You are using an outdated browser. Please upgrade your browser or activate Google Chrome Frame to improve your experience.

Download PDF
To view and print this document, you will need to download Adobe Acrobat Reader .

2015 - Use of Animals in Research
08 May 2015 publication
Research using animals is required to understand how microbes cause disease in human and animal hosts, how hosts respond to infection and the relationship between host microbiomes and health. Such research has been, and continues to be, vital for protecting human and animal health through the discovery and development of safe and effective vaccines, antibiotics and drugs. Through contributing to improved livestock health, research using animals also benefits society through helping to secure food production and the economic impact of agriculture.
Policy Officer , Microbiology Society.
UAR position statement on the use of animals in research
Understanding Animal Research (UAR) supports the humane use of animals in biomedical research, and believes that animal research is a vital part of the scientific process. For over 150 years research using animals has advanced scientific understanding of human and animal health and the impact of the environment on wildlife. This research should never be undertaken lightly and animals should only be used when there is no alternative method available.
Animal research is often used to discover how the basic processes of the body work, and how these can go wrong when the body is affected by disease. This knowledge is vital if we are to develop effective treatments for illnesses affecting humans or animals. Animal research has given us many treatments for critical conditions such as cancer and diabetes, and has allowed the development of preventive measures such as human vaccines. Hundreds of millions of human and animal lives have been saved or improved as a direct result of research on animals. It is sometimes necessary to test substances on animals to understand how they might affect the safety of people, animals and the environment, but only when it is not possible to gather this important information by other means.
We expect the animal research that we support to be of high quality: well-designed, ethically justified, subject to rigorous peer review, and well reported. Animal welfare is important to us, and we expect researchers, animal technologists and their supporting communities to strive towards excellent housing and experimental conditions for their research animals.
UAR is committed to the 3Rs of reduction, replacement and refinement as basic principles of humane animal research, and expects our members to actively employ these to improve animal welfare. This means that animals should be replaced with non-animal research methods wherever possible, that the minimum number of animals needed to give meaningful results should be used, and that research methods should aim to both improve the quality of the data obtained and reduce suffering wherever possible.
Openness and transparency around the use of animals in research is important if we are to have honest conversations about the benefits, harms and limitations of animal research. UAR aims to explain our positions and values clearly, and encourage the life-science sector to be clear about how, when and why animals are used in research. We have worked with the life-sciences community to develop the Concordat on Openness on Animal Research in the UK, driving a culture of greater openness on this issue. We strive to ensure that the scientific community is not at risk of, and does not feel at risk of, harassment or assault because of their association with animal research.
Good research practice and good animal welfare go hand in hand to generate good science. Good animal welfare relies, in turn, on the individuals who care for the animals and who carry out the research being supported, listened to, and educated. A caring institutional culture that allows staff to speak out where they see problems developing, and which supports them in their work-place is important to the well-being of the animals that they work with. We understand that many who work in animal research have chosen a career that allows them to make a real difference to the lives of people or animals, and we aim to support them to succeed.
Featured news

Bird flu in cows: is it a human problem ?

Openness, a powerful tool to support science

Long Covid, can animals provide the answers?
Subscribe to our newsletter.
Get the latest articles and news from Understanding Animal Research in your email inbox every month. For more information, please see our privacy policy .

Position Statement: Use of Animals in Research
April 22, 2021
The American Academy of Otolaryngology—Head and Neck Surgery recognizes the use of animals in research has enabled many of the medical and surgical treatments now available to the field of otolaryngology and surgery of the head and neck. The AAO-HNS support the judicious and appropriate use of animals in research for the advancement of medical knowledge and the development of novel surgical and medical interventions.
The AAO-HNS support for the use of animals in research is predicated on the humane and ethical treatment of the animals. The AAO-HNS stresses adherence to all appropriate federal, state, local, and institutional laws and guidelines that regulate the use of animals in research. Studies employing animals should undergo institutional review and institutions are encouraged to seek certification from a qualified accrediting association.
The AAO HNS recognizes replacing all animal utilization is not feasible while continuing to develop advanced therapies for the most complex disorders of the Head and Neck. When possible, simulation technology, in vitro techniques, and detailed review of available literature should be utilized to reduce the number of animals impacted, optimize the overall investigation, and when possible replace animal usage.
Important Disclaimer Notice (Updated 7/31/14)
Position statements are approved by the American Academy of Otolaryngology—Head and Neck Surgery or Foundation (AAO-HNS/F) Boards of Directors and are typically generated from AAO-HNS/F committees. Once approved by the Academy or Foundation Board of Directors, they become official position statements and are added to the existing position statement library. In no sense do they represent a standard of care. The applicability of position statements, as guidance for a procedure, must be determined by the responsible physician in light of all the circumstances presented by the individual patient. Adherence to these clinical position statements will not ensure successful treatment in every situation. As with all AAO-HNS/F guidance, this position statement should not be deemed inclusive of all proper treatment decisions or methods of care, nor exclusive of other treatment decisions or methods of care reasonably directed to obtaining the same results. Position statements are not intended to and should not be treated as legal, medical, or business advice.
Related Content
Pediatric otolaryngology expert consensus on use and re-use of tracheostomy tubes.
Intended applicable time frame and scope:This statement is intended as a recommendation for the use and re-use of pediatric…
Position Statement: Vaccination Status and Obligation to Provide Care
Background The consequences of the COVID-19 pandemic continue to reverberate. They may disproportionately impact the practice of otolaryngology given…
Position Statement: Tongue Based Procedures
Genioglossus advancement and hyoid myotomy/suspension, whether performed separately or combined, are considered effective and non-investigational with proven c…
Privacy Overview
- Search Menu
- Advance Articles
- Editor's Choice
- Special Issues
- Author Guidelines
- Submission Site
- Open Access
- Self-Archiving Policy
- Reasons to Publish
- About Biology of Reproduction
- Editorial Board
- Advertising & Corporate Services
- Journals Career Network
- Trainee Reviewer Program
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Ssr position statement on the use of animals in research and education.
- Article contents
- Figures & tables
- Supplementary Data
SSR Position Statement on the use of Animals in Research and Education, Biology of Reproduction , Volume 64, Issue 1, 1 January 2001, Page iii, https://doi.org/10.1093/biolreprod/64.1.iii
- Permissions Icon Permissions
The Society for the Study of Reproduction affirms the essential contribution of animals in research and education aimed at improving the health and well being of both humans and animals. The role of animals remains critical in understanding the fundamental processes of life and in developing treatments for injury and disease. The SSR believes that educational objectives are best met when teaching focuses on animals as living, sentient creatures, emphasizing their behavior, life history, and relationships with their environment. The SSR considers that the use of animals in education is a privilege, which imposes a major responsibility on educators to provide for their proper care and humane treatment.
For pre-college biology education, the SSR deems that the educational value of using living animals is not sufficient to justify major manipulations of their behavior or environment or any procedures that cause pain, distress or discomfort. At this level, activities involving live animals should be limited to supervised observations of behavior, growth, and development of domestic mammals, birds, fish, amphibians, reptiles and invertebrates, and their routine care. Educators proposing to involve animals in the classroom or laboratory at this or any level should be familiar with and inform their students of basic animal care and use laws and guidelines with a brief explanation of their value. The SSR supports the use of biological specimens for anatomical or physiological study, provided that their procurement and use are in strict compliance with federal legislation, guidelines and policies of the National Institutes of Health, the US Department of Agriculture, and other such agencies as may be appropriate.
The SSR recognizes that the use of live animals in carefully designed and properly monitored laboratory exercises is an indispensable part of training in certain programs of higher education. Knowledge, experience and insights gained through the responsible use of live animals in the classroom and laboratory are unique, invaluable and irreplaceable elements of a quality education in many basic and clinical disciplines.
In all situations where animal use is envisioned, the SSR advocates both the careful consideration of alternatives, and the highest standards of husbandry and care when animals must be used. In considering alternatives in the design of educational experiences involving animals, the SSR advocates principles embodied by the 3 R’s (replacement, reduction, refinement). Therefore, educators should consider alternative methods that might serve as effective replacements of sentient animal models, adopt practices that will reduce the number of animals needed for effective educational experiences, and refine techniques in order to minimize or eliminate pain, distress or discomfort in animals that must be used. This includes the judicious use of sedation, analgesia, or anesthesia when appropriate. At institutions of higher education, it is expected that all procedures involving vertebrate animal use will be reviewed and approved by an appropriately appointed institutional animal care and use committee (IACUC), and that provision will be made for the training of all personnel involved in the care and use of animals.
Prepared by Drs. Chris Price, Frank F. Bartol and the 1999𡀓2000 SSR Animal Care & Experimentation Committee
Approved by SSR Board of Directors, 2000 Summer Meeting, Dr. John Eppig, President
Email alerts
Citing articles via.
- Recommend to Your Librarian
- Advertising and Corporate Services
Affiliations
- Online ISSN 1529-7268
- Print ISSN 0006-3363
- Copyright © 2024 Society for the Study of Reproduction
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Position Statement
Animals in research, american humane strongly supports the development of alternative methods to the use of animals in biomedical research..
When an animal must be used for research purposes, American Humane endorses the 3 Rs: refinement of tests so animal distress or pain is minimal, reduction of the number of animals used in a study and the replacement, whenever possible, of animal experiments with non-animal experiments. We also support taking the 3 Rs to a higher level by first ensuring that animals are involved only when their use is the most ethically acceptable way to address the question being researched.
- Prior to beginning a study, scientists must demonstrate that they have considered alternative methods to animal research, that their research cannot successfully be done without animal models and that their studies are designed to produce needed results and information.
- Scientists must also consider how to give these animals the best life possible in the lab. Environments must provide safety, comfort, cleanliness and enrichment, and pain and fear should be controlled.
- No animal should be put in a position of experiencing severe suffering.
Laboratory animals should not include those from animal shelters or animal control facilities.
American Humane supports the fields of in vitro and in silico toxicology to promote and develop New Approach Methodologies and other non-animal models, such as 3D organotypic cell models with high-content and high-throughput approaches and metabolomics, proteomics, transcriptomics, miRNA profiling and imaging techniques.
American Humane acknowledges that legal requirements necessitate animal testing prior to the approval of vaccines, drugs and other specific products. Many of these products are needed to advance human and animal health. American Humane supports changes in laws and regulations to eliminate unnecessary animal research and applauds the EPA’s plan to end animal testing by 2035.

- Newsletter Signup
- Advertising
- Board of Directors
- Committee Chairs
- Regional Coordinators
- Section Chairs
- NABT Position Statements
- OBTA Directors
- Sustaining Members
- Leader Nomination Form
- Free ABT Articles
- ABT Archive
- ABT Current Issue
- ABT Digital Edition
- Library Subscriptions
- Manuscript Reviewers Form
- Classroom Resources
- COVID-19 Updates
- Classroom/Laboratory Supplies
- Classroom Technology/Media
- Educational Advancement
- Company Listings
- Downloadable Books
- News & Views
- Education Research Symposium
- Inclusive Teaching Symposium
- Recommend a Resource
- About Awards
- Award Nomination Form
- Past Winners
- 2024 Conference
- 2024 Conference Exhibits
- Future Conferences
- Past Conferences
- CESR Poster Session
- Workshops & Webinars
- BioClub Chapters
- How to Start a Chapter
- New Chapter Application
- Four-Year Guidelines
- Governance & Minutes
- Poster Session
- Research Symposium
- Two-Year Guidelines
- NABT Book Club
- Volunteer at NABT
- Gifts and Logowear
Login Donate

Position Statements
Nabt position statements are regularly updated after adoption and approved by the nabt board of directors. to learn more about this process or to suggest future statements, please contact nabt., the use of animals in biology education.
High quality life science education requires students to be immersed in the study of life and living systems. Educators and schools across the education spectrum should develop programs, policies, and procedures that give students the broadest opportunity to learn the life sciences through field and laboratory experiences that incorporate living and formerly living organisms. NABT strongly supports teaching which allows for student interaction with organisms, both living and dead, that provides enriched, meaningful learning experiences. The involvement of students in first-hand interactions with living animals provides opportunities for increased understanding of content knowledge, the care of living organisms, and appreciation for the value of life. In like manner, the engagement of students in well-crafted dissections is a total sensory experience that removes abstraction as students learn about structure, function, adaptation, and diversity. While the increased quality and accessibility of dissection simulation software has helped address concerns from students and parents opposed to dissection, these alternatives are not without limitations. Utilizing a software-only approach may constitute a disservice to many students and does not acknowledge the well-documented educational benefits of hands-on dissection. Simultaneously, teachers must be sensitive to the beliefs of each student and their right to make informed decisions concerning participation in dissection and, if possible, provide meaningful alternatives in keeping with course goals and objectives. Teachers are the primary role model of respect for living and preserved specimens used in the classroom and for the conservation of organisms both in the classroom and in the field. As such, whenever utilizing organisms for instructional programming, they must employ their expertise to design and execute well-crafted lessons. Teachers have the responsibility to develop and maintain their training on the care and maintenance of living organisms. Likewise, educational institutions have the responsibility to ensure facilities, policies, and procedures are in place for the proper handling of living and non-living organisms. This includes understanding and complying with federal, state, and local laws regarding animal welfare and the use of biological materials, and knowing and utilizing established professional standards and guidelines as applicable. Professional guidelines may include, but are not limited to:
- Guide for the Care and Use of Laboratory Animals from the National Research Council
- Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals from the Office of Laboratory Animal Welfare (OLAW) of the National Institutes of Health
- Principles and Guidelines for the Use of Animals in Precollege Education from the Institute of Laboratory Animal Research (ILAR)
- Guidelines for Ethical Conduct in the Care and Use of Animal s from the American Psychological Association (APA)
The proper and ethical use of animals in science classrooms must always be matched to the stated standards and objectives for the course, the age and previous experiences of the intended student audience, and the expected educational outcomes. It is, therefore, the professional science educators in the classroom and in their professional learning community who are in the best position to make the determination for the use of animals in life science classes. As an organization of professional educators representing classroom teachers as well as professional scientists and education researchers, NABT urges state, provincial, and local school systems to enact and maintain policies concerning animals and other biological materials that allow students the opportunity to learn through the processes and practices of science utilizing all tools and methodologies available to them.
Revised and adopted by the NABT Board of Directors, July 2019. This position supersedes and replaces all previous NABT statements regarding the use of animals in biology education.
Downloadable files.
- Press Release

The National Association of Biology Teachers empowers educators to provide the best possible biology and life science education for all students.
NABT, P.O. Box 3363 Warrenton, VA 20188 [email protected] | Fax: (202) 962-3939 (888) 501-NABT or (703) 264-9696
Privacy Policy
Thank you for visiting the NABT website. Our privacy policy is found here .
Newsletter Sign-up
Announcements for products or services on this website do not imply endorsement of or by NABT.
Website by Morweb.org
Copyright National Association of Biology Teachers

UAR Oceania position statement on the use of animals in research
Understanding Animal Research Oceania (UAR Oceania) supports the humane use of animals in biomedical research, and believes that animal research is a vital part of the scientific process. For over 150 years research using animals has advanced scientific understanding of human and animal health and the impact of the environment on wildlife. This research should never be undertaken lightly and animals should only be used when there is no alternative method available.
Animal research is often used to discover how the basic processes of the body work, and how these can go wrong when the body is affected by disease. This knowledge is vital if we are to develop effective treatments for illnesses affecting humans or animals. Animal research has given us many treatments for critical conditions such as cancer and diabetes, and has allowed the development of preventive measures such as human vaccines. Hundreds of millions of human and animal lives have been saved or improved as a direct result of research on animals. It is sometimes necessary to test substances on animals to understand how they might affect the safety of people, animals and the environment, but only when it is not possible to gather this important information by other means.
We expect the animal research that we support to be of high quality: well-designed, ethically justified, subject to rigorous peer review, and well reported. Animal welfare is important to us, and we expect researchers, animal technologists and their supporting communities to strive towards excellent housing and experimental conditions for their research animals.
UAR Oceania is committed to the 3Rs of reduction, replacement and refinement as basic principles of humane animal research, and expects our members to actively employ these to improve animal welfare. This means that animals should be replaced with non-animal research methods wherever possible, that the minimum number of animals needed to give meaningful results should be used, and that research methods should aim to both improve the quality of the data obtained and reduce suffering wherever possible.
Openness and transparency around the use of animals in research is important if we are to have honest conversations about the benefits, harms and limitations of animal research. UAR Oceania aims to explain our positions and values clearly, and encourage the life-science sector to be clear about how, when and why animals are used in research. We work with the life-sciences community across Oceania to support and provide resource to underpin ANZCCART's Openness agreements in both New Zealand and Australia, driving a culture of greater openness on this issue. We strive to ensure that the scientific community is not at risk of, and does not feel at risk of, harassment or assault because of their association with animal research.
Good research practice and good animal welfare go hand in hand to generate good science. Good animal welfare relies, in turn, on the individuals who care for the animals and who carry out the research being supported, listened to, and educated. A caring institutional culture that allows staff to speak out where they see problems developing, and which supports them in their work-place is important to the well-being of the animals that they work with. We understand that many who work in animal research have chosen a career that allows them to make a real difference to the lives of people or animals, and we aim to support them to succeed.
Featured news

ANZCCART New Zealand releases second annual report on Openness Agreement

Why do 90% of drugs fail?

