- Search Menu
- Sign in through your institution
- Volume 24, Issue 3, May 2024 (In Progress)
- Volume 24, Issue 2, March 2024
- Special Collections
- Author Guidelines
- Submission Site
- Open Access Options
- Self-Archiving Policy
- Reasons to Submit
- ESA Call for Papers
- Benefits of Publishing Open Access
- About Journal of Insect Science
- About the Entomological Society of America
- Editorial Board
- Journals on Oxford Academic
- Books on Oxford Academic

Special Collection: Honey Bee Research in the United States: Investigating Fundamental and Applied Aspects of Honey Bee Biology
The Journal of Insect Science , in partnership with the American Association of Professional Apiculturists, is pleased to publish a special collection of articles featuring the latest research on honey bees.
Honey bees are the most important pollinators in agricultural systems. However, the annual mortality of colonies has been consistently higher than historical records in the United States in recent years. Because of their economic importance and their amenability to management, honey bees provide a unique opportunity to investigate topics that cover a wide range of areas that focus on a mixture of applied and basic questions. The American Association of Professional Apiculturists (AAPA) consists of members whose labs conduct research on a wide range of subjects encompassing genomics, epigenetics, immunity, toxicology, host-parasite interactions, nutrition, evolutionary biology, and population ecology of this model organism. In addition, these labs address key questions about how to develop better management practices that can help the beekeeping industry in the context of the multiple environmental stressors that currently affect honey bee health. This special collection unites honey bee scholars across fields of study to show the state of the most current research on honey bees in the United States. The collection, presented in two parts, will include mini-reviews on topics relating to honey bee health and colony productivity and empirical studies from members associated with AAPA on topics of study including the full range of honey bee research.
The Journal of Insect Science is an open access journal. All articles are freely available to read, download, and re-use in some formats.
*The photograph used in the advertisement images is courtesy of the Florida Division of Plant Industry , Florida Department of Agriculture and Consumer Services, Bugwood.org.
Integrated Pest Management Control of Varroa destructor (Acari: Varroidae), the Most Damaging Pest of (Apis mellifera L. (Hymenoptera: Apidae)) Colonies

To encourage beekeeper adoption, a successful IPM approach to Varroa control in managed colonies must be an improvement over conventional control methods and include cost-effective treatments that can be employed readily by beekeepers. It is our intention to provide the most thorough review of Varroa control options available, ultimately framing our discussion within the context of IPM. We hope this article is a call-to-arms against the most damaging pest managed honey bee colonies face worldwide.
Surfing the Sweet Wave: Migrating Giant Honey Bees (Hymenoptera: Apidae: Apis dorsata) Display Spatial and Temporal Fidelity to Annual Stopover Site in Thailand

Apis dorsata F. (Hymenoptera: Apidae), the giant honey bee of southern Asia, is an important pollinator of crops and non-cultivated angiosperms, and a producer of honey and beeswax. Its populations are in decline in many areas. Here I describe their migratory dances in preparation for departure and their subsequent flights as well as periodic mass flight and defensive behavior. I also describe attributes of the stopover site.
Reproductive and Morphological Quality of Commercial Honey Bee (Hymenoptera: Apidae) Drones in the United States

Our study quantifies the presence of small drones in commercial populations, finding that rates of ‘low-quality’ drones are far higher than theoretically predicted under optimum conditions. Observations from commercial colonies also show significant inter-colony variation among the size and fecundity of drones produced, prompting speculation as to the mechanisms inducing such variation and the potential use of drone-quality variation for the colony- or apiary-level exposure to nutrition, agrichemical, or parasitic stressors.
Testicular Changes of Honey Bee Drones, Apis mellifera (Hymenoptera: Apidae), During Sexual Maturation

We describe the anatomy and sequential histological stages of normal testicular atrophy of drones sampled daily from emergence to sexual maturity in the spring (June) and early summer (July). This description of physiologic testicular atrophy should be useful for future studies investigating potential pathological effects of stressors on drone testes during sexual maturation.
Context-Dependent Effect of Dietary Phytochemicals on Honey Bees Exposed to a Pesticide, Thiamethoxam

Our study aims to understand the role of phytochemicals in pesticide tolerance when worker bees were fed with sublethal doses (1 ppb and 10 ppb) of thiamethoxam (TMX), a neonicotinoid, in 20% (w/v) sugar solution supplemented with 25 ppm of phytochemicals—caffeine, kaempferol, gallic acid, or p-coumaric acid, previously shown to have beneficial impacts on bee health. The effect of phytochemical supplementation during pesticide exposure was context-dependent.
Hygiene-Eliciting Brood Semiochemicals as a Tool for Assaying Honey Bee (Hymenoptera: Apidae) Colony Resistance to Varroa (Mesostigmata: Varroidae)

Here, we tested the hypothesis that hygienic response to a mixture of semiochemicals associated with Varroa -infested honey bee brood can serve as an improved tool for predicting colony-level Varroa resistance. In support of our hypothesis, we demonstrated that a mixture of the compounds (Z)-10-tritriacontene, (Z)-8-hentriacontene, (Z)-8-heptadecene, and (Z)-6-pentadecene triggers hygienic behavior in a two-hour assay, and that high-performing colonies have significantly lower Varroa infestations, remove significantly more introduced Varroa , and are significantly more likely to survive the winter compared to low-performing colonies.
Pollen Treated with a Combination of Agrochemicals Commonly Applied During Almond Bloom Reduces the Emergence Rate and Longevity of Honey Bee (Hymenoptera: Apidae) Queens

To test the individual and combined effects of some pesticides on the survival and emergence of developing queens, we fed worker honey bees in closed queen rearing boxes with pollen artificially contaminated with formulated pesticides as well as the spray adjuvant Dyne-Amic, which contains both organosilicone and alkyphenol ethoxylate. The translocation of pesticides from pesticide-treated pollen into the royal jelly secretions of nurse bees was also measured. The results support recommendations to protect honey bee health by avoiding application of pesticide tank-mixes containing insecticides and adjuvants during almond bloom.
Validation of Diagnostic Methods for European Foulbrood on Commercial Honey Bee Colonies in the United States

In this study, we validate the field use of the lateral flow device compared to microscopic examination and qPCR on larval samples from 78 commercial honey bee colonies in the United States with visual signs of infection. In this study, microscopic diagnosis was more sensitive than the lateral flow device, and we found no false positive results with the lateral flow device. We find high concurrence between the three diagnostic techniques, and all three methods are highly sensitive for diagnosing European foulbrood.
Honey Bee (Hymenoptera: Apidae) Nursing Responses to Cuticular Cues Emanating from Short-term Changes in Larval Rearing Environment

In a series of experiments, we manipulated larval feeding environment by depriving larvae from adult bee contact for four-hour period and examined (i) nurse bee interactions with contact-deprived and non-deprived larvae and larval extracts; (ii) forager bee responses to contact-deprived and non-deprived larval extracts. We also characterized brood ester pheromone of contact-deprived and non-deprived larvae.
Social Apoptosis in Varroa Mite Resistant Western Honey Bees (Apis mellifera)

We tested for the presence of the social apoptosis trait in two Varroa resistant stocks of A. mellifera with different selection histories and compared them to a known Varroa -susceptible stock. We assessed the survival and development of worker brood reared in either highly or lightly infested host colonies, then receiving one of three treatments: uninfested, experimentally inoculated with a Varroa mite, or wounded to simulate Varroa damage . We found that response to treatment was only differentiated in brood reared in lightly infested host colonies, where experimentally infested Russian honey bees had decreased survival relative to the mite-susceptible Italian stock. This is the first evidence that social apoptosis can exist in Western honey bee populations.
Honey Bees and Industrial Agriculture: What Researchers are Missing, and Why it’s a Problem

In this forum article, I unpack the relationship between honey bee health and industrial agriculture. I propose steps we can take to reframe our research to account for the impacts of this destructive system, and I discuss the uncomfortable questions that surface when we engage in this process. The goal of this article is to encourage conversation within the honey bee research community around the impacts of industrial agriculture, so that we can fully engage in the transformative change needed to support honey bee health.
Assessing Repeated Oxalic Acid Vaporization in Honey Bee (Hymenoptera: Apidae) Colonies for Control of the Ectoparasitic Mite Varroa destructor

We tested oxalic acid vaporization in colonies treated with seven applications separated by 5 d (35 d total). We found that adult honey bees and developing brood experienced no adverse impacts from the oxalic vaporization regime. However, we did not find evidence that frequent periodic application of oxalic during brood-rearing periods is capable of bringing V. destructor populations below treatment thresholds.
Honey Bees (Hymenoptera: Apidae) Decrease Foraging But Not Recruitment After Neonicotinoid Exposure

We conducted a feeder experiment with freely flying bees to determine the effects of a sublethal, field-realistic concentration of imidacloprid (IMD) on the foraging and recruitment behaviors of honey bees visiting either a control feeder containing a sucrose solution or a treatment feeder containing the same sucrose solution with IMD. IMD-treated honey bees foraged less frequently and persistently than control foragers. Recruitment behaviors (dance frequency and dance propensity) also decreased with IMD, but nonsignificantly. Our results suggest that neonicotinoids inhibit honey bee foraging, which could potentially decrease food intake and adversely affect colony health.
Impact of Honey Bee Migratory Management on Pathogen Loads and Immune Gene Expression is Affected by Complex Interactions With Environment, Worker Life History, and Season

To test long- and short-term impacts of managed migration on pathogen loads and immunity, experimental honey bee colonies were maintained with or without migratory movement. Age at collection, life-history stage, and season all influenced numerous factors from viral load to immune gene expression. Although the factors that we examined are not independent, the results illuminate potential factors in both migratory and nonmigratory beekeeping that are likely to contribute to colony stress, and also indicate potential mitigation measures.
Understanding the Enemy: A Review of the Genetics, Behavior and Chemical Ecology of Varroa destructor, the Parasitic Mite of Apis mellifera

Given the growing reports of pesticide resistance by Varroa in several countries, a better understanding of the mite’s basic biology is needed to find alternative pest management strategies. This review focuses on the genetics, behavior, and chemical ecology of V. destructor within A. mellifera colonies, and points to areas of research that should be exploited to better control this pervasive honey bee enemy.
Context-Dependent Viral Transgenerational Immune Priming in Honey Bees (Hymenoptera: Apidae)

Here we test for the presence of transgenerational immune priming in honey bees with deformed wing virus (DWV) by injecting pupae from DWV-exposed queens and measuring virus titer and immune gene expression. Our data suggest that there is evidence for viral transgenerational immune priming in honey bees, but it is highly context-dependent based on route of maternal exposure and potentially host genetics or epigenetic factors.
The lifespan and levels of oxidative stress between feral and managed honey bee colonies

In this study, we used paired colony designs to compare the life span of worker bees (foragers) between feral and managed colonies and their levels of oxidative stress. Each pair of colonies shared similar foraging resources. The results indicated that foragers in feral colonies had longer survival times and life spans than those in managed colonies. The levels of oxidative stress from lipid damage and the protein carbonyl content in feral colonies were higher than those in managed colonies, indicating they used a tolerance mechanism rather than a repair mechanism to survive. Our study provides new insights into a colony difference in the physiology and oxidative stress resistance of feral honey bees compared with managed colony stocks.
- Advertising and Corporate Services
- Entomology Today
- Recommend to Your Librarian
Affiliations
- Online ISSN 1536-2442
- Copyright © 2024 Entomological Society of America
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
One Health, One Hive: A scoping review of honey bees, climate change, pollutants, and antimicrobial resistance
Roles Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing
Affiliations Faculty of Agriculture, Life, and Environmental Sciences, Department of Agricultural, Food and Nutritional Science, University of Alberta, Edmonton, Canada, School of Public Health, University of Alberta, Edmonton, Canada, HEAT-AMR (Human-Environment-Animal Transdisciplinary Antimicrobial Resistance) Research Group, School of Public Health, University of Alberta, Edmonton, Canada, Faculty of Veterinary Medicine, University of Calgary, Calgary, Canada, Antimicrobial Resistance–One Health Consortium, Calgary, Canada
Roles Conceptualization, Funding acquisition, Methodology, Resources, Software, Supervision, Writing – review & editing
Affiliation School of Public Health, University of Alberta, Edmonton, Canada
Roles Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing
Roles Conceptualization, Funding acquisition, Methodology, Project administration, Writing – review & editing
Roles Methodology, Supervision, Writing – review & editing
Affiliation Faculty of Agriculture, Life, and Environmental Sciences, Department of Agricultural, Food and Nutritional Science, University of Alberta, Edmonton, Canada
Roles Data curation, Formal analysis, Writing – review & editing
Affiliation HEAT-AMR (Human-Environment-Animal Transdisciplinary Antimicrobial Resistance) Research Group, School of Public Health, University of Alberta, Edmonton, Canada
Roles Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliations School of Public Health, University of Alberta, Edmonton, Canada, HEAT-AMR (Human-Environment-Animal Transdisciplinary Antimicrobial Resistance) Research Group, School of Public Health, University of Alberta, Edmonton, Canada, Antimicrobial Resistance–One Health Consortium, Calgary, Canada, Healthy Environment Lead, Centre for Health Communities, School of Public Health, University of Alberta, Edmonton, Canada
- Etienne J. de Jongh,
- Sherilee L. Harper,
- Shelby S. Yamamoto,
- Carlee J. Wright,
- Craig W. Wilkinson,
- Soumyaditya Ghosh,
- Simon J. G. Otto

- Published: February 16, 2022
- https://doi.org/10.1371/journal.pone.0242393
- See the preprint
- Peer Review
- Reader Comments
Anthropogenic climate change and increasing antimicrobial resistance (AMR) together threaten the last 50 years of public health gains. Honey bees are a model One Health organism to investigate interactions between climate change and AMR. The objective of this scoping review was to examine the range, extent, and nature of published literature on the relationship between AMR and honey bees in the context of climate change and environmental pollutants. The review followed systematic search methods and reporting guidelines. A protocol was developed a priori in consultation with a research librarian. Resulting Boolean search strings were used to search Embase® via Ovid®, MEDLINE®, Scopus®, AGRICOLA™ and Web of Science™ databases. Two independent reviewers conducted two-stage screening on retrieved articles. To be included, the article had to examine honey bees, AMR, and either climate change or environmental pollution. Data, in accordance with Joanna Briggs Institute guidelines, were extracted from relevant articles and descriptively synthesized in tables, figures, and narrative form. A total of 22 articles met the inclusion criteria, with half of all articles being published in the last five years (n = 11/22). These articles predominantly investigated hive immunocompetence and multi-drug resistance transporter downregulation (n = 11/22), susceptibility to pests (n = 16/22), especially American foulbrood (n = 9/22), and hive product augmentation (n = 3/22). This review identified key themes and gaps in the literature, including the need for future interdisciplinary research to explore the link between AMR and environmental change evidence streams in honey bees. We identified three potential linkages between pollutive and climatic factors and risk of AMR. These interconnections reaffirm the necessity of a One Health framework to tackle global threats and investigate complex issues that extend beyond honey bee research into the public health sector. It is integral that we view these “wicked” problems through an interdisciplinary lens to explore long-term strategies for change.
Citation: de Jongh EJ, Harper SL, Yamamoto SS, Wright CJ, Wilkinson CW, Ghosh S, et al. (2022) One Health, One Hive: A scoping review of honey bees, climate change, pollutants, and antimicrobial resistance. PLoS ONE 17(2): e0242393. https://doi.org/10.1371/journal.pone.0242393
Editor: Guy Smagghe, Ghent University, BELGIUM
Received: October 30, 2020; Accepted: January 25, 2022; Published: February 16, 2022
Copyright: © 2022 de Jongh et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the manuscript and its Supporting Information files.
Funding: EJDJ: no number, University of Alberta Undergraduate Research Initiative, https://www.ualberta.ca/current-students/undergraduate-research-initiative/index.html . The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
The global rise of antimicrobial resistance (AMR) over the past 50 years presents troubling health projections for both public and environment sectors [ 1 ]. Antimicrobial resistance has global consequences for human health, resulting in approximately 700,000 deaths each year. By 2050, it is projected that the number of AMR-related deaths could rise to 10 million annually, with an estimated economic impact of $100 trillion USD [ 2 ]. Also at the forefront of global grand challenges lies climate change. The dire consequences of climate change have captured the focus and driven the collaboration of notable organizations such as NASA, the United Nations, and governments the world over [ 3 – 6 ].
Seeded into these critical contemporary issues are complex interactions that necessitate the conduct of interdisciplinary research [ 7 , 8 ]. Reports such as the World Health Organization (WHO) Antimicrobial Resistance Global Report, three recent Special Reports published by the Intergovernmental Panel on Climate Change (IPCC), and the Lancet Commission on Pollution and Health provide detailed insights into AMR, climate change, and environmental quality, respectively [ 1 , 9 – 12 ]. However, these reports neglect to substantially address these components through an interdisciplinary lens that links the three issues. Increasing communication between disciplines is not only helpful in understanding complex multidimensional problems, but is essential for implementing long-term solutions for mitigation [ 13 , 14 ].
While growing interest in areas such as One Health has helped bridge the topics of AMR, climate change, and environmental research, the majority of studies are still concerningly limited to the silo of each individual issue [ 1 ]. One Health is described as an approach to global health that focuses on linkages between the health of humans, animals, and the environment by improving intersectional communication and collaboration through research and policy [ 15 ].
Honey bees can serve as a model One Health organism to investigate the interactions between environmental change and AMR due to their inseparable symbiosis with the determinants of environmental health [ 16 , 17 ]. For example, environmental pollutants in water, soil, and air can negatively impact honey bee and hive health through leaching into pollen and honey foodstuffs [ 18 , 19 ]. Moreover, warming temperatures and other climatic factors related to climate change can increase the prevalence and spread of honey bee diseases and decrease the efficacy of antimicrobials in treating pests and pathogens [ 20 – 22 ]. Drug efficacy is further challenged by years of liberal antibiotic use [ 22 , 23 ], contributing to an increase in multidrug-resistant microorganisms. Apiaries globally are reporting greater colony losses than ever before [ 24 , 25 ]. It is generally believed that complex interactions between multiple environmental, pathogenic, and climatic factors are responsible for the majority of these losses, which have come to be referred to under the umbrella term of “colony collapse disorder” [ 26 , 27 ]. Interdisciplinary research into these interactions is therefore highly beneficial and inherently relevant to honey bee health.
How do environmental and climatic factors interact with each other to exacerbate AMR in honey bees? Given the limited evidence currently available, the objective of this scoping review was to examine the range, extent, and nature of published literature on the relationship between AMR and honey bees in the context of climate change and environmental pollutants through a One Health lens.
Materials and methods
Protocol and search strategy.
The review followed systematic search methods outlined in the Joanna Briggs Institute (JBI) Reviewer’s Manual and is reported according to the PRISMA Scoping Review reporting guidelines [ 28 – 33 ]. A time-stamped protocol was developed a priori in consultation with a research librarian ( S1 File ). The PRISMA-ScR checklist is provided in S1 Checklist .
A comprehensive search strategy was developed to identify articles that discussed AMR in honey bees in the context of environmental or climatic factors. No search restrictions were placed on language, publishing date, or geography. An example search string for Embase® via Ovid® is shown in Table 1 . The complete search strings ( S1 Table ) were used to search Embase® via Ovid®, MEDLINE®, Scopus®, AGRICOLA™ and Web of Science™ databases on July 10, 2019.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0242393.t001
After downloading all retrieved articles within Mendeley© (Elsevier, Amsterdam, Netherlands), articles were collated and de-duplicated in DistillerSR® (Evidence Partners, Ottawa, ON, Canada) and screened for eligibility via a two-stage screening process by two independent reviewers. Article titles, abstracts, and key words were screened in the first stage, followed by full-text screening in the second stage. To be included, the article had to examine honey bees, AMR, and either climate change or environmental pollution ( S2 File ). Antimicrobial resistance was defined as the ability of a pathogen to resist or reduce the effects of a drug or treatment meant to adversely affect its normal function [ 34 ]. Environmental change variables were defined as changes in climate due to natural or anthropogenic causes (climate change), or as an increase in organic or inorganic contaminants of soil, air, or water that alters their natural role or effect in honey bee colonies (environmental pollutants) [ 35 ]. Articles about season, weather, climate, and climate hazards in the context of climate change were also included. Honey bees were defined within the taxum Apis mellifera due to their agricultural importance, though articles using the terms “bees” or “honey bees” were considered relevant if no taxum was mentioned. The initial protocol required articles to include honey bees, AMR, climate change, and environmental pollutants. However, after screening articles to the data extraction level, a lack of articles containing all components prompted a revision of our inclusion criteria. This second round of screening included articles that studied honey bees, AMR and at least one of either climate change or environmental pollutants. This amendment was reflected within the protocol, which was re-time-stamped on December 9, 2019. The amendment was deemed necessary to provide sufficient evidence for discussion, to allow for better identification of gaps in literature, and to provide a more meaningful project outcome as a result. Articles were excluded if they were books, book chapters, theses, dissertations, or commentaries. Conflicts between reviewers were resolved via discussion if necessary.
Data charting process and data items
Data regarding authorship, publication date, location of study, type of antimicrobial and target microbe, environmental and/or climatic factor assessed, research study design type, associated organizations, and outcomes of interest were extracted from relevant articles by two reviewers using DistillerSR®. Article information was exported to a pre-developed data extraction form within Excel® (Microsoft, Redmond, WA) for analysis ( S2 Table ). Articles were partitioned into thematic categories for further exploration, including: immunocompetence and multi-drug resistance (MDR) transporter downregulation, susceptibility to pests, and in-hive products.
Results were synthesized in tables, graphs, and narrative to present the comprehensive scope of current research in a concise and effective manner. Tables and figures present key findings in the results, while supplementary materials provide comprehensive results from the study to allow for replication in future research.
The initial search recovered 1,402 articles, with 1,146 remaining after deduplication ( Fig 1 ). First-stage screening excluded 1,018 articles. 128 articles were eligible for second-stage, full-text screening, which reduced this number to 22. The majority of articles were excluded in this stage due to lacking mention of environmental variables or antibiotic resistance (n = 42), and failure to frame these topics in the context of honey bee health (n = 28). Despite our efforts to locate articles through both the University of Alberta and University of Guelph libraries, we were unable to locate full-text pdfs for 36 articles ( S3 File ). These articles were additionally requested through the University of Alberta and University of Guelph interlibrary loan systems to ensure minimal loss of articles. This process returned six additional articles that were screened, but 36 could not be obtained and were excluded.
https://doi.org/10.1371/journal.pone.0242393.g001
Characteristics of sources of evidence
Twenty-two articles met the inclusion criteria and were included in our analysis. An overview of these articles is included in Table 2 , while a complete listing of included articles and study characteristics is available in S2 Table . Articles were published between 1993 and 2019. Research on AMR and effects of environmental change in honey bees steadily increased in recent years with half (n = 11/22) of included articles published in the last five years alone (2014–2019) ( Fig 2 ).
Articles are organized by year of publication and represented in quantity by the length of the pin above each respective year. The number of articles per year is included inside each pinhead. *Note 2019 was an incomplete year because the article search was conducted in July 2019.
https://doi.org/10.1371/journal.pone.0242393.g002
https://doi.org/10.1371/journal.pone.0242393.t002
Fig 3 shows the study location in a global context. Article publication represented research from ten countries that was distributed globally. While some articles did not specify a geographical origin (n = 4), the majority of publications occurred in high-income nations (n = 13; Czech Republic, Germany, Italy, Japan, Norway, Spain, United States) [ 56 ]. The United States constituted the largest proportion of location-specific publications (n = 6). A large proportion of articles also came from Europe, with a total of seven articles spread over six European countries (Germany, n = 2; Czech Republic, n = 1; Italy, n = 1; Norway, n = 1; Spain, n = 1; Turkey, n = 1.
https://doi.org/10.1371/journal.pone.0242393.g003
Out of the 22 articles, 64% (n = 14/22) followed an experimental study design, with the rest being observational or descriptive studies (n = 16), or review articles (n = 2). There were relatively few studies with broader scope that investigated AMR and environmental change from a global or ecological perspective.
Synthesis of results
Table 3 summarizes environmental factors of interest by climatic or pollutive basis. Environmental factors of interest varied greatly, with environmental insecticides being the most common pollutive factors (n = 7) and indirect geographical differences (different climate zones as a result of different geographical locations) accounting for the majority of climatic factors (n = 6). Although most articles revealed potential indirect links to AMR in honey bees, few articles directly linked specific pollutive variables to AMR, the most common of which was the effect of neonicotinoids (n = 6).
https://doi.org/10.1371/journal.pone.0242393.t003
The 22 articles can be broadly divided into three thematic categories based on the focus of the study and linkage of AMR to environmental factors: 1) immunocompetence and MDR transporter downregulation; 2) interactions with pest susceptibility; and 3) influences on in-hive antimicrobial properties (categorization shown in Table 4 ).
https://doi.org/10.1371/journal.pone.0242393.t004
Immunocompetence and MDR transporter downregulation.
Of these 22 articles, nine focused on immunocompetence [ 20 , 38 , 43 – 45 , 49 , 50 , 53 ] and two investigated the downregulation of MDR transporters [ 41 , 42 ]. Combined, these eleven articles studied the synergistic effects of pesticides and climatic factors on honey bee innate immunity inhibition. Most articles found correlations between exposure to antibiotics or pathogens and decreasing honey bee immune function. One article found an increase in immune function when exposed to contaminants and infection, and one final article noted that dual exposure of pathogens and pesticides may increase transmission of disease [ 38 , 55 ]. Most articles focused on alterations in honey bee immunocompetence resulting from the inhibition of immune-essential endogenous microbiota within the gastrointestinal tract [ 20 , 38 , 43 – 45 , 49 , 50 , 53 ]. These articles described defensive reactions on the part of the biota (e.g. drug efflux, gene expression) to pollutants and environmental contaminants, as well as inhibition of these defensive mechanisms. Several articles explored alteration of MDR transporters, which are natural efflux pumps present in the cells of almost all animal species [ 41 , 42 ]. They pump many different classes of harmful compounds out of the cell, such as heavy metals, pesticides, and in some cases, antimicrobials [ 57 ]. Exposure to one of these compounds can trigger an upregulation of MDR efflux pump expression, thereby increasing resistance to multiple other types of compounds without direct exposure. In this way, MDR transporters can have substantial impact of the efficacy of drug dosages [ 42 ]. No article extrapolated this effect to the development of AMR.
Susceptibility to pests.
Most studies investigated bacterial infections, with almost half of all articles focusing on Paenibacillus larvae , the causative agent of American foulbrood (n = 9/22) [ 23 , 36 , 40 , 41 , 47 – 49 , 51 , 52 ]. Melissococcus plutonius , the causative agent of European foulbrood, and Enterococcus faecalis was also studied [ 23 , 49 , 55 ]. The parasitic mite Varroa destructor (n = 4/22) [ 41 , 49 , 53 , 54 ] and the fungal genus Nosema (n = 2/22) [ 42 , 50 ] received some marginal exploration. These articles linked increased pollutants to reduced honey bee health in the form of antimicrobial peptide (AMP) expression modulation. Antimicrobial peptides are critical to insect immune defence, and by altering their transcription or expression, environmental pollutants may lead to increased infection and transmission of pests and pathogens [ 38 ]. Articles largely neglected to evaluate how this increase in disease may necessitate the need for increased drug treatment in the hive and to the development of AMR. Articles that predominantly focused on V . destructor infection investigated also investigated morbidity as a result of deformed wing virus infection due to the strong association between these two pathogens [ 58 ]. Morbidity as a result of Varroa mite infection often occurs due to secondary infection via deformed wing virus, Escherichia coli , or other bacterial or viral infections [ 58 ]. Therefore, most papers included in this review investigating pest susceptibility explored more than one pathogen at a time. The strong association between pest exposure and immune response, combined with the two-punch approach of most honey bee parasites (destruction of the cuticle followed by secondary viral or bacterial infection), and the broad-spectrum nature of honey bee immune factors resulted in significant overlap between articles binned under pest susceptibility and immunocompetence.
In-hive products.
The third thematic category explored by this study was the self-administration of in-hive antimicrobial products on AMR. Three articles were included on this topic, all of which discussed the effect of the hive product propolis, an antibiotic and sealant made by the honey bees from resinous plant products, beeswax, and salivary enzymes [ 20 , 49 , 51 ]. Two of these three articles focused exclusively on the use of proplis [ 20 , 51 ], while one also investigated all-natural, pharmaceutically active compounds made and used by honey bees in the hive [ 49 ]. In regards to climatic variables, one article investigated seasonality and another investigated geographical origin as factors that impact the efficacy of propolis [ 20 , 51 ]. Together, these found that propolis was more inhibitory to bacteria, particularly P . larvae , when it was sourced from Brazil during the dry season. The remaining article looked how environmental factors influence self medicative behaviour among honey bees [ 49 ].
This study synthesized current interdisciplinary research on AMR, climate change, and environmental pollution in honey bees through a One Health lens in order to characterize past studies and identify potential avenues for future research. The scoping review identified 22 articles published between 1993 and 2019 that examined how interactions between climatic, pollutive, and microbial factors influenced honey bee health through AMR risk and development. Most of these studies were experimental, indicating that research in this area is largely empirical and topically isolated. In general, articles described linkages between environmental factors such as temperature or insecticide pollution and the ability of honey bees to resist or treat hive infection, either at the colony or individual bee level, or at the biological or behavioural level. However, broad research on the linkage between AMR, climate change, and environmental pollutants on honey bee health was generally lacking, indicating a future need for interdisciplinary research in this field.
Honey bee immunity is complex and dependent on both behavioural and biological factors outside of, and within, the honey bee. Our study identified an opportunity for further investigation of immunocompetence and MDR transporter regulation as a consequence of environmental determinants. The relationship between immune function and MDR transporter regulation is pertinent to the field of AMR for a number of potential reasons. Firstly, any resistance acquired by honey bee cells via MDR transport upregulation could possibly increase the risk of AMR in symbiotic microbes [ 59 , 60 ]. Bacterial pathogens can acquire resistance genes through horizontal genetic transfer (HGT) [ 60 ]. There is evidence that insects transfer genetic material bidirectionally through HGT with intracellular primary endosymbiont bacteria within polyploid bacteriocyte cells [ 61 ]. Evidence of exchange of bacterial genes with fungal pathogens by HGT further strengthens this possibility [ 62 ], but specific evidence of the transfer of AMR genes through these mechanisms remains largely unstudied. As this theme did not emerge from the papers included in our scoping review, evaluation of its possibility for honey bees is outside the scope of this paper, but presents an intriguing area of interest for future One Health research.
Secondly, honey bee cell membrane transporters may reduce microbial exposure to administered antimicrobials. Natural honey bee cell membrane transporters remove intracellular compounds from the cytoplasm [ 57 ]. When pesticides are introduced to the hive, these transporters are activated to prevent the compounds from accumulating. Both pesticides and antimicrobials (including vital acaricides such as coumaphos) are substrates of these transporters [ 41 , 42 ]. As a result, pesticide-induced upregulation of these transporters may concurrently accelerate the removal of antimicrobials from the cell and decrease the intracellular concentration. With less antimicrobials circulating within the honey bee cells, intracellular pathogens such as Nosema spp. and pathogens that live within the body cavity such as Ascosphaera apis may be exposed to lower dosages during this upregulation of membrane transporters [ 61 , 62 ]. By “shading” potential pathogens from antimicrobial treatment, there presents an increased risk for AMR development by the microbes. A similar effect has been studied in the public health sector through the use of small colony variants of Staphylococcus aureus , whereby the microbe is theorized to shelter from antimicrobial treatment within host cells to increase resistance against treatment and allow recurring infections [ 63 , 64 ]. One article in our study highlighted the synergistic effect of simultaneous exposure to contaminants and pathogens [ 55 ]. Although this article demonstrates linked immune responses between two distinct etiological agents, the specific pathway was not explored and represents an opportunity for future study [ 55 ].
Lastly, with a decrease in honey bee immunity, pathogens are able to more quickly spread and develop inside the hive. Articles within our study primarily focused on immunity as a factor of honey bee endogenous microbiota, highlighting correlations between environmental pollutants and changes in microbiota function. These microbiota have been found to be exceptionally important both in honey bee pathogenic defence, as well as in recovery [ 65 ]. Small changes in the immune function of the honey bee linked to changes in these microbes can have drastic effects on the ability of honey bees to fight off disease. However, the articles in this study failed to evaluate how an adjustment in immunity may correspond to an increased risk of AMR. Notably, human studies have shown that a compromised immune system increases the risk of AMR emergence [ 66 , 67 ]. This can be due to inhibition of synergistic actions between the immune system and the antimicrobial in reaching an effective minimum inhibitory concentration at the site of infection, an overall increase in disease prevalence, or a higher rate of mutation resulting from unhindered population growth. However, these connections are absent in the articles in this study, and therefore there remains the opportunity to address these connections in the future.
Our scoping review exposed correlations between environmental factors and an increased susceptibility of honey bees to disease. The predominant cause of vulnerability in the hive was due to modulation of AMPs by environmental pollutants. These peptides serve a critical role in innate defences against pathogens in all insects, including honey bees [ 68 ]. The effect of AMP on bacteria and viruses was a key focus of included articles due to the high incidence of American foulbrood (a bacterial infection) and Varroa Mite, which normally increase morbidity in the hive through secondary bacterial and viral infections [ 53 ]. Therefore, because most articles investigated morbidity as a result of bacteria and viruses either directly or indirectly, it follows that AMPs, the primary defence against these organisms, would also be investigated. As shown in human and livestock animal studies, an increase in disease susceptibility inevitably corresponds to an increase in antimicrobial drug treatment, with a subsequent increased risk of AMR [ 69 – 71 ]. Although increased antimicrobial usage is commonly inferred to correlate with an increased risk of AMR, none of the studies in this review investigated this connection. Therefore, there remains an opportunity to holistically connect evidence streams between disease susceptibility, treatment requirement, and risk of AMR to determine their interdependencies.
Although external antimicrobial treatment by beekeepers was the primary focus of research included in this review, our study revealed an increased interest in zoopharmacognostic (self-medicating) behaviours within the hive itself. While normal drug treatment in apiaries occurs once or twice per year in the spring and fall, self-medication processes by honey bees themselves within the hive are continuously implemented [ 72 ]. Additionally, honey bee self-medication utilizes products within the hive that are prone to variable strength and efficacy, partly due to outside factors. Our study exposed some contributors to this antimicrobial variance, namely temperature and seasonality. However, domestication has led to some additional challenges and considerations, such as the mixing of honey bees and antimicrobial products (e.g., honey and propolis) from multiple geographic sources. Given the sensitivity of hive products to climatic conditions, the relocation of honey bees to new climates and environments may alter the antimicrobial properties and efficacy of hive products. There is an opportunity to investigate how the alteration of these products may influence the ability of colonies to appropriately self-medicate. Despite this growing concern, we did not identify any studies that directly correlated honey bee hive product self-medication with an increased threat of AMR. Given that inconsistent antimicrobial strength can lead to AMR, and environmental conditions have been shown to contribute to antimicrobial inconsistency both in bees as well as the general population [ 20 , 73 ], connecting these two areas remains an opportunity for future interdisciplinary research.
Strengths and limitations
While all literature reviews face the possibility of failing to capture all eligible articles, we aimed to minimize this risk by following a rigourous, systematic approach [ 74 ]. We adopted a search strategy without language limitations in order to reflect the global breadth of the issues at hand. However, this global undertaking resulted in the necessary exclusion of 36 articles that were deemed eligible through abstract screening but were not available to us for full-text review ( S3 File ). We recognize that 8/22 included articles were observational/descriptive studies or review articles, and less useful than the 14 experimental studies for identifying causal relationships. We also recognize one article with a questionable link between AMR and climate change or environmental pollution. The Prodelalová et al. (2017) paper used a surrogate virus to assess the effectiveness of disinfectants against the viruses of interest (picornaviruses) at different temperatures. The experimental model itself was tenuous and did not factor largely into our findings. However, the novel insights derived from this study allowed for the identification of multiple literature gaps and future areas of interdisciplinary research and still illustrate the usefulness of honey bees as an organism to determine the One Health impacts of AMR, climate change, and environmental pollution.
Conclusions
This study mapped current literature investigating the relationship between AMR and honey bees in the context of climate change and environmental pollutants through a One Health lens. We identified considerable potential for further interdisciplinary research to holistically correlate environmental influences on honey bee immunity, disease susceptibility, and self medicative behaviours on AMR risk. Despite the immense agricultural and economic significance of honey bees globally, we identified a lack of literature on honey bee health in the context of AMR. Our findings provide the basis for future research to understand the complex linkages of AMR, climate change, environmental pollution and honey bee health in the context of One Health. This study will contribute to the growing body of One Health and interdisciplinary research to find novel solutions for global “wicked” problems beyond the beehive.
Supporting information
S1 checklist. completed checklist..
https://doi.org/10.1371/journal.pone.0242393.s001
S1 Table. Screening questions that define the inclusion and exclusion criteria used in the two-level screening process by two independent reviewers.
https://doi.org/10.1371/journal.pone.0242393.s002
S2 Table. Data extraction table of complete study characteristics of included aritlces.
https://doi.org/10.1371/journal.pone.0242393.s003
S1 File. Protocol outlining the systematic scoping review created using JBI guidelines and following the PRISMA-ScR checklist–time-stamped on December 19, 2019.
https://doi.org/10.1371/journal.pone.0242393.s004
S2 File. Complete search strings for all databases searched in this scoping review.
https://doi.org/10.1371/journal.pone.0242393.s005
S3 File. List of papers excluded due to the inability to obtain full-text documents.
https://doi.org/10.1371/journal.pone.0242393.s006
Acknowledgments
We thank Sandra Campbell from the University of Alberta Library for assistance in developing the search strategy. We also thank Dr Zvonimir Poljak, Dr Philipp Schott, Dr Okan Bulut, Giulia Scarpa, Nia King, and Carina de Micheli for their translating help within this project.
- 1. World Health Organization, editor. Antimicrobial resistance: global report on surveillance. Geneva, Switzerland: World Health Organization; 2014. 232 p.
- View Article
- Google Scholar
- 4. IPCC (Intergovernmental Panel on Climate Change). Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. [Internet]. Stocker TF, Qin D, Plattner GK, Tignor M, Allen S K, Boschung J, et al., editors. Cambridge, United Kingdom and New York, NY, USA: Cambridge University Press; 2013 [cited 2020 Sep 13]. 1535 p. Available from: https://www.ipcc.ch/report/ar5/wg1/ .
- 6. United Nations. Climate Change [Internet]. 2016 [cited 2019 Sep 13]. Available from: https://www.un.org/en/sections/issues-depth/climate-change/ .
- PubMed/NCBI
- 15. World Health Organization. One Health [Internet]. WHO. 2017 [cited 2020 Sep 13]. Available from: http://www.who.int/features/qa/one-health/en/ .
- 24. Bee Informed Partnership. Loss & Management Survey [Internet]. 2020 [cited 2020 Sep 13]. Available from: https://beeinformed.org/citizen-science/loss-and-management-survey/ .
- 25. COLOSS. Honey Bee Research Associaion: Colony losses monitoring [Internet]. COLOSS. 2019 [cited 2020 Sep 13]. Available from: https://coloss.org/core-projects/colony-losses-monitoring/ .
- 56. United Nations. World Economic Situation and Prospects 2014 [Internet]. 2014 [cited 2020 Sep 13]. Available from: https://www.un.org/en/development/desa/policy/wesp/wesp_current/2014wesp_country_classification.pdf .
- 71. Public Health Agency of Canada. Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action [Internet]. 2017 [cited 2020 Sep 13]. Available from: https://www.canada.ca/en/health-canada/services/publications/drugs-health-products/tackling-antimicrobial-resistance-use-pan-canadian-framework-action.html .

MSU Extension Pollinators & Pollination
Special collection of scientific articles on honey bee research from the journal of insect science.
Ana Heck <[email protected]> , Michigan State University Extension - February 22, 2022
A collection of articles on recent honey bee research is available for free online.

