

The Clinical Trials Team - Roles & Responsibilities

In a research study, a clinical trial tests a new medical treatment or a new way of using an existing treatment to see whether it will be a better way to avoid and screen for diagnosing or treating a disease. Purpose of clinical trial:
A research study that is performed on individuals for evaluation of a medical, surgical, or behavioral intervention.
Clinical Research Careers
Clinical Research Associate (CRA)
Clinical Research Coordinator (CRC)
Drug Safety Monitor (PV)
Clinical Trial Assistant (CTA)
Clinical Research Nurse (CRN)
Medical Monitor (MM)
Principal Investigator (PI)
All Research Professionals (ICH GCP)
Types of clinical trials:
Prevention trials
Screening trials
Case control studies
Cohort studies
Cross sectional studies
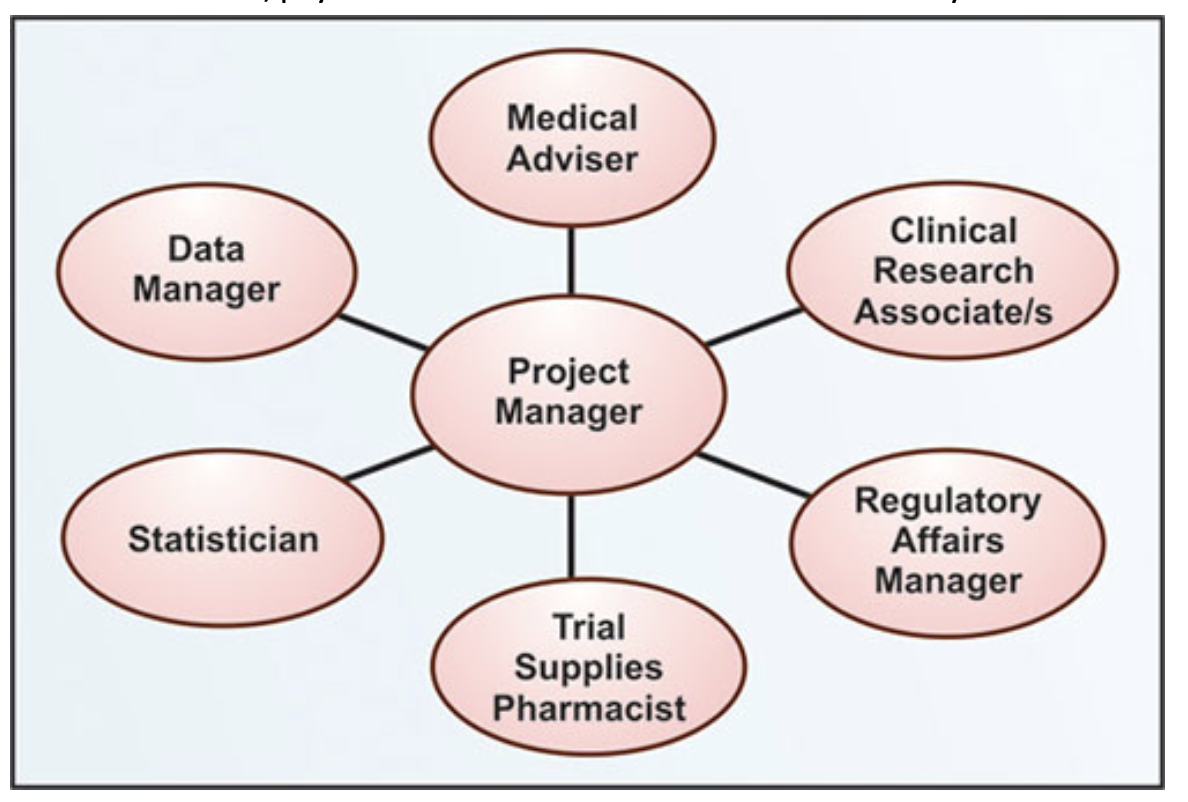
Figure no. 1: clinical trial team flowchart
Clinical research trial team:
The success of a quality clinical research program is essential for developing and maintaining an impeccable clinical research trial team. It is the main component of a research program because total time and effort for conducting a clinical trial; nurses and data managers each contribute more than 30%. On the other hand, physician’s contribution to clinical research is only 9%.
Roles and Responsibilities of clinical trial personnel
Clinical research team:
Participants are provided with information about the clinical trial.
The content of the informed consent is explained.
Reporting of adverse events or drug reactions.
report suspected misconduct.
Protect the integrity and confidentiality of records and data during the clinical study
Responsibilities:
Appropriate training
Following of GCP standard
Following required protocols
Investigator:
• Following ethical principles.
• Provide education programs.
• Design and conduct clinical trials for policies and procedures.
• Refer to GCP course for training.
• Determines the scientific, technical, and administrative aspects of the research project.
Responsibilities:
• Conduction of trial, statement, protocol, and applicable regulations.
• Protection of rights and welfare of participants.
• Obtaining informed consent.
• Maintenance of proper records.
• Management of all safety reports and financial disclosure reports.
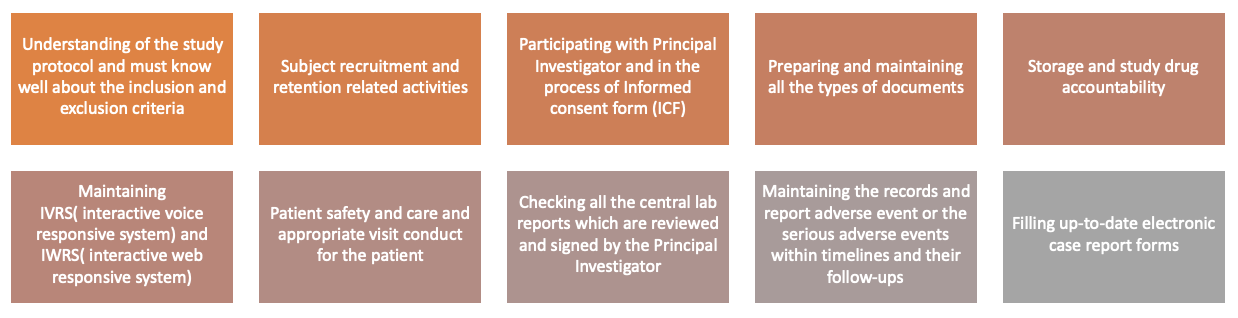
Figure no. 2: Roles of clinical research controller.
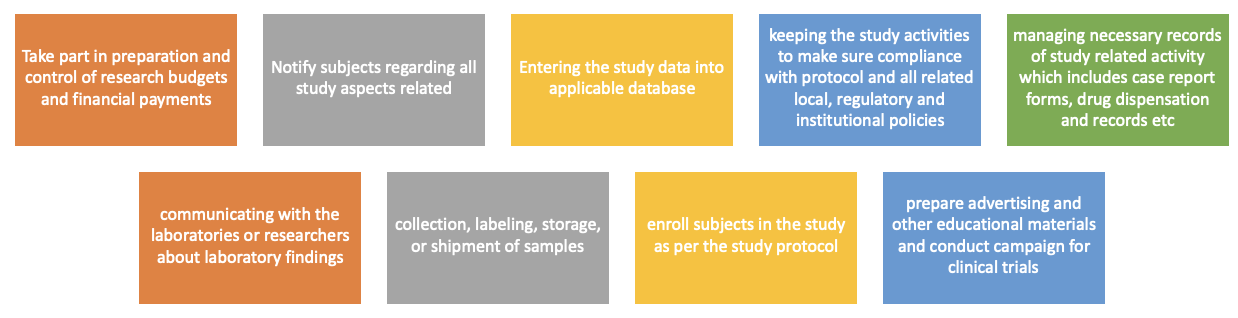
Figure no. 3: Responsibilities of clinical research controller.
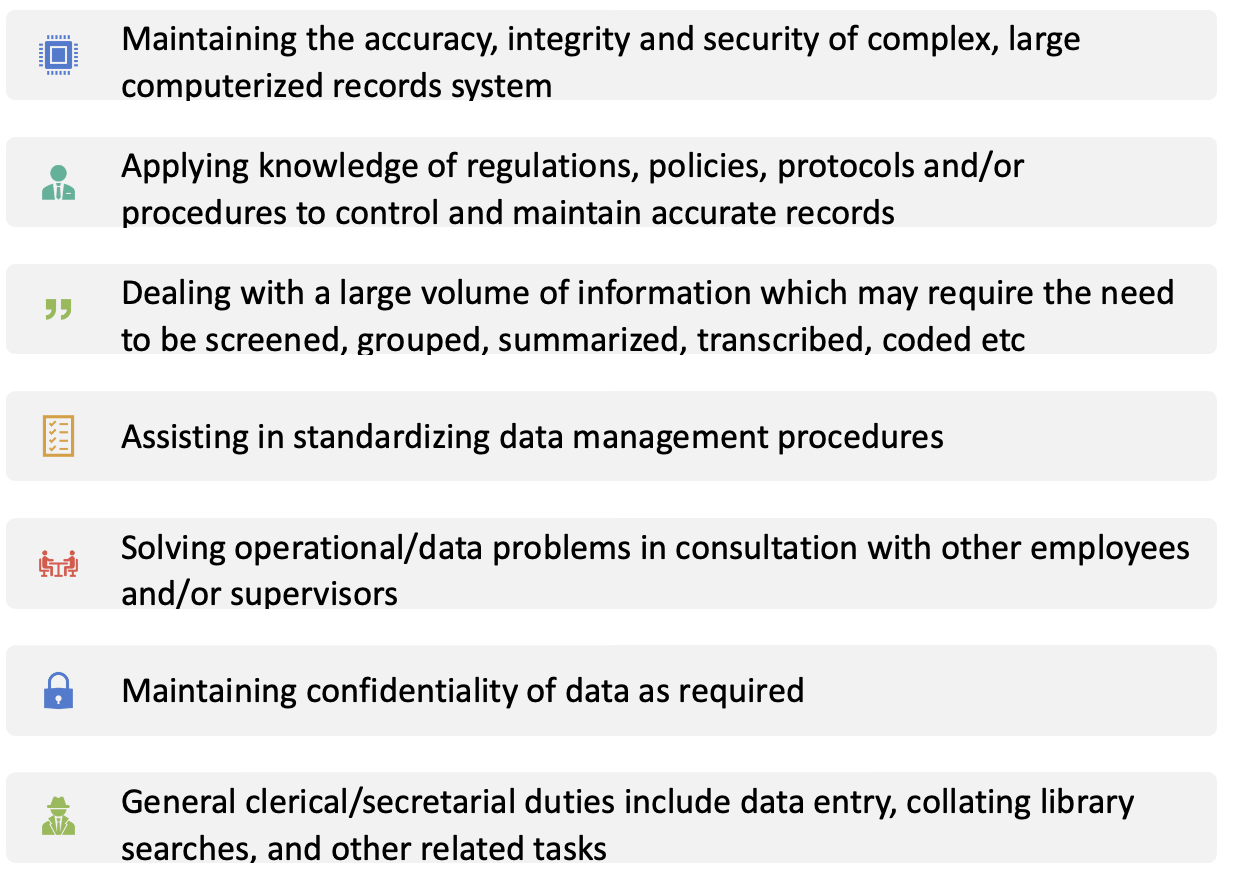
Figure no. 6: Responsibilities of data manager.
Sponsor:
Selection of qualified investigators.
Ensures proper monitoring of the clinical trial.
References: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3092661/ - The Clinical Research Team https://clinicaltrialpodcast.com/clinical-research/ - 15 Clinical Research Job Roles & Responsibilities (2021) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3051859/ - Clinical Investigator Responsibilities https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6042393/ - How to engage stakeholders in research: design principles to support improvement
Efficacy Insight: Revealing its Meaning in Clinical Research
Good documentation in clinical trials.
How Do Clinical Trials Work?
Clinical trials are designed to work in phases that follow strict guidelines, including who can participate. Learning how clinical trials work can help you decide if you want to join.
Who is eligible for clinical trials?

"I want to participate in the clinical trial to advance our research and technology. I want to contribute in saving our women in the coming generations." —Madhu Sood, NCI clinical trial participant
Every clinical trial has requirements that must be met for you to join. These requirements are called eligibility criteria.
Common eligibility criteria address things such as your:
- medical history
- family medical history
- risk factors
- treatment history
- tumor’s genetic changes
These criteria help reduce the medical differences among people in the trial, reduce the risk that people will be harmed, and limit people in the trial to those most likely to benefit.
When people taking part in a trial are alike in specific ways, researchers can be more certain that the results are due to the intervention or drug being tested and not to other factors.
Phases of clinical trials
Clinical trials to test new cancer treatments involve a series of steps, called phases. Depending on the results of each phase, a treatment may move to testing in the next phase.

What Are Clinical Trial Phases?
This video explains the main phases of clinical trials.
The Four Phases of Clinical Trials Early clinical trial phases (phases 1 and 2) test for safety, such as what the side effects are and what a safe dose is. Later phases (phase 3 and 4) compare the treatment to current standard treatments.
In a phase 1 clinical trial, researchers figure out whether a new treatment is safe, what its side effects are, whether people can tolerate it, and the highest dose that people can tolerate. These trials are done in a small group of people (around 15 to 30). They also make sure a treatment affects the cancer.
A phase 2 clinical trial includes more people (50 to 100) to see if the new treatment seems to work against the cancer, such as by shrinking tumors or slowing their growth. Researchers want to see how the new treatment affects the body and fights cancer. In this phase, teams continue to study safety, including short-term side effects.
In a phase 3 clinical trial, researchers compare the treatment to the current standard therapy to see which works better. They also compare the side effects of the treatments. Participants are randomly assigned to one of the treatments to ensure that any differences are real and not the result of differences in the people in each group. Phase 3 trials include large numbers of people (from 100 to several thousand) to make sure that the result is valid.
Results from phase 1–3 trials are used to make decisions about approving new treatments or existing treatments for new conditions by agencies like the US Food and Drug Administration (FDA).
A phase 4 clinical trial looks at long-term safety and effectiveness that take place after a new treatment has been approved by the FDA and is available to the public. Treatment effectiveness and safety are monitored in large, diverse populations. More information is gathered as more people use the drug or device over a longer period of time.
Randomization and bias in clinical trials

Randomization in Clinical Trials
Learn how researchers randomly assign clinical trial participants to different treatment groups in order to prevent bias in the results.
Clinical trial randomization is the process of assigning people by chance to groups that receive different interventions or drugs in later phase trials. A computer is most often used to assign people to groups.
In the simplest trial design, the investigational group receives the study intervention or drug and the control group receives standard treatment.
At several points during and at the end of the clinical trial, researchers compare the groups to see which intervention or drug is more effective or has fewer side effects.
Randomization, in which people are assigned to groups by chance alone, helps prevent bias. Bias occurs when a trial's results are affected by human choices or other factors not related to the treatment being tested.
For example, if doctors could choose which patients to assign to which groups, some might assign healthier patients to the treatment group and sicker patients to the control group even without meaning to. This might make the treatment group appear better than the control group even if it isn’t. Randomization helps avoid biases of this type.
If you are thinking about joining a clinical trial that includes randomization, it is important to understand that neither you nor your doctor can choose which group you will be assigned to.
Placebos in clinical trials
Placebos are rarely used in cancer treatment clinical trials. In most cases, a group of participants will receive the new treatment, and another group will receive the already approved standard treatment. Researchers will then compare the two treatments to see if the new treatment will become the new standard.
If placebos are used, you always will be told ahead of time and can ask questions before deciding to join the trial. The use of placebos in a clinical trial will be covered in the consent form. Learn about consent forms for clinical trials .
When are placebos used in clinical trials? They are used when no standard treatment for a cancer exists. Or they may be used in a trial that compares standard treatment plus a placebo, with standard treatment plus the study treatment.
Placebos may be used in other types of trials, such as prevention trials .
Research team members
Designing and running a clinical trial requires the skills of many experts. Different sites of the same trial may set up their teams differently. Typical team members and their duties include:
Where cancer clinical trials take place
Cancer clinical trials take place in cities and towns across the United States and throughout the world.
They take place in doctors’ offices, cancer centers, medical centers, community hospitals and clinics, and veterans’ and military hospitals. A single trial may take place in one or two places, or at hundreds of different sites.
Trials that are funded in full or in part by NCI, include trials that take place at NCI-Designated Cancer Centers and at the NIH Clinical Center in Bethesda, Maryland .
Cultivating an Effective Research Team Through Application of Team Science Principles

