- Knowledge Base
- Free Resume Templates
- Resume Builder
- Resume Examples
- Free Resume Review
In this dynamic field of clinical research, staying up-to-date with the latest trends and industry expectations is paramount.
Your resume should not only showcase your qualifications but also reflect your ability to adapt and thrive in this ever-changing environment.
Whether you're a seasoned professional looking to climb the career ladder or a newcomer eager to break into the field, our guide will equip you with the knowledge and strategies to excel.
Let's dive in and pave the way to your clinical research career success in 2023 and beyond.

Clinical Research Resume Examples
- Medical research scientist armed with a PhD in Integrated Biological Sciences
- Highly skilled in performing clinical research (lab & site clinical trials), and examining diseases & illnesses to elevate diagnostic & medical treatment procedures
- Demonstrated capability to develop clinical trial protocols and monitor project execution while collecting & scrutinizing data for further analysis and decision-making
- Possesses diverse experience of managing & verifying investigational products, writing trial protocols, and generating insightful medical reports for critical stakeholders in medicine
- Top 2 percentile of the class
- Participated in the RUSH roundtable discussions "The Role of Clinical Biases & Effects in Diagnosis" | Jun '19
- Directed lab research to examine diseases & other illnesses for devising better means of diagnosis & treatment
- Played a key role in developing the clinical protocol and provided inputs in data related to DSMB
- Monitored the progress of projects, reviewed the research performed and drafted operational reports with 100% precision
- Communicated project proposals, researched findings, and provided the status of projects to clients & senior management
- Acted as the first point of contact for clinical and medical clarification from investigative sites
- Interacted with investigators & clinical researchers during conferences , scientific meetings, symposia, webinars, and individual meetings
- Created and wrote trial protocols , and presented the same to senior management
- Identified, evaluated, and established trial sites , and closed sites down upon completion of the trial
- Collaborated with the Ethics committee regarding rights, safety, and well-being of trial subjects
- Trained 5+ site staff members on therapeutic areas , protocol requirements, proper source documentation, and case report form completion
- Ordered, tracked, & managed IP & trial materials while documenting & verifying IP dispensing , inventory, & reconciliation
- Conducted regular site visits, coordinated project meetings , and generated visit reports
- Collected & analyzed data and provided ready access to all the experimental data for the faculty researchers & supervisors
- Convened project meetings , area seminars and other meetings as necessary, and monitored the project budget
- Generated progress reports for the PI & funding agency and created articles, documents & presentations
- Assisted in conducting literature reviews, preparing interview questions, and recruiting and/or interview subjects
- Maintained 100% accurate records of interviews and safeguarded the confidentiality of subjects, as necessary
- Managed & responded to project-related emails and prepared, maintained & updated website content
- Oversaw undergraduate students working on the research projects and to ensure high accuracy :
- Preserved records on assignment completion & acted as a liaison/mediator between them and the faculty researchers
- Member of the Starlight NGO | Jun '19 - Present
- Mentoring 25+ children twice a week and enhancing their mathematical skills by teaching basic concepts
- Organized a 7-day event to increase awareness at the Kids’ Cancer Treatment Division, Victoria Hospital | Dec '19
The chronological format is essential as it effectively showcases your professional journey, skills, and growth over time.
Our featured candidate, armed with a PhD in Integrated Biological Sciences, brings a wealth of expertise to the table.
Their skills include clinical trial protocol development, project monitoring, and data analysis, making them invaluable in the field. Proficiency in tools like SAP and Tableau further enhances their qualifications.
Their professional experience highlights a commitment to advancing medical practices, from examining diseases to contributing to clinical protocols. Effective stakeholder engagement and communication are also part of their skill set.
Additionally, don't underestimate the importance of the Volunteer Experience section, which showcases your dedication beyond regular work duties.
And if you wish to make a similar resume for yourself, visit Hiration’s ChatGPT-powered career activator platform with 24x7 chat support.
Share this blog
Subscribe to Free Resume Writing Blog by Hiration
Get the latest posts delivered right to your inbox
Stay up to date! Get all the latest & greatest posts delivered straight to your inbox
Is Your Resume ATS Friendly To Get Shortlisted?
Upload your resume for a free expert review.

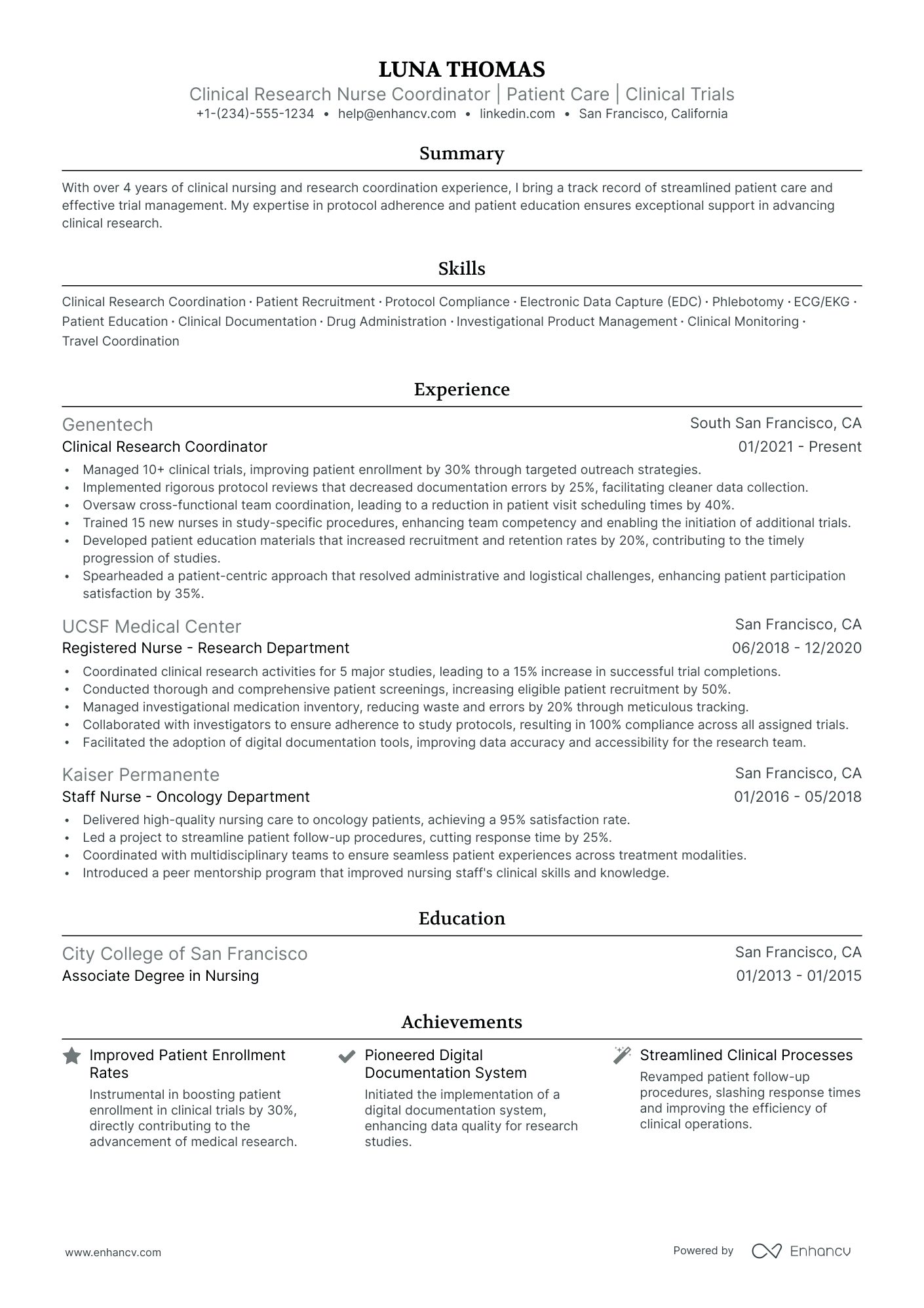
- • Managed 10+ clinical trials, improving patient enrollment by 30% through targeted outreach strategies.
- • Implemented rigorous protocol reviews that decreased documentation errors by 25%, facilitating cleaner data collection.
- • Oversaw cross-functional team coordination, leading to a reduction in patient visit scheduling times by 40%.
- • Trained 15 new nurses in study-specific procedures, enhancing team competency and enabling the initiation of additional trials.
- • Developed patient education materials that increased recruitment and retention rates by 20%, contributing to the timely progression of studies.
- • Spearheaded a patient-centric approach that resolved administrative and logistical challenges, enhancing patient participation satisfaction by 35%.
- • Coordinated clinical research activities for 5 major studies, leading to a 15% increase in successful trial completions.
- • Conducted thorough and comprehensive patient screenings, increasing eligible patient recruitment by 50%.
- • Managed investigational medication inventory, reducing waste and errors by 20% through meticulous tracking.
- • Collaborated with investigators to ensure adherence to study protocols, resulting in 100% compliance across all assigned trials.
- • Facilitated the adoption of digital documentation tools, improving data accuracy and accessibility for the research team.
- • Delivered high-quality nursing care to oncology patients, achieving a 95% satisfaction rate.
- • Led a project to streamline patient follow-up procedures, cutting response time by 25%.
- • Coordinated with multidisciplinary teams to ensure seamless patient experiences across treatment modalities.
- • Introduced a peer mentorship program that improved nursing staff's clinical skills and knowledge.
5 Clinical Research Nurse Resume Examples & Guide for 2024
As a clinical research nurse, your resume must highlight your meticulous attention to detail. Showcase your experience with data collection and patient monitoring protocols. Your resume should also reflect strong communication skills. Demonstrate your ability to liaise between patients and research teams effectively.
All resume examples in this guide
Traditional

Resume Guide
Resume Format Tips
Resume Experience
Skills on Resume
Education & Certifications
Resume Summary Tips
Additional Resume Sections
Key Takeaways

