- Search Menu
- Submission Site
- Author Guidelines
- Open Access Policy
- Self-Archiving Policy
- About the Journal of Sickle Cell Disease
- About the Foundation for Sickle Cell Disease Research
- Editorial Board
- Advertising & Corporate Services
- Journals on Oxford Academic
- Books on Oxford Academic


Editor in Chief
Lanetta Bronté-Hall, MD, MPH, MSPH
Executive Editor
Wally R. Smith, MD
Editorial board
About the journal

The Foundation for Sickle Cell Disease Research
At the Foundation for Sickle Cell Disease Research we believe that everybody is born with the right to a long, healthy, pain-free life. With innovative research, treatments, and education, we can change the conversation and shape the future for this genetic disorder.

Submit your research
Read through the author guidelines and learn about how to submit your work at our submissions site.
Submit your work

Author resources
Learn about how to submit your article, our publishing process, and tips on how to promote your article.
Find out more

- Accessibility
Oxford University Press is committed to making its products accessible to and inclusive of all our users, including those with visual, hearing, cognitive, or motor impairments.
Find out more in our accessibility statement
Related titles

- About The Foundation for Sickle Cell Disease Research
- Recommend to your Library
- Advertising & Corporate Services
- Journals Career Network
Affiliations
- Online ISSN 3029-0473
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Heart-Healthy Living
- High Blood Pressure
- Sickle Cell Disease
- Sleep Apnea
- Information & Resources on COVID-19
- The Heart Truth®
- Learn More Breathe Better®
- Blood Diseases and Disorders Education Program
- Publications and Resources
- Blood Disorders and Blood Safety
- Sleep Science and Sleep Disorders
- Lung Diseases
- Health Disparities and Inequities
- Heart and Vascular Diseases
- Precision Medicine Activities
- Obesity, Nutrition, and Physical Activity
- Population and Epidemiology Studies
- Women’s Health
- Research Topics
- Clinical Trials
- All Science A-Z
- Grants and Training Home
- Policies and Guidelines
- Funding Opportunities and Contacts
- Training and Career Development
- Email Alerts
- NHLBI in the Press
- Research Features
- Past Events
- Upcoming Events
- Mission and Strategic Vision
- Divisions, Offices and Centers
- Advisory Committees
- Budget and Legislative Information
- Jobs and Working at the NHLBI
- Contact and FAQs
- NIH Sleep Research Plan
- < Back To Research Topics
Sickle Cell Disease Research
Language switcher.
The National Institutes of Health (NIH) has supported research on sickle cell disease since before the NHLBI was founded in 1948. With each decade that followed, the NHLBI has kept a sustained focus on advancing the understanding of sickle cell disease and improving clinical care. We lead and support research and programs on sickle cell disease in the United States and around the world. Research and initiatives supported by the NHLBI work to improve treatments and evidence-based clinical care for all individuals living with sickle cell disease. We are committed to building on our legacy of research excellence to find new treatments, cures, and personalized care for the approximately 100,000 Americans and over 20 million people worldwide who have sickle cell disease.
NHLBI research that really made a difference
NHLBI-funded scientists found an effective sickle cell treatment in 1995. Results from the NHLBI Multicenter Study of Hydroxyurea showed that hydroxyurea reduced the number of painful episodes by 50% in severely affected adults with sickle cell disease. Three years later, the U.S. Food and Drug Administration approved hydroxyurea to treat sickle cell disease in adults. Research into optimizing the use of hydroxyurea continued over the next decade. In 2011, results from an NHLBI-funded study called BABY HUG found hydroxyurea to be safe for young children who have sickle cell disease.
Current research funded by the NHLBI
Our Division of Blood Diseases and Resources and the Division of Intramural Research , specifically the Cellular and Molecular Therapeutics Branch and the Sickle Cell Branch , oversee much of the research on sickle cell disease that we fund.
Find funding opportunities and program contacts for sickle cell disease research.
Current research on sickle cell disease treatment
Many current studies are looking at how to use genetic therapies and blood and bone marrow transplants to discover new treatment options for patients.
- Advances in genetics over the last decade may make effective gene-based treatments a reality for people with sickle cell disease. Through funding by the NHLBI and other collaborations , researchers are developing easy-to-administer gene-based interventions.
Read about the Cure Sickle Cell Initiative , a collaborative research effort led by the NHLBI that is accelerating the development of genetic therapies to cure sickle cell disease.
- Many patients with sickle cell disease receive frequent blood transfusions to treat and prevent certain complications. NHLBI-funded researchers are investigating the beneficial and harmful effects of blood transfusions in patients with this condition. In addition, researchers are developing more effective medicine support for blood transfusions when patients undergo bone marrow transplantation. This research is critical because a blood and bone marrow transplant is currently the only cure for many people living with sickle cell disease.
- The NHLBI also funds efforts to improve bone marrow transplantation (BMT) through the BMT Clinical Trials Network . For example, one current trial is investigating treatment of severe sickle cell disease with a BMT procedure that involves either a related or unrelated immune-matched donor.
Find more NHLBI-funded studies on sickle cell disease treatment at NIH RePORTER.

Read more in this news release about NHLBI-funded research on blood transfusions. The study found that using fresh red blood cells — cells that have spent seven days or less in storage — are no more beneficial than older red blood cells in reducing the risk of organ failure or death in critically ill children.
Current research on pain management
- Vaso-occlusive crises are painful episodes that affect many people with sickle cell disease. The crises are the leading cause of emergency department visits and hospitalizations. As part of the Sickle Cell Disease Treatment with Arginine Therapy (STArT) Trial , NHLBI-funded researchers found that patients with sickle cell disease and pain also had low levels of an amino acid called arginine in their blood. The study is investigating whether arginine supplements can reduce pain in patients. Other NHLBI-funded research seeks to determine the best way to calculate dosages of pain medicine for patients with vaso-occlusive episodes (severe pain that occurs when the sickle-shaped cells block blood flow to a part of the body).
- Pain and sickle cell disease often go hand-in-hand, but the exact cause of this pain is not well understood. NHLBI-funded researchers are using animal models of sickle cell disease to better understand the causes and treatments of chronic pain experienced by patients with sickle cell disease.
Find more NHLBI-funded studies on pain management in sickle cell disease at NIH RePORTER.
Current research on improving care for all people with sickle cell disease
The NHLBI is committed to research that will help reduce the barriers patients face when accessing sickle cell disease treatment. Find more NHLBI-funded studies on health disparities and sickle cell disease at NIH RePORTER.
Sickle cell disease research labs at NHLBI
Researchers from the NHLBI Division of Intramural Research , which includes investigators in our Sickle Cell Branch , are focused on developing new treatments for sickle cell disease.
- Intramural NHLBI researchers have developed a new and improved viral vector — a virus-based vehicle that delivers therapeutic genes — for use in genetic therapy for sickle cell disease.
- The NHLBI established the Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) in 2008. Since then, it has grown to include datasets from more than 140 epidemiological studies and clinical trials and about 4 million specimen collections, including from patients with sickle cell disease. Researchers within the NHLBI recently used data from the BioLINCC to reaffirm that hydroxyurea is safe and effective for very young children with sickle cell disease.
Read more about these projects and ongoing clinical trials .
Related sickle cell disease programs
The NHLBI leads and supports many programs and initiatives around the nation and the world as we search for a cure and work to improve the lives of people with sickle cell disease.
- The NHLBI’s annual Sickle Cell Disease Research Meeting brings together investigators, practitioners, and healthcare providers to discuss the progress of ongoing clinical trials and hear presentations about new developments in scientific and clinical aspects of sickle cell disease.
- The NHLBI supports three major programs in sub-Saharan Africa across 9 countries and 11 cities: The Sickle Pan-African Research Consortium , the Sickle Cell Disease Genomics Network of Africa , and the Realizing Effectiveness Across Continents with Hydroxyurea (REACH) Program . All are working to build research capacity and develop an infrastructure to enhance disease surveillance and delivery of care.
- The NHLBI participates in the Stimulating Hematology Investigation: New Endeavors (SHINE) Program, which focuses on basic and early translational hematology research and encourages applications from investigators at all career stages.
- The Recipient Epidemiology and Donor Evaluation Study (REDS) Program evaluates and improves the safety and effectiveness of transfusion therapies. The program also works to proactively address potential emerging threats to the United States’ blood supply and serves as a resource for ongoing work in transfusion research.
- The NHLBI has taken a lead role in managing the Regenerative Medicine Innovation Project (RMIP) under the 21st Century Cures Act . Beginning in 2017, the Act authorized the investment of $30 million in clinical research with adult stem cells to treat diseases including sickle cell disease.
Explore more NHLBI research on sickle cell disease
The sections above provide you with the highlights of NHLBI-supported research on sickle cell disease. You can explore the full list of NHLBI-funded studies on the NIH RePORTER .
To find more studies:
- Type your search words into the Quick Search box and press enter.
- Check Active Projects if you want current research.
- Select the Agencies arrow, then the NIH arrow, then check NHLBI .
If you want to sort the projects by budget size — from the biggest to the smallest — click on the FY Total Cost by IC column heading.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Sickle cell disease articles from across Nature Portfolio
Sickle cell disease is an autosomal recessive blood disorder that can lead to anaemia. It is caused by a mutation in the haemoglobin gene, which leads to deformation of red blood cells. Deformed red blood cells can obstruct small vessels and they are prone to destruction.
Latest Research and Reviews
Brain network hypersensitivity underlies pain crises in sickle cell disease
- Minkyung Kim
Risk factors for acute chest syndrome among children with sickle cell anemia hospitalized for vaso-occlusive crises
- Faisal A. Alghamdi
- Fawaz Al-Kasim
- Rehab Alluqmani
Development of pathophysiologically relevant models of sickle cell disease and β-thalassemia for therapeutic studies
Sickle cell disease (SCD) and β-thalassemia (BT) are globally prevalent inherited blood disorders but, despite extensive research, no ex vivo system exists for SCD and BT. Here, the authors generate pathophysiologically relevant erythroid progenitor models of SCD and BT.
- Pragya Gupta
- Sangam Giri Goswami
- Sivaprakash Ramalingam
The value-based price of transformative gene therapy for sickle cell disease: a modeling analysis
- George Morgan
- Gregory F. Guzauskas
Evaluating sheep hemoglobins with MD simulations as an animal model for sickle cell disease
- Caroline E. Kuczynski
- Christopher D. Porada
- Graça Almeida-Porada
An α-chain modification rivals the effect of fetal hemoglobin in retarding the rate of sickle cell fiber formation
- Eli H. Worth
- Mark K. Fugate
- Frank A. Ferrone
News and Comment
Autologous globin-edited HSCs ameliorate sickle cell disease
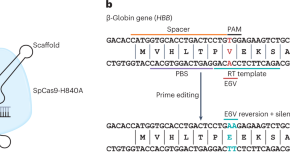
Gene correction for sickle cell disease hits its prime
Prime editing can efficiently rewrite the genetic mutation causing sickle cell disease, in haematopoietic stem cells from patients.
- Sébastien Levesque
- Daniel E. Bauer
Health-related quality of life in sickle cell disease
- Julie A. Panepinto
- Gregory J. Kato
- Wally R. Smith
Comparing health-related quality of life in chronic diseases: the importance of analyzing references
- Christine Maynié-François
- Stéphane Burtey
Omega-3 fatty acids are a potential therapy for patients with sickle cell disease
- Adrian Rabinowicz
- Kebreab Ghebremeskel
Sickle cell solutions in sight
New targets, new drug modalities and new business strategies are drawing long-awaited attention to sickle cell disease.
- Katie Kingwell
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Open access
- Published: 03 March 2022
Advances in the diagnosis and treatment of sickle cell disease
- A. M. Brandow 1 &
- R. I. Liem ORCID: orcid.org/0000-0003-2057-3749 2
Journal of Hematology & Oncology volume 15 , Article number: 20 ( 2022 ) Cite this article
34k Accesses
51 Citations
2 Altmetric
Metrics details
Sickle cell disease (SCD), which affects approximately 100,000 individuals in the USA and more than 3 million worldwide, is caused by mutations in the βb globin gene that result in sickle hemoglobin production. Sickle hemoglobin polymerization leads to red blood cell sickling, chronic hemolysis and vaso-occlusion. Acute and chronic pain as well as end-organ damage occur throughout the lifespan of individuals living with SCD resulting in significant disease morbidity and a median life expectancy of 43 years in the USA. In this review, we discuss advances in the diagnosis and management of four major complications: acute and chronic pain, cardiopulmonary disease, central nervous system disease and kidney disease. We also discuss advances in disease-modifying and curative therapeutic options for SCD. The recent availability of l -glutamine, crizanlizumab and voxelotor provides an alternative or supplement to hydroxyurea, which remains the mainstay for disease-modifying therapy. Five-year event-free and overall survival rates remain high for individuals with SCD undergoing allogeneic hematopoietic stem cell transplant using matched sibling donors. However, newer approaches to graft-versus-host (GVHD) prophylaxis and the incorporation of post-transplant cyclophosphamide have improved engraftment rates, reduced GVHD and have allowed for alternative donors for individuals without an HLA-matched sibling. Despite progress in the field, additional longitudinal studies, clinical trials as well as dissemination and implementation studies are needed to optimize outcomes in SCD.
Introduction
Sickle cell disease (SCD), a group of inherited hemoglobinopathies characterized by mutations that affect the β-globin chain of hemoglobin, affects approximately 100,000 people in the USA and more than 3 million people worldwide [ 1 , 2 ]. SCD is characterized by chronic hemolytic anemia, severe acute and chronic pain as well as end-organ damage that occurs across the lifespan. SCD is associated with premature mortality with a median age of death of 43 years (IQR 31.5–55 years) [ 3 ]. Treatment requires early diagnosis, prevention of complications and management of end-organ damage. In this review, we discuss recent advances in the diagnosis and management of four major complications in SCD: acute and chronic pain, cardiopulmonary disease, central nervous system disease and kidney disease. Updates in disease-modifying and curative therapies for SCD are also discussed.
Molecular basis and pathophysiology
Hemoglobin S (HbS) results from the replacement of glutamic acid by valine in the sixth position of the β-globin chain of hemoglobin (Fig. 1 ). Severe forms of SCD include hemoglobin SS due to homozygous inheritance of HbS and S/β 0 thalassemia due to co-inheritance of HbS with the β 0 thalassemia mutation. Other forms include co-inheritance of HbS with other β-globin gene mutations such as hemoglobin C, hemoglobin D-Los Angeles/Punjab or β + thalassemia. Hb S has reduced solubility and increased polymerization, which cause red blood cell sickling, hemolysis and vaso-occlusion (Table 1 ) that subsequently lead to pain episodes and end-organ damage such as cardiopulmonary, cerebrovascular and kidney disease (Table 2 ).
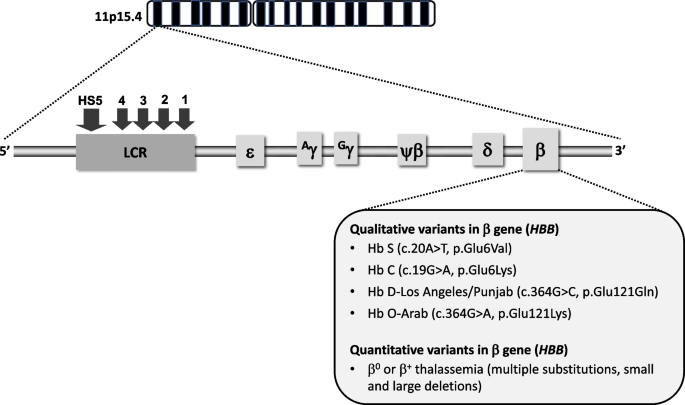
Genetic and molecular basis of sickle cell disease. SCD is caused by mutations in the β globin gene, located on the β globin locus found on the short arm of chromosome 11. The homozygous inheritance of Hb S or co-inheritance of Hb S with the β 0 thalassemia mutation results in the most common forms of severe SCD. Co-inheritance of Hb S with other variants such as Hb C, Hb D-Los Angeles/Punjab, Hb O-Arab or β + thalassemia also leads to clinically significant sickling syndromes (LCR, locus control region; HS, hypersensitivity site)
Acute and chronic pain
Severe intermittent acute pain is the most common SCD complication and accounts for over 70% of acute care visits for individuals with SCD [ 4 ]. Chronic daily pain increases with older age, occurring in 30–40% of adolescents and adults with SCD [ 5 , 6 ]. Acute pain is largely related to vaso-occlusion of sickled red blood cells with ischemia–reperfusion injury and tissue infarction and presents in one isolated anatomic location (e.g., arm, leg, back) or multiple locations. Chronic pain can be caused by sensitization of the central and/or peripheral nervous system and is often diffuse with neuropathic pain features [ 7 , 8 ]. A consensus definition for chronic pain includes “Reports of ongoing pain on most days over the past 6 months either in a single location or multiple locations” [ 9 ]. Disease complications such as avascular necrosis (hip, shoulder) and leg ulcers also cause chronic pain [ 9 ].
Diagnosis of acute and chronic pain
The gold standard for pain assessment and diagnosis is patient self-report. There are no reliable diagnostic tests to confirm the presence of acute or chronic pain in individuals with SCD except when there are identifiable causes like avascular necrosis on imaging or leg ulcers on exam. The effects of pain on individuals’ function are assessed using patient-reported outcome measures (PROs) that determine to what extent pain interferes with individuals’ daily function. Tools shown to be valid, reliable and responsive can be used in clinical practice to track patients’ pain-related function over time to determine additional treatment needs and to compare to population norms [ 10 ]. There are currently no plasma pain biomarkers that improve assessment and management of SCD acute or chronic pain.
Depression and anxiety as co-morbid conditions in SCD can contribute to increased pain, more pain-related distress/interference and poor coping [ 11 ]. The prevalence of depression and anxiety range from 26–33% and 6.5–36%, respectively, in adults with SCD [ 11 , 12 , 13 ]. Adults with SCD have an 11% higher prevalence of depression compared to Black American adults without SCD [ 14 ]. Depression and anxiety can be assessed using self-reported validated screening tools (e.g., Depression: Patient Health Questionnaire (PHQ-9) [ 15 ] for adults, Center for Epidemiologic Studies Depression Scale for Children (CES-DC) [ 16 ], PROMIS assessments for adults and children; Anxiety: Generalized Anxiety Disorder 7-item (GAD-7) scale for adults, State-Trait Anxiety Inventory for Children (STAIC) [ 17 ], PROMIS assessments for adults and children). Individuals who screen positive using these tools should be referred for evaluation by a psychologist/psychiatrist.