Shirley L.T. Helm, MS, CCRP Senior Administrator for Network Capacity & Workforce Strategies
C. Kenneth & Dianne Wright Center for Clinical and Translational Research
Virginia Commonwealth University
Abstract: The practice of team science allows clinical research professionals to draw from theory-driven principles to build an effective and efficient research team. Inherent in these principles are recognizing team member differences and welcoming diversity in an effort to integrate knowledge to solve complex problems. This article describes the basics of team science and how it can be applied to creating a highly-productive research team across the study continuum, including research administrators, budget developers, investigators, and research coordinators. The development of mutual trust, a shared vision, and open communication are crucial elements of a successful research team and research project. A case study illustrates the team science approach.
Introduction
Each research team is a community that requires trust, understanding, listening, and engagement. Stokols, Hall, Taylor, Moser, & Syme said that:
“There are many types of research teams, each one as dynamic as its team members. Research teams may comprise investigators from the same or different fields. Research teams also vary by size, organizational complexity, and geographic scope, ranging from as few as two individuals working together to a vast network of interdependent researchers across many institutions. Research teams have diverse goals spanning scientific discovery, training, clinical translation, public health, and health policy.” 1 1 Stokols D, Hall KL, Taylor BK, Moser RP. The science of team science: overview of the field and introduction to the supplement. Am J Prev Med. 2008 Aug;35(2 Suppl):S77-89. Accessed 8/10/20.
Team science arose from the National Science Foundation and the National Institutes of Health, which fund the work of researchers attempting to solve some of the most complex problems that require a multi-disciplinary approach, such as childhood obesity. 2 Team science is bringing in elements from various disciplines to solve these major problems. 3, 4 This article covers the intersection of team science with effective operationalizing of research teams and how teaming principles can be applied to the functioning of research teams.
Salas and colleagues state that, “a team consists of two or more individuals, who have specific roles, perform interdependent tasks, are adaptable, and share a common goal. . . team members must possess individual and team Knowledge, Skills, Attitudes ….” 5 Great teams have a plan for how people act and work together. There are three elements that must be aligned to ensure success: the individual, the team, and the task. Individuals have their own goals. These goals must align, and not compete with, goals of other individuals and team goals. Task goals are the nuts and bolts of clinical research. Like individuals, the team has an identity. It is necessary to provide feedback both as a team and as individuals.
In a typical clinical research team, the clinical investigator is at the center surrounded by the clinical research coordinators. The coordinator is the person who makes the team function. Other members of the typical clinical research team are:
· Research participant/family
· Financial/administrative staff
· Regulatory body (institutional review board)
· Study staff
· Ancillary services such as radiology or pathology
· Sponsor/monitor.
The Teaming Principles
Bruce Tuckman developed the teaming principles in 1965 and revised them in 1977 (Table 1). 6 Using the teaming principles is not a linear process. These principles start with establishing the team. The team leader does not have to establish the team. Any team member can use teaming principles to provide a framework and structure and systematically determine what the project needs. Storming is establishing roles and responsibilities, communications, and processes. The storming phase, when everybody has been brought together and is on board with the same goal, is a honeymoon period.
Norming is the heavy lifting of the team’s work. This involves working together effectively and efficiently. Team members must develop trust and comfort with each other. Performing focuses on working together efficiently, and satisfaction for team members and the research participants and their families.
Tuckman added adjourning or transforming to the teaming principles in 1977. The team might end or start working on a new project (study) with a new shared goal. Adjourning or transforming involves determining which processes can be transferred from one research study to another research study.
While the teaming principles seem intuitive and like common sense, people are not raised to be fully cooperative. Using the teaming principles provides framework and structure and takes the emotion out of teamwork. The teaming principles empower team members and provide the structure that is necessary for teams, which are constantly evolving and changing.
The shared goal at the center of the teaming principles provides a sense of purpose. This provides commitment, responsibility, and accountability, along with a clear understanding of roles, responsibilities, competencies, expectations, and contributions. In Dare to lead: Brave work. Tough conversations. Whole hearts, Brené Brown coined the phrase, “clear is kind, unclear is unkind.” 7 It is extremely important to define roles and ensure that each team member knows what the other team members are doing. This prevents duplication of effort and ensures that tasks do not fall through the cracks.
How to Use Teaming Principles
Table 2 briefly describes each of the five teaming principles. Forming begins with gathering the team members and involves determining who is needed on the team to ensure success. Each team member must be valued. The team may vary depending upon the study, project, and timelines. During the research study, team members may enter and exit from the team. Forming the team may mean working across boundaries with people and departments that team members do not know. It is also necessary to establish the required competencies and knowledge, skills, and attitudes of team members, and to recognize and celebrate differences. The team must have a shared goal and vision.
Storming the team involves establishing roles, responsibilities, and tasks. This includes determining who has the required competencies to perform tasks such as completing pre-screening logs or consenting research participants. Also, storming involves defining processes, including communication pathways and expectations. Simply sending an email is not an effective way to communicate. Team members need to know whether an email is providing information or requires a response. Expectations for responding to emails should be described and agreed upon by all team members. Emails might be color coded to show whether an email is informational or requires a response. If clinical research sites utilize a clinical trial management system, the process for updating it must be determined and clearly communicated.
Norming is how team members work together. The shared goal is re-visited often under norming. Team members are mutually dependent upon each other and must meet their commitments and established deadlines.
Trust lies at the heart of the team. Building trust takes work and does not come naturally. It is helpful to understand that there are several types of trust. Identity-based trust is based on personal understanding and is usually seen in relationships between partners, spouses, siblings, or best friends. This type of trust does not usually occur in the workplace.
Workplace trust resides in calculus-based trust and competence-based trust. Calculus-based trust is about keeping commitments, meeting deadlines, and meeting expectations. There are some people who can be counted upon to always do what they are supposed to do. These people have earned calculus-based trust. Competence-based trust is confidence in another person’s skills or competencies.
Swift trust is immediate and necessary during extreme situations where there is not time to develop deeper connections with individuals. It relies on personal experiences, stereotypes, and biases. Some people are naturally more trusting than other people.
The teaming principle of performing involves satisfaction in progressing toward the goal and being proactive in preventing issues from arising. There will always be issues; however, the most effective teams learn from issues and have processes for resolving them. This makes a team efficient. Performing also includes revisiting the shared goal, embracing diversity and differences, and continually improving knowledge, skills, and attitudes.
Adjourning/transforming is the completion of tasks and identification of lessons learned. Team members need to circle back and determine what worked well and can be applied to the next study. Celebrating successes and acknowledging the contributions of all team members are also an aspect of adjourning/transforming. When the author was managing a core laboratory, she performed tests for an oncology investigator’s study. Months later, the investigator gave her a thank-you card for her contribution to the study that was unexpected but greatly appreciated.
Strengthening the Team
Without a framework and structure, team dysfunction is likely. In The five dysfunctions of a team: A leadership fable , Lencioni presented team dysfunction as a pyramid. 8 Absence of trust is at the bottom of the pyramid. Absence of trust results in questioning everything people do and results in team members unwilling to share or to ask for help. Without asking for help, mistakes will be made.
Absence of trust leads to a fear of conflict and an inability to resolve issues or improve efficiencies. Fear of conflict leads to lack of commitment. Doubt prevails, team members lack confidence, and the goal is diminished. Team dysfunction leads to avoidance of accountability. Follow-through is poor and mediocrity is accepted, breeding resentment among team members.
At the top of the team dysfunction pyramid is inattention to results, which leads to loss of team members and future research studies. There are some teams where people are constantly moving in and out. This is
a symptom of team dysfunction. Loss of respect and reputation of the team, department, and individual team members is another consequence of inattention to results.
Table 3 highlights ways to strengthen the team. Recognizing the strengths of each team member starts with self-awareness. For example, the author had to understand her communication and learning style and how this is similar to and different than that of other team members. The VIA Institute of Character offers a free assessment that could be a fun activity for research teams.
There is no one road to self-awareness; however, each team member must recognize that other team members do not necessarily share their understanding or perceptions. There are many options and possibilities for how others may understand or perceive an experience, none of which are right or wrong. Each team member should appreciate that different understanding and perceptions of experiences do not have to threaten their identity or relationships.
One quick way to show this is through ambiguous images, in which people see entirely different things in the same image. Once they are aware that there are different ways of seeing the same thing, they can appreciate other perspectives. As Pablo Picasso said, “There is only one way to see things, until someone shows us how to look at them with different eyes.” Strengthening the team requires embracing demographic, educational, and personality diversity.
Open and honest communication should be encouraged. Team members should give and receive constructive feedback. This is a learned skill that is often difficult. However, tools are available for assessing communication and listening styles. Many institutions and human resource departments utilize the Crucial Conversations program by VitalSmarts, LC. One member of the team can participate in Crucial Conversations and bring the knowledge back to the team. Communication must include managing conflict and an awareness of cultural differences.
Opportunities for education and training to acquire new knowledge, skills, and attitudes/competencies should be provided. Education may be transportable across teams or may be study specific. Team members should be cross-trained, which may be accomplished through several methods. Positional clarification is where one person is told what another person is doing, which is primarily for information transfer. Positional modeling is receiving the information but also shadowing the other person while they perform the task/skill. Positional rotation is performing another person’s job. This is best for back-up positions, which are necessary for research teams.
Team success is facilitated by recognizing individual successes and commitment to shared goals. Recognizing individual successes reflects team success. For example, if a team member becomes a certified clinical research professional, this is a success for both the individual and the team. Also, the team must have a shared understanding of the goal or purpose. This shared goal must be linked to the individual goal of each team member.
Teamwork needs constant attention and annual evaluations, and team meetings are not sufficient. It is extremely important to regularly check in with people. Team members can check in with other team members simply to ask how things are going. Misunderstandings should be dealt with immediately. Clear direction, accountability, and rewards are necessary.
The author has a bell on her desk that team members ring when they have a success. This sounds cheesy, however, it is fun and team members really enjoy it. For example, when the author finished her slides for the SOCRA annual conference on time, she rang the bell. Her team members asked what happened, and they had a mini celebration. This small item helps to build and strengthen a team with small successes leading to larger successes.
Case Study Using the Teaming Principles
The following case study illustrates the application of the teaming principles to a team involving four major players. Olivia is a clinician with three clinic days and teaching duties who is a sought-after speaker for international conferences. In addition, Olivia is the clinical investigator for four clinical research studies: two studies are active, one is in long-term follow up, and one is in closeout. The studies are a blend of industry sponsored and investigator initiated. Olivia is also a co-clinical investigator on two additional studies and relies heavily upon Ansh for coordination of all studies and management of two research assistants.
Ansh is the lead research coordinator with seven years of experience in critical care research. Ansh is very detail-oriented and takes pride in error-free case report forms, coordinates with external monitors, and manages two research assistants as well as the day-to-day operations of Olivia’s research studies.
Bernita is a research assistant with six months of work experience in obtaining informed consents, scheduling study visits, and coordinating with ancillary services. Bernita is responsible for contacting participants for scheduled visits and providing participant payments. Bernita is developing coordinating skills, seeks out training and educational opportunities, and is a real people person.
Delroy is the regulatory affairs specialist for the Critical Care Department, which consists of eight clinicians (not all of whom are engaged in research). Studies include one multi-site clinical trial for which the clinical research site is the coordinating site, and one faculty-held Investigational New Drug/Investigational Device Exemption study. The department’s studies are a mixture of federal- and industry-funded studies. Delroy has been with the department for five years in this capacity. However, Delroy’s coworker recently and unexpectedly took family and medical leave, leaving Delroy to manage all regulatory issues for the department. Also, the department chair recently made growing the department’s industry-sponsored study portfolio a priority.
Olivia has received an invitation to be added as a clinical research site for a highly sought-after ongoing Phase II, multisite, industry-sponsored study comparing two asthma medications in an adult outpatient setting. The study uses a central institutional review board (IRB) and has competitive enrollment. It will require the following ancillary services: investigational pharmacy, radiology, and outpatient asthma clinic nursing. For the purposes of this case study, all contracts have been negotiated and all of the regulatory documents are available (e.g., FDA Form 1572, informed consent template, and the current protocol). The institution utilizes a clinical trial management system.
Oliva shares the study information and study enrollment goals with Ansh with the charge of getting this study activated and enrolling within 40 days. What are the potential barriers that might affect this outcome? One potential barrier to the study activation timeline is Delroy’s heavy workload. To ensure that the timeline is met, Ansh might contact Delroy and explain the situation, asking what Ansh can do to help facilitate study start-up to ensure that the timeline is met. Ansh should be clear in determining what Delroy needs for study activation, the deadlines for each item, and assist in facilitation of communicating to other members of the study activation team (e.g., ancillary services, IRB) what is needed. Priorities include the regulatory work and staff training. Barriers include managing the regulatory issues on time. This might be a good opportunity to connect with Bernita for providing Delroy some assistance, as Bernita is knowledgeable and eager to acquire additional skills and training. The shared goal of starting the study on time should be shared with all team members in order to meet the 40 day study activation and enrollment goal.
Nuggets for Success as a Team Member or Leader
Members of a research team must know the other team members and available resources. They need to know who is needed for a particular study. This will change during studies and across studies. Roles and responsibilities among the broader team should be identified.
Table 4 outlines nuggets of success as a team member or leader, starting with using the framework of the teaming principles. Next, the team member or leader should build and create networks for knowledge and access. A knowledge network enables team members to know who to contact to provide an answer to specific questions. Each team member is a knowledge network for someone else. Also, each team member should find a person who they admire to serve as a mentor, even informally.
Team members should take advantage of available training. LinkedIn has many free training programs, and the institution’s human resources department also offers training. Meeting times should be scheduled to set aside time for reflection. Team members should check in often with the team as a whole and individual team members, set realistic boundaries, and establish priorities. Team members should avoid making assumptions, and instead, communicate clearly and often. Other keys to team success are to be respectful and present, participate, and practice humanity.
This work was supported by CTSA award No. UL1TR002649 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Overview of the Teaming Principles
- Establish team (top-down and bottom-up)
- Establish roles and responsibilities, communications, and processes
- Working together effectively and efficiently
- Individuals develop trust and comfort
- Work together efficiently
- Focus on a shared vision
- Resolves issues
- Natural end:dissolution
- New project (study) with a new shared goal
Description of the Teaming Principles
- Team members may vary depending upon the study, project, and timelines
- Work across boundaries
- Appropriate competencies and knowledge, skills, and attitudes
- Recognize and celebrate differences
- Shared goal and vision
- Determining who has the competencies for specific study tasks
- Communication pathways and expectation
- Completing clinical trial management systems updates
- Revisit the shared goal often
- Requires mutual dependence
- Identity-based: personal understanding
- Calculus-based: keep commitments, meet deadlines, meet expectations
- Competence-based: confidence in skills, competencies of another
- Satisfaction in progressing toward goal
- Proactive in preventing issues from arising
- Revisit the shared goal
- Embrace diversity and differences
- Continuous improvement in knowledge, skills, and attitudes
- Completion of tasks
- Identify lessons learned
- Celebrate success and acknowledge the contributions of all
- Self-awareness and assessments
- Demographic
- Educational
- Personality
- Give and receive constructive feedback
- Acquire new knowledge, skills, and attitudes/competencies
- Cross-train
- Recognize individual success, which reflects team success
- Commit to shared goals
Nuggets of Success as a Team Member of Leader
- Use the teaming principles as a framework
- Build and create networks for knowledge and access
- Find a mentor
- Take advantage of training
- Schedule meeting times for reflection
- Check in with the team and team members
- Set boundaries and priorities
- Never make assumptions
- Be respectful and present
- Participate
- Practice humanity
1 Stokols D, Hall KL, Taylor BK, Moser RP. The science of team science: overview of the field and introduction to the supplement. Am J Prev Med. 2008 Aug;35(2 Suppl):S77-89. Accessed 8/10/20.
2 Bennett LM, Gadlin H, Marchand C. Team Collaboration Field Guide. Publication No. 18-7660, 2nd ed., National Institutes of Health; 2018. Accessed 8/10/20.
3 National Research Council. Enhancing the Effectiveness of Team Science. Washington, DC: The National Academies Press; 2015. Accessed 8/10/20.
4 Teambuilding 1: How to build effective teams in healthcare. Nursing Times. Accessed 8/10/20.
5 Salas E, Dickinson TL, Converse SA. Toward an Understanding of Team Performance and Training. In: Swezey R W, Salas E, editors. Teams: Their Training and Performance. Norwood, NJ: Ablex; 1992. pp. 3–29.
6 Tuckman, BW, Jensen MA. Stages of small-group development revisited. Group and Organization Studies, 2. 1977: 419-427.
7 Brown B. Dare to lead: Brave work. Tough conversations. Whole hearts. New York: Random House, 2018.
8 Lencioni P. The five dysfunctions of a team: A leadership fable. San Francisco: Jossey-Bass: 2002.
One thought on “Cultivating an Effective Research Team Through Application of Team Science Principles”
Hey there! I just finished reading your article on cultivating an effective research team through the application of team science principles, and I couldn’t help but drop a comment. First off, kudos to you for sharing such valuable insights. Your article was not only informative but also highly engaging, making it a pleasure to read.
I particularly resonated with your emphasis on the importance of clear communication and collaboration within research teams. It’s incredible how these seemingly simple principles can make such a significant difference in the success of a research project. Your practical tips on fostering trust and encouraging diversity of thought were spot-on. I’ve had my fair share of experiences in research teams, and I can attest that when everyone is on the same page and feels heard, the results are remarkable. Your article has given me a fresh perspective on how to approach team dynamics in my future research endeavors, and I’ll definitely be sharing these insights with my colleagues. Thanks again for sharing your wisdom! Looking forward to more of your articles in the future.
Keep up the fantastic work, and please continue to share your expertise. Your writing style is not only informative but also very relatable, making complex topics like team science principles easy to grasp. I’ll be eagerly awaiting your next piece. Until then, wishing you all the best in your research and writing endeavors! 😊📚
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
This site uses Akismet to reduce spam. Learn how your comment data is processed .
- Clinical Trials
About Clinical Studies
Research: it's all about patients.
Mayo's mission is about the patient, the patient comes first. So the mission and research here, is to advance how we can best help the patient, how to make sure the patient comes first in care. So in many ways, it's a cycle. It can start with as simple as an idea, worked on in a laboratory, brought to the patient bedside, and if everything goes right, and let's say it's helpful or beneficial, then brought on as a standard approach. And I think that is one of the unique characteristics of Mayo's approach to research, that patient-centeredness. That really helps to put it in its own spotlight.
At Mayo Clinic, the needs of the patient come first. Part of this commitment involves conducting medical research with the goal of helping patients live longer, healthier lives.
Through clinical studies, which involve people who volunteer to participate in them, researchers can better understand how to diagnose, treat and prevent diseases or conditions.
Types of clinical studies
- Observational study. A type of study in which people are observed or certain outcomes are measured. No attempt is made by the researcher to affect the outcome — for example, no treatment is given by the researcher.
- Clinical trial (interventional study). During clinical trials, researchers learn if a new test or treatment works and is safe. Treatments studied in clinical trials might be new drugs or new combinations of drugs, new surgical procedures or devices, or new ways to use existing treatments. Find out more about the five phases of non-cancer clinical trials on ClinicalTrials.gov or the National Cancer Institute phases of cancer trials .
- Medical records research. Medical records research involves the use of information collected from medical records. By studying the medical records of large groups of people over long periods of time, researchers can see how diseases progress and which treatments and surgeries work best. Find out more about Minnesota research authorization .
Clinical studies may differ from standard medical care
A health care provider diagnoses and treats existing illnesses or conditions based on current clinical practice guidelines and available, approved treatments.
But researchers are constantly looking for new and better ways to prevent and treat disease. In their laboratories, they explore ideas and test hypotheses through discovery science. Some of these ideas move into formal clinical trials.
During clinical studies, researchers formally and scientifically gather new knowledge and possibly translate these findings into improved patient care.
Before clinical trials begin
This video demonstrates how discovery science works, what happens in the research lab before clinical studies begin, and how a discovery is transformed into a potential therapy ready to be tested in trials with human participants:
How clinical trials work
Trace the clinical trial journey from a discovery research idea to a viable translatable treatment for patients:
See a glossary of terms related to clinical studies, clinical trials and medical research on ClinicalTrials.gov.
Watch a video about clinical studies to help you prepare to participate.
Let's Talk About Clinical Research
Narrator: This presentation is a brief introduction to the terms, purposes, benefits and risks of clinical research.
If you have questions about the content of this program, talk with your health care provider.
What is clinical research?
Clinical research is a process to find new and better ways to understand, detect, control and treat health conditions. The scientific method is used to find answers to difficult health-related questions.
Ways to participate
There are many ways to participate in clinical research at Mayo Clinic. Three common ways are by volunteering to be in a study, by giving permission to have your medical record reviewed for research purposes, and by allowing your blood or tissue samples to be studied.
Types of clinical research
There are many types of clinical research:
- Prevention studies look at ways to stop diseases from occurring or from recurring after successful treatment.
- Screening studies compare detection methods for common conditions.
- Diagnostic studies test methods for early identification of disease in those with symptoms.
- Treatment studies test new combinations of drugs and new approaches to surgery, radiation therapy and complementary medicine.
- The role of inheritance or genetic studies may be independent or part of other research.
- Quality of life studies explore ways to manage symptoms of chronic illness or side effects of treatment.
- Medical records studies review information from large groups of people.
Clinical research volunteers
Participants in clinical research volunteer to take part. Participants may be healthy, at high risk for developing a disease, or already diagnosed with a disease or illness. When a study is offered, individuals may choose whether or not to participate. If they choose to participate, they may leave the study at any time.
Research terms
You will hear many terms describing clinical research. These include research study, experiment, medical research and clinical trial.
Clinical trial
A clinical trial is research to answer specific questions about new therapies or new ways of using known treatments. Clinical trials take place in phases. For a treatment to become standard, it usually goes through two or three clinical trial phases. The early phases look at treatment safety. Later phases continue to look at safety and also determine the effectiveness of the treatment.
Phase I clinical trial
A small number of people participate in a phase I clinical trial. The goals are to determine safe dosages and methods of treatment delivery. This may be the first time the drug or intervention is used with people.
Phase II clinical trial
Phase II clinical trials have more participants. The goals are to evaluate the effectiveness of the treatment and to monitor side effects. Side effects are monitored in all the phases, but this is a special focus of phase II.
Phase III clinical trial
Phase III clinical trials have the largest number of participants and may take place in multiple health care centers. The goal of a phase III clinical trial is to compare the new treatment to the standard treatment. Sometimes the standard treatment is no treatment.
Phase IV clinical trial
A phase IV clinical trial may be conducted after U.S. Food and Drug Administration approval. The goal is to further assess the long-term safety and effectiveness of a therapy. Smaller numbers of participants may be enrolled if the disease is rare. Larger numbers will be enrolled for common diseases, such as diabetes or heart disease.
Clinical research sponsors
Mayo Clinic funds clinical research at facilities in Rochester, Minnesota; Jacksonville, Florida; and Arizona, and in the Mayo Clinic Health System. Clinical research is conducted in partnership with other medical centers throughout the world. Other sponsors of research at Mayo Clinic include the National Institutes of Health, device or pharmaceutical companies, foundations and organizations.
Clinical research at Mayo Clinic
Dr. Hugh Smith, former chair of Mayo Clinic Board of Governors, stated, "Our commitment to research is based on our knowledge that medicine must be constantly moving forward, that we need to continue our efforts to better understand disease and bring the latest medical knowledge to our practice and to our patients."
This fits with the term "translational research," meaning what is learned in the laboratory goes quickly to the patient's bedside and what is learned at the bedside is taken back to the laboratory.
Ethics and safety of clinical research
All clinical research conducted at Mayo Clinic is reviewed and approved by Mayo's Institutional Review Board. Multiple specialized committees and colleagues may also provide review of the research. Federal rules help ensure that clinical research is conducted in a safe and ethical manner.
Institutional review board
An institutional review board (IRB) reviews all clinical research proposals. The goal is to protect the welfare and safety of human subjects. The IRB continues its review as research is conducted.
Consent process
Participants sign a consent form to ensure that they understand key facts about a study. Such facts include that participation is voluntary and they may withdraw at any time. The consent form is an informational document, not a contract.
Study activities
Staff from the study team describe the research activities during the consent process. The research may include X-rays, blood tests, counseling or medications.
Study design
During the consent process, you may hear different phrases related to study design. Randomized means you will be assigned to a group by chance, much like a flip of a coin. In a single-blinded study, participants do not know which treatment they are receiving. In a double-blinded study, neither the participant nor the research team knows which treatment is being administered.
Some studies use an inactive substance called a placebo.
Multisite studies allow individuals from many different locations or health care centers to participate.
Remuneration
If the consent form states remuneration is provided, you will be paid for your time and participation in the study.
Some studies may involve additional cost. To address costs in a study, carefully review the consent form and discuss questions with the research team and your insurance company. Medicare may cover routine care costs that are part of clinical trials. Medicaid programs in some states may also provide routine care cost coverage, as well.
When considering participation in a research study, carefully look at the benefits and risks. Benefits may include earlier access to new clinical approaches and regular attention from a research team. Research participation often helps others in the future.
Risks/inconveniences
Risks may include side effects. The research treatment may be no better than the standard treatment. More visits, if required in the study, may be inconvenient.
Weigh your risks and benefits
Consider your situation as you weigh the risks and benefits of participation prior to enrolling and during the study. You may stop participation in the study at any time.
Ask questions
Stay informed while participating in research:
- Write down questions you want answered.
- If you do not understand, say so.
- If you have concerns, speak up.
Website resources are available. The first website lists clinical research at Mayo Clinic. The second website, provided by the National Institutes of Health, lists studies occurring in the United States and throughout the world.
Additional information about clinical research may be found at the Mayo Clinic Barbara Woodward Lips Patient Education Center and the Stephen and Barbara Slaggie Family Cancer Education Center.
Clinical studies questions
- Phone: 800-664-4542 (toll-free)
- Contact form
Cancer-related clinical studies questions
- Phone: 855-776-0015 (toll-free)
International patient clinical studies questions
- Phone: 507-284-8884
- Email: [email protected]
Clinical Studies in Depth
Learning all you can about clinical studies helps you prepare to participate.
- Institutional Review Board
The Institutional Review Board protects the rights, privacy, and welfare of participants in research programs conducted by Mayo Clinic and its associated faculty, professional staff, and students.
More about research at Mayo Clinic
- Research Faculty
- Laboratories
- Core Facilities
- Centers & Programs
- Departments & Divisions
- Postdoctoral Fellowships
- Training Grant Programs
- Publications
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
New to Clinical Trials?
Get started on the path to finding a trial for you!
- Sponsors & Partners

- Inside Clinical Trials
Home > Blog > Inside Clinical Trials > Who are Clinical Trial Research Team Members?
Who are Clinical Trial Research Team Members?
Published on March 31, 2023
Last Modified on April 3, 2023

Read below to learn about members of the clinical trial research team and what they do.
- National Cancer Institute (NCI): Clinical Trial Research Team Members
- The Center for Information and Study on Clinical Research Participation (Video): The Clinical Research Team Is Similar to a Sports Team
- Javara: What It Means to Be a Clinical Trial Navigator
- Journal of Oncology Navigation and Survivorship: The Navigator’s Role in Supporting Clinical Trials
- Meridian Clinical Research: What is the Role of a Principal Investigator in a Clinical Trial?
- Moffitt Cancer Center: The Role of Nurses in Clinical Trials
Tags: Clinical Trial Navigation Navigating Cancer Care Inside Clinical Trials
Related Content
September 2022
What is it Like to Participate in a Clinical Trial?
Interested in what it's like to be in a clinical trial? Hear first-hand experiences of trial participants...
January 2023
Clinical Trial Participation: The Patient’s Journey
Participating in a clinical trial is a process with many steps. Learn more about what to expect during each step of your clinical trial...
I Found a Trial I’m Interested In. What’s Next?
If you are interested in joining a clinical trial, read about steps to take and questions to ask your doctor and research...
Doctors & Patients on Clinical Trials
Many people diagnosed with cancer never consider entering a clinical trial. Yet clinical trials provide unique opportunities to receive high-quality care while...
Search Our Site
for past articles or specific information.
Explore Past Articles
- Research News
- From the Experts
- Conference Talk
- Special Topics
- Introduction to MBC Clinical Trials
- Trial Search
- Resources & Support
- Personal Stories
- Other MBC Resources
SEARCH OUR SITE
Masks Strongly Recommended but Not Required in Maryland, Starting Immediately
Due to the downward trend in respiratory viruses in Maryland, masking is no longer required but remains strongly recommended in Johns Hopkins Medicine clinical locations in Maryland. Read more .
- Vaccines
- Masking Guidelines
- Visitor Guidelines
Understanding Clinical Trials
Clinical research: what is it.