The Journal of Insect Science partnered with the American Association of Professional Apiculturists to publish a special collection of scientific articles on recent research on honey bees: Honey Bee Research in the United States: Investigating Fundamental and Applied Aspects of Honey Bee Biology.
The articles are open source, so everyone can access them for free. Articles cover topics that affect honey bee health, including varroa mites, pathogens, nutrition, pesticides and many other important topics.
Michigan State University’s Meghan Milbrath and Peter Fowler worked with colleagues Samuel K. Abban, Dawn Lopez, and Jay D. Evans to publish “ Validation of Diagnostic Methods for European Foulbrood on Commercial Honey Bee Colonies in the United States .”
To learn more, visit the Honey Bee Research in the United States: Investigating Fundamental and Applied Aspects of Honey Bee Biology special collection from the Journal of Insect Science.
Michigan State University Extension ’s apiculture team connects beekeepers and pollinator enthusiasts with researched-based information on bees. You can stay connected by subscribing to MSU's Pollinators and Pollination Event's Newsletter .
This article was published by Michigan State University Extension . For more information, visit https://extension.msu.edu . To have a digest of information delivered straight to your email inbox, visit https://extension.msu.edu/newsletters . To contact an expert in your area, visit https://extension.msu.edu/experts , or call 888-MSUE4MI (888-678-3464).
Did you find this article useful?
Check out the MSU Fruit & Vegetable Crop Management Program!
new - method size: 3 - Random key: 0, method: tagSpecific - key: 0
You Might Also Be Interested In

Great Plains Master Beekeeping HapBee Hour
European foulbrood office hours webinar
The Wonders of Wild Bees: Small Organisms, Epic Possibilities
Published on April 6, 2021

Regrow milkweed for monarchs: A citizen science study
Published on May 15, 2020

Bumble bees in greenhouse vegetable production
Published on April 12, 2019

Giant wasps aren’t coming for you
Published on May 8, 2020
- agriculture
- beginning farmer
- home gardening
- integrated pest management
- lawn & garden
- managed pollinator protection plan
- msu extension
- pollinators and pollination
- agriculture,
- beekeeping,
- beginning farmer,
- home gardening,
- integrated pest management,
- lawn & garden,
- managed pollinator protection plan,
- msu extension,

- Research Article
- Computational and Systems Biology
How honey bees make fast and accurate decisions
- James AR Marshall
- Neville Dearden
- Andrew B Barron
- Department of Computer Science, University of Sheffield, United Kingdom ;
- Sheffield Neuroscience Institute, University of Sheffield, United Kingdom ;
- School of Natural Sciences, Macquarie University, Australia ;
- Open access
- Copyright information
Share this article
Cite this article.
- HaDi MaBouDi
- Copy to clipboard
- Download BibTeX
- Download .RIS
Editor's evaluation
Elife digest, introduction, materials and methods, data availability, article and author information.
Honey bee ecology demands they make both rapid and accurate assessments of which flowers are most likely to offer them nectar or pollen. To understand the mechanisms of honey bee decision-making, we examined their speed and accuracy of both flower acceptance and rejection decisions. We used a controlled flight arena that varied both the likelihood of a stimulus offering reward and punishment and the quality of evidence for stimuli. We found that the sophistication of honey bee decision-making rivalled that reported for primates. Their decisions were sensitive to both the quality and reliability of evidence. Acceptance responses had higher accuracy than rejection responses and were more sensitive to changes in available evidence and reward likelihood. Fast acceptances were more likely to be correct than slower acceptances; a phenomenon also seen in primates and indicative that the evidence threshold for a decision changes dynamically with sampling time. To investigate the minimally sufficient circuitry required for these decision-making capacities, we developed a novel model of decision-making. Our model can be mapped to known pathways in the insect brain and is neurobiologically plausible. Our model proposes a system for robust autonomous decision-making with potential application in robotics.
This valuable study elucidates the honeybee's behavioral strategy to associate sensory cues with rewards of different values. Based on solid experimental evidence the study demonstrates how sensory evidence and reward likelihood quantitatively affect the decision-making process and the bees' response time. The behavioral paradigm and the proposed model could provide interesting insights for scientists studying decision-making in higher animal species.
- Decision letter
- Reviews on Sciety
- eLife's review process
In the natural world, decision-making processes are often intricate and challenging. Animals frequently encounter situations where they have limited information on which to rely to guide them, yet even simple choices can have far-reaching impact on survival.
Each time a bee sets out to collect nectar, for example, it must use tiny variations in colour or odour to decide which flower it should land on and explore. Each ‘mistake’ is costly, wasting energy and exposing the insect to potential dangers. To learn how to refine their choices through trial-and-error, bees only have at their disposal a brain the size of a sesame seed, which contains fewer than a million neurons. And yet, they excel at this task, being both quick and accurate. The underlying mechanisms which drive these remarkable decision-making capabilities remain unclear.
In response, MaBouDi et al. aimed to explore which strategies honeybees adopt to forage so effectively, and the neural systems that may underlie them. To do so, they released the insects in a ‘field’ containing artificial flowers in five different colours. The bees were trained to link each colour with a certain likelihood of receiving either a sugary liquid (reward) or bitter quinine (punishment); they were then tested on this knowledge.
Next, MaBouDi et al. recorded how the bees would navigate a ‘reduced evidence’ test, where the colour of the flowers were ambiguous and consisted in various blends of the originally rewarded or punished colours; and a ‘reduced reward likelihood’ test, where the sweet recompense was offered less often than before.
Response times and accuracy rates revealed a complex pattern of decision-making processes. How quickly the insects made a choice, and the types of mistakes they made (such as deciding to explore a non-rewarded flower, or to ignore a rewarded one) were dependent on both the quality of the evidence and the certainty of the reward. Such sophistication and subtlety in decision-making is comparable to that of primates.
Next, MaBouDi et al. developed a computational model which could faithfully replicate the pattern of decisions exhibited by the bees, while also being plausible biologically. This approach offered insights into how a small brain could execute such complex choices ‘on the fly’, and the type of neural circuits that would be required. Going forward, this knowledge could be harnessed to design more efficient decision-making algorithms for artificial systems, and in particular for autonomous robotics.
Decision-making is at the core of cognition. A decision can be considered as the result of an evaluation of possible outcomes ( Mobbs et al., 2018 ; Stevens, 2011 ), and animal lives are full of decisions. What we might consider to be a simple choice, for example choosing the best option from two alternatives, is rarely simple in an ecological setting ( Mobbs et al., 2018 ). Consider the decisions a foraging bee makes. A bee, moment by moment, must decide whether a flower should be explored for pollen and nectar or whether it is not worth landing on. We could suppose that decision to be influenced by what the bee can sense about the flower, her past experiences with that flower type, the context (is a predator nearby?), the state of the bee (does she already carry a full load of nectar and pollen?) and the state of her colony (what does the colony need?) ( Chittka, 2022 ; Conradt and Roper, 2005 ; Stephens, 2008 ). Even this simple decision is a whole-brain activity involving sensory systems, memory systems, motor systems, and the bee’s subjective state. Here, we studied honey bee foraging decisions in controlled conditions to establish their decision-making capacities. We then developed a simple model with the same capacities for decision-making as a bee to assist in hypothesising the necessary neural mechanisms supporting bees’ foraging decisions.
Abstract theories and models of decision-making are well-developed, and these provide frameworks for evaluating animals’ decision-making capacity ( Gold and Shadlen, 2007 ; Mobbs et al., 2018 ; O’Connell et al., 2018 ). Here, we apply signal detection theory to understand how bees make a decision ( Green and Swets, 1966 ; Green and Swets, 1966 ; Sumner and Sumner, 2020 ; Wickens, 2001 ). Signal detection theory helps us think formally about the processes of signal discrimination, which is essential for making decisions ( Wickens, 2001 ). It provides an abstract model and simple logic for how animals should respond given the signal they have received and their prior knowledge. Typically signal detection theory assumes that an individual must choose between two possible actions (acceptance or rejection) after detecting a signal. In such a scenario, there are four possible outcomes, which include two correct actions. These are: 1, correct acceptance when the subject accepts the correct stimulus (‘hit’), 2, correct rejection when the subject rejects the incorrect stimulus (correct rejection), 3, incorrect acceptance when the subject wrongly accepts the incorrect stimulus (‘false positive’, Type I error), 4, incorrect rejection when the subject rejects the correct stimulus (‘false negative’, Type II error). The optimal decision is calculated by considering the expected payoffs of all four outcomes together. Both errors are integral parts of the decision-making process. In an ecological context, both errors typically differ in costs to an animal ( Sumner and Sumner, 2020 ). For example, wrongly rejecting a food item might see an animal missing a meal, but wrongly accepting a food item could see an animal ingesting poison. Signal detection theory emphasises that both acceptance and rejection choices have to be assessed if decision-making is to be understood, but typically in studies of animal behaviour rejection behaviour is ignored ( Ings and Chittka, 2008 ; Sumner and Sumner, 2020 ; Trimmer et al., 2017 ).
Decision-making processes are most often modelled with sequential sampling models, of which there are many variations ( O’Connell and Hofmann, 2012 ; O’Connell et al., 2018 ). Sequential sampling models are built on the biologically realistic assumptions that sensory information on available options is noisy, but evidence for different options accumulates over time through sequential sampling ( Gold and Shadlen, 2007 ). A decision is made when the cumulant reaches a threshold. Variations in sequential sampling models differ in the nature of the threshold for the decision. For example, in the race model ( Vickers, 1970 ) a decision is made when evidence for one alternative reaches an upper threshold. Leaky competing accumulator (LCA) models set the evidence for different options in competition such that as evidence for one option accumulates it inhibits evidence for the alternative and a decision is made when the difference in evidence for the two alternatives reaches a threshold ( Barron et al., 2015 ; Bogacz et al., 2006 ). Sequential sampling models have proved very influential in neuroscience, psychology, and computer science. While they are highly abstract, they capture many features of biological decision-making, particularly a speed/accuracy trade-off ( Barron et al., 2015 ; Bogacz et al., 2006 ; Gold and Shadlen, 2007 ; Pirrone et al., 2014 ).
Investigation of the neural mechanisms of choice in primates has revealed interacting neural systems for the evaluation of different options and the selection of a choice that involve the frontal cortex, the basal ganglia, and the frontal and parietal cortices ( Barron et al., 2015 ; Gurney et al., 2001 ; Seed et al., 2011 ; Shadlen and Kiani, 2013 ; Wang, 2012 ). This is a system of extreme complexity, involving billions of neurons. Most animal brains are orders of magnitude smaller than this. How might smaller brains make effective decisions? To this end, we explored honey bee foraging decisions. We measured bees’ acceptance and rejection of different options under controlled conditions that manipulated the quality of available evidence and the probability of a rewarding outcome. To understand the properties of bee decision-making, we explored our data with signal detection theory and also examined how accuracy varied with decision speed. Having identified the key properties of bee decision-making we then constructed the simplest sequential sampling model capable of the same decision-making capacities as the bee. Finally, we related this abstract model to the known systems of the bee brain to propose a hypothetical brain mechanism for autonomous decision-making in insects.
We individually trained 20 honey bees ( Apis mellifera ) on a colour discrimination task in which they learned to associate five distinct colours each with their visit history of reward and punishment. Over 18 training trials, each colour offered bees a different likelihood of reward and punishment ( Figure 1A , Figure 1—source data 1 ). The five colours offered the reward in 100%, 66%, 50%, 33%, and 0% of training trials ( Figure 1B ) and were otherwise punished. The colour rewarded in 100% of training trials was never punished while the colour rewarded in 0% of training trials was always punished. Each trial offered bees just one pair of colours with one colour in the pair rewarded more often than the other during training (See Materials and methods, Figure 1—source data 1 , Figure 2—figure supplement 1A,B , Table 1 ). Following training, bees were given three tests. In the easy discrimination test , each honey bee was tested with the two colours rewarded at 100% and 0% in training. In the reduced evidence test, bees were tested with two novel colours that were different blends of blue and green (the 100% and 0% rewarded colours) to determine how behaviour changed when the available evidence was degraded. One blend was closer to blue and one closer to green. In the reduced reward likelihood test bees were presented with the 66% and 33% rewarded colours to assess how bees’ behaviour changed when the likelihood of reward offered by a choice was less than 100%. In the easy discrimination and reduced evidence tests, correct choices were considered as acceptance of the more rewarded colour, and rejection of the less rewarded colour. Bee’s acceptance and rejection responses were analysed from videos recorded during the training and tests ( Figure 1D , see Materials and methods section). We employed the Matthew Correlation Coefficient (MCC) ( MaBouDi et al., 2020a ) to measure the performance of the bees in each test. This considered all types of responses (i.e. hit, correct rejection, false positive, and false negative) to calculate decision accuracy such that a positive correlation (with a maximum value of +1) indicates perfect performance accuracy while a value of zero indicates chance-level performance. Values between 0 and +1 demonstrate varying degrees of decision accuracy (see Materials and methods section).
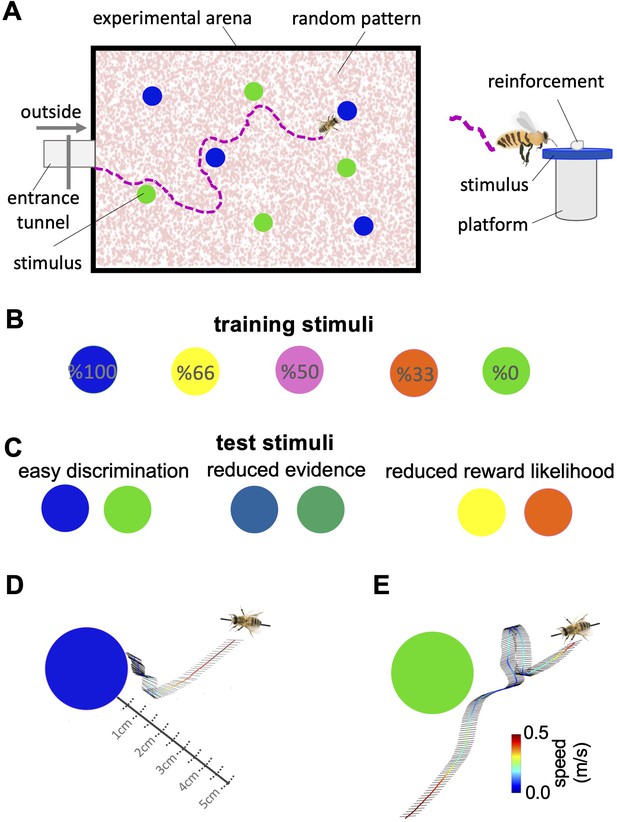
Bees’ behaviour in a colour discrimination task.
( A & B ) Each bee was given 18 training trials in which she could choose between two different colours: one rewarded and the other punished. The bee was free to select each colour and return to the hive when satiated marking the end of a trial. Stimuli positions in the arena were changed in each trial in a pseudo-random manner. Stimuli were 2 cm diameter-coloured disks on a small platform (5 cm tall). On the top of each colour was placed either 10 μl reward (50% sucrose) or punishment (quinine) in training, or distilled water in tests. Two different colours, four disks of each colour, were presented in each training trial and test. Five different colours were used in the training. The colours differed in the proportion of training bouts in which they offered reward and punishment (rewarded at 100, 66, 50, 33, and 0% of training trials). Two groups of bees were trained with different likelihoods of reward and punishment from each colour (see Materials and methods section and Figure 1—source data 1 ). ( C ) Following training, the bee was given three unreinforced tests where the positive or negative reinforcements were replaced with distilled water. Bees’ responses were analysed from video recordings of the first 120 s in the flight arena. In the easy colour discrimination test, bees were presented with three pairs of the 100% and 0% rewarded colours (blue and green). In the reduced reward likelihood test, bees were examined with 66% and 33% rewarded colours (yellow and orange). In the reduced evidence test. bees were given two colours intermediate between green and blue ( D & E ) Examples of flight paths showing the inspection activity of a bee during the easy discrimination test in accepting blue ( D ) and rejecting green ( E ). Each black line on the flight path corresponds to the bee’s body orientation in a single video frame with 4ms intervals between frames. Line colour: flight speed 0.0–0.5 m/s (See Video 1 ).
Figure 1—source data 1
Bees’ choices during the training trials.
Tables show the number of bees’ correct and incorrect choices to the high and low rewarded stimuli during two different sequences of training trials were used (A: Protocol 1; B: Protocol 2). The blue cells indicate the number of reward bees received, whereas the red cells indicate the number of punishment bees received.
Two different sequences of training trials were used.
10 bees were trained with the protocol P1 and 10 with the protocol P2.
In our free-flight choice assay bees learned to prefer the 100% rewarded colour from the 0% rewarded colour ( Figure 2A ; Wilcoxon signed rank test: z=3.62, n=20, p=2.93e-4; see Figure 2—figure supplement 1C for power analysis). Bees’ performance in the reduced evidence test was lower but was still higher than chance ( Figure 2A ; Wilcoxon signed rank test: z=2.10, n=18, p=0.03). In the reduced reward likelihood test, bees selected the 66% reward colour more frequently than chance ( Figure 2—figure supplement 2 ).
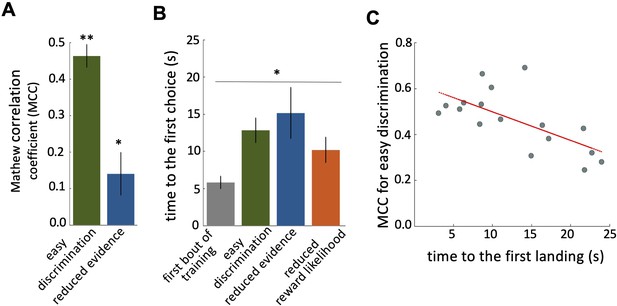
Characteristics of bee decision making.
( A ) Matthew correlation coefficients (MCC) (mean ± SEM) for the easy discrimination and reduced evidence tests. In the both easy discrimination and reduced evidence tests, this correlation is computed with respect to choosing the high-rewarded colours for each bee. A positive correlation (max at +1) indicates perfect correct performance while zero indicates chance level performance. Correlation coefficients were significantly greater than zero for both tests. ( B ) Average time to the first choices for three tests and the first training trial. Bees naive to the stimuli made their first choice faster than bees trained on the stimuli (p=1.55e-3). ( C ) Scatter plot showing a negative correlation between the MCC and the time to first acceptance in the easy discrimination test. A rapid first choice correlated with higher performance. Values for each individual bee are shown by small circles. n=20, **p<0.005 and *p<0.05.
Bees spent longer in flight before their first landing in the tests than in the first training trial ( Figure 2B ; Kruskal-Wallis test, chi-sq=13, df = 7, p=4.60e-3). This shows that during training bees developed a behaviour of assessing the available stimuli in the arena for longer before landing. There was a significant negative correlation between bees’ performance in the easy discrimination test and their time to first landing (assessed by the MCC: Spearman correlation, rho = –0.55, n=20, p=0.02). Poor performance in the test was associated with a longer time before a first choice ( Figure 2C ).
Investigation of bee decision-making using classical signal detection theory
Signal detection theory provides a framework for understanding and predicting how animals make decisions under uncertainty by modelling the relationship between the sensory information they received and their ability to accurately discriminate between stimuli. Hence, the probability of a stimulus being correctly identified is assumed to be a function of the sensory information received. If we have two different stimuli (in our case the high and low rewarded colours) we can model how the probability of identifying them changes as perceived colour information is sampled from two overlapping normal distributions ( Figure 3A ). For each colour, it could be identified correctly or incorrectly. For a trained bee we would recognise this as four types of behavioural response. For the highly rewarded colour, these would be correct acceptance or incorrect rejection. For the low rewarded colour these would be correct rejection or incorrect acceptance ( Figure 3A ). Discriminability (d′) is the difference in the sensory information between the maximal probability of the two different stimuli ( Figure 3A ). From our data, we could calculate discriminability following Sumner and Sumner, 2020 by modelling total accept and reject responses as cumulative distribution functions and considering the hit rate (correct acceptance / total acceptance) and the false positive rate (incorrect rejections/ total rejections; Equation 2 , Materials and methods).
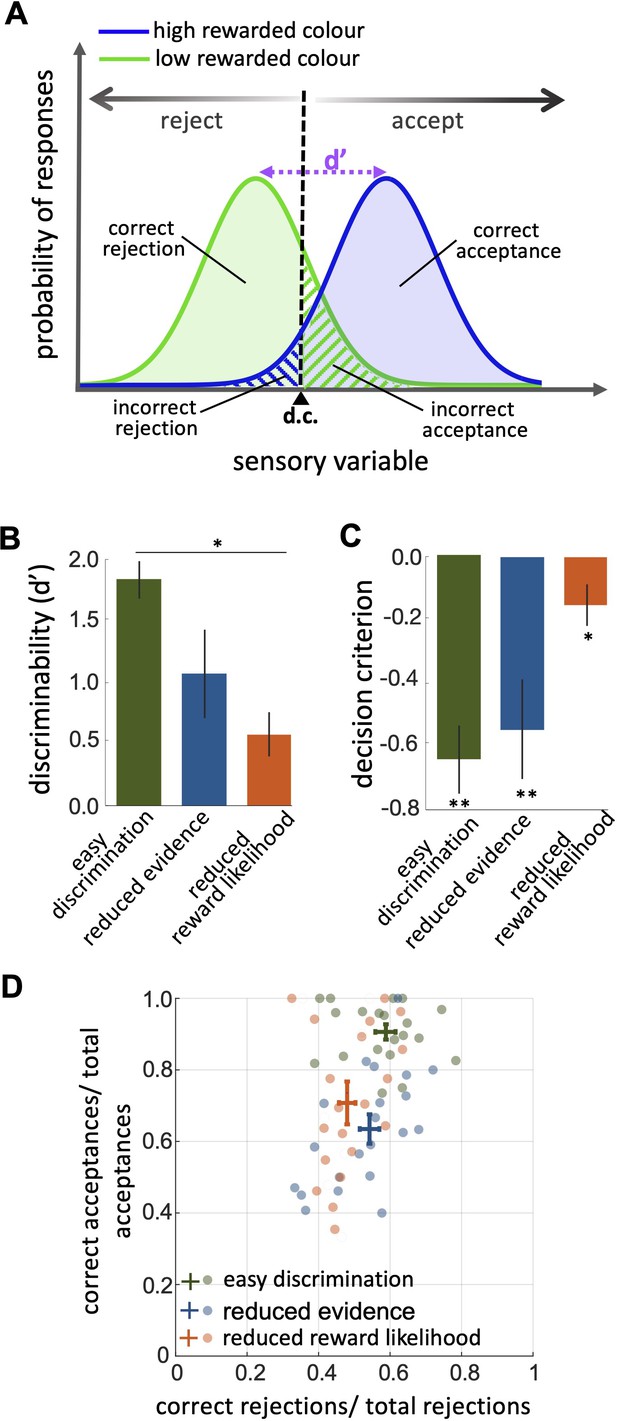
An investigation by classical signal detection theory.
( A ) Probability of responding to the high (blue) and low (green) rewarded stimuli at different levels of sensory input. For a trained bee we recognise a threshold (decision criterion, d.c.) at which their behaviour shifts from rejection to acceptance. As a result, we have four types of behavioural responses. d’ is the discriminability of the two stimuli. ( B ) Discriminability was greatest in the easy discrimination task and was reduced in both reduced evidence and reduced reward likelihood tests. ( C ) The decision criterion was negative for the easy discrimination and reduced evidence tests indicating fewer incorrect acceptances than incorrect rejections in these tests. The decision criterion was closer to zero in the reduced reward likelihood test indicating similar accuracy of acceptance and rejection in this test. ( D ) Plotting the ratio of correct to incorrect acceptances and rejections (crosses show the mean and SEM) for the three tests show that generally, bees were more accurate in acceptance than rejection responses. Acceptance accuracy fell in the reduced evidence and reduced reward likelihood tests. n = 20, **p<0.005 and *p<0.05.
When considering contrasting responses to two different stimuli using signal detection theory we can identify a threshold sensory signal at which behaviour should shift from acceptance to rejection. This is the decision criterion ( d.c ., Figure 3A ). From our experimental data we can estimate the relative location of the d.c . by considering both the hit rate and the false positive rate ( Wickens, 2001 , Equation 3 in the Materials and methods section). A value of 0 for the d.c . indicates that there were as many incorrect rejections as there were incorrect acceptances, or that the acceptance and rejection responses were equally accurate. A negative value for the decision criterion ( d.c .) would move the decision criterion to the left in Figure 3A . This would result in more correct acceptances (i.e. the area under the probability of responding to the high rewarded stimuli (blue) is increased) but fewer correct rejections (i.e. the area under the probability of responding to the low rewarded stimuli [green] is decreased). It would indicate acceptance responses are more precise than rejections.
The reduced evidence test significantly decreased the discriminability of more and less rewarded stimuli ( Figure 3B ; Wilcoxon rank sum test: z=1.81, n=20, p=0.03). Discriminability was also reduced in the reduced evidence test in which the two stimuli were closer in their likelihood of being rewarded ( Figure 3B ; Wilcoxon rank sum test: z=3.94, n=20, p=8.01e-5). This shows that for bees’ discriminability is influenced by both available evidence and reward likelihood.
When the likelihood of reward for the two stimuli was more similar the decision criterion was closer to zero ( Figure 3C ; Wilcoxon signed rank test: z=–2.21, n=20, p=8.4e-3) indicating that the accuracy of acceptance and rejection were more similar when the reward outcomes for the two stimuli were more similar. Otherwise, in both the easy discrimination and reduced evidence tests (in which one stimulus was always rewarded and one punished) acceptance was more accurate than rejection ( Figure 3C ; Wilcoxon signed rank test: z=–3.62, n=20, p=2.93e-4 for easy discrimination test, z=–2.91, n=18, p=3.5e-3 for reduced evidence test). Finally, the comparison of the ratio of correct and incorrect acceptance and rejection in the three tests ( Figure 3D ) revealed that the acceptance accuracy in both reduced evidence and reduced likelihood tests decreased compared to the easy discrimination test, indicating that acceptance accuracy was sensitive to both evidence and reward likelihood. Overall rejection accuracy was lower than acceptance accuracy. Rejection accuracy was lowest in the reduced reward likelihood test than in the reduced evidence test, indicating the rejection accuracy was more influenced by reward likelihood than available evidence ( Figure 3D ). This indicates that the evidence thresholds for accept and reject decisions were distinct, as discussed further in the Discussion section.
How quality of evidence and reward likelihood influence decision accuracy and decision speed
In the easy discrimination test, there were more rejections than acceptances ( Figure 4B ; Wilcoxon signed rank test: z=–3.62, n=20, p=2.9e-4) and bees’ accuracy (the difference between the number of correct and incorrect choices) of acceptance was higher than rejection ( Figure 4B ; Wilcoxon signed rank test: z=3.42, n=20, p=6.1e-4). Also, bees’ accuracy of acceptance in the easy discrimination test was higher than bees’ responses in the reduced evidence test ( Figure 4B and C ; Wilcoxon signed rank test: z=3.77, n=18, p=1.57e-4). While the number of correct rejections is higher than the number of incorrect rejection responses in the easy discrimination test ( Figure 4B ; Wilcoxon signed rank test: z=1.94, n=20, p=0.43), in the reduced evidence test there was no difference in the number of correct and incorrect rejection responses ( Figure 4C ; Wilcoxon signed rank test: z=–0.66, n=20, p=0.50). Hence, we propose that acceptance responses are more accurate than rejection responses, but reducing the available evidence reduced the capacity of bees to distinguish the correct and incorrect options.
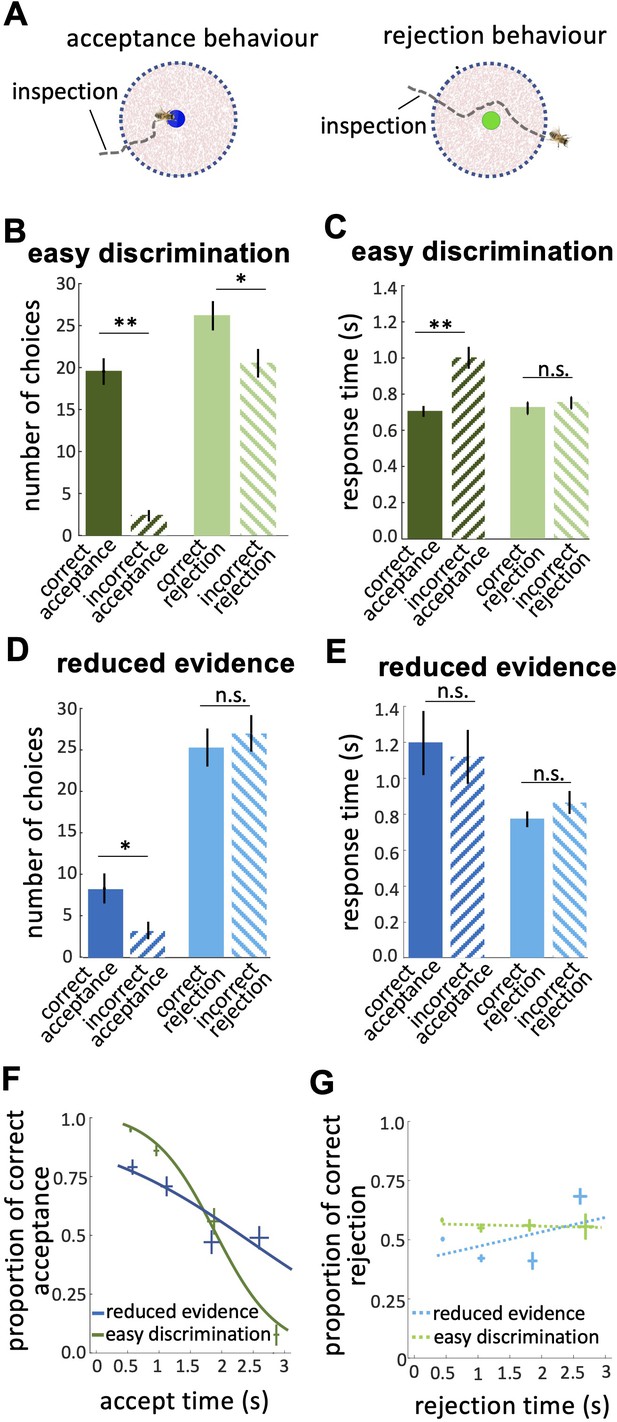
Response times of bee decisions.
( A ) Bees inspected the coloured stimuli prior to accepting or rejecting a colour. ( B ) The number of rejections was higher than the number of acceptances in the easy discrimination test. The difference between the correct and incorrect acceptances was larger than the difference between correct and incorrect rejections. ( C ) In the easy discrimination test bees accepted correct colours faster than incorrect colours, but there was no difference in the response time for correct and incorrect rejections. ( D ) In the reduced evidence test there were still more correct acceptances than incorrect acceptances, but the number of correct acceptances decreased. ( E ) Acceptance times for the correct colour were increased in the reduced evidence test. Bees took longer to accept stimuli with reduced evidence comparing to rejection responses, for both correct or incorrect choices. ( F ) Conditional Accuracy Function (CAF) plot for acceptance responses in the reduced evidence and easy discrimination tests. Lines show the best fit of piece-wise logistic regressions to the bee’s response time. Acceptance accuracy declined with increasing response time. The vertical and horizontal lines at each cross indicate the standard deviation of the proportion of correct acceptance and accept time, respectively. ( G ) CAF curve for rejections in both easy discrimination and reduced evidence tests. The accuracy of rejection did not change significantly with response time. The vertical and horizontal lines at each cross indicate the standard deviation of the proportion of correct rejection and rejection time, respectively. n=20, **p<0.005, *p<0.05 and n.s., p>0.05.
Classical signal detection theory does not consider how signals might be influenced by sampling time, but in our data, we noticed bees differed in the time they spent inspecting stimuli. To explore this, we analysed how bees’ response times influenced their choices.
Prior to bees accepting or rejecting stimuli, we noticed the bees hovered close to and facing the stimulus ( Figure 4A ). We hypothesise bees were sampling information about the stimulus. In the easy discrimination test bees accepted the correct colour faster than the incorrect one ( Figure 4C ; Wilcoxon signed rank test: z=–2.62, n=20, p=8.8e-3), but rejection times did not differ for correct and incorrect colours ( Figure 4C ; Wilcoxon signed rank test: z=–0.40, n=20, p=0.68). This shows the acceptance response was more accurate than the rejection, indicating a higher level of discrimination. In the reduced evidence test there was little difference between correct and incorrect response times ( Figure 4E ; Wilcoxon signed rank test: z=–0.25, n=20, p=0.79 for acceptances; z=–1.28, n=18, p=0.19 for rejections), and longer acceptance times overall ( Figure 4E ; Wilcoxon signed rank test: z=1.98, n=18, p=0.046), suggesting bees struggled to distinguish the correct and incorrect options in the reduced evidence test.
We calculated the Conditional Accuracy Functions (CAF) for acceptance and rejection responses, which is the subject’s accuracy as a function of the decision time ( Figure 4F & G ; Murphy et al., 2016 ). For each bee, we assessed the response time for all acceptance responses (both correct and incorrect) in the reduced evidence and easy discrimination tests. Response times were divided into 0.5 s bins and, for each bin, we calculated the proportion of correct acceptances as the number of correct acceptances / total acceptances in that response time bin. The negative slope of the CAF curves for acceptance indicates that bees made correct acceptances faster than incorrect acceptances ( Figure 4F ; Spearman correlation, rho = –0.43, n=20, p=3.0e-3). However, the CAF for the reduced evidence test was lower than the CAF for the easy discrimination test for almost the entire range of the response time ( Figure 4F ; Spearman correlation, rho = –0.25, n=18, p=6.5e-2). The gradient of the CAF curve was decreased by reducing the available evidence. This shows that decisions based on reduced evidence are slower and less accurate, and accuracy varied less with decision time. The CAF for the rejection response showed that rejection time did not vary with accuracy ( Figure 4G ; Spearman correlation, rho = 0.07, n=20, p=0.87 for easy discrimination test; rho = 0.02, n=18, p=0.81 for reduced evidence test). Collectively our analyses show that acceptance behaviour is very accurate and therefore very sensitive to available evidence, whereas rejection behaviour is less accurate, and hence is less sensitive to changes in evidence (See Discussion section).
Bees' choice strategy is sensitive to the history of reward
In the reduced reward likelihood test bees were more likely to reject than accept stimuli ( Figure 5A ; Wilcoxon signed rank test: z=–3.46, n=20, p=5.35e-4). In the reduced reward likelihood test bees had experienced both stimuli as rewarded and punished (33% and 66% punished) during training. We observed acceptance and rejection responses to both stimuli, most likely because bees were displaying the strategy of matching their choices to the probability each stimulus was rewarded in training ( MaBouDi et al., 2020b ). In the reduced reward likelihood test, there was no difference in times to accept and reject ( Figure 5B ; Wilcoxon signed rank test: z=–0.51, n=20, p=0.60 for acceptances; z=–1.15, n=20, p=0.24). Comparing the acceptance time of the easy discrimination, reduced evidence and reduced likelihood reward tests showed that fast acceptance is associated with more reliable evidence and certainty of outcome, and slower acceptance times are associated with less reliable evidence or less certainty of reward (comparing Figures 4C and 5B ). No negative slope of CAF curves was observed for either acceptance or rejection behaviour in the reduced likelihood reward test ( Figure 5C ). Acceptance time decreased with increasing reward expectation ( Figure 5D ; Spearman correlation, rho = 0.04, n=20, p=0.78 for acceptances; rho = –0.11, n=20, p=0.39 for rejections). Generally, our results show that bees were more likely to reject when either the available evidence or the reward likelihood was reduced.
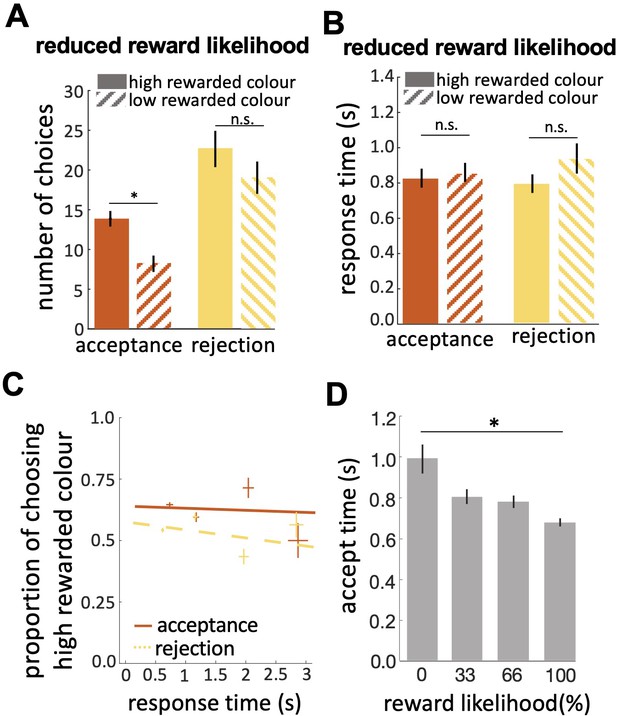
Bees’ performance in the reduced reward likelihood test.
( A ) In the reduced reward likelihood test bees made more rejection than acceptance responses. Bees accepted the highly-rewarded colour more than the low-rewarded colour, but there was no difference in rejections of the two colours. ( B ) Response times did not differ for either colour or response. ( C ) CAF curves for acceptance and rejection response. The accuracy of acceptance or rejection responses did not change with response time in the reduced reward likelihood test (see Figure 4F&G ). ( D ) Comparing acceptance times in the easy discrimination and reduced evidence tests allowed us to compare acceptance times for stimuli with different likelihoods of reward in training. Bees accepted the stimuli with higher reward likelihood faster. n=20, *p<0.05 and n.s., p>0.05.
A minimal model for honey bee decision-making capacity
We assessed various computational sequential sampling models to explore what kinds of computation are necessary for these capacities of decision-making. We used well-established abstract models of decision-making ( Bogacz et al., 2006 ). Our first model had separate accumulators for acceptance or rejection responses ( P a , P r ). Both accumulators receive sensory input and they provide inputs to acceptance ( A ) and rejection ( R ) command cells, respectively ( Figure 6A ). A decision is made either when one of the command cells reaches a predetermined threshold, or when a maximal decision time is exceeded. In this case, the command cell ( A ∨ R ) with the highest activity determines the decision (see Materials and Methods section). It is more common in sequential sampling models to assume accumulators for specific stimuli, with each stimulus channel activating a different specific response. This structure is not biologically feasible as it would demand separate accumulators for every possible visible stimulus. Hence, we modelled accumulators for response (accept and reject) and provided both with sensory input. Simulations showed this model could neither correctly accept nor reject stimuli at above chance levels.
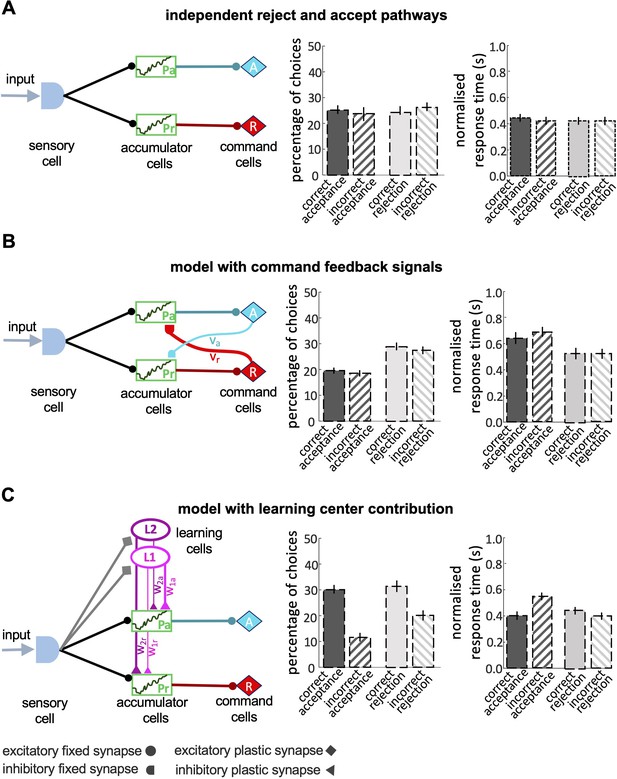
Models of decision-making.
( A ) A simple model with independent accumulators and command cells for acceptance and rejection was not able to reproduce the features of bee decisions. Correct and incorrect choices were made at equal frequency. ( B ) When cross-inhibitory feedback from the command cells was added to the model, the model was still not able to discriminate between the correct and incorrect choices, despite the number of rejections now being higher than acceptances. ( C ) A model with parallel pathways and learning cells that inhibit the accumulators with different values (i.e. w 2 r > w 1 r ∧ w 2 a < w 1 a ) had the ability to discriminate between stimuli, but the proportion of accepting correct colours and rejecting the incorrect colours are equal.
We then added to the model cross-inhibitory feedback signals from command cells back to the accumulators, which are constantly active during accumulating evidence at each accumulator ( Figure 6B ). In this model, as evidence accumulates in one command cell, it dampens the accumulation of evidence in the other accumulator. To build a model with a higher threshold for acceptance than the rejection response we set a stronger inhibitory connection between the reject command cell and the accept accumulator ( v r > v a ). This difference between the strength of cross-inhibitory feedback signals makes the model more likely to reject a stimulus whenever the evidence is insufficient. This model did indeed reject stimuli more often than accept ( Figure 6B ), but it still made an equal number of correct and incorrect choices and therefore could not discriminate between correct and incorrect decisions ( Figure 6B ).
To improve the accuracy of the model in acceptance responses we added learning cells and ( L 1 a n d L 2 ) to the model ( Figure 6C ) that receive input from the sensory cells on the identity of the colours and send different inhibitory outputs to the accumulator cells ( Figure 6C ). Following a model approach by MaBouDi et al., 2020b L 1 is activated when the low rewarded colours were presented to the model. L 2 is activated by the high rewarded colour. The two accumulators receive different levels of inhibition from the learning cells based on the reward likelihood of the presented colour. If a highly-rewarded colour is presented to the model, L 2 is activated and inhibits the reject accumulator more than the accept accumulator. This lowers evidence accumulation in the rejection accumulator. Conversely, a low rewarded colour activates L 1 which inhibits the accept accumulator. The model with learning cells could discriminate between the high-rewarded and low-rewarded colours but in simulations, it made equal numbers of correct acceptance and correct rejection responses ( Figure 6C ). This differed from the behaviour of bees ( Figure 4B ). In summary, none of the classical sequential sampling models in Figure 6 were able to reproduce the experimental data.
Our final model included parallel accumulators for accept and reject, learning cells and the cross-inhibitory feedback signals from the command cells ( Figure 7A ). This model could reproduce the features of bee choice behaviour ( Figures 4 and 5 ): (1) In this model there was a higher threshold for acceptance than rejection, and acceptance was more accurate than rejection ( Figure 7C ); (2) When the available evidence was reduced, the model showed reduced discriminability ( Figure 7D ); (3) The model was sensitive to reward likelihood ( Figure 7E ); (4) Finally, changing evidence and reward likelihood influenced acceptance and rejection response times. By comparing the model outputs with observed bee behaviours, it becomes evident that our final model can appropriately capture the dynamic features of bee decision-making. Comparing the outputs of the different models indicates that a parallel pathway for accept and reject accumulators is crucial in modelling bee decision-making, where both accumulators' evidence is subject to modification through learning and feedback from command cells (see decision letter section).
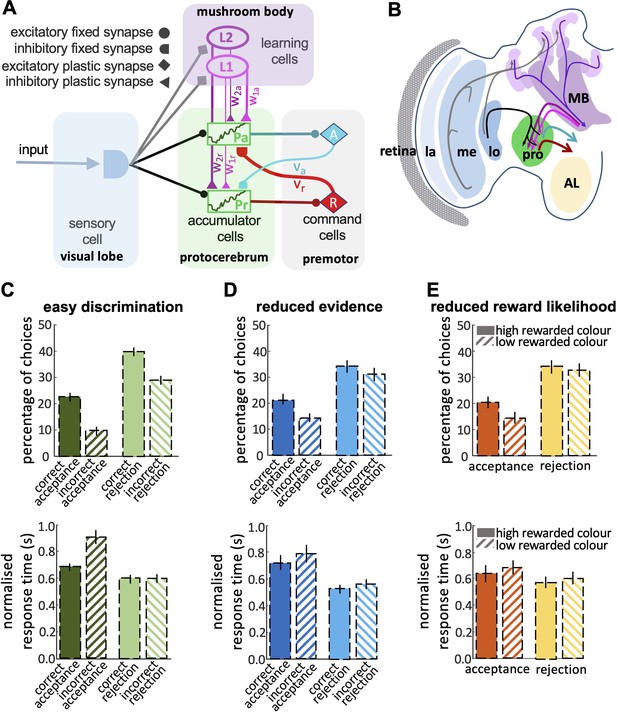
Neurobiologically-plausible model for honey bee foraging choices.
( A ) The model shows the connectivity of the components of the minimum circuitry of bee decision-making, including sensory cells, two parallel accumulators, learning cells and motor commands (See Materials and Methods). Synaptic connection classes are represented at the left-hand side. ( B ) The diagram shows a part of the insect brain involved in the decision-making process. The photoreceptors provide input from the eye to the lamina, which then sends its projections to the medulla. The medulla connects to the protocerebrum and, in parallel, to a third-order visual processing centre, the lobula, which then sends inputs via several tracts into the protocerebrum. In parallel, neurons in the optic lobe region (medulla and lobula) branch in the mushroom body. The anterior portions of the protocerebrum receive outputs from mushroom body output neurons (MBONs), supporting learning and memory. The output from the protocerebrum are premotor neurons. MB: mushroom bodies; AL: antennal lobe; la & me: lamina and medulla neuropils; lo: lobula; pro: protocerebrum. Our model reproduces the bees’ responses to easy discrimination ( C ), reduced evidence ( D ), and reduced reward likelihood tests ( E ). The average percentage of correct choices (acceptance or rejection) made by the model bees within blocks of 25 trials. All non-overlapping SEM error bars are significantly different (p<0.05).
Our study has shown both sophistication and subtlety in honey bee decision-making. Honey bee choice behaviour is sensitive to the quality of the available evidence and the certainty of the outcome ( Figures 3 and 4 ). Acceptance and rejection behaviours each had different relationships with reward quality and the likelihood of reward or punishment as an outcome ( Figure 5 ). Acceptance had a higher evidence threshold than rejection, and the response time to accept was longer than the time to reject ( Figures 3 and 4 ). As a consequence, acceptance was more accurate. We observed a large number of erroneous rejections but far fewer erroneous acceptances ( Figure 4 ). Acceptance behaviour was more sensitive to reductions in reward quality and reductions in the certainty of a rewarding outcome than rejection. Correct acceptance responses were faster than incorrect acceptances ( Figure 4 ) which seems counter to the well-known psychophysical speed/accuracy trade-off ( Chittka et al., 2003 ; Hanks et al., 2014 ; Heitz, 2014 ). The complexity of honey bee decision-making only became apparent because we scored both acceptance and rejection behaviour. Signal detection theory has always highlighted the importance of considering both acceptance and rejection responses to understand choices but typically in animal behaviour studies rejection behaviour is usually ignored ( Figure 3 ; Trimmer et al., 2017 ; Wickens, 2001 ).
How animal decision-making is influenced by sampling time has been studied in species from insects to humans ( Chittka and Niven, 2009 ; O’Connell and Hofmann, 2012 ; O’Connell et al., 2018 ). The sophistication of honey bee decision-making has features in common with primates. For example, for honey bees correct acceptance decisions were faster than incorrect acceptance decisions ( Figure 4 ). A similar phenomenon has been reported for primates Churchland et al., 2008 ; Hanks et al., 2014 ; Murphy et al., 2016 ; Thura and Cisek, 2016 found that for humans in a situation requiring an urgent decision, decision accuracy decreased with increasing response times.
Primates and honey bees then appear to be behaving opposite to the expectation of the well-known speed-accuracy trade-off which predicts greater accuracy for slower decisions ( Chittka et al., 2003 ; Heitz and Schall, 2012 ; Marshall et al., 2006 ; Wickelgren, 1977 ). How can this be? The speed-accuracy trade-off is considered a general psychophysical property of decision-making. It is assumed that if a signal is noisy (for any reason) evidence of the identity of the signal will build up with time. As a consequence of this decision, accuracy should increase with increasing sampling time ( Chittka et al., 2009 ; Heitz, 2014 ). This psychophysical approach to animal decision-making assumes that the threshold of evidence for making a decision is fixed and does not change with the amount of time spent sampling. Ecologically that is rarely the case because sampling time incurs costs; be they energetic costs of sampling, risk of predation or opportunity costs ( McNamara and Houston, 1985 ; McNamara and Trimmer, 2019 ; Mobbs et al., 2018 ). If sampling is costly and the consequences of an error are severe then a better strategy is to vary the evidence threshold for making a decision with sampling time ( Drugowitsch et al., 2012 ; Frazier and Yu, 2007 ; Malhotra et al., 2018 ; Thura et al., 2012 ). One strategy under these conditions is to restrict sampling time, only to accept options for which there is very high confidence in a short sampling interval, and to reject everything else ( Chittka and Osorio, 2007 ; Fawcett et al., 2014 ; Ings and Chittka, 2008 ; Mobbs et al., 2018 ; Murphy et al., 2016 ; Trimmer et al., 2008 ). A consequence of this strategy is that a very high proportion of acceptances made quickly will be correct (because the evidence threshold is high for rapid acceptance). For slower acceptances, the proportion of correct choices will be lower because the evidence threshold is lower for slower decisions. This gives an appearance of a reversed speed/accuracy relationship, but it is a consequence of the dynamic variation of the evidence threshold with increasing sampling time. The strategy of asymmetric errors that bees have taken in their decision is also predictable from the well-known optimal weighting rule from decision theory ( Freund and Schapire, 1997 ; Grofman et al., 1983 ), the drift-diffusion model ( Marshall et al., 2017 ; Ratcliff, 1978 ) and reported neural data ( Kiani and Shadlen, 2009 ; Shadlen and Kiani, 2013 ). With this strategy, the number of rejections should be high overall, the number of erroneous rejections should be high and rejection accuracy less time dependent. These were all features we observed in honey bee decision-making. Hence, we propose in this study bees were following a similar time-dependent decision-making strategy ( Kiani and Shadlen, 2009 ; Malhotra et al., 2018 ; Marshall et al., 2017 ; Murphy et al., 2016 ; O’Connell et al., 2018 ).
Chittka et al., 2003 reported that for bumblebees, accuracy was positively correlated with choice time ( Chittka et al., 2003 ). In this study choice time was the flight time between flowers, and they did not report an actual response time of each decision. Rejections were not reported at all. In our study recording times for all responses (acceptance and rejection) gave a more nuanced interpretation of the honey bee decision-making strategy. This emphasises the importance of recording rejections as well as acceptances.
Acceptance and rejection are fundamental aspects of animal decision-making. While rejection can be complementary to acceptance when an animal has to choose between two simultaneous choices, typically acceptance and rejection are distinct types of choices in nature. For instance, bees scan each flower independently and decide whether to land on it or reject it based on the evidence sampled from the flower, prior knowledge and other factors. Our results emphasize that acceptance and rejection are distinct features of bees’ decision-making. Because rejection behaviour has a lower evidence threshold for the response it operates rather like a ‘default’ response to a stimulus and acceptance of a stimulus is more considered. This could be considered adaptive since accepting a flower is more risky for a bee than rejecting a flower. Rejection is performed in flight and honey bees in flight have high manoeuvrability and are only exposed to aerial predators. Accepting and landing exposes bees to far greater predation risk. Many bee predators, particularly mantids and spiders, have evolved as flower mimics and/or hide in vegetation close to flowers ( Nieh, 1993 ; O’Hanlon et al., 2014 ). A foraging bee feeding on a flower is therefore exposed to greater risks than a bee in flight. Ecologically accept and reject behaviours carry different costs and benefits, and it is beneficial for bees to have separate evidence thresholds and sensitivities to evidence for acceptance and rejection.
The properties of acceptance behaviour were not fixed and were sensitive to the history of reinforcement experienced at a stimulus. Previously we have shown that in response to variable rewards bees match their choice behaviour to the probability a stimulus offers a reward ( MaBouDi et al., 2020b ). Such a probability matching strategy is the most likely ecologically rational strategy, and the best option in circumstances where the rewards offered by different options are unknown and liable to change. Here, we showed that even individual choices were influenced by the history of reinforcement ( MaBouDi et al., 2020b ). Faced with stimuli that offered both reward and punishment in training, bees' acceptance time increased, indicating the threshold for acceptance increased when there was a chance of a negative outcome from the stimulus. This shows that bees adjust how they respond to specific stimuli according to the totality of their prior experience with that stimulus.
A neurobiological model for honey bee decision-making
Our exploration of race and LCA modelling ( Figures 6 and 7A ) showed that the simplest forms of the race model were not sufficient to capture the dynamic features of bee decision-making. Modelling all the properties of bee decisions required two channels for processing stimulus information, one of which was modifiable by learning ( Figure 7A ). These channels interacted with populations of neurons that accumulated evidence for different available options, with feedback from the command cells into the accumulator populations. Our identified model was the simplest found capable of reproducing all the qualitative features of bee decision-making ( Figure 7C, D and E ). There was a striking similarity between the features of this minimal model and our understanding of the sensory-motor transformation in the insect brain ( Figure 7 ).
In the bee brain, visual input is processed by the lamina and medulla in the optic lobes ( Figure 7B ). The medulla projects to the protocerebrum directly, and also indirectly via a third-order visual processing centre, the lobula ( Hertel and Maronde, 1987 ; Paulk et al., 2009 ; Strausfeld, 1976 ; Strausfeld and Okamura, 2007 ). In parallel, the medulla and lobula project to the mushroom bodies ( Strausfeld, 1976 ). The mushroom bodies are considered the cognitive centres of the brain. They receive multimodal input and support learning and classification ( Bräcker et al., 2013 ; Giurfa and Sandoz, 2012 ; Heisenberg, 2003 ; Li et al., 2017 ). The protocerebrum is a complex region that is not completely characterised in honey bees, but in Drosophila the protocerebrum is thought to establish the valence of stimuli, whether attractive or repellent ( Das Chakraborty and Sachse, 2021 ; MaBouDi et al., 2017 ; Parnas et al., 2013 ). The protocerebral regions have been hypothesised to contain ‘action channels’ that help to organise different kinds of behavioural output ( Galizia, 2014 ). We believe the protocerebrum could feasibly contain neural populations acting like accumulators for accept or reject responses ( Aso et al., 2014 ; Dolan et al., 2019 ).
That valence can be modified by learning via the outputs of the mushroom body ( Dolan et al., 2019 ; Eschbach et al., 2020 ; Lewis et al., 2015 ; Sayin et al., 2019 ). These are inhibitory projections to the protocerebrum ( Mauelshagen, 1993 ; Rybak and Menzel, 1993 ; Strausfeld, 2002 ). Finally, protocerebrum interneurons connect with premotor regions such as the lateral accessory lobes and central complex which generate output commands for turning and hence have the capacity to transform an accept or reject signal into an approach or avoid manoeuvre ( Cheong et al., 2020 ; Guo and Ritzmann, 2013 ; Namiki et al., 2018 ; Steinbeck et al., 2020 ; Varela et al., 2019 ).
From these features of the insect brain, we can identify the functional elements needed for our minimal decision model and propose how sophisticated decisions might be possible in the insect brain ( Figure 7B ). Recent evidence from Drosophila has highlighted the role of the fly mushroom body in decision-making ( Groschner et al., 2018 ). In a simple binary choice task, the fly mushroom body accumulated evidence on different available options using separate pools of Kenyon cells that were connected to each other by reciprocal inhibition. These experimental findings lend support to how we have mapped our model against the insect brain, but our results suggest that the fly story may be incomplete. The fly experiments did not score rejection responses, nor did they explore if the properties of the decision were sensitive to evidence quality or reward likelihood, hence the bioassay might not have exposed all the decision-making capabilities of the insect. For bees at least the mushroom body pathway cannot be the only system contributing to the decision, as dual interacting pathways were necessary ( Barron et al., 2015 ; Cheong et al., 2020 ). Further electrophysiological or neurogenetic work is needed to test whether our dual pathway model is an appropriate abstraction of the insect decision system. Our model proposes a simple decision architecture that is capable of responding adaptively to the kinds of variable evidence and circumstances encountered in real-world situations. This type of model could prove of value in autonomous robotics applications ( de Croon et al., 2022 ; Kelly and Barron, 2022 ; Stankiewicz and Webb, 2021 ; Webb, 2020 ).
Our study unveils the remarkable sophistication and subtlety of honey bee decision-making while emphasizing the significance of considering both acceptance and rejection responses in animal behaviour research, an aspect often overlooked in such studies. We provide compelling evidence that honey bee decision-making is influenced by the quality of available evidence and the probability of receiving a reward as an outcome. Notably, acceptance and rejection behaviours exhibit distinct characteristics, with acceptance displaying higher accuracy albeit with greater risk. Interestingly, correct acceptances were found to be faster than incorrect acceptances, contrary to the commonly observed speed/accuracy trade-off in psychophysics. Furthermore, our study, for the first time, introduces a novel and straightforward model that elucidates parallel pathways in decision-making in honey bees. This model aligns with known pathways in the insect brain and holds neurobiological plausibility. By shedding light on the neural mechanisms underlying decision-making, our findings not only provide valuable insights into honey bee behaviour but also propose a potential framework for the development of robust autonomous decision-making systems with applications in the field of robotics.
Bees and flight arena
Experiments were conducted at the Sheffield University Research Apiary with four standard commercial hives of honey bees ( Apis mellifera ). To source honey bees for our experiments, we provide them with a feeder containing 20% sucrose solution (w/w). Some bees visiting the feeder were given individually distinctive marks with coloured paints on their abdomen and/or thorax using coloured Posca marking pens (Uni-Ball, Japan). Experiments were performed in a (100x80 x 80 cm) flight arena made from expanded PVC foam boards with a roof of UV-transparent Plexiglas. To create a natural foraging environment for the bees, we set up the flight arena 5 m away from the gravity feeder and an additional 15 meters away from the hives. A transparent Perspex corridor (20x4 x 4 cm) provided access to the flight arena for bees. The interior walls and floor of the arena were covered with a pink random dot pattern, which created a contrast between the bees' colour and the background. This pattern was specifically designed to aid video analysis in tracking bees ( Figure 1A , Video 1 ). All bees visiting the arena were forager bees motivated to gather sucrose for their colony. In this way, the behavioural state of bees participating in the study was standardised. The flight arena was not connected to the hives, rather for each trial bees visited the flight arena under their own volition when motivated to perform a foraging flight. Forager bees forage for their colony not for themselves and they feed in the colony prior to beginning a foraging flight. Thus, bees visiting the flight arena should have been in similar physiological and motivational states. The typical inter-visit interval by a bee was 5–10 min.

