- Research article
- Open access
- Published: 20 September 2021

A study on knowledge, attitudes and practices regarding dengue fever, its prevention and management among dengue patients presenting to a tertiary care hospital in Sri Lanka
- K. P. Jayawickreme ORCID: orcid.org/0000-0001-9503-2854 1 ,
- D. K. Jayaweera 1 ,
- S. Weerasinghe 1 ,
- D. Warapitiya 1 &
- S. Subasinghe 1
BMC Infectious Diseases volume 21 , Article number: 981 ( 2021 ) Cite this article
18k Accesses
7 Citations
1 Altmetric
Metrics details
The World Health Organization (WHO) has ranked dengue as one of the top ten threats to Global health in 2019. Sri Lanka faced a massive dengue epidemic in 2017, the largest outbreak in the country during the last three decades, consisting of 186,101 reported cases, and over 320 deaths. The epidemic was controlled by intense measures taken by the health sector. However, the reported dengue cases and dengue deaths in 2019 were significantly higher than that of 2018. Deaths were mostly due to delay in hospitalization of severe dengue patients. The mortality of dengue hemorrhagic fever is 2–5% if detected early and treated promptly, but is high as 20% if left untreated.
A descriptive cross-sectional study was done among patients with dengue fever presenting to the Sri Jayawardenepura General Hospital during October 2019. Data was collected using a questionnaire comprising 20 questions based on knowledge, attitudes and practices on dengue, which were categorized into questions on awareness of mortality and severity of dengue burden, prevention of dengue vector mosquito breeding and acquiring the infection, patient’s role in dengue management, and warning signs requiring prompt hospitalization.
The mean KAP score on all questions was 55%, while a majority of 65.2% patients scored moderate KAP scores (50–75%) on all questions, and only 7.6% had high KAP scores (> 75%). The highest categorical mean score of 62% was on awareness of dengue prevention, followed by 54% on awareness of dengue burden, and only 51% on dengue management. Only 5.3% patients scored high scores on awareness of dengue management, followed by 28.5%, and 40.9% patients scoring high scores on awareness of dengue burden, and awareness of prevention of dengue respectively. The mean KAP scores on all questions increased with increasing age category.
The population relatively has a better awareness of dengue prevention, as compared to awareness of dengue mortality and dengue management. The identified weak point is patient awareness of the patients’ role in dengue management, and identifying warning signs requiring prompt hospitalization. This results in delay in treatment, which is a major cause for increased mortality. There was a correlation between those who had good knowledge on dengue burden and those who were aware of patients’ role in dengue management. An action plan should be implemented to improve public awareness through education programs on the role of the public and patients in dengue management to drive a better outcome.
Peer Review reports
The World Health Organization (WHO) has ranked dengue as one of the top ten threats to Global health in 2019 [ 1 ]. Brady et al. estimates a 3.9 billion prevalence of people, accounting to 40%-50% of the world’s population being at risk of infection. 128 countries worldwide are at risk of dengue infection, of which 70% of the global burden being in Asia [ 2 , 3 ]. The reported dengue cases to WHO increased from < 0.5 million in 2000 to > 3.34 million in 2016, characterized by a worldwide outbreak [ 4 ]. Although the world-wide numbers declined in 2017, there was a significant rise again in 2019 with 4.3 million cases worldwide. The highest number of dengue cases worldwide in 2019 in descending order were reported in Brazil, Philippines, Vietnam, Mexico, Nicaragua, Malaysia and India respectively, with Sri Lanka being placed in the 8th place worldwide, and in the 5th place in Asia [ 5 ]. Following a steady rise in annual dengue cases, Sri Lanka faced a massive dengue epidemic in 2017, which was the largest outbreak in the country during the last three decades, consisting of 186,101 reported cases, and over 320 deaths. The epidemic was controlled by intense measures taken by the health sector. However, the reported dengue cases rose again in 2019 reaching 102,746, being twice the number of reported cases of 51,659 in 2018, indicating re-emergence of an outbreak in 2019. A majority of cases being in the western province, with 20% in the Colombo district [ 6 ]. The dengue deaths in 2019 were 90; higher than the total dengue deaths in 2018 being 58, albeit with reduced mortality rate per overall cases [ 6 , 7 ]. The mortality of dengue fever is < 1%, and that of dengue hemorrhagic fever is 2–5% if detected early and treated promptly, but is high as 20% if dengue hemorrhagic fever is left untreated [ 8 ].
Dengue virus is a flavivirus transmitted by mosquito vectors, such as Aedes aegypti and Aedes albopictus. Dengue fever was first serologically confirmed in Sri Lanka in 1962 [ 9 ]. All four serotypes of dengue virus, DENV-1 to DENV-4 have been circulating in the country, and each serotype has many genotypes [ 9 ]. The most common cause for occurrence of new epidemics is the shift of the circulating serotype and genotype of the dengue virus, which is predisposed by increased foreign travel introducing new strains [ 9 ]. The dengue outbreak in 2003 was predominantly due to DENV-3 and DENV-4. The outbreaks in 2006, 2009 and 2010 was predominantly due to DENV-1 [ 9 ]. The predominant serotype in the 2017 epidemic was DENV-2 which was infrequent since 2009 [ 10 ]. The outbreak in 2019 was predominantly due to previously latent serotype DENV-3 [ 11 ].
The WHO published and implemented a “Global Strategy for Dengue Prevention And Control” targeting the years from 2012 to 2020, with the goals of improving dengue mortality, and morbidity by the year 2020, and estimating the true disease burden. The main elements of the global strategy were diagnosis and case management, integrated surveillance and outbreak preparedness, sustainable vector control, future vaccine implementation, basic operational and implementation research [ 12 ].This global strategy follows 10 priority areas for planning dengue emergency response, adapted from Rigau-Pérez and Clark in 2005, which also includes Engaging the community and relevant professional groups about dengue control as well as their participation in dengue prevention and control [ 13 ].
A recent study in Malaysia, showed that the population had only an average knowledge, and poor attitudes and practices on dengue prevention. They identified that a significant percentage had erroneous beliefs, such as fogging being the mainstay of dengue vector control. It had led them to a false sense of security, while evading actual measures that should be taken. They also identified that a proportion of people believed they had no responsibility in preventing dengue breeding, which needed urgent attention. They highlighted that it was impossible to reduce dengue prevalence without community participation, and concluded that measures were urgently required to educate the public to change their attitudes. The Communications for behavioral changes program on dengue prevention were subsequently implemented by Health departments of Malaysia to improve dengue awareness and prevention [ 14 ].
Although there had been a few studies on public awareness on dengue prevention, there was limited evidence focused on public awareness on their role in dengue prevention and management. It is therefore very important to take active measures to reduce the incidence and mortality of dengue, for which the responsibility lies not only with health professionals, but also with the general public. The purpose of this study is to identify the level of awareness in patients on preventing and managing dengue infection, and awareness of the patient’s role and responsibility in the above. Our goals were to identify areas in dengue control and management that need improvement, to implement policies that raise patient participation to deliver a better outcome of dengue infection, its complications and its management.
Study design
This is a descriptive cross-sectional study assessing the knowledge, attitudes, and practices on dengue fever, its prevention and the patient’s role in management, among the dengue patients presenting to a tertiary care hospital in Sri Lanka during the month of October 2019.
Study setting
The study was done among a random sample of 132 patients with dengue fever or dengue hemorrhagic fever who were admitted to adult medical wards for treatment at the Sri Jayawardenepura General Hospital during October 2019. These patients comprised people from draining areas of the western province of Sri Lanka.
Sample size
The number of patients who presented to the Sri Jayawardenepura General hospital in the month of October 2019 was 200. A sample size of 132 was calculated with a confidence interval of 95%, to match the population to assess a statistically significant result.
Participants
The study population was randomly selected among adult patients older than 13 years of age admitted with dengue infection to the medical wards of the Sri Jayawardenepura General Hospital during the month of October 2019.
Participants were not selected from the same family who would likely to be influenced by similar knowledge, to avoid bias of pseudo-replication.
Data collection
Data collection was commenced after obtaining the approval from the institutional Ethical Review committee of the Sri Jayawardenepura General Hospital and Postgraduate Training Centre (SJGH/20/ERC/017). Data was collected using a self-administered validated questionnaire regarding Knowledge, Attitudes, and Practices (KAP) on dengue in languages English, Sinhala, and Tamil which were translated and extensively reviewed for validation (Additional file 1 : Appendix S1, Additional file 2 : Appendix S2, Additional file 3 : Appendix S3).
Data was collected from randomly selected participants, only after informed written consent was obtained. The questionnaires were filled by the participants themselves using the validated questionnaire of the language convenient to them. The study investigators were with them while filling the questionnaire in case the participants needed to clarify any questions in order to ensure quality. The data was collected anonymously, while strict confidentiality of the responses and the results was maintained.
The questionnaire consisted of 20 questions which, comprised 5 questions on knowledge, 6 questions on attitudes, and 9 questions on practices on dengue fever and haemorrhagic fever, its prevention and patient’s role in management. Prior to analysis they were then re-categorized into questions on awareness of:
mortality and severity of dengue burden—5 questions
prevention of dengue vector mosquito breeding and acquiring the infection—5 questions
patient’s role in dengue management, and warning signs requiring prompt hospitalization—10 questions
The responses to each question was analyzed with percentage estimated of correct responses. The total marks scored by each participant to the whole questionnaire was estimated as a percentage, which has been defined as the “KAP score”. KAP score is an abbreviation used for the total score of the questions based on K nowledge, A ttitudes, and P ractices regarding dengue burden, dengue prevention and management in this study. The total results were categorized as “low” when KAP were < 50%, “moderate” when KAP scores were 50–75%, and “high” when KAP scores were > 75%.
Statistical methods
Data was analyzed using the SPSS (Statistical Package for the Social Sciences) software. All the questionnaire sheets were filled completely and none of the sheets were excluded. The mean of the KAP score of each category was calculated. The percentage of the population who scored low, moderate and high KAP scores was calculated separately. The responses to each of the 20 questions were analyzed separately to infer the areas which needed further improvement in awareness of the general public on dengue.
The study population comprised 61% males, and 39% females with a male: female ratio of 3:2. When categorizing by age, 42% of the study population was less than 30 years old, 36% were between 30 and 50 years old, and 22% were more than 50 years old. Of those who were between 30 and 50 years, 35% were graduates or diploma holders. Of those who were more than 50 years old, 21% were graduates or diploma holders. When categorizing by level of education, 10% of the population was currently schooling, 8% were adults educated up to less than ordinary level (O/L) at school who were not graduates or diploma holders, 18% were adults educated up to O/L at school who were not graduates or diploma holders, 34% were adults educated up to advanced level (A/L) at school who were not graduates or diploma holders, 24% were adults who had completed school education and were undergraduates, 6% were adults who had completed school education and were graduates or diploma holders (Table 1 ).
The mean KAP score of the sample population from the questionnaire was 55.04%. When categorizing the KAP scores as low (< 50%), moderate (50–75%), and high (> 75%), a majority of 65.2% of the population had moderate KAP scores. 27.3% had low KAP scores, and only 7.6% had high KAP scores (Fig. 1 ).
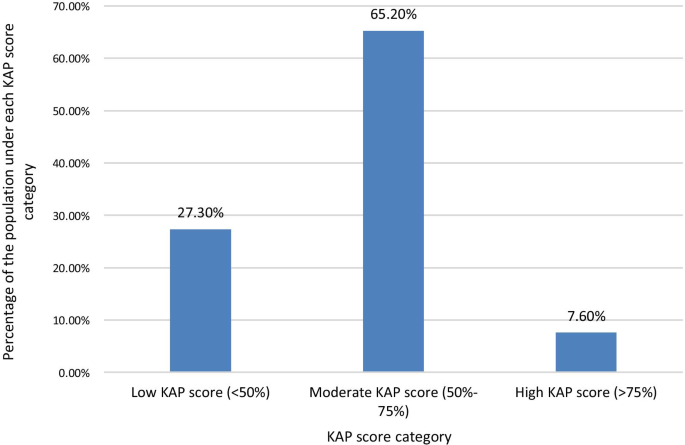
Percentage of the study population who scored under each KAP score level Category. When categorizing the KAP scores as low (< 50%), moderate (50–75%), and high (> 75%) scores, a majority of 65.2% of the population had moderate KAP scores. 27.3% had low KAP scores, and only 7.6% had high KAP scores
The KAP score achieved was higher with increasing age. The highest mean total KAP score of 57.86% was among those > 50 years of age, with those aged < 30 years having a mean KAP score of 53.48% and those aged 30–50 years having a mean KAP score of 55.21% (Fig. 2 ). The mean KAP score on awareness of dengue mortality and burden among the age categories < 30 years, 30–50 years, and > 50 years was 49.29, 56.88, and 58.57% respectively. The mean KAP score on awareness on prevention of dengue vector breeding and acquiring the infection among the age categories < 30 years, 30–50 years, and > 50 years was 63.57, 59.38, and 63.57% respectively. The mean KAP score on awareness of patients’ role in dengue management and warning signs requiring prompt hospital admission among the age categories < 30 years, 30–50 years, and > 50 years was 49.82, 52.08, and 51.79% respectively (Fig. 3 ).
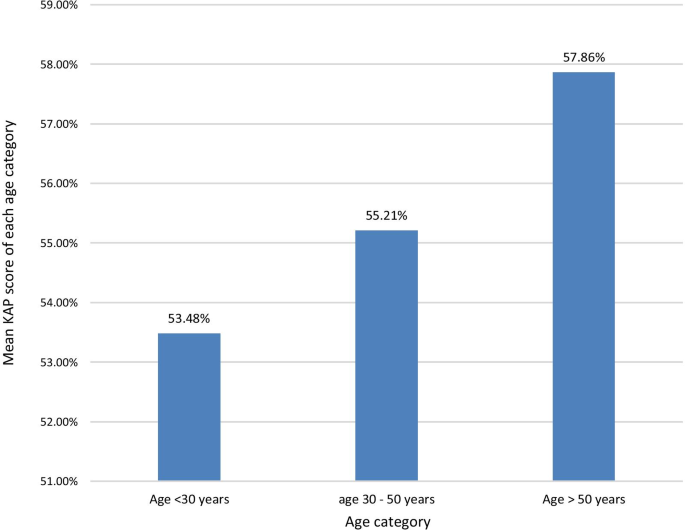
The mean KAP score of each age category. The KAP score achieved was higher with increasing age. The highest mean KAP score of 57.86% was among those > 50 years of age, with those aged < 30 years having a mean KAP score of 53.48% and those aged 30–50 years having a mean KAP score of 55.21%
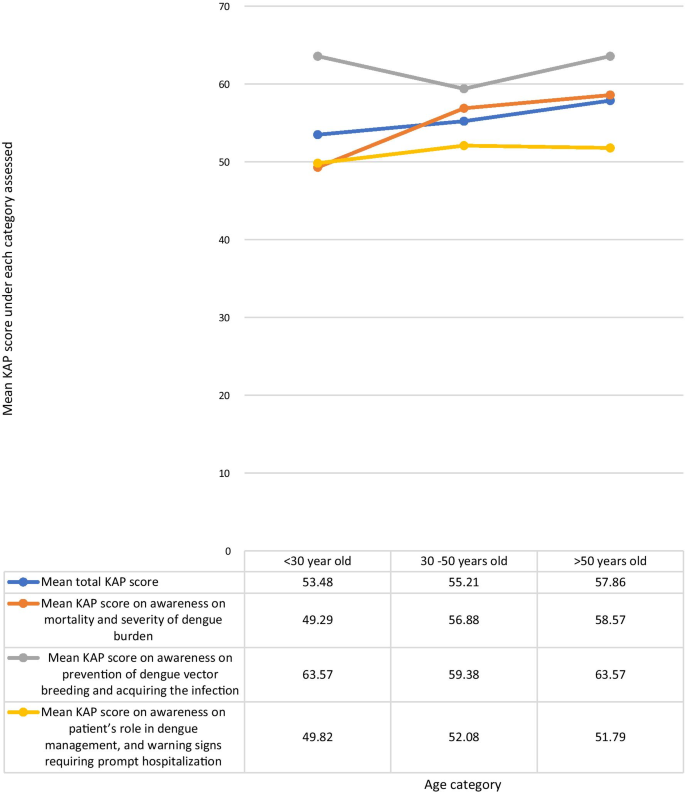
Comparison of the total KAP score, awareness on mortality and severity ofdengue burden, awareness on prevention of dengue vector breeding and acquiring the infection, and awareness on patient’s role in dengue management, and warning signs requiring prompt hospitalization under each age category
The mean KAP score was higher among those with higher educational qualification levels. The highest mean KAP score of 58.13% was among graduates and professional diploma holders of any field, and the lowest score of 49% was among adults educated in school up to below O/L. The mean total KAP score among those currently schooling was 54.62%. Adults who were not undergraduates, graduates, or diploma holders, who were out of school, but were educated at school up to O/L and those who had completed schooling after A/L had mean total KAP scores of 53.96 and 54.67% respectively. The mean KAP score on awareness of dengue mortality and severity of dengue burden among each of the age categories; schooling, adults educated less than O/L, adults educated up to O/L, adults educated up to A/L, under graduates, graduates or diploma holders were 50.77, 42, 60.83, 50.44, 58.75, and 55% respectively. The mean KAP scores on awareness on prevention of dengue vector breeding and acquiring the infection among each of the educational categories in above order were 60, 60, 60, 64, 60.94, 67.5% respectively. The mean KAP scores on awareness of the patient’s role in dengue management and warning signs requiring prompt hospital admission among each of the educational categories in above order were 53.85, 45, 44.58, 51.56, 55, 55% respectively (Fig. 4 ). The mean KAP score among females was 55.48%. and that of males was 54.75%.
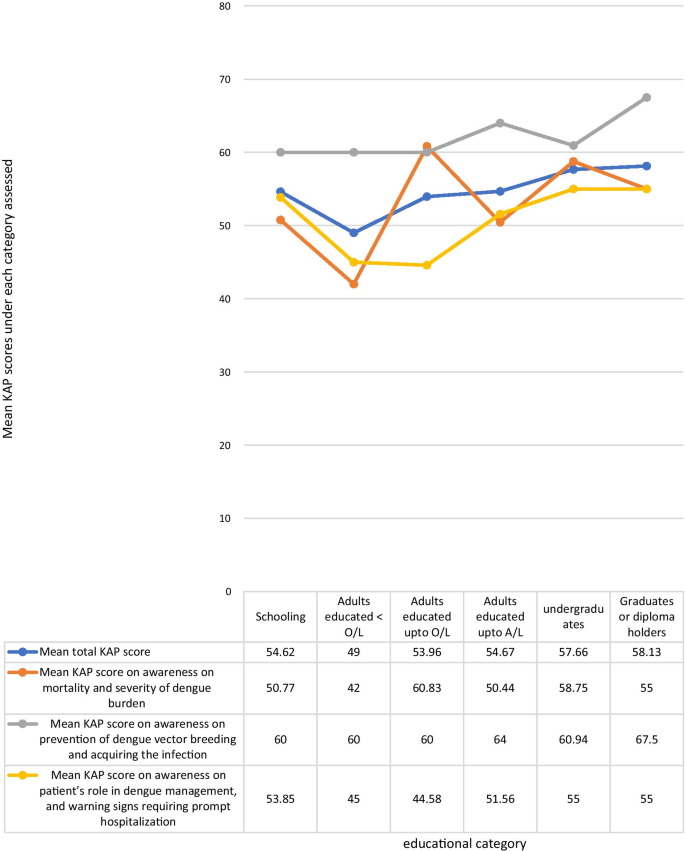
Comparison of the total KAP score, awareness on mortality and severity of dengue burden, awareness on prevention of dengue vector breeding and acquiring the infection, and awareness on patient’s role in dengue management, and warning signs requiring prompt hospitalization under each educational category
When analyzing data by categorizing the questions by the awareness on the area assessed, the highest mean KAP score of 62.05% was on questions on awareness of prevention of dengue vector breeding and acquiring the infection, while the lowest mean KAP score of 51.06% was on questions on awareness of patient’s role in dengue management, and warning signs requiring prompt hospitalization. The mean KAP score on awareness of dengue mortality and severity of burden was 54.02% (Fig. 5 ). On analysis of questions related to awareness of dengue mortality and severity of burden, only 28.8% had high KAP scores, 40.9% had low KAP scores, and 30.3% had moderate KAP scores. On the analysis of questions related to awareness on dengue prevention, an equal percentage of 40.9% had low and high KAP scores respectively, and 18.2% had moderate KAP scores. Analysis of questions related to awareness on patient’s role in dengue management and warning signs prompting hospitalization showed, only 5.3% had high KAP scores, 62.9% had moderate KAP scores, and 31.8% had low KAP scores (Fig. 6 ).
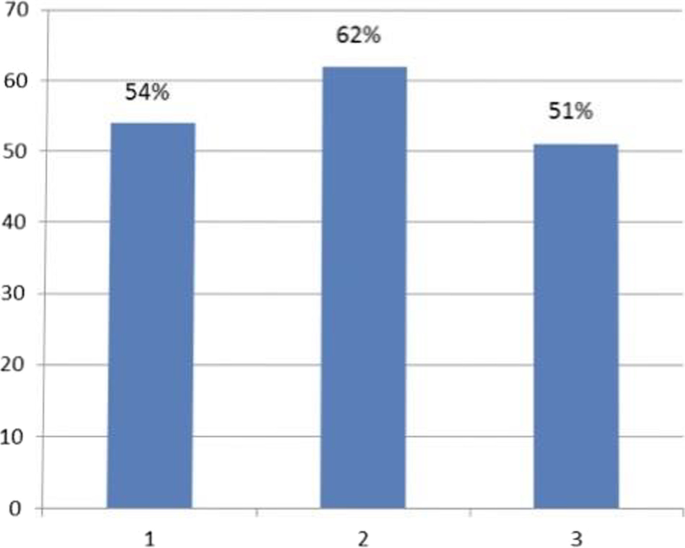
Mean KAP score of each area assessed. 1. Mean KAP score on awareness of mortality and severity of dengue burden- 54%. 2. Mean KAP score on awareness of prevention of dengue breeding and acquiring the infection—62%. 3. Mean KAP score on awareness of patient’s role in dengue management, and warning signs requiring prompt hospitalization—51%
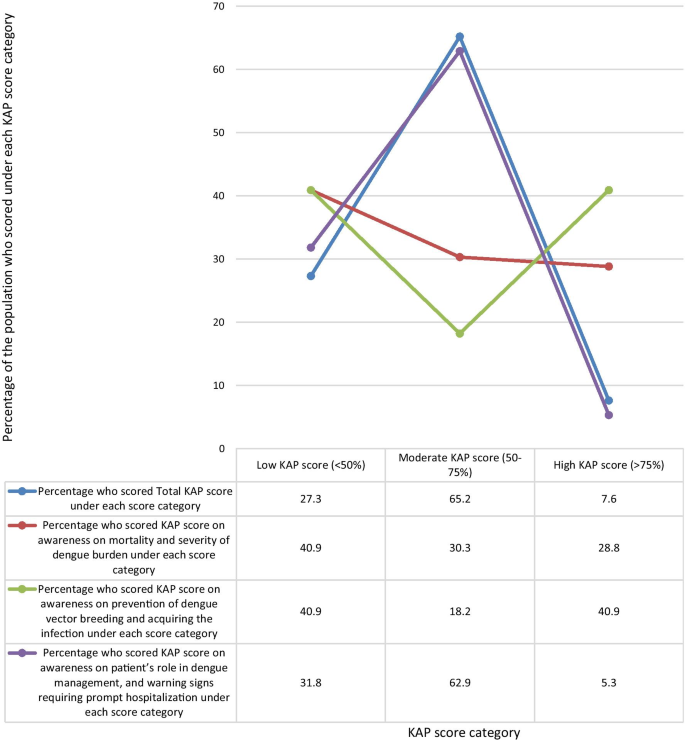
Comparison of percentage of the population who scored low (< 50%), moderate (50%-75%), and high (> 75%) KAP scores under each area assessed
There is no statistically significant correlation between the mean KAP scores on awareness of dengue mortality and severity of dengue burden, and the mean KAP scores on awareness on prevention of dengue vector breeding and acquiring infection according to the spearman’s test (p = 0.084). Although there is a statistically significant correlation between the mean KAP scores on awareness of dengue mortality and severity of dengue burden, and the mean KAP scores on awareness of patient’s role in dengue management and warning signs requiring prompt hospital admission according to the spearman’s test (p = 0.015).
The populations response to each individual question is shown in Table 2 . The percentage of the population who knew the correct answer for the questions on awareness of dengue burden and mortality were as follows: The number of reported dengue cases in Sri Lanka for the year during the outbreak in 2017 was close to 200,000 (42%), The number of reported dengue cases in the year 2019 is higher than that of 2018 (52%), Of 100 persons who get dengue fever only 1 or less persons would die per year when detected early and proper access to medical care (The mortality of dengue fever is < 1%) (60%), The mortality rate of dengue hemorrhagic fever is 2–5%, but is high as 20% if left untreated (60%), The WHO has ranked dengue as one of the top ten threats to Global health in 2019 (56%).
The percentage of the population who knew the correct answer for the questions on awareness of dengue prevention were as follows: all persons with dengue fever do not need to be notified to the Public Health Inspector (PHI) (39%), dengue vector mosquitoes breed in muddy water (52%), The peak biting times of the dengue mosquito is morning and evening (80%), If a person gets dengue fever once in their life, they will be immune to it and will not get dengue fever again (44%), discarded tires, coconut shells, and plastic containers collecting rain water in the garden should be destroyed to prevent dengue vector breeding (96%).
The percentage of the population who knew the correct answer to the questions on awareness of dengue management and warning signs which require prompt hospitalization were as follows: There is a special drug available to treat dengue fever (43%), papaya leaf juice increases the platelet count and thus helps treat dengue fever (33%), dengue patients with a platelet count < 150,000/mm 3 with a rapid drop are recommended to be admitted to hospital (85%), abdominal pain in a dengue patient is not an indication for hospital admission (32%), all pregnant mothers with dengue fever are recommended to be admitted in hospital irrespective of the platelet count (83%), NS1 antigen can be tested on any day since the onset of fever to diagnose dengue fever (23%), a negative report of dengue IgM antibody done on the second day since onset of fever means the patient does not have dengue fever (17%), When a dengue patient has a platelet count > 150,000/mm3 and does not meet criteria which require hospital admission, they should drink 2500 ml of oral fluids per day at home (40%), When a dengue patient has a platelet count > 150,000/mm3 and does not meet criteria which require hospital admission, they should check their Full blood count daily to assess the drop in platelet count (65%), dengue patients should avoid having red or brown drinks (89%).
Dengue virus has four serotypes. Acquisition of dengue infection due to one serotype does not give immunity against a subsequent infection with another serotype, though there is about a two years period of cross-protection [ 15 ]. All four serotypes share only 60–75% identity at amino acid level, and are thus considered as different viruses [ 14 ]. Infection from one serotype gives life-long immunity against that particular serotype [ 10 , 15 ]. Once the cross protection wanes off, secondary dengue infection is more severe than primary dengue infection [ 10 , 15 ]. However only 44% of the study population were aware that occurrence of dengue infection once, does not prevent occurrence of the disease again.
Dengue transmission increases during the rainy season in Sri Lanka, mostly in July, due to increasing dengue vector mosquito breeding places. Other causes for increase in the number of dengue cases is urbanization, climate change, and poor vector control and prevention of disease [ 10 ]. 96% of our cohort were aware of the need to destroy and clean water collecting areas, to prevent breeding of the dengue vector, while 84% of the cohort of a similar study done in the central province of Sri Lanka was aware of this same fact. This is probably because the latter study was done in 2015, prior to the dengue epidemic in 2017 [ 16 ]. Intense measures were taken in the country by which the epidemic in 2017 was controlled. This included clean-up campaigns, awareness programs, National dengue prevention and control, National Strategic framework (2016–2020) to align their action with the WHO Global strategy for dengue prevention and control (2012–2020), The Presidential Task Force on Dengue (PTF) and National dengue control unit of the Ministry of Health launched a rapid inter-sectoral program for prevention and control of dengue [ 7 ]. Awareness programs were held in rural and urban community gatherings, taught in school and institutions, shared on social media, television and radio [ 7 ]. However, data regarding the targeted population for these awareness programs was sparse. Dengue is ranked the third commonest notifiable disease in Sri Lanka, by which means the health sector can implement active vector control measures in the identified areas [ 17 ]. Only 39% of the study population was aware that all persons with dengue fever should be notified to the PHI. The low number of people who were aware of the importance of notifying dengue cases to the PHI, was probably due to the general public being unaware of the PHI’s role in dengue prevention, and lack of awareness of their responsibility in notifying cases, and it’s importance in vector control. Lack of notification of disease hinders action taken for vector control, which gives a falsely lower number of reported cases than the actual number. People should be educated on this to improve notification and vector control. Notification to the PHI of dengue patients managed at home or in the hospital should be made mandatory to avoid negligence in notification. This study population had a relatively good awareness about dengue breeding sites and biting times, probably due to awareness programs during the 2017 epidemic. Literature has shown the importance of improving knowledge on dengue prevention to control dengue outbreaks [ 18 ].
A study in Vietnam during the dengue epidemic in 2017 showed that 91% of the study population considered dengue to be dangerous to very dangerous [ 19 ]. Our study evaluated patients already being admitted for treatment of dengue at the Sri Jayawardenepura general hospital, comprising of patients from the western province, which has the highest dengue burden in the country. A similar study was done in the central province of Sri Lanka by Jayalath et al . among out patients visiting the Peradeniya hospital for reasons other than dengue. Jayalath et al. showed that 95% of their study population knew dengue was a severe disease [ 16 ]. 75% of the cohort of a similar study done among patients being admitted for treatment of dengue fever, in the northern province of Sri Lanka in 2017, knew that dengue was a severe disease [ 20 ]. Our study population had a moderate mean KAP score (54%) on questions on awareness on dengue severity and burden. 40.9% of the population had low awareness on severity and burden of dengue, and only 28.8% had high awareness on its severity and burden. This difference in evidence regarding awareness of severity of dengue in the above studies, could be because the questions by which awareness was evaluated was different in the three studies, and because our study, and the study in the northern province evaluated patients who had already acquired dengue fever and were admitted for treatment at that time. It could also be speculated that these populations acquired dengue infection due to their lack of awareness in prevention of disease.
This lack of awareness on the severity of dengue and it’s burden is probably due to most dengue patients uneventfully recovering from uncomplicated dengue fever, and due to successful dengue management by the healthcare system in the country. This study identified that those who had good awareness on the mortality and severity of the burden of dengue, also had a good awareness about their role in managing dengue, as well as warning signs requiring prompt hospital admission. It can be concluded that there is a strong correlation between those who have an appreciation of the gravity of the symptoms caused by dengue, and the likelihood of them educating themselves on dengue management and their active participation in it. Rozita et al. showed that people who were infected by dengue, or had a family member infected by the disease had better knowledge, attitudes and practices about dengue compared to those who did not [ 21 ]. A study in Singapore in 2017 after the country’s largest dengue epidemic showed that attitudes and practices regarding dengue among primary care physicians significantly improved after experiencing the epidemic [ 22 ]. Chanthalay S et al . showed that those who had better knowledge and attitudes regarding dengue are more likely to take precautions to prevent the disease [ 23 ]. Those who have good awareness will have a good understanding of the gravity and impact of the disease, will know the importance of preventing it, and will be aware of necessary preventive measures.
The mortality of dengue fever is < 1%, and that of dengue hemorrhagic fever is 2–5% if detected early and treated promptly, but is high as 20% if dengue hemorrhagic fever is left untreated [ 8 ]. In 2015 Malhi et al. reported that the presence of comorbidities like diabetes mellitus, hypertension, chronic kidney disease, allergies, asthma, ischemic heart disease and hepatic anomalies, as well as delay in identification and treatment were linked to increased mortality from dengue [ 24 ]. However, in 2017 a study by the same authors showed that 50% of dengue deaths were of previously healthy individuals with no comorbidities [ 25 ]. Therefore, the leading cause for dengue related complications and deaths is delayed identification and treatment of disease. This can be due to delays by the patient or health staff, mostly due to delayed patient presentation to the hospital [ 26 ].Studies have shown that late presentation of dengue fever to the hospital leads to increased development of dengue haemorrhagic fever, dengue shock syndrome, multi-organ involvement like acute kidney injury, and increased mortality [ 26 , 27 , 28 ]. According to the study findings, by identifying areas where the public has misconceptions and misunderstandings about dengue fever, its prevention and management, we can implement steps to improve those loop holes. By following correct practices, avoiding malpractices, and timely hospital admission, his will reduce dengue fatality, improve the outcome, and will also reduce the burden on the healthcare system.
The national Guidelines on dengue management indicates the need for hospital admission in a dengue patient if the platelet count is < 100,000, or platelet count between 100,000- 150,000 with a rapid drop in platelets, fever for three days with any warning signs such as abdominal pain, persistent vomiting, mucosal bleeding, lethargy and restlessness [ 29 ]. Irrespective of the above criteria, admission is required in dengue patients who are pregnant, elderly, obese, with comorbidities, or with adverse social circumstances [ 29 ]. In this study, 85 and 83% patients respectively were aware of the indication for admission as per the platelet count or if pregnant, but only 32% patients knew admission was indicated with warning signs like abdominal pain. Therefore, people need to be educated about warning signs of severe dengue infection. People who do not require admission must be educated about cautious self-management at home until they require admission [ 29 ]. By doing so there will be less likelihood to miss warning signs, will have improved outcome, and there will be less burden to hospital staff. Only 40% of patients knew about fluid management at home, but 89% knew to avoid red drinks.
Serological testing is important to confirm the diagnosis of dengue fever when the presentation is atypical or when unsure of the diagnosis. NS1 antigen is tested in the patient’s blood on the first few days of the disease and has a sensitivity of 60–90%. Dengue IgM antibody will be positive in the patient’s blood only after the 5th day of illness [ 29 ]. Therefore, patients should be educated about the ideal time to do each test to avoid false negatives being reported by doing the test at the wrong time of the illness. However, dengue infection cannot be excluded by a negative serological lab report. Few patients knew about the timing of testing, with only 23% and 17% being aware of the timing of testing, and sensitivity of NS1 antigen and dengue IgM respectively. It is important that health care professionals guide patients on the correct timing to do the serological tests. It would be prudent to do such serological tests only on request by a physician, to avoid patients testing at the wrong time, and getting a report which cannot be interpreted at that time of the illness. False negatives of serological testing can further be avoided by laboratory staff rechecking the patients’ day of the illness, and the physicians request form prior to drawing blood.
This study shows that people had misconceptions about dengue management. Only 43% knew there was no special drug to treat dengue fever. There is no particular drug to treat dengue, but is managed by careful monitoring and fluid tailoring resuscitation [ 29 ]. A tetravalent live attenuated dengue vaccine has been registered for use in several countries [ 15 ]. In sero-negative individuals it is believed that the vaccine mimics a silent natural infection, giving temporary cross-protection against all serotypes, and subsequently causing severe dengue infection when primarily infected [ 15 ]. However, its efficacy varies in different countries and is not currently recommended for use in Sri Lanka [ 15 ]. The use of papaya leaf juice in dengue management had recently gained interest, leading to many people consuming the juice assuming improvement of dengue infection. Research has shown papaya leaf juice to improve platelet counts, but has not shown to prevent or reduce fluid leaking in dengue hemorrhagic fever [ 30 ]. This can adversely cause early rise in platelet count masking the onset of fluid leaking, which can be detrimental in managing dengue hemorrhagic fever. 33% of our cohort believed papaya leaf juice helped treat dengue fever, while 13.4% of the cohort in a study done in Sri Lanka in 2015 believed the same to be true. This is probably because the concept of the effect of papaya leaf juice on platelet count came in to light only later on [ 16 ].
This study demonstrated an increasing trend in awareness on all categories, such as among people with a higher level of education, and maturity by age, indicating that education and maturity are important factors for improved awareness. Kumanan et al. showed a significant association between educational level and knowledge regarding dengue fever, and no significant association between educational level and preventive practices [ 20 ]. The trend in our study demonstrated on Fig. 3 suggests that responses in the awareness on dengue mortality and severity of dengue burden steadily increased with age, and strongly influence the mean total KAP scores. The highest awareness in all age categories was on dengue prevention and the lowest awareness in all categories was on patients’ role in dengue management and warning signs requiring prompt hospitalization (Fig. 3 ).
There was inadequate awareness among adults who dropped out of school prior to completion of the full school education up to advanced level even when they are older. This may demonstrate a population with lower level of understanding of the information given, and those who were not regularly educated at school regarding dengue infection as they dropped out. Those who drop out of school are also those who usually have a poor social background, and they may also have inadequate access to social media and electronic media to receive updates about dengue mortality, prevention and management. This highlights the need for any information to reach the people of all social backgrounds when implementing strategies to improve public awareness on dengue infection. Dissemination of information should be done in various ways targeting different populations of different levels of understanding. People with lower education levels should be the main target group requiring more advice and education regarding the patient’s role in dengue management.
This population has a relatively a better awareness on dengue prevention as compared to awareness of dengue mortality and dengue management. This is possibly due to prior media education of the public on prevention during the previous epidemic in 2017. The identified weak point is patient awareness on the patient’s role in dengue management, as well as identifying warning signs requiring prompt hospitalization. It causes delay in treatment, which is a major cause for increased mortality. The trend demonstrated on Fig. 5 suggests that responses in the dengue management and warning signs prompt hospitalization area strongly influence the total KAP scores. This indicates that patient awareness on the role of the public and patients on dengue management is critical in the outcome of dengue infection. An action plan should be implemented targeting improving public awareness by education programs on the role of the public and patients in dengue management, to improve outcome.
The general public play a major role in prevention and management of dengue fever, and influence the outcome. Jayalath et al. showed that 30% of their population believed the responsibility of dengue prevention lay with the public, while 66% believed both the public and the government were responsible [ 16 ]. In order to improve involvement of patients and the public in dengue prevention, control and management, attention should be paid on educating the public and patients on the disease.
Limitations and recommendations for future research
This study focused on 132 patients from one hospital. Therefore, the conclusions can be relevant only to draining areas in the vicinity of this hospital, and may not represent the knowledge, attitudes and practices in other parts of Sri Lanka. However, since majority of the dengue cases in the country are concentrated in the western province, of which a significant number of patients present to the Sri Jayawardenepura General Hospital, the findings of this study may represent the most dengue dense area in the country. Large scale future research from all parts of the country may be beneficial to infer the knowledge, attitudes, and practices of the country as whole.
The general public was educated about Dengue infection by various means, including messages on social media, electronic media, awareness programs at schools, and village meetings, posters and distribution of leaflets, during the dengue epidemic in 2017. This study did not extensively evaluate whether the study participants had been exposed to these prior teaching about Dengue infection, and if they did, by what means they were educated. However almost all the study participants had access to electronic and social media. This may not be the same when inferring on the population in some rural parts of Sri Lanka who may not have similar access to such media education. Awareness programs and active participation of the general public in dengue prevention and management should be implemented. We suggest future follow up research of the awareness on dengue infection among the public, before and after implementing formal dengue awareness strategies to assess the effectiveness of it. In addition to follow up research before and after implementing disease awareness steps, we also suggest future research to assess an association and comparison of dengue mortality and outcome before and after implementing practices to further educate the public, in order to identify its impact on dengue management and outcome.
The population has relatively a better awareness on dengue prevention, as compared to awareness of dengue mortality and dengue management. The identified weak point is patient awareness on the patient’s role in dengue management, and identifying warning signs requiring prompt hospitalization causing delay in treatment, which is a major cause for increased mortality. There was a correlation between those who had good knowledge on dengue burden and those who were aware of the patients’ role in dengue management. There is also an increasing trend in awareness on all categories, especially among people with a higher level of education, and maturity by age, indicating that education and maturity are important factors for improved awareness. An action plan should be implemented targeting improving public awareness on the role of the public and patients in dengue management to improve outcome.
Availability of data and materials
The raw data sets analyzed during the current study are available on reasonable request from the corresponding author.
Abbreviations
Dengue virus
Knowledge attitudes and practices
Ordinary level at school
Advanced level at school
Ten threats to global health in 2019. World Health Organization. https://www.who.int/emergencies/ten-threats-to-global-health-in-2019 . Accessed 4 Jan 2020.
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–7.
Article CAS Google Scholar
Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6(8):e1760.
Article Google Scholar
Dengue and severe dengue. World health organization.4th November 2019. https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue . Accessed 4 Nov 2019.
Dengue worldwide overview 2019. European Centre for Disease Prevention and Control. https://www.ecdc.europa.eu/en/dengue-monthly . Accessed 4 Jan 2020.
Epidemiology unit, Ministry of Health Sri Lanka. Dengue, disease surveillance trends. http://www.epid.gov.lk . Accessed 4 Jan 2020.
Dengue DREF final report 2017. Dengue Sri Lanka. International federation of red cross and red crescent societies. https://www.chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/viewer.html?pdfurl=https%3A%2F%2Freliefweb.int%2Fsites%2Freliefweb.int%2Ffiles%2Fresources%2FMDRLK007dfr.pdf&clen=1774569&chunk=true . Accessed 4 Jan 2020.
Lahiri M, Fisher D, Tambyah PA. Dengue mortality: reassessing the risks in transition countries. Trans R Soc Trop Med Hyg. 2008;102(10):1011–6.
Sirisena PDNN, Noordeen F. Evolution of dengue in Sri Lanka—changes in the virus, vector, and climate. Int J Infect Dis. 2014;19:6–12.
Jayarajah U, Faizer S, de Zoysa I, Senevirathne SL. A large Dengue epidemic affects Sri Lanka in 2017. IJPSAT. 2017;6(1):84–6.
Google Scholar
National Dengue control unit. Ministry of Health, Nutrition, and Indigenous Medicine. http://www.dengue.health.gov.lk . Accessed 4 Jan 2020.
World Health Organization. Global strategy for dengue prevention and control 2012–2020. Geneva: World Health Organization
Rigau-Pérez JG, Clark GG. Còmo responder a una epidemia de dengue: vision global y experiencia en Puerto Rico [How to respond to a dengue outbreak: global vision and experience in Puerto Rico]. Pan Am J Public Health. 2005;17:282–93.
Selvarajoo S, Liew JWK, Tan W, et al. Knowledge, attitude and practice on dengue prevention and dengue seroprevalence in a dengue hotspot in Malaysia: a cross-sectional study. Sci Rep. 2020;10(1):9534. https://doi.org/10.1038/s41598-020-66212-5 .
Article CAS PubMed PubMed Central Google Scholar
Applicability of dengue vaccines. Weekly epidemiological report. Apublication of the Epidemiological unit, Ministry of Health, nutrition and indigenous medicine, Sri Lanka. 18th - 24th March 2017. Volume 44. no. 12. https://www.chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/viewer.html?pdfurl=http%3A%2F%2Fwww.epid.gov.lk%2Fweb%2Fimages%2Fpdf%2Fwer%2F2017%2Fvol_44_no_12-english.pdf&clen=1799202&chunk=true . Accessed 6 Jan 2020.
Jayalath T, Ralapanawa U, Karunaratne S, Dassanayake UKA, Pathirage M, et al. Knowledge and attitude regarding dengue fever among the outdoor patients of the teaching hospital Peradeniya, Sri Lanka. Int J Med Res Health Sci. 2018;7(1):77–84.
Annual health bulletin of Sri Lanka. Department of Health Services, Colombo, Sri Lanka (2002)
Al-Zurfi BM, Fuad MD, Abdelqaderm MA, Baobaidm MF, Elnajehm M, Ghazim HF, Ibrahim MH, Abdullah MR. Knowledge, attitude and practice of dengue fever and health education programme among students of Alam shah science school, Cheras Malaysia Malays. J Public Health Med. 2006;6:62–7.
Nguye HV, Than PQT, Nguyen TH, Vu GT, et al. Knowledge, attitude and practice about dengue fever among patients experiencing the 2017 Outbreak in Vietnam. Int J Environ Res Public Health. 2017;2019(16):976.
Kumanan T, Logeswaran D. A study on knowledge, attitude and practices regarding dengue among hospitalized patients from Northern Sri Lanka. Sri Lankan J Infect Dis. 2018;8(2):127–32. https://doi.org/10.4038/sljid.v8i2.8220 .
Wan Rozita WM, Yap BW, Veronica S, Muhammad AK, Lim KH, Sumarni MG. Knowledge, attitude and practice (KAP) survey on dengue fever in an urban malay residential area in Kuala Lumpur. Malays J Public Health Med. 2006;6:62–7.
Junxiong P, ZoeJane-Lara H, Tun LH, Jing Y, Yee SL. Assessing changes in knowledge, attitude and practices of dengue diagnosis and management among primary care physicians after the largest dengue epidemic in Singapore. BMC Infect Dis. 2017;17:428.
Chanthalay S, Jiraporn C, Somsak W, Cheerwith R. Knowledge, attitudes and preventive behaviours related to dengue vector breeding control measures among adults in communities of Vientiane, capital of Lao PDR. J Infect Public Health. 2015;8:466–73.
Mallhi TH, Khan AH, Adnan AS, et al. Clinico-laboratory spectrum of dengue viral infection and risk factors associated with dengue hemorrhagic fever: a retrospective study. BMC Infect Dis. 2015. https://doi.org/10.1186/s12879-015-1141-3 .
Article PubMed PubMed Central Google Scholar
Mallhi TH, Khan AH, Sarriff A, Adnan AS, Khan YH. Determinants of mortality and prolonged hospital stay among dengue patients attending tertiary care hospital: a cross-sectional retrospective analysis. BMJ Open. 2017;7(7):e016805.
Mallhi TH, Adnan AS, Khan AH, Habib Y, et al. Patients related diagnostic delay in dengue: an important cause of morbidity and mortality. Clin Epidemiol Glob Health. 2016;4(4):200–1.
Yatra IM. Disease history and delayed diagnosis of dengue infection as risk factors for dengue shock syndrome in Wangaya Hospital Denpasar. Public Health Prev Med Arch. 2015. https://doi.org/10.15562/phpma.v3i2.108 .
Nguyen Thi KT, Nguyen Ngoc AT, Khau MT, Nguyen TT, Luong CQ. Epidemiology analysis of deaths associated with dengue hemorrhagic fever in Southern Viet Nam in 1999–2000. Dengue Bull. 2001;25:28–32.
Guidelines on the management of dengue fever and dengue haemorrhagic fever in adults. National Guidelines 2012. Ministry of Health, Sri Lanka.
Rajapakse S, de Silva NL, Weeratunga P, et al. Carica papaya extract in dengue: a systematic review and meta-analysis. BMC Complement Altern Med. 2019;19:265. https://doi.org/10.1186/s12906-019-2678-2 .
Download references
Acknowledgements
We all express our gratitude to all participants who consented to take part in this study.
Authors’ information
SS is a Consultant Physician [MBBS, MD, FRACP] Medical unit, Sri Jayawardenepura General Hospital. KPJ [MBBS], DKJ [MBBS] and DW [MBBS] are Registrars in Internal medicine, and SW is a Senior Registrar in Medicine at the Sri Jayawardenepura General Hospital.
No funding was obtained for this study.
Author information
Authors and affiliations.
Sri Jayewardenepura General Hospital, Kotte, Sri Lanka
K. P. Jayawickreme, D. K. Jayaweera, S. Weerasinghe, D. Warapitiya & S. Subasinghe
You can also search for this author in PubMed Google Scholar
Contributions
Data collection was done by KPJ, DKJ and DW. Analysis, interpretation of data, literature review and writing of the report was done by KPJ. SS and SW guided the study and corrected the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to K. P. Jayawickreme .
Ethics declarations
Ethics approval and consent to participate.
Ethical clearance was taken from the institutional Ethical Review committee of the Sri Jayawardenepura General Hospital and Postgraduate Training Centre to conduct this study (SJGH/20/ERC/017). Informed written consent was taken from all the participants. All the participants were above the age of 13 years. In the very few participants aged between 13 and 16, informed written consent was obtained from both the participant and the parent or guardian.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: appendix s1..
Questionnaire in English.
Additional file 2: Appendix S2.
Questionnaire in Sinhala.
Additional file 3: Appendix S3.
Questionnaire in Tamil.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Jayawickreme, K.P., Jayaweera, D.K., Weerasinghe, S. et al. A study on knowledge, attitudes and practices regarding dengue fever, its prevention and management among dengue patients presenting to a tertiary care hospital in Sri Lanka. BMC Infect Dis 21 , 981 (2021). https://doi.org/10.1186/s12879-021-06685-5
Download citation
Received : 03 April 2020
Accepted : 13 September 2021
Published : 20 September 2021
DOI : https://doi.org/10.1186/s12879-021-06685-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Dengue fever
BMC Infectious Diseases
ISSN: 1471-2334
- General enquiries: [email protected]
Disclaimer » Advertising
- HealthyChildren.org

