- Open access
- Published: 04 December 2020

RNA sequencing: new technologies and applications in cancer research
- Mingye Hong 1 na1 ,
- Shuang Tao 2 na1 ,
- Ling Zhang 3 ,
- Li-Ting Diao 2 ,
- Xuanmei Huang 1 ,
- Shaohui Huang 1 ,
- Shu-Juan Xie 2 ,
- Zhen-Dong Xiao 2 &
- Hua Zhang ORCID: orcid.org/0000-0001-9731-2737 1
Journal of Hematology & Oncology volume 13 , Article number: 166 ( 2020 ) Cite this article
65k Accesses
213 Citations
27 Altmetric
Metrics details
Over the past few decades, RNA sequencing has significantly progressed, becoming a paramount approach for transcriptome profiling. The revolution from bulk RNA sequencing to single-molecular, single-cell and spatial transcriptome approaches has enabled increasingly accurate, individual cell resolution incorporated with spatial information. Cancer, a major malignant and heterogeneous lethal disease, remains an enormous challenge in medical research and clinical treatment. As a vital tool, RNA sequencing has been utilized in many aspects of cancer research and therapy, including biomarker discovery and characterization of cancer heterogeneity and evolution, drug resistance, cancer immune microenvironment and immunotherapy, cancer neoantigens and so on. In this review, the latest studies on RNA sequencing technology and their applications in cancer are summarized, and future challenges and opportunities for RNA sequencing technology in cancer applications are discussed.
Cancer remains one of the major malignant diseases that endangers human life and health and comprises complex biological systems that require accurate and comprehensive analysis. Since the first appearance of high-throughput sequencing in 2005 [ 1 ], it has become possible to understand life activities at the molecular level and to conduct detailed research to elucidate the genome and transcriptome. As an essential part of high-throughput sequencing, RNA sequencing (RNA-seq), especially single-cell RNA sequencing (scRNA-seq), provides biological information on a single tumor cell, analyzes the determinants of intratumor expression heterogeneity and identifies the molecular basis of formation of many oncological diseases [ 2 , 3 ]. Thus, RNA sequencing offers invaluable insights for cancer research and treatment. With the advent of the era of precision medicine, RNA sequencing will be widely used for research on many different types of cancer. This review summarizes the history of the development of RNA sequencing and focuses on the latest studies of RNA sequencing technology in cancer applications, especially single-cell RNA sequencing and spatial transcriptome sequencing. In addition, we provide a general introduction to the current bioinformatics analysis tools used for RNA sequencing and discuss future challenges and opportunities for RNA sequencing technology in cancer applications.
The development of RNA sequencing technologies
It was not until 1953 when Watson and Crick proposed the double-helix structure did people truly realize at the molecular level that the essence of life is the result of gene interactions [ 4 ]. The continuous development of RNA sequencing has ushered transcriptome analysis into a new era, with higher efficiency and lower cost. The timeline of RNA sequencing technologies is shown in Fig. 1 .

