- Open access
- Published: 18 August 2021

An evaluation of the process of informed consent: views from research participants and staff
- Lydia O’ Sullivan ORCID: orcid.org/0000-0002-7131-5048 1 , 2 ,
- Laura Feeney 1 ,
- Rachel K. Crowley 1 , 3 ,
- Prasanth Sukumar 1 ,
- Eilish McAuliffe 4 &
- Peter Doran 1 , 2
Trials volume 22 , Article number: 544 ( 2021 ) Cite this article
12k Accesses
11 Citations
19 Altmetric
Metrics details
The process of informed consent for enrolment to a clinical research study can be complex for both participants and research staff. Challenges include respecting the potential participant’s autonomy and information needs while simultaneously providing adequate information to enable an informed decision. Qualitative research with small sample sizes has added to our understanding of these challenges. However, there is value in garnering the perspectives of research participants and staff across larger samples to explore the impact of contextual factors (time spent, the timing of the discussion and the setting), on the informed consent process.
Research staff and research participants from Ireland and the UK were invited to complete an anonymous survey by post or online (research participants) and online (research staff). The surveys aimed to quantify the perceptions of research participants and staff regarding some contextual factors about the process of informed consent. The survey, which contained 14 and 16 multiple choice questions for research participants and staff respectively, was analysed using descriptive statistics. Both surveys included one optional, open-ended question, which were analysed thematically.
Research participants (169) and research staff (115) completed the survey. Research participants were predominantly positive about the informed consent process but highlighted the importance of having sufficient time and the value of providing follow-up once the study concludes, e.g. providing results to participants. Most staff (74.4%) staff reported that they felt very confident or confident facilitating informed consent discussions, but 63% felt information leaflets were too long and/or complicated, 56% were concerned about whether participants had understood complex information and 40% felt that time constraints were a barrier. A dominant theme from the open-ended responses to the staff survey was the importance of adequate time and resources.
Conclusions
Research participants in this study were overwhelmingly positive about their experience of the informed consent process. However, research staff expressed concern about how much participants have understood and studies of patient comprehension of research study information would seem to confirm these fears. This study highlights the importance of allocating adequate time to informed consent discussions, and research staff could consider using Teach Back techniques.
Trial Registration
Not applicable
Peer Review reports
Informed consent depends on disclosure of pertinent information, capacity to give consent and a voluntary decision [ 1 , 2 ]. In the clinical research context, the research participant must freely give their informed consent prior to enrolment onto a clinical trial or research study [ 3 ]. While the Participant Information Leaflet (PIL) provides a record of the information disclosed and the informed consent form (ICF) records the participants’ written decision to take part, it is nevertheless recognised that informed consent is a process [ 4 , 5 ], rather than a single event enabled solely by paper or electronic documents. This process involves the researcher approaching the prospective participant, providing some initial information about the research study, and as this information is processed, responding to questions and adjusting the level of information to fit the needs of the individual. The process of informed consent often involves building rapport and trust with the prospective participant and should respect cultural and societal norms, such as involving family members or friends. Finally, once it is evident that the individual has adequately understood the study or trial, they make an informed choice about whether they wish to participate. If they have decided to participate, the participants typically sign a consent form and are given a copy of the signed form and the study/trial information to take away with them. However, while researchers agree that maintaining participant autonomy and supporting their decision-making is important, much debate remains on how the informed consent process can be measured and optimised [ 6 ].
A number of studies report that most research participants are satisfied with the information provided to them [ 7 , 8 , 9 ]. However, there is also ample evidence of participants misunderstanding critical information for good quality consent, such as the risks and the methodological design of the study [ 10 , 11 , 12 ]. It is also recognised that the process of informed consent is complex; for example, the language used during the informed consent process and in the PIL/ICF documents can be too difficult for participants to understand [ 13 , 14 , 15 ]. Furthermore, while it is the researcher’s responsibility to provide the relevant information to the participant, the available evidence suggests that participants’ information needs can vary considerably [ 16 , 17 ], though the reasons are not fully understood. Research participants are often unwell when enrolling in a study and therefore their capacity to absorb information may be diminished, putting them at greater risk of misunderstanding some element of the research study [ 10 , 18 ].
The process of informed consent can be challenging for researchers also. Studies aimed at understanding the consent process from the investigators’ perspective report that the lack of time and difficulty communicating complex concepts are barriers when facilitating informed consent discussions [ 19 , 20 , 21 ]. Several studies also suggest that investigators felt that there was a conflict between their dual role as both a researcher and a clinician [ 22 , 23 , 24 ]—investigators recognised the need to generate data to improve treatments but were also concerned about minimizing risks of experimental treatments to individual participants. Analyses of informed consent discussions and interviews with investigators indicate that investigators seldom confirm a patient’s level of understanding at any point during the conversation [ 25 , 26 ]. Despite this, it seems that investigators remain concerned or in some cases uncertain about how well their patients have understood the study [ 7 , 23 ]. Several studies have also indicated that many research staff do not receive training on how to facilitate an optimal informed consent discussion [ 5 , 27 , 28 ]—this may affect the experience for both staff and participant.
In aggregate, the above studies indicate that the process of informed consent is not straightforward and many factors influence both the participant’s and the investigator’s experience. However, few or no studies have sought to quantify how clinical research participants and staff experience the process of consent and the importance of contextual factors such as the time spent, the setting of the informed consent discussion and the timing at which the participant is approached. Given this gap in our understanding, the aim of this study is to describe how participants and research staff experience the informed consent process and the contextual factors that contribute to their satisfaction with the process.
Survey design and piloting
This study was conducted in accordance with the principles of the Declaration of Helsinki [ 29 ]. Two anonymous surveys were designed to meet the objectives of the study—one for participants (see Additional file 1 ) and one for research staff (see Additional file 2 ). The survey for research participants contained 14 multiple choice questions; the survey for research staff contained 16 multiple-choice questions. Both surveys included an optional, open-ended question for respondents to add any additional opinions related to the topic. Figure 1 illustrates how the survey data were collected and stored. For research participants, a matched paper and electronic version of the survey were available. This maximised accessibility for respondents who may not have access to an electronic device or prefer to complete surveys on paper. To allow for participants who had taken part in more than one study or trial, participants were asked to answer the survey based on the most recent study or trial they were consented to. For research staff, an online survey was exclusively used. In order to record their most usual consent practices, research staff were not asked to answer the survey based on their most recent consent discussion, except for one question about the duration of their last consent discussion. Attempts were made to mitigate acquiescence bias (the tendency of responders to provide positive responses) by including Likert scales, neutral questions and options such as ‘I can’t remember’. Both surveys were piloted among six members of the target groups and the wording of the surveys was adjusted following their feedback, to ensure that the questions were clear. Piloting indicated that the surveys took an average of 5 min to complete. Full ethics approval was granted by the Saint Vincent’s Healthcare Group Ethics and Medical Research Committee, Dublin 4, Ireland; Ref: RS20-026. A low-risk ethics exemption was also granted by the University College Dublin Research Ethics Committee; Ref: LS-E-20-117-OSullivan-Doran.
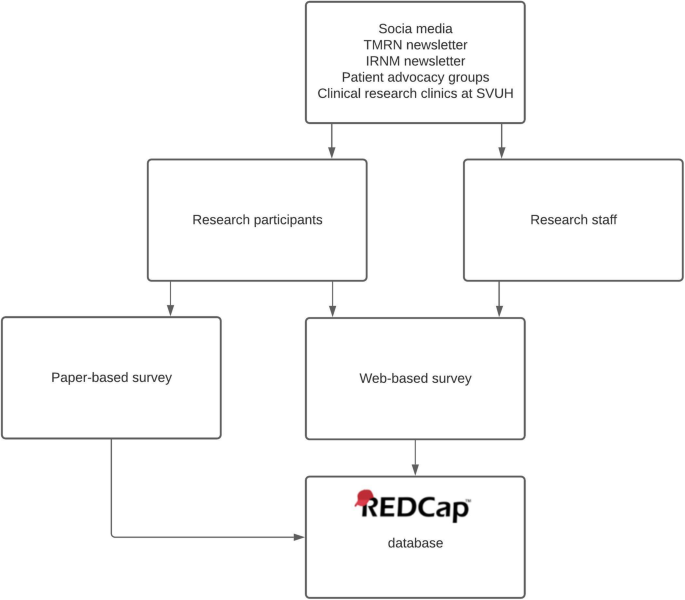
Flowchart describing the dissemination of the surveys. TMRN Trials Methodology Research Network, IRNM Irish Research Nurses and Midwives Network, SVUH Saint Vincent’s University Hospital
Convenience sampling was used. Research participants were eligible to complete the survey if they were > 18 years old and had taken part in a research study in Ireland or the United Kingdom (UK). Research staff were eligible to complete the survey if they facilitate informed consent discussions with an adult, lay research participants in Ireland or the UK. Due to restrictions in place because of the COVID-19 pandemic, some hospital clinics were being conducted by phone, which restricted the distribution of paper surveys. After discussion with the lead research nurse, it was decided that 200 paper surveys could be distributed during the data collection period. Therefore, 200 existing research participants in a single hospital, affiliated with the host university, were offered a paper-based survey by their clinical or research team (research nurse, investigator or clinician) at their routine, in-person, research or clinical visits, with an information leaflet and a stamped addressed envelope to return the survey. This method was used to facilitate participants who prefer a paper-based survey, in order to encourage responses. The surveys were distributed in dermatology, respiratory, oncology, rheumatology, infectious diseases and endocrinology clinics. Requests for research staff were also made via social media (Twitter, Linked In), via the Health Research Board-Trials Methodology Research Network and Clinical Research Coordination Ireland newsletters, and the Irish Research Nurses and Midwives Network. A digital marketing strategy was used to promote the survey to research participants on social media (Facebook, Instagram, Twitter) and through Irish and UK patient advocacy groups. Respondents were also asked to forward the survey to relevant contacts (chain referral sampling). Chain referral sampling provides a swift and cost-effective method of data collection, while ensuring the privacy and confidentiality of prospective respondents [ 30 ]. Due to this method of sampling, it is not possible to accurately estimate the response rate for the online version of the survey. However, the response rate was recorded for the paper-based surveys. Responder bias was minimised by using very short [ 31 , 32 ] and anonymous [ 33 ] surveys. Social desirability bias was minimised by ensuring that research participants returned the survey by post and not to their healthcare or research teams, or by completing the survey online. Respondents were advised in the information provided (either in paper version or on the online survey cover page) that by completing and submitting/returning the survey they were indicating their consent to take part (see Additional Files 3 and 4 ). Data collection took place between September 2020 and February 2021.
Study data were managed using the Research Electronic Data Capture (REDCap) (REDCap 7.4.10, 2019) tool hosted at University College Dublin [ 34 , 35 ]. For the online surveys, responses were inputted directly into REDCap by the respondents. For the paper surveys, the participants’ responses were manually inputted into REDCap by a single researcher (LOS). The same researcher reviewed the data entry for a random 20% of surveys, selected using Microsoft Excel, after an interval of 3 months to ensure accuracy in data entry.
Close-ended questions
Descriptive statistics were used to summarise the following characteristics:
The research participants’ level of satisfaction with the time, timing, location, level of information and explanation provided
The research staff’s level of experience, the training provided to them (if any), approach taken when facilitating informed consent discussions, time spent, confidence level, perceived barriers.
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) (IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.).
Open-ended questions
Responses to the single, optional, open-ended question were extracted—every effort was made to include the verbatim text, but where necessary, some details were omitted to ensure confidentiality. The responses were analysed independently using a thematic approach [ 36 ] by two researchers (LOS and PS). An experienced qualitative researcher (EMcA) reviewed the extracted codes against the quotations to ensure consistency. The consensus was then reached between the two researchers on the extracted themes.
This study is reported in accordance with the Checklist for Reporting Results of Internet E-Surveys (CHERRIES) [ 37 ]—the completed checklist is contained in Additional File 5 .
Research participants
One hundred sixty-nine research participants completed the survey. The response rate for the paper-based survey for research participants was 24% (47 out of 200 offered the survey). The remaining responses were to the online survey. Missing fields were denoted in the results by ‘Didn’t Answer’. Table 1 shows the length of time since the participant signed the consent form and whether they felt the location was comfortable and private. Most participants reported having signed the consent form over a year ago (91 or 45%) or in the last year (30 or 18%). Most participants (160 or 95%) felt the location where they signed the consent form was both comfortable and private. In terms of how participants were informed about the study/trial, the majority of participants (137 or 81%) reported that they were given a verbal explanation by the research team, while only 9 (5%) had watched a video or looked at a website about the research study or trial. Overall, 99 (59%) were Very Satisfied and 59 (35%) were Satisfied with their experience of informed consent. The mean time taken for the research participant’s last informed consent discussion was 51 min (range: 1–300 min; median: 30 min)—see Fig. 2 .

Time taken for informed consent discussion, reported by research participants
Table 2 below indicates that most research participants (149 or 88%) indicated that the time given by the research team to explain the study or trial was about right. The majority of participants also indicated that timing (the day on which they were approached with the study/trial) was a good (74 or 44%) or alright time (41 or 24%). Nearly a third of participants (45 or 27%) responded that the timing would not have made any difference to them. Most respondents (155 or 92%) also felt they had enough time to decide if they wanted to take part.
Table 3 reports how respondents felt about the information, explanation and written information provided to them. The majority of respondents (93%) reported that the amount of information given to them about the study/trial was about right. Similarly, participants said the study/trial was explained to them very well (100 or 60%) or fairly well (58 or 34%). Participants felt that the Participant Information Leaflet was Very Easy (48 or 29%) and Easy (71 or 42%) to understand.
One hundred and forty (83%) participants were encouraged to ask questions during the consent process and 126 (74%) felt that their questions were answered well. Most participants were positive about how well they had understood the study/trial: Very Well 76 (45%), Well 46 (28%) and Fairly Well 34 (20%).
Optional open-ended question
Seventy research participants responded to the optional, open-ended invitation to add any other feedback. The full list of quotations is included in Additional File 6 . The following three themes emerged from these responses:
Reports of positive experiences with the research team.
The value of allowing sufficient time for the participant to consider the study/trial, including time for questions. This may include providing information to the participant in advance of the clinic visit.
The importance of having follow-up after the study/trial has ended, e.g. to be given the results of the trial.
Two sample quotations for each theme are included below:
Research participant 022: ‘ [X Nurse], [X Hospital] is excellent, very helpful and so pleasant to deal with. She explains everything clearly and makes sure all your questions are answered. I know she's always on the end of the phone if needed, which gives me great peace of mind. 10/10’
Research participant 026: ‘ The research staff were very encouraging and open. I felt very involved in the process’.
Research Participant 009: ‘They emailed the document to me before went for study visit. This was really helpful to consider info in my own time in my own surroundings. This meant time could be spent asking for clarification on areas of concern during the meeting without feeling rushed in any way’.
Research participant 068: ‘The research study was introduced during a hospital/clinic appointment. I think that a prior notification that this would happen would have been useful. Normally at a hospital appointment, I would already have questions to ask and information to clarify. So the additional information about research can be difficult to process on day. Prior notification would allow the patient time to mentally prepare, and on a practical note allow them to allocate extra time for hospital visit’.
Research participant 032: ‘Would like to know how the initial results of the trial going’.
Research participant 051: ‘ I would have liked some feedback from the researchers’.
In summary, research participants were positive overall about their experiences of the informed consent process, the time allocated to the process, the amount of information given to them, the environment in which their consent was sought and how well they felt they had understood the study or trial. However, two interesting themes which emerged in response to the open-ended question were the need to allocate sufficient time to the informed consent process and the importance of follow-up or feedback once the trial has finished.
Research staff
One hundred fifteen research staff completed the survey; Fig. 3 and Table 4 describe this cohort. Respondents identified themselves primarily as (respondents could select more than one response):
Research Nurses or Research Midwives (53 or 46%)
Study Coordinators (31 or 27%)
Principal Investigators (22 or 19%).

Research staff—roles of respondents (respondents could select more than one response)
The majority of respondents carried out informed consent discussions in hospitals (99 or 86%). Respondents worked on Clinical Trials of Investigational Medicinal Products (CTIMPs) (83 or 72%), Observational studies (75 or 65%), Translational/Biomarker/Biobanking studies (63 or 55%), Registry trials (40 or 35%), non-CTIMP intervention (surgery, psychology, physiotherapy, radiotherapy, etc.) (45 or 39%) and Medical device studies (17 or 15%). The majority of respondents were experienced: 69 (60%) having greater than 5 years and 13 (11%) having 3–5 years of experience facilitating informed consent discussions. Most research staff (74 or 64%) of research staff had received training on how to facilitate informed consent discussions while 38 (33%) had not. Of those who had received training, 45 (58%) had formal/structured training, 41 (55%) observed a senior colleague and 39 (53%) had informal ‘on-the-job’ training (respondents could select more than one answer). Just over one third of research staff (36 or 31%) had received two forms of training while 23 (20%) of respondents had received all three forms of training. The mean time taken during research staff’s last informed consent discussion was 28 min (range: 3–120 min; median: 20 min)—see Fig. 4 .