Management of acute and chronic pain
The goal of acute pain management is to provide sufficient analgesia to return patients to their usual function, which may mean complete resolution of pain for some or return to baseline chronic pain for others. The goal of chronic pain management is to optimize individuals’ function, which may not mean being pain free. When there is an identifiable cause of chronic pain, treatment of the underlying issue (e.g., joint replacement for avascular necrosis, leg ulcer treatment) is important. Opioids, oral for outpatient management and intravenous for inpatient management, are first line therapy for acute SCD pain. In the acute care setting, analgesics should be initiated within 30–60 min of triage [ 18 ]. Ketamine, a non-opioid analgesic, can be prescribed at sub-anesthetic (analgesic) intravenous doses (0.1–0.3 mg/kg per h, maximum 1 mg/kg per h) as adjuvant treatment for acute SCD pain refractory to opioids [ 18 , 19 ]. In an uncontrolled observational study of 85 patients with SCD receiving ketamine infusions for acute pain, ketamine was associated with a decrease in mean opioid consumption by oral morphine equivalents (3.1 vs. 2.2 mg/kg/day, p < 0.001) and reductions in mean pain scores (0–10 scale) from baseline until discontinuation of the infusion (7.81 vs. 5.44, p < 0.001) [ 20 ]. Nonsteroidal anti-inflammatory drugs (NSAIDs) are routinely used as adjuvant therapy for acute pain treatment [ 18 ]. In a RCT ( n = 20) of hospitalized patients with acute pain, ketorolac was associated with lower total dose of meperidine required (1866.7 ± 12.4 vs. 2804.5 ± 795.1 mg, p < 0.05) and shorter hospitalization (median 3.3 vs. 7.2 days, p = 0.027) [ 21 ]. In a case series of children treated for 70 acute pain events in the ED, 53% of events resolved with ketorolac and hydration alone with reduction in 100 mm visual analog scale (VAS) pain score from 60 to 13 ( p < 0.001) [ 22 ]. Patients at risk for NSAID toxicity (e.g., renal impairment, on anticoagulation) should be identified.
Despite paucity of data, chronic opioid therapy (COT) can be considered after assessing benefits versus harms [ 23 ] and the functional status of patients with SCD who have chronic pain. Harms of COT seen in patient populations other than SCD are dose dependent and include myocardial infarction, bone fracture, increased risk of motor vehicle collisions, sexual dysfunction and mortality [ 23 ]. There are few published studies investigating non-opioid analgesics for chronic SCD pain [ 24 , 25 , 26 ]. In a randomized trial of 39 participants, those who received Vitamin D experienced a range of 6–10 pain days over 24 weeks while those who received placebo experienced 10–16 pain days, which was not significantly different [ 26 ]. In a phase 1, uncontrolled trial of 18 participants taking trifluoperazine, an antipsychotic drug, 8 participants showed a 50% reduction in the VAS (10 cm horizontal line) pain score from baseline on at least 3 assessments over 24 h without severe sedation or supplemental opioid analgesics, 7 participants showed pain reduction on 1 assessment, and the remaining 3 participants showed no reduction [ 24 ]. Although published data are not available for serotonin and norepinephrine reuptake inhibitors (SNRIs), gabapentinoids and tricyclic antidepressants (TCAs) in individuals with SCD, evidence supports their use in fibromyalgia, a chronic pain condition similar to SCD chronic pain in mechanism. A Cochrane Review that included 10 RCTs ( n = 6038) showed that the SNRIs milnacipran and duloxetine, compared to placebo, were associated with a reduction in pain [ 27 ]. A systematic review and meta-analysis of 9 studies ( n = 520) showed the TCA amitriptyline improved pain intensity and function [ 28 ]. Finally, a meta-analysis of 5 RCTs ( n = 1874) of the gabapentinoid pregabalin showed a reduction in pain intensity [ 29 ]. Collectively, the indirect evidence from fibromyalgia supports the conditional recommendation in current SCD practice guidelines to consider these 3 drug classes for chronic SCD pain treatment [ 18 ]. Standard formulary dosing recommendations should be followed and reported adverse effects considered.
Non-pharmacologic therapies (e.g., integrative, psychological-based therapies) are important components of SCD pain treatment. In a case–control study of 101 children with SCD and chronic pain referred for cognitive behavioral therapy (CBT) (57 CBT, 44 no CBT) [ 30 ], CBT was associated with more rapid decrease in pain hospitalizations (estimate − 0.63, p < 0.05) and faster reduction in hospital days over time (estimate − 5.50, p < 0.05). Among 18 children who received CBT and completed PROs pre- and 12 months posttreatment, improvements were seen in mean pain intensity (5.47 vs. 3.76, p = 0.009; 0–10 numeric rating pain scale), functional disability (26.24 vs. 15.18, p < 0.001; 0–60 score range) and pain coping (8.00 vs. 9.65, p = 0.03; 3–15 score range) post treatment [ 30 ]. In 2 uncontrolled clinical trials, acupuncture was associated with a significant reduction in pain scores by 2.1 points (0–10 numeric pain scale) in 24 participants immediately after treatment [ 31 ] or a significant mean difference in pre-post pain scores of 0.9333 (0–10 numeric pain scale) ( p < 0.000) after 33 acupuncture sessions [ 32 ].
Cardiopulmonary disease
Cardiopulmonary disease is associated with increased morbidity and mortality in individuals with SCD. Pulmonary hypertension (PH), most commonly pulmonary arterial hypertension (PAH), is present based on right-heart catheterization in up to 10% of adults with SCD [ 33 ]. Chronic intravascular hemolysis represents the biggest risk factor for development of PAH in SCD and leads to pulmonary arteriole vasoconstriction and smooth muscle proliferation. Based on pulmonary function testing (PFT), obstructive lung disease may be observed in 16% of children and 8% of adults with SCD, while restrictive lung disease may be seen in up to 28% of adults and only 7% of children with SCD [ 34 , 35 ]. Sleep-disordered breathing, which can manifest as obstructive sleep apnea or nocturnal hypoxemia, occurs in up to 42% of children and 46% of adults with SCD [ 36 , 37 ]. Cardiopulmonary disease, including PH or restrictive lung disease, presents with dyspnea with or without exertion, chest pain, hypoxemia or exercise intolerance that is unexplained or increased from baseline. Obstructive lung disease can also present with wheezing.
Diagnosis of cardiopulmonary disease
The confirmation of PH in patients with SCD requires right-heart catheterization. Recently, the mean pulmonary artery pressure threshold used to define PH in the general population was lowered from ≥ 25 to ≥ 20 mm Hg [ 38 ]. Elevated peak tricuspid regurgitant jet velocity (TRJV) ≥ 2.5 m/s on Doppler echocardiogram (ECHO) is associated with early mortality in adults with SCD and may suggest elevated pulmonary artery pressures, especially when other signs of PH (e.g., right-heart strain, septal flattening) or left ventricular diastolic dysfunction, which may contribute to PH, are present [ 39 ]. However, the positive predictive value (PPV) of peak TRJV alone for identifying PH in adults with SCD is only 25% [ 40 ]. Increasing the peak TRJV threshold to at least 2.9 m/s has been shown to increase the PPV to 64%. For a peak TRJV of 2.5–2.8 m/s, an increased N-terminal pro-brain natriuretic peptide (NT-proBNP) ≥ 164.5 pg/mL or a reduced 6-min walk distance (6MWD) < 333 m can also improve the PPV to 62% with a false negative rate of 7% [ 33 , 40 , 41 ].
PFT, which includes spirometry and measurement of lung volumes and diffusion capacity, is standard for diagnosing obstructive and restrictive lung disease in patients with SCD. Emerging modalities include impulse oscillometry, a non-invasive method using forced sound waves to detect changes in lower airway mechanics in individuals unable to perform spirometry [ 42 ], and airway provocation studies using cold air or methacholine to reveal latent airway hyperreactivity [ 43 ]. Formal in-lab, sleep study/polysomnography remains the gold standard to evaluate for sleep-disordered breathing, which may include nocturnal hypoxemia, apnea/hypopnea events and other causes of sleep disruption. Nocturnal hypoxemia may increase red blood cell sickling, cellular adhesion and endothelial dysfunction. In 47 children with SCD, mean overnight oxygen saturation was higher in those with grade 0 compared to grade 2 or 3 cerebral arteriopathy (97 ± 1.6 vs. 93.9 ± 3.7 vs. 93.5 ± 3.0%, p < 0.01) on magnetic resonance angiography and lower overnight oxygen saturation was independently associated with mild, moderate or severe cerebral arteriopathy after adjusting for reticulocytosis (OR 0.50, 95% CI 0.26–0.96, p < 0.05) [ 44 ].
Management of cardiopulmonary disease
Patients with SCD who have symptoms suggestive of cardiopulmonary disease, such as worsening dyspnea, hypoxemia or reduced exercise tolerance, should be evaluated with a diagnostic ECHO and PFT. The presence of snoring, witnessed apnea, respiratory pauses or hypoxemia during sleep, daytime somnolence or nocturnal enuresis in older children and adults is sufficient for a diagnostic sleep study.
Without treatment, the mortality rate in SCD patients with PH is high compared to those without (5-year, all-cause mortality rate of 32 vs. 16%, p < 0.001) [ 33 ]. PAH-targeted therapies should be considered for SCD patients with PAH confirmed by right-heart catheterization. However, the only RCT ( n = 6) in individuals with SCD and PAH confirmed by right-heart catheterization (bosentan versus placebo) was stopped early for poor accrual with no efficacy endpoints analyzed [ 45 ]. In SCD patients with elevated peak TRJV, a randomized controlled trial ( n = 74) of sildenafil, a phosphodiesterase-5 inhibitor, was discontinued early due to increased pain events in the sildenafil versus placebo arm (35 vs. 14%, p = 0.029) with no treatment benefit [ 46 ]. Despite absence of clinical trial data, patients with SCD and confirmed PH should be considered for hydroxyurea or monthly red blood cell transfusions given their disease-modifying benefits. In a retrospective analysis of 13 adults with SCD and PAH, 77% of patients starting at a New York Heart Association (NYHA) functional capacity class III or IV achieved class I/II after a median of 4 exchange transfusions with improvement in median pulmonary vascular resistance (3.7 vs. 2.8 Wood units, p = 0.01) [ 47 ].
Approximately 28% of children with SCD have asthma, which is associated with increased pain episodes that may result from impaired oxygenation leading to sickling and vaso-occlusion as well as with acute chest syndrome and higher mortality [ 48 , 49 , 50 ]. First line therapies include standard beta-adrenergic bronchodilators and supplemental oxygen as needed. When corticosteroids are indicated, courses should be tapered over several days given the risk of rebound SCD pain from abrupt discontinuation. Inhaled corticosteroids such as fluticasone proprionate or beclomethasone diproprionate are reserved for patients with recurrent asthma exacerbations, but their anti-inflammatory effects and impact on preventing pain episodes in patients with SCD who do not have asthma is under investigation [ 51 ]. Finally, management of sleep-disordered breathing is tailored to findings on formal sleep study in consultation with a sleep/pulmonary specialist.
Central nervous system (CNS) complications
CNS complications, such as overt and silent cerebral infarcts, cause significant morbidity in individuals with SCD. Eleven percent of patients with HbSS disease by age 20 years and 24% by age 45 years will have had an overt stroke [ 52 ]. Silent cerebral infarcts occur in 39% by 18 years and in > 50% by 30 years [ 53 , 54 ]. Patients with either type of stroke are at increased risk of recurrent stroke [ 55 ]. Overt stroke involves large-arteries, including middle cerebral arteries and intracranial internal carotid arteries, while silent cerebral infarcts involve penetrating arteries. The pathophysiology of overt stroke includes vasculopathy, increased sickled red blood cell adherence, and hemolysis-induced endothelial activation and altered vasomotor tone [ 56 ]. Overt strokes present as weakness or paresis, dysarthria or aphasia, seizures, sensory deficits, headache or altered level of consciousness, while silent cerebral infarcts are associated with cognitive deficits, including lower IQ and impaired academic performance.
Diagnosis of CNS complications in SCD
Overt stroke is diagnosed by evidence of acute infarct on brain MRI diffusion-weighted imaging and focal deficit on neurologic exam. A silent cerebral infarct is defined by a brain “MRI signal abnormality at least 3 mm in one dimension and visible in 2 planes on fluid-attenuated inversion recovery (FLAIR) T2-weighted images” and no deficit on neurologic exam [ 57 ]. Since silent cerebral infarcts cannot be detected clinically, a screening baseline brain MRI is recommended in school-aged children with SCD [ 58 ]. Recent SCD clinical practice guidelines also suggest a screening brain MRI in adults with SCD to facilitate rehabilitation services, patient and family understanding of cognitive deficits and further needs assessment [ 58 ]. An MRA should be added to screening/diagnostic MRIs to evaluate for cerebral vasculopathy (e.g., moyamoya), which may increase risk for recurrent stroke or hemorrhage [ 59 ].
Annual screening for increased stroke risk by transcranial doppler (TCD) ultrasound is recommended by the American Society of Hematology for children 2–16 years old with HbSS or HbS/β° thalassemia [ 58 ]. Increased stroke risk on non-imaging TCD is indicated by abnormally elevated cerebral blood flow velocity, defined as ≥ 200 cm/s (time-averaged mean of the maximum velocity) on 2 occasions or a single velocity of > 220 cm/s in the distal internal carotid or proximal middle cerebral artery [ 60 ]. Many centers rely on imaging TCD, which results in velocities 10–15% lower than values obtained by non-imaging protocols and therefore, require adjustments to cut-offs for abnormal velocities. Data supporting stroke risk assessment using TCD are lacking for adults with SCD and standard recommendations do not exist.
Neurocognitive deficits occur in over 30% of children and adults with severe SCD [ 61 , 62 ]. These occur as a result of overt and/or silent cerebral infarcts but in some patients, the etiology is unknown. The Bright Futures Guidelines for Health Supervision of Infants, Children and Adolescents or the Cognitive Assessment Toolkit for adults are commonly used tools to screen for developmental delays or neurocognitive impairment [ 58 ]. Abnormal results should prompt referral for formal neuropsychological evaluation, which directs the need for brain imaging to evaluate for silent cerebral infarcts and facilitate educational/vocational accommodations.

Management of CNS complications
Monthly chronic red blood cell transfusions to suppress HbS < 30% are standard of care for primary stroke prevention in children with an abnormal TCD. In an RCT of 130 children, chronic transfusions, compared to no transfusions, were associated with a difference in stroke risk of 92% (1 vs. 10 strokes, p < 0.001) [ 60 ]. However, children with abnormal TCD and no MRI/MRA evidence of cerebral vasculopathy can safely transition to hydroxyurea after 1 year of transfusions [ 63 ]. Lifelong transfusions to maintain HbS < 30% remain standard of care for secondary stroke prevention in individuals with overt stroke [ 64 ]. Chronic monthly red blood cell transfusions should also be considered for children with silent cerebral infarct [ 58 ]. In a randomized controlled trial ( n = 196), monthly transfusions, compared to observation without hydroxyurea, reduced risk of overt stroke, new silent cerebral infarct or enlarging silent cerebral infarct in children with HbSS or HbS/β 0 thalassemia and an existing silent cerebral infarct (2 vs. 4.8 events, incidence rate ratio of 0.41, 95% CI 0.12–0.99, p = 0.04) [ 57 ].
Acute stroke treatment requires transfusion therapy to increase cerebral oxygen delivery. Red blood cell exchange transfusion, defined as replacement of patients’ red blood cells with donor red blood cells, to rapidly reduce HbS to < 30% is the recommended treatment as simple transfusion alone is shown to have a fivefold greater relative risk (57 vs. 21% with recurrent stroke, RR = 5.0; 95% CI 1.3–18.6) of subsequent stroke compared to exchange transfusion [ 65 ]. However, a simple transfusion is often given urgently while preparing for exchange transfusion [ 58 ]. Tissue plasminogen activator (tPA) is not recommended for children with SCD who have an acute stroke since the pathophysiology of SCD stroke is less likely to be thromboembolic in origin and there is risk for harm. Since the benefits and risks of tPA in adults with SCD and overt stroke are not clear, its use depends on co-morbidities, risk factors and stroke protocols but should not delay or replace prompt transfusion therapy.
Data guiding treatment of SCD cerebral vasculopathy (e.g., moyamoya) are limited, and only nonrandomized, low-quality evidence exists for neurosurgical interventions (e.g., encephaloduroarteriosynangiosis) [ 66 ]. Consultation with a neurosurgeon to discuss surgical options in patients with moyamoya and history of stroke or transient ischemic attack should be considered [ 58 ].
Kidney disease
Glomerulopathy, characterized by hyperfiltration leading to albuminuria, is an early asymptomatic manifestation of SCD nephropathy and worsens with age. Hyperfiltration, defined by an absolute increase in glomerular filtration rate, may be seen in 43% of children with SCD [ 67 ]. Albuminuria, defined by the presence of urine albumin ≥ 30 mg/g over 24 h, has been observed in 32% of adults with SCD [ 68 ]. Glomerulopathy results from intravascular hemolysis and endothelial dysfunction in the renal cortex. Medullary hypoperfusion and ischemia also contribute to kidney disease in SCD, causing hematuria, urine concentrating defects and distal tubular dysfunction [ 69 ]. Approximately 20–40% of adults with SCD develop chronic kidney disease (CKD) and are at risk of developing end-stage renal disease (ESRD), with rapid declines in estimated glomerular filtration rate (eGFR) > 3 mL/min/1.73 m 2 associated with increased mortality (HR 2.4, 95% CI 1.31–4.42, p = 0.005) [ 68 ].
Diagnosis of kidney disease in SCD
The diagnosis of sickle cell nephropathy is made by detecting abnormalities such as albuminuria, hematuria or CKD rather than by distinct diagnostic criteria in SCD, which have not been developed. Traditional markers of kidney function such as serum creatinine and eGFR should be interpreted with caution in individuals with SCD because renal hyperfiltration affects their accuracy by increasing both. Practical considerations preclude directly measuring GFR by urine or plasma clearance techniques, which achieves the most accurate results. The accuracy of eGFR, however, may be improved by equations that incorporate serum cystatin C [ 70 ].
Since microalbuminuria/proteinuria precedes CKD in SCD, annual screening for urine microalbumin/protein is recommended beginning at age 10 years [ 71 ]. When evaluating urine for microalbumin concentration, samples from first morning rather than random voids are preferable to exclude orthostatic proteinuria. Recent studies suggest HMOX1 and APOL1 gene variants may be associated with CKD in individuals with SCD [ 72 ]. Potential novel predictors of acute kidney injury in individuals with SCD include urine biomarkers kidney injury molecule 1 (KIM-1) [ 73 ], monocyte chemotactic protein 1 (MCP-1) [ 74 ] and neutrophil gelatinase-associated lipocalin (NGAL) [ 75 ]. Their contribution to chronic kidney disease and interaction with other causes of kidney injury in SCD (e.g., inflammation, hemolysis) are not clear.