One specific resume challenge you might face as a clinical research nurse is effectively highlighting your experience in patient care and protocol compliance across diverse studies. Our guide can provide you with targeted advice to showcase your specialized skills and knowledge, ensuring your resume stands out to hiring managers in the competitive field of clinical research.
- Format your clinical research nurse resume to ensure that it balances professionalism with creativity, and follows the best practices.
- Match the clinical research nurse job requirements by including industry keywords on your resume.
- Use various resume sections to showcase your skills and achievements to answer why you're the best candidate for the clinical research nurse role.
Take inspiration from leading clinical research nurse resume examples to learn how to tailor your experience.
- Ob Nurse Resume Example
- LPN Resume Example
- Nicu Nurse Resume Example
- Clinical Nurse Manager Resume Example
- Clinical Pharmacist Resume Example
- Healthcare Resume Example
- Clinical Trial Manager Resume Example
- Staff Nurse Resume Example
- Assistant Nurse Resume Example
- Ob Gyn Medical Assistant Resume Example
How to style your clinical research nurse resume: layout and format
- Reverse-chronological resume format to highlight your experience;
- Functional skill-based resume format if you have less experience and want to focus on skills;
- Hybrid resume format to guide recruiters through both your experience and skills.
- Make sure your headline is simple and includes the job you're applying for or your current role, an abbreviation of a certificate you have, or even your professional area of interest;
- Always tailor your clinical research nurse resume to the role you're applying for by matching job requirements to your experience via different resume sections;
- Once you've created your resume, download it in PDF (unless otherwise specified). This is to ensure readability and that the layout remains fixed.
Upload & Check Your Resume
Drop your resume here or choose a file . PDF & DOCX only. Max 2MB file size.
List your educational qualifications and certifications in reverse chronological order.
The five (plus) definite sections your resume for a clinical research nurse job should include are:
- Header with your headline, contact details, and/or a preview of your work
- Summary (or objective) to pinpoint how your success aligns with the role
- Experience with bullets of your most relevant achievements in the field
- Skills to integrate vital job requirements (both technical and personal)
- Your further dedication to the field, showcased via relevant higher education and/or certifications
What recruiters want to see on your resume:
- Demonstrated experience in clinical trial management, including patient recruitment, consent, and compliance with study protocols.
- Knowledge of Good Clinical Practice (GCP), FDA regulations, and other regulatory requirements relevant to clinical research.
- Proficient in data collection, management, and reporting, including use of Electronic Data Capture (EDC) systems and proper handling of Case Report Forms (CRFs).
- Strong interpersonal and communication skills, essential for collaboration with multidisciplinary research teams and providing patient education and support.
- Experience in adverse event reporting and management, underscored by a commitment to patient safety and ethical considerations in research.
Advice for your clinical research nurse resume experience section - setting your application apart from other candidates
Your resume experience section needs to balance your tangible workplace achievements with job requirements.
The easiest way to sustain this balance between meeting candidate expectations, while standing out, is to:
- Select really impressive career highlights to detail under each experience and support those with your skills;
- Assess the job advert to define both the basic requirements (which you could answer with more junior roles) and the more advanced requirements - which could play a more prominent role through your experience section;
- Create a separate experience section, if you decide on listing irrelevant experience items. Always curate those via the people or technical skills you've attained that match the current job you're applying for;
- Don't list experience items from a decade ago - as they may no longer be relevant to the industry. That is, unless you're applying for a more senior role: where experience would go to demonstrate your character and ambitions;
- Define how your role has helped make the team, department, or company better. Support this with your skill set and the initial challenge you were able to solve.
Take a look at how real-life clinical research nurse professionals have presented their resume experience section - always aiming to demonstrate their success.
- Spearheaded the clinical trial of a new cardiac drug, overseeing 50+ patients through the trial phases, which improved patient outcomes by 25%.
- Established a compliance framework in alignment with FDA regulations, leading to a 100% audit success rate over the past 2 years.
- Developed and implemented a patient education program that decreased trial attrition rates by 15% due to better patient engagement and understanding.
- Led a cross-functional team through a complex oncology study which resulted in the successful submission of findings to a peer-reviewed journal.
- Managed the data collection and analysis for over 30 clinical trials, using advanced biostatistical tools to enhance data reliability.
- Initiated improvements to patient screening procedures that reduced the time to enroll patients by 20%, accelerating the trial timeline.
- Conducted thorough patient assessments to determine eligibility for participation in neurology trials, increasing enrollment by 30%.
- Collaborated closely with pharmaceutical sponsors to ensure adherence to study protocols and timely delivery of trial materials.
- Authored a patient follow-up process that led to a 10% improvement in long-term study adherence and quality of life measures.
- Managed the logistical aspects of clinical trial operations, successfully coordinating studies across 10 different hospital sites.
- Evaluated and integrated patient-focused technology solutions which increased participant reporting accuracy by 35%.
- Facilitated training for new clinical research staff, resulting in a 40% improvement in trial operation efficiency.
- Oversaw all phases of a landmark diabetes study, which has defined the new standard of care in diabetes management.
- Implemented a robust electronic data capture system that reduced manual errors by 90%, ensuring high-quality trial data.
- Acted as a liaison between the research team and the institutional review board, maintaining ethical standards across all clinical trials.
- Developed a novel patient consent protocol which boosted participation willingness and reduced consent withdrawal by 25%.
- Analyzed and reported on trial data, presenting findings at 3 major national conferences, increasing the visibility of the research institution.
- Established a patient advocacy board that included past clinical trial participants, which informed improvements to study design and patient care.
- Orchestrated the transition to a new clinical trial management system that decreased administrative workload by 50%.
- Pioneered a patient retention strategy that saw a 20% decrease in patient withdrawal, enhancing the integrity of longitudinal studies.
- Enhanced cross-departmental collaboration, establishing efficient communication channels that reduced project lead times by 15%.
- Played a critical role in expanding the hospital's clinical research unit, doubling its capacity and increasing the number of trials conducted annually.
- Led a drive to improve patient-centric care during trials, which increased patient satisfaction scores by 30% and attracted more funding.
- Optimized the use of a centralized monitoring system for adverse events, improving patient safety and reducing severe adverse events by 20%.
Quantifying impact on your resume
- Include the number of clinical trials you have coordinated or assisted with to demonstrate the extent of your experience.
- Specify the amount of funding you have managed for research projects to showcase your financial responsibility.
- Detail the number of patients enrolled in studies to illustrate your recruitment capabilities.
- Mention the quantity of data points collected and analyzed to highlight your meticulous data management skills.
- State the number of research publications and presentations you have contributed to, emphasizing your involvement in knowledge dissemination.
- Count the different therapeutic areas you have worked in to show the breadth of your clinical knowledge.
- Report the number of regulatory audits you have successfully passed to underline your compliance with industry standards.
- Record the percentage of trials you completed on time or ahead of schedule to reflect your efficiency and project management skills.
Action verbs for your clinical research nurse resume
What can candidates do about their resume, if they have no experience
Job requirements can sometimes be answered by other elements you could make more prominent in your clinical research nurse resume.
Thus, you'd be substituting your lack of experience with your relevant:
- Education with details of skills you've obtained that align with the job
- Internships and short-term jobs that are once more dedicated to putting your expertise in the spotlight
- Skills section answering basic and - potentially - more specific job qualifications
- Strengths or accomplishments to show the unique value you present, even as a candidate with less or no professional experience in the industry.
Recommended reads:
- How To Include Your Relevant Coursework On A Resume
- Perfecting the Education Section on Your Resume
If you're in the process of obtaining your certificate or degree, list the expected date you're supposed to graduate or be certified.
Key hard skills and soft skills for your clinical research nurse resume
At the top of any recruiter clinical research nurse checklist, you'd discover a list of technical competencies, balanced with personal skills.
Hard or technical skills are your opportunity to show how you meet the essential responsibilities of the role. The ability to use a particular job-crucial technology or software would also hint to recruiters whether you'd need a prolonged period of on-the-job training - or you'd fit right in the job.
But to land your dream role, you'd also need to demonstrate a variety of soft or people resume skills . Employers care about soft skills as they show how each candidate would fit into the team and company culture.
Both types of skills are specific and to best curate them on your resume, you'd need to:
- Create a skill section within which you showcase your hard and soft skills and present how they help you succeed.
- List specific examples of projects, tasks, or competitions, within which your skill set has assisted your results.
- Soft skills are harder to measure, so think about situations in which they've helped you thrive. Describe those situations concisely, focusing on how the outcome has helped you grow as a professional.
- Metrics of success - like positive ROI or optimized workplace processes - are the best way to prove your technical and people skills.
Take a look at some of clinical research nurse industry leaders' favorite hard skills and soft skills, as listed on their resumes.
Top skills for your clinical research nurse resume:
Clinical monitoring
Patient recruitment and enrollment
Data collection and management
Clinical trial regulatory compliance
Pharmacovigilance
Biomedical ethics
Protocol adherence
Electronic health records (EHR) proficiency
IV therapy administration
Specimen collection and processing
Effective communication
Attention to detail
Critical thinking
Problem-solving
Teamwork and collaboration
Adaptability
Time management
Cultural competence
List all your relevant higher education degrees within your resume in reverse chronological order (starting with the latest). There are cases when your PhD in a particular field could help you stand apart from other candidates.
How to include your education and certifications on your resume
We're taking you back to your college days with this part of our guide, but including your relevant higher education is quite important for your resume.
Your degree shows recruiters your dedication to the industry, your recent and relevant know-how, and some form of experience in the field.
Your clinical research nurse resume education should:
- Include your applicable degrees, college (-s) you've graduated from, as well as start and end dates of your higher education;
- Skip your high school diploma. If you still haven't graduated with your degree, list that your higher education is ongoing ;
- Feature any postgraduate diplomas in your resume header or summary - this is the perfect space to spotlight your relevant MBA degree ;
- Showcase any relevant coursework , if you happen to have less professional experience and think this would support your case in being the best candidate for the role.
As far as your job-specific certificates are concerned - choose up to several of the most recent ones that match the job profile, and include them in a dedicated section.
We've saved you some time by selecting the most prominent industry certificates below.
The top 5 certifications for your clinical research nurse resume:
- Certified Clinical Research Professional (CCRP) - Society of Clinical Research Associates (SOCRA)
- Certified Clinical Research Coordinator (CCRC) - Association of Clinical Research Professionals (ACRP)
- Oncology Certified Nurse (OCN) - Oncology Nursing Certification Corporation (ONCC)
- Certified Research Nurse (CRN) - No specific institution, it's a designation used by nurses who have gained experience and knowledge in the field.
- Advanced Oncology Certified Nurse (AOCN) - Oncology Nursing Certification Corporation (ONCC)
The more trusted the organization you've attained your certificate (or degree) from, the more credible your skill set would be.
- How to List GPA on Your Resume
- When Should You Include Your High School on Your Resume?
Should you write a resume summary or an objective?
No need to research social media or ask ChatGPT to find out if the summary or objective is right for your clinical research nurse resume.
- Experienced candidates always tend to go for resume summaries. The summary is a three to five sentence long paragraph that narrates your career highlights and aligns your experience to the role. In it you can add your top skills and career achievements that are most impressive.
- Junior professionals or those making a career change, should write a resume objective. These shouldn't be longer than five sentences and should detail your career goals . Basically, how you see yourself growing in the current position and how would your experience or skill set could help out your potential employers.
Think of both the resume summary and objective as your opportunity to put your best foot forward - from the get go - answering job requirements with skills.
Use the below real-world clinical research nurse professional statements as inspiration for writing your resume summary or objective.
Resume summaries for a clinical research nurse job
- With a decade of experience in oncology clinical research, I've developed expertise in orchestrating complex trials and advancing patient care through meticulous data analysis. My tenure includes a pivotal trial resulting in an FDA-approved therapy, showcasing my commitment to transformative healthcare outcomes.
- Accomplished pharmacist with 7 years of experience transitioning into clinical research nursing to leverage my extensive knowledge in pharmacology and patient counseling. Responsible for leading medication adherence programs, my expertise will facilitate robust clinical trial management and superior participant support.
- Dedicated Registered Nurse with 15 years in a fast-paced hospital environment, aspiring to apply my clinical expertise and compassionate patient care to the clinical research field. With strong leadership in critical care units, I seek to contribute to groundbreaking studies and patient-centric research methodologies.
- Expert in biostatistics and data management, seeking to pivot into clinical research nursing. Bringing forth 8 years of experience in health data analysis within government agencies, I am poised to employ my quantitative skills and knowledge of health protocols to enhance trial accuracy and efficacy.
- Eager to embark on a career as a clinical research nurse with no prior direct experience in the field but equipped with a solid foundation in health sciences. My academic excellence and unwavering commitment to patient welfare form the cornerstone of my desire to impact clinical trials and advancements in medicine.
- Aspiring clinical research nurse committed to entering the field, bringing a fresh perspective grounded in a BSc Nursing degree and volunteer experience with medical nonprofits. Motivated to integrate my caregiving skills and passion for learning to support innovative clinical studies and improve patient health outcomes.
Other relevant sections for your clinical research nurse resume
Apart from the standard clinical research nurse resume sections listed in this guide, you have the opportunity to get creative with building your profile. Select additional resume sections that you deem align with the role, department, or company culture. Good choices for your clinical research nurse resume include:
- Language skills - always ensure that you have qualified each language you speak according to relevant frameworks;
- Hobbies - you could share more about your favorite books, how you spend your time, etc. ;
- Volunteering - to highlight the causes you care about;
- Awards - for your most prominent clinical research nurse professional accolades and achievements.
Make sure that these sections don't take too much away from your experience, but instead build up your clinical research nurse professional profile.
Key takeaways
- The layout of your resume should take into consideration your professional background while integrating vital sections and design elements;
- Highlight your most pertinent achievements for the role all through different sections;
- Be very specific when selecting your certifications, hard skills, and soft skills to showcase the best of your talents;
- Include within the top one-third of your clinical research nurse resume a header and summary to help recruiters understand your experience and allocate your contact details. A skills box is optional, but it will help you align your expertise with the role;
- Detail the full extent of your professional experience with specific bullets that focus on tasks, actions, and outcomes.

Looking to build your own Clinical Research Nurse resume?

- Resume Examples
Best Fonts to Use On Your Cover Letter
How to answer "why should we hire you", the average length of a job interview: how long does it typically last, how to create a first year elementary school teacher resume, how to name drop in a cover letter, what does a president’s resume look like.
- Create Resume
- Terms of Service
- Privacy Policy
- Cookie Preferences
- Resume Templates
- AI Resume Builder
- Resume Summary Generator
- Resume Formats
- Resume Checker
- Resume Skills
- How to Write a Resume
- Modern Resume Templates
- Simple Resume Templates
- Cover Letter Builder
- Cover Letter Examples
- Cover Letter Templates
- Cover Letter Formats
- How to Write a Cover Letter
- Resume Guides
- Cover Letter Guides
- Job Interview Guides
- Job Interview Questions
- Career Resources
- Meet our customers
- Career resources
- English (UK)
- French (FR)
- German (DE)
- Spanish (ES)
- Swedish (SE)
© 2024 . All rights reserved.
Made with love by people who care.
Clinical Research Associate Resume Examples
Writing a great clinical research associate resume is important because it is one of the first things a potential employer will see when they are considering you for a position. It is your opportunity to make a good first impression and sell yourself as the best candidate for the job.
Create your resume Select from 7 professional resume templates
If you're looking for inspiration when it comes to drafting your own clinical research associate resume, look no further than the samples below. These resumes will help you highlight your experience and qualifications in the most effective way possible, giving you the best chance of landing the clinical research associate job you're after.

or download as PDF
Essential Components of a Clinical Research Associate Resume
A Clinical Research Associate (CRA) plays a pivotal role in the healthcare industry, ensuring the smooth and ethical progression of clinical trials. Crafting a robust CRA resume is crucial to demonstrate your expertise, work history, and commitment to prospective employers. Your resume should not only detail your previous employment but also highlight your capacity to manage significant responsibilities, adhere to regulations, and collaborate effectively with diverse teams. This guide will dissect the critical elements of a Clinical Research Associate resume, providing insights into each section's significance and content, along with tips to enhance your resume's impact.
1. Contact Information
Contact Information is a fundamental section for a Clinical Research Associate (CRA) resume. It's the gateway for hiring managers to initiate contact, so accuracy and currency are paramount. Ensure your contact details are prominently displayed and error-free to facilitate smooth communication for interview arrangements or further discussions.