- Previous Article
- Next Article
A Growing Problem in the United States
Environmental factors contributing to dengue as a public health threat, pathogenesis, clinical considerations, presentation and evaluation, diagnostic testing for symptomatic denv infection, traditional prevention measures, novel vector control efforts, current dengue vaccines, principles of live-attenuated dengue vaccines, history of dengvaxia, safety and efficacy, prevaccination laboratory testing, conclusion and future directions, acknowledgment, dengue: a growing problem with new interventions.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- CME Quiz Close Quiz
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Joshua M. Wong , Laura E. Adams , Anna P. Durbin , Jorge L. Muñoz-Jordán , Katherine A. Poehling , Liliana M. Sánchez-González , Hannah R. Volkman , Gabriela Paz-Bailey; Dengue: A Growing Problem With New Interventions. Pediatrics June 2022; 149 (6): e2021055522. 10.1542/peds.2021-055522
Download citation file:
- Ris (Zotero)
- Reference Manager
Dengue is the disease caused by 1 of 4 distinct, but closely related dengue viruses (DENV-1–4) that are transmitted by Aedes spp. mosquito vectors. It is the most common arboviral disease worldwide, with the greatest burden in tropical and sub-tropical regions. In the absence of effective prevention and control measures, dengue is projected to increase in both disease burden and geographic range. Given its increasing importance as an etiology of fever in the returning traveler or the possibility of local transmission in regions in the United States with competent vectors, as well as the risk for large outbreaks in endemic US territories and associated states, clinicians should understand its clinical presentation and be familiar with appropriate testing, triage, and management of patients with dengue. Control and prevention efforts reached a milestone in June 2021 when the Advisory Committee on Immunization Practices (ACIP) recommended Dengvaxia for routine use in children aged 9 to 16 years living in endemic areas with laboratory confirmation of previous dengue virus infection. Dengvaxia is the first vaccine against dengue to be recommended for use in the United States and one of the first to require laboratory testing of potential recipients to be eligible for vaccination. In this review, we outline dengue pathogenesis, epidemiology, and key clinical features for front-line clinicians evaluating patients presenting with dengue. We also provide a summary of Dengvaxia efficacy, safety, and considerations for use as well as an overview of other potential new tools to control and prevent the growing threat of dengue.
Dengue is the disease caused by 4 closely related but distinct viruses, dengue virus 1–4 (DENV-1–4), referred to as virus types or serotypes. DENVs are most commonly transmitted by the bite of an infected female Aedes spp. mosquito. It is the most common arboviral disease globally, with an estimated 390 million dengue virus infections and 96 million symptomatic cases annually. 1 Global incidence has almost doubled in the last 3 decades and is expected to continue growing in Asia, sub-Saharan Africa, and Latin America. About half of the global population now lives in areas that are suitable for dengue transmission ( Fig 1 ). 2 , 3 Historically, the highest burden of dengue has been in children, adolescents, and young adults. 4 In 2019, countries across the Americas reported more than 3 million dengue cases, the highest number ever recorded, 5 with a greater proportion of severe dengue cases and increased mortality in the pediatric population of children aged 5 to 9 years. 6 Dengue is increasingly common as an etiology of fever in international travelers 7 and has been reported as the leading febrile disease etiology for travelers from some endemic regions during epidemic years. 8 In addition to circulation of all four DENVs worldwide, surveillance of returning travelers with dengue has demonstrated high genetic diversity among circulating DENV genotypes within serotypes, with potential implications for immune or vaccine escape. 9 , 10

Map showing the risk of dengue by country as of 2020. “Frequent or Continuous” risk indicates that there are either frequent outbreaks or ongoing transmission. “Sporadic or Uncertain” indicates that risk is either variable and unpredictable or that data from that country are not available. For updated information, visit https://www.cdc.gov/dengue/areaswithrisk/around-the-world.html .
Increasing numbers of dengue cases in the United States are a growing concern. In parts of the United States and freely associated states with endemic dengue transmission, including American Samoa, Puerto Rico, US Virgin Islands, Federated States of Micronesia, Republic of Marshall Islands, and the Republic of Palau, dengue outbreaks can be explosive, overwhelming the health care system capacity. In Puerto Rico, the largest US territory where dengue is endemic, the highest incidence of dengue cases and hospitalizations from 2010 to 2020 occurred among children aged 10 to 19 years. 11 For the same period, confirmed dengue cases ranged from a minimum of 3 cases in 2018 to a maximum of 10 911 cases in 2010, 11 although suspected case counts during outbreak years were considerably higher. 12
Although local dengue transmission does not occur frequently in most states, increasing numbers of US travelers 13 with dengue have been reported in recent years, with a record 1475 cases in 2019, more than 50% higher than the previous peak in 2016 ( Fig 2 ). 14 Viremia among travel-associated dengue cases can also result in focal outbreaks in nonendemic areas, with competent mosquito vectors for dengue present in approximately half of all US counties. 15 Local dengue cases have been reported in multiple states in recent years, including 70 cases in Florida in 2020, 14 200 cases in Hawaii in 2015, 14 and 53 cases in Texas in 2013. 16

Annual number of travel-associated cases of dengue reported into ArboNET, the national arboviral surveillance system managed by the CDC, from all US jurisdictions from 2010 to 2019 ( n = 6967).
In dengue-endemic areas, environmental factors such as standing water where mosquitoes lay eggs, poor housing quality, lack of air conditioning, and climatic factors (ie, temperature, precipitation, and humidity) increase the abundance, distribution, and risk of exposure to Aedes aegypti , the main vector responsible for dengue transmission, or other Aedes spp. mosquitoes that can also transmit dengue. 2 , 17 – 21 Climate change is predicted to further increase the population at risk for dengue primarily through increased transmission in currently endemic areas and secondarily through expansion of the geographic range of Aedes spp. mosquitoes ( Fig 3 ). 2 , 22 Urbanization, increasing population density, human migration, and growing social and environmental factors associated with poverty and forced displacement are also expected to drive the increase in dengue incidence and force of infection globally. 21 , 23 – 26 Travel is an important driver of dengue expansion by introducing dengue into nonendemic areas with competent vectors 13 , 23 or by introducing new serotypes into endemic areas naïve to the new serotype, thereby increasing the risk for antibody-dependent enhancement (ADE) and severe disease. 27 , 28 Combined environmental effects of poverty and the increased scale and rapidity of human movement can also increase the risk for dengue. 24 , 29 The combined environmental effects of climate change, urbanization, poverty, and human migration together expand the threat of dengue for both individuals and public health systems in the future.

A-C, Projections of average trends in environmental suitability for dengue transmission from 2015 to 2020, 2020 to 2050, and 2050 to 2080. D–F, Areas with expansion or contraction of the Aedes vector range over the same time periods. (Reprinted with permission from Messina JP, Brady OJ, Golding N, Kraemer MUG, Wint GRW, Ray SE, et al. The current and future global distribution and population at risk of dengue. Nature Microbiology. 2019;4(9):1510.)
DENVs belong to the genus Flavivirus in the family Flaviviridae . Because there are 4 dengue serotypes, individuals living in endemic areas can be infected up to 4 times in their life. Although most dengue virus infections are asymptomatic or only cause mild disease, severe disease can occur and is characterized by plasma leakage, a pathophysiologic process by which the protein rich fluid component of blood leaks into the surrounding tissue, leading to extravascular fluid accumulation resulting in shock, coagulopathy, or end organ impairment. 30 , 31
Infection with 1 dengue serotype induces life-long protection against symptomatic infection with that specific serotype (homotypic immunity) 32 , 33 and induces only short-term cross-reactive protection from disease to the other serotypes (heterotypic immunity) for several months to years. 34 , 35 Older children and adults experiencing their second dengue infection are at the highest risk for severe disease because of ADE. ADE has also been observed among infants, in that infants born to mothers with previous dengue virus infection had the lowest risk for dengue shortly after birth and a period of higher risk for severe disease approximately 4 to 12 months after birth, followed by a decrease in risk for severe disease from approximately 12 months after birth. 36 The initial period of lowest risk was correlated with high levels of passively acquired maternal dengue antibodies immediately after birth, and the period of enhanced risk with a decline in these antibodies to subneutralizing levels. After further degradation of these maternal antibodies, there was neither protection from dengue afforded by high levels of antibodies postnatally nor enhanced risk of dengue and severe disease from the intermediate levels of antibodies. 37 Later work showed that lower heterotypic antibody titers are ineffective at neutralizing the virions but still bind them, facilitating binding to Fcγ receptors on circulating monocyte cells, and result in higher viremia than in primary infections ( Fig 4 ). 38 The feared sequela of plasma leakage is believed to be mediated by high levels of DENV nonstructural protein 1 (NS1), a key protein for viral replication and pathogenesis, 39 , 40 that damages endothelial glycocalyces and disrupts endothelial cell junctions. 41 , 42 Cell-mediated immunity through dengue-specific CD8 T cells is thought to protect against ADE and severe disease. 43 , 44

The proposed mechanism of antibody-dependent enhancement with heterotypic antibodies binding to the dengue viruses and entering monocytes through Fcγ receptors. Viral replication occurs in the infected monocyte and releases high levels of virus and dengue virus NS1 protein, which, in turn, lead to increased vascular permeability contributing to severe disease. (Reprinted with permission from Whitehead SS, Blaney JE, Durbin AP, Murphy BR. Prospects for a dengue virus vaccine. Nature Reviews Microbiology. 2007;5(7):524.)
Although ADE occurs in infants due to the interaction between maternal antibodies and primary infection, it is also explanatory for severe disease in older children and adults where the heterotypic antibodies produced after a primary dengue infection will wane over time to subneutralizing levels, resulting in the highest risk for severe disease with secondary infection. Following secondary infection, potent cross-neutralizing/multitypic antibodies are induced that then protect against severe disease in tertiary and quaternary infections. 45 , 46 Although the risk of severe dengue is highest with secondary infection, it can also occur in primary, tertiary, and quaternary infections, and possibly following Zika virus infection. 47 , 48 Identifying cases of severe dengue and understanding the pathogenesis of disease severity is an active area of research with important implications for future vaccines and interventions. 49
DENV infections have a wide range of presentations from asymptomatic infection (approximately 75% of all infections 50 ) to mild to moderate febrile illness to severe disease with associated coagulopathy, shock, or end organ impairment ( Table 1 ). 30 , 31 Symptomatic infections most commonly present with fever accompanied by nonspecific symptoms such as nausea, vomiting, rash, myalgias, arthralgias, retroorbital pain, headache and/or leukopenia. 51 Severe disease develops in as many as 5% of all patients with dengue, although certain populations such as infants aged ≤1 year, pregnant individuals, and adults aged ≥65 years, or individuals with specific underlying conditions such as diabetes, class III obesity, hypertension, asthma, coagulopathy, gastritis or peptic ulcer disease, hemolytic disease, chronic liver disease, anticoagulant therapy, or kidney disease, are at increased risk of severe disease. 52 , 53 In all patients with dengue, warning signs are specific clinical findings that can predict progression to severe disease and are used by the World Health Organization (WHO) to help clinicians in triage and management decisions. Dengue warning signs include abdominal pain or tenderness, persistent vomiting, clinical fluid accumulation, mucosal bleeding, lethargy or restlessness, liver enlargement of >2 cm, and increasing hematocrit concurrent with rapid decrease in platelet count ( Table 1 ). 52
Classification of Dengue Severity and Case Management 51 ,134, 135
Although warning signs are useful for evaluating patients with a high suspicion of dengue (for example, during an outbreak), they are not intended to differentiate dengue from other infectious and noninfectious diseases such as influenza, coronavirus disease 2019, malaria, Zika, measles, leptospirosis, rickettsial disease, typhoid, Kawasaki, or idiopathic thrombocytopenic purpura. Because prompt recognition and early treatment of dengue can greatly reduce morbidity and mortality, 54 , 55 clinicians practicing in the United States and other nonendemic areas should keep dengue in the differential diagnosis for febrile illness in travelers and in areas with competent mosquito vectors.
For symptomatic dengue patients, nucleic acid amplification tests (NAATs) on serum, plasma, or whole blood detect DENV RNA during the first 7 days of illness with high sensitivity and specificity. 56 , 57 Likewise, NS1 antigen can also be detected within the first 7 days and provides confirmatory evidence of DENV infection. 58 For patients with a negative NAAT or patients presenting more than 7 days after symptom onset, a positive anti-DENV immunoblobulin M (IgM) can suggest recent infection, although with less certainty than NAAT or NS1 testing, owing to cross-reactivity with other flaviviruses. Notably, Zika virus is a flavivirus that has been transmitted in most countries where DENV transmission is present. 59 In patients from areas with ongoing transmission of another flavivirus (eg, Zika virus) and whose only evidence of dengue is a positive anti-DENV IgM test, plaque reduction neutralization tests (PRNT) quantifying virus-specific neutralizing antibody titers can distinguish DENV from other flaviviruses, in some but not all cases. PRNTs, however, are rarely available in clinical laboratories and typically do not provide results within a timeframe that is meaningful for clinicians managing acute disease. PRNT’s may be valuable in circumstances where confirming the diagnosis may have important clinical implications, such as distinguishing dengue from a Zika virus infection in a pregnant individual, or epidemiologic implications for a region, such as distinguishing yellow fever from dengue. 60 , 61
The US Food and Drug Administration (FDA) has approved a NAAT for use on serum and whole blood, an NS1 antigen enzyme-linked immunosorbent assay test in serum, and an IgM enzyme-linked immunosorbent assay in serum. 56 , 59 , 62 – 64 Other non–FDA-approved tests for DENV infection are used in clinical practice and are commercially available at accredited laboratories.
Although several medications have been explored as potential therapeutics for dengue, none have demonstrated a reduction in viremia, clinical manifestations, or complications. 30 , 65 As such, dengue treatment focuses on supportive care. Clinicians should evaluate all patients at presentation and in follow-up for warning signs or other signs and symptoms of severe dengue ( Table 1 ). Most patients without warning signs may be treated as outpatients, whereas patients at high risk of progression to severe disease based on age or underlying conditions, patients with warning signs, or patients with challenging social circumstances should be evaluated for observation or inpatient management. 66
For outpatients, fever can be controlled with acetaminophen and physical cooling measures; because of the risk of bleeding and thrombocytopenia, aspirin and nonsteroidal anti-inflammatory drugs are not recommended. Early, abundant oral hydration has been associated with lower hospitalization rates in children with dengue and is a key component of outpatient dengue care. 67 – 69
Early recognition of warning signs or severe dengue is essential for the prompt initiation of systematic intravenous fluid management to restore intravascular volume and avoid related complications and disease progression. 30 , 70 Large-volume resuscitation with isotonic solutions is recommended for patients in shock. 54 , 71 – 73 Fluid management in dengue requires continuous clinical and laboratory monitoring and rate adjustments to maintain adequate volume but also to prevent fluid overload. Mortality for untreated severe dengue can be 13% or higher 74 , 75 but can be reduced to <1% with early diagnosis and appropriate management. 55 Detailed information on systematic fluid management is provided in the current WHO, Pan American Health Organization, and Centers for Disease Control and Prevention (CDC) guidelines. 72 , 73 , 76
Corticosteroids, 77 immunoglobulins, 78 and prophylactic platelet transfusions 79 , 80 have not demonstrated benefits in patients with dengue and are not recommended.
Prevention of dengue involves protection against mosquito bites. Travelers to and residents of endemic areas can prevent mosquito bites by using US Environmental Protection Agency–approved insect repellents ( https://www.epa.gov/insect-repellents ) and wearing clothing that covers arms and legs. The use of screened windows and doors, air conditioning, and bed nets has been associated with protection from dengue infections. 24 , 81 – 87 Sites where mosquitoes lay eggs should be eliminated by emptying and scrubbing, covering, or eliminating standing water receptacles around the house. Mosquito bite prevention measures are important for all persons at risk for dengue, including vaccinated children.
Traditional vector control interventions can be time consuming and inefficient. 88 Furthermore, chemical control is limited by widespread insecticide resistance in endemic areas. 89 In response to these challenges, novel vector control methods have been developed including several strategies employing genetically modified mosquito technology and 2 strategies using Wolbachia pipientis , an intracellular bacterium found in about 60% of all insects but not commonly found in wild Aedes mosquitos. 90 – 92
The first strategy utilizing Wolbachia is Wolbachia -mediated suppression, in which a reduction in wild populations of Aedes mosquitoes is achieved by continuously releasing infected males into the environment. 93 When the infected males mate with wild females, the resultant eggs are inviable, leading to a decline in wild mosquito populations. 94 Some reports have documented reduction of the wild populations that can transmit dengue by more than 80%. 95 , 96
The second strategy is the Wolbachia replacement method, where both Wolbachia -infected male and female mosquitoes are released. Because Wolbachia is transmitted maternally, the mosquitoes that hatch from the eggs of infected females will be infected with Wolbachia from birth. 97 , 98 Wolbachia infection in female mosquitoes taking a bloodmeal reduces transmission of arboviruses, including dengue, chikungunya, and Zika. This method has demonstrated significant reductions of nearly 80% for the outcomes of dengue infection and related hospitalizations in areas where it has been implemented 99 and is currently being deployed in several countries.
Extensive studies have found no evidence of Wolbachia in the plants, soil, or other insects in contact with the Wolbachia -infected mosquitoes or any evidence of Wolbachia transmission to humans from the bites of infected mosquitoes, indicating that safety risks from Wolbachia -based interventions for humans and the environment are low. 100
ACIP made the first recommendation of a dengue vaccine (Dengvaxia) for use in the United States on June 24, 2021, marking an historic moment for dengue control following decades of global efforts to develop a safe and effective vaccine. Two other vaccines, TAK-003 developed by Takeda and TV003 developed by the National Institutes of Health, are in late-stage trials with efficacy results published or expected in 2022.
All 3 are live vaccines and contain 4 different attenuated vaccine viruses (tetravalent) targeting each of the dengue virus serotypes ( Fig 5 ) with the goal of achieving balanced protective immunity against all 4 serotypes, in both those who are DENV naïve and those who have been previously infected with DENV. Vaccine virus replication (infectivity) of each vaccine serotype after immunization will lead to antigenic stimulation, which then results in homotypic immunity. Infectivity by vaccine virus serotype differed among the 3 vaccines ( Table 2 ).

Key features of the 3 live attenuated dengue vaccines. Each DENV serotype is represented by a color (DENV-1 = green, DENV-2 = gray, DENV-3 = crimson, and DENV-4 = blue). Dengvaxia is comprised of 4 chimeric viruses in which the prM and E of each DENV serotype replaces those of yellow fever 17D (yellow). 132 TAK-003 is comprised of 1 full-length DENV-2 and 3 chimeric viruses (prM and E of DENV-1, DENV-3, and DENV-4 on a DENV-2 background). 133 TV003 is comprised of 3 full-length DENV and 1 chimeric virus. 123 The total number of dengue proteins in each vaccine is also shown.
Percentage of Vaccine Recipients with Detectable Vaccine Virus Serotype by RT-PCR after a Single Dose of the Indicated Vaccine in Persons without Previous Dengue Virus Infections
Data are presented as percentage.
These differences in vaccine serotype specific infectivity mirrored the induction of neutralizing homotypic antibody titers. Dengvaxia induced approximately 70% homotypic antibody for DENV-4 but <50% for DENV-1, DENV-2, and DENV-3. 101 Antibodies induced by TAK-003 were 83% homotypic for DENV-2 and 5%, 12%, and 27% homotypic for DENV-1, DENV-3, and DENV-4, respectively. 102 TV003 induced a balanced homotypic antibody response to DENV-1 (62%), DENV-2 (76%), DENV-3 (86%), and DENV-4 (100%). 103 Although homotypic antibody titers are associated with serotype specific vaccine efficacy, immune correlates that reliably predict vaccine efficacy have not yet been identified and remain an area of active research. 46
Dengvaxia uses a 3-dose schedule with each dose given 6 months apart (at months 0, 6, and 12). It was developed by Washington and St Louis Universities and Acambis and licensed to Sanofi Pasteur in the 2000s, entered phase 3 trials in the 2010s, and was first recommended by WHO in 2016 for persons aged 9 years and older living in highly endemic areas. Long-term follow-up data (over 5 years) from the phase 3 trials and further analyses of the efficacy results 104 – 107 demonstrated that children with evidence of previous DENV infection were protected from virologically confirmed dengue illness, including severe dengue if they were vaccinated with Dengvaxia. However, risk of hospitalization for dengue and severe dengue was increased among children without previous dengue infection who were vaccinated with Dengvaxia and had a subsequent dengue infection in the years after vaccination. In children without a previous dengue infection, the vaccine acts as a silent primary dengue infection resulting in a “secondary-like” infection upon their first infection with wild-type DENV and an increased risk of severe disease due to ADE ( Fig 6 ). 108 , 109 After these findings, WHO revised their recommendations for the vaccine to only be given to children with laboratory-confirmed evidence of a past infection. Following WHO’s recommendation, the FDA licensed Dengvaxia in 2019, and in 2021, ACIP recommended routine use of Dengvaxia for children aged 9–16 years with laboratory confirmation of previous DENV infection and living in areas where dengue is endemic. Dengvaxia is the first dengue vaccine recommended for use in the United States.

