- See us on facebook
- See us on twitter
- See us on youtube
- See us on linkedin
- See us on instagram

Experimental depression treatment is nearly 80% effective in controlled study
In a double-blind controlled study, high doses of magnetic brain stimulation, given on an accelerated timeline and individually targeted, caused remission in 79% of trial participants with severe depression.
October 28, 2021 - By Mandy Erickson

Since receiving an experimental depression treatment at Stanford, Tommy Van Brocklin has been walking Scout for "the sheer joy of it." Nellie Van Brocklin
A new type of magnetic brain stimulation brought rapid remission to almost 80% of participants with severe depression in a study conducted at the Stanford University School of Medicine .
The treatment, known as Stanford accelerated intelligent neuromodulation therapy (SAINT) or simply Stanford neuromodulation therapy, is an intensive, individualized form of transcranial magnetic stimulation. In the study, remission typically occurred within days and lasted months. The only side effects were temporary fatigue and headaches.
“It works well, it works quickly and it’s noninvasive,” said Nolan Williams , MD, an assistant professor of psychiatry and behavioral sciences. “It could be a game changer.” Williams is the senior author of the study, which was published Oct. 29 in the American Journal of Psychiatry .
Twenty-nine people with treatment-resistant depression participated in the study: About half received SAINT, and the rest underwent a placebo procedure that mimicked the real treatment. After five days of treatment, 78.6% of the participants in the treatment group were no longer depressed, according to several standard methods of evaluation. “It’s quite a dramatic effect, and it’s quite sustained,” said Alan Schatzberg , MD, the Kenneth T. Norris, Jr. Professor in Psychiatry and Behavioral Sciences, who was a co-author of the study.
A lifetime of depression
Tommy Van Brocklin, 60, has suffered from depression since he was 15. “In 1975, they didn’t have the medication and understanding they do now,” he said. “I was told I wasn’t trying hard enough.”
“I’ve functioned all these years, but it’s been very difficult at times,” the civil engineer added. Talk therapy helped “for about half a day after an appointment.” When selective serotonin reuptake inhibitors became available in the 1990s, he started on paroxetine, commonly sold under the brand name Paxil.
“It worked like a miracle drug,” he said, but after 10 or 15 years it started to lose its effect. After 25 years, it stopped working entirely. He tried other medications, but none helped; one even made him suicidal.
His sister, who lives near Stanford, connected him with the researchers studying SAINT. He flew from his home in Memphis, Tennessee, and underwent the treatment in September. He felt nothing the first day; on day two, he began feeling emotional — “I felt the struggle of what I’d been through all these years.”
“The next day, all of a sudden, it broke through,” he said. “I felt so much better, and it’s stuck with me.”
Specialized magnetic stimulation
The transcranial magnetic stimulation treatment currently approved by the Food and Drug Administration requires six weeks of once-daily sessions. Only about half of patients who undergo the treatment improve, and only about a third experience remission from depression.
SAINT advances that treatment by targeting the magnetic pulses according to each patient’s neurocircuitry and providing a greater number of pulses at a faster pace.
In the study, the researchers first used MRI to locate the best location to target within each participant’s dorsolateral prefrontal cortex, which regulates executive functions, such as problem solving and inhibiting unwanted responses. They applied the stimulation in a subregion that has the strongest relationship with the subgenual cingulate, a part of the brain that is overactive in people experiencing depression. The transcranial magnetic stimulation strengthens the connection between the two regions, facilitating dorsolateral prefrontal cortex control of the activity in the subgenual cingulate.
The researchers also used 1,800 pulses per session instead of 600. (The larger amount has been used safely in other forms of brain stimulation for neurological disorders such as Parkinson’s disease.) And instead of providing one treatment a day, they gave participants 10 10-minute treatments, with 50-minute breaks in between.
For the control group, the researchers disguised the treatment with a magnetic coil that mimicked the experience of the magnetic pulse; both the control and active treatment groups wore noise-canceling earphones and received a topical ointment to dull sensation. Neither the researcher administering the procedure nor the participant knew whether the participant was receiving real treatment.
A hard-to-treat group
The trial participants ranged in age from 22 to 80; on average, they had suffered depression for nine years. They had tried medications, but either they had had no effect or they had stopped working. During the trial, participants who were on medication maintained their regular dosage; participants who weren’t taking medications did not start any.

Nolan Williams demonstrates SAINT, the magnetic brain stimulation therapy he and his colleagues developed, on Deirdre Lehman, a participant in a previous study of the treatment. Steve Fisch
Within four weeks after treatment, 12 of the 14 participants who had received the treatment improved, and 11 of them met FDA criteria for remission. In contrast, only two of the 15 participants who had received the placebo met the criteria for remission.
Because the study participants typically felt better within days of starting SAINT, the researchers are hoping it can be used to quickly treat patients who are at a crisis point. Patients who start taking medication for depression typically don’t experience any reduction of symptoms for a month.
“We want to get this into emergency departments and psychiatric wards where we can treat people who are in a psychiatric emergency,” Williams said. “The period right after hospitalization is when there’s the highest risk of suicide.”
Van Brocklin said that since he returned home following treatment, he’s made some radical changes. “I have a really strong desire to get my life together,” he said.
“I don’t procrastinate anymore,” he added. “I’m sleeping better. I completely quit alcohol. I’m walking my dog and playing the guitar again, for nothing more than the sheer joy of it.”
Most importantly, he said, “I’m remaining positive and being respectful of others. These are big changes in my life.”
Other Stanford scientists who contributed to the study are former postdoctoral scholars Eleanor Cole, PhD, and Angela Phillips, PhD; Brandon Bentzley, MD, PhD, David Carreon, MD, Jennifer Keller, PhD, Kristin Raj, MD, and Flint Espil, PhD, all clinical assistant professors of psychiatry and behavioral sciences; clinical research coordinators Katy Stimpson, Romina Nejad, Clive Veerapal, Nicole Odenwald and Maureen Chang; former clinical research coordinators Fahim Barmak, MD, Naushaba Khan and Rachel Rapier; postdoctoral scholars Kirsten Cherian, PhD, James Bishop, PhD, Azeezat Azeez, PhD, and John Coetzee, PhD; life science research professional Heather Pankow; clinical research manager Jessica Hawkins; Charles DeBattista, MD, professor of psychiatry and behavioral sciences; and Booil Jo, PhD, associate professor of psychiatry and behavioral sciences.
Scientists from the U.S. Department of Veterans Affairs; Palo Alto University; the Centre for Neuroimaging and Cognitive Genomics at the National University of Ireland; and the School of Medicine at Southern Illinois University, Carbondale, contributed to the research.
The research was funded by a Brain and Behavior Research Foundation Young Investigator Award, Charles R. Schwab, the David and Amanda Chao Fund II, the Amy Roth PhD Fund, the Neuromodulation Research Fund, the Lehman Family, the Still Charitable Trust, the Marshall and Dee Ann Payne Fund, and the Gordie Brookstone Fund.
Stanford’s Department of Psychiatry and Behavioral Sciences also contributed to the work.
If you're interested in participating in a study, please email [email protected] .

About Stanford Medicine
Stanford Medicine is an integrated academic health system comprising the Stanford School of Medicine and adult and pediatric health care delivery systems. Together, they harness the full potential of biomedicine through collaborative research, education and clinical care for patients. For more information, please visit med.stanford.edu .
Hope amid crisis
Psychiatry’s new frontiers


- Latest news
- UCL in the media
- Services for media
- Student news
- Tell us your story

Analysis: Depression is probably not caused by a chemical imbalance in the brain – new study
20 July 2022
Writing in The Conversation, Professor Joanna Moncrieff and Dr Mark Horowitz (both UCL Psychiatry) report on their new research showing no clear evidence that serotonin levels or serotonin activity are responsible for depression.

For three decades, people have been deluged with information suggesting that depression is caused by a “chemical imbalance” in the brain – namely an imbalance of a brain chemical called serotonin. However, our latest research review shows that the evidence does not support it.
Although first proposed in the 1960s, the serotonin theory of depression started to be widely promoted by the pharmaceutical industry in the 1990s in association with its efforts to market a new range of antidepressants, known as selective serotonin-reuptake inhibitors or SSRIs. The idea was also endorsed by official institutions such as the American Psychiatric Association, which still tells the public that “differences in certain chemicals in the brain may contribute to symptoms of depression”.
Countless doctors have repeated the message all over the world, in their private surgeries and in the media. People accepted what they were told. And many started taking antidepressants because they believed they had something wrong with their brain that required an antidepressant to put right. In the period of this marketing push, antidepressant use climbed dramatically, and they are now prescribed to one in six of the adult population in England, for example.
For a long time, certain academics, including some leading psychiatrists, have suggested that there is no satisfactory evidence to support the idea that depression is a result of abnormally low or inactive serotonin. Others continue to endorse the theory. Until now, however, there has been no comprehensive review of the research on serotonin and depression that could enable firm conclusions either way.
At first sight, the fact that SSRI-type antidepressants act on the serotonin system appears to support the serotonin theory of depression. SSRIs temporarily increase the availability of serotonin in the brain, but this does not necessarily imply that depression is caused by the opposite of this effect.
There are other explanations for antidepressants’ effects. In fact, drug trials show that antidepressants are barely distinguishable from a placebo (dummy pill) when it comes to treating depression. Also, antidepressants appear to have a generalised emotion-numbing effect which may influence people’s moods, although we do not know how this effect is produced or much about it.
There has been extensive research on the serotonin system since the 1990s, but it has not been collected systematically before. We conducted an “umbrella” review that involved systematically identifying and collating existing overviews of the evidence from each of the main areas of research into serotonin and depression. Although there have been systematic reviews of individual areas in the past, none have combined the evidence from all the different areas taking this approach.
One area of research we included was research comparing levels of serotonin and its breakdown products in the blood or brain fluid. Overall, this research did not show a difference between people with depression and those without depression.
Another area of research has focused on serotonin receptors, which are proteins on the ends of the nerves that serotonin links up with and which can transmit or inhibit serotonin’s effects. Research on the most commonly investigated serotonin receptor suggested either no difference between people with depression and people without depression, or that serotonin activity was actually increased in people with depression – the opposite of the serotonin theory’s prediction.
Research on the serotonin “transporter”, that is the protein which helps to terminate the effect of serotonin (this is the protein that SSRIs act on), also suggested that, if anything, there was increased serotonin activity in people with depression. However, these findings may be explained by the fact that many participants in these studies had used or were currently using antidepressants.
We also looked at research that explored whether depression can be induced in volunteers by artificially lowering levels of serotonin. Two systematic reviews from 2006 and 2007 and a sample of the ten most recent studies (at the time the current research was conducted) found that lowering serotonin did not produce depression in hundreds of healthy volunteers. One of the reviews showed very weak evidence of an effect in a small subgroup of people with a family history of depression, but this only involved 75 participants.
Very large studies involving tens of thousands of patients looked at gene variation, including the gene that has the instructions for making the serotonin transporter. They found no difference in the frequency of varieties of this gene between people with depression and healthy controls.
Although a famous early study found a relationship between the serotonin transporter gene and stressful life events, larger, more comprehensive studies suggest no such relationship exists. Stressful life events in themselves, however, exerted a strong effect on people’s subsequent risk of developing depression.
Some of the studies in our overview that included people who were taking or had previously taken antidepressants showed evidence that antidepressants may actually lower the concentration or activity of serotonin.
The serotonin theory of depression has been one of the most influential and extensively researched biological theories of the origins of depression. Our study shows that this view is not supported by scientific evidence. It also calls into question the basis for the use of antidepressants.
Most antidepressants now in use are presumed to act via their effects on serotonin. Some also affect the brain chemical noradrenaline. But experts agree that the evidence for the involvement of noradrenaline in depression is weaker than that for serotonin.
There is no other accepted pharmacological mechanism for how antidepressants might affect depression. If antidepressants exert their effects as placebos, or by numbing emotions, then it is not clear that they do more good than harm.
Although viewing depression as a biological disorder may seem like it would reduce stigma, in fact, research has shown the opposite, and also that people who believe their own depression is due to a chemical imbalance are more pessimistic about their chances of recovery.
It is important that people know that the idea that depression results from a “chemical imbalance” is hypothetical. And we do not understand what temporarily elevating serotonin or other biochemical changes produced by antidepressants do to the brain. We conclude that it is impossible to say that taking SSRI antidepressants is worthwhile, or even completely safe. People need all this information to make informed decisions about whether or not to take antidepressants.
This article originally appeared in The Conversation on 20 July 2022.
- Article in The Conversation
- Article in The Conversation (Spanish)
- Professor Joanna Moncrieff's academic profile
- Dr Mark Horowitz's academic profile
- UCL Division of Psychiatry
- UCL Faculty of Brain Sciences

Transforming the understanding and treatment of mental illnesses.
Información en español
Celebrating 75 Years! Learn More >>
- Science News
- Meetings and Events
- Social Media
- Press Resources
- Email Updates
- Innovation Speaker Series
Basic Research Powers the First Medication for Postpartum Depression
May 14, 2024 • Feature Story • 75th Anniversary
At a Glance
- Postpartum depression (PPD) is a common mental disorder that many women experience after giving birth.
- Onset of PPD coincides with a dramatic drop in levels of a brain-derived steroid (neurosteroid) known as allopregnanolone.
- Decades of research supported by NIMH illuminated the role of neurosteroids like allopregnanolone in mental illnesses.
- In 2019, brexanolone—a medication that acts by mimicking allopregnanolone—became the first approved drug to treat PPD.
- Able to significantly and rapidly reduce PPD symptoms, brexanolone was a major leap forward in depression treatment.
Joshua A. Gordon, M.D., Ph.D., a practicing psychiatrist at the time, would never forget the call he received one night from a distraught mother.

“She was plagued with a deep, inescapable hopelessness—so depressed she was afraid she was going to hurt her month-old daughter. I helped her get to the hospital, where she spent the next 2 months in an in-patient program trying every available treatment to recover,” said Dr. Gordon, now the Director of the National Institute of Mental Health (NIMH).
Unfortunately, this experience is not uncommon among women and other postpartum people who may feel intense sadness, anxiety, and loss of interest after giving birth. These symptoms can be signs of a clinical disorder known as postpartum depression (PPD) . Unlike the "baby blues" or feelings of sadness many new mothers experience in the days after delivery, PPD is more intense and long-lasting, with damaging impacts on health and well-being.
More than the blues: Impacts of PPD on women's mental health
Depression is a common but serious mood disorder. According to the Centers for Disease Control and Prevention (CDC), rates of depression are high—and rising—among postpartum women. Using data from the 2018 Pregnancy Risk Assessment Monitoring System , the CDC found that about 1 in 8 postpartum women had symptoms of depression, while another CDC study showed rates of PPD that were seven times higher in 2015 compared to 2000.
Depression can happen to anyone, and it's especially tough for new moms dealing with the physical challenges of childbirth and the stresses of caring for a young child. When women experience PPD, they often have strong feelings of sadness, anxiety, worthlessness, and guilt. Their sleep, eating, thoughts, and actions can all change noticeably. These mood and behavior changes can be highly distressing and even life-threatening, making it difficult for a woman to do everyday things and take care of herself or her child. In extreme cases, women with PPD may be at risk of hurting themselves or their child or attempting suicide.
Fast-acting, effective treatment for PPD can be life-changing and potentially lifesaving. However, for too long, such care was hard to reach, leaving many women to struggle with depression at a pivotal point in life. Despite some similarities, PPD is not the same as major depression at other times in life. Because of this, usual depression treatments are much less effective in managing the symptoms of PPD.

“PPD is very difficult to treat,” said Mi Hillefors, M.D., Ph.D., Deputy Director of the NIMH Division of Translational Research. “It is usually treated with medications originally approved for major depression—despite limited evidence that they are effective in treating PPD. Standard depression treatments, including antidepressants, psychotherapy, and brain stimulation therapy, can also take weeks or longer to work.”
PPD’s unique risk factors reflect the physical changes of pregnancy and the postpartum period, which include dramatic changes in levels of many hormones and other molecules.
These biological changes had long been seen as a possible source of postpartum mood disorders like depression. But could they also be a solution?
Unlocking the power of allopregnanolone through basic research
Some psychiatric medications owe their discovery to chance. Not so with brexanolone, the first-ever medication to specifically treat PPD. Brexanolone culminated a long series of research studies, much of it funded by NIMH as part of its commitment to understand and support women’s mental health .
Thanks to NIMH-supported basic research, brexanolone was developed by design—a design centered around a molecule called allopregnanolone .
Allopregnanolone is a steroid naturally produced in the brain and with important actions there, such as regulating neurotransmitter activity and protecting neurons from damage. Its impact extends to mental health, with higher levels linked to better mood, lower anxiety, and reduced depression .
Allopregnanolone is also important to pregnancy , during which its levels are extremely high. This happens because of the enhanced production of a hormone called progesterone, which prepares the body for pregnancy and childbirth.
In the last few months of pregnancy, the ovaries and placenta make more progesterone, causing a huge rise in allopregnanolone levels. These levels then drop rapidly after birth. Because allopregnanolone plays a crucial role in mood, these ups and downs can impact a woman’s mental health during and after pregnancy.
Researchers had been aware of brain-derived steroids like allopregnanolone as far back as the 1940s. But the journey to a new PPD treatment began within NIMH's Intramural Research Program (IRP) . At the helm was the NIMH Scientific Director at that time, Steven Paul, M.D., who collaborated with researchers in the NIMH Clinical Neuroscience Branch and at other NIH institutes, including the National Institute of Neurological Disorders and Stroke (NINDS). The researchers sought to understand how the steroids work, change over time, respond to stress, and ultimately relate to health and disease.
Early discoveries came in the 1980s. Paul, working with Maria Majewska, Ph.D., Jacqueline Crawley, Ph.D., A. Leslie Morrow, Ph.D., and other researchers showed that hormones such as progesterone and molecules derived from them have calming and anxiety-reducing effects . Extensive research by Paul’s lab showed that these anxiolytic effects come from enhancing the activity of GABA by binding to specific sites on its receptor. As the main inhibitory neurotransmitter (chemical messenger), GABA reduces the activity of neurons, making them less likely to fire. When molecules bind to its receptor, GABA becomes more potent at inhibiting electrical activity in the brain, with calming effects on behavior.
Paul and IRP colleague Robert Purdy, Ph.D., used the term “ neuroactive steroids ,” or neurosteroids, to describe these molecules able to bind to receptors in the brain to rapidly alter neuronal excitability. Their work in animals confirmed that allopregnanolone is synthesized in the brain . They also showed the effects of allopregnanolone on GABA receptors in humans. Moreover, they found that allopregnanolone affects the response to stress , with acute stress leading the neurosteroid to increase to levels that alter GABA activity. These findings suggested that neurosteroids play an important role in helping animals “reset” and adaptively respond to stressful life events.
Together, this IRP-conducted research established the importance of neurosteroids via their presence in the brain, ability to reduce neuronal activity, and release during stress. Although much of this work was conducted in animals, it would spotlight neurosteroids—and allopregnanolone in particular—as promising targets for treating mental disorders, eventually opening the door to their therapeutic use in humans.
Bridging the gap to advance clinical intervention
While NIMH intramural researchers were making remarkable strides, researchers at other institutions were also conducting work bolstered by funding from NIMH. Among them were Alessandro Guidotti, M.D., at the University of Illinois at Chicago; Istvan Mody, Ph.D., at the University of California, Los Angeles; and Charles Zorumski, M.D., at Washington University in St. Louis. Their NIMH-funded research propelled understanding of inhibitory neurosteroids and their importance in reducing the adverse effects of stress. This work would be the impetus for homing in on allopregnanolone as a treatment for PPD.

Guidotti and colleagues conducted several NIMH-funded studies. Their research in rodents confirmed that allopregnanolone is produced in the brain and helps regulate neuronal excitability by acting on GABA receptors. They also built on the knowledge that neurosteroids are affected by stress. However, unlike acute stress, a stressor lasting multiple weeks led to a decrease in allopregnanolone in brain areas involved in anxiety- and depression-like behaviors.
Importantly, their NIMH-funded work offered some of the earliest evidence that allopregnanolone contributes to depression by showing significantly lower levels in people with depression compared to people without the disorder, a rise in levels (but not that of other neurosteroids) after treatment with antidepressant medication , and a link between increased levels and reduced depression symptoms .
NIMH and NINDS funded multiple studies by Mody and colleagues on interactions of neurosteroids, stress, and GABA receptors. This research was integral to understanding a mechanism in the brains of mice that might explain why some people become depressed after childbirth. Their NIMH-supported research showed changes in GABA receptors in the brain, where neurosteroids are active, that impaired the body’s ability to adapt to hormonal fluctuations. Animals with an irregular GABA receptor component lacking sensitivity to neurosteroids showed depression-like behaviors and reduced maternal care; treating them with a drug that restored the receptor’s function reversed those changes.
Another study by Mody and colleagues revealed changes in GABA expression during pregnancy that led to greater neuronal activity in the brain—but could be brought down by allopregnanolone. This finding opened the door to future studies exploring whether a postpartum drop in the neurosteroid contributed to the risk for mood disorders after birth.
Zorumski led a team in extensively studying neurosteroids as well. Among their seminal findings was identifying the mechanisms by which inhibitory neurosteroids like allopregnanolone affect GABA receptor activity . Their NIMH-funded work dramatically augmented knowledge of how neurosteroids alter GABA receptors to contribute to the risk for mental disorders like PPD.
“The accumulated evidence from these studies established the necessary bridges to justify examining a potential therapeutic role for allopregnanolone in women with PPD,” said Peter Schmidt, M.D., Chief of the NIMH Behavioral Endocrinology Branch.
By the 2010s, researchers had a much better understanding of how allopregnanolone is linked to PPD. Studies showed decreased allopregnanolone in pregnant and postpartum women with symptoms of depression and higher allopregnanolone associated with a lower risk of PPD . The possibility that PPD might be caused by the downregulation of GABA receptors in response to low levels of allopregnanolone after birth inspired researchers to put that theory to the test in clinical studies with human participants.
Taking allopregnanolone from bench to bedside
Extensive research, supported by NIMH and other NIH institutes, found that neurosteroids play a key role in how people deal with stress. They also contribute to the development of mood disorders like anxiety and depression. For allopregnanolone, evidence that it sharply decreases after pregnancy and regulates GABA activity gave rise to the notion that it contributes to PPD—and inspired hope it could be used to treat the disorder.
The biopharmaceutical company Sage Therapeutics utilized this basic research to develop brexanolone. Administered intravenously by a health care professional in a doctor’s office or clinic, brexanolone mimics the effects of allopregnanolone, increasing the inhibitory actions of GABA receptors.
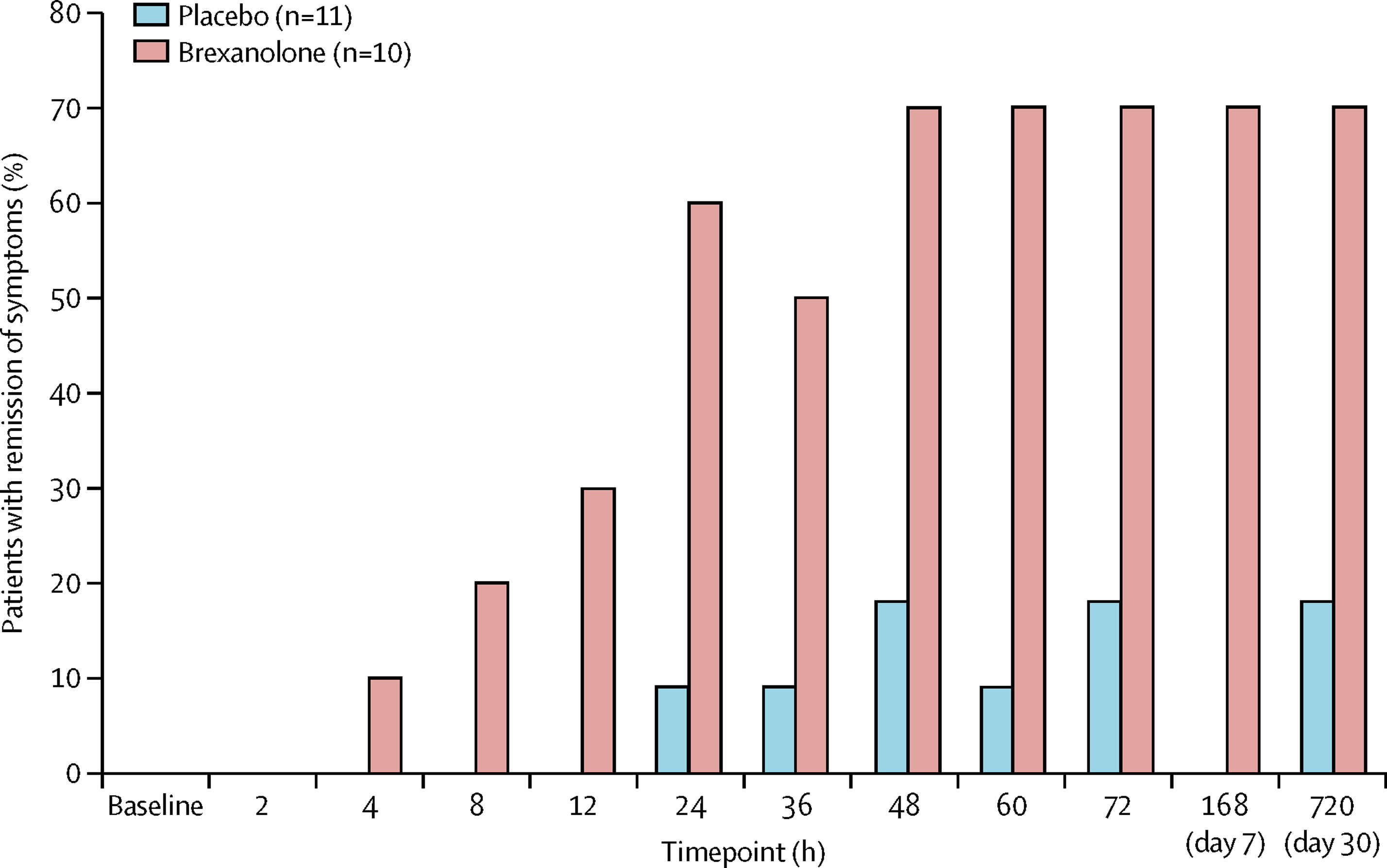
Stephen Kanes, M.D., Ph.D., at Sage Therapeutics and Samantha Meltzer-Brody, M.D., MPH, at the University of North Carolina led several randomized clinical trials to measure the effectiveness of the medication in treating PPD and evaluate its safety and tolerability. The studies, which recruited adult women with PPD from hospitals, research centers, and psychiatric clinics across the United States, measured the effects of brexanolone compared to a placebo over 4 weeks.
The trials were a success. Brexanolone significantly and meaningfully reduced PPD symptoms , and it had only mild side effects. Compared to usual depression treatments, brexanolone brought about a faster response and greater improvement . Whereas most antidepressants take weeks to work, brexanolone improved symptoms and functioning in women with PPD within a few hours to days. And the effects lasted up to a month after the treatment stopped. Not only was brexanolone more effective, but it also worked faster than other depression medications.
“The dramatic impact of basic research on real-world health outcomes has been inspiring. The fact that NIMH-supported studies contributed to successful drug development in a matter of decades is a remarkable feat and a powerful demonstration of the potential of this foundational research,” said Dr. Gordon.
Based on this promising evidence, the U.S. Food and Drug Administration (FDA) gave brexanolone priority review and breakthrough therapy designation in September 2016. Then, in March 2019, the FDA approved brexanolone , making it the first drug to treat PPD.
Brightening the future for women with PPD
For women with PPD, brexanolone was a long-awaited reason to celebrate. For NIMH, it was a testament to discoveries made through the decades of research it supported. Although some barriers to treatment persisted, women now had greater hope for treating depression symptoms after pregnancy.
“The approval of brexanolone was an important milestone. Finally, an effective, fast-acting medication specifically to treat PPD,” said Dr. Hillefors. “It was also a victory for psychiatric neuroscience because basic and translational research—by design, not chance—led to a truly novel and effective treatment for a psychiatric disorder.”
Without NIMH-supported studies providing the foundational knowledge of neurosteroids, researchers may have never made the connection between allopregnanolone and treating PPD. “That’s why the approval of brexanolone is such a cause for celebration for mental health research: It represents a true bench-to-bedside success,” said Dr. Gordon.
The success of brexanolone has continued to open the door to exciting advancements in mental health care. For instance, researchers and clinicians are investigating ways to make brexanolone work better for all postpartum people. Researchers are also testing how neurosteroids can be used to treat other forms of depression and other mental health conditions.
Just the beginning of treatment advances for PPD
Brexanolone is only the start of what will hopefully be a new future for PPD treatment. In August 2023, the FDA approved zuranolone as the first oral medication for PPD. Zuranolone acts via similar biological mechanisms as brexanolone. Its approval reflects the next step in NIMH-supported basic research being translated into clinical practice with real-world benefits.
The success of the drug, which is taken in pill form, was shown in two randomized multicenter clinical trials . Women with severe PPD who received zuranolone showed statistically significant and clinically meaningful improvements in depression symptoms compared to women who received a placebo. These effects were rapid, sustained through 45 days, and seen across a range of clinical measures. The benefits were mirrored in patients’ self-assessment of their depression symptoms.
According to Dr. Schmidt, “The approval of zuranolone to treat PPD provides women with a rapid and effective treatment that avoids some of the limitations of the original intravenous medication.”
And the journey is far from over. Researchers, clinicians, and industry are continuing to innovate new treatments for PPD to increase access and availability to ensure all people can receive help for their postpartum symptoms.
“While I will never forget that phone call from my patient, the development of these effective medications brings us hope for helping people with PPD and for the overall impact of basic research to truly make a difference in people’s lives,” concluded Dr. Gordon.
Publications
Burval, J., Kerns, R., & Reed, K. (2020). Treating postpartum depression with brexanolone. Nursing , 50 (5), 48−53. https://doi.org/10.1097/01.NURSE.0000657072.85990.5a
Cornett, E. M., Rando, L., Labbé, A. M., Perkins, W., Kaye, A. M., Kaye, A. D., Viswanath, O., & Urits, I. (2021). Brexanolone to treat postpartum depression in adult women. Psychopharmacology Bulletin , 51 (2), 115–130. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8146562/pdf/PB-51-2-115.pdf
Deligiannidis, K. M., Meltzer-Brody, S., Maximos, B., Peeper, E. Q., Freeman, M., Lasser, R., Bullock, A., Kotecha, M., Li, S., Forrestal, F., Rana, N., Garcia, M., Leclair, B., & Doherty, J. (2023). Zuranolone for the treatment of postpartum depression. American Journal of Psychiatry , 180 (9), 668−675. https://doi.org/10.1176/appi.ajp.20220785
Deligiannidis, K. M., Kroll-Desrosiers, A. R., Mo, S., Nguyen, H. P., Svenson, A., Jaitly, N., ... & Shaffer, S. A. (2016). Peripartum neuroactive steroid and γ-aminobutyric acid profiles in women at-risk for postpartum depression. Psychoneuroendocrinology , 70 , 98−107. https://doi.org/10.1016/j.psyneuen.2016.05.010
Edinoff, A. N., Odisho, A. S., Lewis, K., Kaskas, A., Hunt, G., Cornett, E. M., Kaye, A. D., Kaye, A., Morgan, J., Barrilleaux, P. S., Lewis, D., Viswanath, O., & Urits, I. (2021). Brexanolone, a GABAA modulator, in the treatment of postpartum depression in adults: A comprehensive review. Frontiers in Psychiatry , 12 , Article 699740. https://doi.org/10.3389/fpsyt.2021.699740
Epperson, C. N., Rubinow, D. R., Meltzer-Brody, S., Deligiannidis, K. M., Riesenberg, R., Krystal, A.D., Bankole, K., Huang, M. Y., Li, H., Brown, C., Kanes, S. J., & Lasser R. (2023). Effect of brexanolone on depressive symptoms, anxiety, and insomnia in women with postpartum depression: Pooled analyses from 3 double-blind, randomized, placebo-controlled clinical trials in the HUMMINGBIRD clinical program. Journal of Affective Disorders , 320 , 353−359. https://doi.org/10.1016/j.jad.2022.09.143
Gilbert Evans, S. E., Ross, L. E., Sellers, E. M., Purdy, R. H., & Romach, M. K. (2005). 3α-reduced neuroactive steroids and their precursors during pregnancy and the postpartum period. Gynecological Endocrinology , 21 (5), 268−279. https://doi.org/10.1080/09513590500361747
Guintivano, J., Manuck, T., & Meltzer-Brody, S. (2018). Predictors of postpartum depression: A comprehensive review of the last decade of evidence. Clinical Obstetrics and Gynecology , 61 (3), 591−603. https://doi.org/10.1097/GRF.0000000000000368
Gunduz-Bruce, H., Koji, K., & Huang, M.-Y. (2022). Development of neuroactive steroids for the treatment of postpartum depression. Journal of Neuroendocrinology , 34 (2), Article e13019. https://doi.org/10.1111/jne.13019
Haight, S. C., Byatt, N., Moore Simas, T. A., Robbins, C. L., & Ko, J. Y. (2019). Recorded diagnoses of depression during delivery hospitalizations in the United States, 2000-2015. Obstetrics and Gynecology , 133 (6), 1216−1223. https://doi.org/10.1097/AOG.0000000000003291
Hellgren, C., Åkerud, H., Skalkidou, A., Bäckström, T., & Sundström-Poromaa, I. (2014). Low serum allopregnanolone is associated with symptoms of depression in late pregnancy. Neuropsychobiology , 69 (3), 147–153. https://doi.org/10.1159/000358838
Hutcherson, T. C., Cieri-Hutcherson, N. E., & Gosciak, M. F. (2023). Brexanolone for postpartum depression. American Journal of Health-System Pharmacy , 77 (5), 336−345. https://doi.org/10.1093/ajhp/zxz333
Kanes, S., Colquhoun, H., Gunduz-Bruce, H., Raines, S., Arnold, R., Schacterle, A., Doherty, J., Epperson, C. N., Deligiannidis, K. M., Riesenberg, R., Hoffmann, E., Rubinow, D., Jonas, J., Paul, S., & Meltzer-Brody, S. (2017). Brexanolone (SAGE-547 injection) in post-partum depression: A randomised controlled trial. The Lancet , 390(10093), 480−489. https://doi.org/10.1016/S0140-6736(17)31264-3
Leader, L. D., O'Connell, M., & VandenBerg, A. (2019). Brexanolone for postpartum depression: Clinical evidence and practical considerations. Pharmacotherapy , 39 (11), 1105–1112. https://doi.org/10.1002/phar.2331
Maguire, J., & Mody, I. (2008). GABAAR plasticity during pregnancy: Relevance to postpartum depression. Neuron , 59 (2), P207–P213. https://doi.org/10.1016/j.neuron.2008.06.019
Maguire, J., & Mody, I. (2016). Behavioral deficits in juveniles mediated by maternal stress hormones in mice. Neural Plasticity , Article 2762518. https://doi.org/10.1155/2016/2762518
Majewska, M. D., Harrison, N. L., Schwartz, R. D., Barker, J. L., & Paul, S. M. (1986). Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science , 232 (4753), 1004−1007. https://doi.org/10.1126/science.2422758
McEvoy, K., & Osborne, L. M. (2019). Allopregnanolone and reproductive psychiatry: An overview. International Review of Psychiatry , 31 (3), 237–244. https://doi.org/10.1080/09540261.2018.1553775
Meltzer-Brody, S., Colquhoun, H., Riesenberg, R., Epperson, C. N., Deligiannidis, K. M., Rubinow, D. R., Li, H., Sankoh, A. J., Clemson, C., Schacterle, A., Jonas, J., & Kanes, S. (2018). Brexanolone injection in post-partum depression: Two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. The Lancet , 392 (10152), 1058−1070. https://doi.org/10.1016/S0140-6736(18)31551-4
Morrison, K. E., Cole, A. B., Thompson, S. M., & Bale, T. L. (2019). Brexanolone for the treatment of patients with postpartum depression. Drugs Today , 55 (9), 537–544. https://doi.org/10.1358/dot.2019.55.9.3040864
Purdy, R. H., Morrow, A. L., Moore, P. H., & Paul, S. M. (1991). Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proceedings of the National Academy of Sciences , 88 (10), 4553−4557. https://doi.org/10.1073/pnas.88.10.4553
Scarff, J. R. (2019). Use of brexanolone for postpartum depression. Innovations in Clinical Neuroscience , 16 (11−12), 32–35.
Schüle, C., Nothdurfter, C., & Rupprecht, R. (2014). The role of allopregnanolone in depression and anxiety. Progress in Neurobiology , 113 , 79−87. https://doi.org/10.1016/j.pneurobio.2013.09.003
Selye, H. (1941). Anesthetic effect of steroid hormones. Experimental Biology and Medicine , 46 (1), 116–121. https://doi.org/10.3181/00379727-46-11907
Shorey, S., Chee, C. Y. I., Ng, E. D., Chan, Y. H., Tam, W. W. S., & Chong, Y. S. (2018). Prevalence and incidence of postpartum depression among healthy mothers: A systematic review and meta-analysis. Journal of Psychiatric Research , 104 , 235–248. https://doi.org/10.1016/j.jpsychires.2018.08.001
Slomian, J., Honvo, G., Emonts, P., Reginster, J. Y., & Bruyère, O. (2019). Consequences of maternal postpartum depression: A systematic review of maternal and infant outcomes. Women's Health , 15 , 1−55. https://doi.org/10.1177/1745506519844044
- Perinatal Depression (NIMH brochure)
- Depression in Women: 4 Things You Should Know (NIMH health topic page)
- Depression (NIMH health topic page)
- Major Depression (NIMH statistics page)
- Women and Mental Health (NIMH health topic page)
- A Bench-to-Bedside Story: The Development of a Treatment for Postpartum Depression (NIMH Director’s Message)
- Bench-to-Bedside: NIMH Research Leading to Brexanolone, First-Ever Drug Specifically for Postpartum Depression (NIIMH press release)
- Population Study Finds Depression Is Different Before, During, and After Pregnancy (NIMH research highlight)
- FDA Approves First Treatment for Post-Partum Depression (FDA news release)
- FDA Approves First Oral Treatment for Postpartum Depression (FDA news release)
Almost 1 in 2 Americans feel they’ve lost time to poor mental health, survey says. It’s worse for people with depression or anxiety

