Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access

Emerging Evidence on the Effectiveness of Tropical Forest Conservation
* E-mail: [email protected]
Affiliation Center for Development Research (ZEF), University of Bonn, and Center for International Forestry Research (CIFOR), Bonn, Germany
Affiliation Department of Agricultural and Consumer Economics, University of Illinois, Urbana, Illinois, United States of America
Affiliation Institute of Environmental Science and Technology (ICTA), Universitat Autònoma de Barcelona, Barcelona, Spain; Department of Economics and Economic History, Universitat Autònoma de Barcelona, Barcelona, Spain
Affiliation Center International en Recherche Agronomique pour le Développement (CIRAD), Montpellier, France
Affiliation Carey Business School & Whiting School of Engineering, Department of Geography and Environmental Engineering, Johns Hopkins University, Baltimore, Maryland, United States of America
Affiliation School of Community and Regional Planning, University of British Columbia, Vancouver, British Columbia, Canada
Affiliation Institut du développement durable et des relations internationales (IDDRI), Paris, France
Affiliation Department of Energy & Environment, Chalmers University of Technology, Göteborg, Sweden
Affiliation Center for International Forestry Research (CIFOR), Lima, Peru
- Jan Börner,
- Kathy Baylis,
- Esteve Corbera,
- Driss Ezzine-de-Blas,
- Paul J. Ferraro,
- Jordi Honey-Rosés,
- Renaud Lapeyre,
- U. Martin Persson,
- Sven Wunder

Published: November 2, 2016
- https://doi.org/10.1371/journal.pone.0159152
- Reader Comments
The PLOS ONE Collection “Measuring forest conservation effectiveness” brings together a series of studies that evaluate the effectiveness of tropical forest conservation policies and programs with the goal of measuring conservation success and associated co-benefits. This overview piece describes the geographic and methodological scope of these studies, as well as the policy instruments covered in the Collection as of June 2016. Focusing on forest cover change, we systematically compare the conservation effects estimated by the studies and discuss them in the light of previous findings in the literature. Nine studies estimated that annual conservation impacts on forest cover were below one percent, with two exceptions in Mexico and Indonesia. Differences in effect sizes are not only driven by the choice of conservation measures. One key lesson from the studies is the need to move beyond the current scientific focus of estimating average effects of undifferentiated conservation programs. The specific elements of the program design and the implementation context are equally important factors for understanding the effectiveness of conservation programs. Particularly critical will be a better understanding of the causal mechanisms through which conservation programs have impacts. To achieve this understanding we need advances in both theory and methods.
Citation: Börner J, Baylis K, Corbera E, Ezzine-de-Blas D, Ferraro PJ, Honey-Rosés J, et al. (2016) Emerging Evidence on the Effectiveness of Tropical Forest Conservation. PLoS ONE 11(11): e0159152. https://doi.org/10.1371/journal.pone.0159152
Editor: Ben Bond-Lamberty, Pacific Northwest National Laboratory, UNITED STATES
Copyright: © 2016 Börner et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: There is no original data contained in or supplement provided along with the paper.
Funding: This work was supported by European Commission – Grant no. DCI-ENV/2011/269520, Robert Bosch Foundation (Grant: 32.5.8043.0012.0), the CGIAR Research Program on Forests, Trees and Agroforestry (FTA), and European Association of Environmental and Resource Economists. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
1 Introduction
Forests provide valuable ecosystem goods and services of local and global significance. According to the latest forest resource assessment of the United Nations Food and Agriculture Organization, our global stock of natural forests continues to shrink, albeit at a slower annual rate than in the past [ 1 ]. Reduced deforestation rates may be the result of slower economic growth, decreasing demand for cleared land in urbanizing economies, or a sign that conservation policies are succeeding [ 2 ]. However, the global drop in rates of tropical tree cover loss is mostly driven by a few countries, such as Brazil. This inter-regional variation represents a major challenge for efforts towards achieving Aichi Target 5 and Sustainable Development Goal 15 on forests [ 3 ]. In the long term, our planet’s forests remain vulnerable to land use changes from increasing demand for agricultural and forest products [ 4 – 6 ].
Multiple policies and programs are being deployed to reduce tropical deforestation, mitigating climate change, and curbing biodiversity loss. Besides actions on forests already included in a number of intended nationally determined contributions to climate change mitigation (INDC), the Paris Agreement, in its Article 5, encourages Parties to the United Framework Convention on Climate Change to implement policy approaches and positive incentives to reduce emissions from deforestation and forest degradation. And yet, our knowledge about how to achieve forest conservation and related development goals is fragmented at best [ 7 – 10 ]. This PLOS ONE Collection contributes to building such a knowledge base and adds to the emerging literature on the effectiveness of conservation policies and measures with a focus on tropical and subtropical biomes.
Section 2 describes the geographic and methodological scope of this Collection, as well as the policy instruments covered in the Collection’s articles. As an open collection, we hope that additional articles will be added in the future. Section 3 synthesizes the main findings from the articles included in the Collection to date and Section 4 identifies potential future research directions.
2 Geographic Scope, Methodological Approaches, and Policy Instruments Covered in the Collection
The Collection as of March 2016 brings together 13 empirical studies covering eight countries across four continents ( Fig 1 and Table 1 ). Four studies evaluate forest conservation policies in Brazil and each presents new insights that help explain the remarkable drop in Amazon deforestation over the past decade. Policies in Costa Rica and Indonesia are addressed by two contributions each, whereas Chile, Colombia, Mexico, Namibia, and Tanzania are covered by one study each.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0159152.g001
https://doi.org/10.1371/journal.pone.0159152.t001
In addition, two studies address methodological issues in the evaluation of conservation policies, one with a focus on payments for environmental services (PES) and one with a focus on defining appropriate spatial scales of analysis.
Table 1 summarizes the methodological approaches used in each contribution as well as the policies or interventions examined. Most studies use some form of matching analysis in their empirical strategies. All authors rely on quasi-experimental evaluation designs when evaluating the effectiveness of forest conservation interventions, either because these interventions do not lend themselves well to experimental evaluation (e.g. protected areas) or because data were obtained only after the policies were rolled out. In both cases, matching procedures have helped researchers identify more realistic control units upon which to develop a possible counterfactual scenario. Matching was also used as a preprocessing step to reduce model dependence in post-matching regression analysis by various studies [ 13 , 14 , 21 , 25 ]. Miteva et al. [ 17 ] employ a matching-based triple difference estimator to exploit the three-period panel structure of their data.
In addition to estimating average treatment effects, post-matching regression analysis (including non-parametric regression techniques) served the purpose of robustness checks, as in Costedoat et al. [ 15 ], or of identifying heterogeneity in treatment effects, as in Shah and Baylis [ 23 ]. Pailler et al. [ 19 ] employ difference-in-difference regression directly. Cisneros et al. [ 14 ] study causal mechanisms behind the average treatment effect of a public disclosure initiative in Brazil, using panel data in a regression and matching-based empirical strategy [ 26 ]. Finally, Sills et al. [ 24 ] use a synthetic control approach [ 27 ] not previously applied to evaluate conservation initiatives.
Most studies in the Collection rely on remote sensing-based indicators of forest cover change to measure conservation effectiveness. Especially in humid tropical climates, such indicators are subject to measurement errors, for example as a result of persistent cloud cover. However, as multi-year remote sensing products measuring land cover change at global scale become increasingly available, new opportunities arise to assess the reliability of quasi-experimental evaluation techniques. Cisneros et al. [ 26 ], for example, use several years of pre-treatment observations to formally test for the parallel time trend assumption in their empirical strategy. Börner et al. [ 9 ] and Costedoat et al. [ 15 ] assess the sensitivity of their results to varying spatial resolutions and Börner et al. [ 13 ] find that treatment effects become insignificant at high spatial resolutions.
The policies and programs evaluated in the Collection range from regulatory disincentives and related enforcement mechanisms (e.g., protected areas, public disclosure, and field inspections) to incentive-based measures (e.g. PES and certification), and enabling institutional arrangements, such as jurisdictional support measures and community-based natural resource management [ 28 ]. Of these interventions, protected areas represent the most frequently studied forest conservation tool in the evaluation literature [ 29 ], whereas counterfactual-based evaluations of incentive-based conservation programs are only slowly emerging [ 30 ]. While a considerable amount of literature exists on community-based natural resource management, few study designs allow for statistically rigorous assessments of effectiveness [ 31 ]. The Collection contributes to filling such gaps in the evidence on the effectiveness of conservation measures.
3 Synthesis of Findings
Here we synthesize the key findings of the Collection papers in terms of broad instrument categories (see also Table 1 for effect sizes and related evaluation periods).
Regulatory disincentives
Collection papers analyzing the conservation effectiveness of protected areas in Brazil, Chile, Costa Rica, and Indonesia found low to moderate forest conservation effects. According to Pfaff et al. [ 20 ], protected areas in the Brazilian Amazon reduced deforestation by 2% on average between 2000 and 2008. However these impacts vary over space and time. They find (1) lower effectiveness of protection as annual rates of forest loss went down in the region as a whole over time, and (2), higher effectiveness of protected areas located close to cities and transport ways, where pressure on forest resources tends to be high. For Costa Rica, Robalino et al. [ 22 ] find average conservation effects of protected areas in a similar range (0.9–1.23% over 2000–2005). For Chile, Arriagada and Echeverria et al. [ 11 ] show that forest loss in protected areas was reduced by 4–5% over 25 years (1986–2011) only vis-à-vis land cover dynamics on private land holdings, but not in comparison with purely public land. Finally in Indonesia, Shah and Baylis [ 23 ] found protected areas to exhibit similarly low conservation effects on average in the period 2000 to 2012 (1.1%), but when examining specific parks, the treatment effects ranged from 5.3% to -3.4%.
Two papers explicitly study alternative forest law enforcement strategies in Brazil. Börner et al. [ 13 ] evaluate the effectiveness of remote sensing-supported field inspections in the Brazilian Amazon, and find that field presence has reduced deforestation by 14% per year on average. However, the effectiveness of field-based enforcement varied across federal states, due to heterogeneous contextual conditions–i.e. the type and intensity of deforestation drivers, and the institutional responses to them. Naming and shaming municipalities with high deforestation rates in the Brazilian Amazon also reduced deforestation by 13–36% on average between 2008 and 2012, according to Cisneros et al. [ 14 ]. This study also explores field enforcement, rural credit provision, and Brazil’s new national land cadaster as potential mechanisms behind the conservation effect of this public disclosure policy. It concludes, nonetheless, that the net effect was primarily driven by local factors.
Conservation incentives
Two Collection papers look at the effectiveness of PES schemes in Costa Rica. Evaluating interactions between PES and protected areas, Robalino et al. [ 22 ] find PES to be marginally more effective than protection if applied separately in space. Combining PES with protection or applying PES to manage buffer areas of protected areas does not substantially alter conservation effectiveness, thus pointing to substitutability rather than complementarity between the two conservation policy options. Arriagada et al. [ 12 ] measure the welfare effects of participating in a PES program in northeastern Costa Rica after having confirmed average conservation effects in the range of 11–17% in a separate study [ 32 ]. Their follow-up analysis finds that participating in PES does not have measurable effects on income and welfare indicators, suggesting that motives other than purely monetary motivations explain why farmers participate in the scheme [ 33 ].
High conservation effects are found by Costedoat et al. for PES in Chiapas (Mexico), where payments increased forest cover in enrolled communities by 12–14.7% in 2007–2013, compared to non-participating communities. The authors, however, also report high levels of non-compliance among participating communities, which leads them to suggest an even higher potential if PES was reinforced by additional conservation policies. In Colombia, Pagiola et al. [ 18 ] examine the long-term impacts of a PES scheme that ended in 2007 and had promoted the adoption of silvopastoral management practices. The initial evaluation had demonstrated that outcomes measured in terms of an environmental service index had increased by roughly 50%. However there was concern that once the program stopped payments, farmers might revert to old practices. Using a control group and controlling for relevant household characteristics, this study finds that the land use systems adopted during the PES program were still in place, even four years after the PES program ceased making payments.
Similarly encouraging, Miteva et al.’s study [ 17 ] of Indonesian timber concessions certified by the Forest Stewardship Council (FSC) demonstrates that certification increased forest cover by 5% on average vis-à-vis non-certified concessions, between 2000–2008. In addition, certification was associated with significant reductions in firewood dependence (33%), air pollution (31%), respiratory infections (32%), and malnutrition in participating villages.
Enabling measures
Two Collection studies covering community-based natural resource management initiatives in Africa focus on welfare outcomes. Pailler et al. [ 19 ] find that collective resource management in Tanzania somewhat improved household food security, but did not affect any of the measured wealth and health outcomes. On the other hand, Riehl et al.’s evaluation [ 21 ] of community-based natural resource management in Namibia finds positive health outcomes. The study, however, also finds that school attendance rates in participating communities did not keep pace with school attendance in non-participating communities.
Finally, Sills et al. [ 24 ] show that annual forest loss in the Brazilian municipality of Paragominas was reduced after the implementation of jurisdictional support for monitoring as well as sustainable transformation of land use systems. The reduction, however, turnes out to be significant only in the fourth out of the five post-treatment years covered in the study.
Forest conservation effectiveness
Horizontal bars and values in brackets represent standard errors. Three letter abbreviations are UN country codes.
https://doi.org/10.1371/journal.pone.0159152.g002
Most studies report effects between 0 and 0.5 percentage points ( Fig 2 ). This effect range corresponds well to that found by Samii et al. for selected PES programs in the tropics, i.e., 0.21 percentage points for studies that measured deforestation and 0.5–1.6% for studies looking at forest cover. Small effects are thus not necessarily a unique feature of PES programs, but instead seem to be a more general characteristic of tropical forest conservation programs. This can be partly explained by the intervention context in which such programs typically occur (see Persson and Alpizar [ 35 ] for a formal treatment of this issue). Since many forest conservation initiatives have a remote location bias, they tend to target a large amount of forest land that is not immediately threatened by deforestation. For many programs, it is thus not surprising to find that large shares of forest would have been conserved even in the absence of the intervention. To judge whether the intervention was worthwhile, we have to assess whether the value of the additional forest cover achieved by the program, whatever the amount, justifies the costs of the intervention. As of yet, few evaluations of forest conservation programs include cost-effectiveness assessments.
Two Collection studies report annual effects on forest cover change that are about one order of magnitude higher than the 0–0.5% effect range, i.e. Costedoat et al. [ 15 ] and Miteva et al. [ 17 ]. While these studies may indeed have evaluated genuinely more effective programs, they also differ from the other six studies in terms of study design and intervention context. Both studies evaluate forest cover change in spatial locations that represent actual decision units, i.e. communities ( ejidos ) in Mexico and villages in Indonesia. In the Mexican case, a large amount of forest remnants exhibited a relatively high risk of deforestation and in the Indonesian case all villages held forests under logging concessions, and thus, are predestined to some form of land cover change.
Methodological insights
Methodological contributions to the Collection provide important insights for grid-based spatial analyses of area-based conservation measures and the evaluation of PES schemes.
For example, researchers’ choice of scale may impact estimates of treatment effects when evaluating forest conservation programs. Spatial aggregation can affect the precision of the estimate as well as the estimate itself. Choosing low resolution will decrease precision and excessively high spatial resolutions can result in downward bias by introducing noise in covariates. The methodological review by Le Velly and Duttily [ 16 ] focuses on the challenges of evaluation PES schemes, but also provides more general lessons for the evaluation of forest conservation measures. Corroborating the lessons from comparing the empirical studies, it highlights the need to carefully characterize the intervention context before applying quantitative evaluation methods.
4 Future Research Directions
Our Collection overview is only a snapshot of the emerging literature using counterfactual-based evaluation to measure the effectiveness of forest conservation initiatives. This literature has a strong focus on protected areas [ 29 ], but also increasingly covers incentive-based conservation measures, such as PES, and enabling community support measures [ 30 ]. By allowing for the construction of observed rather than stated outcome measures, the increased availability of and improved access to remote sensing-based forest cover estimates over the past decade has clearly advanced this line of research.
Vis-à-vis the existing literature on the effectiveness of conservation policies, the new studies in our Collection point to some incipient lessons for future research:
- Beware of location bias: Most conservation policy interventions are implemented in contexts that are not representative and thus suffer from selection bias. However, the direction of bias can change depending on the underlying intervention strategy. For example, several Collection papers show that protected areas tend to be located in remote locations, reflecting lower opportunity costs of land and reduced potential for conflicting land use interests [ 20 ]. In some cases, however, protected areas are also intentionally established in high pressure areas [ 36 ], leading to a bias in the opposite direction. If a forest conservation policy is being systematically implemented in above or below-average pressure contexts, securing internal validity of evaluations is not enough for us to learn about its potential effectiveness.
- Carefully document intervention context: A host of factors including pre-program levels of compliance with intervention goals, policy design, and quality of implementation co-determine outcomes—potentially as strongly as the proper policy instrument choice (see also [ 35 ]). High environmental threats increase the scope for effective counteraction. Careful documentation of context factors and intervention design elements is thus paramount to making sense of comparative analyses within and across policy categories.
- Cautiously interpret early systematic reviews: It is probably too early to derive general lessons on individual policy instruments such as attempted in recent systematic reviews, for example, on PES [ 30 ]. As the studies in this Collection show, the effectiveness of forest conservation instruments in the same category can vary by factor six in terms of effects on annual forest cover change (see Fig 2 ), with high levels of variation particularly between, but even within countries. Until the sources of this variability are better understood, and studies are available from a variety of contexts (see 2.), it is premature to draw generalizable, externally valid conclusions on the effectiveness of individual instruments.
- Push methodological boundaries in quasi-experimental evaluation: Some Collection papers apply heterogeneous treatment effect analysis, or formally measure the contribution of individual causal mechanisms behind average treatment effects. Such analytical extensions require additional assumptions and more careful interpretation, but help us understand where, when, and why interventions work. Moreover, many papers in this Collection show that spatial factors play an important role in affecting the results of empirical analyses. As methods in spatial analysis and statistics are rapidly developing, new and more sophisticated empirical strategies will increasingly become available as ready-to-use software packages for conservation impact evaluation.
- Explore options for randomization: Randomized control trials have been conducted to evaluate conservation management practices, but are virtually absent from the literature on conservation policy effectiveness at the time this Collection was conceptualized [ 10 ]. Not all conservation policy measures lend themselves to randomization, but oversubscription and randomized phase-in clearly represent feasible strategies to evaluate PES and community-based conservation initiatives. Randomization may seem especially appropriate when programs are to be rolled out on a larger scale. Moreover, even if the intervention cannot be entirely randomized, one may still be able to experimentally vary certain contextual conditions or design features of the program in order to evaluate the effectiveness of key mechanisms of the conservation policy according to its theory of change.
- Do not forget intervention costs: Few studies evaluating conservation policy effectiveness, including in this Collection, factor in policy implementation costs as additional performance criterion. Ultimately, however, decision-makers will have to balance policy effectiveness against costs. Especially if conservation policy instruments are part of a much broader environmental policy strategy, quantification of instrument-specific opportunity and implementation cost (including initial investment needs as well as recurrent annual expenditure) can be a daunting task.
It is not enough to ask: “what works and what doesn’t?”. We also need to know where, when, and why forest conservation initiatives failed or worked, and at what cost. While impact evaluation is an important piece of this puzzle, it clearly has shortcomings that require other qualitative and quantitative research approaches to complete the picture [ 37 ]. However, learning from practice for the design of better interventions for conservation, with more cost-effective and equitable outcomes, requires impact evaluation to become an integral part of the policy research cycle, so as to inform theory development and ex-ante impact assessment [ 38 ].
Acknowledgments
This research was financially supported by the European Commission–Grant n° DCI-ENV/2011/269520 and the Robert Bosch Foundation; the CGIAR Research Program on Forests, Trees and Agroforestry (FTA); and European Association of Environmental and Resource Economists. We thank the participants of the International Workshop “Evaluating Forest Conservation Initiatives: New Tools and Policy Needs”, 10–12th December 2013 in Barcelona, Spain, for discussions and comments that have helped putting together this Collection.
Author Contributions
- Wrote the paper: JB KB EC DE PF JH RL UMP SW.
- 1. FAO. Global Forest Resources Assessment 2015. Rome: Food and Agriculture Organization of the United Nations, 2015.
- View Article
- Google Scholar
- 3. CBD. Global Biodiversity Outlook 4. Montréal: Secretariat of the Convention on Biological Diversity, 2014.
- PubMed/NCBI
- 28. Börner J, Vosti S. Managing Tropical Forest Ecosystem Services: An Overview of Options. In: Muradian R, Rival L, editors. Governing the Provision of Ecosystem Services. Studies in Ecological Economics. 4: Springer Netherlands; 2013. p. 21–46.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Philos Trans R Soc Lond B Biol Sci
- v.377(1849); April 25, 2022
Tropical forests in the deep human past
Eleanor m. l. scerri.
1 Pan-African Evolution Research Group, Max Planck Institute for the Science of Human History, Kahlaische Strasse 10, 07745, Jena, Germany
3 Department of Classics and Archaeology, University of Malta, Msida, Malta
4 Department of Prehistoric Archaeology, University of Cologne, 50931 Cologne, Germany
Patrick Roberts
2 Department of Archaeology, Max Planck Institute for the Science of Human History, Kahlaische Strasse 10, 07745, Jena, Germany
5 School of Social Sciences, University of Queensland, Brisbane, Australia
S. Yoshi Maezumi
6 Department of Ecosystem and Landscape Dynamics, Institute for Biodiversity and Ecosystem Dynamics, University of Amsterdam, 1098 XH Amsterdam, The Netherlands
Yadvinder Malhi
7 Environmental Change Institute, School of Geography and the Environment, University of Oxford, South Parks Road, Oxford OX1 3QY, UK
Associated Data
This article has no additional data.
Since Darwin, studies of human evolution have tended to give primacy to open ‘savannah’ environments as the ecological cradle of our lineage, with dense tropical forests cast as hostile, unfavourable frontiers. These perceptions continue to shape both the geographical context of fieldwork as well as dominant narratives concerning hominin evolution. This paradigm persists despite new, ground-breaking research highlighting the role of tropical forests in the human story. For example, novel research in Africa's rainforests has uncovered archaeological sites dating back into the Pleistocene; genetic studies have revealed very deep human roots in Central and West Africa and in the tropics of Asia and the Pacific; an unprecedented number of coexistent hominin species have now been documented, including Homo erectus , the ‘Hobbit’ ( Homo floresiensis ), Homo luzonensis , Denisovans, and Homo sapiens . Some of the earliest members of our own species to reach South Asia, Southeast Asia, Oceania and the tropical Americas have shown an unexpected rapidity in their adaptation to even some of the more ‘extreme’ tropical settings. This includes the early human manipulation of species and even habitats. This volume builds on these currently disparate threads and, for the first time, draws together a group of interdisciplinary, agenda-setting papers that firmly places a broader spectrum of tropical environments at the heart of the deep human past.
This article is part of the theme issue ‘Tropical forests in the deep human past’.
1. The tropics: a frontier for the deep human past
The perception that open grasslands and savannahs were the ecological ‘cradle’ of humans and their ancestors has shaped both the geographical context of fieldwork as well as dominant narratives concerning early hominin evolution, dispersal and cultural development [ 1 , 2 ]. By contrast, tropical forests, where fossil preservation tends to be poorer (e.g. [ 3 , 4 ]), have been presented as relatively pristine environments left free from human influence—habitats deemed too hostile for humans throughout much of prehistory (e.g. [ 5 ], see also [ 6 ] for overview). Indeed, they have often been framed as the primaeval environments we ‘escaped’ from in Africa, leaving behind the lineages of our close Great Ape relatives [ 2 , 7 ]. These attitudes have profoundly impacted narratives of human evolution in Africa and Out of Africa by introducing enormous biases in the construction of global human prehistory and palaeoenvironments. Such biases have meant that the palaeoanthropological record is fundamentally the human history of a narrow set of habitats, notably along coastlines and in open grassland settings, driving a circular argument that such places are the only areas worth investigating—at the expense of others. These settings and habitats have even been elevated to the status of adaptive cruxes, with ‘savannah corridors’ [ 8 ] or coastal ‘highways’ and refugia [ 9 , 10 ] being seen as critical to the cultural efflorescence and dispersal of our species.
As Homo species spread from Africa, they encountered and engaged with tropical forest biomes across South and Southeast Asia, the Pacific and ultimately, in the case of our own species, the tropical Americas ( figure 1 ). Despite popular perception of vast homogeneous green canopies, the tropical forests of these regions comprise an incredibly diverse set of ecosystems. Although wet, lowland evergreen rainforests are often seen as the classic manifestation of this habitat, ecologists have long noted the huge variety of tropical forests that exist on the planet [ 12 – 14 ]. Semi-evergreen forests with a short annual dry season, montane and sub-alpine forests, closed-canopy dry forests and swamp forests all have different characteristics, structures and species compositions that present a series of challenges and opportunities for hominin populations [ 15 ]. In many contexts, tropical forests form mosaic landscapes with open ecosystems such as lowland savannahs or montane grasslands. Furthermore, despite assumptions that tropical forests have been relatively unchanged, there is ample evidence that past fluctuations in precipitation, temperature and CO 2 concentration have impacted forest form and extent in different parts of the tropics throughout the Miocene, Pliocene, Pleistocene and Holocene [ 16 – 18 ]. As we will also see in this volume, the arrival of hominins, particularly Homo sapiens , into these forests may also have introduced further changes to fire dynamics [ 19 – 21 ], species composition [ 22 ] and structure [ 21 ]. Thus, while tropical forests can be defined as sitting between the latitudes of 23.5° N (the Tropic of Cancer) and 23.5° S (the Tropic of Capricorn), covering the tropics of Central and South America, western and central Africa, western India, Southeast Asia and Oceania, they are far from being homogeneous and, in the case of Australia and China [ 13 ], local edaphic and hydrological regimes have led to similar biomes straying beyond the astronomically defined tropics, as they have also done in the past [ 16 ]. Some authors refer here to megathermal forests, defined as forest biomes where the risk of frost damage is non-existent, enabling a proliferation of species diversity [ 16 ]. In warm periods of Earth history, such as the Eocene, such megathermal forests (functionally tropical forests) have extended to the latitudes of Canada and northern Europe.

