- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
June 4, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source

New deep brain stimulation algorithm may help personalize Parkinson's disease treatment
by Mass General Brigham
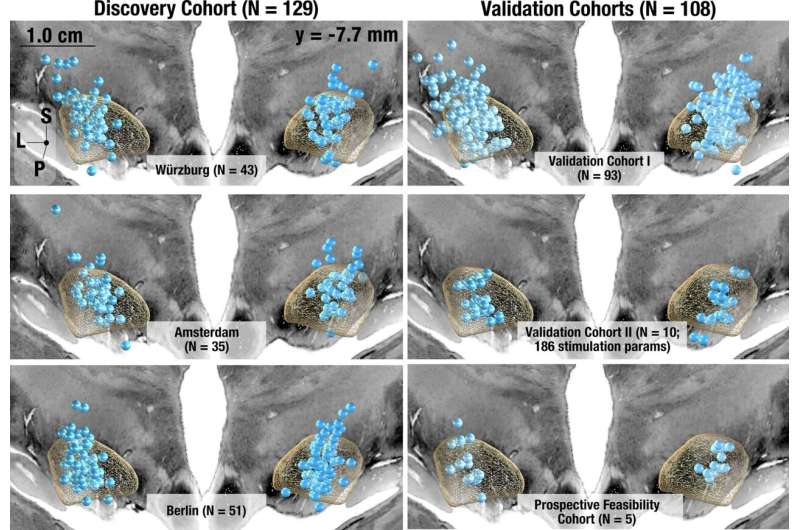
Deep brain stimulation (DBS) has shown promise as a treatment for some symptoms of Parkinson's disease (PD). However, not all symptoms improve equally well with DBS. A better understanding of how different sites of electrical stimulation impact the wide range of motor symptoms associated with PD could help fine tune treatment.
By studying PD patients at five different centers treated with DBS, investigators from Mass General Brigham have created an "atlas" that mapped four major symptoms of PD onto different regions of the brain. Based on these findings, the team created an algorithm capable of generating personalized, symptom-specific DBS treatment plans, which they preliminarily tested in five patients. Findings, published in Nature Communications , demonstrate the algorithm's potential to improve patients' symptoms beyond standard-of-care approaches.
"There is already strong evidence of improved quality of life for PD patients treated with DBS, but currently we still use a 'one-size-fits-all' approach to treatment," said senior author Andreas Horn, MD, Ph.D., a Mass General Brigham neurologist who holds titles at the Center for Brain Circuit Therapeutics in the Department of Neurology at Brigham and Women's Hospital and the Center for Neurotechnology and Neurorecovery at Massachusetts General Hospital. "The techniques we have developed will help us readily tailor DBS to what each patient specifically needs and improve DBS even further."
The researchers from Mass General Brigham studied a total of 237 patients with PD who were treated with DBS to identify tracts associated with four major PD symptoms: tremor (uncontrolled movement), bradykinesia (slow movement), rigidity (freezing), and axial symptoms (such as gait and posture irregularity or instability).
With software developed by Horn's team, the researchers pinpointed the precise location of DBS electrodes in each patient and created a common map of the circuits associated with patients' symptom improvement. Tremor was shown to improve with stimulation of tracts connected to the primary motor cortex and cerebellum, while bradykinesia was associated with the supplementary motor cortex. Rigidity was shown to improve with stimulation of the premotor cortex.
Axial symptoms, which have not received extensive study in relation to DBS, improved with stimulation of tracts connected to the supplementary motor cortex and brainstem. This finding may be especially important given that axial symptoms, such as gait or postural stability problems, typically do not respond well to DBS and existing dopaminergic therapies, such as levodopa.
Based on their findings, the investigators created Cleartune, an algorithm that suggests optimal stimulation parameters for DBS stimulation. The researchers applied Cleartune to inform treatment for five PD patients in Germany undergoing DBS. In four of the five patients, Cleartune settings led to greater improvements in PD symptoms than standard-of-care protocols. The fifth patient showed comparable improvements with Cleartune versus standard treatments.
The researchers are continuing to refine personalized, symptom-specific treatment for PD and other diseases such as OCD, in partnership with Mass General Brigham researchers, who plan to map the brain's circuitry more completely using advanced imaging technologies.
"This was an interdisciplinary effort to create the most precise atlas of symptom-specific pathways that we could," said first and corresponding author Nanditha Rajamani, Ph.D., of Mass General Brigham. "We went a long way to use anatomical information from many different sources and worked with highly skilled neuroanatomists to produce and validate this research. Going forward, this approach can be a framework for improving DBS treatments for other disorders as well."
Explore further
Feedback to editors
Metabolic parameters found to be similar in children born via frozen versus fresh embryo transfer, research shows
6 hours ago
Researchers identify key differences in inner workings of immune cells
Researchers detail molecular pathway that impacts pancreatic cancer progression and treatment response
Epstein-Barr virus and brain cross-reactivity: Possible mechanism for multiple sclerosis detected
Precision laser surgery cuts focal epileptic seizure spread
7 hours ago

Researchers find flavor restrictions affect tobacco buyers differently depending on socioeconomic status

Younger children are more commonly diagnosed with ADHD than their older classmates, says new study
Researchers say AI blood test provides a reliable way to identify lung cancer

New combination therapy shows promise for bladder cancer patients unresponsive to standard treatment

What toilet paper and game shows can teach us about the spread of epidemics
Related stories.
Researchers use deep brain stimulation to map therapeutic targets for four brain disorders
Feb 22, 2024

Brain mapping method illuminates targets for treating neuropsychiatric symptoms
Jul 8, 2021

Study finds that memory complaints can predict biological changes in the brain
May 29, 2024

Deep brain stimulation for Parkinson's disease: New algorithm for the adjustment of stimulation settings developed
Dec 21, 2022

Researchers identify targets in the brain to modulate heart rate and treat depressive disorders
Apr 26, 2024
Electrophysiological signals identify Parkinson's disease subtypes
Aug 23, 2018
Recommended for you

'Artificial lymph node' used to treat cancer in mice
8 hours ago
People feel more connected to 'tweezer-like' bionic hands in virtual reality, study shows
9 hours ago
Clinical study shows zebrafish avatars of cancer patients have high predictive power

Engineered bacteria deliver chemotherapy directly to tumors
10 hours ago
Smart hydrogel injected into intracranial fluid can measure changes in temperature, pH or pressure
Researchers create brain organoid to investigate effects of COVID-19 in people with Down syndrome
12 hours ago
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
- National Headquarters
- 1-800-223-2732
- Select Location

American Parkinson Disease Association
- Mission & Leadership
- Strategic Plan
- Financial Reports
- APDA in the News
- Career Opportunities
- Virtual Events
2023 Update: New Parkinson’s Disease Treatments in the Clinical Trial Pipeline
New Parkinson’s Medication on the Horizon
The development of potential new medications for Parkinson’s disease (PD) medications remains very active, with multiple new medications in various stages of research development that are aiming to treat and slow down PD.
In past blogs, we have reviewed the various mechanisms of action that are being studied to see if they result in successful slowing of disease progression.
These treatment mechanisms include:
Targeting abnormal alpha-synuclein aggregation.
- Increasing activity of GLP-1, a strategy which may block activation of immune cells in the brain
- Other strategies of decreasing inflammation in the brain
- Increasing the activity of the enzyme glucocerebrosidase to enhance the cell’s lysosomal or garbage disposal system
- Decreasing activity of the proteins LRRK2 or c-Abl to decrease neurodegeneration
- Improving function of the mitochondria – the energy-producing element of the nerve cell – to support the health of the neurons
- Increasing neurotrophic factors to enhance nerve survival
- Using cell based therapies to restore healthy nerves in the brain
Decreasing oxidative stress in the brain
Most of the compounds presented in prior blogs are continuing to be studied in various stages of clinical trials.
You can view these past blogs below:
- Neuroprotective strategies in clinical trials – 2020
- Neuroprotective strategies in clinical trials – update 2021
- Medications in clinical trials – 2022
- Therapies for non-motor symptoms in clinical trials
- Repurposed medications being studied for PD
Here are additional medications that we are keeping our eye on in 2023 and into 2024