New statistics show fish were the most widely used species in New Zealand’s animal-based research in 2021
Subscribe to our newsletter.
Get the latest articles and news from Understanding Animal Research in your email inbox every month. For more information, please see our privacy policy .
- Open access
- Published: 22 April 2024
A review of animal models utilized in preclinical studies of approved gene therapy products: trends and insights
- Parham Soufizadeh 1 , 2 ,
- Vahid Mansouri 1 &
- Naser Ahmadbeigi 1
Laboratory Animal Research volume 40 , Article number: 17 ( 2024 ) Cite this article
98 Accesses
Metrics details
Scientific progress heavily relies on rigorous research, adherence to scientific standards, and transparent reporting. Animal models play a crucial role in advancing biomedical research, especially in the field of gene therapy. Animal models are vital tools in preclinical research, allowing scientists to predict outcomes and understand complex biological processes. The selection of appropriate animal models is critical, considering factors such as physiological and pathophysiological similarities, availability, and ethical considerations. Animal models continue to be indispensable tools in preclinical gene therapy research. Advancements in genetic engineering and model selection have improved the fidelity and relevance of these models. As gene therapy research progresses, careful consideration of animal models and transparent reporting will contribute to the development of effective therapies for various genetic disorders and diseases. This comprehensive review explores the use of animal models in preclinical gene therapy studies for approved products up to September 2023. The study encompasses 47 approved gene therapy products, with a focus on preclinical trials. This comprehensive analysis serves as a valuable reference for researchers in the gene therapy field, aiding in the selection of suitable animal models for their preclinical investigations.
In the realm of gene therapy, a pivotal moment arrived with Paul Berg’s groundbreaking identification of the first recombinant DNA in 1972 [ 1 ]. This achievement not only marked a significant milestone but also served as the catalyst for a series of transformative breakthroughs in the field. Berg’s discovery fundamentally altered the landscape of genetic research, opening doors to novel therapeutic possibilities and paving the way for a new era of innovation and advancements in genetic engineering and gene therapy. Given the accelerated development of gene therapy products throughout the past century, this trend is anticipated to persist into the future [ 2 ], with a substantial portion of therapeutic inquiries focusing on preclinical investigations.
The principal objective of this comprehensive review article is to scrutinize and interpret preclinical research about gene therapy products that have garnered current approval and are presently administered to patients. This endeavour aspires to serve as an invaluable reference for researchers embarking on endeavours within the realm of gene therapy, seeking suitable animal models to facilitate their scientific undertakings.
The importance of preclinical studies in gene therapy clinical trials
Preclinical studies in the field of gene therapy play a pivotal role in advancing our understanding of genetic diseases and developing potential treatments. Additionally, all scientific progress and development are intricately intertwined with prior research endeavours. For scientific investigations to pave the way for significant advancements, they should embody three distinct attributes: (1) Adherence to Scientific Standards: The formulation and documentation of a study must strictly adhere to established scientific norms and guidelines. (2) Rigorous Parameterization in Animal Studies: In the realm of animal studies, meticulous attention to parameters is essential to ensure the reliability and validity of such investigations. (3) Transparent and Comprehensive Reporting: Researchers should exert utmost diligence in generating a report that is transparent, comprehensive, and credible in its entirety [ 3 ]. When these fundamental principles are observed in animal studies, they hold the potential to yield profound implications for the development of therapeutic products and our comprehension of disease pathophysiology. For instance, one of the most significant advantages of preclinical gene therapy studies is their ability to address diseases that lack effective avenues for investigation in human subjects, especially in the case of rare genetic diseases. In such instances, the creation of a standardized disease model not only facilitates the examination of all disease stages but also allows for elucidating the initial pathophysiological processes, even before the onset of clinical manifestations. Furthermore, some of these models elucidate genetic interrelationships, thereby uncovering potential modifier genes, a pursuit unfeasible within the confines of human subjects [ 4 ].
However, it is important to note that the success of preclinical gene therapy studies heavily relies on their adherence to scientific rigor, transparency, and meticulous reporting. The lack of these attributes can lead to issues such as irreproducibility and non-reproducibility, which hinder progress in the field [ 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 ]. This predicament often arises due to incomplete or inaccurate descriptions within research protocols, encompassing the allocation of animals among disparate study groups and the criteria underpinning the formation of said groups [ 11 ]. In addition to the formidable challenge of irreproducibility, another substantial hurdle resides in the discordance between the outcomes of animal studies and the results obtained from clinical trials. For example, clinical trials investigating stroke frequently yield results that diverge markedly from those generated in preclinical studies of the same condition. Root causes for this dissonance have been traced to the inability of any animal model to faithfully replicate the intricacies of human patients and the absence of robust, well-documented methodologies in the conduct of animal studies [ 13 ].
Considering the aforementioned quandaries, animal studies that yield congruent results in clinical trials can furnish superior methodologies for advancing subsequent investigations in related domains.
Animal models in gene therapy
The use of animal models in biomedical research, including gene therapy, is essential for gaining insights into complex biological systems and predicting the behaviour of interventions under specific conditions. These models serve as invaluable tools for researchers and can broadly be categorized into two primary functions: elucidating a system or process and predicting the behaviour of the target in question [ 14 ]. The concept of analogical reasoning, as initially introduced by Kant in the “Critique of Judgment”, posits that qualitative similarities between entities can be leveraged to forecast causal relationships, even in the presence of disparities [ 14 ]. With the advent of this concept, the application of models expanded across various scientific disciplines [ 15 ]. For instance, in the field of shipbuilding, scaled-down models are scrutinized to assess their designs, as hydrodynamics principles remain consistent, independent of scale. Conversely, in the biomedical sciences, including gene therapy, scalability lacks relevance [ 14 ] due to the diverse physical and behavioural attributes of organisms that impede such modelling. According to August Krogh’s principle, “For many problems, there is an animal on which it can be most conveniently studied” [ 16 ]. In biological sciences, the concept of analogy has supplanted scale, and its widespread applicability is attributed to the notion of “unity in diversity”, signifying fundamental relationships among organisms in terms of evolution and development [ 14 ]. Consequently, numerous animal models, notably laboratory animals such as mice, have been harnessed in diverse biological research endeavours.
Until 1980, mouse models predominantly comprised wild-type or spontaneously mutant species. Progress in fields such as chemotherapy and DNA-damaging agents owes much to the utilization of these animal models. Over the last four decades, a multitude of models catering to distinct objectives have emerged, thereby fostering advancements across various domains of biological science [ 17 ]. In recent decades, the significance of animal models has burgeoned due to the expansion of therapeutic product development, increased preclinical testing, and clinical trials. Foretelling therapeutic and safety outcomes in humans now constitutes the primary objective of experiments conducted before these products enter development, heavily contingent upon the judicious utilization of animal models [ 18 ].
The classification of animal models in the gene therapy era poses a formidable challenge, given their rapid proliferation and ongoing evolution. Moreover, diverse types of animal models each serve specific purposes, underscoring the critical importance of selecting the ideal model aligned with the research objectives. Meticulous model selection is imperative, as an erroneous choice can lead to inefficient resource allocation, ethical quandaries, and the generation of erroneous and unreliable scientific findings, potentially perpetuating inaccuracies in future experiments [ 19 ]. A 1985 NRC (National Research Council) report outlined various factors for the judicious selection of an appropriate animal model [ 14 ]. Paramount among these factors is the consideration of physiological and pathophysiological similarities between the model and the target of research. Additionally, the model’s capability to emulate desired conditions, such as disease-like states similar to those in the target (e.g., humans), warrants due consideration. Factors encompassing the model’s availability, size, lifespan, and others also play integral roles in this selection process [ 20 ]. Furthermore, individuals should be vigilant about potential mental and unconscious biases when selecting models, as familiarity or ease of use may unduly influence their choices [ 14 ].
One approach to mitigate the risk of inappropriate model selection involves the utilization of models specifically engineered for diverse conditions, such as genetically modified or humanized models closely mirroring human physiology in many aspects [ 21 ]. These models have witnessed substantial growth and find widespread application in research. Additionally, there are instances where a single animal model may prove inadequate to fulfill research objectives, necessitating the concurrent use of multiple models to ensure reliable and desired research outcomes [ 22 ]. Despite the multifaceted aspects elucidated concerning animal models, they are not the panacea for generalizing results and making biomedical predictions. It is essential to recognize that while alternatives to animal models have advanced significantly, they remain the sole practical choice for numerous experiments pertinent to human-related investigations. Numerous studies underscore that, notwithstanding their limitations, animal models persist as the primary resource for a multitude of experiments involving human subjects [ 14 ].
Preclinical gene therapy studies
In this comprehensive analysis, a total of 47 approved gene therapy products, spanning from the inaugural approval of Vitravene to the latest sanctioned product as of September 2023, were meticulously scrutinized. The principal aim of this investigation entailed the retrieval of peer-reviewed publications about the preclinical trials of each product. This endeavour encompassed an extensive exploration through various means, including the pursuit of literature referencing the product’s generic nomenclature, the examination of the backgrounds of the contributing authors, and the scrutiny of pertinent articles from diverse sources. In some instances, official documents released by the regulatory bodies responsible for product approval were also consulted. In certain cases, regrettably, no accessible information concerning preclinical drug investigations was ascertainable. It is noteworthy that references cited within articles linked to the product under study were occasionally examined, even if the specific product was not explicitly mentioned therein. Furthermore, it should be noted that in several instances, multiple animal models were employed for the preclinical assessments. Additionally, a prevalent feature across the majority of these investigations was the reliance on common laboratory animals for safety and pharmacological studies, albeit without explicit specification.
The aggregate findings of this extensive inquiry yielded a corpus of 74 distinct animal models. The classification of animal models can be approached through various taxonomies, such as that delineated by Prabhakar, which delineates four primary categories: inbred strains, disease induction, xenograft, and genetically engineered models. Inbreeding has classically been used to obtain genetically homogeneous animals. Disease induction models are very commonly used to examine pathophysiology and drug development. Disease induction animal models involve manipulating animals to study and replicate specific diseases for research purposes. Xenograft animal models involve transplanting human cells, tissues, or tumour s into immunodeficient animals to study disease and treatment responses. Genetically engineered models are developed by altering the genetic composition of an animal by mutating, deleting, or overexpressing a targeted gene [ 23 ].
In alignment with the research objectives of this study, the “inbred” category within Prabhakar’s taxonomy was omitted, and a novel category denominated “spontaneous or natural occurrence” was introduced. Spontaneous or naturally occurring animal models involve the natural development of a disease in animals without deliberate manipulation for research purposes [ 24 ]. Consequently, the animal models under examination were categorized into four principal groups: disease induction, xenograft, genetically engineered, and spontaneous. In instances where the available information regarding the nature of the animal model utilized in the preclinical investigations of the product was indistinct or inadequately documented, such instances were classified as not applicable or N/A. It is pertinent to highlight that certain animal models were the product of mating between two animals with predetermined genetic attributes. In cases where the parentage of such models was naturally occurring, they were categorized as spontaneous. Conversely, if one or both progenitors had undergone genetic manipulation, their progeny were categorized as genetically engineered ( Fig. 1 ) .
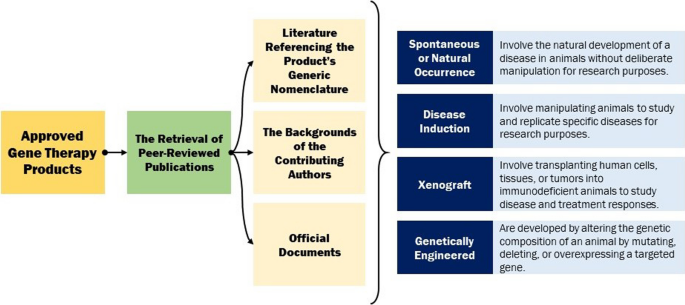
Overview of the study. In this study, by reviewing the available documents about the approved gene therapy products, the animal models used are categorized into 4 main sections
In the broader context, the analysis revealed that the genetically engineered category accounted for 39% of the identified animal models, followed by xenograft, disease induction, and spontaneous categories, with contributions of 19%, 15%, and 5%, respectively (Fig. 2 ). Additionally, 22% of the discerned animal models fell into the N/A category. Among the gamut of models scrutinized, mice emerged as the most frequently employed animal species, constituting 54% of the studies. Nonhuman primates claimed the second position, representing 20% of the investigated studies. Notably, other species were also incorporated into these investigations, including rats, rabbits, dogs, guinea pigs, and cats. A total of 6% of the studies did not involve the utilization of animal models (Fig. 3 ).
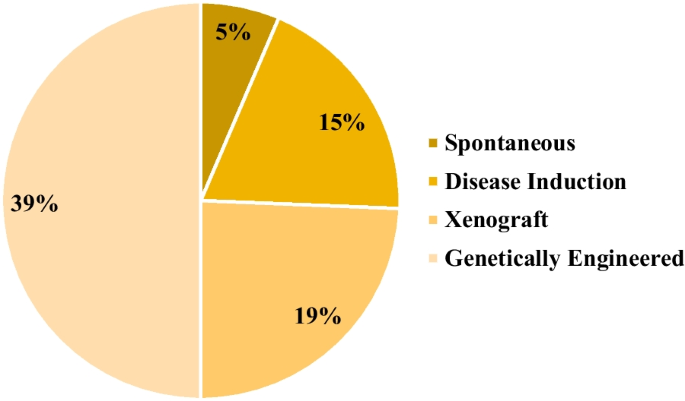
Preclinical studies based on the category of animal model development
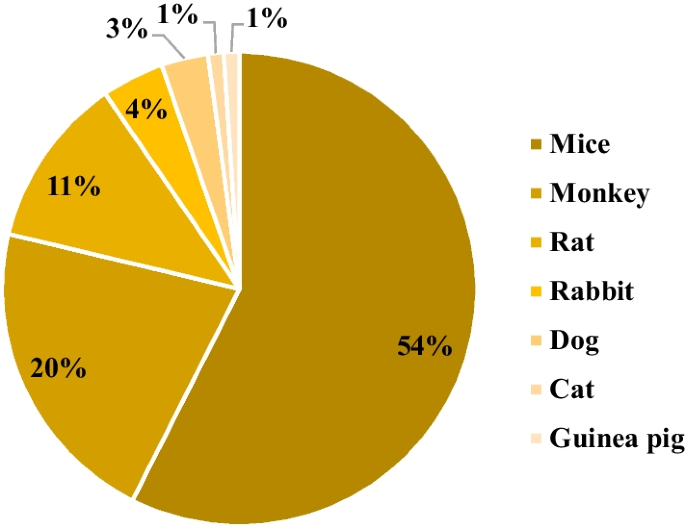
Preclinical studies based on the species of animal model
Furthermore, a granular examination of each category revealed distinctive utilization patterns. In the genetically engineered category, mice predominated, accounting for 79% of the animal species used, trailed by rats at 17%, and nonhuman primates at 7%. In the disease induction category, nonhuman primates emerged as the most frequently employed species, constituting 37% of the cases, with mice and rabbits equally sharing an 18% representation, while rats accounted for 27%. The xenograft category was overwhelmingly dominated by mice, comprising 93% of the animal species employed, with the residual 7% being nonhuman primates. In the spontaneous category, dogs featured 50% of the cases, followed by cats and mice, both with equal prevalence. Consequently, mice held sway in the genetically engineered and xenograft categories, while monkeys took precedence in the disease induction category, albeit with a caveat that 53% of the instances involving monkeys were categorized as uncertain, lacking substantive information regarding their role in the conducted studies. In the genetically engineered and disease induction categories, rats featured prominently (Table 1 ).
Utilization of animal models in preclinical investigations of cancer-related products
Among the 74 scrutinized studies, 18 were pertinent to cancer-related products (Table 2 ). Notably, animal models predominated as a fundamental component of these investigations, with the xenograft methodology being the principal mode of model generation, encompassing 61% of cancer-related animal models. In contrast, the remaining 39% comprised 6% attributed to genetic engineering, and 33% either lacked explicit animal model descriptions or adopted unspecified models. A significant proportion of 67% featured mice as the primary animal model species. Additionally, monkeys were employed in 11% of the studies related to cancer, while a singular study employed guinea pigs. Remarkably, a subset of three studies within this domain dispensed together the use of animal models.
Within the realm of preclinical appraisals about the aforementioned products, cell line-derived xenograft (CDX) models were notably prominent, particularly in the context of bone marrow cancers. It is worth highlighting that nude or immunodeficient mice receiving cancer cell grafts constituted the most frequently employed animal species. Moreover, the products Carvykti and Oncorine uniquely involved the utilization of monkeys and guinea pigs, respectively. In the context of lymphoma, associated with five distinct products, namely, Carteyva, Breyanzi, Tecartus, Kymriah and Yescarta, a conspicuous deficiency in efficient animal models for lymphoma was observed. Consequently, the relevant documentation articulated the absence of animal studies conducted for lymphoma [ 33 , 34 , 37 , 38 ]. However, in the case of Breyanzi, a noteworthy exception emerged, wherein despite the initially stated lack of an efficient model for lymphoma, pharmacological investigations were conducted employing a Raji xenograft animal model [ 37 ]. This model was fashioned based on a distinctive framework devised by Buchsbaum and colleagues [ 38 ], characterized by specific attributes. A solitary instance within this purview featured the application of a conditional knockout mouse model, exclusively pertinent to Gendicine. It is pertinent to note that the spectrum of animal models for this particular drug extends more comprehensively, albeit with limited available information drawn from recent studies [ 25 ].
Utilization of animal models in preclinical investigations of nononcological products
Among the 74 scrutinized studies, 52 were directed toward nononcological products, encompassing a substantial proportion dedicated to genetic disorders (Table 3 ). In contrast to preclinical studies of cancer, 55% of the investigations in this section employed genetically engineered as the primary method for generating animal models. Induction techniques were applied in 17% of instances, while natural occurrences accounted for 8%, and xenografts represented 4%. The preeminent animal model employed in nononcological inquiries paralleled the cancer research sphere, with mice serving as the predominant choice, utilized in 53% of cases. In addition to mice, nonhuman primates featured more prominently, constituting 19% of the studies. Rats were also frequently enlisted, contributing to 16% of the animal models in this category. Other species enlisted in this realm comprised rabbits (4%), dogs (4%), and cats (2%).
Significantly, a substantial portion of the models within this category was rooted in genetically engineered models. Such models in preclinical studies emanated from two principal avenues: procurement from commercial laboratories or in-house generation by researchers. Moreover, in some investigations, the primary model served as a foundation, inheriting genetic alterations from other genetically engineered models, or the foundational disease model emerged through the mating of two distinct genetically modified models (as observed in the EMA (European Medicines Agency) document for Rovtavian) [ 83 ]. Additionally, mice, rats, and nonhuman primates were the prevalent species subjected to genetic engineering, each bearing unique attributes pertinent to specific research objectives. In the majority of cases, animals exhibited specific genetic aberrations, albeit certain exceptions involved the use of highly immunodeficient mice, as exemplified in the Skysona study [ 79 ].
Beyond genetic engineering, induction, natural occurrences, and xenograft methods also found applicability within this category. The induction methodology was multifariously employed to replicate disorders such as adult familial chylomicronemia syndrome and ischemia or arteritis, accomplished through specialized dietary regimens or surgical procedures. Rat and monkey species constituted the primary subjects of experimentation within this domain, although mice and rabbits were sporadically incorporated. In the natural occurrence category, dogs emerged as the primary species of choice, with a solitary instance of cat utilization documented [ 44 ]. A noteworthy case, pertinent to the Libmeldy product, involved the creation of an animal model through the interbreeding of two species with naturally occurring disorders [ 72 ]. In contrast, the adoption of xenograft techniques was relatively limited in this category, with only three investigations resorting to this method. Notably, Vyjuvek and Strimvelis product research incorporated the grafting of cells bearing disease-related defects into severely immunodeficient mice [ 49 , 86 ]. The study associated with the Zalmoxis product similarly employed this method to augment the immune system following the grafting of hematopoietic stem cells.
Of the 74 examined studies, 4 studies were concerned with products about infectious diseases (Table 4 ). In these infectious disease inquiries, the predominant animal models of choice encompassed nonhuman primates and rabbits, primarily induced through techniques such as induction.
Trending approaches in the development of animal models for investigative research
The preeminent method for establishing animal models in cancer research is notably the xenograft approach. Within the purview of xenograft studies, the CDX method stands as the ubiquitous choice. Indeed, the advent of CDX models followed the discernment of metastatic tendencies and their intricate association with the site of tumour cell inoculation in laboratory animals. These models hinge upon the subcutaneous or intravenous injection of human cancer cells into immunocompromised mice, a procedure readily achievable within the confines of a laboratory setting. CDX models have exhibited marked efficacy in the development of cytotoxic cancer therapies [ 92 ]. However, they have proven less efficacious when utilized for drugs targeting specific proteins [ 93 ]. The utility of CDX models is contingent upon the specific objectives of a study. Among their advantages are their suitability for investigating underlying mechanisms, cost-effectiveness, and expeditious development. Additionally, they prove instrumental in the assessment of nonspecific cytotoxic agents. Conversely, their limitations encompass the lack of heterogeneity within models generated through this method, the inability to undertake immunological investigations utilizing these models, and their sole composition of cancer cells, bereft of the rich tumour microenvironment [ 94 , 95 ]. Notwithstanding these drawbacks, CDX models remain the favoured choice for preclinical studies and find extensive use in the majority of scrutinized cases. Furthermore, their utilization in diverse research domains has witnessed a substantial upsurge, underscoring their enduring popularity [ 96 ].
It is imperative to also consider the emergence of patient-derived xenograft (PDX) models, which ameliorate the constraints intrinsic to other methodologies, yielding more efficacious animal models. PDX models preserve not only the tumour microenvironment but also the heterogeneity and mutagenic characteristics of tumours. Furthermore, they facilitate the study of metastasis, with the generated model serving as a suitable biological surrogate. However, it is noteworthy that PDX models can only be generated in severely immunocompromised mice, and their efficiency exhibits variability, rendering them less suitable for early-stage cancer research [ 97 , 98 ]. Thus, a judicious evaluation of the facets of preclinical studies can lead to the adoption of novel and more efficacious models, enhancing the quality of such investigations.
Additionally, as previously mentioned, genetic manipulation has emerged as the preeminent method in investigations of nononcological diseases. This approach affords the potential for creating models that closely mirror the characteristics of the original disease. Recent years have witnessed a substantial proliferation in the usage of such models, attributed to the advent of engineered endonucleases, which enable precise and efficient genome editing [ 99 , 100 , 101 ]. The key step in genome editing is the induction of site-specific double-strand breaks (DSBs) by engineered endonucleases that are subsequently corrected by one of two competing DNA repair pathways, nonhomologous end-joining (NHEJ) and homology-directed repair (HDR) [ 102 ]. Recent advances in genome editing technologies reflect the rapid development of engineered endonucleases, including zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeat (CRISPR) systems [ 103 ]. These endonucleases endow genome editing with two pivotal attributes: 1) the capacity to selectively recognize specific target sequences and 2) a high degree of compatibility for the placement of specified sequences [ 104 ]. Predominantly, the genetic modifications affecting the animal models under scrutiny are knockouts. For instance, in a preclinical study centred on Glybera, a product related to familial lipoprotein lipase deficiency, mice with knockout genomic regions linked to lipoprotein lipase were employed [ 44 ]. Similarly, in the context of the Rovtavian product, which is associated with hemophilia A, knockout mice have been instrumental [ 83 ]. Such instances abound in the corpus of examined research.