Proposed mechanism of Dengvaxia efficacy based on prior dengue antigen exposure. Risk of severe disease is represented by color (low = green, medium = yellow, and high = red). Exposure to dengue antigens is represented by mosquito figure for wild-type exposure and by a syringe for Dengvaxia exposure. The first row shows an unvaccinated individual exposed to 4 different dengue serotypes in their life with highest risk for severe disease with second infection and low risk of severe disease in the third and fourth infection. The second row shows an individual without previous dengue exposure who receives Dengvaxia, which acts as a silent primary infection, and then has higher risk for severe disease upon their first exposure to wildtype dengue, the equivalent of the second exposure to dengue antigen. The third row shows an individual with previous wild-type infection who receives Dengvaxia which acts as a silent second dengue exposure with lower risk for severe disease in subsequent exposures to wild-type dengue.
For children aged 9 to 16 years with evidence of previous dengue infection, Dengvaxia has an efficacy of about 80% against the outcomes of symptomatic virologically confirmed dengue (VCD) followed over 25 months as well as hospitalization for dengue and severe dengue as defined by criteria set by the trial’s independent data monitoring committee and followed over 60 months ( Table 3 ). 105 , 106 The efficacy by serotype mirrored its induction of a homotypic immune response 101 with highest protection against DENV-4 (89%), followed by DENV-3 (80%), and lowest against DENV-1 (67%) and DENV-2 (67%) ( Table 3 ). 106 Protection against mortality could not be reported because there were no dengue-related deaths in the phase 3 trials.
Dengvaxia Efficacy by Outcome and by Serotype in Persons 9–16 Years Old with Evidence of Previous Dengue Virus Infection
Pooled vaccine efficacy data are from CYD14 and CYD15 (clinical trial registration: NCT01373281, NCT01374516). CI, confidence interval; VE, vaccine efficacy. Data are presented as perentages.
Follow-up over 25 mo.
Follow-up over 60 mo.
The most frequently reported side effects (regardless of the dengue serostatus before vaccination) were headache (40%), injection site pain (32%), malaise (25%), asthenia (25%), and myalgia (29%) ( n = 1333). 108 Serious adverse events (ie, life-threatening events, hospitalization, disability or permanent damage, and death) within 28 days were rare in both vaccinated participants (0.6%) and control participants (0.8%) and were not significantly different. At 6 months, fewer severe adverse events were reported in the vaccine (2.8%) than in the control arm (3.2%). 108
Children who were seronegative for dengue at the time of vaccination had increased risk of severe illness on subsequent dengue infections. Risk of dengue-related hospitalization was approximately 1.5 times higher, and risk of severe dengue was approximately 2.5 times higher among seronegative children aged 9 to 16 years who were vaccinated than control participants over a 5-year period. 106
The requirement for a laboratory test before administration creates a unique challenge for Dengvaxia implementation. In areas with ongoing transmission of flaviviruses other than dengue, qualifying laboratory tests include a positive NAAT or NS1 test performed during an episode of acute dengue or a positive result on prevaccination screening tests for serologic evidence of previous infection that meet specific performance characteristics. In areas without other ongoing flavivirus transmission, a positive dengue IgM assay during an episode of acute dengue is also considered a qualifying laboratory test. 11
Prevaccination screening is critical because many DENV infections are asymptomatic or do not result in medical visits and testing. Thus, a significant proportion of previously infected individuals who could benefit from the vaccine will not be aware of or have laboratory documentation of their previous dengue infection. 110 – 113 One of the most challenging aspects in selecting a prevaccination test is defining benchmarks for test performance, as explored by several international working groups. 114 , 115 To reduce the risk of vaccinating someone without previous DENV infection, test specificity is a priority. Although test specificity and sensitivity are independent of seroprevalence, positive predictive value (PPV) and negative predictive value are dependent on seroprevalence and describe the likelihood of a true positive if a patient tests positive or the likelihood of a true negative if a patient tests negative ( Table 4 ). In areas with moderate or low seroprevalence (eg, 30%–50%), high test specificity (>98%) is required to achieve a PPV of 90% and therefore reduce the risk of misclassifying seronegative individuals. In these settings, near-perfect specificity at the expense of sensitivity is preferred to minimize the risk of vaccinating a misclassified negative individual and subsequently increasing their risk of severe dengue. However, high-prevalence areas (eg, >60%) would benefit from a higher test sensitivity and more moderate specificity (eg, 95%), which would increase identification of children who would benefit from the vaccine. 116
Test Performance for a Dengue Prevaccination Screening Test in Different Seroprevalence Scenarios 11
NPV, negative predictive value; PPV, positive predictive value.
CDC recommends that prevaccination screening tests that determine previous dengue infection have a minimum sensitivity of 75% and a minimum specificity of 98%. The recommendations also specify that the tests should be used in populations where they will achieve a positive predictive value (PPV) of ≥90% and a negative predictive value (NPV) of ≥75%. These rows demonstrate that tests with the same CDC recommended minimum sensitivity and specificity will have different PPV and NPV depending on the seroprevalence of the population in which they are used.
Because dengue seroprevalence at age 9 to 16 years is estimated to be approximately 50% in Puerto Rico 117 , 118 (where most of the eligible population for Dengvaxia in the United States and its territories and freely associated states reside), the CDC recommends that tests have a minimum sensitivity of 75% and a minimum specificity of 98%. The recommendations also specify that the test performance in the population should achieve a PPV of ≥90% and a negative predictive value of ≥75%. 11 These test characteristics were used to model the risks and benefits of implementing Dengvaxia. Using Puerto Rico’s population and an estimated seroprevalence of 50%, the model found that Dengvaxia vaccination would avert approximately 4148 symptomatic disease cases and 2956 hospitalizations over a 10-year period. This implementation would also result in an additional 51 hospitalizations caused by vaccination of people without previous dengue infection who were misclassified by the screening test. 119 The most common cause of hospitalization among vaccinated children will be breakthrough disease because the vaccine is not 100% efficacious.
TAK-003, developed by Takeda, consists of 2 doses given 3 months apart. The clinical trial population was primarily composed of children aged 4 to 16 years. At 18 months after vaccination, vaccine efficacy was found to be 80.2% against VCD, which waned to 62.0% by 3 years after vaccination. 120 , 121 Efficacy against hospitalization for dengue remained higher, at 83.6% at 3 years after vaccination. Differences in efficacy were observed by history of previous dengue infection, with higher efficacy among persons with previous infection compared with those without previous infection (65.0%–54.3%), and by age, with higher efficacy in older children. In contrast to findings from Dengvaxia at 25 months, children who were seronegative at the time of TAK-003 vaccination did not show an overall increased risk for hospitalization and severe disease compared with the placebo group at 3 years, although efficacy varied by DENV serotype and an age effect could not be ruled out ( Table 5 ). 106 , 120 Efficacy against both VCD and hospitalization varied by serotype and corresponded to the homotypic antibody titers, 102 with highest efficacy against DENV-2 and lowest against DENV-3 and DENV-4. Among children without previous DENV infection, there was no observed efficacy for VCD against DENV-3 or DENV-4. In the safety analysis, the number of serious adverse events was similar between vaccine (2.9%) and placebo (3.5%) groups.
TAK-003 Efficacy by Serostatus, Outcome, Serotype, and Age Group in Persons Aged 4–16 Years Over 36 Months of Follow-Up 120
Vaccine efficacy data are from clinical trial NCT02747927. CI, confidence interval; VE, vaccine efficacy. Data presented as percentage.
In March 2021, Takeda submitted TAK-003 to the European Medicines Agency for prevention of dengue from any DENV serotype among people aged 4 to 60 years. 122 The company will also be submitting filings to regulatory agencies in Argentina, Brazil, Colombia, Indonesia, Malaysia, Mexico, Singapore, Sri Lanka, and Thailand during 2021 and has future plans to submit to the FDA.
TV003 was developed by the National Institutes of Health and was formulated by selecting serotype-specific components that were determined to provide the most balanced safety and immunogenicity profile based on an evaluation of multiple monovalent and tetravalent candidates. 123 , 124 Because antibody titers failed to predict the efficacy of Dengvaxia, a human infection model was developed to assess the protective immunity induced by TV003 against DENV-2 challenge. Forty-eight volunteers were enrolled and randomized to receive TV003 (24) or placebo (24). Six months later, volunteers were administered a naturally attenuated DENV-2 challenge virus. 125 The primary efficacy endpoint was protection against detectable viremia after challenge. After challenge, DENV-2 was recovered by culture or reverse transcription-polymerase chain reaction (RT-PCR) from 100% of placebo recipients ( n = 20) and 0% of TV003 recipient ( n = 21) ( P < .0001). Postchallenge, rash was observed in 80% of placebo recipients compared with 0% of TV003 recipients ( P < .0001).
TV003 has been licensed to several manufacturers globally, including Merck & Co in the United States and the Instituto Butantan in Brazil. Phase 3 trials in Brazil are underway with efficacy and safety results expected in late 2022 (Clinical trial registration: NCT02406729).
Dengue is the most common arboviral disease worldwide and is projected to increase in range and global burden of disease. Although advancements in the field have progressed incrementally for decades, the recent approval of Dengvaxia for routine use marks a major step forward for control and prevention efforts in the United States and paves the way for future dengue vaccines.
Dengvaxia has several complexities that necessitate future research, including the possibility of fewer doses in the initial schedule followed by booster doses in later years. 30 Because it is the first vaccine to require laboratory testing before administration, public–private partnerships to develop more specific, sensitive, and accessible tests or testing algorithms will be key to minimize vaccination of persons without previous DENV infection and maximize benefit to those with previous infection. Jurisdictions that wish to use Dengvaxia will need to gather seroprevalence data and ensure that prevaccination screening tests meet the requirements for positive and negative predictive values. Furthermore, behavioral science assessments to elicit community-level perceptions and concerns combined with health systems research on optimal “test-and-vaccinate” strategies will result in dengue vaccination programs that are well accepted, efficient, and tailored to individual communities.
TAK-003 and TV003 are in late-stage trials and could soon be approaching licensure. An indication for use in travelers would offer clinicians in nonendemic areas of the United States a prophylactic therapeutic option for their patients. While awaiting the approval of a vaccine with balanced serotype immunity, a mix-and-match strategy guided by differences in serotype-dominant immune responses in each vaccine (TAK-003 followed by Dengvaxia, for example) could potentially lead to higher levels of protection against dengue, but it has yet to be evaluated for safety and efficacy in clinical trials. 126 For all 3 vaccines, studies evaluating efficacy against emerging DENV serotype variants will be important to assess long-term protection induced by the vaccine strains. 10 , 127
Future vaccines against dengue could also benefit from the lessons learned from the COVID-19 pandemic, namely that new vaccine platform technologies plus political will can result in rapid development of safe and effective vaccines and that clear communication with the public is crucial to successful vaccine implementation. 128 – 130 Dengue vaccines based on an mRNA platform are already under investigation. 131
Vaccines are a powerful new tool in our arsenal against dengue, but they are only 1 of many interventions, including novel vector control strategies, to control a virus with a complex epidemiology, immunopathogenesis, and clinical picture influenced by climate change, urbanization, poverty, and human migration. Clinicians should remain vigilant in recognizing and diagnosing patients with dengue, because early treatment remains the cornerstone for reducing morbidity and mortality. However, with the recent approval of Dengvaxia, we are 1 step closer on the path to dengue elimination and can expect exciting new developments in dengue interventions in the near future.
We thank Ms Alexia E. Rodriguez, MPH, for her review of the manuscript.
Drs Wong, Adams, and Paz-Bailey conceptualized and designed the structure of the review, drafted portions of the initial manuscript, and reviewed and revised the manuscript; Drs Durbin, Muñoz-Jordán, Sánchez-González, and Volkman drafted portions of the initial manuscript and reviewed and revised the manuscript; Dr Poehling reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FUNDING: No external funding.
CONFLICT OF INTEREST DISCLOSURES: Dr Durbin is a scientific advisor to Merck & Co on dengue vaccine development. The other authors have no conflicts of interest to disclose.
Advisory Committee on Immunization Practices
antibody dependent enhancement
Centers for Disease Control and Prevention
dengue virus
Food and Drug Administration
immunoglobulin M
nucleic acid amplification test
nonstructural protein 1
positive predictive value
plaque reduction neutralization test
virologically confirmed dengue
World Health Organization

Supplementary data
Advertising Disclaimer »
Citing articles via
Email alerts.

Affiliations
- Editorial Board
- Editorial Policies
- Journal Blogs
- Pediatrics On Call
- Online ISSN 1098-4275
- Print ISSN 0031-4005
- Pediatrics Open Science
- Hospital Pediatrics
- Pediatrics in Review
- AAP Grand Rounds
- Latest News
- Pediatric Care Online
- Red Book Online
- Pediatric Patient Education
- AAP Toolkits
- AAP Pediatric Coding Newsletter
First 1,000 Days Knowledge Center
Institutions/librarians, group practices, licensing/permissions, integrations, advertising.
- Privacy Statement | Accessibility Statement | Terms of Use | Support Center | Contact Us
- © Copyright American Academy of Pediatrics
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Open access
- Published: 30 May 2024
Long-term effects of climate factors on dengue fever over a 40-year period
- Chengdong Xu 1 , 2 ,
- Jingyi Xu 1 , 2 &
- Li Wang 3 , 4
BMC Public Health volume 24 , Article number: 1451 ( 2024 ) Cite this article
144 Accesses
Metrics details
Dengue fever stands as one of the most extensively disseminated mosquito-borne infectious diseases worldwide. While numerous studies have investigated its influencing factors, a gap remains in long-term analysis, impeding the identification of temporal patterns, periodicity in transmission, and the development of effective prevention and control strategies. Thus, we aim to analyze the periodicity of dengue fever incidence and explore the association between various climate factors and the disease over an extended time series.
By utilizing monthly dengue fever cases and climate data spanning four decades (1978–2018) in Guangdong province, China, we employed wavelet analysis to detect dengue fever periodicity and analyze the time-lag relationship with climate factors. Additionally, Geodetector q statistic was employed to quantify the explanatory power of each climate factor and assess interaction effects.
Our findings revealed a prolonged transmission period of dengue fever over the 40-year period, transitioning from August to November in the 1970s to nearly year-round in the 2010s. Moreover, we observed lags of 1.5, 3.5, and 3 months between dengue fever and temperature, relative humidity, and precipitation, respectively. The explanatory power of precipitation, temperature, relative humidity, and the Oceanic Niño Index (ONI) on dengue fever was determined to be 18.19%, 12.04%, 11.37%, and 5.17%, respectively. Dengue fever exhibited susceptibility to various climate factors, with notable nonlinear enhancement arising from the interaction of any two variables. Notably, the interaction between precipitation and humidity yielded the most significant effect, accounting for an explanatory power of 75.32%.
Conclusions
Consequently, future prevention and control strategies for dengue fever should take into account these climate changes and formulate corresponding measures accordingly. In regions experiencing the onset of high temperatures, humidity, and precipitation, it is imperative to initiate mosquito prevention and control measures within a specific window period of 1.5 months.
Peer Review reports
Currently, vector-borne diseases pose a threat to over 80% of the world's population, with mosquito-borne illnesses contributing the most to the overall disease burden [ 1 ]. The most prevalent viral infection transmitted by Aedes mosquitoes, dengue fever affects at least 128 countries and is thought to cause between 50 and 100 million cases annually [ 2 , 3 ]. Aedes mosquitos help spread dengue disease to humans because they are highly adapted to urban settings in tropical and subtropical regions of the world [ 4 ].
A variety of factors influence dengue fever incidence and transmission. Extensive studies have established the importance of climate parameters such as temperature, precipitation, and humidity in driving dengue fever dynamics [ 1 , 5 , 6 ]. The dynamics of mosquito growth, the spread of viruses, and mosquito-human interactions are all influenced by the climate [ 7 ]. Temperature plays a crucial role in vector development, biting rates, and the rate of pathogen development within mosquitoes [ 8 ]. Since stagnant water pools are necessary for mosquito breeding and growth, precipitation is an important element in climate models used to identify dengue fever. The chance of dengue transmission can be impacted by humidity, which can also have an impact on mosquito survival and flight patterns [ 1 , 9 ].
In addition to these commonly studied climate factors, extreme weather occurrences can also significantly affect the incidence of dengue disease [ 10 , 11 ]. The El Niño-Southern Oscillation (ENSO), which has a periodicity of two to seven years and is defined by alternating warm and cool phases in the tropical Pacific, is a persistent and irregular phenomena. Anomalies in the world's temperature and precipitation patterns are brought on by ENSO events. According to several studies, ENSO significantly affects dengue fever dynamics by accounting for about 40% of the fluctuation in temperature and rainfall [ 11 ].
Southern and eastern China are included in the East Asian monsoon region, which is highly sensitive to climate change. The dengue fever epidemic, which has had a considerable influence on these areas over the past few decades and is defined by different temporal and spatial evolution patterns, has had a significant impact on these areas. There were no known dengue fever cases in China until 1977. However, a dengue epidemic in the province of Guangdong in 1978 heralded the start of the illness' intermittent predominance across the country [ 12 ]. Then, dengue fever outbreaks were reported in Hainan, Guangxi, Fujian, and Zhejiang provinces sequentially [ 12 ]. In mainland China, a total of 655,324 cases and 610 fatalities were recorded from 1978 to 2008, and 52,749 cases and 6 fatalities from 2009 to 2014 [ 13 ]. As a result, dengue fever has grown to be a serious illness in China and was classified as an infectious disease of type B in 2004. Despite the fact that there have recently been a few dengue fever cases in inland regions like Henan province [ 14 ], 94.3% total of dengue cases in mainland China were from Guangdong province [ 15 ], where the hot, humid weather conditions are favorable for mosquito breeding and dengue disease transmission [ 16 ]. Especially, in 2014 China has undergone the worst dengue outbreak in the last 20 years [ 17 ], which caused great concern about dengue fever in China.
Many studies have focused on the temporal dynamics of dengue fever incidence over a period of 10 to 25 years [ 6 , 18 , 19 , 20 ]. However, there is limited research on the epidemic patterns of dengue fever and the effect of climate factors on dengue fever under long-term climate change (climate change refers to climate variations typically spanning 30 years or longer). Between the mid-twentieth century and 2018, climate change increased the probability of transmission by 15.0% for Aedes albopictus and 8.9% for Aedes aegypti, the principal vectors of dengue [ 21 ]. As global warming continues, several locations, including China's eastern coast, are expected to become appropriate breeding grounds for the dengue virus by 2050 [ 22 ]. Hence, it is important to conduct a comprehensive analysis of dengue dynamics over a prolonged historical period to understand the relationship between climate and dengue for formulating effective strategies for early warning, prevention, control of dengue fever, and informing public health measures aimed at reducing the burden of dengue fever and guide future research efforts in the field of vector-borne diseases.
This study aimed to analyze the periodicity of dengue fever incidence, evaluate the lag relationship between climate factors and the disease, and assess the explanatory power and interaction effects of these climate variables using dengue fever and climate data from Guangdong province spanning the years 1978 to 2018.
Our study incorporated three distinct datasets. Firstly, we utilized monthly dengue fever cases in Guangdong province spanning the years 1978 to 2018. These data were sourced from previous papers [ 23 , 24 , 25 , 26 ]. Secondly, we obtained meteorological data, including monthly mean temperature, monthly mean relative humidity, and monthly total precipitation, from the China Meteorological Data Sharing Service System ( http://data.cma.cn/ ). Lastly, we incorporated the monthly Oceanic Niño Index (ONI) as an indicator for the El Niño-Southern Oscillation (ENSO) phenomenon. The ONI data, representing the sea surface temperature anomaly index for the Niño region 3.4, were acquired from the Climate Prediction Center of the National Weather Service ( https://origin.cpc.ncep.noaa.gov ). To supplement our analysis, we accessed the monthly population data of Guangdong province from the Guangdong Statistics Yearbook. The multicollinearity test showed that all climate factors had VIF values less than 3, so there the possibility of multicollinearity was ruled out.
Wavelet analysis
When analyzing time series that contain non-stationary power at various frequencies, the wavelet transform is always applied. A wavelet is a zero-mean function that is confined in frequency and temporal space. There are many wavelets that have been characterized, including the Morlet, Paul, and Gaussian derivative, each of which has appropriate application conditions [ 27 ]. In our study, we chose the Morlet wavelet ( \(\omega_0\) = 6), which is considered to provide a good balance between time and frequency localization [ 28 ]. The Morlet is defined as:
where \(w_0\) and \(\eta\) are dimensionless frequency and time, respectively. The wavelet is stretched in time by changing its scale (s) to \(\eta=s\times t\) , and normalized to have unit energy. The wavelet power is defined as \(\left|W_n^X(s)\right|^2\) . The specific formula is as follows:
where \(W_n^X(s)\) is the transformed time series for scale s ; \(\delta_t\) is the time interval; \(n\) is the time and \(n'\) is the reversed time. Then a wavelet power spectrum is generated to explore the periodicity of each time series. The time series were padded with enough zeros to generate a time series of length since working with finite-length time series will result in inaccuracies at the start and end of the wavelet power spectrum. The cone of influence (COI) is used to depict regions that give erroneous results in order to remove the impact of discontinuities at the endpoints and the lowering of edge amplitude influenced by zero-padding [ 27 ].
Cross Wavelet Transform (XWT) is used to probe coincident high power between two time series which is defined as:
where * means complex conjugation. The XWT power is defined as \(\left|W^{XY}\right|\) . There is also a power spectrum in XWT, where the arrows represent the relative phase [ 28 ]. Phase arrows pointing right mean the two variables are in phase, and the left arrows mean anti-phase. Down arrows mean X leads Y by 90 degrees, and up arrows mean Y leads X by 90 degrees [ 29 ].
Geodetector q statistic
In order to explore the association between long-term climate factors and the incidence of dengue fever, and reveal the interaction between these climate factors, Geodetector q statistic was applied. The basic principle of the method is to assume that if the spatial or temporal distribution of two variables tends to be consistent, there is statistical association between them [ 30 ]. Our study aimed to explore the impact of different factors on the variation in time of dengue fever incidence.
In our study, q statistic of Geodetector were used to illustrate the explanatory power of independent variables:
where q is the explanatory power of each factor on dengue fever incidence, which follows a Noncentral-F distribution [ 30 , 31 ] and the range is from 0 to 1. The q value indicates that X explains 100 × q% of Y, the larger the value is, the stronger the explanatory power of independent variable X to Y is, and vice versa. h is the number of the strata of variable X (climate factors); \(N_h\) and N are the sample size in the h- th stratum and the whole regions, respectively; \(\sigma_h^2\) and \(\sigma^2\) are the variance of Y (dengue fever incidence) for the h- th stratum and the whole regions; SSW and SST indicate the within sum of squares and total sum of squares, respectively.
Interaction detector of Geodetector can be used to assess the interaction effect of two variables, e.g., X 1 and X 2 , which can calculate the explanatory power q(X 1 ∩ X 2 ) of two factors and probes whether the explanatory power of two factors is enhanced or weakened when taken together, or whether they are independent by comparing q(X 1 ∩ X 2 ) with q(X 1 ) and q(X 2 ) . The description of each interaction demonstrates in Table 1 [ 32 , 33 ].
Periodicity of dengue fever and climate factors
The 40-year time series analyzed in this study revealed noticeable fluctuations in dengue fever incidence. Specifically, there were seven years during which the number of dengue fever cases exceeded 10,000: 452,674 in 1980, 118,881 in 1986, 45,189 in 2014, 32,830 in 1987, 22,122 in 1978, 19,543 in 1981, and 16,385 in 1985 (Fig. 1 ). In comparison to these high-incidence years, the occurrence of dengue fever in other years was relatively lower.
Temporal dynamics of dengue fever incidence and associated variables, 1978–2018. From top to bottom, the figure shows monthly dengue fever incidence (log 10 ), average temperature, relative humidity, precipitation and ONI
To assess the periodicity of dengue fever incidence and its association with climate factors, we conducted Continuous Wavelet Transform (CWT) analysis on these variables (Fig. 2 ). The wavelet power spectrum revealed distinct scales of periodicity in dengue fever incidence during specific time intervals, namely 1980–1982, 1985–1987, 1994–2004, 2006–2007, and 2011–2016, dengue fever incidence was composed of different scales periods: a distinct one-year cycle and a cycle of half a year. Notably, over the 40-year period, the prevalence of the half-year cycle gradually diminished, transitioning into a one-year cycle. This observation implies an extended transmission period for dengue fever, encompassing nearly the entire year instead of being limited to August to November. Additionally, a five-year cycle was evident during the 1980s, indicating notable inter-annual variation in dengue fever incidence (Fig. 2 a).

Continuous wavelet transform power spectrum of dengue fever incidence and climatic factors, 1978–2018. a - e dengue fever incidence ( a ) average temperature ( b ) average relative humidity ( c ) precipitation ( d ) EI Niño index ( e ). The thick black contour designates the 5% significance level against red noise and the cone of influence (COI) where edge effects might distort the picture is shown as a lighter shade
The wavelet power spectra analysis revealed a consistent one-year cycle in temperature, relative humidity, and precipitation. This cycle showed regular inter-annual variation throughout the entire time series (Fig. 2 b, c, d). Notably, among the analyzed climate factors, the El Niño Index (ONI) exhibited distinct characteristics compared to others. Over the entire time series, the periodicity of the El Niño phenomenon was observed within the range of 2–7 years, with a particular emphasis on the 3–6 year interval, indicating an average occurrence every 3–6 years (Fig. 2 e).
Time-lag association between dengue fever and climate factors
From each two CWTs (one is dengue fever incidence and the other is a climate factor), we performed Cross Wavelet Transform (XWT) to assess their shared power and relative phase in the time–frequency domain, thereby investigating the association between dengue fever incidence and climate factors (Fig. 3 ).

Cross-wavelet power spectrum of dengue fever incidence and climatic factors in Guangdong Province, 1978–2018. a - d dengue fever incidence—average temperature ( a ) dengue fever incidence—average relative humidity ( b ) dengue fever incidence – precipitation ( c ) dengue fever incidence—EI Niño index ( d ). The thick black contour designates the 5% significance level against red noise and the cone of influence (COI) where edge effects might distort the picture is shown as a lighter shade
The XWT of dengue fever incidence and temperature shows that they have common high power in one year over the whole time series, so the temperature is a significant factor that influences the dengue fever. The mean phase of the XWT phase angle outside the COI and inside the 5% significant areas is 45 ± 15°. It indicates that within one year, the two factors are in phase and the incidence of dengue fever lags behind the average temperature by 1/8 cycles, implying that the incidence of dengue fever lags behind the average temperature by about 1.5 months (Fig. 3 a).
The XWT of dengue fever incidence and relative humidity shows that they also have common high power in one year over the whole time series, and the mean phase of the XWT phase angle outside the COI and inside the 5% significant areas is 105 ± 15°. It means that within one year, the incidence of dengue fever lags behind the relative humidity by 7/24 cycles, implying that the incidence of dengue fever lags behind the relative humidity by about 3.5 months (Fig. 3 b).
The XWT of dengue fever incidence and precipitation shows that they are in phase with significant common power in one year during the 40 years. The XWT phase angle has a mean phase of 90 ± 15°, which means that within one year, the incidence of dengue fever lags behind the precipitation by 1/4 cycles, implying that the incidence of dengue fever lags behind the precipitation by about 3 months (Fig. 3 c).
The relationship between dengue fever incidence and the El Niño Index (ONI) exhibits a relatively complex nature. The Cross Wavelet Transform (XWT) analysis reveals a significant shared power in the 3–6 year band, particularly during the period from 1984 to 1992, indicating a strong correlation between the two variables. Furthermore, throughout the entire study period, they also exhibit consistent power in the one-year band, further indicating a correlation between these factors on an annual basis (Fig. 3 d).
The Cross Wavelet Transform (XWT) revealed the degree of correlation between climate change and dengue fever cases in the time–frequency space, providing knowledge about time lags. To further validate these computational results, we used another method, the Spearman coefficient, to calculate the impact of different lag periods of various climate factors on dengue fever cases, as shown in Table 2 . Temperature showed a significant positive correlation with dengue fever incidence at different lag times, with the strongest correlation at a lag of 2 months, with a correlation coefficient of 0.459; this was followed by a lag of 1 month with a correlation coefficient of 0.431. Relative humidity also exhibited a significant positive correlation with dengue fever incidence at different lag times, with the strongest correlation at a lag of 3 months, with a correlation coefficient of 0.333; this was followed by a lag of 4 months with a correlation coefficient of 0.305. Precipitation and the Oceanic Niño Index (ONI) also showed positive correlations with dengue fever incidence at different lag periods. Among them, precipitation exhibited the strongest correlation at lag times of 2 and 3 months, with correlation coefficients of 0.371 and 0.353, respectively. ONI, on the other hand, showed lower correlations with dengue fever incidence in lag periods from 0 to 5 months, indicating longer-term interannual lag effects. These results are consistent with the Cross Wavelet Transform (XWT), demonstrating the reliability of the findings in this study.
Influence of various climate factors on dengue fever incidence
Based on the XWT analysis, it is evident that temperature, relative humidity, precipitation, and the El Niño Index (ONI) play significant roles in influencing the incidence of dengue fever. To quantitatively assess their explanatory power, we employed Geodetector. The geodetector q statistic revealed that precipitation, temperature, and relative humidity accounted for 18.19%, 12.04%, and 11.37% of the heterogeneity in dengue fever incidence, respectively, while the influence of the El Niño Index was measured at 5.17% (Fig. 4 ). All the findings were statistically significant.

