- Employee Self Service

Change Color
- Our Priorities
- Laws & Legislations
- Centre of Excellence
- Organ Donation
- Primary Care
- Medical Education
- Coronavirus - COVID -19
- Medical Tourism
- Research and Innovation Center
- Professionals
- Why Invest?
- Foreign Investment
- Abu Dhabi Health Workforce Masterplan
- Capacity Master Plan
- Scope of Practice
- Lists & Tools
- Medications & Supplements Awareness Material
- Jawda - Quality Metrics
- Unified Healthcare Professional Qualification Requirements (PQR)
- Health Information Exchange standards
Healthcare facilities are required to complete the Training Programme on Abu Dhabi Healthcare Guidelines for Health Media & Advertising System as mentioned in circular number 26/2023, for more information Click Here
DOH urges all healthcare and pharmaceutical facilities & Health professional to Adhere to the circular number(63\2021) by updating the details of the mobile number & e-mail address in order to receive all circulars & letters issued by DOH, for more information Click Here


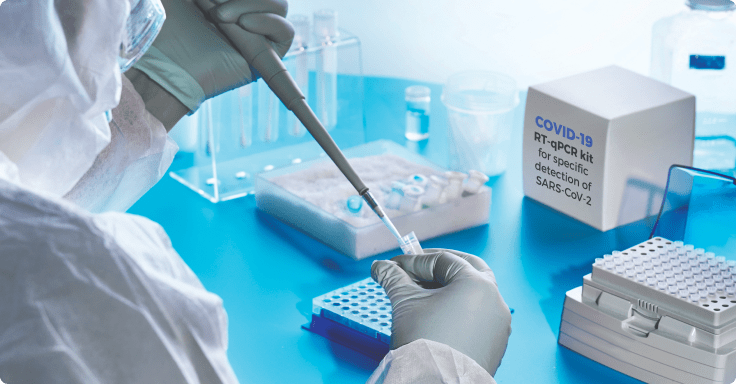
RESEARCH AND INNOVATION CENTER
Abu Dhabi Healthcare Sector has the potential to deliver high quality Medical Research and Innovation
“To be a leading regional center for healthcare & life-science research & innovation for a healthier population”
“to develop and sustain thriving research and innovation ecosystem, across invention, evidence generation and adoption for the regulated products and solutions to position abu dhabi as a regional hub for better healthcare outcomes, knowledge, and socioeconomic prosperity”.

Public-Centered

Transparency

Innovation & Excellence
Department of Health has strategized key priorities that underpin the goal of improving the well-being of Abu Dhabi residents through prevention and access to integrated, innovative, high-quality and cost-effective healthcare. Genomic medicine is a ground-breaking genomics initiative that involves using genomic information about an individual to understand genetics bases, provide accurate diagnosis and treatment and implement precision medicine. A healthier Abu Dhabi community will not be achieved expediently unless innovation and research are at the core of its daily business.
Priority Disease

Areas of Research

Research and Innovation Programs
Publications

Medical Research and Development

This applies to all entities who are interested in conducting a human subject research in Abu Dhabi such as:
- Healthcare facilities
- Academic institutes
- Contract Research Organizations (CRO’s)
- Government/Private institutes

Facilities should form a local Research Ethics Committee in order to submit their application to us

Follow the below requirements

Once approved, research authorization will be granted

Facility name will appear on the list of facilities approved to carry out human subject research
Requirements
- Application for Authorization to Conduct Human Subjects Research
- Research Ethics Committee (REC) members list - Including CVs and DOH health license if applicable
- Research Ethics Training Course For all ethics committee members
- DOH Undertaking letter - Signed by all REC members
Download Available Forms and Documents
Form#1 - Application for Authorization to Conduct Human Subjects Research
Form#2 - REC Details
REC Undertaking Letter
Directly upload the forms or for any inquiries, email us at [email protected]
Maximum file size is 8 MB