Every now and then you may wonder, Where did the time go? Whether mystified at how quickly an afternoon slipped away or reflecting on years gone by at lightning speed, you’ve probably experienced periodic sensations of lost time. Yet 44% of Americans feel they’ve lost time in their lives due to a known culprit: poor mental health .
Among people diagnosed with depression and/or anxiety, this percentage nearly doubles to 78%.
That’s according to a new national survey from Myriad Genetics , dubbed the GeneSight Mental Health Monitor. In February, the genetic testing company and ACUPOLL Precision Research surveyed 1,000 U.S. adults about their mental health. The results, published in April, reveal the chronological toll of mental illness.
Among respondents diagnosed with depression and/or anxiety, 50% said they’ve lost years of their life to poor mental health, while 12% said they’ve lost decades.
“For a patient who is struggling, time ticks a lot slower than it does for the rest of us,” Debbie Thomas, EdD , a psychiatric nurse practitioner in Prospect, Ky., said in a GeneSight news release . “One of my patients told me that when they woke up in the morning, they counted how many hours before they could go back to bed. That’s pretty telling when someone is in the depths of depression and anxiety to that degree.”
Many people reported poor mental health has robbed them of not only time itself, but also fundamental moments. About 71% of respondents said it has kept them from being fully present during important events, and more than half of people with depression and/or anxiety said they’d missed out on a major life event because of their mental health. Respondents with these conditions said they felt guilty, hopeless, useless, worthless, and/or self-critical when missing milestones .
In addition, 33% of respondents with depression and/or anxiety cited ineffective mental health treatments as a reason for missing significant events.
The vast majority of people with depression and/or anxiety, 82%, said their mental health had prevented them from having fun or enjoying themselves in the past year, compared to 78% of all respondents.
Patients with depression and/or anxiety tend to be as upset about the time they feel they lost due to poor mental health as they are about having a mental illness, said Sharon Philbin, MSN , an advanced practice registered nurse in Pawtucket, R.I.
“Patients who have lost time due to depressive episodes or periods of anxiety often feel a sense of loss, which further complicates their mental health situation,” Philbin said in the news release. “Many of my patients say they are thankful they feel better, but they worry that it will happen again.”
Just 16% of survey respondents said they feel “ready to take on the world” following a depressive episode. They also feel:
- Exhausted: 60%
- Coming out of a fog: 50%
- Disappointed to have missed out on life: 47%
The survey relied on respondents to self-report having been diagnosed with depression or anxiety by a medical professional. While polling included mental health screening instruments—the Patient Health Questionnaire-2 (PHQ-2) for depression and the Generalized Anxiety Disorder-2 (GAD-2) questionnaire for anxiety—it’s unclear what types of these disorders respondents had.
If you need immediate mental health support, contact the 988 Suicide & Crisis Lifeline .
For more on mental health:
- Americans are increasingly concerned about their mental health, survey says. Here’s how social media plays a role
- 75% of Americans think mental health issues are treated worse than physical illness, new survey says. Here’s why
- These states and cities have the best—and worst—brain health in America
- These are the 10 worst US states to live in for your mental health, according to a new study
Subscribe to Well Adjusted, our newsletter full of simple strategies to work smarter and live better, from the Fortune Well team. Sign up for free today.
Most Popular

Advertisement
Major Depressive Disorder: Advances in Neuroscience Research and Translational Applications
- Open access
- Published: 13 February 2021
- Volume 37 , pages 863–880, ( 2021 )
Cite this article
You have full access to this open access article

- Zezhi Li 1 , 2 ,
- Meihua Ruan 3 ,
- Jun Chen 1 , 5 &
- Yiru Fang ORCID: orcid.org/0000-0002-8748-9085 1 , 4 , 5
47k Accesses
111 Citations
16 Altmetric
Explore all metrics
A Correction to this article was published on 17 May 2021
This article has been updated
Major depressive disorder (MDD), also referred to as depression, is one of the most common psychiatric disorders with a high economic burden. The etiology of depression is still not clear, but it is generally believed that MDD is a multifactorial disease caused by the interaction of social, psychological, and biological aspects. Therefore, there is no exact pathological theory that can independently explain its pathogenesis, involving genetics, neurobiology, and neuroimaging. At present, there are many treatment measures for patients with depression, including drug therapy, psychotherapy, and neuromodulation technology. In recent years, great progress has been made in the development of new antidepressants, some of which have been applied in the clinic. This article mainly reviews the research progress, pathogenesis, and treatment of MDD.
Similar content being viewed by others

Molecular Mechanisms of Psilocybin and Implications for the Treatment of Depression

Lavender oil preparation Silexan is effective in mild-to-moderate major depression: a randomized, placebo- and reference-controlled trial