Geodetector q statistic of various climate factors
To examine the interactive effect of climate factors on the incidence of dengue fever, we conducted geodetector analysis to calculate the interaction detector for each pair of factors. The results are presented in Fig. 5 . Notably, the analysis reveals that the interaction between any two variables leads to a nonlinear enhancement in their influence. Particularly, when considering the combined effect of temperature, precipitation, and relative humidity, their impact on dengue incidence surpasses 70% of the geodetector q statistic. Furthermore, the explanatory power of the El Niño Index (EI Niño) significantly improves when interacting with any other factor (Fig. 5 ).
The interaction effect of climate factors on dengue fever
Dengue fever is considered to be a disease strongly influenced by climate. Climate change can have both direct and indirect impacts on the ecology of dengue fever. Numerous empirical studies have investigated the relationship between dengue fever and climate factors using various analytical approaches [ 7 , 29 , 34 , 35 ]. However, the long-term effects of climate factors on dengue fever remain unclear. In this study, we conducted wavelet analyses on a time series of monthly reported dengue cases and climate variables spanning from 1978 to 2018. The aim was to detect the periodicity of dengue fever and climate factors and qualitatively demonstrate their phase and time-lag relationship. Additionally, we employed Geodetector to quantitatively assess the relative importance of each climate factor on dengue fever and their interactions. Our findings revealed that dengue fever exhibits noticeable inter-annual and intra-annual variations, with different associations observed between the disease and certain climate factors.
Our study uncovered a novel finding regarding the changing periodicity of dengue fever over time. Specifically, we observed a shift from a half-year cycle to a one-year cycle, indicating a lengthening of the epidemic period for dengue fever. This intriguing observation suggests that the influence of global warming and urbanization might contribute to this phenomenon [ 36 ]. A periodicity of two to three years has been mentioned in a few earlier studies conducted in Southeast Asian nations like Thailand and Vietnam [ 37 , 38 ]. Our study suggests that Guangdong has a relatively low inter-annual variation in dengue fever which is stable in both the short and long term. This also supports the conclusion that when the length of the warm season is short, dengue fever cycles in higher latitudes are shorter than those in lower latitudes [ 20 ]. Guangdong's lower inter-annual variation is most likely caused by the reason that dengue fever in China is still an imported illness rather than an indigenous one. Although Southeast Asian nations were identified as the most likely source of DENV in Guangzhou, strain and genotype alterations were frequent, and neither serotype nor genotype was dominant [ 39 ].
We established a close relationship between climate factors and the incidence of dengue fever. Specifically, we observed a lag of 1.5, 3.5, and 3 months between dengue fever and temperature, relative humidity, and precipitation, respectively. These findings confirm the widely accepted notion that temperature, precipitation, and humidity, as representative climate variables, play a crucial role in influencing the occurrence of dengue fever [ 1 ]. Many previous studies have explored the time-lag relationship between dengue fever and various climate factors, but due to the different time series and regions, there are different results [ 40 , 41 , 42 , 43 , 44 ]. For instance, Taiwan, which is as the same latitude as Guangzhou, had a 3 months lag relationship between dengue and temperature [ 41 ]. Another study also in Guangdong province showed that temperature, precipitation, and humidity are associated with dengue with 2, 3, 4 months lag from 1988 to 2015 [ 40 ]. Compared with these studies, we use a longer time series, which was from 1978 (the first reported outbreak in Guangdong, China) to 2018, to obtain the phase information in time–frequency by using XWT. This provided a more accurate and robust relationship between dengue and climate factors over long-time scales.
In contrast to the evident time-lag observed between temperature, humidity, precipitation, and dengue fever, the relationship between ONI and dengue fever exhibited a more intricate nature. Previous studies have also reported divergent findings regarding their association in various regions [ 40 , 45 , 46 ]. For instance, a study conducted in Guangdong, China from 1992 to 2011 discovered a significant coherence between ONI and dengue fever, with a lag of 12 months [ 40 ]. The reason for the difference from our study may be that seven large-scale outbreaks in Guangdong province (over 10,000 cases per year) were included in our study. These seven years which had abnormally high numbers of cases may be caused by a variety of reasons, and the EI Niño was not the only factor, for example one study conducted in Guangzhou demonstrated that the outbreak was a combination of many factors, including the improved transmission capacity of mosquitoes, increased monitoring due to the high media attention and so on [ 47 ]. In addition, multivariate ENSO Index was utilized in a research in Thailand to discover an association between ENSO and dengue with a 1–11 month lag [ 46 ].
The study revealed that precipitation, temperature, and relative humidity had high explanatory power to the incidence of dengue fever in Guangdong province. This finding aligns with a previous study conducted in Guangzhou, which demonstrated a positive association between cumulative precipitation and the number of days with light or moderate precipitation with dengue fever [ 48 ]. Increased rainfall can contribute to the proliferation of vector breeding habitats, thus influencing the incidence of dengue fever [ 1 ]. Previous studies have found a parabolic relationship between temperature and dengue fever incidence [ 5 , 19 ]. Because within a certain temperature range, an increase in temperature can accelerate virus replication and shorten the external incubation period. Nevertheless, mosquito survival rates drop in extremely hot weather, which reduces the risk of transmitting dengue illness [ 8 , 19 , 49 ]. Prior research has also shown a parabolic pattern indicating a non-linear link between relative humidity and dengue disease. Suitable humidity can influence mosquitoes in many ways, including their life cycle, biting rate, flying distance and so on [ 35 ]. For example, one study discovered that mosquitos bite 19 times per hour in dry settings and 60 times per hour in wet conditions, demonstrating that humidity might affect dengue through modifying mosquito behavior [ 50 ].
Compared to other climate factors, the El Niño phenomenon (ENSO) exerts distinct influences in terms of intensity, duration, and time lag. Several studies have provided evidence of a positive association between the El Niño Index (ONI) and dengue fever, with increased dengue cases occurring during El Niño events in Southern Coastal Ecuador [ 51 ]. El Niño has been found as one of the main causes of dengue fever in Thailand, with ENSO events worldwide responsible for 22% of the monthly incidence variation in eight northern interior provinces [ 46 ]. It is important to note that these differences in findings may be attributed to variations in research areas and time series. The indirect influence of El Niño on mosquito behavior primarily stems from climate variations. For example, the ENSO event in Kaohsiung, Taiwan in 2005 resulted in increased humidity, which gave more ideal conditions for mosquito growth and reproduction, consequently contributing to the rise in dengue disease cases [ 52 ].
Surprisingly, our study not only examines the individual impact of climate factors but also investigates the interaction between them. We discovered that the interaction of any two variables exhibits nonlinear enhancement, with the interaction between precipitation and humidity being the most significant. Furthermore, the explanatory power of the El Niño Index (ONI) is greatly enhanced when it interacts with any climate factor. These findings lead us to comprehend three crucial aspects. Firstly, the transmission of dengue fever is influenced by both common climate factors with regular cycles and extreme climate events with irregular cycles. This finding confirms the close association between El Niño and climate change. Secondly, research should not solely focus on the relationship between individual climate factors and dengue fever but should also consider the combined influence of multiple factors. Thirdly, the water environment plays a critical role in dengue transmission, and this significant impact may be related to various hydrological factors such as soil type and vegetation. Therefore, timely monitoring of El Niño and climate change is imperative for controlling the spread of dengue fever.
The study employed a long-term series analysis to investigate the cyclical patterns of dengue fever, contributing to the body of evidence linking climate and dengue from both qualitative and quantitative perspectives. Our findings reveal notable inter-annual and intra-annual variations in dengue incidence and its susceptibility to various climate factors. Particularly, the interaction between precipitation and relative humidity emerges as the most influential factor. These findings enhance our understanding of dengue ecology and offer valuable insights for early warning and control measures. To prevent a resurgence of dengue fever amid the challenges posed by global warming, such as increased temperatures and precipitation, concerted efforts are required to bolster the public health system's capacity, raise awareness about dengue fever, encourage dengue vaccinations, and foster a healthier living environment.
This study has several limitations that should be acknowledged. As this study is based on a time series analysis, the findings may not capture the spatial variations of dengue fever at more granular levels such as prefecture-level cities, districts, and counties, due to the availability of only provincial-level dengue incidence data. Furthermore, due to data constraints and our specific aims, we did not determine the direct or indirect impact of El Niño's climate variation on dengue incidence. Future studies are expected to obtain more comprehensive data that allows for individual and combined analysis of various factors influencing dengue fever, including spatial variations and its temporal association with climate and weather. Despite these limitations, the findings of this study provide valuable insights for dengue fever warning systems and public health preparedness efforts.
In the context of global climate change, the epidemic period of dengue fever has gradually lengthened over the past 40 years. The incidence of dengue is influenced by a combination of climate factors such as precipitation, temperature, relative humidity and El Niño. Therefore, future dengue prevention and control strategies should take these climate changes into account and develop corresponding measures. In addition, considering the lag relationship between the incidence of dengue fever and climatic factors, mosquito prevention and control should be carried out within a specific window period of 1.5 months in areas with high temperature, high humidity and heavy rainfall.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Oceanic Niño Index
El Niño-Southern Oscillation
Variance inflation factor
Cone of influence
Continuous Wavelet Transform
Cross Wavelet Transform
Dengue virus
Franklinos LHV, Jones KE, Redding DW, Abubakar I. The effect of global change on mosquito-borne disease. Lancet Infect Dis. 2019;19:E302–12.
Article PubMed Google Scholar
Jia P, Chen X, Chen J, Lu L, Liu Q, Tan X. How does the dengue vector mosquito Aedes albopictus respond to global warming? Parasite Vector. 2017;10:140.
Article Google Scholar
Stanaway JD, Shepard DS, Undurraga EA, Halasa YA, Coffeng LE, Brady OJ, et al. The global burden of dengue: an analysis from the global burden of disease study 2013. Lancet Infect Dis. 2016;16:712–23.
Article PubMed PubMed Central Google Scholar
Kraemer MUG, Sinka ME, Duda KA, Mylne AQN, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 2015;4:e08347.
Wu X, Lang L, Ma W, Song T, Kang M, He J, et al. Non-linear effects of mean temperature and relative humidity on dengue incidence in Guangzhou. China Sci Total Environ. 2018;628–629:766–71.
Li R, Xu L, Bjornstad ON, Liu K, Song T, Chen A, et al. Climate-driven variation in mosquito density predicts the spatiotemporal dynamics of dengue. Proc Natl Acad Sci USA. 2019;116:3624–9.
Article CAS PubMed PubMed Central Google Scholar
Morin CW, Comrie AC, Ernst K. Climate and dengue transmission: evidence and implications. Environ Health Perspect. 2013;121:1264–72.
Mordecai EA, Cohen JM, Evans MV, Gudapati P, Johnson LR, Lippi CA, et al. Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models. PLoS Negl Trop Dis. 2017;11:e0005568.
Lo Iacono G, Cunningham AA, Bett B, Grace D, Redding DW, Wood JLN. Environmental limits of Rift Valley fever revealed using ecoepidemiological mechanistic models. Proc Natl Acad Sci. 2018;115:E7448–56.
Cheng J, Bambrick H, Frentiu FD, Devine G, Yakob L, Xu Z, et al. Extreme weather events and dengue outbreaks in Guangzhou, China: a time-series quasi-binomial distributed lag non-linear model. Int J Biometeorol. 2021;65:1033–42.
Hales S, Weinstein P, Woodward A. Dengue fever epidemics in the South Pacific: driven by El Nino Southern Oscillation? Lancet. 1996;348:1664–5.
Article CAS PubMed Google Scholar
James AA, Lun Z-R, Wu J-Y, Chen X-G. Dengue Fever in Mainland China. Am J Trop Med Hyg. 2010;83:664–71.
Chen B, Liu Q. Dengue fever in China. Lancet. 2015;385:1621–2.
Yong HX, Xia MH, Feng WH, Hua DY, Jia S, Le LX, et al. Outbreak of dengue fever in central China, 2013. Biomed Environ Sci. 2014;27:894–7.
Google Scholar
Yue Y, Liu Q, Liu X, Wu H, Xu M. Comparative analyses on epidemiological characteristics of dengue fever in Guangdong and Yunnan, China, 2004–2018. BMC Public Health. 2021;21:1389.
Jin X, Lee M, Shu J. Dengue fever in China: an emerging problem demands attention. Emerging Microbes & Infections. 2015;4:1–3.
Chinese Center for Disease Control and Prevention. Reportable infectious disease statistics. Beijing: CCDC,2014. Available: https://www.chinacdc.cn/tjsj/fdcrbbg/ . Cited 29 Dec 2021.
Xu L, Stige LC, Chan K-S, Zhou J, Yang J, Sang S, et al. Climate variation drives dengue dynamics. Proc Natl Acad Sci U S A. 2017;114:113–8.
Seah A, Aik J, Ng L-C, Tam CC. The effects of maximum ambient temperature and heatwaves on dengue infections in the tropical city-state of Singapore - a time series analysis. Sci Total Environ. 2021;775:145117.
Lai S, Huang Z, Zhou H, Anders KL, Perkins TA, Yin W, et al. The changing epidemiology of dengue in China, 1990–2014: a descriptive analysis of 25 years of nationwide surveillance data. BMC Med. 2015;13:100.
Watts N, Amann M, Arnell N, Ayeb-Karlsson S, Beagley J, Belesova K, et al. The 2020 report of the lancet countdown on health and climate change: responding to converging crises. Lancet. 2021;397:129–70.
Messina JP, Brady OJ, Golding N, Kraemer MUG, Wint GRW, Ray SE, et al. The current and future global distribution and population at risk of dengue. Nat Microbiol. 2019;4:1508–15.
Zeng S, Zhong H, Fang Y, Dai J, Xiao J, Liu T. Analysis of temporal distribution characteristics of dengue fever in Guangdong Province by multi-curve seasonal index model and its application probe. Pract Prev Med. 2018;25:1137–41.
Xi J, Cheng X, Hu H, Liao C, Guo Z, Lu J. Temporal dynamic of dengue fever in Guangdong province from 1990 to 2018. J Trop Med. 2020;20:460–4.
Huang W. Overview of dengue fever epidemic in Guangdong province in. Guangdong J Health Epid Prevent. 1978;1980:7–12.
Zeng Z, He J. Epidemiological analysis of dengue fever in Guangdong province from 1978 to 1999. Sci Travel Med. 2000;6:1–5.
Torrence C, Compo GP. A practical guide to wavelet analysis. Bull Am Meteorol Soc. 1998;79:61–78.
Grinsted A, Moore JC, Jevrejeva S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Processes Geophys. 2004;11:561–6.
Morris A, Gozlan RE, Hassani H, Andreou D, Couppie P, Guegan J-F. Complex temporal climate signals drive the emergence of human water-borne disease. Emerg Microbes Infect. 2014;3:e56.
Wang J, Xu C. Geodetector: principle and prospective. Acta Geogr Sin. 2017;72:116–34.
Wang J, Zhang T, Fu B-J. A measure of spatial stratified heterogeneity. Ecol Indic. 2016;67:250–6.
Wang J-F, Li X-H, Christakos G, Liao Y-L, Zhang T, Gu X, et al. Geographical detectors-based health risk assessment and its application in the neural tube defects study of the Heshun Region. China Int J Geogr Inf Sci. 2010;24:107–27.
Article CAS Google Scholar
Wang J, Hu Y. Environmental health risk detection with GeogDetector. Environ Modell Softw. 2012;33:114–5.
Johansson MA, Cummings DAT, Glass GE. Multiyear climate variability and dengue—El Niño southern oscillation, weather, and dengue incidence in Puerto Rico, Mexico, and Thailand: a longitudinal data analysis. Rohani P, editor. Plos Med. 2009;6:e1000168.
Li C, Lu Y, Liu J, Wu X. Climate change and dengue fever transmission in China: evidences and challenges. Sci Total Environ. 2018;622–623:493–501.
Colón-González FJ, Sewe MO, Tompkins AM, Sjödin H, Casallas A, Rocklöv J, et al. Projecting the risk of mosquito-borne diseases in a warmer and more populated world: a multi-model, multi-scenario intercomparison modelling study. The Lancet Planetary Health. 2021;5:e404–14.
Cazelles B, Chavez M, McMichael AJ, Hales S. Nonstationary Influence of El Niño on the Synchronous Dengue Epidemics in Thailand. Pascual M, editor. Plos Med. 2005;2:e106.
Thai KTD, Cazelles B, Nguyen NV, Vo LT, Boni MF, Farrar J, et al. Dengue dynamics in Binh Thuan province, southern Vietnam: periodicity, synchronicity and climate variability. Gubler DJ, editor. PLoS Negl Trop Dis. 2010;4:e747.
Sang S, Chen B, Wu H, Yang Z, Di B, Wang L, et al. Dengue is still an imported disease in China: a case study in Guangzhou. Infect Genet Evol. 2015;32:178–90.
Xiao J, Liu T, Lin H, Zhu G, Zeng W, Li X, et al. Weather variables and the El Niño Southern oscillation may drive the epidemics of dengue in Guangdong Province, China. Sci Total Environ. 2018;624:926–34.
Chen S-C, Liao C-M, Chio C-P, Chou H-H, You S-H, Cheng Y-H. Lagged temperature effect with mosquito transmission potential explains dengue variability in southern Taiwan: insights from a statistical analysis. Sci Total Environ. 2010;408:4069–75.
Lu L, Lin H, Tian L, Yang W, Sun J, Liu Q. Time series analysis of dengue fever and weather in Guangzhou. China Bmc Public Health. 2009;9:395.
Banu S, Hu W, Guo Y, Hurst C, Tong S. Projecting the impact of climate change on dengue transmission in Dhaka. Bangladesh Environ Int. 2014;63:137–42.
Luz PM, Mendes BVM, Codeco CT, Struchiner CJ, Galvani AP. Time series analysis of dengue incidence in Rio de Janeiro. Brazil Am J Trop Med Hyg. 2008;79:933–9.
Liyanage P, Tozan Y, Overgaard HJ, Tissera HA, Rocklov J. Effect of El Nino-Southern Oscillation and local weather on Aedes vector activity from 2010 to 2018 in Kalutara district, Sri Lanka: a two-stage hierarchical analysis. Lancet Planet Health. 2022;6:E577–85.
Tipayamongkholgul M, Fang C-T, Klinchan S, Liu C-M, King C-C. Effects of the El Niño-Southern Oscillation on dengue epidemics in Thailand, 1996–2005. BMC Public Health. 2009;9:422.
Oidtman RJ, Lai S, Huang Z, Yang J, Siraj AS, Reiner RC, et al. Inter-annual variation in seasonal dengue epidemics driven by multiple interacting factors in Guangzhou. China Nat Commun. 2019;10:1148.
Meng H, Xiao J, Liu T, Zhu Z, Gong D, Kang M, et al. The impacts of precipitation patterns on dengue epidemics in Guangzhou city. Int J Biometeorol. 2021;65:1929–37.
Paaijmans KP, Blanford S, Chan BHK, Thomas MB. Warmer temperatures reduce the vectorial capacity of malaria mosquitoes. Biol Lett. 2012;8:465–8.
Almeida APG, Baptista SSSG, Sousa CAGCC, Novo MTLM, Ramos HC, Panella NA, et al. Bioecology and Vectorial Capacity of Aedes albopictus (Diptera: Culicidae) in Macao, China, in Relation to Dengue Virus Transmission. J Med Entomol. 2005;42:419–28.
Stewart-Ibarra AM, Lowe R. Climate and non-climate drivers of dengue epidemics in southern coastal ecuador. Am J Trop Med Hyg. 2013;88:971–81.
Lai L-W. Influence of environmental conditions on asynchronous outbreaks of dengue disease and increasing vector population in Kaohsiung. Taiwan Int J Environ Heal R. 2011;21:133–46.
Download references
Acknowledgements
Not applicable.
This work was supported by the U2102208, National Natural Science Foundation of China (42130713, 42071375), Natural Science Foundation of Henan Province (242300421361), Strategic Priority Research Program of the Chinese Academy of Sciences(XDB0740100), Innovation Project of LREIS(O88RA205YA).
Author information
Authors and affiliations.
State Key Laboratory of Resources and Environmental Information System, Institute of Geographical Sciences and Natural Resources Research, Chinese Academy of Sciences, Beijing, China
Chengdong Xu & Jingyi Xu
College of Resources and Environment, University of Chinese Academy of Sciences, Beijing, China
College of Geography and Environmental Science, Henan University, Kaifeng, China
Key Laboratory of Geospatial Technology for the Middle and Lower Yellow River Regions (Henan University), Ministry of Education, Kaifeng, China
You can also search for this author in PubMed Google Scholar
Contributions
XCD conceived and designed the study; XJY performed the experiments, collected and processed the data; XCD, XJY and WL were contributors in writing the manuscript.
Corresponding author
Correspondence to Chengdong Xu .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Xu, C., Xu, J. & Wang, L. Long-term effects of climate factors on dengue fever over a 40-year period. BMC Public Health 24 , 1451 (2024). https://doi.org/10.1186/s12889-024-18869-0
Download citation
Received : 05 October 2023
Accepted : 16 May 2024
Published : 30 May 2024
DOI : https://doi.org/10.1186/s12889-024-18869-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
BMC Public Health
ISSN: 1471-2458
- General enquiries: [email protected]
ORIGINAL RESEARCH article
Clinical characteristics and risk factors for severe dengue fever in xishuangbanna, during the dengue outbreak in 2019.
A correction has been applied to this article in:
Corrigendum: Clinical Characteristics and Risk Factors for Severe Dengue Fever in Xishuangbanna, During the Dengue Outbreak in 2019
- Read correction