Management of kidney disease
Managing kidney complications in SCD should focus on mitigating risk factors for acute and chronic kidney injury such as medication toxicity, reduced kidney perfusion from hypotension and dehydration, and general disease progression, as well as early screening and treatment of microalbuminuria/proteinuria. Acute kidney injury, either an increase in serum creatinine ≥ 0.3 mg/dL or a 50% increase in serum creatinine from baseline, is associated with ketorolac use in children with SCD hospitalized for pain [ 76 ]. Increasing intravenous fluids to maintain urine output > 0.5 to 1 mL/kg/h and limiting NSAIDs and antibiotics associated with nephrotoxicity in this setting are important. Despite absence of controlled clinical trials, hydroxyurea may be associated with improvements in glomerular hyperfiltration and urine concentrating ability in children with SCD [ 77 , 78 ]. Hydroxyurea is also associated with a lower prevalence (34.7 vs. 55.4%, p = 0.01) and likelihood of albuminuria (OR 0.28, 95% CI 0.11–0.75, p = 0.01) in adults with SCD after adjusting for age, angiotensin-converting enzyme inhibitor (ACE-I)/angiotensin receptor blockade (ARB) use and major disease risk factors [ 79 ].
ACE-I or ARB therapy reduces microalbuminuria in patients with SCD. In a phase 2 trial of 36 children and adults, a ≥ 25% reduction in urine albumin-to-creatinine ratio was observed in 83% ( p < 0.0001) and 58% ( p < 0.0001) of patients with macroalbuminuria (> 300 mg/g creatinine) and microalbuminuria (30–300 mg/g creatinine), respectively, after 6 months of treatment with losartan at a dose of 0.7 mg/kg/day (max of 50 mg) in children and 50 mg daily in adults [ 80 ]. However, ACE-I or ARB therapy has not been shown to improve kidney function or prevent CKD. Hemodialysis is associated with a 1-year mortality rate of 26.3% after starting hemodialysis and an increase risk of death in SCD patients with ESRD compared to non-SCD patients with ESRD (44.6 vs. 34.5% deaths, mortality hazard ratio of 2.8, 95% CI 2.31–3.38) [ 81 ]. Renal transplant should be considered for individuals with SCD and ESRD because of recent improvements in renal graft survival and post-transplant mortality [ 82 ].
Disease-modifying therapies in SCD
Since publication of its landmark trial in 1995, hydroxyurea continues to represent a mainstay of disease-modifying therapy for SCD. Hydroxyurea induces fetal hemoglobin production through stress erythropoiesis, reduces inflammation, increases nitric oxide and decreases cell adhesion. The FDA approved hydroxyurea in 1998 for adults with SCD. Subsequently, hydroxyurea was FDA approved for children in 2017 to reduce the frequency pain events and need for blood transfusions in children ≥ 2 years of age [ 63 ]. The landscape of disease-modifying therapies, however, has improved with the recent FDA approval of 3 other treatments— l -glutamine and crizanlizumab for reducing acute complications (e.g., pain), and voxelotor for improving anemia (Table 3 ) [ 83 , 84 , 85 ]. Other therapies in current development focus on inducing fetal hemoglobin, reducing anti-sickling or cellular adhesion, or activating pyruvate kinase-R.
l -glutamine
Glutamine is required for the synthesis of glutathione, nicotinamide adenine dinucleotide and arginine. The essential amino acid protects red blood cells against oxidative damage, which forms the basis for its proposed utility in SCD. The exact mechanism of benefit in SCD, however, remains unclear. In a phase 3 RCT of 230 participants (hemoglobin SS or S/β 0 thalassemia), l -glutamine compared to placebo was associated with fewer pain events (median 3 vs. 4, p = 0.005) and hospitalizations for pain (median 2 vs. 3, p = 0.005) over the 48-week treatment period [ 84 ]. The percentage of patients who had at least 1 episode of acute chest syndrome, defined as presence of chest wall pain with fever and a new pulmonary infiltrate, was lower in the l -glutamine group (8.6 vs. 23.1%, p = 0.003). There were no significant between-group differences in hemoglobin, hematocrit or reticulocyte count. Common side effects of l -glutamine include GI upset (constipation, nausea, vomiting and abdominal pain) and headaches.
Crizanlizumab
P-selectin expression, triggered by inflammation, promotes adhesion of neutrophils, activated platelets and sickle red blood cells to the endothelial surface and to each other, which promotes vaso-occlusion in SCD. Crizanlizumab, given as a monthly intravenous infusion, is a humanized monoclonal antibody that binds P-selectin and blocks the adhesion molecule’s interaction with its ligand, P-selectin glycoprotein ligand 1. FDA approval for crizanlizumab was based on a phase 2 RCT ( n = 198, all genotypes), in which the median rate of pain events (primary endpoint) was lower (1.63 vs. 2.68, p = 0.01) and time to first pain event (secondary endpoint) was longer (4.07 vs. 1.38 months, p = 0.001) for patients on high-dose crizanlizumab (5 mg/kg/dose) compared to placebo treated for 52 weeks (14 doses total) [ 83 ]. In this trial, patients with SCD on chronic transfusion therapy were excluded, but those on stable hydroxyurea dosing were not. Adverse events were uncommon but included headache, back pain, nausea, arthralgia and pain in the extremity.
Polymerization of Hb S in the deoxygenated state represents the initial step in red blood cell sickling, which leads to reduced red blood cell deformability and increased hemolysis. Voxelotor is a first-in-class allosteric modifier of Hb S that increases oxygen affinity. The primary endpoint for the phase 3 RCT of voxelotor ( n = 274, all genotypes) that led to FDA approval was an increase in hemoglobin of at least 1 g/dL after 24 weeks of treatment [ 85 ]. More participants receiving 1500 mg daily of oral voxelotor versus placebo had a hemoglobin response of at least 1 g/dL (51%, 95% CI 41–61 vs. 7%, 95% CI 1–12, p < 0.001). Approximately 2/3 of the participants in these trials were on hydroxyurea, with treatment benefits observed regardless of hydroxyurea status. Despite improvements associated with voxelotor in biomarkers of hemolysis (reticulocyte count, indirect bilirubin and lactate dehydrogenase), annualized incidence rate of vaso-occlusive crisis was not significantly different among treatment groups. Adverse events included headaches, GI symptoms, arthralgia, fatigue and rash.
Curative therapies in SCD
For individuals with SCD undergoing hematopoietic stem cell transplantation (HSCT) using HLA-matched sibling donors and either myeloablative or reduced-intensity conditioning regimens, the five-year event-free and overall survival is high at 91% and 93%, respectively [ 86 ]. Limited availability of HLA-matched sibling donors in this population requires alternative donors or the promise of autologous strategies such as gene-based therapies (i.e. gene addition, transfer or editing) (Table 4 ). Matched unrelated donors have not been used routinely due to increased risk of graft-versus-host disease (GVHD) as high as 19% (95% CI 12–28) in the first 100 days for acute GVHD and 29% (95% CI 21–38) over 3 years for chronic GVHD [ 87 ]. Haplo-identical HSCT, using biological parents or siblings as donors, that incorporate post-transplant cyclophosphamide demonstrates acceptable engraftment rates, transplant-related morbidity and overall mortality [ 88 ]. Regardless of allogeneic HSCT type, older age is associated with lower event-free (102/418 vs. 72/491 events, HR 1.74, 95% CI 1.24–2.45) and overall survival (54/418 vs. 22/491 events, HR 3.15, 95% CI 1.86–5.34) in patients ≥ 13 years old compared to < 12 years old undergoing HSCT [ 87 ].
Advancing research in SCD
Despite progress to date, additional high-quality, longitudinal data are needed to better understand the natural history of the disease and to inform optimal screening for SCD-related complications. In the era of multiple FDA-approved therapies with disease-modifying potential, clinical trials to evaluate additional indications and test them in combination with or compared to each other are needed. Dissemination and implementation studies are also needed to identify barriers and facilitators related to treatment in everyday life, which can be incorporated into decision aids and treatment algorithms for patients and their providers [ 89 ]. Lastly, continued efforts should acknowledge social determinants of health and other factors that affect access and disease-related outcomes such as the role of third-party payers, provider and patient education, health literacy and patient trust. Establishing evidence-derived quality of care metrics can also drive public policy changes required to ensure care optimization for this population.
Conclusions
SCD is associated with complications that include acute and chronic pain as well as end-organ damage such as cardiopulmonary, cerebrovascular and kidney disease that result in increased morbidity and mortality. Several well-designed clinical trials have resulted in key advances in management of SCD in the past decade. Data from these trials have led to FDA approval of 3 new drugs, l -glutamine, crizanlizumab and voxelotor, which prevent acute pain and improve chronic anemia. Moderate to high-quality data support recommendations for managing SCD cerebrovascular disease and early kidney disease. However, further research is needed to determine the best treatment for chronic pain and cardiopulmonary disease in SCD. Comparative effectiveness research, dissemination and implementation studies and a continued focus on social determinants of health are also essential.
Availability of data and materials
Not applicable.
Abbreviations
Six-minute walk distance
Angiotensin-converting enzyme inhibitor
Angiotensin receptor blockade
Cognitive behavioral therapy
Chronic kidney disease
Chronic opioid therapy
Echocardiogram
End stage renal disease
Fluid-attenuated inversion recovery
Glomerular filtration rate
Graft-versus-host disease
Hemoglobin S
Hematopoietic stem cell transplant
Nonsteroidal anti-inflammatory drugs
N-terminal pro-brain natriuretic peptide
New York Heart Association
Pulmonary arterial hypertension
Pulmonary function test
Pulmonary hypertension
Positive predictive value
Patient-reported outcomes
Randomized controlled trial
- Sickle cell disease
Serotonin and norepinephrine reuptake inhibitors
Tricyclic antidepressants
Transcranial Doppler
Tissue plasminogen activator
Tricuspid regurgitant jet velocity
Visual Analog Scale
Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Dewi M, et al. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;381(9861):142–51.
Article PubMed PubMed Central Google Scholar
Brousseau DC, Panepinto JA, Nimmer M, Hoffmann RG. The number of people with sickle-cell disease in the United States: national and state estimates. Am J Hematol. 2010;85(1):77–8.
PubMed Google Scholar
Payne AB, Mehal JM, Chapman C, Haberling DL, Richardson LC, Bean CJ, et al. Trends in sickle cell disease-related mortality in the United States, 1979 to 2017. Ann Emerg Med. 2020;76(3S):S28–36.
Article PubMed Google Scholar
Brousseau DC, Owens PL, Mosso AL, Panepinto JA, Steiner CA. Acute care utilization and rehospitalizations for sickle cell disease. JAMA. 2010;303(13):1288–94.
Article CAS PubMed Google Scholar
Sil S, Cohen LL, Dampier C. Psychosocial and functional outcomes in youth with chronic sickle cell pain. Clin J Pain. 2016;32(6):527–33.
Smith WR, Penberthy LT, Bovbjerg VE, McClish DK, Roberts JD, Dahman B, et al. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148(2):94–101.
Ballas SK, Darbari DS. Neuropathy, neuropathic pain, and sickle cell disease. Am J Hematol. 2013;88(11):927–9.
Sharma D, Brandow AM. Neuropathic pain in individuals with sickle cell disease. Neurosci Lett. 2020;714:134445.
Dampier C, Palermo TM, Darbari DS, Hassell K, Smith W, Zempsky W. AAPT diagnostic criteria for chronic sickle cell disease pain. J Pain. 2017;18(5):490–8.
Darbari DS, Hampson JP, Ichesco E, Kadom N, Vezina G, Evangelou I, et al. Frequency of hospitalizations for pain and association with altered brain network connectivity in sickle cell disease. J Pain. 2015;16(11):1077–86.
Levenson JL, Mcclish DK, Dahman BA, Bovbjerg VE, Penberthy LT, et al. Depression and anxiety in adults with sickle cell disease: the PiSCES project. Psychosom Med. 2008;70(2):192–6.
Treadwell MJBB, Kaur K, Gildengorin G. Emotional distress, barriers to care, and health-related quality of life in sickle cell disease. J Clin Outcomes Manag. 2015;22:10.
Google Scholar
Jonassaint CR, Jones VL, Leong S, Frierson GM. A systematic review of the association between depression and health care utilization in children and adults with sickle cell disease. Br J Haematol. 2016;174(1):136–47.
Laurence B, George D, Woods D. Association between elevated depressive symptoms and clinical disease severity in African-American adults with sickle cell disease. J Natl Med Assoc. 2006;98(3):365–9.
PubMed PubMed Central Google Scholar
Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13.
Article CAS PubMed PubMed Central Google Scholar
Faulstich ME, Carey MP, Ruggiero L, Enyart P, Gresham F. Assessment of depression in childhood and adolescence: an evaluation of the Center for Epidemiological Studies Depression Scale for Children (CES-DC). Am J Psychiatry. 1986;143(8):1024–7.
Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the state-trait anxiety inventory. Palo Alto: Consulting Psychologists Press; 1983.
Brandow AM, Carroll CP, Creary S, Edwards-Elliott R, Glassberg J, Hurley RW, et al. American Society of Hematology 2020 guidelines for sickle cell disease: management of acute and chronic pain. Blood Adv. 2020;4(12):2656–701.
Lubega FA, DeSilva MS, Munube D, Nkwine R, Tumukunde J, Agaba PK, et al. Low dose ketamine versus morphine for acute severe vaso occlusive pain in children: a randomized controlled trial. Scand J Pain. 2018;18(1):19–27.
Nobrega R, Sheehy KA, Lippold C, Rice AL, Finkel JC, Quezado ZMN. Patient characteristics affect the response to ketamine and opioids during the treatment of vaso-occlusive episode-related pain in sickle cell disease. Pediatr Res. 2018;83(2):445–54.
Perlin E, Finke H, Castro O, Rana S, Pittman J, Burt R, et al. Enhancement of pain control with ketorolac tromethamine in patients with sickle cell vaso-occlusive crisis. Am J Hematol. 1994;46(1):43–7.
Beiter JL Jr, Simon HK, Chambliss CR, Adamkiewicz T, Sullivan K. Intravenous ketorolac in the emergency department management of sickle cell pain and predictors of its effectiveness. Arch Pediatr Adolesc Med. 2001;155(4):496–500.
Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162(4):276–86.
Molokie RE, Wilkie DJ, Wittert H, Suarez ML, Yao Y, Zhao Z, et al. Mechanism-driven phase I translational study of trifluoperazine in adults with sickle cell disease. Eur J Pharmacol. 2014;723:419–24.
Schlaeger JM, Molokie RE, Yao Y, Suarez ML, Golembiewski J, Wilkie DJ, et al. Management of sickle cell pain using pregabalin: a pilot study. Pain Manag Nurs. 2017;18(6):391–400.
Osunkwo I, Ziegler TR, Alvarez J, McCracken C, Cherry K, Osunkwo CE, et al. High dose vitamin D therapy for chronic pain in children and adolescents with sickle cell disease: results of a randomized double blind pilot study. Br J Haematol. 2012;159(2):211–5.
Hauser W, Urrutia G, Tort S, Uceyler N, Walitt B. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia syndrome. Cochrane Database Syst Rev. 2013;1:CD010292.
Hauser W, Wolfe F, Tolle T, Uceyler N, Sommer C. The role of antidepressants in the management of fibromyalgia syndrome: a systematic review and meta-analysis. CNS Drugs. 2012;26(4):297–307.
Derry S, Cording M, Wiffen PJ, Law S, Phillips T, Moore RA. Pregabalin for pain in fibromyalgia in adults. Cochrane Database Syst Rev. 2016;9:CD011790.
Sil S, Lai K, Lee JL, Gilleland Marchak J, Thompson B, Cohen L, et al. Preliminary evaluation of the clinical implementation of cognitive-behavioral therapy for chronic pain management in pediatric sickle cell disease. Complement Ther Med. 2020;49:102348.
Lu K, Cheng MC, Ge X, Berger A, Xu D, Kato GJ, et al. A retrospective review of acupuncture use for the treatment of pain in sickle cell disease patients: descriptive analysis from a single institution. Clin J Pain. 2014;30(9):825–30.
Mahmood LA, Reece-Stremtan S, Idiokitas R, Martin B, Margulies S, Hardy SJ, et al. Acupuncture for pain management in children with sickle cell disease. Complement Ther Med. 2020;49:102287.
Mehari A, Alam S, Tian X, Cuttica MJ, Barnett CF, Miles G, et al. Hemodynamic predictors of mortality in adults with sickle cell disease. Am J Respir Crit Care Med. 2013;187(8):840–7.
Cohen RT, Strunk RC, Rodeghier M, Rosen CL, Kirkham FJ, Kirkby J, et al. Pattern of lung function is not associated with prior or future morbidity in children with sickle cell anemia. Ann Am Thorac Soc. 2016;13(8):1314–23.
Kassim AA, Payne AB, Rodeghier M, Macklin EA, Strunk RC, DeBaun MR. Low forced expiratory volume is associated with earlier death in sickle cell anemia. Blood. 2015;126(13):1544–50.
Sharma S, Efird JT, Knupp C, Kadali R, Liles D, Shiue K, et al. Sleep disorders in adult sickle cell patients. J Clin Sleep Med. 2015;11(3):219–23.
Rosen CL, Debaun MR, Strunk RC, Redline S, Seicean S, Craven DI, et al. Obstructive sleep apnea and sickle cell anemia. Pediatrics. 2014;134(2):273–81.
Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1).
Chaturvedi S, Labib Ghafuri D, Kassim A, Rodeghier M, DeBaun MR. Elevated tricuspid regurgitant jet velocity, reduced forced expiratory volume in 1 second, and mortality in adults with sickle cell disease. Am J Hematol. 2017;92(2):125–30.
Parent F, Bachir D, Inamo J, Lionnet F, Driss F, Loko G, et al. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med. 2011;365(1):44–53.
Machado RF, Anthi A, Steinberg MH, Bonds D, Sachdev V, Kato GJ, et al. N-terminal pro-brain natriuretic peptide levels and risk of death in sickle cell disease. JAMA. 2006;296(3):310–8.
Mondal P, Yirinec A, Midya V, Sankoorikal BJ, Smink G, Khokhar A, et al. Diagnostic value of spirometry vs impulse oscillometry: a comparative study in children with sickle cell disease. Pediatr Pulmonol. 2019;54(9):1422–30.
Field JJ, Stocks J, Kirkham FJ, Rosen CL, Dietzen DJ, Semon T, et al. Airway hyperresponsiveness in children with sickle cell anemia. Chest. 2011;139(3):563–8.
Dlamini N, Saunders DE, Bynevelt M, Trompeter S, Cox TC, Bucks RS, et al. Nocturnal oxyhemoglobin desaturation and arteriopathy in a pediatric sickle cell disease cohort. Neurology. 2017;89(24):2406–12.
Barst RJ, Mubarak KK, Machado RF, Ataga KI, Benza RL, Castro O, et al. Exercise capacity and haemodynamics in patients with sickle cell disease with pulmonary hypertension treated with bosentan: results of the ASSET studies. Br J Haematol. 2010;149(3):426–35.