The Roles of Serotonin in Neuropsychiatric Disorders
Avoid common mistakes on your manuscript.
Major depressive disorder (MDD) also referred to as depression, is one of the most severe and common psychiatric disorders across the world. It is characterized by persistent sadness, loss of interest or pleasure, low energy, worse appetite and sleep, and even suicide, disrupting daily activities and psychosocial functions. Depression has an extreme global economic burden and has been listed as the third largest cause of disease burden by the World Health Organization since 2008, and is expected to rank the first by 2030 [ 1 , 2 ]. In 2016, the Global Burden of Diseases, Injuries, and Risk Factors Study demonstrated that depression caused 34.1 million of the total years lived with disability (YLDs), ranking as the fifth largest cause of YLD [ 3 ]. Therefore, the research progress and the clinical application of new discoveries or new technologies are imminent. In this review, we mainly discuss the current situation of research, developments in pathogenesis, and the management of depression.
Current Situation of Research on Depression
Analysis of published papers.
In the past decade, the total number of papers on depression published worldwide has increased year by year as shown in Fig. 1 A. Searching the Web of Science database, we found a total of 43,863 papers published in the field of depression from 2009 to 2019 (search strategy: TI = (depression$) or ts = ("major depressive disorder$")) and py = (2009 – 2019), Articles). The top 10 countries that published papers on the topic of depression are shown in Fig. 1 B. Among them, researchers in the USA published the most papers, followed by China. Compared with the USA, the gap in the total number of papers published in China is gradually narrowing (Fig. 1 C), but the quality gap reflected by the index (the total number of citations and the number of citations per paper) is still large, and is lower than the global average (Fig. 1 D). As shown in Fig. 1 E, the hot research topics in depression are as follows: depression management in primary care, interventions to prevent depression, the pathogenesis of depression, comorbidity of depression and other diseases, the risks of depression, neuroimaging studies of depression, and antidepressant treatment.
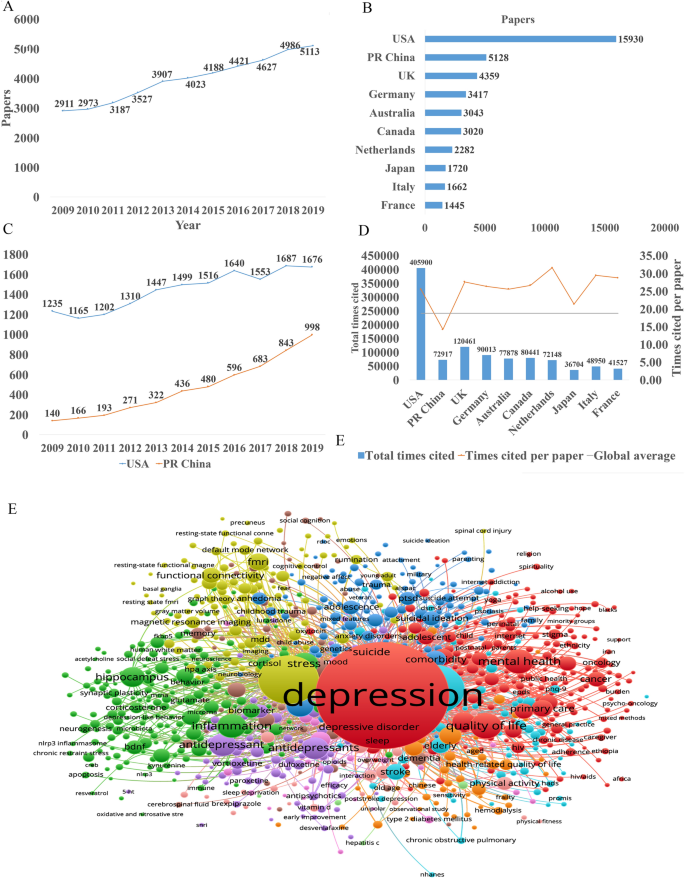
Analysis of published papers around the world from 2009 to 2019 in depressive disorder. A The total number of papers [from a search of the Web of Science database (search strategy: TI = (depression$) or ts = ("major depressive disorder$")) and py = (2009 – 2019), Articles)]. B The top 10 countries publishing on the topic. C Comparison of papers in China and the USA. D Citations for the top 10 countries and comparison with the global average. E Hot topics.
Analysis of Patented Technology Application
There were 16,228 patent applications in the field of depression between 2009 and 2019, according to the Derwent Innovation Patent database. The annual number and trend of these patents are shown in Fig. 2 A. The top 10 countries applying for patents related to depression are shown in Fig. 2 B. The USA ranks first in the number of depression-related patent applications, followed by China. The largest number of patents related to depression is the development of antidepressants, and drugs for neurodegenerative diseases such as dementia comorbid with depression. The top 10 technological areas of patents related to depression are shown in Fig. 2 C, and the trend in these areas have been stable over the past decade (Fig. 2 D).
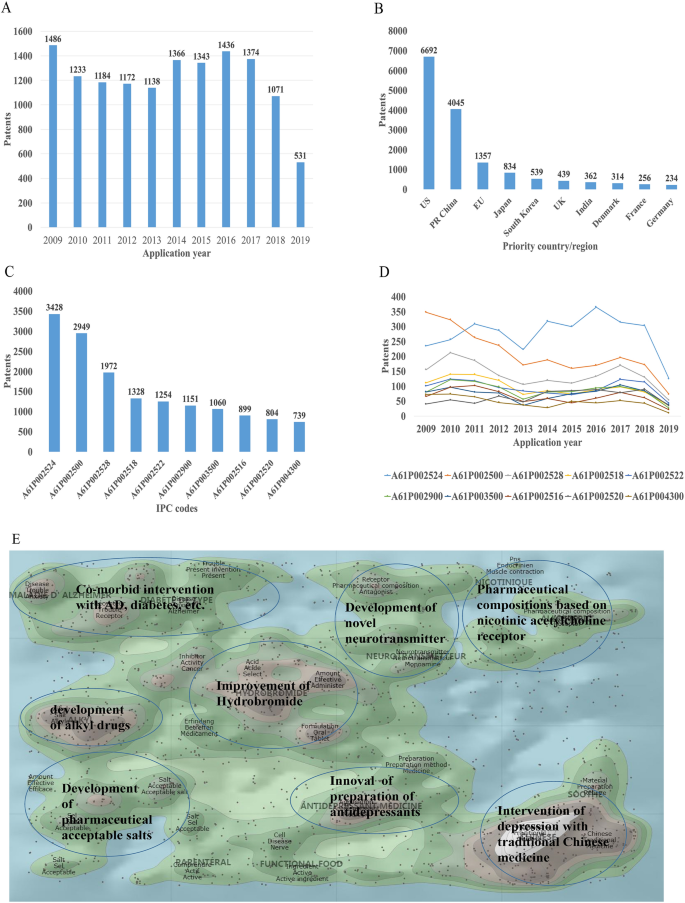
Analysis of patented technology applications from 2009 to 2019 in the field of depressive disorder. A Annual numbers and trends of patents (the Derwent Innovation patent database). B The top 10 countries/regions applying for patents. C The top 10 technological areas of patents. D The trend of patent assignees. E Global hot topic areas of patents.
Analysis of technical hotspots based on keyword clustering was conducted from the Derwent Innovation database using the "ThemeScape" tool. This demonstrated that the hot topic areas are as follows (Fig. 2 E): (1) improvement for formulation and the efficiency of hydrobromide, as well as optimization of the dosage; intervention for depression comorbid with AD, diabetes, and others; (3) development of alkyl drugs; (4) development of pharmaceutical acceptable salts as antidepressants; (5) innovation of the preparation of antidepressants; (6) development of novel antidepressants based on neurotransmitters; (7) development of compositions based on nicotinic acetylcholine receptors; and (8) intervention for depression with traditional Chinese medicine.
Analysis of Clinical Trial
There are 6,516 clinical trials in the field of depression in the ClinicalTrials.gov database, and among them, 1,737 valid trials include the ongoing recruitment of subjects, upcoming recruitment of subjects, and ongoing clinical trials. These clinical trials are mainly distributed in the USA (802 trials), Canada (155), China (114), France (93), Germany (66), UK (62), Spain (58), Denmark (41), Sweden (39), and Switzerland (23). The indications for clinical trials include various types of depression, such as minor depression, depression, severe depression, perinatal depression, postpartum depression, and depression comorbid with other psychiatric disorders or physical diseases, such as schizophrenia, epilepsy, stroke, cancer, diabetes, cardiovascular disease, and Parkinson's disease.
Based on the database of the Chinese Clinical Trial Registry website, a total of 143 clinical trials for depression have been carried out in China. According to the type of research, they are mainly interventional and observational studies, as well as a small number of related factor studies, epidemiological studies, and diagnostic trials. The research content involves postpartum, perinatal, senile, and other age groups with clinical diagnosis (imaging diagnosis) and intervention studies (drugs, acupuncture, electrical stimulation, transcranial magnetic stimulation). It also includes intervention studies on depression comorbid with coronary heart disease, diabetes, and heart failure.
New Medicine Development
According to the Cortellis database, 828 antidepressants were under development by the end of 2019, but only 292 of these are effective and active (Fig. 3 A). Large number of them have been discontinued or made no progress, indicating that the development of new drugs in the field of depression is extremely urgent.
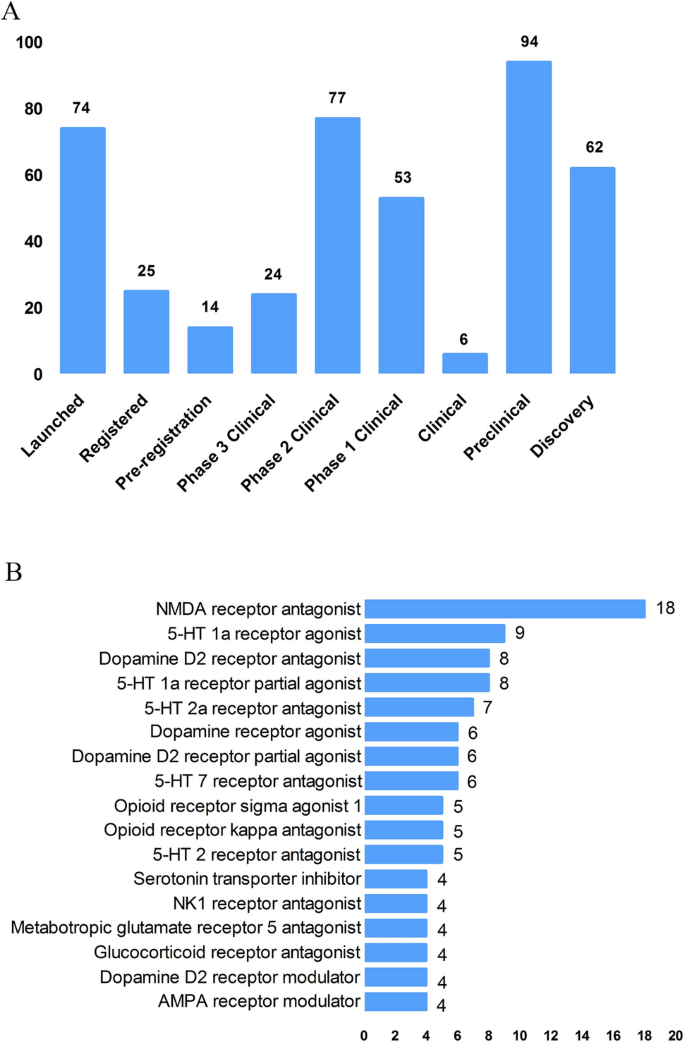
New medicine development from 2009 to 2019 in depressive disorder. A Development status of new candidate drugs. B Top target-based actions.
From the perspective of target-based actions, the most common new drugs are NMDA receptor antagonists, followed by 5-HT targets, as well as dopamine receptor agonists, opioid receptor antagonists and agonists, AMPA receptor modulators, glucocorticoid receptor antagonists, NK1 receptor antagonists, and serotonin transporter inhibitors (Fig. 3 B).
Epidemiology of Depression
The prevalence of depression varies greatly across cultures and countries. Previous surveys have demonstrated that the 12-month prevalence of depression was 0.3% in the Czech Republic, 10% in the USA, 4.5% in Mexico, and 5.2% in West Germany, and the lifetime prevalence of depression was 1.0% in the Czech Republic, 16.9% in the USA, 8.3% in Canada, and 9.0% in Chile [ 4 , 5 ]. A recent meta-analysis including 30 Countries showed that lifetime and 12-month prevalence depression were 10.8% and 7.2%, respectively [ 6 ]. In China, the lifetime prevalence of depression ranged from 1.6% to 5.5% [ 7 , 8 , 9 ]. An epidemiological study demonstrated that depression was the most common mood disorder with a life prevalence of 3.4% and a 12-month prevalence of 2.1% in China [ 10 ].
Some studies have also reported the prevalence in specific populations. The National Comorbidity Survey-Adolescent Supplement (NCS-A) survey in the USA showed that the lifetime and 12-month prevalence of depression in adolescents aged 13 to 18 were 11.0% and 7.5%, respectively [ 11 ]. A recent meta-analysis demonstrated that lifetime prevalence and 12-month prevalence were 2.8% and 2.3%, respectively, among the elderly population in China [ 12 ].
Neurobiological Pathogenesis of Depressive Disorder
The early hypothesis of monoamines in the pathophysiology of depression has been accepted by the scientific community. The evidence that monoamine oxidase inhibitors and tricyclic antidepressants promote monoamine neurotransmission supports this theory of depression [ 13 ]. So far, selective serotonin reuptake inhibitors and norepinephrine reuptake inhibitors are still the first-line antidepressants. However, there remain 1/3 to 2/3 of depressed patients who do not respond satisfactorily to initial antidepressant treatment, and even as many as 15%–40% do not respond to several pharmacological medicines [ 14 , 15 ]. Therefore, the underlying pathogenesis of depression is far beyond the simple monoamine mechanism.
Other hypotheses of depression have gradually received increasing attention because of biomarkers for depression and the effects pharmacological treatments, such as the stress-responsive hypothalamic pituitary adrenal (HPA) axis, neuroendocrine systems, the neurotrophic family of growth factors, and neuroinflammation.
Stress-Responsive HPA Axis
Stress is causative or a contributing factor to depression. Particularly, long-term or chronic stress can lead to dysfunction of the HPA axis and promote the secretion of hormones, including cortisol, adrenocorticotropic hormone, corticotropin-releasing hormone, arginine vasopressin, and vasopressin. About 40%–60% of patients with depression display a disturbed HPA axis, including hypercortisolemia, decreased rhythmicity, and elevated cortisol levels [ 16 , 17 ]. Mounting evidence has shown that stress-induced abnormality of the HPA axis is associated with depression and cognitive impairment, which is due to the increased secretion of cortisol and the insufficient inhibition of glucocorticoid receptor regulatory feedback [ 18 , 19 ]. In addition, it has been reported that the increase in cortisol levels is related to the severity of depression, especially in melancholic depression [ 20 , 21 ]. Further, patients with depression whose HPA axis was not normalized after treatment had a worse clinical response and prognosis [ 22 , 23 ]. Despite the above promising insights, unfortunately previous studies have shown that treatments regulating the HPA axis, such as glucocorticoid receptor antagonists, do not attenuate the symptoms of depressed patients [ 24 , 25 ].
Glutamate Signaling Pathway
Glutamate is the main excitatory neurotransmitter released by synapses in the brain; it is involved in synaptic plasticity, cognitive processes, and reward and emotional processes. Stress can induce presynaptic glutamate secretion by neurons and glutamate strongly binds to ionotropic glutamate receptors (iGluRs) including N-methyl-D-aspartate receptors (NMDARs) and α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptors (AMPARs) [ 26 ] on the postsynaptic membrane to activate downstream signal pathways [ 27 ]. Accumulating evidence has suggested that the glutamate system is associated with the incidence of depression. Early studies have shown increased levels of glutamate in the peripheral blood, cerebrospinal fluid, and brain of depressed patients [ 28 , 29 ], as well as NMDAR subunit disturbance in the brain [ 30 , 31 ]. Blocking the function of NMDARs has an antidepressant effect and protects hippocampal neurons from morphological abnormalities induced by stress, while antidepressants reduce glutamate secretion and NMDARs [ 32 ]. Most importantly, NMDAR antagonists such as ketamine have been reported to have profound and rapid antidepressant effects on both animal models and the core symptoms of depressive patients [ 33 ]. On the other hand, ketamine can also increase the AMPAR pathway in hippocampal neurons by up-regulating the AMPA glutamate receptor 1 subunit [ 34 ]. Further, the AMPAR pathway may be involved in the mechanism of antidepressant effects. For example, preclinical studies have indicated that AMPAR antagonists might attenuate lithium-induced depressive behavior by increasing the levels of glutamate receptors 1 and 2 in the mouse hippocampus [ 35 ].
Gamma-Aminobutyric Acid (GABA)
Contrary to glutamate, GABA is the main inhibitory neurotransmitter. Although GABA neurons account for only a small proportion compared to glutamate, inhibitory neurotransmission is essential for brain function by balancing excitatory transmission [ 36 ]. Number of studies have shown that patients with depression have neurotransmission or functional defects of GABA [ 37 , 38 ]. Schür et al ., conducted a meta-analysis of magnetic resonance spectroscopy studies, which showed that the brain GABA level in depressive patients was lower than that in healthy controls, but no difference was found in depressive patients in remission [ 39 ]. Several postmortem studies have shown decreased levels of the GABA synthase glutamic acid decarboxylase in the prefrontal cortex of patients with depression [ 40 , 41 ]. It has been suggested that a functional imbalance of the GABA and glutamate systems contributes to the pathophysiology of depression, and activation of the GABA system might induce antidepressant activity, by which GABA A receptor mediators α2/α3 are considered potential antidepressant candidates [ 42 , 43 ]. Genetic mouse models, such as the GABA A receptor mutant mouse and conditional the Gad1-knockout mouse (GABA in hippocampus and cerebral cortex decreased by 50%) and optogenetic methods have verified that depression-like behavior is induced by changing the level of GABA [ 44 , 45 ].
Neurotrophin Family
The neurotrophin family plays a key role in neuroplasticity and neurogenesis. The neurotrophic hypothesis of depression postulates that a deficit of neurotrophic support leads to neuronal atrophy, the reduction of neurogenesis, and the destruction of glia support, while antidepressants attenuate or reverse these pathophysiological processes [ 46 ]. Among them, the most widely accepted hypothesis involves brain-derived neurotrophic factor (BDNF). This was initially triggered by evidence that stress reduces the BDNF levels in the animal brain, while antidepressants rescue or attenuate this reduction [ 47 , 48 ], and agents involved in the BDNF system have been reported to exert antidepressant-like effects [ 49 , 50 ]. In addition, mounting studies have reported that the BDNF level is decreased in the peripheral blood and at post-mortem in depressive patients, and some have reported that antidepressant treatment normalizes it [ 51 , 52 ]. Furthermore, some evidence also showed that the interaction of BDNF and its receptor gene is associated with treatment-resistant depression [ 15 ].
Recent studies reported that depressed patients have a lower level of the pro-domain of BDNF (BDNF pro-peptide) than controls. This is located presynaptically and promotes long-term depression in the hippocampus, suggesting that it is a promising synaptic regulator [ 53 ].
Neuroinflammation
The immune-inflammation hypothesis has attracted much attention, suggesting that the interactions between inflammatory pathways and neural circuits and neurotransmitters are involved in the pathogenesis and pathophysiological processes of depression. Early evidence found that patients with autoimmune or infectious diseases are more likely to develop depression than the general population [ 54 ]. In addition, individuals without depression may display depressive symptoms after treatment with cytokines or cytokine inducers, while antidepressants relieve these symptoms [ 55 , 56 ]. There is a complex interaction between the peripheral and central immune systems. Previous evidence suggested that peripheral inflammation/infection may spread to the central nervous system in some way and cause a neuroimmune response [ 55 , 57 ]: (1) Some cytokines produced in the peripheral immune response, such as IL-6 and IL-1 β, can leak into the brain through the blood-brain barrier (BBB). (2) Cytokines entering the central nervous system act directly on astrocytes, small stromal cells, and neurons. (3) Some peripheral immune cells can cross the BBB through specific transporters, such as monocytes. (4) Cytokines and chemokines in the circulation activate the central nervous system by regulating the surface receptors of astrocytes and endothelial cells at the BBB. (5) As an intermediary pathway, the immune inflammatory response transmits peripheral danger signals to the center, amplifies the signals, and shows the external phenotype of depressive behavior associated with stress/trauma/infection. (6) Cytokines and chemokines may act directly on neurons, change their plasticity and promote depression-like behavior.
Patients with depression show the core feature of the immune-inflammatory response, that is, increased concentrations of pro-inflammatory cytokines and their receptors, chemokines, and soluble adhesion molecules in peripheral blood and cerebrospinal fluid [ 58 , 59 , 60 ]. Peripheral immune-inflammatory response markers not only change the immune activation state in the brain that affects explicit behavior, but also can be used as an evaluation index or biological index of antidepressant therapy [ 61 , 62 ]. Li et al . showed that the level of TNF-α in patients with depression prior to treatment was higher than that in healthy controls. After treatment with venlafaxine, the level of TNF-α in patients with depression decreased significantly, and the level of TNF-α in the effective group decreased more [ 63 ]. A recent meta-analysis of 1,517 patients found that antidepressants significantly reduced peripheral IL-6, TNF-α, IL-10, and CCL-2, suggesting that antidepressants reduce markers of peripheral inflammatory factors [ 64 ]. Recently, Syed et al . also confirmed that untreated patients with depression had higher levels of inflammatory markers and increased levels of anti-inflammatory cytokines after antidepressant treatment, while increased levels of pro-inflammatory cytokines were found in non-responders [ 62 ]. Clinical studies have also found that anti-inflammatory cytokines, such as monoclonal antibodies and other cytokine inhibitors, may play an antidepressant role by blocking cytokines. The imbalance of pro-inflammatory and anti-inflammatory cytokines may be involved in the pathophysiological process of depression.
In addition, a recent study showed that microglia contribute to neuronal plasticity and neuroimmune interaction that are involved in the pathophysiology of depression [ 65 ]. When activated microglia promote inflammation, especially the excessive production of pro-inflammatory factors and cytotoxins in the central nervous system, depression-like behavior can gradually develop [ 65 , 66 ]. However, microglia change polarization as two types under different inflammatory states, regulating the balance of pro- and anti-inflammatory factors. These two types are M1 and M2 microglia; the former produces large number of pro-inflammatory cytokines after activation, and the latter produces anti-inflammatory cytokines. An imbalance of M1/M2 polarization of microglia may contribute to the pathophysiology of depression [ 67 ].
Microbiome-Gut-Brain Axis
The microbiota-gut-brain axis has recently gained more attention because of its ability to regulate brain activity. Many studies have shown that the microbiota-gut-brain axis plays an important role in regulating mood, behavior, and neuronal transmission in the brain [ 68 , 69 ]. It is well established that comorbidity of depression and gastrointestinal diseases is common [ 70 , 71 ]. Some antidepressants can attenuate the symptoms of patients with irritable bowel syndrome and eating disorders [ 72 ]. It has been reported that gut microbiome alterations are associated with depressive-like behaviors [ 73 , 74 ], and brain function [ 75 ]. Early animal studies have shown that stress can lead to long-term changes in the diversity and composition of intestinal microflora, and is accompanied by depressive behavior [ 76 , 77 ]. Interestingly, some evidence indicates that rodents exhibit depressive behavior after fecal transplants from patients with depression [ 74 ]. On the other hand, some probiotics attenuated depressive-like behavior in animal studies, [ 78 ] and had antidepressant effects on patients with depression in several double-blind, placebo-controlled clinical trials [ 79 , 80 ].
The potential mechanism may be that gut microbiota can interact with the brain through a variety of pathways or systems, including the HPA axis, and the neuroendocrine, autonomic, and neuroimmune systems [ 81 ]. For example, recent evidence demonstrated that gut microbiota can affect the levels of neurotransmitters in the gut and brain, including serotonin, dopamine, noradrenalin, glutamate, and GABA [ 82 ]. In addition, recent studies showed that changes in gut microbiota can also impair the gut barrier and promote higher levels of peripheral inflammatory cytokines [ 83 , 84 ]. Although recent research in this area has made significant progress, more clinical trials are needed to determine whether probiotics have any effect on the treatment of depression and what the potential underlying mechanisms are.
Other Systems and Pathways
There is no doubt that several other systems or pathways are also involved in the pathophysiology of depression, such as oxidant-antioxidant imbalance [ 85 ], mitochondrial dysfunction [ 86 , 87 ], and circadian rhythm-related genes [ 88 ], especially their critical interactions ( e.g. interaction between the HPA and mitochondrial metabolism [ 89 , 90 ], and the reciprocal interaction between oxidative stress and inflammation [ 2 , 85 ]). The pathogenesis of depression is complex and all the hypotheses should be integrated to consider the many interactions between various systems and pathways.
Advances in Various Kinds of Research on Depressive Disorder
Genetic, molecular, and neuroimaging studies continue to increase our understanding of the neurobiological basis of depression. However, it is still not clear to what extent the results of neurobiological studies can help improve the clinical and functional prognosis of patients. Therefore, over the past 10 years, the neurobiological study of depression has become an important measure to understand the pathophysiological mechanism and guide the treatment of depression.
Genetic Studies
Previous twin and adoption studies have indicated that depression has relatively low rate of heritability at 37% [ 91 ]. In addition, environmental factors such as stressful events are also involved in the pathogenesis of depression. Furthermore, complex psychiatric disorders, especially depression, are considered to be polygenic effects that interact with environmental factors [ 13 ]. Therefore, reliable identification of single causative genes for depression has proved to be challenging. The first genome-wide association studies (GWAS) for depression was published in 2009, and included 1,738 patients and 1,802 controls [ 92 , 93 ]. Although many subsequent GWASs have determined susceptible genes in the past decade, the impact of individual genes is so small that few results can be replicated [ 94 , 95 ]. So far, it is widely accepted that specific single genetic mutations may play minor and marginal roles in complex polygenic depression. Another major recognition in GWASs over the past decade is that prevalent candidate genes are usually not associated with depression. Further, the inconsistent results may also be due to the heterogeneity and polygenic nature of genetic and non-genetic risk factors for depression as well as the heterogeneity of depression subtypes [ 95 , 96 ]. Therefore, to date, the quality of research has been improved in two aspects: (1) the sample size has been maximized by combining the data of different evaluation models; and (2) more homogenous subtypes of depression have been selected to reduce phenotypic heterogeneity [ 97 ]. Levinson et al . pointed out that more than 75,000 to 100,000 cases should be considered to detect multiple depression associations [ 95 ]. Subsequently, several recent GWASs with larger sample sizes have been conducted. For example, Okbay et al . identified two loci associated with depression and replicated them in separate depression samples [ 98 ]. Wray et al . also found 44 risk loci associated with depression based on 135,458 cases and 344,901 controls [ 99 ]. A recent GWAS of 807,553 individuals with depression reported that 102 independent variants were associated with depression; these were involved in synaptic structure and neural transmission, and were verified in a further 1,507,153 individuals [ 100 ]. However, even with enough samples, GWASs still face severe challenges. A GWAS only marks the region of the genome and is not directly related to the potential biological function. In addition, a genetic association with the indicative phenotype of depression may only be part of many pathogenic pathways, or due to the indirect influence of intermediate traits in the causal pathway on the final result [ 101 ].
Given the diversity of findings, epigenetic factors are now being investigated. Recent studies indicated that epigenetic mechanisms may be the potential causes of "loss of heritability" in GWASs of depression. Over the past decade, a promising discovery has been that the effects of genetic information can be directly influenced by environment factors, and several specific genes are activated by environmental aspects. This process is described as interactions between genes and the environment, which is identified by the epigenetic mechanism. Environmental stressors cause alterations in gene expression in the brain, which may cause abnormal neuronal plasticity in areas related to the pathogenesis of the disease. Epigenetic events alter the structure of chromatin, thereby regulating gene expression involved in neuronal plasticity, stress behavior, depressive behavior, and antidepressant responses, including DNA methylation, histone acetylation, and the role of non-coding RNA. These new mechanisms of trans-generational transmission of epigenetic markers are considered a supplement to orthodox genetic heredity, providing the possibility for the discovery of new treatments for depression [ 102 , 103 ]. Recent studies imply that life experiences, including stress and enrichment, may affect cellular and molecular signaling pathways in sperm and influence the behavioral and physiological phenotypes of offspring in gender-specific patterns, which may also play an important role in the development of depression [ 103 ].
Brain Imaging and Neuroimaging Studies
Neuroimaging, including magnetic resonance imaging (MRI) and molecular imaging, provides a non-invasive technique for determining the underlying etiology and individualized treatment for depression. MRI can provide important data on brain structure, function, networks, and metabolism in patients with depression; it includes structural MRI (sMRI), functional MRI (fMRI), diffusion tensor imaging, and magnetic resonance spectroscopy.
Previous sMRI studies have found damaged gray matter in depression-associated brain areas, including the frontal lobe, anterior cingulate gyrus, hippocampus, putamen, thalamus, and amygdala. sMRI focuses on the thickness of gray matter and brain morphology [ 104 , 105 ]. A recent meta-analysis of 2,702 elderly patients with depression and 11,165 controls demonstrated that the volumes of the whole brain and hippocampus of patients with depression were lower than those of the control group [ 106 ]. Some evidence also showed that the hippocampal volume in depressive patients was lower than that of controls, and increased after treatment with antidepressants [ 107 ] and electroconvulsive therapy (ECT) [ 108 ], suggesting that the hippocampal volume plays a critical role in the development, treatment response, and clinical prognosis of depression. A recent study also reported that ECT increased the volume of the right hippocampus, amygdala, and putamen in patients with treatment-resistant depression [ 109 ]. In addition, postmortem research supported the MRI study showing that dentate gyrus volume was decreased in drug-naive patients with depression compared to healthy controls, and was potentially reversed by treatment with antidepressants [ 110 ].
Diffusion tensor imaging detects the microstructure of the white matter, which has been reported impaired in patients with depression [ 111 ]. A recent meta-analysis that included first-episode and drug-naïve depressive patients showed that the decrease in fractional anisotropy was negatively associated with illness duration and clinical severity [ 112 ].
fMRI, including resting-state and task-based fMRI, can divide the brain into self-related regions, such as the anterior cingulate cortex, posterior cingulate cortex, medial prefrontal cortex, precuneus, and dorsomedial thalamus. Many previous studies have shown the disturbance of several brain areas and intrinsic neural networks in patients with depression which could be rescued by antidepressants [ 113 , 114 , 115 , 116 ]. Further, some evidence also showed an association between brain network dysfunction and the clinical correlates of patients with depression, including clinical symptoms [ 117 ] and the response to antidepressants [ 118 , 119 ], ECT [ 120 , 121 ], and repetitive transcranial magnetic stimulation [ 122 ].
It is worth noting that brain imaging provides new insights into the large-scale brain circuits that underlie the pathophysiology of depressive disorder. In such studies, large-scale circuits are often referred to as “networks”. There is evidence that a variety of circuits are involved in the mechanisms of depressive disorder, including disruption of the default mode, salience, affective, reward, attention, and cognitive control circuits [ 123 ]. Over the past decade, the study of intra-circuit and inter-circuit connectivity dysfunctions in depression has escalated, in part due to advances in precision imaging and analysis techniques [ 124 ]. Circuit dysfunction is a potential biomarker to guide psychopharmacological treatment. For example, Williams et al . found that hyper-activation of the amygdala is associated with a negative phenotype that can predict the response to antidepressants [ 125 ]. Hou et al . showed that the baseline characteristics of the reward circuit predict early antidepressant responses [ 126 ].
Molecular imaging studies, including single photon emission computed tomography and positron emission tomography, focus on metabolic aspects such as amino-acids, neurotransmitters, glucose, and lipids at the cellular level in patients with depression. A recent meta-analysis examined glucose metabolism and found that glucose uptake dysfunction in different brain regions predicts the treatment response [ 127 ].
The most important and promising studies were conducted by the ENIGMA (Enhancing NeuroImaging Genetics through Meta Analysis) Consortium, which investigated the human brain across 43 countries. The ENIGMA-MDD Working Group was launched in 2012 to detect the structural and functional changes associated with MDD reliably and replicate them in various samples around the world [ 128 ]. So far, the ENIGMA-MDD Working Group has collected data from 4,372 MDD patients and 9,788 healthy controls across 14 countries, including 45 cohorts [ 128 ]. Their findings to date are shown in Table 1 [ 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 ].
Objective Index for Diagnosis of MDD
To date, the clinical diagnosis of depression is subjectively based on interviews according to diagnostic criteria ( e.g. International Classification of Diseases and Diagnostic and Statistical Manual diagnostic systems) and the severity of clinical symptoms are assessed by questionnaires, although patients may experience considerable differences in symptoms and subtypes [ 138 ]. Meanwhile, biomarkers including genetics, epigenetics, peripheral gene and protein expression, and neuroimaging markers may provide a promising supplement for the development of the objective diagnosis of MDD, [ 139 , 140 , 141 ]. However, the development of reliable diagnosis for MDD using biomarkers is still difficult and elusive, and all methods based on a single marker are insufficiently specific and sensitive for clinical use [ 142 ]. Papakostas et al . showed that a multi-assay, serum-based test including nine peripheral biomarkers (soluble tumor necrosis factor alpha receptor type II, resistin, prolactin, myeloperoxidase, epidermal growth factor, BDNF, alpha1 antitrypsin, apolipoprotein CIII, brain-derived neurotrophic factor, and cortisol) yielded a specificity of 81.3% and a sensitivity of 91.7% [ 142 ]. However, the sample size was relatively small and no other studies have yet validated their results. Therefore, further studies are needed to identify biomarker models that integrate all biological variables and clinical features to improve the specificity and sensitivity of diagnosis for MDD.
Management of Depression
The treatment strategies for depression consist of pharmacological treatment and non-pharmacological treatments including psychotherapy, ECT [ 98 ], and transcranial magnetic stimulation. As psychotherapy has been shown to have effects on depression including attenuating depressive symptoms and improving the quality of life [ 143 , 144 ]; several practice guidelines are increasingly recommending psychotherapy as a monotherapy or in combination with antidepressants [ 145 , 146 ].
Current Antidepressant Treatment
Antidepressants approved by the US Food and Drug Administration (FDA) are shown in Table 2 . Due to the relatively limited understanding of the etiology and pathophysiology of depression, almost all the previous antidepressants were discovered by accident a few decades ago. Although most antidepressants are usually safe and effective, there are still some limitations, including delayed efficacy (usually 2 weeks) and side-effects that affect the treatment compliance [ 147 ]. In addition, <50% of all patients with depression show complete remission through optimized treatment, including trials of multiple drugs with and without simultaneous psychotherapy. In the past few decades, most antidepressant discoveries focused on finding faster, safer, and more selective serotonin or norepinephrine receptor targets. In addition, there is an urgent need to develop new approaches to obtain more effective, safer, and faster antidepressants. In 2019, the FDA approved two new antidepressants: Esketamine for refractory depression and Bresanolone for postpartum depression. Esmolamine, a derivative of the anesthetic drug ketamine, was approved by the FDA for the treatment of refractory depression, based on a large number of preliminary clinical studies [ 148 ]. For example, several randomized controlled trials and meta-analysis studies showed the efficacy and safety of Esketamine in depression or treatment-resistant depression [ 26 , 149 , 150 ]. Although both are groundbreaking new interventions for these debilitating diseases and both are approved for use only under medical supervision, there are still concerns about potential misuse and problems in the evaluation of mental disorders [ 151 ].
To date, although several potential drugs have not yet been approved by the FDA, they are key milestones in the development of antidepressants that may be modified and used clinically in the future, such as compounds containing dextromethorphan (a non-selective NMDAR antago–nist), sarcosine (N-methylglycine, a glycine reuptake inhibitor), AMPAR modulators, and mGluR modulators [ 152 ].
Neuromodulation Therapy
Neuromodulation therapy acts through magnetic pulse, micro-current, or neural feedback technology within the treatment dose, acting on the central or peripheral nervous system to regulate the excitatory/inhibitory activity to reduce or attenuate the symptoms of the disease.
ECT is one of most effective treatments for depression, with the implementation of safer equipment and advancement of techniques such as modified ECT [ 153 ]. Mounting evidence from randomized controlled trial (RCT) and meta-analysis studies has shown that rTMS can treat depressive patients with safety [ 154 ]. Other promising treatments for depression have emerged, such as transcranial direct current stimulation (tDCS) [ 155 ], transcranial alternating current stimulation (tACS)[ 156 ], vagal nerve stimulation [ 157 ], deep brain stimulation [ 158 ] , and light therapy [ 159 ], but some of them are still experimental to some extent and have not been widely used. For example, compared to tDCS, tACS displays less sensory experience and adverse reactions with weak electrical current in a sine-wave pattern, but the evidence for the efficacy of tACS in the treatment of depression is still limited [ 160 ]. Alexander et al . recently demonstrated that there was no difference in efficacy among different treatments (sham, 10-Hz and 40-Hz tACS). However, only the 10-Hz tACS group had more responders than the sham and 40-Hz tACS groups at week 2 [ 156 ]. Further RCT studies are needed to verify the efficacy of tACS. In addition, the mechanism of the effect of neuromodulation therapy on depression needs to be further investigated.
Precision Medicine for Depression
Optimizing the treatment strategy is an effective way to improve the therapeutic effect on depression. However, each individual with depression may react very differently to different treatments. Therefore, this raises the question of personalized treatment, that is, which patients are suitable for which treatment. Over the past decade, psychiatrists and psychologists have focused on individual biomarkers and clinical characteristics to predict the efficiency of antidepressants and psychotherapies, including genetics, peripheral protein expression, electrophysiology, neuroimaging, neurocognitive performance, developmental trauma, and personality [ 161 ]. For example, Bradley et al . recently conducted a 12-week RCT, which demonstrated that the response rate and remission rates of the pharmacogenetic guidance group were significantly higher than those of the non-pharmacogenetic guidance group [ 162 ].
Subsequently, Greden et al . conducted an 8-week RCT of Genomics Used to Improve Depression Decisions (GUIDED) on 1,167 MDD patients and demonstrated that although there was no difference in symptom improvement between the pharmacogenomics-guided and non- pharmacogenomics-guided groups, the response rate and remission rate of the pharmacogenomics-guided group increased significantly [ 163 ].
A recent meta-analysis has shown that the baseline default mode network connectivity in patients with depression can predict the clinical responses to treatments including cognitive behavioral therapy, pharmacotherapy, ECT, rTMS, and transcutaneous vagus nerve stimulation [ 164 ]. However, so far, the biomarkers that predict treatment response at the individual level have not been well applied in the clinic, and there is still a lot of work to be conducted in the future.
Future Perspectives
Although considerable progress has been made in the study of depression during a past decade, the heterogeneity of the disease, the effectiveness of treatment, and the gap in translational medicine are critical challenges. The main dilemma is that our understanding of the etiology and pathophysiology of depression is inadequate, so our understanding of depression is not deep enough to develop more effective treatment. Animal models still cannot fully simulate this heterogeneous and complex mental disorder. Therefore, how to effectively match the indicators measured in animals with those measured in genetic research or the development of new antidepressants is another important challenge.
Change history
17 may 2021.
A Correction to this paper has been published: https://doi.org/10.1007/s12264-021-00694-9
Dadi AF, Miller ER, Bisetegn TA, Mwanri L. Global burden of antenatal depression and its association with adverse birth outcomes: an umbrella review. BMC Public Health 2020, 20: 173
Article PubMed PubMed Central Google Scholar
Zhu S, Zhao L, Fan Y, Lv Q, Wu K, Lang X. Interaction between TNF-alpha and oxidative stress status in first-episode drug-naive schizophrenia. Psychoneuroendocrinology 2020, 114: 104595
Article CAS PubMed Google Scholar
Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390: 1211–1259
Article Google Scholar
Andrade L, Caraveo-Anduaga JJ, Berglund P, Bijl RV, De Graaf R, Vollebergh W, et al. The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) Surveys. Int J Methods Psychiatr Res 2003, 12: 3–21
Article PubMed Google Scholar
Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health 2013, 34: 119–138
Lim GY, Tam WW, Lu Y, Ho CS, Zhang MW, Ho RC. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci Rep 2018, 8: 2861
Liu J, Yan F, Ma X, Guo HL, Tang YL, Rakofsky JJ, et al. Prevalence of major depressive disorder and socio-demographic correlates: Results of a representative household epidemiological survey in Beijing, China. J Affect Disord 2015, 179: 74–81
Zhang YS, Rao WW, Cui LJ, Li JF, Li L, Ng CH, et al. Prevalence of major depressive disorder and its socio-demographic correlates in the general adult population in Hebei province, China. J Affect Disord 2019, 252: 92–98
Ma X, Xiang YT, Cai ZJ, Li SR, Xiang YQ, Guo HL, et al. Prevalence and socio-demographic correlates of major depressive episode in rural and urban areas of Beijing, China. J Affect Disord 2009, 115: 323–330
Huang Y, Wang Y, Wang H, Liu Z, Yu X, Yan J, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry 2019, 6: 211–224
Avenevoli S, Swendsen J, He JP, Burstein M, Merikangas KR. Major depression in the national comorbidity survey-adolescent supplement: prevalence, correlates, and treatment. J Am Acad Child Adolesc Psychiatry 2015, 54(37–44): e32
Google Scholar
Wang F, Zhang QE, Zhang L, Ng CH, Ungvari GS, Yuan Z, et al. Prevalence of major depressive disorder in older adults in China: A systematic review and meta-analysis. J Affect Disord 2018, 241: 297–304
Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature 2008, 455: 894–902
Article CAS PubMed PubMed Central Google Scholar
Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006, 163: 1905–1917
Li Z, Zhang Y, Wang Z, Chen J, Fan J, Guan Y, et al. The role of BDNF, NTRK2 gene and their interaction in development of treatment-resistant depression: data from multicenter, prospective, longitudinal clinic practice. J Psychiatr Res 2013, 47: 8–14
Murphy BE. Steroids and depression. J Steroid Biochem Mol Biol 1991, 38: 537–559
Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci 2008, 31: 464–468
Keller J, Gomez R, Williams G, Lembke A, Lazzeroni L, Murphy GM Jr, et al. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry 2017, 22: 527–536
Gomez RG, Fleming SH, Keller J, Flores B, Kenna H, DeBattista C, et al. The neuropsychological profile of psychotic major depression and its relation to cortisol. Biol Psychiatry 2006, 60: 472–478
Lamers F, Vogelzangs N, Merikangas KR, de Jonge P, Beekman AT, Penninx BW. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry 2013, 18: 692–699
Nandam LS, Brazel M, Zhou M, Jhaveri DJ. Cortisol and major depressive disorder-translating findings from humans to animal models and back. Front Psychiatry 2019, 10: 974
Owashi T, Otsubo T, Oshima A, Nakagome K, Higuchi T, Kamijima K. Longitudinal neuroendocrine changes assessed by dexamethasone/CRH and growth hormone releasing hormone tests in psychotic depression. Psychoneuroendocrinology 2008, 33: 152–161
Mickey BJ, Ginsburg Y, Sitzmann AF, Grayhack C, Sen S, Kirschbaum C, et al. Cortisol trajectory, melancholia, and response to electroconvulsive therapy. J Psychiatr Res 2018, 103: 46–53
Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med 2011, 73: 114–126
Aubry JM. CRF system and mood disorders. J Chem Neuroanat 2013, 54: 20–24
Correia-Melo FS, Leal GC, Vieira F, Jesus-Nunes AP, Mello RP, Magnavita G, et al. Efficacy and safety of adjunctive therapy using esketamine or racemic ketamine for adult treatment-resistant depression: A randomized, double-blind, non-inferiority study. J Affect Disord 2020, 264: 527–534
Duman RS, Voleti B. Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci 2012, 35: 47–56
Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov 2008, 7: 426–437
Hashimoto K. The role of glutamate on the action of antidepressants. Prog Neuropsychopharmacol Biol Psychiatry 2011, 35: 1558–1568
Gray AL, Hyde TM, Deep-Soboslay A, Kleinman JE, Sodhi MS. Sex differences in glutamate receptor gene expression in major depression and suicide. Mol Psychiatry 2015, 20: 1057–1068
Chandley MJ, Szebeni A, Szebeni K, Crawford JD, Stockmeier CA, Turecki G, et al. Elevated gene expression of glutamate receptors in noradrenergic neurons from the locus coeruleus in major depression. Int J Neuropsychopharmacol 2014, 17: 1569–1578
Musazzi L, Treccani G, Mallei A, Popoli M. The action of antidepressants on the glutamate system: regulation of glutamate release and glutamate receptors. Biol Psychiatry 2013, 73: 1180–1188
Kadriu B, Musazzi L, Henter ID, Graves M, Popoli M, Zarate CA Jr. Glutamatergic Neurotransmission: Pathway to Developing Novel Rapid-Acting Antidepressant Treatments. Int J Neuropsychopharmacol 2019, 22: 119–135
Beurel E, Grieco SF, Amadei C, Downey K, Jope RS. Ketamine-induced inhibition of glycogen synthase kinase-3 contributes to the augmentation of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor signaling. Bipolar Disord 2016, 18: 473–480
Gould TD, O’Donnell KC, Dow ER, Du J, Chen G, Manji HK. Involvement of AMPA receptors in the antidepressant-like effects of lithium in the mouse tail suspension test and forced swim test. Neuropharmacology 2008, 54: 577–587
Duman RS, Sanacora G, Krystal JH. Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron 2019, 102: 75–90
Ghosal S, Hare B, Duman RS. Prefrontal Cortex GABAergic Deficits and Circuit Dysfunction in the Pathophysiology and Treatment of Chronic Stress and Depression. Curr Opin Behav Sci 2017, 14: 1–8
Fee C, Banasr M, Sibille E. Somatostatin-positive gamma-aminobutyric acid interneuron deficits in depression: Cortical microcircuit and therapeutic perspectives. Biol Psychiatry 2017, 82: 549–559
Schur RR, Draisma LW, Wijnen JP, Boks MP, Koevoets MG, Joels M, et al. Brain GABA levels across psychiatric disorders: A systematic literature review and meta-analysis of (1) H-MRS studies. Hum Brain Mapp 2016, 37: 3337–3352
Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, et al. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry 2012, 17: 1130–1142
Karolewicz B, Maciag D, O’Dwyer G, Stockmeier CA, Feyissa AM, Rajkowska G. Reduced level of glutamic acid decarboxylase-67 kDa in the prefrontal cortex in major depression. Int J Neuropsychopharmacol 2010, 13: 411–420
Lener MS, Niciu MJ, Ballard ED, Park M, Park LT, Nugent AC, et al. Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biol Psychiatry 2017, 81: 886–897
Chen X, van Gerven J, Cohen A, Jacobs G. Human pharmacology of positive GABA-A subtype-selective receptor modulators for the treatment of anxiety. Acta Pharmacol Sin 2019, 40: 571–582
Ren Z, Pribiag H, Jefferson SJ, Shorey M, Fuchs T, Stellwagen D, et al. Bidirectional homeostatic regulation of a depression-related brain state by gamma-aminobutyric acidergic deficits and ketamine treatment. Biol Psychiatry 2016, 80: 457–468
Kolata SM, Nakao K, Jeevakumar V, Farmer-Alroth EL, Fujita Y, Bartley AF, et al. Neuropsychiatric phenotypes produced by GABA reduction in mouse cortex and hippocampus. Neuropsychopharmacology 2018, 43: 1445–1456
Duman RS, Li N. A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists. Philos Trans R Soc Lond B Biol Sci 2012, 367: 2475–2484
Albert PR, Benkelfat C, Descarries L. The neurobiology of depression—revisiting the serotonin hypothesis. I. Cellular and molecular mechanisms. Philos Trans R Soc Lond B Biol Sci 2012, 367: 2378–2381
Li K, Shen S, Ji YT, Li XY, Zhang LS, Wang XD. Melatonin augments the effects of fluoxetine on depression-like behavior and hippocampal BDNF-TrkB signaling. Neurosci Bull 2018, 34: 303–311
Zhang JJ, Gao TT, Wang Y, Wang JL, Guan W, Wang YJ, et al. Andrographolide exerts significant antidepressant-like effects involving the hippocampal BDNF system in mice. Int J Neuropsychopharmacol 2019, 22: 585–600
Wang JQ, Mao L. The ERK pathway: Molecular mechanisms and treatment of depression. Mol Neurobiol 2019, 56: 6197–6205
Chiou YJ, Huang TL. Serum brain-derived neurotrophic factors in taiwanese patients with drug-naive first-episode major depressive disorder: Effects of antidepressants. Int J Neuropsychopharmacol 2017, 20: 213–218
CAS PubMed Google Scholar
Youssef MM, Underwood MD, Huang YY, Hsiung SC, Liu Y, Simpson NR, et al. Association of BDNF Val66Met polymorphism and brain BDNF levels with major depression and suicide. Int J Neuropsychopharmacol 2018, 21: 528–538
Kojima M, Matsui K, Mizui T. BDNF pro-peptide: physiological mechanisms and implications for depression. Cell Tissue Res 2019, 377: 73–79
Jeon SW, Kim YK. Inflammation-induced depression: Its pathophysiology and therapeutic implications. J Neuroimmunol 2017, 313: 92–98
Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 2016, 16: 22–34
Zhao G, Liu X. Neuroimmune advance in depressive disorder. Adv Exp Med Biol 2019, 1180: 85–98
Li Z, Wang Z, Zhang C, Chen J, Su Y, Huang J, et al. Reduced ENA78 levels as novel biomarker for major depressive disorder and venlafaxine efficiency: Result from a prospective longitudinal study. Psychoneuroendocrinology 2017, 81: 113–121
Ambrosio G, Kaufmann FN, Manosso L, Platt N, Ghisleni G, Rodrigues ALS, et al. Depression and peripheral inflammatory profile of patients with obesity. Psychoneuroendocrinology 2018, 91: 132–141
Mao R, Zhang C, Chen J, Zhao G, Zhou R, Wang F, et al. Different levels of pro- and anti-inflammatory cytokines in patients with unipolar and bipolar depression. J Affect Disord 2018, 237: 65–72
Enache D, Pariante CM, Mondelli V. Markers of central inflammation in major depressive disorder: A systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain Behav Immun 2019, 81: 24–40
Haroon E, Daguanno AW, Woolwine BJ, Goldsmith DR, Baer WM, Wommack EC, et al. Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive disorder. Psychoneuroendocrinology 2018, 95: 43–49
Syed SA, Beurel E, Loewenstein DA, Lowell JA, Craighead WE, Dunlop BW, et al. Defective inflammatory pathways in never-treated depressed patients are associated with poor treatment response. Neuron 2018, 99(914–924): e913
Li Z, Qi D, Chen J, Zhang C, Yi Z, Yuan C, et al. Venlafaxine inhibits the upregulation of plasma tumor necrosis factor-alpha (TNF-alpha) in the Chinese patients with major depressive disorder: a prospective longitudinal study. Psychoneuroendocrinology 2013, 38: 107–114
Kohler CA, Freitas TH, Stubbs B, Maes M, Solmi M, Veronese N, et al. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. Mol Neurobiol 2018, 55: 4195–4206
Innes S, Pariante CM, Borsini A. Microglial-driven changes in synaptic plasticity: A possible role in major depressive disorder. Psychoneuroendocrinology 2019, 102: 236–247
Sochocka M, Diniz BS, Leszek J. Inflammatory response in the CNS: Friend or foe? Mol Neurobiol 2017, 54: 8071–8089
Zhang L, Zhang J, You Z. Switching of the microglial activation phenotype is a possible treatment for depression disorder. Front Cell Neurosci 2018, 12: 306
Sandhu KV, Sherwin E, Schellekens H, Stanton C, Dinan TG, Cryan JF. Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Transl Res 2017, 179: 223–244
Gonzalez-Arancibia C, Urrutia-Pinones J, Illanes-Gonzalez J, Martinez-Pinto J, Sotomayor-Zarate R, Julio-Pieper M, et al. Do your gut microbes affect your brain dopamine? Psychopharmacology (Berl) 2019, 236: 1611–1622
Article CAS Google Scholar
Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Irritable bowel syndrome: a microbiome-gut-brain axis disorder? World J Gastroenterol 2014, 20: 14105–14125
Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology 2002, 122: 1140–1156
Ruepert L, Quartero AO, de Wit NJ, van der Heijden GJ, Rubin G, Muris JW. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev 2011: CD003460.
Marin IA, Goertz JE, Ren T, Rich SS, Onengut-Gumuscu S, Farber E, et al. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci Rep 2017, 7: 43859
Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry 2016, 21: 786–796
Curtis K, Stewart CJ, Robinson M, Molfese DL, Gosnell SN, Kosten TR, et al. Insular resting state functional connectivity is associated with gut microbiota diversity. Eur J Neurosci 2019, 50: 2446–2452
O’Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, et al. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry 2009, 65: 263–267
Garcia-Rodenas CL, Bergonzelli GE, Nutten S, Schumann A, Cherbut C, Turini M, et al. Nutritional approach to restore impaired intestinal barrier function and growth after neonatal stress in rats. J Pediatr Gastroenterol Nutr 2006, 43: 16–24
Hao Z, Wang W, Guo R, Liu H. Faecalibacterium prausnitzii (ATCC 27766) has preventive and therapeutic effects on chronic unpredictable mild stress-induced depression-like and anxiety-like behavior in rats. Psychoneuroendocrinology 2019, 104: 132–142
Messaoudi M, Violle N, Bisson JF, Desor D, Javelot H, Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes 2011, 2: 256–261
Rudzki L, Ostrowska L, Pawlak D, Malus A, Pawlak K, Waszkiewicz N, et al. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019, 100: 213–222
Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci 2013, 36: 305–312
Mittal R, Debs LH, Patel AP, Nguyen D, Patel K, O’Connor G, et al. Neurotransmitters: The critical modulators regulating gut-brain axis. J Cell Physiol 2017, 232: 2359–2372
Diviccaro S, Giatti S, Borgo F, Barcella M, Borghi E, Trejo JL, et al. Treatment of male rats with finasteride, an inhibitor of 5alpha-reductase enzyme, induces long-lasting effects on depressive-like behavior, hippocampal neurogenesis, neuroinflammation and gut microbiota composition. Psychoneuroendocrinology 2019, 99: 206–215
Kiecolt-Glaser JK, Wilson SJ, Bailey ML, Andridge R, Peng J, Jaremka LM, et al. Marital distress, depression, and a leaky gut: Translocation of bacterial endotoxin as a pathway to inflammation. Psychoneuroendocrinology 2018, 98: 52–60
Lindqvist D, Dhabhar FS, James SJ, Hough CM, Jain FA, Bersani FS, et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 2017, 76: 197–205
Czarny P, Wigner P, Galecki P, Sliwinski T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog Neuropsychopharmacol Biol Psychiatry 2018, 80: 309–321
Xie X, Yu C, Zhou J, Xiao Q, Shen Q, Xiong Z, et al. Nicotinamide mononucleotide ameliorates the depression-like behaviors and is associated with attenuating the disruption of mitochondrial bioenergetics in depressed mice. J Affect Disord 2020, 263: 166–174
Wang XL, Yuan K, Zhang W, Li SX, Gao GF, Lu L. Regulation of circadian genes by the MAPK pathway: Implications for rapid antidepressant action. Neurosci Bull 2020, 36: 66–76
Xie X, Shen Q, Yu C, Xiao Q, Zhou J, Xiong Z, et al. Depression-like behaviors are accompanied by disrupted mitochondrial energy metabolism in chronic corticosterone-induced mice. J Steroid Biochem Mol Biol 2020, 200: 105607
Zhang LF, Shi L, Liu H, Meng FT, Liu YJ, Wu HM, et al. Increased hippocampal tau phosphorylation and axonal mitochondrial transport in a mouse model of chronic stress. Int J Neuropsychopharmacol 2012, 15: 337–348
Flint J, Kendler KS. The genetics of major depression. Neuron 2014, 81: 1214
Sullivan PF, de Geus EJ, Willemsen G, James MR, Smit JH, Zandbelt T, et al. Genome-wide association for major depressive disorder: a possible role for the presynaptic protein piccolo. Mol Psychiatry 2009, 14: 359–375
Dunn EC, Brown RC, Dai Y, Rosand J, Nugent NR, Amstadter AB, et al. Genetic determinants of depression: recent findings and future directions. Harv Rev Psychiatry 2015, 23: 1–18
Major Depressive Disorder Working Group of the Psychiatric GC, Ripke S, Wray NR, Lewis CM, Hamilton SP, Weissman MM, et al. A mega-analysis of genome-wide association studies for major depressive disorder. Mol Psychiatry 2013, 18: 497–511.
Levinson DF, Mostafavi S, Milaneschi Y, Rivera M, Ripke S, Wray NR, et al. Genetic studies of major depressive disorder: why are there no genome-wide association study findings and what can we do about it? Biol Psychiatry 2014, 76: 510–512
Sullivan PF, Agrawal A, Bulik CM, Andreassen OA, Borglum AD, Breen G, et al. Psychiatric genomics: An update and an agenda. Am J Psychiatry 2018, 175: 15–27
Schwabe I, Milaneschi Y, Gerring Z, Sullivan PF, Schulte E, Suppli NP, et al. Unraveling the genetic architecture of major depressive disorder: merits and pitfalls of the approaches used in genome-wide association studies. Psychol Med 2019, 49: 2646–2656
Okbay A, Baselmans BM, De Neve JE, Turley P, Nivard MG, Fontana MA, et al. Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nat Genet 2016, 48: 624–633
Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet 2018, 50: 668–681
Howard DM, Adams MJ, Clarke TK, Hafferty JD, Gibson J, Shirali M, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci 2019, 22: 343–352
Ormel J, Hartman CA, Snieder H. The genetics of depression: successful genome-wide association studies introduce new challenges. Transl Psychiatry 2019, 9: 114
Uchida S, Yamagata H, Seki T, Watanabe Y. Epigenetic mechanisms of major depression: Targeting neuronal plasticity. Psychiatry Clin Neurosci 2018, 72: 212–227
Yeshurun S, Hannan AJ. Transgenerational epigenetic influences of paternal environmental exposures on brain function and predisposition to psychiatric disorders. Mol Psychiatry 2019, 24: 536–548
van Eijndhoven P, van Wingen G, Katzenbauer M, Groen W, Tepest R, Fernandez G, et al. Paralimbic cortical thickness in first-episode depression: evidence for trait-related differences in mood regulation. Am J Psychiatry 2013, 170: 1477–1486
Peng W, Chen Z, Yin L, Jia Z, Gong Q. Essential brain structural alterations in major depressive disorder: A voxel-wise meta-analysis on first episode, medication-naive patients. J Affect Disord 2016, 199: 114–123
Geerlings MI, Gerritsen L. Late-life depression, hippocampal volumes, and hypothalamic-pituitary-adrenal axis regulation: A systematic review and meta-analysis. Biol Psychiatry 2017, 82: 339–350
Maller JJ, Broadhouse K, Rush AJ, Gordon E, Koslow S, Grieve SM. Increased hippocampal tail volume predicts depression status and remission to anti-depressant medications in major depression. Mol Psychiatry 2018, 23: 1737–1744
Joshi SH, Espinoza RT, Pirnia T, Shi J, Wang Y, Ayers B, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry 2016, 79: 282–292
Gryglewski G, Baldinger-Melich P, Seiger R, Godbersen GM, Michenthaler P, Klobl M, et al. Structural changes in amygdala nuclei, hippocampal subfields and cortical thickness following electroconvulsive therapy in treatment-resistant depression: longitudinal analysis. Br J Psychiatry 2019, 214: 159–167
Boldrini M, Santiago AN, Hen R, Dwork AJ, Rosoklija GB, Tamir H, et al. Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology 2013, 38: 1068–1077
Liang S, Wang Q, Kong X, Deng W, Yang X, Li X, et al. White matter abnormalities in major depression biotypes identified by diffusion tensor imaging. Neurosci Bull 2019, 35: 867–876
Chen G, Guo Y, Zhu H, Kuang W, Bi F, Ai H, et al. Intrinsic disruption of white matter microarchitecture in first-episode, drug-naive major depressive disorder: A voxel-based meta-analysis of diffusion tensor imaging. Prog Neuropsychopharmacol Biol Psychiatry 2017, 76: 179–187
Brakowski J, Spinelli S, Dorig N, Bosch OG, Manoliu A, Holtforth MG, et al. Resting state brain network function in major depression - Depression symptomatology, antidepressant treatment effects, future research. J Psychiatr Res 2017, 92: 147–159
Dutta A, McKie S, Downey D, Thomas E, Juhasz G, Arnone D, et al. Regional default mode network connectivity in major depressive disorder: modulation by acute intravenous citalopram. Transl Psychiatry 2019, 9: 116
Meyer BM, Rabl U, Huemer J, Bartova L, Kalcher K, Provenzano J, et al. Prefrontal networks dynamically related to recovery from major depressive disorder: a longitudinal pharmacological fMRI study. Transl Psychiatry 2019, 9: 64
Muller VI, Cieslik EC, Serbanescu I, Laird AR, Fox PT, Eickhoff SB. Altered brain activity in unipolar depression revisited: Meta-analyses of neuroimaging studies. JAMA Psychiatry 2017, 74: 47–55
Connolly CG, Ho TC, Blom EH, LeWinn KZ, Sacchet MD, Tymofiyeva O, et al. Resting-state functional connectivity of the amygdala and longitudinal changes in depression severity in adolescent depression. J Affect Disord 2017, 207: 86–94
Godlewska BR, Browning M, Norbury R, Igoumenou A, Cowen PJ, Harmer CJ. Predicting treatment response in depression: The role of anterior cingulate cortex. Int J Neuropsychopharmacol 2018, 21: 988–996
Goldstein-Piekarski AN, Staveland BR, Ball TM, Yesavage J, Korgaonkar MS, Williams LM. Intrinsic functional connectivity predicts remission on antidepressants: a randomized controlled trial to identify clinically applicable imaging biomarkers. Transl Psychiatry 2018, 8: 57
Leaver AM, Vasavada M, Joshi SH, Wade B, Woods RP, Espinoza R, et al. Mechanisms of antidepressant response to electroconvulsive therapy studied with perfusion magnetic resonance imaging. Biol Psychiatry 2019, 85: 466–476
Miskowiak KW, Macoveanu J, Jorgensen MB, Stottrup MM, Ott CV, Jensen HM, et al. Neural response after a single ECT session during retrieval of emotional self-referent words in depression: A randomized, sham-controlled fMRI study. Int J Neuropsychopharmacol 2018, 21: 226–235
Du L, Liu H, Du W, Chao F, Zhang L, Wang K, et al. Stimulated left DLPFC-nucleus accumbens functional connectivity predicts the anti-depression and anti-anxiety effects of rTMS for depression. Transl Psychiatry 2018, 7: 3
Williams LM. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depress Anxiety 2017, 34: 9–24
Williams LM. Precision psychiatry: a neural circuit taxonomy for depression and anxiety. Lancet Psychiatry 2016, 3: 472–480
Williams LM, Korgaonkar MS, Song YC, Paton R, Eagles S, Goldstein-Piekarski A, et al. Amygdala reactivity to emotional faces in the prediction of general and medication-specific responses to antidepressant treatment in the randomized iSPOT-D trial. Neuropsychopharmacology 2015, 40: 2398–2408
Hou Z, Gong L, Zhi M, Yin Y, Zhang Y, Xie C, et al. Distinctive pretreatment features of bilateral nucleus accumbens networks predict early response to antidepressants in major depressive disorder. Brain Imaging Behav 2018, 12: 1042–1052
De Crescenzo F, Ciliberto M, Menghini D, Treglia G, Ebmeier KP, Janiri L. Is (18)F-FDG-PET suitable to predict clinical response to the treatment of geriatric depression? A systematic review of PET studies. Aging Ment Health 2017, 21: 889–894
Schmaal L, Pozzi E, T CH, van Velzen LS, Veer IM, Opel N, et al. ENIGMA MDD: seven years of global neuroimaging studies of major depression through worldwide data sharing. Transl Psychiatry 2020, 10: 172.
Schmaal L, Veltman DJ, van Erp TG, Samann PG, Frodl T, Jahanshad N, et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry 2016, 21: 806–812
Schmaal L, Hibar DP, Samann PG, Hall GB, Baune BT, Jahanshad N, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry 2017, 22: 900–909
Frodl T, Janowitz D, Schmaal L, Tozzi L, Dobrowolny H, Stein DJ, et al. Childhood adversity impacts on brain subcortical structures relevant to depression. J Psychiatr Res 2017, 86: 58–65
Renteria ME, Schmaal L, Hibar DP, Couvy-Duchesne B, Strike LT, Mills NT, et al. Subcortical brain structure and suicidal behaviour in major depressive disorder: a meta-analysis from the ENIGMA-MDD working group. Transl Psychiatry 2017, 7: e1116
Tozzi L, Garczarek L, Janowitz D, Stein DJ, Wittfeld K, Dobrowolny H, et al. Interactive impact of childhood maltreatment, depression, and age on cortical brain structure: mega-analytic findings from a large multi-site cohort. Psychol Med 2020, 50: 1020–1031
de Kovel CGF, Aftanas L, Aleman A, Alexander-Bloch AF, Baune BT, Brack I, et al. No alterations of brain structural asymmetry in major depressive disorder: An ENIGMA consortium analysis. Am J Psychiatry 2019, 176: 1039–1049
Ho TC, Gutman B, Pozzi E, Grabe HJ, Hosten N, Wittfeld K, et al. Subcortical shape alterations in major depressive disorder: Findings from the ENIGMA major depressive disorder working group. Hum Brain Mapp 2020.
Han LKM, Dinga R, Hahn T, Ching CRK, Eyler LT, Aftanas L, et al. Brain aging in major depressive disorder: results from the ENIGMA major depressive disorder working group. Mol Psychiatry 2020.
van Velzen LS, Kelly S, Isaev D, Aleman A, Aftanas LI, Bauer J, et al. White matter disturbances in major depressive disorder: a coordinated analysis across 20 international cohorts in the ENIGMA MDD working group. Mol Psychiatry 2020, 25: 1511–1525
Lv X, Si T, Wang G, Wang H, Liu Q, Hu C, et al. The establishment of the objective diagnostic markers and personalized medical intervention in patients with major depressive disorder: rationale and protocol. BMC Psychiatry 2016, 16: 240
Fabbri C, Hosak L, Mossner R, Giegling I, Mandelli L, Bellivier F, et al. Consensus paper of the WFSBP Task Force on Genetics: Genetics, epigenetics and gene expression markers of major depressive disorder and antidepressant response. World J Biol Psychiatry 2017, 18: 5–28
Li Z, Zhang C, Fan J, Yuan C, Huang J, Chen J, et al. Brain-derived neurotrophic factor levels and bipolar disorder in patients in their first depressive episode: 3-year prospective longitudinal study. Br J Psychiatry 2014, 205: 29–35
Gong Q, He Y. Depression, neuroimaging and connectomics: a selective overview. Biol Psychiatry 2015, 77: 223–235
Papakostas GI, Shelton RC, Kinrys G, Henry ME, Bakow BR, Lipkin SH, et al. Assessment of a multi-assay, serum-based biological diagnostic test for major depressive disorder: a pilot and replication study. Mol Psychiatry 2013, 18: 332–339
Kolovos S, Kleiboer A, Cuijpers P. Effect of psychotherapy for depression on quality of life: meta-analysis. Br J Psychiatry 2016, 209: 460–468
Park LT, Zarate CA Jr. Depression in the primary care setting. N Engl J Med 2019, 380: 559–568
Health N C C F M . Depression: The treatment and management of depression in adults (updated edition) 2010.
Qaseem A, Barry MJ, Kansagara D. Clinical Guidelines Committee of the American College of P. Nonpharmacologic versus pharmacologic treatment of adult patients with major depressive disorder: A clinical practice guideline from the american college of physicians. Ann Intern Med 2016, 164: 350–359
Dodd S, Mitchell PB, Bauer M, Yatham L, Young AH, Kennedy SH, et al. Monitoring for antidepressant-associated adverse events in the treatment of patients with major depressive disorder: An international consensus statement. World J Biol Psychiatry 2018, 19: 330–348
Cristea IA, Naudet F. US Food and Drug Administration approval of esketamine and brexanolone. Lancet Psychiatry 2019, 6: 975–977
Zheng W, Cai DB, Xiang YQ, Zheng W, Jiang WL, Sim K, et al. Adjunctive intranasal esketamine for major depressive disorder: A systematic review of randomized double-blind controlled-placebo studies. J Affect Disord 2020, 265: 63–70
Popova V, Daly EJ, Trivedi M, Cooper K, Lane R, Lim P, et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: A Randomized Double-Blind Active-Controlled Study. Am J Psychiatry 2019, 176: 428–438
Turner EH. Esketamine for treatment-resistant depression: seven concerns about efficacy and FDA approval. Lancet Psychiatry 2019, 6: 977–979
Murrough JW, Abdallah CG, Mathew SJ. Targeting glutamate signalling in depression: progress and prospects. Nat Rev Drug Discov 2017, 16: 472–486
Gill SP, Kellner CH. Clinical practice recommendations for continuation and maintenance electroconvulsive therapy for depression: Outcomes from a review of the evidence and a consensus workshop held in Australia in May 2017. J ECT 2019, 35: 14–20
McClintock SM, Reti IM, Carpenter LL, McDonald WM, Dubin M, Taylor SF, et al. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J Clin Psychiatry 2018, 79.
Chase HW, Boudewyn MA, Carter CS, Phillips ML. Transcranial direct current stimulation: a roadmap for research, from mechanism of action to clinical implementation. Mol Psychiatry 2020, 25: 397–407
Alexander ML, Alagapan S, Lugo CE, Mellin JM, Lustenberger C, Rubinow DR, et al. Double-blind, randomized pilot clinical trial targeting alpha oscillations with transcranial alternating current stimulation (tACS) for the treatment of major depressive disorder (MDD). Transl Psychiatry 2019, 9: 106
Akhtar H, Bukhari F, Nazir M, Anwar MN, Shahzad A. Therapeutic efficacy of neurostimulation for depression: Techniques, current modalities, and future challenges. Neurosci Bull 2016, 32: 115–126
Zhou C, Zhang H, Qin Y, Tian T, Xu B, Chen J, et al. A systematic review and meta-analysis of deep brain stimulation in treatment-resistant depression. Prog Neuropsychopharmacol Biol Psychiatry 2018, 82: 224–232
Li X, Li X. The antidepressant effect of light therapy from retinal projections. Neurosci Bull 2018, 34: 359–368
Shekelle PG, Cook IA, Miake-Lye IM, Booth MS, Beroes JM, Mak S. Benefits and harms of cranial electrical stimulation for chronic painful conditions, depression, anxiety, and insomnia: A systematic review. Ann Intern Med 2018, 168: 414–421
Kessler RC. The potential of predictive analytics to provide clinical decision support in depression treatment planning. Curr Opin Psychiatry 2018, 31: 32–39
Bradley P, Shiekh M, Mehra V, Vrbicky K, Layle S, Olson MC, et al. Improved efficacy with targeted pharmacogenetic-guided treatment of patients with depression and anxiety: A randomized clinical trial demonstrating clinical utility. J Psychiatr Res 2018, 96: 100–107
Greden JF, Parikh SV, Rothschild AJ, Thase ME, Dunlop BW, DeBattista C, et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: A large, patient- and rater-blinded, randomized, controlled study. J Psychiatr Res 2019, 111: 59–67
Long Z, Du L, Zhao J, Wu S, Zheng Q, Lei X. Prediction on treatment improvement in depression with resting state connectivity: A coordinate-based meta-analysis. J Affect Disord 2020, 276: 62–68
Download references
Acknowledgments
This review was supported by the National Basic Research Development Program of China (2016YFC1307100), the National Natural Science Foundation of China (81930033 and 81771465; 81401127), Shanghai Key Project of Science & Technology (2018SHZDZX05), Shanghai Jiao Tong University Medical Engineering Foundation (YG2016MS48), Shanghai Jiao Tong University School of Medicine (19XJ11006), the Sanming Project of Medicine in Shenzhen Municipality (SZSM201612006), the National Key Technologies R&D Program of China (2012BAI01B04), and the Innovative Research Team of High-level Local Universities in Shanghai.
Author information
Authors and affiliations.
Clinical Research Center and Division of Mood Disorders of Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai, 200030, China
Zezhi Li, Jun Chen & Yiru Fang
Department of Neurology, Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200127, China
Shanghai Institute of Nutrition and Health, Shanghai Information Center for Life Sciences, Chinese Academy of Science, Shanghai, 200031, China
Meihua Ruan
Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Science, Shanghai, 200031, China
Shanghai Key Laboratory of Psychotic Disorders, Shanghai, 201108, China
Jun Chen & Yiru Fang
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Yiru Fang .
Ethics declarations
Conflicts of interest.
The authors declare no conflicts of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Li, Z., Ruan, M., Chen, J. et al. Major Depressive Disorder: Advances in Neuroscience Research and Translational Applications. Neurosci. Bull. 37 , 863–880 (2021). https://doi.org/10.1007/s12264-021-00638-3
Download citation
Received : 30 May 2020
Accepted : 30 September 2020
Published : 13 February 2021
Issue Date : June 2021
DOI : https://doi.org/10.1007/s12264-021-00638-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Major depressive disorder
- Pathogenesis
- Find a journal
- Publish with us
- Track your research
New Approach May Help People with Cancer Better Manage Depression, Pain, and Fatigue
May 14, 2024 , by Edward Winstead