The development timeline of RNA sequencing technologies
The first-generation sequencing technology is also called Sanger sequencing. The chain termination method was initiated by Sanger in 1977, followed by the chemical degradation method developed by Maxam and Gilbert [ 5 , 6 ]. The same year, Sanger determined the 5368 bp genome of phage φX174, which is the first DNA genome sequenced [ 7 ]. The DNA microarray has aided significant progress in many fields since it was first introduced. However, microarrays require prior knowledge of gene sequences and are unable to identify novel gene expression [ 8 ]. After the first high-throughput sequencing platform appeared in 2005 [ 1 ], multiple next-generation sequencing platforms followed (Table 1 , Figs. 2 , 3 ). The accuracy and reproducibility among different platforms depended on several factors, including the inherent features of the platform and the corresponding analysis pipelines [ 9 , 10 ]. Pyrosequencing that was no longer supported after 2016, developed by 454 Life Sciences, used a “sequencing by synthesis” method [ 1 , 11 , 12 , 13 ]. The ion torrent sequencing platform is also based on the “sequencing by synthesis” method, which outperforms pyrosequencing with respect to sensitivity. SOLiD (Sequencing by Oligonucleotide Ligation and Detection) exhibits high accuracy, as each base is sequenced twice, but the read length is short [ 11 , 12 , 13 ]. DNBS (DNA nanoball sequencing) enables large collection of DNA nanoballs for simultaneous sequencing. Illumina-based sequencing technology represents a “reversible terminator sequencing” method. High-throughput sequencing has the advantage of fast speed, low sequencing cost and high accuracy, otherwise known as next-generation sequencing (NGS). Compared to microarray, it can detect unknown gene expression sequences but is time intensive [ 14 ].
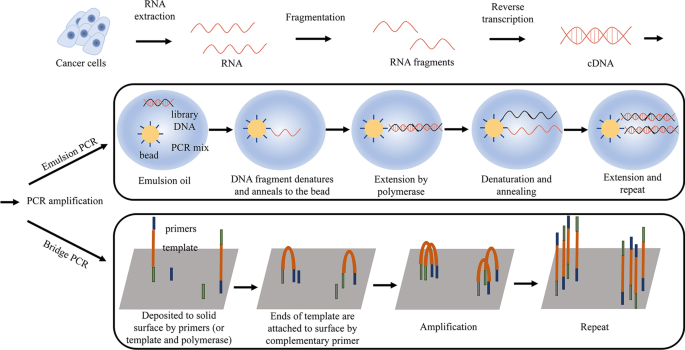
RNA extraction and template preparation before RNA-sequencing. RNA was extracted from tissues, and after fragmentation, fragmented DNA molecules were converted into cDNA by reverse transcription then amplified by emulsion PCR or bridge PCR to prepare sequencing library
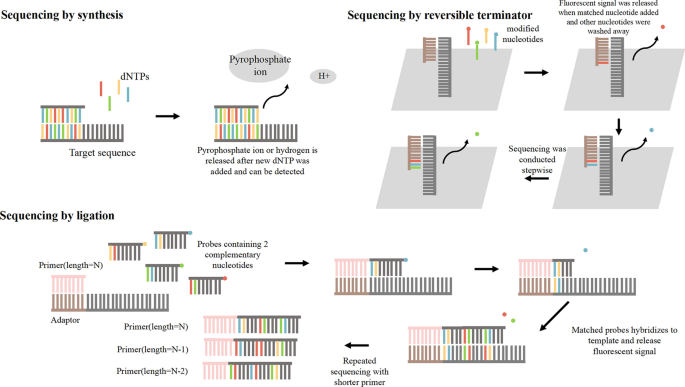
Three kinds of sequencing methods. These methods contain sequencing by synthesis, sequencing by reversible terminator and sequencing by ligation. And their different mechanisms are shown in detail
In addition to NGS, there is third-generation sequencing, which allows for long-read sequencing of individual RNA molecules [ 15 ]. Single-molecule RNA sequencing enables the generation of full-length cDNA transcripts without clonal amplification or transcript assembly. Thus, third-generation sequencing is free from the shortcomings generated by PCR amplification and read mapping. It can greatly reduce the false positive rate of splice sites and capture the diversity of transcript isoforms [ 15 ]. Single-molecule sequencing platforms comprise Pacific Biosciences (PacBio) single-molecule real-time (SMRT) sequencing [ 16 ], Helicos single-molecule fluorescent sequencing [ 17 ] and Oxford Nanopore Technologies (ONT) nanopore sequencing [ 18 ]. Furthermore, RNA-seq recently evolved from bulk sequencing to single-cell sequencing. Single-cell RNA sequencing was first published in 2009 to profile the transcriptome at single-cell resolution [ 19 ]. Drop-Seq and InDrop were initially reported in 2015 by analyzing mouse retina cell and embryonic stem cell transcriptomes, identifying novel cell types. Sci-RNA-seq, single-cell combinatorial indexing RNA sequencing, was developed in 2017, and SPLiT-seq (split-pool ligation-based transcriptome sequencing) was first reported in 2018. Both approaches use a combinatorial indexing strategy in which attached RNAs are labeled with barcodes that indicate their cellular origin [ 20 , 21 ].
Though single-cell data enable single-cell transcriptomics, it may lose spatial information during single-cell isolation. To solve this problem, spatial transcriptomics has emerged. Spatial transcriptomics employs unique positional barcodes to visualize RNA distributions in RNA sequencing of tissue sections and was first published in 2016 [ 22 ]. Slide-seq, reported in 2019, uses DNA barcode beads with specific positional information [ 23 ]. Geo-seq was introduced in 2017 and integrated scRNA-seq with laser capture microdissection (LCM), which can isolate individual cells [ 24 ]. In situ sequencing refers to targeted sequencing of RNA fragments in morphologically preserved tissues or cells without RNA extraction, including in situ cDNA synthesis by padlock probes or stably cross-linked cDNA amplicons in fluorescent in situ RNA sequencing (FISSEQ) and in situ amplification by rolling-circle amplification (RCA) [ 25 , 26 ]. Furthermore, various new technologies based on RNA-seq have been developed for specific applications. For example, a type of targeted RNA sequencing, CaptureSeq, employs biotinylated oligonucleotide probes and results in the enrichment of certain transcripts to identify gene fusion [ 27 , 28 ].
Computational analysis of RNA sequencing data
Computational analysis tools for RNA sequencing have dramatically increased during the past decade. The choice of a particular tool should be based on the purpose and accuracy of application [ 29 , 30 , 31 ]. A general RNA sequencing data analysis process involves the quality control of raw data, read alignment and transcript assembly, expression quantification and differential expression analysis (Fig. 4 ).
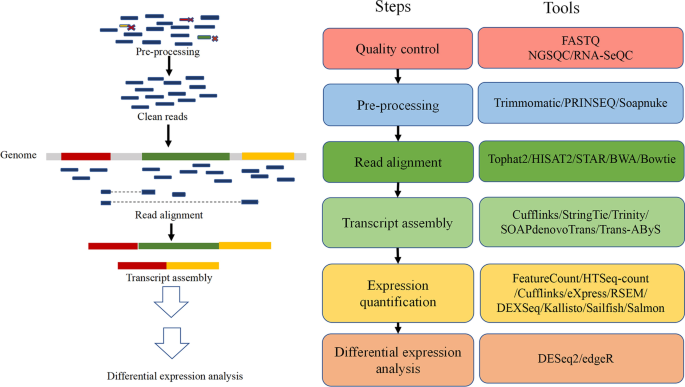
Bioinformatics tools commonly used in RNA-seq data analysis. These tools are primarily used in the four main processes of RNA-seq data analysis, including quality control, read alignment and transcript assembly, expression quantification and differential expression analysis
The first step of data analysis is to assess and clean the raw sequencing data, which is usually provided in the form of FASTQ files [ 32 ]. Quality control visually reflects the quality of the sequencing and purposefully discards low-quality reads, eliminates poor-quality bases and trims adaptor sequences [ 31 ]. Common tools include FASTQ [ 33 ], NGSQC [ 34 ], RNA-SeQC [ 35 ], Trimmomatic [ 36 ], PRINSEQ [ 37 ] and Soapnuke [ 38 ].
The next step is to map the clean reads to either a genome or a transcriptome. There are some mapping tools available, including Tophat2 [ 39 ], HISAT2 [ 40 ], STAR [ 41 ], BWA [ 42 ] and Bowtie [ 43 ]. After alignment, another type of software, such as Cufflinks [ 44 ], StringTie [ 45 ], Trinity [ 46 ], SOAPdenovoTrans [ 47 ] and Trans-AByS [ 48 ] can be used to assemble transcripts from short-reads. When the transcript model is established, its expression can be quantified at the gene, transcript and exon levels. Commonly used software for gene-level quantification includes FeatureCount [ 49 ] and HTSeq-count [ 50 ]. Transcript level quantitative software includes Cufflinks [ 44 ], eXpress [ 51 ] and RSEM [ 52 ]. DEXSeq is a software for exon level quantification [ 53 ]. In addition, there are some alignment-free quantification tools such as Kallisto [ 54 ], Sailfish [ 55 ] and Salmon [ 56 ], which have the advantage of marked computational resource saving. After normalizing, an expression matrix is generated, and statistical methods can be used to identify differentially expressed genes. DESeq2 [ 57 ] and edgeR [ 58 ] are commonly used to perform this task.
Applications of RNA-sequencing in cancer research
Genomic data, such as RNA-seq, have become widely available due to the popularity of high-throughput sequencing technology [ 59 ]. As an important part of next-generation sequencing, RNA sequencing has made great contributions in various fields, especially cancer research, including studies on differential gene expression analysis and cancer biomarkers, cancer heterogeneity and evolution, cancer drug resistance, the cancer microenvironment and immunotherapy, neoantigens, etc. (Fig. 5 ).
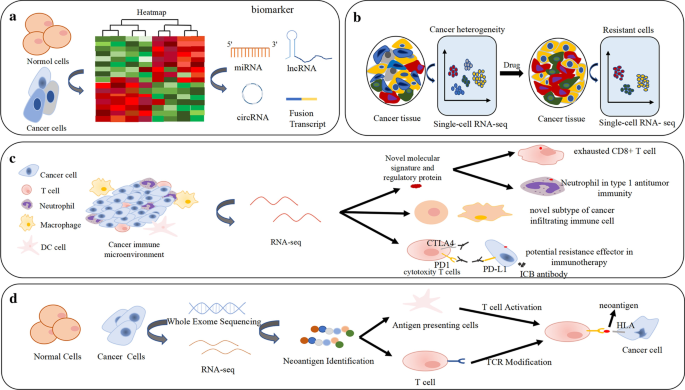
Applications of RNA-seq in differential expression analysis and cancer biomarkers, cancer heterogeneity and drug resistance, cancer immune microenvironment, immunotherapy and neoantigen. a Differential expression analysis by RNA sequencing can identify potential biomarkers, including fusion transcript, lncRNA, miRNA and circRNA. b The heterogeneity and drug resistance of cancer cells identified by RNA-seq. c Novel molecular signature, regulatory protein and unknown subtypes in cancer infiltrating immune cells and potential resistance effector in immunotherapy can be identified by RNA-seq; d Neoantigen profiling by RNA-seq and TCR modification targeted neoantigens
Differential gene expression analysis and cancer biomarkers
Differential gene expression analysis is one of the most common applications of RNA sequencing [ 60 ]. Samples from different backgrounds (different species, tissues and periods) can be used for RNA sequencing to identify differentially expressed genes, revealing their function and potential molecular mechanisms [ 61 ]. More importantly, differential gene expression analysis facilitates the discovery of potential cancer biomarkers [ 62 ]. Many studies have shown that gene fusions are closely related to oncogenesis and are appreciated as both ideal cancer biomarkers and therapeutic targets [ 63 ]. Gene fusions in clinical samples are primarily detected by RNA-CaptureSeq. Compared to whole transcriptome sequencing, RNA-CaptureSeq has significantly higher sequencing depth [ 27 , 64 , 65 ]. It has been reported that the NUP98-PHF23 fusion gene is likely to be a novel therapeutic target in acute myeloid leukemia (AML) [ 66 ]. Recently, a variety of recurrent gene fusions, including ESR1-CCDC170, SEC16A-NOTCH1, SEC22B-NOTCH2 and ESR1-YAP1, have been identified in breast cancer, indicating that recurrent gene fusion is one of the key drivers for cancer [ 67 ]. Several novel configurations of BRAF, NTRK3 and RET gene fusions have been identified in colorectal cancer [ 68 ]. These fusions may promote the development of malignancy and provide new targets for personalized treatment [ 68 ]. In addition, some special genomic factors have been discovered as biomarkers by RNA sequencing, including miRNA, lncRNA and circRNA, which are widely present in various types of cancer [ 69 , 70 , 71 ]. A recent example is circRNA_0001178 and circRNA_0000826, which are biomarkers of colorectal cancer metastasis to the liver [ 72 ]. By applying both RNA sequencing and small RNA sequencing, a study on pancreatic cancer identified differential expression of simple repetitive sequences (SSRs) and demonstrated that the frequency of SSR motifs changed dramatically, which is expected to become a tumor biomarker [ 73 ]. In addition to nucleic acid biomarkers, RNA-seq combined with immunohistochemistry and western blot has also identified certain proteins as cancer biomarkers, such as nuclear COX2 (cyclooxygenase2) in combination with HER2 (human epidermal growth factor receptor type 2), which may serve as potential biomarkers for the diagnosis and prognosis of colorectal cancer [ 74 ]. Similar examples identified using RNA-seq profiling analysis include ISG15 (Interferon-stimulated gene 15) in nasopharyngeal carcinoma [ 75 ] and DMGDH (dimethylglycine dehydrogenase) in hepatocellular carcinoma [ 76 ]. Data-mining analysis of RNA sequencing data and other clinical data has identified that the isoforms of peroxiredoxins also can be expected the prognostic biomarkers for predicting overall survival and relapse-free survival in breast cancer [ 77 ]. Increasing differentially expressed genes are being identified by RNA sequencing, and new potential cancer biomarkers are being continuously discovered (Table 2 ). However, sufficient clinical practice is needed to confirm the diagnostic and predictive applications of these biomarkers in cancer.
RNA-seq could detect early mutations as well as high molecular risk mutations, thus can discover novel cancer biomarkers and potential therapeutic targets, monitoring of diseases and guiding targeted therapy during early treatment decisions. Tumor mutation burden (TMB) is considered as a potential biomarker for immune checkpoint therapy and prognosis [ 78 , 79 ]. RNA-seq can be used to explore the application value of TMB in diffuse glioma [ 78 ]. Through the RNA-seq, MET exon 14 mutation and isocitrate dehydrogenase 1 (IDH1) mutation were identified as new potential therapeutic targets in lung adenocarcinoma and chondrosarcoma patients, respectively [ 80 , 81 ]. Several studies have shown that RNA sequencing can effectively improve the detection rate on the basis of DNA sequencing, provide more comprehensive detection results and achieve a better curative effect for targeted therapy [ 82 ]. In addition, it has been proved that IDH mutation is a good prognostic marker for glioma by RNA-seq [ 83 ]. Targeted therapy is also considered to enhance or replace cytotoxic chemotherapy regimen in cancer including AML [ 84 , 85 , 86 ].
ScRNA-seq also has some new discoveries in diagnosis. For example, scRNA-seq data can be used to infer copy number variations (CNV) and to distinguish malignant from non-malignant cells. The infer CNV algorithm, which was used in the study of glioblastoma, uses averaging relative expression levels over large genomic regions to infer chromosome copy number variation [ 87 ]. Similar examples include head and neck cancer [ 88 ] and human oligodendroglioma [ 89 ]. It is reported that RNA sequence of tumor-educated blood platelets (TEPs) can also become a blood-based cancer diagnosis method [ 90 ]. It should be noted that the lack of detailed functional implications of the identified RNAs in platelets in the field of platelet RNA research is also an urgent problem to be solved [ 91 ].
Cancer heterogeneity and evolution
Heterogeneity has always existed during the transformation of normal cells to cancer cells. The continuous accumulation of heterogeneity may reflect the evolution of cancer [ 109 ]. Early RNA sequencing detected all RNA transcripts in a given tissue or cell group, ignoring differences in individual cells. Transcriptome profiling of single-cell RNA sequencing solves this problem by providing single-cell resolution of the transcriptome [ 3 ]. In melanoma, single-cell RNA-seq was used to analyze 4645 tumor cells from 19 patients, including cancer cells, immune cells, mesenchymal cells and endothelial cells. Transcriptomic data from different single cells revealed that heterogeneity of cells within the same cancer is associated with cell cycle, spatial background and drug resistance [ 110 ]. A recent single-cell RNA-seq study of 49 samples of metastatic lung cancer revealed changes in plasticity induced by non-small cell lung cancer treatment, providing new directions for clinical treatment [ 111 ]. Single-cell RNA sequencing also integrates a variety of information in a single cancer cell, deciphering the secrets of cancer heterogeneity and evolution [ 112 ]. Compared with scRNA-seq, another emerging technology spatial transcriptome sequencing incorporates information on the spatial location of cells. In prostate cancer, using spatial transcriptomics technology, the transcriptome of nearly 6750 tissue regions was analyzed, revealing the whole-tissue gene expression heterogeneity of the entire multifocal prostate cancer and accurately describing the range of cancer foci [ 113 ]. In a study of breast cancer tissues, the results of spatial transcriptome sequencing revealed that gene expression among different regions was surprisingly highly heterogeneous [ 22 ]. In recent years, single-nucleus RNA sequencing (snRNA-seq) has also received extensive attention due to its solving the problem that single-cell RNA sequencing cannot be applied to frozen specimens and cannot obtain all cell types in a given tissue [ 114 , 115 ]. The emerging technology of RNA sequencing will contribute to research on cancer heterogeneity and evolution.
Cancer drug resistance
Drug resistance is a main reason leading to cancer treatment failure. However, the molecular mechanisms underlying drug resistance are still poorly understood [ 116 ]. RNA sequencing became a vital tool for revealing the mechanisms of cancer drug resistance. In breast cancer, single-cell RNA sequencing identified a tumor-infiltrating immunosuppressive immature myeloid cell that leads to drug resistance [ 117 ]. Another study identified a new COX7B gene related to platinum resistance and a surrogate marker CD63 in cancer cells by single-cell RNA-seq [ 118 ]. RNA sequencing has also demonstrated that cancer cells that wake up from a dormant state produce large amounts of BORIS (brother of the regulator of imprinted sites), which can regulate the expression of survival genes in drug-resistant neuroblastoma cells [ 119 ]. Identifying special molecules that mediate these processes could help us understand the occurrence of drug resistance. Single-cell transcriptomics can be used to study different modes of chemoresistance in tumor cells and has shown that pre-existing drug-resistant cells can be selected through higher phenotypic intratumoral heterogeneity, while phenotypic homogeneous cells use other mechanisms to trans-differentiate under drug-selection [ 120 ]. In one study of pancreatic ductal adenocarcinoma, human pancreatic cancer (PANC-1) cells and gemcitabine-resistant PANC-1 cell lines were compared by RNA sequencing, and two circRNAs were identified as both novel biomarkers and potential therapeutic targets for gemcitabine resistant patients [ 121 ]. RNA-seq has also conducted in-depth research on the drug resistance of hematological malignancies. Through RNA-seq, it has been found that non-coding RNAs and fusion genes play an important role in mediating the drug resistance of hematological malignancies [ 122 ]. A good example is to compare the circRNA expression profile of the drug-resistant acute myeloid leukemia cell with its parent cell, and determine the circRNAs involved in drug resistance [ 123 ]. Similarly, the novel MEF2D-BCL9 fusion transcript identified by RNA-seq was found to increase HDAC9 (histone deacetylase 9) expression and to enhance the resistance to dexamethasone in acute lymphocytic leukemia (ALL) [ 124 ]. Leukemia stem cells (LSCs), a rare cell population assumed to be responsible for relapse, is crucial to improve the prognosis of patients [ 125 , 126 ]. RNA-seq analysis showed that LSCs have a unique lncRNA signature with functional relevance and therapeutic potential, providing an explanation for chemotherapy resistance and disease recurrence [ 127 ].
The cancer microenvironment and immunotherapy
The immune system plays a critical role in the cancer microenvironment, affecting several stages of cancer development, including tumorigenesis, progression and metastasis, through tumor-infiltrating lymphocytes (TILs) [ 128 ]. TILs and their interactions with malignant cells and stromal cells make up the cancer immune microenvironment. Due to the heterogeneity of cancer, it is difficult to define the exact pro- or anti-cancer function of certain immune cells. Cancer heterogeneity also causes the varied clinical efficacy observed in patients treated with immunotherapies due to different responses of different subclones [ 129 ]. Transcriptomic profiling by RNA-seq, in particular scRNA-seq, provides comprehensive information on cellular activity and interactions among cells in the tumor microenvironment (TME). ScRNA-seq enables genomic and molecular profiling of high quantity and quality individual immune cells and assessment of cellular heterogeneity to depict the immune system spectrum in the cancer microenvironment [ 130 , 131 , 132 ]. ScRNA-seq data demonstrated that compared to normal tissues, cancer tissues exhibited significantly higher heterogeneity in the immune microenvironment, and a continuity in T cell activation resulting from polyclonal T cells and heterogeneous antigen-presenting cells has been identified [ 133 ].
ScRNA-seq of tumor-infiltrating T cells in metastatic melanoma identified transcription factor NFATC1 (nuclear factor of activated T cells 1) as a potential molecular signature of T cell exhaustion programs and revealed the depletion of low-exhaustion T cells in expanded clones of T cells [ 110 ]. Combining scRNA-seq with assembled T cell receptor (TCR) sequences, 11 T cell subsets, such as CD8 + T cells and CD8 + FOXP3 + regulatory-like cells, and their genomic signatures, were identified in hepatocellular carcinoma (HCC), providing valuable insights for understanding the immune landscape of infiltrating T cells in HCC [ 134 ]. Cancer infiltrating T cells also play an anti-tumor role through impairment of an autophagy protein, LC3 (microtubule-associated protein 1A/1B-light chain 3, often short for LC3)-associated phagocytosis (LAP), demonstrating the role of autophagy in oncogenesis and suppression revealed by scRNA-seq [ 135 ]. In addition to solid tumors, scRNA-seq of acute myeloid leukemia patients detected diverse immunomodulatory genes that suppress T cell function [ 136 ]. By CSOmap, a computational tool for scRNA-seq, the CCL4-CCR8 directed interaction between Tregs and Texs, as well as reduced proliferation of Texs, was characterized [ 137 ]. Notably, findings also revealed that tumor-infiltrating T cells exhibited more interactions among themselves than with T cells from peripheral blood and different interactions between tumors and T cells, indicating a varied response to immunotherapy and a potential trend for immune escape [ 137 ].
In the cancer immune microenvironment, neutrophils, in addition to T cells, are also key components of cancer progression and cancer drug resistance [ 138 , 139 , 140 , 141 ]. Through scRNA-seq of murine sarcomas and certain human cancers, neutrophils with CSF3R (colony stimulating factor 3 receptor) expression were found to be a part of type 1 antitumor immunity associated with unconventional CD4 − CD8 − αβ T cells (UTCαβ) in anti-cancer immunity, indicating better prognosis [ 142 ]. ScRNA-seq of metastatic breast cancer and CD45 cells from primary cancer identified neutrophils as pro- and anti-tumorigenic or metastatic, in which pro-tumorigenic and metastatic neutrophils are induced by IL11 expressing cancer subclones, resulting in polyclonal metastasis [ 143 ]. This observation also provides new insight into anti-cancer immunotherapy by targeting neutrophils [ 143 ]. With scRNA-seq of CD4 and CD8 T cells, several crucial pathways with anti-cancer function were revealed [ 144 ].
The balance between immune reaction and immune tolerance is the basis of immune homeostasis, which is also involved in anti-cancer immunity and oncogenesis. scRNA-seq of monocytes and dendritic cells (DCs) separated from a single lymph node melanoma metastasis revealed a conserved homeostatic module regulated by suppressor-of-cytokine-2 (SOCS2) protein and IFNγ [ 145 ]. SOCS2 serves an essential regulatory role in anti-tumor immunity and T cell priming through DCs. This highly conserved homeostatic program establishes a connection between autoimmune prevention and immune surveillance in cancer [ 145 ].
Immunotherapies, especially immune checkpoint blockade (ICB), has opened a new chapter for anti-cancer therapy with remarkable responses from targeting programmed death 1 (PD1), programmed death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA4) [ 146 , 147 , 148 ]. However, only a few patients benefit from ICB, and severe side effects were observed [ 149 , 150 ]. Obviously, various unknown determinants are correlated with the outcome of immunotherapies in addition to well-known factors such as PD1/PD-L1/CTLA-4 expression and mismatch repair deficiency [ 151 , 152 , 153 , 154 , 155 ]. Therefore, it is paramount to identify potential effectors for ICB efficacy. By analyzing RNA-seq data from melanoma patients who underwent anti-PD1 and anti-CTLA4 treatment, a potential ICB resistance effector SERPINB9 (a member of the serine protease inhibitor (serpin) family) and the connection between cytotoxic T lymphocytes (CTL) infiltration level and ICB response were characterized [ 156 ]. A sub-population cells with immunotherapy persistence have been identified by scRNA-seq and were found to have stem cell-like states with the expression of stem cell antigen-1 (Sca-1) and Snai1 [ 157 ].
Another immunotherapy, myeloid-targeted immunotherapy, is based on the complexity of tumor-infiltrating myeloid cells, including DCs and tumor-associated macrophages (TAMs) revealed by scRNA-seq [ 158 ]. Through scRNA-seq of immune cells from colorectal cancer patients, C1QC + and SPP1 + TAMs, two subsets of TAMs, were identified, and the mechanism of myeloid-targeted immunotherapy, such as anti-CSF1R (colony stimulating factor 1 receptor) and CD40 agonist, was revealed [ 159 ]. Intracellular staining and sequencing (INs-seq), a novel technology integrating scRNA-seq and intracellular protein activity measurements, revealed novel Arg1 + Trem2 + regulatory myeloid (Mreg) cells and demonstrated that depletion of Trem2 led to deduction of exhausted CD8 T cells with increased NK and cytotoxic T cells and cancer suppression by reducing accumulation of intratumoral Mreg cells [ 160 ].
Cancer neoantigens
Neoantigens, human leukocyte antigen (HLA)-bound peptides derived from cancer-specific somatic mutations or gene fusions during tumor growth, are another crucial regulator of the clinical response to immunotherapy [ 161 ]. Higher intratumor neoantigen heterogeneity and clonal neoantigen burden increases sensitivity to ICB and contributes to better clinical outcome in patients with melanoma and advanced non-small cell lung cancer [ 162 ]. This kind of antigen is an optimal target for anti-cancer immunotherapy, enhancing neoantigen-specific T cell activity, and a vaccine targeting personal neoantigens for melanoma patients has been developed [ 163 , 164 ]. Given all these promising features of personalized medicine targeting neoantigens in tumors, massive parallel profiling of tumor neoantigen burden is necessary for improving clinical efficacy and a deeper understanding of the neoantigen landscape. An RNA-seq-based transcriptomic approach is an efficient tool for neoantigen profiling in many studies. It was revealed that homology of neoantigen and somatic-mutation induced pathogens are important in response prediction in anti-CTLA4 treated melanoma [ 165 ]. In addition to melanoma, other studies found reduced neoantigen load in triple-negative and HER2 breast cancers [ 166 ], diverse neoantigen abundance in non-small-cell lung cancer patients with different treatment strategies [ 167 ], a decreased ratio of neoantigen expression to predicted neoantigens in recurrent glioma due to immune selection pressure [ 168 ], a negative correlation between neoantigen abundance and clinical outcome in selected solid tumors [ 169 ] and different neoantigen landscapes in immune filtration and T cell dysfunction based on histology in salivary gland carcinoma (SGC) patients [ 170 ].
A neoantigen prediction program, Neopepsee, based on RNA-seq data and somatic mutation, can be utilized to detect potential neoantigens for personal vaccine development with reduced false-positive rate compared to binding affinity prediction [ 171 ]. ScanNeo is another prediction computational pipeline based on RNA-seq that aims to identify insertion and deletion derived neoantigens, which was validated in prostate cancer [ 172 ], and ASNEO, which identifies personal-specific alternative splicing derived neoantigens [ 173 ]. Several neoantigens have been identified to be related to cancer prognosis and might be potential targets of immunotherapies, such as the TP53 neoantigen for HCC patients [ 174 ]. For the anti-neoantigen immunotherapy in cancer, a new strategy involving neoantigen-specific TCRs modification has been proposed, and scRNA-seq has been applied to isolate neoantigen-specific TCRs for further clinical application [ 175 ].
Conclusions and perspectives
High-throughput RNA-seq technology has been a major tool to explore the transcriptome. The rapid development of RNA-seq technology not only saves time and cost but also sheds light on many new research fields. However, there are still limitations of RNA-seq technology that need to be improved.
For short-read length RNA-seq technologies, bias and imperfections are primarily generated in sequencing library preparation and short read assembly. It is difficult for these methods to correctly identify multiple isoforms from a certain gene. To overcome the disadvantage of short read length, improved read coverage and sequencing depth is required. Long-read length RNA-seq technologies avoid shortcomings in template amplification, reduce the false positive rate in splice junction detection and enable the identification of unannotated longer transcripts, overcoming the common limitations of short-read sequencing [ 176 , 177 ]. However, this method suffers from the drawback of reduced throughput, higher cost and higher sequencing error rate, especially insertion-deletion errors. To reduce random errors, PacBio circular consensus-sequencing (CCS) was developed to increase sequencing depth by rereading molecules several times. However, it also reduces the identification rate of unique isoforms. In addition, the sensitivity of long-read sequencing for identification of differentially expressed genes is lower compared to short-read sequencing [ 178 , 179 , 180 ]. Thus, hybridization of long-read and short-read sequencing has been reported to yield a more comprehensive and accurate analysis [ 181 ].
Improvements in the throughput of RNA sequencing technology have resulted in billions of sequencing reads, bringing great challenges to the computational process, such as data storage, transmission, quality control and data analysis, including read mapping, transcript assembly and read normalization. Therefore, it is important for bioinformatics to keep pace with the continuous developments of RNA-seq technologies. Notably, bias could be produced due to differences in read data handling, necessitating the improvement of current bioinformatics pipelines.
RNA-seq measures gene expression by the read counts, which always containing missing values, thus results in information loss of specific gene and negative impact on downstream analysis. To overcome this problem, missing data need to be imputed and analyzed by several methods, such as optimal clustering with missing values [ 182 ]. For scRNA-seq, the proportion of genes with zero or low expression varies across cells due to biological or technical bias. For example, batch effects can come from cells captured and sequenced in different conditions [ 183 ]. Imputation methods, such as SAVER, MAGIC and kNN-smoothing, are recommended for scRNA-seq [ 184 ]. Another method named batch effects correction with unknown subtypes for scRNA-seq data (BUSseq) utilizes Bayesian hierarchical model and can also be used to correct batch effects and missing data [ 185 ].
Combination of data from multi-omics sequencing can undoubtedly expand the application of RNA-seq. For example, Assay for Transposase-Accessible Chromatin using sequencing (ATAC-seq) was developed by utilizing hyperactive Tn5 transposase to identify open chromatin region and transcriptional factor (TF) binding sites [ 186 ]. The integration of ATAC-seq and RNA-seq enables the reveal of TF-targeted genes and their transcripts [ 187 , 188 ]. Chromatin conformation capture analysis (3C) technology and its several derivatives including circular chromosome conformation capture (4C), carbon copy chromosome conformation capture (5C), ChIP-Loop, Hi-C and capture Hi-C were developed and improved to detect chromatin structure as well as unknown interacting regions [ 189 , 190 , 191 ]. It has been reported that combined analysis of RNA-seq and chromatin structure can detect structure variation-related differentially expressed genes [ 192 , 193 , 194 ].
Epitranscriptomics is a crucial part of gene expression, and methylation of adenosine at the N6 position (m6A) is the most abundant [ 195 ]. Traditional RNA-seq needs reverse transcription before sequencing and thus easily loses the information of transcriptome complexity. This shortcoming can be overcome by directly sequencing native RNA molecules using methods such as nanopore sequencing. Transcript modifications could be inferred from the current signal as the modified RNA molecules passing nanopore cause a characteristic temporary current blockade, which enables the detection of diverse modifications such as m6A or 5-methylcytosine (m5C) [ 196 , 197 , 198 ].
ScRNA-seq is a powerful technology to facilitate further exploration in cancer research and also has been employed in the detection of cancer stem cell subpopulation, metabolic switch in cancer-draining lymph nodes and therapy-induced adaption of cancer cells [ 111 , 199 , 200 ]. Combined with cell sorting or ligand-receptor interaction, scRNA-seq was utilized in cellular interaction, cell spatial organization as well as molecular crosstalk characterization [ 137 , 201 , 202 ]. Coupling of parallel CRISPR (clustered regularly interspaced short palindromic repeats)-pooled screen, scRNA-seq enables the simultaneous analysis of genomic perturbation and transcriptional activity to detect heterogeneous cell type as well as crucial factors of complexity regulatory mechanism [ 203 , 204 , 205 ]. ScNT-seq, single-cell metabolically labeled new RNA tagging sequencing, brings RNA-seq into time resolution by identifying RNAs transcribed at different stage [ 206 ]. Utilizing SNP-based demultiplexing of scRNA-seq data, MIX-Seq was developed to study cancer cell reaction to pharmacologic treatment [ 207 ]. Another technology, snRNA-seq, is invaluable for detecting cellular heterogeneity of cancer and has been employed to identification of a sub-population of adipocytes regulating cancer genesis [ 208 ].
Taken together, RNA-seq has been applied in an impressively wide range of cancer research. All applications in cancer research rely on the boost of advanced RNA-seq technologies, especially the combination of scRNA-seq and spatial transcriptomics as well as data from multi-omics, which will bring RNA-seq technologies into single-cell resolution and tissue-level transcriptomics, providing new insight into cancer diagnosis, treatment and prevention.
Availability of data and materials
Not applicable.
Abbreviations
- RNA sequencing
Single-cell RNA sequencing
Sequencing by Oligonucleotide Ligation and Detection
DNA nanoball sequencing
Next-generation sequencing
Pacific Biosciences single-molecule real-time
Oxford Nanopore Technologies
Single-cell combinatorial indexing RNA sequencing
Split-pool ligation-based transcriptome sequencing
Laser capture microdissection
Fluorescent in situ RNA sequencing
Rolling-circle amplification
Acute myeloid leukemia
Simple repetitive sequences
Cyclooxygenase2
Human epidermal growth factor receptor type 2
Interferon-stimulated gene 15
Dimethylglycine dehydrogenase
Tumor mutation burden
Isocitrate dehydrogenase
Copy number variations
Tumor-educated blood platelets
Single-nucleus RNA-sequencing
Brother of the regulator of imprinted sites
Acute lymphocytic leukemia
Leukemia stem cells
Tumor-infiltrating lymphocytes
Tumor microenvironment
Nuclear factor of activated T cells 1
T cell receptor
Hepatocellular carcinoma
Microtubule-associated protein 1A/1B-light chain 3-associated phagocytosis
Colony stimulating factor 3 receptor
Unconventional CD4 − CD8 − αβ T cells
Dendritic cells
Suppressor-of-cytokine-2 protein
Immune checkpoint blockade
Targeting programmed death 1
Programmed death-ligand 1
Cytotoxic T-lymphocyte-associated protein 4
Cytotoxic T lymphocytes
Stem cell antigen-1
Tumor-associated macrophages
Colony stimulating factor 1 receptor
Intracellular staining and sequencing
Regulatory myeloid cells
Human leukocyte antigen
Salivary gland carcinoma
Circular consensus-sequencing
Assay for Transposase-Accessible Chromatin using sequencing
Transcriptional factor
Chromatin conformation capture analysis
Circular chromosome conformation capture