Map of Late Pleistocene human dispersals showing the dates of earliest suggested arrival in the tropical forests of different regions. Green shading shows an artistic approximation of the current tropical forest distribution based on MODIS (moderate resolution imaging spectroradiometer) Land Cover MCD12Q1 majority landcover type 1, class 2 for 2012. Downloaded from the US Geological Survey Earth Resources Observation System (EROS) Data Center (EDC). See Roberts and Petraglia [ 11 ].
Far from being uninhabited by hominins, African tropical forest habitats seem to have been integral to our hominin ancestors [ 23 ], and Homo erectus notably reached Southeast Asia 1.2 million years ago (Ma), at a time when it has been argued that tropical forest was widespread ([ 24 , 25 ]—although see [ 26 ]). These environments likely formed at least part of the backdrop of local trajectories of evolution, as manifested in species such as Homo floresiensis and Homo luzonensis [ 27 – 29 ]. However, in the history of our genus, it was Homo sapiens that went on to most intensively inhabit and exploit tropical forests [ 6 , 15 ]. For many years, this was thought to have been a relatively recent chapter in the human story. Tropical forests were simply considered too hostile. In this view, the dense vegetation, cryptic fauna and sparsely distributed carbohydrates and fats in rainforests made these ecosystems too resource-poor for humans without recourse to sophisticated technologies, external support and exchange systems ([ 30 , 31 ]; see [ 32 ]). These views have markedly shaped palaeoanthropological research, particularly in Africa, by focusing fieldwork away from vast swathes of dense forest. Indeed, both ecologically and archaeologically, Africa's tropical forests remain the least well-investigated tropical forests in the world. Although anthropologists, human ecologists and archaeologists have repeatedly reiterated that hunter–gatherers can, and do, permanently live in tropical forests, including rainforests (e.g. discussions in [ 11 , 33 ]), they continue to be frequently neglected in deep time archaeological and palaeoanthropological discussions in Africa.
Instead, it is recent research in Asia that has transformed this field of research by firmly pushing back human exploitation and occupation of tropical forests well into the Pleistocene. Research on the island of Sumatra has found evidence for the presence of humans in rainforests dating to 73 thousand years ago (ka) [ 34 ]. In Borneo, a suite of behaviours including the processing of toxic plants, possible alteration of forest edges, and the hunting of forest arboreal fauna has been dated to around 45 ka [ 35 , 36 ]. Seemingly contemporaneously in Sri Lanka, specialist tropical forest adaptations at approximately 45 ka include the hunting of monkeys [ 37 – 39 ], with isotope geochemistry demonstrating a year-round dietary reliance rather than use as seasonal camps [ 38 , 40 ]. These discoveries confirm that intensive exploitation of forest resources has significant antiquity in the human past. Not only that, but they seem to confirm a new, unique ecological adaptability for H. sapiens which repeatedly made specialist niche expansions across a broad ecological spectrum well before the beginning of agriculture [ 41 ]. Similarly, in South America, humans seem to have occupied tropical lowland and montane forest environments soon after their arrival on the continent (12–14 ka). This appears to have initially taken place along river banks and drier fringes of the lowland and montane forest zone. However, within a few millennia, human occupation pushed deeper into the Amazon forest, primarily along river networks, although archaeological evidence may be biased to such accessible sites [ 19 , 20 ]. Human occupation modes ranged from hunting and gathering to agricultural systems which were based either on locally originated domestications such as manioc and squashes, or imported from Mesoamerica, such as maize.
Despite this growing body of research, however, many major questions remain concerning the deep human past in the global tropics: when did hominins first colonize different tropical forest environments and how did this impact evolutionary trajectories? How did diverse tropical environments drive ancient population structure and the emergence of our species? And finally, when did humans begin to significantly impact and alter tropical forests, and how? This volume draws together a set of state-of-the-art papers investigating these questions from around the global tropics. Starting in Africa, the birthplace of our species, they show that these ecosystems have shaped and been shaped by human agency for millennia. The contributions to this volume also highlight the ways in which diverse, and often novel, methodological applications, from geoarchaeology to isotope analysis, from new chronometric programmes to palaeoecology, are coming together to provide a richer picture of tropical human history.
2. African tropical forests
The tropical forests of Africa were the first to be encountered by H. sapiens and its hominin ancestors. Africa's forests have particular structural and floral characteristics including an unusually high biomass of animals, which could potentially act as a food resource for humans. Many areas of Africa's humid forests, for example, are sustained by relatively low rainfall that sits at the edge of rainforest viability, which means that even small changes in precipitation can drive dramatic forest fragmentation [ 17 ]. Throughout the Pleistocene and Holocene, it appears that many African forests have gone through periods of expansion and contraction as climatic conditions fluctuated, and often a mosaic environment of mixed forests and grasslands was the norm over much of the African tropical forest biome; over the prevailing glacial conditions of the Pleistocene, low humidity and carbon dioxide conditions mean that the overall extent of African forests was generally less than in the present. Tree species diversity in Africa is also lower than in Amazonia and Southeast Asian forests, but taller and larger trees mean that Africa's forests store more carbon than for example, Amazonian forests [ 42 ]. These tropical forests also often interdigitate with open grassland regions in a mosaic or patchwork that breaks down a simple dichotomy between open grassland and closed-canopy forest [ 43 ]. Such mosaic landscapes may have prevailed over much of the present forest zone throughout the Pleistocene and provided unique, and critical, opportunities for hominins.
The limited current evidence suggests that humans and their ancestors may have been taking advantage of ecotonal regions for a long time. A hominin tooth from Central Africa indicates that at least some early populations were living in mixed environments at the edges of forests around 2.5 Ma [ 44 ]. Later on in time, following the emergence of our species, the site of Panga ya Saidi in Kenya shows that humans were exploiting mixed tropical forest/grassland environments ca 78 ka, while producing symbolic materials and a variety of technological toolkits [ 45 , 46 ]. If Africa's internal regions hosted the bulk of human populations in the Pleistocene, environments that required humans to flexibly shift between diverse ecotones may have formed the cradle for our species' ecological modernity. In this emerging view, the reliance on different resources may have been the driver that set populations apart, rather than the environments themselves (e.g. [ 47 ]). These processes may sit at the root of our species, which is now thought to have evolved in subdivided populations across much of the continent [ 48 ]. When did this, and a hominin focus on tropical forest occupation, begin?
Braucher et al . [ 49 ] suggest a longer history than previously supposed. They report the oldest evidence of a hominin presence in the Congo Basin, with a minimum age of between 850 and 650 ka. Discovered in 1987, Elarmékora is a high terrace sitting above the Ogooué River within the Lopé National Park in Gabon. The authors present the first absolute dates for the small lithic assemblage found there, including mainly cobble artefacts embedded within alluvial material. Cosmogenic nuclide assessments suggest a minimum age of between 730 and 620 ka for the undiagnostic Earlier Stone Age assemblage. This age is among the oldest documenting a hominin presence in western Central Africa and confirms the long legacy of hominins in this region. These results indicate that the long-held assumption that a hominin presence in tropical forests only emerged following the arrival of agriculture should be rejected, and reorients geographical assessments of human dispersals in and beyond Africa.
This tantalizing picture of a long-term hominin presence in the tropical forest regions of Africa sits within a backdrop of 1 million years of dynamic climatic and environmental change. Here, Gosling and colleagues [ 50 ] synthesize information on Pleistocene and Holocene vegetation changes from long-term terrestrial and marine records, showing how the locations of vegetative resources for hominins shifted geographically over time (see also [ 51 ]). Of particular interest is the fact that the hominin presence in the Congo Basin described by Braucher et al . [ 49 ], coincides with generally humid conditions and therefore likely a period of forest expansion, rather than fragmentation. Furthermore, a profound shift in the hydro-climate in the last 1 Myr in Africa, leading to eastern and western parts of the forest zones being alternately wetter and drier, occurs at a time when the first fossil appearances of our species have been suggested elsewhere in Africa (e.g. [ 52 ]). For later time periods associated with H. sapiens , vegetative changes were clearly asynchronous in different regions, likely producing the conditions for mixed resource acquisition in many regions and necessitating adaptability.
Taylor [ 53 ] specifically pursues the question of mixed resource acquisition through Pleistocene material culture from the Middle Stone Age (MSA), the first and longest-lasting technological repertoire associated with our species. Specifically, he looks at the Lupemban, a stone tool (lithic) technocomplex that has long been associated with Africa's equatorial forests at the site of Kalambo Falls in Zambia. Here, the Lupemban has been best dated to between 270 and 170 ka. Today, Kalambo Falls is dominated by Miombo woodland, and Twin Rivers, another key Lupemban site, by open woodland-bushland. While both sites are just beyond current areas of forest, they may have been within forest zones in the past. Given the frequent interdigitation of open and closed environments in Africa's forests, Taylor argues that H. sapiens may have been adopting a flexible strategy within ecotonal areas that may indicate a partial reliance on forest resources. Taylor concludes that the lanceolate points of the Lupemban may have presented an adaptation to a vegetation mosaic that underscores a potentially unique human niche.
These results complement the work from Blinkhorn et al . [ 54 ] on the availability of refugia in tropical Africa. Refugia are places that remained stable and habitable through various cycles of climate change (see [ 55 ]). As the only continent where H. sapiens have clearly persisted through multiple glacial-interglacial cycles, Africa is a key area where classic refugia models can be formulated and tested. Blinkhorn et al . [ 54 ] apply climatic thresholds on human habitation, rooted in ethnographic studies, in combination with high-resolution model datasets for precipitation and biome distributions to identify persistent refugia spanning the Late Pleistocene (130–10 ka). Remarkably, Blinkhorn and colleagues find that refugia were unlikely to be rare phenomena during the Late Pleistocene, even using conservative estimates. One region that emerges as among the most stable is the modern-day Sene-Gambia region, where MSA assemblages have been remarkably persistent [ 47 , 56 ]. Blinkhorn and colleagues also highlight the broad distributions of stable ecotonal areas, which may have been critical for long-term human habitation [ 45 , 51 , 57 ].
Moving on in time, Orijemie [ 58 ] synthesizes past climatic variability in the forest of West-Central Africa during the Late Pleistocene–Holocene period to understand the interaction of climate on the development and stability of human communities in the region over time. Combining palaeoclimate and vegetation histories, Orijemie highlights the significance of climate variability on the development and survival of early hominin ancestors and humans in the forest regions of West-Central Africa. In response to major climatic fluctuations, West-Central African savannahs expanded at the expense of forests, but did not transit into strictly ‘forest’ or ‘savannah’ blocks. Rather, the forests had a variety of vegetation types and biodiversity ecotones, even during periods of environmental stress. These data suggest heterogeneous and resilient forest ecosystems. Human behaviours exhibited in the form of technological modifications and changes in subsistence strategies, varied independently of climate and vegetation changes, suggesting climate was not the prevailing driver of human behaviour or community stability.
This brings us to the present day, and Boyette and colleagues synthesize genetic, paleoclimatological, and historical linguistic data on the peopling of the Congo Basin and use this to build on their ethnographic work in the northern Republic of Congo with BaYaka foragers living along the Motaba river. They argue that the cultivation of ‘relational wealth’, that is, the forming of strong social ties to enable exchanges of resources and mutual assistance, is key to living in tropical forest environments. This currently includes the cultivation of such wealth among different forest forager groups as well as trading relationships with farmers. Here, Boyette and colleagues argue that it is a mistake to cast this trading as a dependence of foragers on farmers. The BaYaka are seasonally mobile with their own forest gardens, created using knowledge learned from farmers, as well as the creation of spaces for the growth of wild foods such as Dioscorea yams. They are also highly seasonally mobile, with some 82 km being the largest distance between where a parent was born and where their adult child now lives. Indeed, Boyette and colleagues argue that mobility is central to the flow of knowledge throughout the Congo Basin, including subsistence innovations and forest spirit dances. This complements the work of the previous papers that indicate that a high degree of mobility was always required to successfully live in this region. At the same time, Boyette and colleagues review the genetic studies that indicate that western and eastern branches of the forager populations split between 30 and 20 ka, probably following forest fragmentation well before the beginning of agriculture. This implies that significant breaks between different ecosystems may have been major boundaries in the past to populations either adapted to mixed resources or specific habitats.
3. Southeast Asian and pacific forests
Since no continuous tropical forest belt exists between the African and southern and eastern Asian forests ( figure 1 ), moving into other parts of the tropics must have involved repeated adaptation to varied tropical forest ecosystems. In fact, human groups expanding beyond Africa would have encountered significantly drier landscapes that spread into the Thar Desert of India before re-entering tropical zones again [ 59 – 61 ]. Once encountered, the Asian tropical forests presented a completely different set of floral and faunal characteristics compared to those in Africa. In contrast with Africa, Asian tropical forest extent was probably greater throughout the prevailing glacial conditions of the Pleistocene, as low sea levels greatly increased land area and connectivity in Sundaland and Sahul, while the generally maritime climate maintained high rainfall [ 62 ]. Moving into the tropical forests of Wallacea and the Pacific, humans would also have to contend with unique insular tropical ecosystems and the necessity of seafaring (see [ 63 ]).
It is in Asian tropical forests that archaeological and palaeoanthropological evidence began to highlight the critical role of tropical forests in early human adaptations and dispersals. Be it in Sumatra 73 ka [ 34 ], Borneo 50–45 ka [ 35 , 36 ], Sri Lanka 45 ka [ 37 , 39 , 64 ] and perhaps also southern China as early as 100 ka [ 65 ], human populations appear to have repeatedly adapted to tropical forest environments rapidly following their arrival in different parts of tropical Asia. These adaptations do not correspond to a constant wave, with uniform technologies, but rather highlight repeated, variable responses to different forest settings. For example, findings of the bow and arrow and clothing manufacture in Sri Lanka 45 ka [ 66 ] provide a very different context for this innovation than assumptions of its association with drying grasslands or European tundra conditions. Similarly, although the ‘Hoabhinian’ core and flake technologies found across much of Southeast Asia during the Late Pleistocene had been previously considered ‘simple’, more recent work and experimental analyses have highlighted the potential flexibility of these stone tools and their likely association with the manufacture of organic artefacts [ 67 ].
Understanding the exact context of human arrival in Southeast Asia has been plagued by issues of site and artefact preservation, correlation between hominin and palaeontological records, as well as issues with chronology construction. In this volume, Louys et al . [ 68 ] re-examine the fossil deposits of Lida Ajer in Sumatra which documents some of the earliest evidence for the presence of modern humans in tropical forests. Two human teeth from this cave were estimated to be 73–63 kyr old, which is significantly older than estimates of modern human migration out of Africa based on genetic data. The authors provide a new assessment of the available ages and stratigraphic information from the site, confirming its antiquity. The deposits were previously interpreted as rainforest based largely on the presence of abundant orangutan fossils, although their exact ecological preferences remained debatable. The use of stable carbon and oxygen stable isotope analyses of mammalian fossil tooth enamel further demonstrates that early humans likely occupied the site during marine isotope stage 4 (MIS 4; ca 74–60 ka) dominated by a closed-canopy forest very similar to those present in the region today, although the fossil orangutans appear to have occupied a slightly different niche in the rainforest than their modern counterparts.
Similarly, McAdams et al . [ 69 ] undertake geoarchaeological analysis of two archaeological cave sites in Vietnam. By MIS 3, it is clear that our species had dispersed throughout much of Southeast Asia, including the diverse forest systems of upland Vietnam. Here, wetter, sheltered conditions resulted in forest refugia that were attractive to early human populations, with the collection of diverse resources, such as land snails, providing resilience subsistence strategies. Nevertheless, the middens which record such evidence, and the caves in which they are formed, are subject to a series of unique diagenetic and site formation processes that need to be better understood to understand the nature and tempo of human adaptations and settlement patterns. McAdams and colleagues show how thin-section micromorphology is providing more refined insights into depositional and post-depositional sites across tropical zones, providing a basis for wider analysis of our species' interaction with tropical forests around the world.
Finally, moving out into the Pacific realm, Roberts et al . [ 63 ] present new radiocarbon and stable isotope data from the earliest human remains so far excavated in tropical island settings in Near and Remote Oceania. This is a key region for exploring early maritime crossings, human adaptations to insular and coastal environments, and the possibility of interactions between different hominin species. Roberts et al . [ 63 ] show that there is currently a significant gap between the earliest occupation of the portion of Near Oceania beyond the continent of Sahul approximately 45 ka and the oldest human remains from the region approximately 11.8 ka. However, the authors demonstrate that Late Pleistocene–Holocene humans living on islands in the Bismarck Archipelago and Vanuatu had a persistent reliance on tropical forest plants and animals. These habitats, rather than solely coastal settings and arriving domesticates, provided critical settings for human adaptation and landscape manipulation.
4. Neotropical forests
Current archaeological and genomic data suggest that the Americas were colonized sometime between approximately 25 and 15 ka by modern humans likely following the Pacific Rim corridor from northeast Asia into the New World, reaching southern Chile by ca 14.3 ka [ 70 – 72 ]. Early human populations in the Americas have traditionally been portrayed as mobile hunter–gatherers who exploited coastal resources and large savannah game, while avoiding forest habitats as a result of the absence of large mammals and the difficulties of mobility in dense forest vegetation [ 73 – 75 ]. Contrary to this classic paradigm, mounting evidence suggests early colonists were actively exploiting and managing trees of economic importance and quite quickly began practicing early cultivation of annual crops [ 76 – 83 ]. These data have important implications for understanding plant domestication, the long-term legacy of human–plant interactions and the potential role of humans in the current hyperdominance of useful plants in Amazonia [ 22 , 84 , 85 ].
In this volume, Bush et al . [ 19 ] and Nascimento et al . [ 20 ] synthesize paleoecological data to paint detailed pictures of the timing and ecological impacts of early human arrival in the tropical Andes and Amazon lowlands, respectively. In the Andes, the earliest evidence of human occupation occurs around 14–12 ka, coinciding with a time of rapid climate change as species were migrating upslope in response to deglacial warming. The retreat of the glaciers opened up the relatively flat and dry areas of the upper montane Andes (3000–4000 m elevation), and this region seems to have been among the most amenable American tropical regions for first human settlement (see also [ 86 ]). By 12 ka most areas now characterized as high elevation Andean grasslands ( puna and paramo ) were being burned and modified. Bush and colleagues suggest these extensive grasslands should be regarded as long-term anthropogenic Holocene landscapes, and likewise the sharp treeline between the forests of the Andean flank and the grasslands should be regarded as anthropogenic rather than climate-defined. These dense forests of the montane flank were probably less settled than the flatter and drier upland regions for both topographic and climate reasons, though by the mid-Holocene accessible regions of the montane forest zone were substantially modified and settled [ 19 ].
In the extensive Amazon lowlands, the first evidence of human occupation appears around 12 ka, located mainly along the Amazon river and the dry forest-savannah mosaic of the Amazon forest periphery. The more forested areas of southern Amazonia show signs of occupation from 6 ka, with substantial increase in range and density since 4 ka. By the time of European arrival, human occupation had spread across much of the Amazon biome, particularly along its river networks. The earliest human settlers of the Americas encountered continents rich in exotic and now-extinct megafauna, and this is likely true of the tropical Americas as much as for high latitudes. Overall, 34 out of 47 megafaunal species became extinct in South America [ 87 , 88 ]. These megafauna were undoubtedly in the savannah, Andean grassland and savannah-forest transition zones, but the direct evidence of megafaunal occupation of the dense forest zone (as occurs, for example, in African tropical forests) is limited and hampered by poor preservation. The direct cause of the extinction seems to be a confluence of rapid climate change putting wildlife populations under stress, coupled with human pressures through hunting and habitat modification adding additional pressure and preventing the recovery from refugia that occurred after previous periods of environmental variability.
By examining paleoecological evidence from lakes across the Andes, Bush et al . [ 19 ] describe the timing of this transition, with widespread demise of megafauna around 12.5 ka, soon after an increase of fire. They propose the megafauna were stressed by the rapid warming and wet conditions of the deglaciation and population recovery was prevented by hunters who transformed the high Andean landscape through burning. Iriarte et al . [ 89 ] present a compelling picture of this first encounter between Neotropical humans and megafauna, making a detailed case based on rock art found at Serranía de la Lindosa, Colombia, on the present-day ecotone between the northwestern Amazon forest and the Orinoco savannahs. They suggest that this art dates from the Late Pleistocene (around 12.6 ka) and among many other things depicts lost megafauna such as giant sloth (probably Eremotherium ), a camelid (possibly Paleollama ) and a three-toed ungulate (probably Xenorhinotherium ).
Human impacts on Neotropical forests also involved interaction with plant communities [ 90 ], and the region is home to the smallest temporal gap between human arrival and cultivation practices in the tropics. An independent Amazonian origin of agriculture has been a particularly significant discovery in recent years, with manioc ( Manihot ) and squash ( Cucurbita ) cultivation appearing on artificial forest islands in the seasonally flooded savannahs of Beni, Bolivia as early as 10.4 ka [ 78 ]. Cultivation dating to 9 ka also appears in the forest zone north of the savannahs [ 91 ], and there are signs of cultivation near campsites in northwest Amazonia [ 80 ]. In regions away from plant cultivation, early- to mid-Holocene foragers consumed palms, tree fruits and nuts [ 20 ]; many of these species are now hyperdominant in Amazonia and it has been suggested that the elevated abundance of these species across Amazonia may reflect selection and stewardship by indigenous populations over millennia [ 84 ].
The extent to which Amazonia is a cultural landscape with a significant long-term human footprint is still disputed, however [ 19 ]. Nascimento et al . [ 20 ] present an extensive paleoecological synthesis of the ecological effects of early human occupation of Amazonia. Significant vegetation changes are often argued to be found only centuries to millennia after the first signs of human settlement in forests, suggesting that the earliest occupants exerted only a gradual change on the forest. The dry forest-savannah zone seems to have been particularly favoured; as in Africa, this mosaic landscape provides a wide range of resources, and also the possibility of working with and enhancing natural fire regimes to aid vegetation clearance and ecosystem transformation. Maezumi et al . [ 21 ] examine the role of land use, cultural burning and soil enrichment in shaping the composition and structure of the Amazon forest ecotone. They integrate 6000 years of archaeological and palaeoecological data from Laguna Versalles, Bolivia which was dominated by stable forest vegetation throughout the last 10 000 years. These data document the management of forest composition and structure, cultural burning, cultivation of edible plants and the formation of anthropogenic Amazonian Dark (ADE) soils. Frequent cultural burning altered ADE forest composition and structure by controlling ignitions, decreasing fuel loads and increasing the abundance of fire-adapted plants.
With the expanding and varied record of human history in Neotropical forests now established, it remains to explore how human occupation of the varied habitats available, from seasonally dry forests to lowland rainforests, impacted patterns of human settlement, adaptation and culture. In this volume, Sales and colleagues use a statistical approach to explore the spatial distribution of Indigenous populations across the tropical Andes prior to European arrival. They note how variability in elevation, cloud frequency, river proximity and seasonal aridity may have significantly shaped human occupancy. Sales and colleagues present an estimate of the portion of this area occupied by pre-Columbian populations and note how a number of forest ecosystems still document anthropogenic influence centuries later. Further detailed investigations of the tropical forests of the Andes, and elsewhere in tropical North, Central and South America should enable a more detailed understanding as to the tempo and nature of repeated human adaptations to the tropical forests of this region through the Pleistocene and Holocene.
5. Synthesis
Tropical forests clearly represent a key human habitat that can no longer be ignored in the context of deep human history. In particular, the wealth of data, methods and insights emerging from tropical forests in Asia and South America is driving a tropical research agenda that has so far lagged somewhat behind in Africa, the evolutionary home of our species. What can be said so far, and what are the major future research questions and approaches ( figure 2 )?