Time taken for informed consent discussion, reported by research staff
Table 5 reports the difficulties with the informed consent process as reported by research staff. Respondents felt that the PIL/ICF was too long and/or too complex (72 or 63%), the difficulty for participants to understand complex information (64 or 56%), time pressures (46 or 40%), difficulties explaining complex information (44 or 38%), participants being anxious or upset (32 or 28%) and other difficulties (11 or 11%) or no difficulties (4 or 3.5%). In terms of how difficult PILs/ICFs are for participants, 9 (8%) rated them as ‘Very hard’, 44 (39%) as ‘Fairly Hard’, 43 (37%) as ‘Fairly Easy’, 13 (11%) as ‘Easy’, 0 (0%) as ‘Very Easy’ and 6 (5%) did not reply. Research staff reported the following as factors which would deter them from approaching a potential participant:
Patient is too anxious or upset (55 or 48%)
Patient does not have enough time (52 or 45%)
Patient has already received too much information at this visit (44 or 38%)
Do not think participant will understand the study/trial (32 or 28%)
Do not have enough time in clinic (31 or 27%)
Other (21%), did not reply (8 or 7%)
Not applicable—all eligible patients approached (3 or 2.6%)
Table 6 details the approach taken by research staff during the informed consent process: 105 (91%) explain the study verbally, 97 (84%) give participants a PIL and ask them to read it, 55 (48%) read the PIL to participants, 7 (6%) show a participant a video or website and 12 (10%) do ‘Other’, including providing initial information and then following up, working with a translator and summarising important information. Research staff followed a structured approach (i.e., following a checklist or the structure of the PIL): all the time (40 or 35%), often (36 or 31%), occasionally (16 or 14%), never (17 or 15%) and did not reply (6 or 5%). Most research staff (108 or 88%) reported that they check participants’ level of understanding prior to consent, 5 (6%) reported that they did not and 5 (6%) did not answer the question. For those who do check participants’ level of understanding, 88% ask participant if they have understood, 89 (35%) ask the participant to ‘teach back’ or ‘talk back’, 93 (81%) encourage participants to ask questions, 6 (6%) reported using other means, while 2 (2%) did not reply.
Figure 5 provides an overview of the confidence level of research staff to facilitate a good informed consent process. Respondents were ‘Very confident’: 36 (31%), ‘Confident’: 49 (43%), ‘Somewhat confident’: 20 (17%), ‘Not very confident’: 2 (2%), ‘Not at all confident’: 0 (0%) and Did not reply 8 (7%).
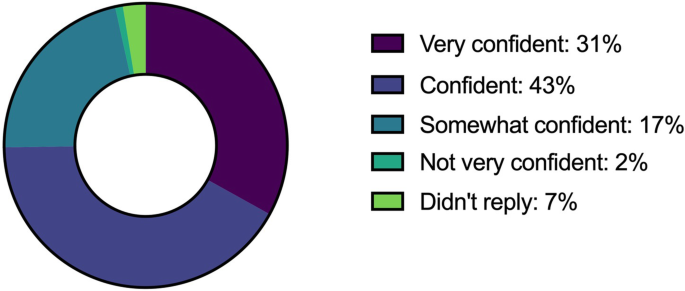
Research staff—confidence level in facilitating informed consent discussions
Table 7 shows the factors which research staff felt would improve the informed consent process for participants: shorter/simpler PIL: (86 or 75%), a PIL with simple diagrams or pictures (68 or 59%), resources like an app or video (56 or 49%), more time with the participant (54 or 47%) and more time with another member of the research team (29 or 25%). Other responses included a quiet, dedicated, uninterrupted space (6 or 5%), PIL with less GDPR information (2 or 2%), PILs in other languages (1 or 0.8%) and miscellaneous (3 or 3%).
Thirty research staff responded to the optional, open-ended request to add any other feedback. The full list of quotations is included in Additional File 7 . A very dominant theme emerged: research staff feel that the time and resources, particularly space, are important to facilitate a good informed consent process and these factors are often limited in supply. The following sample quotations illustrate this theme:
Research staff 003: ‘As a researcher, it feels like the definition of informed consent is constantly changing, the bar is always going up. This is of course, a good thing. But the consequence is the need to have ongoing dialogue. This requires significant resources that the system is not currently providing.’
Research staff 008: ‘Research staff often struggle for dedicated space to conduct informed consent and this can add unnecessary stress and burden to the process. Clinical trials personnel should have dedicated areas for completing this important process with appropriate resources and time availability.’
Research staff 012: ‘Resources badly needed. Dedicated trial clinics. Protected time.’
Research staff 015: ‘Discussing consent in a busy clinical environment is very difficult.’
Three additional themes emerged:
Consent process can be challenging; training on how to facilitate is needed
Technology could be used to improve the informed consent process
An accessible PIL/ICF is important; GDPR/data protection information is often too long, too complicated
Research staff 029: ‘I have had many challenging discussions with collaborators around rates of recruitment which may be lower than others but at least I know I am running my studies with the highest ethical standards….it can be very hard though!’
Research staff 008: ‘Research staff should have more formalised training in the trial and consent process.’
Research staff 005: ‘We need greater facilitation of remote consent (telephone etc.) especially with lack of visiting due to COVID.’
Research staff 028: ‘The issue of informed consent, and electronic consent, is increasingly relevant with the COVID-19 pandemic.’
Research staff 007: ‘PILs much too complex particularly with data protection which patients find cumbersome and excessive.’
Research staff 018: ‘Info leaflets are getting more complicated with GDPR/data protection information. It is almost impossible to make it shorter without risking rejection by ethics committee.’
In summary, research staff in this study reported good levels of experience and confidence in facilitating informed consent discussions. Research staff indicated that a shorter, simpler PIL/ICF or a PIL/ICF with diagrams, the use of technologies and additional time with participants would improve the overall informed consent process.
Summary of key findings
This study quantified a number of factors which are vital to the informed consent process, from the perspectives of both research participants and research staff. Research participants were generally positive about each aspect of the informed consent process explored in the survey. However, they did highlight the importance of having sufficient time and the importance of providing follow-up once the study/trial concludes, e.g. providing the results to research participants. Research staff reported that they felt quite confident with the process of informed consent overall, possibly reflecting that the respondents in this case were generally experienced in facilitating informed consent discussions. Barriers to the informed consent process noted by research staff included lengthy, complex PILs/ICFs, difficulties communicating complex information and time constraints.
Participants report positive experience but have they understood?
Research participants in this study overwhelmingly reported a positive experience of the informed consent process and high levels of understanding of the trial or study. This correlates well with previous studies reporting high levels of satisfaction among research participants [ 9 , 38 , 39 , 40 , 41 ]. It is encouraging to report that the majority of research participants are happy with their experience of the informed consent process, which is testament to the dedication and commitment of research staff. However, other studies consistently demonstrate that when research participants are assessed, they often have a poor understanding of key parts of the study or trial [ 42 , 43 ]. Hietnen’s survey of 261 participants in an oncology trial indicated that while 91% of participants were satisfied with the difficulty level of the information given, 51% had misunderstood randomisation [ 44 ]. It is also concerning to note that Pope et al.’s study found that participants who reported to have received the ‘right amount of information’ were unfortunately not found to have a higher level of understanding of blinding [ 9 ]. Studies have shown the participants’ information needs vary considerably [ 16 , 17 ], and it is possible that research participants feel that they have gained sufficient knowledge to make a decision without understanding key aspects of the trial. However, this jars with the doctrine of informed consent, which states that participants must be informed about the pertinent information prior to providing consent [ 45 ].
The importance of adequate time and resources
Research staff consistently reported in this study that they felt that time and resources such as space were limiting factors in their ability to facilitate an optimal informed consent process—40% reported that time pressures were a difficult component of the informed consent process and 47% felt that more time would improve the informed consent process for participants. This finding also emerged in the responses to the open-ended question from both research staff and research participants. Spaar et al. similarly reported lack of time as the biggest barrier to the process of recruitment to randomised trials [ 20 ]. This is of concern, since two systematic reviews identified additional time as one of the few factors which has been shown to significantly improve participants’ understanding [ 46 , 47 ]. Studies by Aaronson [ 48 ] and Tindall [ 49 ] both found that an additional conversation with a member of the research team improved participants’ understanding. Research participants in this survey study also noted in the open-ended question that it may be helpful to receive the information about a trial in advance of the consent discussion with the research staff, in order to have additional time to consider the information.
The Participant Information Leaflet/Informed Consent Form
Sixty-three percent of research staff in this study felt that the PIL/ICF is too long and complex and 75% reported that a shorter and simpler document would improve the informed consent process. However, it is interesting that research participants did not share the same view, with the majority stating they felt the PIL/ICF was Very Easy, Easy or Fairly Easy to understand. Lynoe’s and Montgomery’s studies with participants of a chronic dialysis and anaesthesia trial similarly found that most participants found the PIL to be helpful and easy to read [ 50 , 51 ]. There is evidence that PILs/ICFs are becoming longer and more complex [ 52 , 53 ], and it is challenging for research teams and sponsors to balance giving the pertinent information without overwhelming potential participants. The General Data Protection legislation introduced in the European Union brought about extra challenges as additional information must now be provided to participants [ 54 , 55 ]. While participant satisfaction with the information provided is undoubtedly important, helping participants understand the key elements needed to provide informed consent is also critical. Regarding multimedia resources, such as an application, website or video: only 5% of research participants were offered them and only 6% of research staff use them—while further empirical research is needed to assess the effect of multimedia on the quality of informed consent, these kinds of supports may be useful, particularly for some groups of participants [ 46 , 56 , 57 ].
Teach Back/Talk Back method
In this study, 88% of research staff reported that they confirm a participant’s level of understanding. This self-reported rate was higher than in Jenkins’ analysis of 82 recordings of actual informed consent discussions which indicated that in nearly 83% of cases participant understanding was not confirmed [ 26 ]. The majority of research staff in this study reported that they confirm participant’s understanding by asking if they have any questions. However, Nusbaum and colleagues were critical of simply asking the participant if they have understood or if they have any questions—how can a participant judge themselves if they have understood? It may also be difficult for participants to ask questions if they have not understood key pieces of information [ 28 ]. In this study, 19% of research participants reported that they did not have any questions to ask. Interestingly, Keller’s semi-structured interviews with 18 research staff indicated that Principal Investigators tend to approach participants who do not ask too many questions and those without a strong personality [ 42 ]. Cox noted that 40% of clinical research participants interviewed regarding their experiences did not feel able to ask questions [ 58 ]. Thirty-five percent of research staff in this study reported that they used Teach Back/Talk Back strategies to ensure that participants have understood. The Teach Back/Talk Back method involves the patient or service user verbally relaying the information that they have been given back to the provider and has been found to have a positive effect on health communication in the clinical (non-research) setting [ 59 ]. Flory and Emanuel’s systematic review of interventions to improve informed consent reported five trials which indicates that test/feedback showed significant improvement in understanding [ 47 ]. Perhaps, therefore, Teach Back/Talk Back strategies should be encouraged when researchers are undergoing training on how to facilitate informed consent. However, it should be noted that the Teach Back/Talk Back method may require additional time, which may already be in short supply.
Limitations
The method of sampling, in particular the use of chain referral sampling is a limitation of this study, as it is non-random in nature [ 30 ]. There may have been enthusiasm bias as both research staff and research participants who are interested in optimizing the informed consent process may have been more likely to respond to the survey. Similarly, there may have been selection bias associated with the distribution of the paper surveys to research participants—due to the COVID-19 restrictions, the surveys had to be given out by the research or clinical staff and not systematically by an independent researcher. The survey was developed specifically to fulfil the aims of this research study and was piloted with representatives from both target groups but was not formally validated. It was not possible to estimate the response rate of the online surveys and approximately a quarter of the research participants were recruited from a single hospital—this makes it difficult to assess the generalisability of the results. Structured, anonymised surveys were used to gather quantitative data regarding specific factors which relate to the process of informed consent. While additional open-ended questions may have explored these factors in more depth, previous qualitative studies have investigated the perceptions of research staff and participants [ 22 , 42 , 60 , 61 , 62 ]. It is also important to note that the research staff in this study had a range of experiences, perhaps in different settings, to report on from when answering the survey, while some of the research participants had only a single consent discussion experience to draw from. Finally, the lack of demographic detail for the research participants, e.g. education level, limits the generalisability of the findings of this study.
Research participants in this study were overwhelmingly positive about their experience of the informed consent process. However, research staff expressed concern about how much participants have understood and studies of patient comprehension of research study information would seem to confirm these fears. Adequate time should be allocated to informed consent discussions and research staff could consider using Teach Back/Talk Back techniques to confirm and enhance understanding.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
General Data Protection Regulation
General Practitioner
Health Research Board – Trials Methodology Research Network
Informed Consent Form
Irish Research Nurses and Midwives Network
Patient Information Leaflet
Research Electronic Data Capture
Statistical Package for the Social Science
United Kingdom
Cahana A, Hurst SA. Voluntary informed consent in research and clinical care: an update. Pain Pract. 2008;8(6):446–51. https://doi.org/10.1111/j.1533-2500.2008.00241.x .
Article PubMed Google Scholar
Gupta UC. Informed consent in clinical research: revisiting few concepts and areas. Perspectives in clinical research. 2013;4(1):26–32. https://doi.org/10.4103/2229-3485.106373 .
Article PubMed PubMed Central Google Scholar
Declaration of Helsinki. Helsinki, Finland: World Medical Association; 2013. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ . Accessed 3 Aug 2021.
Corrigan O. Empty ethics: the problem with informed consent. Sociol Health Illn. 2003;25(7):768–92. https://doi.org/10.1046/j.1467-9566.2003.00369.x .
Grady C. Enduring and emerging challenges of informed consent. New England Journal of Medicine. 2015;372(9):855–62. https://doi.org/10.1056/NEJMra1411250 .
Article CAS Google Scholar
Sugarman J, McCrory DC, Powell D, Krasny A, Adams B, Ball E, et al. Special supplement: empirical research on informed consent: an annotated bibliography. Hastings Center Report. 1999;29(1):S1–S42. https://doi.org/10.2307/3528546 .
Verheggen FW, Jonkers R, Kok G. Patients’ perceptions on informed consent and the quality of information disclosure in clinical trials. Patient Educ Counseling. 1996;29(2):137–53. https://doi.org/10.1016/0738-3991(96)00859-2 .
Fleissig A, Jenkins V, Fallowfield L. Results of an intervention study to improve communication about randomised clinical trials of cancer therapy. Eur J Cancer. 2001;37(3):322–31. https://doi.org/10.1016/S0959-8049(00)00415-9 .
Article CAS PubMed Google Scholar
Pope JE, Tingey DP, Arnold JM, Hong P, Ouimet JM, Krizova A. Are subjects satisfied with the informed consent process? A survey of research participants. J Rheumatol. 2003;30(4):815–24.
PubMed Google Scholar
Tam NT, Huy NT, Thoale TB, Long NP, Trang NT, Hirayama K, et al. Participants’ understanding of informed consent in clinical trials over three decades: systematic review and meta-analysis. Bull World Health Organ. 2015;93(3):186–98h.
Article Google Scholar
Burgess LJ, Gerber B, Coetzee K, Terblanche M, Agar G, Kotze TJ. An evaluation of informed consent comprehension by adult trial participants in South Africa at the time of providing consent for clinical trial participation and a review of the literature. Open Access J Clin Trials. 2019;11:19–35. https://doi.org/10.2147/OAJCT.S145068 .
Knifed E, Lipsman N, Mason W, Bernstein M. Patients’ perception of the informed consent process for neurooncology clinical trials. Neuro-oncology. 2008;10(3):348–54. https://doi.org/10.1215/15228517-2008-007 .
Hamnes B, van Eijk-Hustings Y, Primdahl J. Readability of patient information and consent documents in rheumatological studies. BMC Medical Ethics. 2016;17(1):42. https://doi.org/10.1186/s12910-016-0126-0 .
O'Sullivan L, Sukumar P, Crowley R, McAuliffe E, Doran P. The readability and understandability of clinical research patient information leaflets and consent forms in Ireland and the UK: a retrospective quantitative analysis. BMJ Open. 2020;10(9).
Gillies K, Huang W, Skea Z, Brehaut J, Cotton S. Patient information leaflets (PILs) for UK randomised controlled trials: a feasibility study exploring whether they contain information to support decision making about trial participation. Trials. 2014;15(1):62. https://doi.org/10.1186/1745-6215-15-62 .
Kirkby HM, Calvert M, Draper H, Keeley T, Wilson S. What potential research participants want to know about research: a systematic review. BMJ Open. 2012;2(3).
Smyth RMD, Jacoby A, Elbourne D. Deciding to join a perinatal randomised controlled trial: Experiences and views of pregnant women enroled in the Magpie Trial. Midwifery. 2012;28(4):E478–E85. https://doi.org/10.1016/j.midw.2011.08.006 .
Appelbaum PS, Lidz CW, Grisso T. Therapeutic misconception in clinical research: frequency and risk factors. IRB: Ethics & Human Research. 2004;26(2):1–8. https://doi.org/10.2307/3564231 .
Fallowfield L, Ratcliffe D, Souhami R. Clinicians’ attitudes to clinical trials of cancer therapy. European Journal of Cancer. 1997;33(13):2221–9. https://doi.org/10.1016/S0959-8049(97)00253-0 .
Spaar A, Frey M, Turk A, Karrer W, Puhan MA. Recruitment barriers in a randomized controlled trial from the physicians’ perspective – a postal survey. BMC Medical Research Methodology. 2009;9(1):14. https://doi.org/10.1186/1471-2288-9-14 .
Bernhardt BA, Roche MI, Perry DL, Scollon SR, Tomlinson AN, Skinner D. Experiences with obtaining informed consent for genomic sequencing. American Journal of Medical Genetics Part A. 2015;167(11):2635–46. https://doi.org/10.1002/ajmg.a.37256 .
Donovan JL, de Salis I, Toerien M, Paramasivan S, Hamdy FC, Blazeby JM. The intellectual challenges and emotional consequences of equipoise contributed to the fragility of recruitment in six randomized controlled trials. Journal of Clinical Epidemiology. 2014;67(8):912–20. https://doi.org/10.1016/j.jclinepi.2014.03.010 .
Taylor KM, Kelner M. Interpreting physician participation in randomized clinical trials: the physician orientation profile. J Health Soc Behav. 1987;28(4):389–400. https://doi.org/10.2307/2136792 .
Taylor KM, Margolese RG, Soskolne CL. Physicians’ reasons for not entering eligible patients in a randomized clinical trial of surgery for breast cancer. N Engl J Med. 1984;310(21):1363–7. https://doi.org/10.1056/NEJM198405243102106 .
Schröder Håkansson A, Pergert P, Abrahamsson J, Stenmarker M. Balancing values and obligations when obtaining informed consent: healthcare professionals’ experiences in Swedish paediatric oncology. Acta paediatrica (Oslo, Norway: 1992). 2019.
Jenkins VA, Fallowfield LJ, Souhami A, Sawtell M. How do doctors explain randomised clinical trials to their patients? European Journal of Cancer. 1999;35(8):1187–93. https://doi.org/10.1016/S0959-8049(99)00116-1 .
Boden-Albala B, Carman H, Southwick L, Parikh Nina S, Roberts E, Waddy S, et al. Examining barriers and practices to recruitment and retention in stroke clinical trials. Stroke. 2015;46(8):2232–7. https://doi.org/10.1161/STROKEAHA.114.008564 .
Nusbaum L, Douglas B, Damus K, Paasche-Orlow M, Estrella-Luna N. Communicating risks and benefits in informed consent for research: a qualitative study. Global Qualitative Nursing Research. 2017;4:2333393617732017.
Declaration of Helsinki. Helsinki, Finland: World Medical Organisation; 2013.
Johnson TP. Snowball sampling: introduction. Statistics Reference Online: Wiley StatsRef; 2014.
Google Scholar
Sahlqvist S, Song Y, Bull F, Adams E, Preston J, Ogilvie D, et al. Effect of questionnaire length, personalisation and reminder type on response rate to a complex postal survey: randomised controlled trial. BMC Med Res Methodol. 2011;11(1):62. https://doi.org/10.1186/1471-2288-11-62 .
Jones TL, Baxter MAJ, Khanduja V. A quick guide to survey research. Annals of the Royal College of Surgeons of England. 2013;95(1):5–7. https://doi.org/10.1308/003588413X13511609956372 .
Article CAS PubMed PubMed Central Google Scholar
Sudman S, Bradburn NM. Asking questions: a practical guide to questionnaire design. San Francisco;Oxford: Jossey-Bass; 1982.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–81. https://doi.org/10.1016/j.jbi.2008.08.010 .
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208.
Braun V, Clarke V. Using thematic analysis in psychology. Qualitative Research in Psychology. 2006;3(2):77–101. https://doi.org/10.1191/1478088706qp063oa .
Eysenbach G. Improving the quality of Web surveys: the Checklist for Reporting Results of Internet E-Surveys (CHERRIES). J Med Internet Res. 2004;6(3):e34. https://doi.org/10.2196/jmir.6.3.e34 .
Franck LS, Winter I, Oulton K. The quality of parental consent for research with children: a prospective repeated measure self-report survey. International Journal of Nursing Studies. 2007;44(4):525–33. https://doi.org/10.1016/j.ijnurstu.2006.03.014 .
Kupst MJ, Patenaude AF, Walco GA, Sterling C. Clinical trials in pediatric cancer: parental perspectives on informed consent. Journal of Pediatric Hematology/Oncology. 2003;25(10).
Penman DT, Holland JC, Bahna GF, Morrow G, Schmale AH, Derogatis LR, et al. Informed consent for investigational chemotherapy: patients’ and physicians’ perceptions. Journal of Clinical Oncology. 1984;2(7):849–55. https://doi.org/10.1200/JCO.1984.2.7.849 .
Goodman NW, Cooper GM, Malins AF, Prys-Roberts C. The validity of informed consent in a clinical study. Anaesthesia. 1984;39(9):911–6. https://doi.org/10.1111/j.1365-2044.1984.tb06582.x .
Keller P-H, Grondin O, Tison F, Gonon F. How health professionals conceptualize and represent placebo treatment in clinical trials and how their patients understand it: impact on validity of informed consent. PloS one. 2016;11(5):e0155940. https://doi.org/10.1371/journal.pone.0155940 .
Moynihan C, Lewis R, Hall E, Jones E, Birtle A, Huddart R, et al. The Patient Deficit Model Overturned: a qualitative study of patients’ perceptions of invitation to participate in a randomized controlled trial comparing selective bladder preservation against surgery in muscle invasive bladder cancer (SPARE, CRUK/07/011). Trials. 2012;13(1):228.
Hietanen P, Aro AR, Holli K, Absetz P. Information and communication in the context of a clinical trial. European Journal of Cancer. 2000;36(16):2096–104. https://doi.org/10.1016/S0959-8049(00)00191-X .
Beauchamp TL, Childress JF. Principles of biomedical ethics. 7th ed. New York;Oxford: Oxford University Press; 2013.
Nishimura A, Carey J, Erwin PJ, Tilburt JC, Murad MH, McCormick JB. Improving understanding in the research informed consent process: a systematic review of 54 interventions tested in randomized control trials. BMC Med Ethics. 2013;14(1):28. https://doi.org/10.1186/1472-6939-14-28 .
Flory J, Emanuel E. Interventions to improve research participants’ understanding in informed consent for research: a systematic review. JAMA. 2004;292(13):1593–601. https://doi.org/10.1001/jama.292.13.1593 .
Aaronson NK, Visser-Pol E, Leenhouts GH, Muller MJ, van der Schot AC, van Dam FS, et al. Telephone-based nursing intervention improves the effectiveness of the informed consent process in cancer clinical trials. J Clin Oncol. 1996;14(3):984–96. https://doi.org/10.1200/JCO.1996.14.3.984 .
Tindall B, Forde S, Ross MW, Goldstein D, Barker S, Cooper DA. Effects of two formats of informed consent on knowledge amongst persons with advanced HIV disease in a clinical trial of didanosine. Patient Educ Couns. 1994;24(3):261–6. https://doi.org/10.1016/0738-3991(94)90069-8 .
Lynöe N, Näsström B, Sandlund M. Study of the quality of information given to patients participating in a clinical trial regarding chronic hemodialysis. Scand J Urol Nephrol. 2004;38(6):517–20. https://doi.org/10.1080/00365590410033362 .
Montgomery J, Sneyd J. Consent to clinical trials in anaesthesia. Anaesthesia. 1998;53(3):227–30. https://doi.org/10.1046/j.1365-2044.1998.00309.x .
Beardsley E, Jefford M, Mileshkin L. Longer consent forms for clinical trials compromise patient understanding: so why are they lengthening? J Clin Oncol. 25. United States 2007. p. e13-4.
Hammerschmidt D, Md F, Keane M. Institutional review board (IRB) review lacks impact on the readability of consent forms for research. Am J Med Sci. 1992;304(6):348–51. https://doi.org/10.1097/00000441-199212000-00003 .
Clarke N, Vale G, Reeves EP, Kirwan M, Smith D, Farrell M, et al. GDPR: an impediment to research? Irish J Med Sci. 2019;188(4):1129–35. https://doi.org/10.1007/s11845-019-01980-2 .
General Data Protection Regulation (GDPR) (Regulation (EU) 2016/679 of the European Parliament and of the Council). European Union. 2016.
Synnot A, Ryan R, Prictor M, Fetherstonhaugh D, Parker B. Audio-visual presentation of information for informed consent for participation in clinical trials. Cochrane Database Syst Rev. 2014;(1):Cd003717.
Palmer BW, Lanouette NM, Jeste DV. Effectiveness of multimedia aids to enhance comprehension of research consent information: a systematic review. IRB. 2012;34(6):1–15.
PubMed PubMed Central Google Scholar
Cox K. Informed consent and decision-making: patients’ experiences of the process of recruitment to phases I and II anti-cancer drug trials. Patient Education and Counseling. 2002;46(1):31–8. https://doi.org/10.1016/S0738-3991(01)00147-1 .
Yen PH, Leasure AR. Use and effectiveness of the teach-back method in patient education and health outcomes. Fed Pract. 2019;36(6):284–9.
Durant RW, Wenzel JA, Scarinci IC, Paterniti DA, Fouad MN, Hurd TC, et al. Perspectives on barriers and facilitators to minority recruitment for clinical trials among cancer center leaders, investigators, research staff, and referring clinicians: enhancing minority participation in clinical trials (EMPaCT). Cancer. 2014;120(S7):1097–105. https://doi.org/10.1002/cncr.28574 .
Kost RG, Lee LN, Yessis JL, Wesley R, Alfano S, Alexander SR, et al. Research participant-centered outcomes at NIH-supported clinical research centers. Clinical and translational science. 2014;7(6):430–40. https://doi.org/10.1111/cts.12167 .
Gordon EJ, Knopf E, Phillips C, Mussell A, Lee J, Veatch RM, et al. Transplant candidates’ perceptions of informed consent for accepting deceased donor organs subjected to intervention research and for participating in posttransplant research. Am J Transplantation. 2019.
Download references
Acknowledgements
The research team is grateful to the Health Research Board - Trials Methodology Research Network, Clinical Research Coordination Ireland, the UCD Clinical Research Centre, the Irish Research Nurse’s Network, the Irish Platform for Patient Organisations Science and Industry, Patient Voices in Cancer Research, Patient Voice’s in Arthritis Research and Patient Voices in Diabetes Research for their contributions to this study.
This work was supported by the Health Research Board Trials Methodology Research Network (HRB-TMRM) as part of the HRB-TMRN-2017-1 grant. The funding body was not involved in the design of the study and collection, analysis and interpretation of data.
Author information
Authors and affiliations.
School of Medicine, University College Dublin, Belfield, Dublin 4, Ireland
Lydia O’ Sullivan, Laura Feeney, Rachel K. Crowley, Prasanth Sukumar & Peter Doran
Health Research Board-Trials Methodology Research Network, Galway, Ireland
Lydia O’ Sullivan & Peter Doran
Department of Endocrinology, Saint Vincent’s University Hospital, Dublin 4, Ireland
Rachel K. Crowley
University College Dublin Centre for Interdisciplinary Research, Education and Innovation in Health Systems, Belfield, Dublin 4, Ireland
Eilish McAuliffe
You can also search for this author in PubMed Google Scholar
Contributions
LOS contributed to the study design, carried out data collection and analysis and wrote the manuscript. LF contributed to the study design and data collection and reviewed the manuscript. PS built the REDCap database, contributed to the analysis and reviewed the manuscript. RC and EMcA participated in the study design and review of the manuscript. PD leads the research team, acquired funding for the study, contributed to the design of the research and reviewed the manuscript. The authors approved the final version of the manuscript prior to submission.
Authors’ information
Not applicable.
Corresponding author
Correspondence to Lydia O’ Sullivan .
Ethics declarations
Ethics approval and consent to participate.
Full ethics approval was granted by the Saint Vincent’s Healthcare Group Ethics and Medical Research Committee, Dublin 4, Ireland; Ref: RS20-026. A low-risk ethics exemption was also granted by the University College Dublin Research Ethics Committee; Ref: LS-E-20-117-OSullivan-Doran. Participants self-selected to complete the surveys after they were provided with an information leaflet explaining the study.
Consent for publication
Not applicable—no individual details, images or videos used.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
Research Participant’s Survey.
Additional file 2.
Research Staff Survey.
Additional file 3.
Participant Information Leaflet (Research Participants).
Additional file 4.
Participant Information Leaflet (Research Staff).
Additional file 5.
Checklist for Reporting Results of Internet E-Surveys (CHERRIES)
Additional file 6.
Complete list of responses from research participants to open-ended question
Additional file 7.
Complete list of responses from research staff to open-ended question
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
O’ Sullivan, L., Feeney, L., Crowley, R.K. et al. An evaluation of the process of informed consent: views from research participants and staff. Trials 22 , 544 (2021). https://doi.org/10.1186/s13063-021-05493-1
Download citation
Received : 24 May 2021
Accepted : 27 July 2021
Published : 18 August 2021
DOI : https://doi.org/10.1186/s13063-021-05493-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Informed consent
- Clinical research
- Clinical trials
- Methodology
- Surveys and Questionnaires
ISSN: 1745-6215
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Privacy Policy