Carbon copy chromosome conformation capture
N6 position
5-Methylcytosine
Clustered regularly interspaced short palindromic repeats
Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437:376–80.
Article CAS PubMed PubMed Central Google Scholar
Cieślik M, Chinnaiyan AM. Cancer transcriptome profiling at the juncture of clinical translation. Nat Rev Genet. 2018;19:93–109.
Article PubMed CAS Google Scholar
Suvà ML, Tirosh I. Single-cell RNA sequencing in cancer: lessons learned and emerging challenges. Mol Cell. 2019;75:7–12.
Watson JD, Crick FH. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature. 1953;171:737–8.
Article CAS PubMed Google Scholar
Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–7.
Maxam AM, Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci USA. 1977;74:560–4.
Sanger F, Air GM, Barrell BG, Brown NL, Coulson AR, Fiddes CA, et al. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977;265:687–95.
Russo G, Zegar C, Giordano A. Advantages and limitations of microarray technology in human cancer. Oncogene. 2003;22:6497–507.
Li S, Tighe S, Nicolet C, Grove D, Levy S, Farmerie W, et al. Multi-platform assessment of transcriptome profiling using RNA-seq in the ABRF next-generation sequencing study. Nat Biotechnol. 2014;32:915–25.
Article PubMed PubMed Central CAS Google Scholar
A comprehensive assessment of RNA-seq accuracy, reproducibility and information content by the Sequencing Quality Control Consortium. Nat Biotechnol. 2014;32:903–14.
Mardis E. Next-generation DNA sequencing methods. Annu Rev Genomics Hum Genet. 2008;9:387–402.
Shendure J, Ji H. Next-generation DNA sequencing. Nat Biotechnol. 2008;26:1135–45.
Mardis E. Next-generation sequencing platforms. Annu Rev Anal Chem (Palo Alto Calif). 2013;6:287–303.
Article CAS Google Scholar
Marioni JC, Mason CE, Mane SM, Stephens M, Gilad Y. RNA-seq: an assessment of technical reproducibility and comparison with gene expression arrays. Genome Res. 2008;18:1509–17.
Schadt EE, Turner S, Kasarskis A. A window into third-generation sequencing. Hum Mol Genet. 2010;19:R227–40.
Eid J, Fehr A, Gray J, Luong K, Lyle J, Otto G, et al. Real-time DNA sequencing from single polymerase molecules. Science. 2009;323:133–8.
Hart C, Lipson D, Ozsolak F, Raz T, Steinmann K, Thompson J, et al. Single-molecule sequencing: sequence methods to enable accurate quantitation. Methods Enzymol. 2010;472:407–30.
Bayley H. Nanopore sequencing: from imagination to reality. Clin Chem. 2015;61:25–31.
Tang F, Barbacioru C, Wang Y, Nordman E, Lee C, Xu N, et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nat Methods. 2009;6:377–82.
Cao J, Packer J, Ramani V, Cusanovich D, Huynh C, Daza R, et al. Comprehensive single-cell transcriptional profiling of a multicellular organism. Science. 2017;357:661–7.
Rosenberg A, Roco C, Muscat R, Kuchina A, Sample P, Yao Z, et al. Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science. 2018;360:176–82.
Ståhl P, Salmén F, Vickovic S, Lundmark A, Navarro J, Magnusson J, et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science. 2016;353:78–82.
Rodriques SG, Stickels RR, Goeva A, Martin CA, Murray E, Vanderburg CR, et al. Slide-seq: a scalable technology for measuring genome-wide expression at high spatial resolution. Science. 2019;363:1463–7.
Chen J, Suo S, Tam PP, Han JJ, Peng G, Jing N. Spatial transcriptomic analysis of cryosectioned tissue samples with geo-seq. Nat Protoc. 2017;12:566–80.
Ke R, Mignardi M, Pacureanu A, Svedlund J, Botling J, Wählby C, et al. In situ sequencing for RNA analysis in preserved tissue and cells. Nat Methods. 2013;10:857–60.
Lee JH, Daugharthy ER, Scheiman J, Kalhor R, Yang JL, Ferrante TC, et al. Highly multiplexed subcellular RNA sequencing in situ. Science. 2014;343:1360–3.
Mercer TR, Gerhardt DJ, Dinger ME, Crawford J, Trapnell C, Jeddeloh JA, et al. Targeted RNA sequencing reveals the deep complexity of the human transcriptome. Nat Biotechnol. 2011;30:99–104.
Heyer EE, Deveson IW, Wooi D, Selinger CI, Lyons RJ, Hayes VM, et al. Diagnosis of fusion genes using targeted RNA sequencing. Nat Commun. 2019;10:1388.
Soverini S, Abruzzese E, Bocchia M, Bonifacio M, Galimberti S, Gozzini A, et al. Next-generation sequencing for BCR-ABL1 kinase domain mutation testing in patients with chronic myeloid leukemia: a position paper. J Hematol Oncol. 2019;12:131.
Article PubMed PubMed Central Google Scholar
Chatterjee A, Ahn A, Rodger EJ, Stockwell PA, Eccles MR. A guide for designing and analyzing RNA-seq data. Methods Mol Biol. 2018;1783:35–80.
Article PubMed Google Scholar
Conesa A, Madrigal P, Tarazona S, Gomez-Cabrero D, Cervera A, McPherson A, et al. A survey of best practices for RNA-seq data analysis. Genome Biol. 2016;17:13.
Cock PJ, Fields CJ, Goto N, Heuer ML, Rice PM. The Sanger FASTQ file format for sequences with quality scores, and the Solexa/Illumina FASTQ variants. Nucleic Acids Res. 2010;38:1767–71.
Chen S, Zhou Y, Chen Y, Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–90.
Patel RK, Jain M. NGS QC Toolkit: a toolkit for quality control of next generation sequencing data. PLoS ONE. 2012;7:e30619.
DeLuca DS, Levin JZ, Sivachenko A, Fennell T, Nazaire MD, Williams C, et al. RNA-SeQC: RNA-seq metrics for quality control and process optimization. Bioinformatics. 2012;28:1530–2.
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–20.
Schmieder R, Edwards R. Quality control and preprocessing of metagenomic datasets. Bioinformatics. 2011;27:863–4.
Chen Y, Chen Y, Shi C, Huang Z, Zhang Y, Li S, et al. SOAPnuke: a MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. Gigascience. 2018;7:1–6.
PubMed PubMed Central Google Scholar
Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36.
Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12:357–60.
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21.
Li H, Durbin R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics. 2009;25:1754–60.
Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25.
Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–5.
Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, Salzberg SL. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol. 2015;33:290–5.
Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011;29:644–52.
Hurgobin B. Short read alignment using SOAP2. Methods Mol Biol. 2016;1374:241–52.
Robertson G, Schein J, Chiu R, Corbett R, Field M, Jackman SD, et al. De novo assembly and analysis of RNA-seq data. Nat Methods. 2010;7:909–12.
Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–30.
Anders S, Pyl PT, Huber W. HTSeq: a python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–9.
Roberts A, Pachter L. Streaming fragment assignment for real-time analysis of sequencing experiments. Nat Methods. 2013;10:71–3.
Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011;12:323.
Anders S, Reyes A, Huber W. Detecting differential usage of exons from RNA-seq data. Genome Res. 2012;22:2008–17.
Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol. 2016;34:525–7.
Patro R, Mount SM, Kingsford C. Sailfish enables alignment-free isoform quantification from RNA-seq reads using lightweight algorithms. Nat Biotechnol. 2014;32:462–4.
Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods. 2017;14:417–9.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550.
Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–40.
Moon M, Nakai K. Stable feature selection based on the ensemble L (1)-norm support vector machine for biomarker discovery. BMC Genomics. 2016;17:1026.
Zubovic L, Piazza S, Tebaldi T, Cozzuto L, Palazzo G, Sidarovich V, et al. The altered transcriptome of pediatric myelodysplastic syndrome revealed by RNA sequencing. J Hematol Oncol. 2020;13:135.
Oshlack A, Robinson MD, Young MD. From RNA-seq reads to differential expression results. Genome Biol. 2010;11:220.
Govindarajan M, Wohlmuth C, Waas M, Bernardini MQ, Kislinger T. High-throughput approaches for precision medicine in high-grade serous ovarian cancer. J Hematol Oncol. 2020;13:134.
Wu H, Li X, Li H. Gene fusions and chimeric RNAs, and their implications in cancer. Genes Dis. 2019;6:385–90.
Reeser JW, Martin D, Miya J, Kautto EA, Lyon E, Zhu E, et al. Validation of a targeted RNA sequencing assay for kinase fusion detection in solid tumors. J Mol Diagn. 2017;19:682–96.
Mercer TR, Clark MB, Crawford J, Brunck ME, Gerhardt DJ, Taft RJ, et al. Targeted sequencing for gene discovery and quantification using RNA CaptureSeq. Nat Protoc. 2014;9:989–1009.
Togni M, Masetti R, Pigazzi M, Astolfi A, Zama D, Indio V, et al. Identification of the NUP98-PHF23 fusion gene in pediatric cytogenetically normal acute myeloid leukemia by whole-transcriptome sequencing. J Hematol Oncol. 2015;8:69.
Veeraraghavan J, Ma J, Hu Y, Wang XS. Recurrent and pathological gene fusions in breast cancer: current advances in genomic discovery and clinical implications. Breast Cancer Res Treat. 2016;158:219–32.
Kloosterman WP, Coebergh van den Braak RRJ, Pieterse M, van Roosmalen MJ, Sieuwerts AM, Stangl C, et al. A systematic analysis of oncogenic gene fusions in primary colon cancer. Cancer Res. 2017;77:3814–22.
Sun YM, Chen YQ. Principles and innovative technologies for decrypting noncoding RNAs: from discovery and functional prediction to clinical application. J Hematol Oncol. 2020;13:109.
Zhou X, Zhan L, Huang K, Wang X. The functions and clinical significance of circRNAs in hematological malignancies. J Hematol Oncol. 2020;13:138.
Liu Y, Cheng Z, Pang Y, Cui L, Qian T, Quan L, et al. Role of microRNAs, circRNAs and long noncoding RNAs in acute myeloid leukemia. J Hematol Oncol. 2019;12:51.
Xu H, Wang C, Song H, Xu Y, Ji G. RNA-Seq profiling of circular RNAs in human colorectal Cancer liver metastasis and the potential biomarkers. Mol Cancer. 2019;18:8.
Alisoltani A, Fallahi H, Shiran B, Alisoltani A, Ebrahimie E. RNA-seq SSRs and small RNA-seq SSRs: new approaches in cancer biomarker discovery. Gene. 2015;560:34–43.
Zhou FF, Huang R, Jiang J, Zeng XH, Zou SQ. Correlated non-nuclear COX2 and low HER2 expression confers a good prognosis in colorectal cancer. Saudi J Gastroenterol. 2018;24:301–6.
Chen RH, Du Y, Han P, Wang HB, Liang FY, Feng GK, et al. ISG15 predicts poor prognosis and promotes cancer stem cell phenotype in nasopharyngeal carcinoma. Oncotarget. 2016;7:16910–22.
Liu G, Hou G, Li L, Li Y, Zhou W, Liu L. Potential diagnostic and prognostic marker dimethylglycine dehydrogenase (DMGDH) suppresses hepatocellular carcinoma metastasis in vitro and in vivo. Oncotarget. 2016;7:32607–16.
Mei J, Hao L, Liu X, Sun G, Xu R, Wang H, et al. Comprehensive analysis of peroxiredoxins expression profiles and prognostic values in breast cancer. Biomark Res. 2019;7:16.
Wang L, Ge J, Lan Y, Shi Y, Luo Y, Tan Y, et al. Tumor mutational burden is associated with poor outcomes in diffuse glioma. BMC Cancer. 2020;20:213.
Jiang T, Shi J, Dong Z, Hou L, Zhao C, Li X, et al. Genomic landscape and its correlations with tumor mutational burden, PD-L1 expression, and immune cells infiltration in Chinese lung squamous cell carcinoma. J Hematol Oncol. 2019;12:75.
Seo JS, Ju YS, Lee WC, Shin JY, Lee JK, Bleazard T, et al. The transcriptional landscape and mutational profile of lung adenocarcinoma. Genome Res. 2012;22:2109–19.
Nakagawa M, Nakatani F, Matsunaga H, Seki T, Endo M, Ogawara Y, et al. Selective inhibition of mutant IDH1 by DS-1001b ameliorates aberrant histone modifications and impairs tumor activity in chondrosarcoma. Oncogene. 2019;38:6835–49.
Davies KD, Lomboy A, Lawrence CA, Yourshaw M, Bocsi GT, Camidge DR, et al. DNA-based versus RNA-based detection of MET Exon 14 skipping events in lung cancer. J Thorac Oncol. 2019;14:737–41.
Unruh D, Zewde M, Buss A, Drumm MR, Tran AN, Scholtens DM, et al. Methylation and transcription patterns are distinct in IDH mutant gliomas compared to other IDH mutant cancers. Sci Rep. 2019;9:8946.
Yu J, Jiang PYZ, Sun H, Zhang X, Jiang Z, Li Y, et al. Advances in targeted therapy for acute myeloid leukemia. Biomark Res. 2020;8:17.
Yang X, Wang J. Precision therapy for acute myeloid leukemia. J Hematol Oncol. 2018;11:3.
Gu R, Yang X, Wei H. Molecular landscape and targeted therapy of acute myeloid leukemia. Biomark Res. 2018;6:32.
Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344:1396–401.
Puram SV, Tirosh I, Parikh AS, Patel AP, Yizhak K, Gillespie S, et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell. 2017;171(1611–24):e24.
Google Scholar
Tirosh I, Venteicher AS, Hebert C, Escalante LE, Patel AP, Yizhak K, et al. Single-cell RNA-seq supports a developmental hierarchy in human oligodendroglioma. Nature. 2016;539:309–13.
Best MG, Sol N, Kooi I, Tannous J, Westerman BA, Rustenburg F, et al. RNA-seq of tumor-educated platelets enables blood-based pan-cancer, multiclass, and molecular pathway cancer diagnostics. Cancer Cell. 2015;28:666–76.
Best MG, Vancura A, Wurdinger T. Platelet RNA as a circulating biomarker trove for cancer diagnostics. J Thromb Haemost. 2017;15:1295–306.
Zhu L, Li J, Gong Y, Wu Q, Tan S, Sun D, et al. Exosomal tRNA-derived small RNA as a promising biomarker for cancer diagnosis. Mol Cancer. 2019;18:74.
Nie Y, Jiao Y, Li Y, Li W. Investigation of the clinical significance and prognostic value of the lncRNA ACVR2B-As1 in liver cancer. Biomed Res Int. 2019;2019:4602371.
Gong W, Yang L, Wang Y, Xian J, Qiu F, Liu L, et al. Analysis of survival-related lncRNA landscape identifies a role for LINC01537 in energy metabolism and lung cancer progression. Int J Mol Sci. 2019;20.
Hang D, Zhou J, Qin N, Zhou W, Ma H, Jin G, et al. A novel plasma circular RNA circFARSA is a potential biomarker for non-small cell lung cancer. Cancer Med. 2018;7:2783–91.
Hua Q, Jin M, Mi B, Xu F, Li T, Zhao L, et al. LINC01123, a c-Myc-activated long non-coding RNA, promotes proliferation and aerobic glycolysis of non-small cell lung cancer through miR-199a-5p/c-Myc axis. J Hematol Oncol. 2019;12:91.
Wang Z, Qin B. Prognostic and clinicopathological significance of long noncoding RNA CTD-2510F5.4 in gastric cancer. Gastric Cancer. 2019;22:692–704.
Luo T, Zhao J, Lu Z, Bi J, Pang T, Cui H, et al. Characterization of long non-coding RNAs and MEF2C-AS1 identified as a novel biomarker in diffuse gastric cancer. Transl Oncol. 2018;11:1080–9.
Wang D, Wan X, Zhang Y, Kong Z, Lu Y, Sun X, et al. A novel androgen-reduced prostate-specific lncRNA, PSLNR, inhibits prostate-cancer progression in part by regulating the p53-dependent pathway. Prostate. 2019;79:1362–77.
CAS PubMed Google Scholar
Silva-Fisher JM, Dang HX, White NM, Strand MS, Krasnick BA, Rozycki EB, et al. Long non-coding RNA RAMS11 promotes metastatic colorectal cancer progression. Nat Commun. 2020;11:2156.
Yamada A, Yu P, Lin W, Okugawa Y, Boland CR, Goel A. A RNA-Sequencing approach for the identification of novel long non-coding RNA biomarkers in colorectal cancer. Sci Rep. 2018;8:575.
Bo H, Fan L, Li J, Liu Z, Zhang S, Shi L, et al. High Expression of lncRNA AFAP1-AS1 Promotes the Progression of Colon Cancer and Predicts Poor Prognosis. J Cancer. 2018;9:4677–83.
Chen Q, Hu L, Chen K. Construction of a nomogram based on a hypoxia-related lncRNA signature to improve the prediction of gastric cancer prognosis. Front Genet. 2020;11:570325.
Guo YZ, Sun HH, Wang XT, Wang MT. Transcriptomic analysis reveals key lncRNAs associated with ribosomal biogenesis and epidermis differentiation in head and neck squamous cell carcinoma. J Zhejiang Univ Sci B. 2018;19:674–88.
Yao Y, Chen X, Lu S, Zhou C, Xu G, Yan Z, et al. Circulating long noncoding RNAs as biomarkers for predicting head and neck squamous cell carcinoma. Cell Physiol Biochem. 2018;50:1429–40.
Gong X, Siprashvili Z, Eminaga O, Shen Z, Sato Y, Kume H, et al. Novel lincRNA SLINKY is a prognostic biomarker in kidney cancer. Oncotarget. 2017;8:18657–69.
James AR, Schroeder MP, Neumann M, Bastian L, Eckert C, Gökbuget N, et al. Long non-coding RNAs defining major subtypes of B cell precursor acute lymphoblastic leukemia. J Hematol Oncol. 2019;12:8.
Li S, Ma Y, Tan Y, Ma X, Zhao M, Chen B, et al. Profiling and functional analysis of circular RNAs in acute promyelocytic leukemia and their dynamic regulation during all-trans retinoic acid treatment. Cell Death Dis. 2018;9:651.
Guo M, Peng Y, Gao A, Du C, Herman JG. Epigenetic heterogeneity in cancer. Biomark Res. 2019;7:23.
Tirosh I, Izar B, Prakadan SM, Wadsworth MH 2nd, Treacy D, Trombetta JJ, et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science. 2016;352:189–96.
Maynard A, McCoach CE, Rotow JK, Harris L, Haderk F, Kerr DL, et al. Therapy-induced evolution of human lung cancer revealed by single-cell RNA sequencing. Cell. 2020;182(1232–51):e22.
Nam AS, Chaligne R, Landau DA. Integrating genetic and non-genetic determinants of cancer evolution by single-cell multi-omics. Nat Rev Genet. 2020.
Berglund E, Maaskola J, Schultz N, Friedrich S, Marklund M, Bergenstråhle J, et al. Spatial maps of prostate cancer transcriptomes reveal an unexplored landscape of heterogeneity. Nat Commun. 2018;9:2419.
Bakken TE, Hodge RD, Miller JA, Yao Z, Nguyen TN, Aevermann B, et al. Single-nucleus and single-cell transcriptomes compared in matched cortical cell types. PLoS ONE. 2018;13:e0209648.
Selewa A, Dohn R, Eckart H, Lozano S, Xie B, Gauchat E, et al. Systematic comparison of high-throughput single-cell and single-nucleus transcriptomes during cardiomyocyte differentiation. Sci Rep. 2020;10:1535.
Lim ZF, Ma PC. Emerging insights of tumor heterogeneity and drug resistance mechanisms in lung cancer targeted therapy. J Hematol Oncol. 2019;12:134.
Wang Q, Guldner IH, Golomb SM, Sun L, Harris JA, Lu X, et al. Single-cell profiling guided combinatorial immunotherapy for fast-evolving CDK4/6 inhibitor-resistant HER2-positive breast cancer. Nat Commun. 2019;10:3817.
Tanaka N, Katayama S, Reddy A, Nishimura K, Niwa N, Hongo H, et al. Single-cell RNA-seq analysis reveals the platinum resistance gene COX7B and the surrogate marker CD63. Cancer Med. 2018;7:6193–204.
Debruyne DN, Dries R, Sengupta S, Seruggia D, Gao Y, Sharma B, et al. BORIS promotes chromatin regulatory interactions in treatment-resistant cancer cells. Nature. 2019;572:676–80.
Sharma A, Cao EY, Kumar V, Zhang X, Leong HS, Wong AML, et al. Longitudinal single-cell RNA sequencing of patient-derived primary cells reveals drug-induced infidelity in stem cell hierarchy. Nat Commun. 2018;9:4931.
Shao F, Huang M, Meng F, Huang Q. Circular RNA SIgnature predicts gemcitabine resistance of pancreatic ductal adenocarcinoma. Front Pharmacol. 2018;9:584.
Wang WT, Han C, Sun YM, Chen TQ, Chen YQ. Noncoding RNAs in cancer therapy resistance and targeted drug development. J Hematol Oncol. 2019;12:55.
Shang J, Chen WM, Liu S, Wang ZH, Wei TN, Chen ZZ, et al. CircPAN3 contributes to drug resistance in acute myeloid leukemia through regulation of autophagy. Leuk Res. 2019;85:106198.
Suzuki K, Okuno Y, Kawashima N, Muramatsu H, Okuno T, Wang X, et al. MEF2D-BCL9 fusion gene is associated with high-risk acute B-cell precursor lymphoblastic leukemia in adolescents. J Clin Oncol. 2016;34:3451–9.
Pallarès V, Unzueta U, Falgàs A, Sánchez-García L, Serna N, Gallardo A, et al. An Auristatin nanoconjugate targeting CXCR4+ leukemic cells blocks acute myeloid leukemia dissemination. J Hematol Oncol. 2020;13:36.
Ding Y, Gao H, Zhang Y, Li Y, Vasdev N, Gao Y, et al. Alantolactone selectively ablates acute myeloid leukemia stem and progenitor cells. J Hematol Oncol. 2016;9:93.
Bill M, Papaioannou D, Karunasiri M, Kohlschmidt J, Pepe F, Walker CJ, et al. Expression and functional relevance of long non-coding RNAs in acute myeloid leukemia stem cells. Leukemia. 2019;33:2169–82.
Fan X, Rudensky AY. Hallmarks of tissue-resident lymphocytes. Cell. 2016;164:1198–211.
Burrell RA, Swanton C. Re-evaluating clonal dominance in cancer evolution. Trends Cancer. 2016;2:263–76.
Shalek AK, Satija R, Adiconis X, Gertner RS, Gaublomme JT, Raychowdhury R, et al. Single-cell transcriptomics reveals bimodality in expression and splicing in immune cells. Nature. 2013;498:236–40.
Pan Y, Lu F, Fei Q, Yu X, Xiong P, Yu X, et al. Single-cell RNA sequencing reveals compartmental remodeling of tumor-infiltrating immune cells induced by anti-CD47 targeting in pancreatic cancer. J Hematol Oncol. 2019;12:124.
Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell. 2015;161:1202–14.
Azizi E, Carr AJ, Plitas G, Cornish AE, Konopacki C, Prabhakaran S, et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell. 2018;174:1293–308.
Zheng C, Zheng L, Yoo JK, Guo H, Zhang Y, Guo X, et al. Landscape of infiltrating T cells in liver cancer revealed by single-cell sequencing. Cell. 2017;169:1342–56.
Cunha LD, Yang M, Carter R, Guy C, Harris L, Crawford JC, et al. LC3-associated phagocytosis in myeloid cells promotes tumor immune tolerance. Cell. 2018;175:429–41.
van Galen P, Hovestadt V, Wadsworth Ii MH, Hughes TK, Griffin GK, Battaglia S, et al. Single-cell RNA-seq reveals AML hierarchies relevant to disease progression and immunity. Cell. 2019;176:1265–81.
Ren X, Zhong G, Zhang Q, Zhang L, Sun Y, Zhang Z. Reconstruction of cell spatial organization from single-cell RNA sequencing data based on ligand-receptor mediated self-assembly. Cell Res. 2020;30:763–78.
Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature. 2015;528:413–7.
Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16:431–46.
Singhal S, Bhojnagarwala PS, O’Brien S, Moon EK, Garfall AL, Rao AS, et al. Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell. 2016;30:120–35.
Massara M, Bonavita O, Savino B, Caronni N, Mollica Poeta V, Sironi M, et al. ACKR2 in hematopoietic precursors as a checkpoint of neutrophil release and anti-metastatic activity. Nat Commun. 2018;9:676.
Ponzetta A, Carriero R, Carnevale S, Barbagallo M, Molgora M, Perucchini C, et al. Neutrophils driving unconventional T cells mediate resistance against murine sarcomas and selected human tumors. Cell. 2019;178(346–60):e24.
Janiszewska M, Tabassum DP, Castaño Z, Cristea S, Yamamoto KN, Kingston NL, et al. Subclonal cooperation drives metastasis by modulating local and systemic immune microenvironments. Nat Cell Biol. 2019;21:879–88.
Rath J, Bajwa G, Carreres B, Hoyer E, Gruber I, Martínez-Paniagua M, et al. Single-cell transcriptomics identifies multiple pathways underlying antitumor function of TCR- and CD8αβ-engineered human CD4 T cells. Sci Adv. 2020;6:eaaz7809.
Nirschl CJ, Suárez-Fariñas M, Izar B, Prakadan S, Dannenfelser R, Tirosh I, et al. IFNγ-dependent tissue-immune homeostasis is co-opted in the tumor microenvironment. Cell. 2017;170(127–41):e15.
Wang D, Lin J, Yang X, Long J, Bai Y, Yang X, et al. Combination regimens with PD-1/PD-L1 immune checkpoint inhibitors for gastrointestinal malignancies. J Hematol Oncol. 2019;12:42.
Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–61.
Zhao Z, Zheng L, Chen W, Weng W, Song J, Ji J. Delivery strategies of cancer immunotherapy: recent advances and future perspectives. J Hematol Oncol. 2019;12:126.
Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–23.
Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 2015;14:561–84.
Yi M, Yu S, Qin S, Liu Q, Xu H, Zhao W, et al. Gut microbiome modulates efficacy of immune checkpoint inhibitors. J Hematol Oncol. 2018;11:47.
Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–99.
Li Z, Song W, Rubinstein M, Liu D. Recent updates in cancer immunotherapy: a comprehensive review and perspective of the 2018 China Cancer Immunotherapy Workshop in Beijing. J Hematol Oncol. 2018;11:142.
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20.
Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–87.
Jiang P, Gu S, Pan D, Fu J, Sahu A, Hu X, et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med. 2018;24:1550–8.
Sehgal K, Portell A, Ivanova E, Lizotte P, Mahadevan N, Greene J, et al. Dynamic single-cell RNA sequencing identifies immunotherapy persister cells following PD-1 blockade. J Clin Invest. 2020. https://doi.org/10.1172/JCI135038 .
Article Google Scholar
Zilionis R, Engblom C, Pfirschke C, Savova V, Zemmour D, Saatcioglu HD, et al. Single-cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity. 2019;50:1317–34.
Zhang L, Li Z, Skrzypczynska KM, Fang Q, Zhang W, O’Brien SA, et al. Single-cell analyses inform mechanisms of myeloid-targeted therapies in colon cancer. Cell. 2020;181:442–59.
Katzenelenbogen Y, Sheban F, Yalin A, Yofe I, Svetlichnyy D, Jaitin DA, et al. Coupled scRNA-Seq and intracellular protein activity reveal an immunosuppressive role of TREM2 in cancer. Cell. 2020;182:872–85.
Jiang T, Shi T, Zhang H, Hu J, Song Y, Wei J, et al. Tumor neoantigens: from basic research to clinical applications. J Hematol Oncol. 2019;12:93.
McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–9.
Hacohen N, Fritsch EF, Carter TA, Lander ES, Wu CJ. Getting personal with neoantigen-based therapeutic cancer vaccines. Cancer Immunol Res. 2013;1:11–5.
Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–21.
Nathanson T, Ahuja A, Rubinsteyn A, Aksoy BA, Hellmann MD, Miao D, et al. Somatic mutations and neoepitope homology in melanomas treated with CTLA-4 blockade. Cancer Immunol Res. 2017;5:84–91.
Safonov A, Jiang T, Bianchini G, Győrffy B, Karn T, Hatzis C, et al. Immune gene expression is associated with genomic aberrations in breast cancer. Cancer Res. 2017;77:3317–24.
Karasaki T, Nagayama K, Kuwano H, Nitadori JI, Sato M, Anraku M, et al. Prediction and prioritization of neoantigens: integration of RNA sequencing data with whole-exome sequencing. Cancer Sci. 2017;108:170–7.
Nejo T, Matsushita H, Karasaki T, Nomura M, Saito K, Tanaka S, et al. Reduced neoantigen expression revealed by longitudinal multiomics as a possible immune evasion mechanism in glioma. Cancer Immunol Res. 2019;7:1148–61.
Lv JW, Zheng ZQ, Wang ZX, Zhou GQ, Chen L, Mao YP, et al. Pan-cancer genomic analyses reveal prognostic and immunogenic features of the tumor melatonergic microenvironment across 14 solid cancer types. J Pineal Res. 2019;66:e12557.
Linxweiler M, Kuo F, Katabi N, Lee M, Nadeem Z, Dalin MG, et al. The immune microenvironment and neoantigen landscape of aggressive salivary gland carcinomas differ by subtype. Clin Cancer Res. 2020;26:2859–70.
Kim S, Kim HS, Kim E, Lee MG, Shin EC, Paik S, et al. Neopepsee: accurate genome-level prediction of neoantigens by harnessing sequence and amino acid immunogenicity information. Ann Oncol. 2018;29:1030–6.
Wang TY, Wang L, Alam SK, Hoeppner LH, Yang R. ScanNeo: identifying indel-derived neoantigens using RNA-Seq data. Bioinformatics. 2019;35:4159–61.
Zhang Z, Zhou C, Tang L, Gong Y, Wei Z, Zhang G, et al. ASNEO: Identification of personalized alternative splicing based neoantigens with RNA-seq. Aging (Albany NY). 2020;12:14633–48.
Yang H, Sun L, Guan A, Yin H, Liu M, Mao X, et al. Unique TP53 neoantigen and the immune microenvironment in long-term survivors of Hepatocellular carcinoma. Cancer Immunol Immunother. 2020. https://doi.org/10.1007/s00262-020-02711-8 .
Lu YC, Zheng Z, Robbins PF, Tran E, Prickett TD, Gartner JJ, et al. An efficient single-cell RNA-seq approach to identify neoantigen-specific T cell receptors. Mol Ther. 2018;26:379–89.
Engström P, Steijger T, Sipos B, Grant G, Kahles A, Rätsch G, et al. Systematic evaluation of spliced alignment programs for RNA-seq data. Nat Methods. 2013;10:1185–91.
Sharon D, Tilgner H, Grubert F, Snyder M. A single-molecule long-read survey of the human transcriptome. Nat Biotechnol. 2013;31:1009–14.
Quail MA, Smith M, Coupland P, Otto TD, Harris SR, Connor TR, et al. A tale of three next generation sequencing platforms: comparison of Ion Torrent, Pacific Biosciences and Illumina MiSeq sequencers. BMC Genomics. 2012;13:341.
Tilgner H, Grubert F, Sharon D, Snyder MP. Defining a personal, allele-specific, and single-molecule long-read transcriptome. Proc Natl Acad Sci USA. 2014;111:9869–74.
Stark R, Grzelak M, Hadfield J. RNA sequencing: the teenage years. Nat Rev Genet. 2019;20:631–56.
Antipov D, Korobeynikov A, McLean J, Pevzner P. hybridSPAdes: an algorithm for hybrid assembly of short and long reads. Bioinformatics. 2016;32:1009–15.
Boluki S, Zamani Dadaneh S, Qian X, Dougherty E. Optimal clustering with missing values. BMC Bioinform. 2019;20:321.
Hicks S, Townes F, Teng M, Irizarry R. Missing data and technical variability in single-cell RNA-sequencing experiments. Biostatistics. 2018;19:562–78.
Hou W, Ji Z, Ji H, Hicks S. A systematic evaluation of single-cell RNA-sequencing imputation methods. Genome biol. 2020;21:218.
Song F, Chan G, Wei Y. Flexible experimental designs for valid single-cell RNA-sequencing experiments allowing batch effects correction. Nat Commun. 2020;11:3274.
Buenrostro J, Giresi P, Zaba L, Chang H, Greenleaf W. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods. 2013;10:1213–8.
Yang C, Ma L, Xiao D, Ying Z, Jiang X, Lin Y. Sparassis latifolia Integration of ATAC-Seq and RNA-Seq Identifies Key Genes in Light-Induced Primordia Formation of. Int J Mol Sci. 2019;21.
Wu X, Yang Y, Zhong C, Guo Y, Wei T, Li S, et al. Epinephelus coioides Integration of ATAC-seq and RNA-seq Unravels Chromatin Accessibility during Sex Reversal in Orange-Spotted Grouper ( Epinephelus coioides ). Int J Mol Sci. 2020;21.
Simonis M, Kooren J, de Laat W. An evaluation of 3C-based methods to capture DNA interactions. Nat Methods. 2007;4:895–901.
Lieberman-Aiden E, van Berkum N, Williams L, Imakaev M, Ragoczy T, Telling A, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326:289–93.
Mifsud B, Tavares-Cadete F, Young A, Sugar R, Schoenfelder S, Ferreira L, et al. Mapping long-range promoter contacts in human cells with high-resolution capture Hi-C. Nat Genet. 2015;47:598–606.
Crane E, Bian Q, McCord R, Lajoie B, Wheeler B, Ralston E, et al. Condensin-driven remodelling of X chromosome topology during dosage compensation. Nature. 2015;523:240–4.
Chen H, Seaman L, Liu S, Ried T, Rajapakse I. Chromosome conformation and gene expression patterns differ profoundly in human fibroblasts grown in spheroids versus monolayers. Nucleus. 2017;8:383–91.
Vara C, Paytuví-Gallart A, Cuartero Y, Le Dily F, Garcia F, Salvà-Castro J, et al. Three-dimensional genomic structure and cohesin occupancy correlate with transcriptional activity during spermatogenesis. Cell Rep. 2019;28(352–67):e9.
Zeng C, Huang W, Li Y, Weng H. Roles of METTL3 in cancer: mechanisms and therapeutic targeting. J Hematol Oncol. 2020;13:117.
Jenjaroenpun P, Wongsurawat T, Wadley T, Wassenaar T, Liu J, Dai Q, et al. Decoding the epitranscriptional landscape from native RNA sequences. Nucleic Acids Res. 2020. https://doi.org/10.1093/nar/gkaa620 .
Article PubMed Central Google Scholar
Parker M, Knop K, Sherwood A, Schurch N, Mackinnon K, Gould P, et al. Nanopore direct RNA sequencing maps the complexity of Arabidopsis mRNA processing and mA modification. eLife. 2020;9.
Zhang S, Li R, Zhang L, Chen S, Xie M, Yang L, et al. New insights into Arabidopsis transcriptome complexity revealed by direct sequencing of native RNAs. Nucleic Acids Res. 2020;48:7700–11.
Pan X, Zhang H, Xu D, Chen J, Chen W, Gan S, et al. Identification of a novel cancer stem cell subpopulation that promotes progression of human fatal renal cell carcinoma by single-cell RNA-seq analysis. Int J Biol Sci. 2020;16:3149–62.
Li Y, Chen C, Chen J, Lai Y, Wang S, Jiang S, et al. Single-cell analysis reveals immune modulation and metabolic switch in tumor-draining lymph nodes. Oncoimmunology. 2020;9:1830513.
Giladi A, Cohen M, Medaglia C, Baran Y, Li B, Zada M, et al. Dissecting cellular crosstalk by sequencing physically interacting cells. Nat Biotechnol. 2020;38:629–37.
Caruso F, Garofano L, D'Angelo F, Yu K, Tang F, Yuan J, et al. A map of tumor-host interactions in glioma at single-cell resolution. GigaScience. 2020;9.
Jaitin D, Weiner A, Yofe I, Lara-Astiaso D, Keren-Shaul H, David E, et al. Dissecting immune circuits by linking CRISPR-pooled screens with single-cell RNA-seq. Cell. 2016;167:1883–96.
Datlinger P, Rendeiro A, Schmidl C, Krausgruber T, Traxler P, Klughammer J, et al. Pooled CRISPR screening with single-cell transcriptome readout. Nat Methods. 2017;14:297–301.
Genga R, Kernfeld E, Parsi K, Parsons T, Ziller M, Maehr R. Single-cell RNA-sequencing-based CRISPRi screening resolves molecular drivers of early human endoderm development. Cell Rep. 2019;27:708–18.
Qiu Q, Hu P, Qiu X, Govek K, Cámara P, Wu H. Massively parallel and time-resolved RNA sequencing in single cells with scNT-seq. Nat Methods. 2020;17:991–1001.
McFarland J, Paolella B, Warren A, Geiger-Schuller K, Shibue T, Rothberg M, et al. Multiplexed single-cell transcriptional response profiling to define cancer vulnerabilities and therapeutic mechanism of action. Nat commun. 2020;11:4296.
Sun W, Dong H, Balaz M, Slyper M, Drokhlyansky E, Colleluori G, et al. snRNA-seq reveals a subpopulation of adipocytes that regulates thermogenesis. Nature. 2020;587:98–102.
Download references
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81300398 and 81974436); Key-Area Research and Development Program of Guangdong Province (2020B1111030005); The Program for Guangdong Introducing Innovative and Entrepreneurial Teams (2019ZT08Y485); Key Program of Marine Economy Development (Six Marine Industries) Special Foundation of Department of Natural Resources of Guangdong Province (GDNRC [2020]070); The Fundamental Research Funds for the Central Universities (19ykzd06) and the Opening Fund of Guangdong Key Laboratory of Marine Materia Medica (LMM2020-4).
Author information
Mingye Hong and Shuang Tao have contributed equally to this work
Authors and Affiliations
Institute of Laboratory Medicine, Guangdong Provincial Key Laboratory of Medical Molecular Diagnostics, School of Medical Technology, Guangdong Medical University, Dongguan, 523808, China
Mingye Hong, Xuanmei Huang, Shaohui Huang & Hua Zhang
Biotherapy Center, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, 510630, China
Shuang Tao, Li-Ting Diao, Shu-Juan Xie & Zhen-Dong Xiao
Health Science Center, The University of Texas, Houston, 77030, USA
You can also search for this author in PubMed Google Scholar
Contributions
M.H. and S.T. wrote and edited this manuscript and created figures and tables. L.Z., L-T. D., X-M. H., S-H. H., S-J. X., Z-D. X and H.Z. reviewed and revised the manuscript. Z-D. X and H.Z. provided direction and guidance throughout the preparation of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Zhen-Dong Xiao or Hua Zhang .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Hong, M., Tao, S., Zhang, L. et al. RNA sequencing: new technologies and applications in cancer research. J Hematol Oncol 13 , 166 (2020). https://doi.org/10.1186/s13045-020-01005-x
Download citation
Received : 31 October 2020
Accepted : 22 November 2020
Published : 04 December 2020
DOI : https://doi.org/10.1186/s13045-020-01005-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Application
Journal of Hematology & Oncology
ISSN: 1756-8722
- General enquiries: [email protected]
Cancer Biology Research
Breast cancer cells
The Importance of Cancer Biology Research
Research on the biology of cancer starts with the simplest of questions: What is—and isn’t—normal? To understand how cancer develops and progresses, researchers first need to investigate the biological differences between normal cells and cancer cells. This work focuses on the mechanisms that underlie fundamental processes such as cell growth, the transformation of normal cells to cancer cells, and the spread ( metastasis ) of cancer cells.
Virtually all major advances against cancer originated with discoveries in basic science . Basic research can reveal new ideas about the causes of cancer and how it develops, progresses, and responds to therapy.
Knowledge gained from such studies deepens our understanding of cancer and produces insights that could lead to new clinical interventions. For example, studies of cell signaling pathways in normal cells and cancer cells have contributed greatly to our knowledge about the disease, revealing molecular alterations that are shared among different types of cancer and pointing to possible treatment strategies.
Decades of basic research in cancer biology have created a broad base of knowledge that has been critical to progress against the disease.
Selected NCI Activities in Cancer Biology Research

