editors have been hampered by their genome-wide off-target effects. Recent studies have shown that cytidine deaminases used in CBE induce genome-wide off-target editing independently of sgRNA or Cas9 [ 93 , 94 ]. In addition, both ABE and CBE can cause transcriptome-wide mutations [ 95 , 96 ]. Continuous efforts have been made to reduce off-target effects by engineering the DNA- [ 97 , 98 ] or RNA-binding domain [ 95 , 96 , 99 ], thereby bringing base editors closer to clinical applications.
Prime editing is a newly developed precise genome editing technology that enables all types of base conversion, small deletions, and insertions, as desired. PEs consist of a prime editor protein and prime editing gRNA (pegRNA). The PE protein is constructed by fusing an engineered Cas9 nickase (H840A) with reverse transcriptase, which can be targeted to the genomic locus by pegRNA [ 18 ]. The pegRNA combines a gRNA recognizing the target genomic sequence, a reverse transcriptase template encoding the desired edits, and a primer binding site to initiate reverse transcription [ 18 ] (Fig. 2 d). The newly synthesized edited DNA strand is incorporated into the target locus to generate heteroduplex DNA, in which the non-edited strand is eventually replaced by an edited strand through DNA repair. Compared to base editing, which often introduces bystander editing of extra bases in an activity window, prime editing is more versatile and precise.
A series of PE systems, namely PE2, PE3b, PE4, and PE5b, have been developed and are most widely used. All these systems share a common PE2 protein with an engineered Moloney murine leukemia virus (M-MLV) reverse transcriptase instead of the wild-type M-MLV reverse transcriptase in PE1 to increase editing efficiency [ 18 , 100 ]. The PE3 system contains an additional sgRNA that targets the non-edited strand to increase the editing efficiency [ 18 ]. The PE2 and PE3 systems were further optimized by introducing a DNA mismatch repair-inhibiting domain MLH1dn to generate PE4 and PE5 systems, respectively [ 18 , 100 ]. Systems ending in “b”, namely PE3b and PE5b, use an edit-specific nicking sgRNA to reduce indel levels [ 18 , 100 ]. Constant efforts are being devoted to optimizing PEs, with a primary focus on improving their editing efficiency. Optimization of PE2 protein architecture by codon optimization, SpCas9 mutation, and alterations of the nuclear localization signal and peptide linker sequence results in PEmax protein architecture, which greatly enhances editing efficiency [ 100 ]. They also constructed two types of engineered pegRNAs (epegRNAs) by incorporating 3′ structural motifs, which stabilize pegRNA and increase prime editing efficiency [ 101 ]. Many other groups have adopted similar strategies by optimizing either PE proteins [ 102 , 103 , 104 ] or pegRNAs [ 105 ].
In addition, a dual pegRNA strategy has been used to improve editing efficiency, which can also achieve programmable insertion, deletion, and replacement of large genomic sequences at specific genomic sites [ 106 , 107 , 108 , 109 , 110 ]. Using a pair of pegRNAs, each of which targets a different DNA strand and template the synthesis of complementary DNA flaps, endogenous targeted DNA sequence between the PE-induced nick sites is successfully replaced. This strategy also achieves targeted insertion of gene-sized DNA plasmids (> 5 kb) and targeted inversions of 40 kb in human cells when co-expressing a site-specific serine recombinase, Bxb1 integrase [ 108 ]. The dual pegRNA strategy with expanded capabilities of precision genome editing provides new possibilities for treating genetic disorders caused by large DNA deletions or complex structural mutations.
CRISPR-associated transposon (CAST) systems for large DNA insertion
CAST systems, consisting of transposase subunits and CRISPR effectors, facilitate the RNA-guided transposition of mobile genetic elements, making it a promising system for targeted, precise, and efficient insertion of large DNA segments. Most identified CASTs are derived from Tn7-like transposons that retain the core genes of the transposition machinery, but have no genes for target selection [ 111 , 112 ]. Instead, CASTs co-opt nuclease-deficient CRISPR-Cas proteins to induce RNA-guided transposition [ 111 , 112 ]. Several CAST systems have been experimentally or bioinformatically characterized, including type I-B, type I-C, type I-F, type IV, and type V-K CAST systems [ 111 , 113 , 114 , 115 ]. Bioinformatic analysis of the metagenomic database also revealed a non-Tn7 CAST system that co-opts a nuclease-inactive Cas12 and type I-E cascade [ 111 ].
Type I-F and type V-K CAST systems have been successfully reconstituted to achieve the integration of donor DNA into specific bacterial genome sites [ 113 , 114 ] (Fig. 3 a). An improved version of the type I-F CAST system enables highly specific and effective integration of up to 10 kb DNA fragments in the bacterial genome [ 116 ]. However, the application of these two systems in mammalian cells has not been reported. A very recent study developed an artificial transposon-associated CRISPR-Cas system named find and cut-and-transfer (FiCAT) system by coupling a SpCas9 protein with an engineered piggyBac (PB) transposase (Fig. 3 b), which achieved the targeted integration of multi-kilobase DNA fragments into the genomes of mammalian cell lines and mouse liver [ 117 ]. The discovery of CAST systems has expanded the genome editing toolkit, although CAST systems still require extensive modification and optimization until they can be conveniently and effectively applied to biomedical research.
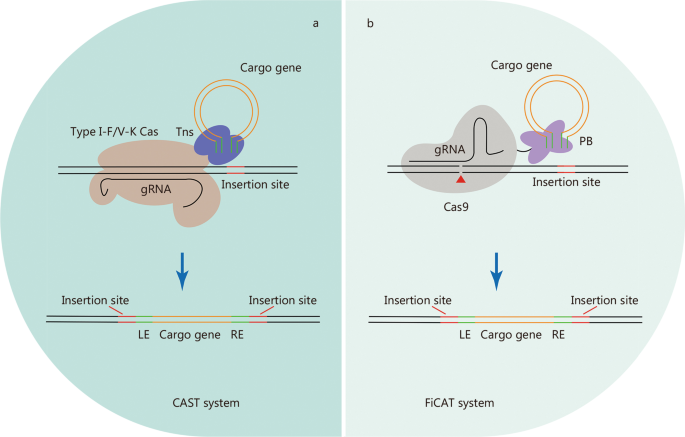
CRISPR-Cas-based transposon systems. Schematic of CRISPR-based transposon systems, CAST system ( a ) and FiCAT system ( b ), which mediate site-specific DNA integration. Tns Tn7-like transposases, PB piggyBac transposase, LE transposon left end sequences, RE transposon right end sequences, CAST CRISPR-associated transposon, FiCAT find and cut-and-transfer
Delivery systems for CRISPRs
The safe, effective, and tissue-specific delivery of CRISPR-Cas tools in vivo determines whether CRISPR-based gene therapy can be used for this tissue. Therapeutic in vivo delivery systems for CRISPR-Cas have recently been discussed [ 118 , 119 ]. CRISPR-Cas tools can be delivered in the form of DNA, mRNA, or ribonucleoprotein complexes (RNP) through ex vivo or in vivo approaches. Various robust methods have been established to deliver genome editing reagents ex vivo, some of which have been used in multiple clinical trials involving different types of diseases [ 120 , 121 , 122 ]. The most efficient method of in vivo delivery of editors reported so far is the use of AAV, which can deliver editor-encoding DNA to target tissues and has been applied in clinical trials [ 118 , 123 , 124 ]. However, AAV-based delivery of DNA-encoding editing agents has a number of disadvantages, such as the possibility of viral vector integration into the transduced cell genome and increased frequency of off-target editing due to prolonged expression [ 75 , 119 , 125 , 126 ], which limits its clinical application. Therefore, safer alternative strategies for in vivo delivery of genome editors must be developed.
As a gene therapy delivery system approved by the Food and Drug Administration (FDA), lipid nanoparticles (LNP) have been demonstrated to safely deliver therapeutic small molecules and nucleic acid drugs to hepatocytes and antigen-presenting cells via systemic administration or intramuscular injection. The LNP system was used to deliver gene editing tools in the first clinical trial involving human gene editing in vivo [ 123 ]. However, because intravenously delivered LNP showed liver tropism, delivering editors to non-hepatocytes has been a huge challenge. Recent studies have shown that high-throughput screening identifies nanoparticles targeting non-hepatocytes, including endothelial cells (ECs) and spleen immune cells [ 127 , 128 ]. In addition, cell-type specificity of LNP-mediated Cas9 therapies could be modified by reducing Cas9-mediated insertions and deletions in hepatocytes using inhibitory oligonucleotides and siRNAs [ 129 ].
Virus-like particle (VLP) systems, which combine the advantages of viral and non-viral delivery systems, are another promising in vivo gene editing delivery vehicle [ 126 ]. VLPs can package genome editing agents in the forms of mRNA or RNP. The short cellular lifespan of RNPs effectively restricts off-target editing. Almost all current VLPs are derived from retroviruses and contain most viral components but no viral genome [ 118 , 130 , 131 , 132 , 133 ]. Very recently, fourth-generation engineered VLPs (eVLPs) based on M-MLV have been developed to deliver Cas9 or base editor RNPs both in vitro and in vivo [ 126 ]. A single intravenous injection of eVLPs carrying a base editor targeting proprotein convertase subtilisin/kexin type 9 ( Pcsk9 ) can achieve base editing in multiple tissues, reduce serum PCSK9 levels by 78%, and partially restore visual function when designed for retinal editing in a mouse model of blindness [ 126 ]. The mammalian endogenous retrovirus-like protein PEG10 has also been programmed as a VLP system called selective endogenous encapsidation for cellular delivery (SEND) platform, which can package and deliver mRNA encoding Cas9 in vivo [ 134 ]. Based on endogenous mammalian proteins, the SEND system may be less immunogenic than bona fide retrovirus-based VLP systems.
Applications of genome editing in modeling and treating CVD
CVD, including heart and vascular diseases, are leading causes of morbidity and mortality at different ages [ 135 , 136 ]. In recent years, a tremendous amount of new genetic information related to CVD has been identified using next-generation sequencing technologies [ 19 , 20 ]. Owing to the advent and development of the CRISPR-Cas system, we can now handle this information and determine CVD-related functions much more easily than ever. The CRISPR-Cas system also provides more possibilities for treating inherited CVD by correcting disease-causing mutations in the patient genome. As the most commonly used Cas proteins, SpCas9 and SaCas9 have been broadly applied in CVD-related modeling and therapeutic purposes, both in vitro [ 137 , 138 , 139 , 140 ] and in vivo [ 141 , 142 , 143 , 144 ]. Newly developed base editing and prime editing systems have also been used [ 145 , 146 ]. Additionally, delivering CRISPR-Cas components to the cardiovascular system remains challenging, and AAV-based systems are currently the most widely used methods [ 147 , 148 , 149 , 150 ].
Modeling CVD using CRISPR
Genetic studies have identified various pathogenic genetic variants associated with the occurrence of CVD [ 151 , 152 ]. Revealing the consequences of specific mutations in CVD-related genes is important for CVD genetic diagnosis and precise medicine. CVD models have played a critical role in establishing causal links between genetic variants and CVD, dissecting the molecular mechanisms underlying CVD, validating therapeutic targets, and preclinical evaluation of therapeutic agents. At present, multiple gene editing tools have been applied to create in vitro and in vivo models of CVD [ 151 ].
In vitro models of CVD
Human induced pluripotent stem cells (hiPSCs) are promising for modeling human cardiomyopathies in vitro because they can differentiate into cardiomyocytes [ 153 ]. Through genome editing of hiPSCs followed by their differentiation into cardiomyocytes (hiPSC-CMs), isogenic hiPSC-CMs have been broadly used to verify causative genes or mutations in cardiomyopathies. Gene disruption induced by CRISPR-Cas9 in hiPSC-CMs is straightforward and suitable for determining the role of a gene in CVD. For example, DNA methyltransferase 3A ( DNMT3A ) gene-deleted hiPSC-CMs generated using CRISPR-Cas9 gene editing showed altered contraction kinetics and impaired glucose/lipid metabolism, suggesting an important role of DNA methylation in cardiac diseases [ 137 ]. The homozygous SCN10A gene (encoding Na V 1.8) knockout hiPSC-CMs help demonstrate that the voltage-gated sodium channel Nav1.8 contributes to late Na + current (I NaL ) formation and displays a harmful proarrhythmogenic function [ 138 ].
The precise introduction of point mutations into hiPSC-CMs facilitates the determination of the causal relationship between genetic mutations and heart diseases. Striated muscle-enriched protein kinase (SPEG) E1680K homozygous mutant hiPSC-CMs recapitulate the hallmarks of dilated cardiomyopathy (DCM), confirming that SPEG E1680K is a novel DCM-causing mutation [ 139 ]. Genome editing of hiPSCs has also been used to identify several causative mutations of arrhythmias. hiPSC-CMs expressing an R211H substitution in the Ras-related associated with diabetes ( RRAD ) gene mimic the single-cell electrophysiological characteristics of Brugada syndrome, a disorder predisposing the patient to ventricular arrhythmias, indicating that RRAD is possibly a novel susceptibility gene for Brugada syndrome [ 154 ]. CRISPR-Cas9-engineered hiPSC-CMs carrying three different mutations of ryanodine receptor 2 (RyR2), R420Q, Q4201R, or F2483I, exhibit various pathological features of catecholaminergic polymorphic ventricular tachycardia 1 (CPVT1)-associated arrhythmia, suggesting that different RyR2 mutations cause varied Ca 2+ signaling consequences and drug sensitivities [ 155 ]. Genome-edited hiPSC-CMs can also be used as high-throughput platforms for scalable functional validation of the pathogenicity and pathophysiology of genetic variants identified in the human population. To determine the functional significance of cardiac troponin T ( TNNT2 ) variants, the endogenous TNNT2 gene was knocked out in hiPSCs using CRISPR-Cas9, and 51 different TNNT2 variants were expressed using lentivirus in differentiated TNNT2 knockout hiPSC-CMs. The results revealed that various TNNT2 variants exhibit different pathogenic mechanisms, greatly expanding the knowledge of which and how TNNT2 variants cause hypertrophic cardiomyopathy (HCM) and DCM [ 156 ].
Genome editing has been used to correct mutations to generate optimal isogenic controls for patient-derived iPSC-CMs, enabling the determination of genotype–phenotype relationships more precisely. Genome editing has been performed to correct the missense mutation T618I in the potassium channel gene KCNH2 in short QT syndrome patience-specific hiPSC-CMs to elucidate the single-cell phenotype of short QT syndrome [ 157 ]. Using iPSC-CMs derived from doxorubicin-treated pediatric patients, cytosine base editing has helped identify the single nucleotide polymorphism rs11140490 in the SLC28A3 locus, which is a novel protector against doxorubicin-induced cardiotoxicity [ 145 ]. Isogenic hiPSC-CM controls generated by CRISPR-based gene correction have also been used as platforms to evaluate other therapeutic methods. Type 1 long QT syndrome is caused by loss-of-function variants in the KCNQ1-encoded Kv7.1 potassium channel α-subunit. iPSC-CMs generated from patients with KCNQ1-V254M and -A344A/spl mutations have recently been corrected using CRISPR-Cas9 to act as isogenic controls, which have been used to evaluate a dual-component suppression-and-replacement gene therapy method [ 158 ]. Base editing and prime editing could possibly be widely used in establishing hiPSC-CM-based CVD models in the near future.
Animal models of CVD
CRISPR-based germline genome editing tools have revolutionized the generation of genetically modified animal models of CVD. Compared to conventional gene targeting technologies using embryonic stem cells, CRISPR-based gene editing technologies are easier to operate, faster, and applicable to most species. One strategy for generating animal models of CVD is to introduce targeted point mutations, insertions, or deletions using HDR-mediated germline genome editing. A mouse model of HCM with a Myh6 R404Q mutation was generated using SpCas9/ssODN-mediated directed genomic DNA editing, and heterozygous mice developed a typical HCM phenotype [ 141 ]. A similar approach was also utilized to insert an additional adenine nucleotide into the lysosomal acid alpha-glucosidase ( Gaa ) gene at the c.1826 locus and generate a novel mouse model of infantile-onset Pompe disease (IOPD), which recapitulates HCM and the skeletal muscle weakness of human IOPD [ 142 ]. A CRISPR-Cas9-generated rat model, with a 9 bp deletion within the hotspot analogous to the novel mutation of the human PDE3A gene, recapitulates arterial hypertension with brachydactyly, demonstrating that mutant PDE3A causes arterial hypertension [ 143 ]. Recently, a 94 bp out of frame deletion was generated in exon 1 of Kcnk3 using SpCas9/ssODN-mediated genome editing, creating a novel rat model of pulmonary arterial hypertension [ 159 ]. Another strategy is to delete exon(s) using two sgRNAs flanking specific exon(s). Exon deletion mutations in the dystrophin are among the most common causes of Duchenne muscular dystrophy (DMD). Several mouse models of DMD have been generated using CRISPR-Cas9 genome editing [ 144 , 160 ], which are discussed further in the next section. CRISPR-Cas9 mediated mosaic inactivation of zebrafish ccm2 led to a lethal multi-cavernous lesion that histologically mimics the typical human hemorrhagic cerebral cavernous malformation [ 161 ].
Compared with germline genome editing, somatic genome editing is a more flexible method for obtaining CVD models, which overcomes the challenges of germline modification, such as embryonic lethality and the cost and time required to establish, reproduce, and maintain these models. It is also suitable for rapid and relatively high-throughput studies on the functions of CVD-related genes. As early as 2016, a cardiomyocyte-specific SpCas9 transgenic mouse model was successfully generated to achieve somatic editing in the heart [ 162 ]. Following this study, intraperitoneal injection of AAV9 encoding sgRNA against three genes critical for the heart, Myh6 , Sav1 , and Tbx20 , in postnatal cardiomyocyte-Cas9 transgenic mice caused a similar degree of DNA disruption and subsequent mRNA downregulation, but only Myh6 disruption induced HCM and heart failure, suggesting that the effect of postnatal cardiac genome editing is target-dependent [ 147 ]. Mouse models can also be generated by activating endogenous gene expression through CRISPR-mediated genome editing in the postnatal heart [ 163 ]. CRISPR-mediated endogenous activation of myocyte enhancer factor 2D ( Mef2d ) leads to cardiac hypertrophy in mice, indicating that CRISPR-mediated genome editing can be used to generate CVD mouse models by controlling transcription in the postnatal heart [ 163 ]. Several recent studies have shown that CRISPR-Cas9 can be used to edit endothelial genes in vivo to obtain vascular disease models and enable reverse genetic studies of gene function in the mammalian vascular endothelium. Co-injection of an adenovirus harboring sgRNAs targeting the Alk1 gene and AAV1-VEGF successfully induced mutations in Alk1 in brain ECs and generated brain arteriovenous malformations in adult mice [ 164 ]. We recently generated a blood–brain barrier (BBB) breakdown mouse model by AAV-BR1-CRISPR mediated somatic genome editing. A single intravenous administration of brain microvascular EC targeting AAV-BR1 encoding sgRNA against the β-catenin ( Ctnnb1 ) gene resulted in a mutation of 36.1% of the Ctnnb1 alleles and dramatically decreased levels of CTNNB1 in brain ECs, leading to BBB breakdown in EC-restricted Tie2 Cas9 mice [ 148 ]. The AAV-BR1-CRISPR system established in this study allowed for the rapid construction of BBB perturbation models in vivo and may be helpful for developing drug delivery systems in the central nervous system. Recently, the nanoparticle-mediated delivery of CRISPR plasmid DNA expressing Cas9 under the control of the Cdh5 promoter resulted in efficient genome editing in the ECs of the peripheral vasculature in adult mice, which provides a powerful tool to construct animal models of peripheral vascular diseases [ 165 ].
Genome editing in CVD treatment
Therapeutic genome editing can be used to treat monogenic CVD, and the technology could permanently correct mutations and eventually eradicate specific CVD. Programmed edits were introduced into the human germline genome [ 166 , 167 , 168 , 169 ]. However, human germline genome editing faces significant ethical concerns and is prohibited in most countries [ 170 ]. Somatic editing is a promising technology for editing CVD-causing mutations without the risk of passing genomic changes to the offspring. Table 1 summarizes the latest applications of genome editing in treating different types of CVD.
DMD is an X-linked disorder characterized by proximal muscle weakness and cardiomyopathy caused by mutations in the largest human gene, dystrophin ( DMD ) [ 182 ]. A variety of mutations exist throughout the DMD gene, most of which are located in the regions crossing exons 43 to 53 and disrupt the ORF, resulting in non-functional truncated proteins.
A single-cut genome editing strategy was applied in both iPSC-CMs and mouse models of DMD bearing an exon 44 deletion mutation (Δ44), one of the most common causative mutations of DMD. The ORF can be restored by disrupting the exon splice site to skip the adjacent exon, inserting one nucleotide, or deleting two nucleotides in exon 44 [ 144 ]. Systemic delivery of gene editing components by a single dose of AAV9 restores ~ 90% dystrophin protein expression in the hearts of Δ44 mice within 4 weeks [ 144 ]. This approach also helps to correct DMD models bearing deletions of exons 43, 45, and 52 (Δ43, Δ45, and Δ52) both in vitro and in vivo [ 171 ]. A dual-AAV system was used to deliver SpCas9 and a single sgRNA targeting the splice donor site of exon 44 (for Δ43 or Δ45 mice) or splice acceptor site of exon 53 (for Δ52 mice) in vivo to restore dystrophin expression. Both exon skipping and reframing were induced in Δ45 and Δ52 mice, and the efficacy of dystrophin in these two models was higher than that in Δ43 mice, in which only exon skipping was generated [ 171 ]. Restoration of dystrophin has also been achieved in hiPSC-CMs from these DMD models [ 171 ]. However, this study did not specify whether exon skipping and/or reframing could subsequently rescue the cardiac phenotypes of DMD models [ 171 ]. Another strategy is to delete exon(s) using two sgRNAs flanking on either side, thus restoring the ORF of the DMD gene. The systemic application of AAV9 carrying an intein-split SpCas9 and a pair of sgRNAs targeting sequences flanking exon 51 in a pig model of DMD lacking exon 52 induced dystrophin expression in the heart and reduced arrhythmogenic vulnerability [ 172 ]. The long-term efficacy and safety of therapeutic editing for DMD have also been studied [ 149 , 150 ]. AAV vectors carrying SaCas9 and a pair of sgRNAs targeting exon 23 or exons 21–23 were administrated for one year or 19 months, respectively, to mdx mice. Cardiac functions were improved without serious adverse effects, indicating that in vivo CRISPR genome editing may be a safe therapeutic strategy for DMD [ 149 , 150 ].
Base editing and prime editing show great promise for treating DMD. Both ABE and PE can restore dystrophin protein expression by inducing exon skipping or exon reframing to correct the Dmd exon 51 deletion mutation in iPSC-CMs, and intramuscular delivery of AAV9 encoding ABE components amends the mutation in ∆E51 DMD mice [ 146 ]. CBE has been shown to rescue dystrophic cardiomyopathy in Dmd E4* mice, which harbor a 4 bp deletion in exon 4 of the Dmd gene and recapitulate many characteristics of human DMD [ 160 ]. A single-dose administration of AAV9-eTAM encoding a fused nuclease-defective SaCas9 (KKH) with activation-induced cytidine deaminase (AID) and UGI, together with AAV9-sgRNA, efficiently induced splice site mutation and exon 4 skipping of the Dmd gene and restored up to 90% of dystrophin proteins in the heart of Dmd E4* mice, resulting in improved cardiac function and an increased life span [ 160 ]. Alternatively, a dual AAV-mediated protein trans-splicing approach was used to deliver a modified ABE-NG to an mdx 4cv mouse model carrying a premature stop codon (CAA-to-TAA) in exon 53 of the Dmd gene. After 10 months of treatment, a near-complete rescue of dystrophin was found in the hearts of mdx 4cv mice without obvious toxicity [ 173 ].
HCM and DCM
Inherited cardiomyopathies, including HCM and DCM, are candidate genetic disorders that are suitable for genome editing-related treatment. ABEmax-NG has been shown to correct a pathogenic R404Q/+ mutation in embryos of the HCM mouse model [ 141 ]. Administration of ABEmax-NG mRNA to Myh6 R404Q/+ embryos corrects the mutant allele at a rate of 62.5% to 70.8%, abolishing the HCM phenotype in postnatal mice and their progeny. Moreover, in utero delivery of intein-split ABEmax-NG induced a high correction rate without introducing indels or off-target editing in Myh6 R404Q/+ fetuses [ 141 ]. Intronic CRISPR repair has been demonstrated as efficient in a preclinical iPSC-CM model of Noonan syndrome-associated HCM [ 174 ]. CRISPR-Cas9-mediated destruction of the mutation-induced additional intronic donor splice site can reverse the hypertrophic phenotypes in Noonan syndrome patient-derived iPSC-CMs carrying biallelic mutations in intron 16 of the leucine zipper-like transcription regulator 1 ( LZTR1 ) gene, indicating new possibilities for personalized therapeutic genome editing in HCM patients [ 174 ]. Notably, CRISPR-based genome editing has been shown to have potential to correct a well-documented heterozygous dominant 4 bp deletion in exon 16 of MYBPC3 , which causes familial HCM, in human embryos [ 175 ]. Co-injection of Cas9 proteins, mutation-specific sgRNAs, and mutant sperm into healthy metaphase II oocytes corrected the deletion by wild-type maternal allele-mediated HDR, resulting in a high yield of homozygous embryos carrying the wild-type MYBPC3 gene without mosaicism or off-target mutations [ 175 ].
Genome editing has also been used to correct DCM-causing mutations. In addition to previous studies using hiPSC-CMs showing that truncated titin (TTNtv) mutations are the most common causes of DCM [ 183 , 184 , 185 ], recently, the pathological mechanisms of TTNtv-associated DCM have been highlighted and a new genome editing strategy has been developed to treat TTNtv-associated DCM. iPSC-CMs with patient-derived or CRISPR-Cas9-generated TTN mutations were corrected using SpCas9/ssODN. Engineered heart muscle generated from corrected hiPSC-CMs shows normalized titin protein levels and contractile function [ 140 ]. Genome editing using SpCas9 and A-band TTNtv -specific sgRNA was also shown to restore the reading frame of TTN protein in hiPSC-CMs, leading to increased full-length TTN protein levels and normalized sarcomere function [ 176 ]. More recently, the application of precise genome editing technology for treating DCM caused by mutations in RBM20 has been reported [ 177 ]. The RBM20 R634Q and RBM20 R636S mutant iPSCs were corrected by ABE and PE, with the efficiency of 92% and 40%, respectively. In addition, AAV9-mediated systemic delivery of ABE components corrected 66% of the RBM20 transcripts expressed in cardiomyocytes of postnatal RBM R636Q/R636Q mice. The corrected mice showed restored cardiac size and function, and prolonged life span [ 177 ].
Cardiac arrhythmia
Cardiac arrhythmia caused by autosomal-dominant mutations can be treated with CRISPR-mediated specific disruption of the mutant allele, which has been validated in several mouse models [ 186 , 187 ]. Recently, humanized mice expressing a human mutant PLN (hPLN-R14del) demonstrated bi-ventricular dilation and a higher propensity for sustained ventricular tachycardia. Disruption of the hPLN-R14del allele by AAV9-CRISPR-Cas9 improved cardiac function and reduced sustained ventricular tachycardia susceptibility in young adult humanized PLN-R14del mice, providing a potential therapeutic strategy for the arrhythmogenic phenotype in human patients with the PLN-R14del mutation [ 178 ].
Atherosclerosis
Atherosclerosis is a chronic disease that refers to the formation of fibrofatty lesions in the arterial wall, and causes ischemic stroke, ischemic cardiomyopathy, myocardial infarction, and peripheral arterial disease. Blood concentration of low-density lipoprotein cholesterol (LDL-C) is one of the best-established causal risk factors for atherosclerosis.
The lipid metabolism-related gene, Pcsk9 , is specifically expressed in the liver and functions primarily as an antagonist to the LDL receptor. Disruption of PCSK9 activity can reduce circulating LDL-C levels, thereby lowering the risk of atherosclerosis [ 188 ]. Several clinical trials have investigated monoclonal antibodies targeting PCSK9. However, even if these antibody-based drugs are effective, their effect on LDL-C is short-lived. Genome editing using CRISPR systems provides an alternative method for reducing PCSK9 levels. A single administration of adenovirus co-expressing SpCas9 and sgRNA targeting exon 1 of the mouse Pcsk9 gene can efficiently introduce loss-of-function mutations into endogenous Pcsk9 genes in vivo and chronically decrease plasma cholesterol levels in the blood [ 179 ]. The AAV-SaCas9 system has also been proven to be effective in editing the Pcsk9 gene in vivo, leading to significantly decreased serum PCSK9 and cholesterol levels [ 30 ]. Single injections of engineered DNA-free VLPs targeting the Pcsk9 gene into adult mice demonstrated 63% base editing in the liver, resulting in 78% reduction in serum PCSK9 levels [ 126 ]. In addition to these studies carried out in rodents, somatic Pcsk9 gene editing has also been validated in nonhuman primates [ 180 , 181 ]. CRISPR base editors delivered using LNPs proved highly effective in editing the Pcsk9 gene in the liver of macaques and cynomolgus monkeys. A single-dose treatment of LNPs carrying CRISPR base editors leads to stable Pcsk9 knockdown in the liver and a 60% reduction in blood LDL-C for at least 8 months [ 180 ]. To ensure the safety of gene editing in comparatively more proliferative organs, such as the liver, the proportion of edited cells that remain stable over time must be investigated. All these genome editing approaches offer the potential for once-and-done therapies for the lifelong treatment of atherosclerosis-associated CVD.
Perspectives
CRISPR-based genome editing technology has been rapidly applied in almost all fields, from basic biology to translational medicine. The development of novel systems and tools for more accurate, efficient, and faster genome editing and tighter control of the duration, efficiency, and specificity of genome editors will further benefit their translational applications. Newly uncovered thousands of phage-encoded CRISPR systems provide a valuable resource for searching novel miniature single-effector CRISPR-Cas systems [ 39 ]. In addition, newly developed Cas13a-based RNA editing tools can achieve RNA knockdown and precise base editing of mammalian transcripts without causing DNA damage, providing a promising potential therapeutic strategy in translational cardiovascular medicine [ 11 , 189 ]. Notably, type III-E CRISPR-Cas7-11 effector has recently been shown to cleavage protein under target RNA guidance [ 190 , 191 ], bringing new potential CRISPR tools for CVD diagnosis and treatment.
Genome editing technologies have been successfully translated into human clinical trials for enhanced chimeric antigen receptor (CAR) T-cell therapy, cell-based regenerative medicine, and treatment of monogenic diseases, such as transfusion-dependent beta thalassemia (TDT) and sickle cell disease (SCD) [ 192 , 193 ]. Researchers have used in vivo genome editing to target the transthyretin (TTR) gene to treat transthyretin amyloidosis and have achieved very encouraging results in phase 1 clinical trials, taking the most critical step towards applying CRISPR-based genome editing technology to treat human genetic diseases [ 123 ]. Taking the most optimistic view, CVD with known causal genes can theoretically be treated with CRISPR technology. However, there are still several important challenges. Recently, CRISPR-based genome editing in human embryos was shown to cause unpredictable genomic alterations, including DNA rearrangements, large deletions, and even loss of allele-specific chromosomes [ 168 , 194 , 195 ]. Therefore, potential technical safety concerns, including mosaicism, off-target effects, and long-term risks caused by genome editing, need to be addressed before the therapeutic applications of CRISPR technology in treating CVD. Perhaps striking a balance between the efficiency and safety of genome editing is crucial. At present, efficient delivery of CRISPR-Cas systems to human cardiovascular system remains a challenge. In addition, the efficacy and safety of each therapeutic gene editing strategy for each CVD need to be confirmed by clinical trials. Although this paper uses the CVD as example to illustrate the progress of CRISPR-based genome editing in modeling and treating diseases, the same strategies could also be used for numerous diseases in other tissues.
Like any cutting-edge technology, gene editing technology could be a double-edged sword. Genome editing has been listed as a potential weapon of mass destruction in the 2016 annual worldwide threat assessment report of the U.S. intelligence community, indicating a high risk of extreme misuse. Recent rapid advances have made genome editing technologies more accessible and difficult to control, which may further lower the threshold for genome editing misuse and increase biosecurity threats. The possible misuse of genome editing technology and biosecurity risks may include, but are not limited to creating (1) pathogens with increased virulence, (2) new pathogens and biotoxins, and (3) gene-driven animals that may have irreversible effects on specific populations and the environment. Regulations and guidelines should be developed after extensive consultation to ensure that the development of gene editing technologies will not harm living organisms, including humans, or the environment.
Conclusions
The emerging novel Cas nucleases and their extended applications have greatly expanded the CRISPR-based genome editing toolbox and promoted the development of life science and medicine. CRISPR-based genome editing technology has also revolutionized cardiovascular research, accelerating the generation of genetically modified models of CVD and its application in the treatment of different types of CVD. However, this technology may also bring huge potential biological threats, which should be strictly controlled to prevent its abuse.
Availability of data and materials
Not applicable.
Abbreviations
Adeno-associated virus
Adenine base editor
Blood–brain barrier
Clustered regularly interspaced short palindromic repeats
CRISPR-associated protein
- Cardiovascular disease
CRISPR-associated complex for antiviral defense
Cytosine base editor
C-to-G base editor
CRISPR-associated transposon
Chimeric antigen receptor
Dilated cardiomyopathy
Duchenne muscular dystrophy
Double-strand break
Endothelial cell
Find and cut-and-transfer
Human induced pluripotent stem cell
Hypertrophic cardiomyopathy
Homology-directed repair
Lipid nanoparticle
Low-density lipoprotein cholesterol
Moloney murine leukemia virus
Non-homologous end-joining
Open reading frame
Obligate mobile element-guided activity
Protospacer adjacent motif
Prime editor
Prime editing gRNA
Ribonucleoprotein complexes
Single guide RNA
Single-stranded oligodeoxynucleotide
Sickle cell disease
Trans-activating CRISPR RNA
Transposon-associated motif
Transfusion-dependent beta thalassemia
Uracil-DNA glycosylase inhibitor
Uracil-DNA glycosylase
Virus-like particle
Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346(6213):1258096.
Article PubMed Google Scholar
Doudna JA. The promise and challenge of therapeutic genome editing. Nature. 2020;578(7794):229–36.
Article CAS PubMed PubMed Central Google Scholar
Nambiar TS, Baudrier L, Billon P, Ciccia A. CRISPR-based genome editing through the lens of DNA repair. Mol Cell. 2022;82(2):348–88.
Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157(6):1262–78.
Komor AC, Badran AH, Liu DR. CRISPR-based technologies for the manipulation of eukaryotic genomes. Cell. 2017;169(3):559.
Article CAS PubMed Google Scholar
Knott GJ, Doudna JA. CRISPR-Cas guides the future of genetic engineering. Science. 2018;361(6405):866–9.
Li G, Li X, Zhuang S, Wang L, Zhu Y, Chen Y, et al. Gene editing and its applications in biomedicine. Sci China Life Sci. 2022;65(4):660–700.
Yang X. Applications of CRISPR-Cas9 mediated genome engineering. Mil Med Res. 2015;2:11.
PubMed PubMed Central Google Scholar
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–23.
Liu G, Lin Q, Jin S, Gao C. The CRISPR-Cas toolbox and gene editing technologies. Mol Cell. 2022;82(2):333–47.
Pickar-Oliver A, Gersbach CA. The next generation of CRISPR-Cas technologies and applications. Nat Rev Mol Cell Biol. 2019;20(8):490–507.
Porteus MH. A new class of medicines through DNA editing. N Engl J Med. 2019;380(10):947–59.
Yeh CD, Richardson CD, Corn JE. Advances in genome editing through control of DNA repair pathways. Nat Cell Biol. 2019;21(12):1468–78.
Clark JF, Dinsmore CJ, Soriano P. A most formidable arsenal: genetic technologies for building a better mouse. Genes Dev. 2020;34(19–20):1256–86.
Nishiga M, Liu C, Qi LS, Wu JC. The use of new CRISPR tools in cardiovascular research and medicine. Nat Rev Cardiol. 2022;19(8):505–21.
Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature. 2016;533(7603):420–4.
Gaudelli NM, Komor AC, Rees HA, Packer MS, Badran AH, Bryson DI, et al. Programmable base editing of A · T to G · C in genomic DNA without DNA cleavage. Nature. 2017;551(7681):464–71.
Anzalone AV, Randolph PB, Davis JR, Sousa AA, Koblan LW, Levy JM, et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature. 2019;576(7785):149–57.
Watkins WS, Hernandez EJ, Wesolowski S, Bisgrove BW, Sunderland RT, Lin E, et al. De novo and recessive forms of congenital heart disease have distinct genetic and phenotypic landscapes. Nat Commun. 2019;10(1):4722.
Article PubMed PubMed Central Google Scholar
Jin SC, Homsy J, Zaidi S, Lu Q, Morton S, Depalma SR, et al. Contribution of rare inherited and de novo variants in 2,871 congenital heart disease probands. Nat Genet. 2017;49(11):1593–601.
Makarova KS, Wolf YI, Alkhnbashi OS, Costa F, Shah SA, Saunders SJ, et al. An updated evolutionary classification of CRISPR-Cas systems. Nat Rev Microbiol. 2015;13(11):722–36.
Dolan AE, Hou Z, Xiao Y, Gramelspacher MJ, Heo J, Howden SE, et al. Introducing a spectrum of long-range genomic deletions in human embryonic stem cells using type I CRISPR-Cas. Mol Cell. 2019;74(5):936-50.e5.
Morisaka H, Yoshimi K, Okuzaki Y, Gee P, Kunihiro Y, Sonpho E, et al. CRISPR-Cas3 induces broad and unidirectional genome editing in human cells. Nat Commun. 2019;10(1):5302.
Osakabe K, Wada N, Murakami E, Miyashita N, Osakabe Y. Genome editing in mammalian cells using the CRISPR type I-D nuclease. Nucleic Acids Res. 2021;49(11):6347–63.
Tan R, Krueger RK, Gramelspacher MJ, Zhou X, Xiao Y, Ke A, et al. Cas11 enables genome engineering in human cells with compact CRISPR-Cas3 systems. Mol Cell. 2022;82(4):852-67.e5.
Liu TY, Doudna JA. Chemistry of class 1 CRISPR-Cas effectors: binding, editing, and regulation. J Biol Chem. 2020;295(42):14473–87.
Altae-Tran H, Kannan S, Demircioglu FE, Oshiro R, Nety SP, Mckay LJ, et al. The widespread IS200/IS605 transposon family encodes diverse programmable RNA-guided endonucleases. Science. 2021;374(6563):57–65.
Karvelis T, Druteika G, Bigelyte G, Budre K, Zedaveinyte R, Silanskas A, et al. Transposon-associated TnpB is a programmable RNA-guided DNA endonuclease. Nature. 2021;599(7886):692–6.
Schuler G, Hu C, Ke A. Structural basis for RNA-guided DNA cleavage by IscB-ωRNA and mechanistic comparison with Cas9. Science. 2022;376(6600):1476–81.
Ran FA, Cong L, Yan WX, Scott DA, Gootenberg JS, Kriz AJ, et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520(7546):186–91.
Hou Z, Zhang Y, Propson NE, Howden SE, Chu LF, Sontheimer EJ, et al. Efficient genome engineering in human pluripotent stem cells using Cas9 from Neisseria meningitidis . Proc Natl Acad Sci U S A. 2013;110(39):15644–9.
Kim E, Koo T, Park SW, Kim D, Kim K, Cho HY, et al. In vivo genome editing with a small Cas9 orthologue derived from Campylobacter jejuni . Nat Commun. 2017;8:14500.
Edraki A, Mir A, Ibraheim R, Gainetdinov I, Yoon Y, Song CQ, et al. A compact, high-accuracy Cas9 with a dinucleotide PAM for in vivo genome editing. Mol Cell. 2019;73(4):714-26 e4.
Liu JJ, Orlova N, Oakes BL, Ma E, Spinner HB, Baney KLM, et al. CasX enzymes comprise a distinct family of RNA-guided genome editors. Nature. 2019;566(7743):218–23.
Kim DY, Lee JM, Moon SB, Chin HJ, Park S, Lim Y, et al. Efficient CRISPR editing with a hypercompact Cas12f1 and engineered guide RNAs delivered by adeno-associated virus. Nat Biotechnol. 2022;40(1):94–102.
Wu Z, Zhang Y, Yu H, Pan D, Wang Y, Wang Y, et al. Programmed genome editing by a miniature CRISPR-Cas12f nuclease. Nat Chem Biol. 2021;17(11):1132–8.
Xu X, Chemparathy A, Zeng L, Kempton HR, Shang S, Nakamura M, et al. Engineered miniature CRISPR-Cas system for mammalian genome regulation and editing. Mol Cell. 2021;81(20):4333-45.e4
Pausch P, Al-Shayeb B, Bisom-Rapp E, Tsuchida CA, Li Z, Cress BF, et al. CRISPR-CasΦ from huge phages is a hypercompact genome editor. Science. 2020;369(6501):333–7.
Al-Shayeb B, Skopintsev P, Soczek KM, Stahl EC, Li Z, Groover E, et al. Diverse virus-encoded CRISPR-Cas systems include streamlined genome editors. Cell. 2022;185(24):4574-86.e16.
Collias D, Beisel CL. CRISPR technologies and the search for the PAM-free nuclease. Nat Commun. 2021;12(1):555.
Miller SM, Wang T, Randolph PB, Arbab M, Shen MW, Huang TP, et al. Continuous evolution of SpCas9 variants compatible with non-G PAMs. Nat Biotechnol. 2020;38(4):471–81.
Walton RT, Christie KA, Whittaker MN, Kleinstiver BP. Unconstrained genome targeting with near-PAMless engineered CRISPR-Cas9 variants. Science. 2020;368(6488):290–6.
Kleinstiver BP, Sousa AA, Walton RT, Tak YE, Hsu JY, Clement K, et al. Engineered CRISPR-Cas12a variants with increased activities and improved targeting ranges for gene, epigenetic and base editing. Nat Biotechnol. 2019;37(3):276–82.
Tóth E, Varga É, Kulcsár PI, Kocsis-Jutka V, Krausz SL, Nyeste A, et al. Improved LbCas12a variants with altered PAM specificities further broaden the genome targeting range of Cas12a nucleases. Nucleic Acids Res. 2020;48(7):3722–33.
Chatterjee P, Jakimo N, Lee J, Amrani N, Rodriguez T, Koseki SRT, et al. An engineered ScCas9 with broad PAM range and high specificity and activity. Nat Biotechnol. 2020;38(10):1154–8.
Chatterjee P, Lee J, Nip L, Koseki SRT, Tysinger E, Sontheimer EJ, et al. A Cas9 with PAM recognition for adenine dinucleotides. Nat Commun. 2020;11(1):2474.
Ma D, Xu Z, Zhang Z, Chen X, Zeng X, Zhang Y, et al. Engineer chimeric Cas9 to expand PAM recognition based on evolutionary information. Nat Commun. 2019;10(1):560.
Liu RM, Liang LL, Freed E, Chang H, Oh E, Liu ZY, et al. Synthetic chimeric nucleases function for efficient genome editing. Nat Commun. 2019;10(1):5524.
Slaymaker IM, Gao L, Zetsche B, Scott DA, Yan WX, Zhang F. Rationally engineered Cas9 nucleases with improved specificity. Science. 2016;351(6268):84–8.
Kleinstiver BP, Pattanayak V, Prew MS, Tsai SQ, Nguyen NT, Zheng Z, et al. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature. 2016;529(7587):490–5.
Chen JS, Dagdas YS, Kleinstiver BP, Welch MM, Sousa AA, Harrington LB, et al. Enhanced proofreading governs CRISPR-Cas9 targeting accuracy. Nature. 2017;550(7676):407–10.
Casini A, Olivieri M, Petris G, Montagna C, Reginato G, Maule G, et al. A highly specific SpCas9 variant is identified by in vivo screening in yeast. Nat Biotechnol. 2018;36(3):265–71.
Vakulskas CA, Dever DP, Rettig GR, Turk R, Jacobi AM, Collingwood MA, et al. A high-fidelity Cas9 mutant delivered as a ribonucleoprotein complex enables efficient gene editing in human hematopoietic stem and progenitor cells. Nat Med. 2018;24(8):1216–24.
Lee JK, Jeong E, Lee J, Jung M, Shin E, Kim YH, et al. Directed evolution of CRISPR-Cas9 to increase its specificity. Nat Commun. 2018;9(1):3048.
Tan Y, Chu AHY, Bao S, Hoang DA, Kebede FT, Xiong W, et al. Rationally engineered Staphylococcus aureus Cas9 nucleases with high genome-wide specificity. Proc Natl Acad Sci U S A. 2019;116(42):20969–76.
Xie H, Ge X, Yang F, Wang B, Li S, Duan J, et al. High-fidelity SaCas9 identified by directional screening in human cells. PLoS Biol. 2020;18(7):e3000747.
Bak SY, Jung Y, Park J, Sung K, Jang HK, Bae S, et al. Quantitative assessment of engineered Cas9 variants for target specificity enhancement by single-molecule reaction pathway analysis. Nucleic Acids Res. 2021;49(19):11312–22.
Kim N, Kim HK, Lee S, Seo JH, Choi JW, Park J, et al. Prediction of the sequence-specific cleavage activity of Cas9 variants. Nat Biotechnol. 2020;38(11):1328–36.
Schmid-Burgk JL, Gao L, Li D, Gardner Z, Strecker J, Lash B, et al. Highly parallel profiling of Cas9 variant specificity. Mol Cell. 2020;78(4):794-800.e8.
Bravo JPK, Liu MS, Hibshman GN, Dangerfield TL, Jung K, Mccool RS, et al. Structural basis for mismatch surveillance by CRISPR-Cas9. Nature. 2022;603(7900):343–7.
Zhang L, Rube HT, Vakulskas CA, Behlke MA, Bussemaker HJ, Pufall MA. Systematic in vitro profiling of off-target affinity, cleavage and efficiency for CRISPR enzymes. Nucleic Acids Res. 2020;48(9):5037–53.
Tsuchida CA, Zhang S, Doost MS, Zhao Y, Wang J, O’brien E, et al. Chimeric CRISPR-CasX enzymes and guide RNAs for improved genome editing activity. Mol Cell. 2022;82(6):1199-209.e6.
Richardson CD, Ray GJ, Dewitt MA, Curie GL, Corn JE. Enhancing homology-directed genome editing by catalytically active and inactive CRISPR-Cas9 using asymmetric donor DNA. Nat Biotechnol. 2016;34(3):339–44.
Maruyama T, Dougan SK, Truttmann MC, Bilate AM, Ingram JR, Ploegh HL. Increasing the efficiency of precise genome editing with CRISPR-Cas9 by inhibition of nonhomologous end joining. Nat Biotechnol. 2015;33(5):538–42.
Nishiyama J, Mikuni T, Yasuda R. Virus-mediated genome editing via homology-directed repair in mitotic and postmitotic cells in mammalian brain. Neuron. 2017;96(4):755-68.e5.
Ishizu T, Higo S, Masumura Y, Kohama Y, Shiba M, Higo T, et al. Targeted genome replacement via homology-directed repair in non-dividing cardiomyocytes. Sci Rep. 2017;7(1):9363.
Zheng Y, Vandusen NJ, Butler CE, Ma Q, King JS, Pu WT. Efficient in vivo homology-directed repair within cardiomyocytes. Circulation. 2022;145(10):787–9.
Kosicki M, Tomberg K, Bradley A. Repair of double-strand breaks induced by CRISPR-Cas9 leads to large deletions and complex rearrangements. Nat Biotechnol. 2018;36(8):765–71.
Cullot G, Boutin J, Toutain J, Prat F, Pennamen P, Rooryck C, et al. CRISPR-Cas9 genome editing induces megabase-scale chromosomal truncations. Nat Commun. 2019;10(1):1136.
Liu M, Zhang W, Xin C, Yin J, Shang Y, Ai C, et al. Global detection of DNA repair outcomes induced by CRISPR-Cas9. Nucleic Acids Res. 2021;49(15):8732–42.
Yu C, Liu Y, Ma T, Liu K, Xu S, Zhang Y, et al. Small molecules enhance CRISPR genome editing in pluripotent stem cells. Cell Stem Cell. 2015;16(2):142–7.
Riesenberg S, Maricic T. Targeting repair pathways with small molecules increases precise genome editing in pluripotent stem cells. Nat Commun. 2018;9(1):2164.
Genomes Project C, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. 2015;526(7571):68–74.
Article Google Scholar
Eichler EE. Genetic variation, comparative genomics, and the diagnosis of disease. N Engl J Med. 2019;381(1):64–74.
Anzalone AV, Koblan LW, Liu DR. Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat Biotechnol. 2020;38(7):824–44.
Despres PC, Dube AK, Seki M, Yachie N, Landry CR. Perturbing proteomes at single residue resolution using base editing. Nat Commun. 2020;11(1):1871.
Hanna RE, Hegde M, Fagre CR, Deweirdt PC, Sangree AK, Szegletes Z, et al. Massively parallel assessment of human variants with base editor screens. Cell. 2021;184(4):1064-80.e20.
Cuella-Martin R, Hayward SB, Fan X, Chen X, Huang JW, Taglialatela A, et al. Functional interrogation of DNA damage response variants with base editing screens. Cell. 2021;184(4):1081-97.e19.
Komor AC, Zhao KT, Packer MS, Gaudelli NM, Waterbury AL, Koblan LW, et al. Improved base excision repair inhibition and bacteriophage Mu Gam protein yields C:G-to-T: A base editors with higher efficiency and product purity. Sci Adv. 2017;3(8):eaao4774.
Zafra MP, Schatoff EM, Katti A, Foronda M, Breinig M, Schweitzer AY, et al. Optimized base editors enable efficient editing in cells, organoids and mice. Nat Biotechnol. 2018;36(9):888–93.
Koblan LW, Doman JL, Wilson C, Levy JM, Tay T, Newby GA, et al. Improving cytidine and adenine base editors by expression optimization and ancestral reconstruction. Nat Biotechnol. 2018;36(9):843–6.
Kim YB, Komor AC, Levy JM, Packer MS, Zhao KT, Liu DR. Increasing the genome-targeting scope and precision of base editing with engineered Cas9-cytidine deaminase fusions. Nat Biotechnol. 2017;35(4):371–6.
Li X, Wang Y, Liu Y, Yang B, Wang X, Wei J, et al. Base editing with a Cpf1-cytidine deaminase fusion. Nat Biotechnol. 2018;36(4):324–7.
Thuronyi BW, Koblan LW, Levy JM, Yeh WH, Zheng C, Newby GA, et al. Continuous evolution of base editors with expanded target compatibility and improved activity. Nat Biotechnol. 2019;37(9):1070–9.
Huang TP, Zhao KT, Miller SM, Gaudelli NM, Oakes BL, Fellmann C, et al. Circularly permuted and PAM-modified Cas9 variants broaden the targeting scope of base editors. Nat Biotechnol. 2019;37(6):626–31.
Richter MF, Zhao KT, Eton E, Lapinaite A, Newby GA, Thuronyi BW, et al. Phage-assisted evolution of an adenine base editor with improved Cas domain compatibility and activity. Nat Biotechnol. 2020;38(7):883–91.
Gaudelli NM, Lam DK, Rees HA, Sola-Esteves NM, Barrera LA, Born DA, et al. Directed evolution of adenine base editors with increased activity and therapeutic application. Nat Biotechnol. 2020;38(7):892–900.
Tan J, Zhang F, Karcher D, Bock R. Expanding the genome-targeting scope and the site selectivity of high-precision base editors. Nat Commun. 2020;11(1):629.
Kurt IC, Zhou R, Iyer S, Garcia SP, Miller BR, Langner LM, et al. CRISPR C-to-G base editors for inducing targeted DNA transversions in human cells. Nat Biotechnol. 2021;39(1):41–6.
Zhao D, Li J, Li S, Xin X, Hu M, Price MA, et al. Glycosylase base editors enable C-to-A and C-to-G base changes. Nat Biotechnol. 2021;39(1):35–40.
Koblan LW, Arbab M, Shen MW, Hussmann JA, Anzalone AV, Doman JL, et al. Efficient C · G-to-G · C base editors developed using CRISPRi screens, target-library analysis, and machine learning. Nat Biotechnol. 2021;39(11):1414–25.
Yuan T, Yan N, Fei T, Zheng J, Meng J, Li N, et al. Optimization of C-to-G base editors with sequence context preference predictable by machine learning methods. Nat Commun. 2021;12(1):4902.
Zuo E, Sun Y, Wei W, Yuan T, Ying W, Sun H, et al. Cytosine base editor generates substantial off-target single-nucleotide variants in mouse embryos. Science. 2019;364(6437):289–92.
Mcgrath E, Shin H, Zhang L, Phue JN, Wu WW, Shen RF, et al. Targeting specificity of APOBEC-based cytosine base editor in human iPSCs determined by whole genome sequencing. Nat Commun. 2019;10(1):5353.
Grunewald J, Zhou R, Garcia SP, Iyer S, Lareau CA, Aryee MJ, et al. Transcriptome-wide off-target RNA editing induced by CRISPR-guided DNA base editors. Nature. 2019;569(7756):433–7.
Zhou C, Sun Y, Yan R, Liu Y, Zuo E, Gu C, et al. Off-target RNA mutation induced by DNA base editing and its elimination by mutagenesis. Nature. 2019;571(7764):275–8.
Doman JL, Raguram A, Newby GA, Liu DR. Evaluation and minimization of Cas9-independent off-target DNA editing by cytosine base editors. Nat Biotechnol. 2020;38(5):620–8.
Zuo E, Sun Y, Yuan T, He B, Zhou C, Ying W, et al. A rationally engineered cytosine base editor retains high on-target activity while reducing both DNA and RNA off-target effects. Nat Methods. 2020;17(6):600–4.
Wang L, Xue W, Zhang H, Gao R, Qiu H, Wei J, et al. Eliminating base-editor-induced genome-wide and transcriptome-wide off-target mutations. Nat Cell Biol. 2021;23(5):552–63.
Chen PJ, Hussmann JA, Yan J, Knipping F, Ravisankar P, Chen PF, et al. Enhanced prime editing systems by manipulating cellular determinants of editing outcomes. Cell. 2021;184(22):5635-52.e29.
Nelson JW, Randolph PB, Shen SP, Everette KA, Chen PJ, Anzalone AV, et al. Engineered pegRNAs improve prime editing efficiency. Nat Biotechnol. 2022;40(3):402–10.
Song M, Lim JM, Min S, Oh JS, Kim DY, Woo JS, et al. Generation of a more efficient prime editor 2 by addition of the Rad51 DNA-binding domain. Nat Commun. 2021;12(1):5617.
Park SJ, Jeong TY, Shin SK, Yoon DE, Lim SY, Kim SP, et al. Targeted mutagenesis in mouse cells and embryos using an enhanced prime editor. Genome Biol. 2021;22(1):170.
Zong Y, Liu Y, Xue C, Li B, Li X, Wang Y, et al. An engineered prime editor with enhanced editing efficiency in plants. Nat Biotechnol. 2022;40(9):1394–402.
Jiang YY, Chai YP, Lu MH, Han XL, Lin Q, Zhang Y, et al. Prime editing efficiently generates W542L and S621I double mutations in two ALS genes in maize. Genome Biol. 2020;21(1):257.
Choi J, Chen W, Suiter CC, Lee C, Chardon FM, Yang W, et al. Precise genomic deletions using paired prime editing. Nat Biotechnol. 2022;40(2):218–26.
Lin Q, Jin S, Zong Y, Yu H, Zhu Z, Liu G, et al. High-efficiency prime editing with optimized, paired pegRNAs in plants. Nat Biotechnol. 2021;39(8):923–7.
Anzalone AV, Gao XD, Podracky CJ, Nelson AT, Koblan LW, Raguram A, et al. Programmable deletion, replacement, integration and inversion of large DNA sequences with twin prime editing. Nat Biotechnol. 2021;40(5):731–40.
Jiang T, Zhang XO, Weng Z, Xue W. Deletion and replacement of long genomic sequences using prime editing. Nat Biotechnol. 2022;40(2):227–34.
Zhuang Y, Liu J, Wu H, Zhu Q, Yan Y, Meng H, et al. Increasing the efficiency and precision of prime editing with guide RNA pairs. Nat Chem Biol. 2022;18(1):29–37.
Rybarski JR, Hu K, Hill AM, Wilke CO, Finkelstein IJ. Metagenomic discovery of CRISPR-associated transposons. Proc Natl Acad Sci U S A. 2021;118(49):e2112279118.
Klompe SE, Jaber N, Beh LY, Mohabir JT, Bernheim A, Sternberg SH. Evolutionary and mechanistic diversity of type I-F CRISPR-associated transposons. Mol Cell. 2022;82(3):616-28.e5.
Klompe SE, Vo PLH, Halpin-Healy TS, Sternberg SH. Transposon-encoded CRISPR-Cas systems direct RNA-guided DNA integration. Nature. 2019;571(7764):219–25.
Strecker J, Ladha A, Gardner Z, Schmid-Burgk JL, Makarova KS, Koonin EV, et al. RNA-guided DNA insertion with CRISPR-associated transposases. Science. 2019;365(6448):48–53.
Saito M, Ladha A, Strecker J, Faure G, Neumann E, Altae-Tran H, et al. Dual modes of CRISPR-associated transposon homing. Cell. 2021;184(9):2441-53.e18.
Vo PLH, Ronda C, Klompe SE, Chen EE, Acree C, Wang HH, et al. CRISPR RNA-guided integrases for high-efficiency, multiplexed bacterial genome engineering. Nat Biotechnol. 2021;39(4):480–9.
Pallarès-Masmitjà M, Ivančić D, Mir-Pedrol J, Jaraba-Wallace J, Tagliani T, Oliva B, et al. Find and cut-and-transfer (FiCAT) mammalian genome engineering. Nat Commun. 2021;12(1):7071.
Raguram A, Banskota S, Liu DR. Therapeutic in vivo delivery of gene editing agents. Cell. 2022;185(15):2806–27.
Wang D, Zhang F, Gao G. CRISPR-based therapeutic genome editing: strategies and in vivo delivery by AAV vectors. Cell. 2020;181(1):136–50.
Newby GA, Yen JS, Woodard KJ, Mayuranathan T, Lazzarotto CR, Li Y, et al. Base editing of haematopoietic stem cells rescues sickle cell disease in mice. Nature. 2021;595(7866):295–302.
Lattanzi A, Camarena J, Lahiri P, Segal H, Srifa W, Vakulskas CA, et al. Development of β-globin gene correction in human hematopoietic stem cells as a potential durable treatment for sickle cell disease. Sci Transl Med. 2021;13(598):eabf2444.
Zhang J, Hu Y, Yang J, Li W, Zhang M, Wang Q, et al. Non-viral, specifically targeted CAR-T cells achieve high safety and efficacy in B-NHL. Nature. 2022;609(7926):369–74.
Gillmore JD, Gane E, Taubel J, Kao J, Fontana M, Maitland ML, et al. CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis. N Engl J Med. 2021;385(6):493–502.
Mendell JR, Al-Zaidy SA, Rodino-Klapac LR, Goodspeed K, Gray SJ, Kay CN, et al. Current clinical applications of in vivo gene therapy with AAVs. Mol Ther. 2021;29(2):464–88.
Chandler RJ, Sands MS, Venditti CP. Recombinant adeno-associated viral integration and genotoxicity: insights from animal models. Hum Gene Ther. 2017;28(4):314–22.
Banskota S, Raguram A, Suh S, Du SW, Davis JR, Choi EH, et al. Engineered virus-like particles for efficient in vivo delivery of therapeutic proteins. Cell. 2022;185(2):250-65.e16.
Sago CD, Lokugamage MP, Paunovska K, Vanover DA, Monaco CM, Shah NN, et al. High-throughput in vivo screen of functional mRNA delivery identifies nanoparticles for endothelial cell gene editing. Proc Natl Acad Sci U S A. 2018;115(42):E9944–52.
Ni H, Hatit MZC, Zhao K, Loughrey D, Lokugamage MP, Peck HE, et al. Piperazine-derived lipid nanoparticles deliver mRNA to immune cells in vivo. Nat Commun. 2022;13(1):4766.
Sago CD, Lokugamage MP, Loughrey D, Lindsay KE, Hincapie R, Krupczak BR, et al. Augmented lipid-nanoparticle-mediated in vivo genome editing in the lungs and spleen by disrupting Cas9 activity in the liver. Nat Biomed Eng. 2022;6(2):157–67.
Lyu P, Wang L, Lu B. Virus-like particle mediated CRISPR/Cas9 delivery for efficient and safe genome editing. Life (Basel). 2020;10(12):366.
CAS PubMed Google Scholar
Lu Z, Yao X, Lyu P, Yadav M, Yoo K, Atala A, et al. Lentiviral capsid-mediated streptococcus pyogenes Cas9 ribonucleoprotein delivery for efficient and safe multiplex genome editing. CRISPR J. 2021;4(6):914–28.
Hamilton JR, Tsuchida CA, Nguyen DN, Shy BR, Mcgarrigle ER, Sandoval Espinoza CR, et al. Targeted delivery of CRISPR-Cas9 and transgenes enables complex immune cell engineering. Cell Rep. 2021;35(9):109207.
Lyu P, Lu Z, Cho SI, Yadav M, Yoo KW, Atala A, et al. Adenine base editor ribonucleoproteins delivered by lentivirus-like particles show high on-target base editing and undetectable RNA off-target activities. CRISPR J. 2021;4(1):69–81.
Segel M, Lash B, Song J, Ladha A, Liu CC, Jin X, et al. Mammalian retrovirus-like protein PEG10 packages its own mRNA and can be pseudotyped for mRNA delivery. Science. 2021;373(6557):882–9.
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021.
Leopold JA, Loscalzo J. Emerging role of precision medicine in cardiovascular disease. Circ Res. 2018;122(9):1302–15.
Madsen A, Höppner G, Krause J, Hirt MN, Laufer SD, Schweizer M, et al. An important role for DNMT3A-mediated DNA methylation in cardiomyocyte metabolism and contractility. Circulation. 2020;142(16):1562–78.
Bengel P, Dybkova N, Tirilomis P, Ahmad S, Hartmann N, Mohamed BA, et al. Detrimental proarrhythmogenic interaction of Ca 2+ /calmodulin-dependent protein kinase II and NaV1.8 in heart failure. Nat Commun. 2021;12(1):6586.
Levitas A, Muhammad E, Zhang Y, Perea Gil I, Serrano R, Diaz N, et al. A novel recessive mutation in SPEG causes early onset dilated cardiomyopathy. PLoS Genet. 2020;16(9):e1009000.
Fomin A, Gärtner A, Cyganek L, Tiburcy M, Tuleta I, Wellers L, et al. Truncated titin proteins and titin haploinsufficiency are targets for functional recovery in human cardiomyopathy due to TTN mutations. Sci Transl Med. 2021;13(618):eabd3079.
Ma S, Jiang W, Liu X, Lu WJ, Qi T, Wei J, et al. Efficient correction of a hypertrophic cardiomyopathy mutation by ABEmax-NG. Circ Res. 2021;129(10):895–908.
Huang JY, Kan SH, Sandfeld EK, Dalton ND, Rangel AD, Chan Y, et al. CRISPR-Cas9 generated Pompe knock-in murine model exhibits early-onset hypertrophic cardiomyopathy and skeletal muscle weakness. Sci Rep. 2020;10(1):10321.
Ercu M, Markó L, Schächterle C, Tsvetkov D, Cui Y, Maghsodi S, et al. Phosphodiesterase 3A and arterial hypertension. Circulation. 2020;142(2):133–49.
Min YL, Li H, Rodriguez-Caycedo C, Mireault AA, Huang J, Shelton JM, et al. CRISPR-Cas9 corrects Duchenne muscular dystrophy exon 44 deletion mutations in mice and human cells. Sci Adv. 2019;5(3):eaav4324.
Magdy T, Jouni M, Kuo HH, Weddle CJ, Lyra-Leite D, Fonoudi H, et al. Identification of drug transporter genomic variants and inhibitors that protect against doxorubicin-induced cardiotoxicity. Circulation. 2022;145(4):279–94.
Chemello F, Chai AC, Li H, Rodriguez-Caycedo C, Sanchez-Ortiz E, Atmanli A, et al. Precise correction of Duchenne muscular dystrophy exon deletion mutations by base and prime editing. Sci Adv. 2021;7(18):eabg4910.
Johansen AK, Molenaar B, Versteeg D, Leitoguinho AR, Demkes C, Spanjaard B, et al. Postnatal cardiac gene editing using CRISPR/Cas9 with AAV9-mediated delivery of short guide RNAs results in mosaic gene disruption. Circ Res. 2017;121(10):1168–81.
Song X, Cui Y, Wang Y, Zhang Y, He Q, Yu Z, et al. Genome editing with AAV-BR1-CRISPR in postnatal mouse brain endothelial cells. Int J Biol Sci. 2022;18(2):652–60.
Xu L, Lau YS, Gao Y, Li H, Han R. Life-long AAV-mediated CRISPR genome editing in dystrophic heart improves cardiomyopathy without causing serious lesions in mdx mice. Mol Ther. 2019;27(8):1407–14.
Nelson CE, Wu Y, Gemberling MP, Oliver ML, Waller MA, Bohning JD, et al. Long-term evaluation of AAV-CRISPR genome editing for Duchenne muscular dystrophy. Nat Med. 2019;25(3):427–32.
Liu N, Olson EN. CRISPR modeling and correction of cardiovascular disease. Circ Res. 2022;130(12):1827–50.
Heimlich JB, Bick AG. Somatic mutations in cardiovascular disease. Circ Res. 2022;130(1):149–61.
Strong A, Musunuru K. Genome editing in cardiovascular diseases. Nat Rev Cardiol. 2017;14(1):11–20.
Article CAS Google Scholar
Belbachir N, Portero V, Al Sayed ZR, Gourraud JB, Dilasser F, Jesel L, et al. RRAD mutation causes electrical and cytoskeletal defects in cardiomyocytes derived from a familial case of Brugada syndrome. Eur Heart J. 2019;40(37):3081–94.
Zhang XH, Wei H, Xia Y, Morad M. Calcium signaling consequences of RyR2 mutations associated with CPVT1 introduced via CRISPR/Cas9 gene editing in human-induced pluripotent stem cell-derived cardiomyocytes: comparison of RyR2-R420Q, F2483I, and Q4201R. Heart Rhythm. 2021;18(2):250–60.
Pettinato AM, Ladha FA, Mellert DJ, Legere N, Cohn R, Romano R, et al. Development of a cardiac sarcomere functional genomics platform to enable scalable interrogation of uhman TNNT2 variants. Circulation. 2020;142(23):2262–75.
Guo F, Sun Y, Wang X, Wang H, Wang J, Gong T, et al. Patient-specific and gene-corrected induced pluripotent stem cell-derived cardiomyocytes elucidate single-cell phenotype of short QT syndrome. Circ Res. 2019;124(1):66–78.
Dotzler SM, Kim CSJ, Genfrom WAC, Zhou W, Ye D, Bos JM, et al. Suppression-replacement KCNQ1 gene therapy for type 1 long QT syndrome. Circulation. 2021;143(14):1411–25.
Lambert M, Capuano V, Boet A, Tesson L, Bertero T, Nakhleh MK, et al. Characterization of Kcnk3-mutated rat, a novel model of pulmonary hypertension. Circ Res. 2019;125(7):678–95.
Li J, Wang K, Zhang Y, Qi T, Yuan J, Zhang L, et al. Therapeutic exon skipping through a CRISPR-guided cytidine deaminase rescues dystrophic cardiomyopathy in vivo. Circulation. 2021;144(22):1760–76.
Li W, Tran V, Shaked I, Xue B, Moore T, Lightle R, et al. Abortive intussusceptive angiogenesis causes multi-cavernous vascular malformations. Elife. 2021;10:e62155.
Carroll KJ, Makarewich CA, Mcanally J, Anderson DM, Zentilin L, Liu N, et al. A mouse model for adult cardiac-specific gene deletion with CRISPR/Cas9. Proc Natl Acad Sci U S A. 2016;113(2):338–43.
Schoger E, Carroll KJ, Iyer LM, Mcanally JR, Tan W, Liu N, et al. CRISPR-mediated activation of endogenous gene expression in the postnatal heart. Circ Res. 2020;126(1):6–24.
Zhu W, Saw D, Weiss M, Sun Z, Wei M, Shaligram S, et al. Induction of brain arteriovenous malformation through CRISPR/Cas9-mediated somatic Alk1 gene mutations in adult mice. Transl Stroke Res. 2019;10(5):557–65.
Zhang X, Jin H, Huang X, Chaurasiya B, Dong D, Shanley TP, et al. Robust genome editing in adult vascular endothelium by nanoparticle delivery of CRISPR-Cas9 plasmid DNA. Cell Rep. 2022;38(1): 110196.
Liang P, Xu Y, Zhang X, Ding C, Huang R, Zhang Z, et al. CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein Cell. 2015;6(5):363–72.
Kang X, He W, Huang Y, Yu Q, Chen Y, Gao X, et al. Introducing precise genetic modifications into human 3PN embryos by CRISPR/Cas-mediated genome editing. J Assist Reprod Genet. 2016;33(5):581–8.
Zuccaro MV, Xu J, Mitchell C, Marin D, Zimmerman R, Rana B, et al. Allele-specific chromosome removal after Cas9 cleavage in human embryos. Cell. 2020;183(6):1650-64.e15.
Turocy J, Adashi EY, Egli D. Heritable human genome editing: research progress, ethical considerations, and hurdles to clinical practice. Cell. 2021;184(6):1561–74.
Baylis F, Darnovsky M, Hasson K, Krahn TM. Human germ line and heritable genome editing: the global policy landscape. CRISPR J. 2020;3(5):365–77.
Min YL, Chemello F, Li H, Rodriguez-Caycedo C, Sanchez-Ortiz E, Mireault AA, et al. Correction of three prominent mutations in mouse and human models of Duchenne muscular dystrophy by single-cut genome editing. Mol Ther. 2020;28(9):2044–55.
Moretti A, Fonteyne L, Giesert F, Hoppmann P, Meier AB, Bozoglu T, et al. Somatic gene editing ameliorates skeletal and cardiac muscle failure in pig and human models of Duchenne muscular dystrophy. Nat Med. 2020;26(2):207–14.
Xu L, Zhang C, Li H, Wang P, Gao Y, Mokadam NA, et al. Efficient precise in vivo base editing in adult dystrophic mice. Nat Commun. 2021;12(1):3719.
Hanses U, Kleinsorge M, Roos L, Yigit G, Li Y, Barbarics B, et al. Intronic CRISPR repair in a preclinical model of noonan syndrome-associated cardiomyopathy. Circulation. 2020;142(11):1059–76.
Ma H, Marti-Gutierrez N, Park SW, Wu J, Lee Y, Suzuki K, et al. Correction of a pathogenic gene mutation in human embryos. Nature. 2017;548(7668):413–9.
Romano R, Ghahremani S, Zimmerman T, Legere N, Thakar K, Ladha FA, et al. Reading frame repair of TTN truncation variants restores titin quantity and functions. Circulation. 2022;145(3):194–205.
Nishiyama T, Zhang Y, Cui M, Li H, Sanchez-Ortiz E, Mcanally JR, et al. Precise genomic editing of pathogenic mutations in RBM20 rescues dilated cardiomyopathy. Sci Transl Med. 2022;14(672):eade1633.
Dave J, Raad N, Mittal N, Zhang L, Fargnoli A, Oh JG, et al. Gene editing reverses arrhythmia susceptibility in humanized PLN-R14del mice: modelling a European cardiomyopathy with global impact. Cardiovasc Res. 2022;118(15):3140–50.
Ding Q, Strong A, Patel KM, Ng SL, Gosis BS, Regan SN, et al. Permanent alteration of PCSK9 with in vivo CRISPR-Cas9 genome editing. Circ Res. 2014;115(5):488–92.
Musunuru K, Chadwick AC, Mizoguchi T, Garcia SP, Denizio JE, Reiss CW, et al. In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates. Nature. 2021;593(7859):429–34.
Rothgangl T, Dennis MK, Lin PJC, Oka R, Witzigmann D, Villiger L, et al. In vivo adenine base editing of PCSK9 in macaques reduces LDL cholesterol levels. Nat Biotechnol. 2021;39(8):949–57.
Hoffman EP, Brown RH Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51(6):919–28.
Hinson JT, Chopra A, Nafissi N, Polacheck WJ, Benson CC, Swist S, et al. Heart disease. Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy. Science. 2015;349(6251):982–6.
Chopra A, Kutys ML, Zhang K, Polacheck WJ, Sheng CC, Luu RJ, et al. Force generation via β-cardiac myosin, titin, and α-actinin drives cardiac sarcomere assembly from cell-matrix adhesions. Dev Cell. 2018;44(1):87-96.e5.
Zaunbrecher RJ, Abel AN, Beussman K, Leonard A, Von Frieling-Salewsky M, Fields PA, et al. Cronos titin is expressed in human cardiomyocytes and necessary for normal sarcomere function. Circulation. 2019;140(20):1647–60.
Xie C, Zhang YP, Song L, Luo J, Qi W, Hu J, et al. Genome editing with CRISPR/Cas9 in postnatal mice corrects PRKAG2 cardiac syndrome. Cell Res. 2016;26(10):1099–111.
Pan X, Philippen L, Lahiri SK, Lee C, Park SH, Word TA, et al. In vivo Ryr2 editing corrects catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2018;123(8):953–63.
Libby P. The changing landscape of atherosclerosis. Nature. 2021;592(7855):524–33.
Kannan S, Altae-Tran H, Jin X, Madigan VJ, Oshiro R, Makarova KS, et al. Compact RNA editors with small Cas13 proteins. Nat Biotechnol. 2022;40(2):194–7.
Kato K, Okazaki S, Schmitt-Ulms C, Jiang K, Zhou W, Ishikawa J, et al. RNA-triggered protein cleavage and cell growth arrest by the type III-E CRISPR nuclease-protease. Science. 2022;378(6622):882–9.
Strecker J, Demircioglu FE, Li D, Faure G, Wilkinson ME, Gootenberg JS, et al. RNA-activated protein cleavage with a CRISPR-associated endopeptidase. Science. 2022;378(6622):874–81.
Frangoul H, Altshuler D, Cappellini MD, Chen YS, Domm J, Eustace BK, et al. CRISPR-Cas9 gene editing for sickle cell disease and beta-thalassemia. N Engl J Med. 2021;384(3):252–60.
Stadtmauer EA, Fraietta JA, Davis MM, Cohen AD, Weber KL, Lancaster E, et al. CRISPR-engineered T cells in patients with refractory cancer. Science. 2020;367(6481):eaba7365.
Grunwald HA, Gantz VM, Poplawski G, Xu XS, Bier E, Cooper KL. Super-Mendelian inheritance mediated by CRISPR-Cas9 in the female mouse germline. Nature. 2019;566(7742):105–9.
Alanis-Lobato G, Zohren J, McCarthy A, Fogarty NME, Kubikova N, Hardman E, et al. Frequent loss of heterozygosity in CRISPR-Cas9-edited early human embryos. Proc Natl Acad Sci U S A. 2021;118(22):e2004832117.
Download references
This work was supported by the National Natural Science Foundation of China (82270355, 82270354, 81970134, 82030011, 31630093), and the National Key Research and Development Program of China (2019YFA0801601, 2021YFA1101801).
Author information
Zhen-Hua Li and Jun Wang contributed equally to this work
Authors and Affiliations
State Key Laboratory of Proteomics, Beijing Proteome Research Center, National Center for Protein Sciences, Beijing Institute of Lifeomics, Beijing, 100071, China
Zhen-Hua Li, Jun Wang, Jing-Ping Xu, Jian Wang & Xiao Yang
Yaneng BIOScience (Shenzhen) Co., Ltd., Shenzhen, 518102, Guangdong, China
Jing-Ping Xu
You can also search for this author in PubMed Google Scholar
Contributions
ZHL, JW, JW and XY drafted the manuscript. ZHL and JPX prepared figures. JW and XY conceived and supervised the study. All authors have read and approved the final manuscript.
Corresponding authors
Correspondence to Jian Wang or Xiao Yang .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Li, ZH., Wang, J., Xu, JP. et al. Recent advances in CRISPR-based genome editing technology and its applications in cardiovascular research. Military Med Res 10 , 12 (2023). https://doi.org/10.1186/s40779-023-00447-x
Download citation
Received : 12 September 2022
Accepted : 14 February 2023
Published : 10 March 2023
DOI : https://doi.org/10.1186/s40779-023-00447-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Genome editing
- CRISPR-Cas system
- Base editing
- Prime editing
- Transposon-associated genome editing
- Blood vessel
- Gene therapy
Military Medical Research
ISSN: 2054-9369
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Reference Manager
- Simple TEXT file
People also looked at
Review article, crispr genome editing using computational approaches: a survey.

