- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution


Search form
- Advanced search
- Search responses
- Search blogs
- The PRISMA 2020...
The PRISMA 2020 statement: an updated guideline for reporting systematic reviews
PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews
- Related content
- Peer review
- Matthew J Page , senior research fellow 1 ,
- Joanne E McKenzie , associate professor 1 ,
- Patrick M Bossuyt , professor 2 ,
- Isabelle Boutron , professor 3 ,
- Tammy C Hoffmann , professor 4 ,
- Cynthia D Mulrow , professor 5 ,
- Larissa Shamseer , doctoral student 6 ,
- Jennifer M Tetzlaff , research product specialist 7 ,
- Elie A Akl , professor 8 ,
- Sue E Brennan , senior research fellow 1 ,
- Roger Chou , professor 9 ,
- Julie Glanville , associate director 10 ,
- Jeremy M Grimshaw , professor 11 ,
- Asbjørn Hróbjartsson , professor 12 ,
- Manoj M Lalu , associate scientist and assistant professor 13 ,
- Tianjing Li , associate professor 14 ,
- Elizabeth W Loder , professor 15 ,
- Evan Mayo-Wilson , associate professor 16 ,
- Steve McDonald , senior research fellow 1 ,
- Luke A McGuinness , research associate 17 ,
- Lesley A Stewart , professor and director 18 ,
- James Thomas , professor 19 ,
- Andrea C Tricco , scientist and associate professor 20 ,
- Vivian A Welch , associate professor 21 ,
- Penny Whiting , associate professor 17 ,
- David Moher , director and professor 22
- 1 School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia
- 2 Department of Clinical Epidemiology, Biostatistics and Bioinformatics, Amsterdam University Medical Centres, University of Amsterdam, Amsterdam, Netherlands
- 3 Université de Paris, Centre of Epidemiology and Statistics (CRESS), Inserm, F 75004 Paris, France
- 4 Institute for Evidence-Based Healthcare, Faculty of Health Sciences and Medicine, Bond University, Gold Coast, Australia
- 5 University of Texas Health Science Center at San Antonio, San Antonio, Texas, USA; Annals of Internal Medicine
- 6 Knowledge Translation Program, Li Ka Shing Knowledge Institute, Toronto, Canada; School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, Canada
- 7 Evidence Partners, Ottawa, Canada
- 8 Clinical Research Institute, American University of Beirut, Beirut, Lebanon; Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, Ontario, Canada
- 9 Department of Medical Informatics and Clinical Epidemiology, Oregon Health & Science University, Portland, Oregon, USA
- 10 York Health Economics Consortium (YHEC Ltd), University of York, York, UK
- 11 Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada; School of Epidemiology and Public Health, University of Ottawa, Ottawa, Canada; Department of Medicine, University of Ottawa, Ottawa, Canada
- 12 Centre for Evidence-Based Medicine Odense (CEBMO) and Cochrane Denmark, Department of Clinical Research, University of Southern Denmark, Odense, Denmark; Open Patient data Exploratory Network (OPEN), Odense University Hospital, Odense, Denmark
- 13 Department of Anesthesiology and Pain Medicine, The Ottawa Hospital, Ottawa, Canada; Clinical Epidemiology Program, Blueprint Translational Research Group, Ottawa Hospital Research Institute, Ottawa, Canada; Regenerative Medicine Program, Ottawa Hospital Research Institute, Ottawa, Canada
- 14 Department of Ophthalmology, School of Medicine, University of Colorado Denver, Denver, Colorado, United States; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA
- 15 Division of Headache, Department of Neurology, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts, USA; Head of Research, The BMJ , London, UK
- 16 Department of Epidemiology and Biostatistics, Indiana University School of Public Health-Bloomington, Bloomington, Indiana, USA
- 17 Population Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK
- 18 Centre for Reviews and Dissemination, University of York, York, UK
- 19 EPPI-Centre, UCL Social Research Institute, University College London, London, UK
- 20 Li Ka Shing Knowledge Institute of St. Michael's Hospital, Unity Health Toronto, Toronto, Canada; Epidemiology Division of the Dalla Lana School of Public Health and the Institute of Health Management, Policy, and Evaluation, University of Toronto, Toronto, Canada; Queen's Collaboration for Health Care Quality Joanna Briggs Institute Centre of Excellence, Queen's University, Kingston, Canada
- 21 Methods Centre, Bruyère Research Institute, Ottawa, Ontario, Canada; School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, Canada
- 22 Centre for Journalology, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada; School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, Canada
- Correspondence to: M J Page matthew.page{at}monash.edu
- Accepted 4 January 2021
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement, published in 2009, was designed to help systematic reviewers transparently report why the review was done, what the authors did, and what they found. Over the past decade, advances in systematic review methodology and terminology have necessitated an update to the guideline. The PRISMA 2020 statement replaces the 2009 statement and includes new reporting guidance that reflects advances in methods to identify, select, appraise, and synthesise studies. The structure and presentation of the items have been modified to facilitate implementation. In this article, we present the PRISMA 2020 27-item checklist, an expanded checklist that details reporting recommendations for each item, the PRISMA 2020 abstract checklist, and the revised flow diagrams for original and updated reviews.
Systematic reviews serve many critical roles. They can provide syntheses of the state of knowledge in a field, from which future research priorities can be identified; they can address questions that otherwise could not be answered by individual studies; they can identify problems in primary research that should be rectified in future studies; and they can generate or evaluate theories about how or why phenomena occur. Systematic reviews therefore generate various types of knowledge for different users of reviews (such as patients, healthcare providers, researchers, and policy makers). 1 2 To ensure a systematic review is valuable to users, authors should prepare a transparent, complete, and accurate account of why the review was done, what they did (such as how studies were identified and selected) and what they found (such as characteristics of contributing studies and results of meta-analyses). Up-to-date reporting guidance facilitates authors achieving this. 3
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement published in 2009 (hereafter referred to as PRISMA 2009) 4 5 6 7 8 9 10 is a reporting guideline designed to address poor reporting of systematic reviews. 11 The PRISMA 2009 statement comprised a checklist of 27 items recommended for reporting in systematic reviews and an “explanation and elaboration” paper 12 13 14 15 16 providing additional reporting guidance for each item, along with exemplars of reporting. The recommendations have been widely endorsed and adopted, as evidenced by its co-publication in multiple journals, citation in over 60 000 reports (Scopus, August 2020), endorsement from almost 200 journals and systematic review organisations, and adoption in various disciplines. Evidence from observational studies suggests that use of the PRISMA 2009 statement is associated with more complete reporting of systematic reviews, 17 18 19 20 although more could be done to improve adherence to the guideline. 21
Many innovations in the conduct of systematic reviews have occurred since publication of the PRISMA 2009 statement. For example, technological advances have enabled the use of natural language processing and machine learning to identify relevant evidence, 22 23 24 methods have been proposed to synthesise and present findings when meta-analysis is not possible or appropriate, 25 26 27 and new methods have been developed to assess the risk of bias in results of included studies. 28 29 Evidence on sources of bias in systematic reviews has accrued, culminating in the development of new tools to appraise the conduct of systematic reviews. 30 31 Terminology used to describe particular review processes has also evolved, as in the shift from assessing “quality” to assessing “certainty” in the body of evidence. 32 In addition, the publishing landscape has transformed, with multiple avenues now available for registering and disseminating systematic review protocols, 33 34 disseminating reports of systematic reviews, and sharing data and materials, such as preprint servers and publicly accessible repositories. To capture these advances in the reporting of systematic reviews necessitated an update to the PRISMA 2009 statement.
Summary points
To ensure a systematic review is valuable to users, authors should prepare a transparent, complete, and accurate account of why the review was done, what they did, and what they found
The PRISMA 2020 statement provides updated reporting guidance for systematic reviews that reflects advances in methods to identify, select, appraise, and synthesise studies
The PRISMA 2020 statement consists of a 27-item checklist, an expanded checklist that details reporting recommendations for each item, the PRISMA 2020 abstract checklist, and revised flow diagrams for original and updated reviews
We anticipate that the PRISMA 2020 statement will benefit authors, editors, and peer reviewers of systematic reviews, and different users of reviews, including guideline developers, policy makers, healthcare providers, patients, and other stakeholders
Development of PRISMA 2020
A complete description of the methods used to develop PRISMA 2020 is available elsewhere. 35 We identified PRISMA 2009 items that were often reported incompletely by examining the results of studies investigating the transparency of reporting of published reviews. 17 21 36 37 We identified possible modifications to the PRISMA 2009 statement by reviewing 60 documents providing reporting guidance for systematic reviews (including reporting guidelines, handbooks, tools, and meta-research studies). 38 These reviews of the literature were used to inform the content of a survey with suggested possible modifications to the 27 items in PRISMA 2009 and possible additional items. Respondents were asked whether they believed we should keep each PRISMA 2009 item as is, modify it, or remove it, and whether we should add each additional item. Systematic review methodologists and journal editors were invited to complete the online survey (110 of 220 invited responded). We discussed proposed content and wording of the PRISMA 2020 statement, as informed by the review and survey results, at a 21-member, two-day, in-person meeting in September 2018 in Edinburgh, Scotland. Throughout 2019 and 2020, we circulated an initial draft and five revisions of the checklist and explanation and elaboration paper to co-authors for feedback. In April 2020, we invited 22 systematic reviewers who had expressed interest in providing feedback on the PRISMA 2020 checklist to share their views (via an online survey) on the layout and terminology used in a preliminary version of the checklist. Feedback was received from 15 individuals and considered by the first author, and any revisions deemed necessary were incorporated before the final version was approved and endorsed by all co-authors.
The PRISMA 2020 statement
Scope of the guideline.
The PRISMA 2020 statement has been designed primarily for systematic reviews of studies that evaluate the effects of health interventions, irrespective of the design of the included studies. However, the checklist items are applicable to reports of systematic reviews evaluating other interventions (such as social or educational interventions), and many items are applicable to systematic reviews with objectives other than evaluating interventions (such as evaluating aetiology, prevalence, or prognosis). PRISMA 2020 is intended for use in systematic reviews that include synthesis (such as pairwise meta-analysis or other statistical synthesis methods) or do not include synthesis (for example, because only one eligible study is identified). The PRISMA 2020 items are relevant for mixed-methods systematic reviews (which include quantitative and qualitative studies), but reporting guidelines addressing the presentation and synthesis of qualitative data should also be consulted. 39 40 PRISMA 2020 can be used for original systematic reviews, updated systematic reviews, or continually updated (“living”) systematic reviews. However, for updated and living systematic reviews, there may be some additional considerations that need to be addressed. Where there is relevant content from other reporting guidelines, we reference these guidelines within the items in the explanation and elaboration paper 41 (such as PRISMA-Search 42 in items 6 and 7, Synthesis without meta-analysis (SWiM) reporting guideline 27 in item 13d). Box 1 includes a glossary of terms used throughout the PRISMA 2020 statement.
Glossary of terms
Systematic review —A review that uses explicit, systematic methods to collate and synthesise findings of studies that address a clearly formulated question 43
Statistical synthesis —The combination of quantitative results of two or more studies. This encompasses meta-analysis of effect estimates (described below) and other methods, such as combining P values, calculating the range and distribution of observed effects, and vote counting based on the direction of effect (see McKenzie and Brennan 25 for a description of each method)
Meta-analysis of effect estimates —A statistical technique used to synthesise results when study effect estimates and their variances are available, yielding a quantitative summary of results 25
Outcome —An event or measurement collected for participants in a study (such as quality of life, mortality)
Result —The combination of a point estimate (such as a mean difference, risk ratio, or proportion) and a measure of its precision (such as a confidence/credible interval) for a particular outcome
Report —A document (paper or electronic) supplying information about a particular study. It could be a journal article, preprint, conference abstract, study register entry, clinical study report, dissertation, unpublished manuscript, government report, or any other document providing relevant information
Record —The title or abstract (or both) of a report indexed in a database or website (such as a title or abstract for an article indexed in Medline). Records that refer to the same report (such as the same journal article) are “duplicates”; however, records that refer to reports that are merely similar (such as a similar abstract submitted to two different conferences) should be considered unique.
Study —An investigation, such as a clinical trial, that includes a defined group of participants and one or more interventions and outcomes. A “study” might have multiple reports. For example, reports could include the protocol, statistical analysis plan, baseline characteristics, results for the primary outcome, results for harms, results for secondary outcomes, and results for additional mediator and moderator analyses
PRISMA 2020 is not intended to guide systematic review conduct, for which comprehensive resources are available. 43 44 45 46 However, familiarity with PRISMA 2020 is useful when planning and conducting systematic reviews to ensure that all recommended information is captured. PRISMA 2020 should not be used to assess the conduct or methodological quality of systematic reviews; other tools exist for this purpose. 30 31 Furthermore, PRISMA 2020 is not intended to inform the reporting of systematic review protocols, for which a separate statement is available (PRISMA for Protocols (PRISMA-P) 2015 statement 47 48 ). Finally, extensions to the PRISMA 2009 statement have been developed to guide reporting of network meta-analyses, 49 meta-analyses of individual participant data, 50 systematic reviews of harms, 51 systematic reviews of diagnostic test accuracy studies, 52 and scoping reviews 53 ; for these types of reviews we recommend authors report their review in accordance with the recommendations in PRISMA 2020 along with the guidance specific to the extension.
How to use PRISMA 2020
The PRISMA 2020 statement (including the checklists, explanation and elaboration, and flow diagram) replaces the PRISMA 2009 statement, which should no longer be used. Box 2 summarises noteworthy changes from the PRISMA 2009 statement. The PRISMA 2020 checklist includes seven sections with 27 items, some of which include sub-items ( table 1 ). A checklist for journal and conference abstracts for systematic reviews is included in PRISMA 2020. This abstract checklist is an update of the 2013 PRISMA for Abstracts statement, 54 reflecting new and modified content in PRISMA 2020 ( table 2 ). A template PRISMA flow diagram is provided, which can be modified depending on whether the systematic review is original or updated ( fig 1 ).
Noteworthy changes to the PRISMA 2009 statement
Inclusion of the abstract reporting checklist within PRISMA 2020 (see item #2 and table 2 ).
Movement of the ‘Protocol and registration’ item from the start of the Methods section of the checklist to a new Other section, with addition of a sub-item recommending authors describe amendments to information provided at registration or in the protocol (see item #24a-24c).
Modification of the ‘Search’ item to recommend authors present full search strategies for all databases, registers and websites searched, not just at least one database (see item #7).
Modification of the ‘Study selection’ item in the Methods section to emphasise the reporting of how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process (see item #8).
Addition of a sub-item to the ‘Data items’ item recommending authors report how outcomes were defined, which results were sought, and methods for selecting a subset of results from included studies (see item #10a).
Splitting of the ‘Synthesis of results’ item in the Methods section into six sub-items recommending authors describe: the processes used to decide which studies were eligible for each synthesis; any methods required to prepare the data for synthesis; any methods used to tabulate or visually display results of individual studies and syntheses; any methods used to synthesise results; any methods used to explore possible causes of heterogeneity among study results (such as subgroup analysis, meta-regression); and any sensitivity analyses used to assess robustness of the synthesised results (see item #13a-13f).
Addition of a sub-item to the ‘Study selection’ item in the Results section recommending authors cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded (see item #16b).
Splitting of the ‘Synthesis of results’ item in the Results section into four sub-items recommending authors: briefly summarise the characteristics and risk of bias among studies contributing to the synthesis; present results of all statistical syntheses conducted; present results of any investigations of possible causes of heterogeneity among study results; and present results of any sensitivity analyses (see item #20a-20d).
Addition of new items recommending authors report methods for and results of an assessment of certainty (or confidence) in the body of evidence for an outcome (see items #15 and #22).
Addition of a new item recommending authors declare any competing interests (see item #26).
Addition of a new item recommending authors indicate whether data, analytic code and other materials used in the review are publicly available and if so, where they can be found (see item #27).
PRISMA 2020 item checklist
- View inline
PRISMA 2020 for Abstracts checklist*

PRISMA 2020 flow diagram template for systematic reviews. The new design is adapted from flow diagrams proposed by Boers, 55 Mayo-Wilson et al. 56 and Stovold et al. 57 The boxes in grey should only be completed if applicable; otherwise they should be removed from the flow diagram. Note that a “report” could be a journal article, preprint, conference abstract, study register entry, clinical study report, dissertation, unpublished manuscript, government report or any other document providing relevant information.
- Download figure
- Open in new tab
- Download powerpoint
We recommend authors refer to PRISMA 2020 early in the writing process, because prospective consideration of the items may help to ensure that all the items are addressed. To help keep track of which items have been reported, the PRISMA statement website ( http://www.prisma-statement.org/ ) includes fillable templates of the checklists to download and complete (also available in the data supplement on bmj.com). We have also created a web application that allows users to complete the checklist via a user-friendly interface 58 (available at https://prisma.shinyapps.io/checklist/ and adapted from the Transparency Checklist app 59 ). The completed checklist can be exported to Word or PDF. Editable templates of the flow diagram can also be downloaded from the PRISMA statement website.
We have prepared an updated explanation and elaboration paper, in which we explain why reporting of each item is recommended and present bullet points that detail the reporting recommendations (which we refer to as elements). 41 The bullet-point structure is new to PRISMA 2020 and has been adopted to facilitate implementation of the guidance. 60 61 An expanded checklist, which comprises an abridged version of the elements presented in the explanation and elaboration paper, with references and some examples removed, is available in the data supplement on bmj.com. Consulting the explanation and elaboration paper is recommended if further clarity or information is required.
