- Research article
- Open access
- Published: 29 January 2021

Molecular characterization of lumpy skin disease virus (LSDV) emerged in Bangladesh reveals unique genetic features compared to contemporary field strains
- Shukes Chandra Badhy 1 , 2 ,
- Mohammad Golam Azam Chowdhury 1 , 2 ,
- Tirumala Bharani Kumar Settypalli 3 ,
- Giovanni Cattoli 3 ,
- Charles Euloge Lamien 3 ,
- Mohammad Aflak Uddin Fakir 1 , 2 ,
- Shamima Akter 1 , 2 ,
- Mozaffar Goni Osmani 2 ,
- Faisol Talukdar 2 ,
- Noorjahan Begum 2 ,
- Izhar Ahmed Khan 1 , 2 ,
- Md Bazlur Rashid 1 , 2 &
- Mohammad Sadekuzzaman ORCID: orcid.org/0000-0002-5530-2829 1 , 2
BMC Veterinary Research volume 17 , Article number: 61 ( 2021 ) Cite this article
20k Accesses
50 Citations
27 Altmetric
Metrics details
Lumpy skin disease (LSD) is a contagious viral disease of cattle caused by lumpy skin disease virus (LSDV). LSD has recently spread in Asia following outbreaks in the Middle East and Europe. The disease emerged in Bangladesh in July 2019 in the Chattogram district, then rapidly spread throughout the entire country. We investigated six LSD outbreaks in Bangladesh to record the clinical signs and collect samples for diagnostic confirmation. Furthermore, we performed the molecular characterization of Bangladesh isolates, analyzing the full RPO30 and GPCR genes and the partial EEV glycoprotein gene.
Clinical observations revealed common LSD clinical signs in the affected cattle. PCR and real-time PCR, showed the presence of the LSDV genome in samples from all six districts. Phylogenetic analysis and detailed inspection of multiple sequence alignments revealed that Bangladesh isolates differ from common LSDV field isolates encountered in Africa, the Middle East, and Europe, as well as newly emerged LSDV variants in Russia and China. Instead, they were closely related to LSDV KSGP-0240, LSDV NI2490, and LSDV Kenya.
Conclusions
These results show the importance of continuous monitoring and characterization of circulating strains and the need to continually refine the strategies for differentiating vaccine strains from field viruses.
Lumpy skin disease (LSD) is a viral disease of cattle, caused by lumpy skin disease virus (LSDV) within the genus Capripoxvirus , family Poxviridae . The genus Capripoxvirus also comprises goatpox virus (GTPV) and sheeppox virus (SPPV). LSD is a notifiable disease by the World Organization for Animal Health (OIE) because of its potential rapid spread and substantial economic importance.
LSDV has a limited host range and does not infect non-ruminant hosts [ 1 ]. Both sexes and all ages of cattle breeds are susceptible to LSDV. However, younger animals may be more susceptible to the severe form of the disease [ 2 ]. Even in close contact with infected cattle, sheep and goats never developed LSD [ 3 ], with one noted exception, LSDVKSGP-0240 also known as LSDV KS1.
There is a significant variation of clinical signs with LSDV infections ranging from subclinical infection to death [ 4 ]. The main clinical signs include fever, the appearance of nodules in the skin, lesions in the mouth, pharynx, enlarged superficial lymph nodes, edema in the skin, and sometimes death [ 3 , 4 , 5 ]. There is an initial incubation period of 6 to 9 days during acute cases followed by a fever that may exceed 41 °C [ 6 ].
LSD is one of the most economically significant viral diseases of cattle because of the loss of production, permanent damage of hides, infertility, and death. Although the mortality rate is usually less than 10%, the disease morbidity rate can be as high as 100% [ 7 ].
For many years, the LSDV genome appeared to be stable. Indeed, following its first description in Zambia in 1929 [ 8 ], LSDV field isolates recovered for decades in Africa showed only minor genomic differences [ 9 , 10 , 11 , 12 ]. As the disease further spread into the Middle East from 2012 [ 13 ] and Europe in 2015 [ 7 ], the recovered LSDV field isolates showed little variability to contemporary African LSDV field isolates [ 14 , 15 , 16 ]. This genetic stability was exploited for the differentiation of LSDV live attenuated vaccines from contemporary field isolates [ 16 , 17 , 18 , 19 , 20 , 21 ].
However, this dynamic has shifted following the discovery of field LSDVs in Russia in 2017 and 2019 showing vaccine-like profiles [ 22 , 23 , 24 , 25 ]. Some of these LSDV variants, presented a 12-nucleotide insertion in the GPCR gene, like vaccine strains, and others presented a27-nucleotide deletion, similar to the LSDV Neethling vaccine strain, in the ORF LSDV 126. The authors attributed these variants’ emergence to recombination events between the Neethling vaccine strain and field isolates [ 23 ]. This has prompted researchers [ 26 ] to question the relevance of current strategies to differentiate LSDV vaccine strains from viral field strains. Similarly, the LSDV strains involved in the outbreaks in China present the GPCR profile of LSDV vaccines with the 27-nucleotide insertion in their EEV glycoprotein gene. Interestingly, Kononova et al. [ 27 ], also showed in vitro and in vivo that the recombinant LSDVs could induce more severe disease than the typical field isolates.
The increased variability of LSDV in recent years makes it crucial to adapt the molecular DIVA strategies based on the knowledge of the circulating strain of LSDV. This requires the constant monitoring and characterization of LSDV field isolates.
Several PCR, real-time PCR, and HRM based methods are available for the detection of the LSDV genome, [ 12 , 14 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 ] and molecular epidemiological studies of LSDV rely on analyzing various genomic regions, such as the GPCR, the RPO30, the P32, and the EEV glycoprotein genes [ 11 , 12 , 17 , 18 , 19 , 37 ].
On September 15, 2019, Bangladesh notified to OIE the first outbreak of LSD in the country. The disease started in July 2019 in the Southeast (Chattogram district) of the country, then progressively spread throughout the country. Because of the wide distribution and large cattle population in Bangladesh, LSD is now one of the most economically important emerging livestock disease in Bangladesh.
This study aimed to investigate and confirm the recent outbreaks and provide LSDV molecular characterization in different regions in Bangladesh.
Outbreak investigation
All affected cattle in different districts in Bangladesh (Chottogram, Dhaka, Gazipur, Narayanganj, Pabna, and Satkhira) showed the following common clinical signs: fever (40–41 °C), depression, loss of appetite, nasal and ocular discharges, salivation, circumscribed nodules with different sizes on the skin covering their head, neck, trunk, perineum, udder, and teats. Figure 1 illustrates the skin lesions of affected cattle. In many infected animals, the necrotic nodules were ulcerated and formed deep scabs (sitfast). Moreover, a decrease in body weight and reduced milk production were prominent signs observed in cattle affected by LSD. The total cattle population, reported morbidities and mortalities in the six districts of this study (Fig. 2 ) are sumarised in Table 1 .
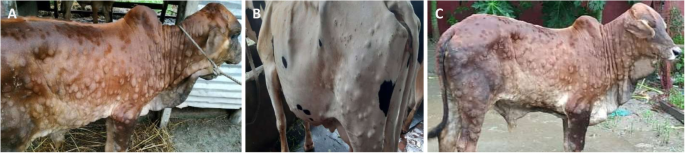
Skin lesions characteristics of lumpy skin disease in 3 animals in Bangladesh. The generalized circumscribed active nodular skin lesions covering the entire body are visible. Source: own
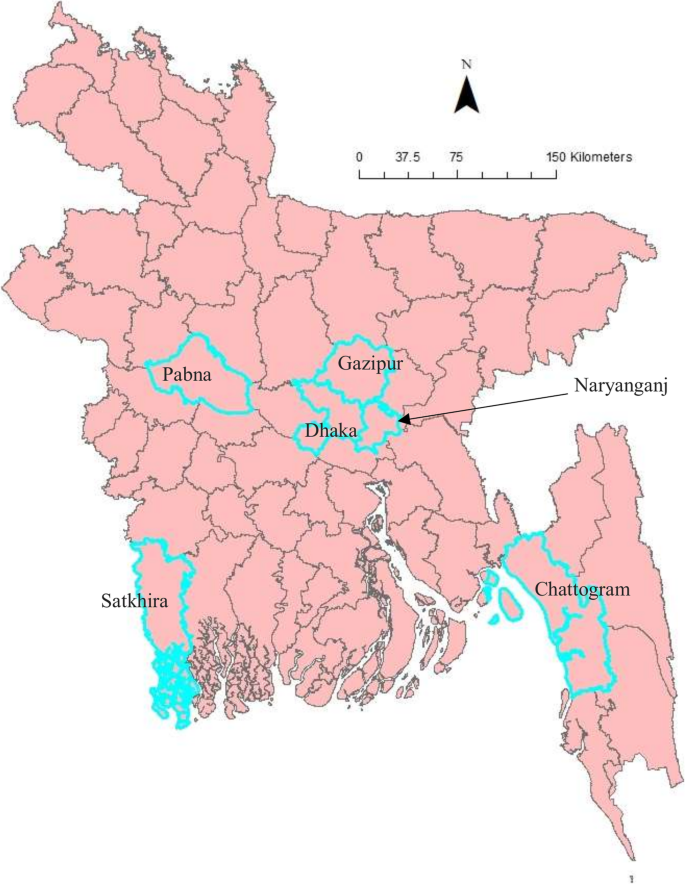
Map of Bangladesh showing the sample collection area. The map is an own creation using Arc GIS software version 13.2
Molecular detection of LSDV
Gel electrophoresis of the P32 amplicons showed a 390 bp product in all fifty (50) samples collected in six districts, as illustrated for selected samples in Fig. 3 .
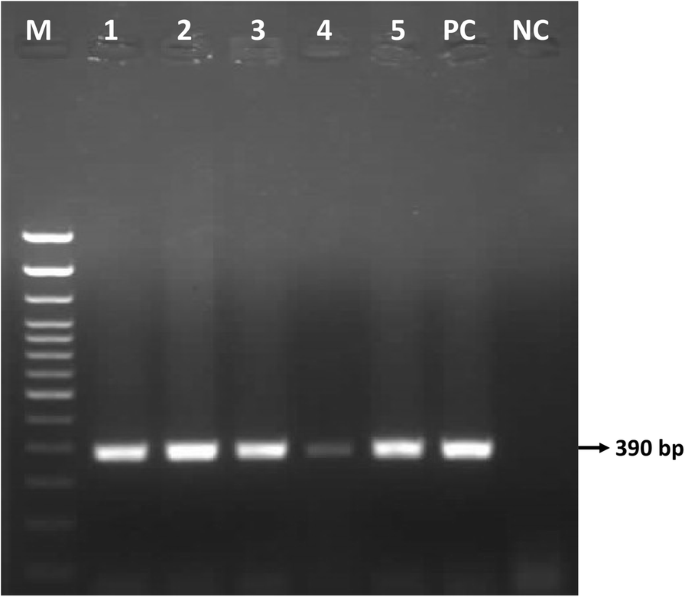
Agarose gel electrophoresis showing the 390 bp amplicon of P32 gene for selected samples of Bangladesh. Lane M: 100 bp DNA ladder, Lane 1–5: LSDV field samples, Lane PC: positive control, lane NC: negative control
The real-time PCR result confirmed capripoxvirus DNA in all samples. Six representative samples, one per district with a Cq value between 19.17 and 25.31, were selected for sequencing (Table 2 ).
Amplification and sequencing of the RPO30, GPCR, and EEV glycoprotein genes
We have successfully amplified and sequenced two fragments for the RPO30 gene (554 bp and 520 bp) in 6 samples and three for the GPCR (617 bp, 603 bp, and 684 bp) in 5 samples out of 6. We also amplified and sequenced the partial EEV glycoprotein gene in 6 samples. The complete RPO30 and GPCR genes and the partial EEV glycoprotein gene sequences were submitted to the GenBank database under accession numbers MT448690 to MT448701.
Phylogenetic analysis
For each of the targeted genes, the sequences of the Bangladesh LSDVs showed 100% identity among each other. On the phylogenetic trees for both RPO30 (Fig. 4 ) and GPCR (Fig. 5 ), all the Bangladesh LSDVs clustered together.
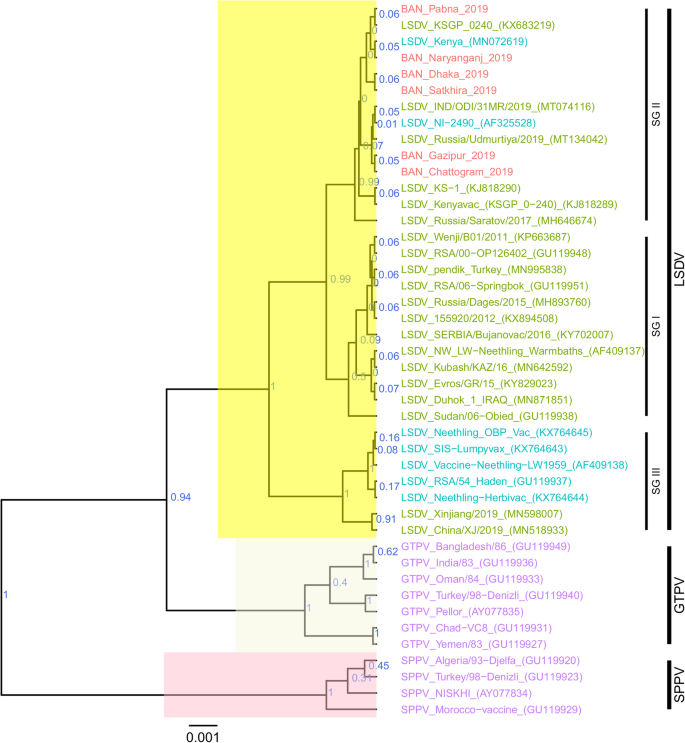
Maximum clade credibility (MCC) tree based on the complete RPO30 complete gene sequences of capripoxviruses. The posterior probabilities are plotted as respective nodes labels. LSDVs from Bangladesh are highlighted in red and reference sequences are represented with their accession numbers
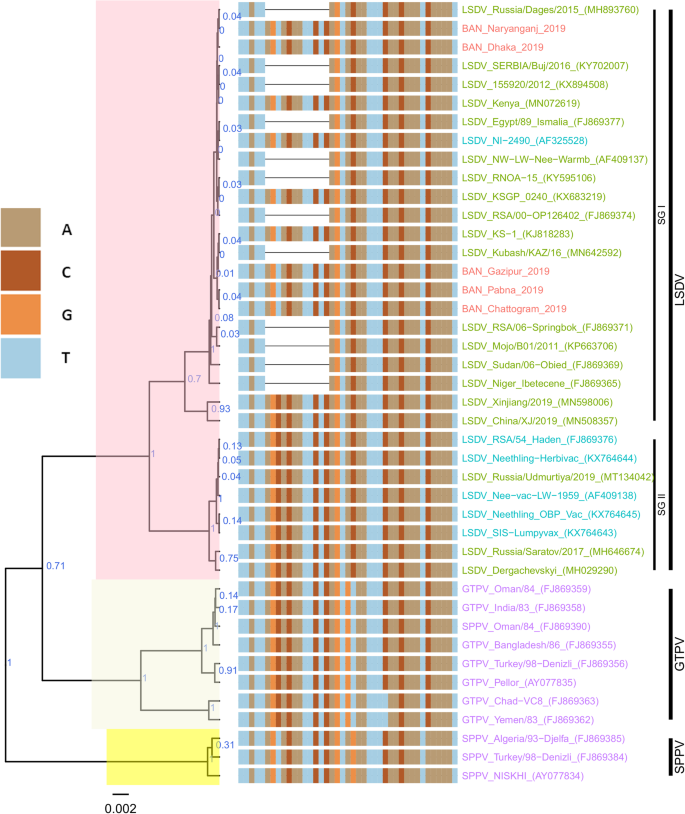
Maximum clade credibility (MCC) tree based on the complete GPCR gene sequences of Capripoxviruses, plotted together with multiple sequence alignment. Only the portion of the alignment between positions 80 and 120 is shown. The posterior probabilities are plotted as respective nodes labels. LSDVs from Bangladesh are highlighted in red and reference sequences are represented with their accession numbers
On the RPO30 tree (Fig. 4 ), Bangladesh isolates clustered within subgroup I, tightly with LSDV KSGP 0240 (KX683219), known as LSDV KS1, LSDV NI-2490 (AF325528), Indian LSDV field isolates, and two recombinant LSDV field isolates from Russia, LSDV Russia/Udmurtiya/2019 (MT134042), and LSDV Russia/Saratov/2017 (MH646674). The commonly circulating field isolates from Africa, the Middle East, and Europe are segregated from the Bangladesh isolates, clustering within subgroup II. A third subgroup contained mainly LSDV Neethling derived vaccine strains, the historical field LSDV RSA/54 Haden, and the LSDV field isolates from China. On the GPCR tree (Fig. 5 ), there were only two sub-groups. Bangladesh LSDV isolates clustered within subgroup I, together with LSDV SGP_O-240 (KJ818288), LSDV NI-2490 (AF325528), LSDV Kenya (MN072619), common LSDV field isolates from Africa, the Middle East, and Europe, LSDV Xinjiang/2019 (MN598006), and LSDV China/XJ/2019(MN508357) from China. The second subgroup of the GPCR consisted of LSDV Neethling derived vaccines, LSDV RSA/54 Haden (FJ869376), and three recombinant LSDVs from Russia: LSDV Russia/Udmurtiya/2019 (MT134042), LSDV Russia/Saratov/2017 (MH646674) and LSDV Dergachevskyi (MH029290). The multiple sequence alignments of the GPCR gene showed that the Bangladesh LSDV contained the 12-nucleotide insertion (Fig. 5 ). This 12-nucleotide insertion is also present in the two common LSDV vaccine strains (LSDV KSGP 0240 and LSDV Neethling) and a few historical field isolates (collected before 1960) such as LSDV NI-2490 (AF325528), LSDV Kenya (MN072619), and LSDV RSA/54Haden (GU119937). This insertion is also present in recombinant LSDVs from Russia (LSDV Russia/Udmurtiya/2019, LSDV Russia/Saratov/2017, and LSDV Dergachevskyi) and in recent LSDV isolates from China.
Alignment of the EEV glycoprotein gene sequence showed a 27-nucleotide insertion in all LSDVs from Bangladesh (Fig. 6 ), which is characteristic of common field isolates and also present in the LSDV KSGP-0240 derived vaccines and historical LSDVs, LSDV NI2490 (1958) and LSDV Kenya (1950), both from Kenya.
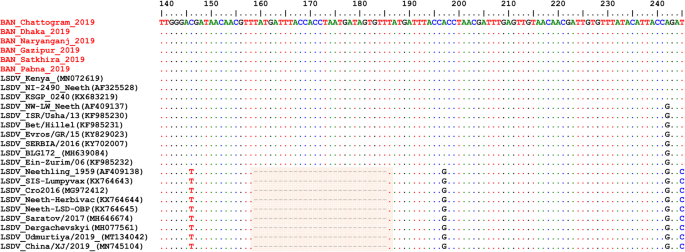
Multiple sequence alignments of the partial nucleotide sequences of the of EEV glycoprotein gene. LSDVs from Bangladesh were aligned with representative LSDVs’ sequences retrieved from GenBank. A unique sequence signature of 27-nucleotide only in LSDV Neethling like viruses is highlighted in the box. Identical nucleotides are indicated with dots
Taken together, the analyses of all three targets suggest that the Bangladesh LSDVs were more related to LSDV KSGP-0240, LSDV NI-2490, and LSDV Kenya. They differed from all recent LSDV field isolates, including the LSDVs from China and the recombinant LSDVs described in Russia, and LSDV Neethling vaccine strain.
The diagnosis of LSD was confirmed by real time PCR and the viruses in samples collected from outbreaks between July and September 2019 in Bangladesh were molecularly characterized.
LSD emerged in Bangladesh in July 2019, hitting the district of Chattogram, before quickly spreading to Dhaka, Naryanganj, Gazipur, Satkhira, and Pabna regions, between July and September 2019 [ 38 ]. The affected cattle exhibited common clinical manifestations of LSD in cattle, including nasal and ocular discharges and skin lesions [ 39 ]. The epidemiology unit data shows a very high incidence of LSD in the Chattogram province compared to other regions in the country. A plausible explanation is that Chattogram, a port city of Bangladesh, is a major coastal city and financial center in southeastern Bangladesh, with more cattle movement due to trade. It was also the first affected province.
The Bangladesh LSDVs presented the 12-nucleotide insertion found in the GPCR gene of LSDV KSGP-0240, LSDV Neethling vaccines, and a few historical LSDVs such as the LSDV NI2490 and LSDV Kenya, LSDVs from China and the recombinant LSDVs described in Russia. The presence of this 12-nucleotide insertion makes them different from commonly circulating field LSDVs encountered in Africa, Europe, and the Middle East [ 11 , 16 , 19 , 24 , 40 ]. However, a 27-nucleotide insertion in the EEV glycoprotein gene of Bangladesh isolates, and the RPO30 and GPCR gene trees’ analysis differentiated them from LSDV Neethling derived vaccines. This 27-nucleotide insertion in the EEV glycoprotein also makes them different from Chinese LSDV isolates as well as from the recombinant LSDVs described in Russia in recent years.
A close inspection of the sequence alignment of the EEV glycoprotein, the GPCR, and RPO30 genes showed 100% identity to the LSDV KSGP 0240, LSDV NI2490, and LSDV Kenya at the nucleotide level. These features make them unique, as the commonly circulating LSDV isolates have not demonstrated that level of closeness to LSDV KSGP 0240, LSDV NI2490, and LSDV Kenya. It is worth noting that the Bangladesh LSDV RPO30 sequence was 100% identical with the Indian isolate, however, as no GPCR and EEV glycoprotein gene sequences were available from India, it was not possible to extend the comparison.
The existence of vaccine-like field isolates with mixed characteristics between common field viruses and the LSDV Neethling vaccine has been reported in Russia [ 24 ]. A more recent report described a field LSDV isolate in Kurgan, Russia, exhibiting similarities to LSDV KSGP 0240 and LSDV NI2490 based on the analysis of GPCR and RPO30 gene fragments [ 26 ]. Although the complete RPO30 and GPCR sequences were not available for a full comparison, we noticed a nucleotide difference between the partial RPO30 sequence of the Kurgan isolate and those of this study. Our findings support the circulation of LSDV KS1 or LSDV NI2490-like virus in the field.
How such a virus has emerged suddenly in Bangladesh remains unknown. An extensive characterization of LSDV in neighboring countries could help resolve the emergence of these isolates.
Previous studies showed that the use of the LSDV KSGP 0240 for vaccination could lead to the appearance of generalized lesions [ 15 , 41 ]. The lesions in cattle in Bangladesh showed pathogenic virus-like lesions, especially the presence of deep “sit fast,” not usually observed with KSGP 0240-induced disease [ 15 ]. It is also worth noting that Bangladesh was not vaccinating cattle against LSD before these outbreaks but later started vaccination using a goat poxvirus strain. Therefore, it is unlikely that the administration of a good quality LSDV KSGP 0240 vaccine caused these outbreaks. Furthermore, LSDV KSGP 0240-induced disease manifests only as an adverse reaction in vaccinated animals and shows no signs of animal to animal spread [ 15 , 41 ].
Historically, viruses resembling LSDV KSGP 0240, the LSDV NI2490 (1958), and LSDV Kenya (1950, but sequenced only recently), caused LSD outbreaks in Kenya [ 10 ]; however, these viruses were never detected in subsequent LSDV epidemics in Africa, the Middle East, and Europe. Whether Bangladesh isolates, and presumably those described in Kurgan, Russia, relate to LSDV NI2490 and LSDV Kenya is unclear. Further investigation through full genome sequencing is warranted, as none of the three targeted genes of this study could provide differentiation between LSDV KSGP-0240 and LSDV NI2490.
The reason for the emergence of such LSDV variants remains uncertain. However, recent reports from Russia suggest the possibility of recombination events [ 24 ].
In conclusion, using a multi-targeted approach,we discovered that the viruses causing outbreaks in Bangladesh were different from common contemporary LSDV field isolates circulating worldwide, including the Chinese isolates and the recombinant LSDVs described between 2017 and 2019 in Russia. Full genome analysis will elucidate whether these viruses are LSDV KSGP-0240 or LSDV NI2490/LSDV Kenya. This study highlights the importance of continuous monitoring and characterization of circulating strains and the need to continually refine the strategies for differentiating vaccine strains from field viruses.
Outbreak investigations and sample collection
Fifty (50) biopsies of skin nodules were collected from 6 different districts of Bangladesh (Fig. 2 ) between July and September 2019. Table 2 shows the location, source, and collection period of LSDV samples. The samples were collected aseptically and transported in a cool box to the Central Disease Investigation Laboratory (CDIL) at Dhaka, Bangladesh. Samples were stored at − 80 °C for further processing.
Sample preparation and DNA extraction
Biopsy nodule samples were cut with a scalpel into small pieces. Pieces were macerated with pestle and mortar, then transferred to sterile tubes with 10 ml sterile phosphate-buffered saline (PBS) to prepare tissue homogenates. Tubes were centrifuged at 1000 g, and 200 μl of supernatant was transferred to an Eppendorf tube for DNA extraction.
DNA extraction from skin samples was performed using DNeasy Blood & Tissuekit (Qiagen, Germany) according to the manufacturer’s recommendations. The DNA was eluted using 70 ul elution buffer and stored at − 20 °C until further use.
Molecular detection
A conventional PCR was carried out to amplify a 390 bp fragment within the P32 gene of capripoxviruses [ 28 ]. The PCR was performed using the Platinum™ Taq DNA Polymerase kit (cat# 10966–026) in a reaction volume of 25 μl containing 2.5 μl 10 X PCR buffer, 0.75 μl Magnesium chloride (50 mM), 0.5 ul dNTPs (10 mM), 0.1 μl Platinum Taq DNA polymerase, 400 nM of each primer and 1 μl template DNA. The PCR tubes containing the above mixture were transferred into a thermal cycler (T-1000, Bio-Rad, USA), and amplification was conducted with the following program: initial denaturation at 94 °C for 5 min, 38 cycles denaturation at 94 °C for 30 s, annealing at 50 °C for 30 s, extension at 72 °C for 30 s; and a final extension phase run at 72 °C for 5 min.
The PCR products were separated by gel electrophoresis on a 1.5% agarose for 60 min and visualized with a gel documentation system (UVP GelStudio PLUS Gel Documentation Imaging Systems, Analytik Jena, Germany).
A real-time PCR for the detection of capripoxvirus DNA was performed as previously described [ 31 ] with some modifications.
Briefly, the PCR mixture was set up in a reaction volume of 25 μl, containing 12.5 μl of the iQsupermix (Bio-Rad, USA), 400 nM of each primer, 250 nM of the fluorogenic probe and 5 μl of template. The reaction consisted of an initial denaturation step at 95 °C for 10 min, followed by 45 cycles at 95 °C for 15 s and 60 °C for 60 s with the fluorescence recording at the end of the combined annealing elongation step. The assaywasperformed using the CFX real-time PCR detection system (Bio-Rad).
Amplification and sequencing of the RPO30, GPCR, and EEV glycoprotein genes
The RPO30 and the GPCR were amplified as previously described [ 19 ].
A pair of primers; EEVGly F- 5′- ATGGGAATAGTATCTGTTGTATACG-3′ and EEVGly R-5′- CGAACCCCTATTTACTTGAGAA-3′ were designed for the amplification of fragments containing the partial EEV glycoprotein (encoded by ORF LSDV126) and hypothetical protein LSDV 127 gene [ 18 ]. The PCR reaction was performed in a reaction volume of 20 μl containing 500 nM of each of the forward and reverse primers, 0.2 mM of dNTPs, 1x buffer (Qiagen), 2.5 U of Taq DNA polymerase (Qiagen), and 2 μl template DNA. The amplification consisted of an initial denaturation at 95 °C for 4 min followed by 35 cycles of 95 °C for 40 s, 55 °C for 30 s, and 72 °C for 1 min, and a final extension step at 72 °C for 7 min.
The PCR products were separated by electrophoresis on a 1.5% agarose gel at 100 V for 60 min and visualized using a Gel Documentation System (Bio-Rad, USA).