In a clinical trial, people with cancer who received cognitive behavioral therapy via telehealth reported improvements in their quality of life.
Many people who are being treated for cancer experience symptoms of depression, pain, and fatigue. But scientists are still studying how best to manage these symptoms in people with cancer.
One approach is to integrate the assessment and treatment of symptoms as a part of routine cancer care. With this approach, people who need support are offered weekly cognitive behavioral therapy sessions from a trained counselor and/or medicine for their symptoms provided by their medical teams.
The strategy, called integrated screening and stepped collaborative care, has now shown promise in a large clinical trial involving people with different types and stages of cancer.
In the NCI-supported study, participants were randomly assigned to receive integrated screening and stepped collaborative care or standard care, which consisted of referring patients to health care providers for treatment.
The stepped collaborative care group had a greater improvement in health-related quality of life, including their emotional, physical, and functional well-being , during the first 6 months of treatment, according to results published March 12 in The Lancet .
The improvement was maintained for up to a year. In addition, participants who received stepped collaborative care also reported reductions in the three most common symptoms.
“Our results highlight the importance of integrating screening and treatment with routine cancer care and offering this at no cost to patients,” said the trial’s lead researcher, Jennifer Steel, Ph.D., a clinical health psychologist at the University of Pittsburgh Medical Center (UPMC).
“We need to revisit our current approach of screening patients for symptoms and referring them for treatment,” Dr. Steel said. “Our hope is that this research could lead to a shift in care that improves patient quality of life.”
Getting started with treatment for symptoms
The study occurred during the pandemic, and the stepped collaborative care intervention was delivered via telehealth. The trained counselors worked closely with the cancer care team to manage people’s symptoms.
With standard care, people being treated for cancer are screened for symptoms of depression, pain, and fatigue. Those who need treatment for these symptoms are referred to a specialist within or outside their health care facilities. Patients then follow up to arrange an appointment and may be responsible for some or all the treatment costs.
But this approach often fails to give people the full support they need, the researchers noted. Just getting started can be a challenge. By contrast, offering patients an integrated approach to screening for the symptoms and automated referral to receive stepped collaborative care increases the likelihood that they will begin treatment, Dr. Steel said.
In the trial, about 75% of the patients who were offered support began treatment with a trained counselor, compared with only about 4% of the patients in the standard treatment group.
Some of the participants in the stepped collaborative group said they were willing to try the therapy because it was free and part of their routine cancer care.
Being invited to participate in therapy was “helpful,” one participant said. The counselor “walks you through the process and knows your doctor,” the person added. “That makes you feel comfortable because you’re already scared to death.”
Another participant said, “Being reached out to absolutely influenced my decision” to receive treatment.
Reducing the use of health care resources
The trial included 459 people being treated for cancer who had certain levels of depression, pain, or fatigue (or all of these). They were treated at one of 29 cancer outpatient clinics affiliated with UPMC. The vast majority of participants were White and over age 60.
The researchers randomly assigned participants to receive stepped collaborative care or standard care, which included referring patients who showed evidence of depression, pain, or fatigue on screening to a health care professional.
In the stepped collaborative care group, participants were contacted to begin cognitive behavioral therapy for an hour once a week through telehealth. Patients received 8 to 12 sessions initially but could continue therapy for up to 6 months if needed. Medicine for depression, pain, and fatigue was also available if the patient preferred or did not respond to cognitive behavioral therapy.
After a median follow-up of 6 months, people in the stepped collaborative care group had clinically meaningful improvements in emotional, functional, and physical well-being, whereas those in the standard treatment group didn’t. The improvements lasted up to 1 year, which is how long the participants were followed.
In addition, people in the stepped collaborative care group had fewer emergency room visits, fewer hospital readmissions within 90 days, and shorter hospital stays than the standard care group.
Dr. Steel noted that reducing the use of health care resources might be important for patients. Fewer hospitalizations and emergency room visits “could lower cancer care costs to the patient, as well as stress associated with those visits for both the patient and the family,” she said.
“The trial highlights what can be achieved using telehealth,” said Paige A. Green, Ph.D., a health psychologist and behavioral medicine researcher in NCI’s Division of Cancer Control and Population Sciences who was not involved in the trial.
Dr. Green called the findings “promising” but noted that the trial had limitations, such as a study population that was more than 90% White.
“The lack of meaningful inclusion of patient populations that are often underrepresented in cancer research might limit the relevance of the study findings to those groups,” said Dr. Green.
How is cognitive behavior therapy used to manage symptoms?
The trial participants who underwent cognitive behavioral therapy were taught strategies to deal with their symptoms, including relaxation techniques and ways to alter core beliefs about themselves and their environment.
Participants who were experiencing pain and fatigue were taught strategies to positively affect their thinking, improve sleep hygiene, and increase physical activity.
With stepped care, health care providers continually monitor a person’s response to treatment until the symptoms are adequately addressed.
“If the person were not responding, the providers could ‘step up’ the care by increasing the frequency or the intensity of the treatment they were providing or by trying another treatment approach,” Dr. Steel explained.
Cost savings from stepped collaborative care
According to the researchers, if health care systems offered an integrated screening and treatment program at no cost to the patient, such systems would save about $16,000 per patient per year. Their estimate was based on the savings from shorter hospital stays, fewer emergency room visits, and fewer 90-day readmissions.
“This study is an important contribution” to the data on stepped collaborative care as part of cancer treatment, said Barbara L. Andersen, Ph.D., a clinical psychologist who studies biobehavioral aspects of cancer at The Ohio State University.
“The inclusion of cost data, I hope, will significantly strengthen the case for [providing] psychological care for patients in need,” added Dr. Andersen, who is also a member of an expert panel on the management of anxiety and depression in adult survivors of cancer .
For some participants in the trial, receiving mental health support at no cost played a role in their decisions to try cognitive behavioral therapy. One participant said cost was “a very big factor.”
“Seniors my age are on a budget,” the person explained. The cost of talking with a therapist on the phone “is the number one thing that anybody’s going to look at, especially if they’re 65 or older.”
Testing stepped collaborative care in up to 100 cancer clinics
For the study, Dr. Steel and her colleagues screened nearly 1,600 patients. Only 481 (30%) did not report any of the three symptoms targeted by the intervention.
Susanne Oksbjerg Dalton, Ph.D., and Christoffer Johansen, M.D., Ph.D., of Copenhagen University wrote in an accompanying editorial that the finding highlights the need to scale up collaborative care for nearly all people with cancer.
“Such a high symptom prevalence in patients treated for cancer seems overwhelming, but this underpins the negative effect of treatment on patient quality of life,” they wrote.
Dr. Steel and her colleagues are planning to develop a training institute to prepare mental health professionals to deliver the intervention. They are also planning a clinical trial to evaluate the new approach in nearly 100 clinics at UPMC Hillman Cancer Center.
Featured Posts
March 27, 2024, by Edward Winstead
March 21, 2024, by Elia Ben-Ari
March 5, 2024, by Carmen Phillips
- Biology of Cancer
- Cancer Risk
- Childhood Cancer
- Clinical Trial Results
- Disparities
- FDA Approvals
- Global Health
- Leadership & Expert Views
- Screening & Early Detection
- Survivorship & Supportive Care
- February (6)
- January (6)
- December (7)
- November (6)
- October (7)
- September (7)
- February (7)
- November (7)
- October (5)
- September (6)
- November (4)
- September (9)
- February (5)
- October (8)
- January (7)
- December (6)
- September (8)
- February (9)
- December (9)
- November (9)
- October (9)
- September (11)
- February (11)
- January (10)
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Environ Res Public Health

An Examination of Depression, Anxiety, and Self-Esteem in Collegiate Student-Athletes
Samantha r. weber.
1 Department of Nursing and Health Science, Limestone University, Gaffney, SC 29340, USA
Zachary K. Winkelmann
2 Department of Exercise Science, University of South Carolina, Columbia, SC 29208, USA
Eva V. Monsma
3 Department of Physical Education, University of South Carolina, Columbia, SC 29208, USA
Shawn M. Arent
Toni m. torres-mcgehee, associated data.
Not available based on Institutional Review Board policies.
Mental health research exists for student-athletes in the areas of depression, anxiety, and self-esteem prevalence. However, updated prevalence rates and assessment of risks across sports, academic status, and genders are needed. Filling the gaps in research assists in the creation of patient-centered mental health screening and interventions designed for student-athletes. Therefore, the purpose is to examine the prevalence of depression, anxiety, and self-esteem in collegiate student-athletes and differences between sex, academic status, and sport type, and identify associations for risks. Using a cross-sectional design, collegiate student-athletes were surveyed to assess for risks of depression, anxiety, and self-esteem. With the use of SPSS, Chi-square analyses and multinomial logistic regressions were used. Student-athletes (22.3%) were at risk for depression, anxiety (12.5%), and low self-esteem (8%). No significant differences were found for sex, academic status, and sport type for depression or self-esteem; however, significant differences occurred for state and trait anxiety by sex. A significant association for depression and anxiety risk was found with females at risk. Depression and anxiety are present within student-athletes, regardless of sport type. Females are at a higher risk; however, all student-athletes would benefit from the creation of validated, patient-centered mental health screenings and psychotherapeutic interventions.
1. Introduction
There are approximately a half-million collegiate student-athletes in the National Collegiate Athletic Association (NCAA), attending over 1000 colleges and universities in over 100 athletic conferences. According to the National Institute of Mental Health, approximately 8.4% of adults aged 18 or older experienced a depressive episode, and 19.1% had an anxiety disorder in the past year [ 1 ]. More specifically, within college-age students ranging from 18–25, the prevalence was highest for depression at 17.0% and 22.3% for anxiety disorders [ 1 ]. College is considered an at-risk period for the development of mental health illnesses. According to the American College Health Association, over 30% of students reported significant signs of depression [ 1 , 2 , 3 ]. College student-athletes are a subset of the young adult population and may be at risk for stressors linked to mental health issues (e.g., disordered eating, substance or alcohol abuse). With many student-athletes participating in sport, it is reasonable to believe that numerous student-athletes participate in their sport while managing the signs, symptoms, and known risk factors of depression and anxiety. With an increase in mental health visibility, an updated examination on the prevalence of depression, anxiety, and low self-esteem for this population is warranted.
Depression is characterized by mood changes, loss of interest or pleasure in daily activities, and the associated symptoms of sleep and eating problems, low energy, lack of concentration and self-worth [ 1 ]. With participation in sport, student-athletes are thought to be immune to mental health disorders like depression; however, research demonstrates that the general college student population and student-athletes are comparable [ 4 , 5 ]. Research directly investigating the prevalence and severity of depression symptoms in collegiate student-athletes varies by instruments used and by sports and sex examined. The prevalence of the risk of depression in collegiate student-athletes ranges from 15.6% to 33.2%, with first-year students and females typically reporting more symptoms [ 4 , 6 , 7 ]. When examining the depression risk prevalence in specific sports including but not limited to football, baseball, wrestling, track and field, and lacrosse, the range was from 12.1% to 35.4%, with higher rates consistent with females [ 8 , 9 , 10 ]. In the current literature, sports have been categorized as individual and team sports when examining risk of depression in collegiate student-athletes, indicating that individual sports may be at an increased risk over team sports [ 10 , 11 ]. With a younger population, a lower prevalence rate of 8% was found for depression and anxiety, and specifically 13% for individual sports and 7% for team sports, further supporting sport type as being associated with the risk of depression and anxiety in student-athletes [ 11 ]. The previous research on prevalence rates for team versus individual sports is based on a younger population, and there are different validated measures used throughout the research to assess for the presence of the risk of depression.
Anxiety is commonly known as a reaction to a perceived stressful or dangerous situation that can have debilitating effects on daily activities and performance. State anxiety refers to a temporary response to a stressful advent and trait anxiety is defined as a personality feature or predisposition [ 12 ]. Athletes often experience state anxiety during situations that create pressure, for example if a free throw determines the outcome of a basketball game. However, trait anxiety refers to characteristics of a person, where an individual is anxious about general unknown outcomes. Researchers have identified that high levels of trait anxiety may lead to an increase in state anxiety during performance [ 13 ]. However, there is limited research focusing on the examination of state and trait anxiety prevalence in student-athletes. According to current literature by Li and colleagues [ 14 ], one-third of student-athletes reported anxious symptoms prior to the season beginning with a significantly higher risk for sport injury. Furthermore, previous studies examining student-athletes primarily occurred during preseason training and did not find significance for gender, sport, or academic status differences and the state and trait anxiety scores [ 8 , 14 ]. However, both studies by Yang et. al. [ 8 ] and Li et. al. [ 14 ], indicated that the link between depression and anxiety is associated with higher levels of pain and injury incidence. Therefore, examining anxiety prevalence rates in student-athletes by sex, academic status, and sport type and determining additional risks for depression and anxiety is warranted to help clinicians prevent additional injury. Without further research on depression and anxiety prevalence, it is difficult to develop preventative mental health programs and interventions for current conditions.
Participation in sport facilitates positive mental health behaviors, including self-confidence, positive self-esteem, and social support [ 15 ]. Individuals with positive mental health behaviors may be utilizing their social support systems to cope and manage stress in helpful ways to lower risks for depression and anxiety. However, student-athletes may be more susceptible to mental health issues due to the demands of sport participation (e.g., sports injury, coach expectations) [ 9 ]. Student-athletes are thought to be protected from mental health issues because of increased self-esteem, a sense of connectedness, and social support from their teammates [ 15 ]. There is an established relationship between self-esteem and depression, indicating that self-esteem is associated with depression. In those with lower self-esteem, depression rates tend to be higher, whereas in student-athletes with a higher self-esteem a lower rate of depression was found [ 15 ]. However, this study is out of date, and updated research for student-athletes is needed.
Clinicians providing medical services within the collegiate sports setting should be mindful of comorbidities of mental illnesses and which student-athletes are at the highest risk. Additionally, being able to recognize the common mental health illnesses and risks among student-athletes can help guide clinicians to utilize validated patient-centered screenings to identify those who may need referral for psychotherapeutic intervention [ 16 ]. Early screening, identification, and intervention can allow healthcare professionals to gain more information on their patients; furthermore, this allows providers to ask in-depth questions tailored to their patients’ needs, and to develop strategies (i.e., goal setting, coping mechanisms) to support their mental and physical needs [ 17 ]. Student-athletes who have an individualized approach to their needs are more likely to communicate with their providers and trust the clinician providing healthcare [ 18 ], and with a tailored approach to healthcare, specifically mental health care, student athletes are able to receive help before signs and symptoms begin to manifest. While all student-athletes have unique personal stressors and individual experiences, understanding the associations between depression, anxiety, self-esteem, sport type, and sex may help clinicians choose to use validated screenings and further develop interventions for managing symptoms for their patients. Therefore, the purpose of this study was to examine the overall prevalence of depression, anxiety, and self-esteem in NCAA Division I and II collegiate student-athletes, with a secondary purpose to examine differences between depression and anxiety risk, and low self-esteem with demographic variables such as sex, academic status (e.g., freshman, sophomore, etc.) and sport type (e.g., power, ball sports, technical, endurance, etc.); and lastly to identify associations for depression, anxiety, and low self-esteem.
2. Materials and Methods
2.1. participants.
Participants were NCAA Division I and II student-athletes ( n = 615; age 20 ± 1 years; males: n = 233, height: 184.1 ± 0.5 cm, weight: 91.5 ± 0.15 kg; females: n = 382, height = 168.4 ± 0.39 cm, weight: 63.25 ± 19.8 kg) from across 40 institutions. To be included in the cross-sectional study, the student-athletes had to be between the ages of 18–26 and on an active roster during the time of the survey. The Institutional Review Board approved the study, and all participants consented prior to completing the survey. The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the University of South Carolina (protocol code Pro00092702 and date of approval 1 November 2019).
2.2. Instruments
2.2.1. demographic information.
The demographic information collected included age, sex, self-reported height, weight, body mass index (BMI), academic status, and sport. Academic status was defined as a first-year, sophomore, junior, or senior. Participants that were considered fifth-year seniors or graduate students were coded as seniors. Sport type was classified using prior classification by Sundgot-Borgen [ 19 ], by sorting sports into groups of endurance (i.e., cross country, track, swimming), aesthetic (i.e., cheerleading, diving, dance, equestrian), power (i.e., football), ball (i.e., baseball, softball, basketball, soccer, volleyball, beach volleyball), and technical sports (i.e., golf, tennis) [ 19 ].
2.2.2. Center for Epidemiologic Studies Depression Scale
The Center for Epidemiologic Studies Depression Scale (CESD) is a self-report measure of depressive symptoms. There are eight different subscales, including sadness, loss of interest, appetite, sleep, thinking/concentration, guilt, worthlessness, being tired, fatigue, movement, and suicidal ideation over the past week. Student-athletes selected how often they have felt or behaved in a particular way on a scale of 1 (rarely or none of the time) to 4 (most or all the time) during the past week. Any scores higher than 16 indicates an individual is at risk for depression [ 20 ]. The internal consistency for the CESD is r = 0.85 to 0.90, with a test-retest reliability of r = 0.45–0.7, and r = 0.91 for this study [ 20 , 21 ].
2.2.3. State Trait Anxiety Inventory
The State Trait Anxiety Inventory (STAI) is a self-report tool that indicates anxiety and distinguishes between state and trait anxiety. The first 20 questions consist of statements examining how individuals feel “right now at this moment”, and individuals respond on a scale of 1 (not at all) to 4 (very much so). The second 20 questions examine how individuals generally feel on a scale of 1 (almost never) to 4 (almost always) [ 12 , 22 ]. State and Trait Anxiety were measured using the State-Trait Anxiety Inventory. The college student norms were used for analyses with state anxiety for females being 38.76 ± 11.96 and males at 46.47 ± 10.02, and trait anxiety for females 40.40 ± 10.15 and males 38.30 ± 9.18. For the STAI, the internal consistency coefficients range from r = 0.86 to 0.95 and the test-retest reliability ranges from r = 0.65 to 0.75 [ 12 ]. The reliability for the STAI in this study r = 0.95.