You can read more about each of the clinical trials mentioned by following the links provided. Each is associated with an NCT number on clinicaltrials.gov, a database of all the clinical trials for all diseases worldwide. Each link also provides the contact information for each trial if you would like to find out more about the possibility of participating in the trial.)
Decreasing activity of LRRK2
BIIB122: One compound that is successfully moving through the research pipeline is BIIB122. We previously reported on a Phase 1 study of a small molecule LRRK2 inhibitor known at the time as DNL151. The results of that study were published , and this molecule now called BIIB122, is being tested to see its efficacy in a much larger group of people.
Mutations (a change in the DNA sequence) in the LRRK2 (Leucine-rich repeat kinase 2) gene represent a common genetic cause of PD. LRRK2 plays several roles in the cell and mutations that increase its enzymatic activity are thought to cause neurodegeneration. BIIB122 is a small molecule that decreases the activity of LRRK2. The current study NCT05418673 is evaluating whether taking BIIB122 slows the progression of PD more than placebo in the early stages of PD. The study will focus on participants with specific genetic variants in their LRRK2 gene.
Butanetap : Buntanetap is a small molecule that suppresses the translation of DNA into messenger RNA of several neurotoxic proteins. This group of neurotoxic proteins produces insoluble clumps that accumulate in nerve cells, disrupting the cell’s normal function. One of these proteins is alpha-synuclein, which abnormally accumulates in PD. In early studies, Buntanetap showed reduction of inflammation and preservation of axonal integrity and synaptic function. The current study NCT05357989 is designed tomeasure safety and efficacy of Buntanetap compared with placebo in participants with early PD.
Sulfuraphane : Sulfuraphane is an antioxidant, found in dark green vegetables such as broccoli and brussel sprouts. It is currently being studied NCT05084365 to see if it improves motor and cognitive function in PD.
Decreasing activity of the c-Abl kinase
IKT-148009 : IKT-148009 is a small molecule that decreases the activity of c-Abl, an enzyme that acts on a wide range of targets within the cell, supporting many different cellular functions. Research suggests that overactivation of c-Abl is a downstream effect of oxidative stress and may play a role in neurodegeneration in PD. There is also research to suggest that increased c-Abl activation correlates with alpha-synuclein aggregation. These findings and others led to the possibility that inhibiting c-Abl may be a helpful strategy in PD therapy. The current study NCT05424276 is investigating whether decreasing the activity of c-Abl in early, untreated people with PD is safe and tolerable, and whether it improves motor and non-motor features of the disease.
Cell-based therapy
Bemdaneprocel (BRT-DA01, previously known as MSK-DA01): A recently-completed Phase 1 study investigated the surgical transplantation of dopaminergic neuron precursor cells into the brains of people with PD. In an open label study (one without a control group) of 12 people, the treatment was found to be safe and well-tolerated. Transplantation of the cells was feasible and resulted in successful cell survival and engraftment. A phase 2 study is currently being planned for early 2024.
Decreasing inflammation
RO-7486967/selnoflast: – RO-7486967 is a small molecule that inhibits the NLRP3 inflammasome, a complex of proteins involved in inflammation that is thought to be overactive in PD. The current study NCT05924243 will investigate whether this molecule is safe and tolerable in early stages of PD.
New mechanism of action: Targeting cell death
KM819: Apoptosis, a series of organized molecular steps that leads to programmed cell death, is a normal part of cell function. When this system goes awry however, cells may die when they are not supposed to. KM819 is a small molecule inhibitor of Fas-associated factor1 (FAF1), a key regulator of cell death. It is being investigated to see if decreasing the process of cell death will protect neurons in PD. The current study NCT05670782 is testing this compound in both healthy adults and people with PD.
The Parkinson’s Hope List
We continue to thank Dr. Kevin McFarthing, a biochemist and person with Parkinson’s for his efforts in creating and maintaining The Parkinson’s Hope List — a collation of all the compounds that are being explored as new therapies for PD at all stages of the research pipeline and is updated frequently. It is an excellent source of information for those interested in the current state of PD research focused on new potential treatments. APDA was privileged to host Dr. McFarthing as a special guest on our broadcast entitled Dr. Gilbert Hosts:Taking Research From the Lab to our Lives .
Dr. McFarthing and his colleagues put together a yearly review of the medications for Parkinson’s disease in clinical trials. The year 2023’s review can be accessed here . Dr. McFarthing and colleagues reported that as of January 2023, there were nearly 139 Parkinson’s therapies active in the clinical trial pipeline as registered on the www.clinicaltrials.gov website involving almost 17,000 participants. Of these drugs tested, 76 (55%) trials were focused on symptomatic treatment (STs), medications that attempt ameliorate the symptoms of PD; and 63 (45%) were disease-modifying therapies (DMTs), medications that attempt to slow the progression of the disease. The pipeline grew in the past year, with 35 newly registered trials (18 ST and 17 DMT trials). Most of these clinical trials (34%) are in Phase 1 (early-stage of clinical testing, primarily performed to assess for safety), while 52% have progressed to Phase 2 testing stage (mid-stage, performed in small numbers of people with PD to assess for efficacy), followed by 14% currently in Phase 3 (late-stage trials, performed in larger numbers of people with PD to assess for efficacy).
APDA proudly funds innovative work
APDA recently announced its newly-funded research grantees for the 2023-2024 academic year. Our new pool of grantees are working on many of the strategies discussed above and will continue to push the field of PD research forward, introducing new ideas to the field and new possibilities in PD therapy.
Here are some examples:
- Dr. Nikhil Panicker is investigating the NRLP3 inflammasome. He is exploring whether reducing the activation of the inflammasome within microglia can protect neurons from accumulating alpha-synuclein in a cell model of PD.
- Dr. William Zeiger is studying the mechanisms by which the abnormal accumulation of alpha-synuclein cause thinking and memory problems in PD.
- Dr. Naemeh Pourshafie is studying the relationship between tau and alpha-synuclein, two proteins that abnormally accumulate in neurodegenerative diseases.
We are so proud to help make this vital work possible!
Tips and takeaways
- There is hope in progress, with multiple treatment strategies in the PD research pipeline.
- Potential treatments are generally divided into two large categories: disease modifying therapies and symptomatic treatments.
- Mechanisms of action that are being studied to alter the progression of PD include: decreasing activity of LRRK2, decreasing aggregation of alpha-synuclein, decreasing oxidative stress in the brain, decreasing activity of c-Abl, introducing dopaminergic neurons into the brain, decreasing inflammation, and inhibiting programmed cell death.
- APDA supports essential research, bringing new ideas to fruition in the treatment of PD. Read more about past work we have funded, and the projects that we are funding this year.
- We need your support in order to continue this extremely valuable research. Click here to make a donation.
Support Our Mission
To support your local 2023 Update: New Parkinson’s Disease Treatments in the Clinical Trial Pipeline chapter please click the button below:
chapter-content-page
training-courses
apda-in-the-news
css_apda_events
css_apda_event_recur
tutor_assignments
popupbuilder
Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Social justice
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
Ultrasound offers a new way to perform deep brain stimulation
Press contact :.
Previous image Next image
Deep brain stimulation, by implanted electrodes that deliver electrical pulses to the brain, is often used to treat Parkinson’s disease and other neurological disorders. However, the electrodes used for this treatment can eventually corrode and accumulate scar tissue, requiring them to be removed.
MIT researchers have now developed an alternative approach that uses ultrasound instead of electricity to perform deep brain stimulation, delivered by a fiber about the thickness of a human hair. In a study of mice, they showed that this stimulation can trigger neurons to release dopamine, in a part of the brain that is often targeted in patients with Parkinson’s disease.
“By using ultrasonography, we can create a new way of stimulating neurons to fire in the deep brain,” says Canan Dagdeviren, an associate professor in the MIT Media Lab and the senior author of the new study. “This device is thinner than a hair fiber, so there will be negligible tissue damage, and it is easy for us to navigate this device in the deep brain.”
In addition to offering a potentially safer way to deliver deep brain stimulation, this approach could also become a valuable tool for researchers seeking to learn more about how the brain works.
MIT graduate student Jason Hou and MIT postdoc Md Osman Goni Nayeem are the lead authors of the paper, along with collaborators from MIT’s McGovern Institute for Brain Research, Boston University, and Caltech. The study appears today in Nature Communications .
Deep in the brain
Dagdeviren’s lab has previously developed wearable ultrasound devices that can be used to deliver drugs through the skin or perform diagnostic imaging on various organs . However, ultrasound cannot penetrate deeply into the brain from a device attached to the head or skull.
“If we want to go into the deep brain, then it cannot be just wearable or attachable anymore. It has to be implantable,” Dagdeviren says. “We carefully customize the device so that it will be minimally invasive and avoid major blood vessels in the deep brain.”
Deep brain stimulation with electrical impulses is FDA-approved to treat symptoms of Parkinson’s disease. This approach uses millimeter-thick electrodes to activate dopamine-producing cells in a brain region called the substantia nigra. However, once implanted in the brain, the devices eventually begin to corrode, and scar tissue that builds up surrounding the implant can interfere with the electrical impulses.
The MIT team set out to see if they could overcome some of those drawbacks by replacing electrical stimulation with ultrasound. Most neurons have ion channels that are responsive to mechanical stimulation, such as the vibrations from sound waves, so ultrasound can be used to elicit activity in those cells. However, existing technologies for delivering ultrasound to the brain through the skull can’t reach deep into the brain with high precision because the skull itself can interfere with the ultrasound waves and cause off-target stimulation.
“To precisely modulate neurons, we must go deeper, leading us to design a new kind of ultrasound-based implant that produces localized ultrasound fields,” Nayeem says. To safely reach those deep brain regions, the researchers designed a hair-thin fiber made from a flexible polymer. The tip of the fiber contains a drum-like ultrasound transducer with a vibrating membrane. When this membrane, which encapsulates a thin piezoelectric film, is driven by a small electrical voltage, it generates ultrasonic waves that can be detected by nearby cells.
“It’s tissue-safe, there’s no exposed electrode surface, and it’s very low-power, which bodes well for translation to patient use,” Hou says.
In tests in mice, the researchers showed that this ultrasound device, which they call ImPULS (Implantable Piezoelectric Ultrasound Stimulator), can provoke activity in neurons of the hippocampus. Then, they implanted the fibers into the dopamine-producing substantia nigra and showed that they could stimulate neurons in the dorsal striatum to produce dopamine.
“Brain stimulation has been one of the most effective, yet least understood, methods used to restore health to the brain. ImPULS gives us the ability to stimulate brain cells with exquisite spatial-temporal resolution and in a manner that doesn’t produce the kind of damage or inflammation as other methods. Seeing its effectiveness in areas like the hippocampus opened an entirely new way for us to deliver precise stimulation to targeted circuits in the brain,” says Steve Ramirez, an assistant professor of psychological and brain sciences at Boston University, and a faculty member at B.U.’s Center for Systems Neuroscience, who is also an author of the study.
A customizable device
All of the components of the device are biocompatible, including the piezoelectric layer, which is made of a novel ceramic called potassium sodium niobate, or KNN. The current version of the implant is powered by an external power source, but the researchers envision that future versions could be powered a small implantable battery and electronics unit.
The researchers developed a microfabrication process that enables them to easily alter the length and thickness of the fiber, as well as the frequency of the sound waves produced by the piezoelectric transducer. This could allow the devices to be customized for different brain regions.
“We cannot say that the device will give the same effect on every region in the brain, but we can easily and very confidently say that the technology is scalable, and not only for mice. We can also make it bigger for eventual use in humans,” Dagdeviren says.
The researchers now plan to investigate how ultrasound stimulation might affect different regions of the brain, and if the devices can remain functional when implanted for year-long timescales. They are also interested in the possibility of incorporating a microfluidic channel, which could allow the device to deliver drugs as well as ultrasound.
In addition to holding promise as a potential therapeutic for Parkinson’s or other diseases, this type of ultrasound device could also be a valuable tool to help researchers learn more about the brain, the researchers say.
“Our goal to provide this as a research tool for the neuroscience community, because we believe that we don’t have enough effective tools to understand the brain,” Dagdeviren says. “As device engineers, we are trying to provide new tools so that we can learn more about different regions of the brain.”
The research was funded by the MIT Media Lab Consortium and the Brain and Behavior Foundation Research (BBRF) NARSAD Young Investigator Award.
Share this news article on:
Related links.
- Canan Dagdeviren
- Conformable Decoders Group
- School of Architecture and Planning
Related Topics
- Neuroscience
- Brain and cognitive sciences
- Medical devices
- Parkinson's
Related Articles

A new ultrasound patch can measure how full your bladder is
Soft optical fibers block pain while moving and stretching with the body

A wearable ultrasound scanner could detect breast cancer earlier
Previous item Next item
More MIT News

A data-driven approach to making better choices
Read full story →

Paying it forward

John Fucillo: Laying foundations for MIT’s Department of Biology

Researchers demonstrate the first chip-based 3D printer

The unexpected origins of a modern finance tool

Exotic black holes could be a byproduct of dark matter
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram
New therapeutic target for Parkinson’s disease discovered
- Feinberg School of Medicine
Northwestern Medicine scientists have uncovered a new mechanism by which mutations in a gene parkin contribute to familial forms of Parkinson's disease. The discovery opens a new avenue for Parkinson’s therapeutics, scientists report in a new study.
The Northwestern scientists discovered that mutations in parkin result in a breakdown of contacts between two key workers in the cell — lysosomes and mitochondria.
Mitochondria are the main producers of energy in cells, and lysosomes recycle cellular debris that accumulates during normal function of our cells. These organelles are especially important in our brains because neurons are highly dependent on energy production by mitochondria, and because of their activity, neurons produce an abundance of cellular debris that must be cleared by lysosomes.
In a prior study, published in Nature, Dr. Dimitri Krainc, chair of neurology and director of Simpson Querrey Center for Neurogenetics at Northwestern University Feinberg School of Medicine, and his group discovered that lysosomes and mitochondria form contacts with each other. After the initial discovery, Northwestern scientists tried to understand the function of these contacts in Parkinson’s disease.
In the new study published July 19 in Science Advances, the investigators report that lysosomes help mitochondria by providing key metabolites for their function. Mitochondria must import many of their essential ingredients, but it has not been well known where some of these metabolites come from. On the other hand, lysosomes serve as recycling factories in cells and, therefore, produce many breakdown products that could be used by other organelles such as mitochondria.
In this work, scientists found that lysosomes provide important amino acids that support the function of mitochondria. However, they also found that in some forms of Parkinson’s disease, lysosomes cannot serve as a “helping hand” to mitochondria because the contacts between the two organelles are disrupted. This results in dysfunctional mitochondria and ultimately degeneration of vulnerable neurons in Parkinson’s disease.
“Findings from this study suggest that dysregulation of mitochondria-lysosome contacts contributes to the Parkinson's disease pathophysiology,” said Krainc, the study’s corresponding author. “We propose that restoring such mitochondria-lysosome contacts represents an important new therapeutic opportunity for Parkinson’s disease.”
From a broader perspective, this study opens a new avenue of research in neurodegenerative disorders, by highlighting the importance of direct communication and collaboration between cellular organelles in the pathogenesis of these disorders.
The first author of the study is Dr. Wesley Peng who recently completed the medical scientist training program (MD-PhD) at Northwestern and currently serves as a neurology resident at Mass General Brigham and Harvard Medical School. Other contributors to the study include Leonie Schroder, Pingping Song and Yvette Wong.
Editor’s Picks

This algorithm makes robots perform better
‘the night watchman’ named next one book selection, six northwestern faculty elected to american academy of arts and sciences, related stories.

As we age, we grow more lonely
Rogan grant named a schmidt science fellow, these workers can be pivotal in improving u.s. health equity.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Recent developments in the treatment of Parkinson's Disease
Thomas b stoker.
1 John van Geest Centre for Brain Repair, Department of Clinical Neurosciences, University of Cambridge, Forvie Site, Robinson Way, Cambridge, CB2 0PY, UK
2 Department of Neurology, Norfolk and Norwich University Hospital, Norwich, UK
Roger A Barker
3 Wellcome Trust – Medical Research Council Stem Cell Institute, University of Cambridge, Cambridge, UK
Parkinson’s disease (PD) is a common neurodegenerative disease typified by a movement disorder consisting of bradykinesia, rest tremor, rigidity, and postural instability. Treatment options for PD are limited, with most of the current approaches based on restoration of dopaminergic tone in the striatum. However, these do not alter disease course and do not treat the non-dopamine-dependent features of PD such as freezing of gait, cognitive impairment, and other non-motor features of the disorder, which often have the greatest impact on quality of life. As understanding of PD pathogenesis grows, novel therapeutic avenues are emerging. These include treatments that aim to control the symptoms of PD without the problematic side effects seen with currently available treatments and those that are aimed towards slowing pathology, reducing neuronal loss, and attenuating disease course. In this latter regard, there has been much interest in drug repurposing (the use of established drugs for a new indication), with many drugs being reported to affect PD-relevant intracellular processes. This approach offers an expedited route to the clinic, given that pharmacokinetic and safety data are potentially already available. In terms of better symptomatic therapies that are also regenerative, gene therapies and cell-based treatments are beginning to enter clinical trials, and developments in other neurosurgical strategies such as more nuanced deep brain stimulation approaches mean that the landscape of PD treatment is likely to evolve considerably over the coming years. In this review, we provide an overview of the novel therapeutic approaches that are close to, or are already in, clinical trials.
Introduction
Parkinson’s disease (PD) is a common neurodegenerative disease characterised by a movement disorder consisting of bradykinesia, rest tremor, and rigidity, along with postural instability, a range of other more-subtle motor features, and many non-motor features 1 . Many of the core motor features result from the loss of a specific population of neurons: the dopaminergic neurons of the substantia nigra pars compacta, which project axons to the striatum 2 , 3 . As such, most of the current pharmacological treatment approaches for PD aim to restore dopaminergic tone in the striatum.
Whilst often effective at improving motor function, current treatments are associated with significant side effects due to delivery of dopamine to extra-striatal regions, variability in their absorption and transit across the blood–brain barrier, and the non-physiological continuous release of dopamine and its effects on the dopamine receptors within the basal ganglia 4 , 5 . Patients frequently develop cognitive problems, levodopa-induced dyskinesias, and on-off fluctuations, which we have estimated to occur in 46%, 56%, and 100% of cases, respectively, at 10 years from diagnosis based on data from our ongoing community-based incident study in PD 6 , 7 . All of these factors coupled with some of the neuropsychiatric features of PD have a significant impact on quality of life in advancing PD. Many features of PD (such as cognitive impairment and autonomic dysfunction) have a mainly non-dopaminergic basis, resulting from neurodegeneration at other sites in the central nervous system as well as the enteric and autonomic nervous systems 3 , 8 . It is often these features that have the most detrimental impact on the quality of life of patients with PD, yet treatment options remain limited for these elements of disease.
Levodopa, the precursor of dopamine, was first developed for the treatment of PD in the 1960s and continues to be the most-effective therapeutic agent for PD in 2020 9 . Other dopaminergic drugs have since been used, including inhibitors of dopamine metabolism as well as dopamine receptor agonists, but these are generally less well tolerated and less effective. Thus, there is an urgent need for better therapies, including disease-modifying treatments. However, the requirement for relevant pre-clinical disease models for testing such agents and the lack of robust biomarkers for diagnosing PD and the identification of prodromal disease, which would allow for treatment before significant neuronal loss had occurred, pose barriers to drug discovery.
It is on this background that a number of new developments are emerging that may transform the management of PD over the coming years, and we will now discuss those that are in, or soon to be in, clinical trials ( Figure 1 ).