Home » Informed Consent in Research – Types, Templates and Examples
Informed Consent in Research – Types, Templates and Examples
Table of Contents

Informed Consent in Research
Informed consent is a process of communication between a researcher and a potential participant in which the researcher provides adequate information about the study, its risks and benefits, and the participant voluntarily agrees to participate. It is a cornerstone of ethical research involving human subjects and is intended to protect the rights and welfare of participants.
Types of Informed Consent in Research
There are different types of informed consent in research , which may vary depending on the nature of the study, the type of participants, and the context. Some of the common types of informed consent in research include:
Written Consent
This is the most common type of informed consent, where participants are provided with a written document that explains the study and its requirements. The document typically includes information about the purpose of the study, procedures involved, risks and benefits, confidentiality, and participant rights. Participants are asked to sign the document as an indication of their willingness to participate.
Oral Consent
In some cases, oral consent may be used when a written document is not practical or feasible. Oral consent involves explaining the study and its requirements to participants verbally and obtaining their consent. This method may be used for studies with illiterate or visually impaired participants or when conducting research remotely.
Implied Consent
Implied consent is used in studies where participants’ actions are taken as an indication of their willingness to participate. For example, a participant may be considered to have given implied consent if they show up for a scheduled appointment for the study.
Opt-out Consent
This method is used when participants are given the opportunity to decline participation in a study. Participants are provided with information about the study and are given the option to opt-out if they do not wish to participate. This method is commonly used in population-based studies or surveys.
Assent is used in studies involving minors or participants who are unable to provide informed consent due to cognitive impairment or disability. Assent involves obtaining the agreement of the participant to participate in the study, along with the consent of a legally authorized representative.
Informed Consent Format in Research
Here’s a basic format for informed consent that can be customized for specific research studies:
- Introduction : Begin by introducing yourself and the purpose of the study. Clearly state that participation is voluntary and that participants can withdraw at any time without penalty.
- Study Overview : Provide a brief overview of the study, including its purpose, methods, and expected outcomes.
- Procedures : Describe the procedures involved in the study in clear, concise language. Include information about the types of data that will be collected, how they will be collected, and how long the study will take.
- Risks and Benefits : Outline the potential risks and benefits of participating in the study. Be honest and upfront about any discomfort, inconvenience, or potential harm that may be involved, as well as any potential benefits.
- Confidentiality and Privacy : Explain how participant data will be collected, stored, and used, and what measures will be taken to ensure confidentiality and privacy.
- Voluntary Participation: Emphasize that participation is voluntary and that participants can withdraw at any time without penalty. Explain how to withdraw from the study and who to contact if participants have questions or concerns.
- Compensation and Incentives: If applicable, explain any compensation or incentives that will be offered to participants for their participation.
- Contact Information: Provide contact information for the researcher or a representative from the research team who can answer questions and address concerns.
- Signature : Ask participants to sign and date the consent form to indicate their voluntary agreement to participate in the study.
Informed Consent Templates in Research
Here is an example of an informed consent template that can be used in research studies:
Introduction
You are being invited to participate in a research study. Before you decide whether or not to participate, it is important for you to understand why the research is being done, what your participation will involve, and what risks and benefits may be associated with your participation.
Purpose of the Study
The purpose of this study is [insert purpose of study].
If you agree to participate, you will be asked to [insert procedures involved in the study].
Risks and Benefits
There are several potential risks and benefits associated with participation in this study. Some of the risks include [insert potential risks of participation]. Some of the benefits include [insert potential benefits of participation].
Confidentiality
Your participation in this study will be kept confidential to the extent allowed by law. All data collected during the study will be stored in a secure location and only accessed by authorized personnel. Your name and other identifying information will not be included in any reports or publications resulting from this study.
Voluntary Participation
Your participation in this study is completely voluntary. You have the right to withdraw from the study at any time without penalty. If you choose not to participate or if you withdraw from the study, there will be no negative consequences.
Contact Information
If you have any questions or concerns about the study, you can contact the investigator(s) at [insert contact information]. If you have questions about your rights as a research participant, you may contact [insert name of institutional review board and contact information].
Statement of Consent
By signing below, you acknowledge that you have read and understood the information provided in this consent form and that you freely and voluntarily consent to participate in this study.
Participant Signature: _____________________________________ Date: _____________
Investigator Signature: ____________________________________ Date: _____________
Examples of Informed Consent in Research
Here’s an example of informed consent in research:
Title : The Effects of Yoga on Stress and anxiety levels in college students
Introduction :
We are conducting a research study to investigate the effects of yoga on stress and anxiety levels in college students. We are inviting you to participate in this study.
If you agree to participate, you will be asked to attend four yoga classes per week for six weeks. Before and after the six-week period, you will be asked to complete surveys about your stress and anxiety levels. Additionally, we will measure your heart rate variability at the beginning and end of the six-week period.
Risks and Benefits:
There are no known risks associated with participating in this study. However, the benefits of practicing yoga may include decreased stress and anxiety levels, increased flexibility and strength, and improved overall well-being.
Confidentiality:
All information collected during this study will be kept strictly confidential. Your name will not be used in any reports or publications resulting from this study.
Voluntary Participation:
Participation in this study is completely voluntary. You are free to withdraw from the study at any time without penalty.
Contact Information:
If you have any questions or concerns about this study, you may contact the principal investigator at (phone number/email address).
By signing this form, I acknowledge that I have read and understood the above information and agree to participate in this study.
Participant Signature: ___________________________
Date: ___________________________
Researcher Signature: ___________________________
Importance of Informed Consent in Research
Here are some reasons why informed consent is important in research:
- Protection of participants’ rights : Informed consent ensures that participants understand the nature and purpose of the research, the risks and benefits of participating, and their rights as participants. It empowers them to make an informed decision about whether to participate or not.
- Ethical responsibility : Researchers have an ethical responsibility to respect the autonomy of participants and to protect them from harm. Informed consent is a crucial way to uphold these principles.
- Legality : Informed consent is a legal requirement in most countries. It is necessary to protect researchers from legal liability and to ensure that research is conducted in accordance with ethical standards.
- Trust : Informed consent helps build trust between researchers and participants. When participants understand the research process and their role in it, they are more likely to trust the researchers and the study.
- Quality of research : Informed consent ensures that participants are fully informed about the research and its purpose, which can lead to more accurate and reliable data. This, in turn, can improve the quality of research outcomes.
Purpose of Informed Consent in Research
Informed consent is a critical component of research ethics, and it serves several important purposes, including:
- Respect for autonomy: Informed consent respects an individual’s right to make decisions about their own health and well-being. It recognizes that individuals have the right to choose whether or not to participate in research, based on their own values, beliefs, and preferences.
- Protection of participants : Informed consent helps protect research participants from potential harm or risks that may arise from their involvement in a study. By providing participants with information about the study, its risks and benefits, and their rights, they are able to make an informed decision about whether to participate.
- Transparency: Informed consent promotes transparency in the research process. It ensures that participants are fully informed about the research, including its purpose, methods, and potential outcomes, which helps to build trust between researchers and participants.
- Legal and ethical requirements: Informed consent is a legal and ethical requirement in most research studies. It ensures that researchers obtain voluntary and informed agreement from participants to participate in the study, which helps to protect the rights and welfare of research participants.
Advantages of Informed Consent in Research
The advantages of informed consent in research are numerous, and some of the most significant benefits include:
- Protecting participants’ autonomy: Informed consent allows participants to exercise their right to self-determination and make decisions about whether to participate in a study or not. It also ensures that participants are fully informed about the risks, benefits, and implications of participating in the study.
- Promoting transparency and trust: Informed consent helps build trust between researchers and participants by providing clear and accurate information about the study’s purpose, procedures, and potential outcomes. This transparency promotes open communication and a positive research experience for all parties involved.
- Reducing the risk of harm: Informed consent ensures that participants are fully aware of any potential risks or side effects associated with the study. This knowledge enables them to make informed decisions about their participation and reduces the likelihood of harm or negative consequences.
- Ensuring ethical standards are met : Informed consent is a fundamental ethical requirement for conducting research involving human participants. By obtaining informed consent, researchers demonstrate their commitment to upholding ethical principles and standards in their research practices.
- Facilitating future research : Informed consent enables researchers to collect high-quality data that can be used for future research purposes. It also allows participants to make an informed decision about whether they are willing to participate in future studies.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

Appendix in Research Paper – Examples and...

Scope of the Research – Writing Guide and...

Literature Review – Types Writing Guide and...

Implications in Research – Types, Examples and...

Delimitations in Research – Types, Examples and...