The PCR amplicons were purified using the Wizard SV Gel and PCR clean-up system kit (Promega) according to the manufacturer’s instructions. LGC Genomics (Germany) performed the sequencing of the purified PCR amplicons. Vector NTI 11.5 software (Invitrogen, USA) was used for sequencing data analysis and assembly.
Nucleotide sequences were aligned using the Muscle algorithm and the codon option implemented in MEGA7 [ 42 ]. The complete RPO30 and GPCR gene sequences of additional CaPVs (LSDVs, GTPVs, and SPPVs), retrieved from GenBank, were included for comparative analyses.
The file with aligned sequences in FASTA was converted to a Nexus format using Seaview. The Bayesian phylogenetic inference was performed with BEAST v1.8.4 [ 43 ]. First, the BEAUti module was used to generate BEAST files using the HKY substitution +G nucleotide substitution and a UPGMA starting tree option. The Markov Chain Monte Carlo method was run with BEAST, for 10,000,000 generations with a sample taken each 10,000 generations. The TRACER program was used to inspect the log files and determine the optimum number of burn-in based on the Effective Sample Sizes (ESS > 200).
TreeAnnotator was used to generate the Maximum Clade Credibility (MCC) after discarding the 3% burn-in. The tree was visualized with the associated meta-data using the ggtree package in R [ 44 ]. Additionally, for the GPCR tree, the multiple sequence alignment file of the nucleotide sequences was imported. A specific slice of the alignment, between positions 80 and 120, was visualized together with the tree [ 44 ].
Availability of data and materials
DNA sequences generated and analyzed under the current study are available in GenBank under accession numbers MT448690 to MT448701. All the remaining datasets generated during this study are available from the corresponding author on request.
Abbreviations
Central Disease Investigation Laboratory
Deoxyribonucleic acid
Deoxynucleotide triphosphate
Extracellular enveloped virus
Effective Sample Sizes
G protein-coupled receptor
Goatpox virus
High-resolution melting
Lumpy skin disease
Lumpy skin disease virus
Maximum clade credibility
World Organization for Animal Health
Open reading frame
Phosphate-buffered saline
Polymerase chain reaction
RNA polymerase 30 kDa subunit
Sheeppox virus
Shen YJ, Shephard E, Douglass N, Johnston N, Adams C, Williamson C, et al. A novel candidate HIV vaccine vector based on the replication deficient Capripoxvirus, lumpy skin disease virus (LSDV). Virol J. 2011;8:1–2.
Article Google Scholar
Tageldin MH, Wallace DB, Gerdes GH, Putterill JF, Greyling RR, Phosiwa MN, et al. Lumpy skin disease of cattle: an emerging problem in the Sultanate of Oman. Trop Anim Health Prod. 2014;46:241–6.
Article PubMed Google Scholar
Davies FG. Lumpy skin disease, an African capripox virus disease of cattle. Br Vet J. 1991;147:489–503.
Article CAS PubMed Google Scholar
Carn VM, Kitching RP. An investigation of possible routes of transmission of lumpy skin disease virus (Neethling). Epidemiol Infect. 1995;114:219–26.
Article CAS PubMed PubMed Central Google Scholar
OIE. Lumpy skin disease. In: Manual of diagnostic tests and vaccines for terrestrial animals; 2017. http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.04.13_LSD.pdf .
Google Scholar
USDA. Lumpy skin disease standard operating procedures: 1. overview of etiology and ecology. 2016. https://www.aphis.usda.gov/animal_health/emergency_management/downloads/sop/lsdv_fadprep_ee.pdf . Accessed 19 July 2020.
Tuppurainen ESM, Venter EH, Shisler JL, Gari G, Mekonnen GA, Juleff N, et al. Review: Capripoxvirus diseases: current status and opportunities for control. Transbound Emerg Dis. 2017;64:729–45.
Tuppurainen ESM, Stoltsz WH, Troskie M, Wallace DB, Oura CAL, Mellor PS, et al. A potential role for Ixodid (hard) tick vectors in the transmission of lumpy skin disease virus in cattle. Transbound Emerg Dis. 2011;58:93–104.
Kara PD, Afonso CL, Wallace DB, Kutish GF, Abolnik C, Lu Z, et al. Comparative sequence analysis of the south African vaccine strain and two virulent field isolates of lumpy skin disease virus. Arch Virol. 2003;148:1335–56.
Tulman ER, Afonso CL, Lu Z, Zsak L, Kutish GF, Rock DL. Genome of lumpy skin disease virus. J Virol. 2001;75:7122–30.
Le Goff C, Lamien CE, Fakhfakh E, Chadeyras A, Aba-Adulugba E, Libeau G, et al. Capripoxvirus G-protein-coupled chemokine receptor: a host-range gene suitable for virus animal origin discrimination. J Gen Virol. 2009;90:1967–77.
Article PubMed CAS Google Scholar
Lamien CE, Le Goff C, Silber R, Wallace DB, Gulyaz V, Tuppurainen E, et al. Use of the Capripoxvirus homologue of Vaccinia virus 30 kDa RNA polymerase subunit (RPO30) gene as a novel diagnostic and genotyping target: development of a classical PCR method to differentiate goat poxvirus from sheep poxvirus. Vet Microbiol. 2011;149:30–9.
Alkhamis MA, VanderWaal K. Spatial and temporal epidemiology of lumpy skin disease in the Middle East, 2012–2015. Front Vet Sci. 2016;3:19.
Article PubMed PubMed Central Google Scholar
Stram Y, Kuznetzova L, Friedgut O, Gelman B, Yadin H, Rubinstein-Guini M. The use of lumpy skin disease virus genome termini for detection and phylogenetic analysis. J Virol Methods. 2008;151:225–9.
Abutarbush SM, Hananeh WM, Ramadan W, Al Sheyab OM, Alnajjar AR, Al Zoubi IG, et al. Adverse reactions to field vaccination against lumpy skin disease in Jordan. Transbound Emerg Dis. 2016;63:e213–9.
Agianniotaki EI, Tasioudi KE, Chaintoutis SC, Iliadou P, Mangana-Vougiouka O, Kirtzalidou A, et al. Lumpy skin disease outbreaks in Greece during 2015–16, implementation of emergency immunization and genetic differentiation between field isolates and vaccine virus strains. Vet Microbiol. 2017;201:78–84.
Menasherow S, Rubinstein-Giuni M, Kovtunenko A, Eyngor Y, Fridgut O, Rotenberg D, et al. Development of an assay to differentiate between virulent and vaccine strains of lumpy skin disease virus (LSDV). J Virol Methods. 2014;199:95–101.
Menasherow S, Erster O, Rubinstein-Giuni M, Kovtunenko A, Eyngor E, Gelman B, et al. A high-resolution melting (HRM) assay for the differentiation between Israeli field and Neethling vaccine lumpy skin disease viruses. J Virol Methods. 2016;232:12–5.
Gelaye E, Belay A, Ayelet G, Jenberie S, Yami M, Loitsch A, et al. Capripox disease in Ethiopia : Genetic differences between field isolates and vaccine strain , and implications for vaccination failure. Antivir Res. 2015;119:28–35.
Agianniotaki EI, Chaintoutis SC, Haegeman A, Tasioudi KE, De Leeuw I, Katsoulos PD, et al. Development and validation of a TaqMan probe-based real-time PCR method for the differentiation of wild type lumpy skin disease virus from vaccine virus strains. J Virol Methods. 2017;249:48–57.
Pestova Y, Byadovskaya O, Kononov A, Sprygin A. A real time high-resolution melting PCR assay for detection and differentiation among sheep pox virus, goat pox virus, field and vaccine strains of lumpy skin disease virus. Mol Cell Probes. 2018;41:57–60.
Kononov A, Byadovskaya O, Kononova S, Yashin R, Zinyakov N, Mischenko V, et al. Detection of vaccine-like strains of lumpy skin disease virus in outbreaks in Russia in 2017. Arch Virol. 2019;164:1575–85.
Sprygin A, Pestova Y, Bjadovskaya O, Prutnikov P, Zinyakov N, Kononova S, et al. Evidence of recombination of vaccine strains of lumpy skin disease virus with field strains, causing disease. PLoS One. 2020;15:e0232584.
Sprygin A, Babin Y, Pestova Y, Kononova S, Wallace DB, Van Schalkwyk A, et al. Analysis and insights into recombination signals in lumpy skin disease virus recovered in the field. PLoS One. 2018;13:e0207480.
Sprygin A, Pestova Y, Prutnikov P, Kononov A. Detection of vaccine-like lumpy skin disease virus in cattle and Musca domestica L. flies in an outbreak of lumpy skin disease in Russia in 2017. Transbound Emerg Dis. 2018;65:1137–44.
Aleksandr K, Pavel P, Olga B, Svetlana K, Vladimir R, Yana P, et al. Emergence of a new lumpy skin disease virus variant in Kurgan oblast, Russia, in 2018. Arch Virol. 2020;165:1343–56.
Kononova S, Kononov A, Shumilova I, Byadovskaya O, Nesterov A, Prutnikov P, et al. A lumpy skin disease virus which underwent a recombination event demonstrates more aggressive growth in primary cells and cattle than the classical field isolate. Transbound Emerg Dis. 2020;00:1–7. https://doi.org/10.1111/tbed.13798 .
Heine HG, Stevens MP, Foord AJ, Boyle DB. A capripoxvirus detection PCR and antibody ELISA based on the major antigen P32, the homolog of the vaccinia virus H3L gene. J Immunol Methods. 1999;227:187–96.
Ireland DC, Binepal YS. Improved detection of capripoxvirus in biopsy samples by PCR. J Virol Methods. 1998;74:1–7.
Balinsky CA, Delhon G, Smoliga G, Prarat M, French RA, Geary SJ, et al. Rapid preclinical detection of sheeppox virus by a real-time PCR assay. J Clin Microbiol. 2008;46:438–42.
Bowden TR, Babiuk SL, Parkyn GR, Copps JS, Boyle DB. Capripoxvirus tissue tropism and shedding: a quantitative study in experimentally infected sheep and goats. Virology. 2008;371:380–93.
Stubbs S, Oura CAL, Henstock M, Bowden TR, King DP, Tuppurainen ESM. Validation of a high-throughput real-time polymerase chain reaction assay for the detection of capripoxviral DNA. J Virol Methods. 2012;179:419–22.
Haegeman A, Zro K, Vandenbussche F, Demeestere L, Van Campe W, Ennaji MM, et al. Development and validation of three Capripoxvirus real-time PCRs for parallel testing. J Virol Methods. 2013;193:446–51.
Lamien CE, Lelenta M, Goger W, Silber R, Tuppurainen E, Matijevic M, et al. Real time PCR method for simultaneous detection, quantitation and differentiation of capripoxviruses. J Virol Methods. 2011;171:134–40.
Gelaye E, Lamien CE, Silber R, Tuppurainen ESM, Grabherr R, Diallo A. Development of a cost-effective method for Capripoxvirus genotyping using snapback primer and dsDNA intercalating dye. PLoS One. 2013;8:e75971.
Gelaye E, Mach L, Kolodziejek J, Grabherr R, Loitsch A, Achenbach JE, et al. A novel HRM assay for the simultaneous detection and differentiation of eight poxviruses of medical and veterinary importance. Sci Rep. 2017;7:42892.
Hosamani M, Mondal B, Tembhurne PA, Bandyopadhyay SK, Singh RK, Rasool TJ. Differentiation of sheep pox and goat poxviruses by sequence analysis and PCR-RFLP of P32 gene. Virus Genes. 2004;29:73–80.
Anonymous. Situation Report: Lumpy Skin Disease in Bangladesh Background. 2019. https://www.oie.int/fileadmin/Home/eng/Animal_Health_in_the_World/docs/pdf/Disease_cards/LUMPY_SKIN_DISEASE_FINAL.pdf . Accessed 19 July 2020.
Babiuk S, Bowden TR, Parkyn G, Dalman B, Manning L, Neufeld J, et al. Quantification of lumpy skin disease virus following experimental infection in cattle. Transbound Emerg Dis. 2008;55:299–307.
Şevik M, Avci O, Doǧan M, Ince ÖB. Serum biochemistry of lumpy skin disease virus-infected cattle. Biomed Res Int. 2016;2016:6257984.
Article PubMed PubMed Central CAS Google Scholar
Yeruham I, Perl S, Nyska A, Abraham A, Davidson M, Haymovitch M, et al. Adverse reactions in cattle to a capripox vaccine. Vet Rec. 1994;135:330–2.
Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–4.
Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol. 2012;29:1969–73.
Yu G, Smith DK, Zhu H, Guan Y, Lam TTY. Ggtree: an R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol Evol. 2017;8:28–36.
Download references
Acknowledgments
We are very grateful to the veterinarian of the Department of Livestock Services, who helped a lot in the sample collection.
This study was supported by VETLAB network initiative of the Joint FAO/IAEA Division, funded through the African Renaissance and International Cooperation fund of South Africa and the Peaceful Uses Initiatives (PUI) by Japan and the United States of America. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and affiliations.
Central Disease Investigation Laboratory (CDIL), 48, KaziAlauddin Road, Dhaka, People’s Republic of Bangladesh
Shukes Chandra Badhy, Mohammad Golam Azam Chowdhury, Mohammad Aflak Uddin Fakir, Shamima Akter, Izhar Ahmed Khan, Md Bazlur Rashid & Mohammad Sadekuzzaman
Department of Livestock Services, Dhaka, People’s Republic of Bangladesh
Shukes Chandra Badhy, Mohammad Golam Azam Chowdhury, Mohammad Aflak Uddin Fakir, Shamima Akter, Mozaffar Goni Osmani, Faisol Talukdar, Noorjahan Begum, Izhar Ahmed Khan, Md Bazlur Rashid & Mohammad Sadekuzzaman
Animal Production and Health Laboratory, Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture, Department of Nuclear Sciences and Applications, International Atomic Energy Agency, Wagramer Strasse 5, P.O. Box 100, A-1400, Vienna, Austria
Tirumala Bharani Kumar Settypalli, Giovanni Cattoli & Charles Euloge Lamien
You can also search for this author in PubMed Google Scholar
Contributions
Conceived and designed the experiments: SCB, MS, IAK; Performed the experiments: SCB, MGAC, TBKS, SA; Analyzed the data: CEL, TBKS, SA; Contributed reagents/materials/analysis tools: MAUF, MGAC, TABMMGO, FT; Wrote the paper: MS, CEL, NB; Supervised the study. MBR, GC, IAK; Edited the final manuscript: TABMMGO, FT, NB, MBR, GC, MAUF. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Mohammad Sadekuzzaman .
Ethics declarations
Ethics approval and consent to participate.
Approval of this study was obtained through the approval of Central Disease Investigation Laboratory (CDIL), Bangladesh. The samples were summited to the Central Disease Investigation Laboratory (CDIL) for diagnostic confirmation and the results reported to the OIE. Sampling was carried out under the owner’s consent.
Consent for publication
Not applicable.
Competing interests
All authors declared that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Badhy, S.C., Chowdhury, M.G.A., Settypalli, T.B.K. et al. Molecular characterization of lumpy skin disease virus (LSDV) emerged in Bangladesh reveals unique genetic features compared to contemporary field strains. BMC Vet Res 17 , 61 (2021). https://doi.org/10.1186/s12917-021-02751-x
Download citation
Received : 30 September 2020
Accepted : 08 January 2021
Published : 29 January 2021
DOI : https://doi.org/10.1186/s12917-021-02751-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Lumpy skin disease virus;Capripoxvirus;RPO30
- EEV glycoprotein
BMC Veterinary Research
ISSN: 1746-6148
- General enquiries: [email protected]
REVIEW article
Understanding the research advances on lumpy skin disease: a comprehensive literature review of experimental evidence.

- State Key Laboratory of Veterinary Etiological Biology, College of Veterinary Medicine, Lanzhou University, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Lanzhou, China
Lumpy skin disease is caused by lumpy skin disease virus (LSDV), which can induce cattle with high fever and extensive nodules on the mucosa or the scarfskin, seriously influencing the cattle industry development and international import and export trade. Since 2013, the disease has spread rapidly and widely throughout the Russia and Asia. In the past few decades, progress has been made in the study of LSDV. It is mainly transmitted by blood-sucking insects, and various modes of transmission with distinct seasonality. Figuring out how the virus spreads will help eradicate LSDV at its source. In the event of an outbreak, selecting the most effective vaccine to block and eliminate the threat posed by LSDV in a timely manner is the main choice for farmers and authorities. At present, a variety of vaccines for LSDV have been developed. The available vaccine products vary in quality, protection rate, safety and side effects. Early detection of LSDV can help reduce the cost of disease. In addition, because LSDV has a huge genome, it is currently also used as a vaccine carrier, forming a new complex with other viral genes through homologous recombination. The vaccine prepared based on this can have a certain preventive effect on many kinds of diseases. Clinical detection of disease including nucleic acid and antigen level. Each method varies in convenience, accuracy, cost, time and complexity of equipment. This article reviews our current understanding of the mode of transmission of LSDV and advances in vaccine types and detection methods, providing a background for further research into various aspects of LSDV in the future.
Introduction
Lumpy skin disease (LSD) is an emerging viral transboundary disease which can spread beyond the outbreak area and become epidemic ( Jordi et al., 2018 ; K, 2014 ; K et al., 2021 ). The most common clinical symptoms are nodular lesions on the surface of the skin and mucous membranes. Skin nodule lesions often appear on the outside of infected cattle, such as head, neck, back, perineum, breast, and other areas of the cattle ( Molla et al., 2017 ). The affected cattle have varying degrees of edema and lameness in their legs ( Salib and Osman, 2011 ). In vivo , they often present with mucosal ulcerations high fever and enlarged lymph nodes ( Moyer et al., 2000 ). It is often manifested as mucopurulent nasal discharge ( Lubinga et al., 2015 ; Elhaig et al., 2017 ). But this is not the characteristic clinical symptom of LSD. Although a large majority of the affected cattle could recover after a long period of illness, they will have long-term symptoms of mastitis, pneumonia, and deep holes in the hide ( Selim et al., 2021a ). As it is a fulminating infectious disease, the World Organization for Animal Health (OIE) stipulates LSD is the communicable disease that must be reported. LSDV can spread in many ways, such as indirect contact transmission between animals through vectors, lactation spread, blooding feeding insects, semen spread and iatrogenic transmission ( Weiss, 1968 ; Carn and Kitching, 1995 ; Mullen and Durden, 2002 ; Annandale et al., 2010 ; European Food Safety Authority, 2018 ). Some researchers have conducted experiments to confirm that the disease is difficult to spread through direct contact ( Carn and Kitching, 1995 ; Magori-Cohen et al., 2012 ; Mulatu and Feyisa, 2018 ). LSD was first reported in Zambia, South Africa in 1929 ( Rhodesia, 1932 ). During the past decades, LSDV has spread widely and rapidly throughout the North Africa, Middle Eastern, Asia and other areas of the world, seriously influence the development of the cattle and water buffalo industry ( Rgbe, 2014 ). Among them, the cattle with fine-skinned such as Holstein-Friesian and Jersey are the most susceptible to the virus. However, thick-skinned Bos indicus breeds including the Afrikander show less severe signs of the disease ( Coetzer, 2004 ).
However, there are fewer effective preventive measures for LSD. Restricting the movement of the sick cattle, quarantine, sacrifice the cattle infected with LSDV are heavily recommended ( Wolff et al., 2020b ). Control and prevention of LSD in the countries like Albania, Bulgaria, Greece, Montenegro, FYROM, Serbia, Ethiopia relies extremely on vaccination ( Gari et al., 2015 ; Klement et al., 2020 ). Because the LSDV has an intricate immune escape mechanism, no safe and efficient vaccines have been developed for this disease till now. Sheeppox virus (SPPV) and goatpox virus (GTPV) have antigenic homology and cross protection with LSDV; therefore, the vaccines of these two viruses can be used to prevented the LSD. Inevitably, the above two vaccines may have some potential risks because they are live-attenuated vaccines that derived from strains isolated in the field ( Tuppurainen et al., 2014 ; Liu L. et al., 2021a ). It is not recommended to use in the disease-free areas.
The diagnostic measures for LSD are mainly aimed at its nucleic acid sequence or corresponding antigen and antibody ( Ireland and Binepal, 1998 ). The accuracy of each diagnostic method varies in a variety of occasions.
The aim of this study is to summarize the research progress of LSDV transmission modes, the types of vaccines used, and detection methods, and to sort out the characteristics of each vaccine and detection method. It will provide a reference for cutting off the spread of diseases, research on safe and efficient vaccines and the development of efficient and fast detection methods.
LSD is a viral contagious cattle disease caused by Lumpy skin disease virus (LSDV; Murphy et al., 2008 ). The virus is a large linear double-stranded DNA genomes of 151 kb and belongs to one of the Capripoxvirus genus, subfamily Chordopoxvirniae , family Poxviridae ( Tulman et al., 2001 ; Bhanuprakash et al., 2006 ; Moss, 2007 ; K, 2014 ). Viruses of the Poxviridae family are very similar in morphological characteristics ( Mcfadden, 2005 ). Since the researchers have not yet resolved the particle pattern diagram of LSDV, we draw the prediction diagram of LSDV structural pattern based on the pattern diagram of poxvirus for reference ( Figure 1 ). The Capripoxvirus genus consists of SPPV, GTPV and LSDV ( Tulman et al., 2001 ; Zare, 2010 ). These three viruses could cause transboundary disease with serious consequences among the ruminants, causing a major threat to the global animal husbandry ( Sprygin et al., 2018a ). They all have their own specific natural reservoir. The main hosts of the first two viruses are sheep and goat, while the LSDV mainly affects the cattle and water buffalo ( Afonso et al., 2012 ; Fagbo et al., 2014 ; Lefkowitz et al., 2018 ). In addition, LSDV can also infect giraffes, impalas, and wildebeest ( Young et al., 1970 ; Dao et al., 2022 ). Capripoxvirus genus is the most harmfully significant in the Poxviridae family affecting domestic ruminants in Africa and Asia. LSDV has more than 97% nucleotide sequence homology with GTPV ( Gershon et al., 1989a ; Upton, 2004 ). It is generally acknowledged that, the original pox virus may have originated from one or more basic species. They adapted by spreading the disease among the different kind of susceptible animals ( Seet et al., 2003 ; Sohier et al., 2019 ). Homologous recombination is the key towards the evolution of the virion. A lot of viruses may have evolved from a common ancestor through genetic recombination within the virus itself to expand their host range and virulence ( Gershon et al., 1989b ). As a result, poxviruses of various animals were formed. The morphology, structure, biochemistry, and antigenicity of mammalian poxviruses in each genus are similar with each other. After infecting cells with LSDV in the 1960s, Alexander et al. (1957) and Plowright and Witcomb (1959) observed that the inclusion bodies produced in the cytoplasm were highly similar to those produced by other members of the Poxviridae family. Later, Munz and Owen (1966) observed that the morphological structure of LSDV and vaccinia virus was also very similar under the electron microscope. In recent years, researchers have observed the appearance of LSDV under the electron microscope, which is indeed similar to the appearance of other virus members in the Poxviridae that have been published ( Sanz-Bernardo et al., 2020 ). The length of the virus genome is 151 kb, which consists of a central coding area and a 2.4 kb inverted terminal repeat sequence on both wings. According to the scientific prediction, LSDV has 156 putative genes. It has nine more genes than the other two viruses in the genus. Its morphological characteristics are similar to poxvirus, about 350 nanometers in length and 300 nanometers in width, with envelopes, but no clotting activity. This virus can be proliferated in primary cells, such as lamb and calf kidney or testicular cells, sheep embryonic kidney and lung cells, and chicken embryo fibroblasts. It also can multiply in Madin-Darby bovine kidney cells and baby hamster kidney cells (BHK-21), but the pathological changes are slower. Recent investigations have found that LSDV can hardly proliferate in African green monkey kidney (Vero) cells ( Wang et al., 2022 ). Kumar et al. (2021) treated Vero cells with the product of virus amplification from primary goat kidney (PGK) cells, and it could obtain higher viral titer only after adapting to LSDV and continuing to pass generations. Higher virus titers can be produced in Vero which are cell adapted LSDV ( Kumar et al., 2021 ). This also has new implications for the production of vaccines. In some experiments that need to ensure virulence stability, for example, the construction of recombinant viruses, we choose to perform in Vero cells. LSDV can proliferate on the chorioallantoic membrane of chicken embryo and cause acne-spots, and the virus does not cause the death of the embryo ( Black et al., 1986 ; Upton, 2004 ; Kumar et al., 2021 ). The next year, Chinese scientists found that LSDV could produce higher viral titers in primary cattle testicular cells ( Wang et al., 2022 ). When the wild-type virus was attenuated to prepare the vaccine strains, the state of the chicken embryo can be observed as a reference. Because it is a double stranded DNA virus, it has a certain thermal stability. Research shows that LSDV can be completely inactivated at 56°C, making it lose its infectivity ( Wolff et al., 2020a ).
Figure 1 . The prediction diagram of LSDV structure mode. Mature virion of LSDV (MV), sometimes mature virion is surrounded by a lipid membrane derived from the endoplasmic reticulum (EV). The surface of the virus is envelope, which contains some entry-fusion complex. The virus contains lateral body, capsid and core. The surface of the EV has many surface microtubules.
Epidemiology
Geographical distribution of lsd.
In 1929, LSD was found in the Zambia, Southern Africa, then it spread north to the Middle East ( Weiss, 1968 ). By the 1940s, the disease had spread across the southern African countries, affecting plenty of livestock. During the following decades, LSD transferred slowly northwards, and it is currently present throughout virtually the entire African continent, including Madagascar. Libya, Algeria, Morocco and Tunisia are the only African countries unaffected by the disease. The first LSD outbreak in Egypt was reported in May 1988 ( Ali et al., 1990 ). In 1989 there was an LSD outbreak in Israel ( Yeruham et al., 1994 ). This outbreak was the first instance of LSD north of the Sahara Desert and outside of the African continent. After the year 2000, more and more outbreaks were reported by Middle Eastern countries and currently LSD is considered as an endemic disease in the region. At the end of 2013, the disease invaded into Turkey and Iraq. Incursion the disease into Iran and Azerbaijan was reported in the year after that. In the late 2014, the first LSDV cases were reported in the northern part of an island in the eastern Mediterranean, Cyprus. Turkey serves as an important crossroads connecting the Eurasian continent, facilitated the spread of LSD to the Balkans and some parts of the European countries. And it eventually spread to the northeast countries in Asia. Spread to the Russian Federation in 2015, followed by Kazakhstan in 2016. Then it was spread into Yi li, Xinjiang Province, China in 2019. In the following 2 years, the disease spread to southern and eastern parts of China and the countries in South Asia, including Nepal, Bhutan, Vietnam, Thailand and Myanmar ( Pseudo-urticaria, 1931 ; Mulatu and Feyisa, 2018 ; Arjkumpa et al., 2021 ; Punyapornwithaya et al., 2022 ). By 2022, the disease had spread east and north to Mongolia and Eastern Siberia ( Figure 2 ).