- 1 Department of Computer Engineering, University of Zanjan, Zanjan, Iran
- 2 Department of Neurozentrum, Universitätsklinikum Freiburg, Freiburg, Germany
Clustered regularly interspaced short palindromic repeats (CRISPR)-based gene editing has been widely used in various cell types and organisms. To make genome editing with Clustered regularly interspaced short palindromic repeats far more precise and practical, we must concentrate on the design of optimal gRNA and the selection of appropriate Cas enzymes. Numerous computational tools have been created in recent years to help researchers design the best gRNA for Clustered regularly interspaced short palindromic repeats researches. There are two approaches for designing an appropriate gRNA sequence (which targets our desired sites with high precision): experimental and predicting-based approaches. It is essential to reduce off-target sites when designing an optimal gRNA. Here we review both traditional and machine learning-based approaches for designing an appropriate gRNA sequence and predicting off-target sites. In this review, we summarize the key characteristics of all available tools (as far as possible) and compare them together. Machine learning-based tools and web servers are believed to become the most effective and reliable methods for predicting on-target and off-target activities of Clustered regularly interspaced short palindromic repeats in the future. However, these predictions are not so precise now and the performance of these algorithms -especially deep learning one’s-depends on the amount of data used during training phase. So, as more features are discovered and incorporated into these models, predictions become more in line with experimental observations. We must concentrate on the creation of ideal gRNA and the choice of suitable Cas enzymes in order to make genome editing with Clustered regularly interspaced short palindromic repeats far more accurate and feasible.
1 Introduction
Over the last decade, the Clustered regularly interspaced short palindromic repeats (CRISPR)/Cas system has become the dominant tool for genome editing due to its simplicity, high performance, accuracy, and programmability ( Gaj et al., 2013 ; Jacquin et al., 2019 ; Afzal et al., 2020 ). In addition, other influential factors such as ease of use, low cost, high speed, multiplex potential, and higher specific DNA targeting ability have increased the success and popularity of CRISPR across the global scientific community ( Mali et al., 2013 ). The unique characteristics of this technology have made it one of the broad topics in molecular biology, synthetic biology, and genetic engineering ( Jinek et al., 2012 ). Gene activation (CRISPRa), gene repression, CRISPR interference (CRISPRi), and epigenome editing are popular tasks in genome engineering using CRISPER. The basic overflow of the CRISPR systems is illustrated in Figure 1 .
FIGURE1 . Basic overflow of CRISPR systems.
As shown in Figure 2 , CRISPR systems have three main components. The first one is a short synthetic guide RNA sequence (gRNA) necessary for Cas binding. The gRNA targets the Cas9 endonuclease (a protein which can cleave the DNA sequences) to define DNA. The gRNA can be supplied as a two-part system consisting of crRNA and tracrRNA, or as a single guide RNA (sgRNA), where the crRNA and tracrRNA are connected by a linker. The target’s recognition is facilitated by the protospacer-adjacent motif (PAM). Cleavage occurs on both strands 3 bp upstream of the PAM.
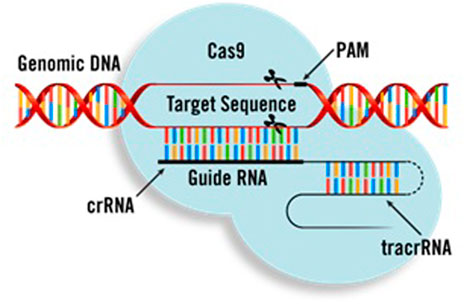
FIGURE 2 . Main components of CRISPR ( Duan et al., 2021 ).
To use CRISPR for genome engineering, we need to select two components: Cas9 and gRNA ( Gasiunas et al., 2012 ; Cox et al., 2015 ). Once a genome modification is decided, the first step is to identify the best site/sites for targeting Cas-induced DSBs ( Jinek et al., 2014 ). The second step is to design the appropriative gRNA ( Cui et al., 2018 ).
After designing gRNA, the only requirement for cleaving a CRISPR target site is finding a 3-base pair (3 bp) PAM. The form of PAM varies depending on the bacterial species of the Cas9 gene. For example, the most commonly used Cas9 nuclease, derived from S.pyogenes, recognizes a PAM sequence of NGG ( Rabinowitz et al., 2020 ). Using the frequency of “GG” = 5.21% in the reference human genome, there would be an expected 161,284,793 NGG PAM sites in the human genome, or roughly one “GG” dinucleotide every 42 bases. So, cleaving unwanted sites, called off-target sites, is very common ( Duan et al., 2021 ). Therefore, CRISPR target sites should be selected in such a way that minimizes potential off-target cleavage ( Herai, 2019 ; Rabinowitz et al., 2020 ). But this is not always straightforward as it is not guaranteed that the desired cleaves will appear on just the selected site. Unfortunately, the existence of these unwanted cleaves is possible in every experiment. Therefore, activity (on-target) and specificity (off-target) are two critical factors considered when designing a genomic edition with CRISPR ( Herai, 2019 ).
According to research, the accuracy of CRISPR-based genomic edition depends on two issues: 1) the choice of Cas enzyme with suitable cutting power, 2) the choice of the appropriate cutting site, which relies on the performance of the gRNA. To achieve this, in the first step, we must select the optimal gRNAs contains high on-target activity and low (no) off-target efficiency ( Moreno-Mateos et al., 2015 ; Luo et al., 2019 ; Manibalan et al., 2020 ). We will discuss this issue later. In the second step, we must select a suitable Cas enzyme [15]. In recent years, different variants of the Cas enzyme have been discovered. We can proceed according to Figure 3 to choose the proper Cas, depending on the type of editing. The choice of the Cas enzyme is effective on the PAM and the gRNA design.
FIGURE 3 . Selection of Cas enzyme.
In recent years, researchers have taken two main approaches for designing gRNAs, including experimental and machine learning-based methods (ML) ( Lin and Luo, 2019 ). ML-based methods utilize the results of computational algorithms trained with real data to predict the effects of gRNAs instead of designing an actual experiment. Experimental methods are very costly and time-consuming ( Chuai et al., 2017 ; Lin and Luo, 2019 ). In contrast, ML models are inexpensive and manageable. However, in terms of accuracy, they are still very different from experimental methods ( Höijer et al., 2020 ). The accuracy of ML methods is highly dependent on the training process and the availability of adequate training data. Recent advances in the genome-wide analyses help researchers to discover all off-target sites, while the detection methods like Polymerase Chain Reaction (PCR) based methods, cannot find all of these sites. Using new sequencing technology, such as next-generation sequencing (NGS), and third generation sequencing which based on long-reads, can help us to detect more off-target sites. Mainly, single-molecule real-time sequencing (SMRT), has shown promising performance in genome sequencing. Researchers use these techniques to find more accurate information about off-target sites and use them in training their computational models ( Lin and Wong, 2018 ; Höijer et al., 2020 ). Also, there are some repetitive, low complexity, AT/GC-rich regions, known as dark, in which ML-based tools cannot predict on-target and off-target sites in these areas. But amplification-free long-read sequencing technology helps to reveal Cas9 target sites even in these dark regions ( Höijer et al., 2020 ). As the number of available features about on-target and off-target sites and the creation of large databases in this field increases, the predictions of ML-based methods become closer to experimental observations ( Jiang et al., 2016 ; Abadi et al., 2017 ).
Some recent research has shown that ML-based methods can determine the extent of effective interactions and side-effects (changing unwanted sites) of each gRNA precisely ( Abadi et al., 2017 ; Lin and Wong, 2018 ). Such a process can significantly accelerate the process of gRNA design for any part of human DNA, thus allowing us to edit anywhere in DNA ( Jiang et al., 2016 ). However, existing models still have challenging issues, such as data imbalance, data heterogeneity, insufficient training data, generalizability, and cross-species inefficiency ( Chuai et al., 2017 ).
We described the basic concepts of CRISPR systems and introduced activity and specificity as two main challenges in this area ( Moreno-Mateos et al., 2015 ; Herai, 2019 ). In the rest of the paper, we provide an overview of computational approaches, especially machine and deep learning (MDL) algorithms, which we believe are the most effective and reliable methods for predicting gRNAs effects. The summary of our review is presented in Tables 1 – Tables3 , only for tools with active access link. Table 1 illustrates computational tools and software packages related to CRISPR systems; Table 2 summarizes tools and software packages related to finding off-target sites; Table 3 shows those related to gRNA design; and finally, Table 4 reports MDL-based tools and software packages related to CRISPR systems.
TABLE 1 . Tools and software packages related to CRISPR systems.
TABLE 2 . Tools and software packages related to finding off-target sites.
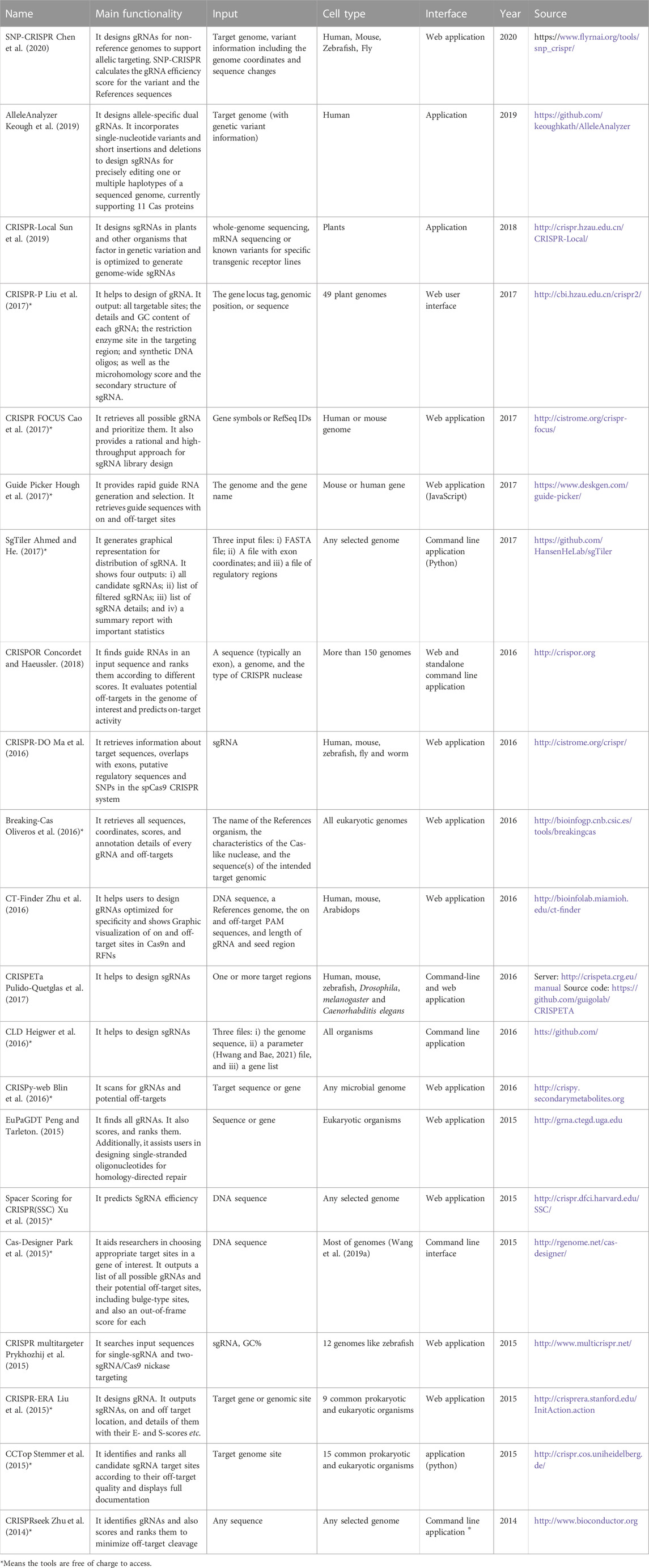
TABLE 3 . Tools and software packages related to gRNA design.
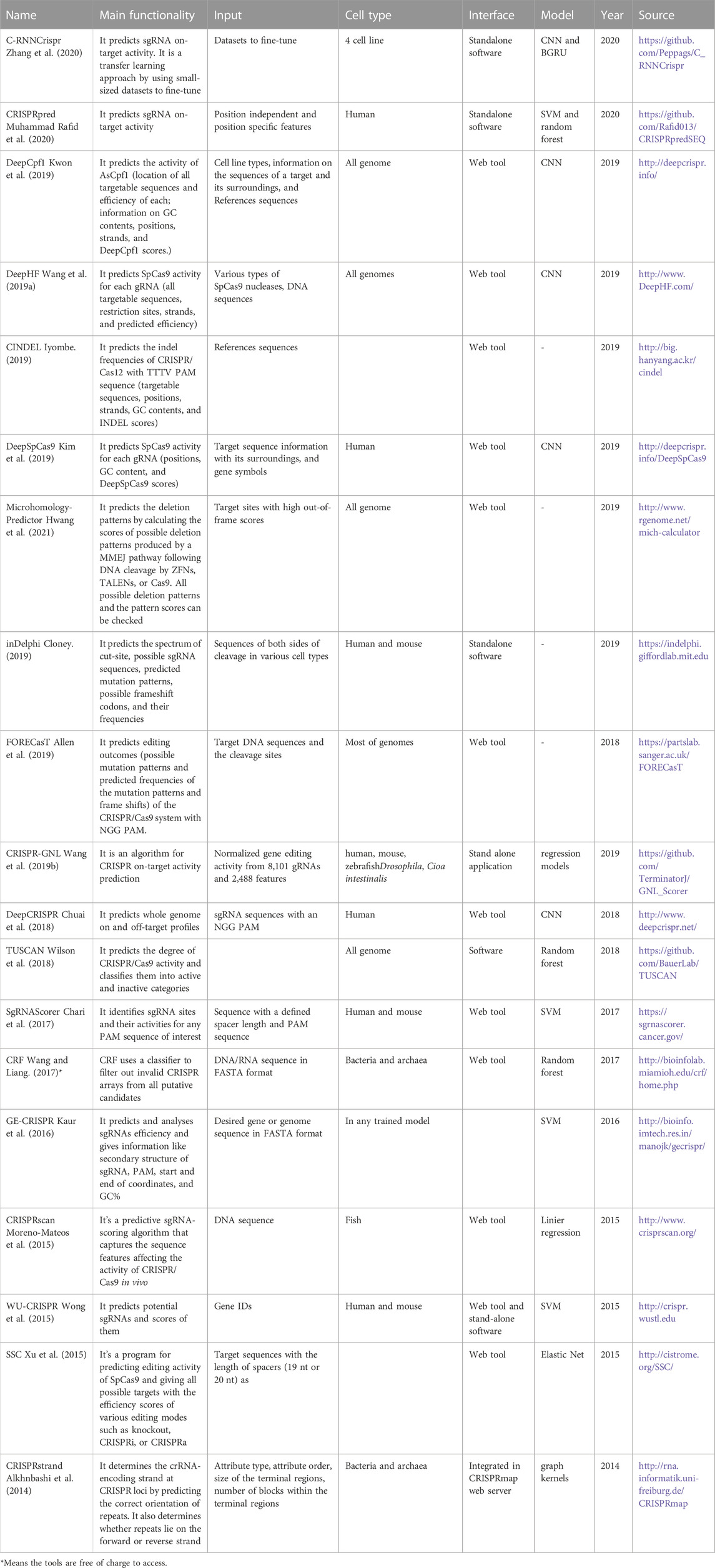
TABLE 4 . MDL-based tools and software packages related to CRISPR systems.
2 Computational approaches in CRISPR
Computational approaches are an essential part of CRISPR research. The bioinformatics studies have made significant contributions to the initial discovery of CRISPR ( Alkhnbashi et al., 2014 ; Makarova et al., 2015 ). We summarize some of them in Table 1 . Bioinformatics tools play a significant role in these fields: 1) determination of the specific differences between the CRISPR/Cas systems from archaeal and bacterial sources; 2) determination of required repeat spacer sequences for processing the mature CRISPR RNA (crRNA); 3) prediction of the transcribed strand of CRISPR arrays; 4) determination of CRISPR leader sequences; 5) classification of Cas proteins; 6) prediction of proper gRNA; 7) prediction of on-target and off-target effects; and so on ( Listgarten et al., 2016 ; Lin and Wong, 2018 ; Listgarten et al., 2018 ; Herai, 2019 ; Alkhnbashi et al., 2020 ; Smith et al., 2020 ).
According to our review, low cleavage efficiency and off-target effects hamper CRISPR development and application. So, prediction of proper gRNA and prediction of on-target and off-target effects is so critical. In the rest of the paper, we will focus on the tools that have been developed for designing optimal gRNA with low off-target effects.
2.1 gRNA design
There are two fundamental questions in CRISPR researches. The first question is: what are the targets of the given gRNA? Some methods, such as CRISPResso ( Pinello et al., 2016 ) and CRISPRTarget ( Biswas et al., 2013 ), try to calculate potential targets by taking a gRNA as input and using computational algorithms (more details are described in Table 3 ). Tools like CRISPRTarget ( Biswas et al., 2013 ) offer a way to answer this question using a ML-based approach ( Table 4 shows more details). The second important question is how to be confident about the accuracy of CRISPR edits. Most of the tools or methods in CRISPR’s field have been developed to answer these two questions. In Tables 2 , 3 , we tried to collect all of them and describe their details.
Also, we realized that most of researches in CRISPR area mainly focus on increasing cleavage activity (more on-targets) and cleavage efficiency (low off-target sites). As known, low efficiency makes CRISPR editing unreliable and also hampers CRISPR development and application ( Wang et al., 2019a ). Unfortunately, the high focus on more activity induces more off-target cleavage, which can be toxic. Therefore, we must maintain a balance between these two criteria. These issues can be resolved by designing successful CRISPR gRNA and choosing an appropriate Cas protein ( Kuscu et al., 2014 ; Shen et al., 2018 ).
As mentioned earlier, cleavage efficiency varies significantly among different target sites and cell lines ( Yan et al., 2018 ). Several features can influence the gRNA binding ability and the Cas enzyme cutting efficacy. Sequence composite features (nucleotide position, GC content), genetic and epigenetic features (chromatin accessibility, gene expression), and energetic properties (RNA secondary structure, melting temperature, free energy) are the most important influential features on cleavage efficiency ( Pallarès Masmitjà et al., 2019 ; Wang et al., 2020 ). Based on these features, many computational tools have been developed for designing highly efficient gRNAs. In the rest of this section, we will discuss the most popular ones.
Rule set 1 ( Liu et al., 2020 ) is a ML-based model that uses a support vector machine (SVM), a supervised ML method, and contains a linear regression method for classifying gRNAs. Rule set 1 uses sequence-based features, and its predictive data is highly correlated with experimental results ( Xu et al., 2015 ). Rule set 2 ( Liu et al., 2020 ) is an improved version of Rule set 1 and counts the nucleotides independent location of the gRNA target site within the gene to improve results ( Doench et al., 2016 ). It is a powerful model, used for both CRISPR Knock Out (CRISPR KO) and CRISPR activation/interference (CRISPRa/i) experiments. Another powerful model-based package has been developed and implemented at the Broad Institute to predict gRNA efficiency, named sgRNA Designer ( Pallarès Masmitjà et al., 2019 ).
Elastic Net is another ML-based and regularized regression-based method ( Li and Lin, 2010 ). Although there are significant differences in nucleotide preference between CRISPR KO and CRISPRa/I, the Elastic Net algorithm is used to construct models for both CRISPR KO and CRISPRa/i. Also, this practical algorithm has been applied in Spacer Scoring for CRISPR (SSC) software to predict the gRNA efficiency ( Qin et al., 2019 ). Additionally, well-known platforms such as E-CRISP ( Heigwer et al., 2014 ), CHOPCHOP ( Labun et al., 2019 ), and CRISPRFOCUS ( Cao et al., 2017 ) have applied this method.
Moreno and his colleagues designed another logistic regression-based method and integrated it into CRISPRscan to predict the gRNA precision ( Moreno-Mateos et al., 2015 ). Additionally, they have applied extra features such as guanine enrichment and adenine depletion, which increase the gRNA activity ( Cui et al., 2018 ).
Another ML-based method is WU-CRISPR ( Wong et al., 2015 ) which uses sequence composite features like guanine enrichment and adenine depletion, and some other novel features to build a higher precision model. The CRISPR/Cas9 target online predictor (CCTop) ( Stemmer et al., 2015 ), a platform for CRISPR target prediction, takes advantage of this model. The SgRNAScorer is another software that uses SVM to calculate gRNA on-target scores. The new version of this software can predict other Cas systems such as SaCas9 ( Qin et al., 2019 ) and AsCpf1 [94].
To avoid unwanted effects in other sites except for desired target sites (off-target), researchers try to modify a spacer sequence that does not adopt other sites in the genome. Tools such as CRISPRpred ( Hwang and Bae, 2021 ), DeepSpCas9, and SgRNAScorer are usually limited to the set of preprocessed genomes used when training ML models. To build good gRNAs in genomes other than those used in the training process, researchers can use web-based tools such as CRISPy ( Blin et al., 2016 ). Looking at Tables 1 – Tables 4 , we have listed the genome in which the editing takes place (named target genome) as a significant feature for all tools. The existence of target genome is even more critical for deep learning-based (DL) methods, because they are usually unpractical in genomes other than the ones from which training data was extracted. Basically, being used in all genomes is a significant strength for ML-based tools. But one tool may not have the same accuracy over all genomes or even all regions of a genome (see Figure 7 ) ( Kim et al., 2021 ). Furthermore, structural correctness and base-level accuracy of the target genome are important. The accuracy of a genome differs not only between genome sequencing technologies but also across genomic regions, as some stretches of the genome are inherently more difficult to read ( Kim et al., 2021 ). It is commonly known that certain genomic regions are more difficult for sequencing and extracting features. AT-rich or GC-rich regions, which are important for detecting off-target sites, are tough because they respond poorly to the amplification protocols required by some platforms. Palindromic sequences or hairpin structures similar to gRNA structures are difficult to denature, making such regions challenging for sequencing tools ( Selvakumar et al., 2022 ).
2.1.1 Selecting the best gRNA
There may be several gRNAs for an experiment, in which case we have to pick the best one. Many computational approaches have been developed for scoring and selecting the best gRNAs. Some of them use experimental data to score a gRNA. According to the different criteria, these methods consider a specific score for each gRNA. The criteria and final score calculation are different in each algorithm. CHOPCHOP ( Labun et al., 2019 ) provides multiple scores for users, such as Rule Set 1 and Rule set 2, SSC ( Xu et al., 2015 ), CRISPRscan [13], and deepCpf1 ( Kim et al., 2018 ). E-CRISP ( Heigwer et al., 2014 ) uses a particular score to determine the quality of each gRNA, named SAE, which combines three scores: specificity, annotation and efficacy. E-CRISP uses Rule Set 1 and SSC too. CCTop ( Stemmer et al., 2015 ) calculates the CRISPRater score to predict the efficiency of gRNAs. CCTop also calculates off-target scores for each sequence. The CRISPOR ( Concordet and Haeussler, 2018 ) ranks gRNAs according to different scores, such as on-target activity and protentional off-targets scores.
To score a gRNA or determine whether it is suitable for the desired genome editing or not, we need to determine potential targets of a gRNA in the selected genome and determine which of these potential targets are desirable. Hence, the number of on-target and off-target sites is critical in gRNA evaluation. In other words, since genomic edits are permanent and very sensitive, it is crucial to determine potential targets before the main editing occurs and then remove or reduce them ( Yan et al., 2018 ). Therefore, many researchers have focused on this issue. Furthermore, many developers have attempted to develop practical tools for this purpose. We will discuss these tools in the next section.
2.2 Prediction of CRISPR specificity (off-target sites)
The prediction of off-target mutations in CRISPR/Cas9 is a hot topic owing to its relevance to gene-editing research. Cas nucleases may cleave unintended genomic sites and cause unexpected mutations called off-target cleavage ( Listgarten et al., 2018 ). Even though the CRISPR/Cas9 system is routinely used in a large variety of tasks, there is also a significant concern that off-target effects may reduce its effectiveness of CRISPR. In response to this concern, researchers have concluded that the best way to mitigate off-target effects is to know when and where they occur and then design guides to avoid them while balancing for on-target efficiency. By predicting CRISPR cutting specificity and designing optimal gRNAs, off-target effects can be effectively relieved. As noted earlier, careful CRISPR target selection and low concentrations of CRISPR components can reduce off-target cleavage ( Zetsche et al., 2020 ).
The off-target predictive modelling problem can be broken down into three main tasks. Given a gRNA to evaluate off-target activity, one needs to ( Afzal et al., 2020 ) search the whole genome for potential targets; in other words, search those regions of the genome matching the guide sequence with up to X number of mismatches ( Gaj et al., 2013 ); score each potential target found in step 1 according to its activity ( Jacquin et al., 2019 ); collect the second stage scores and evaluate the final score of a gRNA. Several solutions have been presented for these tasks, including Cas-OFFinder ( Bae et al., 2014 ), CRISPOR ( Concordet and Haeussler, 2018 ), CHOPCHOP ( Labun et al., 2019 ), and e-CRISPR ( Tarasava et al., 2018 ). These models differ in their search algorithms and the completeness of the search process. Completeness is dictated by options such as the maximum number of mismatches, allowed PAMs, and the search algorithm used.
There are two basic methods to predict the specificity of CRISPR gRNAs: the alignment-based and the scoring-based methods. In the following, we will explain these approaches and give successful examples of each one. Also, the overview of these approaches is depicted in Figure 4 .
FIGURE 4 . Basic methods to predict the specificity of CRISPR gRNAs.
2.2.1 Alignment-based methods
In the alignment-based method, gRNAs are aligned to a given genome, and off-target sequences and sites are returned. These methods are mainly used to find out all potential off-target sites in silico . Choosing a search engine and setting search parameters plays an important role in evaluating these tools ( Liu et al., 2020 ). For example, if we set the maximum number of mismatches to a large number, like four or more, we will probably find all possible off-targets. The observed rate of off-target activity is about 59% when there is one mismatch between the target DNA and gRNA sequences and decreases toward 0% when four or more mismatches exist ( Kim et al., 2021 ). So, it can be concluded that an increased number of mismatches decreases the likelihood of off-target activity.
Common sequence alignment tools use BLAST, BLAT, Bowtie, Bowtie2, BWA or customized search engines. Table 5 summarizes the search engine of famous alignment-based tools in CRISPR.
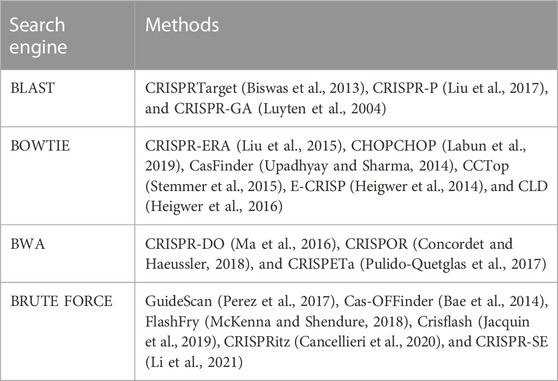
TABLE 5 . The most popular alignment-based methods and related search engines.
Compared to methods which use BLAST, Bowtie and BWA as search engine, methods like GuideScan ( Perez et al., 2017 ), Cas-OFFinder ( Bae et al., 2014 ), FlashFry ( McKenna and Shendure, 2018 ), Crisflash ( Jacquin et al., 2019 ), CRISPRitz ( Cancellieri et al., 2020 ), and finally, CRISPR-SE ( Li et al., 2021 )are faster due to the use of Brute force search engine. In addition, unlike most methods that support only a limited number of mismatches (mostly 3 or 4), Cas-OFFinder, CRISPRitz and CRISPR-SE have more preference due to their support of any number of mismatches.
The Bowtie and BWA are traditional tools for short sequence alignment that can be used for off-target sites detection ( de Ruijter and Guldenmund, 2016 ). However, they cannot identify small PAMs since they were developed for NGS read alignment. Moreover, these tools allow very limited mismatches with default parameters, so they cannot identify all potential off-target sites.
Most tools, like CCTop ( Stemmer et al., 2015 ), modify default algorithms and parameters and utilize Bowtie ( de Ruijter and Guldenmund, 2016 ) to find off-target sites. CCTop follows three main steps. In the first step, CCTop identifies PAM sites; In the second step, it modifies default parameters (up to five mismatches against one in default) of Bowtie, and uses them to search for matches and mismatches in protospacer sequences. In the third step, it evaluates the off-target score for each candidate gRNA.
SeqMAp ( Jiang and Wong, 2008 ) is an ultrafast short sequence mapping tool used in sgRNAcas9 ( Xie et al., 2014 ) to find off-target sites. The sgRNAcas9 classifies all off-target sites into three categories and scores them to choose the best gRNA.
CasOT ( Xiao et al., 2014 ) is another tool that can find Cas9 on-target and off-target sites with up to six mismatches in the seed region (12 nucleotides adjacent to the PAM). This tool can also determine whether off-targets are within a coding exon ( Listgarten et al., 2016 ) or not. FlashFry ( McKenna and Shendure, 2018 ) is another alignment-based method that defines off-targets with high speed. Additionally, it chooses the best gRNA and provides useful information such as annotating off-target sites, on and off-target scores, GC content, etc. FlashFry is a good choice for many applications because of its high speed and comprehensive output. Crisflash ( Jacquin et al., 2019 ) is another one that belongs to the alignment-based approaches group. Crisflash designs gRNAs with a tree-based algorithm and uses user-supplied variant data to optimizes gRNA accuracy. It uses an N-ary tree structure, which searches up to four mismatches. CRISPRitz ( Cancellieri et al., 2020 ) used a four-bit-based encoding to represent each nucleotide to allow for efficient bitwise operations. CRISPRitz supports off-targets with both mismatches and indels.
CALITAS ( Fennell et al., 2021 ) is a new CRISPR-Cas-aware aligner tool which uses a modified and CRISPR-tuned version of the Needleman–Wunsch algorithm, supports an unlimited number of mismatches and gaps, and allows PAM mismatches or PAM-less searches. CALITAS returns a single best alignment for a given off-target site and it enables off-targets to be referenced directly using alignment coordinate.
CHOPCHOP v3.0 ( Labun et al., 2019 ), a well-known model, is another tool that uses Bowtie with parameters–V and–L to detect off-target sites [90]. But, CRISPOR uses BWA to find all potential off-target sites iteratively and can find all validated off-targets as well as Cas-OFFinder ( Bae et al., 2014 ).
Sequence alignment tools like CRISPy ( Qin et al., 2019 ) and CRISPRdirect ( Heigwer et al., 2016 ) rely on a minimum of one K-mer exact match. They are likely to miss some off-targets, spatially with a high number of mismatches and ultra-short gRNAs (20-mer). So, the accuracy of these methods cannot be very high.
In recent years, some tools like GuideScan ( Perez et al., 2017 ), Cas-OfFinder ( Bae et al., 2014 ), and CRISPR-SE ( Li et al., 2021 ) have been developed with Brute force algorithm as their search engine. GuideScan uses a “tree” data structure with a brute-force algorithm that guarantees the search accuracy. Another tool in this category is Cas-OFFinder. Cas-OFFinder is one of the most popular tools for detecting potential off-target sites, with no limit to the number of mismatches, PAM types, or gRNA length. In our opinion, the most significant advantage of Cas-OFFinder is its high running speed due to using GPUs. It can also predict off-target sites with one-bp deletions or insertions.
OffScan ( Cui et al., 2020 ) is the last one we considered in this study that is, belongs to the alignment-based approaches group. OffScan is not limited by the number of mismatches and allows custom PAM. Besides, OffScan adopts the FM-index, which efficiently improves query speed and reduce memory consumption.
Here, we discussed several alignment-based methods for the prediction of the gRNA output and realized that Cas-OFFinder may be the best option for identifying all potential off-targets with any Cas nucleases among these tools. Although users can reduce the number of outputs by restricting the maximum mismatches while exploring off-target cleavage, there are always redundant outputs; many are false positives.
On the whole, all nucleotide positions containing mismatches do not have the same decisive effect on off-target cleavage, but this issue is not considered in alignment-based methods. Because of this problem, and in order to increase the accuracy of the off-target detection methods, adding the features that influence the non-specific binding of CRISPR gRNAs to the methods is essential. As a result, another group of approaches emerged called scoring-based methods, which are discussed in the following sub-section.
2.2.2 Scoring-based methods
In the scoring-based method, the gRNAs identified in the alignment process are scored and ranked, and the sgRNA with the highest score is selected. There are two groups of scoring-based approaches: 1) hypothesis-driven-based approaches, where off-targets are scored based on the contribution of specific genome context factors to gRNA specificity; 2) learning-based approaches, where gRNAs are scored and predicted from a training model that considers the different features affecting specificity.
MIT ( Hsu et al., 2013 ) is the first popular score-based tool for CRISPR off-target evaluation. To score the off-target efficiency of each gRNA, it counts and evaluates the contributions made by different mismatch positions. It also calculates a weight matrix to determine off-target efficiency for each gRNA ( Chuai et al., 2017 ). The MIT score has been integrated into many CRISPR gRNA design tools, such as CHOPCHOP v3.0 CHOP ( Labun et al., 2019 ) and CRISPOR ( Concordet and Haeussler, 2018 ).
Another popular score-based tool for off-target evaluation is CFD (Cutting Frequency Determination). It is noticeable that gRNA can bind genome loci with non-canonical PAMs such as NAG, NCG, and NGA. So, CFD has added PAM features to their scoring metrics ( Abadi et al., 2017 ). Also, for examining correlations between RNAs and off-targets, gRNAs with mismatches and indels in target sequences are added. GUIDE-seq ( Tsai et al., 2015 ) validated the CFD score and proved that it performs better than the MIT score. The CFD score has been integrated into CRISPRscan ( Moreno-Mateos et al., 2015 ), GuideScan ( Perez et al., 2017 ), CRISPOR ( Concordet and Haeussler, 2018 ), and others. CRISPRoff ( Carlson-Stevermer et al., 2020 ) and uCRISPR ( Carlson-Stevermer et al., 2020 ) integrated energetic properties into their scoring metrics. They both yielded better accuracy than MIT and CFD in off-target prediction.
Scoring-based methods consider only a few features, and unfortunately, all practical features cannot be considered. Also, most features are not understood yet, while learning-based methods use combinations of multiple features to build complex models for better prediction of off-target sites. These models are based on ML and, more recently, DL methods.
DL-based methods are attractive for CRISPR gRNA target efficacy prediction. They are mainly based on CNNs. Table 4 introduces some famous models that use MDL models for gRNA on-target prediction. These models used neural networks to extract features from the input genomic sequence. Generally, they are superior to models that use classical ML tools in prediction accuracy.
DeepCRISPR ( Chuai et al., 2018 ) is a DL-based platform that combines gRNA on-target and off-target site predictions. As mentioned, in DL-based models, we do not need to identify all effective features, as they are detected automatically using the deep neural network. DeepCRISPR learns all possible sequence and epigenetic features that may affect gRNA Knock Out (KO) efficacy ( Hana et al., 2021 ) in its learning process with a large dataset that is, gathered for it.
CRISPR-Cpf1 ( Kim et al., 2017 ) is a ML-based model that achieved high efficiency, although it suffers minor off-target effects. DeepCpf1 ( Kwon et al., 2019 ) is another highly used DL-based algorithm, mainly used in predicting Cpf1 activity. It uses chromatin accessibility data. It showed a significant improvement in the accuracy of Cpf1 activity prediction. CRISPR-DT ( Zhu and LiangCRISPR-, 2019 ) is a recently developed platform for predicting the Cpf1 target efficiency. This model has been implemented with the SVM algorithm and displays better performance than the DL-based models such as DeepCpf1.
CRISPOR ( Concordet and Haeussler, 2018 ) may be the best tool for designing gRNAs. CRISPOR combines multiple tools and gathers a large dataset to develop a highly efficient CRISPR gRNA design. CRISPOR contains 417 genomes and 19 PAM types, making it useful in almost all genomes. CRISPOR calculates two specificity scores: MIT and CFD. Additionally, it calculates ten efficiency scores, including Rule Set 2, CRISPRscan, microhomology, Lindel scores ( Chen et al., 2019 ) and others for outcome prediction. CRISPOR designs primers for each gRNA as well as off-target sites. These primers are helpful when conducting on and off-target validation. CRISPOR enables the filtering of gRNAs with genomic variants based on well-known variant databases.
Some computational tools use CNNs for feature extraction or classification of CRISPR Cas. For instance, Seq-deepCpf1 ( Kim et al., 2018 ; Kwon et al., 2019 ) has used CNN to extract features from the input gRNA sequence. And DeepCRISPR incorporates a CNN for predicting CRISPR/Cas9 gRNA on-target knockout efficiency and whole-genome off-target profiles. Also, DeepCas9 uses CNN to automatically learn the sequence determinants and predict the activities of gRNAs across multiple species genomes ( Bhagwat and Khuri, 2021 ). Deeper-Bind ( Hassanzadeh and Wang, 2016 ) used a LSTM layer to learn the dependencies between sequence features; this helps improve the prediction of protein binding specificity ( Zhang et al., 2020 ). C-RNNCrispr ( Zhang et al., 2020 ) has used a hybrid architecture combining CNN with bidirectional GRU (BGRU) to predict sgRNA cleavage efficacy ( Sledzinski et al., 2020 ).
The performance of these tools is quantitatively assessed with two commonly used evaluation metrics, including accuracy and Spearman Correlation Coefficient (SCC) between predicted and real detected off-target activity. However, other evaluation metrics like Precision and Sensitivity (Eqs 2 , 3 ) are used in some research as well. Spearman correlation seems to be a more reliable criterion. Most of these tools achieve promising accuracy in off-target prediction. Figures 5 , 6 compare the off-target prediction efficacy of some popular tools. Due to their importance, we compare the accuracy of DL-based tools in separate diagram. The average accuracy of these tools is illustrated in the figures, as their accuracy differs among different genomes. For example, DeepCRISPR was the most accurate tool in the HEL cell line but performed poorly in the others. More details can be found in ( Wang et al., 2019a ; Zhu and LiangCRISPR-, 2019 ). Also, as a ML method, the accuracy differs between the train and test datasets. Unfortunately, for DeepCas9 and DeepSpCas9 ( Chen et al., 2019 ), there is no report in their primary reference for the training dataset and the test dataset in CRISPRLearner ( Bhagwat and Khuri, 2021 ). Accuracy, Precision, and Sensitivity are defined as follows, where TP, FP, TN, and FN represent true positive, false positive, true negative, and false negative, respectively.
FIGURE 5 . Average accuracy of off-target prediction.
FIGURE 6 . Average accuracy of off-target prediction in DL-based methods.
SCC evaluates the ability of the models to predict the actual efficiency of each gRNA sequence ( Konstantakos et al., 2022 ). While some models are trained to minimize the mean squared error (MSE), the comparison between models on different datasets is necessarily made using Spearman correlation. Figure 7 compares the predictive ability of off-target sites in some ML-based tools over five datasets named Zebrafish_G, Zebrafish_S, HEL, A375, and mESC. In general, the larger the polygon area, the better the overall performance of the tool. Figure 7 clearly illustrates the better and more robust performance of the DeepHF, DeepSpCas9, and DeepCas9 models. As shown, classic ML-based tools such as Azimuth 2.0 achieve comparable performance to DL-based tools. Also, even though E-CRISP is more accurate than some learning-based tools, it does not achieve high enough correlations. However, E-CRISP has stable performance across all datasets. In addition, as it is clear from Figure 7 , DeepCRISPR outperforms the other tools on the HEL dataset, and E-CRISP and CRISPRLearner achieve better results based on this metric.
FIGURE 7 . Spearman correlation for ML-based tools over the different datasets. Each polygon represents a tool, and the edges illustrate the obtained correlation over the respective dataset.
As mentioned, gRNAs are typically designed by computational tools which compare gRNA sequence with a reference genome to predict the activity of on-target and potential off-targets. However, these tools can yield false-positive (FP) or false-negative (FN) results. Furthermore, the DNA in clinical experiments can differ from the reference genome used in the computational modeling, which means they would be more false predictions. Therefore, the accuracy is less than the values shown in Figure 7 in the actual experiment. To resolve this problem, in-vitro based tools have been developed for the experimental detection of off-target sites in a particular DNA sample. Tools like SMRT-OTS and Nano-OTS ( Höijer et al., 2020 ) use long-read single-molecule sequencing.
In this article, we review both traditional and ML-based approaches for gRNA designing and predicting off-target sites. As mentioned before, experimental methods which use third-generation sequencing technology, have a better performance in Cas9 target detection on dark genomic regions ( Höijer et al., 2020 ). This new technology helps us to detect more on-target and off-target sites and to design optimal gRNA. Furthermore, collected data in experimental methods, could improve the accuracy of DL-based tools.
Also, we have presented a comprehensive list of available tools. Each tool has merits and demerits, and the performance of different tools differs in different situations. According to our studies, some tools can be a better choice in some situations; However, others may be more popular in the scientific community. So, choosing the right tool depends on the conditions and limitations of an application.
Among the alignment-based methods, tools like CRISPR-P, Flycrispr, CRISPRseek, Cas-OFFinder, CasOT, sgRNACas9, and Flashfly have high accuracy and efficiency; however, CRISPR-P and Flycrispr are only used in specific genomes. Other tools such as CRISPRseek, Cas-Offinder, and CasOT, are used in almost all genomes. Moreover, they support only particular types of PAMs, while methods such as sgRNACas9 and Flashfly are compatible with all types of PAMs and seem to be a better option for designing gRNAs.
Among the learning-based methods, DL-based methods, including C-RNNCrispr, DeepCpf1, DeepHF, DeepSpCas9, and DeepCRISP, have drawn much interest recently. However, learning-based methods such as CLD, CRISPR-ERA, sgRNA-design, E-CRISP are significant due to their high accuracy and use in all genomes. Finally, based on our study, methods such as CRISPR-SE and E-CRISP are the best options to be used in all genomes with high accuracy.
3 Conclusion
CRISPR systems have been developed for accurate genome editing. Since genomic modifications are permanent ( Ding et al., 2018 ), it is crucial to make precise edits. Most of the tools or methods in CRISPR’s field have been developed to help users design proper gRNA with fewer off-target effects. It is considered that the efficiency of one gRNA may differ among different models and databases. Users must evaluate several gRNAs using multiple models and select the best one for their experiments.
The previous successes of CNN and RNN architectures in bioinformatics motivated other researchers to extend their applications with a DL platform, which we believe is the best solution for predicting off-target effects. DL methods are inexpensive and fast compared to experimental methods. However, their accuracy depends on the amount of available data for a model’s training. Additionally, most of existing methods have three big problems, which means their predictions are not exact. First, they calculate scores based on mismatches to the guide sequence. However, DL-based methods can extract more efficient features hidden in the input data. In other words, DL-based methods can capture features other than gRNA sequence-based features. These features can be utilized and encoded in the input sequence to improve the performance of the existing DL architectures. In addition, most proposed DL-based methods use a one-hot vector representation to encode the input data. ( Charlier et al., 2021 ). The use of newer encoding and embedding methods proposed in the field of DL can enhance the efficiency of existing DL-based methods. Also, the use of gRNA-DNA pair encoding can be helpful. Second, there is a rapid expansion in experimental data in CRISPR research. Most methods cannot scale and improve their performance with this new data. As known, DL-based methods achieve better performance by training on large datasets, but they require a pre-processing step to prepare and aggregate data obtained from diverse sources based on different experimental methods. This step requires enough knowledge about the type of input data, the operation mechanism of CRISPR, and the architecture of the deep neural network. Finally, the most severe issue is that existing DL-based methods still need to be improved in providing sufficient precision for clinical practice usage. NGS-based whole-genome sequencing technologies help to discover almost all off-target sites in the target genome and create a large and more accurate train dataset. As the number of instances in a train dataset increases, the predictions of DL-based methods become closer to experimental observations.
Author contributions
RA: supplied acquisition of data, analysis, interpretation of data and drafting the paper. LS: provided the conception and design of the study, analysis and interpretation of data, revised it critically for important intellectual content, and final approval of the version to be submitted. AK: provided the conception and design of the study, analysis and interpretation of data, revised it critically for important intellectual content, and final approval of the version to be submitted. RA has the first authorship right. LS and AK contributed equally to this work and share senior authorship.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abadi, S., Yan, W. X., Amar, D., and Mayrose, I. (2017). A machine learning approach for predicting CRISPR-Cas9 cleavage efficiencies and patterns underlying its mechanism of action. PLoS Comput. Biol. 13 (10), e1005807. doi:10.1371/journal.pcbi.1005807
PubMed Abstract | CrossRef Full Text | Google Scholar
Afzal, S., Sirohi, P., and Singh, N. K. (2020). A review of CRISPR associated genome engineering: Application, advances and future prospects of genome targeting tool for crop improvement. Biotechnol. Lett. 42, 1611–1632. doi:10.1007/s10529-020-02950-w
Ahmed, M., and He, H. H. (2017). SgTiler: A fast method to design tiling sgRNAs for CRISPR/cas9 mediated screening, BioRxiv. 217166 .
Google Scholar
Alkhnbashi, O. S., Costa, F., Shah, S. A., Garrett, R. A., Saunders, S. J., and Backofen, R. (2014). CRISPRstrand: Predicting repeat orientations to determine the crRNA-encoding strand at CRISPR loci. Bioinformatics 30 (17), i489–i496. doi:10.1093/bioinformatics/btu459
Alkhnbashi, O. S., Meier, T., Mitrofanov, A., Backofen, R., and Voß, B. (2020). CRISPR-Cas bioinformatics. Methods 172, 3–11. doi:10.1016/j.ymeth.2019.07.013
Alkhnbashi, O. S., Mitrofanov, A., Bonidia, R., Raden, M., Tran, V. D., Eggenhofer, F., et al. (2021). CRISPRloci: Comprehensive and accurate annotation of CRISPR–cas systems. Nucleic Acids Res. 49, W125–W130. doi:10.1093/nar/gkab456
Allen, F., Crepaldi, L., Alsinet, C., Strong, A. J., Kleshchevnikov, V., De Angeli, P., et al. (2019). Predicting the mutations generated by repair of Cas9-induced double-strand breaks. Nat. Biotechnol. 37 (1), 64–72. doi:10.1038/nbt.4317
CrossRef Full Text | Google Scholar
Bae, S., Park, J., and Kim, J-S. (2014). Cas-OFFinder: A fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 30 (10), 1473–1475. doi:10.1093/bioinformatics/btu048
Bhagwat, N., and Khuri, N. (2021). “Predicting targets for genome editing with long short term memory networks,” in Advances in computer vision and computational biology (Berlin, Germany: Springer ), 657–670.
Biswas, A., Gagnon, J. N., Brouns, S. J., Fineran, P. C., and Brown, C. M. (2013). CRISPRTarget: Bioinformatic prediction and analysis of crRNA targets. RNA Biol. 10 (5), 817–827. doi:10.4161/rna.24046
Biswas, A., Staals, R. H., Morales, S. E., Fineran, P. C., and Brown, C. M. (2016). CRISPRDetect: A flexible algorithm to define CRISPR arrays. BMC genomics 17 (1), 356–370. doi:10.1186/s12864-016-2627-0
Blin, K., Pedersen, L. E., Weber, T., and Lee, S. Y. (2016). CRISPy-web: An online resource to design sgRNAs for CRISPR applications. Synthetic Syst. Biotechnol. 1 (2), 118–121. doi:10.1016/j.synbio.2016.01.003
Boel, A., Steyaert, W., De Rocker, N., Menten, B., Callewaert, B., De Paepe, A., et al. (2016). BATCH-GE: Batch analysis of Next-Generation Sequencing data for genome editing assessment. Sci. Rep. 6 (1). doi:10.1038/srep30330
Cancellieri, S., Canver, M. C., Bombieri, N., Giugno, R., and Pinello, L. (2020). CRISPRitz: Rapid, high-throughput and variant-aware in silico off-target site identification for CRISPR genome editing. Bioinformatics 36 (7), 2001–2008. doi:10.1093/bioinformatics/btz867
Cao, Q., Ma, J., Chen, C-H., Xu, H., Chen, Z., Li, W., et al. (2017). CRISPR-FOCUS: A web server for designing focused CRISPR screening experiments. PLoS One 12 (9), e0184281. doi:10.1371/journal.pone.0184281
Carlson-Stevermer, J., Kelso, R., Kadina, A., Joshi, S., Rossi, N., Walker, J., et al. (2020). CRISPRoff enables spatio-temporal control of CRISPR editing. Nat. Commun. 11 (1), 5041–5047. doi:10.1038/s41467-020-18853-3
Chari, R., Yeo, N. C., Chavez, A., and Church, G. M. (2017). sgRNA Scorer 2.0: a species-independent model to predict CRISPR/Cas9 activity. ACS Synth. Biol. 6 (5), 902–904. doi:10.1021/acssynbio.6b00343
Charlier, J., Nadon, R., and Makarenkov, V. (2021). Accurate deep learning off-target prediction with novel sgRNA-DNA sequence encoding in CRISPR-Cas9 gene editing. Bioinformatics 37 (16), 2299–2307. doi:10.1093/bioinformatics/btab112
Chen, C-L., Rodiger, J., Chung, V., Viswanatha, R., Mohr, S. E., Hu, Y., et al. (2020). SNP-CRISPR: A web tool for SNP-specific genome editing. G3 Genes, Genomes, Genet. 10 (2), 489–494. doi:10.1534/g3.119.400904
Chen, W., McKenna, A., Schreiber, J., Haeussler, M., Yin, Y., Agarwal, V., et al. (2019). Massively parallel profiling and predictive modeling of the outcomes of CRISPR/Cas9-mediated double-strand break repair. Nucleic acids Res. 47 (15), 7989–8003. doi:10.1093/nar/gkz487
Chuai, G., Ma, H., Yan, J., Chen, M., Hong, N., Xue, D., et al. (2018). DeepCRISPR: Optimized CRISPR guide RNA design by deep learning. Genome Biol. 19 (1), 80–18. doi:10.1186/s13059-018-1459-4
Chuai, G-h., Wang, Q-L., and Liu, Q. (2017). In silico meets in vivo : Towards computational CRISPR-based sgRNA design. Trends Biotechnol. 35 (1), 12–21. doi:10.1016/j.tibtech.2016.06.008
Cloney, R. (2019). The oracle of inDelphi predicts Cas9 repair outcomes. Nat. Rev. Genet. 20 (1), 4–5. doi:10.1038/s41576-018-0077-z
Concordet, J-P., and Haeussler, M. (2018). Crispor: Intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic acids Res. 46 (W1), W242–W245. doi:10.1093/nar/gky354
Cox, D. B. T., Platt, R. J., and Zhang, F. (2015). Therapeutic genome editing: Prospects and challenges. Nat. Med. 21 (2), 121–131. doi:10.1038/nm.3793
Cradick, T. J., Qiu, P., Lee, C. M., Fine, E. J., and Bao, G. (2014). COSMID: A web-based tool for identifying and validating CRISPR/cas off-target sites. Mol. Therapy-Nucleic Acids. 3, e214. doi:10.1038/mtna.2014.64
Cui, Y., Liao, X., Peng, S., Tang, T., Huang, C., and Yang, C. (2020). OffScan: A universal and fast CRISPR off-target sites detection tool. BMC genomics 21 (1), 872–876. doi:10.1186/s12864-019-6241-9
Cui, Y., Xu, J., Cheng, M., Liao, X., and Peng, S. (2018). Review of CRISPR/Cas9 sgRNA design tools. Interdiscip. Sci. Comput. Life Sci. 10 (2), 455–465. doi:10.1007/s12539-018-0298-z
Dampier, W., Chung, C-H., Sullivan, N. T., Atkins, A. J., Nonnemacher, M. R., and Wigdahl, B. (2018). CRSeek: A Python module for facilitating complicated CRISPR design strategies, PeerJ Prepr. Report No, 2167–9843.
de Ruijter, A., and Guldenmund, F. (2016). The bowtie method: A review. Saf. Sci. 88, 211–218. doi:10.1016/j.ssci.2016.03.001
Ding, W., Mao, W., Shao, D., Zhang, W., and Gong, H. (2018). DeepConPred2: An improved method for the prediction of protein residue contacts. Comput. Struct. Biotechnol. J. 16, 503–510. doi:10.1016/j.csbj.2018.10.009
Doench, J. G., Fusi, N., Sullender, M., Hegde, M., Vaimberg, E. W., Donovan, K. F., et al. (2016). Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol. 34 (2), 184–191. doi:10.1038/nbt.3437
Duan, L., Ouyang, K., Xu, X., Xu, L., Wen, C., Zhou, X., et al. (2021). Nanoparticle delivery of CRISPR/Cas9 for genome editing. Front. Genet. 12, 673286. doi:10.3389/fgene.2021.673286
Fennell, T., Zhang, D., Isik, M., Wang, T., Gotta, G., Wilson, C. J., et al. (2021). CALITAS: A CRISPR-cas-aware ALigner for in silico off-TArget search. CRISPR J. 4 (2), 264–274. doi:10.1089/crispr.2020.0036
Gaj, T., Gersbach, C. A., and Barbas, C. F. (2013). ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 31 (7), 397–405. doi:10.1016/j.tibtech.2013.04.004
Gasiunas, G., Barrangou, R., Horvath, P., and Siksnys, V. (2012). Cas9–crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc. Natl. Acad. Sci. 109 (39), E2579–E2586. doi:10.1073/pnas.1208507109
Ge, R., Mai, G., Wang, P., Zhou, M., Luo, Y., Cai, Y., et al. (2016). CRISPRdigger: Detecting CRISPRs with better direct repeat annotations. Sci. Rep. 6 (1). doi:10.1038/srep32942
Güell, M., Yang, L., and Church, G. M. (2014). Genome editing assessment using CRISPR Genome Analyzer (CRISPR-GA). Bioinformatics 30 (20), 2968–2970. doi:10.1093/bioinformatics/btu427
Hana, S., Peterson, M., McLaughlin, H., Marshall, E., Fabian, A. J., McKissick, O., et al. (2021). Highly efficient neuronal gene knockout in vivo by CRISPR-Cas9 via neonatal intracerebroventricular injection of AAV in mice. Gene Ther. 28, 646–658. doi:10.1038/s41434-021-00224-2
Hassanzadeh, H. R., and Wang, M. D. (2016). “DeeperBind: Enhancing prediction of sequence specificities of DNA binding proteins,” in 2016 IEEE International Conference on Bioinformatics and Biomedicine (BIBM) , Shenzhen, China , 15-18 December 2016 ( IEEE ).
Heigwer, F., Kerr, G., and Boutros, M. E-C. R. I. S. P. (2014). E-CRISP: Fast CRISPR target site identification. Nat. methods 11 (2), 122–123. doi:10.1038/nmeth.2812
Heigwer, F., Zhan, T., Breinig, M., Winter, J., Brügemann, D., Leible, S., et al. (2016). CRISPR library designer (CLD): Software for multispecies design of single guide RNA libraries. Genome Biol. 17 (1), 55–10. doi:10.1186/s13059-016-0915-2
Herai, R. H. (2019). Avoiding the off-target effects of CRISPR/cas9 system is still a challenging accomplishment for genetic transformation. Gene 700, 176–178. doi:10.1016/j.gene.2019.03.019
Höijer, I., Johansson, J., Gudmundsson, S., Chin, C-S., Bunikis, I., Häggqvist, S., et al. (2020). Amplification-free long-read sequencing reveals unforeseen CRISPR-Cas9 off-target activity. Genome Biol. 21 (1), 290. doi:10.1186/s13059-020-02206-w
Hough, S. H., Kancleris, K., Brody, L., Humphryes-Kirilov, N., Wolanski, J., Dunaway, K., et al. (2017). Guide Picker is a comprehensive design tool for visualizing and selecting guides for CRISPR experiments. BMC Bioinforma. 18 (1), 167–210. doi:10.1186/s12859-017-1581-4
Hsu, P. D., Scott, D. A., Weinstein, J. A., Ran, F. A., Konermann, S., Agarwala, V., et al. (2013). DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 31 (9), 827–832. doi:10.1038/nbt.2647
Hwang, G-H., and Bae, S. (2021). Computational methods in synthetic biology . Berlin, Germany: Springer , 81–88.Web-based base editing toolkits: BE-Designer and BE-analyzer
Hwang, G-H., Song, B., and Bae, S. (2021). Current widely-used web-based tools for CRISPR nucleases, base editors, and prime editors. Gene Genome Ed. 1, 100004. doi:10.1016/j.ggedit.2021.100004
Iyombe, J-P. (2019). Correction du gène de la dystrophine avec la méthode CRISPR induced deletion . Québec: CinDel .
Jacquin, A. L., Odom, D. T., and Lukk, M. (2019). Crisflash: Open-source software to generate CRISPR guide RNAs against genomes annotated with individual variation. Bioinformatics 35 (17), 3146–3147. doi:10.1093/bioinformatics/btz019
Jeong, H-H., Kim, S. Y., Rousseaux, M. W., Zoghbi, H. Y., and Liu, Z. (2017). CRISPRcloud: A secure cloud-based pipeline for CRISPR pooled screen deconvolution. Bioinformatics 33 (18), 2963–2965. doi:10.1093/bioinformatics/btx335
Jiang, F., Taylor, D. W., Chen, J. S., Kornfeld, J. E., Zhou, K., Thompson, A. J., et al. (2016). Structures of a CRISPR-Cas9 R-loop complex primed for DNA cleavage. Science 351 (6275), 867–871. doi:10.1126/science.aad8282
Jiang, H., and Wong, W. H. (2008). SeqMap: Mapping massive amount of oligonucleotides to the genome. Bioinformatics 24 (20), 2395–2396. doi:10.1093/bioinformatics/btn429
Jinek, M., Chylinski, K., Fonfara, I., Hauer, M., Doudna, J. A., and Charpentier, E. (2012). A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. science 337 (6096), 816–821. doi:10.1126/science.1225829
Jinek, M., Jiang, F., Taylor, D. W., Sternberg, S. H., Kaya, E., Ma, E., et al. (2014). Structures of Cas9 endonucleases reveal RNA-mediated conformational activation. Science 343 (6176), 1247997. doi:10.1126/science.1247997
Kaur, K., Gupta, A. K., Rajput, A., and Kumar, M. (2016). ge-CRISPR-An integrated pipeline for the prediction and analysis of sgRNAs genome editing efficiency for CRISPR/Cas system. Sci. Rep. 6 (1). doi:10.1038/srep30870
Keough, K. C., Lyalina, S., Olvera, M. P., Whalen, S., Conklin, B. R., and Pollard, K. S. (2019). AlleleAnalyzer: A tool for personalized and allele-specific sgRNA design. Genome Biol. 20 (1), 167–169. doi:10.1186/s13059-019-1783-3
Kim, D., Kang, B. C., and Kim, J. S. (2021). Identifying genome-wide off-target sites of CRISPR RNA-guided nucleases and deaminases with Digenome-seq. Nat. Protoc. 16 (2), 1170–1192. doi:10.1038/s41596-020-00453-6
Kim, H., Kim, S-T., Ryu, J., Kang, B-C., Kim, J-S., and Kim, S-G. (2017). CRISPR/Cpf1-mediated DNA-free plant genome editing. Nat. Commun. 8 (1), 14406–14407. doi:10.1038/ncomms14406
Kim, H. K., Kim, Y., Lee, S., Min, S., Bae, J. Y., Choi, J. W., et al. (2019). SpCas9 activity prediction by DeepSpCas9, a deep learning–based model with high generalization performance. Sci. Adv. 5 (11), eaax9249. doi:10.1126/sciadv.aax9249
Kim, H. K., Min, S., Song, M., Jung, S., Choi, J. W., Kim, Y., et al. (2018). Deep learning improves prediction of CRISPR–Cpf1 guide RNA activity. Nat. Biotechnol. 36 (3), 239–241. doi:10.1038/nbt.4061
Konstantakos, V., Nentidis, A., Krithara, A., and Paliouras, G. (2022). CRISPR-Cas9 gRNA efficiency prediction: An overview of predictive tools and the role of deep learning. Nucleic acids Res. 50 (7), 3616–3637. doi:10.1093/nar/gkac192
Kuscu, C., Arslan, S., Singh, R., Thorpe, J., and Adli, M. (2014). Genome-wide analysis reveals characteristics of off-target sites bound by the Cas9 endonuclease. Nat. Biotechnol. 32 (7), 677–683. doi:10.1038/nbt.2916
Kwon, K. H., Seonwoo, M., Myungjae, S., Soobin, J., Woo, C. J., Younggwang, K., et al. (2019). DeepCpf1: Deep learning-based prediction of CRISPR-Cpf1 activity atendogenous sites. Proc. Annu. Meet. Jpn. Pharmacol. Soc. 92, JKL-05.
Labun, K., Montague, T. G., Krause, M., Torres Cleuren, Y. N., Tjeldnes, H., and Valen, E. (2019). CHOPCHOP v3: Expanding the CRISPR web toolbox beyond genome editing. Nucleic acids Res. 47 (W1), W171–W174. doi:10.1093/nar/gkz365
Li, B., Chen, P. B., and DiaoCRISPR-, Y. S. E. (2021). CRISPR-SE: A brute force search engine for CRISPR design. NAR genomics Bioinforma. 3 (1), lqab013. doi:10.1093/nargab/lqab013
Li, Q., and Lin, N. (2010). The Bayesian elastic net. Bayesian anal. 5 (1), 151–170. doi:10.1214/10-ba506
Lin, J., and Wong, K-C. (2018). Off-target predictions in CRISPR-Cas9 gene editing using deep learning. Bioinformatics 34 (17), i656–i663. doi:10.1093/bioinformatics/bty554
Lin, L., and Luo, Y. (2019). Tracking CRISPR’s footprints. CRISPR Gene Ed. 1961, 13–28. doi:10.1007/978-1-4939-9170-9_2
Listgarten, J., Weinstein, M., Elibol, M., Hoang, L., Doench, J., and Fusi, N. (2016) Predicting off-target effects for end-to-end CRISPR guide design. bioRxiv.:078253.
Listgarten, J., Weinstein, M., Kleinstiver, B. P., Sousa, A. A., Joung, J. K., Crawford, J., et al. (2018). Prediction of off-target activities for the end-to-end design of CRISPR guide RNAs. Nat. Biomed. Eng. 2 (1), 38–47. doi:10.1038/s41551-017-0178-6
Liu, G., Zhang, Y., and Zhang, T. (2020). Computational approaches for effective CRISPR guide RNA design and evaluation. Comput. Struct. Biotechnol. J. 18, 35–44. doi:10.1016/j.csbj.2019.11.006
Liu, H., Ding, Y., Zhou, Y., Jin, W., Xie, K., and Chen, L-L. (2017). CRISPR-P 2.0: An improved CRISPR-cas9 tool for genome editing in plants. Mol. plant 10 (3), 530–532. doi:10.1016/j.molp.2017.01.003
Liu, H., Wei, Z., Dominguez, A., Li, Y., Wang, X., and Qi, L. S. (2015). CRISPR-ERA: A comprehensive design tool for CRISPR-mediated gene editing, repression and activation: Fig. 1. Bioinformatics 31 (22), 3676–3678. doi:10.1093/bioinformatics/btv423
Luo, J., Chen, W., Xue, L., and Tang, B. (2019). Prediction of activity and specificity of CRISPR-Cpf1 using convolutional deep learning neural networks. BMC Bioinforma. 20 (1). doi:10.1186/s12859-019-2939-6
Luyten, H., Plijter, J. J., and Van Vliet, T. (2004). Crispy/crunchy crusts of cellular solid foods: A literature review with discussion. J. texture Stud. 35 (5), 445–492. doi:10.1111/j.1745-4603.2004.35501.x
Ma, J., Köster, J., Qin, Q., Hu, S., Li, W., Chen, C., et al. (2016). CRISPR-DO for genome-wide CRISPR design and optimization. Bioinformatics 32 (21), 3336–3338. doi:10.1093/bioinformatics/btw476
Makarova, K. S., Wolf, Y. I., Alkhnbashi, O. S., Costa, F., Shah, S. A., Saunders, S. J., et al. (2015). An updated evolutionary classification of CRISPR–Cas systems. Nat. Rev. Microbiol. 13 (11), 722–736. doi:10.1038/nrmicro3569
Mali, P., Yang, L., Esvelt, K. M., Aach, J., Guell, M., DiCarlo, J. E., et al. (2013). RNA-guided human genome engineering via Cas9. Science 339 (6121), 823–826. doi:10.1126/science.1232033
Manibalan, S., Thirukumaran, K., Varshni, M., Shobana, A., and Achary, A. (2020). Report on biopharmaceutical profile of recent biotherapeutics and insilco docking studies on target bindings of known aptamer biotherapeutics. Biotechnol. Genet. Eng. Rev. 36 (2), 57–80. doi:10.1080/02648725.2020.1858395
McKenna, A., and Shendure, J. (2018). FlashFry: A fast and flexible tool for large-scale CRISPR target design. BMC Biol. 16 (1), 74–76. doi:10.1186/s12915-018-0545-0
Mitrofanov, A., Alkhnbashi, O. S., Shmakov, S. A., Makarova, K. S., Koonin, E. V., and Backofen, R. (2021). CRISPRidentify: Identification of CRISPR arrays using machine learning approach. Nucleic acids Res. 49 (4), e20–e. doi:10.1093/nar/gkaa1158
Moreno-Mateos, M. A., Vejnar, C. E., Beaudoin, J-D., Fernandez, J. P., Mis, E. K., Khokha, M. K., et al. (2015). CRISPRscan: Designing highly efficient sgRNAs for CRISPR-cas9 targeting in vivo . Nat. methods 12 (10), 982–988. doi:10.1038/nmeth.3543
Muhammad Rafid, A. H., Toufikuzzaman, M., Rahman, M. S., and Rahman, M. S. (2020). CRISPRpred (SEQ): A sequence-based method for sgRNA on target activity prediction using traditional machine learning. BMC Bioinforma. 21. doi:10.1186/s12859-020-3531-9
Naito, Y., Hino, K., Bono, H., and Ui-Tei, K. (2015). CRISPRdirect: Software for designing CRISPR/cas guide RNA with reduced off-target sites. Bioinformatics 31 (7), 1120–1123. doi:10.1093/bioinformatics/btu743
O’Brien, A., and BaileyGT-Scan, T. L. (2014). GT-scan: Identifying unique genomic targets. Bioinformatics 30 (18), 2673–2675. doi:10.1093/bioinformatics/btu354
Oliveros, J. C., Franch, M., Tabas-Madrid, D., San-León, D., Montoliu, L., Cubas, P., et al. (2016). Breaking-Cas—Interactive design of guide RNAs for CRISPR-cas experiments for ENSEMBL genomes. Nucleic acids Res. 44 (W1), W267–W271. doi:10.1093/nar/gkw407
Pallarès Masmitjà, M., Knödlseder, N., and Güell, M. (2019). CRISPR gene editing . Berlin, Germany: Springer , 3–11.CRISPR-gRNA design
Park, J., Bae, S., and Kim, J-S. (2015). Cas-Designer: A web-based tool for choice of CRISPR-cas9 target sites. Bioinformatics 31 (24), 4014–4016. doi:10.1093/bioinformatics/btv537
Park, J., Lim, K., Kim, J-S., and Bae, S. (2017). Cas-analyzer: An online tool for assessing genome editing results using NGS data. Bioinformatics 33 (2), 286–288. doi:10.1093/bioinformatics/btw561
Peng, D., and Tarleton, R. (2015). EuPaGDT: A web tool tailored to design CRISPR guide RNAs for eukaryotic pathogens. Microb. genomics 1 (4), e000033. doi:10.1099/mgen.0.000033
Perez, A. R., Pritykin, Y., Vidigal, J. A., Chhangawala, S., Zamparo, L., Leslie, C. S., et al. (2017). GuideScan software for improved single and paired CRISPR guide RNA design. Nat. Biotechnol. 35 (4), 347–349. doi:10.1038/nbt.3804
Pinello, L., Canver, M. C., Hoban, M. D., Orkin, S. H., Kohn, D. B., Bauer, D. E., et al. CRISPResso: Sequencing analysis toolbox for CRISPR genome editing. bioRxiv. 2016:031203.
Pinello, L., Canver, M. C., Hoban, M. D., Orkin, S. H., Kohn, D. B., Bauer, D. E., et al. (2015). CRISPResso: Sequencing analysis toolbox for CRISPR-cas9 genome editing, bioRxiv. 031203 .
Prykhozhij, S. V., Rajan, V., Gaston, D., and Berman, J. N. (2015). CRISPR multitargeter: A web tool to find common and unique CRISPR single guide RNA targets in a set of similar sequences. PloS onee0119372 10 (3). doi:10.1371/journal.pone.0119372
Pulido-Quetglas, C., Aparicio-Prat, E., Arnan, C., Polidori, T., Hermoso, T., Palumbo, E., et al. (2017). Scalable design of paired CRISPR guide RNAs for genomic deletion. PLoS Comput. Biol. 13 (3), e1005341. doi:10.1371/journal.pcbi.1005341
Qin, R., Li, J., Li, H., Zhang, Y., Liu, X., Miao, Y., et al. (2019). Developing a highly efficient and wildly adaptive CRISPR-SaCas9 toolset for plant genome editing. Plant Biotechnol. J. 17 (4), 706–708. doi:10.1111/pbi.13047
Rabinowitz, R., Almog, S., Darnell, R., and Offen, D. (2020). CrisPam: SNP-Derived PAM analysis tool for allele-specific targeting of genetic variants using CRISPR-cas systems. Front. Genet. 11, 851. doi:10.3389/fgene.2020.00851
Rastogi, A., Murik, O., Bowler, C., and Tirichine, L. (2016). PhytoCRISP-ex: A web-based and stand-alone application to find specific target sequences for CRISPR/CAS editing. BMC Bioinforma. 17 (1), 261–264. doi:10.1186/s12859-016-1143-1
Selvakumar, S. C., Preethi, K. A., Ross, K., Tusubira, D., Khan, M. W. A., Mani, P., et al. (2022). CRISPR/Cas9 and next generation sequencing in the personalized treatment of Cancer. Mol. Cancer 21 (1), 83. doi:10.1186/s12943-022-01565-1
Shen, M. W., Arbab, M., Hsu, J. Y., Worstell, D., Culbertson, S. J., Krabbe, O., et al. (2018). Predictable and precise template-free CRISPR editing of pathogenic variants. Nature 563 (7733), 646–651. doi:10.1038/s41586-018-0686-x
Skennerton, C. T., Imelfort, M., and Tyson, G. W. (2013). Crass: Identification and reconstruction of CRISPR from unassembled metagenomic data. Nucleic acids Res. 41 (10), e105–e. doi:10.1093/nar/gkt183
Sledzinski, P., Nowaczyk, M., and Olejniczak, M. (2020). Computational tools and resources supporting CRISPR-Cas experiments. Cells 9 (5), 1288. doi:10.3390/cells9051288
Smith, R. H., Chen, Y-C., Seifuddin, F., Hupalo, D., Alba, C., Reger, R., et al. (2020). Genome-wide analysis of off-target CRISPR/Cas9 activity in single-cell-derived human hematopoietic stem and progenitor cell clones. Genes 11 (12), 1501. doi:10.3390/genes11121501
Stemmer, M., Thumberger, T., del Sol Keyer, M., Wittbrodt, J., and Mateo, J. L. (2015). CCTop: An intuitive, flexible and reliable CRISPR/Cas9 target prediction tool. PloS one 10 (4), e0124633. doi:10.1371/journal.pone.0124633
Sun, J., Liu, H., Liu, J., Cheng, S., Peng, Y., Zhang, Q., et al. (2019). CRISPR-local: A local single-guide RNA (sgRNA) design tool for non-reference plant genomes. Bioinformatics 35 (14), 2501–2503. doi:10.1093/bioinformatics/bty970
Tarasava, K., Liu, R., Garst, A., and Gill, R. T. (2018). Combinatorial pathway engineering using type I-E CRISPR interference. Biotechnol. Bioeng. 115 (7), 1878–1883. doi:10.1002/bit.26589
Tsai, S. Q., Zheng, Z., Nguyen, N. T., Liebers, M., Topkar, V. V., Thapar, V., et al. (2015). GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat. Biotechnol. 33 (2), 187–197. doi:10.1038/nbt.3117
Upadhyay, S. K., and Sharma, S. (2014). SSFinder: High throughput CRISPR-cas target sites prediction tool. BioMed Res. Int. 2014, 1–4. doi:10.1155/2014/742482
Wang, D., Zhang, C., Wang, B., Li, B., Wang, Q., Liu, D., et al. (2019). Optimized CRISPR guide RNA design for two high-fidelity Cas9 variants by deep learning. Nat. Commun. 10 (1). doi:10.1038/s41467-019-12281-8
Wang, J., Xiang, X., Cheng, L., Zhang, X., and Luo, Y. (2020). CRISPR-GNL: An improved model for predicting CRISPR activity by machine learning and featurization. bioRxiv. 2019:605790.
Wang, J., Zhang, X., Cheng, L., and Luo, Y. (2020). An overview and metanalysis of machine and deep learning-based CRISPR gRNA design tools. RNA Biol. 17 (1), 13–22. doi:10.1080/15476286.2019.1669406
Wang, K., and Liang, C. C. R. F. (2017). CRF: Detection of CRISPR arrays using random forest. PeerJ 5, e3219. doi:10.7717/peerj.3219
Wang, X., Tilford, C., Neuhaus, I., Mintier, G., Guo, Q., Feder, J. N., et al. (2017). CRISPR-DAV: CRISPR NGS data analysis and visualization pipeline. Bioinformatics 33 (23), 3811–3812. doi:10.1093/bioinformatics/btx518
Wilson, L. O., Reti, D., O'Brien, A. R., Dunne, R. A., and Bauer, D. C. (2018). High activity target-site identification using phenotypic independent CRISPR-Cas9 core functionality. CRISPR J. 1 (2), 182–190. doi:10.1089/crispr.2017.0021
Winter, J., Schwering, M., Pelz, O., Rauscher, B., Zhan, T., Heigwer, F., et al. CRISPRAnalyzeR: Interactive analysis, annotation and documentation of pooled CRISPR screens. BioRxiv. 2017:109967.
Wong, N., Liu, W., and WangWU-Crispr, X. (2015). Wu-CRISPR: Characteristics of functional guide RNAs for the CRISPR/Cas9 system. Genome Biol. 16 (1), 218–8. doi:10.1186/s13059-015-0784-0
Xiao, A., Cheng, Z., Kong, L., Zhu, Z., Lin, S., Gao, G., et al. (2014). CasOT: A genome-wide cas9/gRNA off-target searching tool. Bioinformatics 30 (8), 1180–1182. doi:10.1093/bioinformatics/btt764
Xie, S., Shen, B., Zhang, C., Huang, X., and Zhang, Y. (2014). sgRNAcas9: a software package for designing CRISPR sgRNA and evaluating potential off-target cleavage sites. PloS one 9 (6), e100448. doi:10.1371/journal.pone.0100448
Xu, H., Xiao, T., Chen, C-H., Li, W., Meyer, C. A., Wu, Q., et al. (2015). Sequence determinants of improved CRISPR sgRNA design. Genome Res. 25 (8), 1147–1157. doi:10.1101/gr.191452.115
Yan, J., Chuai, G., Zhou, C., Zhu, C., Yang, J., Zhang, C., et al. (2018). Benchmarking CRISPR on-target sgRNA design. Briefings Bioinforma. 19 (4), 721–724. doi:10.1093/bib/bbx001
Yu, S-H., Vogel, J., and Förstner, K. U. (2018). ANNOgesic: A Swiss army knife for the RNA-seq based annotation of bacterial/archaeal genomes. GigaScience 7 (9), giy096. doi:10.1093/gigascience/giy096
Zetsche, B., Abudayyeh, O. O., Gootenberg, J. S., Scott, D. A., and Zhang, F. (2020). A survey of genome editing activity for 16 Cas12a orthologs. Keio J. Med. 69 (3), 59–65. doi:10.2302/kjm.2019-0009-oa
Zhang, G., Dai, Z., and Dai, X. (2020). C-RNNCrispr: Prediction of CRISPR/Cas9 sgRNA activity using convolutional and recurrent neural networks. Comput. Struct. Biotechnol. J. 18, 344–354. doi:10.1016/j.csbj.2020.01.013
Zhu, H., and LiangCRISPR-, C. D. T. (2019). CRISPR-DT: Designing gRNAs for the CRISPR-cpf1 system with improved target efficiency and specificity. Bioinformatics 35 (16), 2783–2789. doi:10.1093/bioinformatics/bty1061
Zhu, H., Misel, L., Graham, M., Robinson, M. L., and LiangCT-Finder, C. (2016). CT-finder: A web service for CRISPR optimal target prediction and visualization. Sci. Rep. 6 (1), 25516–25518. doi:10.1038/srep25516
Zhu, H., Richmond, E., and LiangCRISPR-Rt, C. (2018). CRISPR-RT: A web application for designing CRISPR-C2c2 crRNA with improved target specificity. Bioinformatics 34 (1), 117–119. doi:10.1093/bioinformatics/btx580
Zhu, L. J., Holmes, B. R., Aronin, N., and Brodsky, M. H. (2014). CRISPRseek: A bioconductor package to identify target-specific guide RNAs for CRISPR-cas9 genome-editing systems. PloS onee108424 9 (9). doi:10.1371/journal.pone.0108424
Keywords: CRiSPR/Cas, gRNA design, on-target, off-target, computational approach, machine learning
Citation: Alipanahi R, Safari L and Khanteymoori A (2023) CRISPR genome editing using computational approaches: A survey. Front. Bioinform. 2:1001131. doi: 10.3389/fbinf.2022.1001131
Received: 22 July 2022; Accepted: 19 December 2022; Published: 11 January 2023.
Reviewed by:
Copyright © 2023 Alipanahi, Safari and Khanteymoori. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leila Safari, [email protected]
† This author share first authorship
‡ These authors have contributed equally to this work and share senior authorship
This article is part of the Research Topic
Transparent Machine Learning in Bio-Medicine
- Open access
- Published: 01 October 2021
Application of the CRISPR/Cas9-based gene editing technique in basic research, diagnosis, and therapy of cancer
- Huimin Zhang 1 na1 ,
- Chunhong Qin 1 , 2 na1 ,
- Changming An 3 na1 ,
- Xiwang Zheng 1 , 4 ,
- Shuxin Wen 5 ,
- Wenjie Chen 5 ,
- Xianfang Liu 6 ,
- Zhenghua Lv 6 ,
- Pingchang Yang 7 , 8 ,
- Wei Xu ORCID: orcid.org/0000-0002-9977-7535 6 ,
- Wei Gao ORCID: orcid.org/0000-0001-7836-2851 1 , 4 , 9 &
- Yongyan Wu ORCID: orcid.org/0000-0003-1669-3860 1 , 2 , 4
Molecular Cancer volume 20 , Article number: 126 ( 2021 ) Cite this article
50k Accesses
85 Citations
5 Altmetric
Metrics details
The 2020 Nobel Prize in Chemistry was awarded to Emmanuelle Charpentier and Jennifer Doudna for the development of the Clustered regularly interspaced short palindromic repeats/CRISPR-associated nuclease9 (CRISPR/Cas9) gene editing technology that provided new tools for precise gene editing. It is possible to target any genomic locus virtually using only a complex nuclease protein with short RNA as a site-specific endonuclease. Since cancer is caused by genomic changes in tumor cells, CRISPR/Cas9 can be used in the field of cancer research to edit genomes for exploration of the mechanisms of tumorigenesis and development. In recent years, the CRISPR/Cas9 system has been increasingly used in cancer research and treatment and remarkable results have been achieved. In this review, we introduced the mechanism and development of the CRISPR/Cas9-based gene editing system. Furthermore, we summarized current applications of this technique for basic research, diagnosis and therapy of cancer. Moreover, the potential applications of CRISPR/Cas9 in new emerging hotspots of oncology research were discussed, and the challenges and future directions were highlighted.
Cancer is a refractory disease with high mortality and global attention. The malignant tumor causes 1 out of 6 deaths globally thus threatening lives of thousands of human beings [ 1 ]. Despite many exciting achievements in the field of cancer therapy, including surgery, radiotherapy, chemotherapy, targeted biotherapy, and new combination therapies, high post-operative recurrence rates and radiation/chemotherapy resistance and harmful toxic side effects continue to be barriers to survival time and quality of life [ 2 ]. Studies have shown that cancer is a potentially fatal disease that accumulates multiple genes and alters epigenetics throughout the genome [ 3 ]. The mutation of genes in cancer usually drives the proceeding of cancer and impacts the future of tumorigenesis [ 4 ]. During the past two decades, a large number of genes related to cancer initiation and progression have been identified by high-throughput sequencing technology [ 5 , 6 ]. Based on these progressions, gene editing technology holds a big promise for cancer treatment via adjustment of the expression and correction of mutations of genes, which may lead to further breakthroughs in the field of precision oncology.
A number of techniques including zinc finger endonuclease (ZFN) [ 7 ], transcription activator-like effector nuclease (TALEN) [ 8 ], and the Clustered regularly interspaced short palindromic repeats/CRISPR associated nuclease (CRISPR/Cas) system, are applied to achieve gene editing. Due to its advantages of simple design, rapid implementation, low cost, and strong scalability, researchers consider CRISPR/Cas system as a revolutionary gene editing toolbox that has expanded to almost all genomic targets [ 9 ]. Particularly, this system has been widely used in cancer research, and has become a potential approach for cancer diagnosis and treatment [ 10 , 11 ]. Winning the 2020 Nobel Prize in chemistry is a strong indication of the superiority of CRISPR gene editing.
In this review, we focused on how CRISPR/Cas gene editing technology opens new avenues for cancer basic research, diagnosis, and therapy. We also discussed the current limitations and speculated future directions of the CRISPR/Cas technology in cancer biology.
Development of the CRISPR/Cas9-based gene editing tools
Mechanism of the classical crispr/cas9 system.
The CRISPR/Cas9 system is a heritable adaptive antiviral immune system of prokaryotes that targets infectious invading viruses and bacteriophages and uses RNA-guided nucleases to cut foreign genetic components [ 12 , 13 ]. It contains two compartments, one for Cas9 endonuclease and one for single-stranded guide RNA (sgRNA) [ 14 ]. The sgRNA directs the Cas9 endonuclease to cleave both DNA strands of the target gene in a sequence-specific manner. DNA cleavage occurs at a sequence 3 base pairs upstream of an “NGG” protospacer adjacent motif (PAM). The genome DNA is repaired by double-strand break (DNA-DSB) repair mechanisms after the cleavage [ 15 ]. Therefore, the utilization of the CRISPR/Cas9 gene editing system achieves genome modifications by the introduction of small insertions or deletions (indels) through the relatively error-prone non-homologous end-joining (NHEJ) or the high-fidelity homology-directed repair (HDR) [ 16 ] (Fig. 1 ).
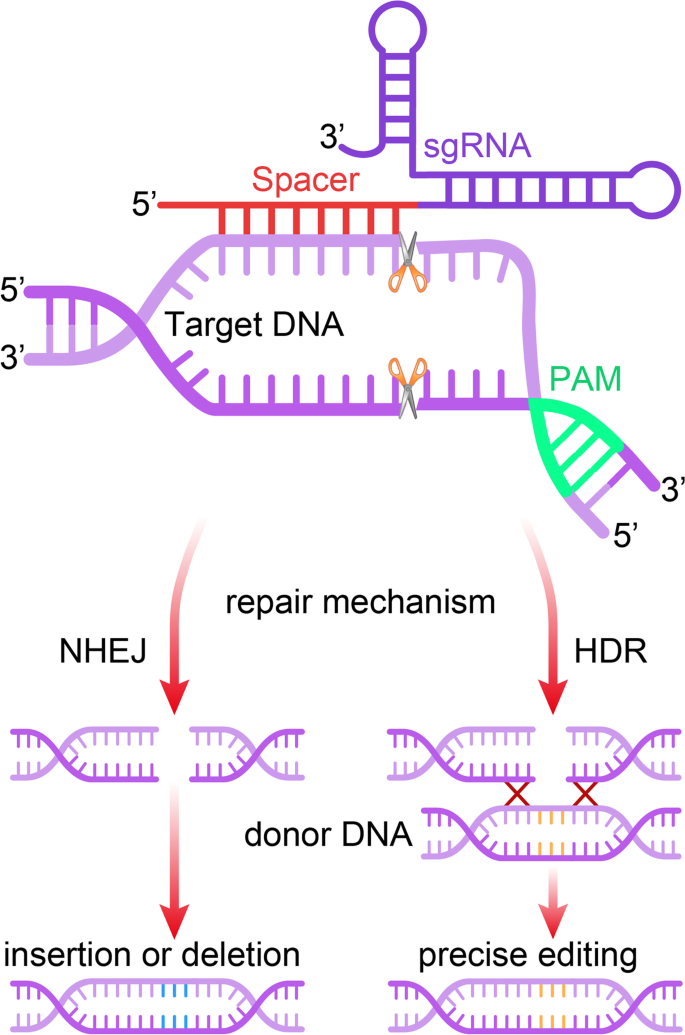
Mechanism of the CRISPR/Cas9 gene editing system. The single guide RNA (sgRNA) directs the Cas9 nuclease to a complementary sequence in the genome where Cas9 will induce a double strand break (DSB). The target genomic locus must be followed by a 5′-NGG-3′motif (protospacer adjacent motif, PAM) for Cas9 to function. DSB are repaired by non-homologous end joining (NHEJ), or by homology directed repair (HDR) in the presence of a DNA repair template, which can be exploited to introduce precise genetic modifications or exogenous sequences
Development of the CRISPR/Cas9 system and related gene editing tools
In 1987, Japanese scientists discovered some unknown tandem repeats in the Escherichia coli genome but did not explore their biological significance [ 17 ]. In 2002, these sequences were named as Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR), but the significance of these sequences remained unknown [ 18 ]. In 2005, three research teams independently discovered the role of CRISPR loci in adaptive immunity [ 19 , 20 , 21 ], then speculated that protospacer-adjacent motifs (PAMs) might direct the type II Cas9 nuclease to cleave DNA [ 21 ]. In 2007, Barrangou et al. proved that the CRISPR system is indeed an adaptive immune system and found that phage gene sequences incorporated by bacteria could change the bacteria’s resistance to phages [ 22 ]. In 2008, Brouns et al. found that non-coding RNA transcribed from the CRISPR proto-interregional sequence could guide Cas protein to the target-specific part of DNA to play a defensive role [ 23 ]. In 2011, Deltcheva et al. revealed that trans-coding crRNA (tracrRNA) was involved in the pre-crRNA processing and maturation process and their study revealed new pathways for crRNA maturation [ 24 ]. In 2012, in vitro experiments demonstrated that mature crRNA formed a special double-stranded RNA structure with tracrRNA through base complementary pairing, thus directing Cas9 protein to cause double-stranded fracture on the target DNA [ 15 ]. In 2013, Cong and Mali et al. applied the type II Cas system to the cutting of DNA in mammalian cells [ 14 , 25 ], which paved the way for the application of the CRISPR/Cas9 system in genome modification. In the same year, the Cas9 protein mutant dCas9 (endonuclease-deficient Cas9), which had lost nuclease activity, was first developed [ 26 ]. Subsequently, the CRISPR activation (CRISPRa) [ 27 ] (Fig. 2 a) and interference (CRISPRi) [ 28 ] (Fig. 2 b) tools were developed by fusing the dCas9 protein with transcription regulators that activate or inhibit gene transcription.
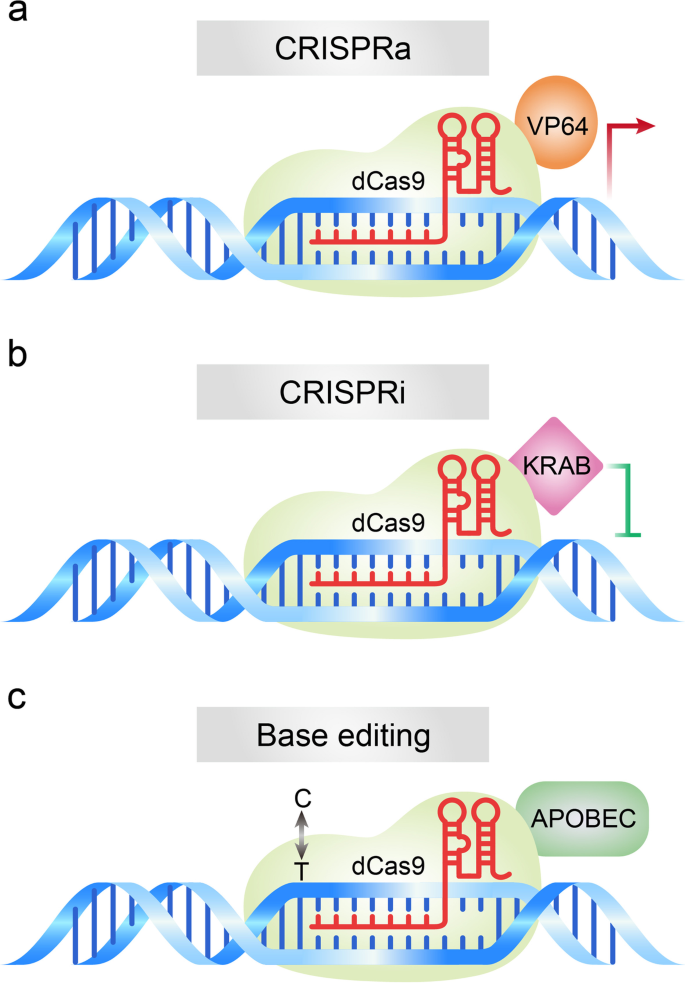
Schematic diagram of dCas9-based gene editing tools. a CRISPRa: Fusion of deactivated Cas9 (dCas9) with activation domain VP64 induces the expression of gene of interest. b CRISPRi: Fusion of dCas9 with repressor domain KRAB leads to inhibition of gene of interest. c Base editing: Fusion of dCas9 with adenosine deaminase or cytidine deaminase enable introduction of point mutation in the genome
To overcome the problem of unexpected disruptions observed in the CRISPR/Cas9 gene editing system, Komor et al. fused APOBEC (cytosine deaminase) with CRISPR/Cas9. This modified Cas9 makes the C → T (or G → A) conversion under the guidance of gRNA without causing DSB. This base editor could effectively correct a variety of point mutations in the genome [ 29 ] (Fig. 2 c). Furthermore, the adenine bases Editor (ABE) that converts A - T Base pairs to G - C Base pairs was developed [ 30 ]. The team further improved the single-base editing and the CRISPR/Cas9 systems, thus reducing greatly the miss rate of the single-base editor and improving the target range of spCas9 [ 31 , 32 ]. Interestingly, Gilpatrick et al. used Cas9 and the adapter ligation to develop a nanopore Cas9-targeted sequence (nCATS) for third-generation nanopore sequencing through modifying the target DNA region and change the structure of the target genome, which can read long fragments at low cost [ 33 ]. Recently, a caged RNA strategy was developed that allows Cas9 to bind to DNA but does not cleave before light-induced activation. This method was named very fast CRISPR (vfCRISPR) and it creates double-strand breaks (DSBs) at the submicrometer and second scales. The vfCRISPR is very accurate and can edit only one allele at a time, providing a basis for investigating complex genetic traits [ 34 ] (Fig. 3 ). Collectively, these improvements allowed for the transformation of the CRISPR/Cas9 editing tool from a blunt instrument to a precision instrument.
Development history of the CRISPR/Cas9-based gene editing tools. The “CRISPR” repeat sequence was reported in 1987 and named in 2002. In 2012, in vitro experiments demonstrated that mature crRNA formed a special double-stranded RNA structure with tracrRNA by base complementary pairing, thus directing Cas9 protein to cause double-stranded fracture on the target DNA. In 2013, the type II Cas system was applied to the cutting of DNA in mammalian cells, which paved the way for the application of the CRISPR/Cas9 system for gene editing. Since then, the CRISPR/Cas9 technology developed rapidly, and several CRISPR/Cas9-based tools were generated for gene editing at both DNA and RNA levels by 2020
CRISPR/Cas9 variations
Streptococcus pyogenes Cas9 (SpCas9) was the first to be used outside prokaryotic cells [ 15 ] and reprogrammed for genome editing in mammalian cells [ 14 ]. SpCas9 targeting of DNA relies on the 20-nucleotide-long spacer and on the PAM 5′-NGG (N represents any nucleotide) [ 35 ]. In 2015, Kleinstiver et al. obtained the SpCas9-VRER variant with NGA and the SpCas9-VRER variant with NGCG recognition through the error-prone PCR strategy [ 36 ]. In the same year, Nishimasu et al. identified the gene editing role of SaCas9 in mammalian cells; it recognizes the PAM of NNGRRT (N refers to A, T, C, G; R refers to A and G) and has excellent cutting activity and targeting accuracy [ 37 , 38 ]. In 2018, Hu et al. built xCas9 3.7 variants that could identify NGG, NG, GAA, and GAT [ 39 ]. Furthermore, a more active SpCas9-NG variant was constructed in the Nureki laboratory, and the identified PAM sequence was extended to NG [ 40 ]. Recently, Walton et al. developed a SpCas9 variant SpRY that was almost completely liberated from the constraints of the sequence of PAM [ 41 ] (Table 1 ). These advancements expanded the application scope and accuracy of the CRISPR/Cas9 system in genome editing.
Like CRISPR/Cas9, Cas12a (also known as Cpf1) has been applied to genome editing for its ability to generate targeted DSB [ 46 ] (Fig. 4 a). However, Cas12a requires only crRNA guidance for DNA targeting, and its enzyme recognizes a T-rich PAM upstream of the target region and cleaves DNA at the PAM-distal site [ 47 ]. Chen et al. combined isothermal amplification of recombinase polymerase with LbCas12a to create a method called DNA Endonuclease Targeted CRISPR Trans Reporter (DETECTR), which achieves attomolar sensitivity for nucleic acid detection. They proved that DETECTR could rapidly and specifically detect HPV in human patient samples, providing a more efficient platform for nucleic acid-based diseases [ 46 ]. Therefore, Cas12a offers a novel approach to genome editing with its unique cutting mechanism that enhances and extends the CRISPR toolkit.
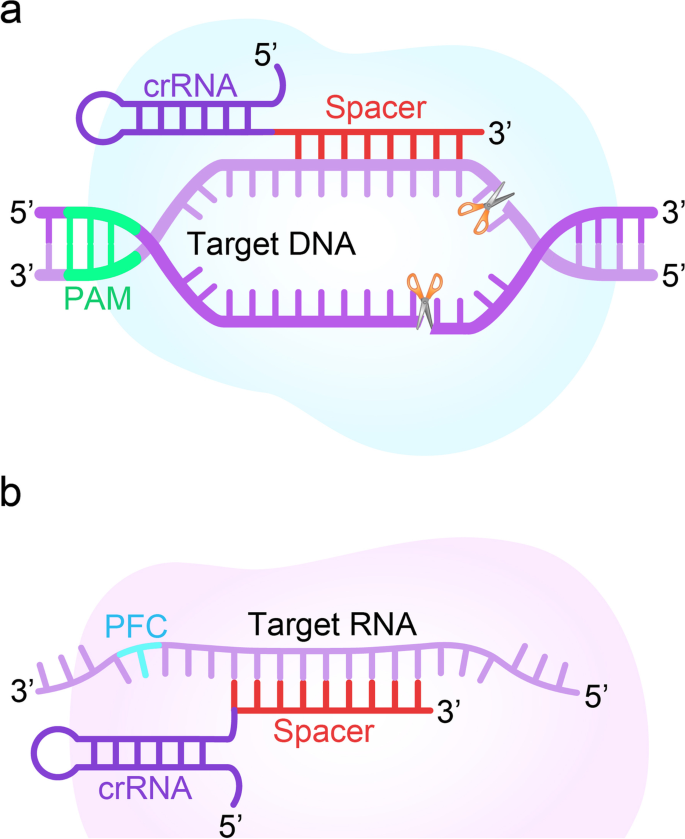
Schematic diagram of CRISPR/Cas12 and CRISPR/Cas13 systems. a CRISPR/Cas12: the crRNA directs the Cas12 nuclease to a complementary sequence in the genome where Cas12 will induce a double-strand break (DSB) of DNA. The target genomic locus must be followed by PAM for Cas12. b CRISPR/Cas13: the crRNA directs the Cas13 nuclease to a complementary sequence in the genome where Cas13 will induce a single-strand break of RNA. The target sequence must be followed by a protospacer flanking sequence (PFS) for Cas13
The type-V CRISPR effector Cas12b (formerly known as C2c1), is a dual-RNA-guided nuclease containing a single RuvC domain and requiring both crRNA and tracrRNA [ 48 ]. Cas12b generates staggered ends distal to the protospacer adjacent motif site in vitro when reconstituted with the crRNA/tracrRNA duplex [ 49 ]. Importantly, Cas12b offers a smaller size than the most widely-used SpCas9 and Cas12a, making it suitable for in vivo delivery of adeno-associated virus (AAV)-mediated gene therapy [ 47 , 50 ]. Moreover, Cas12b recognizes simpler PAM sequences than the small-sized Cas9, such as SaCas9 and CjCas9 [ 48 ], which can markedly expand the targeting range of the genome. Teng et al. reported that AaCas12b can be used for mammalian genome engineering, enabling a variety of functional applications such as single and multiple genome-editing, gene activation, and generation of mutant mouse models [ 51 ]. Recently, a CRISPR/Cas12-based diagnostic tool for the rapid and visual detection of SARS-CoV-2 from extracted RNA from the patient sample has been reported [ 52 ]. These studies revealed the functional diversity achieved in different pathways of CRISPR/Cas evolution that further extends the application of the CRISPR/Cas9.
The class II type-VI CRISPR/Cas13 system was found to be useful for RNA editing of eukaryotic cells [ 53 , 54 ]. Cas13(C2c2) is guided by a single CRISPR RNA and can be programmed to cleave single-stranded RNA targets carrying complementary protospacer [ 55 ] (Fig. 4 b). Furthermore, the RNA Editing for Programmable Adenosine to Inosine Replacement (REPAIR) system has been developed through the improvement of the Cas13 effector and can edit full-length transcripts containing pathogenic mutations [ 56 ]. Functionally, the CRISPR effector Cas13 has effective antiviral ability for single-stranded RNA (ssRNA) viruses [ 57 ]. Besides, Cas13 is used as a tool to target and knockdown viruses and to investigate their replication, localization, and evolution. Particularly, the catalytic death mutant of Cas13 (dCas13) can be used to study viral RNA localization, and dCas13 fusion proteins with RNA editing capabilities used to functionally characterize specific viral polymorphisms [ 48 , 58 ]. These studies provided tools to visualize and interfere with virus replication with high precision.
In 2017, Gootenberg et al. developed a viral detection technology based on the CRISPR/Cas13 system that could make the cut RNA bands form visual clues and display them visually. They named it Specific High Sensitivity Enzymatic Reporter Unlocking (SHERLOCK) [ 59 ]. SHERLOCK has since been applied in detecting RNA virus outbreaks such as Lassa fever, Ebola, Zika, and Dengue. It is cheaper and faster with improved efficiency of virus detection, hence helping to reduce the lethality of viruses. In the recent outbreak of novel coronavirus SARS-COV-2 that posed a major challenge to global health, Zhang Feng’s team used SHERLOCK to design a new virus detection method to maximize the specificity and accuracy of detection. Moreover, Freije et al. combined the antiviral activity of Cas13 with its diagnostic capability to build a powerful and rapidly programmable diagnostic and antiviral system, named CARVER (cas13-assisted restriction of virus expression and readout), to detect and eliminate RNA viruses in human cells [ 57 ]. Notably, researchers found that the CRISPR/Cas13d gene editing system delivered by adeno-associated virus AAV could edit and clear the new coronavirus, and this method could effectively deal with the possible mutation of the virus [ 60 ]. Taken together, Cas13 may be a potential tool for clinical diagnosis and treatment of diseases caused by viral infections.
Application of the CRISPR/Cas system in cancer research
Cancer initiation and progression involved in mutations and dysregulated expression of a series of genes [ 61 ], including oncogenes, tumor suppressor-genes, chemoresistant genes, metabolism-related genes, and cancer stem cell-related genes. The ultimate goal of cancer treatment is to restrain tumor growth and progression by specifically correcting mutations and restoring expression of dysregulated genes [ 62 ]. The CRISPR/Cas9 gene editing system has been widely used in the basic research of cancer, and some encouraging progress has been achieved.
DNA-based knockout/in
Oncogenes are regulated differently from normal genes and can promote malignant transformation of normal cells [ 63 ]. The CRISPR/Cas9 system provides a valid measure for deletion, interfering with the expression, and changing the activity of oncogenes, thereby inhibiting tumor growth [ 64 ]. The CRISPR/Cas9-mediated knockout of CD133 downregulated vimentin expression in colon cancer cells, significantly reduced cell proliferation and colony formation, and showed significant inhibitory effects on cell migration and invasion [ 65 ]. Knockdown of miR-3064 using CRISPR/Cas9 technology significantly inhibited the proliferation, invasion, and tumorigenic capacity of pancreatic cancer (PC) cells [ 66 ]. Besides, using CRISPR/Cas9 knockdown of oncogenic mutant EGFR alleles, the growth and proliferation of lung cancer cell lines H1975, A549, and H1650 were found to be inhibited, and the tumor size of xenografted mice implanted with H1975 or A549 cells was reduced [ 67 ]. Knockdown of the FAK gene in NSCLC cells with KRAS mutations using CRISPR/Cas9 resulted in detectable DNA damage and increased sensitivity to radiotherapy [ 68 ]. Furthermore, the CRISPR/Cas9-mediated deletion of the E3 ubiquitin ligase UBR5 in an experimental murine model of triple-negative breast cancers (TNBC) remarkably inhibited tumor growth and metastasis in vivo [ 69 ] (Fig. 5 a). These studies indicated that the CRISPR/Cas9 gene editing system is an effective tool for identifying oncogene and evaluating the therapeutic potential of oncogene targeting.
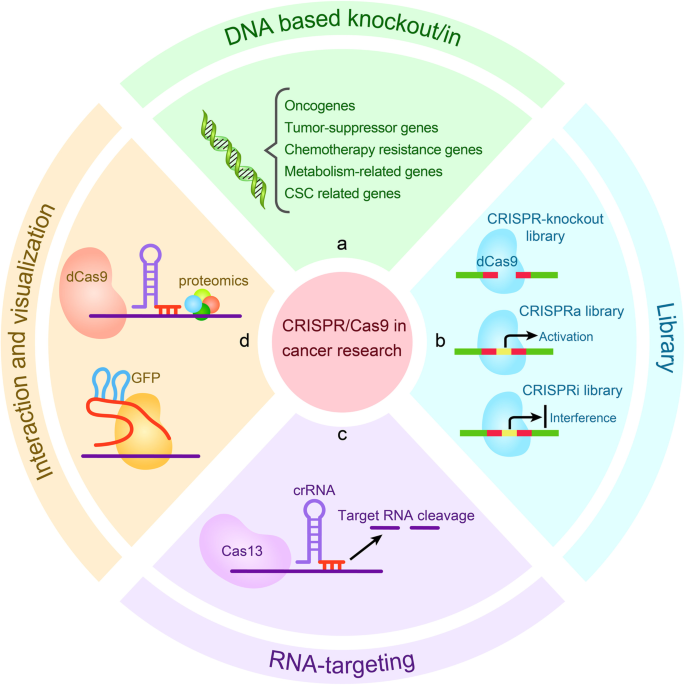
Applications of CRISPR/Cas9-based gene editing tools for basic research of cancer. a CRISPR/Cas9-mediated knockout or knockin was applied to identify and verify of functional gene, including oncogene, tumor-suppressor gene, chemoresistant gene, metabolism-related gene, and cancer stem cell-related gene. b CRISPR/Cas9 library screen for drug target and functional gene. c Application of the CRISPR/Cas13 system for RNA targeting. d In vivo biotinylated dCas9 protein was used for interaction research, and dCas9 protein fused with fluorescent markers such as GFP was used for imaging analysis
Tumor-suppressor genes
Inactivation of tumor suppressor genes is a significant feature in the initiation and progression of cancer [ 70 ]. The silencing, deficiency, or mutation of tumor-suppressor gene activates oncogenes, leading to tumor initiation and progression [ 71 ]. Notably, the application of the CRISPR/Cas9 system has reformed a revolution in cancer research by enabling the rapid validation of tumor-suppressor gene in vitro and in vivo.
The mutation of NF2 (Neurofibromin 2), a gene to inhibit tumorigenesis, was found in malignant pleural mesothelioma (MPM). Compared to the untreated cells, an NF2-knockout human mesothelial cell line, MeT-5A (NF2-KO) showed enhanced migration and invasion abilities [ 72 ]. Similar phenomenon was observed on MFN2 (mitochondrial GTPase mitofusin-2) knockout cells in lung cancer via CRISPR/Cas9 [ 73 ]. Deletion of multiple tumor suppressor genes (including p53, Nf1, Pten, and Ptch1) in the mouse brain by CRISPR/cas9 resulted in the development of glioblastoma [ 11 ]. The loss of LATS1/2 in many mouse cancer cell lines through CRISPR/Cas9 significantly increased anchorage-independent growth in pancreatic cancer Panc02, prostate cancer MyC-Cap, breast cancer 168FARN and 67NR cells, and growth of colon cancer CT26, glioma GL261, and bladder cancer MB49 [ 74 ] (Fig. 5 a). These studies showed that the use of CRISPR/Cas9 technology is able to identify tumor-suppressor gene.