Thesis Statement – Examples, Writing Guide
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Process consent: a model for enhancing informed consent in mental health nursing
Affiliation.
- 1 School of Nursing Sciences, James Cook University of North Queensland, Townsville, Australia.
- PMID: 9578197
- DOI: 10.1046/j.1365-2648.1998.00589.x
Informed consent, essentially a legal doctrine, is designed to protect the rights of patients. However, in an area of practice such as psychiatry, informed consent imposes many problems if one considers it to be a static process. In this paper we propose that process consent, the type of consent considered essential in qualitative research projects, is not only appropriate but necessary for mental health nursing practice. This type of consent is an ongoing consensual process that involves the nurse and patient in mutual decision making and ensures that the patient is kept informed at all stages of the treatment process. We have used neuroleptic medications as an example throughout the paper and have suggested that seeking informed consent should be added to the role of the nurse in the mental health setting.
PubMed Disclaimer
Similar articles
- Informed consent in the elderly: assessing decisional capacity. Artnak KE. Artnak KE. Semin Perioper Nurs. 1997 Jan;6(1):59-64. Semin Perioper Nurs. 1997. PMID: 9087123 Review.
- The nurse's role as patient advocate for mentally ill people. Duxbury J. Duxbury J. Nurs Stand. 1996 Feb 7;10(20):36-9. doi: 10.7748/ns.10.20.36.s48. Nurs Stand. 1996. PMID: 8695429
- The right to refuse: informed consent and the psychosocial nurse. Weiss FS. Weiss FS. J Psychosoc Nurs Ment Health Serv. 1990 Aug;28(8):25-30. doi: 10.3928/0279-3695-19900801-09. J Psychosoc Nurs Ment Health Serv. 1990. PMID: 2398483 No abstract available.
- Intrusion into patient privacy: a moral concern in the home care of persons with chronic mental illness. Magnusson A, Lützén K. Magnusson A, et al. Nurs Ethics. 1999 Sep;6(5):399-410. doi: 10.1177/096973309900600506. Nurs Ethics. 1999. PMID: 10696187
- Approaches (and possible contraindications) to enhancing patients' autonomy. Howe EG. Howe EG. J Clin Ethics. 1994 Fall;5(3):179-88. J Clin Ethics. 1994. PMID: 7841467 Review. No abstract available.
- A qualitative study of organisational resilience in care homes in Scotland. Ross A, Anderson JE, Selveindran S, MacBride T, Bowie P, Sherriff A, Young L, Fioratou E, Roddy E, Edwards H, Dewar B, Macpherson LM. Ross A, et al. PLoS One. 2022 Dec 20;17(12):e0279376. doi: 10.1371/journal.pone.0279376. eCollection 2022. PLoS One. 2022. PMID: 36538564 Free PMC article.
- Respect for Autonomy and Dementia Care in Nursing Homes: Revising Beauchamp and Childress's Account of Autonomous Decision-Making. Soofi H. Soofi H. J Bioeth Inq. 2022 Sep;19(3):467-479. doi: 10.1007/s11673-022-10195-7. Epub 2022 Jun 24. J Bioeth Inq. 2022. PMID: 35749025 Free PMC article.
- Diagnostic accuracy and clinical applicability of the Swedish version of the 4AT assessment test for delirium detection, in a mixed patient population and setting. Johansson YA, Tsevis T, Nasic S, Gillsjö C, Johansson L, Bogdanovic N, Kenne Sarenmalm E. Johansson YA, et al. BMC Geriatr. 2021 Oct 18;21(1):568. doi: 10.1186/s12877-021-02493-3. BMC Geriatr. 2021. PMID: 34663229 Free PMC article.
- Factors impacting the implementation of a psychoeducation intervention within the mental health system: a multisite study using the consolidation framework for implementation research. Higgins A, Murphy R, Downes C, Barry J, Monahan M, Hevey D, Kroll T, Doyle L, Gibbons P. Higgins A, et al. BMC Health Serv Res. 2020 Nov 9;20(1):1023. doi: 10.1186/s12913-020-05852-9. BMC Health Serv Res. 2020. PMID: 33168003 Free PMC article.
- A Proposed Process for Risk Mitigation During the COVID-19 Pandemic. Cox DJ, Plavnick JB, Brodhead MT. Cox DJ, et al. Behav Anal Pract. 2020 Apr 23;13(2):299-305. doi: 10.1007/s40617-020-00430-1. eCollection 2020 Jun. Behav Anal Pract. 2020. PMID: 32328220 Free PMC article. Review.
Publication types
- Search in MeSH
LinkOut - more resources
Full text sources.
- Ovid Technologies, Inc.
- MedlinePlus Health Information

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Informed consent
Information and guidance for researchers, what is informed consent.
Informed consent is one of the founding principles of research ethics. Its intent is that human participants can enter research freely (voluntarily) with full information about what it means for them to take part, and that they give consent before they enter the research.
Consent should be obtained before the participant enters the research (prospectively), and there must be no undue influence on participants to consent. The minimum requirements for consent to be informed are that the participant understands what the research is and what they are consenting to.
There are two distinct stages to a standard consent process for competent adults:
- Stage 1 (giving information) : the person reflects on the information given; they are under no pressure to respond to the researcher immediately.
- Stage 2 (obtaining consent): the researcher reiterates the terms of the research, often as separate bullet points or clauses; the person agrees to each term (giving explicit consent) before agreeing to take part in the project as a whole. Consent has been obtained.
Researchers should ensure that they comply with the General Data Protection Regulation (GDPR) during and after the consent process, especially if they will be collecting 'special category' (ie sensitive) data or personal data in the course of their research (also refer to the advice on consent in research involving children ). See also the guidance on data protection and research and the data protection checklist for use when preparing an application for ethical review.
Where your research includes filming or photography, you should refer to specific guidance in the Photography and GDPR toolkit .
Written or oral consent – which process suits your project?
Which process to use depends on the research project (its context, design and participants), though an oral process is usually only appropriate where a written process is not feasible. Any consent process must be understandable to the participants concerned. Please see the sections below to find out about different processes which may be used depending on the context, as well as informed consent templates for each process.
Written informed consent process
A written process is used where:
- Reading and signing forms is not problematic.
- The research is complex or has multiple stages.
- First access to the research participants is by providing written information.
Though opinions differ about the legal force of signed consent forms, they provide extra proof that the terms of consent have been understood. This can be especially important when seeking consent for copyright over data, or for future uses of data. Also, future funders or regulators may want written proof of the terms of original consent.
For literate participants who are not put off by written information, a written process is often a straightforward way of communicating the 'research contract'.
Between the provision of information and obtaining consent, the participant should be given a reasonable amount of time to consider whether to consent and to ask questions, though the time given depends on the project design, the context of the research and the participants.
The written consent templates below can be adapted to suit your study.
Oral informed consent process
An oral consent process is where researcher and participant have a conversation to give information and obtain consent. There is no paper form to sign. It is normally used:
- where literacy is a problem
- where there are cultural or political concerns with signing contract-like documents
- where either the researcher and/or the participant could be put at risk by existence of a paper record
- where time for consent is limited, eg a chance interaction between researcher and participant (although you should not use an oral process merely to correct poor planning of research)
- for research conducted via remote video conferencing software
It may also be more appropriate when interviewing elite participants as part of the research.
For all other research, how you arrange the oral process depends on how you will encounter your participants (for example email, phone, an on-the-street-meeting by chance). Between the information-giving and consent stage the participant should be given a reasonable amount of time to consider whether to consent, though this depends on the project design, the type of participants and the context of the research.
When obtaining oral consent, please ensure you are recording the consent process either using a recording device (for example audio recorder if you are conducting an interview that needs to be recorded) or, if participants do not agree to audio recording or if using or keeping audio records is unsafe, by using a researcher record of oral consent template or completing a written consent form on their behalf.
The oral consent templates below can be adapted to suit your study, but careful consideration is required to ensure that these are appropriate for the research and the participants.
Informed consent templates
Written informed consent process (including online surveys).
| Description | File download |
|---|---|
| Any relevant advertising or recruiting material (poster, email text, social media advert) | |
| Template information sheet | |
| Template written consent form | |
| Template information sheet for online research For online tasks only, where there is no face-to-face contact with human participants | |
| Template information sheet for research involving children |
|
| Template assent form for research involving children |
|
| Participant information sheet for CUREC 3 studies | |
| Consent form for CUREC 3 studies | |
| Template email consent form – for low-risk research in the social sciences and humanities that does not involve face-to-face contact with participants |
| Description | File download |
|---|---|
| Any relevant advertising or recruiting material (poster, email text, social media advert) | |
| Oral script | |
| Written information sheet | |
| Assent template for studies recruiting children | |
| Alongside your script, the use of an oral consent record form is recommended. This is a form only you fill in, and helps you keep track of any specific conditions attached to an oral consent |
Template agreements
| Description | File download | |
|---|---|---|
| This legal contribution template should be signed by research participants and Oxford researchers if the latter wish to upload participants’ photographs, videos, films, podcasts, audio recordings, etc to University of Oxford archives. This should be completed in addition to the above informed consent templates | ||
| This legal confidentiality agreement template can be adapted for research assistants/ interpreters/ translators/ transcribers/ local fieldworkers who are non-University employees | ||
| This legal confidentiality agreement template can be adapted for research assistants/ interpreters/ translators/ transcribers/ local fieldworkers who are University employees |
Cases where 'implied consent' may be acceptable (for example online surveys)
Researchers should always aim to inform people fully and obtain appropriate consent. However, in some cases the research may be straightforward enough that a separate, deliberate process for obtaining consent is not needed. In these cases participants, by their actions, imply consent. This is seen most often in research:
BPG 06 Internet-mediated research
Template information sheet for online research
- where no participant personal details are obtained
- where the topic of research is very low risk and no special category data will be collected
- where participation is confined to one small task, eg completing a survey or simple pencil or computer task
Please note: consent cannot be inferred from inaction (eg failure to move away from a camera).
When consent works against the aims of research
If your research employs deception, you will not be able to inform participants fully about your project’s true aims. In this case please check if you can fully apply the CUREC approved procedure on research involving the deception of participants . If the deception raises ethical concerns such that the application is not covered by this procedure please complete a CUREC 2 application form.
Some research settings evolve very rapidly (for example in conflict studies). Similarly, some research participants may only be revealed in time-poor or emergency settings (eg heart attack patients). This infringes on the standard information-giving stage of research. The vulnerability of participants in those settings may justify an expedited or fully waived consent process. Again it is important to describe the research setting clearly. You may need to complete a CUREC 2 application to the relevant committee in these cases: please check with your DREC or your IDREC .
Last updated Thursday 2 December 2021
Related links
- Where and how to apply for ethical review
- Committee information
- Research ethics FAQs and glossary
- Research integrity and ethics policy
- Ethics committee contacts
Qualitative vs. quantitative data in research: what's the difference?

If you're reading this, you likely already know the importance of data analysis. And you already know it can be incredibly complex.
At its simplest, research and it's data can be broken down into two different categories: quantitative and qualitative. But what's the difference between each? And when should you use them? And how can you use them together?
Understanding the differences between qualitative and quantitative data is key to any research project. Knowing both approaches can help you in understanding your data better—and ultimately understand your customers better. Quick takeaways:
Quantitative research uses objective, numerical data to answer questions like "what" and "how often." Conversely, qualitative research seeks to answer questions like "why" and "how," focusing on subjective experiences to understand motivations and reasons.
Quantitative data is collected through methods like surveys and experiments and analyzed statistically to identify patterns. Qualitative data is gathered through interviews or observations and analyzed by categorizing information to understand themes and insights.
Effective data analysis combines quantitative data for measurable insights with qualitative data for contextual depth.
What is quantitative data?
Qualitative and quantitative data differ in their approach and the type of data they collect.
Quantitative data refers to any information that can be quantified — that is, numbers. If it can be counted or measured, and given a numerical value, it's quantitative in nature. Think of it as a measuring stick.
Quantitative variables can tell you "how many," "how much," or "how often."
Some examples of quantitative data :
How many people attended last week's webinar?
How much revenue did our company make last year?
How often does a customer rage click on this app?
To analyze these research questions and make sense of this quantitative data, you’d normally use a form of statistical analysis —collecting, evaluating, and presenting large amounts of data to discover patterns and trends. Quantitative data is conducive to this type of analysis because it’s numeric and easier to analyze mathematically.
Computers now rule statistical analytics, even though traditional methods have been used for years. But today’s data volumes make statistics more valuable and useful than ever. When you think of statistical analysis now, you think of powerful computers and algorithms that fuel many of the software tools you use today.
Popular quantitative data collection methods are surveys, experiments, polls, and more.
Quantitative Data 101: What is quantitative data?
Take a deeper dive into what quantitative data is, how it works, how to analyze it, collect it, use it, and more.
Learn more about quantitative data →
What is qualitative data?
Unlike quantitative data, qualitative data is descriptive, expressed in terms of language rather than numerical values.
Qualitative data analysis describes information and cannot be measured or counted. It refers to the words or labels used to describe certain characteristics or traits.
You would turn to qualitative data to answer the "why?" or "how?" questions. It is often used to investigate open-ended studies, allowing participants (or customers) to show their true feelings and actions without guidance.
Some examples of qualitative data:
Why do people prefer using one product over another?
How do customers feel about their customer service experience?
What do people think about a new feature in the app?
Think of qualitative data as the type of data you'd get if you were to ask someone why they did something. Popular data collection methods are in-depth interviews, focus groups, or observation.
Start growing with data and Fullstory.
Request your personalized demo of the Fullstory behavioral data platform.
What are the differences between qualitative vs. quantitative data?
When it comes to conducting data research, you’ll need different collection, hypotheses and analysis methods, so it’s important to understand the key differences between quantitative and qualitative data:
Quantitative data is numbers-based, countable, or measurable. Qualitative data is interpretation-based, descriptive, and relating to language.
Quantitative data tells us how many, how much, or how often in calculations. Qualitative data can help us to understand why, how, or what happened behind certain behaviors .
Quantitative data is fixed and universal. Qualitative data is subjective and unique.
Quantitative research methods are measuring and counting. Qualitative research methods are interviewing and observing.
Quantitative data is analyzed using statistical analysis. Qualitative data is analyzed by grouping the data into categories and themes.
As you can see, both provide immense value for any data collection and are key to truly finding answers and patterns.
More examples of quantitative and qualitative data
You’ve most likely run into quantitative and qualitative data today, alone. For the visual learner, here are some examples of both quantitative and qualitative data:
Quantitative data example
The customer has clicked on the button 13 times.
The engineer has resolved 34 support tickets today.
The team has completed 7 upgrades this month.
14 cartons of eggs were purchased this month.
Qualitative data example
My manager has curly brown hair and blue eyes.
My coworker is funny, loud, and a good listener.
The customer has a very friendly face and a contagious laugh.
The eggs were delicious.
The fundamental difference is that one type of data answers primal basics and one answers descriptively.
What does this mean for data quality and analysis? If you just analyzed quantitative data, you’d be missing core reasons behind what makes a data collection meaningful. You need both in order to truly learn from data—and truly learn from your customers.
What are the advantages and disadvantages of each?
Both types of data has their own pros and cons.
Advantages of quantitative data
It’s relatively quick and easy to collect and it’s easier to draw conclusions from.
When you collect quantitative data, the type of results will tell you which statistical tests are appropriate to use.
As a result, interpreting your data and presenting those findings is straightforward and less open to error and subjectivity.
Another advantage is that you can replicate it. Replicating a study is possible because your data collection is measurable and tangible for further applications.
Disadvantages of quantitative data
Quantitative data doesn’t always tell you the full story (no matter what the perspective).
With choppy information, it can be inconclusive.
Quantitative research can be limited, which can lead to overlooking broader themes and relationships.
By focusing solely on numbers, there is a risk of missing larger focus information that can be beneficial.
Advantages of qualitative data
Qualitative data offers rich, in-depth insights and allows you to explore context.
It’s great for exploratory purposes.
Qualitative research delivers a predictive element for continuous data.
Disadvantages of qualitative data
It’s not a statistically representative form of data collection because it relies upon the experience of the host (who can lose data).
It can also require multiple data sessions, which can lead to misleading conclusions.
The takeaway is that it’s tough to conduct a successful data analysis without both. They both have their advantages and disadvantages and, in a way, they complement each other.
Now, of course, in order to analyze both types of data, information has to be collected first.
Let's get into the research.

Quantitative and qualitative research
The core difference between qualitative and quantitative research lies in their focus and methods of data collection and analysis. This distinction guides researchers in choosing an appropriate approach based on their specific research needs.
Using mixed methods of both can also help provide insights form combined qualitative and quantitative data.
Best practices of each help to look at the information under a broader lens to get a unique perspective. Using both methods is helpful because they collect rich and reliable data, which can be further tested and replicated.
What is quantitative research?
Quantitative research is based on the collection and interpretation of numeric data. It's all about the numbers and focuses on measuring (using inferential statistics ) and generalizing results. Quantitative research seeks to collect numerical data that can be transformed into usable statistics.
It relies on measurable data to formulate facts and uncover patterns in research. By employing statistical methods to analyze the data, it provides a broad overview that can be generalized to larger populations.
In terms of digital experience data, it puts everything in terms of numbers (or discrete data )—like the number of users clicking a button, bounce rates , time on site, and more.
Some examples of quantitative research:
What is the amount of money invested into this service?
What is the average number of times a button was dead clicked ?
How many customers are actually clicking this button?
Essentially, quantitative research is an easy way to see what’s going on at a 20,000-foot view.
Each data set (or customer action, if we’re still talking digital experience) has a numerical value associated with it and is quantifiable information that can be used for calculating statistical analysis so that decisions can be made.
You can use statistical operations to discover feedback patterns (with any representative sample size) in the data under examination. The results can be used to make predictions , find averages, test causes and effects, and generalize results to larger measurable data pools.
Unlike qualitative methodology, quantitative research offers more objective findings as they are based on more reliable numeric data.
Quantitative data collection methods
A survey is one of the most common research methods with quantitative data that involves questioning a large group of people. Questions are usually closed-ended and are the same for all participants. An unclear questionnaire can lead to distorted research outcomes.
Similar to surveys, polls yield quantitative data. That is, you poll a number of people and apply a numeric value to how many people responded with each answer.
Experiments
An experiment is another common method that usually involves a control group and an experimental group . The experiment is controlled and the conditions can be manipulated accordingly. You can examine any type of records involved if they pertain to the experiment, so the data is extensive.
What is qualitative research?
Qualitative research does not simply help to collect data. It gives a chance to understand the trends and meanings of natural actions. It’s flexible and iterative.
Qualitative research focuses on the qualities of users—the actions that drive the numbers. It's descriptive research. The qualitative approach is subjective, too.
It focuses on describing an action, rather than measuring it.
Some examples of qualitative research:
The sunflowers had a fresh smell that filled the office.
All the bagels with bites taken out of them had cream cheese.
The man had blonde hair with a blue hat.
Qualitative research utilizes interviews, focus groups, and observations to gather in-depth insights.
This approach shines when the research objective calls for exploring ideas or uncovering deep insights rather than quantifying elements.
Qualitative data collection methods
An interview is the most common qualitative research method. This method involves personal interaction (either in real life or virtually) with a participant. It’s mostly used for exploring attitudes and opinions regarding certain issues.
Interviews are very popular methods for collecting data in product design .
Focus groups
Data analysis by focus group is another method where participants are guided by a host to collect data. Within a group (either in person or online), each member shares their opinion and experiences on a specific topic, allowing researchers to gather perspectives and deepen their understanding of the subject matter.
Digital Leadership Webinar: Accelerating Growth with Quantitative Data and Analytics
Learn how the best-of-the-best are connecting quantitative data and experience to accelerate growth.
So which type of data is better for data analysis?
So how do you determine which type is better for data analysis ?
Quantitative data is structured and accountable. This type of data is formatted in a way so it can be organized, arranged, and searchable. Think about this data as numbers and values found in spreadsheets—after all, you would trust an Excel formula.
Qualitative data is considered unstructured. This type of data is formatted (and known for) being subjective, individualized, and personalized. Anything goes. Because of this, qualitative data is inferior if it’s the only data in the study. However, it’s still valuable.
Because quantitative data is more concrete, it’s generally preferred for data analysis. Numbers don’t lie. But for complete statistical analysis, using both qualitative and quantitative yields the best results.
At Fullstory, we understand the importance of data, which is why we created a behavioral data platform that analyzes customer data for better insights. Our platform delivers a complete, retroactive view of how people interact with your site or app—and analyzes every point of user interaction so you can scale.
Unlock business-critical data with Fullstory
A perfect digital customer experience is often the difference between company growth and failure. And the first step toward building that experience is quantifying who your customers are, what they want, and how to provide them what they need.
Access to product analytics is the most efficient and reliable way to collect valuable quantitative data about funnel analysis, customer journey maps , user segments, and more.
But creating a perfect digital experience means you need organized and digestible quantitative data—but also access to qualitative data. Understanding the why is just as important as the what itself.
Fullstory's DXI platform combines the quantitative insights of product analytics with picture-perfect session replay for complete context that helps you answer questions, understand issues, and uncover customer opportunities.
Start a free 14-day trial to see how Fullstory can help you combine your most invaluable quantitative and qualitative insights and eliminate blind spots.
About the author
Our team of experts is committed to introducing people to important topics surrounding analytics, digital experience intelligence, product development, and more.
Related posts
Quantitative data is used for calculations or obtaining numerical results. Learn about the different types of quantitative data uses cases and more.
Discover how data discovery transforms raw data into actionable insights for informed decisions, improved strategies, and better customer experiences.
Learn the 3 key benefits democratized data can achieve, and 3 of the most pertinent dangers of keeping data (and teams) siloed.
Learn the essentials of behavioral data and its transformative impact on customer experience. Our comprehensive guide provides the tools and knowledge to harness this power effectively.
Discover how Fullstory leverages AI to turn raw data into actionable insights, transforming user experiences and driving business growth.
Discover how just-in-time data, explained by Lane Greer, enhances customer insights and decision-making beyond real-time analytics.