This video cannot be played in place because your browser does support HTML5 video. You may still download the video for offline viewing.
Sample video of honeybee in the test.
The video was captured from an overhead perspective, providing a clear view of the bees' movements within the flight arena, showcasing their reactions to various stimuli. The black lines depict the orientation of the bee’s body at each frame of the video, offering further observations of their positioning and behaviour during the experiment.
Training and testing stimuli
Bees were trained to visit coloured stimuli inside the arena. Stimuli were disks (2.5 cm in diameter) of coloured paper covered with transparent laminate ( Figure 1A ) placed on small inverted transparent plastic cups (5 cm in height). Two additional colours intermediate between green and blue were designed for the reduced evidence test ( Figure 1B ). All colours were distinguishable for bees ( MaBouDi et al., 2020b ).
Training protocol
During the pre-training phase, marked bees were attracted to the entrance of the arena from the gravity feeder using a cotton bud soaked in a 50% sucrose solution (w/w). Once at the gravity feeder, the bees were given more 50% sucrose solution and were gently moved to the entrance of the corridor. This process was repeated until the bee was able to fly independently to the entrance of the corridor. The bees were then trained to fly into the arena via the entrance corridor to locate drops of 50% sucrose placed on transparent disks of laminate top plastic cups. The roof of the arena was lifted to release the bees from the arena every time they were satiated. Only those bees that flew independently into the arena to feed were selected for the training phase.
Each bee was separately trained with five different coloured stimuli in a colour discrimination task for 18 bouts of training. In each trial a bee was presented with a pair of colours selected from the training stimuli ( Figure 1B ); one rewarded colour and the other punished ( Figure 1A ). During each trial, the bees were presented with four stimuli of each colour of the pair and given multiple opportunities to choose each colour until they reached satiation. Stimuli were placed randomly in the arena. Based on the reward or punishment assigned to each colour listed in the Protocol 1 and 2 during 18 trials ( Table 1 ), the five different colours were each assigned a different likelihood of reward during the training trials: 100%, %66, %50, %33, and %0 of training trials ( Figure 1 ). Colour pairs were organised such that in every trial one colour was rewarded and one punished ( Table 1 ). For example, bees were rewarded with stimulus S%66 in four trials (#4, #8, #13, #17 in the protocol P1; #4, #9, #12, #15 in protocol P2) and punished in two trials (#1, #10 in protocol P1; #2, #17 in the protocol P2) ( Table 1 ). Thus, the likelihood of receiving a reward for stimulus S%66 during the training trials was 4/6=66%. Stimuli were rewarded with 10 μl sucrose solution 50% (w/w) or punished with 10 μl of saturated quinine hemisulphate solution.
To evaluate any effect of the innate colour preference of bees on their decision, bees were randomly assigned to one of two groups: A and B. For group A, colours were ordered as: blue = 100%, yellow = 66%, pink = 50%, orange = 33%, green = 0%. For group B, the colours were ordered as: green = 100%, orange = 66%, white = 50%, yellow = 33%, blue = 0%. Specific details on the reflectance spectrum of each colour are given in MaBouDi et al., 2020a .
Over 18 training trials, bees experienced all combinations of the five colours twice, with the exception that bees in training never experienced %66 rewarded paired with %33 rewarded colours. This pairing was excluded from training so that in the post-training, reduced reward likelihood test, we could examine how trained bees evaluate a colour pair based on the reward likelihood of colours. To control the effect of the training sequence on bees’ colour preferences, bees were randomly assigned to one colour group (A or B) and one of two different sequences of training bouts (protocols P1 and P2; Table 1 ). In each training bout, bees were able to freely choose and feed from rewarded stimuli. 10 μL drops of 50% sucrose solution were replaced on depleted rewarded stimuli until the bee had fed to satiation and left the arena via the roof. Between trials, all stimuli and the arena were cleaned with soap water and then 70% ethanol and water to remove any possible pheromonal cues left by the bee. They finally were air-dried before reuse.
Each bee was given three tests. Each test was video recorded for 120 s. In all tests, all stimuli provided 10 μl water. The easy colour discrimination test presented bees with the colours that had been rewarded in 100% and 0% of training trials. The reduced reward likelihood test presented bees with 66% and 33% rewarded colours – a combination they never experienced in training. In the reduced evidence test bees were given two novel colours that were similar to but intermediate to the 100% and 0% rewarded colours. The sequence of the three tests was pseudo-randomised for each bee. To maintain the bees’ motivation to visit the arena, one or two refreshment trials were given between tests. In a refreshment trial, the bees were allowed to feed from 10 μL sucrose drops placed on eight disks of transparent laminate positioned in the arena. As in training, stimuli and the arena were cleaned between each test.
Automatic bee tracking algorithm
The flight arena was equipped with an iPhone 6 camera placed at the top of the arena, 1 meter distance from the floor, facing down that captured the full base of the flight arena in the field of view ( Figure 1A ). The camera was configured to record at 30 FPS (at a resolution of 1080 pixels) in the training phase, and 240 FPS at 720 pixels in the testing phase. The first 120 s of the test and the first bout of the training phase were used to analyse bees’ flights. Examples of a recorded flight path are shown in Video 1 .
A bee’s flight path was determined frame by frame extracting the x, y coordinates of the bee’s body and its body orientation. From each frame, the background was subtracted using the average of the previous 50 frames. By modifying MATLAB’s blob detection function with a threshold set close to the size of the bee very few candidate positions for the bee were found in each frame. We associated each pixel in each frame of the video with either a bee or the background. The bee’s position at each frame obtained from the algorithm became a single point in the trajectory over time. The obtained trajectory represents the position of the bee as a function of time. An elliptic filter was applied to the frame at the position of the detected bee to evaluate the bee’s body orientation. The smoothing function, ‘smoothdata’, was used to exclude outlier locations from the trajectory.
The flight path began when the bee entered the arena. Hovering time prior to accepting or rejecting a stimulus was assessed as the total time the bee’s body was within a 5 cm radius of the centre of the stimulus ( Figure 1D ). We assumed that bees did not attend to the stimuli when flying over them at high speeds (above the height of the cups) as they did when flying between the stimuli at a similar speed, opposed to when bees were approaching the stimuli at the same height as the plastic cups. Thus, 7% of all paths with length <0.2 s close to the edge of the focal area were excluded from analyses. A bee accepted a colour when it made contact with the colour (antennae at least contacting the platform; Figure 1C ). This translated to an automatically count bees’ landings algorithm. This algorithm counts bee’s landing and utilises a threshold flight speed classifier based on the k-means algorithm that was applied to flight paths that crossed over the stimuli ( MaBouDi et al., 2021 ). In this dynamic threshold determination, the speed of bees within the border of the colours was clustered into two groups: acceptance (very low-speed paths) and reject (high-speed paths). The boundary between the two groups obtained by the K-means algorithm was set as a defined rule to determine whether the bee chose or did not choose the colour.
Flight analysis and statistics
In each test, we evaluated bees’ performance from their choices during their first 120 s in the arena. Choices were scored as accepting (made a contact with colour) or rejecting a stimulus (flying away without landing). If the bee accepted the colour more likely to be rewarded in training, we considered this a correct choice. If the bee rejected the colour more likely to be punished in training, we also considered this a correct choice. Hence the bees’ decision was classified into four distinct responses: (1) correct acceptance (CA), landing on the more rewarded colour (2) incorrect acceptance (IA), landing on the less rewarded colour (3) correct rejection (CR), rejecting the less rewarded colour and (4) incorrect rejection (IR), rejecting the more rewarded colour. To summarise the bees’ performance in the tests, the Matthew correlation coefficient (MCC) was used as follows MaBouDi et al., 2020a ; Matthews, 1975 :
where n C A , n C R , n I A and n I R represent the number of CA, CR, IA and IRs for a bee in a test. The MCC has a scale from –1 to +1. High positive values indicate mostly correct acceptance and rejection choices. Negative values correspond to bees making mostly incorrect choices. Zero indicates bees choose colours randomly. A Wilcoxon signed rank test was applied to the MCC values to compare bees’ performance. Finally, the relationship between bees’ MCC and their scanning behaviours in the tests was evaluated by the Spearman’s correlation tests. All statistical tests were performed in MATLAB 2019 (MathWorks, Natick, MA, USA). Also, to ensure the validity of our conclusions, we conducted a power analysis on the bees' performance in the experimental tests, which helped us to confirm that our sample size was sufficient ( Figure 2—figure supplement 1C ). This approach allowed us to have greater confidence in the statistical significance of our findings and to draw more accurate conclusions from our data.
Signal detection theory
Signal detection theory ( Wickens, 2001 ) was used to analyse bee decisions. Signal detection theory proposes that bees evaluate a signal (stimulus with strength x) as either rewarded or punished. We assume that the probability of either accepting or rejecting a perceived signal can be described by two distributions that are normal in shape with equal variance ( Figure 3A ). We also assume a decision criterion ( d.c .) of the perceived signal at which the response changes from accept to reject ( Figure 3A ). From the positions of the distributions and the location of the criterion, we can estimate the expected probabilities of correct acceptances (hits) correct rejections, incorrect acceptance (false negative), and incorrect rejections (false positive; Figure 3A ). The location of d.c . can be influenced by training and the experience of each signal as either punished or rewarded as well as the consequences of correct and incorrect acceptance and rejection choices ( Wickens, 2001 ). Discriminability (d’) is the difference in signal between the maximum likelihood of acceptance and rejection responses ( Figure 3A ). If d’ is low the acceptance and rejection distributions overlap. Hence more errors are made.
Discriminability ( d ` ) and the decision criteria ( d . c . ) can be calculated from the empirical measurements of hit and false positive rates as follows
where the function Z . is the inverse of the standard normal cumulative distribution function (CDF). The hit rate is the ratio of correct acceptance to all acceptances ( n C A / n C A + n I A ) and the false positive rate is the ratio of incorrect rejections to all rejections ( n I R / n I R + n C R ).
Modelling honey bee decision-making
We started with the simple and well-defined sequential sampling model ( Bogacz et al., 2006 ; Pike, 1966 ; Vickers, 1970 ) which we adjusted to provide a better fit to experimental data for both accuracy and reaction times ( Figures 4 and 5 ). Our adjustments to the sequential sampling model were constrained by the types of processing considered plausible to derive both acceptance and rejection responses through two parallel pathways.
In the model, evidence favouring each alternative ( I ) accumulated in separate accept ( P a ) or reject ( P r ) accumulators over time ( Figure 6A ). Biologically plausible leaky accumulators (with decay rate, k ) were used to model the decision time which represent the duration that bees spend accumulating evidence in favour of or against a stimulus. At each time step, accept and reject accumulators send signals to the accept ( A ) and rejection ( R ) command cells, respectively. The output of command cells of accept and rejection was calculated by A = m a x 0.1 , P a and R = m a x 0.1 , P r with the baseline activity at 0.1. A decision was made either when one of the command cells reached a predetermined threshold, or when a decision was forced by exceeding a maximal assessment time in which case the decision associated with the command cell with the highest activity was chosen. The accumulation of evidence in the model is governed according to the following stochastic ordinary differential equations:
At time zero, the evidence accumulated P a and P r are set to zero; P a 0 = P r 0 = 0 . Brownian random motions d W a and d W r are added to represent noise in input and model the random walk behaviour.
To add inhibitory feedback signals from the command cells into the accumulators ( Figure 6B ), both accept and reject accumulators actively received feedback inhibitory signals from the opposite command cells while simultaneously receiving inputs from their respective accumulators as:
Here v a and v r are the fraction of command outputs that inhibit the alternative accumulator.
In a previous studies ( MaBouDi et al., 2020b ; Vasas et al., 2019 ), we developed a model for the five-armed bandit task, which showed that plasticity in both the input (calyx) and output (lobes) of the mushroom body can effectively learn the history of reinforcement for different colours. This implies that the mushroom body output neurons can provide distinct inhibitory signals to the accumulator cells based on the reinforcement history of each colour. In the current study, we utilized the abstract version of learning cells from our previous work, which underwent 18 training trials for the five different colours in the five-armed bandit task, identical to what the bees experienced in this study. Building upon the model proposed in MaBouDi et al., 2020b , we incorporated two types of learning cells ( L 1 , L 2 ) into the model and presented the modified version in Figure 6C . Both learning cells received the sensory input and sent different inhibitory outputs to the accumulators based on the reward likelihood of the colours. w 1 a , w 1 r , w 2 a and w 2 r are the value of inhibitory signals that the accept and reject accumulators received from the learning cells ( L 1 , L 2 ) such that w 1 a > w 2 a and w 1 r < w 2 r . The model activates the first learning cell, r L 1 = α I , if the high rewarded colour is presented to the model, and activates the second learning cell, r L 2 = α I , if the low rewarded colour is presented to the model. 0 ≤ α ≤ 1 represent the rate of the learning cells activity based on the input signal ( I ). The behaviour of learning cells and the value of the alpha were assumed and inspired by the model presented in our previous research ( MaBouDi et al., 2020b ), that demonstrated how the reinforcement neurons modulates the strengths of the synaptic connectivity in mushroom bodies in response to both reward and punishment. Synaptic weights w 1 a , w 1 r , w 2 a , and w 2 r were updated for each presented stimulus during training such that the accumulation of evidence in the model proceed according to the following equations:
where r L 1 and r L 2 represent the activity of learning cells L 1 , L 2 , respectively. Our final model, ( Figure 7A ) accumulated evidence following Equations 8 and 9 . The accumulators received cross-inhibitory signals from the command cells according to Equations 6 and 7 .
Model evaluation
The models are presented with 25 trials in which high-rewarded and low-rewarded stimuli were randomly presented. Each model responded after each trial by accepting or rejecting the presented stimulus. The performance of the model was evaluated by counting the number of correct and incorrect acceptances or rejections and their corresponding response times. In addition, we normalised the time response of the model to the maximum time response of all model bees, which allowed us to make meaningful comparisons between the relative time responses of different experimental conditions and the observed time responses. This approach helped us to identify significant differences in the bees' responses to different stimuli and to gain a deeper understanding of the factors that influence their behaviour. Twenty different model bees with different random factors were examined and reported in this study. The final model could be simplified to emphasise the effect of the contributions of learning and feedback from command cells. In this way, the final model ( Figure 7A ) was also examined with learning cells inactive ( α = 0 ) or without the contribution of command cells by synaptic weights v a and v r set to zero. We assumed the accept and reject pathways process the input interdependently (i.e. no interaction between pathways) if α = 0 , v a = 0 and v r = 0 .
Collected data have been deposited in figshare via link GitHub , (copy archived at MaBouDi, 2023 ).
- Marshall JAR
- Sitaraman D
- Belliart-Guérin G
- Schnaitmann C
- Johnston RM
- Nitabach MN
- Heberlein U
- Google Scholar
- Vasconcelos ML
- Grunwald Kadow IC
- Siwanowicz I
- Skorupski P
- Churchland AK
- Das Chakraborty S
- de Croon GCHE
- Dupeyroux JJG
- Christoforou C
- Sutcliffe B
- Meissner GW
- Jefferis GS
- Drugowitsch J
- Moreno-Bote R
- Schneider-Mizell CM
- Valdes-Aleman J
- Litwin-Kumar A
- Fallenstein B
- Higginson AD
- Mallpress DEW
- McNamara JM
- Modelling Animal Decisions Group
- Schapire RE
- Groschner LN
- Chan Wah Hak L
- Miesenböck G
- Ritzmann RE
- Prescott TJ
- Heisenberg M
- Friedrich AB
- Bulteel AJB
- Shimazaki H
- Galpayage Dona HS
- Onoufriou PD
- Matthews BW
- Mauelshagen J
- Blumstein DT
- Nieuwenhuis S
- Dickinson MH
- O’Connell LA
- O’Connell RG
- O’Hanlon JC
- Herberstein ME
- Huetteroth W
- Phillips-Portillo J
- Gronenberg W
- De Backer J-F
- Wosniack ME
- Edmondson-Stait A
- Calle-Schuler SA
- Lauritzen JS
- Jefferis GSXE
- Gjorgjieva J
- Carruthers P
- Dickinson A
- Glimcher PW
- Güntürkün O
- Stankiewicz J
- Steinbeck F
- Stephens DW
- Strausfeld NJ
- Beauregard-Racine J
- Wickelgren WA
Author details
- Department of Computer Science, University of Sheffield, Sheffield, United Kingdom
- Sheffield Neuroscience Institute, University of Sheffield, Sheffield, United Kingdom
Present address
Contribution, for correspondence, competing interests.
- School of Natural Sciences, Macquarie University, North Ryde, Australia
Engineering and Physical Sciences Research Council (EP/P006094/1)
Australian research council (ft140100452), leverhulme trust (vp1-2017-026), templeton world charity foundation (twcf-2020-20539).
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
We thank Michael Port from Sheffield Robotics for assistance in building the testing arena. We thank Amy Bullivant for her assistance in analysing the video. HM, ND and JARM were supported by the Engineering and Physical Sciences Research Council (grant no EP/P006094/1). ABB is supported by funding by a Future Fellowship from the Australian Research Council (FT140100452), a Leverhulme Visiting Fellowship from the Leverhulme Trust and the Templeton World Charity Foundation (grant no. TWCF-2020–20539).
Version history
- Preprint posted: January 3, 2023 (view preprint)
- Received: January 14, 2023
- Accepted: May 24, 2023
- Version of Record published: June 27, 2023 (version 1)
- Version of Record updated: September 29, 2023 (version 2)
© 2023, MaBouDi et al.
This article is distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use and redistribution provided that the original author and source are credited.
- 3,362 views
- 522 downloads
- 2 citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as pdf).
- Article PDF
- Figures PDF
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools), categories and tags.
- action selection
- decision-making
- mushroom bodies
- sequential sampling model
- protocerebrum
Research organism
- Apis mellifera
Further reading
- Genetics and Genomics
Spotless, a reproducible pipeline for benchmarking cell type deconvolution in spatial transcriptomics
Spatial transcriptomics (ST) technologies allow the profiling of the transcriptome of cells while keeping their spatial context. Since most commercial untargeted ST technologies do not yet operate at single-cell resolution, computational methods such as deconvolution are often used to infer the cell type composition of each sequenced spot. We benchmarked 11 deconvolution methods using 63 silver standards, 3 gold standards, and 2 case studies on liver and melanoma tissues. We developed a simulation engine called synthspot to generate silver standards from single-cell RNA-sequencing data, while gold standards are generated by pooling single cells from targeted ST data. We evaluated methods based on their performance, stability across different reference datasets, and scalability. We found that cell2location and RCTD are the top-performing methods, but surprisingly, a simple regression model outperforms almost half of the dedicated spatial deconvolution methods. Furthermore, we observe that the performance of all methods significantly decreased in datasets with highly abundant or rare cell types. Our results are reproducible in a Nextflow pipeline, which also allows users to generate synthetic data, run deconvolution methods and optionally benchmark them on their dataset ( https://github.com/saeyslab/spotless-benchmark ).
Phantasus, a web-application for visual and interactive gene expression analysis
Transcriptomic profiling became a standard approach to quantify a cell state, which led to accumulation of huge amount of public gene expression datasets. However, both reuse of these datasets or analysis of newly generated ones requires significant technical expertise. Here we present Phantasus - a user-friendly web-application for interactive gene expression analysis which provides a streamlined access to more than 96000 public gene expression datasets, as well as allows analysis of user-uploaded datasets. Phantasus integrates an intuitive and highly interactive JavaScript-based heatmap interface with an ability to run sophisticated R-based analysis methods. Overall Phantasus allows users to go all the way from loading, normalizing and filtering data to doing differential gene expression and downstream analysis. Phantasus can be accessed on-line at https://alserglab.wustl.edu/phantasus or can be installed locally from Bioconductor (https://bioconductor.org/packages/phantasus). Phantasus source code is available at https://github.com/ctlab/phantasus under MIT license.
- Evolutionary Biology
CoCoNuTs are a diverse subclass of Type IV restriction systems predicted to target RNA
A comprehensive census of McrBC systems, among the most common forms of prokaryotic Type IV restriction systems, followed by phylogenetic analysis, reveals their enormous abundance in diverse prokaryotes and a plethora of genomic associations. We focus on a previously uncharacterized branch, which we denote co iled- co il nu clease t andems (CoCoNuTs) for their salient features: the presence of extensive coiled-coil structures and tandem nucleases. The CoCoNuTs alone show extraordinary variety, with three distinct types and multiple subtypes. All CoCoNuTs contain domains predicted to interact with translation system components, such as OB-folds resembling the SmpB protein that binds bacterial transfer-messenger RNA (tmRNA), YTH-like domains that might recognize methylated tmRNA, tRNA, or rRNA, and RNA-binding Hsp70 chaperone homologs, along with RNases, such as HEPN domains, all suggesting that the CoCoNuTs target RNA. Many CoCoNuTs might additionally target DNA, via McrC nuclease homologs. Additional restriction systems, such as Type I RM, BREX, and Druantia Type III, are frequently encoded in the same predicted superoperons. In many of these superoperons, CoCoNuTs are likely regulated by cyclic nucleotides, possibly, RNA fragments with cyclic termini, that bind associated CARF ( C RISPR- A ssociated R ossmann F old) domains. We hypothesize that the CoCoNuTs, together with the ancillary restriction factors, employ an echeloned defense strategy analogous to that of Type III CRISPR-Cas systems, in which an immune response eliminating virus DNA and/or RNA is launched first, but then, if it fails, an abortive infection response leading to PCD/dormancy via host RNA cleavage takes over.
Be the first to read new articles from eLife

Subscribe or renew today
Every print subscription comes with full digital access
Science News
A vaccine for bees has an unexpected effect.
Honeybees immunized against bacteria also fought off a virus

Honeybees are under stress from pesticides, mites and a variety of diseases. Now, a new vaccine aimed at protecting bees from a serious bacterial infection may do double duty by warding off a virus.
© Jackie Bale/Moment/Getty Images
Share this:
By Tina Hesman Saey
April 24, 2024 at 8:30 am
WASHINGTON — The first vaccine designed for insects may make honeybees healthier overall.
Honeybee hives vaccinated against a bacterial disease had much lower levels of an unrelated viral disease than did unvaccinated hives, veterinarian Nigel Swift of Dalan Animal Health reported April 3 at the World Vaccine Congress .
Researchers at Dalan, based in Athens, Ga., designed the bee vaccine to protect against American foulbrood — a fatal disease caused by a spore-forming bacterium called Paenibacillus larvae . Adult bees don’t get sick but can spread spores in the hive, where the disease infects and kills larvae. Spores can remain viable for more than 50 years, so beekeepers with infected colonies must destroy hives by irradiating or burning them to keep the disease in check. A vaccine may save bee lives and beekeepers’ livelihoods.
Foulbrood disease is just one of many problems plaguing bees, Swift said. “Pesticides, parasites, climate change, nutritional stress — these all make bees more susceptible to infectious diseases.” From April 2022 to April 2023, U.S. beekeepers lost an estimated 48 percent of their colonies, according to the Bee Informed Partnership, a nonprofit research organization.
Dalan’s vaccine against foulbrood disease doesn’t rely on tiny syringes. Instead, bees are inoculated through a sugar paste that researchers spike with heat-killed P. larvae . Worker bees eat the candy and incorporate it into their royal jelly, which they feed to the queen. Inside the queen’s gut, bits of the bacteria attach to a protein, which in turn transports the vaccine fragments to the ovaries where they can be deposited in eggs. Larvae that hatch from the eggs should be protected from the disease.
Testing the vaccine wasn’t easy. One larvae-producing site in Florida was hit by a hurricane, “another was taken out by bears,” Swift said. But the team persisted. In lab tests, the company infected larvae from both vaccinated and placebo-treated hives with P. larvae . About twice as many placebo larvae died as vaccinated larvae , the researchers reported in 2022.
Based on that evidence, the U.S. Department of Agriculture gave conditional approval for the bee vaccine, Dalan announced in 2023. The Canadian Food Inspection Agency authorized use of the vaccine later that year.
Beekeepers who had been using the vaccine told Dalan that vaccinated colonies seemed to have all-around improvements in health that couldn’t be explained just by reducing the incidence of foulbrood disease. The company decided to look at a variety of diseases, honey production and other measures of bee health along with the efficacy of the vaccine in a real-world setting. An apiary called Vidalia Apicultural Services & Bee Co. in Lyons, Ga., let Dalan use 400 hives for the study, which lasted for one season. Half of the hives got a new vaccinated queen and half got a new unvaccinated one.
In one sense, the test was a bust. No cases of foulbrood disease were found in any of the hives. “This apiary was just too good” at controlling the disease, Swift said. So the company couldn’t determine how effective the vaccine was against its intended target.
Yet the researchers found a surprising result: Vaccinated hives were protected from a viral disease spread by varroa mites ( SN: 3/7/16 ). Both vaccinated and unvaccinated hives started the study with the same number of mites and a baseline level of virus, as measured by a PCR test. Virus levels continued to rise in the unvaccinated hives but declined in the vaccinated ones. At the end of the study, vaccinated hives had accumulated 83 percent less virus than unvaccinated hives did, Swift said. The number of mites per hive remained the same.
“It’s an important finding for sure, if it’s repeatable,” says biochemist Andrea Gwyn of the biopharmaceutical company GSK, based in Middlesex, England. Gwyn, who works on vaccines for people, is a hobbyist beekeeper. She is particularly interested in whether queen bees can pass on defenses against American foulbrood and perhaps other infections for more than one egg-laying season and whether a queen’s drone sons and daughter queens could pass on the protections to a second generation.
The results are still preliminary, and the researchers aren’t sure exactly why immunizing bees against bacteria might also protect against viruses, Swift said. It may be because bees’ immune systems aren’t as specific as those of humans and other mammals: Anything that revs up bees’ immune responses may help them take on multiple intruders.
“We’re just trying to think it through: What is really happening?” Swift says, “It’s humbling.… You get these results sometimes that weren’t what you were expecting. This could be somebody’s Ph.D. now to go and tackle this particular topic.”
More Stories from Science News on Life

Biological puzzles abound in an up-close look at a human brain

Two distinct neural pathways may make opioids like fentanyl so addictive

Human body lice could harbor the plague and spread it through biting

‘The High Seas’ tells of the many ways humans are laying claim to the ocean

Sumatran orangutans start crafting their engineering skills as infants

The heart plays a hidden role in our mental health

Genetic analyses of the bird flu virus unveil its evolution and potential

How smart was T. rex ?
Subscribers, enter your e-mail address for full access to the Science News archives and digital editions.
Not a subscriber? Become one now .
November 4, 2020
The Problem with Honey Bees
They’re important for agriculture, but they’re not so good for the environment
By Alison McAfee