Your doctor may have said that you are eligible for a clinical trial, or you may have seen an ad for a clinical research study. What is clinical research, and is it right for you?
Clinical research is the comprehensive study of the safety and effectiveness of the most promising advances in patient care. Clinical research is different than laboratory research. It involves people who volunteer to help us better understand medicine and health. Lab research generally does not involve people — although it helps us learn which new ideas may help people.
Every drug, device, tool, diagnostic test, technique and technology used in medicine today was once tested in volunteers who took part in clinical research studies.
At Johns Hopkins Medicine, we believe that clinical research is key to improve care for people in our community and around the world. Once you understand more about clinical research, you may appreciate why it’s important to participate — for yourself and the community.
What Are the Types of Clinical Research?
There are two main kinds of clinical research:
Observational Studies
Observational studies are studies that aim to identify and analyze patterns in medical data or in biological samples, such as tissue or blood provided by study participants.
Clinical Trials
Clinical trials, which are also called interventional studies, test the safety and effectiveness of medical interventions — such as medications, procedures and tools — in living people.
Clinical research studies need people of every age, health status, race, gender, ethnicity and cultural background to participate. This will increase the chances that scientists and clinicians will develop treatments and procedures that are likely to be safe and work well in all people. Potential volunteers are carefully screened to ensure that they meet all of the requirements for any study before they begin. Most of the reasons people are not included in studies is because of concerns about safety.
Both healthy people and those with diagnosed medical conditions can take part in clinical research. Participation is always completely voluntary, and participants can leave a study at any time for any reason.
“The only way medical advancements can be made is if people volunteer to participate in clinical research. The research participant is just as necessary as the researcher in this partnership to advance health care.” Liz Martinez, Johns Hopkins Medicine Research Participant Advocate
Types of Research Studies
Within the two main kinds of clinical research, there are many types of studies. They vary based on the study goals, participants and other factors.
Biospecimen studies
Healthy volunteer studies.
Clinical trials study the safety and effectiveness of interventions and procedures on people’s health. Interventions may include medications, radiation, foods or behaviors, such as exercise. Usually, the treatments in clinical trials are studied in a laboratory and sometimes in animals before they are studied in humans. The goal of clinical trials is to find new and better ways of preventing, diagnosing and treating disease. They are used to test:
Drugs or medicines

New types of surgery

Medical devices

New ways of using current treatments

New ways of changing health behaviors

New ways to improve quality of life for sick patients

Goals of Clinical Trials
Because every clinical trial is designed to answer one or more medical questions, different trials have different goals. Those goals include:
Treatment trials
Prevention trials, screening trials, phases of a clinical trial.
In general, a new drug needs to go through a series of four types of clinical trials. This helps researchers show that the medication is safe and effective. As a study moves through each phase, researchers learn more about a medication, including its risks and benefits.
Is the medication safe and what is the right dose? Phase one trials involve small numbers of participants, often normal volunteers.
Does the new medication work and what are the side effects? Phase two trials test the treatment or procedure on a larger number of participants. These participants usually have the condition or disease that the treatment is intended to remedy.
Is the new medication more effective than existing treatments? Phase three trials have even more people enrolled. Some may get a placebo (a substance that has no medical effect) or an already approved treatment, so that the new medication can be compared to that treatment.
Is the new medication effective and safe over the long term? Phase four happens after the treatment or procedure has been approved. Information about patients who are receiving the treatment is gathered and studied to see if any new information is seen when given to a large number of patients.
“Johns Hopkins has a comprehensive system overseeing research that is audited by the FDA and the Association for Accreditation of Human Research Protection Programs to make certain all research participants voluntarily agreed to join a study and their safety was maximized.” Gail Daumit, M.D., M.H.S., Vice Dean for Clinical Investigation, Johns Hopkins University School of Medicine
Is It Safe to Participate in Clinical Research?
There are several steps in place to protect volunteers who take part in clinical research studies. Clinical Research is regulated by the federal government. In addition, the institutional review board (IRB) and Human Subjects Research Protection Program at each study location have many safeguards built in to each study to protect the safety and privacy of participants.
Clinical researchers are required by law to follow the safety rules outlined by each study's protocol. A protocol is a detailed plan of what researchers will do in during the study.
In the U.S., every study site's IRB — which is made up of both medical experts and members of the general public — must approve all clinical research. IRB members also review plans for all clinical studies. And, they make sure that research participants are protected from as much risk as possible.
Earning Your Trust
This was not always the case. Many people of color are wary of joining clinical research because of previous poor treatment of underrepresented minorities throughout the U.S. This includes medical research performed on enslaved people without their consent, or not giving treatment to Black men who participated in the Tuskegee Study of Untreated Syphilis in the Negro Male. Since the 1970s, numerous regulations have been in place to protect the rights of study participants.
Many clinical research studies are also supervised by a data and safety monitoring committee. This is a group made up of experts in the area being studied. These biomedical professionals regularly monitor clinical studies as they progress. If they discover or suspect any problems with a study, they immediately stop the trial. In addition, Johns Hopkins Medicine’s Research Participant Advocacy Group focuses on improving the experience of people who participate in clinical research.
Clinical research participants with concerns about anything related to the study they are taking part in should contact Johns Hopkins Medicine’s IRB or our Research Participant Advocacy Group .
Learn More About Clinical Research at Johns Hopkins Medicine
For information about clinical trial opportunities at Johns Hopkins Medicine, visit our trials site.
Video Clinical Research for a Healthier Tomorrow: A Family Shares Their Story
Clinical Research for a Healthier Tomorrow: A Family Shares Their Story

Responsibilities of the Research Team
A research team is comprised of several key individuals., principal investigator (pi).
The Principal Investigator has the primary responsibility for ensuring the ethical conduct of the research study. This includes protecting human subjects’ rights, safety and welfare, protocol compliance, and adherence to institutional, state and federal regulations and guidance. The PI is responsible for ensuring informed consent is appropriately obtained from each participant and for appropriately maintaining study records. The PI is also responsible for complying with the financial and administrative policies and regulations associated with the award, overall fiscal management of the project, and conflict of interest disclosure.
- 1572 Statement of the Investigator
- 21 CFR 312.50: General Responsibilities of Investigators
- 21 CFR 812.100: Responsibilities of Investigators: Biologics
- 21 CFR 812.110: Responsibilities of Investigators: Devices
- DHHS: Office of Human Research Protections (OHRP): Frequently Asked Questions
- ICH E6: Good Clinical Practice
- OSU HRPP: PI Responsibilities at OSU
The PI oversees all aspects of a clinical trial from protocol design, recruitment, data collection, analysis and interpretation of results, but some tasks can be delegated to other research team members (Co-Investigators and Key Personnel). The PI is responsible for ensuring that all research team members have appropriate education, training and qualifications to assume delegated study tests. All study team members are responsible for ensuring that the conduct of the study is compliant with institutional, state, federal and industry guidance and regulations.
Sub-Investigator (Sub-I) / Co-Investigator (Co-I)
The Sub-Investigator/Co-Investigator may perform all or some of the PI functions, but they do not accept primary responsibility for the research study.The sub-investigator/co-Investigator is under the supervision of the PI and is responsible for performing study–related procedures and /or to make important study-related decisions in compliance with the ethical conduct of the study.
Regulatory Coordinator
The Regulatory Coordinator is typically responsible for drafting or editing the protocol document and submitting new protocols, protocol amendments, continuing reviews and safety reports to the appropriate IRB for review. They are responsible for maintaining regulatory binders in accordance with sponsor specifications and general industry standards. They often are the keepers of the delegation of authority log for key personnel involved in the study.
Data Coordinator
The Data Coordinator is responsible for the overall data management of a research study. Data points for analysis must be extracted from multiple source documents and entered into specific databases. Data coordinators ensure accurate and timely data entry in electronic databases, electronic case report forms (eCRFs) or paper case report forms (CRF). They work closely with sponsor monitors and resolve any data queries that may be generated. They also work closely with the research team in the study development process to identify key data points for collection and analysis for investigator initiated trials.
Research Coordinator/ Research Nurse
The Research Coordinator/Nurse oversees and coordinates the daily activities of clinical research studies. They work closely with the clinical teams and investigators to ensure that all protocol required procedures and visits occur according to protocol specified guidelines. Research Coordinators/ Research Nurses generally manage participant enrollment and ensure compliance with the protocol and other applicable regulations. This includes but is not limited to; participant recruitment, obtaining informed consent, educating participants on the details of the research study, assessing participant eligibility, facilitating participant care and follow-up per protocol, creating source documentation, assisting in the assessment of toxicities/adverse events and reporting serious adverse events per IRB and sponsor requirements.
If you have a disability and experience difficulty accessing this content, please submit an email to [email protected] for assistance.
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- For Patients
- Clinical Trials: What Patients Need to Know
Basics About Clinical Trials
What are clinical trials.
Clinical trials are research studies in which people volunteer to help find answers to specific health questions. When carefully conducted, they are the safest and fastest way to find new treatments and ways to improve health.
Clinical trials are conducted according to a plan, called a protocol, which describes:
- the types of patients who may enter the study
- the schedules of tests and procedures
- the drugs involved
- the dosages, or amount of the drug
- the length of the study
- what the researchers hope to learn from the study.
Volunteers who participate in the study must agree to the rules and terms outlined in the protocol. Similarly, researchers, doctors, and other health professionals who manage the clinical trials must follow strict rules set by the FDA. These rules make sure that those who agree to participate are treated as safely as possible.
Learn more about the basics of clinical trial participation, read first hand experiences from actual clinical trial volunteers, and see explanations from researchers at the NIH Clinical Research Trials and You Web site.
Why are clinical trials done?
Clinical trials are conducted for many reasons:
- to determine whether a new drug or device is safe and effective for people to use.
- to study different ways to use standard treatments or current, approved treatments so that they will be more effective, easier to use, or decrease certain side effects.
- to learn how to safely use a treatment in a population for which the treatment was not previously tested, such as children.
Who should consider clinical trials and why?
Some people participate in clinical trials because none of the standard (approved) treatment options have worked, or they are unable to tolerate certain side effects. Clinical trials provide another option when standard therapy has failed. Others participate in trials because they want to contribute to the advancement of medical knowledge.
Ensuring people from diverse backgrounds join clinical trials is key to advancing health equity. Participants in clinical trials should represent the patients that will use the medical products. This is often not the case—people from racial and ethnic minority and other diverse groups are underrepresented in clinical research. This is a concern because people of different ages, races, and ethnicities may react differently to certain medical products. Learn more about the clinical trial diversity initiative from the Office of Minority Health and Health Equity.
All clinical trials have guidelines, called eligibility criteria, about who can participate. The criteria are based on such factors as age, sex, type and stage of disease, previous treatment history, and other medical conditions. This helps to reduce the variation within the study and to ensure that the researchers will be able to answer the questions they plan to study. Therefore, not everyone who applies for a clinical trial will be accepted.
It is important to test drugs and medical products in the people they are meant to help. It is also important to conduct research in a variety of people, because different people may respond differently to treatments. FDA seeks to ensure that people of different ages, races, ethnic groups, and genders are included in clinical trials. Learn more about FDA’s efforts to increase diversity in clinical trials .
Where are clinical trials conducted?
Clinical trials can be sponsored by organizations (such as a pharmaceutical company), Federal offices and agencies (such as the National Institutes of Health or the U.S. Department of Veterans Affairs), or individuals (such as doctors or health care providers). The sponsor determines the location(s) of the trials, which are usually conducted at universities, medical centers, clinics, hospitals, and other Federally or industry-funded research sites.
Are clinical trials safe?
FDA works to protect participants in clinical trials and to ensure that people have reliable information before deciding whether to join a clinical trial. The Federal government has regulations and guidelines for clinical research to protect participants from unreasonable risks. Although efforts are made to control the risks to participants, some may be unavoidable because we are still learning more about the medical treatments in the study.
The government requires researchers to give prospective participants complete and accurate information about what will happen during the trial. Before joining a particular study, you will be given an informed consent document that describes your rights as a participant, as well as details about the study, including potential risks. Signing it indicates that you understand that the trial is research and that you may leave at any time. The informed consent is part of the process that makes sure you understand the known risks associated with the study.
What should I think about before joining a clinical trial?
Before joining a clinical trial, it is important to learn as much as possible. Discuss your questions and concerns with members of the health care team conducting the trial. Also, discuss the trial with your health care provider to determine whether or not the trial is a good option based on your current treatment. Be sure you understand:
- what happens during the trial
- the type of health care you will receive
- any related costs once you are enrolled in the trial
- the benefits and risks associated with participating.
What is FDA’s role in approving new drugs and medical treatments?
FDA makes sure medical treatments are safe and effective for people to use. We do not develop new therapies or conduct clinical trials. Rather, we oversee the people who do. FDA staff meet with researchers and perform inspections of clinical trial study sites to protect the rights of patients and to verify the quality and integrity of the data.
Learn more about the Drug Development Process .
Where can I find clinical trials?
One good way to find out if there are any clinical trials that might help you is to ask your doctor. Other sources of information include:
- FDA Clinical Trials Search. Search a database of Federally and privately supported studies available through clinicaltrials.gov. Learn about each trial’s purpose, who can participate, locations, and who to contact for more information.
- Clinicaltrials.gov. Conduct more advanced searches
- National Cancer Institute or call 1–800–4–CANCER (1–800–422–6237). Learn about clinical trials for people with cancer.
- AIDS Clinical Trials and Information Services (ACTIS) or call 1–800–TRIALS–A (1–800–874–2572). Locate clinical trials for people with HIV.
- AIDSinfo. Search a database of HIV/AIDS trials, sponsored by the National Institutes of Health’s National Library of Medicine.
What is a placebo and how is it related to clinical trials?
A placebo is a pill, liquid, or powder that has no treatment value. It is often called a sugar pill. In clinical trials, experimental drugs are often compared with placebos to evaluate the treatment’s effectiveness.
Is there a chance I might get a placebo?
In clinical trials that include placebos, quite often neither patients nor their doctors know who is receiving the placebo and how is being treated with the experimental drug. Many cancer clinical trials, as well as trials for other serious and life-threatening conditions, do not include placebo control groups. In these cases, all participants receive the experimental drug. Ask the trial coordinator whether there is a chance you may get a placebo rather than the experimental drug. Then, talk with your doctor about what is best for you.
How do I find out what Phase a drug is in as part of the clinical trial?
Talk to the clinical trial coordinator to find out which phase the clinical trial is in. Learn more about the different clinical trial phases and whether they are right for you.
What happens to drugs that don't make it out of clinical trials?
Most drugs that undergo preclinical (animal) research never even make it to human testing and review by the FDA. The drug developers go back to begin the development process using what they learned during with their preclinical research. Learn more about drug development .

Transforming the understanding and treatment of mental illnesses.
Información en español
Celebrating 75 Years! Learn More >>
- Health Topics
- Brochures and Fact Sheets
- Help for Mental Illnesses
- Clinical Trials
Clinical Research Trials and You: Questions and Answers

- Download PDF
- Order a free hardcopy
What is a clinical trial?
A clinical trial is a research study that involves people like you. Researchers conduct clinical trials to find new or better ways to prevent, detect, or treat health conditions. Often, researchers want to find out if a new test, treatment, or preventive measure is safe and effective. Tests can include ways to screen for, diagnose, or prevent a disease or condition. Treatments and preventive measures can include medications, surgeries, medical devices, and behavioral therapies.
Clinical trials are important because they serve as the foundation for most medical advances. Without clinical trials, many of the medical treatments and cures we have today wouldn’t exist.
Why should I volunteer for a clinical trial?
People volunteer for clinical trials for many reasons. Some want to advance science or help doctors and researchers learn more about disease and improve health care. Others, such as those with an illness, may join to try new or advanced treatments that aren’t widely available.
Whatever your reason for joining a clinical trial, researchers generally need two types of volunteers: those without specific illnesses or conditions and those with them.
A healthy volunteer is someone in a clinical trial with no known related health problems. Researchers need healthy volunteers to establish a healthy or optimal reference point. They use data from healthy volunteers to test new treatments or interventions, not to provide direct benefit to participants.
A patient volunteer is someone in a clinical trial who has the condition being studied. Researchers need patient volunteers to learn if new tests, treatments, or preventive measures are safe and effective. Not all trial participants will receive experimental medications or treatments; sometimes, participants may receive a placebo. Researchers need to vary medications and treatments so they can compare results and learn from their differences.
While a study’s treatment or findings may help patients directly, sometimes participants will receive no direct benefit. However, in many cases, study results can still serve as building blocks that are used to help people later.
What would I experience during a clinical trial?
During a clinical trial, the study team will track your health. Participating in a clinical trial may take more time than standard treatment, and you may have more tests and treatments than you would if you weren’t in a clinical trial. The study team also may ask you to keep a log of symptoms or other health measures, fill out forms about how you feel, or complete other tasks. You may need to travel or reside away from home to take part in a study.
What are the risks and benefits of my participation in a clinical trial?
Clinical trials can provide many benefits to participants and society. However, before volunteering for a clinical trial, you should talk with your health care provider and the study team about the risks and benefits.
Potential Risks
When weighing the risks of volunteering, you should consider:
- The likelihood of any harm occurring
- How much harm could result from your participation in the study
Researchers try to limit patient discomfort during clinical trials. However, in some cases, volunteers have complications that require medical attention. In rare cases, volunteers have died when participating in clinical trials.
Potential Benefits
The benefits of volunteering can include:
- Treatment with study medications that may not be available elsewhere
- Care from health care professionals who are familiar with the most advanced treatments available
- The opportunity to learn more about an illness and how to manage it
- Playing an active role in your health care
- Helping others by contributing to medical research
Where can I find a mental health clinical trial?
The National Institute of Mental Health (NIMH) is the lead federal agency for research on mental disorders. While NIMH supports research around the world, it also conducts many clinical trials at the National Institutes of Health (NIH) campus in Bethesda, Maryland.
To learn more about NIMH studies conducted on the NIH campus, visit NIMH's Join a Study webpage . These studies enroll volunteers from the local area and across the nation. In some cases, participants receive free study-related evaluations, treatment, and transportation to NIH.
To learn more about NIMH-funded clinical trials at universities, medical centers, and other institutions, visit NIMH's clinical trials webpage .
What is the next step after I find a clinical trial?
To learn more about a specific clinical trial, contact the study coordinator. You can usually find this contact information in the trial’s description.
If you decide to join a clinical trial, let your health care provider know. They may want to talk to the study team to coordinate your care and ensure the trial is safe for you. Find tips to help prepare for and get the most out of your visit .
How do I know if I can join a clinical trial?
People of all ages, ethnicities, and racial backgrounds can volunteer for clinical trials. If you want to join a clinical trial, you must be eligible to participate in that specific trial. Your eligibility can usually be determined by phone or online screening.
All clinical trials have eligibility guidelines called inclusion and exclusion criteria. These criteria may include:
- The type and stage of an illness
- Treatment history
- Other medical conditions
Researchers use these guidelines to find suitable study participants, maximize participant safety, and ensure trial data are accurate.
What kinds of questions should I ask the study team before deciding if I want to take part in a clinical trial?
It can be helpful to write down any questions or concerns you have. When you speak with the study team, you may want to take notes or ask to record the conversation. Bringing a supportive friend or family member may also be helpful.
The following topics may give you some ideas for questions to ask:
- The study’s purpose and duration
- The possible risks and benefits
- Your participation and care
- Personal and cost concerns
For a list of specific questions, check out Questions to Ask About Volunteering for a Research Study from the U.S. Department of Health and Human Services’ Office for Human Research Protections.
How is my safety protected if I choose to take part in a clinical trial?
Strict rules and laws help protect participants in research studies, and the study team must follow these rules to conduct research. Below are some measures that can help ensure your safety.
Ethical Guidelines
Ethical guidelines protect volunteers and ensure a study’s scientific integrity. Regulators created these guidelines primarily in response to past research errors and misconduct. Federal policies and regulations require that researchers conducting clinical trials obey these ethical guidelines.
Informed Consent
Before joining a trial, you should understand what your participation will involve. The study team will provide an informed consent document with detailed information about the study. The document will include details about the length of the trial, required visits, medications, and medical procedures. It will also explain the expected outcomes, potential benefits, possible risks, and other trial details. The study team will review the informed consent document with you and answer any questions you have. You can decide then or later if you want to take part in the trial.
If you choose to join the trial, you will be asked to sign the informed consent document. This document is not a contract; it verifies you understand the study and describes what your participation will include and how your data will be used. Your consent in a clinical trial is ongoing and your participation is voluntary. You may stop participating at any time.
Institutional Review Board Review
Institutional review boards (IRBs) review and monitor most clinical trials in the United States. An IRB works to protect the rights, welfare, and privacy of human subjects. An IRB usually includes a team of independent doctors, scientists, and community members. The IRB’s job is to review potential studies, weigh the risks and benefits of studies, and ensure that studies are safe and ethical.
If you’re thinking about volunteering for a clinical trial, ask if an IRB reviewed the trial.
What happens when a clinical trial ends?
When a clinical trial ends, researchers will analyze the data to help them determine the results. After reviewing the findings, researchers often submit them to scientific journals for others to review and build on.
Before your participation ends, the study team should tell you if and how you’ll receive the results. If this process is unclear, be sure to ask about it.
Where can I find more information?
This fact sheet covers the basics of clinical trials. To find more details and resources, visit NIMH's clinical trials webpage .
For More Information
MedlinePlus (National Library of Medicine) ( en español )
ClinicalTrials.gov ( en español )
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES National Institutes of Health NIH Publication No. 23-MH-4379 Revised 2023
The information in this publication is in the public domain and may be reused or copied without permission. However, you may not reuse or copy images. Please cite the National Institute of Mental Health as the source. Read our copyright policy to learn more about our guidelines for reusing NIMH content.

Better. Sooner. Together.
We build solutions that connect and empower the clinical trial ecosystem.
Introducing StudyTeam, the Patient Enrollment Management Platform
The latest from onestudyteam.
6 Key Clinical Trial Metrics to Evaluate Your Patient Recruitment Campaigns
3 Benefits of StudyTeam’s Online Learning Portal for Research Sites
How to Optimize Patient Recruitment and Pre-Screening, According to Patient Advertising Guru/CSSi and OneStudyTeam
3 Ways to Design a Clinical Trial That’s Patient-Centric
Research Sites Can Now Use StudyTeam Technology to Enroll Clinical Trials in China
3 Benefits of eSource for the Principal Investigator in a Clinical Trial
The Difference Between Screening and Enrollment in Clinical Trials
Reify Health Announces Global Expansion of StudyTeam Platform to Over 2,700 Clinical Research Sites
Trusted by the most innovative sponsors and sites around the world
of the sponsors with the most clinical trials, including Amgen, AstraZeneca, and Eli Lilly & Co. use StudyTeam
StudyTeam is available in more than 100 countries, and counting
StudyTeam is being used by 7,000+ clinical research sites to manage patient recruitment and enrollment

I was immediately impressed with how StudyTeam was built. There's a simplicity to the usability that's really smooth for integration. It’s built to give us the capacity to multitask and the workflow makes sense for those of us that manage patient recruitment on a daily basis.
Tyler Whitfield
Research Coordinator
Clinical Integrative Research Center of Atlanta

We left Excel completely out. StudyTeam has greatly improved our recruitment. StudyTeam is complete and helps us in all stages of our work.
Marinez Pinheiro Pugsley Jucovski
Clinical Research Coordinator and Regulatory Affairs
Centro de Diabetes Curitiba Ltda

Based on what you see in StudyTeam, you can give a sponsor feedback on why patients screen fail, and the sponsor amends the study. StudyTeam has made it so much easier for us to exchange that information with sponsors so they can make those changes.
Natalie Marlen
Center of Excellence in Diabetes and Endocrinology

The user-friendly platform, ease of use, and real-time patient updates are what captured our attention for our company. Immediately, our company felt the benefits of StudyTeam and we were confident no potential patients fell through the cracks.
Rebecca Goldfaden, PharmD, CCRP
Vice President of Clinical Operations
East Coast Institute for Research
Loved by sites.
Indispensable to sponsors., sites can focus on what matters most.
Sites spend less time on redundant and manual tasks so that they can focus on patient care to move enrollment forward.
Sites and sponsors can streamline workflows
Reduce duplicate work for sites and enable more focused communication between sites and sponsors.
Sponsors can take control of patient enrollment
Sponsors can optimize patient enrollment proactively with earlier access to novel pre-screening and enrollment data.
StudyTeam for Research Sites
Surprisingly simple patient recruitment and patient enrollment solution trusted by more than 7,000 sites and counting, globally.

StudyTeam for Sponsors
Access powerful patient recruitment and patient enrollment insights to run faster, more predictable clinical trials.


Get StudyTeam Today
OneStudyTeam, a member of the Reify Health portfolio, provides the cloud-based platform StudyTeam to accelerate the development of new and life-saving therapies. StudyTeam brings research site workflows online and enables sites, sponsors, and other key stakeholders to work together more effectively using common technology. The suite of StudyTeam solutions reduces site burden and helps sites pre-screen and enroll more patients, provides sponsors with end-to-end visibility into recruitment activity across all channels, and guides sites in conducting the trial for patients who have been enrolled. StudyTeam is trusted by the largest global biopharmaceutical companies, used in over 7,000 research sites, and is available in over 100 countries. One mission. One team. That’s OneStudyTeam.
Get OneStudyTeam news and clinical trial enrollment insights delivered directly to your inbox.