The primary objective of knockout is to supplant a specific genomic segment with one that is either nonfunctional, modified, or irrelevant. This substitution can precipitate alterations in the phenotype of the animal model, thereby manifesting unique disease characteristics. The development of these models represents a watershed moment in the realm of animal models and therapeutic product development. The field has witnessed a plethora of advances that permit increasingly specific and temporally controlled genetic manipulations, in addition to confining mutations to designated tissues [ 105 ]. Notwithstanding these commendable strides, challenges persist in the handling of these models. For instance, target genes may not always be amenable to genetic manipulation, and genetic editing in these models is a complex endeavour that may engender metabolic perturbations within the animal’s pathways, precipitating phenotypic anomalies [ 106 ]. Nonetheless, the usage of genetically modified animal models is burgeoning, with the advent of novel technologies that hold the potential to ameliorate the limitations of prior models, thereby engendering models of greater aptitude than their predecessors.
Trending species in the animal models for investigative research
As indicated by the findings of this study, the preclinical investigation of gene therapy products predominantly employs the mouse model, which stands as the most prevalent species of choice. Furthermore, upon closer scrutiny, it becomes evident that mice are extensively employed in the development of genetically modified animal models. The utilization of mice as an animal model boasts several merits, including cost-effectiveness in maintenance. In addition, their rapid reproduction rate and comparatively short lifespan render them ideal for genetic inquiries. Significantly, mice exhibit an estimated genetic similarity to humans in the range of 99% [ 107 ]. Furthermore, the extensive research conducted on their genetic resources, which are publicly accessible [ 108 , 109 ], underscores their prominence as a preferred model for conducting preclinical investigations.
Consequently, following mice, nonhuman primates emerge as the second most utilized species in the research endeavours under review. Phylogenetically, nonhuman primates share the closest genetic proximity to humans and find widespread application in diverse domains, encompassing psychiatric, metabolic, reproductive, and immunological studies [ 52 ]. In the specific context of the studies under consideration, nonhuman primates were predominantly deployed for disease induction purposes. However, some instances featured their deployment as noncompliant subjects, likely chosen for safety and toxicity assessments. It is worth noting that despite the marked desirability of employing this species, limitations such as restricted availability, associated expenses, and ethical concerns regarding genetic manipulation serve as constraining factors [ 110 ].
Within the third category of animal models, rats were also included. Rats serve as apt animal models extensively employed in the examination of physiology and pathophysiology, and they constitute a suitable choice for evaluating the efficacy and toxicity of clinical trials [ 111 , 112 , 113 ]. In the studies scrutinized, rats were most frequently employed in genetic manipulations.
Last, it is noteworthy that dogs were solely featured in the studies under consideration as models with naturally occurring traits. Specifically, hereditary diseases in dogs, classified as naturally occurring, bear the highest clinical resemblance to human diseases [ 114 ]. This congruence has engendered substantial demand for the use of dogs in these particular contexts.
Conclusions
The selection of an appropriate animal model constitutes a pivotal and fundamental step in the execution of animal studies, particularly within the domain of preclinical research. This selection process necessitates strict adherence to established scientific criteria and standards, as it holds the key to attaining optimal outcomes not only in the present investigation but also in subsequent research endeavours. An effective strategy for model selection involves recourse to prior studies that have traversed all requisite phases, culminating in the approval of resultant products. By doing so, one can confidently employ the chosen animal model and extend the generalizability of its findings to forthcoming investigations. Moreover, this retrospective approach enables the identification of successful methodologies for generating animal models and the identification of species suitable for the intended research purposes.
In the context of the current study, we focused on the examination of animal models employed in preclinical assessments of gene therapy products. Our findings have illuminated that the xenograft methodology, predominantly implemented through the CDX technique, stands as the most prevalent approach in preclinical studies about cancer therapeutics. Furthermore, in the realm of generating animal models for diverse pathologies, with a particular emphasis on genetic disorders, genetic manipulation emerges as the predominant technique, particularly in the creation of knockout models. Within this landscape, mice and nonhuman primates have emerged as the two most frequently utilized species.
Notably, recent trends underscore a discernible upswing in the utilization of mice and genetic manipulation methodologies as we approach the contemporary era. It is imperative not to overlook the transformative potential inherent in emerging technologies for the creation of these animal models, as the incorporation of state-of-the-art innovations undoubtedly holds promise for the generation of models of superior quality and fidelity.
Availability of data and materials
All datasets on which the conclusions of this article rely are presented within the article. No additional data repositories are required as all relevant data can be found within the manuscript itself. We have taken care to ensure that the data is easily accessible to readers in the main paper.
Abbreviations
National research council
Cell line-derived xenograft
European medicines agency
Patient-derived xenograft
Double-strand breaks
Nonhomologous end-joining
Homology-directed repair
Zinc finger nucleases
Transcription activator-like effector nucleases
Clustered regularly interspaced short palindromic repeat
Shchaslyvyi AY, Antonenko SV, Tesliuk MG, Telegeev GD. Current state of human gene therapy: approved products and vectors. Pharmaceuticals. 2023;16(10):1416.
Article CAS PubMed PubMed Central Google Scholar
Arabi F, Mansouri V, Ahmadbeigi N. Gene therapy clinical trials, where do we go? An overview. Biomed Pharmacother. 2022;153: 113324.
Article CAS PubMed Google Scholar
Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490:187–91.
Guénet JL. Animal models of human genetic diseases: do they need to be faithful to be useful? Mol Genet Genomics. 2011;286:1–20.
Article PubMed Google Scholar
Begley CG. Raising standards for preclinical research. Evid Based Preclin Med. 2014;1: e00003.
Google Scholar
Hess KR. Statistical design considerations in animal studies published recently in cancer research. Cancer Res. 2011;71:625.
Kilkenny C, Parsons N, Kadyszewski E, Festing MFW, Cuthill IC, Fry D, et al. Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PLoS ONE. 2009;4: e7824.
Article PubMed PubMed Central Google Scholar
Moher D, Simera I, Schulz KF, Hoey J, Altman DG. Helping editors, peer reviewers and authors improve the clarity, completeness and transparency of reporting health research. BMC Med. 2008;6:13.
Prinz F, Schlange T, Asadullah K. Believe it or not: how much can we rely on published data on potential drug targets? Nat Rev Drug Discov. 2011;10:712.
Sena E, van der Worp HB, Howells D, Macleod M. How can we improve the pre-clinical development of drugs for stroke? Trends Neurosci. 2007;30:433–9.
Steward O, Popovich PG, Dietrich WD, Kleitman N. Replication and reproducibility in spinal cord injury research. Exp Neurol. 2012;233:597–605.
Van der Worp HB, Macleod MR. Preclinical studies of human disease: time to take methodological quality seriously. J Mol Cell Cardiol. 2011;51:449–50.
Fisher M. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke Stroke Therapy Academic Indus Roundtable. 1999;30:2752–8.
Wall RJ, Shani M. Are animal models as good as we think? Theriogenology. 2008;69:2–9.
Popper KR. The logic of scientific discovery. Central works of philosophy v4: Twentieth Century: Moore to Popper. 2015. pp.262–286.
Krebs HA. The August Krogh principle: “For many problems there is an animal on which it can be most conveniently studied.” J Exp Zool. 1975;194:221–6.
Lee H. Genetically engineered mouse models for drug development and preclinical trials. Biomol Ther. 2014;22:267–74.
Article CAS Google Scholar
Pehlivanovic B, Smajic NZ, Belma P, Dina F, Emina A, Nermina Ž, et al. Animal Models in Modern Biomedical Research. Eur J Pharm Med Res. 2019
Connors TA. Animal models in toxicology. J Pharm Pharmacol. 1993;45:1015.
Article Google Scholar
Mukherjee P, Roy S, Ghosh D, Nandi SK. Role of animal models in biomedical research: a review. Lab Anim Res. 2022;38:1–18.
Simmons D. The Use of Animal Models in Studying Genetic Disease. Nature Education. 2008;1–10.
Van Dam D, De Deyn PP. Animal models in the drug discovery pipeline for Alzheimer’s disease. Br J Pharmacol. 2011;164:1285–300.
Prabhakar S. Translational research challenges: finding the right animal models. J Investig Med. 2012;60:1141–6.
Hansen K, Khanna C. Spontaneous and genetically engineered animal models: Use in preclinical cancer drug development. Eur J Cancer. 2004;40:858–80.
Engelmann D, Pützer BM. Emerging from the shade of p53 mutants: N-terminally truncated variants of the p53 family in EMT signaling and cancer progression. Sci Signal. 2014;7:re9.
Li Y, Li B, Li CJ, Li LJ. Key points of basic theories and clinical practice in rAd-p53 (Gendicine™) gene therapy for solid malignant tumors. Expert Opin Biol Ther. 2015;15:437–54.
Liang M. Oncorine, the world first oncolytic virus medicine and its update in China. Curr Cancer Drug Targets. 2018;18:171–6.
Gordon EM, Hall FL. Rexin-G, a targeted genetic medicine for cancer. Expert Opin Biol Ther. 2010;10:819–32.
Kohlhapp FJ, Kaufman HL. Molecular pathways: Mechanism of action for talimogene laherparepvec, a new oncolytic virus immunotherapy. Clin Cancer Res. 2016;22:1048–54.
Liu BL, Robinson M, Han ZQ, Branston RH, English C, Reay P, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Therapy. 2003;10:292–303.
Elsallab M, Levine BL, Wayne AS, Abou-el-enein M, Abou-el- PM, Berlin U, et al. Before and after marketing authorisation. Lancet Oncol. 2020;21:104–16.
Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17:1453–64.
SUMMARY OF PRODUCT CHARACTERISTICS—Yescarta. In: European Medicines Agency. 2022. https://www.ema.europa.eu/en/documents/product-information/yescarta-epar-product-information_en.pdf .
SUMMARY OF PRODUCT CHARACTERISTICS—Tecartus. In: European Medicines Agency. 2022. https://www.ema.europa.eu/en/documents/product-information/tecartus-epar-product-information_en.pdf .
Assessment report of Abecma. In: European Medicines Agency. 2021. https://www.ema.europa.eu/en/documents/assessment-report/abecma-epar-public-assessment-report_en.pdf .
Maldonado-Pérez N, Tristán-Manzano M, Justicia-Lirio P, Martínez-Planes E, Muñoz P, Pavlovic K, et al. Efficacy and safety of universal (TCRKO) ARI-0001 CAR-T cells for the treatment of B-cell lymphoma. Front Immunol. 2022;13:1–17.
Assessment report of Breyanzi. In: European Medicines Agency. 2022. https://www.ema.europa.eu/en/documents/assessment-report/breyanzi-epar-public-assessment-report_en.pdf
Buchsbaum DJ, Wahl RL, Normolie DP, Kaminski MS. Therapy with unlabeled and 131I-labeled pan-B-cell monoclonal antibodies in nude mice bearing Raji Burkitt’s Lymphoma Xenografts. Cancer Res. 1992;52:6476–81.
CAS PubMed Google Scholar
Cheema TA, Wakimoto H, Fecci PE, Ning J, Kuroda T, Jeyaretna DS, et al. Multifaceted oncolytic virus therapy for glioblastoma in an immunocompetent cancer stem cell model - Supporting Information. Proc Natl Acad Sci. 2013;110:12006–11.
Todo T, Martuza RL, Rabkin SD, Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci USA. 2001;98:6396–401.
Summary Basis for Regulatory Action—ADSTILADRIN. In: U.S. Food and Drug Administration. 2022. https://www.fda.gov/media/164532/download .
Seckinger A, Delgado JA, Moser S, Moreno L, Neuber B, Grab A, et al. Target expression, generation, preclinical activity, and pharmacokinetics of the BCMA-T cell bispecific antibody EM801 for multiple myeloma treatment. Cancer Cell. 2017;31:396–410.
Bozo IY, Drobyshev AY, Redko NA, Komlev VS, Isaev AA, Deev RV. Bringing a gene-activated bone substitute into clinical practice: from bench to bedside. Front Bioeng Biotechnol. 2021;9:1–14.
Bryant LM, Christopher DM, Giles AR, Hinderer C, Rodriguez JL, Smith JB, et al. Lessons learned from the clinical development and market authorization of Glybera. Hum Gene Ther Clin Dev. 2013;24:55–64.
Ross CJD, Twisk J, Meulenberg JM, Liu G, Van Den Oever K, Moraal E, et al. Long-term correction of murine lipoprotein lipase deficiency with AAV1-mediated gene transfer of the naturally occurring LPL S447X beneficial mutation. Hum Gene Ther. 2004;15:906–19.
Hair P, Cameron F, McKeage K. Mipomersen sodium: first global approval. Drugs. 2013;73:487–93.
Lim KRQ, Maruyama R, Yokota T. Eteplirsen in the treatment of Duchenne muscular dystrophy. Drug Des Dev Ther. 2017;11:533–45.
Singh NN, Howell MD, Androphy EJ, Singh RN. How the discovery of ISS-N1 led to the first medical therapy for spinal muscular atrophy. Gene Ther. 2017;24:520–6.
Aiuti A, Roncarolo MG, Naldini L. Gene therapy for ADA-SCID, the first marketing approval of an ex vivo gene therapy in Europe: paving the road for the next generation of advanced therapy medicinal products. EMBO Mol Med. 2017;9:737–40.
Ferrari G, Rossini S, Giavazzi R, Maggioni D, Nobili N, Soldati M, et al. An in vivo model of somatic cell gene therapy for human severe combined immunodeficiency. Science. 1991;251:1363–6.
Assessment report of Zalmoxis. In: European Medicines Agency. 2016. https://www.ema.europa.eu/en/documents/assessment-report/zalmoxis-epar-public-assessment-report_en.pdf .
Lee H, Choi K, Kim H, Kim D, Lee Y, Lee B, et al. INVOSSA-K induces an anti-inflammatory environment in a rat mia model via macrophage polarization. Osteoarthritis Cartilage. 2018;26:S125.
CharlesRiver. Osteoarthritis Model. Encyclopedia of Pain. 2013. pp. 2558–2558.
Ciulla TA, Hussain RM, Berrocal AM, Nagiel A. Voretigene neparvovec-rzyl for treatment of RPE65-mediated inherited retinal diseases: a model for ocular gene therapy development. Expert Opin Biol Ther. 2020;20:565–78.
Butler JS, Chan A, Costelha S, Fishman S, Willoughby JLS, Borland TD, et al. Preclinical evaluation of RNAi as a treatment for transthyretin-mediated amyloidosis. Amyloid. 2016;23:109–18.
Ackermann EJ, Guo S, Benson MD, Booten S, Freier S, Hughes SG, et al. Suppressing transthyretin production in mice, monkeys and humans using 2nd-Generation antisense oligonucleotides. Amyloid. 2016;23:148–57.
Benson MD, Kluve-Beckerman B, Zeldenrust SR, Siesky AM, Bodenmiller DM, Showalter AD, et al. Targeted suppression of an amyloidogenic transthyretin with antisense oligonucleotides. Muscle Nerve. 2006;33:609–18.
Suda H, Murakami A, Kaga T, Tomioka H, Morishita R. Beperminogene perplasmid for the treatment of critical limb ischemia. Expert Rev Cardiovasc Ther. 2014;12:1145–56.
Taniyama Y, Morishita R, Hiraoka K, Aoki M, Nakagami H, Yamasaki K, et al. Therapeutic angiogenesis induced by human hepatocyte growth factor gene in rat diabetic hind limb ischemia model. Circulation. 2001;104:2344–50.
Van Putten M, Hulsker M, Young C, Nadarajah VD, Heemskerk H, Van Der Weerd L, et al. Low dystrophin levels increase survival and improve muscle pathology and function in dystrophin/utrophin double-knockout mice. FASEB J. 2013;27:2484–95.
Servais L, Mercuri E, Straub V, Guglieri M, Seferian AM, Scoto M, et al. Long-term safety and efficacy data of golodirsen in ambulatory patients with duchenne muscular dystrophy amenable to exon 53 skipping: a first-in-human, multicenter, two-part, open-label, phase 1/2 trial. Nucleic Acid Ther. 2022;32:29–39.
D’Erasmo L, Gallo A, Di Costanzo A, Bruckert E, Arca M. Evaluation of efficacy and safety of antisense inhibition of apolipoprotein C-III with volanesorsen in patients with severe hypertriglyceridemia. Expert Opin Pharmacother. 2020;00:1675–84.
Graham MJ, Lee RG, Bell TA, Fu W, Mullick AE, Alexander VJ, et al. Antisense oligonucleotide inhibition of apolipoprotein c-iii reduces plasma triglycerides in rodents, nonhuman primates, and humans. Circ Res. 2013;112:1479–90.
Assessment report of Zolgensma. In: European Medicines Agency. 2020. https://www.ema.europa.eu/en/documents/assessment-report/zolgensma-epar-public-assessment-report_en.pdf .
SUMMARY OF PRODUCT CHARACTERISTICS—Zolgensma. In: European Medicines Agency. 2022. https://www.ema.europa.eu/en/documents/product-information/zolgensma-epar-product-information_en.pdf .
SUMMARY OF PRODUCT CHARACTERISTICS—Zynteglo. In: European Medicines Agency. 2022. https://www.ema.europa.eu/en/documents/product-information/zynteglo-epar-product-information_en.pdf
Scott LJ. Givosiran: first approval. Drugs. 2020;80:335–9.
SUMMARY OF PRODUCT CHARACTERISTICS—Givlaari. In: European Medicines Agency. 2020. https://www.ema.europa.eu/en/documents/product-information/givlaari-epar-product-information_en.pdf .
Chan A, Liebow A, Yasuda M, Gan L, Racie T, Maier M, et al. Preclinical development of a subcutaneous ALAS1 RNAi therapeutic for treatment of hepatic porphyrias using circulating RNA quantification. Mol Ther Nucleic Acids. 2015;4: e263.
Assessment report of Leqvio. In: European Medicines Agency. 2020. https://www.ema.europa.eu/en/documents/assessment-report/leqvio-epar-public-assessment-report_en.pdf .
Fitzgerald K, White S, Borodovsky A, Bettencourt BR, Strahs A, Clausen V, et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N Engl J Med. 2017;376:41–51.
Biffi A, De Palma M, Quattrini A, Del Carro U, Amadio S, Visigalli I, et al. Correction of metachromatic leukodystrophy in the mouse model by transplantation of genetically modified hematopoietic stem cells. J Clin Investig. 2004;113:1118–29.
Messina M, Gissen P. Atidarsagene autotemcel for metachromatic leukodystrophy. Drugs of Today. 2023;59:63–70.
Liebow A, Li X, Racie T, Hettinger J, Bettencourt BR, Najafian N, et al. An investigational RNAi therapeutic targeting glycolate oxidase reduces oxalate production in models of primary hyperoxaluria. J Am Soc Nephrol. 2017;28:494–503.
Scott LJ, Keam SJ. Lumasiran: first approval. 2021;277–82.
Shimatsu Y, Katagiri K, Furuta T, Nakura M, Tanioka Y, Yuasa K, et al. Canine X-linked muscular dystrophy in Japan (CXMDJ). Exp Anim. 2003;52:93–7.
Yucel N, Chang AC, Day JW, Rosenthal N, Blau HM. Humanizing the mdx mouse model of DMD: the long and the short of it. npj Regenerative Medicine. 2018;3.
Brolin C, Shiraishi T. Antisense mediated exon skipping therapy for duchenne muscular dystrophy (DMD). Artif DNA PNA XNA. 2011;2:6–15.
Assessment report of Skysona. In: European Medicines Agency. 2021. https://www.ema.europa.eu/en/documents/assessment-report/skysona-epar-public-assessment-report_en.pdf .
Summary Basis for Regulatory Action—SKYSONA. In: U.S. Food and Drug Administration. 2022. https://www.fda.gov/media/162098/download .
SUMMARY OF PRODUCT CHARACTERISTICS—Hemgenix. In: European Medicines Agency. 2023. https://www.ema.europa.eu/en/documents/product-information/hemgenix-epar-product-information_en.pdf .
Summary Basis for Regulatory Action—HEMGENIX. In: U.S. Food and Drug Administration. 2022. https://www.fda.gov/media/164094/download .
Assessment report of Roctavian. In: European Medicines Agency. 2022. https://www.ema.europa.eu/en/documents/assessment-report/roctavian-epar-public-assessment-report_en.pdf .
Assessment report of Upstaza. In: European Medicines Agency. 2022. https://www.ema.europa.eu/en/documents/assessment-report/upstaza-epar-public-assessment-report_en.pdf .
Summary Basis for Regulatory Action—ELEVIDYS. In: U.S. Food and Drug Administration. 2023. https://www.fda.gov/media/169746/download .
Gurevich I, Agarwal P, Zhang PP, Dolorito JA, Oliver S, Liu H, et al. In vivo topical gene therapy for recessive dystrophic epidermolysis bullosa: a phase 1 and 2 trial. Nat Med. 2022;28:780–8.
DRAFT U.S. PACKAGE INSERT—Vitravene. In: U.S. Food and Drug Administration. 1998. https://www.accessdata.fda.gov/drugsatfda_docs/nda/98/20961_Vitravene_prntlbl.pdf .
Dix RD, Cousins SW. AIDS-related cytomegalovirus retinitis: Lessons from the laboratory. Curr Eye Res. 2004;29:91–101.
Oh JJ, Carter JJ, Dix RD. A mouse model that mimics aids-related cytomegalovirus retinitis: Insights into pathogenesis. Pathogens. 2021;10:1–15.
Khehra N, Mahtani A, Rehman O, Jaferi U, Kipker N. Elasomeran (mRNA1273) Vaccine: The Journey from Preclinical Research to Clinical Trials, Authorization, and FDA Approval. 2022.
Khehra N, Padda I, Jaferi U, Atwal H, Narain S, Parmar MS. Tozinameran (BNT162b2) Vaccine: The Journey from Preclinical Research to Clinical Trials and Authorization. AAPS PharmSciTech. 2021. 172.
Day CP, Merlino G, Van Dyke T. Preclinical mouse cancer models: a maze of opportunities and challenges. Cell. 2015;163:39–53.
Johnson JI, Decker S, Zaharevitz D, Rubinstein LV, Venditti JM, Schepartz S, et al. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001;84:1424–31.
Ajith A, Mulloy LL, Musa MA, Bravo-Egana V, Horuzsko DD, Gani I, et al. Humanized mouse model as a novel approach in the assessment of human allogeneic responses in organ transplantation. Front Immunol. 2021;12: 687715.
Georges LMC, De Wever O, Galván JA, Dawson H, Lugli A, Demetter P, et al. Cell line derived xenograft mouse models are a suitable in vivo model for studying tumor budding in colorectal cancer. Front Med. 2019;6:139.
Abdolahi S, Ghazvinian Z, Muhammadnejad S, Saleh M, Asadzadeh Aghdaei H, Baghaei K. Patient-derived xenograft (PDX) models, applications and challenges in cancer research. J Transl Med. 2022;20(1):206.
Jung J, Seol HS, Chang S. The generation and application of patient-derived xenograft model for cancer research. Cancer Res Treat. 2018;50:1–10.
Pearson AT, Finkel KA, Warner KA, Nör F, Tice D, Martins MD, et al. Patient-derived xenograft (PDX) tumors increase growth rate with time. Oncotarget. 2016;7:7993–8005.
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 1979;2013(339):819–23.
Gaj T, Gersbach CA, Barbas CF. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31:397–405.
Shao M, Xu TR, Chen CS. The big bang of genome editing technology: development and application of the CRISPR/Cas9 system in disease animal models. Dongwuxue Yanjiu. 2016;37:191–204.
CAS PubMed PubMed Central Google Scholar
Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2014;32:347–50.
Lee JG, Sung YH, Baek IJ. Generation of genetically-engineered animals using engineered endonucleases. Arch Pharm Res. 2018;41:885–97.
Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD. Genome editing with engineered zinc finger nucleases. Nat Rev Genet. 2010;11:636–46.
Majzoub JA, Muglia LJ. Knockout Mice. N Engl J Med. 1996;334:904–7.
Houdebine LM. Transgenic animal models in biomedical research. Methods Mol Biol. 2007. p. 163–202.
Boguski MS. The mouse that roared. Nature. 2002;420:515–6.
Eppig JT. Mouse genome informatics (MGI) resource: genetic, genomic, and biological knowledgebase for the laboratory mouse. ILAR J. 2017;58:17–41.
Paigen K. A miracle enough: the power of mice. Nat Med. 1995;1:215–20.
Chan AWS. Progress and prospects for genetic modification of nonhuman primate models in biomedical research. ILAR J. 2013;54:211–23.
Aitman TJ, Critser JK, Cuppen E, Dominiczak A, Fernandez-Suarez XM, Flint J, et al. Progress and prospects in rat genetics: a community view. Nat Genet. 2008;40:516–22.
Jacob HJ. Functional genomics and rat models. Genome Res. 1999;9:1013–6.
Jacob HJ, Kwitek AE. Rat genetics: attaching physiology and pharmacology to the genome. Nat Rev Genet. 2002;3:33–42.
Shearin AL, Ostrander EA. Leading the way: canine models of genomics and disease. DMM Dis Models Mech. 2010;3:27–34.
Download references
Acknowledgements
We would like to acknowledge that there are no specific individuals or organizations to acknowledge for their contributions to this research.
The article lacks any sources of funding that require declaration.
Author information
Authors and affiliations.
Gene Therapy Research Center, Digestive Diseases Research Institute, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran
Parham Soufizadeh, Vahid Mansouri & Naser Ahmadbeigi
Biomedical Research Institute, University of Tehran, Tehran, Iran
Parham Soufizadeh
You can also search for this author in PubMed Google Scholar
Contributions
PS contributed to data collection, analysis, and interpretation and also drafted the article. VM revised it critically for important intellectual content. NA contributed to the conception and design of the study. All authors have read and approved the final version of the manuscript.
Corresponding author
Correspondence to Naser Ahmadbeigi .
Ethics declarations
Competing interests.
The authors declare that they have no financial or non-financial competing interests related to this research.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Soufizadeh, P., Mansouri, V. & Ahmadbeigi, N. A review of animal models utilized in preclinical studies of approved gene therapy products: trends and insights. Lab Anim Res 40 , 17 (2024). https://doi.org/10.1186/s42826-024-00195-6
Download citation
Received : 09 November 2023
Revised : 18 February 2024
Accepted : 23 February 2024
Published : 22 April 2024
DOI : https://doi.org/10.1186/s42826-024-00195-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Animal model
- Preclinical study
- Gene therapy
Laboratory Animal Research
ISSN: 2233-7660
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Minimal criteria for reporting mesenchymal stem cells in veterinary regenerative medicine
- Correspondence
- Published: 27 April 2024
Cite this article