Organizations and researchers who are interested in conducting human subject research in the below streams, are welcomed to apply directly to DOH Medical Research Team
- Clinical Trials
- Multi-centers
- Processing of medical data outside UAE,
- Pharmaceutical/companies sponsored research, and Academic sponsored research.
- Others (COVID, etc)
Research proposals for the above streams will be evaluated by DOH ADHRTC (Abu Dhabi Research and Technology Committee)

Click here to download application form

Send your application form and all the required documents (Detailed proposal- Research team CVs and GCPs- Data collection Sheet- Questionnaire/Survey- Consent Form) to Medical Research team at [email protected]

ADHRTC Committee will review the application

Final outcome letter will be shared

Browse the Research Registry
Please check below:
- Medical Research Policies
- Medical Research Circulars
- Medical Research Guidelines
For any inquiries, email : [email protected]
The Showcase of Medical Research Publications from Abu Dhabi is a collection of valuable medical research which appeared in peer-reviewed journals from healthcare community within Abu Dhabi’s healthcare sector. The Showcase includes scientific literature on a wide range of medical topics.
Find out more about the medical research publications from Abu DHabi
INNOVATION SERVICES

Health technology assessment (HTA) refers to the systematic evaluation of properties, effects, and/or impacts of health technology.

Submit HTA application (please find Forms and Documents below)

Once received, an evaluation process will take place based on safety, efficiency and effectiveness

An Official letter will be sent to applicants (Approved or Disapproved)

Codes/pricing, product/therapy registration and publication
Browse the Technology Registry
Form#1 - New Health Technology New therapeutic practices Submission Form
Form#2 - New Health technology New therapeutic practices Application Check list
Form #3 - Information Validity Form HTA
Fill the forms and email us at [email protected]
For disapproval decisions: HTA applicants appealing submission to [email protected] (Within 30 days of receiving disapproval decision) to start the same journey.

Shape the future of healthcare through innovation. HealthTech Hub offers a comprehensive support network for the testing, implementation and commercialization of health technologies.

Initial interview & meeting of startups

Startup submission of business plan and requirements for ecosystem support

Agreement of joint project plan and timeline

Startups application of ADGM Tech License

DoH support in regulation, Proof of Concept Design, Coaching, Access to Investor Networks, Access to Partners and Health Technology Assessment

Graduation from DoH HealthTech program (maximum 24 months)
For any inquiries, email us at: [email protected]
Drug and Medical Products

Through this service, as drug agents and healthcare providers (hospitals), you can apply to code drugs, including conventional and general sale products. These codes are essential for drug product reimbursement by insurance and applicants can apply to amend coded drug details.

Submit the application and all required documents through TAMM portal

Receive DoH’s approval, rejection or request for additional details

Obtain the drug code.
Required Documents:
- MOH Drug/Product Registration Certificate; From the Ministry of Health and Prevention for registered drugs (drug agents)
- MOH Price Certificate; From the Ministry of Health and Prevention for registered drugs (drug agents)
- Price Quotation; For non-registered drugs (hospitals)
- Good Manufacturing Practice (GMP) Certificate; For non-registered drugs (hospitals)
- Artwork/Product Image
- Product Image
- Product Leaflet
For any inquiries, email : [email protected]

Through this service, you can apply for laboratory analysis of any medical product, such as pharmaceuticals, dietary supplements, herbal or cosmetic products to ensure the absence of any dangerous substances and that it is safe for use.
Conditions The applicant must hand in the sample in person.