Journals and publishers might impose word and section limits, and limits on the number of tables and figures allowed in the main report. In such cases, if the relevant information for some items already appears in a publicly accessible review protocol, referring to the protocol may suffice. Alternatively, placing detailed descriptions of the methods used or additional results (such as for less critical outcomes) in supplementary files is recommended. Ideally, supplementary files should be deposited to a general-purpose or institutional open-access repository that provides free and permanent access to the material (such as Open Science Framework, Dryad, figshare). A reference or link to the additional information should be included in the main report. Finally, although PRISMA 2020 provides a template for where information might be located, the suggested location should not be seen as prescriptive; the guiding principle is to ensure the information is reported.
Use of PRISMA 2020 has the potential to benefit many stakeholders. Complete reporting allows readers to assess the appropriateness of the methods, and therefore the trustworthiness of the findings. Presenting and summarising characteristics of studies contributing to a synthesis allows healthcare providers and policy makers to evaluate the applicability of the findings to their setting. Describing the certainty in the body of evidence for an outcome and the implications of findings should help policy makers, managers, and other decision makers formulate appropriate recommendations for practice or policy. Complete reporting of all PRISMA 2020 items also facilitates replication and review updates, as well as inclusion of systematic reviews in overviews (of systematic reviews) and guidelines, so teams can leverage work that is already done and decrease research waste. 36 62 63
We updated the PRISMA 2009 statement by adapting the EQUATOR Network’s guidance for developing health research reporting guidelines. 64 We evaluated the reporting completeness of published systematic reviews, 17 21 36 37 reviewed the items included in other documents providing guidance for systematic reviews, 38 surveyed systematic review methodologists and journal editors for their views on how to revise the original PRISMA statement, 35 discussed the findings at an in-person meeting, and prepared this document through an iterative process. Our recommendations are informed by the reviews and survey conducted before the in-person meeting, theoretical considerations about which items facilitate replication and help users assess the risk of bias and applicability of systematic reviews, and co-authors’ experience with authoring and using systematic reviews.
Various strategies to increase the use of reporting guidelines and improve reporting have been proposed. They include educators introducing reporting guidelines into graduate curricula to promote good reporting habits of early career scientists 65 ; journal editors and regulators endorsing use of reporting guidelines 18 ; peer reviewers evaluating adherence to reporting guidelines 61 66 ; journals requiring authors to indicate where in their manuscript they have adhered to each reporting item 67 ; and authors using online writing tools that prompt complete reporting at the writing stage. 60 Multi-pronged interventions, where more than one of these strategies are combined, may be more effective (such as completion of checklists coupled with editorial checks). 68 However, of 31 interventions proposed to increase adherence to reporting guidelines, the effects of only 11 have been evaluated, mostly in observational studies at high risk of bias due to confounding. 69 It is therefore unclear which strategies should be used. Future research might explore barriers and facilitators to the use of PRISMA 2020 by authors, editors, and peer reviewers, designing interventions that address the identified barriers, and evaluating those interventions using randomised trials. To inform possible revisions to the guideline, it would also be valuable to conduct think-aloud studies 70 to understand how systematic reviewers interpret the items, and reliability studies to identify items where there is varied interpretation of the items.
We encourage readers to submit evidence that informs any of the recommendations in PRISMA 2020 (via the PRISMA statement website: http://www.prisma-statement.org/ ). To enhance accessibility of PRISMA 2020, several translations of the guideline are under way (see available translations at the PRISMA statement website). We encourage journal editors and publishers to raise awareness of PRISMA 2020 (for example, by referring to it in journal “Instructions to authors”), endorsing its use, advising editors and peer reviewers to evaluate submitted systematic reviews against the PRISMA 2020 checklists, and making changes to journal policies to accommodate the new reporting recommendations. We recommend existing PRISMA extensions 47 49 50 51 52 53 71 72 be updated to reflect PRISMA 2020 and advise developers of new PRISMA extensions to use PRISMA 2020 as the foundation document.
We anticipate that the PRISMA 2020 statement will benefit authors, editors, and peer reviewers of systematic reviews, and different users of reviews, including guideline developers, policy makers, healthcare providers, patients, and other stakeholders. Ultimately, we hope that uptake of the guideline will lead to more transparent, complete, and accurate reporting of systematic reviews, thus facilitating evidence based decision making.
Acknowledgments
We dedicate this paper to the late Douglas G Altman and Alessandro Liberati, whose contributions were fundamental to the development and implementation of the original PRISMA statement.
We thank the following contributors who completed the survey to inform discussions at the development meeting: Xavier Armoiry, Edoardo Aromataris, Ana Patricia Ayala, Ethan M Balk, Virginia Barbour, Elaine Beller, Jesse A Berlin, Lisa Bero, Zhao-Xiang Bian, Jean Joel Bigna, Ferrán Catalá-López, Anna Chaimani, Mike Clarke, Tammy Clifford, Ioana A Cristea, Miranda Cumpston, Sofia Dias, Corinna Dressler, Ivan D Florez, Joel J Gagnier, Chantelle Garritty, Long Ge, Davina Ghersi, Sean Grant, Gordon Guyatt, Neal R Haddaway, Julian PT Higgins, Sally Hopewell, Brian Hutton, Jamie J Kirkham, Jos Kleijnen, Julia Koricheva, Joey SW Kwong, Toby J Lasserson, Julia H Littell, Yoon K Loke, Malcolm R Macleod, Chris G Maher, Ana Marušic, Dimitris Mavridis, Jessie McGowan, Matthew DF McInnes, Philippa Middleton, Karel G Moons, Zachary Munn, Jane Noyes, Barbara Nußbaumer-Streit, Donald L Patrick, Tatiana Pereira-Cenci, Ba’ Pham, Bob Phillips, Dawid Pieper, Michelle Pollock, Daniel S Quintana, Drummond Rennie, Melissa L Rethlefsen, Hannah R Rothstein, Maroeska M Rovers, Rebecca Ryan, Georgia Salanti, Ian J Saldanha, Margaret Sampson, Nancy Santesso, Rafael Sarkis-Onofre, Jelena Savović, Christopher H Schmid, Kenneth F Schulz, Guido Schwarzer, Beverley J Shea, Paul G Shekelle, Farhad Shokraneh, Mark Simmonds, Nicole Skoetz, Sharon E Straus, Anneliese Synnot, Emily E Tanner-Smith, Brett D Thombs, Hilary Thomson, Alexander Tsertsvadze, Peter Tugwell, Tari Turner, Lesley Uttley, Jeffrey C Valentine, Matt Vassar, Areti Angeliki Veroniki, Meera Viswanathan, Cole Wayant, Paul Whaley, and Kehu Yang. We thank the following contributors who provided feedback on a preliminary version of the PRISMA 2020 checklist: Jo Abbott, Fionn Büttner, Patricia Correia-Santos, Victoria Freeman, Emily A Hennessy, Rakibul Islam, Amalia (Emily) Karahalios, Kasper Krommes, Andreas Lundh, Dafne Port Nascimento, Davina Robson, Catherine Schenck-Yglesias, Mary M Scott, Sarah Tanveer and Pavel Zhelnov. We thank Abigail H Goben, Melissa L Rethlefsen, Tanja Rombey, Anna Scott, and Farhad Shokraneh for their helpful comments on the preprints of the PRISMA 2020 papers. We thank Edoardo Aromataris, Stephanie Chang, Toby Lasserson and David Schriger for their helpful peer review comments on the PRISMA 2020 papers.
Contributors: JEM and DM are joint senior authors. MJP, JEM, PMB, IB, TCH, CDM, LS, and DM conceived this paper and designed the literature review and survey conducted to inform the guideline content. MJP conducted the literature review, administered the survey and analysed the data for both. MJP prepared all materials for the development meeting. MJP and JEM presented proposals at the development meeting. All authors except for TCH, JMT, EAA, SEB, and LAM attended the development meeting. MJP and JEM took and consolidated notes from the development meeting. MJP and JEM led the drafting and editing of the article. JEM, PMB, IB, TCH, LS, JMT, EAA, SEB, RC, JG, AH, TL, EMW, SM, LAM, LAS, JT, ACT, PW, and DM drafted particular sections of the article. All authors were involved in revising the article critically for important intellectual content. All authors approved the final version of the article. MJP is the guarantor of this work. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: There was no direct funding for this research. MJP is supported by an Australian Research Council Discovery Early Career Researcher Award (DE200101618) and was previously supported by an Australian National Health and Medical Research Council (NHMRC) Early Career Fellowship (1088535) during the conduct of this research. JEM is supported by an Australian NHMRC Career Development Fellowship (1143429). TCH is supported by an Australian NHMRC Senior Research Fellowship (1154607). JMT is supported by Evidence Partners Inc. JMG is supported by a Tier 1 Canada Research Chair in Health Knowledge Transfer and Uptake. MML is supported by The Ottawa Hospital Anaesthesia Alternate Funds Association and a Faculty of Medicine Junior Research Chair. TL is supported by funding from the National Eye Institute (UG1EY020522), National Institutes of Health, United States. LAM is supported by a National Institute for Health Research Doctoral Research Fellowship (DRF-2018-11-ST2-048). ACT is supported by a Tier 2 Canada Research Chair in Knowledge Synthesis. DM is supported in part by a University Research Chair, University of Ottawa. The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/conflicts-of-interest/ and declare: EL is head of research for the BMJ ; MJP is an editorial board member for PLOS Medicine ; ACT is an associate editor and MJP, TL, EMW, and DM are editorial board members for the Journal of Clinical Epidemiology ; DM and LAS were editors in chief, LS, JMT, and ACT are associate editors, and JG is an editorial board member for Systematic Reviews . None of these authors were involved in the peer review process or decision to publish. TCH has received personal fees from Elsevier outside the submitted work. EMW has received personal fees from the American Journal for Public Health , for which he is the editor for systematic reviews. VW is editor in chief of the Campbell Collaboration, which produces systematic reviews, and co-convenor of the Campbell and Cochrane equity methods group. DM is chair of the EQUATOR Network, IB is adjunct director of the French EQUATOR Centre and TCH is co-director of the Australasian EQUATOR Centre, which advocates for the use of reporting guidelines to improve the quality of reporting in research articles. JMT received salary from Evidence Partners, creator of DistillerSR software for systematic reviews; Evidence Partners was not involved in the design or outcomes of the statement, and the views expressed solely represent those of the author.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient and public involvement: Patients and the public were not involved in this methodological research. We plan to disseminate the research widely, including to community participants in evidence synthesis organisations.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/ .
- Gurevitch J ,
- Koricheva J ,
- Nakagawa S ,
- Liberati A ,
- Tetzlaff J ,
- Altman DG ,
- PRISMA Group
- Tricco AC ,
- Sampson M ,
- Shamseer L ,
- Leoncini E ,
- de Belvis G ,
- Ricciardi W ,
- Fowler AJ ,
- Leclercq V ,
- Beaudart C ,
- Ajamieh S ,
- Rabenda V ,
- Tirelli E ,
- O’Mara-Eves A ,
- McNaught J ,
- Ananiadou S
- Marshall IJ ,
- Noel-Storr A ,
- Higgins JPT ,
- Chandler J ,
- McKenzie JE ,
- López-López JA ,
- Becker BJ ,
- Campbell M ,
- Sterne JAC ,
- Savović J ,
- Sterne JA ,
- Hernán MA ,
- Reeves BC ,
- Whiting P ,
- Higgins JP ,
- ROBIS group
- Hultcrantz M ,
- Stewart L ,
- Bossuyt PM ,
- Flemming K ,
- McInnes E ,
- France EF ,
- Cunningham M ,
- Rethlefsen ML ,
- Kirtley S ,
- Waffenschmidt S ,
- PRISMA-S Group
- ↵ Higgins JPT, Thomas J, Chandler J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions : Version 6.0. Cochrane, 2019. Available from https://training.cochrane.org/handbook .
- Dekkers OM ,
- Vandenbroucke JP ,
- Cevallos M ,
- Renehan AG ,
- ↵ Cooper H, Hedges LV, Valentine JV, eds. The Handbook of Research Synthesis and Meta-Analysis. Russell Sage Foundation, 2019.
- IOM (Institute of Medicine)
- PRISMA-P Group
- Salanti G ,
- Caldwell DM ,
- Stewart LA ,
- PRISMA-IPD Development Group
- Zorzela L ,
- Ioannidis JP ,
- PRISMAHarms Group
- McInnes MDF ,
- Thombs BD ,
- and the PRISMA-DTA Group
- Beller EM ,
- Glasziou PP ,
- PRISMA for Abstracts Group
- Mayo-Wilson E ,
- Dickersin K ,
- MUDS investigators
- Stovold E ,
- Beecher D ,
- Noel-Storr A
- McGuinness LA
- Sarafoglou A ,
- Boutron I ,
- Giraudeau B ,
- Porcher R ,
- Chauvin A ,
- Schulz KF ,
- Schroter S ,
- Stevens A ,
- Weinstein E ,
- Macleod MR ,
- IICARus Collaboration
- Kirkham JJ ,
- Petticrew M ,
- Tugwell P ,
- PRISMA-Equity Bellagio group

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

This strategy is intended to retrieve citations to systematic reviews in PubMed and encompasses: citations assigned the "Systematic Review" publication type during MEDLINE indexing; citations that have not yet completed MEDLINE indexing; and non-MEDLINE citations. This filter can be used in a search as systematic [sb].
Example: exercise hypertension AND systematic [sb]
This filter is also available on the Filters sidebar under "Article types." It is also available on the Clinical Queries screen.
Strategy last modified December 2018.
Last Reviewed: February 20, 2019
Literature Searching
In this guide.
- Introduction
- Steps for searching the literature in PubMed
- Step 1 - Formulate a search question
- Step 2- Identify primary concepts and gather synonyms
- Step 3 - Locate subject headings (MeSH)
- Step 4 - Combine concepts using Boolean operators
- Step 5 - Refine search terms and search in PubMed
- Step 6 - Apply limits

Steps for Searching the Literature
Searching is an iterative process and often requires re-evaluation and testing by adding or changing keywords and the ways they relate to each other. To guide your search development, you can follow the search steps below. For more information on each step, navigate to its matching tab on the right menu.
1. Formulate a clear, well-defined, answerable search question
Generally, the basic literature search process begins with formulating a clear, well-defined research question. Asking the right research question is essential to creating an effective search. Your research question(s) must be well-defined and answerable. If the question is too broad, your search will yield more information than you can possibly look through.
2. Identify primary concepts and gather synonyms
Your research question will also help identify the primary search concepts. This will allow you to think about how you want the concepts to relate to each other. Since different authors use different terminology to refer to the same concept, you will need to gather synonyms and all the ways authors might express them. However, it is important to balance the terms so that the synonyms do not go beyond the scope of how you've defined them.
3. Locate subject headings (MeSH)
Subject databases like PubMed use 'controlled vocabularies' made up of subject headings that are preassigned to indexed articles that share a similar topic. These subject headings are organized hierarchically within a family tree of broader and narrower concepts. In PubMed and MEDLINE, the subject headings are called Medical Subject Headings (MeSH). By including MeSH terms in your search, you will not have to think about word variations, word endings, plural or singular forms, or synonyms. Some topics or concepts may even have more than one appropriate MeSH term. There are also times when a topic or concept may not have a MeSH term.
4. Combine concepts using Boolean operators AND/OR
Once you have identified your search concepts, synonyms, and MeSH terms, you'll need to put them together using nesting and Boolean operators (e.g. AND, OR, NOT). Nesting uses parentheses to put search terms into groups. Boolean operators are used to combine similar and different concepts into one query.
5. Refine search terms and search in PubMed
There are various database search tactics you can use, such as field tags to limit the search to certain fields, quotation marks for phrase searching, and proximity operators to search a number of spaces between terms to refine your search terms. The constructed search string is ready to be pasted into PubMed.
6. Apply limits (optional)
If you're getting too many results, you can further refine your search results by using limits on the left box of the results page. Limits allow you to narrow your search by a number of facets such as year, journal name, article type, language, age, etc.
Depending on the nature of the literature review, the complexity and comprehensiveness of the search strategies and the choice of databases can be different. Please contact the Lane Librarians if you have any questions.
The type of information you gather is influenced by the type of information source or database you select to search. Bibliographic databases contain references to published literature, such as journal articles, conference abstracts, books, reports, government and legal publications, and patents. Literature reviews typically synthesis indexed, peer-reviewed articles (i.e. works that generally represent the latest original research and have undergone rigorous expert screening before publication), and gray literature (i.e. materials not formally published by commercial publishers or peer-reviewed journals). PubMed offers a breadth of health sciences literature and is a good starting point to locate journal articles.
What is PubMed?
PubMed is a free search engine accessing primarily the MEDLINE database of references and abstracts on life sciences and biomedical topics. Available to the public online since 1996, PubMed was developed and is maintained by the National Center for Biotechnology Information (NCBI) , at the U.S. National Library of Medicine (NLM) , located at the National Institutes of Health (NIH) .
MEDLINE is the National Library of Medicine’s (NLM) premier bibliographic database that contains more than 27 million references to journal articles from more than 5,200 worldwide journals in life sciences with a concentration on biomedicine. The Literature Selection Technica Review Committee (LSTRC) reviews and selects journals for MEDLINE based on the research quality and impact of the journals. A distinctive feature of MEDLINE is that the records are indexed with NLM Medical Subject Headings (MeSH).
PubMed also contains citations for PubMed Central (PMC) articles. PMC is a full-text archive that includes articles from journals reviewed and selected by NLM for archiving (current and historical), as well as individual articles collected for archiving in compliance with funder policies. PubMed allows users to search keywords in the bibliographic data, but not the full text of the PMC articles.

How to Access PubMed?
To access PubMed, go to the Lane Library homepage and click PubMed in "Top Resources" on the left. This PubMed link is coded with Find Fulltext @ Lane Library Stanford that links you to Lane's full-text articles online.

- << Previous: Introduction
- Next: Step 1 - Formulate a search question >>
- Last Updated: Jan 9, 2024 10:30 AM
- URL: https://laneguides.stanford.edu/LitSearch
- Open access
- Published: 29 May 2024
Heterotopic ossification following COVID-19 infections: systematic literature review of case reports and case series
- Hachem Chaitani 1 ,
- Laurent Fabeck 2 &
- Simon Koulischer 2
BMC Musculoskeletal Disorders volume 25 , Article number: 421 ( 2024 ) Cite this article
Metrics details
This review aims to study the clinical characteristics, diagnostic results, treatments, and outcomes in patients with heterotopic ossification following COVID-19 infection.
A literature search for eligible articles was conducted using MEDLINE/Pubmed, Global Health, and Scopus databases (January 12th, 2023), including all case reports and case series from any country and language. The criteria for inclusion in this review were cases of COVID-19 infection subsequently developing heterotopic ossification.
This systematic review analysed 15 reports ( n = 20 patients) documenting cases of heterotopic ossification following COVID-19 infection. 80% of the patients were male, with a median age of 59 years. All patients required intensive care unit stay with an average duration of 48.5 days. Mechanical ventilation was necessary for all patients and 30% of them underwent tracheostomy. Common symptoms included stiffness and pain, most frequently affecting multiple locations (70%), with the hips and shoulders being predominantly involved. X-rays were the most commonly used imaging modality, followed by computed tomography. Although treatment was given, some of the patients continued to experience symptoms, particularly stiffness.
20 patients who developed heterotopic ossification after COVID-19 have been reported, the majority of which had at least two independent risk factors for this condition. The link between those two clinical entities is therefore uncertain, requiring further investigation. It is nonetheless important to suspect heterotopic ossification in patients with severe COVID-19 infection, prolonged immobilisation, mechanical ventilation, who develop joint pain and stiffness, as this condition can significantly impact patients’ quality of life.
Protocol registration
CRD42023393516.
Peer Review reports
Introduction
The coronavirus disease 2019 (COVID-19) global pandemic erupted in December 2019, resulting in numerous infections caused by severe acute respiratory syndrome coronavirus two (SARS-CoV-2). Although the viral infection affected mostly the lower respiratory tract causing acute respiratory distress syndrome (ARDS), many extrapulmonary complications have been described after COVID-19 infections. They may be the result of the viral infection itself, systemic inflammation, or other factors including intensive care unit (ICU) stay and prolonged bed rest [ 1 , 2 ]. The aetiology of heterotopic ossification (HO) is still not clearly understood. It can be defined as the emergence of bone tissue in ectopic tissue such as muscles. It occurs most commonly following traumatic brain or spinal cord injuries, intense trauma, severe thermal injuries, surgeries (e.g. hip arthroplasty), and immobilisation [ 3 , 4 ].
No link has been described yet between COVID-19 infection and HO, as the two phenomena remain unclear generally. We conducted a systematic review of case reports to summarise the evidence in the literature of the association between severe COVID-19 infections and HO development. Although a systematic review cannot demonstrate a causal relationship between these two processes, it can help make a few hypotheses requiring further research investigations. One potential hypothesis is that the systemic inflammatory response triggered by COVID-19 infection may contribute to dysregulation in bone formation pathways, thus predisposing individuals to HO. This study aims to give a better understanding of the clinical features in patients who get HO after contracting COVID-19. It serves as a starting point for delving deeper into the potential reasons behind this connection.
Materials and methods
This systematic review was conducted (protocol registration: CRD42023393516 ) following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [ 5 ], as shown in Fig. 1 .
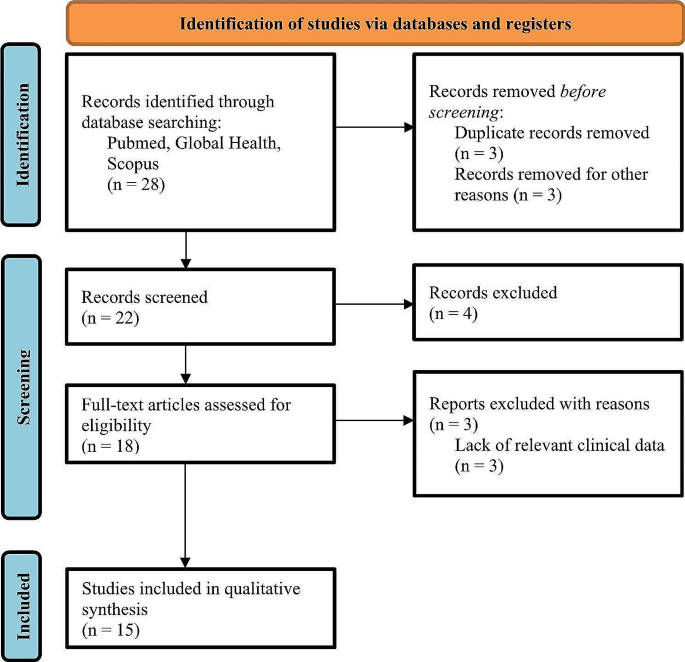
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart
Search strategy
We conducted a literature search for eligible articles published until January 12th, 2023, using three databases (MEDLINE/Pubmed, Global Health, and Scopus). The search strategy used the following keywords: “heterotopic ossification” or “ectopic ossification” or “myositis ossificans” and “COVID-19” or “SARS-CoV-2” or “coronavirus”. The title and abstract determined the eligibility of the case report.
Eligibility criteria
We searched case reports and case series of HO and COVID-19. No language restriction was applied. Cases reporting COVID-19 infection and a diagnosis of HO were included. Review articles, commentaries, articles concerning fibrodysplasia ossificans progressiva, and articles with a lack of relevant clinical data were excluded.
Study selection
Two authors reviewed independently the titles, abstracts, and full articles. These authors confirmed articles with predetermined eligibility criteria.
Data collation and quality assessment
One author extracted data and another cross checked it. Physician collaborators helped in extracting data from articles written in other languages than English (French, Spanish, Portuguese, German, and Dutch). Subsequent details were drawn out for each case report: author, origin country, patient’s age, gender, past medical history (including other complications that the patient has developed during hospitalisation), presenting symptoms, ICU stay duration (if not specified, mechanical ventilation duration was taken into consideration), mechanical ventilation or tracheostomy use, time to HO diagnosis after COVID-19 onset (if the duration was not explicitly stated, we calculated an approximative duration in months by considering the hospitalisation duration and the time of HO symptom onset or imaging), HO diagnosis technique, HO location(s), serum alkaline phosphatase value, treatment of HO, and follow-up.
Data analysis and synthesis
We summarised the extracted information qualitatively. Methods for synthesising qualitative data such as meta-analysis were not used, as we are providing a summary of case reports. Thus, no effect measures were calculated.
This review included 20 patients diagnosed with HO following COVID-19 infection, as reported in 15 published studies [ 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 ]. Table 1 provides a compilation of the extracted data from each article.
The first case was reported in September 2020 [ 6 ], and the last one in October 2022 [ 20 ]. The majority of the cases were reported from Europe (13 patients, 65%), followed by the Americas (6 patients, 30%), and finally from Asia (1 patient, 5%).
The age of the patients ranged from 23 to 76 years with a median age of 59 years and a standard deviation of 14.5 years. 80% of the patients were male.
63.2% of the patients with known past medical history ( n = 19) had hypertension, 15.8% of them had chronic obstructive pulmonary disease (COPD), and 21.1% of them had polyneuropathy of which one patient developed tetraparesis. One patient had a recent left humerus head fracture and multilocular cerebral infarction with left hemiparesis, and one patient had recent shoulder surgery. Other patients had deep vein thrombosis, pulmonary embolism, sepsis, and septic shock.
All patients required ICU care, with an average length of stay of 48.5 days (standard deviation: 26.7 days). Additionally, all patients required mechanical ventilation, and 30% underwent tracheostomy.
Of the patients for whom the presenting symptoms are known ( n = 19), stiffness of the affected joint and pain were the predominant symptoms of HO. One patient presented soft tissue swelling [ 12 ] and one patient had a palpable mass in the knee [ 19 ]. Symptoms prompted imaging to diagnose HO in 90% of the patients, while in two cases (10%), HO was incidentally detected during imaging requested for other purposes. On average, HO was diagnosed 2.8 months after the onset of COVID-19, with a standard deviation of 1.6 months.
X-rays were the most used diagnosis technique (80%), followed by computed tomography scans (CT-scans) (60%). Bone scintigraphy was carried out in 30% of cases. 15% of the patients underwent magnetic resonance imaging (MRI) and one patient underwent a single-photon emission computed tomography (SPECT). Of the 12 cases for which serum alkaline phosphatase fluctuations were reported, 11 of them showed elevated levels.
Across all patients, HO was found to be unilocular in 30% of cases and multilocular in 70% of cases. The most common location of HO was the hip joint, accounting for 65%. Additionally, the bilateral presentation of HO in the hip was more common (69.2%) than the unilateral presentation (30.8%). Following the hip, the shoulder joint was the second most common location for HO (55%) and was also more commonly found bilaterally (72.7%) than unilaterally (27.2%).
Table 2 summarises treatments received by the patients and their follow-up. For patients with known treatment ( n = 15), 86.7% of them received physical therapy. Three patients underwent surgery or had a surgical excision planned, and four patients were treated with nonsteroidal anti-inflammatory drugs (NSAIDs). Other therapeutic measures included corticosteroids and radiotherapy (refer to Table 3 for an overview of patient characteristics and trends). Out of 20 cases, follow-up data was available for seven patients. Among these, three experienced persistent mobility restriction, while three showed improvement in joint mobility.
HO is frequently divided into two groups: acquired HO, which is the most common, and rare genetic cases of fibrodysplasia ossificans progressiva and progressive osseous heteroplasia [ 3 , 4 ]. Three conditions are required for HO to develop: a local environment compatible with osteogenesis, an osteogenic precursor, and a triggering event [ 4 , 21 ]. Factors influencing the environment are pH, oxygen tension, micronutrients availability, and mechanical stimuli [ 22 ]. An insult triggers local inflammation with the recruitment of inflammatory cells including macrophages, lymphocytes, and mast cells, damaging skeletal muscle cells, which launches HO formation by inducing undifferentiated cell proliferation [ 21 ].
The prevalence of HO in patients with severe COVID-19 infection remains undetermined. Nevertheless, in a study by Stoira et al. [ 23 ]. which focused on a cohort of 52 COVID-19 infected patients admitted to the ICU and subjected to CT-scans, a notably high prevalence of 19.2% was observed. According to published case reports and case series, males were more commonly affected by HO, potentially due to sex-related differences that may influence predisposition [ 22 ]. 70% of the patients developed HO in multiple locations, with the hips and shoulders being the most frequently affected joints. Interestingly, these joints are also frequently affected in conditions such as traumatic brain injuries, spinal cord injuries, and burns [ 22 ].
Traumatic brain injury and spinal injury are known causes of HO formation [ 3 , 4 , 22 ]. Non-traumatic brain injuries were described as possible aetiologies for HO, such as vascular or anoxic brain injuries. This risk of developing HO is correlated to the severity of the brain lesions, and a higher occurrence in diffuse brain lesions was pointed out rather than focal brain lesions. It might be the result of mesenchymal cell differentiation into osteoblasts in ectopic tissues such as muscles, due to an anoxic insult [ 24 ]. Dahmen A. et al. [ 9 ] indicated that the patient had a prior history of multilocular cerebral infarction resulting in left hemiparesis, which could have contributed to the triggering of HO in addition to a humeral head fracture, with COVID-19 potentially confounding the situation.
Prolonged immobilisation and hypoxia have been identified in the literature as potential risk factors for HO [ 3 , 22 ]. Since the majority of reported cases involve prolonged stays in the ICU with mechanical ventilation or tracheostomy, this is a potential confounding factor for HO development. This finding is consistent with the study conducted by Stoira et al. where HO was linked to extended periods of mechanical ventilation and prolonged hospital stays [ 23 ]. Additionally, mechanical ventilation can induce a proinflammatory state [ 25 ], which may further contribute to the development of HO.
Mesenchymal cell function is influenced by type two diabetes, which can contribute to bone emergence [ 21 ]. Two patients were reported with diabetes mellitus in their past medical history, which could exacerbate the development of HO.
SARS-CoV-2 affects mostly the higher respiratory tract but can also affect the lower respiratory tract causing pneumonia, and an ARDS in severe infections. Disease severity is not only correlated to the viral infection, but to the inflammatory response as well [ 2 ]. In severe COVID-19 infections, uncontrolled inflammation can spread and result in multi-organ damage. It implicates macrophages, monocytes, and lymphocytes generating a cytokine storm. The angiotensin-converting enzyme two (ACE2) receptor, in conjunction with the transmembrane protease, serine two (TMPRSS2) allows the entry of SARS-CoV-2 into specific cell types, in particular type two pneumocytes. While other cells, such as smooth muscle cells, synovial cells, and articular cartilage, have been found to express these proteins, the musculoskeletal system is also a potential target for the viral infection. In addition to cytokines and a proinflammatory condition, it could possibly lead to muscle and joint diseases [ 25 ]. A clinical trial is necessary in order to demonstrate the relevance of this hypothesis. Furthermore, Davis et al. reported a case in 2012 of HO after prolonged intubation due to H1N1 influenza, highlighting the potential link between HO and ARDS caused by H1N1 infection. This underscores the need for further investigation into the association between HO and infection-related ARDS, offering potential avenues for future research in understanding the underlying mechanisms and developing targeted interventions for prevention and treatment [ 26 ].
HO’s diagnosis is based on the clinical history and on radiographic imaging, which has been performed in the majority of the reported cases. The most commonly reported patient complaints were joint stiffness, restriction of mobility, and pain. Radiography and CT-scans are the gold standards for diagnosis, although three-phase bone scintigraphy is the most sensitive medical imaging to detect HO, it is also recommended for follow-up and to determine the accurate stage for surgical excision. Moreover, ultrasonography (US) is an imaging technique that is safe, affordable and easy to use. It is sensitive for detecting soft tissue lesions and calcification. Its bedside application is particularly beneficial for bed-confined patients, while also enabling quantitative assessment of HO progression during rehabilitation through variations in grey-scale values across different stages of HO maturation [ 4 ]. Serum alkaline phosphatase levels, calcium, and phosphorus are not reliable markers for diagnosis, nor for prognostication of HO [ 22 ]. We note an elevation of serum alkaline phosphatase in 91.7% of the cases. This could serve as a potential indicator of HO development. However, further confirmation is required.
Treatment for HO is divided into two categories: prophylaxis for high-risk patients, and management of already developed ectopic bone. In prophylaxis, low-dose radiation and NSAIDs tend to deliver the same result, the latter being less costly. Physical therapy is controversial in the management of formed HO but is the most commonly used treatment in the patients included in the case reports of this review. Surgical excision is recommended when the ectopic bone growth has matured and a functional deficit persists [ 3 , 4 , 22 ]. Although there are no specific guidelines for treating patients who have developed HO after contracting severe COVID-19 infections, they present several risk factors that predispose them to HO. Therefore, prophylaxis could be employed in such cases. Some of the treated patients had residual effects, particularly reduced mobility, which could ultimately result in a decreased quality of life. New therapy lines targeting specific mediators are being tested and are giving promising effects like targeting the hypoxia-inducible factor 1-alpha that normally stimulates endothelial cell precursors subsequently to ischemia [ 22 ], or stimulating the retinoic acid receptor (RAR) that is a chondrogenesis’ inhibitor, or inhibiting the bone morphogenic protein (BMP) pathway implicated in the differentiation of the progenitor cells to endochondral differentiation or chondrogenesis lineage [ 21 ].
It is important to acknowledge several limitations of our study. Firstly, our study primarily relies on a review of case reports, which inherently presents limitations related to data consistency and comprehensiveness. Secondly, due to the nature of our study, we lack an accurate count of these cases, which impedes our ability to calculate the prevalence of HO within the population of COVID-19 patients. Additionally, the absence of quantitative analysis, including outcome and effect measures, limits our capacity to draw definitive conclusions about the clinical impact and outcomes associated with HO in this context. Furthermore, none of the included case reports provided data on bone density, which could have shed light on the relationship between bone resorption following immobilisation and HO development. In light of these limitations, our study serves as a preliminary exploration of HO in the context of COVID-19, emphasizing the need for more extensive and rigorous research in the future to address these shortcomings and provide a more comprehensive understanding of this phenomenon.
This systematic review provides a comprehensive overview of the clinical characteristics, diagnostic results, treatment options, and outcomes related to HO in COVID-19 patients. The study included 20 COVID-19 patients who developed HO. Most of them had at least two independent risk factors for developing HO, such as prolonged immobilisation and mechanical ventilation. The link between SARS-CoV-2 and HO remains uncertain, and multivariate analysis with adjustment for these risk factors are required. Although there is some evidence suggesting that SARS-CoV-2 might be targeting cells of the musculoskeletal system, it is unclear whether this is related to the development of HO. HO should nonetheless be suspected in patients with prolonged immobilisation, mechanical ventilation, and presenting joint pain and stiffness. This condition can have a significant impact on the patient’s quality of life, and its diagnosis is typically confirmed through radiographic imaging, which is considered the gold standard. The treatments of HO are controversial, and new studies are being conducted to explore new therapy lines. Therefore, it is important to continue investigating this pathology to identify effective treatment options and improve patient outcomes.
Data availability
All data generated or analysed during this study are included in this published article.
Abbreviations
coronavirus disease 2019
severe acute respiratory syndrome coronavirus two
intensive care unit
heterotopic ossification
chronic obstructive pulmonary disease
computed tomography
magnetic resonance imaging
single-photon emission computed tomography
nonsteroidal anti-inflammatory drugs
angiotensin-converting enzyme 2
hemagglutinin 1 neuraminidase 1
transmembrane protease, serine 2
retinoic acid receptor
bone morphogenic protein
Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–32. https://doi.org/10.1016/S0140-6736(20)32656-8
Article CAS PubMed PubMed Central Google Scholar
Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–74. https://doi.org/10.1038/s41577-020-0311-8
Meyers C, Lisiecki J, Miller S, Levin A, Fayad L, Ding C, et al. Heterotopic ossification: a Comprehensive Review. JBMR Plus. 2019;3(4):e10172. https://doi.org/10.1002/jbm4.10172
Article PubMed PubMed Central Google Scholar
Mujtaba B, Taher A, Fiala MJ, Nassar S, Madewell JE, Hanafy AK, et al. Heterotopic ossification: radiological and pathological review. Radiol Oncol. 2019;53(3):275–84. https://doi.org/10.2478/raon-2019-0039
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71
Ploegmakers DJM, Zielman-Blokhuis AM, van Duijnhoven HJR, de Rooy JWJ, Geurts ACH, Nonnekes J. Heterotope ossificatie na een covid-19-pneumonie [Heterotopic ossifications after COVID-19 pneumonia]. Ned Tijdschr Geneeskd. 2020;164:D5357.
PubMed Google Scholar
Meyer C, Haustrate MA, Nisolle JF, Deltombe T. Heterotopic ossification in COVID-19: a series of 4 cases. Ann Phys Rehabil Med. 2020;63(6):565–7. https://doi.org/10.1016/j.rehab.2020.09.010
Aziz A, Choudhari R, Alexander AJ, Allam E. Heterotopic ossification post COVID-19: report of two cases. Radiol Case Rep. 2021;16(2):404–9. https://doi.org/10.1016/j.radcr.2020.12.002
Article PubMed Google Scholar
Dahmen A, Roukens R, Lindenberg S, Peters KM. Heterotope Ossifikationen Nach Langzeitbeatmung Bei COVID-19. Osteologie. 2021;30(02):182–6. https://doi.org/10.1007/s15002-021-3269-4
Article Google Scholar
Peters J, Köhler HC, Oltmanns K, Besselmann M, Zwaan M, Gutcke A, et al. Heterotope Ossifikationen Nach Langzeitbeatmung Bei Covid-19 Erkrankung. Rehabil. 2021;60(4):231–4. https://doi.org/10.1055/a-1339-5365
Nieto Morales ML, Lara Martínez MF, Luna Gómez C, Bello Báez A, Allende Riera AJ. Osificación heterotópica en paciente con SARS-CoV-2: imágenes gammagráficas y radiológicas [Heterotopic ossification in SARS-CoV-2: Scintigraphic and radiological images]. Rehabilitacion. 2022;56(4):399–403. https://doi.org/10.1016/j.rh.2021.09.003
Article CAS PubMed Google Scholar
Brance ML, Cóccaro NM, Casalongue AN, Durán A, Brun LR. Extensive progressive heterotopic ossification post-covid-19 in a man. Bone. 2022;155:116287. https://doi.org/10.1016/j.bone.2021.116287
da Nóbrega Danda GJ. Ossificação heterotópica na COVID-19: relato de caso e revisão da literatur. Braz J Infect Dis. 2022;26(1):102046–102046. https://doi.org/10.1016/j.bjid.2021.102046
Grosjean D, Dekoster M, Beaudart C, Kaux JF. Ossifications hétérotopiques après une hospitalisation aux soins intensifs liée à Une Pneumopathie à SARS-CoV-2 [Heterotopic ossifications after hospitalisation in intensive care for SARS-CoV-2 pneumopathy]. Rev Med Liege. 2022;77(1):13–7.
CAS PubMed Google Scholar
Minjauw C, Wautier D, Mundama M. Mono-articular idiopathic heterotopic ossification in a coronavirus infected patient admitted in the intensive care unit. Acta Orthop Belg. 2022;88(1):206–10. https://doi.org/10.52628/88.1.26
Van Ochten N, Shori A, Benert J, Puderbaugh M, Krishnamurthy M. Heterotopic ossification in Post-COVID-19 patient on Anticoagulation with Limited Treatment options. Arch Phys Med Rehabil. 2022;103(3):e34. https://doi.org/10.1016/j.apmr.2022.01.094
Article PubMed Central Google Scholar
Vardar S, Özsoy Ünübol T, Ata E, Yılmaz F. A case report of a patient with COVID-19 infection and widespread heterotopic ossification. Turk J Phys Med Rehabil. 2022;68(1):149–53. https://doi.org/10.5606/tftrd.2022.8172
Micolich Vergara A, Marsico S, Solano López A, Zuccarino F. Bilateral intercostal, subscapular and teres major heterotopic ossifications in a 63-year-old male with COVID-19. Oxf Med Case Rep. 2022;2022(3):omac024. https://doi.org/10.1093/omcr/omac024
Article CAS Google Scholar
Liu J, Luther L, Dwivedi S, Evans AR. Long-term Orthopedic Manifestations of COVID-19: Heterotopic Ossification and Digital Necrosis. R I, Med J. 2013. 2022;105(7):31–5.
Castro JM, De-la-hoz JJ, Valiente JM, Feliu E, Llamas A. Osificación heterotópica masiva en un paciente con infección por SARS-CoV-2. Reporte De caso [Massive heterotopic ossification in a patient with SARS-COV-2 infection. Case report]. Rev Chil Radiol. 2022;28(3):109–12. https://doi.org/10.24875/rchrad.21000007
Łęgosz P, Drela K, Pulik Ł, Sarzyńska S, Małdyk P. Challenges of heterotopic ossification-molecular background and current treatment strategies. Clin Exp Pharmacol Physiol. 2018;45(12):1229–35. https://doi.org/10.1111/1440-1681.13025
Ranganathan K, Loder S, Agarwal S, Wong VW, Forsberg J, Davis TA, et al. Heterotopic ossification: Basic-Science principles and clinical correlates. J Bone Jt Surg. 2015;97(13):1101–11. https://doi.org/10.2106/JBJS.N.01056
Stoira E, Elzi L, Puligheddu C, Garibaldi R, Voinea C, Chiesa AF, et al. High prevalence of heterotopic ossification in critically ill patients with severe COVID-19. Clin Microbiol Infect. 2021;27(7):1049–50. https://doi.org/10.1016/j.cmi.2020.12.037
Bargellesi S, Cavasin L, Scarponi F, De Tanti A, Bonaiuti D, Bartolo M, et al. Occurrence and predictive factors of heterotopic ossification in severe acquired brain injured patients during rehabilitation stay: cross-sectional survey. Clin Rehabil. 2018;32(2):255–62. https://doi.org/10.1177/0269215517723161
Disser NP, De Micheli AJ, Schonk MM, Konnaris MA, Piacentini AN, Edon DL, et al. Musculoskeletal consequences of COVID-19. J Bone Joint Surg Am. 2020;102(14):1197–204. https://doi.org/10.2106/JBJS.20.00847
Davis C, Kolovich GP, Scharschmidt TJ. Atraumatic heterotopic ossification in the setting of prolonged intubation because of H1N1 influenza: a case report. Orthop Surg. 2012;4(4):258–62. https://doi.org/10.1111/os.12009
Download references
Acknowledgements
Not applicable.
The authors declare that no financial assistance or other forms of support were received while preparing the manuscript.
Author information
Authors and affiliations.
Université Libre de Bruxelles, 808 route de Lennik, Anderlecht, 1070, Belgium
Hachem Chaitani
Department of Orthopaedic Surgery, Saint-Pierre University Hospital, 105 rue aux Laines, Brussels, 1000, Belgium
Laurent Fabeck & Simon Koulischer
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the study conception and design. HC and SK conducted the material preparation and the literature review. Data collection and analysis were performed by HC and cross checked by LF. All authors contributed to the interpretation of data. All authors contributed to writing the manuscript, read and approved the final version.
Corresponding author
Correspondence to Hachem Chaitani .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Chaitani, H., Fabeck, L. & Koulischer, S. Heterotopic ossification following COVID-19 infections: systematic literature review of case reports and case series. BMC Musculoskelet Disord 25 , 421 (2024). https://doi.org/10.1186/s12891-024-07537-4
Download citation
Received : 18 March 2023
Accepted : 23 May 2024
Published : 29 May 2024
DOI : https://doi.org/10.1186/s12891-024-07537-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Heterotopic ossification
- Ectopic ossification
- Myositis ossificans
- Coronavirus
BMC Musculoskeletal Disorders
ISSN: 1471-2474
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Advertisement
Extra-skeletal intracranial mesenchymal chondrosarcoma: systematic-literature review
- Published: 19 May 2024
Cite this article

- Sivaraman Kumarasamy 1 ,
- Kanwaljeet Garg 1 ,
- Ajay Garg 2 ,
- M. C. Sharma 3 ,
- Manmohanjit Singh 1 ,
- Poodipedi Sarat Chandra 1 &
- Shashank Sharad Kale 1
61 Accesses
1 Altmetric
Explore all metrics
Intracranial mesenchymal chondrosarcoma (IMC) is a rare malignant tumor in pediatric population. IMC can present as extra- or intra-axial lesion in pediatric patients, though the former is commoner causing raised intracranial pressure (ICP). Radiological diagnosis is a challenge in these cases, as is it difficult to differentiate these from other extra-axial neoplasms due to the wide differential diagnosis in pediatric population. We aim to systematically review the literature and present a rare case of extraskeletal intracranial mesenchymal chondrosarcoma treated with safe maximal resection.
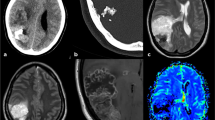
A systematic review of literature was conducted in accordance with PRISMA guidelines. PubMed and Scopus databases were queried using the search terms, “primary intracranial chondrosarcoma”, “extraskeletal mesenchymal chondrosarcoma”, “mesenchymal chondrosarcoma” and “pediatric”. Presentation, surgical management and outcome of a 15-year-old male with an extraskeletal IMC are also described.
The search yielded 25 articles which met the inclusion criteria. These published records consisted of 33 IMC cases with mean age at presentation of 9.81 ± 5.2 years (range 2 months to 18 years). Frontal region was the commonest locations (11, 33.3%). Most common presentation was headache (14, 42.4%). All patients underwent surgical intervention: gross total resection (20, 60.6%), subtotal resection (9, 27.3%) and no extent mentioned (4, 12.1%). No adjuvant therapy was received in 15 patients (45.5%). On latest follow-up, 11 patients (33.3%) are on remission, 5 patients (15.2%) are symptom free, 3 patients (9.1%) had recurrence, 2 patients (6.1%) had metastasis and 9 patients (27.3%) expired.
IMC is a rare entity in pediatric population with imaging findings which are non-characteristic leading to its diagnostic challenge. It can masquerade as other extra-axial intracranial neoplasm (meningioma or hemangiopericytoma). Combination of clinico-radiological and pathological examination can help in accurate diagnosis. Safe Maximal resection followed by radiotherapy is the preferred treatment strategy.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Intracranial extra-axial mesenchymal chondrosarcoma in a 16-month-old patient with a literature review of pediatric patients
A multicenter retrospective analysis of clinical outcomes of intracranial chondrosarcoma in 26 patients
Primary extraosseous dural chondrosarcoma: a case report
Data availability.
Data is provided within the manuscript.
Beena D, Kattoor J, Mathews A (2021) Mesenchymal chondrosarcoma-a retrospective study. Gulf J Oncol 1(35):54–58
Google Scholar
Bloch OG, Jian BJ, Yang I, Han SJ, Aranda D, Ahn BJ et al (2009) A systematic review of intracranial chondrosarcoma and survival. J Clin Neurosci 16(12):1547–1551
Article PubMed PubMed Central Google Scholar
Chen J-Y, Hsu S-S, Ho J-T (2004) Extraskeletal intracranial mesenchymal chondrosarcoma: case report and literature review. Kaohsiung J Med Sci 20(5):240–246
Article PubMed Google Scholar
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D et al (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23(8):1231–1251
Article CAS PubMed PubMed Central Google Scholar
Lightenstein L, Bernstein D (1959) Unusual benign and malignant chondroid tumors of bone. A survey of some mesenchymal cartilage tumors and malignant chondroblastic tumors, including a few multicentric ones, as well as many atypical benign chondroblastomas and chondromyxoid fibromas. Cancer 12:1142–1157
Article CAS PubMed Google Scholar
Dahlin DC, Henderson ED (1962) Mesenchymal chondrosarcoma. Further observations on a new entity. Cancer 15(2):410–417
Oruckaptan HH, Berker M, Soylemezoglu F, Ozcan OE (2001) Parafalcine chondrosarcoma: an unusual localization for a classical variant: case report and review of the literature. Surg Neurol 55(3):174–179
Aigner T, Loos S, Müller S, Sandell LJ, Unni KK, Kirchner T (2000) Cell differentiation and matrix gene expression in mesenchymal chondrosarcomas. Am J Pathol 156(4):1327–1335
Shabani S, Kaushal M, Kaufman B, Knipstein J, Lawlor MW, Lew S et al (2019) Intracranial extraskeletal mesenchymal chondrosarcoma: case report and review of the literature of reported cases in adults and children. World Neurosurgery 129:302–310
Scheithauer BW, Rubinstein LJ (1978) Meningeal mesenchymal chondrosarcoma. Report of 8 cases with review of the literature. Cancer 42(6):2744–2752
Ostlere S, McDonald B, Athanasou N (1999) Mesenchymal chondrosarcoma associated with Goldenhar’s syndrome. Arch Orthop Trauma Surg 119:347–348
Ventura E, Dionísio S, Ferreira Â, Saleiro R, Marques H, Magalhães M et al (2020) Maxillary mesenchymal chondrosarcoma leading to a diagnosis of Li-Fraumeni syndrome. J Surg Case Rep 2020(1):rjz386
Dini LI, Isolan GR, Saraiva GA, Dini SA, Gallo P (2007) Maffucci’s syndrome complicated by intracranial chondrosarcoma: Two new illustrative cases. Arq Neuropsiquiatr 65:816–821
Frezza AM, Cesari M, Baumhoer D, Biau D, Bielack S, Campanacci DA et al (2015) Mesenchymal chondrosarcoma: Prognostic factors and outcome in 113 patients. A European Musculoskeletal Oncology Society study. Eur J Cancer 51(3):374–81
Chu J, Ma H, Wang Y, Li K, Liao C, Ding Y (2022) CT and MRI findings of intracranial extraskeletal mesenchymal chondrosarcoma—a case report and literature review. Transl Cancer Res 11(9):3409
Sadashiva N, Sharma A, Shukla D, Rajalakshmi P, Mahadevan A, Devi BI (2016) Intracranial extraskeletal mesenchymal chondrosarcoma. World Neurosurg 95:618–e1
Chi J, Zhang M, Kang J (2016) Classical intracranial chondrosarcoma: a case report. Oncol Lett 12(5):4051–4053
Yang MJ, Whelan R, Madden J, Mulcahy Levy JM, Kleinschmidt-DeMasters B, Hankinson TC et al (2018) Intracranial Ewing sarcoma: four pediatric examples. Childs Nerv Syst 34:441–448
Chen Q, Chen X-Z, Wang J-M, Li S-W, Jiang T, Dai J-P (2012) Intracranial meningeal hemangiopericytomas in children and adolescents: CT and MR imaging findings. Am J Neuroradiol 33(1):195–199
Kumarasamy S, Garg K, Sharma MC, Chandra PS (2023) Intracranial myeloid sarcoma as the first presentation of acute myeloid leukemia and literature review. Childs Nerv Syst 39(12):3607–3612
Kumarasamy S, Satyarthee GD (2024) Supratentorial extra-ventricular ependymoma as a mass lesion in a child: report and literature review. Childs Nerv Syst 2:1–7
Choi JH, Ro JY (2021) The 2020 WHO classification of tumors of bone: an updated review. Adv Anat Pathol 28(3):119–138
Shakked RJ, Geller DS, Gorlick R, Dorfman HD (2012) Mesenchymal chondrosarcoma: Clinicopathologic study of 20 cases. Arch Pathol Lab Med 136(1):61–75
Arora K, Riddle ND (2018) Extraskeletal mesenchymal chondrosarcoma. Arch Pathol Lab Med 142(11):1421–1424
Yapıcıer Ö, Nanah AR, Taskapılıoglu MÖ, Demir MK (2021) Intracranial extra-axial mesenchymal chondrosarcoma in a 16-month-old patient with a literature review of pediatric patients. Childs Nerv Syst 37(2):649–657
El Beaino M, Roszik J, Livingston JA, Wang W-L, Lazar AJ, Amini B et al (2018) Mesenchymal chondrosarcoma: a review with emphasis on its fusion-driven biology. Curr Oncol Rep 20:1–11
Article Google Scholar
Neff B, Sataloff RT, Storey L, Hawkshaw M, Spiegel JR (2002) Chondrosarcoma of the skull base. Laryngoscope 112(1):134–139
Sikdar D, Gupta S, Roshan R, Agarwal S, Joseph DM, Gupta M (2023) Primary intracranial extraskeletal mesenchymal chondrosarcoma of the brain in a pediatric patient: A case report and review of literature. Indian J Med Paediat Oncol 14
Isaacson B, Kutz JW, Roland PS (2007) Lesions of the petrous apex: diagnosis and management. Otolaryngol Clin North Am 40(3):479–519
Flyger G, Freidenfeldt H, Orell S (1963) Intracerebral, possibly malignant osteochondrofibroma in a child. Acta Pathol Microbiol Scand 58(3):299–305
Wu WQ, Lapi A (1970) Primary non-skeletal intracranial cartilaginous neoplasms: report of a chondroma and a mesenchymal chondrosarcoma. J Neurol Neurosurg Psychiatry 33(4):469–475
Salvador AH, Beabout JW, Dahlin DC (1971) Mesenchymal chondrosarcoma—observations on 30 new cases. Cancer 28(3):605–615
Lynch P, Uriburu E (1973) An intracranial cartilage-containing meningeal tumor: case report. J Neurosurg 39(2):261–264
Rollo J, Green W, Kahn L (1979) Primary meningeal mesenchymal chondrosarcoma. Arch Pathol Lab Med 103(5):239–243
CAS PubMed Google Scholar
Kobayashi T, Yoshida J, Kageyama N, Makita Y, Aoyama I, Yamashita J (1980) Mesenchymal chondrosarcoma arising from dura mater (author’s transl). No Shinkei Geka Neurol Surg 8(9):881–887
CAS Google Scholar
Rodda R, Franklin C (1984) Intracranial meningeal chondrosarcoma—probable mesenchymaltype. Aust N Z J Surg 54(4):387–390
Nagata S, Sawada K, Kitamura K (1986) Chondrosarcoma arising from the falx cerebri. Surg Neurol 25(5):505–509
Chhem R, Bui B, Calderon-Villar H, Fontaine S (1992) Case report: primary mesenchymal chondrosarcoma of the brain. Clin Radiol 45(6):422–423
Cho B-K, Chi JG, Wang KC, Chang KH, Choi KS (1993) Intracranial mesenchymal chondrosarcoma: a case report and literature review. Childs Nerv Syst 9:295–299
Schut L, Canady AI, Sutton LN, Bruce DA (1994) Meningeal tumors in children. Pediatr Neurosurg 20(3):207–213
Malik SN, Farmer PM, Hajdu SI, Rosenthal A (1996) Mesenchymal chondrosarcoma of the cerebellum. Ann Clin Lab Sci 26(6):496–500
Rushing EJ, Armonda RA, Ansari Q, Mena H (1996) Mesenchymal chondrosarcoma: a clinicopathologic and flow cytometric study of 13 cases presenting in the central nervous system. Cancer: Interdiscip Int J Am Cancer Soc 77(9):1884–1891
Article CAS Google Scholar
Nozaki K, Nagata I, Takahashi J, Hashimoto N (1999) Intracranial mesenchymal chondrosarcoma associated with a left transverse sigmoid dural AV fistule. Acta Neurochir 141(3):327
Crosswell H, Buchino JJ, Sweetman R, Reisner A (2000) Intracranial mesenchymal chondrosarcoma in an infant. Med Pediatr Oncol: Official J SIOP—Int Soc Pediatr Oncol (Soc Int Oncol Pédiatr) 34(5):370–374
La Spina M, Dollo C, Giangaspero F, Bertolini P, Russo G (2003) Intracranial mesenchymal chondrosarcoma with osteoid formation: report of a pediatric case. Childs Nerv Syst 19:680–682
Huang Y-L, Lai P-H, Lin S-L, Yuan M-K, Pan H-B (2004) Mesenchymal chondrosarcoma at the falx cerebri. 中華放射線醫學雜誌 29(6):337–341
Waliuddin A, Jamjoom AB, Thomas J (2006) Intracranial extraskeletal mesenchymal chondrosarcoma. Neurosci J 11(3):205–209
De Cecio R, Migliaccio I, Falleti J, Del De Caro MB, Pettinato G (2008) Congenital intracranial mesenchymal chondrosarcoma: case report and review of the literature in pediatric patients. Pediatr Dev Pathol 11(4):309–313
Fanburg-Smith JC, Auerbach A, Marwaha JS, Wang Z, Santi M, Judkins AR et al (2010) Immunoprofile of mesenchymal chondrosarcoma: aberrant desmin and EMA expression, retention of INI1, and negative estrogen receptor in 22 female-predominant central nervous system and musculoskeletal cases. Ann Diagn Pathol 14(1):8–14
Sardi I, Massimino M, Genitori L, Buccoliero AM, Giangaspero F, Ferrari A (2011) Intracranial mesenchymal chondrosarcoma: report of two pediatric cases. Pediatr Blood Cancer 56(4):685–686
Fu L-Y, Han Q, Cheng P, Yang H-J, Zhao Y (2021) Rare case report and literature review of intracranial mesenchymal chondrosarcoma. Ann Palliat Med 10(11):12012–12017
Download references
Author information
Authors and affiliations.
Department of Neurosurgery, All India Institute of Medical Sciences, Room No 720, CNC, New Delhi, India
Sivaraman Kumarasamy, Kanwaljeet Garg, Manmohanjit Singh, Poodipedi Sarat Chandra & Shashank Sharad Kale
Department of Neuroradiology, All India Institute of Medical Sciences, New Delhi, India
Department of Neuropathology, All India Institute of Medical Sciences, New Delhi, India
M. C. Sharma
You can also search for this author in PubMed Google Scholar
Contributions
Sivaraman Kumarasamy — Conception & design of work, acquisition, analysis, interpretation of data and drafted the work. Kanwaljeet Garg — Analysis, interpretation of data and revised the work. Ajay Garg — Interpretation of data and revised the work. M C Sharma — Interpretation of data and revised the work. Manmohanjit singh — Interpretation of data and revised the work. Poodipedi Sarat Chandra — Interpretation of data and revised the work. Shashank Sharad Kale — Interpretation of data and revised the work. All authors have approved the submitted version (and any substantially modified version that involves the author’s contribution to the study) AND agreed both to be personally accountable for the contributions and to ensure that questions related to the accuracy or integrity of any part of the work.
Corresponding author
Correspondence to Kanwaljeet Garg .
Ethics declarations
Ethical approval.
Consent to participate and consent for publication was taken from the patient using written informed consent to publish from the parent. Ethics committee approval was obtained.
Conflict of interest
The authors declare no competing interests
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Kumarasamy, S., Garg, K., Garg, A. et al. Extra-skeletal intracranial mesenchymal chondrosarcoma: systematic-literature review. Childs Nerv Syst (2024). https://doi.org/10.1007/s00381-024-06452-2
Download citation
Received : 06 February 2024
Accepted : 09 May 2024
Published : 19 May 2024
DOI : https://doi.org/10.1007/s00381-024-06452-2

Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Intracranial mesenchymal chondrosarcoma
- Extra-axial
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 27 May 2024
Patients’ satisfaction with cancer pain treatment at adult oncologic centers in Northern Ethiopia; a multi-center cross-sectional study
- Molla Amsalu 1 ,
- Henos Enyew Ashagrie 2 ,
- Amare Belete Getahun 2 &
- Yophtahe Woldegerima Berhe ORCID: orcid.org/0000-0002-0988-7723 2
BMC Cancer volume 24 , Article number: 647 ( 2024 ) Cite this article
22 Accesses
Metrics details
Patient satisfaction is an important indicator of the quality of healthcare. Pain is one of the most common symptoms among cancer patients that needs optimal treatment; rather, it compromises the quality of life of patients.
To assess the levels and associated factors of satisfaction with cancer pain treatment among adult patients at cancer centers found in Northern Ethiopia in 2023.
After obtaining ethical approval, a multi-center cross-sectional study was conducted at four cancer care centers in northern Ethiopia. The data were collected using an interviewer-administered structured questionnaire that included the Lubeck Medication Satisfaction Questionnaire (LMSQ). The severity of pain was assessed by a numerical rating scale from 0 to 10 with a pain score of 0 = no pain, 1–3 = mild pain, 4–6 = moderate pain, and 7–10 = severe pain Binary logistic regression analysis was employed, and the strength of association was described in an adjusted odds ratio with a 95% confidence interval.
A total of 397 cancer patients participated in this study, with a response rate of 98.3%. We found that 70.3% of patients were satisfied with their cancer pain treatment. Being married (AOR = 5.6, CI = 2.6–12, P < 0.001) and being single (never married) (AOR = 3.5, CI = 1.3–9.7, P = 0.017) as compared to divorced, receiving adequate pain management (AOR = 2.4, CI = 1.1–5.3, P = 0.03) as compared to those who didn’t receive it, and having lower pain severity (AOR = 2.6, CI = 1.5–4.8, P < 0.001) as compared to those who had higher level of pain severity were found to be associated with satisfaction with cancer pain treatment.
The majority of cancer patients were satisfied with cancer pain treatment. Being married, being single (never married), lower pain severity, and receiving adequate pain management were found to be associated with satisfaction with cancer pain treatment. It would be better to enhance the use of multimodal analgesia in combination with strong opioids to ensure adequate pain management and lower pain severity scores.
Peer Review reports
Introduction
Pain is defined as an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage [ 1 ]. The prevalence of pain in cancer patients is 44.5-66%. with the prevalence of moderate to severe pain ranging from 30 to 38%, and it can persist in 5-10% of cancer survivors [ 2 ]. Using the World Health Organization’s (WHO) cancer pain management guidelines can effectively reduce cancer-related pain in 70-90% of patients [ 3 , 4 ]. Compared to traditional pain states, the mechanism of cancer-related pain is less understood; however, cancer-specific mechanisms, inflammatory, and neuropathic processes have been identified [ 5 ]. Uncontrolled pain can negatively affect patients’ daily lives, emotional health, social relationships, and adherence to cancer treatment [ 6 ]. Patients with moderate to severe pain have a higher fatigue score, a loss of appetite, and financial difficulties [ 7 ]. Patients fear the pain caused by cancer more than dying from the disease since pain affects their physical and mental aspects of life [ 8 ]. A meta-analysis of 30 studies stated that pain was found to be a significant prognostic factor for short-term survival in cancer patients [ 9 ]. Many cancer patients have a very poor prognosis. However, adequate pain treatment prevents suffering and improves their quality of life. Although the WHO suggested non-opioids for mild pain, weak opioids for moderate pain, and strong opioids for severe pain, pain treatment is not yet adequate in one-third of cancer patients [ 10 ].
Patient satisfaction with pain management is a valuable measure of treatment effectiveness and outcome. It could be used to evaluate the quality of care [ 11 , 12 , 13 ]. Patient satisfaction affects treatment compliance and adherence [ 12 ]. Studies have reported that 60-76% of patients were satisfied with pain treatment, and a variety of factors were found associated with levels of satisfaction [ 3 , 14 , 15 , 16 ]. Studies conducted in Ethiopia reported the prevalence of pain ranging from 59.9 to 93.4% [ 17 , 18 ]. These studies indicate that cancer pain is inadequately treated. Assessment of pain treatment satisfaction can help identify appropriate treatment modalities and further its effectiveness. We conducted this study since there was limited research-based evidence on cancer pain management in low-income countries like Ethiopia. Our research questions were: how satisfied are adult cancer patients with pain treatment, and what are the factors associated with the satisfaction of adult cancer patients with pain treatment?
Methodology
Study design, area, period, and population.
A multi-center cross-sectional study was conducted at four cancer care centers in Amhara National Regional State, Northern Ethiopia from March to May 2023. Those cancer care centers were found in the University of Gondar Comprehensive Specialized Hospital (UoGCSH), Felege-Hiwot Comprehensive Specialized Hospital (FHCSH), Tibebe-Ghion Comprehensive Specialised Hospital (TGCSH) and Dessie Comprehensive Specialized Hospital (DCSH). We selected these centers as they were the only institutions providing oncologic care in the region during the study period.
The UoGCSH had 28 beds in its adult oncology ward and serves 450 cancer patients every month. Three specialist oncologists and 12 nurses provide services in the ward. The FHCSH had 22 beds and provides services for 325 cancer patients every month. Two specialist oncologists, two oncologic nurses, and 7 comprehensive nurses provide services. The TGCSH had eight beds and serves 300 cancer patients every month. There were three specialist oncologists and four oncologic nurses at the care center. The cancer care center at DCSH had 10 beds. It serves 350 cancer patients every month. There was one specialist oncologist, three oncologic nurses, and three comprehensive nurses.
All cancer patients who attended those cancer care centers were the source population, and adult (18+) cancer patients who were prescribed pain treatment for a minimum of one month were the study population. Unconscious patients, patients with psychiatric problems, patients with advanced cancer who were unable to cooperate, and patients with oncologic emergencies were excluded from this study.
Variables and operational definitions
The outcome variable was patient satisfaction with cancer pain treatment, which was measured by the Lubeck Medication Satisfaction Questionnaire. The independent variables were sociodemographic (age, sex, marital status, monthly income, and level of education), clinical (site of tumor, stage of cancer, metastasis), cancer treatment (surgery, chemotherapy, radiotherapy), level of pain, and analgesia (type of analgesia, severity of pain, adequacy of pain treatment, adjuvant analgesic).
- Patient satisfaction
perceptions of the patients regarding the outcome of pain management and the extent to which it meets their needs and expectations. It was measured by a 4-point Likert scale (1 = strongly disagree, 2 = disagree, 3 = agree, 4 = strongly agree) using the LMSQ which has 18 items within 6 subscales that have 3 items in each (effectivity, practicality, side-effects, daily life, healthcare providers, and overall satisfaction) [ 19 ]. Final categorization was done by dichotomizing into satisfied and dissatisfied by using the demarcation threshold formula.
\((\frac{\text{T}\text{o}\text{t}\text{a}\text{l}\,\,\text{h}\text{i}\text{g}\text{h}\text{e}\text{s}\text{t}\,\,\text{s}\text{c}\text{o}\text{r}\text{e} - \text{T}\text{o}\text{t}\text{a}\text{l}\,\, \text{l}\text{o}\text{w}\text{e}\text{s}\text{t}\,\, \text{s}\text{c}\text{o}\text{r}\text{e} }{2}\) ) + Total lowest score [ 20 ]. The highest patient satisfaction score was 70 and the lowest satisfaction score was 26. A score < 48 was classified as dissatisfied, and a score ≥ 48 was classified as satisfied.
The Numeric rating scale (NRS) is a validated pain intensity assessment tool that helps to give patients a subjective feeling of pain with a numerical value between 0 and 10, in which 0 = no pain, 1–3 = mild pain, 4–6 = moderate pain, 7–10 = severe pain [ 21 ].
The Adequacy of cancer pain treatment was measured by calculating the Pain Management Index (PMI) according to the recommendations of the WHO pain management guideline [ 22 ]. The PMI was calculated by considering the prescribed most potent analgesic agent and the worst pain reported in the last 24 h [ 23 ]. The prescribed analgesics were scored as follows: 0 = no analgesia, 1 = non-opioid analgesia, 2 = weak opioids, and 3 = strong opioids. The PMI was calculated by subtracting the reported NRS value from the type of most potent analgesics administered. The calculated values of PMI ranged from − 3 (no analgesia therapy for a patient with severe pain) to + 3 (strong opioid for a patient with no pain). Patients with a positive PMI value were considered to be receiving adequate analgesia, whereas those with a negative PMI value were considered to be receiving inadequate analgesia.
Sample size determination and sampling technique
A single population proportion formula was used to determine the sample size by considering 50% satisfaction with cancer pain treatment and a 5% margin of error at a 95% confidence interval (CI). A non-probability (consecutive) sampling technique was employed to attain a sample size within two months of data collection period. After adjusting the proportional allocation for each center and adding 5% none response, a total of 404 study participants were included in the study: 128 from the University of Gondar Comprehensive Specialized Hospital, 99 from Dessie Comprehensive Specialized Hospital, 92 from Felege Hiwot Comprehensive Specialized Hospital, and 85 from Tibebe Ghion Comprehensive Specialized Hospital.
Data collection, processing, and analysis
Ethical approval.
was obtained from the Ethical Review Committee of the School of Medicine at the University of Gondar ( Reference number: CMHS/SM/06/01/4097/2015) . Data were collected using an interviewer-administered structured questionnaire and chart review during outpatient and inpatient hospital visits by four trained data collectors (one for every center). Written informed consent was obtained from each participant after detailed explanations about the study. Informed consent with a fingerprint signature was obtained from patients who could not read or write after detailed explanations by the data collectors as approved by the Ethical Review Committee of the School of Medicine, at the University of Gondar.
Questions to assess the severity of pain and pain relief were taken from the American Pain Society patient outcome questionnaire [ 24 ]. Patients were asked to report the worst and least pain in the past 24 h and the current pain by using a numeric rating scale from 0 to 10, with a pain score of 0 = no pain, 1–3 = mild pain, 4–6 = moderate pain, 7–10 = severe pain.
The Pain Management Index (PMI) based on WHO guidelines, was used to quantify pain management by measuring the adequacy of cancer pain treatment [ 25 ]. The following scores were given (0 = no analgesia, 1 = non-opioid analgesia, 2 = weak opioid 3 = strong opioid). Pain Management Index was calculated by subtracting self-reported pain level from the type of analgesia administered and ranges from − 3 (no analgesic therapy for a patient with severe pain) to + 3 (strong opioid for a patient with no pain). The level of pain was defined as 0 with no pain, 1 for mild pain, 2 for moderate pain, and 3 for severe pain. Patients with negative PMI scores received inadequate analgesia.
The pain treatment satisfaction was measured by the Lübeck Medication Satisfaction Questionnaire (LMSQ) consisting of 18 items [ 19 ]. Lübeck Medication Satisfaction Questionnaire (LMSQ) has six subclasses each consisting of equally waited and similar context of three items. The subclass includes satisfaction with the effectiveness of pain medication, satisfaction with the practicality or form of pain medication, satisfaction with the side effect profile of pain medication, satisfaction with daily life after receiving pain treatment, satisfaction with healthcare providers, and overall satisfaction. Satisfaction was expressed by a four-point Likert scale (4 = Strongly Agree, 3 = Agree, 2 = Disagree, 1 = Strongly Disagree). The side effect subclass was phrased negatively, marked with Asterix, and reverse-scored in STATA before data analysis.
Data were collected with an interviewer-administered questionnaire. The reliability of the questionnaire was assessed by using 40 pretested participants and the reliability coefficient (Cronbach’s alpha value) of the questionnaire was 91.2%. The collected data was checked for completeness, accuracy, and clarity by the investigators. The cleaned and coded data were entered in Epi-data software version 4.6 and exported to STATA version 17. The Shapiro-Wilk test, variance inflation factor, and Hosmer-Lemeshow test were used to assess distribution, multicollinearity, and model fitness, respectively. Descriptive, Chi-square and binary logistic regression analyses were performed to investigate the associations between the independent and dependent variables. The independent variables with a p-value < 0.2 in the bivariable binary logistic regression were fitted to the final multivariable binary logistic regression analysis. Variables with p-value < 0.05 in the final analysis were considered to have a statistically significant association. The strength of associations was described in adjusted odds ratio (AOR) at a 95% confidence interval.
Sociodemographic and clinical characteristics
A total of 397 patients were involved in this study (response rate of 98.3%). Of the participants, 224 (56.4%) were female, and over half were from rural areas ( n = 210, 52.9%). The median (IQR) age was 48 (38–59) years [Table 1 ]. The most common type of cancer was gastrointestinal cancer 114 (28.7%). Most of the study participants, 213 (63.7%), were diagnosed with stage II to III cancer. The majority of the participants were taking chemotherapy alone (292 (73.6%)) [Table 2 ]. Over 90% of patients reported pain; 42.3% reported mild pain, 39.8% reported moderate pain, and 10.1% reported severe pain. Pain treatment adequacy was assessed by self-reports from study participants following pain management guidelines, and 17.1% of patients responded to having inadequate pain treatment. The majority of patients, 132 (33.3%), were prescribed combinations of non-opioid and weak opioid analgesics for cancer pain treatment. Only 34 (8.6%) cancer patients used either strong opioids alone or in combination with non-opioid analgesics.
Patients’ satisfaction with cancer pain treatment and correlation among the subscales
Most participants strongly agree (243, (61.2%)) with item LMSQ18 in the “overall satisfaction” subscale and strongly disagree (206, (51.9%)) for item LMSQ2 in the “side-effect” subscale respectively [Table 3 ]. The highest satisfaction score was observed in the side-effect subscale, with a median (IQR) of 10 (9–11) [Table 4 ].
Two hundred and seventy-nine (70.3%) cancer patients were found to be satisfied with cancer pain treatment (CI = 65.6−74.6%). The highest satisfaction rate was observed in the “side-effects” subscale, to which 343 (86.4%) responded satisfied [Fig. 1 ]. A Spearman’s correlation test revealed that there were correlations among the subscales of LMSQ; and the strongest positive correlation was observed between effectivity and healthcare workers subscale (r s = 0.7, p < 0.0001). The correlation among the subscales is illustrated in a heatmap [Fig. 2 ].
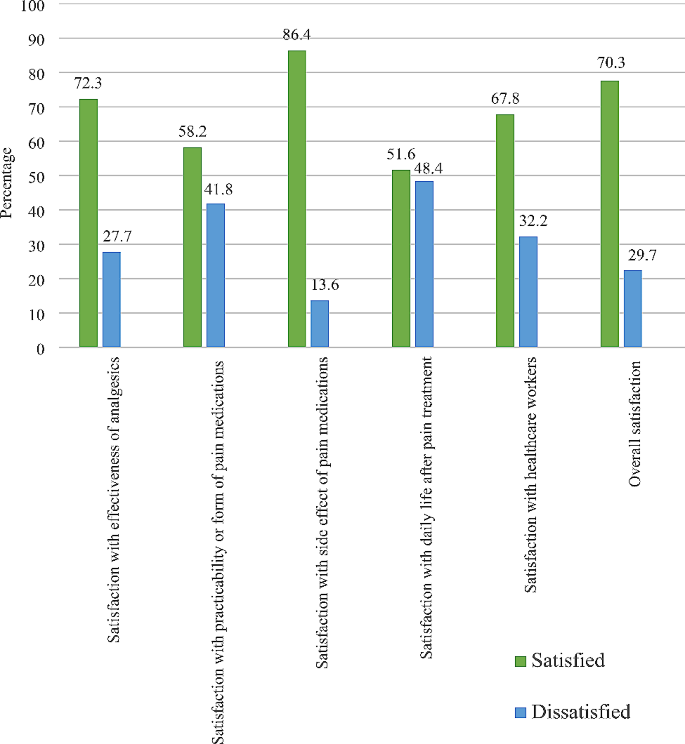
Patient satisfaction with cancer pain treatment with each LMSQ subclass, n = 397
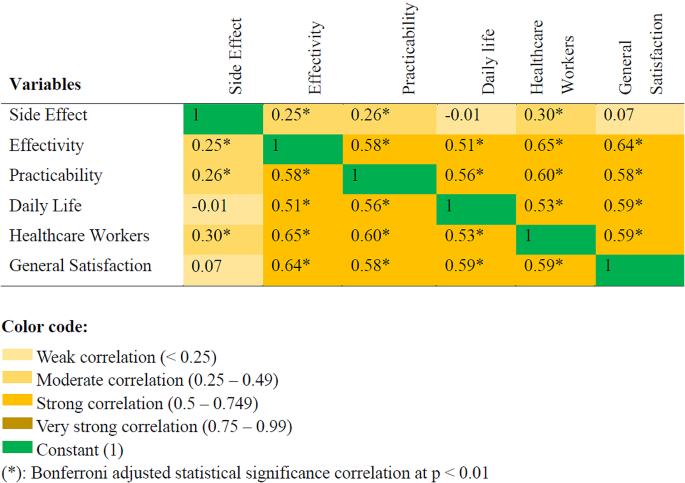
A heatmap showing the Spearman correlation of each subclass of pain treatment satisfaction, n = 397
Factors associated with patient satisfaction with cancer pain treatment
In the bivariable binary logistic regression analysis, marital status, stage of cancer, types of cancer treatment, severity of pain in the last 24 h, current pain severity, types of analgesics, and pain management index met the threshold of P-value < 0.2 to be included into the final multivariable binary logistic regression analysis. In the final analysis, marital status, current pain severity, and pain management index were significantly associated with patient satisfaction (P-value < 0.05). Married and single cancer patients had higher odds of being satisfied with cancer pain treatment compared to divorced patients (AOR = 5.6, CI, 2.6–12.0, P < 0.001), (AOR = 3.5, CI = 1.3–9.7, P = 0.017), respectively. The odds of being satisfied with cancer pain treatment among patients who received adequate pain management were more than two times greater than those who received inadequate pain management (AOR = 2.4, CI = 1.1–5.3, P = 0.03). Patients who reported a lesser severity of current pain were nearly three times more likely to be satisfied with cancer pain treatment (AOR = 2.6, CI = 1.5–4.8, P < 0.001) [Table 5 ].
The objective of the present study was to assess patients’ satisfaction with cancer pain treatment at adult oncologic centers. Our study revealed that most cancer patients (70.3%) have been satisfied with cancer pain treatment. This is consistent with studies done by Kaggwa et al. and Mazzotta et al. [ 16 , 26 ]. Whereas, it is a higher rate of satisfaction compared to other studies that reported 33.0% [ 27 ] and 47.7% [ 28 ] of satisfaction. The differences might be possibly explained by the use of different pain and satisfaction assessment tools, the greater inclusion (about 70%) of patients with advanced stages of cancer, the duration of cancer pain treatment, and the adequacy of pain management. In the current study, only 19.6% of patients have been diagnosed with stage IV cancer: patients should take treatment at least for a month, and over 80% of patients have received adequate pain management according to PMI. However, some studies have reported higher rates of satisfaction with cancer pain treatment [ 15 , 29 ]. The possible reason for the discrepancy might be the greater (over 40%) use of strong opioid analgesics in the previous studies. Strong opioids were prescribed only for 8.6% of patients in our study. Due to the complex pathophysiology, cancer pain involves multiple pain pathways. Hence, multimodal analgesia in combination with strong opioids is vital in cancer pain management [ 30 ]. Furthermore, the use of epidural analgesia could be another reason for higher rates of satisfaction [ 29 ].
Regarding satisfaction with subscales of LMSQ, about 80% of patients were satisfied with the information provided by the healthcare providers [ 27 ]. In our study; 67.8% of patients were satisfied with the education provided by healthcare providers about their disease and treatment. In contrast, a higher proportion of participants were satisfied with information provision in a study conducted by Kharel et al. [ 29 ]. Furthermore, we observed the lowest satisfaction rate in the daily life subscale. About 48% of cancer patients were not satisfied with their daily lives after receiving analgesic treatment for cancer pain.
Married and single (never married) cancer patients were found to have higher odds of being satisfied with cancer pain treatment as compared to divorced cancer patients. These findings could be explained by the presence of better social support from family or loved ones. Better social support can enhance positive coping mechanisms, increase a sense of well-being, and decrease anxiety and depression. It also improves a sense of societal vitality and results in higher patient’ satisfaction [ 31 , 32 ].
Patients who had a lower pain score were satisfied compared to those who reported a higher pain score, and this is supported by multiple previous studies [ 16 , 26 , 27 , 29 , 33 , 34 ]. This could be explained by the negative impacts of pain on physical function, sleep, mood, and wellbeing [ 35 ]. Moreover, higher pain severity scores could increase financial expenses because of unnecessary or avoidable emergency department visits; and has a consequence of dissatisfaction [ 23 ]. On the contrary, there are studies that state pain severity does not affect patients’ satisfaction [ 36 , 37 ].
Positive PMI scores were significantly associated with cancer pain treatment satisfaction. Patients who received adequate pain management were highly likely to be satisfied with cancer pain treatment. This finding is similar to that of a study done in Taiwan [ 38 ]. However, a study conducted by Kaggwa et al. has denied any association between PMI scores and cancer pain satisfaction [ 16 ].
Satisfaction with healthcare workers and effectivity of analgesics
This study showed that there was a moderately positive correlation between satisfaction with healthcare workers and satisfaction with patients’ perceived effectiveness of analgesics. This might be explained by a positive relationship between healthcare professionals and patients receiving cancer pain treatment. Healthcare providers who provide health education regarding the effectiveness of analgesics may improve patients’ adherence to the prescribed analgesic agent and improve patients’ perceived satisfaction with the effectiveness of analgesics. A systematic review showed that the hope and positivity of healthcare professionals were important for patients to cope with cancer and increase satisfaction with care [ 39 ]. Increased patient satisfaction with care provided by healthcare workers may change attitude of patients who accepted cancer pain as God’s wisdom or punishment and create a positive attitude toward the effectiveness of analgesics [ 40 ]. Another study supported this finding and stated that healthcare providers who deliver health education regarding the prevention of drug addiction, side effects of analgesics, timing, and dosage of analgesics improve patient attitude and cancer pain treatment [ 41 ].
Correlation of each subclass of cancer pain treatment satisfaction
A Spearman correlation was run to assess the correlation of each subclass of LMSQ using the total sample. There was strong positive correlation (r s = 0.5–0.64) between most of LMSQ subclass at p < 0.01.
A cross-sectional study stated that the effectiveness of analgesic, efficacy of medication and patient healthcare provider communication were associated with patient satisfaction [ 42 ]. In this study, 58.2% of patients were satisfied with the practicability of analgesic medications. Comparable to this study, a cross-sectional study stated that patients who were prescribed convenient, fast-acting medications were more satisfied [ 43 ]. Another study stated that 100% of patients who received sufficient information on analgesic treatment and 97.9% of patients who received sufficient information about the side effects of analgesic treatment were satisfied with cancer pain management [ 44 ]. Patients who were satisfied with their pain levels reported statistically lower mean pain scores (2.26 ± 1.70) compared to those not satisfied (4.68 ± 2.07) or not sure (4.21 ± 2.21) [ 27 ]. This may be explained by the impact of pain on daily activity. Patients who report a lower average pain score may have a lower impact of pain on physical activity compared to those who report a higher mean pain score. Another study also supports this evidence and states that patients who reported a severe pain score and lower quality of life had lower satisfaction with the treatment received [ 45 ].
As a secondary outcome, only 16% of patients were diagnosed to have stage I cancer. This finding could indirectly indicate that there were delays in cancer diagnosis at earlier stage. Further studies may be required to underpin this finding.
In this study, baseline pain before analgesic treatment was not assessed and documented. As a cross-sectional study, we could not draw a cause-and-effect conclusion. Since questions that were used to measure oncologic pain treatment satisfaction were self-reported, answers to each question might not be trustful. The expectation and opinion of the interviewer also might affect the result of the study. These could be potential limitations of the study.
Conclusions
Despite the fact that most cancer patients reported moderate to severe pain, there was a high rate of satisfaction with cancer pain treatment. It would be better if hospitals, healthcare professionals, and administrators took measures to enhance the use of multimodal analgesia in combination with strong opioids to ensure adequate pain management, lower pain severity scores, and better daily life. We also urge the arrangement of better social support mechanisms for cancer patients, the improvement of information provision, and the deployment of professionals who have trained in pain management discipline at cancer care centres.
Data availability
Data and materials used in this study are available and can be presented by the corresponding author upon reasonable request.
Abbreviations
Adjusted Odds Ratio
Crude Odds Ratio
Confidence Interval
Dessie Compressive and Specialized Hospital
Felege-Hiwot Compressive and Specialized Hospital
Inter-quartile Range
Lubeck Medication Satisfaction Questionnaire
Numerical Rating Scale
Pain Management Index
Standard Deviation
Tibebe-Ghion Compressive and Specialized Hospital
University of Gondar Compressive and Specialized Hospital
World health organization
Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, et al. The revised International Association for the study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161(9):1976–82.
Article PubMed PubMed Central Google Scholar
Brown MR, Ramirez JD, Farquhar-Smith P. Pain in cancer survivors. Br J pain. 2014;8(4):139–53.
Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on Prevalence of Pain in Patients With Cancer: Systematic Review and Meta-Analysis. Journal of pain and symptom management. 2016;51(6):1070-90.e9.
Snijders RAH, Brom L, Theunissen M, van den Beuken-van Everdingen MHJ. Update on Prevalence of Pain in patients with Cancer 2022: a systematic literature review and Meta-analysis. Cancers. 2023;15(3).
Falk S, Dickenson AH. Pain and nociception: mechanisms of cancer-induced bone pain. J Clin Oncol. 2014;32(16):1647–54.
Article CAS PubMed Google Scholar
Gibson S, McConigley R. Unplanned oncology admissions within 14 days of non-surgical discharge: a retrospective study. Support Care Cancer. 2016;24:311–7.
Article PubMed Google Scholar
Oliveira KG, von Zeidler SV, Podestá JRV, Sena A, Souza ED, Lenzi J, et al. Influence of pain severity on the quality of life in patients with head and neck cancer before antineoplastic therapy. BMC Cancer. 2014;14(1):39.
Smith MD, Meredith PJ, Chua SY. The experience of persistent pain and quality of life among women following treatment for breast cancer: an attachment perspective. Psycho-oncology. 2018;27(10):2442–9.
Zylla D, Steele G, Gupta P. A systematic review of the impact of pain on overall survival in patients with cancer. Support Care Cancer. 2017;25(5):1687–98.
Greco MT, Roberto A, Corli O, Deandrea S, Bandieri E, Cavuto S, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32(36):4149–54.
Baker TA, Krok-Schoen JL, O’Connor ML, Brooks AK. The influence of pain severity and interference on satisfaction with pain management among middle-aged and older adults. Pain Research and Management. 2016;2016.
Baker TA, O’Connor ML, Roker R, Krok JL. Satisfaction with pain treatment in older cancer patients: identifying variants of discrimination, trust, communication, and self-efficacy. J Hospice Palliat Nursing: JHPN: Official J Hospice Palliat Nurses Association. 2013;15(8).
Naidu A. Factors affecting patient satisfaction and healthcare quality. Int J Health care Qual Assur. 2009.
Davies A, Zeppetella G, Andersen S, Damkier A, Vejlgaard T, Nauck F, et al. Multi-centre European study of breakthrough cancer pain: pain characteristics and patient perceptions of current and potential management strategies. Eur J Pain. 2011;15(7):756–63.
Thinh DHQ, Sriraj W, Mansor M, Tan KH, Irawan C, Kurnianda J et al. Patient and physician satisfaction with analgesic treatment: findings from the analgesic treatment for cancer pain in Southeast Asia (ACE) study. Pain Research and Management. 2018;2018.
Kaggwa AT, Kituyi PW, Muteti EN, Ayumba RB. Cancer-related Bone Pain: patients’ satisfaction with analgesic Pain Control. Annals Afr Surg. 2022;19(3):144–52.
Article Google Scholar
Adugna DG, Ayelign AA, Woldie HF, Aragie H, Tafesse E, Melese EB et al. Prevalence and associated factors of cancer pain among adult cancer patients evaluated at the Oncology unit in the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. Front Pain Res.3:231.
Tuem KB, Gebremeskel L, Hiluf K, Arko K, Hailu HG. Adequacy of cancer-related pain treatments and factors affecting proper management in Ayder Comprehensive Specialized Hospital, Mekelle, Ethiopia. Journal of Oncology. 2020;2020.
Matrisch L, Rau Y, Karsten H, Graßhoff H, Riemekasten G. The Lübeck medication satisfaction Questionnaire—A Novel Measurement Tool for Therapy satisfaction. J Personalized Med. 2023;13(3):505.
Bayable SD, Ahmed SA, Lema GF, Yaregal Melesse D. Assessment of Maternal Satisfaction and Associated Factors among Parturients Who Underwent Cesarean Delivery under Spinal Anesthesia at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia, 2019. Anesthesiology research and practice. 2020;2020:8697651.
Breivik H, Borchgrevink P-C, Allen S-M, Rosseland L-A, Romundstad L, Breivik Hals E, et al. Assessment of pain. BJA: Br J Anaesth. 2008;101(1):17–24.
Tegegn HG, Gebreyohannes EA. Cancer Pain Management and Pain Interference with Daily Functioning among Cancer patients in Gondar University Hospital. Pain Res Manage. 2017;2017:5698640.
Shen W-C, Chen J-S, Shao Y-Y, Lee K-D, Chiou T-J, Sung Y-C, et al. Impact of undertreatment of cancer pain with analgesic drugs on patient outcomes: a nationwide survey of outpatient cancer patient care in Taiwan. J Pain Symptom Manag. 2017;54(1):55–65. e1.
Gordon DB, Polomano RC, Pellino TA, Turk DC, McCracken LM, Sherwood G, et al. Revised American Pain Society Patient Outcome Questionnaire (APS-POQ-R) for quality improvement of pain management in hospitalized adults: preliminary psychometric evaluation. J Pain. 2010;11(11):1172–86.
Thronæs M, Balstad TR, Brunelli C, Løhre ET, Klepstad P, Vagnildhaug OM, et al. Pain management index (PMI)—does it reflect cancer patients’ wish for focus on pain? Support Care Cancer. 2020;28:1675–84.
Mazzotta M, Filetti M, Piras M, Mercadante S, Marchetti P, Giusti R. Patients’ satisfaction with breakthrough cancer pain therapy: A secondary analysis of IOPS-MS study. Cancer Manage Res. 2022:1237–45.
Golas M, Park CG, Wilkie DJ. Patient satisfaction with Pain Level in patients with Cancer. Pain Manage Nursing: Official J Am Soc Pain Manage Nurses. 2016;17(3):218–25.
Tang ST, Tang W-R, Liu T-W, Lin C-P, Chen J-S. What really matters in pain management for terminally ill cancer patients in Taiwan. J Palliat Care. 2010;26(3):151–8.
Kharel S, Adhikari I, Shrestha K. Satisfaction on Pain Management among Cancer patient in selected Cancer Care Center Bhaktapur Nepal. Int J Med Sci Clin Res Stud. 2023;3(4):597–603.
Breivik H, Eisenberg E, O’Brien T. The individual and societal burden of chronic pain in Europe: the case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health. 2013;13:1–14.
Gonzalez-Saenz de Tejada M, Bilbao A, Baré M, Briones E, Sarasqueta C, Quintana J, et al. Association between social support, functional status, and change in health‐related quality of life and changes in anxiety and depression in colorectal cancer patients. Psycho‐oncology. 2017;26(9):1263–9.
Yoo H, Shin DW, Jeong A, Kim SY, Yang H-k, Kim JS, et al. Perceived social support and its impact on depression and health-related quality of life: a comparison between cancer patients and general population. Jpn J Clin Oncol. 2017;47(8):728–34.
Hanna MN, González-Fernández M, Barrett AD, Williams KA, Pronovost P. Does patient perception of pain control affect patient satisfaction across surgical units in a tertiary teaching hospital? Am J Med Qual. 2012;27(5):411–6.
Naveh P. Pain severity, satisfaction with pain management, and patient-related barriers to pain management in patients with cancer in Israel. Number 4/July 2011. 2011;38(4):E305–13.
Google Scholar
Black B, Herr K, Fine P, Sanders S, Tang X, Bergen-Jackson K, et al. The relationships among pain, nonpain symptoms, and quality of life measures in older adults with cancer receiving hospice care. Pain Med. 2011;12(6):880–9.
Kelly A-M. Patient satisfaction with pain management does not correlate with initial or discharge VAS pain score, verbal pain rating at discharge, or change in VAS score in the emergency department. J Emerg Med. 2000;19(2):113–6.
Lin J, Hsieh RK, Chen JS, Lee KD, Rau KM, Shao YY, et al. Satisfaction with pain management and impact of pain on quality of life in cancer patients. Asia-Pac J Clin Oncol. 2020;16(2):e91–8.
Su W-C, Chuang C-H, Chen F-M, Tsai H-L, Huang C-W, Chang T-K, et al. Effects of Good Pain Management (GPM) ward program on patterns of care and pain control in patients with cancer pain in Taiwan. Support Care Cancer. 2021;29(4):1903–11.
Prip A, Møller KA, Nielsen DL, Jarden M, Olsen M-H, Danielsen AK. The patient–healthcare professional relationship and communication in the oncology outpatient setting: a systematic review. Cancer Nurs. 2018;41(5):E11.
Orujlu S, Hassankhani H, Rahmani A, Sanaat Z, Dadashzadeh A, Allahbakhshian A. Barriers to cancer pain management from the perspective of patients: a qualitative study. Nurs open. 2022;9(1):541–9.
Uysal N. Clearing barriers in Cancer Pain Management: roles of nurses. Int J Caring Sci. 2018;11(2).
Beck SL, Towsley GL, Berry PH, Lindau K, Field RB, Jensen S. Core aspects of satisfaction with pain management: cancer patients’ perspectives. J Pain Symptom Manag. 2010;39(1):100–15.
Wada N, Handa S, Yamamoto H, Higuchi H, Okamoto K, Sasaki T, et al. Integrating Cancer patients’ satisfaction with Rescue Medication in Pain assessments. Showa Univ J Med Sci. 2020;32(3):181–91.
Antón A, Montalar J, Carulla J, Jara C, Batista N, Camps C, et al. Pain in clinical oncology: patient satisfaction with management of cancer pain. Eur J Pain. 2012;16(3):381–9.
Valero-Cantero I, Casals C, Espinar-Toledo M, Barón-López FJ, Martínez-Valero FJ, Vázquez-Sánchez MÁ. Cancer Patients’ Satisfaction with In-Home Palliative Care and Its Impact on Disease Symptoms. Healthcare. 2023;11(9):1272.
Download references
Acknowledgements
We would like to acknowledge the University of Gondar Comprehensive Specialized Hospital, Tibebe-Ghion Comprehensive Specialized Hospital, Felege-Hiwot Comprehensive Specialized Hospital, Dessie Comprehensive Specialized Hospital. We would also want to acknowledge Ludwig Matrisch from the Department of Rheumatology and Clinical Immunology, Universität zu Lübeck, 23562 Lübeck, Germany for supporting us on the utilization of the Lübeck Medication Satisfaction Questionnaire (LMSQ) [email protected],
This study was supported by University of Gondar and Debre Birhan University with no conflict of interest. The support did not include publication charges.
Author information
Authors and affiliations.
Department of Anesthesia, Debre Birhan University, Debre Birhan, Ethiopia
Molla Amsalu
Department of Anaesthesia, University of Gondar, Gondar, Ethiopia
Henos Enyew Ashagrie, Amare Belete Getahun & Yophtahe Woldegerima Berhe
You can also search for this author in PubMed Google Scholar
Contributions
‘’M.A. has conceptualized the study and objectives; and developed the proposal. Y.W.B., H.E.A., and A.B.G. criticized the proposal. All authors had participated in the data management and statistical analyses. Y.W.B, M.A., and H.E.A. have prepared the final manuscript. All authors read and approved the final manuscript.‘’.
Corresponding author
Correspondence to Yophtahe Woldegerima Berhe .
Ethics declarations
Ethics approval and consent to participate.
Ethical approval was obtained from the Ethical Review Committee of the School of Medicine, at the University of Gondar ( Reference number: CMHS/SM/06/01/4097/2015, Date: March 24, 2023 ). Permission support letters were obtained from FHCSH, TGCSH, and DCSH. Written informed consent was obtained from each participant after detailed explanations about the study. Informed consent with a fingerprint signature was obtained from patients who could not read or write after detailed explanations by the data collectors as approved by the Ethical Review Committee of the School of Medicine, at the University of Gondar.
Consent for publication
Not applicable; this article does not include any personal details of any participant.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Amsalu, M., Ashagrie, H.E., Getahun, A.B. et al. Patients’ satisfaction with cancer pain treatment at adult oncologic centers in Northern Ethiopia; a multi-center cross-sectional study. BMC Cancer 24 , 647 (2024). https://doi.org/10.1186/s12885-024-12359-7
Download citation
Received : 17 October 2023
Accepted : 08 May 2024
Published : 27 May 2024
DOI : https://doi.org/10.1186/s12885-024-12359-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cancer pain treatment
- Treatment satisfaction
- Cancer pain
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Defining the process to literature searching in systematic reviews: a literature review of guidance and supporting studies
Affiliations.
- 1 Institute of Health Research, University of Exeter Medical School, Exeter, UK. [email protected].
- 2 HEDS, School of Health and Related Research (ScHARR), University of Sheffield, Sheffield, UK.
- 3 Institute of Health Research, University of Exeter Medical School, Exeter, UK.
- 4 European Centre for Environment and Human Health, University of Exeter Medical School, Truro, UK.
- PMID: 30107788
- PMCID: PMC6092796
- DOI: 10.1186/s12874-018-0545-3
Background: Systematic literature searching is recognised as a critical component of the systematic review process. It involves a systematic search for studies and aims for a transparent report of study identification, leaving readers clear about what was done to identify studies, and how the findings of the review are situated in the relevant evidence. Information specialists and review teams appear to work from a shared and tacit model of the literature search process. How this tacit model has developed and evolved is unclear, and it has not been explicitly examined before. The purpose of this review is to determine if a shared model of the literature searching process can be detected across systematic review guidance documents and, if so, how this process is reported in the guidance and supported by published studies.
Method: A literature review. Two types of literature were reviewed: guidance and published studies. Nine guidance documents were identified, including: The Cochrane and Campbell Handbooks. Published studies were identified through 'pearl growing', citation chasing, a search of PubMed using the systematic review methods filter, and the authors' topic knowledge. The relevant sections within each guidance document were then read and re-read, with the aim of determining key methodological stages. Methodological stages were identified and defined. This data was reviewed to identify agreements and areas of unique guidance between guidance documents. Consensus across multiple guidance documents was used to inform selection of 'key stages' in the process of literature searching.
Results: Eight key stages were determined relating specifically to literature searching in systematic reviews. They were: who should literature search, aims and purpose of literature searching, preparation, the search strategy, searching databases, supplementary searching, managing references and reporting the search process.
Conclusions: Eight key stages to the process of literature searching in systematic reviews were identified. These key stages are consistently reported in the nine guidance documents, suggesting consensus on the key stages of literature searching, and therefore the process of literature searching as a whole, in systematic reviews. Further research to determine the suitability of using the same process of literature searching for all types of systematic review is indicated.
Publication types
- Research Support, Non-U.S. Gov't
- Databases, Bibliographic / classification
- Databases, Bibliographic / standards
- Databases, Bibliographic / statistics & numerical data*
- Guidelines as Topic / standards
- Information Storage and Retrieval / methods
- Information Storage and Retrieval / standards
- Information Storage and Retrieval / statistics & numerical data*
- Review Literature as Topic
- Systematic Reviews as Topic*
Grants and funding
- 16/54/11/Department of Health/United Kingdom
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Family Med Prim Care
- v.2(1); Jan-Mar 2013
Systematic Reviews and Meta-analysis: Understanding the Best Evidence in Primary Healthcare
S. gopalakrishnan.
Department of Community Medicine, SRM Medical College, Hospital and Research Centre, Kattankulathur, Tamil Nadu, India
P. Ganeshkumar
Healthcare decisions for individual patients and for public health policies should be informed by the best available research evidence. The practice of evidence-based medicine is the integration of individual clinical expertise with the best available external clinical evidence from systematic research and patient's values and expectations. Primary care physicians need evidence for both clinical practice and for public health decision making. The evidence comes from good reviews which is a state-of-the-art synthesis of current evidence on a given research question. Given the explosion of medical literature, and the fact that time is always scarce, review articles play a vital role in decision making in evidence-based medical practice. Given that most clinicians and public health professionals do not have the time to track down all the original articles, critically read them, and obtain the evidence they need for their questions, systematic reviews and clinical practice guidelines may be their best source of evidence. Systematic reviews aim to identify, evaluate, and summarize the findings of all relevant individual studies over a health-related issue, thereby making the available evidence more accessible to decision makers. The objective of this article is to introduce the primary care physicians about the concept of systematic reviews and meta-analysis, outlining why they are important, describing their methods and terminologies used, and thereby helping them with the skills to recognize and understand a reliable review which will be helpful for their day-to-day clinical practice and research activities.
Introduction
Evidence-based healthcare is the integration of best research evidence with clinical expertise and patient values. Green denotes, “Using evidence from reliable research, to inform healthcare decisions, has the potential to ensure best practice and reduce variations in healthcare delivery.” However, incorporating research into practice is time consuming, and so we need methods of facilitating easy access to evidence for busy clinicians.[ 1 ] Ganeshkumar et al . mentioned that nearly half of the private practitioners in India were consulting more than 4 h per day in a locality,[ 2 ] which explains the difficulty of them in spending time in searching evidence during consultation. Ideally, clinical decision making ought to be based on the latest evidence available. However, to keep abreast with the continuously increasing number of publications in health research, a primary healthcare professional would need to read an insurmountable number of articles every day, covered in more than 13 million references and over 4800 biomedical and health journals in Medline alone. With the view to address this challenge, the systematic review method was developed. Systematic reviews aim to inform and facilitate this process through research synthesis of multiple studies, enabling increased and efficient access to evidence.[ 1 , 3 , 4 ]
Systematic reviews and meta-analyses have become increasingly important in healthcare settings. Clinicians read them to keep up-to-date with their field and they are often used as a starting point for developing clinical practice guidelines. Granting agencies may require a systematic review to ensure there is justification for further research and some healthcare journals are moving in this direction.[ 5 ]
This article is intended to provide an easy guide to understand the concept of systematic reviews and meta-analysis, which has been prepared with the aim of capacity building for general practitioners and other primary healthcare professionals in research methodology and day-to-day clinical practice.
The purpose of this article is to introduce readers to:
- The two approaches of evaluating all the available evidence on an issue i.e., systematic reviews and meta-analysis,
- Discuss the steps in doing a systematic review,
- Introduce the terms used in systematic reviews and meta-analysis,
- Interpret results of a meta-analysis, and
- The advantages and disadvantages of systematic review and meta-analysis.
Application
What is the effect of antiviral treatment in dengue fever? Most often a primary care physician needs to know convincing answers to questions like this in a primary care setting.
To find out the solutions or answers to a clinical question like this, one has to refer textbooks, ask a colleague, or search electronic database for reports of clinical trials. Doctors need reliable information on such problems and on the effectiveness of large number of therapeutic interventions, but the information sources are too many, i.e., nearly 20,000 journals publishing 2 million articles per year with unclear or confusing results. Because no study, regardless of its type, should be interpreted in isolation, a systematic review is generally the best form of evidence.[ 6 ] So, the preferred method is a good summary of research reports, i.e., systematic reviews and meta-analysis, which will give evidence-based answers to clinical situations.
There are two fundamental categories of research: Primary research and secondary research. Primary research is collecting data directly from patients or population, while secondary research is the analysis of data already collected through primary research. A review is an article that summarizes a number of primary studies and may draw conclusions on the topic of interest which can be traditional (unsystematic) or systematic.
Terminologies
Systematic review.
A systematic review is a summary of the medical literature that uses explicit and reproducible methods to systematically search, critically appraise, and synthesize on a specific issue. It synthesizes the results of multiple primary studies related to each other by using strategies that reduce biases and random errors.[ 7 ] To this end, systematic reviews may or may not include a statistical synthesis called meta-analysis, depending on whether the studies are similar enough so that combining their results is meaningful.[ 8 ] Systematic reviews are often called overviews.
The evidence-based practitioner, David Sackett, defines the following terminologies.[ 3 ]
- Review: The general term for all attempts to synthesize the results and conclusions of two or more publications on a given topic.
- Overview: When a review strives to comprehensively identify and track down all the literature on a given topic (also called “systematic literature review”).
- Meta-analysis: A specific statistical strategy for assembling the results of several studies into a single estimate.
Systematic reviews adhere to a strict scientific design based on explicit, pre-specified, and reproducible methods. Because of this, when carried out well, they provide reliable estimates about the effects of interventions so that conclusions are defensible. Systematic reviews can also demonstrate where knowledge is lacking. This can then be used to guide future research. Systematic reviews are usually carried out in the areas of clinical tests (diagnostic, screening, and prognostic), public health interventions, adverse (harm) effects, economic (cost) evaluations, and how and why interventions work.[ 9 ]
Cochrane reviews
Cochrane reviews are systematic reviews undertaken by members of the Cochrane Collaboration which is an international not-for-profit organization that aims to help people to make well-informed decisions about healthcare by preparing, maintaining, and promoting the accessibility of systematic reviews of the effects of healthcare interventions.