Typically, your Contact Information should include your full name, address, phone number, and a professional email address. Opt for an email that reflects professionalism, often a variation of your first and last names.
Consider adding your LinkedIn profile , personal website, or portfolio if they provide additional value to your application. However, these are supplementary and should only be included if they strengthen your candidacy.
Important: Avoid including sensitive personal information such as your social security number or driver's license number at this stage of the application process.
In essence, the Contact Information section is vital for facilitating communication between you and potential employers, so ensure it's clear, accessible, and contains all necessary details.
2. Professional Summary or Objective Statement
The Professional Summary or Objective Statement is a prominent feature of a Clinical Research Associate (CRA) resume. It's often the first section hiring managers encounter, so it must be compelling, concise, and tailored to the position you're targeting.
In the Professional Summary, showcase your core competencies, experiences, and notable achievements in clinical research. This is your opportunity to highlight your expertise in areas such as:
- Conducting clinical trials
- Data collection and analysis
- Regulatory compliance
- Patient recruitment
- Other relevant expertise.
Utilize this section to demonstrate your proficiency with Good Clinical Practice (GCP) guidelines or your familiarity with specific clinical data management systems.
For those new to the field or transitioning into clinical research, an Objective Statement may be more appropriate. Here, you can articulate your career objectives and how you intend to contribute to the organization, showcasing your enthusiasm and readiness to embrace new challenges.
Whether you opt for a Professional Summary or Objective Statement depends on your career stage.
- Your summary or statement should be precise and focused, emphasizing what you bring to the table as a potential CRA.
- Given that hiring managers often skim resumes due to time constraints, ensure this section captures their attention immediately by demonstrating your suitability for the role.
Further Reading: Top Clinical Research Associate Resume Objective Examples
3. Education and Certifications
The " Education and Certifications " section is a cornerstone of a Clinical Research Associate (CRA) resume, showcasing your academic background and professional qualifications, which are crucial in this field.
Employers typically require a minimum of a bachelor’s degree in life sciences or related fields such as biology, nursing, biochemistry, or pharmacy. Possessing a master's degree or Ph.D. can significantly enhance your employability and distinguish you from other candidates.
Certifications are highly regarded in the clinical research arena. The Association of Clinical Research Professionals (ACRP) offers the Certified Clinical Research Associate (CCRA) credential, signifying expertise in overseeing clinical trials. Similarly, the Society of Clinical Research Associates (SoCRA) provides the Certified Clinical Research Professional (CCRP) certification to individuals who meet specific criteria and pass an examination.
Include any relevant training courses you've completed, such as Good Clinical Practice (GCP) training, which is often a requirement due to its standardization of clinical trial conduct involving human subjects.
List your educational achievements starting with the most recent, including the institution's name and graduation year. For certifications, mention the title, issuing organization, and validity period, if applicable.
Effectively presenting your education and certifications can underscore your commitment to continuous learning and adherence to industry standards—attributes highly sought after by employers in a CRA candidate.
Further Reading: Clinical Research Associate Certifications
4. Relevant Work Experience
Your Clinical Research Associate (CRA) resume should emphasize your relevant work experience prominently. This section provides potential employers with a comprehensive view of your previous roles and responsibilities in clinical research, affirming your capability to excel in the position.
Focus on positions that have equipped you with the skills and knowledge pertinent to a CRA role. You may have previous experience as a Clinical Trial Assistant, Research Coordinator, or Research Nurse .
For each role listed, provide specific details about your responsibilities and accomplishments. For instance, if you coordinated clinical trials, mention the trial phases (I-IV), the number of sites managed, the therapeutic areas (such as oncology or cardiology), and key tasks like data collection, patient recruitment, or regulatory document management.
- Highlight any experience across various medical specialties or managing studies in multiple regions to demonstrate adaptability.
- Emphasize any familiarity with different study designs and methodologies.
It's crucial to show your knowledge of Good Clinical Practice (GCP) guidelines and other regulatory standards. If your work has been audited or inspected by regulatory bodies like the FDA or EMA, include these experiences as well.
Quantifying your achievements can make them more impactful . For example: "Oversaw 5 clinical trials concurrently across 10 sites" or "Achieved 100% compliance in all regulatory audits."
If you're new to the field but possess transferable skills from other healthcare or research roles that are applicable to a CRA position, be sure to highlight these experiences. Skills in patient care, data analysis, project management, or regulatory affairs can be highly relevant.
In summary, the 'Relevant Work Experience' section is where you can demonstrate your practical understanding of clinical research operations and your ability to manage the multifaceted responsibilities of a CRA.
5. Skills and Competencies
The " Skills and Competencies " section is a critical part of a Clinical Research Associate's (CRA) resume, showcasing the abilities you bring to the role. This section should highlight both hard and soft skills that qualify you for the position.
- Clinical Trial Management: CRAs must have a thorough understanding of the clinical trial process.
- Data Management: Proficiency in collecting, handling, and interpreting data is crucial.
- Regulatory Knowledge: Familiarity with GCP, FDA regulations, and other guidelines is essential.
- Medical Terminology: Knowledge in medical terminology, pharmacology, or related areas is beneficial.
- Technical Proficiency: CRAs often utilize specialized software for data collection and analysis.
- Communication Skills: CRAs must communicate effectively with various stakeholders, including doctors, patients, and sponsors.
- Attention to Detail: Precision is critical in clinical trials to ensure accuracy and compliance.
- Problem-Solving: CRAs must be adept at quickly identifying and resolving issues during trials.
- Organizational Skills: Managing multiple tasks simultaneously requires excellent time management and organizational abilities.
- Ethical Judgment: Upholding patient safety in clinical trials necessitates strong ethical decision-making.
Listing these skills on your resume can demonstrate your readiness for the role. However, it's also crucial to provide real-world examples of how you've applied these skills, allowing potential employers to envision how you could positively impact their team. Related: Clinical Research Associate Skills: Definition and Examples
6. Research Projects and Publications
The " Research Projects and Publications " section is an integral part of a Clinical Research Associate (CRA) resume. It allows you to showcase your practical experience and theoretical knowledge in the field.
Detail the research projects you've been involved in, starting with the project title, followed by a brief description of its objectives. Highlight your specific role, focusing on your responsibilities and any methodologies you utilized. If your involvement in a project led to significant findings or advancements in the field, emphasize these outcomes.
Publications are also a critical aspect of this section. If your research has been published in scientific journals or presented at conferences, list these accomplishments, including the title, publication or conference name, and date. Where possible, provide a link to the publication.
Quality is more important than quantity; it's better to thoroughly describe a few significant projects and publications rather than list many with minimal detail.
By effectively showcasing your research projects and publications, you can demonstrate not only your expertise and skills but also your commitment to advancing the field of clinical research—a trait highly valued by employers.
7. References or Affiliations
References: The "References" section of your Clinical Research Associate (CRA) resume is crucial. It provides potential employers with contacts who can vouch for your competencies, experience, and work ethic. These references should ideally be former supervisors, colleagues, or collaborators who are well-acquainted with your CRA-related work and can provide detailed insights into your capabilities. Always secure permission before listing someone as a reference.
Affiliations: The "Affiliations" section demonstrates your engagement with the clinical research community and your commitment to professional development. Membership in organizations such as the Association of Clinical Research Professionals (ACRP) or the Society of Clinical Research Associates (SoCRA) indicates your dedication to upholding industry standards and best practices.
Certifications: Include any relevant certifications from these professional bodies or other recognized institutions in this section. Credentials like the Certified Clinical Research Associate (CCRA) or Certified Clinical Research Professional (CCRP) highlight your specialized expertise and skills.
Considerations:
- Customize this section for each job application to ensure relevance.
- Only include references and affiliations that are pertinent to the position you are applying for.
- Your aim is to present yourself as a knowledgeable, active participant in the clinical research community, recognized by peers for your contributions and proficiency.
Related Resume Examples
- Clinical Research Assistant
- Clinical Research Nurse
- Clinical Research Coordinator
- Clinical Research Manager
- Clinical Researcher
- Clinical Trial Associate
Clinical Research Resume Sample
The resume builder.
Create a Resume in Minutes with Professional Resume Templates
Work Experience
- Acts as the primary site contact for the sponsor’s CRA for assigned industry trials
- Independently prepares for and participates in NCI, FDA, pharmaceutical and other audits for assigned trials, as well as any other trials assigned to the CISO CRA’s Team
- Strong IT skills in appropriate software and company systems. Willingness to travel with occasional overnight stay away from home
- Proficient in speaking and writing the country language and English. Good written and oral communication skills
- A = Assess visit type, assess protocol specific and UBC SOP knowledge and assess individual’s ability to perform visit type independently
- Prepare and assisting in initiating implementation of clinical studies including preparation of Ethics Committee/Institutional Review Board materials and communicating approval
- Provide site personnel training including preparation/maintenance of study documents, completion of forms, and understanding of examinations/assessments required
- Maintain site and internal documentation in accordance with established Standard Operate Procedures (SOP´s) for agency/internal auditing
- Provide documentation, monitoring and distribution of contract obligations including financial obligations
- Ensure that trial contract obligations are met in a timely manner
- Serve as key contact to assigned clinical sites throughout the study process until site closeout
- Facilitate and support audit activities both in-house and at sites
- Prepare site visit reports
- Remain trained on EW SOPs and applicable clinical regulations
- Be available for monthly Clinical Team meetings in EW EMEA office, Nyon, Switzerland
- OTHER: Assume responsibility for additional assignments as directed by the CTO Director of Operations
- Maintain contact with key institutional departments as necessary
- Monitor and reporting site enrollment progress including device accountability in registration studies
- Identify & documenting enrollment deviations (inclusion/exclusion criteria, eligible not enrolled patients)
- Collect completed Case Report Forms (CRF) and collecting, confirming and auditing supporting documentation (paper CRF)
- Review, confirming and auditing documentation of the eCRF
- Monitor identifying and reporting adverse events and device-related complications in accordance with regulatory and internal requirements
- Deliver monitored CRF´s to Clinical Affairs or European Data Management if applicable
- Resolve identified CRF/eCRF queries and follow-up with the specific study sites to resolve them
Professional Skills
- Take initiative and demonstrate the ability to work effectively pro-active on cross-functional teams with strong leadership skills
- Possess excellent strategic planning skills, good judgment and strong decision making capabilities
- Excellent organizational, interpersonal, verbal, and written communication skills, (including experience in making presentations)
- Demonstrated excellent communication skills in speaking and writing
- Strong interpersonal skills, customer focused, and ability to work independently, managing multiple priorities across a highly matrixed global organization
- Strong leadership skills with proven success in people management
- Position requires composition ability, data analysis skills, scientific writing and presentation skills
How to write Clinical Research Resume
Clinical Research role is responsible for research, clinical, organizational, interpersonal, training, advanced, computer, clear, technical, software. To write great resume for clinical research job, your resume must include:
- Your contact information
- Work experience
- Skill listing
Contact Information For Clinical Research Resume
The section contact information is important in your clinical research resume. The recruiter has to be able to contact you ASAP if they like to offer you the job. This is why you need to provide your:
- First and last name
- Telephone number
Work Experience in Your Clinical Research Resume
The section work experience is an essential part of your clinical research resume. It’s the one thing the recruiter really cares about and pays the most attention to. This section, however, is not just a list of your previous clinical research responsibilities. It's meant to present you as a wholesome candidate by showcasing your relevant accomplishments and should be tailored specifically to the particular clinical research position you're applying to. The work experience section should be the detailed summary of your latest 3 or 4 positions.
Representative Clinical Research resume experience can include:
- Experience with solid understanding of current Good Manufacturing Practices, Good Tissue Practices, and Quality Programs
- Strong organizational skills, good at multi-tasking
- Have excellent interpersonal skills, including but not limited to: communication, problem-solving, teamwork development, leadership, mentorship
- Excellent understanding and demonstrated application of FDA guidelines, Good Clinical Practices and applicable Standard Operating Procedures
- Strong communication skills (written, verbal, listening) with all levels of the organization
- Demonstrated effectiveness in working in a multidisciplinary, matrix team situation, and demonstrated ability to manage change
Education on a Clinical Research Resume
Make sure to make education a priority on your clinical research resume. If you’ve been working for a few years and have a few solid positions to show, put your education after your clinical research experience. For example, if you have a Ph.D in Neuroscience and a Master's in the same sphere, just list your Ph.D. Besides the doctorate, Master’s degrees go next, followed by Bachelor’s and finally, Associate’s degree.
Additional details to include:
- School you graduated from
- Major/ minor
- Year of graduation
- Location of school
These are the four additional pieces of information you should mention when listing your education on your resume.
Professional Skills in Clinical Research Resume
When listing skills on your clinical research resume, remember always to be honest about your level of ability. Include the Skills section after experience.
Present the most important skills in your resume, there's a list of typical clinical research skills:
- Knowledge and skills with Microsoft Office, Microsoft Word and Excel along with strong organization skills and attention to detail are essential
- Excellent interpersonal skills in establishing and maintaining effective working relationships
- Demonstrates effective written and oral communication skills to a varied audience including, but not limited to; medical staff, hospital staff, and patients
- Excellent follow up skills required, including excellent follow through with colleagues and families
- Demonstrated experience in writing skills and understanding of research grant application process
- Excellent attention to details in data collections, strong written and oral communication skills
List of Typical Experience For a Clinical Research Resume
Experience for manager, clinical research resume.
- Effective verbal and written communication skills enhancing BCBST business processes, and provider credential satisfaction.10%
- Demonstrated analytical, negotiation, meeting management, cross-functional team and leadership skills at a management level are required
- Demonstrated planning skills; ability to create and track detailed project plans
- Excellent problem-‐solving skills
- Excellent Finnish, English & Swedish communication skills
- Strong experience in using and developing EDC and CTMS systems
- Computer skills vital including Word, Excel, PowerPoint
- Some experience with database software/strong interest in learning more
- Validate data using FITBIR Validation Tool
Experience For Clinical Research Monitor Resume
- Communicate effectively with sponsors and clinical sites
- Excellent understanding of Serious Adverse Event (SAE) reporting
- Needs experience developing and managing EDC, CTMS or QMS systems
- Good understanding of medical device regulatory requirements and documents, device accountability and adverse events reporting
- Desired experience with software packages for neuroimaging processing and statistics, such as FreeSurfer, SPM, FSL and R
- Experience with programming in Matlab and/or other related computing languages
- Previous Oncology onsite monitoring experience
- Experience in Immunology GI monitoring
- Clinical trial experience (CRO, healthcare setting and industry acceptable)
Experience For Clinical Research Coordinator Associate Resume
- Maintain a good working knowledge of interventional clinical practices, new devices and outcome measures as related to assigned work
- Understand the study protocol and accompanying background information (e.g., Investigator Brochure, Report of Prior Investigations etc.)
- Experience/knowledge of neuroimaging
- Experience creating clinical evaluation reports and templates
- Provide assistance to research center staff in their daily work related to prioritizing projects and tasks
- To guide researchers through the process of forming a good data request
Experience For Clinical Research Supervisor Resume
- Experience using Medidata RAVE system
- Strong understanding of cardiovascular anatomy, pathology and physiology required
- Related experience in subject recruitment, assessment, and analysis related to neuroimaging research
- Previous experience with human subject (behavioral/neuroimaging) research
- Proven track record in personnel/team management and team building
- At least seven (7) years experience in clinical research and in handling subjects and patients in a clinical research setting
- Independent monitoring experience – at least 2 years
- Knowing, implementing, monitoring and assuring the Program’s compliance with University of Florida institutional regulations regarding research
Experience For Clinical Research RN Resume
- Authoring or assisting in the creation of Monitoring Plans, monitoring section of Data Management Plans, and source document worksheets
- Coordinating with Investigational Drug Services (Pharmacy) for study visits requiring study drugs and arranging for orders and dispensing
- Working with the monitor from the sponsoring company during site initiation
- Ensuring Monitoring Plan requirements are being met and escalates risks
- Participating in the strategic planning for clinical trials team and assisting with development and implementation of the overall divisional plan
- Assisting with the preparation, organization and follow-up of investigator, site communications and clinical monitoring tracking
- Assisting in the creation of corrective and preventive action plans and trending reports
- Overseeing action item tracking and resolution status
- Performing co-monitoring visits as needed
Experience For Manager Clinical Research Resume
- Conducting investigations and audits of potential research compliance violations and implementing corrective action
- Reporting & writing data reports
- Interacting with sponsors on developing analysis plans
- Screening potential patients for protocol eligibility and presenting trial concepts and details to patients
- Participating in the informed consent process and enrolling patients on protocol
- Understanding of financial implications of the department and ability to analyze financial data, reports, project budgets, staffing, and expenditures
Experience For In-house Clinical Research Associate Resume
- Using existing FITBIR form structures, as applicable, for the ORION study
- Preparing and managing research protocols
- Working knowledge of US Federal government regulations regarding the conduct of human clinical research
- Working knowledge of information systems, including PC and Microsoft Office (Word, Access, PowerPoint, and Excel) and statistical software
- Providing input on study protocol, CRFs and other study documents
- Managing site assignments in collaboration with manager
- Facilitating communication and information flow between assigned project teams and assigned monitors on project
- Ensuring monitors are trained, current with project requirements and understand study milestones
- Reviewing data and source documentation from investigational sites for accuracy and completeness
Experience For Clinical Research Analyst Resume
- Attending Core/Study team meetings
- Explaining the ideal outcome of analysis plans to sponsors
- Coordinating patient care in compliance with protocol requirements
- Assisting with the maintenance of the Trial Master File to ensure completeness
- Working knowledge of dashboard software such as Tableau
- Recruiting and entering eligible candidates into research studies by obtaining informed consent and maintaining a clinical database
- Acting as liaison with members of internal and external units and reporting on progress
Experience For Clinical Research Coordinator Healthcare Resume
- Preparing reports to the Institutional Review Board in a timely manner
- Maintaining timely, accurate and thorough records of the program’s research activities
- Conducts hiring, training, directing, developing, and evaluating of staff
- Arranges meeting logistics. Drafts meeting agendas and assists in preparing meeting minutes
- Liaises with protocol principal investigators to establish pharmacy’s role in the trial for study drug compounding, packaging, and dispensing
- Help prepare manuscripts and reports, including preparing tables and formatting references
Experience For Field Clinical Research Associate Resume
- Work with the department to organize and improve health care services, including the tracking and reporting metrics for performance, program outcomes
- Manages action items resulting from meetings through resolution, including developing action plans
- Provide initial and ongoing training to site personnel regarding the study protocol, applicable policies/procedures, and GCP
- Ensure the site is entering data according to the CRF Completion Guidelines and meeting data entry and query resolution deadlines
- Conduct certain human resource functions such as evaluation of staffing need, interviewing, performance evaluations, timekeeping and conflict resolution
- Thorough and first-hand knowledge of all clinical research processes including IRB submission, recruiting, collecting patient data and specimens
List of Typical Skills For a Clinical Research Resume
Skills for manager, clinical research resume.
- Strong attention to detail, and competence to manage multiple priorities and respond effectively with limited guidance
- Solid working knowledge, skills, and understanding of project and program management techniques and technologies
- Excellent organizational skills and knowledge of federal legislation regarding human subject research and the provision of health care
- Effective leadership, people management, interpersonal, communication, and problem solving skills
- Effective verbal and written communication skills in relating to colleagues and associates both inside and outside the organization
- Plans, manages time and prioritises effectively
- Excellent verbal, written, interpersonal and written communication skills
- Well-organized, detail-oriented and excellent follow-up skills
Skills For Clinical Research Monitor Resume
- Demonstrated organizational skills and ability to manage complex IT projects
- Excellent interpersonal, communication, and presentation skills are required
- Strong communication, organizational skills, and self-motivated
- Work independently and proactively, with good organizational skills
- Strong interpersonal skills and flexibility for work in diverse settings
- Excellent analytical skills with the ability to collect, organize, analyze, and disseminate significant amounts of data efficiently and accurately
- + Experience working in a team/matrix environmentrequiring strong working relationships
Skills For Clinical Research Coordinator Associate Resume
- Strong working knowledge of statistics and experience using statistical packages for analyzing datasets (SAS, Excel, SPSS, etc.)
- Demonstrates strong leadership, team building and problem solving ability
- Prior research experience, including interaction with human subjects, ideally in a mental health setting
- Successfully manage multiple competing priorities and adapt quickly to changing priorities
- Adequate word processing skills with advanced MS Word and MS Excel are required
- Proven experience working with computers
- Skilled at providing excellent customer service
- Excellent working knowledge of all applicable ICH/GCP regulations and Good Documentation Practices and ability to work within these guidelines
- Technical writing skills (protocols, CRF development, study tools)
Skills For Clinical Research Supervisor Resume
- Proven experience in strategic planning, risk and change management
- Prior clinical trial experience, including source document and regulatory document maintenance
- Related quality assurance experience, including supervisory experience
- Competencies and essential skills in the following: cross functional Last
- Basic computer skills and working knowledge of Windows, Word, Excel
Skills For Clinical Research RN Resume
- Technical skills and familiarity with imaging software packages such as SPM, FSL and/or FreeSurfer
- In depth critical thinking skills to evaluate issues and identify a potential solution. Creatively address complex or new problems
- Interpersonal skills, detailed -oriented and meticulous
- Current medical device industry experience, people management, project management and budget management experience required
- Communicate effectively with the public, staff, faculty and students with proper English usage
- Organizational skills and management of large number of electronic forms and data points
- Detail-oriented person with the ability to work independently on multiple tasks and manage time effectively
- Proven experience in resource and expectations management in a matrix organization
Skills For Manager Clinical Research Resume
- Effective experience required
- Demonstrated experience
- Prior Experience in clinical study management or research collaborations is required
- Strong knowledge of Therapeutic Area(s), subject matter expertise and international experience are desirable
- Clinical experience with psychiatric populations and familiarity with DSM-IV diagnoses are very important. Experience with SCID helpful
Skills For In-house Clinical Research Associate Resume
- Proven ability to operate in a home office environment with flexibility to travel and report to corporate offices, as appropriate
- Effectively work in a fast paced environment with multiple projects and timelines
- Beginning to Intermediate level Metlab experience
- Experience with direct line management of staff including hiring, training, oversight and mentoring
- Previous clinical research monitoring Experience (including pre-study, initiation, routine monitoring and closeout visits) in Russia
- Previous clinical research monitoring Experience (including pre-study, initiation, routine monitoring and closeout visits) in South Africa
- Experience designing and overseeing a clinical development program, with emphasis on studies in humans
- Clinical research experience and/or basic science research including involvement with clinical protocols and participating in clinical trials
- Previous experience working with MRI scanning and data analysis
Skills For Clinical Research Analyst Resume
- Clinical research experience including involvement with clinical protocols and participating in clinical trials
- Two years’ experience in working within and/or conducting pragmatic, observational, and community-based health care projects
- Related hands-on experience in acquiring and/or processing fMRI data
- Extensive experience in conducting and managing Clinical Research Projects
- Over 5 years and up to and including 7 years of experience in clinical research preferably in a managed care setting
- Oversee supervisors and/or subordinate managers, including establishing priorities
Skills For Clinical Research Coordinator Healthcare Resume
- Substantial clinical development and execution experience, handling multiple parallel programs
- Experience working with documentation systems
- Able to prioritize work with an appreciation and understanding of organizational drivers, mission, critical objectives and budgetary expectations
- Cost-effectiveness analysis, financial accounting, or microeconomics
- Expertise in and excellent working knowledge of core trial management systems and tools
- Experience with clinical trial management systems/tracking databases
- One (1) year of experience working as a Registered Nurse (RN)
- Experience in Women’s Health monitoring
- Oncology monitoring experience required
Skills For Field Clinical Research Associate Resume
- Ideally 2 years’ monitoring experience in clinical development phase II-IV
- Valid Driver’s License and acceptable driving record at the time of hire
- Experience as a field clinical research monitor (Traveling CRA)
- Research experience in a healthcare setting required
- Experience in managing staff
List of Typical Responsibilities For a Clinical Research Resume
Responsibilities for manager, clinical research resume.
- Effective verbal and written communication skills enhancing BCBST business processes, and provider credential satisfaction
- Demonstrated experience working in clinical research as an In-house Clinical Research Associate, Clinical Research Coordinator, or Regulatory Affairs Associate
- Prior experience in a healthcare environment of clinical research setting
- Experience teaching, orienting and mentoring new employees to clinical research
- Experience using Enterprise Reporting for reconciling grant finances
- Previous clinical research monitoring Experience (including pre-study, initiation, routine monitoring and closeout visits) in Bulgaria
- Nursing experience, preferably in a hospital setting
- Experience in acquiring and/or processing fMRI data
- Experience with programming software including Matlab and Python
Responsibilities For Clinical Research Monitor Resume
- Experience managing grants using PeopleSoft tools
- Demonstrate expertise in leading-edge theories, techniques and/or technologies currently used in clinical trial design and conduct
- Work collaboratively with investigative sites to develop strong, long-term, working relationships
- Previous experience working on issues related to nutrition and/or obesity prevention
- Experience assessing clinical research protocols
- Independent field monitoring experience for clinical trials