2.2.4. Rosenberg Self-Esteem Scale
The Rosenberg Self-Esteem Scale (RSES) is one of the most widely used measures of self-esteem [ 23 , 24 ]. The scale consists of 10 items that are rated on a 4-point Likert scale (strongly agree = 3, agree = 2, disagree = 1, and strongly disagree = 0), and assesses how an individual thinks and feels about themselves. Each participant’s responses are summed across the 10 items and further categorized as low self-esteem or at risk (below 15) or not at risk or high self-esteem (above 15) [ 23 , 24 ]. The scale has been validated for college populations with a test-retest reliability ranging from 0.85 to 0.88 [ 23 ], with excellent stability and a reliability of 0.90 for this study [ 24 ].
2.3. Procedures
Upon receiving approval from the University of South Carolina’s Institution Review Board, participants were recruited using a snowball sampling method. Athletic trainers who worked directly with student-athletes at NCAA Division I or II intuitions were contacted with an invitation letter and a survey link and asked to forward the invitation to their current student-athletes. There was no incentive for completion of the survey. The web-based online survey (SurveyMonkey, San Mateo, CA, USA) included an invitation/consent letter, and the demographic items were followed by the CESD, STAI, and the RSES. The survey was available for 30 days, with a follow-up reminder sent to the participant every 10 days until the window closed.
2.4. Statistical Analysis
We used SPSS statistical software (Version 27; SPSS Inc. Armonk, NY, USA) with an alpha set at p < 0.05 for all analyses. We used G*Power 3 (version 3.1.9.2., Heinrich Heine University, Dusseldorf, Germany) software to calculate power [ 25 ]. Using an alpha of 0.05 and a small effect size (0.2), our power calculation indicated that we needed a sample of 495 completed surveys to achieve an estimated power of 0.95 [ 25 , 26 ]. For sport type, power was estimated at 0.05 and large effect size (0.6), and our power calculation indicated a sample size of 46 per sport group to achieve an estimated power of 0.90 [ 25 , 26 ]. We performed basic descriptive statistics to examine the demographic information (e.g., height, weight, age, body mass index (BMI), sex, academic status, etc.) Chi-squared analyses were used to determine differences between depression, anxiety, and self-esteem risk, sex, academic status, and sport type. The results of the chi-squared tests were used to select association variables to assess each outcome using multinomial logistic regression. Education, sport, and ethnicity were not analyzed due to results from the chi-squared analyses, and sex was examined as an association variable for the outcome.
A total of 821 student-athletes initiated the survey, 675 partially completed the survey, and 615 student-athletes fully completed the survey (75% completion rate). Student-athletes included in this study were from 40 institutions across 22 teams which were categorized into endurance sports ( n = 171), aesthetic sports ( n = 102), power sports, ( n = 117), ball sports ( n = 194), and technical sports ( n = 31). Detailed demographic information can be found in Table 1 .
Participant Demographics (Mean ± SD).
3.1. Prevalence of Depression Risk
Overall, 22.3% ( n = 137/615) of student-athletes were classified as being at risk for depression. A Chi-squared analysis revealed no significant differences between the CES-D and sex (Χ 2 1,615 = 0.00, p = 0.99, with females (13.8.%) reporting the same risk as males (8.5%). Please refer to Table 2 for sex distribution. No significant differences were identified for depression risk and academic status ((Χ 2 3,615 = 6.36, p = 0.095), Table 3 ), with sophomores ( n = 45/154, 29.2%) and juniors ( n = 33/149, 22.1%) reporting the highest depression risk. A Chi-squared analysis revealed no significant differences between the CES-D and sport type (Χ 2 4,615 = 3.427, p = 0.489), with ball ( n = 48/194, 24.7%) and power ( n = 28/117, 23.9%) sports reporting the highest risk. The distribution for CES-D risk and sport type can be found in Table 3 .
Depression, Anxiety and Low Self-Esteem Prevalence Rates by Sex.
a p Values ≤ 0.05. State and trait anxiety were measured using the State-Trait Anxiety Inventory. The college student norms were used for analyses. State anxiety means and standard deviation for females was 38.76 (11.96) and for males it was 46.47 (10.02), and trait anxiety for females was 40.40 (10.15), while for males it was 38.30 (9.18).
Depression Prevalence by Academic Status and Sport.
3.2. Prevalence of Anxiety Risk
Overall, 8.5% ( n = 52/615) of student-athletes were classified as over the norm means for college-aged students for state anxiety, and 12.5% ( n = 77/615) for trait anxiety. However, most of the participants were within the norms for state (66.7%, n = 410/615) and trait (57.4%, n = 353/615) anxiety, respectively. The overall raw mean scores and standard deviations by sex are presented in Table 4 . A Chi-squared analysis revealed a significant difference for state anxiety and sex (Χ 2 2,615 = 10.46, p = 0.005), and for trait anxiety and sex (Χ 2 2,615 = 10.32, p = 0.006). Table 2 provides the distribution of state and trait anxiety by sex. There were no differences found for state and trait anxiety for academic status (Χ 2 6,615 = 3.53, p = 0.740), (Χ 2 6,615 = 4.42, p = 0.620) or for sport type (Χ 2 8,615 = 12.25, p = 0.141), (Χ 2 6,158 = 4.27, p = 0.832), as demonstrated in Table 5 .
State and Trait Anxiety Raw Scores. [M (SD)].
State and trait anxiety were measured using the State-Trait Anxiety Inventory. The college student norms were used for analyses. State anxiety means and standard deviation for females was 38.76 (11.96) and for males it was 46.47 (10.02), and trait anxiety for females was 40.40 (10.15), while for males it was 38.30 (9.18).
Anxiety Prevalence by Academic Status and Sport.
3.3. Prevalence of Low-Self Esteem
Overall, 8.0% ( n = 49/615) of student-athletes were classified as being at risk for low self-esteem. A Chi-squared analysis revealed no significant differences between the RSES and sex (Χ 2 1,615 = 1.112, p = 0.292), with females (7.1%) reporting a slightly lower risk than males (9.4%). The distribution for RSES by sex is found in Table 2 . No significant differences were identified for low self-esteem and academic status (Χ 2 3,615 = 0.394, p = 0.942), with sophomores ( n = 14/154, 9.1%) and juniors ( n = 10/125, 8.0%) reporting scores indicating low self-esteem. A Chi-squared analysis revealed no significant differences between the RSES and sport type (Χ 2 4,615 = 4.094, p = 0.393), with power ( n = 12/117, 10.3%) and endurance ( n =17/171, 9.9%) sports reporting low self-esteem. The distribution for the RSES and sport type can be found in Table 6 .
Low Self-Esteem Prevalence by Academic Status and Sport.
3.4. Multinomial Logistic Regression
Based off of the prevalence data, the results of the multinomial analysis indicated that sex is associated with depression risk, and state and trait anxiety risk ( Table 2 ). For depression risk, females are more likely to be at risk for depression when compared to males, with an odds ratio of 1.795 (CI: 1.184, 2.722). Females are more likely to be within the average college student mean for state anxiety as compared to males, with an odds ratio of 1.771 (CI: 1.214, 2.585) with an increase in odds by 77.1%. As for trait anxiety, females are more likely to be within or above the average college student mean for trait anxiety as compared to males, with an odds ratio of 1.427 (CI: 0.994, 2.048) and 2.539 (CI: 1.402, 4.596), respectively.
4. Discussion
This study examined prevalence rates for depression, anxiety, and low self-esteem risk in a large NCAA Division I and II collegiate student-athlete sample. In addition, the differences of sex, academic status and sport were explored. This study also expands on earlier research by examining sport type classifications and investigates sex and academic status for the association with depression or anxiety risk. Furthermore, this study compares findings with previous sport categorizations and provides suggestions for future interventions.
4.1. Depression
Despite increased recognition of the importance of mental health in student-athletes, prevalence rates are still high when compared with previous literature. We found an overall prevalence of 22.3% for depression risk, similar to previous research in student-athletes using the CES-D [ 7 , 8 , 14 ]. With the risk for depression at 22.3%, nearly one in every four student-athletes report signs and symptoms of depression. Collegiate student-athletes not only have an expectation of being successful athletically but are also required to succeed personally and academically. Student-athletes are required to maintain a balance between academics and specific sport requirements, placing undue stress on the student-athletes, increasing their risk of depression. While there were no significant differences for sex, this study demonstrated that more females reported signs and symptoms of depression with the CES-D. Our findings support the suggestion that females may be at a higher risk for depression than males, further reinforcing previous efforts examining depression prevalence in student-athletes [ 6 , 7 , 8 , 9 , 27 ]. When considering the distribution of sex in the sport classification, power and ball sport groups had the highest percentage for reported symptoms. For the examination of sex with depression, significant associations were found, indicating that females are more likely to report depressive symptoms than males, which is consistent with prior research [ 8 ]. Although sport type was not found to be significant, each sport type has its own associated risks.
It has been previously suggested that individual sports are at a higher risk for depression than team sports, and, furthermore, indicating that sport type is a risk for depression [ 10 , 11 ]. When examining sport in our study, we categorized our sports by the recommendations of Sundgot–Borgen [ 19 ]. With this categorization, we found no differences for sport type and depression risk; however, it is important to note that ball (basketball, soccer) and power sports (football) were the highest among sport types. Ball and power sports would fall into the category of a team sport, and while not significant, both sport groups were higher for depression risk than others that would be categorized as individual sports. The ball sport category included both females and males, while power sports only included football athletes. This finding is contrary to earlier research indicating that individual sports are more likely to report symptoms, however it should not be overlooked. The team aspect of sport is thought to be a supportive community [ 11 ], and with higher numbers from our study this could indicate possible a contention within the team community.
In addition, no significant differences were found for academic status, indicating that the risk of depression was consistent across education levels. Contrary to previous research, first-year and sophomore students have been identified to be more at risk when compared to the other education levels [ 6 , 8 ]. Our results have also indicated that academic status was not a significant factor for depression risk. While the results are insignificant, it is essential to note that mental health interventions may be beneficial for all academic levels. Each academic class experiences unique individual stressors. For example, first-year students must learn to adapt to new friends, classes, and living situations while adjusting to the new team, coach, and expectations, whereas seniors are preparing to finish their athletic careers, graduate, and become working professionals in their field of study. Mental health interventions tailored to the student-athletes’ mental and physical needs such as the instruction of coping mechanisms, time management skills, and self-care may help reduce mental health prevalence among all student-athletes regardless of academic status. In addition, to ensure appropriate patient-centered care identifying and intervening with individuals that present with symptomology early can result in appropriate referrals, improving patient relationships, and outcomes.
4.2. Anxiety
In collegiate student-athletes, there is limited research on the prevalence of state and trait anxiety [ 8 , 14 ]. Prior research has examined state and trait anxiety in student-athletes during their preseason and found no differences for sex [ 8 , 14 ] or collegiate class [ 8 ]. Our results are consistent with those of Yang and colleagues [ 8 ], and demonstrate that student-athletes’ state and trait scores are significantly lower than that of typical college students [ 22 ]. While student-athletes are reporting scores lower than regular college students, our results demonstrate that student-athletes are still demonstrating signs and symptoms of anxiety. In addition, when examining sex and anxiety, females are more likely to be at or above the average mean for both state and trait anxiety. It has been suggested that with an increase in trait anxiety, state anxiety scores may increase and affect performance [ 13 ].
In addition, no significant differences were found for state or trait anxiety scores across academic status, which is supported by Yang and colleagues [ 8 ], who revealed similar findings for anxiety scores. While not significant, it was suggested that female, freshman, or juniors had a higher tendency to report symptoms than their counterparts [ 8 ]. With increased stress to maintain academic standards, it is interesting that the scores for state anxiety were not higher. Student-athletes are required to maintain a specific grade point average each semester to maintain their scholarships and ability to participate. Similarly, a stable or general fear and worry of maintaining these requirements does not seem to be higher than that of a typical college student, which would indicate trait anxiety. Furthermore, academic status was not significant for anxiety scores in our study. Although not significant, student-athletes are still reporting anxiety symptoms. Support programs and more options for tutoring and academic success may be beneficial for all student-athletes to be successful both in the classroom and in their athletic performance.
Similar to academic status, no significant differences were found for sport type and anxiety risks. When considering state anxiety and sport, athletes would typically experience symptoms of anxiety directly after or during stressful situations [ 28 , 29 ]. The participants in our study were able to complete the survey at their convenience; therefore, it is possible that the student-athletes were completing the survey in a non-stressful environment and were truly not experiencing state anxiety. In the context of sport competition, state anxiety scores are known to increase during or directly after situations that are perceived as stressful. Therefore, future research in student-athletes may benefit from an examination of anxiety states throughout a competitive season.
4.3. Low Self-Esteem
Low self-esteem is not a mental health disorder, but rather a behavior that can be a risk for depression and anxiety [ 15 , 30 ]. Individuals experiencing low self-esteem may have an inadequate perception of their performance, possibly predisposing them to mental health problems. Our study indicated that self-esteem was above the threshold for nearly all athletes sampled (92%), reflecting prior research, indicating that student-athletes have a higher sense of self-esteem when compared to non-athletes [ 15 ]. Furthermore, in a study examining college nursing students, over 70% reported low self-esteem and high academic stress [ 31 ]. From the results of our study, more student-athletes have a higher sense of self-esteem when compared to non-athletes and college nursing students. While our sample indicated a high prevalence of high self-esteem, we still had a higher prevalence for depression risk in the same sample. This contradicts previous research that demonstrates an inverse relationship with depression and self-esteem [ 15 ]. In our study, no differences were found for academic status or sport type, while previous studies did not broadly stratify participants by sport-types when examining self-esteem. Student-athletes participating in “lean” sports tend to have lower self-esteem which becomes a predictor for other mental health issues, such as eating disorders [ 32 ]. However, in our study, power and endurance athletes reported higher scores, indicating a lower self-esteem when compared to the other sport types. Endurance athletes would fall into the category of lean sports; however, power sports would not. These results demonstrate that low self-esteem occurs in both females and males, although at a low rate. While self-esteem does not seem to be a risk for student-athletes, the prevalence rates for depression and anxiety are consistent with previous research indicating that change is not occurring.
4.4. Limitations and Future Research
The current findings emphasize the importance of investigating the prevalence of depression and anxiety risk in collegiate student-athletes. However, the study is not without limitations. First, it is important to note that the data is self-reported by the athletes and depends on the participants’ honest answers. Second, the self-report tools are not diagnostic, and instead indicate whether an individual is at risk. Additionally, when the data were categorized by sport, it is important to recognize that there were no females in the power sport category. We were also unable to examine the data by NCAA Division level due to the design of the survey and level of anonymity; an examination by division level may provide additional insight into risk differences. Lastly, as current mental health interventions are loosely focused on improving mental health awareness and decreasing stigma, future research should focus on creating mental health screenings from already validated screenings that can be tailored to the student-athlete population and on developing psychotherapeutic mental health interventions for student-athletes that reduces mental health symptomology and improves coping skills.
5. Conclusions
The present study sought to establish the prevalence of depression risk, anxiety risk, low self-esteem risk, and determine if sex, academic status, or sport type were associated. It suggests that depression and anxiety signs and symptoms are present in the student-athlete population, with females predominantly more at risk than males. Further examination into risks that may result in student-athlete mental health symptomology can help practitioners preemptively refer to mental health professionals. The student-athletes in the present sample do not appear to be at risk for low self-esteem, suggesting that other factors may be at play among the athletes that are. Furthermore, we suggest that future research create mental health screenings from validated screenings that can be tailored for the student-athlete population and the implementation of interventions to help reduce symptomology for anxiety and depression.
Funding Statement
This research received no external funding.
Author Contributions
Conceptualization, S.R.W. and T.M.T.-M.; methodology, S.R.W. and T.M.T.-M.; validation, S.R.W., T.M.T.-M.; formal analysis, S.R.W.; investigation, T.M.T.-M.; data curation, S.R.W. and T.M.T.-M.; writing—original draft preparation, S.R.W.; writing—review and editing, S.R.W., Z.K.W. and T.M.T.-M.; visualization, T.M.T.-M.; supervision, Z.K.W., E.V.M., S.M.A.; T.M.T.-M.; project administration, S.R.W., Z.K.W., T.M.T.-M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the University of South Carolina (protocol code Pro00092702 and date of approval 11/1/2019).
Informed Consent Statement
The survey contained an invitation and consent letter. Therefore, participants who completed the survey provided their consent.
Data Availability Statement
Conflicts of interest.
The authors declare that they have no conflict of interest.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
- Skip to main content
- Keyboard shortcuts for audio player
- Your Health
- Treatments & Tests
- Health Inc.
- Public Health
As the FDA evaluates ecstasy treatment for PTSD, questions mount about the evidence

Research on MDMA has shown it can be effective for PTSD, but approval of the treatment isn't yet guaranteed. The Washington Post via Getty Images hide caption
Research on MDMA has shown it can be effective for PTSD, but approval of the treatment isn't yet guaranteed.
In a matter of months, the Food and Drug Administration is expected to decide whether the drug commonly known as ecstasy can be used as a treatment for post-traumatic stress disorder.
An approval by the agency would represent an enormous milestone for the movement to bring psychedelics into the mainstream of mental health care. An FDA rejection of MDMA, the abbreviation of the drug's chemical name, would deal a major setback to the effort.
Clinical trials have inspired optimism in the drug for its potential to help the millions of Americans who experience PTSD. Accounts from some of those who've participated in the trials describe the treatment as transformational.
But new and troubling questions about this research are now threatening to upset the final stretch in the drug's path to market.
The allegations surfaced in a draft report released in March by the Institute for Clinical and Economic Review , a nonprofit that evaluates clinical trials and drug prices, which found "substantial concerns about the validity of the results" of the MDMA clinical trials.
The ICER report was followed in April by a citizen petition to the FDA. In that document, a group of concerned people allege possible misconduct and ethical violations that could compromise the MDMA research. The petition asked the agency to hold a public meeting to address the concerns.
If true, the claims could jeopardize the drug's chances of receiving FDA approval, a decision that is expected to come by early August .
"There's the possibility that the data might not be representative of what's actually happened in clinical trials," says Neşe Devenot , one of the authors of the citizen petition and a senior lecturer in the writing program at Johns Hopkins University who is involved in psychedelic research. "I don't think this has been publicly reckoned with."
That may soon happen. The FDA announced Thursday that it plans to hold a public advisory committee meeting on MDMA-assisted therapy on June 4.
At the heart of the controversy are the organizations that have pioneered research into MDMA: the Multidisciplinary Association for Psychedelic Studies , and the public benefit corporation incubated by MAPS, which was recently rebranded as Lykos Therapeutics .
Lykos sponsored the clinical trials of MDMA. The results are included in the company's application to the FDA seeking approval to market the drug for therapy-assisted PTSD treatment.
Researchers and clinicians involved in the trials have pushed back strongly against the accusations that their clinical data isn't sound.
Jennifer Mitchell , lead author of the published papers from the Phase 3 trials, says she stands behind their findings.
"I didn't feel any pressure from the sponsor to come up with anything different than what the data was providing," says Mitchell, a professor of neurology and psychiatry at the University of California, San Francisco and associate chief of staff for research at the San Francisco VA Medical Center. "I wouldn't have continued to work with them if I had felt that."
Promising MDMA research for PTSD
The Phase 3 trials evaluated MDMA-assisted therapy, a protocol in which the drug is given under the supervision of two therapists.
In the second stage of the trials , 94 people with moderate and severe PTSD received either the drug or a placebo during three sessions, each spaced a month apart. There were also follow up "integration" sessions to help people process their experiences while on MDMA.
By the end of the trial, about 71% of participants in the MDMA arm no longer met the diagnostic criteria for PTSD, compared to about 48% who underwent the same therapy but took a placebo instead. Those findings built on promising results from earlier studies .
The study documented various adverse events in both groups — ranging from nausea and anxiety to heart palpitations — but none of them qualified as serious. The treatment was "generally well tolerated."
"Consistent with PTSD, suicidal ideation was observed in both groups," the authors reported in the journal Nature Medicine , "MDMA did not appear to increase this risk, and no suicidal behavior was observed."
Casey Tylek, a participant in the Phase 3 trials, says he had no experience with the drug prior to the study.
Tylek was in the placebo group, but was later given the opportunity to undergo the therapy with MDMA.
"It was incredibly powerful," says Tylek, a veteran who lives in Massachusetts, "I truly don't know if I would be alive today if I hadn't gone through that trial."
ICER report raises concerns
In its report , ICER acknowledges that the MDMA data suggest it would be an "important addition to treatment options for PTSD," but it questions whether the published findings tell the full story.
Among the concerns, the ICER report details a well-known challenge in psychedelic research around how to make sure study participants don't know if they got the experimental treatment or a placebo. Most of those in the MDMA group were able to identify they had received the drug. It also suggests the method used to assess PTSD — considered the gold-standard — showed improvements in symptoms after the treatment, even though some people were worse overall.
Beyond that, however, the report brings up the possibility that "very strong prior beliefs" among therapists, investigators and patients influenced the results.
"Concerns have been raised by some that therapists encouraged favorable reports by patients and discouraged negative reports by patients including discouraging reports of substantial harms, potentially biasing the recording of benefits and harms," the report states.
ICER does not identify the sources who were interviewed, although it did include two trial participants, a "trial therapist" and those who worked on a podcast called Cover Story , says Dr. David Rind , the chief medical officer for ICER.
"This was a very unusual review," says Rind.
The podcast, produced by New York Magazine and the nonprofit media organization Psymposia , brought to light claims by a participant named Meaghan Buisson, who appeared in a video of two therapists , a married couple, engaged in what Buisson described as inappropriate physical contact while she was under the influence of MDMA at a Phase 2 trial site in Canada.

Enlighten Me with Rachel Martin
This psychedelics researcher approached his death with calm and curiosity.
MAPS determined the therapists "substantially deviated" from the treatment manual. The organization also barred the two therapists from becoming providers of MDMA-assisted therapy in affiliation with MAPS, and health authorities were notified in Canada and the U.S.
The podcast also interviewed two people (their full names were not revealed) who said they received MDMA in the large-scale, or Phase 3, trials and experienced feelings of suicidality and other distress after the studies.
The ICER report is yet to be finalized, but Rind says their analysis showed "there's still a lot of uncertainty" about the treatment.
"You have a group of people who are very upset about how these trials went," he says. "We couldn't tell, even though we talked with people where this happened, whether that represents a tiny fraction of bad events or a number of bad events large enough to have rendered the trial just not believable."
Pushback against the allegations
According to Rind, MAPS and Lykos had "very little" engagement with ICER on the draft report.
But since then, a group of more than 70 clinicians, investigators and others involved in the Phase 3 MDMA trials have published a detailed response , saying that certain aspects of the trials were "misrepresented" and that a number of assertions amount to "hearsay."
Willa Hall , a clinical psychologist in the Phase 3 trials, says she and her colleagues were shocked by how ICER described their work.
"I didn't recognize the study that they were talking about," Hall told NPR. "I think a lot of us felt quite insulted actually by that characterization. I saw nothing like that. I only saw professionalism ."
In their response, Hall and her colleagues write that "[ICER] does not note the many measures taken to train, support, and evaluate therapists on those trials—measures that met, and in some cases exceeded, the accepted standards in the field of psychotherapy research."
They also take issue with ICER relying on "a small number of undisclosed study participants and unnamed 'experts' rather than validated research outcomes." The critiques that participants knew they received the treatment or that the measure of PTSD symptoms might not capture someone's overall condition would also apply to other clinical trials, unrelated to MDMA, they say.
UCSF's Jennifer Mitchell says the clinical trial was designed to safeguard against bias.
Therapists on site were not collecting key data from participants about their PTSD symptoms following the sessions. Instead, that was being done online by "independent assessors" who didn't know who had received the treatment or a placebo.
Hall says therapists "meticulously" captured any adverse events. "We encouraged our participants to be very honest," she says. "We're all invested in knowing how it works and what are the risks for people."
Still, Mitchell acknowledges she doesn't have full insight into what was going on at each trial site on a given day.
"This is my own frustration," she says. "I can't attest to what was happening at one of the sites in Israel on a day to day basis, or on one of the sites in Canada."
But she contends that ICER tried to conduct an investigation without access to the full data.
Luminous: A Series About Psychedelics from 'To The Best Of Our Knowledge'
The FDA granted MDMA "breakthrough therapy" status, she says, which means the agency was involved in the study design and "many aspects" of the trial.
"So there's no keeping things from the FDA," Mitchell says.
The ICER report points out that therapists and participants in the study were "pulled heavily from the existing community of those interested and involved in the use of psychedelics for possible psychological benefits."
For her part though, Mitchell says she's not what some would call a "true believer."
"My personal feeling is that psychedelics are complicated compounds and they do not work for everyone," she says.
Petition adds to controversy ahead of the FDA meeting
On the heels of the ICER report, Neşe Devenot and four others, including Meaghan Buisson, submitted the citizen petition to the FDA calling for a public advisory meeting about the Lykos' application for MDMA.
In it, they ask for extended time for stakeholders who are concerned about the "risks and shortcomings" of the research.
"Evidence from multiple sources indicates that the sponsor has engaged in a pattern of systematic and deliberate omission of adverse events from the public record while minimizing documented harms," the petition states.
It continues: "We cannot rule out the possibility that MAPS/Lykos manipulated clinical trial data to hide adverse events from regulatory agencies."
The petition cites media reports and public statements from figures at Lykos and MAPS — and disclosures from a former employee of the MAPS public benefit corporation "who prefers to maintain anonymity at this juncture."
In addition, the petition alleges that clinical trial investigators would phone MAPS in the event of an incident during the trial to see whether that should be reported as an adverse event and that a suicide attempt during a dosing session was discouraged from being reported.
In an email to NPR, a spokesperson for MAPS rejects the claim.
It's not clear if the FDA's decision to hold a meeting was influenced by Devenot's petition, which has over 80 signatures .
Alaina Jaster , who has a doctorate in pharmacology and toxicology, is another author of the petition.
"We need to listen to people [in the trials] who are having these experiences, instead of telling them that they are liars and that they're going to ruin the psychedelic renaissance," says Jaster who hosts a podcast on psychedelics.
"None of us are against this as a useful tool, or none of us are against treating mental health. We don't have any monetary interests in this not going through," she says.
Neşe Devenot and Brian Pace, another author of the petition, are affiliated with Psymposia , the media organization that produced the podcast, but Devenot says they were not involved in the podcast and are unpaid board members.
In response to the petition's call for a public meeting, a spokesperson for Lykos sent NPR a statement in April saying the company supports holding an advisory meeting. "The voice of the PTSD patient is incredibly important," the email reads.