Cochrane Primary Health Care Field is a systematic review of primary healthcare research on prevention, treatment, rehabilitation, and diagnostic test accuracy. The overall aim and mission of the Primary Health Care Field is to promote the quality, quantity, dissemination, accessibility, applicability, and impact of Cochrane systematic reviews relevant to people who work in primary care and to ensure proper representation in the interests of primary care clinicians and consumers in Cochrane reviews and review groups, and in other entities. This field would serve to coordinate and promote the mission of the Cochrane Collaboration within the primary healthcare disciplines, as well as ensuring that primary care perspectives are adequately represented within the Collaboration.[ 10 ]
Meta-analysis
A meta-analysis is the combination of data from several independent primary studies that address the same question to produce a single estimate like the effect of treatment or risk factor. It is the statistical analysis of a large collection of analysis and results from individual studies for the purpose of integrating the findings.[ 11 ] The term meta-analysis has been used to denote the full range of quantitative methods for research reviews.[ 12 ] Meta-analyses are studies of studies.[ 13 ] Meta-analysis provides a logical framework to a research review where similar measures from comparable studies are listed systematically and the available effect measures are combined wherever possible.[ 14 ]
The fundamental rationale of meta-analysis is that it reduces the quantity of data by summarizing data from multiple resources and helps to plan research as well as to frame guidelines. It also helps to make efficient use of existing data, ensuring generalizability, helping to check consistency of relationships, explaining data inconsistency, and quantifies the data. It helps to improve the precision in estimating the risk by using explicit methods.
Therefore, “systematic review” will refer to the entire process of collecting, reviewing, and presenting all available evidence, while the term “meta-analysis” will refer to the statistical technique involved in extracting and combining data to produce a summary result.[ 15 ]
Steps in doing systematic reviews/meta-analysis
Following are the six fundamental essential steps while doing systematic review and meta-analysis.[ 16 ]
Define the question
This is the most important part of systematic reviews/meta-analysis. The research question for the systematic reviews may be related to a major public health problem or a controversial clinical situation which requires acceptable intervention as a possible solution to the present healthcare need of the community. This step is most important since the remaining steps will be based on this.
Reviewing the literature
This can be done by going through scientific resources such as electronic database, controlled clinical trials registers, other biomedical databases, non-English literatures, “gray literatures” (thesis, internal reports, non–peer-reviewed journals, pharmaceutical industry files), references listed in primary sources, raw data from published trials and other unpublished sources known to experts in the field. Among the available electronic scientific database, the popular ones are PUBMED, MEDLINE, and EMBASE.
Sift the studies to select relevant ones
To select the relevant studies from the searches, we need to sift through the studies thus identified. The first sift is pre-screening, i.e., to decide which studies to retrieve in full, and the second sift is selection which is to look again at these studies and decide which are to be included in the review. The next step is selecting the eligible studies based on similar study designs, year of publication, language, choice among multiple articles, sample size or follow-up issues, similarity of exposure, and or treatment and completeness of information.
It is necessary to ensure that the sifting includes all relevant studies like the unpublished studies (desk drawer problem), studies which came with negative conclusions or were published in non-English journals, and studies with small sample size.
Assess the quality of studies
The steps undertaken in evaluating the study quality are early definition of study quality and criteria, setting up a good scoring system, developing a standard form for assessment, calculating quality for each study, and finally using this for sensitivity analysis.
For example, the quality of a randomized controlled trial can be assessed by finding out the answers to the following questions:
- Was the assignment to the treatment groups really random?
- Was the treatment allocation concealed?
- Were the groups similar at baseline in terms of prognostic factors?
- Were the eligibility criteria specified?
- Were the assessors, the care provider, and the patient blinded?
- Were the point estimates and measure of variability presented for the primary outcome measure?
- Did the analyses include intention-to-treat analysis?
Calculate the outcome measures of each study and combine them
We need a standard measure of outcome which can be applied to each study on the basis of its effect size. Based on their type of outcome, following are the measures of outcome: Studies with binary outcomes (cured/not cured) have odds ratio, risk ratio; studies with continuous outcomes (blood pressure) have means, difference in means, standardized difference in means (effect sizes); and survival or time-to-event data have hazard ratios.
Combining studies
Homogeneity of different studies can be estimated at a glance from a forest plot (explained below). For example, if the lower confidence interval of every trial is below the upper of all the others, i.e., the lines all overlap to some extent, then the trials are homogeneous. If some lines do not overlap at all, these trials may be said to be heterogeneous.
The definitive test for assessing the heterogeneity of studies is a variant of Chi-square test (Mantel–Haenszel test). The final step is calculating the common estimate and its confidence interval with the original data or with the summary statistics from all the studies. The best estimate of treatment effect can be derived from the weighted summary statistics of all studies which will be based on weighting to sample size, standard errors, and other summary statistics. Log scale is used to combine the data to estimate the weighting.
Interpret results: Graph
The results of a meta-analysis are usually presented as a graph called forest plot because the typical forest plots appear as forest of lines. It provides a simple visual presentation of individual studies that went into the meta-analysis at a glance. It shows the variation between the studies and an estimate of the overall result of all the studies together.
Forest plot
Meta-analysis graphs can principally be divided into six columns [ Figure 1 ]. Individual study results are displayed in rows. The first column (“study”) lists the individual study IDs included in the meta-analysis; usually the first author and year are displayed. The second column relates to the intervention groups and the third column to the control groups. The fourth column visually displays the study results. The line in the middle is called “the line of no effect.” The weight (in %) in the fifth column indicates the weighting or influence of the study on the overall results of the meta-analysis of all included studies. The higher the percentage weight, the bigger the box, the more influence the study has on the overall results. The sixth column gives the numerical results for each study (e.g., odds ratio or relative risk and 95% confidence interval), which are identical to the graphical display in the fourth column. The diamond in the last row of the graph illustrates the overall result of the meta-analysis.[ 4 ]

Interpretation of meta-analysis[ 4 ]
Thus, the horizontal lines represent individual studies. Length of line is the confidence interval (usually 95%), squares on the line represent effect size (risk ratio) for the study, with area of the square being the study size (proportional to weight given) and position as point estimate (relative risk) of the study.[ 7 ]
For example, the forest plot of the effectiveness of dexamethasone compared with placebo in preventing the recurrence of acute severe migraine headache in adults is shown in Figure 2 .[ 17 ]

Forest plot of the effectiveness of dexamethasone compared with placebo in preventing the recurrence of acute severe migraine headache in adults[ 17 ]
The overall effect is shown as diamond where the position toward the center represents pooled point estimate, the width represents estimated 95% confidence interval for all studies, and the black plain line vertically in the middle of plot is the “line of no effect” (e.g., relative risk = 1).
Therefore, when examining the results of a systematic reviews/meta-analysis, the following questions should be kept in mind:
- Heterogeneity among studies may make any pooled estimate meaningless.
- The quality of a meta-analysis cannot be any better than the quality of the studies it is summarizing.
- An incomplete search of the literature can bias the findings of a meta-analysis.
- Make sure that the meta-analysis quantifies the size of the effect in units that you can understand.
Subgroup analysis and sensitivity analysis
Subgroup analysis looks at the results of different subgroups of trials, e.g., by considering trials on adults and children separately. This should be planned at the protocol stage itself which is based on good scientific reasoning and is to be kept to a minimum.
Sensitivity analysis is used to determine how results of a systematic review/meta-analysis change by fiddling with data, for example, what is the implication if the exclusion criteria or excluded unpublished studies or weightings are assigned differently. Thus, after the analysis, if changing makes little or no difference to the overall results, the reviewer's conclusions are robust. If the key findings disappear, then the conclusions need to be expressed more cautiously.
Advantages of Systematic Reviews
Systematic reviews have specific advantages because of using explicit methods which limit bias, draw reliable and accurate conclusions, easily deliver required information to healthcare providers, researchers, and policymakers, help to reduce the time delay in the research discoveries to implementation, improve the generalizability and consistency of results, generation of new hypotheses about subgroups of the study population, and overall they increase precision of the results.[ 18 ]
Limitations in Systematic Reviews/Meta-analysis
As with all research, the value of a systematic review depends on what was done, what was found, and the clarity of reporting. As with other publications, the reporting quality of systematic reviews varies, limiting readers’ ability to assess the strengths and weaknesses of those reviews.[ 5 ]
Even though systematic review and meta-analysis are considered the best evidence for getting a definitive answer to a research question, there are certain inherent flaws associated with it, such as the location and selection of studies, heterogeneity, loss of information on important outcomes, inappropriate subgroup analyses, conflict with new experimental data, and duplication of publication.
Publication Bias
Publication bias results in it being easier to find studies with a “positive” result.[ 19 ] This occurs particularly due to inappropriate sifting of the studies where there is always a tendency towards the studies with positive (significant) outcomes. This effect occurs more commonly in systematic reviews/meta-analysis which need to be eliminated.
The quality of reporting of systematic reviews is still not optimal. In a recent review of 300 systematic reviews, few authors reported assessing possible publication bias even though there is overwhelming evidence both for its existence and its impact on the results of systematic reviews. Even when the possibility of publication bias is assessed, there is no guarantee that systematic reviewers have assessed or interpreted it appropriately.[ 20 ]
To overcome certain limitations mentioned above, the Cochrane reviews are currently reported in a format where at the end of every review, findings are summarized in the author's point of view and also give an overall picture of the outcome by means of plain language summary. This is found to be much helpful to understand the existing evidence about the topic more easily by the reader.
A systematic review is an overview of primary studies which contains an explicit statement of objectives, materials, and methods, and has been conducted according to explicit and reproducible methodology. A meta-analysis is a mathematical synthesis of the results of two or more primary studies that addressed the same hypothesis in the same way. Although meta-analysis can increase the precision of a result, it is important to ensure that the methods used for the reviews were valid and reliable.
High-quality systematic reviews and meta-analyses take great care to find all relevant studies, critically assess each study, synthesize the findings from individual studies in an unbiased manner, and present balanced important summary of findings with due consideration of any flaws in the evidence. Systematic review and meta-analysis is a way of summarizing research evidence, which is generally the best form of evidence, and hence positioned at the top of the hierarchy of evidence.
Systematic reviews can be very useful decision-making tools for primary care/family physicians. They objectively summarize large amounts of information, identifying gaps in medical research, and identifying beneficial or harmful interventions which will be useful for clinicians, researchers, and even for public and policymakers.
Source of Support: Nil
Conflict of Interest: None declared.

IMAGES
VIDEO
COMMENTS
The best reviews synthesize studies to draw broad theoretical conclusions about what a literature means, linking theory to evidence and evidence to theory. This guide describes how to plan, conduct, organize, and present a systematic review of quantitative (meta-analysis) or qualitative (narrative review, meta-synthesis) information.
SLR, as the name implies, is a systematic way of collecting, critically evaluating, integrating, and presenting findings from across multiple research studies on a research question or topic of interest. SLR provides a way to assess the quality level and magnitude of existing evidence on a question or topic of interest.
Systematic reviews retrieve, appraise and summarise all the available evidence on a specific health question. They are designed to reduce the effect of the reviewers' own bias, and a full protocol should be written to define and guide the process. ... Systematic literature reviews Complement Ther Med. 2005 Mar;13(1):54-60. doi: 10.1016/j.ctim ...
Introduction. A systematic review collects secondary data, and is a synthesis of all available, relevant evidence which brings together all existing primary studies for review (Cochrane 2016).A systematic review differs from other types of literature review in several major ways.
Background. A systematic review, as its name suggests, is a systematic way of collecting, evaluating, integrating, and presenting findings from several studies on a specific question or topic.[] A systematic review is a research that, by identifying and combining evidence, is tailored to and answers the research question, based on an assessment of all relevant studies.[2,3] To identify assess ...
Systematic reviews that summarize the available information on a topic are an important part of evidence-based health care. There are both research and non-research reasons for undertaking a literature review. It is important to systematically review the literature when one would like to justify the need for a study, to update personal ...
Literature reviews establish the foundation of academic inquires. However, in the planning field, we lack rigorous systematic reviews. In this article, through a systematic search on the methodology of literature review, we categorize a typology of literature reviews, discuss steps in conducting a systematic literature review, and provide suggestions on how to enhance rigor in literature ...
A systematic review is a type of study that synthesises research that has been conducted on a particular topic. Systematic reviews are considered to provide the highest level of evidence on the hierarchy of evidence pyramid. Systematic reviews are conducted following rigorous research methodology. To minimise bias, systematic reviews utilise a ...
A literature search is distinguished from, but integral to, a literature review. Literature reviews are conducted for the purpose of (a) locating information on a topic or identifying gaps in the literature for areas of future study, (b) synthesising conclusions in an area of ambiguity and (c) helping clinicians and researchers inform decision-making and practice guidelines.
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement, published in 2009, was designed to help systematic reviewers transparently report why the review was done, what the authors did, and what they found. Over the past decade, advances in systematic review methodology and terminology have necessitated an update to the guideline. The PRISMA 2020 statement ...
A systematic literature review (SLR) summarizes the results of all available studies on a specific topic and provides a high level of evidence. ... Often, the authors use the PubMed/MEDLINE, Cochrane Library, and Embase databases. In most reviews, two or more databases are searched, and common limits are language (usually restricted to English ...
A systematic review is a literature review that follows a rigorous process to find all of the research conducted on a topic and then critically appraises the research methods of the highest quality reports. ... A thorough PubMed search must identify the author words likely to be in the title and abstract or the indexer's selected MeSH (Medical ...
Learn how to develop a PubMed literature search strategy for a systematic review.For details on the search building blocks, check this video playlist:https:/...
When performing literature searches for a systematic review it's important to use a wide range of resources and searching methods in order to identify all relevant studies. As expert searchers, librarians play an important role in making sure your searches are comprehensive and reproducible. Standard 3.1.1 of the Institute of Medicine's Finding ...
Search Strategy Used to Create the PubMed Systematic Reviews Filter. This strategy is intended to retrieve citations to systematic reviews in PubMed and encompasses: citations assigned the "Systematic Review" publication type during MEDLINE indexing; citations that have not yet completed MEDLINE indexing; and non-MEDLINE citations.
Among the various types of reviews, the systematic review of the literature is ranked as the most rigorous since it is a high-level summary of existing … Acad Radiol . 2018 Nov;25(11):1481-1490. doi: 10.1016/j.acra.2018.04.025.
Purpose: Nowadays, systematic literature reviews (SLRs) and meta-analyses are often placed at the top of the study hierarchy of evidence. The main objective of this paper is to evaluate the trends in SLRs of randomized controlled trials (RCTs) throughout the years. ... The PubMed search for the SLR of RCTs yielded 86,765 results (Figure 1).
Included SRs evaluated 24 unique methodological approaches used for defining the review scope and eligibility, literature search, screening, data extraction, and quality appraisal in the SR process. Limited evidence supports the following (a) searching multiple resources (electronic databases, handsearching, and reference lists) to identify ...
The Literature Selection Technica Review Committee (LSTRC) reviews and selects journals for MEDLINE based on the research quality and impact of the journals. A distinctive feature of MEDLINE is that the records are indexed with NLM Medical Subject Headings (MeSH). PubMed also contains citations for PubMed Central (PMC) articles. PMC is a full ...
This review aims to study the clinical characteristics, diagnostic results, treatments, and outcomes in patients with heterotopic ossification following COVID-19 infection. A literature search for eligible articles was conducted using MEDLINE/Pubmed, Global Health, and Scopus databases (January 12th, 2023), including all case reports and case series from any country and language.
The steps for implementing a systematic review include (i) correctly formulating the clinical question to answer (PICO), (ii) developing a protocol (inclusion and exclusion criteria), (iii) performing a detailed and broad literature search and (iv) screening the abstracts of the studies identified in the search and subsequently of the selected ...
A systematic review of literature was conducted in accordance with PRISMA guidelines. PubMed and Scopus databases were queried using the search terms, "primary intracranial chondrosarcoma", "extraskeletal mesenchymal chondrosarcoma", "mesenchymal chondrosarcoma" and "pediatric". Presentation, surgical management and outcome of a ...
Fink has defined research literature review as a "systematic, explicit and reproducible method for identifying, evaluating, ... Although reviews are available in PubMed, for systematic reviews and meta-analysis, Cochrane library is a much better resource. The Cochrane library is a collection of full length systematic reviews, which can be ...
A systematic review showed that the hope and positivity of healthcare professionals were important for patients to cope with cancer and increase satisfaction with care . Increased patient satisfaction with care provided by healthcare workers may change attitude of patients who accepted cancer pain as God's wisdom or punishment and create a ...
Background: Systematic literature searching is recognised as a critical component of the systematic review process. It involves a systematic search for studies and aims for a transparent report of study identification, leaving readers clear about what was done to identify studies, and how the findings of the review are situated in the relevant evidence.
Introduction and background. A literature review provides an important insight into a particular scholarly topic. It compiles published research on a topic, surveys different sources of research, and critically examines these sources [].A literature review may be argumentative, integrative, historical, methodological, systematic, or theoretical, and these approaches may be adopted depending ...
A systematic review is a summary of the medical literature that uses explicit and reproducible methods to systematically search, critically appraise, and synthesize on a specific issue. ... the popular ones are PUBMED, MEDLINE, and EMBASE. Sift the studies to select relevant ones. ... Systematic reviews have specific advantages because of using ...