Applicants to submit form and required documents through TAMM portal

Submit the sample of the product At the Department of Health counter

Obtain the results from DoH through SMS or a phone call, if needed
Call 800555 (Abu Dhabi Government Portal)

Through this service, healthcare facilities in Abu Dhabi may apply to assign or replace a person that is responsible for narcotics and controlled drugs

Once received, an evaluation process will take place

Receive the approved electronic application form
- Application Form; Complete and sign the “Approving a person to be responsible for narcotics and controlled drugs” form
- Official Letter From the Medical Director to authorize the current person in-charge
- Signed handover Report on Controlled and Narcotic Drugs; in case of changing responsible person

Through this service, Facility’s Narcotic in-charge should submit the consumption report for controlled drugs on a monthly basis and quarterly for narcotic drugs. Process and required documents:

Click here to download the report form.

Send the monthly controlled report to DoH through
Send the Narcotic quaterly report to DoH through
For any inquiries, email : [email protected]

Through this service, Facility’s Narcotic in charge should report to DoH any lost/broken ampoules; or any narcotic or controlled drugs incidences.
Download the Narcotic/ Controlled Drug Incident Report Form.

Enclose and submit the required documents as required.

Send the form to DoH Narcotic officer through e-mail address:

Through this service you can access the unified electronic platform approved at the country level for prescribing and dispensing of narcotic, controlled and semi controlled medicines. The federal law obligates all healthcare facilities and providers to use the system for prescribing and dispensing narcotic, controlled and semi-controlled medicines. Also, all licensed healthcare facilities and pharmacies must provide Emirates ID card reader to all their physicians and pharmacists who are dealing with narcotic and controlled medicines. Register the health facility on the Electronic Platform for Controlled Medicines, or log-in the platform if the facility is registered, as mentioned in the links below.
New user - register on the Electronic Platform for Controlled Medicines

Existing user - log in to the platform
For more information or technical support, please contact: Tel. 600566635 Email: [email protected]

Through this service, concerned stakeholders including hospitals, pharmacies and drug suppliers are required to report all anticipated and /or actual drug shortages to DOH immediately in order to avert and mitigate the problem. Process:
Download the Drug Shortage Reporting Form.
Send the Reporting Form through e-mail address
DoH will work closely with the drug suppliers, manufacturers & health care facilities to identify root causes of the reported shortages and fill any gaps in the supply chain to ensure ease of access and availability of necessary medicines to all residents of Abu Dhabi.

Through this service, healthcare professionals can report any suspected Adverse Reaction (AR), Medication Error (ME) and Adverse Event Following Immunization (AEFI) experienced from any of the drug product within the stipulated timeframe as defined in DoH policies, even if reporters are not certain that the particular medicinal product was the cause. Unintended adverse effects, drug abuse, overdose, interaction (including drug-drug and drug-food interactions) and unusual lack of therapeutic efficacy are all considered to be reportable suspected Adverse Reactions (AR).
Click the button below to access the e-Notification system
Log in using your professional or facility account information
Select notification then Pharmacovigilance then select type of report
Fill out all the required information
Click ‘’Submit “
Reference documents:
- DOH Standard on Reporting Medication Errors
- DOH Standard on Reporting suspected Adverse Drug Reactions and Adverse Events following Immunization
- Guidance on How to Access Pharmacovigilance reporting tool
For any enquiries send an email to: [email protected]

Through this service Healthcare facilities are required to submit their antidote stock report on monthly basis . The service allows the hospital user to report the stock of antidote by the 5th of each month. Process:

Click the here to access the e-Notification system.

Log in using your professional or facility account information.
Select notification then Antidote Monthly Stock Report.

The Antidote Monthly Stock Report Form will be displayed to be filled by user.
For enquiries e-mail to [email protected]
Subscribe for updates
Thanks for subscribing, last updated: 03 may 2024.