- 1 Institute of Medical Biology, Chinese Academy of Medical Sciences, Peking Union Medical College, Kunming, China
- 2 Yunnan Key Laboratory of Vaccine Research & Development on Severe Infectious Diseases, Kunming, China
- 3 Yunnan Key Laboratory of Vector-Borne Infectious Disease, Kunming, China
- 4 Xishuangbanna Dai Autonomous Prefecture People’s Hospital, Jinhong, China
- 5 Kunming Medical University, Kunming, China
Background: Dengue poses a large burden on the public health systems worldwide. severe dengue (SD) could lead to more serious clinical symptoms and even death. This study aimed to identify the cause of SD in a clinical trial during the dengue outbreak in Xishuangbanna in 2019, and could provide new insights into the pathogenic mechanisms of SD.
Methods: Mosquito-borne viral (DENV, JEV, and CHIKV) infections were identified. The epidemiological factors and clinical symptoms of inpatients in Xishuangbanna were recorded. The IgG and IgM levels in the serum of dengue inpatients were evaluated, and secondary infections were identified. Then, the structural proteins (C/PrM/E) were sequenced and compared with those of the same type of DENV in the same area as before, and their structures were predicted by the SWISS-MODEL ( expasy.org ). The full-length viral genomes were sequenced and aligned with representative strains by BioEidt or MEGA 5.0.
Results: In this outbreak, the clinical symptoms were more serious in SD. The proportion of SD inpatients of male and Han nationality was larger than that of dengue fever (DF) inpatients ( p < 0.05). DENV-2 infection was the majority in DF, with 45 inpatients. However, DENV-1 infection was the most common SD, with 54 inpatients. There were 3 DENV-3-positive inpatients in the DF group and 6 ZIKV-positive inpatients in the SD group. A secondary infection accounted for 76.47% (78 cases) of SD inpatients, but secondary infections were only in 20% (17 cases) of DF inpatients. In the three-dimensional structure of protein analysis, the C/PrM/E of DENV-1 and DENV-2 showed more stability than previous epidemic strains, while DENV-3 in 2019 showed a looser spatial structure. After a complete genome sequencing and analysis, all six DENV-2 strains belonged to cosmopolitan, five of which clustered into one branch. The GC/AT of the five strains decreased from 2014 to 2018. Compared with DF strains, SD strains had no mutations of commonness.
Conclusions: SD may related to secondary heteromorphic dengue in Xishuangbanna in 2019. The coinfection of ZIKV could be another related factor for SD. The currently datas were very limited and only suggestive.
Introduction
Dengue virus (DENV) is a mosquito-borne flavivirus that is transmitted by the vectors, Aedes aegypti and Aedes albopictus , and is a vector-borne infectious disease virus ( Hawley et al., 1987 ). Dengue virus is a single stranded, positive RNA virus with an envelope genome of approximately 11 kb. The genome encodes a polyprotein, which is processed into three structural proteins [the capsid (C), premembrane (prM), and envelope (E) protein] and seven non-structural proteins (NS1-NS5) ( Guzman and Harris, 2015 ). There are currently four circulating serotypes (DENV-1 to DENV-4) that exhibit up to 70% sequence homology ( Bhatt et al., 2020 ). The incubation period of dengue virus infection is 4–7 days ( Bhatt et al., 2020 ). The disease spectrum ranges from asymptomatic infection and moderate febrile illness (DF) to more severe dengue (SD), such as dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS; Chaturvedi et al., 2000 ). The clinical symptoms of SD patients mainly include high fever, severe pain in the bones, joints and muscles, headache, skin rash, lymph node enlargement, bleeding, shock and even death ( World Health Organization, 2009 ).
Dengue was listed as a potential threat among ten diseases by the WHO in 2019 ( Norshidah et al., 2021 ). The global incidence has been estimated at 390 million infected individuals each year. In China, no case was reported from 1949 to 1977 until an outbreak occurred in Guangdong Province in 1978 ( Yue et al., 2020 ). In recent years, dengue cases have been reported in almost all provinces (autonomous regions) in China ( Liu, 2020 ). Southeast Asia is an important area for Aedes aegypti and Aedes albopictus and has always been the main epidemic area of dengue disease. Yunnan, as one of the border provinces of China, is adjacent to the Southeast Asian countries Laos, Myanmar and Vietnam. A total of 15,572 dengue cases were recorded in Yunnan Province from 2013 to 2019, as shown in Figure 1 . Dengue cases were concentrated in the border areas, and a total of 8,477 dengue cases were recorded in Xishuangbanna Prefecture (red circle in Figure 1 ), bordering Laos and Myanmar, including 568 imported cases (6.70%) and 7,909 local cases (93.30%) ( Zhang, 2021 ). In Xishuangbanna, few cases of dengue virus infection were reported before 2013. The number of reported dengue virus infections (DENV-3) rose to 1,319 in 2013, 1,132 in 2015 (DENV-2) and 1348 in 2017 (DENV-1). As of November 2019, the number of dengue virus NS1 positive infections exceeded 3,900 ( Zhang et al., 2021 ). With the increase in the number of infections, the number of inpatients with SD increased to 102 in 2019. According to previous reports, 70 of 634 inpatients (11.04%) had SD in 2013 ( Ma et al., 2016 ). Among the 109 inpatients in 2015, 13 (11.9%) had SD ( Cui et al., 2016 ).
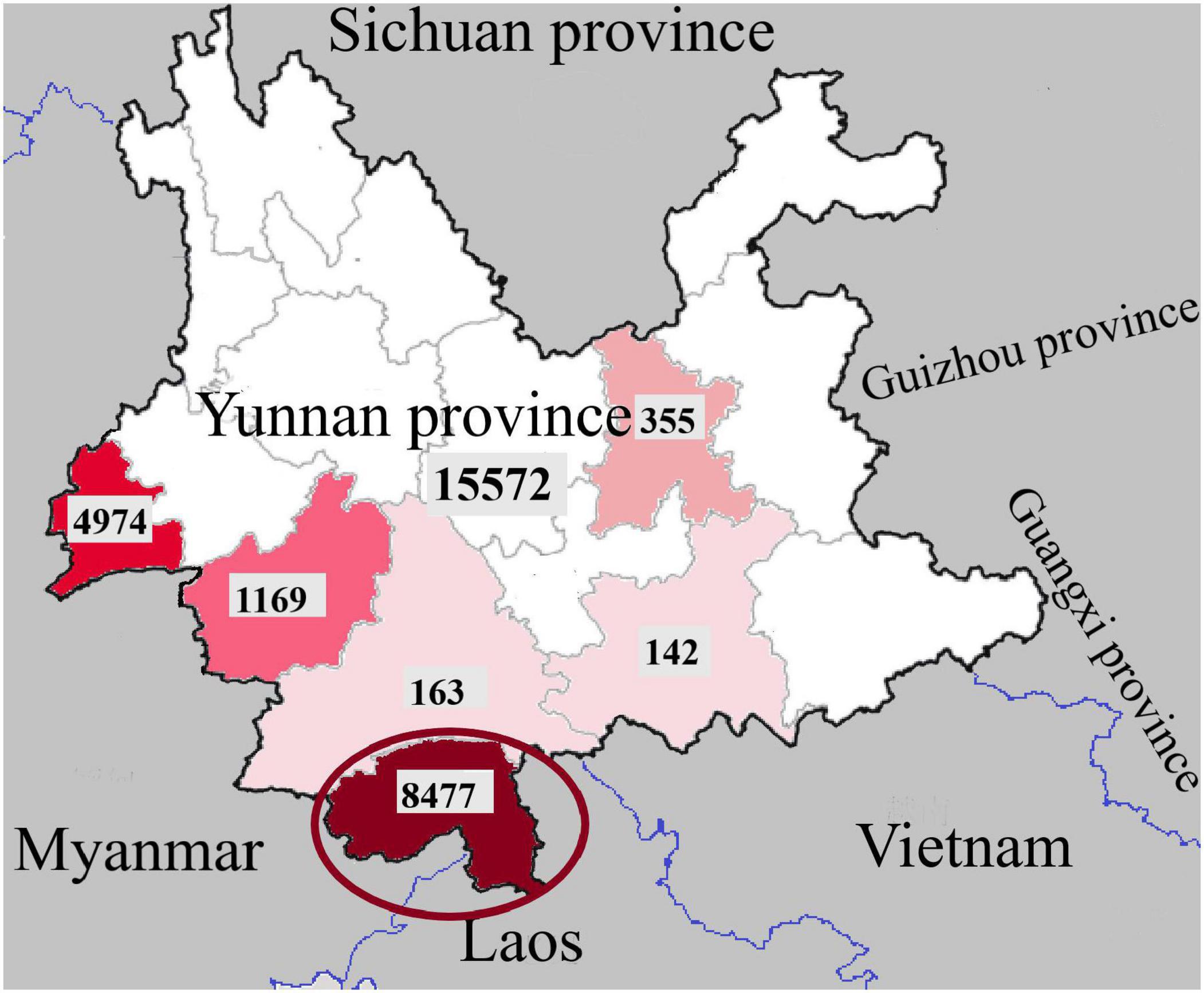
Figure 1. Regional distribution of dengue fever cases in Yunnan Province, China, 2013-2019 (the dates in the picture are from Zhang, 2021 ).
As a more serious form of dengue infection, SD is directly life-threatening. In some areas, the mortality rate of pediatric patients is as high as 5% ( Wang et al., 2016 ). Former studies suggest that age, gender, social status, genetic background, chronic diseases might adversely influence the clinical presentation of dengue infection ( Htun et al., 2015 ). The aim of this article is to study the factors associated with SD. The infection of mosquito-borne viruses [including Zika virus (ZIKV), Japanese encephalitis virus (JEV), and Chikungunya virus (CHIV)] and the serotype of DENV were identified, and the epidemiological factors and clinical symptoms of inpatients in Xishuangbanna were recorded. Then, IgG and IgM in the serum of dengue inpatients were detected, and secondary infections were identified. The structural proteins (C-PrM-E) were sequenced and compared with those of the same type of DENV in the same region. The three-dimensional structure of dengue virus structural proteins was predicted by the SWISS-MODEL ( expasy.org ). Finally, whole genome sequences of 6 inpatients (including 3 SD and 3 DF) were obtained and compared with the sequences of different viruses from different years to detect the homology of the sequence.
Materials and Methods
Study design and participants.
Laboratory-confirmed dengue fever inpatients admitted to the People’s Hospital of Dai Autonomous Prefecture of Xishuangbanna from September to November 2019 were enrolled in this study. Patients were diagnosed based on the Guidelines for the Diagnosis, Treatment, Prevention and Control of Dengue Fever ( World Health Organization, 2009 ). Data on clinical symptoms and laboratory tests were collected for the analysis. Laboratory test data included the white blood cell count (WBC) and the platelet count (PLT). The overall study design is shown in Figure 2 .
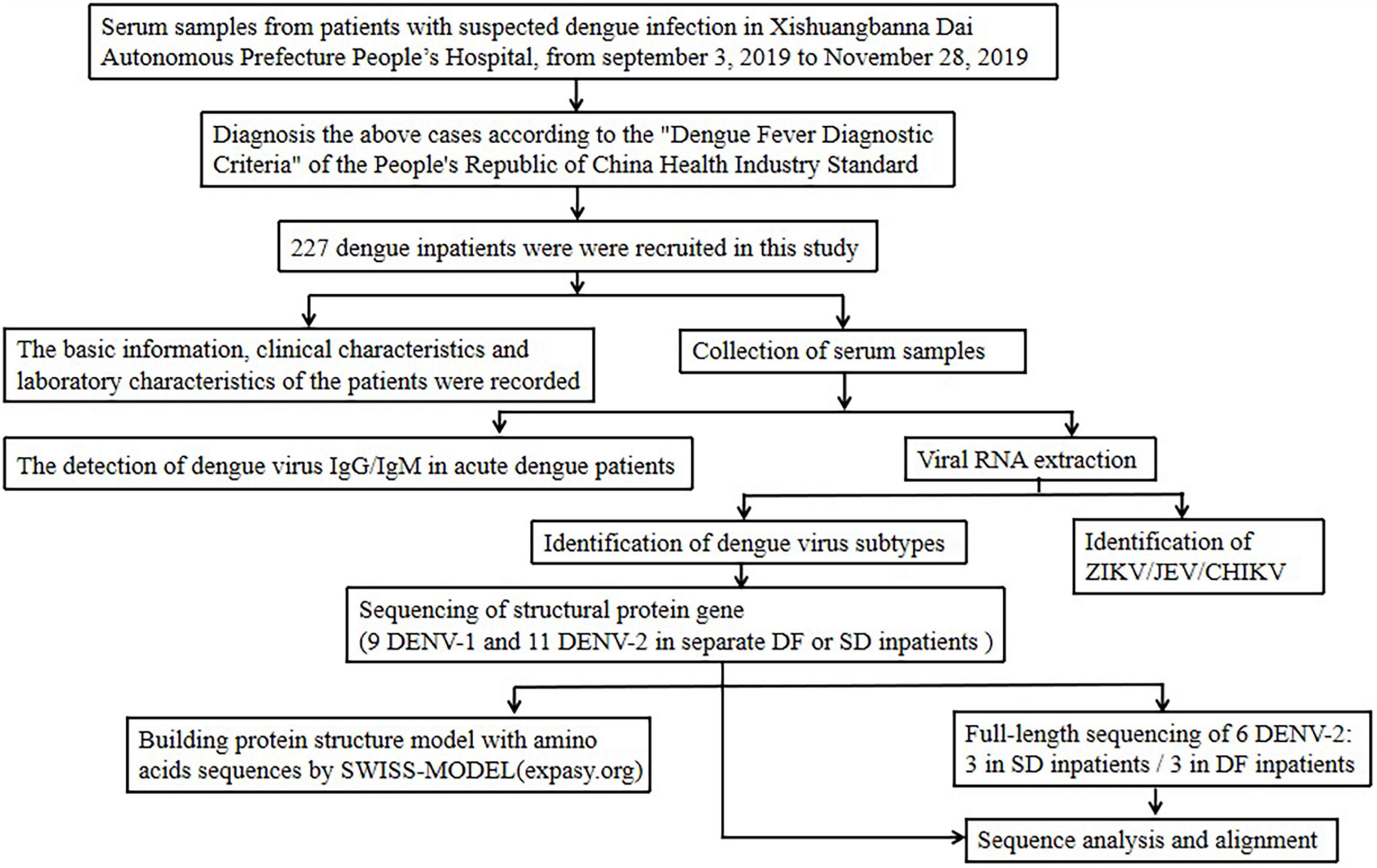
Figure 2. Study design and participants.
Mosquito-Borne Virus Identification
A total of 225 DENV-positive serum samples of inpatients were collected from inpatients in the People’s Hospital of Dai Autonomous Prefecture of Xishuangbanna. Dengue NS1 antigen was detected using a DF NS1 test kit (Blue Cross, Beijing, China). Viral RNA was extracted from 140 μL of serum using a QIAamp Viral RNA Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions and stored at −80°C. Viral RNA was used for PCR the identification of the dengue viral subtypes, Zika virus (ZIKV), Japanese encephalitis virus (JEV) and Chikungunya virus (CHIKV), following the identification of flavivirus. The primers were as Supplementary Table 1 shown ( Wang et al., 2016 ). All dengue virus PCR positive samples were labeled for the next step, which included the amplification of the whole gene or the structural protein nucleic acid sequence.
IgG and IgM Antibodies of DENV Detection
DENV IgG and IgM antibodies were detected by enzyme-linked immunosorbent assay (ELISA) (Order Nr: IB05044 or IB05045, Immuno-Biological Laboratories, Inc., Minneapolis, MN, United States) in 225 DENV-positive serum samples of inpatients, according to the instructions of the manufacturer.
Determination of Primary and Secondary Dengue Virus Infection
The judgment basis of primary and secondary infection was defined as follows ( Wei et al., 2016 ): for specimens taken less than 7 days after the onset, both IgM and IgG antibodies were negative, the judgment could not be made. If the IgM antibody was positive, IgG antibody was negative, the judgment was primary infection. The judgment of secondary infection is that both antibodies are positive, or if IgM antibody was negative, IgG antibody and the DENV RNA are both positive.
Analysis of the Amino Acid Sequence of DENV Structural Proteins (C-PrM-E)
Twenty DENV nucleic acid-positive samples were randomly selected to sequence the nucleic acid sequence of DENV structural proteins and then translated into amino acids with BioEdit 7.0. The C-PrM-E structure was used to build a protein structure model with amino acid sequences by the SWISS-MODEL ( expasy.org ).
Amplification of the Full-Length Genome and Analysis of Isolated DENV Nucleotides
Serum from 3 DF inpatients and 3 SD inpatients was selected for sequencing the full-length genome of DENV, all the six samples were from the same twenty DENV nucleic acid-positive samples used. The primers were used in this study were from our former study ( Jiang et al., 2018 ). Sequences were analyzed using BioEdit 7.0 and compared with sequences available from the BLAST database ( blast.ncbi.nlm.nih.gov/Blast.cgi ). Phylogenetic analyses were performed using the neighbor-joining method with the Tajima-Nei model (MEGA, version 6.0 1 ). The DENV genotype was analyzed using the related reference sequences in NCBI (National Center for Biotechnology Information, Minneapolis, MN, United States) and with known genotypes in the phylogenetic tree. The information of reference sequences were shown in Supplementary Table 2 .
Statistical Analysis
The continuous variables were described by the mean ± standard deviation, and the categorical variables were described by the constituent ratio. Differences or associations with p -values <0.05 were considered significant. All data analyses were performed using SPSS 22.0 software (IBM, Armonk, NY, United States) and GraphPad Prism 7.
Ethical Approval
Institutional Review Board approval was obtained from the Ethics Committee of the Institute of Medical Biology, Chinese academy of Medical Sciences, China. All procedures that were performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Basic Characteristics of Dengue Inpatients
In this study, there were 102 SD patients among the 225 dengue inpatients. Compared with the general dengue patients, the proportions of male and Han nationality inpatients with SD were larger ( p < 0.05) ( Table 1 ). The mean age of DF inpatients was 48.67, the median was 49 (2–97). The youngest is 2 years old and the oldest is 92 years old in DF. The mean age of SD inpatients was 46.14, and the median was 43 (13–88). The youngest is 13 years old and the oldest is 88 years old in SD. The ratio of males to females in SD was 1.76, which was higher than that in DF (0.81). The clinical symptoms were more serious in SD. Compared with DF, the number of low platelet counts (PLT) in SD was greater than that in dengue patients ( p < 0.01). Unexpectedly, other clinical sympt o ms, including fever, vomiting, muscle pain, bleeding, coma, convulsions, and white blood cell counts (WBT), between DF and DHF were no significant differences.
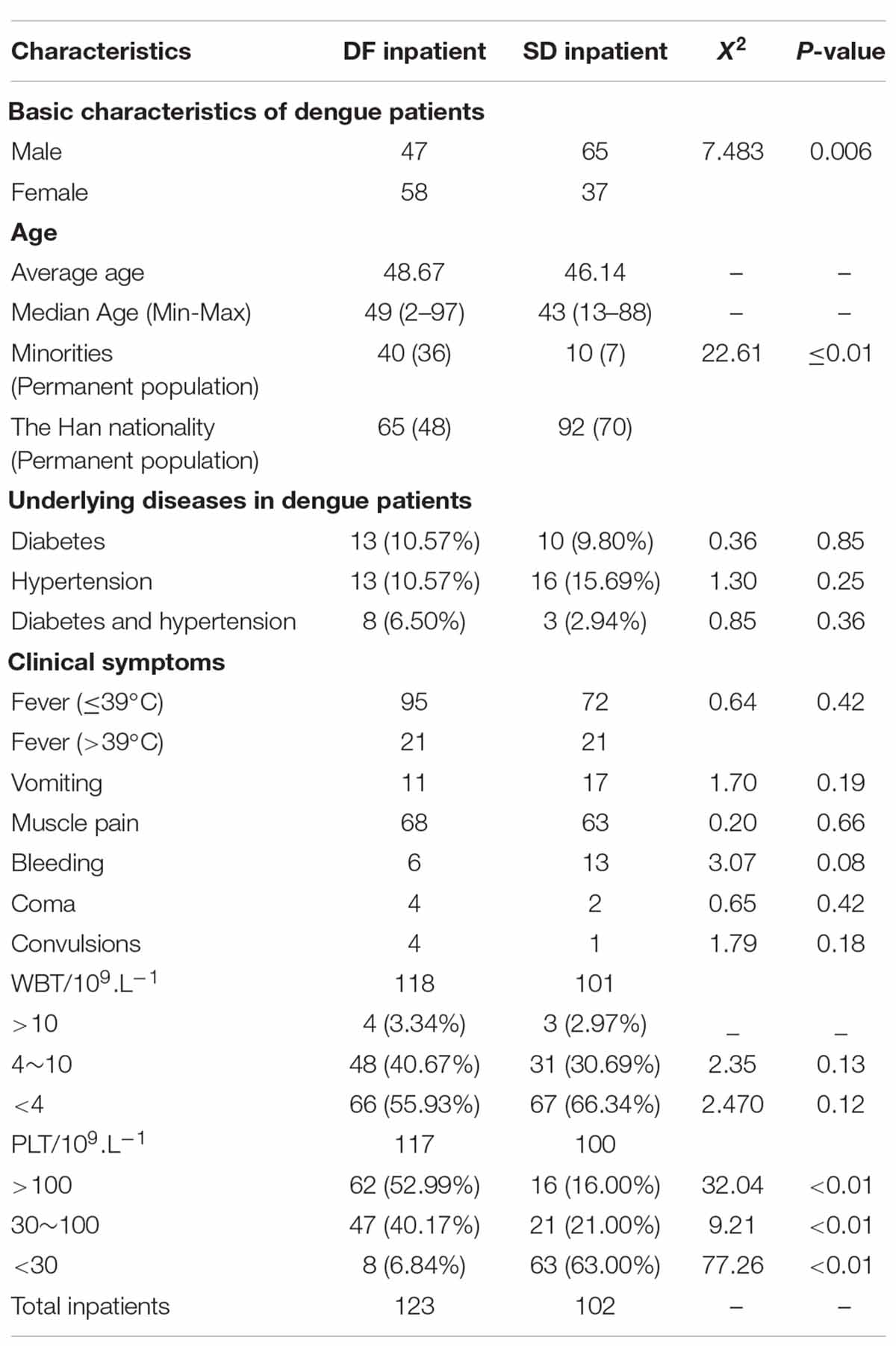
Table 1. Comparison of characteristics of moderate and severe dengue fever inpatients.
A total of 23 diabetics and 29 hypertension patients were found in 225 dengue inpatients. There were 11 inpatients suffering from both two diseases, and 3 of them are SD inpatients. As Table 1 shown, in DF inpatients, 10.57% of patient have diabetes or hypertension, 4.89% have both two diseases. As for SD inpatients, 9.80% of patient have diabetes, 15.69% have hypertension, and only 2.94% have both two diseases. There is no statistical difference between the two groups.
Mosquito-Borne Virus or Virus Coinfection Identification
After nucleic acid samples were extracted and evaluated with specific primers for the presence of different viruses (DENV 1-4, ZIKV, CHIKV, JEV), 54 DENV-1 and 22 DENV-2 were found in 102 samples of sera of SD inpatients. There was one coinfected DENV-1 patient with ZIKV and five coinfected DENV-2 patients with ZIKV. All other viruses were negative. Similarly, among 122 common DF inpatients, there were 19 DENV-1, 45 DENV-2 and 3 DENV-3. The main epidemic serotype of DF inpatients was DENV-2, but the main serotype of SD patients was DENV-1, as shown in Table 2 .
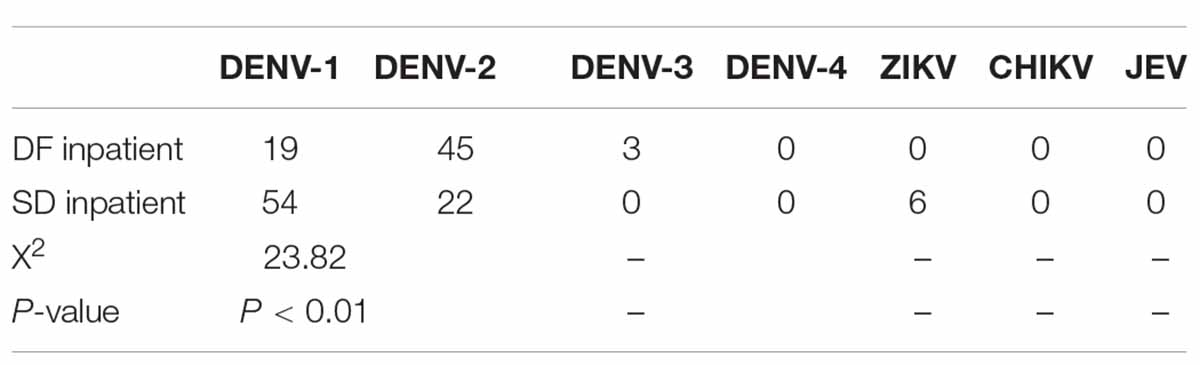
Table 2. Mosquito-borne virus (DENV 1-4, ZIKV, CHIKV, JEV) or virus coinfection identification.
IgG and IgM were detected in 85 DF inpatients, of which 44 were IgM positive and 19 were IgG positive, as shown in Table 3 . In all IgG-positive inpatients, the onset time was less than or equal to 7 days in 17 cases and more than 7 days in 2 cases. IgG and IgM were detected in 95 patients with SD. Among them, 90 patients were IgM positive, and 84 patients were IgG positive. Among all the IgG-positive patients, 78 had an onset time less than or equal to 7 days. According to the judgment basis of primary and secondary infections as previously described in the methods, 78 were secondary infections in SD inpatients and 17 were secondary infections in DF inpatients.
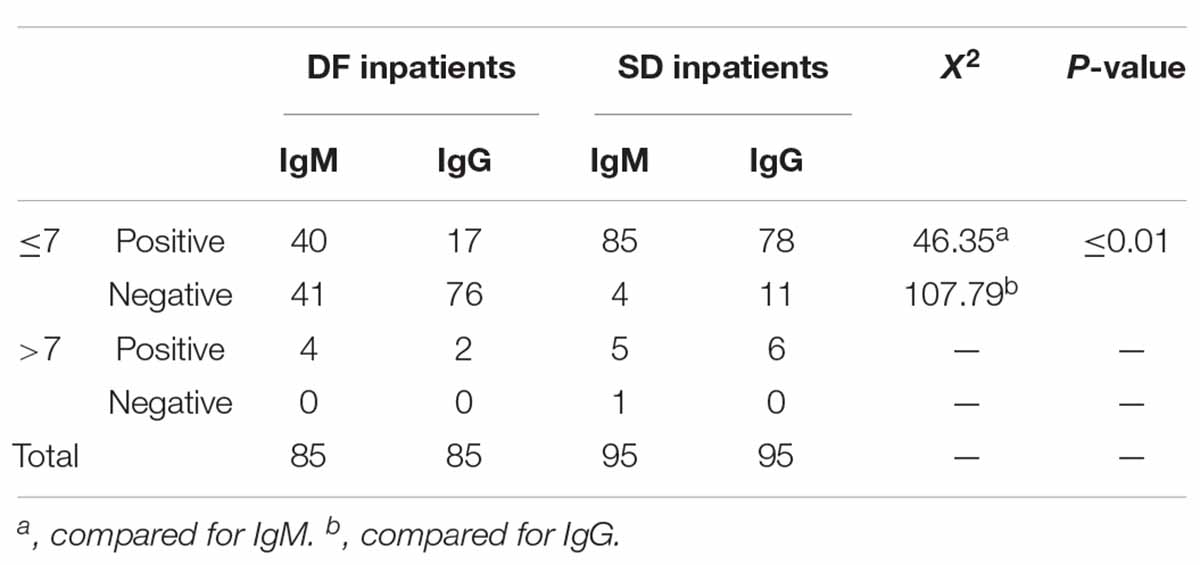
Table 3. IgG and IgM antibodies for DENV detection in dengue inpatients.
Analysis of the Amino Acid Sequence of DENV Structural Proteins (C/PrM/E)
The amino acid sequences of DENV-1, DENV-2 and DENV-3 structural proteins (C/prM/E) were compared by BioEdit. In DENV-1s, compared with the strain KY672931.1 (2015), there were three amino acid mutations of the C protein in strain 5 (2019), from proline(P) to Serine (S), Arginine (R) to Lysine (K) and K to R. Two amino acid mutations were observed in the E protein, including a Leucine (L) to Isoleucine (I), Valine (V) to Alanine (A), and but there were no mutations in the PrM protein. In DENV-2s, compared with strain KY672955.1 (2015), there was one amino acid mutation in the C protein in 15 (2019), K to R. Two amino acid mutations in the PrM protein included K to R, V to A, and one amino acid (L to I) mutation in the E protein. In DENV-3s, compared with strain KR296743.1 (2013), there were two amino acid mutations in the C protein, K to R, asparagine (N) to I, five amino acid mutations in the PrM protein, and ten amino acid mutations in the E protein.
The possible three-dimensional structures of the structural proteins of DENV-1, DENV-2 and DENV-3 in 2019 were later predicted and compared with those of the same type of DENV in Xishuangbanna Prefecture as previously ( Table 4 ). Homology modeling revealed that four strains of DENV-1/DENV-2 had the same three-dimensional structure. However, the two strains of DENV-3 were different. Among the 21 mutation sites in C/prM/E, there were 11 hydrophobic amino acids and 10 hydrophilic amino acids in 2013. However, there were 8 hydrophobic amino acids and 13 hydrophilic amino acids in 2019. The decrease in hydrophobic amino acids in 2019 led to a looser structure than the strain in 2013.
Table 4. The amino acid sequences of DENV-1, DENV-2 and DENV-3 structural proteins (C/prM/E) were compared by BioEdit, and the protein structure was predicted by SWISS-MODEL ( expasy.org ).
Phylogenetic Analysis of Isolated DENV Nucleotide
To analyze whether the sequence is a factor for the severity of dengue patients, we randomly selected three DENV-2 strains from each of the severe and mild patients for whole genome sequencing. After sequencing, nucleic acid and amino acid sequences were analyzed.
In the nucleotide composition analysis, the sequences from DF inpatients (DF10, DF11, and DF15) or SD inpatients (SD9, SD92, and SD106) were compared with other sequences in China from different years, including the strains, MF940237.1 (China Yunnan Province 2015), MN018339.1 (China Guangdong Province 2014), MN018337.1 (China Guangdong Province 2015), MN018340.1 (China Guangdong Province 2016), MN018341.1 (China Guangdong Province 2017), MK783207.1 (China Guangdong Province 2018), and the DENV-2 standard strain NCBI Reference Sequence (NC 001474.2). The results showed that the GC/AT of DENV-2 in China decreased from 2014 to 2018, except the MN018341.1 strain (China Guangdong Province 2017) ( Table 5 ). However, the GC/AT in five of six strains increased in 2019, and the portion rose to the level of 2014. Compared with the other five strains, the GC/AT of DF15 was closer to that of MK783207.1 (China Guangdong Province 2018). Compared with DF strains, SD strains had no mutations of commonness. Although DF15 is different from other virus strains, an evolutionary tree analysis showed that it belongs to the cosmopolitan type, similar to the other five viruses. According to the phylogenetic analysis, DENV-2 of the 2019 dengue outbreak in Yunnan most likely originated from the China Guangdong Province or Thailand, not Yunan Province ( Figure 3 ).
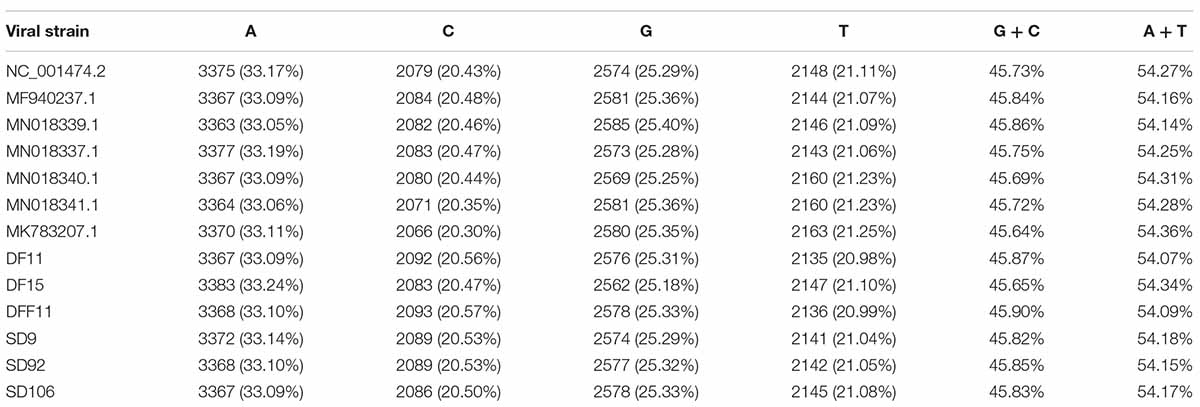
Table 5. Basic information on the DENV-2 sequences was analyzed by BioEdit.
Figure 3. Neighbor-joining phylogenetic tree generated using the nucleotide sequences of complete dengue virus sequences. Study sequences are labeled in black triangles or hollow circles. Others are standard sequences, including sequences of the DENV-2 subgenotype retrieved from the NCBI GenBank. Phylogenetic trees were constructed by the neighbor-joining method and the Kimura 2-parameter model by the MEGA package.
Dengue poses a large burden on the public health systems worldwide. Due to the lack of an ideal animal model, the pathogenesis of dengue has not yet been elucidated. SD is currently believed to be mainly related to secondary heteromorphic DENV infection, coinfection of mosquito-borne viruses, viral variation and host immune response ( Simmons et al., 2012 ; St. John et al., 2013 ). The purpose of this study was to investigate the clinical characteristics and risk factors for severe dengue fever in Xishuangbanna, during the dengue outbreak in 2019.
As a more serious consequence of dengue, SD patients often have more serious clinical symptoms. However, in this outbreak, the only significant difference between SD and dengue patients is the platelet counts. The number of low platelet counts in SD was greater than that in dengue patients ( p < 0.01). But there were no significant differences between DF and DHF in other clinical symptoms. The small sample size was the main reason for those results and the basic characteristics of certain groups were also very important for the pathogenesis of SD. In South America, Southeast Asia and other countries, SD is considered to occur in children and infants ( Sharp et al., 2017 ). However, in this study, there were only two SD inpatients (13 and 18 years old) younger than 18 years old. The mean ages of SD and DF were 46.14 and 48.67, respectively, which were not significantly different. Compared with DF inpatients, SD inpatients were more likely to be male and of Han nationality ( p < 0.05). The formation of SD is not related to age but is related to gender. The reason may be that the spread of dengue is related to the population mobility. Compared with females, males have a larger proportion of migrant workers and are more vulnerable to mosquito bites, which are more likely to lead to SD.
In 2019, there were 22599 cases of dengue fever, with an incidence rate of 1.63/10 million ( Liu, 2020 ). As a typical dengue epidemic area, in 2019, the main epidemic type in Xishuangbanna Prefecture was DENV-1, with an incidence rate of up to 67%. DENV-2 accounted for 32%, and only one patient had DENV-3, which was consistent with our assumption that the epidemic trend was dengue virus. Although DENV-1 was prevalent in Xishuangbanna in 2017, DENV-1 was still prevalent in Xishuangbanna in 2019. According to former studies, DENV-2 and DENV-3 are more likely to cause SD than DENV-1 and DENV-4 ( Fried et al., 2010 ). Among the first infections of this outbreak, 6 SD inpatients were infected with DENV-2, and 3 SD inpatients were infected with DENV-1. After infection with one DENV serotype for the first time, the serogroup cross reactive antibody produced by the host usually can only protect from other serotypes of DENV infection for 3–6 months. When the host is reinfected with heterotypic DENV, the E or PrM antibody produced by the first infection causes a subneutralization titer in the body and forms an immune complex with the virus being infected, increasing the infection rate and replication of the virus ( Murphy and Whitehead, 2011 ). Therefore, we compared the nucleic acid sequence of the PrM protein of the DENV strain in 2019 with that of the dengue virus strain in the same area. Compared with the previous same virus strain genotype, little difference was observed in the primary structure of DENV-1 and DENV-2, but the higher structures were the same. DENV-3 had some differences in the primary structure, which led to different higher structures. These results indicated that DENV-1 and DENV-2 may be more stable than DENV-3. Previous epidemiological studies have shown that the outbreak of DENV-3 and DENV-2 occurred earlier than that of DENV-1 and was more able to lead to subneutralization titers in first infected people. Thus, DENV-1 should be the main serotype in subsequent secondary infections. Among the secondary infection SD inpatients in this study, 50 (%) were DENV-1 and 13 (%) were DENV-2, which was consistent with our study.
ZIKV, CHKIV, and JEV are also transmitted by the mosquito. When there were multiple arboviruses in one place at the same time, humans may be infected with different types of arboviruses at the same time through mosquito bites. In this study, we compared the coinfection of ZIKV, CHIKV, and JEV in SD or DF to explore whether coinfection can lead to an increase in SD. After detection, six inpatients were infected with ZIKV in the SD group, and 0 were infected in the DF group. However, the clinical symptoms of these six coinfected inpatients were not obviously different from those of other SD patients. These results suggested that coinfection may not lead to an aggravation of the symptoms. Unfortunately, among the six ZIKV infected patients, the serum collection time of five of them is longer than 7 days, and the possibility of secondary dengue infection cannot be ruled out. Due to the small number of coinfections in this cohort, more patients needed to be enrolled, to study the role of ZIKV coinfection in SD patients.
The virulence of viruses could influence the occurrence of SD ( Tuiskunen et al., 2011 ). In this study, we selected three DENV-2 epidemic strains from DF or DHF patients and performed whole genome sequencing and analysis. Compared with the sequences before 2019, the GC/AT in five of six strains increased in 2019. However, there was no regularities of the mutations between SD and DF sequences. The results showed that all the sequences from DF and SD belonged to cosmopolitan, and five of them were in a cluster.
The aim of this study was to investigate the causes of SD through the demographic information of SD patients, the co-infection of mosquito-borne viruses, the identification of DENV serotypes, the presence of DENV secondary infections, and the characteristics of the samples of the DENV complete genomes in Xishuangbanna, 2019 ( Zhang et al., 2021 ). The prevalence of three dengue virus serotypes before 2019 might mediate subneutralization titer antibodies and lead to secondary infections, increasing the number of severe dengue patients in Xishuangbanna in 2019. The results of this study might provide insight into early prognostic factors associated with a severe disease progression and improve the rates of early diagnosis and successful treatment. The currently datas were very limited and only suggestive. More dengue patients should be recruited for those study. More other risk factors, especially environmental factors, the basic situation of patients should be included in those studys.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/ , MZ452990-MZ453011.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Institute of Medical Biology, Chinese academy of Medical Sciences, China. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
XW and QS have drafted and revised the manuscript. XW contributed to the sequencing, major experiment and analysis of data. QS and PL have designed and administered the study. TL, YS, JZ, XS, and DL contributed to sample collection. All other co-authors contributed to its finalization and approval for publication.
This research was supported by the National Natural Science Foundation of China (31970868), the Youth Project in Yunnan Province (2019FD082), the Foundation of Yunnan Innovation Team (202105AE160020), Major Projects and Key Research and Development Plans of Yunnan Province (2019ZF004), and Yunnan health training project of high level talents (L-2019030, H-2017052).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.739970/full#supplementary-material
- ^ http://www.megasoftware.net/
Bhatt, P., Sasidharan, S. P., Varma, M., and Arunkumar, G. (2020). Current understanding of the pathogenesis of dengue virus infection. Curr. Microbiol. 78, 17–32. doi: 10.1007/s00284-020-02284-w
PubMed Abstract | CrossRef Full Text | Google Scholar
Chaturvedi, U. C., Agarwal, R., Elbishbishi, E. A., and Mustafa, A. S. (2000). Cytokine cascade in dengue hemorrhagic fever: implications for pathogenesis. FEMS Immunol. Med. Microbiol. 28, 183–188. doi: 10.1111/j.1574-695X.2000.tb01474.x
Cui, X. G., Li, L. H., Zhou, H. N., Shan, X. Y., and Bai, C. H. (2016). Clinical characteristics of 109 dengue virus type 2 infected cases in Xishuangbanna. Chin. J. Infect. Dis. 34, 231–232.
Google Scholar
Fried, J. R., Gibbons, R. V., Kalayanarooj, S., Thomas, S. J., Srikiatkhachorn, A., Yoon, I.-K., et al. (2010). Serotype-specific differences in the risk of dengue hemorrhagic fever: an analysis of data collected in Bangkok, Thailand from 1994 to 2006. PLoS Negl. Trop. Dis. 4:e617. doi: 10.1371/journal.pntd.0000617
Guzman, M. G., and Harris, E. (2015). Dengue. Lancet 385, 453–465. doi: 10.1016/S0140-6736(14)60572-9
Hawley, W. A., Reiter, P., Copeland, R. S., Pumpuni, C. B., and Craig, G. B. (1987). Aedes albopictus in North America: probable introduction in used tires from northern Asia. Science 236, 1114–1116. doi: 10.1126/science.3576225
Htun, N. S. N., Odermatt, P., Eze, I. C., Boillat-Blanco, N., D’Acremont, V., and Probst-Hensch, N. (2015). Is diabetes a risk factor for a severe clinical presentation of dengue? – review and metaanalysis. PLoS Negl. Trop. Dis. 9:e0003741. doi: 10.1371/journal.pntd.0003741
Jiang, L. M., Ma, D. H., Ye, C., Li, L., Li, X., Yang, J., et al. (2018). Molecular characterization of dengue virus serotype 2 cosmospolitan genotype from 2015 dengue outbreak in Yunnan, China. Front. Cell. Infect. Microbiol. 8:219. doi: 10.3389/fcimb.2018.00219
Liu, Q. Y. (2020). Dengue fever in China: new epidemical trend, challengesand strategies for prevention and control. Chin. J. Vector Biol. Control 31, 1–6.
Ma, D. H., Shan, X. Y., Li, L. H., Li, D., Ma, D., Li, T., et al. (2016). An analysis of clinical features of dengue fever in the border areas of Xishuangbanna, China, Laos and Myanmar. J. Trop. Dis. Parasitol. 14, 231–232.
Murphy, B. R., and Whitehead, S. S. (2011). Immune response to dengue virus and prospects for a vaccine. Annu. Rev. Immunol. 29, 587–619. doi: 10.1146/annurev-immunol-031210-101315
Norshidah, H., Vignesh, R., and Lai, N. S. (2021). Updates on dengue vaccine and antiviral: where are we heading? Molecules 26, 6768. doi: 10.3390/molecules26226768
Sharp, T. M., Tomashek, K. M., Read, J. S., Margolis, H. S., and Waterman, S. H. (2017). A new look at an old disease: recent insights into the global epidemiology of dengue. Curr. Epidemiol. Rep. 4, 11–21. doi: 10.1007/s40471-017-0095-y
Simmons, C. P., Farrar, J. J., Nguyen, V., and Wills, B. (2012). Dengue. N. Engl. J. Med. 366, 1423–1432. doi: 10.1056/NEJMra1110265
St. John, A. L., Abraham, S. N., and Gubler, D. J. (2013). Barriers to preclinical investigations of anti-dengue immunity and dengue pathogenesis. Nat. Rev. Microbiol. 11, 420–426. doi: 10.1038/nrmicro3030
Tuiskunen, A., Monteil, V., Plumet, S., Boubis, L., Wahlström, M., Duong, V., et al. (2011). Phenotypic and genotypic characterization of dengue virus isolates differentiates dengue fever and dengue hemorrhagic fever from dengue shock syndrome. Arch. Virol. 156, 2023–2032. doi: 10.1007/s00705-011-1100-2
Wang, J., Xie, L., Xie, X. L., Jiang, J. Y., Yang, B., Li, C. M., et al. (2016). Dengue vectors and the natural infection in border with Laos, Jiangcheng County, China. Chin. J. Zoonoses 32, 843–849.
Wei, H. Y., Shu, P. Y., and Hung, M. N. (2016). Characteristics and risk factors for fatality in patients with dengue hemorrhagic fever, Taiwan, 2014. Am. J. Trop. Med. Hyg. 95, 322–327. doi: 10.4269/ajtmh.15-0905
World Health Organization (2009). Dengue Guidelines for Diagnosis, Treatment, Prevention and Control R. Geneva: World Health Organization, 14.
Yue, Y. J., Ren, D. S., Liu, X. B., Wu, H. X., Guo, Y. H., Zhou, N., et al. (2020). A study on spatial characteristics and correlations of different types of dengue cases in mainland China, 2014-2018. Chin. J. Vector Biol. Control 31, 517–520.
Zhang, H. L. (2021). Cross-border spread, indigenous transmission, development trend, and control strategy for dengue fever and chikungunya fever in Yunnan Province, China. Chin. J. Vector Biol. Control 32, 12–18.
Zhang, J., Shu, Y., Shan, X. Y., Li, D., Ma, D., Li, T., et al. (2021). Co-circulation of three dengue virus serotypes led to a severe dengue outbreak in Xishuangbanna, a border area of China, Myanmar, and Laos, in 2019. Int. J. Infect. Dis. 107, 15–17. doi: 10.1016/j.ijid.2021.04.010
Keywords : severe dengue fever, IgG, IgM, dengue inpatients, dengue gene sequence
Citation: Wang X, Li T, Shu Y, Zhang J, Shan X, Li D, Ma D, Long S, Pan Y, Chen J, Liu P and Sun Q (2022) Clinical Characteristics and Risk Factors for Severe Dengue Fever in Xishuangbanna, During the Dengue Outbreak in 2019. Front. Microbiol. 13:739970. doi: 10.3389/fmicb.2022.739970
Received: 12 July 2021; Accepted: 25 January 2022; Published: 10 March 2022.
Reviewed by:
Copyright © 2022 Wang, Li, Shu, Zhang, Shan, Li, Ma, Long, Pan, Chen, Liu and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiangming Sun, [email protected] ; Pinghua Liu, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Loading metrics
Open Access
Peer-reviewed
Research Article
Diabetes mellitus as a risk factor for severe dengue fever and West Nile fever: A meta-analysis
Contributed equally to this work with: Hong-Zheng Lu, Yu-Zhuang Xie
Roles Data curation, Methodology, Writing – original draft
Affiliations Department of Pathogen Biology, the Key Laboratory of Microbiology and Parasitology of Anhui Province, the Key Laboratory of Zoonoses of High Institutions in Anhui, School of Basic Medical Sciences, Anhui Medical University, Hefei, China, Department of Epidemiology and Biostatistics, School of Public Health, Anhui Medical University, Hefei, Anhui China
Affiliation Department of Pathogen Biology, the Key Laboratory of Microbiology and Parasitology of Anhui Province, the Key Laboratory of Zoonoses of High Institutions in Anhui, School of Basic Medical Sciences, Anhui Medical University, Hefei, China
Roles Data curation
Roles Data curation, Funding acquisition
Affiliation Department of Tropical Medicine, College of Military Preventive Medicine, Army Medical University, Chongqing, China
Affiliation Department of Endocrinology, The First Affiliated Hospital of Anhui Medical University, Hefei, China
Roles Supervision, Writing – review & editing
* E-mail: [email protected] (FD); [email protected] (D-QW); [email protected] , [email protected] (S-QD)
Affiliation National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention (Chinese Center for Tropical Diseases Research), National Health Commission Key Laboratory of Parasite and Vector Biology; WHO Collaborating Center for Tropical Diseases; National Center for International Research on Tropical Diseases, Shanghai, China
Roles Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing
- Hong-Zheng Lu,
- Yu-Zhuang Xie,
- Chen Gao,
- Ying Wang,
- Ting-Ting Liu,
- Xing-Zhe Wu,
- Fang Dai,
- Duo-Quan Wang,
- Sheng-Qun Deng

- Published: May 31, 2024
- https://doi.org/10.1371/journal.pntd.0012217
- Reader Comments
This is an uncorrected proof.
Dengue fever (DF) and West Nile fever (WNF) have become endemic worldwide in the last two decades. Studies suggest that individuals with diabetes mellitus (DM) are at a higher risk of developing severe complications from these diseases. Identifying the factors associated with a severe clinical presentation is crucial, as prompt treatment is essential to prevent complications and fatalities. This article aims to summarize and assess the published evidence regarding the link between DM and the risk of severe clinical manifestations in cases of DF and WNF.
Methodology/Principal findings
A systematic search was conducted using the PubMed and Web of Science databases. 27 studies (19 on DF, 8 on WNF) involving 342,873 laboratory-confirmed patients were included in the analysis. The analysis showed that a diagnosis of DM was associated with an increased risk for severe clinical presentations of both DF (OR 3.39; 95% CI: 2.46, 4.68) and WNF (OR 2.89; 95% CI: 1.89, 4.41). DM also significantly increased the risk of death from both diseases (DF: OR 1.95; 95% CI: 1.09, 3.52; WNF: OR 1.74; 95% CI: 1.40, 2.17).
Conclusions/Significance
This study provides strong evidence supporting the association between DM and an increased risk of severe clinical manifestations in cases of DF and WNF. Diabetic individuals in DF or WNF endemic areas should be closely monitored when presenting with febrile symptoms due to their higher susceptibility to severe disease. Early detection and appropriate management strategies are crucial in reducing the morbidity and mortality rates associated with DF and WNF in diabetic patients. Tailored care and targeted public health interventions are needed to address this at-risk population. Further research is required to understand the underlying mechanisms and develop effective preventive and therapeutic approaches.
Author summary
In our study, we investigated the association between diabetes mellitus (DM) and the risk of severe clinical manifestations in cases of dengue fever (DF) and West Nile fever (WNF). By analyzing 27 studies involving over 342,000 laboratory-confirmed patients, we found compelling evidence supporting a link between DM and an increased risk of severe complications in both DF and WNF. Moreover, DM was found to significantly raise the risk of mortality from these diseases.
Our findings emphasize the importance of early detection and appropriate management strategies for diabetic individuals residing in endemic areas. Healthcare providers should be vigilant in monitoring diabetic patients with febrile symptoms, as they are more susceptible to developing severe disease. Tailored care and targeted interventions are crucial to minimize the morbidity and mortality rates associated with DF and WNF in diabetic individuals.
These findings have significant implications for public health, highlighting the need for awareness campaigns and preventive measures aimed at diabetic individuals. Further research is needed to understand the underlying mechanisms and develop effective strategies for prevention and treatment. By addressing these issues, we can reduce the impact of DF and WNF on individuals with DM.
Citation: Lu H-Z, Xie Y-Z, Gao C, Wang Y, Liu T-T, Wu X-Z, et al. (2024) Diabetes mellitus as a risk factor for severe dengue fever and West Nile fever: A meta-analysis. PLoS Negl Trop Dis 18(5): e0012217. https://doi.org/10.1371/journal.pntd.0012217
Editor: Christopher M. Barker, University of California, Davis, UNITED STATES
Received: December 11, 2023; Accepted: May 14, 2024; Published: May 31, 2024
Copyright: © 2024 Lu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the manuscript and its Supporting Information files.
Funding: This research was supported by the National Natural Science Foundation of China (82102432 to SQD, 81971971 to YW) and the Anhui Provincial Natural Science Foundation Project (2108085QH347 to SQD). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Flaviviridae are a family of small enveloped viruses comprising four genera: Flavivirus , Pegivirus , Pestivirus , and Hepativirus , which are host-specific and pathogenic, mostly infecting mammals and birds. Clinical signs of flaviviral infection range from asymptomatic to severe or fatal hemorrhagic fever or neurologic disease [ 1 ]. Many flaviviruses are transmitted through the bite of infected arthropod vectors, primarily the Aedes genus and Culex genus. Human-to-human transmission from infected blood and tissues is also possible [ 2 ]. Flaviviruses have caused serious public health problems over the past decades, with epidemics of dengue virus (DENV), Japanese encephalitis virus (JEV), West Nile virus (WNV), Zika virus (ZIKV), and yellow fever virus (YFV) occurring worldwide [ 3 ]. A study of DENV prevalence estimated that 3.9 billion people worldwide are at risk of DENV infection. Over the past 20 years, the number of dengue cases reported to the World Health Organization (WHO) has increased more than sevenfold, from 505,430 in 2000 to more than 2.4 million in 2010 and 5.2 million in 2019 [ 4 , 5 ]. WNV first appeared in the northeastern United States in 1999 and is now distributed throughout most of the United States and southern Canada [ 6 ].
Although most people infected with DENV and WNV present asymptomatically or with undifferentiated febrile illnesses, a small number of infected individuals develop acute fever that may progress to severe clinical manifestations such as hemorrhage, vascular leakage, and encephalitis [ 7 ]. Because dengue fever (DF) and West Nile fever (WNF) are characterized by dynamic clinical changes over time, it is of great practical importance to identify predictive factors that measure the evolution of the disease into severe illness in the early clinical stages [ 8 , 9 ]. Available evidence suggests that age, sex, genetic background, and comorbidities may adversely affect the clinical presentation of the infection [ 9 – 11 ]. However, current knowledge of the risk factors for both diseases is insufficient to predict whether a patient will develop more severe clinical symptoms or even die. Early indicators of dengue progression to a severe stage (including abdominal pain or pressure, bleeding from mucous membranes, liver enlargement of more than 2 centimeters, and erythrocyte pressurization accompanied by a rapid drop in platelet count) are described in the 2009 WHO dengue guidelines [ 12 ]. In areas where the disease has been prevalent for a long time, the monitoring cost of these indicators is high, and unnecessary medical resources may be wasted. In addition, some of the warning signs may appear after the disease has progressed, lacking sensitivity and clinical value [ 13 ]. For WNF, there is also a lack of a clinical presentation that has been shown to be specific enough to predict severe illness, and it is now generally accepted that preexisting chronic conditions such as obesity, asthma, diabetes mellitus (DM), and hypertension are risk predictors of severe illness in WNF [ 14 , 15 ]. Movement disorders (muscle spasms, Parkinson’s syndrome) in patients and multifocal chorioretinitis have also been reported to be predictive of the development of WNF [ 16 , 17 ].
When infection occurs, elucidating the factors influencing disease severity is critical to identifying populations at high risk for severe illness, and effective intervention programmes and individualized clinical surveillance practices should specifically target these populations. DM is a multifaceted disease involving chronic metabolic disorders and immune dysfunction that leads to a wide range of clinical complications [ 18 , 19 ]. Additionally, DM is one of the most common and efficient predictors of potential clinical deterioration of flaviviruses [ 20 , 21 ]. The purpose of this study was to systematically review the available literature on the course of DF and WNF diseases associated with DM, to further determine whether DM promotes flavivirus infections (DENV and WNV) and to assess the magnitude of its role in serious versus nonserious clinical outcomes of disease infections.
Literature search
For this systematic review and meta-analysis, we followed the protocol described in the PRISMA statement [ 22 – 24 ]. We searched two databases (PubMed, Web of Science) to access all relevant published articles as of August 1, 2023 describing the association between DM and DENV and WNV. We used the following keywords: (("diabetes") OR ("mellitus") OR ("glycuresis") OR ("alloxan diabetes") OR ("alloxandiabetes") OR ("maturity-onset diabetes")) AND (("mosquito") OR ("mosquito-borne disease") OR ("MBD") OR ("dengue") OR ("breakbone fever") OR ("DENV") OR ("classical dengues") OR ("West Nile")). Only publications in English were included in this study. Conference abstracts were not included due to the lack of detailed descriptions of the study methods, and thus, the subsequent quality assessment could not be performed.
Inclusion and exclusion criteria
Two investigators (HZ Lu and YZ Xie) reviewed the titles and abstracts independently to identify the potentially eligible studies, for which the full texts were retrieved, and further assessment by reviewing the full text was conducted to identify the eligible studies. All discrepancies were resolved by discussion with the third investigator (C Gao).
The inclusion criteria of the studies in this meta-analysis were as follows: (1) DENV and WNV were clearly defined in the text; (2) both experimental and control groups were patients; and (3) reliable case identification methods were available.
The exclusion criteria were as follows: (1) no available full text or no extracted data; (2) fewer than 3 cases in each study or animal/cell study; (3) data from before 2000; and [ 4 ] when there were multiple publications on the same population or based on overlapping data, the latest or the largest study was included.