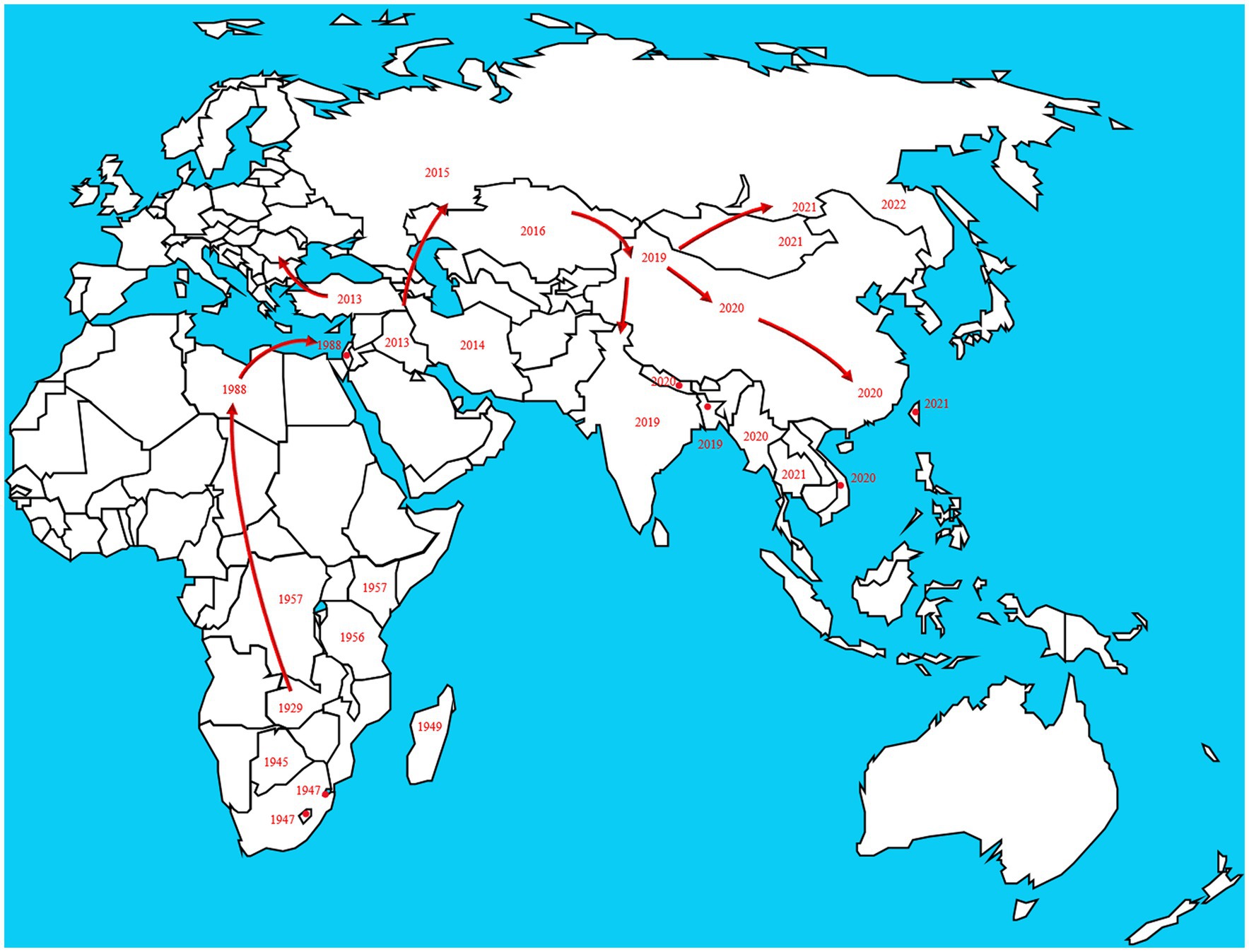
Figure 2 . The disease originated in Africa and subsequently spread to European and Asian countries. The transmission route is based on the time and location of LSD which reported by the OIE in the past two decades. https://wahis.woah.org/#/dashboards/country-or-disease-dashboard/ (accessed 26 September 2022).
Risk factors for LSD transmission
In early Africa, LSD may be widely spread due to long-distance migration of cattle. There is plenty of experimental data to support that LSDV is transmitted through the arthropods such as mosquitoes or midges, and the hematophagus such as hard ticks ( Burdin and Prydie, 1959 ; Carn and Kitching, 1995 ; Tuppurainen et al., 2011 ; El-Ansary et al., 2022 ). The latter are the main vector of the virus. Amblyomma hebraeum ticks can transmit the virus by the mechanical/intrastadial and transstadial transmission modes ( Lubinga et al., 2015 ). LSDV can live in Aedes aegypti female mosquitoes for a minimum of 2 to 8 days and infect other healthy cattle by themselves ( Chihota et al., 2001 ; Sanz-Bernardo et al., 2021 ). In addition to the blood-borne virus transmitted by tick bites, it can also be taken by the female insects of Amblyomma hebraeum and Rhipicephalus appendiculatus passing through their eggs ( Jongejan et al., 1980 ; Lubinga et al., 2014 ). The virus DNA can be detected in blood samples and nodular lesion area near the skin of susceptible cattle bitten by the larva of the Rhipicehalus decoloratus ticks. The larvae came from female ticks that had fed the blood from experimentally infected donors, and then the healthy experimentally cattle bitten by these small worms have mild symptoms typical of LSD ( Tuppurainen et al., 2013 ). LSDV can protect itself from being destroyed by the wintering habits of the individual tick species ( Bryson et al., 2002 ). LSDV can be transmitted by Stomoxys calcitrans and Haematopota spp., which are tiny blood-sucking insects ( Sohier et al., 2019 ; Issimov et al., 2020 ). They are the most probably LSDV transmission vectors ( Gubbins, 2019 ; Sanz-Bernardo et al., 2021 ). No direct studies have shown that LSDV can further multiply in vectors, but the basic reproductive number of LSDV in hosts varies greatly after the virus transmitted by different vectors reaches susceptible animals. Stomoxys Calcitrans has the highest reproductive number of 19.1, while Aedes aegypti has the lowest reproductive number of 2.4. That’s nearly eight-fold difference. However, it has been suggested that LSDV can survive in vitro culture of tick cell lines for 35 days without loss of infectivity ( Tuppurainen et al., 2015 ). In addition to the bite of ticks, the bite of some species of mosquitoes can transmit the virus too. The latest British study confirmed that LSDV can exist in the mouthparts of four blood sucking insects including Stomoxys calcitrans , Aedes aegypti , Culex quinquefasciatus , and Cubicoides nubeculosus , for about 9 days, and then spread the disease by biting healthy cattle. This is the main way for mosquitoes to transmit LSDV ( Sanz-Bernardo et al., 2022 ). According to early investigation in South Africa, it also can be spread by the direct contact, but at a significantly lower transmission rates and efficiency ( Mulatu and Feyisa, 2018 ). Due to the limitation of tick’s mobility, flies, which are good at flying, have become one of the most harmful arthropod pests to the cattle worldwide ( Gubbins, 2019 ). During the dry and cold seasons, the rate of this disease infection drops obviously, however, in the warm and wet period, the rate increases, which is closely related to the plummeting insect population and mobility. There is no significant association between sex or different cattle populations and seroprevalence of LSD infection. Furthermore, the variety of cattle, age, season, water supply and feeding system, introduction of breeding stock, and exposure to other species such as birds and insects all play important role in the occurrence of LSDV infection of LSD ( Burdin and Prydie, 1959 ; Selim et al., 2021b ). In addition to the direct contact and bites from blood-sucking insects, close-range transmission may also occur through LSDV-contaminated medical devices ( Ali et al., 2012 ). Some viruses of the Poxviridae family can be transmitted through aerosols ( Aleksandr et al., 2020 ). As a member of it, LSDV has also been reported that it may spread to other areas through air transport ( Klausner et al., 2017 ). This is the cause of repeated LSD outbreaks in some countries and regions in the Middle East. It is also possible that the blood-sucking insects travel long distances with the help of air currents ( Greenberg et al., 2012 ). But there is little chance of it spreading further into Russia and parts of Europe ( Klausner et al., 2017 ; Saegerman et al., 2018 ). When sick cattle which carrying LSDV share a food tank with the healthy cattle to drink water or feed troughs, the healthy cattle will have typical symptoms of this disease ( Ali et al., 2012 ). Researchers have pointed out that LSDV is difficult to spread through direct contact between cattle ( Weiss, 1968 ; Carn and Kitching, 1995 ; Mulatu and Feyisa, 2018 ). Aleksandr et al. (2020) found that LSDV could be transmitted without the presence of flying insects and ticks. They speculated that it might be the contact between the skin and mucous membrane of healthy cattle and infected cattle that caused the transmission of the virus. The complexity of communication has not been fully analyzed, which can be used as a future research direction. Researchers has confirmed that it should be caused by the polluted snot and saliva of the sick livestock. The reason why the cattle with symptoms are different is that the virulence level of the virus is low, and the symptoms will be more severe if they come into contact with more viruses, while eating the food with less virus, they will show mild fever, the surface of skin does not even appear nodular lesions ( Dietze et al., 2018 ). In another study, the experimenters reported that the viral loads in oral and nasal mucosa are comparable to those in skin nodules. The virus is most likely to be found in droplets and aerosols formed by the infected cattle and spread further by air currents. Therefore, saliva and nasal swabs can be a more convenient sampling method for the detection of this disease ( Babiuk et al., 2008 ; Dietze et al., 2018 ). Even though experiments have shown that LSDV may be transmitted vertically from mother to offspring through the uterus ( Şevik and Doğan, 2017 ). However, there were wounds on the surface of the born calf, which did not rule out the infection of the virus after birth. So the conclusion of vertical transmission needs to be verified ( Rouby et al., 2016 ). Vaccinated cows could detect vaccine virus shedding in secreted milk ( Bedeković et al., 2018 ). Therefore, vaccinated cows cannot breastfeed during the withdrawal period. An earlier study had confirmed that the LSDV in bovine semen for a long-term excretion. The experimental animals in this study, including azoospermic or severely oligozoospermic bull, can also detect the nucleic acid of LSDV by PCR, indicating that LSDV may exist in other body fluids than the semen fraction ( Osuagwuh et al., 2007 ; Figure 3 ). Even if the clinical signs of the bulls are not obvious, they will continue to expel LSDV to the outside environment ( Irons et al., 2005 ; Annandale et al., 2014 ). The testicles and lymph nodes of infected cattle can carry the virus, which can spread the disease. If these unsterilized animal products are transported over long distance by plane or truck, or live cattle with asymptomatic infection, or even infected cattle with obvious symptoms, as mentioned above, the disease can spread to other countries and regions ( Kononov et al., 2019 ; Table 1 ). Recently, a new technology was developed to forecast the incidence of LSDV infection by assessing meteorological and geological attributes ( Afshari Safavi, 2022 ). If this technology can be improved to predict and prevent the infection with antiparasitic drugs or vaccines in time, the losses from infection can be greatly reduced.
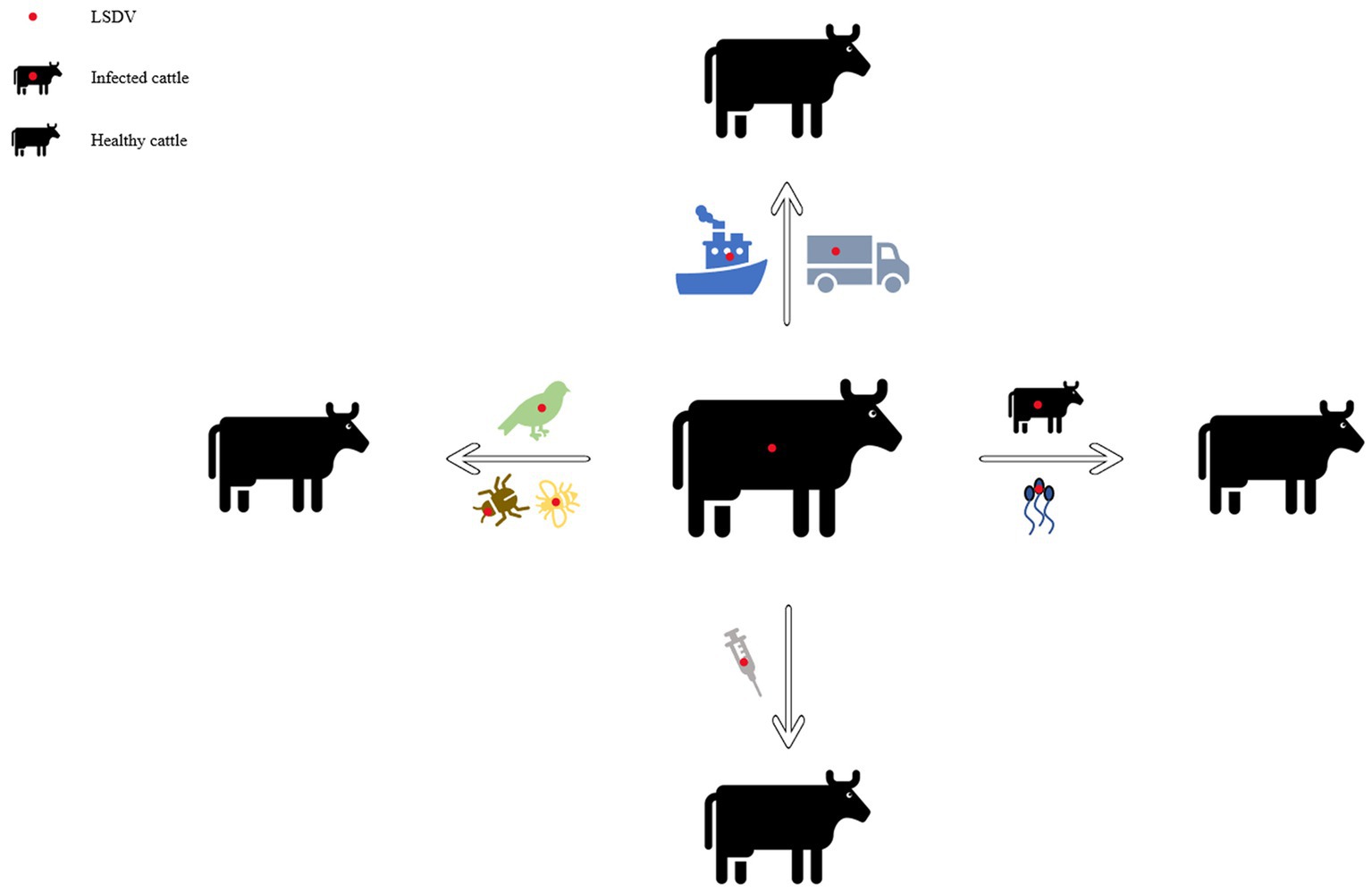
Figure 3 . Diagram of the transmission modes of lumpy skin disease. This picture shows the propagation mode of LSDV more intuitively. Each infection mode corresponds to the one which was introduced in the article.
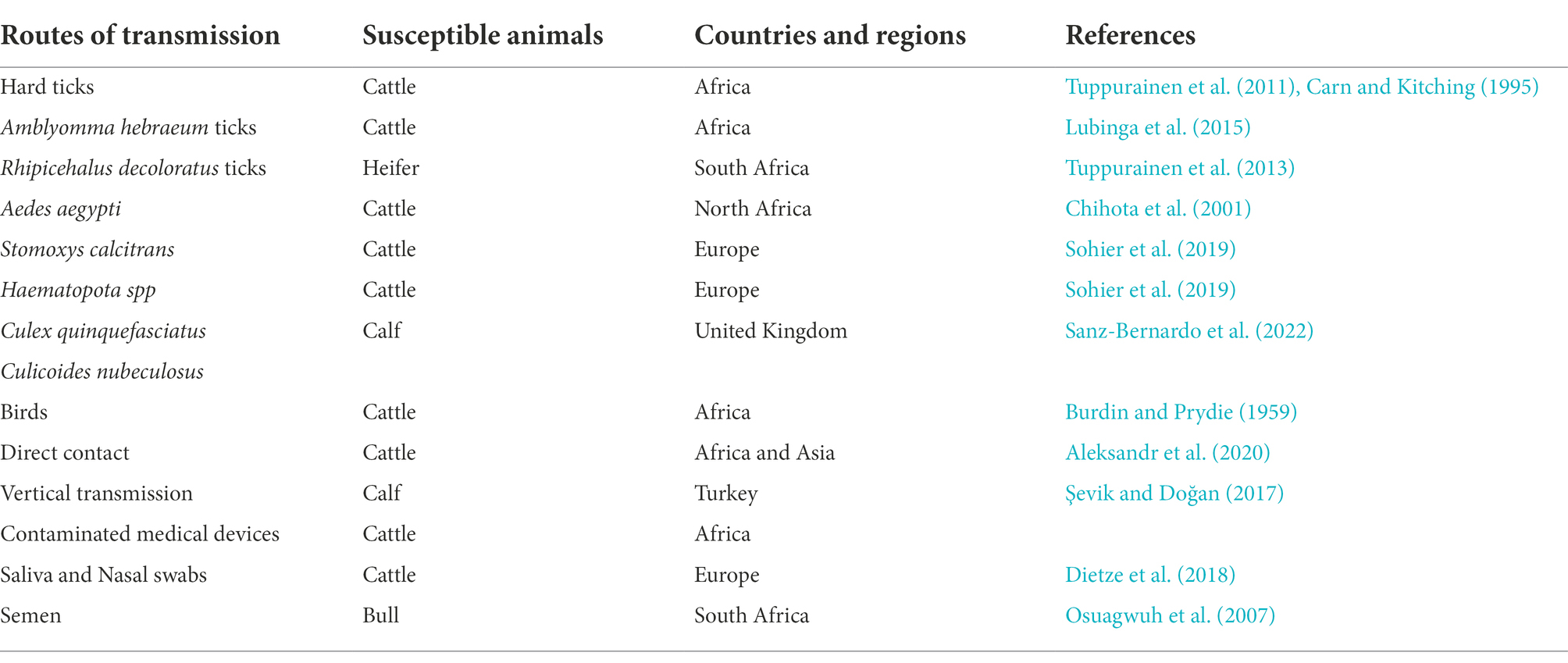
Table 1 . The modes of transmission of lumpy skin disease and the summary of the countries and regions.
The effects of LSD on cattle health
The diseased cattle infected with LSD showed some clinical symptoms that could affect their health with naked eyes, such as edema of skin mucosa, decrease milk yield of cows, enlargement of lymph nodes, nodular lesions of different sizes on the skin surface, lameness of legs, etc. ( Awad et al., 2010 ; Salib and Osman, 2011 ; Molla et al., 2017 ; Okur-Gumusova et al., 2020 ). The pathological changes of organs and tissues caused by LSDV infection in their bodies also affect their health.
Studies have shown that most of the organs and tissues of infected animals have pathological changes such as orchitis, cow mastitis, necrotic hepatitis, disseminated vasculitis, lymphadenitis, etc. A small number of cattle are accompanied by tracheitis, myocardial damage and other pathological changes, and can produce different intensity of injury induction in the affected animals, making LSDV more aggressive to the body ( Ali et al., 2021 ). Ahmed et al. found in the clinical trial that the imbalance of oxidation antioxidation status in diseased cattle resulted in excessive increase of proinflammatory cytokine content and adverse effects on animals. Subsequently, the accumulation of metabolites in the liver, kidney and heart makes the organ function impaired, which leads to the occurrence of hypophosphatemia, and further aggravates the symptoms of hemolytic anemia ( Ahmed and Dessouki, 2013 ). Abutarbush (2015) and Jalali et al. (2017) carried out the hematological and biochemical effect of LSD, the results showed that the blood of the affected cattle had pancytopenia, hyperproteinemia, hyperkalemia, hyperchloremia, and reduced creatinine concentration. It can be used as an index to evaluate the severity of the disease and to judge the prognosis.
Severe nodular lesions on the body surface of the cattle will cause holes in the skin, exposing the wound to the air. Affected cattle lack the effective protection of the first line of defense, and are prone to secondary infection with other bacterial or viral diseases, which may directly lead to their death. For susceptible cattle, timely prevention of disease and disinfection of diseased parts should be handled in place. Studies have shown that some nucleoside and nucleotide analogues can be used as anti-poxviruses drugs ( De Clercq and Neyts, 2004 ). In the future, specific anti LSDV drugs should be developed. Under the premise of vaccination, drug assisted prevention will achieve better effects against the epidemic.
The infective sensitivity of the host animals
As mentioned above, LSDV mainly infects cattle and water buffalo, but also has been reported to infect wildebeest, impala and giraffe ( Young et al., 1970 ; Dao et al., 2022 ). After all, the number of infections is very small compared with the first two. As the main host animal of LSDV, cattle and buffalo have different susceptibility to this virus.
House et al. (1990) pointed out in an investigation report that all sheep, goats and buffaloes survived the LSD outbreak in Suez and Ismalia, showing no clinical symptoms. In recent years, there is also experimental evidence that buffaloes have low susceptibility to this virus ( Neamat-Allah and Mahmoud, 2019 ). Researchers speculate that the reason is that buffalo have thick skin, and the mouthparts of blood sucking insects such as mosquitoes, flies and ticks cannot easily pass through the skin of buffalo, so the transmission rate and susceptibility of this disease are low ( Chihota et al., 2003 ; Neamat-Allah and Mahmoud, 2019 ). It is also possible that because buffalo have been living in the pond for a long time, their skin is exposed to the air for a much shorter time than other breeds of cattle. This makes it difficult for blood sucking insects to touch their skin, resulting in a lower susceptibility to LSD. Barnard (1997) detected that there was no LSD antibody in wild buffaloes in South Africa, which may also indicate that buffaloes are not sensitive to LSD. However, the number of subjects is not large enough, the reliability of this study remains to be discussed. Some clinical trials also showed that buffalo inoculated with LSD vaccine could not effectively stimulate the body to produce anti LSD antibodies ( Okur-Gumusova et al., 2020 ). This requires national veterinary authorities to timely and effectively assess the effectiveness of vaccines and develop vaccination strategies.
For cattle with strong resistance, such as buffalo, it may be able to resist the invasion of LSDV. The researchers said that this may be due to the insensitivity of buffalo to LSDV, which is only its non-adapted host. It may also be caused by the life habits of buffalo and the structure of their skin is different from that of ordinary domestic cattle. They also found that there was capripoxviruses in the buffaloes of the test group, which may be the symptom caused by other viruses rather than LSDV, making the laboratory staff not accurate enough to detect the content of LSDV antibody ( Elhaig et al., 2017 ). Therefore, the future research direction should be to confirm the true pathogen in buffaloes with LSDV symptoms. If LSDV is indeed the culprit, scientists need to develop LSDV vaccines that specifically target buffalo and can induce high-level antibodies.
Research advances in LSD vaccines
The prevention and elimination of infectious diseases ultimately depend on the largescale use of corresponding vaccines. Smallpox virus, a member of Poxviridae family that damages human life and health, has been eradicated in the last century after high-density mass inoculation of live vaccinia vaccines ( Bhanuprakash et al., 2012 ). LSD is no exception as an infectious disease that seriously endangers the development of cattle industry ( Wolff et al., 2020b ). As far as the development of vaccines is concerned, it has roughly gone through live attenuated vaccines, inactivated vaccines, recombinant vaccines, combined vaccines, genetic engineering vaccines ( Francis, 2018 ; Wang et al., 2020 ). The various vaccines that are developed by scientists over the years have their own characteristics, advantages and disadvantages.
Live attenuated vaccines
Live attenuated vaccines, also known as attenuated vaccines, refer to natural virulent strains of microorganisms that have lost or weakened their pathogenicity to the original host animal through physical, chemical or biological treatment, and have been continuously passaged and screened. Vaccines prepared from strains that maintain good immunogenicity and genetic characteristics, or attenuated strains selected and multiplied from nature and culture conditions with good immunogenicity ( Gershon et al., 1984 ). However, the live vaccine itself also has limitations, such as clinical side-effects, the risk of detoxification, and the risk of contracting new diseases due to homologous recombination with other viruses of this genus ( Lee et al., 2012 ; Sprygin et al., 2018b ; Krotova et al., 2022 ). Therefore, it is not recommended to be used in the areas without this disease. Immunosuppression is a factor to be considered after vaccination with attenuated vaccine, and its consequences can lead to a weak immune response to the vaccinated vaccine, while increasing the risk of secondary infection ( Harland et al., 1992 ).
As the most representative strain of LSDV, the Neethling strain in South Africa was originally known as a virus similar to vaccinia virus, and it was the real pathogen that caused the outbreaks in Botswana in 1943 and South Africa in 1945 and then it was purified and named Neethling type ( Stephens, 1966 ; Hunter and Wallace, 2001 ). Weiss (1968) investigated in clinical trials that the live vaccine made of this strain attenuated could play a certain prevention role against LSD. Then Weiss serially passaged this strain in the challocyst membrane of chicken embryos, resulting in the attenuation of the virus virulence. By the 20th passage, the virus did not cause systemic rash or other typical symptoms, and only half of the inoculated cattle localized swelling at the site that resolves within the next 4 to 6 weeks without signs of necrosis. Some mild side effects from this vaccine are called Neethling disease. The disease was also reported after vaccinating cattle with Neethling strain ( Yeruham et al., 1994 ). Those vaccinated with the live attenuated Neethling strain produced a local response and the antibodies in the cattle were maintained for more than 3 years, and both cattle were resistant to the virulent strain even in cattle without a local response. Field study in Israel in 2012 concluded that Neethling had a lower incidence of morbidity after vaccination ( Ben-Gera et al., 2015 ). The use of passaging and attenuation methods should be appropriate. If the virulence is excessively weakened, the immune effect will be counterproductive. The Neethling vaccine produced in Ethiopia could not protect vaccinated cattle against the virus challenge in clinical trials, with a protection rate of only 30% ( Gari et al., 2015 ). One survey on a dairy farm in Northern Greece showed that after inoculation of adult cows with the Neethling strain, swelling was seen in 12% of immunized cows, which then subsided. Small skin nodules less than 0.5 cm in 9% of them, not in calves. Mild viremia occurs in vaccinated herds, luckily this condition is of short duration ( Katsoulos et al., 2018 ). According to the ( European Food Safety Authority, 2017 ), in Croatia, less than 1% of the cattle were vaccinated with Neethling vaccine and had adverse reactions ( Calistri et al., 2019 ). It has been reported that Ethiopian Neethling vaccine was not protective against the disease ( Gari et al., 2015 ). A study carried out by Bedeković et al. (2018) found that the vaccine virus could be detected in milk from cows vaccinated with this vaccine strain. Adverse reactions may occur when using the Neethling vaccine. Therefore, vaccine efficacy and safety should be fully evaluated to achieve the desired immune effect.
Haegeman et al. (2021) conducted numerous clinical trials and compared LSDV homologous live attenuated vaccines including Lumpy Skin Disease Vaccine (South-Africa), Lumpyvax (South-Africa), Kenyavac (South-Africa), Herbivac LS (South-Africa) and Vaccin LSD Neethling O vivant (Morocco). The above vaccines could cause the body to have a fever, but none negatively affected feed intake and daily activities and general health in all groups. Swollen lymph nodes in the group receiving the Herbivac LS vaccine. The remaining three vaccines from South Africa showed symptoms of Neethling disease after being vaccinated. Small nodules developed in the group vaccinated with the Moroccan Neethling vaccine, not as large as those found in infected animals ( Haegeman et al., 2021 ). Considering the aforementioned Greek study, the subjects of the two experiments are very different, so there is a certain deviation in the data of clinical symptoms ( Katsoulos et al., 2018 ). After the first three vaccines were inoculated and challenged by virulent strains, the virus can be detected in the blood. It is considered that the virus is detected in the blood after being challenged with the virulent strains, which is not the true viremia. None virus was detected in the blood of the cattle which were vaccinated with the latter two vaccines. The pathogen of contagious bovine pleuropneumonia (CBPP) is Mycoplasma mycoides subsp. mycoides (Mmm). Safini et al. (2022) made a bivalent vaccine by attenuated this pathogen (strain obtained from CIRAD AF262936) and Neethling strain (ID: AF409138), which can induce inoculated cattle to produce high-level neutralizing antibodies against the two diseases without clinical adverse reactions. It is predicted that the vaccine can protect the two diseases. However, there is no challenge with virulent strains, and the future test direction should be inclined to verify the protection after the challenge of virulent strains.
LSDV shares more than 97% nucleic acid sequence homology with GTPV and SPPV ( Tulman et al., 2001 , 2002 ). Therefore, cross-immunization of goat pox or sheep pox live attenuated vaccine is usually used clinically to prevent LSD. Back in the 1990s, veterinarians in Egypt controlled outbreaks of LSD using a vaccine against a Romanian poxvirus strain ( Ali et al., 1990 ). Gari et al. (2015) verified that a sheep and goat pox (KSGP) 0–180 strain vaccines prepared in Kenya did not provide LSDV protection in cattle ( Gari et al., 2015 ). Brenner et al. (2009) developed a clinical response after re-exposure to LSDV infection in Yugoslav RM65-vaccinated cattle during an epidemic in 2006–2007. Bamouh et al. (2021) demonstrated that the KSGP O-180 and KSGP O-240 vaccine strains may have the problem of vaccine virus shedding, thereby infecting other unvaccinated or other healthy cattle. The Gorgan goatpox vaccine (Gorgan vaccines) developed by the Jordan Biological Center was used to prevent goat pox virus in the Middle East in 2010 ( Abbas, 2010 ), and then Gari et al. (2015) used this vaccine to study against LSDV and found that it can significantly stimulate the cellular immune response of vaccinated cattle, proved that the vaccine is highly immunogenic against LSDV. For two decades from 1989 to 2009, the Israeli authorities had used the RM-65 vaccine strain to control LSD and sheeppox, but the vaccine did not eliminate both diseases ( Brenner et al., 1992 ; Yeruham et al., 1995 ).