NCI Research and the National Cancer Plan
NCI supports a broad variety of research that aligns with the goals of the National Cancer Plan. Read about the plan and explore each goal.
Federal funding for cancer biology is essential because this area of research receives relatively little funding from entities that are driven by profit. NCI supports and directs cancer biology research through a variety of programs and approaches. For example:
- The Metastasis Research Network (MetNet) supports research to improve our understanding of how cancer spreads. Cancer metastasis is a complex, dynamic, nonlinear process. The network supports several specialized centers working collaboratively on multidisciplinary projects focused on several themes of the metastatic process, including mechanisms of early dissemination, cellular and physical microenvironment crosstalk, dormancy, and mechanisms of responses to therapy by metastatic cells.
- The Translational and Basic Science Research in Early Lesions (TBEL) Program is advancing the understanding of the mechanisms driving, or restraining, the development of precancers and early cancers, as well as informing the development of precision prevention approaches. The program supports multidisciplinary research centers that are integrating basic and translational research to investigate the interactions of an early lesion, its microenvironment, and host factors as “co-organizers” of tumor initiation and the development of cancer.
- The Human Tumor Atlas Network is constructing 3-dimensional atlases of the cellular, morphological, and molecular features of human cancers as they evolve from precancerous lesions to advanced disease. The atlases, which represent a diverse patient population, will also be used to study how tumors respond to treatment and develop resistance to drugs.
- The Cancer Tissue Engineering Collaborative (TEC) supports the development and characterization of state-of-the-art biomimetic tissue-engineered technologies for cancer research. This program advances innovative, well-characterized in vitro and ex vivo systems available for cancer research, expands the breadth of these systems to several cancer types, and promotes investigations of cancer with tissue-engineered systems.