Machado RF, Barst RJ, Yovetich NA, Hassell KL, Kato GJ, Gordeuk VR, et al. Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV and low exercise capacity. Blood. 2011;118(4):855–64.
Turpin M, Chantalat-Auger C, Parent F, Driss F, Lionnet F, Habibi A, et al. Chronic blood exchange transfusions in the management of pre-capillary pulmonary hypertension complicating sickle cell disease. European Respiratory Journal. 2018;52(4).
Knight-Madden JM, Barton-Gooden A, Weaver SR, Reid M, Greenough A. Mortality, asthma, smoking and acute chest syndrome in young adults with sickle cell disease. Lung. 2013;191(1):95–100.
Strunk RC, Cohen RT, Cooper BP, Rodeghier M, Kirkham FJ, Warner JO, et al. Wheezing symptoms and parental asthma are associated with a physician diagnosis of asthma in children with sickle cell anemia. J Pediatr. 2014;164(4):821-6e1.
Article Google Scholar
Takahashi T, Okubo Y, Handa A. Acute chest syndrome among children hospitalized with vaso-occlusive crisis: a nationwide study in the United States. Pediatr Blood Cancer. 2018;65(3):e26885.
Glassberg J, Minnitti C, Cromwell C, Cytryn L, Kraus T, Skloot GS, et al. Inhaled steroids reduce pain and sVCAM levels in individuals with sickle cell disease: a triple-blind, randomized trial. Am J Hematol. 2017;92(7):622–31.
Ohene-Frempong K, Weiner SJ, Sleeper LA, Miller ST, Embury S, Moohr JW, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91(1):288–94.
CAS PubMed Google Scholar
Bernaudin F, Verlhac S, Arnaud C, Kamdem A, Vasile M, Kasbi F, et al. Chronic and acute anemia and extracranial internal carotid stenosis are risk factors for silent cerebral infarcts in sickle cell anemia. Blood. 2015;125(10):1653–61.
Kassim AA, Pruthi S, Day M, Rodeghier M, Gindville MC, Brodsky MA, et al. Silent cerebral infarcts and cerebral aneurysms are prevalent in adults with sickle cell anemia. Blood. 2016;127(16):2038–40.
Powars D, Wilson B, Imbus C, Pegelow C, Allen J. The natural history of stroke in sickle cell disease. Am J Med. 1978;65(3):461–71.
Switzer JA, Hess DC, Nichols FT, Adams RJ. Pathophysiology and treatment of stroke in sickle-cell disease: present and future. Lancet Neurol. 2006;5(6):501–12.
DeBaun MR, Gordon M, McKinstry RC, Noetzel MJ, White DA, Sarnaik SA, et al. Controlled trial of transfusions for silent cerebral infarcts in sickle cell anemia. N Engl J Med. 2014;371(8):699–710.
DeBaun MR, Jordan LC, King AA, Schatz J, Vichinsky E, Fox CK, et al. American Society of Hematology 2020 guidelines for sickle cell disease: prevention, diagnosis, and treatment of cerebrovascular disease in children and adults. Blood Adv. 2020;4(8):1554–88.
Dobson SR, Holden KR, Nietert PJ, Cure JK, Laver JH, Disco D, et al. Moyamoya syndrome in childhood sickle cell disease: a predictive factor for recurrent cerebrovascular events. Blood. 2002;99(9):3144–50.
Adams RJ, McKie VC, Hsu L, Files B, Vichinsky E, Pegelow C, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339(1):5–11.
Vichinsky EP, Neumayr LD, Gold JI, Weiner MW, Rule RR, Truran D, et al. Neuropsychological dysfunction and neuroimaging abnormalities in neurologically intact adults with sickle cell anemia. JAMA. 2010;303(18):1823–31.
Hijmans CT, Fijnvandraat K, Grootenhuis MA, van Geloven N, Heijboer H, Peters M, et al. Neurocognitive deficits in children with sickle cell disease: a comprehensive profile. Pediatr Blood Cancer. 2011;56(5):783–8.
Ware RE, Davis BR, Schultz WH, Brown RC, Aygun B, Sarnaik S, et al. Hydroxycarbamide versus chronic transfusion for maintenance of transcranial doppler flow velocities in children with sickle cell anaemia-TCD With Transfusions Changing to Hydroxyurea (TWiTCH): a multicentre, open-label, phase 3, non-inferiority trial. Lancet. 2016;387(10019):661–70.
Scothorn DJ, Price C, Schwartz D, Terrill C, Buchanan GR, Shurney W, et al. Risk of recurrent stroke in children with sickle cell disease receiving blood transfusion therapy for at least five years after initial stroke. J Pediatr. 2002;140(3):348–54.
Hulbert ML, Scothorn DJ, Panepinto JA, Scott JP, Buchanan GR, Sarnaik S, et al. Exchange blood transfusion compared with simple transfusion for first overt stroke is associated with a lower risk of subsequent stroke: a retrospective cohort study of 137 children with sickle cell anemia. J Pediatr. 2006;149(5):710–2.
Hall EM, Leonard J, Smith JL, Guilliams KP, Binkley M, Fallon RJ, et al. Reduction in overt and silent stroke recurrence rate following cerebral revascularization surgery in children with sickle cell disease and severe cerebral vasculopathy. Pediatr Blood Cancer. 2016;63(8):1431–7.
Lebensburger JD, Aban I, Pernell B, Kasztan M, Feig DI, Hilliard LM, et al. Hyperfiltration during early childhood precedes albuminuria in pediatric sickle cell nephropathy. Am J Hematol. 2019;94(4):417–23.
Niss O, Lane A, Asnani MR, Yee ME, Raj A, Creary S, et al. Progression of albuminuria in patients with sickle cell anemia: a multicenter, longitudinal study. Blood Adv. 2020;4(7):1501–11.
Cazenave M, Audard V, Bertocchio JP, Habibi A, Baron S, Prot-Bertoye C, et al. Tubular acidification defect in adults with sickle cell disease. Clin J Am Soc Nephrol. 2020;15(1):16–24.
Yee MEM, Lane PA, Archer DR, Joiner CH, Eckman JR, Guasch A. Estimation of glomerular filtration rate using serum cystatin C and creatinine in adults with sickle cell anemia. Am J Hematol. 2017;92(10):E598–9.
Yawn BP, Buchanan GR, Afenyi-Annan AN, Ballas SK, Hassell KL, James AH, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312(10):1033–48.
Saraf SL, Zhang X, Shah B, Kanias T, Gudehithlu KP, Kittles R, et al. Genetic variants and cell-free hemoglobin processing in sickle cell nephropathy. Haematologica. 2015;100(10):1275–84.
Hamideh D, Raj V, Harrington T, Li H, Margolles E, Amole F, et al. Albuminuria correlates with hemolysis and NAG and KIM-1 in patients with sickle cell anemia. Pediatr Nephrol. 2014;29(10):1997–2003.
dos Santos TE, Goncalves RP, Barbosa MC, da Silva GB, Jr., Daher Ede F. Monocyte chemoatractant protein-1: a potential biomarker of renal lesion and its relation with oxidative status in sickle cell disease. Blood Cells Mol Dis. 2015;54(3):297–301.
Audard V, Moutereau S, Vandemelebrouck G, Habibi A, Khellaf M, Grimbert P, et al. First evidence of subclinical renal tubular injury during sickle-cell crisis. Orphanet J Rare Dis. 2014;9:67.
Baddam S, Aban I, Hilliard L, Howard T, Askenazi D, Lebensburger JD. Acute kidney injury during a pediatric sickle cell vaso-occlusive pain crisis. Pediatr Nephrol. 2017;32(8):1451–6.
Zahr RS, Hankins JS, Kang G, Li C, Wang WC, Lebensburger J, et al. Hydroxyurea prevents onset and progression of albuminuria in children with sickle cell anemia. Am J Hematol. 2019;94(1):E27–9.
Alvarez O, Miller ST, Wang WC, Luo Z, McCarville MB, Schwartz GJ, et al. Effect of hydroxyurea treatment on renal function parameters: results from the multi-center placebo-controlled BABY HUG clinical trial for infants with sickle cell anemia. Pediatr Blood Cancer. 2012;59(4):668–74.
Laurin LP, Nachman PH, Desai PC, Ataga KI, Derebail VK. Hydroxyurea is associated with lower prevalence of albuminuria in adults with sickle cell disease. Nephrol Dial Transplant. 2014;29(6):1211–8.
Quinn CT, Saraf SL, Gordeuk VR, Fitzhugh CD, Creary SE, Bodas P, et al. Losartan for the nephropathy of sickle cell anemia: a phase-2, multicenter trial. Am J Hematol. 2017;92(9):E520–8.
McClellan AC, Luthi JC, Lynch JR, Soucie JM, Kulkarni R, Guasch A, et al. High one year mortality in adults with sickle cell disease and end-stage renal disease. Br J Haematol. 2012;159(3):360–7.
Gérardin C, Moktefi A, Couchoud C, Duquesne A, Ouali N, Gataut P, et al. Survival and specific outcome of sickle cell disease patients after renal transplantation. Br J Haematol. 2019;187(5):676–80.
Ataga KI, Kutlar A, Kanter J, Liles D, Cancado R, Friedrisch J, et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med. 2017;376(5):429–39.
Niihara Y, Miller ST, Kanter J, Lanzkron S, Smith WR, Hsu LL, et al. A phase 3 trial of l -glutamine in sickle cell disease. N Engl J Med. 2018;379(3):226–35.
Vichinsky E, Hoppe CC, Ataga KI, Ware RE, Nduba V, El-Beshlawy A, et al. A phase 3 randomized trial of voxelotor in sickle cell disease. N Engl J Med. 2019;381(6):509–19.
Gluckman E, Cappelli B, Bernaudin F, Labopin M, Volt F, Carreras J, et al. Sickle cell disease: an international survey of results of HLA-identical sibling hematopoietic stem cell transplantation. Blood. 2017;129(11):1548–56.
Eapen M, Brazauskas R, Walters MC, Bernaudin F, Bo-Subait K, Fitzhugh CD, et al. Effect of donor type and conditioning regimen intensity on allogeneic transplantation outcomes in patients with sickle cell disease: a retrospective multicentre, cohort study. Lancet Haematol. 2019;6(11):e585–96.
Bolanos-Meade J, Cooke KR, Gamper CJ, Ali SA, Ambinder RF, Borrello IM, et al. Effect of increased dose of total body irradiation on graft failure associated with HLA-haploidentical transplantation in patients with severe haemoglobinopathies: a prospective clinical trial. Lancet Haematol. 2019;6(4):e183–93.
Krishnamurti L, Ross D, Sinha C, Leong T, Bakshi N, Mittal N, et al. Comparative effectiveness of a web-based patient decision aid for therapeutic options for sickle cell disease: randomized controlled trial. J Med Internet Res. 2019;21(12):e14462.
Download references
Acknowledgements
We would like to acknowledge Lana Mucalo, MD, for supporting data collection for this manuscript.
Author information
Authors and affiliations.
Department of Pediatrics, Section of Pediatric Hematology/Oncology/Bone Marrow Transplantation, Medical College of Wisconsin, Milwaukee, WI, USA
A. M. Brandow
Division of Hematology, Oncology and Stem Cell Transplantation, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL, USA
You can also search for this author in PubMed Google Scholar
Contributions
AB and RL contributed equally to the writing and editing of this manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to R. I. Liem .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors have no competing interests to declare.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Brandow, A.M., Liem, R.I. Advances in the diagnosis and treatment of sickle cell disease. J Hematol Oncol 15 , 20 (2022). https://doi.org/10.1186/s13045-022-01237-z
Download citation
Received : 30 September 2021
Accepted : 15 February 2022
Published : 03 March 2022
DOI : https://doi.org/10.1186/s13045-022-01237-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Sickle cell anemia
Journal of Hematology & Oncology
ISSN: 1756-8722
- General enquiries: [email protected]
Featured Clinical Reviews
- Screening for Atrial Fibrillation: US Preventive Services Task Force Recommendation Statement JAMA Recommendation Statement January 25, 2022
- Evaluating the Patient With a Pulmonary Nodule: A Review JAMA Review January 18, 2022
- Download PDF
- CME & MOC
- Share X Facebook Email LinkedIn
- Permissions
Sickle Cell Disease : A Review
- 1 Division of General Pediatrics, Boston University School of Medicine, Boston Medical Center, Boston, Massachusetts
- 2 Division of Hematology-Oncology, Department of Pediatrics, Baylor College of Medicine and Texas Children’s Hospital, Houston
- 3 School of Medicine, Division of Hematology and Oncology, University of California Davis, Sacramento
- Comment & Response A Review of Sickle Cell Disease—Reply Patricia L. Kavanagh, MD; Titilope Fasipe, MD, PhD; Ted Wun, MD JAMA
- Comment & Response A Review of Sickle Cell Disease Nikolaos Vlachadis, MD, DMD, MPH, MSc, DSc; Nikolaos Vrachnis, MD, PhD JAMA
- JAMA Clinical Guidelines Synopsis Diagnosis and Management of Priapism Richard J. Fantus, MD; Robert E. Brannigan, MD; Andrew M. Davis, MD, MPH JAMA
Importance Sickle cell disease (SCD) is an inherited disorder of hemoglobin, characterized by formation of long chains of hemoglobin when deoxygenated within capillary beds, resulting in sickle-shaped red blood cells, progressive multiorgan damage, and increased mortality. An estimated 300 000 infants are born annually worldwide with SCD. Most individuals with SCD live in sub-Saharan Africa, India, the Mediterranean, and Middle East; approximately 100 000 individuals with SCD live in the US.
Observations SCD is diagnosed through newborn screening programs, where available, or when patients present with unexplained severe atraumatic pain or normocytic anemia. In SCD, sickling and hemolysis of red blood cells result in vaso-occlusion with associated ischemia. SCD is characterized by repeated episodes of severe acute pain and acute chest syndrome, and by other complications including stroke, chronic pain, nephropathy, retinopathy, avascular necrosis, priapism, and leg ulcers. In the US, nearly all children with SCD survive to adulthood, but average life expectancy remains 20 years less than the general population, with higher mortality as individuals transition from pediatric to adult-focused health care systems. Until 2017, hydroxyurea, which increases fetal hemoglobin and reduces red blood cell sickling, was the only disease-modifying therapy available for SCD and remains first-line therapy for most individuals with SCD. Three additional therapies, L-glutamine, crizanlizumab, and voxelotor, have been approved as adjunctive or second-line agents. In clinical trials, L-glutamine reduced hospitalization rates by 33% and mean length of stay from 11 to 7 days compared with placebo. Crizanlizumab reduced pain crises from 2.98 to 1.63 per year compared with placebo. Voxelotor increased hemoglobin by at least 1 g/dL, significantly more than placebo (51% vs 7%). Hematopoietic stem cell transplant is the only curative therapy, but it is limited by donor availability, with best results seen in children with a matched sibling donor. While SCD is characterized by acute and chronic pain, patients are not more likely to develop addiction to pain medications than the general population.
Conclusions and Relevance In the US, approximately 100 000 people have SCD, which is characterized by hemolytic anemia, acute and chronic pain, acute chest syndrome; increased incidence of stroke, nephropathy, and retinopathy; and a life span that is 20 years shorter than the general population. While hydroxyurea is first-line therapy for SCD, L-glutamine, crizanlizumab, and voxelotor have been approved in the US since 2017 as adjunctive or second-line treatments, and hematopoietic stem cell transplant with a matched sibling donor is now standard care for severe disease.
Read More About
Kavanagh PL , Fasipe TA , Wun T. Sickle Cell Disease : A Review . JAMA. 2022;328(1):57–68. doi:10.1001/jama.2022.10233
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Advertisement
Competing Interests
Sickle cell disease in 2023: laying the foundation for future breakthroughs.
- Request Permissions
Samuel Wilson; Sickle Cell Disease in 2023: Laying the Foundation for Future Breakthroughs. The Hematologist 2024; 21 (1): No Pagination Specified. doi: https://doi.org/10.1182/hem.V21.1.202419
Download citation file:
- Ris (Zotero)
- Reference Manager
Despite arising from alterations in just one gene (beta-globin), sickle cell disease (SCD) manifests in a plethora of complex, varied, and challenging-to-manage phenotypes. Globally, there are more than 7.5 million individuals living with SCD, with unacceptably high mortality concentrated in lower-income countries. 1 Common complications include vasculopathy, vaso-occlusion leading to periodic pain episodes, and damage to end-organs — all of which can lead to strokes, renal disease, bone osteonecrosis, and cardiopulmonary disease. This culminates in a high rate of healthcare utilization, significant morbidity, diminished quality of life, and ultimately, a shortened lifespan.
SCD was initially described over a century ago in 1910. Despite this, research and therapeutic development in SCD has historically been hampered by inadequate/disparate funding. 2 Thankfully, the landscape is changing as policy-makers, patients and family members, advocacy organizations, researchers, and pharmaceutical companies all place a heavy emphasis on improving the lives of individuals living with SCD.
The year drew to a close with an incredible breakthrough in SCD management: approval by the U.S. Food and Drug Administration (FDA) of the first gene therapies for SCD after a decade of painstaking research and clinical trials. Two products were approved in early December: Exagamglogene autotemcel (exa-cel), a CRISPR-based gene therapy, and lovotibeglogene autotemcel (lovo-cel), a lentiviral based gene therapy. The enthusiasm for these therapies is tempered by the unknown long-term side effects and durability of the edited cells, uncertainty about affordability and access (a likely, major barrier to widespread adoption), and the effects of myeloablative conditioning on fertility.
Novel, alternative gene editing approaches for SCD are also in various stages of clinical development, portending more curative-intent options in the future. 3 , 4 One of the most innovative approaches relies on a non-integrating, prime-editor-expressing viral vector after granulocyte colony-stimulating factor (G-CSF) mobilization to correct the beta-globin gene mutation in vivo in a mouse model of SCD. 5 If further developed, this approach may obviate or reduce the need for chemotherapy, expanding the eligibility pool and improving the safety of gene editing for SCD.
The exuberant focus on curative-intent therapies often overshadows other interesting and foundational research necessary for further advances in SCD treatment. Erica M. Sparkenbough, PhD, and colleagues established that coagulation factor XII contributes to inflammation and thrombin generation in a mouse model of SCD, further demonstrating factor XII inhibition as a potential target for reducing thrombosis and vaso-occlusion in SCD. 6 In their recent study, Huihui Li, PhD, and colleagues highlighted the role of iron hemostasis and the gut microbiome in acute vaso-occlusion episodes in SCD mice, suggesting the need for further study of iron restriction as a novel therapy for SCD. 7 Building on previous work linking the endocannabinoid pain modulator 2-arachidonoylglycerol (2-AG) to hyperalgesia in SCD, Iryna Khasabova, PhD, and colleagues demonstrated that maintaining physiological levels of 2-AG reduces hyperalgesia in SCD mice, a promising and unique target for treating chronic pain in SCD. 8
On the epidemiology front, the Global Burden of Disease Study 2021 yielded the most comprehensive assessment of SCD burden to date using data from over 200 countries. Their recent report highlighted the rising population of individuals living with SCD over the past two decades, which has increased from 5.46 million to 7.74 million, largely due to population growth in the Caribbean and the western and central regions of sub-Saharan Africa. Importantly, this study documents the disparate outcomes of SCD worldwide, particularly in mortality rates for children under 5. 1 Studies like this are critical for policymakers and stakeholders in improving SCD outcomes globally.