Ed Peeters Getty Images
To many people, honey bees symbolize prosperity, sustainability and environmentalism. But as a honey bee researcher, I have to tell you that only the first item on that list is defensible. Although they are important for agriculture, honey bees also destabilize natural ecosystems by competing with native bees—some of which are species at risk.
The rise in hobby beekeeping , now a trendy activity for hundreds of thousands of Americans, followed strong awareness campaigns to “save the bees.” But as a species, honey bees are least in need of saving. Media attention disproportionately covers them over native pollinators, and murky messaging has led many citizens—myself once included—to believe they are doing a good thing for the environment by putting on a beekeeper’s veil. Unfortunately, they are probably doing more harm than good.
“Beekeeping is for people; it's not a conservation practice,” says Sheila Colla, an assistant professor and conservation biologist at Toronto’s York University, Canada. “People mistakenly think keeping honey bees, or helping honey bees, is somehow helping the native bees, which are at risk of extinction."
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Colla recently published an analysis of nearly a thousand comments submitted by citizens in response to Ontario’s draft Pollinator Health Action Plan —a proposal that involved a plan for stricter neonicotinoid pesticide regulations. Despite intense public interest in bees and pollination and strong support of tighter pesticide regulations, Colla and her colleagues found that citizens had a surprisingly poor understanding of the diversity of pollinators and their roles in pollination.
“The focus on neonics [a kind of pesticide] and honey bees has taken a ton of resources away from conserving wild pollinators from their most important threats,” Colla says. She is justifiably frustrated at the misappropriated attention on saving honey bees when, from a conservationist’s point of view, native bees are the ones in more dire need of support.
And while honey bee–centric businesses often support initiatives that benefit native bees, such as developing bee-friendly habitat, the financial contributions pale in comparison to what could be achieved if funds were applied to these initiatives directly. “Beekeeping companies and various non-science-based initiatives have financially benefitted from the decline of native pollinators,” Colla explains. “These resources thus were not allocated to the actual issue people are concerned about.”
For some reason, maybe because they are small, honey bees are not generally viewed as the massively distributed livestock animal that they are. There are millions of honey bee colonies in North America, 2.8 million of which are in the U.S. Approximating around 30,000 bees per colony (the size of a pollination unit ), that’s roughly a billion honey bees in Canada and the U.S. alone—almost triple the number of people.
High densities of honey bee colonies increase competition between native pollinators for forage, putting even more pressure on the wild species that are already in decline. Honey bees are extreme generalist foragers and monopolize floral resources, thus leading to exploitative competition—that is, where one species uses up a resource, not leaving enough to go around.
But determining honey bees' influence on natural ecosystems requires empirical testing. It is possible, for example, that alternate foraging habits of native bees—differences in their active times of day or preferred plants, for example—could lead to little effective competition. Honey bees are so ubiquitous, though, that it has been hard to test exactly how their introduction, and subsequent resource monopolization, affects ecosystem networks.
Not so for the Canary Islands. Alfredo Valido and Pedro Jordano, researchers from the Spanish National Research Council in Tenerife and Sevilla, respectively, saw an opportunity to use these islands—a Spanish archipelago off the northwestern coast of Africa—to study how the introduction of honey bees affects the native pollinating community.
In the highlands of the islands’ Teide National Park, thousands of honey bee colonies are introduced seasonally for honey production and removed again at the end of the nectar flow, creating an excellent scenario for experimentation. Their results, published in Scientific Reports , do not make honey bees look like the sustainability celebrities they have become.
Bringing in honey bees reduced the connectedness of the plant-pollinator networks. Nestedness and modularity, two indicators of ecosystem resilience, also declined. While some plant species enjoyed higher fruit set, fruits sampled nearest the apiaries contained only aborted seeds. “The impact of the beehives is so dramatic,” says Valido, “You can detect disruption between plants and pollinators just the day after beehive installation.”
“By introducing tens or hundreds of beehives, the relative density of honey bees increases exponentially compared with wild native pollinators,” Valido explains. This causes a drastic reduction of flower resources—pollen and nectar—within the foraging range. “Beekeeping appears to have more pervasive, negative impacts on biodiversity than it was previously assumed,” says Jordano.
Valido and Jordano suspect that their findings on the Canary Islands are generally applicable to other ecosystems where honey bees are introduced, but they note that the specific impact of beekeeping in other locations may differ.
Indeed, honey bees are not always the top competitor in a pollinator network: Whether they succeed at outcompeting the native bees depends on other factors. For example, Nicholas Balfour and his colleagues at the University of Sussex, England, found that native bumble bees were superior competitors on the tubular flowers of lavender, owing in part to their longer proboscis (tongue).
In still other ecosystems , honey bees appear not to be as influential as in the Canary Islands. After introduction in northern Patagonia, nonnative bumble bees and honey bees overtook the native bees as the most frequent floral visitors, but this had no effect on the native bees’ actual visitation rates.
While every ecosystem has its own quirks—with different pollinator players and participating plants—pollination network studies conducted closer to home tend to agree with the findings in the Canary Islands. “There have been studies in North America showing pollination system disruptions by honey bees,” says Colla. “Honey bees also are very effective at pollinating certain weedy species, which changes the overall plant communities.”
Many of those weedy species are also invasive, including Scotch broom, dandelions, Himalayan blackberry and Japanese knotweed, among others. And beekeepers secretly love invasive plants. Their intense proliferation provides a lucrative and predictable nectar flow—perfect for the honey bees, and beekeepers, to capitalize on—but the plants, too, disrupt native ecosystems.
Even with this boost of forage, there is sometimes still not enough to go around amongst honey bees, let alone native bees. In the lower mainland surrounding Vancouver, Canada, I kept a small research apiary with 15–20 hives. It was my first year keeping research colonies in a high-density area, and I have never struggled so much to keep my bees alive.
The hives were riddled with diseases. I even euthanized one colony with symptoms of American foulbrood—standard protocol, as it’s one of the most destructive, contagious diseases that honey bees face. Despite being entirely free of Varroa destructor —a devastating parasitic mite—at the start of the season, the hives required miticide treatments by late summer. And the colonies did not produce a crop of honey.
Colony densities in some locations have become too high, facilitating the spread of disease and exacerbating problems with poor nutrition. If it was this hard to keep my honey bees healthy, I’m not sure I can bear to think about the wild bees.
But think about them, we must. I used to believe that honey bees were a gateway species, and that concern over their health and prosperity would spill over onto native bees, benefitting them, too. While this may have happened in some cases, evidence is mounting that misguided enthusiasm for honey bees has likely been to the native bees’ detriment. Beekeeping doesn’t make me feel good, anymore. In fact, quite the opposite.
Variation in Pesticide Toxicity in the Western Honey Bee ( Apis mellifera ) Associated with Consuming Phytochemically Different Monofloral Honeys
- Open access
- Published: 18 May 2024
Cite this article
You have full access to this open access article

- Ling-Hsiu Liao 1 ,
- Wen-Yen Wu 1 &
- May R. Berenbaum 1
346 Accesses
Explore all metrics
Insecticide toxicity to insect herbivores has long been known to vary across different host plants; this phenomenon has been widely documented in both foliage-feeders and sap-feeders. Species-specific phytochemical content of hostplant tissues is assumed to determine the pattern of induction of insect enzymes that detoxify insecticides, but specific phytochemicals have rarely been linked to host plant-associated variation in pesticide toxicity. Moreover, no studies to date have examined the effects of nectar source identity and phytochemical composition on the toxicity of insecticides to pollinators. In this study, we compared LD 50 values for the insecticide bifenthrin, a frequent contaminant of nectar and pollen in agroecosystems, in the western honey bee, Apis mellifera , consuming three phytochemically different monofloral honeys: Nyssa ogeche (tupelo), Robinia pseudoacacia (black locust), and Fagopyrum esculentum (buckwheat). We found that bifenthrin toxicity (LD 50 ) values for honey bees across different honey diets is linked to their species-specific phytochemical content. The profiles of phenolic acids and flavonoids of buckwheat and locust honeys are richer than is the profile of tupelo honey, with buckwheat honey containing the highest total content of phytochemicals and associated with the highest bifenthrin LD 50 in honey bees. The vector fitting in the ordination analysis revealed positive correlations between LD 50 values and two honey phytochemical richness estimates, Chao1 and Abundance-based Coverage Estimator (ACE). These findings suggest unequal effects among different phytochemicals, consistent with the interpretation that certain compounds, including ones that are rare, may have a more pronounced effect in mitigating pesticide toxicity.
Similar content being viewed by others

Behavioral responses of honey bees (Apis mellifera) to natural and synthetic xenobiotics in food

Toxicological status changes the susceptibility of the honey bee Apis mellifera to a single fungicidal spray application
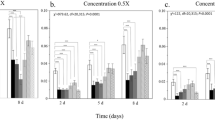
Fungicide ingestion reduces net energy gain and microbiome diversity of the solitary mason bee
Avoid common mistakes on your manuscript.
Introduction
Insecticide toxicity to insect herbivores has long been known to vary across different host plants. Among the mechanisms proposed by which host plant identity can influence the toxicity of an insecticide to an insect herbivore is that the species-specific phytochemical content of hostplant tissues determines the pattern of induction of detoxification enzymes, particularly the cytochrome P450 monooxygenases and carboxylesterases, capable of detoxifying the insecticide. This phenomenon was first described in interactions between polyphagous folivores and their host plants. Berry et al. ( 1980 ), e.g., showed that induction of aldrin epoxidase in Peridroma saucia (variegated cutworm) (Lepidoptera: Noctuidae) differed depending on hostplant identity and that this induction in turn affected the level of tolerance of three organophosphate insecticides.
Since then, host plant effects on insecticide toxicity have been demonstrated in other polyphagous (e.g., Karuppaiah et al. 2016 ; Saeed et al. 2019 ; Xue et al. 2010 ) as well as oligophagous (Prouty et al. 2021 ) lepidopterans. At least one coleopteran folivore, the oligophagous Colorado potato beetle, Leptinotarsa decemlineata , is known to experience differential toxicity in response to insecticide exposure depending on host plant identity (Ghidiu et al. 1990 ; Mahdavi et al. 1991 ). Mechanistically, differential activity of cytochrome P450 monooxygenases (P450s) can mediate the differential toxicity of insecticides to generalist lepidopterans. In the polyphagous tobacco cutworm, Spodoptera litura , with more than 120 recorded hostplant species, hostplant identity affected LD 50 values for the organophosphate profenophos and the pyrethroid cypermethrin, with LD 50 values for profenophos higher for larvae consuming castor than for larvae consuming soybean and the LD 50 for cypermethrin lower for larvae consuming castor than for larvae consuming soybean. Activity levels of P450s were positively correlated with the LD 50 of cypermethrin (Karuppaiah et al. 2016 ). Moreover, Guo et al. ( 2023 ) found that host-plant switching by the rice leaf-folder Cnaophalocrocis medinalis affected its susceptibility to abamectin and chlorpyrifos as well as activity of its detoxification enzymes [glutathione- S -transferases, “multifunctional oxidases” (including P450s) and carboxylesterases].
Although most extensively documented in folivores, hostplant identity can also influence the toxicity of insecticides to sap-sucking herbivores. Castle et al. ( 2008 ) demonstrated that the highly polyphagous silverleaf whitefly, Bemisia tabaci , recorded on more than 600 hostplant species, displayed higher LC 50 values for a bifenthrin-endosulfan mixture when raised on broccoli or related cole crops than on cantaloupes or cotton. Similarly, Xie et al. ( 2011 ), comparing performance of B. tabaci across multiple host plants, demonstrated that all insecticides tested displayed lower toxicity on one host species, poinsettia ( Euphorbia pulcherrima ), relative to three other host plants, with the LC 50 values for acetamiprid approximately 15-fold, tenfold, and 7.3-fold higher than for whiteflies on tomato, cucumber, and cabbage, respectively. In terms of mechanisms, Xie et al. ( 2011 ) linked induction effects of host plants to insecticide susceptibility in B. tabaci ; glutathione- S -transferase (GST) and cytochrome P450 activity levels were lowest in the population on cucumber. Liang et al. ( 2007 ) linked the hostplant effects on insecticide toxicity to the induction of carboxyesterase activity in Bemisia tabaci biotype B and greenhouse whitefly, Trialeurodes vaporariorum . Njiru et al. ( 2023 ) found host plant modulation of acaricide resistance in the two-spottedspider mite, Tetranychus urticae , which use their mouthparts to pierce individual cells to remove their contents, to 13 acaricides with different modes of action and, with piperonyl butoxide synergism assays, demonstrated enhancement of toxicity of cyflumetofen in tomato but not bean, implicating P450s in detoxification. In addition, Dermauw et al. ( 2013 ) observed that when T. urticae mites were adapted from bean to a challenging host plant (tomato), their differentially expressed genes increased over generations, including P450 genes. Moreover, expression profiles of adapted mites resembled those of multipesticide-resistant strains, and this adaptation reduced their susceptibility to pesticides. This finding links host plant adaptation to pesticide resistance.
Despite the broad recognition of impacts of hostplant identity on pesticide toxicity to foliage-feeding or piercing-sucking herbivores, there is virtually no literature on the effects of plant food source identity on insecticide toxicity to pollinators. As well, although enzymatic responses to host plant identity have been well-characterized, phytochemical traits of host plants that differentially affect pesticide toxicity have almost never been documented. As a consumer of plant nectar, pollen and their processed forms honey and beebread, the western honey bee, Apis mellifera , in particular should be susceptible to effects, positive and negative, of phytochemicals due to the considerable diversity of nectar sources exploited by this highly polylectic species and to its ability to concentrate and convert nectar into honey as a storable food resource. Some nectars, e.g., contain phytochemicals toxic to bees; conversion of these nectars into honey can lead to hive collapse (Bischoff and Moiseff 2023 ). Others contain phytochemicals that can be beneficial, providing antioxidant and antimicrobial activities (Berenbaum and Calla 2021 ). Most importantly, certain phytochemicals found in honey can ameliorate pesticide toxicity in bees by upregulating cytochrome P450 enzymes (Mao et al. 2011 , 2013 ).
In this study, we set out to determine whether pollinators, like other herbivores, experience differential toxicity of insecticides depending on the species identity of the nectar sources used to make honey. Apis mellifera , the western honey bee, was selected for this study as a test case due to its ability to collect and process into honey nectars from many different plant species, encountering a broad range of nectar phytochemicals in the process. As well, because bees concentrate nectar in converting it to honey, effects of nectar source identity on pesticide toxicity should be more likely to be detectable, particularly if induction of detoxification enzymes is dose-dependent. To date, individual honey constituents have been tested for their effects on insecticide toxicity (Arathi and Bernklau 2021 ; Liao et al. 2020 , 2017 ; Mao et al. 2011 , 2013 ; Mitton et al. 2020 ; Wong et al. 2018 ), but honey bees encounter phytochemicals in complex mixtures, not in isolation, when they eat honey, and there are few if any studies of the effects of the phytochemical composition of honey on insecticide tolerance. Accordingly, we tested the toxicity of a pyrethroid insecticide, bifenthrin, on adult honey bees consuming three types of monofloral honeys–i.e., honeys that derive 50% or more of their constituent nectar from a single nectar source. Bifenthrin is both highly toxic to honey bees (USEPA OPP Pesticide Ecotoxicity Database) and frequently encountered in agroecosystems in which bees forage and in the hive environment; accordingly, we selected it to serve as a representative pesticide to determine effects of honey identity on pesticide toxicity. In a recent study of pesticide residues in bee-attractive border plantings, e.g., bifenthrin was the most frequently detected among 33 pesticides, found in 44 percent of all samples (Ward et al. 2022 ). As well, in a four-year monitoring survey of honey bee exposure to pesticide residues in hives in China’s main honey-producing areas, bifenthrin had the third-highest detection rate, 19.7% (Xiao et al. 2022 ), behind only the fungicide carbendazim, with a detection rate of 45%, and the in-hive acaricide tau-fluvalinate, with a detection rate of 36.8%. In addition, we analyzed the correlations between alpha diversity metrics, which measure the richness and evenness of the “community” of phytochemicals contained in honey, and the LD 50 values of bees consuming a pesticide administered in five honey diets. This analysis allowed us to measure correlations between the identity and diversity of honey phytochemicals consumed and observed toxic effects of pesticide exposure.
Identification and Quantification of Phenolic Components of Honeys
Monofloral honeys from each of three plant families known to differ in phytochemical content and composition (Gheldof et al. 2002 ) were selected for this study: white tupelo (Nyssaceae: Nyssa ogeche ; commercial tupelo honey from Wewahitchka, FL, USA), black locust (Fabaceae: Robinia pseudoacacia ; commercial locust blossom raw honey, from Plains, PA, USA), and buckwheat (Polygonaceae: Fagopyrum esculentum ; commercial buckwheat honey, from Plains, PA, USA). Methods for honey sample preparation and high-pressure liquid chromatography (HPLC) analysis were adapted from those reported by Gheldof et al. ( 2002 ) and Michalkiewicz et al. ( 2008 ). Twenty grams of tupelo or locust honey or 10 g of buckwheat honey were dissolved in 100 mL of acidified deionized water (pH 2.0) and filtered through solid-phase extraction (SPE) cartridges (186008718, Waters Corporation, Milford, MA) on a vacuum station at flow rate < 5 mL/min. The loading quantity of buckwheat honey was halved to avoid saturating and blocking SPE cartridges. After washing each cartridge with an additional 100 mL acidified water to remove sugars and polar compounds, 50 mL methanol were eluted to recover the adsorbed phenolic acids and flavonoids. The methanol extract was concentrated using a rotary evaporator at 30°C and the solid extracts were then redissolved in 1 mL (tupelo and locust) or 0.5 mL (buckwheat) methanol containing methyl 4-hydroxybenzoate (200 μg/mL) as internal standard. The supernatant of reconstituted extract, centrifuged at 18,000 g RCF for 30 s, was used for HPLC analysis.
HPLC analysis was performed on a Phenomenex® Gemini C18 column (150 mm by 2 mm, 5 μm) with a Shimadzu Prominence SPD-M20A photodiode array detector (PDA; scanning range: 190–450 nm, slit of 1.2 nm, acquisition rate of 1.5625 Hz, and flow in the cell temperature of 40 °C). The column oven temperature was maintained at 40°C as well. Gradient elution and variable total flow rate of the mobile phase were carried out for obtaining an optimized chromatographic peak separation and for keeping the operating pressure below the upper limit of the pump and system. The mobile phase consisted of 0.5% formic acid in water (phase A) and methanol (phase B). Before the sample injection, the mobile phase was kept at 20% B for 15 min at 0.2 mL/min flow rate. After the injection (0 min), the mobile phase was delivered in linear gradient mode as follows: in 0.01 min decreasing 15% B, 0.01–5 min 15% B, 9–16 min 25% B, 30–34 min 45% B, 44 min 48% B, 50–65 min 60% B, 66–71 min 95% B, and holding for 4 min. The flow rate was also changed linearly after sample injection, decreasing from 0.2 to 0.1 mL/min over four min, maintained from 4–7 min at 0.1 mL/min, from 9–16 min at 0.15 mL/min, from 17–24 min at 0.18 mL/min, from 28–38 min at 0.16 mL/min, for 39 min at 0.18 mL/min, from 43–71 min at 0.2 mL/min; and holding for 4 min.
Components were identified and quantified by comparing with reference standard retention time, absorbance spectral characteristics, and integrated area of absorbance peaks detected at their best detection wavelength (Table S1 ). The quantification was calibrated via normalization of the peak areas by referring to the internal standard and calibration curves established with known concentrations of standard chemicals.
Effects of Honey Phytochemicals on Acute Pesticide Toxicity
For bioassays assessing pesticide toxicity to bees on different monofloral honey diets, the method of Wong et al ( 2018 ) was used to evaluate the impact of consuming three different monofloral honeys on bifenthrin median lethal dose (LD 50 ) values. Honey bees were obtained from apiaries of the University of Illinois Bee Research Facility located in Urbana, Champaign County, IL (40°07′52"N 88°08′43"W and 40°07′38"N 88°10′31"W) in summer 2018. Frames of capped brood were collected from three naturally mated queen colonies and then incubated in a dark room at 34°C to obtain newly emerged worker bees. The day-old bees, collected within 24 h of eclosion, were introduced into cages in groups of 10 individuals (except for two cages, which inadvertently contained 11 bees). Each cage, following methods used in earlier studies (Liao et al. 2020 , 2017 , 2019 ), was equipped with four 2-mL microcentrifuge tube feeders; three feeders provided a formulated honey diet and one provided water. The experiment comprised five diet treatments: tupelo, locust, and buckwheat honey in separate cages, a choice treatment (TLB-Choice) offering three honey options, and a sugar control that represents the average sugar proportions in the honeys (40% fructose: 29% glucose: 1% sucrose) as documented in previous studies (Gardiner 2015 ; Pasini et al. 2013 ; White and Doner 1980 ). All diets contained casein (C3400, Sigma–Aldrich Co. LLC., St. Louis, MO) at a ratio of 1:12 protein to carbohydrate as a phytochemical-free protein source. Three days after caging, surviving bees (9–11 bees per cage) within their cages were chilled with ice to keep them immobilized and were then individually treated topically with bifenthrin in acetone or acetone alone as a solvent control. We evaluated the effects of honey on the bifenthrin (LD 50 ) with 1 µl acetone containing concentrations of bifenthrin encompassing 0 ppb, 120 ppb, 150 ppb, 240 ppb, 300 ppb, 600 ppb, 1200 ppb, 1500 ppb, 2400 ppb, and 3000 ppb. Three to nine replicates of each concentration in each treatment were tested from each of three naturally mated queen colonies, except for 120 ppb, which had two replicates for two colonies, for a total of 5159 bees. All three hives and cage replicates were carried out within a 24-day period.
Probit analysis was conducted to estimate LD 50 values using IBM SPSS Statistics (version 24, SPSS Inc., Chicago, IL, USA). A heterogeneity factor was included in the calculation of 95% confidence limits if the significance level of Pearson Goodness-of-Fit Test was below 0.15 (Norušis 2007 ). Significant differences between LD 50 values were determined by estimation of confidence intervals of the relative median potency (RMP) when values of the 95% confidence interval of relative median potency did not include “1”.
Analysis of the Phytochemical Composition of Honeys and their Associations with Honey Bees
In phytochemical studies, alpha diversity indices have been used to assess of phytochemical diversity to provide a quantitative measure of the composition of naturally occurring mixtures (Hilker 2014 ; Wetzel and Whitehead 2020 ). The indices have facilitated comparisons of phytochemical diversity among host samples and have been used as quantitative indices to develop models to study the effects of phytochemical diversity on herbivore performance (Glassmire et al. 2020 ), ecological interactions (Cacho et al. 2015 ; Doyle 2009 ; Richards et al. 2015 ), and evolutionary processes (Morris et al. 2014 ; Tewes et al. 2018 ). We used several common diversity indices for a comprehensive characterization of phytochemical richness and evenness, including Richness, Shannon–Wiener diversity Index (Shannon), Inverse Simpson diversity Index (inv_ Simpson), Pielou's Evenness Index (Pielou), and extrapolated richness estimators (Chao et al. 2014 ), including the Chao1 richness estimator (Chao1) and the Abundance-based Coverage Estimator (ACE), using the 'vegan' package (Dixon 2003 ; Oksanen et al. 2022 ) in R (R Core Team 2023 ). The Richness index quantified the total number of phytochemicals in each honey sample; the Shannon index and the inverse Simpson index were used to measure both richness and evenness; the Pielou index measured the evenness of the compound distribution; and Chao1 and ACE estimated the total number of phytochemicals, considering both detected and undetected ones.
To assess the differences in phytochemical diversity among the three honey samples, we first used Levene's test for equality of variances to evaluate the homogeneity of variances. If homogeneity of variances was confirmed (Levene's test, p > 0.05), we used analysis of variance (ANOVA) followed by Scheffé's post-hoc analysis. If homogeneity of variances was violated, indicating unequal variances, we performed the Kruskal–Wallis rank test with Dunn's test for pairwise comparisons. A significance level of α = 0.05 was used in the tests. For the analysis of the phytochemical composition of honey and its effects on pesticide toxicity, we employed multivariate analysis with non-metric multidimensional scaling (NMDS) and the envfit function from the R package 'vegan' (Dixon 2003 ; Oksanen et al. 2022 ). Prior to analysis, the phytochemical units in honey were converted to μM to assess the bioavailable concentrations of the phytochemicals in the honey diet. NMDS plots, in conjunction with a stress value and the Adonis index, were utilized to evaluate the clustering of honey samples based on phytochemistry (Bray–Curtis distance, k = 5). A stress value close to 0 indicated a good fit to the NMDS plot, while the Adonis test provided R-square and p values to assess the significance of the observed group differences. Additionally, the envfit function for multiple regression with 999 permutations was used to fit variables (vectors) to the NMDS ordination, regardless of whether explained variables were part of the original analysis that generated the plot. This function facilitated the visualization and quantification of relationships between variables by aligning environmental factors with the ordination plot (Dixon 2003 ). This approach revealed associations of the variables with the phytochemicals present in the honey samples by correlating them with the underlying ordination axes. It also helped to characterize relationships between variables; for example, angles between vectors (variables) on the NMDS ordination plot indicate their correlations (Šmilauer and Lepš 2014 ). These variables included individual phytochemicals, alpha diversity metrics of honey phytochemical composition, and average 24-h LD 50 values to represent toxicity of pesticides to bees on different honey diets.
Monofloral Honey Characteristics: Phytochemicals and Alpha-Diversity
The major phytochemical constituents of the three monofloral honey are presented in Table 1 . Buckwheat honey is characterized by its richness in phenolic acids, especially p -hydroxybenzoic acid, and surpassed tupelo and locust honey in its levels of pinobanksin and pinocembrin (Table 1 ). Tupelo honey contained high levels of the sesquiterpene abscisic acid and greater concentrations of quercetin and kaempferol, while locust honey was characterized by its higher hyperoside content.
As reflected by diversity indices, buckwheat and locust honeys exhibited a phytochemical profile with higher richness than tupelo honey. Buckwheat honey had the highest Chao1 estimation at 21.89 ± 1.46 of total phytochemicals, followed by locust honey at 19.38 ± 0.20, and tupelo honey at 16.50 ± 0.13. Both buckwheat and locust honeys had significantly higher estimation of phytochemicals than does tupelo honey (Table 2 and S2 ) (p < 0.05, Dunn's test after Kruskal–Wallis rank test). In the ACE estimation of honey phytochemicals, a similar pattern was observed, with buckwheat honey having the highest value at 20.51 ± 0.48, followed by locust honey at 19.68 ± 0.20, and tupelo honey at 18.47 ± 0.17. Both buckwheat and locust honeys showed significantly higher values than tupelo honey (p < 0.05, Scheffé post hoc test after ANOVA), with no significant difference between buckwheat and locust honeys. The average phytochemical richness values were 19.33, 18, and 15.17 for locust, buckwheat, and tupelo honeys, respectively, with locust honey having a significantly higher richness value than tupelo honey (p < 0.05, Dunn’s test after Kruskal–Wallis rank test), while no significant difference was observed between locust and buckwheat honey. However, with respect to phytochemical evenness, locust honey had the highest evenness (Pielou index: 0.60); Pielou evenness values for buckwheat (0.50) and tupelo honey (0.53) were lower. In addition, locust honey was characterized by the highest values for the Shannon and Inverse Simpson indices.
Effects of Honey on Honey Bees: Pesticide Acute Toxicity
In terms of acute toxicity, the median lethal dose (LD 50 ) values for bifenthrin in bees on each of the four honey-containing diets were higher than those for bees on the phytochemical-free diet at both 24 h and 48 h (Table 3 ). The LD 50 values for bifenthrin for bees on the buckwheat honey diet were greater than the LD 50 values for bees on the phytochemical-free sugar diet [the relative median potency (RMP) = 0.73 (0.61–0.87, 95% CI) at 24 h and RMP = 0.77 (0.63–0.93, 95% CI) at 48 h]. Bees on the TLB-Choice diet also had greater LD 50 values at 24 h than the bees on the sugar diet [RMP = 0.80 (0.67–0.95, 95% CI)].