Responsibilities For Clinical Research Coordinator Associate Resume
- Strong knowledge and understanding of application of GCP, specifically as it applies to investigative site conduct of clinical trials
- Professional attitude. Ability to respond well to changing priorities
- Demonstrated technical expertise in data models, database design development, data mining, and data segmentation
- Adept at queries, report writing, and presentation of findings with a strong drive for process optimization and data integrity
- Experience/knowledge of quantitative sensory testing
- Ensures patient questions and concerns are answered prior to obtaining consent. Explains in detail protocol requirements to the patient
- Experience assisting in the development of policy, procedure or patient education materials related to clinical research
Responsibilities For Clinical Research Supervisor Resume
- Substantial experience with standard software packages including Word, Excel, and PowerPoint
- Clinical Research Coordinator experience in an academic setting
- Experience working with IRB and research compliance regulations
- Clinical Monitoring experience
- Troubleshooting, contact, account, document and site management, monitoring visit report process and troubleshooting
Responsibilities For Clinical Research RN Resume
- Implementing digital and site facing clinical trial innovation to improve clinical trial efficiencies, reduced timelines and costs
- Understanding of statistics and familiarity with STATA or similar data analysis software
- Ensuring significant participation by all centers across the portfolio to positively impact recruitment performance and reduce study timelines and cost
- Ongoing portfolio surveillance and cross departmental collaboration with the CSUs to ensure network membership adequately supports program needs
- Providing seamless communication related to feasibility outcomes, study timelines to the CSU Network Site Liaisons
Responsibilities For Manager Clinical Research Resume
- Ensuring the reliable recruitment commitment of the Network sites against targets
- Understanding of mental health prevention and treatment
- Assists the Development Manager with disseminating funding opportunities and updating PI’s with status of funding and award notices
- Data collection and recording of everything surrounding the trial (doctor and patient information)
- Assist the investigators in evaluating, documenting, and reporting adverse events and other safety related data
- Oversee the collection and processing for specimens to meet study requirements, including saliva specimens and intravenous blood samples
- Understands and keeps a current understanding of FDA and Medicare guidelines for clinical trial billing
- Involvement in the activities required for the preparation, setting up, conducting and completion of clinical studies
- Acts as a resource and assists in the development of and provides training related to audit, monitoring, and QA/QI findings
Responsibilities For In-house Clinical Research Associate Resume
- Assist in obtaining outside documents and study specimen procurement and handling
- Prepare for monitoring/auditing visits, work with monitors when on site, and address all inquiries and follow-up as required by policies
- Proficiency in using computers, software, and web-based applications in a previous administrative setting
- Completion of an accredited Medical Assistant training program and/or an accredited Phlebotomy training program required
- Available to support site staff for studies not currently involved in performing on-site monitoring
- Audit monitor performance for studies not currently performing on-site monitoring, as requested
Responsibilities For Clinical Research Analyst Resume
- Advises Principal Investigator on funding source instructions regarding the grant budget, application, and allowable costs
- Conduct telephone screening calls for patients inquiring about their participation in clinical trials
- Interact and educate patients and families to help them gain an understanding and alleviate any apprehension regarding the studies
- Obtain written informed consent from patient allowing for free expression of fears, questions, etc., to ensure patient understanding
- Serve as a resource to others for all aspects of conducting a complex and multi-site clinical study, and lead a robust and expansive team
Responsibilities For Clinical Research Coordinator Healthcare Resume
- Writes informed consents following IRB requirements
- Submits application and applicable documents (protocol, informed consent, etc.) to overseeing Institutional Review Board (IRB)
- Conduct audit of selected participant’s source documentation including review of the electronic medical record, research files, labs etc
- Primary point of contact to support planning and execution of Celgene-sponsored clinical trials (phase’s I-IV)
- High level of exposure to cutting edge clinical trials
- Coordinates preparation of responses to regulatory agencies’ questions regarding the clinical study
- Represent clinical development function for other teams’ need including business development and legal related projects
- Serves as CRA back up providing verbal and written communication with study personnel
- Apply SOPs, Clinical Monitoring Plan (CMP), study manuals and other materials and guidelines as applicable
Responsibilities For Field Clinical Research Associate Resume
- Assist with start-up activities, including essential document review and collection as requested
- Perform Interim Monitoring Visits for assigned studies
- Review research specimen sample documentation, storage and processing and ensure shipments are sent to central lab as required
- Assist study team as necessary in resolving lab queries and other issues
- Collaborate with the drug safety group to ensure site compliance with serious adverse event reporting requirements
- Track and report progress of study, data monitoring, protocol variations, issue resolution, and follow up compliance
Related to Clinical Research Resume Samples
Clinical research associate resume sample, clinical research coord resume sample, director, clinical research resume sample, engineer, research resume sample, senior clinical research resume sample, post-doctoral research resume sample, resume builder.
Resume Worded | Proven Resume Examples
- Resume Examples
- Research & Science Resumes
- Clinical Research Resume Guide & Examples
Clinical Research Coordinator Resume Examples: Proven To Get You Hired In 2024