When live vaccines are used to protect animals against viral and bacterial infections, the exact nature of the genetic transformation that results in attenuation is unknown. Since attenuating mutations occur randomly, a single point mutation that causes a virulence return in animals will occur. These uncontrollable factors make the attenuated vaccine a time bomb that can be detonated at any time ( Minor et al., 1986 ). The previous description mentioned that the effects of some vaccine strains in the last century and this century were significantly different, which may be due to the base pair mutation of the vaccine strains during the production process. The incidence of homologous recombination of double-strand DNA viruses is high, and the vaccine may not exert its original effect due to the enhanced virulence of the virus after inoculation and other viruses of the Poxviridae family. Therefore, in the clinical application of live attenuated vaccines, specific problems should be analyzed in detail, and more suitable vaccines should be selected according to the actual situation of the cows to be vaccinated ( Table 2 ).
Table 2 . The live attenuated vaccines of LSD.
Inactivated vaccines
Inactivated vaccines refer to the inactivation of complete viruses (or bacteria) by physical, chemical and biological methods, so that the pathogens are sufficiently killed, infectivity or virulence is lost, and their immunogenicity is maintained. It has the advantages of low production cost, short development cycle and good usage effect. Compared with live attenuated vaccines, inactivated vaccines usually require booster immunization to prevent virus invasion ( Bhanuprakash et al., 2004 ). Blackall (1988) reported in 1988 that the use of vaccine adjuvants can effectively enhance the effect of inactivated vaccines.
There are no reports of inactivated LSD vaccines circulating on the market. It was found that the use of Bi-ethylimine bromure (BEI) to inactivate the attenuated Neethling strain also provided good protection. A variety of antibodies can be detected and the virus neutralization test demonstrated that the antibody response rate of the inactivated vaccine was 37% higher than that of the live attenuated vaccine on the 28th day after vaccination ( Hamdi et al., 2020 ). Y Es-sadeqy et al. developed a bivalent inactivated vaccine with oil adjuvants against the LSDV and Bluetongue virus (BTV) in 2020, and stimulated the production of high levels of neutralizing antibodies considered animals welfare and animal ethics, no challenge test to be conducted, so the specific clinical effect needs to be verified by further experiments ( Es-Sadeqy et al., 2021 ). Wolff et al. (2020b) pointed out that different vaccine adjuvants can make inactivated vaccines more effective. The use of adjuvant A, whose main component is a low molecular weight copolymer, and adjuvant B is composed of amphotericin, Quil A and cholesterol, both adjuvants were used in the inactivated Neethling vaccine and Serbia vaccine have no adverse reactions. Adjuvant A can effectively stimulate the humoral immune response and the production of IFN-γ in vaccinated cattle, so it has become a clinically preferred adjuvant. Matsiela et al. (2022) inactivated Neethling strain with low concentration of BEI, used Montanide™ Gel-01 as vaccine adjuvant, and immunized rabbits to obtain a high level of neutralizing antibody. It has not been tested in cattle, and the new adjuvant developed can be used as a reference.
After the inactivated vaccine is injected into the animal, other proteins and antigen components in it will also induce the body to react. Therefore, in addition to interfering with the host’s immune response, it will also induce unwanted immune responses, which facilitates the antigen extraction process, optimizations and improvements ( Stephens et al., 1984 ). One disadvantage of inactivated vaccines is that they generate a narrow immune spectrum, although they are excellent in inducing humoral immunity, they are rarely effective in inducing cell-mediated immune responses and mucosal immune responses. Therefore, it is still necessary to prepare a safer and more efficient inactivated vaccine against LSDV ( Table 3 ).
Table 3 . The inactive vaccines of LSD.
Recombinant vaccines
Because the live attenuated vaccine can keep all relevant antigens in the vaccine, and the pathogen can replicate in the host, it can stimulate the host’s cellular immunity and humoral immunity, so it is considered to be the most ideal method. Unfortunately, traditional methods cannot attenuate all pathogens. As mentioned above, even if the virus is attenuated, virulence return may occur. In order to overcome these problems, some scientists had tried to identify the virulence genes of different pathogens, change the virulence of pathogens by directional mutation or deletion of these virulence genes, and achieve attenuated strain in a recombination way ( Liang et al., 1991 ).
Deletion of the thymidine kinase and glycoprotein genes of herpes virus did not change its normal replication in vitro ( Kitching et al., 1987 ). Then Romero et al. pointed out that the recombinant capripox virus vaccine expressing the rinderpest fusion protein gene was prepared by using homologous recombination to knock out the thymidine kinase gene of pox virus and then recombine with the fusion protein gene of rinderpest virus, which can effectively protect vaccinated cows from rinderpest and the threat of lumpy skin disease ( Romero et al., 1993 ). Ten years later, Ngichabe et al. (2002) confirmed the reliability of the recombinant vaccine for rinderpest and goat pox through successive research results. A single vaccination protected cattle from rinderpest and lumpy skin disease virus for up to a year and the cattle in other experimental group can be protected for up to 3 years. Johnston and McFadden (2003) found that poxviruses can encode a homologue of interferon gamma to competitively block the binding of interferon gamma produced in the host to its natural receptor, thereby achieving the purpose of immune escape. Wendy et al. (2002) found that the aphthous virus gene can encode the production of interleukin-10-like, which subsequently produces immunosuppressive effects on host cells. On this basis, D. Kara et al. used homologous recombination technology to construct deletion of LSDV open reading frames 005 and 008, and then constructed recombinant vaccines, including LSDV-WB005KO and LSDV-WB008KO. Clinical trials after that, the aggregation of the two can effectively stimulate the neutralizing antibody level of the vaccinated cattle, which can effectively resist the invasion of LSDV. However, in the early stage of vaccination, there will be clinical reactions that are small and can be subsided ( Kara et al., 2018 ). It was previously reported that LSDV-WB005KO also protected vaccinated animals from SPPV and GTPV ( Boshra et al., 2015 ). Then the LSDV-WB005KO may be a better choice in clinical practice.
Recombinant poxviruses, like other vaccines, are concerned by regulatory authorities that vaccines made from recombinant viruses will also be released into the environment, posing a safety hazard to healthy animals. The solution to this problem is to study suicidal or non-replicating recombinant viruses. Graham and Prevec (1992) developed replication-deficient adenoviruses. These viruses can replicate in vitro in cells containing the E1 region, but cannot replicate in normal cells. Even if healthy animals are exposed to the virus, it is safe. In the following 2 years, Heffner and Peeters et al. reported that some herpes viruses must delete their glycoprotein genes to replicate in cell lines containing glycoprotein genes, but not in normal cells. The development in a direction of safe use also provides a new idea for the preparation of vaccines after recombination of LSDV with other viruses ( Table 4 ; Heffner et al., 1993 ; Peeters et al., 1994 ).
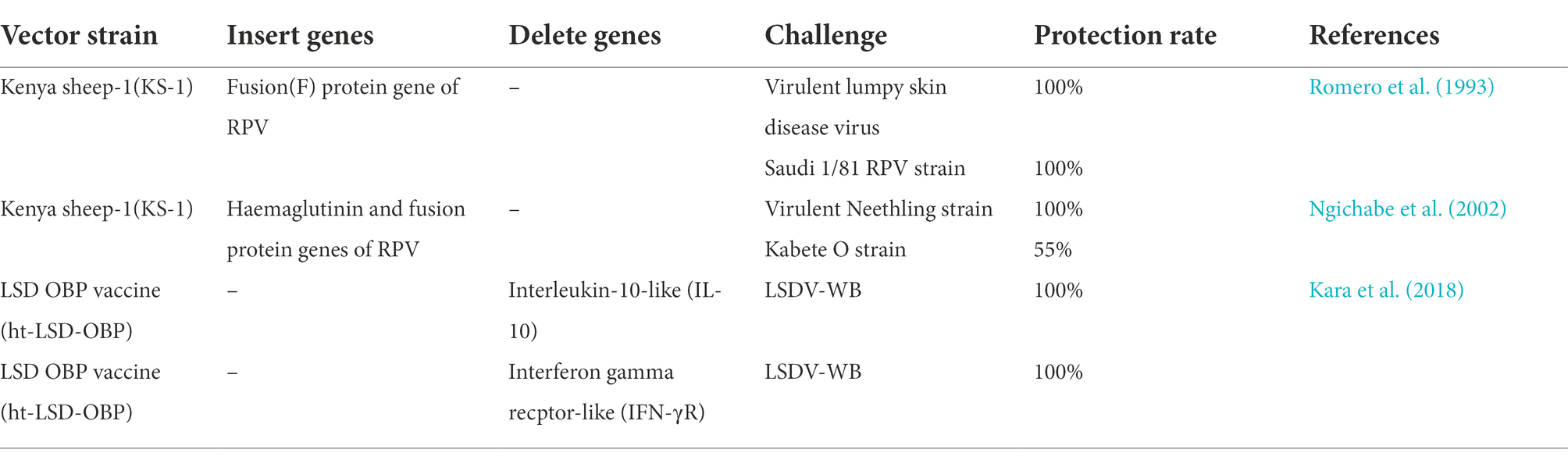
Table 4 . The recombinant vaccines of LSD.
Vaccine carrier
Therapeutics that deliver DNA into the body to express the corresponding protein in some way is a long-term goal that will require the efforts of generations. Miller and Dusty (1992) . published an article saying that from more difficult techniques such as transplantation of transfected cells (lymphocytes, myoblasts, hematopoietic stem cells), to more direct methods, such as the use of viral vectors (reverse transcription Viruses, adenoviruses, herpesviruses, parvoviruses) deliver DNA to target tissues in the body, thereby stimulating the activation of the body’s immune system. Poxviruses have been widely studied as vaccine vectors. Due to their large genome, vary in size from 130 to 375 kb ( Holowczak, 1982 ). Such innate conditions allow them to tolerate the insertion of foreign genes of more than 25,000 base pairs. In the presence of a highly active promoter, the simultaneous expression of multiple exogenous genes can be achieved, and the humoral immunity and cellular immunity can be effectively activated ( Zavala et al., 2001 ; Willey et al., 2003 ). In addition, it has a narrow host spectrum and is safe as a vaccine carrier ( Hunter and Wallace, 2001 ). The virus is heat-resistant, which can reduce the cost of refrigerated storage. At the same time, a multivalent vaccine can prevent multiple diseases after injection, which is far safer and more cost-effective than multiple injections of a single vaccine to achieve the same effect of preventing multiple diseases ( Prow et al., 2018 ).
Aspden et al. (2002) recombined the glycoprotein gene of rabies virus into LSDV as a vector (rLSDV-RG), this recombinant virus can stimulate the humoral immune response after clinical trials, the results show that 75% of cattle can resist rabies virus significantly stronger than the control group. Subsequently, the same author in 2003 published a paper confirming that the vaccination of non-ruminant animals such as rabbits and mice with this vaccine induce rabies virus-neutralizing antibodies twice as high as reported by the World Health Organization (WHO). The level of neutralizing antibodies produced after vaccination of the mice was comparable to that of the commercially available rabies vaccine ( Aspden et al., 2003 ). Wallace and Viljoen (2005) found that two viral recombinants were constructed by using lumpy skin disease virus as a vector and the thymidine kinase gene located on it as the insertion site of foreign genes. They are the structural glycoprotein gene expressing Bovine Epizootic fever (LSDV-BEFV) and the two genes expressing Rift Valley fever glycoprotein (LSDV-RVFV). These two virus recombinants can induce the production of neutralizing antibodies in experimental animals. After LSDV-BEFV stimulation test, high levels of neutralizing antibodies can be stimulated. Mice inoculated with LSDV-RVFV are resistant to RVFV up to 100%.
The genes recombined with LSDV listed above are all from RNA viruses (Rabies virus, Bovine Epizootic fever virus, Rift Valley fever virus), so this other multivalent vaccine brings new ideas. At the same time, it also provides a reference for the development of vaccines for lumpy skin disease.
Research advances on the diagnostic methods of LSD
The on-site diagnosis of LSD often relies on clinical symptoms to determine whether the cattle are infected with the disease. However, in the early stages of the disease, affected cattle often show only fever and very few skin lesions, which greatly reduces the accuracy of the diagnosis. According to the researchers’ findings, the clinical symptoms of lumpy skin disease are similar to those of bovine herpesvirus infection, demodicosis, bovine viral diarrhea-mucosal disease and bovine malignant catarrhal fever ( Reid et al., 1984 ; Deregt and Loewen, 1995 ; Reddy and Sivajothi, 2016 ). The presence of these factors complicates field diagnosis. Therefore, more accurate detection methods such as directly targeting the pathogen in the laboratory are needed ( Perry, 2010 ). Sometimes after vaccination, animals still suffer from the disease. In consequence, judging whether animals were infected with the more virulent wild strain or the vaccine strain adversely affected the animals. Then it is particularly important to distinguish whether there is a wild virus or a vaccine strain in the animal ( Chibssa et al., 2018 ; Fawzi et al., 2022 ).
Diagnostic methods of The LSD
Heine et al. (1999) found that SPPV and LSDV in the genus Capripoxvirus have a P32 gene with a nucleic acid sequence similarity of more than 98%. Two nucleotide site mutations in the P32 gene of LSDV lead to two Eco R V sites are missing. Therefore, the researchers used this idea for clinical testing, using specific primers B68 and B69 to amplify the P32 gene by polymerase chain reaction (PCR) in the viral samples collected from the field, followed by restriction endonuclease digestion susceptibility to determine whether the cattle infected with LSDV or SPPV. If this feature is used as a diagnostic method, more Capripoxviruses need to be sequenced to confirm that the Eco R V locus is specific for all SPPV. Babiuk et al. found that the presence of LSDV could be detected using real-time PCR. Compared with the oral and nasal mucosa, the detection rate of the diseased material in the skin nodule injury site was higher. This provides a reference for the collection of clinical patient materials in the future ( Babiuk et al., 2008 ). In October of the same year, Stram et al. (2008) reported that they analyzed the non-homologous sequences of the three viruses (GTPV, SPPV and LSDV) in the genus Capripoxvirus , found a nucleic acid sequence that only exists in LSDV, and then designed PCR primers for this sequence to distinguish LSDV. A comparative experiment by Awad et al. (2010) in October of the following year showed that the detection rate of virus in the blood of infected cow skin biopsy specimens and infected animals by PCR can reach 100%, and the detection rate of virus in the blood of febrile cows can reach 77.8%. All were significantly higher than the virus detection rate of virus isolation, dot blot hybridization and indirect enzyme-linked immunosorbent assay (ELISA). Therefore, PCR method can be used as a fast and efficient tool for LSDV field infection diagnosis ( Awad et al., 2010 ). In 2016, a portable, simple, and rapid method called recombinase polymerase amplification (RPA) assay for the field detection of the genome of LSDV ( Shalaby et al., 2016 ). Yana et al. (2018) published a technique based on real-time high-resolution fusing PCR. The nucleic acid sequence of the viruses (SPPV, GTPV, LSDV) in the disease materials collected on site was amplified by PCR, and then the three viruses were distinguished according to the melting temperature of the generated amplicons. The gene that specifically targets LSDV is LSDV-ORF010, which has the unique species-specific nucleotide differences. Subsequently, Modise et al. (2021) found on this basis that the type of virus can be analyzed by the high-resolution melting (HRM) assay of PCR amplification products generated after genus-specific primers amplify sample viral DNA and bind dyes. Some farms do not have expensive and high-precision instruments, such as PCR machines, and the staff that on the farm may not have the skills to operate PCR machines, so a cheaper, convenient, reliable and easy-to-operate method is needed to replace PCR. Mwanandota et al. (2018) explored a novel method for the detection of LSDV, named loop-mediated isothermal amplification (LAMP). This method target the poly (A) polymerase small subunit (VP39) gene because of its higher detection rate and sensitivity. It is possible to detect extremely small amounts of nucleic acid substances present in the air with experimental error. Zeedan et al. (2019) compared PCR, real-time PCR (qPCR), fluorescent antibody technology (FAT), indirect FAT (iFAT) and indirect ELISA (iELISA) for the detection of LSDV and the positive rate of LSDV antibodies. In the skin disease material detection group, the detection rate of qPCR was better than that of conventional PCR, which could reach 39.13%. The virus detection rate of the FAT method was the lowest at 32.6%. All were higher than the antibody positive rate detected in blood. For the detection of LSDV antibodies in serum, iELISA was 3.45 percentage points higher than iFAT. This also confirms what was mentioned above, suggesting that when collecting disease materials on the spot, the skin nodule lesion area is preferentially collected as a test sample ( Zeedan et al., 2019 ). Then, Haegeman et al. (2020) developed a novel high-sensitivity, high-specificity assay, the Immunoperoxidase Monolayer Assay (IPMA), which can detect LSDV antibodies early in vaccination and disease infection. This technology can be used in simple and crude environment detection, with high safety and can be processed in ordinary biosafety level laboratory. The same year, a novel study was reported by Milovanovic et al. (2020) using ELISA to detect LSDV-specific antibodies in milk. The advantage of this new technology is non-invasive sampling, which can collect a wide range of samples and can be used for large-scale screening. Sequence differences in three genes, RPO30, P32, and GPCR, were analyzed using single-nucleotide polymorphism using nanopore sequencing technology to build a database to distinguish GTPV, SPPV, and LSDV. The ease of replication of this database makes it more widely used. The advantages of this technology are that it is suitable for complex clinical environments, short detection time, and strong portability ( Eltom et al., 2021 ). Lesions caused by LSDV in other species can also be used as a differential diagnosis method, for example, the isolation of the virus into embryonated chicken eggs (ECEs) can cause characteristic pitting lesions on the chorioallantoic membrane ( El-Ansary et al., 2022 ). The pathological sections made from the skin lesions can be diagnosed by immunohistochemical (IHC) methods, and the distribution of pathogenic antigens can be detected by specific anti-LSDV antibodies. Changes in the dermis and epidermis of the skin after infection with the virus can be observed under the microscope, including watery degeneration, granulomatous reaction, dystrophic calcification of the dermis, and the formation of inflammatory cells ( El-Neweshy et al., 2013 ; Sanz-Bernardo et al., 2020 ; Amin et al., 2021 ). Ali et al. observed inclusion bodies in the cytoplasm of bovine skin capsule through histopathological examination, and confirmed that these inclusion bodies were characteristic pathological lesions related to LSD. In recent years, some researchers confirmed this view in clinical tests ( Ali et al., 1990 ; Ahmed and Dessouki, 2013 ; Neamat-Allah and Mahmoud, 2019 ). A new rapid diagnostic technique for LSDV-ORF068 gene targeting using recombinase polymerase amplification assay (RPA) combined with CRISPR-Cas12a-based fluorescence assay (RPA-Cas12a-fluorescence assay). It can be detected in trace amounts with excellent accuracy and sensitivity. There is no cross-reactivity with other common bovine viruses ( Jiang et al., 2022 ). A rapid diagnostic tool of colorimetric sandwich-type lateral flow immunoassay (LFIA) was established using two monoclonal antibodies against different epitopes of P32 structural protein of LSDV and gold nanoparticles ( Cavalera et al., 2022 ). The sensitivity of this new method is similar to that of ELISA, but it has not been widely used in clinical diagnosis, and its specificity needs to be determined after clinical trials. All the above methods require instruments and power equipment to detect. Korthase et al. (2022) invented a method of extracting nucleic acid without electricity, namely Triple E , which can extract nucleic acid from 8 samples within 10 min and ensure sensitivity. It can be applied to the place without good experimental conditions for diagnosis. For the diagnostic methods of detecting a specific gene to determine the type of virus, we need to screen a large number of viral nucleic acid types to ensure that the above-mentioned specific gene is not in the local affected cows with similar symptoms due to the homologous recombination or gene mutation ( Table 5 ).
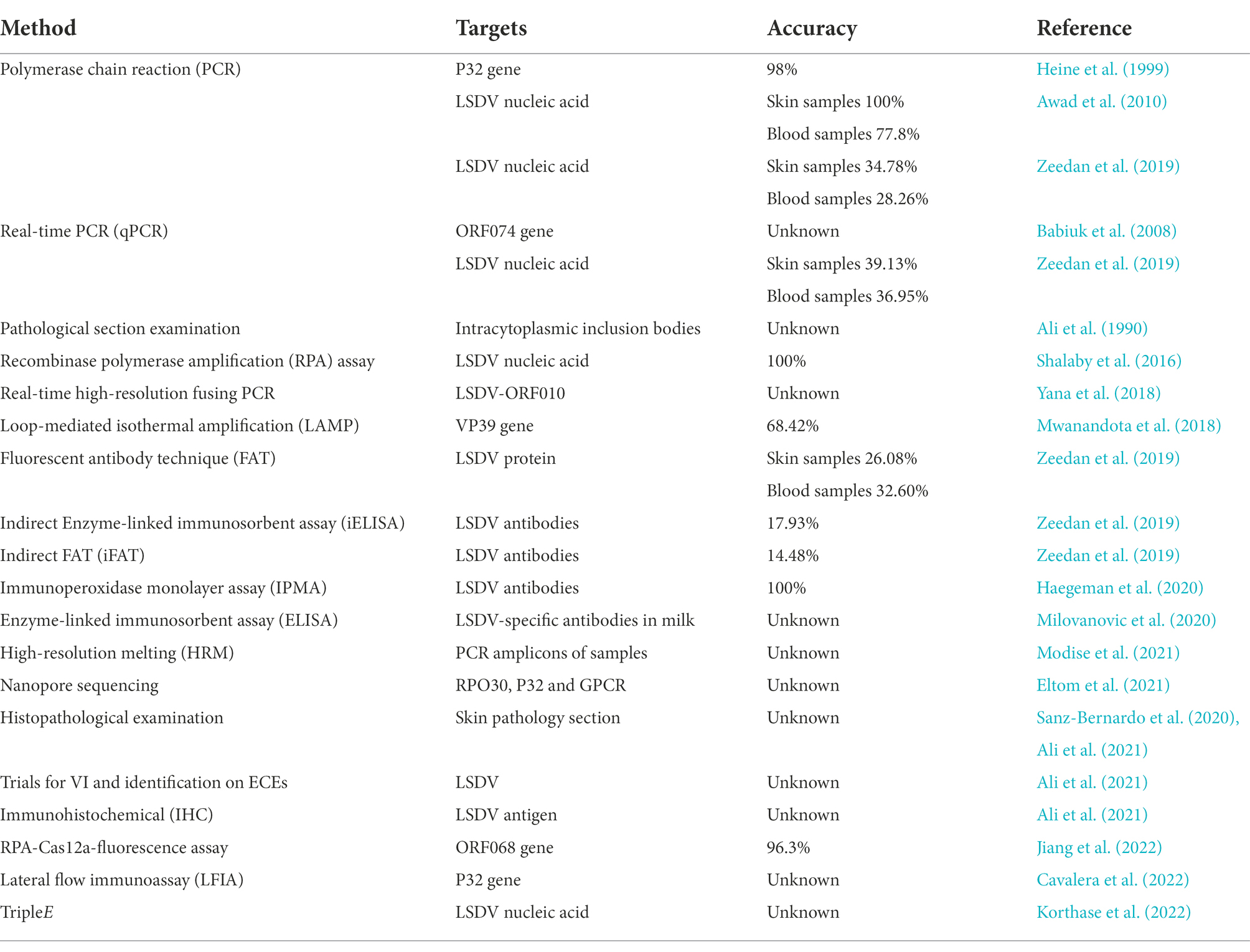
Table 5 . The diagnostic methods of LSDV.
Different diagnostic methods can be selected according to the stage of the disease in clinical application. In the early stage of LSDV infection, when the clinical symptoms are not obvious, some highly sensitive detection methods can be used, such as nucleic acid level, antigen antibody level detection methods. Early diagnosis, detection and treatment can effectively prevent and treat in advance. When the clinical symptoms obviously, the characteristic pathological changes can be used for differential diagnosis.
Distinguish between wild-type and vaccine viruses of LSDV
Menasherow et al. (2014) found three methods to detect vaccine strains versus wild strains. First, the Neethling vaccine strain is 27 bases shorter than the Israeli virulent strain of the enveloped virions (EEV) gene, which can be distinguished by genetic sequencing based on this finding. The second method is to use primers to amplify the genome of the virus, and then use specific upstream and downstream primers to amplify by nested PCR, and determine the composition of the virus according to the difference in annealing temperature. The last method is to digest the amplicon of the PCR reaction according to the Mbo I enzyme cleavage site. The vaccine strain samples can be digested by Mbo I enzyme, but the virulent strain cannot be digested. Clinically, the second and third identification methods are more rapidly and widely used ( Menasherow et al., 2014 ). Agianniotaki et al. (2017) developed a dual real-time PCR method in 2017, targeting GPCR genes, and the amplification efficiency of wild-type virus and the vaccine virus was 91.3% and 90.7%. According to the amplification efficiency of the sample to identify whether it is a wild-type virus or a vaccine virus. Several detection methods that have been researched and discovered so far need to analyze the obtained data to get the results. Möller et al. (2019) developed a new technology based on TaqMan probe-based independent double-stranded real-time qPCR (real-time quantitative PCR) detection method to distinguish virulent strains from vaccine strains with 100% analytical sensitivity and specificity. Also targeting the EEV gene, Agianniotaki et al. (2021) developed a novel surveillance tool, duplex real-time PCR, to specifically detect the presence of wild-type LSDV in samples containing high titer vaccine against LSDV. In the presence and absence of LSDV vaccine virus, the amplification efficiencies of virus samples were 99.0% and 98.6%. The experimenters set β-actin as an internal amplification control to increase the detection accuracy ( Table 6 ).
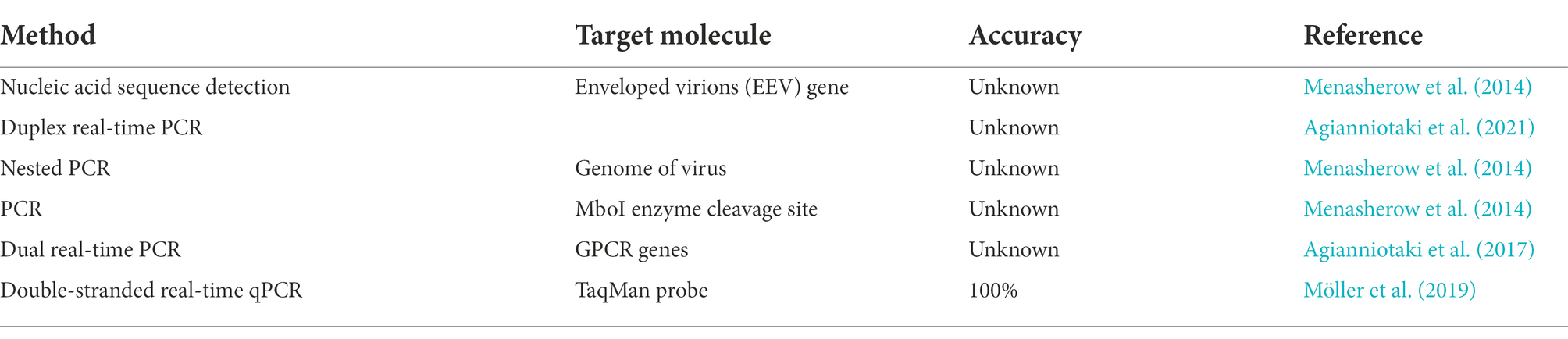
Table 6 . The methods of distinguishing the vaccine and wild-type viruses of LSDV.
Prevention and control should be carried out according to the local disease transmission mechanism and the living habits of species that are often used as transmission vectors. Eliminate those species in the right season to reduce their exposure to susceptible animals. Although there is substantial evidence of widespread vector-borne disease, LSD also spreads during periods when insects are inactive. Studies by Klausner et al. (2017) have reported that the virus may be transmitted through the atmosphere with aerosols, but it is not completely certain. Therefore, further investigation of the role of airflow on the transmission of LSDV is required. After the epidemic, there may be problems such as immune recessive infection and continuous detoxification, and it is necessary to further clarify and standardize the treatment of the same herd of cattle. High temperature fumigation can also effectively avoid the breeding of LSDV and eliminate the hotbed of virus.