Honey Phytochemical Composition and Associations with Honey Bees
The non-metric multidimensional scaling (NMDS) plot (Fig. 1 ), based on the Bray–Curtis distance, illustrated the grouping of honey samples according to their phytochemicals (Adonis: 0.99, p < 0.001). With the exception of rutin and chlorogenic acid, phytochemicals showed statistically significant associations with honey types (p < 0.01; based on 999 permutations, Fig. 1 A; Table S3 ). Similarly, LD 50 values and alpha diversity metrics of phytochemicals showed statistically significant associations with honey phytochemical composition (Fig. 1 B; Table S3 ).
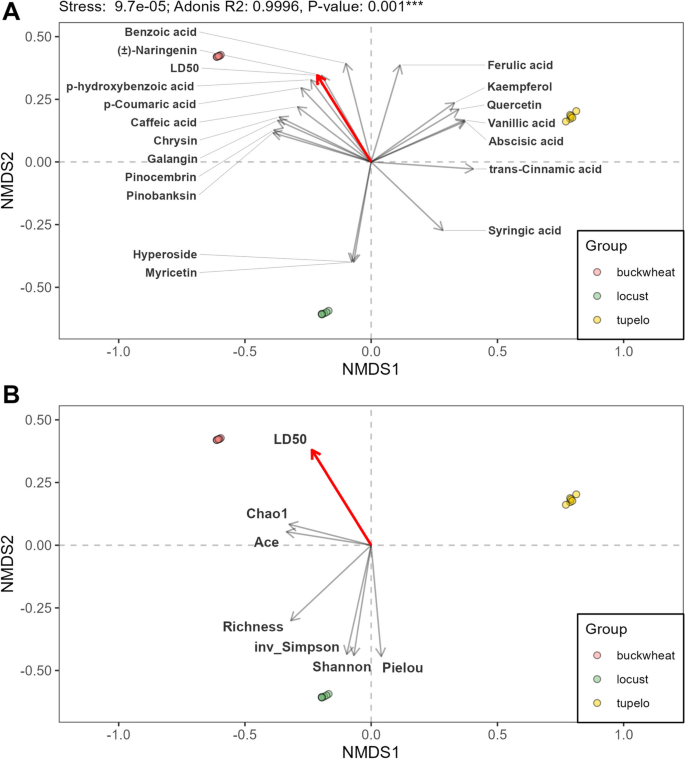
The nonmetric multidimensional scaling (NMDS) plot illustrates the phytochemical distributions among samples from three monofloral honey, based on the Bray–Curtis distance (n = 18; Adonis: 0.99, p < 0.001). The NMDS plot also displays vectors for phytochemicals ( A ), alpha diversity metrics of honey phytochemical composition ( B ), and LD 50 of bifenthrin for honey bees (red), as determined by the envfit function. The direction of the vector arrows indicates the maximum gradient direction of the variable (the direction of the most rapid change in the variable), and the arrow length is proportional to the squared correlation coefficient with honey samples. The angle between two vectors indicates the direction of the relationship between them, with an acute angle indicating a positive correlation, a perpendicular angle indicating an uncorrelated relationship, and obtuse angles indicating a negative correlation. Only variables that are statistically significant are shown (p < 0.05; based on 999 permutations; Table S3)
In addition, our analysis using envfit evaluated the relationships between honey bee LD 50 and phytochemical variables within the NMDS ordination plots (Fig. 1 ), suggesting that certain phytochemicals influence susceptibility to bifenthrin pesticide toxicity more than others. For example, some phytochemical variables, such as p -hydroxybenzoic acid, p -coumaric acid, benzoic acid, caffeic acid, naringenin, pinobanksin, pinocembrin, galangin, and chrysin, pointed in a similar direction to the LD 50 variable, suggesting positive correlations, while others showed negative correlations (hyperoside, myricetin, syringic acid and trans-cinnamic acid) or were uncorrelated (Fig. 1 ).
Because honey bees are exposed to phytochemicals in mixtures rather than in isolation, our analysis examined the correlations between honey bee LD 50 and alpha-diversity metrics of phytochemicals in honey. Acute angles reflected the relationship between honey bee LD 50 and the Chao1 richness estimate (Chao1), as well as between LD 50 and the Abundance-based Coverage Estimator (ACE), suggesting a positive correlation (Fig. 1 B). This finding indicates that, as the richness estimate increases, so does the LD 50 value. Both Chao1 and ACE estimate phytochemical richness by weighting more heavily compounds present in low abundance to account for compounds that may have been missed during the analysis (Chao et al. 2014 ; Xia and Sun 2023 ). By contrast, the observed nearly perpendicular angles between LD 50 and the richness index indicate a lack of correlation. However, when alpha diversity metrics that incorporate evenness, such as Pielou's Evenness Index, Shannon's Index, and inverse Simpson's Index, are considered, we observed inverted angles. This finding indicates a negative correlation between honey bee LD 50 and these metrics, suggesting that, as the evenness of phytochemical distribution increases, the LD 50 tends to decrease.
In our study, the significant associations between LD 50 with honey phytochemical composition suggest multiple phytochemicals of honey influence susceptibility to bifenthrin. Although in honeys some properties are associated with sugars (Berenbaum and Calla 2021 ), in most honeys biological activity results from their phytochemical profile, which varies substantially according to availability of floral sources for foragers. Examining only three monofloral honeys to evaluate functional differences among honeys varying in phytochemical diversity is a limitation of our study in that it captures only a minuscule sample of honey phytochemical diversity. As well, accurately estimating phytochemical ingestion by bees is challenging. The TLB-Choice diet, designed to allow bees to choose among the three honeys to simulate behavioral regulation of phytochemical ingestion, did not allow us to determine the exact amounts of each honey type consumed and thus to estimate the diversity of phytochemicals ingested by the bees choosing their food. Notwithstanding these limitations, we were able to document differences in biological activities of these three honeys that are directly relevant to bee health—that is, sensitivity to a pesticide of agricultural importance.
A well-documented property of honey relevant to bee health is its ability to up-regulate specific detoxification enzymes (Johnson et al. 2012 ; Mao et al. 2013 ). Such activity is reflected in the diet-dependent differences in bifenthrin LD 50 we observed; relative to the sugar diet, the median lethal dose of bifenthrin increased with consumption of honey with greater diversity of phytochemicals (Chao1 richness estimate and Abundance-based Coverage Estimator (ACE)). This finding is consistent with increased pesticide detoxification after ingestion of individual phytochemicals found in honey. Multiple studies have demonstrated amelioration of pesticide toxicity by consumption of certain phytochemicals individually (Arathi and Bernklau 2021 ; Liao et al. 2020 , 2017 ; Mao et al. 2013 ; Mitton et al. 2020 ; Wong et al. 2018 ). Along the same lines, Ardalani et al. ( 2021a ) demonstrated that bees consuming quercetin displayed reduced residual concentrations of ingested imidacloprid. To date, however, Ardanali et al. ( 2021b ) is the only study of impacts of diets containing natural mixtures of phytochemicals on pesticide metabolism; these authors reported that flavonoids in nectar and pollen diets reduce the residual concentrations of imidacloprid and tau-fluvalinate.
Fully characterizing the beneficial non-nutritive effects of honey phytochemicals will require a multifactorial approach. The main mechanism for increased pesticide detoxification by complex mixtures of phytochemicals in honey relative to a phytochemical-free sugar diet is ostensibly the collective induction of detoxification pathways, particularly CYP6AS and CYP9Q subfamilies (Haas et al. 2022a ; Mao et al. 2009 ). Induction of cytochrome P450s occurs in honey bees consuming individual phytochemicals, including p -coumaric acid, pinocembrin, pinobanksin and pinobanksin 5-methyl ether (Mao et al. 2013 ); of these, present in all three honeys were p -coumaric acid and pinobanksin, albeit in different concentrations. CYP6AS subfamily enzymes and CYP9Q3 metabolize quercetin and are induced by p -coumaric acid (Mao et al. 2009 , 2013 , 2015 ); phytochemical-rich honeys induced four CYP6AS transcripts and CYP9Q3 transcripts, which likely also increased the overall capacity for detoxification of natural and synthetic xenobiotics (Liao et al., in preparation). Additionally, CYP9Q3 is involved in the detoxification of multiple insecticides, including the pyrethroid tau-fluvalinate and the organophosphate coumaphos (Mao et al. 2011 ), the N-cyanoamidine neonicotinoid thiacloprid (Manjon et al. 2018 ), the butenolide flupyradifurone (Belden 2022 ), and the anthranilic diamide chlorantraniliprole (Haas et al. 2022a ). A phylogenomic analysis showed that functional CYP9Q orthologs are generally conserved across bee families (Haas et al. 2022b ), suggesting their importance in the adaptation of bees to environmental stress. Rather than consuming phytochemicals individually, however, honey bees ingest mixtures of phytochemicals while feeding on honey. Herbivore-plant ecological interactions correlate with mixtures of host phytochemicals (Marion et al. 2015 ; Petrén et al. 2023 ), suggesting that studies of the effects of phytochemicals of honey on bees should take into account the overall diversity of honey phytochemicals. Our multivariate analysis revealed the relationship between bifenthrin toxicity and the diversity of phytochemicals of the three honeys, reflected by positive correlations between LD 50 and richness estimates (Chao1 and ACE), indicating higher LD 50 with increased richness, and negative correlations with alpha diversity metrics incorporating evenness of phytochemical composition. These results suggest that certain specific compounds may have a more pronounced effect than others in reducing pesticide toxicity. In addition, the positive correlation observed between richness estimates (Chao1 and ACE) and LD 50 suggests that low-abundance phytochemicals, which are weighted more heavily in these estimates (Chao et al. 2014 ; Xia and Sun 2023 ), may also contribute to the reduction of bifenthrin pesticide toxicity.
Conclusions
Although up-regulation of xenobiotic detoxification pathways in honey bees in response to honey likely evolved in response to potentially toxic phytochemicals, induction of detoxification pathways by phytochemical-rich honeys is likely beneficial in contemporary pesticide-contaminated environments. Impacts of reduced phytochemical diversity in the diet provide insights into the consequences of reduced floral resource diversity and intensively farmed agroecosystems (Decourtye et al. 2010 ). It is important to note, however, that phytochemicals that are not derived from floral nectars are also found in honey (Nešović et al. 2020 ). As Soler et al. ( 1995 ) point out, honeys contain not only phytochemicals derived from nectar but also “the characteristic flavonoids from propolis and/or beeswax (chrysin, galangin, tectochrysin, pinocembrin and pinobanksin)”, which in our study have a positive correlation with the LD 50 variable. Truchado et al. ( 2008 ) specifically point out that the flavonoid aglycones in acacia, or locust, honey ( R. pseudoacacia ) derive from propolis, the substance made by bees from resins collected from plants that are mixed with wax and saliva. The phenolic acid p -coumaric acid is a frequent component of European propolis (Hegazi et al. 2000 ). Propolis-derived flavonoids, including pinocembrin, pinobanksin, and galangin, are absent or present in very low concentrations in our tupelo honey samples relative to the amounts in locust and buckwheat honey. Buckwheat honey in particular is rich in these flavonoids. Mao et al. ( 2013 ) reported that p -coumaric acid is the strongest inducer of the detoxification enzyme CYP9Q3 among phenolic acids, and, among flavonoids, chrysin and naringenin were more effective at inducing CYP9Q3 than were pinocembrin and galangin; pinobanksin 5-methyl ether is “highly effective.” Thus, for bees, plant diversity of landscapes other than that representing nectar sources may have hitherto unrecognized or underestimated health benefits in terms of pesticide toxicity challenges.
In conclusion, honey, the principal stored food product during a substantial proportion of the lifecycle of the honey bee, likely has greater importance in honey bee health than previously recognized, particularly if bees can self-regulate induction of detoxification enzymes as they apparently self-medicate in the presence of pathogens (Gherman et al. 2014 ; Spivak et al. 2019 ; Tihelka 2018 ). Variation in honey phytochemical content may help equip bees with defenses against both natural and synthetic xenobiotics. Potential applications arising from our findings may include landscape diversification plans aimed at optimizing the phytochemical content of non-crop flora to increase the likelihood of occurrence of honey phytochemicals, particularly those introduced into the hive via resin-collecting and propolis production, that can upregulate detoxification enzymes, to promote year-round good health.
Data accessibility
The datasets generated and analyzed in this study are available on the Illinois Data Bank ( https://doi.org/10.13012/B2IDB-6733018_V1 ) (Liao et al. 2024 ).
Arathi HS, Bernklau E (2021) Context-dependent effect of dietary phytochemicals on honey bees exposed to a pesticide, thiamethoxam. J Insect Sci 21:11. https://doi.org/10.1093/jisesa/ieab053
Article CAS Google Scholar
Ardalani H, Vidkjær NH, Laursen BB, Kryger P, Fomsgaard IS (2021a) Dietary quercetin impacts the concentration of pesticides in honey bees. Chemosphere 262:127848. https://doi.org/10.1016/j.chemosphere.2020.127848
Article CAS PubMed Google Scholar
Ardalani H, Vidkjær NH, Kryger P, Fiehn O, Fomsgaard IS (2021b) Metabolomics unveils the influence of dietary phytochemicals on residual pesticide concentrations in honey bees. Environ Int 152:106503. https://doi.org/10.1016/j.envint.2021.106503
Belden JB (2022) The acute toxicity of pesticide mixtures to honeybees. Integr Environ Assess Manage 18:1694–1704. https://doi.org/10.1002/ieam.4595
Berenbaum MR, Calla B (2021) Honey as a functional food for Apis mellifera . Annu Rev Entomol 66:185–208. https://doi.org/10.1146/annurev-ento-040320-074933
Berry RE, Yu SJ, Terriere LC (1980) Influence of host plants on insecticide metabolism and management of variegated cutworm. J Econ Entomol 73:771–774. https://doi.org/10.1093/jee/73.6.771
Bischoff K, Moiseff J (2023) The role of the veterinary diagnostic toxicologist in apiary health. J Vet Diagn Invest 35:597–616. https://doi.org/10.1177/10406387231203965
Article PubMed Google Scholar
Cacho NI, Kliebenstein DJ, Strauss SY (2015) Macroevolutionary patterns of glucosinolate defense and tests of defense-escalation and resource availability hypotheses. New Phytol 208:915–927. https://doi.org/10.1111/nph.13561
Castle SJ, Prabhaker N, Henneberry TJ, Toscano NC (2008) Host plant influence on susceptibility of Bemisia tabaci (Hemiptera: Aleyrodidae) to insecticides. Bull Entomol Res 99:263–273. https://doi.org/10.1017/s0007485308006329
Chao A, Gotelli NJ, Hsieh TC, Sander EL, Ma KH, Colwell RK, Ellison AM (2014) Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol Monogr 84:45–67. https://doi.org/10.1890/13-0133.1
Article Google Scholar
Decourtye A, Mader E, Desneux N (2010) Landscape enhancement of floral resources for honey bees in agro-ecosystems. Apidologie 41:264–277. https://doi.org/10.1051/apido/2010024
Dermauw W, Wybouw N, Rombauts S et al (2013) A link between host plant adaptation and pesticide resistance in the polyphagous spider mite Tetranychus urticae . Proc Natl Acad Sci USA 110:E113–E122. https://doi.org/10.1073/pnas.1213214110
Dixon P (2003) VEGAN, a package of R functions for community ecology. J Veg Sci 14:927–930. https://doi.org/10.1658/1100-9233(2003)014[0927:vaporf]2.0.co;2
Doyle L (2009) Quantification of information in a one-way plant-to-animal communication system. Entropy 11:431–442. https://doi.org/10.3390/e110300431
Gardiner SR (2015) Physicochemical and Flavor Characterization of Tupelo Honey. Master thesis, University of Illinois at Urbana-Champaign
Gheldof N, Wang X-H, Engeseth NJ (2002) Identification and quantification of antioxidant components of honeys from various floral sources. J Agric Food Chem 50:5870–5877. https://doi.org/10.1021/jf0256135
Gherman BI, Denner A, Bobiş O et al (2014) Pathogen-associated self-medication behavior in the honeybee Apis mellifera . Behav Ecol Sociobiol 68:1777–1784. https://doi.org/10.1007/s00265-014-1786-8
Ghidiu GM, Carter C, Silcox CA (1990) The effect of host plant on colorado potato beetle (Coleoptera: Chrysomelidae) susceptibility to pyrethroid insecticides. Pestic Sci 28:259–270. https://doi.org/10.1002/ps.2780280305
Glassmire AE, Zehr LN, Wetzel WC (2020) Disentangling dimensions of phytochemical diversity: alpha and beta have contrasting effects on an insect herbivore. Ecology 101:e03158. https://doi.org/10.1002/ecy.3158
Guo J, Cheng Y, Zhao X et al (2023) Host-Plant switching impacts susceptibility and biochemical responses of Cnaphalocrocis medinalis to abamectin and chlorpyrifos. Agronomy 13:1245. https://doi.org/10.3390/agronomy13051245
Haas J, Glaubitz J, Koenig U, Nauen R (2022a) A mechanism-based approach unveils metabolic routes potentially mediating chlorantraniliprole synergism in honey bees, Apis mellifera L., by azole fungicides. Pest Manag Sci 78:965–973. https://doi.org/10.1002/ps.6706
Haas J, Hayward A, Buer B et al (2022b) Phylogenomic and functional characterization of an evolutionary conserved cytochrome P450-based insecticide detoxification mechanism in bees. Proc Natl Acad Sci USA 119:e2205850119. https://doi.org/10.1073/pnas.2205850119
Article CAS PubMed PubMed Central Google Scholar
Hegazi AG, Hady FKAE, Allah FAMA (2000) Chemical composition and antimicrobial activity of European propolis. Zeitschrift Für Naturforschung C 55:70–75. https://doi.org/10.1515/znc-2000-1-214
Hilker M (2014) New synthesis: Parallels between biodiversity and chemodiversity. J Chem Ecol 40:225–226. https://doi.org/10.1007/s10886-014-0402-8
Johnson RM, Mao W, Pollock HS, Niu G, Schuler MA, Berenbaum MR (2012) Ecologically appropriate xenobiotics induce cytochrome P450s in Apis mellifera . PLoS ONE 7:e31051. https://doi.org/10.1371/journal.pone.0031051
Karuppaiah V, Srivastava C, Subramanian S (2016) Effect of host plants on insecticide susceptibility and detoxification enzymes activity in Spodoptera litura Fabricius (Noctuidae: Lepidoptera). Proc Natl Acad Sci India Sect B Biol Sci 86:715–721. https://doi.org/10.1007/s40011-015-0515-z
Liang P, Cui JZ, Yang XQ, Gao XW (2007) Effects of host plants on insecticide susceptibility and carboxylesterase activity in Bemisia tabaci biotype B and greenhouse whitefly, Trialeurodes vaporariorum . Pest Manag Sci 63:365–371. https://doi.org/10.1002/ps.1346
Liao L-H, Wu W-Y, Berenbaum MR (2017) Impacts of dietary phytochemicals in the presence and absence of pesticides on longevity of honey bees ( Apis mellifera ). Insects 8:22. https://doi.org/10.3390/insects8010022
Article PubMed PubMed Central Google Scholar
Liao L-H, Wu W-Y, Berenbaum M (2024) Data: Variation in pesticide toxicity in the western honey bee ( Apis mellifera ) associated with consuming phytochemically different monofloral honeys. Illinois Data Bank, Urbana, IL. https://doi.org/10.13012/B2IDB-6733018_V1
Book Google Scholar
Liao L-H, Wu W-Y, Dad A, Berenbaum MR (2019) Fungicide suppression of flight performance in the honeybee ( Apis mellifera ) and its amelioration by quercetin. Proc R Soc B 286:20192041. https://doi.org/10.1098/rspb.2019.2041
Liao L-H, Pearlstein DJ, Wu W-Y, Kelley AG, Montag WM, Hsieh EM, Berenbaum MR (2020) Increase in longevity and amelioration of pesticide toxicity by natural levels of dietary phytochemicals in the honey bee. Apis Mellifera Plos ONE 15:e0243364. https://doi.org/10.1371/journal.pone.0243364
Mahdavi A, Solomon KR, Hubert JJ (1991) Effect of solanaceous hosts on toxicity and synergism of permethrin and fenvalerate in Colorado potato beetle (Coleoptera: Chrysomelidae) larvae. Environ Entomol 20:427–432. https://doi.org/10.1093/ee/20.2.427
Manjon C, Troczka BJ, Zaworra M et al (2018) Unravelling the molecular determinants of bee sensitivity to neonicotinoid insecticides. Curr Biol 28:1137–1143. https://doi.org/10.1016/j.cub.2018.02.045
Mao W, Schuler MA, Berenbaum MR (2011) CYP9Q-mediated detoxification of acaricides in the honey bee ( Apis mellifera ). Proc Natl Acad Sci USA 108:12657–12662. https://doi.org/10.1073/pnas.1109535108
Mao W, Schuler MA, Berenbaum MR (2013) Honey constituents up-regulate detoxification and immunity genes in the western honey bee Apis mellifera . Proc Natl Acad Sci USA 110:8842–8846. https://doi.org/10.1073/pnas.1303884110
Mao W, Schuler MA, Berenbaum MR (2015) A dietary phytochemical alters caste-associated gene expression in honey bees. Sci Adv 1:e1500795. https://doi.org/10.1126/sciadv.1500795
Mao W, Rupasinghe SG, Johnson RM, Zangerl AR, Schuler MA, Berenbaum MR (2009) Quercetin-metabolizing CYP6AS enzymes of the pollinator Apis mellifera (Hymenoptera: Apidae). Comp Biochem Physiol b: Biochem Mol Biol 154:427–434. https://doi.org/10.1016/j.cbpb.2009.08.008
Marion ZH, Fordyce JA, Fitzpatrick BM (2015) Extending the concept of diversity partitioning to characterize phenotypic complexity. Am Nat 186:348–361. https://doi.org/10.1086/682369
Michalkiewicz A, Biesaga M, Pyrzynska K (2008) Solid-phase extraction procedure for determination of phenolic acids and some flavonols in honey. J Chromatogr A 1187:18–24. https://doi.org/10.1016/j.chroma.2008.02.001
Mitton GA, Szawarski N, Mitton FM, Iglesias A, Eguaras MJ, Ruffinengo SR, Maggi MD (2020) Impacts of dietary supplementation with p-coumaric acid and indole-3-acetic acid on survival and biochemical response of honey bees treated with tau-fluvalinate. Ecotoxicol Environ Saf 189:109917. https://doi.org/10.1016/j.ecoenv.2019.109917
Morris EK, Caruso T, Buscot F et al (2014) Choosing and using diversity indices: insights for ecological applications from the German Biodiversity Exploratories. Ecol Evol 4:3514–3524. https://doi.org/10.1002/ece3.1155
Nešović M, Gašić U, Tosti T et al (2020) Polyphenol profile of buckwheat honey, nectar and pollen. R Soc Open Sci 7:201576. https://doi.org/10.1098/rsos.201576
Njiru C, Vandenhole M, Jonckheere W, Wybouw N, Leeuwen TV (2023) The host plant strongly modulates acaricide resistance levels to mitochondrial complex II inhibitors in a multi-resistant field population of Tetranychus urticae . Pestic Biochem Physiol 196:105591. https://doi.org/10.1016/j.pestbp.2023.105591
Norušis MJ (2007) IBM SPSS Statistics 15.0 Advanced Statistical Procedures Companion. Prentice Hall Press, Hoboken, NJ, USA
Oksanen J, Simpson G, Blanchet F et al. (2022) vegan: Community Ecology Package. R package version 2.6–4.
Pasini F, Gardini S, Marcazzan G, Caboni M (2013) Buckwheat honeys: Screening of composition and properties. Food Chem 141:2802–2811. https://doi.org/10.1016/j.foodchem.2013.05.102
Petrén H, Köllner TG, Junker RR (2023) Quantifying chemodiversity considering biochemical and structural properties of compounds with the R package chemodiv. New Phytol 237:2478–2492. https://doi.org/10.1111/nph.18685
Prouty C, Barriga P, Davis AK, Krischik V, Altizer S (2021) Host plant species mediates impact of neonicotinoid exposure to monarch butterflies. Insects 12:999. https://doi.org/10.3390/insects12110999
R Core Team (2023) R: A language and environment for statistical computing. Austria, Vienna
Google Scholar
Richards LA, Dyer LA, Forister ML, Smilanich AM, Dodson CD, Leonard MD, Jeffrey CS (2015) Phytochemical diversity drives plant–insect community diversity. Proc Natl Acad Sci USA 112:10973–10978. https://doi.org/10.1073/pnas.1504977112
Saeed Q, Ahmad F, Iqbal N, Zaka SM (2019) Chemical control of polyphagous pests on their auxiliary hosts can minimize insecticide resistance: A case study of Spodoptera exigua Hübner (Lepidoptera: Noctuidae) in cotton agroecosystem. Ecotoxicol Environ Saf 171:721–727. https://doi.org/10.1016/j.ecoenv.2019.01.038
Šmilauer P, Lepš J (2014) Visualising multivariate data. In: Šmilauer P, Lepš J (eds) Multivariate Analysis of Ecological Data Using CANOCO 5. 2 edn. Cambridge University Press, Cambridge, UK, pp 184–207. https://doi.org/10.1017/cbo9781139627061.012
Soler C, Gil MI, García-Viguera C, Tomás-Barberán FA (1995) Flavonoid patterns of French honeys with different floral origin. Apidologie 26:53–60. https://doi.org/10.1051/apido:19950107
Spivak M, Goblirsch M, Simone-Finstrom M (2019) Social-medication in bees: the line between individual and social regulation. Curr Opin Insect Sci 33:49–55. https://doi.org/10.1016/j.cois.2019.02.009
Tewes LJ, Michling F, Koch MA, Müller C (2018) Intracontinental plant invader shows matching genetic and chemical profiles and might benefit from high defence variation within populations. J Ecol 106:714–726. https://doi.org/10.1111/1365-2745.12869
Tihelka E (2018) The immunological dependence of plant-feeding animals on their host’s medical properties may explain part of honey bee colony losses. Arthropod-Plant Interact 12:57–64. https://doi.org/10.1007/s11829-017-9553-1
Truchado P, Ferreres F, Bortolotti L, Sabatini AG, Tomás-Barberán FA (2008) Nectar flavonol rhamnosides are floral markers of acacia ( Robinia pseudacacia ) honey. J Agric Food Chem 56:8815–8824. https://doi.org/10.1021/jf801625t
Ward LT, Hladik ML, Guzman A, Winsemius S, Bautista A, Kremen C, Mills NJ (2022) Pesticide exposure of wild bees and honey bees foraging from field border flowers in intensively managed agriculture areas. Sci Total Environ 831:154697. https://doi.org/10.1016/j.scitotenv.2022.154697
Wetzel WC, Whitehead SR (2020) The many dimensions of phytochemical diversity: linking theory to practice. Ecol Lett 23:16–32. https://doi.org/10.1111/ele.13422
White JW, Doner LW (1980) Honey composition and properties. In: Beekeeping in the United States, vol 335. United States Department of Agriculture, Washington, DC, USA, pp 82–91
Wong MJ, Liao L-H, Berenbaum MR (2018) Biphasic concentration-dependent interaction between imidacloprid and dietary phytochemicals in honey bees ( Apis mellifera ). PLoS ONE 13:e0206625. https://doi.org/10.1371/journal.pone.0206625
Xia Y, Sun J (2023) Alpha diversity. In: Bioinformatic and Statistical Analysis of Microbiome Data, From Raw Sequences to Advanced Modeling with QIIME 2 and R. Springer, Cham, Switzerland, pp 289–333. https://doi.org/10.1007/978-3-031-21391-5_19
Xiao J, He Q, Liu Q et al (2022) Analysis of honey bee exposure to multiple pesticide residues in the hive environment. Sci Total Environ 805:150292. https://doi.org/10.1016/j.scitotenv.2021.150292
Xie W, Wang S, Wu Q et al (2011) Induction effects of host plants on insecticide susceptibility and detoxification enzymes of Bemisia tabac i (Hemiptera: Aleyrodidae). Pest Manag Sci 67:87–93. https://doi.org/10.1002/ps.2037
Xue M, Pang YH, Li QL, Liu TX (2010) Effects of four host plants on susceptibility of Spodoptera litura (Lepidoptera: Noctuidae) larvae to five insecticides and activities of detoxification esterases. Pest Manag Sci 66:1273–1279. https://doi.org/10.1002/ps.2005
Download references
Acknowledgements
We thank Daniel J. Pearlstein, and Allison G. Kelley for their assistance with conducting honey bee bioassays, Terry Harrison, Alison Sankey, and other staff at the University of Illinois Bee Research Facility for assistance, Bernarda Calla for helpful discussions, and Gene Robinson for access to the UIUC apiaries and advice.
This work was supported by USDA-AFRI AG2017-67013–265337 to MRB and USDA-AFRI 2021–67013-33557 to MRB. It was also supported through the Research Training Program in Toxicology and Environmental Health (5T32ES007326-09) at UIUC supported to W.W.
Author information
Authors and affiliations.
Department of Entomology, University of Illinois Urbana-Champaign, Urbana, IL, USA
Ling-Hsiu Liao, Wen-Yen Wu & May R. Berenbaum
You can also search for this author in PubMed Google Scholar
Contributions
LL and MB conceived the idea; LL and WW performed phytochemical assays, conducted bee bioassays, and analyzed the data; all authors contributed to writing the manuscript.
Corresponding author
Correspondence to Ling-Hsiu Liao .
Ethics declarations
Competing interests.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported here.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 30.4 KB)
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Liao, LH., Wu, WY. & Berenbaum, M.R. Variation in Pesticide Toxicity in the Western Honey Bee ( Apis mellifera ) Associated with Consuming Phytochemically Different Monofloral Honeys. J Chem Ecol (2024). https://doi.org/10.1007/s10886-024-01495-w
Download citation
Received : 08 March 2024
Revised : 05 April 2024
Accepted : 12 April 2024
Published : 18 May 2024
DOI : https://doi.org/10.1007/s10886-024-01495-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Phenolic acid
- Find a journal
- Publish with us
- Track your research
share this!
May 15, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Bees and butterflies on the decline in western and southern North America
by Public Library of Science

Bee and butterfly populations are in decline in major regions of North America due to ongoing environmental change, and significant gaps in pollinator research limit our ability to protect these species, according to a study published May 15, 2024, in the open-access journal PLOS ONE by Sara Souther of Northern Arizona University, US, and colleagues.
Recent research has detected declines in populations of pollinator species, sparking alarm from scientists and policymakers concerned about negative impacts on ecosystems and agriculture. These declines have been linked to various factors including climate change , habitat loss , and invasive species , but reports are often limited to well-studied species in easily accessible regions.
In this study, Souther and colleagues used data compiled on four major families of bees and butterflies to construct species distribution models , enabling them to assess changes over time and space across North America.
The highest species richness was found along North America's West Coast, especially California and the Rocky Mountains. However, the models revealed declining species richness in all four families over the past century in western North America.
In contrast, there were disproportionate increases in eastern North America. The authors also assessed similar data for a broader sample of potential pollinator species, including both invertebrate and vertebrate species of conservation concern, and found similar trends.
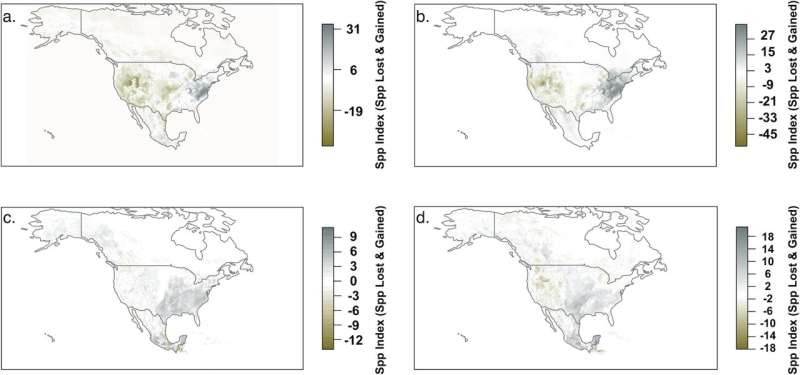
Comparisons with climate data indicate that these population changes are at least partly related to the impacts of recent climate change—such as prolonged drought and habitat degradation —and regions experiencing apparent population declines have also been heavily impacted by anthropogenic land use.
The authors note too that the apparent increases in the eastern U.S. may partly reflect increased detections in these populous areas, given an increase in citizen science and similar data collection efforts.
Overall, this study detects broad trends of population changes in bees and butterflies, as well as other potential pollinators. These results help to identify regions of declining populations where researchers and policymakers can prioritize conservation efforts.
This study also identifies gaps in existing knowledge of pollinators, including regions that are more poorly sampled and species that are less well studied, limitations that might be overcome by improved monitoring methods and enhanced citizen science efforts.
The authors add, "Existing records of North American pollinators suggest that diversity has broadly declined in the western US and southern Mexico in recent decades. Losses are consistent with changes in climate and suggest a need for increased monitoring to inform conservation and mitigation actions."
Journal information: PLoS ONE
Provided by Public Library of Science
Explore further
Feedback to editors
Researchers develop organic photoredox catalysts with enhanced stability and recyclability
9 hours ago

Theory and experiment combine to shine a new light on proton spin

On repeat: Biologists observe recurring evolutionary changes, over time, in stick insects
New findings on fertility: Sperm can adapt to sexually transmitted microbes
10 hours ago
Observing mammalian cells with superfast soft X-rays

New study challenges conventional wisdom that Americans are 'pocketbook voters'
Carbon dioxide, the main culprit of global warming, reborn as an antioxidant substance
11 hours ago

New study offers a cleaner path for controlling water, transforming greenhouse gases
12 hours ago

Heavy water: How melting ice sheets and pumped groundwater can lower local sea levels—and boost them elsewhere

Unveiling a novel AAK1 inhibitor: How chemical proteomics unlock therapeutic potential
13 hours ago
Relevant PhysicsForums posts
And now, here comes covid-19 version ba.2, ba.4, ba.5,....
8 hours ago
DNA-maternity test - could you see other relationship than mother?
May 19, 2024
Is it usual for vaccine injection site to hurt again during infection?
May 16, 2024
A Brief Biography of Dr Virgina Apgar, creator of the baby APGAR test
May 12, 2024
Who chooses official designations for individual dolphins, such as FB15, F153, F286?
May 9, 2024
The Cass Report (UK)
May 1, 2024
More from Biology and Medical
Related Stories

Potential regional declines in species richness of tomato pollinators under climate
Feb 22, 2021

Climate change negatively impacting bumblebees, study finds
Jun 24, 2022
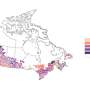
Q&A: Where the wild bees are—and aren't—impacts food supply
Apr 3, 2024

Warning sting on Asia's bee pollinators
Aug 7, 2023

Human activities appear to drive insect declines in Europe
Aug 23, 2023

Decades-long butterfly study shows common species on the decline
Jul 9, 2019
Recommended for you
Artificial intelligence resolves conflicts impeding animal behavior research

Desert locusts' jaws sharpen themselves, materials scientist discovers
Heatmaps show trematodes congregate in certain parts of amphibians' bodies, often to dire physical consequences

Crows can deliberately plan how many calls to make, study shows
May 23, 2024
Birdsong and human voice built from same genetic blueprint
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 14 May 2024
Quantitative microbiome profiling of honey bee ( Apis mellifera ) guts is predictive of winter colony loss in northern Virginia (USA)
- David B. Carlini 1 ,
- Sundre K. Winslow 1 ,
- Katja Cloppenborg-Schmidt 2 &
- John F. Baines 2 , 3
Scientific Reports volume 14 , Article number: 11021 ( 2024 ) Cite this article
272 Accesses
9 Altmetric
Metrics details
- Microbiology
For the past 15 years, the proportion of honey bee hives that fail to survive winter has averaged ~ 30% in the United States. Winter hive loss has significant negative impacts on agriculture, the economy, and ecosystems. Compared to other factors, the role of honey bee gut microbial communities in driving winter hive loss has received little attention. We investigate the relationship between winter survival and honey bee gut microbiome composition of 168 honey bees from 23 hives, nine of which failed to survive through winter 2022. We found that there was a substantial difference in the abundance and community composition of honey bee gut microbiomes based on hive condition, i.e ., winter survival or failure. The overall microbial abundance, as assessed using Quantitative Microbiome Profiling (QMP), was significantly greater in hives that survived winter 2022 than in those that failed, and the average overall abundance of each of ten bacterial genera was also greater in surviving hives. There were no significant differences in alpha diversity based on hive condition, but there was a highly significant difference in beta diversity. The bacterial genera Commensalibacter and Snodgrassella were positively associated with winter hive survival. Logistic regression and random forest machine learning models on pooled ASV counts for the genus data were highly predictive of winter outcome, although model performance decreased when samples from the location with no hive failures were excluded from analysis. As a whole, our results show that the abundance and community composition of honey bee gut microbiota is associated with winter hive loss, and can potentially be used as a diagnostic tool in evaluating hive health prior to the onset of winter. Future work on the functional characterization of the honey bee gut microbiome’s role in winter survival is warranted.
Similar content being viewed by others

A short exposure to a semi-natural habitat alleviates the honey bee hive microbial imbalance caused by agricultural stress