The relationship between theory and research goals for understanding the role of tropical forests in the deep human past. (Online version in colour.)
Perhaps the most obvious outcome of increasing archaeological research in tropical forests is that we can no longer afford to think about them as peripheral areas to the main stage of human evolution and the early human past. Despite the persistence of various hypotheses tied to savannahs, grasslands and coasts across both the Old and the New World, humans are fundamentally plastic in their behaviour [ 92 ]. This plasticity is seen among earlier Homo species, as well as our own. As an extreme example, it is remarkable how humans adapted from being Arctic hunter–gatherers to Amazonian cultivators within a few millennia. It therefore seems unlikely that humans ever restricted themselves to any single narrow set of resources [ 41 ]. Indeed, it seems unlikely that the pan-African distribution of the MSA—the earliest and longest-lasting cultural phase associated with our species—was only ever present in grasslands and savannahs. Building on this, researchers must begin to abandon simple dichotomies between ‘rainforest’ and ‘savannah’ as mutually exclusive areas of human habitation.
Along a spectrum of adaptation, it may well be that various human groups found specialist solutions to their particular habitat of choice; however, in many cases specialization is likely found in the ability to remain flexible and exploit a range of habitats and their resources [ 41 ]. Indeed, it is the clear, repeated ability of our species to adapt in different ways to these habitats, among others, that might be what sets us apart from our closest relatives. As we have seen, for example in Africa, tropical forests are not themselves homogeneous blocks. Instead, forests for example, can interdigitate with clearings, drier forest types, palm swamps, gallery forests, grassy floodplains and savannahs that invite such flexible exploitation. To investigate this further, it seems clear that vast swathes of tropical forests remain to be investigated. Despite the emerging work in Southeast Asia and Amazonia, substantial areas remain near completely unexplored, particularly in Africa, for what they can say about the deep human past. What expectations should we have, and what methods should we be using?
The papers of this volume also highlight that many of the most recent advances in our understanding of early human encounters with tropical forests have involved the application of varied methodologies that cut across the social and natural sciences. Resolving the role of tropical forests in the deep human past is clearly a truly interdisciplinary endeavour, often involving ‘an archaeology of the invisible’. For example, traces of human activities may be found in the current distributions and community composition of wild plants and trees (such as palm nuts in the Congo Basin and brazil nuts in the Amazon Basin), in patterns of charcoal accumulation [ 93 ] and in alterations of soil composition in palaeoenvironmental cores and archaeological sites [ 83 ], and faunal communities [ 39 ] ( figure 2 ). The study of the growth rings of living trees (dendrochronology) has even been shown to track human management of forests in more recent periods [ 94 ]. In warm and wet ecosystems where organic preservation is low and sites difficult to find and locate, the traces of human impact on the environment may sometimes be the only evidence of past occupation. Stable isotope analysis of human tooth enamel has also emerged as a means of assessing overall dietary reliance in the face of incomplete plant and animal assemblages [ 38 , 40 , 63 ]. Such sensitive approaches must be combined with traditional archaeological investigations in order to fully appreciate the context of past human engagement with tropical forests.
When it comes to the archaeology of Pleistocene tropical forests, we should not necessarily always expect radically transformed stone tool types, but also more generalist and flexible tools capable of dealing with a dynamic contextual environment ( figure 3 ). In Africa, regionalization of the MSA may shed more light on the degree of isolation between groups rather than purely environmental determinants, and clearly a range of MSA tools can be used in a wide variety of contexts. Ubiquitous, generic elements of MSA toolkits are also found across Africa for over 300 thousand years, suggesting they met flexible and dynamic needs in a variety of environments. Indeed, examples from the rainforests of Southeast Asia suggest that specialist adaptations can be found beyond simply lithics, in the form of the development of organic tools involving bamboo and other materials, in the type of prey targeted, in possible trapping techniques that may leave no trace, and in the treatment of carbohydrates such as the detoxification of tubers [ 95 ]. Southeast Asian ‘Hoabhinian’ technologies (see [ 96 ]) may provide an interesting comparison to MSA technologies in West and Central Africa in future, in this regard, although lithics analyses have often retained a local and regional focus. Meanwhile, microliths and bone tools found in Sri Lanka, argued to be part of early bow and arrow technologies [ 66 ], indicate another route towards specialized tropical adaptation.