For this year’s best, I must spotlight the growing emphasis on improving reproductive health during SCD management. In a poignant article, Lydia Pecker, MD, and colleagues argued that inadequate access, knowledge, and counseling surrounding assisted reproductive technologies contributes to health disparities in SCD and is a barrier for individuals considering curative-intent therapies. 9 A recent study demonstrated suboptimal contraception counseling and STI management in transitioning adolescents and young adults with SCD. 10 To improve awareness and education for women with SCD, ASH collaborated with the CDC, the Foundation for Women & Girls with Blood Disorders, and the Sickle Cell Reproductive Health Education Directive to release fact sheets on preconception, prenatal, and postpartum care. 11 – 13 These efforts will, over time, improve fertility and pregnancy outcomes in people living with SCD.
Overall, 2023 has been another year of steady progress in SCD and transformative breakthroughs. The future has never been brighter for individuals living with SCD, as the combined efforts of key stakeholders and patients continually yields tangible progress for those living with SCD.
Dr. Wilson indicated no relevant conflicts of interest.
- Previous Article
- Next Article
Email alerts
Affiliations.
- Current Issue
- About The Hematologist
- Advertising in The Hematologist
- Editorial Board
- Permissions
- Submissions
- Email Alerts
- ASH Publications App
American Society of Hematology
- 2021 L Street NW, Suite 900
- Washington, DC 20036
- TEL +1 202-776-0544
- FAX +1 202-776-0545
ASH Publications
- Blood Advances
- Blood Neoplasia
- Blood Vessels, Thrombosis & Hemostasis
- Hematology, ASH Education Program
- ASH Clinical News
- The Hematologist
- Publications
- Privacy Policy
- Cookie Policy
- Terms of Use
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Submit Abstract / Manuscript
Please click "submit" below, to submit abstract and/or manuscript.

FSCDR's 18th Annual Sickle Cell Disease Research and Educational Symposium and 47th National Sickle Cell Disease Scientific Meeting
June 7 - 9, 2024.
Submission & Notification Deadlines:
Abstract Scorecard Site opens- January 8, 2024
Abstract Scorecard Site closes for general submissions- February 19, 2024 Notifications sent out- March 11, 2024
Late-breaking abstract submission deadline- April 1, 2024 Late-breaking notifications sent out- April 22, 2024
This website also serves as the submission location for the Grant Writing Institute Grant submissions. If you are submitting a grant for review, please select the “Grant Writing Institute” option once you are logged in
Accepted submissions are published on the first day of the symposium you are submitting for. For example, if the symposium is June 7 – June 9, 2024 the Journal of Sickle Cell Disease and Hemoglobinopathies publication date is June 7, 2024.

Santosh Saraf, MD
Scientific chair.
Dr. Santosh L. Saraf received his medical degree from the Temple University School of Medicine and completed an internal medicine residency and hematology & oncology fellowship at the University of Illinois at Chicago (UIC). Dr. Saraf joined the Division of Hematology & Oncology at UIC in 2012 and completed a Master of Science in Clinical and Translational Research through the University of Illinois School of Public Health in 2014. He currently serves as the Director of Translational Research for the Sickle Cell Center and the Fellowship Program Director for Hematology & Oncology. Dr. Saraf focuses his clinical care and research on understanding the mechanisms of kidney disease in patients with sickle cell disease and on developing curative therapies through hematopoietic stem cell transplantation for patients with clinically aggressive sickle cell disease.

Alan Anderson, MD
Scientific co-chair.
Dr. Anderson is an Associate Professor of Clinical Pediatrics at the University of South Carolina School of Medicine in Greenville, SC. He completed his pediatric residency at the Medical University of SC in Charleston, SC and pediatric hematology/oncology fellowship training at Emory University. He is the director of the Comprehensive “Lifespan” Sickle Cell Disease Program at Prisma Health-Upstate in Greenville, SC. His program provides care for over 450 individuals with sickle cell disease and includes services for patients of all ages within the same dedicated clinical space. Dr. Anderson is involved in both cooperative and industry-sponsored research trials for SCD and his program is part of the American Society of Hematology SCD Clinical Trials Network (ASH SCD-CTN). In addition to his work in the United States, Dr. Anderson partners with clinicians in W. Africa to establish newborn screening programs for SCD. He believes strongly in engaging the voice of the patient and the community in all aspects of care.
- ASH Foundation
- Log in or create an account
- Publications
- Diversity Equity and Inclusion
- Global Initiatives
- Resources for Hematology Fellows
- American Society of Hematology
- Hematopoiesis Case Studies
Case Study: Sickle Cell Disease A 25-Year-Old in Transition
- Agenda for Nematology Research
- Precision Medicine
- Genome Editing and Gene Therapy
- Immunologic Treatment
- Research Support and Funding
A 25-year-old woman with a history of sickle cell disease (SCD) presents to the clinic for follow-up after a hospitalization for a vaso-occlusive pain crisis complicated by influenza A. She has a history of an acute ischemic stroke at age 5 years and has received monthly, simple red cell transfusions since the stroke. Her last transfusion was approximately four months prior. She is taking deferasirox 20 mg/kg daily but occasionally misses doses.
Laboratory results show the following:
Which of the following is the next best step in diagnosis
- Restart scheduled red blood cell transfusions
- Start prophylactic penicillin
- Discontinue transfusions and start hydroxyurea
- Order transcranial doppler ultrasonography (TCD) to assess risk of stroke
- Increase dose of deferasirox to 25 mg/kg/day
Explanation
The incidence of primary stroke in children with SCD is 0.6 to 0.8 events per 100 patient-years, with a cumulative incidence of 7.8 percent by age 14 years in the Jamaican cohort and 11 percent by age 20 years in the U.S. Cooperative Study of Sickle Cell Disease. Once stroke has occurred, the incidence of recurrent (secondary) stroke ranges from 47 to 93 percent in patients not started on regular transfusions. The Stroke Prevention Trial in SCD (STOP) randomized 130 high-risk children with SCD to either transfusion therapy (to maintain HbS 30%) or observation. These high-risk children had an increased blood flow in the internal carotid or middle cerebral artery by TCD. This study showed a 92 percent reduction in incidence of first stroke in transfused high-risk patients. A follow-up study, STOP2, randomly assigned 72 children whose TCD had normalized after 30 months of transfusion therapy to either ongoing or discontinued transfusions. The study was closed early due to a significant increase in abnormal TCD velocity and stroke risk for those who halted transfusion therapy.
The multicenter phase III TWiTCH trial evaluated children with SCA and abnormal TCD velocities without a history of stroke on chronic transfusions. Data showed that hydroxyurea at maximal tolerated dose was noninferior to chronic transfusions for maintaining TCD velocities as primary stroke prophylaxis (choice C). This patient has a history of ischemic stroke, so the results of TWiTCH do not apply to her.
The Stroke with Transfusions Changing to Hydroxyurea (SWiTCH) study was designed as a phase III multicenter trial to determine the efficacy of hydroxyurea/phlebotomy, compared with transfusions/chelation for children with SCA, stroke, and iron overload in secondary stroke prophylaxis. The primary endpoint was a composite of noninferiority for stroke prevention and superiority for reduction of liver iron content. The trial was terminated at the first scheduled interim analysis for futility for the composite endpoint, which required superiority of phlebotomy over iron chelation for reducing excess iron stores. The incidence of stroke on the hydroxyurea plus phlebotomy arm was higher (7 of 67 patients; 10.4%) than in the transfusion plus chelation arm (1 of 66 patients; 1.5%). These results, though not powered for inferiority, showed a trend towars increased stroke risk with transition to hydroxyurea. In patients with prior stroke, cessation of transfusion therapy is currently not recommended.
Whether chronic transfusion therapy can be stopped after a longer period of transfusions in a patient with a prior stroke remains unclear even though risk of recurrent stroke remains high in adolescence and young adulthood. In patients older than 16 years, TCD velocity criteria to determine stroke risk is not reliable (choice D).
In the Prophylaxis with Oral Penicillin in Children with Sickle Cell Anemia trial, children with SCA were randomly assigned to receive oral prophylactic penicillin or placebo PROPS 1986 ). The trial ended eight months early after the occurrence of 15 cases of pneumococcal sepsis, 13 in the placebo group and two in the penicillin group, showing an 84 percent reduction in pneumococcal sepsis with penicillin prophylaxis. The follow-up study, PROPS II, did not show an increased risk in pneumococcal infections with discontinuation of prophylactic penicillin after age 5 years. Therefore, prophylactic penicillin is not recommended in adults with SCA (choice B).
The trajectory of ferritin in this patient has not been established and an increase in oral iron chelation is not indicated at this time.
Case Study submitted by Marquita Nelson, MD, of University of Chicago, Chicago, IL.
- Hirst C, Owusu-Ofori S Prophylactic antibiotics for preventing pneumococcal infection in children with sickle cell disease . Cochrane Database Syst Rev. 2014 6:CD003427.
- Valadi N, Silva GS, Bowman LS, et al Transcranial Doppler ultrasonography in adults with sickle cell disease . Neurology. 2006 22:572-574.
- Ware RE, Davis BR, Schultz WH, et al Stroke with transfusions changing to hydroxyurea (SWiTCH) . Blood. 2012 119:3925-3932.
- Kumar N, Gross JB Jr, Ahlskog JE TCD with transfusions changing to hydroxyurea (TWiTCH): hydroxyurea therapy as an alternative to transfusions for primary stroke prevention in children with sickle cell anemia . Blood. 2015 126:3.
American Society of Hematology. (1). Case Study: Sickle Cell Disease A 25-Year-Old in Transition. Retrieved from https://www.hematology.org/education/trainees/fellows/case-studies/sickle-cell-disease-a-25-year-old-in-transition .
American Society of Hematology. "Case Study: Sickle Cell Disease A 25-Year-Old in Transition." Hematology.org. https://www.hematology.org/education/trainees/fellows/case-studies/sickle-cell-disease-a-25-year-old-in-transition (label-accessed April 24, 2024).
"American Society of Hematology." Case Study: Sickle Cell Disease A 25-Year-Old in Transition, 24 Apr. 2024 , https://www.hematology.org/education/trainees/fellows/case-studies/sickle-cell-disease-a-25-year-old-in-transition .
Citation Manager Formats
Safety of a potential new treatment to manage complications from sickle cell disease
Treatment for lung condition could help patients with sickle cell disease control complications from hypertension and kidney damage.
A drug approved to treat pulmonary arterial hypertension may be effective at managing hypertension and end-organ damage in patients with sickle cell disease, according to a new study published in Lancet Haematology . An early phase randomized clinical trial involving 130 patients with sickle cell disease found that the drug, called riociguat, was found to be safe to use and well tolerated in these patients and significantly improved their blood pressure. Preliminary efficacy data suggested the medication might improve heart function.
An estimated 100,000 Americans have sickle cell disease, and the disease occurs in about 1 out of every 365 Black or African-American births, according to the Centers for Disease Control and Prevention. People with sickle cell disease are at high risk for vascular complications that can lead to pulmonary hypertension, stroke, and kidney failure as well as severe pain when red blood cells block blood flow through tiny blood vessels in the chest, abdomen, and joints. These complications can be worsened by hypertension.
Unfortunately, previous research found that sildenafil, an effective treatment for pulmonary hypertension, caused unacceptable side effects in patients with sickle cell disease. It found that those who took this drug experienced high levels of pain that caused increased admissions to the hospital compared to those who took a placebo treatment.
This new study was designed to test the safety of riociguat and how well it works in preventing or reducing the clinical complications for patients with sickle cell disease.
In the study, patients with sickle cell disease and mild hypertension or protein in their urine (an early sign of kidney disease) were randomly assigned to receive either riociguat or a placebo in a double-blind clinical trial. Both groups received the study drug at a starting dose of 1 milligram, which was gradually increased up to 2.5 milligram, taken three times a day for 12 weeks. The researchers found that among the participants who took riociguat, 22.7 percent experienced at least one serious adverse event related to the treatment. In comparison, in the group that received the placebo, 31.3 percent of participants had at least one serious adverse event during the study.
The differences were not statistically significant. There were no differences between the two groups in the rates of pain severity, pain interference in their daily lives, and in vascular events related to their sickle cell disease. When it comes to the effectiveness of the drug treatment, participants who took riociguat had their blood pressure drop by 8.20 mmHg, while those who took a placebo only saw a decrease of about 1.24 mmHg. The result was highly statistically significant, meaning riociguat was much more effective at lowering blood pressure compared to the placebo, with a difference of approximately 6.96 mmHg. In summary, riociguat was found to be safe and led to a significant improvement of blood pressure over the duration of the study.
"Our results are encouraging and open the door to larger clinical trials involving this class of drugs in patients with sickle cell disease who have pulmonary hypertension or kidney disease. Having a drug that's easy to tolerate can help them better manage their blood pressure and help prevent serious complications down the road," said study leader Mark T. Gladwin, MD, who is the John Z. and Akiko K. Bowers Distinguished Professor and Dean of UMSOM, and Vice President for Medical Affairs at University of Maryland, Baltimore.
Bayer Pharmaceuticals, manufacturer of riociguat, provided funding (as well as the drug and placebo) for the study.
The study was led by the clinical and data coordinating centers at the University of Pittsburgh. Study co-authors included faculty from the University of Illinois at Chicago, Albert Einstein College of Medicine, University of Pittsburgh, Emory University, Duke University, Johns Hopkins School of Medicine, and other institutions.
- Sickle Cell Anemia
- Hypertension
- Heart Disease
- Pharmacology
- Diseases and Conditions
- Blood Clots
- Sickle-cell disease
- Pharmaceutical company
- Clinical trial
- Stem cell treatments
- Drug discovery
- Deep brain stimulation
Story Source:
Materials provided by University of Maryland School of Medicine . Original written by Deborah Kotz. Note: Content may be edited for style and length.
Journal Reference :
- Mark T Gladwin, Victor R Gordeuk, Payal C Desai, Caterina Minniti, Enrico M Novelli, Claudia R Morris, Kenneth I Ataga, Laura De Castro, Susanna A Curtis, Fuad El Rassi, Hubert James Ford, Thomas Harrington, Elizabeth S Klings, Sophie Lanzkron, Darla Liles, Jane Little, Alecia Nero, Wally Smith, James G Taylor, Ayanna Baptiste, Ward Hagar, Julie Kanter, Amy Kinzie, Temeia Martin, Amina Rafique, Marilyn J Telen, Christina M Lalama, Gregory J Kato, Kaleab Z Abebe. Riociguat in patients with sickle cell disease and hypertension or proteinuria (STERIO-SCD): a randomised, double-blind, placebo controlled, phase 1–2 trial . The Lancet Haematology , 2024; DOI: 10.1016/S2352-3026(24)00045-0
Cite This Page :
Explore More
- Fossil Frogs Share Their Skincare Secrets
- Fussy Eater? Most Parents Play Short Order Cook
- Precise Time Measurement: Superradiant Atoms
- Artificial Cells That Act Like Living Cells
- Affordable and Targeted Anticancer Agent
- This Alloy Is Kinky
- Giant Galactic Explosion: Galaxy Pollution
- Flare Erupting Around a Black Hole
- Two Species Interbreeding Created New Butterfly
- Warming Antarctic Deep-Sea and Sea Level Rise
Trending Topics
Strange & offbeat.
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Sickle cell anemia.
Ankit Mangla ; Moavia Ehsan ; Nikki Agarwal ; Smita Maruvada .
Affiliations
Last Update: September 4, 2023 .
- Continuing Education Activity
Sickle cell anemia is an inherited disorder of the globin chains that causes hemolysis and chronic organ damage. Sickle cell anemia is the most common form of sickle cell disease (SCD), with a lifelong affliction of hemolytic anemia requiring blood transfusions, pain crises, and organ damage. Since the first description of the irregular sickle-shaped red blood cells (RBC) more than 100 years ago, our understanding of the disease has evolved tremendously. Recent advances in the field, more so within the last three decades, have alleviated symptoms for countless patients, especially in high-income countries. This activity reviews the pathophysiology, presentation, complications, diagnosis, and treatment of sickle cell anemia and also highlights the role of the interprofessional team in the management of these patients.
- Describe the pathophysiology of sickle cell anemia.
- Summarize the epidemiology of sickle-cell anemia.
- List the management options for sickle cell anemia.
- Outline the importance of cooperation among healthcare professionals to educate the patients on getting vaccinated, remaining hydrated, and timely follow-up to prevent the development of complications in those with sickle cell disease.
- Introduction
Sickle cell disease (SCD) refers to a group of hemoglobinopathies that include mutations in the gene encoding the beta subunit of hemoglobin. The first description of SCA 'like' disorder was provided by Dr. Africanus Horton in his book The Disease of Tropical Climates and their treatment (1872). However, it was not until 1910 when Dr. James B Herrick and Dr. Ernest Irons reported noticing 'sickle-shaped' red cells in a dental student (Walter Clement Noel from Grenada). [1] In 1949, independent reports from Dr. James V Neel and Col. E. A. Beet described the patterns of inheritance in patients with SCD. In the same year, Dr. Linus Pauling described the molecular nature of sickle hemoglobin (HbS) in his paper 'Sickle Cell Anemia Hemoglobin.' Ingram Vernon, in 1956, used a fingerprinting technique to describe the replacement of negatively charged glutamine with neutral valine and validated the findings of Linus Pauling. [2]
Within the umbrella of SCD, many subgroups exist, namely sickle cell anemia (SCA), hemoglobin SC disease (HbSC), and hemoglobin sickle-beta-thalassemia (beta-thalassemia positive or beta-thalassemia negative). Several other minor variants within the group of SCDs also, albeit not as common as the varieties mentioned above. Lastly, it is essential to mention the sickle cell trait (HbAS), which carries a heterozygous mutation and seldom presents clinical signs or symptoms. Sickle cell anemia is the most common form of SCD, with a lifelong affliction of hemolytic anemia requiring blood transfusions, pain crises, and organ damage. [3]
Since the first description of the irregular sickle-shaped red blood cells (RBC) more than 100 years ago, our understanding of the disease has evolved tremendously. Recent advances in the field, more so within the last three decades, have alleviated symptoms for countless patients, especially in high-income countries. In 1984, Platt et al. first reported the use of hydroxyurea in increasing the levels of HbF. [4] Since then, the treatment of sickle cell has taken to new heights by introducing several new agents (voxelotor, crinzalizumab, L-glutamine) and, most recently, gene therapy.