Gut microbiota structure differs between honeybees in winter and summer
Nutritional status of honey bee (Apis mellifera L.) workers across an agricultural land-use gradient
Introduction.
The honey bee is one of the most important species in domestic agriculture: one-third of the food eaten in the United States is derived from honey bee pollinated crops 1 . In addition to agricultural crops, a large number of ecologically important plant species are pollinated by honey bees 2 . Since 2006–2007, the proportion of honey bee hives that do not survive through the winter months has averaged roughly 30% in the United States 3 . Winter colony loss can be attributed to many factors, but when the worker bees suddenly depart from the hive and leave behind a queen, some nurse bees, immature bees, brood, and ample food, the phenomenon is termed Colony Collapse Disorder (CCD). CCD first appeared in the United States and Europe during the winter of 2006–2007 and was responsible for roughly 50% of hive loss that year. Fortunately, rates of CCD have decreased somewhat over the past five years, accounting for 15–25% of all winter hive loss 4 .
Regardless of the reason, hive loss is a serious threat to agriculture and ecosystem function, and any research that increases our understanding of contributing factors will help in developing approaches to reduce its frequency of occurrence. Winter hive loss has been attributed to abiotic factors, biotic factors, nutrition, beekeeping practices, or some combination thereof. Abiotic factors include pesticide use 5 as well as temperature and precipitation 6 . Biotic factors include viral 7 , bacterial 8 , or fungal pathogens 9 , mite infestation 10 , or hive invasions by small hive beetles 11 , wax moths 12 , or wasps 13 . Relative to mite loads and pathogens, one biotic factor that has received comparatively little attention is the composition of honey bee gut microbiomes.
The first large scale study to exam microbiome composition in relation to hive loss did not find any clear-cut differences in microbial abundance related to hive loss 14 . However, this early study revealed that, across geographically diverse populations, the honey bee gut microbiome composition is simple, consisting of a core group of less than a dozen taxa. Subsequent studies characterized the microbiota of the honey bee gut community in more detail and established honey bees as a model for gut microbiome research 15 , 16 , 17 , 18 . The eusocial behavior of honey bees is an important determinant of their gut microbiome since the microbiota are transmitted vertically between the generations rather than by seeding from the environment 15 . While a core group of microbial taxa are common to honey bees from diverse environments, the proportional composition of each taxon can vary from one colony to the next 19 , and differences in microbial composition might impact colony health through a variety of mechanisms (endocrine signaling and behavior, metabolism, and immune function). Antibiotic-induced alteration of honey bee gut microbiota negatively impacted survival and increased susceptibility to opportunistic pathogens and Nosema ceranae 20 , 21 . Thus, given that experimentally induced changes in the composition of the honey bee gut microbiome can negatively impact hive health, additional research is required to determine the extent to which natural variation in gut microbiomes contributes to colony loss. Toward that end, here we investigate the relationship between winter hive loss, the most common time for hives to fail 22 , and honey bee gut microbiome composition.
Hive sampling
Adult forager bees were collected in July 2021 from 23 hives in three geographically proximate locations in northern Virginia, twelve hives from Gainesville, VA (38.82° N, 77.60° W), nine hives from Upperville, VA (38.97° N, 77.85° W), and two hives from Vienna, VA (38.92° N, 77.25° W) (Table S1 ). Between 5 and 8 foragers (average 7.3 bees per hive) were collected from the front entrance of each hive, immediately placed in conical tubes containing 50 mL of 100% ethanol (one 50 mL conical tube per hive), transported back to laboratory, and stored at 4 °C until further processing. Hive condition was classified as “survived” or “failed” according to the status of the hive on March 31, 2022, with all hive failures having occurred during the period between December 2021 and March 2022. Eight of the twelve hives sampled from the Gainesville location failed, none of the nine hives sampled from the Upperville location failed, and one of the two hives sampled from the Vienna location failed. All hives had been treated for mites in the spring and fall prior to sampling, and all hives had received supplementary feeding of sugar syrup during the late autumn and winter months; none of the 23 hives had ever been treated with antibiotics, and no visible pathogens such as chalkbrood, foulbrood, or symptoms of Nosema infection were detected.
DNA extraction
Prior to dissection of the gastrointestinal tract, each bee was rinsed with 100% ethanol and then placed in its own sterile disposable petri dish for dissection. The entire intestinal tract was removed from each bee using sterile dissection tools and placed in an individual microcentrifuge tube containing 100% ethanol, and the remaining carcass of each bee, to be used for RNA extractions, was placed in a separate microcentrifuge tube containing 100% ethanol. Prior to DNA extraction, the ethanol was removed, and the intestinal tract was washed twice in sterile PBS. The PBS wash was then removed, and 100 μL of sterile PBS added prior to homogenization with a sterile micropestle. Following homogenization, an additional 900 μL of sterile PBS was added and the tubes were gently mixed and centrifuged at 10,000 × g for one minute. Microbial genomic DNA was extracted from the supernatant using the DNeasy® UltraClean® Microbial Kit (Qiagen) following the manufacter’s protocol using a final elution volume of 50 μL EB buffer. DNA was quantified with a NanoVue™ Plus spectrophotometer (GE Healthcare), and stored at − 80 °C.
16S rRNA gene sequencing and sequence processing
The hypervariable V1-V2 region of the 16S rRNA gene from each sample was amplified using 27F and 338R primers barcoded with unique octameric multiplex identifiers using a dual indexing approach on an Illumina MiSeq sequencing platform, as previously described 23 . Eight negative extraction controls and three ZymoBIOMICS Microbial Community DNA Standards (Zymo Research) were also sequenced. DADA2 v 1.22 24 was employed to quality filter sequencing reads, infer amplicon sequence variants (ASVs), and determine taxonomic classification based on the Silva Project v138.1 reference database 25 . The ratio of microbe DNA to honey bee host DNA was determined via quantitative PCR using eubacterial 16S rRNA primers Eub338F and Eub518R 26 and honey bee acetylcholinesterase 2 primers 27 Total bacterial load estimates, obtained from the qPCR data, along with the negative extraction controls, were used as input to identify and remove contaminants using the decontam R software package (v1.10.0) 28 using the “prevalence” method with a threshold of 0.05. Rare ASVs not detected in more than 3 reads in at least 20% of the samples were also excluded from analysis, resulting in 77 retained ASVs. The 16S rRNA gene copy number for each ASV was obtained based on genus assignment using the Ribosomal RNA Database 29 . Sixty-four of the 77 ASVs had a 16S rRNA gene copy number of four. Of the remaining 13 ASVs, ten had a 16S rRNA copy number of two, one had a copy number of three, and two had a copy number of five. ASV counts were weighted by copy number (i.e., counts from high copy number ASVs were reduced, and counts from low copy number ASVs were increased), and the values scaled so that the sum of the scaled counts for each bee sample were the same after copy number correction.
Quantitative microbiome profiling and microbial community analysis
The qPCR-based bacterial load estimates were used to determine the relative abundance of each sample for quantitative microbiome profiling (QMP) 30 . The sampling depth of reads from each bee gut was calculated as the total number sequencing reads divided by the sample relative abundance. Pooled reads from each bee gut were then rarefied to the sampling depth of the bee gut with minimal sampling depth (bee gut 21-116S-2, sampling depth = 25,348), which rarefied all to an even sampling depth, the ratio between sequencing depth and bacterial load 30 . Sampling depth rarefaction was repeated 1000 times, and the average of the 1000 replicates was used for relative total abundance of each ASV for each bee gut.
Microbial community analysis was conducted in R (v4.2.3) 31 using the following software packages. Phyloseq (v.1.42.0) 32 was used to conduct sampling depth rarefaction, and to calculate pairwise distances. DECIPHER (v2.0) 33 was used to align sequences and Phangorn (v2.11.1) 34 employed to calculate distances and construct neighbor-joining phylogenetic trees for use in Phyloseq. The Shannon ( H ), Simpson ( D ), and Pielou’s Evenness ( J ) alpha diversity metrics were calculated with the software package vegan (v2.6.4) 35 , which was also used to conduct Permutational Multivariate Analysis of Variance (PERMANOVA) tests on beta diversity, as well as to perform ordination using non-metric multidimensional scaling (NMDS). The software packages DESeq2 (v.1.38.3) 36 and indicspecies (v1.7.13) 37 were used to conduct differential abundance analyses on ASVs. Two-way ANOVAs were performed in base R. Two-way ANOVAs were performed using both hive condition and hive location as categorical factors, allowing us to determine how both factors in combination relate to microbial abundance and diversity, and also whether there is any interaction between those factors. For all tests involving multiple comparisons, P values were corrected using a Benjamini and Hochberg adjustment for a type I error rate of 0.05 38 . MikropML (v1.6.0) 39 was employed to conduct supervised machine learning for classification of the hive condition of individual honey bee samples using L2 logistic regression and random forest algorithms based on the ASVs annotated to the genus level, as fine resolution ASV level analysis has been found to be too individualized for accurate classification 40 .
RNA extraction and deformed wing virus quantitative PCR
Deformed wing virus (DWV), vectored by the ectoparasitic mite Varroa destructor , has been identified as a major biological causative agent of colony loss, and the most regularly detected virus in western honey bees 41 , 42 . We quantified viral loads of the three main DWV strains via qPCR. Total RNA was extracted from the same bees from which the gut microbiome 16S rRNA gene sequence data was obtained. RNA was extracted using the RNeasy® Plus Universal Mini Kit (Qiagen) and stored at − 80 °C. cDNA was synthesized from extracted RNA using the oligo(dT) primer supplied with the AffinityScript qPCR cDNA Synthesis Kit (Agilent), and qPCR was performed using the Brilliant III Ultra-Fast SYBR Green qPCR Master Mix (Agilent) on the Mx3005P qPCR System (Agilent). To characterize deformed wing virus (DWV) viral loads we used the DWV strain specific A, B, and C primer sequences developed by Kevill et al. 42 along with GAPDH primers as the control gene 43 . All four genes were assayed in triplicate on the same qPCR run for each sample, along with triplicate negative controls (with water as template for cDNA synthesis) for each gene. The relative abundances of DWV-A, DWV-B, and DWV-C were calculated as DC T (C T GAPDH −C T DWV ), the difference between the GAPDH threshold cycle and that of the DWV-A, DWV-B, or DWV-C threshold cycle. Two-way nested ANOVAs, with hive condition and location as factors, were used to assess the statistical significance of DC T values for each DWV strain.
Gut microbial composition and abundance
The microbial gut communities of 168 bees from 23 hives were sequenced, yielding an average of 36,178 reads (range: 9461–73,402) and 62 ASVs (range: 13–333) per sample after error correction, quality filtering, and removal of potential contaminants. After removal of rare ASVs, a total of 77 ASVs were retained, with an average of 28,098 reads (range: 4148–63,441) and 35 ASVs (range: 10–52) per sample. After adjusting for differences due to different 16S rRNA gene copy number among different taxa and rarefying samples to an even sampling depth (the ratio between sequencing depth and bacterial load), overall QMP abundance was significantly greater in samples from hives that survived winter 2022 than in those that failed ( P = 0.0136, nested two-way ANOVA), whereas hive location and hive condition × location interaction effects were not statistically significant. Each of the nine hives in the Upperville location survived Winter 2022, and the average QMP sampling abundance of those hives (307 ± 33) was over twofold greater than the average QMP abundance of the eight hives that failed in the Gainesville location (109 ± 38), which was also less than the average of the four hives that survived in the Gaineville location (184 ± 94) (Fig. 1 A). The average QMP abundance of the single failed hive in the Vienna location (142 ± 31) was less than the average of the surviving hives from both other locations, but greater than that of the single surviving hive from the Vienna location (103 ± 25). However, there was substantial variation in QMP abundance among hives within each sampling location and hive condition category, particularly at the Gainesville location (Fig. 1 B).
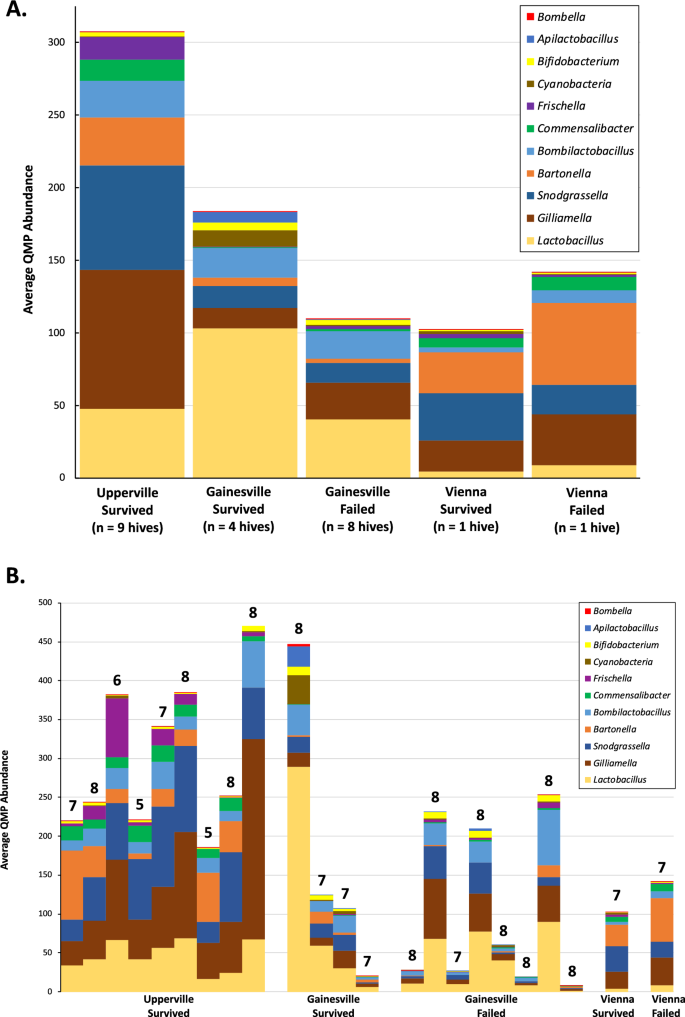
Community composition of honey bee gastrointestinal tracts deduced from quantitative microbiome profiling (QMP). Relative bacterial loads for QMP were obtained via qPCR, with the sampling depth of reads from each bee gut calculated as the total number sequencing reads divided by the sample relative abundance. Pooled reads from each bee gut were then rarefied to the sampling depth of the bee gut with minimal sampling depth, which rarefied all to an even sampling depth, the ratio between sequencing depth and bacterial load. ( A ) Average overall QMP sampling abundance of bacterial genera in hives that survived or failed in each sampling location. ( B ) Average QMP sampling abundance of bacterial genera in each of the 23 sampled hives. The number of bees sampled per hive is given above each stacked bar (5–8 bees per hive, average = 7.3).
The honey bee gut microbiome is dominated by five core genera, Bifidobacterium , Bombilactobacillus , Gilliamella , Lactobacillus , and Snodgrassella , which have high abundance and prevalence, and four to five noncore genera which are typically found at lower frequencies and prevalence than the core genera 19 . Each of the five core genera had high prevalence, being present in at least 98% of the 168 individual honey bee samples. The noncore genera Apilactobacillus , Bartonella , Bombella , Commensalibacter , and Frischella had prevalences in individual bees of 58, 71, 42, 89, and 68%, respectively. For each of these ten bacterial genera, the average quantitative sampling abundance was greater in honey bees from surviving hives than in those from failed hives (Fig. 2 ). In addition to these ten bacterial genera, most hives contained 16rRNA gene hits to “Cyanobacteria”, sequences that are likely derived from the chloroplasts of consumed pollen 15 , 44 . Sequences of Paenibacillus larvae and Streptococcus pluton , the causative agents of American and European foulbrood (diseases of bee larvae), were not detected among the 77 ASVs or among the inclusive set of 1465 ASVs recovered prior to filtering.
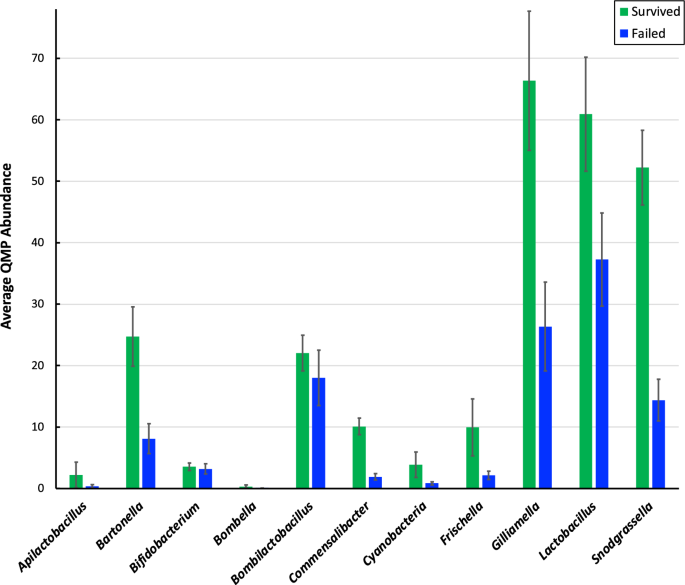
Pairwise comparisons of average QMP sampling abundance of ten most abundance bacterial genera of hives that survived (green) or failed to survive (blue) winter 2022. Error bars indicate standard error of the mean.
Microbial diversity
Within subject species diversity (alpha diversity) was not significantly different between hives that survived and those that failed for Shannon Diversity ( P = 0.24, two-way ANOVA), Simpson Diversity ( P = 0.53) or Pielou’s Evennness ( P = 0.15) (Table S2 ). Alpha diversity based on hive location was not significant for Shannon or Simpson Diversity ( P = 0.065, 0.17, respectively), but was significant for Pielou’s Evennness ( P = 0.015). However, when samples from the Upperville location (where no winter hive loss occurred) were excluded from analysis of alpha diversity, the differences based on hive condition or hive location were not statistically significant for any of the three measures of alpha diversity (Table S2 ).
Next, we visualized the difference in bacterial species composition (beta diversity) by NMDS ordination (Fig. 3 ) based on the weighted UniFrac distance (ordination stress = 0.122). To compare community composition among hives, we conducted permutational multivariate analysis of variance (PERMANOVA) implemented with the adonis method with 10 6 permutations in the R vegan package (v.2.6.4) 35 . For all three distance metrics used (weighted UniFrac, Bray Curtis, and Jaccard), the effects of hive condition, as well as hive location, were highly significant (PERMANOVA test: P < 10 –6 ), indicating that microbial community composition differed based on both hive condition and hive location (Table 1 ). Permutation tests for the assumption of homogeneity of dispersions 45 confirmed that the assumption was met for all three distance metrics (PERMDISP, adjusted P > 0.05). Since each of the hives in the Upperville location survived, it could be argued that most of that signal was due to hive location. We therefore conducted the PERMANOVA analysis after excluding the Upperville samples, but found that hive condition remained significant (P < 10 –4 , Table 2 ; Supplementary Fig. S1 ), indicating that the differences in bacterial community composition can be attributed to hive condition.
NMDS ordination of honey bee gut microbiota from hives that survived (green) or hives that failed to survive (blue) winter 2022. Weighted UniFrac dissimilarity was calculated using the absolute abundance of 77 ASVs. PERMANOVA performed on the weighted UniFrac distances showed significant effect of hive condition on beta-diversity ( P < 10 –6 , stress = 0.122).
Differential abundance
Differential abundance analysis was conducted using DESeq2 and indicspecies on QMP ASV counts from all locations, as well as by performing nested two-way ANOVAs on genus QMP abundance. DESeq2 identified 12 ASVs that were associated with hive condition, seven of which were positively associated and five of which were negatively associated with winter survival (Table 3 ). Indicspecies identified nine ASVs that were positively associated with hive survival (Table 4 ), six of which were also identified by DESeq2: ASV1 ( Snodgrassella alvi ), ASV2 ( Lactobacillus apis ), ASV3 ( Giliamella apicola ), ASV9 ( Commensalibacter melissae ), ASV13 ( Bartonella apis ), and ASV25 ( Giliamella ).
Nested two-way ANOVAs on pooled QMP ASV counts for each genus identified that two genera were positively associated with winter survival while accounting for location, Commensalibacter and Snodgrassella (Table 5 ).
Quantitative PCR assays on DWV levels
There were no significant differences in DWV loads based on hive condition (Table S3 ). For Gainesville and Vienna (the two locations containing both hives that survived and failed winter 2022), hives that failed winter 2022 exhibited higher average DWV-A, DWV-B, and DWV-C loads than hives that survived winter 2022, although these differences were not statistically significant. There were also no significant differences in DWV-A and DWV-B loads based on hive location. DWV-C loads were significantly different among locations ( P = 0.0087, two-way ANOVA), due to lower loads of DWV-C in the Gainesville location (average DC T = − 0.94) compared to the Upperville (average DC T = 0.55) and Vienna (average DC T = 0.42) locations.
Machine learning classification of winter hive loss
To gain a better understanding of the relative importance of each factor contributing to hive loss, we employed supervised machine learning, as implemented in the R software package MikropML 39 , using two machine learning (ML) models, L2 logistic regression and random forest. We performed a grid search for hyperparameter settings when training the L2 logistic regression models. For each model, we ran 100 iterations, with a split of 80% of the data for the training and 20% for testing. The performance of the two methods, as measured by the area under the receiver operator characteristic curve (AUROC), illustrate the predictive power of a binary classifier, in this case whether a particular sample comes from a hive which failed or survived winter 2022 (Fig. 4 ). The overall performance of the two methods was similar, where the median AUROC was 0.908 and 0.903 for the L2 logistic regression and random forest models, respectively. For comparison, we also employed a L2 logistic regression model excluding the samples from the Upperville location, where no hive failures occurred, which resulted in a substantial drop in performance (median AUROC = 0.673).
Model performance of machine learning methods, as measured by the area under the receiver operator characteristic curve (AUROC). Strip plots of AUROC values on the test data set for each of the 100 seeds using a L2 logistic regression model (blue), random forest model (purple), or L2 logistic regression model with the Upperville samples excluded (orange), with the median AUROC values for each depicted as filled circles. An AUROC value of 0.5 corresponds to random classification, whereas an AUROC value of 0.9, the approximate median of the 100 seeds for both the L2 logistic regression and random forest ML models with all locations excluded, corresponds to a classification system that correctly identifies a sample as coming from a surviving or failed hive with 90% probability.
For L2 logistic regression, an intuitive way to evaluate the relative contributions of each feature ( i.e ., genus or location) to model performance is to compare the regression coefficients ( i.e ., weights) for each feature, where the magnitude is proportional to feature importance in the ML model. As expected, given that each of the hives from the Upperville location survived and that two-thirds of the hives from the Gainesville location failed, the average regression coefficients of these two location features were of the greatest magnitude, 0.97 and − 0.93, respectively (Fig. 5 ). Of the 10 taxonomic features, four were positively associated (average regression coefficient > 0.1) with hive survival ( Bartonella , Commensalibacter , Gilliamella , Snodgrassella ), two were negatively associated (average regression coefficient < − 0.1) with hive survival ( Apilactobacillus , Bfidobacterium ), and four were weakly associated (− 0.1 < average regression coefficient < 0.1) with hive survival ( Bombella , Bombilactobacillus , Frischella , Lactobacillus ). Another means of determining the relative importance of each feature is through permutation, where model performance is compared between the inclusive data and when each feature is omitted. As expected, exclusion of the Upperville and Gainesville location features had the greatest impact on model performance (~ 8.5–10% drop in median AUROC), followed by the taxonomic features Bfidobacterium , Commensalibacter , Snodgrassella , and Gilliamella (~ 2–5% drop in median AUROC), with the remaining taxonomic features having a lesser to negligible effect on model performance (< 2% drop in median AUROC).
Boxplot of L2 regression model feature coefficients ( i.e ., weights) for each feature. Features associated with winter survival have positive regression coefficients ( e.g ., genera Commensalibacter and Snodgressella , location Upperville), and those associated with winter failure have negative regression coefficients (e.g. genus Bfidobacterium , locations Gainesville and Vienna), with the magnitude proportional to feature importance in the ML model. The medians and interquartile ranges of the 100 seeds are depicted as horizontal black lines and boxes, respectively.
In this study, we compared the gut microbiota in honey bees from hives in three sampling locations that either survived or failed to survive through winter 2022. The bacterial taxa found in this study included the six Gram-negative genera Bartonella , Bombella (formerly Parasaccharibacter ), Commensalibacter , Frischella , Gilliamella , and Snodgrassella , as well as the four Gram-positive genera Apilactobacillus (formerly Lactobacillus ), Bifidobacterium , Bombilactobacillus (formerly Lactobacillus ), and Lactobacillus . These taxa are consistent with previous work demonstrating that the honey bee gut microbiome is simple and consists of less than a dozen species clusters 16 , 17 , 19 , 46 .
The most salient difference between the bacteriomes of honey bees from hives that survived versus those that failed is in total bacterial abundance, where bacterial abundance of hives that survived consistently exceeded that of hives that failed. Importantly, for each of the ten bacterial genera the average QMP abundance was greater in honey bees from surviving hives (Fig. 2 ). Given that all 10 genera are well known commensals of honey bees, perhaps an overall dearth of gut commensals, which are critical for metabolism, endocrine signaling/growth, immune function, and pathogen resistance 47 , led to various forms of stress (e.g., lack of nutrition, increased susceptibility to pathogens), contributing to winter hive failure. Consistent with the hypothesis that greater abundance of members of the core bee gut microbiome is protective, a survey of the gut microbiota of thriving versus non-thriving honey bees found higher relative abundances of Bartonella , Bifidobacterium , Bombella , Commensalibacter , and Snodgrassella in thriving bees 48 . In another recent study, winter bees, which are crucial for colony survival, were found to have roughly tenfold higher total gut bacterial loads than summer foragers 49 , which suggests a relationship between the health of winter hives and total bacterial abundance under a scenario in which hives with particularly low total bacterial abundance in their summer foragers are more likely to exhibit low bacterial abundance in the winter.
Whereas the difference wasn’t statistically significant for two-way ANOVAs performed on most genera, the pooled ASVs from two genera, Commensalibacter and Snodgrassella , exhibited significantly greater abundances based on hive condition. The average abundance of Commensalibacter , a member of the acetic acid bacteria (family Acetobacteraceae), among honey bees from hives that survived was, on average, over five-fold higher than that from those from hives that failed. In addition, the average regression coefficient for Commensalibacter as a feature in a logistic regression ML model was of greater magnitude (positive or negative) than that of any other genus, indicating that a greater abundance of the Commensalibacter was more predictive of hive success than any other gut commensal. In a comparative study of thriving versus non-thriving honey bees, Commensalibacter was found to be significantly more abundant in the gut of thriving bees compared to non-thriving bees 48 , and Commensalibacter abundance has been found to increase in winter bees relative to summer foragers 49 . In Drosophila , Commensalibacter suppresses the proliferation of a pathogenic commensal Gluconobacter morbifer 50 .
The average abundance of Snodgrassella among honey bees from hives that survived was, on average, almost four-fold higher than that from honey bees from failed hives and, similar to Commensalibacter , Snodgrassella abundance was predictive of hive success in a logistic regression ML model. Snodgressella alvi is a member of the core gut microbiome, it has been found in almost every adult honey bee worker worldwide, and is most abundant in the ileum region of the hindgut 19 . S. alvi has been found to protect against E. coli hemolymph infection 51 , and is known to play a very important role in maintaining anoxia in the gut, a condition required by the metabolism of other gut symbionts 52 . After infection with E. coli , honey bees mono-inoculated with S. alvi cleared more E. coli from the hemolymph after infection, and they had higher levels of antimicrobial peptide, so it has been proposed that S. alvi may have a role in immune priming 49 . Consistent with this finding, recent studies determined that S. alvi , which forms a dense biofilm in the ileum, triggered an immune response against the opportunistic and harmful microbial pathogen Serratia marescens , protecting them against infection 53 , 54 . Serratia marescens infection is unlikely to be a factor influencing hive survival in our study given that Serratia was only detectable in three samples prior to filtering out rare ASVs. Serratia hits accounted for < 1% of the total reads from each of the three individual honey bee samples in which it was detected, and two of the three samples were from two different hives that survived winter 2022. S. alvi has also been shown to protect against Paenibacillus larvae 55 , the microbial pathogen responsible for American foulbrood, the most widespread disease affecting honey bee larvae. We did not detect any reads from American foulbrood prior to filtering out rare ASVs, therefore, similar to Serratia , any protection S. alvi conferred against P. larvae was probably not a factor influencing hive survival in our study. However, S. alvi may protect against a variety of pathogens, not all of which would be detectable using bacterial 16SrRNA gene sequencing. A seasonal shift in the abundance of S. alvi occurs in the midgut, where it becomes more abundant in summer, possibly due to changes in diet 56 . It is possible that lower levels of S. alvi are indicative of poor nutrition, resulting in higher disease susceptibility and mortality.
Contrary to results from a previous study 48 that found higher alpha diversity in the gut microbiomes of thriving hives (rapid hive population growth, high honey production) compared to non-thriving hives (slow hive population growth, low honey production), we found no difference in the alpha diversity of hives that survived or failed winter 2022. Whether increased microbial diversity is a signature of hive health depends on which species most contribute to increased diversity, e.g ., the presence of rarer pathogenic bacteria could increase species richness but may have a detrimental effect on hive health. There was a marginally significant difference in species evenness based on hive location, with the Gainesville location having higher diversity (average Pielou’s evenness = 0.87) than the Upperville and Vienna locations (average Pielou’s evenness = 0.72 and 0.78, respectively). Given that the Gainesville location had the highest rates of hive failure, this is a case where higher gut microbial diversity is not necessarily a sign of hive health (average Shannon and Simpson diversity were highest for the Gainesville location as well, but the differences among locations were not statistically significant).
In contrast to some previous studies on colony loss 41 , 44 , 57 , we did not find any evidence that any of the three DWV strains was a significant driver of colony failure. The lack of any significant differences in DWV levels based on hive condition may be attributed to the fact that each of the hives we sampled had been treated during the spring and fall for parasitic Varroa destructor mites, an important vector of DWV and other viruses. It is possible that, in the ensuing months between the fall mite treatment and winter, mite loads and the concomitant DWV levels accrued in some hives, perhaps contributing to some hive failures.
In addition to differences in specific taxa, the difference in overall composition of hives that survived versus those that failed was highly significant ( P < 10 –6 ) as determined from a PERMANOVA test of beta diversity differences based on hive condition. Although the effect of hive location was also highly significant ( P < 10 –6 ), the PERMANOVA test remained highly significant ( P < 10 –4 ) even after excluding samples from the Upperville location, where all hives survived winter 2022. These results indicate that there are consistent differences in the abundances of individual taxa in surviving versus failed hives, although the specific nature of these differences is difficult to discern due to the multidimensionality of the beta diversity data.
To aid in the interpretation of PERMANOVA results we used ML models to determine if the microbial community composition of honey bee guts were predictive of winter hive survival or failure, as well as to determine which taxonomic features were most strongly associated with survival or failure. Both the logistic regression and random forest ML models performed very well (median AUROC > 0.9) when all features were included, however, since all samples from the Upperville location were obtained from hives that survived the winter and two-thirds of the hives from the Gainesville location failed, the location features contributed significantly to ML performance. Despite the predictive importance of the Upperville location feature, when the Upperville samples were excluded the logistic regression ML model remained predictive of winter survival (median AUROC = 0.673), indicating that the taxonomic composition of the honey bee gut microbiome alone was an important determinant of winter failure and could be used to correctly predict whether a particular honey bee sample came from a failed hive ~ 67% of the time. In accordance with the results from statistical analysis of individual genus abundance data discussed above, the Commensalibacter and Snodgrassella genera were the taxonomic features that were most predictive of hive success, having the largest regression coefficients in the logistic regression model, and having the greatest impact on model performance when excluded as features in the logistic regression and random forest ML models.
Given that (1) most colony loss occurs during the winter months, that honey bee gut microbial communities undergo seasonal fluctuations 47 , (2) there are clear differences in the gut microbial communities of thriving versus non-thriving bees during the summer 46 , (3) there is a significant difference between the gut microbial communities of honey bees from hives that were destined for winter failure and those that survived (this study), and (4) that this difference has predictive power (this study), further analysis and a functional characterization of the honey bee gut microbiome’s role in winter survival is clearly warranted.
This study suggests that honey bee gut microbial abundance and community composition may play a significant role in winter hive loss. Honey bees from hives that survived winter 2022 had significantly higher microbial loads, and there was a highly significant difference in the beta diversity based on hive condition. Two bacterial genera previously demonstrated to be beneficial, Commensalibacter and Snodgrassella , were also found to be positively associated with winter survival in our study. Machine learning models were predictive of hive outcome, indicating that in the future the community composition honey bee gut microbiota can potentially be used as a diagnostic tool in evaluating hive health prior to the onset of winter.
Data availability
The 16S rRNA gene reads supporting the conclusions of this article are available in the NIH Sequence Read Archive repository ( https://www.ncbi.nlm.nih.gov/sra ) under the Bioproject ID PRJNA1010874. R scripts used for microbiome profiling are available upon request.
Calderone, N. W. Insect pollinated crops, insect pollinators and US agriculture: Trend analysis of aggregate data for the period 1992-2009. PLoS ONE 7 , 5 (2012).
Article Google Scholar
Hung, K.-L.J., Kingston, J. M., Albrecht, M., Holway, D. A. & Kohn, J. R. The worldwide importance of honey bees as pollinators in natural habitats. Proc. R. Soc. Ser. B 285 , 20172140 (2018).
Steinhauer, N., Wilson, M., Aurell, D., Bruckner, S. & Williams, G. United States Honey Bee Colony Losses 2022–2023: Preliminary Results from the Bee Informed Partnership . https://beeinformed.org/2023/06/22/united-states-honey-bee-colony-losses-2022-23-preliminary-results-from-the-bee-informed-partnership (2023).
Honey Bee Colonies. National Agricultural Statistics Service, USDA . (2023, accessed 27 Oct 2023). https://downloads.usda.library.cornell.edu/usda-esmis/files/rn301137d/4m90gc28p/gq67m7401/hcny0823.pdf .
Traynor, K. S. et al. Pesticides in honey bee colonies: Establishing a baseline for real world exposure over seven years in the USA. Environ. Pollut. 279 , 116566 (2021).
Article CAS PubMed Google Scholar
Overturf, K. A. et al. Winter weather predicts honey bee colony loss at the national scale. Ecol. Indic. 145 , 109709 (2022).
Beaurepaire, A. et al. Diversity and global distribution of viruses of the western honey bee, Apis mellifera . Insects 11 , 239 (2020).
Article PubMed PubMed Central Google Scholar
Evans, J. D. & Schwarz, R. S. Bees brought to their knees: Microbes affecting honey bee health. Trends Microbiol. 19 , 614–620 (2011).
Steinhauer, N. et al. Drivers of colony losses. Curr. Opin. Insect Sci. 26 , 142–148 (2018).
Article PubMed Google Scholar
Guzmán-Novoa, E. et al. Varroa destructor is the main culprit for the death and reduced populations of overwintered honey bee ( Apis mellifera ) colonies in Ontario, Canada. Apidologie 41 , 443–450 (2010).
Roth, M., Wilson, J. M. & Gross, A. D. Biology and management of small hive beetles (Coleoptera: Nitidulidae): A pest of European honey bee (Hymenoptera: Apidae) colonies. J. Integr. Pest Manag. 13 , 7 (2022).
Kwadha, C. A., Ongamo, G. O., Ndegwa, P. N., Raina, S. K. & Fombong, A. T. The biology and control of the greater wax moth, Galleria mellonella . Insects 8 , 61 (2017).
Requier, F. et al. Predation of the invasive Asian hornet affects foraging activity and survival probability of honey bees in Western Europe. J. Pest Sci. 92 , 567–578 (2019).
Cox-Foster, D. L. et al. A metagenomic survey of microbes in honey bee colony collapse disorder. Science 318 , 283–287 (2007).
Article ADS CAS PubMed Google Scholar
Martinson, V. G. et al. A simple and distinctive microbiota associated with honey bees and bumble bees. Mol. Ecol. 20 , 619–628 (2011).
Moran, N. A., Hansen, A. K., Powell, J. E. & Sabree, Z. L. Distinctive gut microbiota of honey bees assessed using deep sampling from individual worker bees. PLoS ONE 7 , e36393 (2012).
Article ADS CAS PubMed PubMed Central Google Scholar
Sabree, Z. L., Hansen, A. K. & Moran, N. A. Independent studies using deep sequencing resolve the same set of core bacterial species dominating gut communities of honey bees. PLoS ONE 7 , e41250 (2012).
Zheng, H., Steele, M. I., Leonard, S. P., Motta, E. V. S. & Moran, N. A. Honey bees as models for gut microbiota research. Lab. Anim. 47 , 317–325 (2018).
Kwong, W. K. & Moran, N. A. Gut microbial communities of social bees. Nat. Rev. Microbiol. 14 , 374–384 (2016).
Article CAS PubMed PubMed Central Google Scholar
Raymann, K., Shaffer, Z. & Moran, N. A. Antibiotic exposure perturbs the gut microbiota and elevates mortality in honeybees. PLoS Biol. 15 , e2001861 (2017).
Li, J. H. et al. New evidence showing that the destruction of gut bacteria by antibiotic treatment could increase the honey bee’s vulnerability to Nosema infection. PLoS ONE 12 , e0187505 (2017).
Döke, M. A., Frazier, M. & Grozinger, C. M. Overwintering honey bees: Biology and management. Curr. Opin. Insect Sci. 10 , 185–193 (2015).
Belheouane, M., Gupta, Y., Künzel, S., Ibrahim, S. & Baines, J. F. Improved detection of gene-microbe interactions in the mouse skin microbiota using high-resolution QTL mapping of 16S rRNA transcripts. Microbiome 5 , 59 (2017).
Callahan, B. J. et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13 , 581–583 (2016).
Quast, C. et al. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 41 , D590-596 (2013).
Fierer, N., Jackson, J. A., Vilgalys, R. & Jackson, R. B. Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Appl. Environ. Microbiol. 71 , 4117–4120 (2005).
Moon, K., Lee, S. H. & Kim, Y. H. Validation of quantitative real-time PCR reference genes for the determination of seasonal and labor-specific gene expression profiles in the head of Western honey bee, Apis mellifera . PLoS ONE 13 , e0200369 (2018).
Davis, N. M. et al. Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data. Microbiome 6 , 226 (2018).
Stoddard, S. F., Smith, B. J., Hein, R., Roller, B. R. & Schmidt, T. M. rrnDB: Improved tools for interpreting rRNA gene abundance in bacteria and archaea and a new foundation for future development. Nucleic Acids Res. 43 , 593–598 (2015).
Vandeputte, D. et al. Quantitative microbiome profiling links gut community variation to microbial load. Nature 551 , 507–511 (2017).
R Core Team. R: A Language and Environment for Statistical Computing (2023).
McMurdie, P. J. & Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8 , e61217 (2013).
Wright, E. S. Using DECIPHER v2.0 to analyze big biological sequence data in R. R J. 8 , 352–359 (2016).
Schliep, K. P. phangorn: Phylogenetic analysis in R. Bioinformatics 27 , 592–593 (2011).
Oksanen, J. et al . Vegan: Community Ecology Package. https://CRAN.R-project.org/package=vegan (2022).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15 , 550 (2014).
De Cáceres, M. & Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 90 , 3566–3574 (2009).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 57 , 289–300 (1995).
Article MathSciNet Google Scholar
Topçuoğlu, B. D. et al. mikropml: User-friendly R package for supervised machine learning pipelines. J. Open Source Softw. 6 , 3073 (2021).
Article ADS PubMed PubMed Central Google Scholar
Armour, C. R., Topçuoğlu, B. D., Garretto, A. & Schloss, P. D. A Goldilocks principle for the gut microbiome: Taxonomic resolution matters for microbiome-based classification of colorectal cancer. mBio 13 , e0316121 (2022).
Dainat, B. & Neumann, P. Clinical signs of deformed wing virus infection are predictive markers for honey bee colony losses. J. Invertebr. Pathol. 112 , 278–280 (2013).
Kevill, J. L., Highfield, A., Mordecai, G. J., Martin, S. J. & Schroeder, D. C. ABC Assay: Method development and application to quantify the role of three DWV master variants in overwinter colony losses of European honey bees. Viruses 9 , 314 (2017).
Reim, T., Thamm, M., Rolke, D., Blenau, W. & Scheiner, R. Suitability of three common reference genes for quantitative real-time PCR in honey bees. Apidologie 44 , 342–350 (2013).
Article CAS Google Scholar
Cornman, R. S. et al. Pathogen webs in collapsing honey bee colonies. PLoS ONE 7 , e43562 (2012).
Anderson, M. J. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62 , 245–253 (2006).
Article MathSciNet PubMed Google Scholar
Ellegaard, K. M. & Engel, P. Genomic diversity landscape of the honey bee gut microbiota. Nat. Commun. 10 , 446 (2019).
Raymann, K. & Moran, N. A. The role of the gut microbiome in health and disease of adult honey bee workers. Curr. Opin. Insect Sci. 26 , 97–104 (2018).
Ribière, C., Hegarty, C., Stephenson, H., Whelan, P. & O’Toole, P. W. Gut and whole-body microbiota of the honey bee separate thriving and non-thriving hives. Microb Ecol. 78 , 195–205 (2019).
Article ADS PubMed Google Scholar
Kešnerová, L. et al. Gut microbiota structure differs between honeybees in winter and summer. ISME J. 14 , 801–814 (2020).
Ryu, J. H. et al. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila . Science 319 , 777–782 (2008).
Kwong, W. K., Mancenido, A. L. & Moran, N. A. Immune system stimulation by the native gut microbiota of honey bees. R. Soc. Open Sci. 4 , 170003 (2017).
Zheng, H., Powell, J. E., Steele, M. I., Dietrich, C. & Moran, N. A. Honeybee gut microbiota promotes host weight gain via bacterial metabolism and hormonal signaling. Proc. Natl. Acad. Sci. U. S. A. 114 , 4775–4780 (2017).
Burritt, N. L. et al. Sepsis and hemocyte loss in honey bees ( Apis mellifera ) infected with Serratia marcescens strain Sicaria. PLoS ONE 11 , e0167752 (2016).
Horak, R. D., Leonard, S. P. & Moran, N. A. Symbionts shape host innate immunity in honeybees. Proc. Biol. Sci. 287 , 20201184 (2023).
Google Scholar
Erban, T. et al. Honeybee ( Apis mellifera )-associated bacterial community affected by American foulbrood: Detection of Paenibacillus larvae via microbiome analysis. Sci. Rep. 7 , 5084 (2017).
Ludvigsen, J. et al. Shifts in the midgut/pyloric microbiota composition within a honey bee apiary throughout a season. Microbes Environ. 30 , 235–244 (2015).
McMahon, D. P. et al. Elevated virulence of an emerging viral genotype as a driver of honeybee loss. Proc. Biol. Sci. 283 , 20160811 (2016).
PubMed PubMed Central Google Scholar
Download references
Acknowledgements
We thank Karla Eisen for generously providing access to her hives at the Upperville and Gainesville locations and for her kind assistance in sampling honey bees from those hives.
Open Access funding enabled and organized by Projekt DEAL. This work was supported by a Mellon Grant from American University awarded to DBC, and by the German Science Foundation (DFG) CRC 1182 Project Z03 awarded to JFB.
Author information
Authors and affiliations.
Department of Biology, American University, 4400 Massachusetts Ave. NW, Washington, DC, 20016, USA
David B. Carlini & Sundre K. Winslow
Section of Evolutionary Medicine, Institute for Experimental Medicine, Kiel University, Arnold-Heller-Str. 3, 24105, Kiel, Germany
Katja Cloppenborg-Schmidt & John F. Baines
Max Planck Institute for Evolutionary Biology, August-Thienemann-Str. 2, 24306, Plön, Germany
John F. Baines
You can also search for this author in PubMed Google Scholar
Contributions
DBC – funding, study design, hive sampling, wet lab work, data analysis, writing of manuscript. SKW – wet lab work. KCS – wet lab work. JFB – funding, study design, data analysis, critical review of manuscript.
Corresponding authors
Correspondence to David B. Carlini or John F. Baines .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Carlini, D.B., Winslow, S.K., Cloppenborg-Schmidt, K. et al. Quantitative microbiome profiling of honey bee ( Apis mellifera ) guts is predictive of winter colony loss in northern Virginia (USA). Sci Rep 14 , 11021 (2024). https://doi.org/10.1038/s41598-024-61199-9
Download citation
Received : 08 January 2024
Accepted : 02 May 2024
Published : 14 May 2024
DOI : https://doi.org/10.1038/s41598-024-61199-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Microbiology newsletter — what matters in microbiology research, free to your inbox weekly.
Researchers find unique adaptations of fungus associated with bee bread
The past attempts of honey bee researchers to inventory the fungal diversity in honey bee colonies revealed that Aspergillus flavus is frequently found in hives. In a new study, researchers have discovered that this fungus is uniquely adapted to survive in bee colonies.
The western honey bee, Apis mellifera, stores large quantities of food in the form of bee bread, which is used as a main food source for the hive. The abundant nutritional value of this food source also makes it an appealing target for microorganisms. However, bee bread is acidic with little moisture, and is doused with the antimicrobial chemical propolis.
Despite the inhospitable nature of bee bread, the microbiome in hives consists of several bacterial and fungal species that are important to honey bee food preparation, storage, and digestion.
"Most of the research on bee bread has been focused on bacteria and it was assumed that fungi didn't play a big role because the bacteria made it too inhospitable to them," said Daniel Bush, a graduate student in the Berenbaum (IGOH/GEGC/GNDP) lab. "After talking to mycologists, I suspected that wasn't the case and I set out to demonstrate that fungi were capable of living successfully in bee bread."
In the study, the researchers used three strains of A. flavus : one that is not found in bee hives, a strain that was isolated from hives in central Illinois, and a pathogenic strain from a honey bee colony that had a stonebrood infection.
They first tested whether the strains showed any differences in their responses to pH and temperature. The latter was looked at because hives are characterized by higher year-round temperatures compared to the outside environments, which is a challenge for many microbes. Although the strains were all able to grow across different temperature ranges, they had visible growth differences under different pH conditions. The strain that was isolated from the hives was able to withstand low pH, while the other two could not.
The strains were also tested under different matric potential, which measures how much moisture is available, and response to propolis. "We saw that the strain from the hive was capable of dealing with extreme levels of environmental pressure from colony-specific sources," Bush said. "It was interesting that it could deal with propolis, which is believed to have fungicidal properties."
To better understand how the hive-associated fungal species were able to adapt, the researchers also sequenced the A. flavus strain and found that it had several genetic mutations that allowed it to tolerate the harsh conditions of the bee bread environment.
"We believe that these are signs that there is a level of adaptation for the fungus that helps it cohabitate with the bees," Bush said. "We suspect that there is some mutual benefit to both organisms, but we haven't found sufficient evidence yet."
The researchers are now hoping to study how the fungus performs on different compositions of bee bread during their life cycle. They hope that their work will shed light on how fungicides that are routinely used to protect the bee hives will affect these microbes.
The study "An Aspergillus flavus strain from bee bread of the Western honey bee ( Apis mellifera ) displays adaptations to distinctive features of the hive environment" was published in Ecology and Evolution and can be found at 10.1002/ece3.10918. The study was supported by the Agriculture and Food Research Initiative.
- Food and Agriculture
- Agriculture and Food
- Microbes and More
- Exotic Species
- Geochemistry
- Environmental Policy
- Environmental Science
- Pollination management
- Egg (biology)
- Microorganism
Story Source:
Materials provided by Carl R. Woese Institute for Genomic Biology, University of Illinois at Urbana-Champaign . Original written by Ananya Sen. Note: Content may be edited for style and length.
Journal Reference :
- Daniel S. Bush, Bernarda Calla, May R. Berenbaum. An Aspergillus flavus strain from bee bread of the Western honey bee (Apis mellifera) displays adaptations to distinctive features of the hive environment . Ecology and Evolution , 2024; 14 (2) DOI: 10.1002/ece3.10918
Cite This Page :
Explore More
- Symbiotic Bacteria Communicate With Plants
- Birdsong and Human Voice: Same Genetic Blueprint
- Molecular Dysregulations in PTSD and Depression
- Look, Then Listen to 1 Person in a Crowd: AI
- Predicting Individual Cancer Risk
- Sexual Parasitism Helped Anglerfish Enter ...
- Treating Cataracts and Other Eye Conditions
- Early Arrival of Palaeolithic People On Cyprus
- Networks Regulating Gene Function in Human Brain
- Birth of Universe's Earliest Galaxies
Trending Topics
Strange & offbeat.
The Great Honeybee Fallacy
For years, people have understood them to be at imminent risk of extinction, despite evidence to the contrary. Why?

Updated at 10:15 a.m. ET on May 16, 2024
E veryone , for so long, has been worried about the honeybees. Governments, celebrities, social-media users, small businesses, multinational conglomerates—in the two decades or so since news emerged that American honeybees were disappearing, all manner of entities with a platform or a wallet have taken up and abandoned countless other causes, but they can’t quit trying to save the bees.
In 2022, at least 18 states enacted bee-related legislation. Last year, a cryptocurrency launched with the intention of raising “awareness and support for bee conservation.” If you search Etsy right now for “save the bees,” you’ll be rewarded with thousands of things to buy. Bees and Thank You, a food truck in suburban Boston, funds bee sanctuaries and gives out a packet of wildflower seeds—good for the bees!—with every grilled cheese sandwich it sells. A company in the United Kingdom offers a key ring containing a little bottle of chemicals that can purportedly “revive” an “exhausted bee” should you encounter one, “so it can continue its mission pollinating planet Earth.”
All of the above is surprising for maybe a few different reasons, but here’s a good place to start: Though their numbers have fluctuated, honeybees are not in trouble. Other bees are. But the movement’s poster child, biggest star, and attention hound is not at risk of imminent extinction, and never has been. “There are more honeybees on the planet now than there probably ever have been in the history of honeybees,” Rich Hatfield, a biologist at the Xerces Society for Invertebrate Conservation, told me. “They are in no threat of going endangered. It’s not an issue.”
The idea that honeybees need our help is one of our most curiously persistent cultural myths. It is well intended. But it is also unhelpful: a distraction from more urgent biodiversity problems, and an object lesson in the limits of modern environmentalism and the seductiveness of modern consumerism. That the misconception has survived for so long may tell us less about bees than it does about the species that has, for centuries, adored, influenced, and exploited them more than any other. “Save the bees” rhetoric has turned them into something unspoiled, a miracle of mother nature’s ingenious machinery. But everything about the modern American honeybee has been shaped by humans, including its sustained existence.
A true truth about the bees: The modal American honeybee is, essentially, a farm animal—part of a $200-billion-a-year industry that’s regulated by the U.S. Department of Agriculture and is as sophisticated and professionalized as any other segment of the sprawling system that gets food on our plates. The nation’s largest beekeeping operation, Adee Honey Farms, has more than 80,000 colonies, facilities in five states, and nearly 100 employees. Its bees, and those at other large-scale apiaries, do produce honey, but more and more, the real money is in what the industry calls “pollination services”: the renting-out of bees to fertilize the farms of Big Ag, which have seen their indigenous pollinators decline with urbanization and industrialization.
Every February, right before the almond trees start blooming powdery and white across California’s San Joaquin Valley, bees from all over the country pack onto semitrucks and head west, where they participate in the largest supervised pollination event on Earth, doing their part to ensure that America’s most beloved nut makes its way again into snack packs and candy bars. Throughout the spring and early summer, they do the same for other crops—watermelons, pumpkins, cucumbers, alfalfas, onions—before heading home to the honey farm, where the most ambitious among them can expect to make a 12th of a teaspoon of the gooey, golden stuff over their lifetime. In the early 1990s, when Adee started renting out bees for industrial fertilization, that income accounted for about a third of its revenue, with honey making up the rest. Now the ratio is flipped.
Read: A uniquely French approach to environmentalism
As that transition was happening, another force threatened to rearrange the industry even more dramatically. Worker bees were flying away for pollen and never coming back, abandoning their hives’ queens and young like a lousy husband in an enduring cliché. No one could figure out why. Some blamed a common class of pesticides called neonicotinoids, which are toxic to bees. Others zeroed in on the stress incurred by all that trucking of beehives around the country for pollination. Maybe it was warmer winters, or malnutrition, or the parasitic Varroa mite, or a sign of the Rapture.
This was not the first time bees had gone missing en masse. In 1869, and in 1918, and in 1965, farmers had reported similar phenomena, given names such as “spring dwindle” and “disappearing disease” in the scientific literature. But it was the first time that such an event reached full-scale public crisis, or that knowledge of it spread much beyond the insular world of farmers, beekeepers, entomologists, and agriculture regulators.
In retrospect, it was a perfect moment for a predicament like this to effloresce into panic. Social media had recently birthed an immensely powerful way of both disseminating information and performing one’s values loudly and publicly. An Inconvenient Truth , Al Gore’s feature-length climate-change call to arms, had become one of the highest-grossing documentaries of all time. Michael Pollan was at the peak of his powers, having just published The Omnivore’s Dilemma , which laid out the consequence and quantity of choices facing contemporary eaters. Americans were newly aware of the terrifying fragility of our food systems, and newly in possession of robust ways to talk about it. Brands were interested in aligning themselves with noncontroversial, blandly feel-good causes. Plus, humans were already primed to love bees; we have since biblical times . “We think of bees as being very pure,” Beth Daly, an anthrozoology professor at the University of Windsor, in Canada, told me. They are honey and flowers and sunshine, beauty and abundance, communitarianism and hard work.
By 2007, the mystery thing making these lovely creatures go away had a scary-sounding new name: colony collapse disorder. Within a decade, bee panic was everywhere. A spate of nonfiction books warned of the imminent threat of a Fruitless Fall and A Spring Without Bees . The White House convened a task force. General Mills temporarily removed the cartoon-bee mascot from boxes of Honey Nut Cheerios, enacting a high-concept allegory meant, I guess, to stun Americans into action. The cosmetics company Burt’s Bees released a limited-edition lip-balm flavor (strawberry), some of whose proceeds went to one of the approximately gazillion honeybee-conservation nonprofits that had recently sprung up. Samuel L. Jackson gave Scarlett Johansson and Ryan Reynolds “10 pounds of bees” as a wedding gift. Laypeople started keeping backyard hives. Häagen-Dazs created an awareness-raising ice-cream flavor and funded a VR short film shot from the perspective of a bee; in it, Alex, our apian protagonist, warns that “something terrible is happening.”
She (it?) was not entirely wrong. Colony collapse was an actual problem, a scientific whodunit with genuinely high stakes. Honeybees are responsible for pollinating roughly every third bite Americans eat. Scientists were correct to think back then that if colonies were to keep collapsing, our food system would need to change in painful, potentially catastrophic ways.
Much more worrying, though, and more real: The population of wild bees—the non-honey-producing, non-hive-dwelling relatives of the species humans have been intent on saving—has been decreasing steadily, for years. Insects of all kinds are declining in record numbers , and their deaths will have repercussions we cannot even imagine.
Read: The illogical relationship Americans have with animals
Yet heads have been turned mostly toward the honeybee. That’s because, unlike so many other imperiled animals, honeybees are part of a huge industry quite literally invested in their survival. Apis mellifera are living things, but they are also revenue-generating assets; the thousands of people who rely on bees’ uncompensated labor to buy groceries and pay the cable bill had every incentive to figure out colony collapse. So they found better agrochemicals and bred mite-resistant bees. They gave their bees nutritional supplements, fats and proteins and minerals ground as fine as pollen and snuck into the food supply. They moved hives into atmospherically controlled warehouses. They adapted.
All told, it was kind of the Y2K of environmental disasters. Not that colony collapse was a hoax, or that the panic surrounding it was an overreaction. Rather, it was an appropriate reaction—a big problem made smaller thanks to the difficult, somewhat unglamorous, behind-the-scenes labor of trained professionals with a vested interest in averting disaster. In 2019, an economist-entomologist team published a study analyzing the effects of colony collapse on the managed-pollinator industry; they found “cause for considerable optimism, at least for the economically dominant honey bee.” According to the most recent data from the USDA Census of Agriculture , honeybees have been the country’s fastest-growing livestock category since 2007. Also, very clearly, our food system has not fallen to pieces.
This doesn’t mean honeybee keepers aren’t struggling—some are. But as Hatfield, the Xerces Society biologist, told me, that’s an issue for the business of honeybee keeping, not the moral and practical project of pollinator conservation. He finds a useful comparison in a different domesticated animal: chickens. “When we get bird flu,” he said, “we leave that up to USDA scientists to develop immunizations and other things to help these chickens that are suffering in these commercial chicken coops. We don’t enlist homeowners to help the chicken populations in their backyard.”
I n 2018 , Seirian Sumner, a wasp scientist and fan , conducted a survey of 748 people, mostly in the United Kingdom, on their perceptions of various insects. She and her collaborators, she told me, “were absolutely flabbergasted” by their results: Bees are roughly as adored as butterflies and significantly more liked than wasps—their wilder cousins—which serve various important roles in ecosystem regulation, and which are in genuine, fairly precipitous decline.
Sumner was born in 1974 and doesn’t recall much love for bees when she was growing up. You weren’t “buying your bee slippers and your bee socks and your bee scarf and your bee mug and everything else,” she told me. Today’s craze for bees, her research suggests, is a mutually reinforcing phenomenon. People love bees because they understand their importance as pollinators. People understand their importance as pollinators because it is easier to fund research and write magazine articles and publish children’s books and engage in multi-platform brand campaigns about animals that people are already fond of.
Honeybees are, in point of fact, amazing. They have five eyes, two stomachs, and a sense of smell 50 times more sensitive than a dog’s. They do a little dance when they find good pollen and want to tell their friends about it. They are feminists, and obviously, they dress well. They produce a near-universally-liked substance, and they do not have to die to do it. Loving bees, and wanting more of them in our food system, is simple. Engaging meaningfully with the cruel, complicated reality of industrial food production, or the looming, life-extinguishing horror of climate change, is not.
To save the bees is to participate in an especially appealing kind of environmental activism, one that makes solutions seem straightforward and buying stuff feel virtuous. Worried about vanishing biodiversity? Save the bees. Feeling powerless about your mandatory participation, via the consumption required to stay alive, in agriculture systems that produce so much wreckage, so much waste, so much suffering for so many living things? Save the bees. Tired of staring at the hyperobject ? Save the bees. When we are grasping for ways to help, we tend to land on whatever is within arm’s reach.
In the 17th century, when the bees that now pollinate America’s crops and occupy Americans’ imaginations were first imported from Europe, large-scale industrial agriculture did not exist. Farms were surrounded by wild flora and powered by non-machine labor, without pesticides and chemical fertilizers, which also did not exist. Bees lived, ate, and pollinated all in the same place; they built their nests in untilled soil and unchopped trees. Even if farmers could have trucked them in, they didn’t have to. But as farming changed, bees became livestock, then itinerant laborers—there to meet the needs of the industrial systems that created those needs in the first place. Their numbers have always oscillated based on our demands: In the 1940s, when sugar rationing made beekeeping extraordinarily profitable, the bee population swelled; as soon as the war was over, it fell again. In 2024, thanks to the efforts of professional beekeepers and (to a lesser extent) backyard hobbyists, they’re faring better than ever.
Now the industrialized world that made, and saved, the honeybee as we know it is being called on to save other insects—the ones that really are in trouble. This will be trickier. When you ask experts what a layperson should do for all pollinators in 2024, they have a lot to say: Use fewer insecticides, inside and outside. Convert mowed lawn into habitat that can feed wild animals. Reconsider your efforts to save the honeybee—not just because it’s a diversion, but because honeybees take resources from wild bees . Buy organic, and look for food grown using agricultural practices that support beneficial insects. Get involved with efforts to count and conserve bees of all species. (The experts do not think you should buy a lip balm.)
What they are getting at is … an inconvenient truth: America does have an insect-biodiversity crisis. It is old and big—much older and much bigger than colony collapse disorder—and so are the solutions to it. The best require returning our environment into something that looks much more like the place the first American honeybees encountered. Having a backyard beehive isn’t the answer to what’s ailing our ecosystem, because having a backyard is the problem. Buying ice cream from a global food conglomerate isn’t the answer, because buying ice cream from a global food conglomerate is the problem . The movement to save the honeybee is a small attempt at unwinding centuries of human intervention in our natural world, at undoing the harms of the modern food system, without having to sacrifice too much. No wonder so many of us wanted to believe.
This article has been updated to clarify a colloquial reference to Apis mellifera as “the American honeybee.”
When you buy a book using a link on this page, we receive a commission. Thank you for supporting The Atlantic.
- Skip to main content
- Keyboard shortcuts for audio player
Research News
We've been trying to save the wrong bees.
Popular slogans and ad campaigns have urged the public to save honeybees. But reports suggest those efforts were directed at saving the wrong bees.
Copyright © 2024 NPR. All rights reserved. Visit our website terms of use and permissions pages at www.npr.org for further information.
NPR transcripts are created on a rush deadline by an NPR contractor. This text may not be in its final form and may be updated or revised in the future. Accuracy and availability may vary. The authoritative record of NPR’s programming is the audio record.