NCI Fiscal Year 2025 Professional Judgment Budget Proposal
Each year, NCI prepares a professional judgment budget to lead progress against cancer.
- The consortium of tumor glycomics laboratories and their research partners that make up the Alliance of Glycobiologists for Cancer Research are investigating the cancer-related dynamics of complex carbohydrates. The alliance, which NCI sponsors with the National Institute of General Medical Sciences and the National Heart, Lung, and Blood Institute, aims to study the structure and function of glycans in relation to cancer.
- The NCI RNA Biology Initiative facilitates the exchange of information and expertise among investigator studying the structure, function, and biological roles of RNA for the purpose of developing new cancer diagnostics and therapies.
- NCI’s Centers of Excellence bring together the institute’s intramural researchers to collaborate on new projects and initiatives in various areas of cancer biology, including Chromosome Biology and Genitourinary Malignancies .
Recent Research Findings in Cancer Biology
- Loss of Y Chromosome in Men Makes Bladder Cancer More Aggressive
- Cells’ decision to divide is reversible
- How Fatty Liver Disease Helps Cancer Thrive in the Liver
- No Glucose? Pancreatic Cancer May Have a Ready Energy Alternative
- How Some Brain Tumors Hijack the Mind to Grow
- Researchers discover the multiple shapes of RNA, a boon for drug design
- Vulnerability in Brain Tumors May Open Door to New Treatments
- Preventing Chemo Brain? Study Identifies Potential Approach for Common Problem
- Research & Faculty
- Offices & Services
- Information for:
- Faculty & Staff
- News & Events
- Contact & Visit
- Student Resources
- Curriculum Overview
- Course Schedules
- Minors and Certificates
- Nanobiotechnology
- Course Listings
- Student Research Overview
- Research Areas
- Biomaterials
- Cancer Biotechnology
- Cardiovascular Biology & Transplantation Biology
- Cell & Molecular Biology
- Developmental Biology & Neurobiology
- Diagnostics & Medical Devices
- Drug Discovery & Delivery
- Microbial & Environmental Biotechnology
- Stem Cell Biology
- Sustainability & Global Health Biotechnology
- Synthetic & Systems Biology
- Research Papers
- Admissions Overview
- Information Sessions
- Core Faculty and Staff
- Adjunct Faculty and Staff
- Research Advisors
- Current Students
- Association of Biotechnology Students
- Industrial Advisory Board
- Program Benefactors
- Class of 2021
- Class of 2020
- Class of 2019
- Class of 2018
- Class of 2017
- Class of 2016
- Class of 2015
- Class of 2014
- Class of 2013
- Class of 2012
- Class of 2011
- Class of 2010
- Class of 2009
- Class of 2008
- Class of 2007
- Class of 2006
- Class of 2005
- Class of 2004
- Career Development
- Co-op / Internship
- Where to Work in Biotech
- Philadelphia
- Research Triangle
- San Francisco
- Washington D.C.
- Stories Archive
- Seminars & Events
- Upcoming Events
- Biotech Nexus
- Site Visits
- Northwestern Engineering
Student Research / Research Areas Cancer Biotechnology
Research focused on understanding the molecular mechanisms of cancer, and developing diagnostics and drugs for its cure. The most cross-disciplinary of contemporary research areas, cancer biotechnology research includes scientists from medicine, biology, physics and engineering disciplines.
Faculty members in cancer biotechnology include:
- Irina Budunova
- Elizabeth Eklund
- Qingshen Gao
- Roger Kroes
- Joshua Leonard
- Hidayatulah Munshi
- Marcus Peter
- Leonidas Platanias
- Alexander Stegh
- Teresa Woodruff
Student Publications
Fu-nien tsai '09.
- “The significant role of mast cells in cancer.” Cancer and Metastasis Reviews. March 2011.
- “PI3K/AKT Signaling Is Essential for Communication between Tissue-Infiltrating Mast Cells, Macrophages, and Epithelial Cells in Colitis-Induced Cancer.” Clinical Cancer Research. May 1, 2013.
- “Methods for the study of mast cells in cancer.” Methods Molecular Biology. 2015.
Chintan Chheda '10
- “Characterization of mouse models of early pancreatic lesions induced by alcohol and chronic pancreatitis.” Pancreas. November 1, 2014.
Kenichi Iwadate '10
- “Differential targeting of androgen and glucocorticoid receptors induces ER stress and apoptosis in prostate cancer cells: A novel therapeutic modality.” Cell Cycle. January 15, 2012.
Sayali Kale Kandari '10
- “Loss of the F-BAR protein CIP4 reduces platelet production by impairing membrane-cytoskeleton remodeling.” Blood. June 3, 2013.
Stefanie Kall '10
- “Genes That Mediate Metastasis Organotropism.” Metastatic Cancer: Clinical and Biological Perspectives. 2013.
Yuanming Xu '11
- “Use of an organotypic mammalian in vitro follicle growth (IVFG) assay to facilitate female reproductive toxicity screening.” Reproduction, Fertility and Development. February 18, 2015.
- “Engineering the ovarian cycle using in vitro follicle culture.” Human reproduction. March 16, 2015.
- “The inorganic anatomy of the mammalian preimplantation embryo and the requirement of zinc during the first mitotic divisions.” Development Dynamics. July 16, 2015.
More in this section
- Engineering Home
- MBP Program
- Student Research
How To Apply
Related links.
- International Office
- Graduate Housing
- Meet Our Faculty
- Our Whole-Brain Engineering Philosophy
Contact Info
Master of Biotechnology Program Northwestern University Tech Institute, E136 2145 Sheridan Road Evanston, Illinois 60208 Phone: 847-491-7399 Fax: 847-491-3728 Email the program
Request Info
Request your program & application guide.