Jump to a template:
- Clinical Research Coordinator
- Clinical Trials Coordinator
- Clinical Research Manager
Get advice on each section of your resume:
Jump to a resource:
- Clinical Research Coordinator Resume Tips
Clinical Research Coordinator Resume Template
Download in google doc, word or pdf for free. designed to pass resume screening software in 2022., clinical research coordinator resume sample.
In this position, you will not be conducting the trials themselves but rather coordinating to ensure everything that needs to be done for the trial is done. This may include ensuring the finances and personnel needed are available, and that all related regulations and laws are followed. You will be reporting to a clinical principal investigator.

We're just getting the template ready for you, just a second left.
Recruiter Insight: Why this resume works in 2022
Tips to help you write your clinical research coordinator resume in 2024, include your strongest and most relevant abilities in the introduction..
Start your resume strong by including your strongest and most relevant skills and abilities in the introduction section. It sets a strong and impressive tone for the rest of your resume.
Include relevant awards or recognition.
Awards show that not only do you do excellent work but that your work is good enough to receive recognition over others. This applicant has effectively added 2 relevant awards.

Clinical Trials Coordinator Resume Sample
Clinical research manager resume sample.
We spoke with hiring managers at top clinical research organizations like IQVIA, PPD, and Syneos Health to understand what they look for in Clinical Research Coordinator resumes. The following tips will help your resume stand out and increase your chances of landing an interview in this competitive field.
Highlight your clinical research experience
Hiring managers want to see that you have relevant experience in clinical research. Be specific about your roles and responsibilities in each position.
- Coordinated 10+ phase II and III clinical trials for oncology drugs, ensuring compliance with FDA regulations and GCP guidelines
- Managed all aspects of clinical trial execution, including patient recruitment, data collection, and adverse event reporting for a 200-patient cardiovascular study
If you lack direct clinical research experience, emphasize any transferable skills from related fields such as healthcare or research.
- Worked as a research assistant in a biology lab
- Conducted research on the efficacy of a new antibiotic, resulting in a 25% reduction in infection rates among test subjects

Demonstrate your knowledge of regulations and guidelines
Clinical research is heavily regulated, so it's crucial to show your understanding of relevant regulations and guidelines.
- Ensured compliance with FDA 21 CFR Part 11, HIPAA, and ICH GCP guidelines across 5 global clinical trials
- Completed training in Good Clinical Practice (GCP), Good Documentation Practice (GDP), and IATA Dangerous Goods Regulations
Avoid simply listing regulations without context:
- Familiar with FDA, HIPAA, and GCP
Quantify your achievements
Use numbers and metrics to highlight your accomplishments and show the impact of your work.
- Increased patient recruitment rates by 30% through the implementation of targeted social media campaigns
- Reduced average data query resolution time from 5 days to 2 days by streamlining communication between sites and the CRO
Avoid vague or unquantified statements:
- Improved patient recruitment
- Resolved data queries quickly
Showcase your project management skills
Clinical Research Coordinators often manage multiple tasks and stakeholders simultaneously. Demonstrate your ability to effectively manage projects and collaborate with cross-functional teams.
- Led a team of 5 Clinical Research Associates in the successful execution of a 500-patient, multi-center clinical trial
- Collaborated with investigators, study coordinators, and data management to ensure timely completion of a 12-month oncology study, resulting in a 95% data accuracy rate
Avoid generic statements that don't provide specific examples:
- Managed clinical trials
- Worked with different teams
Tailor your resume to the job description
Customize your resume to highlight the skills and experience that align with the specific Clinical Research Coordinator position you're applying for.
For example, if the job description emphasizes experience with oncology trials:
- Coordinated 3 phase II oncology trials, focusing on solid tumors and hematologic malignancies
- Collaborated with oncologists and study nurses to ensure proper administration of investigational products and adherence to study protocols
Avoid using a generic resume that doesn't address the specific requirements of the position:
- Coordinated clinical trials in various therapeutic areas
Include relevant certifications and training
Listing relevant certifications and training can help demonstrate your expertise and commitment to professional development in the clinical research field.
- Certified Clinical Research Coordinator (CCRC) - Association of Clinical Research Professionals (ACRP)
- Completed NIH Clinical Research Training Course
Only include certifications and training that are directly applicable to the Clinical Research Coordinator role. Avoid listing irrelevant or outdated certifications:
- CPR certification (expired)
- Microsoft Office Specialist
By following these tips and tailoring your resume to the specific Clinical Research Coordinator position, you'll be well-positioned to showcase your qualifications and stand out from other applicants.
Writing Your Clinical Research Coordinator Resume: Section By Section
summary.
A resume summary for a Clinical Research Coordinator is optional, but it can be a valuable addition if you want to provide context or highlight key details that may not be immediately apparent from the rest of your resume. It's especially useful if you're changing careers and your past experience doesn't directly align with the Clinical Research Coordinator role, or if you're an experienced professional with a lot of relevant experience to showcase. However, it's important to avoid using an objective statement, as these are outdated and often ineffective.
When crafting your summary, focus on providing a concise overview of your most relevant skills, experiences, and achievements. Avoid repeating information that's already covered in other sections of your resume, and keep it brief - aim for no more than a short paragraph. While it can be tempting to mention soft skills like 'hard-working' or 'team player', it's best to avoid doing so directly. Instead, focus on highlighting specific examples or achievements that demonstrate these qualities.