For remote areas, it may not be financially feasible to fully vaccinate and eradicate disease, but vaccinated cattle should be permanently marked at least. These vaccinated and non-vaccinated cattle can be managed together, and using herd immunity to reduce the losses from outbreaks. At present, live attenuated vaccines are more commonly used. However, such live vaccines have the risk of homologous recombination with other viruses, causing the vaccinated animals to be infected with other new viruses. An inactivated vaccine targeting LSDV has not yet been developed. Researchers have developed a live attenuated vaccine that can be inactivated and used with adjuvants to achieve a good preventive effect, but it has not been widely used in clinical practice ( Hamdi et al., 2020 ). For countries and regions without this disease, the problem of inactivated vaccines still needs to be solved urgently. There are no reports on subunit vaccines with higher safety profile. LSDV has been used in the laboratory as a vector for recombinant subunit vaccines for some diseases ( Aspden et al., 2002 ; Wallace and Viljoen, 2005 ). It has been studied that the H3L gene of vaccinia virus is the main immunodominant envelope protein of the mature virus in cells, which can induce the production of neutralizing antibodies ( Kumar et al., 2017 ). In addition, the LSDV14 gene is similar to the K3L gene of vaccinia virus and the M156 gene of myxoma virus, which inhibit the phosphorylation of protein kinases, thereby inhibiting the production of interferon ( Beattie et al., 1995 ; Peng et al., 2016 ). These may prompt the production of safe and efficient vaccines in other ways. In order to overcome the risk of virulence reversion and homologous recombination of attenuated vaccines, the development of safer and more efficient recombinant vaccines may be the direction of LSDV vaccine development in the future. By inserting a gene at a specific location in the LSDV genome, it can replicate in a specific cell line, but not in the host, resulting in higher safety ( Klupp et al., 1992 ; Heffner et al., 1993 ). Therefore, it is necessary to deeply study the pathogenic mechanism of LSDV, and explore the virulence genes and immunosuppressive genes, so that the pathogen can be weakened to different degrees through targeted mutation or deletion. Studies have shown that ivermectin (IVM) can inhibit the viral titer of LSDV and attenuate the transmission of LSDV ( Toker et al., 2022 ). As an insect-borne disease, LSDV can be targeted to study whether other anti-parasitic drugs can be used in conjunction with vaccines to effectively block the spread of LSDV.
Currently used diagnostic methods with highly sensitivity, such as LAMP and RPA-Cas12a-fluorescence assay, are all targeting viral genes. However, these detection methods are easily affected by aerosol contamination, which affects the accuracy of the diagnostic results ( Liu S. et al., 2021 ). Therefore, future research should focus on how to overcome the pollution caused by these aerosols.
Since 1929, LSD has endangered the healthy development of the global cattle industry for nearly a 100 years. Scientists are doing everything they can to eliminate this pathogen. The feasible prevention and diagnosis methods that have been developed need to be verified by a large number of clinical trials. Therefore, cutting off effective transmission routes, large-scale safe and efficient use of vaccines, and correct detection methods are the directions of efforts in the future. However, we still do not have a deep understanding of the pathogenic mechanism of this disease. This review highlighted the current research progress of this disease, and puts forward prospects for the insufficiency of research and future research trends, providing information for the elimination of the disease.
Author contributions
ZL, YS, XY and XW conceived and designed the study. ZL, KY, SW, JY, XM, YS, XY wrote the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This study was supported by the grants from the Key Development and Research Foundation of Gansu (grant no. 21YF5WA153), Natural Science Foundation of Gansu Province (21JR7RA022), and Natural Science Foundation Project of China (grant no. 32202779).
Acknowledgments
We appreciate the other members of the Y. S. lab for their constructive comments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbas, F. (2010). Production of goat pox virus vaccine from a live attenuated goat pox virus strain. JAPS 20, 315–317.
Google Scholar
Abutarbush, S. M. (2015). Hematological and serum biochemical findings in clinical cases of cattle naturally infected with lumpy skin disease. J. Infect. Dev. Ctries. 9, 283–288. doi: 10.3855/jidc.5038
CrossRef Full Text | Google Scholar
Afonso, P. P., Silva, P. M., Schnellrath, L. C., Jesus, D. M., Hu, J., Yang, Y., et al. (2012). Biological characterization and next-generation genome sequencing of the unclassified Cotia virus SPAn232 ( Poxviridae ). J. Virol. 86, 5039–5054. doi: 10.1128/JVI.07162-11
PubMed Abstract | CrossRef Full Text | Google Scholar
Afshari Safavi, E. (2022). Assessing machine learning techniques in forecasting lumpy skin disease occurrence based on meteorological and geospatial features. Trop. Anim. Health Prod. 54:55. doi: 10.1007/s11250-022-03073-2
Agianniotaki, E. I., Chaintoutis, S. C., Haegeman, A., De Clercq, K., Chondrokouki, E., and Dovas, C. I. (2021). A Taq man probe-based multiplex real-time PCR method for the specific detection of wild type lumpy skin disease virus with beta-actin as internal amplification control. Mol. Cell. Probes 60:101778. doi: 10.1016/j.mcp.2021.101778
Agianniotaki, E. I., Chaintoutis, S. C., Haegeman, A., Tasioudi, K. E., De Leeuw, I., Katsoulos, P. D., et al. (2017). Development and validation of a Taq Man probe-based real-time PCR method for the differentiation of wild type lumpy skin disease virus from vaccine virus strains. J. Virol. Methods 249, 48–57. doi: 10.1016/j.jviromet.2017.08.011
Ahmed, A. M., and Dessouki, A. A. (2013). Abattoir-based survey and histopathological findings of lumpy skin disease in cattle at Ismailia abattoir. Int. J. Biosci. Biochem. Bioinformat. 3. doi: 10.7763/IJBBB.2013.V3.235
Aleksandr, K., Olga, B., David, W. B., Pavel, P., Yana, P., Svetlana, K., et al. (2020). Non-vector-borne transmission of lumpy skin disease virus. Sci. Rep. 10:7436. doi: 10.1038/s41598-020-64029-w
Alexander, R., Plowright, W., and Haig, D. (1957). Cytopathogenic agents associated with lumpy skin disease of cattle. Bull. Epizoot. Dis. Afr. 5, 489–492.
Ali, H., Ali, A. A., Atta, M. S., and Cepica, A. (2012). Common, eEmerging, vector-borne and infrequent abortogenic virus infections of cattle. Transbound. Emerg. Dis. 59, 11–25. doi: 10.1111/j.1865-1682.2011.01240.x
Ali, A. A., Esmat, M., Attia, H., Selim, A., and Abdel-Hamid, Y. M. (1990). Clinical and pathological studies on lumpy skin disease in Egypt. Vet. Rec. 127, 549–550.
Ali, A. A., Neamat-Allah, A. N. F., Sheire, H. A. E., and Mohamed, R. I. (2021). Prevalence, intensity, and impacts of non-cutaneous lesions of lumpy skin disease among some infected cattle flocks in Nile Delta governorates, Egypt. Comp. Clin. Path. 30, 693–700. doi: 10.1007/s00580-021-03264-7
Al-Salihi, K. A. (2014). Lumpy skin disease: review of the literature. Mirror Res. Vet. Sci. Ani. 3, 6–23.
Amin, D. M., Shehab, G., Emran, R., Hassanien, R. T., Alagmy, G. N., Hagag, N. M., et al. (2021). Diagnosis of naturally occurring lumpy skin disease virus infection in cattle using virological, molecular, and immunohistopathological assays. Vet. World 14, 2230–2237. doi: 10.14202/vetworld.2021.2230-2237
Annandale, C. H., Holm, D. E., Ebersohn, K., and Venter, E. H. (2014). Seminal transmission of lumpy skin disease virus in heifers. Transbound. Emerg. Dis. 61, 443–448. doi: 10.1111/tbed.12045
Annandale, C. H., Irons, P. C., Bagla, V. P., Osuagwuh, U. I., and Venter, E. H. (2010). Sites of persistence of lumpy skin disease virus in the genital tract of experimentally infected bulls. Reprod. Domest. Anim. 45, 250–255. doi: 10.1111/j.1439-0531.2008.01274.x
Arjkumpa, O., Suwannaboon, M., Boonrod, M., Punyawan, I., Liangchaisiri, S., Laobannue, P., et al. (2021). The first lumpy skin disease outbreak in Thailand (2021): epidemiological features and spatio-temporal analysis. Front. Vet. Sci. 8:799065. doi: 10.3389/fvets.2021.799065
Aspden, K., Dijk, A., Bingham, J., Cox, D., and Williamson, A. L. (2002). Immunogenicity of a recombinant lumpy skin disease virus (neethling vaccine strain) expressing the rabies virus glycoprotein in cattle. Vaccine 20, 2693–2701. doi: 10.1016/S0264-410X(02)00203-7
Aspden, K., Passmore, J. A., Tiedt, F., and Williamson, A. L. (2003). Evaluation of lumpy skin disease virus, a capripoxvirus, as a replication-deficient vaccine vector. J. Gen. Virol. 84, 1985–1996. doi: 10.1099/vir.0.19116-0
Awad, W. S., Ibrahim, A. K., Mahran, K., Fararh, K. M., and Abdel Moniem, M. I. (2010). Evaluation of different diagnostic methods for diagnosis of lumpy skin disease in cows. Trop. Anim. Health Prod. 42, 777–783. doi: 10.1007/s11250-009-9486-5
Babiuk, S., Bowden, T. R., Parkyn, G., Dalman, B., Manning, L., Neufeld, J., et al. (2008). Quantification of lumpy skin disease virus following experimental infection in cattle. Transbound. Emerg. Dis. 55, 299–307. doi: 10.1111/j.1865-1682.2008.01024.x
Bamouh, Z., Hamdi, J., Fellahi, S., Khayi, S., and Elharrak, M. (2021). Investigation of post vaccination reactions of two live attenuated vaccines against lumpy skin disease of cattle. Vaccine (Basel) 6, 5–17. doi: 10.3390/vaccines9060621
Barnard, B. J. (1997). Antibodies against some viruses of domestic animals in southern African wild animals. Onderstepoort J. Vet. Res. 64, 95–110.
Beattie, E., Paoletti, E., and Tartaglia, J. (1995). Distinct patterns of IFN sensitivity observed in cells infected with vaccinia K3L-and E3L-mutant viruses. Virology 210, 254–263. doi: 10.1006/viro.1995.1342
Bedeković, T., Šimić, I., Krešić, N., and Lojkić, I. (2018). Detection of lumpy skin disease virus in skin lesions, blood, nasal swabs and milk following preventive vaccination. Transbound. Emerg. Dis. 65, 491–496. doi: 10.1111/tbed.12730
Ben-Gera, J., Klement, E., Khinich, E., Stram, Y., and Shpigel, N. Y. (2015). Comparison of the efficacy of Neethling lumpy skin disease virus and x10RM65 sheep-pox live attenuated vaccines for the prevention of lumpy skin disease—the results of a randomized controlled field study. Vaccine 33, 4837–4842.
Bhanuprakash, V., Hosamani, M., Venkatesan, G., Balamurugan, V., Yogisharadhya, R., and Singh, R. K. (2012). Animal poxvirus vaccines: a comprehensive review. Expert Rev. Vaccines 11, 1355–1374. doi: 10.1586/erv.12.116
Bhanuprakash, V., Indrani, B. K., Hegde, R., Kumar, M. M., and Moorthy, A. (2004). A classical live attenuated vaccine for sheep pox. Trop. Anim. Health. Prod. 36, 307–320. doi: 10.1023/b:trop.0000026661.88631.50
Bhanuprakash, V., Indrani, B. K., Hosamani, M., and Singh, R. K. (2006). The current status of sheep pox disease. Comp. Immunol. Microbiol. Infect. Dis. 29, 27–60. doi: 10.1016/j.cimid.2005.12.001
Black, D. N., Hammond, J. M., and Kitching, R. P. (1986). Genomic relationship between capripoxviruses. Virus Res. 5, 277–292. doi: 10.1016/0168-1702(86)90024-9
Blackall, P. J. (1988). Further comparison of adjuvants for an inactivated infectious coryza vaccine. Avian Dis. 32.
Boshra, H., Truong, T., Nfon, C., Bowden, T. R., Gerdts, V., Tikoo, S., et al. (2015). A lumpy skin disease virus deficient of an IL-10 gene homologue provides protective immunity against virulent capripoxvirus challenge in sheep and goats. Antivir. Res. 123, 39–49. doi: 10.1016/j.antiviral.2015.08.016
Brenner, J., Bellaiche, M., Gross, E., Elad, D., Oved, Z., Haimovitz, M., et al. (2009). Appearance of skin lesions in cattle populations vaccinated against lumpy skin disease: statutory challenge. Vaccine 27, 1500–1503. doi: 10.1016/j.vaccine.2009.01.020
Brenner, J., David, D., Avraham, A., Klopferorgad, U., Samina, I., and Peleg, B.A. (1992). Experimental infection with local lumpy skin disease virus in cattle vaccinated with sheep pox vaccine .
Bryson, N. R., Tice, G. A., Horak, I. G., Stewart, C. G., and du Plessis, B. J. (2002). Ixodid ticks on cattle belonging to small-scale farmers at 4 communal grazing areas in South Africa. J. S. Afr. Vet. Assoc. 73, 98–103. doi: 10.4102/jsava.v73i3.568
Burdin, M., and Prydie, J. (1959). Observations on the first outbreak of lumpy skin disease in Kenya. Bull. Epizoot. Dis. Afr. 7, 21–26.
Calistri, P., Declercq, K., Gubbins, S., Klement, E., Stegeman, A., Abrahantes, J. C., et al. (2019). Lumpy skin disease: III. Data collection and analysis. EFSA J. 17. doi: 10.2903/j.efsa.2019.5638
Carn, V. M., and Kitching, R. P. (1995). An investigation of possible routes of transmission of lumpy skin disease virus (Neethling). Epidemiol. Infect. 114, 219–226. doi: 10.1017/S0950268800052067
Cavalera, S., Pezzoni, G., Grazioli, S., Brocchi, E., Baselli, S., Lelli, D., et al. (2022). Investigation of the “antigen hook effect,” in lateral flow Sandwich immunoassay: the case of lumpy skin disease virus detection. Biosensors (Basel) 12:739. doi: 10.3390/bios12090739
Chibssa, T. R., Grabherr, R., Loitsch, A., Settypalli, T. B. K., Tuppurainen, E., Nwankpa, N., et al. (2018). A gel-based PCR method to differentiate sheeppox virus field isolates from vaccine strains. Virol. J. 15:59. doi: 10.1186/s12985-018-0969-8
Chihota, C. M., Rennie, L. F., Kitching, R. P., and Mellor, P. S. (2003). Attempted mechanical transmission of lumpy skin disease virus by biting insects. Med. Vet. Entomol. 17, 294–300. doi: 10.1046/j.1365-2915.2003.00445.x
Chihota, C. M., Rennie, L. F., Kitching, R. P., and Mellor, P. S. (2001). Mechanical transmission of lumpy skin disease virus by Aedes aegypti (Diptera: Culicidae). Epidemiol. Infect. 126, 317–321.
Coetzer, J.A.W. (2004). Infectious diseases of livestock . Cape Town, South Africa: Oxford University Press pp. 1268–1276.
Dao, T. D., Tran, L. H., Nguyen, H. D., Hoang, T. T., Nguyen, G. H., Tran, K. V. D., et al. (2022). Characterization of lumpy skin disease virus isolated from a giraffe in Vietnam. Transbound. Emerg. Dis. e3268–e3271. doi: 10.1111/tbed.14583
De Clercq, E., and Neyts, J. (2004). Therapeutic potential of nucleoside/nucleotide analogues against poxvirus infections. Rev. Med. Virol. 14, 289–300. doi: 10.1002/rmv.439
Deregt, D., and Loewen, K. G. (1995). Bovine viral diarrhea virus: biotypes and disease. Can. Vet. J. 36, 371–378.
Dietze, K., Moritz, T., Alexandrov, T., Krstevski, K., Schlottau, K., Milovanovic, M., et al. (2018). Suitability of group-level oral fluid sampling in ruminant populations for lumpy skin disease virus detection. Vet. Microbiol. 221, 44–48. doi: 10.1016/j.vetmic.2018.05.022
El-Ansary, R. E., El-Dabae, W. H., Bream, A. S., and El Wakil, A. (2022). Isolation and molecular characterization of lumpy skin disease virus from hard ticks, Rhipicephalus (Boophilus) annulatus in Egypt. BMC Vet. Res. 18:302. doi: 10.1186/s12917-022-03398-y
Elhaig, M. M., Selim, A., and Mahmoud, M. (2017). Lumpy skin disease in cattle: frequency of occurrence in a dairy farm and a preliminary assessment of its possible impact on Egyptian buffaloes. Onderstepoort J. Vet. Res. 84, e1–e6. doi: 10.4102/ojvr.v84i1.1393
El-Neweshy, M. S., El-Shemey, T. M., and Youssef, S. A. (2013). Pathologic and immunohistochemical findings of natural lumpy skin disease in Egyptian cattle. Pak. Vet. J. 33, 60–64.
Eltom, K. H., Althoff, A. C., Hansen, S., Böhlken-Fascher, S., Yousif, A., El-Sheikh, H. A., et al. (2021). Differentiation of Capripox viruses by Nanopore sequencing. Vaccines (Basel) 9:351. doi: 10.3390/vaccines9040351
Es-Sadeqy, Y., Bamouh, Z., Ennahli, A., Safini, N., and Harrak, M. E. (2021). Development of an inactivated combined vaccine for protection of cattle against lumpy skin disease and bluetongue viruses. Vet. Microbiol. 256:109046. doi: 10.1016/j.vetmic.2021.109046
European Food Safety Authority (2017). Lumpy skin disease: I. Data collection and analysis. Efsa J 15:e04773. doi: 10.2903/j.efsa.2017.4773
European Food Safety Authority (2018). Lumpy skin disease II. Data collection and analysis. EFSA J. 16:e05176. doi: 10.2903/j.efsa.2018.5176
Fagbo, S., Coetzer, J. A., and Venter, E. H. (2014). Seroprevalence of Rift Valley fever and lumpy skin disease in African buffalo (Syncerus caffer) in the Kruger National Park and Hluhluwe-iMfolozi park, South Africa. J. S. Afr. Vet. Assoc. 85, e1–e7. doi: 10.4102/jsava.v85i1.1075
Fawzi, E. M., Morsi, A. M., and Abd-Elfatah, E. B. (2022). Molecular diagnosis of three outbreaks during three successive years (2018, 2019, and 2020) of lumpy skin disease virus in cattle in Sharkia governorate. Egypt. Open Vet J 12, 451–462. doi: 10.5455/OVJ.2022.v12.i4.6
Francis, M. J. (2018). Recent advances in vaccine technologies. Vet. Clin. North Am. Small Anim. Pract. 48, 231–241. doi: 10.1016/j.cvsm.2017.10.002
Gari, G., Abie, G., Gizaw, D., Wubete, A., Kidane, M., Asgedom, H., et al. (2015). Evaluation of the safety, immunogenicity and efficacy of three capripoxvirus vaccine strains against lumpy skin disease virus. Vaccine 33, 3256–3261. doi: 10.1016/j.vaccine.2015.01.035
Gershon, P. D., Ansell, D. M., and Black, D. N. (1989a). A comparison of the genome organization of capripoxvirus with that of the orthopoxviruses. J. Virol. 63, 4703–4708.
Gershon, P. D., Paul Kitching, R., Hammond, J. M., and Black, D. N. (1989b). Poxvirus genetic recombination during natural virus transmission. J. Gen. Virol. 70:485.
Gershon, A. A., Steinberg, S. P., Gelb, L., Galasso, G., Borkowsky, W., LaRussa, P., et al. (1984). Live attenuated varicella vaccine. Efficacy for children with leukemia in remission. JAMA 252:355.
Graham, F. L., and Prevec, L. (1992). Adenovirus-based expression vectors and recombinant vaccines. Biotechnology 20, 363–390.
Greenberg, J. A., DiMenna, M. A., Hanelt, B., and Hofkin, B. V. (2012). Analysis of post-blood meal flight distances in mosquitoes utilizing zoo animal blood meals. J. Vector Ecol. 37, 83–89. doi: 10.1111/j.1948-7134.2012.00203.x
Gubbins, S. (2019). Using the basic reproduction number to assess the risk of transmission of lumpy skin disease virus by biting insects. Transbound. Emerg. Dis. 66, 1873–1883. doi: 10.1111/tbed.13216
Haegeman, A., De Leeuw, I., Mostin, L., Van Campe, W., Aerts, L., Vastag, M., et al. (2020). An Immunoperoxidase monolayer assay (IPMA) for the detection of lumpy skin disease antibodies. J. Virol. Methods 277:113800. doi: 10.1016/j.jviromet.2019.113800
Haegeman, A., Leeuw, I. D., Mostin, L., Campe, W. V., and Clercq, K. D. (2021). Comparative evaluation of lumpy skin disease virus-based live attenuated vaccines. Vaccines (Basel) 9:473. doi: 10.3390/vaccines9050473
Hamdi, J., Boumart, Z., Daouam, S., Arkam, A. E., and Harrak, M. E. (2020). Development and evaluation of an inactivated lumpy skin disease vaccine for cattle. Vet. Microbiol. 245:108689. doi: 10.1016/j.vetmic.2020.108689
Harland, R. J., Potter, A. A., Donkersgoed, J. V., Parker, M. D., Zamb, T. J., and Janzen, E. D. (1992). The effect of subunit or modified live bovine herpesvirus-1 vaccines on the efficacy of a recombinant Pasteurella haemolytica vaccine for the prevention of respiratory disease in feedlot calves. Can. Vet. J. 33, 734–741.
Heffner, S., Kovacs, F., Klupp, B. G., and Mettenleiter, T. C. (1993). Glycoprotein gp 50-negative pseudorabies virus: a novel approach toward a nonspreading live herpesvirus vaccine. J. Virol. 67, 1529–1537. doi: 10.1128/jvi.67.3.1529-1537.1993
Heine, H. G., Stevens, M. P., Foord, A. J., and Boyle, D. B. (1999). A capripoxvirus detection PCR and antibody ELISA based on the major antigen P32, the homolog of the vaccinia virus H3L gene. J. Immunol. Methods. 187–196. doi: 10.1016/s0022-1759(99)00072-1
Holowczak, J. A. (1982). Poxvirus DNA. Curr. Top. Microbiol. Immunol. 97, 27–79.
House, J. A., Wilson, T. M., el Nakashly, S., Karim, I. A., Ismail, I., el Danaf, N., et al. (1990). The isolation of lumpy skin disease virus and bovine herpesvirus-4 from cattle in Egypt. J. Vet. Diagn. Investig. 2, 111–115. doi: 10.1177/104063879000200205
Hunter, P., and Wallace, D. (2001). Lumpy skin disease in southern Africa: a review of the disease and aspects of control. J. S. Afr. Vet. Assoc. 72, 68–71. doi: 10.4102/jsava.v72i2.619
Ireland, D. C., and Binepal, Y. S. (1998). Improved detection of capripoxvirus in biopsy samples by PCR. J. Virol. Methods 74, 1–7.
Irons, P. C., Tuppurainen, E. S., and Venter, E. H. (2005). Excretion of lumpy skin disease virus in bull semen. Theriogenology 63, 1290–1297. doi: 10.1016/j.theriogenology.2004.06.013
Issimov, A., Kutumbetov, L., Orynbayev, M. B., Khairullin, B., Myrzakhmetova, B., Sultankulova, K., et al. (2020). Mechanical transmission of lumpy skin disease virus by Stomoxys Spp ( Stomoxys calsitrans , Stomoxys sitiens , Stomoxys indica ), Diptera: Muscidae. Animals (Basel) 10:477. doi: 10.3390/ani10030477
Jalali, S. M., Rasooli, A., Seifi Abad Shapuri, M., and Daneshi, M. (2017). Clinical, hematologic, and biochemical findings in cattle infected with lumpy skin disease during an outbreak in Southwest Iran. Arch. Razi Inst. 72, 255–265. doi: 10.22092/ari.2017.113301
Jiang, C., Tao, D., Geng, Y., Yang, H., Xu, B., Chen, Y., et al. (2022). Sensitive and specific detection of lumpy skin disease virus in cattle by CRISPR-Cas 12a fluorescent assay coupled with Recombinase polymerase amplification. Genes (Basel) 13:734. doi: 10.3390/genes13050734
Johnston, J. B., and McFadden, G. (2003). Poxvirus immunomodulatory strategies: current perspectives. J. Virol. 77, 6093–6100.
Jongejan, F., Perié, N., Franssen, F. F., and Uilenberg, G. (1980). Artificial infection of Rhipicephalus appendiculatus with Theileria parva by percutaneous injection. Res. Vet. Sci. 29, 320–324.
Jordi, C., Alberto, A., Aleksandra, M., Ledi, P., Blagojco, T., Dimitar, T., et al. (2018). Economic cost of lumpy skin disease outbreaks in three Balkan countries: Albania, Bulgaria and the former Yugoslav Republic of Macedonia (2016–2017). Transbound. Emerg. Dis. 65, 1680–1688. doi: 10.1111/tbed.12926
K, A.-S. (2014). Lumpy skin disease: Review of the literature. Mirror Res. Vet. Sci. Ani. 3, 6–23.
Kara, P. D., Mather, A. S., Alri, P., Thireshni, C., Shawn, B., and Wallace, D. B. (2018). Characterisation of putative immunomodulatory gene knockouts of lumpy skin disease virus in cattle towards an improved vaccine. Vaccine 36, 4708–4715. doi: 10.1016/j.vaccine.2018.06.017
Katsoulos, P. D., Chaintoutis, S. C., Dovas, C. I., Polizopoulou, Z. S., Brellou, G., Agianniotaki, E. I., et al. (2018). Investigation on the incidence of adverse reactions, viraemia and haematological changes following field immunization of cattle using a live attenuated vaccine against lumpy skin disease. Transbound. Emerg. Dis. 174–185. doi: 10.1111/tbed.12646
Kitching, R. P., Hammond, J. M., and Taylor, W. P. (1987). A single vaccine for the control of capripox infection in sheep and goats. Res. Vet. Sci. 42, 53–60.
K, L. K., B, S., and K, R. (2021). Clinico-molecular diagnosis and characterization of bovine lumpy skin disease virus in Andhra Pradesh, India. Trop. Anim. Health Prod. 53:424. doi: 10.1007/s11250-021-02872-3
Klausner, Z., Fattal, E., and Klement, E. (2017). Using synoptic Systems' typical wind trajectories for the analysis of potential atmospheric long-distance dispersal of lumpy skin disease virus. Transbound. Emerg. Dis. 64, 398–410. doi: 10.1111/tbed.12378
Klement, E., Broglia, A., Antoniou, S. E., Tsiamadis, V., Plevraki, E., Petrovic, T., et al. (2020). Neethling vaccine proved highly effective in controlling lumpy skin disease epidemics in the Balkans. Prev. Vet. Med. 181:104595. doi: 10.1016/j.prevetmed.2018.12.001
Klupp, B. G., Visser, N., and Mettenleiter, T. C. (1992). Identification and characterization of pseudorabies virus glycoprotein H. J. Virol. 66:3048.