- Khan Sharun 1 , 2 ,
- S. Amitha Banu 1 ,
- A. M. Pawde 1 ,
- Kuldeep Dhama 3 &
- Amar Pal 1
11 Accesses
Explore all metrics
The widespread application of mesenchymal stem cells (MSCs) in veterinary regenerative medicine highlights their promising therapeutic potential. However, the lack of standardized characterization and reporting practices across studies poses a significant challenge, compromising the assessment of their safety and efficacy. While criteria established for human MSCs serve as a foundation, the unique characteristics of animal-derived MSCs warrant updated guidelines tailored to veterinary medicine. A recent position statement outlining minimal reporting criteria for MSCs in veterinary research reflects efforts to address this need, aiming to enhance research quality and reproducibility. Standardized reporting criteria ensure transparency, facilitate evidence synthesis, and promote best practices adoption in MSC isolation, characterization, and administration. Adherence to minimal reporting criteria is crucial for maintaining scientific rigor and advancing the field of veterinary regenerative medicine. Ongoing collaboration among stakeholders is essential for effective implementation and adherence to updated guidelines, fostering excellence and innovation in MSC-based therapies for animal patients.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Data availability
No datasets were generated or analysed during the current study.
Bahsoun S, Coopman K, Akam EC (2019) The impact of cryopreservation on bone marrow-derived mesenchymal stem cells: a systematic review. J Transl Med 17:397. https://doi.org/10.1186/s12967-019-02136-7
Article PubMed PubMed Central Google Scholar
Cristóbal JI, Duque FJ, Usón-Casaús J et al (2024) Oxidative stress in dogs with chronic inflammatory enteropathy treated with allogeneic mesenchymal stem cells. Vet Res Commun 48:901–910. https://doi.org/10.1007/s11259-023-10265-0
Article PubMed Google Scholar
Devireddy LR, Boxer L, Myers MJ et al (2017) Questions and challenges in the development of mesenchymal stromal/stem cell-based therapies in veterinary medicine. Tissue Eng Part B Rev 23:462–470. https://doi.org/10.1089/ten.TEB.2016.0451
Dominici M, Le Blanc K, Mueller I et al (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317. https://doi.org/10.1080/14653240600855905
Article CAS PubMed Google Scholar
Guest DJ, Dudhia J, Smith RKW et al (2022) Position statement: minimal criteria for reporting veterinary and animal medicine research for mesenchymal stromal/stem cells in orthopedic applications. Front Vet Sci 9:817041. https://doi.org/10.3389/fvets.2022.817041
Ivanovska A, Wang M, Arshaghi TE et al (2022) Manufacturing mesenchymal stromal cells for the treatment of osteoarthritis in canine patients: challenges and recommendations. Front Vet Sci 9:897150. https://doi.org/10.3389/fvets.2022.897150
Li J, Wu Z, Zhao L et al (2023) The heterogeneity of mesenchymal stem cells: an important issue to be addressed in cell therapy. Stem Cell Res Ther 14:381. https://doi.org/10.1186/s13287-023-03587-y
Linkova DD, Rubtsova YP, Egorikhina MN (2022) Cryostorage of mesenchymal stem cells and biomedical cell-based products. Cells 11:2691. https://doi.org/10.3390/cells11172691
Sharun K, Pawde AM, Manjusha KM et al (2021) Classification and coding of platelet-rich plasma derived from New Zealand white rabbits for tissue engineering and regenerative medicine applications. Expert Opin Biol Ther 21:1473–1482. https://doi.org/10.1080/14712598.2021.1955099
Sharun K, Chandran D, Manjusha KM et al (2023) Advances and prospects of platelet-rich plasma therapy in veterinary ophthalmology. Vet Res Commun 47:1031–1045. https://doi.org/10.1007/s11259-022-10064-z
Download references
Acknowledgements
The authors thank the Director, ICAR-Indian Veterinary Research Institute, Izatnagar, Bareilly, India, and the All-India Network Program on Diagnostic Imaging and Management of Surgical Conditions in Animals (AINP-DIMSCA) for providing the necessary facilities to carry out this work.
This compilation is a correspondence article written, analyzed, and designed by its authors, and no substantial funding is needed.
Author information
Authors and affiliations.
Division of Surgery, ICAR-Indian Veterinary Research Institute, Izatnagar, Bareilly, Uttar Pradesh, India
Khan Sharun, S. Amitha Banu, A. M. Pawde & Amar Pal
Graduate Institute of Medicine, Yuan Ze University, 32003, Taoyuan, Taiwan
Khan Sharun
Division of Pathology, ICAR-Indian Veterinary Research Institute, Izatnagar, Bareilly, Uttar Pradesh, India
Kuldeep Dhama
You can also search for this author in PubMed Google Scholar
Contributions
Khan Sharun: Writing– review & editing, Writing– original draft, Investigation, Conceptualization; S. Amitha Banu: Writing– review & editing, Writing– original draft; A. M. Pawde: Writing– review & editing, Writing– original draft; Kuldeep Dhama: Writing– review & editing, Writing– original draft; Amar Pal: Writing– review & editing, Writing– original draft. All authors read and approved the fnal manuscript.