It’s OK to mow in May − the best way to help pollinators is by adding native plants
Professor of Entomology and Director, Center for Pollinator Research, Penn State
Assistant Research Professor of Entomology, Penn State
Disclosure statement
Christina Grozinger has received funding to study pollinator nutrition and plant-pollinator interactions from the National Science Foundation, US Department of Agriculture, Foundationn for Food and Agricultural Research, and Human Science Frontiers Program.
Harland Patch has received funding to study pollinator heath, floral traits and plant-pollinator interactions from the National Science Foundation, US Department of Agriculture, Gates Foundation and the Horticultural Research Institute.
Penn State provides funding as a founding partner of The Conversation US.
View all partners
It’s a simple idea: Stop mowing your lawn in the month of May to let flowers in the lawn, such as dandelions and clover, grow and support bees and other pollinators.
“No Mow May” was started in 2019 by Plantlife, a conservation charity based in the United Kingdom, in response to a well-documented loss of meadows and an alarming decline of native plants and animals there. Since then, it has been taken up by many gardeners and conservation advocates in North America .
Studies have shown that many flowers that grow in unmown British lawns do support British pollinators . But North America has vastly different ecological communities, composed of unique flora and fauna.
If you are interested in supporting pollinators, it is important to consider the ecological context of your yard – and #NoMowMay may not be an effective strategy. As entomology researchers who run programs on pollinators , we see better ways for people in North America to help pollinators flourish in their yards.
What grows in North American lawns?
Most common lawn flowers in North America are not native to this continent but were brought here from Europe and Asia. Many, such as bull thistle , are noxious weeds that can displace native plants and contribute to problems such as soil erosion . Others, such as ground ivy , are aggressive, invasive weeds in natural areas.
Allowing these weeds to grow can increase their numbers in the landscape and potentially reduce native biodiversity by creating near-monocultures. Not mowing your lawn and allowing these plants to spread can create weed pest problems that people on neighboring properties likely will have to manage with herbicides.
You will find pollinators on lawn flowers, but looks can be deceiving.
Some nonnative lawn plants are very attractive to pollinators. Thistle, crown vetch and, to a lesser degree, dandelion and white clover are commonly visited by bees. This attractiveness helps invasive plants get pollinated, set seed and spread effectively.
But the pollinators you see on these nonnative plants are already the most common in the landscape. Adding these plants to a landscape does not improve North American pollinator communities or support biodiversity.

The dominance of a few pollinator species on these plants may indicate that human influence has reduced the number of species in that ecosystem. Typical human-altered landscapes have a small number of cosmopolitan weedy plants – species found in a broad range of habitats in many parts of the world – and a handful of pollinator species.
For example, a 2014 study that examined urban and suburban lawns in Kentucky found that 90% of spring insects visiting dandelions there belonged to one bee species, the nonnative honeybee ; one butterfly species, the common branded skipper ; and a few hoverfly species . Honeybees represented nearly 50% of pollinators visiting white clover in spring, followed by hoverflies and a few bumblebee species.
Surrounding landscapes matter too
Few home gardens are large enough to support pollinator populations. A dandelion meadow in a city neighborhood dominated by steel and concrete would look like a pollinator haven, simply because there would be nothing else nearby for bees to feed on. But very few bees would visit the dandelions, and they would be species of bee that were common across the whole landscape, just as most birds in the area would be pigeons or house sparrows.
In a nature reserve, that same dandelion meadow would attract a more diverse community of pollinators. But it still would be dominated by the common generalist species that visit many types of flowers and are not very picky. When more specialized pollinators appear in backyards, they are spilling over from adjoining landscapes that don’t include lawn plants.
A 2016 study found this pattern on suburban lawns in Springfield, Massachusetts . Researchers collected 5,331 bees belonging to 111 species flying around small suburban lots over a two-year period. Just 13 species accounted for 4,442 individual bees they collected, while 81 species were each represented by 10 or fewer individual bees.
Plant diverse native plants
Instead of taking a pause on mowing and letting nonnative plants dominate the spring landscape, we recommend planting a diverse range of native trees, shrubs and herbaceous flowering plants.
Native North American plants and pollinators have evolved together over time. The plants have traits that allow for specific interactions, the right bloom times and the right kind of nectar and pollen for specific native insects.
Researchers at Penn State’s Center for Pollinator Research , where we both work, have examined the pollen that wild bees and managed honeybees collect to see which plants provided the most nutritional resources for bees. They found that in April and May, flowering trees – including maple, oak and willow – provided the most pollen for bees.
Other North American native plants that pollinators visit, such as Virginia bluebells, columbine and phlox, have evolved to grow in partial shade as trees leaf out above them.

More reasons to mow
Leaving grass unmowed in May or June is also problematic because it creates a favorable habitat for ticks and for wild animals such as deer and rodents that carry ticks. This can increase local risk of tick infestations and tick-borne diseases .
Maintaining shorter grass provides a drier environment that is unfavorable for ticks. It also limits wildlife habitat and food sources, which reduces tick populations.
Finally, letting weeds grow in unmowed lawns can create conflict with neighbors. Whether the concern is aesthetics, local property values or public health, many cities have ordinances that set limits on lawn height and will fine residents who don’t comply .
While we agree that mowed lawns don’t provide much food or support for native species, skipping mowing for a short period doesn’t do much either. Pollinators need flowering plants for the entire growing season, from early spring to winter.
We recommend converting your yard into a true pollinator haven, adding native plants and flower beds over time and potentially turning your entire lawn into a garden. For lists of North American pollinator plants that are native to your region, visit the Xerces Society and Pollinator Partnership . State Master Gardener groups also have detailed information on developing pollinator gardens for your region, such as the Pollinator Garden Certification Program from the Penn State Master Gardeners .
- Biodiversity
- Invasive species
- Pollinators
- Urban wildlife
- Native plants

Research Fellow

Senior Research Fellow - Women's Health Services

Lecturer / Senior Lecturer - Marketing

Assistant Editor - 1 year cadetship

Executive Dean, Faculty of Health

A gentle tap on the hive reveals honeybee health
K eeping tabs on honeybee colonies can be tricky, especially during the winter months when they become inactive. Traditionally, beekeepers have relied on opening the hives to assess their health, which can be disruptive to the honeybees.
But now, a study from Nottingham Trent University offers a bee-friendly alternative: a gentle tap that can reveal a hive's health without needing to peek inside.
Monitoring honeybee health with vibrations
The method involves gently introducing a vibration to the hive using a special device called an electromagnetic shaker.
The shaker basically gives the hive a small nudge to get the bees' attention. To see how the bees react to this nudge, the researchers use an accelerometer - which can pick up even the tiniest movements inside the hive.
By measuring these movements, the researchers can tell how active the bees are after feeling the vibration.
Honeybees' reaction to the vibrations
At first, the bees tend to slow down their activity, likely because they are being cautious and want to figure out what's going on. However, after a while, they become more active as they try to understand what caused the vibration.
By looking at these patterns of how active the honeybees become, the scientists can actually figure out the overall health and well-being of the entire bee colony.
Signs of a healthy honeybee colony
In the summer, honeybees do not react much when researchers introduce vibrations. This is because they are focused on essential tasks like collecting food and raising young bees , which are crucial for the survival of the colony.
However, things change in the winter. As the weather gets colder, the bees huddle together to stay warm and become less active. During this time, the bees become more sensitive to the same vibrations and react more strongly.
This increased sensitivity and the resulting "whooping" sound, which the researchers observed earlier, are signs that a healthy colony is adapting to the cold temperatures by clustering together.
Evaluating the health of a honeybee colony
The new method not only measures bee activity but also provides clues about the overall health of the honeybee colony. The researchers monitored several beehives and noticed that one colony buzzed constantly throughout the summer , even during periods when bees are typically very active.
This particular colony turned out to be the only unhealthy group in the study, suggesting that the continuous buzzing might be a sign of poor health.
Further investigation revealed that the struggling colony had lost its queen in the spring. The abnormal buzzing pattern stopped and returned to normal after the colony was successfully reintroduced to a healthy new queen.
Revolutionizing beekeeping
"Our measurements reveal, non-invasively, colony mobility, the clustering of the colony and its restfulness, and can detect the absence of the queen in the active season," noted lead researcher Dr. Martin Bencsik, a scientist at Nottingham Trent University.
"We believe our work might also help to give beekeepers an indication of the size of their colonies, based upon the strength of the signal."
In the future, the technology might even be used to create tools that constantly monitor beehive health. This would give beekeepers immediate information about their bees, allowing them to address problems quickly.
Even beyond beekeeping, the research could be helpful in understanding how the environment affects bee health. This information would be valuable for scientists who are studying ecology and conservation.
The study is published in the journal Scientific Reports .
Like what you read? Subscribe to our newsletter for engaging articles, exclusive content, and the latest updates.
Check us out on EarthSnap , a free app brought to you by Eric Ralls and Earth.com.