Data extraction
The following data were independently extracted from the studies by two investigators (Y Wang and TT Liu): (1) information about the publication (article title, first author, year of publication, year of data, region, study design); (2) data acquisition methods and case identification methods; (3) number of participants in the experiment; and (4) criteria for case definition.
Case definitions
Combining the 1997 and 2009 WHO guidelines for the classification of critical illnesses, we classified dengue progression into two groups [ 5 , 12 ]:
(1) Based on routine clinical data collected by the WHO’s 1997 guidelines, these guidelines classify symptomatic dengue virus infections into three clinical categories: undifferentiated fever, DF, and denguehemorrhagic fever (DHF). DHF was further categorized into four severity levels, of which levels III and IV were defined as dengue shock syndrome (DSS). We refer to DHF/DSS as “severe clinical presentation of dengue”.
(2) Applying the WHO 2009 classification criteria for dengue, a severe dengue case is defined as a suspected dengue patient with one or more of the following diseases: (i) severe plasma leakage that leads to shock (dengue shock) and/or fluid accumulation with respiratory distress; (ii) severe bleeding; and (iii) severe organ impairment.
West Nile fever
We refer to those who developed West Nile Neuroinvasive Disease (WNND) as having a “severe clinical presentation of WNV”, such as cases of WN encephalitis (WNE), WN meningitis (WNM), poliomyelitis or acute flaccid paralysis, WNV-associated retinopathy (WNVR), chorioretinitis or fatal cases [ 15 , 25 – 31 ].
Quality assessment
The qualities of the included studies were assessed by the Newcastle Ottawa Quality Scale (NOS) [ 32 ]. This scale evaluates the quality of the study by 8 questions from three aspects, namely, adequate case definition; representativeness of the cases; selection of controls; definition of controls; comparability of cases and controls on the basis of the design or analysis; ascertainment of exposure; same method of ascertainment for cases and controls; and nonresponse rate. For each trial, the results of the assessment were given. The quality assessment was performed by two investigators independently (LHZ and GC), and discrepancies were resolved by discussion with the third investigator (DSQ).
Statistical analysis
Meta-analyses were performed using Stata 16.0. We used odds ratios as the “primary model” and used random-effects or fixed-effects meta-analysis across all studies. The results were visualized in forest plots. Subgroup analyses were used to address heterogeneity and variability in the dependent variable and age of patients in the control and experimental groups. Heterogeneity between studies was assessed by using the I 2 test, the chi-squared test and forest plots. Heterogeneity was considered statistically significant when the P value < 0.05 or I 2 values > 50% [ 33 , 34 ]. A random-effects model was used when heterogeneity was considered; otherwise, a fixed-effects model was used. In addition, sensitivity analyses were used to assess the robustness.
General characteristics of the included studies
In total, 1700 studies were retrieved from the database in the initial search, of which 278 were considered potentially eligible after reviewing the titles and abstracts. After reading the full text of the articles, 31 articles were eligible, of which 4 studies used the same dataset as the others, and 27 articles were included in this meta-analysis [ 15 , 20 , 25 – 31 , 35 – 52 ]. The processes of study screening are shown in Fig 1 , and the general characteristics of the included studies and the corresponding NOS scores are shown in Tables 1 and 2 . Of these 27 studies, 19 were studies on dengue, 1 study was only able to extract data on deaths [ 49 ], and the remaining 8 were studies on WNF. In addition, most of these studies were case–control studies based on hospital administrative records. Both dengue and WNF were diagnosed by ELISA or RT–PCR, whereas DM was mostly derived from case records or self-reported by patients, and nine of these studies did not report the source of the DM diagnosis. Seven studies on dengue defined severe illness according to the WHO 1997 criteria, 7 studies defined it according to the WHO 2009 criteria, and the remaining studies combined both criteria. Subsequently, we divided the severe cases of both diseases into DHF (8 studies) and DSS (2 studies) according to the WHO 1997 criteria, and the remaining 8 studies used the WHO 2009 criteria and therefore were not included in the subgroup analysis. WNF was classified as WNM (1 study), WNE (2 studies), and WNVR (1 study). Finally, we tried to extract mortality data to analyze the effect of DM on mortality. We extracted 10 studies from 27 studies, 6 studies on dengue and 4 studies on WNF( S1 Table ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pntd.0012217.g001
https://doi.org/10.1371/journal.pntd.0012217.t001
https://doi.org/10.1371/journal.pntd.0012217.t002
Risk of bias assessment
All 31 studies were evaluated by the NOS tool. Five data points scored 9, nine data points scored 8, four data points scored 7, and eight data points scored 6. The findings of the funnel plot were confirmed by Egger’s test, indicating no significant publication bias in the analysis except for the results of two studies on dengue ( P >0.05) ( Table 3 ).
https://doi.org/10.1371/journal.pntd.0012217.t003
Effects of DM on dengue and WNF
Eighteen and 8 studies reported the effect of DM on dengue and West Nile fever severity, respectively. Heterogeneity tests showed a high degree of heterogeneity in the effect of DM on the two diseases, with I 2 ( P ) values of 76% ( P < 0.001) and 66% ( P = 0.003), respectively. Therefore, a random effects model was used to estimate the combined effect of DM on both diseases. The results showed that DM significantly increased the risk of severe dengue and WNF, with ORs of 3.39 (95% CI: 2.46, 4.68) and 2.89 (95% CI: 1.89, 4.41), respectively ( Table 3 and Figs 2 and 3 ). The funnel plot showed publication bias ( P <0.05) on the effect of DM on dengue fever, while there was no publication bias ( P <0.05) on WNF ( Table 3 and S1 , S2 , S3 and S4 Figs). Sensitivity analysis showed a significant change in the study results when two articles, Mallhi (2015) and Mirza (2016), were excluded, and the heterogeneity was reduced from 76% to 47%.
The size of the black square corresponding to each study is proportional to the sample size, and the center of each square represents the OR. Horizontal line shows the corresponding 95% CI of the OR. Pooled OR is represented by hollow diamond.
https://doi.org/10.1371/journal.pntd.0012217.g002
https://doi.org/10.1371/journal.pntd.0012217.g003
We then stratified dengue (DHF, DSS) and WNF (WNM, WNE, WNVR) by disease progression or clinical symptoms to estimate the effect of DM on both. The combined effect of DM on DHF (n = 8) (OR = 2.73, 95% CI: 1.68, 4.44) and DSS (n = 2) (OR = 7.29, 95% CI: 3.09, 17.20) was statistically significant in both classifications of dengue ( Table 3 and Fig 4 ). Among the 3 classifications of WNF, the combined effect of DM on WNE (n = 2) (OR = 3.29, 95% CI: 1.15, 9.40) and WNVR (n = 1) (OR = 11.00, 95% CI: 1.13, 106.84) was statistically significant, whereas WNM (n = 1) (OR = 1.15, 95% CI: 0.38, 3.49) did not show statistical significance ( Table 3 and Fig 5 ). We then extracted mortality data for subgroup analyses to analyze the impact of DM on mortality from both diseases. The results showed a statistically significant combined effect of DM on dengue deaths (n = 6) (OR = 1.95, 95% CI: 1.09, 3.52) and West Nile fever deaths (n = 4) (OR = 1.74, 95% CI: 1.40, 2.17) ( Table 3 and Fig 6 ). With the exception of all studies ( P = 0.006) and DHF ( P = 0.032), the funnel plot did not show publication bias in several stratified studies ( P >0.05) ( S5 , S6 , S7 , S8 , S9 and S10 Figs).
Forest plot of denguehemorrhagic fever (A). Forest plot of dengue shock syndrome (B). The size of the black square corresponding to each study is proportional to the sample size, and the center of each square represents the OR. Horizontal line shows the corresponding 95% CI of the OR. Pooled OR is represented by hollow diamond.
https://doi.org/10.1371/journal.pntd.0012217.g004
Forest plot of West Nile meningitis (A). Forest plot of West Nile encephawlitis (B). Forest plot of West Nile virus-associated retinopathy (C). The size of the black square corresponding to each study is proportional to the sample size, and the center of each square represents the OR. Horizontal line shows the corresponding 95% CI of the OR. Pooled OR is represented by hollow diamond.
https://doi.org/10.1371/journal.pntd.0012217.g005
Forest plot of diabetes mellitus on death of dengu (A). Forest plot of diabetes mellitus on death of West Nile fever (B). The size of the black square corresponding to each study is proportional to the sample size, and the center of each square represents the OR. Horizontal line shows the corresponding 95% CI of the OR. Pooled OR is represented by hollow diamond.
https://doi.org/10.1371/journal.pntd.0012217.g006
In this meta-analysis, we found that having DM was a demographic risk factor for the progression of DF and WNF to severe disease. Additionally, the results of the subgroup analyses showed that differences in the dependent variable (deterioration or death) did not lead to changes in the conclusions.
Our study also suffers from a number of shortcomings that cannot be addressed at this time. Most of the studies selected were retrospective, had different clinical and laboratory diagnostic criteria and control groups and were heterogeneous in terms of exposure and outcome. Some studies only used ELISA for diagnosis, which may result in serological cross-reactivity [ 57 ]. Most patients with DM have other comorbidities (e.g., hypertension, coronary artery disease), and we were unable to assess the impact of DM on the conditions of DF and WNF in isolation. At the same time, the judgment and inclusion criteria of DM in these articles are not clear, and only three studies showed a specific typology of DM (type 2) [ 39 , 43 , 44 ]. The diagnosis of DM mostly comes from case records, and some patients report themselves. It is difficult to distinguish whether DM is diagnosed before, after or during the onset of infection. Based on the literature included, we found that the majority of WNF studies originated in the United States, which may be related to the repeated outbreaks of WNF in North America over the past decade, which have resulted in the continued spread of WNF in communities [ 58 – 60 ]. This strong geographic trend may lead to impeded extrapolation of results. Most of the studies in dengue have adopted the new case classification of the WHO in 2009 to improve case management in the clinical setting. However, a proportion of reports still used the 1997 version, which was developed by the WHO based on a model of DF in Thai children and lacked clarity in the description of the measurement of outcome endpoints [ 61 ].
DM has been listed as a significant predictor of flavivirus-caused disease in the past, and this is supported by the findings of our meta-analysis. The underlying pathophysiologic mechanisms regarding the role of DM in the progression of DF and WNF are not completely clear at this time. Lee et al. found that patients with difficult glycemic control (HbA1c >7%) had a higher risk of dengue exacerbation than diabetic patients with better glycemic control (both without additional comorbidities) [ 44 ]. Hyperglycemia in diabetic patients is thought to lead to immune response dysfunction, with suppression of cytokine production, defective phagocytosis, and immune cell dysfunction, in addition to the risk of natural barrier impairment due to neuropathy [ 62 , 63 ]. This provides more opportunities for viruses to invade. In addition, platelet activity is increased to varying degrees in both type 1 and type 2 diabetic patients [ 64 ]. Platelets can interact with neutrophils to promote their activation and release of platelet factor (CXCL4), which has been shown to significantly inhibit the interferon pathway and enhance DENV replication in cells both in vitro and in vivo [ 65 ]. In addition, there is some biological evidence that can prove that patients with DM who are infected with DENV and WNV are more likely to aggravate infection. A study has shown that blood sugar is conducive to DENV replication, and it promotes virus transmission in mosquitoes through AKT and TOR signaling [ 66 ]. Furthermore, the study reveals that mosquito cells incubated in a high glucose medium exhibit upregulated levels of DENV proteins NS1, NS5, E, and prM, as well as AKT signaling (AKT phosphorylation) and TOR signaling (S6K phosphorylation) [ 66 ]. Some articles have found that monocytes infected with DENV in type 2 DM increase the production of interleukin-4 (IL-4), interleukin-10 (IL-10) and granulocyte-macrophage colony stimulating factor (GM-CSF) [ 67 ]. According to records, T helper (Th) cells play an important role in the immune pathogenesis of DHF [ 68 ]. According to the type of cytokine produced during activation, Th cells are divided into Th1 and Th2 cells. Activated Th1 cells produce IFN-γ (interferon), IL-2 and IL-12, while Th2 cells produce IL-4, IL-5, IL-10 and IL-13 [ 69 ]. Among the various mechanisms of the pathogenesis of DF, it has been reported that in secondarily infected hosts, a high DENV load is indirectly associated with DHF [ 70 ], and the overwhelming activation of Th2 cytokines has been documented in the development of DHF in patients with primary and secondary infections with DF [ 68 , 71 ]. Specifically, in the Th2 cytokine spectrum, IL-4 is the most effective cytokine for inducing Th2 cell differentiation, and IL-10 is responsible for the anti-inflammatory response in host immune activity [ 72 ]. In addition, it has been reported that compared with mild DF patients, the serum GM-CSF of severe DF patients is significantly increased [ 73 ]. Our stratified analysis similarly confirms this observation, with the OR value for DSS (7.29) being significantly higher than that for DHF (2.73).
Mukesh Kumar and others performed experiments on WNV infection in diabetic mouse models. The diabetic mouse model showed a high susceptibility to WNV disease, showing higher tissue tropism and mortality than wild-type mice. This is related to WNV infection and increased inflammation in diabetic mice and severely impaired and delayed specific immune response, which is characterized by delayed induction of IFN-α (interferon), and the concentration of WNV-specific IgM and IgG antibodies decreased in viremia [ 74 ]. Later, they discovered that the presence of DM significantly changed the recruitment of white blood cells in the brain, resulting in failure to clear the WNV infection in the brains of diabetic mice [ 75 ]. These findings are consistent with our study, emphasizing the importance of researching the role of DM in DF and WNF infections, and highlighting that DM could worsen the symptoms and severity of these diseases. Thus, our study further supports the need to focus on DM in the management of DF and WNF infections, as well as the importance of individualized intervention measures for patients with DM.
In addition to dengue and WNF, several studies have shown the effect of DM on Zika virus disease, Japanese encephalitis, and yellow fever. Azar et al. demonstrated increased susceptibility of Aedes aegypti that fed on “diabetic” bloodmeals to ZIKV by in vitro and in vivo modeling of type II DM and suggested that the prevalence of type II DM in the population may have a significant impact on ZIKV transmission [ 76 ]. Ahlers et al. showed that mammalian insulin can trigger AKT and ERK signaling in mosquitoes, leading to the transcription of JAK/STAT-associated antiviral genes [ 77 ]. DM was one of the most common comorbidities in the study patients (9.94%), and patients with comorbid JEV had higher medical costs than patients without DM [ 78 ]. Studies have shown that JEV comorbid with DM significantly increased the risk of death by 2.47 times ( P <0.05) [ 74 ]. Our results indicate that diabetes has a statistically significant combined impact on dengue fever mortality (OR = 1.95) and West Nile fever mortality (OR = 1.74). Patients with YFV and DM had a higher case fatality rate (CFR) of 80% compared with 65% in patients without DM [ 79 ]. In addition, DM attenuates the YFV vaccine effect by reducing 2’,5’-oligoadenylate synthase levels. Basal 2’,5’-oligoadenylate activity increased several-fold in response to YFV vaccination. In DM subjects, this increase was significantly lower ( P = 0.025) [ 80 ]. Based on these reports, DM can be shown to increase the risk of adverse outcomes of mosquito-borne flavivirus infection [ 14 , 76 , 80 ].
Overall, studying DM for DF and WNF infections is important to reduce the burden of disease by guiding approaches to improve patient prognosis or differential case management. We provide evidence that the prevalence of DM is higher in severe cases of dengue and WNF infection than in nonsevere cases. This means that DM may exacerbate the symptoms of DF and WNF infections. A further study with more focus on DM, DENV and WNV is therefore suggested. Examples include longitudinal studies of DM, DF and WNF, i.e., the effect of blood glucose concentration on the clinical symptoms of the disease. In addition, standardized prospective cohort studies in areas with high rates of infection will help to better understand the etiological role of DM in serious disease outcomes and to evaluate the causal relationship between them. This study can also provide some warnings for doctors who have DENV or WNV patients. For example, when a DENV or WNV patient with DM appears, the doctor should promptly decide whether it needs close observation, adequate treatment or hospitalization, and when a patient with DF has severe clinical symptoms, the doctor should promptly ask about past medical history, especially the history of DM. Patients with DM living in areas with high rates of DENV and WNV infection should be given a higher level of attention after diagnosis.
Supporting information
S1 fig. egger’s test of dengue..
https://doi.org/10.1371/journal.pntd.0012217.s001
S2 Fig. Trim and fill analysis of dengue.
https://doi.org/10.1371/journal.pntd.0012217.s002
S3 Fig. Egger’s test of West Nile fever.
https://doi.org/10.1371/journal.pntd.0012217.s003
S4 Fig. Trim and fill analysis of West Nile fever.
https://doi.org/10.1371/journal.pntd.0012217.s004
S5 Fig. Egger’s test of dengue hemorrhagic fever.
https://doi.org/10.1371/journal.pntd.0012217.s005
S6 Fig. Trim and fill analysis of dengue hemorrhagic fever.
https://doi.org/10.1371/journal.pntd.0012217.s006
S7 Fig. Egger’s test of death of dengue.
https://doi.org/10.1371/journal.pntd.0012217.s007
S8 Fig. Trim and fill analysis of death of dengue.
https://doi.org/10.1371/journal.pntd.0012217.s008
S9 Fig. Egger’s test of death of West Nile fever.
https://doi.org/10.1371/journal.pntd.0012217.s009
S10 Fig. Trim and fill analysis of death of West Nile fever.
https://doi.org/10.1371/journal.pntd.0012217.s010
S1 Table. Data collection of the studies included in the meta-analysis.
https://doi.org/10.1371/journal.pntd.0012217.s011
- View Article
- Google Scholar
- PubMed/NCBI
- 12. WHO. Dengue guidelines for diagnosis t, prevention and control: new edition. Geneva: World Health Organization, 2009. [cited August 1, 2023]. Available from: https://www.who.int/publications/i/item/9789241547871
- 17. Abroug F, Ouanes-Besbes L, Letaief M, Romdhane FB, Khairallah M, Triki H, et al., editors. A cluster study of predictors of severe West Nile virus infection. Mayo Clinic Proceedings; 2006: Elsevier.
Dengue fever, once confined to the tropics, now threatens the U.S.
M eg Norris was traveling in Argentina in April when the first signs of dengue fever hit her. The weather in Salta, just south of the Bolivian border, was warm, but Norris, a 33-year-old from Boulder, Colorado, zipped a fleece sweatshirt around her body to stop herself from shivering.
“I thought it was sun poisoning,” she said.
She woke that night in a sweat and spent the hours alternately burning up then freezing. In the morning, her eyes were sore and her lymph nodes were swollen. For the following week, there was nothing to do but sleep, stay hydrated and wait for the body aches that give the illness the moniker “break-bone fever” to pass.
Latin America is experiencing its worst dengue fever outbreak on record. Case numbers in the first 4 ½ months of 2024 are already 238% higher than they were by this time last year, which itself ended with a record 4.1 million cases, according to the Pan American Health Organization . Cases are more than 400% higher than the five-year average.
An unusually wet and warm summer season brought by the El Niño weather pattern has created ideal conditions for the mosquitoes that spread dengue to hatch en masse and carry higher amounts of the virus.
Experts warn this could be a preview of what dengue fever will look like in the future. Climate change is creating unusually balmy conditions, which are already expanding the range of mosquito-borne diseases.
“That’s concerning for places where dengue hasn’t occurred before in recent history: North America and Europe,” said Dr. Albert Ko, a professor of epidemiology of microbial diseases at the Yale School of Public Health.
Dengue is a viral fever caused by four different viruses and spread through mosquito bites. It’s common in many tropical regions across the globe, but has begun to appear in more temperate climates. The mosquitoes that carry dengue fever, Aedes aegypti are now regularly found in the southern parts of the U.S., but recently, the insects have been found as far north as the Bay Area and Washington, D.C. One 2019 study predicted an additional 2 billion people will be at risk for dengue fever by 2080.
“We are definitely worried,” Ko said.
Why are dengue cases rising around the world?
Dengue outbreaks have historically occurred in the Americas every three to four years, said Dr. Gabriela Paz-Bailey, dengue branch chief in the division of vector-borne diseases at the Centers for Disease Control and Prevention. “But now we are seeing them every year,” she said.
Part of the reason for that is tied to climate change.
A warming climate expands the mosquitoes’ habitat and allows them to breed all year long, rather than only in the warmer months. The hotter temperatures also cause the viruses to replicate faster, meaning mosquitoes end up carrying many more viral copies, increasing the likelihood that a person will become infected if bitten.
“We are also seeing dengue cause outbreaks at times when they usually don’t occur,” Ko said.
South America’s dengue cases weren’t just unusually high this year, but they also came unusually early in the season. Similarly, Puerto Rico, a place where dengue outbreaks can occur in the summer and fall, declared a public health emergency in late March after the U.S. territory was overtaken by dengue fever cases and more than 400 people were hospitalized .
In recent years, the epidemic has spread to parts of southern Brazil and northern Argentina, where dengue hasn’t previously been a big problem, Ko said.
“That gives us a snapshot of what we may see here in North America in the coming decades,” Ko said.
How would dengue get a foothold in the U.S.?
The fact that Aedes aegypti mosquitoes are found in places outside their normal range doesn’t mean the mosquitoes are carrying dengue viruses, but those first insects are a warning of what may be to come, Ko added
Locally transmitted dengue fever infections — meaning the infected person didn’t get sick abroad — are still rare in the continental U.S., but have recently been seen for the first time in some states. Last October, California health officials reported the state’s first case of locally transmitted dengue in Pasadena. Local transmission has also occurred in Arizona, Florida and the southern coast of Texas. Last summer brought record-breaking heat waves to Europe, where cases of local dengue transmission were seen in France, Italy and Spain.
“I think this means dengue will become more common,” said Paz-Bailey, adding that the main concern is still the significant increase in cases where the virus is already endemic.
This summer, she does not expect to see significant dengue outbreaks on the U.S. mainland, but she said there is likely to be some people who travel to regions that have higher-than-usual cases and bring the virus back home.
“Travel-associated cases do result in small chains of outbreaks,” Paz-Bailey said.
Humans are reservoirs for dengue, so in order to have widespread transmission, enough people must be infected for the mosquitoes to reliably bite someone with the virus so that they can spread it to another person.
“That’s why we’re seeing an outbreak of dengue in Puerto Rico right now,” said Michael von Fricken, director of the One Health Center of Excellence at the University of Florida in Gainesville. “They’ve reached this tipping point where there are enough infected humans that they’re subsequently infecting other mosquitoes that are continuing to transmit disease.”
Florida has logged 176 dengue cases so far this year, the vast majority in people who were infected in other countries, most frequently Brazil or Cuba. The Florida Health Department has recorded only seven cases of locally transmitted dengue transmission in the state so far this year. In all of 2023, the department documented 173 locally transmitted cases, most of them in Miami-Dade County.
What are the symptoms of dengue fever?
Dengue fever is caused by four viruses, so a person can be infected four times in their lifetime. Only about 1 in 4 people are symptomatic the first time they’re infected, according to the CDC.
Ko said a person’s initial symptoms are usually a fever and headaches. Fatigue, nausea, vomiting, a rash that looks like measles, as well as the extremely painful body aches.
Most people recover in a week or two, but about 1 in 20 people develop severe dengue, which can be fatal. The more times a person is infected with dengue, the higher risk they are for complications.
“After you’ve had your first exposure, your risk of having dengue hemorrhagic fever or severe symptoms increases exponentially,” Von Fricken said. Dengue also becomes deadlier with each infection.
While the U.S. does have a dengue vaccine, it’s approved only for children ages 9 to 16 who live in places where dengue is endemic, including Puerto Rico, American Samoa or the U.S. Virgin Islands.
What’s more, children can get the vaccine only if they’ve previously had a dengue infection. That’s because if a person were to get vaccinated and then get their first dengue infection, they still run the risk of getting very sick, just as someone gets sicker from their second infection. Since most Americans have not had dengue, “that vaccine is not very useful” for most, Ko said.
There’s no specific drug to treat dengue. Instead, doctors just aim to treat the symptoms and keep the patient comfortable until the virus runs its course. That means resting and drinking a lot of fluids. Ko said people should try to take acetaminophen (Tylenol) for pain and fever if they can, since nonsteroidal anti-inflammatory drugs (NSAIDs), which include ibuprofen and aspirin, can make bleeding worse if someone develops hemorrhagic dengue, in which their blood vessels are damaged and become leaky.
Paz-Bailey said it’s important for people traveling to places with dengue to stay in places with air conditioning when possible, use insect-repellant and wear long sleeves and pants to avoid mosquito bites.
Bed nets can be helpful, but the mosquitoes that carry dengue typically bite during the day, so they may be less helpful than they are at preventing other mosquito-borne diseases like malaria, Ko said.
At home, people can make their yards less appealing to mosquitos by reducing the amount of standing water, especially after a bout of rain.
“It’s difficult to control the mosquito population, so we need to hit it with all we have and layer our strategies,” Paz-Bailey said. “No single strategy will be good enough.”
This article was originally published on NBCNews.com

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- PLoS Negl Trop Dis
- v.12(7); 2018 Jul

Dengue infection in India: A systematic review and meta-analysis
Parasuraman ganeshkumar.
1 Department of Epidemiology, National Institute of Epidemiology, Chennai, Tamil Nadu, India
Manoj V. Murhekar
Veeraraghavadoss poornima.
2 School of Public Health, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India
Velusamy Saravanakumar
Krishnendu sukumaran, anandan anandaselvasankar.
3 Campbell Collaboration, New Delhi, India
Sanjay M. Mehendale
4 Division of Epidemiology and Communicable Diseases, Indian Council of Medical Research, New Delhi, India
Associated Data
All relevant data are within the paper and its Supporting Information files.
Introduction
Dengue is the most extensively spread mosquito-borne disease; endemic in more than 100 countries. Information about dengue disease burden, its prevalence, incidence and geographic distribution is critical in planning appropriate control measures against dengue fever. We conducted a systematic review and meta-analysis of dengue fever in India
We searched for studies published until 2017 reporting the incidence, the prevalence or case fatality of dengue in India. Our primary outcomes were (a) prevalence of laboratory confirmed dengue infection among clinically suspected patients, (b) seroprevalence in the general population and (c) case fatality ratio among laboratory confirmed dengue patients. We used binomial–normal mixed effects regression model to estimate the pooled proportion of dengue infections. Forest plots were used to display pooled estimates. The metafor package of R software was used to conduct meta-analysis.
Of the 2285 identified articles on dengue, we included 233 in the analysis wherein 180 reported prevalence of laboratory confirmed dengue infection, seven reported seroprevalence as evidenced by IgG or neutralizing antibodies against dengue and 77 reported case fatality. The overall estimate of the prevalence of laboratory confirmed dengue infection among clinically suspected patients was 38.3% (95% CI: 34.8%–41.8%). The pooled estimate of dengue seroprevalence in the general population and CFR among laboratory confirmed patients was 56.9% (95% CI: 37.5–74.4) and 2.6% (95% CI: 2–3.4) respectively. There was significant heterogeneity in reported outcomes (p-values<0.001).
Conclusions
Identified gaps in the understanding of dengue epidemiology in India emphasize the need to initiate community-based cohort studies representing different geographic regions to generate reliable estimates of age-specific incidence of dengue and studies to generate dengue seroprevalence data in the country.
Author summary
Dengue fever, an extensively spread mosquito-borne disease, is endemic in more than 100 countries. Information about dengue disease burden, its prevalence and incidence and geographic distribution is necessary to guide in planning appropriate control measures including the dengue vaccine that has recently been licensed in a few countries. We performed a systematic review and meta-analysis of published studies in India on dengue. The overall estimate of the prevalence of laboratory confirmed dengue infection based on testing of more than 200,000 clinically suspected patients from 180 Indian studies was 38.3%. The pooled estimate of dengue seroprevalence in the general population and CFR among laboratory confirmed dengue patients was 56.9% and 2.6% respectively. There were no community-based studies reporting incidence of dengue. Our review also identified certain knowledge gaps about dengue epidemiology in the country. Identified gaps in the understanding of dengue epidemiology in India emphasize the need to initiate community-based cohort studies representing different geographic regions to generate reliable estimates of age-specific incidence of dengue and studies to generate dengue seroprevalence data in the country.
Dengue is the most extensively spread mosquito-borne disease, transmitted by infected mosquitoes of Aedes species. Dengue infection in humans results from four dengue virus serotypes (DEN-1, DEN-2, DEN-3, and DEN-4) of Flavivirus genus. As per the WHO 1997 classification, symptomatic dengue virus infection has been classified into dengue fever (DF), dengue haemorrhagic fever (DHF) and dengue shock syndrome (DSS). The revised WHO classification of 2009 categorizes dengue patients according to different levels of severity as dengue without warning signs, dengue with warning signs (abdominal pain, persistent vomiting, fluid accumulation, mucosal bleeding, lethargy, liver enlargement, increasing haematocrit with decreasing platelets) and severe dengue [ 1 , 2 , 3 ]. Dengue fever is endemic in more than 100 countries with most cases reported from the Americas, South-East Asia and Western Pacific regions of WHO [ 1 ]. In India, dengue is endemic in almost all states and is the leading cause of hospitalization. Dengue fever had a predominant urban distribution a few decades earlier, but is now also reported from peri-urban as well as rural areas [ 4 , 5 ]. Surveillance for dengue fever in India is conducted through a network of more than 600 sentinel hospitals under the National Vector Borne Disease Control Program (NVBDCP) [ 6 ], Integrated Disease Surveillance Program (IDSP) [ 7 ] and a network of 52 Virus Research and Diagnostic Laboratories (VRDL) established by Department of Health Research [ 8 ]. In 2010, an estimated 33 million cases had occurred in the country [ 9 ]. During 2016, the NVBDCP reported more than 100,000 laboratory confirmed cases of dengue [ 6 ]. It is therefore possible that dengue disease burden is grossly under-estimated in India.
High dengue disease burden and frequent outbreaks result in a serious drain on country’s economy and stress on the health systems. In India, case detection, case management, and vector control are the main strategies for prevention and control of dengue virus transmission [ 6 ]. A new dengue vaccine is now available and several vaccines are in the process of development [ 10 , 11 , 12 ]. Information about dengue disease burden, its prevalence, incidence and geographic distribution is necessary in decisions on appropriate utilization of existing and emerging prevention and control strategies. With this background, we conducted a systematic review and meta-analysis to estimate the disease burden of dengue fever in India. We also reviewed serotype distribution of dengue viruses in circulation, and estimated case fatality ratios as well as proportion of secondary infections.
Search strategy and selection criteria
This systematic review is registered in PROSPERO (Reg. No. CRD 42017065625). We searched Medline (PubMed), Cochrane Central, WHOLIS, Scopus, Science Direct, Ovid, Google Scholar, POPLINE, Cost-Effectiveness Analysis (CEA) Registry and Paediatric Economic Database Evaluation (PEDE) databases for articles published up to 2017. The main search terms included incidence, prevalence, number of reported cases, mortality, disease burden, cost of illness, or economic burden of dengue in India. The complete search strategy is described in S1 Appendix . Back referencing of included studies in bibliography was also done to identify additional studies.
Review approach
The search results were initially imported to Zotero software (Version 4.0.29.5) and duplicate records were removed. During title screening, we examined relevant studies from various databases. Our inclusion criterion was studies reporting dengue infection in India, not restricted to setting, design, purpose and population. Titles thus selected were subjected to abstract screening. Studies were considered eligible for further examination in full text if their abstracts reported incidence, prevalence, number of reported cases, mortality or the burden of dengue fever anywhere in India. Studies reporting complications of dengue, serotype details of dengue virus as well as seroprevalence of dengue were also included. Using a pre-designed data extraction form, two reviewers extracted details from selected studies independently. The data, which differed between the reviewers, were resolved by consensus. Information about the year of publication, study setting (hospital/laboratory based, or community-based), study location, study period, laboratory investigations, number of suspected patients tested and positives, age distribution of cases, and details of dengue serotypes were abstracted ( S1 Dataset ).
The primary outcome measures of interest were (a) prevalence (proportion) of laboratory confirmed dengue infection among clinically suspected patients in hospital/laboratory based or community-based studies, (b) seroprevalence of dengue in the general population and (c) case fatality ratio among laboratory confirmed dengue patients. The diagnosis of acute dengue infection among the clinically suspected patients was based on any of the following laboratory criteria: (a) detection of non-structural protein-1 (NS1) antigen, (b) Immunoglobulin M (IgM) antibodies against dengue virus (c) haemagglutination inhibition (HI) antibodies against dengue virus, (d) Real-time polymerase chain reaction (RT-PCR) positivity or (e) virus isolation. Seroprevalence of dengue was based on detection of IgG or neutralizing antibodies against dengue virus. Studies providing prevalence (proportion) of laboratory confirmed dengue infection among clinically suspected patients were classified into (a) hospital/laboratory-based surveillance studies and (b) outbreak investigations or hospital/laboratory-based surveillance studies when the outbreak was ongoing in the area, as mentioned in the original research paper. Studies regarding outbreak investigations considered an increase in number of reported cases of febrile illness in a geographical area, as the criteria for defining an outbreak. The outbreak investigations included one or more of the following activities: active search for case-patients in the community, calculation of attack rates for suspected case-patients, confirmation of aetiology and entomological investigations. For the case fatality ratio, the numerator included reported number of deaths due to dengue and denominator as laboratory confirmed dengue patients.
Our secondary outcomes of interest were the following: (a) proportion of primary and secondary infections among the laboratory confirmed dengue patients. This classification was made based on the information about dengue serology provided in the paper. Primary dengue infection was defined as acute infection, as indicated by qualitative detection of NS1 antigen, and/or IgM or HI antibodies or RT-PCR positivity and absence of IgG antibodies against dengue virus. A case of acute infection as defined above, in presence of IgG antibodies, was considered as secondary dengue infection [ 2 , 13 , 14 ]. Some of the studies used the ratio of IgG to IgM antibodies as the criteria for differentiating primary and secondary infections [ 14 ]; (b) distribution of predominant and co-circulating dengue virus serotypes; (c) proportion of severe dengue infections based on WHO 1997 or WHO 2009 criteria [ 1 , 2 ]. The category of severe dengue infection included patients with DHF and DSS as per the WHO 1997 classification as well as severe dengue infections classified as per the WHO 2009 classification and (d) cost of illness, which included reported direct and indirect costs associated with dengue hospitalization.
Risk of bias
The risk of bias was assessed using a modified Joanna Briggs Institute (JBI) appraisal checklist for studies reporting prevalence data [ 15 ] and essential items listed in the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist [ 16 ]. The criteria for assessing bias primarily included methods for selecting participants, methods for laboratory testing, and outcome variables (Supplementary file S2 Appendix ).
Statistical analysis
We conducted quantitative synthesis to derive meta-estimates of primary and secondary outcomes (severity of disease and primary/ secondary infections) and qualitative synthesis to describe the serotype distribution and economic burden due to dengue. We followed Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines [ 17 ]. For each study, primary outcomes (prevalence of acute infection, seroprevalence and CFR) were summarized as proportion and their 95% confidence intervals were computed. We used logit and inverse logit transformations for variance stabilization of proportions [ 18 ]. Binomial–Normal mixed effects regression model was used to estimate the pooled proportion of dengue infections. Forest plots were used to display pooled estimates. Heterogeneity was tested using likelihood ratio test. Funnel plots with logit prevalence on x-axis and standard errors on y-axis and Egger’s test were used to evaluate publication bias. Independent variables potentially associated with the prevalence of laboratory confirmed dengue were included as fixed-effects in univariate and multivariate binomial meta-regression models. P <0.05 was considered statistically significant. Sensitivity analysis was carried out by leaving out one study at a time in the order of publication to check for consistency of pooled estimates. Analyses were performed in the R statistical programming language using the ‘metafor’ package [ 19 , 20 ].
Characteristics of included studies
The search strategy initially identified 2,285 articles from different databases. After removal of duplicates, 1,259 articles were considered for title and abstract screening. Seven hundred and forty-six articles were excluded for reasons provided in Fig 1 . Thus, 513 articles were found to be eligible for full-text review. After the review of full-text articles, 233 studies were included for the analysis [ 21 – 253 ]. The details of the studies included in the review are provided in the PRISMA flowchart ( Fig 1 ). None of the studies reported incidence of dengue fever.

Primary outcomes
Prevalence (proportion) of laboratory confirmed dengue fever.
Of the 233 studies included in the analysis, 180 provided information about proportion of laboratory confirmed dengue cases among clinically suspected patients [ 21 – 200 ]. This included 154 studies conducted in hospital or laboratory setting [ 21 – 174 ] and 26 studies reporting outbreak investigations [ 175 – 200 ]. Of the 154 studies conducted in hospital/ laboratory setting, 40 were conducted when an outbreak was ongoing in the area [ 135 – 74 ]. The diagnosis of acute dengue infection was based on a single assay in 86 studies (IgM antibodies = 68, RT-PCR = 11, HI antibodies = 4, virus isolation = 2, detection of NS1 antigen = 1) and more than one assay in 95 studies.
Case definitions used : Of the 154 studies conducted in hospital settings, WHO or NVBDCP case definitions were used by 39 and 2 studies respectively. The remaining studies used case definitions such as acute febrile illness/acute undifferentiated illness (n = 20), and clinically suspected dengue fever (n = 93). Similarly, of the 26 reported outbreaks, investigators used WHO or NVBDCP case definitions in 7 and 2 settings respectively, whereas acute febrile illness and clinically suspected dengue fever case definitions were used in 5 and 12 settings respectively.
Place and time distribution of studies : Of the 154 studies conducted in hospital setting, 75, 41, 27 and 7 were from north, south, east and western Indian states respectively, whereas 3 studies were from north-eastern states. One study reported data from VRDL network, covering multiple regions in India [ 65 ]. Of the 26 outbreaks, most (10, 38.5%) were reported from Southern states, followed by 9 (34.6%) in the north, 4 (15.4%) in the east, and 3 (11.5%) in the north-eastern Indian states. Most (65, 42.2%) studies conducted in hospital settings were between 2011–2017, while 48 (31.2%) were conducted between 2006–2010 and 41 (26.6%) were conducted before 2006. Eighteen (69.2%) of the 26 outbreaks were reported after 2000.
Of the 180 studies which reported proportion of dengue cases, 74 studies (30%) provided the details of laboratory confirmed cases by month with most (n = 60, 81%) reporting higher dengue positivity between August and November months.
Age distribution of dengue cases : The age distribution of laboratory confirmed dengue patients was available from 52 out of 180 studies. The pooled median age of laboratory confirmed dengue cases in these studies was 22 years ( Fig 2 ). Fifteen (28.8%) studies reported the median age of dengue cases below 15 years.