To learn how to write an effective resume summary for your Clinical Research Coordinator resume, or figure out if you need one, please read Clinical Research Coordinator Resume Summary Examples , or Clinical Research Coordinator Resume Objective Examples .
1. Tailor your summary to the clinical research coordinator role
When writing your summary for a Clinical Research Coordinator position, it's crucial to tailor it to the specific role and industry. A generic summary that could apply to any job won't effectively capture the hiring manager's attention or demonstrate your suitability for the position. Instead, focus on highlighting your most relevant skills and experiences that directly relate to the responsibilities of a Clinical Research Coordinator.
For example, rather than using a generic summary like this:
Experienced professional seeking a challenging position in a dynamic organization where I can utilize my skills and contribute to the company's success.
Tailor your summary to the Clinical Research Coordinator role:
Detail-oriented Clinical Research Coordinator with 5+ years of experience in managing clinical trials, ensuring compliance with protocols, and coordinating with cross-functional teams. Skilled in data management, patient recruitment, and regulatory documentation. Seeking to leverage my expertise to contribute to groundbreaking research at XYZ Company.
2. Highlight your clinical research skills and achievements
When crafting your Clinical Research Coordinator resume summary, focus on showcasing your most impressive and relevant skills and achievements. This is your opportunity to make a strong first impression and entice the hiring manager to read the rest of your resume.
Some key skills and experiences to highlight in your summary include:
- Experience in managing and coordinating clinical trials
- Knowledge of regulatory requirements and guidelines (e.g., FDA, GCP)
- Proficiency in data management and analysis
- Successful patient recruitment and retention strategies
- Collaboration with cross-functional teams (e.g., investigators, sponsors, IRBs)
In addition to mentioning your skills, try to include a quantifiable achievement that demonstrates your impact. For example:
Skilled Clinical Research Coordinator with 7+ years of experience in managing phase I-IV clinical trials. Proficient in data management, patient recruitment, and regulatory compliance. Spearheaded a patient retention initiative that resulted in a 95% completion rate for a complex, multi-site trial.
Experience
Your work experience section is the most important part of your clinical research coordinator resume. It's where you show hiring managers how you've applied your skills and knowledge to solve problems, exceed goals, and make an impact in your previous roles.
In this section, we'll break down everything you need to know to write a compelling work experience section step-by-step, with plenty of examples to inspire you.
1. Lead with a strong action verb
When describing your clinical research coordinator experience, choose powerful action verbs that showcase your contributions and accomplishments. Consider verbs like:
- Coordinated a multi-center Phase III clinical trial with 250+ participants across 6 sites
- Managed all aspects of a clinical study, including protocol development, IRB submission, and site management
- Collaborated with cross-functional teams to ensure successful study execution and on-time delivery of milestones
- Implemented a new electronic data capture (EDC) system, resulting in a 30% reduction in query resolution time
Avoid bland, overused verbs like "responsible for" or "participated in" that don't convey the specifics of what you actually did.
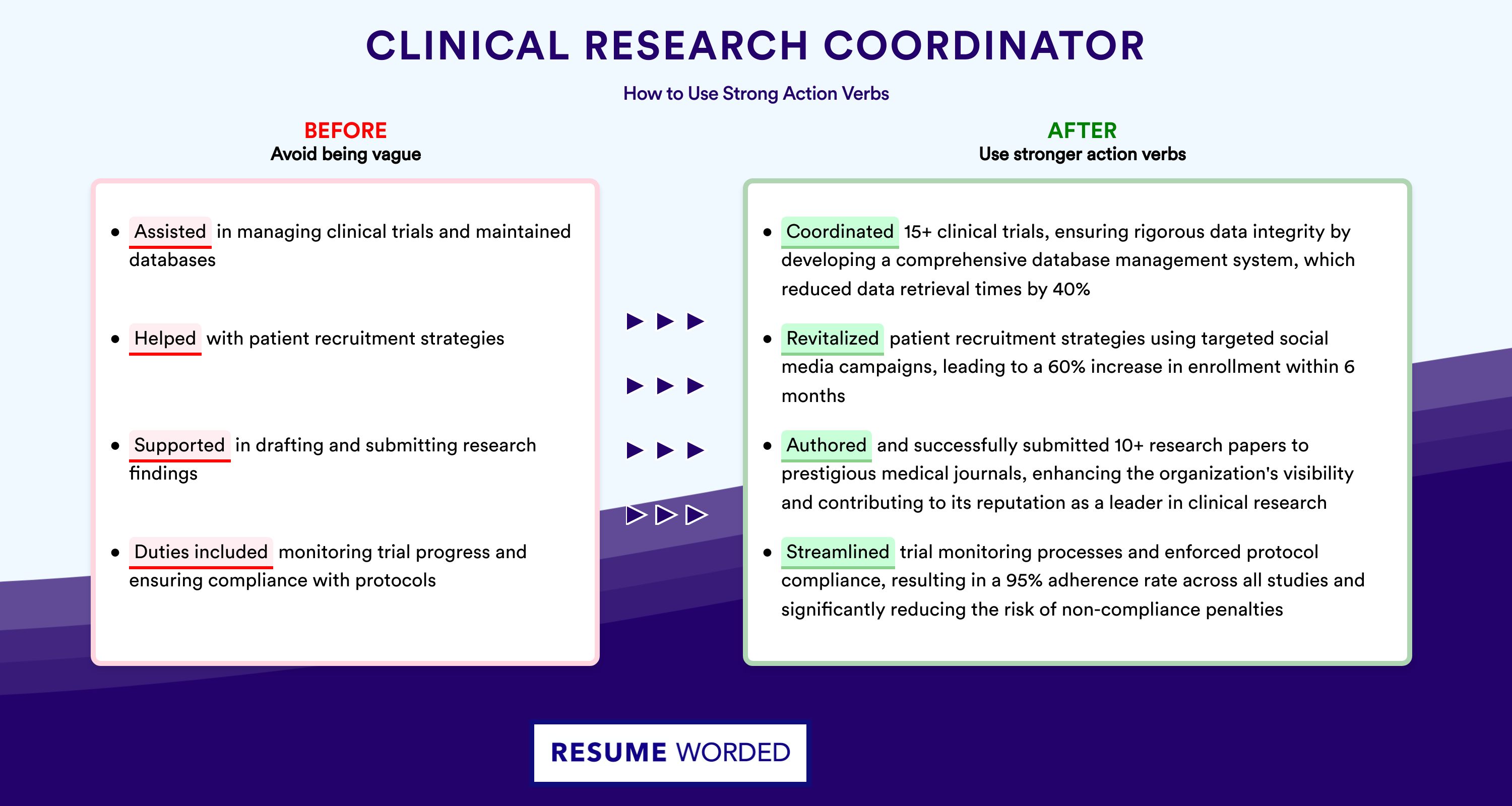
2. Quantify your accomplishments with metrics
Numbers are a powerful way to demonstrate your achievements and stand out to hiring managers. Whenever possible, include specific metrics that show the scope and impact of your work, like in these examples:
- Screened and enrolled 95 study participants in under 60 days, exceeding enrollment targets by 20%
- Managed a site budget of $1.2M, consistently tracking and reporting on spend to ensure on-budget delivery
- Trained and supervised a team of 5 clinical research assistants and 10+ site personnel
If you don't have exact metrics, estimates are okay too. Even general numbers provide helpful context, like:
- Reviewed and processed 100+ case report forms (CRFs) per week to ensure clean, accurate data
3. Highlight your clinical research skills and tools
The best clinical research coordinator resumes showcase the specific skills and tools needed for the job. As you describe your experience, weave in the technical skills, therapeutic areas, and research tools you've used, like:
- Coordinated 3+ oncology studies, managing all aspects of the clinical trial lifecycle
- Managed study data in EDC systems like Medidata Rave and Oracle InForm
- Utilized CTMS systems like Veeva Vault to manage site documents and monitor progress
Avoid simply listing skills without context, like:
- Clinical research
- EDC systems
Instead, provide specific examples of how you've applied those skills to manage clinical trials successfully.
4. Show career growth and promotions
Hiring managers love to see candidates who have progressed and taken on more responsibility in their career. If you've been promoted, make it stand out in your work experience section:
Clinical Research Coordinator II, ABC Pharma (2019-Present) Clinical Research Coordinator I, ABC Pharma (2017-2019)
If your title hasn't changed but your role has evolved, you can still showcase your growth:
- Promoted to Lead CRC for a global Phase III study after successfully managing a Phase II study
- Selected to train and mentor junior CRCs based on strong performance and subject matter expertise
However, avoid exaggerating your contributions or making promotions up. Stick to the facts and let your real career progression shine.
Education
Your education section is a crucial part of your clinical research coordinator resume. It shows hiring managers that you have the necessary educational background and training to excel in the role. In this section, we'll cover key tips for crafting a compelling education section that will help you stand out from other candidates.
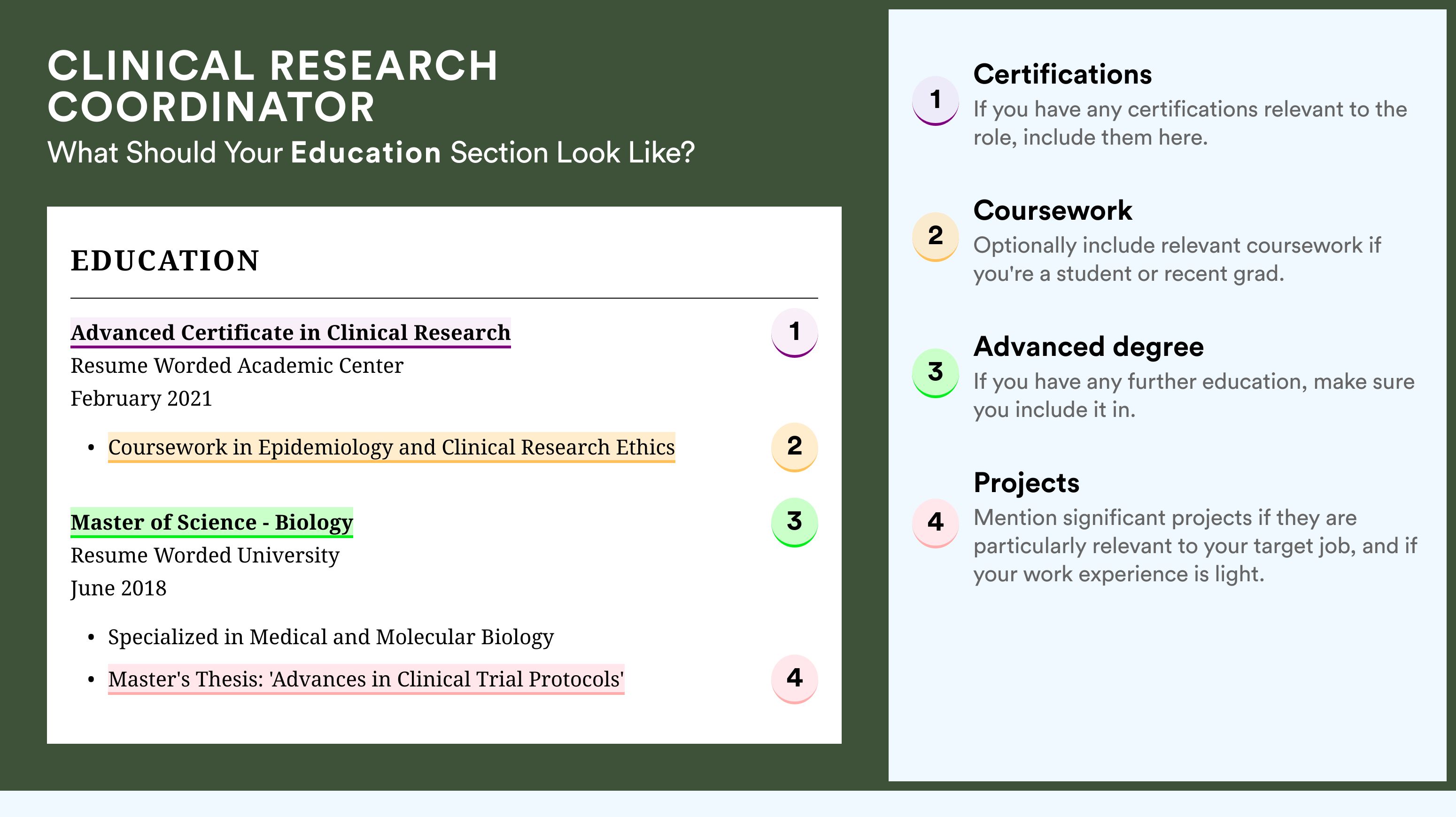
1. List your degrees in reverse chronological order
Start with your most recent degree and work backwards. This format helps hiring managers quickly identify your highest level of education and see your educational progression over time.
Here's an example of how to list your degrees in reverse chronological order:
Master of Science in Clinical Research, XYZ University, 2019 Bachelor of Science in Biology, ABC College, 2015
2. Include relevant coursework for entry-level candidates
If you are a recent graduate or have limited work experience, highlighting relevant coursework can help demonstrate your knowledge and skills to potential employers. List courses that are directly related to clinical research, such as:
- Clinical Trial Design and Management
- Biostatistics
- Pharmacology
- Research Ethics
However, avoid listing irrelevant or general education courses, as they can clutter your resume and distract from your relevant qualifications. For example:
- Introduction to Psychology
- English Composition
- Art History
3. Keep it concise for experienced clinical research coordinators
If you have several years of experience in clinical research, your education section should be brief and to the point. Hiring managers will be more interested in your professional accomplishments and skills.
For example, an experienced clinical research coordinator's education section might look like this:
M.S., Clinical Research, XYZ University B.S., Biology, ABC College
Notice how the graduation years are omitted to prevent potential age discrimination. This concise format allows you to dedicate more space to your work experience and achievements.
4. Include certifications to showcase your expertise
In addition to your formal education, certifications demonstrate your commitment to professional development and can set you apart from other candidates. Some relevant certifications for clinical research coordinators include:
- Certified Clinical Research Coordinator (CCRC)
- Certified Clinical Research Professional (CCRP)
- Certified Research Administrator (CRA)
You can list your certifications in your education section or create a separate section titled "Certifications" if you have several to showcase. Be sure to include the full name of the certification, the issuing organization, and the year you obtained it.
Skills
The skills section is a critical part of your clinical research coordinator resume. It's where you showcase your expertise and qualifications to potential employers. When writing this section, focus on highlighting the most relevant and impressive skills that match the job description. Here are some tips to help you craft a compelling skills section:
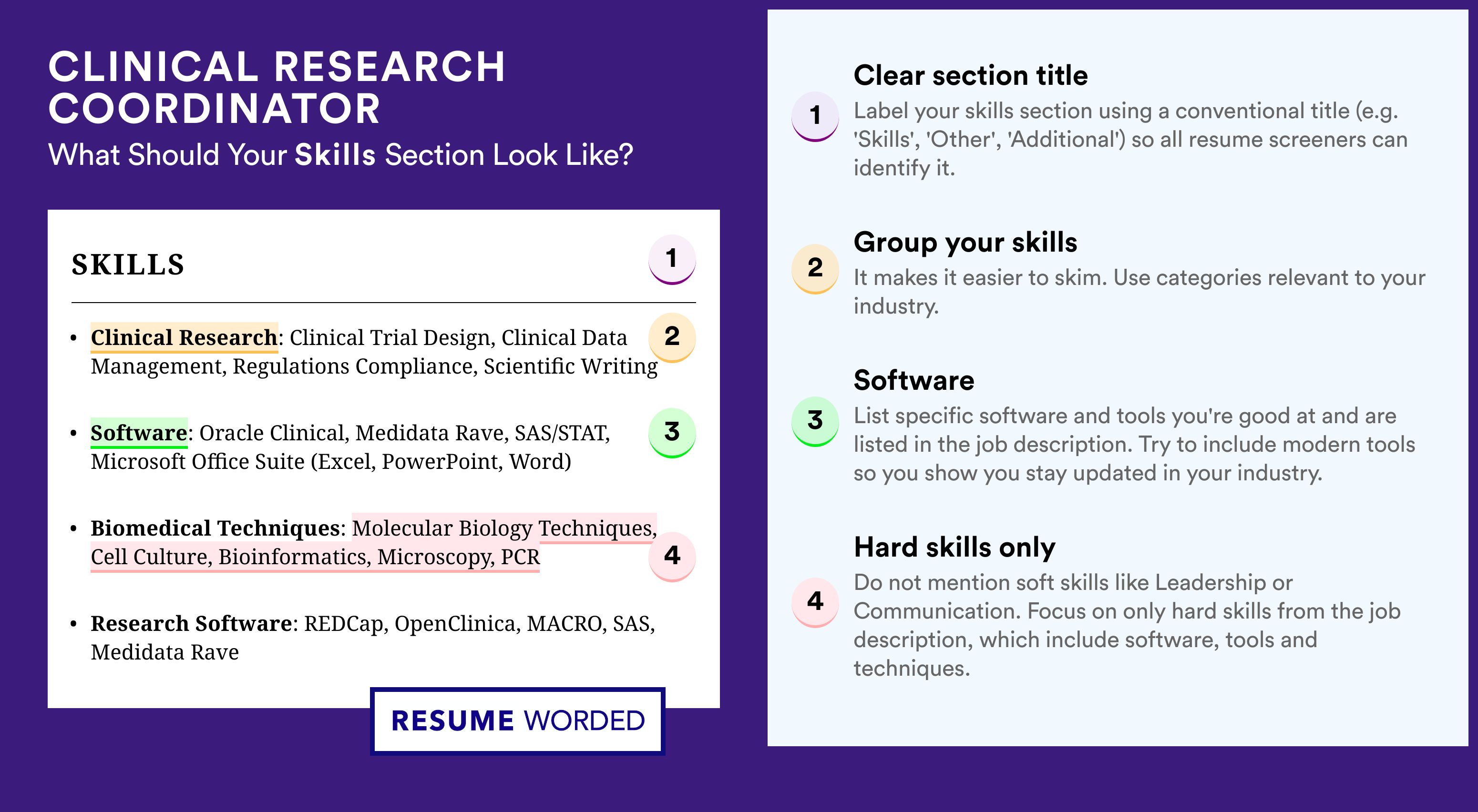
1. Highlight your clinical research expertise
As a clinical research coordinator, you likely have a wide range of skills related to managing clinical trials. When listing your skills, prioritize those that are most relevant to the specific position you're applying for. Consider including:
- Clinical trial management
- Protocol development
- Regulatory compliance (e.g., FDA, IRB)
- Patient recruitment and retention
- Data management and analysis
By showcasing your expertise in these areas, you'll demonstrate to employers that you have the knowledge and experience necessary to excel in the role.
2. Categorize your skills for clarity
To make your skills section easy to read and navigate, consider grouping your skills into categories. This is particularly helpful if you have a diverse skill set that spans multiple areas. For example:
Clinical Research Skills Clinical trial management Protocol development Regulatory compliance (FDA, IRB) Data Management Skills Electronic data capture (EDC) systems Database management Statistical analysis (SAS, R)
By organizing your skills in this way, you'll make it easier for hiring managers to quickly assess your qualifications and determine if you're a good fit for the role.
3. Focus on hard skills over soft skills
When it comes to your skills section, it's best to prioritize hard skills over soft skills. Hard skills are specific, measurable abilities that are directly related to the job, such as proficiency in certain software or experience with particular research techniques. Soft skills, on the other hand, are more general traits like communication or teamwork.
While soft skills are important, they're better demonstrated through your work experience and achievements. In your skills section, focus on concrete, technical skills that showcase your expertise. For example:
Skills: Strong communication Detail-oriented Proficient in Microsoft Office
Instead, highlight specific skills like:
Skills: Electronic data capture (EDC) systems (Medidata Rave, Oracle InForm) Clinical trial management systems (CTMS) Good Clinical Practice (GCP)
4. Avoid outdated or irrelevant skills
When crafting your skills section, be selective about which skills you choose to include. Avoid listing outdated or irrelevant skills that don't directly relate to the clinical research coordinator role. For example, while proficiency in Microsoft Office may be useful, it's a basic skill that's expected of most professionals and doesn't particularly set you apart.
Similarly, if you have experience with older clinical trial management systems or software that's no longer widely used, it's best to leave those off your resume. Stick to current, relevant skills that demonstrate your ability to thrive in the role.
Skills: Microsoft Office (Word, Excel, PowerPoint) Clinical trial management (paper-based) Data entry
Instead, focus on up-to-date, job-specific skills:
Skills: Electronic data capture (EDC) systems (Medidata Rave, Oracle InForm) Clinical trial management systems (CTMS) Risk-based monitoring
Skills For Clinical Research Coordinator Resumes
Here are examples of popular skills from Clinical Research Coordinator job descriptions that you can include on your resume.
- Regulatory Submissions
- Data Analysis
- Good Clinical Practice (GCP)
- Clinical Trial Management System (CTMS)
- Public Health
- Oncology Clinical Research
- Life Sciences
- Informed Consent
Skills Word Cloud For Clinical Research Coordinator Resumes
This word cloud highlights the important keywords that appear on Clinical Research Coordinator job descriptions and resumes. The bigger the word, the more frequently it appears on job postings, and the more likely you should include it in your resume.