Kononov, A., Prutnikov, P., Shumilova, I., Kononova, S., Nesterov, A., Byadovskaya, O., et al. (2019). Determination of lumpy skin disease virus in bovine meat and offal products following experimental infection. Transbound. Emerg. Dis. 66, 1332–1340. doi: 10.1111/tbed.13158
Korthase, C., Elnagar, A., Beer, M., and Hoffmann, B. (2022). Easy express extraction (triple E)-A universal, electricity-free nucleic acid extraction system for the lab and the pen. Microorganisms 10:1074. doi: 10.3390/microorganisms10051074
Krotova, A., Byadovskaya, O., Shumilova, I., van Schalkwyk, A., and Sprygin, A. (2022). An in-depth bioinformatic analysis of the novel recombinant lumpy skin disease virus strains: from unique patterns to established lineage. BMC Genomics 23:396. doi: 10.1186/s12864-022-08639-w
Kumar, N., Chander, Y., Kumar, R., Khandelwal, N., Riyesh, T., Chaudhary, K., et al. (2021). Isolation and characterization of lumpy skin disease virus from cattle in India. PLoS One 16:e0241022. doi: 10.1371/journal.pone.0241022
Kumar, A., Yogisharadhya, R., Venkatesan, G., Bhanuprakash, V., Pandey, A. B., and Shivachandra, S. B. (2017). Co-administration of recombinant major envelope proteins (rA27L and rH3L) of buffalopox virus provides enhanced immunogenicity and protective efficacy in animal models. Antivir. Res. 141, 174–178. doi: 10.1016/j.antiviral.2017.02.017
Lee, S. W., Markham, P. F., Coppo, M. J., Legione, A. R., Markham, J. F., Noormohammadi, A. H., et al. (2012). Attenuated vaccines can recombine to form virulent field viruses. Science 337:188. doi: 10.1126/science.1217134
Lefkowitz, E. J., Dempsey, D. M., Hendrickson, R. C., Orton, R. J., Siddell, S. G., and Smith, D. B. (2018). Virus taxonomy: the database of the international committee on taxonomy of viruses (ICTV). Nucleic Acids Res. 46, D708–D717. doi: 10.1093/nar/gkx932
Liang, X. P., Babiuk, L. A., Hurk, L., Fitzpatrick, D. R., and Zamb, T. J. (1991). Bovine herpesvirus 1 attachment to permissive cells is mediated by its major glycoproteins gI, gIII, and gIV. J. Virol. 65, 1124–1132. doi: 10.1128/jvi.65.3.1124-1132.1991
Liu, S., Tao, D., Liao, Y., Yang, Y., Sun, S., Zhao, Y., et al. (2021). Highly sensitive CRISPR/Cas12a-based fluorescence detection of porcine reproductive and respiratory syndrome virus. ACS Synth. Biol. 10, 2499–2507. doi: 10.1021/acssynbio.1c00103
Liu, L., Wang, X., Mao, R., Zhou, Y., Yin, J., Sun, Y., et al. (2021). Research progress on live attenuated vaccine against African swine fever virus. Microb. Pathog. 158:105024. doi: 10.1016/j.micpath.2021.105024
Lubinga, J. C., Tuppurainen, E. S., Coetzer, J. A., Stoltsz, W. H., and Venter, E. H. (2014). Transovarial passage and transmission of LSDV by Amblyomma hebraeum, Rhipicephalus appendiculatus and Rhipicephalus decoloratus. Exp. Appl. Acarol. 62, 67–75. doi: 10.1007/s10493-013-9722-6
Lubinga, J. C., Tuppurainen, E. S., Mahlare, R., Coetzer, J. A., Stoltsz, W. H., and Venter, E. H. (2015). Evidence of transstadial and mechanical transmission of lumpy skin disease virus by Amblyomma hebraeum ticks. Transbound. Emerg. Dis. 62, 174–182. doi: 10.1111/tbed.12102
Magori-Cohen, R., Louzoun, Y., Herziger, Y., Oron, E., Arazi, A., Tuppurainen, E., et al. (2012). Mathematical modelling and evaluation of the different routes of transmission of lumpy skin disease virus. Vet. Res. 43:1. doi: 10.1186/1297-9716-43-1
Matsiela, M. S., Naicker, L., Dibakwane, V. S., Ntombela, N., Khoza, T., and Mokoena, N. (2022). Improved safety profile of inactivated Neethling strain of the lumpy skin disease vaccine. Vaccine X 12:100209. doi: 10.1016/j.jvacx.2022.100209
Mcfadden, G. (2005). Poxvirus tropism. Nat. Rev. Microbiol. 3:201. doi: 10.1038/nrmicro1099
Menasherow, S., Rubinstein-Giuni, M., Kovtunenko, A., Eyngor, Y., Fridgut, O., Rotenberg, D., et al. (2014). Development of an assay to differentiate between virulent and vaccine strains of lumpy skin disease virus (LSDV). J. Virol. Methods 199, 95–101. doi: 10.1016/j.jviromet.2013.12.013
Miller,, and Dusty, A. (1992). Human gene therapy comes of age. Nature 357, 455–460. doi: 10.1038/357455a0
Milovanovic, M., Milicevic, V., Radojicic, S., Valcic, M., Hoffmann, B., and Dietze, K. (2020). Suitability of individual and bulk milk samples to investigate the humoral immune response to lumpy skin disease vaccination by ELISA. Virol. J. 17:28. doi: 10.1186/s12985-020-01298-x
Minor, P. D., John, A., Ferguson, M., and Icenogle, J. P. (1986). Antigenic and molecular evolution of the vaccine strain of type 3 poliovirus during the period of excretion by a primary vaccinee. J. Gen. Virol. 67, 693–706. doi: 10.1099/0022-1317-67-4-693
Modise, B. M., Settypalli, T. B. K., Kgotlele, T., Xue, D., Ntesang, K., Kumile, K., et al. (2021). First molecular characterization of poxviruses in cattle, sheep, and goats in Botswana. Virol. J. 18:167. doi: 10.1186/s12985-021-01634-9
Molla, W., de Jong Mart, C. M., Gari, G., and Frankena, K. (2017). Economic impact of lumpy skin disease and cost effectiveness of vaccination for the control of outbreaks in Ethiopia. Prev. Vet. Med. 147, 100–107. doi: 10.1016/j.prevetmed.2017.09.003
Möller, J., Moritz, T., Schlottau, K., Krstevski, K., Hoffmann, D., Beer, M., et al. (2019). Experimental lumpy skin disease virus infection of cattle: comparison of a field strain and a vaccine strain. Arch. Virol. 164, 2931–2941. doi: 10.1007/s00705-019-04411-w
Moss, B. (2007). Poxviridae: The Viruses and Their Replication .
Moyer, R.W., Arif, B.M., Black, D.N., Boyle, D.B., Buller, R.M., Dumbell, K.R., et al. (2000). Family Poxviridae .
Mulatu, E., and Feyisa, A. (2018). Review: Lumpy Skin Disease. J. Vet. Sci. Technol. 09:3. doi: 10.4172/2157-7579.1000535
Mullen, G.R., and Durden, L.A. (2002). Medical and Veterinary Entomology . New York: Elsevier M, 15–27.
Munz, E. K., and Owen, N. C. (1966). Electron microscopic studies on lumpy skin disease virus type "Neethling". Onderstepoort J. Vet. Res. 33, 3–8.
Murphy, F. A., Fauquet, C. M., Bishop, D., Ghabrial, S. A., and Summers, M. D. (2008). Virus taxonomy: classification and nomenclature of viruses. Encyclopedia Virol. 140, 9–23.
Mwanandota, J. J., Macharia, M., Ngeleja, C. M., Sallu, R. S., Yongolo, M. G., Mayenga, C., et al. (2018). Validation of a diagnostic tool for the diagnosis of lumpy skin disease. Vet. Dermatol. 29, 532–e178. doi: 10.1111/vde.12690
Neamat-Allah, A. N. F., and Mahmoud, E. A. (2019). Assessing the possible causes of hemolytic anemia associated with lumpy skin disease naturally infected buffaloes. Comp. Clin. Pathol. 28, 747–753. doi: 10.1007/s00580-019-02952-9
Ngichabe, C. K., Wamwayi, H. M., Ndungu, E. K., Mirangi, P. K., Bostock, C. J., Black, D. N., et al. (2002). Long term immunity in African cattle vaccinated with a recombinant capripox-rinderpest virus vaccine. Epidemiol. Infect 128, 343–349.
Okur-Gumusova, S., Tamer, C., Ozan, E., Cavunt, A., Kadi, H., Muftuoglu, B., et al. (2020). An investigation of the seroprevalence of Crimean Congo hemorrhagic fever and lumpy skin disease in domesticated water buffaloes in northern Turkey. Trop. Biomed. 37, 165–173.
Osuagwuh, U. I., Bagla, V., Venter, E. H., Annandale, C. H., and Irons, P. C. (2007). Absence of lumpy skin disease virus in semen of vaccinated bulls following vaccination and subsequent experimental infection. Vaccine 25, 2238–2243. doi: 10.1016/j.vaccine.2006.12.010
Peeters, B., Bouma, A., Bruin, T. D., Moormann, R., and Kimman, T. (1994). Non-transmissible pseudorabies virus gp 50 mutants: a new generation of safe live vaccines. Vaccine 12, 375–380. doi: 10.1016/0264-410X(94)90104-X
Peng, C., Haller, S. L., Rahman, M. M., McFadden, G., and Rothenburg, S. (2016). Myxoma virus M156 is a specific inhibitor of rabbit PKR but contains a loss-of-function mutation in Australian virus isolates. Proc. Natl. Acad. Sci. U. S. A. 113, 3855–3860. doi: 10.1073/pnas.1515613113
Perry, B. (2010). Infectious diseases of livestock with special reference to southern Africa: J.A.W. Coetzer, G.R. Thomson and R.C. Tustin (editors), Oxford university press, 1994, 2 vols, 1, 605 + viiipp, $245.00, ISBN 0-1957-0506-8. Aust. Vet. J. 73:198.
Plowright, W., and Witcomb, M. A. (1959). The growth in tissue cultures of a virus derived from lumpy-skin disease of cattle. J. Pathol. Bacteriol. 78, 397–407. doi: 10.1002/path.1700780207
Prow, N. A., Jimenez Martinez, R., Hayball, J. D., Howley, P. M., and Suhrbier, A. (2018). Poxvirus-based vector systems and the potential for multi-valent and multi-pathogen vaccines. Expert Rev. Vaccines 17, 925–934. doi: 10.1080/14760584.2018.1522255
Pseudo-urticaria (1931). Northern Rhodesia Department of Animal Health. Morris JPA 12.
Punyapornwithaya, V., Seesupa, S., Phuykhamsingha, S., Arjkumpa, O., Sansamur, C., and Jarassaeng, C. (2022). Spatio-temporal patterns of lumpy skin disease outbreaks in dairy farms in northeastern Thailand. Front. Vet. Sci. 9:957306. doi: 10.3389/fvets.2022.957306
Reddy, B. S., and Sivajothi, S. (2016). Clinical management of demodicosis in Ongole cattle. J. Parasit. Dis. 40, 1311–1312. doi: 10.1007/s12639-015-0677-x
Reid, H. W., Buxton, D., Berrie, E., Pow, I., and Finlayson, J. (1984). Malignant catarrhal fever. Vet. Rec. 114, 581–583. doi: 10.1136/vr.114.24.581
Rgbe, H. (2014). Lumpy skin disease (LSD): Outbreak investigation, isolation and molecular detection of lumpy skin disease in selected areas of eastern Shewa, Ethiopia. Doctoral dissertation. AAU, 72.
Rhodesia, N. (1932). Annual bulletin, Department of Animal Health 1931. Annual bulletin Department of Animal Health .
Romero, C. H., Barrett, T., Evans, S. A., Kitching, R. P., Gershon, P. D., Bostock, C., et al. (1993). Single capripoxvirus recombinant vaccine for the protection of cattle against rinderpest and lumpy skin disease. Vaccine 11, 737–742. doi: 10.1016/0264-410X(93)90258-Y
Rouby, S., and Aboulsoud, E. (2016). Evidence of intrauterine transmission of lumpy skin disease virus. Vet. J. 193–195. doi: 10.1016/j.tvjl.2015.11.010
Saegerman, C., Bertagnoli, S., Meyer, G., Ganiere, J. P., Caufour, P., De Clercq, K., et al. (2018). Risk of introduction of lumpy skin disease in France by the import of vectors in animal trucks. PLoS One 13:e0198506. doi: 10.1371/journal.pone.0198506
Safini, N., Elmejdoub, S., Bamouh, Z., Jazouli, M., Hamdi, J., Boumart, Z., et al. (2022). Development and evaluation of a combined contagious bovine Pleuropneumonia (CBPP) and lumpy skin disease (LSD) live vaccine. Viruses 14:372. doi: 10.3390/v14020372
Salib, F. A., and Osman, A. H. (2011). Incidence of lumpy skin disease among Egyptian cattle in Giza governorate. Egypt. Veterinary World 4, 162–167.
Sanz-Bernardo, B., Haga, I. R., Wijesiriwardana, N., Basu, S., Larner, W., Diaz, A. V., et al. (2021). Quantifying and modeling the acquisition and retention of lumpy skin disease virus by Hematophagus insects reveals clinically but not subclinically affected cattle are promoters of viral transmission and key targets for control of disease outbreaks. J. Virol. 95:e02239-20. doi: 10.1128/JVI.02239-20
Sanz-Bernardo, B., Haga, I. R., Wijesiriwardana, N., Hawes, P. C., and Beard, P. M. (2020). Lumpy skin disease is characterized by severe multifocal dermatitis with necrotizing Fibrinoid Vasculitis following experimental infection. Vet. Pathol. 57, 388–396. doi: 10.1177/0300985820913268
Sanz-Bernardo, B., Suckoo, R., Haga, I. R., Wijesiriwardana, N., Harvey, A., Basu, S., et al. (2022). The acquisition and retention of lumpy skin disease virus by blood-feeding insects is influenced by the source of virus, the insect body part, and the time since feeding. J. Virol. 96:e0075122. doi: 10.1128/jvi.00751-22
Seet, B. T., Johnston, J. B., Brunetti, C. R., Barrett, J. W., Everett, H., Cameron, C., et al. (2003). Poxviruses and immune evasion. Annu. Rev. Immunol. 21, 377–423. doi: 10.1146/annurev.immunol.21.120601.141049
Selim, A., Manaa, E., and Khater, H. (2021a). Molecular characterization and phylogenetic analysis of lumpy skin disease in Egypt. Comp. Immunol. Microbiol. Infect. Dis. 79:101699. doi: 10.1016/j.cimid.2021.101699
Selim, A., Manaa, E., and Khater, H. (2021b). Seroprevalence and risk factors for lumpy skin disease in cattle in northern Egypt. Trop. Anim. Health Prod. 53:350. doi: 10.1007/s11250-021-02786-0
Şevik, M., and Doğan, M. (2017). Epidemiological and molecular studies on lumpy skin disease outbreaks in Turkey during 2014-2015. Transbound. Emerg. Dis. 64, 1268–1279. doi: 10.1111/tbed.12501
Shalaby, M. A., El-Deeb, A., El-Tholoth, M., Hoffmann, D., Czerny, C. P., Hufert, F. T., et al. (2016). Recombinase polymerase amplification assay for rapid detection of lumpy skin disease virus. BMC Vet. Res. 12:244. doi: 10.1186/s12917-016-0875-5
Sohier, C., Haegeman, A., Mostin, L., De Leeuw, I., Campe, W. V., De Vleeschauwer, A., et al. (2019). Experimental evidence of mechanical lumpy skin disease virus transmission by Stomoxys calcitrans biting flies and Haematopota spp. horseflies. Sci. Rep. 9:20076. doi: 10.1038/s41598-019-56605-6
Sprygin, A., Artyuchova, E., Babin, Y., Prutnikov, P., Kostrova, E., Byadovskaya, O., et al. (2018a). Epidemiological characterization of lumpy skin disease outbreaks in Russia in 2016. Transbound. Emerg. Dis. 65, 1514–1521. doi: 10.1111/tbed.12889
Sprygin, A., Babin, Y., Pestova, Y., Kononova, S., Wallace, D. B., Van Schalkwyk, A., et al. (2018b). Analysis and insights into recombination signals in lumpy skin disease virus recovered in the field. PLoS One 13:e0207480. doi: 10.1371/journal.pone.0207480
Stephens, W. H. (1966). Emerging diseases of animals. Aust. Vet. J. 42.
Stephens, L. R., Little, P. B., Wilkie, B. N., and Barnum, D. A. (1984). Isolation of Haemophilus somnus antigens and their use as vaccines for prevention of bovine thromboembolic meningoencephalitis. Am. J. Vet. Res. 45, 234–239.
Stram, Y., Kuznetzova, L., Friedgut, O., Gelman, B., Yadin, H., and Rubinstein-Guini, M. (2008). The use of lumpy skin disease virus genome termini for detection and phylogenetic analysis. J. Virol. Methods 151, 225–229. doi: 10.1016/j.jviromet.2008.05.003
Toker, E. B., Ates, O., and Yeşilbağ, K. (2022). Inhibition of bovine and ovine capripoxviruses (lumpy skin disease virus and Sheeppox virus) by ivermectin occurs at different stages of propagation in vitro. Virus Res. 310:198671. doi: 10.1016/j.virusres.2021.198671
Tulman, E. R., Afonso, C. L., Lu, Z., Zsak, L., Kutish, G. F., and Rock, D. L. (2001). Genome of lumpy skin disease virus. J. Virol. 75, 7122–7130. doi: 10.1128/JVI.75.15.7122–7130.2001
Tulman, E. R., Afonso, C. L., Lu, Z., Zsak, L., Sur, J. H., Sandybaev, N. T., et al. (2002). The genomes of Sheeppox and Goatpox viruses. J. Virol. 76:6054. doi: 10.1128/jvi.76.12.6054-6061.2002
Tuppurainen, E. S., Lubinga, J. C., Stoltsz, W. H., Troskie, M., Carpenter, S. T., Coetzer, J. A., et al. (2013). Evidence of vertical transmission of lumpy skin disease virus in Rhipicephalus decoloratus ticks. Ticks Tick Borne Dis. 4, 329–333. doi: 10.1016/j.ttbdis.2013.01.006
Tuppurainen, E. S., Pearson, C. R., Bachanek-Bankowska, K., Knowles, N. J., Amareen, S., Frost, L., et al. (2014). Characterization of sheep pox virus vaccine for cattle against lumpy skin disease virus. Antivir. Res. 109, 1–6. doi: 10.1016/j.antiviral.2014.06.009
Tuppurainen, E. S., Stoltsz, W. H., Troskie, M., Wallace, D. B., Oura, C. A., Mellor, P. S., et al. (2011). A potential role for ixodid (hard) tick vectors in the transmission of lumpy skin disease virus in cattle. Transbound. Emerg. Dis. 58, 93–104. doi: 10.1111/j.1865-1682.2010.01184.x
Tuppurainen, E. S., Venter, E. H., Coetzer, J. A., and Bell-Sakyi, L. (2015). Lumpy skin disease: attempted propagation in tick cell lines and presence of viral DNA in field ticks collected from naturally-infected cattle. Ticks Tick Borne Dis. 6, 134–140. doi: 10.1016/j.ttbdis.2014.11.002
Upton, C. (2004). Poxvirus bioinformatics. Methods Mol. Biol. 269, 347–370. doi: 10.1385/1-59259-789-0:347
Wallace, D. B., and Viljoen, G. J. (2005). Immune responses to recombinants of the south African vaccine strain of lumpy skin disease virus generated by using thymidine kinase gene insertion. Vaccine 23, 3061–3067. doi: 10.1016/j.vaccine.2004.10.006
Wang, C. L., Li, M., and Lyu, X. J. (2020). Classification and production process of human vaccine. Zhonghua Yu Fang Yi Xue Za Zhi 54, 1017–1025. doi: 10.3760/cma.j.cn112150-20200520-00756
Wang, J., Xu, Z., Wang, Z., Li, Q., Liang, X., Ye, S., et al. (2022). Isolation, identification and phylogenetic analysis of lumpy skin disease virus strain of outbreak in Guangdong, China. Transbound. Emerg. Dis. 69:e2291-e 2301. doi: 10.1111/tbed.14570
Weiss, K.E. (1968). Lumpy Skin Disease Virus . Springer Berlin Heidelberg.
Wendy, I., Catherine, A. M., Andrew, A. M., David, H., and Stephen, B. F. (2002). Orf virus-encoded interleukin-10 stimulates the proliferation of murine mast cells and inhibits cytokine synthesis in murine peritoneal macrophages. J. Gen. Virol. 83, 1049–1058. doi: 10.1099/0022-1317-83-5-1049
Willey, R. L., Byrum, R., Piatak, M., Kim, Y. B., Cho, M. W., Rossio, J. L., et al. (2003). Control of viremia and prevention of simian-human immunodeficiency virus-induced disease in rhesus macaques immunized with recombinant vaccinia viruses plus inactivated simian immunodeficiency virus and human immunodeficiency virus type 1 particles. J. Virol. 77, 1163–1174. doi: 10.1128/jvi.77.2.1163-1174.2003
Wolff, J., Beer, M., and Hoffmann, B. (2020a). Thermal inactivation of different Capripox virus isolates. Microorganisms 8:2053. doi: 10.3390/microorganisms8122053
Wolff, J., Moritz, T., Schlottau, K., Hoffmann, D., Beer, M., and Hoffmann, B. (2020b). Development of a safe and highly efficient inactivated vaccine candidate against lumpy skin disease virus. Vaccines (Basel) 9:4. doi: 10.3390/vaccines9010004
Yana, P., Olga, B., Alexander, K., and Alexander, S. (2018). A real time high-resolution melting PCR assay for detection and differentiation among sheep pox virus, goat pox virus, field isolates and vaccine strains of lumpy skin disease virus. Mol. Cell. Probes 41:S0890850818301488. doi: 10.1016/j.mcp.2018.08.003
Yeruham, I., Nir, O., Braverman, Y., Davidson, M., Grinstein, H., Haymovitch, M., et al. (1995). Spread of lumpy skin disease in Israeli dairy herds. Vet. Rec. 137:91.
Yeruham, I., Perl, S., Nyska, A., Abraham, A., and Grinstein, H. (1994). Adverse reactions in cattle to a capripox vaccine. Vet. Rec. 135, 330–332. doi: 10.1136/vr.135.14.330
Young, E., Basson, P. A., and Weiss, K. E. (1970). Experimental infection of game animals with lumpy skin disease virus (prototype strain Neethling). Onderstepoort J. Vet. Res. 37, 79–87.
Zare, P. (2010). Concise Review of Veterinary Microbiology (P.J. Quinn, B.K. Markey) .
Zavala, F., Rodrigues, M., Rodriguez, D., Rodriguez, J. R., Nussenzweig, R. S., and Esteban, M. (2001). A striking property of recombinant poxviruses: efficient inducers of in vivo expansion of primed CD8+ T cells. Virology 280, 155–159. doi: 10.1006/viro.2000.0792
Zeedan, G. S. G., Mahmoud, A. H., Abdalhamed, A. M., El-Razik, K., Khafagi, M. H., and Zeina, H. (2019). Detection of lumpy skin disease virus in cattle using real-time polymerase chain reaction and serological diagnostic assays in different governorates in Egypt in 2017. Vet World 12, 1093–1100. doi: 10.14202/vetworld.2019.1093-1100
Keywords: lumpy skin disease, lumpy skin disease virus, epidemiology, vaccine, etiology, diagnosis
Citation: Liang Z, Yao K, Wang S, Yin J, Ma X, Yin X, Wang X and Sun Y (2022) Understanding the research advances on lumpy skin disease: A comprehensive literature review of experimental evidence. Front. Microbiol . 13:1065894. doi: 10.3389/fmicb.2022.1065894
Received: 10 October 2022; Accepted: 27 October 2022; Published: 28 November 2022.
Reviewed by:
Copyright © 2022 Liang, Yao, Wang, Yin, Ma, Yin, Wang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuefeng Sun, [email protected] ; Xiangwei Wang, [email protected] ; Xiangping Yin, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Lumpy skin disease, an emerging transboundary viral disease: A review
Affiliation.
- 1 Department of Pathobiology, School of Veterinary Medicine, Shiraz University, Shiraz, Iran.
- PMID: 33522708
- PMCID: PMC8136940
- DOI: 10.1002/vms3.434
Lumpy skin disease is an emerging bovine viral disease, which is endemic in most African countries and some Middle East ones, and the elevated risk of the spread of disease into the rest of Asia and Europe should be considered. The recent rapid spread of disease in currently disease-free countries indicates the importance of understanding the limitations and routes of distribution. The causative agent, Capripoxvirus, can also induce sheeppox and goatpox. The economic significance of these diseases is of great concern, given that they threaten international trade and could be used as economic bioterrorism agents. The distribution of capripoxviruses seems to be expanding due to limited access to effective vaccines and poverty within farming communities. This is largely due to the economic effects of the Covid-19 pandemic and the imposition of crippling sanctions in endemic regions, as well as an increase in the legal and illegal trade of live animals and animal products, and also global climate change. The present review is designed to provide existing information on the various aspects of the disease such as its clinicopathology, transmission, epidemiology, diagnosis, prevention and control measures, and the potential role of wildlife in the further spread of disease.
Keywords: capripox; epidemiology; lumpy skin disease; transboundary disease.
© 2021 The Authors Veterinary Medicine and Science Published by John Wiley & Sons Ltd.
Publication types
- COVID-19 / economics
- COVID-19 / epidemiology
- Communicable Diseases, Emerging / economics
- Communicable Diseases, Emerging / epidemiology
- Communicable Diseases, Emerging / veterinary*
- Communicable Diseases, Emerging / virology
- Lumpy Skin Disease / economics
- Lumpy Skin Disease / epidemiology
- Lumpy Skin Disease / virology*
Deep Learning Based Lumpy Skin Disease (LSD) Detection
Ieee account.
- Change Username/Password
- Update Address
Purchase Details
- Payment Options
- Order History
- View Purchased Documents
Profile Information
- Communications Preferences
- Profession and Education
- Technical Interests
- US & Canada: +1 800 678 4333
- Worldwide: +1 732 981 0060
- Contact & Support
- About IEEE Xplore
- Accessibility
- Terms of Use
- Nondiscrimination Policy
- Privacy & Opting Out of Cookies
A not-for-profit organization, IEEE is the world's largest technical professional organization dedicated to advancing technology for the benefit of humanity. © Copyright 2024 IEEE - All rights reserved. Use of this web site signifies your agreement to the terms and conditions.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.24(3); 2023 May
- PMC10244130


Lumpy skin disease as an emerging infectious disease
Hye jin eom.
Department of Infectious Diseases, BK21 FOUR Future Veterinary Medicine Leading Education and Research Center, Research Institute for Veterinary Science and College of Veterinary Medicine, Seoul National University, Seoul 08826, Korea.
Eun-Seo Lee
Han sang yoo, associated data.
Lumpy skin disease (LSD) is one of the most important emerging transboundary diseases. Recently, LSD has emerged in many countries in the northern hemisphere. The LSD virus has a huge genome and is highly resistant to environmental conditions. The virus is also host-specific and large ruminants, such as cattle and domestic water buffalo, are particularly susceptible. In addition, wild ruminants can serve as potential reservoirs for spreading the LSD virus. The emergence might be related to climate change in various regions because LSD is an arthropod-borne infectious disease. This disease causes enormous economic losses, such as leather damage, decreased milk production, abortion, and death in infected ruminants. The economic importance of LSD in the bovine industry has forced countries to develop and implement control strategies against the disease. With the recent global spread and the economic impact, LSD will be discussed intensively. In addition, effective preventive measures are suggested based on the presence or absence of LSD outbreaks.
INTRODUCTION
The lumpy skin disease (LSD) virus is a member of the family Poxviridae , genus Capripoxvirus that causes LSD. This disease has recently emerged in most east European and Asian countries [ 1 , 2 , 3 , 4 ]. Bovine species ( Bos taurus and Bos indicus ) and water buffalo ( Bubalus bubalis ) are the primary hosts of this disease. In addition, the virus can infect some wild animals, such as giraffes, impalas, wildebeest, springboks, and oryxes [ 1 , 5 ]. Natural infections and antibodies against the LSD virus have been found in African wildlife species. Although the prevalence of LSD in wildlife is low, the role of wildlife as potential reservoirs for transmission to domestic ruminants should not be ignored [ 6 ]. LSD is a vector-borne disease transmitted by bloodsucking arthropods: mosquitoes ( Culex mirificens and Aedes natrionus ), biting flies ( Stomoxys calcitrans and Biomyia fasciata ), and male ticks ( Riphicephalus appendiculatus and Amblyomma hebraeum ) [ 1 , 5 ]. This disease is characterized by high fever, emaciation, enlarged superficial lymph nodes, lacrimation, conjunctivitis, and notable nodules on the skin and mucous membranes of the mouth, respiratory tract, and genitalia [ 1 , 2 , 3 , 4 ]. It can also cause reduced milk production, abortion in pregnant animals, and death. LSD has a high morbidity of up to 90% and less than 10% mortality in infected cattle [ 1 , 5 , 7 ]. The World Organization for Animal Health (WOAH) has listed LSD as a notifiable disease because of its clinical and economic importance.