Furthermore, CRISPR/Cas9 technology has shown cancer therapeutic benefit by repairing inactivated tumor-suppressor genes. Moses et al. used the CRISPR/dCas9 in combination with the trans-activator VP64-p65-Rta (VPR) to activate PTEN expression in cancer cells with low-level PTEN expression. Results showed that the PTEN expression level was increased in melanoma and TNBC cell lines by the dCas9-VPR system, and the activation of PTEN obviously inhibited downstream oncogenic pathways [ 75 ]. Artegiani et al. found that after loss-of-function of BAP1 by CRISPR/Cas9 in normal human cholangiocyte organoids, the cells became more motile and fused with other organoids, features that resemble the metastatic invasion of cancer. Interestingly, they restored the catalytic activity of BAP1 in the nucleus and rescue the cellular and molecular alterations [ 76 ].
Chemotherapy resistance genes
The development of resistance of cancer cells to chemotherapeutic drugs is a major obstacle to cancer treatment. The main mechanism of chemoresistance is dysregulation of chemoresistance related genes [ 77 ]. Therefore, identifying chemoresistance related genes and modulating their expression levels or functions are key to the elimination of chemoresistant cancer cells.
CRISPR/Cas9-mediated knockdown of NRF2 in a lung cancer cell xenograft mouse model had enhanced sensitivity to cisplatin and carboplatin [ 78 ]. Furthermore, Gao et al. found that CRISPR/Cas9-mediated knockdown of SKA3 enhanced the sensitivity of laryngeal cancer cells to cisplatin in preclinical model [ 79 ]. Knockdown of RSF1 using CRISPR/Cas9 technology significantly increased the sensitivity of H460 cells to paclitaxel [ 80 ]. In contrast, knockdown of ERCC1 decreased the sensitivity of lung cancer cell lines to cisplatin [ 81 ]. The knockdown of aurora B (AURKB) in NSCLC cell lines by CRISPR/Cas9 also restored the expression of the tumor suppressor gene TP53 and sensitivity to cisplatin and paclitaxel [ 82 ]. Therefore, the use of CRISPR/Cas9 technology enable to identify and validate chemoresistant genes, which are of great significance in the clinical treatment of cancer.
Metabolism-related genes
Tumor cells are dependent on an adequate supply of energy to support proliferation, migration, and invasion as occurs with metastatic cells. The research suggested that metabolic reprogramming, the regulation of energy metabolism to promote rapid cell growth and proliferation, is the new Hallmark of cancer [ 63 ]. Cancer cells tend to favor the “Warburg effect”, which promotes glycolysis or aerobic glycolysis, even in the adequate supply of oxygen [ 83 ]. In addition to glucose metabolism disorders, abnormal lipid metabolism, amino acid metabolism, mitochondrial biogenesis, and other bioenergy metabolism pathways also exist in cancer cells [ 84 ]. Therefore, understanding the energy metabolism mechanism might provide us with new ideas to target energy production pathways in cancer treatment.
Common markers expressed in high levels in cellular hypoxia, glucose transporter-1 (GLUT-1) and hypoxia-inducible factor-1α (HIF-1α), have been associated with the biological behavior of cancer [ 85 , 86 ]. Under the hypoxic stress condition, these two proteins are important for glucose uptake and glycolysis in laryngeal cancer cells [ 87 ]. Lu et al. conducted HIF-1α and GLUT − 1 gene knockout in HEp-2 cells by CRISPR/Cas 9 system, leading to decreased proliferation, migration, and invasion. They found that HIF-1α and GLUT-1 gene knockout resulted in a significant reduction in glucose uptake and lactic acid of HEp-2 cells [ 88 ].
Using an impartial genome-wide CRISPR/Cas9 screen, Gallipoli et al. revealed that glutaminase (GLS) existing in glutamine metabolism as a primary enzyme had a synthetically lethal effect with FLT3 tyrosine kinase inhibitors (TKI) treatment. Subsequently, combined with complementary metabolomic and gene-expression analysis, they indicated that there is a metabolic dependency relationship between glutamine metabolism and FLT3 internal tandem duplication (FLT3ITD) cells in acute myeloid leukemia (AML) after FLT3 TK inhibition. They used these approaches to explore AML subtypes driven by other tyrosine kinases (TK) activating mutations and verified the possibility of GLS as a clinically therapeutic target in AML [ 89 ].
The chemotherapeutic drug methotrexate is cytotoxic through inhibition of the synthesis of nucleotides which inhibit the DHFR enzyme (dihydrofolate reductase) that produces tetrahydrofolate (THF) and reduces its potential efficiency thereby leading to cell death. Kanarek et al. conducted a CRISPR/Cas9-based screening that generated FTCD, an encoded enzyme that is essential for histidine catabolism (formimidoyltransferase cyclodeaminase). They found that the absence of multiple genes in the histidine catabolism pathway significantly reduced sensitivity to methotrexate in cultured cancer cells. Thus, the flow through the histidine degradation pathway could be increased by dietary supplementation of histidine in vivo, which enhances the sensitivity of leukemic xenografts to methotrexate [ 90 ] (Fig. 5 a). This application of CRISPR/Cas technology in tumor metabolism has brought new insights into the treatment of cancer.
Cancer stem cell-related genes
Cancer stem cells (CSC) are self-renewing cells in tumors that can produce heterogeneous tumor cells, which play critical roles in cancer initiation, progression, recurrence, and therapeutic resistance [ 91 ]. Since CSC may be derived from oncogene reprogramming and the dynamic characteristics of cancer cells, the identification of CSC-related gene is speculated to generate new cancer treatment targets [ 92 ]. Nowadays, the application of CRISPR technology in tumor stem cells has provided a new direction for clinical tumor treatment.
Ovarian cancer stem cells (OCSC) lead to a poor prognosis of ovarian cancer. Nanog has been identified as the key gene that maintains CSC pluripotency and self-renewal capability [ 93 ]. Androgen receptors (AR) are involved in the malignant behavior of other tumors [ 94 ]. Ling et al. constructed a green fluorescent protein (GFP) labeled Nanog cell model in ovarian cell lines (A2780 and SKOV3) using the CRISPR/Cas9 system, they found that the interaction of Nanog with the AR signaling axis may induce or contribute to the regulation of OCSC [ 95 ]. In another study, Yang et al. used CRISPR/Cas9 technology to knockdown the transcription factor YB-1 gene in cancer stem cells. They observed that the absence of YB-1 inhibited the proliferation of breast cancer and melanoma stem cells, leading to cell cycle arrest, apoptosis, and irreversible differentiation [ 96 ]. In colorectal cancer, aberrant Wnt signaling is critical for the development and maintenance of cancer stem cells [ 97 ]. Zhan et al. found that APC truncation mutations generated by CRISPR/Cas9 and MEK inhibitors synergistically enhanced Wnt responses in a CRC model. Using CRC-like organs derived from patients, they demonstrated that MEK inhibition leads to an increased Wnt activity and enhanced genetic markers associated with stemness and cancer recurrence [ 98 ]. This provides a potential solution for the treatment of colorectal cancer. Hwang et al. used CRISPR/Cas9 to knockdown REG4 in colorectal cancer spheres containing both APC and KRAS mutations, and results showed that knockdown of REG4 inhibited Wnt/β-linked protein signaling and thus effectively suppressed CSCs properties [ 99 ].(Fig. 5 a). These findings explored the regulatory mechanisms of cancer stem cell stemness from multiple perspectives and provide new ideas for the CSC targeting treatment of cancer.
Using CRISPR/Cas9 library for screening functional genes in cancer cells
Cancer cell genomes carry a diversity of genetic aberrations that accumulate from congenital and acquired mutations and are triggered by successive clonal expansions [ 63 ]. Identifying the genes that drive tumor evolution can clarify the initiation and development of cancer [ 3 ]. Large-scale genomic screening is a powerful tool to detect the mutated genes that cause various cancers [ 63 ]. Using CRISPR to perform functional genomic screening can reveal phenotype changes after drug treatment or other stimulation, thereby identifying new target for cancer treatment [ 100 ].
Large-scale screening using CRISPR/Cas9 knockout libraries is widely used in gene loss-of-function studies. In 2014, Shalem et al. constructed a sgRNA library targeting 18,080 genes in the human genome and named it Genome-wide CRISPR/Cas9 knockout Library (GecKO). Using this system, they screened candidate genes that respond to vemurafenib (a therapeutic RAF inhibitor) in the human melanoma cell line A375, and six candidate genes were identified, including NF1, MED12, NF2, Cul3, TADA2B, and TADA1 [ 101 ]. In 2015, Chen et al. performed a genome-wide CRISPR/Cas9-mediated loss-of-function screen in a mouse model of tumor evolution. They used a lentiviral mouse sgRNA library named mGeCKOa, which containing 67,405 sgRNAs targeting 20,611 protein-coding genes and1175 microRNA precursors. A total of 5 protein-coding genes and 2 microRNAs were identified as metastasis-suppressive genes, including Nf2, Pten, Cdkn2a, Trim72, Fga, miR345, and miR-152 [ 102 ].
The tumor suppressor gene ATRX is frequently mutated in a variety of tumors, including hepatocellular carcinoma (HCC) and glioma [ 103 , 104 ], and has a minimal response to current therapies. Liang et al. revealed that the checkpoint kinase WEE1 was a potential therapeutic target for ATRX mutant cancers using CRISPR/Cas9 whole-genome screening technology. Subsequent experiments revealed that treatment with the WEE1 inhibitor AZD1775 robustly inhibited the growth of several ATRX-deficient HCC cell lines in vitro, as well as in vivo xenografting. Thus, the discovery of a synthetic lethal relationship between WEE1 and ATRX could be widely used for therapeutic applications in human tumors [ 105 ]. Additionally, researchers used the CRISPR/Cas9 library screening technique to knockout more than 3000 genes involved in T-cell metabolism. They tested the function of these genes in a mouse anti-tumor ACT (Adoptive cell therapy) model and found that damage to the gene encoding the REGNASE-1 enzyme caused more T-cells to infiltrate tumor tissue. Subsequently, they used CRISPR/Cas9 knockout library to destroy about 20,000 genes in REGNASE-1-deficient CD8+ T cells. Their study indicated that the loss of REGNASE-1 protein prolongs the survival of anti-tumor CD8+ T cells, enhances their function, and enable T cells to fight cancer better and more effectively [ 106 ]. Moreover, Zhu et al. developed a CRISPR/Cas9 strategy using paired gRNAs (pgRNAs) for large segment deletions to identify functional long non-coding RNAs in cancer cells. By applying this method, the researchers identified 51 lncRNAs that positively or negatively regulate the growth of human cancer cells [ 107 ]. Subsequently, the same team developed an alternative loss-of-function screen for 10,996 multiple exon lncRNA splice sites in chronic myeloid leukemia cells K562 and identified 230 lncRNAs associated with cell survival or proliferation [ 108 ] (Fig. 5 b).
CRISPRi (CRISPR inhibition) is another important CRISPR/Cas9-based technology that was developed for loss-of-function screening in cancer research. Since CRISPRi functions only in a small range (1 kb) around the target transcription start site (TSS) [ 109 ], and dCas9 blocks only 23 bp of the targeted sequence [ 37 ], CRISPRi can interfere precisely with any lncRNA gene. Liu et al. developed a CRISPRi library targeting 16,401 lncRNA loci to screen cell lines, including iPSCs (human induced pluripotent stem cells), and transformed cell lines. They identified 499 loci that are required for robust cell growth [ 110 ]. Jost et al. used a CRISPRi/a-mediated chemogenetic screen to identify a target of anticancer drug Rigosertib. Their results indicated that tubulin with structure-directed mutations at the interface with rigosertib developed resistance to rigosertib, and it was determined that rigosertib kills cancer cells by destabilizing microtubules [ 111 ]. This work confirmed the importance of CRISPR-based chemical gene screening in identifying physiologically relevant targets for drugs. Raffeiner et al. used a pooled library screen of dCas9 fused to the efficient transcriptional repression domain of the MXD1 protein to identify non-coding sites required for the growth of the human lymphoblastoid cell lines P493–6 and RAMOS. The study also provides additional CRISPRi-based tools to facilitate genetic perturbation of noncoding targets [ 112 ] (Fig. 5 b). In summary, these approaches open the door to both coding and non-coding RNA screening.
In contrast to pooled libraries for knockdown screening, sgRNAs in CRISPR activation (CRISPRa) libraries target the promoter sites of target genes. CRISPRa in particular has enormous potential for the elucidation of drug resistance mechanisms in cancer cells, which are thought to arise frequently from gain-of-function events. The use of CRISPRa screening in BRAF (V600E) melanoma cells for resistance to the BRAF inhibitor PLX-4720 not only reproduced previously known resistance mechanisms, such as EGFR and ERK pathway activation but also revealed novel resistance mechanisms regarding G protein-coupled receptors [ 113 ]. Furthermore, Melanoma was screened for CRISPRa library and positively selected by the BRAF protein kinase inhibitor vemurafenib (PLX). EGFR, PCDH7, ITGB5, ARHGFE1, BCAR3, GPR35, and TFAP2C were identified as PLX-resistant genes. Activation of these genes may be related to the ERK pathway, leading to PLX resistance [ 114 ]. Moreover, researchers constructed a genome-wide CRISPRa library targeting 14,701 lncRNA genes. By screening with this library, lncRNA GAS6-AS2 was found to lead to Ara-C resistance in multiple cancers, including AML [ 100 ]. In a recent study, Wang et al. developed new cancer immunotherapy, MAEGI (Multiple Activation of Endogenous Genes as Immunotherapy), which uses CRISPR activation technology to directly activate endogenous mutant genes, amplify specific signals from cancer cells, and induce effective adaptive anti-tumor immunity. This is a versatile and highly scalable strategy that is effective against multiple cancer types, including those currently resistant to immunotherapy [ 115 ] (Fig. 5 b). Collectively, CRISPRa library screening provides more advanced strategies for tumor research and is a good guide for the clinical treatment of cancer.
Application of CRISPR/Cas13 for RNA targeting in cancer
With the development of gene editing technology, researchers demonstrated that the class 2 type VI RNA-guided RNA-targeting CRISPR/Cas effector Cas13 (previously known as C2c2) can be engineered for mammalian cell RNA knockdown [ 58 ] (Fig. 5 c). This technology is also increasingly being used in cancer research. Qi et al. built a light sensor that effectively induced Cas13a protein expression after blue light irradiation. They select the lncRNA Metastasis-associated Lung Adenocarcinoma Transcript 1 (MALAT1) as the functional target. Their results showed that the expression of MALAT1 was significantly downregulated by the light-switchable CRISPR/Cas13a system in bladder cancer cells [ 116 ]. Recently, Wang et al. overexpressed the LwCRISPR/Cas13a by lentivirus in glioma cells reveals that crRNA-EGFP induces a “collateral effect” after knocking down the target gene in EGFP-expressing cells. This study expands the application scope of the CRISPR/Cas13a system [ 117 ]. Taken together, these studies demonstrated that the CRISPR/Cas13a system provides a new approach for RNA manipulation in cancer cells.
Using CRISPR/dCas9 for interactions and visualization research
Importantly, identifying molecules associated with genomic regions of interest in vivo helps to understand locus function (Fig. 5 d). Researchers have established an enChIP (engineered DNA-binding molecule-mediated ChIP) system that uses catalytically inactive dCas9 to purify genomic sequences designated by specific gRNAs [ 118 ]. For example, enChIP was used for biochemical analysis of epigenetic regulation and transcription at specific genomic loci in living cell lines including HT1080 (a human fibrosarcoma cell line) and K562 (a human leukemia cell line) [ 119 ]. Recently, a CRISPR affinity purification in situ of regulatory elements (CAPTURE) system has been developed to identify locus-specific chromatin-regulating protein complexes and long-range DNA interactions. The CAPTURE system can isolate chromatin interactions at a single-copy genomic locus using an in vivo biotinylated dCas9 protein and sequence-specific guide RNAs [ 120 ] (Fig. 6 a). The ability of CAPTURE to allow the isolation and analysis of the factors that regulate DNA offers multiple possibilities for studying how different proteins control genomic function in cancer cells and stem cells. It also provides an entirely new avenue for discovering new drug targets.
Schematic diagram of dCas9-based methods for molecular interactions and visualization research. a dCas9 mediated capture of chromatin complex and downstream analysis. b dCas9-mediated imaging of genomic elements in living cells
Visualization of chromosome dynamics and shapes in a live cell is very important in the field of cell biology. The copy number of a specific chromosome in cancer cells is usually abnormal, so detecting the chromosome copy number can help cancer diagnosis. In the interphase, each chromosome exists in its nuclear region and can be imaged by fluorescence in situ hybridization (FISH) using sequence-specific probes of different colors [ 121 ]. Nonetheless, such chromosome mapping is only applicable to fixed cells and cannot be dynamically monitored in live cells. Recently researchers fused catalytically inactivated Cas9 (dCas9) with fluorescent markers (such as GFP) turning dCas9 into a customizable DNA marker that is compatible with live cell fluorescence microscopy [ 121 ] (Fig. 6 b). CRISPR-based imaging has many advantages over other imaging technologies because gRNA is easy to design and implement, thereby it can be programmed for different genomic sites and can detect multiple genomic sites at the same time [ 122 ]. Alternatively, gRNA and protein-interacting RNA aptamers can be fused, the latter will recruit specific RNA binding proteins (RBP) labeled with fluorescent proteins to visualize target genomic sites [ 123 ]. Therefore, the fluorescent CRISPR system has been used for the dynamic tracking of genomic loci and mapping of chromosomes in living cells. In a recent study, Artegiani et al. developed a new tool called CRISPR-hot (CRISPR/Cas9-mediated homology-independent organoid transgenesis) that fluoresces and visualizes specific genes in human organs. They used CRISPR-hot to insert fluorescent tags into the DNA of human-like organs [ 124 ]. These new techniques open a wide outlook for studying the real-time dynamic cellular processes in the cell, tissues, and even organs.
Application of CRISPR/Cas9 in cancer diagnostics
Early detection and treatment of cancer can reduce mortality and improve the life quality of patients after treatment. Although several techniques are widely used for cancer detection, they need improvements in terms of sensitivity, specificity, and speed. Therefore, identifying sensitive genes through genetic diagnosis is key to the prevention of cancer [ 125 ] (Fig. 7 a). To overcome these problems, Gootenberg et al. established a CRISPR-based diagnostic system called SHERLOCK (Specific High Sensitivity Enzymatic Reporter UnLOCKing). This system consists of the RNA-guided RNase Cas13a (induces robust non-specific single-stranded DNA (ssDNA) trans-cleavage as a collateral effect) and a reporter signal (released after RNA cleavage), which showed high sensitivity in mutation detection of BRAF V600E and EGFR L858R in mammalian cells [ 59 ]. Another similar system named DETECTR (DNA endonuclease-targeted CRISPR trans reporter) consists of Cas12a and recombinase polymerase amplification (RPA) and is used as a detection tool to screen for viral infections in cancer and to amplify micro-samples. The system is rapid and inexpensive for detecting high-risk HPV types, such as HPV16 and HPV18, in samples infected with many different types of HPV [ 46 , 126 ].
Applications of CRISPR/Cas9 gene editing tools for diagnosis and therapy of cancer. a Using CRISPR/Cas9-based diagnostic system SHERLOCK and DETECTR for detecting cancer. b CRISPR/Cas9 edits immune cells in vitro, and then these cells were administrated to patients to combat against cancer. c Knockout of inhibitory receptors PD-1 by CRISPR/Cas9 technology improves the efficacy of cancer immunotherapy. d Viral genome-specific Cas9-sgRNA eliminates oncogenic virus. e Establishing in vivo tumor model with multiple gene mutations with CRISPR/Cas9 gene editing tools
miRNAs are involved into many diseases and have potential value in the diagnosis and treatment of cancer. However, in view of the complexity, the available miRNAs detection methods have low sensitivity and are of high cost thus, need for improvement. It has been shown that a new isothermal amplification platform based on CRISPR/Cas9 technologies can be used for efficient detection of miRNAs [ 127 ]. This was the first time the CRISPR/Cas9 method has been used for miRNA detection. The further development of CRISPR detection technology will provide a rapid, scalable, and high-resolution platform for the diagnosis of cancer.
Application of CRISPR/Cas9 in cancer therapy
In addition to making rapid progress in basic research of oncology, CRISPR/Cas-mediated genome editing also has broad prospects in cancer treatment. Tumorigenesis is a multistep process involving complex interactions between cancer cells and the host immune system [ 128 ]. The combination of the CRISPR/Cas technique with cancer immunotherapy and its application to combating carcinogenic virus infection offers great promise.
Chimeric antigen receptor T(CART) cells
Cancer immunotherapy is another widely recognized treatment after surgery, chemotherapy, and radiation therapy. Adoptive T cell immunotherapy, especially chimeric antigen receptor (CAR) T cell therapy, has ushered in a new era of cancer therapy, especially after the FDA approved Kymriah and Yescarta (CD19-oriented CAR T cells in B-cell leukemia and lymphoma) [ 129 ] (Fig. 7 b). Clinical trials have shown that CAR T-cell therapy can alleviate clinical symptoms in patients with a variety of hematologic and solid cancers, particularly in relapsed/refractory acute lymphoblastic leukemia (ALL) and multiple myeloma, providing hope of a cure for patients with relapsed and refractory hematologic cancers [ 130 , 131 , 132 ]. Although the widely used autologous CAR T cells have shown promising results in cancer treatment, there are still limitations that affect their therapeutic efficacy. The combination with CRISPR/Cas9 technology would bridge the gap in T-cell engineering.
An MSKCC (Memorial Sloan Kettering Cancer Center) group has used CRISPR/Cas9 technology to construct more effective CAR-T cells using targeted insertion of the CAR gene delivered to specific locations. It is a precise method that kills cancer cells in the long term, is safer, and enhances the effectiveness of T cells [ 133 ]. Researchers have developed novel antigen-specific immunotherapies using CAR-T cell-based combined with CRISPR/Cas9 technology. They used the CRISPR/Cas9 tool to remove a specific protein called CD33 from healthy cells. Healthy stem cells that lacked CD33 were able to function normally as well, making CD33 a unique marker for leukemia cells and enabling CAR-T cell therapy to easily identify and attack cancer cells [ 134 ].
Researchers at the University of Pennsylvania conducted a human clinical trial in which they used CRISPR technology to delete two genes, TCRα (TRAC) and TCRβ (TRBC), encoding the endogenous T cell receptor (TCR) chain in T cells to reduce TCR mismatch and enhance the expression of a synthetic cancer-specific TCR transgene (NY-ESO − 1). They then deleted a third gene encoding PD-1 (PDCD1) to enhance antitumor immunity. This approach may help avoid host-mediated immunity and thus provide patients with an anti-leukemic effect without the fear of graft-versus-host disease (GVHD). This was the first demonstration of the ability of CRISPR/Cas9 technology to target multiple human genes simultaneously [ 135 ]. In a study using CRISPR/Cas9 to disrupt GM-CSF, CART19 cells deficient in GM-CSF were constructed with enhanced cellular function, increased antitumor activity, and improved overall survival. Additionally, after neutralizing GM-CSF using lenzilumab, there was durable control of leukemia and reduced cytokine release syndrome (CRS) and neuroinflammation in patient-derived xenografts. They plan to conduct a phase II clinical study using a combination of lenzilumab and CART19 cell therapy [ 136 ]. These studies confirm the feasibility of CRISPR/Cas9 gene editing in cancer immunotherapy.
PD-1/PD-L1 targeted therapy
Programmed death ligand 1 (PD-L1) is an important immunosuppressive molecule, which can down-regulate the immune system’s response to avoid autoimmune diseases [ 137 , 138 ] (Fig. 7 c). PD-L1 is expressed in a variety of immune and cancer cells. By interacting with PD-1 on T cells, PD-L1 inhibits the activity and growth of T cells, promotes the exhaustion of T cells, and induces apoptosis of activated T cells to help tumor cells to escape host immunity [ 139 , 140 ]. Therefore, inhibiting the interaction between PD-1 and PD-L1 through inhibitors can allow T cells to kill and eliminate tumor cells normally, a treatment strategy that is effective for anti-tumor immunity. Cyranoski et al. conduct the first CRISPR human trial to treat patients with metastatic non-small cell lung cancer who had failed to respond to chemotherapy, radiation, and other therapies with CRISPR-edited T cells (knockout PD-1 gene) [ 141 , 142 ]. They recently published the results of the latest trial demonstrating the safety and feasibility of CRISPR gene-edited T cells targeting PD-1 in a cohort of patients with advanced lung cancer(NCT02793856) [ 143 ]. Besides, there are clinical trials (NCT02867345, NCT02863913, NCT04417764, NCT03081715, NCT02867332) of CRISPR-mediated PD-1 gene knockout in patients with prostate cancer, bladder cancer, hepatocellular Carcinoma, advanced esophageal cancer and metastatic renal cell carcinoma associated with treatment [ 144 ]. Additionally, it is reported that destroying PD-1 enhances the anti-tumor activity of CAR-T cells against hepatocellular carcinoma in vivo, and improves the persistence and infiltration of CAR T cells in tumors [ 145 ]. He et al. delivered CRISPR/Cas9 plasmids to the tumor nucleus through the use of an Aptamer/peptide-functionalized vector to knockout the β-catenin, thus downregulating the expression of PD-L1 on tumor cells. They found that the PD-L1 mediated immune escape and immunosuppression of cancer was reversed [ 146 ]. These studies provided strategies to reverse tumor immune escape and immunosuppression. The developed genome-editing delivery system has broad research and application prospects for cancer treatment.
Combating carcinogenic virus infection
The CRISPR/Cas9 system has an antiviral role in bacterial adaptive immunity and thus great potential for the defense and clearance of infected viruses [ 147 ]. Carcinogenic viral infections are one of the causes of cancer and commonly include hepatitis B virus (HBV) and hepatitis C virus (HCV) in liver cancer, human papillomavirus (HPV) in laryngeal cancer [ 126 ], and Epstein-Barr virus (EBV) in nasopharyngeal carcinoma, Hodgkin’s lymphoma, and Burkitt’s lymphoma [ 148 ]. The use of Cas9-sgRNA, which can specifically recognize the viral genome, directly targets oncogenic viral genes or genes required to maintain viral replication. This results in mutations in the viral genome and suppression of oncogenic viral gene expression thereby inducing cancer cell death. Consequently, fighting viral infection and eliminating cancer through CRISPR/Cas technology provides new ideas for the treatment of cancers associated with viral infection (Fig. 7 d).
The occurrence of cervical cancer is mainly caused by HPV. HPV-related tumors are attributed to HPV E6 and E7 proteins, which are involved in the malignant transformation of cervical cancer and the maintenance of the malignant phenotype [ 149 ]. In HPV16 and HPV-18 positive cervical cancer cells, knockdown of the E6 and E7 genes with CRISPR/Cas9 restored cellular tumor suppressor p53 and retinoblastoma (Rb) protein levels, resulting in cell death and apoptosis [ 150 ]. Similar results were also observed when the HPV-16 E6 and E7 genes were knockdown in a nude mouse model of cervical cancer cells, and tumor growth was inhibited [ 151 ].
The development of hepatocellular carcinoma (HCC) is closely related to HBV infection of hepatocytes, in which HBV covalent closed-loop DNA (cccDNA) plays a crucial role [ 152 ]. Therefore, the removal of cccDNA from hepatocytes is necessary to cure HBV infection. A number of studies have demonstrated that CRISPR/Cas9-mediated HBV DNA editing can effectively reduce cccDNA in cells and mouse models and inhibit viral production [ 153 , 154 , 155 , 156 ]. Recently, using CRISPR/Cas9 technology, researchers found that the lncRNA PCNAP1 enhances HBV replication by regulating miR-154/PCNA/HBV cccDNA signaling, explaining the mechanism of the effect of lncRNAs on HBV replication and HCC [ 157 ]. Besides, the targeted cut mouse p53 and Pten genes were delivered to the liver tissue of adult HBV transgenic mice through the CRISPR/Cas9 system, and tumors were found in the liver of the mice. This observation proved that CRISPR/Cas9-mediated somatic p53 and Pten mutations can accelerate the occurrence of primary hepatocellular carcinoma in adult HBV transgenic mice [ 158 ]. HCV can also promote the development of HCC. Price et al. designed Francisella novicida Cas9 (FnCas9) to precisely target the viral RNA genome of HCV through RNA-guided RNA recognition, to reduce the production of viral proteins and inhibit HCV infection [ 159 ]. Since FnCas9 can target both RNA and DNA, this multifunctional endonuclease may be able to fight multiple types of viruses at the same time, such as HBV and HCV.
EBV is related to a variety of human malignancies, and studies have used the CRISPR/Cas9 system to target EBV. When using Cas9/gRNA transfected Burkitt lymphoma human cells to target EBNA-1, LMP-1, or EBNA-3C genes, the cell proliferation and the viral load are significantly reduced [ 160 ] and almost a complete clearance of the latent EBV infection occurs [ 161 ]. Moreover, the researchers conducted a human genome-wide CRISPR/Cas9 screen in Burkitt’s lymphoma B cells to systematically analyze the host factors regulating the EBV proliferative infection cycle for the first time and deeply characterized the molecular mechanism of EBV switching from latent infection to viral proliferative infection cycle. Multiple drug therapeutic targets were identified, which laid the foundation for tumor cell-transient immunotherapy targeting EBV infection [ 162 ]. These studies proved the potential of CRISPR/Cas9 in preventing and treating cancer virus infections.
Application of CRISPR/Cas9 in cancer modeling
CRISPR/Cas9 enables in vivo construction of tumor models with multiple genetic mutations to better model complex human diseases (Fig. 7 e). Since the emergence of homologous recombination or random transgenic integration, transgenic mice have been the gold standard for cancer modeling studies [ 163 ]. The use of laboratory mice to mimic human cancers through remodeling (xenografting) can be used for both basic oncology research to functionally infer cancer genes and for anti-cancer drug screening [ 164 ]. The fast and precise CRISPR/Cas9 technology enables researchers to create mouse models of cancer with specific genetic modifications, allowing a more objective study of multistep carcinogenesis. In 2017, Huang et al. created a mouse model of sarcoma using CRISPR/Cas9 technology successfully [ 165 ]. Blasco et al. created a mouse model that mimics non-small-cell lung cancers (NSCLC). A specific chromosomal translocation involving the genes Eml4-Alk, which is present in approximately 5–7% of these tumors, was achieved, using a lentiviral CRISPR/Cas9 vector. Employing this system, almost all mice developed lung cancer 8 weeks after the procedure [ 166 ]. Disruption of the tumor suppressor genes Pten and P53 in mouse liver by CRISPR/Cas9 has created a mouse model that generates liver tumors with a cancer phenotype similar to that of mice made with conventional Cre-loxP technology [ 167 ]. These models allow for the study of potential mechanisms of tumorigenesis and progression while exploring various therapeutic approaches.
Patient-derived xenograft (PDX) animal models can enable exogenous growing of human tumors and provide an indispensable preclinical tool for oncology research [ 168 ]. Mice are the most widely used host for human PDX models; nevertheless, the small size of mice limits the growth of xenografts, which in turn affects sample collection and drug evaluation. Thus, the researchers used the CRISPR/Cas9 technique to knockout Rag1, Rag2, and n Il2 in Sprague Dawley (SD) rats to develop a new rat model with significantly impaired lymphoid organ development. The SD-RG rats with severe immunodeficiency overcame the above shortcomings and have successfully been developed into a PDX model of lung squamous cell carcinoma in which the grafts reproduced the histopathological features of the primary tumor in multiple passages. This has great potential to be used as a new model for cancer research [ 169 ].
Zebrafish have been used for the study of different types of cancers, such as skin cancer [ 170 , 171 ] pancreatic cancer [ 172 ], breast cancer [ 173 ], leukemia [ 174 ], glioma [ 175 ], and lung cancer [ 176 ]. With the help of the CRISPR/Cas9 system, zebrafish has obtained a flexible, cheap, and easy tool in research that can generate mosaic knockout or lines on demand. This will improve the analysis of tumor suppressor genes that are difficult to study and make the development of a more complicated Zebrafish cancer model possible [ 177 ]. Ablain et al. used CRISPR technology in their study to create a zebrafish model of genetic SPRED1-deficient mucosal melanoma and found that SPRED1 plays a tumor suppressor role. These findings provide a rationale for MAPK (mitogen-activated protein kinase) inhibition in SPRED1-deficient melanoma and suggest a new zebrafish modeling approach that can be used to rapidly study genetic mutations occurring in human cancers [ 178 ]. These emerging modeling approaches could allow for a more efficient investigation of cancer genes in vitro and in vivo.
Application of CRISPR/Cas system in a new emerging hotspot of oncology
RNA modification mediated by N6-methyladenosine (m6A) influences practically the whole post-transcriptional processes. Emerging evidence reveals that m6A modification is correlated with tumor initiation, proliferation, differentiation, invasion, metastasis, and survival rate [ 179 ]. Furthermore, the regulators of m6A modification function as oncogenes or tumor suppressors in various cancers [ 180 , 181 ]. However, previous RNA biology methods cannot distinguish the effect of individual m6A modifications. Recently, based on the CRISPR/Cas9 technology, Liu et al. developed a robust approach that enables m6A accession and erasure at the specific site via recognition of homologous sequence from cellular RNAs. They engineered fusions of dCas9 with m6A methyltransferases METTL3 and METTL14 and found that the resultant m6A ‘writers’ enable comparison of the action of single-site methylation by distinct mRNA regions. In a further study, they used m6A ‘erasers’ via fusing CRISPR/Cas9 with ALKBH5 or FTO to achieve RNAs demethylation in a specific site [ 182 ]. Latest, Wilson et al. developed a targeted RNA methylation (TRM) system based on the RNA-targeting ability of CRISPR/Cas13 in combination with RNA methyltransferases generated m6A. They indicated that site-specific incorporation of m6A into different cellular compartments is guided by fusion of dCas13 with the METTL3 methyltransferase domain. They also established that the cytoplasmic localization of the METTL3:METTL14 methyltransferase complexes domain and cytoplasm-localized fusions with a modified METTL3:METTL14 methyltransferase complex can direct site-specific m6A incorporation in distinct cellular compartments [ 183 ]. The TRM is a targeted epigenome engineering tool used to reveal and analyze the functions of individual m6A modifications. Utilization of these tools will facilitate mechanistic understanding and clinical trials of malignant tumors in a way of epitranscriptome.
Long non-coding RNAs (lncRNA) are considered to be the main “dark matter” in the genome. Numerous studies have shown that these RNAs may participate in a series of important physiological activities in cells and are also closely related to the initiation and progression of cancers [ 184 ]. The interaction between RNA binding proteins (RBP) and lncRNAs determines the function and fate of RNA molecules. Therefore, accurate identification of lncRNA interacting proteins in living cells can help us in revealing the molecular mechanism of some complex human diseases better [ 185 ]. Recently, using CRISPR/CasRx-based RNA targeting and proximity labeling to characterize specific long non-coding RNAs (lncRNAs) binding proteins in primary cells, Yi et al. established the CRISPR-assisted RNA-protein interaction detection method (CARPID). The detection method was applied in nuclear lncRNA XIST and captured a range of known interacting proteins and plentiful unidentified binding proteins [ 186 ]. This tool can be used to detect the RNA-binding proteins that are significantly involved in tumorigenesis, and these protein molecules may serve as drug targets for treating tumor-related diseases.
The CRISPR/Cas adaptive immune defense systems in some cases can protect against specific sequences of foreign DNA or RNA. However, phages have not been eliminated by the evolution of defense systems in the bacterial host, suggesting that there are genes in some phages that encode antagonistic bacterial CRISPR/Cas immune system products. Expectedly, recent studies have discovered a group of bacteriophage proteins, anti-CRISPRs (Acrs), that can inactivate certain CRISPR systems [ 187 , 188 ]. Nakamura et al. evaluated the activity of a panel of Acrs in mammalian cells using both CRISPRi and CRISPRa. Their results clearly suggested that AcrIIA4 is a potent regulator of (d) Cas9 activity in various cell types. Moreover, AcrIIA4 benefits from its small size which enables easy incorporation into a range of environments and it can efficiently inhibit CRISPR activity when it is fused to other gene products via abounding linkers [ 189 ]. These studies indicated that anti-CRISPR proteins could act as preferable tools for the generation of more advanced dynamic control over gene regulation by regulating CRISPR activity in cancer research, thus providing more approaches for overcoming the complex features of cancer.
Conclusions and perspectives
In just a few years, the CRISPR/Cas9 has emerged and advanced rapidly as a stable, efficient, simple, and extensively used gene editing technology. Actually, the CRISPR/Cas9 has fundamentally impacted many fields, such as agriculture, biotech, and biomedicine, but no field has felt a more profound impact than cancer research as evidenced by the accumulating data in the fast-growing publications. The capacity to implement the genome rapidly and accurately opened the windows for a new and more elaborate outlook on the mechanisms of tumorigenesis and progression. More importantly, CRISPR/cas9 gene editing technology hold big promise for cancer therapy. However, several challenges remain before this technology can be used in clinical treatment of cancer safely and efficiently.
Firstly, the off-target effect of CRISPR/Cas9 gene editing technology has always been a major concern. Therefore, improvement of the specificity and the tools for off-target detection is required for safe therapeutic uses of CRISPR/Cas9. Many efforts have been made for increasing specificity, including development of more prioritizing sgRNA designer by integrating of multiple factors [ 190 ], discovery and use of more specific Cas9 variants, limiting the time of CRISPR/Cas9 activity, use of inducible Cas9 variants, and use of anti-CRISPR proteins [ 191 ]. Furthermore, techniques such as GUIDE-Seq [ 192 ], CIRCLE-Seq [ 193 ], and CHANGE-seq [ 194 ] that can detect low-frequency mutations have been developed. Further studies are needed to fully understand the principles that govern CRISPR/Cas9 specificity, and to improve off-target detection sensitivity.
Secondly, on-target mutagenesis was occurred frequently in double-strand breaks induced by single-guided RNA/Cas9, such as large deletions (over many kilobases) and complex genomic rearrangements at the targeted sites, thus will elicit long-range transcriptional consequences and may have pathogenic consequences [ 195 ] . Therefore, the technology of precise spatiotemporal control of CRISPR/Cas9 activity in cells and complex conditions will be beneficial, such as cell-specific promoters, small molecule activation/inhibition, bioresponsive delivery carriers and optical/ultrasonic/thermal/magnetic activation of the CRISPR/Cas9 system [ 196 ]. In addition, it is necessary to perform comprehensive genomic analysis to identify cells with normal genomes before clinical applications.
Thirdly, efficient, safe and targetable delivery of the CRISPR/Cas9 system in vivo is also a huge challenge for clinical application. To overcome this problem, novel delivery strategies and control mechanisms is required. Fortunately, a series of viral and nonviral delivery systems were developed for gene editing in diverse tissues, and these methods all show certain advantages and disadvantages [ 197 , 198 , 199 ]. Recently, multifunctional nanoparticles with tumor pH response, active EGFR targeting, and nuclear localization provided new idea for overcoming the delivery problem of the CRISPR/Cas system [ 200 ]. Looking ahead, a delivery system that enable to deliver the CRISPR/Cas9 components for tissue- and cell-type-specific gene editing with safety and efficiency is ideal for clinical translation.
Fourthly, another challenge for CRISPR/Cas9 application is the human body’s immune response to the bacteria-derived Cas9 protein. Charlesworth et al. detected antibodies against Cas9 in human serum by ELISA (enzyme-linked immunosorbent assay). Results showed that both SaCas9 and SpCas9 antibodies were existed in 78 and 58% of subjects, respectively. Furthermore, they also found anti-SaCas9 and anti-SpCas9 T cells in 78 and 67% of subjects, respectively [ 201 ]. These data indicated that there are preexisting humoral and cell-mediated adaptive immune responses to Cas9 in humans that may compromise efficiency of gene editing. Therefore, optimizing vector, dose, administration route, and immune suppression are potential approaches to perfect the CRISPR/Cas9 gene editing in vivo.
Finally, the DNA double-strand break caused by CRISPR/Cas9 can activate the p53 pathway, induce a p53-mediated DNA damage response and cell cycle arrest, thus leading to failure of gene editing [ 202 , 203 ] . Nevertheless, recent studies revealed that although Cas9-induced p53 pathway activation alters cellular sensitivity to both genetic and chemical perturbations [ 204 ], careful experimental design and thorough data analysis made it is possible to get useful results even in cells with functional p53 protein [ 205 ]. In addition, it has been demonstrated that the single Cas9 nickase approach, which does not rely on double-stranded DNA breaks, is expected to circumvent this risk [ 206 ]. To usher a golden age of the CRISPR/Cas technology in cancer research, diagnosis and treatment, continuous efforts are needed to overcome the above challenges in future.
Availability of data and materials
Not applicable.
Abbreviations
Clustered regularly interspaced short palindromic repeats/CRISPR-associated nuclease9
Zinc finger endonuclease
Transcription activator-like effector nuclease
Single-stranded guide RNA
Protospacer adjacent motif
Double-strand break
Non-homologous end-joining
Homology-directed repair
Endonuclease-deficient Cas9
CRISPR activation
CRISPR inhibition
Adenine bases Editor
Nanopore Cas9-targeted sequence
Very fast CRISPR
Streptococcus pyogenes Cas9
DNA Endonuclease Targeted CRISPR Trans Reporter
RNA Editing for Programmable Adenosine to Inosine Replacement
Single-stranded RNA
Specific High Sensitivity Enzymatic Reporter Unlocking
Cas13-assisted restriction of virus expression and readout
Pancreatic cancer
Triple-negative breast cancer
Tyrosine kinase inhibitor
Acute myeloid leukemia
Ovarian cancer stem cell
- Cancer stem cell
Genome-wide CRISPR/Cas9 knockout
Hepatocellular carcinoma
Adoptive cell therapy
CRISPR affinity purification in situ of regulatory element
Fluorescence in situ hybridization
Chimeric antigen receptor T cell
Acute lymphoblastic leukemia
Cytokine release syndrome
Hepatitis B virus
Hepatitis C virus
Human papillomavirus
Epstein-Barr virus
Francisella novicida Cas9
Non-small-cell lung cancer
Patient-derived xenograft
Targeted RNA methylation
Long non-coding RNA
CRISPR-assisted RNA-protein interaction detection
Genome-wide, unbiased identification of DSBs enabled by sequencing
Circularization for in vitro reporting of cleavage effects by sequencing
Circularization for high-throughput analysis of nuclease genome-wide effects by sequencing
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66.
Article CAS PubMed Google Scholar
Garraway LA, Lander ES. Lessons from the cancer genome. Cell. 2013;153:17–37.
Sánchez-Rivera FJ, Jacks T. Applications of the CRISPR-Cas9 system in cancer biology. Nat Rev Cancer. 2015;15:387–95.
Article PubMed PubMed Central CAS Google Scholar
Pon JR, Marra MA. Driver and passenger mutations in cancer. Annu Rev Pathol. 2015;10:25–50.
Shen P, Jing Y, Zhang R, Cai MC, Ma P, Chen H, et al. Comprehensive genomic profiling of neuroendocrine bladder cancer pinpoints molecular origin and potential therapeutics. Oncogene. 2018;37:3039–44.
Carroll D. Genome engineering with zinc-finger nucleases. Genetics. 2011;188:773–82.
Article CAS PubMed PubMed Central Google Scholar
Joung JK, Sander JD. TALENs: a widely applicable technology for targeted genome editing. Nat Rev Mol Cell Biol. 2013;14:49–55.
Zhang F, Wen Y, Guo X. CRISPR/Cas9 for genome editing: progress, implications and challenges. Hum Mol Genet. 2014;23:R40–6.
Rupp LJ, Schumann K, Roybal KT, Gate RE, Ye CJ, Lim WA, et al. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci Rep. 2017;7:737.
Zuckermann M, Hovestadt V, Knobbe-Thomsen CB, Zapatka M, Northcott PA, Schramm K, et al. Somatic CRISPR/Cas9-mediated tumour suppressor disruption enables versatile brain tumour modelling. Nat Commun. 2015;6:7391.
Horvath P, Barrangou R. CRISPR/Cas, the immune system of bacteria and archaea. Science. 2010;327:167–70.
Garneau JE, Dupuis MÈ, Villion M, Romero DA, Barrangou R, Boyaval P, et al. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature. 2010;468:67–71.
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–23.
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–21.
Pawelczak KS, Gavande NS, VanderVere-Carozza PS, Turchi JJ. Modulating DNA repair pathways to improve precision genome engineering. ACS Chem Biol. 2018;13:389–96.
Ishino Y, Shinagawa H, Makino K, Amemura M, Nakata A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J Bacteriol. 1987;169:5429–33.
Jansen R, Embden JD, Gaastra W, Schouls LM. Identification of genes that are associated with DNA repeats in prokaryotes. Mol Microbiol. 2002;43:1565–75.
Mojica FJ, Díez-Villaseñor C, García-Martínez J, Soria E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J Mol Evol. 2005;60:174–82.
Pourcel C, Salvignol G, Vergnaud G. CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies. Microbiology (Reading). 2005;151:653–63.
Article CAS Google Scholar
Bolotin A, Quinquis B, Sorokin A, Ehrlich SD. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology (Reading). 2005;151:2551–61.
Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315:1709–12.
Brouns SJ, Jore MM, Lundgren M, Westra ER, Slijkhuis RJ, Snijders AP, et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science. 2008;321:960–4.
Deltcheva E, Chylinski K, Sharma CM, Gonzales K, Chao Y, Pirzada ZA, et al. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature. 2011;471:602–7.
Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, et al. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–6.
Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152:1173–83.
Maeder ML, Linder SJ, Cascio VM, Fu Y, Ho QH, Joung JK. CRISPR RNA-guided activation of endogenous human genes. Nat Methods. 2013;10:977–9.
Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE, et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013;154:442–51.
Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature. 2016;533:420–4.
Gaudelli NM, Komor AC, Rees HA, Packer MS, Badran AH, Bryson DI, et al. Programmable base editing of a•T to G•C in genomic DNA without DNA cleavage. Nature. 2017;551:464–71.
Doman JL, Raguram A, Newby GA, Liu DR. Evaluation and minimization of Cas9-independent off-target DNA editing by cytosine base editors. Nat Biotechnol. 2020;38:620–8.
Miller SM, Wang T, Randolph PB, Arbab M, Shen MW, Huang TP, et al. Continuous evolution of SpCas9 variants compatible with non-G PAMs. Nat Biotechnol. 2020;38:471–81.
Gilpatrick T, Lee I, Graham JE, Raimondeau E, Bowen R, Heron A, et al. Targeted nanopore sequencing with Cas9-guided adapter ligation. Nat Biotechnol. 2020;38:433–8.
Liu Y, Zou RS, He S, Nihongaki Y, Li X, Razavi S, et al. Very fast CRISPR on demand. Science. 2020;368:1265–9.
Mojica F, Díez-Villaseñor C, García-Martínez J, Almendros C. Short motif sequences determine the targets of the prokaryotic CRISPR defence system. Microbiology (Reading). 2009;155:733–40.
Kleinstiver BP, Prew MS, Tsai SQ, Topkar VV, Nguyen NT, Zheng Z, et al. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature. 2015;523:481–5.
Nishimasu H, Cong L, Yan WX, Ran FA, Zetsche B, Li Y, et al. Crystal structure of Staphylococcus aureus Cas9. Cell. 2015;162:1113–26.
Ran FA, Cong L, Yan WX, Scott DA, Gootenberg JS, Kriz AJ, et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520:186–91.
Hu JH, Miller SM, Geurts MH, Tang W, Chen L, Sun N, et al. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity. Nature. 2018;556:57–63.
Nishimasu H, Shi X, Ishiguro S, Gao L, Hirano S, Okazaki S, et al. Engineered CRISPR-Cas9 nuclease with expanded targeting space. Science. 2018;361:1259–62.
Walton RT, Christie KA, Whittaker MN, Kleinstiver BP. Unconstrained genome targeting with near-PAMless engineered CRISPR-Cas9 variants. Science. 2020;368:290–6.
Morsy SG, Tonne JM, Zhu Y, Lu B, Budzik K, Krempski JW, et al. Divergent susceptibilities to AAV-SaCas9-gRNA vector-mediated genome-editing in a single-cell-derived cell population. BMC Res Notes. 2017;10:720.
Article PubMed PubMed Central Google Scholar
Koo T, Lu-Nguyen NB, Malerba A, Kim E, Kim D, Cappellari O, et al. Functional Rescue of Dystrophin Deficiency in mice caused by Frameshift mutations using campylobacter jejuni Cas9. Mol Ther. 2018;26:1529–38.
Fujii W, Ito H, Kanke T, Ikeda A, Sugiura K, Naito K. Generation of genetically modified mice using SpCas9-NG engineered nuclease. Sci Rep. 2019;9:12878.
Casini A, Olivieri M, Petris G, Montagna C, Reginato G, Maule G, et al. A highly specific SpCas9 variant is identified by in vivo screening in yeast. Nat Biotechnol. 2018;36:265–71.
Chen JS, Ma E, Harrington LB, Da Costa M, Tian X, Palefsky JM, et al. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science. 2018;360:436–9.
Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, Essletzbichler P, et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 2015;163:759–71.
Shmakov S, Abudayyeh OO, Makarova KS, Wolf YI, Gootenberg JS, Semenova E, et al. Discovery and functional characterization of diverse class 2 CRISPR-Cas systems. Mol Cell. 2015;60:385–97.
Yang H, Gao P, Rajashankar KR, Patel DJ. PAM-Dependent Target DNA Recognition and Cleavage by C2c1 CRISPR-Cas Endonuclease. Cell. 2016;167:1814–28.e12.
Koonin EV, Makarova KS, Zhang F. Diversity, classification and evolution of CRISPR-Cas systems. Curr Opin Microbiol. 2017;37:67–78.
Teng F, Cui T, Feng G, Guo L, Xu K, Gao Q, et al. Repurposing CRISPR-Cas12b for mammalian genome engineering. Cell Discov. 2018;4:63.
Broughton JP, Deng X, Yu G, Fasching CL, Servellita V, Singh J, et al. CRISPR-Cas12-based detection of SARS-CoV-2. Nat Biotechnol. 2020;38:870–4.
Mahas A, Neal Stewart C Jr, Mahfouz MM. Harnessing CRISPR/Cas systems for programmable transcriptional and post-transcriptional regulation. Biotechnol Adv. 2018;36:295–310.
Abudayyeh OO, Gootenberg JS, Konermann S, Joung J, Slaymaker IM, Cox DB, et al. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science. 2016;353:aaf5573.
East-Seletsky A, O'Connell MR, Knight SC, Burstein D, Cate JH, Tjian R, et al. Two distinct RNase activities of CRISPR-C2c2 enable guide-RNA processing and RNA detection. Nature. 2016;538:270–3.
Granados-Riveron JT, Aquino-Jarquin G. CRISPR-Cas13 precision Transcriptome engineering in Cancer. Cancer Res. 2018;78:4107–13.
Freije CA, Myhrvold C, Boehm CK, Lin AE, Welch NL, Carter A, et al. Programmable Inhibition and Detection of RNA Viruses Using Cas13. Mol Cell. 2019;76:826–37.e11.
Abudayyeh OO, Gootenberg JS, Essletzbichler P, Han S, Joung J, Belanto JJ, et al. RNA targeting with CRISPR-Cas13. Nature. 2017;550:280–4.
Gootenberg JS, Abudayyeh OO, Lee JW, Essletzbichler P, Dy AJ, Joung J, et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science. 2017;356:438–42.
Nguyen TM, Zhang Y, Pandolfi PP. Virus against virus: a potential treatment for 2019-nCov (SARS-CoV-2) and other RNA viruses. Cell Res. 2020;30:189–90.
Vogelstein B, Kinzler KW. The multistep nature of cancer. Trends Genet. 1993;9:138–41.
Liu Y, Hu X, Han C, Wang L, Zhang X, He X, et al. Targeting tumor suppressor genes for cancer therapy. Bioessays. 2015;37:1277–86.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Jiang C, Meng L, Yang B, Luo X. Application of CRISPR/Cas9 gene editing technique in the study of cancer treatment. Clin Genet. 2020;97:73–88.
Li W, Cho MY, Lee S, Jang M, Park J, Park R. CRISPR-Cas9 mediated CD133 knockout inhibits colon cancer invasion through reduced epithelial-mesenchymal transition. PLoS One. 2019;14:e0220860.
Yan J, Jia Y, Chen H, Chen W, Zhou X. Long non-coding RNA PXN-AS1 suppresses pancreatic cancer progression by acting as a competing endogenous RNA of miR-3064 to upregulate PIP4K2B expression. J Exp Clin Cancer Res. 2019;38:390.
Koo T, Yoon AR, Cho HY, Bae S, Yun CO, Kim JS. Selective disruption of an oncogenic mutant allele by CRISPR/Cas9 induces efficient tumor regression. Nucleic Acids Res. 2017;45:7897–908.
Tang KJ, Constanzo JD, Venkateswaran N, Melegari M, Ilcheva M, Morales JC, et al. Focal adhesion kinase regulates the DNA damage response and its inhibition Radiosensitizes mutant KRAS lung Cancer. Clin Cancer Res. 2016;22:5851–63.
Liao L, Song M, Li X, Tang L, Zhang T, Zhang L, et al. E3 ubiquitin ligase UBR5 drives the growth and metastasis of triple-negative breast Cancer. Cancer Res. 2017;77:2090–101.
Chen ML, Chang JH, Yeh KT, Chang YS, Chang JG. Epigenetic changes in tumor suppressor genes, P15, P16, APC-3 and E-cadherin in body fluid. Kaohsiung J Med Sci. 2007;23:498–503.
Morris LG, Chan TA. Therapeutic targeting of tumor suppressor genes. Cancer. 2015;121:1357–68.
Wahiduzzaman M, Karnan S, Ota A, Hanamura I, Murakami H, Inoko A, et al. Establishment and characterization of CRISPR/Cas9-mediated NF2−/− human mesothelial cell line: molecular insight into fibroblast growth factor receptor 2 in malignant pleural mesothelioma. Cancer Sci. 2019;110:180–93.
Xu K, Chen G, Li X, Wu X, Chang Z, Xu J, et al. MFN2 suppresses cancer progression through inhibition of mTORC2/Akt signaling. Sci Rep. 2017;7:41718.
Pan WW, Moroishi T, Koo JH, Guan KL. Cell type-dependent function of LATS1/2 in cancer cell growth. Oncogene. 2019;38:2595–610.
Moses C, Nugent F, Waryah CB, Garcia-Bloj B, Harvey AR, Blancafort P. Activating PTEN tumor suppressor expression with the CRISPR/dCas9 system. Mol Ther Nucleic Acids. 2019;14:287–300.
Artegiani B, van Voorthuijsen L, Lindeboom R, Seinstra D, Heo I, Tapia P, et al. Probing the Tumor Suppressor Function of BAP1 in CRISPR-Engineered Human Liver Organoids. Cell Stem Cell. 2019;24:927–43.e6.
Maji S, Panda S, Samal SK, Shriwas O, Rath R, Pellecchia M, et al. Bcl-2 Antiapoptotic family proteins and Chemoresistance in Cancer. Adv Cancer Res. 2018;137:37–75.
Bialk P, Wang Y, Banas K, Kmiec EB. Functional gene knockout of NRF2 increases Chemosensitivity of human lung Cancer A549 cells in vitro and in a Xenograft mouse model. Mol Ther Oncolytics. 2018;11:75–89.
Gao W, Zhang Y, Luo H, Niu M, Zheng X, Hu W, et al. Targeting SKA3 suppresses the proliferation and chemoresistance of laryngeal squamous cell carcinoma via impairing PLK1-AKT axis-mediated glycolysis. Cell Death Dis. 2020;11:919.
Chen X, Sun X, Guan J, Gai J, Xing J, Fu L, et al. Rsf-1 influences the sensitivity of non-small cell lung Cancer to paclitaxel by regulating NF-κB pathway and its downstream proteins. Cell Physiol Biochem. 2017;44:2322–36.
Heyza JR, Lei W, Watza D, Zhang H, Chen W, Back JB, et al. Identification and characterization of synthetic viability with ERCC1 deficiency in response to Interstrand crosslinks in lung Cancer. Clin Cancer Res. 2019;25:2523–36.
Yu J, Zhou J, Xu F, Bai W, Zhang W. High expression of Aurora-B is correlated with poor prognosis and drug resistance in non-small cell lung cancer. Int J Biol Markers. 2018;33:215–21.
Pavlova NN, Thompson CB. The emerging hallmarks of Cancer metabolism. Cell Metab. 2016;23:27–47.
Li Z, Zhang H. Reprogramming of glucose, fatty acid and amino acid metabolism for cancer progression. Cell Mol Life Sci. 2016;73:377–92.
Yasuda M, Miyazawa M, Fujita M, Kajiwara H, Iida T, Hirasawa T, et al. Expression of hypoxia inducible factor-1alpha (HIF-1alpha) and glucose transporter-1 (GLUT-1) in ovarian adenocarcinomas: difference in hypoxic status depending on histological character. Oncol Rep. 2008;19:111–6.
CAS PubMed Google Scholar
Pez F, Dayan F, Durivault J, Kaniewski B, Aimond G, Le Provost GS, et al. The HIF-1-inducible lysyl oxidase activates HIF-1 via the Akt pathway in a positive regulation loop and synergizes with HIF-1 in promoting tumor cell growth. Cancer Res. 2011;71:1647–57.
Wu XH, Chen SP, Mao JY, Ji XX, Yao HT, Zhou SH. Expression and significance of hypoxia-inducible factor-1α and glucose transporter-1 in laryngeal carcinoma. Oncol Lett. 2013;5:261–6.
Article PubMed Google Scholar
Lu ZJ, Yu Q, Zhou SH, Fan J, Shen LF, Bao YY, et al. Construction of a GLUT-1 and HIF-1α gene knockout cell model in HEp-2 cells using the CRISPR/Cas9 technique. Cancer Manag Res. 2019;11:2087–96.
Gallipoli P, Giotopoulos G, Tzelepis K, Costa A, Vohra S, Medina-Perez P, et al. Glutaminolysis is a metabolic dependency in FLT3ITD acute myeloid leukemia unmasked by FLT3 tyrosine kinase inhibition. Blood. 2018;131:1639–53.
Kanarek N, Keys HR, Cantor JR, Lewis CA, Chan SH, Kunchok T, et al. Histidine catabolism is a major determinant of methotrexate sensitivity. Nature. 2018;559:632–6.
Papaccio F, Paino F, Regad T, Papaccio G, Desiderio V, Tirino V. Concise review: Cancer cells, Cancer stem cells, and Mesenchymal stem cells: influence in Cancer development. Stem Cells Transl Med. 2017;6:2115–25.
Zomer A, Ellenbroek SI, Ritsma L, Beerling E, Vrisekoop N, Van Rheenen J. Intravital imaging of cancer stem cell plasticity in mammary tumors. Stem Cells. 2013;31:602–6.
Noh KH, Kim BW, Song KH, Cho H, Lee YH, Kim JH, et al. Nanog signaling in cancer promotes stem-like phenotype and immune evasion. J Clin Invest. 2012;122:4077–93.
Chang C, Lee SO, Yeh S, Chang TM. Androgen receptor (AR) differential roles in hormone-related tumors including prostate, bladder, kidney, lung, breast and liver. Oncogene. 2014;33:3225–34.
Ling K, Jiang L, Liang S, Kwong J, Yang L, Li Y, et al. Nanog interaction with the androgen receptor signaling axis induce ovarian cancer stem cell regulation: studies based on the CRISPR/Cas9 system. J Ovarian Res. 2018;11:36.
Yang F, Cui P, Lu Y, Zhang X. Requirement of the transcription factor YB-1 for maintaining the stemness of cancer stem cells and reverting differentiated cancer cells into cancer stem cells. Stem Cell Res Ther. 2019;10:233.
Krausova M, Korinek V. Wnt signaling in adult intestinal stem cells and cancer. Cell Signal. 2014;26:570–9.
Zhan T, Ambrosi G, Wandmacher AM, Rauscher B, Betge J, Rindtorff N, et al. MEK inhibitors activate Wnt signalling and induce stem cell plasticity in colorectal cancer. Nat Commun. 2019;10:2197.
Hwang JH, Yoon J, Cho YH, Cha PH, Park JC, Choi KY. A mutant KRAS-induced factor REG4 promotes cancer stem cell properties via Wnt/β-catenin signaling. Int J Cancer. 2020;146:2877–90.
Bester AC, Lee JD, Chavez A, Lee YR, Nachmani D, Vora S, et al. An Integrated Genome-wide CRISPRa Approach to Functionalize lncRNAs in Drug Resistance. Cell. 2018;173:649–64.e20.
Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelson T, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343:84–7.
Chen S, Sanjana NE, Zheng K, Shalem O, Lee K, Shi X, et al. Genome-wide CRISPR screen in a mouse model of tumor growth and metastasis. Cell. 2015;160:1246–60.
Totoki Y, Tatsuno K, Covington KR, Ueda H, Creighton CJ, Kato M, et al. Trans-ancestry mutational landscape of hepatocellular carcinoma genomes. Nat Genet. 2014;46:1267–73.
Schwartzentruber J, Korshunov A, Liu XY, Jones DT, Pfaff E, Jacob K, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482:226–31.
Liang J, Zhao H, Diplas BH, Liu S, Liu J, Wang D, et al. Genome-wide CRISPR-Cas9 screen reveals selective vulnerability of ATRX-mutant cancers to WEE1 inhibition. Cancer Res. 2020;80:510–23.
Wei J, Long L, Zheng W, Dhungana Y, Lim SA, Guy C, et al. Targeting REGNASE-1 programs long-lived effector T cells for cancer therapy. Nature. 2019;576:471–6.
Zhu S, Li W, Liu J, Chen CH, Liao Q, Xu P, et al. Genome-scale deletion screening of human long non-coding RNAs using a paired-guide RNA CRISPR-Cas9 library. Nat Biotechnol. 2016;34:1279–86.
Liu Y, Cao Z, Wang Y, Guo Y, Xu P, Yuan P, et al. Genome-wide screening for functional long noncoding RNAs in human cells by Cas9 targeting of splice sites. Nat Biotechnol. 2018;36:1203–10.
Gilbert LA, Horlbeck MA, Adamson B, Villalta JE, Chen Y, Whitehead EH, et al. Genome-scale CRISPR-mediated control of gene repression and activation. Cell. 2014;159:647–61.
Liu SJ, Horlbeck MA, Cho SW, Birk HS, Malatesta M, He D, et al. CRISPRi-based genome-scale identification of functional long noncoding RNA loci in human cells. Science. 2017;355:aah7111.
Jost M, Chen Y, Gilbert LA, Horlbeck MA, Krenning L, Menchon G, et al. Combined CRISPRi/a-Based Chemical Genetic Screens Reveal that Rigosertib Is a Microtubule-Destabilizing Agent. Mol Cell. 2017;68:210–23.e6.
Raffeiner P, Hart JR, García-Caballero D, Bar-Peled L, Weinberg MS, Vogt PK. An MXD1-derived repressor peptide identifies noncoding mediators of MYC-driven cell proliferation. Proc Natl Acad Sci U S A. 2020;117:6571–9.
Konermann S, Brigham MD, Trevino AE, Joung J, Abudayyeh OO, Barcena C, et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature. 2015;517:583–8.
Kurata M, Yamamoto K, Moriarity BS, Kitagawa M, Largaespada DA. CRISPR/Cas9 library screening for drug target discovery. J Hum Genet. 2018;63:179–86.
Wang G, Chow RD, Bai Z, Zhu L, Errami Y, Dai X, et al. Multiplexed activation of endogenous genes by CRISPRa elicits potent antitumor immunity. Nat Immunol. 2019;20:1494–505.
Qi F, Tan B, Ma F, Zhu B, Zhang L, Liu X, et al. A synthetic light-switchable system based on CRISPR Cas13a regulates the expression of LncRNA MALAT1 and affects the malignant phenotype of bladder Cancer cells. Int J Biol Sci. 2019;15:1630–6.
Wang Q, Liu X, Zhou J, Yang C, Wang G, Tan Y, et al. The CRISPR-Cas13a Gene-Editing System Induces Collateral Cleavage of RNA in Glioma Cells. Adv Sci (Weinh). 2019;6:1901299.
Fujita T, Fujii H. Isolation of specific genomic regions and identification of associated molecules by engineered DNA-binding molecule-mediated chromatin immunoprecipitation (enChIP) using CRISPR. Methods Mol Biol. 2015;1288:43–52.
Fujita T, Fujii H. Identification of proteins associated with an IFNγ-responsive promoter by a retroviral expression system for enChIP using CRISPR. PLoS One. 2014;9:e103084.
Liu X, Zhang Y, Chen Y, Li M, Zhou F, Li K, et al. In Situ Capture of Chromatin Interactions by Biotinylated dCas9. Cell. 2017;170:1028–43.e19.
Zhou Y, Wang P, Tian F, Gao G, Huang L, Wei W, et al. Painting a specific chromosome with CRISPR/Cas9 for live-cell imaging. Cell Res. 2017;27:298–301.
Ma H, Naseri A, Reyes-Gutierrez P, Wolfe SA, Zhang S, Pederson T. Multicolor CRISPR labeling of chromosomal loci in human cells. Proc Natl Acad Sci U S A. 2015;112:3002–7.
Shao S, Zhang W, Hu H, Xue B, Qin J, Sun C, et al. Long-term dual-color tracking of genomic loci by modified sgRNAs of the CRISPR/Cas9 system. Nucleic Acids Res. 2016;44:e86.
Artegiani B, Hendriks D, Beumer J, Kok R, Zheng X, Joore I, et al. Fast and efficient generation of knock-in human organoids using homology-independent CRISPR-Cas9 precision genome editing. Nat Cell Biol. 2020;22:321–31.
Rahman N. Mainstreaming genetic testing of cancer predisposition genes. Clin Med (Lond). 2014;14:436–9.
Article Google Scholar
Yang D, Shi Y, Tang Y, Yin H, Guo Y, Wen S, et al. Effect of HPV infection on the occurrence and development of laryngeal Cancer: a review. J Cancer. 2019;10:4455–62.
Qiu XY, Zhu LY, Zhu CS, Ma JX, Hou T, Wu XM, et al. Highly effective and low-cost MicroRNA detection with CRISPR-Cas9. ACS Synth Biol. 2018;7:807–13.
Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–58.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44.
Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24:20–8.
Susanibar Adaniya SP, Cohen AD, Garfall AL. Chimeric antigen receptor T cell immunotherapy for multiple myeloma: A review of current data and potential clinical applications. Am J Hematol. 2019;94:S28–28S33.
Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380:1726–37.
Eyquem J, Mansilla-Soto J, Giavridis T, van der Stegen SJ, Hamieh M, Cunanan KM, et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543:113–7.
Kim MY, Yu KR, Kenderian SS, Ruella M, Chen S, Shin TH, et al. Genetic Inactivation of CD33 in Hematopoietic Stem Cells to Enable CAR T Cell Immunotherapy for Acute Myeloid Leukemia. Cell. 2018;173:1439–53.e19.
Stadtmauer EA, Fraietta JA, Davis MM, Cohen AD, Weber KL, Lancaster E, et al. CRISPR-engineered T cells in patients with refractory cancer. Science. 2020;367:eaba7365.
Sterner RM, Sakemura R, Cox MJ, Yang N, Khadka RH, Forsman CL, et al. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood. 2019;133:697–709.
Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, Zang X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med. 2015;21:24–33.
Hamanishi J, Mandai M, Matsumura N, Abiko K, Baba T, Konishi I. PD-1/PD-L1 blockade in cancer treatment: perspectives and issues. Int J Clin Oncol. 2016;21:462–73.
Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125:3384–91.
Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800.
Cyranoski D. Chinese scientists to pioneer first human CRISPR trial. Nature. 2016;535:476–7.
Cyranoski D. CRISPR gene-editing tested in a person for the first time. Nature. 2016;539:479.
Lu Y, Xue J, Deng T, Zhou X, Yu K, Deng L, et al. Safety and feasibility of CRISPR-edited T cells in patients with refractory non-small-cell lung cancer. Nat Med. 2020;26:732–40.
Yi L, Li J. CRISPR-Cas9 therapeutics in cancer: promising strategies and present challenges. Biochim Biophys Acta. 1866;2016:197–207.
Google Scholar
Guo X, Jiang H, Shi B, Zhou M, Zhang H, Shi Z, et al. Disruption of PD-1 enhanced the anti-tumor activity of chimeric antigen receptor T cells against hepatocellular carcinoma. Front Pharmacol. 2018;9:1118.
He XY, Ren XH, Peng Y, Zhang JP, Ai SL, Liu BY, et al. Aptamer/peptide-functionalized genome-editing system for effective immune restoration through reversal of PD-L1-mediated Cancer immunosuppression. Adv Mater. 2020;32:e2000208.
Article PubMed CAS Google Scholar
Saayman S, Ali SA, Morris KV, Weinberg MS. The therapeutic application of CRISPR/Cas9 technologies for HIV. Expert Opin Biol Ther. 2015;15:819–30.
Gaglia MM, Munger K. More than just oncogenes: mechanisms of tumorigenesis by human viruses. Curr Opin Virol. 2018;32:48–59.
Mirabello L, Yeager M, Yu K, Clifford GM, Xiao Y, Zhu B, et al. HPV16 E7 Genetic Conservation Is Critical to Carcinogenesis. Cell. 2017;170:1164–74.e6.
Kennedy EM, Kornepati AV, Goldstein M, Bogerd HP, Poling BC, Whisnant AW, et al. Inactivation of the human papillomavirus E6 or E7 gene in cervical carcinoma cells by using a bacterial CRISPR/Cas RNA-guided endonuclease. J Virol. 2014;88:11965–72.
Zhen S, Hua L, Takahashi Y, Narita S, Liu YH, Li Y. In vitro and in vivo growth suppression of human papillomavirus 16-positive cervical cancer cells by CRISPR/Cas9. Biochem Biophys Res Commun. 2014;450:1422–6.
Lucifora J, Xia Y, Reisinger F, Zhang K, Stadler D, Cheng X, et al. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science. 2014;343:1221–8.
Ramanan V, Shlomai A, Cox DB, Schwartz RE, Michailidis E, Bhatta A, et al. CRISPR/Cas9 cleavage of viral DNA efficiently suppresses hepatitis B virus. Sci Rep. 2015;5:10833.
Seeger C, Sohn JA. Targeting hepatitis B virus with CRISPR/Cas9. Mol Ther Nucleic Acids. 2014;3:e216.
Seeger C, Sohn JA. Complete Spectrum of CRISPR/Cas9-induced mutations on HBV cccDNA. Mol Ther. 2016;24:1258–66.
Dong C, Qu L, Wang H, Wei L, Dong Y, Xiong S. Targeting hepatitis B virus cccDNA by CRISPR/Cas9 nuclease efficiently inhibits viral replication. Antivir Res. 2015;118:110–7.
Feng J, Yang G, Liu Y, Gao Y, Zhao M, Bu Y, et al. LncRNA PCNAP1 modulates hepatitis B virus replication and enhances tumor growth of liver cancer. Theranostics. 2019;9:5227–45.
Liu Y, Qi X, Zeng Z, Wang L, Wang J, Zhang T, et al. CRISPR/Cas9-mediated p53 and Pten dual mutation accelerates hepatocarcinogenesis in adult hepatitis B virus transgenic mice. Sci Rep. 2017;7:2796.
Price AA, Sampson TR, Ratner HK, Grakoui A, Weiss DS. Cas9-mediated targeting of viral RNA in eukaryotic cells. Proc Natl Acad Sci U S A. 2015;112:6164–9.
Wang J, Quake SR. RNA-guided endonuclease provides a therapeutic strategy to cure latent herpesviridae infection. Proc Natl Acad Sci U S A. 2014;111:13157–62.
Yuen KS, Chan CP, Wong NM, Ho CH, Ho TH, Lei T, et al. CRISPR/Cas9-mediated genome editing of Epstein-Barr virus in human cells. J Gen Virol. 2015;96:626–36.
Guo R, Jiang C, Zhang Y, Govande A, Trudeau SJ, Chen F, et al. MYC Controls the Epstein-Barr Virus Lytic Switch. Mol Cell. 2020;78:653–69.e8.
Lampreht Tratar U, Horvat S, Cemazar M. Transgenic mouse models in Cancer research. Front Oncol. 2018;8:268.
Day CP, Merlino G, Van Dyke T. Preclinical mouse cancer models: a maze of opportunities and challenges. Cell. 2015;163:39–53.
Huang J, Chen M, Whitley MJ, Kuo HC, Xu ES, Walens A, et al. Generation and comparison of CRISPR-Cas9 and Cre-mediated genetically engineered mouse models of sarcoma. Nat Commun. 2017;8:15999.
Blasco RB, Karaca E, Ambrogio C, Cheong TC, Karayol E, Minero VG, et al. Simple and rapid in vivo generation of chromosomal rearrangements using CRISPR/Cas9 technology. Cell Rep. 2014;9:1219–27.
Xue W, Chen S, Yin H, Tammela T, Papagiannakopoulos T, Joshi NS, et al. CRISPR-mediated direct mutation of cancer genes in the mouse liver. Nature. 2014;514:380–4.
Byrne AT, Alférez DG, Amant F, Annibali D, Arribas J, Biankin AV, et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat Rev Cancer. 2017;17:254–68.
He D, Zhang J, Wu W, Yi N, He W, Lu P, et al. A novel immunodeficient rat model supports human lung cancer xenografts. FASEB J. 2019;33:140–50.
Kaufman CK, Mosimann C, Fan ZP, Yang S, Thomas AJ, Ablain J, et al. A zebrafish melanoma model reveals emergence of neural crest identity during melanoma initiation. Science. 2016;351:aad2197.
Liu CJ, Xie L, Cui C, Chu M, Zhao HD, Yao L, et al. Beneficial roles of melanoma cell adhesion molecule in spinal cord transection recovery in adult zebrafish. J Neurochem. 2016;139:187–96.
Weiss FU, Marques IJ, Woltering JM, Vlecken DH, Aghdassi A, Partecke LI, et al. Retinoic acid receptor antagonists inhibit miR-10a expression and block metastatic behavior of pancreatic cancer. Gastroenterology. 2009;137:2136–45.e1–7.
Drabsch Y, He S, Zhang L, Snaar-Jagalska BE, ten Dijke P. Transforming growth factor-β signalling controls human breast cancer metastasis in a zebrafish xenograft model. Breast Cancer Res. 2013;15:R106.
Zhang B, Shimada Y, Kuroyanagi J, Umemoto N, Nishimura Y, Tanaka T. Quantitative phenotyping-based in vivo chemical screening in a zebrafish model of leukemia stem cell xenotransplantation. PLoS One. 2014;9:e85439.
Yang XJ, Cui W, Gu A, Xu C, Yu SC, Li TT, et al. A novel zebrafish xenotransplantation model for study of glioma stem cell invasion. PLoS One. 2013;8:e61801.
Moshal KS, Ferri-Lagneau KF, Haider J, Pardhanani P, Leung T. Discriminating different cancer cells using a zebrafish in vivo assay. Cancers (Basel). 2011;3:4102–13.
Mayrhofer M, Mione M. The toolbox for conditional Zebrafish Cancer models. Adv Exp Med Biol. 2016;916:21–59.
Ablain J, Xu M, Rothschild H, Jordan RC, Mito JK, Daniels BH, et al. Human tumor genomics and zebrafish modeling identify SPRED1 loss as a driver of mucosal melanoma. Science. 2018;362:1055–60.
Dai F, Wu Y, Lu Y, An C, Zheng X, Dai L, et al. Crosstalk between RNA m6A modification and non-coding RNA contributes to Cancer growth and progression. Mol Ther Nucleic Acids. 2020;22:62–71.
Chen XY, Zhang J, Zhu JS. The role of m6A RNA methylation in human cancer. Mol Cancer. 2019;18:103.
Lan Q, Liu PY, Haase J, Bell JL, Hüttelmaier S, Liu T. The critical role of RNA m6A methylation in Cancer. Cancer Res. 2019;79:1285–92.
Liu XM, Zhou J, Mao Y, Ji Q, Qian SB. Programmable RNA N6-methyladenosine editing by CRISPR-Cas9 conjugates. Nat Chem Biol. 2019;15:865–71.
Wilson C, Chen PJ, Miao Z, Liu DR. Programmable m6A modification of cellular RNAs with a Cas13-directed methyltransferase. Nat Biotechnol. 2020;38:1431–40.
Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1:391–407.
Yao RW, Wang Y, Chen LL. Cellular functions of long noncoding RNAs. Nat Cell Biol. 2019;21:542–51.
Yi W, Li J, Zhu X, Wang X, Fan L, Sun W, et al. CRISPR-assisted detection of RNA-protein interactions in living cells. Nat Methods. 2020;17:685–8.
Liu Q, Zhang H, Huang X. Anti-CRISPR proteins targeting the CRISPR-Cas system enrich the toolkit for genetic engineering. FEBS J. 2020;287:626–44.
Pawluk A, Davidson AR, Maxwell KL. Anti-CRISPR: discovery, mechanism and function. Nat Rev Microbiol. 2018;16:12–7.
Nakamura M, Srinivasan P, Chavez M, Carter MA, Dominguez AA, La Russa M, et al. Anti-CRISPR-mediated control of gene editing and synthetic circuits in eukaryotic cells. Nat Commun. 2019;10:194.
He W, Wang H, Wei Y, Jiang Z, Tang Y, Chen Y, et al. GuidePro: a multi-source ensemble predictor for prioritizing sgRNAs in CRISPR/Cas9 protein knockouts. Bioinformatics. 2021;37:134–6.
Tasan I, Zhao H. Targeting specificity of the CRISPR/Cas9 system. ACS Synth Biol. 2017;6:1609–13.
Tsai SQ, Zheng Z, Nguyen NT, Liebers M, Topkar VV, Thapar V, et al. GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat Biotechnol. 2015;33:187–97.
Tsai SQ, Nguyen NT, Malagon-Lopez J, Topkar VV, Aryee MJ, Joung JK. CIRCLE-seq: a highly sensitive in vitro screen for genome-wide CRISPR-Cas9 nuclease off-targets. Nat Methods. 2017;14:607–14.
Lazzarotto CR, Malinin NL, Li Y, Zhang R, Yang Y, Lee G, et al. CHANGE-seq reveals genetic and epigenetic effects on CRISPR-Cas9 genome-wide activity. Nat Biotechnol. 2020;38:1317–27.
Kosicki M, Tomberg K, Bradley A. Repair of double-strand breaks induced by CRISPR-Cas9 leads to large deletions and complex rearrangements. Nat Biotechnol. 2018;36:765–71.
Zhuo C, Zhang J, Lee JH, Jiao J, Cheng D, Liu L, et al. Spatiotemporal control of CRISPR/Cas9 gene editing. Signal Transduct Target Ther. 2021;6:238.
Lee S, Kim YY, Ahn HJ. Systemic delivery of CRISPR/Cas9 to hepatic tumors for cancer treatment using altered tropism of lentiviral vector. Biomaterials. 2021;272:120793.
Wei T, Cheng Q, Farbiak L, Anderson DG, Langer R, Siegwart DJ. Delivery of tissue-targeted scalpels: opportunities and challenges for in vivo CRISPR/Cas-based genome editing. ACS Nano. 2020;14:9243–62.
Mashel TV, Tarakanchikova YV, Muslimov AR, Zyuzin MV, Timin AS, Lepik KV, et al. Overcoming the delivery problem for therapeutic genome editing: current status and perspective of non-viral methods. Biomaterials. 2020;258:120282.
Wang CS, Chang CH, Tzeng TY, Lin AM, Lo YL. Gene-editing by CRISPR-Cas9 in combination with anthracycline therapy via tumor microenvironment-switchable, EGFR-targeted, and nucleus-directed nanoparticles for head and neck cancer suppression. Nanoscale Horiz. 2021;6:729–43.
Charlesworth CT, Deshpande PS, Dever DP, Camarena J, Lemgart VT, Cromer MK, et al. Identification of preexisting adaptive immunity to Cas9 proteins in humans. Nat Med. 2019;25:249–54.
Ihry RJ, Worringer KA, Salick MR, Frias E, Ho D, Theriault K, et al. p53 inhibits CRISPR-Cas9 engineering in human pluripotent stem cells. Nat Med. 2018;24:939–46.
Haapaniemi E, Botla S, Persson J, Schmierer B, Taipale J. CRISPR-Cas9 genome editing induces a p53-mediated DNA damage response. Nat Med. 2018;24:927–30.
Enache OM, Rendo V, Abdusamad M, Lam D, Davison D, Pal S, et al. Author correction: Cas9 activates the p53 pathway and selects for p53-inactivating mutations. Nat Genet. 2020;52:748–9.
Bowden AR, Morales-Juarez DA, Sczaniecka-Clift M, Agudo MM, Lukashchuk N, Thomas JC, et al. Parallel CRISPR-Cas9 screens clarify impacts of p53 on screen performance. Elife. 2020;9:e55325.
Cullot G, Boutin J, Toutain J, Prat F, Pennamen P, Rooryck C, et al. CRISPR-Cas9 genome editing induces megabase-scale chromosomal truncations. Nat Commun. 2019;10:1136.
Download references
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 82073101, 81872210, 81802948), The Excellent talent science and technology innovation project of Shanxi Province (No. 201805D211007), Shanxi Province Scientific and Technological Achievements Transformation Guidance Foundation (No. 201804D131043), Shanxi Province Science Foundation for Excellent Young Scholars (No. 201901D211486), Applied Basic Research project of Shanxi province (No. 201801D221421, 201901D211490), Research Project Supported by Shanxi Scholarship Council of China (No. 2020165), Fund for the Scientific Activities of Selected Return Overseas Professionals in Shanxi Province (No.20200034), Shenzhen Key Laboratory Foundation (No. ZDSYS20200811143757022), “1111” medical innovation project of Shanxi Province (No. 2020TD26, 2020RC13, 2020RC20), Youth Top Talent Program Fund of Shanxi Province.
Author information
Huimin Zhang, Chunhong Qin and Changming An contributed equally to this work.
Authors and Affiliations
Shanxi Key Laboratory of Otorhinolaryngology Head and Neck Cancer, Shanxi Province Clinical Medical Research Center for Precision Medicine of Head and Neck Cancer, Department of Otolaryngology Head & Neck Surgery, First Hospital of Shanxi Medical University, Taiyuan, 030001, Shanxi, China
Huimin Zhang, Chunhong Qin, Xiwang Zheng, Wei Gao & Yongyan Wu
Department of Biochemistry & Molecular Biology, Shanxi Medical University, Taiyuan, 030001, Shanxi, China
Chunhong Qin & Yongyan Wu
Department of Head and Neck Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, China
Changming An
General Hospital, Clinical Medical Academy, Shenzhen University, Shenzhen, 518055, Guangdong, China
Xiwang Zheng, Wei Gao & Yongyan Wu
Department of Otolaryngology Head & Neck Surgery, Shanxi Bethune Hospital, Taiyuan, 030032, Shanxi, China
Shuxin Wen & Wenjie Chen
Department of Otolaryngology-Head and Neck Surgery, Shandong Provincial ENT Hospital, Cheeloo College of Medicine, Shandong University, Jinan, 250022, Shandong, China
Xianfang Liu, Zhenghua Lv & Wei Xu
Research Center of Allergy and Immunology, Shenzhen University School of Medicine, Shenzhen, 518055, Guangdong, China
Pingchang Yang
Guangdong Provincial Key Laboratory of Regional Immunity and Diseases, Shenzhen, 518055, Guangdong, China
Department of Cell biology and Genetics, Basic Medical School of Shanxi Medical University, Taiyuan, 030001, Shanxi, China
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: YYW, WG, and WX. Compilation of literature: HMZ, CHQ, CMA, SXW, WJC, XFL, ZHL, and PCY. Article writing and editing: HMZ, CHQ, CMA, and PCY. Figure organization: HMZ, CHQ, and CMA. Supervision: YYW, WG, and WX. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Wei Xu , Wei Gao or Yongyan Wu .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Zhang, H., Qin, C., An, C. et al. Application of the CRISPR/Cas9-based gene editing technique in basic research, diagnosis, and therapy of cancer. Mol Cancer 20 , 126 (2021). https://doi.org/10.1186/s12943-021-01431-6
Download citation
Received : 10 July 2021
Accepted : 19 September 2021
Published : 01 October 2021
DOI : https://doi.org/10.1186/s12943-021-01431-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cancer research
- CRISPR/Cas9
- Gene editing technology
- Cancer therapy
- Diagnosis of cancer
Molecular Cancer
ISSN: 1476-4598
- General enquiries: [email protected]
Advances In Research On Genome Editing Crispr-Cas9 Technology
Affiliations.
- 1 Centre of Excellence in Molecular Biology, University of the Punjab, Lahore, Pakistan.
- 2 School of Biological Sciences, University of Punjab, Lahore, Pakistan.
- 3 Department of Biotechnology, Quaid-i-Azam University, Islamabad, Pakistan.
- 4 Ayub Medical College, Abbottabad, Pakistan.
- 5 Institute of Biochemistry and Biotechnology, University of the Punjab, Lahore, Pakistan.
- PMID: 30868795
Background: The current era of genome engineering has been revolutionized by the evolution of a bacterial adaptive immune system, CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) into a radical technology that is making an expeditious progress in its mechanism, function and applicability..
Methods: A systematic literature review study was carried out with the help of all available information and online resources..
Results: In this review, we intend to elucidate different aspects of CRISPR in the light of current advancements. Utilizing a nonspecific Cas9 nuclease and a sequence specific programmable CRISPR RNA (crRNA), this system cleaves the target DNA with high precision. With a vast potential for profound implications, CRISPR has emerged as a mainstream method for plausible genomic manipulations in a range of organisms owing to its simplicity, accuracy and speed. A modified form of CRISPR system, known as CRISPR/Cpf1 that employs a smaller and simpler endonuclease (Cpf1) than Cas9, can be used to overcome certain limitations of CRISPR/Cas9 system. Despite clear-cut innovative biological applications, this technology is challenged by off-target effects and associated risks, thus safe and controlled implementation is needed to enable this emerging technique assist both biological research and translational applications.
Conclusions: CRISPR/Cas9 systems will undoubtedly revolutionize the study and treatment of both immunologic and allergic diseases. Concerned authorities should formulate and authorize such laws and regulations that permit the safe and ethical use of this emerging technology for basic research and clinical purposes.
Keywords: Anti-CRISPR activity; Implications; CRISPR-Cas9 technology; Off-target effects.
Publication types
- CRISPR-Cas Systems*
- Gene Editing*
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Comput Struct Biotechnol J
CRISPR-Cas systems: Overview, innovations and applications in human disease research and gene therapy
Genome editing is the modification of genomic DNA at a specific target site in a wide variety of cell types and organisms, including insertion, deletion and replacement of DNA, resulting in inactivation of target genes, acquisition of novel genetic traits and correction of pathogenic gene mutations. Due to the advantages of simple design, low cost, high efficiency, good repeatability and short-cycle, CRISPR-Cas systems have become the most widely used genome editing technology in molecular biology laboratories all around the world. In this review, an overview of the CRISPR-Cas systems will be introduced, including the innovations, the applications in human disease research and gene therapy, as well as the challenges and opportunities that will be faced in the practical application of CRISPR-Cas systems.
1. Introduction
Genome editing is the modification of genomic DNA at a specific target site in a wide variety of cell types and organisms, including insertion, deletion and replacement of DNA, resulting in inactivation of target genes, acquisition of novel genetic traits and correction of pathogenic gene mutations [1] , [2] , [3] . In recent years, with the rapid development of life sciences, genome editing technology has become the most efficient method to study gene function, explore the pathogenesis of hereditary diseases, develop novel targets for gene therapy, breed crop varieties, and so on [4] , [5] , [6] , [7] .
At present, there are three mainstream genome editing tools in the world, zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs) and the RNA-guided CRISPR (clustered regularly interspaced short palindromic repeats)-Cas (CRISPR-associated) nucleases systems [8] , [9] , [10] . Due to the advantages of simple design, low cost, high efficiency, good repeatability and short-cycle, CRISPR-Cas systems have become the most widely used genome editing technology in molecular biology laboratories all around the world [11] , [12] . In this review, an overview of the CRISPR-Cas systems will be introduced, including the innovations and applications in human disease research and gene therapy, as well as the challenges and opportunities that will be faced in the practical application of CRISPR-Cas systems.
2. Overview of CRISPR-Cas systems
CRISPR-Cas is an adaptive immune system existing in most bacteria and archaea, preventing them from being infected by phages, viruses and other foreign genetic elements [13] , [14] . It is composed of CRISPR repeat-spacer arrays, which can be further transcribed into CRISPR RNA (crRNA) and trans -activating CRISPR RNA (tracrRNA), and a set of CRISPR-associated (cas) genes which encode Cas proteins with endonuclease activity [15] . When the prokaryotes are invaded by foreign genetic elements, the foreign DNA can be cut into short fragments by Cas proteins, then the DNA fragments will be integrated into the CRISPR array as new spacers [16] . Once the same invader invades again, crRNA will quickly recognize and pair with the foreign DNA, which guides Cas protein to cleave target sequences of foreign DNA, thereby protecting the host [16] .
CRISPR-Cas systems can be classified into 2 classes (Class 1 and Class 2), 6 types (I to VI) and several subtypes, with multi-Cas protein effector complexes in Class 1 systems (Type I, III, and IV) and a single effector protein in Class 2 systems (Type II, V, and VI) [17] , [18] . The classification, representative members, and typical characteristics of each CRISPR-Cas system are summarized in Table 1 [10] , [12] , [15] , [16] , [17] , [18] .
Summary of CRISPR-Cas systems.
Type II CRISPR-Cas9 system derived from Streptococcus pyogene s (SpCas9) is one of the best characterized and most commonly used category in numerous CRISPR-Cas systems [18] , [19] . The main components of CRISPR-Cas9 system are RNA-guided Cas9 endonuclease and a single-guide RNA (sgRNA) [20] . The Cas9 protein possesses two nuclease domains, named HNH and RuvC, and each cleaves one strand of the target double-stranded DNA [21] . A single-guide RNA (sgRNA) is a simplified combination of crRNA and tracrRNA [22] . The Cas9 nuclease and sgRNA form a Cas9 ribonucleoprotein (RNP), which can bind and cleave the specific DNA target [23] . Furthermore, a protospacer adjacent motif (PAM) sequence is required for Cas9 protein’s binding to the target DNA [20] .
During genome editing process, sgRNA recruits Cas9 endonuclease to a specific site in the genome to generate a double-stranded break (DSB), which can be repaired by two endogenous self-repair mechanisms, the error-prone non-homologous end joining (NHEJ) pathway or the homology-directed repair (HDR) pathway [24] . Under most conditions, NHEJ is more efficient than HDR, for it is active in about 90% of the cell cycle and not dependent on nearby homology donor [25] . NHEJ can introduce random insertions or deletions (indels) into the cleavage sites, leading to the generation of frameshift mutations or premature stop codons within the open reading frame (ORF) of the target genes, finally inactivating the target genes [26] , [27] . Alternatively, HDR can introduce precise genomic modifications at the target site by using a homologous DNA repair template [28] , [29] ( Fig. 1 ). Furthermore, large fragment deletions and simultaneous knockout of multiple genes could be achieved by using multiple sgRNAs targeting one single gene or more [30] , [31] .