Who’s Who in Clinical Research – A Complete Guide (2022)
- by Kunal Sampat
- February 25, 2022
- in Clinical Operations , General

Almost everyone I’ve met in clinical research has accidentally discovered this hidden profession.
Your interest in science and medicine somehow got you involved in clinical research and clinical trial management.
But didn’t you wish you had a crystal clear understanding of “Who’s Who In Clinical Research?”
That’s when this post comes handy.
Clinical Research is sometimes also referred to as Medical Affairs or Clinical Affairs.
Aside from the naming nuances, the pillars of clinical research remain the same. This is true for pharmaceuticals, medical devices, and biologics.
In this post, you’ll learn the functional areas that form an ideal clinical research team at a sponsor or clinical research organization (CRO).
For each function, I’ve highlighted why the group exists and their general mindset.
We’ll wrap up our discussion with adjacent departments that interact with clinical research professionals.
So let’s get started:
Clinical Project Management
I’d like to start with my personal favorite clinical research role, Project Management.
The reason why the project management role is so interesting is because it provides a holistic view of clinical trial management and clinical research.
“General Contractor” for the study
The project management team is made up of Clinical Project Managers and Program Managers.
In some companies, Clinical Project Managers (CPM) may be referred to as Clinical Trial Managers (CTM) or Study Managers.
Project managers are the “General Contractors” of clinical research. They are accountable of all aspects of a clinical trial.
Go-to person for clinical trial budget, timelines, resources
Developing and actively managing clinical trial finances, timelines, and resource allocation is necessary for a smooth trial execution. This responsibility falls under the project management team.
After mapping out trial assumptions such as number of sites, patients, enrollment period, monitoring, a project manager will develop the study budget and timeline with key milestones.
Depending on the size and reporting structure of the company, project resources are either managed by the project manager or by functional managers.
Clinical project manager is able to connect the dots
A great project manager is able to connect two seemingly unrelated issues and assess the impact on the project.
Let me give you an example.
Say you’re in the midst of securing FDA approval to start a new clinical trial. You find out that the FDA has follow-up questions. This is going to delay the First Patient In (FPI) date. Your investigational devices with limited shelf-life are ready to be shipped to the sites.
A great PM will recognize the downstream effects of study startup delays. For example, she’ll start planning for the additional devices. Not only that but the PM will also adjust the study budget.
Clinical project manager anticipates issues
In addition, to be able to connect the dots, a project manager also anticipates issues.
For instance, a clinical site may be enrolling at a rapid pace. During a periodic data review meeting, the project manager finds out that the site has a significant number of protocol deviations.
Rather than letting the clinical site continue enrollment, the PM decides to put the site enrollment on hold until the compliance issues are fully addressed and resolved.
The PM anticipates the negative impact of compliance issues on the overall trial. If the PM does not anticipate the issue, it could have negative consequences on the final results and major audit findings for both the sponsor and the clinical site.
Clinical project manager can visualize and paint the big picture
A clinical trial has many moving parts. The objective of most trials is secure product approval, indication approval or assess the long term safety of the product after it is approved.
Keeping this end goal in mind, a clinical project manager needs to have a thorough understanding of the key milestones. If challenges arise (which they will), the project manager needs to communicate the challenges effectively to the right stakeholders.
It is also not uncommon for a project manager to paint the big picture for the team. This is necessary to prevent the team from digressing on irrelevant topics or getting distracted.
Clinical project manager is not afraid to get his/her hands dirty
When I started my career, I felt that the project manager’s primary job was to tell other people what to do and by when it needs to be done.
That certainly did not turn out to be true.
Most of us desire a “perfect” project with no issues or challenges. This is however not reality.
There will be obstacles along the way that a project manager will need to overcome.
For example, let’s consider a clinical site that urgently needs investigational devices to enroll a patient the next morning. Unfortunately, the FedEx package drop-off time is 4:00 pm and the pick-up truck has left for the day. The only way to get the device to the site in time for the procedure is to drop off the device package to the airport. A great project manager would drive to the airport and drop off that package.
Operations may be further subdivided in Internal Operations and External Operations. Members of the operations team usually have titles such as clinical research associate or clinical research manager.
CRA champions site start-up
The in-house operations team is primarily responsible for clinical trial site start-up. This team ensures that each site has the most recent version of the site start-up packet. The start-up packet consists of the protocol, ICF template, and other study specific documents.
The in-house operations team also facilitates clinical contract negotiation with the site, which includes the study budget.
CRA understands devil is in the details
Once the study-specific ICF template is sent to the sites, the site’s personnel review and edit the ICF to meet their site-specific IRB/ Ethics Committee requirements. The redlined ICF is then sent to the in-house operations team for final review and approval.
Similar to the ICF redlines, the CRAs also receive redlines on the clinical contracts.
This means that in-house operation is constantly inundated with multiple documents and redlines. This requires the in-house team to be detailed oriented and organized. An error can have significant ramifications including potential audit findings or lawsuits.
CRA’s life revolves site management and support
The in-house operations team is available for site management and support. Aside from ICF and clinical contract questions, it is usual for sites to contact their in-house CRA with questions about clinical protocol, study specific requirements, investigational device restocking, and more.
Field CRAs are also known as monitors.
In-house operations and field operations roles can sometimes overlap. In some organizations, field and in-house operations teams are combined into one group, namely Clinical Operations.
CRA is the face of the study for clinical sites
A field team is the “face of a study” for a clinical site. Most field operation team members work remotely from their home office. They travel to clinical sites on a regular basis for monitoring and site support.
The field CRA is also responsible for conducting pre-study visits, site initiation visits and study close-out visits.
A field CRA is responsible for monitoring clinical trial data at the site.
A field CRA needs to have thorough understanding of the study protocol, Good Clinical Practice (GCP) and regulations applicable to the conduct of clinical trials.
A clinical trial database has numerous fields. Depending on the monitoring plan, a CRA needs to review and verify the accuracy of the source data at the site.
For example, the CRA ensures the patient informed consent was signed prior to the procedure, serious adverse events were reported on time or protocol deviations were addressed appropriately. This work requires attention to details and deep focus to be done right.
CRA helps site succeed on all fronts – start-up, enrollment, compliance
Site coordinators can get overwhelmed with multiple trials. Many clinical sites are also nonprofit organization with limited funding and resources to support clinical research.
A field CRA can help their clinical site be successful by promptly answering all questions during study start-up, enrollment and follow-up.
In addition, it’s not uncommon for a CRA to prep sites prior to any scheduled audits.
The job of the field CRA is not “police” the site but rather be the site’s champion and help a site be successful in their research efforts.
Products are brought to market based on clinical data. Government agencies, medical community, and patients believe in the power of data.
It is no easy task to collect and clean hundreds of clinical trial data points in a compliant manner. This is where the data management team comes to play.
Data manager decides how data will be collected and cleaned
One of the key responsibilities of a data manager is to develop case report forms (CRF) for the collection of clinical trial data. These days most data is collected via electronic data capture (EDC) forms.
A well-designed and thought out CRF can be of great value to the sponsor in the long run.
For example, data collected in a case report form can not only help secure product or indication approvals but also forms the basis of publications and presentations.
Data manager’s life revolves around database locks
Once all patients have completed their primary endpoint visit and the data has been cleaned, the final step is to lock the database.
Database lock is a very important milestone not only for data management but also for the entire organization.
Database locks can be a very stressful time for data managers, sites and CRAs. The CRAs are frantically working with the clinical sites to resolve open queries. A database is usually not locked till open queries are resolved.
Once the database lock occurs, the biostatistics is able to analyze the data and generate tables and graphs.
Data manager communicates study metrics
Without data metrics, you would have no way of knowing whether your sites are completing the CRFs in a timely manner.
Let’s consider a 1000 patient clinical study at 65 sites with 3-year patient follow-up. For each patient there are 20-30 case report forms with multiple fields in each form. It becomes increasingly complex to determine what data is missing, incorrectly entered or not available.
A data management team can create custom reports that can provide data metrics by site, by visit or by patient. This information makes it efficient for sites and CRAs to address data entry gaps and query resolution.
Biostatistics (or Biometrics)
Statisticians are the numerical brain behind a clinical study. Statistics is a very broad field with numerous data analysis methods.
Biostatistician is the key driver behind trial design
One of the key components of clinical trial design is the sample size. Simply speaking, you want to know how many patients are needed in order for the clinical study to be statistically sound.
For many studies, regulatory agencies review the statistical analysis plan (SAP) as part of the trial design review.
Life revolves around p-values, trial power and performance goals
P-values, power and performance goals are the geeky pieces of information that statisticians care about.
Statisticians want to understand if the trial results (good or bad) are replicable in the real world.
They attempt to understand the probability of the certain benefits or risks to re-occur in real world once the medical products are commercially available.
Shares clinical study results using tables and graphs
The Statistical Analysis Plan (SAP) specifies how the data will be analyzed.
Once the clinical data is analyzed, it is presented beautifully in the form of tables and graphs. This is a key task as it forms the foundation of how the trial results will be communicated to the outside world.
Tables are graphs utilized in clinical summary reports, annual updates, presentations and publications. Tables and graphs are also included on product Information For Use (IFU) documents and Patient Guides.
When the product is approved to be sold commercially, sales and marketing teams use government approved tables and graphs to promote the use of the drug or device.
Clinical Safety
This is a very interesting role for anyone who wants to be closest to the medical aspects of any clinical trial.
Safety touches most aspects of a clinical trial including the protocol, patient informed consent, patient safety outcomes in the final study report, Instructions for Use (IFU) document and more.
Understands regulatory requirements around patient safety
Regulatory agencies are most concerned about the safety of the clinical trial procedure and investigational device or drug.
The safety team has a thorough understanding of regulatory requirements pertaining to the safety of any clinical trial.
When patients are enrolled in a clinical trial, they may experience an adverse event. Clinical sites, sponsors and CROs are required to meet strict adverse event reporting requirements. Adverse event reporting requirements can vary by country.
A safety team member has a clear understanding of regulatory requirements and helps ensure safety compliance.
Life revolves around adverse events management
A safety monitor champions all safety aspects of a clinical trial such as adverse events, clinical trial procedure risks, and device/ drug risks.
When a patient participates in a clinical trial, he or she may experience adverse events, also known as AEs. These AEs are reviewed by the safety team.
In some cases, there may be unexpected serious adverse events (SAEs) that may impact patient safety. When such events occur, the safety team evaluates and communicates the adverse event information with stakeholders such as clinical trial sites, patients, and regulatory agencies.
Manages Clinical Events Committee (CEC) and Data Monitoring Committee (DMC)
When patients experience AEs, the clinical sites reports the AEs to the trial sponsor or CRO. The safety team reviews reported AEs and collects relevant medical records.
A subset of these AEs and the corresponding relevant medical records are sent to a physician committee, known as the Clinical Events Committee (CEC). The safety team manages the selection and operation of a CEC.
The basic premise of a clinical trial is that it’s a drug or medical device experiment on human beings. Participation in a clinical trial usually involves safety risks. Some safety risks are anticipated and others aren’t.
For unanticipated risks during the enrollment phase, a sponsor may recruit a data safety monitoring board (DMC). The DMC ensures there is no risk to patients in the trial.
Not all trials require DMC as the duration of enrollment might be too small to detect a safety signal. Similar to CEC, the safety team leads the selection and management of a DMC.
Clinical Quality
Throughout my career, I’ve worked with clinical quality experts that either “police” every step in the clinical process or serve as a partner and resource to other clinical research functions.
In both cases, the ultimate goal of any clinical quality personnel is to keep you, your clinical trial and your organization out of trouble.
Ensures compliance in all aspects of a clinical trial
As we’ve discussed earlier, clinical research is a highly regulated industry. With regulations, comes compliance. Clinical research professionals need to comply with government regulations, Good Clinical Practice (GCP) and operating procedures.
You may ask, “What’s the purpose of all this compliance”?
Well, the simple answer is that the outcome of a clinical trial sets a new medical standard or leads to an update of an existing medical standard.
Would you trust a drug or device that is brought to market based on a non-compliant clinical trial?
Probably not.
Clinical quality wants no audit Findings
Passing an audit is the ultimate test of any clinical research organization. This is especially true for audits by regulatory agencies.
Audit findings are painful not just for the quality team but for the entire clinical team. Significant audit findings also raise doubts about the robustness of a clinical trial.
Life revolves around Standard Operating Procedures (SOPs)
For instance, in the US, FDA regulations dictate how a clinical trial should be conducted. These regulations form the basis of standard operating procedures (SOPs).
A great quality associate helps a clinical research organization create and maintain a practical set of SOPs.
It is important to note that the burden of creating SOPs does not lie with clinical quality. Other clinical research functions are responsible for drafting and finalizing their own role-specific SOPs.
Understands Corrective and Preventive Action Plan (CAPA)
Since many aspects of clinical trials are managed by humans, the process is prone to errors. Errors can happen due to oversight, gap in a SOP, or mismanagement. A quality personnel initiates a CAPA when errors are discovered.
The goal of a CAPA is to document the error and take necessary steps prevent the error from happening in the future.
CAPAs are frowned up and are never a good thing for any organization. More CAPAs means there is greater risk, which in turn reduces the public’s confidence in the clinical trial outcomes, product or company.
Medical Writing
Clinical trial information is communicated with all stakeholders via clinical documents such as the protocol, clinical reports and manuscripts.
A medical writer leads the task for assimilating trial information and putting it together in a logical way.
Understands scientific content
A medical writer has an in-depth understanding of the therapeutic area and previous clinical and preclinical information on the drug or device.
Given the countless number of medical products and trials, it’s not unusual to learn trial or product specific information after joining the project.
Life revolves around writing protocols, reports or manuscripts
As a medical writer, you need to enjoy the process of writing. If you don’t enjoy writing, it will be painful to write several hundred pages of clinical trial documents on a regular basis.
A medical writer has a unique ability to communicate high-level clinical trial strategy vision in a document such a protocol or report.
Regulatory decisions are made based on these documents. Therefore it is extremely important for a writer to understand what regulators are looking for.
Detailed oriented with focus on punctuation, grammar and formatting
A medical writer is responsible for writing key clinical documents such as the protocol, clinical reports, safety charters, and more.
Punctuation, grammatical errors or typos can reduce the reviewers trust in the final document. Such errors make the company look sloppy in front of regulators, clinical sites or trial sponsor.
Additionally, a medical writer needs to master writing software such as Microsoft Word. Inability to effectively use such software will cause issues with document formatting, resulting in delays and frustration.
Medical Science
A scientist is responsible for the clinical trial strategy.
In some organizations, the role of the scientist may be combined with that of the medical writer or that of project management.
Brainchild behind the overall clinical strategy
Prior to the start of any clinical trial, a lot of work is put into designing a cost-effective, safe and effective study.
Depending on the trial design, there may be quite a bit of back-and-forth between the regulator and the sponsor or CRO.
A clinical trial can begin enrolling patients only after the regulatory alignment is obtained.
The scientist champions the development of clinical documents and communication necessary to secure this regulatory alignment.
Life revolves around designing a trial that meets the primary endpoint
If you remember your life as a student (or maybe you’re still a student), your final grade on the test depends on how well you do on the finals.
Along the same lines, the success of any clinical trial is determined on whether or not it meets a pre-specified primary endpoint.
The trial endpoints are clearly stated in the protocol. Once the primary endpoint is met, the medical product will most likely be approved for commercialization.
Medical science wants high-quality clinical trial data
The success of a clinical trial is hinged on high-quality clinical trial data. But what does “high quality” mean?
High-quality data is error-free, accurate, and complete. Missing or incomplete data due to missed visits or sloppy data entry can cause headaches for many clinical stakeholders, primarily the clinical scientist.
Also, there can be a tendency to collect more data than needed for “just in case” scenarios such as unanticipated requests from regulators or potential for interesting publications in future years.
Clinical Systems and Solutions
Not too long ago, clinical trial data was collected on paper case report forms. Then it became incredibly hard to manage data queries, keep track of complete forms and manual entry of data from paper into an electronic database.
With the help of technology, electronic data capture (EDC) solutions were developed. Now site staff can directly enter data in the case report forms.
The Systems and Solutions team is responsible for managing clinical technology solutions such as the EDC. They are also responsible for onboarding new technology solutions and retiring old solutions that no longer add value to the organization.
Technology backbone for clinical trials
More than ever before, technology is becoming an integral part of clinical research. The system and solutions group is the technology backbone for clinical trials.
Want to incorporate wearable technology in a clinical study?
Or build an iPhone app to organize study team contact?
Clinical systems and solutions group is your go-to team.
Depending on the size of the company, this role may be a separate function within clinical research or part of the information technology (IT) organization.
Focused on clinical research software and applications
Clinical research teams utilize a few core technology solutions such as the Trial Master File (TMF), Clinical Trial Management System (CTMS), Electronic Data Capture (EDC), Integrated Web/Voice Response System (IxRS), and electronic Informed Consent (eConsent).
The TMF allows you to store study and site level documents in electronic format.
CTMS is useful for managing study contact information, monitoring trip reports, site and vendor payments and more.
EDC allows sponsors to design electronic case report forms and allows sites to remotely enter data into the system.
IxRS serves to register patients to a specific treatment.
In addition, there may be other systems for safety management and reporting, protocol deviation and monitoring visit management.
Understands regulatory requirements for technology solutions
Regulators are interested in ensuring clinical trial technology is compliant with the law.
At the most basic level, technology solutions need to maintain an audit trail, protect patient personal health information (PHI) and encrypt sensitive clinical trial information.
Systems and solutions teams need to have an in-depth understanding of regulatory requirements pertaining to technology solutions.
Regulators such as the FDA frequently publish guidance documents related to a specific technology product.
Guidance documents can be easily accessed via the FDA website and can serve as a great resource for anyone wanting to dig deep in a specific clinical technology area.
Senior Management
Senior management is ultimately accountable for the success (or failure) of any clinical trial.
Key senior management functions in a clinical research organization include directors of each of the functional teams described above.
Depending on how the organization is structured, a clinical director may oversee more than one clinical trial or clinical function.
Accountable for all aspects of a clinical program
Providing adequate oversight is the key role of a senior manager.
Oversight is not the same thing as “micro managing” an employee or a task. For certain critical deliverables, a senior manager may be more hands-on.
Strategic focus – Budget, Timelines, Resource Allocation, Procedure Compliance, Scientific Robustness
Clinical trial costs can run into millions of dollars. By controlling project timelines and resources, a senior manager can control expenses.
In addition, it is crucial to ensure the trial is conducted in compliance with regulations and internal procedures.
Finally, if the study is not scientifically robust, the clinical trial may not get regulatory approval.
It is not possible for one individual to be competent in all these areas. For this reason, a senior management team is needed to collaboratively achieve strategic goals.
Interested in Key Performance Indicators (KPIs)
Do you have a retirement account?
Or go to the doctor for an annual check-up?
If so, you’re probably paying attention to the performance of your retirement fund or your health over a period of time.
Along the same lines, senior managers are interested in a set of KPIs that track the performance of a clinical trial against a standard or threshold.
For example, regulators want to ensure patients sign the correct version of the informed consent form (ICF). Signing an incorrect ICF version is a major compliance issue. Thus ICF deviation rate can be a KPI.
Since senior managers are not involved in the day-to-day operations of the trial, they assess the health of a project by reviewing KPI metrics. If KPI metrics trend towards non-compliance, senior managers will take steps to address the issues.
Other Clinical Trial Stakeholders
A clinical department frequently collaborates and seeks help from other teams. The section below covers the key teams that interact and support clinical teams on a regular basis.
Since the focus of this post is clinical research, the role of these adjacent functions has been described keeping in mind the clinical context.
Regulatory Affairs (RA)
Regulatory Affairs (RA) is responsible for regulatory strategy and submissions. For a clinical trial, a RA team member is generally the primary contact person for the regulator such as the US FDA, Japan PMDA, China FDA.
Deep understanding of regulatory process and requirements
Ready to start a clinical trial? Or address clinical trial deficiencies with the regulator?
You’ll want to start with your regulatory affairs team. Clinical trials are conducted in compliance with regulations. RA can educate you on the clinical documents needed to meet regulatory requirements in a given geography.
A regulatory affairs expert has an in-depth understanding of the regulatory process and requirements.
Adept at reviewing and editing clinical submission documents
Once the clinical team has created the necessary documents such as the protocol, statistical analysis plan or the clinical summary report, a regulatory affairs associate performs a detailed review of the documents.
In addition, regulators have specific formatting requirements such as cover letters and forms that need to be completed with every submission.
Focus is on regulatory agency alignment on all aspects of clinical research
There is a specific way of communicating clinical trial information with regulators. This communication style is very different from the day-to-day communication amongst clinical research team members. A seasoned regulatory affairs expert understands this nuance and is able to communicate effectively with regulators.
Seeks new medical product/indication approvals
Success of a regulatory affairs team is measured by product and indication approvals. It’s no easy task to get a product to market, especially for new therapies.
Health Economics and Outcomes Research (HEOR)
Getting medical products or indications approvals is always great news.
But here’s the deal.
You need someone to pay for the approved drug or device. If payors such as insurance companies or Medicare are not willing to provide the reimbursement, it can significantly hurt a company’s bottom line.
This is where HEOR comes into play. A HEOR team helps create a body of evidence that the new drug or device has health benefits such as improved quality of life.
Deep understanding of payor process and requirements
A HEOR expert can help you develop a robust protocol and case report, so you can proactively collect the clinical data needed for reimbursement.
HEOR has an in-depth understanding of country-specific requirements. Their work leads to maximum medical product reimbursement for the company.
Understands medical product impact on Quality of Life (QoL)
Let’s say your friend experienced a heart attack and got a stent. Now 10 years have passed. Your friend has not experienced any new heart problems. He feels great, goes for a morning run and is able to spend quality time with his family.
How would you rate his quality of life? Excellent, right?
HEOR pays much attention to a patient’s quality of life. In addition to being safe and effective, medical products need to have a positive impact on a patient’s quality of life.
There are many tools available to measure such quality of life. Clinical trials include quality of life questionnaires for patients to complete. This data is then analyzed and utilized to secure reimbursement approvals.
Seeks to maximize medical product reimbursement
One of the primary roles of the HEOR team is to ensure the medical product receives maximum reimbursement possible.
Reimbursement can vary significantly based on country laws or availability of new health data. For example, Japan offers higher reimbursement for newer medical products but then decreases reimbursement in future years.
Clinical Research Organizations (CROs) and Consultants
Some sponsors hire full-time staff to manage their trials. Some outsource all clinical research tasks to CROs and consultants. While others may use a combination of internal and external resources.
Supports sponsor by filling in resource and talent gaps
It is expensive to build a full clinical team, especially for an organization with limited financial resources. CROs can help fill the talent gap for each of the clinical research roles discussed in this post.
Work is billable
A major chunk of clinical trial costs is full-time salaries paid for each of the clinical roles explained in this post.
CROs and consultants charge by the hour or by deliverable. The “pay by the hour” or “pay by deliverable” model gives sponsors greater flexibility and control on expenses.
People that work for a CRO may be full time employees or contractors. At any given time, a CRO or a consultant may be working on multiple projects for different sponsors.
Can help reduce fixed overhead expenses
For smaller organizations, steady cash flow can be an issue. Therefore having a full clinical team means paying for employee salaries, office space, health insurance and other benefits. This can be cost prohibitive.
Hiring a CRO or consultant can reduce fixed expenses, giving companies greater flexibility with their limited resources.
Clinical research cannot be conducted without legal support.
You’d open yourself to all sorts of risks if you didn’t have a lawyer on your team.
Complete understanding of legal landscape
A lawyer with clinical research experience has an understanding of intellectual property law (patents, trademarks), legal entities i.e. how the hospitals or suppliers are structured from legal standpoint, supplier contracts, subject injury and liabilities, and more.
Seeks legal compliance in a clinical trial
A lawyer will be extremely happy if a company is successfully able to conduct a clinical trial in a legally compliant manner.
For instance, there is great risk to patients participating in clinical trials. If the legal language on the informed consent form or clinical trial contract between the site and sponsor is inadequate or unclear, it can lead to lawsuits.
Lawsuits impact the company’s finances, brand and reputation. A legal team ensures the company does not end up in legal troubles with any of the clinical trial stakeholders.
By now you have an in-depth understanding of all clinical research functions and teams that support clinical trials.
Clinical research and clinical trial management involve cross-functional teams. The chart below summarizes the roles of different clinical research team members.
Which clinical research function interests you the most? Leave your response in the comments section below.
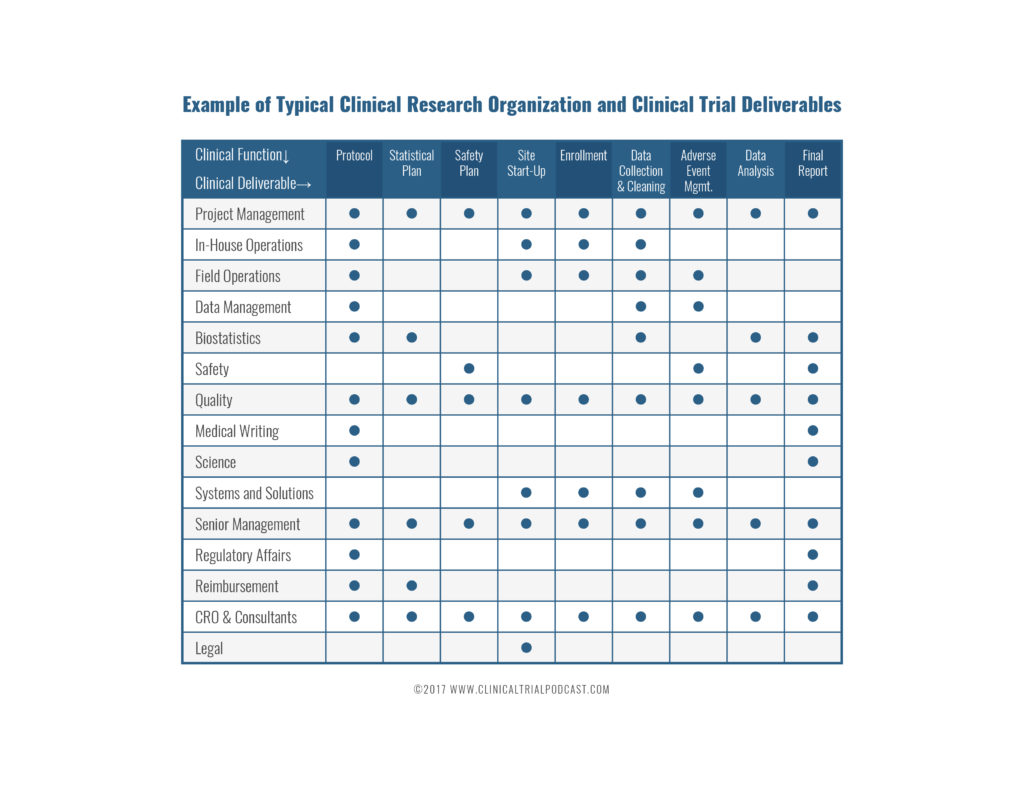
Clinical Data Management with Mariam Mirgoli
Clinical research billing for small to medium sites with kristi etchberger, 25 thoughts on "who’s who in clinical research – a complete guide (2022)", ultimate guide to clinical trial costs - clinical trial podcast, 25 soft skills to boost your clinical research career – clinical trial podcast, 10 smart strategies to getting response from clinical site coordinators – clinical trial podcast, 5 ways to get clinical research associate experience – clinical trial podcast, 5 ways to engage clinical trial sites with technology | florence healthcare, conversation with clinical research expert | marshall cool, how to get a clinical research job | clinical trial podcast, how to get clinical research associate (cra) experience – clinical trial podcast, how to become a cra – career keg, 9 essential components of a clinical trial agreement - clinical trials arena.
Sonia Menezes
I have a masters in clinical research and work experience at an oncology site. I’m looking to get into to industry and medical writing is the most interesting to me. I wanted opinions on what people in the industry think about the field and whether it is too niche to start your with?
Kunal Sampat
Hi Sonia, I would suggest starting as a medical writer in clinical research. There is a huge demand for great medical writers. There are several medical writing groups on LinkedIn that you may want to consider joining. Reach to people in those groups and find out how you can get started. It’s not a small niche and the opportunities are endless. You can always move in different directions as long as you remain curious and interested in other roles. Goodluck!
mamtha srinivas
hi I have worked in clinicalresearch for last 4 years. i have an opportunity to work under investigator directly .I need to search for new projects.Guide me how to go about consulting for projects.
Hi Mamtha, I would recommend you follow the steps outlined in the blog post. https://clinicaltrialpodcast.com/get-a-clinical-research-job/ . Let me know if you have any additional questions. Goodluck!
Subuloye Olufunke
Hi, Please for someone thinking of going into the clinical research field with no experience, would you suggest getting an online training or getting a certification in one of the Universities? Which one has a better chance of getting a job considering the fact that there is no experience. Thanks.
An informative read. As an clinical research novice it imparts more knowledge about the field.
great, thanks for the feedback
Kunal, you’ve missed the Risk Manager, a critical role per ICH E6 R2, and expect even more important in upcoming R3. Love your work!!!
Thank you, Fran for your thoughtful comment. Safety monitors and quality associates can also serve as Risk Managers
This is such an informative post. I’m a Registered Dietitian and another RD informed me that RDs make good Project Managers in research; she works as one. I came across this post when looking for the “job ladder” and responsibilities involved in the field and its exactly what I needed. Thank you for compiling it!
raveena aher
Hey thanks for sharing this blog over here. It seems useful to start career in clinical research. We will look forward for more updates.
It is a pleasure to hear you share such useful information.
Moses Musitwa
Safety monitors and quality associates is some fascinating role,as a medical doctor, medical educator and manager which role would suite me because am spoiled for choice.
Safety monitors and quality associates are fantastic clinical research positions for doctors to pursue.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
- Português Br
- Journalist Pass
Clinical trials team brings hope and dedication to colorectal cancer study
Vincent Jacobbi
Share this:

Clinical trials can be a beacon of hope for many patients and can fuel the discovery of new advancements in healthcare. They represent the power of collaboration between volunteer participants and research teams who carefully collect data.
One clinical trials team at Mayo Clinic is working to test a screening tool for colorectal cancer , a leading cause of cancer deaths in the U.S.
Early detection of colorectal cancer may hold the key to saving lives.
In the Voyage study , the team is gathering real-world data on Cologuard, a noninvasive stool DNA screening test for colorectal cancer, and now has 150,000 participants enrolled. The participants will be tracked over a seven-year period.
The team conducting this study includes data librarians, statisticians and clinical research coordinators, who ensure the research is thorough and conducted responsibly. The team has worked in lockstep throughout the trial, now in its fifth year.
"The purpose of this study is to gather real-world evidence on Cologuard's impact on long-term outcomes in a large, diverse population, and so the overarching goal is to examine the colorectal cancer-related incidence and mortality in a cohort of people who had an order for the test kit," says clinical research coordinator Allison Berry.
"It is a prospective survey-based study, so we're looking at people going forward in time," she adds.
The participants enrolled in the study answered a baseline questionnaire about demographics and their health history. From there, Berry says, the team is building a data repository that documents any changes in their health or personal information over time.
Labor of love
The team notes that this study is a labor of love.
"People send us notes all the time; one person said they are doing this because their mother passed away, and they do not want anyone to go through this pain," says Berry. "I think just seeing the care, attention and investment that participants have makes our job rewarding."
The team notes that the work being conducted today within the study has implications for now and well into the future when it comes to those considering preventive measures such as early screening for colorectal cancer. They hope the data points they are collecting will provide lifesaving information and result in positive outcomes for patients.
"We're not just collecting data and keeping it," says clinical research coordinator Angie Kostreba. "We're concentrating on following up and continuing to validate what the participants have reported by checking in with their healthcare providers."
Senior program coordinator Jessica O’Neill emphasized that the effort put into the details of the clinical trial makes all the difference.
"When people hear about survey studies, they don't realize how much work goes into it," says O'Neill. "The research coordinators have fielded over 24,000 phone calls, 6,000 voicemails, 10,000 emails, 500,000 incoming documents and one million mailed outgoing documents."
The clinical research coordinators are critical to the work, their colleagues say.
"The clinical research coordinators have played a hugely important role in the success of the Voyage study,” says Janet Olson, Ph.D. , the study's principal investigator. "As advocates for the study and the participants, the study coordinator team has been well prepared to ensure that all tasks were done with quality and integrity and that participants received timely responses to their inquiries about the study."
Members say that flexibility and cohesive team dynamics also are key to ensuring the study's success.
"We would not be here without everyone who helped get us to this point," says clinical research coordinator Jen Fondakowski. "We've been lucky to have such a phenomenal team for many years."
Mayo Clinic has a financial interest in the technology referenced in this news article. Mayo Clinic will use any revenue it receives to support its not-for-profit mission in patient care, education and research.
- Golfer’s future back on course following hip surgery In case you missed it: This week’s Top 5 stories on social media
Related Articles

Building Trust in Clinical Research
Tuesday, May 28, 2024 • Neph Rivera : contact

For public health researchers, involving community members in a study is critical to finding meaningful results. Sometimes, involvement means responding to questionnaires and surveys. Other times, it means giving blood samples or other biomaterials.
For some prospective participants, though, submitting samples is a red flag. A team of researchers at University of Texas at Arlington wanted to find out why.
In an article for the peer-reviewed Journal of Racial and Ethnic Health Disparities , a team led by Kyrah Brown, assistant professor of kinesiology in the College of Nursing and Health Innovation, explored Black women’s willingness to provide blood samples for clinical research.
The idea for the research was sparked when Liao Yue, co-author on the study and assistant professor of kinesiology, was discussing a potential project with Brown, one that would include the possibility of asking for blood samples from Black women.
“To me, that’s a very standard procedure in research. I never thought too much that this would be a problem,” Liao said.
That’s when Brown mentioned the general feeling of mistrust that some Black women may feel when it comes to submitting sensitive information about themselves. This is especially true among generally healthy women who are not used to giving blood, compared to those who may have chronic conditions and be more familiar with the process.
“Prior research with older Black cancer patients has reported generally positive attitudes toward biospecimen donation, but little is known about younger individuals,” Brown said.
She and her team asked nearly 500 Black women ages 18-49 to complete an online survey about their attitude toward giving blood for clinical health research. They found that less than half, 44%, would be willing to take part.
Among those who were not willing, some reported concerns about the transparency of the work and feared for their privacy. Others said their mistrust in providing samples stemmed from the institutional racism they believe exists in the medical community. And still others cited a need to trust the research team fully before participating.
Some of the findings contradicted older studies of Black adults’ willingness to donate samples. Brown believes that part of the reason for the disparity may be the age of participants, as this was one of the first studies to focus on the younger Black population and their thoughts.
“I think there’s a generational piece that came to light,” she said. “Some research suggests that Millennial and Gen Z members tend to have a heightened, more sensitive awareness of historical and contemporary racial injustices in the medical field. This shined through with the results.”
“I was very surprised to see the thorough answers participants submitted,” Liao added. “Typically, people skip right to saying yes or no without explaining. But here, people wrote and shared a lot.”
Brown emphasized the importance for research teams to be transparent and trustworthy when working with potential study participants.
“We need to have representative samples that are reflective of a diverse population,” she said. “There are differences in lived experiences and how those experiences shape one’s biomarkers and health. That’s why it matters.”
To increase transparency and trust, Liao suggests that both researchers and participants open up dialogue, ask questions and avoid making assumptions.
“We all have the same goal: to be healthy and improve health outcomes. We might just not be talking to each other as often as we should.”
National Cancer Institute - Cancer.gov
Patient Care Coordinator - scheduling, clinical trials
Job description.
Within Leidos Biomedical Research Inc., the Clinical Monitoring Research Program Directorate (CMRPD) provides high-quality comprehensive and strategic operational support to the high-profile domestic and international clinical research initiatives of the National Cancer Institute (NCI), National Institute of Allergy and Infectious Diseases (NIAID), Clinical Center (CC), National Heart, Lung and Blood Institute (NHLBI), National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), National Center for Advancing Translational Sciences (NCATS), National Institute of Neurological Disorders and Stroke (NINDS), and the National Institute of Mental Health (NIMH). Since its inception in 2001, CMRPD’s ability to provide rapid responses, high-quality solutions, and to recruit and retain experts with a variety of backgrounds to meet the growing research portfolios of NCI, NIAID, CC, NHLBI, NIAMS, NCATS, NINDS, and NIMH has led to the considerable expansion of the program and its repertoire of support services. CMRPD’s support services are strategically aligned with the program’s mission to provide comprehensive, dedicated support to assist National Institutes of Health (NIH) researchers in providing the highest quality of clinical research in compliance with applicable regulations and guidelines, maintaining data integrity, and protecting human subjects. For the scientific advancement of clinical research, CMRPD services include comprehensive clinical trials monitoring, regulatory, pharmacovigilance, protocol navigation and development, and programmatic and project management support for facilitating the conduct of 400+ Phase I, II, and III domestic and international trials on a yearly basis. These trials investigate the prevention, diagnosis, treatment of, and therapies for cancer, influenza, HIV, and other infectious diseases and viruses such as hepatitis C, tuberculosis, malaria, and Ebola virus; heart, lung, and blood diseases and conditions; parasitic infections; rheumatic and inflammatory diseases; and rare and neglected diseases. CMRPD’s collaborative approach to clinical research and the expertise and dedication of staff to the continuation and success of the program’s mission has contributed to improving the overall standards of public health on a global scale.
The Clinical Monitoring Research Program Directorate (CMRPD) provides comprehensive clinical support research and study coordination in support of the National Cancer Institute’s (NCI’s) and the Center for Cancer Research (CCR).
KEY ROLES/RESPONSIBILITIES
- Provides patient care coordination of all clinic-related functions and administrative support for the study team
- Consults with patients to schedule appointments, enters patient ID and demographic data into the system to update clinic and physician schedules
- Communicates with various clinical administrative support offices/clinics/diagnostic centers concerning the scheduling of patient appointments, new and existing work scopes and clinical protocols (Surgery, X-ray, etc.)
- Helps to ensures protocol compliance using established guidelines
- Provides patients with information about their appointments including medical materials the patient will need to bring, dates and times, clinic information, hospital maps, travel and hotel information
- Arranges Admission Travel Voucher (ATV) travel including lodging, meals and direct bill requests
- Obtains up-to-date patient records and other pertinent information prior to appointment or admission
- Maintains electronic rosters of all patients and tracks their appointments
- Attends weekly meetings; weekly clinics, and other team meetings as required by the study team.
- Helps coordinate new research patient screening appointments between protocol investigators and the outpatient clinic scheduling personnel
- Assesses clinic and/or physician appointment schedule availability using the central appointment computer
- Composes correspondence and communications for various clinical trial administrative issues or topics.
- Organizes film consults and files in the LabMatrix database
- Acts as a liaison between physicians, nursing staff and other departments
- Collects outside CT scans and pathology slides, records arrival times, and fills out appropriate requests to be read by NIH personnel
- Delivers slides/blocks to pathology for review and films to the film library, 21) designs and sets up filing systems and office procedures
- Participates in biospecimen training (packaging/handling)
- Maintains electronic calendars with current updates for the weekly clinic meeting
- Interacts with Branch teams and clinic personnel to ensure clinical trial processes and NIH/NCI regulations are followed
- This position is hybrid with in-person work located in Bethesda, Maryland. Days working in-person at Bethesda are subject to change and are done at the request of the study team
Qualifications and Job Details
Required and preferred skills.
- Must have experience scheduling patient appointments and maintaining patient records
- Knowledge of record keeping and medical terminology
- Ability to understand clinical operations in order to provide adequate patient care arrangements
- Ability to efficiently compose correspondence, prepare reports from raw data, design and set up filing systems
- Experience with Microsoft software applications
- Excellent oral and written communication skills
- Must be able to obtain and maintain a security clearance
- Familiarity with regulatory requirements and guidelines for clinical research
- Oncology experience
- General familiarity with requirements of clinical protocols
- General knowledge of scientific, safety, or medical technologies
About the NCI Center for Cancer Research
The Center for Cancer Research (CCR) is home to nearly 250 basic and clinical research groups located on two campuses just outside of Washington, D.C. CCR is part of the National Cancer Institute (NCI) and makes up the largest component of the research effort at the National Institutes of Health (NIH). Centrally supported by long-term funding and a culture of complete intellectual freedom, CCR scientists are able to pursue the most important and challenging problems in cancer research. We collaborate with academic and commercial partners and advocacy groups across the world in efforts to prevent, diagnose and treat cancer and HIV/AIDS. The CCR research portfolio covers the full spectrum of biological and biomedical research. Our work ranges from basic to translational and clinical, and our clinical trials are conducted in the NIH Clinical Center, the world’s largest hospital dedicated to clinical research that offers a robust infrastructure to support CCR’s patients on an estimated 250 open studies. The success of CCR is grounded in an exceptionally strong discovery research program that provides the foundation for the seamless translation of insights from bench to bedside. Read more about CCR , the benefits of working at CCR and hear from our staff on their CCR experiences.
Bethesda is one of the most highly educated communities in the United States and has a nationally renowned school system. The city is a thriving suburban center close to Washington, D.C., and home to many restaurants, retailers and a flourishing arts and entertainment district.
- 3 References
- Cover Letter
Apply at: https://leidosbiomed.csod.com/ux/ats/careersite/4/home/requisition/3950?c=leidosbiomed
"Please reference job id REQ3950 for all inquiries”
Hiring for this position will be through Leidos Biomedical Research, Inc., a wholly owned subsidiary of Leidos, Inc. Leidos Biomedical is the operations and technical support contractor for the Frederick National Laboratory for Cancer Research, a federal national laboratory.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.15(2); 2023 Feb
- PMC10023071

Clinical Trials and Clinical Research: A Comprehensive Review
Venkataramana kandi.
1 Clinical Microbiology, Prathima Institute of Medical Sciences, Karimnagar, IND
Sabitha Vadakedath
2 Biochemistry, Prathima Institute of Medical Sciences, Karimnagar, IND
Clinical research is an alternative terminology used to describe medical research. Clinical research involves people, and it is generally carried out to evaluate the efficacy of a therapeutic drug, a medical/surgical procedure, or a device as a part of treatment and patient management. Moreover, any research that evaluates the aspects of a disease like the symptoms, risk factors, and pathophysiology, among others may be termed clinical research. However, clinical trials are those studies that assess the potential of a therapeutic drug/device in the management, control, and prevention of disease. In view of the increasing incidences of both communicable and non-communicable diseases, and especially after the effects that Coronavirus Disease-19 (COVID-19) had on public health worldwide, the emphasis on clinical research assumes extremely essential. The knowledge of clinical research will facilitate the discovery of drugs, devices, and vaccines, thereby improving preparedness during public health emergencies. Therefore, in this review, we comprehensively describe the critical elements of clinical research that include clinical trial phases, types, and designs of clinical trials, operations of trial, audit, and management, and ethical concerns.
Introduction and background
A clinical trial is a systematic process that is intended to find out the safety and efficacy of a drug/device in treating/preventing/diagnosing a disease or a medical condition [ 1 , 2 ]. Clinical trial includes various phases that include phase 0 (micro-dosing studies), phase 1, phase 2, phase 3, and phase 4 [ 3 ]. Phase 0 and phase 2 are called exploratory trial phases, phase 1 is termed the non-therapeutic phase, phase 3 is known as the therapeutic confirmatory phase, and phase 4 is called the post-approval or the post-marketing surveillance phase. Phase 0, also called the micro-dosing phase, was previously done in animals but now it is carried out in human volunteers to understand the dose tolerability (pharmacokinetics) before being administered as a part of the phase 1 trial among healthy individuals. The details of the clinical trial phases are shown in Table Table1 1 .
This table has been created by the authors.
MTD: maximum tolerated dose; SAD: single ascending dose; MAD: multiple ascending doses; NDA: new drug application; FDA: food and drug administration
Clinical research design has two major types that include non-interventional/observational and interventional/experimental studies. The non-interventional studies may have a comparator group (analytical studies like case-control and cohort studies), or without it (descriptive study). The experimental studies may be either randomized or non-randomized. Clinical trial designs are of several types that include parallel design, crossover design, factorial design, randomized withdrawal approach, adaptive design, superiority design, and non-inferiority design. The advantages and disadvantages of clinical trial designs are depicted in Table Table2 2 .
There are different types of clinical trials that include those which are conducted for treatment, prevention, early detection/screening, and diagnosis. These studies address the activities of an investigational drug on a disease and its outcomes [ 4 ]. They assess whether the drug is able to prevent the disease/condition, the ability of a device to detect/screen the disease, and the efficacy of a medical test to diagnose the disease/condition. The pictorial representation of a disease diagnosis, treatment, and prevention is depicted in Figure Figure1 1 .

This figure has been created by the authors.
The clinical trial designs could be improvised to make sure that the study's validity is maintained/retained. The adaptive designs facilitate researchers to improvise during the clinical trial without interfering with the integrity and validity of the results. Moreover, it allows flexibility during the conduction of trials and the collection of data. Despite these advantages, adaptive designs have not been universally accepted among clinical researchers. This could be attributed to the low familiarity of such designs in the research community. The adaptive designs have been applied during various phases of clinical trials and for different clinical conditions [ 5 , 6 ]. The adaptive designs applied during different phases are depicted in Figure Figure2 2 .