Hemoglobin (Hb) is a significant protein within the red blood cell (RBC). It comprises four globin chains, two derived from alpha-globin (locus on chromosome 16) and two from beta-globin (locus on chromosome 11). There are many subtypes of Hb. The most common ones that are found in adults without hemoglobinopathies are listed here:
- HbA1- comprises two chains of the alpha-globin and two chains of the beta-globin (a2b2) - This constitutes 95% of the adult hemoglobin.
- HbA2- comprises two chains of the alpha-globin and two chains of the delta-globin (a2d2) - This constitutes less than 4% of the adult hemoglobin.
- HbF- comprises two chains of the alpha-globin and two chains of the gamma-globin (a2g2) - This Hb is more prevalent in the fetus (due to the high oxygen binding affinity that helps extract oxygen from maternal circulation).
The sickle cell mutation occurs when negatively charged glutamine is replaced by a neutral valine at the sixth position of the beta-globin chain. The mutation is transmitted via Mendelian genetics and is inherited in an autosomal codominant fashion. [5] A homozygous mutation leads to the severest form of SCD, i.e., SCA- also called HBSS disease. The coinheritance of beta-naught thalassemia and sickle cell mutation leads to HBS-Beta-0 disease, which phenotypically behaves like HBSS disease.
A heterozygous inheritance leads to HbAS. Patients with HbAS are not considered within the spectrum of SCD as most of them never present with typical symptoms of SCA. They might only be detected during childbirth, blood donation, or screening procedures.
Several other compound heterozygotes exist where a single copy of the mutated beta-globin gene is coinherited with a single copy of another mutated gene. The second most common variant of SCD is the HbSC disease, where the sickle cell gene is coinherited with a single copy of the mutated hemoglobin C gene. HbC is formed when lysine replaces glutamine at the sixth position on the beta-globin chain. HbSC disease accounts for 30% of patients in the United States.
- Epidemiology
The epidemiological data on SCD is scarce. It is well known that SCD and HbAS are more prevalent in sub-Saharan Africa, where the carrier of HbAS is afforded natural protection against severe Plasmodium falciparum malaria. It is estimated that ~230,000 children were born with SCA, and more than 3.5 million neonates were born with HbAS in sub-Saharan Africa in 2010. an estimated 75% of SCD-related births take place in sub-Saharan Africa. West Africa is home to the largest population of individuals with HbSC disease. [3]
The United States (US) Center for Disease Control (CDC) estimates that approximately 100,000 Americans have SCD. The CDC also estimates that 1 in 13 babies born to African-American parents have sickle cell trait, and 1 in 365 African-Americans have SCD. The estimated ratio of Hispanic Americans with SCD is 1 in 16,300. Children and adolescents make up to 40% of all SCD patients in the US. The incidence varies by state and geographical concentration of ethnicities. Besides, migration within the country and immigration from foreign countries alter the prevalence of SCD and HbAS. This is true for several countries where patients with SCD and SCA are living. Genetic studies in Brazil have also tied the origin of such patients to the slave trade originating from West Africa (Mina Coast and Angola). [6] With the improvement in technology and ease of international migration, the incidence of SCA is predicted to rise. It is estimated that the annual number of newborns with SCA will exceed 400,000 by 2050.
There is also a stark difference in mortality and morbidity in high-income and low-income countries. Adopting vaccination guidelines for children with SCD and intensive screening procedures has sharply reduced the mortality of kids with SCD between 0 to 4 years (68% drop noted from 1999 to 2002 compared to 1983 to 1986). On the other hand, in sub-Saharan Africa, 50 to 90% of children born with SCD will die before their fifth birthday. Improving the care afforded in high-income countries and targeted training of healthcare providers have improved life expectancy. However, it still lags by decades compared to matched non-SCD cohorts (54 versus 76 years - projected life expectancy, and 33 years versus 67 years- quality-adjusted life expectancy). [7]
HbSC disease accounts for 30% of all patients with SCD in the US. As with HbAS, patients with the Hb C trait (heterozygous mutation) also remain asymptomatic for most of their lives. Although considered a milder variant of SCD, HbSC disease may present with severe morbidities. [8]
- Pathophysiology
Sickle cell anemia is characterized by two major components: Hemolysis and vaso-occlusive crises (VOC). The defect in the beta-globin gene makes the sickle hemoglobin (HbS) molecule susceptible to converting into rigid, elongated polymers in a deoxygenated state. The sickling process is cyclical initially, where sickle erythrocytes oscillate between the normal biconcave shape and the abnormal crescent shape (acquired under low oxygen pressure). However, there comes a time when the change becomes irreversible, and the sickle erythrocytes develop a permanent sickle shape increasing the risk for hemolysis and VOC. All variants of SCD share the same pathophysiology leading to polymerization of the HbS component. [3]
Multiple factors inherent to sickle erythrocytes, like low affinity of HbS to oxygen, physiologically high 2,3-diphosphoglycerate, and increased sphingokinase-1 activity, lead to deoxygenation, which promotes polymerization of HbS. In addition to this, high concentration of HbS, abnormal activity of Gados channel leading to dehydration, and repeated damage to red blood cell (RBC) membrane also increase the risk of polymerization of HbS.
Oxidative stress contributes to hemolysis by auto-oxidation of HbS, leading to erythrocyte cell membrane damage. The increased expression of xanthine dehydrogenase, xanthine oxidase, and decreased expression of NADPH oxidase increase the oxidative stress within sickle RBC. A hemolyzed cell releases free hemoglobin (scavenges nitrous oxide) and arginase 1 (competes for L-arginine) that prevent the action and formation of nitrous oxide and contribute to oxidative stress and vascular remodeling (arginase-1 converts arginine to ornithine). [3]
Besides the polymerization of the HbS and intravascular hemolysis, several other factors also contribute to vaso-occlusion. For example, the sickle RBC (expresses several adhesion molecules on the surface), free heme and Hb, reactive oxygen species, and endothelium interact with each other and with neutrophils and platelets to promote vaso-occlusion and thrombosis.
- Histopathology
In patients with SCA, peripheral blood smear shows elongated RBC with tapering ends that look like a sickle (also called drepanocytes). Additional findings are present in a few patients.
- Howell-Jolly bodies- Remnants of DNA are seen in the RBC and commonly seen in patients in whom the spleen has been removed. Therefore, patients with SCA have auto-splenectomy.
- Target cells (Leptocytes)- Most commonly seen in patients with Thalassemia. They are seen frequently in sickle-thalassemia syndromes and are sometimes noted in patients with SCA.
- Polychromatic cells - these are reticulocytes that signify marrow response to hemolysis.
- Nucleated red blood cells can sometimes be visible on the peripheral smear.
None of these findings are confirmatory. Confirmation is obtained only through hemoglobin electrophoresis, high-performance liquid chromatography, or isoelectric focusing. DNA-based techniques are not used routinely. Instead, they are used in patients with uncertain diagnoses. Pre-natal fetal testing involves using fetal DNA obtained through amniocentesis. Techniques to capture the fetal DNA in maternal blood remain investigational.
- History and Physical
Most patients with HbSS phenotype do not present with classical 'sickle cell crises' soon after birth. HbF is still present in the blood, helping maintain adequate tissue oxygenation, and it takes around 6-9 months to wean off completely. Not all SCA have the same phenotype, and multiple phenotypes exist that can either co-exist or present as a spectrum of the disease. [3]
- Vaso-occlusive subphenotype - Distinguished by higher hematocrit (Hct) compared to other SCA. A higher Hct leads to higher viscosity that promotes frequent vaso-occlusive crises and acute chest syndrome.
- Higher risk of gallstones, pulmonary hypertension, ischemic stroke, priapism, and nephropathy
- Severe anemia increases cardiac workload and blood flow through organs, making them susceptible to damage
- Higher free heme and Hb in blood vessels cause oxidative damage
- High Hb F subtype- A 10 to 15% level of HbF alleviates the symptoms of SCA. However, the distribution of HbF is not consistent throughout the body.
- Pain-sensitive subphenotype- Altered neurophysiology amongst various individuals make them susceptible to pain. Some individuals are more susceptible to pain compared to others with SCA.
The patients with SCA present wither with acute or chronic complications associated with the disease. The most common acute complication of SCA is Vaso-occlusive crisis (VOC). The treatment section below discusses the management of acute and chronic issues.
Important points to be noted in the history of patients with SCA
- All patients with SCA will experience VOC during their lives. The earliest presentation is dactylitis in kids as young as six months of age.
- Any body organ can develop VOC (head, eyes, etc.), although extremities and the chest are most commonly involved. If a VOC pain sounds atypical, obtain a history to rule out other causes.
- When was the last pain crisis, and how many times in the previous year have they been admitted to the hospital with pain crises?
- If they take analgesics daily, it is prudent to know the type and quantity of the analgesic (opioid or non-opioid), the last use of analgesics, and whether they take the analgesics before coming to the ER/office
- History of taking disease-modifying drugs (hydroxyurea, voxelotor, crinzalizumab, etc.)
- A history of substance abuse, psychiatric disorders, and use of psychotropic medications must be obtained.
- History of receiving blood transfusions and exchange transfusions- helps assess the risk of iron overload, presence of alloantibodies (multiple transfusions in the past can lead to the development of alloantibodies, which will help assess the risk of transfusion reactions), and previous transfusion reactions.
- History of any other diseases that may or may not be associated with SCA - previous history of stroke, thrombosis, priapism, etc.
- It is also advised to get in touch with the primary hematologist taking care of the patient- it is valuable to have their input in understanding the patient's normal physiology.
- History of previous surgeries.
- History of life-threatening crises in the past- if present, should alert the clinician to ensure that a similar event is not occurring again. For example, fat embolism may occur more frequently in patients with SCA.
The physical exam should focus on the general system exam to determine the need for oxygen requirements, pain management, and blood/exchange transfusion. However, a focused exam is also necessary to rule out any organ-specific problem. For example, a rapidly enlarging liver or spleen should alert the physician about sequestration crises.
Patients with SCA are usually diagnosed in childhood. Intensive newborn screening programs in developed countries can identify patients in the neonatal stage. In the US, universal screening for SCA was implemented in all states by 2007. High-performance liquid chromatography and isoelectric focusing are the methods used in the US. In Europe, most countries deploy targeted screening in high-risk areas (where SCA is more common) and not a universal screen. In sub-Saharan Africa, no country has adopted a screening program. In India, the solubility test is used as the first step- if positive, then high-performance liquid chromatography is used to confirm at the reference center. [3]
Acute Complications in Patients with SCA
Acute Chest Syndrome (ACS): ACS is the most common complication of SCA. It is also the most common cause of death and the second most common cause of hospital admission. A patient can either present with ACS or may develop it during hospitalization for any other reason. Hence, it is prudent to monitor all patients with SCA admitted to the hospital for ACS. It is important to recognize ACS early and act upon it to prevent respiratory failure.
- The risk factors for ACS include a previous history of ACS, asthma, or recent events like recent surgical procedures, pulmonary embolism, fluid overload, infection, etc.
- The clinical features include sudden onset of cough and shortness of breath. Fever may or may not be a part of the spectrum of presentation. If present, then it usually points towards infection.
- Laboratory evaluation includes a complete blood count with differential chemistries, including liver and kidney evaluation, blood cultures, and sputum cultures.
- Chest X-ray shows a new pulmonary infiltrate- this is a quintessential feature of defining ACS. CT and perfusion mismatch scans are only used if there is a strong clinical suspicion of pulmonary embolism or fat embolism. Therefore, they are not usually helpful in acute settings.
Sequestration Crises: This can either be hepatic or splenic sequestration.
- Patients experience rapid spleen enlargement associated with pain in the left upper quadrant. In children with SCA, it is common in children between 1 to 4 years of age, as the spleen is still intact.
- Patients with non-SCA variants (HbSC, HbS-beta+ thalassemia) are not prone to 'auto-splenectomy' commonly seen in patients with SCA. Hence they can develop splenic sequestration later in life. Such patients may have baseline splenomegaly, causing hypersplenism. Parents and patients must receive counseling regarding the signs and symptoms of an enlarging spleen.
- Younger patients present with acute anemia and hypovolemic shock due to smaller circulating volumes, whereas adults may present with a more insidious onset.
- Pain occurs due to stretching of the splenic capsule and new infarcts.
- Blood count shows a drop in Hb by more than 2gm/dL, increased reticulocyte count, and nucleated red blood cells.
- Hepatic sequestration: Hepatic sequestration can occur across all phenotypes of SCA. Like the spleen, patients may have a baseline enlargement of the liver. Hepatic sequestration is also defined as rapid enlargement of the liver with stretching of the capsule. The hemoglobin shows a drop of more than 2gm/dL. Liver enzymes may not get elevated.
Acute Stroke: Stroke is the most devastating complication of SCA. Since the advent of transcranial doppler (TCD) and the institution of primary prevention programs, the incidence of stroke has gone down in patients with SCA. In the absence of primary prevention, ~10% of children suffer from overt stroke, and approximately 20 to 35% have silent cerebral infarcts. TCD is not useful for adults.
- Severe headache, altered mental status, slurred speech, seizures, and paralysis- are signs of stroke.
- Urgent neurological consultation and CT scan followed by MRI/MRA must be done.
Aplastic crises: It is usually precipitated by parvovirus B-19 and is defined as a rapid drop in Hb at least 3 to 6 gm/dL below the baseline. Patients present with severe fatigue, anemia, shortness of breath, and even syncope. Blood counts show severely low hemoglobin with near-absent reticulocytes. Bone marrow biopsy shows arrest in the pro-normoblast stage in patients with acute parvovirus infections. [9]
Acute intrahepatic cholestasis (AIC): Presents with sudden onset right upper quadrant pain. Physical exam shows worsening jaundice, enlarging and tender liver, and clay-colored stools. Labs show very high bilirubin levels, elevated alkaline phosphatase, and coagulopathy. The hemolysis parameters may be normal. AIC is a medical emergency.
Infections in patients with SCA can be a harbinger of infection with Streptococcus pneumoniae infection or osteomyelitis.
- The use of prophylactic antibiotics and pneumococcal vaccinations has reduced their incidence. However, loss of splenic function in SCA patients puts them at risk of invasive bacterial species.
- Osteomyelitis can be unifocal or multifocal- Staphylococcus aureus , Salmonella , and other enteric organisms can cause osteomyelitis in SCA patients.
Priapism is defined as a sustained, unwanted painful erection lasting more than 4 hours. It is a common condition among patients with SCA, affecting 35% of all men/boys.
Acute Ocular Complications
- The complication presents similarly in patients with SCA and sickle cell trait.
- The low oxygen pressure and acidotic nature of the aqueous humor promote sickling of the RBC, leading to blockage of the trabecular network leading to an acute rise in intraocular pressure (IOP).
- High IOP is poorly handled in patients with SCA - which can lead to CRAO and secondary hemorrhages.
- Central Retinal Artery Occlusion (CRAO)- Results from thrombus formation in the retinal artery leading to infarction of the retina, macular ischemia, or macular infarction. CRAO can occur spontaneously or secondary to increased IOP (from hyphema), Moyamoya syndrome, or ACS in patients with SCA.
- Patients present with proptosis, local pain, and edema of the lid or orbit.
- The exam shows reduced extraocular motility and decreased visual acuity.
- CT scan helps in distinguishing this from orbital cellulitis/ infection.
- Orbital Compression Syndrome (OCS) - also called orbital apex syndrome, is characterized by ophthalmoplegia and vision loss secondary to events occurring at the orbital apex. Cranial nerves II, III, IV, VI, and the first division of CN V can be involved. MRI of the orbits is the best modality for diagnosis.
Chronic Complications in Patients with SCA
Iron Overload: Iron (Fe) overload is a common problem in SCA patients due to repeated transfusions and chronic hemolysis. Each unit of packed RBC contains 200 to 250 mg of iron. Excessive iron mainly affects the heart, lungs, and endocrine glands. [10] Hepatic cirrhosis from excessive iron is a major cause of death in patients with SCA. Clinical trials in patients with thalassemia have shown that systemic iron load correlates directly with survival and cardiac incidents. [11]
Avascular Necrosis (AVN) of Joints: AVN of the femoral head is a common cause of chronic pain and disability in SCA patients. Although the hip joint is the most common joint to be involved, other joints can also be affected. AVN occurs at the distal portion of the bone, where collateral circulation is poor. The capillaries get occluded by sickle RBCs leading to hypoxia and bone death. Risk factors for AVN of the femoral head include age, frequency of painful episodes, hemoglobin level, and alpha-gene deletion. In patients with HbSS, the overall prevalence is 50 percent by age 33. HbSS-alpha thalassemia and HbSS-Beta-0 thalassemia are at higher risk of developing AVN early in life.
Leg Ulcers : More common in SCA compared to other SCD genotypes. Approximately 2.5% of patients with SCA above ten years of age have leg ulcers. Leg ulcers are more common in men and older people and less common in people with high total hemoglobin, alpha-gene deletion, and high levels of HbF. Trauma, infections, and severe anemia also increase the risk of leg ulcers. The ulcers occur more commonly on the medial and lateral surfaces of the ankles. They vary in size and depth, and chronic ulcers may lead to osteomyelitis, especially if they are deep enough to expose the bone.
Pulmonary Artery Hypertension (PAH) : Affects 6 to 11% of patients with SCA. PAH in SCA is classified under World Health Organization (WHO) group V. However; chronic hemolysis leads to pulmonary vascular changes classified under WHO group 1 in up to 10% of all SCA patients. PAH in SCA can also occur due to left heart dysfunction (Group II), chronic lung disease from SCA (Group III), chronic thromboembolism (Group IV), or extrathoracic causes (Group V).
The patient may complain of dyspnea on exertion, swelling in the legs, or present with symptoms of underlying disease (like a history of thrombosis, heart failure, etc.). An echocardiogram helps in estimating the tricuspid regurgitant jet velocity (TRV). Elevated TRV is associated with increased mortality in adults. However, TRV can be transiently elevated during acute chest syndrome. Serum NT-pro-BNP is directly correlated with mortality as well. The final diagnosis is made with a right heart catheterization.
Renal complications: Chronic kidney disease (CKD) occurs in approximately 30% of adult patients with SCA. The acidotic, osmotic, and hypoxic environment of the kidney increases the risk of polymerization of HbS, leading to the sickling of RBC. SCA patients secrete excessive creatinine in their proximal tubules. Hence, it becomes challenging to identify early signs of kidney disease, as creatinine takes a longer time to rise. Microalbuminuria (30-300mg albumin in 24-hour urine collection) is often the first manifestation of CKD. Spot urine-creatinine ratio is not validated in SCA patients due to hypersecretion of creatinine.