Mechanism of genome editing. Double-strand break (DSB) induced by nucleases can be repaired by non-homologous end joining (NHEJ) or homology-directed repair (HDR) pathways. NHEJ can introduce random insertions or deletions (indels) of varying length at the site of the DSB. Alternatively, HDR can introduce precise genomic modifications at the target site by using a homologous DNA donor template.
3. Innovations of CRISPR-Cas systems
CRISPR-Cas systems have become the most favorite genome editing tool in the molecular biology laboratory since they were confirmed to have genome editing capabilities in 2012 [23] . They have made numerous achievements in the field of correcting pathogenic mutations, searching for essential genes for cancer immunotherapy, and solving key problems in organ xenotransplantation [5] , [32] , [33] . Unfortunately, there are still some limitations which need to solve in CRISPR-Cas systems, such as potential off-target effects, limited genome-targeting scope restricted by PAM sequences, and low efficiency and specificity [34] , [35] . Therefore, many research teams have been trying to improve this tool.
3.1. Dead-Cas9 system
By introducing two point mutations, H840A and D10A, into HNH and RuvC nuclease domain, researchers have obtained a nuclease dead Cas9 (dCas9) [36] . The dCas9 lacks DNA cleavage activity, but DNA binding activity is not affected. Then, by fusing transcriptional activators or repressors to dCas9, the CRISPR-dCas9 system can be used to activate (CRISPRa) or inhibit (CRISPRi) transcription of target genes [37] , [38] . Additionally, dCas9 can be fused to various effector domains, which enables sequence-specific recruitment of fluorescent proteins for genome imaging and epigenetic modifiers for epigenetic modification [39] , [40] . Furthermore, this system is easy to operate and allows simultaneous manipulation of multiple genes within a cell [38] .
3.2. Base editing system
In order to improve the efficiency of site-directed mutagenesis, base editing systems containing dCas9 coupled with cytosine deaminase (cytidine base editor, CBE) or adenosine deaminase (adenine base editor, ABE) have been developed [41] , [42] . It can introduce C·G to T·A or A·T to G·C point mutations into the editing window of the sgRNA target sites without double-stranded DNA cleavage [41] , [42] . Since base editing systems avoid the generation of random insertions or deletions to a great extent, the results of gene mutation are more predictive. However, owing to the restriction of base editing window, base editing systems are not suitable for any target sequence in the genome. Accordingly, C-rich sequences, for example, would produce a lot of off-target mutations [43] . Therefore, researchers have always been trying to develop and optimize novel base editing systems to overcome this drawback [44] . At present, base editing systems have been widely used in various cell lines, human embryos, bacteria, plants and animals for efficient site-directed mutagenesis, which may have broad application prospects in basic research, biotechnology and gene therapy [45] , [46] , [47] . In theory, 3956 gene variants existing in Clin var database could be repaired by base substitution of C-T or G-A [42] , [48] .
3.3. Cas9 variant system
An NGG PAM at the 3′ end of the target DNA site is essential for the recognization and cleavage of the target gene by Cas9 protein [20] . Besides classical NGG PAM sites, other PAM sites such as NGA and NAG also exist, but their efficiency of genome editing is not high [49] . However, such PAM sites only exist in about one-sixteenth of the human genome, thereby largely restricting the targetable genomic loci. For this purpose, several Cas9 variants have been developed to expand PAM compatibility.
In 2018, David Liu et al. [50] developed xCas9 by phage-assisted continuous evolution (PACE), which can recognize multiple PAMs (NG, GAA, GAT, etc.). In the latter half of the same year, Nishimasu et al. developed SpCas9-NG, which can recognize relaxed NG PAMs [51] . In 2020, Miller et al. developed three new SpCas9 variants recognizing non-G PAMs, such as NRRH, NRCH and NRTH PAMs [52] . Later in the same year, Walton et al. developed a SpCas9 variant named SpG, which is capable of targeting an expanded set of NGN PAMs [53] . Subsequently, they optimized the SpG system and developed a near-PAMless variant named SpRY, which is capable of editing nearly all PAMs (NRN and NYN PAMs) [53] .
By using these Cas9 variants, researchers have repaired some previously inaccessible disease-relevant genetic variants [51] , [52] , [53] . However, there are still some drawbacks in these variants, such as low efficiency and cleavage activity [50] , [51] . Therefore, they should be further improved by molecular engineering in order to expand the applications of SpCas9 in disease-relevant genome editing.
3.4. RNA editing system
In addition to editing DNA, CRISPR-Cas systems can also edit RNA. Class 2 Type VI CRISPR-Cas13 systems contain a single RNA-guided Cas13 protein with ribonuclease activity, which can bind to target single-stranded RNA (ssRNA) and specifically cleave the target [54] . To date, four Cas13 proteins have been identified: Cas13a (also known as C2c2), Cas13b, Cas13c and Cas13d [55] . They have successfully been applied in RNA knockdown, transcript labeling, splicing regulation and virus detection [56] , [57] , [58] . Later, Feng Zhang et al. developed two RNA base edting systems (REPAIR system, enables A-to-I (G) replacement; RESCUE system, enables C-to-U replacement) by fusing catalytically inactivated Cas13 (dCas13) with the adenine/cytidine deaminase domain of ADAR2 (adenosine deaminase acting on RNA type 2) [59] , [60] .
Compared with DNA editing, RNA editing has the advantages of high efficiency and high specificity. Furthermore, it can make temporary, reversible genetic edits to the genome, avoiding the potential risks and ethical issues caused by permanent genome editing [61] , [62] . At present, RNA editing has been widely used for pre-clinical studies of various diseases, which opens a new era for RNA level research, diagnosis and treatment.
3.5. Prime editing system
Recently, Anzalone et al. developed a novel genome editing technology, named prime editing, which can mediate targeted insertions, deletions and all 12 types of base substitutions without double-strand breaks or donor DNA templates [63] . This system contains a catalytically impaired Cas9 fused to a reverse transcriptase and a prime editing guide RNA (pegRNA) with functions of specifying the target site and encoding the desired edit [63] . After Cas9 cleaves the target site, the reverse transcriptase uses pegRNA as a template for reverse transcription, and then, new genetic information can be written into the target site [63] . Prime editing can effectively improve the efficiency and accuracy of genome editing, and significantly expand the scope of genome editing in biological and therapeutic research. In theory, it is possible to correct up to 89% known disease-causing gene mutations [63] . Nevertheless, as a novel genome editing technique, more research is still needed to further understand and improve prime editing system.
4. Applications of CRISPR-Cas systems in human disease research
4.1. applications of crispr-cas systems in establishing animal and cell models of human diseases.
So far, as a rapid and efficient genome editing tool, CRISPR-Cas systems have been extensively used in a variety of species, including bacteria, yeast, tobacco, Arabidopsis, sorghum, rice, Caenorhabditis elegans, Drosophila, zebrafish, Xenopus laevis, mouse, rat, rabbit, dog, sheep, pig and monkey [64] , [65] , [66] , [67] , [68] , [69] , [70] , [71] , [72] , [73] , [74] , [75] , [76] , [77] , [78] , as well as various human cell lines, such as tumor cells, adult cells and stem cells [79] , [80] . In medical field, the most important application of CRISPR-Cas systems is to establish genetically modified animal and cell models of many human diseases, including gene knockout models, exogenous gene knock-in models, and site directed mutagenesis models [80] , [81] .
Animal models are crucial tools for understanding gene function, exploring pathogenesis of human diseases and developing new drugs. However, traditional methods for generating animal models are complex, costly and time-consuming, which severely limit the application of animal models in basic medical research and preclinical studies [82] . Since the discovery of CRISPR-Cas systems, a series of genetically modified animal models have successfully been generated in a highly efficient manner [72] , [73] , [74] , [75] , [76] , [77] , [78] .
Among numerous model animals, mice are widely used for scientific studies and recognized as the most important model animals in human disease research [83] . So far, researchers have successfully generated many genetically modified mouse models, such as cancer, cardiovascular disease, cardiomyopathy, Huntington's disease, albino, deafness, hemophilia B, obesity, urea cycle disorder and muscular dystrophy [84] , [85] , [86] , [87] , [88] , [89] , [90] , [91] , [92] , [93] . Nevertheless, owing to the great species differences between humans and rodents, they can’t provide effective assessment and long-term follow-up for research and treatment of human diseases [94] . Therefore, the application of larger model animals, such as rabbits, pigs and non-human primates, is becoming more and more widespread [74] , [77] , [78] . With the development of CRISPR-Cas systems, generating larger animal models for human diseases has become a reality, which greatly enriches the disease model resource bank.
Our research focuses on the generation of genetically modified rabbit models using CRISPR-Cas systems. Compared with mice, rabbits are closer to humans in physiology, anatomy and evolution [95] . In addition, rabbits have a short gestation period and less breeding cost. All these make them suitable for studies of the cardiovascular, pulmonary and metabolism diseases [95] , [96] . Nowadays, we have generated a series of rabbit models for simulating human diseases, including congenital cataracts, duchenne muscular dystrophy (DMD), X-linked hypophosphatemia (XLH), etc (summarized in Table 2 ) [97] , [98] , [99] , [100] , [101] , [102] , [103] , [104] , [105] , [106] , [107] , [108] , [109] , [110] , [111] , [112] , [113] , [114] . Take the generation of PAX4 gene knockout rabbits as an example, the procedure we used to establish genetically modified rabbit models is summarized in Fig. 2 and Table 3 .
CRISPR-Cas system mediated rabbit models of human diseases.

Generation of PAX4 gene knockout (KO) rabbits using CRISPR-Cas9 system. (A) Schematic diagram of the sgRNA target sites located in the rabbit PAX4 locus. PAX4 exons are indicated by yellow rectangles; target sites of the two sgRNA sequences, sgRNA1 and sgRNA2, are highlighted in green; protospacer-adjacent motif (PAM) sequence is highlighted in red. Primers F and R are used for mutation detection in pups. (B) Microinjection and embryo transfer. First a mixture of Cas9 mRNA and sgRNA is microinjected into the cytoplasm of the zygote at the pronuclear stage. Then the injected embryos are transferred into the oviduct of recipient rabbits. After 30 days gestation, PAX4 KO rabbits are born. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Summary of the PAX4 KO rabbits generated by CRISPR-Cas9 system.
In addition, the pig is an important model animal extensively used in biomedical research. Compared with mice, their body/organ size, lifespan, anatomy, physiology, metabolic profile and immune characteristics are more similar to those of humans, which makes the pig an ideal model for studying human cardiovascular diseases and xenotransplantation [115] . At present, several genetically modified pig models have been successfully generated, including neurodegenerative diseases, cardiovascular diseases, cancer, immunodeficiency and xenotransplantation model [116] , [117] , [118] , [119] , [120] , [121] , [122] .
To date, non-human primates are recognized as the best human disease models. Their advantage is that their genome has 98% homology with the human genome; also, they are highly similar to humans in tissue structure, immunity, physiology and metabolism [123] . What’s more, they can be infected by human specific viruses, which makes them very important models in infectious disease research [124] . Nowadays, researchers have generated many genetically modified monkey models, such as cancer, muscular dystrophy, developmental retardation, adrenal hypoplasia congenita and Oct4-hrGFP knockin monkeys [125] , [126] , [127] , [128] , [129] .
It was found that the efficiency of CRISPR-Cas mediated genome editing is higher in vitro than in vivo , thus the use of genetically modified cell models can greatly shorten the research time in medical research [130] . Until now, researchers have used CRISPR-Cas systems to perform genetic manipulations on various cell lines, such as tumor cells, adult cells and stem cells, in order to simulate a variety of human diseases [79] , [80] .
Fuchs et al. generated the RPS25-deficient Hela cell line by knocking out ribosomal protein eS25 (RPS25) gene using CRISPR-Cas9 system [131] . Drost et al. edited four common colorectal cancer-related genes (APC, P53, KRAS and SMAD4) in human intestinal stem cells (hISCs) by CRISPR-Cas9 technology [132] . The genetically modified hISCs with 4 gene mutations possessed the biological characteristics of intestinal tumors and could simulate the occurrence of human colorectal cancer [132] . Jiang et al. induced site-specific chromosome translocation in mouse embryonic stem cells by CRISPR-Cas9, in order to establish a cell and animal model for subsequent research on congenital genetic diseases, infertility, and cancer related to chromosomal translocation [133] .
In addition, induced pluripotent stem cells (iPSCs) have shown great application prospect in disease model establishment, drug discovery and patient-specific cellular therapy development [134] . iPSCs have the ability of self-renewal and multiple differentiation potential, which are of great significance in disease model establishment and regenerative medicine research [135] . In recent years, by combining CRISPR-Cas systems with iPSC technology, researchers have generated numerous novel and reliable disease models with isogenic backgrounds and provided new solutions for cell replacement therapy and precise therapy in a variety of human diseases, including neurodegenerative diseases, acquired immunodeficiency syndrome (AIDS), β-thalassemia, etc [134] , [135] , [136] .
4.2. Applications of CRISPR-Cas systems in disease diagnosis
With the development of CRISPR-Cas systems and the discovery of novel Cas enzymes (Cas12, Cas13, etc.), CRISPR-based molecular diagnostic technology is rapidly developing and has been selected as one of the world's top ten science and technology advancements in 2018 [137] .
Unlike Cas9, Cas13 enzymes possess a ‘collateral cleavage’ activity, which can induce cleavage of nearby non-target RNAs after cleavage of target sequence [54] . Based on the ‘collateral cleavage’ activity of Cas13, Feng Zhang et al. [138] developed a Cas13a-based in vitro nucleic acid detection platform, named SHERLOCK (Specific High Sensitivity Enzymatic Reporter UnLOCKing). It is composed of Cas13a, sgRNA targeting specific RNA sequences and fluorescent RNA reporters. After Cas13a protein recognizes and cleaves the target RNA, it will cut the report RNA and release the detectable fluorescence signal, so as to achieve the purpose of diagnosis [138] . Researchers have used this method to detect viruses, distinguish pathogenic bacteria, genotype human DNA and identify tumor DNA mutations [137] , [138] . Later, Feng Zhang et al. improved SHERLOCK system and renamed it as SHERLOCKv2, which can detect four virus at the same time [139] .
In addition to Cas13, Cas12 enzymes are also found to possess collateral cleavage activity [140] . Doudna et al. [141] developed a nucleic acid detection system based on Cas12a (also known as Cpf1), named DETECTR (DNA endonuclease-targeted CRISPR trans reporter) . DETECTR has been used to detect cervical cancer associated HPV subtypes (HPV16 and HPV18) in either virus-infected human cell lines or clinical patient samples [141] . Furthermore, Doudna et al. are trying to use the newly discovered Cas14 and CasX proteins in molecular diagnosis, which may further enrich the relevant techniques of CRISPR-based molecular diagnosis [142] , [143] .
CRISPR-based molecular diagnostic technology has incomparable advantages over traditional molecular diagnostic methods, such as high sensitivity and single-base specificity, which is suitable for early screening of cancer, detection of cancer susceptibility genes and pathogenic genes [137] , [144] . Meanwhile, CRISPR diagnostics is inexpensive, simple, fast, without special instrument, and is suitable for field quick detection and detection in less-developed areas [137] , [144] . At present, many companies are trying to develop CRISPR diagnostic kits for family use, to detect HIV, rabies, Toxoplasma gondi, etc.
4.3. Applications of CRISPR-Cas systems in genome-scale screening
CRISPR-Cas9 system enables genome-wide high-throughput screening, making it a powerful tool for functional genomic screening [145] . The high efficiency of genome editing with CRISPR-Cas9 system makes it possible to edit multiple targets in parallel, thus a mixed cell population with gene mutation can be produced, and the relationship between genotypes and phenotypes could be confirmed by these mutant cells [146] . CRISPR-Cas9 library screening can be divided into two categories: positive selection and negative selection [147] . It has been utilized to identify genes associated with cancer cell survival, drug resistance and virus infection in various models [148] , [149] , [150] . Compared with RNAi-based screening, high-throughput CRISPR-Cas9 library screening has the advantages of higher transfection efficiency, minimal off-target effects and higher data reproducibility [151] . At present, scientists have constructed human and mouse genome-wide sgRNA libraries, and they have been increasingly improved according to different requirements [152] , [153] . In the future, CRISPR-Cas9-based high-throughput screening technology will definitely get unprecedented development and application.
4.4. Applications of CRISPR-Cas systems in gene therapy
Gene therapy refers to the introduction of foreign genes into target cells to treat specific diseases caused by mutated or defective genes [154] . Target cells of gene therapy are mainly divided into two categories: somatic cells and germ line cells. However, since germ line gene therapy is complicated in technique as well as involves ethical and security issues, today gene therapy is limited to somatic cell gene therapy [155] . Traditional gene therapy is usually carried out by homologous recombination or lentiviral delivery. Nevertheless, the efficiency of homologous recombination is low, and lentiviral vectors are randomly inserted into the recipient genome, which may bring potential security risks to clinical applications [156] . Currently, with the rapid development of CRISPR-Cas systems, they have been widely applied in gene therapy for treating various of human diseases, monogenic diseases, infectious diseases, cancer, etc [155] , [156] , [157] . Furthermore, some CRISPR-mediated genome-editing therapies have already reached the stage of clinical testing. Table 4 briefly summarizes the ongoing clinical trials of gene therapy using genome-editing technology, including ZFN, TALEN and CRISPR-Cas systems.
Monogenic diseases refer to the genetic diseases caused by mutations of a single allele or a pair of alleles on a pair of homologous chromosomes [158] . There are more than 6600 known monogenic diseases around the world, β-thalassaemia, sickle cell disease (SCD), hemophilia B (HB), retinitis pigmentosa (RP), leber congenital amaurosis type 10 (LCA10), duchenne muscular dystrophy (DMD), hutchinson-gilford progeria syndrome (HGPS), hereditary tyrosinemia (HT), cystic fibrosis (CF), etc [159] . Most of the monogenic diseases are rare diseases lacking of effective treatment, which will greatly affect the life quality of patients. Nowadays, many animal models of monogenic diseases have been treated with CRISPR-mediated gene therapy. Furthermore, even some CRISPR clinical trials for monogenic diseases are going on [160] .
Summary of clinical trials of gene therapy using genome-editing technology.
β-Thalassaemia, a hereditary hemolytic anemia disease, is one of the most common and health-threatening monogenic diseases in the world. It is characterized by mutations in the β-globin (HBB) gene, leading to severe anemia caused by decreased hemoglobin (Hb) level [161] . For the moment, the only way to cure β-thalassemia is hematopoietic stem cell transplantation (HSCT). Yet, high cost of treatment and shortage of donors limit its clinical application [162] . Other therapy, for example, blood transfusion, can only sustain the life of patients but can’t cure the disease [161] . To better treat β-thalassemia, researchers have turned their attention to gene therapy. A major technical idea is to repair the defective β-globin gene of iPSCs from patients with β-thalassemia by CRISPR-Cas9 technology, then red blood cells can be produced normally and the disease could be cured [163] , [164] . Besides, reactivating fetal hemoglobin (HbF) expression has also been proposed to be an effective method to treat β-thalassemia through knockout of BCL11A gene, which suppresses the expression of fetal hemoglobin [165] , [166] .
Additionally, CRISPR-Cas systems have also been used for the treatment of other hematologic diseases, such as sickle cell disease (SCD) and hemophilia B (HB). SCD is a monogenic disease caused by a single-nucleotide mutation in human β-globin gene, leading to a substitution of glutamic acid by valine and the production of an abnormal version of β-globin, which is known as hemoglobin S (HbS) [167] . CRISPR-Cas9 system has been used to treat SCD by repairing the β-globin gene mutation or reactivating HbF expression [168] , [169] . HB is an X-linked hereditary bleeding disorder caused by deficiency of coagulation factor IX, and the most common treatment for hemophilia B is supplement blood coagulation factor [170] , [171] . Huai et al. injected naked Cas9-sgRNA plasmid and donor DNA into the adult mice of F9 mutation HB mouse model for gene correction [172] . Meanwhile, Cas9/sgRNA were also microinjected into germline cells of this HB mouse model for gene correction. Both in vivo and ex vivo experiment were sufficient to remit the coagulation deficiency [172] . Guan et al. corrected the F9 Y371D mutation in HB mice using CRISPR-Cas9 mediated in situ genome editing, which greatly improved the hemostatic efficiency and increased the survival of HB mice [173] .
Duchenne muscular dystrophy (DMD) is an X-chromosome recessive hereditary disease, with clinical manifestations of muscle weakness or muscle atrophy due to a progressive deterioration of skeletal muscle function [174] . It is usually caused by mutations in the DMD gene, a gene encoding dystrophin protein [174] . Deletions of one or more exons of the DMD gene will result in frameshift mutations or premature termination of translation, thereby normal dystrophin protein can not be synthesized [175] . Currently, there is no effective treatment for DMD. Conventional drug treatment can only control the disease to a certain extent, but can not cure it. It was found that a functional truncated dystrophin protein can be obtained by removing the mutated transcripts with CRISPR-Cas9 system [176] , [177] , [178] . In addition, base editing systems can also be applied in DMD treatment by repairing single base mutation or inducing exon skipping by introducing premature termination codons (PTCs) [179] .
Retinitis pigmentosa (RP) is a group of hereditary retinal degenerative diseases characterized by progressive loss of photoreceptor cells and retinal pigment epithelium (RPE) function [180] . RP has obvious genetic heterogeneity, and the inheritance patterns include autosomal dominant, autosomal recessive, and X-linked recessive inheritance [180] . To date, there is still no cure for RP. In recent years, with the rapid development of gene editing technology, there has been some progress in the treatment of RP. Several gene mutations causing RP have been corrected by CRISPR-Cas9 in mouse models to prevent retinal degeneration and improve visual function, for example, RHO gene, PRPF31 gene and RP1 gene [181] , [182] .
Leber Congenital Amaurosis type 10 (LCA10) is an autosomal retinal dystrophy with severe vision loss at an early age. The most common gene mutation found in patients with LCA10 is IVS26 mutation in the CEP290 gene, which disrupts the coding sequence by generating an aberrant splice site [183] . Ruan et al. used CRISPR-Cas9 system to knock out the intronic region of the CEP290 gene and restored normal CEP290 expression [184] . In addition, subretinal injection of EDIT-101 in humanized CEP290 mice showed rapid and sustained CEP290 gene editing [185] , [186] .
Hutchinson-Gilford Progeria Syndrome (HGPS) is a rare lethal genetic disorder with the characteristic of accelerated aging [187] . A point mutation within exon 11 of lamin A gene activates a cryptic splice site, leading to the production of a truncated lamin A called progerin [188] . However, CRISPR-Cas based gene therapy has opened up a broad prospect in HGPS treatment. Administration of AAV-delivered CRISPR-Cas9 components into HGPS mice can reduce the expression of progerin, thereby improved the health condition and prolonged the lifespan of HGPS mice [189] , [190] . In addition, Suzuki et al . repaired G609G mutation in a HGPS mouse model via single homology arm donor mediated intron-targeting gene integration (SATI), which ameliorated aging-associated phenotypes and extended the lifespan of HGPS mice [191] .
CRISPR-Cas systems have also showed their advantages in gene therapy of hereditary tyrosinemia (HT) and cystic fibrosis (CF). HT is a disorder of tyrosine metabolism caused by deficiency of fuarylacetoacetate hydrolase (Fah) [192] . Yin et al. corrected a Fah mutation in a HT mouse model by injecting CRISPR-Cas9 components into the liver of the mice [193] . Then, the wild-type Fah protein in the liver cells began to express and the body weight loss phenotype was rescued [193] . CF, an autosomal recessive inherited disease with severe respiratory problems and infections, has a high mortality rate at an early age [194] . It is caused by mutations in the CFTR gene, which encodes an epithelial chloride anion channel, the cystic fibrosis transmembrane conductance regulator (CFTR) [194] . Until now, genome editing strategies have been carried out in cell models to correct CFTR mutations. In cultured intestinal stem cells and induced pluripotent stem cells from cystic fbrosis patients, the CFTR homozygous Δ508 mutation has been corrected by CRISPR-Cas9 technology, leading to recovery of normal CFTR expression and function in differentiated mature airway epithelial cells and intestinal organoids [195] , [196] .
In recent years, gene therapy has gradually been applied to the treatment of viral infectious diseases. Transforming host cells to avoid viral infection or preventing viral proliferation and transmission are two main strategies for gene therapy of viral infectious diseases [197] .
Human immunodeficiency virus (HIV), a kind of retrovirus, mainly attacks the human immune system, especially the CD4 + T lymphocytes. When human cells are invaded by HIV, the viral sequences can be integrated into the host genome, blocking cellular and humoral immunity while causing acquired immunodeficiency syndrome (AIDS) [198] . There is still no known cure for AIDS but it could be treated. Although antiretroviral therapy can inhibit HIV-1 replication, the viral sequences still exist in the host genome, and they could be reactivated at any time [199] . CRISPR-Cas9 system can target long terminal repeat (LTR) and destruct HIV-1 proviruses, thus it is possible to completely eliminate HIV-1 from genome of infected host cells [200] , [201] . In addition, resistance to HIV-1 infection could be induced by knockout of the HIV co-receptor CCR5 gene in CD4 + T cells [202] , [203] .
Cervical cancer is the second most common gynecologic malignant tumor. The incidence is increasing year by year and young people are especially prone to this disease. It was found that the occurrence of cervical cancer is closely related to HPV (human papillomavirus) infection [204] . HPV is a double-stranded cyclic DNA virus, E6 and E7 genes located in HPV16 early regions are carcinogenic genes [205] . Researchers designed sgRNAs targeting E6 and E7 genes to block the expression of E6 and E7 protein, subsequently the expression of p53 and pRb was restored to normal, finally increasing tumor cells apoptosis and suppressing subcutaneous tumor growth in in vivo experiments [206] , [207] , [208] . Moreover, HPV virus proliferation was blocked through cutting off E6/E7 genes, and the virus in the bodies could be eliminated [206] , [207] , [208] .
Cancer is the second leading cause of death worldwide after cardiovascular diseases, and it is also a medical problem that needs to be solved urgently. A variety of genetic or epigenetic mutations have been accumulated in the cancer genome, which can activate proto-oncogenes, inactivate tumor suppressors and produce drug resistance [209] , [210] . So far, CRISPR-Cas systems have been used to correct the oncogenic genome/epigenome mutations in tumor cells and animal models, resulting in inhibition of tumor cell growth and promotion of cell apoptosis, thereby inhibiting tumor growth [211] , [212] , [213] .
In addition, immunotherapy is considered to be a major breakthrough in cancer treatment, especially chimeric antigen receptor-T (CAR-T) cell therapy, which has a significantly therapeutic effect on leukemia, lymphoma and certain types of solid tumors [214] , [215] , [216] . CAR-T cells are genetically manipulated, patient-specific T cells, which express receptors targeting antigens specially expressed on tumor cells, for example, CD19 CAR-T cells for B cell malignancies. Then these cells will be transfused back to patients to fight against cancer [217] . However, CAR-T cell therapy is complex, time-consuming and expensive, and it is greatly limited by the quality and quantity of autologous T cells. Therefore, researchers have used CRISPR-Cas9 system to develop universal CAR-T cells, such as simultaneously removing endogenous T cell receptor gene and HLA class I encoding gene on T cells of healthy donors and introducing CAR sequence [218] , [219] , [220] . Thereby, it could be used in multiple patients without causing graft versus host reaction (GVHR). In addition, CRISPR-Cas mediated genome editing has also been used to enhance the function of CAR-T cells by knocking out genes encoding signaling molecules or T cell inhibitory receptors, such as programmed cell death protein 1 (PD-1) and cytotoxic T lymphocyte antigen 4 (CTLA-4) [221] , [222] .
5. Challenges and perspectives
Though CRISPR-Cas mediated efficient genome editing technologies have been broadly applied in a variety of species and different types of cells, there are still some important issues needed to be addressed during the process of application, such as off-target effects, delivery methods, immunogenicity and potential risk of cancer.
5.1. Off-target effects
It was found that designed sgRNAs will mismatch with non-target DNA sequences and introduce unexpected gene mutations, called off-target effects [223] . Off-target effects seriously restrict the widespread application of CRISPR-Cas mediated genome editing in gene therapy, for it might lead to genomic instability and increase the risk of certain diseases by introducing unwanted mutations at off-target sites [224] . At present, several strategies have been used to predict and detect off-target effects, online prediction software, whole genome sequencing (WGS), genome-wide, unbiased identification of DSBs enabled by sequencing (GUIDE-seq), discovery of in situ cas off-targets and verification by sequencing (DISCOVER-Seq), etc [225] . Furthermore, to minimize off-target effects, researchers have systematically studied the factors affecting off-target effects and developed a number of effective approaches.
The specific binding of sgRNA with the target sequence is the key factor in CRISPR-Cas mediated genome editing. Rational design of highly specific sgRNAs might minimize off-target effects [224] . The length and GC content of sgRNAs, and mismatches between sgRNA and its off-target site will all affect the frequency of off-target effects [226] . In addition, on the basis of rational design of sgRNAs, the specificity of CRISPR-Cas systems can be further improved by modifying sgRNAs, such as engineered hairpin sgRNAs and chemical modifications of sgRNAs [227] , [228] .
As we know, the interaction between Cas9 and DNA affects the stability of DNA-Cas9/sgRNA complex as well as tolerance to mismatch [229] . Therefore, high-fidelity SpCas9 variants have been developed by introducing amino substitution(s) into Cas9 protein in order to destabilize the function structure of the CRISPR complex [230] . Researchers have developed several highly effective Cas9 mutants, high-fidelity Cas9 (SpCas9-HF1), enhanced specificity Cas9 (eSpCas9), hyper-accurate Cas9 (HypaCas9), etc [231] , [232] , [233] . All of them can significantly reduce off-target effects while retain robust target cleavage activity.
Recently, a double-nicking strategy has been developed to minimize off-target effects, which employs two catalytic mutant Cas9-D10A nickases and a pair of sgRNAs to produce a cleavage on each strand of the target DNA, thus forming a functional double strand break [234] . Additionally, it was proven that the fusion protein generated by combining dCas9 with Fok Ⅰ nuclease can also reduce off-target effects [235] . Only when the two fusion protein monomers are close to each other to form dimers, can they perform the cleavage function [235] . This strategy could greatly reduce DNA cleavage at non-target sites.
“Off switches” for CRISPR-Cas9 system was first discovered by Pawluk et al. in 2016. They identified three naturally existing protein families, named as “anti-CRISPRs”, which can specifically inhibit the CRISPR-Cas9 system of Neisseria meningitidis [236] . Later, Rauch et al. discovered four unique type IIA CRISPR-Cas9 inhibitor proteins encoded by Listeria monocytogenes prophages, and two of them (AcrllA2 and AcrllA4) can block SpCas9 when assayed in Escherichia coli and human cells [237] . Recently, Doudna et al. discovered two broad-spectrum inhibitors of CRISPR-Cas9 system (AcrllC1 and AcrllC3) [238] . Therefore, in order to reduce off-target effects, the “anti-CRISPRs” could be used to prevent the continuous expression of Cas9 protein in cells to be edited.
The concentration of Cas9/sgRNA can also affect the frequency of off-target mutations [239] . Thus, the optimal concentration of Cas9 and sgRNA needs to be determined by pre-experiment. Besides, the formulation of CRISPR-Cas9 can affect the frequency of off-target mutations as well. Cas9 nucleases can be delivered into target cells in 3 different forms: DNA expression plasmid, mRNA or recombination protein [240] . Currently, the use of Cas9/sgRNA ribonucleoprotein complexes (Cas9-RNPs), which are composed of purified Cas9 proteins in combination with sgRNA, is becoming more and more widespread. It was found that delivery as plasmid usually produces more off-targets than delivery as RNPs, since the CRISPR-Cas system is active for a shorter time without Cas9 transcription and translation stages [241] , [242] .
5.2. Delivery methods
Nowadays, how to effectively deliver CRISPR-Cas components to specific cells, tissues and organs for precisely directed genome editing is still a major problem in gene therapy. Ideal delivery vectors should have the advantages of non-toxicity, well targeting property, high efficiency, low cost, and biodegradability [35] , [156] . At present, three main delivery methods have been employed in delivering CRISPR-Cas components, including physical, viral and non-viral methods [243] . Physical methods are the simplest way to deliver CRISPR-Cas components, including electroporation, microinjection and mechanical cell deformation. They are simple and efficient, which can also improve the expression of genes, and being widely applied in in vitro experiments [243] , [244] . In addition, viral vectors, such as adenovirus, adeno-associated virus (AAV) and lentivirus viral vectors, are being widely used for both in vitro / ex vivo and in vivo delivery due to their high delivery efficiency. They are commonly used for gene delivery in gene therapy, and some of them have been approved for clinical use [245] , [246] . However, safety issue of viral vectors is still a major problem needed to be solved in pre-clinical trials. Therefore, researchers have turned their attention to non-viral vectors, for instance, liposomes, polymers and nanoparticles [247] . Based on the advantages of safety, availability and cost-effectiveness, they are becoming a hotspot for the delivery of CRISPR-Cas components [248] .
Since all these delivery methods have both advantages and disadvantages, it’s necessary to design a complex of viral vectors and non-viral vectors, which combines the advantages of both vectors. Along with the deepening of research, various carriers could be modified by different methods to increase the delivery efficiency and reduce the toxicity [249] . In addition, more novel vectors, such as graphene and carbon nanomaterials (CNMs), could also be applied in the delivery of CRISPR-Cas components [250] , [251] .
5.3. Immunogenicity
Since the components of CRISPR-Cas systems are derived from bacteria, host immune response to Cas gene and Cas protein is regarded as one of the most important challenges in the clinical trials of CRISPR-Cas system [156] , [252] . It was found that in vivo delivery of CRISPR-Cas components can elicit immune responses against the Cas protein [252] , [253] . Furthermore, researchers also found that there were anti-Cas9 antibodies and anti-Cas9 T cells existing in healthy humans, suggesting the pre-existing of humoral and celluar immune responses to Cas9 protein in humans [254] . Therefore, how to detect and reduce the immunogenicity of Cas proteins is a major challenge will be faced in clinical application of CRISPR-Cas systems. Researchers are trying to handle this problem by modifying Cas9 protein or using Cas9 homologues [255] .
5.4. Potential risk of cancer
Recently, two independent research groups found that CRISPR-Cas mediated double-stranded breaks (DSBs) can activate the p53 signaling pathway [256] , [257] . This means that genetically edited cells are likely to become potential cancer initiating cells, and clinical treatment with CRISPR-Cas systems might inadvertently increase the risk of cancer [256] , [257] , [258] . Although there is still no direct evidence to confirm the relationship between CRISPR-Cas mediated genome editing and carcinogenesis, these studies once again give a warning on the application of CRISPR-Cas systems in gene therapy. It reminds us that there is still a long way to go before CRISPR-Cas systems could be successfully applied to humans.
5.5. Ethical issues
CRISPR-Cas mediated genome editing has attracted much attention since its advent in 2012. In theory, each gene can be edited by CRISPR-Cas systems, even genes in human germ cells [259] . However, germline gene editing is forbidden in many countries including China, for it could have unintended consequences and bring ethical and safety concerns [260] .
However, in March 2015, a Chinese scientist, Junjiu Huang, published a paper about gene editing in human tripronuclear zygotes in the journal Protein & Cell, which brings the ethical controversy of human embryo gene editing to a climax [261] . Since then, genome editing has been challenged by ethics and morality, and legal regulation of genome editing has triggered a heated discussion all around the world.
Then, on Nov. 28, 2018, the day before the opening of the second international human genome editing summit, Jiankui He, a Chinese scientist from the Southern University of Science and Technology, announced that a pair of gene-edited babies, named Lulu and Nana, were born healthy in China this month. They are the world’s first gene-edited babies, whose CCR5 gene has been modified, making them naturally resistant to HIV infection after birth [262] . The announcement has provoked shock, even outrage among scientists around the world, causing widespread controversy in the application of genome editing.
The society was shocked by this breaking news, for it involves genome editing in human embryos and propagating into future generations, triggering a chorus of criticism from the scientific community and bringing concerns about ethics and security in the use of genome editing. Therefore, scientists call on Chinese government to investigate the matter fully and establish strict regulations on human genome editing. Global supervisory system is also needed to ensure genome editing of human embryos moving ahead safely and ethically [263] .
5.6. Conclusions
Since CRISPR-Cas mediated genome editing technologies have provided an accessible and adaptable means to alter, regulate, and visualize genomes, they are thought to be a major milestone for molecular biology in the 21st century. So far, CRISPR-Cas systems have been broadly applied in gene function analysis, human gene therapy, targeted drug development, animal model construction and livestock breeding, which fully prove their great potential for further development. However, there are still some limitations to overcome in the practical applications of CRISPR-Cas systems, and great efforts still need to be made to evaluate their long-term safety and effectiveness.
CRediT authorship contribution statement
Yuanyuan Xu: Conceptualization, Writing - original draft. Zhanjun Li: Supervision, Validation, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was financially supported by the National Key Research and Development Program of China Stem Cell and Translational Research (2017YFA0105101). The Program for Changjiang Scholars and Innovative Research Team in University (No.IRT_16R32). The Strategic Priority Research Program of the Chinese Academy of Sciences (XDA16030501, XDA16030503), Key Research & Development Program of Guangzhou Regenerative Medicine and Health Guangdong Laboratory(2018GZR110104004).
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.