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Saudi J Biol Sci
- v.28(1); 2021 Jan
Viral impacts on honey bee populations: A review
Amjad ullah.
a Department of Zoology, Kohat University of Science and Technology, Kohat-26000, Khyber Pakhtunkhwa, Pakistan
Ivana Tlak Gajger
b Department for Biology and Pathology of Fish and Bees, Faculty of Veterinary Medicine University of Zagreb, Zagreb, Croatia
Arnold Majoros
c Veterinary Ambulance LunaVet, Novi Sad, Serbia
Showket Ahmad Dar
d Division of Agricultural Entomology, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, India
Sanaullah Khan
e Department of Zoology, University of Peshawar, Khyber Pakhtunkhwa, Pakistan
Ayesha Haleem Shah
f Institute of Biological Sciences, Gomal University, Dera Ismail Khan, Pakistan
Muhammad Nasir Khabir
Riaz hussain, hikmat ullah khan, mehwish hameed, syed ishtiaq anjum.
Honey bee is vital for pollination and ecological services, boosting crops productivity in terms of quality and quantity and production of colony products: wax, royal jelly, bee venom, honey, pollen and propolis. Honey bees are most important plant pollinators and almost one third of diet depends on bee’s pollination, worth billions of dollars. Hence the role that honey bees have in environment and their economic importance in food production, their health is of dominant significance. Honey bees can be infected by various pathogens like: viruses, bacteria, fungi, or infested by parasitic mites. At least more than 20 viruses have been identified to infect honey bees worldwide, generally from Dicistroviridae as well as Iflaviridae families, like ABPV (Acute Bee Paralysis Virus), BQCV (Black Queen Cell Virus), KBV (Kashmir Bee Virus), SBV (Sacbrood Virus), CBPV (Chronic bee paralysis virus), SBPV (Slow Bee Paralysis Virus) along with IAPV (Israeli acute paralysis virus), and DWV (Deformed Wing Virus) are prominent and cause infections harmful for honey bee colonies health. This issue about honey bee viruses demonstrates remarkably how diverse this field is, and considerable work has to be done to get a comprehensive interpretation of the bee virology.
1. Introduction
The Western honey bee ( Apis mellifera ) belongs to genus Apis, mainly recognized by production of honeybee products ( Engel, 1999 ), distributed globally and well known for efficient plant pollination ( Chapman et al., 2019 ). A. mellifera had been exploited by humans about 5000 years ( Gisder & Genersch, 2017 ). The native range of A. mellifera comprises Africa, Europe and Middle East ( Chapman et al., 2019 , Tihelka et al., 2020 ), whereas other nine species of Apis are present exclusively in Asia ( Han et al., 2012 ). Honey bees are the most important crop and wild plant pollinators ( Bacandritsos et al., 2010 , Cirkovic et al., 2018 , Klein et al., 2006 ) and almost one-third (T O’Neal et al., 2018 ) of distinctive agricultural crops need bees pollination. A. mellifera is the key pollinator of several food crops ( Gisder and Genersch, 2017 , Tantillo et al., 2015 ) including vegetable, nuts, fruit as well as oilseeds ( Brutscher et al., 2016 , Geldmann and González-Varo, 2018 ). The value of these pollination services is commonly measured in billions of dollars ( Goulson et al., 2015a ), adding about 9.5% to the value of crops across the world ( Klein et al., 2006 , Potts et al., 2010a , Tantillo et al., 2015 ). Although wild insect pollinators as well as reared honey bees provide significant ecosystem maintenance (as pollinators of flowering plants), whereas the number of colonies (managed honey bees) has been improved globally (more than 45%) since preceding 60 years ( Breeze et al., 2014 , Brutscher et al., 2016 , Gisder and Genersch, 2017 , Goulson et al., 2015a , McMenamin and Genersch, 2015 , Potts et al., 2010a ). According to available published results, in the last 15 years, dramatic honey bee winter colony losses have been reported frequently from different regions all over the world ( Breeze et al., 2014 , Carreck and Neumann, 2010 ).
Pollinating insect declines portend human food safety and can affect not only apiculture ( Francis et al., 2013 ) or agriculture but also has anthropological threats ( Gisder and Genersch, 2015 , McMenamin and Flenniken, 2018 ). It was documented that the reasons for global bees-decline are pesticides ( Alburaki et al., 2018 , Becher et al., 2014 , Branchiccela et al., 2019 , Francis et al., 2013 , Goulson et al., 2015a , Klein et al., 2017 ; T O’Neal and S., Anderson, T. D., & Wu-Smart, J. Y. , 2018 , Wells et al., 2016 ), destruction of habitat ( Brown and Paxton, 2009 , Brutscher et al., 2016 , Goulson et al., 2008 , Goulson et al., 2015a ), industries, agriculture, parasites/pathogens ( Alburaki et al., 2018 , McMenamin and Flenniken, 2018 ), climate change ( Klein et al., 2017 , Sánchez-Bayo and Wyckhuys, 2019 , Sihag, 2014 ) as well as inadequate food supply ( Alburaki et al., 2018 , Becher et al., 2014 , Branchiccela et al., 2019 , Carreck and Neumann, 2010 , Chagas et al., 2019 , Dolezal et al., 2019 , McMenamin and Genersch, 2015 ; T O’Neal and S., Anderson, T. D., & Wu-Smart, J. Y. , 2018 , Tantillo et al., 2015 , Wells et al., 2016 ). Investigators demonstrated that viral infections in honey bee colonies are the main source of septicity and considered as a key risk for their health ( Chagas et al., 2019 , Levin et al., 2019 , Remnant et al., 2017 , Tantillo et al., 2015 ) at individual and colony level ( Beaurepaire et al., 2020 , Gisder and Genersch, 2015 ). In the 20th century, Dr. White first described honey bee viruses, while studying the filtrate of infected honey bee larvae with causative agent of Sacbrood disease ( Tantillo et al., 2015 ). Furthermore, at least 24 viruses were known to be associated with honey bees ( Chagas et al., 2019 , Dolezal et al., 2016 , Ellis and Munn, 2005 , Gisder and Genersch, 2015 , Gisder and Genersch, 2017 , Remnant et al., 2017 , Runckel et al., 2011 ) which remained an important hazard to the fitness and well‐being of A. mellifera ( Dolezal et al., 2016 ) as well as to other honey bees worldwide ( Allen and Ball, 1996 , Chen and Siede, 2007 , Gisder and Genersch, 2017 ).
Studies revealed that single strand positive (SS+) sense RNA viruses’ constitute the major assemblage of honey bees infectious agents ( Brutscher et al., 2015 , Chen and Siede, 2007 , Gisder and Genersch, 2017 ). Moreover, most common viruses that cause damage to bees health are: Dicistro viruses: Acute bee paralysis virus (ABPV), Kashmir bee virus (KBV), Israeli acute paralysis virus (IAPV), Black queen cell virus (BQCV) ( Chagas et al., 2019 ); Iflaviruses such as: Deformed wing virus (DWV), Slow bee paralysis virus (SBPV), Varroa destructor virus (VDV1) and Sacbrood virus (SBV); and taxonomically unsystematic viruses such as: Lake Sinai viruses (LSV) and Chronic bee paralysis virus (CBPV). Last two mentioned viruses are evolutionary exclusive besides universally dispersed with LSV1-7, as well as other alternates ( Brutscher et al., 2016 , McMenamin and Flenniken, 2018 ). Similarly, A. mellifera rhabdovirus-1 (ARV-1) and A. mellifera rhabdovirus-2 (ARV-2) from the order Rhabdovirus, as well as a single double stranded DNA Filamentous virus (AmFV) were described in honey bees ( Chagas et al., 2019 ). In this review we provided a current data and recent progress in honey bee viruses research.
2. Viral infections in honey bee colonies
Viruses are possibly the hidden enemies of honey bees ( Ray et al., 2020 ) as compared to other pathogens, because most of infections pass without clinical manifestation of characteristic disease signs ( Chen et al., 2006b , Martin et al., 2012 ). However, viral infestations had great concern ( Wilfert et al., 2016 ), as it cause damage at various developmental stages of honey bees, like egg, larvae, pupa, adult worker, drone or queen ( Chen et al., 2006a , Chen et al., 2004 ). In addition, clinically visible symptoms of honey bee virus diseases are mostly associated with another infectious agents, like the presence of microsporidia Nosema apis and strong infestations with Varroa destructor mites ( Evans & Spivak, 2010 ). Viral particles spread in honey bees by two ways: by vertical and horizontal transmission ( Beaurepaire et al., 2020 , Chagas et al., 2019 , Chen and Siede, 2007 , De Miranda et al., 2012 ). In vertical transmission route, viruses proliferate from queen ( trans -ovarial) or drones ( trans -spermal) or during their mating (venereal) to the offspring’s while in horizontal transmission route viral particles spread amongst colony members of same age generation ( Chagas et al., 2019 , Chen et al., 2006b , De Miranda et al., 2012 ) and between same and different castes (oral or by contact). According to Bowen-Walker et al. V. destructor mites got DWV from infested bees and behave as a carrier to spread viruses in healthy bees, when they feed upon it (vector-borne transmission) ( Chen et al., 2006b , Gisder and Genersch, 2017 ). Researchers studied that the process of infecting or feeding on bee larvae or adult bees, these ectoparasites would appear to create an opportunity for viral particles to enter the larvae or adult bee. Under these conditions bee virus infections can be lethal ( de Miranda et al., 2013 ). Whereas, other factors which may increase viral negative impact on bees could include environmental and nutritional stresses ( Evans et al., 2009 ). Although, honey bee viruses typically continue as hidden contagions while showing no signs of disease but destroy bee fitness and health during favorable conditions ( Tantillo et al., 2015 ).
3. Viral impacts
3.1. colony level infection.
Honey bee colonies are challenged with a wide range of diseases caused by various pathogens ( Alburaki et al., 2018 , Chen et al., 2006b , Gisder and Genersch, 2017 ) and changed environmental conditions in different ways ( Hao & Li, 2016 ). Scientists documented that areas of the world that are accountable for universal food supply had large number of colony losses annually, therefore, it is important to recognize the responsible agents. Various environmental factors having negative effect on bees fitness are: intensive agriculture with included regular pesticides use, deficiency of eminence food and loss of habitat, pathogens and pests ( Brutscher et al., 2016 , Goulson et al., 2015a , McMenamin and Genersch, 2015 , Tantillo et al., 2015 ). In addition, viruses are significant threats to the honeybee colony strength ( Chen and Siede, 2007 , Gisder and Genersch, 2017 ). Colony level research points out related viruses such as ABPV, KBV, IAPV, DWV as well as LSV2 present in feeble or CCD-affected hives, however these links are not globally detected ( Brutscher et al., 2016 , McMenamin and Genersch, 2015 ). CCD (Colony collapse disorder) is categorized with quick extinction of adult bees from hive and finally queen remains with few newly emerged bees ( Evans et al., 2009 , McMenamin and Genersch, 2015 ) while consequent damage was valued about 75 billion dollars, both to agriculture as well as apiculture, globally ( Tantillo et al., 2015 ). Studies revealed that IAPV was related to CCD ( Tantillo et al., 2015 ) because its presence in a honey bee colony is linked with high threat to colony collapse ( Genersch and Aubert, 2010 , Meixner, 2010 ). Similarly, KBV was also detected in colonies showing CCD signs ( Brutscher et al., 2016 , de Miranda et al., 2010 , McMenamin and Flenniken, 2018 , Tantillo et al., 2015 ).
The severe infection of KBV and ABPV results in the excessive loss of adult bees. Consequently, it results in the emergence of infected pupae and larvae and lack of adults to look after the young brood. ABPV aggregates in the hypopharyngeal glands ( Chagas et al., 2019 ) and brain, while KBV and ABPV were determined in fecal material and were transmitted through various routes, such as oral communication (larvae, adults, cannibalism, fecal material or contaminated diet) ( de Miranda et al., 2010 ). Furthermore, transmission through live vectors as V. destructor or Tropilaelaps spp. mites results in the remarkable spread of viruses ( De Miranda et al., 2012 ), e.g. DWV in an individual bee ( Tehel et al., 2016 ) as well as in the whole colony ( Amiri et al., 2015 , de Miranda et al., 2010 , Genersch et al., 2010 , Goulson et al., 2015a , McMenamin and Flenniken, 2018 ). Due to high spread rates, DWV is more extremely described virus in A. mellifera ( Goulson et al., 2015a , Schittny et al., 2020 ) if compared to other bee species ( Tehel et al., 2016 ). Similarly, DWV-A (deformed wing virus type A) and DWV-B (deformed wing virus type B) were responsible for winter colony losses ( Highfield et al., 2009 ).
According to published results, KBV and IAPV were initially linked with CCD but further research revealed that not a single virus was responsible for it ( Barron, 2015 , Cornman et al., 2012 , McMenamin and Flenniken, 2018 ). In the same way, data collected in Spain, Belgium and US (both affected and non-affected CCD colonies) have shown numerous LSVs which are spread worldwide and occasionally responsible for infections of honey bee colonies ( McMenamin and Flenniken, 2018 , Remnant et al., 2017 ). Likewise, long time disclosure to various stresses causes fall of wild bee pollinators populations number and colony destruction. But the effect of these factors is different and depends on geographical area ( Goulson et al., 2015b ). Research has exposed that SBPV was correlated with colony collapse in England, but mostly less prevalent in other European apiaries ( Carreck et al., 2010 , De Miranda et al., 2012 ). SBPV not only primarily affects the fore legs (paralyses) of honey bees but also found in head, salivary gland, mandibular and hypopharyngeal glands, crop, fat body, while present in thorax, midgut, hindlegs and rectum in low quantity ( De Miranda et al., 2012 ). ABPV and DWV were present in honey bee colonies before the appearance of parasitic mites in UK and were responsible for colony destruction but rarely ( Sánchez-Bayo et al., 2016 ). Furthermore, V. destructor mites as well as viral pathogens were both responsible for colony failure ( Francis et al., 2013 , Genersch, 2010 ), as without mites, viruses were unable to cause honey bee colony collapse ( Sánchez-Bayo et al., 2016 ).
Moreover, research also has revealed that CCD in USA was caused by IAPV ( De Miranda et al., 2012 , Genersch, 2010 , Hou and Chejanovsky, 2014 ) while it was not mentioned in Australia which is free of V. destructor mites. Likewise, KBV also enhance in the presence of strong infestations of V. destructor mites but causes losses in beekeeping also in Australia ( Sammataro et al., 2000 ). Moreover, V. destructor together with DWV were deliberated as evolving infection and showed dangerous results ( Chagas et al., 2019 , McMenamin and Genersch, 2015 , Tantillo et al., 2015 ) both in individual bees and colonies ( Chen and Siede, 2007 , Dalmon et al., 2019 , Francis et al., 2013 , Genersch and Aubert, 2010 , Gisder and Genersch, 2017 ). An inquiry from Thailand showed that in varroa mites’ samples, DWV were present in 100%. In the same way, parasitic mites collected from honey bee colonies in France were 100% and in Poland 69% positive for DWV ( Tantillo et al., 2015 ). According to GBMP (German bee monitoring project) the existence of DWV in hives had the better indication of colony impermanence as compared to KBV and ABPV ( Francis et al., 2013 ). CBPV is highly distributed in England ( Chen & Siede, 2007 ) and reports from Austria acknowledged that because CBPV were present in diverse geographic area and virus was determinated in 10% of different disease-ridden colonies ( Berényi et al., 2006 , Chen and Siede, 2007 ). Another study from Thailand suggested that V. destructor was accountable for various viral infections (DWV, ABPV, BQCV, KBV and SBV) in beehives ( Chantawannakul et al., 2006 ).
It was demonstrated that BQCV had been highly spread and responsible for colony losses of Apis cerana in Thailand, South Korea, Japan, China and Vietnam ( Chantawannakul et al., 2016 , Yang et al., 2013 ) as well as of A. mellifera in Vietnam, South Korea, Thailand, China, Japan ( Ai et al., 2012 ) and also caused infection in Apis dorsata and Apis floreae populations in Thailand and China ( Chantawannakul et al., 2016 , Mookhploy et al., 2015 , Zhang et al., 2012 ). Additionally, parasitic mite associated viruses such as DVW, IAPV ( Francis et al., 2013 ) and ABPV caused colony mortality ( Chen et al., 2006a , Hou and Chejanovsky, 2014 , Martin, 2001 , Tentcheva et al., 2004 ) and their virulence might be enhanced when V. destructor acted as vector for their transmission ( Molineri et al., 2017 ). After infection, these viruses cause shaking, restrain the movement of bees and loss of workers within two days ( Amiri et al., 2017 ). Furthermore, IAPV was reported in every developmental stage of honey bee and its infection was observed almost in all tissues, but mostly existed in hypopharyngeal glands, alimentary canal and nervous system ( Amiri et al., 2019 , Chen et al., 2014 , De Miranda et al., 2012 ). It was documented that V. destructor feeds upon bee brood as well as adults and behaves as a carrier for various honey bee viruses ( Chagas et al., 2019 , Tantillo et al., 2015 ).
3.2. Declines of reared honey bees
Managed honey bees are in decline because of numerous interacting agents including parasite and pathogen pressure ( Carr-Markell et al., 2020 , Hellerstein et al., 2017 ), pesticide exposure ( Carr-Markell et al., 2020 , Ferrier et al., 2018 , McMenamin and Genersch, 2015 , Ratnieks and Carreck, 2010 , Tantillo et al., 2015 , Wells et al., 2016 , Williams et al., 2010 ) and territory demolition as well ( Chagas et al., 2019 , Goulson et al., 2015a , Potts et al., 2010a , Smith et al., 2015 ). It has been reported that viruses of honey bee can be transmitted to another bee species ( Gisder & Genersch, 2017 ) and are responsible to their declines ( Graystock et al., 2015 ). Studies have shown that population of European reared honey bees are in decreasing trend ( Potts et al., 2010b ) while there is regular fall of colony number in middle European states ( Amiri et al., 2015 , Antúnez et al., 2017 , Van der Zee et al., 2012 ) whereas slightly increase in Mediterranean countries ( Ball, 1996 , Goulson et al., 2015b ). BQCV was common in managed honey bee population in North America ( Desai et al., 2016 ) and Europe ( McMahon et al., 2016 ). A study of 26 sites from England revealed that, BQCV was more prevalent in the honey bees as compared to bumble bee species ( McMahon et al., 2015 ). Similarly, IAPV was widespread in honey bees in North America, while it was occasionally noticed in European apiaries ( Cox-Foster et al., 2007 , Tehel et al., 2016 ). Research stated that viruses can be transmitted among wild bees and managed bees and opposite ( Brutscher et al., 2016 , Galbraith et al., 2018 , Graystock et al., 2016 , McMenamin and Flenniken, 2018 , Murray et al., 2019 ). Furthermore, viral particles can be multiplied within and spread among different bee species and their infection level depends on either viral strain or host ( Tehel et al., 2016 ) as well as some other features, including host sex, nutritive status, genetic makeup and age ( McMenamin & Flenniken, 2018 ).
The consequence of viral infection in wild bees include body malformation ( Genersch et al., 2006 ), diminish reproductive capability, efficient septicity and susceptibility to death ( McMenamin & Flenniken, 2018 ). It has been reported that transmission of IAPV from reared honey bees to wild bees can be the consequence of common usage of flowers during foraging ( Tehel et al., 2016 ). IAPV was noticed to be spread from A. mellifera to A. cerana ( Theisen-Jones & Bienefeld, 2016 ) . Conversely, ABPV most commonly present in wild bees as compared to managed honey bees, was possibly transmitted from wild bees to the A. mellifera ( Tehel et al., 2016 ). Additionally, Chines SBV had triggered severe damage in A. cerana managed colonies in Asia ( Steinhauer et al., 2018 ). ABPV was prevalent commonly in South America and Europe, while IAPV in Australia and Middle East alongside KBV in North America and New Zealand. Besides, the titers and frequency of KBV and ABPV enhances in late summer while ABPV particles increased their titer earlier than KBV, depend upon the colony progression level ( de Miranda et al., 2010 ). Main symptoms of honey bees CBPV Type I disease are trembling wings and bodies, ataxia, circling, inability for fly, crawling on ground and up grass streams and mortality; and CBPV Type II syndrome include blackening and withdrawal of hair from the thorax and abdomen, small-shiny-dark bees and finally loss of highly paralyzed adults ( de Miranda et al., 2010 , Tantillo et al., 2015 ). In south American countries like Uruguay, Brazil and Chile, many viruses of honey bee had been detected in apiaries, also exist in moderate environment ( Molineri et al., 2017 ). Study from Denmark revealed that, winter colony losses exceeds near 32% from 2007 to 2008, that were mostly correlated with V. destructor infestations and viral particles association ( Amiri et al., 2015 , Nielsen et al., 2008 , Vejsnæs et al., 2010 ).
Moreover, about 14% colony losses were reported in Greece in 2007 to 2008 ( Bacandritsos et al., 2010 ). Similarly, Thailand Sacbrood virus (TSBV) was responsible for the loss of 90% of managed A. cerana bees in India ( Theisen-Jones & Bienefeld, 2016 ), Kashmir, Japan, South Korea, Thailand, Nepal, Vietnam, China ( Forsgren et al., 2015 ) and also observed to cause infestation in A. florae and A. dorsata in India ( Allen & Ball, 1996 ). In addition, DWV was more widespread than SBV in A. mellifera population, reported from Thailand, Sri Lanka, Japan, Nepal, Vietnam, and China ( Ai et al., 2012 , Forsgren et al., 2015 ). While Uganda and Turkey were considered to be SBV free and no particle of SBV was reported in honey bee population ( Beaurepaire et al., 2020 ). Besides, DWV infestation in A. cerana has been determinated in Japan, China, Vietnam, and South Korea ( Forsgren et al., 2015 ) in A. dorsata , and A. florae population in China ( Chantawannakul et al., 2016 , Zhang et al., 2012 ). DWV was isolated from A. dorsata, A. florae and A. mellifera revealed that it is transmitted from A. mellifera to A . dorsata and A. florae in China through common pollinating resources ( Forsgren et al., 2015 ). Moreover, Serbian apiaries showed high level of DWV incidence as compared to further countries like Slovenia, Hungary, Austria, Uruguay and France ( Cirkovic et al., 2018 ). In Croatia before affiliation to European Union most of honeybee viruses were detected in lower levels than in its neighboring countries ( Gajger et al., 2014 ; Tlak Gajger et al., 2014 ). In last few decades many different diagnostic methods were developed or improved ( Schurr et al., 2017 ). Also, two plant infecting viruses such as Turnip ring spot virus (TuRSV) and Tobacco ring spot virus (TRSV) were also detected in honey bees ( Granberg et al., 2013 , Li et al., 2014 , McMenamin and Genersch, 2015 ).
3.3. Impact of viruses on nutritional stress honey bees
The impact of quality and amount of food in mammals mark their exposure to environmental stressors and pathogens ( Dolezal & Toth, 2018 ). In the same way, this fact in honey bees is not well known ( Goulson et al., 2015a ). However, nectar and pollen contain different ingredients: carbohydrates, proteins and lipids, other phytonutrients and essential substances (vitamins and minerals) having optimistic influence on their immune system ( Di Pasquale et al., 2013 , Goulson et al., 2015a ). Additionally, various food resources hold important effect on bees health ( Dolezal & Toth, 2018 ). Furthermore, nectar and pollen are necessary for larvae and adult bees providing vital nutrient’s for survival. Whereas, poor nutrition results in less bee foraging capability and has negative impact on individual and colony level ( Leach and Drummond, 2018 , Steinhauer et al., 2018 ). Studies have shown that, variety of pollen has great effect to standardize of the inborn immune system and decreases mortality rate which are the consequences of visible IAPV infections ( Dolezal & Toth, 2018 ) and N. ceranae attack ( Leach & Drummond, 2018 ). Other nutrients like proteins, vitamins and minerals play an important role and enhance bee’s life span ( Dolezal & Toth, 2018 ). The access of bees to food resources is now alarming because alteration of land practice marks the change of floral properties, retain negative impact on bee physiology and health. Moreover, hives bounded by more cultivated land result high colony losses ( Dolezal et al., 2019 , Steinhauer et al., 2018 ) and decrease fat assemblage arriving winter time ( Dolezal & Toth, 2018 ).
Though, this fact is not always true because in some cases higher crops growing consequently decrease pollen storage as well as production of honey ( Sande et al., 2009 ). Whereas, a study from western United State of America revealed higher honey production in cultivated areas as compared to urban regions ( Brodschneider and Crailsheim, 2010 , Dolezal and Toth, 2018 ). N. apis and N. ceranae infections of honey bees cause the disturbance in digestion and also effect immunity of the host, which consequently lead to bees malnutrition as well as immunosuppression and susceptibility to additional viral pathogens infections, like SBV ( Dolezal & Toth, 2018 ) and large number of DWV particles ( Dolezal et al., 2019 ). The infection of pathogens (e.g. SBV and Nosema spp.) cause decline of nutritional value in a honey bee colony like lower collection of pollen and less storage of vitellogenin, and enhance immature bee’s hormone level (causes premature foraging behavior) ( Dolezal and Toth, 2018 , Goblirsch et al., 2013 ). Various environmental factors such as imbalance nutrition and pathogens infection cause honey bee colony disorganization, depopulation and mortality ( Dolezal & Toth, 2018 ).
Additionally, the reduction of protein level in honey bees, weakens their immune system and vulnerable to viral attack ( Evans & Schwarz, 2011 ). In another study, it was reported that like Nosemosis, viruses also associated with digestive system of bee’s, interrupt digestion, physiology and nutritional requirements of host ( Dolezal & Toth, 2018 ). As digestive system acts as a reservoir for viral titers because most activities occur there like pollen storing, nectar dispensation and other metabolic activities ( De Miranda et al., 2012 ). Moreover, lower quality of food intake consequently higher DWV titer in bees as compare to higher quality of pollen holding food ( DeGrandi-Hoffman et al., 2010 ). Though, the correlation between food quality and DWV contamination need additional research, as other studies revealed that, DWV titer were found more in pollen containing diet consuming by bees as compare to nutrients comprising sucrose foodstuff ( Alaux et al., 2011 , Grozinger and Flenniken, 2019 ). Due to lack of pollen supply, bees cannibalize their young brood to get proteins ( De Miranda et al., 2012 ) while feed on old larvae upon them ( Brodschneider & Crailsheim, 2010 ) that lead to the possible cause of oral exchange of viral particles ( De Miranda et al., 2012 ).
3.4. Effect of viruses on foraging performance of honey bees
Honey bees are the major managed insect pollinator worldwide ( Abou-Shaara, 2014 , Calderone, 2012 , Gisder and Genersch, 2017 , Joseph et al., 2020 ) while wild bees can also improve yields of various crops and are a key part of natural ecosystems ( Aizen et al., 2009 , Garibaldi et al., 2013 ). Honey bee ( A. mellifera ) is an important player in farming and pollinate a large number of food crops ( Ferrier et al., 2018 ), which add more than 15 billion dollars to the market per year ( Chen et al., 2006a , Chen et al., 2006b , Morse and Calderone, 2000 ). Nowadays since there is gradual decline in bee populations over the world, there is great emphasis on bee survival because bees are beneficial and famous for their pollination services, to supply foodstuff (e.g. fruits, nuts, berries, seeds, leaves and roots) ( Hung et al., 2018 ). Besides human, they also pollinate foods eaten by other animals and birds worldwide ( Chagas et al., 2019 , Hung et al., 2018 ). Varroa mites feed upon honey bees and introduces high concentrations of viruses to their host, and causes different alterations such as body weight loss, suppressed immunity, short life span and diminishes foraging capacity ( Amiri et al., 2017 ). Additionally, DWV attack on those areas of brain, that controls sense of smell and had negative influence in the foraging behavior of honey bees ( Klein et al., 2017 ). DWV infected bees without physical deformities, fly to a short distance and flight duration as compared to non-infected bees. DWV decrease the gene countenance which control memory as well as wing development together with olfactory learning ( Wells et al., 2016 ).
Viruses from Dicistroviridae family and CBPV create severe manifestations in adult bees and decline the life span of workers if viral titers increased up to maximum level ( de Miranda et al., 2010 , Ribière et al., 2010 ). Whereas, SBV and DWV as well as BQCV are present and persist in honey bees with no outward affliction signs. But turn out to be troublesome when they get suitable conditions (biotic or abiotic) for replication ( Amiri et al., 2015 , Chen and Siede, 2007 , Dainat et al., 2012 , Prisco et al., 2011 ). In addition, CBPV aggregate, multiplies, damage and disturb the brain area (CNS) of honey and their neuronal function responsible for sensory perception ( Ribière et al., 2010 ). As result the infected honey bee vibrating, crawling at the hive entrance and is unable to fly ( De Miranda et al., 2012 ). Scientists discovered a new virus called Kakugo virus (KV) which was isolated in Japan from the brain of worker bees in 2004 ( Fujiyuki et al., 2004 ) that had similarities with DWV (about 98% at the nucleotide level) ( Terio et al., 2008 ). In addition, the brain tropism was noticed (specifically in antennas, in optic neuropils and in mushroom bodies) ( Shah et al., 2009 ), which controlled sense experiences and organized the performance in bees ( De Miranda and Genersch, 2010 , Tantillo et al., 2015 ).
3.5. Viral effects on queen bee health
Pathogenic viruses are extremely hazardous to honey bee health and can cause colony losses ( Martin et al., 2012 , Mondet et al., 2014 , Remnant et al., 2017 ). Besides, disease causative agents, the death of queen has been considered as one of the major factors for colony failure. Because presence of queen for a colony is crucial, not only to lay eggs but to coordinate colony members tasks and behavior through pheromone secretion ( Brutscher et al., 2019 ). Likewise, other colony members, the queen bee also acquires and faces the attack of pathogenic viruses ( Amiri et al., 2017 ) and contain many viruses (up to six) at a time ( Beaurepaire et al., 2020 ). It was documented that drones were able to transmit DWV to queen when infected drones come to the mating area and introduced contaminated semen to queens ( Amiri et al., 2017 , Brutscher et al., 2019 , Chen et al., 2006b ). Additionally, the presence of viral titers in the reproductive system of drone and queen ( Chen et al., 2006a , Chen et al., 2006b ) and semen, demonstrate viral transference through sexual intercourse in bees ( De Miranda et al., 2012 , Tantillo et al., 2015 ). Infected queen can transfer viral particles directly (vertically) to her brood ( Amiri et al., 2017 , Schittny et al., 2020 , Tantillo et al., 2015 ). Vertical conveyance of viruses occur through already infected ovarian tissue of queen bee or by fertilization of egg by infected sperms ( De Miranda et al., 2012 , Tantillo et al., 2015 ). Study directed by Chen and collaborators revealed the existence of BQCV and DWV in the digestive tract of queen bee ( Amiri et al., 2017 , Tantillo et al., 2015 ). DWV can infect young and adult queens, but is less prevalent in young ones ( Delaney et al., 2011 ). It infects queens gut, head, ovaries, fat body ( Amiri et al., 2016 ) and can cause clinical visible characteristic disease sign in form of deformed wings ( Amiri et al., 2017 ). As a result, due to high contamination of viruses in queen ovaries, causes ovary degeneration and may the source for deposited sperm mortality ( Gauthier et al., 2011 ).
Consequently, failure in reproductive capability might be effect hive accomplishment, efficiency and replacement of queen ( Amiri et al., 2017 ). Furthermore, SBV was also observed in the embowelled body and ovary of queen bee but its transmission and negative impact was not well understood ( Amiri et al., 2017 , Ravoet et al., 2015 ). BQCV can be responsible for the decease of honey bee queen larvae, and cause infection in queen pupa ( Tantillo et al., 2015 ). BQCV particles were detected in the ovary, and gut of queen bee ( Amiri et al., 2017 ). Additionally, like other viruses BQCV was also detected in A. mellifera globally ( Allen and Ball, 1996 , Ellis and Munn, 2005 ), where it caused covert infestation in brood and adult worker bees ( De Miranda et al., 2012 ). The presence of DWV and BQCV in queen bee digestive tract showed that viral titer must ingested by queen through contaminated food ( Tantillo et al., 2015 ), that was the suitable environment for viral proliferation and can be easily transmitted to other tissues (spermatheca, ovaries, hemolymph) by penetrating wall of the tract ( Chen et al., 2006b ). Recent finding have shown that IAPV was transmitted from infected workers to queen bee through prophylaxes and direct contact with infected bees ( Amiri et al., 2019 ).
BQCV is currently the most important and widespread virus in A. mellifera ( Chagas et al., 2019 , Gauthier et al., 2007 ) globally. Additionally, BQCV was reported in Japan, China and Thailand in local honey bee populations of A. c. japonica, A. c. indica, A. florea and A. dorsata ( Gisder & Genersch, 2017 ). Furthermore, BQCV has partial stress on worker as well as on drone bees ( Amiri et al., 2015 , Retschnig et al., 2014 ). Experimental evidences confirmed the simultaneous presence of DWV and BQCV in queen bee, and were also detected in her eggs, larva’s as well as adult workers and suggested that, it can be transferred vertically ( Chen et al., 2006b , Tantillo et al., 2015 ). Similarly, ABPV particles in outwardly normal drone bee’s semen indicated that, the virus can be transmitted from drone to queen bee through mating. Furthermore, CBPV was also determinated in queen bee as well as in its developing stages and it was assumed that CBPV can be transmitted vertically ( Tantillo et al., 2015 ). These results showed that most viruses were problematic for queen health but some revealed no visible signs of infection and they were easily spread by queen to their offspring. Generally, viruses had less effect on queen health but these prognosis require further research to investigate their association with colony fitness and queen health ( Amiri et al., 2017 ).
3.6. Effect of viruses on the honey bee’s immunity
The transmission of viral particles from parasitic mites to honey bees suppresses the immunity of bees or increase the virus virulence and infectiveness. Additionally, the V. destructor saliva contain immunosuppressive proteins and intimidate the immune system of infested honey bees ( Meixner, 2010 , Tantillo et al., 2015 ). The parasitic mites infestation on honey bee, destroy its body's mechanical defenses and it becomes exposed to further viral attack ( Tantillo et al., 2015 ). The strong virus infection encourages virulence increase in honey bees, suppress their immunity, and consequently make them vulnerable after exposures for other environmental stresses ( Sánchez-Bayo et al., 2016 ). Similarly, organosilicon spray complementary (used in several pesticide preparations) destroy honey bees olfactory learning and enhance viral multiplication (T O’Neal et al., 2018 ). The neonicotinoids also disrupt the immune and reproductive system functions of honey bees ( Gajger et al., 2017 , Piiroinen and Goulson, 2016 ), consequently enhances multiplication of DWV in bees ( Di Prisco et al., 2013 , Goulson et al., 2015a ). It was also reported that high amount of DWV titers in honey bees inhibits their immune responses and melanin production, leading to propagate V. destructor mites and enhances its progeny as well ( Gisder & Genersch, 2017 ). Furthermore, V. destructor septicity subsidize to the suppression of bee host immunity, and enhances the infections with DWV ( Genersch, 2010 , Gisder and Genersch, 2017 , López-Uribe and Simone-Finstrom, 2019 ) and ABPV ( Molineri et al., 2017 ).
4. Conclusion and future recommendation
Beekeeping is more dependent on complex environmental factors than any other animal or food production industry. Although knowledge of honey bee viruses is still limited compared to other well- studied insect viruses, such as Baculo viruses, hence understanding of virus infections in honey bees are of great concern. In last few decades a global uncontrolled inter- and intranational exchanges and trade of honey bees and other goods have led to the spread of viral diseases to new geographical areas. Much work has been done regarding honey bee viruses’ identification, spread, history, physiochemical characteristics and infections etc. Observation of pollinators is instantly crucial to notify managing approaches and both beginning as well as advanced beekeepers should learn to recognize and control honey bee diseases. By implementing of One health approach thrugh good veterinary, beekeeping and environmental practices can be guarantee for safety of beehive products, as well as, sustainable apiculture and insects pollinators health protection patterns. Scientists and researchers from all over the world, recommend special suggestions for consistent policies that should be implemented globally to support beekeeping and its management in future.
Declaration of Competing Interest
The authors declare that they have no known competing interests.
Acknowledgements
The authors from Department of Zoology, KUST acknowledge the financial support provided by the Higher Education Commission of Pakistan under the project entitled “Exploring Pathogen Web Affecting Honey Bee Health and its Effective Treatment in Pakistan”.
Peer review under responsibility of King Saud University.
- Abou-Shaara H. The foraging behaviour of honey bees, Apis mellifera : a review. Vet. Med. 2014; 59 [ Google Scholar ]
- Ai H., Yan X., Han R. Occurrence and prevalence of seven bee viruses in Apis mellifera and Apis cerana apiaries in China. J. Invertebr. Pathol. 2012; 109 :160–164. [ PubMed ] [ Google Scholar ]
- Aizen M.A., Garibaldi L.A., Cunningham S.A., Klein A.M. How much does agriculture depend on pollinators? Lessons from long-term trends in crop production. Ann. Bot. 2009; 103 :1579–1588. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Alaux C., Dantec C., Parrinello H., Le Conte Y. Nutrigenomics in honey bees: digital gene expression analysis of pollen's nutritive effects on healthy and varroa-parasitized bees. BMC Genomics. 2011; 12 :496. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Alburaki M., Chen D., Skinner J., Meikle W., Tarpy D., Adamczyk J. Honey bee survival and pathogen prevalence: from the perspective of landscape and exposure to pesticides. Insects. 2018; 9 :65. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Allen M., Ball B. The incidence and world distribution of honey bee viruses. Bee world. 1996; 77 :141–162. [ Google Scholar ]
- Amiri E., Meixner M., Nielsen S.L., Kryger P. Four categories of viral infection describe the health status of honey bee colonies. PLoS ONE. 2015; 10 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Amiri E., Meixner M.D., Kryger P. Deformed wing virus can be transmitted during natural mating in honey bees and infect the queens. Sci. Rep. 2016; 6 :33065. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Amiri E., Seddon G., Zuluaga Smith W., Strand M.K., Tarpy D.R., Rueppell O. Israeli acute paralysis virus: Honey bee queen–worker interaction and potential virus transmission pathways. Insects. 2019; 10 :9. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Amiri E., Strand M., Rueppell O., Tarpy D. Queen quality and the impact of honey bee diseases on queen health: potential for interactions between two major threats to colony health. Insects. 2017; 8 :48. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Antúnez K., Invernizzi C., Mendoza Y., Zunino P. Honeybee colony losses in Uruguay during 2013–2014. Apidologie. 2017; 48 :364–370. [ Google Scholar ]
- Bacandritsos N., Granato A., Budge G., Papanastasiou I., Roinioti E., Caldon M. Sudden deaths and colony population decline in Greek honey bee colonies. J. Invertebr. Pathol. 2010; 105 :335–340. [ PubMed ] [ Google Scholar ]
- Ball B. Honey bee viruses: a cause for concern? Bee World. 1996; 77 :117–119. [ Google Scholar ]
- Barron A.B. Death of the bee hive: understanding the failure of an insect society. Curr. Opin. Insect Sci. 2015; 10 :45–50. [ PubMed ] [ Google Scholar ]
- Beaurepaire A., Piot N., Doublet V., Antunez K., Campbell E., Chantawannakul P. Diversity and Global Distribution of Viruses of the Western Honey Bee. Apis mellifera . Insects. 2020; 11 :239. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Becher M.A., Grimm V., Thorbek P., Horn J., Kennedy P.J., Osborne J.L. BEEHAVE: a systems model of honeybee colony dynamics and foraging to explore multifactorial causes of colony failure. J. Appl. Ecol. 2014; 51 :470–482. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Berényi O., Bakonyi T., Derakhshifar I., Köglberger H., Nowotny N. Occurrence of six honeybee viruses in diseased Austrian apiaries. Appl. Environ. Microbiol. 2006; 72 :2414–2420. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Branchiccela B., Castelli L., Corona M., Díaz-Cetti S., Invernizzi C., de la Escalera G.M. Impact of nutritional stress on the honeybee colony health. Scientific reports. 2019; 9 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Breeze T.D., Vaissière B.E., Bommarco R., Petanidou T., Seraphides N., Kozák L. Agricultural policies exacerbate honeybee pollination service supply-demand mismatches across Europe. PLoS ONE. 2014; 9 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Brodschneider R., Crailsheim K. Nutrition and health in honey bees. Apidologie. 2010; 41 :278–294. [ Google Scholar ]
- Brown M.J., Paxton R.J. The conservation of bees: a global perspective. Apidologie. 2009; 40 :410–416. [ Google Scholar ]
- Brutscher L., Baer B., Niño E. Putative drone copulation factors regulating honey bee ( Apis mellifera ) queen reproduction and health: A review. Insects. 2019; 10 :8. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Brutscher L.M., Daughenbaugh K.F., Flenniken M.L. Antiviral defense mechanisms in honey bees. Curr. Opin. Insect Sci. 2015; 10 :71–82. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Brutscher L.M., McMenamin A.J., Flenniken M.L. The buzz about honey bee viruses. PLoS Pathog. 2016; 12 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Calderone N.W. Insect pollinated crops, insect pollinators and US agriculture: trend analysis of aggregate data for the period 1992–2009. PLoS ONE. 2012; 7 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Carr-Markell M.K., Demler C.M., Couvillon M.J., Schürch R., Spivak M. Do honey bee ( Apis mellifera ) foragers recruit their nestmates to native forbs in reconstructed prairie habitats? PLoS ONE. 2020; 15 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Carreck N., Neumann P. Honey bee colony losses. J Apic Res. 2010; 49 :1–6. [ Google Scholar ]
- Carreck N.L., Ball B.V., Martin S.J. Honey bee colony collapse and changes in viral prevalence associated with Varroa destructor. J. Apic. Res. 2010; 49 :93–94. [ Google Scholar ]
- Chagas D.B., Monteiro F.L., Hübner S.d.O., Lima M.D., Fischer G. Viruses that affect Apis mellifera and their occurrence in Brazil. Ciência Rural. 2019; 49 [ Google Scholar ]
- Chantawannakul P., de Guzman L.I., Li J., Williams G.R. Parasites, pathogens, and pests of honeybees in Asia. Apidologie. 2016; 47 :301–324. [ Google Scholar ]
- Chantawannakul P., Ward L., Boonham N., Brown M. A scientific note on the detection of honeybee viruses using real-time PCR (TaqMan) in Varroa mites collected from a Thai honeybee ( Apis mellifera ) apiary. J. Invertebr. Pathol. 2006; 91 :69–73. [ PubMed ] [ Google Scholar ]
- Chapman N.C., Sheng J., Lim J., Malfroy S.F., Harpur B.A., Zayed A. Genetic origins of honey bees ( Apis mellifera ) on Kangaroo Island and Norfolk Island (Australia) and the Kingdom of Tonga. Apidologie. 2019; 50 :28–39. [ Google Scholar ]
- Chen Y., Evans J., Feldlaufer M. Horizontal and vertical transmission of viruses in the honey bee, Apis mellifera. J. Invertebr. Pathol. 2006; 92 :152–159. [ PubMed ] [ Google Scholar ]
- Chen Y., Pettis J.S., Collins A., Feldlaufer M.F. Prevalence and transmission of honeybee viruses. Appl. Environ. Microbiol. 2006; 72 :606–611. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chen Y., Zhao Y., Hammond J., Hsu H.-T., Evans J., Feldlaufer M. Multiple virus infections in the honey bee and genome divergence of honey bee viruses. J. Invertebr. Pathol. 2004; 87 :84–93. [ PubMed ] [ Google Scholar ]
- Chen Y.P., Pettis J.S., Corona M., Chen W.P., Li C.J., Spivak M. Israeli acute paralysis virus: epidemiology, pathogenesis and implications for honey bee health. PLoS Pathog. 2014; 10 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chen Y.P., Siede R. Honey bee viruses. Adv. Virus Res. 2007; 70 :33–80. [ PubMed ] [ Google Scholar ]
- Cirkovic D., Stevanovic J., Glavinic U., Aleksic N., Djuric S., Aleksic J. Honey bee viruses in Serbian colonies of different strength. PeerJ. 2018; 6 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cornman R.S., Tarpy D.R., Chen Y., Jeffreys L., Lopez D., Pettis J.S. Pathogen webs in collapsing honey bee colonies. PLoS ONE. 2012; 7 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cox-Foster D.L., Conlan S., Holmes E.C., Palacios G., Evans J.D., Moran N.A. A metagenomic survey of microbes in honey bee colony collapse disorder. Science. 2007; 318 :283–287. [ PubMed ] [ Google Scholar ]
- Dainat B., Evans J.D., Chen Y.P., Gauthier L., Neumann P. Predictive markers of honey bee colony collapse. PLoS ONE. 2012; 7 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dalmon A., Peruzzi M., Le Conte Y., Alaux C., Pioz M. Temperature-driven changes in viral loads in the honey bee Apis mellifera . J. Invertebr. Pathol. 2019; 160 :87–94. [ PubMed ] [ Google Scholar ]
- De Miranda J., Gauthier L., Ribiere M., Chen Y. Challenges and sustainable solutions; Honey bee colony health: 2012. Honey bee viruses and their effect on bee and colony health; pp. 71–102. [ Google Scholar ]
- de Miranda J.R., Bailey L., Ball B.V., Blanchard P., Budge G.E., Chejanovsky N. Standard methods for virus research in Apis mellifera . J. Apic. Res. 2013; 52 :1–56. [ Google Scholar ]
- de Miranda J.R., Cordoni G., Budge G. The acute bee paralysis virus–Kashmir bee virus–Israeli acute paralysis virus complex. J. Invertebr. Pathol. 2010; 103 :S30–S47. [ PubMed ] [ Google Scholar ]
- De Miranda J.R., Genersch E. Deformed wing virus. J. Invertebr. Pathol. 2010; 103 :S48–S61. [ PubMed ] [ Google Scholar ]
- DeGrandi-Hoffman G., Chen Y., Huang E., Huang M.H. The effect of diet on protein concentration, hypopharyngeal gland development and virus load in worker honey bees (Apis mellifera L.) J. Insect Physiol. 2010; 56 :1184–1191. [ PubMed ] [ Google Scholar ]
- Delaney D.A., Keller J.J., Caren J.R., Tarpy D.R. The physical, insemination, and reproductive quality of honey bee queens ( Apis mellifera L.) Apidologie. 2011; 42 :1–13. [ Google Scholar ]
- Desai S.D., Kumar S., Currie R.W. Occurrence, detection, and quantification of economically important viruses in healthy and unhealthy honey bee (Hymenoptera: Apidae) colonies in Canada. The Canadian Entomologist. 2016; 148 :22–35. [ Google Scholar ]
- Di Pasquale G., Salignon M., Le Conte Y., Belzunces L.P., Decourtye A., Kretzschmar A. Influence of pollen nutrition on honey bee health: do pollen quality and diversity matter? PLoS ONE. 2013; 8 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Di Prisco G., Cavaliere V., Annoscia D., Varricchio P., Caprio E., Nazzi F. Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proc. Natl. Acad. Sci. 2013; 110 :18466–18471. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dolezal A.G., Carrillo-Tripp J., Judd T.M., Allen Miller W., Bonning B.C., Toth A.L. Interacting stressors matter: diet quality and virus infection in honeybee health. R. Soc. Open Sci. 2019; 6 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dolezal A.G., Hendrix S.D., Scavo N.A., Carrillo-Tripp J., Harris M.A., Wheelock M.J. Honey bee viruses in wild bees: viral prevalence, loads, and experimental inoculation. PLoS ONE. 2016; 11 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dolezal A.G., Toth A.L. Feedbacks between nutrition and disease in honey bee health. Curr. Opin. Insect Sci. 2018; 26 :114–119. [ PubMed ] [ Google Scholar ]
- Ellis J.D., Munn P.A. The worldwide health status of honey bees. Bee World. 2005; 86 :88–101. [ Google Scholar ]
- Engel M.S. Apidae; Apis; Hymenoptera: 1999. The taxonomy of recent and fossil honey bees. [ Google Scholar ]
- Evans J.D., Saegerman C., Mullin C., Haubruge E., Nguyen B.K., Frazier M. Colony collapse disorder: a descriptive study. PLoS ONE. 2009; 4 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Evans J.D., Schwarz R.S. Bees brought to their knees: microbes affecting honey bee health. Trends Microbiol. 2011; 19 :614–620. [ PubMed ] [ Google Scholar ]
- Evans J.D., Spivak M. Socialized medicine: individual and communal disease barriers in honey bees. J. Invertebr. Pathol. 2010; 103 :S62–S72. [ PubMed ] [ Google Scholar ]
- Ferrier, P. M., Rucker, R. R., Thurman, W. N., & Burgett, M. (2018). Economic effects and responses to changes in honey bee health.
- Forsgren E., Wei S., Guiling D., Zhiguang L., Van Tran T., Tang P.T. Preliminary observations on possible pathogen spill-over from Apis mellifera to Apis cerana. Apidologie. 2015; 46 :265–275. [ Google Scholar ]
- Francis R.M., Nielsen S.L., Kryger P. Varroa-virus interaction in collapsing honey bee colonies. PLoS ONE. 2013; 8 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fujiyuki T., Takeuchi H., Ono M., Ohka S., Sasaki T., Nomoto A. Novel insect picorna-like virus identified in the brains of aggressive worker honeybees. J. Virol. 2004; 78 :1093–1100. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gajger I.T., Kolodziejek J., Bakonyi T., Nowotny N. Prevalence and distribution patterns of seven different honeybee viruses in diseased colonies: a case study from Croatia. Apidologie. 2014; 45 :701–706. [ Google Scholar ]
- Gajger I.T., Sakač M., Gregorc A. Impact of thiamethoxam on honey bee queen ( Apis mellifera carnica ) reproductive morphology and physiology. Bulletin of environmental contamination and toxicology. 2017; 99 :297–302. [ PubMed ] [ Google Scholar ]
- Galbraith D.A., Fuller Z.L., Ray A.M., Brockmann A., Frazier M., Gikungu M.W. Investigating the viral ecology of global bee communities with high-throughput metagenomics. Sci. Rep. 2018; 8 :8879. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Garibaldi L.A., Steffan-Dewenter I., Winfree R., Aizen M.A., Bommarco R., Cunningham S.A. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science. 2013; 339 :1608–1611. [ PubMed ] [ Google Scholar ]
- Gauthier L., Ravallec M., Tournaire M., Cousserans F., Bergoin M., Dainat B. Viruses associated with ovarian degeneration in Apis mellifera L. queens. PLoS ONE. 2011; 6 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gauthier L., Tentcheva D., Tournaire M., Dainat B., Cousserans F., Colin M.E. Viral load estimation in asymptomatic honey bee colonies using the quantitative RT-PCR technique. Apidologie. 2007; 38 :426–435. [ Google Scholar ]
- Geldmann J., González-Varo J.P. Conserving honey bees does not help wildlife. Science. 2018; 359 :392–393. [ PubMed ] [ Google Scholar ]
- Genersch E. Honey bee pathology: current threats to honey bees and beekeeping. Appl. Microbiol. Biotechnol. 2010; 87 :87–97. [ PubMed ] [ Google Scholar ]
- Genersch E., Aubert M. Emerging and re-emerging viruses of the honey bee ( Apis mellifera L.) Vet. Res. 2010; 41 :54. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Genersch E., Von Der Ohe W., Kaatz H., Schroeder A., Otten C., Büchler R. The German bee monitoring project: a long term study to understand periodically high winter losses of honey bee colonies. Apidologie. 2010; 41 :332–352. [ Google Scholar ]
- Genersch E., Yue C., Fries I., de Miranda J.R. Detection of deformed wing virus, a honey bee viral pathogen, in bumble bees (Bombus terrestris and Bombus pascuorum) with wing deformities. J. Invertebr. Pathol. 2006; 91 :61–63. [ PubMed ] [ Google Scholar ]
- Gisder S., Genersch E. Multidisciplinary Digital Publishing Institute; 2015. Honey bee viruses. [ Google Scholar ]
- Gisder S., Genersch E. Viruses of commercialized insect pollinators. J. Invertebr. Pathol. 2017; 147 :51–59. [ PubMed ] [ Google Scholar ]
- Goblirsch M., Huang Z.Y., Spivak M. Physiological and behavioral changes in honey bees ( Apis mellifera ) induced by Nosema ceranae infection. PLoS ONE. 2013; 8 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Goulson D., Lye G.C., Darvill B. Decline and conservation of bumble bees. Annu. Rev. Entomol. 2008; 53 :191–208. [ PubMed ] [ Google Scholar ]
- Goulson D., Nicholls B., Botias Talamantes C., Rotheray E. Combined stress from parasites, pesticides and lack of flowers drives bee declines. Science. 2015; 347 [ PubMed ] [ Google Scholar ]
- Goulson D., Nicholls E., Botías C., Rotheray E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science. 2015; 347 :1255957. [ PubMed ] [ Google Scholar ]
- Granberg F., Vicente-Rubiano M., Rubio-Guerri C., Karlsson O.E., Kukielka D., Belák S. Metagenomic detection of viral pathogens in Spanish honeybees: co-infection by aphid lethal paralysis, Israel acute paralysis and Lake Sinai viruses. PLoS ONE. 2013; 8 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Graystock P., Blane E.J., McFrederick Q.S., Goulson D., Hughes W.O. Do managed bees drive parasite spread and emergence in wild bees? International Journal for Parasitology: Parasites and Wildlife. 2016; 5 :64–75. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Graystock P., Goulson D., Hughes W.O. Parasites in bloom: flowers aid dispersal and transmission of pollinator parasites within and between bee species. Proceedings of the Royal Society B: Biological Sciences. 2015; 282 :20151371. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Grozinger C.M., Flenniken M.L. Bee viruses: Ecology, pathogenicity, and impacts. Annu. Rev. Entomol. 2019; 64 :205–226. [ PubMed ] [ Google Scholar ]
- Han F., Wallberg A., Webster M.T. From where did the W estern honeybee ( A pis mellifera ) originate? Ecol. Evol. 2012; 2 :1949–1957. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hao Y., Li J. Volume 2. Springer; 2016. Proteomic Research on Honeybee Diseases; pp. 289–298. (Agricultural Proteomics). [ Google Scholar ]
- Hellerstein, D., Hitaj, C., Smith, D., & Davis, A. (2017). Land use, land cover, and pollinator health: A review and trend analysis.
- Highfield A.C., El Nagar A., Mackinder L.C., Laure M.-L.N., Hall M.J., Martin S.J. Deformed wing virus implicated in overwintering honeybee colony losses. Appl. Environ. Microbiol. 2009; 75 :7212–7220. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hou C., Chejanovsky N. Acute paralysis viruses of the honey bee. Virol Sin. 2014; 29 :324–326. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hung K.-L.J., Kingston J.M., Albrecht M., Holway D.A., Kohn J.R. The worldwide importance of honey bees as pollinators in natural habitats. Proceedings of the Royal Society B: Biological Sciences. 2018; 285 :20172140. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Joseph J., Santibáñez F., Laguna M.F., Abramson G., Kuperman M.N., Garibaldi L.A. A spatially extended model to assess the role of landscape structure on the pollination service of Apis mellifera . Ecol. Model. 2020; 431 [ Google Scholar ]
- Klein A.-M., Vaissiere B.E., Cane J.H., Steffan-Dewenter I., Cunningham S.A., Kremen C. Importance of pollinators in changing landscapes for world crops. Proceedings of the royal society B: biological sciences. 2006; 274 :303–313. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Klein S., Cabirol A., Devaud J.-M., Barron A.B., Lihoreau M. Why bees are so vulnerable to environmental stressors. Trends Ecol. Evol. 2017; 32 :268–278. [ PubMed ] [ Google Scholar ]
- Leach, M. E., & Drummond, F. 2018. A review of native wild bee nutritional health. International Journal of Ecology. 2018,
- Levin S., Sela N., Erez T., Nestel D., Pettis J., Neumann P. New viruses from the ectoparasite mite Varroa destructor Infesting Apis mellifera and Apis cerana . Viruses. 2019; 11 :94. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Li J.L., Cornman R.S., Evans J.D., Pettis J.S., Zhao Y., Murphy C. Systemic spread and propagation of a plant-pathogenic virus in European honeybees, Apis mellifera. MBio. 2014; 5 :e00898–00813. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- López-Uribe M.M., Simone-Finstrom M. Multidisciplinary Digital Publishing Institute; 2019. Honey bee research in the US: Current state and solutions to beekeeping problems. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Martin S.J. The role of Varroa and viral pathogens in the collapse of honeybee colonies: a modelling approach. J. Appl. Ecol. 2001; 38 :1082–1093. [ Google Scholar ]
- Martin S.J., Highfield A.C., Brettell L., Villalobos E.M., Budge G.E., Powell M. Global honey bee viral landscape altered by a parasitic mite. Science. 2012; 336 :1304–1306. [ PubMed ] [ Google Scholar ]
- McMahon D.P., Fürst M.A., Caspar J., Theodorou P., Brown M.J., Paxton R.J. A sting in the spit: widespread cross-infection of multiple RNA viruses across wild and managed bees. J. Anim. Ecol. 2015; 84 :615–624. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- McMahon D.P., Natsopoulou M.E., Doublet V., Fürst M., Weging S., Brown M.J. Elevated virulence of an emerging viral genotype as a driver of honeybee loss. Proceedings of the Royal Society B: Biological Sciences. 2016; 283 :20160811. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- McMenamin A.J., Flenniken M.L. Recently identified bee viruses and their impact on bee pollinators. Curr. Opin. Insect Sci. 2018; 26 :120–129. [ PubMed ] [ Google Scholar ]
- McMenamin A.J., Genersch E. Honey bee colony losses and associated viruses. Curr. Opin. Insect Sci. 2015; 8 :121–129. [ PubMed ] [ Google Scholar ]
- Meixner M.D. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 2010; 103 :S80–S95. [ PubMed ] [ Google Scholar ]
- Molineri A., Giacobino A., Pacini A., Cagnolo N.B., Fondevila N., Ferrufino C. Risk factors for the presence of Deformed wing virus and Acute bee paralysis virus under temperate and subtropical climate in Argentinian bee colonies. Preventive veterinary medicine. 2017; 140 :106–115. [ PubMed ] [ Google Scholar ]
- Mondet F., de Miranda J.R., Kretzschmar A., Le Conte Y., Mercer A.R. On the front line: quantitative virus dynamics in honeybee ( Apis mellifera L.) colonies along a new expansion front of the parasite Varroa destructor . PLoS Pathog. 2014; 10 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mookhploy W., Kimura K., Disayathanoowat T., Yoshiyama M., Hondo K., Chantawannakul P. Capsid gene divergence of black queen cell virus isolates in Thailand and Japan honey bee species. J. Econ. Entomol. 2015; 108 :1460–1464. [ PubMed ] [ Google Scholar ]
- Morse R.A., Calderone N.W. The value of honey bees as pollinators of US crops in 2000. Bee culture. 2000; 128 :1–15. [ Google Scholar ]
- Murray E.A., Burand J., Trikoz N., Schnabel J., Grab H., Danforth B.N. Viral transmission in honey bees and native bees, supported by a global black queen cell virus phylogeny. Environ. Microbiol. 2019; 21 :972–983. [ PubMed ] [ Google Scholar ]
- Nielsen S.L., Nicolaisen M., Kryger P. Incidence of acute bee paralysis virus, black queen cell virus, chronic bee paralysis virus, deformed wing virus, Kashmir bee virus and sacbrood virus in honey bees ( Apis mellifera ) in Denmark. Apidologie. 2008; 39 :310–314. [ Google Scholar ]
- Piiroinen S., Goulson D. Chronic neonicotinoid pesticide exposure and parasite stress differentially affects learning in honeybees and bumblebees. Proceedings of the Royal Society B: Biological Sciences. 2016; 283 :20160246. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Potts S.G., Biesmeijer J.C., Kremen C., Neumann P., Schweiger O., Kunin W.E. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 2010; 25 :345–353. [ PubMed ] [ Google Scholar ]
- Potts S.G., Roberts S.P., Dean R., Marris G., Brown M.A., Jones R. Declines of managed honey bees and beekeepers in Europe. J. Apic. Res. 2010; 49 :15–22. [ Google Scholar ]
- Prisco G.D., Zhang X., Pennacchio F., Caprio E., Li J., Evans J.D. Dynamics of persistent and acute deformed wing virus infections in honey bees, Apis mellifera. Viruses. 2011; 3 :2425–2441. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ratnieks F.L., Carreck N.L. Clarity on honey bee collapse? Science. 2010; 327 :152–153. [ PubMed ] [ Google Scholar ]
- Ravoet J., De Smet L., Wenseleers T., de Graaf D.C. Vertical transmission of honey bee viruses in a Belgian queen breeding program. BMC veterinary research. 2015; 11 :61. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ray A.M., Lopez D.L., Iturralde Martinez J.F., Galbraith D.A., Rose R., Van Engelsdorp D. Distribution of recently identified bee-infecting viruses in managed honey bee ( Apis mellifera ) populations in the USA. Apidologie. 2020; 1–10 [ Google Scholar ]
- Remnant E.J., Shi M., Buchmann G., Blacquière T., Holmes E.C., Beekman M. A diverse range of novel RNA viruses in geographically distinct honey bee populations. J. Virol. 2017; 91 :e00158–00117. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Retschnig G., Williams G.R., Mehmann M.M., Yañez O., De Miranda J.R., Neumann P. Sex-specific differences in pathogen susceptibility in honey bees ( Apis mellifera ) PLoS ONE. 2014; 9 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ribière M., Olivier V., Blanchard P. Chronic bee paralysis: a disease and a virus like no other? J. Invertebr. Pathol. 2010; 103 :S120–S131. [ PubMed ] [ Google Scholar ]
- Runckel C., Flenniken M.L., Engel J.C., Ruby J.G., Ganem D., Andino R. Temporal analysis of the honey bee microbiome reveals four novel viruses and seasonal prevalence of known viruses, Nosema, and Crithidia. PLoS ONE. 2011; 6 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sammataro D., Gerson U., Needham G. Parasitic mites of honey bees: life history, implications, and impact. Annu. Rev. Entomol. 2000; 45 :519–548. [ PubMed ] [ Google Scholar ]
- Sánchez-Bayo F., Goulson D., Pennacchio F., Nazzi F., Goka K., Desneux N. Are bee diseases linked to pesticides?—A brief review. Environ. Int. 2016; 89 :7–11. [ PubMed ] [ Google Scholar ]
- Sánchez-Bayo F., Wyckhuys K.A. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019; 232 :8–27. [ Google Scholar ]
- Sande S.O., Crewe R.M., Raina S.K., Nicolson S.W., Gordon I. Proximity to a forest leads to higher honey yield: Another reason to conserve. Biol. Conserv. 2009; 142 :2703–2709. [ Google Scholar ]
- Schittny D., Yañez O., Neumann P. Honey Bee Virus Transmission via Hive Products. Veterinary Sciences. 2020; 7 :96. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Schurr F., Cougoule N., Rivière M.-P., Ribière-Chabert M., Achour H., Ádám D. Trueness and precision of the real-time RT-PCR method for quantifying the chronic bee paralysis virus genome in bee homogenates evaluated by a comparative inter-laboratory study. J. Virol. Methods. 2017; 248 :217–225. [ PubMed ] [ Google Scholar ]
- Shah K.S., Evans E.C., Pizzorno M.C. Localization of deformed wing virus (DWV) in the brains of the honeybee. Apis mellifera Linnaeus. Virology journal. 2009; 6 :182. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sihag, R. C. 2014. Phenology of migration and decline in colony numbers and crop hosts of giant honeybee (Apis dorsata F.) in semiarid environment of Northwest India. Journal of Insects. 2014,
- Smith M.R., Singh G.M., Mozaffarian D., Myers S.S. Effects of decreases of animal pollinators on human nutrition and global health: a modelling analysis. The Lancet. 2015; 386 :1964–1972. [ PubMed ] [ Google Scholar ]
- Steinhauer N., Kulhanek K., Antúnez K., Human H., Chantawannakul P., Chauzat M.-P. Drivers of colony losses. Curr. Opin. Insect Sci. 2018; 26 :142–148. [ PubMed ] [ Google Scholar ]
- O’Neal T.S., Anderson T.D., Wu-Smart J.Y. Interactions between pesticides and pathogen susceptibility in honey bees. Curr. Opin. Insect Sci. 2018; 26 :57–62. [ PubMed ] [ Google Scholar ]
- Tantillo G., Bottaro M., Di Pinto A., Martella V., Di Pinto P., Terio V. Virus infections of honeybees Apis Mellifera . Italian journal of food safety. 2015; 4 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tehel A., Brown M.J., Paxton R.J. Impact of managed honey bee viruses on wild bees. Current opinion in virology. 2016; 19 :16–22. [ PubMed ] [ Google Scholar ]
- Tentcheva D., Gauthier L., Zappulla N., Dainat B., Cousserans F., Colin M.E. Prevalence and seasonal variations of six bee viruses in Apis mellifera L. and Varroa destructor mite populations in France. Appl. Environ. Microbiol. 2004; 70 :7185–7191. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Terio V., Martella V., Camero M., Decaro N., Testini G., Bonerba E. Detection of a honeybee iflavirus with intermediate characteristics between kakugo virus and deformed wing virus. New Microbiol. 2008; 31 :439–444. [ PubMed ] [ Google Scholar ]
- Theisen-Jones H., Bienefeld K. The Asian honey bee ( Apis cerana ) is significantly in decline. Bee World. 2016; 93 :90–97. [ Google Scholar ]
- Tihelka E., Cai C., Pisani D., Donoghue P.C. Mitochondrial genomes illuminate the evolutionary history of the Western honey bee ( Apis mellifera ) Sci. Rep. 2020; 10 :1–10. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tlak Gajger I., Bičak J., Belužić R. The occurrence of honeybee viruses in apiaries in the Koprivnica-Križevci district in Croatia. Veterinarski arhiv. 2014; 84 :421–428. [ Google Scholar ]
- Van der Zee R., Pisa L., Andonov S., Brodschneider R., Charrière J.-D., Chlebo R. Managed honey bee colony losses in Canada, China, Europe, Israel and Turkey, for the winters of 2008–9 and 2009–10. J. Apic. Res. 2012; 51 :100–114. [ Google Scholar ]
- Vejsnæs F., Nielsen S.L., Kryger P. Factors involved in the recent increase in colony losses in Denmark. J. Apic. Res. 2010; 49 :109–110. [ Google Scholar ]
- Wells T., Wolf S., Nicholls E., Groll H., Lim K.S., Clark S.J. Flight performance of actively foraging honey bees is reduced by a common pathogen. Environmental microbiology reports. 2016; 8 :728–737. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wilfert L., Long G., Leggett H., Schmid-Hempel P., Butlin R., Martin S. Deformed wing virus is a recent global epidemic in honeybees driven by Varroa mites. Science. 2016; 351 :594–597. [ PubMed ] [ Google Scholar ]
- Williams G.R., Tarpy D.R., Vanengelsdorp D., Chauzat M.P., Cox-Foster D.L., Delaplane K.S. Colony collapse disorder in context. BioEssays. 2010; 32 :845–846. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yang B., Peng G., Li T., Kadowaki T. Molecular and phylogenetic characterization of honey bee viruses, Nosema microsporidia, protozoan parasites, and parasitic mites in China. Ecol. Evol. 2013; 3 :298–311. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zhang X., He S., Evans J., Pettis J., Yin G., Chen Y. New evidence that deformed wing virus and black queen cell virus are multi-host pathogens. J. Invertebr. Pathol. 2012; 109 :156–159. [ PubMed ] [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
The collection, presented in two parts, will include mini-reviews on topics relating to honey bee health and colony productivity and empirical studies from members associated with AAPA on topics of study including the full range of honey bee research. The Journal of Insect Science is an open access journal. All articles are freely available to ...
The western honey bee (Apis mellifera) is the most frequent floral visitor of crops worldwide, but quantitative knowledge of its role as a pollinator outside of managed habitats is largely lacking.Here we use a global dataset of 80 published plant-pollinator interaction networks as well as pollinator effectiveness measures from 34 plant species to assess the importance of A. mellifera in ...
Crane, E. Recent research on the world history of beekeeping. Bee World 80, 174-186 (1999). Article Google Scholar Jaffé, R. et al. Estimating the density of honeybee colonies across their ...
Further, the research reports are focused on managed honey bees, Apis mellifera in particular, with little or no information on non-managed bees 5. Hence, extrapolation of findings from these ...
The combined behaviours of individuals within insect societies determine the survival and development of the colony. For the western honey bee (Apis mellifera), individual behaviours include nest building, foraging, storing and ripening food, nursing the brood, temperature regulation, hygiene and defence. However, the various behaviours inside the colony, especially within the cells, are ...
Honey bee foragers must supply their colony with a balance of pollen and nectar to sustain optimal colony development. ... were obtained from the research apiary of Macquarie University (Sydney ...
1. Introduction. The European honey bee (Apis mellifera) is the most important managed species for agricultural pollination across the world.Despite their importance, managed honey bee colonies are experiencing annual mortality rates that now typically range between 30 to 40% in North America and Europe [1,2].These high overwintering losses have been linked to a myriad of stressors—including ...
Anthropogenic climate change and increasing antimicrobial resistance (AMR) together threaten the last 50 years of public health gains. Honey bees are a model One Health organism to investigate interactions between climate change and AMR. The objective of this scoping review was to examine the range, extent, and nature of published literature on the relationship between AMR and honey bees in ...
In this delicate and timely scenario, Molecules welcomes original research articles and reviews on bee research, with special reference to the identification of novel compounds to boost bee health, including products to fight their parasites and pathogens. In addition, as mentioned above, beekeeping is a major source of important products of ...
2. Constraints of honeybee and its products. There are many abiotic and biotic factors that that weaken honeybee-keeping and its values. Among the abiotic factors, climate change is a major factor (Langowska et al., Citation 2016).It caused the decline of honeybees by asynchronizing the season of flowering plants (Le Conte & Navajas, Citation 2008; Hegland et al., Citation 2009; Lever et al ...
1. Introduction. Honey is a natural substance produced by honey bees (Apis mellifera).They collect flower nectar, plant secretions or excretions of plant-sucking insects from plants and transform it into honey [].Worldwide, 1779.6 metric tons of honey are produced, and the market value of honey is expected to grow by 2028 [].China produces almost 28% of the world's honey, followed by Turkey ...
Still, the articles aim at providing deeper understanding to beekeepers on honey bee genetics, selection and breeding better bees. We start with two review articles. The first one is written by a team of French and Swiss authors lead by Matthieu Guichard and Benjamin Dainat from the Swiss Bee Research Centre, Agroscope, Bern, Switzerland. Their ...
The most relevant articles on honey bee parasites, fungi and their treatment, published between January 1960 and December 2020, were selected based on the keywords searched, the rigor of the methodology used, and the conclusions drawn. ... In the light of the data obtained from the present research, the honey bee is affected by several ...
The Journal of Insect Science partnered with the American Association of Professional Apiculturists to publish a special collection of scientific articles on recent research on honey bees: Honey Bee Research in the United States: Investigating Fundamental and Applied Aspects of Honey Bee Biology.. The articles are open source, so everyone can access them for free.
We individually trained 20 honey bees (Apis mellifera) on a colour discrimination task in which they learned to associate five distinct colours each with their visit history of reward and punishment.Over 18 training trials, each colour offered bees a different likelihood of reward and punishment (Figure 1A, Figure 1—source data 1).The five colours offered the reward in 100%, 66%, 50%, 33% ...
United States honey bee colony losses 2022-23: ... membership organization dedicated to public engagement in scientific research and education (EIN 53-0196483). Science News Explores;
Although they are important for agriculture, honey bees also destabilize natural ecosystems by competing with native bees—some of which are species at risk. The rise in hobby beekeeping, now a ...
Bees play a crucial role as pollinators, contributing significantly to ecosystems. However, the honeybee population faces challenges such as global warming, pesticide use, and pathogenic microorganisms. Promoting bee growth using several approaches is therefore crucial for maintaining their roles. To this end, the bacterial microbiota is well-known for its native role in supporting bee growth ...
In conclusion, honey, the principal stored food product during a substantial proportion of the lifecycle of the honey bee, likely has greater importance in honey bee health than previously recognized, particularly if bees can self-regulate induction of detoxification enzymes as they apparently self-medicate in the presence of pathogens (Gherman ...
Bee and butterfly populations are in decline in major regions of North America due to ongoing environmental change, and significant gaps in pollinator research limit our ability to protect these ...
Adult forager bees were collected in July 2021 from 23 hives in three geographically proximate locations in northern Virginia, twelve hives from Gainesville, VA (38.82° N, 77.60° W), nine hives ...
The past attempts of honey bee researchers to inventory the fungal diversity in honey bee colonies revealed that Aspergillus flavus is frequently found in hives. In a new study, researchers have ...
Source: Getty. May 7, 2024. Everyone, for so long, has been worried about the honeybees. Governments, celebrities, social-media users, small businesses, multinational conglomerates—in the two ...
SCOTT DETROW, HOST: For years now, conventional wisdom, popular slogans and ad campaigns have heralded the same message - we need to save the honeybees. Products from peanut butter to shampoo were ...
Researchers at Penn State's Center for Pollinator Research, where we both work, have examined the pollen that wild bees and managed honeybees collect to see which plants provided the most ...
Artificial insemination in queen honey bees is the only tool that provides complete control over mating for research and breeding purposes, making it essential in genetic improvement and conservation programs in this species. The aims of this study were to characterize drone semen bacterial loads by culture-dependent and independent methods and to describe their variation depending on the ...
Monitoring honeybee health with vibrations. The method involves gently introducing a vibration to the hive using a special device called an electromagnetic shaker. The shaker basically gives the ...
Viral effects on queen bee health. Pathogenic viruses are extremely hazardous to honey bee health and can cause colony losses ( Martin et al., 2012, Mondet et al., 2014, Remnant et al., 2017 ). Besides, disease causative agents, the death of queen has been considered as one of the major factors for colony failure.
According to research done by the USDA, at least 98% of wild honeybee nests in the Grand Canyon area were found to have African honeybee genes. Research in Texas found similar numbers.
Join the UF/IFAS Honey Bee Research and Extension Laboratory at the 2024 University of Florida Bee College in Panama City, Florida, for one or two days of all things honey beekeeping.Those new to beekeeping can follow the beginner track, while more experienced beekeepers can participate in hands-on training in the apiary, classes on honey bee research, and other advanced topics in beekeeping.