Abu Dhabi opens new clinical trials unit to boost medical research
The new unit will conduct research and trials focused on key disease areas
Abu Dhabi has inaugurated a new clinical trials unit to help respond to pressing local health challenges and position the emirate as a research and innovation hub.
The new unit was launched by Sheikh Shakhbout Medical City (SSMC), a joint-venture partnership between Mayo Clinic and Abu Dhabi Health Services Company (SEHA).
to enabling the health sector in the expansion of research and development. The newly launched Clinical Trials Unit, brings medical research and development to the forefront in finding innovative solutions to the treatment of patients with complex care requirements. pic.twitter.com/Qfx8LtQsOZ — Sheikh Shakhbout Medical City (@SSMCAbuDhabi) January 17, 2023
The centre will conduct complex research and trials focused on key disease areas like neurology, rheumatology, hematology, oncology, cardiology, ophthalmology, gastroenterology and pediatrics.
SSMC’s researchers will also collaborate with national research institutions across the country, including hospitals and universities.
Meanwhile, the unit will offer greater opportunities for local and regional researchers, helping to develop the next generation of doctors aspiring to pursue medical research, a statement said.
The launch of the trials unit is part of a multi-phase approach, spread over a period of 10 years. The unit’s core infrastructure and initial operations will be established within the first two years of operations, marking its first phase. Phase 2 will see the creation of a dedicated physical space.
H.E. Sheikh Abdulla bin Mohamed Al Hamed, H.E. Dr. Jamal Al Kaabi, Dr. Naser Ammash, chief executive officer at #SSMC , cut the ribbon at the opening ceremony of the Clinical Trials Unit. pic.twitter.com/MKJtqqCJQ2 — Sheikh Shakhbout Medical City (@SSMCAbuDhabi) January 17, 2023
The unit, in its third phase, will reach its innovation development capabilities, including business development and entrepreneurship.
“The groundbreaking launch of our clinical trials unit illustrates our commitment to investing in research and finding new and innovative ways to treat our patients and respond to the region’s most concerning health challenges today and in the future,” said Dr Naser Ammash, chief executive officer of SSMC.
“SSMC’s clinical trials unit is a foundational component of what we’re building to become a leading medical research institute in the region performing unparalleled translational research at the interface of medicine, science, and technology,” added Dr. Shahrukh Hashmi, acting medical director of research at SSMC.
“At this moment in time, we have over 55 industry and investigator-initiated trials and over 30 registries that are ongoing or in development.”
Meanwhile, Dr Asma Al Mannaei, executive director of the research and innovation center at the Department of Health – Abu Dhabi (DoH), noted that currently, there were around 400 clinical research trials under way in Abu Dhabi.
Abu Dhabi has been home to research and innovative medical projects. In July of last year, the Abu Dhabi Early Childhood Authority (ECA) had announced Dhs1.2m worth of funding for five research projects related to early childhood development (ECD).
Read: Abu Dhabi Early Childhood Authority awards Dhs1.2m to fund research projects
G42 Healthcare signed a strategic agreement with US-based ProPhase Precision Medicine to explore collaborative opportunities focusing on, but not limited to, genomic sequencing, artificial intelligence, sharing genomic data insights, and obtaining advanced certifications.
Read: G42 Healthcare, ProPhase to collaborate on genomic sequencing capabilities, data insights
Also read: Role of biotechnology and life sciences in UAE’s healthcare ambitions
In early 2021, the UAE had launched a project to begin manufacturing the Covid-19 vaccine within the country.
Read: UAE to begin manufacturing ‘Hayat-Vax’ Covid-19 vaccine
You might also like