Exercise cuts heart disease risk in part by lowering stress, study finds

Women rarely die from heart problems, right? Ask Paula.

When will patients see personalized cancer vaccines?
How to realize immense promise of gene editing.

Nobel laureate Jennifer Doudna at Harvard Medical School.
Photos by Niles Singer/Harvard Staff Photographer
Alvin Powell
Harvard Staff Writer
Nobel-winning CRISPR pioneer says approval of revolutionary sickle-cell therapy shows need for more efficient, less expensive process
The world stands on the edge of an era when gene editing can address many serious ills plaguing humankind, according to a pioneer of the revolutionary gene editing technique known as CRISPR-Cas9. But first, she said, there is a problem to solve.
Jennifer Doudna , whose work on CRISPR earned her the 2020 Nobel Prize in chemistry , applauded the recent approval of a CRISPR-based gene-editing therapy to help those struggling with sickle-cell disease. The therapy, developed by Boston-based Vertex Pharmaceuticals and CRISPR Therapeutics, was approved by the FDA in 2023. Preapproval studies showed it was very effective at reducing the severe pain that accompanies the life-threatening blood disorder.
Doudna, who visited Harvard Medical School last week to deliver the century-old Dunham Lectures, said the advance shows how CRISPR-based therapies can address hard-to-treat ailments, but it also highlights the hurdles that still stand in the way of widespread use. The therapy, she said, uses a process similar to that of a bone-marrow transplant. Blood stem cells are extracted from a patient’s bone marrow, genetically engineered, and then reinfused into the marrow to produce blood cells that greatly reduce disease symptoms and dangerous complications.
That process, while groundbreaking, is physically challenging for patients, and expensive, with each treatment costing more than $1 million. Together, those factors explain why only 250 people have received the therapy so far, Doudna said, even though the condition afflicts 90,000 to 100,000 in the U.S. and millions worldwide.
“It’s exciting, but that’s quite a small number,” Doudna said.

Doudna delivered her talk, “Rewriting the Future of Health Care with Genome Editing,” on Thursday in a packed Joseph B. Martin Amphitheater on HMS’ Longwood Campus in Boston.
She said that if CRISPR is to match its promise to reduce human suffering, new delivery methods are essential. She described several efforts underway in her lab and those of colleagues to create nanoparticle delivery systems that could, if perfected, relatively simply and cheaply deliver the CRISPR-based gene editor to target cells in various tissues.
That would allow the gene-editing process to occur inside the patient’s body rather than in the lab, as occurs with the new sickle-cell treatment. That would avoid the expensive and arduous process of extracting cells from a patient’s body, engineering them to address a condition’s genetic causes, and then reinjecting them into the patient.
“How we can achieve in vivo genome editing, I increasingly think this is the bottleneck in this field,” Doudna said. “Broadly speaking, what we need to be addressing is how these editors are going to get into target cells in the body. It’s a really interesting, really big challenge, and there’s many people working on it.”
The discovery of CRISPR/Cas9 in 2012 stemmed from basic scientific research into how bacteria fight off viruses. Researchers realized that a portion of the bacterial immune system contains molecules that precisely snip DNA at specific locations, and developed that into the molecular scissors of CRISPR/Cas9 that allow the precise editing of human, plant, and animal DNA at specific locations.
The technique was immediately seen as a major advance and other scientists began using it in their own research.
“Her groundbreaking development of CRISPR/Cas9 genome editing technology, with collaborator Emmanuelle Charpentier, earned the two of them the Nobel Prize in chemistry in 2020 and forever changed the course of human, animal, and agricultural research,” said Stephen Blacklow , chair of the HMS Department of Biological Chemistry and Molecular Pharmacology , who introduced Doudna. He added that Doudna has an “unsurpassed capacity to engage and inspire the next generation.”
Doudna, who received her Ph.D. from HMS’ Biological Chemistry and Molecular Pharmacology Department in 1989 under Nobel laureate Jack Szostak, expressed confidence that the problem of delivering gene-editing therapy directly to patients’ cells is a solvable one. Her talk dealt with strategies to tackle the problem including lentiviruses, lipid nanoparticles, and something called EDV — enveloped delivery vehicles.
“It makes me think that ultimately … we can come up with a strategy for a particle that will be both easy to make, easy to program, and be effective at delivering in vivo,” Doudna said.
Share this article
You might like.
Benefits nearly double for people with depression

New book traces how medical establishment’s sexism, focus on men over centuries continues to endanger women’s health, lives

Sooner than you may think, says researcher who recently won Sjöberg Prize for pioneering work in field
Harvard announces return to required testing
Leading researchers cite strong evidence that testing expands opportunity
Finding right mix on campus speech policies
Legal, political scholars discuss balancing personal safety, constitutional rights, academic freedom amid roiling protests, cultural shifts
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
CRISPR-Cas9 genome editing articles from across Nature Portfolio
CRISPR-Cas9 genome editing exploits the CRISPR-Cas system to modify a genome in a targeted manner. Guided by RNA, the Cas9 endonuclease breaks DNA at a target sequence. Imprecise repair of the double strand break can result in insertion or deletion mutations, while repair pathways can be engineered to introduce specific point mutations or insertions.
Latest Research and Reviews
Splice modulators target PMS1 to reduce somatic expansion of the Huntington’s disease-associated CAG repeat
Somatic expansion of a CAG repeat in HTT drives onset of Huntington’s disease. Using a human cell line model and splice modulators, here the authors show that PMS1 is an enhancer of CAG repeat expansion, making it a target for therapeutic intervention.
- Zachariah L. McLean
- James F. Gusella
An in vitro CRISPR screen of cell-free DNA identifies apoptosis as the primary mediator of cell-free DNA release
A novel approach using whole-genome CRISPR-Cas9 screen indicates that apoptosis is the most important mediator of cfDNA release.
- Brad. A. Davidson
- Adam X. Miranda
- Ben Ho Park
Developmental progression of DNA double-strand break repair deciphered by a single-allele resolution mutation classifier
DNA double-strand breaks (DSBs) are repaired by a hierarchically regulated network of pathways. Here, authors develop ICP for deciphering somatic DSB repair patterns in multicellular organisms and discover developmental regulation in flies and mosquitoes, enabling tracking of mutant alleles and interhomolog copying of gene cassettes.
Identifying regulators of aberrant stem cell and differentiation activity in colorectal cancer using a dual endogenous reporter system
Aberrant stem cell-like activity and impaired differentiation are central to the development of colorectal cancer. Here, authors develop a dual endogenous reporter system to identify functional regulators of aberrant stem cell and differentiation programs, showing that SMARCB1 restricts differentiation, and nominating other regulators with therapeutic potential.
- Sandor Spisak
- Nilay S. Sethi
Enhancing prime editor activity by directed protein evolution in yeast
Compared to traditional Cas9 nucleases prime editors (PEs) are less active. Here the authors use OrthoRep, a yeast-based platform for directed protein evolution to enhance the editing efficiency of PEs: they identify mutations that have a positive effect on kinetics and use this knowledge to generate an efficient in vivo PE.
- Yanik Weber
- Desirée Böck
- Gerald Schwank
Cas9-assisted biological containment of a genetically engineered human commensal bacterium and genetic elements
Engineered biosensing bacteria can potentially probe the human gut microbiome to prevent, diagnose, or treat disease. Here the authors present a robust biocontainment assisted by Cas9 and an engineered gene expression control combined in a genetically engineered human commensal bacterium that successfully functioned in a mouse intestinal tract as well as cell culture condition.
- Naoki Hayashi
- Timothy K. Lu
News and Comment
Genome editing by self-deliverable ribonucleoproteins
An article in Nature Communications reports the development of self-deliverable ribonucleoproteins for genome editing in the brain.
- Nesma El-Sayed Ibrahim
MEGA-CRISPR tool gives a power boost to cancer-fighting cells
A system that edits RNA rather than DNA can give new life to exhausted CAR T cells.
- Sara Reardon
Move over, CRISPR: RNA-editing therapies pick up steam
Two RNA-editing therapies for genetic diseases have in the past few months gained approval for clinical trials, raising hopes for safer treatments.
- Mariana Lenharo
Turbocharged CAR-T cells melt tumours in mice — using a trick from cancer cells
Immune cells armed with a mutation first identified in cancer cells gain potency but don’t turn cancerous themselves.
- Asher Mullard

CRISPR-edited crops break new ground in Africa
Scientists in the global south use the popular technique to protect local crops against local threats.
- Heidi Ledford
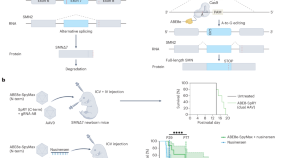
Mutation corrections in spinal muscular atrophy
Base editors can restore the expression of survival motor neuron protein to therapeutically beneficial levels in animal and cell models of spinal muscular atrophy.
- Andrew Portell
- Prashant Mali
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
A journey back in time helps solve a modern gene editing conundrum
April 18, 2024
By Christopher Vaughan

Hiro Nakauchi, MD, PhD
The gene editing technology known as CRISPR-Cas9 has given researchers the opportunity to precisely edit genes, an ability that can potentially lead to prevention or cures for genetic diseases that have long been incurable. However, researchers at the Institute for Stem Cell Biology and Regenerative Medicine found—somewhat by accident—that the gene editing technology is often not as precise as scientists think, and that some of the errors that it produces are difficult to spot using current technologies. Straightening things out required institute scientists to use older genetic technology common a generation ago.
The problem was first noticed by Fabian Suchy, PhD, a postdoctoral fellow in the laboratory of Professor Hiro Nakauchi, MD, PhD. After using the CRISPR system with engineered viral particles to carefully insert new genes into cells, he noticed that the cells’ appearance and behavior (the phenotype) was inconsistent with indications of whether the right genes had been successfully inserted (the genotype). Cells that showed fluorescent labels indicating that they had successfully taken up the inserted gene were not acting like it.
“We weren’t sure what was going on,” Suchy said. “At first we thought that the genes were sometimes being randomly inserted (and were therefore not active), but that turned out not to be the case.”
Sequencing some gene-edited cells’ DNA showed that the new genes were indeed inserted where they should be. Suchy did some digging into the historical record and found something interesting. Normally, the new genes the researchers intend to edit into a cell’s genome are nested in the middle of leftover viral DNA sequences that are intended to drop away as the gene is inserted into its target position. But through a biochemical quirk, the bits of viral DNA that were used to carry the new gene into the genome could link up with each other to form long chains. If the bits of viral DNA link up with each other, it could create an insertion that includes multiple copies of the new gene interspersed with bits of viral DNA between each one.
“The linkage of this viral DNA was shown in the 1970s, but these genetic chains have not been shown to frequently insert by researchers doing gene editing now,” Suchy said.
Proving that this linkage was happening in the lab—and that the scientists were inserting multiple genes and viral material instead of single genes—was not simple, however. “It was very hard to spot the insertion of multiple genes using common, modern methods,” Suchy said.
To find out whether this was happening, Suchy enlisted the help of senior research scientist Katja Pekrun, PhD. Pekrun is familiar with Southern blots, a technique invented in the 1970s that was well known to any genetic researcher practicing 40 years ago, but which is now largely unused. Southern blots break up DNA into pieces and then use an electric current to pull those pieces through a gel. Large pieces of DNA move more slowly than small pieces, so if multiple copies of the gene and viral material are inserted, that section of DNA will not advance as far in the gel over time.
“The Southern blots were tedious to do, and required a lot of DNA,” Suchy said. “But once we finished, it was very obvious that multiple genes were being inserted.” They eventually developed easier detection methods and found that around 50 percent of the edited cells had these hidden, repeat insertions.
Once this viral DNA linkage and insertion was identified in one system, Suchy teamed up with postdoctoral fellow Daiki Karigane MD, PhD in the lab of institute director Ravi Majeti MD, PhD to investigate this phenomenon in many cell types and genes. Indeed, the team found these linked insertions could occur in multiple genes and cell types, including pluripotent stem cells and blood stem cells, indicating this to be a broad problem in gene editing, the researchers said.
Luckily, the team also went on to find ways to avoid the viral DNA linking that lead to multiple gene insertions. A key element of editing with CRISPR is the use of a guide RNA that directs a protein called Cas9 to cut the cell’s DNA at a precise location. The team found that they could use a different guide RNA to cut out the viral DNA before the new gene was inserted. This reduced the incidence of multiple insertions by more than ten-fold, they said.
“These findings might help researchers around the world who are making gene edits understand why they may not be getting the results they expect, and give them tools to correct the problem,” Nakauchi said.
The research was published in the Journal Nature Biotechnology. Suchy and Karigane were co-first authors on the paper. Nakauchi and Majeti were co-senior authors on the paper.
Learn about the many ways to support the institute for Stem Cell Biology and Regenerative Medicine
CRISPR-Cas System: A New Dawn to Combat Antibiotic Resistance
- Review Article
- Published: 11 April 2024
Cite this article
- Muhammad Shahzad Rafiq 1 ,
- Muhammad AbuBakar Shabbir 2 ,
- Ahmed Raza 3 ,
- Shoaib Irshad 3 ,
- Andleeb Asghar 4 ,
- Muhammad Kashif Maan 5 ,
- Mushtaq Ahmed Gondal 6 &
- Haihong Hao 1
49 Accesses
2 Altmetric
Explore all metrics
Antimicrobial resistance (AMR) can potentially harm global public health. Horizontal gene transfer (HGT), which speeds up the emergence of AMR and increases the burden of drug resistance in mobile genetic elements (MGEs), is the primary method by which AMR genes are transferred across bacterial pathogens. New approaches are urgently needed to halt the spread of bacterial diseases and antibiotic resistance. Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR), an RNA-guided adaptive immune system, protects prokaryotes from foreign DNA like plasmids and phages. This approach may be essential in limiting horizontal gene transfer and halting the spread of antibiotic resistance. The CRISPR-Cas system has been crucial in identifying and understanding resistance mechanisms and developing novel therapeutic approaches. This review article investigates the CRISPR-Cas system’s potential as a tool to combat bacterial AMR. Antibiotic-resistant bacteria can be targeted and eliminated by the CRISPR-Cas system. It has been proven to be an efficient method for removing carbapenem-resistant plasmids and regaining antibiotic susceptibility. The CRISPR-Cas system has enormous potential as a weapon against bacterial AMR. It precisely targets and eliminates antibiotic-resistant bacteria, facilitates resistance mechanism identification, and offers new possibilities in diagnostics and therapeutics.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
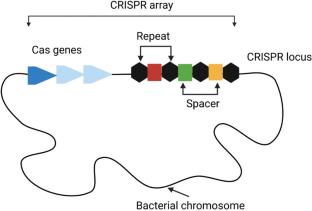
Similar content being viewed by others

Precise Sequence-Specific Antimicrobials Based on CRISPR: Toward Prevailing Over Bacterial Antibiotic Resistance