Estimates of prevalence (proportion) : The overall estimate of the prevalence of laboratory confirmed dengue infection in the random effects model based on testing of 213,285 clinically suspected patients from 180 studies was 38.3% (95% CI: 34.8%–41.8%) ( Fig 3 ). There was a significant heterogeneity in the prevalence reported by the 180 studies (LRT p<0.001). The prevalence of laboratory confirmed dengue infection was higher in studies reporting outbreaks or hospital-based surveillance studies during outbreaks (47.3%, 95% CI: 40.9–53.8) as compared to hospital-based surveillance studies (33.6%, 95% CI: 29.9–37.5) ( S1A and S1B Fig ). The attack rates of suspected dengue case patients were available in 8 out of the 26 outbreak investigations reports. The attack rates ranged between 1.9% and 19.5%.

Error bars indicate 95% confidence intervals. Diamonds show the pooled estimates with 95% confidence intervals based on random effects (RE) model.
In the univariate mixed effect meta-regression model, odds of laboratory confirmation were higher in case of outbreaks or hospital-based studies conducted during outbreaks (OR = 1.8, 95% CI: 1.3–2.4). Studies which used WHO/ NVBDCP case definitions for enrolment of patients also had higher odds of detecting laboratory confirmed dengue compared to studies which used acute febrile illness/ clinically suspected dengue cases as case definitions. Compared to studies conducted before 2006–10, studies conducted between 2011 and 2017 had higher odds of identifying laboratory confirmed patients (OR = 1.33, 95% CI: 0.93–1.9). The odds of laboratory confirmation did not differ by region ( Table 1 ). In the multivariate meta-regression model constructed by including all covariates, case definition (WHO/NVBDCP), type of study (hospital-based surveillance studies conducted during outbreaks or outbreaks) and period of study (prior to 2005 and 2011–2017) were associated with higher odds of dengue cases being laboratory confirmed.
Ref—Reference category; CI—Confidence interval; P*–P value.
Seroprevalence of dengue among healthy individuals
We included 7 studies reporting seroprevalence of dengue based on detection of IgG (n = 5), neutralizing antibodies (n = 1) or HI antibodies (n = 1) against dengue in the analysis [ 201 – 207 ]. These studies, conducted in 12 Indian states [Andaman and Nicobar islands (n = 1), Andhra Pradesh (n = 2), Tamil Nadu (n = 3), Delhi (n = 4), West Bengal (n = 1), and Maharashtra (n = 1)], surveyed 6,551 individuals. The study population surveyed in these studies included healthy children (n = 2), general population (n = 3), blood donors (n = 1) and neighbourhood contacts of dengue confirmed cases (n = 1). The overall seroprevalence of dengue fever based on these studies was 56.9% (95% CI: 37.5–74.4) ( Fig 4 ). The age-specific prevalence of IgG antibodies was available in three studies [ 201 , 204 , 206 ]. There was a significant heterogeneity in the seroprevalence reported by the seven studies (LRT p<0.001). In the 3 studies which provided age specific seroprevalence, by the age of 9 years, 47.6% -73.4% children were reported to have developed IgG or neutralizing antibodies against dengue ( Table 2 ).

Figure in square bracket indicate reference
Case fatality ratios (CFR)
Seventy-seven studies provided information about case fatality ratios; most of them (n = 72, 93.5%) were conducted after 2000. The reported CFRs in these studies ranged from 0% to 25%. There was a significant heterogeneity in the CFRs reported by the 74 studies (LRT p<0.001). Twenty (25.9%) studies reported CFR of 2% or more. Three studies [ 30 , 239 , 195 ] which affected overall meta-estimates due to small denominator and hence were excluded from analysis. The pooled estimate of CFR was 2.6% (95% CI: 2.0–3.4) ( Fig 5 ).

Secondary outcomes
Primary and secondary dengue infection.
A total of 49 studies provided data which enabled classification of laboratory confirmed dengue into primary and secondary dengue infections. The number of patients with acute dengue infections in these studies ranged between 13 and 1752. Only two studies estimated the proportion of secondary infection based on IgG to IgM ratio [ 174 , 237 ]. The prevalence of secondary dengue infection was <10% in 6 studies, 10–25% in 9 studies, 26–50% in 12 studies, 51–75% in 17 studies and >75% in 5 studies. The overall proportion of secondary dengue infection among laboratory confirmed patients was 42.9% (95%CI: 33.7–52.6) ( Fig 6 ).

Proportion of severe cases
Information about severity of dengue was available in 49 studies. Most studies (n = 46, 93.9%) used the WHO 1997 classification while 3 studies used the WHO 2009 classification for dengue severity. The reported proportion of severe dengue cases among laboratory confirmed patients ranged between 1.4% and 97.4%. The overall proportion of severe dengue among laboratory confirmed studies in the random effects model was 28.9% (95% CI: 22.2–36.6) ( Fig 7 ).

Serotypes of dengue virus
Information about dengue serotypes was available in 51 studies. These studies were conducted in 19 Indian states; with a regional distribution of north (n = 28), south (n = 13), east (n = 4), northeast (n = 4), and west (n = 2). Thirty-eight (75%) of the 51 studies reported circulation of more than one serotype. The predominant serotypes reported in these studies were DEN-2 and DEN-1 in the northern region, DEN-2 and DEN-3 in the southern region, and DEN-1 and DEN-2 in the eastern and the western regions. In the four studies reported from the north-eastern region, the predominant serotypes was DEN-3 followed by DEN-1 and DEN-2 serotypes ( Table 3 ).
Key for coloured cell: Blue—One circulating serotype, Yellow—two co-circulating serotypes, Green—three co- circulating serotypes, Orange—four co- circulating serotypes. Numbers mentioned in the cell indicate predominant serotypes, in descending order.
Economic burden
Direct and Indirect cost analysis : An estimate of direct and indirect costs was reported in three studies. The average direct cost per case of dengue ranged between USD 23.5 and USD 161 and the indirect cost was around USD 25 whereas the average cost of hospitalization ranged between USD 186 and USD 432.2 [range 249 -252]. The cost of dengue treatment in the private health sector was two to four times higher than that in the public sector hospitals [ 249 , 253 ].
Economic impact of dengue on National Economy : Three macro-level studies addressed the economic impact of dengue faced by India [ 250 , 251 , 253 ]. It was estimated that the average total economic burden due to dengue in India was USD 27.4 million [ 251 ]. Another study estimated that the total direct medical cost of dengue in 2012 was USD 548 million [ 253 ]. The overall economic burden of dengue would be even higher if the cost borne by individual patients is combined with the society level cost of dengue prevention, vector control, disease control and its management, dengue surveillance as well as the cost of research and development [ 250 , 251 , 253 ].
Publication bias and sensitivity analysis
Funnel plots and Egger’s test revealed no publication bias in the estimates of dengue prevalence in hospital-based surveillance studies, hospital-based surveillance studies during outbreaks and outbreak investigations. CFR estimates, however, showed a significant publication bias, and studies with high prevalence were more likely to be published. In the sensitivity analysis, the estimated pooled proportions were found to be consistent for all study outcomes. ( S3 Appendix )
The present study has estimated the burden of dengue fever based on published literature from India spanning over five decades. Most of the published literature included in the analysis were hospital/ laboratory-based surveillance studies or reports of dengue outbreak investigations. Additionally the published data from VRDL network has been included in the analysis [ 65 , 96 ]. The data from the other two nationally representative surveillance platforms could not be used for the analysis because surveillance data from NVBDCP only reports the number of laboratory confirmed dengue cases, while the IDSP data is not available in the public domain.
There was no community-based epidemiological study reporting the incidence of dengue fever. Our analysis revealed that among the clinically suspected dengue fever patients, the estimated prevalence of laboratory-confirmed dengue infection was 38%. The burden of dengue was also variable in studies conducted in different settings. Our findings indicated that most of the laboratory confirmed dengue cases in India occurred in young adults. Dengue positivity was higher between the months of August and November, corresponding to monsoon and post-monsoon season in most states in India.
In the meta-regression, studies that had used WHO/NVBDCP case definitions and the hospital based studies conducted during outbreaks or studies reporting outbreaks were more likely to have laboratory confirmation of dengue. The odds of laboratory confirmation were also higher among studies conducted during the period of 2011 to 2017, as compared to studies conducted prior to the year 2000.
Information about seroprevalence of dengue in the general population is a useful indicator for measuring endemicity of dengue fever. The dengue vaccine (CYD-TDV) manufactured by Sanofi Pasteur has been introduced in two sub-national programs in Philippines and Brazil [ 254 ] and it has been suggested that vaccine acts by boosting the naturally acquired immunity [ 255 ]. WHO SAGE conditionally recommends the use of this vaccine for areas in which dengue is highly endemic as defined by seroprevalence in the population targeted for vaccination [ 12 , 256 ]. The results of the two vaccine trials and mathematical modelling suggest that optimal benefits of vaccination if seroprevalence in the age group targeted for vaccination was in the range of ≥70% [ 255 , 256 ]. In 2018, WHO revised the recommendation from population sero-prevalence criteria to pre-vaccination screening strategy [ 257 ]. The pooled estimate based on the seven studies conducted in India indicated a dengue seroprevalence of 57%. However, this estimated seroprevalence is not representative of the country, as these studies were conducted only in 12 Indian states, and some had used a convenience sampling method [ 201 ].
The computed pooled estimate of case fatality due to dengue in India was 2.6% with a high variability in the reported CFRs. The CFR estimated in our study was higher than the estimate of 1.14% (95% CI: 0.82–1.58) reported in the meta-analysis of 77 studies conducted globally; in the 69 studies which adopted WHO 1997 dengue case classification, the pooled CFR was 1.1% (0.8–1.6) while the pooled CFR for 8 studies which used the WHO 2009 case definition, the pooled CFR was 1.6% (95% CI: 0.64–4.0) [ 258 ]. Higher CFR observed in our analysis could be due to smaller sample sizes as 14 of the 35 studies that reported CFR of 2.6 or higher had a sample size of 100 or less, while in the remaining 21 studies the denominator ranging between 101 and 400. Also, we only considered laboratory confirmed dengue cases in the denominator for the calculation of CFR. As per the NVBDCP surveillance data, a total of 683,545 dengue cases and 2,576 deaths were reported in India during 2009–2017 giving a CFR of 0.38% [ 6 ]. The lower CFR estimates from NVBDCP data could probably be on account of under-reporting of deaths due to dengue, or inclusion of higher number of mild cases in the denominator [ 259 ]. As per the NVBDCP surveillance data, an average of 28,227 dengue cases and 154 deaths were reported annually during 2009–2012. The number of dengue cases reported increased thereafter, with an average of 100,690 cases per year during 2013–2017. However, the reported number of deaths did not increase proportionately. The information about severity of dengue cases is not available from NVBDCP surveillance data.
The published studies from India indicated circulation of all the four-dengue serotypes, with DEN-2 and DEN-3 being the more commonly reported serotypes. Two third of the studies reported circulation of more than one serotype. Co-circulation of multiple serotypes was particularly evident from the published studies in Delhi. More than two third (16/19) studies from Delhi reported circulation of more than one serotype; and most of the studies conducted in the last 10 years identified co-circulation of more than one serotype [ Table 3 ]. Our review also revealed that more than two-fifth of the laboratory confirmed infections were secondary dengue infections and nearly one-fourth of the cases were severe in nature. Circulation of numerous dengue serotypes is known to increase the probability of secondary infection, leading to a higher risk of severe dengue disease [ 260 ].
Our systematic review has certain limitations. First, our study included only peer-reviewed literature from selected databases and we excluded grey literature which may have provided additional data. Second, most of the studies on disease burden were hospital-based, with no community-based studies estimating incidence. Hospital-based studies do not provide any information about the community level transmission as hospitalization is a function of health-seeking behaviour of the population. In absence of the information about health seeking behaviour provided in these studies, we estimated the prevalence of dengue using number of patients tested in the hospitals as the denominator. Third, the hospital-based studies used varying case definitions and laboratory tests to confirm dengue infection. Fourth, information about the type of health facility (public or private), or residential status of patients (urban or rural), and age was not uniformly reported and hence we did not estimate the dengue prevalence by these variables.
In conclusion, the findings of our systematic review indicate that dengue continues to be an important public health problem in India, as evidenced by the high proportion of dengue positivity, severity and case fatality as well as co-circulation of multiple dengue virus serotypes. Our review also identified certain research gaps in the understanding on dengue epidemiology in the country. There is a need to initiate well planned community-based cohort studies representing different geographic regions of the country in order to generate reliable estimates of age-specific incidence of dengue fever in India. As such studies are cost intensive, a national level survey to estimate age-stratified dengue seroprevalence rates could be an alternative. Such estimates could be used to derive the relative proportions of primary and secondary infections using mathematical models [ 261 ]. Well planned studies in different geographic settings are also needed to generate reliable data about economic burden from India. Although the existing dengue surveillance platforms of NVBDCP, IDSP and VRDL are generating data about dengue disease burden, these systems could be strengthened to also generate data about dengue serotypes, severity, and primary and secondary infection from India.
Supporting information
S1 appendix, s2 appendix, s3 appendix, s1 checklist, funding statement.
The study was funded by the Department of Bio-technology, Govt of India. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 12 June 2020
Knowledge, attitude and practice on dengue prevention and dengue seroprevalence in a dengue hotspot in Malaysia: A cross-sectional study
- Sivaneswari Selvarajoo ORCID: orcid.org/0000-0002-1518-3335 1 ,
- Jonathan Wee Kent Liew ORCID: orcid.org/0000-0001-9456-8104 1 ,
- Wing Tan 1 ,
- Xin Ying Lim 1 ,
- Wardha F. Refai 2 ,
- Rafdzah Ahmad Zaki 3 ,
- Neha Sethi 4 ,
- Wan Yusoff Wan Sulaiman 1 ,
- Yvonne Ai Lian Lim 1 ,
- Jamuna Vadivelu 5 &
- Indra Vythilingam 1
Scientific Reports volume 10 , Article number: 9534 ( 2020 ) Cite this article
30k Accesses
64 Citations
1 Altmetric
Metrics details
- Viral infection
Dengue has become a global public health problem. Despite reactive efforts by the government in Malaysia, the dengue cases are on the increase. Adequate knowledge, positive attitude and correct practice for dengue control are essential to stamp out the disease. Hence, this study aims to assess the factors associated with dengue knowledge, attitude and practice (KAP), as well as the association with dengue IgM and IgG seropositivity. A community-based cross-sectional study was conducted in a closed, dengue endemic area with multi-storey dwellings . Five hundred individuals (aged 18 years and above) were approached for pre-tested KAP and seroprevalences assessment. The study showed only half of the total participants have good knowledge (50.7%) but they had insufficient knowledge about dengue during pregnancy. 53.2% of people had poor attitude and 50.2% reported poor practice for dengue control. Out of 85 respondents who agreed to participate in the dengue seroprevalence study, 74.1% (n = 63) were positive for dengue IgG and 7.1% (n = 6) were positive for dengue IgM. Among all sociodemographic variable, race is the only independent predicator for all KAP levels (P < 0.05). In conclusion, proactive and sustainable efforts are needed to bring a behavioural change among communities in order to fight dengue outbreaks in endemic areas.
Similar content being viewed by others

Knowledge, attitudes and practices of dengue prevention between dengue sustained hotspots and non-sustained hotspots in Singapore: a cross-sectional study
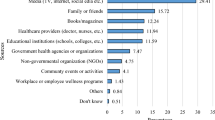
Assessment of perceived dengue risk and prevention practices among youth in Bangladesh