American Psychological Association

The impact of transgender legislation
Psychological science points to an increased risk of suicide and poor mental health amid a record number of bills aimed at restricting the rights of the LGBTQ+ population
APA policy supporting transgender, gender diverse, nonbinary individuals
Membership in APA

APA Community
A new exclusive destination tailored for APA members

Membership benefits
Unlock the tools, discounts, and services included with your membership

Renew your membership
Keep your benefits and access to leading psychological information
Psychology topics spotlight

Misinformation and disinformation

Resources to navigate trauma

Tips to foster a healthy workplace
Science and practice of psychology

Ethics Code

Continuing Education

Grants, Awards, and Funding

Standards and Guidelines
Networks and communities

Network with peers, enhance your professional development, expand your personal growth, and more

APA Divisions

High school teachers

Undergraduate educators

Graduate students

Early career psychologists

Managing your career
Resources to help you throughout your career in psychology, including finding a job, salary data, finances and money management, mentoring and supervision, and training and professional development

Explore career paths

Psychologist profiles

How did you get that job?

Events and training
Featured jobs
Apa publications and products.

Write with clarity, precision, and inclusion
Children’s books
Monitor on Psychology
Newsletters
Reports and surveys
Continuing education
Merchandise

Real Siblings

Jacob's Missing Book

Harper Becomes a Big Sister
Attachment-Based Family Therapy for Sexual and Gender Minority Young Adults and Their Non-Accepting Parents
Dismantling Everyday Discrimination
APA Services

Learn how you can help APA advocate for psychology-informed federal policy and legislation, and support psychological research

APA Services, Inc.
A companion professional organization to APA, serving all members and advocating for psychology
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Neurol Res Pract

How to use and assess qualitative research methods
Loraine busetto.
1 Department of Neurology, Heidelberg University Hospital, Im Neuenheimer Feld 400, 69120 Heidelberg, Germany
Wolfgang Wick
2 Clinical Cooperation Unit Neuro-Oncology, German Cancer Research Center, Heidelberg, Germany
Christoph Gumbinger
Associated data.
Not applicable.
This paper aims to provide an overview of the use and assessment of qualitative research methods in the health sciences. Qualitative research can be defined as the study of the nature of phenomena and is especially appropriate for answering questions of why something is (not) observed, assessing complex multi-component interventions, and focussing on intervention improvement. The most common methods of data collection are document study, (non-) participant observations, semi-structured interviews and focus groups. For data analysis, field-notes and audio-recordings are transcribed into protocols and transcripts, and coded using qualitative data management software. Criteria such as checklists, reflexivity, sampling strategies, piloting, co-coding, member-checking and stakeholder involvement can be used to enhance and assess the quality of the research conducted. Using qualitative in addition to quantitative designs will equip us with better tools to address a greater range of research problems, and to fill in blind spots in current neurological research and practice.
The aim of this paper is to provide an overview of qualitative research methods, including hands-on information on how they can be used, reported and assessed. This article is intended for beginning qualitative researchers in the health sciences as well as experienced quantitative researchers who wish to broaden their understanding of qualitative research.
What is qualitative research?
Qualitative research is defined as “the study of the nature of phenomena”, including “their quality, different manifestations, the context in which they appear or the perspectives from which they can be perceived” , but excluding “their range, frequency and place in an objectively determined chain of cause and effect” [ 1 ]. This formal definition can be complemented with a more pragmatic rule of thumb: qualitative research generally includes data in form of words rather than numbers [ 2 ].
Why conduct qualitative research?
Because some research questions cannot be answered using (only) quantitative methods. For example, one Australian study addressed the issue of why patients from Aboriginal communities often present late or not at all to specialist services offered by tertiary care hospitals. Using qualitative interviews with patients and staff, it found one of the most significant access barriers to be transportation problems, including some towns and communities simply not having a bus service to the hospital [ 3 ]. A quantitative study could have measured the number of patients over time or even looked at possible explanatory factors – but only those previously known or suspected to be of relevance. To discover reasons for observed patterns, especially the invisible or surprising ones, qualitative designs are needed.
While qualitative research is common in other fields, it is still relatively underrepresented in health services research. The latter field is more traditionally rooted in the evidence-based-medicine paradigm, as seen in " research that involves testing the effectiveness of various strategies to achieve changes in clinical practice, preferably applying randomised controlled trial study designs (...) " [ 4 ]. This focus on quantitative research and specifically randomised controlled trials (RCT) is visible in the idea of a hierarchy of research evidence which assumes that some research designs are objectively better than others, and that choosing a "lesser" design is only acceptable when the better ones are not practically or ethically feasible [ 5 , 6 ]. Others, however, argue that an objective hierarchy does not exist, and that, instead, the research design and methods should be chosen to fit the specific research question at hand – "questions before methods" [ 2 , 7 – 9 ]. This means that even when an RCT is possible, some research problems require a different design that is better suited to addressing them. Arguing in JAMA, Berwick uses the example of rapid response teams in hospitals, which he describes as " a complex, multicomponent intervention – essentially a process of social change" susceptible to a range of different context factors including leadership or organisation history. According to him, "[in] such complex terrain, the RCT is an impoverished way to learn. Critics who use it as a truth standard in this context are incorrect" [ 8 ] . Instead of limiting oneself to RCTs, Berwick recommends embracing a wider range of methods , including qualitative ones, which for "these specific applications, (...) are not compromises in learning how to improve; they are superior" [ 8 ].
Research problems that can be approached particularly well using qualitative methods include assessing complex multi-component interventions or systems (of change), addressing questions beyond “what works”, towards “what works for whom when, how and why”, and focussing on intervention improvement rather than accreditation [ 7 , 9 – 12 ]. Using qualitative methods can also help shed light on the “softer” side of medical treatment. For example, while quantitative trials can measure the costs and benefits of neuro-oncological treatment in terms of survival rates or adverse effects, qualitative research can help provide a better understanding of patient or caregiver stress, visibility of illness or out-of-pocket expenses.
How to conduct qualitative research?
Given that qualitative research is characterised by flexibility, openness and responsivity to context, the steps of data collection and analysis are not as separate and consecutive as they tend to be in quantitative research [ 13 , 14 ]. As Fossey puts it : “sampling, data collection, analysis and interpretation are related to each other in a cyclical (iterative) manner, rather than following one after another in a stepwise approach” [ 15 ]. The researcher can make educated decisions with regard to the choice of method, how they are implemented, and to which and how many units they are applied [ 13 ]. As shown in Fig. 1 , this can involve several back-and-forth steps between data collection and analysis where new insights and experiences can lead to adaption and expansion of the original plan. Some insights may also necessitate a revision of the research question and/or the research design as a whole. The process ends when saturation is achieved, i.e. when no relevant new information can be found (see also below: sampling and saturation). For reasons of transparency, it is essential for all decisions as well as the underlying reasoning to be well-documented.

Iterative research process
While it is not always explicitly addressed, qualitative methods reflect a different underlying research paradigm than quantitative research (e.g. constructivism or interpretivism as opposed to positivism). The choice of methods can be based on the respective underlying substantive theory or theoretical framework used by the researcher [ 2 ].
Data collection
The methods of qualitative data collection most commonly used in health research are document study, observations, semi-structured interviews and focus groups [ 1 , 14 , 16 , 17 ].
Document study
Document study (also called document analysis) refers to the review by the researcher of written materials [ 14 ]. These can include personal and non-personal documents such as archives, annual reports, guidelines, policy documents, diaries or letters.
Observations
Observations are particularly useful to gain insights into a certain setting and actual behaviour – as opposed to reported behaviour or opinions [ 13 ]. Qualitative observations can be either participant or non-participant in nature. In participant observations, the observer is part of the observed setting, for example a nurse working in an intensive care unit [ 18 ]. In non-participant observations, the observer is “on the outside looking in”, i.e. present in but not part of the situation, trying not to influence the setting by their presence. Observations can be planned (e.g. for 3 h during the day or night shift) or ad hoc (e.g. as soon as a stroke patient arrives at the emergency room). During the observation, the observer takes notes on everything or certain pre-determined parts of what is happening around them, for example focusing on physician-patient interactions or communication between different professional groups. Written notes can be taken during or after the observations, depending on feasibility (which is usually lower during participant observations) and acceptability (e.g. when the observer is perceived to be judging the observed). Afterwards, these field notes are transcribed into observation protocols. If more than one observer was involved, field notes are taken independently, but notes can be consolidated into one protocol after discussions. Advantages of conducting observations include minimising the distance between the researcher and the researched, the potential discovery of topics that the researcher did not realise were relevant and gaining deeper insights into the real-world dimensions of the research problem at hand [ 18 ].
Semi-structured interviews
Hijmans & Kuyper describe qualitative interviews as “an exchange with an informal character, a conversation with a goal” [ 19 ]. Interviews are used to gain insights into a person’s subjective experiences, opinions and motivations – as opposed to facts or behaviours [ 13 ]. Interviews can be distinguished by the degree to which they are structured (i.e. a questionnaire), open (e.g. free conversation or autobiographical interviews) or semi-structured [ 2 , 13 ]. Semi-structured interviews are characterized by open-ended questions and the use of an interview guide (or topic guide/list) in which the broad areas of interest, sometimes including sub-questions, are defined [ 19 ]. The pre-defined topics in the interview guide can be derived from the literature, previous research or a preliminary method of data collection, e.g. document study or observations. The topic list is usually adapted and improved at the start of the data collection process as the interviewer learns more about the field [ 20 ]. Across interviews the focus on the different (blocks of) questions may differ and some questions may be skipped altogether (e.g. if the interviewee is not able or willing to answer the questions or for concerns about the total length of the interview) [ 20 ]. Qualitative interviews are usually not conducted in written format as it impedes on the interactive component of the method [ 20 ]. In comparison to written surveys, qualitative interviews have the advantage of being interactive and allowing for unexpected topics to emerge and to be taken up by the researcher. This can also help overcome a provider or researcher-centred bias often found in written surveys, which by nature, can only measure what is already known or expected to be of relevance to the researcher. Interviews can be audio- or video-taped; but sometimes it is only feasible or acceptable for the interviewer to take written notes [ 14 , 16 , 20 ].
Focus groups
Focus groups are group interviews to explore participants’ expertise and experiences, including explorations of how and why people behave in certain ways [ 1 ]. Focus groups usually consist of 6–8 people and are led by an experienced moderator following a topic guide or “script” [ 21 ]. They can involve an observer who takes note of the non-verbal aspects of the situation, possibly using an observation guide [ 21 ]. Depending on researchers’ and participants’ preferences, the discussions can be audio- or video-taped and transcribed afterwards [ 21 ]. Focus groups are useful for bringing together homogeneous (to a lesser extent heterogeneous) groups of participants with relevant expertise and experience on a given topic on which they can share detailed information [ 21 ]. Focus groups are a relatively easy, fast and inexpensive method to gain access to information on interactions in a given group, i.e. “the sharing and comparing” among participants [ 21 ]. Disadvantages include less control over the process and a lesser extent to which each individual may participate. Moreover, focus group moderators need experience, as do those tasked with the analysis of the resulting data. Focus groups can be less appropriate for discussing sensitive topics that participants might be reluctant to disclose in a group setting [ 13 ]. Moreover, attention must be paid to the emergence of “groupthink” as well as possible power dynamics within the group, e.g. when patients are awed or intimidated by health professionals.
Choosing the “right” method
As explained above, the school of thought underlying qualitative research assumes no objective hierarchy of evidence and methods. This means that each choice of single or combined methods has to be based on the research question that needs to be answered and a critical assessment with regard to whether or to what extent the chosen method can accomplish this – i.e. the “fit” between question and method [ 14 ]. It is necessary for these decisions to be documented when they are being made, and to be critically discussed when reporting methods and results.
Let us assume that our research aim is to examine the (clinical) processes around acute endovascular treatment (EVT), from the patient’s arrival at the emergency room to recanalization, with the aim to identify possible causes for delay and/or other causes for sub-optimal treatment outcome. As a first step, we could conduct a document study of the relevant standard operating procedures (SOPs) for this phase of care – are they up-to-date and in line with current guidelines? Do they contain any mistakes, irregularities or uncertainties that could cause delays or other problems? Regardless of the answers to these questions, the results have to be interpreted based on what they are: a written outline of what care processes in this hospital should look like. If we want to know what they actually look like in practice, we can conduct observations of the processes described in the SOPs. These results can (and should) be analysed in themselves, but also in comparison to the results of the document analysis, especially as regards relevant discrepancies. Do the SOPs outline specific tests for which no equipment can be observed or tasks to be performed by specialized nurses who are not present during the observation? It might also be possible that the written SOP is outdated, but the actual care provided is in line with current best practice. In order to find out why these discrepancies exist, it can be useful to conduct interviews. Are the physicians simply not aware of the SOPs (because their existence is limited to the hospital’s intranet) or do they actively disagree with them or does the infrastructure make it impossible to provide the care as described? Another rationale for adding interviews is that some situations (or all of their possible variations for different patient groups or the day, night or weekend shift) cannot practically or ethically be observed. In this case, it is possible to ask those involved to report on their actions – being aware that this is not the same as the actual observation. A senior physician’s or hospital manager’s description of certain situations might differ from a nurse’s or junior physician’s one, maybe because they intentionally misrepresent facts or maybe because different aspects of the process are visible or important to them. In some cases, it can also be relevant to consider to whom the interviewee is disclosing this information – someone they trust, someone they are otherwise not connected to, or someone they suspect or are aware of being in a potentially “dangerous” power relationship to them. Lastly, a focus group could be conducted with representatives of the relevant professional groups to explore how and why exactly they provide care around EVT. The discussion might reveal discrepancies (between SOPs and actual care or between different physicians) and motivations to the researchers as well as to the focus group members that they might not have been aware of themselves. For the focus group to deliver relevant information, attention has to be paid to its composition and conduct, for example, to make sure that all participants feel safe to disclose sensitive or potentially problematic information or that the discussion is not dominated by (senior) physicians only. The resulting combination of data collection methods is shown in Fig. 2 .

Possible combination of data collection methods
Attributions for icons: “Book” by Serhii Smirnov, “Interview” by Adrien Coquet, FR, “Magnifying Glass” by anggun, ID, “Business communication” by Vectors Market; all from the Noun Project
The combination of multiple data source as described for this example can be referred to as “triangulation”, in which multiple measurements are carried out from different angles to achieve a more comprehensive understanding of the phenomenon under study [ 22 , 23 ].
Data analysis
To analyse the data collected through observations, interviews and focus groups these need to be transcribed into protocols and transcripts (see Fig. 3 ). Interviews and focus groups can be transcribed verbatim , with or without annotations for behaviour (e.g. laughing, crying, pausing) and with or without phonetic transcription of dialects and filler words, depending on what is expected or known to be relevant for the analysis. In the next step, the protocols and transcripts are coded , that is, marked (or tagged, labelled) with one or more short descriptors of the content of a sentence or paragraph [ 2 , 15 , 23 ]. Jansen describes coding as “connecting the raw data with “theoretical” terms” [ 20 ]. In a more practical sense, coding makes raw data sortable. This makes it possible to extract and examine all segments describing, say, a tele-neurology consultation from multiple data sources (e.g. SOPs, emergency room observations, staff and patient interview). In a process of synthesis and abstraction, the codes are then grouped, summarised and/or categorised [ 15 , 20 ]. The end product of the coding or analysis process is a descriptive theory of the behavioural pattern under investigation [ 20 ]. The coding process is performed using qualitative data management software, the most common ones being InVivo, MaxQDA and Atlas.ti. It should be noted that these are data management tools which support the analysis performed by the researcher(s) [ 14 ].

From data collection to data analysis
Attributions for icons: see Fig. Fig.2, 2 , also “Speech to text” by Trevor Dsouza, “Field Notes” by Mike O’Brien, US, “Voice Record” by ProSymbols, US, “Inspection” by Made, AU, and “Cloud” by Graphic Tigers; all from the Noun Project
How to report qualitative research?
Protocols of qualitative research can be published separately and in advance of the study results. However, the aim is not the same as in RCT protocols, i.e. to pre-define and set in stone the research questions and primary or secondary endpoints. Rather, it is a way to describe the research methods in detail, which might not be possible in the results paper given journals’ word limits. Qualitative research papers are usually longer than their quantitative counterparts to allow for deep understanding and so-called “thick description”. In the methods section, the focus is on transparency of the methods used, including why, how and by whom they were implemented in the specific study setting, so as to enable a discussion of whether and how this may have influenced data collection, analysis and interpretation. The results section usually starts with a paragraph outlining the main findings, followed by more detailed descriptions of, for example, the commonalities, discrepancies or exceptions per category [ 20 ]. Here it is important to support main findings by relevant quotations, which may add information, context, emphasis or real-life examples [ 20 , 23 ]. It is subject to debate in the field whether it is relevant to state the exact number or percentage of respondents supporting a certain statement (e.g. “Five interviewees expressed negative feelings towards XYZ”) [ 21 ].
How to combine qualitative with quantitative research?
Qualitative methods can be combined with other methods in multi- or mixed methods designs, which “[employ] two or more different methods [ …] within the same study or research program rather than confining the research to one single method” [ 24 ]. Reasons for combining methods can be diverse, including triangulation for corroboration of findings, complementarity for illustration and clarification of results, expansion to extend the breadth and range of the study, explanation of (unexpected) results generated with one method with the help of another, or offsetting the weakness of one method with the strength of another [ 1 , 17 , 24 – 26 ]. The resulting designs can be classified according to when, why and how the different quantitative and/or qualitative data strands are combined. The three most common types of mixed method designs are the convergent parallel design , the explanatory sequential design and the exploratory sequential design. The designs with examples are shown in Fig. 4 .