Conceptual figure of land use in: ( a ) hominins using the forest edge, ( b ) early humans exploiting forest resources, ( c ) specialized adaptations in the forests of Sri Lanka/South Asia and ( d ) polyculture agroforestry in Amazonia. (Online version in colour.)
When it comes to unravelling this global tropical record of human evolution, the unknowns are numerous. Despite the work done to date, we still often have no clear ideas of when H. sapiens first began to intensively exploit different types of tropical forests in a given region, and whether such behaviour can also be observed in ancestral species ( figure 3 ). We also do not know how this may have specifically been characterized. Did past Pleistocene forest foragers rely on high mobility and strong social networks? How did they navigate the forest, for example, using forest elephant trails in Africa, as well as the river networks that may have been key for human mobility in Amazonia? How may they have used the forest seasonally, for example, by controlling the distribution and location of preferred wild foods at certain times of year, such as the land snails of North Vietnam? Many of these questions have long been asked of Pleistocene sites in temperate Eurasia and southern and eastern Africa. However, a general absence of tropical forests in wider theoretical discussions in human evolution means these themes are only just starting to become accessible for these environments. At a broader level, it is still unclear whether tropical forests could sometimes still represent significant barriers, for example, driving population structure. How important were forest edges and ecotonal regions in human evolution?
Moving into the Holocene, the evidence is a little less sparse, but many questions remain. Many of the biases can also still be found. For example, research in forests continues to be dominated by geographic biases, for example, focusing on rivers or dry margins. In Africa, forest research in the Holocene still lags behind similar work in the Americas in particular. Yet the Late Pleistocene and Holocene also provide opportunities through which human adaptations to forests can be better understood, as there are multiple cases of human colonization of tropical forest environments that can be compared and contrasted. How was the ecology of the tropical forests that humans occupied in the Late Pleistocene and early Holocene different from those of the late Holocene, given changing atmospheric carbon dioxide and dynamic shifting mosaic landscapes? How did megafauna either hinder or facilitate forest occupation? How do biogeographical differences across the tropics, such as the relatively low-fruit abundance in the wind-dispersal-dominated dipterocarp forests of southeast Asia, affect how early humans used forest resources? And how may the long history of human occupation of these forests have also shaped the species composition of modern-day tropical forests?
The ‘big questions’ that remain are summarized in box 1 . Addressing these will require the continuation and expansion of foundational research across the global tropics, alongside the recognition that there is a whole spectrum of tropical forest habitats, not just ‘rainforests’. The pursuit of these goals will require the investment of funding agencies and a commitment of risk to further research. These are, after all, not the ‘well-trodden’ regions of grassland and savannah, where a wealth of previous discoveries robustly attests to future potential. In particular, funding for local researchers to lead multidisciplinary investigation of tropical regions will be essential to boosting tropical forest archaeological and palaeoanthropological research. Investment in tropical forests, and researchers within the tropics, will lead to a new and enriched understanding of the deep human past: the accumulation as well as the importance of evidence to date unmistakably supports this view. This volume represents a substantial step in furthering this goal and represents a call to scholars and funders alike to give new attention to how our collective human prehistory interweaves with this globally important ecological region.
Big Questions.
- 1. What is the time depth of human and even hominin engagement with tropical forests?
- 2. How many times did humans adapt to tropical forests?
- 3. How do repeated adaptations to tropical forests compare across the global tropical belt, and are they underpinned by any commonalities specific to these environments?
- 4. What was the speed of transition from dry/open to tropical forest environments, and how did forest-savannah transition zones act as entry points?
- 5. How can we characterize the dynamism of tropical forest climate and distribution throughout the Pleistocene, and which were the mosaics favoured by early humans?
- 6. Were dense tropical forests largely barriers or corridors?
- 7. How does the varying ecological biogeography of tropical forests affect how they have been used and stewarded by humans in the past?
- 8. Do mosaic forest environments generate new resources greater than the sum of forest and savannahs alone (e.g. edge specialist species)?
- 9. How have the diverse forests shaped adaptations, from foraging to agriculture, e.g. discussions of seasonal environments as critical to early tropical cultivation? How far does this hold, and what were the legacies of diminishing megafauna?
- 10. How has the long history of human interaction with tropical forests influenced the modern ecology and function of these forests?
Data accessibility
Authors' contributions.
E.M.L.S.: conceptualization, writing—original draft and writing—review and editing; P.R.: conceptualization, writing—original draft and writing—review and editing; Y.M.: conceptualization, writing—original draft and writing—review and editing; S.Y.M.: conceptualization, writing—original draft and writing—review and editing. All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
This theme issue was put together by the Guest Editor team under supervision from the journal's Editorial staff, following the Royal Society's ethical codes and best-practice guidelines. The Guest Editor team invited contributions and handled the review process. Individual Guest Editors were not involved in assessing papers where they had a personal, professional or financial conflict of interest with the authors or the research described. Independent reviewers assessed all papers. Invitation to contribute did not guarantee inclusion.
Open access funding provided by the Max Planck Society.
E.M.L.S. and P.R. thank the Max Planck Society for funding. P.R. is funded by the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement number 850709; PANTROPOCENE). Y.M. is supported by the Jackson Foundation. S.Y.M. was supported by the European Commission (Marie CurieFellowship 792197).
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 14 February 2024
Rainforest transformation reallocates energy from green to brown food webs
- Anton M. Potapov ORCID: orcid.org/0000-0002-4456-1710 1 , 2 , 3 ,
- Jochen Drescher ORCID: orcid.org/0000-0002-5162-9779 1 ,
- Kevin Darras 4 ,
- Arne Wenzel 5 ,
- Noah Janotta 1 ,
- Rizky Nazarreta 6 ,
- Kasmiatun 6 ,
- Valentine Laurent 1 ,
- Amanda Mawan ORCID: orcid.org/0000-0003-1820-7432 1 ,
- Endah H. Utari 6 ,
- Melanie M. Pollierer ORCID: orcid.org/0000-0002-1498-2362 1 ,
- Katja Rembold ORCID: orcid.org/0000-0001-9019-1530 7 , 8 ,
- Rahayu Widyastuti 9 ,
- Damayanti Buchori ORCID: orcid.org/0000-0002-2843-0737 6 , 10 ,
- Purnama Hidayat ORCID: orcid.org/0000-0001-9507-6275 6 ,
- Edgar Turner 11 ,
- Ingo Grass ORCID: orcid.org/0000-0001-7788-1940 12 ,
- Catrin Westphal 5 ,
- Teja Tscharntke 4 &
- Stefan Scheu ORCID: orcid.org/0000-0003-4350-9520 1 , 13
Nature volume 627 , pages 116–122 ( 2024 ) Cite this article
10k Accesses
142 Altmetric
Metrics details
- Ecological networks
- Ecosystem ecology
Terrestrial animal biodiversity is increasingly being lost because of land-use change 1 , 2 . However, functional and energetic consequences aboveground and belowground and across trophic levels in megadiverse tropical ecosystems remain largely unknown. To fill this gap, we assessed changes in energy fluxes across ‘green’ aboveground (canopy arthropods and birds) and ‘brown’ belowground (soil arthropods and earthworms) animal food webs in tropical rainforests and plantations in Sumatra, Indonesia. Our results showed that most of the energy in rainforests is channelled to the belowground animal food web. Oil palm and rubber plantations had similar or, in the case of rubber agroforest, higher total animal energy fluxes compared to rainforest but the key energetic nodes were distinctly different: in rainforest more than 90% of the total animal energy flux was channelled by arthropods in soil and canopy, whereas in plantations more than 50% of the energy was allocated to annelids (earthworms). Land-use change led to a consistent decline in multitrophic energy flux aboveground, whereas belowground food webs responded with reduced energy flux to higher trophic levels, down to −90%, and with shifts from slow (fungal) to fast (bacterial) energy channels and from faeces production towards consumption of soil organic matter. This coincides with previously reported soil carbon stock depletion 3 . Here we show that well-documented animal biodiversity declines with tropical land-use change 4 , 5 , 6 are associated with vast energetic and functional restructuring in food webs across aboveground and belowground ecosystem compartments.
Similar content being viewed by others

Silvopastoral systems and remnant forests enhance carbon storage in livestock-dominated landscapes in Mexico
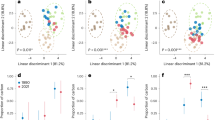
Climate warming restructures food webs and carbon flow in high-latitude ecosystems
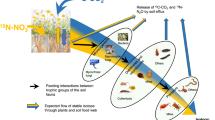
Intensive grassland management disrupts below-ground multi-trophic resource transfer in response to drought
Losses of biodiversity in terrestrial ecosystems have been documented across continents, biomes, clades and ecosystem compartments 1 . Tropical ecosystems are among the most threatened globally, with losses driven primarily by land-use change, such as the conversion towards commodity crops 2 . However, understanding of these transformations is hampered by the complexity and enormous biodiversity of tropical ecosystems. On first approximation, the spread of agricultural monocultures causes drastic declines in plant diversity in comparison to rainforests 4 . These effects cascade beyond basal trophic levels through food webs and also affect higher trophic-level invertebrate and vertebrate consumers 2 , 5 , 6 . Thus, to mechanistically understand the consequences of land-use changes for animal biodiversity and related functions, we need to know the resulting complex changes in food webs across multiple trophic levels and along different food chains.
Losses of animal diversity may be explained by reduced primary ecosystem productivity 7 and by changes in the structure of, and interactions in, consumer communities, as has been shown in studies on the impacts of invasive species, climate or other environmental changes 8 , 9 . Energy, as a common currency which sustains life 10 , can impose limits on the total number of species in an ecosystem 7 , whereas shifts in community structure can change energy pathways through ecological networks (energy flux), which is closely associated with the distribution of biodiversity across different trophic levels and ecosystem compartments 11 . For instance, under tropical land-use change, large declines in the number of species were correlated with a simultaneous reduction in total energy flux in litter invertebrate communities 12 , demonstrating that biodiversity loss is associated with a loss in available energy. In soil, however, a similar decline in biodiversity was not associated with reduced total energy flux but with a redistribution of energy across the food web 8 , 12 . This indicates that biodiversity loss is associated with exclusion of specific functional groups, rebalancing the system energetically. Disentangling total available energy changes from shifts in its distribution may help us to determine appropriate measures for restoration of ecosystem functioning.
The distribution of biomass and energy fluxes in terrestrial ecosystems is largely structured in ‘green’ (aboveground) and ‘brown’ (belowground) food-web compartments, which jointly shape ecosystem functioning and stability 13 . Redirection of energy across aboveground and belowground compartments is of interest to agricultural management, including, for example, nutrient availability 14 , yield 3 , 15 , soil carbon storage 3 and pest control 16 . However, despite close linkages of these two compartments by means of common primary producers (plant shoots and roots) and mobile animals, including generalist predators 17 , belowground and aboveground tropical food webs have been studied independently of each other and the distribution of energy across aboveground–belowground and invertebrate–vertebrate food webs has never been quantified. This non-integrated perspective hampers understanding of the consequences of conversion of rainforest into agricultural production systems on total animal energy flux and, accordingly, on animal biodiversity and ecosystem functioning.
Here, we quantified energy fluxes across earthworms, birds and arthropods in soil and canopies of tropical rainforests in Sumatra, Indonesia to describe the energetic structure of tropical animal food webs across aboveground and belowground ecosystem compartments. Our group selection represents most animal biomass in these systems (arthropods and earthworms) 18 , 19 , including ecosystem engineers (earthworms and ants) and animals at different trophic levels—from detritivores, microbivores and herbivores (various arthropod groups) to top predators (for example, spiders and birds)—thus reliably reflecting the composition of the food web as a whole. We further assessed changes in the energy flux distribution after rainforest transformation into plantation systems, including jungle rubber (selectively logged rainforest with planted rubber trees), as well as rubber and oil palm monoculture plantations, to show how altered land use changes the trophic functioning of aboveground versus belowground food webs. Our main hypothesis was that there are different keystone animal groups which channel most of the energy in rainforest and plantations and that energy distribution changes with land use: (1) across strata more energy is allocated to aboveground food webs in plantations because plantation management commonly aims to maximize aboveground production; (2) across trophic levels less energy is channelled to higher trophic levels in plantations because monocultures cannot sustain abundant and diverse predator communities; and (3) across resources at the base of the food web living plants are more important, whereas leaf litter is less important in plantations because of lower predation pressure, monodominant plant species and a reduction in litterfall. Such energy re-allocation is associated with changes in animal trophic functions across aboveground and belowground ecosystem compartments, with functional consequences at the ecosystem level.
To test our hypotheses, we estimated abundance and biomass of canopy arthropods using insecticide fogging, of birds using audio recorders and point counts and of soil arthropods and earthworms using high-gradient heat extraction from soil cores across 32 sites representing rainforests and plantations 20 . We linked collected body mass and biomass data to literature data on traits and feeding preferences of taxa to define 62 trophic guilds across all animal groups and to reconstruct food-web topologies at each site. We further used steady-state food-web modelling, which assumes that energetic demands of each trophic guild (including metabolic rate, losses during food assimilation and consumption by higher trophic levels) are compensated by energy uptake from lower trophic levels. Metabolic rates of each guild per biomass unit were estimated from body masses using metabolic regressions and multiplied by the observed biomasses. Resulting energy fluxes were used as quantitative measures of the distribution of energy and consumption of different resources (living plants, litter, bacteria, fungi, soil organic matter and other animals) in aboveground and belowground food webs 11 , 12 . We validate our results with another independent survey at the same sites (except jungle rubber) 4 years after the main survey, to prove the generality of our findings.
Aboveground and belowground rainforest food webs
We found that most of the energy in rainforests was channelled in belowground, rather than in aboveground, animal food webs. The total aboveground energy flux (sum of all energy fluxes to canopy arthropods and birds) was 21.6 ± 9.7 (1 s.d.) mW m −2 with a total fresh animal biomass of 0.8 ± 0.6 g m −2 , whereas the total belowground energy flux (sum of all energy fluxes to litter and soil arthropods and earthworms) was 295.8 ± 125.5 mW m −2 and the biomass was 9.5 ± 7.1 g m −2 (Figs. 1 and 2 ). These figures question the existing research focus on aboveground tropical food webs and animal biomass 21 . This energetic dominance of soil over canopy animals in rainforest is unexpected because about 95% of the energy channelled belowground is assumed to be processed by microorganisms 22 . The soil biomass numbers generally resembled those reported previously for animals in rainforests 8 , 22 but for canopy arthropods they were slightly lower 6 , 21 , 23 . Because canopy fogging may result in potential undersampling (suggested numbers span from twofold 21 to sixfold 23 ), we also ran a sensitivity analysis, assuming that canopy height affected the effectiveness of this method ( Methods ; Extended Data Fig. 2 ). This analysis suggested that the real energy flux aboveground (assuming uniform distribution of arthropods in canopies) could be 62.0 ± 24.5 mW m −2 in the most-severe undersampling scenario but could still not explain the 14-fold aboveground–belowground difference in energy flux we recorded. The belowground energetic dominance could be related to plant production, animal metabolism and resource quality: (1) tropical trees allocate twice as much produced organic matter belowground, in the form of litter and root biomass, as they store aboveground 3 , 24 ; (2) soil is inhabited by numerous small animals which have high metabolic rates per unit biomass 10 and together make up the biggest share of energy channelling across aboveground and belowground compartments; and (3) basal food resources belowground (litter and soil organic matter) are of poor palatability which results in a low assimilation efficiency. Thus, more resource consumption belowground than aboveground is needed to gain the same amount of energy 25 . This finding also indicates a perceived ‘biomass/energy flux—diversity discrepancy’ between aboveground and belowground tropical communities, with tropical canopies being extremely species-rich but having relatively low animal biomass and energy flux in comparison to soil and litter communities. However, very little is known about species diversity of arthropods in tropical soils 3 , 12 , 26 , so it is possible that biodiversity levels are much higher in rainforest soils than is estimated at present.
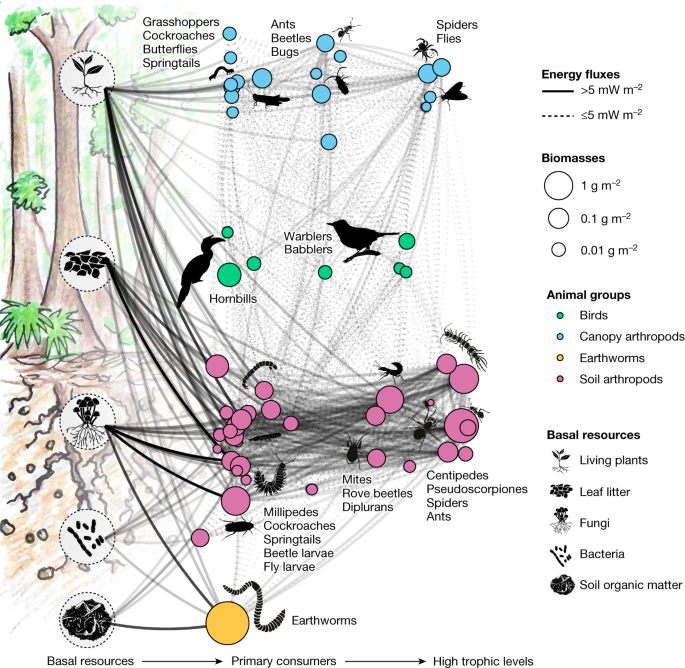
Connecting lines on the food-web diagram represent average energy fluxes. Fluxes are classified into strong (solid lines) and weak (dotted lines), on the basis of an arbitrary threshold of 5 mW m −2 . The opacity of the lines scales with flux values. Food-web nodes include basal resources (displayed with black drawings/diagrams on the left) and consumer trophic guilds (coloured points), grouped into canopy arthropods (blue), birds (green), soil arthropods (pink) and earthworms (yellow). Sizes of consumer nodes are proportional to node fresh biomasses (square root scale). Nodes are ordered horizontally according to the trophic position (continuous variable; nodes were slightly jittered to avoid overlaps but the general order remains) and vertically according to the ecosystem stratification (positions within the four major animal groups/colours are random). Exemplary dominant taxonomic groups in the major trophic levels (primary consumers, omnivores and primary predators, top predators) are shown with text. The scheme summarizes data across all rainforest sites ( n = 8). Illustrations of a plant seedling, litter, fungi, bacteria, soil organic matter, ant, spider, springtail, mite, diptera larvae, millipede, earthworm, centipede and bird were drawn by S. Meyer.
Source Data
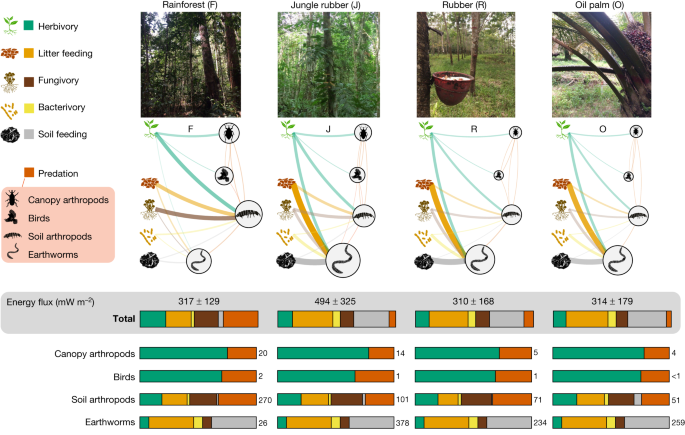
Food-web nodes include basal resources shown in different colours (living plants, green; plant litter, orange; fungi, brown; bacteria, yellow; soil organic matter, grey) and consumers merged into four major groups according to their ecological niches (canopy arthropods, birds, soil arthropods and earthworms). Sizes of consumer nodes are proportional to node biomasses. Connecting lines on the food-web diagram represent average energy fluxes, quantified in mW m −2 (represented by line thickness). Colours of energy fluxes reflect colours of the donor nodes and represent associated ‘trophic functions’: herbivory (on leaves or roots), litter transformation, bacterivory, fungivory, soil transformation and predation. Average trophic functions for each major group of consumers and for the food web in total are summarized as stacked proportional bar charts ( n = 8 sites per system). Estimated mean energy fluxes are shown with numbers to the right of the bars; total energy flux (sum of all fluxes) is given as mean ± 1 s.d. Illustrations of a plant seedling, litter, fungi, bacteria, soil organic matter, springtail, earthworm and bird were drawn by S. Meyer.
Rainforest canopy arthropods and birds
We found that arthropods dominated energetically over birds in rainforest canopies. Energy flux to canopy arthropods was 18.0 ± 9.7 mW m −2 , whereas birds contributed only 1.6 ± 1.9 mW m −2 (Figs. 1 and 2 ). The bird biomass estimate (0.3 g m −2 ) matches a previous detailed inventory in the neotropics 27 , suggesting that our estimates are realistic. As we did not measure contributions by other vertebrate groups (for example, bats and amphibians), we cannot be certain about the relative contributions of vertebrates versus invertebrates based on our data. However, including more vertebrate groups would also increase invertebrate energy flux, as many of them feed on invertebrates, making it unlikely that this would compensate for the 12-fold difference in energy flux we detected. Overall, it is evident that rainforest food webs are energetically dominated by invertebrates and are largely ‘brown’.
Keystone groups across land uses
We found strong community shifts in plantations in comparison to rainforest, which supports our main hypothesis that different taxa play key energetic roles in different systems (Extended Data Fig. 6 ). These shifts were not associated with total animal energy flux decline but mainly with its re-allocation. The total animal energy flux was similar in rainforest and monoculture plantations (310–317 mW m −2 ) and was about 50% higher in jungle rubber, although the variation was very high (the total system effect was not significant; Fig. 2 and Extended Data Table 1 ). Differences were strongest in earthworms, which were responsible for an average of 13% of the energy flux per site in rainforest (29.4 ± 37.1 mW m −2 ) but for 60–79% of the energy flux across plantations (group × system interaction χ 2 9 = 50.1, P < 0.0001; Extended Data Table 1 ). The high energy flux in jungle rubber may be explained by intermediate disturbance of the ecosystem combined with favourable conditions for earthworms (for example, higher pH due to liming and ashes after burning 8 ), which are able to exploit earlier accumulated soil organic matter as an extra resource and incorporate it into the food web (Fig. 1 ). The increase in the earthworm-associated energy flux was mirrored by a decline in the soil arthropod-associated energy flux (Fig. 1 ). It is known that earthworms may negatively affect soil and litter arthropods through direct (consumption of small fauna) and indirect trophic interactions and environmental modifications (litter removal and microbial feeding) 8 , 28 , but the arthropod decline may also have been a result of reduced leaf litter input and reduced soil organic carbon and nitrogen in plantations 29 . Energetically important arthropod groups in rainforest included springtails (12%), beetles (9%) and ants (7%; belowground food webs; Fig. 1 ), whereas in plantations they included springtails (3–5%), beetles (1–5%) and termites, symphylans, butterfly larvae, millipedes and dipterans, depending on specific ecosystem type (belowground food webs; Supplementary Table 1 ). These shifts illustrate different susceptibility of animal taxa to ecosystem transformation 30 , 31 . Tropical land-use change has been found to result in an 18–70% decline in species richness in arthropods, birds and other taxa 30 , 31 , 32 , 33 . Our findings show that this species decline is associated with fundamental changes in the energy distribution across food webs, rather than overall energy flux decline in converted tropical ecosystems.
Aboveground-to-belowground shift with land use
Plantation management commonly aims to maximize yield and associated aboveground production. Therefore, it is likely that energy flux will be higher in aboveground compared to belowground food webs in plantation systems. In support of this, a previous study found that biomass of canopy arthropods declined less than that of soil arthropods after rainforest transformation to oil palm monoculture plantations 6 . Thus, we initially proposed that belowground energy flux (sum of all energy fluxes belowground) would be stronger in rainforests, whereas aboveground energy flux (sum of all energy fluxes aboveground) would be stronger in plantations. However, contrary to our hypothesis, rainforest transformation resulted in a relative increase in belowground compared to aboveground fluxes. The belowground energy flux was higher than the aboveground in rainforest (about 14-fold) and this difference increased in jungle rubber (about 30-fold), rubber (55-fold) and oil palm monocultures (68-fold), with an even higher difference in biomass (Fig. 3a,b ; significant system:compartment interactions). This change in the ratios resulted from reduction of the total aboveground energy flux by −75% to −79% in both monoculture plantation types in comparison to rainforest (up to −92% considering potential undersampling of canopy arthropods; Extended Data Fig. 2 ), whereas belowground energy flux changed little. This change may be because of a delayed impact of land-use change on belowground compared to aboveground biodiversity, which could be explained by legacy effects due to the high inertia of soils 34 , for example, exploitation of earlier accumulated soil organic matter. The differing energetic responses of aboveground and belowground systems to land-use change in tropical landscapes echo the recently demonstrated differences in aboveground and belowground biodiversity responses observed in temperate grasslands 35 . This implies that such diverging responses might be universal, fitting the ‘green–brown imbalance’ hypothesis, which suggests a higher resistance of belowground than aboveground food webs owing to a lower number of specialized links in the former 13 (because of restricted mobility of organisms and thus a more opportunistic food selection). At present, belowground processes in plantations seem to be stabilized by earthworms which energetically compensate for losses in arthropod communities 36 . However, earthworms in plantations are mainly represented by invasive species 37 and their dominance reduces the entire food web to a detritus–microbe–animal or detritus–animal scheme. The number of trophic interactions in both aboveground and belowground webs in plantation systems decreased by 13% to 37%, reflecting reduced biodiversity aboveground and belowground (Fig. 3c ). Therefore, soil animal communities in plantations rely on fewer interactions (on average −21%), reflecting documented losses of biodiversity and multifunctionality 8 , 12 , 30 , 38 but nevertheless process a similar amount of energy as soil animal communities in rainforests. This demonstrates a remarkable adaptability of belowground food-web functioning to perturbations 35 .
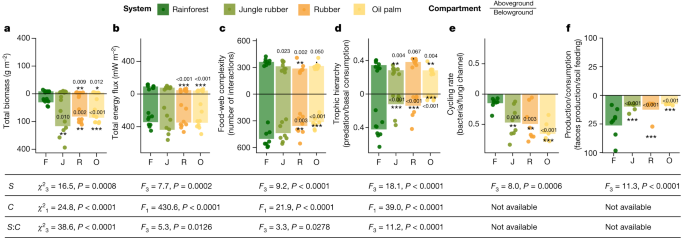
a – d , Bulk indicators were calculated separately for aboveground (canopy arthropods and birds, above the zero line) and belowground food webs (soil arthropods and earthworms, below the zero line) for total biomass ( a ), total energy flux ( b ), food-web complexity ( c ) and trophic hierarchy ( d ). Trophic hierarchy was calculated as the ratio of all ‘predatory’ energy fluxes to all ‘basal resource consumption’ energy fluxes. e , Carbon cycling rate was calculated as the ratio of all outgoing fluxes from bacteria to all outgoing fluxes from fungi. f , Carbon balance was calculated as the ratio of all produced faeces (unassimilated food) to all outgoing fluxes from soil organic matter. Each point is a site, bars represent means ( n = 8 sites per system). Colours denote land-use systems (dark green, rainforest; light green, jungle rubber; orange, rubber; yellow, oil palm). Units for each parameter are given in brackets; note square root scale in a , b and f . Asterisks mark significant differences of mean values for the given parameter aboveground or belowground from that in rainforest (generalized linear mixed-effects models; two-tailed *** P < 0.001, ** P < 0.01, * P < 0.05). Effects of land-use system ( S ) and aboveground/belowground ecosystem compartment ( C ) and their interaction ( S : C ) on the tested parameters are given below the corresponding bar charts. F, rainforest; J, jungle rubber; R, rubber; O, oil palm.
Predation decline in plantations
It has been suggested that diverse plant communities avoid resource concentrations and promote nutrient heterogeneity, which prevent (specialized) herbivores from being very abundant; at the same time, diverse plant communities provide greater refuge and resources for (generalist) predators than do monocultures, which jointly sustain higher predation-to-herbivory rates 16 , 39 . Indeed, previous studies have shown that proportionally less energy flows to predators in soil and litter food webs in plantations than in rainforests 8 , 12 . Thus, we also suggested that predation to primary consumption rates would be lower across aboveground and belowground food webs in plantations than in rainforest. In agreement with this, the predation/consumption ratio declined by 18% aboveground and by up to 90% belowground with rainforest transformation to jungle rubber and oil palm. However, in monoculture rubber plantations the proportion of predation in canopy and soil arthropods (but not in birds) was similar or even slightly higher than that in rainforest (increase of 11% aboveground; Fig. 3d ). High predation in rubber canopies might be associated with a simple canopy structure 40 but this does not explain low predation in oil palm. Because the high predation in rubber canopies was mainly associated with a large biomass of blood-sucking gnats and mosquitoes, it may be explained by the presence of small water bodies (rubber sap collection buckets) in rubber plantations which can host aquatic dipteran larvae. The different effects of oil palm and rubber cultivation on relative predation suggest that tropical land-use choices can have a predictable impact on specific food-web functions. Our results illustrate that decline in predation is a common trend across aboveground and belowground compartments and taxa with agricultural transformation 8 , 12 . Agroecosystems often have a weaker natural control of pests in comparison to more natural ecosystems 41 , which may partly explain pest outbreaks in plantation systems such as oil palm 42 . Reduced natural pest control in oil palm is also supported by a lower predation-to-herbivory ratio (0.37 ± 0.16 in birds, 0.28 ± 0.05 in canopy arthropods and 1.14 ± 0.63 in soil arthropods) in comparison to rainforest (0.64 ± 0.29 in birds, 0.34 ± 0.05 in canopy arthropods and 1.95 ± 0.74 in soil arthropods).
Changes in belowground carbon cycling
We classified non-predatory energy fluxes according to five major basal resource classes, corresponding to the ‘trophic functions’ of herbivory, litter feeding, fungivory, bacterivory and soil feeding (Fig. 2 ) 43 . We proposed that the dominant trophic functions would change with land use, indicating different carbon pathways at the ecosystem scale—specifically, we expected proportionally higher use of primary basal food resources, especially living plants, in plantations, resulting from a decrease in alternative resources, such as microbial biomass and leaf litter 44 . We found that land-use change to plantations consistently altered energy distribution at the base of food webs by reducing total herbivory and fungivory, while increasing bacterivory and soil feeding (function × system interaction χ 2 15 = 111.1, P < 0.0001; Fig. 2 and Extended Data Table 1 ). We recorded a 3.2- to 4.4-fold increase in bacteria/fungi energy flux ratio across plantation systems (Fig. 3e ). This increase was explained mostly by the high abundance of earthworms in plantations, which can effectively assimilate bacterial carbon from old soil organic matter 45 . However, an almost twofold increase in bacteria/fungi energy flux ratio was also observed in soil arthropods in oil palm monocultures (Fig. 2 ). These results are in line with previous studies showing that disturbance associated with agriculture and high fertilization rates may change the balance from slow (for example, fungal) to fast (for example, bacterial) energy fluxes in soil food webs 15 , 46 . At the same time, these results are in contrast to the existing evidence of higher bacteria consumption by soil animal communities in rainforests, as indicated by bacteria-specific fatty acid biomarkers 47 . However, the same study reported an increase in non-specific bacterial biomarkers 47 . The likely increase in bacterivory therefore indicates that there is accelerated energy processing (faster turnover rates) in these systems. A shift from the naturally observed balance to food webs dominated by fast energy channelling may make the system more susceptible to perturbations (resulting from an increase in strong interactions 48 ) and may accelerate depletion of carbon stocks 15 ; the latter has been observed in rubber and oil palm plantations 3 . This depletion is associated with high soil feeding by earthworms, which can effectively use old soil carbon resources 49 . However, the net effect of earthworm feeding activity on carbon sequestration and emission remains a controversial topic in soil ecology 50 , 51 . To quantify animal effects on soil carbon stocks, we here calculated the ratio between the production of faeces (unassimilated food) and the consumption of soil organic matter by all soil invertebrates. It has been shown that conversion of plant materials into faeces by soil invertebrates increases microbial biomass production 52 , which is the key process contributing to soil organic matter formation and stabilization 53 . In turn, invertebrates are able to mobilize and recycle this stored carbon while feeding on bulk soil. Supporting the link between the belowground food-web structure and net carbon loss in plantations, we found that the production-to-consumption ratio decreased by more than 75% from 27.6 ± 29.6 in rainforest to 3.8 ± 2.9 in jungle rubber, 6.2 ± 10.4 in rubber and 2.3 ± 0.3 in oil palm plantations (Fig. 3f ). Overall, our analysis suggests that changes in energy flux distribution due to habitat transformation have large functional consequences for carbon cycling. However, the exact mechanisms involved and quantification of these animal effects over time requires dynamic ecosystem-level modelling and targeted experiments.
Methodological caveats
There are few empirical studies on tropical invertebrate food webs and food-web analysis can be sensitive to assignment of trophic guilds and interactions 54 . Here, we based our reconstruction on a recent review 55 and empirical data collected from our study sites 36 , which make our food webs as close to reality as possible at the current state of knowledge. Sensitivity tests of our food-web reconstruction model revealed feeding specialization/omnivory as the main characteristic affecting absolute estimates of belowground-to-aboveground energy balance but none of the possible coefficients affected our conclusions (Extended Data Fig. 1 ). Our aboveground energy flux estimates could also be biased because we did not sample all vertebrate animal groups. Amphibians, reptiles, bats and other mammals are important invertebrate predators in tropical rainforests. However, as discussed above, this is unlikely to change our conclusions which are based on more than tenfold differences in energy fluxes, with the same applying to the potential undersampling of canopy invertebrates (Extended Data Fig. 2 ). Finally, our plantation systems were 14–18 years old and were unlikely to be at a stable state, especially considering higher rates of change in the aboveground than in the belowground ecosystem compartments. We therefore call for studies evaluating tropical land-use systems in the longer term. To prove the generality of our findings, we performed another survey at the same sites (except jungle rubber) in 2016–2017. This validation survey showed lower estimates of the absolute biomass and energy flux but validated energetic dominance of the belowground over the aboveground energy flux, canopy arthropods over birds, energetic decline in canopies, re-allocation of energy to belowground food webs in plantations and shifts in trophic functions, such as an increase in bacteria-to-fungi and a decrease in faeces production-to-soil consumption ratios. However, it did not validate the general loss of trophic links across aboveground and belowground compartments (Extended Data Figs. 5 , 6 and 7 ; Supplementary Notes ). Potentially, some trophic links were restored as plantation aged (from about 15 years old in the main survey to about 19 years old in the validation survey) but future plantation replanting (normally done at 25 years) will probably result in a second wave of biodiversity decline 56 , which may lead to further food-web disassembly. Overall, it is clear that our assumptions and approaches do not affect our main conclusions.
Conclusions
Our study provides an energetic description of tropical rainforest and plantation food webs across aboveground and belowground compartments, demonstrating generalities of land-use effects previously observed only in temperate ecosystems. In addition, we report new and nuanced patterns of food-web responses depending on specific land uses and ecosystem compartments. Overall, we conclude that (1) rainforest animal communities are energetically dominated by arthropods in belowground food webs; (2) animal communities in tropical canopies suffer higher total energetic losses due to rainforest transformation than those in belowground food webs but the energy in belowground food webs in plantations is reallocated from functionally diverse arthropod communities to invasive earthworms 8 ; (3) land-use change is associated with a decline in predation and an increase in relative herbivory both aboveground and belowground in jungle rubber and oil palm, however, the high predation in rubber suggests that crop choices can have predictable outcomes for trophic functions in food webs; and (4) belowground food webs in plantations rely on different basal resources than those in rainforest, promoting faster energy channelling and shifting carbon balance from production of faeces to consumption of soil organic matter. These changes are associated with previously observed depletion of carbon stocks 3 but the mechanisms driving animal effects in this context remain to be tested experimentally.
It is well documented that tropical land-use change results in animal biodiversity losses both aboveground and belowground 30 , 31 . We show here that biodiversity losses are associated with changes in food-web structure, consumption of different pools of organic matter and energy fluxes and these changes are distinctly different between the aboveground and belowground realm. We suggest that restoration and management practices in the tropics which alter the energetic balance across ecosystem compartments, taxa, size classes and trophic levels, need to be more closely considered and trialled. Plantations, especially oil palm, are very productive 3 but the available energy for maintaining multitrophic biodiversity is disproportionately low, which is associated with re-allocation of energy fluxes to basal trophic levels in belowground food webs. The high total energy flux indicates that energy is not a limiting factor for animal biodiversity in plantations and restoration measures should focus on other ecosystem aspects. Improving belowground habitat structure through mulching 38 , 57 and reducing herbicide use 58 could be sufficient to partly restore soil biodiversity and energetic balance in belowground food webs. However, it may take time for the effects of these measures to become visible as a result of high historical inertia of the soil system. Aboveground, measures directly affecting vegetation are needed. For example, increasing canopy complexity by planting trees in monoculture plantations 59 , 60 and designing diverse landscapes 30 could provide more ecological niches, probably resulting in re-allocation of more energy to aboveground food webs. In the absence of restoration measures, intensive tropical land use may foster earthworm invasion belowground, further depletion of soil organic stocks and increase risks of aboveground pest outbreaks. This is likely to result in intensification of fertilizer, herbicide and pesticide use. Experimental studies exploring the effect of restoration measures on the energy distribution and trophic functions of food webs across aboveground and belowground compartments of tropical ecosystems will be crucial for better management of the energy of tropical ecosystems, to sustain tropical biodiversity and ecosystem services.
Study region and design
The study was carried out in Jambi Province, Sumatra, Indonesia in the framework of the Collaborative Research I 990 ‘EFForTS’ 20 . Over the last few decades, lowlands in this region have experienced drastic land-use change from rainforests to smallholder-dominated cash crop agriculture of mainly rubber ( Hevea brasiliensis ) and oil palm plantations ( Elaeis guineensis ) 30 . We studied four common land-use systems: primary but slightly degraded lowland rainforest 61 , jungle rubber, rubber monocultures and oil palm monocultures. Forest plots were located in the Bukit Duabelas National Park and the Harapan Rainforest Restoration concession (PT REKI) and had a 90th percentile tree height of 29.5 m and tree density of 556 trees ha −1 (diameter at breast height ≥ 10 cm). Jungle rubber plots represented an extensively managed agroforest system, which is established by planting rubber trees into secondary or disturbed forest and had a 90th percentile tree height of 21.0 m and tree density of 580 trees ha −1 . Rubber and oil palm monocultures represented smallholder plantations, often with intensive management (fertilizers and herbicides) and a 90th percentile tree height of 17.0 and 12.7 m and tree density of 467 and 138 trees ha −1 , respectively. At the time of the main survey (May–November 2013), the age of all monoculture plantations was between 8 and 17 years. In total, 32 sampling sites were surveyed in an area of about 80 × 80 km spanning two regions (with loamy Acrisol and clayey Acrisol soils) 62 ; each land-use system was replicated eight times, four in each of the two regions 20 , 30 . Statistical methods were not used to predetermine the sample size, no blinding and randomization were used. Each plot measured 50 × 50 m and had five permanent 5 × 5 m subplots. More information is provided in the introductory EFForTS paper 20 . In each of the 32 plots, we applied a combination of collection methods to assess bird, canopy arthropod, soil arthropod and earthworm communities. Our assessment is a snapshot which cannot represent all animal species at the study sites. However, the functional composition of communities is typically more stable than the species composition 38 ; that is, despite species turnover, different species will perform similar roles in the food web. This turnover, however, is expected to be moderate because of a limited seasonality at the study region, with a rainier period during December–March and a dryer period during July–August 20 . Although we were not able to fully cover the spatial heterogeneity in each plot, our sampling design compensates for this with true replication of n = 8 plots per system. To account for the temporal variation, validate results of the main survey and prove the generality of our findings, we did another independent survey with the same approach at the same sites (except jungle rubber; that is, 24 plots) in 2016–2017. Data from both surveys were processed in the same way to reconstruct food webs across aboveground and belowground compartments 43 . To do this, we estimated densities and biomasses of taxa, classified trophic guilds and assigned body masses, habitat preferences and feeding preferences to each guild 43 . Feeding preferences at the base of the food web were assigned to the five major basal resource classes 43 : living plants (leaves and roots), leaf litter, fungi, bacteria and soil organic matter (dead organic matter mainly associated with the mineral soil fraction). The biomass of basal resources was not used in the food-web modelling because we focussed on consumption/energy flux 63 .
Birds were sampled with point counts as well as automated sound recordings from May to July 2013. All plots were visited three times for 20 min point counts. The observer stood in the plot middle and all birds detected in the plot were recorded. Point counts took place between 6:00 and 10:00 and the timing for individual plots alternated between early and late morning 32 . We excluded detections from fly-overs and bird vocalizations that could not be identified immediately were recorded using a directional microphone (Sennheiser ME-66) to compare with recordings from the xeno-canto online bird call database ( http://xeno-canto.org/ ). In addition to point counts, we recorded stereo sound at 44,100 Hz sampling frequency (SMX-II microphones, SM2+ recorder, Wildlife acoustics); the recorders were attached to the central tree of the plot at 2.0–2.5 m height. We recorded sound in eight plots simultaneously; sampling all 32 plots took 4 days (10 and 13 May and the 3 and 7 June 2013). We uploaded the first 20 min of recording after sunrise to the online eco-acoustics platform BioSounds 64 so that two independent ornithologists could identify all audible bird calls and calls visible on the spectrogram (within an estimated 35 m radius) to species. For each plot, bird species identified by both ornithologists in the recordings were subsequently merged with the species obtained from the point counts to generate the dataset used in the analysis. In total, 418 bird occurrences were detected in 2013 and 542 in 2016 (validation survey). Guilds were defined on the basis of feeding preferences of species (five levels: fruits and nectar, plants and seeds, invertebrates, vertebrates and scavenging, omnivores), spatial distribution (canopy, ground foraging or both) and body masses; following information obtained from a public database 65 . In total, 11 guilds were distinguished (raw data are available from figshare; Data availability).
Canopy arthropods
Canopy arthropods were collected by fogging (the application of a knockdown insecticide) in three locations per plot between May and October 2013 (main survey) and 2017 (validation survey). Target locations were randomly positioned in the plot; fallen trees and canopy gaps were avoided. Fogging was conducted immediately after sunrise, in dry conditions to avoid small arthropods sticking to precipitation. A mixture of 50 ml of DECIS 25 EC (Bayer Crop Science, deltamethrine 25 g l −1 ) and 4 l of petroleum white oil was applied to each target canopy, about 20 min per fogging event. Underneath each target canopy, square 1 × 1 m funnel traps were placed at about 1.5 m above ground level using ropes and each funnel was fitted with a 250 ml plastic bottle containing 100 ml of 96% ethanol. Sixteen funnels were used during the main survey in 2013, whereas eight funnels were used for the validation survey in 2017. Two hours after the application of the insecticide, stunned or dead arthropods were collected and cleaned from debris, the ethanol was exchanged and the samples were stored at −20 °C until further analysis. The data used in this study were based on combined abundances of canopy arthropods across the three subsamples per plot, resulting in one abundance value per plot. More details on the sampling are provided elsewhere 66 . Overall, 366,975 individual canopy arthropods were collected during the main survey and 179,334 during the validation survey. Arthropods were then sorted to 12 major arthropod orders (Acarina, Araneae, Blattodea, Coleoptera, Collembola, Diptera, Hemiptera, Hymenoptera, Lepidoptera, Orthoptera, Psocoptera and Thysanoptera). As large flying taxa such as Apoidea and Vespoidea in part actively evaded the insecticide fog at the time of application (J.D., personal observation), the order Hymenoptera in this study is represented by Formicidae (ants) and Braconidae (a family of parasitoid wasps), both of which were highly abundant in the samples 66 , 67 . Also, four abundant beetle families with contrasting feeding strategies were analysed separately from the rest of the order Coleoptera (henceforth termed ‘other Coleoptera’)—Chrysomelidae, Curculionidae, Elateridae and Staphylinidae. Arthropod taxa listed above were used as trophic guilds (17 in total), each assigned with feeding preferences to living plants or other invertebrates and vertebrates according to existing literature 55 and unpublished data on stable isotope composition measured in the collected animals. We extrapolated general knowledge on the trophic ecology of high-rank taxa (for example, Chrysomelidae are herbivores whereas Staphylinidae are predators) to all collected individuals in these taxa assuming phylogenetic signal in trophic niches and because information on the feeding preferences of most tropical invertebrate species is lacking. Average body mass of each guild at each plot was estimated using group-specific length–mass regressions 63 ; body lengths were measured for all animal groups in each sample (up to ten random individuals per sample per group to estimate the mean). Density of canopy arthropods per square metre was calculated by dividing the total abundance of collected arthropods by the number of traps used. Detailed biodiversity declines over the investigated land-use change gradient are published for arboreal communities of ants 67 , beetles 68 , springtails 69 , spiders 33 and parasitoid wasps 66 .