Dengue virus infection in people residing in Africa: a systematic review and meta-analysis of prevalence studies
Introduction.
Dengue fever is a mosquito-borne viral disease caused by a flavivirus. There are four distinct serotypes of dengue virus, namely DEN-1, 2, 3 and 4. Female Aedes aegypti and Aedes albopictus mosquitoes are the primary and secondary vectors in Malaysia, respectively. Evidently, dengue is the most rapidly spreading arboviral disease in the world. The Global Burden of Disease reported that dengue incidence has multiplied to six-folds from 1990 to 2013, with Southeast Asia region contributing 52% of the disease burden 1 . World Health Organisation (WHO) estimates that 50 million to 100 million cases occur annually 2 .The disease is currently endemic in more than 100 countries, with South-East Asia being among the worst affected region.
Dengue fever was established in Malaysia ever since the first reported case of dengue in 1902. From then on, the numbers of cases continued to rise despite numerous initiatives undertaken by the Ministry of Health to curb the disease 3 , 4 . According to WHO, the recent cumulative case count in Malaysia from 1 Jan to 2 Mac 2019 was 157% higher than that of the same period in 2018 5 . In addition, a total of 79,151 dengue cases have been reported until end of July 2019 nationwide, with Selangor state contributing more than 50% of the cases (n = 40,849, 51.6%) 6 .
Vector control and surveillance is still the mainstay of dengue prevention strategies since there is no specific treatment for disease and vaccination remains a non-viable option 7 . Local programs like Communications for Behavorial Changes (COMBI) in Malaysia have proved their potential effect in reducing dengue morbidity 8 but it requires understanding from community as well 9 . Besides, vector control measures eg. larval survey, fogging, ULV sprays and laws such as the Destruction of Disease Bearing Insects (Amendment) Act 2000, require support, cooperation and participation from the community 10 . Therefore, an understanding of the society’s baseline knowledge, attitudes and practices (KAP) of dengue is essential for effective vector control. Health education is equally important in the prevention of dengue 11 , 12 , 13 . Hence, apart from evaluating the KAP of the community with regards to dengue, providing basic knowledge of the disease and its preventive methods is of paramount importance.
In 1997, two cases of vertical transmission of dengue fever in Malaysia were reported for the first time 14 . A total of 16 dengue cases in pregnancy were reported in a study conducted in Malaysia from 2000 to 2004, which concluded that dengue infection in pregnancy may lead to poor maternal and foetal outcomes 15 , 16 , 17 . A hospital-based prospective study conducted in Vientiane, Laos found dengue to be the most common infection among febrile pregnant women 18 . Symptomatic dengue infection during pregnancy or delivery may even lead to preterm births, infants with low birth weight 19 , haemorrhagic complications, maternal death, vertical transmission of dengue to symptomatic infants, and other neonatal complications 20 . Since prompt treatment and admission are needed, the community should be more aware of the impact of dengue during pregnancy. However, to date no data has been reported on the community’s knowledge regarding dengue in pregnancy. Therefore, this study also provides an opportunity to assess and clarify any misconceptions regarding dengue infection in pregnancy among the community.
Of the 390 million DENV infections per year, 300 million are asymptomatic 21 . As primary infections by the virus are usually asymptomatic, the actual magnitude of dengue infection in Malaysia is likely to be greater than expected. Interestingly, a nationwide cohort study found that 91.6% of the adults aged 35 to 74 years were dengue IgG positive 22 . Considering, the entire discourse above, this study also aimed to investigate the factors which affect the dengue related KAP of an endemic community, as well as the association of the KAP with dengue IgM and IgG seropositivity.
Various studies which have been conducted in different parts of Malaysia primarily focused on sociodemographic data and KAP of study populations 23 . Previous studies performed in Selangor, where the current study took place, demonstrated that the general communities have good knowledge on dengue and positive attitudes when it comes to dengue prevention 23 , 24 , 25 , 26 . These factors are further differentially influenced by age group, ethnicity, level of education, employment status and marital status 27 , 28 . KAP aside, several other researches have also evaluated the association between the health belief model construct and IgG seropositivity in Malaysians 29 . To the best of our knowledge, there are hardly any studies on KAP and associated factors of dengue infections in closed, endemic communities. Also, in view of the recent rise in dengue deaths within the study area, it is a necessity to assess the dengue related KAP of the endemic community to devise effective vector control and surveillance strategies.
Sociodemographic characteristics of study population
Out of 500 questionnaires distributed among residents of eight apartments, 474 responded, giving a response rate of 94.8%. Table 1 shows the demographic status of the participants. Briefly, the study population is predominated by females (60.6%) with mean age of 36 ± 11.62. Majority of the respondents were Malay (62.1%) and married (74.4%). More than half of the participants (56.6%) were residing in low rise apartments. Almost all participants had received primary education (91.8%). Of the total respondents, 51.8% of them were working and earning an individual monthly income of less than Malaysian Ringgit (MYR) 5,000 (94.1%) (1 USD = MYR 4.15).
Despite being marked as a hotspot area for dengue, 83.3% respondents self-reported that they never had any history of dengue, only 16.7% had been infected with dengue in the past. Among 474 respondents, only 85 respondents agreed to participate in the dengue seroprevalence study. Based on this, it is a mere 18% of the total respondents, out of which 74.1% (n = 63) were positive for dengue IgG and 7.1% (n = 6) were positive for dengue IgM. Dengue IgM positivity demonstrates a recent or acute dengue infection, while dengue IgG positive individuals have had previous exposure to dengue. Therefore, in this community where mostly did not report any symptom at the time of sampling, 74.1% of the participants had previous exposure to dengue, while 7.1% had recent or acute dengue infection.
Knowledge about dengue
Table 2 summarizes the correct knowledge of respondents on dengue. Among the endemic study population, level of knowledge regarding dengue disease and transmission is high. Almost all the questions in the “knowledge” category were answered correctly by more than half of the study population. Most respondents knew dengue to be a viral disease (82.2%) and is transmitted by mosquito bites (97.2%). Furthermore, majority of participants were able to respond correctly to questions on vector behaviour, regarding active biting time and breeding preferences. However, knowledge regarding dengue during pregnancy was lacking among the study participants. Only 54.6% were aware that dengue virus can be transmitted from an infected pregnant mother to the foetus. Moreover, knowledge regarding the life cycle of dengue vectors was also not satisfactory. Only 18% were able to answer correctly the number of days required by the vector mosquitoes to complete their life cycle. In addition, only two-thirds (66.7%) were aware that dengue virus was able to infect the same person multiple times. Regarding symptoms of dengue, high grade fever was a popular response among study subjects followed by muscle pain (92.6%), headache (92.5%) and joint pain (91.0%). However, rapid breathing (70.7%) and restlessness (71.1%) were less identified as associated symptoms of dengue fever. Overall, based on 80% cut-off value, 50.7% participants (n = 213) were identified to have substantial knowledge of dengue.
Factors associated with good knowledge (Table 3 ) were age, race, marital status, monthly income, education and employment (P < 0.05). Whereas, gender and previous history of dengue have no association with participant’s knowledge. Our findings revealed participants with higher income and education background have good knowledge compared to those with lower income and education. Moreover, participants more than 35 years and married have better knowledge about dengue. Interestingly, Malays are 2.3 times more likely to have good knowledge compared to non-Malays. After all significant factors (P ≤ 0.25) were included in analysis, multivariable model (Table 4 ) revealed race and occupation as the independent predictors for good knowledge.
Attitude toward dengue prevention
Table 5 shows the participants’ attitude towards dengue prevention. 89.7% of participants would like to reduce dengue cases in their area. However, not all of them (70.5%) regularly check the dengue situation in their areas. Around half of them have the wrong perception that chemical fogging by health authorities is enough for dengue prevention. Only 78.0% would like to actively engage in removal of breeding sites. Based on 80% cut off value, 46.8% of the study population possess an appropriate and acceptable attitude towards dengue prevention and this is associated with age, race, marital status and employment (Table 3 ). In multivariable model, employment, marital status and race were independent factors significantly associated with good attitude (Table 4 ).
Practices toward dengue prevention
Table 6 shows participants’ practices towards preventing dengue. In general, 50.2% of participants have unsatisfactory practice towards dengue prevention based on 80% cut-off. The response “search and destroy mosquito breeding sites” (95.0%) followed by “usage of mosquito spray” (94.5%) were much preferred methods of control by participants whenever there was a high abundance of mosquitoes. About 53.5% of them were aware of scrubbing containers before discarding the containers with water collections to get rid of mosquito eggs attached to containers. The best self-protection method was chosen as removal of mosquito breeding sites (80.4%) followed by 58.3% preferring to use mosquito repellents. Race is the only factor correlated with good prevention practice for dengue (Table 4 ). Malay participants were three times more likely to have good prevention practice for dengue compared to non-Malays.
Factors associated with dengue IgG seropositivity
Table 7 shows the sociodemographic factors associated with dengue IgG seropositivity. Type of residential units and ethnicity were significantly associated with IgG seropositivity. IgG seropositivity in 73.2% of 85 participants suggests they were unknowingly infected with dengue virus previously. Interestingly, 75.0% of the participants with positive dengue IgG possessed poor knowledge, attitude and practice toward dengue disease.
Correlation between knowledge, attitude and practice
The correlation test found a significant positive correlation between knowledge-attitude (r s = 0.384, P < 0.001), knowledge-practice (r s = 0.319, P < 0.001) and attitude-practice (r s = 0.457, P < 0.001). However, the degree of correlation was fair (r s < 0.5). With further analysis, it was found that participants who had good knowledge were 2.8 times more likely have good attitude (OR:2.89; 95% CI:1.94–4.29) but no strong association was found with good practice (OR:1.96 ;95% CI:1.33–2.89) regarding dengue prevention. Nonetheless, participants with good attitude are 2.5 times more likely to have good dengue preventive practice.
This study provides the first description of KAP and seroprevalence in a closed, dengue endemic urban community in Malaysia, where an upward trend of dengue cases has been reported for more than a decade. Currently, Malaysia like many other countries in the region, are plagued by dengue. Dengue has no cure, only symptomatic management, while the current vaccine has moderate efficacy and does not provide equal protection against all four serotypes 1 . Hence, vector control remains the current mainstay for dengue control. More effort is needed on health campaign programs to educate the populace about prevention of dengue infection and to actively put these into action. Human practice is known to play a crucial role in maintaining dengue vector and transmission of virus because Ae. aegypti , the primary dengue vector depends on human to provide a suitable environment and for a blood meal. The latest KAP study in Malaysia by Ghani et al . (2019) reported that participants from non-dengue hotspot areas have better knowledge and attitude than those from hotspot areas but factors associated with this were unanswered 27 . The current study focuses more on the KAP level of residents from dengue hotspot and their dengue seroprevalence, along with factor associated with these variables. The findings from this study may contribute to the development of a proactive program to protect the health of vulnerable groups in the community.
It is noteworthy that interpretation and comparison of the data from this current study and from others have to be made cautiously. This is due to the methodological differences between studies including different modes of analyses of the data, varying focus of the questions in the questionnaire, dissimilar demographic background of the respondents, different scoring systems or cut-off points for “poor” and “good” KAP, etc. In view of the above, results from the current study were compared to those of previous studies on adults from urban/suburban settings similar to the current study. In our study, our cut-off values can be considered high i.e. 80% score, compared to studies which uses mean or other arbitrary cut-offs. Therefore, despite obtaining correct and positive answers (average of >75%) in each of the knowledge, attitude and practice component, only 50.7%, 46.8%, and 49.8% of the community living in the 8 apartments in Damansara Damai, a dengue hotspot, had good knowledge of dengue, attitude and practices in dengue prevention, respectively. Results of previous Malaysian studies showed that the urban/suburban communities generally have good knowledge of dengue and its symptoms and positive attitude on dengue prevention, but these are not translated to good practice in dengue prevention 25 , 30 , 31 , 32 . However, there are other studies which demonstrated various levels of KAP different from those reported above 26 , 28 , 33 , with few others citing good dengue prevention practices among the urban/suburban communities 24 , 34 , 35 .
This KAP study revealed that only half of the study population has exemplary and adequate knowledge regarding dengue. Nonetheless, it should be noted that the overall community did have exemplary knowledge of dengue infection per se and the signs and symptoms. This is consistent with earlier cross-sectional studies in Malaysia 25 , 36 , Jamaica 37 , Philippines 38 and Thailand 39 . But it differs from some studies conducted in Nepal 40 and India 41 . The difference may be due to intensified education and awareness campaign by the department of health in our endemic area which can be reflected in the communities’ level of knowledge. However, knowledge of the vector’s habit, behaviour and life-cycle is still lacking. This has been equally expressed in other similar studies 42 , 43 .
In addition, the current study found a lack of knowledge on transmission of dengue during pregnancy from mother to foetus among the study population. Hitherto, no KAP studies have assessed knowledge of vertical transmission of dengue, thus creating a dire void of knowledge on this issue. From a systematic review on maternal dengue and pregnancy outcome, most case reports or case series concluded that dengue infection before and even during pregnancy leads to adverse foetal outcomes due to vertical transmission of antibodies 44 . This is extremely worrying as seroprevalence analysis revealed 74.1% of 85 adults in the study population had previous exposure to dengue, without them knowing. Putting the two and two together, pregnant women may be unknowingly infected with dengue, but this vulnerable population and the foetus are at risk of complications during pregnancy, due to inapparent dengue. Indeed, a 2.5–3.4% rate of recent dengue infection (based on dengue IgM serology or RT-PCR) has been reported among pregnant women or parturient in Malaysia 15 , 45 , 46 and New Caledonia 20 . Thus, it is important to heighten education among parents and future parents regarding the risks of dengue infection during pregnancy.
In univariate analysis, factor associated with good knowledge on dengue fever were being married, higher education level, being employed with higher monthly income and being Malay. However, the multivariate analysis revealed only race and employment were independent predictors of good knowledge. Many studies support a significant positive association between education and good knowledge of dengue 27 , 33 , 34 , 43 , while several studies support the effect of employment on knowledge 28 , 42 . This could be because working adults are more likely to be involved in health campaigns and education in their workplace and have more information on dengue fever compared to the unemployed. The Communications for Behavioural Changes (COMBI) programme on dengue prevention have also been implemented by Health Departments and developers at construction sites while the Department of Occupational Safety and Health of Malaysia do promote dengue awareness and prevention 47 . Obviously, there is a direct relationship between economic statuses (e.g. owning a house, better income) and having good knowledge of dengue 28 , 33 , 35 , 48 . People with better economic status may have better access and appreciation for reliable information. Other studies have also described significant associations between good knowledge scores of dengue with being married 28 , 42 , increasing age 43 and history of dengue infection 33 , 34 .
Poor attitude (53.2%) and poor practice (50.2%) were observed among participants in the study area. Many (49.6%) have an erroneous belief that chemical fogging by the health personnel is adequate to reduce dengue transmission, similar to results reported by Kamel et al . (2017). They do not realise it is important to carry out source reduction since in Malaysia, fogging is always carried out after cases are reported. Chemical fogging itself has many pros and cons as a control measure 49 , 50 . From the ongoing cluster randomised trial using GOS trap and NS1 antigen test kit (unpublished data, 2020), we were still able to detect mosquitoes with dengue virus after fogging was carried out. This concept must change, and more proactive measures are needed. Vector control cannot rely on fogging alone, effective vector control requires reduction of vector breeding habitats to reduce disease transmission and prevalence. Moreover, evidence has been provided that ULV is not effective 51 , yet it seems to be the main strategy used during outbreaks. Perhaps, that is the reason why residents think that it is the best method for getting rid of the mosquitoes.
Furthermore, there was a small proportion of participants who believed that it is not their responsibility to remove mosquito breeding sites in their residence. This is similar to that of Zaki et al . (2019) and of another study which states that 32.7% believed removing larvae breeding is a complete waste of time 23 . In order to curb dengue, reducing the vector population and prevention of virus transmission are equally important. Without community participation, it is impossible to reduce dengue prevalence. In multi-storey apartments, all residents should a play role to clean up their housing units to ensure it is free of mosquito breeding sites. As people are living very close to each other, they can be bitten by infected mosquitoes which can easily fly from one house to another. Also, since most of the dengue epidemics are occurring in high-rise apartments, the management bodies of the apartments should take the responsibility of keeping the surroundings free of breeding sites while the residents should take care of their homes. Previous study has reported that search and destroy practice requires good knowledge and skills in order to remove breeding sites efficiently 52 . Therefore, the health authorises have a role in spearheading this effort too. Nonetheless, it is encouraging to note that the general public, including those in the current study support dengue control programs and believe that the public also has the responsibility in preventing and controlling dengue 10 , 25 , 26 , 36 , 43 .
In univariate analysis, factors associated with good attitudes were age, race, marital status, employment status and monthly individual income. These factors can be categorised as good socioeconomic background. The findings support the notion that participants with better socioeconomic background have good attitude regarding dengue fever. Other Malaysian studies have reported income 27 , 31 , 35 , employment status 27 , marital status 53 and ethnicity (especially being Malay) 43 , 54 to be associated with good attitude of dengue prevention. Similarly, in Aceh, different areas have reported that good attitude was associated with socioeconomic status. Better socioeconomic status does provide better access to dengue information 55 , 56 . However, excluding all insignificant variables, race, marital status and employment are independent predictor of good attitudes. Married couples possess good attitudes towards dengue control than single individuals. This may be due to greater sense of responsibility towards their family compared to single inhabitants who may be staying there temporarily for working purposes. A study in Lao PDR described that families tend to possess more resources to make sure their house and surroundings are comfortable, safe for the children and clear of Aedes breeding sites 57 . Conversely, findings from Singapore have further shown that a high incidence of dengue fever was associated with living alone and having no family nucleus 58 .
Interestingly, in multivariate analysis, race was a strong significant predicator for good knowledge, attitudes and practices. The Malay race possessed good KAP in comparison to other races. Two studies have shown Malays outperformed other ethnic groups in this context 30 , 54 . These may be due to the main language used in most mass media. Mass media is a powerful tool in disseminating health information and consistent with previous studies done in Malaysia 23 , 44 , Indonesia 56 and Thailand 59 . In Malaysia, most mass media advertisements about health awareness are mainly in the national language understood by the Malay race more so than the other races. Other studies have also indicated the Malay race to be associated with good practice of dengue prevention 43 , 54 . Nonetheless, there might be underlying actual characteristics behind the variable race for being associated with good KAP. For example, being pious to religion or having a strong sense of community. Perhaps there is a better, ingrained sense of community attributed to the more religious and cultural upbringing of the Malays than among the other races. Interestingly, this phenomenon is most apparent in rural settings, where the villagers are mostly Malays with a strong sense of neighbourliness, and they often demonstrate good dengue prevention practices 36 , 60 , 61 .
From seroprevalence results, it was noted that dengue IgG seropositivity was significantly associated with race and type of buildings. Additionally, 75.0% of the participants with positive dengue IgG possessed poor knowledge, attitude and practice toward dengue disease and prevention. 83.0% of residents from shop houses were dengue IgG seropositive in this study area. This can be explained by population density and mobilization effects, as shop houses are typically surrounded by shops where high population movement occurs and thus residents are at higher risk of imported dengue. In fact, mosquitoes tend to remain in the same location throughout their lifetime. This means that people, rather than mosquitoes are responsible for dengue transmission in and outside of their communities 62 . Dense population has been postulated as one of the factors causing transmission of dengue virus 29 , 63 . Hence, a significant positive association between type of residential unit with dengue IgG seropositivity found in this study could be linked to the interplay of abundant mosquito breeding sites 64 , 65 , high population density and human mobility, leading to high dengue incidence attributed to import dengue. Wong et al . (2014) showed that significantly higher proportion of people living in high-rise residential buildings are IgG seropositive, compared to people living in single or terraced houses and they experience more frequent fogging and mosquito problems 29 .
These self-reported questionnaires do show a fair correlation between means score of knowledge, attitude and practice. As knowledge improves, attitude and practice among participants also improve in the study area. Moreover, a significant positive association was observed between knowledge, attitude and practice. Our results are most similar to some earlier studies 26 , 43 . Whereas other studies only reported correlation between knowledge on dengue with positive attitude for dengue control 53 and between positive attitude and good practice of dengue prevention 33 , 35 . However, the level of translation from knowledge to attitude and practice of the community in this study is still low. Even with good knowledge, poor attitude and practice were observed in the study population in some instances. Evidence of association between knowledge with practice is varying. While some observed a positive association in Malaysia 29 , 31 , 42 , Cuba 48 and Laos 66 ,others saw no correlation between good knowledge and practices 28 , 32 , 67 . In our current study, an effective and sustainable strategy is required to translate the community’s knowledge into good practices.
Overall, to tackle, poor attitude and practice in our study population, change in their behaviour towards dengue prevention is very crucial. The residents have fairly good knowledge, but practice and attitude need improvement. Perhaps, the residents have always been used to reactive methods since they were only informed when dengue cases occurred. And actions are taken only when being monitored or when there is a death case. Also, possibly some people do perform good practices but quickly became demotivated when they realise their efforts are not matched by their immediate community 68 . Therefore, heightening awareness and good attitude and positive encouragement of subsequent good practices are important. Seeing results (eg reduction in Aedes population or dengue cases) of their preventive behaviours may fuel continuous motivation to perform these activities 69 . Besides, it is envisaged that with the new proactive paradigms like informing residents when dengue positive mosquitoes are obtained may improve their attitude and practice and to carry out source reduction 70 .
Knowledge and attitude are associated with practice, and these two are easier to improve than to improve other factors associated with good practice such as economic status 71 . Besides, ingrained, negative habits are difficult to discourage with plain knowledge sharing. Perhaps more personal and practical approaches in the health education programmes are needed to influence a change in behaviour. This has been a common recommendation by previous studies since the early 1990’s 34 , 64 , 70 . House-to-house inspection by health personnel should not be done for Aedes surveillance only, but to convey information and educate the residents in a more personal manner. Health personnel and religious bodies should be encouraged to influence and motivate change in habit and spur social mobilization 10 , 23 , 72 . Furthermore, the provision of adequate and relevant information should be made readily available to all layers of the communities. For this matter, television/radio has been cited as the main source of information, followed by newspaper/magazine in both the urban and rural communities 30 , 31 , 32 , 43 .
Finally, some limitations of this study is that the subjects were recruited with more than one approaches such as from house to house and community event like clean-ups causing non-probabilistic sampling. Their KAP levels are also assessed only at one time point, so the overall dynamic might change according to time. Moreover, as a self-reporting questionnaire was used, respondents might had provided answers not reflective of their actual attitude and practices, to appear socially desirable, which may contribute to reporting bias.
Therefore, this study revealed knowledge regarding dengue symptoms, transmission (except vertical transmission and dengue during pregnancy) and vector control measures to be generally high among the residents of the 8 apartments in the dengue hotspot, Damansara Damai, Petaling Jaya, Selangor, Malaysia. Furthermore, dengue IgG seropositivity was significantly associated with race and living in shop houses. However, this knowledge on dengue and prevention is not translated to positive attitude and practice. In this sort of setting, conventional health education campaigns should be adapted to encourage social mobilization among the people. Bottom-up approaches are more likely to be successful and sustainable 73 . What is essential is a multi-disciplinary approach to change the attitudes and behaviour of the people. This can be achieved by having many different stakeholders coming together, synergising their efforts to fight a common cause. To combat dengue successfully without greater harm to the ecosystem, integrated vector control measures will rely mostly on the success of community and household practices to eliminate vector breeding sites.
Methodology
Study setting.
A community-based, cross-sectional study was conducted at Damansara Damai (3.1930°N, 101.5923°E), a residential area located at the northernmost part of Petaling Jaya, the district, which accounted for the largest number of dengue cases in the state of Selangor 6 . Damansara Damai (Fig. 1 ) has a population of 61,615 in an area of 3.45 km 2 which translates into a population density of approximately 17,859 inhabitants/km 2 . Overpopulation in the low and high-rise residential buildings may be responsible for the major dengue hotspots in the study site. A detailed description of the study site has been published 70 . This cross-sectional study was conducted during a cluster randomized controlled trial study investigating the efficacies of Gravid Ovipositing Sticky Trap (GOS) and dengue NS1 antigen test kit as early surveillance tool for dengue. This trial has been registered at ClinicalTrials.gov (ID: NCT03799237) on 8th January 2019. A detailed study protocol is available online 70 .
Map of Selangor state, Malaysia showing study district, Petaling district. Damansara Damai is one of the sub-district which is a closed area with only one main entrance and exit.
Ethic approval
This research received the ethical approval from Medical Research Ethics Committee, University of Malaya Medical Centre, Malaysia (MRECID No: 2018525-6321).
Participation and sample size
Sample size estimation was calculated to achieve power of 80% with a 5% margin of error and 95% confidence level, by using the Cochran (1963) formulae 72 . Since the prevalence of KAP in the study population is unknown, the estimated sample size was calculated by assuming that 50% of the population have baseline knowledge about dengue. A minimum of 384 residents were required for this study; a further 20% was added to account for anticipated loss of participants and this increased the sample size to 460 residents. Data was collected from September 2018 to January 2019. The residents were approached during office hours on weekdays by a maximum of two research teams, each of which has at least a well-trained phlebotomist. Specifically, house-to-house visits were performed following receipt of written approval by the joint management bodies of respective apartments. All residents who were aged at least 18 years and could provide informed consent were included in the study. The research followed the guidelines stated in the ethics form and written informed consent was obtained from all participants.
Knowledge, attitude, and practice questionnaire
A self-administered close-ended questionnaire was used as the instrument to measure the residents’ baseline KAP. The questionnaire has been adapted from previous study 40 and used in a pilot study which involved similar Malaysian community 25 . There are three sections in the questionnaire. Section A concerns the sociodemographic data which covers the basic information of respondents. Section B contains 28 questions to assess the residents’ knowledge on dengue. The knowledge possessed by a community refers to their understanding towards dengue including its vectors and symptoms of dengue. Section C has 9 attitude-related items referring to their feelings as well as any preconceived idea of dengue, and 7 items on common dengue prevention practices of the respondents. Practices refer to the ways in which they demonstrate their knowledge and attitude through their actions.
Dengue seroprevalence test
In addition to the KAP survey, the respondents were also given the choice to participate in the seroprevalence study. Respondents name, identification number and consent were taken before 3 ml of venous blood was withdrawn by the trained personnel. The samples were collected in EDTA blood tubes and transported to the laboratory at room temperature within 4 hours. The blood was then centrifuged at 4,000 rpm for 4 min and the resulting serum aliquoted, coded and stored at −80 °C pending further use. Later, Dengue IgM and IgG ELISA kit (Focus Diagnostics Inc., Cypress, CA, USA) were used to detect the presence of anti-dengue immunoglobulin in the sera according to manufacturer’s instructions. Each individual’s serum was tested in duplicates.
Data management and analysis
Each resident’s KAP questionnaire and sample were coded with a unique identification number based on their apartments. Data analysis was performed using Statistical Package for Social Science version 23 (SPSS, Inc., Chicago, IL). A scoring system was utilized in the evaluation of the KAP data. Specifically, all correct and positive answer was scored 1 while “Don’t Know” and wrong answers as 0. The total number of correct answers in each section was used to determine the KAP level. If a participant scored at least 80% in a category, he/she would be labelled as “good”; if the converse was true, then he/ she would be labelled as “poor”. Scoring system and cut-off points for the KAP survey were in accordance with those of Dhimal et al . (2014) 40 .
Descriptive statistics of the sociodemographic factors comprised frequencies and percentages. The association of independent variable with KAP levels (good/poor) were determined using the chi-square test or Fisher’s exact test as appropriate. Meanwhile, Spearmen’s rank correlation was used to determine the extent of correlation between KAP scores since data was not normally distributed as per outcome of the Kolmogorov-Smirnov normality test. The level of statistical significance was set at 0.05. Univariate analysis was performed to examine the association between good KAP and the demographic and socioeconomic factors. Then, multivariable logistic regression model was developed with all possible associations, variable that showed an association with P ≤ 0.25 were included in the model, as suggested by Bendel and Afifi 74 .
Data availability
The analysed data is all in the manuscript. However, the dataset of this study is available upon request from the corresponding author.
Stanaway, J. D. et al . The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect. Dis. 16 , 712–723 (2016).
Article PubMed PubMed Central Google Scholar
Wilder-Smith, A., Ooi, E.-E., Horstick, O. & Wills, B. J. T. L. Dengue. Lancet. 393 , 350–363 (2019).
Article PubMed Google Scholar
Ahmad, R. et al . Factors determining dengue outbreak in Malaysia. PloS One. 13 , e0193326 (2018).
Article PubMed PubMed Central CAS Google Scholar
Nani, R. Dengue incidence and the prevention and control program in Malaysia. Int. Med. J. Mal. 14 , 1–9 (2015).
Google Scholar
World Health Organisation (WHO). Dengue Situation Update 2019. http://iris.wpro.who.int/handle/10665.1/14329h (2019).
Crisis Preparedness and Response Centre (CPRC). Dengue in Malaysia, http://idengue.remotesensing.gov.my/idengue/ (2019).
Scott, T. W. & Morrison, A. C. Vector dynamics and transmission of dengue virus: implications for dengue surveillance and prevention strategies: vector dynamics and dengue prevention. Curr. Top. Microbiol. Immunol. 338 , 115–128 (2010).
PubMed Google Scholar
Rozhan, S., Jamsiah, M., Rahimah, A. & Ang, K. T. The COMBI (Communication for Behavioural Impact) program in the prevention and control of dengue-The Hulu Langat experience. J. Kesihatan Masyarakat. 12 , 1–13 (2006).
Vythilingam, I. & Wan-Yusoff, W. S. Dengue vector control in Malaysia: are we moving in the right direction? Trop. Biomed. 34 , 746–758 (2017).
Hairi, F. et al . A knowledge, attitude and practices (KAP) study on dengue among selected rural communities in the Kuala Kangsar district. Asia Pac. J. Public Health. 15 , 37–43 (2003).
Article ADS PubMed Google Scholar
Horstick, O., Runge-Ranzinger, S., Nathan, M. B. & Kroeger, A. Dengue vector-control services: how do they work? A systematic literature review and country case studies. Trans. R. Soc. Trop. Med. Hyg. 104 , 379–386 (2010).
Kroeger, A., Nathan, M. & Hombach, J. Focus: Dengue. Nat. Rev. Microbiol. 2 , 360–361 (2004).
Article CAS PubMed Google Scholar
Nguyen, H. V. et al . Knowledge, attitude and practice about dengue fever among patients experiencing the 2017 outbreak in Vietnam. Int. J. Environ. Res. Public Health. 16 , 976 (2019).
Article PubMed Central Google Scholar
Chye, J. K. et al . Vertical transmission of dengue. Clin. Infect. Dis. 25 , 1374–1377 (1997).
Ismail, M. et al . Seropositivity of dengue antibodies during pregnancy. Sci. World J. 2014 , 436975 (2014).
Sharma, S., Jain, S. & Rajaram, S. Spectrum of maternofetal outcomes during dengue infection in pregnancy: an insight. Infect. Dis. Obstet. Gynecol. 2016 , 5046091 (2016).
Paixão, E. S., Teixeira, M. G., Maria da Conceição, N. C. & Rodrigues, L. C. Dengue during pregnancy and adverse fetal outcomes: a systematic review and meta-analysis. Lancet Infect. Dis. 16 , 857–865 (2016).
Chansamouth, V. et al . The aetiologies and impact of fever in pregnant inpatients in Vientiane, Laos. PLoS Negl. Trop. Dis. 10 , e0004577 (2016).
Friedman, E. E. et al . Symptomatic dengue infection during pregnancy and infant outcomes: a retrospective cohort study. PLoS Negl. Trop. Dis. 8 , e3226 (2014).
Arragain, L. et al . Vertical transmission of dengue virus in the peripartum period and viral kinetics in newborns and breast milk: new data. J. Pediat. Inf. Dis. Soc. 6 , 324–331 (2017).
Bhatt, S. et al . The global distribution and burden of dengue. Nature. 496 , 504–507 (2013).
Article ADS CAS PubMed PubMed Central Google Scholar
Azami, N. A. M., Salleh, S. A., Neoh, H., Zakaria, S. Z. S. & Jamal, R. Dengue epidemic in Malaysia: Not a predominantly urban disease anymore. BMC Res. Notes. 4 , 216 (2011).
Article Google Scholar
Al-Dubai, S. A., Ganasegeran, K., Mohanad Rahman, A., Alshagga, M. A. & Saif-Ali, R. Factors affecting dengue fever knowledge, attitudes and practices among selected urban, semi-urban and rural communities in Malaysia. Southeast Asian . J. Trop. Med. Public Health. 44 , 37–49 (2013).
Abas, B. H., Sahani, M., Nordin, R. H. & Azlan, S. Knowledge and practices regarding Aedes control amongst residents of dengue hotspot areas in Selangor: a cross-sectional study. Sains Malays. 48 , 841–849 (2019).
Zaki, R. et al . Public perception and attitude towards dengue prevention ativity and response to dengue early warning in Malaysia. PloS One. 14 , e0212497 (2019).
Article CAS PubMed PubMed Central Google Scholar
Kamel, M. N. A. M. et al . The KAP study on dengue among community in Taman Salak Baiduri, Sepang, Selangor. Int . J. Sci. Healthcare Res. 2 , 19–25 (2017).
ADS Google Scholar
Ghani, N. A. et al . Comparison of knowledge, attitude, and practice among communities living in hotspot and non-hotspot areas of dengue in Selangor, Malaysia. Trop. Med. Int. Health. 4 , 37 (2019).
Azfar, M. et al . Knowledge, attitude and practice of dengue prevention among sub urban community in Sepang, Selangor. International J. Pub. Health Clin. Sci. 4 , 73–83 (2017).
Wong, L. P., AbuBakar, S. & Chinna, K. Community knowledge, health beliefs, practices and experiences related to dengue fever and its association with IgG seropositivity. PLoS Negl. Trop. Dis. 8 , e2789 (2014).
Ayyamani, U. D., Ying, G. C. & San, O. G. A knowledge attitude and practice (KAP) study on dengue/dengue haemorrhagic fever and the Aedes mosquitoes. Med. J. Malaysia. 41 , 108–115 (1986).
Alhoot, M. A. et al . Knowledge, attitude, and practice towards dengue fever among patients in Hospital Taiping. Malaysian J. Pub. Health Med. 17 , 66–75 (2017).
Mahyiddin, N. S., Mohamed, R., Mohamed, H. J. J. & Ramly, N. High knowledge on dengue but low preventive practices among residents in a low cost flat in Ampang, Selangor. Malaysian J. Nurs. 8 , 39–48 (2016).
Wan Rozita, W., Yap, B., Veronica, S., Mohammad, A. & Lim, K. Knowledge, attitude and practice (KAP) survey on dengue fever in an urban Malay residential area in Kuala Lumpur. Malaysian J. Pub. Health Med. 6 , 62–67 (2006).
Wong, L. P., Shakir, S. M. M., Atefi, N. & AbuBakar, S. Factors affecting dengue prevention practices: nationwide survey of the Malaysian public. PloS One. 10 , e0122890 (2015).
Lugova, H. & Wallis, S. Cross-sectional survey on the dengue knowledge, attitudes and preventive practices among students and staff of a public university in Malaysia. J. Community Health. 42 , 413–420 (2017).
Abdul Aziz, K. H. et al . Knowledge, attitude and practice on dengue among adult population in Felda Sungai Pancing Timur, Kuantan, Pahang. IIUM Medical Journal of Malaysia. 16 , 2 (2017).
Shuaib, F., Todd, D., Campbell-Stennett, D., Ehiri, J. & Jolly, P. E. Knowledge, attitudes and practices regarding dengue infection in Westmoreland, Jamaica. W. Indian Med. J. 59 , 139–146 (2010).
CAS PubMed Google Scholar
Yboa, B. C. & Labrague, L. J. Dengue knowledge and preventive practices among rural residents in Samar province, Philippines. Am. J. Public Health Res. 1 , 47–52 (2013).
Koenraadt, C. J. et al . Dengue knowledge and practices and their impact on Aedes aegypti populations in Kamphaeng Phet, Thailand. Am . J. Trop. Med. Hyg. 74 , 692–700 (2006).
Dhimal, M. et al . Knowledge, attitude and practice regarding dengue fever among the healthy population of highland and lowland communities in central Nepal. PLoS One. 9 , e102028 (2014).
Article ADS PubMed PubMed Central Google Scholar
Acharya, A., Goswami, K., Srinath, S. & Goswami, A. Awareness about dengue syndrome and related preventive practices amongst residents of an urban resettlement colony of south Delhi. J. Vector Dis. 42 , 122–127 (2005).
Naing, C. et al . Awareness of dengue and practice of dengue control among the semi-urban community: a cross sectional survey. J. Community Health. 36 , 1044–1049 (2011).
Nasaruddin, N. K., A. Rahman, N.A. & Mamat, S. Knowledge, Attitude and Practice regarding Dengue: A Case Study in Taman Temerloh Jaya, Malaysia . 2-68 (LAP Lambert Academic Publishing, 2014).
Pouliot, S. H. et al . Maternal dengue and pregnancy outcomes: a systematic review. Obstet. Gynecol. Surv. 65 , 107–118 (2010).
Tan, P. C. et al . Dengue infection and miscarriage: a prospective case control study. PLoS Negl. Trop. Dis. 6 , e1637 (2012).
Tan, P. C., Rajasingam, G., Devi, S. & Omar, S. Z. Dengue infection in pregnancy: prevalence, vertical transmission, and pregnancy outcome. Obstet. Gynecol. 111 , 1111–1117 (2008).
Ismail, A., Nawi, A. M. & Mohamed, A. Communication for behavioural impact (COMBI) program in dengue prevention evaluation: Mixed methods approach. Int. Med. J. 22 , 367–370 (2015).
Castro, M. et al . The relationship between economic status, knowledge on dengue, risk perceptions and practices. PLoS One. 8 , e81875 (2013).
Zahir, A., Ullah, A., Shah, M. & Mussawar, A. Community participation, dengue fever prevention and control practices in Swat, Pakistan. Int. J. MCH AIDS. 5 , 39–45 (2016).
Ong, S.-Q. Dengue vector control in Malaysia: a review for current and alternative strategies. Sains Malays. 45 , 777–785 (2016).
CAS Google Scholar
Esu, E., Lenhart, A., Smith, L. & Horstick, O. Effectiveness of peridomestic space spraying with insecticide on dengue transmission: systematic review. Trop. Med. Int. Health. 15 , 619–631 (2010).
Carandang, R. R., Valones, A., Valderama, M. T., Cotoco, K. & Asis, E. Community-based approach for dengue prevention and control in Sta. Cruz, Laguna, Philippines. Int. J. Community Med. Public Health. 2 , 627 (2015).
Zamri, S. N. Z. B. M., Rahman, N. A. A. & Haque, M. Knowledge, attitude, and practice regarding dengue among Kuantan medical campus students of international Islamic university of Malaysia. Bangladesh . J. Med. Sci. 19 , 245–253 (2020).
Leong, T. K. Knowledge, attitude and practice on dengue among rural communities in Rembau and Bukit Pelanduk, Negeri Sembilan, Malaysia. Int . J. Trop. Dis. Health. 4 , 841–848 (2014).
Itrat, A. et al . Knowledge, awareness and practices regarding dengue fever among the adult population of dengue hit cosmopolitan. PloS One. 3 , e2620 (2008).
Article ADS PubMed PubMed Central CAS Google Scholar
Harapan, H. et al . Knowledge, attitude, and practice regarding dengue virus infection among inhabitants of Aceh, Indonesia: a cross-sectional study. BMC Infect. Dis. 18 , 96–96 (2018).
Sayavong, C., Chompikul, J., Wongsawass, S. & Rattanapan, C. Knowledge, attitudes and preventive behaviors related to dengue vector breeding control measures among adults in communities of Vientiane, capital of the Lao PDR. J. Infect. Public Heal. 8 , 466–473 (2015).
Ma, S., Ooi, E. E. & Goh, K. T. Socioeconomic determinants of dengue incidence in Singapore. WHO Regional Office for South-East Asia. https://apps.who.int/iris/handle/10665/170464 (2008).
Kittigul, L., Suankeow, K., Sujirarat, D. & Yoksan, S. Dengue hemorrhagic fever: knowledge, attitude and practice in Ang Thong Province, Thailand. Southeast Asian . J. Trop. Med. Public Health. 34 , 385–392 (2003).
Aung, M. M. T. et al . Knowledge, attitude, practices related to dengue fever among rural population in Terengganu, Malaysia. Malaysian . J. Public Heal. Med. 16 , 15–23 (2016).
Firdous, J. et al . Knowledge, attitude and practice regarding dengue infection among Ipoh community, Malaysia. J. Appl. Pharm. Sci. 7 , 099–103 (2017).
World Health Organization (WHO). Dengue: guidelines for diagnosis, treatment, prevention and control. https://apps.who.int/iris/handle/10665/44188 (2009).
Barreto, F. R., Teixeira, M. G., Maria da Conceição, N. C., Carvalho, M. S. & Barreto, M. L. Spread pattern of the first dengue epidemic in the city of Salvador, Brazil. BMC Public Health. 8 , 51 (2008).
Honório, N. A. et al . Spatial evaluation and modeling of dengue seroprevalence and vector density in Rio de Janeiro, Brazil. PLoS Negl. Trop. Dis. 3 , e545 (2009).
Chen, C. et al . Mixed breeding of Aedes aegypti (L.) and Aedes albopictus Skuse in four dengue endemic areas in Kuala Lumpur and Selangor, Malaysia. Trop. Biomed. 23 , 224–227 (2006).
Mayxay, M. et al . Dengue in peri-urban Pak-Ngum district, Vientiane capital of Laos: a community survey on knowledge, attitudes and practices. BMC Public Health. 13 , 434 (2013).
Lozano, E. B., Isok, B. T. & Greif, M. M. People’s knowledge, attitude and practices on dengue in two barangays with high dengue incidences in Cebu city, Philippines. J. Entomol. 6 , 218–223 (2018).
Jaramillo Ramírez, G. I. & Álvarez, L. S. B. Knowledge, attitudes and practices regarding dengue, chikungunya, and Zika and their vector in Villavicencio, Colombia. Open Public Health J. 10 , 80–89 (2017).
Sulistyawati, S. et al . Dengue vector control through community empowerment: lessons learned from a community-based study in Yogyakarta, Indonesia. Int. J. Environ. Res. Public Health. 16 , 1013 (2019).
Liew, J. W. K., Selvarajoo, S., Tan, W., Zaki, R. A. & Vythilingam, I. Gravid oviposition sticky trap and dengue non-structural 1 antigen test for early surveillance of dengue in multi-storey dwellings: study protocol of a cluster randomized controlled trial. Infect. Dis. Poverty. 8 , 71 (2019).
Harapan, H. et al . Dengue prevention: confirmatory factor analysis of relationships between economic status, knowledge, attitudes and practice, vaccine acceptance and willingness to participate in a study. Southeast Asian . J. Trop. Med. Public Health. 48 , 297–305 (2017).
Cochran, W. G. Sampling techniques: 2nd edition. 300-303 (John Wiley & Sons, 1963).
Spiegel, J. et al . Barriers and bridges to prevention and control of dengue: the need for a social–ecological approach. EcoHealth. 2 , 273–290 (2005).
Bendel, R. B. & Afifi, A. A. Comparison of stopping rules in forward “stepwise” regression. J. Am. Stat. Assoc. 72 , 46–53 (1977).
MATH Google Scholar
Download references
Acknowledgements
The authors would like to thank the management bodies of all participating apartments and residents of Damansara Damai, Selangor for support and willingness to participate in this study. We would like to express our gratitude to Fumakilla Malaysia Berhad and OPC Resources Sdn Bhd, Malaysia for sponsoring the Fumakilla Nobite Lotion and NATMOS anti mosquito spray. Special thanks are also extended to the research group members and interns who were involved in data collection at study area and also to Prof. Hesham Al-Mekhlafi (Jazan University, Saudi Arabia) for his constructive comments on the manuscript. This study was funded by Ministry of Education Malaysia under Fundamental Research Grant Scheme (FRGS) (Project No. MRSA MO013–2017).
Author information
Authors and affiliations.
Department of Parasitology, Faculty of Medicine, University of Malaya, 50603, Kuala Lumpur, Malaysia
Sivaneswari Selvarajoo, Jonathan Wee Kent Liew, Wing Tan, Xin Ying Lim, Wan Yusoff Wan Sulaiman, Yvonne Ai Lian Lim & Indra Vythilingam
Postgraduate Institute of Medicine (PGIM), Colombo, 7, Sri Lanka
Wardha F. Refai
Centre for Epidemiology and Evidence Based Practice, Department of Social and Preventive Medicine, Faculty of Medicine, University of Malaya, 50603, Kuala Lumpur, Malaysia
Rafdzah Ahmad Zaki
Department of Obstetrics and Gynaecology, University of Malaya Medical Centre (UMMC), 50603, Kuala Lumpur, Malaysia
Department of Medical Microbiology, Faculty of Medicine, University of Malaya, 50603, Kuala Lumpur, Malaysia
Jamuna Vadivelu
You can also search for this author in PubMed Google Scholar
Contributions
Conception and design of experiment: I.V., J.W.K.L., N.S., W.Y.W.S., Y.A.L.L. and J.V. Questionnaire designing and analysis: S.S., R.A.Z. Data entry: S.S., W.T. Data collection: J.W.K.L., W.T., W.F.R., N.S. Drafting manuscript: S.S., X.Y.L. Review and editing: I.V., J.W.K.L., S.S. All authors revised and approved the final manuscript.
Corresponding author
Correspondence to Indra Vythilingam .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Selvarajoo, S., Liew, J.W.K., Tan, W. et al. Knowledge, attitude and practice on dengue prevention and dengue seroprevalence in a dengue hotspot in Malaysia: A cross-sectional study. Sci Rep 10 , 9534 (2020). https://doi.org/10.1038/s41598-020-66212-5
Download citation
Received : 07 October 2019
Accepted : 18 May 2020
Published : 12 June 2020
DOI : https://doi.org/10.1038/s41598-020-66212-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
- Abu Bakkar Siddique
- Nishat Tamanna Omi
- Md. Tajuddin Sikder
Scientific Reports (2024)
Knowledge, attitude, and practices of adults and children towards the Asian tiger mosquito, Aedes albopictus (Diptera: Culicidae), in a recently invaded municipality of Valencia, Spain
- Pedro María Alarcón-Elbal
- Marcos López-de-Felipe
- Rubén Bueno-Marí
International Journal of Tropical Insect Science (2024)
Dengue in the urban slums of Pakistan: health costs, adaptation practices, and the role of dengue-diagnosis and surveillance in controlling the epidemic
- Yasir Mehmood
- Muhammad Arshad
GeoJournal (2024)
Fogging to combat dengue: factors influencing stakeholders' attitudes in Malaysia
- Ahmad Firdhaus Arham
- Latifah Amin
- Noor Sharizad Rusly
BMC Public Health (2023)
Comparing the performance of dengue virus IgG and IgG-capture enzyme-linked immunosorbent assays in seroprevalence study
- Jih-Jin Tsai
- Ching-Yi Tsai
- Wei-Kung Wang
BMC Infectious Diseases (2023)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
COMMENTS
Dengue is an acute viral illness caused by RNA virus of the family Flaviviridae and spread by Aedes mosquitoes. Presenting features may range from asymptomatic fever to dreaded complications such as hemorrhagic fever and shock. A cute-onset high fever, muscle and joint pain, myalgia, cutaneous rash, hemorrhagic episodes, and circulatory shock ...
In addition, dengue fever may present with extended and unusual manifestations affecting any organ, including the heart, liver, kidney and brain. Studies on vaccine development and vector control are ongoing to prevent this infection of global importance. In this article, the clinicopathological features and management aspects of dengue are ...
Dengue is a mosquito-transmitted virus and is the leading cause of arthropod-borne viral disease worldwide, posing a significant global health concern. This disease is also known by various monikers, such as breakbone or 7-day fever, and is characterized by intense muscle spasms, joint pain, and high fever, reflecting both the severity and the duration of symptoms. Although most dengue fever ...
Dengue, caused by four closely related viruses, is a growing global public health concern, with outbreaks capable of overwhelming health-care systems and disrupting economies. Dengue is endemic in more than 100 countries across tropical and subtropical regions worldwide, and the expanding range of the mosquito vector, affected in part by climate change, increases risk in new areas such as ...
Background The World Health Organization (WHO) has ranked dengue as one of the top ten threats to Global health in 2019. Sri Lanka faced a massive dengue epidemic in 2017, the largest outbreak in the country during the last three decades, consisting of 186,101 reported cases, and over 320 deaths. The epidemic was controlled by intense measures taken by the health sector. However, the reported ...
Basic research includes a wide range of studies focused on learning how the dengue virus is transmitted and how it infects cells and causes disease. This type of research investigates many aspects ...
Defeating dengue with Wolbachia. A recent study reports the efficacy of Wolbachia -infected mosquito deployments for the control of dengue fever in Indonesia. Ashley York. Research Highlights 18 ...
Infection with any one of the four dengue virus serotypes can cause disease that ranges from a mild febrile illness through to haemorrhagic fever and shock. This Primer outlines the epidemiology ...
Dengue virus (DENV) is responsible for an estimated 100 million symptomatic cases of infection and 10,000 deaths annually. The incidence of dengue has been doubling every decade since 1990.1 Dengue...
Introduction. Dengue is an infectious disease transmitted between humans by Aedes mosquitoes, especially across the tropical and subtropical latitudes afflicting ~400 million people annually, of which 100 million manifests clinically ( 1 ). Estimates by the World Health Organization (WHO) suggest that the global consequence of dengue is ...
Dengue is the disease caused by 1 of 4 distinct, but closely related dengue viruses (DENV-1-4) that are transmitted by Aedes spp. mosquito vectors. It is the most common arboviral disease worldwide, with the greatest burden in tropical and sub-tropical regions. In the absence of effective prevention and control measures, dengue is projected to increase in both disease burden and geographic ...
Studies were included if they met the eligibility criteria: (1) peer-reviewed original research articles, (2) quantitative observational time series, case-crossover or case series human studies in the general population with dengue infection cases or incidence as the outcome of interest and (3) the exposures of interest were high temperatures ...
The transmission of dengue virus (DENV) from an infected Aedes mosquito to a human, causes illness ranging from mild dengue fever to fatal dengue shock syndrome. The similar conserved structure and sequence among distinct DENV serotypes or different flaviviruses has resulted in the occurrence of cross reaction followed by antibody-dependent enhancement (ADE).
Background Dengue fever stands as one of the most extensively disseminated mosquito-borne infectious diseases worldwide. While numerous studies have investigated its influencing factors, a gap remains in long-term analysis, impeding the identification of temporal patterns, periodicity in transmission, and the development of effective prevention and control strategies. Thus, we aim to analyze ...
In adults, primary dengue 1 and 3 infections result in high rates of classic dengue fever but dengue 2 and 4 infections cause milder disease and are often ... Dengue hemorrhagic fever in infants: research opportunities ignored. Emerg Infect Dis. 2002; 8 (12):1474-9. 10.3201/eid0812.020170 [PMC free article] [Google Scholar] 15. ...
Dengue, or dengue fever, is a disease caused by infection with the dengue virus (DENV), a member of the Flavivirus genus (Flaviviridae) that also includes yellow fever and Japanese encephalitis. Milder cases can range from asymptomatic to clinical manifestations that include high fever, severe headaches, retro-orbital pain, joint and muscle pains, vomiting and rash. DENV is classified into ...
2 Yunnan Key Laboratory of Vaccine Research & Development on Severe Infectious Diseases, Kunming, China; 3 Yunnan Key Laboratory of Vector-Borne Infectious Disease, Kunming, ... In 2019, there were 22599 cases of dengue fever, with an incidence rate of 1.63/10 million . As a typical dengue epidemic area, in 2019, the main epidemic type in ...
Interest in containment has only become more acute as dengue's global burden has ballooned 9, recent serious Zika 10 and yellow fever 11 outbreaks have attracted global attention, and new dengue ...
Dengue fever virus. Dengue fever (DF) is one of the most common widespread vector borne diseases in the world , , , .There are currently 2.5 billion people living in areas at risk of DF transmission, with 100 million cases reported annually , .DF is a flaviviral disease caused by one of four serotypes of dengue virus (DEN 1-4) which are transmitted by mosquito vectors, in particular the ...
Author summary In our study, we investigated the association between diabetes mellitus (DM) and the risk of severe clinical manifestations in cases of dengue fever (DF) and West Nile fever (WNF). By analyzing 27 studies involving over 342,000 laboratory-confirmed patients, we found compelling evidence supporting a link between DM and an increased risk of severe complications in both DF and WNF.
Dengue fever (DF) is an acute, self-limiting systemic viral illness caused by the dengue virus (Flaviviridae), ... This research also implicates that virus-induced destruction or suppression of myeloid progenitor cells may cause leukopenia in dengue fever. Bradycardia, one of the major dengue manifestations, was found to occur in 46.15% of ...
Dengue Fever. Dengue fever is an infectious disease carried by mosquitoes and caused by any of four related dengue viruses. This disease used to be called "break-bone" fever because it sometimes causes severe joint and muscle pain that feels like bones are breaking. Health experts have known about dengue fever for more than 200 years.
As of 30 April 2024, over 7.6 million dengue cases have been reported to WHO in 2024, including 3.4 million confirmed cases, over 16 000 severe cases, and over 3000 deaths. While a substantial increase in dengue cases has been reported globally in the last five years, this increase has been particularly pronounced in the Region of the Americas, where the number of cases has already exceeded ...
A key challenge for vaccine development is that dengue is caused by four distinct viral subtypes, or serotypes: DENV-1, DENV-2, DENV-3 and DENV-4. "The perfect dengue vaccine would have 90% ...
A total of 48,884 adults with dengue (54.05% men; mean age, 43.08) were identified from the National Health Insurance Research Database in Taiwan. A comparator group of 195,536 individuals without ...
To read the full-text of this research, you can request a copy directly from the authors. ... Data on monthly-reported cases of dengue fever over the period 2004-2010 were obtained from the ...
Dengue is a viral fever caused by four different viruses and spread through mosquito bites. It's common in many tropical regions across the globe, but has begun to appear in more temperate climates.
Dengue is the most extensively spread mosquito-borne disease; endemic in more than 100 countries. Information about dengue disease burden, its prevalence, incidence and geographic distribution is critical in planning appropriate control measures against dengue fever. We conducted a systematic review and meta-analysis of dengue fever in India
Dengue fever is a mosquito-borne viral disease caused by a flavivirus. There are four distinct serotypes of dengue virus, namely DEN-1, 2, 3 and 4. ... Research articles News & Comment Collections ...
Their work further supports research in developing a germline-targeting strategy for priming the immune system to elicit a bNAb called VRC01. This bNAb was discovered by NIAID researchers almost 15 years ago. The goal of this line of research is to develop an HIV vaccine that generates multiple classes of bNAbs to prevent HIV. ARTICLE: