WAEC Chemistry Questions and Answers 2023: OBJ/Essay
The West African Examination Council (WAEC) conducts the West African Senior School Certificate Examination (WASSCE) for Chemistry. This examination tests students’ knowledge and skills in the subject, preparing them for higher education and career opportunities. In this comprehensive guide, we provide you with essential information, sample questions, and answers to help you excel in the Objectives and Essay sections of the WAEC Chemistry exam in 2023.


Overview of WAEC Chemistry Examination
The WAEC Chemistry examination consists of two main sections: Objectives and Essay. The Objectives section contains multiple-choice questions that test students’ basic understanding of Chemistry concepts. On the other hand, the Essay section requires students to provide detailed explanations and solutions to various Chemistry problems.
To excel in the WAEC Chemistry examination, you need to have a solid understanding of the subject matter. This guide will provide you with valuable insights and sample questions, covering both the Objectives and Essay sections of the exam.
WAEC Chemistry Objectives
The Objectives section consists of multiple-choice questions, which test your basic understanding of Chemistry concepts. In this section, we provide you with some sample questions and answers to help you prepare for the 2023 WAEC Chemistry Objectives.
Sample Objective Questions
- Which of the following elements will burn in excess oxygen to form a product that is neutral to litmus?
A. Carbon B. Hydrogen C. Sulphur D. Sodium
Answer: B. Hydrogen
- A solution of salt formed from HCl and NH3 solutions is:
A. Acidic B. Basic C. Complex D. Neutral
Answer: D. Neutral
- The boiling points of water, ethanol, methylbenzene, and butan-2-ol are 373.0K, 351.3K, 383.6K, and 372.5K respectively. Which liquid has the highest vapor pressure at 323.0K?
A. Water B. Methylbenzene C. Ethanol D. Butan-2-ol
Answer: C. Ethanol
WAEC Chemistry Essay
The Essay section requires students to provide detailed explanations and solutions to various Chemistry problems. In this section, we provide you with a comprehensive list of sample essay questions and answers to help you prepare for the 2023 WAEC Chemistry Essay.
Sample Essay Questions and Answers
- (a) Define the following terms:
(i) Atomicity (ii) Isotopes
(b) Elements P, Q, and R have atomic numbers 9, 16, and 20, respectively. Which of these elements would gain electron(s) during ionic bonding?
(c) Write the electron configuration of the following elements:
(i) 9F (ii) 20Ca
(a) (i) Atomicity refers to the number of atoms present in a molecule of an element or compound. For example, the atomicity of oxygen (O2) is 2, and that of ozone (O3) is 3.
(ii) Isotopes are varieties of an element that have the same atomic number but different mass numbers due to the different number of neutrons in their nuclei. For example, the isotopes of hydrogen are protium (1H), deuterium (2H), and tritium (3H).
(b) Elements with atomic numbers 9 (P) and 16 (Q) would gain electron(s) during ionic bonding because they have fewer than four electrons in their outermost shells.
(c) Electron configuration of:
(i) 9F: 1s² 2s² 2p⁵ (ii) 20Ca: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s²
(i) Standard electrode potential (ii) Redox reaction
(b) Balance the following redox equation: MnO₄⁻ + I⁻ + H⁺ → Mn²⁺ + I₂ + H₂O
(a) (i) Standard electrode potential is the potential difference (voltage) between an electrode and its respective half-cell when all the species involved are in their standard states (298 K temperature, 1 atm pressure, and 1 mol/dm³ concentration).
(ii) A redox reaction is a chemical reaction in which the oxidation states of the atoms involved in the reaction change. One species is reduced (gains electrons), and another is oxidized (loses electrons).
(b) The balanced redox equation is: 2MnO₄⁻ + 10I⁻ + 16H⁺ → 2Mn²⁺ + 5I₂ + 8H₂O
Tips for Success in WAEC Chemistry Examination
- Understand the concepts: Develop a strong foundation in Chemistry by thoroughly understanding the basic concepts, principles, and theories.
- Practice regularly: Solve numerous practice questions and problems to improve your problem-solving skills and enhance your understanding of the subject.
- Manage your time effectively: Allocate adequate time for each section of the exam, ensuring you have enough time to read and understand the questions and provide well-thought-out answers.
- Learn from your mistakes: Review your practice questions and identify areas where you may be struggling, then focus on improving in these areas.
- Stay updated: Keep abreast of the latest developments in Chemistry and apply your knowledge to real-life situations.
In conclusion, preparing for the Objectives and Essay sections of the WAEC Chemistry examination requires dedication, persistence, and a strong understanding of the subject matter. By following the tips provided in this guide and practicing with the sample questions and answers, you will be well on your way to achieving success in the 2023 WAEC Chemistry examination.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Related Articles

Yadda Zaka Sami Sakon Jamb Profile Code a Sawwake 2021

NYSC Releases 2023 Batch B Stream 1 Call-Up Letter – Print Your NYSC Call-Up Letter Here

WAEC Further Maths Questions and Answers 2022

Read: Jamb Approves 140 Cut-Off Mark For University Admission And 140 For Poly And College Of Education

YADDA ZAKA CIKE BUK SCREENING ECERCISE 2021/2022

Delta State University First Batch JUPEB Admission List For 2022/2023

WAEC GCE Civic Education Answers Objectives and Essays 2023
NECO results for 2023 are officially out – NECO Results Portal

Kano State Polytechnic Na Cigaba Da Bada Admission Na 2019/2020

Charge climb: BUK extends understudies’ enlistment by one month

Kano Science and Technical 2023 Exam Result Is Out

NYSC Online Registration for 2023 Batch ‘C’ Stream II Begins: See How
St Charles Edu Services
Genuine Exam Past Questions and Answers Online Bookshop – PDF and MS Word Download
WAEC Chemistry Past Questions and Answers in 2023 PDF Download Objective & Theory
Are you writing the West Africa Examination Council WAEC Internal or External examination, if yes you need the WAEC Past Questions on Chemistry
we at stcharlesedu.com has compiled a good number of Chemistry WAEC Past Questions and Answers in Pdf Chemistry 2 – Theory/Essay Questions. Chemistry 1 – Objective Test Questions.
Our research has confirm that candidate that uses WASSCE Chemistry past questions to prepare is ten times better than those who do not.
Table of Contents
- 1.1 Chemistry WAEC Objective Questions
- 2 SSCE WAEC Chemistry Theory Questions
- 3 Chemistry WAEC Essay Questions
- 4 Free WAEC Chemistry Exam Past Questions Download
- 5 How to Get WASSCE Chemistry Exam Past Questions and Answers
SSCE WAEC Chemistry Objective Questions and Answers
CHEMISTRY Paper 1 (Objective Test Questions) Paper 1 will last for 1 hours Use HB pencil throughout.
Answer All Questions Each question is followed by four options lettered A to D. Find out the correct options for each question and shade in pencil on your answer sheet, the answer space which bears the same letter as the option you Chosen. Give only one answer to each question. An example is given below
What others are downloading WAEC Past Questions for all Subjects
Chemistry WAEC Objective Questions
Which of the following elements reacts with water? A. Carbon B. Iodine C. Sodium D. Sulphur
The correct answer is Sodium, which is lettered C and therefore answer space C would be shaded. [A] [ B ] [C] [ D ]
Think carefully before you shade the answer spaces; erase completely any answer you wish to change.
Which of the following raw materials is used in the plastic industry? A. Ethene B. Methane C. Sulphur D. Hydrogen
Which of the following organic compounds can undergo both addition and substitution reactions? A. Petane B. Benzene C. Propane D. Hexane
Which of the following equations represents a redox reaction? A. AgNO 3 (aq) + KCl(ag)->AgCl(s)+ KNO 3 (aq) B. HNO 3 (aq)+ NaOH(aq) -> NaNO 3 (aq) + H 2 O(l) C. CaCO 3 (s) -> CaO(s) + CO 2 (g) D. 2H 2 S(g) + SO 2 (g) -> 2H 2 O(I) + 3S(g)
T he process of extraction of iron from its ore is A. decomposition. B. oxidation. C. reduction. D. sublimation.
What is the solubility of a salt if 0.4 g of it is obtained on evaporating 200 cm3 of its saturated solution to dryness? A. 0.08 gdm -3 B. 2.00 gdm -3 C. 8.00 gdm -3 D. 80.00 gdm -3
An acidic salt has A. double anions in its aqueous solution. B. a single cation in its aqueous solution. C. hydrogen ions in its aqueous solution. D. hydrogen atoms in its aqueous solution.
A reaction is endothermic if the A. reaction vessel feels cool during the reaction. B. enthalpy change is negative. C. bond forming energy exceeds bond breaking energy. D. heat of formation of reactants exceeds heat of formation of products.
In which of the following compounds does hydrogen form ionic compounds? A. CH 4 B. HCl C. NH 3 D. NaH
Consider the following reaction equation: Br 2 + 2KI -> 2KBr + I 2 . Bromine is acting as A. an oxidizing agent. B. a reducing agent. C. an acid. D. a base.
An organic compound has the empirical formula CH 2 . If its molar mass is 42 gmol-1 what is its molecular formula? [H = 1.0, C = 12.0] A. C 2 H 4 B. C 3 H 4 C. C 3 H 6 D. C 4 H 8
Ethene is produced from ethanol by A. decomposition. B. hydrolysis. C. ozonolysis. D. dehydration.
Consider the following equilibrium reaction: 2 AB(g) + B 2 (g) -><- 2AB 3 (g) AH = -XkJmol -1 The backward reaction will be favored by A. a decrease in pressure. B. an increase in pressure. C. a decrease in temperature. D. an introduction of a positive catalyst.
What is the mass of solute in 500 cm 3 of 0.005 moldm -3 H 2 SO 4 ? [H =1.0, O = 16.0, S = 32.0] A. 0.490 g B. 0.049 g C. 0.245 g D. 0.0245 g
Pure water can be made to boil at a temperature lower than 100 °C by A. reducing its quantity. B. decreasing the external pressure. C. distilling it. D. increasing the external pressure.
Consider the following sketch of the solubility curve of some substances. Note: scroll down to download the free chemistry waec questions in pdf copy to view the sketch
At what temperature does the solubility of KNO 3, equal that of NaNO 3 ? A. 0°C B. 20 °C C. 30 °C D. 40 °C
When a salt is added to its saturated solution, the salt A. dissolves and the solution becomes super saturated. B. dissolves and the solution becomes unsaturated. C. precipitates and the solution remains unchanged. D. dissolves and crystals are formed.
When substance X was added to a solution of bromine water, the solution became colorless. X is likely to be A. propane. B. propanoic acid. C. propyne. D. propanol.
The preferential discharge of ions during electrolysis is influenced by the A. mechanism of electrolysis. B. electrolytic reactions. C. nature of the electrode. D. type of electrolytic cell.
The valence electrons of 12 Mg are in the A. 3s orbital. B. 2px orbital. C. 2s orbital. D. 1s orbital.
Stainless Steel is an alloy comprising of A. Fe and C. B. Fe and Ni. C. Fe, C and Ni. D. Fe, C and Al.
The number of hydrogen ions in 1.0 dm 3 of 0.02 moldm -3 tetraoxosulphate(VI) acid is [NA = 6.02 x 1023] A. 1.2 x 10 22 B. 1.2 x 10 23 . C. 2.4 x 10 22 . D. 2.4 x 10 23 .
The most suitable substance for putting out petrol fire is A. water. B. carbon(IV)oxide. C. fire blanket. D. sand.
The following factors would contribute to environmental pollution except A. production of ammonia. B. manufacture of cement. C. photosynthesis. D. combustion.
The position of equilibrium in a reversible reaction is affected by A. particle size of the reactants. B. vigorous stirring of the reaction mixture. C. presence of a catalyst. D. change in concentration of the reactants.
The diagram below illustrates a conical flask containing water and ice.
NOTE: scroll down and download the free chemistry pdf past questions to see the diagram
Which of the following statements about the diagram is correct? A. The water is at a lower temperature than the ice B. Energy is absorbed when the ice changes to water C. Energy is released when the ice changes to water D. The water molecules vibrate about a fixed point
Which of the following statements best explains the differences between a gas and a vapor? A. Unlike gases, vapors are liquids at room temperature B. Unlike gases, vapor can easily be condensed into liquids C. Unlike gases, vapour is readily converted into solids D. Vapours are generally denser than gases
Consider the following reaction equation: 2HCl + Ca(OH) 2 –> CaCl 2 + H 2 O. What is the volume of 0.1 moldrn -3 HCl that would completely neutralize 25cm 3 of 0.3 moldm -3 Ca(OH) 2 ? A. 150 cm 3 B. 75 cm 3 C. 30 cm 3 D. 25 cm 3
Cu and HNO 3 are not suitable for preparing hydrogen gas because of their A. reactivity and oxidation respectively. B. conductivity and corrosiveness respectively. C. melting point and reduction respectively. D. electro negativity and solubility respectively.
Which of the following formulae cannot be an empirical formula? A. CH B. CH2 C. P2O5 D. N204
One of the criteria for confirming the purity of benzene is to determine its A. heat capacity. B. boiling point. C. mass. D. colour.
Want more Chemistry Objective Test Questions like this? Get the Complete WAEC Chemistry Exam Past Questions and Answers (Obj and Essay) in PDF Format from us.
SSCE WAEC Chemistry Theory Questions
Chemistry Paper 2 Paper 2 will last for 2 hours This paper consists of two sections A and B. Answer one questions from Section A and three questions from Section B.
Credit will be given for clarity of expression and orderly presentation of material.
SECTION A (1ai) Define the term fermentation. (1aii) Name the catalyst that can be used for this process.
(b) Name two factors which determines the choice of an indicator for an acid-base titration. (c) Consider the following reaction equation: [Fe + H2S04 ] FeS04 + H2. Calculate the mass of unreacted iron when 5.0g of iron reacts with 10cm3 of 1.0 moldrrv3 H SO [Fe = 56.0] (d) Name one: (di) Heavy chemical used in electrolytic cells; (dii) Fine chemical used in textile industries.
(e) Explain briefly how a catalyst increases the rate of a chemical reaction. (f) (i) Write the chemical formula for the product formed when ethanoic acid reacts with ammonia. (ii) Give the name of the product formed in 1 (f) (i)..
(g) List three properties of aluminum that makes it suitable for the manufacture of drink can. (h) State two industrial uses of alkylalkanoates. (i) List two effects of global warming. (j) Name two steps involved in the crystallization of a salt from its solution.
Chemistry WAEC Essay Questions
SECTION B. 2ai. State the collision theory of reaction rates. 2aii.Using the collision theory, explain briefly how temperature can affect the rate of a chemical reaction.
bi. Sketch a graphical representation of Charles’s law. bii. Calculate the volume of oxygen that would be required for the complete combustion of 2.5moles of ethanol at s.t.p. [ molar volume at s.t.p = 22.4dm3]
ci. Define esterification. cii. Give two uses of alkanoates. ciii. Give the products of the alkaline hydrolysis of ethyl ethanoate.
d. A tin coated plate and a galvanized plate were exposed for the same length of time. di. Which of the two plates corrodes faster? dii. Explain briefly your answer in 2 (d) (i).
Want more Chemistry Theory Questions like this? Get the Complete WAEC Chemistry Exam Past Questions and Answer (Obj and Essay) in PDF Format from us.
Free WAEC Chemistry Exam Past Questions Download
Click to Download your free NECO Past Question on Painting and Decorating Paper 2 and 3
Link 1: WASSCE Chemistry Questions Booklet Link 2: WASSCE Chemistry Questions Booklet
How to Get WASSCE Chemistry Exam Past Questions and Answers
To get the complete and more recent copy of the West Africa Examination Council WAEC Past Questions and answer
Take Note of the following step
Make a Call Call or whatsapp us on 08051311885 for the account number to make payment and how to received your complete copy of the past questions to be sent directly to your email address or whatsapp number.
Mode of Payment. Mobile Transfer or Direct Bank Deposit.
After Payment send us the following Depositor Name: Name of Product Paid for: Valid email address.
DELIVERY ASSURANCE We will deliver the past question to you 10 mins after confirmation of payment to the email you will send to us.
Related Posts:
- WAEC Technical Drawing Past Questions PDF Download – Objective, Essay, Building Plan/Practical Drawing
- WAEC Government Past Questions and Answers in 2023 PDF Download Objective & Theory
- WAEC Financial Accounting Past Questions and Answer 2023 – Objective & Essay
- WAEC Visual Art Past Questions and Answers – Objective, Theory in 2023
- WASSCE/WAEC Electrical Installation & Maintenance Past Questions PDF – Objective/Essay
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *

Home » EXAM NEWS » NOV/DEC 2023 WAEC GCE Chemistry (Essay & OBJ) Answers For All GCE Candidates
NOV/DEC 2023 WAEC GCE Chemistry (Essay & OBJ) Answers For All GCE Candidates

TODAY ANSWERS ON 2023 WAEC GCE CHEMISTRY OBJ AND THEORY ANSWERS WE BE UPLOADED HERE….
The Time Of the WAEC GCE Chemistry Essay | Chemistry Objective | Chemistry Practical Exam is 11:30pm – 3:30pm for all the 2023 WAEC GCE Chemistry exams.
A. 2023 WAEC GCE CHEMISTRY ESSAY | (THEORY) ANSWERS
Kindly bookmark the website for the answers that will be released here, or better still reload the site to check if the answers for the 2023 Waec GCE Chemistry Questions and Answers have dropped.
2023 WAEC GCE CHEMISTRY OBJ AND THEORY ANSWERS
Loading……………………………………………
B. 2023 WAEC GCE CHEMISTRY OBJECTIVES (OBJ) ANSWERS
WAEC GCE Chemistry Questions and Answers Answers Loading……………………………………………
C. 2023 WAEC GCE CHEMISTRY PRACTICAL ANSWERS
Feel free to see the 2023 Waec Gce questions and answers we’ve shared already on Waec Gce Igbo, Waec Gce Hausa, Waec Gce Yoruba for free below. Many students/candidates who regularly visit our site find helpful answers for their subjects.
Related Content For You:
- 2023 Free WAEC GCE Answers For All WAEC GCE Subjects – Obj and Theory
- JOIN THE GCE WHATSAPP GROUP LINK FOR 2023-2024 FOR FREE
If you have any doubts about whether the questions you’ve come across are indeed the 2023 WAEC GCE questions and answers, you can easily confirm by asking your friends who took the exam to share their questions. Compare their questions with our provided answers to ensure accuracy.
You can Join our Whatsapp here .
WAEC GCE IGBO | WAEC GCE YOURUBA | WAEC GCE LANGUAGE QUESTIONS AND ANSWERS – 16th November 2023
2023 WAEC GCE YORUBA OBJ === You can confirm it yourself.
1-10: BABCDBBDAC 11-20: BBDDABCDAD 21-30: BAACDDAAAA 31-40: DBACCACDAC 41-50: CADBBCDACC 51-60: DBABACDDBB
WAEC GCE YORUBA THEORY ANSWERS ======= You can confirm it yourself.
(12) Dandogo jẹ ẹwu nla kan, ti o de awọn eekun ti awọn ọlọrọ ati awọn agbalagba wọ, ti o wọpọ laarin awọn olori ilu, lati jade. Aṣọ yii le jẹ kedere ni gbangba nipa sisọpọ awọn ohun elo pẹlẹbẹ pẹlu ohun elo akọkọ tabi o kan lati dara aṣọ naa ni iṣẹ-ọnà le ṣee ṣe lori rẹ. eyi yoo fun ni irisi ọlọrọ. Dandogo jẹ aṣa Yoruba ati pe o gba akoko ati ifarada lati ran.” Òwe Yorùbá gbajúmọ ‘Dandogo kọjá aso a binu da’ ( A kì í kánjú ra aṣọ gígùn ) fi èyí hàn, bẹẹ ni ọrọ náà ṣe ń lọ, tí ẹ bá fẹ ran Dandogo a nílò 36yards, ó sì gba àkókò. lati ran.
A Le wo Dandogo pẹlu awọn oriṣiriṣi sokoto…..
WAEC GCE HAUSA OBJ ANSWERS === You can confirm it yourself.

WAEC GCE IGBO OBJECTIVE ANSWERS === You can confirm it yourself.
1-10: BCDCDCBBDB 11-20: BCADCDBCCC 21-30: ABDCADCDDB 31-40: CACDBDDABC 41-50: DDBAACBCCD 51-60: BACCBDAAAC
WAEC GCE IGBO THEORY ANSWERS========== You can confirm it yourself.
(1a) Izunna: Mmaduka enyim! Anyi aga ekwugodi okwu obere oge
Mmaduka: Izuu nwannem, ọfuma! ka anyi kpaa. Kedu ihe di gi n’obi
Izunna: E nwere kepukepu m na-anụ gbasara gi.
Mmaduka: Hahaha, kedu ihe i nụrụ maka m?
Izunna: A nụrụm na i na-añuzi anwụrụ ike.
Mmaduka: Kedu ebe inukwara asiri nke a? Nke a bụ asiri, ọ bughi eziokwu.
Izunna: Ya burukwa asiri. Mana achọputaram na agwa gi gbanweziri na nke ikpeazu a. Ujọ na-atụm maka na-amaram ọghọm di na anwụrụ ike.
Mmaduka: Izunna nwannem, m ga-eletawu onwe m anya ọfuma. Echena oke uche maka m. A maram ebe ihe osise m jedebere.
Izunna: Enweghi kwa njede na-abia na ebe anwụrụ ike nọ. O nwere ike ighasa gi aru, ma ghasakwaa gi ụbụlụ. O nwekwara ike ibutere gi ọgba-aghara n’etiti gi na ndi be ụnụ.
Mmaduka: Echegbuna onwe gi, Izuu. Ihe m ji wee na-añuchalu obere bu ka m wee wepụ echiche.
Izunna: Mmadu, aghọtaram na ụwa nke anyi nọ n’ime ya siri ike mana obughi anwụrụ ike ka e ji eso ije uwa. Kama, anwụrụ ike ka enyere gi aka, otisaara gi ihe. Tinyezie gi nsogbu kariri nke inobụ na ya. O nwere ike ime ka igaa nga, maobu gbaa gi ara.
Mmaduka: Echebeyikwam ya otu a isi wee kwuo
Izunna: Nwanne, lee anya, obim di ebe inọ oge ọbụla. Aghaghim achọ ka ihe ọjọọ dakwasa gi.
Mmaduka: O bụ eziokwu ka ikwụrụ. Dalu na ihe nkea igwagasirim n’ehihie a. Chukwu gozie gi nwannem.
Izunna: Chukwu gozikwaa gi ezi oyim. oge ọbụla odi ka i ga-aba n;echiche, kpoturum ka mụna gi nọrọ, ka anyi tụkọọ aro ọnu, na anyụkọọ mamiri ọnụ ọ gbọọ ụfụfụ.
(5a) Nnochiaha bu mkpuruokwu na-anochite anya aha n’ahiriokwu maobu ebe obula o no. O bukwa umu irighiri mkpuruokwu e ji edochi anya aha ihe n’ahiriokwu ka a ghara ide ya ugboro ugboro.
NNOCHIONYE: (i) Nochionwe na-anochite anya onye(mmadu) (ii) O na-anochite anya ihe a kporo aha ya (iii) Omumatu bu ndi a: m, mụ anyi, gi, ụnụ, ọ, o, ya na ha
NNOCHIMPESIN: (i) O na-anaghi aru aka kpomkwem onye mere ihe (ii) O na-anaghi aru aka kpomkwem onye a na-ekwu maka ya (iii) Omumatụ bụ ndi a: A kụrụ aka, A sara efere, E riri nri ahụ.
(5bii) NNOCHIONYE: (i) Emeka bu di ya. (ii) O na-esi nri. (iii) Nnewi bu obodo m.
NNOCHIMPESIN: (i) A kụrụ aka (ii) A sara efere (iii) E riri nri ahụ
(8) (i) Nne na nna ga ekuziri umu ha otu esi aza ụlo (ii) Ha ga-ekuziri ha otu esi asu asusu Igbo (iii) Ha ga-ekuziri umu ha otu esi ekene aha (iv) Ha ga-azukwa ha n’uzo eziokwu (v) Ha ga-apiakwa ha ihe oge ha mere ihee ọjọọ (vi) Ha ga-ekuzikwara ihe gbasara mmekọ nwoke na nwanyi na ha tolite (vii) Ha ga-ekuzikwara ha ọghọm di na ibi ajọ ndu (viii) Ha ha ekuzikwara ha otu esi ekpe ekpere (ix) Ha ga-akuzikwara umu ha otu esi asopuru ndi tọrọ ha ato (x) Ha ga-ekuzikwara maka ihu mmadu ibe ha n’anya.
Kindly bookmark this website for quick access to the released WAEC GCE answers. Alternatively, you can reload the site periodically to check if the answers for the 2023 WAEC GCE Chemistry questions have been posted. Stay tuned for the latest updates.
You may also like

MAY/JUNE 2024 WAEC Chemistry Practical Specimen For...

May/June WAEC Agricultural Practical Specimen For 2024

2024 WAEC EXAM TIMETABLE FOR ALL WAEC CANDIDATES PDF...

2024 JAMB DAY1 EXAM QUESTIONS AND ANSWERS FOR JAMB...

Approved Jamb Cut-off Mark For Law Into Nigeria Law...

How Many Candidates Registered For 2024 UTME JAMB Exam...
About the author.
Leave a Comment X
Save my name, email, and website in this browser for the next time I comment.
You cannot copy content of this page

Home » WAEC Chemistry Answers 2024 Essay/OBJ Questions Out

2024 WAEC Chemistry Questions & Answers for Essay and Objective Released.
The Waec Chemistry Answers 2024 essay and objective questions for the West African Examination Council (WAEC) Chemistry SSCE exam paper scheduled to be written on Wednesday, 22nd May 2024 can now be studied here.
The 2024 Chemistry Essay answer paper will start at 9:30 am and will last for 2hrs while the Objective exam will commence at 11:30 pm and will last for 1hr.
In this post, we will be posting the West African Senior School Certificate Examinations (WASSCE) Chemistry questions for candidates who will participate in the examination from past questions.

Continue reading below.
WAEC Chemistry Answers 2024.
PAPER 2 [Essay] Answer any FOUR questions. Write your answers on the answer booklet provided.
1. (a) (i) What is the common name given to the group VII elements? (ii) Name the hydrides of the first two elements in group VII. (iii) State three chemical properties of group VII elements.
(b) Copy and complete the following table
(c) (i) Define each of the following processes: nuclear fission; nuclear fusion. (ii) Give one use of each process in 1(c)(i)
(d) (i) List three types of radiation that are produced during radioactivity. (ii) Arrange the radiations listed in 1(d)(i) in order of increasing: penetrating power; ionizing power.
ANS: (a) (i) Halogens (ii) Hydrogen fluoride; Hydrogen chloride (iii) high electron affinity/strong oxidizing agents/electron acceptor – highly electronegative; – react with hydrogen to form acid; – form salts with metals; – react with alkalis to form salts; – react with water to form acids; – displacement of lower halogens from their acids/salts; – react with hydrocarbon to form alkyl halides. (b)
(c)(i) I. Nuclear fission – splitting of a heavy nucleus into two smaller nuclei of similar mass with the release of a large amount of energy and radiation. II. Nuclear fusion – a combination of smaller nuclei to form a large nucleus with the release of large amounts of energy and radiation. (ii) I. Used to generate electricity/nuclear bomb/production of new elements/production of radioisotopes. II. Used to produce nuclear weapons/atomic bombs/production of new elements/production radioisotopes. (d) (i) alpha, beta, gamma OR α , β, (ii) I. α < β < increasing penetrating power II. <β < α increasing ionizing power.
2. (a) (i) What is the structure of the atom as proposed by Rutherford? (ii) Distinguish between the atomic number and the mass number of an element. (iii) Explain briefly why the relative atomic mass of chlorine is not a whole number.
(b) (i) What is meant by first ionization energy? (ii) List three properties of electrovalent compounds (iii) Consider the following pairs of elements: 9F and 17CL; 12Mg and 20Ca. Explain briefly why the elements in each pair have similar chemical properties.
(c) Explain briefly the following terms using an appropriate example in each case (i) homologous series; (ii) heterolytic fission.
(d) State the indicator(s) which could be used to determine the end-point of the following titrations: (i) dilute hydrochloric acid against sodium hydroxide solution; (ii) dilute hydrochloric acid against ammonium hydroxide solution; (iii) ethanoic acid against sodium hydroxide solution.
(e) A solid chloride E which sublimed on heating reacted with an alkali F to give a choking gas G. G turned moist red litmus paper blue. Identify E, F and G.
ANS: (a) (i) The atom has a small/ tiny positively charged centre /nucleus with electrons surrounding the space around the centre. (ii) Atomic number of an element is the number of protons/electrons in an atom of the element while the mass number is the sum of the protons and neutrons in the atom of the element. (iii) Chlorine atom is made up of a mixture of isotopes and the relative atomic mass of chlorine is the average of its isotopic masses.
(b) (i) Is the (minimum) energy required to remove one mole of an electron from one mole of gaseous atom (to form one mole gaseous charged ion (ii) High melting /boiling point; Ability to conduct electricity in the molten state or in solution; Solid at room temperature; Soluble in water or polar solvents /insoluble in non-polar solvents. (iii) Atoms of the elements in each pair have the same number of electrons in their outer-most shell therefore similar chemical properties.
(c) (i) Is a family of organic compounds: – where successive members differ by –CH2 of the molar mass of 14; – with similar chemical properties; – which conform to the same general formula; – which show a gradation of physical properties; – which have the same general method of preparation. e.g alkanes, alkenes , alkanols. (ii) Is a process in which a (covalent) bond is broken in such a way that the electron pair is completely transferred to one of the atoms (resulting in the formation of ions) H ÷ CI → H+ + Cl-/ HCl ® H+ + Cl- (d)(i) Methyl orange/ methyl red/ phenolphthalein; (ii) Methyl orange/ methyl red; (iii) Phenolphthalein. (e) E – NH4Cl F – NaOH, KOH, or Ca (OH)2, Li OH, CsOH, Ba(oH)2, Mg(OH)2 G – NH3.
3. (a) (i) Define saturated solution. (ii) The solubility of KN03 at 20°C was 3.00 mol dm-3 If 67.0g of KN03 was added to 250cm3 of water and stirred at 20°C, determine whether the solution formed was saturated or not at that temperature.
(b) (i) Distinguish between the dative bond and covalent bond. (ii) Explain why sugar and common salt do not conduct electricity in the solid state. (iii) State the type of intermolecular forces present in I. hydrogen fluoride; II. argon. (iv) Consider the compounds with the following structures: S – H —-N and 0 – H —–N In which of the compounds is the hydrogen bond stronger? Give a reason for your answer.
(c) (i) State Dalton’s Law of Partial Pressure. (ii) If 200cm3 of carbon(IV) oxide were collected over water at 18°C and 700 mmHg, determine the volume of the dry gas at s.t.p.[ standard vapour pressure of water at 18°C = 15 mmHg]
ANS: (a) (i) Is a solution that contains the maximum amount of solute it can dissolve at a given temperature (in the presence of undissolved solute). (ii) Solubility of KN03 in in g dm-3 = 3.00 x 101 = 303 .. 1000cm3 of saturated solution = 303g 250cm3 of the solution = 303 x 250 1000 = 75.8 g Since the quantity of KN03 added (67.0) to 250 cnr’ of water is less than the maximum amount required to form a saturated solution, then the solution is unsaturated.
(b)(i) In a dative bond, only one of the participating atoms/ species donated electrons to be shared by both atoms while in a covalent bond both participating atoms/ species contribute equally to the electrons being shared. (ii) Sugar is covalent while common salt (NaCl) is electrovalent/ ionic. Electrical conductivity (in compounds) depends on the presence of mobile ions. (iii) The intermolecular forces present in hydrogen fluoride and argon were hydrogen bond and van der Waal’s forces respectively. (iv) 0 – H —- N has a stronger hydrogen bond because oxygen is more electronegative and smaller in size than sulphur.
(c)(i) the total pressure exerted by a mixture of gases that do not react chemically is equal to the sum of the individual partial pressures of the gases in the mixture. (ii) pressure of the dry gas (P 1) = 700 – 15 = 685 mmHg VI = 200cm 3 , TI = 18°C = 273 + 18 = 291K, P2 = 760 mmHg, T2 = 273 P1V1 = P2V2 T1 T2 V2 = P1V1T 2 = P2T1
= 685 x 200 x 273 760 x 291
= 169.1cm 3
4. (a) (i) Define nuclear fission (ii) A certain natural decay series starts with and ends with. Each step involves the loss of an alpha or a beta particle. Using the given information, deduce how many alpha and beta particles were emitted. (b) Consider the equilibrium reaction represented by the following equation: A2(g) + 3B2(g) 2AB3(g); H = + kJmol-1 Explain briefly the effect of each of the following changes on the equilibrium composition; (i) increase in the concentration of B; (ii) decrease in pressure of the system; (iii) addition of catalyst. (c) The lattice energies of three sodium halides are as follows:
Explain briefly the trend.
(d) State the property exhibited by nitrogen (IV) oxide in each of the following reactions: (i) 4Cu + 2NO2 4CuO + N2; (ii) H2O+ 2NO2 HNO3 + HNO2
(e) Iron is manufactured in a blast furnace using iron ore (Fe2O3), coke and limestone. Write the equation for the reaction(s) at the: (i) top of the furnace; (ii) middle of the furnace; (iii) bottom of the furnace. (f) (i) Name two products of destructive distillation of coal. (ii) Give one use of each product in 3(f)(i).
5. Copy and complete the following table:
(i) Mention one compound that makes water I. temporarily hard; II. permanently hard. (ii) State one method that could be used to remove I. only temporary hardness; II. permanent hardness. (iii) Write an equation to show the removal of: I. temporary hardness; II. permanent hardness. (c) (i) List three sources of water pollution. (ii) Mention two ways by which water pollution can be controlled. (d) state the function of each of the following substances in the purification of water for town supply: I. sodium aluminate (III) (NaAIO2); II. lime (calcium hydroxide); III. calculated mount of iodine; IV. sand bed.
6. (a) (i) What is meant by atomicity? (ii) Mention one element in each case which is I. monatomic, II. diatomic, III. tetratomic. (iii) Write the orbital electron configuration of I. 20Ca, II. 9F. a. In which group does each of the elements belong? b. How many unpaired electrons are present in 9F? c. How many electrons are present in 20Ca2+?
(b) (i) Write a balanced equation for the thermal decomposition of KCƖO3. (ii) Mention the catalyst that could be used to increase the rate of reaction in 6(b)(i). (ii) If 5.0 g of KCƖO3 was decomposed by heat, determine the volume of oxygen produced at s.t.p. [Molar gas volume at s.t.p. = 22.4dm3, K = 39, Cl = 35.5, O = 16]
(c) (i) Mention the products formed when each of the following substances is heated strongly: I. ZnCO3; II. CuSO4.5H2O. (ii) State the colour change observed when each of the residues in 1(d)(i) above is allowed to cool.
7. (a) Describe briefly how each of the following aqueous solutions could be identified in the laboratory: (i) Ammonium trioxocarbonate (IV); (ii)Ammonium chloride. (b) Arrange the following compounds in order of increasing boiling point and give reasons for your answer: CS2, NaF and CO2. (c) List two gases each that are: (i) acidic; (ii) highly soluble in water; (iii) oxidized by acidified KMnO4(aq). (d) In a tabular form, compare the elements silicon and sulphur under the following properties: (i) metallic character; (ii) physical state; (iii) conduction of electricity. (e) A cuboid piece of sodium metal measures 3 cm x 4 cm x 10 cm. If the density of sodium is 0.971 g cm-3, calculate the number of atoms in the sodium metal. [ Na = 23; Avogadro constant = 6.02 x 1023 mol_1 ]
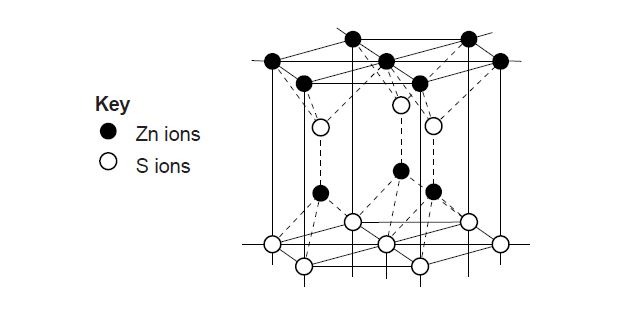
8. (a) (i) Define standard electrode potential. (ii) State two factors that affect the value of standard electrode potential. (iii) Give two uses of the values of standard electrode potential. (iv) Draw and label a diagram for an electrochemical cell made up of Cu2+/Cu; = + 0.34 Zn2+/Zn; = – 0.76 (v) Calculate the e.m.f of the cell in 8(a)(iv) above (b) (i) In terms of electron transfer, define I. oxidation; II. oxidizing agent. (ii) Balance the following redox reaction: MnO4- + I- H+ I2Mn2+
(c) Classify each of the following oxides as basic, amphoteric, acidic or neutral: (i) Carbon (II) oxide; (ii) Sulphur(IV) oxide; (iii) Aluminium oxide; (iv) Lithium oxide.
(d) What is hydrogen bonding?
WAEC Chem Objective Questions 202 4 .
PAPER 1 [Essay] Answer All questions in this section. Write your answers on the answer sheet provided.
1. Two immiscible liquids with different boiling points can be separated by _____ A. The use of separating funnel B. Evaporation C. Distillation D. Decantation.
2. A mixture of CaCl2 and CaCO3 in water can be separated by ______ A. Evaporation B. Sublimation C. Distillation D. Decantation.
3. What is responsible for metallic bonding? A. sharing of electrons between the metal atoms B. attraction between the atomic nuclei and the cloud of electrons C. Transfer of electrons from one atom to another D. attraction between positive and negative ions.
4. 25cm3 of 1.5M solution of NaCl are added to 50cm3 of 3M NaCl. The molar concentration of the resulting solution is ________ A. 2.5M B. 3M C. 2.25M D. 4.5M
5. A solution of salt formed from HCl and NH3 solutions is _____ A. Acidic B. Basic C. complex D. Neutral
6. Which of the following elements will burn in excess oxygen to form a product that is neutral to litmus? A. carbon B. Hydrogen C. Sulphur D. Sodium
7. A current was passed for 10 mins and 0.2mole of Cu was deposited. How many grammes of Ag will it deposit? (Cu = 64, Ag = 108) A. 43.2g B. 21.6g C. 10.8g D. 5.4g
8. Pollution of underground water by metal ions is very likely in a soil that has high ________ A. Acidity B. Alkalinity C. Chloride content D. Nitrate content
9. Producer gas is a gas with low caloric value because it contains more ____ A. CO2 than O2 B. N2 than CO C. CO2 than N2 D. N2 than CO2
10. Silver chloride turns grey when exposed to sunlight because _____ A. The silver ion is reduced to silver B. The silver ion is oxidized to silver C. Silver is a transition metal D. The silver chloride forms complexes in the sun.
11. Which of these compounds exhibits resonance? A. Benzene B. Ethanol C. Propene D. Butyne
15. Hydrolysis of CH3COOCH2CH3 in dilute HCl produces ______ A. CH3COOH + CH3CH3 B. CH3CH2OH + CH3COCl C. CH3COOH + CH3CH2OH D. CH3COOH + CH3CH3
16. Calculate the volume of CO2 measured at s.t.p produced on heating 250g of potassium hydrogen trioxocarbonate (IV) strongly. (K = 39, H = 1, C = 12, O = 16) A. 28dm3 B. 2.8dm3 C.5.6dm3 D. 11.2dm3
17. The boiling points of water, ethanol, methylbenzene and butan-2-ol are 373.0K, 351.3K, 383.6K and 372.5K respectively. Which liquid has the highest vapour pressure at 323.0K? A. Water B. Methylbenzene C. Ethanol D. Butan-2-ol
18. The conclusion from Rutherford’s alpha scattering experiment is that ______ A. Atoms are mostly empty space with a small nucleus B. Emissions from radioactive substances consist of three main components C. There is a nuclear pull on orbital electrons D. Electrons are deflected by both magnetic and electric fields.
19. Elements P, Q and R have atomic numbers 9, 16 and 20 respectively. Which of them would gain electron(s) during ionic bonding A. Q and R B. P and R C. P and Q D. P, Q and R.
20. Which of the following has the lowest PH? A. 5cm3 of M/10 HCl B. 10cm3 of M/10 HCl C. 20cm3 of M/8 HCl D. 15cm3 of M/2 HCl
21. Which of the following is an acid salt? A. (NH4)2CO3 B. CHCOONa C. KHSO4 D. MgSO4.7H2O
22. Cr2O2-7 + 14H+ + 6I → 2Cr3+ + 3I2 + 7H2O. The change in the oxidation number of oxygen in the equation above is _______ A. 0 B. 1 C. 2 D. 7
23. During electrolysis of CuSO4 solution using platinum electrodes, which of the following occurs ______ A. Acidity increases at the cathode B. Oxygen is liberated at the cathode C. PH decreases at the cathode D. PH of solution increases.
24. Which of the following ions is a pollutant in drinking water even in trace quantities? A. Ca2+ B. Pb2+ C. Mg2+ D. Fe2+
25. The solubility of a salt of molar mass 100g at 20oC is 0.34mol/dm3. If 3.4g of that salt dissolved completely 250cm3 of water at that temperature, the resulting solution is ________ A. A suspension B. Saturated C. Unsaturated D. Supersaturated
26. Catalyst is important in in chemical industry in that ______ A. it affects the purity of the products B. it affects the quantity of the products C. it increases the time for reaching equilibrium D. Bond breaking is slowed down.
27. An alkanioc acid has a molecular mass of 88. Name the acid. (C = 12, O =16, H = 1) A. Propanioc acid B. Botanioc acid C. Pentanioc acid D. But-2-ionic acid
28. Ethyne undergoes the following reactions EXCEPT A. Polymerization B. Addition C. Combustion D. Etherification
Keep following, more questions and answers will be added soon.
PS: Once again, there is nothing like waec chemistry expo. Do not fall victim to scammers online trying to obtain money from you with fake promises of having access to live question paper before the exam. What we have on this page are likely exam questions from waec past questions and answers to serve as a revision guide.
Get the Most Legit Information and Guide on the Latest Jobs in Nigeria, Facebook and Education Here
NECO Chemistry Questions and Answers 2023/2024 (Essay and Objectives)
NECO Chemistry Questions and Answers 2023. I will be showing you past Chemistry objectives and theory repeated questions for free in this post. You will also understand how NECO Chemistry questions are set and how to answer them.
The National Examinations Council (NECO) is an examination body in Nigeria that conducts the Senior Secondary Certificate Examination and the General Certificate in Education in June/July and December/January respectively.
Table of Contents
NECO Chemistry Objectives and Essay Answers 2023 (Expo)
The 2023 NECO Chemistry expo will be posted here today 24th July during the NECO Chemistry examination. Keep checking and reloading this page for the answers.
NECO 2023 Chemistry Answers Loading.
OBJ Answers:
1-10: DEADADECAD
11-20: BAEDDBDBAE
21-30: CCDCABDDCD
31-40: EBEECEBCEE
41-50: BCCECDDADD
51-60: DABBDEAECA
————————————————————————————————————-
NECO Chemistry Questions and Answers For Practice
The following NECO Chemistry questions are questions to expect in the 2023 NECO examination.
1. The minimum amount of energy required for effective collisions between reacting particles is known A) Activation energy B) Bond energy C) Kinetic energy D) Potential energy
2. The bond formed between H2OH2O and H+H+ to form the hydroxonium H3O+H3O+ is A) Dative B) Covalent C) Electrovalent D) Ionic
3. An element XX forms the following oxides X2O,XOX2O,XO and XO2.XO2. This phenomenon illustrates the law of ________. A) Conservation of mass B) Definite proportion C) Mass action D) Multiple proportion
4.. How many moles of oxygen would contain 1.204×10241.204×1024 molecules? NB: Avogadro’s constant (NA) =6.02×1023=6.02×1023 A) 1 B) 2 C) 3 D) 4
See: NECO Timetable
5. Which of the following statements about solids is correct? A) Solid particles are less orderly than those of a liquid B) Solid have lower densities than liquids C) Solid particles have greater kinetic energies than those of liquids D) Solid particles cannot be easily compressed
6. Which of the following apparatus can be used to measure a specific volume of a liquid accurately? A) Beaker B) Conical flask C) Measuring cyclinder D) Pipette
7. The general gas equation PVT=KPVT=K is a combination of A) Boyle’s and Charles’ laws B) Boyle’s and Graham’s laws C) Charles’ and Graham’s laws D) Dalton’s and Graham’s laws
8. The spreading of the scent of a flower in a garden is an example of? A) Brownian motion B) Diffusion C) Osmosis D) Tynadal effect
9. Propane and carbon (IV) oxide diffuse at the same rate because [H = 1.00, C = 12.0, O = 16.0] Options A) They are both gases B) Their molecules contain carbon C) They have the same relative molecular mass D) Both are denser than air
1O. The energy which accompanies the addition of an electron to an isolated gaseous atom is A) Atomization B) Electronegativity C) Electron affinity D) Ionization
11. A sample of hard water contains some calcium sulphate and calcium hydrogen carbonate. The total hardness may therefore be removed by A. boiling the water B. adding excess calcium hydroxide C. adding a calculated amount of calcium hydroxide D. adding sodium carbonate E. adding magnesium hydroxide
12. During the electrolysis of copper II sulphate between platinum electrodes, if litmus solution is added to the anode compartment, A. the litmus turns blue but no gas is evolved B. the litmus turns blue and oxygen is evolved C. the litmus turns blue and hydrogen is evolved D. the litmus turns red and oxygen is evolved E. the litmus turns red and then becomes colourless
13. The reaction between an organic acid and an alcohol in the presence of an acid catalyst is known as; A. saponification B. dehydration C. esterification D. hydrolysis E. hydration
14. The IUPAC names of the compounds CH3COOH and CH2=CH2 are respectively; A. acetic acid and ethane B. ethanoic acid and ethene C. methanoic acid and ethylene D. ethanol and ethene E. acetic acid and ethylene
15. If 30cm3 of oxygen diffuses through a porous pot in 7 seconds, how long will it take 60cm3 of chlorine to diffuse through the same pot, if the vapour densities of oxygen and chlorine are 16 and 36 respectively? A. 9.3 sec B. 14 sec C. 21 sec D. 28 sec E. 30.3 sec
16. When heat is absorbed during a chemical reaction, the reaction is said to be A. thermodynamic B. exothermic C. isothermal D. endothermic E. thermostatic
17. When large hydrocarbon molecules are heated at high temperature in the presence of a catalyst to give smaller molecules, the process is known as A. disintegration B. polymerization C. cracking D. degradation E. distillation
18. The pH of four solutions W, X, Y, Z are 4, 6, 8, 10 respectively, therefore A. none of these solutions is acidic B. the pH of Y is made more acidic by addition of distilled water C. Z is the most acidic solution D. W is the most acidic solution E. X is neutral
19. When each of the nitrates of Potassium, Magnesium and iron is heated, A. all the nitrates decompose to their oxides B. the nitrate of magnesium gives the nitrite and oxygen C. the nitrates of iron magnesium and iron give the oxides D. the nitrate of iron gives the nitrite and oxygen E. the nitrate of the magnesium is not decomposed
2O. Which of the following metals cannot replace hydrogen from water or steam? A. Sodium B. Magnesium C. Iron D. Calcium E. Copper
21. small quantity of solid ammonium chloride (NH4Cl) was heated gently in a test tube, the solid gradually disappears to produce two gases. Later, a white cloudy deposit was observed on the cooler part of the test tube. The ammonium chloride is said to have undergone A. distillation B. sublimation C. precipitation D. evaporation E. decomposition
22. Elements P, Q, R, S have 6, 11, 15, 17 electrons respectively, therefore, A. P will form an electrovalent bond with R B. Q will form a covalent bond with S C. R will form an electrovalent bond with S D. Q will form an electrovalent bond with S E. Q will form a covalent bond with R
23. An element X forms the following compounds with chlorine; XCl4, XCl3, XCl2. This illustrates the A. law of multiple proportions B. law of chemical proportions C. law of simple proportions D. law of conservation of mass E. law of definite proportions
24. The oxidation state of chlorine in potassium chlorate is A. +1 B. +2 C. +3 D. +5 E. +7
25. 10 When air which contains the gases Oxygen, nitrogen, carbondioxide, water vapour and the rare gases, is passed through alkaline pyrogallol and then over quicklime, the only gases left are; A. nitrogen and carbondioxide B. the rare gases C. nitrogen and oxygen D. nitrogen and the rare gases E. nitrogen, carbondioxide and the rare
26. Which of the following statements is NOT correct? A. The average kinetic energy of a gas is directly proportional to its temperature B. At constant tempearture, the volume of a gas increases as the pressure increases C. The pressure of a gas is inversely proportional to its volume D. The temperature of a gas is directly proportional to its volume E. The collisions of molecules with each other are inelastic
27. Zinc Oxide is a A. Basic Oxide B. Acidic Oxide C. Amphoteric Oxide D. Neutral Oxide E. Reactive Oxide
28. When sodium chloride and metallic sodium are each dissolved in water A. both processes are exothermic B. both processes are endothermic C. the dissolution of metallic sodium is endothermic D. the dissolution of metallic sodium is exothermic E. the dissolution of sodium chloride is explosive
29. The periodic classification of elements is an arrangement of the elements in order of their A. Atomic Weights B. Isotopic Weights C. Molecular Weights D. Atomic Numbers E. Atomic Masses
3O. In the reaction between sodium hydroxide and sulphuric acid solutions, what volume of 0.5 molar sodium hydroxide would exactly neutralise 10cm3 of 1.25 molar sulphuric acid? A. 5cm3 B. 10cm3 C. 20cm3 D. 25cm3 E. 50cm3
Recommended: How to check NECO Result
NECO Chemistry Questions And Answers 2023 (Paper 2)
Don’t worry about these NECO Chemistry Questions And Answers 2023. All you need to do is to keep on refreshing this page for the 2023 NECO Chemistry Questions And Answers for this year. It will be posted here in few minutes.
Tips on How to Pass 2023 NECO Chemistry Examinations
The following guidelines will help you pass the 2023 NECO Chemistry examination with flying colours.
Have a Target and Work Towards Actualizing it
You have decided to pass NECO Chemistry 2023 and I am sure of that. Now, the next thing you should do is set targets.
You have told yourself, “I will score A in NECO Chemistry 2023”, that’s not all. You need to plan on how to make it happen. Create a timetable and master plan to achieve your goals.
Get the Recommended Textbook on Chemistry for 2023 NECO Examination
Normally, NECO recommends books for the examination. But apart from NECO Literature in English where certain novels are compulsory, you are free to use any good Chemistry textbook to prepare for NECO 2023 exam.
Some textbooks are more difficult to understand. If you have any topic you are finding difficult to understand, then get a textbook that will simplify the topics and make life better for you.
Do not Skip Chemistry Examples and Exercise you Will Come Across While Reading:
Many candidates are fond of skipping exercises and even examples while studying textbooks. In fact, we like notebooks so much that we could ask, “can I read my notebook and pass NECO Chemistry 2023?” Don’t be scared of attempting exercises in Biology. Face the challenges.
If you have any questions about the NECO Chemistry Questions and Answers 2023 , kindly drop your question in the comment box.
Last Updated on July 25, 2023 by Admin
Related posts:

122 thoughts on “NECO Chemistry Questions and Answers 2023/2024 (Essay and Objectives)”
Please when will the answer come up
I just need questions for the two
I am really greatfull for the coperation you showed to us most especially me if not for you guys thisneco would have been trouble so sincear thank you
WHERE IS THE ANSWER FOR THE OBJ QUESTIONS THAT IS UP THERE
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Notify me of follow-up comments by email.
Notify me of new posts by email.

NECO Chemistry Questions and Answers 2023 Objectives and Essay
- Post author: Study Admin
- Post published: October 26, 2023
- Post category: School News
- Post comments: 0 Comments
NECO chemistry 2023 answers are now available. NECO chemistry questions and answers 2023/2024 objective and essay and other exam details for NECO 2023 are on this page. See the 2023 NECO chemistry answers for both objective and theory below. Get the NECO chemistry objective and essay answers here.
The 2023 chemistry NECO OBJ and theory questions and answers are provided here for free. All you have to do is to go through the questions and take note of the NECO chemistry answers 2023. Read on to find out.
NECO Chemistry Questions and Answers 2023 Objective and Essay
Have you been searching on Google in order to get the NECO chemistry questions and answers 2023? If so, we have got you covered!
We have the 2023 NECO chemistry questions and our team of experts will soon upload the NECO chemistry questions and their accurate answers to help you pass the 2023 NECO chemistry examination.
The 2023 NECO chemistry theory questions and OBJ will be uploaded any moment from now. So if you are searching for the NECO chemistry answers 2023 for objective and theory, then you are on the right page. See NECO chemistry objective and essay questions and answers below.
NECO Chemistry Answers 2023 Objective and Theory
The National Examination Council (NECO) is an examination body in Nigeria that conducts the Senior Secondary Certificate Examination and the General Certificate in Education in June/July and November/December respectively.
The 2023 NECO chemistry questions are set from the SS1 to SS3 chemistry syllabus. So all the questions you will encounter in this year’s examination are in the syllabus, and nearly 95% of the questions are repeated.
You don’t have to worry about the 2023 NECO chemistry questions and answers PDF (essay and objective). The NECO chemistry answers 2023 will be uploaded any moment from now. All you need to do is to keep refreshing this page so as not to miss out.
Once again, keep refreshing this page because we will upload the original NECO chemistry questions and answers for this year’s exams on this page at any moment from now. Also, to download the past questions and answers, click on this link NECO chemistry past questions .
If you have any questions about the NECO chemistry questions 2023 and answers, feel free to use the comment box below or use the Chat With Us button and we will respond immediately.
The 2023 NECO chemistry answers will be posted here. Be patient. Keep checking and reloading this page for the correct answers. NECO 2023 chemistry answers loading…….
There is nothing like NECO chemistry expo 2023 online. All students are advised to avoid all patronizing online fraudsters/vendors who claim to provide such services.
You Might Also Like
Ibbu post utme form 2024/2025 academic session, greenfield university post utme form 2023/2024 academic session, waec auto mechanical work questions and answers 2024 objective and essay, leave a reply cancel reply.
Save my name, email, and website in this browser for the next time I comment.
EXAMCLASS.NET BEST WEBSITE FOR JAMB RUNZ,WAEC RUNZ, NECO RUNZ,SCHOOL NEWS, UPDATES
Your #no 1 best website for waec runz | jamb cbt runz | neco runz | gce runz and school news, for easy & fast contact +2349068045697 || call 09068045697, 2023 neco gce chemistry (essay & obj) answers.
December 6, 2023 Mr Class Neco Gce 0

NECO GCE CHEMISTRY OBJECTIVES (OBJ) ANSWERS 2023
Neco GCE 2023 Chemistry Expo > All NECO GCE candidates are hereby informed that our platform has given go ahead for 2022 Neco GCE per subject subscription for the 2023/2024 ongoing Neco SSCE November/December External exam.
This is to say that Neco Gce Chemistry Expo 2023 Runs Questions and Answers Subscription has commenced online so you can pay. Be part of your success by following the guidelines provided on this article.
NECO GCE 2023/2024 chemistry theory EXPO SUBSCRIPTION
Our subscription price list for whatsapp message..
(i) Per Subject: N600 each (ii) (iii) Maths & English: N1200 each
Our Subscription Price List For Password Answers.
(i) Per Subject: N500 each (ii) practicals Per Subject: N500 each (iii) Maths & English: N1000 each
CLICK HERE FOR ANSWERPAGE
Send (i) Payment name/Your name. (ii) Subject Name (e.g Chemistry) (iii) MTN Recharge Card Pin(s) (iv) Phone number to 09068045697
With MR classic man , Your Success In Sure.
Remember Success is not a must, is an Option. Don’t let this little amount of money make you fail this exam. NECO GCE 2023 EXPO On today Chemistry ANSWERS
WARNING :-Please Don’t Even Come for Free Answers, because it Would’nt be Posted. Take me Serious This Time.
Make Sure you Subscribe if you Don’t Want to be on Hot Seat
Neco Gce Chemistry Expo 2023 – Neco Gce Chemistry Runs 2023 / Neco Gce Chemistry Objective and Essay Answers / Neco Gce Chemistry Questions and Answers Runz 2023/2024.
If you discovered this amusing webpage, you must be very lucky enough, because a lot of people have been seeking to know us, but it’s not easy as you think. Regardless of wherever you got our attention from, you are welcome to the best Nigeria exam solution provider portal.
This article is written to expose our mode of operation regarding the ongoing Neco SSCE November/December External exam. Mr classic mission is for you to make excellent results in the Neco SSCE exam. I have not seen where our Candidates after writing various exams we help, reportedly fails, its never our motto.
Get Neco Gce Chemistry Objective and Essay Answers sent to you via Whatsapp or direct text message few hours before the commencement of any exam as stated in the timetable.
NB : We have Password link Answers delivery, but The best we provide our selfs is via Whatsapp platform. 2023 NECO GCE Chemistry (Essay & OBJ) Answers
On Wednesday December 6th 2023, chemistry papers will be written as confirmed by the Timetable released by Neco exam board. Are you one of the candidates seeking external help for the above mentioned subjects, don’t hesitate to reckon on Examclass.net for the assistance. We have not failed and can never fail.
Thinking of how to Get Neco Gce Chemistry Expo 2023 Runs Questions and Answers, Mr classic website is the best to give you all the necessary, unique and verified answers to them.
In order to be part of the candidates who wish to subscribe, Neco Gce Chemistry Objective and Essay will cost the sum 600 only. It is left for the candidate to choose method of payment. NECO GCE CHEMISTRY OBJECTIVES (OBJ) ANSWERS 2023
How to Get NECO Gce Chemistry Answers Contact Mr classic on 090 6804 5697.
Send your Mtn Recharge Card Pin to our line 09068045697 on WhatsApp or do mobile transfer or pos vtu by requesting for Mr classic mtn number on Same WhatsApp.

Be the first to comment
Leave a reply cancel reply.
Your email address will not be published.
Save my name, email, and website in this browser for the next time I comment.
Copyright © 2024 | WordPress Theme by MH Themes
Content protected!!
EXAMKING.NET
The king of all exams.
EXAMKING MOBILE APP
Ads; Click Here Now to Download All WAEC 2024 Questions And Answers
Ads 2; download all jamb 2024 questions and answers now click here download now, neco 2023 chemistry essay and obj answers.
April 1, 2024 ExamKing CEO NECO FORUM 0

NECO 2023 CHEMISTRY ESSAY AND OBJ ANSWERS – EXAMKING.NET ••••••••••••••••••••••••••••••••••••••••••••••••••••••••••• CLICK HERE TO JOIN OUR TELEGRAM CHANNEL FOR ALL 2023 EXAM ANSWERS FOR FREE ••••••••••••••••••••••••••••••••••••••••••••••••••••••••••• CHEMISTRY-Obj 01-10: DEADADECAD 11-20: BAEDDBDBAE 21-30: CCDCADDECD 31-40: EBEEBEBCEE 41-50: BCCECEDADD 51-60: DABBDEAECA Solved By Examking.Net Completed Solved by Examking.net COMPLETED!!! ••••••••••••••••••••••••••••••••••••••••••••••••••••••••••• CHEMISTRY ESSAY-Answers (1ai) (i) Manufacturing sulfuric acid (ii) Vulcanization of rubber (iii) Formulation of Pesticides and fungicides
(1aii) (i) It is a colorless gas that has a distinct smell of rotten eggs (ii) Hydrogen sulphide is soluble in water to some extent
(1aiii) Soaps are made from natural products while detergents are made from synthetic products.
(1aiv) Detergents is for household cleaning and laundry purposes
(1bi) Number of neutrons = Mass number (A) – Atomic number (Z)
I. ²³₁₁X A = 23 (mass number) Z = 11 (atomic number)
Number of neutrons = 23 – 11 = 12
II. ³⁹₁₉Y A = 39 (mass number) Z = 19 (atomic number)
Number of neutrons = 39 – 19 = 20
(1bii) Molar mass: Na = 22.99 g/mol O₂ = 2 * 16.00 g/mol = 32.00 g/mol
Now, let’s calculate the mass of oxygen needed:
First, calculate the number of moles of sodium (Na) in 9.2g: Number of moles = Mass / Molar mass Number of moles of Na = 9.2g / 22.99 g/mol ≈ 0.4002 mol
Since the mole ratio of Na to O₂ is 4:1, the number of moles of O₂ needed is: Number of moles of O₂ = 0.4002 mol / 4 ≈ 0.1001 mol
Now, calculate the mass of oxygen needed: Mass of O₂ = Number of moles of O₂ * Molar mass of O₂ Mass of O₂ = 0.1001 mol * 32.00 g/mol ≈ 3.204 g
Therefore, approximately 3.204 grams of oxygen are needed to burn 9.2 grams of sodium.
(1biii) CaCO₃(s) + 2 HCl(aq) —> CaCl₂(aq) + CO₂(g) + H₂O(l)
From the balanced equation, 1 mole of calcium carbonate (CaCO₃) reacts with 2 moles of HCl to produce 1 mole of calcium chloride (CaCl₂).
Molar masses: CaCO₃ = Ca(40.08) + C(12.01) + 3O(16.00) = 100.09 g/mol CaCl₂ = Ca(40.08) + 2Cl(35.45) = 110.98 g/mol
Now, let’s calculate the number of moles of CaCO₃ in 50g:
Number of moles of CaCO₃ = Mass / Molar mass Number of moles of CaCO₃ = 50g / 100.09 g/mol ≈ 0.4998 mol
Since the mole ratio of CaCO₃ to CaCl₂ is 1:1, the number of moles of CaCl₂ that can be obtained is also approximately 0.4998 mol.
Thus, about 0.4998 moles of calcium chloride can be obtained from 50g of limestone in the presence of excess hydrogen chloride.
(1ci) (i) Sol: A sol is a colloidal solution in which solid particles are dispersed in a liquid medium. (ii) Aerosol: An aerosol is a colloidal solution in which liquid or solid particles are dispersed in a gas medium.
(1cii) The law of definite proportions, also known as the law of constant composition, states that a given chemical compound always contains its constituent elements in fixed and definite proportions by mass. This means that the ratio of the masses of the elements in a compound is constant, regardless of the compound’s origin or method of preparation.
(I) Sodium trioxonitrate (V) is also known as sodium nitrate, with the chemical formula NaNO₃.
The atomic masses are as follows: Na (Sodium) = 22.99 g/mol N (Nitrogen) = 14.01 g/mol O (Oxygen) = 16.00 g/mol
Relative molecular mass of NaNO₃ = (1 * Na) + (1 * N) + (3 * O) Relative molecular mass of NaNO₃ = (1 * 22.99 g/mol) + (1 * 14.01 g/mol) + (3 * 16.00 g/mol) Relative molecular mass of NaNO₃ = 22.99 g/mol + 14.01 g/mol + 48.00 g/mol Relative molecular mass of NaNO₃ = 85.00 g/mol
Therefore, the relative molecular mass of sodium nitrate (NaNO₃) is 85.00 g/mol.
(II) Copper (II) trioxosulphate (VI) pentahydrate is also known as copper (II) sulfate pentahydrate, with the chemical formula CuSO₄ · 5H₂O.
The atomic masses are as follows: Cu (Copper) = 63.55 g/mol S (Sulfur) = 32.06 g/mol O (Oxygen) = 16.00 g/mol H (Hydrogen) = 1.01 g/mol
Relative molecular mass of CuSO₄ · 5H₂O = (1 * Cu) + (1 * S) + (4 * O) + (10 * H) + (5 * O)
Relative molecular mass of CuSO₄ · 5H₂O = (1 * 63.55 g/mol) + (1 * 32.06 g/mol) + (4 * 16.00 g/mol) + (10 * 1.01 g/mol) + (5 * 16.00 g/mol)
Relative molecular mass of CuSO₄ · 5H₂O = 63.55 g/mol + 32.06 g/mol + 64.00 g/mol + 10.10 g/mol + 80.00 g/mol
Relative molecular mass of CuSO₄ · 5H₂O = 249.71 g/mol
Therefore, the relative molecular mass of copper (II) sulfate pentahydrate (CuSO₄ · 5H₂O) is 249.71 g/mol.
(2ai) Mass of silver deposited (in grams) = (Current in Amperes × Time in seconds × Atomic mass of silver) / (1 Faraday)
Given: Current = 4.6 A Time = 90 minutes = 90 × 60 seconds = 5400 seconds Atomic mass of silver (Ag) = 108g/mol 1 Faraday = 96,500C
Substituting the values to calculate the mass of silver deposited:
Mass of silver deposited = (4.6 A × 5400 s × 108 g/mol) / 96,500 C
Mass of silver deposited ≈ (2,682,720 g·s/mol) / 96,500 C
Mass of silver deposited ≈ 27.8g •••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
(2aii) (i) Electrode surface area (ii) Electrolyte temperature
(2aiii) (i) The oxidizing agent is MnO₄⁻(aq) (ii) The reducing agent is Fe²⁺(aq)
(2aiv) MnO₄⁻(aq) + 8H⁺(aq) + 5e⁻ —-> Mn²⁺ + 4H₂O(l)

(2bii) (i) Gases have no fixed shape or volume. (ii) Gases have low density compared to solids and liquids. (iii) Gases have high kinetic energy and are in constant motion.
(2biii) Faraday’s second law of electrolysis states that the mass of a substance deposited (or liberated) during electrolysis is directly proportional to the quantity of electric charge passed through the electrolyte.
(2biv) (i) Charcoal (ii) Coal
(2bv) Na (Sodium) > Ca (Calcium) > Mg (Magnesium) > Al (Aluminum) •••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
(3ai) (i) Butan-2-ol – Secondary alkanol (ii) 2-methylpropanol – Primary alkanol (iii) 2-methylpropan-2-ol – Tertiary alkanol
(3aii) (i) Fermentation (ii) Ethylene hydration
(3aiii) Let the relative molecular mass of gas Z be M. (Rate of diffusion of hydrogen)/(Rate of diffusion of gas Z) = √(molar mass of gas Z)/√(molar mass of hydrogen) 6/1 = (√M)/(√2) 36 = M/2 M = 2×36 M = 72
(3bi) 1s², 2s², 2p⁴
(3bii) (i) It is a colorless (ii) It is soluble in water. (iii) It is tasteless
(3biii) (i) Identify the longest chain. (ii) Name the substituents alphabetically
(3biv) C₂H₄ + O₂ —> 2CO₂ + 2H₂O
(3ci) Endothermic reaction can be defined as a form of heat reaction in which heat is absorbed from the surrounding into the reacting system.
(3cii) Zn(s) + H₂SO₄(aq) —> ZnSO₄(aq) + H₂(g)
(3ciii) Redox reaction.
(3iv) (i) For refining petrol (ii) For food processing (iii) For producing fertilizer
•••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
(4ai) A super saturated solution is a solution that contains more than the maximum amount of solute that is capable of being dissolved at a given temperature.
(4aii) 15/345 = Solubility *25/1000
Solubility =1000*15/25*345=15000/8625
Solubility = 1.79mol/dm³
(4aiii) (i) H₃0⁺ (ii) NH₄⁺ (iii) [CN]⁻₆
(4bi) (i) It has no chemical formula (ii) It can be separated physically (iii) Freezing air slowly yields different liquids at different temperatures
(4bii) (i) Noble gases (ii) Carbon (iv) oxide
(4biii) H₂SO₄ —-> 2H+ + SO₄²⁻
1 mole of H₂SO₄ = 2 mole of H⁺
0.1 mole of H₂SO₄ = 0.2 mole of H⁺ Mole = no. of H⁺/Avogadro’s constant
No. of H⁺ = Mole * Avogadro’s constant = 0.2 * 6.0*10²³ = 1.2*10²³ ions
(4biv) (i) Dative bonding (ii) Hydrogen bonding
(4bv) (i) BRASS: Constituent: Copper and zinc. Use: Brass is used in the production of musical instruments decorative items and plumbing fixtures.
(ii) BRONZE: Constituent: Copper and tin. Use: Bronze is used in the production of statues coins and various machinery. •••••••••••••••••••••••••••••••••••••••••••••••••••••••••••
(5ai) A base is a substance which when disolve produce hydroxyl ion (OH⁻) as the only negative ion
(5aii) (i) K₂O (ii) MgO
(5aiii) (i) it is used in printing inks and dyes (ii) it is used in making photographic chemicals
(5aiv) Aliphatic does not have good odour while an aromatic hydrocarbon has
(5av) M.m of XCl₃=10-8+(35-5*3) =10.8+106.5 =117.3 Vapour density =117.3/2=58.65
(5bi) (i) Temperature (ii) concentration (iii) surface area
(5bii) The law states that energy can neither be created nor destroyed in and isolated system.
(5biii) (i) burning of wood (ii) neutralization reaction

Be the first to comment
Leave a reply.
Your email address will not be published.
Save my name, email, and website in this browser for the next time I comment.
ExamKing.Net is the Best exam runz website for Waec, Neco, Nabteb and Gce runz website for exam student’s this website has been in existence for over 11 years and it is known for its excellency.
USEFUL LINKS
HOMEPAGE OUR ANSWER PAGE OUR DATABASE
CONNECT WITH US
(Send Us Sms Direct) (WhatsApp Us) Follow us on Facebook Follow Us On Instagram Follow Us On Twitter
Copyright © 2024 | Designed by Examking.Net Team
EXAMKING APP FOR FREE EXAM ANSWERS
NECO Chemistry Questions and Answers 2023 (100% Sure) Theory & Obj Solution
Get free Verified NECO Chemistry Questions and Answers 2023. NECO June/July Free Chemistry EXPO answers. National Examination Council Chemistry Theory and Objective Answers for you to have good NECO result. You will also understand how NECO Chemistry questions are set and how to answer them. The National Examination Council is an examination body in Nigeria that conducts the Senior Secondary Certificate Examination and the General Certificate in Education in June/July and November/December respectively.
Click Here Now To Chat Us on WhatsApp For Subscription Details

Click Here To See All NECO Chemistry Question Paper 2023
Please Note that the NECO 2023 Chemistry Questions and Answers and any other NECO expo is provided by us for free. We understand that a lot of website charge of collect money from student to provide NECO expo Chemistry Answers to them. NECO questions and answers are provided for free. We will do same during Other Exam like NECO GCE.
NECO 2023 Chemistry Questions will be posted in this page. Our Team are right now with the question paper. It is under verification and once the verification process complete, we will go ahead to upload it here.
Be at alert! Keep refreshing this page for NECO Chemistry Theory Questions and Answers
2023 Chemistry Essay Questions Loading 93.8%
Also Read: How To Check Your JAMB Result
Click Here To Join The 2023 NECO Direct Link answers Group
NECO Chemistry Questions and Answers 2023 OBJECTIVES (OBJ) ANSWERS Loading
2023 VERIFIED Chemistry OBJ:…Loading
CHEMISTRY-OBJ
Please Click Here To See The NECO Chemistry OBJ Answers
CHEMISTRY-Obj! 1-10 DEADADECAD 11-20 BAEDDBDBAE 21-30 CCDCABDDCD 31-40 EBEECEBCEE 41-50 BCCECDDADD 51-60 DABBDEAECA Solved By beafans.com Completed!!
NECO Chemistry Questions and Answers 2023 loading…
Please do not panic and fall into the right hand. 2023 Chemistry Answers will be posted for free here once ready.
We are right now getting things ready for you. The NECO 2023 Chemistry theory questions and answers will be posted any moment from now. All you need do is to keep refreshing this page until you see the answers.
NECO Chemistry -ESSAY-ANSWERS-joberplanet.com
Answers Loading….
CHEMISTRY-ANSWERS
(1ai) (i) Manufacturing sulfuric acid (ii) Vulcanization of rubber (iii) Formulation of Pesticides and fungicides
(1aii) (i) It is a colorless gas that has a distinct smell of rotten eggs (ii) Hydrogen sulphide is soluble in water to some extent
(1aiii) Soaps are made from natural products while detergents are made from synthetic products.
(1aiv) Detergents is for household cleaning and laundry purposes
(1bi) Number of neutrons = Mass number (A) – Atomic number (Z)
I. ²³₁₁X A = 23 (mass number) Z = 11 (atomic number)
Number of neutrons = 23 – 11 = 12
II. ³⁹₁₉Y A = 39 (mass number) Z = 19 (atomic number)
Number of neutrons = 39 – 19 = 20
(1bii) Molar mass: Na = 22.99 g/mol O₂ = 2 * 16.00 g/mol = 32.00 g/mol
Now, let’s calculate the mass of oxygen needed:
First, calculate the number of moles of sodium (Na) in 9.2g: Number of moles = Mass / Molar mass Number of moles of Na = 9.2g / 22.99 g/mol ≈ 0.4002 mol
Since the mole ratio of Na to O₂ is 4:1, the number of moles of O₂ needed is: Number of moles of O₂ = 0.4002 mol / 4 ≈ 0.1001 mol
Now, calculate the mass of oxygen needed: Mass of O₂ = Number of moles of O₂ * Molar mass of O₂ Mass of O₂ = 0.1001 mol * 32.00 g/mol ≈ 3.204 g
Therefore, approximately 3.204 grams of oxygen are needed to burn 9.2 grams of sodium.
(1biii) CaCO₃(s) + 2 HCl(aq) —> CaCl₂(aq) + CO₂(g) + H₂O(l)
From the balanced equation, 1 mole of calcium carbonate (CaCO₃) reacts with 2 moles of HCl to produce 1 mole of calcium chloride (CaCl₂).
Molar masses: CaCO₃ = Ca(40.08) + C(12.01) + 3O(16.00) = 100.09 g/mol CaCl₂ = Ca(40.08) + 2Cl(35.45) = 110.98 g/mol
Now, let’s calculate the number of moles of CaCO₃ in 50g:
Number of moles of CaCO₃ = Mass / Molar mass Number of moles of CaCO₃ = 50g / 100.09 g/mol ≈ 0.4998 mol
Since the mole ratio of CaCO₃ to CaCl₂ is 1:1, the number of moles of CaCl₂ that can be obtained is also approximately 0.4998 mol.
Thus, about 0.4998 moles of calcium chloride can be obtained from 50g of limestone in the presence of excess hydrogen chloride.
(1ci) (i) Sol: A sol is a colloidal solution in which solid particles are dispersed in a liquid medium. (ii) Aerosol: An aerosol is a colloidal solution in which liquid or solid particles are dispersed in a gas medium.
(1cii) The law of definite proportions, also known as the law of constant composition, states that a given chemical compound always contains its constituent elements in fixed and definite proportions by mass. This means that the ratio of the masses of the elements in a compound is constant, regardless of the compound’s origin or method of preparation.
(I) Sodium trioxonitrate (V) is also known as sodium nitrate, with the chemical formula NaNO₃.
The atomic masses are as follows: Na (Sodium) = 22.99 g/mol N (Nitrogen) = 14.01 g/mol O (Oxygen) = 16.00 g/mol
Relative molecular mass of NaNO₃ = (1 * Na) + (1 * N) + (3 * O) Relative molecular mass of NaNO₃ = (1 * 22.99 g/mol) + (1 * 14.01 g/mol) + (3 * 16.00 g/mol) Relative molecular mass of NaNO₃ = 22.99 g/mol + 14.01 g/mol + 48.00 g/mol Relative molecular mass of NaNO₃ = 85.00 g/mol
Therefore, the relative molecular mass of sodium nitrate (NaNO₃) is 85.00 g/mol.
(II) Copper (II) trioxosulphate (VI) pentahydrate is also known as copper (II) sulfate pentahydrate, with the chemical formula CuSO₄ · 5H₂O.
The atomic masses are as follows: Cu (Copper) = 63.55 g/mol S (Sulfur) = 32.06 g/mol O (Oxygen) = 16.00 g/mol H (Hydrogen) = 1.01 g/mol
Relative molecular mass of CuSO₄ · 5H₂O = (1 * Cu) + (1 * S) + (4 * O) + (10 * H) + (5 * O)
Relative molecular mass of CuSO₄ · 5H₂O = (1 * 63.55 g/mol) + (1 * 32.06 g/mol) + (4 * 16.00 g/mol) + (10 * 1.01 g/mol) + (5 * 16.00 g/mol)
Relative molecular mass of CuSO₄ · 5H₂O = 63.55 g/mol + 32.06 g/mol + 64.00 g/mol + 10.10 g/mol + 80.00 g/mol
Relative molecular mass of CuSO₄ · 5H₂O = 249.71 g/mol
If you have any questions about the NECO Chemistry theory & Obj 2023, kindly let us know in the comment box.
We are happy you are here for the 2023 Chemistry answers. The complete solution will be made free in some minutes before the Chemistry examination.
NECO Chemistry questions 2023, NECO 2023 Chemistry questions and answers, Chemistry NECO 2023, NECO questions 2023/2023, Chemistry 2023,
Therefore, the relative molecular mass of copper (II) sulfate pentahydrate (CuSO₄ · 5H₂O) is 249.71 g/mol.
(2ai) Mass of silver deposited (in grams) = (Current in Amperes × Time in seconds × Atomic mass of silver) / (1 Faraday)
Given: Current = 4.6 A Time = 90 minutes = 90 × 60 seconds = 5400 seconds Atomic mass of silver (Ag) = 108g/mol 1 Faraday = 96,500C
Substituting the values to calculate the mass of silver deposited:
Mass of silver deposited = (4.6 A × 5400 s × 108 g/mol) / 96,500 C
Mass of silver deposited ≈ (2,682,720 g·s/mol) / 96,500 C
Mass of silver deposited ≈ 27.8g
(2aii) (i) Electrode surface area (ii) Electrolyte temperature
(2aiii) (i) The oxidizing agent is MnO₄⁻(aq) (ii) The reducing agent is Fe²⁺(aq)
(2aiv) MnO₄⁻(aq) + 8H⁺(aq) + 5e⁻ —-> Mn²⁺ + 4H₂O(l)
(2bi) Coming
(2bii) (i) Gases have no fixed shape or volume. (ii) Gases have low density compared to solids and liquids. (iii) Gases have high kinetic energy and are in constant motion.
(2biii) Faraday’s second law of electrolysis states that the mass of a substance deposited (or liberated) during electrolysis is directly proportional to the quantity of electric charge passed through the electrolyte.
(2biv) (i) Charcoal (ii) Coal
(2bv) Na (Sodium) > Ca (Calcium) > Mg (Magnesium) > Al (Aluminum)
(3ai) (i) Butan-2-ol – Secondary alkanol (ii) 2-methylpropanol – Primary alkanol (iii) 2-methylpropan-2-ol – Tertiary alkanol
(3aii) (i) Fermentation (ii) Ethylene hydration
(3aiii) Let the relative molecular mass of gas Z be M. (Rate of diffusion of hydrogen)/(Rate of diffusion of gas Z) = √(molar mass of gas Z)/√(molar mass of hydrogen) 6/1 = (√M)/(√2) 36 = M/2 M = 2×36 M = 72
(3bi) 1s², 2s², 2p⁴
(3bii) (i) It is a colorless (ii) It is soluble in water. (iii) It is tasteless
(3biii) (i) Identify the longest chain. (ii) Name the substituents alphabetically
(3biv) C₂H₄ + O₂ —> 2CO₂ + 2H₂O
(3ci) Endothermic reaction can be defined as a form of heat reaction in which heat is absorbed from the surrounding into the reacting system.
(3cii) Zn(s) + H₂SO₄(aq) —> ZnSO₄(aq) + H₂(g)
(3ciii) Redox reaction.
(3iv) (i) For refining petrol (ii) For food processing (iii) For producing fertilizer
(4ai) A super saturated solution is a solution that contains more than the maximum amount of solute that is capable of being dissolved at a given temperature.
(4aii) 15/345 = Solubility *25/1000
Solubility =1000*15/25*345=15000/8625
Solubility = 1.79mol/dm³
(4aiii) (i) H₃0⁺ (ii) NH₄⁺ (iii) [CN]⁻₆
(4bi) (i) It has no chemical formula (ii) It can be separated physically (iii) Freezing air slowly yields different liquids at different temperatures
(4bii) (i) Noble gases (ii) Carbon (iv) oxide
(4biii) H₂SO₄ —-> 2H+ + SO₄²⁻
1 mole of H₂SO₄ = 2 mole of H⁺
0.1 mole of H₂SO₄ = 0.2 mole of H⁺ Mole = no. of H⁺/Avogadro’s constant
No. of H⁺ = Mole * Avogadro’s constant = 0.2 * 6.0*10²³ = 1.2*10²³ ions
(4biv) (i) Dative bonding (ii) Hydrogen bonding
(4bv) (i) BRASS: Constituent: Copper and zinc. Use: Brass is used in the production of musical instruments decorative items and plumbing fixtures.
(ii) BRONZE: Constituent: Copper and tin. Use: Bronze is used in the production of statues coins and various machinery.
(5ai) A base is a substance which when disolve produce hydroxyl ion (OH⁻) as the only negative ion
(5aii) (i) K₂O (ii) MgO
(5aiii) (i) it is used in printing inks and dyes (ii) it is used in making photographic chemicals
(5aiv) Aliphatic does not have good odour while an aromatic hydrocarbon has
(5av) M.m of XCl₃=10-8+(35-5*3) =10.8+106.5 =117.3 Vapour density =117.3/2=58.65
(5bi) (i) Temperature (ii) concentration (iii) surface area
(5bii) The law states that energy can neither be created nor destroyed in and isolated system.
(5biii) (i) burning of wood (ii) neutralization reaction