An expanding number of drugs are being considered for their ability to influence the pathogenic processes of PD. These include novel agents and technologies, such as active and passive immunisation and RNA interference techniques to limit the propagation, and synthesis, of α-synuclein. Additionally, several drugs used for other conditions are of interest for potential use in PD given their ability to influence pathways such as the lysosome–autophagy system, mitochondrial function, and neuroinflammation, for example. Abbreviations: α-syn, α-synuclein; ASO, anti-sense oligonucleotide; GCase, glucocerebrosidase; PD, Parkinson’s disease; RNA, ribonucleic acid; UDCA, ursodeoxycholic acid.
Immunotherapies
The pathological hallmark of PD is the presence of abnormal aggregates of α-synuclein 10 . The role of α-synuclein in PD is not clear, but it is presumed to play a central pathogenic role, as demonstrated by the fact that mutations or duplications/triplications of the gene ( SNCA ) cause rare familial forms of PD 11 , coupled with many independent studies showing the detrimental effects of manipulating α-synuclein in cell and animal models 12 , 13 . Potential pathogenic mechanisms of α-synuclein include dysfunction of vesicular transport, perturbations in the lysosome–autophagy system, mitochondrial dysfunction, and oxidative stress, for example 14 . It has also been proposed that pathological forms of α-synuclein can act in a prion-like fashion, allowing pathology to spread from cell to cell, and the “strains” underlying this are now being identified 15 . This in turn means the disease follows a pattern of pathology that results from the sequential involvement of a number of anatomical structures. All of this suggests that therapies designed to reduce levels of α-synuclein or the propagation of toxic “strains” may limit PD progression 8 .
One experimental approach to restricting the propagation of α-synuclein is to use antibodies to target and degrade extracellular α-synuclein and thus prevent it from “infecting” neighbouring cells. Passive and active immunisation techniques against α-synuclein have been shown to convey neuroprotective effects in animal models, with the results of early clinical trials in humans starting to emerge 14 . Other approaches to reducing α-synuclein levels include anti-sense oligonucleotide and ribonucleic acid (RNA) interference techniques to reduce its synthesis, though these remain in pre-clinical stages and are thus not discussed in detail here 16 – 18 .
A humanised monoclonal antibody targeting the C-terminus of aggregated α-synuclein (prasinezumab or PRX002, Prothena) has been shown to reduce free serum α-synuclein by approximately 97% and to be well tolerated in phase I clinical trials 19 , 20 , with a phase II trial currently underway ( {"type":"clinical-trial","attrs":{"text":"NCT03100149","term_id":"NCT03100149"}} NCT03100149 ). Another antibody, BIIB054 (Biogen), targeting the N-terminal portion of α-synuclein reduces the propagation of α-synuclein pathology and improves the motor phenotype in a PD model involving injection of α-synuclein pre-formed fibrils into mice 21 . This antibody has also been found to be well tolerated in humans 22 and is under investigation in a phase II clinical trial ( {"type":"clinical-trial","attrs":{"text":"NCT03318523","term_id":"NCT03318523"}} NCT03318523 ).
The company AFFiRiS are approaching this problem in a different way by investigating a range of treatments consisting of α-synuclein fragments or α-synuclein-mimicking epitopes designed to induce an active immune response against α-synuclein, with phase I trials completed ( {"type":"clinical-trial","attrs":{"text":"NCT01568099","term_id":"NCT01568099"}} NCT01568099 and {"type":"clinical-trial","attrs":{"text":"NCT02267434","term_id":"NCT02267434"}} NCT02267434 ). These products have been administered subcutaneously in early trials and seem to be well tolerated. One of these, AFFITOPE PD01A, conveyed a dose-dependent immune response to the peptide itself and to α-synuclein and is now being taken forward to phase II trials 14 .
The use of immunotherapies to limit the propagation of PD pathology is an interesting avenue for further exploration, but important questions remain, not least the extent to which PD in the clinic is driven by protein spread. In addition, the ability of these antibodies to cross the blood–brain barrier and influence α-synuclein homeostasis in the brain is potentially an obstacle for their use in the clinic. Furthermore, neuroprotective effects of such immunotherapies appear in part to be due to intracellular effects, and their ability to enter cells may influence their efficacy. Engineered fragments (intrabodies and nanobodies) may allow for greater central nervous system penetration and entry to the cell, but these are yet to enter clinical trials 23 . Another concern is the potential consequences of suppressing the physiological function of α-synuclein, an abundant protein whose function is incompletely understood. Suppression of α-synuclein levels in some models has been shown to be detrimental 24 – 27 , and evaluation of the long-term safety of this approach will be important. It is for this reason that some groups have sought to reduce α-synuclein through drug therapies, including the repurposing of β-agonists (see below).
Drug repurposing
An alternative approach to limit PD pathology and disease progression is through the use of drugs that reduce α-synuclein pathology or have beneficial effects on other processes implicated in PD ( Table 1 ). In particular, there is a great deal of interest in drug repurposing (using established drugs for a new indication), which would potentially lead to an expedited path to the clinic, given that safety and pharmacokinetic data may already be available. Here we discuss some of the most promising agents being considered for the treatment of PD ( Figure 1 ).
Abbreviations: ATP, adenosine triphosphate; G-CSF, granulocyte colony-stimulating factor; GLP-1, glucagon-like peptide-1; HSP90, heat shock protein 90; PGK1, phosphoglycerate kinase-1; Treg, regulatory T cell; UPDRS, Unified Parkinson’s Disease Rating Scale.
One class that is under consideration, but yet to enter clinical trials, is the β-adrenergic receptor agonists, given recent epidemiological and in vitro work demonstrating an association with reduced α-synuclein levels and risk of PD, thought to be mediated through modulation of SNCA transcription 28 . Given that such agents are widely used in the treatment of reversible airway obstruction, and have been for many years, moving this to the clinic should be relatively straightforward.
Of those that have gone to clinical trials, the glucagon-like peptide-1 (GLP-1) analogue exenatide, which is used for the treatment of type two diabetes mellitus, has advanced the most. This agent has been trialled in PD patients after a similar compound (exendin-4) was found to convey neuroprotective effects in cell and animal models of nigral degeneration 29 – 31 . Several mechanisms have been proposed to mediate this effect through GLP-1 receptor activation, including inhibition of apoptosis, reduced microglial activation and neuroinflammation, reduced oxidative stress, and promotion of neurogenesis 32 . In an initial open-label trial, exenatide was found to be safe in PD patients (though some experienced problems with weight loss), and there was an associated improvement in cognitive and motor function, which persisted after cessation of treatment 33 . This was followed by a double-blind randomised placebo-controlled trial, which reported that once-weekly exenatide was associated with a reduction in Unified Parkinson’s Disease Rating Scale (UPDRS) motor scores in comparison to the placebo group 40 . A multicentre phase III trial is currently in set-up, in which participants will receive weekly exenatide or placebo ( {"type":"clinical-trial","attrs":{"text":"NCT04232969","term_id":"NCT04232969"}} NCT04232969 ). A pegylated form of exenatide (NLY01), which harbours enhanced pharmacokinetic properties, has also recently been taken to a phase I trial in healthy volunteers, with results awaited ( {"type":"clinical-trial","attrs":{"text":"NCT03672604","term_id":"NCT03672604"}} NCT03672604 ).
Another repurposed drug that has been trialled for PD is nilotinib. This is an ABL tyrosine kinase inhibitor used in the treatment of chronic myelogenous leukaemia. ABL activity inhibits the activity of Parkin, which is important in the initiation of mitophagy, and nilotinib is proposed to enhance autophagy activity, potentially reducing the accumulation of α-synuclein aggregates 34 . An initial phase I trial reported that the drug was well tolerated and safe, with preliminary reports of benefits on motor and cognitive function 43 . However, there was no placebo group in this study, and some of the clinical effects observed may have been due to baseline differences between the groups and withdrawal of monoamine oxidase inhibitors in a number of subjects 44 . Nevertheless, nilotinib has now progressed to randomised placebo-controlled trials ( {"type":"clinical-trial","attrs":{"text":"NCT03205488","term_id":"NCT03205488"}} NCT03205488 and {"type":"clinical-trial","attrs":{"text":"NCT02954978","term_id":"NCT02954978"}} NCT02954978 ), and it appears to reduce the ratio of pathogenic oligomeric α-synuclein to total α-synuclein in the cerebrospinal fluid (CSF) 45 . However, a recent press release for the NILO-PD trial showed that, while safe and tolerable, nilotinib did not offer any clinical benefit.
Terazosin, an α 1 -adrenergic antagonist used in benign prostatic hypertrophy, has recently emerged as a putative treatment for PD. Terazosin has been found to activate phosphoglycerate kinase-1 and the chaperone protein HSP90, which is involved in multiple intracellular stress responses 46 . It has been shown to have neuroprotective effects in neurotoxin models of nigrostriatal degeneration in invertebrates and rodents, including after delayed administration 35 . Additionally, terazosin reduced α-synuclein levels in transgenic mice and in neurons derived from patients with LRRK2 mutation-associated PD 35 . Furthermore, a retrospective epidemiological study found that people taking terazosin have a reduced relative risk of PD 35 . These promising findings have led to terazosin rapidly progressing to a randomised placebo-controlled trial, which will involve 20 patients with Hoehn and Yahr stage 3 PD ( {"type":"clinical-trial","attrs":{"text":"NCT03905811","term_id":"NCT03905811"}} NCT03905811 ). However, terazosin reduces blood pressure and can cause orthostatic hypotension, which is a problem in many patients with advancing PD and may limit its applicability in this disease.
In addition to targeting α-synuclein clearance pathways, drugs that target other intracellular pathways may be useful in PD. For example, ursodeoxycholic acid (UCDA), a drug used to treat primary biliary cirrhosis, has been found to restore mitochondrial function in cells derived from patients carrying PARKIN and LRRK2 mutations as well as in invertebrate and rodent models of PD 47 – 49 . UCDA has recently progressed to a randomised placebo-controlled phase II trial, which is currently in the process of recruiting 30 patients with early PD ( {"type":"clinical-trial","attrs":{"text":"NCT03840005","term_id":"NCT03840005"}} NCT03840005 ). A number of other agents are currently in, or have recently completed, clinical trials, which are summarised in Table 1 .
Advances in our understanding of pathogenic subtypes of PD may allow for the targeting of specific pathogenic mechanisms in subgroups of PD patients. One such group is patients carrying GBA1 mutations, found in approximately 5% of so-called sporadic PD patients 50 – 52 . The GBA1 gene encodes the lysosomal enzyme glucocerebrosidase, the activity of which has been found to be reduced in PD patients without GBA1 mutations, making it an interesting therapeutic target for a wider PD population. These mutations are associated with dysfunction of the lysosome–autophagy system, important in α-synuclein clearance 53 , 54 . Some GBA1 mutations have been shown to lead to misfolding of glucocerebrosidase, which impairs its delivery to the lysosomal compartment, leading to perturbations in α-synuclein processing 54 . Ambroxol, historically used as an expectorant, has recently been trialled in patients with GBA1 mutation-associated PD, as it has been shown to facilitate the re-folding of glucocerebrosidase and increase its activity in human cells and transgenic mice with subsequent reduction in α-synuclein levels 55 , 56 . The results of the first open-label clinical trial of ambroxol in PD patients with and without GBA1 mutations (AiM-PD) have recently been published, where the drug was found to be well tolerated over 6 months, with an associated rise in CSF glucocerebrosidase levels 57 .
Alternatively, the glucocerebrosidase pathway may be targeted through glucosylceramide synthase inhibitors, which reduce levels of the glucocerebrosidase substrates glucosylceramide and glucosylsphingosine. Such substrate reduction therapies have been used in Gaucher disease (caused by biallelic mutations in the GBA1 gene), but the role of these substrates in PD pathogenesis is disputed 58 . A phase II clinical trial of a glucosylceramide synthase inhibitor (venglustat) in PD patients with GBA1 variants is currently underway (MOVES-PD, {"type":"clinical-trial","attrs":{"text":"NCT02906020","term_id":"NCT02906020"}} NCT02906020 ).
Targeting non-dopaminergic neurotransmitter systems
Though many of the motor features of PD are dopamine responsive, for others, such as freezing of gait and tremor, dopamine offers little benefit. It is now understood that deficiencies in other neurotransmitter systems underlie some of these features 59 . As such, there is interest in modulating their function to treat specific dopamine-resistant aspects of PD.
One novel drug that has recently received approval for use in PD is safinamide, a drug that is proposed to have multi-modal actions. It is a potent reversible monoamine oxidase B inhibitor, conveying a benefit for the treatment of dopaminergic aspects of PD. It also modulates glutamate transmission, which may be implicated in some of the non-motor features of PD 60 , 61 . In a multicentre phase III clinical trial involving 669 patients with moderate to advanced PD, safinamide resulted in improved UPDRS motor scores, reduced off-time, and improvements in depression and communication scores 62 . Safinamide is now becoming more widely available for clinical use, though its exact role is yet to be determined. Currently, it is most likely to be used as an adjunct to levodopa-based therapies, particularly in those who experience problematic dyskinesias and fluctuations.
Additionally, the cholinesterase inhibitors rivastigmine and donepezil have been trialled for their ability to reduce falls in PD, with promising preliminary results 63 , 64 . The noradrenaline reuptake inhibitors methylphenidate and atomoxetine are also currently being investigated for their effects on balance and gait in PD in an ongoing trial ( {"type":"clinical-trial","attrs":{"text":"NCT02879136","term_id":"NCT02879136"}} NCT02879136 ). Serotoninergic neurons in the dorsal raphe nucleus have been proposed to contribute to levodopa-induced dyskinesias, and the use of serotonin agonists has been seen to reduce such dyskinesias in animal models 65 – 67 . However, their use has been accompanied by worsening of other motor features of PD in some clinical studies 68 . However, advances in our understanding of the role of the serotoninergic system in the development of levodopa-induced dyskinesias means that there is ongoing interest in modulation of this system as a therapeutic option 69 .