Abu Dhabi’s ADNOC says oil production capacity hits 4.85 million bpd

Abu Dhabi’s ADQ lists debut $2.5bn bond on London Stock Exchange

UAE leaders mourn passing of Sheikh Tahnoun bin Mohammed

UAE weather: NCM issues orange alert as heavy rains hit Abu Dhabi, Dubai
Abu dhabi police’s 80kph lower speed limit for bad weather: what you should know, weather forecast: more rain on the way for abu dhabi, dubai says ncm, major schengen visa update for bahrain, oman, saudi arabia and india, latest issue.
- Saudi Arabia
- Real Estate
- Special Report
- Art & Culture
Advertise With Us
Privacy policy.
© 2021 MOTIVATE MEDIA GROUP. ALL RIGHTS RESERVED.
- Refer a Patient
- Our Leadership
- CEO Message
- Facts and Figures
- Awards and Accreditations
- Annual Reports
- Corporate and Community Outreach
- Appointments
- Executive Health Program
- International Patient Services
- Patient Stories
- Visiting Guidelines
- How to get to SSMC
- Child Safety Survey
- Patient Education
- Health Blogs
- Our Doctors
- Adolescent Medicine
- General Pediatrics
- Pediatric Endocrinology
- Pediatric Intensive Care
- Pediatric Neurology
- Pediatric Rheumatology
- Pediatrics Neonatology
- General surgery
- Neurosurgery
- Ophthalmology
- Oral and Maxillofacial Surgery (OMF)
- Orthopedics
- Otolaryngology – Head and Neck Surgery (ENT)
- Pediatric Surgery and Pediatric Urology
- Plastic and Reconstructive Surgery
- Thoracic Surgery
- Vascular Surgery
- Dermatology
- Endocrinology
- Gastroenterology and Hepatology
- Internal Medicine
- Physical Medicine and Rehabilitation
- Pulmonary and Sleep Medicine
- Rheumatology
- Tropical and Infectious Diseases
- Department of Pathology and Laboratory
- Department of Critical Care
- Department of Emergency Medicine
- Department of Clinical Imaging
- Department of Obstetrics and Gynecology
- Department of Anesthesiology
- Department of Radiation Therapy
- Introduction to Education
- Graduate Medical Education Center
- Simulation Center
- Nursing Education Center
- Medical Student Education Center
- Health Sciences Education Center
- Staff Development Center
- Clinical Trials Unit
- Scientific Advisory Committee
- Institutional Review Board (IRB)
- Request an Appointment
Jan. 18, 2023
Sheikh shakhbout medical city inaugurates a dedicated clinical trials unit.
- In partnership with Mayo Clinic, SSMC opens a dedicated Clinical Trials Unit to set a new benchmark for innovative research and development in the UAE
- The inauguration comes in line with DoH’s ongoing efforts to enable the healthcare sector to expand R&D operations in the Emirate and elevate the sector’s outcomes
- SSMC’s Clinical Trials Unit will conduct complex research and trials focused on key disease areas like Neurology, Cardiology and Oncology
Sheikh Shakhbout Medical City (SSMC), one of the UAE’s largest hospitals for serious and complex care and a joint-venture partnership between Mayo Clinic and Abu Dhabi Health Services Company (SEHA), launched today one of the most advanced Clinical Trials Units in Abu Dhabi. By conducting research on areas with unmet medical needs, the Unit will help the UAE respond to pressing local health challenges, contributing to a more sustainable health care system and supporting the ongoing efforts to position Abu Dhabi as a leading life-science research and innovation hub.
In the presence of H.E. Abdulla bin Mohamed Al Hamed, Chairman of the Department of Health – Abu Dhabi (DoH), a delegation from the Department witnessed the inauguration, including H.E. Dr. Jamal Al Kaabi, Undersecretary of DoH.
The newly inaugurated specialist Unit will be led by a dedicated team of researchers set to conduct cutting-edge research and complex trials to help reduce the impact of some of the greatest health care challenges in Neurology, Rheumatology, Hematology, Oncology, Cardiology, Ophthalmology, Gastroenterology and Pediatrics. SSMC’s researchers will also collaborate with national research institutions across the country, including hospitals and universities in the UAE.
H.E Dr. Jamal Al Kaabi, Undersecretary of the Department of Health, said: “Under the directives of the UAE’s wise leadership, we are proud to witness Sheikh Shakhbout Medical City lead the way of complex research and trials from the heart of Abu Dhabi. Supported by the Department of Health, the inauguration comes in line with DoH’s ongoing efforts to enable the healthcare sector to expand R&D operations in the Emirate and elevate the sector’s outcomes. Reinforcing Abu Dhabi’s position as a leading destination for life sciences, we are confident that this milestone will fruit innovative solutions and expose insights to some of the most pressing healthcare challenges.”
Through localizing research, SSMC’s team of researchers will be able to address the immediate and future needs of the UAE’s population and help optimize processes and standards when it comes to delivering care. Looking towards the future, the Unit is also set to provide greater opportunities for local and regional researchers, helping to develop the next generation of doctors aspiring to pursue medical research.
Dr. Naser Ammash , Chief Executive Officer of SSMC, said: “As part of SSMC’s three shield vision with clinical practice, research and education being interlocked disciplines working together to find definitive solutions to unmet patients’ needs, research is a fundamental pillar that brings new discoveries to the bedside and adds value to the local health care ecosystem. The groundbreaking launch of our Clinical Trials Unit illustrates our commitment to investing in research and finding new and innovative ways to treat our patients and respond to the region’s most concerning health challenges today and in the future. This would not be possible without our partnership with Abu Dhabi’s Department of Health and Mayo Clinic.”
Dr. Shahrukh Hashmi , Acting Medical Director of Research at SSMC, said: “Research is a distinguishing element for Mayo Clinic, and here at SSMC, we share that commitment to solving complex research questions for the benefit of our patients. SSMC’s Clinical Trials Unit is a foundational component of what we’re building to become a leading medical research institute in the region performing unparalleled translational research at the interface of medicine, science, and technology. We are extremely proud to have the specialized infrastructure for complex trials and research to address key healthcare areas right here at the hospital. At this moment in time, we have over 55 industry and investigator-initiated trials and over 30 registries that are ongoing or in development.”
Dr. Asma Al Mannaei, Executive Director of the Research and Innovation Center at the Department of Health – Abu Dhabi (DoH), said: “Inspired by the UAE’s visionary leadership, Abu Dhabi is primed to be a major clinical trial capital and our healthcare ambitions are limitless. We are proud to witness leading healthcare bodies such as Sheikh Shakhbout Medical City excel in the field of research and clinical trials, further solidifying the Emirate’s position as a leading destination for life sciences. Currently, there are around 400 clinical research trials under way in Abu Dhabi, and we are confident the newly inaugurated unit will further elevate efforts and accelerate findings and innovative solutions.”
With Abu Dhabi’s visionary leadership fully supporting SSMC’s Clinical Trials Unit to conduct innovative medical research, SSMC is one step closer to meeting patients’ complex care needs. SSMC will continue leveraging SEHA’s long-standing legacy, understanding of local demands, and Mayo Clinic’s expertise and extensive medical experience.
The launch of SSMC’s Clinical Trials Unit is part of a multi-phase approach, which sets the center on a 10-year growth course. In the first phase, the Unit’s core infrastructure and initial operations will be established within the first two years of operations. Phase 2 will see the creation of a dedicated physical space, and finally in a third phase, the Unit will reach its mature innovation development capabilities, including business development and entrepreneurship.