Resistance and survival of the LSD virus are well documented [ 1 ]. The resistance to heat is variable. The LSD virus can be inactivated at 55°C for 2 h and at 65°C for 30 min. On the other hand, the virus can be recovered from skin nodules after storage for 10 yr at −80°C. The virus remains viable for less than 42 d in the nodules on the animal [ 1 ]. It has excellent resistance to cold. In addition, the LSD virus is very sensitive to light. Therefore, the virus can persist in the dark in livestock buildings for several months [ 1 ]. The virus is stable at neutral pH, even though resistance to the pH depends on temperature. The virus is susceptible to high alkaline or acidic pH, but can survive at pH 6.6–8.6 for 5 d at 37°C [ 1 ]. This LSD virus is susceptible to several disinfectants, especially organic matter and detergents [ 1 ].
After the first outbreak of LSD in Zambia in 1929, the disease spread in most African countries until the first report in Israel in 1989. Since the outbreak in Israel, more outbreaks of the disease were reported in Middle Eastern countries, and LSD is currently considered an endemic disease in the region [ 1 , 5 , 7 ]. From the end of 2013, LSD invaded neighboring countries, such as Türkiye, Iraq, Iran, and Azerbaijan. Türkiye may serve as a crossroads connecting the Eurasian continent, facilitating the spread of LSD to the Balkans and some European countries [ 1 ]. After spreading to the Russian Federation in 2015, LSD has invaded several Asian countries, such as Kazakhstan, China, Nepal, Pakistan, India, Bhutan, Vietnam, Thailand, Myanmar, and Taiwan [ 3 , 4 , 8 , 9 ]. Finally, the disease spread east and north to Mongolia and Eastern Siberia [ 1 , 4 ].
In wildlife, natural infections were reported in five Asian water buffalos ( B. bubalis ) during the LSD outbreak in Egypt in 1988 [ 6 ]. Antibodies to LSDV have been found in six out of 44 wildlife species in Africa: African buffalo, greater kudu Tragelaphus strepsiceros , waterbuck Kobus ellipsiprymnus , reedbuck Redunca arundinum , impala, springbok Antidorcas marsupialis , and giraffe [ 6 ]. The presence of antibodies in wildlife represents its susceptibility and epidemiological potential in LSD. Moreover, infected wildlife shows symptoms ranging from asymptomatic to mild clinical signs but may not always show detectable antibody levels. Hence, the actual number of wild ruminants infected with the LSD virus may be significantly higher than currently known.
Each country has implemented control measures to prevent the spread of LSD, considering the differences between countries, such as cattle distribution situation, region, and climate. In most countries, the control measures of LSD were surveillance, expert training, stamping-out, movement restriction, hygiene management, quarantine and bio-security, vector control, and vaccination [ 1 , 5 ]. The purpose of this survey was to enhance the awareness of LSD spread and provide information on control measures based on the country.
RECENT OUTBREAKS OF LSD
According to the WOAH report, outbreaks of LSD in susceptible animals have occurred and are increasing consistently ( Fig. 1 ). LSD is endemic in most African countries. Since 2013, it has spread rapidly through the Middle East and Southeast Europe. Since 2019, several thousand outbreaks of LSD have been reported in Southeast Asian countries [ 1 , 7 ]. The number of LSD outbreaks in Asia reported was 28, 317, and 1,088 cases in 2019, 2020, and 2021, respectively [ 10 ] ( Supplementary Fig. 1 ). Specifically, China first reported LSD in August 2019 and reported it in adjacent areas in June 2020 [ 8 ]. LSD first occurred in July 2020 in Kinmen island, Taiwan, neighboring China, and then in New Taipei City in April 2021 [ 2 ]. In addition, it first occurred in Thailand in March 2021 and spread rapidly throughout Thailand [ 9 ].

The spread of LSD outbreaks in Southeast Asian countries has been faster compared to the transmission period in African and Middle Eastern outbreaks. This phenomenon might be affected by the high temperature and humidity of the tropical climate in the region, which is a favorable environment for the reproduction of arthropod vectors [ 1 ]. The speed of LSD spread might differ according to the pattern of cattle raising and cattle movements. The speed at which the infection transmits was calculated to be 7.3 km per week, based on the spread pattern from Western Türkiye to the countries in the Balkans peninsula, from May 2015 to August 2016 [ 5 ]. On the other hand, a survey in Thailand showed that calculating the distance to the reported area was 50 and 100 d after the initial outbreak, the speed of LSD transmission in Thailand was estimated to be approximately 2 to 14 km/day [ 10 ].
CONTROL STRATEGIES AGAINST LSD
The principle of control strategies against LSD is similar regardless of the LSD outbreak. On the other hand, the implemented actions differ according to the countries with or without the LSD outbreak. In most countries with outbreaks, stamping out the positive cases, emergency vaccination, movement control of cattle, surveillance, and monitoring are the main control strategies ( Supplementary Table 1 ) [ 11 , 12 ]. On the other hand, legislation and risk assessment are the most important preparation against the LSD outbreak in countries without LSD outbreaks. Legislation includes surveillance and monitoring, stamping out policy, movement and disease control, and emergency vaccination policy. More detailed action plans, such as preparing vaccine banks and defining disease control zone, have been implemented based on the risk assessment in each country ( Supplementary Table 2 ).
Although reports of LSD in wildlife have a low incidence, this disease should be controlled in wildlife because of the possibility of transmission and the difficulty of vaccinating wildlife. The presence of LSD in wildlife can be easily missed because monitoring the clinical signs, such as skin lesions, is difficult due to wildlife migration. Therefore, surveillance of the clinical signs in wildlife and strict monitoring of wildlife movements are needed.
LSD CONTROL STRATEGIES BY COUNTRY WITH THE OUTBREAK
The current global situation of LSD outbreaks was intensively reviewed by the European Food Safety Authority, the Food Agriculture Organization of the United Nations (FAO), and other studies. In particular, the two organizations focused on the LSD outbreak in the Balkan and Caucasus countries [ 12 ]. On the other hand, epidemiological aspects and control strategies related to LSD in Asian countries remain to be analyzed, even though some studies have been done and reported [ 1 , 2 , 3 , 4 , 8 , 13 ].
As shown in Supplementary Table 1 , the control measures implemented and applied to LSD outbreaks differed according to the economic, political, and social situation of each country. Most countries with outbreaks implemented the following control policies: stamping-out, animal movement control, vaccination, active surveillance, vector control, and compensation. The total stamping-out policy in the infected area was more effective in disease control than the partial stamping-out policy, which culled cattle with clinical signs and LSD virus positive. Regarding a vaccination strategy, live attenuated homologous vaccines (Neethling strain) were more effective than heterologous ones (sheeppox/goatpox virus-based vaccines), and the vaccination area was also very important in preventing the spread of LSD [ 14 ]. The area to be vaccinated should be determined according to the probability of LSD virus infection and LSD transmission [ 5 ]. Considering the above facts, stamping-out and vaccination policies should be conducted. Long-distance movement by infected or asymptomatic cattle is a rapid transmission factor and has been suspected of causing rapid spread throughout Thailand. Therefore, movement restrictions should be thoroughly enforced for disease management. Vector control might also use systemic or local insecticides (Ivermectin) and tick repellent (DEET and Permethrin). Compensation and awareness of the disease were also important factors in controlling LSD.
LSD CONTROL STRATEGIES BY COUNTRY WITHOUT THE OUTBREAK
In the countries without LSD outbreaks, as the first step, an LSD control strategy should be developed based on the status of LSD outbreaks in the surrounding countries. In addition, building up a vaccine bank or preventive vaccination is recommended in those countries.
The control strategy should include legislation, surveillance and monitoring, stamping-out policy, movement control, disease control zone, vaccination strategy, and education to related workers. The size of the vaccine bank should be determined based on the related factors of each country, such as disease control area, taking the incubation period into account, the distance of cattle movement, duration of full immunity development, the time needed to carry out vaccination, density of susceptible animals, and vector activities [ 5 ]. On the other hand, only a few countries have a contingency plan. France carried out a risk assessment of LSD and estimated the size of the vaccine bank ( Supplementary Table 2 ).
S. calcitrans , a bloodsucking arthropod, can travel up to 255 km by wind. After the outbreak of Türkiye, a safety quarantine zone of up to 10 km along the border was established to strengthen the monitoring of LSD in Greece [ 12 ]. Those reports suggest that control strategies should be considered in preparing for the spread by neighboring countries.
Several vaccines with homologous or heterologous strains against LSD are commercially available. Hence, each country should build up a vaccine bank for the emergence of LSD based on their current epidemiological situation.
CONCLUSION AND FUTURE PROSPECTIVE
LSD is a devastating infectious disease to the bovine industry and wildlife, both clinically and economically. Currently, LSD has invaded most Asian countries and is a hot spot of the outbreak, particularly in Southeastern Asian countries. The current situation of the disease makes LSD an emerging transboundary infectious disease worldwide, especially in Asian countries. Therefore, world organizations, such as FAO and WOAH, have intensively reviewed the outbreaks epidemiologically, clinically, and economically and provided guidelines to develop contingency plans for LSD. This survey summarized the control measures and contingency plans in various countries. Considering this survey, the author believes that vaccination and movement control of large ruminants are important factors for effective methods of LSD control. Vaccines should be established in advance in countries where there have been no LSD outbreaks, and in the outbreak of LSD, immediate total stamping-out in holdings and establishing a disease control zone should be done. Rapid vaccination and strict movement control of susceptible animals should be carried out according to the control area. Therefore, many countries should prepare a control plan for LSD regardless of the devastating infectious disease outbreak.
Funding: This subject is supported by the National Institute of Wildlife Disease Control and Prevention as “Specialized Graduate School Support Project for Wildlife Disease Specialists,” the Agriculture, Food and Rural Affairs Convergence Technologies Program for Educating Creative Global Leader (No. 320005-4); the Ministry of Agriculture, Food and Rural Affairs, BK21FOUR Program, and the Research Institute for Veterinary Science, Seoul National University, Republic of Korea.
Conflict of Interest: The authors declare no conflicts of interest.
Author Contributions:
- Conceptualization: Yoo HS, Eom HJ, Lee ES.
- Data curation: Eom HJ, Lee ES.
- Formal analysis: Eom HJ.
- Investigation: Eom HJ, Lee ES.
- Methodology: Yoo HS, Eom HJ.
- Project administration: Yoo HS, Eom HJ, Lee ES.
- Resources: Eom HJ, Lee ES.
- Supervision: Yoo HS, Eom HJ.
- Validation: Yoo HS.
- Visualization: Eom HJ.
- Writing - original draft: Eom HJ.
- Writing - review & editing: Yoo HS.
SUPPLEMENTARY MATERIALS
Control measures implemented in LSD outbreak countries
Recommendations legislated in countries without LSD outbreaks and international organizations
Annual reports of lumpy skin disease outbreaks by the World Organization for Animal Health.
Advertisement
Assessing machine learning techniques in forecasting lumpy skin disease occurrence based on meteorological and geospatial features
- Regular Articles
- Published: 14 January 2022
- Volume 54 , article number 55 , ( 2022 )
Cite this article

- Ehsanallah Afshari Safavi ORCID: orcid.org/0000-0001-7022-0867 1
6166 Accesses
17 Citations
1 Altmetric
Explore all metrics
Lumpy skin disease virus (LSDV) causes an infectious disease in cattle. Due to its direct relationship with the survival of arthropod vectors, geospatial and climatic features play a vital role in the epidemiology of the disease. The objective of this study was to assess the ability of some machine learning algorithms to forecast the occurrence of LSDV infection based on meteorological and geological attributes. Initially, ExtraTreesClassifier algorithm was used to select the important predictive features in forecasting the disease occurrence in unseen (test) data among meteorological, animal population density, dominant land cover, and elevation attributes. Some machine learning techniques revealed high accuracy in predicting the LSDV occurrence in test data (up to 97%). In terms of area under curve (AUC) and F1 performance metric scores, the artificial neural network (ANN) algorithm outperformed other machine learning methods in predicting the occurrence of LSDV infection in unseen data with the corresponding values of 0.97 and 0.94, respectively. Using this algorithm, the model consisted of all predictive features and the one which only included meteorological attributes as important features showed similar predictive performance. According to the findings of this research, ANN can be used to forecast the occurrence of LSDV infection with high precision using geospatial and meteorological parameters. Applying the forecasting power of these methods could be a great help in conducting screening and awareness programs, as well as taking preventive measures like vaccination in areas where the occurrence of LSDV infection is a high risk.
Similar content being viewed by others
Application of machine learning models for risk estimation and risk prediction of classical swine fever in Assam, India
Comparison of machine learning methods to predict udder health status based on somatic cell counts in dairy cows

Machine learning based forest fire susceptibility assessment of Manavgat district (Antalya), Turkey
Avoid common mistakes on your manuscript.
Introduction
Lumpy skin disease virus (LSDV) infection is a major challenge to cattle production, causing acute or subacute disease in cattle and water buffalo population. Cattle of all breeds can become infected, and cows that are around the peak of milk production and calves are particularly susceptible to LSDV infection (Namazi and Khodakaram Tafti 2021 ).
The LSDV is a double-stranded DNA virus belonging to the Capripoxvirus genus. Fever, inappetence, a significant drop in milk production, swollen lymph nodes, and the appearance of hard, slightly elevated skin nodules quickly after the onset of fever are the main clinical signs of the infection. Despite the availability of a variety of diagnostic tests, the diagnosis is generally confirmed using a traditional or real-time PCR (polymerase chain reaction) approach (Namazi and Khodakaram Tafti 2021 ).
In 1929, the first case of LSDV infection was recorded in Zambia (Von Backstrom 1945 ). LSDV has gradually expanded through Africa, the Middle East, Southeastern Europe, Central Asia, and, most recently, South Asia and China. The disease is now endemic in many African countries, as well as areas of the Middle East (Iraq, Saudi Arabia, and the Syrian Arab Republic) and Turkey (Roche et al. 2020 ). The disease has resulted in major economic losses in the affected countries. Due to high fever and secondary mastitis, it causes a substantial drop in milk production. Other consequences of the disease include damaged skin, a reduction in the growth rate of beef cattle, transient or lifelong infertility, abortion, treatment and vaccination costs, and the mortality in infected animals (Alemayehu et al. 2013 ; Namazi and Khodakaram Tafti 2021 ).
LSDV is transmitted by insects, in particular blood-sucking arthropods, contaminated food and drink, and at the later stages of the disease through saliva, nasal secretions, and semen (Sprygin et al. 2018 ; Tuppurainen et al. 2017 ). Due to its direct relationship with the survival of vectors, climatic conditions play an important role in the epidemiology of the disease. A warm and humid climate, environmental conditions that support an influx of vector populations, such as those seen during seasonal rains, and the introduction of new animals to a herd are all risk factors for the spread of LSDV. Furthermore, the wind’s direction and intensity may play a role in the spread of the virus (Chihota et al. 2003 ).
The association between LSDV infection and meteorological and geospatial factors has been studied in many studies, and they have discovered that factors like temperature, precipitation, land cover, humidity, and wind speed can predict or influence the occurrence of the disease (Alkhamis and VanderWaal 2016 ; Allepuz et al. 2019 ; Machado et al. 2019 ; Molla et al. 2017 ; Sprygin et al. 2018 ; Tuppurainen and Oura 2012 ).
Due to the introduction of new technologies and analytical techniques such as big data, remote sensing, and Earth observation, many digital Earth researches are now employing big spatiotemporal data to track and define the dynamic Earth climate system, (Kovacs-Györi et al. 2020 ; Yang et al. 2017 ).
Nowadays, machine learning (ML) offers highly valuable resources for intelligent geospatial and environmental data analysis, synthesis, and visualization. ML methods, particularly deep learning approaches, have become more common as the availability of more and different types of big data has grown (Xu and Jackson 2019 ). These techniques use general purpose learning algorithms to look for similarities in often complex and unwieldy data (Bzdok et al. 2018 ). In general, they can be used effectively at all levels of environmental data mining: exploratory spatial data processing, identification and modeling of spatial–temporal patterns, and decision-driven mapping. Traditional geostatistical methods have been replaced greatly by machine learning techniques especially in big data analyses (Kanevski et al. 2008 ). However, ML techniques should be implemented accurately and effectively from pre-processing data to analysis and justification of the findings (Kanevski et al. 2008 ).
ML techniques have been evaluated in several studies for predicting the occurrence of infectious diseases in human or animals using various climatic and geospatial features.
Wang et al. ( 2015 ) developed a feed-forward back-propagation neural network model to predict the weekly number of human cases of infectious diarrhea in China (Shanghai) using meteorological factors as predictive features. Non-linear models including neural networks, support vector regression, and random forests regression showed better performance than multiple linear regression. Neural networks showed most satisfactory results when all performance evaluation criteria were considered simultaneously.
Malki et al. ( 2020 ) explored various regressor machine learning models to predict confirmed and death cases of COVID-19 in various countries. In forecasting the COVID-19 confirmed cases, the highest performance was obtained by the KNN (K-nearest neighbors) regressor. Decision tree algorithm showed best performance in predicting the rate of COVID-19 mortality. Weather variables such as temperature and humidity were more important in predicting the mortality rate when compared to the other census variables such as population, age, and urbanization.
Golden et al. ( 2019 ) collected soil and feces samples from 11 pastured poultry farms from 2014 to 2017 in the USA. They generated random forest and gradient boosting machine predictive models to predict Listeria spp. prevalence in samples based on meteorological factors such as temperature, wind speed, gust speed, humidity, and precipitation at the farming location. AUC performance metric for the random forest and gradient boosting machine models of fecal samples was 0.905 and 0.855, respectively. The soil gradient boosting machine model outperformed the random forest model with AUCs of 0.873 and 0.700, respectively.
Liang et al. ( 2020 ) used machine learning methods to forecast African swine fever outbreaks around the world using bio-climatic variables. The random forest algorithm outperformed other techniques with 80.4% accuracy in the dataset containing all predictive variables, and the support vector machine algorithm showed the best accuracy in the subset dataset containing only important climatic features (76.02%).
The accuracy score of prediction varied between 47.8 and 99.6% in the study by Niu et al. ( 2020 ), which used various machine learning algorithms to forecast Peste des Petits ruminants (PPR) outbreaks based on certain bio-climatic variables and altitude data. The random forest algorithm performed best in a test dataset consisting of data from three countries that were not included in the training process.
To the best of the author’s knowledge, no related research has been undertaken in terms of evaluating ML techniques in building models to forecast the incidence of LSDV infection using meteorological and/or geospatial attributes.
Because of the importance of insects in LSDV transmission and their reliance on climatic and geographical features, the key objective of this research was to develop predictive models using some robust ML algorithms based on meteorological and geospatial features to predict the incidence of LSDV infection in countries with a prior history of disease outbreak reported between 2011 and 2021.
Materials and methods
Figure 1 depicts the summary of steps taken in the materials and methods, and the details of each step are explained in the following sections.
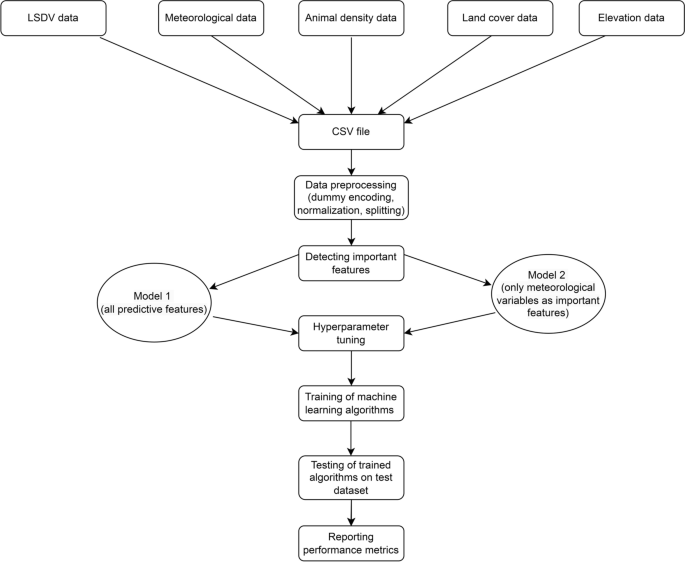
Summary of steps taken in the materials and methods section
Data sources
Lumpy skin disease outbreak data.
Geographic coordinates of Lumpy Skin Disease outbreaks were obtained from Global Animal Disease Information System of FAO (Food and Agriculture Organization) ( https://empres-i.review.fao.org/ /). Relevant information between January 2011 and March 2021 including the specific time of the outbreak and the longitude and latitude of the outbreak point were downloaded.
Meteorological data
Monthly cloud cover (percentage), diurnal temperature range (degrees Celsius), frost day frequency (days per month), wet day frequency (days), potential evapotranspiration (millimeters per day), precipitation (millimeters per months), daily mean temperature (degrees Celsius), monthly average maximum and minimum temperature (degrees Celsius), and vapor pressure (hectopascal) data for the period January 2011–December 2019 were obtained from the University of East Anglia’s Climatic Research Unit (CRU TS4.04) (Harris et al. 2020 ).
Animal density data
Cattle and buffalo population density data were obtained from Gridded Livestock of the World (GLW 3) database (Gilbert et al. 2018 ).
Land cover data
GLC-SHARE Beta-Release v1.0 (Latham et al. 2014 ) was used to extract global land cover data (spatial information on various forms of physical covering of the Earth’s surface) including artificial surfaces, cropland, grassland, tree covered areas, shrubs covered areas, herbaceous vegetation, aquatic or regularly flooded areas, mangroves, sparse vegetation, bare soil, snow and glaciers, and waterbodies coverage.
Elevation data
Global geospatial elevation dataset (GRAY_50M_SR.VERSION 2.1.0) was downloaded from Natural Earth database (free vector and raster map data @ naturalearthdata.com).
Data preprocessing
Only data in countries which reported the LSDV infection during the study time period (2011–2021) were extracted in all downloaded data and map files. In order to prepare data values to be used by ML algorithms, categorical variables were converted to numeric values using one-hot encoding technique. Moreover, the values of different predictive features were normalized using min–max scaling. Finally, the dataset was split into train and test sets using train_test_split class from scikit-learn library (Pedregosa et al. 2011 ). The training dataset which was used during model development and the test set which was not seen by the model were used for validation. Repeated stratified K-Fold cross-validation using 3 splits and 2 repeats was also used to validate the machine learning models during training step.
Selecting of features based on importance
The Scikit-learn module’s ExtraTreesClassifier and SelectFromModel classes were used to select features that are most useful for prediction. The ExtraTreesClassifier class implements a meta estimator that employs averaging to control over-fitting by fitting a number of randomized decision trees (extra-trees) on different sub-samples of the dataset (Geurts et al. 2006 ). SelectFromModel class is a meta-transformer for selecting features based on importance weights. SelectFromModel accepts a threshold parameter and will select the features whose importance (defined by the coefficients) are above this threshold. SelectFromModel requires the underlying estimator to expose a coef_ attribute or a feature_importances_ attribute which in this case was provided by ExtraTreesClassifier class. The net results of the cooperation of these two classes are choosing the important predictive features among all predictive variables.
Hyperparameter tuning
To choose a set of optimal parameters for each machine learning techniques, RandomizedSearchCV method from the scikit-learn library was used. This method can test a given number of candidates from a parameter set with a specified distribution.
Machine learning algorithms used in training and testing phases
Logistic regression.
Logistic regression is one of the machine learning classification techniques, which is utilized for anticipating the categorical dependent variable employing a given set of dependent variables and gives the probabilistic values which lie between 0 and 1 (Cox 1958 ).
Support vector machine
Support vector machines (SVMs) are a group of supervised learning techniques which are effective in high dimensional spaces. It creates the best decision boundary to separate multi-dimensional space into subclasses using the extreme cases which are called support vectors (Scholkopf 1998 ).
Decision tree
A decision tree classifier is a tree-like structure that creates a training model to predict the target class through learning simple decision rules inferred from prior data (training data). Internal nodes represent features (or attributes), the branches represent decision rules, and each leaf node represents the outcome (Safavian and Landgrebe 1991 ).
Random forest
Random forest is an ensemble decision tree-based classification method that acts through building a number of trees and each tree is dependent on the values of an independently sampled random vector with the same distribution for all trees within the forest (Breiman 2001 ).
AdaBoost is an ensemble algorithm in which subsequent weak learners are adjusted adaptively in favor of those instances misclassified by previous classifiers (Freund and Schapire 1997 ).
As another ensemble method, bagging (short for bootstrap aggregating) uses the same training algorithm for every predictor and train them on different random subsets of the training set with replacement (Breiman 1996 ).
XGBoost is a popular and efficient open-source implementation of the gradient boosted trees algorithm (Chen and Guestrin 2016 ). XGBoost stands for extreme gradient boosting, which uses decision trees as base learners, merging several weak learners to create a more powerful learner. Therefore, it is referred to as an ensemble learning algorithm since the final prediction incorporates the output of several models.
Artificial neural network (multilayer perceptron)
A simple form of artificial neural network (ANN) is the multilayer perceptron (MLP). In most cases, it has three layers: input, output, and a hidden layer. The input layer is where the data to be processed is received. The output layer is in charge of classification. The true computational engine of the MLP is an arbitrary number of hidden layers located between the input and output layers (Chollet 2018 ).
Evaluating the performance of predictive models
Accuracy score, precision, recall, F1 score, and area under curve (AUC) were used as performance metrics to measure the power of different classifiers in predicting unseen data (test set) (Géron 2019 ).
Accuracy score is one of the common performance metrics which is calculated by dividing the number of correct predictions by total number of predictions.
Another useful metric is precision, or the accuracy of the positive predictions:
Recall is the ratio of positive instances that are correctly detected by the classifier:
F1 score is the harmonic mean of precision and recall which gives much more weight to low values:
Receiver operating characteristic (ROC) curve plots the true positive rate (recall) against the false positive rate. The area under curve (AUC) of ROC curves used as its summary and assesses a classifier’s ability to discriminate between classes.
Analysis tools
QGIS software (version 3.16 – Hannover) was applied to analyze and edit spatial data files. Machine learning techniques were implemented using the Python programming language (version 3.8) and the Anaconda navigator platform (as a package manager; version 1.10.0). Scikit-learn 0.24.1 (Pedregosa et al. 2011 ) was used to implement logistic regression, SVM, decision tree, random forest, AdaBoost, and bagging algorithms. The XGBoost library was utilized to run the XGBoost technique (Chen and Guestrin 2016 ). Keras API (Chollet 2018 ) running as an abstraction layer on top of TensorFlow 2 framework (Abadi et al. 2016 ) was used for building multilayer perceptron (ANN).
Distribution of outbreaks points
Between January 2011 and March 2021, 3039 LSDV infection outbreaks were recorded across Africa, Asia, and Europe. Figure 2 indicates the distribution of outbreaks points along with 21,757 free points.
The distribution of reported LSDV infection points during 2011–2021
The highest incidence of the disease during the study period was reported in Europe (2172 outbreaks), Asia (777 outbreaks), and Africa (90 outbreaks), respectively.
The highest incidence of the disease was recorded in 2016 (Fig. 3 ).
Reported LSDV infection outbreaks in each year during 2011–2021
Important features
Based on the results of applying ExtraTreesClassifier and SelectFromModel algorithms on the dataset, only meteorological variables were considered as important features. Therefore, two independent analyses were carried out: one involving all predictive variables including all meteorological, elevation, animal population density, and land cover features (model 1) and the other consisting of only meteorological features (model 2).
Tuned parameters of algorithms
In Tables 1 and 2 , some of the most important tuned parameters in each algorithms in model 1 and 2 are shown, respectively.
The predictive ability of various machine learning algorithms
Depending on the type of ML algorithm and performance metric used, the predictive ability of techniques using two subsets of features was different (Table 3 ).