Shots - Health News
Psychedelic drugs may launch a new era in psychiatric treatment, brain scientists say.
MAPS "remains fully supportive of comprehensive, high-quality research; careful analysis of safety and efficacy; and stringent regulatory oversight of any psychedelic-assisted therapy research or delivery," according to the statement it also sent to NPR in April. "We stand behind Lykos' execution of the clinical program and support the clinical results."
One of those who signed the petition after seeing it posted online is Dr. Boris Heifets , an anesthesiologist at Stanford University whose lab studies psychedelics, including MDMA.
"I don't know if the allegations are true, it just makes me deeply sad if there was actually malfeasance for such an important trial," says Heifets. "The MDMA Phase 3 trials were very important for mental health, important for a lot of people who may benefit from this therapy."
Learning about MAPS several decades ago was, in part, what inspired Heifets to get involved in this type of research.
He says he donates $100 a year to MAPS and that they have supplied his lab with MDMA. He also consults for one company that's developing a derivative of MDMA.
Heifets says the petition contains some "very strong allegations," particularly the claim that certain adverse events were not disclosed.
"I want to hear MAPS respond," he says. " I would really like to understand the risk profile of this drug before it's approved, not after."
- psychedelic drugs
- psychedelics
- clinical trials
- mdma therapy
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article-Invited
- Open access
- Published: 03 April 2019
Prognosis and improved outcomes in major depression: a review
- Christoph Kraus ORCID: orcid.org/0000-0002-7144-2282 1 , 2 ,
- Bashkim Kadriu ORCID: orcid.org/0000-0002-3809-9451 2 ,
- Rupert Lanzenberger ORCID: orcid.org/0000-0003-4641-9539 1 ,
- Carlos A. Zarate Jr. 2 &
- Siegfried Kasper ORCID: orcid.org/0000-0001-8278-191X 1
Translational Psychiatry volume 9 , Article number: 127 ( 2019 ) Cite this article
72k Accesses
236 Citations
26 Altmetric
Metrics details
- Human behaviour
- Predictive markers
- Scientific community
Treatment outcomes for major depressive disorder (MDD) need to be improved. Presently, no clinically relevant tools have been established for stratifying subgroups or predicting outcomes. This literature review sought to investigate factors closely linked to outcome and summarize existing and novel strategies for improvement. The results show that early recognition and treatment are crucial, as duration of untreated depression correlates with worse outcomes. Early improvement is associated with response and remission, while comorbidities prolong course of illness. Potential biomarkers have been explored, including hippocampal volumes, neuronal activity of the anterior cingulate cortex, and levels of brain-derived neurotrophic factor (BDNF) and central and peripheral inflammatory markers (e.g., translocator protein (TSPO), interleukin-6 (IL-6), C-reactive protein (CRP), tumor necrosis factor alpha (TNFα)). However, their integration into routine clinical care has not yet been fully elucidated, and more research is needed in this regard. Genetic findings suggest that testing for CYP450 isoenzyme activity may improve treatment outcomes. Strategies such as managing risk factors, improving clinical trial methodology, and designing structured step-by-step treatments are also beneficial. Finally, drawing on existing guidelines, we outline a sequential treatment optimization paradigm for selecting first-, second-, and third-line treatments for acute and chronically ill patients. Well-established treatments such as electroconvulsive therapy (ECT) are clinically relevant for treatment-resistant populations, and novel transcranial stimulation methods such as theta-burst stimulation (TBS) and magnetic seizure therapy (MST) have shown promising results. Novel rapid-acting antidepressants, such as ketamine, may also constitute a paradigm shift in treatment optimization for MDD.
Similar content being viewed by others

Major depressive disorder: hypothesis, mechanism, prevention and treatment

Psilocybin microdosers demonstrate greater observed improvements in mood and mental health at one month relative to non-microdosing controls