- Methodology
- Open access
- Published: 25 April 2024
The research trends and future prospects of nanomaterials in breast cancer
- Yue Li 1 , 3 ,
- Xiaoqing Li 1 , 2 ,
- Aoqun Li 1 , 2 ,
- Jingyan Zhu 1 , 2 ,
- Zhenhua Lin 1 , 2 &
- Yang Yang 1 , 2
Cancer Nanotechnology volume 15 , Article number: 24 ( 2024 ) Cite this article
129 Accesses
Metrics details
Breast cancer is the most common cause of cancer-related deaths among women globally and the most deadly illness for them. New advances in nanotechnology have led to the development of strategies intended to target breast cancer cells more precisely while causing the least amount of damage to healthy cells. We retrieved articles about nanomaterials for the diagnosis and treatment of breast cancer from the Web of Science Core Collection (WoSCC) database between 2008 and 2023. Our research aims to assess publications on the use of nanomaterials for breast cancer treatment and diagnosis to predict future research directions.
A total of 457 papers on nanomaterials in breast cancer were discovered from various nations, with China being the primary source and the United States having the highest H index. The number of papers in this discipline is increasing on an annual basis. The Egyptian Knowledge Bank is an important research center in this sector. The International Journal of Nanomedicine has the most papers, and Kesharwani P is the most frequently referenced author. The most quoted article was written by Miele, Evelina of India in 2009. Topics such as drug delivery may be emerging areas of research.
Our findings predict that the use of nanomaterials in medication delivery will become a significant research area in the future, and provide valuable references for scholars investigating the role of nanotechnology in breast cancer.
Introduction
Breast cancer is the most common malignant tumor among women, with the highest incidence rate (Thomas et al. 2024 ; Siegel et al. 2023 ). According to the GLOBACON database, there were 22,968,840 new cases and 666,103 deaths of breast cancer between 2022 and 2024 (8th February). Early diagnosis of breast cancer is challenging due to the limited sensitivity of existing methods in detecting small lesions (Ha et al. 2018 ). Various therapeutic modalities have been used to treat breast cancer, including the combination of radiotherapy and chemotherapy to inhibit tumor progression and recurrence. However, the low therapeutic efficacy of these drugs is due to their poor target and affinity (Liu et al. 2023 ; Zhang et al. 2022 ). Therefore, new and effective methods for diagnosing and treating breast cancer are needed.
Nanoparticles can interact with a variety of organelles and biomolecules and range in size from 1 to 100 nm (Nikalje 2015 ). These properties make them suitable for various applications, from chemical reactions to biomedicine (Mujahid et al. 2022 ). Quantum dots (QDs) and gold nanoparticles are used at the molecular level for cancer diagnosis (Kher and Kumar 2022 ). Molecular diagnostic techniques based on nanoparticles can be used for biomarker discovery and rapid tumor diagnosis (Yan et al. 2019 ). The rapid growth of tumors results in the epidermal cell gap of intra-tumor blood vessels being larger than that of normal blood vessels, and the lack of a lymphatic system within the tumor makes it easy for nanoparticles to “leak” into the tumor from the gap in the tumor blood vessels and accumulate in the tumor. Some investigations have shown that nanomaterials can be targeted against the endothelial cells of tumor blood vessels, releasing anti-angiogenic medicines, successfully suppressing tumor blood vessel growth and reducing the oxygen supply. (Chakraborty et al. 2019 ). The high surface-to-volume ratio of magnetic nanoparticles allows them to assemble with biomolecules or residues, which can enhance the specificity of chemical drug complexes in targeted therapy (Moloudi et al. 2023 ; Londhe et al. 2023 ). In addition, nanotechnology-enhanced photodynamic therapy and immunotherapy are emerging as exciting cancer therapeutic methods with significant potential for improving patient outcomes (Jia et al. 2023 ). The addition of nanomaterials enhances the responsive release, depth of tissue penetration and precise targeting of phototherapy, enabling precise treatment of specific cancer tissues and cells through photodynamic therapy, photothermal therapy and combination therapy (Mosleh-Shirazi et al. 2022 ). Some studies have indicated that immune checkpoint blockade therapy does not become noticeably more effective when drugs are delivered using nanoparticles. On the other hand, when paired with chemotherapy and other treatments, nanoparticles can enhance anti-cancer immune responses as well as improve medication transport and usage efficiency. However, no bibliometric analysis has been published on the use of nanomaterials in the diagnosis and treatment of breast cancer.
Hence, this study aims to analyze the use of nanoparticles in breast cancer therapy through quantitative methods. Scientometric analyses aid academics in comprehending the effectiveness of nanomaterials in treating breast cancer, familiarising themselves with published research results, and understanding collaborations and links between countries, institutions, and authors. Additionally, we objectively reveal the current status and future directions of nanotechnology in breast cancer; review current research hotspots; and anticipate research trends and future prospects in this field.
Browse and search
Web of Science is a crucial database for accessing global academic information (Pei et al. 2022 ). It can rapidly identify high-impact papers, reveal research directions that domestic and foreign authorities focus on, and expose the trend of subject development (Wang and Maniruzzaman 2022 ). A search was conducted in the WOSCC database for literature related to the use of nanomaterials in breast cancer diagnosis and treatment from 1 January 2008 to 31 December 2023. The search formula used was as follows: TS = (“nanostructured materials” OR “nanomaterials” OR “nanotechnology”) AND TS = (“breast” OR “breast cancer”) AND TS = (“treatment” OR “diagnosis” OR “cure” OR “therapy”).
Screening procedures
The inclusion criteria are as follows: (1) the full text of the available papers focused on the diagnosis and treatment of nanomaterials in breast cancer; (2) the papers were written in English; (3) only articles and reviews were permitted; (4) the papers were sourced from the Social Science Citation Index (SSCI) and the Science Citation Index Expanded (SCI-E) databases; and (5) the timeframe was from 2008 to 2023. The study’s inclusion criteria were as follows: The following criteria were excluded: (1) This paper will focus on nanomaterials that are not related to breast cancer diagnosis and treatment. (2) This study will consider all types of publications, including reports, theses, and conference abstracts.
Data analytics
The publications and citations were exported as plain text for bibliometric analysis and visualization. VOSviewer (version 1.6.19) and CiteSpace (version 6.2) were used to create visualizations. Line graphs were generated using GraphPad Prism (version 9.5.1) to display the number of publications, citations, and h-index per year. To analyze the most prolific/collaborative countries, institutions, authors, co-cited journals, and co-occurring keywords, VOSviewer was used.
CiteSpace can be used to construct keyword timeline charts and keyword bursts. The visualization chart displays dots representing countries, institutions, authors, or journals, which are clustered into different groups based on their collaboration (Liu et al. 2022 ). The size of the dots corresponds to the number of publications. The thickness of the lines connecting the nodes represents the link strength (LS) and reflects the strength of collaboration between them (Xia et al. 2022 ). The Total Link Strength (TLS) metric measures the level of collaboration between nodes (Jin et al. 2023 ). We improved the keyword analysis by excluding irrelevant keywords and merging those with similar meanings to provide a better perspective. The graph generated by CiteSpace shows significant and reasonable clustering, with a modularity value greater than 0.3 and an average silhouette value greater than 0.7.
Selection and characterization of literature
A search for keywords related to breast cancer and nanomaterials in the Web of Science database retrieved a total of 463 papers. In the first stage of selection, 6 articles were excluded due to type restriction. Next, we screened 457 publications published during the decade from 2008 to 2023 to do the study (Fig. 1 ). The results show an overall increasing trend in the number of annual publications (Fig. 2 A), indicating an increased interest in the field of breast cancer and its treatment and nanomaterials. The number of publications peaked in 2022 with 72 publications, which accounted for 16.74% of the total publications (Fig. 2 B). The year with the highest number of citations was 2020, with a total of 1269 citations (Fig. 2 C). The number of publications has steadily increased from 2008 to 2024. The annual H-index increased from 2 in 2008 to 20 in 2020 (Fig. 2 D).
Flowchart of the literature screening proces
Publication trends in breast cancer and nanomaterials research. A . The number of the publications; B .The number of cumulative publications; 15-year publication rate with an exponential trend line, 2008 to 2023. C . The total citations of the publications; D . The H‑index values of the publications
Country/region and institutional analysis of publications
VOSviewer analysis shows that a total of 59 countries/regions have cooperated in this area (Fig. 3 A, B ). India had the most robust international cooperation network (TLS = 84) and cooperated most closely with Saudi Arabia (TLS = 65). The country with the highest centrality is Iran (0.53), followed by USA (0.34) and India (0.32). The USA (5.9) has the highest burst strength next to Spain (2.85) and South Korea (2.42). Next, we analyzed the top 10 productive countries/regions in terms of number of publications, total citations, and H-index. China published the most papers (109, 22.61%), followed by India (85, 18.53%) and USA (79, 40.14%). In addition, the USA has the highest number of citations (3171) and the highest H-index (30).
Diagram of the Nanomaterials and Breast Cancer Collaboration Network. A-B . The coauthorship network map of countries; C-D . The coauthorship network map of authors; E . The coauthorship network map of institutions; F . The cocitation network map of journals
The institutional collaboration network diagram is shown in Fig. 3 E, which contains 99 institutions. Jamia Hamdard has the strongest total connectivity strength (TLS = 51). Statistically, the institution with the highest centrality is the Egyptian Knowledge Bank (EKB) (0.14) followed by Islamiv Azda University (IAU) (0.07). Table 1 shows the top 10 most productive institutions. The highest number of published papers was from EKB (20), next to IAU (16). EKB had the highest total number of citations (393) and the highest h-index (10).
Analyzing the authors of publications
The network of inter-authors and collaborative relationships is shown in Fig. 3 C, D . In this study, we examined the top ten contributors in terms of posting volume. Kesharwani P (TLS = 60) works most closely with other authors. The highest number of posts is Kesharwani P (11, 25.09%), followed by Chorilli M (7, 5.71%). Kesharwani P has not only the highest number of citations (276), but also has the highest H-index (9).
Analysis of the publication journal
For this study, we selected the top ten most productive journals from the communication network diagram (Fig. 3 F, Table 2 ). The most partnerships with other journals are the Biomaterials (TLS = 108,995). The top three journals with the most published research in the field are the International Journal of Nanomedicine , the Journal of Controlled Release , and the Journal of Drug Delivery Science and Technology . The most cited is the International Journal of Nanomedicine , which has the highest H-index. Furthermore, the Impact Factor (IF) of journal is a crucial parameter for assessing its worth and that of its published works. Journal of Controlled Release has the highest IF (10.8) and JCR category (Q1).
Analysis of the most cited articles
The nanomaterials analyzed in the most cited publications of each year during the study period are shown in Fig. 4 (Karathanasis et al. 2008 ; Eghtedari et al. 2009 ; Chen et al. 2010 ; Deng et al. 2011 ; Dreaden et al. 2012 ; You et al. 2013 ; Lee et al. 2014 ; Gurunathan et al. 2015 ; Kumar et al. 2017 ; Mu et al. 2017 ; Zhang et al. 2018 ; Gao et al. 2019 ; Wang et al. 2020 ; Dubey et al. 2021 ; Fatima et al. 2022 ; Ashrafizadeh et al. 2023 ). Gold nanoparticles were commonly used in these studies. Table 3 shows the top 10 most cited articles as of 18 November 2023, along with the information associated with them, which we will examine in the next section. Four of the ten studies are from the USA, two are from India, and the remaining four are from Singapore, China, South Korea, and Italy. The study titled “Albumin-bound formulation of paclitaxel (Abraxane ® ABI-007) in the treatment of breast cancer” by Miele E, published in the International Journal of Nanomedicine in Italy in 2009, which was cited 652 times, making it the most cited publication in the field. (Miele et al. 2009 ).
The timeline of nanomaterials and breast cancer research