The Bayesian adaptive trial design has gained popularity, especially during the Coronavirus Disease-19 (COVID-19) pandemic. Such designs could operate under a single master protocol. It operates as a platform trial wherein multiple treatments can be tested on different patient groups suffering from disease [ 7 ].
In this review, we comprehensively discuss the essential elements of clinical research that include the principles of clinical research, planning clinical trials, practical aspects of clinical trial operations, essentials of clinical trial applications, monitoring, and audit, clinical trial data analysis, regulatory audits, and project management, clinical trial operations at the investigation site, the essentials of clinical trial experiments involving epidemiological, and genetic studies, and ethical considerations in clinical research/trials.
A clinical trial involves the study of the effect of an investigational drug/any other intervention in a defined population/participant. The clinical research includes a treatment group and a placebo wherein each group is evaluated for the efficacy of the intervention (improved/not improved) [ 8 ].
Clinical trials are broadly classified into controlled and uncontrolled trials. The uncontrolled trials are potentially biased, and the results of such research are not considered as equally as the controlled studies. Randomized controlled trials (RCTs) are considered the most effective clinical trials wherein the bias is minimized, and the results are considered reliable. There are different types of randomizations and each one has clearly defined functions as elaborated in Table Table3 3 .
Principles of clinical trial/research
Clinical trials or clinical research are conducted to improve the understanding of the unknown, test a hypothesis, and perform public health-related research [ 2 , 3 ]. This is majorly carried out by collecting the data and analyzing it to derive conclusions. There are various types of clinical trials that are majorly grouped as analytical, observational, and experimental research. Clinical research can also be classified into non-directed data capture, directed data capture, and drug trials. Clinical research could be prospective or retrospective. It may also be a case-control study or a cohort study. Clinical trials may be initiated to find treatment, prevent, observe, and diagnose a disease or a medical condition.
Among the various types of clinical research, observational research using a cross-sectional study design is the most frequently performed clinical research. This type of research is undertaken to analyze the presence or absence of a disease/condition, potential risk factors, and prevalence and incidence rates in a defined population. Clinical trials may be therapeutic or non-therapeutic type depending on the type of intervention. The therapeutic type of clinical trial uses a drug that may be beneficial to the patient. Whereas in a non-therapeutic clinical trial, the participant does not benefit from the drug. The non-therapeutic trials provide additional knowledge of the drug for future improvements. Different terminologies of clinical trials are delineated in Table Table4 4 .
In view of the increased cost of the drug discovery process, developing, and low-income countries depend on the production of generic drugs. The generic drugs are similar in composition to the patented/branded drug. Once the patent period is expired generic drugs can be manufactured which have a similar quality, strength, and safety as the patented drug [ 9 ]. The regulatory requirements and the drug production process are almost the same for the branded and the generic drug according to the Food and Drug Administration (FDA), United States of America (USA).
The bioequivalence (BE) studies review the absorption, distribution, metabolism, and excretion (ADME) of the generic drug. These studies compare the concentration of the drug at the desired location in the human body, called the peak concentration of the drug (Cmax). The extent of absorption of the drug is measured using the area under the receiver operating characteristic curve (AUC), wherein the generic drug is supposed to demonstrate similar ADME activities as the branded drug. The BE studies may be undertaken in vitro (fasting, non-fasting, sprinkled fasting) or in vivo studies (clinical, bioanalytical, and statistical) [ 9 ].
Planning clinical trial/research
The clinical trial process involves protocol development, designing a case record/report form (CRF), and functioning of institutional review boards (IRBs). It also includes data management and the monitoring of clinical trial site activities. The CRF is the most significant document in a clinical study. It contains the information collected by the investigator about each subject participating in a clinical study/trial. According to the International Council for Harmonisation (ICH), the CRF can be printed, optical, or an electronic document that is used to record the safety and efficacy of the pharmaceutical drug/product in the test subjects. This information is intended for the sponsor who initiates the clinical study [ 10 ].
The CRF is designed as per the protocol and later it is thoroughly reviewed for its correctness (appropriate and structured questions) and finalized. The CRF then proceeds toward the print taking the language of the participating subjects into consideration. Once the CRF is printed, it is distributed to the investigation sites where it is filled with the details of the participating subjects by the investigator/nurse/subject/guardian of the subject/technician/consultant/monitors/pharmacist/pharmacokinetics/contract house staff. The filled CRFs are checked for their completeness and transported to the sponsor [ 11 ].
Effective planning and implementation of a clinical study/trial will influence its success. The clinical study majorly includes the collection and distribution of the trial data, which is done by the clinical data management section. The project manager is crucial to effectively plan, organize, and use the best processes to control and monitor the clinical study [ 10 , 11 ].
The clinical study is conducted by a sponsor or a clinical research organization (CRO). A perfect protocol, time limits, and regulatory requirements assume significance while planning a clinical trial. What, when, how, and who are clearly planned before the initiation of a study trial. Regular review of the project using the bar and Gantt charts, and maintaining the timelines assume increased significance for success with the product (study report, statistical report, database) [ 10 , 11 ].
The steps critical to planning a clinical trial include the idea, review of the available literature, identifying a problem, formulating the hypothesis, writing a synopsis, identifying the investigators, writing a protocol, finding a source of funding, designing a patient consent form, forming ethics boards, identifying an organization, preparing manuals for procedures, quality assurance, investigator training and initiation of the trial by recruiting the participants [ 10 ].
The two most important points to consider before the initiation of the clinical trial include whether there is a need for a clinical trial, if there is a need, then one must make sure that the study design and methodology are strong for the results to be reliable to the people [ 11 ].
For clinical research to envisage high-quality results, the study design, implementation of the study, quality assurance in data collection, and alleviation of bias and confounding factors must be robust [ 12 ]. Another important aspect of conducting a clinical trial is improved management of various elements of clinical research that include human and financial resources. The role of a trial manager to make a successful clinical trial was previously reported. The trial manager could play a key role in planning, coordinating, and successfully executing the trial. Some qualities of a trial manager include better communication and motivation, leadership, and strategic, tactical, and operational skills [ 13 ].
Practical aspects of a clinical trial operations
There are different types of clinical research. Research in the development of a novel drug could be initiated by nationally funded research, industry-sponsored research, and clinical research initiated by individuals/investigators. According to the documents 21 code of federal regulations (CFR) 312.3 and ICH E-6 Good Clinical Practice (GCP) 1.54, an investigator is an individual who initiates and conducts clinical research [ 14 ]. The investigator plan, design, conduct, monitor, manage data, compile reports, and supervise research-related regulatory and ethical issues. To manage a successful clinical trial project, it is essential for an investigator to give the letter of intent, write a proposal, set a timeline, develop a protocol and related documents like the case record forms, define the budget, and identify the funding sources.
Other major steps of clinical research include the approval of IRBs, conduction and supervision of the research, data review, and analysis. Successful clinical research includes various essential elements like a letter of intent which is the evidence that supports the interest of the researcher to conduct drug research, timeline, funding source, supplier, and participant characters.
Quality assurance, according to the ICH and GCP guidelines, is necessary to be implemented during clinical research to generate quality and accurate data. Each element of the clinical research must have been carried out according to the standard operating procedure (SOP), which is written/determined before the initiation of the study and during the preparation of the protocol [ 15 ].
The audit team (quality assurance group) is instrumental in determining the authenticity of the clinical research. The audit, according to the ICH and GCP, is an independent and external team that examines the process (recording the CRF, analysis of data, and interpretation of data) of clinical research. The quality assurance personnel are adequately trained, become trainers if needed, should be good communicators, and must handle any kind of situation. The audits can be at the investigator sites evaluating the CRF data, the protocol, and the personnel involved in clinical research (source data verification, monitors) [ 16 ].
Clinical trial operations are governed by legal and regulatory requirements, based on GCPs, and the application of science, technology, and interpersonal skills [ 17 ]. Clinical trial operations are complex, time and resource-specific that requires extensive planning and coordination, especially for the research which is conducted at multiple trial centers [ 18 ].
Recruiting the clinical trial participants/subjects is the most significant aspect of clinical trial operations. Previous research had noted that most clinical trials do not meet the participant numbers as decided in the protocol. Therefore, it is important to identify the potential barriers to patient recruitment [ 19 ].
Most clinical trials demand huge costs, increased timelines, and resources. Randomized clinical trial studies from Switzerland were analyzed for their costs which revealed approximately 72000 USD for a clinical trial to be completed. This study emphasized the need for increased transparency with respect to the costs associated with the clinical trial and improved collaboration between collaborators and stakeholders [ 20 ].
Clinical trial applications, monitoring, and audit
Among the most significant aspects of a clinical trial is the audit. An audit is a systematic process of evaluating the clinical trial operations at the site. The audit ensures that the clinical trial process is conducted according to the protocol, and predefined quality system procedures, following GCP guidelines, and according to the requirements of regulatory authorities [ 21 ].
The auditors are supposed to be independent and work without the involvement of the sponsors, CROs, or personnel at the trial site. The auditors ensure that the trial is conducted by designated professionally qualified, adequately trained personnel, with predefined responsibilities. The auditors also ensure the validity of the investigational drug, and the composition, and functioning of institutional review/ethics committees. The availability and correctness of the documents like the investigational broacher, informed consent forms, CRFs, approval letters of the regulatory authorities, and accreditation of the trial labs/sites [ 21 ].
The data management systems, the data collection software, data backup, recovery, and contingency plans, alternative data recording methods, security of the data, personnel training in data entry, and the statistical methods used to analyze the results of the trial are other important responsibilities of the auditor [ 21 , 22 ].
According to the ICH-GCP Sec 1.29 guidelines the inspection may be described as an act by the regulatory authorities to conduct an official review of the clinical trial-related documents, personnel (sponsor, investigator), and the trial site [ 21 , 22 ]. The summary report of the observations of the inspectors is performed using various forms as listed in Table Table5 5 .
FDA: Food and Drug Administration; IND: investigational new drug; NDA: new drug application; IRB: institutional review board; CFR: code of federal regulations
Because protecting data integrity, the rights, safety, and well-being of the study participants are more significant while conducting a clinical trial, regular monitoring and audit of the process appear crucial. Also, the quality of the clinical trial greatly depends on the approach of the trial personnel which includes the sponsors and investigators [ 21 ].
The responsibility of monitoring lies in different hands, and it depends on the clinical trial site. When the trial is initiated by a pharmaceutical industry, the responsibility of trial monitoring depends on the company or the sponsor, and when the trial is conducted by an academic organization, the responsibility lies with the principal investigator [ 21 ].
An audit is a process conducted by an independent body to ensure the quality of the study. Basically, an audit is a quality assurance process that determines if a study is carried out by following the SPOs, in compliance with the GCPs recommended by regulatory bodies like the ICH, FDA, and other local bodies [ 21 ].
An audit is performed to review all the available documents related to the IRB approval, investigational drug, and the documents related to the patient care/case record forms. Other documents that are audited include the protocol (date, sign, treatment, compliance), informed consent form, treatment response/outcome, toxic response/adverse event recording, and the accuracy of data entry [ 22 ].
Clinical trial data analysis, regulatory audits, and project management
The essential elements of clinical trial management systems (CDMS) include the management of the study, the site, staff, subject, contracts, data, and document management, patient diary integration, medical coding, monitoring, adverse event reporting, supplier management, lab data, external interfaces, and randomization. The CDMS involves setting a defined start and finishing time, defining study objectives, setting enrolment and termination criteria, commenting, and managing the study design [ 23 ].
Among the various key application areas of clinical trial systems, the data analysis assumes increased significance. The clinical trial data collected at the site in the form of case record form is stored in the CDMS ensuring the errors with respect to the double data entry are minimized.
Clinical trial data management uses medical coding, which uses terminologies with respect to the medications and adverse events/serious adverse events that need to be entered into the CDMS. The project undertaken to conduct the clinical trial must be predetermined with timelines and milestones. Timelines are usually set for the preparation of protocol, designing the CRF, planning the project, identifying the first subject, and timelines for recording the patient’s data for the first visit.
The timelines also are set for the last subject to be recruited in the study, the CRF of the last subject, and the locked period after the last subject entry. The planning of the project also includes the modes of collection of the data, the methods of the transport of the CRFs, patient diaries, and records of severe adverse events, to the central data management sites (fax, scan, courier, etc.) [ 24 ].
The preparation of SOPs and the type and timing of the quality control (QC) procedures are also included in the project planning before the start of a clinical study. Review (budget, resources, quality of process, assessment), measure (turnaround times, training issues), and control (CRF collection and delivery, incentives, revising the process) are the three important aspects of the implementation of a clinical research project.
In view of the increasing complexity related to the conduct of clinical trials, it is important to perform a clinical quality assurance (CQA) audit. The CQA audit process consists of a detailed plan for conducting audits, points of improvement, generating meaningful audit results, verifying SOP, and regulatory compliance, and promoting improvement in clinical trial research [ 25 ]. All the components of a CQA audit are delineated in Table Table6 6 .
CRF: case report form; CSR: clinical study report; IC: informed consent; PV: pharmacovigilance; SAE: serious adverse event
Clinical trial operations at the investigator's site
The selection of an investigation site is important before starting a clinical trial. It is essential that the individuals recruited for the study meet the inclusion criteria of the trial, and the investigator's and patient's willingness to accept the protocol design and the timelines set by the regulatory authorities including the IRBs.
Before conducting clinical research, it is important for an investigator to agree to the terms and conditions of the agreement and maintain the confidentiality of the protocol. Evaluation of the protocol for the feasibility of its practices with respect to the resources, infrastructure, qualified and trained personnel available, availability of the study subjects, and benefit to the institution and the investigator is done by the sponsor during the site selection visit.
The standards of a clinical research trial are ensured by the Council for International Organizations of Medical Sciences (CIOMS), National Bioethics Advisory Commission (NBAC), United Nations Programme on Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome (HIV/AIDS) (UNAIDS), and World Medical Association (WMA) [ 26 ].
Recommendations for conducting clinical research based on the WMA support the slogan that says, “The health of my patient will be my first consideration.” According to the International Code of Medical Ethics (ICME), no human should be physically or mentally harmed during the clinical trial, and the study should be conducted in the best interest of the person [ 26 ].
Basic principles recommended by the Helsinki declaration include the conduction of clinical research only after the prior proof of the safety of the drug in animal and lab experiments. The clinical trials must be performed by scientifically, and medically qualified and well-trained personnel. Also, it is important to analyze the benefit of research over harm to the participants before initiating the drug trials.
The doctors may prescribe a drug to alleviate the suffering of the patient, save the patient from death, and gain additional knowledge of the drug only after obtaining informed consent. Under the equipoise principle, the investigators must be able to justify the treatment provided as a part of the clinical trial, wherein the patient in the placebo arm may be harmed due to the unavailability of the therapeutic/trial drug.
Clinical trial operations greatly depend on the environmental conditions and geographical attributes of the trial site. It may influence the costs and targets defined by the project before the initiation. It was noted that one-fourth of the clinical trial project proposals/applications submit critical data on the investigational drug from outside the country. Also, it was noted that almost 35% of delays in clinical trials owing to patient recruitment with one-third of studies enrolling only 5% of the participants [ 27 ].
It was suggested that clinical trial feasibility assessment in a defined geographical region may be undertaken for improved chances of success. Points to be considered under the feasibility assessment program include if the disease under the study is related to the population of the geographical region, appropriateness of the study design, patient, and comparator group, visit intervals, potential regulatory and ethical challenges, and commitments of the study partners, CROs in respective countries (multi-centric studies) [ 27 ].
Feasibility assessments may be undertaken at the program level (ethics, regulatory, and medical preparedness), study level (clinical, regulatory, technical, and operational aspects), and at the investigation site (investigational drug, competency of personnel, participant recruitment, and retention, quality systems, and infrastructural aspects) [ 27 ].
Clinical trials: true experiments
In accordance with the revised schedule "Y" of the Drugs and Cosmetics Act (DCA) (2005), a drug trial may be defined as a systematic study of a novel drug component. The clinical trials aim to evaluate the pharmacodynamic, and pharmacokinetic properties including ADME, efficacy, and safety of new drugs.
According to the drug and cosmetic rules (DCR), 1945, a new chemical entity (NCE) may be defined as a novel drug approved for a disease/condition, in a specified route, and at a particular dosage. It also may be a new drug combination, of previously approved drugs.
A clinical trial may be performed in three types; one that is done to find the efficacy of an NCE, a comparison study of two drugs against a medical condition, and the clinical research of approved drugs on a disease/condition. Also, studies of the bioavailability and BE studies of the generic drugs, and the drugs already approved in other countries are done to establish the efficacy of new drugs [ 28 ].
Apart from the discovery of a novel drug, clinical trials are also conducted to approve novel medical devices for public use. A medical device is defined as any instrument, apparatus, appliance, software, and any other material used for diagnostic/therapeutic purposes. The medical devices may be divided into three classes wherein class I uses general controls; class II uses general and special controls, and class III uses general, special controls, and premarket approvals [ 28 ].
The premarket approval applications ensure the safety and effectiveness, and confirmation of the activities from bench to animal to human clinical studies. The FDA approval for investigational device exemption (IDE) for a device not approved for a new indication/disease/condition. There are two types of IDE studies that include the feasibility study (basic safety and potential effectiveness) and the pivotal study (trial endpoints, randomization, monitoring, and statistical analysis plan) [ 28 ].
As evidenced by the available literature, there are two types of research that include observational and experimental research. Experimental research is alternatively known as the true type of research wherein the research is conducted by the intervention of a new drug/device/method (educational research). Most true experiments use randomized control trials that remove bias and neutralize the confounding variables that may interfere with the results of research [ 28 ].
The variables that may interfere with the study results are independent variables also called prediction variables (the intervention), dependent variables (the outcome), and extraneous variables (other confounding factors that could influence the outside). True experiments have three basic elements that include manipulation (that influence independent variables), control (over extraneous influencers), and randomization (unbiased grouping) [ 29 ].
Experiments can also be grouped as true, quasi-experimental, and non-experimental studies depending on the presence of specific characteristic features. True experiments have all three elements of study design (manipulation, control, randomization), and prospective, and have great scientific validity. Quasi-experiments generally have two elements of design (manipulation and control), are prospective, and have moderate scientific validity. The non-experimental studies lack manipulation, control, and randomization, are generally retrospective, and have low scientific validity [ 29 ].
Clinical trials: epidemiological and human genetics study
Epidemiological studies are intended to control health issues by understanding the distribution, determinants, incidence, prevalence, and impact on health among a defined population. Such studies are attempted to perceive the status of infectious diseases as well as non-communicable diseases [ 30 ].
Experimental studies are of two types that include observational (cross-sectional studies (surveys), case-control studies, and cohort studies) and experimental studies (randomized control studies) [ 3 , 31 ]. Such research may pose challenges related to ethics in relation to the social and cultural milieu.
Biomedical research related to human genetics and transplantation research poses an increased threat to ethical concerns, especially after the success of the human genome project (HGP) in the year 2000. The benefits of human genetic studies are innumerable that include the identification of genetic diseases, in vitro fertilization, and regeneration therapy. Research related to human genetics poses ethical, legal, and social issues (ELSI) that need to be appropriately addressed. Most importantly, these genetic research studies use advanced technologies which should be equally available to both economically well-placed and financially deprived people [ 32 ].
Gene therapy and genetic manipulations may potentially precipitate conflict of interest among the family members. The research on genetics may be of various types that include pedigree studies (identifying abnormal gene carriers), genetic screening (for diseases that may be heritable by the children), gene therapeutics (gene replacement therapy, gene construct administration), HGP (sequencing the whole human genome/deoxyribonucleic acid (DNA) fingerprinting), and DNA, cell-line banking/repository [ 33 ]. The biobanks are established to collect and store human tissue samples like umbilical tissue, cord blood, and others [ 34 ].
Epidemiological studies on genetics are attempts to understand the prevalence of diseases that may be transmitted among families. The classical epidemiological studies may include single case observations (one individual), case series (< 10 individuals), ecological studies (population/large group of people), cross-sectional studies (defined number of individuals), case-control studies (defined number of individuals), cohort (defined number of individuals), and interventional studies (defined number of individuals) [ 35 ].
Genetic studies are of different types that include familial aggregation (case-parent, case-parent-grandparent), heritability (study of twins), segregation (pedigree study), linkage study (case-control), association, linkage, disequilibrium, cohort case-only studies (related case-control, unrelated case-control, exposure, non-exposure group, case group), cross-sectional studies, association cohort (related case-control, familial cohort), and experimental retrospective cohort (clinical trial, exposure, and non-exposure group) [ 35 ].
Ethics and concerns in clinical trial/research
Because clinical research involves animals and human participants, adhering to ethics and ethical practices assumes increased significance [ 36 ]. In view of the unethical research conducted on war soldiers after the Second World War, the Nuremberg code was introduced in 1947, which promulgated rules for permissible medical experiments on humans. The Nuremberg code suggests that informed consent is mandatory for all the participants in a clinical trial, and the study subjects must be made aware of the nature, duration, and purpose of the study, and potential health hazards (foreseen and unforeseen). The study subjects should have the liberty to withdraw at any time during the trial and to choose a physician upon medical emergency. The other essential principles of clinical research involving human subjects as suggested by the Nuremberg code included benefit to the society, justification of study as noted by the results of the drug experiments on animals, avoiding even minimal suffering to the study participants, and making sure that the participants don’t have life risk, humanity first, improved medical facilities for participants, and suitably qualified investigators [ 37 ].
During the 18th world medical assembly meeting in the year 1964, in Helsinki, Finland, ethical principles for doctors practicing research were proposed. Declaration of Helsinki, as it is known made sure that the interests and concerns of the human participants will always prevail over the interests of the society. Later in 1974, the National Research Act was proposed which made sure that the research proposals are thoroughly screened by the Institutional ethics/Review Board. In 1979, the April 18th Belmont report was proposed by the national commission for the protection of human rights during biomedical and behavioral research. The Belmont report proposed three core principles during research involving human participants that include respect for persons, beneficence, and justice. The ICH laid down GCP guidelines [ 38 ]. These guidelines are universally followed throughout the world during the conduction of clinical research involving human participants.
ICH was first founded in 1991, in Brussels, under the umbrella of the USA, Japan, and European countries. The ICH conference is conducted once every two years with the participation from the member countries, observers from the regulatory agencies, like the World Health Organization (WHO), European Free Trade Association (EFTA), and the Canadian Health Protection Branch, and other interested stakeholders from the academia and the industry. The expert working groups of the ICH ensure the quality, efficacy, and safety of the medicinal product (drug/device). Despite the availability of the Nuremberg code, the Belmont Report, and the ICH-GCP guidelines, in the year 1982, International Ethical Guidelines for Biomedical Research Involving Human Subjects was proposed by the CIOMS in association with WHO [ 39 ]. The CIOMS protects the rights of the vulnerable population, and ensures ethical practices during clinical research, especially in underdeveloped countries [ 40 ]. In India, the ethical principles for biomedical research involving human subjects were introduced by the Indian Council of Medical Research (ICMR) in the year 2000 and were later amended in the year 2006 [ 41 ]. Clinical trial approvals can only be done by the IRB approved by the Drug Controller General of India (DGCI) as proposed in the year 2013 [ 42 ].
Current perspectives and future implications
A recent study attempted to evaluate the efficacy of adaptive clinical trials in predicting the success of a clinical trial drug that entered phase 3 and minimizing the time and cost of drug development. This study highlighted the drawbacks of such clinical trial designs that include the possibility of type 1 (false positive) and type 2 (false negative) errors [ 43 ].
The usefulness of animal studies during the preclinical phases of a clinical trial was evaluated in a previous study which concluded that animal studies may not completely guarantee the safety of the investigational drug. This is noted by the fact that many drugs which passed toxicity tests in animals produced adverse reactions in humans [ 44 ].
The significance of BE studies to compare branded and generic drugs was reported previously. The pharmacokinetic BE studies of Amoxycillin comparing branded and generic drugs were carried out among a group of healthy participants. The study results have demonstrated that the generic drug had lower Cmax as compared to the branded drug [ 45 ].
To establish the BE of the generic drugs, randomized crossover trials are carried out to assess the Cmax and the AUC. The ratio of each pharmacokinetic characteristic must match the ratio of AUC and/or Cmax, 1:1=1 for a generic drug to be considered as a bioequivalent to a branded drug [ 46 ].
Although the generic drug development is comparatively more beneficial than the branded drugs, synthesis of extended-release formulations of the generic drug appears to be complex. Since the extended-release formulations remain for longer periods in the stomach, they may be influenced by gastric acidity and interact with the food. A recent study suggested the use of bio-relevant dissolution tests to increase the successful production of generic extended-release drug formulations [ 47 ].
Although RCTs are considered the best designs, which rule out bias and the data/results obtained from such clinical research are the most reliable, RCTs may be plagued by miscalculation of the treatment outcomes/bias, problems of cointerventions, and contaminations [ 48 ].
The perception of healthcare providers regarding branded drugs and their view about the generic equivalents was recently analyzed and reported. It was noted that such a perception may be attributed to the flexible regulatory requirements for the approval of a generic drug as compared to a branded drug. Also, could be because a switch from a branded drug to a generic drug in patients may precipitate adverse events as evidenced by previous reports [ 49 ].
Because the vulnerable population like drug/alcohol addicts, mentally challenged people, children, geriatric age people, military persons, ethnic minorities, people suffering from incurable diseases, students, employees, and pregnant women cannot make decisions with respect to participating in a clinical trial, ethical concerns, and legal issues may prop up, that may be appropriately addressed before drug trials which include such groups [ 50 ].
Conclusions
Clinical research and clinical trials are important from the public health perspective. Clinical research facilitates scientists, public health administrations, and people to increase their understanding and improve preparedness with reference to the diseases prevalent in different geographical regions of the world. Moreover, clinical research helps in mitigating health-related problems as evidenced by the current Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) pandemic and other emerging and re-emerging microbial infections. Clinical trials are crucial to the development of drugs, devices, and vaccines. Therefore, scientists are required to be up to date with the process and procedures of clinical research and trials as discussed comprehensively in this review.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
- Earn $10 rewards on $35+ healthy summer essentials
- BOGO 50% off select skin care & sun care
- Up to 60% off clearance
- Your Account
- Walgreens Cash Rewards
- Prescription Refills & Status
- Vaccination Records
- Order Status & History
- Buy It Again
Select a store
Home > Health Services
Clinical Trials
Everyone's welcome to join a clinical trial—no experience required..

Featured clinical trials

Major depressive disorder
Learn more about a clinical study for adults diagnosed with depression.

Lung cancer screening
Help develop a test that detects lung cancer early, when more treatment options are available.