April 25, 2024
Sheikh shakhbout medical city team successfully removes 30.5kg ovarian tumor.

April 23, 2024
Uae president recognizes ssmc doctor among honorees at abu dhabi awards 2024.

March 6, 2024
Transformation expo – 2024.
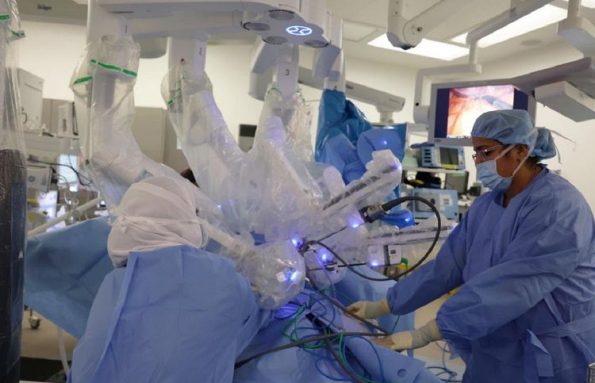
Feb. 8, 2024
Robotic surgery in the uae provides pain relief and improves mobility for patients.
- Request an appointment
Care of the Critically Ill Surgical Patient (CCrISP) is a two-day, interactive course, which includes mandatory, pre-course e-learning. It covers the practical skills and knowledge that health care professionals need to effectively care for surgical patients, including those who are deteriorating or at risk of doing so.