Now Loading…94.5%
We want to hear from you concerning the NECO Chemistry Questions and Answers 2023 , kindly use the comment section below to get to us.
Related Posts
Grange School Recruitment 2023/2024 Jobs Vacancies Application Form (7 Positions)

Nile University of Nigeria Recruitment 2023, Job Vacancies & Application Form (Over 240 Positions)

Wellspring College Recruitment 2023/2024, Job Vacancies & Application Form (4 Positions)

Modibbo Adama University Recruitment 2023/2024, Job Vacancies & Application Form (3 Positions)

St. Mary Dedication British International High Schools Recruitment 2023/2024, Job vacancies & Application Form (3 Positions)

Pulse Recruitment 2023/2024, Job Vacancies & Application Form (4 Positions)
Comments are closed.
Type above and press Enter to search. Press Esc to cancel.
- Now Trending:
- 2024 WAEC Yoruba Questio...
- 2024 WAEC Igbo Questions...
- WAEC Food And Nutrition ...
- WAEC Data Processing 202...
2023 Neco Chemistry Essay and Objective Questions and Answers Expo
Table of Contents
2023 Neco Chemistry Essay and Objective Questions and Answers
The National Examination Council (NECO) Has Scheduled The 2023 Neco Chemistry Essay and Objective Questions and Answers Paper To Kick of on Monday 24th July 2023.
This brings the attention of candidates writing the exam in to searching for 2023 Neco Chemistry Essay and Objective Questions and Answers , 2023 NECO Chemistry Essay and Objective Expo 2023, Neco chemistry essay and objective 2023 and etc.
In this section, you will read the steps and requirements needed for you to get 2023 Neco Chemistry Essay and Objective 2023 Questions And Answers before exam.
NECO Chemistry Essay and Objective 2023 Paper is Categorized in to 2 parts;
- NECO Chemistry Essay 2023
- Neco Chemistry Objective 2023
Here on zamgist, we have solved all the questions. That is 2023 Neco Chemistry Essay and Objective Questions and Answers.

CLICK HERE TO CHAT ME UP ON WHATSAPP & GET IT NOW
NECO Chemistry Essay and Objective Expo 2023
==> Direct SMS: N1000 MTN CARD Direct SMS MEANS all answers(theory & obj) will come direct to ur phone as sMs.
==> Online PIN: N500 MTN CARD Online PIN MEANS The Pin to access our answers online via https://examdubs.com.ng/ will be sent to u at least 4 hours before the exam to access our answers.
==> WhatsApp: N500 MTN CARD Whatsapp MEANS The answer will be sent to you on WhatsApp after we confirm your subscription. Add Us On WhatsApp at 08023429251.
Send The Following details:-
(i) MTN CARD Pin(s) (ii) Your Name (iii) Subject (iv) Phone number ===> 08023429251 via WhatsApp
RELATED POST:
Civic Education NECO 2023 Questions and Answers Expo
2023 NECO Essay and Objective Agric Questions and Answers Expo
IJMB Chemistry Paper 1 2023 Questions And Answers
2023 NECO Physics practical Questions and Answers Expo
2023 NECO Chemistry practical Questions and Answers Expo
2023 NECO History Questions and Answers Expo
Neco Physics Practical Specimen 2023
NECO Chemistry Specimen 2023
2023 NECO Data processing practical Questions and Answers Expo
NECO Physics Specimen 2023: Apparatus and Instructions
NECO Timetable 2023 For Art Student
NECO 2023 Timetable for Science students
NOTE:- All whatsapp messages sent to the above number are attended to. Always send us whatsapp message of your complaint, your message(s) will get to us and we will reply immediately.
Neco Chemistry Theory Questions 2023

CLICK HERE TO CHAT ME UP ON WHATSAPP TO PAY N500 & GET THE COMPLETE SOLUTIONS NOW
Related Posts

IMAGES
VIDEO
COMMENTS
In conclusion, preparing for the Objectives and Essay sections of the WAEC Chemistry examination requires dedication, persistence, and a strong understanding of the subject matter. By following the tips provided in this guide and practicing with the sample questions and answers, you will be well on your way to achieving success in the 2023 WAEC ...
WAEC 2023 Chemistry (CHEM) OBJ & Essay Answers . NB: Online WAEC chemistry answers arrive 3 hour Before the exam starts (keep refreshing this page). Visit This Link to Join Our Whatsapp Group. NB: Please only share this page with reputable students. This page will be removed as soon as the exam is over and a new one will be made for the next exam.
The 2023 Waec Chemistry Theory questions and answers are loading! 2023 Chemistry Essay answers Loading!! 2023 Waec Chemistry Theory Answers Loading!!! Kindly bookmark the website for the answers that will be released. or better still reload the site to check if the answers for the 2023 Waec Chemistry questions and answers have dropped. 3d)
(1f) Van der Waals forces between chlorine molecules are stronger than those between fluorine molecules because chlorine atoms have more electrons, leading to a larger electron cloud. This larger electron cloud results in stronger temporary dipoles and thus stronger Van der Waals forces. (1g) A reversible reaction is a chemical reaction that can proceed in both the forward and reverse directions.
SSCE WAEC Chemistry Objective Questions and Answers. CHEMISTRY Paper 1 (Objective Test Questions) Paper 1 will last for 1 hours Use HB pencil throughout. Answer All Questions Each question is followed by four options lettered A to D. Find out the correct options for each question and shade in pencil on your answer sheet, the answer space which bears the same letter as the option you Chosen.
TO SUBSCRIBE FOR 2023 WAEC CHEMISTRY ESSAY & OBJ ANSWERS VIA LINK. JUST GO OUT AND BUY MTN CARDS OF N600 ( 400 + 400 = 800) GO TO YOUR MESSAGE, TYPE THE CARD PINS CORRECTLY AND SEND TO 08107431933. DON'T CALL, JUST TEXT, IF THE CARDS PINS ARE VALID, A REPLY WILL BE SENT TO YOU CONFIRMING THAT YOU HAVE BEEN SUBSCRIBED.
The Time Of the WAEC GCE Chemistry Essay | Chemistry Objective | Chemistry Practical Exam is 11:30pm - 3:30pm for all the 2023 WAEC GCE Chemistry exams. A. 2023 WAEC GCE CHEMISTRY ESSAY | (THEORY) ANSWERS. Kindly bookmark the website for the answers that will be released here, or better still reload the site to check if the answers for the ...
A. 2023 WAEC GCE CHEMISTRY ESSAY (THEORY) ANSWERS | 12TH DECEMBER, 2023. (i) Ensure that both copper (II) oxide and dilute trioxonitrate (V) acid used in the reaction are of high purity. (ii) Ensure that the beakers and stirring rods used are clean and dry. OR.
Waec Gce Chemistry Expo 2023 - Waec Gce Chemistry Runs 2023 / Waec Gce Chemistry Objective and Essay Answers / Waec Gce Chemistry Questions and Answers Runz 2023/2024. If you discovered this amusing webpage, you must be very lucky enough, because a lot of people have been seeking to know us, but it's not easy as you think.
The 2024 WAEC chemistry questions are set from the SS1 to SS3 chemistry syllabus. So all the questions you will encounter in this year's examination are in the syllabus, and nearly 90% of the questions are repeated. You don't have to worry about the 2024 WAEC chemistry questions and answers PDF (essay and objective).
The National Examination Council, NECO Chemistry SSCE paper is scheduled to be written on Monday, 24th July 2023 from 10:00 am to 1:00 pm. This NECO Chemistry questions paper is for Papers III & II: Objective & Essay and will take a total of 3hrs to write. Here, we will be posting the neco chemistry questions for candidates that will ...
The NECO Chemistry Practical Syllabus is similar to the WAEC Syllabus, if not the same. This video, which is the detailed solution to past WAEC and NECO ques...
The 2024 Chemistry Essay answer paper will start at 9:30 am and will last for 2hrs while the Objective exam will commence at 11:30 pm and will last for 1hr. In this post, we will be posting the West African Senior School Certificate Examinations (WASSCE) Chemistry questions for candidates who will participate in the examination from past questions.
The following NECO Chemistry questions are questions to expect in the 2023 NECO examination. 1. The minimum amount of energy required for effective collisions between reacting particles is known. A) Activation energy. B) Bond energy. C) Kinetic energy. D) Potential energy. 2.
The 2023 NECO chemistry questions are set from the SS1 to SS3 chemistry syllabus. So all the questions you will encounter in this year's examination are in the syllabus, and nearly 95% of the questions are repeated. You don't have to worry about the 2023 NECO chemistry questions and answers PDF (essay and objective).
B. 2023 NECO CHEMISTRY (ESSAY) ANSWERS: TO SUBSCRIBE FOR NECO CHEMISTRY OBJ & THEORY ANSWERS VIA LINK ONLY. JUST GO OUT AND BUY MTN CARDS OF N600 ( 200 + 200 + 200 = 600) GO TO YOUR MESSAGE, TYPE THE MTN CARD PINS CORRECTLY AND SEND TO 08107431933. DON'T CALL THE NUMBER, JUST TEXT, IF THE CARDS PINS ARE VALID, A REPLY WILL BE SENT TO YOU ...
2023 NECO GCE Chemistry (Essay & OBJ) Answers. On Wednesday December 6th 2023, chemistry papers will be written as confirmed by the Timetable released by Neco exam board. Are you one of the candidates seeking external help for the above mentioned subjects, don't hesitate to reckon on Examclass.net for the assistance. We have not failed and ...
Now, let's calculate the mass of oxygen needed: First, calculate the number of moles of sodium (Na) in 9.2g: Number of moles = Mass / Molar mass Number of moles of Na = 9.2g / 22.99 g/mol ≈ 0.4002 mol Since the mole ratio of Na to O₂ is 4:1, the number of moles of O₂ needed is: Number of moles of O₂ = 0.4002 mol / 4 ≈ 0.1001 mol Now, calculate the mass of oxygen needed: Mass of ...
NECO GCE CHEMISTRY ESSAY (THEORY) ANSWERS 2023: TO SUBSCRIBE FOR NECO CHEMISTRY ANSWERS VIA LINK ONLY. JUST GO OUT AND BUY MTN CARDS OF N600 ( 200+ 200 + 200 = 600) GO TO YOUR MESSAGE, TYPE THE CARD PINS CORRECTLY AND SEND TO 08107431933. DON'T CALL, JUST TEXT, IF THE CARDS PINS ARE VALID, A REPLY WILL BE SENT TO YOU CONFIRMING THAT YOU HAVE ...
Relative molecular mass of CuSO₄ · 5H₂O = 63.55 g/mol + 32.06 g/mol + 64.00 g/mol + 10.10 g/mol + 80.00 g/mol. Relative molecular mass of CuSO₄ · 5H₂O = 249.71 g/mol. If you have any questions about the NECO Chemistry theory & Obj 2023, kindly let us know in the comment box. We are happy you are here for the 2023 Chemistry answers.
In this section, you will read the steps and requirements needed for you to get 2023 Neco Chemistry Essay and Objective 2023 Questions And Answers before exam. NECO Chemistry Essay and Objective 2023 Paper is Categorized in to 2 parts; NECO Chemistry Essay 2023. Neco Chemistry Objective 2023. Here on zamgist, we have solved all the questions.