HIV prevention
Study to improve HIV prevention for Black women.
All clinical trials
Medical breakthroughs wouldn’t be possible without clinical trials..
By including people of various communities, ages, races, genders and ethnicities, researchers can better understand how people respond to treatments. This helps to ensure that medical advancements are safe, effective, and accessible, ultimately leading to more equitable healthcare for all.
Joining a clinical trial doesn’t mean you’ll be a guinea pig or receive low-quality care.
Patient care and safety is prioritized above all else. You’re able to stop participation at any time, and you’ll never be treated as a lab rat or guinea pig. Here are just a few of the additional benefits research participants experience by joining clinical trials:
Better understand your health & condition
Access to the latest treatments & technology
Condition-specific care from trusted sources
Directly contribute to medical breakthroughs
Do you have questions about clinical trials or want to share your participant story?
We want to hear from you! Email us at [email protected] or connect with us on any of the social channels below.
Note: This contact form is for general inquiries only. Do not use the form to share medical information or communicate about specific ongoing studies. Contact the research team directly if you have questions about a specific study.
Clinical trials are research studies that look at new ways to prevent, detect or treat diseases. They rely on human volunteers, known as participants , and aim to find out if a new medical approach is safe and effective. Clinical trials often test new drugs or new combinations of drugs, new surgical procedures or devices, or different ways to use existing treatments. Clinical trials can also test other aspects of care, such as ways to improve the quality of life for people with chronic conditions.
People of all ages, genders, races and ethnic groups can volunteer to participate, and all participants must qualify for a study before joining a clinical trial. Each clinical trial has specific eligibility criteria , which help identify appropriate participants and ensure their safety. Eligibility criteria consist of both inclusion criteria (required for participation) and exclusion criteria (which prevent participation). These criteria are based on factors such as age, gender, disease type and stage, treatment history and other medical conditions. Some studies look for patient volunteers who have a known health condition and can help improve understanding, diagnosis or treatment, while others need healthy volunteers who don’t have significant health problems and may be needed to help test new treatments.
Each clinical trial has a unique set of requirements and screening process. Before joining a trial, you’ll receive an informed consent form detailing the study’s purpose, duration, compensation, contact information, and required tests or procedures. The form also explains the study’s known benefits and risks and can help participants make an informed decision about whether to join a study. Study participation is a personal decision. The research team will be prepared to answer questions to help you fully understand what to expect before, during and after the study
- Phase 1 – Testing begins with a small group of people (typically 20–80) to help evaluate safety and identify side effects.
- Phase 2 – More people are included (typically 100–300) to determine effectiveness and continue evaluating safety.
- Phase 3 – The new drug or treatment is given to a larger group of people (typically 1,000–3,000) to help confirm effectiveness, monitor side effects and compare it with current treatments.
- Phase 4 – After a drug or device is approved by the FDA and made available to the public, researchers continue monitoring its safety and effectiveness in the general population.
A clinical trial sponsor is the person, institution, company or organization responsible for initiating, managing or financing the clinical trial. A study’s principal investigator (PI) , often a medical doctor, works closely with the clinical research coordinator (CRC) to lead the trial and help ensure compliance and safety. The PI and CRC typically work for the sponsor and leads a team of research staff that could include doctors , nurses , technicians and other healthcare professionals. Members of the research team regularly monitor participants’ health to determine the study’s safety and effectiveness.
Walgreens has partnered with trial sponsors and researchers to help make clinical trials more accessible. Browse our active clinical trials and select “Learn more” to see how you can get involved.
Insurance is generally not required to participate in a clinical trial.
Participants may be paid, but the amount varies based on the study. Some clinical trials will provide compensation for the time and effort involved with participation, and reimbursement for costs associated with research-related travel, such as parking fees or meals.
Yes. You can decide to end your participation and withdraw at any time. Just tell the study researcher if you wish to stop being in the study. You don’t need to give a reason why you want to stop participating.
There are different types of clinical trials; they don’t always test medicine or include a placebo , which is a pill or substance that has no therapeutic effect. Placebos aren’t used in research if an effective treatment is already available or if the lack of effective therapy would put a person at risk. Participants will always be notified if a trial uses a placebo during the informed consent process .
Clinical trial participant privacy and confidentiality are protected by law. For example, trials that use or disclose a participant’s protected health information (PHI) for research purposes must be conducted in accordance with the Privacy Rule of the Health Insurance Portability and Accountability Act (HIPAA) . During the informed consent process, participants will receive information on personal information collected during the trial, how it will be used, who will have access to the information, and rights regarding personal information.
Factors like age, race and gender influence the risk and likelihood of developing a disease and responses to treatment. By including more diverse groups of people in clinical trials, we can better understand the therapy effectiveness and safety for the broader population.
Better understand how you can engage with clinical trials, no matter where you are in their health journey, from trusted sources.
National Institutes of Health: Clinical Research Trials and You
FDA: Clinical Trial Diversity
Patient Advocate Foundation: Education Resource Library
FDA: "Medical Device Clinical Trials" (video)
The Center for Information and Study on Clinical Research Participation: Education Center
NoiseFilter: "Clinical Trials: The Heart and Soul of Science" (video)
NoiseFilter: "Clinical Trials: Lights, Camera, Take Action!" (video)
University at Buffalo Clinical and Translational Science Institute: "Sofia Learns About Research" (children's activity book)
Walgreens Clinical Trials for sponsors: Clinical Trials for Healthcare Companies
https://www.nih.gov/health-information/nih-clinical-research-trials-you/basics
https://www.nia.nih.gov/health/clinical-trials-and-studies/what-are-clinical-trials-and-studies
https://www.nhlbi.nih.gov/research/clinical-trials/participating
https://www.nih.gov/health-information/nih-clinical-research-trials-you/glossary-common-terms
https://clinicaltrials.gov/study-basics/learn-about-studies
https://clinicaltrials.gov/study-basics/glossary
https://www.hhs.gov/hipaa/for-professionals/special-topics/research/index.html
https://www.nimhd.nih.gov/resources/understanding-health-disparities/diversity-and-inclusion-in-clinical-trials.html
In a conversation with Ramita Tandon, Walgreens’ Chief Clinical Trials Officer, we explore the company’s cutting-edge strategies to maximize its expansive pharmacy network for clinical trials conducted at retail pharmacies. This interview illuminates the ways in which Walgreens integrates technological advancements and engagement tactics to revolutionize the process of enrolling participants and decentralizing clinical trials.
Collaborating with community partners, we design and build outreach and engagement activations to break down barriers e.g. the lack of awareness about trials, mistrust of research, and logistical obstacles that prevent equitable access across sub-groups.
The 2024 SCOPE Summit, a pivotal event in clinical trials, showcased innovative strategies to transform patient recruitment and trial conduct. At the forefront of these discussions was John Campbell, Head of Decentralized Trials for Walgreens, who shared profound insights
Less than two years after entering the clinical trials business, Walgreens has made strides in increasing diverse representation in pharmaceutical trials, according to Ramita Tandon, the company’s chief clinical trials officer. The retail pharmacy giant opened a clinical trials arm in June 2022 with the goal of diversifying trial participants, which have historically been overwhelmingly white and male.
Clinical trial recruitment has historically been centered in larger urban areas near established medical institutions, leaving out the nearly 61 million Americans that live in rural locations where healthcare access is limited. Walgreens is proud to be an industry leader in increasing access to clinical research across the country, and is committed to reaching vulnerable and marginalized populations that are often excluded from
Diverse participation in clinical trials is critically low. At this year’s Black Women’s Expo, Walgreens sponsorship centered on educating the community and sparking change for better health outcomes.
In this session at DIA, John Campbell, head of decentralized trials, Walgreens began with emphasizing that much of industry does not yet understand the role of pharmacy in clinical trials.
Retail pharmacy giant Walgreens inked another partnership to recruit participants for research as it continues to build out its clinical trials business. The company signed a deal with biotech startup Freenome to advance clinical trials of its blood-based tests for the early detection of cancer.
The commitment of pharmacy to address clinical trials has been a recurring topic this week. Craig Lipset was joined by Kendal K. Whitlock, MPH, Head of Digital Optimization, RWE Clinical Trials from Walgreens to discuss the state of the industry, commitment to the field, and progress to-date.
In the digital age, technology has become an important tool to improve involvement in clinical trials. Watch this discussion with Kendal Whitlock, Head of Digital Operations at Walgreens Boot Alliance
Walgreens Clinical Trials Events
Leveraging rich real world data (RWD), Walgreens delivered patient referrals that improve on the national averages of diverse participation, also demonstrating that our referrals are 20% more likely to enroll in study compared to other recruitment providers' referrals.
Walgreens leveraged rich and diverse real world data (RWD) and was able to deliver referrals that exceeded the targeted goal of our study partner by 23%, all within a two-week recruitment period.
Walgreens identified and engaged the right patients using our unparalleled RWD to enable a significant recruitment of Black/African American (17%) and Hispanic/Latino (19%) population in this clinical trial. These results are a significant improvement when compared to nationwide historical averages of clinical trial participation (as of 2020 of 8% and 11% respectively).
Walgreens leveraged their unparalleled real-world data insights to identify eligible patient populations within 20 miles of study sites.
Walgreens leveraged our unparalleled patient insights and community presence to identify and engage potential participants so efficiently that we outperformed other recruitment partners--64% of qualified referrals and 62% of randomized patients.
You're leaving Walgreens
Any information you provide will be subject to our partner’s privacy and security policies.
You're about to enter W en español
NIH VideoCasting
CIT can broadcast your seminar, conference or meeting live to a world-wide audience over the Internet as a real-time streaming video. The event can be recorded and made available for viewers to watch at their convenience as an on-demand video or a downloadable file. CIT can also broadcast NIH-only or HHS-only content.
VideoCast Send Live Feedback
INCLUDE Webinar- Community Engagement in Clinical Trials: Lessons from PCORI
- Open access
- Published: 24 May 2024
Efficacy and safety of autologous or allogeneic mesenchymal stromal cells from adult adipose tissue expanded and combined with tricalcium phosphate biomaterial for the surgical treatment of atrophic nonunion of long bones: a phase II clinical trial
- Lluís Orozco Delclós ORCID: orcid.org/0000-0002-9339-8702 1 ,
- Robert Soler Rich 1 ,
- Rafael Arriaza Loureda 2 ,
- Alonso Moreno García 3 &
- Enrique Gómez Barrena 3 , 4
Journal of Translational Medicine volume 22 , Article number: 493 ( 2024 ) Cite this article
Metrics details
Autologous bone grafting is the standard treatment for the surgical management of atrophic nonunion of long bones. Other solutions, such as bone marrow mesenchymal stem cells (BM-MSC) combined with phospho-calcium material, have also been used. Here we evaluate the safety and early efficacy of a novel procedure using autologous or allogenic adipose tissue mesenchymal stromal cells (AT-MSC) seeded in a patented tricalcium phosphate-based biomaterial for the treatment of bone regeneration in cases of atrophic nonunion.
This was a prospective, multicentric, open-label, phase 2 clinical trial of patients with atrophic nonunion of long bones. Biografts of autologous or allogenic AT-MSC combined with a phosphate substrate were manufactured prior to the surgical procedures. The primary efficacy was measured 6 months after surgery, but patients were followed for 12 months after surgery and a further year out of the scope of the study. All adverse events were recorded. This cohort was compared with a historical cohort of 14 cases treated by the same research team with autologous BM-MSC.
A total of 12 patients with atrophic nonunion of long bones were included. The mean (SD) age was 41.2 (12.1) years and 66.7% were men. Bone healing was achieved in 10 of the 12 cases (83%) treated with the AT-MSC biografts, a percentage of healing similar (11 of the 14 cases, 79%) to that achieved in patients treated with autologous BM-MSC. Overall, two adverse events, in the same patient, were considered related to the procedure.
Conclusions
The results of this study suggest that AT-MSC biografts are safe for the treatment of bone regeneration in cases of atrophic nonunion and reach high healing rates.
Trial registration
Study registered with EUDRA-CT (2013-000930-37) and ClinicalTrials.gov (NCT02483364).
About 2.2 million bone grafting procedures are performed annually worldwide [ 1 ]. Patients with atrophic nonunion (fractures which do not heal within 8 months, characterized by the absence of “bone callus” and the interposition of fibrous tissue between both segments) often require autologous bone grafting, as this treatment provides an osteoconductive structure, progenitor cells, morphogenic proteins, and growth factors that induce angiogenesis and osteogenesis [ 2 ]. These elements, together with mechanical stability, are essential for the healing of fractures [ 3 ]. During the past decade various surgical approaches have been tested to treat atrophic nonunions, although there is no consensus on which one is the best technique for any specific situation [ 4 , 5 , 6 , 7 , 8 , 9 ]. Autologous cancellous bone grafting is still considered the ‘gold standard’ [ 10 , 11 , 12 ], but the process of obtaining the autologous graft increases the surgery time and has an associated morbidity, such as persistent pain in the donor area [ 13 , 14 ]. The iliac bone is usually the autologous bone graft donor site, but it can only provide a limited volume of tissue, especially if it has been previously used [ 15 ]. To solve the inconveniences of the surgical procedure and the low content of progenitor cells of autologous bone grafting, other alternatives such as allogeneic, cryopreserved, or lyophilized bone have been used. These options, although acellular, have osteoconducting properties and carry osteoinductive molecules.
Some clinical trials have focused on the application of mesenchymal stromal cells (MSCs) or MSC-based bone tissue on bone regeneration [ 16 , 17 ]. Systems based on processing and enrichment of autologous or allogenic progenitor cells could potentially achieve bone regeneration at the nonunion site in a shorter time, avoiding the risks and disadvantages of graft-obtaining surgery. Recently, a new therapeutic alternative was developed consisting of an osteoconductive structure colonized by high doses of cultured adipose tissue-derived mesenchymal stromal cells (AT-MSC) pre-differentiated to the osteoblastic line. Adipose tissue is considered an easy-to-access tissue and can be obtained with a low incidence of comorbidity. AT-MSC have a multi-lineage potential that is very similar to BM-MSC [ 18 , 19 ], have been found to have similar expression of phenotypic markers [ 20 ]. However, in some patients it is not possible to obtain sufficient adipose tissue to have an adequate amount of AT-MSC with therapeutic potential (in the differentiation phase and releasing secretome). The alternative to an autologous graft procedure is the use of cryopreserved banks of allogeneic AT-MSC from adipose tissue of healthy donors. This alternative system based on biomaterial designed for bone regeneration purposes, totally recovered with allogenic AT-MSC, would provide a tissue-engineering biograft product with which to accelerate the formation of new and stable bone at the site of the nonunion. The use of this system would avoid the disadvantages of surgery for obtaining the graft. Here we present the results of a multicenter phase II trial to evaluate this approach and the feasibility and safety of a novel biograft product based on these principles in humans.
Materials and methods
This was a prospective, multicentric, open-label, phase 2 clinical trial for the surgical treatment of atrophic nonunion of long bones using adult autologous or allogeneic AT-MSC associated with tricalcium phosphate biomaterial. See Supplementary Materials for brief additional information on the pre-clinical studies on which this study was based. The study was conducted from 2 June 2015 to 20 October 2020 at the Institut de Teràpia Regenerativa Tissular (Centro Médico Teknon, Barcelona, Spain) and the Hospital Universitario La Paz (Madrid, Spain). The trial followed the criteria of the Osteosynthesis Association for the Study of Internal Fixation (AO-ASIF) and was authorized by the Ethics Committee of the Quirón Salud Hospital Group (protocol STEMQUIRI/12ES01, on 24 May 2018) and the Spanish Agency for Medicines and Health Products (AEMPS). Informed consent was obtained from all patients. The trial was conducted in accordance with the US Health Insurance Portability and Accountability Act (HIPAA) and the Declaration of Helsinki. It was registered with EudraCT number as 2013-000930-37 and with ClinicalTrials.gov as NCT02483364.
Subjects were recruited between 18 and 65 years of age of both sexes, diagnosed with radiographically confirmed atrophic nonunion of a long bone. Patients were excluded if presenting any infection, other lesions interfering with weight bearing, open pseudoarthrosis at the time of inclusion, congenital bone diseases (hypophosphatemia), metabolic bone disease associated with primary or secondary hypoparathyroidism, or other conditions or circumstances according to medical criteria.
Study procedures
See Supplementary Methods for full details of scheduled visits applicable to the 6 patients treated with the autologous (Table S1 ) and the 6 patients treated with allogeneic (Table S2 ) biografts. For patients treated with the autologous biograft product an outpatient surgery visit was arranged for the aspiration of adipose tissue plus biograft application surgery 36 days later (time required for isolation, expansion and seeding the cells in the biomaterials, final product preparation and referral to the hospital). Patients who were treated with the allogeneic biograft product did not have the outpatient surgery visit. There was a period of 15–18 days from the screening visit to the visit for the surgical implantation (time required to process the product and have the appropriate dose of biograft). Once they have had the surgery, the patients were followed up for safety and efficacy (by way of X-rays) as established in the visit schedule (Tables S1 and S2 ).
Autologous AT-MSC processing
The AT-MSC were obtained from 100 mL of lipoaspirate from the abdominal wall obtained under local anesthesia and sedation. The cells were processed under controlled temperature conditions (2–6ºC) at the Histocell laboratories (Derio, Spain) under good manufacturing practice (GMP) standards for clinical application. The AT-MSC were isolated through an enzymatic digestion process and their number amplified using the usual media in cell culture processes. When the culture in passage 3 reaches semiconfluency, a specific osteogenic pre-differentiating medium (STEMPRO® Osteogenic Differentiation Kit; Gibco Life Technologies) was added to the cells and the culture maintained for 8 days.
Allogeneic AT-MSC processing
All the implanted allogeneic cells derived from a Master Cell Bank (MCB) of MSC obtained from the same donor (See Supplementary Methods for details on donor selection, and Table S3 ). The manufacturing process included the generation of a cryopreserved working cell bank (WCB) at passage 3. Once the patient was recruited and surgery scheduled, enough cells for product manufacturing were thawed, and once reached semiconfluency, cells were pre-differentiated for 8 days using specific GMP culture media (STEMPRO® Osteogenic Differentiation Kit, Gibco Life Technologies) (See Supplementary Methods for determination of differentiation time).
Final product preparation and quality control
The pre-differentiated cells were seeded on cylindrical matrices (CMT) 3 to 5 mm in size and kept for 4 days to ensure adherence. The matrices were composed of β-tricalcium phosphate (F 1088 standard specification for β-tricalcium phosphate for surgical implantation, Sigma-Aldrich), monobasic calcium phosphate (Budenheim Aldrich), calcium carbonate (Ph. Eur. Specifications, Sigma-Aldrich) and sodium pyrophosphate authorized as a food additive by the FDA (BK Giulini GmbH Aldrich). The porosity of the biomaterial was adequate for the colonization of each CMT with 4 ± 0.8 × 10 5 AT-MSC. Pre-differentiated AT-MSCs maintained the phenotypic and immunophenotypic characteristics required by the criteria defined by the International Society for Cellular Therapy for MSC: (1) in vitro adherent fibroblastoid morphology; (2) Expression of the CD105, CD73 and CD90 antigens with the absence of hematopoietic markers such as CD45, CD34, CD14 or CD11b, CD79 or CD19 and HLA-DR; and (3) Ability to differentiate in vitro into osteoblasts, adipoblasts, and chondroblasts (See Supplementary Methods for details on characterization) [ 21 ]. Subsequent steps included determining the immunophenotype and the viability of the cells in their final container (See Supplementary Materials Table S4 for immunophenotypic characterization and support adhesion of batches used), carrying out genetic stability tests, calculating the total number of generations and ensuring the microbiological quality by means of sterility, mycoplasma, bacterial endotoxin and GRAM control tests. The cells were supplied to the surgical team ready for application in syringes containing 10 CMT in a gelled medium (Ringer’s lactate, 82.8%; Gelita-Spon® gelatine, 5.7%; 33 g/L glucosaline solution, 2.9%; 1.4% sodium bicarbonate, 8.6%) which ensures the viability of the cells that colonize the interior and exterior of the biomaterial for which they show great affinity. Each syringe with 10 CMT provided a dose of 4 ± 0.8 × 10 6 AT-MSC (See Fig. S1 ). The total dose to be used was determined by the volume of the lesion (Table S5 ). A representative example of the cellular response in the matrix over time is shown in Fig. S2 . This product is patented (PCT/ES2009/000358) by Histocell SL (Spain).
Endpoints and assessments
The primary objective of this study was to assess safety of the intervention and, additionally, the efficacy by radiographic criteria within 6 months after surgery. The assessment included evaluation of the clinical evolution of the lesion, distal trophism, vascular status, healing, joint balance, comparative measurement of muscle circumference in both legs or arms, deviations, and rotations. The evolution of the lesion was determined by radiographs in frontal and profile projections and computed tomography scans were conducted only when necessary to avoid unnecessary exposure given the accumulation of previous radiographic examinations of the patients in the trial. The radiographs were consistently assessed for efficacy by the surgeon following the accepted radiographic criteria. The efficacy of the intervention using radiographic criteria was determined by evidence of “callus” formation in 3 of 4 cortices (humerus, ulna, radius or femur) or 2 of 3 cortices (tibia) within 6 months of surgery. The variable used was the time in weeks from surgery to “callus” formation determined using radiographic criteria for fracture healing. Any adverse events (AEs) occurring during the conducting of the trial, either observed by the investigator or reported by subjects themselves, were recorded.
The secondary objective was the comparison of the incidence of AEs, complications, and healing times, between the 12 patients in this study and the data from a historic cohort of 14 consecutive cases of refractory long bone nonunion who have been treated since 2009 with a fixed cell dose of 40 × 10 6 autologous bone marrow MSC (BM-MSC), expanded at the Institute of Molecular Biology and Genetics (IBGM) of Valladolid (Spain), corresponding to authorized compassionate use treatments, and subject to AEMPS control after the clinical trial on nonunion treated with stem progenitor cells (registered with EUDRA-CT number 2005-001755-38). The BM-MSC were included in 3–5 mm chips of lyophilized allogeneic cancellous bone in a quantity adjusted to the specific treatment and the whole included in an autologous fibrin clot. To comply with the characteristics required by the International Society for Cell & Gene Therapy [ 21 ], they were delivered to the surgical team in a suspension of lactated Ringer, 0.2% human albumin and 5 mM glucose. The components were mixed in the operating room just prior to implantation.
A total of 12 patients with atrophic nonunion were included in the trial ( Table 1 ) . Of the 12 patients, 8 (66.7%) were men. Mean (± SD) age was 41.2 ± 12.11 years with a range of 19 to 58 years. On average the patients had had 1.9 surgeries prior to the inclusion to the study. The most common site of the lesion for the randomized patients overall was the tibia (41.7%); the rest of locations were femur and humerus with a 16.7% each, and ulna, clavicle and first metatarsal with an 8.3% each. Autologous AT-MSC were applied in the first 6 cases treated, which involved a first stage consisting of cell obtention through liposuction. Allogeneic AT-MSC were used in the other 6 cases. The maximum applied cell dose was 56 × 10 6 AT-MSC in a femur and the lowest 6 × 10 6 AT-MSC in a metatarsal.
Safety was evaluated throughout the 12 months of study duration, with a total of 36 AEs reported (Table 2 ). Only 2 of them were considered related to the procedure (2 cases of device expulsion in the same patient). One patient withdrew from the study after 9 months due a non-related serious AE (device loosening). No other serious AE was observed in any study patient. In the cases involving liposuction, the patients tolerated the procedure well in terms of pain and the only remarkable AE was a diffuse ecchymosis along the abdominal wall that persisted for a few weeks. In the case of allogenic biografts, the designed procedure was shown to be viable and no relevant AEs attributable to the biografts were observed.
The main efficacy variable was bone healing, assessed by radiographic criteria within 6 months after surgery, and was achieved in 10 of the 12 treated cases (83%). A representative example of a case treated with allogenic AT-MSC is shown in Fig. 1 .