The serotonin theory of depression: a systematic umbrella review of the evidence
Depression: a major and relentless burden.
Major depressive disorder (MDD) is the most common psychiatric disease and a worldwide leading cause of years lived with disability 1 , 2 . In addition, the bulk of suicides are linked to a diagnosis of MDD. Despite the high prevalence rate of MDD and ongoing efforts to increase knowledge and skills for healthcare providers, the illness remains both underdiagnosed and undertreated 3 . Many novel strategies with potentially broad impact are not yet ready for ‘prime time’, as they are either in early experimental stages or undergoing regulatory processes for approval. This review sought to: (1) provide a synopsis of key factors associated with outcomes in MDD, and (2) synthesize the existing literature on novel treatment strategies for depression. A literature search was conducted using the search terms ‘depression’, ‘antidepressant’, ‘outcome’, ‘predictor’, ‘(bio)marker’, ‘treatment-resistant depression (TRD)’, and ‘chronic depression’ in addition to combinations of these terms. The search was conducted in PubMed, Scopus, and Google Scholar with no restrictions on time period and concluded in October 2018. Notably, we defined ‘outcomes’ loosely, as either disease course (i.e., treatment resistance, chronic depression) or response/remission to treatment.
Prognostic variables for treatment outcomes in MDD
Clinical variables.
Clear evidence of an inverse relationship between duration of episode and treatment outcome (either response or remission) underscores the importance of early intervention in MDD 4 (Table 1 ). In particular, replicable prospective and retrospective studies indicate that shorter duration of untreated disease—both in terms of first and recurrent episodes—is a prognostic factor indicating better treatment response and better long-term outcomes 5 , 6 , 7 , 8 , 9 , 10 , although not all studies have found such an association 11 . Another important clinical variable is time to antidepressant response. For instance, one meta-analysis found that early improvement was positively linked to antidepressant treatment outcome in 15 of 16 studies 9 . Early response to antidepressant treatment appears to occur independently of treatment modality 12 , 13 or outcome parameters 14 , 15 . Another study found that early improvement in work productivity was a significant positive predictor of higher remission rates after three and seven months of treatment 16 . Similarly, imaging studies found that early response to treatment correlated with default mode network deactivation in the posterior cingulate 17 , as well as thickening of gray matter in the anterior cingulate cortex (ACC) 18 . Interestingly, two recent meta-analyses found that initial improvement was linked to response and outcome but failed to be associated with treatment resistance 19 , 20 . This suggests that TRD—defined loosely here as non-response to at least two adequate antidepressant trials—and chronic depression (roughly defined here as non-response to any treatment) may have similar response slopes in the earliest treatment stages.
In addition, lower baseline function and quality of life—including longer duration of the current index episode—have been associated with lower remission rates to various types of antidepressant treatments 21 , 22 . This is in line with results from a previous study that found that baseline function predicted antidepressant response in TRD patients 23 . Worse outcomes in more severely ill patients at baseline were also reported in elderly patients treated in primary-care settings 24 . In contrast, several controlled clinical studies found that elevated baseline severity correlated with improved response and remission rates 25 . Two naturalistic studies with broad inclusion criteria similarly found that remission correlated with higher baseline scores 4 , 26 . However, this discrepancy might be explained by variations in outcome according to parameter. It was noted earlier that studies that defined remission as percent change of baseline values might be biased in favor of higher baseline scores, while absolute endpoints (e.g., remission defined below a cutoff score) favor less sick patients 4 .
Psychosocial variables
The influence of sociodemographic factors such as age, age of onset, gender, and number of previous episodes on treatment outcome has been investigated with mixed results 4 , 27 , 28 . One study found that females had higher remission rates 21 , but this was not confirmed by another prospective study 27 . Others have found that stress related to high occupational levels might impair outcomes 29 . The European “Group for the Study of Resistant Depression” (GSRD) multi-site study found that age at first treatment (i.e., early-onset and early treatment), age, timespan between first and last episode (i.e., duration of illness), suicidality, and education level were all important variables for outcome 30 . Notably, authors of long-lasting longitudinal studies have suggested that recall bias may influence the age of onset variable 31 , 32 ; given the cognitive deficits associated with acute episodes of MDD, retrospective studies must hence address the factor of memory bias in data collection.
Environmental stress and stressful life events (SLEs)
High stress levels significantly influence outcomes in MDD patients who are prone to vulnerable states, such as those with high levels of neuroticism 33 , 34 . A meta-analysis found that history of childhood maltreatment was associated with elevated risk of developing recurrent and persistent depressive episodes, as well as with lack of response or remission during treatment 35 . Another meta-analysis confirmed the detrimental impact of childhood maltreatment (emotional physical or sexual maltreatment or neglect) as a predisposing risk factor for severe, early-onset, and treatment-resistant depression 36 , 37 . Studies also found gender-specific effects; in particular, at lower stress levels females were at higher risk of MDD than males 34 . Moreover, twin studies have suggested a differential reactivity of gender in response to type of SLE 38 . For instance, a treatment study using escitalopram and nortriptyline investigated the association between number of SLEs (e.g., job loss, psychological trauma, loss of a loved one) and antidepressant treatment. Subjects with more SLEs exhibited greater cognitive symptoms at baseline but not significantly more mood or neurovegetative symptoms. These patients also had greater cognitive symptom reduction in response to escitalopram but not nortriptyline 39 . This suggests that SLEs may have a cognitive domain-specific impact in MDD, but more data are needed to elucidate this issue.
Psychiatric and physical comorbidities
Psychiatric comorbidity has been shown to influence outcome in both treated and untreated patients 40 , 41 . Studies have found that elevated baseline anxiety symptoms or comorbid anxiety disorder are associated with worse antidepressant response to first-line selective serotonin reuptake inhibitors (SSRIs) or second-line treatment strategies 42 , 43 . Worse outcomes have also been reported for MDD patients with comorbid drug or alcohol use disorders, post-traumatic stress disorder (PTSD), and “double depression” (depression and dysthymia) 26 , 41 . Data from the Sequential Treatment Alternatives to Relieve Depression (STAR*D) study, which included patients who were seeking medical care in routine medical or psychiatric outpatient treatment, indicate that roughly one-third (34.8%) of all MDD patients are free of any comorbidity; the most frequent comorbid Axis-I disorders are social phobia (31.3%), generalized anxiety disorder (23.6%), PTSD (20.6%), and obsessive-compulsive disorder (14.3%) 21 . A large recent study found that clinically diagnosed personality disorder was associated with negative outcomes (with regard to remission and persistent depressive symptoms) six months after diagnosis in MDD subjects enrolled in primary care 44 . Moreover, meta-analytic studies indicate that comorbid personality disorder increases the likelihood of poorer outcomes 45 , 46 ; it should be noted, though, that negative studies have also been reported 40 .
MDD and several physical diseases—including cardiovascular disease and diabetes—appear to have bidirectional effects on disease trajectory 47 , 48 , yet pathophysiologic links are most likely complex and have to be elucidated. In addition, depression appears to be linked to hormonal diseases, including hypothyroidism 49 . A number of physical disabilities and medical comorbidities have been shown to significantly impact outcome measures in MDD 50 , particularly in elderly subjects 51 . This connection appears to be relevant at any stage of the disease, as number of physical comorbidities did not separate TRD from non-TRD patients 52 . Links between MDD and pain have also been noted; subjects with elevated levels of baseline pain due to chronic conditions had longer depressive episodes, delayed remission 53 and, most importantly, elevated suicide risk 54 , 55 . Interestingly, a prospective, 12-month study of older patients found that elderly patients with atrial fibrillation exhibited better remission rates 56 . Patients with chronic pulmonary diseases had worse outcomes in uncontrolled treatment settings than those without these diseases. This difference was absent in the intervention group, in which depression care managers helped physicians with guideline-concordant recommendations and helped patients adhere to treatment 56 . Further longitudinal studies on shared pathophysiology with physical diseases are needed to confirm such associations.
Neuroimaging markers of treatment outcomes
Structural markers of antidepressant treatment outcomes suggest that hippocampal volumes are related to response and remission 57 , 58 . One study found that low baseline hippocampal volumes were related to impaired treatment outcomes after 3 years 59 ; a meta-analysis confirmed that low baseline hippocampal volumes are associated with negative outcomes 60 . However, negative studies have also been reported 61 , 62 . The volume of other brain regions, including the anterior cingulate or orbitofrontal cortices, have also been shown to be decreased in MDD subjects 63 , but more longitudinal neuroimaging trials with antidepressants are needed to clarify this association. Interestingly, several studies, including one meta-analysis 64 , found significant hippocampal volume increases after ECT 65 , 66 , 67 , although the relationship to antidepressant response has yet to be confirmed 64 , 68 .
The largest functional magnetic resonance imaging (fMRI) study of MDD patients conducted to date reported neurophysiological subtypes based on connectivity patterns within limbic and frontostriatal brain areas 69 . In subset analyses, connectivity patterns plus subtype classifications predicted response to repetitive transcranial magnetic stimulation (rTMS) treatment with higher accuracy (89.6%) than clinical characteristics alone. Other task-based and resting-state fMRI studies found that ACC activity (including pregenual activity) predicted treatment response 70 , a finding corroborated by an expanded electroencephalography study 71 as well as a meta-analysis 60 . While these interesting results suggest that fMRI measures could ultimately help classify biological subtypes of depression, these methods are far from ready for clinical application and results will have to be reproduced. However, given its easy implementation and the short time needed to acquire measurements, fMRI appears to be a promising tool for identifying imaging biomarkers.
Positron emission tomography (PET) studies have identified altered serotonin-1A (5-HT 1A ) receptor and 5-HT transporter (SERT) binding potentials, an index of protein concentration, at baseline and in TRD patients 72 , 73 , 74 , 75 . Most of these results found reduced baseline SERT levels and elevated baseline 5-HT 1A heteroreceptors in MDD patients (depending on PET methodology for 5-HT 1A ); non-remitters had lower 5-HT 1A autoreceptor binding in the serotonergic raphe nuclei 75 , as well as lower SERT 76 . Reduced global 5-HT 1A receptor binding has also been observed after ECT 77 . High costs, technical and methodological challenges, lack of dedicated PET centers with 11 C-radiochemistry, small sample sizes, small effect sizes, and unclear cutoff values have heretofore prevented the broader clinical application of these tools in MDD compared to disorders such as Alzheimer’s and Parkinson’s disease. An earlier [ 18 F]FDG PET study of unmedicated MDD patients was consistent with the aforementioned fMRI results, demonstrating increased glucose turnover in the orbitofrontal and posterior cingulate cortices and amygdala and decreased turnover in the subgenual ACC and dorsolateral prefrontal cortex 78 . A later study corroborated these results and found that glucose turnover was differentially affected by cognitive behavioral therapy or venlafaxine 79 . Interestingly, several studies detected microglial activation by labeling translocator protein (TSPO) with PET, using TSPO radioligands like 18 F-FEPPA. Microglial activation is closely linked to brain tissue damage, traumatic brain injury, neuroinflammation, and increased metabolic demands. Increased TSPO binding in MDD patients has been observed in the ACC, insula, and prefrontal cortex 80 . In addition, TSPO binding has also been shown to positively correlate with length of illness and time without antidepressant treatment, and to negatively correlate with SSRI treatment 80 . Elevated TSPO levels in unmedicated, acutely ill MDD patients have now been reported in at least two independent datasets 81 , 82 . However, TSPO-positive MDD patients may reflect a specific subtype (i.e., associated with neuroinflammation) and may, thus, respond better to treatments that target neuroinflammation. For a graphical summary of these findings see Fig. 1 .
Imaging findings exhibiting unidirectional (left) relationships with outcome in MDD vs. bidirectional (right). fMRI, functional magnetic resonance imaging; PET, positron emission tomography; EEG electroencephalography; 5-HT1A, serotonin-1A receptor; SERT, serotonin transporter; MAO-A monoamine oxidase-A; BP ND , nondisplaceable binding potential; V T , volume of distribution
Blood-based markers of disease outcomes
Consistent with neuroinflammatory processes, elevated levels of C-reactive protein (CRP), tumor necrosis factor alpha (TNFα), and interleukin-6 (IL-6) have been reported in a subset of MDD patients. In particular, elevated levels of CRP, a well-established marker of increased proinflammatory state in blood, was shown to be associated with MDD and increased risk for psychological distress in cross-sectional samples of the general population 83 . A longitudinal study found that lower CRP levels were associated with quicker response to SSRIs, an association not observed for SSRI-bupropion combination therapy 84 . Interestingly, elevated CRP levels have been shown to be more pronounced in female versus male MDD patients 85 . Similar findings have been observed for IL-6 and TNFα. One meta-analysis found that all three were significantly elevated at baseline in MDD patients, but their treatment trajectories differed 86 ; IL-6 levels decreased with antidepressant treatment, but outcomes were indistinguishable. In the same meta-analysis, persistingly high TNFα levels identified TRD patients 86 . Notably, heterogeneity was high within the pooled studies. Another study noted that levels of acute phase protein complement C3 significantly differentiated between atypical and melancholic MDD subtypes 87 . MDD patients have also been shown to have altered levels of peripheral adipokines and bone inflammatory markers; these deficits were corrected with ketamine treatment 88 , 89 .
Given the importance of neuroplasticity in the pathophysiology and treatment of depression, interest has grown in studying brain-derived neurotrophic factor (BDNF), a neurotrophin involved in the structural adaptation of neuronal networks and a prerequisite for neuronal reactions to stressors. BDNF blood levels most likely stem from peripheral tissue. While these peripheral levels are linked to central levels, the question of whether BDNF is actively transported through the blood–brain barrier remains controversial 90 . Compelling evidence suggests that BDNF levels are decreased at baseline in MDD patients and elevated in response to pharmacological 90 , 91 treatments as well as ECT 92 . A meta-analysis found that increased BDNF levels in response to treatment successfully stratified responders and remitters compared to non-responders 93 .
Outcome and genetic and epigenetic links
Heritable risk for MDD is between 30 and 40%, with higher rates in women. A large, collaborative genome-wide association study (GWAS) detected 44 significant loci associated with MDD 94 . Specific analyses identified neuronal genes (but not microglia or astrocytes), gene-expression regulating genes (such as RBFOX1 ), genes involved in gene-splicing, as well as genes that are the targets of antidepressant treatment. The authors suggested that alternative splicing could lead to shifts in the proportion of isoforms and altered biological functions of these proteins 94 .
Hypothesis-driven approaches with candidate genes have provided initial insights into the influence of single-nucleotide polymorphisms (SNPs). It is beyond the scope of this manuscript to review the large number of candidate genes; here, we outline only several representative genes (see Table 1 for meta-analytic evidence of treatment outcomes). These include synaptic proteins involved in stress response, antidepressant binding structures, or neuroplasticity (e.g., CRH receptor 1 ( CRHR1 )), the sodium-dependent serotonin transporter ( SLC6A4 ), and BDNF 95 . The aforementioned multicenter GSRD study also found that combining clinical and genetic variables explained antidepressant response better than SNPs alone in a random forest algorithm 96 . In that study, regulatory proteins such as ZNF804A (associated with response) and CREB1 (associated with remission), as well as a cell adhesion molecule (CHL1, associated with lower risk of TRD), were linked to antidepressant treatment outcomes. Another interesting candidate gene is FK506 binding protein 5 ( FKBP5 ), which was found to moderate the influence of standard treatments in an algorithm lasting up to 14 weeks 97 ; FKBP5 is known to influence HPA axis reactivity 98 , treatment response 99 , and epigenetic mechanisms in response to environmental stressors 100 . Another relevant avenue of research is drug-drug interactions and gene isoforms in the cytochrome P450 pathway (CYP450), which could account for insufficient amounts of a given drug reaching the brain or, conversely, result in exceedingly high plasma values, making subjects more vulnerable to treatment side effects 101 , 102 . Several commercially available kits categorize patients according to their phenotypic status (e.g., CYP2D6, 2C19, CYP3A4). This led to the introduction of phenotype categories—“poor”, “intermediate”, “extensive (normal)”, and “ultrarapid” metabolizers—based on CYP450 isoenzyme status and their relationship to plasma levels at fixed doses 102 . A large naturalistic study of CYP2C19 isoforms found that treatment success with escitalopram was less frequent in “poor” (CYP2C19Null/Null) and “ultrarapid” metabolizers (CYP2C19*1/*17 or CYP2C19*17/*17) 103 .
Clinical subgroups, TRD, and treatment outcomes
While some studies have suggested that depressive subtypes in MDD—including anxious, mixed, melancholic, atypical, and psychotic depression—respond differently to antidepressant treatment, this literature is mixed. For instance, some studies found that melancholic patients initially present with high levels of severity and may respond less well to SSRI treatment than to venlafaxine or tricyclic antidepressants 104 , but other studies did not support this finding 105 . No association was found between subgroups and clinical outcomes in a parallel design, uncontrolled study investigating sertraline, citalopram, and venlafaxine 106 , which found that near equal percentages of patients who met criteria for a pure-form subtype (39%) also had more than one subtype (36%), making these psychopathological subtypes difficult to classify.
It should be noted that treatment success might have more discriminatory power for identifying subgroups than psychopathological subgroup stratification. Although a wide range of definitions exists specifying the number of failed trials necessary to diagnose TRD 107 , the core definition of TRD centers around a lack of improvement in response to consecutive, adequate antidepressant treatments. Resistance occurs at alarmingly high rates and is thought to affect 50–60% of all treated patients 107 . Unsurprisingly, this group of patients has dramatically worse outcomes than those who respond to antidepressants, and factors that are associated with TRD overlap with many of those presented above 28 . Cross-sectional data from the GSRD 108 identified a number of risk factors linked to TRD, including comorbidity (particularly anxiety and personality disorders), suicide risk, episode severity, number of hospitalizations, episode recurrence, early-onset, melancholic features, and non-response at first treatment 28 . Most importantly, TRD is life-threatening, and associated with a two- to threefold increased risk of suicide attempts compared to responding patients, and a 15-fold increased risk compared to the general population 109 . Taken together, the evidence indicates that TRD patients need special attention, as outcomes in these individuals are significantly worse.
Novel and existing strategies to improve treatment outcomes
Early identification, prevention, and early treatment.
Numerous programs for suicide prevention exist 110 , and recognizing acute depressive symptoms is just one of many important facets of such work. Screening tools for early identification of depressed patients can be helpful 111 , and such instruments can start with as few as two items—for instance, the Patient Health Questionnaire-2 112 or Ask Suicide-Screening Questions (asQ’em) 113 —and proceed to more detailed instruments if initial screens are positive. Positive screening should be followed by a diagnostic interview to determine whether patients meet criteria for MDD 111 . In the general population, two large independent studies that used only clinical variables were nevertheless able to accurately predict depression within 1–3 years 114 . In addition, long-term monitoring of vulnerable subjects with known SLEs may further improve the ability to identify at-risk individuals early in their course of illness. As noted above, duration of untreated disease is a negative predictor of treatment outcomes. Because the advantages of early intervention in MDD have been demonstrated 115 , efforts to achieve early treatment might also help slow disease progression in individuals with TRD; however, this hypothesis has not been sufficiently tested.
Modeling environmental impact on predisposition
As noted above, severe SLEs constitute an important risk factor. Elegantly designed studies have demonstrated that genetic predisposition, in concert with SLEs, might account for increased vulnerability to MDD 100 . In this manner, the presence of ‘weak alleles’ in candidate genes such as BDNF, SERT , and others would be increasingly detrimental in the presence of SLEs 116 , 117 . However, studies have been quite inconsistent and yielded small effect sizes, including a negative result in 252 patients enrolled in the GSRD study 118 . It should be noted that counter-regulatory mechanisms or resilience factors, such as social support, may exist that counter SLEs. Nevertheless, preliminary research suggests that the impact of SLEs on MDD may depend on measurable factors such as gender and the timing of exposure 119 . Both genes and the environment are complex systems with frequent opportunity for interaction and elaborate compensatory mechanisms. While the complexity of genetic susceptibility in MDD can be tackled through enormous collaborative projects 94 , the interactions between genetic susceptibility and environmental factors have yet to be determined. Properly powered gene×environment interaction projects may exceed current research capabilities, and large longitudinal studies will certainly be needed 120 .
Developing markers for subgroup identification and disease course
Pioneering research on biological differences—for instance, between patients with atypical versus melancholic depression—suggests differential HPA axis or autonomous nervous system reactivity 121 , 122 , though the subtype results have been only moderately consistent across time and are prone to low group specificity 123 , 124 , 125 . However, at least one study demonstrated the more reliable stability of extreme types over a 2-year period 87 . Interestingly, one study found that individuals with atypical depression had significantly higher body-mass index, waist circumference, levels of inflammatory markers, and triglyceride levels, and lower levels of high-density lipid cholesterol than those with melancholic depression or controls 126 . Using fMRI and biological variables, another study found that MDD subjects could be divided into low/high appetite groups with distinctive correlations between neuronal activity and endocrine, metabolic, and immune states 127 . Other research groups have tried to overcome conventional psychopathological subgroups and model biotypes using resting-state fMRI 69 . Molecular and functional neuroimaging, as well as epigenetic studies, are promising approaches for separating subgroups and may be better suited to identifying screening markers (see Fig. 2 ) that are exclusively valid in certain subgroups with higher predictive power.
These approaches highlight the feasibility of linking and stratifying psychopathological categories with biological variables, a goal further supported by the Research Domain Criteria (RDoc), which seek to link dimensions of observable behavior with neurobiological systems 128 . In the search for biomarkers, subgroup- or domain-specific classifications using unidimensional variables might improve subgroup stratification 129 . Moreover, applying markers to other categories could boost the utility of existing markers that have failed in any given category (see Fig. 2 for established markers). As a field, the focus is largely on staging and prediction markers, but ‘predisposition’ or ‘recurrence’ markers may equally be worth investigating. Presently, however, the relative lack of biologically defined MDD subgroups and their stratification are key obstacles to finding and establishing treament outcome predictors appropriate for broader clinical applications.
Candidate disease markers can be applied in clinically meaningful ways. While only candidate markers are presently available, sorting these according to their potential applications may facilitate the development of clinically applicable disease markers. The outline follows the classification of markers as suggested by others 200 (modified and reprinted with permission from Springer)
The most important outcome of successful subgroup stratification and staging markers would be that patients and their relatives would receive valuable information at treatment onset about how their disease is likely to improve or worsen. Toward this end, the development of staging methods provides promising solutions. Currently, at least five different methods exist 130 that, to date, have not been evaluated thoroughly enough for clinical implementation. Continuous variables—as obtained by the Maudsley Method and Massachusetts General Hospital Staging Model—appear to provide greater staging advantages than categorical variables. It should be noted here that data indicate that research in severely ill, suicidal, and TRD subjects is safe to conduct in controlled inpatient settings 131 . Presently, patients in various stages of disease and/or treatment history are lumped together and compared in statistical analyses. We propose that staging should be more thoroughly integrated into clinical trial design.
Algorithm- and guideline-based treatments
Despite the availability and distribution of a variety of expert-based guidelines, only a fraction of patients are actually treated according to guidelines 132 (see Table 2 for current guidelines (≤10 years)). New guidelines – particularly for TRD – and more rigorous implementation of guideline-based care are needed. Improvements in currently available treatments have been conducted using treatment algorithms and following sequential treatment strategies, with standardized instructions for therapeutic decision-making. In the past two decades, large, collaborative studies using treatment-based algorithms have introduced standardized, sequential treatments; these include the Texas Medication Algorithm Project 133 , the STAR*D trial 21 , and the German algorithm project 134 . Indeed, evidence suggests that algorithm-based treatments improve treatment outcomes 135 and are cost effective 136 . Here, we considered current clinical treatment guidelines to create a sequential treatment optimization scheme of recommended treatments. While there is no fixed time-frame, first- and second-line treatments are recommended sequentially during the first episode and within 3 months (see Fig. 3 , which also illustrates the need for more third- and fourth-stage treatment options). Figure 4 , illustrates potential reasons for “pseudoresistance” 42 that should be ruled out during this time-frame.
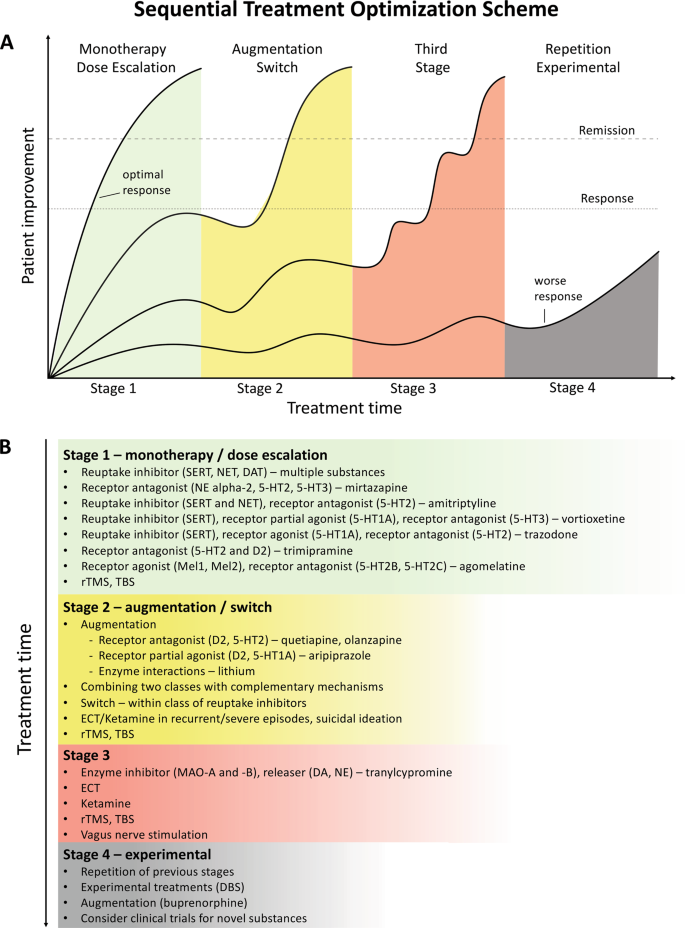
A sequential treatment optimization scheme was generated based on antidepressant treatment guidelines (see Table 2 ). Treatment optimization is possible for patients being treated for the first time but also for patients with insufficient response to first- or second-stage therapies. a Treatment response curves for four common types of patients highlight the importance of sequentially introducing the next step upon non-response to previous steps. b Currently available treatments are listed in neuroscience-based nomenclature 201 with treatment lines corresponding to improvement curves in a . Although current classifications vary, patients classified as having treatment-resistant depression (TRD) are eligible for second- or third-stage therapies. 5-HT1A and similar: serotonin receptor subtypes; DBS: deep brain stimulation; DAT: dopamine transporter; D2: dopamine receptor D2; ECT: electroconvulsive therapy; MAO: monoamine oxidase; NET: noradrenaline transporter; SERT: serotonin transporter; TBS: theta-burst stimulation; rTMS: repetitive transcranial magnetic stimulation; DA: dopamine; NE: norepinephrine.
Points—in random order—follow earlier suggestions by Dold and Kasper (2017) 202
Reducing placebo response in clinical trials while harnessing placebo effects in clinical treatment
The issue of placebo response in antidepressant trials has become increasingly important 137 , 138 . Indeed, the contribution of placebo effects to early response needs to be systematically studied in order to disentangle biological therapy-induced effects from psychologically induced effects. Strikingly, in the brain, anatomically similar regions that mediate placebo response are affected by MDD (for a comprehensive review, see ref. 139 ). Several mechanisms contribute to placebo response, including patients’ expectations of benefits, behavioral conditions, and the quality of patient-physician interactions 139 . Strategies for reducing placebo response could lead to better discrimination between effective treatments in clinical trials; such strategies include extending trial duration, excluding placebo responders by including a placebo run-in, or using randomized run-in and withdrawal periods 138 , 139 . Others have suggested using more thorough criteria to select study participants 140 . On the other hand, when antidepressant agents are used clinically, placebo effects must be taken advantage of by harnessing patients’ expectations and learning mechanisms to improve treatment outcomes 141 .
Novel antidepressant treatments
The recent discovery that glutamatergic-based drugs are uniquely capable of rapidly and robustly treating mood disorders has ushered in a new era in the quest to develop novel and effective antidepressants 142 , 143 , 144 . In this regard, the prototypic glutamatergic modulator ketamine has catalyzed research into new mechanistic approaches and offered hope for the development of novel, fast-acting antidepressants. While ketamine’s underlying mechanism of action remains the subject of active investigation, several theories have been propsed 144 . These include N-methyl- d -aspartate receptor (NMDAR)-dependent mechanisms, such as the inhibition of NMDARs on gamma aminobutyric acid (GABA)-ergic interneurons, the inhibition of spontaneous NMDAR-mediated transmission, the inhibition of extrasynaptic NMDARs, the inhibition of lateral habenula neurons, and GABA B receptor expression/function 144 . Substantial evidence also supports additional NMDAR-independent mechanisms, including the stabilization of glutamate release/excitatory transmission, active metabolites such as hydroxynorketamine, regulation of the dopaminergic system, G-alpha subunit translocation, and activation of cyclic adenosine monophosphate, as well as potential sigma-1 and mu-opioid receptor activation 145 . Among those theories, a leading hypothesis remains that NMDAR antagonism increases BDNF synthesis, a process mediated by decreased phosphorylation of eukaryotic elongation factor-2 and the subsequent activation of the mammalian target of rapamycin pathway by BDNF activation of the TrkB receptor 146 , 147 . These putative mechanisms of action are not mutually exclusive and may complement each other to induce potentiation of excitatory synapses in affective-regulating brain circuits, resulting in improved depressive symptoms.
The initial serendipitous discovery that a single, subanesthetic-dose ketamine infusion has rapid-acting antidepressant effects in MDD 148 , a finding subsequently confirmed by numerous randomized trials, has been hailed as one of the most important discoveries in psychiatry in the last decades 149 . The initial proof-of-concept studies demonstrated that a single dose of ketamine (0.5 mg/kg, IV) administered over 40 min led to rapid, robust, and relatively sustained antidepressant effects in TRD—both MDD 150 , 151 , 152 , 153 and bipolar depression 154 , 155 . In research settings, studies of TRD patients found response rates of >70% within 24 h post-infusion 153 , with about 50–70% of participants exhibiting a variable duration of response 156 , 157 . Ketamine has also been shown to be superior to any blinding counterpart 158 . Off-label ketamine use has also been associated with significant and rapid (one to four hours) antisuicidal effects 150 , 159 , 160 , a finding supported by a large, recent metanalysis showing that ketamine exerted rapid (within hours) and sustained (up to 7 days) improvements in suicidal thoughts compared to placebo 161 .
Esketamine hydrochloride
The ketamine enantiomer esketamine received approval by the FDA for TRD and is currently undergoing further Phase III clinical trials. A Phase II, 10-week, clinical trial of flexibly dosed intranasal esketamine (28 mg/56 mg or 84 mg) found that, in TRD patients, this agent demonstrated rapid and clinically relevant improvements in depressive symptoms compared to placebo 162 . Strikingly, 65% of TRD patients met response criteria through Day 57. In another Phase II proof-of-concept, multi-site, 4-week, double-blind study, standard treatment plus intranasal esketamine (84 mg) was compared to standard treatment plus placebo in individuals with MDD at imminent risk of suicide 163 . The authors found a rapid antisuicidal effect, as assessed via the Montgomery-Åsberg Depression Rating Scale Suicide Item score at 4 h.
Other rapid acting and novel antidepressants
Based on the success of ketamine, other rapid-acting or novel antidepressant substances within the glutamatergic/GABA neurotransmitter systems are being developed, several of which are in Phase III clinical trials. A prototype novel substance is AV-101 (L-4-cholorkynurenine). This is a potent selective antagonist at the glycine-binding site of the NMDAR NR1 subunit and has demonstrated antidepressant-like effects in animal models, while human Phase II studies are currently ongoing 164 . Brexanolone is a formulation of the endogenous neurosteroid allopregnanolone, which modulates neuronal activation of GABA A receptors and has met positive endpoints in Phase III, leading to FDA approval for postpartum depression. A comparable substance is under development for MDD 165 . In addition, serotonergic agonists have been studied as our understanding of their mechanism of action (e.g., their effects on glutamate release or plasticity) has increased 166 . Encouraging results have been seen for the serotonin 2A receptor agonist psilocybin 167 , but these findings need to be replicated in larger systematic clinical trials. Initial positive trials of add-on agents—such as buprenorphine 168 , 169 , rapastinel 170 , or scopolamine 145 —have also been conducted. However, it is beyond the scope of this manuscript to review all of these findings, and we refer the interested reader to recent comprehensive reviews of this subject 144 , 145 , 165 , 171 .
Transcranial stimulation paradigms
In contrast to pharmaceutical treatments that exert their efficacy at the molecular level, electrical stimulation techniques target entire neuronal circuits. TMS of the (left) dorsolateral prefrontal cortex has been FDA-approved since 2008 to treat depression in patients who failed to respond to one standard antidepressant treatment. Apart from transient local skin and muscle irritation at the stimulation site and headaches, it is a very safe technique with few side effects. Studies have repeatedly demonstrated the superiority of rTMS over sham procedures, though effect sizes have been moderate 172 , 173 , 174 . Initial studies suggest that rTMS is also effective in TRD but the data are too few to draw definitive conclusions 175 , 176 . Improvements in rTMS techniques known as theta-burst stimulation (TBS) provide significantly shortened treatment times (3 min for TBS versus 37 min for rTMS) and hence allow more patients to be treated per day. A large non-inferiority trial of 414 moderately resistant MDD patients found that TBS was at least as effective as rTMS in reducing depressive symptoms 177 .
Electroconvulsive therapy (ECT)
Regarded as the ‘gold standard’, ECT has been successfully used for many years to treat severe TRD and exhibits both relatively rapid and sustained onset of efficacy; approximately 50% of all patients reach response criteria at the third treatment, typically within 1 week. It is also one of the most effective antidepressant therapies 178 , yielding response rates of ~80%, remission rates of ~75% 179 , and antisuicidal effects 180 . Remission is achieved by about 30% of patients within six ECT sessions 179 . ECT also reduces the risk of readmission 181 and is likewise safe to use in depressed elderly subjects 182 . The side effects of ECT include intermediate disorientation, impaired learning, and retrograde amnesia, all of which usually resolve 183 . The optimal anatomic location of the stimulus electrodes is a topic of current debate 184 , 185 . Recent evidence suggests that all three methods for electrode placement (bifrontal, bitemporal, and unilateral) show clinically significant effects 186 . While no difference in cognitive side effects was observed, bitemporal placement should be considered the first-line choice for urgent clinical situations. Despite its clinical efficacy, ECT remains underutilized. Its use is declining 187 because it needs to be administered in hospital settings under anesthesia, and partly because of misleading portrayals of the procedure itself. Adjusting the dose of electrical stimuli (e.g., through refined electrode placement or individually adjusted pulse amplitudes) may improve ECT’s side effect profile.
Magnetic seizure therapy (MST)
MST uses high doses of rTMS to induce seizures 188 . The electromagnetically induced electrical field generated by MST is unifocal and variable, as there are individual differences in the degree to which the skull provides electrical resistance 189 . As an advantage over ECT, MST is associated with a more superficial stimulation, which exerts less impact on the medial-temporal lobe where cognitive side effects are thought to be elicited. To date, few research sites across the world have used MST, with a concomitant dearth of open-label trials. Nevertheless, the preliminary treatment data suggest that results obtained with MST are similar to those obtained with ECT but with a more favorable side effect profile 190 , 191 .
Vagus nerve stimulation (VNS)
VNS is a surgically implanted pacemaker-like device attached to a stimulating wire threaded along the left vagus nerve. Since 2005, the FDA has approved VNS use for the adjunctive long-term treatment of long-lasting recurrent depression in patients 18 years and older who are experiencing a major depressive episode and have failed to respond to four or more previous adequate standard antidepressant treatment trials. In such cases, it has been shown to have superior long-term effects over conventional psychopharmacological treatment 192 . A recent, large, observational, adjunctive, open-label, naturalistic study followed TRD patients over 5 years 193 . In this group, adjunctive VNS led to significantly better clinical outcomes and higher remission rates than treatment as usual (67.6% vs. 40.9%, respectively).
Deep-brain stimulation (DBS)
DBS involves the neurosurgical implantation of electrodes and has become clinically routine in the treatment of Parkinson’s disease and Dystonia. The technique is safe, removable, and does not cause lasting neuronal lesions. In TRD, anatomical targets include the subgenual cingulate, nucleus accumbens, habenula, and medial forebrain bundle. Clinical trials typically only enroll severely ill TRD patients whose current episode has lasted >12 months, whose age of onset is <45 years, and who have failed to respond to at least four adequate prior treatment trials of standard antidepressants, ECT, and/or psychotherapy. Initial open-label or single-blind trials found that DBS had both rapid and sustained antidepressant effects 194 , 195 , 196 . In contrast, one large and one smaller sham-controlled clinical study both failed to achieve their primary endpoints of symptom reduction 197 , 198 . To date, the number of MDD patients treated with DBS has been very small compared to other treatment options, including ECT and TMS. Nevertheless, brain-electrode interfaces are evolving quickly and it is possible that next generation brain-responsive stimulation devices will be able to adjust stimulation on-demand only when abnormal biological marker impulses (e.g., pulse amplitude) are detected 199 .
Conclusions
Although enormous progress has been made in measuring, predicting, and improving outcomes, depression remains a relentless disease that places a heavy burden on both individuals and society. The research reviewed above indicates that early recognition and early adequate treatment at illness onset are preferable to watch-and-wait strategies. The studies reviewed above also underscore the manner in which SLEs, as well as physical and psychiatric comorbidities, contribute to impaired outcomes. Together, these factors contribute toward treatment resistance, which has gained a substantial amount of importance as a patient-stratifying variable.
This paper also reviewed biological markers, where research has grown exponentially to encompass enormous projects with potentially tens of thousands of subjects enrolled in real world studies. In parallel, studies exploring the underlying genetics of depression have evolved from early candidate gene studies of neurotransmitters, stress, or gene-regulatory systems to large GWAS that help reveal potential new pathways and treatment targets. Moreover, the burgeoning field of proteomics has found promising target molecules. Nevertheless, despite the wealth of recent work in this area, no single biomarker has yet been used in clinical applications. A substantial need exists for replication and, because many biomarker studies are currently open-label, for controlled studies. In combination with neuroimaging techniques such as fMRI, genes or blood-based markers have a high potential of future implementation in stratification of MDD or serve as prognostic marker on treatment outcome.
Above, we also outlined efforts to optimize outcomes. We argue that disease-inherent heterogeneity, in concert with inaccurate group stratification tools, might have contributed to the lack of clinically applicable stratification and response prediction markers. Successful subgroup identification, and the ability to use this information in clinical settings, is crucial to improving future treatment paradigms. While recent research has increasingly focused on TRD, we wish to reiterate that no standard definition of TRD presently exists. Thus, based on currently available guidelines, we have outlined a sequential treatment optimization scheme that includes options for TRD; such work highlights the substantial need to develop and improve “third-line-and-beyond” therapeutics. In this context, this manuscript also reviews novel treatments and brain stimulation techniques that have demonstrated rapid antidepressant effects in TRD, including ketamine, esketamine, ECT, MST, TMS/TBS, VNS, DBS, and others. When treating TRD patients, physicians should consider illness severity, the chronicity of past and recent depressive episodes, the side effect profile of available treatment options, as well as previous refractoriness to particular treatment approaches. If acuity supersedes chronicity, one could consider fast-acting interventions such as ketamine or ECT/MST.
This review, though comprehensive, was not able to consider several lines of evidence on outcome prediction and treatment improvement. In particular, we focused on clinical outcomes in humans and were, thus, unable to fully explore the highly valuable advances made in translational science. Similarly, it was beyond the scope of this manuscript to review the richness of results from animal research and their relevance to MDD. Moreover, given the amount of literature, we were not able to incorporate many proteomic, genetic, or psychopharmacological findings.
Taken together, this review outlines important clinical, psychosocial, and biological factors associated with response and remission to antidepressant treatment (see Table 3 ). Recent studies have led to important insights into neurobiological disease markers that could result in improved disease stratification and response prediction in the near future. Key discoveries into novel rapid-acting substances, in concert with improvements in brain stimulation techniques, may also result in significantly improved treatment outcomes in formerly hard-to-treat patients.
Wittchen, H. U. et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol.: J. Eur. Coll. Neuropsychopharmacol. 21 , 655–679 (2011).
Article CAS Google Scholar
Lim, S. S. et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380 , 2224–2260 (2012).
Article PubMed PubMed Central Google Scholar
Lecrubier, Y. Widespread underrecognition and undertreatment of anxiety and mood disorders: results from 3 European studies. J . Clin . Psychiatry 68 Suppl 2 , 36–41 (2007).
Riedel, M. et al. Clinical predictors of response and remission in inpatients with depressive syndromes. J. Affect. Disord. 133 , 137–149 (2011).
Article PubMed Google Scholar
Rost, K. et al. Persistently poor outcomes of undetected major depression in primary care. Gen. Hosp. Psychiatry 20 , 12–20 (1998).
Article CAS PubMed Google Scholar
Ghio, L., Gotelli, S., Marcenaro, M., Amore, M. & Natta, W. Duration of untreated illness and outcomes in unipolar depression: a systematic review and meta-analysis. J. Affect. Disord. 152–154 , 45–51 (2014).
Hung, C. I., Liu, C. Y. & Yang, C. H. Untreated duration predicted the severity of depression at the two-year follow-up point. PLoS ONE 12 , e0185119 (2017).
Article PubMed PubMed Central CAS Google Scholar
Bukh, J. D., Bock, C., Vinberg, M. & Kessing, L. V. The effect of prolonged duration of untreated depression on antidepressant treatment outcome. J. Affect. Disord. 145 , 42–48 (2013).
Habert, J. et al. Functional recovery in major depressive disorder: Focus on early optimized treatment. Prim Care Companion CNS Disord. 18 (2016).
Kautzky, A. et al. Clinical factors predicting treatment resistant depression: affirmative results from the European multicenter study. Acta Psychiatr. Scand. 139 , 78–88 (2018).
Furukawa, T. A., Kitamura, T. & Takahashi, K. Time to recovery of an inception cohort with hitherto untreated unipolar major depressive episodes. Br. J. Psychiatry.: J. Ment. Sci. 177 , 331–335 (2000).
Feffer, K. et al. Early symptom improvement at 10 sessions as a predictor of rTMS treatment outcome in major depression. Brain Stimul. 11 , 181–189 (2018).
Martinez-Amoros, E. et al. Early improvement as a predictor of final remission in major depressive disorder: New insights in electroconvulsive therapy. J. Affect. Disord. 235 , 169–175 (2018).
Soares, C. N., Endicott, J., Boucher, M., Fayyad, R. S. & Guico-Pabia, C. J. Predictors of functional response and remission with desvenlafaxine 50 mg/d in patients with major depressive disorder. CNS Spectr. 19 , 519–527 (2014).
Lam, R. W. et al. Predictors of functional improvement in employed adults with major depressive disorder treated with desvenlafaxine. Int Clin. Psychopharmacol. 29 , 239–251 (2014).
Jha, M. K. et al. Early improvement in work productivity predicts future clinical course in depressed outpatients: Findings from the CO-MED trial. Am. J. Psychiatry 173 , 1196–1204 (2016).
Spies, M. et al. Default mode network deactivation during emotion processing predicts early antidepressant response. Transl. Psychiatry 7 , e1008 (2017).
Article CAS PubMed PubMed Central Google Scholar
Bartlett, E. A. et al. Pretreatment and early-treatment cortical thickness is associated with SSRI treatment response in major depressive disorder. Neuropsychopharmacol.: Off. Publ. Am. Coll. Neuropsychopharmacol. 43 , 2221–2230 (2018).
Olgiati, P. et al. Early improvement and response to antidepressant medications in adults with major depressive disorder. Meta-analysis and study of a sample with treatment-resistant depression. J. Affect Disord. 227 , 777–786 (2018).
de Vries, Y. A. et al. Predicting antidepressant response by monitoring early improvement of individual symptoms of depression: individual patient data meta-analysis. Br. J. Psychiatry.: J. Ment. Sci. 214 , 1–7 (2018).
Google Scholar
Trivedi, M. H. et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am. J. Psychiatry 163 , 28–40 (2006).
Blom, M. B. et al. Severity and duration of depression, not personality factors, predict short term outcome in the treatment of major depression. J. Affect. Disord. 104 , 119–126 (2007).
Kautzky, A. et al. Refining prediction in treatment-resistant depression: results of machine learning analyses in the TRD III sample. J. Clin. Psychiatry 79 , 16m11385 (2018).
Katon, W., Unutzer, J. & Russo, J. Major depression: the importance of clinical characteristics and treatment response to prognosis. Depress Anxiety 27 , 19–26 (2010).
Papakostas, G. I. Surrogate markers of treatment outcome in major depressive disorder. Int. J. Neuropsychopharmacol./Off. Sci. J. Coll. Int. Neuropsychopharmacol. 15 , 841–854 (2012).
Friedman, E. S. et al. Baseline depression severity as a predictor of single and combination antidepressant treatment outcome: results from the CO-MED trial. Eur. Neuropsychopharmacol.: J. Eur. Coll. Neuropsychopharmacol. 22 , 183–199 (2012).
Balestri, M. et al. Socio-demographic and clinical predictors of treatment resistant depression: A prospective European multicenter study. J. Affect Disord. 189 , 224–232 (2016).
Souery, D. et al. Clinical factors associated with treatment resistance in major depressive disorder: results from a European multicenter study. J. Clin. Psychiatry 68 , 1062–1070 (2007).
Mandelli, L. et al. Opinion paper: poor response to treatment of depression in people in high occupational levels. Psychol. Med. 49 , 49–54 (2019).
Kautzky, A. et al. A new prediction model for evaluating treatment-resistant depression. J. Clin. Psychiatry 78 , 215–222 (2017).
Paksarian, D. et al. Stability and change in reported age of onset of depression, back pain, and smoking over 29 years in a prospective cohort study. J. Psychiatr. Res. 88 , 105–112 (2017).
Wells, K. et al. Five-year impact of quality improvement for depression: results of a group-level randomized controlled trial. Arch. Gen. Psychiatry 61 , 378–386 (2004).
Vinkers, C. H. et al. Stress exposure across the life span cumulatively increases depression risk and is moderated by neuroticism. Depress Anxiety 31 , 737–745 (2014).
Kendler, K. S., Kuhn, J. & Prescott, C. A. The interrelationship of neuroticism, sex, and stressful life events in the prediction of episodes of major depression. Am. J. Psychiatry 161 , 631–636 (2004).
Nanni, V., Uher, R. & Danese, A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am. J. Psychiatry 169 , 141–151 (2012).
Thompson, A. E. & Kaplan, C. A. Childhood emotional abuse. Br. J. Psychiatry.: J. Ment. Sci. 168 , 143–148 (1996).
Nelson, J., Klumparendt, A., Doebler, P. & Ehring, T. Childhood maltreatment and characteristics of adult depression: meta-analysis. Br. J. Psychiatry.: J. Ment. Sci. 210 , 96–104 (2017).
Article Google Scholar
Kendler, K. S. & Gardner, C. O. Sex differences in the pathways to major depression: a study of opposite-sex twin pairs. Am. J. Psychiatry 171 , 426–435 (2014).
Keers, R. et al. Stressful life events, cognitive symptoms of depression and response to antidepressants in GENDEP. J. Affect. Disord. 127 , 337–342 (2010).
Henriksen, C. A. et al. Identifying factors that predict longitudinal outcomes of untreated common mental disorders. Psychiatr. Serv. 66 , 163–170 (2015).
Dennehy, E. B., Marangell, L. B., Martinez, J., Balasubramani, G. K. & Wisniewski, S. R. Clinical and functional outcomes of patients who experience partial response to citalopram: secondary analysis of STAR*D. J. Psychiatr. Pract. 20 , 178–187 (2014).
Dold, M. et al. Clinical characteristics and treatment outcomes of patients with major depressive disorder and comorbid anxiety disorders—results from a European multicenter study. J. Psychiatr. Res. 91 , 1–13 (2017).
Fava, M. et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am. J. Psychiatry 165 , 342–351 (2008).
Angstman, K. B. et al. Personality disorders in primary care: impact on depression outcomes within collaborative care. J. Prim. Care Community Health 8 , 233–238 (2017).
Zeeck, A. et al. Prognostic and prescriptive predictors of improvement in a naturalistic study on inpatient and day hospital treatment of depression. J. Affect. Disord. 197 , 205–214 (2016).
Newton-Howes, G., Tyrer, P. & Johnson, T. Personality disorder and the outcome of depression: meta-analysis of published studies. Br. J. Psychiatry.: J. Ment. Sci. 188 , 13–20 (2006).
Whooley, M. A. et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA 300 , 2379–2388 (2008).
Ducat, L., Philipson, L. H. & Anderson, B. J. The mental health comorbidities of diabetes. JAMA 312 , 691–692 (2014).
Fugger, G. et al. Comorbid thyroid disease in patients with major depressive disorder—results from the European Group for the Study of Resistant Depression (GSRD). Eur. Neuropsychopharmacol. 28 , 752–760 (2018).
Iosifescu, D. V. et al. The impact of medical comorbidity on acute treatment in major depressive disorder. Am. J. Psychiatry 160 , 2122–2127 (2003).
Oslin, D. W. et al. Association between medical comorbidity and treatment outcomes in late-life depression. J. Am. Geriatr. Soc. 50 , 823–828 (2002).
Amital, D. et al. Physical co-morbidity among treatment resistant vs. treatment responsive patients with major depressive disorder. Eur. Neuropsychopharmacol. 23 , 895–901 (2013).
Karp, J. F. et al. Pain predicts longer time to remission during treatment of recurrent depression. J. Clin. Psychiatry 66 , 591–597 (2005).
Ohayon, M. M. & Schatzberg, A. F. Using chronic pain to predict depressive morbidity in the general population. Arch. Gen. Psychiatry 60 , 39–47 (2003).
Racine, M. Chronic pain and suicide risk: A comprehensive review. Prog. Neuropsychopharmacol. Biol. Psychiatry 87 , 269–280 (2018).
Bogner, H. R. et al. The role of medical comorbidity in outcome of major depression in primary care: the PROSPECT study. Am. J. Geriatr. Psychiatry 13 , 861–868 (2005).
MacQueen, G. M., Yucel, K., Taylor, V. H., Macdonald, K. & Joffe, R. Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biol. Psychiatry 64 , 880–883 (2008).
Phillips, J. L., Batten, L. A., Tremblay, P., Aldosary, F. & Blier, P. A prospective, longitudinal study of the effect of remission on cortical thickness and hippocampal volume in patients with treatment-resistant depression. Int. J. Neuropsychopharmacol. / Off. Sci. J. Coll. Int. Neuropsychopharmacol. 18 , pyv037 (2015).
Frodl, T. et al. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. J. Psychiatry Neurosci. 33 , 423–430 (2008).
PubMed PubMed Central Google Scholar
Fu, C. H., Steiner, H. & Costafreda, S. G. Predictive neural biomarkers of clinical response in depression: a meta-analysis of functional and structural neuroimaging studies of pharmacological and psychological therapies. Neurobiol. Dis. 52 , 75–83 (2013).
Frodl, T. et al. Reduced hippocampal volumes associated with the long variant of the serotonin transporter polymorphism in major depression. Arch. Gen. Psychiatry 61 , 177–183 (2004).
Vythilingam, M. et al. Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biol. Psychiatry 56 , 101–112 (2004).
Schmaal, L. et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol. Psychiatry 22 , 900–909 (2017).
Wilkinson, S. T., Sanacora, G. & Bloch, M. H. Hippocampal volume changes following electroconvulsive therapy: a systematic review and meta-analysis. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2 , 327–335 (2017).
Nordanskog, P. et al. Increase in hippocampal volume after electroconvulsive therapy in patients with depression: a volumetric magnetic resonance imaging study. J. ECT 26 , 62–67 (2010).
Dukart, J. et al. Electroconvulsive therapy-induced brain plasticity determines therapeutic outcome in mood disorders. Proc. Natl Acad. Sci. USA 111 , 1156–1161 (2014).
Gryglewski, G. et al. Structural changes in amygdala nuclei, hippocampal subfields and cortical thickness following electroconvulsive therapy in treatment-resistant depression: longitudinal analysis. Br. J. Psychiatr 214 , 159–167 (2019).
Oltedal, L. et al. Volume of the human hippocampus and clinical response following electroconvulsive therapy. Biol. Psychiatry 84 , 574–581 (2018).
Drysdale, A. T. et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med. 23 , 28–38 (2017).
Dunlop, B. W. et al. Functional connectivity of the subcallosal cingulate cortex and differential outcomes to treatment with cognitive-behavioral therapy or antidepressant medication for major depressive disorder. Am. J. Psychiatry 174 , 533–545 (2017).
Pizzagalli, D. A. et al. Pretreatment rostral anterior cingulate cortex theta activity in relation to symptom improvement in depression: A randomized clinical trial. JAMA Psychiatry 75 , 547–554 (2018).
Gryglewski, G., Lanzenberger, R., Kranz, G. S. & Cumming, P. Meta-analysis of molecular imaging of serotonin transporters in major depression. J. Cereb. Blood Flow. Metab. 34 , 1096–1103 (2014).
Spies, M., Knudsen, G. M., Lanzenberger, R. & Kasper, S. The serotonin transporter in psychiatric disorders: insights from PET imaging. Lancet Psychiatry 2 , 743–755 (2015).
Wang, L. et al. Serotonin-1A receptor alterations in depression: a meta-analysis of molecular imaging studies. BMC Psychiatry 16 , 319 (2016).
Miller, J. M. et al. Brain serotonin 1A receptor binding as a predictor of treatment outcome in major depressive disorder. Biol. Psychiatry 74 , 760–767 (2013).
Miller, J. M., Oquendo, M. A., Ogden, R. T., Mann, J. J. & Parsey, R. V. Serotonin transporter binding as a possible predictor of one-year remission in major depressive disorder. J. Psychiatr. Res. 42 , 1137–1144 (2008).
Lanzenberger, R. et al. Prediction of SSRI treatment response in major depression based on serotonin transporter interplay between median raphe nucleus and projection areas. NeuroImage 63 , 874–881 (2012).
Drevets, W. C. Neuroimaging studies of mood disorders. Biol. Psychiatry 48 , 813–829 (2000).
Kennedy, S. H. et al. Differences in brain glucose metabolism between responders to CBT and venlafaxine in a 16-week randomized controlled trial. Am. J. Psychiatry 164 , 778–788 (2007).
Setiawan, E. et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 72 , 268–275 (2015).
Richards, E. M. et al. PET radioligand binding to translocator protein (TSPO) is increased in unmedicated depressed subjects. EJNMMI Res. 8 , 57 (2018).
Holmes, S. E. et al. Elevated translocator protein in anterior cingulate in major depression and a role for inflammation in suicidal thinking: a positron emission tomography study. Biol. Psychiatry 83 , 61–69 (2018).
Wium-Andersen, M. K., Orsted, D. D. & Nordestgaard, B. G. Elevated plasma fibrinogen, psychological distress, antidepressant use, and hospitalization with depression: two large population-based studies. Psychoneuroendocrinology 38 , 638–647 (2013).
Jha, M. K. et al. Can C-reactive protein inform antidepressant medication selection in depressed outpatients? Findings from the CO-MED trial. Psychoneuroendocrinology 78 , 105–113 (2017).
Kohler-Forsberg, O. et al. Association between C-reactive protein (CRP) with depression symptom severity and specific depressive symptoms in major depression. Brain Behav. Immun. 62 , 344–350 (2017).
Strawbridge, R. et al. Inflammation and clinical response to treatment in depression: A meta-analysis. Eur. Neuropsychopharmacol.: J. Eur. Coll. Neuropsychopharmacol. 25 , 1532–1543 (2015).
Lamers, F. et al. Serum proteomic profiles of depressive subtypes. Transl. Psychiatry 6 , e851 (2016).
Kadriu, B. et al. Acute ketamine administration corrects abnormal inflammatory bone markers in major depressive disorder. Mol. Psychiatry 23 , 1626–1631 (2017).
Machado-Vieira, R. et al. The role of adipokines in the rapid antidepressant effects of ketamine. Mol. Psychiatry 22 , 127–133 (2017).
Schmidt, H. D., Shelton, R. C. & Duman, R. S. Functional biomarkers of depression: diagnosis, treatment, and pathophysiology. Neuropsychopharmacology 36 , 2375–2394 (2011).
Molendijk, M. L. et al. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N = 9484). Mol. Psychiatry 19 , 791–800 (2014).
Brunoni, A. R., Baeken, C., Machado-Vieira, R., Gattaz, W. F. & Vanderhasselt, M. A. BDNF blood levels after electroconvulsive therapy in patients with mood disorders: a systematic review and meta-analysis. World J. Biol. Psychiatry 15 , 411–418 (2014).
Polyakova, M. et al. BDNF as a biomarker for successful treatment of mood disorders: a systematic & quantitative meta-analysis. J. Affect. Disord. 174 , 432–440 (2015).
Wray, N. R. et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 50 , 668–681 (2018).
Fabbri, C. et al. Consensus paper of the WFSBP Task Force on Genetics: Genetics, epigenetics and gene expression markers of major depressive disorder and antidepressant response. World J. Biol. Psychiatry 18 , 5–28 (2017).
Kautzky, A. et al. The combined effect of genetic polymorphisms and clinical parameters on treatment outcome in treatment-resistant depression. Eur. Neuropsychopharmacol. 25 , 441–453 (2015).
Stamm, T. J. et al. The FKBP5 polymorphism rs1360780 influences the effect of an algorithm-based antidepressant treatment and is associated with remission in patients with major depression. J. Psychopharmacol. 30 , 40–47 (2016).
Binder, E. B. et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat. Genet. 36 , 1319–1325 (2004).
Fabbri, C. et al. Pleiotropic genes in psychiatry: Calcium channels and the stress-related FKBP5 gene in antidepressant resistance. Prog. Neuropsychopharmacol. Biol. Psychiatry 81 , 203–210 (2018).
Klengel, T. & Binder, E. B. Gene x environment interactions in the prediction of response to antidepressant treatment. Int. J. Neuropsychopharmacol./Off. Sci. J. Coll. Int. Neuropsychopharmacol. 16 , 701–711 (2013).
CAS Google Scholar
Schosser, A. & Kasper, S. The role of pharmacogenetics in the treatment of depression and anxiety disorders. Int. Clin. Psychopharmacol. 24 , 277–288 (2009).
Zeier, Z. et al. Clinical implementation of pharmacogenetic decision support tools for antidepressant drug prescribing. Am. J. Psychiatry 175 , 873–886 (2018).
Jukic, M. M., Haslemo, T., Molden, E. & Ingelman-Sundberg, M. Impact of CYP2C19 genotype on escitalopram exposure and therapeutic failure: A retrospective study based on 2,087 patients. Am. J. Psychiatry 175 , 463–470 (2018).
Bauer, M. et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J. Biol. Psychiatry 14 , 334–385 (2013).
Uher, R. et al. Melancholic, atypical and anxious depression subtypes and outcome of treatment with escitalopram and nortriptyline. J. Affect. Disord. 132 , 112–120 (2011).
Arnow, B. A. et al. Depression subtypes in predicting antidepressant response: A report from the iSPOT-D trial. Am. J. Psychiatry 172 , 743–750 (2015).
Kasper, S. & Montgomery, S. A. Ohio Library and Information Network, Wiley Online Library (Online service). Treatment-resistant Depression , 1 online resource (2013).
Schosser, A. et al. European Group for the Study of Resistant Depression (GSRD)–where have we gone so far: review of clinical and genetic findings. Eur. Neuropsychopharmacol. 22 , 453–468 (2012).
Bergfeld, I. O. et al. Treatment-resistant depression and suicidality. J. Affect. Disord. 235 , 362–367 (2018).
Mann, J. J. et al. Suicide prevention strategies: a systematic review. JAMA 294 , 2064–2074 (2005).
Nimalasuriya, K., Compton, M. T. & Guillory, V. J., Prevention Practice Committee of the American College of Preventive M. Screening adults for depression in primary care: A position statement of the American College of Preventive Medicine. J. Fam. Pract. 58 , 535–538 (2009).
PubMed Google Scholar
Kroenke, K., Spitzer, R. L. & Williams, J. B. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med. Care 41 , 1284–1292 (2003).
Horowitz, L. M. et al. Ask suicide-screening questions to everyone in medical settings: the asQ’em Quality Improvement Project. Psychosomatics 54 , 239–247 (2013).
King, M. et al. Predicting onset of major depression in general practice attendees in Europe: extending the application of the predictD risk algorithm from 12 to 24 months. Psychol. Med. 43 , 1929–1939 (2013).
Kupfer, D. J., Frank, E. & Perel, J. M. The advantage of early treatment intervention in recurrent depression. Arch. Gen. Psychiatry 46 , 771–775 (1989).
Lopizzo, N. et al. Gene-environment interaction in major depression: focus on experience-dependent biological systems. Front. Psychiatry 6 , 68 (2015).
Gutierrez, B. et al. The risk for major depression conferred by childhood maltreatment is multiplied by BDNF and SERT genetic vulnerability: a replication study. J. Psychiatry Neurosci.: JPN 40 , 187–196 (2015).
Serretti, A. et al. The impact of adverse life events on clinical features and interaction with gene variants in mood disorder patients. Psychopathology 46 , 384–389 (2013).
Herbison, C. E., Allen, K., Robinson, M., Newnham, J. & Pennell, C. The impact of life stress on adult depression and anxiety is dependent on gender and timing of exposure. Dev. Psychopathol. 29 , 1443–1454 (2017).
Keers, R. & Uher, R. Gene-environment interaction in major depression and antidepressant treatment response. Curr. Psychiatry Rep. 14 , 129–137 (2012).
Gold, P. W. & Chrousos, G. P. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol. Psychiatry 7 , 254–275 (2002).
Carroll, B. J. et al. A specific laboratory test for the diagnosis of melancholia. Standardization, validation, and clinical utility. Arch. Gen. Psychiatry 38 , 15–22 (1981).
Musil, R. et al. Subtypes of depression and their overlap in a naturalistic inpatient sample of major depressive disorder. Int. J. Methods Psychiatr. Res . 27 (2018).
Angst J., Gamma A., Benazzi F., Ajdacic V., Rossler W. Melancholia and atypical depression in the Zurich study: epidemiology, clinical characteristics, course, comorbidity and personality. Acta Psychiatr. Scand. Suppl. 72–84 (2007).
Ionescu, D. F., Niciu, M. J., Henter, I. D. & Zarate, C. A. Defining anxious depression: a review of the literature. CNS Spectr. 18 , 252–260 (2013).
Lamers, F. et al. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol. Psychiatry 18 , 692–699 (2013).
Simmons, W. K. et al. Appetite changes reveal depression subgroups with distinct endocrine, metabolic, and immune states. Mol. Psychiatry , https://doi.org/10.1038/s41380-018-0093-6 (2018).
Woody, M. L. & Gibb, B. E. Integrating NIMH research domain criteria (RDoC) into depression. Res. Curr. Opin. Psychol. 4 , 6–12 (2015).
Ballard, E. D. et al. Parsing the heterogeneity of depression: An exploratory factor analysis across commonly used depression rating scales. J. Affect. Disord. 231 , 51–57 (2018).
Ruhe, H. G., van Rooijen, G., Spijker, J., Peeters, F. P. & Schene, A. H. Staging methods for treatment resistant depression. A systematic review. J. Affect. Disord. 137 , 35–45 (2012).
Nugent, A. C. et al. Safety of research into severe and treatment-resistant mood disorders: analysis of outcome data from 12 years of clinical trials at the US National Institute of Mental Health. Lancet Psychiatry 3 , 436–442 (2016).
Herzog, D. P. et al. Guideline adherence of antidepressant treatment in outpatients with major depressive disorder: a naturalistic study. Eur. Arch. Psychiatry Clin. Neurosci. 267 , 711–721 (2017).
Trivedi, M. H. et al. Clinical results for patients with major depressive disorder in the Texas Medication Algorithm Project. Arch. Gen. Psychiatry 61 , 669–680 (2004).
Adli, M. et al. How effective is algorithm-guided treatment for depressed inpatients? results from the randomized controlled multicenter german algorithm project 3 trial. Int J. Neuropsychopharmacol. 20 , 721–730 (2017).
Bauer, M. et al. Efficacy of an algorithm-guided treatment compared with treatment as usual: a randomized, controlled study of inpatients with depression. J. Clin. Psychopharmacol. 29 , 327–333 (2009).
Ricken, R. et al. Algorithm-guided treatment of depression reduces treatment costs–results from the randomized controlled German Algorithm Project (GAPII). J. Affect. Disord. 134 , 249–256 (2011).
Khan, A. & Brown, W. A. Antidepressants versus placebo in major depression: an overview. World Psychiatry 14 , 294–300 (2015).
Fava, M., Evins, A. E., Dorer, D. J. & Schoenfeld, D. A. The problem of the placebo response in clinical trials for psychiatric disorders: culprits, possible remedies, and a novel study design approach. Psychother. Psychosom. 72 , 115–127 (2003).
Enck, P., Bingel, U., Schedlowski, M. & Rief, W. The placebo response in medicine: minimize, maximize or personalize? Nat. Rev. Drug Discov. 12 , 191–204 (2013).
Desseilles, M. et al. Massachusetts general hospital SAFER criteria for clinical trials and research. Harv. Rev. Psychiatry 21 , 269–274 (2013).
Finniss, D. G., Kaptchuk, T. J., Miller, F. & Benedetti, F. Biological, clinical, and ethical advances of placebo effects. Lancet 375 , 686–695 (2010).
Henter, I. D., de Sousa, R. T. & Zarate, C. A. Jr. Glutamatergic modulators in depression. Harv. Rev. Psychiatry 26 , 307–319 (2018).
Ionescu, D. F. & Papakostas, G. I. Current trends in identifying rapidly acting treatments for depression. Curr. Behav. Neurosci. Rep. 3 , 185–191 (2016).
Kadriu, B. et al. Glutamatergic neurotransmission: pathway to developing novel rapid-acting antidepressant treatments. Int. J. Neuropsychopharmacol. / Off. Sci. J. Coll. Int. Neuropsychopharmacol. 22 , 119–135 (2018).
Zanos, P. et al. Convergent mechanisms underlying rapid antidepressant action. CNS Drugs 32 , 197–227 (2018).
Li, N. et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329 , 959–964 (2010).
Autry, A. E. et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475 , 91–95 (2011).
Berman, R. M. et al. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 47 , 351–354 (2000).
Duman, R. S. & Aghajanian, G. K. Synaptic dysfunction in depression: potential therapeutic targets. Science 338 , 68–72 (2012).
Diazgranados, N. et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J. Clin. Psychiatry 71 , 1605–1611 (2010).
Iadarola, N. D. et al. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther. Adv. Chronic Dis. 6 , 97–114 (2015).
Ibrahim, L. et al. Rapid decrease in depressive symptoms with an N-methyl-d-aspartate antagonist in ECT-resistant major depression. Progress. neuro-Psychopharmacol. Biol. Psychiatry 35 , 1155–1159 (2011).
Zarate, C. A. et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 63 , 856–864 (2006).
Diazgranados, N. et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch. Gen. Psychiatry 67 , 793–802 (2010).
Zarate, C. A. et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol. Psychiatry 71 , 939–946 (2012).
aan het Rot, M. et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol. Psychiatry 67 , 139–145 (2010).
Wan, L. B. et al. Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J. Clin. Psychiatry 76 , 247–252 (2015).
Murrough, J. W. et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol. Psychiatry 74 , 250–256 (2013).
Murrough, J. W. et al. Ketamine for rapid reduction of suicidal ideation: a randomized controlled trial. Psychol. Med. 45 , 3571–3580 (2015).
Price, R. B., Nock, M. K., Charney, D. S. & Mathew, S. J. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol. Psychiatry 66 , 522–526 (2009).
Wilkinson, S. T. et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am. J. Psychiatry 175 , 150–158 (2018).
Daly, E. J. et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: A randomized clinical trial. JAMA Psychiatry 75 , 139–148 (2018).
Canuso, C. M. et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am. J. Psychiatry 175 , 620–630 (2018).
Zanos, P. et al. The prodrug 4-chlorokynurenine causes ketamine-like antidepressant effects, but not side effects, by NMDA/glycineB-site inhibition. J. Pharmacol. Exp. Ther. 355 , 76–85 (2015).
Wilkinson, S. T. & Sanacora, G. A new generation of antidepressants: an update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems. Drug Discov. Today 24 , 606–615 (2018).
Article PubMed CAS PubMed Central Google Scholar
Vollenweider, F. X. & Kometer, M. The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat. Rev. Neurosci. 11 , 642–651 (2010).
Carhart-Harris, R. L. et al. Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry 3 , 619–627 (2016).
Karp, J. F. et al. Safety, tolerability, and clinical effect of low-dose buprenorphine for treatment-resistant depression in midlife and older adults. J. Clin. Psychiatry 75 , e785–e793 (2014).
Fava, M. et al. Opioid modulation with buprenorphine/samidorphan as adjunctive treatment for inadequate response to antidepressants: A randomized double-blind placebo-controlled trial. Am. J. Psychiatry 173 , 499–508 (2016).
Preskorn, S. et al. Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J. Psychiatr. Pract. 21 , 140–149 (2015).
Garay, R. P. et al. Investigational drugs in recent clinical trials for treatment-resistant depression. Expert Rev. Neurother. 17 , 593–609 (2017).
Kolbinger, H. M., Hoflich, G., Hufnagel, A., Moller, H. J. & Kasper, S. Transcranial magnetic stimulation (TMS) in the treatment of major depression—a pilot study. Human. Psychopharmacol. 10 , 305–310 (1995).
Lisanby, S. H. et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacol.: Off. Publ. Am. Coll. Neuropsychopharmacol. 34 , 522–534 (2009).
Brunoni, A. R. et al. Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: A systematic review with network meta-analysis. JAMA Psychiatry 74 , 143–152 (2017).
Benadhira, R. et al. A randomized, sham-controlled study of maintenance rTMS for treatment-resistant depression (TRD). Psychiatry Res 258 , 226–233 (2017).
Cusin, C. & Dougherty, D. D. Somatic therapies for treatment-resistant depression: ECT, TMS, VNS, DBS. Biol. Mood Anxiety Disord. 2 , 14 (2012).
Blumberger, D. M. et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet 391 , 1683–1692 (2018).
Fava, M. Diagnosis and definition of treatment-resistant depression. Biol. Psychiatry 53 , 649–659 (2003).
Husain, M. M. et al. Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a Consortium for Research in ECT (CORE) report. J. Clin. Psychiatry 65 , 485–491 (2004).
Kellner, C. H. et al. Relief of expressed suicidal intent by ECT: a consortium for research in ECT study. Am. J. Psychiatry 162 , 977–982 (2005).
Slade, E. P., Jahn, D. R., Regenold, W. T. & Case, B. G. Association of electroconvulsive therapy with psychiatric readmissions in US hospitals. JAMA Psychiatry 74 , 798–804 (2017).
Kellner, C. H. et al. Right unilateral ultrabrief pulse ECT in geriatric depression: phase 1 of the PRIDE study. Am. J. Psychiatry 173 , 1101–1109 (2016).
McClintock, S. M. et al. Multifactorial determinants of the neurocognitive effects of electroconvulsive therapy. J. ECT 30 , 165–176 (2014).
Fink, M. & Taylor, M. A. Electroconvulsive therapy: evidence and challenges. JAmA 298 , 330–332 (2007).
American Psychiatric Association. Task Force on Electroconvulsive Therapy. The practice of ECT: recommendations for treatment, training and privileging. Convuls. Ther. 6 , 85–120 (1990).
Kellner, C. H. et al. Bifrontal, bitemporal and right unilateral electrode placement in ECT: randomised trial. Br. J. Psychiatry.: J. Ment. Sci. 196 , 226–234 (2010).
Wilkinson, S. T., Agbese, E., Leslie, D. L. & Rosenheck, R. A. Identifying recipients of electroconvulsive therapy: data from privately insured Americans. Psychiatr. Serv. 69 , 542–548 (2018).
Lisanby, S. H., Schlaepfer, T. E., Fisch, H. U. & Sackeim, H. A. Magnetic seizure therapy of major depression. Arch. Gen. Psychiatry 58 , 303–305 (2001).
Deng, Z. -D., Lisanby, S. H. & Peterchev, A. V. Electric field strength and focality in electroconvulsive therapy and magnetic seizure therapy: a finite element simulation study. J. Neural Eng. 8 , 016007 (2011).
Fitzgerald, P. B. et al. Pilot study of the clinical and cognitive effects of high-frequency magnetic seizure therapy in major depressive disorder. Depress Anxiety 30 , 129–136 (2013).
Kayser, S. et al. Magnetic seizure therapy in treatment-resistant depression: clinical, neuropsychological and metabolic effects. Psychol. Med. 45 , 1073–1092 (2015).
Nahas, Z. et al. Two-year outcome of vagus nerve stimulation (VNS) for treatment of major depressive episodes. J. Clin. Psychiatry 66 , 1097–1104 (2005).
Aaronson, S. T. et al. A 5-Year observational study of patients with treatment-resistant depression treated with vagus nerve stimulation or treatment as usual: comparison of response, remission, and suicidality. Am. J. Psychiatry 174 , 640–648 (2017).
Schlaepfer, T. E., Bewernick, B. H., Kayser, S., Madler, B. & Coenen, V. A. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol. Psychiatry 73 , 1204–1212 (2013).
Bewernick, B. H. et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol. Psychiatry 67 , 110–116 (2010).
Bergfeld, I. O. et al. Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression: A randomized clinical trial. JAMA Psychiatry 73 , 456–464 (2016).
Dougherty, D. D. et al. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol. Psychiatry 78 , 240–248 (2015).
Holtzheimer, P. E. et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry 4 , 839–849 (2017).
Widge, A. S., Malone, D. A. Jr. & Dougherty, D. D. Closing the loop on deep brain stimulation for treatment-resistant depression. Front Neurosci. 12 , 175 (2018).
Jain, K. K. in The Handbook of Biomarkers . 1–26 (Springer New York, New York, NY, 2017).
Zohar, J. et al. A review of the current nomenclature for psychotropic agents and an introduction to the Neuroscience-based Nomenclature. Eur. Neuropsychopharmacol.: J. Eur. Coll. Neuropsychopharmacol. 25 , 2318–2325 (2015).
Dold, M. & Kasper, S. Evidence-based pharmacotherapy of treatment-resistant unipolar depression. Int J. Psychiatry Clin. Pract. 21 , 13–23 (2017).
Colle, R. et al. BDNF/TRKB/P75NTR polymorphisms and their consequences on antidepressant efficacy in depressed patients. Pharmacogenomics 16 , 997–1013 (2015).
Porcelli, S., Fabbri, C. & Serretti, A. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with antidepressant efficacy. Eur. Neuropsychopharmacol.: J. Eur. Coll. Neuropsychopharmacol. 22 , 239–258 (2012).
Download references
Acknowledgements
We thank the 7SE research unit and staff for their support. Ioline Henter (NIMH) provided invaluable editorial assistance. We also thank E. Acevedo-Diaz, Z.D. Deng, and J.W. Evans for scientific input.
Author information
Authors and affiliations.
Department of Psychiatry and Psychotherapy, Medical University of Vienna, Vienna, Austria
Christoph Kraus, Rupert Lanzenberger & Siegfried Kasper
Section on Neurobiology and Treatment of Mood Disorders, Intramural Research Program, National Institute of Mental Health, National Institutes of Health, Bethesda, MD, USA
Christoph Kraus, Bashkim Kadriu & Carlos A. Zarate Jr.
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Siegfried Kasper .
Ethics declarations
Conflict of interest.
Funding for this work was supported in part by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIA MH002927). All support given to authors was not related to the design of the manuscript or the ideas stated in this review. Dr. Kasper received grants/research support, consulting fees, and/or honoraria within the last 3 years from Angelini, AOP Orphan Pharmaceuticals AG, AstraZeneca, Eli Lilly, Janssen, KRKA-Pharma, Lundbeck, Neuraxpharm, Pfizer, Pierre Fabre, Schwabe, and Servier. Dr. Lanzenberger received travel grants and/or conference speaker honoraria from AstraZeneca, Lundbeck A/S, Dr. Willmar Schwabe GmbH, Orphan Pharmaceuticals AG, Janssen-Cilag Pharma GmbH, and Roche Austria GmbH. Dr. Kraus has received travel grants from Roche Austria GmbH and AOP Orphan. Dr. Zarate is a full-time U.S government employee. He is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation; as a co-inventor on a patent for the use of (2 R ,6 R )-hydroxynorketamine, ( S )-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of ( R,S )-ketamine metabolites in the treatment of depression and neuropathic pain; and as a co-inventor on a patent application for the use of (2 R ,6 R )-hydroxynorketamine and (2 S ,6 S )-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. He has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Kraus, C., Kadriu, B., Lanzenberger, R. et al. Prognosis and improved outcomes in major depression: a review. Transl Psychiatry 9 , 127 (2019). https://doi.org/10.1038/s41398-019-0460-3
Download citation
Received : 07 November 2018
Revised : 10 January 2019
Accepted : 11 February 2019
Published : 03 April 2019
DOI : https://doi.org/10.1038/s41398-019-0460-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Cannabinoid type 2 receptor inhibition enhances the antidepressant and proneurogenic effects of physical exercise after chronic stress.
- R. S. Rodrigues
- J. B. Moreira
Translational Psychiatry (2024)
Developing an individualized treatment rule for Veterans with major depressive disorder using electronic health records
- Nur Hani Zainal
- Robert M. Bossarte
- Ronald C. Kessler
Molecular Psychiatry (2024)
Multiomics and blood-based biomarkers of electroconvulsive therapy in severe and treatment-resistant depression: study protocol of the DetECT study
- Iven-Alex von Mücke-Heim
- Julius C. Pape
- Elisabeth B. Binder
European Archives of Psychiatry and Clinical Neuroscience (2024)
Perspectives in treatment-resistant depression: esketamine and electroconvulsive therapy
- Pia Baldinger-Melich
- Marie Spies
- Richard Frey
Wiener klinische Wochenschrift (2024)
Functional and structural alterations in different durations of untreated illness in the frontal and parietal lobe in major depressive disorder
- Xiaowei Jiang
- Yanqing Tang
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Significant gaps between science of obesity and the care patients receive
As research continues to produce evidence about the underlying causes of obesity and optimal strategies to treat and manage obesity have evolved, there are disparities in application of the latest scientific advances in the clinical care that people with obesity receive. Widespread adoption of current findings, consistency of care and expertise in obesity care varies by health care professional and institution. These findings are detailed in a new American Heart Association scientific statement, "Implementation of Obesity Science Into Clinical Practice," published today in the Association's flagship scientific journal Circulation .
"Obesity is undeniably a critical public health concern in the U.S. and around the world, affecting nearly all populations and straining our health care systems," said Deepika Laddu, Ph.D., FAHA, chair of the statement writing committee and a senior research scientist at Arbor Research Collaborative for Health in Ann Arbor, Michigan. "As a major risk factor for heart disease, obesity has significantly hindered progress in reducing heart disease rates. Despite advancements in understanding the complexities of obesity and newer treatment options, major gaps remain between obesity research and real-world implementation in clinical practice."
Studies show intensive lifestyle therapy is considerably more effective for weight loss than brief advice from a health care professional. However, general educational information is offered more frequently by health professionals rather than referrals to classes, programs or tangible resources for lifestyle changes. One study revealed that only 16% of health care professionals had working knowledge about evidence-based lifestyle treatments for obesity, including diet and nutrition, physical activity and intensive behavioral therapy referral. Other barriers to addressing weight loss are exacerbated by socioeconomic and racial or ethnic inequities. People of diverse races and ethnicities and people who are covered by Medicare or Medicaid are less likely to be referred to weight management programs or to have them covered by insurance.
The number of people living with obesity is increasing worldwide. For about 30 years, the prevalence of obesity in the United States and around the world has been escalating. Recent estimates indicate more than 40% of U.S. adults ages 20 and older are living with obesity, according to the U.S. Centers for Disease Control and Prevention.
Research has led experts to unlock the multifactorial causes of obesity, including sociological and physiological determinants of health. Treatments for obesity have also evolved with more strategies for lifestyle modifications, medication therapy and bariatric (weight-loss) surgery. However, each treatment approach comes with challenges.
"While significant strides have been made in advancing the science to help us understand obesity, there remains a considerable gap between what we know and what happens in the doctor's office," said Laddu. "Health care professionals and health care systems need to find better ways to put what we know about obesity into action so more people can get the right support and treatment. Adopting new technologies and telemedicine, making referrals to community-based weight management programs to encourage behavioral change, providing social support and increasing reach and access to treatments are just some of the promising methods we could implement to unlock successful, evidence-based obesity care."
Weight loss medications
Glucagon-like peptide-1 (GLP-1) agonists, such as high-dose semaglutide and tirzepatide, are the most recently FDA-approved medications for long-term weight management, and both are associated with an average weight loss of more than 10% at six months in clinical studies. However, despite half of adults in the U.S. meeting the BMI criteria for obesity and being eligible for these medications, a small proportion of this population is currently taking them. Until recently, the primary barriers to greater use of anti-obesity medications were lack of insurance coverage and high out-of-pocket costs for these medications.
Since the beginning of the Medicare (Part D) program in 2006, all medications taken for weight loss have been excluded from basic coverage. In March, the Centers for Medicare and Medicaid Services (CMS) determined that Medicare and Medicaid can cover the anti-obesity medication semaglutide when it is approved by the FDA for an additional use. That decision included high-dose semaglutide, which is FDA-approved for weight loss and to reduce the risk of cardiovascular death, heart attack and stroke in adults with cardiovascular disease and either obesity or overweight. State Medicaid programs, which provide health care coverage for people in low-income populations and who are disproportionately affected by obesity and heart disease, are required to cover nearly all FDA-approved anti-obesity medications for people meeting the health and body mass index (BMI) criteria. However, state health plans may require step therapy with other treatments or medications prior to approving use of GLP-1 medications.
"FDA approval and insurance coverage of the latest treatments, including GLP-1 medications, are integral to improving access to care and outcomes for people who need these therapies the most. This is especially true for high-risk, high-need patients for the prevention of adverse cardiovascular events. It is encouraging that these steps in increasing access may lead to reduced risk of CVD and improved outcomes for potentially millions of adults in the U.S.," said the scientific statement's Vice Chair Ian J. Neeland, M.D., FAHA, director of cardiovascular prevention, director of the Center for Integrated and Novel Approaches in Vascular-Metabolic Disease at University Hospitals Harrington Heart and Vascular Institute at Institute, and an associate professor of medicine at the Case Center for Diabetes, Obesity and Metabolism at Case Western Reserve University School of Medicine, both in Cleveland.
Weight loss surgery
In the decades since bariatric (weight loss) surgery was first introduced as an option for people with severe obesity, there have been advances in the expertise and safety of the procedures, as well as an increased understanding of the health benefits that often result after bariatric surgery. A comprehensive review of studies focused on weight loss surgeries showed that patients who underwent bariatric surgery had lower risks of cardiovascular disease and decreased risks for multiple other obesity-associated conditions, including Type 2 diabetes and high blood pressure. One challenge facing health care professionals is ensuring that the populations with the greatest needs have access to bariatric surgery in terms of costs, resources and social support.
The statement describes strategies that both address these challenges and improve how obesity-based research is incorporated into clinical care. The statement also identifies the need to develop solutions across populations in order to manage obesity at the community level. Potential improved public health policies and future research to expand patient care models and optimize the delivery and sustainability of equitable obesity-related care are suggested.
Specific approaches are highlighted in the statement to help bridge the gap between the science about obesity and clinical care, such as:
- To reach and successfully impact populations in need, health care professionals may consider how social determinants of health, including insurance type, household income, race and ethnicity, environment, health literacy, access to health-promoting resources and social supports all influence the likelihood of successful patient treatment.
- Education for health care professionals explaining the complex origins and clinical consequences of obesity is discussed. Such training should emphasize information about diagnosis, prevention and treatment of obesity. Despite the high prevalence of obesity around the world, there is a lack of education programs centered on obesity for medical professionals.
- Further evaluation of health policy changes that health care systems and insurance plans can implement and scale in order to make obesity treatment affordable for patients, especially those at high risk for adverse outcomes such as cardiovascular disease.
- A framework for delivering obesity care into clinical practice settings is reviewed, as well as efforts by some professional societies for developing interventions that make obesity treatment more accessible.
"The statement emphasizes the importance of a comprehensive approach across different levels of health care delivery and public policy, along with the adoption of feasible, evidence-based strategies in clinical settings," said Laddu. "It also underscores the need for future research and policy changes to improve current patient care models and ensure equitable access to obesity-related care for people in underrepresented groups."
The scientific statement also provides possible solutions for how to help people in their day-to-day lives, including interventions with digital technology and access through telemedicine. However, more research is needed in obesity science and treatment. Limited understanding of the cost-effectiveness of obesity prevention and the long-term health outcomes for established therapies has hindered the implementation of obesity science into clinical settings. Cross-collaborative obesity science research between stakeholders and health economists may serve as the bridge to developing and scaling cost-effective prevention programs.
Further research into Food Is Medicine approaches in health care, such as medically tailored meals and produce prescriptions, to prevent and treat cardiovascular disease and other diet-related diseases are also being explored in several settings including the Association's Health Care by Food TM initiative.
This scientific statement was prepared by the volunteer writing group on behalf of the American Heart Association Obesity Committee of the Council On Lifestyle and Cardiometabolic Health; the Council on Epidemiology and Prevention; the Council on Clinical Cardiology, the Council on Hypertension; the Council on the Kidney in Cardiovascular Disease; and the Council on Cardiovascular and Stroke Nursing. American Heart Association scientific statements promote greater awareness about cardiovascular diseases and stroke issues and help facilitate informed health care decisions. Scientific statements outline what is currently known about a topic and what areas need additional research. While scientific statements inform the development of guidelines, they do not make treatment recommendations. American Heart Association guidelines provide the Association's official clinical practice recommendations.
Additional co-authors and members of the writing group are Mercedes Carnethon, Ph.D., FAHA; Fatima C. Stanford, M.D., M.P.H., M.P.A., M.B.A., FAHA; Morgana Mongraw-Chaffin, Ph.D., FAHA; Bethany Barone Gibbs, Ph.D., FAHA; Chiadi E. Ndumele, M.D., Ph.D., FAHA; Chris T. Longenecker, M.D., FAHA; Misook L. Chung, Ph.D., R.N., FAHA; and Goutham Rao, M.D.
- Diet and Weight Loss
- Health Policy
- Diseases and Conditions
- Dieting and Weight Control
- Mental Health
- Nutrition Research
- Anti-obesity drug
- Physical exercise
- Atkins Diet
- Low-carb diets
- Stretch marks
- Palliative care
Story Source:
Materials provided by American Heart Association . Note: Content may be edited for style and length.
Journal Reference :
- Deepika Laddu, Ian J. Neeland, Mercedes Carnethon, Fatima C. Stanford, Morgana Mongraw-Chaffin, Bethany Barone Gibbs, Chiadi E. Ndumele, Chris T. Longenecker, Misook L. Chung, Goutham Rao. Implementation of Obesity Science Into Clinical Practice: A Scientific Statement From the American Heart Association . Circulation , 2024; DOI: 10.1161/CIR.0000000000001221
Cite This Page :
Explore More
- Record Low Antarctic Sea Ice: Climate Change
- Brain 'Assembloids' Mimic Blood-Brain Barrier
- 'Doomsday' Glacier: Catastrophic Melting
- Blueprints of Self-Assembly
- Meerkat Chit-Chat
- First Glimpse of an Exoplanet's Interior
- High-Efficiency Photonic Integrated Circuit
- Life Expectancy May Increase by 5 Years by 2050
- Toward a Successful Vaccine for HIV
- Highly Efficient Thermoelectric Materials
Trending Topics
Strange & offbeat.