Neurotrophic factors
Neurotrophic factors such as glial cell line-derived neurotrophic factor (GDNF) have beneficial effects on dopaminergic neurons in pre-clinical models, and there has been much interest in developing neuroprotective therapies based on these 70 , 71 .
Open-label studies of intraputaminal GDNF infusion have seen improvements in motor UPDRS scores 72 , 73 , with some evidence of restoration of the nigrostriatal pathway pathologically and on imaging 74 . However, randomised double-blind trials have failed to recapitulate these results, including a recent study in the UK 75 , 76 . However, there has been much discussion about why these open and double-blind studies have produced such varying results, which led to a workshop in 2019 where these issues were addressed; the conclusions of which have recently been published 77 . Whilst GDNF studies have thus far yielded mixed results, this remains an exciting experimental approach with ongoing interest. Variable results in these trials may in part be due to the involvement of patients with moderately advanced PD, inadequate follow-up times, and the large placebo effect (which is often seen in clinical trials for PD).
Neurturin, a GDNF analogue, has also been trialled in patients, with similar results to those seen with GDNF, namely promising open-label trials that have failed to translate to clinical benefit in larger trials 78 – 81 . Nevertheless, determination of the most-appropriate patients, improvement in delivery systems, and development of novel neurotrophic factor analogues mean that this approach remains an avenue of interest and is currently being explored in a new EU-funded trial looking at cerebral dopamine neurotrophic factor (CDNF, Herantis Pharma). It has recently been reported in a press release that the agent can be delivered without major side effects, although it is too early to say whether it has therapeutic benefits for patients.
Regenerative treatments
As well as the pharmacological approaches described above, there is considerable interest in the use of cell-based and gene therapies to replace the function of the lost dopaminergic neurons. The aim of these treatments is to restore dopaminergic tone in a more targeted and physiological manner than can be achieved with current dopaminergic therapies. Several of these approaches are now entering clinical trials 82 .
Gene therapies may be used to increase dopamine levels in the striatum through the introduction of genes that mediate dopamine synthesis. Tyrosine hydroxylase (TH) is needed for the production of the dopamine precursor levodopa, which in turn is converted to dopamine by DOPA decarboxylase, also termed aromatic L-amino acid decarboxylase (AADC). Two gene therapies involving the genes encoding these enzymes are currently undergoing clinical trials for PD.
Voyager Therapeutics have developed an adeno-associated virus (AAV) therapy containing the gene for AADC (VY-AADC). This therapy has entered a phase I clinical trial, in which 15 patients with advanced PD are receiving the treatment at three different doses. It is introduced into the putamen, with preliminary reports suggesting that the treatment is well tolerated. The effects seem encouraging, particularly given that the volume of agent delivered covers a large part of the target structure (the putamen), with corresponding increases in enzyme activity. These benefits correlated with a dose-dependent reduction in levodopa dose 83 . A randomised sham-surgery controlled phase II trial is also ongoing ( {"type":"clinical-trial","attrs":{"text":"NCT03562494","term_id":"NCT03562494"}} NCT03562494 ).
A tricistronic lentivirus vector is also currently undergoing clinical trials. This treatment consists of the genes encoding AADC, TH, and GTP cyclohydrolase 1 (which catalyses the rate-limiting step of tetrahydrobiopterin synthesis, a cofactor required for the synthesis of dopamine and serotonin). The first iteration of this treatment to enter trials, OXB-101 or ProSavin®, was assessed in an open-label phase I trial involving 15 patients with advanced PD 84 . The treatment was well tolerated, with no serious adverse effects related to the treatment, with improvements in “off” state UPDRS scores at 12 months. However, the extent of improvement was not sufficient to make this therapy competitive. However, an improved version of this gene therapy with greater potency, OXB-102 or AXO-Lenti-PD, is currently in a two-part clinical trial in which safety will be assessed at multiple doses before progression to a randomised double-blind trial ( {"type":"clinical-trial","attrs":{"text":"NCT03720418","term_id":"NCT03720418"}} NCT03720418 ).
Cell-based therapies offer another emerging approach for the targeted replacement of dopamine to treat the dopamine-dependent aspects of PD. Cell-grafting with human foetal ventral mesencephalon has been taking place since the 1980s, and whilst this has been seen to be effective in some cases with patients able to come off dopaminergic medication for sustained periods, it has become clear that logistical barriers regarding the supply of adequate tissue will prevent this from ever being a useful treatment in itself 85 – 88 . Nevertheless, a renewable source of dopaminergic cells would make cell-based therapies potentially feasible, assuming they can be shown to have sustained clinical benefits to patients.
Stem cells offer a renewable source of dopaminergic neuron progenitor cells that can be grafted into patients, and clinical trials of such products are now underway ( Table 2 ). Whilst controversial trials involving parthenogenetic stem cell-derived neural stem cells have been ongoing for several years 89 , new stem cell products developed on the back of robust pre-clinical data are now progressing to trials 82 . A clinical trial of dopaminergic progenitors derived from induced pluripotent stem cells (iPSCs) has begun (Center for iPS Cell Research and Application, Kyoto University, Japan). In this trial, seven patients will receive bilateral grafts of allogenic iPSC-derived cells. Trials involving embryonic stem cell (ESC)-derived cells are underway in China ( {"type":"clinical-trial","attrs":{"text":"NCT03119636","term_id":"NCT03119636"}} NCT03119636 ) 90 and in set-up in the USA (NYSTEM-PD) and the UK/Sweden (STEM-PD trial). A number of other trials using ESC-derived neurons and allogenic and autologous iPSC-derived neurons are expected to commence over the next 2 to 3 years.
Abbreviations: ESC, embryonic stem cell; FDA, US Food and Drug Administration; iPSC, induced pluripotent stem cell.
Advances in deep brain stimulation
Deep brain stimulation (DBS) is another established treatment for PD that is useful in treating dopamine-dependent motor symptoms when levodopa-induced side effects become particularly problematic. DBS involves the surgical implantation of electrodes that stimulate subcortical structures including the subthalamic nucleus and globus pallidus internus 91 – 94 . DBS offers significant improvements in motor symptoms and fluctuations in comparison to best medical therapy in some advanced PD patients, but dopamine-resistant symptoms other than tremor (e.g. gait disturbance and postural instability) respond poorly 95 . It has also been suggested in an open-label trial that DBS is beneficial in early PD patients, with improved tremor scores and reduced development of de novo tremor 96 . In addition to surgical complications, DBS strategies may cause cognitive and neuropsychiatric adverse effects as well as speech dysfunction. Novel DBS approaches, including adaptive DBS, targeting different regions, and refined intra-operative imaging techniques promise to offer improved clinical applicability and reduce the impact of adverse effects 97 .
The pedunculopontine nucleus has recently been trialled as a new target for DBS, particularly for the gait problems seen in PD. While initial trials reported positive impacts on gait and postural instability, more rigorous subsequent trials were less promising, in part because of variability in the anatomical definition of the pedunculopontine nucleus in the human brain, suboptimal programming settings, and low patient numbers 61 , 98 . More recently, stimulation of the substantia nigra reticularis has shown promising effects on axial symptoms in preliminary studies 99 along with stimulation of the basal forebrain (with STN) for some of the cognitive deficits in PD 100 . In another pilot study, thoracic spinal cord stimulation significantly reduced the frequency of freezing episodes in patients with advanced PD, with trials ongoing 101 .
There is great interest in adaptive DBS, a system in which the stimulation delivered to the target is adjusted in response to physiological signals 61 . This approach theoretically limits adverse effects, improves clinical response, and reduces the requirements for battery changes and the associated cost. Further work is required in identifying and validating a reliable host signal 102 , but it is hoped that such technologies will enhance the clinical utility of DBS in the future. Non-invasive DBS techniques involving the use of external devices delivering electric fields to deep structures would circumvent the need for neurosurgery and its associated risks 103 . One such approach that has been used more for patients with essential tremor than PD involves using magnetic resonance imaging-focussed ultrasound lesioning of discrete brain structures. Reports on the long-term efficacy of these therapies are awaited 104 .
A wide variety of experimental treatment approaches for PD have progressed towards the clinic over recent years. Many previous putative treatments have fallen by the wayside when taken to clinical trials, despite being backed up by promising pre-clinical results, emphasising the need for robust trial design. A greater understanding of the pathogenic mechanisms and anatomical basis for PD symptoms has opened up avenues for new treatment modalities, and it now seems probable that the management of PD will evolve significantly over the coming years.
[version 1; peer review: 2 approved]
Funding Statement
The authors acknowledge financial support from the following organisations: Medical Research Council and Wellcome Trust Stem Cell Institute (Cambridge 203151/Z/16/Z), National Institute for Health Research (NIHR) (NF-SI-0616-10011), NIHR Biomedical Research Centre (reference number 146281), and the Cure Parkinson’s Trust. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
- Fredric P. Manfredsson , Parkinson's Disease Research Unit, Department of Neurobiology, Barrow Neurological Institute, Phoenix, Arizona, USA No competing interests were disclosed.
- Tipu Z. Aziz , Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, UK No competing interests were disclosed.
Queensland researchers target gut health to slow or stop progression of Parkinson's disease
Australian researchers are working on developing drugs that target bugs in the guts of Parkinson's disease patients in a radical new treatment approach they hope will slow or even stop the progression of the debilitating illness.
Queensland University of Technology neuroscientist Richard Gordon said the research followed emerging evidence suggesting the gut was as important as the brain in the development of Parkinson's.
Associate Professor Gordon, based at the Translational Research Institute in Brisbane, said studies showed differences in the complex gut ecosystems of Parkinson's disease patients compared with healthy people.
He said people with Parkinson's disease were known to experience persistent inflammation and activation of the immune system, believed to be closely linked to an imbalance of microbes in their guts.
"The inflammation, over a prolonged period, has been shown to damage the vulnerable dopamine-producing neurons that are gradually lost in people with Parkinson's," Associate Professor Gordon said.
Two years ago, Ross Martin began to notice a tremor in his hand.
The 66-year-old Queenslander is not the first in his family to be diagnosed with Parkinson's — his uncle also had the disease.
"Being on a computer, you go to play a game or something [and] your left hand is hitting all the wrong keys," he said.
"It's the little things at the moment, but the longer-term things and knowing where I'm heading and what life I'm heading towards is something I don't want to think about too much."
He said the the study gave him hope.
"We are pro-science, and it's obvious the good work is being done," Mr Martin said.
Rise in cases linked to 'chemical exposure'
Parkinson's disease is a progressive movement disorder, characterised by degeneration of dopamine-producing neurons in the brain.
The decrease in dopamine levels results in impaired mobility – including tremors, stiffness of the arms and legs, slow movement, and poor balance.
Other symptoms can include an impaired sense of smell, disturbed sleep, anxiety and depression, fatigue, gut problems, and speech changes.
Drug treatments, such as levodopa, which increases the amount of dopamine in the brain, help alleviate some patients' symptoms rather than slow the progression of the illness.
In what he described as "a radical new way of thinking" about Parkinson's disease, Associate Professor Gordon's team has been awarded $4 million over four years by the US Department of Defense to work on new therapeutics targeting the gut microbiome.
He said military personnel were considered at increased risk of developing neurological conditions, such as Parkinson's disease, because of chemical exposures during their service.
"There is this rapid increase in the prevalence of Parkinson's globally," Associate Professor Gordon said.
"We believe it's linked to … chemical exposures."
The gut is a new target
The Queensland research will involve both human and animal studies to identify new classes of therapeutics to treat Parkinson's disease, first described more than 200 years ago by London doctor, James Parkinson.
Scientists will study blood, urine, and faecal samples from at least 70 Parkinson's patients and compare them to those of similarly aged healthy volunteers.
"One of the ways we study the gut microbiome is by sequencing the bacteria that's present in people's guts," Associate Professor Gordon said.
They hope to be able to identify so-called "healthy bugs" that may disappear in people with Parkinson's.
"Then we're going to use that knowledge to develop drugs, or improve the drugs that we have, to target the microbes rather than just target the brain, which we've done in the past," Associate Professor Gordon said.
'Bugs as drugs'
In what he termed a "bugs as drugs" approach, he said the team would also engineer bacteria and test their potential to slow or stop Parkinson's progression by altering the gut ecosystem.
"These studies would be done in animals initially," he said.
"Once we know that it's safe and it's effective the next phase of this work will take that towards clinical trials."
The research team includes scientists from QUT's School of Biomedical Sciences and neurologists from the Royal Brisbane and Women's and Princess Alexandra hospitals.
They will partner with researchers at the University of Georgia in the US.
An estimated 200,000 Australians have Parkinson's disease.
- X (formerly Twitter)
Related Stories
Deep brain electrodes, adjusted by wi-fi, are bringing 'miraculous' results to parkinson's patients.
Perfect Match helped them find each other 31 years ago. Now they're facing their greatest challenge
Parkinson's can show up in the way you move '15 to 20 years' before a diagnosis — could motion capture tech spot it?
- Academic Research
- Brain and Nervous System
- Doctors and Medical Professionals
- Medical Research
- Parkinson s Disease
JavaScript is currently disabled. Please enable JavaScript for an optimal experience.
- ${result.term}
Research News
Read the latest developments, reporting and analysis from the world of Parkinson's research, including progress made in studies, tools and collaborations funded by The Michael J. Fox Foundation.
- Alpha-synuclein