Soil arthropods and earthworms
Soil invertebrates were collected using a high-gradient extraction method. In each plot, three soil samples were taken (one in each of three subplots) during October and November 2013. Samples measured 16 × 16 cm and comprised the litter layer and the underlying mineral soil layer to a depth of 5 cm. Litter and soil were extracted separately but merged in the food-web analysis. Animals were extracted from litter and soil for 6–8 days under a heat gradient from 40 to 50 °C above the sample to 15 °C below the sample and collected in dimethyleneglycol:water solution (1:1) and thereafter transferred to 70% ethanol. More details on the sampling and extraction procedure are given elsewhere 70 . In total, 29,956 soil invertebrate individuals were collected in 2013 and 50,401 individuals in 2016 (validation survey). The lower total number of collected individuals in 2013 is because mites and springtails were counted in only two out of three samples per plot. Collected animals, including earthworms were sorted to high-rank taxa (orders and families) under a dissecting microscope, allowing allocation to trophic guilds 55 . Soil invertebrate taxa are generally consistent in their trophic niches 71 . However, to reflect widespread omnivory, most of them were assigned to feed on multiple basal resources (living plants, litter, bacteria, fungi, soil organic matter and other invertebrates) on the basis of existing knowledge 55 and stable isotope composition previously measured in the collected animals 36 . Average body mass of each guild at each plot was estimated using group-specific length–mass regressions 63 , 72 , 73 , 74 , with body lengths measured from all individuals in each sample (for ants and symphylans we measured only the first ten individuals per sample). Vertical distribution across soil, litter and ground for each trophic guild of soil arthropods was estimated using the relative abundance of this guild in litter (litter and ground layers) or soil 10 . In total, 33 guilds of soil arthropods and one guild of earthworms were distinguished (Extended Data Fig. 6 ). Density of soil invertebrates per square metre was calculated by recalculating the abundance from the sample to the metre scale.
Food-web reconstruction
All data manipulations and statistical analyses were done in R v.4.2.0 with R studio interface v.1.4.1103 (RStudio, PBC). We used a ‘multichannel’ food-web reconstruction approach 43 . We combined all trophic guilds across birds, canopy and soil arthropods and earthworms into a single table which included the following traits of each guild: feeding preferences to plants (including phototrophic microorganisms and endophytic/epiphytic microorganisms aboveground), litter, fungi, bacteria, soil organic matter or animal food (predation on invertebrates or vertebrates), mean body mass, body mass variation (standard deviation), biomass per square metre and spatial niche (soil, litter, ground and canopy). The table was complemented with published information on protection traits and C and N content for each guild 55 (full data with all traits are available from figshare; Data availability). Because species-level biology of tropical invertebrates is poorly known and we did not have species-level information for about 50% of the studied arthropods, traits were assigned to supraspecific taxa assuming their general trophic and functional consistency 71 . Generic rules of food-web reconstruction based on food-web theory were used to infer weighted trophic interactions among all nodes with the following assumptions 43 : (1) there are phylogenetically inherited differences in feeding preferences for various basal resources and predation capability among soil animal taxa which define their feeding interactions (reflected as resource preferences in the raw data table) 55 ; (2) predator–prey interactions are primarily defined by the optimum predator–prey mass ratio (PPMR) 75 , 76 —typically, a predator is larger than its prey but certain predator traits (hunting traits and behaviour, parasitic lifestyle) can considerably modify the optimum PPMR 43 . We measured body mass distribution overlap for each potential pair of predator and prey in each food web to determine the most plausible trophic interactions; (3) strength of the trophic interaction between predator and prey is defined by the overlap in their spatial niches related to vertical differentiation, with greater overlap leading to stronger interactions (no overlap among specialized canopy and soil arthropods and full overlap between ‘canopy’ birds and arthropods collected using canopy fogging); (4) predation is biomass-dependent 77 —because of higher encounter rate, predators will preferentially feed on prey that are locally abundant; and (5) strength of the trophic interaction between predator and prey can be considerably reduced by prey protective traits—prey with physical, chemical or behavioural protection are consumed less 78 . All these assumptions are applied together to infer the most plausible trophic interaction matrix. For example, feeding preferences of omnivorous nodes to basal resources or other invertebrates were assigned on the basis of literature (assumption 1), whereas prey selection among other invertebrates was based on size, spatial niche, total biomass and protection of prey (assumptions 2–5). The reconstruction R script is available from figshare (Code availability). Food-web reconstruction was carried out separately for each plot; collected data were averaged across subplots. Plots were assumed to represent local food webs and were used as biological replicates in statistical analyses (Extended Data Figs. 3 and 4 ).
Energy flux estimation
To calculate energy fluxes among food-web nodes we used reconstructed interaction networks, biomasses, body mass-dependent metabolic losses and environmental temperature and applied the fluxweb package 77 . In brief, per-biomass metabolic rates were calculated from average fresh body masses using the equation and coefficients for corresponding phylogenetic groups of invertebrates 13 , 79 and endothermic vertebrates 12 (the used metabolic regressions typically have R 2 > 95% if calculated for a wide range of body masses; Extended Data Table 2 ). The mean annual soil temperature was taken from meteorological measurements at our study sites (forest 25.0 °C, jungle rubber 25.6 °C, rubber and oil palm 26.1 °C) 80 . The energy flux to each node was calculated from per-biomass metabolism, accounting for assimilation efficiencies (proportion of energy from food that is metabolized by the consumer) and losses to predation assuming a steady-state energetic system (energetic losses from each node are compensated by the lower trophic levels; for example, if herbivores are present in the system there is enough plant biomass to sustain them) 13 , 81 . Although the steady-state assumption is unlikely to be fully supported in most real-world ecosystems, this assumption allows for comparison of dominant energy processes across different ecosystems that are stable at the time of consideration (years) and thus was appropriate for our aims. We used diet‐specific assimilation efficiencies which we calculated from nitrogen content of each prey/basal resource node using a published equation 25 . Assimilation efficiencies for basal resources were calculated as 21% from plant material, 18% from leaf litter, 13% from soil organic matter, 96% from bacteria and 36% from fungi and from 61% in millipedes to 97% in centipedes, earthworms and several other animal groups 43 . Then, we applied the fluxing function to the reconstructed interaction networks, which delivered energy flux estimations among all food-web nodes. Data were expressed in mW m −2 . Because the absolute estimates of the energy flux can be biased as a result of the abovementioned assumptions and regression-based conversions, we focus mainly on comparisons in our main conclusions. Detailed information on the approach can be found in the energy flux methodology paper ref. 63 .
Food-web parameters
To analyse food-web structure and energetics and test our hypotheses, we calculated bulk parameters for each food web and classified energy fluxes according to ‘trophic functions’. Trophic functions were primarily linked to the consumed food: herbivory represented a sum of outgoing fluxes from living plants (leaves/shoots and roots); litter and soil feeding represented a sum of outgoing fluxes from plant litter and soil organic matter, respectively; bacterivory and fungivory represented a sum of outgoing fluxes from bacteria and fungi, respectively; predation represented a sum of outgoing fluxes from all animal nodes. Six bulk parameters were calculated: (1) total biomass of all studied animal groups per square metre; (2) total energy flux (in mW) across all studied animal groups per square metre; (3) number of trophic links among trophic guilds in the reconstructed food web (a proxy for food-web complexity); (4) ratio of all energy fluxes from prey to predators to all energy fluxes from basal resources to primary consumers (a proxy for trophic hierarchy and predation control 18 ); (5) ratio of all energy fluxes from bacteria versus all energy fluxes from fungi (a proxy for carbon cycling rate 48 ); and (6) ratio between the production of faeces and the consumption of soil organic matter (a proxy for soil organic matter/carbon balance). The last indicator is new and is based on three main lines of evidence: (i) conversion of plant material into faeces by soil invertebrates increases microbial biomass production 52 ; (ii) microbial biomass production is the key process contributing to soil organic matter formation and stabilization 50 ; (iii) consumption of soil organic matter by invertebrates (a sum of outgoing fluxes from soil organic matter) leads to consumption of associated microbial biomass 50 and thus has opposite effects to the first two lines of evidence. To calculate the production of faeces, we multiplied all energy fluxes by inverted assimilation efficiency and summed them up, thus quantifying all unassimilated food in the food web. We highlight that this parameter is new and should be validated through controlled experiments, as the effect of soil feeders on soil organic matter sequestration is context-dependent (although often negative as predicted) 50 . All parameters, except (5) and (6), and all trophic functions were calculated for the entire food web, separately for aboveground and belowground food-web compartments and for individual animal groups (birds, canopy arthropods, soil arthropods and earthworms).
Statistical analyses
To analyse the overall distribution of energy flux across animal groups and trophic functions, we first ran two mixed-effect models testing the effect of land-use system (rainforest, jungle rubber, rubber and oil palm), region (two regions included in the design) and either major animal group or trophic function on energy fluxes in food webs (the lme4 package) 82 . Two models were run separately for groups and functions because not all functions are performed by all groups. Chi-square, significance and degrees of freedom were approximated using Wald Chi-square tests (the car package) 83 . We allowed for random intercepts depending on the plot to account for interdependence of groups and functions in the same site. The model code was lmer(Flux ~ Group (or Function) * Landuse + Region + (1 | Plot), data). To test specific hypotheses related to changes in trophic functions (first, more energy allocated to aboveground food webs in plantations; second, lower predation in plantations; third, a shift in basal resource feeding and carbon cycling across land-use systems), generalized linear models were run for each of the four bulk food-web parameters calculated separately for aboveground and belowground food-web compartments (response variables: total biomass, total energy flux, number of trophic links and trophic hierarchy) and two indicators of carbon cycling in belowground food webs (response variables: bacteria-to-fungi ratio and faeces production-to-soil consumption ratio). Data distribution selection followed visual inspection of the frequency distributions of raw data and homogeneity in the residuals of the model. Gaussian distribution was used for the number of trophic links, bacteria-to-fungi ratio, trophic hierarchy and production-to-soil consumption ratio and log-normal distribution was used for the total energy flux. The model code was lm(Flux ~ Landuse * Above/belowground + Region, data). Owing to a strong heteroscedasticity of variance across aboveground and belowground compartments, we used generalized least-squares models to analyse the total biomass 84 (the nlme package) 85 . The model code was gls(Flux ~ Landuse * Above/belowground + Region, weights = vf, data), where vf <- varIdent(form = ~ 1 | Above/belowground * Landuse). To test for significant differences between rainforest and other land-use systems, we applied the same types of models for aboveground and belowground food-web compartments separately, testing the effect of the land-use system and reported P values of the linear model coefficients.
Sensitivity analyses
We ran two further analyses to evaluate sensitivity of our conclusions to food-web reconstruction assumptions and undersampling of canopy arthropods. To test if the revealed patterns are robust to our food-web reconstruction assumptions (see section on Food-web reconstruction ), we re-ran food-web reconstructions and energy flux calculations 50 times, varying the following parameters from 0 to 1 with the step of 0.1 (Extended Data Fig. 1 ): (1) omnivory, where 0 is full resource specialization and 1 is full trophic generalism; (2) self-predation, where 0 is no self-predation and 1 is no limits on self-predation; (3) size-structured predation, where 0 is strictly size-structured predation and 1 is trophic interactions independent of body masses; (4) spatial-structured predation, where 0 is predation strictly defined by spatial niche overlaps and 1 is trophic interactions independent of spatial niche overlaps; and (5) protection, where 0 is all protection considered and 1 is no protection considered. Results are presented in Extended Data Fig. 1 . Overall, we found that our absolute energy flux estimations in belowground food webs were most sensitive to the degree of omnivory. This effect was driven by a low assimilation efficiency of specific food resources (for example, soil organic matter). Degree of omnivory used in the main analysis (auxiliary resources were assumed to be five times less important than the main ones) seems realistic considering that multichannel feeding has repeatedly been reported in soil invertebrates 55 , 86 , 87 . None of the tested settings undermined our main conclusions.
To test if our results were biased because canopy fogging underestimated the canopy arthropod biomasses, we used data on canopy heights. For that, during February 2013 to August 2014 we measured all trees in all plots with a minimum diameter at breast height of 10 cm, allowing us to calculate the average tree height and 90th quantile per plot. We assumed that canopy fogging was efficient until a certain tree height but failed to assess arthropods above this height. Ten different heights were tested, starting from 14 m maximum fogging efficiency (high undersampling) to 22 m maximum fogging efficiency (low undersampling). In each iteration, we used the range from 5 m (lower canopy) to the height of maximum efficiency as the ‘assessed community’ and everything above that height as ‘unassessed community’. Assuming the same density and community composition in the unassessed community, we multiplied canopy arthropod biomass by the ratio of unassessed to assessed community. Final multiplication coefficients varied from 1.0 in most of the plantation plots (no undersampling) to 3.5–3.8 in several rainforest plots (only about 30% of arthropods were sampled). Food-web reconstructions and energy flux calculations were re-run using new canopy arthropod biomasses. Results are presented in Extended Data Fig. 2 . Overall, we found that under ‘high undersampling’ scenario energy fluxes in aboveground food webs in rainforest increased almost threefold in comparison to our initial model. This increase was less evident in jungle rubber and almost not present in plantations in which the canopy height was low. Thus, the ‘high undersampling’ scenario exacerbated land-use effects on the total energy fluxes aboveground (−87% decline in rubber and −92% decline in oil palm in comparison to −75% and −79% decline in our initial analysis, correspondingly). Despite these pronounced differences, none of the tested settings undermined our main conclusions.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Raw data used in the analysis are available from figshare: https://doi.org/10.6084/m9.figshare.24648438 . The following datasets were used for bird identification and assignment of traits to invertebrates: xeno-canto online bird call database ( http://xeno-canto.org/ ); Elton Traits 65 ; feeding habits of invertebrates 60 ; and stable isotope data 14 . Source data are provided with this paper.
Code availability
Food-web reconstruction code is available from figshare: https://doi.org/10.6084/m9.figshare.24648438 . Statistical models are specified in Methods .
IPBES. Global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. Zenodo https://doi.org/10.5281/ZENODO.3831673 (2019).
Laurance, W. F., Sayer, J. & Cassman, K. G. Agricultural expansion and its impacts on tropical nature. Trends Ecol. Evol. 29 , 107–116 (2014).
Article PubMed Google Scholar
Guillaume, T. et al. Carbon costs and benefits of Indonesian rainforest conversion to plantations. Nat. Commun. 9 , 2388 (2018).
Article ADS PubMed PubMed Central Google Scholar
Rembold, K., Mangopo, H., Tjitrosoedirdjo, S. S. & Kreft, H. Plant diversity, forest dependency and alien plant invasions in tropical agricultural landscapes. Biol. Conserv. 213 , 234–242 (2017).
Article Google Scholar
Barnes, A. D. et al. Direct and cascading impacts of tropical land-use change on multi-trophic biodiversity. Nat. Ecol. Evol. 1 , 1511–1519 (2017).
Turner, E. C. & Foster, W. A. The impact of forest conversion to oil palm on arthropod abundance and biomass in Sabah, Malaysia. J. Trop. Ecol. 25 , 23–30 (2009).
Currie, D. J. Energy and large-scale patterns of animal- and plant-species richness. Am. Nat. 137 , 27–49 (1991).
Potapov, A. M., Klarner, B., Sandmann, D., Widyastuti, R. & Scheu, S. Linking size spectrum, energy flux and trophic multifunctionality in soil food webs of tropical land-use systems. J. Anim. Ecol. 88 , 1845–1859 (2019).
Schwarz, B. et al. Warming alters the energetic structure and function but not resilience of soil food webs. Nat. Clim. Change 7 , 895–900 (2017).
Article ADS Google Scholar
Brown, J. H., Gillooly, J. F., Allen, A. P., Savage, V. M. & West, G. B. Toward a metabolic theory of ecology. Ecology 85 , 1771–1789 (2004).
Barnes, A. D. et al. Energy flux: the link between multitrophic biodiversity and ecosystem functioning. Trends Ecol. Evol. 33 , 186–197 (2018).
Article PubMed PubMed Central Google Scholar
Barnes, A. D. et al. Consequences of tropical land use for multitrophic biodiversity and ecosystem functioning. Nat. Commun. 5 , 5351 (2014).
Article ADS CAS PubMed Google Scholar
Thakur, M. P. Climate warming and trophic mismatches in terrestrial ecosystems: the green–brown imbalance hypothesis. Biol. Lett. 16 , 20190770 (2020).
Article PubMed Central Google Scholar
Wardle, D. A. et al. Ecological linkages between aboveground and belowground biota. Science 304 , 1629–1633 (2004).
de Vries, F. T. et al. Soil food web properties explain ecosystem services across European land use systems. Proc. Natl Acad. Sci. USA 110 , 14296–14301 (2013).
Barnes, A. D. et al. Biodiversity enhances the multitrophic control of arthropod herbivory. Sci. Adv. 6 , eabb6603 (2020).
Scheu, S. Plants and generalist predators as links between the below-ground and above-ground system. Basic Appl. Ecol. 2 , 3–13 (2001).
Rosenberg, et al. The global biomass and number of terrestrial arthropods. Sci. Adv. 9 , eabq4049 (2023).
Article ADS CAS PubMed PubMed Central Google Scholar
Bar-On, Y. M., Phillips, R. & Milo, R. The biomass distribution on Earth. Proc. Natl Acad. Sci. USA 115 , 6506–6511 (2018).
Drescher, J. et al. Ecological and socio-economic functions across tropical land use systems after rainforest conversion. Phil. Trans. R. Soc. B 371 , 20150275 (2016).
Ellwood, M. D. F. & Foster, W. A. Doubling the estimate of invertebrate biomass in a rainforest canopy. Nature 429 , 549–551 (2004).
Petersen, H. & Luxton, M. A comparative analysis of soil fauna populations and their role in decomposition processes. Oikos 39 , 288–388 (1982).
Dial, R. J., Ellwood, M. D. F., Turner, E. C. & Foster, W. A. Arthropod abundance, canopy structure and microclimate in a Bornean lowland tropical rain forest. Biotropica 38 , 643–652 (2006).
Raich, J. W., Clark, D. A., Schwendenmann, L. & Wood, T. E. Aboveground tree growth varies with belowground carbon allocation in a tropical rainforest environment. PLoS ONE 9 , e100275 (2014).
Jochum, M. et al. Decreasing stoichiometric resource quality drives compensatory feeding across trophic levels in tropical litter invertebrate communities. Am. Nat. 190 , 131–143 (2017).
Stork, N. E. How many species of insects and other terrestrial arthropods are there on Earth? Annu. Rev. Entomol. 63 , 31–45 (2018).
Article CAS PubMed Google Scholar
Terborgh, J., Robinson, S. K., Parker, T. A., Munn, C. A. & Pierpont, N. Structure and organization of an Amazonian forest bird community. Ecol. Monogr. 60 , 213–238 (1990).
Mueller, K. E. et al. Light, earthworms and soil resources as predictors of diversity of 10 soil invertebrate groups across monocultures of 14 tree species. Soil Biol. Biochem. 92 , 184–198 (2016).
Article CAS Google Scholar
Krashevska, V., Klarner, B., Widyastuti, R., Maraun, M. & Scheu, S. Impact of tropical lowland rainforest conversion into rubber and oil palm plantations on soil microbial communities. Biol. Fertil. Soil. 51 , 697–705 (2015).
Grass, I. et al. Trade-offs between multifunctionality and profit in tropical smallholder landscapes. Nat. Commun. 11 , 1186 (2020).
Edwards, D. P. et al. Selective-logging and oil palm: multitaxon impacts, biodiversity indicators and trade-offs for conservation planning. Ecol. Appl. 24 , 2029–2049 (2014).
Article MathSciNet PubMed Google Scholar
Prabowo, W. E. et al. Bird responses to lowland rainforest conversion in Sumatran smallholder landscapes, Indonesia. PLoS ONE 11 , e0154876 (2016).
Ramos, D. et al. Rainforest conversion to rubber and oil palm reduces abundance, biomass and diversity of canopy spiders. PeerJ 10 , e13898 (2022).
Kulmatiski, A. & Beard, K. H. Long-term plant growth legacies overwhelm short-term plant growth effects on soil microbial community structure. Soil Biol. Biochem. 43 , 823–830 (2011).
Le Provost, G. et al. Contrasting responses of above- and belowground diversity to multiple components of land-use intensity. Nat. Commun. 12 , 3918 (2021).
Zhou, Z., Krashevska, V., Widyastuti, R., Scheu, S. & Potapov, A. Tropical land use alters functional diversity of soil food webs and leads to monopolization of the detrital energy channel. eLife 11 , e75428 (2022).
Article CAS PubMed PubMed Central Google Scholar
Potapov, A. et al. Oil palm and rubber expansion facilitates earthworm invasion in Indonesia. Biol. Invasions 23 , 2783–2795 (2021).
Potapov, A. M. et al. Functional losses in ground spider communities due to habitat structure degradation under tropical land-use change. Ecology 101 , e02957 (2020).
Rakotomalala, A. A. N. A., Ficiciyan, A. M. & Tscharntke, T. Intercropping enhances beneficial arthropods and controls pests: a systematic review and meta-analysis. Agric. Ecosyst. Environ. 356 , 108617 (2023).
Camarretta, N. et al. Using airborne laser scanning to characterize land-use systems in a tropical landscape based on vegetation structural metrics. Remote Sens. 13 , 4794 (2021).
Tscharntke, T. et al. Conservation biological control and enemy diversity on a landscape scale. Biol. Control 43 , 294–309 (2007).
Corley, R. H. V. & Tinker, P. B. H. The Oil Palm (John Wiley & Sons, 2015).
Potapov, A. M. Multifunctionality of belowground food webs: resource, size and spatial energy channels. Biol. Rev. Camb. Philos. Soc. 97 , 1691–1711 (2022).
Krashevska, et al. Micro-decomposer communities and decomposition processes in tropical lowlands as affected by land use and litter type. Oecologia 187 , 255–266 (2018).
Article ADS PubMed Google Scholar
Hyodo, F. et al. Gradual enrichment of 15 N with humification of diets in a below-ground food web: relationship between 15 N and diet age determined using 14 C. Funct. Ecol. 22 , 516–522 (2008).
Hannula, S. E. & Morriën, E. Will fungi solve the carbon dilemma? Geoderma 413 , 115767 (2022).
Article ADS CAS Google Scholar
Susanti, W. I., Pollierer, M. M., Widyastuti, R., Scheu, S. & Potapov, A. Conversion of rainforest to oil palm and rubber plantations alters energy channels in soil food webs. Ecol. Evol. 9 , 9027–9039 (2019).
Rooney, N. & McCann, K. S. Integrating food web diversity, structure and stability. Trends Ecol. Evol. 27 , 40–46 (2012).
Hyodo, F., Uchida, T., Kaneko, N. & Tayasu, I. Use of radiocarbon to estimate diet ages of earthworms across different climate regions. Appl. Soil Ecol. 62 , 178–183 (2012).
Garnier, P., Makowski, D., Hedde, M. & Bertrand, M. Changes in soil carbon mineralization related to earthworm activity depend on the time since inoculation and their density in soil. Sci. Rep. 12 , 13616 (2022).
Angst, G. et al. Earthworms as catalysts in the formation and stabilization of soil microbial necromass. Glob. Change Biol. 28 , 4775–4782 (2022).
Joly, F.-X. et al. Detritivore conversion of litter into faeces accelerates organic matter turnover. Commun. Biol. 3 , 660 (2020).
Tao, F. et al. Microbial carbon use efficiency promotes global soil carbon storage. Nature 618 , 981–985 (2023).
Buchkowski, R. W. & Lindo, Z. Stoichiometric and structural uncertainty in soil food web models. Funct. Ecol. 35 , 288–300 (2021).
Potapov, A. M. et al. Feeding habits and multifunctional classification of soil-associated consumers from protists to vertebrates. Biol. Rev. Camb. Philos. Soc. 97 , 1057–1117 (2022).
Ashton‐Butt, A. et al. Replanting of first‐cycle oil palm results in a second wave of biodiversity loss. Ecol. Evol. 9 , 6433–6443 (2019).
Tao, H.-H. et al. Application of oil palm empty fruit bunch effects on soil biota and functions: a case study in Sumatra, Indonesia. Agric. Ecosyst. Environ. 256 , 105–113 (2018).
Darras, K. F. A. et al. Reducing fertilizer and avoiding herbicides in oil palm plantations—ecological and economic valuations. Front. For. Glob. Change 2 , 65 (2019).
Teuscher, M. et al. Experimental biodiversity enrichment in oil-palm-dominated landscapes in Indonesia. Front. Plant Sci. 7 , 1538 (2016).
Ashraf, M. et al. Alley-cropping system can boost arthropod biodiversity and ecosystem functions in oil palm plantations. Agric. Ecosyst. Environ. 260 , 19–26 (2018).
Margono, B. A., Potapov, P. V., Turubanova, S., Stolle, F. & Hansen, M. C. Primary forest cover loss in Indonesia over 2000–2012. Nat. Clim. Change 4 , 730–735 (2014).
Allen, K., Corre, M. D., Kurniawan, S., Utami, S. R. & Veldkamp, E. Spatial variability surpasses land-use change effects on soil biochemical properties of converted lowland landscapes in Sumatra, Indonesia. Geoderma 284 , 42–50 (2016).
Sohlström, E. H. et al. Applying generalized allometric regressions to predict live body mass of tropical and temperate arthropods. Ecol. Evol. 8 , 12737–12749 (2018).
Darras, K. et al. BioSounds: an open-source, online platform for ecoacoustics. F1000 Res. 9 , 1224 (2020).
Wilman, H. et al. EltonTraits 1.0: species-level foraging attributes of the world’s birds and mammals. Ecology 95 , 2027–2027 (2014).
Azhar, A. et al. Rainforest conversion to cash crops reduces abundance, biomass and species richness of parasitoid wasps in Sumatra, Indonesia. Agric. For. Entomol. 24 , 506–515 (2022).
Article MathSciNet Google Scholar
Nazarreta, R. et al. Rainforest conversion to smallholder plantations of rubber or oil palm leads to species loss and community shifts in canopy ants (Hymenoptera: Formicidae). Myrmecol. News 30 , 175–186 (2020).
Kasmiatun, et al. Rainforest conversion to smallholder cash crops leads to varying declines of beetles (Coleoptera) on Sumatra. Biotropica 55 , 119–131 (2023).
Mawan, A. et al. Response of arboreal Collembola communities to the conversion of lowland rainforest into rubber and oil palm plantations. BMC Ecol. Evol. 22 , 144 (2022).
Klarner, B. et al. Trophic niches, diversity and community composition of invertebrate top predators (Chilopoda) as affected by conversion of tropical lowland rainforest in Sumatra (Indonesia). PLoS ONE 12 , e0180915 (2017).
Potapov, A. M., Scheu, S. & Tiunov, A. V. Trophic consistency of supraspecific taxa in below-ground invertebrate communities: comparison across lineages and taxonomic ranks. Funct. Ecol. 33 , 1172–1183 (2019).
Petersen, H. Estimation of dry weight, fresh weight and calorific content of various collembolan species. Pedobiologia 15 , 222–243 (1975).
Mercer, R. D., Gabriel, A. G. A., Barendse, J., Marshall, D. J. & Chown, S. L. Invertebrate body sizes from Marion Island. Antarct. Sci. 13 , 135–143 (2001).
Hale, C. M., Reich, P. B. & Frelich, L. E. Allometric equations for estimation of ash-free dry mass from length measurements for selected European earthworm species (Lumbricidae) in the Western Great Lakes region. Am. Midl. Nat. 151 , 179–185 (2004).
Brose, U. et al. Foraging theory predicts predator–prey energy fluxes. J. Anim. Ecol. 77 , 1072–1078 (2008).
Brose, U. et al. Predator traits determine food-web architecture across ecosystems. Nat. Ecol. Evol. 3 , 919–927 (2019).
Gauzens, B. et al. fluxweb: an R package to easily estimate energy fluxes in food webs. Methods Ecol. Evol. 10 , 270–279 (2019).
Peschel, K., Norton, R., Scheu, S. & Maraun, M. Do oribatid mites live in enemy-free space? Evidence from feeding experiments with the predatory mite Pergamasus septentrionalis . Soil Biol. Biochem. 38 , 2985–2989 (2006).
Ehnes, R. B., Rall, B. C. & Brose, U. Phylogenetic grouping, curvature and metabolic scaling in terrestrial invertebrates. Ecol. Lett. 14 , 993–1000 (2011).
Meijide, A. et al. Impact of forest conversion to oil palm and rubber plantations on microclimate and the role of the 2015 ENSO event. Agric. For. Meteorol. 252 , 208–219 (2018).
Jochum, M. et al. For flux’s sake: general considerations for energy-flux calculations in ecological communities. Ecol. Evol. 11 , 12948–12969 (2021).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67 , 1–48 (2015).
Fox, J. & Weisberg, S. An R Companion to Applied Regression (Sage, 2011).
Zuur, A. F., Ieno, E. N., Walker, N., Saveliev, A. A. & Smith, G. M. Mixed Effects Models and Extensions in Ecology with R (Springer Science & Business Media, 2009).
Pinheiro, J. & Bates, D. M. Mixed-Effects Models in S and S-PLUS (Springer, 2000).
Digel, C., Curtsdotter, A., Riede, J., Klarner, B. & Brose, U. Unravelling the complex structure of forest soil food webs: higher omnivory and more trophic levels. Oikos 123 , 1157–1172 (2014).
Wolkovich, E. M. Reticulated channels in soil food webs. Soil Biol. Biochem. 102 , 18–21 (2016).
Download references
Acknowledgements
This study was funded by the Deutsche Forschungsgemeinschaft (DFG), project number 192626868–SFB 990 in the framework of the collaborative German-Indonesian research project CRC990. We thank PT REKI for granting us access and use of their properties. A.M.P. acknowledges support of the DFG Emmy Noether programme (project no. 493345801) and of iDiv (DFG–FZT 118, 202548816). C.W. is grateful for support by the DFG Heisenberg programme (project no. 493487387). We thank B. Gauzens for advice on energy flux calculations and discussion about the index for carbon balance. We thank S. Meyer and contributors of PhyloPic.org for the animal and resource silhouettes used in Figs. 1 and 2 .
Author information
Authors and affiliations.
Animal Ecology, University of Göttingen, Göttingen, Germany
Anton M. Potapov, Jochen Drescher, Noah Janotta, Valentine Laurent, Amanda Mawan, Melanie M. Pollierer & Stefan Scheu
German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig, Leipzig, Germany
Anton M. Potapov
Insitute of Biology, University of Leipzig, Leipzig, Germany
Agroecology, University of Göttingen, Göttingen, Germany
Kevin Darras & Teja Tscharntke
Functional Agrobiodiversity, University of Göttingen, Göttingen, Germany
Arne Wenzel & Catrin Westphal
Department of Plant Protection, IPB University, Bogor, Indonesia
Rizky Nazarreta, Kasmiatun, Endah H. Utari, Damayanti Buchori & Purnama Hidayat
Botanical Garden of University of Bern, University of Bern, Bern, Switzerland
Katja Rembold
Biodiversity, Macroecology & Biogeography, University of Göttingen, Göttingen, Germany
Department of Soil Science, IPB University, Bogor, Indonesia
Rahayu Widyastuti
Centre for Transdisciplinary and Sustainability Sciences, IPB University, Bogor, Indonesia
Damayanti Buchori
Department of Zoology, University of Cambridge, Cambridge, UK
Edgar Turner
Ecology of Tropical Agricultural Systems, University of Hohenheim, Stuttgart, Germany
Centre of Biodiversity and Sustainable Land Use, University of Göttigen, Göttingen, Germany
Stefan Scheu
You can also search for this author in PubMed Google Scholar
Contributions
A.M.P. developed the idea and led the writing. A.M.P., J.D., K.D., A.W., R.N., K.R., K., E.H.U., N.J., V.L. and A.M. provided the data. S.S., T.T., C.W., I.G., E.T., A.M.P., J.D., K.D., R.W., D.B. and P.H. contributed to coordination of the data collection. S.S., T.T., C.W., I.G., E.T., M.M.P., A.M.P., J.D., K.D. and A.W. contributed to conceptualization of the idea. All authors revised the manuscript.
Corresponding author
Correspondence to Anton M. Potapov .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature thanks John Moore, Peter de Ruiter and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended data fig. 1 sensitivity analysis of the food-web reconstruction..
Effect of the food-web reconstruction coefficients (i.e. food-web topology) on aboveground (solid line) and belowground energy fluxes (dashed line) in four land-use systems (dark green – forest, light green – jungle rubber, orange – rubber, yellow – oil palm; n = 8 sites per system, standard error is shown as the variation measure). Coefficients used in the main analysis are shown with black vertical lines. Omnivory controls how important auxiliary resources are for the consumers (a). Self-predation controls for the extent to which each node feeds on itself (cannibalism), where 1 means that individuals of their own guild are considered in the same way as those in all other nodes (b). Size-structured predation controls for deviations from the rules of predator–prey mass ratios (c). Spatial predation controls for deviations from the rules of spatial niche overlap (d). Protection controls for the importance of prey protection (e).
Extended Data Fig. 2 Sensitivity analysis of the canopy fogging undersampling.
Effect of canopy arthropod undersampling on the energy fluxes in four land-use systems (dark green – forest, light green – jungle rubber, orange – rubber, yellow – oil palm; n = 8 sites per system, standard error is shown as the variation measure). We tested how the maximum tree height for efficient fogging affected our results. Average and 90th quantile (Q90) of tree height were calculated for each plot (a). The quantiles were further used to estimate bias in energy flux estimations aboveground (solid line) and belowground (dashed line), assuming that fogging works efficiently to assess canopy arthropods up to a certain tree height (models were run for each additional metre starting from 14 m as a ‘large bias’ and up to 22 m as a ‘small bias’; b). Land-use effect was more pronounced if we assumed a large bias due to higher trees in rainforest, than in plantations (n = 8, 1 SD variation; c).
Extended Data Fig. 3 Reconstructed food webs in each land-use system from the main survey in 2013.
Eight food webs per system representing eight sites are shown. “B” and “H” in the site codes refer to the “Bukit Duabelas” and “Harapan” regions, correspondingly. Food-web nodes include basal resources displayed with black labels (living plants – P, plant litter – L, fungi – F, bacteria – B, soil organic matter – S) and consumer trophic guilds shown with circles. Consumer nodes are clustered in four major groups according to their vertical distribution and ecological niches (canopy arthropods – light green, birds – dark green, soil arthropods – light red, earthworms – beige). Horizontal distribution of consumer nodes represent trophic positions (trophic level increases from left to right). Connecting lines on the food-web diagram represent energy fluxes in mW m −2 (represented by the thickness of the lines).
Extended Data Fig. 4 Reconstructed food webs in each land-use system from the validation survey in 2016–2017.
Eight food webs per system representing eight sites are shown. “B” and “H” in the site codes refer to the “Bukit Duabelas” and “Harapan” regions, respectively. Food-web nodes include basal resources displayed with black labels (living plants – P, plant litter – L, fungi – F, bacteria – B, soil organic matter – S) and consumer trophic guilds shown with circles. Consumer nodes are clustered in four major groups according to their vertical distribution and ecological niches (canopy arthropods – light green, birds – dark green, soil arthropods – light red, earthworms – beige). Horizontal distribution of consumer nodes represent trophic positions (trophic level increases from left to right). Connecting lines on the food-web diagram represent energy fluxes in mW m −2 (represented by the thickness of the lines).
Extended Data Fig. 5 Validation of the main survey from 2013 with the results of the validation survey from 2016–2017.
Effects of land use and above/belowground compartment identity on bulk food-web parameters compared between the main survey in 2013 and the validation survey in 2016–2017 (a). Each point is a site, bars represent means. Colours denote land-use systems (dark green – forest, light green – jungle rubber, orange – rubber, yellow – oil palm; n = 8 sites per system). Note square root scale in biomass, total energy flux and carbon balance charts. Asterisks mark significant differences between means for the given parameter above- or belowground from that in rainforest (two-tailed; ***p < 0.001, **p < 0.01, *p < 0.05). Effects of land-use system and above/belowground on the tested parameters are given below the corresponding bar charts. Average trophic functions for each major group of consumers and for the food web in total are summarized as stacked proportional bar charts (b). Refer to Figs. 2 and 3 in the main text for more detailed explanations. Note that jungle rubber was not assessed in 2016–2017.
Extended Data Fig. 6 Biomasses of trophic guilds in different land-use systems.
Guilds of aboveground (birds, canopy arthropods) and belowground (earthworms and soil arthropods) animals are shown separately and ordered according to average biomass across systems. Mean fresh biomasses from the main survey in 2013 are displayed with bars. Full definitions and abbreviations (for example Ew – earthworms, Av – birds) of the trophic guilds are given in the data tables available from Figshare (see Data availability).
Extended Data Fig. 7 Biomasses of trophic guilds in different land-use systems in the validation survey.
Guilds of aboveground (birds, canopy arthropods) and belowground (earthworms and soil arthropods) animals are shown separately and ordered according to average biomass across systems. Mean fresh biomasses from the validation survey in 2016–2017 are displayed with bars. Full definitions and abbreviations (for example Ew – earthworms, Av – birds) of the trophic guilds are available from Figshare (see Data availability).
Supplementary information
Supplementary notes.
Validation survey.
Reporting Summary
Peer review file, supplementary table 1.
Fluxes groups: contribution of different consumer trophic guilds to the total energy flux in different land-use systems. Guilds are given in descending order according to their mean proportion in the total energy flux.
Supplementary Table 2
BirdGuilds: classification of detected bird species to feeding guilds based on their diet and foraging habitat preferences. The information is taken from Elton Traits ( Methods ).
Source data
Source data figs. 1 and 2, source data fig. 3, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Potapov, A.M., Drescher, J., Darras, K. et al. Rainforest transformation reallocates energy from green to brown food webs. Nature 627 , 116–122 (2024). https://doi.org/10.1038/s41586-024-07083-y
Download citation
Received : 21 October 2022
Accepted : 16 January 2024
Published : 14 February 2024
Issue Date : 07 March 2024
DOI : https://doi.org/10.1038/s41586-024-07083-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