How to use these skills?
Similar resume templates, care coordinator.

Equity Research

Human Resources (HR)
- Clinical Research Resume Guide
- Quality Control Resume Guide
- Chemistry Resume Guide
- Health and Safety Resume Guide
- Environmental Scientist Resume Guide
Resume Guide: Detailed Insights From Recruiters
- Clinical Research Resume Guide & Examples for 2022
Improve your Clinical Research Coordinator resume, instantly.
Use our free resume checker to get expert feedback on your resume. You will:
• Get a resume score compared to other Clinical Research Coordinator resumes in your industry.
• Fix all your resume's mistakes.
• Find the Clinical Research Coordinator skills your resume is missing.
• Get rid of hidden red flags the hiring managers and resume screeners look for.
It's instant, free and trusted by 1+ million job seekers globally. Get a better resume, guaranteed .
Clinical Research Coordinator Resumes
- Template #1: Clinical Research Coordinator
- Template #2: Clinical Research Coordinator
- Template #3: Clinical Trials Coordinator
- Template #4: Clinical Research Manager
- Skills for Clinical Research Coordinator Resumes
- Free Clinical Research Coordinator Resume Review
- Other Research & Science Resumes
- Clinical Research Coordinator Interview Guide
- Clinical Research Coordinator Sample Cover Letters
- Alternative Careers to a Clinical Research Coordinator
- All Resumes
- Resume Action Verbs
Download this PDF template.
Creating an account is free and takes five seconds. you'll get access to the pdf version of this resume template., choose an option..
- Have an account? Sign in
E-mail Please enter a valid email address This email address hasn't been signed up yet, or it has already been signed up with Facebook or Google login.
Password Show Your password needs to be between 6 and 50 characters long, and must contain at least 1 letter and 1 number. It looks like your password is incorrect.
Remember me
Forgot your password?
Sign up to get access to Resume Worded's Career Coaching platform in less than 2 minutes
Name Please enter your name correctly
E-mail Remember to use a real email address that you have access to. You will need to confirm your email address before you get access to our features, so please enter it correctly. Please enter a valid email address, or another email address to sign up. We unfortunately can't accept that email domain right now. This email address has already been taken, or you've already signed up via Google or Facebook login. We currently are experiencing a very high server load so Email signup is currently disabled for the next 24 hours. Please sign up with Google or Facebook to continue! We apologize for the inconvenience!
Password Show Your password needs to be between 6 and 50 characters long, and must contain at least 1 letter and 1 number.
Receive resume templates, real resume samples, and updates monthly via email
By continuing, you agree to our Terms and Conditions and Privacy Policy .
Lost your password? Please enter the email address you used when you signed up. We'll send you a link to create a new password.
E-mail This email address either hasn't been signed up yet, or you signed up with Facebook or Google. This email address doesn't look valid.
Back to log-in
These professional templates are optimized to beat resume screeners (i.e. the Applicant Tracking System). You can download the templates in Word, Google Docs, or PDF. For free (limited time).
access samples from top resumes, get inspired by real bullet points that helped candidates get into top companies., get a resume score., find out how effective your resume really is. you'll get access to our confidential resume review tool which will tell you how recruiters see your resume..

Writing an effective resume has never been easier .
Upgrade to resume worded pro to unlock your full resume review., get this resume template (+ 9 others), plus proven bullet points., for a small one-time fee, you'll get everything you need to write a winning resume in your industry., here's what you'll get:.
- 📄 Get the editable resume template in Google Docs + Word . Plus, you'll also get all 9 other templates .
- ✍️ Get sample bullet points that worked for others in your industry . Copy proven lines and tailor them to your resume.
- 🎯 Optimized to pass all resume screeners (i.e. ATS) . All templates have been professionally designed by recruiters and 100% readable by ATS.
Buy now. Instant delivery via email.
instant access. one-time only., what's your email address.

I had a clear uptick in responses after using your template. I got many compliments on it from senior hiring staff, and my resume scored way higher when I ran it through ATS resume scanners because it was more readable. Thank you!

Thank you for the checklist! I realized I was making so many mistakes on my resume that I've now fixed. I'm much more confident in my resume now.

Clinical Research Associate CV example (CRA)
Landing a good Clinical Research Associate role can be tough in today’s competitive job market, but having a strong CV will make it much easier.
This guide will show you how to write a winning CV, and even includes a CRA CV example to help you get started.
Guide contents
- Clinical Research Associate CV example
- CV layout and format
- Your CV profile
- Work experience
Education section
CV templates

This CV example demonstrates the type of info you should be including within your Clinical Research Associate CV, as well as how to format and structure the information in a way which looks professional and is easy for time-strapped recruiters to read.
This is the look and feel you should be aiming for, so remember to refer back to it throughout your CV writing process.