AUC metric ranged between 0.53% to 0.97% and 0.63% to 0.97% in model 1 and model 2, respectively. In both models, ANN algorithm outperformed other algorithms in terms of AUC and F1 score. ROC curves of different ML algorithms for model 1 and model 2 are shown in Figs. 4 and 5 , respectively.
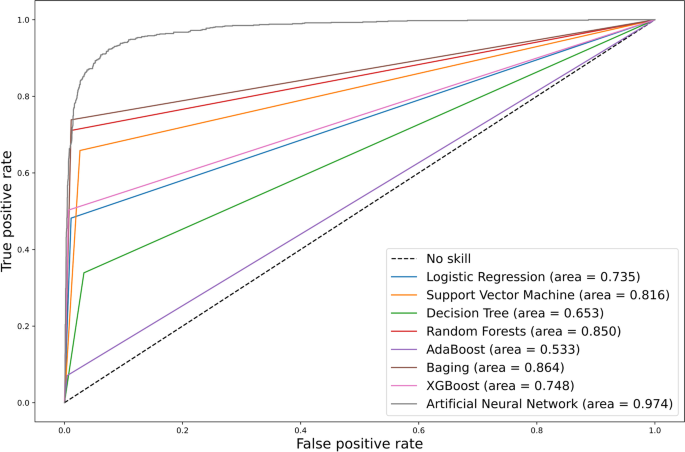
Receiver operating characteristic (ROC) curves of various machine learning algorithms for model 1 (including all predictors)
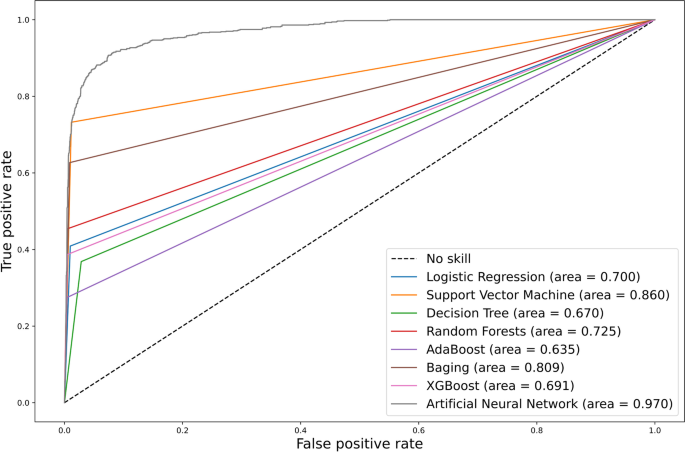
Receiver operating characteristic (ROC) curves of various machine learning algorithms for model 2 (including only predictive meteorological variables)
The findings of current study demonstrated that by applying machine learning methods and using climatic and geospatial features as predictive variables, the occurrence of LSDV infection could be predicted in test set (unseen data) with high accuracy. For instance, ANN algorithm indicated 97% accuracy score. However, the accuracy score is not the preferred performance measure for classifiers, particularly where certain classes are more frequent than others (Géron 2019 ). As a result, when assessing the predictive power of algorithms, it makes more sense to consider performance metrics such as precision, recall, F1 score, and AUC. Regarding AUC metric and by incorporating all predictive variables in the model or using only meteorological variables as predictors, the highest performance was associated with ANN algorithm (97% in both models) (Table 3 ).
Artificial neural networks have been widely used in different fields including medical and health field, such as medical diagnosis and disease prediction and obtained the very good prediction results (Abbass 2002 ; Al-Shayea 2011 ; Baxt 1995 ; Fang et al. 2014 ; Flores-Fernández et al. 2012 ; Kara and Dirgenali 2007 ; Kia et al. 2013 ; Ma and Wang 2010 ; Wang and Gupta 2013 ; Wang et al. 2001 ; Zhu and Wang 2010 ).
The reason for better performance of ANN could be attributed to the fact that this algorithm is a universal approximator which can approximate a large class of functions with a high degree of accuracy (Y. Wang et al. 2015 ).
The predictive performance of ANN was almost the same in both models (using all predictor variables vs only climatic predictive variables) with AUC of 0.97. The literature shows that feature selection can boost the classifier’s prediction accuracy, scalability, and generalization capability. This technique is critical in information discovery because it reduces computational complexity, storage, and cost (Gutkin et al. 2009 ). It should be noted, however, that any predictive feature may be irrelevant individually, but when combined with others, it becomes relevant (Gheyas and Smith 2010 ). As a result, feature selection does not always imply improved results, and in some cases, eliminating features could be detrimental (Guyon et al. 2008 ).
To the best of the author’s knowledge, no other study has used machine learning algorithms to forecast the incidence of LSDV infection using geospatial and meteorological predictive parameters. However, some similar studies utilized machine learning methods to predict the occurrence of some viral livestock diseases based on climatic data.
Liang et al. ( 2020 ) used machine learning methods to forecast African swine fever outbreaks around the world using bio-climatic variables, and Niu et al. ( 2020 ) applied various machine learning algorithms to forecast Peste des Petits ruminants (PPR) outbreaks based on certain bio-climatic variables and altitude data. Nevertheless, the time frame during which climate data (WordClim database which contains data for 1970–2000) used in these studies was before the time period during which disease outbreaks data utilized and this could be a potential source of bias. In contrast, in the present study, meteorological data were downloaded for the period 2011–2019 from CRU TS4.04 database (Harris et al. 2020 ) to provide better time concordance with event data of LSDV infection.
According to the feature selection algorithm, out of meteorological, animal density, land cover, and elevation data, only meteorological variables were chosen as significant predictive factors in the present study. Similarly, wet and warm climates which are prime habitat for blood-feeding arthropods have been linked to the occurrence of LSDV infection previously (Alkhamis and VanderWaal 2016 ; Chihota et al. 2003 ; Weiss 1968 ). Some studies which used statistical methods have found a connection between land cover characteristics and/or animal density and disease incidence. For instance, Alkhamis and VanderWaal ( 2016 ) examined LSDV outbreak records in the Middle East between 2012 and 2015. The most important environmental predictors that contributed to the ecological niche of LSDV were annual precipitation, land cover, mean diurnal range, type of livestock production system, and global livestock densities, according to ecological niche modeling. Allepuz et al. ( 2019 ) investigated the relationship between confirmed LSDV infection outbreaks and climatic factors, land cover, and cattle density in the Balkans, Caucasus, and Middle East between 2012 and 2018. The findings revealed that the likelihood of disease incidence was considerably higher in areas dominated by croplands, grassland, or shrub land. Higher cattle populations, as well as regions with a higher annual mean temperature and a larger diurnal temperature range, increased the odds. In contrast to areas covered mostly by forest, areas with sparse vegetation have a lower risk of infection.
Gari et al. ( 2010 ) conducted a questionnaire survey to perform a cross-sectional analysis to assess the distribution of LSDV infection and related risk factors in Ethiopia’s three major agro-climatic areas. Across agro-climate zones, herd-level prevalence of LSDV infection was slightly higher in the midland agro-climate than in the highland and lowland agro-climate zones. The odds ratio of LSDV infection incidence was 3.86 (95% confidence interval: 2.61–5.11) in the midland vs. highland region and 4.85 (95% confidence interval: 2.59–7.1) in the lowland vs. highland zone. The introduction of new animal, as well as communal grazing and watering management, was correlated with a significantly increased risk of LSDV infection incidence.
Molla et al. ( 2017 ) conducted a research between 2000 and 2015 with the goals of determining the geographical and temporal spread of LSDV infection outbreaks and forecasting the possible outbreaks in Ethiopia. The incidence varied by region, with the lowest in hot dry lowlands and the highest in wet moist highlands. They discovered that outbreaks were seasonal, occurring most often in the months after a long rainy season.
All the mentioned researches used statistical methods which are designed for inference about the relationships between variables and not making predictions. On the contrary, prediction made by machine learning algorithms aims at forecasting unobserved outcomes (Bzdok et al. 2018 ) which is what has been used in the present study. In addition to the different methods used, discrepancies in the results of similar researches could also be caused by the use of different independent variables (risk factors) and different study locations.
Howerver, it is worth mentioning that the LSDV outbreak data used in the present study were mainly passive accounts from veterinary facilities in various countries. There are some drawbacks of using passive monitoring data that should be addressed when analyzing the findings. The presence or quality of compensation schemes, the capability and transparency of veterinary facilities, the remoteness of some regions, and farmer visibility all impede reporting in some countries. Nevertheless, the lack of LSDV reports in some areas of the surveyed countries could be attributed to a lack of suitable environmental conditions for the dissemination of the disease in the area.
Other limitations of the current study include the small amount of data used, the small number of predictor variables used, and the possibility that the disease has spread to other regions of the studied countries with different climatic and geographical conditions since conducting this research.
In conclusion, some machine learning algorithms like ANN could be potentially used to accurately forecast the occurrence of LSDV infection based on some geospatial and meteorological parameters. Using this approach could be extremely beneficial to implement monitoring and awareness schemes, as well as preventive measures such as vaccine campaigns in areas where LSDV infection is a high risk.
Data availability
The datasets generated during and/or analyzed during the current study are available in the Mendeley repository, https://data.mendeley.com/datasets/7pyhbzb2n9/1 .
Abadi, M., Agarwal, A., Barham, P., Brevdo, E., Chen, Z., Citro, C., . . . Devin, M. (2016). Tensorflow: Large-scale machine learning on heterogeneous distributed systems. arXiv preprint arXiv:1603.04467.
Abbass, H. A. (2002). An evolutionary artificial neural networks approach for breast cancer diagnosis. Artificial intelligence in Medicine, 25 (3), 265-281.
Article Google Scholar
Alemayehu, G., Zewde, G., & Admassu, B. (2013). Risk assessments of lumpy skin diseases in Borena bull market chain and its implication for livelihoods and international trade. Tropical Animal Health and Production, 45 (5), 1153–1159. https://doi.org/10.1007/s11250-012-0340-9
Alkhamis, M. A., & VanderWaal, K. (2016). Spatial and temporal epidemiology of lumpy skin disease in the Middle East, 2012–2015. Frontiers in veterinary science, 3 , 19. https://doi.org/10.3389/fvets.2016.00019
Article PubMed PubMed Central Google Scholar
Allepuz, A., Casal, J., & Beltrán‐Alcrudo, D. (2019). Spatial analysis of lumpy skin disease in Eurasia—Predicting areas at risk for further spread within the region. Transboundary and emerging diseases, 66 (2), 813-822. https://doi.org/10.1111/tbed.13090
Article PubMed Google Scholar
Al-Shayea, Q. K. (2011). Artificial neural networks in medical diagnosis. International Journal of Computer Science Issues, 8 (2), 150-154.
Google Scholar
Von Backstrom, U. (1945). Ngamiland cattle disease: preliminary report on a new disease, the etiological agent being probably of an infectious nature. Journal of the South African Veterinary Association, 16 (1), 29–35. https://hdl.handle.net/10520/AJA00382809_377
Baxt, W. G. (1995). Application of artificial neural networks to clinical medicine. The lancet, 346 (8983), 1135-1138.
Article CAS Google Scholar
Breiman, L. (1996). Bagging predictors. Machine learning, 24 (2), 123-140. https://doi.org/10.1023/A:1018054314350
Breiman, L. (2001). Random forests. Machine learning, 45 (1), 5-32. https://doi.org/10.1023/A:1010933404324
Bzdok, D., Altman, N., & Krzywinski, M. (2018). Points of significance: statistics versus machine learning. In: Nature Publishing Group.
Chen, T., & Guestrin, C. (2016). Xgboost: A scalable tree boosting system. Paper presented at the Proceedings of the 22nd acm sigkdd international conference on knowledge discovery and data mining.
Chihota, C., Rennie, L., Kitching, R., & Mellor, P. (2003). Attempted mechanical transmission of lumpy skin disease virus by biting insects. Medical and Veterinary Entomology, 17 (3), 294-300. https://doi.org/10.1046/j.1365-2915.2003.00445.x
Article CAS PubMed Google Scholar
Chollet, F. (2018). Deep learning with Python (Vol. 361): Manning New York.
Cox, D. R. (1958). The regression analysis of binary sequences. Journal of the Royal Statistical Society: Series B (Methodological), 20 (2), 215-232. https://doi.org/10.1111/j.2517-6161.1958.tb00292.x
Fang, Y., Fataliyev, K., Wang, L., Fu, X., & Wang, Y. (2014). Improving the genetic-algorithm-optimized wavelet neural network for stock market prediction. Paper presented at the 2014 International Joint Conference on Neural Networks (IJCNN).
Flores-Fernández, J. M., Herrera-López, E. J., Sánchez-Llamas, F., Rojas-Calvillo, A., Cabrera-Galeana, P. A., Leal-Pacheco, G., . . . Martínez-Velázquez, M. (2012). Development of an optimized multi-biomarker panel for the detection of lung cancer based on principal component analysis and artificial neural network modeling. Expert Systems with Applications, 39 (12), 10851-10856.
Freund, Y., & Schapire, R. E. (1997). A decision-theoretic generalization of on-line learning and an application to boosting. Journal of computer and system sciences, 55 (1), 119-139. https://doi.org/10.1006/jcss.1997.1504
Gari, G., Waret-Szkuta, A., Grosbois, V., Jacquiet, P., & Roger, F. (2010). Risk factors associated with observed clinical lumpy skin disease in Ethiopia. Epidemiology & Infection, 138 (11), 1657-1666. https://doi.org/10.1017/s0950268810000506
Géron, A. (2019). Hands-on machine learning with Scikit-Learn, Keras, and TensorFlow: Concepts, tools, and techniques to build intelligent systems : O'Reilly Media.
Geurts, P., Ernst, D., & Wehenkel, L. (2006). Extremely randomized trees. Machine learning, 63 (1), 3-42.
Gheyas, I. A., & Smith, L. S. (2010). Feature subset selection in large dimensionality domains. Pattern recognition, 43 (1), 5-13. https://doi.org/10.1016/j.patcog.2009.06.009
Gilbert, M., Nicolas, G., Cinardi, G., Van Boeckel, T. P., Vanwambeke, S. O., Wint, G. W., & Robinson, T. P. (2018). Global distribution data for cattle, buffaloes, horses, sheep, goats, pigs, chickens and ducks in 2010. Scientific data, 5 (1), 1-11. https://doi.org/10.1038/sdata.2018.227
Golden, C. E., Rothrock Jr, M. J., & Mishra, A. (2019). Comparison between random forest and gradient boosting machine methods for predicting Listeria spp. prevalence in the environment of pastured poultry farms. Food research international, 122 , 47-55.
Gutkin, M., Shamir, R., & Dror, G. (2009). SlimPLS: a method for feature selection in gene expression-based disease classification. PloS one, 4 (7), e6416. https://doi.org/10.1371/journal.pone.0006416
Guyon, I., Gunn, S., Nikravesh, M., & Zadeh, L. A. (2008). Feature extraction: foundations and applications (Vol. 207): Springer.
Harris, I., Osborn, T. J., Jones, P., & Lister, D. (2020). Version 4 of the CRU TS monthly high-resolution gridded multivariate climate dataset. Scientific data, 7 (1), 1-18. https://doi.org/10.6084/m9.figshare.11980500
Kanevski, M., Pozdnukhov, A., & Timonin, V. (2008). Machine learning algorithms for geospatial data. Applications and software tools.
Kara, S., & Dirgenali, F. (2007). A system to diagnose atherosclerosis via wavelet transforms, principal component analysis and artificial neural networks. Expert Systems with Applications, 32 (2), 632-640.
Kia, S., Setayeshi, S., Shamsaei, M., & Kia, M. (2013). Computer-aided diagnosis (CAD) of the skin disease based on an intelligent classification of sonogram using neural network. Neural Computing and Applications, 22 (6), 1049-1062.
Kovacs-Györi, A., Ristea, A., Havas, C., Mehaffy, M., Hochmair, H. H., Resch, B., . . . Blaschke, T. (2020). Opportunities and Challenges of Geospatial Analysis for Promoting Urban Livability in the Era of Big Data and Machine Learning. ISPRS International Journal of Geo-Information, 9 (12), 752. https://doi.org/10.3390/ijgi9120752
Latham, J., Cumani, R., Rosati, I., & Bloise, M. (2014). Global land cover share (GLC-SHARE) database beta-release version 1.0–2014. FAO: Rome, Italy .
Liang, R., Lu, Y., Qu, X., Su, Q., Li, C., Xia, S., . . . Chen, Q. (2020). Prediction for global African swine fever outbreaks based on a combination of random forest algorithms and meteorological data. Transboundary and emerging diseases, 67 (2), 935-946. https://doi.org/10.1111/tbed.13424
Ma, Y.-x., & Wang, S.-g. (2010). The application of artificial neural network in the forecasting on incidence of a disease. Paper presented at the 2010 3rd International Conference on Biomedical Engineering and Informatics.
Machado, G., Korennoy, F., Alvarez, J., Picasso‐Risso, C., Perez, A., & VanderWaal, K. (2019). Mapping changes in the spatiotemporal distribution of lumpy skin disease virus. Transboundary and emerging diseases, 66 (5), 2045-2057. https://doi.org/10.1111/tbed.13253
Malki, Z., Atlam, E.-S., Hassanien, A. E., Dagnew, G., Elhosseini, M. A., & Gad, I. (2020). Association between weather data and COVID-19 pandemic predicting mortality rate: Machine learning approaches. Chaos, Solitons & Fractals, 138 , 110137.
Molla, W., de Jong, M., & Frankena, K. (2017). Temporal and spatial distribution of lumpy skin disease outbreaks in Ethiopia in the period 2000 to 2015. BMC veterinary research, 13 (1), 1-9. https://doi.org/10.1186/s12917-017-1247-5
Namazi, F., & Khodakaram Tafti, A. (2021). Lumpy skin disease, an emerging transboundary viral disease: A review. Veterinary Medicine and Science . https://doi.org/10.1002/vms3.434
Niu, B., Liang, R., Zhou, G., Zhang, Q., Su, Q., Qu, X., & Chen, Q. (2020). Prediction for global Peste des petits ruminants outbreaks based on a combination of random forest algorithms and meteorological data. Frontiers in veterinary science, 7 . https://doi.org/10.3389/fvets.2020.570829
Pedregosa, F., Varoquaux, G., Gramfort, A., Michel, V., Thirion, B., Grisel, O., . . . Dubourg, V. (2011). Scikit-learn: Machine learning in Python. the Journal of machine Learning research, 12 , 2825–2830.
Roche, X., Rozstalnyy, A., TagoPacheco, D., Pittiglio, C., Kamata, A., Beltran Alcrudo, D., . . . Larfaoui, F. (2020). Introduction and spread of lumpy skin disease in South, East and Southeast Asia: Qualitative risk assessment and management : Food & Agriculture Org.
Safavian, S. R., & Landgrebe, D. (1991). A survey of decision tree classifier methodology. IEEE transactions on systems, man, and cybernetics, 21 (3), 660-674. https://doi.org/10.1109/21.97458
Scholkopf, B. (1998). Support vector machines: a practical consequence of learning theory. IEEE Intelligent systems, 13 . https://doi.org/10.1041/X4018s-1998
Sprygin, A., Artyuchova, E., Babin, Y., Prutnikov, P., Kostrova, E., Byadovskaya, O., & Kononov, A. (2018). Epidemiological characterization of lumpy skin disease outbreaks in Russia in 2016. Transboundary and emerging diseases, 65 (6), 1514-1521. https://doi.org/10.1111/tbed.12889
Tuppurainen, E., Venter, E. H., Shisler, J., Gari, G., Mekonnen, G., Juleff, N., . . . Bowden, T. (2017). Capripoxvirus diseases: current status and opportunities for control. Transboundary and emerging diseases, 64 (3), 729–745. https://doi.org/10.1111/tbed.12444
Tuppurainen, E., & Oura, C. (2012). lumpy skin disease: an emerging threat to Europe, the Middle East and Asia. Transboundary and emerging diseases, 59 (1), 40-48. https://doi.org/10.1111/j.1865-1682.2011.01242.x
Wang, Y., Li, J., Gu, J., Zhou, Z., & Wang, Z. (2015). Artificial neural networks for infectious diarrhea prediction using meteorological factors in Shanghai (China). Applied Soft Computing, 35 , 280-290.
Wang, L., & Gupta, S. (2013). Neural networks and wavelet de-noising for stock trading and prediction. In Time Series Analysis, Modeling and Applications (pp. 229–247): Springer.
Wang, L., Teo, K. K., & Lin, Z. (2001). Predicting time series with wavelet packet neural networks. Paper presented at the IJCNN'01. International Joint Conference on Neural Networks. Proceedings (Cat. No. 01CH37222).
Weiss, K. (1968). Lumpy skin disease virus. In Cytomegaloviruses. Rinderpest Virus. Lumpy Skin Disease Virus (pp. 111–131): Springer.
Xu, C., & Jackson, S. A. (2019). Machine learning and complex biological data. In: Springer.
Yang, C., Huang, Q., Li, Z., Liu, K., & Hu, F. (2017). Big Data and cloud computing: innovation opportunities and challenges. International Journal of Digital Earth, 10 (1), 13-53. https://doi.org/10.1080/17538947.2016.1239771
Zhu, M., & Wang, L. (2010). Intelligent trading using support vector regression and multilayer perceptrons optimized with genetic algorithms. Paper presented at the The 2010 International Joint Conference on Neural Networks (IJCNN).
Download references
Author information
Authors and affiliations.
Department of Clinical Sciences, Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran
Ehsanallah Afshari Safavi
You can also search for this author in PubMed Google Scholar
Contributions
Study conception and design, material preparation, data collection and analysis, and writing the manuscript were all performed by E.A.S.. E.A.S. read and approved the final manuscript.
Corresponding author
Correspondence to Ehsanallah Afshari Safavi .
Ethics declarations
Ethics approval.
The manuscript does not contain clinical studies or patient data.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (TXT 0 KB)
Rights and permissions.
Reprints and permissions
About this article
Afshari Safavi, E. Assessing machine learning techniques in forecasting lumpy skin disease occurrence based on meteorological and geospatial features. Trop Anim Health Prod 54 , 55 (2022). https://doi.org/10.1007/s11250-022-03073-2
Download citation
Received : 25 June 2021
Accepted : 10 January 2022
Published : 14 January 2022
DOI : https://doi.org/10.1007/s11250-022-03073-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Lumpy skin disease
- Forecasting
- Meteorological parameters
- Geospatial features
- Machine learning techniques
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
Abstract. Lumpy skin disease is an emerging bovine viral disease, which is endemic in most African countries and some Middle East ones, and the elevated risk of the spread of disease into the rest of Asia and Europe should be considered. The recent rapid spread of disease in currently disease‐free countries indicates the importance of ...
1. Introduction. The previous decade has seen an unprecedented increase in scientific reports and publications on lumpy skin disease virus (LSDV), mainly due to its spread into new geographic regions and its adverse economic impacts on a global scale leading to greater interest in the disease and the causative agent (Bianchini et al., 2023).LSDV is a double-stranded DNA (dsDNA) poxvirus virus ...
1 INTRODUCTION. Lumpy skin disease (LSD), a major threat to stockbreeding, can cause acute or subacute disease in cattle and water buffalo (Givens, 2018; Tuppurainen, Venter, et al., 2017).All ages and breeds of cattle are affected, but especially the young and cattle in the peak of lactation (Tuppurainen et al., 2011).The reason why the World Organization for Animal Health (OIE) has placed ...
Special Issue Information. Dear Colleagues, Lumpy skin disease (LSD) is still on the rise and continues to challenge research communities worldwide. A great body of data has been published since the first incursions of LSD into Turkey, Balkans, and Russia in 2015-2017, followed by the emergence of recombinant vaccine-like strains formed by an ...
Lumpy skin disease (LSD) is a contagious viral disease of cattle caused by lumpy skin disease virus (LSDV). LSD has recently spread in Asia following outbreaks in the Middle East and Europe. The disease emerged in Bangladesh in July 2019 in the Chattogram district, then rapidly spread throughout the entire country. We investigated six LSD outbreaks in Bangladesh to record the clinical signs ...
Lumpy skin disease is caused by lumpy skin disease virus (LSDV), which can induce cattle with high fever and extensive nodules on the mucosa or the scarfskin, seriously influencing the cattle industry development and international import and export trade. Since 2013, the disease has spread rapidly and widely throughout the Russia and Asia.
In recent years, lumpy skin disease virus has extended its geographical range outside of endemic sub-Saharan countries to the Middle East and Asia indicating transboundary spread. Recently, lumpy skin disease (LSD) outbreaks have been reported in Asian countries such as Bangladesh, India, China, Nepal, Bhutan, Vietnam, Myanmar, Sri Lanka, Thailand, Malaysia, Laos and for the first time and ...
Lumpy Skin disease: Review of. literature. MRSVA. 3 (3), 6-23. Introduction. Lumpy skin disease (LSD, Pseudo-urticaria, Neethling virus disease, exanthema. nodularis bovis, and knopvelsiekte) is a ...
The spread of lumpy skin disease (LSD) to free countries over the last 10 years, particularly countries in Europe, Central and South East Asia, has highlighted the threat of emergence in new areas or re-emergence in countries that achieved eradication. This review aimed to identify studies on LSD epidemiology. A focus was made on hosts, modes of transmission and spread, risks of outbreaks and ...
Lumpy skin disease is one of the fast-spreading viral diseases of cattle and buffalo that can potentially cause severe economic impact. Lesotho experienced LSD for the first time in 1947 and episodes of outbreaks occurred throughout the decades. In this study, eighteen specimens were collected from LSD-clinically diseased cattle between 2020 and 2022 from Mafeteng, Leribe, Maseru, Berea, and ...
Abstract. Lumpy skin disease is an emerging bovine viral disease, which is endemic in most African countries and some Middle East ones, and the elevated risk of the spread of disease into the rest of Asia and Europe should be considered. The recent rapid spread of disease in currently disease-free countries indicates the importance of ...
Lumpy skin disease (LSD) is a contagious viral disease that spreads through vectors and is characterized by persistent fever; skin nodules; edema of subcutaneous tissue and organs; and damage to ...
The lumpy skin disease virus (LSDV), which can cause serious infections and inflict significant economic losses, is the cause of lumpy skin disease (LSD), a viral condition affecting cattle. LSD is a fast-spreading disease that has lately expanded from Africa to Asia and spread to Europe, raising growing concerns on a worldwide scale.
Lumpy skin disease (LSD) is a viral disease caused by lumpy skin disease virus (LSDV), a member of Capripoxvirus genus of Poxviridae family. It is a transboundary disease of the economic importance affecting cattle and water buffaloes. The disease is transmitted by arthropod vectors and causes high morbidity and low mortality. LSD has recently been reported first time in India with 7.1% ...
The emergence of the lumpy skin disease has become a major threat to the livestock industry in recent years, causing high economic losses and health risks to both animals and humans. This virus is difficult to detect due to its complexity, making the early detection and accurate diagnosis of this virus essential. This study will explore the utilization of convolutional neural networks (CNNs ...
2. Geographic distribution of LSDV. Lumpy skin disease (LSD) was first reported in 1929 in the Mazabuka, Lusaka, and Kufue districts of Northern Rhodesia (Zambia), where it was called pseudo-urticaria (MacDonald, 1931; Morris, 1931).In the absence of the known causative agent, the disease was predominantly characterized by firm circumscribed skin lesions, considered to be caused by insect ...
INTRODUCTION. The lumpy skin disease (LSD) virus is a member of the family Poxviridae, genus Capripoxvirus that causes LSD. This disease has recently emerged in most east European and Asian countries [1,2,3,4].Bovine species (Bos taurus and Bos indicus) and water buffalo (Bubalus bubalis) are the primary hosts of this disease.In addition, the virus can infect some wild animals, such as ...
Paper statistics. If you need immediate assistance, call 877-SSRNHelp (877 777 6435) in the United States, or +1 212 448 2500 outside of the United States, 8:30AM to 6:00PM U.S. Eastern, Monday - Friday. Lumpy skin disease is a viral cross-border sickness that affects cattle and domestic water buffaloes across Africa.
Lumpy skin disease virus (LSDV) causes an infectious disease in cattle. Due to its direct relationship with the survival of arthropod vectors, geospatial and climatic features play a vital role in the epidemiology of the disease. The objective of this study was to assess the ability of some machine learning algorithms to forecast the occurrence of LSDV infection based on meteorological and ...