Episode 16: Studying Basal Ganglia Circuits and Developing Deep Brain Stimulation Protocols for Longer-Lasting Effects with Aryn Gittis
June 4, 2024
In Memoriam: MJFF Mourns Loss of Parkinson’s Research Leader Ira Shoulson
May 22, 2024

Episode 15: Innovative Fellowship Program is Training Tomorrow's Parkinson's Leaders with Rachel Dolhun
May 21, 2024
Episode 14: Evidence Linking Parkinson's Disease Risk and Environmental Exposure to Trichloroethylene (TCE) with Sam Goldman
May 7, 2024

What We Fund: $34.2M Toward Light Therapy for Sleep, Freezing of Gait and More
April 29, 2024
Episode 13: New Advances in Neurosurgical Interventions for Parkinson's Disease with Doris Wang
April 16, 2024

Results of Parkinson’s Trial for Diabetes Drug Lixisenatide Published
April 5, 2024

Join the Study that's Changing Everything
The Parkinson's Progression Markers Initiative is changing how patients, families, doctors and scientists think about brain disease. Now it needs you.

Patients First
Our Foundation exists for one reason: to speed breakthroughs patients can feel in their everyday lives.

- Immediate family member has PD
- Immediate family member had PD
- Extended family member or friend has PD
- Extended family member or friend had PD
- I am a researcher, clinician or work with the PD community
New therapeutic target for Parkinson's disease discovered
Restoring contacts between mitochondria and lysosomes improves neuronal function.
Northwestern Medicine scientists have uncovered a new mechanism by which mutations in a gene parkin contribute to familial forms of Parkinson's disease. The discovery opens a new avenue for Parkinson's therapeutics, scientists report in a new study.
The Northwestern scientists discovered that mutations in parkin result in a breakdown of contacts between two key workers in the cell -- lysosomes and mitochondria.
Mitochondria are the main producers of energy in cells, and lysosomes recycle cellular debris that accumulates during normal function of our cells. These organelles are especially important in our brains because neurons are highly dependent on energy production by mitochondria, and because of their activity, neurons produce an abundance of cellular debris that must be cleared by lysosomes.
In a prior study, published in Nature, Dr. Dimitri Krainc, chair of neurology and director of Simpson Querrey Center for Neurogenetics at Northwestern University Feinberg School of Medicine, and his group discovered that lysosomes and mitochondria form contacts with each other. After the initial discovery, Northwestern scientists tried to understand the function of these contacts in Parkinson's disease.
In the new study published in Science Advances, the investigators report that lysosomes help mitochondria by providing key metabolites for their function. Mitochondria must import many of their essential ingredients, but it has not been well known where some of these metabolites come from. On the other hand, lysosomes serve as recycling factories in cells and, therefore, produce many breakdown products that could be used by other organelles such as mitochondria.
In this work, scientists found that lysosomes provide important amino acids that support the function of mitochondria. However, they also found that in some forms of Parkinson's disease, lysosomes cannot serve as a "helping hand" to mitochondria because the contacts between the two organelles are disrupted. This results in dysfunctional mitochondria and ultimately degeneration of vulnerable neurons in Parkinson's disease.
"Findings from this study suggest that dysregulation of mitochondria-lysosome contacts contributes to the Parkinson's disease pathophysiology," said Krainc, the study's corresponding author. "We propose that restoring such mitochondria-lysosome contacts represents an important new therapeutic opportunity for Parkinson's disease."
From a broader perspective, this study opens a new avenue of research in neurodegenerative disorders, by highlighting the importance of direct communication and collaboration between cellular organelles in the pathogenesis of these disorders.
The first author of the study is Dr. Wesley Peng who recently completed the medical scientist training program (MD-PhD) at Northwestern and currently serves as a neurology resident at Mass General Brigham and Harvard Medical School. Other contributors to the study include Leonie Schroder, Pingping Song and Yvette Wong.
The study was supported by the following National Institute on Aging grant AG066333, National Institute of Neurological Disorders and Stroke (NINDS) grants NS109252 and NS122257, all from the National Institutes of Health.
- Parkinson's Research
- Chronic Illness
- Nervous System
- Diseases and Conditions
- Parkinson's
- Disorders and Syndromes
- Schizophrenia
- Alzheimer's disease
- Gene therapy
- Parkinson's disease
- Drug discovery
- Excitotoxicity and cell damage
- Vector (biology)
Story Source:
Materials provided by Northwestern University . Note: Content may be edited for style and length.
Journal Reference :
- Wesley Peng, Leonie F. Schröder, Pingping Song, Yvette C. Wong, Dimitri Krainc. Parkin regulates amino acid homeostasis at mitochondria-lysosome (M/L) contact sites in Parkinson’s disease . Science Advances , 2023; 9 (29) DOI: 10.1126/sciadv.adh3347
Cite This Page :
Explore More
- Babies and AI Both Learn Key Foundation Models
- Myelination May Drive Drug Addiction
- Freshwater On Earth 4 Billion Years Ago
- Extended Battle: 3,500-Year-Old Mycenaean Armor
- Oral Insulin Drops: Relief for Diabetes Patients
- AIs Are Irrational, but Not Like Humans
- Bronze Age Cuisine of Mongolian Nomads
- Poor Quality Diet Makes Our Brains Sad
- AI Improves Performance Across Fusion Devices
- New Explanation for Why Earthquakes Happen
Trending Topics
Strange & offbeat.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
Progression subtypes in Parkinson’s disease identified by a data-driven multi cohort analysis.
- Tamara Raschka
- Holger Fröhlich
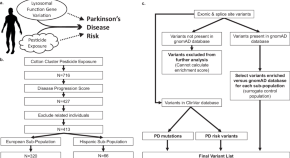
Lysosomal genes contribute to Parkinson’s disease near agriculture with high intensity pesticide use
- Kathie J. Ngo
- Kimberly C. Paul
- Brent L. Fogel
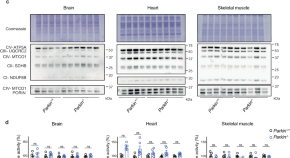
PARKIN is not required to sustain OXPHOS function in adult mammalian tissues
- Roberta Filograna
- Jule Gerlach
- Nils-Göran Larsson
Announcements
5 questions with our deputy editor.
Get to know our new Deputy Editor, Dr. David Breen, as he answers 5 questions about his research and experience and shares his thoughts about becoming involved with the journal.
Neuromodulation in movement disorders
Submit your work to our Collection edited by Aparna Shukla and Muthuraman Muthuraman
npj Parkinson's Disease Journal Metrics
npj Parkinson’s Disease has a 2-year impact factor of 8.7 (2022), article downloads of 468,446 (2022) and 12 days from submission to first editorial decision (2022).
Part of the npj Series

Published in partnership with

Advertisement
Browse articles
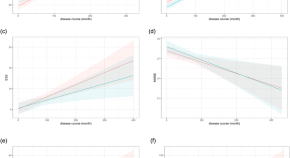
Disease progression in proposed brain-first and body-first Parkinson’s disease subtypes
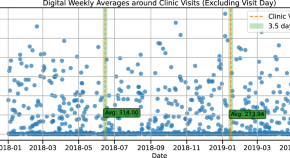
Digital outcome measures from smartwatch data relate to non-motor features of Parkinson’s disease
- Ann-Kathrin Schalkamp
- Neil A. Harrison
- Cynthia Sandor
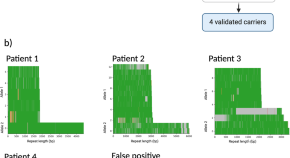
Profiling complex repeat expansions in RFC1 in Parkinson’s disease
- Pilar Alvarez Jerez
- Kensuke Daida
- Kimberley J. Billingsley
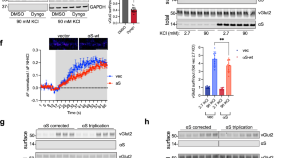
A stem cell-based assay platform demonstrates alpha-synuclein dependent synaptic dysfunction in patient-derived cortical neurons
- Andrew J. White
- Karis A. Clark
- Gary P. H. Ho
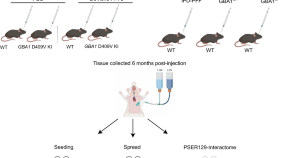
Neither alpha-synuclein fibril strain nor host murine genotype influences seeding efficacy
- Sara Walton
- Alexis Fenyi
- Jeffrey H. Kordower
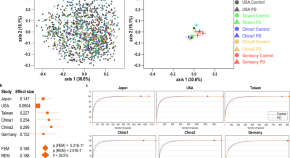
Meta-analysis of shotgun sequencing of gut microbiota in Parkinson’s disease
- Hiroshi Nishiwaki
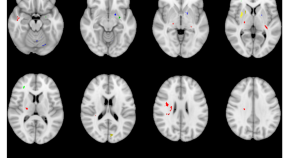
Microstructure predicts non-motor outcomes following deep brain stimulation in Parkinson’s disease
- Philipp A. Loehrer
- Miriam H. A. Bopp
- Marcus Belke
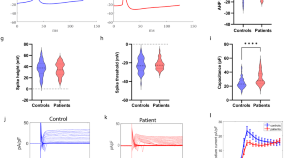
Upregulated ECM genes and increased synaptic activity in Parkinson’s human DA neurons with PINK1 / PRKN mutations
- Utkarsh Tripathi
- Shani Stern
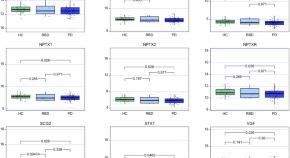
Lysosomal and synaptic dysfunction markers in longitudinal cerebrospinal fluid of de novo Parkinson’s disease
- Michael Bartl
- Johanna Nilsson
- Brit Mollenhauer
Trending - Altmetric
Glucocerebrosidase activity and lipid levels are related to protein pathologies in Parkinson’s disease
Misfolded protein deposits in Parkinson’s disease and Parkinson’s disease-related cognitive impairment, a [11C]PBB3 study
Science jobs
Postdoc fellow / senior scientist.
The Yakoub and Sulzer labs at Harvard Medical School-Brigham and Women’s Hospital and Columbia University
Boston, Massachusetts (US)
Harvard Medical School and Brigham and Women's Hospital
Postdoctoral Research Associate
Qualifications: PhD degree in chemistry, radiochemistry, or nuclear medicine technology with at least 3 years of PET radiochemistry work experience i
Charlottesville, Virginia
University of Virginia Health
Physician-Scientist in Neurology
About us...The Luxembourg Centre for Systems Biomedicine (LCSB) is an interdisciplinary research centre of the University of Luxembourg. We conduct fu
LCSB – Luxembourg Centre for Systems Biomedicine
Postdoctoral Researcher (f/m/x)
Become part of the DZNE and support us in realizing our research strategy! Support us full-time at the site Magdeburg as Postdoctoral Researcher.
Magdeburg, Sachsen-Anhalt (DE)
Deutsches Zentrum für Neurodegenerative Erkrankungen e.V. (DZNE)
Researcher at the Department of Biomedicine
At the Faculty of Medicine, Department of Biomedicine, a full-time permanent position as researcher is available.
Bergen (By), Vestland (NO)
University of Bergen
This journal is a member of and subscribes to the principles of the Committee on Publication Ethics.

Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

Parkinson's Disease
What is parkinson's disease.
Parkinson's disease (PD) is movement disorder of the nervous system that gets worse over time. As nerve cells (neurons) in parts of the brain weaken, are damaged, or die, people may begin to notice problems with movement, tremor, stiffness in the limbs or the trunk of the body, or impaired balance. As symptoms progress, people may have difficulty walking, talking, or completing other simple tasks. Not everyone with one or more of these symptoms has PD, as the symptoms appear in other diseases as well.
There is no cure for PD, but research is ongoing and medications or surgery can often provide substantial improvement with motor symptoms.
The four primary symptoms of PD are:
- Tremor—Tremor (shaking) often begins in a hand, although sometimes a foot or the jaw is affected first. The tremor associated with PD has a characteristic rhythmic back-and-forth motion that may involve the thumb and forefinger and appear as a “pill rolling.” It is most obvious when the hand is at rest or when a person is under stress. This tremor usually disappears during sleep or improves with a purposeful, intended movement.
- Rigidity—Rigidity (muscle stiffness), or a resistance to movement, affects most people with PD. The muscles remain constantly tense and contracted so that the person aches or feels stiff. The rigidity becomes obvious when another person tries to move the individual's arm, which will move only in short, jerky movements known as “cogwheel” rigidity.
- Bradykinesia—This is a slowing down of spontaneous and automatic movement that can be particularly frustrating because it may make simple tasks difficult. Activities once performed quickly and easily—such as washing or dressing—may take much longer. There is often a decrease in facial expressions (also known as "masked face").
- Postural instability—Impaired balance and changes in posture can increase the risk of falls.
PD does not affect everyone the same way. The rate of progression and the particular symptoms differ among individuals. PD symptoms typically begin on one side of the body. However, the disease eventually affects both sides, although symptoms are often less severe on one side than on the other.
People with PD often develop a so-called parkinsonian gait that includes a tendency to lean forward, taking small quick steps as if hurrying (called festination), and reduced swinging in one or both arms. They may have trouble initiating movement (start hesitation), and they may stop suddenly as they walk (freezing).
Other problems may accompany PD, such as:
- Depression—Some people lose their motivation and become dependent on family members.
- Emotional changes—Some people with PD become fearful and insecure, while others may become irritable or uncharacteristically pessimistic.
- Difficulty with swallowing and chewing—Problems with swallowing and chewing may occur in later stages of the disease. Food and saliva may collect in the mouth and back of the throat, which can result in choking or drooling. Getting adequate nutrition may be difficult.
- Speech changes—About half of all individuals with PD have speech difficulties that may be characterized as speaking too softly or in a monotone. Some may hesitate before speaking, slur, or speak too fast.
- Urinary problems or constipation—Bladder and bowel problems can occur due to the improper functioning of the autonomic nervous system, which is responsible for regulating smooth muscle activity.
- Skin problems—The skin on the face may become oily, particularly on the forehead and at the sides of the nose. The scalp may become oily too, resulting in dandruff. In other cases, the skin can become very dry.
- Sleep problems—Common sleep problems in PD include difficulty staying asleep at night, restless sleep, nightmares and emotional dreams, and drowsiness or sudden sleep onset during the day. Another common problem is “REM behavior disorder,” in which people act out their dreams, potentially resulting in injury to themselves or their bed partners. The medications used to treat PD may contribute to some sleep issues. Many of these problems respond to specific therapies.
- Dementia or other cognitive problems—Some people with PD develop memory problems and slow thinking. Cognitive problems become more severe in the late stages of PD, and some people are diagnosed with Parkinson's disease dementia (PDD). Memory, social judgment, language, reasoning, or other mental skills may be affected.
- Orthostatic hypotension—Orthostatic hypotension is a sudden drop in blood pressure when a person stands up from a lying down or seated position. This may cause dizziness, lightheadedness, and, in extreme cases, loss of balance or fainting. Studies have suggested that, in PD, this problem results from a loss of nerve endings in the sympathetic nervous system, which controls heart rate, blood pressure, and other automatic functions in the body. The medications used to treat PD may also contribute.
- Muscle cramps and dystonia—The rigidity and lack of normal movement associated with PD often causes muscle cramps, especially in the legs and toes. PD can also be associated with dystonia—sustained muscle contractions that cause forced or twisted positions. Dystonia in PD is often caused by fluctuations in the body's level of dopamine (a chemical in the brain that helps nerve cells communicate and is involved with movement).
- Pain—Muscles and joints may ache because of the rigidity and abnormal postures often associated with the disease.
- Fatigue and loss of energy—Many people with PD often have fatigue, especially late in the day. Fatigue may be associated with depression or sleep disorders, but it may also result from muscle stress or from overdoing activity when the person feels well. Fatigue may also result from akinesia, which is trouble initiating or carrying out movement.
- Sexual dysfunction—Because of its effects on nerve signals from the brain, PD may cause sexual dysfunction. PD-related depression or use of certain medications may also cause decreased sex drive and other problems.
- Hallucinations, delusions, and other psychotic symptoms can be caused by the drugs prescribed for PD.
Diseases and conditions that resemble PD
PD is the most common form of parkinsonism, which describes disorders of other causes that produce features and symptoms that closely resemble Parkinson's disease. Many disorders can cause symptoms similar to those of PD, including:
- Multiple system atrophy (MSA) refers to a set of slowly progressive disorders that affect the central and autonomic nervous systems. The protein alpha-synuclein forms harmful filament-like aggregates in the supporting cells in the brain called oligodendroglia. MSA may have symptoms that resemble PD. It may also take a form that primarily produces poor coordination and slurred speech, or it may involve a combination of these symptoms. MSA with parkinsonian symptoms is sometimes referred to as MSA-P (or striatonigral degeneration).
- Lewy body dementia is associated with the same abnormal protein deposits (Lewy bodies) found in Parkinson's disease but appears in areas throughout the brain. Symptoms may range from primary parkinsonian symptoms such as bradykinesia, rigidity, tremor, and shuffling walk, to symptoms similar to those of Alzheimer's disease (memory loss, poor judgment, and confusion). These symptoms may fluctuate (vary) dramatically. Other symptoms may include visual hallucinations, psychiatric disturbances such as delusions and depression, and problems with cognition.
- Progressive supranuclear palsy (PSP) is a rare, progressive brain disorder caused by a gradual deterioration of cells in the brain stem. Symptoms may include problems with control of gait and balance (people often tend to fall early in the course of PSP), an inability to move the eyes, and alterations of mood and behavior, including depression and apathy as well as mild dementia. PSP is characterized by clumps of a protein called tau.
- Corticobasal degeneration (CBD) results from atrophy of multiple areas of the brain, including the cerebral cortex and the basal ganglia. Initial symptoms may first appear on one side of the body, but eventually affect both sides. Symptoms include rigidity, impaired balance, and problems with coordination. Other symptoms may include dystonia that affects one side of the body, cognitive and visual-spatial impairments, apraxia (loss of the ability to make familiar, purposeful movements), hesitant and halting speech, myoclonus (muscular jerks), and dysphagia (difficulty swallowing). CBD also is characterized by deposits of the tau protein.
Several diseases, including MSA, CBD, and PSP, are sometimes referred to as “Parkinson's-plus” diseases because they have the symptoms of PD plus additional features.
In very rare cases, parkinsonian symptoms may appear in people before the age of 20. This condition is called juvenile parkinsonism. It often begins with dystonia and bradykinesia, and the symptoms often improve with levodopa medication.
Who is more likely to get Parkinson's disease ?
Risk factors for PD include:
- Age—The average age of onset is about 70 years, and the incidence rises significantly with older age. However, a small percent of people with PD have “early-onset” disease that begins before the age of 50.
- Biological sex—PD affects more men than women.
- Heredity—People with one or more close relatives who have PD have an increased risk of developing the disease themselves. An estimated 15 to 25 percent of people with PD have a known relative with the disease. Some cases of the disease can be traced to specific genetic mutations.
- Exposure to pesticides—Studies show an increased risk of PD in people who live in rural areas with increased pesticide use.
The precise cause of PD is unknown, although some cases are hereditary and can be traced to specific genetic mutations. Most cases are sporadic—that is, the disease does not typically run in families. It is thought that PD likely results from a combination of genetics and exposure to one or more unknown environmental factors that trigger the disease.
Parkinson's disease occurs when nerve cells, or neurons, in the brain die or become impaired. Although many brain areas are affected, the most common symptoms result from the loss of neurons in an area near the base of the brain called the substantia nigra. The neurons in this area produce dopamine. Dopamine is the chemical messenger responsible for transmitting signals between the substantia nigra and the next “relay station” of the brain, the corpus striatum, to produce smooth, purposeful movement. Loss of dopamine results impaired movement.
Studies have shown that most people with Parkinson's have lost 60 to 80 percent or more of the dopamine-producing cells in the substantia nigra by the time symptoms appear. People with PD also lose the nerve endings that produce the neurotransmitter norepinephrine—the main chemical messenger to the part of the nervous system that controls many automatic functions of the body, such as pulse and blood pressure. The loss of norepinephrine might explain several of the non-motor features seen in PD, including fatigue and abnormalities of blood pressure regulation.
The protein alpha-synuclein—The affected brain cells of people with PD contain Lewy bodies—deposits of the protein alpha-synuclein. Researchers do not yet know why Lewy bodies form or what role they play in the disease. Some research suggests that the cell's protein disposal system may fail in people with PD, causing proteins to build up to harmful levels and trigger cell death. Additional studies have found evidence that clumps of protein that develop inside brain cells of people with PD may contribute to the death of neurons.
Genetics—Several genetic mutations are associated with PD, including the alpha-synuclein gene, and many more genes have been tentatively linked to the disorder. The same genes and proteins that are altered in inherited cases may also be altered in sporadic cases by environmental toxins or other factors.
Environment—Exposure to certain toxins has caused parkinsonian symptoms in rare circumstances (such as exposure to MPTP, an illicit drug, or in miners exposed to the metal manganese). Other still-unidentified environmental factors may also cause PD in genetically susceptible individuals.
Mitochondria—Mitochondria are the energy-producing components of the cell and abnormalities in the mitochondria are major sources of free radicals—molecules that damage membranes, proteins, DNA, and other parts of the cell. This damage is often referred to as oxidative stress. Oxidative stress-related changes, including free radical damage to DNA, proteins, and fats, have been detected in the brains of individuals with PD. Some mutations that affect mitochondrial function have been identified as causes of PD.
Genes linked to PD
Several genes have been definitively linked to PD:
- SNCA—This gene, which makes the protein alpha-synuclein, was the first gene identified to be associated with Parkinson's. Research findings by the National Institutes of Health (NIH) and other institutions prompted studies of the role of alpha-synuclein in PD, which led to the discovery that Lewy bodies seen in all cases of PD contain clumps of alpha-synuclein. This discovery revealed the link between hereditary and sporadic forms of the disease.
- LRRK2—Mutations in LRRK2 were originally identified in several English and Basque families as a cause of a late-onset PD. Subsequent studies have identified mutations of this gene in other families with PD (such as European Ashkenazi Jewish families) as well as in a small percentage of people with apparently sporadic PD. LRRK2 mutations are a major cause of PD in North Africa and the Middle East.
- DJ-1—This gene helps regulate gene activity and protect cells from oxidative stress and can cause rare, early forms of PD.
- PRKN (Parkin)—The parkin gene is translated into a protein that helps cells break down and recycle proteins.
- PINK1—PINK1 codes for a protein active in mitochondria. Mutations in this gene appear to increase susceptibility to cellular stress. PINK1 has been linked to early forms of PD.
- GBA (glucocerebrosidase-beta)—Mutations in GBA cause Gaucher disease (in which fatty acids, oils, waxes, and steroids accumulate in the brain), but different changes in this gene are associated with an increased risk for Parkinson's disease as well.
How is Parkinson's disease diagnosed and treated?
Diagnosing PD
There are currently no specific tests that diagnose PD. The diagnosis is based on:
- Medical history and a neurological examination
- Blood and laboratory tests to rule out other disorders that may be causing the symptoms
- Brain scans to rule out other disorders. However, computed tomography (CT) and magnetic resonance imaging (MRI) brain scans of people with PD usually appear "normal" or "unremarkable"
In rare cases, where people have a clearly inherited form of PD, researchers can test for known gene mutations as a way of determining an individual's risk of developing the disease. However, this genetic testing can have far-reaching implications and people should carefully consider whether they want to know the results of such tests.
Treating PD
Currently, there is no cure for PD, but medications or surgery can often provide improvement in the motor symptoms.
Drug Therapy
Medications for PD fall into three categories:
- Drugs that increase the level of dopamine in the brain. The most common drugs for PD are dopamine precursors—substances like levodopa that cross the blood-brain barrier and are then changed into dopamine. Other drugs mimic dopamine or prevent or slow its breakdown.
- Drugs that affect other neurotransmitters in the body to ease some of the symptoms of the disease. For example, anticholinergic drugs interfere with production or uptake of the neurotransmitter acetylcholine. These can be effective in reducing tremors.
- Medications that help control the non-motor symptoms of the disease, or the symptoms that don't affect movement. For example, people with PD-related depression may be prescribed antidepressants.
Symptoms may significantly improve at first with medication but can reappear over time as PD worsens and drugs become less effective.
Medications
Levodopa-Carbidopa—The cornerstone of PD therapy is the drug levodopa (also known as L-dopa). Nerve cells can use levodopa to make dopamine and replenish the brain's reduced supply. People cannot simply take dopamine pills because dopamine does not easily pass through the blood-brain barrier, which is a protective lining of cells inside blood vessels that regulate the transport of oxygen, glucose, and other substances in the brain. People are given levodopa combined with another substance called carbidopa. When added to levodopa, carbidopa prevents the conversion of levodopa into dopamine except for in the brain; this stops or diminishes the side effects due to dopamine in the bloodstream. Levodopa-carbidopa is often very successful at reducing or eliminating the tremors and other motor symptoms of PD during the early stages of the disease. People may need to increase their dose of levidopa gradually for maximum benefit. Levodopa can reduce the symptoms of PD but it does not replace lost nerve cells or stop its progression. Initial side effects of levodopa-carbidopa may include:
- Low blood pressure
- Restlessness
- Drowsiness or sudden sleep
Side effects of long-term or extended use of levodopa may include:
- Hallucinations and psychosis
- Dyskinesia, or involuntary movements such as mild to severe twisting and writhing
Later in the course of the disease, people with PD may begin to notice more pronounced symptoms before their first dose of medication in the morning and between doses as the period of effectiveness after each dose begins to shorten, called the wearing-off effect. People experience sudden, unpredictable “off periods,” where the medications do not seem to be working. One approach to alleviating this is to take levodopa more often and in smaller amounts. People with PD should never stop taking levodopa without their physician's input, because rapidly withdrawing the drug can have potentially serious side effects.