Three common mixed methods designs
In the convergent parallel design, a qualitative study is conducted in parallel to and independently of a quantitative study, and the results of both studies are compared and combined at the stage of interpretation of results. Using the above example of EVT provision, this could entail setting up a quantitative EVT registry to measure process times and patient outcomes in parallel to conducting the qualitative research outlined above, and then comparing results. Amongst other things, this would make it possible to assess whether interview respondents’ subjective impressions of patients receiving good care match modified Rankin Scores at follow-up, or whether observed delays in care provision are exceptions or the rule when compared to door-to-needle times as documented in the registry. In the explanatory sequential design, a quantitative study is carried out first, followed by a qualitative study to help explain the results from the quantitative study. This would be an appropriate design if the registry alone had revealed relevant delays in door-to-needle times and the qualitative study would be used to understand where and why these occurred, and how they could be improved. In the exploratory design, the qualitative study is carried out first and its results help informing and building the quantitative study in the next step [ 26 ]. If the qualitative study around EVT provision had shown a high level of dissatisfaction among the staff members involved, a quantitative questionnaire investigating staff satisfaction could be set up in the next step, informed by the qualitative study on which topics dissatisfaction had been expressed. Amongst other things, the questionnaire design would make it possible to widen the reach of the research to more respondents from different (types of) hospitals, regions, countries or settings, and to conduct sub-group analyses for different professional groups.
How to assess qualitative research?
A variety of assessment criteria and lists have been developed for qualitative research, ranging in their focus and comprehensiveness [ 14 , 17 , 27 ]. However, none of these has been elevated to the “gold standard” in the field. In the following, we therefore focus on a set of commonly used assessment criteria that, from a practical standpoint, a researcher can look for when assessing a qualitative research report or paper.
Assessors should check the authors’ use of and adherence to the relevant reporting checklists (e.g. Standards for Reporting Qualitative Research (SRQR)) to make sure all items that are relevant for this type of research are addressed [ 23 , 28 ]. Discussions of quantitative measures in addition to or instead of these qualitative measures can be a sign of lower quality of the research (paper). Providing and adhering to a checklist for qualitative research contributes to an important quality criterion for qualitative research, namely transparency [ 15 , 17 , 23 ].
Reflexivity
While methodological transparency and complete reporting is relevant for all types of research, some additional criteria must be taken into account for qualitative research. This includes what is called reflexivity, i.e. sensitivity to the relationship between the researcher and the researched, including how contact was established and maintained, or the background and experience of the researcher(s) involved in data collection and analysis. Depending on the research question and population to be researched this can be limited to professional experience, but it may also include gender, age or ethnicity [ 17 , 27 ]. These details are relevant because in qualitative research, as opposed to quantitative research, the researcher as a person cannot be isolated from the research process [ 23 ]. It may influence the conversation when an interviewed patient speaks to an interviewer who is a physician, or when an interviewee is asked to discuss a gynaecological procedure with a male interviewer, and therefore the reader must be made aware of these details [ 19 ].
Sampling and saturation
The aim of qualitative sampling is for all variants of the objects of observation that are deemed relevant for the study to be present in the sample “ to see the issue and its meanings from as many angles as possible” [ 1 , 16 , 19 , 20 , 27 ] , and to ensure “information-richness [ 15 ]. An iterative sampling approach is advised, in which data collection (e.g. five interviews) is followed by data analysis, followed by more data collection to find variants that are lacking in the current sample. This process continues until no new (relevant) information can be found and further sampling becomes redundant – which is called saturation [ 1 , 15 ] . In other words: qualitative data collection finds its end point not a priori , but when the research team determines that saturation has been reached [ 29 , 30 ].
This is also the reason why most qualitative studies use deliberate instead of random sampling strategies. This is generally referred to as “ purposive sampling” , in which researchers pre-define which types of participants or cases they need to include so as to cover all variations that are expected to be of relevance, based on the literature, previous experience or theory (i.e. theoretical sampling) [ 14 , 20 ]. Other types of purposive sampling include (but are not limited to) maximum variation sampling, critical case sampling or extreme or deviant case sampling [ 2 ]. In the above EVT example, a purposive sample could include all relevant professional groups and/or all relevant stakeholders (patients, relatives) and/or all relevant times of observation (day, night and weekend shift).
Assessors of qualitative research should check whether the considerations underlying the sampling strategy were sound and whether or how researchers tried to adapt and improve their strategies in stepwise or cyclical approaches between data collection and analysis to achieve saturation [ 14 ].
Good qualitative research is iterative in nature, i.e. it goes back and forth between data collection and analysis, revising and improving the approach where necessary. One example of this are pilot interviews, where different aspects of the interview (especially the interview guide, but also, for example, the site of the interview or whether the interview can be audio-recorded) are tested with a small number of respondents, evaluated and revised [ 19 ]. In doing so, the interviewer learns which wording or types of questions work best, or which is the best length of an interview with patients who have trouble concentrating for an extended time. Of course, the same reasoning applies to observations or focus groups which can also be piloted.
Ideally, coding should be performed by at least two researchers, especially at the beginning of the coding process when a common approach must be defined, including the establishment of a useful coding list (or tree), and when a common meaning of individual codes must be established [ 23 ]. An initial sub-set or all transcripts can be coded independently by the coders and then compared and consolidated after regular discussions in the research team. This is to make sure that codes are applied consistently to the research data.
Member checking
Member checking, also called respondent validation , refers to the practice of checking back with study respondents to see if the research is in line with their views [ 14 , 27 ]. This can happen after data collection or analysis or when first results are available [ 23 ]. For example, interviewees can be provided with (summaries of) their transcripts and asked whether they believe this to be a complete representation of their views or whether they would like to clarify or elaborate on their responses [ 17 ]. Respondents’ feedback on these issues then becomes part of the data collection and analysis [ 27 ].
Stakeholder involvement
In those niches where qualitative approaches have been able to evolve and grow, a new trend has seen the inclusion of patients and their representatives not only as study participants (i.e. “members”, see above) but as consultants to and active participants in the broader research process [ 31 – 33 ]. The underlying assumption is that patients and other stakeholders hold unique perspectives and experiences that add value beyond their own single story, making the research more relevant and beneficial to researchers, study participants and (future) patients alike [ 34 , 35 ]. Using the example of patients on or nearing dialysis, a recent scoping review found that 80% of clinical research did not address the top 10 research priorities identified by patients and caregivers [ 32 , 36 ]. In this sense, the involvement of the relevant stakeholders, especially patients and relatives, is increasingly being seen as a quality indicator in and of itself.
How not to assess qualitative research
The above overview does not include certain items that are routine in assessments of quantitative research. What follows is a non-exhaustive, non-representative, experience-based list of the quantitative criteria often applied to the assessment of qualitative research, as well as an explanation of the limited usefulness of these endeavours.
Protocol adherence
Given the openness and flexibility of qualitative research, it should not be assessed by how well it adheres to pre-determined and fixed strategies – in other words: its rigidity. Instead, the assessor should look for signs of adaptation and refinement based on lessons learned from earlier steps in the research process.
Sample size
For the reasons explained above, qualitative research does not require specific sample sizes, nor does it require that the sample size be determined a priori [ 1 , 14 , 27 , 37 – 39 ]. Sample size can only be a useful quality indicator when related to the research purpose, the chosen methodology and the composition of the sample, i.e. who was included and why.
Randomisation
While some authors argue that randomisation can be used in qualitative research, this is not commonly the case, as neither its feasibility nor its necessity or usefulness has been convincingly established for qualitative research [ 13 , 27 ]. Relevant disadvantages include the negative impact of a too large sample size as well as the possibility (or probability) of selecting “ quiet, uncooperative or inarticulate individuals ” [ 17 ]. Qualitative studies do not use control groups, either.
Interrater reliability, variability and other “objectivity checks”
The concept of “interrater reliability” is sometimes used in qualitative research to assess to which extent the coding approach overlaps between the two co-coders. However, it is not clear what this measure tells us about the quality of the analysis [ 23 ]. This means that these scores can be included in qualitative research reports, preferably with some additional information on what the score means for the analysis, but it is not a requirement. Relatedly, it is not relevant for the quality or “objectivity” of qualitative research to separate those who recruited the study participants and collected and analysed the data. Experiences even show that it might be better to have the same person or team perform all of these tasks [ 20 ]. First, when researchers introduce themselves during recruitment this can enhance trust when the interview takes place days or weeks later with the same researcher. Second, when the audio-recording is transcribed for analysis, the researcher conducting the interviews will usually remember the interviewee and the specific interview situation during data analysis. This might be helpful in providing additional context information for interpretation of data, e.g. on whether something might have been meant as a joke [ 18 ].
Not being quantitative research
Being qualitative research instead of quantitative research should not be used as an assessment criterion if it is used irrespectively of the research problem at hand. Similarly, qualitative research should not be required to be combined with quantitative research per se – unless mixed methods research is judged as inherently better than single-method research. In this case, the same criterion should be applied for quantitative studies without a qualitative component.
The main take-away points of this paper are summarised in Table Table1. 1 . We aimed to show that, if conducted well, qualitative research can answer specific research questions that cannot to be adequately answered using (only) quantitative designs. Seeing qualitative and quantitative methods as equal will help us become more aware and critical of the “fit” between the research problem and our chosen methods: I can conduct an RCT to determine the reasons for transportation delays of acute stroke patients – but should I? It also provides us with a greater range of tools to tackle a greater range of research problems more appropriately and successfully, filling in the blind spots on one half of the methodological spectrum to better address the whole complexity of neurological research and practice.
Take-away-points
• Assessing complex multi-component interventions or systems (of change) • What works for whom when, how and why? • Focussing on intervention improvement | • Document study • Observations (participant or non-participant) • Interviews (especially semi-structured) • Focus groups | • Transcription of audio-recordings and field notes into transcripts and protocols • Coding of protocols • Using qualitative data management software |
• Combinations of quantitative and/or qualitative methods, e.g.: • : quali and quanti in parallel • : quanti followed by quali • : quali followed by quanti | • Checklists • Reflexivity • Sampling strategies • Piloting • Co-coding • Member checking • Stakeholder involvement | • Protocol adherence • Sample size • Randomization • Interrater reliability, variability and other “objectivity checks” • Not being quantitative research |
Acknowledgements
Abbreviations.
| EVT | Endovascular treatment |
| RCT | Randomised Controlled Trial |
| SOP | Standard Operating Procedure |
| SRQR | Standards for Reporting Qualitative Research |
Authors’ contributions
LB drafted the manuscript; WW and CG revised the manuscript; all authors approved the final versions.
no external funding.
Availability of data and materials
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- Open access
- Published: 27 June 2023
Exploring a competency framework for the chief financial officer of a hospital: a qualitative study from China
- Hongzhi Wang 1 na1 ,
- Xin Xiang 2 na1 &
- Luping Dong 3
BMC Health Services Research volume 23 , Article number: 692 ( 2023 ) Cite this article
1331 Accesses
1 Citations
Metrics details
Hospital chief financial officer (CFO) plays a vital role in supporting the effective management of organization. Understanding their competencies is essential to improve hospital development and health care services in China. This paper aims to explore competencies necessary for hospital CFOs to fulfil their management responsibilities and develop a competency framework for hospital CFOs in China.
A qualitative study was applied by conducting in-depth interviews with 151 participants from 15 Chinese provinces, comprising 89 individuals from 67 hospitals, and 62 individuals from 39 medical universities. Interviews were anonymised, recorded and transcribed. Qualitative thematic analysis was applied through a multi-stage review process and modified via the Delphi process using a national panel of 36 experts.
Using content analysis, we identified 17 competencies organized into three themes (personal attitudes, leadership competencies and managerial competencies) to conduct a competency framework for hospital CFO to fulfil their management practices. Those competencies emphasized the integration of different competencies required by the hospital CFO.
Conclusions
This paper identified the detailed expertise, abilities and personal traits required by hospital CFOs in China, expanding the insights and perspectives of hospital CFOs currently working in China to literature. The proposed framework will help hospitals establish selection criteria, coaching tools, and development plans for CFOs.
Peer Review reports
Chief financial officers (CFOs) play a pivotal role in dealing with organization environments, strategy management and resources allocation [ 1 , 2 , 3 ]. Most studies have focused on corporate CFOs [ 4 , 5 , 6 , 7 ], but there has been less research on hospital CFOs. In the Chinese context, with the growing complexity of the health system environment and the demands of health services, hospitals began to develop the CFO position, expecting them to demonstrate capabilities to meet the demands of the position. However, our knowledge of CFO competencies in this context is limited. This paper aims to address this unfilled space and proposes an exploratory study to identify competencies required by CFO to fulfil their responsibilities in China’s hospitals.
The CFO role is established through appointment, serving as an oversight function for an organization’s financial matters and reporting. Typically, the CFO holds a leadership position as the second-in-command within the organizational hierarchy [ 8 ]. In corporate settings, the CFO’s responsibilities often include analysing the performance of business units, engaging in strategic planning, and handling stock exchange reporting [ 9 ]. Scholars have identified four roles of the CFO, including finance expert, generalist, performance leader, and growth champion [ 10 ]. Non-profit organizations, such as health care, education and social services, are increasingly assuming responsibility for services that were traditionally considered the domain of the government [ 11 ]. Non-profit CFOs may face challenges due to the comparatively lesser emphasis historically placed on the economic approach underlying accounting for non-profit organizations [ 12 ]. Therefore, the responsibilities of CFOs and the competencies required by them to perform their roles vary with the type of organization.
In addition to efficiently and autonomously handling cost control and performance budgeting matters [ 13 ], CFOs from health service organizations play a significant role in shaping the strategic direction of their respective organizations [ 14 ]. Advanced medical systems have previously established the position of CFO to improve management of the health care system by playing pivotal roles in corporate leadership and management, stewardship and accountability, financial management, commercial acumen, professional leadership and management. For example, NHS CFOs play a crucial role in the efficient delivery of safe, effective and financially sustainable services by providing strategic focus, corporate management, decision-making and leadership; additionally, they bear the responsibility of establishing the overall financial direction of the institution and fostering a culture that promotes collaboration, innovation, quality, and cost-effectiveness while ensuring prudent and efficient use of public funds [ 15 ], thus needing practical capabilities and technical knowledge, such as communication, influencing and negotiating, analysing information and solving problems, and working in and leading teams.
In recent years, the hospital CFO position has emerged in China. The “Hospital financial system” issued by the Ministry of Finance and the Ministry of Health in China proposes that tertiary public hospitals should establish the position of CFO, while other hospitals may determine the necessity based on their specific circumstances [ 16 ]. Hospital CFOs in China must be qualified accountants who are members of either the senior accountant or the certified public accountant classification. Due to a shortage of talent in the position of CFO, many hospitals have not appointed CFOs, and even in hospitals where CFO is present, there are often issues with inadequate fulfilment of their responsibilities.
Since the COVID-19 pandemic, China’s hospitals have faced serious challenges in organizational operations, resource allocation and efficiency [ 17 ], leading to an increase in the demand of capable CFOs. To address this problem, China’s central government has published several documents, such as “Opinions on accelerating the construction of chief accountant system in tertiary public hospitals” [ 18 ] and “The guidelines opinions of building modern hospital management system” [ 19 ], to highlight the importance of CFOs and offer further guidance in fostering their managerial competencies. However, there is limited research devoted to understanding the competencies required by hospital CFOs.
In China, as reported by China Association of Chief Financial Officers, the responsibilities of hospital CFOs are primarily reflected in the following: assisting the hospital director in achieving the overall goals of the hospital, supervising and overseeing the responsibilities of the financial department, coordinating with various departments to ensure diligent compliance with financial regulations, and participating in the analysis, recommendations, and decision-making processes related to significant financial matters within the hospital [ 20 ]. Hospital CFO faces a range of different expectations from their stakeholders and are expected to fulfil various managerial functions [ 21 , 22 ]. Specifically, CFOs are generally expected to be involved in organizing and planning financial and operation management, revealing risks, providing suggestions and financial information to support decision-making, and supervising hospitals to implement the relevant national laws and regulations [ 23 , 24 , 25 ]. CFOs also play a pivotal role in identifying opportunities and threats in strategy management and take an active part in strategy formulation [ 26 , 27 ]. Several studies have stressed the urgency of developing managerial competencies to achieve efficient and effective management among China’s hospital CFOs [ 28 , 29 ]. Additionally, other members of the executive team in China’s hospitals are medical experts and commonly lack management knowledge and skills [ 30 ], resulting in an increased demand for professional CFOs in the hospital system. This further reinforces the importance of CFO competency to meet the requirements of fulfilling management functions in daily management.
Competency is typically described as a set of knowledge, skills, abilities and other characteristics necessary for people to meet a range of job needs more effectively than others [ 31 , 32 , 33 , 34 ]. In terms of human resource management, the competency framework is fundamental to assessing the demands within a specific organization, evaluating performance assessments and monitoring career development [ 35 , 36 ], as well as providing guidelines for individual self-development [ 37 , 38 , 39 ]. Hitherto, there has been limited research on hospital CFOs in the Chinese context. The unexplored areas in the literature indicate a lack of understanding of CFO positions and suggests that CFOs may be poorly prepared for management roles. The competency of CFOs in China’s hospitals have not been widely researched; in this paper, we pursue this challenge.
This paper aims to identify specific competencies necessary for China’s hospital CFOs then develop a competency framework of hospital CFOs to fulfil their responsibilities and achieve organization objectives in the Chinese health services context. It will develop our understanding of professional development needs of health service management in China.
Hitherto, the competency of CFO remains an underexplored topic in the literature; hence, a qualitative approach was considered the most suitable method for this research. This research was a cross-sectional, in-depth interview based and descriptive study. Figure 1 displays the process of the multi-stage method.
Multi-stage process
Participants
From both theoretical and practical perspectives, we considered public hospital executives and CFOs as well as researchers focusing on hospital management research to be relevant in this study. The hospital executive is the chief executive officer (CEO) who is responsible for nominating candidates for the position of CFO, thus possessing a clear understanding of the competencies required for the role to aid in making appropriate personnel judgment. Researchers focusing on the health system, health economics and hospital management have a deeper exploration of the combination of theory and practice in hospital management. They also possess in-depth and specific knowledge of competencies that enable CFOs effectively manage hospital financial operations. Candidates are selected in accordance with the following four principles: 1) hospital candidates are from tertiary hospitals and have more than 10 years of management experience; 2) researchers come from medical universities and have been engaged in health system or hospital management research for more than 5 years; 3) the geographical distribution of candidates covers different provinces; 4) we are able to establish contact with them; and 5) we tried to split the percentage of respondents from academia and practice equally but adjusted for the actual situation. We invited 210 potential interviewees comprising 124 CEOs and CFOs from 82 hospitals and 86 researchers from 52 medical universities. A total of 71.90% (151 individuals, dram from 15 provinces across mainland China) of them responded to the invitation to participate. The final sample comprised 89 individuals from 67 hospitals, and an additional 62 individuals from 39 medical universities. Of the 67 hospitals included in this study, all were tertiary hospitals (AAA, the highest level of hospital certification), and 88.06% (59 hospitals) were large, with more than 4000 beds. Most respondents (115, 76.16%) were males, and the average age was 45.79 years. Ph.D degree holders were larger in number, with 103 (68.21%). Table 1 reports the demographic characteristics of the study participants.
Data collection
To explore and develop indicators and a framework of competency for CFOs in China’s hospitals, we adopted in-depth interview (semi-structured interviews) to collect data. At the beginning of interview, researchers introduced the study and participation in the interview, including assurances of anonymity and confidentiality, and answered any questions. Interviews was conducted at the workplace in a conference room during working hours. All interviews were audio recorded, transcribed and checked by interviewers. Interviews were conducted between December 2020 and January 2022, and the average length was approximately 50 min each.
The semi-structured interviews were conducted using a topic guide that outlines the topics to be covered and the main question to be asked. The interview involved questions about “How do you understand the CFO position in China’s hospitals?” and “What knowledge, skills, attitudes, values and traits are necessary for hospital CFOs to practice and fulfil their position responsibilities and requirements?”.
Data analysis
The original research was intended to focus on application rather than theoretical construction [ 40 ]; therefore, a thematic description was considered the most appropriate analysis for the original study [ 41 ]. All interview transcripts were analysed using thematic analysis and coded in NVivo. The coding framework was informed by a priori knowledge reflected in the topic guide, initial readings and preliminary coding of the transcripts [ 40 ]. Peer debriefing (extensive discussion of transcript data meaning and codes)was undertaken by the team. After the initial coding of five interviews, two researchers coded the same transcripts separately to ensure consistency in the interpretation of the data. Finally, the initial thematic template containing three themes and 23 items was developed, as presented in Table 2 .
Delphi process
We applied a Delphi study, which is a systematic research method that uses the judgement of an expert panel, to reach consensus. The criteria we employed to develop a list of potential panel members were academic qualifications, experience in operating hospital or studying hospital management, and level of appointment (e.g., professor, hospital CEO or CFO). We sent email invitations to a selected group of academics and practitioners, providing them with information about the study’s purpose and the estimated time commitment expected from their participation. Out of 51 invited experts, 36 (70.59%) agreed to participate in the Delphi study. The 36 experts originated from 13 different provinces and have an average of 17.27 years of experience in hospital management.
The Delphi study was conducted in three rounds comprising one qualitative and two quantitative rounds. Delphi round 1 was completed by 36 experts, and three experts withdrew due to unexpected unavailability to attend, resulting in a response rate of 91.67% in rounds 2 and 3. Table 3 displays the Delphi study round overview.
Delphi rounds
In round 1, the initial template (as reported in Table 2 ) was sent to the panel, who was required to modify key themes and potential components obtained from the interviews. Two open-ended questions were set as follows: 1) Please kindly provide comments or modifications regarding the proposed themes and components in relation to the competencies expected of hospital CFO; 2) Please add additional competencies that are crucial but missing from the proposed template. The responses were collected and used to generate a comprehensive set of modified and newly proposed components encompassing all themes.
In round 2, self-administered questionnaires were developed based on results derived from round 1. The questionnaire used a five-point Likert scale (1 = strongly disagree; 2 = disagree; 3 = undecided; 4 = agree; s = strongly agree), and experts rated the extent to which they agreed or disagreed with those competencies required by CFO to fulfil position responsibilities.
In round 3, we provided feedback on the results obtained from round 2. The same questionnaire and the feedback as a simple statistical summary of responses from round 2 were sent back to the same group. Experts were encouraged to review and reconsider their initial voting based on the consolidated results. This facilitated the opportunity for members to modify their responses in consideration of the collective opinion with the group.
Consensus was determined by considering the percentage of participants who either “agree” or “strongly agree” with the recommendation statements in both rounds 2 and 3. Following previous studies (e.g., [ 42 ]), consensus was defined as a threshold of over 70% agreement among the participants. The data analysis process involved two main steps. First, the comments from round 1 were manually analysed using thematic analysis. Second, standard descriptive statistical analysis for data obtained from rounds 2 and 3 was conducted using SPSS. The reliability and coherence between components in questionnaires were assessed independently using Cronbach’s alpha coefficient. The calculated coefficients ranged from 0.85 to 0.97, indicating sufficient reliability and inter-item correlation.
According to the meaningful comments that emerged from Round 1, several components from the initial template were renamed and integrated. For example, three items were renamed (e.g., ‘personality’ was changed to ‘personal attitudes’; ‘responsibility’ was changed to ‘dependability’; ‘knowledge sharing’ was changed to ‘knowledge management’). Several items were integrated (e.g., ‘patient services care’ and ‘employee benefits care’ were transformed to ‘compassion’; ‘financial budget and program analysis’ and ‘budget formulation’ were transformed to ‘budget and fiscal management’; ‘strategy management’ and ‘change management’ were integrated as ‘applied strategic thinking’). After rounds 2 and 3, three items in total were excluded, namely, managerial courage, performance measurement and technical proficiency. Considering this expert vetting, eventually, the competencies framework of the hospital CFO was established, covering three themes and 17 items.
Ethical considerations
All participants in the in-deep interviews and the Delphi process gave informed consent in response to a letter that explicitly stated that participation was voluntary and guaranteed complete confidentiality.
In China, the prerequisite for becoming a hospital CFO is to possess a senior professional and technical qualification in finance or accounting or to have a certified public accountant qualification and work in financial accounting for five years or more. In addition to professional qualifications, hospital CFOs also require a range of competencies to meet the demands of job responsibilities. By interviewed 151 participants from 15 Chinese provinces and adopting a Delphi study with three rounds, we identified three themes in terms of personal attitudes, leadership competencies and managerial competencies, involving 17 items, to develop competency framework for hospital CFOs. Table 4 reports the proposed competency framework necessary for CFOs to fulfil their responsibilities and achieve objectives in China’s hospitals. It also presents statistics for each item in Round 2 (in brackets) and Round 3, and the results of testing the statistical significance for the differences in the mean scores in the two rounds are also reported in Table 4 .
Personal attitudes
This theme describes individual traits and personality required to determine CFOs’ professionally and ethical behaviour. It comprises five items: honesty and trustworthiness, dependability, integrity, social responsibility and compassion. Participants suggested that this theme is fundamental to the professional attitudes of CFOs to participate in hospital management.
Honesty and trustworthiness
CFOs should promote a climate of openness and honesty as well as enabling building trust with organizational members and stakeholders. Participants mentioned that by only building trust with employees, the strategy proposed by CFO can be implemented by organization members. In addition, the supervisory role played by COF also require them to maintain honesty and trustworthiness:
As a member of the hospital executive team, a CFO has the responsibility to supervise the hospital’s operation management and financial behaviours. A CFO, as a moral modelling for financial personnel, must create an honest atmosphere and ensure the authenticity of financial information. (Participant 3)
Dependability
The CFO is expected to take full responsibility in his or her position, to deal with problems quickly, to display a strong commitment to organizational success and to demonstrate a commitment to delivering on his or her public duty. As participants noted, CFO plays multiple roles in hospital management and requires taking his or her responsibilities seriously and consistently meeting the public’s expectations for health care development and professionalism:
The functional roles of the CFO refer to developing organizational strategy, allocating resources, monitoring process and evaluating performance. We expect CFOs to hold themselves and others accountable for making principled decisions. (Participant 11)
It refers to the CFO behaving in an ethical manner and treating others fairly and with respect. Participants emphasized that hospitals, as providers of health care services, are faced with more ethical choices; therefore, CFOs need to identify ethical dilemmas and conflicts of interest and take action to prevent them when developing organizational strategies. Moreover, CFO should remain fair and objective when communicating with employees:
The CFO is the value manager of organizational resources, and his or her actions directly affect the development of the organization and the interests of stakeholders; hence, the CFO should remain fair and objective in the allocation of resources and projects. (Participant 7)
Social responsibility
CFOs must be aware of social responsibility undertaken by the hospital and the self, ensure that the decisions and actions made are based on the public interest, and rationally used social resources to improve the efficiency of the hospital. Participants mentioned that CFOs should establish plans and programs for satisfying the needs and expectations of public health care services:
Hospitals are non-profit organizations responsible for providing health care service to the public, undertaking the social responsibilities of participating in the rescue of major disasters and accidents, and managing public health emergencies. As a member of the executive team of the hospital, the CFO should formulate a development strategy that complies with social responsibility of the hospital. (Participant 9)
It describes genuinely caring about others, being helpful when others are in trouble, and showing genuine empathy for others’ emotions. CFOs should understand the emotional components of working in the hospital context and have the remarkable ability to empathize with and demonstrate healthy concern for others. Specifically, compassion emphasizes that CFO pays attention to employee benefits care and patient services care. As participants mentioned:
Every hospital employee must have a strong sense of empathy, being able to understand the situation of others, and know to put himself or herself in others’ shoes. We expect capable CFOs to put others’ feelings first when necessary and provide help when people approach a problem. (Participant 12)
Leadership competencies
This theme refers to the abilities and skills to lead, direct and inspire others to achieve organization goals through teamwork and collaboration, as well as the abilities to motivate and support others to self-development. It comprises seven items: planning and organizing, critical thinking, learning, creativity, communication, analytical thinking and flexibility.
Planning and organizing
This references ways CFOs are able to plan and organize daily works, using goal setting, creating work schedules and work plans with associated budgets and resources to achieve established goals. Participants indicated that a capable CFO could integrate resources and apply “best practices” to improve effectiveness and efficiency:
CFOs should carry out strategic planning and financial planning for the development of the hospital and participate in major decision-making of the hospital; hence, they need abilities to organize work, set priorities and determine resource requirements. (Participant 27)
Critical thinking
It refers to the ability to consider situations from multiple perspectives and to systematically analyse and organize parts. Participants stressed that CFOs can provide creative solutions and identify the link between action and outcomes. A competent CFO can assess the quality of evidence and reasoning, as well as consider the big picture and impact on results:
In China’s hospitals, most of executive teams are medical experts, and they are professional in the medical field rather than in hospital management. As the professional manager, a CFO must critically compare different points of view and examine situations from multiple or different perspectives when making decisions. (Participant 33)
It describes the ability to learn actively and continuously, to summarize past achievements and failures to improve productivity, and to identify technological changes and update information reserves. Participants emphasized that the functional role of CFOs involves multiple aspects, such as finance, accounting, strategy and operations, hence, requiring him or her to be able to continuously learn and improve to meet the demands of the position. Without the learning ability, the CFO makes decisions from a single perspective:
Medicine refers to fields of technological and innovation and require the most cutting-edge knowledge and information. If CFOs do not have the ability to lead and understand the latest medical technology and trends, they will be unscientific when formulating hospital strategies. (Particiapant 37)
It refers to the ability to generate original and unique ideas, connecting previously unrelated concepts, to address complex challenges. Participants mentioned that a CFO needs to develop new methods/procedures/processes that are proven to work and encourage new ideas and motivate others to be proactive and resourceful. They highlighted:
CFOs are different from financial director; they need to work creatively, continuously promote the improvement of hospital operation and processes in accordance with the principle of maximizing benefits, and break the convention to find more effective ways to allocate and use resources. (Participant 2)
Communication
It refers to listening respectfully to others to gain a full understanding of issues and to ensure that important managerial information is shared with employees and others in the appropriate way. Participants emphasized CFOs should communicate clearly and effectively and have responsibility to understand others. A competent CFO can apply skills to present information, analysis and opinions to audiences in a clear, accurate and compelling manner:
CFOs should not only pay attention to establishing contacts with the financial team, executive team and other departments within the hospital but also be good at establishing contacts with higher authorities, relevant units inside and outside of the health care system and other peers and coordinate the relationship between all parties according to the needs of the work. (Participant 40)
Analytical thinking
This describes the ability to identify and define problems, analyse and verify the cause of the problem by extracting key information, and develop feasible solutions to address the identified problem. Participants indicated that a competent CFO could analyse the situation of the hospital, identify the problem affecting the development of the hospital and select the most effective solution to solve the problem. They noted that:
The CFO is responsible for making logical deductions from information and identifying a number of solutions to complex problems integrating findings from several different disciplines. (Participant 41)
Flexibility
This refers to the ability to recognize and accept change and new information and rapidly adapt to changing conditions. Participants stressed that CFOs should be aware of the requirements of different situations and be able to effectively adjust their actions to adapt to changing environment. Moreover, CFO is responsible for providing new approaches or solutions to meed the different expectations in changing organizational environment:
COFs should have the ability to change plan based on input from staff and stakeholders and adjust organizational priorities quickly as situations change. (Participant 31)
Managerial competencies
This theme refers to the ability and expertise needed by CFOs to fulfil their responsibilities to ensure that they make more professional judgments and choices. It includes expertise, applied strategic thinking, budget and fiscal management, knowledge management, risk management and problem solving.
This describes that the needs of a CFO to have professional knowledge in term of finance, accounting, medical system, legal system, rules and regulations to meet the different demands of the position. Participants emphasized expertise contribute to CFO consideration of organizational strategy from a big picture. They highlighted:
CFOs should be familiar with the professional knowledge in terms of operation management, accounting, finance, law and information technology. Meanwhile, they should understand the development of the health industry and economic society. (Participant 1)
Applied strategic thinking
It refers to the ability to interpret health care policies and analyse organization strategy, as well as to design change management to implement strategic and political directions. Participants stressed that the CFO is responsible for designing for designing performance measures and determining the resource allocation to achieve strategy. They emphasized that:
CFOs should have a long-term vision to formulate hospital strategic goals and plans while making overall plans and allocating resources to achieve those goals. (Participant 5)
Budget and fiscal management
This describes the ability to plan the work-unit budget, to monitor and evaluate the financial procedures and systems, and to provide suggestions on financial condition. Participants described a capable CFO should develop, monitor and evaluate procedures and standards to ensure the good financial condition of the hospital. Participants also emphasized that a CFO needs to analyze budget statements and financial reports while making recommendation to improve the financial performance of hospitals:
One of responsibilities for the CFO is to use financial and other quantitative information to manage the hospital and understand overall financial performance of hospital, as well as analyze financial information to evaluate strategic opportunities and options. (Participant 51)
Knowledge management
It refers to the ability to identify and provide useful information to others to increase organizational effectiveness. Participants highlighted the CFOs can collect and obtain external and internal information through novel approaches and then share that information with others in a timely manner to reduce information asymmetry. They stated that:
CFOs are expected to plan, develop and manage information storage and retrieval systems and to promote organizational development by sharing knowledge and information. (Participant 43)
Risk management
It refers to the ability to plan and implement measures to avoid, overcome or compensate for risk factors. Participants emphasized that CFOs should manage work and information within a strategic framework, establish a proven approach to risk management, identify risks of negative outcome, and develop solutions to reduce risk and maximize value. A capable CFO can communicate the impact of identified risks and apply corrective action. Participants stressed the following:
CFOs should carefully sort out and manage the operation process of hospitals to ensure effective management of various risks for hospitals. (Participant 20)
Emerging evidence highlights the significant role played by CFOs in improving hospital operation and management [ 14 ], especially during the COVID-19 pandemic. A growing number of public hospitals in China have established the position of CFO; however, competent hospital CFOs are scarce. Like CFOs in the NHS who must be qualified accountants who are members of either the Chartered Institute of Management Accountants (CIMA) or a member of one of the five accountancy institute members of the Consultative Committee of Accountancy Bodies, hospital CFOs in China need to hold the professional qualification of senior accountant or certified public accountant. The responsibilities of hospital CFOs are complex and varied, such as protector, supporter, innovator and strategist [ 24 ], thus requiring various competencies to fulfil position responsibilities.
This study aims to explore competencies necessary for management practice by CFOs in China’s hospitals and develop a competency framework for hospital CFOs. Using a qualitative research method, by interviewing 151 participants (hospital executive, CFO and researcher) from 15 Chinese provinces, we identified the initial thematic template covering three themes and 23 competencies. Subsequently, we applied a three-rounds Delphi process to verify expert validity. The ultimate competency framework for hospital CFOs composed of 17 competencies and organized into three themes: personal attitudes, leadership competencies and managerial competencies. The proposed framework highlighted that fulfilling complex responsibilities in hospital management practice requires the integration of different competencies.
This competencies framework highlighted the role played by hospital CFOs and their responsibilities proposed in previous studies. For example, competencies such as social responsibility and dependability reflect that the hospital CFO should fulfil a position based on the organization’s mission and expectations, which is consistent with Bish and Becker [ 43 ], who stated that leadership and management are different in nonprofit, as nonprofits have unique strategic and operational characteristics. Critical thinking, analytical thinking, creativity and flexibility may be indicators of the growing emphasis of CFOs involved in formulating and executing organizational strategy [ 44 ]. Furthermore, the competencies of communication and flexibility encourage hospital CFOs to lead others to achieve objective in a more effective way [ 45 ]. The ability of budget and fiscal management indicates the growing emphasis on the role played by CFOs in the accountability and financial performance of hospitals. The element of risk management reflects CFO’s responsibilities, such as advising on project evaluations both for financial and nonfinancial impacts, forecasting based on financial performance, identifying risk and providing suggestions [ 46 ].
In the comparison with the competency models in other countries, there are some similarities. The previous competency framework for public CFOs [ 47 ] emphasized the domains of strategic and financial, which were similar to our results. Additionally, the description of the NHS CFO position highlights the value in terms of compassion and integrity, essential CFO attributes such as planning, communication, flexibility and analytical thinking [ 15 ]. Our study also emphasized the competencies necessary for China’s hospital CFOs to fulfil their responsibilities. Additionally, our proposed framework expanded the theme of personal attitudes of CFOs by adding dependability, honesty and trustworthiness, which are commonly valued in Chinese culture.
This paper provides several theoretical contributions and practical implications. First, this qualitative research contributes to deeply exploring the competency required by CFOs in China’s hospitals. Gaining the insights and perspectives of CFOs currently working in hospital sector expands our understanding and knowledge of competency requirements in an understudied context. The findings indicate that the uniqueness of hospital affect the requirements of CFO competency, which will have an impact on organizational processes in terms of recruitment, selection and human resource management. In practical terms, the proposed competency framework clarifies the management requirements of hospital CFOs and can be used as the cornerstone for establishing selection criteria, guidance tools and development programs. Finally, the analysis of the required competencies for CFO provides significant practical implications for designing human resource management practices for hospital management in China.
This study has some limitations and provides opportunities for future research to strengthen and develop our findings. As the qualitative approach was adopted in this study, this research was limited by the sample size ( N = 151, from 15 Chinese provinces), which may limit the comprehensive identification of competencies required for CFO practice in China’s hospital. Furthermore, the extent to which we can generalize these findings to all hospitals in China is limited, and our findings need to be validated in other hospital settings (e.g., secondary hospitals and private hospitals). It seems the good opportunities for future research. Compared with our cross-sectional design, using a longitudinal design would provide stronger evidence of the expected competencies requirements of CFOs over time. The proposed competency framework was conducted based on respondents’ perceptions of the expectations of their CFOs. Future research could consider adding the objective measured of performance expectations for CFO position to enhance this research. Additionally, it cannot be overlooked to develop assessment approaches that can be used to assess the competency of the current hospital CFO in China, and future research could fill this gap.
The role played by hospital CFO in strategy, performance and risk management is gaining increasing attention from practitioners and scholars. China’s hospital seeks competent CFOs to take on their responsibilities, especially in an increasingly complex organizational environment. This paper provides insights into the competencies required by CFOs to achieve their responsibilities. The proposed framework identifies the integration of different competencies in terms of personal attitudes, leadership competencies and managerial competencies as the core competencies for CFO in hospital management. It expands an underexplored topic in prior studies and provides a systematic framework to develo hospital CFO in China, including establishing clear management competency requirements to guide management position recruitment, development and performance management.
Availability of data and materials
The data and materials analysed during the current study are not publicly available because they are part of datasets for three further papers, but the interview materials (questions) are available from the corresponding author on reasonable request.
Chahyadi C, Abusalim B. The role of education and experience in CFO career and compensation. J Account Financ. 2011;11:26–35.
Google Scholar
Daff L. Chief financial officers’ perceptions of their roles inside nonprofit organizations. Financ Account Manage. 2020;3:1–17. https://doi.org/10.1111/faam.12233 .
Article Google Scholar
Hiebl M, Neubauer H, Duller C. The chief financial officer’s role in medium-sized firms: Exploratory evidence from Germany. J Int Bus Econ. 2013;13:83–92.
Habib A, Hossain M. CEO/CFO characteristics and financial reporting quality: A review. Res Account Regul. 2013;25:88–100.
Kathy F, Tomas J, Mccumber WR. CFO social capital and private debt. J Corp Finan. 2018;52:28–52.
Ferramosca S, Allegrini M. Impairment or amortization of goodwill? An analysis of CFO perceptions of goodwill accounting. Eur Manag J. 2021;39:816–28.
Gupta VK, Mortal S, Chakrabarty B, Guo X, Turban DB. CFO gender and financial statement irregularities. Acad Manag J. 2019;63:802–31.
Bremer D. The effect of stakeholder influence on CFO and CEO turnover in German corporate governance. Remshalden, Germany: Pressel; 2010.
Lee A, Zhang T. Building the CFO function: roles and responsibilities. 2012. Available from: https://ink.library.smu.edu.sg/soa_research/924 .
Agrawal A, Goldie J, Huyett B. Today’s CFO: Which profile best suits your company? Corp Finance Pract. 2013;1:1–6.
Unerman J, O’Dwyer B. Accounting and accountability for NGOs. In Hopper T, Tsamenji M, Uddin S, Wickremasinghe D (Eds.). Handbook of accounting and development. Cheltenham: Edward Elgar Publishing. 2012. pp.143–161.
Lightbody M. On being a financial manager in a church organisation: Understanding the experience. Financ Account Manage. 2003;19:117–38.
Jakobsen MLF, Lapsley I. Performance budgeting in practice: The case of Danish hospital management. Public Organ Rev. 2017;17(2):255–73.
Malmmose M, Dahl Pedersen L. “Money is not an issue!”: hospital CFOs’ narratives about handling a sudden shift in managerial focus. Int Rev Admin Sci . 2023. https://doi.org/10.1177/00208523231169913 .
Healthcare Financial Management Association. The role of the NHS Chief Finance Officer. 2017. Available from: www.hfma.org.uk .
Misnistry of Finance of the People’s Republic of China, Ministry of Health of the People’s Republic of China. Hospital financial system. 2010. Available from: https://www.gov.cn/gongbao/content/2011/content_1852409.htm .
Liang ZM, Blackstock FC, Howard P, Liu CJ, Leggat G, Ma H, Zhang ZJ, Bartram T. Managers in the publicly funded health services in China - characteristics and responsibilities. BMC Health Serv Res. 2020;20:721.
Article PubMed PubMed Central Google Scholar
National Health Commission of the People’s Republic of China. Opinions on accelerating the construction of chief accountant system in tertiary public hospitals. 2017. Available from: http://www.nhc.gov.cn/caiwusi/s7785t/201706/98069506ead348caa5d23e1adf9edfd3.shtml .
National Health Commission of the People’s Republic of China. The guidelines opinions of building modern hospital management system. 2017. http://www.gov.cn/xinwen/2017-07/25/content_5213309.htm .
China Association of Chief Financial Officers. The role of Hospital CFOs. 2014. Available from: www.cacfo.com .
Pan JJ. The competence and value improvement of the chief account of public hospital in China. China Chief Financ Off. 2018;08:106–8.
Lin QY. Research on the countermeasures to improve the competence and quality of hospital chief accountant. Chinese Int Bus. 2017; 21:48–49. https://doi.org/10.19516/j.cnki.10-1438/f.2017.21.036 .
Yang XH. Development of the CFOs status in China and Western countries. East China Econ Manage. 2007;21:61–4.
Daff L. Chief financial officers’ perceptions of their roles inside nonprofit organizations. Financ Account Manage. 2021;37:3–19.
Donatella P, Tagesson T. CFO characteristics and opportunistic accounting choice in public sector organization. J Manage Governance. 2021;25:509–34.
Zoni L, Pippo F. CFO and finance function: what matters in value creation. J Account Organ Chang. 2017;13:216–38.
Caglio A, Dossi A, Van der Stede WA. CFO role and CFO compensation: An empirical analysis of their implications. J Account Public Policy. 2018;37:265–81. https://doi.org/10.1016/j.jaccpubpol.2018.07.002 .
Lei ZQ, Li JJ, Zhang GS, Deng P, Cui WP, Zhao ZN, Gao YB, Ma XL. Research on chief accountant system in tertiary hospital under the new medical reform. Chinese Hosp Manage. 2016;36:69–71.
Chen ZJ, Zhang GP, Fang SZ. The responsibility and competence of hospital general accountant. Health Econ Res. 2011;10:14–6.
Liang ZM, Howard PF, Wang J, Xu M, Zhao M. Developing senior hospital managers: does ‘one size fit all’? - evidence from the evolving Chinese health system. BMC Health Serv Res. 2020;20:281.
Frank JR, Mungroo R, Ahmad Y, Wang M, De Rossi S, Horsley T. Toward a definition of competency-based education in medicine: a systematic review of published definitions. Med Teach. 2010;32:631–7.
Article PubMed Google Scholar
Carraccio C, Wolfsthal SD, Englander R, Ferentz K, Martin C. Shifting paradigms: from Flexner to competencies. Acad Med. 2002;77:361–7.
Ten Cate TO, Snell L, Carraccio C. Medical competence: The interplay between individual ability and the health care environment. Med Teach. 2010;32:669–75.
Hager P, Gonczi A. What is competence? Med Teach. 1996;18:15–8.
Milner RJ, Gusic ME, Thorndyke LE. Perspective: toward a competency framework for faculty. Acad Med. 2011;86:1204–10.
Daouk-Oyry L, Zaatari G, Sahakian T, Alameh BR, Mansour N. Developing a competency framework for academic physicians. Med Teach. 2016;39:269–77.
Khan K, Ramachandran S. Conceptual framework for performance assessment: competency, competence and performance in the context of assessments in healthcare - deciphering the terminology. Med Teach. 2012;34:920–8.
Mdidhat Ali M, Qureshi SM, Memon MS, Mari SI, Ramzan MB. Competency framework development for effective human resource management. SAGE Open. 2021;11:1–15.
Johansson P, Grimm B, Abdel-Monem T, Hoffman SJ, DeKraai M, McMillan A. Perceived impacts of a public health training center field placement program among trainees: findings from a small group externship experience. Front Public Health. 2014;2:67. https://doi.org/10.3389/fpubh.2014.00067 .
Ritchie J, Spencer L. Qualitative data analysis for applied policy research. Analyzing qualitative data. Routledge; 2002. p. 187–208.
Sandelowski M, Barroso J. Classifying the findings in qualitative studies. Qual Health Res. 2003;13:905–23.
Maassen SM, Van Oostveen C, Vermeulen H, Weggelaar AM. Defining a positive work environment for hospital healthcare professionals: A Delphi study. PLoS ONE. 2021;16(2): e0247530.
Article CAS PubMed PubMed Central Google Scholar
Bish A, Becker K. Exploring expectations of nonprofit management capabilities. Nonprofit Volunt Sect Q. 2016;45:437–57.
Datta S, Datta MI. Upper-echelon executive human capital and compensation: generalist vs Specialist skills. Strateg Manage J. 2014;35:1853–66.
Yukl G, Mahsud R. Why flexible and adaptive leadership is essential. Consult Psychol J Pract Res. 2010;62:81–93.
IFAC. Enabling the accountant’s role in effective enterprise. 2018.
Schobel K, Pond G. Public CFO competencies–a national defence case study examining the balance between financial and strategic priorities. Can Public Adm. 2020;63(2):229–46.
Download references
Acknowledgements
We would like to thank Guangxi Academy of Medical Sciences and Shandong Academy of Social Sciences for their support of our research projects. We would also like to thank all participants who participated in this study.
This paper did not receive any specific grant from funding agencies in the public, commercial or nonprofit organizations.
Author information
Hongzhi Wang and Xin Xiang are first-authors.
Authors and Affiliations
Research Center of Hospital Management and Medical Prevention, Guangxi Academy of Medical Sciences, The People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, Guangxi, 530021, China
Hongzhi Wang
Institute of Fiscal and Finance, Shandong Academy of Social Sciences, Jinan, China
Department of Neurology, The People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, China
Luping Dong
You can also search for this author in PubMed Google Scholar
Contributions
H.W. and X.X. conceptualized the study and wrote the main manuscript text, and L.D. invited participants. All authors reviewed the manuscript.
Authors’ information
Dr Hongzhi Wang is a senior researcher working at Research Center of Hospital Management and Medical Prevention, Guangxi Academy of Medical Sciences. Dr Wang gained a PhD from Henley Business School, University of Reading. His main research areas include leadership, medical leadership, medical management, competency development, Chinese philosophy, and talent management.
Dr Xin Xiang is currently a senior researcher at the Institute of Fiscal and Finance, Shandong Academy of Social Sciences. Xin gained a PhD from Henley Business School, University of Reading. Her research primarily focuses on medical leadership, human resource management, leadership development, Chinese philosophy, organizational studies and talent management.
Ms Luping Dong is attending doctor in Department of Neurology, The People’s Hospital of Guangxi Zhuang Autonomous Region. She is interested in neurology and qualitative research.
Corresponding author
Correspondence to Hongzhi Wang .
Ethics declarations
Ethics approval and consent to participate.
This study was conducted with the approval of the ethics committee of Guangxi Academy of Medical Sciences. Participation in this study was fully anonymous and voluntary, and all participants signed a written informed consent form. All interviews were performed in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Wang, H., Xiang, X. & Dong, L. Exploring a competency framework for the chief financial officer of a hospital: a qualitative study from China. BMC Health Serv Res 23 , 692 (2023). https://doi.org/10.1186/s12913-023-09711-1
Download citation
Received : 25 March 2023
Accepted : 16 June 2023
Published : 27 June 2023
DOI : https://doi.org/10.1186/s12913-023-09711-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- China’s hospital
- Competency framework
- Qualitative research
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]

COMMENTS
Abstract. This article examines ways of approaching informed consent as a relationally constituted process in qualitative research practices. It argues that a researcher's operationalization of informed consent should be coherent with the overall epistemological framework of the project. Based on empirical examples from an ethnographic ...
Qualitative research with small sample sizes has added to our understanding of these challenges. However, there is value in garnering the perspectives of research participants and staff across larger samples to explore the impact of contextual factors (time spent, the timing of the discussion and the setting), on the informed consent process ...
The article's conceptualization of a continuous, situated and relational approach to informed consent is also supported by the concepts of response-ability and thinking with care in research ...
The informed consent process. The voluntary expression of the consent by a competent subject and the adequate information disclosure about the research are critical and essential elements of the informed consent process [].Competent subjects able to comprehend the research-related information should personally decide and provide the consent on research participation.
This article examines ways of approaching informed consent as a relationally constituted process in qualitative research practices. It argues that a researcher's operationalization of informed consent should be coherent with the overall epistemological framework of the project.
The informed consent process respects a person's right to decide whether participating in the research will be compatible with his or her values, beliefs, and interests ... Qualitative research methods, for example, in-depth interviews grant researchers the privilege of viewing study participants' lives and experiences in great detail ...
Informed consent is an essential part of all research endeavors that involve human participants. The human rights of research participants must be protected. It is incumbent upon the qualitative researcher to provide a dynamic informed consent when study outcomes change. The violation of privacy is more apt to occur with in-depth interviews ...
The process of informed consent for enrolment to a clinical research study can be complex for both participants and research staff. Challenges include respecting the potential participant's autonomy and information needs while simultaneously providing adequate information to enable an informed decision. Qualitative research with small sample sizes has added to our understanding of these ...
Qualitative research with small sample sizes has added to our understanding of these challenges. However, there is value in garnering the perspectives of research participants and staff across larger samples to explore the impact of contextual factors (time spent, the timing of the discussion and the setting), on the informed consent process ...
A "meaningful" informed consent. An "Informed" consent emphasizes a process where the clinical research participant must receive and comprehend information appropriately to make an autonomous decision. An informed consent process can be termed as complete, valid, and meaningful if all four criteria of information disclosure, competence ...
Informed Consent in Research. Informed consent is a process of communication between a researcher and a potential participant in which the researcher provides adequate information about the study, its risks and benefits, and the participant voluntarily agrees to participate. It is a cornerstone of ethical research involving human subjects and is intended to protect the rights and welfare of ...
Clandinin and Connelly's ethics guidelines widely cited in the qualitative research literature claim ethical considerations can and must be negotiated throughout the research process. Process consent is the most common definition of this negotiation. It is an active form of consent and taking the participant's right to withdraw beyond a ...
In this paper we propose that process consent, the type of consent considered essential in qualitative research projects, is not only appropriate but necessary for mental health nursing practice. This type of consent is an ongoing consensual process that involves the nurse and patient in mutual decision making and ensures that the patient is ...
In this paper we propose that process consent, the type of consent considered essential in qualitative research projects, is not only appropriate but necessary for mental health nursing practice. This type of consent is an ongoing consensual process that involves the nurse and patient in mutual decision making and ensures that the patient is ...
When qualitative researchers attend to and discuss informed consent, emphasis is often on the recruitment phase, before the fieldwork or data collection has begun (Gallagher et al., 2010). In this phase, researchers obtain formal access by sharing information and soliciting individuals' consent to participate in the research project.
An effective informed consent process involves these elements: Conducting the process in a manner and location that ensures participant privacy. Obtaining the prospective subject voluntary agreement to participate. Giving adequate information about the study in a language understandable to the potential subject.
Informed consent is one of the founding principles of research ethics. Its intent is that human participants can enter research freely (voluntarily) with full information about what it means for them to take part, and that they give consent before they enter the research. Consent should be obtained before the participant enters the research ...
Qualitative research plays a pivotal role in health psychology, offering insights into the intricacies of health-related issues. However, the specificity of qualitative methodology presents challenges in adhering to standard open science principles, including data sharing.
Qualitative research, characterized by substantially inductive and open-ended methods, is an important and accepted approach in the social sciences. Four specific features of qualitative research - understanding research participants' meanings, investigating the influence of the specific contexts in which the individuals and activities ...
Study Aim and Questions. The aim of this qualitative study was to explore the perspectives of key stakeholder experts in informed consent provision, representing clinicians, regulators, researchers, and patients advocates, about conveying risks and benefits messages to potential research participants during the informed consent process.
Consent theoretically threads through the whole qualitative research method, so getting this right can set the tone for person-centred relationships between researcher and participants. However, most attention has been given in the UK to cognitively biased informed consent and to consent taking place at the beginning of projects; and in North ...
Document the informed consent conversation and the patient's (or surrogate's) decision in the medical record in some manner. When the patient/surrogate has provided specific written consent, the consent form should be included in the record. In emergencies, when a decision must be made urgently, the patient is not able to participate in ...
Methods. The study used a qualitative research design to explore what implementation strategies were selected and the justifications for selecting these strategies. Workshops were used because this qualitative method is particularly well suited for studying co-design processes that involve substantial attention to social interaction and the ...
Qualitative research methods are interviewing and observing. Quantitative data is analyzed using statistical analysis. Qualitative data is analyzed by grouping the data into categories and themes. As you can see, both provide immense value for any data collection and are key to truly finding answers and patterns. ...
The American Psychological Association (APA) is a scientific and professional organization that represents psychologists in the United States. APA educates the public about psychology, behavioral science and mental health; promotes psychological science and practice; fosters the education and training of psychological scientists, practitioners and educators; advocates for psychological ...
We obtained informed consent from participants prior to the onset of interviews. We maintained confidentiality through secure storage of interview data (e.g., audio recordings), password-protection of sensitive documents, and the de-identification of transcripts. ... Qualitative research methods for the social sciences: 2nd ed. Bostan, MA ...
Abstract. There is always a debate around consent in the context of research. Given the expansion of different approaches to qualitative research within dementia care, there is increasing consideration around consent in this context; particularly in research concerning the experiences of living with dementia and the care of persons with dementia.
The JBI Qualitative Assessment and Review Instrument (QARI) software was utilized to analyze and categorize findings as either unequivocal ... All the included studies demonstrated congruity between the research methodology and the research questions, methods used to collect data, analysis, representation, and the conclusions from the data ...
Abstract. This paper aims to provide an overview of the use and assessment of qualitative research methods in the health sciences. Qualitative research can be defined as the study of the nature of phenomena and is especially appropriate for answering questions of why something is (not) observed, assessing complex multi-component interventions ...
All participants in the in-deep interviews and the Delphi process gave informed consent in response to a letter that explicitly stated that participation was voluntary and guaranteed complete confidentiality. ... Using a qualitative research method, by interviewing 151 participants (hospital executive, CFO and researcher) from 15 Chinese ...