For the study of keywords and research hotspots
In a paper, keywords can help us get the topic and theme of the research more accurately and quickly (Tang et al. 2023 ). The division of all keywords into six clusters in Fig. 5 A. As shown in Fig. 5 B, terms in purple indicate that their average publication year is in 2018 and earlier, while terms in bright yellow indicate that their average publication year is after 2021. “quantum dots”, “resistance” and “therapeutics” were the main focus in the early days. Keywords such as “immunotherapy”, “nanomaterials” and “anticancer activity” will not start to attract widespread attention until after 2021. The largest clusters are in red and include keywords like “diagnosis” and “metastasis” in breast cancer. The second cluster is in purple and contains keywords such as “nanotechnology”, “nanoparticles” and “cells”. The third cluster is in yellow and includes keywords such as “drug delivery”, “in vitro” and “co-delivery”. The fourth cluster, in blue, contains keywords like “doxorubicin”, “apoptosis” and “gold nanoparticles”. The fifth cluster in pink contains the keywords “delivery” and “immunotherapy”.
Keyword analysis of research hotspots. A-B . The network A and overlay B of keyword co-occurring; C . The timeline map of keyword co-occurring; D . The Top 25 keywords with the strongest citation bursts
In addition, we have used CiteSpace to analyze the similarities and differences in the keywords over time. Until 2014, the main areas of research were breast cancer, metastasis, antitumor therapy, anti-tumour agents and chemotherapy. Breast cancer, photodynamic therapy, resistance and gold nanoparticles continue to be topics of high interest in 2023. The strength of the keyword bursts is another important indicator of the frontiers and hotspots of the study over time. The top ten keywords with the highest outbreak values are iron oxide nanoparticles (5.47), nanotechnology (3.62), quantum dots (3.5), metastatic breast cancer (3.44), transmission electron microscopy (3.2), reactive oxygen species (3.11), scanning electron microscopy (3,04), metastasis (3.04), chemotherapy (2.85), gold nanoparticles (2.84).
In recent years, there has been a surge in the bibliometric analysis of articles across various fields (Tang et al. 2023 ). Tools such as CiteSpace and the VOSviewer enable raw data visualization, offering comprehensive and intuitive data representation (Zhou et al. 2020 ). Our findings demonstrate an overall increased trend in the number of annual publications in the discipline. 2020 was the most significant year in the field, with the most citations and the highest H-index.
China leads in the number of publications per nation with 109 publications, accounting for 25.35% of the total. It also has the highest number of international partnerships, publications, and citations, as well as the highest h-index. China has a large population base and a large number of researchers and institutions in related fields, resulting in higher literature production. In particular, the efficacy of nanomedicines in breast cancer has been a hot topic of research in recent years. The International Journal of Nanomedicine , Journal of Controlled Release , and Journal of Drug Delivery Science and Technology are widely regarded as the most influential publications in this field. International Journal of Nanomedicine has the highest number of published papers, the highest h-index, the highest number of citations and the highest average number of citations of the papers, while Biomaterials has the highest JCR category and IF.
Subsequently, we found that one of the most cited papers used nanocarriers to improve the solubility of paclitaxel in water to increase the solubility and stability of the drug, thereby enhancing its efficacy against breast cancer. Furthermore, we have mapped the mechanism of nanomedicine therapy for breast cancer based on this article (Fig. 6 ). The study points to the development of so-called “third generation” nanocarriers and is based on a multi-stage strategy that addressing all the possible therapies in a more specific manner. The article states that human serum albumin stabilizes the drug particle at an average size of 130 nm (Stinchcombe 2007 ). This prevents any risk of capillary obstruction and eliminates the need for any particular infusion systems or steroid/antihistamine premedication before the infusion (Okuyama et al. 2021 ). Preclinical studies have shown that Abraxane® ABI-007 has higher tumor cell penetration and anti-tumor activity compared to equivalent doses of standard paclitaxel (Miele et al. 2009 ). The most highly cited article published in 2023 focuses on the therapeutic effects of Superparamagnetic Iron-Oxide Nanoparticles (SPIONs) on breast cancer (Dongsar et al. 2023 ). Controlled drug release from SPIONs can also aid in overcoming non-specific targeting and reducing the need for large therapeutic doses. This is because drug-loaded SPIONs can specifically target certain cells, tissues, or organs, minimizing damage to healthy tissues and improving therapeutic efficacy. (Fakayode et al. 2018 ). Hoekman et al. demonstrated that liposomes have a stable and robust closed membrane that can carry hydrophilic small molecule drugs, as well as protein, RNA, and DNA drugs within aqueous chambers. This membrane protects the drugs from plasma scavenging and degrading enzymes, prolonging their retention time in the circulation of the body (Xinchen et al. 2023 ). Overall, by increasing treatment efficacy and lowering harmful side effects, nanocarriers can be a useful drug delivery method.
Mechanistic map of nanochemical combination therapy in breast cancer (represented by the combination of paclitaxel and nanomaterials)
Moreover, keyword analysis has reviewed the development of nanomaterials in breast cancer. Before 2018, the main focus of research was still on the mechanisms of treatment resistance in people with breast cancer. Loading curcumin into solid lipid nanoparticles promotes the Bax/Bcl-2 ratio but reduces the expression of the cell cycle proteins CyclinD1 and cyclin-dependent kinase 4 (CDK4), which in turn improves the therapeutic outcome of breast cancer (Mitra and Dash 2018 ). It shows that research on nanomaterials and breast cancer was at a more macroscopic and superficial stage. In the medium term (2018–2021), the research focus gradually expands to the level of magnetic nanoparticles and gold nanoparticles, which have important applications in the diagnosis and treatment of breast cancer. Magnetic nanoparticles can be used in magnetic resonance imaging to help doctors diagnose tumors more accurately (Boosz et al. 2021 ). On the other hand, gold nanoparticles as the drug carriers can be used to deliver drugs precisely to the tumor site, improving therapeutic effects and reducing side effects (Javan Nikkhah and Thompson 2021 ). From here, the significance of nanomaterials in detecting and treating breast cancer will become clearer. Nanomaterials will play an important role in the immunotherapy of breast cancer between 2021 and 2024. They can act as carriers for immunomodulators or anticancer drugs, which contribute to increasing the concentration of the drug locally and reducing damage to healthy tissue (Vincenzo et al. 2023 ). Furthermore, nanomaterials can be designed the specific immunogenic properties that activate the immune system to identify and attack breast cancer cells. Tailored Nano-immunotherapy is expected to enhance therapeutic efficacy and decrease adverse effects in patients with breast cancer.
While there is no denying the importance that nanomaterials play in the detection and management of breast cancer, we think that there are still certain limitations to them. The selectivity and specificity of nanomaterials might have negative effects on cells other than cancer. The usefulness of nanomaterials in therapy and diagnosis may be impacted by their unknown biodistribution and metabolic pathways in vivo (Park et al. 2017 ). At the same time, not all nanomaterials can be effectively translated into clinical applications, and the process of translating nanomaterials into clinical applications involves extensive clinical trials that demand time and money (Shirwaiker et al. 2013 ). Additionally, there is still some literature that was not included, such as grey literature and relevant literature that was not included in the WoSCC database. Some recently published articles may receive more citations in the future, which may have led to the exclusion of some high-quality studies from this analysis. And the number of citations does not fully reflect the importance of an article, as some important articles may have few citations and self-citations may introduce bias (Brown et al. 2021 ).
The findings indicate that the number of publications on this subject increased significantly starting in 2015, with China and the USA exhibiting the highest productivity levels. In the treatment of breast cancer, nanomedicines have demonstrated improved drug uptake and retention, resulting in more effective targeting of the tumor tissue and reduced systemic toxicity. The treatment of metastatic breast cancer may benefit greatly from the use of multifunctional nanotherapeutic medications. Nanotechnology holds the significant potential in improving drug transport, releasing, and targeting, which could lead to higher treatment efficacy and fewer side effects. Targeting nanomaterial-based drugs could have a beneficial effect on breast cancer patients and could be a milestone in the diagnosis and treatment of breast cancer.
Availability of data and materials
All data generated or analyzed during this study are included in this article and additional data are available from the corresponding author upon reasonable request.
Abbreviations
Web of science core collection
Quantum dots
Social science citation index
Science citation index expanded
Link strength
The total link strength
Egyptian knowledge bank
Islamiv Azda University
Albumin-bound formulation of paclitaxel
Superparamagnetic iron-oxide nanoparticles
Cyclin-dependent kinase 4
Ashrafizadeh M, Zarrabi A, Bigham A, Taheriazam A, Saghari Y, Mirzaei S et al (2023) (Nano) platforms in breast cancer therapy: drug/gene delivery, advanced nanocarriers and immunotherapy. Med Res Rev 43(6):2115–2176
Article CAS PubMed Google Scholar
Boosz P, Pfister F, Stein R, Friedrich B, Fester L, Band J et al (2021) Citrate-coated superparamagnetic iron oxide nanoparticles enable a stable non-spilling loading of T cells and their magnetic accumulation. Cancers 13(16):4143
Article CAS PubMed PubMed Central Google Scholar
Brown AC, Ross PP, Brown SM, Mulcahey MK (2021) The 50 most cited articles on meniscus injuries and surgery from 2000 to 2019 focus on arthroscopic repair or removal, originate from institutions within the United States and were published before 2010. Arthrosc Sports Med Rehabil 3(6):e2103–e2116
Article PubMed PubMed Central Google Scholar
Chakraborty D, Mohan L, Alex SA, Chandrasekaran N, Mukherjee A (2019) Bimetallic gold nanorods with enhanced biocorona formation for doxorubicin loading and sustained release. Biomater Sci 7(1):63–75
Article CAS Google Scholar
Chen C, Peng J, Xia H, Wu Q, Zeng L, Xu H et al (2010) Quantum-dot-based immunofluorescent imaging of HER2 and ER provides new insights into breast cancer heterogeneity. Nanotechnology 21(9):095101
Article PubMed Google Scholar
Deng Z, Zhen Z, Hu X, Wu S, Xu Z, Chu PK (2011) Hollow chitosan–silica nanospheres as pH-sensitive targeted delivery carriers in breast cancer therapy. Biomaterials 32(21):4976–4986
Di Vincenzo F, Yadid Y, Petito V, Emoli V, Masi L, Gerovska D et al (2023) Circular and circulating DNA in inflammatory bowel disease: from pathogenesis to potential molecular therapies. Cells 12(15):1953
Dongsar TT, Dongsar TS, Abourehab MA, Gupta N, Kesharwani P (2023) Emerging application of magnetic nanoparticles for breast cancer therapy. Eur Polymer J 187:111898
Dreaden EC, Mwakwari SC, Austin LA, Kieffer MJ, Oyelere AK, El-Sayed MA (2012) Small molecule–gold nanorod conjugates selectively target and induce macrophage cytotoxicity towards breast cancer cells. Small 8(18):2819–2822
Dubey SK, Kali M, Hejmady S, Saha RN, Alexander A, Kesharwani P (2021) Recent advances of dendrimers as multifunctional nano-carriers to combat breast cancer. Eur J Pharm Sci 164:105890
Eghtedari M, Liopo AV, Copland JA, Oraevsky AA, Motamedi M (2009) Engineering of hetero-functional gold nanorods for the in vivo molecular targeting of breast cancer cells. Nano Lett 9(1):287–291
Fakayode OJ, Kruger CA, Songca SP, Abrahamse H, Oluwafemi OS (2018) Photodynamic therapy evaluation of methoxypolyethyleneglycol-thiol-SPIONs-gold-meso-tetrakis (4-hydroxyphenyl) porphyrin conjugate against breast cancer cells. Mater Sci Eng, C 92:737–744
Fatima M, Abourehab MA, Aggarwal G, Jain GK, Sahebkar A, Kesharwani P (2022) Advancement of cell-penetrating peptides in combating triple-negative breast cancer. Drug Discovery Today 27(11):103353
Gao F, Xie W, Miao Y, Wang D, Guo Z, Ghosal A et al (2019) Magnetic hydrogel with optimally adaptive functions for breast cancer recurrence prevention. Adv Healthc Mater 8(14):1900203
Article Google Scholar
Gurunathan S, Park JH, Han JW, Kim JH (2015) Comparative assessment of the apoptotic potential of silver nanoparticles synthesized by Bacillus tequilensis and Calocybe indica in MDA-MB-231 human breast cancer cells: targeting p53 for anticancer therapy. Int J Nanomed 4203–4223
Ha GH, Kim DY, Breuer EK, Kim CK (2018) Combination treatment of polo-like kinase 1 and tankyrase-1 inhibitors enhances anticancer effect in triple-negative breast cancer cells. Anticancer Res 38(3):1303–1310
CAS PubMed Google Scholar
Javan Nikkhah S, Thompson D (2021) Molecular modelling guided modulation of molecular shape and charge for design of smart self-assembled polymeric drug transporters. Pharmaceutics 13(2):141
Jia J, Wu X, Long G, Yu J, He W, Zhang H et al (2023) Revolutionizing cancer treatment: Nanotechnology-enabled photodynamic therapy and immunotherapy with advanced photosensitizers. Front Immunol 14:1219785
Jin X, Zhao J, Li H, Zheng M, Shao J, Chen Z (2023) Research trends and hot spots in global nanotechnology applications in liver cancer: a bibliometric and visual analysis (2000–2022). Front Oncol 13
Karathanasis E, Chan L, Balusu SR, D’Orsi CJ, Annapragada AV, Sechopoulos I, Bellamkonda RV (2008) Multifunctional nanocarriers for mammographic quantification of tumor dosing and prognosis of breast cancer therapy. Biomaterials 29(36):4815–4822
Kher C, Kumar S (2022) The application of nanotechnology and nanomaterials in cancer diagnosis and treatment: a review. Cureus 14(9)
Kumar N, George BPA, Abrahamse H, Parashar V, Ngila JC (2017) Sustainable one-step synthesis of hierarchical microspheres of PEGylated MoS2 nanosheets and MoO3 nanorods: their cytotoxicity towards lung and breast cancer cells. Appl Surf Sci 396:8–18
Lee J, Chatterjee DK, Lee MH, Krishnan S (2014) Gold nanoparticles in breast cancer treatment: promise and potential pitfalls. Cancer Lett 347(1):46–53
Liu C, Deng C, Li Z, Liu Y, Wang S (2022) Optimization of spatial pattern of land use: progress, frontiers, and prospects. Int J Environ Res Public Health 19(10):5805
Liu S, Zhang HL, Li J, Ye ZP, Du T, Li LC et al (2023) Tubastatin A potently inhibits GPX4 activity to potentiate cancer radiotherapy through boosting ferroptosis. Redox Biol 62:102677
Londhe S, Haque S, Tripathy S, Bojja S, Patra CR (2023) Silver nitroprusside nanoparticles for breast cancer therapy: in vitro and in vivo approach. Nanoscale 15(23):10017–10032
Miele E, Spinelli GP, Miele E, Tomao F, Tomao S (2009) Albumin-bound formulation of paclitaxel (Abraxane ® ABI-007) in the treatment of breast cancer. Int J Nanomed 99–105
Mitra S, Dash R (2018) Natural products for the management and prevention of breast cancer. Evid-Based Complement Alternat Med 2018
Moloudi K, Abrahamse H, George BP (2023) Photodynamic therapy induced cell cycle arrest and cancer cell synchronization. Front Oncol 13:1225694
Mosleh-Shirazi S, Abbasi M, Reza Moaddeli M, Vaez A, Shafiee M, Kasaee SR et al (2022) Nanotechnology advances in the detection and treatment of cancer: an overview. Nanotheranostics 6(4):400
Mu Q, Wang H, Zhang M (2017) Nanoparticles for imaging and treatment of metastatic breast cancer. Expert Opin Drug Deliv 14(1):123–136
Mujahid MH, Upadhyay TK, Khan F, Pandey P, Park MN, Sharangi AB et al (2022) Metallic and metal oxide-derived nanohybrid as a tool for biomedical applications. Biomed Pharmacother 155:113791
Nikalje AP (2015) Nanotechnology and its applications in medicine. Med Chem 5(2):081–089
Okuyama H, Kagawa Y, Masuishi T, Mishima S, Shirasu H, Ando K et al (2021) Infusion-related reaction to ramucirumab plus FOLFIRI in patients with advanced colorectal cancer. Int J Clin Oncol 26(11):2025–2028
Park SM, Aalipour A, Vermesh O, Yu JH, Gambhir SS (2017) Towards clinically translatable in vivo nanodiagnostics. Nat Rev Mater 2(5):1–20
Pei Z, Chen S, Ding L, Liu J, Cui X, Li F, Qiu F (2022) Current perspectives and trend of nanomedicine in cancer: a review and bibliometric analysis. J Control Release 352:211–241
Shirwaiker RA, Samberg ME, Cohen PH, Wysk RA, Monteiro-Riviere NA (2013) Nanomaterials and synergistic low-intensity direct current (LIDC) stimulation technology for orthopedic implantable medical devices. Wiley Interdiscip Rev Nanomed Nanobiotechnol 5(3):191–204
Siegel RL, Miller KD, Wagle NS, Jemal A (2023) Cancer statistics, 2023. CA Cancer J Clin 73(1):17–48
Stinchcombe TE (2007) Nanoparticle albumin-bound paclitaxel: a novel Cremphor-EL ® -free formulation of paclitaxel
Tang K, Cai Z, Lv Y, Liu R, Chen Q, Gu J (2023) Scientometric research on trend analysis of nano-based sustained drug release systems for wound healing. Pharmaceutics 15(4):1168
Thomas NS, Scalzo RL, Wellberg EA (2024) Diabetes mellitus in breast cancer survivors: metabolic effects of endocrine therapy. Nat Rev Endocrinol 20(1):16–26
Wang J, Maniruzzaman M (2022) A global bibliometric and visualized analysis of bacteria-mediated cancer therapy. Drug Discovery Today 27(10):103297
Wang Z, Li Z, Sun Z, Wang S, Ali Z, Zhu S et al (2020) Visualization nanozyme based on tumor microenvironment “unlocking” for intensive combination therapy of breast cancer. Sci Adv 6(48):e8733
Xia Y, Yao RQ, Zhao PY, Tao ZB, Zheng LY, Zhou HT et al (2022) Publication trends of research on COVID-19 and host immune response: a bibliometric analysis. Front Public Health 10:939053
Xinchen Y, Jing T, Jiaoqiong G (2023) Lipid-based nanoparticles via nose-to-brain delivery: a mini review. Front Cell Dev Biol 11:1214450
Yan C, Wang J, Yang Y, Ma W, Chen X (2019) Molecular biomarker-guided anti-angiogenic targeted therapy for malignant glioma. J Cell Mol Med 23(8):4876–4882
You JO, Guo P, Auguste DT (2013) A drug-delivery vehicle combining the targeting and thermal ablation of HER2+ breast-cancer cells with triggered drug release. Angew Chem Int Ed 52(15):4141–4146
Zhang YJ, Li BA, Li ZY, Yu HY, Zhang YZ (2018) Synthesis and characterization of Tamoxifen citrate modified reduced graphene oxide nano sheets for breast cancer therapy. J Photochem Photobiol, B 180:68–71
Zhang R, Yang Y, Dong W, Lin M, He J, Zhang X et al (2022) D-mannose facilitates immunotherapy and radiotherapy of triple-negative breast cancer via degradation of PD-L1. Proc Natl Acad Sci 119(8):e2114851119
Zhou P, Adeel M, Shakoor N, Guo M, Hao Y, Azeem I et al (2020) Application of nanoparticles alleviates heavy metals stress and promotes plant growth: an overview. Nanomaterials 11(1):26
Download references
Acknowledgements
We thank Professor Zhenhua Lin [Key Laboratory of Pathobiology (Yanbian University), State Ethnic Affairs Commission] for revising this manuscript.
This research was supported by the National Natural Science Foundation of China (No.82160552), the National Natural Science Foundation of Jilin Province (YDZJ202201ZYTS245).
Author information
Authors and affiliations.
Key Laboratory of Pathobiology (Yanbian University), State Ethnic Affairs Commission, Yanji, 133000, China
Yue Li, Xiaoqing Li, Aoqun Li, Jingyan Zhu, Zhenhua Lin & Yang Yang
Department of Pathology and Cancer Research Center, Yanbian University, Gong Yuan Road No.977, Yanji, 133002, China
Xiaoqing Li, Aoqun Li, Jingyan Zhu, Zhenhua Lin & Yang Yang
Integration College, Yanbian University, Yanji, 133000, China
You can also search for this author in PubMed Google Scholar
Contributions
LY and LXQ performed the literature search and collected the data. LY performed the statistical analysis and wrote the manuscript, and prepared Figs. 1 , 2 , 3 . LAQ and ZJY prepared Figs. 4 , 5 . LZH revised the manuscript. LY and YY designed the study and conceived and revised the manuscript. All authors contributed to the article and approved the final manuscript.
Corresponding author
Correspondence to Yang Yang .
Ethics declarations
Consent for publication.
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Li, Y., Li, X., Li, A. et al. The research trends and future prospects of nanomaterials in breast cancer. Cancer Nano 15 , 24 (2024). https://doi.org/10.1186/s12645-024-00261-7
Download citation
Received : 25 March 2024
Accepted : 18 April 2024
Published : 25 April 2024
DOI : https://doi.org/10.1186/s12645-024-00261-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Nanomaterials
- Clinical value
- Breast cancer
- Visualized analysis
Cancer Nanotechnology
ISSN: 1868-6966
- Submission enquiries: [email protected]
We couldn’t find any results matching your search.
Please try using other words for your search or explore other sections of the website for relevant information.
We’re sorry, we are currently experiencing some issues, please try again later.
Our team is working diligently to resolve the issue. Thank you for your patience and understanding.
News & Insights