Dopamine agonists—These mimic the role of dopamine in the brain and can be given alone or with levodopa. They are somewhat less effective than levodopa in treating PD symptoms but work for longer periods of time. Many of the potential side effects are similar to those associated with the use of levodopa, including drowsiness, sudden sleep onset, hallucinations, confusion, dyskinesias, edema (swelling due to excess fluid in body tissues), nightmares, and vomiting. In rare cases, they can cause an uncontrollable desire to gamble, hypersexuality, or compulsive shopping. Dopamine agonist drugs include apomorphine, pramipexole, ropinirole, and rotigotine. MAO-B inhibitors—These drugs block or reduce the activity of the enzyme monoamine oxidase B, or MAO-B, which breaks down dopamine in the brain. MAO-B inhibitors cause dopamine to accumulate in surviving nerve cells and reduce the symptoms of PD. These medications include selegiline and rasagiline. Studies supported by the NINDS have shown that selegiline (also called deprenyl) can delay the need for levodopa therapy by up to a year or more. When selegiline is given with levodopa, it appears to enhance and prolong the response to levodopa and thus may reduce wearing-off. Selegiline is usually well-tolerated, although side effects may include nausea, orthostatic hypotension, or insomnia. The drug rasagiline is used in treating the motor symptoms of PD with or without levodopa.
COMT inhibitors—COMT stands for catechol-O-methyltransferase and is another enzyme that breaks down dopamine. The drugs entacapone, opicapone, and tolcapone prolong the effects of levodopa by preventing the breakdown of dopamine. COMT inhibitors can decrease the duration of “off periods” of one's dose of levodopa. Side effects may include diarrhea, nausea, sleep disturbances, dizziness, urine discoloration, abdominal pain, low blood pressure, or hallucinations. In a few rare cases, tolcapone has caused severe liver disease, and people taking tolcapone need regular monitoring of their liver function.
Amantadine—This antiviral drug can help reduce symptoms of PD and levodopa-induced dyskinesia. It can be prescribed alone in the early stages of the disease and can be used with an anticholinergic drug or levodopa. After several months, amantadine's effectiveness wears off in up to half of the people taking it. Amantadine's side effects may include insomnia, mottled skin, edema, agitation, or hallucinations. Researchers are not certain how amantadine works in PD, but it may increase the effects of dopamine.
Anticholinergics—These drugs, which include trihexyphenidyl, benztropine, and ethopropazine, decrease the activity of the neurotransmitter acetylcholine and can be particularly effective for tremor associated with PD. Side effects may include dry mouth, constipation, urinary retention, hallucinations, memory loss, blurred vision, and confusion.
When recommending a course of treatment, a doctor will assess how much the symptoms disrupt the person's life and then tailor therapy to the person's particular condition. Since no two people will react the same way to a given drug, it may take time and patience to get the dose right. Even then, symptoms may not be completely alleviated.
Medications to treat motor symptoms of PD
Before the discovery of levodopa, surgery was an option for treating PD. Studies in the past few decades have led to great improvements in surgical techniques, and surgery is again considered for people with PD for whom drug therapy is no longer sufficient.
Pallidotomy and Thalamotomy
The earliest types of surgery for PD involved selectively destroying specific parts of the brain that contribute to PD symptoms. Surgical techniques have been refined and can be very effective for the motor symptoms of PD. The most common lesion surgery is called pallidotomy. In this procedure, a surgeon selectively destroys a portion of the brain called the globus pallidus. Pallidotomy can improve symptoms of tremor, rigidity, and bradykinesia, possibly by interrupting the connections between the globus pallidus and the striatum or thalamus. Some studies have also found that pallidotomy can improve gait and balance and reduce the amount of levodopa people require, thus reducing drug-induced dyskinesias.
Another procedure, called thalamotomy, involves surgically destroying part of the thalamus; this approach is useful primarily to reduce tremor.
Because these procedures cause permanent destruction of small amounts of brain tissue, they have largely been replaced by deep brain stimulation for treatment of PD. However, a method using focused ultrasound from outside the head is being tested because it creates lesions without the need for surgery.
Deep Brain Stimulation
Deep brain stimulation (DBS) uses an electrode surgically implanted into part of the brain, typically the subthalamic nucleus or the globus pallidus. Similar to a cardiac pacemaker, a pulse generator (battery pack) that is implanted in the chest area under the collarbone sends finely controlled electrical signals to the electrode(s) via a wire placed under the skin. When turned on using an external wand, the pulse generator and electrodes painlessly stimulate the brain in a way that helps to block signals that cause many of the motor symptoms of PD. (The signal can be turned off using the wand.) Individuals must return to the medical center frequently for several months after DBS surgery in order to have the stimulation adjusted very carefully to give the best results. DBS is approved by the U.S. Food and Drug Administration (FDA) and is widely used as a treatment for PD.
- DBS is primarily used to stimulate one of three brain regions: the subthalamic nucleus, the globus pallidus interna, or the thalamus. Stimulation of either the globus pallidus or the subthalamic nucleus can reduce tremor, bradykinesia, and rigidity. Stimulation of the thalamus is useful primarily for reducing tremor.
- DBS does not stop PD from progressing, and some problems may gradually return. While the motor function benefits of DBS can be substantial, it usually does not help with speech problems, “freezing,” posture, balance, anxiety, depression, or dementia.
- DBS is generally appropriate for people with levodopa-responsive PD who have developed dyskinesias or other disabling “off” symptoms despite drug therapy. DBS has not been demonstrated to be of benefit for “atypical” parkinsonian syndromes such as multiple system atrophy, progressive supranuclear palsy, or post-traumatic parkinsonism, which also do not improve with Parkinson's medications.
Complementary and Supportive Therapies
A wide variety of complementary and supportive therapies may be used for PD, including:
A healthy diet—At this time there are no specific vitamins, minerals, or other nutrients that have any proven therapeutic value in PD. The National Institute of Neurological Disorders and Stroke ( NINDS ) and other components of the NIH are funding research to determine if caffeine, antioxidants, and other dietary factors may be beneficial for preventing or treating PD. A healthy diet can promote overall well-being for people with PD just as it would for anyone else. Eating a fiber-rich diet and drinking plenty of fluids also can help alleviate constipation. A high protein diet, however, may limit levodopa's absorption.
Exercise—Exercise can help people with PD improve their mobility, flexibility, and body strength. It also can improve well-being, balance, minimize gait problems, and strengthen certain muscles so that people can speak and swallow better. General physical activity, such as walking, gardening, swimming, calisthenics, and using exercise machines, can have other benefits. People with PD should always check with their doctors before beginning a new exercise program.
Alternative approaches that are used by some individuals with PD include:
- A NINDS-funded clinical trial demonstrated the benefit of tai chi exercise compared to resistance or stretching exercises in people with PD
- Massage therapy to reduce muscle tension
- Yoga to increase stretching and flexibility
- Hypnosis acupuncture
- The Alexander Technique to optimize posture and muscle activity
Coping with PD
While PD usually progresses slowly, eventually daily routines may be affected—from socializing with friends to earning a living and taking care of a home. These changes can be difficult to accept. Support groups can help people cope with the disease's emotional impact. These groups can provide valuable information, advice, and experience to help people with PD, their families, and their caregivers deal with a wide range of issues, including locating doctors familiar with the disease and coping with physical limitations. A list of national organizations that can help people locate support groups in their communities appears at the end of this information. Individual or family counseling may also help people find ways to cope with PD.
People with PD may also benefit from being proactive and finding out as much as possible about the disease in order to alleviate fear of the unknown and to take a proactive role in maintaining their health. Many people with PD continue to work either full- or part-time, although they may need to adjust their schedule and working environment to accommodate their symptoms.
The average life expectancy of a person with PD is generally the same as for people who do not have the disease. Fortunately, there are many treatment options available for people with PD. However, in the late stages, PD may no longer respond to medications and can become associated with serious complications such as choking, pneumonia, and falls.
Because PD is a slow, progressive disorder, it is not possible to predict what course the disease will take for an individual person.
What are the latest updates on Parkinson's disease ?
The mission of the National Institute of Neurological Disorders and Stroke ( NINDS ) is to seek fundamental knowledge about the brain and nervous system and to use the knowledge to reduce the burden of neurological disease. NINDS, a component of the National Institutes of Health ( NIH ), conducts and supports research on Parkinson's disease are to better understand and diagnose PD, develop new treatments, and ultimately, prevent PD. NINDS also supports training for the next generation of PD researchers and clinicians and serves as an important source of information for people with PD and their families.
Establishing PD research priorities
The NINDS-organized Parkinson's Disease 2014: Advancing Research, Improving Lives conference brought together researchers, clinicians, patients, caregivers, and nonprofit organizations to develop 31 prioritized recommendations for research on PD. These recommendations are being implemented through investigator-initiated grants and several NINDS programs. NINDS and the NIH 's National Institute of Environmental Health Sciences held the Parkinson's Disease: Understanding the Environment and Gene Connection workshop to identify priorities for advancing research on environmental contributors to PD.
Research recommendations for Lewy Body Dementia, including Parkinson's disease dementia, were updated during the NIH Alzheimer's Disease-Related Dementias Summit 2019 (pdf, 2180 KB) .
Key programs and resources
- The Parkinson's Disease Biomarkers Programs (PDBP) , a major NINDS initiative, is aimed at discovering ways to identify individuals at risk for developing PD and Lewy Body Dementia and to track the progression of the diseases. It funds research and collects human biological samples and clinical data to identify biomarkers (signs that may indicate risk of a disease and improve diagnosis) that will speed the development of novel therapeutics for PD. Goals are improving clinical trials and earlier diagnosis and treatment. Projects are actively recruiting volunteers at sites across the U.S. NINDS also collaborates with the Michael J. Fox Foundation for Parkinson's Research (MJFF) on BioFIND, a project collecting biological samples and clinical data from healthy volunteers and those with PD.
- The NINDS Morris K. Udall Centers of Excellence for Parkinson's Disease Research program supports research centers across the country that work collaboratively to study PD disease mechanisms, the genetic contributions to PD, and potential therapeutic targets and treatment strategies.
- The Accelerating Medicines Partnership ® for Parkinson's Disease (AMP ® -PD) program is a partnership between the NIH, multiple biopharmaceutical and life sciences communities, and nonprofit advocacy organizations to identify and validate biomarkers that can track the progression of PD. It allows scientists to perform large-scale genetic analyses and share data to more quickly bring new medicines to people either with or at risk of PD.
- The NINDS Intramural Research Program conducts clinical studies to better understand PD mechanisms and develop novel and improve treatments.
- The NINDS Biospecimens Repositories store and distribute DNA, cells, blood samples, cerebrospinal fluid, and autopsy tissue to PD researchers around the world.
Disease research
NINDS research looks at all aspects of the mechanisms of PD, identifying clues to PD development and its processes, and improving current therapies and testing new ones. Research efforts include:
Cellular Processes—Mutations in alpha-synuclein and dozens of genes (some of which were discovered at NIH ) are known to either cause PD or modify a person's risk of developing it. Researchers are investigating how the cellular processes controlled by these genes contribute to neurodegeneration, including the toxic accumulation of alpha-synuclein and how the loss of dopamine impairs communication between nerve cells.
Deep Brain Stimulation (DBS)—NINDS has been a pioneer in the study and development of DBS, which is now considered a standard treatment response to PD medications. Current NINDS-funded projects include research to fine-tune the optimal site within the brain to implant the DBS electrode, studies to better understand the therapeutic effect of DBS on neural circuitry and brain regions affected by PD, and different forms of brain stimulation on different areas of the brain.
Environmental studies—Risk factors such as repeated occupational exposure to certain pesticides and chemical solvents may influence who develops PD. A NINDS-funded research consortium is hunting for environmental risk factors that increase susceptibility to developing PD.
Genetic studies—A better understanding of genetic risk factors is playing a critical role in revealing PD disease mechanisms. A NINDS workshop contributed to the development of NeuroX, the first DNA chip that can identify genetic changes in persons at risk for a number of late-onset neurodegenerative diseases, including PD. Another NINDS collaborative, the Consortium On Risk for Early-onset Parkinson's Disease (CORE PD), is trying to identify the genetic factors that contribute to the development of early-onset PD. Current clinical studies include the genetic connection to memory and motor behavior, the search for genes that may increase the risk of PD and related neurodegenerative disorders, and identifying biomarkers for PD.
Motor complications— NINDS scientists have studied the safety and effectiveness of drugs and interventions in alleviating motor symptoms in people with PD. For example, basic research using adenosine found it could improve motor complications associated with PD. A current NINDS clinical study of motor complications is testing an at-home device to evaluate PD movement symptoms while performing different tasks.
Exercise—Exercise routines are often recommended to help individuals with PD maintain movement and balance necessary for everyday living. Research continues on the role of exercise in slowing the decline of motor function and modifying the course of PD.
Neuroprotective Drugs— NINDS supports basic, clinical, and translational research aimed at protecting nerve cells from the damage caused by PD. The NINDS-funded NeuroNext Network is designed to test new therapies and to validate biomarkers in a number of neurological disorders, including Parkinson's disease.
How can I or a loved one help improve care for people with Parkinson's disease ?
Consider participating in a clinical trial so clinicians and scientists can learn more about PD and related disorders. Clinical research uses human volunteers to help researchers learn more about a disorder and perhaps find better ways to safely detect, treat, or prevent disease.
All types of volunteers are needed— those who are healthy or may have an illness or disease— of all different ages, sexes, races, and ethnicities to ensure that study results apply to as many people as possible, and that treatments will be safe and effective for everyone who will use them.
For information about participating in clinical research visit NIH Clinical Research Trials and You . Learn about clinical trials currently looking for people with PD at Clinicaltrials.gov .
Where can I find more information about Parkinson's disease ? Information may be available from the following organizations and resources: American Parkinson Disease Association Phone: 718-981-8001 or 800-223-2732 Bachmann-Strauss Dystonia & Parkinson Foundation Phone: 212-509-0995 Davis Phinney Foundation Phone: 303-733-3340 or 866-358-0285 Lewy Body Dementia Association Phone: 404-935-6444 Michael J. Fox Foundation for Parkinson's Research Phone: 800-708-7644 Parkinson Alliance Phone: 609-688-0870 or 800-579-8440 Parkinson's Foundation Phone: 800-473-4636 Parkinson's Resource Organization Phone: 760-773-5628 or 877-775-4111 The Parkinson's Institute and Clinical Center Phone: 408-734-2800 or 800-655-2273
Learn about related topics
- Corticobasal Degeneration
- Lewy Body Dementia
- Multiple System Atrophy
- Progressive Supranuclear Palsy (PSP)
Advertisement
Parkinson’s disease could be prevented by a recent tetanus vaccine
People who have had a recent vaccine against tetanus appear to be less likely to develop Parkinson’s disease, suggesting that the bacterial infection is involved in the condition
By Clare Wilson
29 May 2024