Clinical Research Associate CV layout and format
Think your CV is just about the content within it? Think again.
Your CV needs to look professional and be easy for recruiters to read, meaning the structure and format of your CV are just as important as the written content within it.
Facilitate ease of reading by using a simple structure which allows anybody to easily navigate your experience.
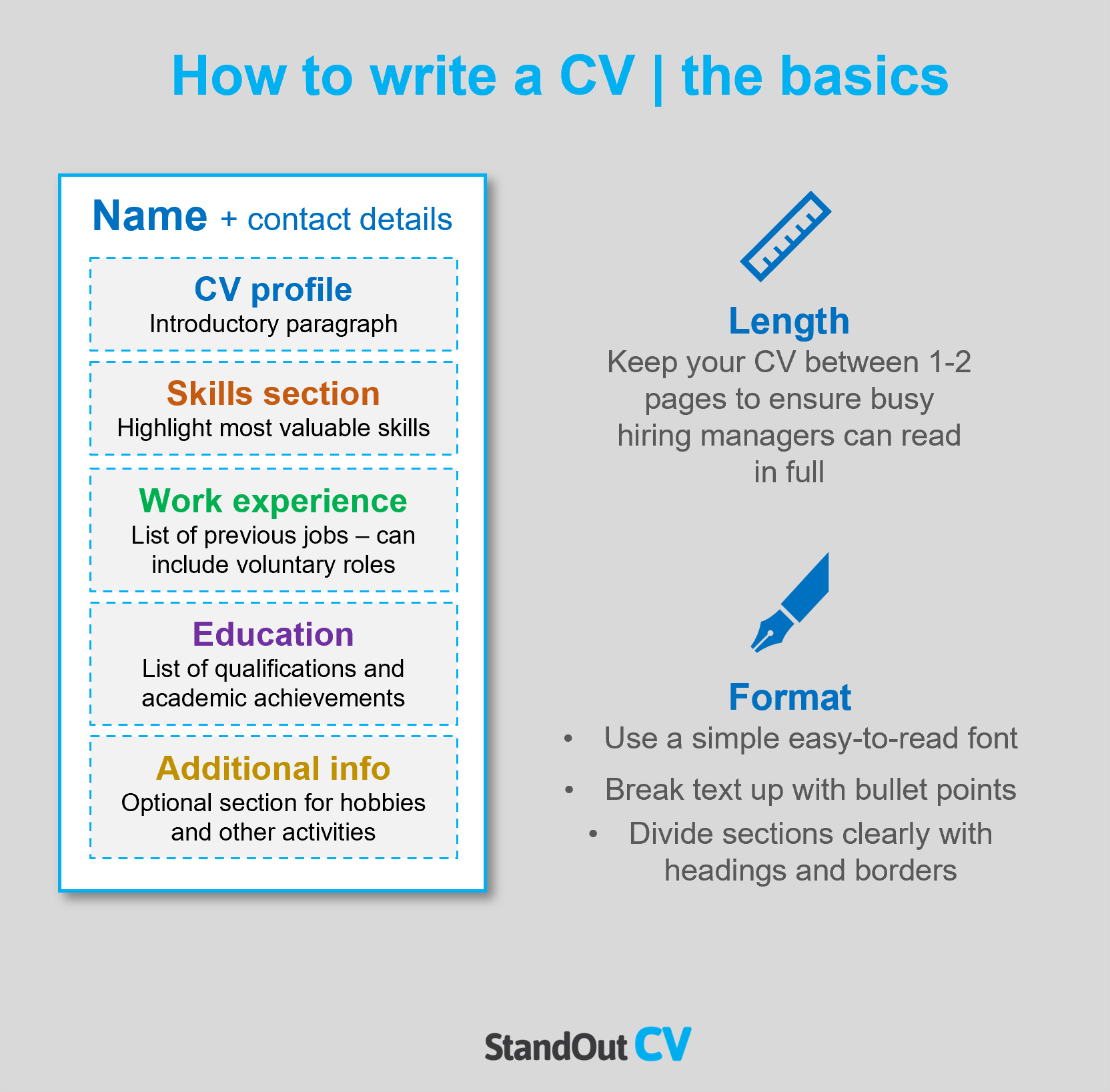
Formatting advice
- Length: Recruiters will be immediately put off by lengthy CVs – with hundreds of applications to read through, they simply don’t have the time! Grabbing their attention with a short, snappy and highly relevant CV is far more likely to lead to success. Aim for two sides of A4 or less.
- Readability : By clearly formatting your section headings (bold, or a different colour font, do the trick) and breaking up big chunks of text into snappy bullet points, time-strapped recruiters will be able to skim through your CV with ease.
- Design: While it’s okay to add your own spin to your CV, avoid overdoing the design. If you go for something elaborate, you might end up frustrating recruiters who, above anything, value simplicity and clarity.
- Avoid photos: Ditch logos, images or profile photos . Not only do they take up valuable space, but they may even distract recruiters from your important written content.
CV structure
For easy reading, write your CV to the following CV structure:
- Contact details – Make it easy for recruiters to get in touch with you by listing your contact details at the top of your CV.
- Profile – A short and snappy summary of your experience and skills, showcasing what makes you a good fit for the position.
- Work experience / career history – Note down all your work history, with your current position first, then working backwards.
- Education – A short list of your academic background and professional/vocational qualifications.
- Interest and hobbies – This is an optional section, which you can use to highlight any relevant hobbies or interests.
Now I’ll tell you exactly what you should include in each CV section.
CV Contact Details

Write your contact details in the top corner of your CV, so that they’re easy to find but don’t take up too much space.
You only need to list your basic details, such as:
- Mobile number
- Email address
- Location – Don’t list your full address. Your town or city, such as ‘Norwich’ or ‘Coventry’ is perfect.
- LinkedIn profile or portfolio URL – Remember to update these before listing them on an application.
Clinical Research Associate CV Profile
Grab the reader’s attention by kick-starting your CV with a powerful profile (or personal statement , if you’re a junior applicant).
This is a short introduction paragraph which summarises your skills, knowledge and experience.
It should paint you as the perfect match for the job description and entice recruiters to read through the rest of your CV.

Tips for creating an strong CV profile:
- Keep it concise: When it comes to CV profile length, less is more, as recruiters are often time-strapped. Aim for around of 3-5 persuasive lines.
- Tailor it: No matter how much time you put into your CV profile, it won’t impress if it’s irrelevant to the role you’re applying for. Before you start writing, make a list of the skills, knowledge and experience your target employer is looking for. Then, make sure to mention them in your CV profile and throughout the rest of your application.
- Don’t add an objective: If you want to discuss your career objectives, save them for your cover letter , rather than wasting valuable CV profile space.
- Avoid cliches: If there’s one thing that’ll annoy a recruiter, it’s a clichè-packed CV. Focus on showcasing your hard skills, experience and the results you’ve gained in previous roles, which will impress recruiters far more.
Example CV profile for Clinical Research Associate
What to include in your clinical research associate cv profile.
- Summary of experience: Start with a brief summary of your relevant experience so far. How many years experience do you have? What type of companies have you worked for? What industries/sectors have you worked in? What are your specialisms?
- Relevant skills: Highlight your skills which are most relevant to CRA jobs, to ensure that recruiters see your most in-demand skills as soon as they open your CV.
- Essential qualifications: Be sure to outline your relevant Clinical Research Associate qualifications, so that anyone reading the CV can instantly see you are qualified for the jobs you are applying to.
Quick tip: If spelling and grammar are not a strong point of yours, Use our quick-and-easy CV Builder to add pre-written content that has been created by recruitment experts, and proofread by our team.
Core skills section
Underneath your profile, write a core skills section to make your most relevant skills jump off the page at readers.
It should be made up of 2-3 columns of bullet points of your relevant skills.
Before you do this, look over the job description and make a list of any specific skills, specialisms or knowledge required.
Then, make sure to use your findings in your list. This will paint you as the perfect match for the role.

Work experience/Career history
By now, you’ll have hooked the reader’s attention and need to show them how you apply your skills and knowledge in the workplace, to benefit your employers.
So, starting with your most recent role and working backwards to your older roles, create a thorough summary of your career history to date.
If you’ve held several roles and are struggling for space, cut down the descriptions for your oldest jobs.

Structuring your roles
If you don’t pay attention to the structure of your career history section, it could quickly become bulky and overwhelming.
Get in recruiters’ good books by creating a pleasant reading experience, using the 3-step structure below:

Begin with a summary of your role, detailing what the purpose of your job was, who you reported to and what size of team you were part of (or led).
Key responsibilities
Next, write up a punchy list of your daily duties and responsibilities, using bullet points.
Wherever you can, point out how you put your hard skills and knowledge to use – especially skills which are applicable to your target role.
Key achievements
Round up each role by listing 1-3 key achievements , accomplishments or results.
Wherever possible, quantify them using hard facts and figures, as this really helps to prove your value.
After your work experience, your education section should provide a detailed view of your academic background.
Begin with those most relevant to CRA jobs, such as vocational training or degrees. If you have space, you can also mention your academic qualifications, such as A-Levels and GCSEs.
Focus on the qualifications that are most relevant to the jobs you are applying for.
Interests and hobbies
This section is entirely optional, so you’ll have to use your own judgement to figure out if it’s worth including.
If your hobbies and interests could make you appear more suitable for your dream job, then they are definitely worth adding.
Interests which are related to the industry, or hobbies like sports teams or volunteering, which display valuable transferable skills might be worth including.
Writing your Clinical Research Associate CV
An interview-winning CV for a Clinical Research Associate role, needs to be both visually pleasing and packed with targeted content.
Whilst it needs to detail your experience, accomplishments and relevant skills, it also needs to be as clear and easy to read as possible.
Remember to research the role and review the job ad before applying, so you’re able to match yourself up to the requirements.
If you follow these guidelines and keep motivated in your job search, you should land an interview in no time.
Best of luck with your next application!

IMAGES
VIDEO
COMMENTS
Template 1 of 10: Clinical Research Associate Resume Example. A clinical research associate (CRA) is in charge of planning and coordinating the execution of clinical trials aimed at testing products. You will also serve as the contact person for doctors, patients, pharmacists, etc.
1. Write a dynamic profile summarizing your clinical qualifications. Start your resume with a powerful opening summary that encapsulates your strongest qualifications as a CRA. List your title, years of experience, and three to four specializations in your opening sentence that match the job description.
Here are seven steps for writing a clinical research associate resume: 1. Study the job description. Study the job description carefully to check if you fulfill all the job requirements, as it may require specific certifications or a certain number of years of experience.
Professional Summary. Creative Clinical Research Associate with extensive project experience from concept to development. Talents include in-depth knowledge of ICH guidelines, SAE reporting, and GCP auditing. Integral team player with excellent communication skills; fluent in French. Core Qualifications.
Clinical Research Monitor Resume Examples & Samples. Bachelor's Degree in life sciences, health related disciplines or nursing. 2-3 years Clinical Research experience (preferably with Clinical Monitoring experience) Expertise in GCP, ISO 14155 (preferably) and regulatory requirements in Greece.
Clinical Research Associates test drugs before they are released on the market and assess their benefits and risks. Usual work activities described in a Clinical Research Associate resume example include creating trial protocols, collaborating with ethics committees, recruiting assistants, verifying data, writing visit reports, presenting results, and writing final reports.
Example for a clinical research associate resume Here is an example of a clinical research associate resume: Amala Dhavaak 1311 Flower street #4B, 10005 New York City, New York 444-444-4444 [email protected] Professional summary Diligent professional with five years of experience in the medical industry. Excellent communicator with attention to detail and time management skills.
Senior Clinical Research Associate Resume Examples & Samples. 4-6 years industry experience, including a minimum 4 years clinical research experience. Competent in the skills for the CRA II. Experience in line management and/or mentoring of CR Assistants and CRAs. Contributes in process improvement teams.
Unlock the secrets to building a standout clinical research resume with our comprehensive 2023 guide. Let our expert insights and up-to-date industry trends be your roadmap to success and elevate your career to new heights today. ... These resume examples build into crafting the perfect insurance agent resume, arming you with industry-specific ...
Clinical Researcher Resume Examples. Clinical Researchers run clinical trials to test drugs for effectiveness and safety. They are typically involved in all stages of the clinical trial and complete duties such as writing trial protocols, designing data collection forms, collaborating with the ethics committee, setting up trial sites, closing ...
5 Clinical Research CV Examples - Here's What Works In 2024. If you want to work in clinical research, you need a CV that shows your skills and experience. This article can help with that. It's full of examples and templates for you to use. Each one is designed to catch an employer's eye.
The Guide To Resume Tailoring. Guide the recruiter to the conclusion that you are the best candidate for the clinical research scientist job. It's actually very simple. Tailor your resume by picking relevant responsibilities from the examples below and then add your accomplishments. This way, you can position yourself in the best way to get ...
Now, here's how to write the job-winning resume for a clinical research coordinator: 1. Format Your Clinical Research Coordinator Resume Right. Clinical research coordinators lead drug trials and medical research. Being in a coordinating role, they are also responsible for recruiting staff and ensuring smooth operation in research labs.
For example, "Created a comprehensive training program for clinical trial associates, resulting in a 40% increase in employee productivity.". Use action words such as coordinate, maintain and perform to make an impact on your clinical trial associate resume. Tailor your resume to your target clinical trial associate job.
Sample clinical research coordinator resume objective: Enthusiastic and detail-oriented recent graduate with a Bachelor of Science in Public Health. Eager to leverage academic foundation in clinical research methodologies and understanding of regulatory compliance to contribute to the success of research initiatives at a dynamic health care ...
The five (plus) definite sections your resume for a clinical research nurse job should include are: Header with your headline, contact details, and/or a preview of your work. Summary (or objective) to pinpoint how your success aligns with the role. Experience with bullets of your most relevant achievements in the field.
Further Reading: Top Clinical Research Associate Resume Objective Examples. 3. Education and Certifications. The " Education and Certifications " section is a cornerstone of a Clinical Research Associate (CRA) resume, showcasing your academic background and professional qualifications, which are crucial in this field.
Position requires composition ability, data analysis skills, scientific writing and presentation skills. Create a Clinical Research Resume. Find and customize career-winning Clinical Research resume samples and accelerate your job search. All clinical research resume samples have been written by expert recruiters.
The Guide To Resume Tailoring. Guide the recruiter to the conclusion that you are the best candidate for the clinical research specialist job. It's actually very simple. Tailor your resume by picking relevant responsibilities from the examples below and then add your accomplishments. This way, you can position yourself in the best way to get ...
Here are eight steps to help you write a resume for this position, including some ideas on what to include in the document to capture a hiring manager's attention: 1. Research the position. Review the job description to better understand what the employer seeks in clinical research coordinator candidates.
EXPERIENCE. Resume Worded - Austin, USA February 2022 - Present. Coordinator. Led the Oncology Clinical Research division, overseeing 12 trials and resulting in a 35% increase in successful outcomes. Developed streamlined patient recruitment strategies, leading to a 40% boost in patient enrollment in trials.
The Guide To Resume Tailoring. Guide the recruiter to the conclusion that you are the best candidate for the clinical research physician job. It's actually very simple. Tailor your resume by picking relevant responsibilities from the examples below and then add your accomplishments. This way, you can position yourself in the best way to get ...
CV templates This CV example demonstrates the type of info you should be including within your Clinical Research Associate CV, as well as how to format and structure the information in a way which looks professional and is easy for time-strapped recruiters to read.. This is the look and feel you should be aiming for, so remember to refer back to it throughout your CV writing process.