Corresponding author
Correspondence to Khan Sharun .
Ethics declarations
Ethical approval.
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Sharun, K., Banu, S.A., Pawde, A.M. et al. Minimal criteria for reporting mesenchymal stem cells in veterinary regenerative medicine. Vet Res Commun (2024). https://doi.org/10.1007/s11259-024-10398-w
Download citation
Received : 08 April 2024
Accepted : 25 April 2024
Published : 27 April 2024
DOI : https://doi.org/10.1007/s11259-024-10398-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Mesenchymal stem cells
- Regenerative medicine
- Reporting guidelines
- Minimum requirements
- Characterization
- Find a journal
- Publish with us
- Track your research
- Share full article

How Do We Know What Animals Are Really Feeling?
Animal-welfare science tries to get inside the minds of a huge range of species — in order to help improve their lives.
Credit... Photo Illustration by Zachary Scott
Supported by
By Bill Wasik and Monica Murphy
Bill Wasik is the magazine’s editorial director and Monica Murphy is a veterinarian and writer.
- April 23, 2024
What makes a desert tortoise happy? Before you answer, we should be more specific: We’re talking about a Sonoran desert tortoise, one of a few species of drab, stocky tortoises native to North America’s most arid landscapes. Adapted to the rocky crevices that striate the hills from western Arizona to northern Mexico, this long-lived reptile impassively plods its range, browsing wildflowers, scrub grasses and cactus paddles during the hours when it’s not sheltering from the brutal heat or bitter cold. Sonoran desert tortoises evolved to thrive in an environment so different from what humans find comfortable that we can rarely hope to encounter one during our necessarily short forays — under brimmed hats and layers of sunblock, carrying liters of water and guided by GPS — into their native habitat.
Listen to this article, read by Gabra Zackman
This past November, in a large, carpeted banquet room on the University of Wisconsin’s River Falls campus, hundreds of undergraduate, graduate and veterinary students silently considered the lived experience of a Sonoran desert tortoise. Perhaps nine in 10 of the participants were women, reflecting the current demographics of students drawn to veterinary medicine and other animal-related fields. From 23 universities in the United States and Canada, and one in the Netherlands, they had traveled here to compete in an unusual test of empathy with a wide range of creatures: the Animal Welfare Assessment Contest.
That morning in the banquet room, the academics and experts who organize the contest (under the sponsorship of the American Veterinary Medical Association, the nation’s primary professional society for vets) laid out three different fictional scenarios, each one involving a binary choice: Which animals are better off? One scenario involved groups of laying hens in two different facilities, a family farm versus a more corporate affair. Another involved bison being raised for meat, some in a smaller, more managed operation and others ranging more widely with less hands-on human contact.
Then there were the tortoises. On screens along the room’s outer edge, a series of projected slides laid out two different settings: one, a desert museum exhibiting seven Sonoran specimens together in a large, naturalistically barren outdoor enclosure; the other, a suburban zoo housing a group of four tortoises, segregated by sex, in small indoor and outdoor pens furnished with a variety of tortoise toys and enticements. Into the slides had been packed an exhausting array of detail about the care provided for the tortoises in each facility. Only contestants who had prepared thoroughly for the competition — by researching the nutritional, environmental, social and medical needs of the species in question — would be able to determine which was doing a better job.
“Animal welfare” is sometimes misused as a synonym for “animal rights,” but in practice the two worldviews can sometimes be at cross purposes. From an animal rights perspective, nearly every human use of animals is morally suspect, but animal-welfare thinkers take it as a given that animals of all kinds do exist in human care, for better or worse, and focus on how to treat them as well as possible. In the past half century, an interdisciplinary group of academics, working across veterinary medicine and other animal-focused fields, have been trying to codify what we know about animal care in a body of research referred to as “animal-welfare science.”
The research has unlocked riddles about animal behavior, spurred changes in how livestock are treated and even brought about some advances in how we care for our pets: Studies of domestic cats, for example, have found that “puzzle feeders,” which slow consumption and increase mental and physical effort while eating, can improve their health and even make them friendlier. The discipline has begun to inform policy too, including requirements for scientists receiving federal grants for their animal-based research, regulations governing transport and slaughter of livestock, accreditation standards for zoos and aquariums and guidelines for veterinarians performing euthanasia.
Contest organizers hope to help their students, who might someday go into a range of animal-related jobs — not just as vets but in agribusiness, conservation, government and more — employ data and research to improve every aspect of animal well-being. Americans own an estimated 150 million dogs and cats, and our policies and consumption patterns determine how hundreds of millions of creatures from countless other species will live and die. The Animal Welfare Assessment Contest tries to introduce students to that enormous collective responsibility, and to the complexity of figuring out what each of these animals needs, especially when — as in the case of reptiles living in a shell — their outlook differs radically from our own.
The effort to improve the lives of America’s animals began more than 150 years ago. As it happens, a hundred or so turtles figured in one of the most important events in the early history of animal activism in America. It was May 1866 — the heyday of turtle soup, a dish so beloved at the time that restaurants in New York would take out newspaper ads, or even maintain special outdoor signage, declaring the hour at which the day’s batch would be ready. And so this group of unlucky sea turtles, after being captured by hunters in Florida, was brought to New York upside down on a schooner. To further immobilize them, holes were pierced through their fins with cords run through them.
The turtles would have assumed a tranquil, passive demeanor under such conditions, perhaps making it possible for the ship’s crew to believe that the creatures weren’t suffering. But there is every reason to believe they were. Evolution has equipped the marine turtle for a life afloat, with a large lung capacity filling the space beneath the shell, to enable long dives. When the turtles were on their backs, the weight of their organs would have put pressure on these lungs, forcing their breathing to become deliberate and deep.
The American Society for the Prevention of Cruelty to Animals started up the month before. Its president and founder, a Manhattan shipbuilding heir named Henry Bergh, spent its early weeks focusing on domestic species — above all, horses, the rough treatment of which in 19th-century streets was the main inspiration for his activism. But when he became aware of these suffering sea turtles for sale at Fulton Market, he decided to extend his campaign to a wildlife species that then barely rated more consideration than a cockroach, if not a cabbage.
Bergh made a case that the infliction of prolonged pain and distress upon sea turtles bound for the soup pot was illegal as well as immoral. As with other “mute servants of mankind” providing labor, locomotion, meat or milk to human beings, the turtle was entitled to be treated with compassion. But when Bergh hauled the ship’s captain in front of a judge, the defense argued (successfully!) that turtles were not even “animals,” but rather a form of fish, and thereby did not qualify under the new animal-cruelty law that Bergh succeeded in passing earlier that year.