CRISPR and CAS Editing Tools Employent in the Control of AMR Pathogens

Role of Gene Editing Tool CRISPR-Cas in the Management of Antimicrobial Resistance
de Kraker MEA, Stewardson AJ, Harbarth S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLOS Med. 2016;13: e1002184.
Article PubMed PubMed Central Google Scholar
Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet Lond Engl. 2022;399:629–55.
Article Google Scholar
Murray, C. J. L. et al.Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet Lond Engl. 2022;399:629–55.
Chung M, Yeh I, Sung L, Wu M, Chao Y, Ng I, et al. Enhanced integration of large DNA into E. coli chromosome by CRISPR/Cas9. Biotechnol Bioeng. 2017;114:172–83.
Article CAS PubMed Google Scholar
Tao S, Chen H, Li N, Liang W. The application of the CRISPR-Cas system in antibiotic resistance. Infect Drug Resist. 2022;15:4155–68.
Shetty VP, Akshay SD, Rai P, Deekshit VK. Integrons as the potential targets for combating multidrug resistance in Enterobacteriaceae using CRISPR- Cas9 technique. J Appl Microbiol. 2023;134: lxad137.
Article PubMed Google Scholar
Wu Z-Y, Huang Y-T, Chao W-C, Ho S-P, Cheng J-F, Liu P-Y. Reversal of carbapenem-resistance in Shewanella algae by CRISPR/Cas9 genome editing. J Adv Res. 2019;18:61–9.
Article CAS PubMed PubMed Central Google Scholar
Lin DM, Koskella B, Lin HC. Phage therapy: an alternative to antibiotics in the age of multi-drug resistance. World J Gastrointest Pharmacol Ther. 2017;8:162–73.
Janik E, Niemcewicz M, Ceremuga M, Krzowski L, Saluk-Bijak J, Bijak M. Various aspects of a gene editing system—crispr–cas9. Int J Mol Sci. 2020;21:9604.
He Y-Z, Kuang X, Long T-F, Li G, Ren H, He B, et al. Re-engineering a mobile-CRISPR/Cas9 system for antimicrobial resistance gene curing and immunization in Escherichia coli . J Antimicrob Chemother. 2022;77:74–82.
Article CAS Google Scholar
Lone BA, Karna SKL, Ahmad F, Shahi N, Pokharel YR. CRISPR/Cas9 system: a bacterial tailor for genomic engineering. Genet Res Int. 2018. https://doi.org/10.1155/2018/3797214 .
Kim J-S, Cho D-H, Park M, Chung W-J, Shin D, Ko KS, et al. CRISPR/Cas9-mediated re-sensitization of antibiotic-resistant Escherichia coli harboring extended-spectrum β-lactamases. J Microbiol Biotechnol. 2016;26:394–401.
Mojica FJ, Díez-Villaseñor C, Soria E, Juez G. Biological significance of a family of regularly spaced repeats in the genomes of Archaea, Bacteria and mitochondria. Mol Microbiol. 2000;36:244–6.
Jansen R, van Embden JD, Gaastra W, Schouls LM. Identification of genes that are associated with DNA repeats in prokaryotes. Mol Microbiol. 2002;43:1565–75.
Hille F, Charpentier E. CRISPR-Cas: biology, mechanisms and relevance. Philos Trans R Soc B Biol Sci. 2016;371:20150496.
Nuñez JK, Kranzusch PJ, Noeske J, Wright AV, Davies CW, Doudna JA. Cas1–Cas2 complex formation mediates spacer acquisition during CRISPR–Cas adaptive immunity. Nat Struct Mol Biol. 2014;21:528–34.
Le Rhun A, Escalera-Maurer A, Bratovič M, Charpentier E. CRISPR-Cas in Streptococcus pyogenes . RNA Biol. 2019;16:380–9.
Teng M, Yao Y, Nair V, Luo J. Latest advances of virology research using CRISPR/Cas9-based gene-editing technology and its application to vaccine development. Viruses. 2021;13:779.
Koonin EV, Makarova KS, Zhang F. Diversity, classification and evolution of CRISPR-Cas systems. Curr Opin Microbiol. 2017;37:67–78.
Abudayyeh OO, Gootenberg JS, Konermann S, Joung J, Slaymaker IM, Cox DB, et al. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science. 2016;353: aaf5573.
Alduhaidhawi AHM, AlHuchaimi SN, Al-Mayah TA, Al-Ouqaili MT, Alkafaas SS, Muthupandian S, et al. Prevalence of CRISPR-cas systems and their possible association with antibiotic resistance in Enterococcus faecalis and Enterococcus faecium collected from hospital wastewater. Infect Drug Resist. 2022;15:1143–54.
Hullahalli K, Rodrigues M, Schmidt BD, Li X, Bhardwaj P, Palmer KL. Comparative analysis of the orphan CRISPR2 locus in 242 Enterococcus faecalis strains. PLoS One. 2015;10: e0138890.
Hullahalli K, Rodrigues M, Nguyen UT, Palmer K. An attenuated CRISPR-Cas system in Enterococcus faecalis permits DNA acquisition. MBio. 2018. https://doi.org/10.1128/mbio.00414-18 .
Burley KM, Sedgley CM. CRISPR-Cas, a prokaryotic adaptive immune system, in endodontic, oral, and multidrug-resistant hospital-acquired Enterococcus faecalis . J Endod. 2012;38:1511–5.
Hullahalli K, Rodrigues M, Palmer KL. Exploiting CRISPR-Cas to manipulate Enterococcus faecalis populations. Elife. 2017;6: e26664.
Gholizadeh P, Aghazadeh M, Ghotaslou R, Rezaee MA, Pirzadeh T, Cui L, et al. Role of CRISPR-Cas system on antibiotic resistance patterns of Enterococcus faecalis . Ann Clin Microbiol Antimicrob. 2021;20:1–12.
Zhou Y, Yang Y, Li X, Tian D, Ai W, Wang W, et al. Exploiting a conjugative endogenous CRISPR-Cas3 system to tackle multidrug-resistant Klebsiella pneumoniae . EBioMedicine. 2023;88: 104445.
Sinkunas T, Gasiunas G, Fremaux C, Barrangou R, Horvath P, Siksnys V. Cas3 is a single-stranded DNA nuclease and ATP-dependent helicase in the CRISPR/Cas immune system. EMBO J. 2011;30:1335–42.
Mousseau G, Kessing CF, Fromentin R, Trautmann L, Chomont N, Valente ST. The Tat inhibitor didehydro-cortistatin A prevents HIV-1 reactivation from latency. MBio. 2015. https://doi.org/10.1128/mbio.00465-15 .
Selle K, Fletcher JR, Tuson H, Schmitt DS, McMillan L, Vridhambal GS, et al. In Vivo Targeting of Clostridioides difficile Using Phage-Delivered CRISPR-Cas3 Antimicrobials. MBio. 2020;11: e00019-20.
Yosef I, Manor M, Kiro R, Qimron U. Temperate and lytic bacteriophages programmed to sensitize and kill antibiotic-resistant bacteria. Proc Natl Acad Sci. 2015;112:7267–72.
Wu X, Kriz AJ, Sharp PA. Target specificity of the CRISPR-Cas9 system. Quant Biol. 2014;2:59–70.
Hao M, He Y, Zhang H, Liao X-P, Liu Y-H, Sun J, et al. CRISPR-Cas9-mediated carbapenemase gene and plasmid curing in carbapenem-resistant enterobacteriaceae. Antimicrob Agents Chemother. 2020;64:e00843-e920.
Kyrou K, Hammond AM, Galizi R, Kranjc N, Burt A, Beaghton AK, et al. A CRISPR–Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nat Biotechnol. 2018;36:1062–6.
Shahriar SA, Islam MN, Chun CNW, Rahim MA, Paul NC, Uddain J, et al. Control of plant viral diseases by CRISPR/Cas9: resistance mechanisms, strategies and challenges in food crops. Plants. 2021;10:1264.
Hazafa A, Mumtaz M, Farooq MF, Bilal S, Chaudhry SN, Firdous M, et al. CRISPR/Cas9: a powerful genome editing technique for the treatment of cancer cells with present challenges and future directions. Life Sci. 2020;263: 118525.
Yao R, Liu D, Jia X, Zheng Y, Liu W, Xiao Y. CRISPR-Cas9/Cas12a biotechnology and application in bacteria. Synth Syst Biotechnol. 2018;3:135–49.
Hamilton TA, Pellegrino GM, Therrien JA, Ham DT, Bartlett PC, Karas BJ, et al. Efficient inter-species conjugative transfer of a CRISPR nuclease for targeted bacterial killing. Nat Commun. 2019;10:4544.
Pouillot F, Chomton M, Blois H, Courroux C, Noelig J, Bidet P, et al. Efficacy of bacteriophage therapy in experimental sepsis and meningitis caused by a clone O25b:H4-ST131 Escherichia coli strain producing CTX-M-15. Antimicrob Agents Chemother. 2012;56:3568–75.
Aslam B, Rasool M, Idris A, Muzammil S, Alvi RF, Khurshid M, et al. CRISPR-Cas system: a potential alternative tool to cope antibiotic resistance. Antimicrob Resist Infect Control. 2020;9:131.
Meeske AJ, Nakandakari-Higa S, Marraffini LA. Cas13-induced cellular dormancy prevents the rise of CRISPR-resistant bacteriophage. Nature. 2019;570:241–5.
Watanabe S, Cui B, Kiga K, Aiba Y, Tan X-E, Sato’o Y, et al. Composition and diversity of CRISPR-Cas13a systems in the genus leptotrichia. Front Microbiol. 2019. https://doi.org/10.3389/fmicb.2019.02838 .
Kiga K, Tan X-E, Ibarra-Chávez R, Watanabe S, Aiba Y, Sato’o Y, et al. Development of CRISPR-Cas13a-based antimicrobials capable of sequence-specific killing of target bacteria. Nat Commun. 2020;11:2934.
Wan F, Draz MS, Gu M, Yu W, Ruan Z, Luo Q. Novel strategy to combat antibiotic resistance: a sight into the combination of CRISPR/Cas9 and nanoparticles. Pharmaceutics. 2021;13:352.
Pyne ME, Moo-Young M, Chung DA, Chou CP. Coupling the CRISPR/Cas9 system with lambda red recombineering enables simplified chromosomal gene replacement in Escherichia coli . Appl Environ Microbiol. 2015;81:5103–14.
Asmamaw Mengstie M. Viral Vectors for the in vivo delivery of CRISPR components: advances and challenges. Front Bioeng Biotechnol. 2022. https://doi.org/10.3389/fbioe.2022.895713 .
Yang W, Yan J, Zhuang P, Ding T, Chen Y, Zhang Y, et al. Progress of delivery methods for CRISPR-Cas9. Expert Opin Drug Deliv. 2022;19:913–26.
Yip BH. Recent advances in CRISPR/Cas9 delivery strategies. Biomolecules. 2020;10:839.
Wang Y, Qi T, Liu J, Yang Y, Wang Z, Wang Y, et al. A highly specific CRISPR-Cas12j nuclease enables allele-specific genome editing. Sci Adv. 2023;9: eabo6405.
Pantoja Angles A, Ali Z, Mahfouz M. CS-cells: a CRISPR-Cas12 DNA device to generate chromosome-shredded cells for efficient and safe molecular biomanufacturing. ACS Synth Biol. 2022;11:430–40.
Khambhati K, Bhattacharjee G, Gohil N, Dhanoa GK, Sagona AP, Mani I, et al. Phage engineering and phage-assisted CRISPR-Cas delivery to combat multidrug-resistant pathogens. Bioeng Transl Med. 2022;8: e10381.
Rodrigues M, McBride SW, Hullahalli K, Palmer KL, Duerkop BA. Conjugative delivery of CRISPR-Cas9 for the selective depletion of antibiotic-resistant enterococci. Antimicrob Agents Chemother. 2019;63:e01454-e1519.
Nguyen GT, Dhingra Y, Sashital DG. Miniature CRISPR-Cas12 endonucleases–programmed DNA targeting in a smaller package. Curr Opin Struct Biol. 2022;77: 102466.
Yeh T-K, Jean S-S, Lee Y-L, Lu M-C, Ko W-C, Lin H-J, et al. Bacteriophages and phage-delivered CRISPR-Cas system as antibacterial therapy. Int J Antimicrob Agents. 2022;59: 106475.
Lam KN, Spanogiannopoulos P, Soto-Perez P, Alexander M, Nalley MJ, Bisanz JE, et al. Phage-delivered CRISPR-Cas9 for strain-specific depletion and genomic deletions in the gut microbiome. Cell Rep. 2021;37: 109930.
Fage C, Lemire N, Moineau S. Delivery of CRISPR-Cas systems using phage-based vectors. Curr Opin Biotechnol. 2021;68:174–80.
Deng H, Huang W, Zhang Z. Nanotechnology based CRISPR/Cas9 system delivery for genome editing: progress and prospect. Nano Res. 2019;12:2437–50.
Rodríguez-Rodríguez DR, Ramírez-Solís R, Garza-Elizondo MA, Garza-Rodríguez MDL, Barrera-Saldaña HA. Genome editing: a perspective on the application of CRISPR/Cas9 to study human diseases (Review). Int J Mol Med. 2019;43:1559–74.
PubMed PubMed Central Google Scholar
Givens BE, Naguib YW, Geary SM, Devor EJ, Salem AK. Nanoparticle-based delivery of CRISPR/Cas9 genome-editing therapeutics. AAPS J. 2018;20:1–22.
Huang J, Zhou Y, Li J, Lu A, Liang C. CRISPR/Cas systems: Delivery and application in gene therapy. Front Bioeng Biotechnol. 2022;10: 942325.
Huang K, Zapata D, Tang Y, Teng Y, Li Y. In vivo delivery of CRISPR-Cas9 genome editing components for therapeutic applications. Biomaterials. 2022;291: 121876.
Qin W, Wang H. Delivery of CRISPR-Cas9 into mouse zygotes by electroporation. Methods Mol Biol Clifton NJ. 2019;1874:179–90.
Wu Y, Battalapalli D, Hakeem MJ, Selamneni V, Zhang P, Draz MS, et al. Engineered CRISPR-Cas systems for the detection and control of antibiotic-resistant infections. J Nanobiotechnology. 2021;19:401.
Dhasmana N, Ram G, McAllister KN, Chupalova Y, Lopez P, Ross HF, et al. Dynamics of antibacterial drone establishment in Staphylococcus aureus : unexpected effects of antibiotic resistance genes. MBio. 2021;12:e02083-e2121.
Abavisani M, Khayami R, Hoseinzadeh M, Kodori M, Kesharwani P, Sahebkar A. CRISPR-Cas system as a promising player against bacterial infection and antibiotic resistance. Drug Resist Updat. 2023;68: 100948.
Huemer M, Mairpady Shambat S, Brugger SD, Zinkernagel AS. Antibiotic resistance and persistence—implications for human health and treatment perspectives. EMBO Rep. 2020;21: e51034.
Senthilnathan R, Ilangovan I, Kunale M, Easwaran N, Ramamoorthy S, Veeramuthu A, et al. An update on CRISPR-Cas12 as a versatile tool in genome editing. Mol Biol Rep. 2023;50:2865–81.
Agarwal C. A review: CRISPR/Cas12-mediated genome editing in fungal cells: advancements, mechanisms, and future directions in plant-fungal pathology. Sci Prepr. 2023. https://doi.org/10.14293/S2199-1006.1.SOR.2023.0001.v1
Song X, Liu C, Wang N, Huang H, He S, Gong C, et al. Delivery of CRISPR/Cas systems for cancer gene therapy and immunotherapy. Adv Drug Deliv Rev. 2021;168:158–80.
Taati Moghadam M, Amirmozafari N, Shariati A, Hallajzadeh M, Mirkalantari S, Khoshbayan A, et al. How phages overcome the challenges of drug resistant bacteria in clinical infections. Infect Drug Resist. 2020;13:45–61.
Wang Z, Cui W. CRISPR-Cas system for biomedical diagnostic platforms. View. 2020;1:20200008.
Tang Y, Gao L, Feng W, Guo C, Yang Q, Li F, et al. The CRISPR–Cas toolbox for analytical and diagnostic assay development. Chem Soc Rev. 2021;50:11844–69.
Padmanaban V, Ranganathan UDK. CRISPR–Cas system and its use in the diagnosis of infectious diseases. Microbiol Res. 2022;263: 127100.
Quan J, Langelier C, Kuchta A, Batson J, Teyssier N, Lyden A, et al. FLASH: a next-generation CRISPR diagnostic for multiplexed detection of antimicrobial resistance sequences. Nucleic Acids Res. 2019;47:e83–e83.
Haeussler M, Schönig K, Eckert H, Eschstruth A, Mianné J, Renaud J-B, et al. Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR. Genome Biol. 2016;17:1–12.
Yang H, Zhang Y, Teng X, Hou H, Deng R, Li J. CRISPR-based nucleic acid diagnostics for pathogens. Trends Anal Chem. 2023;160: 116980.
Wang C, Liu M, Wang Z, Li S, Deng Y, He N. Point-of-care diagnostics for infectious diseases: from methods to devices. Nano Today. 2021;37: 101092.
Kellner MJ, Koob JG, Gootenberg JS, Abudayyeh OO, Zhang F. SHERLOCK: nucleic acid detection with CRISPR nucleases. Nat Protoc. 2019;14:2986–3012.
Arizti-Sanz J, Freije CA, Stanton AC, Petros BA, Boehm CK, Siddiqui S, et al. Streamlined inactivation, amplification, and Cas13-based detection of SARS-CoV-2. Nat Commun. 2020;11:5921.
Bonini A, Poma N, Vivaldi F, Biagini D, Bottai D, Tavanti A, et al. A label-free impedance biosensing assay based on CRISPR/Cas12a collateral activity for bacterial DNA detection. J Pharm Biomed Anal. 2021;204: 114268.
Wang F, Wang L, Chen H, Li N, Wang Y, Li Y, et al. Rapid detection of blaKPC, blaNDM, blaOXA-48-like and blaIMP carbapenemases in enterobacterales using recombinase polymerase amplification combined with lateral flow strip. Front Cell Infect Microbiol. 2021. https://doi.org/10.3389/fcimb.2021.772966 .
Nguyen LT, Macaluso NC, Pizzano BLM, Cash MN, Spacek J, Karasek J, et al. A thermostable Cas12b from Brevibacillus leverages one-pot detection of SARS-CoV-2 variants of concern. MedRxiv Prepr Serv Health Sci. 2021;2021.10.15.21265066.
Ge X, Meng T, Tan X, Wei Y, Tao Z, Yang Z, et al. Cas14a1-mediated nucleic acid detectifon platform for pathogens. Biosens Bioelectron. 2021;189: 113350.
Wei Y, Tao Z, Wan L, Zong C, Wu J, Tan X, et al. Aptamer-based Cas14a1 biosensor for amplification-free live pathogenic detection. Biosens Bioelectron. 2022;211: 114282.
Abavisani M, Khayami R, Hoseinzadeh M, Kodori M, Kesharwani P, Sahebkar A. CRISPR-Cas system as a promising player against bacterial infection and antibiotic resistance. Drug Resist Updat Rev Comment Antimicrob Anticancer Chemother. 2023;68: 100948.
CAS Google Scholar
Huang M, Zhou X, Wang H, Xing D. Clustered regularly interspaced short palindromic repeats/Cas9 triggered isothermal amplification for site-specific nucleic acid detection. Anal Chem. 2018;90:2193–200.
Xiao G, Zhang S, Liang Z, Li G, Fang M, Liu Y, et al. Identification of Mycobacterium abscessus species and subspecies using the Cas12a/sgRNA-based nucleic acid detection platform. Eur J Clin Microbiol Infect Dis. 2020;39:551–8.
Duan C, Cao H, Zhang L-H, Xu Z. Harnessing the CRISPR-Cas systems to combat antimicrobial resistance. Front Microbiol. 2021. https://doi.org/10.3389/fmicb.2021.716064 .
Pouillot F, Chomton M, Blois H, Courroux C, Noelig J, Bidet P, et al. Efficacy of bacteriophage therapy in experimental sepsis and meningitis caused by a clone O25b: H4-ST131 Escherichia coli strain producing CTX-M-15. Antimicrob Agents Chemother. 2012;56:3568–75.
Sun Q, Wang Y, Dong N, Shen L, Zhou H, Hu Y, et al. Application of CRISPR/Cas9-based genome editing in studying the mechanism of pandrug resistance in Klebsiella pneumoniae . Antimicrob Agents Chemother. 2019;63:e00113-e119.
Wu X, Zha J, Koffas MA, Dordick JS. Reducing Staphylococcus aureus resistance to lysostaphin using CRISPR-dCas9. Biotechnol Bioeng. 2019;116:3149–59.
Ram G, Ross HF, Novick RP, Rodriguez-Pagan I, Jiang D. Conversion of staphylococcal pathogenicity islands to CRISPR-carrying antibacterial agents that cure infections in mice. Nat Biotechnol. 2018;36:971–6.
Park JY, Moon BY, Park JW, Thornton JA, Park YH, Seo KS. Genetic engineering of a temperate phage-based delivery system for CRISPR/Cas9 antimicrobials against Staphylococcus aureus . Sci Rep. 2017;7:44929.
Kang YK, Kwon K, Ryu JS, Lee HN, Park C, Chung HJ. Nonviral genome editing based on a polymer-derivatized CRISPR nanocomplex for targeting bacterial pathogens and antibiotic resistance. Bioconjug Chem. 2017;28:957–67.
Bikard D, Euler CW, Jiang W, Nussenzweig PM, Goldberg GW, Duportet X, et al. Exploiting CRISPR-Cas nucleases to produce sequence-specific antimicrobials. Nat Biotechnol. 2014;32:1146–50.
Walflor HSM, Lucena ARC, Tuon FF, Medeiros LCS, Faoro H. Resensitization of fosfomycin-resistant Escherichia coli using the CRISPR system. Int J Mol Sci. 2022;23:9175.
Wan P, Cui S, Ma Z, Chen L, Li X, Zhao R, et al. Reversal of mcr-1-mediated colistin resistance in Escherichia coli by CRISPR-Cas9 system. Infect Drug Resist. 2020;13:1171–8.
Reuter A, Hilpert C, Dedieu-Berne A, Lematre S, Gueguen E, Launay G, et al. Targeted-antibacterial-plasmids (TAPs) combining conjugation and CRISPR/Cas systems achieve strain-specific antibacterial activity. Nucleic Acids Res. 2021;49:3584–98.
Tagliaferri TL, Guimarães NR, Pereira MPM, Vilela LFF, Horz H-P, Dos Santos SG, et al. Exploring the potential of CRISPR-Cas9 under challenging conditions: facing high-copy plasmids and counteracting beta-lactam resistance in clinical strains of Enterobacteriaceae. Front Microbiol. 2020;11:578.
Li P, Wan P, Zhao R, Chen J, Li X, Li J, et al. Targeted elimination of blaNDM-5 gene in Escherichia coli by conjugative CRISPR-Cas9 system. Infect Drug Resist. 2022;15:1707–16.
Citorik RJ, Mimee M, Lu TK. Sequence-specific antimicrobials using efficiently delivered RNA-guided nucleases. Nat Biotechnol. 2014;32:1141–5.
Liu H, Li H, Liang Y, Du X, Yang C, Yang L, et al. Phage-delivered sensitisation with subsequent antibiotic treatment reveals sustained effect against antimicrobial resistant bacteria. Theranostics. 2020;10:6310–21.
Wang T, Liu Y, Sun H-H, Yin B-C, Ye B-C. An RNA-guided Cas9 nickase-based method for universal isothermal DNA amplification. Angew Chem. 2019;131:5436–40.
Guk K, Keem JO, Hwang SG, Kim H, Kang T, Lim E-K, et al. A facile, rapid and sensitive detection of MRSA using a CRISPR-mediated DNA FISH method, antibody-like dCas9/sgRNA complex. Biosens Bioelectron. 2017;95:67–71.
Wang X, Xiong E, Tian T, Cheng M, Lin W, Wang H, et al. Clustered regularly interspaced short palindromic repeats/Cas9-mediated lateral flow nucleic acid assay. ACS Nano. 2020;14:2497–508.
You Y, Zhang P, Wu G, Tan Y, Zhao Y, Cao S, et al. Highly specific and sensitive detection of Yersinia pestis by portable Cas12a-UPTLFA platform. Front Microbiol. 2021;12: 700016.
Ai J-W, Zhou X, Xu T, Yang M, Chen Y, He G-Q, et al. CRISPR-based rapid and ultra-sensitive diagnostic test for Mycobacterium tuberculosis . Emerg Microbes Infect. 2019;8:1361–9.
Wang Y, Liang X, Xu J, Nan L, Liu F, Duan G, et al. Rapid and ultrasensitive detection of methicillin-resistant Staphylococcus aureus based on CRISPR-Cas12a combined with recombinase-aided amplification. Front Microbiol. 2022;13: 903298.
Liu X, Qiu X, Xu S, Che Y, Han L, Kang Y, et al. A CRISPR-Cas12a-assisted fluorescence platform for rapid and accurate detection of Nocardia cyriacigeorgica . Front Cell Infect Microbiol. 2022;12: 835213.
Wang Y, Ke Y, Liu W, Sun Y, Ding X. A one-pot toolbox based on Cas12a/crRNA enables rapid foodborne pathogen detection at attomolar level. Acs Sens. 2020;5:1427–35.
Lu P, Chen J, Li Z, Li Z, Zhang J, Kan B, et al. Visual identification and serotyping of toxigenic Vibrio cholerae serogroups O1 and O139 with CARID. Front Cell Infect Microbiol. 2022;12: 863435.
Xiao X, Lin Z, Huang X, Lu J, Zhou Y, Zheng L, et al. Rapid and sensitive detection of Vibrio vulnificus using CRISPR/Cas12a combined with a recombinase-aided amplification assay. Front Microbiol. 2021;12: 767315.
Qiu E, Jin S, Xiao Z, Chen Q, Wang Q, Liu H, et al. CRISPR-based Detection of Helicobacter pylori in Stool Samples. Helicobacter. 2021;26: e12828.
Li C, Chen X, Wen R, Ma P, Gu K, Li C, et al. Immunocapture magnetic beads enhanced the LAMP-CRISPR/Cas12a method for the sensitive, specific, and visual detection of Campylobacter jejuni . Biosensors. 2022;12:154.
Wu H, Cao X, Meng Y, Richards D, Wu J, Ye Z, et al. DropCRISPR: a LAMP-Cas12a based digital method for ultrasensitive detection of nucleic acid. Biosens Bioelectron. 2022;211: 114377.
Schultzhaus Z, Wang Z, Stenger D. Systematic analysis, identification, and use of CRISPR/Cas13a–associated crRNAs for sensitive and specific detection of the lcrV gene of Yersinia pestis . Diagn Microbiol Infect Dis. 2021;99: 115275.
Gootenberg JS, Abudayyeh OO, Lee JW, Essletzbichler P, Dy AJ, Joung J, et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science. 2017;356:438–42.
Zhou J, Yin L, Dong Y, Peng L, Liu G, Man S, et al. CRISPR-Cas13a based bacterial detection platform: sensing pathogen Staphylococcus aureus in food samples. Anal Chim Acta. 2020;1127:225–33.
Zhan Y, Gao X, Li S, Si Y, Li Y, Han X, et al. Development and evaluation of rapid and accurate CRISPR/Cas13-based RNA diagnostics for Pneumocystis jirovecii pneumonia. Front Cell Infect Microbiol. 2022. https://doi.org/10.3389/fcimb.2022.904485 .
Gao S, Liu J, Li Z, Ma Y, Wang J. Sensitive detection of foodborne pathogens based on CRISPR-Cas13a. J Food Sci. 2021;86:2615–25.
Song F, Wei Y, Wang P, Ge X, Li C, Wang A, et al. Combining tag-specific primer extension and magneto-DNA system for Cas14a-based universal bacterial diagnostic platform. Biosens Bioelectron. 2021;185: 113262.
WAI CC. Genome-wide CRISPR Screen for Host Factors Associated With Norovirus Infections in Stem Cell-derived Human Intestinal Enteroid Model [Internet]. clinicaltrials.gov; 2018 Sep. Report No.: NCT03342547. Available from: https://clinicaltrials.gov/study/NCT03342547
Chinese Medical Association. Species-specific Bacterial Detector for Fast Pathogen Diagnosis of Severe Pneumonia Patients in Intensive Care Uint: a Multicentre, Randomised Controlled Trial [Internet]. clinicaltrials.gov; 2022 May. Report No.: NCT05143593. Available from: https://clinicaltrials.gov/study/NCT05143593
Zhang W. Evaluation of CRISPR-based Test for the Rapid Identification of Mycobacterium Tuberculosis Complex in Pulmonary Tuberculosis Suspects [Internet]. clinicaltrials.gov; 2019 Aug. Report No.: NCT04074369. Available from: https://clinicaltrials.gov/study/NCT04074369
Diagnostic trial: Human Enterovirus Infections, HEV, (NCT04535648) [Internet]. CRISPR Med. [cited 2024 Feb 22]. Available from: https://crisprmedicinenews.com/diagnostic-trial/human-enterovirus-infections-hev-nct04535648-1/
Chinese Medical Association. Effect of PCR-CRISPR/Cas12a on the Early Anti-infective Schemes in Patients With Open Air Pneumonia [Internet]. clinicaltrials.gov; 2019 Nov. Report No.: NCT04178382. Available from: https://clinicaltrials.gov/study/NCT04178382
Children’s Hospital of Fudan University. Establishment a Nucleic Acid Rapid Detection Technology Platform for Detecting Pathogenic Bordetella and Its Drug Resistance Genes [Internet]. clinicaltrials.gov; 2022 Sep. Report No.: NCT04535505. Available from: https://clinicaltrials.gov/study/NCT04535505
Download references
Author information
Authors and affiliations.
MOA Laboratory for Risk Assessment of Quality and Safety of Livestock and Poultry Products, Huazhong Agricultural University, Wuhan, 430070, China
Muhammad Shahzad Rafiq & Haihong Hao
Institute of Microbiology, University of Veterinary and Animal Sciences, Lahore, Pakistan
Muhammad AbuBakar Shabbir
Livestock and Dairy Development Department, Punjab, Pakistan
Ahmed Raza & Shoaib Irshad
Institute of Pharmaceutical Sciences, University of Veterinary and Animal Sciences, Lahore, Pakistan
Andleeb Asghar
Department of Veterinary Surgery and Pet Sciences, University of Veterinary and Animal Sciences, Lahore, Pakistan
Muhammad Kashif Maan
Institute of Continuing Education and Extension, Cholistan University of Veterinary and Animal Sciences, Bahawalpur, Pakistan
Mushtaq Ahmed Gondal
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Haihong Hao .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for Publication
Availability of data and materials.
Financial support for this study was provided by the National Natural Science Foundation of China (Grant No. 32172914), the National Key Research and Development Program (Grant No. 2021YFD1800600), and the Fundamental Research Funds for the Central Universities (Grant No. 2662022DKYJC005).
Competing Interests
All authors report no conflicts of interest.
Code Availability
Author contributions.
Shahzad Rafiq contributed to the original conceptualization and initial draft of the manuscript. Muhammad AbuBakr Shabbir, Ahmed Raza, Shoaib Irshad, Andleeb Asghar, Muhammad Kashif Maan, and Mushtaq Ahmed Gondal carried out the review, editing, and validation tasks. Haihong Hao played a role in the article's conceptualization, review, editing, and supervision. All the authors have thoroughly read and unanimously agreed to the final version of the published manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Rafiq, M., Shabbir, M.A., Raza, A. et al. CRISPR-Cas System: A New Dawn to Combat Antibiotic Resistance. BioDrugs (2024). https://doi.org/10.1007/s40259-024-00656-3
Download citation
Accepted : 08 March 2024
Published : 11 April 2024
DOI : https://doi.org/10.1007/s40259-024-00656-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Advertisement
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 15 April 2024
Developing hydrogels for gene therapy and tissue engineering
- Chunyu Su 1 , 2 , 3 na1 ,
- Dini Lin 2 na1 ,
- Xinyu Huang 3 ,
- Jiayin Feng 1 ,
- Anqi Jin 1 ,
- Fangyan Wang 1 ,
- Qizhuang Lv 3 ,
- Lanjie Lei 1 &
- Wenjie Pan 2
Journal of Nanobiotechnology volume 22 , Article number: 182 ( 2024 ) Cite this article
227 Accesses
1 Altmetric
Metrics details
Hydrogels are a class of highly absorbent and easily modified polymer materials suitable for use as slow-release carriers for drugs. Gene therapy is highly specific and can overcome the limitations of traditional tissue engineering techniques and has significant advantages in tissue repair. However, therapeutic genes are often affected by cellular barriers and enzyme sensitivity, and carrier loading of therapeutic genes is essential. Therapeutic gene hydrogels can well overcome these difficulties. Moreover, gene-therapeutic hydrogels have made considerable progress. This review summarizes the recent research on carrier gene hydrogels for the treatment of tissue damage through a summary of the most current research frontiers. We initially introduce the classification of hydrogels and their cross-linking methods, followed by a detailed overview of the types and modifications of therapeutic genes, a detailed discussion on the loading of therapeutic genes in hydrogels and their characterization features, a summary of the design of hydrogels for therapeutic gene release, and an overview of their applications in tissue engineering. Finally, we provide comments and look forward to the shortcomings and future directions of hydrogels for gene therapy. We hope that this article will provide researchers in related fields with more comprehensive and systematic strategies for tissue engineering repair and further promote the development of the field of hydrogels for gene therapy.
Graphical abstract
Introduction
The human body inevitably encounters tissue damage owing to occurrences such as car accidents, burns, and accidental damage. Part of the persistent infection caused by chronic injury will lead to uncontrolled immune regulation. Infected bone defects and traditional surgical resection of tumors are prone to recurrence, while tissue transplantation will cause immune rejection, among other issues, which will further aggravate the patient’s symptoms and even lead to death [ 1 ]. Moreover, the human body’s self-repair ability is very limited; therefore, regulating and invoking gene therapy is a very promising method to further improve the therapeutic effect. Gene therapy is the introduction of genes into cells to up-regulate the expression of target genes to treat or repair tissue defects [ 2 , 3 ]. It exhibits high specificity, low immune rejection, and durability; it is widely used in the treatment of cystic fibrosis, hereditary retinopathy, multiple sclerosis, bone defect repair, and cancer [ 4 , 5 ].
Direct injection of gene drugs has shortcomings, such as rapid degradation, easy off-target effects, and low bioavailability. The hydrogel is an excellent class of carrier material; it is a porous hydrophilic three-dimensional network structure with good biocompatibility, biodegradability, and water retention. It can improve the stability of therapeutic genes, which can enhance cell adhesion, transfection efficiency, and targeting precision. Hydrogels have good mechanical properties and are less invasive, injectable, and easy to modify and customize, which is conducive to the efficient delivery of gene therapy [ 6 ]; thus, the combination of gene therapy with hydrogels presents a promising avenue for tissue damage repair.
An increasing number of gene therapy hydrogels for tissue engineering have been developed. However, several unresolved problems remain [ 7 , 8 ]. For example, the poor mechanical properties and stability of some gene therapy hydrogels can lead to immune reactions and toxic effects; their safety and effectiveness are not guaranteed. Poor transfection efficiency may hinder the efficient delivery of gene drugs. To fully realize the potential of gene therapy hydrogels in tissue engineering repair, continual optimization of the key steps is required such as more efficient loading of genes into the hydrogel, improving the stability and activity of genes in vivo, and refining the efficiency of gene transfection.
Despite these challenges, considerable progress has been made in the development of gene therapy hydrogels. Therefore, this review initially introduced the classification of hydrogels and their cross-linking methods. Subsequently, we provide a detailed overview of the types and modifications of therapeutic genes, followed by a detailed discussion on the loading of therapeutic genes in hydrogels and their characterization features, a summary of the design of hydrogels for therapeutic gene release, and an overview of their applications in tissue engineering. Finally, we provide comments on the shortcomings and future directions of hydrogels for gene therapy. We envisage that this article will provide researchers in related fields with more comprehensive and systematic strategies for tissue engineering repair to promote the development of the field of hydrogels for gene therapy.
Hydrogels can absorb large amounts of water, have high water swelling, and maintain their original structure without dissolution, even after substantial expansion. This attribute is advantageous to improve cell adhesion and transfection efficiency and promote gene stability by preventing degradation [ 9 ]. Currently, hydrogels are less invasive, injectable, and customizable, and are suitable for efficient delivery of gene therapy drugs, showing great potential for tissue repair (Fig. 1 ). In the following section, we explore the types and properties of hydrogels. Furthermore, we summarize the commonly used crosslinking methods, with detailed descriptions (Table 1 ).
Types of hydrogels and their crosslinking modes and therapeutic gene types and their modification strategies. Created with BioRender.com. (Agreement number: EC26NU6OOQ)
Classification of hydrogels
Natural polysaccharide hydrogels.
Sodium alginate (SA) is a by-product of the extraction of iodine from brown algae, which is formed by the connection of α-L-guluronic and β-D-mannuronic [ 38 ]. Its hydrogels exhibit good biocompatibility, biodegradability, stability, and high viscosity, which can provide a moist environment for wound repair. However, their lack of mechanical strength limits their application, and intermolecular crosslinking can be induced by the addition of ionic crosslinking agents, for example, Ca 2+ and Al 3+ , which improves the mechanical strength [ 39 ]. The applications of SA hydrogel-based gene therapy are well established. For example, Li et al. [ 40 ] used the 45S5 Bioglass® (BG)/SA hydrogel to encapsulate small interfering RNA (siRNA) from matrix metalloproteinase 9 (MMP9), which can directly inhibit the overexpression of MMP-9 in cells, thus reducing inflammation, accelerating traumatic angiogenesis, and promoting the synthesis of collagen and fibroblast extracellular matrix proteins (ECMs). Owing to the high viscosity of the SA hydrogel, it covers and adheres more firmly to and better protects the damaged tissue area, especially in the treatment of skin wounds [ 39 ].
Chitosan is derived from the partial deacetylation of chitin, an alternate link between N-acetylglucosamine and glucosamine [ 40 ]. Chitosan hydrogels are bacteriostatic, compression-resistant, have good adhesion, and can be easily chemically modified. Through modification, this material can significantly improve the binding capacity and delivery efficiency to genes, thus promoting tissue wound healing more effectively [ 41 ]. Cai et al. [ 15 ] used MMP-2 to modify carboxymethyl chitosan (CMCS-AGE) and prepared a hydrogel by loading siRNA complexes that target transforming growth factor-β1 (TGF-β1). The resulting hydrogel upregulated MMP-2 expression in tendon tissues, allowing controlled release of siRNA, which largely reduced the negative effects on tendon healing. Gene therapy often results in decreased therapeutic effects owing to off-target effects and low transfection efficiency. To address these issues, multifunctionality has emerged as a strategy. Chitosan can easily be chemically modified, significantly enhancing cell targeting, which greatly improves the targeting and efficiency of gene therapy. Therefore, the synergistic effect of pluripotent chitosan and gene therapy may be an important topic for future research [ 41 ].
Hyaluronic acid (HA) can be obtained by microbial fermentation and extraction of animal tissues. It comprises alternately attached N-acetylglucosamine and glucuronic acid [ 42 ]. Hyaluronic acid hydrogels have good biocompatibility and pH response and can be customized according to the size, shape, position, and other factors of the wound of the patient [ 43 ]. Sun et al. [ 16 ] prepared a THH-3/Exos-microRNA (miRNA) 24-3p hydrogel composite. The gel promotes migration and accelerates the rate of wound healing in rabbit corneal epithelial cells. An inhibitory effect on keratitis and corneal fibrosis is also evident in animal burn models. Its functionality is outstanding compared with those of other materials owing to the natural repair effect of HA hydrogels on tissues, although the mechanical properties of these hydrogels are poor, and the degradation rate is difficult to control; therefore, HA hydrogels require further improvement. This might be achieved by adjusting the concentration of cross-linking agents, the timing of the cross-linking reaction, or the conditions to improve their mechanical properties. Moreover, the modulation of the degradation rate could be improved by intelligent responsive design [ 43 ].
Agarose (AG) is a linear polysaccharide found in red algae, comprising alternating arrangements of 1,3-linked β-D-galactose and 1,4-linked 3,6-endo-ether-L-galactose [ 44 ]. Agarose hydrogels possess exceptional biocompatibility, antiadhesion, degradability, and thermo-reversible properties, allowing them to protect genes from the external environment and enhance the efficiency and stability of gene delivery [ 17 ]. siRNA targeting the mRNA encoded by the Col1a1 gene (Col1a1 siRNA) is a crucial regulatory substance for invasive chondrocyte therapy. Chondrocytes transfected with Col1a1 siRNA and encapsulated in an AG hydrogel effectively promote long-term proliferation and expression of the corresponding proteins [ 17 ]. The AG hydrogel has low immunogenicity and mitigates the risk of immune rejection, rendering it indispensable for gene therapy after tissue damage. However, its limitation lies in the inability to specifically target cells for gene transfection, particularly impeding their application in tissue traumas that require high specificity in gene therapy. Thus, future advances in cell-specific improvements are crucial for the continued development of AG hydrogels [ 17 ].
Chondroitin sulfate (CS) is widely distributed in animal cartilaginous tissues and comprises N-acetylgalactose linked to D-glucose via a 1,4-glucosidic linkage [ 45 ]. Chondroitin sulfate hydrogels are biocompatible and degradable, promote cell adhesion and proliferation, and reduce serum cholesterol and triglyceride levels in patients with hyperlipidemia, thus decreasing the incidence of coronary heart disease [ 46 ]. Zhang et al. [ 47 ] prepared hydrogels with strong adhesion and biocompatibility by modifying CS and alginate-dopamine with enbucrilate. The loading of exosomes (Exos) efficiently promotes the proliferation, differentiation, expansion, and migration of bone marrow mesenchymal stem cells (BMSCs). In situ injection of this hydrogel into the rat patellofemoral groove resulted in the recruitment of BMSCs into newborn cartilage under the guidance of the chemokine signaling pathway. This accelerated extracellular matrix (ECM) remodeling and regeneration of cartilage defects. Although the CS hydrogel protects therapeutic genes from degradation factors in vivo, it lacks efficient gene transfection capabilities, causing the additional use of viral vectors or other gene transfection reagents to enhance entry into cells and expression levels.
Dextran derived from the cell walls of cereals such as wheat, oats, and yeast constitutes homopolysaccharides with glucose units linked by glycosidic bonds and serves as a substitute for plasma in medicine [ 48 ]. Dextran hydrogels exhibit good biocompatibility, biodegradability, high gene transfection efficiency, and immunoisolation effects, reducing the immune response of the body and protecting therapeutic genes during gene transfection. The hydrogel obtained by coupling MAES, dextran, and siRNA exhibits good degradability and tunability, continuously presents siRNA to enhance osteogenic differentiation of encapsulated human mesenchymal stem cells (hMSCs), and exhibits controlled cell adhesion and outstanding siRNA delivery capacity [ 49 ]. Although dextran hydrogels are not considerably toxic to cells, residual substances (including incompletely polymerized monomers) are present during preparation, which can have a toxic effect on cells. Thus, surface modification and the addition of bioactive substances are necessary to further the gene therapy applications of chitosan hydrogels [ 49 ].
Protein hydrogels
Gelatin is a product of collagen breakdown composed of proline, hydroxyproline, and oxyribonucleic acid linked by peptides or hydrogen bonds [ 50 ]. Photo crosslinking promotes the crosslinking of gelatin and methacryloyl anhydride under UV irradiation to form GelMA, resulting in the controlling the release or localization of therapeutic genes/growth factors, and improving gene therapy accuracy. Gelatin hydrogels exhibit good cell adhesion, biocompatibility, and processing properties, and can be prepared into different shapes and sizes of hydrogels, which makes them more suitable for different damaged tissue repairs [ 51 ]. Pan et al. [ 52 ] prepared miR-29b/gold nanoparticle (AuNP) hydrogel composites using 3D printing technology, which can mimic the bone healing process in vitro, achieve a slow release of miRNAs, and gradually replace the gel surface with new tissue, which is suitable for bone repair. Research on gelatin hydrogels has matured, and the gelatin material after photo crosslinking can better load the therapeutic drug, which is suitable for tissue trauma. However, excessive UV irradiation can lead to a mutation of the gene therapy drug, which will reduce the therapeutic effect. Future studies should investigate ways to reduce the number of mutations.
Collagen is the most abundant class of proteins in mammals and is widely distributed in the connective tissues of animals; it comprises three intertwined peptide chains [ 53 ]. Collagen modification by photo crosslinking can significantly increase its tensile strength and tensile coefficient and reduce the elongation at break, which can better meet the needs of gene therapy; however, the mutation and other problems caused by photo crosslinking modification must be emphasized. Collagen hydrogels exhibit good bioactivity, biocompatibility, and low immunogenicity, and maintain stability in vivo without causing strong immune and inflammatory reactions, with improved efficacy and safety. Weißenberger et al. [ 54 ] used an adenoviral vector containing BMP-2 cDNA and TGFB 1 to modify hMSCs and placed them in a type I collagen hydrogel. Within the cartilage medium, the gel significantly promoted glycosaminoglycan (GAG) synthesis and effectively induced cartilage formation in mesenchymal stem cells (MSCs). The low immunogenicity of collagen hydrogel is excellent for organismal safety and renders it more suitable as a gene delivery vector, although the degradation rate of collagen hydrogel is difficult to control. The future degradation rate of collagen hydrogels might be improved by surface modification, addition of cross-linking agents, and introduction of specific enzymatic sequences [ 54 ].
Silk proteins (SF) are macromolecular fibrous proteins of silkworm silk comprised of several protein subunits, including α-serin and β-serin subunits [ 55 ]. Silk protein hydrogels show good biocompatibility, degradability, mechanical properties, and ease of modification, integrate well with host tissues, and reduce inflammatory reactions and immune rejection during gene therapy [ 27 ]. Han et al. [ 56 ] prepared hydrogels by coupling silk gum (SS) with SF and used them to encapsulate human umbilical cord MSC-derived exosomes (UMSC-Exos). The wound closure assay and the in vitro and in vivo inflammatory responses showed that the hydrogel had a significant effect on improving skin fibroblast viability, reduced TNF-α secretion, and macrophage inflammatory response, and accelerating vascular regeneration and wound healing efficiency. The synergistic effect of multifunctionalized SF hydrogels with gene therapy presents a promising research avenue for introducing additional therapeutic functions or improving targeting, rendering them ideal gene delivery carriers, and improving the relevance and efficiency of gene therapy.
Sericin is a globulin produced by silkworms during the production process and comprises amino acids such as serine, aspartic acid, and glycine [ 57 ]. Sericin hydrogels have good elasticity, tensile strength, biocompatibility, safety, and mechanical properties, which can enhance the efficacy and safety of gene therapy and repair damaged tissues. Kanazawa et al. [ 29 ] used a sericin hydrogel to load siRNA targeting RelA (anti-RelA siRNA) to achieve sustained release of siRNA in vitro, which was not degraded for a long time in the rat knee joint. The treatment of collagen-induced arthritis (CIA) mice with a sericin hydrogel containing anti-RelA siRNA significantly improved the incidence rate, clinical severity, and thickness of the knee joint compared with the control group. Furthermore, sericin has natural anti-infective properties and can be used for wound suturing. Moreover, sericin hydrogels have good mechanical strength, which can provide sufficient support to satisfy the strength requirements for tissue engineering, improve the efficacy and stability of gene therapy, and accelerate the healing and functional recovery of tissues.
Albumin is a predominant protein in human plasma; it is synthesized as prealbumin and plays a role in maintaining plasma osmolality, transport, detoxification, and anti-inflammatory effects [ 58 ]. Albumin hydrogels usually exhibit good biocompatibility, degradability, adhesion, and mechanical properties, which significantly reduce adverse reactions when fused with host tissues. However, its lack of bioactivity limits its application, which can be enhanced by the addition of bioactive molecules, surface functionalization, gene transduction, and complexation with other biomaterials [ 30 ]. Chen et al. [ 31 ] loaded adipose stem cell-derived Exos onto Ag@bovine serum albumin (BSA) nanoflowers (Exos-Ag@BSA NFs) to form a protective delivery structure that precisely released Exos to remodel the trauma environment, protected Exos from oxidative denaturation and elimination of bacteria, and induced apoptosis of damaged oxidized cells. It can accelerate the rate of diabetic wound healing by promoting angiogenesis and epithelial regeneration, collagen deposition, tissue granule formation, and blood perfusion. The albumin hydrogel adheres more firmly to the damaged area, delays gene therapy, and greatly improves tissue wound repair to exhibit a good mucoadhesive effect.
Synthetic hydrogels
N-isopropyl acrylamide (NIPAM) is an acrylamide derivative monomer that is used to prepare poly(N-isopropyl acrylamide) (PNIPAM) [ 59 ]. NIPAM hydrogels have better biocompatibility, degradability, and thermal sensitivity and are widely used to prepare smart response materials capable of localized drug release at specific sites in gene therapy [ 60 ]. Yang et al. [ 61 ] used layered double hydroxides (LDHs) to modify PNIPAM to prepare hydrogels that are non-inhibitory to cells, can be used as thermotropic telescopic injection carriers for siRNA transduction, and can specifically modulate several factors in cartilage tissue, thus realizing in vivo gene therapy. Moreover, the PNIPAM composite hydrogel effectively inhibited degenerative factors and supported the cells. Although NIPAM hydrogels exhibit excellent thermal sensitivity, they do not have a good swelling rate. This requires alteration of the crosslinking agents and initiators or the addition of bioactives to improve their deficiencies and meet the needs of gene therapy.
Polyethylene glycol (PEG) is a commonly used reagent by gradual addition polymerization of ethylene glycol with ethylene oxide [ 62 , 63 ]. Polyethylene glycol hydrogels have good biocompatibility, biodegradability, and processing properties, as well as good mechanical strength and elasticity, which protect therapeutic genes from the complex environment of the body and improve the rate of tissue engineering repair (Fig. 2 A) [ 64 ]. Wang et al. [ 65 ] coupled difunctionalized polyethylene glycol, coralline hydroxyapatite, glycol chitosan, and SF into a hydrogel and loaded human umbilical cord mesenchymal stem cells (hucMSC)-derived Exos. The hydrogel has outstanding biocompatibility, stability, and mechanical properties that can effectively accelerate vascular regeneration and promote BMP-2 deposition, osteogenic differentiation of mouse osteoblast progenitor cells (mOPCSs), and proliferation and migration (Fig. 5 B). As PEG hydrogels have good processing properties, they can be prepared according to the target shape and size, which meets the customization needs of different gene therapy applications and broadens the selection and application of gene therapy materials.
In vivo localization and distribution of therapeutic genes. A In vivo localization of M2-Exogels. (i) Ex vivo fluorescent images of some rat organs; (ii) Fluorescent images of biodistribution of cy5.5-labeled M2-Exos in BALB/c nude mice. (Reproduced with permission from Ref. [ 64 ], Copyright 2022, John Wiley and Sons). B Distribution diagram of FAM-siRNAs. (i) Distribution of FAM-siRNA in selected organs; (ii) Fluorescent images of FAM-siRNA after in situ transplantation in nude mice. (Reproduced with permission from Ref. [ 66 ], Copyright 2023, American Chemical Society)
Acrylic acid (AA) is the simplest unsaturated carboxylic acid, which can be prepared by the oxidation of acrolein or the hydrolysis of acrylonitrile [ 67 ]. It is known for its corrosive, unstable nature, and susceptibility to easy polymerization. Acrylic acid hydrogels offer controllable biodegradability, ease of processing, and customization, rendering them suitable for creating materials with diverse properties to address various gene therapy applications. Nuhb [ 68 ] copolymerized tri(ethylene glycol)methyl ether methacrylate with pentafluorophenyl methacrylate via RAFT and subsequently converted it into nanohydrogel particles using an amine-containing crosslinking agent for siRNA delivery. Champeau et al. [ 69 ] developed hydrogels using polyacrylic acid-coupled PEO-PPO-PEO (F127). These hydrogels promote the expression of IL-10, SDF-1, IGF-1, and TGF-β, leading to increased fibrous collagen tissue and angiogenesis, ultimately aiding in wound repair. However, AA hydrogels exhibit instability and are susceptible to factors such as temperature, pH, and ionic strength, affecting the delivery of therapeutic genes and resulting in abnormal cell growth and proliferation in gene therapy. Strategies for enhancement include UV irradiation, the use of chemical crosslinking agents (such as ethylene glycol diacrylate), and the addition of nanoparticles to enhance mechanical strength and stability. Furthermore, the presence of acetone in AA hydrogels (which possess some toxicity) can be mitigated by introducing biocompatible groups or molecular chains, optimizing the polymerization reaction conditions, and increasing the amount of crosslinking agent.
Acrylamide is an unsaturated linear polymer that serves as a crucial water treatment agent, thickener, and flocculant synthesized by catalytic hydration. Acrylamide hydrogels exhibit commendable biocompatibility, gene transfection ability, and facile chemical modification, effectively shielding genes from in vivo degradation factors and significantly improving transfection efficiency. Chang et al. [ 70 ] developed a DNA-based acrylamide hydrogel microcapsule to achieve miRNA-responsive effects for the detection of cancer-associated miRNAs. To meet clinical requirements, they combined strand displacement polymerization/nicking amplification machinery (SDP/NA) with a hydrogel sensor, enhancing the sensitivity of miRNA detection. However, polyacrylamide hydrogels are typically cytotoxic, may induce immune reactions upon repeated use, and possess a slow degradation rate, potentially affecting the healing speed of gene therapy tissue engineering. Therefore, more research is imperative to address these limitations.
Crosslinking of hydrogels
Crosslinking is essential to enhance hydrogel functionalization, alter surface properties, and improve characteristics. Hydrogel crosslinking falls primarily into two categories: physical and chemical. Physically cross-linked hydrogels are pure and exhibit minimal side effects, high elasticity, and plasticity. In contrast, chemically cross-linked hydrogels offer mechanical stability, adjustability, and biocompatibility. The subsequent sections detail different types of crosslinking, presenting a comparison of their advantages and disadvantages (Table 2 ).
Physical crosslinking
Ionic crosslinking involves the combination of a charged polymer with another charged polymer or multivalent ion through ionic bonding. Some natural polysaccharides and their derivatives lack high thermal stability and mechanical strength, often causing ionic crosslinking to enhance their properties [ 71 ]. Rezvanian et al. [ 11 ] prepared alginate-pectin hydrogels by ionic crosslinking, facilitating the release of simvastatin for trauma repair. Li et al. [ 72 ] designed an SA/Hardystonite (HS) hydrogel to accelerate the rate of epithelial tissue and vascular regeneration using Ca 2+ as a crosslinking agent to promote wound healing. Ren et al. [ 12 ] obtained shape-memory hydrogels (SM) under Ca 2+ crosslinking, wherein Ca 2+ modification improved the mechanical strength of the gels and imparted SM to the hydrogels so that they could be restored to their initial state, allowing controlled growth at different locations. The synergistic effect of ionic crosslinking contributes to the remarkable toughening of hydrogels with no specific structural design or the addition of other reinforcing agents. This enhancement renders hydrogels capable of meeting the mechanical properties required for tissue engineering, making them more suitable for gene therapy.
Base pairing involves the interaction between two double-stranded DNA molecules via hydrogen bonds [ 73 ]. Simple base-based hydrogels have poor mechanical properties and often interact with other crosslinks. Gao et al. [ 74 ] constructed multifunctional DNA hydrogels to accelerate trauma repair. Precise base pairings between sticky ends can be constructed quickly, and cytimidine–Ag + –cytimidine bridging ligands complexed with Ag + significantly improve the mechanical properties of hydrogels and provide slow-release, long-lasting properties outstanding for gene therapy for tissue trauma. Furthermore, the hydrogel acted on M2 macrophages through passive recruitment and release of endogenous chemokines by G-coupled protein receptors, promoting the early transition from the inflammatory phase to the proliferative phase. Tang et al. [ 75 ] used enzymatic amplification synthesis to prepare DNA strands and constructed hydrogels for tissue engineering applications using the base-pairing principle. Base pairing has excellent recognition ability and can build highly sensitive and selective biosensing and diagnostic platforms; however, its mechanical properties may be affected by environmental factors, such as temperature, pH, and ionic strength, which are detrimental to the stability of gene therapy.
A thermotropic phase transition is used to prepare hydrogels by changing the temperature of the polymer solution. The entanglement of polymer molecular chains to form a cross-linked network structure also depends on the change in solution temperature [ 76 ]. Thermotropic phase-transition-prepared hydrogels do not require chemical crosslinking agents and can be injected into the body as a liquid to achieve in situ curing. The co-existence of hydrophilic/hydrophobic groups is a key feature of thermotropic phase-transition hydrogels [ 77 ]. Wu et al. [ 78 ] prepared sEV@CS/β-GP hydrogels and used them to piggyback BMSCs-derived sEVs. These hydrogels have excellent temperature-sensitive properties, biocompatibility, and stability, with a volume that responds to changes in temperature and exosomal miR-21 that can promote vascular regeneration by targeting SPRY2. Mao et al. [ 79 ] prepared a PEG-PDLA/PLLA-PEG-PLLA hybrid hydrogel that exhibited 20 diverse thermo-responsive phase-transition behaviors suitable for the encapsulation and release of gene therapy drugs. As the thermotropic phase transition depends on temperature change, the temperature change should always be considered in hydrogel preparation, and most therapeutic genes will be degraded at an accelerated rate under the effect of high temperature. Therefore, it is crucial to consider the effect of temperature on the activity of therapeutic genes, which is necessary for the high efficiency of gene therapy.
Molecular-specific recognition involves bonding through van der Waals forces or electrostatic interactions [ 80 ]. It is highly sensitive, recognizes a wide range of molecules, and mediates biological processes essential for biochemical reactions [ 81 ]. For example, specific binding between receptors and ligands is the basis of signaling and drug action, whereas specific binding between enzymes and substrates is the basis of biocatalytic reactions [ 82 , 83 ]. Hu et al. [ 84 ] prepared the amorphous CMC-ALG-EGF hydrogel by treating ALG, N-carboxymethyl chitosan (CMC), and calcium chloride with divalent chelation with epidermal growth factor (EGF) and static electricity, d. The porous structure of the hydrogels facilitates the loading and release of EGF, promoting cell proliferation and wound repair. Although the sensitivity of molecular-specific recognition is outstanding—which renders it possible to deliver therapeutic genes to damaged parts of the body with greater precision—, it binds weakly and is sensitive to environmental factors. Therefore, the preparation must be strictly controlled, and these points are worth focusing on and improving.
Chemical crosslinking
The Schiff base reaction is a nucleophilic addition elimination reaction that involves nitrogen atoms with lone pairs of electrons in nucleophilic reagent ammonia compounds attacking a positive charge carbonyl carbon atom, forming an α-hydroxy-amine compounds intermediate, and obtaining the Schiff base through the dehydration reaction [ 85 ]. The Schiff base reaction does not undergo a solution–gel phase transition, which makes it favorable for the filling of organ defects. Yang et al. [ 86 ] prepared HA-PEI@siRNA-29a hydrogels based on Schiff base bonds to improve the wound healing rate. Fan et al. [ 20 ] prepared CMC-oxidized chondroitin sulfate (OCS) hydrogels using the Schiff base reaction. However, the prepared hydrogels exhibited high release rates and poor mechanical properties. Therefore, they prepared BSA-loaded chitosan microspheres (CMs) using the emulsion crosslinking method and embedded them in prepared hydrogels to improve their bioactivity and mechanical properties. The survival of chondrocytes cultured in vitro significantly improved. The Schiff base reaction has excellent characteristics, such as multiple responsiveness, low energy loss, and process simplicity; however, its poor mechanical properties, poor biological activity, sensitivity to environmental factors, and biosafety deficiencies can limit its use.
The Diels–Alder (DA) reaction is a typical cycloaddition reaction used for the synthesis of cyclic and heterocyclic compounds [ 87 ]. This reaction involves the simultaneous participation of four π-electrons in the dienophile and two π-electrons in the allylic reagent, resulting in the formation of a conjugated system of six-membered rings [ 87 ]. Fujisawa et al. [ 88 ] synthesized a drug-release system using a DA reaction designed to enhance drug release in locally heated tissues. The system targets drug release, thus improving therapeutic efficiency and overcoming side effects associated with conventional drugs used in the treatment of pancreatic cancer. Li et al. [ 89 ] prepared a pectin-chitosan hydrogel using the DA reaction. The hydrogel exhibited an initial increase and a subsequent decrease in solubilization at elevated pH. The MTT assay and the cytotoxicity analysis of fibroblast L929 cells indicated that the gel showed good cytocompatibility and a favorable crosslinking density. It is effective in targeting the release of therapeutic gene drugs, enhancing the transfection efficiency of gene therapy, and is suitable for treating tissue trauma owing to the thermal responsiveness, mild reaction conditions, the absence of byproducts, and stability of the DA reaction.
Michael addition is a conjugate addition reaction that involves a nucleophilic conjugate system and a nucleophilic negative carbon ion under the catalytic action of a base. This reaction is characterized by mild conditions, strong selectivity, good stability, and high yield, making it a common method for growing carbon chains in organic synthesis [ 90 ]. Jin et al. [ 33 ] used Michael’s addition to crosslink PEG vinyl sulfone and thiolated HA in hydrogels to promote bovine chondrocyte proliferation. Tao et al. [ 14 ] prepared Gel-PDA@Cur hydrogels through the coupling of curcumin (Cur)-loaded mesoporous polydopamine (PDA@Cur) nanoparticles with PEGDA modified with chitosan by Michael addition. The gel can promote the expression of VEGF, TGF-β1, Arg I anti-inflammatory factors and inhibit the expression of TNF-α, IL-6, and CCR7 pro-inflammatory factors, as well as reduce the inflammatory response and accelerate wound healing. Furthermore, the hydrogel causes bacterial death by inducing exocytosis of K + , causing leakage of DNA, RNA, and proteins inside the bacteria, and disrupting the bacterial membrane. Despite the stability and high yield of the Michael addition reaction, its sensitivity to environmental factors and low mechanical properties may hinder the hydrogel from meeting tissue engineering needs, diminishing the effectiveness of gene therapy. Future research should focus on functional modification to address these shortcomings.
Click chemistry involves the Cu-catalyzed reaction of an azide with an alkynyl compound to form an enimide intermediate, which then reacts with another alkynyl compound to form a 1,2,3-triazole compound [ 91 ]. Click chemistry offers mild reaction conditions, fast reaction speed, good selectivity, and easy purification, providing additional options for the preparation of hydrogels with various specifications [ 92 ]. Ren et al. [ 93 ] prepared the glycopolypeptide by coupling 3-(4-hydroxyphenyl) propanamide (HPPA) to poly(γ-propargyl-L-glutamate) (PPLG) by click chemistry, followed by H 2 O 2 and horseradish peroxidase (HRP) to obtain PPLG-grafted with mannose and HPPA (PPLG-g-Man/HPPA) hydrogels. Chondrocyte cultures showed strong cell viability and the content of proliferation rates, and type II collagen and GAG were significantly increased on this gel, which also showed good biocompatibility with L929 cells in vitro. However, the poor mechanical properties of chemically responsive hydrogels and their sensitivity to environmental factors (which can trigger an immune response or be potentially toxic) are detrimental to the realization of gene therapy in vivo and need to be improved and optimized.
In the presence of heat or light, precursors with unsaturated or photosensitive functional groups undergo free-radical polymerization. This method is characterized by high selectivity, fast reaction rate, modulation, and easy serialization, and is well-suited for gene therapy [ 94 ]. Zou et al. [ 95 ] prepared a Gel@Exo system with hucMSC-derived Exos that resulted in a substantial increase in retention time and a large decrease in the fibrotic area in myocardial tissues after introduction into injured rat hearts. Real-time polymerase chain reaction (RT-PCR) and immunofluorescence staining revealed that the expression of TGF-β1 , VEGF-A , VEGF-B , vWF , and Serca2a genes, as well as myocardium-associated proteins, such as CD31, Cx43, α-SMA, and Ki67, were significantly upregulated in damaged cardiomyocytes, which had a significant therapeutic effect on myocardial infarction (MI). Free-radical polymerization is characterized by a strong modulation that can precisely control the structure and performance of hydrogels by adjusting the polymerization conditions (such as light intensity, polymerization temperature, initiator concentration, and monomer concentration), thus better adapting to different gene therapy requirements.
Enzymes are highly specific catalytic RNA or proteins produced by living cells [ 96 ]. Enzymes can significantly reduce the activation energy required for a chemical reaction or facilitate or inhibit the reaction by binding products [ 97 ]. The development of enzyme crosslinking is well established. Cai et al. [ 15 ] cross-linked MMP-2 with allyl ethylene oxide (AGE)-modified carboxymethyl chitosan (CMC) and loaded siRNA complexes targeting TGF-β1 onto it to make hydrogels. The hydrogel responded to MMP-2 upregulation in tendon tissues, resulting in the controlled release of siRNA, which reduced the negative impact on tendon healing. Furthermore, the gel has a silencing effect on the gene related to TGF-β1, which can inhibit fibroblast proliferation and differentiation, thus preventing adhesion around the tendon. The enzyme is susceptible to external factors (such as pH and temperature); therefore, it can be unstable, which requires modification of the additive material to improve its deficiencies and achieve a higher level of gene therapy effects.
Chemical crosslinkers increase the stability, hardness, strength, and durability of substances by crosslinking hydrocarbon bonds in the molecular chains [ 98 ]. Common chemical crosslinking agents include glutaraldehyde (GTA), epoxy resin, phenolic resin, isocyanate, and glutaralose. Yu et al. [ 99 ] coupled CMC with a poloxamer to form a hydrogel using GTA as a chemical crosslinker. The composite hydrogel exhibits a reversible sol–gel transition regarding the pH and temperature. Cell Counting Kit-8 experiments confirmed that the hydrogel was non-toxic to human corneal epithelial cells, confirming it as a potential material for ophthalmic drug delivery. Despite the numerous advantages of chemical crosslinkers in the materials field, there are challenges and limitations. The crosslinking process may cause shrinkage of the material or side reactions that can lead to a decrease in product quality for some specific polymer systems. Furthermore, some chemical crosslinking agents may pose non-negligible risks to humans or the environment, and these are barriers that need to be overcome.
Natural polysaccharide hydrogels have poor mechanical properties, controllability, and permeability, and are therefore not strong enough to withstand high mechanical loads, often needing to be modified with other materials. At the same time, the degradation products of some natural polysaccharide hydrogels can trigger allergic reactions, thus limiting their application. Protein hydrogels have excellent mechanical strength and biocompatibility, but their degradation behavior, bioactivity, and stability are insufficient and they are susceptible to external factors (e.g. pH, temperature). Synthetic hydrogels have good stability and maneuverability and can be well adapted to the needs of different scenarios, but their poor biocompatibility, slow degradation, and high biotoxicity need to be addressed. Researchers have tried various methods, such as introducing bioactive molecules and changing the crosslinking method, to solve these problems, but further research is still needed. Hydrogel cross-linking requires harsh external conditions, cross-linking agents, and chemical initiators, and the toxicity of chemical cross-linking has led to further discussion [ 6 ]. Future improvements of physically crosslinked hydrogels can be achieved by developing dual or multi-network structures that incorporate different types of physical cross-linking to enhance overall performance; smart material design, enhancement of the intrinsic stability and functionality of physically crosslinked hydrogels, and the realization of controllability of their performance under complex conditions. Future improvements of chemically crosslinked hydrogels should focus on smart crosslinker design, green and non-toxic synthetic routes, and enhancement of their biological activities to improve their performance [ 84 , 86 ].
Therapeutic genes
Gene therapy is closely related to therapeutic genes. RNA, microRNA, small plasmids, interfering fragments, exosomes, and aptamers are commonly used therapeutic genes, but they often suffer from easy degradation and poor stability. For instance, RNA is frequently influenced by extracellular and cellular barriers, as well as enzymes. SiRNA can activate the immune system although its cellular uptake is low. Therefore, modifications are often necessary, and zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and CRISPR-Cas9 are commonly used modifications.
Types of therapeutic genes
Ribonucleic acid (RNA) is a single-stranded molecule made up of ribonucleotides. It contains the genetic information present in cells and some viruses and virus-like organisms [ 100 ]. Transferring RNA to endoplasmic reticulum ribosomes directs protein synthesis and regulates target genes, and some RNA can be used as nucleases to catalyze intracellular reactions [ 101 ]. Although targeted RNA delivery allows disease treatment, RNA is susceptible to degradation by ribonucleases (RNases) and is hindered by extracellular and cellular barriers [ 102 ]. Therefore, researchers have attempted to encapsulate therapeutic genes in liposomes, inorganic nanomaterials, and polymeric nanoparticles (NPs) with additional hydrogel loading to improve the transfection efficiency of gene therapy [ 103 ]. The topical application of hydrogel-dispersed lyophilized TLNκ (a polymeric nanocarrier) encapsulated with locked nucleic acid (LNA) anti-miR-107 accelerates wound healing [ 104 ]. Lipid nanoparticles (LNPs) are commonly used as RNA inclusions, with outstanding gene therapy effects after additional hydrogel loading. Chitosan-SA hydrogels containing mRNA lipoplexes induce localized transfection in vivo and increase T-cell proliferation and antibody production [ 102 ]. However, liposomes are thermodynamically unstable and prone to aggregation in charged hydrogels, and published studies on RNA liposome-loaded hydrogels remain limited. Further improvement of their deficiencies is needed to develop new countermeasures for tissue trauma from gene therapy.
MicroRNAs (miRNAs) are single-stranded RNAs encoded by endogenous genes involved in processes such as biological growth and development, differentiation, and metabolism; they also play a substantial role in gene regulation [ 105 ]. In recent years, miRNAs have been increasingly explored in tissue engineering, which has greatly expanded the value of their use [ 106 ]. Yang et al. [ 86 ] prepared composite hydrogels based on Schiff base bonds by mixing HA-ADH, siRNA-29a gene-loaded HA-polyethyleneimine (PEI) complex (HA-PEI@siRNA-29a), and oridonin (ori) loading alginate microspheres (Alg@ori) to prepare composite hydrogels. In vitro mouse fibroblast L929 experiments confirmed a good biocompatibility of the gel, which significantly shortened the inflammatory phase. In vivo experiments confirmed that it promotes the production of angiogenic factors such as CD31 and α-SMA, increasing the rate of wound healing. Pan et al. [ 52 ] used PEI-coated AuNPs coupled with miR-29b to prepare a hydrogel. Hydrogels were prepared by mixing alginate with modified gelatin with GTA and Ca 2+ , and the prepared miR-29b/AuNPs were loaded onto them. The gel scaffold can achieve bone healing; miRNA is slowly and continuously released during the process and the scaffold is gradually replaced by new tissue, which is a promising bone repair material. The miRNA hydrogel has excellent biocompatibility and natural degradation in vivo, which makes it a highly superior material; however, miRNAs are prone to mutation (since they are single-stranded fragments) which prompts a decrease in the effectiveness of gene therapy. Future studies should investigate the prevention of miRNA mutation.
Small plasmids
Small plasmids are independent of the cell chromosome outside the ring DNA; they exist in bacteria, archaea, and other prokaryotic organisms; carry a certain amount of genetic information; and have some autonomy and genetic characteristics through transfer, replication, and other means of transmission between cells, making them suitable carriers for genetic engineering [ 107 ]. Some small plasmids can deliver antibiotic-resistant drugs and play an important role in medicine [ 108 ]. The BMP-2 plasmid (pBMP-2) is a good osteogenic differentiation gene and promotes bone differentiation [ 109 ]. Ding et al. [ 110 ] combined chitosan microspheres (CS-MS) with hydroxyapatite lime (HAp) using an emulsification method and loaded infectious PEI/pBMP-2 onto them to prepare hydrogels. The good biosolubility and wide range of surface areas of the hydrogel allowed the sustained release of plasmids for their continuous uptake by cells adhering to the material. This system also continuously secretes relevant target proteins to promote osteogenic differentiation of bone-deficient tissues. Small plasmids are cyclic DNA molecules and are easily degraded by DNA enzymes; therefore, they often require an external carrier. The hydrogel itself is superior, and complex small plasmids can improve the efficiency and stability of gene transfection and better achieve gene therapy for tissue trauma.
Interference fragments
Interference fragments mainly refer to siRNAs, which can silence almost any target gene after entering the cell, and are important for gene regulation [ 111 ]. siRNA specifically cleaves and degrades mRNA with the complementary sequence of a gene substrate, targeting a specific mRNA to inhibit the expression of target genes when linked to the RNA-induced complex (RISC) [ 112 , 113 ]. RNA interference (RNAi) is a process involving short double-stranded RNAs (dsRNAs) that leads to sequence-specific degradation. During RNAi degradation, the imported strand is recognized and binds to the complementary sequence of the target mRNA, resulting in mRNA degradation, affecting gene expression and regulatory functions [ 111 ]. Tang et al. [ 66 ] synthesized the SeMSN(siCFL1)@ P(MNs)Ang2 complex and used it to initiate RNAi under X-ray irradiation to inhibit radiotherapy-resistant glioblastoma (rrGBM) invasion in situ (Fig. 2 B). Wang et al. [ 114 ] prepared a siRNA/NP complex and embedded it in a PEGb-PLA-b-DM hydrogel, which silenced Wwp1 expression and accelerated fracture repair. However, interfering fragments activate the immune system and exhibit instability and low cellular uptake, thus posing challenges for practical applications.
Exosomes are small membrane vesicles produced by cells and released outside the cell; they contain RNA, lipids, and proteins, are widely considered key carriers of information transfer between cells, and are widely used in tissue engineering [ 115 ]. Human mesenchymal stem cell-derived Exos slow degeneration and seal annulus fibrosus damage during disk degeneration [ 86 ]. Kuang et al. [ 116 ] prepared Exo@miR-26a with microRNA-26a (miR-26a) and BMSC-sourced Exos and piggybacked it onto a hydrogel. The hydrogel had a significant inhibitory effect on osteoblast differentiation genes, achieved miR-26a-targeted release, and demonstrated an efficient ability to induce osteogenic differentiation in a rat model with cranial bone defect. Yu et al. [ 117 ] isolated antagomiR-708-5p-loaded exosomes from patient plasma and loaded them onto HA hydrogels to treat femoral fracture nonunion in mice. Compared with the blank group, the Exos-equipped HA hydrogel was much more antibacterial, anti-inflammatory, biocompatible, and mechanically supportive, and significantly promoted bone differentiation and accelerated fracture healing. Although Exos have a cellular messenger function and can participate in cellular communication, they are not specific enough for target cells and may have toxic effects on non-targeted cells; thus, further research and optimization are needed to improve these deficiencies.
Aptamers are single-stranded oligonucleotides screened in vitro that can fold into a defined structure and bind to a target protein, inhibiting protein–protein interactions for disease treatment [ 118 ]. Aptamers are easy to screen and chemically modify, have low-ionic-strength dependence, exhibit good stability in vivo and in vitro, and can serve as drug delivery carriers to precisely deliver drugs into cells, such as chemotherapeutic agents, therapeutic RNAs, and radioisotopes. Systematic evolution of ligands by exponential enrichment (SELEX) is a common method for the large-scale in vitro synthesis of aptamers [ 119 ]. Mesenchymal stem cell-specific aptamers enable gene therapy in osteoporotic bone regeneration [ 120 ]. Liang et al. [ 121 ] used an osteosarcoma (OS) cell-specific aptamer (LC09) to promote the selective distribution of in situ OS and inhibit the effects of in situ OS malignancy and metastasis, which significantly increased bone mineral density in osteoporotic mice. The aptamer is unstable in vivo, has a short half-life, and is prone to degradation, which can compromise the effectiveness of gene therapy. The aptamer can be modified by chemical or genetic modification to improve its stability, biocompatibility, and function in the field of gene therapy tissue engineering.
Modifying therapeutic genes
Gene therapy is inseparable from processing and modification, and ZFNs, TALENs, and CRISPR-Cas9 are commonly used modifications [ 122 ]. TALENs and ZFNs rely on DNA interactions to edit genomic sites, and CRISPR-Cas is designed to achieve specific localization of genomic DNA sequences through complementary RNA strands [ 123 ]. ZFNs are artificially constructed proteins comprising several zinc finger structural domains fused to a type IIS restriction endonuclease FokI, which are used for specific DNA triplexes, DNA sequence recognition, and editing of targeted genomes (Fig. 3 A). However, the ZFN structural domains suffer from limited modularity, lack of specificity, and off-target cleavage, which results in poor performance [ 124 ]. TALENs avoid these limitations by significantly increasing the amount of chromosomal double-strand breaks at the locus and joining the broken double-strand to form the desired genetic modification through homologous recombination (HR) and non-homologous end joining (NHEJ) (Fig. 3 B); thus, they exhibit a higher editing efficiency compared to ZFNs [ 125 ]. TALENs act as a pair of “DNA scissors” that can precisely edit genomic loci and were widely used in the genetic studies of most species [ 126 ]. Chen et al. [ 127 ] designed TALENs that are applicable to DNA-conserved regions of different genotypes of hepatitis B virus (HBV) to promote the transcription of HBV inhibitor interferon (IFN)-stimulated responsive elements. The synergistic effect of IFN-α opens a new avenue for gene therapy of chronic hepatitis B (CHB).
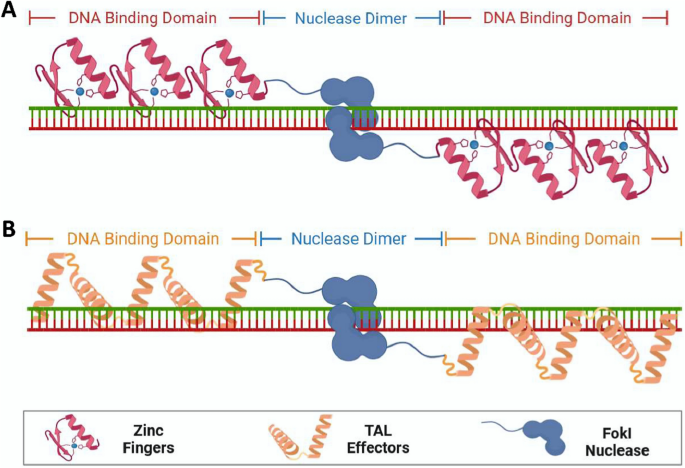
Mechanism of the action of therapeutic gene modification modality. A ZFNs. B TALENs. Created with BioRender.com. (Agreement number: LO26NU7V24)
Although TALENs are already an excellent gene modification technology, they are limited by the complexity of the preparation process, the large production cost, and the high number of amino acids involved [ 125 ]. Thus, the more versatile CRISPR-Cas9 has become the new generation of “DNA scissors” [ 128 , 129 ]. CRISPR, known as “Clustered Regularly Interspaced Short Palindromic Repeats,” is a specialized sequence found in bacteria and some archaea that plays an active role in defending the immune system against invasion by exogenous substances (such as phages) [ 130 ]. Cas9 refers to “CRISPR-associated protein 9,” an enzyme used to cut specific DNA sequences. Fujikura et al. [ 131 ] added a medium-chain triglyceride-containing ketogenic diet (MCTKD) to promote skeletal muscle regeneration in CRISPR-Cas9 gene-edited Duchenne muscular dystrophy rats. Furthermore, Freitas et al. [ 132 ] gene-edited MSCs with CRISPR-Cas9 to enhance gene expression of genes related to the BMP/TGF-β signaling pathway to overexpress BMP-9. CRISPR-Cas9 is a powerful genomic editing technology widely recognized for its broad range of applications, precise gene editing, and low cost (Fig. 4 ). However, this method has limitations such as immunotoxicity, low HR efficiency, PAM limitations, off-target effects, and differences in editing efficiency. Therefore, it is worthwhile to further improve it for therapeutic gene modification.
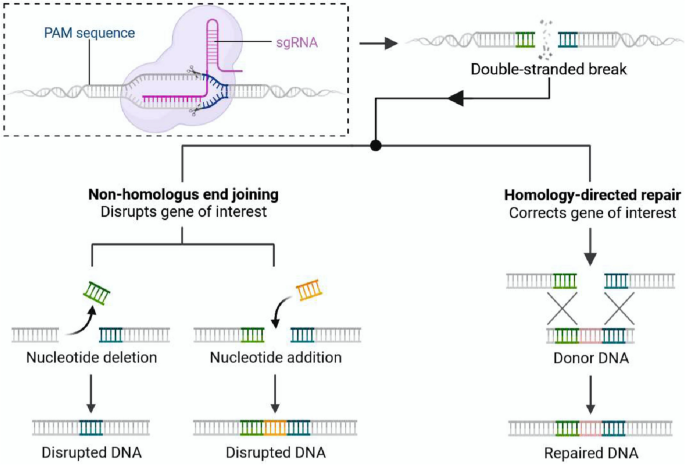
Principles of CRISPR-Cas9 gene editing technology. Created with BioRender.com. (Agreement number: ZT26NU7MMY)
The development of therapeutic genes has brought great hope for treating human diseases; however, they usually exhibit problems such as easy degradation, instability, mutability, and safety, and some may trigger immune responses, interference with healthy cells (such as neuronal cell damage or death), and the risk of tumors. Although ZFNs, TALENs, and CRISPR-Cas9 modifications are widely used in gene therapy, they have shortcomings. The activity of ZFNs is affected by the ability of zinc finger proteins to bind to DNA, as well as by factors such as the DNA sequence and the intracellular environment, rendering their action unreliable and limited. Although TALENs and CRISPR-Cas9 are characterized by high efficiency and precision, the construction and design of TALENs require customized modifications for different genomes, which are costly and suffer from immune responses and off-target effects. CRISPR-Cas9 still has limitations, such as off-target effects, immune responses, and gene polymorphisms. Therefore, future research should focus on improving the deficiencies of therapeutic genes and their modification.
Loading of therapeutic genes into hydrogels
Therapeutic genes are often affected by cellular barriers, enzyme sensitivity, and difficulty in transport to cellular compartments; therefore, carrier loading of therapeutic genes is necessary. Physical and chemical loadings are commonly used strategies; the former is stable, controllable, and free from the addition of chemical reagents, manifesting low or no toxicity. The chemical loading varies in toxicity owing to the addition of chemical reagents with flexible adjustability, which has attracted the interest of researchers. In the following sections, we organized the loading methods, principles, and characteristics of gene therapy (Table 3 ).
Physical loading
Freeze-drying method.
Freeze drying is a widely used method of gene loading that involves mixing of solvents and polymers, followed by freezing and lyophilization to force a pressure drop, which removes the water in the solution that drives the mixture to sublime, resulting in the formation of an interconnected porous structure [ 133 ]. During freeze drying, therapeutic genes are encapsulated in the hydrogel skeleton, thus avoiding the problem of gene activity being easily damaged by normal drying methods. Simultaneously, low-temperature drying conditions can reduce the possibility of gene mutations and maintain biological activity [ 134 ]. Fiorica et al. [ 145 ] obtained HA-EDA-g-α-elastin/HA hydrogels by freeze drying, which were outstanding mechanically and rheologically and compensated for the poor adhesion of HA cells. The sustained release of VEGF was effectively controlled in endothelial cell cultures to promote adsorption, proliferation, growth, and induction of tubular structure formation in human vascular endothelial cells (HUVECs), which are suitable for vascular neovascularization and soft tissue regeneration. Although freeze drying can reduce the mutation rate, the therapeutic genes in the hydrogel are encapsulated in a solid skeleton and pores or cracks may form after freeze drying, which can lead to poor release of therapeutic genes.
Injection method
The injection method is a loading method wherein genes are introduced into a hydrogel using high-pressure gas jet injection [ 135 ]. This injection method can evenly disperse therapeutic genes in the backbone of the hydrogel in a short time, improving the loading efficiency and reducing the loss of activity by avoiding direct contact with the external environment. Spraying siRNA-containing hydrogels onto arthritic mice specifically silenced genes related to the pathogenesis of rheumatoid arthritis (RA) and achieved an anti-RA induction effect [ 29 ]. Wang et al. [ 136 ] introduced a hydrogel containing siRNA against MMP-2 (siMMP2) in a mouse model of MI by injection. The hydrogel was eroded by proteases, resulting in the release of active siRNAs, which reduced the MMP2 content of cardiac fibroblasts and improved myocardial thickness in MI. Moreover, the hydrogel improved myocardial blood output, ejection fraction, and cardiac output in the MI region. The spraying process may lead to damage or deformation of the hydrogel structure since it requires the use of high-pressure or high-speed airflow, thus affecting its stability and release performance and reducing the effect of gene therapy.
Centrifugal coprecipitation method
Centrifugal coprecipitation enables efficient loading of therapeutic genes. Centrifugation allows the coprecipitation of therapeutic genes with hydrogel particles, thus achieving uniform distribution in the hydrogel. This method can improve the efficiency of gene loading and stability of genes, which is conducive to improving therapeutic effects [ 137 ]. The advantages of the centrifugal coprecipitation method are as follows: the loading process is simple, does not require complex equipment and operations, does not require the addition of chemical reagents, and has a relatively low impact on the integrity and activity of therapeutic genes. Furthermore, the centrifugal coprecipitation method loads gene therapy hydrogels with a denser structure, a higher degree of crosslinking, and greater controllability, which helps to realize the sustained release of therapeutic genes [ 138 , 146 ]. However, centrifugation can lead to structural changes in the hydrogel that affect its performance and release behavior. Therefore, the properties and structure of the hydrogel should be considered, optimized, and adjusted accordingly when centrifugal coprecipitation is used.
Chemical loading
Hydrogen bonds.
A hydrogen bond is an electrostatic interaction between H + and an extremely electronegative atom (typically nitrogen, oxygen, or fluorine) [ 139 ]. Hydrogen bonds are important for stabilizing the structure between molecules, catalyzing chemical reactions, aiding the intermolecular transfer of substances, and changing melting and boiling points [ 147 ]. For example, the rate of in vitro release of siRNA from HA-poly(vinyl alcohol) hydrogels is slower than that from poly (vinyl alcohol) hydrogels owing to the presence of more hydrogen bonds between the HA backbone and siRNAs [ 148 ]. Chen et al. [ 140 ] prepared siRNA@G5-PBA@Gel hydrogels by modifying therapeutic siRNA to silence the expression of P65 with G5-PBA under the effect of hydrogen bonding, which had a slow release of siRNA in vitro/in vivo and an inhibitory effect on inflammatory factors and is suitable for precision therapy of intervertebral disc (IVD) generation (IVDD). The different materials of the gene therapy hydrogel indicate that the material and molecular weight may affect the ability and stability of hydrogen bond formation and that the gene release behavior of the hydrogen-bonded hydrogel may be affected by environmental factors, leading to further destabilization of the hydrogen bond that can affect the efficacy of the final tissue engineering application.
Ionic bonds
Ionic bonds are chemical bonds formed by charge interactions between metal and nonmetal ions and have high melting and boiling points [ 149 ]. Ren et al. [ 12 ] prepared SM hydrogels by crosslinking with Ca 2+ to compensate for this deficiency. Li et al. [ 40 ] demonstrated that an ionic product synergized with a small interfering RNA for MMP-9 (MMP-9-siRNA) improved the synthesis of the ECM protein and decreased the expression of MMP-9 in cells. Chitosan particles loaded with MMP-9-siRNA in 45S5 Bioglass®/SA hydrogels significantly reduced MMP-9 expression, promoted collagen synthesis, and accelerated vascular regeneration and wound healing rate, showing great potential in diabetic wounds. However, the gene release of ionic bonding can be affected by environmental factors such as pH, ionic strength, and temperature, and a small portion of the ionic bonding process can produce harmful byproducts that can have toxic effects on cells; the ionic bonding of the hydrogel lacks resilience and mechanical strength, which can affect the effectiveness of gene therapy.
Covalent bonds
Covalent bonds form stable structures by sharing one or more electron pairs to fill the valence electron layers [ 150 ]. Cai et al. [ 43 ] prepared nanoparticles with siRNA and modified them to prepare GelMA MS. Subsequently, HA-ADH and HA-CHO were coupled and used to modify siRNA@MS using the hydrazone bond as a linkage to obtain the siRNA@MS @HA hydrogel, which showed good elasticity, obvious self-repair in the rheology test, and release of on-demand siRNA, which was effective in preventing tendon sheath adhesion in the damaged part of the rat tendon. Covalent bonds are more stable; however, the release of therapeutic genes in the covalent bond is usually irreversible, which limits the effectiveness and durability of gene therapy, contrary to the sustained slow release required for traumas such as bone defects, rendering it difficult to achieve good therapeutic effects.
Hydrophobic interactions
Hydrophobic interactions occur when a substance is in contact with water and repels and prevents water molecules from entering its interior. Substances with similar hydrophobic properties attract each other, whereas those with different properties repel each other [ 151 ]. Hydrophobic interactions mediate the self-assembly of succinylbutanediol nanomaterials to prevent breast cancer metastasis by inhibiting the expression of VCAM-1 [ 144 ]. Kim et al. [ 152 ] prepared polyethyleneimine-poly(organophosphoronitrile) conjugates coupled with siRNA to form composites and transformed them through hydrophobic interactions into complex hydrogels, which induced slow release of polyplexes through solubilization and degradation and achieved antitumor effects through cell cycle protein B1 gene silencing. However, the ratio of the hydrophobic to hydrophilic regions of such hydrogels must be strictly adjusted to maintain the stability of their hydrophobicity, thus making the loading modification of gene therapy hydrogels extremely stringent.
The loading of therapeutic genes into hydrogels has made progress, but it still faces challenges. Physical loading is a notable approach owing to its relative simplicity of operation, biocompatibility, and controllability; however, it suffers from shortcomings such as low loading efficiency and difficulty in controlling release behavior. The physical loading of hydrogels may lead to gene aggregation or precipitation, which is insufficient for efficient gene therapy. Although chemical loading is characterized by controllability and high loading efficiency, its immunogenicity, cytotoxicity, and stability still do not meet the requirements; chemical loading can inactivate and denature, and the stability and durability of chemical bonding cannot meet the demand. Smart-responsive cross-linking agents, cell-affinity modifications, development of novel non-toxic, low-immunogenic materials, and combining gene therapy with other therapeutic modalities (photothermal therapy, immunomodulation, etc.) should be the key strategies for future research on loading therapeutic genes into hydrogels.
Characterization of gene-loaded hydrogels
To enhance therapeutic gene protection, improve delivery efficiency, and ensure transfection efficiency, detailed characterization of loaded gene hydrogels is essential. Parameters such as the aperture size, mechanical properties, and transfection efficiency of hydrogels need to be evaluated.
Aperture size
Aperture size is a key parameter for loading gene hydrogels and is an important indicator of the hydrogel pore structure, loading capacity, and gene release rate. A large aperture size provides more space for the loading of therapeutic genes but leads to an excessively fast release, whereas a small aperture limits the amount of gene loading and the release rate [ 153 ]. The aperture size can be measured using the nitrogen adsorption–desorption technique, the pressed mercury method, and the aperture size analyzer [ 102 , 154 ]. Gene therapy hydrogels are commonly desired for slow release in tissue engineering applications that include cartilage repair, spinal cord repair, and tumor therapy; therefore, the aperture size is more demanding. In cardiac repair, the aperture size of the hydrogel should be appropriate to avoid being too large, which results in blood vessel blockage. Similarly, eye treatment also requires an appropriate selection of aperture sizes. Therefore, the aperture size should be planned according to the expected loading and release requirements when designing gene carriers. The design and regulation of aperture size must be further optimized based on a more comprehensive understanding of its mechanisms of action to achieve more efficient, safe, and precise gene therapy [ 153 ].
Mechanical properties
Mechanical properties refer to the mechanical properties of a material under force, including strength, toughness, hardness, and ductility. The mechanical properties of hydrogels play prominent roles in cell proliferation, differentiation, and organ recovery. Hydrogels often have poor mechanical properties; thus, they are difficult to meet the requirements of tissue compression. Therefore, researchers have made significant efforts to improve these properties of hydrogels [ 13 ]. The mechanical properties of hydrogels can help researchers assess how stable and deforming they become. A high level of stiffness and stability provides better results for the encapsulation and protection of genes and ensures the stability and continuity of gene delivery. Bone-regeneration hydrogels with excellent mechanical properties can effectively support damaged bones. The mechanical properties of gene-loaded hydrogels are significant factors that affect their use as gene carriers. The hydrogel mechanical properties and preparation process need to be further optimized to provide more suitable and stable carriers for gene therapy.
Biocompatibility
Biocompatibility refers to the ability of a material to co-exist with tissue cells in vivo without causing significant immune or inflammatory reactions. Good biocompatibility is a prerequisite for efficient gene delivery and tissue repair in gene therapy hydrogels. In gene therapy, commonly used hydrogel materials (including natural macromolecules such as collagen and HA and synthetic macromolecules such as polyvinyl alcohol and polyacrylamide) have good biocompatibility and can interact with tissue cells and promote cell adhesion and differentiation [ 18 ]. While biocompatibility primarily concerns the interactions between the material and the organism, safety is further refined to the potential risks or side effects that may result from these interactions. Therefore, at the level of material safety, gene-loaded hydrogels must undergo an exhaustive toxicological evaluation. The loaded genes must also be rigorously screened and validated to ensure that they do not contain harmful sequences and to avoid adverse reactions [ 155 ]. Therefore, the biocompatibility of gene-loaded hydrogels influences their applications in tissue engineering.
Degradability
Degradability refers to the ability of a material to break down and be excreted from the internal environment of the body, thus ensuring that the hydrogel does not produce toxic and inflammatory reactions in the human body. It directly affects the rate of hydrogel decomposition; therefore, it is important to control gene release and maintain its duration. Bone-regeneration hydrogels should not degrade too quickly and should have adequate mechanical support and excellent mechanical properties [ 115 ]. Researchers have designed degradable hydrogels to piggyback exosomes to maximize the efficacy of skin wound healing (Fig. 5 A) [ 64 ]. In wound repair, hydrogel degradation maximizes the conditions for vascular infiltration and provides advantages for painless gel removal; therefore, good degradability is necessary. The rate of degradation of gene therapy hydrogels is determined by the type of material, the crosslinking method, and tissue coordination. Meanwhile, the degradability required for tissue repair also varies, which undoubtedly highlights the flexible, customizable, and controllable advantages of synthetic hydrogels. Degradation products of hydrogels must be harmless to the human body from the point of view of degradability. Moreover, the speed of degradation rate is also critical. Degradation that is too fast may result in the loaded genes not functioning adequately; degradation that is too slow may trigger long-term inflammatory or foreign body reactions. Therefore, long-term safety assessment is necessary. Animal and clinical trials can help visualize the degradation of hydrogels in vivo, the effect of degradation products on surrounding tissues, and possible immune responses.
Characterization of gene therapy hydrogels. A Characterization of 8P-TS Exogel. (i) Transmission electron microscopy images; (ii) Expression of exosomal tetraspanin; (iii) Cellular uptake of M2-Exos or RM2-Exos. (Reproduced with permission from Ref. [ 64 ], Copyright 2022, John Wiley and Sons). B Characterization of the CHA/SF/GCS/DF-PEG hydrogel. (i) Self-healing of hydrogels; (ii) Flow cytometry analysis; (iii) LIVE/DEAD staining. (Reproduced with permission from Ref. [ 65 ], Copyright 2020, Frontiers). C Scanning electron microscopy image. (i) Scanning electron microscopy image of microsphere hydrogels; (ii) SEM image of hair after several days of microsphere hydrogel treatment. (Reproduced with permission from Ref. [ 156 ], Copyright 2022, Royal Society of Chemistry). D Correlation characterization of Exos-Ag@BSA NFs/Col hydrogels. (i) Masson’s trichrome staining; (ii) Exos loaded on Ag@BSA NFs. (Reproduced with permission from Ref. [ 31 ], Copyright 2023, John Wiley and Sons)
Mechanism of hydrogels
Hydrogels can be cross-linked by physical or chemical means, as described in Sect. “ Crosslinking of hydrogels ”. Physically cross-linked hydrogels can be formed through interactions, but they usually exhibit instability and reversible crosslinking; they also cause morphological and structural changes when subjected to external influences (such as temperature, pH, oxidative stress, light, and ionic strength). Therefore, they are mainly used in self-healing hydrogels and responsive hydrogels, which are also useful in bone regeneration, skin trauma, and tumor therapy [ 157 ]. Chemically cross-linked hydrogels exhibit excellent stability and mechanical properties, and the mechanism of the gel can be adjusted by the addition of chemical crosslinking agents during the gelation process. However, this can reduce gel water absorption and cause toxicity to the realization of therapeutic genes, which cannot be ignored before the applications of chemical cross-linked hydrogels in tissue engineering [ 10 ]. Physical and chemical crosslinking has its advantages and disadvantages; however, they also provide a variety of options for different areas of tissue engineering.
Transfection efficiency
Transfection is the introduction of an exogenous gene into a cell, and transfection efficiency is a measure of the degree of success of the introduction of an exogenous gene during transfection. Transfection efficiency directly affects gene delivery and expression. Methods used to assess transfection efficiency include fluorescence staining, PCR analysis, western blotting, and flow cytometry [ 158 , 159 ]. RNA is highly susceptible to degradation by RNase in vitro, and there is a problem of low transfection efficiency when it is directly mixed with the hydrogel. Therefore, researchers encapsulated therapeutic genes in liposomes and polymeric NP vectors and loaded them onto a hydrogel to improve their transfection efficiency (Fig. 5 C) [ 156 ]. Hydrogel microspheres (MSs) have attracted considerable attention in the field of minimally invasive tissue repair, and amino acid coupling is a popular method for enhancing gene transfection efficiency [ 160 ]. The high transfection potency of the complexes prepared for the release of miR-140 nanoparticles compensated for the short half-life, low transfection rate, and susceptibility to inactivation caused by direct injection of crude miRNAs [ 161 ]. During hydrogel transfection, the transfection efficiency is affected by numerous factors, including the composition, structure, and property of the hydrogel and the type and state of the introduced recipient cells, which affect the final effect of gene therapy.
The safety assurance of gene therapy hydrogels requires comprehensive consideration in several dimensions. In terms of material selection, preference should be given to hydrogel materials with superior biocompatibility and low immunogenicity, aiming to minimize potential irritation and immune response to the body. At the same time, the degradation rate of the material needs to be compatible with the natural process of wound healing to ensure that it does not adversely affect the neoplastic tissue. Secondly, optimization of the preparation process is also a key step in ensuring safety. By improving the preparation technique, the impurities and residues in the hydrogel can be effectively reduced, and its purity and stability can be enhanced [ 6 ]. Furthermore, the design of gene carriers (e.g., hydrogels) should also take safety and efficacy into full consideration. The use of vectors with specific targeting can accurately deliver genes to target cells and avoid interference with non-target cells. At the same time, factors such as toxicity, immunogenicity, and stability of the vector should also be fully evaluated to ensure its safety in vivo. Moreover, a personalized treatment plan should be developed according to the patient’s specific condition and wound healing needs. In the selection of genes, the use of genes with well-proven efficacy and high safety should be favored, and the regulatory mechanism of gene expression should be taken into account to prevent the possible risk of gene overexpression or abnormal expression. Finally, gene therapy hydrogels must be thoroughly evaluated and tested before clinical application. This includes, but is not limited to, an in-depth evaluation of its biocompatibility, degradation rate, gene transfection efficiency, drug release kinetics, and other properties, as well as a rigorous prediction and assessment of potential risks and side effects [ 54 ]. These efforts will help reduce potential risks and enhance the safety and efficacy of gene therapy hydrogels in tissue engineering repair, leading to safer and more effective treatment options for patients [ 155 ].
Other characterizations
Hydrogel chargeability affects the loading and release of genes, and the adsorption and release properties affect the loading and release efficiency of genes. In tumor therapy, excessive hydrogel crosslinking hinders the release of RNA and reduces the therapeutic efficiency. Hydrogels with controlled crosslinking can be released on demand for gene therapy and continue to act on target tissues [ 161 ]. The attractiveness of the bone tissue hydrogel should be sufficient to mimic high-water content ECM to provide the environment needed for bioactive molecules or damaged cells. Controlled drug release should also be noted. Hydrogels used in trauma repair should be safe, anti-infectious, and appropriately dosed to ensure safe and effective therapeutic results (Fig. 5 D) [ 32 ]. Gene therapy hydrogels have great potential for cartilage repair, although careful consideration should be given to the type of gene, the delivery system, the tissue engineering scaffold, safety, efficacy, and durability before use. In spinal cord therapy, attention should be paid to gene type, delivery system, treatment time, dosage, long-term efficacy, and monitoring. To achieve gene therapy more efficiently, it is important to understand and evaluate its various characteristics to guide tissue repair. The demands for different hydrogels with different physicochemical properties and characterization parameters are also different and worth discussing.
The characterizations of the gene-loaded hydrogel affect its use as a gene carrier, and the physicochemical properties of the hydrogel can be understood to optimize its preparation process and performance through characterization tests. The biocompatibility and safety of the gene therapy hydrogel in cells and organisms can be evaluated using this method. Gene therapy hydrogels should be designed and applied with a greater focus on the need for personalized treatment. Different diseases and different patients have different gene expression and treatment needs. Therefore, hydrogels with specific gene drug and release properties should be tailored to achieve personalized and precise treatment. In order to achieve this goal, new hydrogel materials with excellent biocompatibility, stability, and controlled degradation should be developed; gene drugs with high efficiency, low toxicity, and high specificity should be screened; and the structure and properties of hydrogels should be modulated to achieve precise drug release and targeting.
Hydrogel designs to enable therapeutic gene release
The release of therapeutic genes cannot be achieved without the design of the carrier. Hydrogels are a class of materials suitable for this purpose. The design of smart-responsive hydrogels is a priority because these hydrogels respond to changes in temperature, pH, light, and other factors. Furthermore, nanomaterials/microspheres loaded with genes and combined with hydrogels are a key design strategy that can increase the carrying capacity of the genes, protect the genes, and slow the release of the genes to better realize gene therapy for damaged tissues.
Responsive hydrogels
Temperature-responsive hydrogels.
Temperature-responsive hydrogels were extensively studied for piggybacking and the release of therapeutic genes, which do not require additional copolymerization agents or chemicals [ 162 ]. Hong et al. [ 163 ] prepared temperature-responsive hydrogels with imidazole-poly(organophosphazenes) that interact with macrophages and histamine receptors to produce MMP-9, remodel the fibrotic ECM, and reduce the bridging effect. Liu et al. [ 164 ] reported that miR-130a secretion modulates BMSC-sEVs, which stimulate the PTEN/AKT signaling pathway to promote bone regeneration. Wu et al. [ 78 ] prepared a temperature-responsive hydrogel with β-glycerophosphate-modified chitosan to rely on BMSC-derived sEVs, which had excellent temperature-sensitive properties, biocompatibility, and stability, and the BMSC-derived miR-21 exosome miR-21 promoted vascular regeneration by targeting SPRY2.
pH-responsive hydrogels
pH-responsive hydrogels comprise cross-linked polymers containing specific functional groups that absorb excess water under acidic conditions, resulting in volume expansion [ 22 ]. pH-responsive hydrogels can be created by atom transfer radical polymerization (ATRP). pH-responsive carboxymethylcellulose hydrogels activate the VEGF signaling pathway by loading plasma exosomes, leading to wound repair in type 1 diabetic mice [ 165 ]. Khaled et al. [ 166 ] reported a pH-responsive hydrogel on which siRNA loading could be used to slow the release of siRNA by cation-adjusting the pH of the hydrogel. The siRNA was safeguarded against enzymatic degradation and in vivo microenvironmental influences. After intravenous administration, CXCR4 siRNA was delivered to the cytoplasm of breast cancer cells to silence the expression of the CXCR4 protein.
Hydrogels responsive to oxidative stress
Oxidative stress-responsive hydrogels typically comprise polymers containing oxidation-sensitive functional groups that react with oxides, resulting in changes in the volume and structure of the hydrogel and responsive behavior by releasing specific substances. In gene therapy hydrogels, oxidative stress responsiveness allows site-specific targeting of genes to treat tissue damage [ 40 ]. For example, atopic dermatitis can be treated by modulating oxidative stress [ 167 ]. Lei et al. [ 168 ] prepared a biodegradable tannic acid-siRNA nanohydrogel based on environmental reactive oxygen species (ROS) stress, the release of therapeutic agents into cells to silence the expression of the MMP-9 gene, synergistically advancing macrophage polarization and vascular regeneration to reduce the degree of chronic inflammation in wounds and promote wound healing.
Pressure-responsive hydrogels
Pressure-responsive hydrogels that respond to changes in external pressure typically comprise cross-linked polymer networks containing polymer chains that slide over each other. Hydrogels that respond to pressure contain a drug-containing substance released after pressure stimulation [ 169 ]. Zhao et al. [ 170 ] screened siRNA that effectively interferes with TGFβ1 expression and used it to prepare an siRNA-TGFβ1-337 transdermal patch, which is a combination of siRNA-TGFβ1-337 and pressure-responsive adhesive hydrogel. It downregulates the expression of type I collagen, promotes scar fibroblasts, and reduces scar size. In conclusion, pressure-responsive hydrogels are smart materials with important applications; however, their properties and applications require further exploration.
Photo-responsive hydrogels
Photo-responsive hydrogels comprise polymeric materials (polyvinyl alcohol, acrylamide, and amphoteric polymers). When the material is exposed to specific wavelengths, its crosslinks break, leading to gel dissolution [ 171 ]. Photo-responsive hydrogels are suitable for cell culture and controlled release of therapeutic genes and can be used for tissue engineering therapies [ 26 ]. Huynh et al. [ 171 ] reported a light-responsive hydrogel for on-demand delivery of genes that modulated siRNA release without impairing the biological activity upon exposure to UV light. Yang et al. [ 172 ] designed a photo-responsive hydrogel based on a photoinduced imine crosslinking (PIC) reaction. Under light irradiation, an antiadhesion barrier attached to the tissue surface was formed, and L929 cells showed excellent cytocompatibility. Light-responsive hydrogels have important applications in tissue engineering; however, excessive UV irradiation can lead to mutations in gene therapy drugs, reducing the therapeutic efficacy.