Top 3 Canadian Biotech Stocks of 2024

April 29, 2024 — 04:15 pm EDT
Written by Meagen Seatter for Investing News Network ->
Biotech is a dynamic industry that is driving scientific advancements and innovation in healthcare.
According to Grandview Research , the global biotech market was worth US$1.55 trillion in 2023, and the firm expects it to grow at a CAGR of 13.96 percent between 2024 and 2030 to reach a value of US$3.08 trillion.
In Canada, the biotech sector is home to companies pursuing cutting-edge therapies and medical technologies, and the Investing News Network has identified the top three biotech stocks based on their year-on-year gains.
Data was collected on April 16, 2024, using TradingView's stock screener and companies listed had market capitalizations of over C$50 million at that time. Companies on the TSX, TSXV and CSE were considered, but no TSXV-listed stocks made the list this time. Read on to learn what's been driving these biotech firms.
1. ME Therapeutics Holdings (CSE:METX)
Year-on-year gain : 7,240 percent; market cap: C$86.23 million; current share price:C$3.67
ME Therapeutics Holdings is a biotechnology company developing cancer-fighting drug candidates that can increase the efficacy of current immuno-oncology drugs by targeting suppressive myeloid cells, which have been found to hinder the effectiveness of existing immuno-oncology treatments. Immuno-oncology is a relatively new area of cancer drug research , and has shown promising results when used to treat cancer with low survival rates.
In December 2023, ME Therapeutics announced that its most advanced preclinical asset, h1B11-12, an antibody targeting G-CSF, had been found to bind to and neutralize G-CSF in lab tests and animal studies. Subsequent studies conducted with Dr. Kenneth Harder’s laboratory at the University of British Columbia revealed that G-CSF is involved in many different processes influencing how breast and colon cancers grow and spread.
In a January update, ME Therapeutics shared that preliminary results for trials of h1B11-12 on non-human primates were tolerated well up to a dose of 10 milligrams per kilogram. The next step is to study how the drug behaves inside the body, which will help the company plan future research and decide how to continue developing h1B11-12.
ME Therapeutics saw a major share price boost on February 27, when it announced a non-brokered private placement to raise gross proceeds of up to C$1.55 million. It said it was unaware of any other change that would account for the rise.
2. Cardiol Therapeutics (TSX:CRDL)
Year-on-year gain : 230.14 percent; market cap: C$160.18 million; current share price: C$2.41
Cardiol Therapeutics is a biopharma company developing innovative treatments for inflammation and fibrosis in cardiovascular conditions. Its research is concentrated on pericarditis, which is inflammation of the membrane surrounding the heart; myocarditis, or inflammation of the heart muscle; and heart failure.
Cardiol currently has two drug candidates in its pipeline. CardiolRX, the company's lead candidate, is being clinically developed for use in rare heart diseases . Aside from those efforts, Cardiol is developing a drug formulation of cannabidiol, called CRD-38 , for its efficacy in treating heart conditions subcutaneously.
The company's share price began a significant rise in mid-February, when the US Food and Drug Administration granted it orphan drug designation for CardiolRx. Less than a week later, Cardiol completed patient enrollment in a Phase II open-label pilot study investigating the safety, tolerability and efficacy of CardiolRx in patients with recurrent pericarditis. It said it was expecting top-line results in the second quarter of this year.
3. Medicenna (TSX:MDNA)
Year-on-year gain: 130 percent; market cap: C$131.61 million; current share price: C$1.84
Medicenna is a clinical-stage immuno-oncology company specializing in the development of innovative therapies for patients with challenging unmet needs. Its focus is on creating novel, highly selective versions of cytokines, such as IL-2, IL-4, and IL-13, which it refers to as "Superkines" and "empowered superkines."
Cytokines are small proteins that play a crucial role in regulating immune responses and helping cells communicate. Interleukins, which Medicenna says are at the core of its therapies, are groups of cytokines. The company's interleukins are engineered to fuse with specific molecules to optimize their function.
Medicenna's mission is to leverage its expertise in cytokine biology to design life-changing therapies that can potentially transform people's lives. Its therapies treat solid tumors, which have a low response rate to conventional cancer treatments, and autoimmune and neuroinflammatory diseases.
In February, the company shared the news that its lead candidate, MDNA11, had been used in combination with Keytruda in a study testing the effectiveness of the two drugs in treating patients with advanced solid tumors.
Don’t forget to follow us @INN_LifeScience for real-time news updates!
Securities Disclosure: I, Meagen Seatter, hold no direct investment interest in any company mentioned in this article.
Editorial Disclosure: Cardiol Therapeutics is a client of the Investing News Network. This article is not paid-for content.
The views and opinions expressed herein are the views and opinions of the author and do not necessarily reflect those of Nasdaq, Inc.

More Related Articles
This data feed is not available at this time.
Sign up for the TradeTalks newsletter to receive your weekly dose of trading news, trends and education. Delivered Wednesdays.
To add symbols:
- Type a symbol or company name. When the symbol you want to add appears, add it to My Quotes by selecting it and pressing Enter/Return.
- Copy and paste multiple symbols separated by spaces.
These symbols will be available throughout the site during your session.
Your symbols have been updated
Edit watchlist.
- Type a symbol or company name. When the symbol you want to add appears, add it to Watchlist by selecting it and pressing Enter/Return.
Opt in to Smart Portfolio
Smart Portfolio is supported by our partner TipRanks. By connecting my portfolio to TipRanks Smart Portfolio I agree to their Terms of Use .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
Latest science news, discoveries and analysis
Who’s making chips for AI? Chinese manufacturers lag behind US tech giants

France’s research mega-campus faces leadership crisis

'Orangutan, heal thyself': First wild animal seen using medicinal plant

UTIs make life miserable — scientists are finding new ways to tackle them
Found: the dial in the brain that controls the immune system, controversial virus-hunting scientist skewered at us covid-origins hearing, chinese virologist who was first to share covid genome sleeps on street after lab shuts, why is exercise good for you scientists are finding answers in our cells, how to meet africa’s grand challenges with african know-how alfred r. bizoza.

Sex and gender discussions don't need to be toxic

Plagiarism in peer-review reports could be the ‘tip of the iceberg’
Dad’s microbiome can affect offspring’s health — in mice
‘Shut up and calculate’: how Einstein lost the battle to explain quantum reality
Dark energy is tearing the universe apart. what if the force is weakening, mount etna’s spectacular smoke rings and more — april’s best science images, scientists tried to give people covid — and failed, the science of 3 body problem: what’s fact and what’s fiction.
Why it’s essential to study sex and gender, even as tensions rise

Male–female comparisons are powerful in biomedical research — don’t abandon them

We need more-nuanced approaches to exploring sex and gender in research
Daniel kahneman obituary: psychologist who revolutionized the way we think about thinking, allen j. bard obituary: electrochemist whose techniques underpin clinical diagnostics, materials discovery and more, current issue.

Support communities that will lose out in the energy transition
Do cutting-edge car-t-cell therapies cause cancer what the data say, marsupial genomes reveal how a skin membrane for gliding evolved, a recently quenched galaxy 700 million years after the big bang, a magnetar giant flare in the nearby starburst galaxy m82, research analysis.
Endurance exercise causes a multi-organ full-body molecular reaction
Dad’s gut microbes matter for pregnancy health and baby’s growth
Genomics reveal unknown mutation-promoting agents at global sites
Cells destroy donated mitochondria to build blood vessels
Intel brings quantum-computing microchips a step closer, resilience lessons from ancient societies are still relevant today, how to stop students cramming for exams send them to sea, robust optical clocks promise stable timing in a portable package.

I strive to make the Great Barrier Reef more resilient to heat stress

Scientists urged to collect royalties from the ‘magic money tree’

Breaking ice, and helicopter drops: winning photos of working scientists
Hunger on campus: why us phd students are fighting over food, my pi yelled at me and i’m devastated. what do i do, books & culture.

How volcanoes shaped our planet — and why we need to be ready for the next big eruption

Dogwhistles, drilling and the roots of Western civilization: Books in brief

The AI tuner
From multiverses to cities: books in brief, smarty plants controversial plant-intelligence studies explored in new book, nature podcast.

Latest videos
Nature briefing.
An essential round-up of science news, opinion and analysis, delivered to your inbox every weekday.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
VIDEO
COMMENTS
The Biology of Cancer. Cancer is a disease that begins with genetic and epigenetic alterations occurring in specific cells, some of which can spread and migrate to other tissues. 4 Although the biological processes affected in carcinogenesis and the evolution of neoplasms are many and widely different, we will focus on 4 aspects that are particularly relevant in tumor biology: genomic and ...
Cancer therapies have evolved considerably in recent decades, substantially improving the quality of life and survival of patients with cancer. In this issue, we launch our Series on Cancer ...
Cancer care has advanced at an impressive pace in recent years. New insights into tumor immunology and biology, combined with advances in artificial intelligence, nano tools, genetic engineering ...
Technology in Cancer Research & Treatment. Technology in Cancer Research & Treatment (TCRT) is a peer-reviewed, gold open access journal (featured in SCIE (Web of Science), PMC, and PubMed: MEDLINE) which focuses on the prevention, diagnosis, treatment, and monitoring of cancer. View full journal description.
Recent preclinical and clinical research has led to exciting advances related to high-grade serous ovarian cancer, from examining its cellular origins to gaining insight into DNA-damage-repair ...
Over the past few decades, RNA sequencing has significantly progressed, becoming a paramount approach for transcriptome profiling. The revolution from bulk RNA sequencing to single-molecular, single-cell and spatial transcriptome approaches has enabled increasingly accurate, individual cell resolution incorporated with spatial information. Cancer, a major malignant and heterogeneous lethal ...
cancer therapy such as chemotherapy and radiotherapy. In this paper we have focused on biotechnology techniques. for cancer contro l and discussed th e r ole of each t echnique with e xample and ...
July 27, 2020 , by NCI Staff. CRISPR is a highly precise gene editing tool that is changing cancer research and treatment. Credit: Ernesto del Aguila III, National Human Genome Research Institute. Ever since scientists realized that changes in DNA cause cancer, they have been searching for an easy way to correct those changes by manipulating DNA.
Our scientists pursue every aspect of cancer research—from exploring the biology of genes and cells, to developing immune-based treatments, uncovering the causes of metastasis, and more. Programs & Centers; Sloan Kettering Institute Pursuing basic and translational research across 9 programs and 100+ labs.
The Cancer Systems Biology Consortium (CSBC), which includes cancer biologists, engineers, mathematicians, physicists, and oncologists, aims to tackle the most perplexing issues in cancer to increase our understanding of tumor biology, treatment options, and patient outcomes.The initiative takes an integrative approach to cancer research, with the goal of improving the lives of people with cancer.
The Nature Portfolio editors who handle cancer primary research, methods, protocols and reviews bring you the latest articles, covering all aspects from disease mechanisms to therapeutic ...
Research focused on understanding the molecular mechanisms of cancer, and developing diagnostics and drugs for its cure. The most cross-disciplinary of contemporary research areas, cancer biotechnology research includes scientists from medicine, biology, physics and engineering disciplines. Faculty. Faculty members in cancer biotechnology include:
Browse and search. Web of Science is a crucial database for accessing global academic information (Pei et al. 2022).It can rapidly identify high-impact papers, reveal research directions that domestic and foreign authorities focus on, and expose the trend of subject development (Wang and Maniruzzaman 2022).A search was conducted in the WOSCC database for literature related to the use of ...
This Special Collection will feature articles from attendees of ICBEB 2023 that report on novel applications, design, basic research, and other aspects related to the use of biomedical engineering and biotechnology to detect, diagnose, or treat cancer. Research papers covering all aspects of biomedical engineering and biotechnology as they ...
2. Literature review. The PubMed biomedical repository and the dblp computer science bibliography were selected to perform a literature overview on ML-based studies in cancer towards disease diagnosis, disease outcome prediction and patients' classification. We searched and selected original research journal papers excluding reviews and technical reports between 2016 (January) and 2020 ...
An mRNA cancer vaccine quickly reprogrammed the immune system to attack the most aggressive type of brain tumor in a first-ever human clinical trial. In a first-ever human clinical trial of four ...
NK cells are innate lymphocytes critical for surveillance of viruses and tumors, however the mechanisms underlying NK cell dysfunction in cancer are incompletely understood. We assessed the effector function of NK cells from bladder cancer patients and found severe dysfunction in NK cells derived from tumors versus peripheral blood. While both peripheral and tumor-infiltrating NK cells ...
Browse the archive of articles on Nature Biotechnology. ... Research Article (441) Research Paper (510) Review Article (284) This Month in Biotechnology (328) Year. All. All; 2024 (179)
Biotech vets raise $67 million to develop targeted activators for cancer, Endpoints Regeneron's blockbuster eye drug posts weaker sales due to inventory impact, Reuters About the Author Reprints
G1 Therapeutics is based in Research Triangle Park. mehmet demirci. ... The biotech is focused on liver cancer and has the rights to develop the molecule in all indications except for certain ...
Cancer genomics is the study of the totality of DNA sequence and gene expression differences between tumour cells and normal host cells. It aims to understand the genetic basis of tumour cell ...
Biotech is a dynamic industry that is driving scientific advancements and innovation in healthcare.According to Grandview Research, the global biotech market was worth US$1.55 trillion in 2023 ...
Read the latest Research articles from Nature Biotechnology. ... Research Note (8) Research Paper (510) Resource (72) Roundtable (3) Speakers (61) Technical Report (92) Technologies (11)
Find breaking science news and analysis from the world's leading research journal.