Still, the case became a media sensation — and signaled to New Yorkers that Bergh’s campaign on behalf of animals was going to force them to account for the suffering of all animals, not just the ones they already chose to care about.
It’s perhaps no accident that Bergh turned to activism after a failed career as a dramatist. There’s something irreducibly imaginative in considering questions of animal welfare, regardless of how much science we marshal to back up our conclusions. George Angell of Boston, his fellow titan of that founding generation of animal advocates, pirated a 13-year-old British novel called “Black Beauty” and turned it into one of the century’s best-selling books, touting it as “the ‘Uncle Tom’s Cabin’ of the Horse” — though its real innovation was its use of an animal as a first-person narrator, thrusting readers into a working horse’s perspective and forcing them to contemplate how the equines all around them might see the world differently.
But how far does imagination really get us? The philosopher Thomas Nagel famously explored this problem in an essay called “What Is It Like to Be a Bat?” which took up that question only to dramatize the impossibility of answering it to anyone’s satisfaction. “It will not help,” he wrote, “to try to imagine that one has webbing on one’s arms, which enables one to fly around at dusk and dawn catching insects in one’s mouth; that one has very poor vision, and perceives the surrounding world by a system of reflected high-frequency sound signals; and that one spends the day hanging upside down by one’s feet in an attic. Insofar as I can imagine this (which is not very far), it tells me only what it would be like for me to behave as a bat behaves.”
In the case of chelonians like turtles — and their encarapaced brethren, the tortoises — we may know even less about how they experience the world than we do about bats. Take their vision, for example: Among those species that have been studied, scientists have found evidence of broad-spectrum color vision, sometimes including ultraviolet wavelengths invisible to the human eye. And while chelonians can see well beyond their pointed beaks, where edible vegetation or predators may await notice, their brains process these visual signals slowly — a quality of certain animal brains that might, some experts have theorized, result in a sped-up perception of time. (In chelonian eyes, do grasses wave frenetically in the breeze and clouds race across the sky?)
Next to vision, smell is probably the sense turtles and tortoises rely upon most. Their sensitive nasal epithelium, distributed between two chambers, can detect odors diffused in a warm desert breeze or dissolved in a cold ocean current. Chelonian ears are where you’d expect them to be, but buried beneath their scaled reptilian skin. They hear well at low frequencies, even if they don’t register the high notes of twittering birds, humming mosquitoes or the whistling wind. Some chelonians seem to have the power of magnetoreception, which means that somewhere in their anatomy — perhaps their eyes, or their nervous systems, or elsewhere — there are chemicals or structures that allow them to sense the earth’s geomagnetic field and navigate by it.
The chelonian sense of touch presents fewer mysteries. Specialized receptors in the skin and on the shell detect mechanical, temperature and pain stimuli and send messages to the nervous system — just as they do in humans and a wide variety of other species. Recognition of pain, in particular, is considered a primordial sense, essential to the survival of animals on every limb of the evolutionary tree. But even here, there are differences separating species: What does the nervous system do with signals from its nociceptors? Does the desert tortoise withdraw its foot from the scorpion’s tail only reflexively, or does it consciously register the pain of the sting? What suffering does a turtle endure when its shell is struck by the sharp edges of a boat propeller?
As Nagel argued, there is no way to meaningfully narrow the gulch in understanding that exists around “what it is like to be” such a creature. The strategy of animal-welfare science is to patiently use what we can observe about these other kinds of minds — what they choose to eat and to do, how they interact with their environments, how they respond to certain forms of treatment — looking for objective cues to show experts what imagination cannot.
Upstairs from the banquet hall, student competitors nervously milled around carpeted corridors. One by one they were called into conference rooms to face a judge, who sat at a table beside a digital chronograph. In one room, a neatly dressed young woman in owlish glasses took a breath as the display began counting up hundredths of seconds in bright red digits. Catherine LeBlond, a second-year student at Atlantic Veterinary College at Canada’s University of Prince Edward Island, began her presentation about the bison scenario.
She was allowed to refer only to a single 3-by-5 index card, which she had packed with information based on a “summary sheet” of takeaways that she and her teammates worked up together, with key phrases emphasized and sources cited, all of it broken down by category: social behavior (“Bison are a very social species with strong matriarchal divisions”), handling guidelines (“Prods should not be used to move bison unless safety is an issue”), facility design (“Ensure that there is a sufficient number of gates within facilities to slow the animals”), euthanasia (“The recommended euthanasia method of a bison is gunshot”) and more.
LeBlond began by declaring her choice: The wilder facility provided a more humane environment for its animals. She felt it was helping bison “live a more natural life”: The more spacious grounds would support wallowing behavior, she reasoned, and allow the animals to choose their social grouping, an important policy given bisons’ strong sense of social structure. She also praised the operation for enabling bison to avoid “aversive life events,” by using drones, rather than ranchers on horseback, to monitor the animals in the field, and also by slaughtering the animals on-site to avoid the distress they experience in transport. As she ran through her presentation, she took care to hit two important rhetorical notes that judges look for: “granting” some areas in which the other institution excelled and offering positive advice about how it might improve.
One way to think about her reasoning is through the lens of “the five freedoms,” a rubric that animal-welfare thinkers have long embraced to consider all the different obligations that humans have to the animals in their care. They are: 1. the freedom from hunger and thirst; 2. the freedom from discomfort; 3. the freedom from pain, injury or disease; 4. the freedom to express normal behavior; and 5. the freedom from fear and distress. In fact, it was arguably the articulation of these five freedoms — in the Brambell Report, a document put out by a British government committee in 1965 to assess the welfare conditions of the nation’s livestock — that inaugurated the whole field of animal-welfare science.
What made this list of “freedoms” so influential, in retrospect, was that it created a context for other, more targeted thinking about how a species might experience each freedom or its violation. What sort of environment will offer “freedom from discomfort” to a beef steer, on the one hand, and a freedom “to express normal behavior” on the other? Trying to answer such questions in a rigorous way involves considerations of veterinary medicine but also of evolutionary history, behavioral observation, physiology (scientists have begun using proxies like cortisol levels as an indication of animal stress), neuroscience and more.
In her bison presentation, by citing “a more natural life” and avoiding “aversive life events,” LeBlond was emphasizing Freedoms 4 and 5, the freedom to express normal behavior and the freedom from distress. In the scenario about tortoises, though, Freedoms 4 and 5 seemed to be at odds. When LeBlond addressed the judge for that category, she awarded the edge to the zoo — weighing its better health outcomes and stimulating enrichments over the more naturalistic setting at the museum. She zeroed in on the zoo’s visitor program, which gave the tortoises a novel method of choosing whether or not they wanted to interact with humans: Staff put out a transport crate, and over the course of 20 minutes, tortoises could decide to climb into the crate to be taken to the human guests, and later receive a special biscuit for their service.
And she linked this to a behavioral difference, illustrated by a set of charts comparing how readily each set of tortoises moved toward a “novel object” (like an enrichment toy) or a “novel person” in their midst. The numbers showed that the zoo’s tortoises were far more drawn to interactions with people. “This indicates that they have less fear of humans,” LeBlond pointed out, “which could be because they are given a choice about whether or not they get to participate in educational programs, and those that do are positively reinforced with high-value rewards.”
Most of the students followed a similar logic and chose the zoo. The judges, however, disagreed. As one of them explained later at the awards ceremony — at which LeBlond took second place among vet students — the facility may have seemed to be offering their tortoises a consensual choice, but it was more accurate to see it as heavy-handed operant conditioning, which lured them into submitting to human contact with the promise of a biscuit. In scenarios involving domestic animals, a documented comfort around humans is a sign of positive treatment, but when it comes to wild animals, the goal is the opposite: to acclimate them as little to human contact as possible. Another way of putting it is this: Biscuits might make a desert tortoise “happy,” insofar as we can even imagine what that means, but happiness isn’t ultimately what humane treatment is about.
Each year at the contest, competitors are asked to perform one “live” assessment: a situation with real animals in it. This time, the species of choice was the laboratory rat. We joined Kurt Vogel, head of the Animal Welfare Lab at University of Wisconsin-River Falls, on a tour of the scenario that he and a colleague, Brian Greco, had constructed in a warren of rooms a few buildings over from the competition site.
They had brought a great deal of brio to the task. In the first room, where several rats snoozed in containers, Vogel and Greco had left a panoply of welfare infractions for eagle-eyed students to find. One cage was missing a water bottle, while others housed only a single rat, a violation of best practices (rats prefer to be housed in groups). Feed bags sat on the floor with detritus all around, and a note in a lab journal indicated that pest rodents had been observed snacking on it.
In subsequent rooms, the horrors became more baroque. A euthanasia chamber had the wrong size lid on it, and a nearby journal described a rat escaping in the middle of its extermination. Paperwork in an office laid out the nature of the study being performed, which involved prolonged deprivation of food and water, forced swimming and exposure to wet bedding. Diagrams showed that the rats’ brains were being studied through physical implants, and students could see that the operating room was a nightmare, littered with unsterile implements and the researchers’ food trash (the remnants of Vogel’s bagel sandwich, deliberately left behind). None of the abuse was real — Vogel and Greco were even taking care to cycle the rats in and out of the fake scenario, in order to avoid undue stress from the parade of students who came through taking notes.
Happiness isn’t ultimately what humane treatment is about.
Rodents did not always play the role in labs that they do today. In the late 19th century, experiments were carried out on a whole host of species, including a high proportion of dogs — a fact that animal-welfare activists publicized to turn the “vivisection” debate into a political issue, to the point that even some prominent doctors became galvanized to restrict or ban the practice. In the 20th century, as research shifted to carefully bred rats and mice, optimized for predictability and uniformity, animal experimentation receded as an issue in the public discourse. Today animal-welfare advocates struggle to motivate their base on the matter of rodents: the Humane Society’s website illustrates its section on “Taking Suffering Out of Science” (which sits at the very end in its list of the group’s current “fights”) with a picture of a beagle in a cage, despite the fact that roughly 95 percent of all lab mammals are now rats or mice.
Lab rodents are maybe the most vivid example of a species whose suffering is hard to know how to weigh against the benefits it provides us. Studies using rat and mouse models have sought to answer basic scientific questions across diverse fields of inquiry: psychology, physiology, pathology, genetics. Look into any new advance in human health care, and you’re likely to find that it’s built on years of experimentation that consumed the lives of literally thousands of rodents. We may now be on the cusp of innovations that could allow that toll to be radically reduced — by essentially replacing animal models with some combination of virtual simulations and lab-grown tissue and organs — but it’s hard to imagine a world anytime soon where human patients would be subject to therapies that have never been tested on hundreds of animals. No one even reliably counts how many rodents are killed in U.S. labs every year, but the estimates range from 10 million up to more than 100 million.
This question of scale especially haunts the problem of livestock, which is an area where many of the contest’s student competitors will eventually find jobs. America is currently home to roughly 87 million cattle and 75 million pigs: populations that exceed those of dogs and cats in scale, but the welfare of which commands so much less of our moral attention.
When the practice of centralized, industrialized livestock management began in earnest after the Civil War, the treatment of the animals, especially during slaughter, could be barbaric. Pigs were simply hoisted up and their throats cut, and after some point were assumed to be dead enough to dump into boiling water so that the sharp bristles on their skin could be scraped away. There was little doubt that some of them were still conscious at the point that they were plunged into the water, as was reported in a broad exposé in 1880 by The Chicago Tribune: “Not infrequently,” the reporter noted, “a hog reaches the scalding-tub before life is extinct; in fact, they sometimes are very full of life when they reach the point whence they are dumped into the seething tub.”
After 1906, when Upton Sinclair’s “The Jungle” exposed the industry’s unsanitary practices, a series of reforms did lead to significant improvements in the lives and deaths of American livestock. Thanks to the Humane Slaughter Act of 1958, federal law now requires that animals be “rendered insensible to pain” before the act of killing; with pigs, this is generally done either with electrocution or by suffocation in a carbon-dioxide chamber, while with cattle, the method of choice is the captive-bolt gun. And since the 1970s, animal-welfare science has led to some considerable reforms. Perhaps the most transformational work has been done by Temple Grandin, the animal behaviorist whose research into how food animals experience and respond to their environment — particularly during transport and slaughter — has changed the way that meat and dairy producers operate.
Still, despite years of promises to end the practice, many sows are still kept almost permanently in 7-feet-by-2-feet “gestation crates,” too small to turn around in. And the rise of concentrated animal feeding operations (CAFOs) has doomed millions of pigs, cattle and chickens to lives spent cheek to jowl in the stench of their own waste — waste that also threatens the health of nearby communities and ecosystems.
At the contest, many attendees were excited about the gains that artificial intelligence could bring to the animal-welfare field. Pilot studies have indeed shown great promise: For example, with A.I. assistance, 24-hour video surveillance can help pinpoint sick or injured animals much more quickly so they can be pulled out for veterinary care. Last year, a group of European researchers announced that based on 7,000 recordings of more than 400 pigs, they had made significant progress in understanding the meaning of their grunts. “By training an algorithm to recognize these sounds, we can classify 92 percent of the calls to the correct emotion,” one of the scientists remarked.
That well may be, but given what we know about pigs — specifically, their remarkable intelligence, which rivals (if not exceeds) that of a dog, to the point that a group of scientists recently trained some to play video games — there is no amount of A.I.-driven progress that can reconcile their short, crowded life as an American industrial food animal with any definition of what a “good” life looks like for such brainy creatures, all 75 million of them.
The laying hen, among the four species considered at the contest, is the one that lives among us in the largest numbers: There are an estimated 308 million of them in the United States alone, or nine for every 10 Americans. In a backyard flock, these hens could be expected to live six to eight years, but a vast majority of them toil in industrial operations that will slaughter them after only two to three years, once their productivity (six eggs a week) declines — and chickens, notably, are not covered by the Humane Slaughter Act. Poor air quality, soiled litter, nutritional stress and conflict with other chickens can contribute to dietary deficiencies, infectious diseases, egg-laying complications, self-mutilation, even cannibalism. And even in the best laying-hen operations, including the “cage-free” ones imagined in the contest scenario, these are short lives spent under 16 hours a day of artificial lighting in extremely close quarters with other birds.
More than in the other scenarios, the organizers had made the laying-hen choice a straightforward one. The corporate farm offered fewer amenities for the birds, which were also observed rarely to use the dirt-floored, plastic-covered “veranda” that was supposed to serve as a respite from their long hours laying in the aviary. The more commodious verandas of the family farm, covered with synthetic grass, proved more popular with their chickens, and in warm weather, its birds made use of a screened “garden” as well.
In her presentation, Catherine LeBlond correctly picked the family farm, for many of the same reasons that the judges did. Again, she “granted” some positive qualities of the corporate farm and offered it some advice — reflecting, after all, the values of the veterinary profession that she was training to enter, a field that takes on the advising of everyone who has animals in their care, not only the most conscientious.
Even so, at the very end, LeBlond briefly stepped back to ask a true ethical question, one that troubled the entire premise of a multibillion-dollar global industry: “whether or not it is ethical to keep these hens for the sole purpose of egg-laying, only to have them slaughtered at the end.” Among the scores of students we watched over the course of a weekend, LeBlond and her teammates from the Atlantic Veterinary College were the only ones who, in the final seconds of their talks, raised deep questions about the scenario’s entire premise — about whether, in the end, these fictional animals should have been put in these fictional situations in the first place.
It was a question that the judges of the Animal Welfare Assessment Contest had no doubt considered, but it also was one that seemed to lie outside the contest’s purview: In its either-or structure, the contest is helping train future professionals how to improve, rather than remove, the ties that bind animals into human society. Unless the day arrives when there is no need for laboratory rats, or poultry barns, or facilities to house desert tortoises and other captive wildlife, the animals of North America will be in the hands of veterinarians and animal scientists like LeBlond and her classmates, to help shape their situations the very best way they can.
Parts of this article are adapted from “Our Kindred Creatures: How Americans Came to Feel the Way They Do About Animals,” by Bill Wasik and Monica Murphy, published this month by Knopf.
Read by Gabra Zackman
Narration produced by Krish Seenivasan and Emma Kehlbeck
Engineered by Lance Neal
Explore The New York Times Magazine
A Playwright Reimagines America: In her new play, “Sally & Tom,” Suzan-Lori Parks brings exuberant provocation to the gravest historical questions.
The New Mood Music: The Texan trio Khruangbin’s vibes have spawned countless imitators, but their magic isn’t so easy to replicate.
Inside a Media-Fighting Law Firm: Tensions had been brewing for years inside Clare Locke, a top defamation law firm. Then came the biggest defamation case of them all : a case against Fox News.
Is Corporate America in Denial?: Despite Donald Trump’s populist promises , many bigwigs are keeping the faith that it couldn’t really happen here.
Larry David’s Rule Book: He’s a wild, monomaniacal jerk . He’s also our greatest interpreter of American manners since Emily Post.
Inside The National Enquirer : An ex-editor at the tabloid reveals the story of the notorious “catch and kill” campaign that now stands at the heart of Donald Trump’s’s legal trial.
Advertisement