Magnetic resonance imaging scan showing plaques (dark areas) in the centre of the brain of someone with Parkinson’s disease
ZEPHYR/SCIENCE PHOTO LIBRARY
Parkinson’s disease could be caused by an infection with tetanus bacteria, according to a radical new idea.
We may finally know how cognitive reserve protects against Alzheimer's
The claim stems from the finding that people who have recently been vaccinated against tetanus to prevent a wound infection are half as likely to later be diagnosed with Parkinson’s. “The closer to the vaccine date, the less likely individuals are…
Sign up to our weekly newsletter
Receive a weekly dose of discovery in your inbox! We'll also keep you up to date with New Scientist events and special offers.
To continue reading, subscribe today with our introductory offers
No commitment, cancel anytime*
Offer ends 2nd of July 2024.
*Cancel anytime within 14 days of payment to receive a refund on unserved issues.
Inclusive of applicable taxes (VAT)
Existing subscribers
More from New Scientist
Explore the latest news, articles and features
Pet dogs smell Parkinson's disease with almost 90 per cent accuracy
Subscriber-only
Beeping shoes help people with Parkinson's disease walk further
Parkinson's disease progression slowed by antibody infusions, spine stimulator lets man with severe parkinson's walk without falling, popular articles.
Trending New Scientist articles

Parkinson’s mitochondria-targeting research nets $12.5M more funding
Lucy Therapeutics plans to submit IND applications in early 2025

by Patricia Inácio, PhD | June 4, 2024
Share this article:

Lucy Therapeutics ‘ (LucyTx) overall funding haul has grown to $36 million with the addition of $12.5 million more to support the company’s mitochondrial platform to develop new therapies for Parkinson’s disease.
The total funding will also buttress the development of Lucy’s therapeutic pipeline for Alzheimer’s disease and Rett syndrome , a rare neurodevelopmental disorder marked by cognitive, emotional, sensory, and motor disturbances. Plans are underway to submit investigational new drug (IND) applications in early 2025, which would allow the company to start clinical trials, according to a press release .
“This latest funding will advance our work pioneering a new class of therapeutics designed to address mitochondrial dysfunction and provide potentially curative treatments for people suffering from Alzheimer’s, Parkinson’s and Rett syndrome,” said Amy Ripka, PhD, founder and CEO of LucyTx. Mitochondria are the cells’ powerhouses and are important for nerve cells. Mitochondrial dysfunction is common in neurological diseases such as Parkinson’s.
Transplanting mitochondria shows promise in lab models of Parkinson’s
Mitochondrial function and neuronal health.
LucyTx is developing small molecules that restore mitochondria function to normalize neuronal health in people with these diseases.
“To make meaningful progress in developing treatments for complex diseases, we need to embrace nontraditional methods. LucyTx is doing just that, identifying new drug targets based on a deep understanding of the chemical and biological interplay at work in disease, and ultimately linking neurodegenerative disease to dysfunctional mitochondria,” Ripka said.
Lucy’s pipeline includes two small molecules — LucyTx-1209 and LucyTx-1212 — which have been shown to improve mitochondrial function and reduce alpha-synuclein, a protein that forms toxic clumps in the brain of people with Parkinson’s disease.
The compounds were also deemed safe in cell and rodent models of the disease. The company is working to select the best candidate to bring into clinical testing.
The new round of funding was led by Engine Ventures and Safar Partners, along with new investors, including Bill Gates, Parkinson’s UK , and the Michael J. Fox Foundation , which provided a $2 million nondilutive grant.
“The LucyTx team is creating a cutting-edge platform to identify drug targets by understanding mitochondria’s impact. This platform is already enhancing their drug discovery programs,” said Mike Poole, LucyTx’s newest board member and a senior advisor to Gates Ventures.
About the Author

Recent Posts
- AI algorithm uses smartwatch data to predict Parkinson’s emotions
- Coffee intake before brain imaging may influence results, study says
- G2019S mutation carriers in LRRK2 gene have higher Parkinson’s risk
- Depression linked to low cognitive function, sex drive in Parkinson’s
- Identifying AAK1 inhibitor could aid therapy discovery: Study
Recommended reading

Method generates dopaminergic neurons lost in Parkinson’s disease

Risvodetinib shows potential to slow disease progression in clinical trial

Monitor COVID-19 patients for Parkinson’s symptoms, scientists say
Subscribe to our newsletter.
Get regular updates to your inbox.

IMAGES
VIDEO
COMMENTS
In this latest randomized, double-blind trial on 20 patients diagnosed with Parkinson's disease, the antibodies were shown to bind exclusively to aggregated forms of α-syn. Analysis of the spinal fluid of those given UB-312 revealed a 20 percent drop in their usual α-syn aggregate levels, compared with a 3 percent decline in those who ...
Parkinson's disease is a progressive neurodegenerative disorder, which is characterized by motor symptoms such as tremor, rigidity, slowness of movement and problems with gait. Motor symptoms are ...
— New clinical trial data for bemdaneprocel, a promising stem cell-derived therapy for Parkinson's disease, continues to show positive results 18 months after treatment, scientists reported today at an international conference in Portugal. ... Henchcliffe, an internationally recognized Parkinson's expert whose research has included stem ...
"We hope that research like this will provide mechanistic, molecular-based therapies that can actually slow or halt the progression of Parkinson's disease." Parkinson's disease symptoms, including a variety of motor and cognitive deficits that worsen over time, result from the death of neurons that produce the chemical messenger dopamine.
At least six GLP-1 receptor agonists have been or are being tested as potential treatments in persons with Parkinson's disease. 9 A small single-center, randomized, controlled trial of the GLP-1 ...
Introduction. Parkinson's disease (PD) is the second most common neurodegenerative disorder after Alzheimer's disease and is currently imposing a heavy economic and social burden on society as the population continues to age [1, 2].PD is clinically characterized by motor dysfunctions (including resting tremor, bradykinesia, rigidity, and postural instability) and various non-motor symptoms ...
Researchers have found a way to better control the preclinical generation of key neurons depleted in Parkinson's disease, pointing toward a new approach for a disease with no cure and few ...
Credit: Nature Communications (2024). DOI: 10.1038/s41467-024-48731-1. Deep brain stimulation (DBS) has shown promise as a treatment for some symptoms of Parkinson's disease (PD). However, not all ...
Because Parkinson's stems from the dysfunction of one type of cell in a concentrated spot in the brain, the disease has long been viewed as an ideal candidate for stem-cell therapy. Restore ...
Jan. 23, 2024 — Scientists argue that Parkinson's disease complexity demands a new way of classifying the disease for research purposes, one based not on clinical diagnosis but biology. The ...
Developments in levodopa formulations and the standardisation of deep brain stimulation (DBS) substantially improved clinical management of patients with Parkinson's disease before the turn of the century. As a result of these developments, Parkinson's disease has become a chronic disorder and it is associated with a plethora of non-motor disabling complications. Cognitive impairment is now a ...
The American Parkinson Disease Association (APDA) is a nationwide grassroots network dedicated to fighting Parkinson's disease (PD) and works tirelessly to help the approximately one million with PD in the United States live life to the fullest in the face of this chronic, neurological disorder. Founded in 1961, APDA has raised and invested more than $282 million to provide outstanding ...
The new approach uses ultrasound delivered by a fiber about the thickness of a human hair. ... by implanted electrodes that deliver electrical pulses to the brain, is often used to treat Parkinson's disease and other neurological disorders. However, the electrodes used for this treatment can eventually corrode and accumulate scar tissue ...
A hallmark of Parkinson's disease is the presence of misfolded aggregates of α-synuclein in the brain. ... Parkinson's disease; Biomarkers; Latest on: ... The Research University in the ...
The discovery opens a new avenue for Parkinson's therapeutics, scientists report in a new study. The Northwestern scientists discovered that mutations in parkin result in a breakdown of contacts between two key workers in the cell — lysosomes and mitochondria. Mitochondria are the main producers of energy in cells, and lysosomes recycle ...
D.G. StandaertN Engl J Med 2024;390:1233-1234. Parkinson's disease is a common and debilitating disorder. The best-known features are resting tremor, rigidity, and slowness, but recently a ...
The pathological hallmarks of PD and the interplay of aging, environmental hazards, and genetics in the pathogenesis of PD. (A) The pathological hallmarks of Parkinson's disease include dopaminergic neuronal death, α-synuclein aggregates, mitochondrial dysfunction, reactive oxygen species, apoptosis, and neuroinflammation.(B) PD is a multifactorial disorder involving aging, genetics, and ...
Parkinson's disease is a recognisable clinical syndrome with a range of causes and clinical presentations. Parkinson's disease represents a fast-growing neurodegenerative condition; the rising prevalence worldwide resembles the many characteristics typically observed during a pandemic, except for an infectious cause. In most populations, 3-5% of Parkinson's disease is explained by genetic ...
Introduction. Parkinson's disease (PD) is a common neurodegenerative disease characterised by a movement disorder consisting of bradykinesia, rest tremor, and rigidity, along with postural instability, a range of other more-subtle motor features, and many non-motor features 1.Many of the core motor features result from the loss of a specific population of neurons: the dopaminergic neurons of ...
New deep brain stimulation algorithm may help personalize Parkinson's disease treatment. ScienceDaily . Retrieved June 6, 2024 from www.sciencedaily.com / releases / 2024 / 06 / 240604132249.htm
The gut is a new target. The Queensland research will involve both human and animal studies to identify new classes of therapeutics to treat Parkinson's disease, first described more than 200 ...
Read the latest developments, reporting and analysis from the world of Parkinson's research, including progress made in studies, ... Episode 14: Evidence Linking Parkinson's Disease Risk and Environmental Exposure to Trichloroethylene (TCE) with Sam Goldman. May 7, 2024.
Date: July 19, 2023. Source: Northwestern University. Summary: Scientists have uncovered a new mechanism by which mutations in a gene parkin contribute to familial forms of Parkinson's disease ...
Find the latest research in npj Parkinson's Disease, an open access, peer reviewed journal with a 8.7 Impact Factor and 15 days to first decision. ... npj Parkinson's Disease has a 2-year impact ...
Parkinson's disease (PD) is movement disorder of the nervous system that gets worse over time. As nerve cells (neurons) in parts of the brain weaken, are damaged, or die, people may begin to notice problems with movement, tremor, stiffness in the limbs or the trunk of the body, or impaired balance. As symptoms progress, people may have ...
Researchers based at Australia's Translational Research Institute (TRI) have received major funding from the United States Department of Defense (DoD) to develop Parkinson's disease (PD ...
In the latest study, the scientists recruited 163 people with early Parkinson's disease and 40 healthy controls. Each participant underwent a single photon emission computed tomography scan, a ...
Jun 3 2024 University of Turku. Regular high caffeine consumption affects dopamine function in patients with Parkinson's disease, shows a new international study led by the University of Turku ...
Parkinson's disease could be caused by an infection with tetanus bacteria, according to a radical new idea. The claim stems from the finding that people who have recently been vaccinated against ...
Lucy Therapeutics ' (LucyTx) overall funding haul has grown to $36 million with the addition of $12.5 million more to support the company's mitochondrial platform to develop new therapies for Parkinson's disease. The total funding will also buttress the development of Lucy's therapeutic pipeline for Alzheimer's disease and Rett ...