IMAGES
VIDEO
COMMENTS
A new type of magnetic brain stimulation brought rapid remission to almost 80% of participants with severe depression in a study conducted at the Stanford University School of Medicine. The treatment, known as Stanford accelerated intelligent neuromodulation therapy (SAINT) or simply Stanford neuromodulation therapy, is an intensive, individualized form of transcranial magnetic stimulation.
Its severe form, major depression is classified as a mood disorder. Latest Research and Reviews Improved implicit self-esteem is associated with extended antidepressant effects following a novel ...
The primary aim of this literature review: to address the aforementioned challenges, we have synthesized recent research on the biological, psychological, and social determinants of depression and we have reviewed research from fields including genetics, immunology, neurology, psychology, public health, and epidemiology, among others.
Depression is common, costly, debilitating, and associated with increased risk of suicide. It is one of the leading global public health problems. Although existing available pharmacological treatments can be effective, their onset of action can take up to 6 weeks, side-effects are common, and recovery can require treatment with multiple different agents. Although psychosocial interventions ...
VOL. 390 NO. 17. In this first episode of a four-part Double Take video miniseries on depression from the New England Journal of Medicine, Dr. Scott-Vernaglia recounts her personal experiences ...
Analysis of published papers around the world from 2009 to 2019 in depressive disorder. A The total number of papers [from a search of the Web of Science database (search strategy: TI = (depression$) or ts = ("major depressive disorder$")) and py = (2009-2019), Articles)].B The top 10 countries publishing on the topic.C Comparison of papers in China and the USA.
The current lack of guidelines about the therapeutic monitoring of ketamine treatment for depression further complicates the expanding use of this treatment. Even though ketamine might never reach the market, it has stimulated research in the neurobiology of depression, including studies on potential fast and long-lasting antidepressants.
The idea that depression is the result of abnormalities in brain chemicals, particularly serotonin (5-hydroxytryptamine or 5-HT), has been influential for decades, and provides an important ...
The current speed of progress in depression research is simply remarkable. We have therefore been able to create a second special issue of Molecular Psychiatry, 2020, focused on depression, with ...
Recent numbers from the National Institute of Mental Health show that depression causes major distress and life disruption for more 63% of adults and more than 70% of teens with the disorder ...
Newly discovered trigger for major depression opens new possibilities for treatments. ScienceDaily . Retrieved May 19, 2024 from www.sciencedaily.com / releases / 2023 / 03 / 230330172127.htm
In this 6-week, double-blind study, patients with major depression remained on their antidepressant treatment and also received either placebo, cariprazine 1.5 mg/day, or cariprazine 1.5 mg for 2 weeks and then increased to 3 mg/day. A total of 751 patients were included in the modified intention-to-treat analysis.
FDA approval of the postpartum depression treatment brexanolone represents the final phase of a bench-to-bedside journey for this drug — a journey that began in the NIMH Intramural Research Program. NIMH experts are available to provide information on postpartum depression and the importance of, and the science underlying, this new drug.
R. McShaneN Engl J Med 2023;389:1333-1334. Depression can become self-perpetuating as occupational, relationship, and social losses accrue. Sustained remission is achieved in less than half of ...
Market research quota sampling is a non-probabilistic sampling strategy whereby participants are identified from a database to match the population ... [574·1-807·2] new cases per 100 000 population, an increase of 27·6% [25·1-30·3 ... Major depression in the era of economic crisis: a replication of a cross-sectional study across ...
Research round-up: depression Why depression in women is so misunderstood Subjects. Depression; Psychiatric disorders; Health care; Psychology; Latest on: Depression. Interpersonal therapy can be ...
Research on the most commonly investigated serotonin receptor suggested either no difference between people with depression and people without depression, or that serotonin activity was actually increased in people with depression - the opposite of the serotonin theory's prediction.
Read the latest research findings and in-depth information on clinical depression and stress in adults, teens, and children. Expand your understanding of the symptoms and available treatment for ...
Postpartum depression (PPD) is a common mental disorder that many women experience after giving birth. Onset of PPD coincides with a dramatic drop in levels of a brain-derived steroid (neurosteroid) known as allopregnanolone. Decades of research supported by NIMH illuminated the role of neurosteroids like allopregnanolone in mental illnesses.
The results, published in April, reveal the chronological toll of mental illness. Among respondents diagnosed with depression and/or anxiety, 50% said they've lost years of their life to poor ...
Analysis of published papers around the world from 2009 to 2019 in depressive disorder. A The total number of papers [from a search of the Web of Science database (search strategy: TI = (depression$) or ts = ("major depressive disorder$")) and py = (2009-2019), Articles)].B The top 10 countries publishing on the topic.C Comparison of papers in China and the USA.
The depression rate recorded between January and May 2020 — after the COVID-19 outbreak had begun in each country — ranged from 7% to 48%, with an average of about 25%. Even the lowest rates ...
Stepped collaborative care is an approach for managing symptoms such as depression, pain, and fatigue in people with cancer. It includes psychotherapy and medication if the symptoms are not reduced by psychotherapy alone. A person's symptoms are assessed every 4 weeks. If the symptoms are not in the normal range, health care providers change ...
Without further research on depression and anxiety prevalence, it is difficult to develop preventative mental health programs and interventions for current conditions. Participation in sport facilitates positive mental health behaviors, including self-confidence, positive self-esteem, and social support . Individuals with positive mental health ...
Another recent review summarized the available research evaluating the effects of the KD on cognition, depression symptoms, and anxiety-associated behaviors, as well as social and dietary behaviors, in laboratory rodents . The majority of the included research in diverse disease animal models shows that the KD may beneficially impact cognitive ...
Lykos sponsored the clinical trials of MDMA. The results are included in the company's application to the FDA seeking approval to market the drug for therapy-assisted PTSD treatment. Researchers ...
In this regard, the prototypic glutamatergic modulator ketamine has catalyzed research into new mechanistic approaches and offered hope for the development of novel, fast-acting antidepressants.
For about 30 years, the prevalence of obesity in the United States and around the world has been escalating. Recent estimates indicate more than 40% of U.S. adults ages 20 and older are living ...