- Login/Settings

Animal Lab Technician
The University of North Dakota is currently not hiring remote employees in the following states: AR, CA, CO, HI, MD, ME, OR, PA, WA, and any country outside of the United States.
- Grand Forks, North Dakota, United States
- Center for Biomedical Research
- Laboratory Support
- Full-time Staff
- Closing on: May 12 2024
Salary/Position Classification
- $17.40+ Hourly, Non-Exempt (Eligible for overtime)
- 40 hours per week
- 100% Remote Work Availability: No
- Hybrid Work Availability: No
Purpose of Position
This position ensures that the husbandry procedures according to the National Research Council “Guide for the Care and Use of Laboratory Animals" are properly performed. The animal care technician provides all levels of services ranging from custodial duties, preparation and sanitation of equipment involved in housing, animal husbandry duties, as well as technical assistance to researchers. This position is responsible for caring and maintaining experimental animals, observing animal behaviors and monitoring animal health status. They oversee animal breeding colonies as well as monitor the Sentinel mice used in the health monitoring program. Successful teamwork depends upon reliability, interdependency among co-workers and good communication. CBR staff are responsible for the Center for Biomedical Research (CBR), the CBR2 Vivarium located at the School of Medicine and Health Sciences and the BRCF located in Columbia Hall. Animals must be properly cared for 365 days per year; therefore, this position sometimes requires the individual to work during weekends, emergencies, and closures.
Duties & Responsibilities
- Provide animal care in line with requirements in the Guide for the Care and Use of Laboratory Animals (The Guide) and IACUC-approved research protocols, including preparing and monitoring special diets, monitoring water consumption, and monitoring animal health throughout their time in the facility.
- Monitor animal rooms to ensure they are appropriate for the safety of research animals.
- Monitor individually ventilated cage (IVC) racks to ensure racks are operating normally.
- Provide specialty care for animals as needed based upon protocols
- Serve on call on weekends and after hours based on a rotating scheduled.
- Prepare and sanitize equipment used in animal housing and husbandry.
- Assist in medical treatment of animals
- Responsible for recording all pertinent data from breeding colonies including: Litter sizes, date of birth, sex, etc.
- Accurately record daily inventory of animals
- Observe pregnant dams and report unusual signs/behavior. Notify investigator upon litter delivery.
- Observation/maintenance of animal neonatal care.
- Maintain animal weaning
- Foster animals as needed including choosing foster mom to give pups the best chance of survival.
- Communicate in a professionalism manner and have excellent teamwork.
Required Competencies
- Must be dependable and have an excellent attendance record
- Strong emphasis will be placed on the ability to prioritize, organize and multi-task while working under time constraints
- Must take the initiative to work independently as well as part of a team
- Respectfulness, cooperation, and following orders are imperative
- Must have strong communication skills
- Must have strong observational skills
- Must be punctual, dependable, honest, and trustworthy
- Understanding of the need for the use of animals in biomedical research, and empathy and compassion towards research animals
Minimum Requirements
- Associate degree or three years of directly related work experience or combination of education and work experience
- Experience utilizing Microsoft - Excel, word
- Successful completion of a Criminal History Background Check
In compliance with federal law, all persons hired will be required to verify identity and eligibility to work in the US and to complete the required employment eligibility verification form upon hire. This position does not support visa sponsorship for continued employment.
Preferred Qualifications
- Familiarity with and/or have experience in a laboratory setting
- Experience with handling mice and rats
Submit a resume and cover letter showing how you meet each required qualification, any preferred qualifications, and competencies.
Please note, all employment postings close at 11:55pm CST.
Position Benefits
Benefits include single or family health care coverage (UND pays the full premium), life insurance, employee assistance program, retirement plans with generous employer contributions, annual & sick leave in addition to 10 paid holidays.
Optional benefits include supplemental life, dental, vision, flexible spending account, supplemental retirement plans.
UND also offers an employee tuition waiver and a variety of professional development opportunities .
Find out more about UND's great benefits and perks here !
Want to be notified of similar opportunities?
Thank you for your interest in applying to the University of North Dakota
Other UND Career Openings
Warehouse delivery specialist, eerc custodian, instructional accessibility specialist, additional information.
Find out why Grand Forks is Cooler .
All information listed in this position announcement will be used by Human Resources, the Hiring Department, and EO/Title IX for screening, interviewing and selection purposes.
Please email the Human Resources Department at [email protected] or contact us by phone at 701.777.4226. If you anticipate needing any type of accommodation to participate in any portion of the University's employment process, including completion of the online application process, please contact our office in advance of your participation or visit.
Veteran’s Preference
Veterans claiming preference must submit all proof of eligibility by the closing date. Proof of eligibility includes a DD-214 or a copy of NGB 22 from National Guard or Reserve (with a unit located in ND) or certification from the applicant's unit command that the individual is expected to be discharged or released from active duty in the uniformed services under other than dishonorable conditions not later than one hundred twenty days after the date of the submission of the certification. If claiming disabled veteran status, proof of eligibility includes a DD-214 and a current letter of disability dated within the past year.
Confidentiality of Application Materials
Pursuant to NDCC 44-04-18.27, applications and any records related to the applications that identify an applicant are confidential, except records related to the finalists of the position, which are open to the public after the search committee has identified the top three or more finalists who will be invited to campus.
EEO Statement
The University of North Dakota is an Affirmative Action/Equal Opportunity Employer. All qualified applicants will receive consideration for employment without regard to race, color, religion, sex, sexual orientation, gender identity, national origin, disability or other protected characteristic. Women, minorities, veterans, individuals with disabilities, and members of other underrepresented groups are especially encouraged to apply. Applicants are invited to provide information regarding their gender, race and/or ethnicity, veteran’s status and disability status as part of the application process. This information will remain confidential and separate from your application.
Clery Statement
In compliance with the Jeanne Clery Disclosure of Campus Security Policy and Campus Crime Statistics Act, the University of North Dakota publishes an Annual Security and Fire Safety Report. The report includes the university’s policies, procedures, and programs concerning safety and security, as well as three years of crime statistics for our campus. As a prospective employee, you are entitled to a copy of this report. The report and statistical data can be found online at UND.edu. You may also request a paper copy of the report from the UND Police Department located at 3851 Campus Road, Grand Forks, ND, 58202.

This website uses cookies.
We use cookies to personalize content such as job recommendations, and to analyze our traffic. You consent to our cookies if you click "I Accept". If you click on "I Do Not Accept", then we will not use cookies but you may have a deteriorated user experience. You can change your settings by clicking on the Settings link on the top right of the device
An official website of the United States government Here's how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
Biden-Harris Administration Announces Final Rule Requiring Automatic Refunds of Airline Tickets and Ancillary Service Fees
Rule makes it easy to get money back for cancelled or significantly changed flights, significantly delayed checked bags, and additional services not provided
WASHINGTON – The Biden-Harris Administration today announced that the U.S. Department of Transportation (DOT) has issued a final rule that requires airlines to promptly provide passengers with automatic cash refunds when owed. The new rule makes it easy for passengers to obtain refunds when airlines cancel or significantly change their flights, significantly delay their checked bags, or fail to provide the extra services they purchased.
“Passengers deserve to get their money back when an airline owes them - without headaches or haggling,” said U.S. Transportation Secretary Pete Buttigieg . “Our new rule sets a new standard to require airlines to promptly provide cash refunds to their passengers.”
The final rule creates certainty for consumers by defining the specific circumstances in which airlines must provide refunds. Prior to this rule, airlines were permitted to set their own standards for what kind of flight changes warranted a refund. As a result, refund policies differed from airline to airline, which made it difficult for passengers to know or assert their refund rights. DOT also received complaints of some airlines revising and applying less consumer-friendly refund policies during spikes in flight cancellations and changes.
Under the rule, passengers are entitled to a refund for:
- Canceled or significantly changed flights: Passengers will be entitled to a refund if their flight is canceled or significantly changed, and they do not accept alternative transportation or travel credits offered. For the first time, the rule defines “significant change.” Significant changes to a flight include departure or arrival times that are more than 3 hours domestically and 6 hours internationally; departures or arrivals from a different airport; increases in the number of connections; instances where passengers are downgraded to a lower class of service; or connections at different airports or flights on different planes that are less accessible or accommodating to a person with a disability.
- Significantly delayed baggage return: Passengers who file a mishandled baggage report will be entitled to a refund of their checked bag fee if it is not delivered within 12 hours of their domestic flight arriving at the gate, or 15-30 hours of their international flight arriving at the gate, depending on the length of the flight.
- Extra services not provided: Passengers will be entitled to a refund for the fee they paid for an extra service — such as Wi-Fi, seat selection, or inflight entertainment — if an airline fails to provide this service.
DOT’s final rule also makes it simple and straightforward for passengers to receive the money they are owed. Without this rule, consumers have to navigate a patchwork of cumbersome processes to request and receive a refund — searching through airline websites to figure out how make the request, filling out extra “digital paperwork,” or at times waiting for hours on the phone. In addition, passengers would receive a travel credit or voucher by default from some airlines instead of getting their money back, so they could not use their refund to rebook on another airline when their flight was changed or cancelled without navigating a cumbersome request process.
The final rule improves the passenger experience by requiring refunds to be:
- Automatic: Airlines must automatically issue refunds without passengers having to explicitly request them or jump through hoops.
- Prompt: Airlines and ticket agents must issue refunds within seven business days of refunds becoming due for credit card purchases and 20 calendar days for other payment methods.
- Cash or original form of payment: Airlines and ticket agents must provide refunds in cash or whatever original payment method the individual used to make the purchase, such as credit card or airline miles. Airlines may not substitute vouchers, travel credits, or other forms of compensation unless the passenger affirmatively chooses to accept alternative compensation.
- Full amount: Airlines and ticket agents must provide full refunds of the ticket purchase price, minus the value of any portion of transportation already used. The refunds must include all government-imposed taxes and fees and airline-imposed fees, regardless of whether the taxes or fees are refundable to airlines.
The final rule also requires airlines to provide prompt notifications to consumers affected by a cancelled or significantly changed flight of their right to a refund of the ticket and extra service fees, as well as any related policies.
In addition, in instances where consumers are restricted by a government or advised by a medical professional not to travel to, from, or within the United States due to a serious communicable disease, the final rule requires that airlines must provide travel credits or vouchers. Consumers may be required to provide documentary evidence to support their request. Travel vouchers or credits provided by airlines must be transferrable and valid for at least five years from the date of issuance.
The Department received a significant number of complaints against airlines and ticket agents for refusing to provide a refund or for delaying processing of refunds during and after the COVID-19 pandemic. At the height of the pandemic in 2020, refund complaints peaked at 87 percent of all air travel service complaints received by DOT. Refund problems continue to make up a substantial share of the complaints that DOT receives.
DOT’s Historic Record of Consumer Protection Under the Biden-Harris Administration
Under the Biden-Harris Administration and Secretary Buttigieg, DOT has advanced the largest expansion of airline passenger rights, issued the biggest fines against airlines for failing consumers, and returned more money to passengers in refunds and reimbursements than ever before in the Department’s history.
- Thanks to pressure from Secretary Buttigieg and DOT’s flightrights.gov dashboard, all 10 major U.S. airlines guarantee free rebooking and meals, and nine guarantee hotel accommodations when an airline issue causes a significant delay or cancellation. These are new commitments the airlines added to their customer service plans that DOT can legally ensure they adhere to and are displayed on flightrights.gov .
- Since President Biden took office, DOT has helped return more than $3 billion in refunds and reimbursements owed to airline passengers – including over $600 million to passengers affected by the Southwest Airlines holiday meltdown in 2022.
- Under Secretary Buttigieg, DOT has issued over $164 million in penalties against airlines for consumer protection violations. Between 1996 and 2020, DOT collectively issued less than $71 million in penalties against airlines for consumer protection violations.
- DOT recently launched a new partnership with a bipartisan group of state attorneys general to fast-track the review of consumer complaints, hold airlines accountable, and protect the rights of the traveling public.
- In 2023, the flight cancellation rate in the U.S. was a record low at under 1.2% — the lowest rate of flight cancellations in over 10 years despite a record amount of air travel.
- DOT is undertaking its first ever industry-wide review of airline privacy practices and its first review of airline loyalty programs.
In addition to finalizing the rules to require automatic refunds and protect against surprise fees, DOT is also pursuing rulemakings that would:
- Propose to ban family seating junk fees and guarantee that parents can sit with their children for no extra charge when they fly. Before President Biden and Secretary Buttigieg pressed airlines last year, no airline committed to guaranteeing fee-free family seating. Now, four airlines guarantee fee-free family seating, and the Department is working on its family seating junk fee ban proposal.
- Propose to make passenger compensation and amenities mandatory so that travelers are taken care of when airlines cause flight delays or cancellations.
- Expand the rights for passengers who use wheelchairs and ensure that they can travel safely and with dignity . The comment period on this proposed rule closes on May 13, 2024.
The final rule on refunds can be found at https://www.transportation.gov/airconsumer/latest-news and at regulations.gov , docket number DOT-OST-2022-0089. There are different implementation periods in this final rule ranging from six months for airlines to provide automatic refunds when owed to 12 months for airlines to provide transferable travel vouchers or credits when consumers are unable to travel for reasons related to a serious communicable disease.
Information about airline passenger rights, as well as DOT’s rules, guidance and orders, can be found at https://www.transportation.gov/airconsumer .

Statement of the Royal Society’s position on the use of animals in research
The Royal Society believes that all research should be carried out with a high regard for animal welfare. At present the use of animals remains the only way for some areas of research to progress. The Society believes that where this research offers considerable benefits, it should go ahead under rigorous review to ensure it is absolutely necessary and there are no alternatives. At the same time steps must be taken to replace the use of animals, reduce the numbers used and refine procedures so the degree of suffering for animals is kept to the absolute minimum (the 3Rs).
Funding research that uses animals
The Society requires that the research it supports in the UK must comply with UK legislation and endorses the principle of the 3Rs (replace, refine and reduce). Researchers in receipt of Society funding report annually on their work, including their use of animals.
Animal research in the UK is regulated by the Animals (Scientific Procedures) Act 1986 which was updated in 2013. Everyone conducting animal research, as well as the facilities and the projects that they work on, must be licensed to do so. As part of the licensing procedures, research on animals is subjected to rigorous independent review in order to ensure that the use of animals is absolutely necessary and there are no alternatives. This review also considers whether any steps can be taken to replace, refine or reduce the number of animals used.
Find out more about UK regulation or licensing procedures on the Home Office website
International research supported by the Society must, as a minimum standard, be carried out in accordance with the principles of UK legislation as well as complying with all local legislation and ethical review procedures. Read the full policy .
The Society has over 20 award schemes for researchers , some of which may involve research with animals. The number of grants awarded each year that involve animals are detailed in this downloadable table (XLS) . The proportion of grants awarded each year by the Society that involve the use of animals has grown over the past eleven years and has fluctuated around 10% for the past five years.
How the Royal Society ensures high standards in the research using animals that it funds
Implementation of the principles in the following guidance is a condition of receiving funds from the Royal Society:
- Responsibility for the Use of Animals in Bioscience Research guidelines
- NC3Rs Guidelines: Primate Accommodation, Care and Use documents produced by the National Centre for the Replacement, Refinement and Reduction of Animals in Research ( NC3Rs ).
These guidelines set out the expectations for the use of animals in research and is therefore also useful to ethics committees, referees and Panel/Committee/Board members involved in reviewing research proposals.
The Royal Society must be satisfied that:
- The simplest possible, or least sentient, species of animal appropriate is used
- Distress and pain are avoided wherever possible
- Appropriate experimental design to ensure the minimum number of animals possible is used, consistent with ensuring that scientific objectives will be met
- There are no feasible alternatives
- All reasonable efforts have been made to address the principles (replace, reduce, refine) of the 3Rs
- All proposals using animals should explain not only why the use of animals is necessary and the ethical implications of the planned experiments, but also clearly describe how the planned experimental design is appropriate to give robust results
- All animal work will be done in strict compliance with local Animal Welfare Ethical Review Board (AWERB) committee requirements, sector standards and applicable law including, in the UK, the Animals (Scientific Procedures) Act 1986
- Welfare standards are consistent with the principles of UK legislation and that the guidance documents set out above are applied and maintained, even where the funded research is to be performed outside the UK
- The research has been independently peer reviewed, and applications which propose the use of non-human primates (NHPs), cats, dogs or equines, which are specially protected species under the Animals (Scientific Procedures) Act 1986, are subject to additional peer review by the NC3Rs
How the Royal Society implements its policy
The Royal Society is committed to ensuring that our policy on animal research is implemented effectively to help reduce animal use in research, improve welfare standards, and due to the scientific benefits, ensure that appropriate models are used, and that experimental design is scientifically robust and reproducible.
This is achieved through the following mechanisms:
- Applicants - applicants who submit an application proposing research involving the use of animals must provide detailed information to allow for appropriate review and assessment of the proposed research. Applicants are expected to detail how the number of animals to be used was decided, plans to minimise experimental bias, and provide information on statistical aspects of the study including statistical power and appropriate statistical analysis. Applicants are encouraged to use the NC3Rs Experimental Design Assistant when designing their experiments, and the ARRIVE guidelines for improving the reproducibility and reporting of research involving animals.
- Peer review - all research involving the use of animals is rigorously assessed by appropriately qualified independent peer reviewers. All Panels and panel members, including independent peer reviewers are instructed to consider whether the principles of the 3Rs have been followed by the applicant in their response. Applications specifying the use of non-human primates (NHPs), cats, dogs or equines are subject to additional peer review by the NC3Rs with applicants expected to provide an adequate response to any concerns raised by the NC3Rs, which is shared with Panels members for consideration prior to making award recommendations. Issues raised by the NC3Rs may be included as a condition of funding.
- Conditions of award - it is a condition of funding that the Host Organisation and the Award Holder must ensure that research involving the use of animals falls within the regulations laid down in the UK Animals (Scientific Procedures) Act 1986 and subsequent amendments. Award holders using animals must implement and adhere to the principles detailed in the Responsibility in the Use of Animals in Bioscience Research guidelines , and where an uses non-human primates they must also comply with the NC3Rs guidelines on Primate Accommodation, Care and Use . Any element of research funded by the Award that is conducted outside the UK must, as a minimum standard, be conducted in accordance with the principles of UK legislation.
- Improving standards - the Royal Society takes an active role in policy discussions on the use of animals in research. The Society is a member of the Society of Biology’s Animals in Science Group. This Group feeds into the UK Bioscience Sector Coalition which engages directly with the Home Office and Department for Business, Innovation and Skills. The Society endorses the work of the National Centre for the replacement, refinement and reduction of animals in research ( NC3Rs ). NC3Rs supports national and international efforts to improve conditions for laboratory animals and welcomes attempts to maintain and strengthen an ethical approach to the use of animals in research through discussion and debate.
- Increasing transparency - the Royal Society is a signatory to the Concordat on Openness in Animal Research , and is working to fulfil the commitments of the Concordat as they apply to the Society. More information about the Concordat and the actions taken by other signatories can be found on the Understanding Animal Research website .
Publishing research findings
Papers published in the Society’s journals which involve work with animals must meet set conditions and will be accepted only if the procedures used are clearly described - we encourage all authors to comply with the Animal Research: Reporting in vivo Experiments (ARRIVE) guidelines - and conform to the legal requirements of the country in which the work was carried out and to all institutional guidelines.
In addition, referees are required to express any ethical concerns they may have about the animal experimentation under review. Papers will be accepted for publication only if they are considered to be ethically sound in the judgement of the editor. Read the full policy .
Royal Society publications on animal research
Response to the Nuffield Council on Bioethics consultation on the ethics of research involving animals (PDF) (December 2003)
The use of animals in research (June 2006)
The use of genetically modified animals (May 2001, ISBN 0 85403 556 7)
Read our 2015 statement on animal research , or our 2006 statement .
For further information please contact [email protected] .
Page last updated: 24 May 2023
Email updates
We promote excellence in science so that, together, we can benefit humanity and tackle the biggest challenges of our time.
Subscribe to our newsletters to be updated with the latest news on innovation, events, articles and reports.
What subscription are you interested in receiving? (Choose at least one subject)