Ionic-strength responsive hydrogels
Based on the interaction of specific functional groups on the polymer chain with ions in solution, the functional groups adsorb or desorb ions when the concentration of ions in the environment changes, thus causing changes in the gel volume and structural rearrangements. Zhou et al. [ 10 ] prepared SA-polyacrylamide hydrogels by crosslinking them with different concentrations of Cu 2+ , Ca 2+ , and Zn 2+ . This has substantially improved the antimicrobial and mechanical strength of the hydrogel. The in vitro molecular biology assay showed good expression of VEGF and TGF-β, and the compatibility in Escherichia coli was outstanding, which is suitable for trauma repair. Li et al. [ 72 ] designed an SA/HS hydrogel to accelerate the rate of epithelial tissue and vascular regeneration for wound healing using Ca 2+ as a crosslinking agent. However, there are few specific applications for ionic-strength-responsive hydrogels, and their specific properties and potential applications require further investigation.
Nanomaterials loaded with genes and combined with hydrogels
Nanomaterials typically range in size from 1 to 100 nm, and the biological properties of the material significantly change (Fig. 6 A) [ 14 ]. RNA loading into nanomaterials combined with hydrogels can improve the retention time around tumors and the uptake of RNA into cancer cells [ 173 ]. Qin et al. [ 174 ] prepared miR-29b/GO-PEG-PEI@chitosan hydrogel that shows excellent miR-29b loading capacity and delivery efficiency. In vivo experiments showed that transfection of BMSCs significantly promoted their osteogenic differentiation and did not trigger inflammatory responses. Kim et al. [ 175 ] coupled protamine with poly(organophos-phazene) and loaded siRNA nanocomplexes onto it to create a nanohydrogel that was injected into the organism. The loaded siRNA complexes crossed the cellular barrier to achieve high efficiency of delivery and reduce the expression of the corresponding genes and proteins. Furthermore, NPs containing RNA therapeutic agents (such as miRNAs, siRNA, and siRNAs) are embedded in the hydrogel matrix, which is then implanted at the tumor site for tumor gene therapy [ 176 ]. However, there are challenges and problems associated with this combination strategy. First, the combination of nanomaterials and hydrogels may affect their biocompatibility and drug-release properties. Second, this combination may increase the complexity and cost of treatment. More research is needed to validate the safety of this binding strategy for the treatment of tissue trauma.
Hydrogel design for therapeutic gene release. A Nanomaterials loaded with genes and combined with hydrogels. (Reproduced with permission from Ref. [ 14 ], Copyright 2021, Elsevier). B Microspheres loaded with genes and combined with hydrogels. (Reproduced with permission from Ref. [ 177 ], Copyright 2017, Royal Society of Chemistry)
Microspheres loaded with genes and combined with hydrogels
Microspheres are a class of tiny spheres formed by the dissolution of drugs in polymeric materials, with a particle size of 1–250 μm, suitable for gene encapsulation or adsorption on their surface to ensure slow drug-release efficiency [ 178 ]. They have low toxicity, are degradable and safe, have a special affinity for some cell tissues, and can be endocytosed by the reticuloendothelial system (RES) to achieve targeting. Moreover, the route of administration of microspheres is mainly injection. This can bypass the first-pass effect, improve utilization, and reduce the dose of administration (Fig. 6 B) [ 177 ]. Phase separation, hot-melt extrusion, emulsification, volatilization, and spray drying are common methods of microsphere preparation [ 179 , 180 ]. Microspheres play an important role in numerous fields. Gan et al. [ 181 ] used MSC-derived exosomes (MSC-Exos) externally encapsulated in SA hydrogel microspheres to protect against degradation in the intestinal environment as an agent to treat intestinal inflammation. Furthermore, the microspheres reduced the levels of pro-inflammatory cytokines in damaged colonic epithelial cells and inflammatory macrophages, which is highly effective in treating acute colitis. DiStefano et al. [ 182 ] embedded MSC-Exos in a poly(lactic-co-glycolic acid) microsphere and combined it with a hydrogel, a system that sustainably delivers MSC-Exos for fibrotic repair under the protection of cells from a denaturing and pro-inflammatory environment. The system stimulates cell proliferation and migration by promoting sustained treatment of IVDD by promoting stimulation of cell proliferation and migration. The combination of microsphere-loaded genes with hydrogels represents an advanced strategy for creating hydrogel gene therapy systems with adaptive and self-healing properties. This system has the advantage of enabling sustained and controlled gene release while safeguarding them from the in vivo environment. Hydrogels can also be used as reservoirs for drugs and genes to further enhance their therapeutic effects.
The release of therapeutic genes cannot be separated from the carriers, and smart-responsive hydrogels are the preferred design strategy. Some hydrogels are characterized by high sensitivity and an adjustable structure, especially physically cross-linked hydrogels that can respond to external stimuli by changing the pathways of swelling, contraction, and sol–gel phase transition to achieve targeted gene release at specific sites. However, there is a need to emphasize the improvement in controlling environmental stimuli in some performances [ 72 ]. Microspheres/nanomaterials loaded with genes and then combined with hydrogels are a key design strategy that increases the gene-carrying capacity, protects the genes, and facilitates the slow release of genes. However, the microsphere production process is complicated, the sterilization cost is high, the encapsulation rate is low, and the safety of the nanomaterials and the preparation cost must be considered. Therefore, the release of therapeutic genes needs to be further investigated. We hope that with continued scientific research these difficulties can be solved.
Tissue engineering applications
Hydrogel biocompatibility, encapsulability, and plasticity are suitable for repair after tissue damage. This review focused on gene therapy hydrogels within bone regeneration, cardiac repair, cartilage repair, spinal cord injuries, skin trauma, eye disorders, tumor treatment, osteoarthritis, brain disorders, inflammatory bowel disorders, Rheumatoid arthritis, and hair regeneration. The delivery gene, hydrogels, and their therapeutic effects in tissue engineering applications are shown in Table 4 .
Bone regeneration
An increasing number of patients are experiencing fractures, bone defects, and osteoporosis, often owing to trauma, car accidents, and tumor ablation [ 195 ]. A fracture is defined as a fracture of the bone caused by trauma or pathology, presenting with clinical manifestations such as localized pain, swelling, and ecchymosis. Stem cell and biofactor therapies are commonly used for these conditions [ 196 ]. Demineralized bone matrix (DBM) is a biomaterial produced by removing the mineral content of the bone and is used to repair fractures and bone defects. However, commercially available DBM products often employ calcium sulfate, SA, and HA as delivery vehicles. These substances can spread throughout the body, causing inflammation and diminishing bone regeneration [ 197 ]. Studies have highlighted the effectiveness of Exos hydrogel in treating fracture nonunion. The hydrogel’s excellent adjustability and hydrophilicity make it suitable for mimicking the natural bone tissue environment. Yu et al. [ 117 ] demonstrated sustained release of miRNA antagonists using a sensitized hydrogel, incorporating Exos loaded with antagomiR-708-5p onto HA hydrogels to treat femoral fracture nonunion in mice. AntagomiR-708-5p promotes the Wnt/β-catenin signaling pathway, enhancing the osteogenic differentiation of organelle cells. Compared with the blank group, the hydrogel exhibited improved antibacterial and anti-inflammatory properties, biocompatibility, and mechanical support, leading to enhanced bone differentiation and accelerated fracture healing (Fig. 7 A).
Applications of therapeutic gene hydrogels in bone regeneration. A Effect of Exos hydrogel on osteogenesis of bone marrow mesenchymal stem cells. (i) Exosomal miR-708-5p inhibits osteogenic differentiation of BMSCs; (ii) Exos hydrogel to treat infected bone nonunion. (Reproduced with permission from Ref. [ 117 ], Copyright 2022, American Chemical Society). B Accelerated bone repair in rats with uMSC-derived Exos. (i) Schematic diagram of uMSC-derived Exos accelerating bone repair in rats; (ii) Masson’s trichrome staining; (iii) promoting neovascularization; (iv) realizing bone repair. (Reproduced with permission from Ref. [ 183 ], Copyright 2021, American Chemical Society)
Bone regeneration is influenced by factors such as osteoblasts, osteogenic spiking factors, and osteogenic scaffolds. The HA hydrogel is an outstanding osteogenic scaffold [ 42 , 198 ]. Exosomes contain a variety of RNAs and are a friendly osteogenic factor [ 16 ]. Zhang et al. [ 183 ] developed uMSCEXOs/Gel/nanohydroxyapatite/poly-ε-caprolactone (nHP) hydrogels by wrapping uMSCEXOs on an HA-Gel modified with nHP. In a rat cranial bone defect model, the gel induced increased expression of angiogenesis-promoting factors, such as VEGFA and HF-1α, through upregulation of the NOTCH1/DLL4 pathway, which promotes vascular neovascularization and osteogenic differentiation. The hydrogel also promoted endothelial progenitor cell proliferation and angiogenesis, providing strong mechanical support for improved bone regeneration (Fig. 7 B). However, gene therapy hydrogels must meet a number of stringent requirements if they are to be effective in the field of bone regeneration. These include suitable biocompatibility, sufficient mechanical strength, excellent osteogenic properties, excellent integration ability, and long-lasting slow drug release. In particular, hydrogels must meet certain standards in terms of mechanical strength, otherwise, it will be difficult to meet the needs of the bone regeneration field. Therefore, the application of gene therapy hydrogels in the field of bone regeneration still needs to undergo in-depth research and exploration to promote the progress of material science and bring substantial benefits to patients.
Heart repair
Heart injury is abnormal damage to the structure or function of the heart, blood vessels, nerves, and other tissues caused by external or internal factors [ 199 ]. Common manifestations include MI, heart valve disease, cardiomyopathy, coronary artery disease, post-surgical cardiac adhesions, and other related diseases [ 200 ]. Although medication, surgery, and intervention are commonly used repair methods, some drugs may cause side effects with persistent effects on the organism. Gene therapy hydrogels offer a solution by achieving stable and continuous delivery of therapeutic genes to the target location to repair injuries [ 201 ]. Zhu et al. [ 184 ] prepared MA-HA hydrogels with methacrylic anhydride (MA)-cross-linked HA and loaded Exos secreted by MCSs onto the hydrogels. Exos injected through iPC had a prolonged residence time in the cardiac organ, inducing the proliferation and differentiation of epicardial-derived cells, increasing epicardial thickness, and reducing fibrosis after MI.
Myocardial infarction (MI) results from myocardial hypoxia and ischemia owing to coronary blood flow obstruction leading to necrosis of myocardial tissue. Its treatment focuses on promoting functional recovery of the myocardium and local blood circulation [ 202 ]. Wang et al. [ 185 ] developed a miR-302-targeted HA hydrogel for cardiomyocyte proliferation and repair after cardiac ischemic injury. The hydrogel combined large tumor suppressor 2 and macrophage-stimulating 1 to inhibit Hippo signaling and activate the expression of cell proliferation-related genes. Spectral tracing, fluorescence scanning images, and clonal cell analysis showed that the MI mouse hearts injected with the hydrogel reactivated the cell cycle of cardiomyocytes and promoted its sustained proliferation, which is suitable for cardiac regeneration and improving related problems after MI. In conclusion, gene therapy hydrogels emerge as an excellent option for heart repair owing to their minimally invasive, long-lasting, and relatively safe nature. Non-toxicity is a prerequisite for cardiac repair, and good biocompatibility is essential to prevent immune rejection. The therapeutic gene hydrogel should offer suitable mechanical support, with characteristics similar to those of rheological suitability, ensuring that it does not excessively affect blood flow. As medical advances continue, there is hope for a new era in heart repair.
Cartilage repair
Cartilage is a translucent, elastic, and tough tissue that plays a supportive and protective role in organisms [ 26 ]. There are several causes of cartilage damage, including ischemia, traumatic injury, and degenerative diseases [ 203 ]. Osteoarthritis (OA) causes cartilage cells in the joints to age, causing cartilage damage [ 42 , 204 ]. miR-29b-5p upregulates TET1 to repress the expression of P16INK4a/P21, senescence-associated genes, and MMPs, slowing chondrocyte senescence and repairing defective cartilage by improving the microbial milieu [ 205 ]. Zhu et al. [ 186 ] loaded agomir-29b-5p onto a self-assembled nanofiber hydrogel to induce the homing of MSCs. The anterior cruciate ligament transection in rats showed that the hydrogel had outstanding mechanical properties, good biocompatibility, moderate pore size, and could slow chondrocyte senescence through the P16INK4a/P21 pathway. Zhang et al. [ 47 ] prepared an injectable alginate-dopamine (AD), CS, and regenerated silk fibroin (AD/CS/RSF) hydrogel that has strong adhesion and can efficiently promote efficient proliferation of BMSCs after loading with Exos. This is an innovative strategy for the minimally invasive treatment of cartilage defects.
Exosomes contain various nucleic acids commonly used for cell proliferation, cartilage repair, and other purposes. Bone marrow stem cell-derived Exos can secrete trophic factors such as TIMPs and TFG-β through a paracrine mechanism to promote articular chondrocyte functionalization [ 206 ]. Although TEM did not reveal morphological differences between hypoxia-preconditioned exosomes (H-Exos) and normoxia-preconditioned exosomes (N-Exos), the former was more easily internalized in articular chondrocytes and more effective in promoting migration, proliferation, anti-inflammation, and matrix synthesis. Furthermore, it upregulated Exos miR-205-5p expression, efficiently activated the PTEN/AKT pathway, inhibited the expression of RUNX2, inhibited inflammation, promoted chondrocyte proliferation, migration, and metabolism, and repaired defective cartilage [ 207 ]. However, Exos are locally administered and easily removed. Shen et al. [ 187 ] modified the H-Exos hydrogel to achieve an efficient and sustainable release of Exos and achieved significant results in the repair of cartilage defects in rat models. Hydrogels as carriers for gene therapy can protect and deliver genes, ensuring that they act on damaged cartilage.
Spinal cord injury
The spinal cord located within the spinal column is an important neural structure that connects the brain to the trunk, transmits information, and coordinates body functions. Spinal cord injury (SCI) causes neurons and nerve fibers to break down, resulting in the inability of nerve signals to be properly transmitted, affecting the body’s sensory, motor, and autonomic functions of the body [ 208 ]. It can be subdivided into primary and secondary SCI, with the first being damage owing to external forces acting on the spinal cord and the second being further damage to the spinal cord resulting from compression of the spinal cord owing to hemorrhage of small blood vessels in the spinal canal to form hematomas, spinal cord edema, compression fracture, and fragmentation of intervertebral disk tissue [ 209 ]. Bone MSC-derived exosomes were used as therapeutic agents for SCI. Cheng et al. [ 210 ] prepared a GelMA-Exo delivery system by loading Exos with GelMA, which significantly improved Exo retention, promoted neuron differentiation and extension, induced neurogenesis, and significantly reduced scar formation.
Bone marrow stem cell-derived H-Exos-encapsulated miR-216a-5p repairs traumatic spinal cord by inducing glial cell M1/M2 polarization to sustain inhibition of TLR4/NF-κB and activation of PI3K/AKT signaling pathway [ 188 ]. Pluronic F-127 hydrogel-mediated shRNA induces the proliferation of neuronal and myeloid cells by inhibiting Lingo-1 expression after bone marrow fracture in rats [ 211 ]. The USC-Exo hydrogel injected into the SCI model crossed the spinal cord blood–brain barrier and delivered ANGPTL3 to damaged areas. Therefore, it mediates the PI3K/AKT signaling pathway, promotes vascular regeneration, and restores the neurological function of the spinal cord [ 189 ]. miR-124 is associated with pro-neuronal differentiation of neural precursor cells, which inhibit inflammatory cytokine production and prevent deterioration of the damaged spinal cord. Louw et al. [ 212 ] intraperitoneally injected chitosan hydrogel with miR-124 into rat SCI, which significantly inhibited the expression of MHC-II and TNF-α. Reduced ED-1 macrophage content in damaged spinal cord inhibits secondary neuronal damage and inflammatory effects caused by microglia/macrophage secretion proteins.
Gene therapy hydrogels are an effective SCI treatment strategy, whereby the delivery of the target gene to the targeted location is achieved with the advantageous action of the hydrogel, leading to treatment of the spinal cord treatment. The selection of appropriate genes (such as exosomes or miRNAs) that promote nerve cell growth and connectivity for better functional recovery of the damaged spinal cord is critical for spinal cord therapy. Safety, long-term efficacy, monitoring, and biosolubility are also issues of concern.
Skin trauma
The skin comprises three layers: the epidermis, dermis, and subcutis. It is the largest organ of the body, covering the surface of our body and protecting it from the environment [ 36 , 213 ]. However, the human body often experiences skin trauma owing to external forces. Medical advances have led to the development of effective treatments for skin trauma. Gene therapy hydrogel is an emerging treatment method with the advantage of realizing the efficient delivery of therapeutic genes and inducing the regeneration of damaged skin (Fig. 8 A) [ 214 ]. Exosomes derived from human adipose-derived MSCs (hADSCs) are effective for skin trauma and improve Exo retention after in vivo transplantation. Zhou et al. [ 215 ] used the heat-sensitive Pluronic F-127 hydrogel to encapsulate hADSCs-Exos to promote wound healing and regeneration, which significantly increased the expression of α-SMA, CD31, and Ki67, and reduced the inflammatory response by upregulating the expression of AQP3 and KRT1 skin barrier proteins. This led to the repair of the skin wounds.
Applications of therapeutic gene hydrogels in skin trauma. A Exosomes promote skin wound healing. (i) Schematic diagram of Exos and macrophage (ϕs) preparation; (ii) promotion of wound healing. (Reproduced with permission from Ref. [ 214 ], Copyright 2019, John Wiley and Sons). B The 8P-TS Exogel was used for wound repair. (i) Schematic diagram of the gel formation strategy for hydrogels; (ii) accelerated wound healing. (Reproduced with permission from Ref. [ 64 ], Copyright 2022, John Wiley and Sons)
Efficient and sustained delivery of siRNA is necessary for skin trauma repair. Therefore, a hydrogel containing anti-RelA siRNA and functional peptides was prepared. This hydrogel showed anti-atopic dermatitis effects and intradermal penetration in an AD mouse model and improvement in severe skin trauma [ 190 ]. Cao et al. [ 216 ] prepared a composite hydrogel that mimicked the ECM, provided attachment points and nutrients to cells, and promoted VEGF expression. The hydrogel exhibited good skin regeneration in terms of total skin defects. During trauma healing, macrophages (Mφs) shift from pro-inflammatory (M1) to anti-inflammatory (M2), which is critical for acute inflammation relief and guided tissue repair. Kwak et al. [ 64 ] extracted Exos (M2-Exos) from M2-Mφs and used them as local microenvironment signals to induce reprogramming of M1-Mφs to M2-Mφs. Sustained results were achieved by designing a PEG hydrogel and using it to piggyback on M2-Exos to maximize the rate of skin wound healing. Localized M2-Exos can induce a rapid transition of M1 macrophages to the M2 state in damaged and diseased skin tissues, thus accelerating the rate of damage. These benefits include faster wound closure and better healing rates (Fig. 8 B).
Hydrogels perform exogenous debridement and promote autolytic debridement during skin wound repair. Simultaneously, hydrogels can improve the regeneration of wound granulation tissue, promote epithelial cell division and migration, and accelerate wound healing. In terms of wound healing transformation, gene therapy is able to promote wound healing from the source by directly regulating cell growth, differentiation, and metabolic processes. In addition, a patient’s wound healing process is unique and can be interfered with by a variety of factors such as environment, genetics, and age. Gene therapy allows for a personalized treatment plan, thus providing a more precise and effective treatment. Traditional wound treatment often faces problems such as rapid degradation and uneven release of drugs [ 43 ]. A hydrogel, as a drug delivery system, can effectively protect the gene drug from the damage of the external environment, and at the same time achieve a continuous and stable release of the drug. This can not only improve the utilization rate of the drug but also prolong its action time, thus enhancing the therapeutic effect. The biocompatibility and degradation rate of hydrogels are key factors that limit their application [ 155 ]. Although hydrogels are designed to mimic the microenvironment of the extracellular matrix, their biocompatibility is not always perfect. In some cases, hydrogels may cause an immune or inflammatory response that can interfere with the normal wound-healing process. If the degradation rate of the hydrogel is too fast, it may not provide adequate support to the wound; if the degradation rate is too slow, it may hinder the formation of new tissue and wound healing. The stability of therapeutic genes in hydrogels is affected by a variety of factors, such as temperature, humidity, and pH. If the treatment is degraded or inactivated in the hydrogel, the therapeutic effect will be greatly reduced. Therefore, how to ensure the stability and activity of therapeutic genes in hydrogels is an urgent problem to be solved.
Eye diseases
The eye is an important part of the visual system of humans and animals, and is the main organ that senses the outside world. The eye consists of the eyeball, cornea, pupil, lens, and retina, among others. Therefore, the organizational integrity of the eye’s component structures is a prerequisite for visual function. However, eye diseases often occur due to excessive and inappropriate eye use and trauma [ 217 ]. With the growing interest in gene therapy, hydrogel materials, and tissue engineering techniques, and also based on the advantages of high water retention and stability of hydrogels, gene therapy hydrogels are a very advanced strategy for the treatment of eye diseases. Exosomes promote corneal epithelial healing. Sun et al. [ 16 ] prepared a THH-3/Exos-miRNA 24-3p hydrogel that improves corneal epithelial cell migration and induces post-healing of corneal epithelial defects, effectively preventing macrophage activation and corneal stromal fibrosis (Fig. 9 A). Tang et al. [ 218 ] used temperature-sensitive chitosan hydrogels to sustain the release of iPSC-MSC-derived Exos to induce repair of the stromal layer and corneal epithelial cells and downregulate the mRNA expression of collagen to reduce scar formation. Furthermore, the hydrogel inhibited TRAM2 to inhibit ECM deposition, which is effective for corneal repair after injury (Fig. 9 B). Effective control of the gene therapy process can be achieved through precise control of the temperature to improve therapeutic effects since both hydrogels are temperature-responsive.
Applications of therapeutic gene hydrogels in eye diseases. A HA hydrogel loaded with miRNA 24-3p exosome promotes corneal epithelial healing. (i) Exos-miRNA 24-3p hydrogel for repair after corneal epithelial defects; (ii) Evaluation of in vitro release of Exos-miRNA in a rabbit corneal epithelial defect model. (Reproduced with permission from Ref. [ 16 ], Copyright 2022, Elsevier). B Temperature-sensitive hydrogel regenerates corneal epithelium after piggybacking on Exos. (i) Extracellular matrix (ECM) remodeling after anterior corneal plate injury; (ii) Repair of the anterior corneal lamina propria by the CHI hydrogel. (Reproduced with permission from Ref. [ 218 ], Copyright 2022, Elsevier)
Age-related macular degeneration (AMD) is an eye disease that affects the central vision and is associated with choroidal neovascularization (CNV). Cells and tissues in the macular region of patients with AMD gradually degenerate, leading to blurred vision and blindness [ 177 ]. siRNA therapy is indicated for this class of diseases, but its efficacy is limited by a short working time, TLR3-related undesirable pathway activation, and poor delivery to the desired subretinal target tissue [ 219 ]. Ryoo et al. [ 212 ] prepared an anti-VEGF nanoball (siVEGF NB) encapsulated by an siRNA hydrogel. In a CNV mouse tissue model, the nanospheres overcame the vitreous to enter the retina and subchoroidal space through endocytosis of CD44 receptors on the inner rim, bypassing the TLR3-induced siRNA-like effector pathway, thereby showing good tissue therapeutic effects. The DNA-based composite hydrogel system supports the water-soluble ophthalmic treatment of allergic conjunctivitis [ 220 ].
In conclusion, gene therapy hydrogels have powerful therapeutic effects on eye diseases and can provide new methods and ideas to treat various eye diseases. Gene therapy safety and biocompatibility are crucial. Therefore, clinicians should pay special attention to the characterization of their composition, crosslinking degree, and degradability when selecting and using hydrogels. Temperature-sensitive hydrogels may gain popularity in the treatment of eye diseases.
Tumor treatment
A tumor is a disease caused by the abnormal proliferation of cells in the body owing to gene mutations, which can be classified as benign or malignant [ 221 ]. Benign tumors grow slowly, are not easy to metastasize, are surrounded by a peripheral membrane made of proliferating connective tissues, have obvious boundaries with the surrounding tissues, do not cause pressure and damage to the surrounding tissues, and generally have little effects; only in vital organs (intracranial, thoracic cavity) will they threaten life. Malignant tumors grow rapidly, have no envelope, the boundary between tissues is blurred, and the tumor cells will spread to the surrounding tissues and invade the interstices, ducts, and cavities of the surrounding tissues, which will cause compression and damage to the neighboring organs and tissues [ 222 ]. Local infiltration and distant metastasis are the most important characteristics of malignant tumors and are the main causes of death from malignant tumors [ 223 ]. Tumors can be treated using a variety of methods, such as surgery, radiation therapy, and chemotherapy [ 223 ]. Paclitaxel is an excellent anticancer agent that effectively induces apoptosis and inhibits the Akt1 signaling pathway and the growth of human papillary carcinoma cells [ 224 ]. The MMP-sensitive PEG hydrogel vectors had a significant sensitizing effect on tumor necrosis factor α-related apoptosis-inducing ligand (TRAIL) when mediating glioblastoma (GBM) cells in synergizing with the induction of apoptosis by GBM [ 225 ]. Hydrogels can carry therapeutic genes for local delivery to attack primary tumors or inhibit primary tumor recurrence, which is a novel tumor therapy strategy.
siRNA hydrogels exert antitumor effects by silencing the cell cycle protein B1 [ 152 ]. Peng et al. [ 226 ] used collagen hydrogels as carriers to achieve local, efficient, and sustained targeted delivery of Id1-targeted siRNAs, confirming the feasibility of their use for gastric cancer inhibition. Nanoparticles containing RNA therapeutic agents (such as miRNAs, siRNAs, and siRNAs) were embedded in hydrogel matrices, which were then implanted near the tumor for tumor gene therapy [ 176 ]. However, excessive hydrogel crosslinking can hinder the release of RNA therapeutic agents and reduce their therapeutic efficiency. Therefore, single-component injectable RNA hydrogels are preferred [ 227 ]. RNA hydrogels can manipulate immunomodulatory factors and silence oncogenes during cancer immunotherapy [ 102 , 173 ].
In conclusion, gene therapy hydrogels are excellent materials for tumor treatment. However, numerous factors must be considered, such as tumor size, location, and morphology, as well as the effect of injection on surrounding tissues and organs. Furthermore, the injection route is limited by the type and distribution of the tumor. Moreover, clinicians should ensure that hydrogels are compatible and coordinated with other therapeutic approaches (such as surgery, radiotherapy, or chemotherapy) to improve treatment efficacy before using gene therapy hydrogels as part of a comprehensive treatment strategy.
Osteoarthritis
Osteoarthritis (OA) is a non-inflammatory and degenerative joint disease that is more common in people over 50 and women, with symptoms of joint stiffness, pain, and swelling [ 228 ]. The main pathologies include cartilage degeneration or disappearance, joint limb ligament attachment, and cartilage-bone hyperplasia to form osteophytes [ 229 ]. Zhang et al. [ 47 ] prepared a composite hydrogel with enbucrilate modified with regenerated SF, CS, and AD, and its cross-linked network was highly bound to the wet surface, demonstrating high adhesive strength and biocompatibility. The loaded exosomes efficiently promoted the proliferation, differentiation, expansion, and migration of BMSCs. In situ injection of the hydrogel into the rat patellofemoral groove released exosomes that recruited BMSCs into newborn cartilage through the chemokine signaling pathway, accelerating the rate of the remodeling of ECM and OA treatment (Fig. 10 A). The cause of OA is unknown but may be related to obesity and aging. Excess joint activity, joint trauma, intraosseous hypertension, and osteoporosis can lead to the development of OA. In the early stages of OA, the surface layer of the body is deficient owing to cartilage degradation and loss of adhesion to wet tissue, resulting in a lack of cartilage regeneration [ 230 ].
Applications of therapeutic gene hydrogels in OA. A Injectable exosome hydrogel for cartilage defect regeneration. (i) Promoting superficial cartilage regeneration; (ii) Repair of cartilage defects by the AD/CS/RSF/EXO hydrogel; (iii) Gross observation of cartilage integrity; (iv) MRI images of rat knees. (Reproduced with permission from Ref. [ 47 ], Copyright 2021, Elsevier). B MS@G5-AHP/miR-140 to treat OA. (i) Gene-hydrogel MSs; (ii) Articular cavity injection of MS@G5-AHP/miR-140 to alleviate OA; (iii) Release of miR-140 and endocytosis of G5-AHP/miR-140. (Reproduced with permission from Ref. [ 161 ], Copyright 2022, Springer Nature)
Osteoarthritis is a non-inflammatory degenerative disease caused by tissue degeneration, chronic injury, and reduced cartilage elasticity, and is caused by increased production of MMPs [ 231 ]. Hydrogels are injectable, highly compatible, and of great interest for minimally invasive tissue repair. Amino acid coupling improves gene transfection efficiency [ 160 ]. Li et al. [ 161 ] constructed an MS@G5-AHP/miR-140 hydrogel that exhibits a high transfection efficiency for the release of G5-AHP/miR-140 nanoparticles. It maintains the metabolic balance of the cartilage matrix, alleviates cartilage matrix degradation, and inhibits the overexpression of MMP5 and MMP13. The hydrogel prevents articular cartilage degeneration, reduces osteophyte formation, and alleviates OA symptoms. It also compensates for the short half-life, low transfection rate, and susceptibility to inactivation caused by direct injection of crude miRNAs; this demonstrates the excellent therapeutic ability of gene therapy hydrogels (Fig. 10 B).
The advantage of gene therapy hydrogels is that they can be injected into the joint through a minimally invasive procedure with sustained and controlled release of the agent at the implantation site. This local delivery is ideal for patients with OA since it reduces systemic side effects and can be personalized according to the specific needs of each patient.
Brain diseases
Brain diseases are commonly caused by inflammation of intracranial tissues and organs (cerebrum, cerebellum, brainstem, cranial nerves, and meningeal blood vessels), vascular diseases, parasitic diseases, and trauma [ 232 ]. The extrapyramidal system is an integral part of the locomotor system, and the striatal pallidum is the main structure of the extrapyramidal system. Structures of the thalamic floor nucleus, red nucleus, substantia nigra, and reticularis are also included. Damage to the striatal pallidocellular system causes hypertonic hypokinesia and hypotonic hyperkinesia syndrome. The former is like Parkinson’s disease (PD) and involves lesions located in the substantia nigra and nigrostriatal pathways, whereas the latter is like chorea, bradykinesia, and torsion spasticity, and involves lesions located in the striatum [ 233 , 234 ]. Hydrogels combined with MSCs, and glial cell line-derived neurotrophic factor (GDNF) are good materials for the delivery of cellular drugs and the treatment of PD [ 235 ]. RNAi-loaded plasmid nanoparticles achieved effective repair in a PD model (Fig. 11 A) [ 236 ]. Exosomes derived from human endometrial stem cells inhibit S protein aggregation and prevent neuronal cell death [ 217 ].
Applications of therapeutic gene hydrogels in brain diseases. A Schematic representation of the inhibitory effect of RNAi plasmid on Parkinson’s disease. (Reproduced with permission from Ref. [ 236 ], Copyright 2017, Ivyspring). B Exosomal hydrogels and microglia work together for nerve repair after TBI. (Reproduced with permission from Ref. [ 191 ], Copyright 2022, Elsevier)
Traumatic Brain Injury (TBI) is a highly morbid brain tissue damage disease, and the number of deaths caused by it is increasing each year. Oxidative stress and local inflammation at the site of TBI damage are key factors determining the outcome of the treatment, and SHED-Exo is able to effectively ameliorate the inflammation in the neural tissues and promote the repair of TBI tissues [ 237 ]. However, instability, intermittency, and induction of oxidative stress during Exo release can exacerbate damage to the original site. Li et al. [ 191 ] modulated TBI nerve repair by preparing a hybrid hydrogel based on poly(citrate-gallic acid) (FPGEGa) by hydrogel modification and conjugation with microglia. FPGEGa exhibited self-healing, thermo-sensitizing, and antioxidant effects, and the sustained release of SHED-Exos reduces ROS levels in the central nervous system. The hydrogel can also induce microglia to exhibit stronger anti-inflammatory effects by promoting anti-inflammatory M2 polarization and inhibiting pro-inflammatory M1 polarization, providing a new therapeutic strategy for TBI and other tissue engineering fields (Fig. 11 B).
Gene therapy hydrogels may also treat other brain diseases, such as Alzheimer’s disease and Huntington’s disease. These diseases are often associated with amyloid deposits and genetic mutations. Gene therapy drugs for these diseases can be delivered directly to the brain to promote nerve cell repair and regeneration with the use of gene therapy hydrogels.
Enteric diseases
Enteritis diseases are inflammatory diseases of the intestines, including infectious and non-infectious enteritis, and the symptoms of this type of disease include diarrhea, abdominal pain, nausea, and vomiting [ 238 ]. Oral administration of siRNA-containing nanolipids is a highly effective siRNA delivery method to treat ulcerative colitis (UC) [ 239 ]. The diagnosis of enteritis requires tests. Routine stool examination is a basic procedure that can detect white blood cell and red blood cell indicators in the stool. Enteroscopy is a method used to visually inspect the intestinal mucosa, directly observe changes, and obtain tissue samples for pathological examination. Furthermore, blood tests, radiological imaging, and immunological tests are often necessary. Infectious enteritis is mainly treated with antibiotics and other medications, hydration, and electrolytes, whereas non-infectious enteritis needs to be treated with immunomodulators, hormones, and other medications, and sometimes with surgery [ 238 ].
Ulcerative colitis is the main type of inflammatory bowel disease that causes diarrhea, blood in the stool, colon atrophy, and colorectal cancer. Daily oral medications only provide short-term relief from the inflammatory response, and biologics in the antibody drug class were effective in suppressing colitis. However, this requires large doses of antibodies, has low bioavailability, and triggers systemic toxicity [ 240 ]. In contrast, siRNAs are significantly more valuable because they are more efficient, non-toxic, and safer. It is difficult for siRNAs to exert therapeutic effects on UC by oral administration owing to their degradation in the gastrointestinal environment [ 241 ]. Gao et al. [ 192 ] designed a SA@MOF-siRNATNFα drug delivery system to avoid oral degradation. The stability of the system was demonstrated in the acidic environment of the small intestine and its successful uptake by inflammatory macrophages, resulting in increased siRNATNFα release. Furthermore, siRNA had a concentration and infiltrating effect, which led the system to reduce the severity of enteritis more efficiently and provided a prospective therapeutic option for treating the colon.
Rheumatoid arthritis
Rheumatoid arthritis (RA) is a systemic inflammatory disease whose etiology is not yet clear, with mostly symmetric, chronic, multi-synovial arthritis, extra-articular lesions as clinical manifestations. RA occurs in the small joint tissues of the feet, wrists, and hands, and is often recurrent, with red, swollen joints and hot pain at early stages, and stiff and deformed joints at late stages, accompanied by atrophy of the bones and skeletal muscles. Damaged tissues are easily disfigured and disabled if not treated in a timely manner [ 242 ]. siRNA is commonly used to treat RA and specifically silences genes involved in the pathogenesis of RA. Specific silencing of genes involved in the pathogenesis of RA can be achieved by spraying arthritic mice with siRNA-containing hydrogels [ 29 ]. Kanazawa et al. [ 29 ] used sericin hydrogels to load anti-RelA siRNA hydrogels to achieve sustained-release siRNA in vitro, and the knee joints of the rats were continuously degraded for a long time in vivo. CIA mice treated with a silk glycoprotein hydrogel containing anti-RelA siRNA showed significant improvements in morbidity, knee joint thickness, and clinical severity compared to controls.
Exosomes are small membrane vesicles produced by cells and released outside the cell; they contain proteins, nucleic acids, and lipids and are widely recognized as important carriers of information transfer between cells [ 115 , 243 ]. Exotherapy is a recognized and highly promising strategy for RA treatment; however, it still presents serious challenges in the long-term modulation of the specific pathogenesis of RA and the protection of reduced joint damage. Therefore, Rui et al. [ 193 ] prepared a photo-cross-linked SF hydrogel and encapsulated olfactory ecto-MSC-derived Exos to form the Exos@SFMA hydrogel for continuous treatment of the immune microenvironment of RA. Hydrogels have flexible mechanical properties, excellent biocompatibility, and adsorption, and are suitable for the protection of joint tissue surfaces. Furthermore, the hydrogel promotes PD-L1 expression, which inhibits the PI3K/AKT pathway to achieve inhibition of polarization of T-follicular helper cells (Tfh). The hydrogels inhibited the differentiation of germinal center B cells into plasma cells by decreasing the Tfh cell response, which prevented joint damage and alleviated synovial inflammation. In summary, gene therapy hydrogels are an efficient and effective therapeutic strategy for RA with great potential for clinical applications.
Hair regeneration
Hair regrowth specifically stimulates hair growth, increasing hair density and length. Hair regeneration is achieved through the activation and differentiation of stem cells to form newborn hair cells, which are then transformed into hair fibers [ 244 ]. Some growth factors (such as hepatocyte growth factor, insulin-like growth factor, vascular endothelial growth factor, platelet-derived growth factor, and transforming growth factor beta) promote hair regrowth. They promote hair regeneration by stimulating hair follicle cell proliferation, improving the follicular periphery and vascular microenvironment, inhibiting apoptosis, and repairing endothelial damage. However, excessive growth factors can induce adverse effects [ 245 ]. Many approaches were used for hair regeneration, with cell therapy and tissue engineering being the most promising. Exosomes with internal miR-218-5p promote hair regeneration by modulating β-catenin signaling (Fig. 12 A) [ 246 ]. Cell therapy involves autologous or allogeneic cells, such as hair follicle stem cells and fibroblasts for hair regeneration. Tissue engineering involves the use of biomaterials and stem cells to build hair structures that are then transplanted, and these methods have yielded remarkable results [ 247 ].
Applications of therapeutic gene hydrogels in hair regeneration. A miR-218-5p-containing exosomes modulate the β-catenin signaling pathway to promote hair regrowth. (i) Schematic representation of dermal papilla globules promoting hair by secreting exosomes; (ii) Exosome hydrogel promotes back hair regeneration in mice. (Reproduced with permission from Ref. [ 246 ], Copyright 2020, American Association for the Advancement of Science). B LPN microneedle patch delivers miR-218 to promote hair regrowth. (i) Schematic representation of the preparation of MN/LPNs/miR-218; (ii) Schematic representation of hair regeneration in mice administered at different times. (Reproduced with permission from Ref. [ 156 ], Copyright 2022, Royal Society of Chemistry)
Tao et al. [ 194 ] stimulated human dermal microvascular endothelial cells (HMEC-1) and human dermal fibroblasts stimulated proliferation in a dose-dependent manner in miR-126-3p overexpressing SMSCs (SMSC-126-Exos). miR-218 down-regulates SFRP2 to activate the Wnt/β-catenin channel, which is a key channel in the transformation of the follicular cycle for hair regeneration. Therefore, it can achieve hair regeneration by delivering the miR-218 gene. However, miRNA targeting the target site is limited owing to skin barrier restrictions (such as stratum corneum obstruction) or insufficient resistance of miR-218 to enzymatic degradation. Zhao et al. [ 156 ] used HA microneedles to enhance the permeability of the stratum corneum and then used lipid polymer hybrid nanoparticles (LPNs) as the delivery vehicle of miR-218 to avoid enzymatic degradation, which efficiently promoted the diffusion of LPNs/miR-218 in the dermal region and maximized the utilization of miR-218 (Fig. 12 B). In conclusion, this system enables the targeted delivery of miRNAs for hair regeneration.
Gene therapy hydrogels have numerous applications for tissue engineering. First, they can function as a gene carrier to deliver therapeutic genes accurately to target organs, enhance the effects of gene therapy, and minimize side effects. Second, they promote cell proliferation for tissue repair. Finally, they can control the rate and mode of gene release to optimize the efficacy and repair of damaged tissues. Despite its superior biocompatibility, the hydrogel can cause immune and inflammatory reactions. Some hydrogels are difficult to degrade naturally, and this may cause tissue damage. Bone remodeling can be achieved through autografts and xenografts, but it is difficult owing to its adverse immune response, potential risk of infection, and restricted sources. Some hydrogels have shortcomings such as low mechanical strength, an inhomogeneous crosslinking structure, slow response, and low crosslinking density. Furthermore, the ease of degradation of therapeutic genes, stability, and safety issues also affect the final efficacy. Therefore, measures such as the introduction of biologically active components, the improvement of the preparation process, and the development of novel crosslinking agents are needed to overcome these problems.
Summary and future directions
Delivery strategies targeting tissue have received significant attention in recent decades owing to their ability to effectively avoid complications associated with therapeutic gene delivery. Hydrogels that serve as carriers of therapeutic genes exhibit excellent biocompatibility, biodegradability, and solubility. These properties shield therapeutic genes from enzymatic degradation and microenvironmental effects, facilitating sustained and slow gene release and enhancing therapeutic outcomes. Hydrogels can also enable responsive drug release through functional modifications. Gene therapy hydrogels find widespread application in bone, heart, trauma, cartilage, spinal cord, skin, eyes, and tumors, demonstrating robust scientific and clinical efficacy across diverse tissues and organs.
Although gene therapy hydrogels offer considerable advantages, they face several challenges. Natural polysaccharide hydrogels exhibit inadequate control, mechanical properties, and permeability. Furthermore, the degradation products of natural polysaccharide hydrogels can incite immune reactions. Protein hydrogels suffer from insufficient degradation rates, bioactivity, and stability, and are susceptible to external pH and temperature influences. Despite the chemical stability and controllability of synthetic hydrogels, issues like poor biocompatibility, slow degradation, and biotoxicity persist and cause resolution. Moreover, optimizing the crosslinking of hydrogels remains an ongoing challenge. Therapeutic genes are prone to degradation, instability, mutations, and safety concerns, demanding continuous refinement and modification. While ZFNs, TALENs, and CRISPR-Cas9 modifications have found wide application in gene therapy, their limitations require further improvement. The loading of therapeutic genes into hydrogels presents both advantages and disadvantages, requiring enhancement.
Certain gene therapy hydrogels exhibit inadequate mechanical properties and stability, potentially triggering immune reactions and toxic effects. Consequently, safety and efficacy are primary considerations. Furthermore, poor transfection efficiency can hinder the efficient delivery of gene drugs and all these factors affect tissue engineering applications. Gene therapy, as a therapeutic means of intervening directly against genetic defects or abnormalities, is of great potential therapeutic efficacy and clinical value. Before gene therapy is carried out, the treatment strategy needs to be comprehensively evaluated and optimized to ensure the safety and effectiveness of the treatment process. Secondly, the safety of gene therapy also needs to be ensured through strict clinical trials and regulation. However, we must also be aware that there are still challenges and uncertainties regarding the safety of gene therapy. For example, the assessment of long-term therapeutic effects and potential side effects needs to be supported by longer and larger-scale clinical studies; issues such as immune rejection and genetic instability that may be triggered by gene therapy also need to be further researched and resolved.
In summary, gene therapy hydrogels have shown significant advantages in tissue engineering and play an active role in a wide range of organs and tissues. However, its application still faces numerous areas for improvement and requires continuous exploration and effort. With a deeper understanding of gene function and regulatory mechanisms, gene therapy strategies will become more precise and personalized. This means that future gene therapy hydrogels will need to be able to precisely deliver therapeutic genes to target cells or tissues while achieving fine regulation of gene expression levels. Future research should be devoted to optimizing material types, improving cross-linking methods, and broadening gene modification methods in order to continuously improve the key properties of gene therapy hydrogels, such as mechanical properties, biocompatibility, and transfection efficiency. Moreover, with the deepening of crossovers between disciplines, we believe that more advanced and effective gene therapy hydrogels and tissue engineering products can be developed. The close integration of gene therapy and hydrogels will bring brand new possibilities to the field of tissue engineering, inject new vitality into materials science, provide more effective means of clinical treatment, and bring better therapeutic effects and quality of life to patients.
Availability of data and materials
The data used to support this review are included within the article.
Li J, Lai Y, Li M, Chen X, Zhou M, Wang W, Li J, Cui W, Zhang G, Wang K, Liu L, Lin Y. Repair of infected bone defect with clindamycin-tetrahedral DNA nanostructure complex-loaded 3D bioprinted hybrid scaffold. Chem Eng J. 2022;435: 134855.
Article CAS Google Scholar
Zha Y, Lin T, Li Y, Zhang X, Wang Z, Li Z, Ye Y, Wang B, Zhang S, Wang J. Exosome-mimetics as an engineered gene-activated matrix induces in-situ vascularized osteogenesis. Biomaterials. 2020;247: 119985.
Article CAS PubMed Google Scholar
Wolf DP, Mitalipov PA, Mitalipov SM. Principles of and strategies for germline gene therapy. Nat Med. 2019;25:890–7.
Collon K, Gallo MC, Lieberman JR. Musculoskeletal tissue engineering: regional gene therapy for bone repair. Biomaterials. 2021;275: 120901.
Yu Y, Gao Y, He L, Fang B, Ge W, Yang P, Ju Y, Xie X, Lei L. Biomaterial-based gene therapy. MedComm. 2023;4: e259.
Article CAS PubMed PubMed Central Google Scholar
Balakrishnan B, Banerjee R. Biopolymer-based hydrogels for cartilage tissue engineering. Chem Rev. 2011;111:4453–74.
Madry H, Gao L, Rey-Rico A, Venkatesan JK, Müller-Brandt K, Cai X, Goebel L, Schmitt G, Speicher-Mentges S, Zurakowski D, Menger MD, Laschke MW, Cucchiarini M. Thermosensitive hydrogel based on PEO-PPO-PEO poloxamers for a controlled in situ release of recombinant adeno-associated viral vectors for effective gene therapy of cartilage defects. Adv Mater. 2020;32:1906508.
Chen Z, Liu F, Chen Y, Liu J, Wang X, Chen AT, Deng G, Zhang H, Liu J, Hong Z, Zhou J. Targeted delivery of CRISPR/Cas9-mediated cancer gene therapy via liposome-templated hydrogel nanoparticles. Adv Funct Mater. 2017;27:1703036.
Article PubMed PubMed Central Google Scholar
Huang H, Dong Z, Ren X, Jia B, Li G, Zhou S, Zhao X, Wang W. High-strength hydrogels: fabrication, reinforcement mechanisms, and applications. Nano Res. 2023;16:3475–515.
Article Google Scholar
Zhou Q, Kang H, Bielec M, Wu X, Cheng Q, Wei W, Dai H. Influence of different divalent ions cross-linking sodium alginate-polyacrylamide hydrogels on antibacterial properties and wound healing. Carbohydr Polym. 2018;197:292–304.
Rezvanian M, Ahmad N, Amin MCIM, Ng SF. Optimization, characterization, and in vitro assessment of alginate-pectin ionic cross-linked hydrogel film for wound dressing applications. Int J Biol Macromol. 2017;97:131–40.
Article PubMed Google Scholar
Ren Z, Zhang Y, Li Y, Xu B, Liu W. Hydrogen bonded and ionically crosslinked high strength hydrogels exhibiting Ca 2+ -triggered shape memory properties and volume shrinkage for cell detachment. J Mater Chem B. 2015;3:6347–54.
Shao J, Ding Z, Li L, Chen Y, Zhu J, Qian Q. Improved accumulation of TGF-β by photopolymerized chitosan/silk protein bio-hydrogel matrix to improve differentiations of mesenchymal stem cells in articular cartilage tissue regeneration. J Photochem Photobiol B. 2020;203: 111744.
Tao B, Lin C, Yuan Z, He Y, Chen M, Li K, Hu J, Yang Y, Xia Z, Cai K. Near infrared light-triggered on-demand Cur release from Gel-PDA@Cur composite hydrogel for antibacterial wound healing. Chem Eng J. 2021;403: 126182.
Cai C, Wang W, Liang J, Li Y, Lu M, Cui W, Fan C, Deng L, Li Y, Wang F, Liu S. MMP-2 responsive unidirectional hydrogel-electrospun patch loading TGF-β1 siRNA polyplexes for peritendinous anti-adhesion. Adv Funct Mater. 2021;31:2008364.
Sun X, Song W, Teng L, Huang Y, Liu J, Peng Y, Lu X, Yuan J, Zhao X, Zhao Q, Xu Y, Shen J, Peng X, Ren L. MiRNA 24–3p-rich exosomes functionalized DEGMA-modified hyaluronic acid hydrogels for corneal epithelial healing. Bioact Mater. 2023;25:640–56.
CAS PubMed Google Scholar
Ninan N, Forget A, Shastri VP, Voelcker NH, Blencowe A. Antibacterial and anti-inflammatory pH-responsive tannic acid-carboxylated agarose composite hydrogels for wound healing. ACS Appl Mater Interfaces. 2016;8:28511–21.
Ma M, Zhong Y, Jiang X. Thermosensitive and pH-responsive tannin-containing hydroxypropyl chitin hydrogel with long-lasting antibacterial activity for wound healing. Carbohydr Polym. 2020;236: 116096.
Li H, Cheng F, Wei X, Yi X, Tang S, Wang Z, Zhang YS, He J, Huang Y. Injectable, self-healing, antibacterial, and hemostatic N, O-carboxymethyl chitosan/oxidized chondroitin sulfate composite hydrogel for wound dressing. Mater Sci Eng C. 2021;118: 111324.
Fan M, Ma Y, Tan H, Jia Y, Zou S, Guo S, Zhao M, Huang H, Ling Z, Chen Y, Hu X. Covalent and injectable chitosan-chondroitin sulfate hydrogels embedded with chitosan microspheres for drug delivery and tissue engineering. Mater Sci Eng C. 2017;71:67–74.
Su H, Zheng R, Jiang L, Zeng N, Yu K, Zhi Y, Shan S. Dextran hydrogels via disulfide-containing schiff base formation: synthesis, stimuli-sensitive degradation and release behaviors. Carbohydr Polym. 2021;265: 118085.
Ding H, Li B, Liu Z, Liu G, Pu S, Feng Y, Jia D, Zhou Y. Decoupled pH- and thermo-responsive injectable Chitosan/PNIPAM hydrogel via thiol-ene click chemistry for potential applications in tissue engineering. Adv Healthcare Mater. 2020;9: e2000454.
Goodarzi H, Jadidi K, Pourmotabed S, Sharifi E, Aghamollaei H. Preparation and in vitro characterization of cross-linked collagen–gelatin hydrogel using EDC/NHS for corneal tissue engineering applications. Int J Biol Macromol. 2019;126:620–32.
Wang C, Hashimoto K, Tamate R, Kokubo H, Watanabe M. Controlled sol–gel transitions of a thermoresponsive polymer in a photoswitchable azobenzene ionic liquid as a molecular trigger. Angew Chem Int Edit. 2018;57:227–30.
Pham L, Dang LH, Truong MD, Nguyen TH, Le L, Le VT, Nam ND, Bach LG, Nguyen VT, Tran NQ. A dual synergistic of curcumin and gelatin on thermal-responsive hydrogel based on Chitosan-P123 in wound healing application. Biomed Pharmacother. 2019;117: 109183.
Yang K, Sun J, Wei D, Yuan L, Yang J, Guo L, Fan H, Zhang X. Photo-crosslinked mono-component type II collagen hydrogel as a matrix to induce chondrogenic differentiation of bone marrow mesenchymal stem cells. J Mater Chem B. 2017;5:8707–18.
Ju J, Hu N, Cairns DM, Liu H, Timko BP. Photo-cross-linkable, insulating silk fibroin for bioelectronics with enhanced cell affinity. Proc Natl Acad Sci U S A. 2020;117:15482–9.
Kim HA, Kim S, Chang SH, Hwang HJ, Choi YN. Anti-arthritic effect of ginsenoside Rb1 on collagen induced arthritis in mice. Int Immunopharmacol. 2007;7:1286–91.
Kanazawa T, Tamano K, Sogabe K, Endo T, Ibaraki H, Takashima Y, Seta Y. Intra-articular retention and anti-arthritic effects in collagen-induced arthritis model mice by injectable small interfering RNA containing hydrogel. Biol Pharm Bull. 2017;40:1929–33.
Deng L, Xia T, Cheng W, Yang M, Zhu W, Chen X. Injectable redox albumin-based hydrogel with in-situ loaded dihydromyricetin. Colloids Surf B Biointerfaces. 2022;220: 112871.
Chen Y, Younis MR, He G, Zheng Z, Wang Y, Xue K, Sun J, Liu K, Huang P, Wang X. Oxidative stimuli-responsive “pollen-like” exosomes from silver nanoflowers remodeling diabetic wound microenvironment for accelerating wound healing. Adv Healthc Mater. 2023;12: e2300456.
Karg M, Hellweg T. New, “smart” poly(NIPAM) microgels and nanoparticle microgel hybrids: properties and advances in characterisation. Curr Opin Colloid Interface Sci. 2009;14:438–50.
Jin R, Teixeira LSM, Krouwels A, Dijkstra PJ, Blitterswijk CAV, Karperien M, Feijen J. Synthesis and characterization of hyaluronic acid-poly(ethylene glycol) hydrogels via Michael addition: an injectable biomaterial for cartilage repair. Acta Biomater. 2010;6:1968–77.
Hou K, Hu Z, Mugaanire IT, Li C, Chen G, Zhu M. Fiber forming mechanism and reaction kinetics of novel dynamic-crosslinking-spinning for poly(ethylene glycol) diacrylate fiber fabrication. Polymer. 2019;183: 121903.
Theis A, Davis TP, Stenzel MH, Barner-Kowollik C. Mapping chain length and conversion dependent termination rate coefficients in methyl acrylate free radical polymerization. Macromolecules. 2005;38:10323–7.
Chen S, Shi J, Xu X, Ding J, Zhong W, Zhang L, Xing M, Zhang L. Study of stiffness effects of poly(amidoamine)–poly(n-isopropyl acrylamide) hydrogel on wound healing. Colloid Surface B. 2016;140:574–82.
Yu LMY, Kazazian K, Shoichet MS. Peptide surface modification of methacrylamide chitosan for neural tissue engineering applications. J Biomed Mater Res Part A. 2007;82:243–55.
Wang L, Zhang HJ, Liu X, Liu Y, Zhu X, Liu X, You X. A physically cross-linked sodium alginate–gelatin hydrogel with high mechanical strength. ACS Appl Polym Mater. 2021;3:3197–205.
Wei Q, Zhou J, An Y, Li M, Zhang J, Yang S. Modification, 3D printing process and application of sodium alginate based hydrogels in soft tissue engineering: a review. Int J Biol Macromol. 2023;232: 123450.
Li Y, Zhang X, He D, Ma Z, Xue K, Li H. 45S5 Bioglass® works synergistically with siRNA to downregulate the expression of matrix metalloproteinase-9 in diabetic wounds. Acta Biomater. 2022;145:372–89.
Ouyang Y, Su X, Zheng X, Zhang L, Chen Z, Yang Q, Qian Q, Zhao J, Li P, Wang S. Mussel-inspired, “all-in-one” sodium alginate/carboxymethyl chitosan hydrogel patch promotes healing of infected wound. Int J Biol Macromol. 2024;261: 129828.
Zhai P, Peng X, Li B, Liu Y, Sun H, Li X. The application of hyaluronic acid in bone regeneration. Int J Biol Macromol. 2020;151:1224–39.
Cai C, Zhang X, Li Y, Liu X, Wang S, Lu M, Yan X, Deng L, Liu S, Wang F, Fan C. Self-healing hydrogel embodied with macrophage-regulation and responsive-gene-silencing properties for synergistic prevention of peritendinous adhesion. Adv Mater. 2022;34: e2106564.
Khanarian NT, Haney NM, Burga RA, Lu HH. A functional agarose-hydroxyapatite scaffold for osteochondral interface regeneration. Biomaterials. 2012;33:5247–58.
Liu J, Su C, Chen Y, Tian S, Lu C, Huang W, Lv Q. Current understanding of the applications of photocrosslinked hydrogels in biomedical engineering. Gels. 2022;8:216.
Yang J, Shen M, Wen H, Luo Y, Huang R, Rong L, Xie J. Recent advance in delivery system and tissue engineering applications of chondroitin sulfate. Carbohydr Polym. 2020;230: 115650.
Zhang FX, Liu P, Ding W, Meng QB, Su DH, Zhang QC, Lian RX, Yu BQ, Zhao MD, Dong J, Li YL, Jiang LB. Injectable mussel-Inspired highly adhesive hydrogel with exosomes for endogenous cell recruitment and cartilage defect regeneration. Biomaterials. 2021;278: 121169.
Chen L, Zhao N, McClements DJ, Hamaker BR, Miao M. Advanced dendritic glucan-derived biomaterials: from molecular structure to versatile applications. Compr Rev Food Sci Food Saf. 2023;22:4107–46.
Nguyen MK, McMillan A, Huynh CT, Schapira DS, Alsberg E. Photocrosslinkable, biodegradable hydrogels with controlled cell adhesivity for prolonged siRNA delivery to hMSCs to enhance their osteogenic differentiation. J Mater Chem B. 2017;5:485–95.
Gupta B, Tummalapalli M, Deopura BL, Alam MS. Preparation and characterization of in-situ crosslinked pectin–gelatin hydrogels. Carbohydr Polym. 2014;106:312–8.
Li J, Lv Y, Chen Z, Zhao J, Wang S. Citric acid loaded hydrogel-coated stent for dissolving pancreatic duct calculi. Gels. 2024;10:125.
Pan T, Song W, Xin H, Yu H, Wang H, Ma D, Cao X, Wang Y. MicroRNA-activated hydrogel scaffold generated by 3D printing accelerates bone regeneration. Bioact Mater. 2022;10:1–14.
PubMed Google Scholar
Parenteau-Bareil R, Gauvin R, Berthod F. Collagen-based biomaterials for tissue engineering applications. Materials. 2010;3:1863–87.
Article CAS PubMed Central Google Scholar
Weißenberger M, Weißenberger MH, Wagenbrenner M, Heinz T, Reboredo J, Holzapfel BM, Rudert M, Groll J, Evans CH, Steinert AF. Different types of cartilage neotissue fabricated from collagen hydrogels and mesenchymal stromal cells via SOX9, TGFB1 or BMP2 gene transfer. PLoS ONE. 2020;15: e0237479.
Xiao W, He J, Nichol JW, Wang L, Hutson CB, Wang B, Du Y, Fan H, Khademhosseini A. Synthesis and characterization of photocrosslinkable gelatin and silk fibroin interpenetrating polymer network hydrogels. Acta Biomater. 2011;7:2384–93.
Han C, Liu F, Zhang Y, Chen W, Luo W, Ding F, Lu L, Wu C, Li Y. Human umbilical cord mesenchymal stem cell derived exosomes delivered using silk fibroin and sericin composite hydrogel promote wound healing. Front Cardiovasc Med. 2021;8: 713021.
Rahimpour S, Jabbari H, Yousofi H, Fathi A, Mahmoodi S, Jafarian MJ, Shomali N, Shotorbani SS. Regulatory effect of sericin protein in inflammatory pathways; a comprehensive review. Pathol Res Pract. 2023;243: 154369.
Su C, Chen Y, Tian S, Lu C, Lv Q. Natural materials for 3D printing and their applications. Gels. 2022;8:748.
Zarrin NK, Mottaghitalab F, Reis RL, Kundu SC, Farokhi M. Thermosensitive chitosan/poly(N-isopropyl acrylamide) nanoparticles embedded in aniline pentamer/silk fibroin/polyacrylamide as an electroactive injectable hydrogel for healing critical-sized calvarial bone defect in aging rat model. Int J Biol Macromol. 2022;213:352–68.
Tang L, Wang L, Yang X, Feng Y, Li Y, Feng W. Poly(N-isopropylacrylamide)-based smart hydrogels: design, properties and applications. Prog Mater Sci. 2021;115: 100702.
Yang HY, van Ee RJ, Timmer K, Craenmehr EGM, Huang JH, Öner FC, Dhert WJA, Kragten AHM, Willems N, Grinwis GCM, Tryfonidou MA, Papen-Botterhuis NE, Creemers LB. A novel injectable thermoresponsive and cytocompatible gel of poly(N-isopropylacrylamide) with layered double hydroxides facilitates siRNA delivery into chondrocytes in 3D culture. Acta Biomater. 2015;23:214–28.
D’souza AA, Shegokar R. Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications. Expert Opin Drug Deliv. 2016;13:1257–75.
Sharma B, Fermanian S, Gibson M, Unterman S, Herzka DA, Cascio B, Coburn J, Hui AY, Marcus N, Gold GE, Elisseeff JH. Human cartilage repair with a photoreactive adhesive-hydrogel composite. Sci Transl Med. 2013;5:167ra6.
Kwak G, Cheng J, Kim H, Song S, Lee SJ, Yang Y, Jeong JH, Lee JE, Messersmith PB, Kim SH. Sustained exosome-guided macrophage polarization using hydrolytically degradable PEG hydrogels for cutaneous wound healing: identification of key proteins and MiRNAs, and sustained release formulation. Small. 2022;18: e2200060.
Wang L, Wang J, Zhou X, Sun J, Zhu B, Duan C, Chen P, Guo X, Zhang T, Guo H. A new self-healing hydrogel containing hucMSC-derived exosomes promotes bone regeneration. Front Bioeng Biotechnol. 2020;8: 564731.
Tang X, Wang Z, Xie Y, Liu Y, Yang K, Li T, Shen H, Zhao M, Jin J, Xiao H, Liu H, Gu N. Radiation-triggered selenium-engineered mesoporous silica nanocapsules for RNAi therapy in radiotherapy-resistant glioblastoma. ACS Nano. 2023;17:4062–76.
Hermens JGH, Jensma A, Feringa BL. Highly efficient biobased synthesis of acrylic acid. Angew Chem Int Edit. 2022;61: e202112618.
Nuhn L, Hirsch M, Krieg B, Koynov K, Fischer K, Schmidt M, Helm M, Zentel R. Cationic nanohydrogel particles as potential siRNA carriers for cellular delivery. ACS Nano. 2012;6:2198–214.
Champeau M, Póvoa V, Militão L, Cabrini FM, Picheth GF, Meneau F, Jara CP, de Araujo EP, de Oliveira MG. Supramolecular poly(acrylic acid)/F127 hydrogel with hydration-controlled nitric oxide release for enhancing wound healing. Acta Biomater. 2018;74:312–25.
Chang WH, Lee YF, Liu YW, Willner I, Liao WC. Stimuli-responsive hydrogel microcapsules for the amplified detection of microRNAs. Nanoscale. 2021;13:16799–808.
Henderson KJ, Zhou TC, Otim KJ, Shull KR. Ionically cross-linked triblock copolymer hydrogels with high strength. Macromolecules. 2010;43:6193–201.
Li Y, Han Y, Wang X, Peng J, Xu Y, Chang J. Multifunctional hydrogels prepared by dual ion cross-linking for chronic wound healing. ACS Appl Mater Interfaces. 2017;9:16054–62.
Ahmed EM. Hydrogel: preparation, characterization, and applications: a review. J Adv Res. 2015;6:105–21.
Gao F, Ma X, Wang F, Zhou F, Ye J, Yang D, Li M, Wang P. Injectable multifunctional DNA hydrogel for accelerated wound healing. Chem Eng J. 2023;470: 144347.
Tang J, Jia X, Li Q, Cui Z, Liang A, Ke B, Yang D, Yao C. A DNA-based hydrogel for exosome separation and biomedical applications. Proc Natl Acad Sci U S A. 2023;120: e2303822120.
Xia M, Cheng Y, Theato P, Zhu M. Thermo-induced double phase transition behavior of physically cross-linked hydrogels based on oligo(ethylene glycol) methacrylates. Macromol Chem Phys. 2015;216:2230–40.
Song M, Wang L, Shao F, Xie H, Xu H, Yu W. Thermally induced flexible phase change hydrogels for solar thermal storage and human thermal management. Chem Eng J. 2023;464: 142682.
Wu D, Qin H, Wang Z, Yu M, Liu Z, Peng H, Liang L, Zhang C, Wei X. Bone mesenchymal stem cell-derived sEV-encapsulated thermosensitive hydrogels accelerate osteogenesis and angiogenesis by release of exosomal miR-21. Front Bioeng Biotechnol. 2022;9: 829136.
Mao H, Wang C, Chang X, Cao H, Shan G, Bao Y, Pan P. Poly(lactic acid)/poly(ethylene glycol) stereocomplexed physical hydrogels showing thermally-induced gel–sol–gel multiple phase transitions. Mater Chem Front. 2018;2:313–22.
Bergmann NM, Peppas NA. Molecularly imprinted polymers with specific recognition for macromolecules and proteins. Prog Polym Sci. 2008;33:271–88.
Kim M, Jo H, Jung GY, Oh SS. Molecular complementarity of proteomimetic materials for target-specific recognition and recognition-mediated complex functions. Adv Mater. 2023;35: e2208309.
Carmon KS, Gong X, Lin Q, Thomas A, Liu Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/β-catenin signaling. Proc Natl Acad Sci U S A. 2011;108:11452–7.
Vocadlo DJ. O-GlcNAc processing enzymes: catalytic mechanisms, substrate specificity, and enzyme regulation. Curr Opin Chem Biol. 2012;16:488–97.
Hu Y, Zhang Z, Li Y, Ding X, Li D, Shen C, Xu FJ. Dual-crosslinked amorphous polysaccharide hydrogels based on chitosan/alginate for wound healing applications. Macromol Rapid Commun. 2018;39: e1800069.
Mo C, Xiang L, Chen Y. Advances in injectable and self-healing polysaccharide hydrogel based on the Schiff base reaction. Macromol Rapid Commun. 2021;42: e2100025.
Yang L, Zhang L, Hu J, Wang W, Liu X. Promote anti-inflammatory and angiogenesis using a hyaluronic acid-based hydrogel with miRNA-laden nanoparticles for chronic diabetic wound treatment. Int J Biol Macromol. 2021;166:166–78.
Wessig P, Müller G. The dehydro-Diels-Alder reaction. Chem Rev. 2008;108:2051–63.
Fujisawa N, Takanohashi M, Chen L, Uto K, Matsumoto Y, Takeuchi M, Ebara M. A Diels-Alder polymer platform for thermally enhanced drug release toward efficient local cancer chemotherapy. Sci Technol Adv Mater. 2021;22:522–31.
Li D, Wang S, Meng Y, Guo Z, Cheng M, Li J. Fabrication of self-healing pectin/chitosan hybrid hydrogel via Diels-Alder reactions for drug delivery with high swelling property, pH-responsiveness, and cytocompatibility. Carbohydr Polym. 2021;268: 118244.
Hiemstra C, van der Aa LJ, Zhong Z, Dijkstra PJ, Feijen J. Novel in situ forming, degradable dextran hydrogels by Michael addition chemistry: synthesis, rheology, and degradation. Macromolecules. 2007;40:1165–73.
Jiang Y, Chen J, Deng C, Suuronen EJ, Zhong Z. Click hydrogels, microgels and nanogels: emerging platforms for drug delivery and tissue engineering. Biomaterials. 2014;35:4969–85.
Lutz JF, Zarafshani Z. Efficient construction of therapeutics, bioconjugates, biomaterials and bioactive surfaces using azide-alkyne “click” chemistry. Adv Drug Deliv Rev. 2008;60:958–70.
Ren K, He C, Xiao C, Li G, Chen X. Injectable glycopolypeptide hydrogels as biomimetic scaffolds for cartilage tissue engineering. Biomaterials. 2015;51:238–49.
Das D, Pham TTH, Noh I. Characterizations of hyaluronate-based terpolymeric hydrogel synthesized via free radical polymerization mechanism for biomedical applications. Colloids Surf B Biointerfaces. 2018;170:64–75.
Zou Y, Li L, Li Y, Chen S, Xie X, Jin X, Wang X, Ma C, Fan G, Wang W. Restoring cardiac functions after myocardial infarction–ischemia/reperfusion via an exosome anchoring conductive hydrogel. ACS Appl Mater Interfaces. 2021;13:56892–908.
van Beilen JB, Li Z. Enzyme technology: an overview. Curr Opin Biotechnol. 2002;13:338–44.
Ju Y, Liu X, Ye X, Dai M, Fang B, Shen X, Liu L. Nanozyme-based remodeling of disease microenvironments for disease prevention and treatment: a review. ACS Appl Nano Mater. 2023;6:13792–823.
Oryan A, Kamali A, Moshiri A, Baharvand H, Daemi H. Chemical crosslinking of biopolymeric scaffolds: current knowledge and future directions of crosslinked engineered bone scaffolds. Int J Biol Macromol. 2018;107:678–88.
Yu S, Zhang X, Tan G, Tian L, Liu D, Liu Y, Yang X, Pan W. A novel pH-induced thermosensitive hydrogel composed of carboxymethyl chitosan and poloxamer cross-linked by glutaraldehyde for ophthalmic drug delivery. Carbohydr Polym. 2017;155:208–17.
Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15:541–55.
Mendes BB, Conniot J, Avital A, Yao D, Jiang X, Zhou X, Sharf-Pauker N, Xiao Y, Adir O, Liang H, Shi J, Schroeder A, Conde J. Nanodelivery of nucleic acids. Nat Rev Methods Primers. 2022;2:24.
Zhong R, Talebian S, Mendes BB, Wallace G, Langer R, Conde J, Shi J. Hydrogels for RNA delivery. Nat Mater. 2023;22:818–31.
Patel S, Athirasala A, Menezes PP, Ashwanikumar N, Zou T, Sahay G, Bertassoni LE. Messenger RNA delivery for tissue engineering and regenerative medicine applications. Tissue Eng Part A. 2019;25:91–112.
Li J, Ghatak S, El Masry MS, Das A, Liu Y, Roy S, Lee RJ, Sen CK. Topical lyophilized targeted lipid nanoparticles in the restoration of skin barrier function following burn wound. Mol Ther. 2018;26:2178–88.
Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immun. 2018;141:1202–7.
Lim J, Hwang JS, Seo SB, Kang B, Jang S, Son SU, Ki J, Kim JS, Kang T, Jung J, Han TS, Lim EK. Janus hydrogel-based fuel stimulant powered amplification for multiple detections of miRNA biomarkers in gastric cancer. Chem Eng J. 2022;448: 137637.
Søndergaard JN, Geng K, Sommerauer C, Atanasoai I, Yin X, Kutter C. Successful delivery of large-size CRISPR/Cas9 vectors in hard-to-transfect human cells using small plasmids. Commun Biol. 2020;3:319.
Castañeda-Barba S, Top EM, Stalder T. Plasmids, a molecular cornerstone of antimicrobial resistance in the One Health era. Nat Rev Microbiol. 2024;22:18–32.
Xu X, Qiu S, Zhang Y, Yin J, Min S. PELA microspheres with encapsulated arginine-chitosan/pBMP-2 nanoparticles induce pBMP-2 controlled-release, transfected osteoblastic progenitor cells, and promoted osteogenic differentiation. Artif Cells Nanomed Biotechnol. 2017;45:330–9.
Ding R, Liu Y, Cheng D, Yang G, Wu W, Du H, Jin X, Chen Y, Wang Y, Heng BC, Yang Q, Xu J. A novel gene-activated matrix composed of PEI/plasmid-BMP2 complexes and hydroxyapatite/chitosan-microspheres promotes bone regeneration. Nano Res. 2022;15:6348–60.
Wang LL, Burdick JA. Engineered hydrogels for local and sustained delivery of RNA-interference therapies. Adv Healthcare Mater. 2017;6:1601041.
Lam JKW, Chow MYT, Zhang Y, Leung SWS. siRNA versus miRNA as therapeutics for gene silencing. Mol Ther Nucleic Acids. 2015;4: e252.
Blake TR, Haabeth OAW, Sallets A, McClellan RL, Del Castillo TJ, Vilches-Moure JG, Ho WC, Wender PA, Levy R, Waymouth RM. Lysine-derived charge-altering releasable transporters: targeted delivery of mRNA and siRNA to the lungs. Bioconjugate Chem. 2023;34:673–85.
CAS Google Scholar
Wang Y, Malcolm DW, Benoit DSW. Controlled and sustained delivery of siRNA/NPs from hydrogels expedites bone fracture healing. Biomaterials. 2017;139:127–38.
Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–79.
Kuang H, Ma J, Chi X, Fu Q, Zhu Q, Cao W, Zhang P, Xie X. Integrated osteoinductive factors─exosome@microRNA-26a hydrogel enhances bone regeneration. ACS Appl Mater Interfaces. 2023;15:22805–16.
Yu C, Chen L, Zhou W, Hu L, Xie X, Lin Z, Panayi AC, Zhan X, Tao R, Mi B, Liu G. Injectable bacteria-sensitive hydrogel promotes repair of infected fractures via sustained release of miRNA antagonist. ACS Appl Mater Interfaces. 2022;14:34427–42.
Jain S, Kaur J, Prasad S, Roy I. Nucleic acid therapeutics: a focus on the development of aptamers. Expert Opin Drug Discov. 2021;16:255–74.
Ning Y, Hu J, Lu F. Aptamers used for biosensors and targeted therapy. Biomed Pharmacother. 2020;132: 110902.
Garcia-Garcia P, Reyes R, García-Sánchez D, Pérez-Campo FM, Rodriguez-Rey JC, Évora C, Díaz-Rodríguez P, Delgado A. Nanoparticle-mediated selective Sfrp-1 silencing enhances bone density in osteoporotic mice. J Nanobiotechnology. 2022;20:462.
Liang C, Li F, Wang L, Zhang ZK, Wang C, He B, Li J, Chen Z, Shaikh AB, Liu J, Wu X, Peng S, Dang L, Guo B, He X, Au DWT, Lu C, Zhu H, Zhang BT, Lu A, Zhang G. Tumor cell-targeted delivery of CRISPR/Cas9 by aptamer-functionalized lipopolymer for therapeutic genome editing of VEGFA in osteosarcoma. Biomaterials. 2017;147:68–85.
Lino CA, Harper JC, Carney JP, Timlin JA. Delivering CRISPR: a review of the challenges and approaches. Drug Deliv. 2018;25:1234–57.
Behboudi E, Zeynali P, Hamidi-Sofiani V, Nakstad B, Tahamtan A. Transcription activator-like effector nuclease (TALEN) as a promising diagnostic approach for COVID-19. Expert Rev Mol Diagn. 2022;22:395–7.
Pattanayak V, Ramirez CL, Joung JK, Liu DR. Revealing off-target cleavage specificities of zinc-finger nucleases by in vitro selection. Nat Methods. 2011;8:765–70.
Chen S, Sun H, Miao K, Deng CX. CRISPR-Cas9: from genome editing to cancer research. Int J Biol Sci. 2016;12:1427–36.
Zhang Y, Zhang F, Li X, Baller JA, Qi Y, Starker CG, Bogdanove AJ, Voytas DF. Transcription activator-like effector nucleases enable efficient plant genome engineering. Plant Physiol. 2013;161:20–7.
Chen J, Zhang W, Lin J, Wang F, Wu M, Chen C, Zheng Y, Peng X, Li J, Yuan Z. An efficient antiviral strategy for targeting hepatitis B virus genome using transcription activator-like effector nucleases. Mol Ther. 2014;22:303–11.
Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–78.
Liu C, Zhang L, Liu H, Cheng K. Delivery strategies of the CRISPR-Cas9 gene-editing system for therapeutic applications. J Control Release. 2017;266:17–26.
Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315:1709–12.
Fujikura Y, Kimura K, Yamanouchi K, Sugihara H, Hatakeyama M, Zhuang H, Abe T, Daimon M, Morita H, Komuro I, Oishi K. A medium-chain triglyceride containing ketogenic diet exacerbates cardiomyopathy in a CRISPR/Cas9 gene-edited rat model with Duchenne muscular dystrophy. Sci Rep. 2022;12:11580.
Freitas GP, Lopes HB, Souza ATP, Gomes MPO, Quiles GK, Gordon J, Tye C, Stein JL, Stein GS, Lian JB, Beloti MM, Rosa AL. Mesenchymal stem cells overexpressing BMP-9 by CRISPR-Cas9 present high in vitro osteogenic potential and enhance in vivo bone formation. Gene Ther. 2021;28:748–59.
Asghari F, Samiei M, Adibkia K, Akbarzadeh A, Davaran S. Biodegradable and biocompatible polymers for tissue engineering application: a review. Artif Cells Nanomed Biotechnol. 2017;45:185–92.
Kumar N, Desagani D, Chandran G, Ghosh NN, Karthikeyan G, Waigaonkar S, Ganguly A. Biocompatible agarose-chitosan coated silver nanoparticle composite for soft tissue engineering applications. Artif Cells Nanomed Biotechnol. 2018;46:637–49.
Parmar H, Tucci F, Carlone P, Sudarshan TS. Metallisation of polymers and polymer matrix composites by cold spray: state of the art and research perspectives. Int Mater Rev. 2022;67:385–409.
Wang LL, Chung JJ, Li EC, Uman S, Atluri P, Burdick JA. Injectable and protease-degradable hydrogel for siRNA sequestration and triggered delivery to the heart. J Control Release. 2018;285:152–61.
Natterodt JC, Shirole A, Sapkota J, Zoppe JO, Weder C. Polymer nanocomposites with cellulose nanocrystals made by co-precipitation. J Appl Polym Sci. 2017;135:45648.
Ni JF, Zhou HH, Chen JT, Zhang XX. LiFePO4 doped with ions prepared by co-precipitation method. Mater Lett. 2005;59:2361–5.
Shao C, Chang H, Wang M, Xu F, Yang J. High-strength, tough, and self-healing nanocomposite physical hydrogels based on the synergistic effects of dynamic hydrogen bond and dual coordination bonds. ACS Appl Mater Interfaces. 2017;9:28305–18.
Chen J, Zhu H, Xia J, Zhu Y, Xia C, Hu Z, Jin Y, Wang J, He Y, Dai J, Hu Z. High-performance multi-dynamic bond cross-linked hydrogel with spatiotemporal siRNA delivery for gene-cell combination therapy of intervertebral disc degeneration. Adv Sci. 2023;10: e2206306.
Godoy-Gallardo M, Eckhard U, Delgado LM, de Roo Puente YJD, Hoyos-Nogués M, Gil FJ, Perez RA. Antibacterial approaches in tissue engineering using metal ions and nanoparticles: from mechanisms to applications. Bioact Mater. 2021;6:4470–90.
CAS PubMed PubMed Central Google Scholar
Kramer RK, Belgacem MN, Carvalho AJF, Gandini A. Thermally reversible nanocellulose hydrogels synthesized via the furan/maleimide Diels-Alder click reaction in water. Int J Biol Macromol. 2019;141:493–8.
Wilson A, Gasparini G, Matile S. Functional systems with orthogonal dynamic covalent bonds. Chem Soc Rev. 2014;43:1948–62.
Cao H, Zhang Z, Zhao S, He X, Yu H, Yin Q, Zhang Z, Gu W, Chen L, Li Y. Hydrophobic interaction mediating self-assembled nanoparticles of succinobucol suppress lung metastasis of breast cancer by inhibition of VCAM-1 expression. J Controlled Release. 2015;205:162–71.
Fiorica C, Palumbo FS, Pitarresi G, Allegra M, Puleio R, Giammona G. Hyaluronic acid and α-elastin based hydrogel for three dimensional culture of vascular endothelial cells. J Drug Delivery Sci Technol. 2018;46:28–33.
Paidikondala M, Kadekar S, Varghese OP. Innovative strategy for 3D transfection of primary human stem cells with BMP-2 expressing plasmid DNA: a clinically translatable strategy for ex vivo gene therapy. Int J Mol Sci. 2018;20:56.
Bi S, Pang J, Huang L, Sun M, Cheng X, Chen X. The toughness chitosan-PVA double network hydrogel based on alkali solution system and hydrogen bonding for tissue engineering applications. Int J Biol Macromol. 2020;146:99–109.
Paidikondala M, Nawale GN, Varghese OP. Insights into siRNA transfection in suspension: efficient gene silencing in human mesenchymal stem cells encapsulated in hyaluronic acid hydrogel. Biomacromol. 2019;20:1317–24.
Lee J, Chang K, Kim S, Gite V, Chung H, Sohn D. Phase controllable hyaluronic acid hydrogel with iron(III) ion-catechol induced dual cross-linking by utilizing the gap of gelation kinetics. Macromolecules. 2016;49:7450–9.
Chakma P, Konkolewicz D. Dynamic covalent bonds in polymeric materials. Angew Chem Int Ed Engl. 2019;58:9682–95.
Meyer EE, Rosenberg KJ, Israelachvili J. Recent progress in understanding hydrophobic interactions. Proc Natl Acad Sci U S A. 2006;103:15739–46.
Kim YM, Park MR, Song SC. Injectable polyplex hydrogel for localized and long-term delivery of siRNA. ACS Nano. 2012;6:5757–66.
Chen YB, Qiao T, Wang YQ, Cui YL, Wang QS. Hydrogen bond-enhanced nanogel delivery system for potential intranasal therapy of Parkinson’s disease. Mater Des. 2022;219: 110741.
Flégeau K, Puiggali-Jou A, Zenobi-Wong M. Cartilage tissue engineering by extrusion bioprinting utilizing porous hyaluronic acid microgel bioinks. Biofabrication. 2022;14: 034105.
Patras G, Qiao GG, Solomon DH. Characterization of the pore structure of aqueous three-dimensional polyacrylamide gels with a novel cross-linker. Electrophoresis. 2000;21:3843–50.
Zhao Y, Tian Y, Ye W, Wang X, Huai Y, Huang Q, Chu X, Deng X, Qian A. A lipid–polymer hybrid nanoparticle (LPN)-loaded dissolving microneedle patch for promoting hair regrowth by transdermal miR-218 delivery. Biomater Sci. 2022;11:140–52.
Shen X, Shamshina JL, Berton P, Gurau G, Rogers RD. Hydrogels based on cellulose and chitin: fabrication, properties, and applications. Green Chem. 2016;18:53–75.
Ju Y, Hu Y, Yang P, Xie X, Fang B. Extracellular vesicle-loaded hydrogels for tissue repair and regeneration. Mater Today Bio. 2022;18: 100522.
Perrier-Groult E, Pasdeloup M, Malbouyres M, Galéra P, Mallein-Gerin F. Control of collagen production in mouse chondrocytes by using a combination of bone morphogenetic protein-2 and small interfering RNA targeting Col1a1 for hydrogel-based tissue-engineered cartilage. Tissue Eng Part C Methods. 2013;19:652–64.
Wang F, Hu K, Cheng Y. Structure–activity relationship of dendrimers engineered with twenty common amino acids in gene delivery. Acta Biomater. 2016;29:94–102.
Li B, Wang F, Hu F, Ding T, Huang P, Xu X, Liang J, Li C, Zhou Q, Lu M, Deng L, Guo L, Cui W. Injectable, “nano-micron” combined gene-hydrogel microspheres for local treatment of osteoarthritis. NPG Asia Mater. 2022;14:1.
He C, Kim SW, Lee DS. In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery. J Controlled Release. 2008;127:189–207.
Hong LTA, Kim YM, Park HH, Hwang DH, Cui Y, Lee EM, Yahn S, Lee JK, Song SC, Kim BG. An injectable hydrogel enhances tissue repair after spinal cord injury by promoting extracellular matrix remodeling. Nat Commun. 2017;8:533.
Liu L, Liu Y, Feng C, Chang J, Fu R, Wu T, Yu F, Wang X, Xia L, Wu C, Fang B. Lithium-containing biomaterials stimulate bone marrow stromal cell-derived exosomal miR-130a secretion to promote angiogenesis. Biomaterials. 2019;192:523–36.
Huang L, Shi Y, Li M, Wang T, Zhao L. Plasma exosomes loaded pH-responsive carboxymethylcellulose hydrogel promotes wound repair by activating the vascular endothelial growth factor signaling pathway in type 1 diabetic mice. J Biomed Nanotechnol. 2021;17:2021–33.
Khaled SZ, Cevenini A, Yazdi IK, Parodi A, Evangelopoulos M, Corbo C, Scaria S, Hu Y, Haddix SG, Corradetti B, Salvatore F, Tasciotti E. One-pot synthesis of pH-responsive hybrid nanogel particles for the intracellular delivery of small interfering RNA. Biomaterials. 2016;87:57–68.
Kim YE, Choi SW, Kim MK, Nguyen TL, Kim J. Therapeutic hydrogel patch to treat atopic dermatitis by regulating oxidative stress. Nano Lett. 2022;22:2038–47.
Lei H, Fan D. A combination therapy using electrical stimulation and adaptive, conductive hydrogels loaded with self-assembled nanogels incorporating short interfering RNA promotes the repair of diabetic chronic wounds. Adv Sci. 2022;9: e2201425.
Chen W, Fu X, Ge S, Sun T, Zhou G, Jiang D, Sheng Z. Ontogeny of expression of transforming growth factor-beta and its receptors and their possible relationship with scarless healing in human fetal skin. Wound Repair Regen. 2005;13:68–75.
Zhao R, Yan Q, Huang H, Lv J, Ma W. Transdermal siRNA-TGFβ1-337 patch for hypertrophic scar treatment. Matrix Biol. 2013;32:265–76.
Huynh CT, Nguyen MK, Tonga GY, Longé L, Rotello VM, Alsberg E. Photocleavable hydrogels for light-triggered siRNA release. Adv Healthc Mater. 2016;5:305–10.
Yang Y, Liu X, Li Y, Wang Y, Bao C, Chen Y, Lin Q, Zhu L. A postoperative anti-adhesion barrier based on photoinduced imine-crosslinking hydrogel with tissue-adhesive ability. Acta Biomater. 2017;62:199–209.
Duong HTT, Thambi T, Yin Y, Kim SH, Nguyen TL, Phan VHG, Kim J, Jeong JH, Lee DS. Degradation-regulated architecture of injectable smart hydrogels enhances humoral immune response and potentiates antitumor activity in human lung carcinoma. Biomaterials. 2020;230: 119599.
Qin H, Ji Y, Li G, Xu X, Zhang C, Zhong W, Xu S, Yin Y, Song J. MicroRNA-29b/graphene oxide-polyethyleneglycol-polyethylenimine complex incorporated within chitosan hydrogel promotes osteogenesis. Front Chem. 2022;10: 958561.
Kim YM, Park MR, Song SC. An injectable cell penetrable nano-polyplex hydrogel for localized siRNA delivery. Biomaterials. 2013;34:4493–500.
Gilam A, Conde J, Weissglas-Volkov D, Oliva N, Friedman E, Artzi N, Shomron N. Local microRNA delivery targets Palladin and prevents metastatic breast cancer. Nat Commun. 2016;7:12868.
Ryoo NK, Lee J, Lee H, Hong HK, Kim H, Lee JB, Woo SJ, Park KH, Kim H. Therapeutic effects of a novel siRNA-based anti-VEGF (siVEGF) nanoball for the treatment of choroidal neovascularization. Nanoscale. 2017;9:15461–9.
Lei L, Zhu Y, Qin X, Chai S, Liu G, Su W, Lv Q, Li D. Magnetic biohybrid microspheres for protein purification and chronic wound healing in diabetic mice. Chem Eng J. 2021;425: 130671.
Lei L, Lv Q, Jin Y, An H, Shi Z, Hu G, Yang Y, Wang X, Yang L. Angiogenic microspheres for the treatment of a thin endometrium. ACS Biomater Sci Eng. 2021;7:4914–20.
Lei L, Wang X, Zhu Y, Su W, Lv Q, Li D. Antimicrobial hydrogel microspheres for protein capture and wound healing. Mater Des. 2022;215: 110478.
Gan J, Sun L, Chen G, Ma W, Zhao Y, Sun L. Mesenchymal stem cell exosomes encapsulated oral microcapsules for acute colitis treatment. Adv Healthc Mater. 2022;11: e2201105.
DiStefano TJ, Vaso K, Panebianco CJ, Danias G, Chionuma HN, Kunnath K, Karoulias SZ, Wang M, Xu P, Davé RN, Sahoo S, Weiser JR, Iatridis JC. Hydrogel-embedded poly(lactic-co-glycolic acid) microspheres for the delivery of hMSC-derived exosomes to promote bioactive annulus fibrosus repair. Cartilage. 2022;13:19476035221113960.
Zhang Y, Xie Y, Hao Z, Zhou P, Wang P, Fang S, Li L, Xu S, Xia Y. Umbilical mesenchymal stem cell-derived exosome-encapsulated hydrogels accelerate bone repair by enhancing angiogenesis. ACS Appl Mater Interfaces. 2021;13:18472–87.
Zhu D, Li Z, Huang K, Caranasos TG, Rossi JS, Cheng K. Minimally invasive delivery of therapeutic agents by hydrogel injection into the pericardial cavity for cardiac repair. Nat Commun. 2021;12:1412.
Wang LL, Liu Y, Chung JJ, Wang T, Gaffey AC, Lu M, Cavanaugh CA, Zhou S, Kanade R, Atluri P, Morrisey EE, Burdick JA. Sustained miRNA delivery from an injectable hydrogel promotes cardiomyocyte proliferation and functional regeneration after ischemic injury. Nat Biomed Eng. 2017;1:983–92.
Zhu J, Yang S, Qi Y, Gong Z, Zhang H, Liang K, Shen P, Huang YY, Zhang Z, Ye W, Yue L, Fan S, Shen S, Mikos AG, Wang X, Fang X. Stem cell-homing hydrogel-based miR-29b-5p delivery promotes cartilage regeneration by suppressing senescence in an osteoarthritis rat model. Sci Adv. 2022;8:eabk0011.
Shen K, Duan A, Cheng J, Yuan T, Zhou J, Song H, Chen Z, Wan B, Liu J, Zhang X, Zhang Y, Xie R, Liu F, Fan W, Zuo Q. Exosomes derived from hypoxia preconditioned mesenchymal stem cells laden in a silk hydrogel promote cartilage regeneration via the miR-205-5p/PTEN/AKT pathway. Acta Biomater. 2022;143:173–88.
Liu W, Rong Y, Wang J, Zhou Z, Ge X, Ji C, Jiang D, Gong F, Li L, Chen J, Zhao S, Kong F, Gu C, Fan J, Cai W. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J Neuroinflammation. 2020;17:47.
Cao Y, Xu Y, Chen C, Xie H, Lu H, Hu J. Local delivery of USC-derived exosomes harboring ANGPTL3 enhances spinal cord functional recovery after injury by promoting angiogenesis. Stem Cell Res Ther. 2021;12:20.
Kanazawa T, Shizawa Y, Takeuchi M, Tamano K, Ibaraki H, Seta Y, Takashima Y, Okada H. Topical anti-nuclear factor-kappa B small interfering RNA with functional peptides containing sericin-based hydrogel for atopic dermatitis. Pharmaceutics. 2015;7:294–304.
Li Y, Wang M, Sun M, Wang X, Pei D, Lei B, Li A. Engineering antioxidant poly (citrate-gallic acid)-exosome hybrid hydrogel with microglia immunoregulation for traumatic brain Injury-post neuro-restoration. Compos Part B Eng. 2022;242: 110034.
Gao M, Yang C, Wu C, Chen Y, Zhuang H, Wang J, Cao Z. Hydrogel–metal-organic-framework hybrids mediated efficient oral delivery of siRNA for the treatment of ulcerative colitis. J Nanobiotechnol. 2022;20:404.
Rui K, Tang X, Shen Z, Jiang C, Zhu Q, Liu S, Che N, Tian J, Ling J, Yang Y. Exosome inspired photo-triggered gelation hydrogel composite on modulating immune pathogenesis for treating rheumatoid arthritis. J Nanobiotechnol. 2023;21:111.
Tao SC, Guo SC, Li M, Ke QF, Guo YP, Zhang CQ. Chitosan wound dressings incorporating exosomes derived from microRNA-126-overexpressing synovium mesenchymal stem cells provide sustained release of exosomes and heal full-thickness skin defects in a diabetic rat model. Stem Cells Transl Med. 2017;6:736–47.
Wang D, Liu Y, Liu Y, Yan L, Zaat SA, Wismeijer D, Pathak JL, Wu G. A dual functional bone-defect-filling material with sequential antibacterial and osteoinductive properties for infected bone defect repair. J Biomed Mater Res A. 2019;107:2360–70.
Yang L, Liu Y, Sun L, Zhao C, Chen G, Zhao Y. Biomass microcapsules with stem cell encapsulation for bone repair. Nano-Micro Lett. 2021;14:4.
Zhang H, Yang L, Yang XG, Wang F, Feng JT, Hua KC, Li Q, Hu YC. Demineralized bone matrix carriers and their clinical applications: an overview. Orthop Surg. 2019;11:725–37.
Su C, Chen Y, Tian S, Lu C, Lv Q. Research progress on emerging polysaccharide materials applied in tissue engineering. Polymers. 2022;14:3268.
Li Y, Chen X, Jin R, Chen L, Dang M, Cao H, Dong Y, Cai B, Bai G, Gooding JJ, Liu S, Zou D, Zhang Z, Yang C. Injectable hydrogel with MSNs/microRNA-21-5p delivery enables both immunomodification and enhanced angiogenesis for myocardial infarction therapy in pigs. Sci Adv. 2021;7:eabd6740.
Zhang Y, Wang J, Zhao J, Huang G, Liu K, Pan W, Sun L, Li J, Xu W, He C, Zhang Y, Li S, Zhang H, Zhu J, He Y. Current status and challenges in prenatal and neonatal screening, diagnosis, and management of congenital heart disease in China. Lancet Child Adolesc Health. 2023;7:479–89.
Correa S, Grosskopf AK, Lopez Hernandez H, Chan D, Yu AC, Stapleton LM, Appel EA. Translational applications of hydrogels. Chem Rev. 2021;121:11385–457.
Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–35.
Lee H, Park TG. Photo-crosslinkable, biomimetic, and thermo-sensitive pluronic grafted hyaluronic acid copolymers for injectable delivery of chondrocytes. J Biomed Mater Res A. 2009;88:797–806.
Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745–59.
Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33.
Bekkers JEJ, Tsuchida AI, van Rijen MHP, Vonk LA, Dhert WJA, Creemers LB, Saris DBF. Single-stage cell-based cartilage regeneration using a combination of chondrons and mesenchymal stromal cells: comparison with microfracture. Am J Sports Med. 2013;41:2158–66.
Zhang Y, Xie RL, Croce CM, Stein JL, Lian JB, van Wijnen AJ, Stein GS. A program of microRNAs controls osteogenic lineage progression by targeting transcription factor Runx2. Proc Natl Acad Sci U S A. 2011;108:9863–8.
McDonald JW, Sadowsky C. Spinal-cord injury. Lancet. 2002;359:417–25.
Yu Q, Jin S, Wang S, Xiao H, Zhao Y. Injectable, adhesive, self-healing and conductive hydrogels based on MXene nanosheets for spinal cord injury repair. Chem Eng J. 2023;452: 139252.
Cheng J, Chen Z, Liu C, Zhong M, Wang S, Sun Y, Wen H, Shu T. Bone mesenchymal stem cell-derived exosome-loaded injectable hydrogel for minimally invasive treatment of spinal cord injury. Nanomedicine. 2021;16:1567–79.
Wu HF, Cen JS, Zhong Q, Chen L, Wang J, Deng DYB, Wan Y. The promotion of functional recovery and nerve regeneration after spinal cord injury by lentiviral vectors encoding Lingo-1 shRNA delivered by Pluronic F-127. Biomaterials. 2013;34:1686–700.
Louw AM, Kolar MK, Novikova LN, Kingham PJ, Wiberg M, Kjems J, Novikov LN. Chitosan polyplex mediated delivery of miRNA-124 reduces activation of microglial cells in vitro and in rat models of spinal cord injury. Nanomed Nanotechnol. 2016;12:643–53.
Jang HJ, Lee JB, Yoon JK. Advanced in vitro three-dimensional skin models of atopic dermatitis. Tissue Eng Regen Med. 2023;20:539–622.
Kim H, Wang SY, Kwak G, Yang Y, Kwon IC, Kim SH. Exosome-guided phenotypic switch of M1 to M2 macrophages for cutaneous wound healing. Adv Sci. 2019;6:1900513.
Zhou Y, Zhang XL, Lu ST, Zhang NY, Zhang HJ, Zhang J, Zhang J. Human adipose-derived mesenchymal stem cells-derived exosomes encapsulated in pluronic F127 hydrogel promote wound healing and regeneration. Stem Cell Res Ther. 2022;13:407.
Cao J, Wang P, Liu Y, Zhu C, Fan D. Double crosslinked HLC-CCS hydrogel tissue engineering scaffold for skin wound healing. Int J Biol Macromol. 2022;155:625–35.
Mobahat M, Sadroddiny E, Nooshabadi VT, Ebrahimi-Barough S, Goodarzi A, Malekshahi ZV, Ai J. Curcumin-loaded human endometrial stem cells derived exosomes as an effective carrier to suppress alpha-synuclein aggregates in 6OHDA-induced Parkinson’s disease mouse model. Cell Tissue Bank. 2023;24:75–91.
Tang Q, Lu B, He J, Chen X, Fu Q, Han H, Luo C, Yin H, Qin Z, Lyu D, Zhang L, Zhou M, Yao K. Exosomes-loaded thermosensitive hydrogels for corneal epithelium and stroma regeneration. Biomaterials. 2022;280: 121320.
Hu B, Zhong L, Weng Y, Peng L, Huang Y, Zhao Y, Liang XJ. Therapeutic siRNA: state of the art. Sig Transduct Target Ther. 2020;5:101.
Ren N, Sun R, Xia K, Zhang Q, Li W, Wang F, Zhang X, Ge Z, Wang L, Fan C, Zhu Y. DNA-based hybrid hydrogels sustain water-insoluble ophthalmic therapeutic delivery against allergic conjunctivitis. ACS Appl Mater Interfaces. 2019;11:26704–10.
Sun Z, Song C, Wang C, Hu Y, Wu J. Hydrogel-based controlled drug delivery for cancer treatment: a review. Mol Pharm. 2020;17:373–91.
Yang Q, Li M, Yang X, Xiao Z, Tong X, Tuerdi A, Li S, Lei L. Flourishing tumor organoids: history, emerging technology, and application. Bioeng Transl Med. 2023;8: e10559.
Lüönd F, Tiede S, Christofori G. Breast cancer as an example of tumour heterogeneity and tumour cell plasticity during malignant progression. Br J Cancer. 2021;125:164–75.
Guo DD, Hong SH, Jiang HL, Kim JH, Minai-Tehrani A, Kim JE, Shin JY, Jiang T, Kim YK, Choi YJ, Cho CS, Cho MH. Synergistic effects of Akt1 shRNA and paclitaxel-incorporated conjugated linoleic acid-coupled poloxamer thermosensitive hydrogel on breast cancer. Biomaterials. 2012;33:2272–81.
Erkoc P, Cingöz A, Onder TB, Kizilel S. Quinacrine mediated sensitization of glioblastoma (GBM) cells to TRAIL through MMP-sensitive PEG hydrogel carriers. Macromol Biosci. 2017;17:1600267.
Peng H, Yang H, Song L, Zhou Z, Sun J, Du Y, Lu K, Li T, Yin A, Xu J, Wei S. Sustained delivery of siRNA/PEI complex from in situ forming hydrogels potently inhibits the proliferation of gastric cancer. J Exp Clin Cancer Res. 2016;35:57.
Chen X, Wang YW, Xing AY, Xiang S, Shi DB, Liu L, Li YX, Gao P. Suppression of SPIN1-mediated PI3K-Akt pathway by miR-489 increases chemosensitivity in breast cancer. J Pathol. 2016;239:459–72.
Lei Y, Zhang Q, Kuang G, Wang X, Fan Q, Ye F. Functional biomaterials for osteoarthritis treatment: from research to application. Smart Med. 2022;1: e20220014.
Yue L, Berman J. What is osteoarthritis? JAMA. 2022;327:1300.
Chen D, Shen J, Zhao W, Wang T, Han L, Hamilton JL, Im HJ. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 2017;5:16044.
Kim JH, Jeon J, Shin M, Won Y, Lee M, Kwak JS, Lee G, Rhee J, Ryu JH, Chun CH, Chun JS. Regulation of the catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis. Cell. 2014;156:730–43.
Wang J, Li Z, Pan M, Fiaz M, Hao Y, Yan Y, Sun L, Yan F. Ultrasound-mediated blood-brain barrier opening: an effective drug delivery system for theranostics of brain diseases. Adv Drug Deliv Rev. 2022;190: 114539.
Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912.
Ascherio A, Schwarzschild MA. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. 2016;15:1257–72.
Torres-Ortega PV, Del Campo-Montoya R, Plano D, Paredes J, Aldazabal J, Luquin MR, Luquin MR, Santamaría E, Sanmartín C, Blanco-Prieto MJ, Garbayo E. Encapsulation of MSCs and GDNF in an injectable nanoreinforced supramolecular hydrogel for brain tissue engineering. Biomacromol. 2022;23:4629–44.
Niu S, Zhang LK, Zhang L, Zhuang S, Zhan X, Chen WY, Du S, Yin L, You R, Li CH, Guan YQ. Inhibition by multifunctional magnetic nanoparticles loaded with alpha-synuclein RNAi plasmid in a Parkinson’s disease model. Theranostics. 2017;7:344–56.
Capizzi A, Woo J, Verduzco-Gutierrez M. Traumatic brain injury: an overview of epidemiology, pathophysiology, and medical management. Med Clin North Am. 2020;104:213–38.
Jian Y, Zhang D, Liu M, Wang Y, Xu ZX. The impact of gut microbiota on radiation-induced enteritis. Front Cell Infect Microbiol. 2021;11: 586392.
Zhang M, Wang X, Han MK, Collins JF, Merlin D. Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Nanomedicine. 2017;12:1927–43.
Xu J, Tam M, Samaei S, Lerouge S, Barralet J, Stevenson MM, Cerruti M. Mucoadhesive chitosan hydrogels as rectal drug delivery vessels to treat ulcerative colitis. Acta Biomater. 2017;48:247–57.
Danese S, Roda G, Peyrin-Biroulet L. Evolving therapeutic goals in ulcerative colitis: towards disease clearance. Nat Rev Gastroenterol Hepatol. 2020;17:1–2.
Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388:2023–38.
Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, Shi H, Wu L, Zhu W, Qian H, Xu W. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells. 2015;33:2158–68.
Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–94.
Ji S, Zhu Z, Sun X, Fu X. Functional hair follicle regeneration: an updated review. Signal Transduct Target Ther. 2021;6:66.
Hu S, Li Z, Lutz H, Huang K, Su T, Cores J, Dinh PC, Cheng K. Dermal exosomes containing miR-218-5p promote hair regeneration by regulating β-catenin signaling. Sci Adv. 2020;6:eaba1685.
Clevers H, Loh KM, Nusse R. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:1248012.
Download references
Acknowledgements
Not applicable.
The study was supported by the Zhejiang Shuren University research project (2023R053 and 2023KJ237).
Author information
Chunyu Su and Dini Lin contributed equally to this work.
Authors and Affiliations
Key Laboratory of Artificial Organs and Computational Medicine in Zhejiang Province, Institute of Translational Medicine, Zhejiang Shuren University, Hangzhou, 310015, China
Chunyu Su, Jiayin Feng, Anqi Jin, Fangyan Wang & Lanjie Lei
The Third Affiliated Hospital of Wenzhou Medical University, Wenzhou, 325200, China
Chunyu Su, Dini Lin & Wenjie Pan
College of Biology & Pharmacy, Yulin Normal University, Yulin, 537000, China
Chunyu Su, Xinyu Huang & Qizhuang Lv
You can also search for this author in PubMed Google Scholar
Contributions
QL, LL, and WP accomplished the conception of the research work and designed the manuscript. CS, DL, XH, JF, AJ and FW wrote the main manuscript and drew the figures. QL, LL and WP reviewed and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Qizhuang Lv , Lanjie Lei or Wenjie Pan .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors have no competing interests as defined by BMC, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Su, C., Lin, D., Huang, X. et al. Developing hydrogels for gene therapy and tissue engineering. J Nanobiotechnol 22 , 182 (2024). https://doi.org/10.1186/s12951-024-02462-z
Download citation
Received : 20 February 2024
Accepted : 04 April 2024
Published : 15 April 2024
DOI : https://doi.org/10.1186/s12951-024-02462-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Gene therapy
- Tissue engineering
Journal of Nanobiotechnology
ISSN: 1477-3155
- Submission enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
A decade of CRISPR. In the decade since the publication of CRISPR-Cas9 as a genome-editing technology, the CRISPR toolbox and its applications have profoundly changed basic and applied biological research. Wang and Doudna now review the origins and utility of CRISPR-based genome editing, the successes and current limitations of the technology ...
The genome-editing technologies and CRISPR tools have come to the current exciting stage through years of basic science research and progress from a large number of researchers. This review will ...
The CRISPR/Cas-9 genome-editing tool has a wide number of applications in many areas including medicine, agriculture, and biotechnology. In agriculture, it could help in the design of new grains to improve their nutritional value. In medicine, it is being investigated for cancers, HIV, and gene therapy such as sickle cell disease, cystic ...
This paper reviews the current developments in three aspects, namely, gene-editing type, delivery vector, and disease characteristics. ... Previous research on CRISPR/Cas9 has focused on ...
CRISPR/Cas9 is a simple two-component system used for effective targeted gene editing. The first component is the single-effector Cas9 protein, which contains the endonuclease domains RuvC and HNH. RuvC cleaves the DNA strand non-complementary to the spacer sequence and HNH cleaves the complementary strand.
A comprehensive understanding of these limitations guides ongoing research toward the creation of secure and efficient nanomaterials for gene editing purposes [64]. To conclude, nanomaterials exhibit significant potential for optimizing CRISPR-Cas9 gene editing through tailored modifications and innovative strategies [[65], [66], [67]]. 5.
The authors of this paper, also state this methodology can be applied to ... Beyond off-target effects in CRISPR editing and DNA interaction, recent research has found unanticipated effects in protein expression and function after ... CRISPR-based gene editing tools, in particular, are quickly developing and have been used to generate diverse ...
In this Review, we discuss the current state of CRISPR gene editing technologies in both research and therapy, highlighting limitations that constrain them and the technological innovations that have been developed in recent years to address them. ... Consequently, the application potential of first-generation CRISPR-based gene editing tools is ...
Abstract. The recent development of the CRISPR/Cas9 system as an efficient and accessible programmable genome-editing tool has revolutionized basic science research. CRISPR/Cas9 system-based technologies have armed researchers with new powerful tools to unveil the impact of genetics on disease development by enabling the creation of precise ...
It's CRISPR. Two scientists who pioneered the revolutionary gene-editing technology are the winners of this year's Nobel Prize in Chemistry. The Nobel Committee's selection of Emmanuelle ...
CRISPR-Cas9 Gene Editing for SCD and TDT. 3m 25s. Transfusion-dependent β-thalassemia (TDT) and sickle cell disease (SCD) are the most common monogenic diseases worldwide, with an annual ...
CRISPR (clustered regularly interspaced short palindromic repeats) is a Nobel Prize-winning technology that holds significant promise for revolutionizing the prevention and treatment of human disease through gene editing. However, CRISPR's public health implications remain relatively uncertain and underdiscussed because (1) targeting genetic factors alone will have limited influence on ...
The rapid development of genome editing technology has brought major breakthroughs in the fields of life science and medicine. In recent years, the clustered regularly interspaced short palindromic repeats (CRISPR)-based genome editing toolbox has been greatly expanded, not only with emerging CRISPR-associated protein (Cas) nucleases, but also novel applications through combination with ...
The prediction of off-target mutations in CRISPR/Cas9 is a hot topic owing to its relevance to gene-editing research. Cas nucleases may cleave unintended genomic sites and cause unexpected mutations called off-target cleavage (Listgarten et al., 2018). Even though the CRISPR/Cas9 system is routinely used in a large variety of tasks, there is ...
The 2020 Nobel Prize in Chemistry was awarded to Emmanuelle Charpentier and Jennifer Doudna for the development of the Clustered regularly interspaced short palindromic repeats/CRISPR-associated nuclease9 (CRISPR/Cas9) gene editing technology that provided new tools for precise gene editing. It is possible to target any genomic locus virtually using only a complex nuclease protein with short ...
Cystic Fibrosis* / therapy. DNA End-Joining Repair. Gene Editing*. Genetic Therapy. Humans. Cystic fibrosis (CF) is a life-limiting genetic disorder affecting approximately 70,000 people worldwide. Current burden of treatment is high. While the latest pharmaceutical innovation has benefitted many, patients with certain genotypes remain excluded.
A modified form of CRISPR system, known as CRISPR/Cpf1 that employs a smaller and simpler endonuclease (Cpf1) than Cas9, can be used to overcome certain limitations of CRISPR/Cas9 system. Despite clear-cut innovative biological applications, this technology is challenged by off-target effects and associated risks, thus safe and controlled ...
The discovery of CRISPR-Cas9 in 2012 stemmed from basic scientific research into how bacteria fight off viruses. Researchers realized that a portion of the bacterial immune system contains molecules that precisely snip DNA at specific locations and developed that into the molecular scissors of CRISPR-Cas9 that allow the precise editing of human, plant, and animal DNA at specific locations.
Efforts have been directed towards genome editing in cyanobacteria, yet achieving genome reduction in cyanophages remains a challenging task. In this study, we utilized the CRISPR-Cas12a system to successfully delete multiple genes within A-1(L) and A-4(L) cyanophages. Through careful manipulation, we generated a deletion mutant cyanophage with a 2,778 bp reduction in genome size, representing ...
Recently, two independent research groups found that CRISPR-Cas mediated double-stranded breaks (DSBs) can activate the p53 signaling pathway , . This means ... published a paper about gene editing in human tripronuclear zygotes in the journal Protein & Cell, which brings the ethical controversy of human embryo gene editing to a climax . Since ...
Jennifer Doudna, whose work on CRISPR earned her the 2020 Nobel Prize in chemistry, applauded the recent approval of a CRISPR-based gene-editing therapy to help those struggling with sickle-cell disease. The therapy, developed by Boston-based Vertex Pharmaceuticals and CRISPR Therapeutics, was approved by the FDA in 2023.
One of the greatest strengths of Drosophila genetics is its easily observable and selectable phenotypic markers. The mini-white marker has been widely used as a transgenic marker for Drosophila transgenesis. Flies carrying a mini-white construct can exhibit various eye colors ranging from pale orange to intense red, depending on the insertion site and gene dosage. Because the two copies of the ...
While gene editing is already in use, CRISPR is still in the clinical trials phase, Dr. Chan says. "It's used all the time in research laboratories and industries," he notes.
CRISPR-Cas9 genome editing exploits the CRISPR-Cas system to modify a genome in a targeted manner. Guided by RNA, the Cas9 endonuclease breaks DNA at a target sequence. Imprecise repair of the ...
CRISPR-Cas systems have proven effective in a variety of applications due to their ease of use and relatively high editing efficiency. Yet, any individual CRISPR-Cas system has inherent limitations, necessitating a diversity of RNA-guided nucleases to suit applications with distinct needs. We searched through metagenomic sequences to identify RNA-guided nucleases and found enzymes from diverse ...
DOI: 10.1007/s11816-024-00901-9 Corpus ID: 269045609; Optimization of CRISPR/Cas9 ribonucleoprotein delivery into cabbage protoplasts for efficient DNA-free gene editing @article{Lee2024OptimizationOC, title={Optimization of CRISPR/Cas9 ribonucleoprotein delivery into cabbage protoplasts for efficient DNA-free gene editing}, author={Sora Lee and Su Hyun Park and Yu Jeong Jeong and Soyoung Kim ...
The gene editing technology known as CRISPR-Cas9 has given researchers the opportunity to precisely edit genes, an ability that can potentially lead to prevention or cures for genetic diseases that have long been incurable. ... Suchy enlisted the help of senior research scientist Katja Pekrun, PhD. Pekrun is familiar with Southern blots, a ...
Antimicrobial resistance (AMR) can potentially harm global public health. Horizontal gene transfer (HGT), which speeds up the emergence of AMR and increases the burden of drug resistance in mobile genetic elements (MGEs), is the primary method by which AMR genes are transferred across bacterial pathogens. New approaches are urgently needed to halt the spread of bacterial diseases and ...
Furthermore, Freitas et al. gene-edited MSCs with CRISPR-Cas9 to enhance gene expression of genes related to the BMP/TGF-β signaling pathway to overexpress BMP-9. CRISPR-Cas9 is a powerful genomic editing technology widely recognized for its broad range of applications, precise gene editing, and low cost (Fig. 4). However, this method has ...
During the past decade, numerous viral and nonviral delivery systems have been developed for Cas9 RNP-mediated gene editing. Viral vectors usually have high delivery efficiency but are associated with potential risks of immune responses, genome integration, and the activation of cancer-related genes (11, 12).Alternatively, nonviral carriers such as lipids (13, 14), inorganic nanoparticles ...