- Hypoesthenuria- Inability to concentrate urine due to loss of deep juxtamedullary nephrons. It is the most common complication in SCA patients. It leads to frequent urination and increases the risk of dehydration. It also increases the risk of enuresis in children.
- Renal papillary necrosis occurs due to obstruction of the vessels supplying the vasa recta resulting in medullary infarction. It presents with hematuria. It is more common in patients with HbSC disease.
- Asymptomatic Proteinuria: It is present in 15 to 50% of patients. It develops early in life due to hyperfiltration and loss of selectivity for albumin.
Ophthalmologic Complications: Chronic eye complications are more common in patients with HbSC and HbSS disease. They are found in up to 50% of patients.
- Proliferative Sickle Retinopathy occurs due to vaso-occlusion of vitreal arterioles leading to ischemia which leads to neovascularization. Neovascular tissue is predisposed to hemorrhage and vitreal traction forces resulting in vitreal hemorrhage (the most severe complication of proliferative sickle retinopathy).
- Treatment / Management
Patients with SCA present with acute and chronic complications.
Management of Acute Complications
Pain management is a critical part of SCA. It is challenging for clinicians to accurately assess patients' needs, especially if they meet them for the first time. Patients with SCA often suffer from the stigma of requiring high doses of opioids for pain control, which leads to them being labeled as 'opioid abusers,' 'manipulators,' or even' drug seekers.' [12]
- Analgesic administration starts simultaneous with evaluating the cause, ideally within 30 minutes of triage and 60 minutes of registration.
- Develop individualized pain management plans - this should be made available to the emergency room and should be implemented each time the patient presents with VOC and pain.
- NSAIDs are used in patients with mild to moderate pain who report prior episodes of relief with NSAIDs
- Any patient presenting with severe pain- preferably used parenteral opioids. An intravenous route is preferred; however, if access is difficult, use the subcutaneous route.
- The dose of parenteral opioids is calculated based on the total dose of short-acting oral opioids taken at home.
- Pain should be reassessed every 15 to 30 minutes, and readminister opioids if needed. The escalation of opioids is done in 25% increments.
- Patient-controlled analgesia (PCA) is preferred. If an "on-demand" setting is used in PCA, then continue long-acting analgesia.
- When pain control is achieved, "wean off" parenteral opioids before converting to oral medications.
- Calculate the inpatient analgesic requirement at discharge and adjust home doses of short and long-acting opioids accordingly.
- Meperidine is not used in managing VOC-related pain unless this is the only medication that controls the pain.
- Antihistamines only help in controlling opioid-related itching. When required, use oral formulations only—readminister every 4 to 6 hours as needed.
- Incentive spirometry
- Intravenous hydration
- Supplemental oxygen is needed only if saturation drops below 95% on the room air.
Management of Chronic Pain
Chronic pain management in SCA patients focuses on the safe and adequate use of pain medications, particularly opioids. A comprehensive assessment of the patient's ailment, the kind and doses of pain medicine required to control pain, and the functional outcomes of using these medications are made at each encounter. The process involves collaboration with multiple specialties, like psychiatry, social work, etc., to administer the right pain medicine in the proper doses.
The strategy adopted in the clinic to prescribe pain medicine involves:
- One person must be assigned to prescribe long-term opioids. They should document all encounters extensively involving the physical exam, lab work, etc.
- Assess each patient for non-SCA-related pain and treat/refer to the appropriate specialty for managing this pain.
- Limit prescribing pain medicines without meeting the patient- every patient must be physically assessed every 2 to 3 months or sooner.
- Develop an individualized pain management plan for each patient, reassess this plan annually, and modify it accordingly.
- Encourage patients to explore alternative methods of controlling pain, like direct massage, self-hypnosis, and music therapy.
Acute Chest Syndrome (ACS): It is an emergency regardless of the sickle cell disease phenotype. It can lead to respiratory failure and death if not managed as an emergency.
- All patients must be hospitalized-
- Upon admission, start treatment with antibiotics, including coverage for atypical bacteria.
- Supplemental oxygen is provided to those with oxygen saturation of less than 95% at room air.
- "Early" administration of simple blood transfusion is recommended for hypoxic patients. However, exchange transfusion is recommended at the earliest opportunity.
- Close monitoring for worsening respiratory status, increasing oxygen requirement, worsening anemia, and bronchospasm (use of beta-adrenergic dilators is encouraged in asthmatics) must be done. Intensive care units must be on standby to receive such patients who experience worsening respiratory status.
- Closely monitor predictors of severity- increasing respiratory rate, worsening hypoxia, decreasing hemoglobin or platelet count, multilobar involvement on chest X-ray, and developing neurological complications.
- Incentive spirometry and hydration (intravenous or oral) must always be encouraged.
- ACS is a strong indicator for initiating disease-modifying therapy (hydroxyurea, etc.) or starting the patient on a chronic blood transfusion program.
Sequestration Crises
- Intravenous fluids for hydration, pain control, and simple/exchange blood transfusion are central to managing sequestration crises.
- Never correct anemia completely- when the crises resolve, and the organs shrink, the sequestered blood re-enters the circulation, leading to increased hematocrit and viscosity, increasing the risk of thrombotic and ischemic events.
- Splenectomy is recommended for patients with life-threatening episode splenic sequestration crises or with recurrent splenic sequestration. It is also offered to those who have baseline hypersplenism.
- Instruct patients and parents in monitoring the size of the liver and spleen regularly.
Acute Stroke: Urgent neurology and transfusion medicine consultation are needed to provide optimal care and prevent long-term damage.
- Simple or exchange blood transfusion emergently.
- Start a program of chronic exchanges or blood transfusion.
- Where blood transfusion cannot be used (iron overload, excessive alloantibodies) or is unavailable, start on long-term disease-modifying therapy. SWiTCH trial demonstrated that chronic transfusions are a better way to manage patients with stroke.
Aplastic Crises: Parvovirus infections cause a transient drop in hemoglobin. Humoral immunity develops within 7 to 10 days that stays for life. The patient is extremely susceptible to developing ACS or stroke during the acute period. Initiate exchange/simple transfusion to bring Hb to a safe level, not necessarily to normal/baseline level.
Infections presenting with fever: Oral empiric antibiotics are given promptly while evaluating the reason for the fever. For ill-appearing patients, admit them and administer intravenous antibiotics.
Priapism: Early recognition is the key to management. Delayed management can lead to impotence. Urologists need to be involved early on in the care of such patients.
- Conservative measures include using analgesics, hydration, and sedation - which usually leads to detumescence and retains potency. Most experts would call for upfront urologic management rather than losing time trying conservative measures. [13]
- Urologists can perform penile aspiration or irrigation of corpora cavernosa with alpha-adrenergic drugs.
- Blood transfusion/ exchange transfusion is not useful - few authors have reported neurological complications with the use of blood transfusion (ASPEN syndrome). Hence it is best to avoid blood transfusion.
Acute ocular Complications: All ocular complications must be managed in consultation with ophthalmologists and hematologists to prevent vision loss.
- Hyphema- Anterior chamber paracentesis or surgical intervention to manage the thrombus must be done promptly.
- Reducing intraocular pressure helps prevent CRAO and other compression issues.
- Infections are managed with prompt administration of antibiotics.
- Corticosteroids are used to relieve excessive pressure in patients with OCS.
Chronic Complications
Avascular Necrosis: About 40 to 80% of cases of hip joint AVN are bilateral; therefore, both joints should be investigated simultaneously. Pain management and physical therapy are to be initiated as early as possible. Advanced cases may require hip arthroplasty.
Leg Ulcer: Conservative measures involve wound care, wet-to-dry dressings, and pain control. Hydroxyurea is avoided in patients with open leg ulcers, as it may prevent healing. Frequent evaluation for the stage of healing or lack of infection, osteomyelitis must be done. Local and systemic antibiotics are used for infected ulcers.
Pulmonary Hypertension: Patients with higher TRV are referred to pulmonologists for management. Small studies have shown increased mortality with sildenafil.
Renal Complications: Refer SCA patients with micro- or microalbuminuria to nephrologists for detailed workup and consideration of angiotensin-converting enzyme inhibitor (ACE-inhibitor). Follow patients closely who have modest elevation in creatinine (>0.7 mg/dL in children, >1.0 mg/dL in adults), and refer to a nephrologist at the earliest sign of worsening creatinine.
Ophthalmologic Complications: Refer SCA patients regularly for ophthalmologic evaluation, especially if they complain of slow vision changes. Direct and indirect ophthalmoscopy, slit-lamp biomicroscopy, and fluorescein angiography are used to evaluate SCA patients. Laser photocoagulation therapy is used to manage proliferative sickle retinopathy. A vitrectomy or retinal repair may be needed in the rare event of vitreal hemorrhage or retinal detachment.
Iron Overload
Unlike hemochromatosis, phlebotomy is not an option in patients with SCA. Preventing iron overload with good transfusion practices is the best way to deal with iron overload. Patients with SCA need not follow the rule of having hemoglobin close to 7gm/dL. Packed RBC transfusion should be restricted to the management of symptoms. Choosing exchange transfusion over simple transfusion also helps to reduce/prevent iron overload.
Indications to start iron chelation therapy
- A liver iron concentration (LIC) greater than 3 mg iron (Fe)/gm dry weight
- Cardiac T2* < 20 milliseconds
- Serum ferritin greater than 1000 on two different occasions 15 days apart
- Age greater than two years
- Expected survival beyond one year
- Number of transfusion of Packed RBC in 1 year- > 10 in pediatric patients OR > 20 in adults.
Goals of therapy
- Serum ferritin < 1000 mcg/L,
- LIC <7mg Fe/gm dry weight
- Cardiac T2* > 20 milliseconds
When do patients need modification of treatment?
- Treatment needs to be intensified if LIC > 15 mg Fe/gm dry weight and deescalated when LIC < 3 mg Fe/gm dry weight.
- Treatment needs to be intensified if serum ferritin > 2500 IU/L and deescalated when serum ferritin < 300 IU/L
- Treatment needs to be intensified when cardiac MRI shows T2* < 15 milliseconds or when cardiac symptoms occur (like heart failure, arrhythmias)
Iron Chelators
- Disperse tab formulation: Initial dose: 10mg/kg/day. Maximum dose: 20mg/kg/day
- Tablet or granule formulation: Initial dose: 7mg/kg/day. Maximum dose: 14mg/kg/day
- It does not interfere with the pharmacodynamics of hydroxyurea; hence it can be used simultaneously.
- Adverse effects- gastrointestinal intolerance, dose-dependent rise in serum creatinine, liver dysfunction.
- Daily subcutaneous infusions via portable infusion pump given over 8 to 24 hours; 1 to 2 gm/day
- It can be given as a daily IV infusion also. 40 to 50 mg/kg/day (max dose 60 mg/kg/day) over 8 to 12 hours (max rate 15 mg/kg/hour)
- IM route is acceptable for children but not preferred for adults. 0.5 to 1mg/day
- Adverse effects- Injection site reactions, cardiovascular shock (if administered too fast), blood dyscrasias, growth retardation.
- Adverse effects - agranulocytosis, hepatotoxicity, gastrointestinal symptoms, and arthralgia.
Blood transfusion: Blood transfusions form an integral part of the management of SCA. The goal of transfusion is to increase the oxygen-carrying capacity of blood and reduce the HbS component. A blood transfusion (simple or exchange) is given to keep the HbS level below 30% (STOP 1 and 2 trials). [14] In patients receiving regular exchange transfusions (history of stroke, intolerance, or contraindication to hydroxyurea), a more practical target for HbS is 25% to prevent a rise of HbS beyond 30%.
What types of blood transfusion are used in SCA?
- Simple transfusion: Transfusion of matched packed red blood cells (PRBC)
- Exchange transfusion: Transfusion of PRBC while removing blood from the patient at the same time.
Who should receive blood transfusions?
- Hb < 7gm/dL or drop of >2 gm/dL from baseline- consider simple or exchange transfusion.
- Twin pregnancy- consider prophylactic exchange transfusion
- Hb less than 9 gm/dL- Simple transfusion
- Hb more than 9gm/dL- Partial exchange transfusion
What kind of transfusion practice should be followed?
- Severe ACS - oxygen saturation less than 90% even when started on supplemental oxygen.
- Multiorgan Failure
- Acute ischemic stroke
- Splenic sequestration - never corrects the anemia completely.
- Acute anemia
Complications from Chronic Transfusions
- Alloimmunization- increases the risk of transfusion reactions, especially delayed hemolytic transfusion reactions.
- Iron overload
- Transmission of blood-borne diseases like hepatitis B, C, and HIV; extremely low risk due to intensive screening of donors and blood products.
- Differential Diagnosis
In general, globin gene mutations affecting hemoglobin are common and affect 7% of the entire world population. [15] Over 1000 variations of hemoglobin exist. However, only a handful of variations are significant clinically.
Common Variants of SCA or HbSS Disease
- Hemoglobin S-beta-0 thalassemia (Clinically behaves exactly like HbSS disease)
- Hemoglobin SC (a milder variant of SCD) - can have a phenotypic presentation of sickle cell anemia
- Hemoglobin S-beta+ thalassemia (a milder variant of SCD)
Several other hemoglobin variants are present that can mimic SCA if they are inherited along with HbS.
- Hemoglobin Jamaica-Plain (beta-68 [E12] Leu -> Phe)
- Hemoglobin Quebec-Chori (beta-87 [F3] Thr > Ile)
- Hemoglobin D-Punjab (beta-globin, codon 121, glutamine to glutamic acid)
- Hemoglobin O-Arab
- Hemoglobin E
Other conditions that can present with hemolysis, where SCA can be ruled out with history, examination, hemoglobin electrophoresis, and study of the peripheral smear
- Antibody-mediated autoimmune hemolytic anemia (both warm and cold antibodies)
- Other hemoglobinopathies- alpha or beta-thalassemia
- Paroxysmal nocturnal hemoglobinuria
- RBC-membrane defects (hereditary spherocytosis, hereditary elliptocytosis)
- Enzyme defects (pyruvate kinase deficiency, glucose-6-phosphate deficiency)
- Drug-induced hemolysis
- Transfusion-related hemolysis (acute or delayed hemolytic reaction)
- Microangiopathic hemolytic anemia (atypical or typical hemolytic uremic syndrome, thrombotic thrombocytopenic purpura)
- Infectious causes (malaria, babesiosis, Rickettsia , Clostridia , Bartonella )
- Vasculitis-induced hemolysis
- Medical Oncology
The goal of disease-modifying therapy in sickle cell anemia is to reduce the frequency of vaso-occlusive crises (VOC) and pain crises and prevent organ damage. These medications usually do not have a role "during" acute crises. Hydroxycarbamide, or hydroxyurea, was the first drug approved by the FDA for use in patients with SCA. However, the USFDA approved hydroxyurea for pediatric patients two years and above only in 2017 (based on the ESCORT HU trial).
Disease-Modifying Drugs/Therapy
The goal of disease-modifying therapy in patients with SCA is to alter the kinetics of sickle erythrocytes. Hydroxyurea does this by increasing the concentration of fetal hemoglobin (HbF).
Hydroxyurea: This is a ribonucleotide reductase inhibitor that increases the concentration of HbF in patients with SCD. It not only increases the intracellular concentration of HbF but also increases the number of erythrocytes containing HbF. In addition to this, hydroxyurea also reduces the number of circulating reticulocytes and leukocytes, raises the volume of an RBC (high MCV is noted in patients receiving hydroxyurea), reduces the deformability of RBC, improves the flow of blood through capillaries, and alters the expression of adhesion molecules hence preventing vaso-occlusive crises. The initial trials with hydroxyurea (Phase-III Multicenter Study of Hydroxyurea in Sickle Cell Anemia (MSH)) demonstrated a clear benefit over placebo in reducing the incidence of pain crises and the cost of care. Long term, the MSH study also showed a mortality benefit. In the pediatric age group, two seminal trials (HUG-KIDS-Phase I/II and BABY HUG-phase III) demonstrated good tolerability and led to the drug's approval. [16] [17]
- Having three or more sickle cell-associated moderate to severe pain crises within a 12-month period; treat with hydroxyurea
- Those with sickle cell-associated pain that interferes with daily activities of living and quality of life
- History of severe and/or recurrent ACS
- Severe symptomatic chronic anemia that interferes with daily activities or quality of life
- Infants 9 months of age and older, children, and adolescents with SCA, offer hydroxyurea regardless of clinical severity to reduce SCA-related complications (e.g., pain, dactylitis, ACS, anemia)
- For those with chronic kidney disease taking erythropoietin and hydroxyurea can be added to improve anemia
- DO NOT give hydroxyurea to pregnant women and lactating mothers who choose to breastfeed their babies
- Dosing for adults: Start with 15 mg/kg/day. Round up to the closest 500 mg. For patients with CKD- start at 5 to 10 mg/kg/day.
- Dosing for infants and children: start at 20 mg/kg/day
- Target absolute neutrophil count (ANC) of above 2000/microL and platelet count above 80,000/microL. In younger patients, an ANC of 1250/microL is allowed if baseline counts are low.
- Monitor blood counts every four weeks when increasing the dose of hydroxyurea.
- Clinical response takes 3 to 6 months to come. Hence a minimal trial of 6 months of daily continued use of hydroxyurea is done before considering alternative therapies.
- Daily adherence is a must. It must be emphasized to the patient.
- If a positive response is seen, then hydroxyurea must be continued indefinitely.
- Myelotoxicity is the most common and most substantiated adverse effect of hydroxyurea. The rest of the adverse effects reported in the literature, especially carcinogenesis and leukemia, have never been demonstrated in large studies.
- Avoid the use of hydroxyurea in patients with leg ulcers.
Voxelotor: Voxelotor acts by inhibiting the polymerization of HbS and increasing the affinity for oxygen. It is dosed at 1500 mg by mouth daily and is approved for SCA treatment in patients 12 years of age and older. Voxelotor can be given with or without hydroxyurea. USFDA approved it in 2019 based on the results of the phase 3 HOPE trial (Hemoglobin Oxygen Affinity Modulation to Inhibit HbS Polymerization) evaluating voxelotor (1500 mg versus 900 mg versus placebo in 1:1:1 design). [18] [19]
The most common adverse reactions are headache, diarrhea, abdominal pain, nausea, fatigue, rash, and pyrexia. Voxelotor interferes with high-performance liquid chromatography (HPLC). Hence the hemoglobin quantification is not accurate when the patient is on voxelotor. HPLC should be done when the patient is off therapy. Also, the use of voxelotor may increase the Hb, but there is no evidence to suggest discontinuation of exchange transfusion in patients receiving this for stroke prophylaxis.