An official website of the United States government
Here’s how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
Take action
- Report an antitrust violation
- File adjudicative documents
- Find banned debt collectors
- View competition guidance
- Competition Matters Blog
New HSR thresholds and filing fees for 2024
View all Competition Matters Blog posts
We work to advance government policies that protect consumers and promote competition.
View Policy
Search or browse the Legal Library
Find legal resources and guidance to understand your business responsibilities and comply with the law.
Browse legal resources
- Find policy statements
- Submit a public comment

Vision and Priorities
Memo from Chair Lina M. Khan to commission staff and commissioners regarding the vision and priorities for the FTC.
Technology Blog
Consumer facing applications: a quote book from the tech summit on ai.
View all Technology Blog posts
Advice and Guidance
Learn more about your rights as a consumer and how to spot and avoid scams. Find the resources you need to understand how consumer protection law impacts your business.
- Report fraud
- Report identity theft
- Register for Do Not Call
- Sign up for consumer alerts
- Get Business Blog updates
- Get your free credit report
- Find refund cases
- Order bulk publications
- Consumer Advice
- Shopping and Donating
- Credit, Loans, and Debt
- Jobs and Making Money
- Unwanted Calls, Emails, and Texts
- Identity Theft and Online Security
- Business Guidance
- Advertising and Marketing
- Credit and Finance
- Privacy and Security
- By Industry
- For Small Businesses
- Browse Business Guidance Resources
- Business Blog
Servicemembers: Your tool for financial readiness
Visit militaryconsumer.gov
Get consumer protection basics, plain and simple
Visit consumer.gov
Learn how the FTC protects free enterprise and consumers
Visit Competition Counts
Looking for competition guidance?
- Competition Guidance
News and Events
Latest news, ftc finalizes changes to the health breach notification rule.
View News and Events
Upcoming Event
Older adults and fraud: what you need to know.
View more Events
Sign up for the latest news
Follow us on social media
--> --> --> --> -->

Playing it Safe: Explore the FTC's Top Video Game Cases
Learn about the FTC's notable video game cases and what our agency is doing to keep the public safe.
Latest Data Visualization
FTC Refunds to Consumers
Explore refund statistics including where refunds were sent and the dollar amounts refunded with this visualization.
About the FTC
Our mission is protecting the public from deceptive or unfair business practices and from unfair methods of competition through law enforcement, advocacy, research, and education.
Learn more about the FTC

Meet the Chair
Lina M. Khan was sworn in as Chair of the Federal Trade Commission on June 15, 2021.
Chair Lina M. Khan
Looking for legal documents or records? Search the Legal Library instead.
- Cases and Proceedings
- Premerger Notification Program
- Merger Review
- Anticompetitive Practices
- Competition and Consumer Protection Guidance Documents
- Warning Letters
- Consumer Sentinel Network
- Criminal Liaison Unit
- FTC Refund Programs
- Notices of Penalty Offenses
- Advocacy and Research
- Advisory Opinions
- Cooperation Agreements
- Federal Register Notices
- Public Comments
- Policy Statements
- International
- Office of Technology Blog
- Military Consumer
- Consumer.gov
- Bulk Publications
- Data and Visualizations
- Stay Connected
- Commissioners and Staff
- Bureaus and Offices
- Budget and Strategy
- Office of Inspector General
- Careers at the FTC
FTC Announces Rule Banning Noncompetes
- Competition
- Office of Policy Planning
- Bureau of Competition
Today, the Federal Trade Commission issued a final rule to promote competition by banning noncompetes nationwide, protecting the fundamental freedom of workers to change jobs, increasing innovation, and fostering new business formation.
“Noncompete clauses keep wages low, suppress new ideas, and rob the American economy of dynamism, including from the more than 8,500 new startups that would be created a year once noncompetes are banned,” said FTC Chair Lina M. Khan. “The FTC’s final rule to ban noncompetes will ensure Americans have the freedom to pursue a new job, start a new business, or bring a new idea to market.”
The FTC estimates that the final rule banning noncompetes will lead to new business formation growing by 2.7% per year, resulting in more than 8,500 additional new businesses created each year. The final rule is expected to result in higher earnings for workers, with estimated earnings increasing for the average worker by an additional $524 per year, and it is expected to lower health care costs by up to $194 billion over the next decade. In addition, the final rule is expected to help drive innovation, leading to an estimated average increase of 17,000 to 29,000 more patents each year for the next 10 years under the final rule.
Noncompetes are a widespread and often exploitative practice imposing contractual conditions that prevent workers from taking a new job or starting a new business. Noncompetes often force workers to either stay in a job they want to leave or bear other significant harms and costs, such as being forced to switch to a lower-paying field, being forced to relocate, being forced to leave the workforce altogether, or being forced to defend against expensive litigation. An estimated 30 million workers—nearly one in five Americans—are subject to a noncompete.
Under the FTC’s new rule, existing noncompetes for the vast majority of workers will no longer be enforceable after the rule’s effective date. Existing noncompetes for senior executives - who represent less than 0.75% of workers - can remain in force under the FTC’s final rule, but employers are banned from entering into or attempting to enforce any new noncompetes, even if they involve senior executives. Employers will be required to provide notice to workers other than senior executives who are bound by an existing noncompete that they will not be enforcing any noncompetes against them.
In January 2023, the FTC issued a proposed rule which was subject to a 90-day public comment period. The FTC received more than 26,000 comments on the proposed rule, with over 25,000 comments in support of the FTC’s proposed ban on noncompetes. The comments informed the FTC’s final rulemaking process, with the FTC carefully reviewing each comment and making changes to the proposed rule in response to the public’s feedback.
In the final rule, the Commission has determined that it is an unfair method of competition, and therefore a violation of Section 5 of the FTC Act, for employers to enter into noncompetes with workers and to enforce certain noncompetes.
The Commission found that noncompetes tend to negatively affect competitive conditions in labor markets by inhibiting efficient matching between workers and employers. The Commission also found that noncompetes tend to negatively affect competitive conditions in product and service markets, inhibiting new business formation and innovation. There is also evidence that noncompetes lead to increased market concentration and higher prices for consumers.
Alternatives to Noncompetes
The Commission found that employers have several alternatives to noncompetes that still enable firms to protect their investments without having to enforce a noncompete.
Trade secret laws and non-disclosure agreements (NDAs) both provide employers with well-established means to protect proprietary and other sensitive information. Researchers estimate that over 95% of workers with a noncompete already have an NDA.
The Commission also finds that instead of using noncompetes to lock in workers, employers that wish to retain employees can compete on the merits for the worker’s labor services by improving wages and working conditions.
Changes from the NPRM
Under the final rule, existing noncompetes for senior executives can remain in force. Employers, however, are prohibited from entering into or enforcing new noncompetes with senior executives. The final rule defines senior executives as workers earning more than $151,164 annually and who are in policy-making positions.
Additionally, the Commission has eliminated a provision in the proposed rule that would have required employers to legally modify existing noncompetes by formally rescinding them. That change will help to streamline compliance.
Instead, under the final rule, employers will simply have to provide notice to workers bound to an existing noncompete that the noncompete agreement will not be enforced against them in the future. To aid employers’ compliance with this requirement, the Commission has included model language in the final rule that employers can use to communicate to workers.
The Commission vote to approve the issuance of the final rule was 3-2 with Commissioners Melissa Holyoak and Andrew N. Ferguson voting no. Commissioners Rebecca Kelly Slaughter , Alvaro Bedoya , Melissa Holyoak and Andrew N. Ferguson each issued separate statements. Chair Lina M. Khan will issue a separate statement.
The final rule will become effective 120 days after publication in the Federal Register.
Once the rule is effective, market participants can report information about a suspected violation of the rule to the Bureau of Competition by emailing [email protected] .
The Federal Trade Commission develops policy initiatives on issues that affect competition, consumers, and the U.S. economy. The FTC will never demand money, make threats, tell you to transfer money, or promise you a prize. Follow the FTC on social media , read consumer alerts and the business blog , and sign up to get the latest FTC news and alerts .
Press Release Reference
Contact information, media contact.
Victoria Graham Office of Public Affairs 415-848-5121

IMAGES
VIDEO
COMMENTS
The Guide was prepared to assist. researchers in maintaining high quality care for all commonly-used. laboratory animals. It includes the Government principles for animal. care and use adopted by all agencies and institutions that conduct. federally-supported animal research. This guide also applies under.
Position Statement on the Use of Animals in Research 1. Summary The Microbiology Society supports the replacement, refinement and reduction of animals in research. However, when no alternative is available, the use of animals within an approved regulatory framework remains essential. The Society is committed to openness in the reporting of
In recognition of the need for the appropriate and humane use of animals in research, and in response to the growing pressure from other organizations that would deny Americans the health benefits evolving from research using animals, the APA joins with other scientific and medical organizations in support of the following position statement: 1.
Updated: September 2020 Medical research charities are dedicated to improving patient lives and outcomes through high quality research to better understand and treat disease. To achieve this, many types of research methods are harnessed in AMRC charity strategies. These include clinical trials, use of tissues samples, computer models and, when appropriate, animals. All approaches, including ...
Position Statement on Use of Animals in Research. This search input has a predictive search function. When 3 letters or more are entered, a number of predictive results appear in a dropdown.
2015 - Use of Animals in Research. 08 May 2015 publication. Research using animals is required to understand how microbes cause disease in human and animal hosts, how hosts respond to infection and the relationship between host microbiomes and health. Such research has been, and continues to be, vital for protecting human and animal health ...
Understanding Animal Research (UAR) supports the humane use of animals in biomedical research, and believes that animal research is a vital part of the scientific process. For over 150 years research using animals has advanced scientific understanding of human and animal health and the impact of the environment on wildlife.
The University of Rochester places a high priority on "The Three R's" - reduction, replacement, and refinement, and is committed to supporting the development of techniques that: Reduce the number of animals used. Replace animals with other models whenever possible. Refine procedures to ensure the best possible care and comfort. The ...
of animals for these purposes. This position statement asserts that the appropriate use of animals in conducting biomedical and veterinary research and education is justified to enhance the quality of life for both humans and animals. Numerous medical advances, many of which today are taken for granted, were the results of research that ...
POSITION STATEMENT ON ANIMAL EXPERIMENTATION Laboratory animal veterinarians play a vital role in the conduct and oversight of research utilizing animals. Their ... § Recognizes that the use of animals in research, teaching and testing is a privilege; § Supports regulation of the care and use of animals used, or intended for use, in ...
The American Academy of Otolaryngology—Head and Neck Surgery recognizes the use of animals in research has enabled many of the medical and surgical treatments now available to the field of otolaryngology and surgery of the head and neck. The AAO-HNS support the judicious and appropriate use of animals in research for the advancement of medical knowledge and the development of […]
The Society for the Study of Reproduction affirms the essential contribution of animals in research and education aimed at improving the health and well being o ... SSR Position Statement on the use of Animals in Research and Education, Biology of Reproduction, Volume 64, Issue 1, 1 January 2001, Page iii, ...
Statement of the Royal Society's position on the use of human biological material in research (2 page position statement, November 2004, ISBN 0 85403 607 5) The use of non-human animals in research: a guide for scientists (28 page document, February 2004, ISBN 0 85403 598 2) Response to the Nuffield Council on Bioethics
3.4 The discovery of alternatives to animal research requires the use of animals to validate the new, non‐animal methods. The development of alternatives is driven by the bioscience sector itself, based on its understanding of the benefits, limitations and costs of using animals in research.
Animals in Research American humane strongly supports the development of alternative methods to the use of animals in biomedical research. When an animal must be used for research purposes, American Humane endorses the 3 Rs: refinement of tests so animal distress or pain is minimal, reduction of the number of animals used in a study and the replacement, whenever possible, of animal experiments ...
Statement of the Royal Society's position on the use of animals in research. 12 May 2015. From antibiotics and insulin to blood transfusions and treatments for cancer or HIV, virtually every medical achievement in the past century has depended directly or indirectly on research using animals, including veterinary medicine.
the responsible use of animals in biomedical research conducted with the goal of advancing biomedical science in service to animal and human health. It is essential that this research be performed in accordance with all laws, regulations and policies governing the care and use of animals in research. This position affirms the essential role
The use of animals in research. The Council of the Royal Society, the UK national academy of science, today (28 January 2002) publishes a joint statement about the use of animals in research. The statement points out that everybody has benefited immensely from scientific research involving animals and that virtually every medical achievement in ...
The Use of Animals in Biology Education. High quality life science education requires students to be immersed in the study of life and living systems. Educators and schools across the education spectrum should develop programs, policies, and procedures that give students the broadest opportunity to learn the life sciences through field and ...
Animals provide a way to study the fundamental workings of the human body and explore how its basic building blocks—molecules and cells—work in health and disease. In doing so, researchers can unravel the most basic mechanisms that fuel illness. Animal models help researchers understand how the normal processes in molecules, cells, and ...
UAR Oceania position statement on the use of animals in research. Understanding Animal Research Oceania (UAR Oceania) supports the humane use of animals in biomedical research, and believes that animal research is a vital part of the scientific process. For over 150 years research using animals has advanced scientific understanding of human and ...
Scientific progress heavily relies on rigorous research, adherence to scientific standards, and transparent reporting. Animal models play a crucial role in advancing biomedical research, especially in the field of gene therapy. Animal models are vital tools in preclinical research, allowing scientists to predict outcomes and understand complex biological processes. The selection of appropriate ...
The Society strongly endorses the principles of the 'three Rs' which means that every effort must be made: to replace the use of live animals by non-animal alternatives; to reduce the number of animals used in research to the minimum required for meaningful results; and to refine the procedures so that the degree of suffering is kept to a ...
A recent position statement outlining the minimal criteria that should be employed for reporting MSCs in veterinary and animal medicine research has been published (Fig. 2) (Guest et al. 2022). This initiative reflects a concerted effort to establish standardized protocols and enhance the quality and reproducibility of research in the field of ...
They are: 1. the freedom from hunger and thirst; 2. the freedom from discomfort; 3. the freedom from pain, injury or disease; 4. the freedom to express normal behavior; and 5. the freedom from ...
Salary/Position Classification $17.40+ Hourly, Non-Exempt (Eligible for overtime) 40 hours per week 100% Remote Work Availability: No Hybrid Work Availability: No Purpose of Position This position ensures that the husbandry procedures according to the National Research Council "Guide for the Care and Use of Laboratory Animals" are properly performed. The animal care technician provides all ...
Media Contact. Press Office. US Department of Transportation 1200 New Jersey Ave, SE Washington, DC 20590 United States. Email: [email protected] Phone: 1 (202) 366-4570 If you are deaf, hard of hearing, or have a speech disability, please dial 7-1-1 to access telecommunications relay services.
The Royal Society supports animal research that is necessary, ethical and complies with UK legislation and the 3Rs principles. It funds research involving animals under strict review and conditions, and promotes high standards and transparency in the sector.
The FTC estimates that the final rule banning noncompetes will lead to new business formation growing by 2.7% per year, resulting in more than 8,500 additional new businesses created each year. The final rule is expected to result in higher earnings for workers, with estimated earnings increasing for the average worker by an additional $524 per ...