Example of use of allogeneic AT-MSC in a tibia nonunion (patient 1-14-10). X-ray anteroposterior ( A and C ) and lateral projections ( B and D ). A and B show fracture before and after surgery; C and D show the follow up until month 12. The 54-year-old female patient initially presented an open tibial pilon fracture treated with external fixation and minimal osteosynthesis with Kirschner wires. Sepsis was confirmed three weeks later, which was treated with debridement, circumferential fixation, and antibiotics. After three months the external fixation was withdrawn and replaced by a cast, and subsequently by a removable orthosis without load. The fracture evolved into a nonunion. Thirteen months after the initial fracture, and after ruling out a septic process, curettage of the wound was conducted. Once an acceptable anatomical realignment had been achieved, a large space lost bone was observed inside the metaphysis; the contact area between segments was very limited and only on the internal slope of the tibia. Stability was achieved with a lag screw and an anterolateral distal tibial plate overlying it. An allogenic biograft consisting of 98 monetites was applied, with a total dose of 39 × 10 6 AT-MSC. The patient presented a good postoperative course, without inflammatory signs, and was discharged from the hospital after 4 days without immobilization. Healing was verified at 6 months, with gait being painless and presenting only limited joint balance in dorsiflexion (5º)
The historical cohort of 14 consecutive cases was treated by the same research group with a fixed cell dose of 40 × 10 6 MSC from autologous bone marrow mesenchymal stromal cells (BM-MSC) ( Table 3 ) . In this cohort, bone healing was observed in 11 of the 14 treated cases (79%).
The study included an additional 24-month safety visit requested by the regulatory agency. During this period, one non-related AE (removal of two long screws of the osteosynthesis) and one non-related serious AE (tibial fracture) were observed. This last case was considered radiographically healed at the 6-month visit, but it could have been a misinterpretation of the diagnosis of healing or a refracture due to the fragility of the achieved bone callus. These 2 AEs do not change the global safety conclusions of the study.
The results from this phase 2 study suggest that the treatment of atrophic nonunion of long bones by means of cultured MSC derived from autologous or allogeneic fat cells is safe and offers an efficacy rate comparable to that achieved with surgical techniques involving other techniques, such as the use of cultured BM-MSC. With this alternative system based on the amplification in culture of AT-MSC and its inclusion in biomaterial the drawbacks of surgery to obtain bone autograft were avoided.
In this study we used autologous and allogenic AT-MSC biografts. The manufacturing process of the allogeneic bone obtained from a healthy donor was identical to the autologous one. The difference lies in the use of a MCB and WCB from the same donor that includes cryopreservation and thawing steps before final product preparation, which was not carried out for the autologous product. The obvious advantage of using allogeneic MSC is that it spared for the patient the surgical procedure associated with the adipose tissue sample obtention, performed under anesthesia, and the 36-day treatment delay, which is the time invested in the production of the biograft. As the autologous AT-MSC biografts require considerable time before they are available for use, they are not suitable for acute diseases or those requiring rapid treatment [ 22 ]. The alternative use of allogeneic AT-MSC, obtained from healthy donor adipose tissue and cryopreserved, overcomes these problems. In either case, the autologous or the allogeneic AT-MSC biografts contain a high dose of progenitor cells. Also, in the case of allogenic biografts, the possibility of an immune reaction is reduced, as it has been widely demonstrated that MSC evade antigenic recognition and also inhibit immune responses, being tolerated even if the cells are intravenously administered at a large dose. Due to their immunomodulatory potential, AT-MSC have been applied in graft versus host disease or autoimmune diseases, including against the cytokine storm linked to SARS-CoV-2 infection [ 23 ].
The procedure to obtain autologous adipose tissue can be more complex in elderly patients or patients with a low body fat index, or in patients with diabetes, rheumatoid arthritis, or systemic lupus erythematosus.
The autologous or allogenic AT-MSC were included in a biomaterial whose composition and form was designed to exert a retentive effect on the cells and facilitate their proliferation. The biomaterial was suspended in a gelatinous solution with compacting effect and supplied to the surgeons in a ready-to-use syringe. Therefore, it did not require any additional manipulation during surgery, except for appropriate distribution in the nonunion site treated, avoiding excessive compaction that would compromise cell viability and the conductivity and revascularization of the biograft.
The structure and composition of the biomaterial are key factors to achieve bone regeneration. Unlike TCP granules already on the market, monetite (TCP derivative) interacts better with host bone cells and their physical structure includes homogeneously distributed microporosity and transversal macro channels that go across entire cylinders and that are totally colonized by MSC. This strategy provides a better interaction in the implanted area, creating a microenvironment that facilitates new tissue revascularization [ 24 , 25 ].
The comparison between the cases treated with AT-MSC and the historical series treated by the same surgical team with a fixed dose of 40 × 10 6 autologous BM-MSC revealed a similar rate of efficacy and safety, even though a significantly lower cell dose was implanted in 9 of the 12 cases. Positive results were also obtained in the international, non-comparative multicenter clinical trial that included 28 patients in which much higher cell doses (100 to 200 × 10 6 ) of autologous BM-MSC were used in 5–10 cc of phospho-calcium material [ 26 ]. The variability of the cases, protocols and surgical procedures preclude any precise comparison between the present work and other pilot studies, but favorable results may be obtained by different techniques. Hernigou reported good results by simply inoculating a bone marrow concentrate at the focus of the atrophic nonunion of the tibia without further surgery such as osteosynthesis or even the replacement of osteosynthesis material, which are necessary actions in most nonunion cases [ 27 ].
One of the limitations of this study is the small sample size and the heterogeneity in the distribution of cases, but this is a common problem in early studies that treat a condition such as atrophic nonunion that involves a surgical intervention.
The described procedure using both autologous and allogeneic AT-MSC, pre-differentiated to the osteoblastic line and incorporated into special designed calcium phosphate-based biomaterials, suggests that it is safe for use in humans for the treatment of atrophic nonunion. A major advantage of this technology is to avoid a bone graft, which is the standard therapy for long bone nonunion. We believe that the results from this clinical trial also confirm the feasibility of manufacturing and delivering AT-MSC-based biografts. The analysis of the adverse effects reported suggest that their use is safe in cases requiring bone grafting. Taken together, the results suggest that further studies are needed to explore of the efficacy of the procedures described.
Data availability
The datasets supporting the findings of this study are available from the corresponding author upon reasonable request.
Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005;36(Suppl 3):S20–27.
Article PubMed Google Scholar
Nicholson JA, Makaram N, Simpson A, Keating JF. Fracture nonunion in long bones: a literature review of risk factors and surgical management. Injury. 2021;52(Suppl 2):S3–11.
Giannoudis PV, Kontakis G. Treatment of long bone aseptic non-unions: monotherapy or polytherapy? Injury. 2009;40:1021–2.
Yin P, Zhang L, Li T, Zhang L, Wang G, Li J, et al. Infected nonunion of tibia and femur treated by bone transport. J Orthop Surg Res. 2015;10:49.
Article PubMed PubMed Central Google Scholar
Han F, Peter L, Lau ETC, Thambiah J, Murphy D, Kagda FHY. Reamer Irrigator Aspirator bone graft harvesting: complications and outcomes in an Asian population. Injury. 2015;46:2042–51.
Napora JK, Weinberg DS, Eagle BA, Kaufman BR, Sontich JK. Hexapod Stacked Transport for Tibial infected Nonunions with Bone loss: long-term functional outcomes. J Orthop Trauma. 2018;32:e12–8.
Raven TF, Moghaddam A, Ermisch C, Westhauser F, Heller R, Bruckner T, et al. Use of Masquelet technique in treatment of septic and atrophic fracture nonunion. Injury. 2019;50(Suppl 3):40–54.
Le Baron M, Vivona J-P, Maman P, Volpi R, Flecher X. Can the Reamer/Irrigator/Aspirator System replace anterior iliac crest grafting when treating long bone nonunion? Orthop Traumatol Surg Res. 2019;105:529–33.
Pak H, Kim SH, Won SG, Ri SG, Jang MG, Ri JS, et al. Tibialis Anterior muscle: pedicled bone graft for defect of the Tibia. Indian J Orthop. 2022;56:862–6.
Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol. 2015;11:45–54.
Fillingham Y, Jacobs J. Bone grafts and their substitutes. Bone Joint J. 2016;98–B:6–9.
Schmidt AH. Autologous bone graft: is it still the gold standard? Injury. 2021;52(Suppl 2):S18–22.
Myeroff C, Archdeacon M. Autogenous bone graft: donor sites and techniques. J Bone Joint Surg Am. 2011;93:2227–36.
Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011;42(Suppl 2):S3–15.
Kamal M, Gremse F, Rosenhain S, Bartella AK, Hölzle F, Kessler P et al. Comparison of Bone Grafts From Various Donor Sites in Human Bone Specimens: Journal of Craniofacial Surgery [Internet]. 2018 [cited 2022 Dec 2];29:1661–5. http://journals.lww.com/00001665-201809000-00067 .
Marolt Presen D, Traweger A, Gimona M, Redl H. Mesenchymal stromal cell-based bone regeneration therapies: from cell transplantation and tissue Engineering to Therapeutic Secretomes and Extracellular vesicles. Front Bioeng Biotechnol. 2019;7:352.
Zha K, Tian Y, Panayi AC, Mi B, Liu G. Recent advances in enhancement strategies for osteogenic differentiation of mesenchymal stem cells in bone tissue Engineering. Front Cell Dev Biol. 2022;10:824812.
Musina RA, Bekchanova ES, Sukhikh GT. Comparison of mesenchymal stem cells obtained from different human tissues. Bull Exp Biol Med. 2005;139:504–9.
Article CAS PubMed Google Scholar
Sharma S, Muthu S, Jeyaraman M, Ranjan R, Jha SK. Translational products of adipose tissue-derived mesenchymal stem cells: bench to bedside applications. World J Stem Cells. 2021;13:1360–81.
De Ugarte DA, Alfonso Z, Zuk PA, Elbarbary A, Zhu M, Ashjian P, et al. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunol Lett. 2003;89:267–70.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7.
Zhang J, Huang X, Wang H, Liu X, Zhang T, Wang Y, et al. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res Ther. 2015;6:234.
Vega A, Martín-Ferrero MA, Del Canto F, Alberca M, García V, Munar A, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a Randomized Controlled Trial. Transplantation. 2015;99:1681–90.
Zhang W, Wray LS, Rnjak-Kovacina J, Xu L, Zou D, Wang S et al. Vascularization of hollow channel-modified porous silk scaffolds with endothelial cells for tissue regeneration. Biomaterials [Internet]. 2015 [cited 2023 May 29];56:68–77. https://linkinghub.elsevier.com/retrieve/pii/S0142961215003312 .
Zhang M, Lin R, Wang X, Xue J, Deng C, Feng C et al. 3D printing of Haversian bone–mimicking scaffolds for multicellular delivery in bone regeneration. Sci Adv [Internet]. 2020 [cited 2023 May 29];6:eaaz6725. https://www.science.org/doi/ https://doi.org/10.1126/sciadv.aaz6725 .
Gómez-Barrena E, Rosset P, Gebhard F, Hernigou P, Baldini N, Rouard H, et al. Feasibility and safety of treating non-unions in tibia, femur and humerus with autologous, expanded, bone marrow-derived mesenchymal stromal cells associated with biphasic calcium phosphate biomaterials in a multicentric, non-comparative trial. Biomaterials. 2019;196:100–8.
Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87:1430–7.
PubMed Google Scholar
Download references
Acknowledgements
The authors thank Neus Sarobé, MD; David Roca, MD; Joan Rius, MD; Manuel Blanco, MD; Marta Domínguez; Pablo Hernández, MD; Maite del Olmo, PhD; Jone Herrero, surgical nurses Alejandra Jiménez and Claudia Quera and experimental laboratories for their contributions to this work. Francisco López de Saro (Trialance SCCL) provided medical writing support, funded by Laboratorios Salvat, S.A.
This work was supported by Laboratorios Salvat, S.A. This report represents the results of the clinical trial sponsored by Laboratorios Salvat, S.A.
Author information
Authors and affiliations.
Institut de Teràpia Regenerativa Tissular, Centro Médico Teknon, Barcelona, Spain
Lluís Orozco Delclós & Robert Soler Rich
Instituto Médico Arriaza, Grupo INCIDE Universidad da Coruña, A Coruña, Spain
Rafael Arriaza Loureda
Department of Orthopaedic Surgery and Traumatology, Hospital Universitario La Paz-IdiPaz, Madrid, Spain
Alonso Moreno García & Enrique Gómez Barrena
School of Medicine, Universidad Autónoma de Madrid, Madrid, Spain
Enrique Gómez Barrena
You can also search for this author in PubMed Google Scholar
Contributions
Lluís Orozco Delclós: designed the study, collected data, wrote the manuscript, Robert Soler Rich: designed the study, collected data, wrote the manuscript. Rafael Arriaza Loureda: collected data. Alonso Moreno García: collected data. Enrique Gómez Barrena: collected data. All authors revised and approved the final version of the manuscript.
Corresponding author
Correspondence to Lluís Orozco Delclós .
Ethics declarations
Ethics approval and consent to participate.
The trial followed the criteria of the Osteosynthesis Association for the Study of Internal Fixation (AO-ASIF) and was authorized by the Ethics Committee of the Quirón Salud Hospital Group and the Spanish Agency for Medicines and Health Products (AEMPS). Informed consent was obtained from all patients.
Consent for publication
Consent for publication was obtained from all patients.
Competing interests
The authors declare no competing interests. The biograft product is patented (PCT/ES2009/000358) by Histocell SL (Spain).
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Orozco Delclós, L., Soler Rich, R., Arriaza Loureda, R. et al. Efficacy and safety of autologous or allogeneic mesenchymal stromal cells from adult adipose tissue expanded and combined with tricalcium phosphate biomaterial for the surgical treatment of atrophic nonunion of long bones: a phase II clinical trial. J Transl Med 22 , 493 (2024). https://doi.org/10.1186/s12967-024-05280-x
Download citation
Received : 18 December 2023
Accepted : 07 May 2024
Published : 24 May 2024
DOI : https://doi.org/10.1186/s12967-024-05280-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Atrophic nonunion
- Bone marrow mesenchymal stem cells
- Adipose tissue mesenchymal stromal cells
Journal of Translational Medicine
ISSN: 1479-5876
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Switch language:

Evrima pilots new patient recruitment approach for trials
Using the technology, 39 suitable participants were identified and matched with trials via regional pharmacies in days.
- Share on Linkedin
- Share on Facebook

Evrima Technologies has executed a pilot project with StrongRoomAI and Paratus Clinical, to offer new avenues for patients to discover and participate in clinical trials.
This pilot approach integrated networks of general practitioners (GPs), pharmacies and clinical trial sites in Australia.
Go deeper with GlobalData

LOA and PTSR Model - EVT-401 in Inflammation
Loa and ptsr model - etrumadenant in melanoma, premium insights.
The gold standard of business intelligence.
Find out more
The collaborative effort utilised Evrima’s GP and pharmacy network, Evripath, to identify patients potentially eligible for clinical trials.
These patients are then contacted through StrongRoom’s pharmacy channels and connected with the onsite team of Paratus Clinical for further assessment.
The project marks a significant shift in the Australian healthcare sector, offering patients the chance to consider clinical trials as a viable option in their healthcare journey.
Early results from the partnership are claimed to have indicated a positive impact.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
Using this technology, 39 suitable subjects were identified and matched with clinical trials via regional pharmacies in just a few days.
The objective of the alliance is to create Australia’s most extensive referral network by capitalising on StrongRoomAI’s pharmacy network and Paratus Clinical’s expanding trial site network.
This strategy aims to integrate research into the community, removing obstacles to trial participation and accelerating the clinical development process.
The approach could broaden the potential participant base as well as promote diversity and inclusivity in clinical research.
Evrima Technologies founder and CEO Charlotte Bradshaw said: “Evrima successfully completed a pilot project in New South Wales, along with StrongRoom AI, a medication management platform, and Paratus Clinical, a clinical trial site network, to identify eligible participants via pharmacies and refer them to local clinical trials in their community.
“This project saw rapid adoption by healthcare professionals and provided a number of patients with the opportunity to participate in clinical trials they wouldn’t normally have been aware of. Evrima plans to expand this network to reach five million people by the end of the year.”
Sign up for our daily news round-up!
Give your business an edge with our leading industry insights.
More Relevant
Neuren Pharmaceuticals’ NNZ-2591 shows promise in trial for Pitt Hopkins syndrome
Transcenta’s osemitamab succeeds in phase iia g/gej cancer triple combo trial, tilt biotherapeutics reports data from phase i trial of ovarian cancer treatment, innovent's psoriasis treatment picankibart meets all endpoints in phase iii trial, sign up to the newsletter: in brief, your corporate email address, i would also like to subscribe to:.
I consent to Verdict Media Limited collecting my details provided via this form in accordance with Privacy Policy
Thank you for subscribing
View all newsletters from across the GlobalData Media network.

COMMENTS
The success of a quality clinical research program is essential for developing and maintaining an impeccable clinical research trial team. It is the main component of a research program because total time and effort for conducting a clinical trial; nurses and data managers each contribute more than 30%. ... In a research study, a clinical trial ...
Principal investigator (PI) Also called a primary investigator, this person oversees all aspects of a clinical research study. They develop the study concept; write a detailed description of how the study will be conducted; and submit it for approval to the site's institutional review board (IRB). In addition, this person oversees participant ...
The Four Phases of Clinical Trials. Early clinical trial phases (phases 1 and 2) test for safety, such as what the side effects are and what a safe dose is. Later phases (phase 3 and 4) compare the treatment to current standard treatments. In a phase 1 clinical trial, researchers figure out whether a new treatment is safe, what its side effects ...
A research team may include clinical research associates (CRAs), research nurses, data managers, and study coordinators. Depending on a program's organizational structure, the responsibilities assigned to each may differ greatly from one research program to another, both within and outside the United States.
What roles perform study team members. ... "Regardless of … the size of the trial, each member of the team must be educated in the conduct of clinical trials, the regulations that govern trials, and the protocol document that describes the trial to be conducted." (Tompkins, A., 2007 in: Gallin, J.I. & Ognibene, p.67). ...
Participates in discussions regarding feasibility of protocol implementation based on knowledge of institutional capabilities and limitations, therapy, or population of interest. Collaborates with the research team to implement procedures for maintaining patient study participation from enrollment through completion.
Shirley L.T. Helm, MS, CCRP Senior Administrator for Network Capacity & Workforce Strategies C. Kenneth & Dianne Wright Center for Clinical and Translational Research Virginia Commonwealth University Abstract: The practice of team science allows clinical research professionals to draw from theory-driven principles to build an effective and efficient research team. Inherent in these principles are
When considering participation in a research study, carefully look at the benefits and risks. Benefits may include earlier access to new clinical approaches and regular attention from a research team. Research participation often helps others in the future. Risks/inconveniences. Risks may include side effects.
In 2003, leaders in medicine and clinical studies gathered at the Institute of Medicine's Clinical Research Roundtable to address the issue of the shrinking field of clinical research, and to identify challenges facing researchers. 1,2 Many of their solutions were focused on improving the dynamic of a clinical research team. 1 A successful research team typically consists of numerous members ...
Each member has a specific role to play. Read below to learn about members of the clinical trial research team and what they do. National Cancer Institute (NCI): Clinical Trial Research Team Members. The Center for Information and Study on Clinical Research Participation (Video): The Clinical Research Team Is Similar to a Sports Team.
Since the 1970s, numerous regulations have been in place to protect the rights of study participants. Many clinical research studies are also supervised by a data and safety monitoring committee. This is a group made up of experts in the area being studied. These biomedical professionals regularly monitor clinical studies as they progress.
Dec 15, 2023. --. Photo by Canva. A clinical research team is a group of people who work together on a research study to learn about human health or determine the safety and effectiveness of new ...
They also work closely with the research team in the study development process to identify key data points for collection and analysis for investigator initiated trials. Research Coordinator/ Research Nurse. The Research Coordinator/Nurse oversees and coordinates the daily activities of clinical research studies.
Learn about clinical trials for people with cancer. AIDS Clinical Trials and Information Services (ACTIS) or call 1-800-TRIALS-A (1-800-874-2572). Locate clinical trials for people ...
A clinical research coordinator is an integral part of the research team for medical studies. They conduct and manage clinical trials, providing outcomes that shape medical advances in preventative care, curing diseases, and immunizations, among other areas. With employment options available in hospitals, pharmaceutical companies, and private ...
A clinical trial is a research study that involves people like you. Researchers conduct clinical trials to find new or better ways to prevent, detect, or treat health conditions. Often, researchers want to find out if a new test, treatment, or preventive measure is safe and effective. Tests can include ways to screen for, diagnose, or prevent a ...
The study management templates are a University of Michigan resource available to all study team members. These templates are designed to help meet requirements for FDA-regulated clinical trials. They may be useful, but not required, to organize study documentation for other studies as well.
Clinical Research Coordinator and Regulatory Affairs. Centro de Diabetes Curitiba Ltda. Based on what you see in StudyTeam, you can give a sponsor feedback on why patients screen fail, and the sponsor amends the study. StudyTeam has made it so much easier for us to exchange that information with sponsors so they can make those changes.
The project management team is made up of Clinical Project Managers and Program Managers. In some companies, Clinical Project Managers (CPM) may be referred to as Clinical Trial Managers (CTM) or Study Managers. Project managers are the "General Contractors" of clinical research. They are accountable of all aspects of a clinical trial.
Introduction. In clinical research, our aim is to design a study, which would be able to derive a valid and meaningful scientific conclusion using appropriate statistical methods that can be translated to the "real world" setting. 1 Before choosing a study design, one must establish aims and objectives of the study, and choose an appropriate target population that is most representative of ...
The clinical research coordinators are critical to the work, their colleagues say. "The clinical research coordinators have played a hugely important role in the success of the Voyage study," says Janet Olson, Ph.D., the study's principal investigator. "As advocates for the study and the participants, the study coordinator team has been well ...
There are no guarantees in the outcomes of clinical studies. That's why we conduct the trials! However, on the path of any study-related project there are essential steps that can contribute to a greater potential for success. Best practice efforts to enhance effective team interactions and communications are a part of these essentials.
ResearchMatch helps you find a clinical trial or research study near you, or across the country, by matching you with researchers from leading medical research institutions. Whether you are a healthy volunteer or have a health condition, ResearchMatch connects you to research opportunities so you can make a difference and advance scientific discoveries by participating in research studies ...
"Prior research with older Black cancer patients has reported generally positive attitudes toward biospecimen donation, but little is known about younger individuals," Brown said. She and her team asked nearly 500 Black women ages 18-49 to complete an online survey about their attitude toward giving blood for clinical health research.
For the scientific advancement of clinical research, CMRPD services include comprehensive clinical trials monitoring, regulatory, pharmacovigilance, protocol navigation and development, and programmatic and project management support for facilitating the conduct of 400+ Phase I, II, and III domestic and international trials on a yearly basis.
Clinical research could be prospective or retrospective. It may also be a case-control study or a cohort study. Clinical trials may be initiated to find treatment, prevent, observe, and diagnose a disease or a medical condition. ... is an independent and external team that examines the process (recording the CRF, analysis of data, and ...
Clinical trials are research studies that look at new ways to prevent, detect or treat diseases. They rely on human volunteers, known as participants, and aim to find out if a new medical approach is safe and effective. Clinical trials often test new drugs or new combinations of drugs, new surgical procedures or devices, or different ways to use existing treatments.
Description: The NIH INCLUDE (Investigation of Co-occurring conditions across the Lifespan to Understand Down syndromE) Project Team invites you to join us for a dynamic webinar on Community Engagement in Clinical Trials: Lessons from PCORI (Patient Centered Outcomes Research Institute) Learn effective strategies for engagement and partnership ...
The primary efficacy was measured 6 months after surgery, but patients were followed for 12 months after surgery and a further year out of the scope of the study. All adverse events were recorded. This cohort was compared with a historical cohort of 14 cases treated by the same research team with autologous BM-MSC.
Evrima Technologies has executed a pilot project with StrongRoomAI and Paratus Clinical, to offer new avenues for patients to discover and participate in clinical trials. This pilot approach integrated networks of general practitioners (GPs), pharmacies and clinical trial sites in Australia. The collaborative effort utilised Evrima's GP and ...