Paying people to replant tropical forests − and letting them harvest the timber − can pay off for climate, justice and environment
Staff Scientist and Director, Agua Salud Project, Smithsonian Institution
Postdoctoral Fellow, Agua Salud Project, Smithsonian Tropical Research Institute, Smithsonian Institution
Associate Professor of Environmental Science, National University of Singapore
Disclosure statement
Jefferson S. Hall receives funding from the US government via the Smithsonian Institution, Stanly Motta, Frank and Kristin Levinson, the Hoch family, U-Trust, and the Mark and Rachel Rohr Foundation.
Katherine Sinacore receives funding from the Mark and Rachel Rohr Foundation, Stanly Motta, Frank and Kristin Levinson, the Hoch family, and the Smithsonian.
Michiel van Breugel receives funding from Singapore’s Ministry of Education and the Future Cities Lab Global Program of the ETH-Singapore Centre, which is funded by National Research Foundation Singapore.
Smithsonian Institution provides funding as a member of The Conversation US.
View all partners
Tropical forest landscapes are home to millions of Indigenous peoples and small-scale farmers . Just about every square meter of land is spoken for, even if claims are not formally recognized by governments .
These local landholders hold the key to a valuable solution as the world tries to slow climate change – restoring deforested tropical landscapes for a healthier future.
Tropical forests are vital to Earth’s climate and biodiversity , but a soccer field-size area of mature tropical forest is burned or cut down about every 5 seconds to clear space for crops and cattle today.
While those trees may be lost, the land still has potential. Tropical forests’ combination of year-round sunshine and high rainfall can lead to high growth rates, suggesting that areas where tropical forests once grew could be valuable sites for reforestation . In fact, a host of international agreements and declarations envision just this.