Crizanlizumab: A humanized immunoglobulin G2-Kappa monoclonal antibody inhibits P-selectin, thereby blocking its interaction with P-selecting glycoprotein-1. This leads to reduced interaction between activated endothelium, platelets, leukocytes, and sickled RBCs, leading to reduced VOC. [20] The phase II SUSTAIN trial demonstrated a clinical benefit of Crizanlizumab by demonstrating a reduction in pain crises, VOC, emergency room visits, and increased median time to first crises. Although the hospitalization rate was numerically lower in the intervention group, the difference was not statistically significant compared to the placebo group. [21]
It is approved for the treatment of SCA in patients 16 years of age and older. It is dosed as a 5mg/kg intravenous infusion administered over 30 minutes at weeks 0, 2, and then every four weeks. The most common adverse reactions are nausea, arthralgia, back pain, and pyrexia. Infusion-related reactions can occur. Crizanlizumab can interfere with platelet counts; send the blood immediately before administration or send blood in citrated tubes.
L-Glutamine: Glutamine is the most abundant amino acid in the body. It is not an essential amino acid under normal circumstances, but in patients with SCA, a high hemolysis rate increases the demand for glutamine. L-glutamine is available in a medical formulation. The exact mechanism of action of L-glutamine remains anecdotal. It is believed to work by scavenging for reactive oxygen species and acting as a substrate for the regeneration of nitrous oxide, NAD, and NADH. [22] The USFDA approved L-glutamine in 2017 after positive results from the phase III trial. The authors demonstrated a statistically lower number of pain crises, fewer hospitalizations, fewer cumulative days in the hospital, prolonged time to first and second pain crises, and a reduced number of ACS. [23] Adverse events include constipation, nausea, headache, abdominal pain, cough, extremity pain, back pain, and chest pain. There is an additional concern that L-glutamine may increase mortality and the rate of multiorgan failure. However, these are yet exploratory.
Hematopoietic Stem Cell Transplant
Allogeneic hematopoietic stem cell transplant (HSCT) is a potentially curative option in SCA patients where cure rates approach approximately 90%. Improving the quality of life and reducing the cost of managing long-term complications trumps the cost of performing allogeneic HSC. Pre-school age is considered the best time to perform HSCT, with increased mortality recorded in older patients. A myeloablative or a non-myeloablative regimen can be used; however, myeloablative regimens are not recommended for adults. Matched sibling donor is preferred for performing allogeneic HSCT. Due to the lack of matched sibling donors, other approaches like a matched unrelated donor, umbilical cord blood transplant, and haploidentical transplant are also being explored. [24] [25]
Potential barriers to performing allogeneic HSCT
- Alloimmunization due to repetitive transfusions (exchange of blood)
- Organ dysfunction due to SCA (possibly a reason why younger patients do better)
- Lack of matched sibling donors/ insurance.
Indications for performing allogeneic HSCT
- Stroke (most common and strongest indication to perform allogeneic HSCT.
- Abnormal transcranial doppler
- Acute chest syndrome
- Recurrent VOC not controlled with medical therapy or chronic transfusions
The complications with allogeneic HSCT:
- Transplant-related mortality approaches 7 to 10%, comparable with SCD-related mortality
- Graft rejection OR graft failure - less with myeloablative regimens (7 to 11%) compared to non-myeloablative regimens (11 to 50%)
- Graft-versus-host disease and related morbidity
- Transplant-related complications like lung injury, endocrine, and metabolic adverse events
The recent approvals of newer agents and the emergence of gene-editing techniques have expanded the options for SCA patients. Also, extending the benefit of HSCT to low-income countries remains a significant challenge.
Future Perspectives
Gene editing is a new therapy focus whereby researchers attempt to increase the HbF level in patients with SCA. This technique is being developed alongside HSCT. Many approaches to gene editing are in clinical trials right now. [26] [27]
- Viral gene addition using lentivirus: The technique aims to add a modified beta or gamma-globin gene to reduce the HbS component and increase the HbA (beta-globin gene) or the HbF (gamma-globin gene).
- CRISPR (Clustered regularly interspaced short palindromic repeats): Targets the expression of BCL11A, which normally downregulates gamma-globin expression. By introducing insertions and deletions in the BCL11A erythroid lineage-specific enhancer on chromosome 2, BCL11A is downregulated, resulting in increased expression of the gamma-globin gene, which subsequently increases HbF.
Cost Factor
The annual cost of the voxelotor is approximately $125,000. Each vial of crizanlizumab costs approximately $2400, with a yearly cost of $84,852 and $113,136 per year for most patients. The monthly cost of the L-glutamine formulation is $3000 for adults and up to $1000 for the pediatric age group. A myeloablative regimen for HSCT can lead to a cost of approximately $280,000 at 100 days of care/admission. [28] In addition, the advanced level of expertise and dedicated infrastructure required to deliver such care also comes at a considerably high cost. Considering such high costs for the newer therapies, bringing them to lower-income regions like sub-Saharan Africa is a challenge, where approximately 6 million suffer from sickle cell anemia.
Most of the survival data in patients with SCA does not factor in the advent of new medications. The Cooperative Study of Sickle Cell Disease (CSSCD) (between 1978-88) reported the median age of death for women and men as 42 and 48 years, respectively. This study also showed that acute chest syndrome, renal failure, seizures, high leukocyte count, and low levels of HbF were associated with an increased risk of early death in patients with SCA. [29] More recent studies have shown that elevated tricuspid regurgitant jet velocity on echocardiography, prolonged QTc interval, pulmonary hypertension, high N-terminal pro-brain natriuretic peptide, history of asthma and/or wheezing, history of end-stage renal disease requiring dialysis, and the severity of hemolysis are independent risk factors towards early death in patients with SCA. [30]
More recent data combining nine studies from Europe and North America (evaluating 3257 patients) listed the following as predictors of mortality:
- Age (per 10-year increase in age)
- Tricuspid regurgitant jet velocity 2.5 m/s or more
- Reticulocyte count
- Log(N-terminal-pro-brain natriuretic peptide)
- Fetal hemoglobin [30]
With the approval of newer drugs in 2019 (voxelotor and crizanlizumab), increased use of hematopoietic stem cell transplant, and exploring newer techniques like gene therapy, survival is bound to increase along with the quality of life.
- Complications
SCA can lead to acute complications and chronic complications
Acute complications: Most acute complications are associated with occlusion of the small to medium-sized vessels (sometimes large-sized vessels) due to polymerization of HbS and hemolysis.
- Sequestration crises: splenic or hepatic sequestration
- Fat embolism
- Bone infarction/necrosis
- Coagulopathy: increases the risk of both arterial and venous clots- stroke, myocardial infarction, venous thrombosis
- Ophthalmic: vitreous hemorrhage, retinal detachment, retinal artery/vein occlusion
- Aplastic crises: in association with parvovirus infection
- Papillary necrosis
- Delayed growth and development and growth retardation
- Cardiac: cardiomegaly, cardiomyopathy, left ventricular hypertrophy, arrhythmia, congestive heart failure
- Pulmonary: pulmonary edema, sickle cell lung disease, pulmonary hypertension
- Hepatobiliary: Hepatomegaly, intrahepatic cholestasis, cholelithiasis, viral hepatitis
- Splenic complications: splenomegaly, hyposplenia, asplenia
- Renal: acute and chronic renal failure, pyelonephritis, renal medullary carcinoma
- Musculoskeletal: degenerative changes, osteomyelitis, septic arthritis, osteonecrosis, osteopenia/osteoporosis
- Neurologic: aneurysm, mental retardation
- Ophthalmic: proliferative sickle retinopathy, vitreous hemorrhage, retinal detachment, nonproliferative retinal changes
- Endocrine: primary hypogonadism, hypopituitarism, hypothalamic insufficiency
- Iron overload due to repeated transfusions and chronic hemolysis
- Deterrence and Patient Education
SCA is a debilitating disease that affects a patient physically and has significant emotional and psychiatric consequences. The stigma of being diagnosed with SCA has been well documented. Many SCA patients are inaccurately labeled as drug seekers and opioid abusers due to the need for an inordinately high amount of opioids for pain control. In addition, frequent interactions with different providers (in the emergency rooms, hospital admissions, etc.) can lead to inconsistent care. In such a scenario, the patients need to be an advocate for themselves. The following points can act as a guide for patient education.
- Show consistency in outpatient clinics and show up for your appointments. Regularity in visits to your providers helps to build trust within the system.
- Discuss pain requirements for pain medications with your provider with an open mindset- They may appear restrictive in prescribing pain medications, especially opioids. Still, they are trying to help you by protecting you against overdosing.
- Try and use the same emergency room, or at least the ER within the same hospital system. It is useful and helps in developing familiarity with the people who work in that ER. It also allows easy access to your individualized plan of care, which your provider develops for such situations.
- Adherence to disease-modifying therapy will help reduce the events of pain crises and prevent long-term organ damage.
- Always be receptive to alternative ways of getting control over pain - including music therapy, self-hypnosis, and deep muscle relaxation.
- Patients can adopt protective measures- stay warm and avoid exposure to extreme temperatures, adequate hydration, and breathing exercises at home.
- Enhancing Healthcare Team Outcomes
SCA is a systemic disorder that affects the entire body. The disease not only manifests with physical symptoms (pain crises, organ damage, etc.) but also has numerous psycho-social implications. Most patients with SCA belong to the African-American community and a minority to Hispanic and other communities, which makes them prone to certain prejudices. Besides, the high demand for opioids to manage chronic pain makes the situation even more challenging. [31] All providers must keep aside their inherent prejudice when caring for a patient with SCA, working collaboratively as an interprofessional team. Almost all specialties need to be involved in managing patients with SCA. However, the hematology team dedicated to taking care of SCA patients must be the primary physicians for these patients.
Specialties like ophthalmology, orthopedics, psychiatry, gastroenterology, and cardiovascular medicine interact closely with SCA patients. However, this does not diminish the importance of other specialties. Pharmacy and nursing also play a vital role. With the advent of newer drugs and infusions and SCA affecting liver and kidney function, pharmacists and nursing experts are required to ensure safe dosage and medication delivery to the patient.
The data presented here is derived mostly from large and small randomized clinical trials. [Level 1 and 2] Few aspects of care presented here are from cohort and case-control studies. [Level 3]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Sickle Cell Anemia, Hemoglobin C Contributed by Ed Uthman (CC BY 2.0 https://creativecommons.org/licenses/by/2.0)
Disclosure: Ankit Mangla declares no relevant financial relationships with ineligible companies.
Disclosure: Moavia Ehsan declares no relevant financial relationships with ineligible companies.
Disclosure: Nikki Agarwal declares no relevant financial relationships with ineligible companies.
Disclosure: Smita Maruvada declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Mangla A, Ehsan M, Agarwal N, et al. Sickle Cell Anemia. [Updated 2023 Sep 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Review Sickle Cell Disease. [GeneReviews(®). 1993] Review Sickle Cell Disease. Bender MA, Carlberg K. GeneReviews(®). 1993
- Review Potential role of LSD1 inhibitors in the treatment of sickle cell disease: a review of preclinical animal model data. [Am J Physiol Regul Integr Comp...] Review Potential role of LSD1 inhibitors in the treatment of sickle cell disease: a review of preclinical animal model data. Rivers A, Jagadeeswaran R, Lavelle D. Am J Physiol Regul Integr Comp Physiol. 2018 Oct 1; 315(4):R840-R847. Epub 2018 Aug 1.
- Hematopoietic Stem Cell Transplantation in Sickle Cell Disease. [StatPearls. 2024] Hematopoietic Stem Cell Transplantation in Sickle Cell Disease. Ashorobi D, Naha K, Bhatt R. StatPearls. 2024 Jan
- Sickle Cell Trait. [StatPearls. 2024] Sickle Cell Trait. Ashorobi D, Ramsey A, Yarrarapu SNS, Bhatt R. StatPearls. 2024 Jan
- Rheological properties of sickle erythrocytes in patients with sickle-cell anemia: The effect of hydroxyurea, fetal hemoglobin, and α-thalassemia. [Eur J Haematol. 2018] Rheological properties of sickle erythrocytes in patients with sickle-cell anemia: The effect of hydroxyurea, fetal hemoglobin, and α-thalassemia. Ballas SK, Connes P, Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Eur J Haematol. 2018 Dec; 101(6):798-803. Epub 2018 Oct 9.
Recent Activity
- Sickle Cell Anemia - StatPearls Sickle Cell Anemia - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

IMAGES
VIDEO
COMMENTS
INTRODUCTION. In 1910, sickle cell disease burst onto the Western medical scene as a "strange" or, as Herrick termed it, a "new, unknown disease." 1 Physicians were intrigued by the sickled appearance of the red cells in this disorder, and case reports and analytical papers detailing the clinical features of this disorder appeared to almost always involve people of color. 2-6 The ...
This review provides an overview of the current and emerging techniques for sickle cell disease detection and highlights the different potential methods that could be applied to help the early diagnosis of SCD. Keywords: sickle cell anemia, hemoglobinopathies, detection, diagnosis, point of care. 1.
2 Objective: To identify, critically appraise and synthesise primary research exploring adolescents' experiences of living with sickle cell disease to make recommendations for practice and research. Design: Integrative narrative review Data sources: A systematic search of 10 electronic databases and key journals was conducted to identify studies from the inception of databases to September 2016.
1. Introduction. Sickle cell disease (SCD), an inherited red blood cell disorder, is associated with numerous complications that result in increased morbidity and mortality, and estimated annual medical costs exceeding $1.1 billion. 1-4 Acute vaso-occlusive pain episodes are the main complication of SCD and the most common reason for healthcare encounters. 5 However, some adults with SCD ...
The Journal of Sickle Cell Disease (JSCD) is a leading peer-reviewed, open access journal focusing on the realms of basic, translational, and clinical sciences associated with sickle cell disease. Our primary mission is to publish innovative research findings, fostering an interdisciplinary environment that bridges laboratory research with ...
The Foundation for Sickle Cell Disease Research . At the Foundation for Sickle Cell Disease Research we believe that everybody is born with the right to a long, healthy, pain-free life. With innovative research, treatments, and education, we can change the conversation and shape the future for this genetic disorder. Learn more
Over a lifetime, sickle cell disease can harm a patient's spleen, brain, eyes, lungs, liver, heart, kidneys, penis, joints, bones, or skin. Sickle cell disease is a life-long illness, but the severity of the disease varies widely from person to person. In the early 1970s, the average lifespan was only 14 years.
The National Institutes of Health (NIH) has supported research on sickle cell disease since before the NHLBI was founded in 1948. With each decade that followed, the NHLBI has kept a sustained focus on advancing the understanding of sickle cell disease and improving clinical care. We lead and support research and programs on sickle cell disease ...
Sickle cell disease is an autosomal recessive blood disorder that can lead to anaemia. It is caused by a mutation in the haemoglobin gene, which leads to deformation of red blood cells. Deformed ...
Sickle cell disease (SCD), which affects approximately 100,000 individuals in the USA and more than 3 million worldwide, is caused by mutations in the βb globin gene that result in sickle hemoglobin production. Sickle hemoglobin polymerization leads to red blood cell sickling, chronic hemolysis and vaso-occlusion. Acute and chronic pain as well as end-organ damage occur throughout the ...
Sickle cell disease and its variants constitute the most common inherited blood disorders affecting millions of individuals worldwide. ... Discover the world's research. 25+ million members; 160 ...
Abstract. Sickle cell disease (SCD) is a monogenetic disorder due to a single base-pair point mutation in the β-globin gene resulting in the substitution of the amino acid valine for glutamic acid in the β-globin chain. Phenotypic variation in the clinical presentation and disease outcome is a characteristic feature of the disorder.
In SCD, PhenX efforts have focused on selecting high-quality SCD-related outcome measures to be included in the Toolkit (consensus measures; www.phenxtoolkit.org), guided by the Sickle Cell Disease Research and Scientific Panel. 19 Finally, the Cure Sickle Cell Initiative (CureSCi), led by the NHLBI, has centered on innovating genetic therapies ...
CRISPR-Cas9 Gene Editing for SCD and TDT. 3m 25s. Transfusion-dependent β-thalassemia (TDT) and sickle cell disease (SCD) are the most common monogenic diseases worldwide, with an annual ...
Abstract. ImportanceSickle cell disease (SCD) is an inherited disorder of hemoglobin, characterized by formation of long chains of hemoglobin when deoxygenated within capillary beds, resulting in sickle-shaped red blood cells, progressive multiorgan damage, and increased mortality. An estimated 300 000 infants are born annually worldwide with SCD.
Novel, alternative gene editing approaches for SCD are also in various stages of clinical development, portending more curative-intent options in the future. 3, 4 One of the most innovative approaches relies on a non-integrating, prime-editor-expressing viral vector after granulocyte colony-stimulating factor (G-CSF) mobilization to correct the beta-globin gene mutation in vivo in a mouse ...
Abstract: This paper reviews Sickle cell anaemia.Sickle cell anaemia is a homozygous form of HbS (HbSS).This result. from sin gle point replacement of glut amine by valine at position 6 of β ...
Abstract. Sickle cell disease (SCD) is a hereditary blood disorder characterized by the production of abnormal hemoglobin molecules that cause red blood cells to take on a crescent or sickle shape. This condition affects millions of people worldwide, particularly those of African, Mediterranean, Middle Eastern, and South Asian descent.
passed the National Sickle Cell Disease Control Act in 1972 which called for the establishment of the National Sickle Cell Disease Program. Over the years, this program and others like the Cooperative Study of Sickle Cell Disease (CSSCD), established in 1979, has funded research that has elucidated much of what we know about the disease today [4].
FSCDR's 18th Annual Sickle Cell Disease Research and Educational Symposium and 47th National Sickle Cell Disease Scientific Meeting June 7 - 9, 2024. SUBMIT ABSTRACT. ... For example, if the symposium is June 7 - June 9, 2024 the Journal of Sickle Cell Disease and Hemoglobinopathies publication date is June 7, 2024.
Pathophysiology of Sickle Cell Disease. Sickle cell disease is caused by an abnormal HbS (α 2 β S 2) in which glutamic acid at position 6 of the β-globin chain of hemoglobin is changed to valine. Goldstein et al. (1963) showed that this amino acid substitution arose from a single base change (A>T) at codon 6 (rs334).The genetic causes of SCD include homozygosity for the rs334 mutation (HbSS ...
Explanation. The incidence of primary stroke in children with SCD is 0.6 to 0.8 events per 100 patient-years, with a cumulative incidence of 7.8 percent by age 14 years in the Jamaican cohort and 11 percent by age 20 years in the U.S. Cooperative Study of Sickle Cell Disease. Once stroke has occurred, the incidence of recurrent (secondary ...
A drug approved to treat pulmonary arterial hypertension may be effective at managing hypertension and end-organ damage in patients with sickle cell disease, according to a new study. An early ...
Sickle cell disease (SCD) refers to a group of hemoglobinopathies that include mutations in the gene encoding the beta subunit of hemoglobin. The first description of SCA 'like' disorder was provided by Dr. Africanus Horton in his book The Disease of Tropical Climates and their treatment (1872). However, it was not until 1910 when Dr. James B Herrick and Dr. Ernest Irons reported noticing ...