For reforestation projects to make a dent in climate change, however, they have to work with and for the people who live there.
As forest ecologists involved in tropical forest restoration, we have been studying effective ways to compensate people for the ecosystem services flowing from their land. In a new study , we show how compensation that also allows landholders to harvest and sell some of the trees could provide powerful incentives and ultimately benefit everyone.
The extraordinary value of ecosystem services
Tropical forests are celebrated for their extraordinary biodiversity, with their preservation seen as essential for protecting life on Earth . They are reservoirs of vast carbon stocks, slowing down climate change. However, when tropical forests are cleared and burned, they release copious amounts of carbon dioxide , a greenhouse gas that drives climate change.
Programs offering payments for ecosystem services are designed to help keep those forests and other ecosystems healthy by compensating landholders for goods and services produced by nature that are often taken for granted. For example, forests moderate stream flows and reduce flood risks , support bees and other pollinators that benefit neighboring croplands, and help regulate climate .

In recent years, a cottage industry has grown up around paying people to reforest land for the carbon it can hold. It has been driven in part by corporations and other institutions looking for ways to meet their commitments to cut greenhouse gas emissions by paying projects to reduce or prevent emissions elsewhere.
Early iterations of projects that pay landholders for ecosystem services have been criticized for focusing too much on economic efficiency, sometimes at the expense of social and environmental concerns .

Win-win solutions – where environmental and social concerns are both accounted for – may not be the most economically efficient in the short term, but they can lead to longer-term sustainability as participants feel a sense of pride and responsibility for the project’s success.
That longer-term sustainability is essential for trees’ carbon storage, because many decades of growth is required to build up stored carbon and combat climate change.
Why timber can be a triple win
In the study, we looked at ways to maximize all three priorities – environmental, economic and social benefits – in forest restoration, focusing on infertile land.
It may come as a surprise, but most soils in the tropics are extraordinarily infertile , with concentrations of phosphorus and other essential nutrients an order of magnitude or more lower than in crop-producing areas of the northern hemisphere. This makes restoring tropical forests through reforestation more complex than simply planting trees – these areas also require maintenance.

In our study we used some 1.4 million tree measurements taken over 15 years at the Smithsonian Tropical Research Institute ’s Agua Salud site in Panama to project carbon sequestration and potential timber revenues. We looked at naturally regrowing forests, native tree species plantations and an effort to rehabilitate a failed teak plantation by planting high-value native trees known to grow on low-fertility soils to test routes to profitability .
One set of solutions stood out: We found that giving landholders both payments for carbon storage and the ability to generate revenue through timber production on the land could lead to vibrant forests and financial gains for the landholder.

It may seem counterintuitive to suggest timber harvesting when the goal is to restore forests, but allowing landholders to generate timber revenue can give them an incentive to protect and manage planted forests over time.
Regrowing trees on a deforested landscape, whether natural regrowth or plantations, is a net win for climate change, as trees take vast amounts of carbon out of the atmosphere . New forests that are selectively logged or plantations that are harvested in 30 to 80 years can help slow climate change while the world cuts emissions and expands carbon capture technologies.
Reliable payments matter
The structure of the payments is also important. We found that reliable annual carbon payments to rural landlords to regrow forests could match or surpass the income they might otherwise get from clearing land for cattle, thus making the transition to raising trees possible.
When cash payments are based instead on measurements of tree growth, they can vary widely year to year and among planting strategies. With the costs involved, that can stand in the way of effective land management to combat climate change.
Using flat annual payments instead guarantees a stable income and will help encourage more landholders to enroll. We are now using that method in Panama’s Indigenous Ngäbe-Buglé Comarca . The project pays residents to plant and nurture native trees over 20 years.
Shifting risk to buyers of carbon offsets
From a practical perspective, flat annual carbon payments and other cost-sharing strategies to plant trees shift the burden of risk from participants to carbon buyers, often companies in wealthy countries.
The landholders get paid even if actual growth of the trees falls short, and everyone benefits from the ecosystem services provided.
While win-win solutions may not initially appear to be economically efficient, our work helps to illustrate a viable path forward – where environmental, social and economic objectives can be met.
- Climate change
- Deforestation
- Payment for Ecosystem Services
- Smithsonian
- Tropical forests
- Reforestation
- Greenhouse gas emissions (GHG)

Case Management Specialist

Lecturer / Senior Lecturer - Marketing

Assistant Editor - 1 year cadetship

Executive Dean, Faculty of Health

Lecturer/Senior Lecturer, Earth System Science (School of Science)

IMAGES
VIDEO
COMMENTS
Tropical forests may be vulnerable to climate change1-3 if photosynthetic carbon uptake currently operates near a high temperature limit4-6. Predicting tropical forest function requires ...
Tropical forests are critically important for the global climate because of their impact on the radiation, hydrology, and biogeochemical cycles [].Tropical forests are large pools of global carbon, with about 360 Pg of carbon in forest vegetation, that with soil carbon adds up to 800 PgC, almost as much as is stored in the atmosphere [].In addition, forests are responsible for much of the ...
Abstract. Tropical forests disappear rapidly because of deforestation, yet they have the potential to regrow naturally on abandoned lands. We analyze how 12 forest attributes recover during secondary succession and how their recovery is interrelated using 77 sites across the tropics. Tropical forests are highly resilient to low-intensity land ...
Tropical forests encompass less than one-fifth of Earth's terrestrial area (Dinerstein et al. 2017), yet directly influence the well-being of approximately 1.5 billion people through the provisioning of a multitude of ecosystem services (Lewis et al. 2015) and indirectly benefit much of the rest of humanity through their role in climate regulation (Lawrence and Vandecar 2015).
This study identifies recent research on tropical forest monitoring using a systematic literature review. The research explores whether the location of these studies is in the countries where ...
The PLOS ONE Collection "Measuring forest conservation effectiveness" brings together a series of studies that evaluate the effectiveness of tropical forest conservation policies and programs with the goal of measuring conservation success and associated co-benefits. This overview piece describes the geographic and methodological scope of these studies, as well as the policy instruments ...
This paper presents how tropical forests cycle carbon, and the physiological basis of how this will change with climate change. ... The next generation of scenarios for climate change research and ...
Global climate change will affect the hysteresis of tropical forests by the end of the 21st century. Notably, we find a large reduction of 66% to 1.66 million km 2 in minimal forest area for South ...
Tropical Forests and People Research Centre, Forest Research Institute, University of Sunshine Coast, 90 Sippy Downs Drive, Sippy Downs, 4556, Queensland, Australia. ... We screened 373 papers in total to identify studies that included monitoring of both natural regeneration and planted restoration plot(s) that were established within the same ...
Tropical forests play a disproportionate role in global ecosystem services, including carbon sequestration, climate and water cycle regulation, and maintenance of biodiversity (1-3).However, increasing rates of tropical forest cover loss due to land use change threaten the continued functioning of many of these services (4, 5).Spatial patterns of forest loss could amplify the adverse effects ...
The Journal of Tropical Forest Science (JTFS) is an international peer-reviewed journal concerning the science, technology and development of tropical forests and forest products.First published in 1988, the journal welcomes articles reporting original fundamental or applied research on tropical forest biology, ecology, chemistry, management, silviculture, conservation, utilization and product ...
Protected areas can conserve tropical forests, but most are underfunded and therefore underprotected. ... Discover the world's research. 25+ million members; 160+ million publication pages; 2.3 ...
The BCI forest dynamics research project was founded by S. P. Hubbell and R. B. Foster and is now managed by R. Condit, S. Lao, and R. Perez under the Center for Tropical Forest Science and the Smithsonian Tropical Research Institute in Panama. Numerous organizations have provided funding, principally the U.S. National Science Foundation.
Tropical ecosystems play an important role in the environment. They provide multiple ecosystem services, such as carbon capture and sequestration, food supply, and climate regulation. Studying land use and land cover change makes it possible to understand the land's alterations associated with deforestation, degradation, erosion, soil desertification, and biodiversity loss. The objective of ...
The climate of lowland tropical rain forests is warm, humid, and relatively stable. Tropical rain. forests are characterized by mean annual temperatures ranging from 23 C to 28C, with mean ...
Agricultural expansion is recognized as a major driver of forest loss in the tropics. However, accurate data on the links between agriculture and tropical deforestation are lacking. Pendrill et al. synthesized existing research and datasets to quantify the extent to which tropical deforestation from 2011 to 2015 was associated with agriculture.
Tropical forests are global epicentres of biodiversity and important modulators of the rate of climate change. Recent research on deforestation rates and ecological changes within intact forests, both areas of recent research and debate, are reviewed, and the implications for biodiversity (species loss) and climate change (via the global carbon cycle) addressed.
The increase in the frequency of fires has been widely explored in fire-prone temperate and Mediterranean forests, where the plants are adapted to fire (Ganteaume et al., 2013), but the research on tropical forests fires is not sufficient. Ecologists and foresters are currently interested in exploring the causes and effects of forest fires in ...
Methods in Ecology and Evolution is an open access journal publishing papers across a wide range of subdisciplines, disseminating new methods in ecology and evolution. Abstract Large-scale data compilation is increasing steadily in tropical forest research, but the lack of standardized methods for data collection limits drawing inference from ...
ForestGEO, Smithsonian Tropical Research Institute, Ancón, 0843-03092 Panama, Panama. Department of Geography, National University of Singapore, Singapore, 119077 Singapore. Yale-NUS College, Singapore, 138527 Singapore. Search for more papers by this author
Abstract. Rainforests are widely known to be the most biodiverse (in species per square hectare) of terrestrial ecosystem types on Earth. Home to an estimated half of the Earth's plant and animal species, rainforests cover approximately 7% of total land area. Yet many rainforests worldwide are still in descriptive stages and have not yet been ...
As temperature rises, net carbon uptake in tropical forests decreases, but the underlying mechanisms are not well understood. High temperatures can limit photosynthesis directly, for example by reducing biochemical capacity, or indirectly through rising vapor pressure deficit (VPD) causing stomatal closure.
New Phytologist is an international journal owned by the New Phytologist Foundation publishing original research in plant science and its ... Unraveling the drivers and impacts of leaf phenological diversity in a subtropical forest: A fine-scale analysis using PlanetScope CubeSats ... Search for more papers by this author. Buhang Li, Buhang Li ...
Instead, it is recent research in Asia that has transformed this field of research by firmly pushing back human exploitation and occupation of tropical forests well into the Pleistocene. Research on the island of Sumatra has found evidence for the presence of humans in rainforests dating to 73 thousand years ago (ka) [ 34 ].
The dataset produced by the European Commission's Joint Research Center (JRC) on forest cover change in tropical moist forests (TMF dataset) is a highly structured and comprehensive record of forest cover and land use change in these ecosystems (Vancutsem et al. Citation 2021). It is compiled from optical satellite imagery spanning several ...
These figures question the existing research focus on aboveground tropical food webs and ... introductory EFForTS paper 20. In each of the 32 plots, we applied a combination of collection methods ...
In recent years, large areas of cash crops, such as rubber and oil palm, have expanded in tropical areas, replacing a large proportion of natural forest and other traditional agricultural land (Warren-Thomas et al., 2018; Xu et al., 2022), For example, rubber plantations have been established on former natural forests or paddy fields along the ...
Tropical forests are vital to Earth's climate and biodiversity, but a soccer field-size area of mature tropical forest is burned or cut down about every 5 seconds to clear space for crops and ...
Abstract and Figures. Tropical Forest and Biodiversity Assessment for Sri Lanka was prepared in 2012 by Richard Volk, H.M.Bandaratillake and Shamen P. Vidanage for USAID as part of their mandatory ...