- Environment
- Road to Net Zero
- Art & Design
- Film & TV
- Music & On-stage
- Pop Culture
- Fashion & Beauty
- Home & Garden
- Things to do
- Combat Sports
- Horse Racing
- Beyond the Headlines
- Trending Middle East
- Business Extra
- Culture Bites
- Year of Elections
- Pocketful of Dirhams
- Books of My Life
- Iraq: 20 Years On
Abu Dhabi launches grant programme to promote research and innovation in healthcare
Funding will support clinical research projects that have the potential to fight rare diseases, cancer, alzheimer's and other ailments.
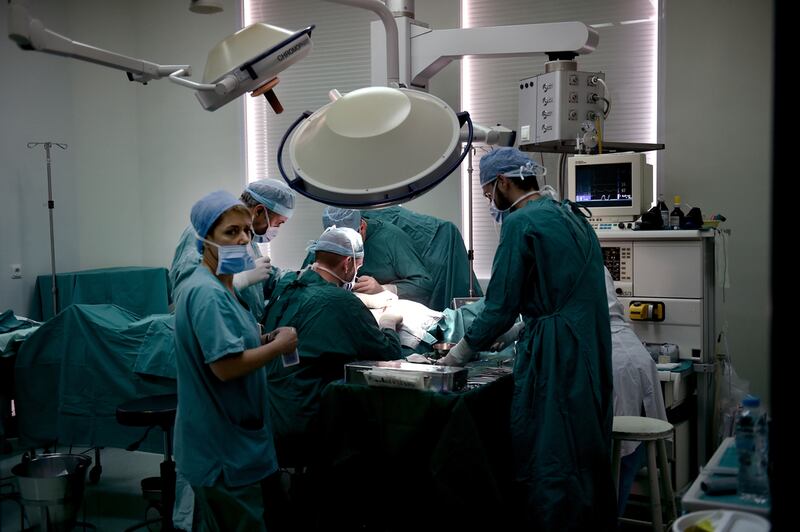
The Department of Health has teamed up with the emirate's healthcare sector, government and non-government entities in the UAE to offer a grant that will support clinical research. AFP

Abu Dhabi has launched a new programme of funding aimed at supporting research and innovation to fight life-threatening diseases.
The emirate's Department of Health said the grant will be offered to those who carry out clinical research projects in the areas of cardio-metabolic and vascular disorders, cancer , Alzheimer's disease , rare diseases, emerging infectious disease and antimicrobial resistance .
It is also open to people who have innovative technological ideas and solutions focusing on smart hospitals and the prevention and management of chronic diseases, a news release said.
The winners will also receive guidance to ensure project continuity and sustainability.
Applicants can submit their entries on www.doh.gov.ae but they should partner with a healthcare facility in Abu Dhabi to finalise and submit their entries.
Applications will be accepted during the next six weeks but the grant amount has not been revealed.
A committee of experts from the Department of Health - Abu Dhabi (DoH) and Abu Dhabi Health Research & Technology Committee will evaluate and assess submissions. It will be headed by Dr Asma Al Mannaei, executive director of the research and innovation centre at the DoH.
"As part of the DoH’s efforts to [support] the capital’s position as a leading destination for life sciences and innovation, we are committed to enabling and empowering innovators and researchers within our community to take their passion projects to the next stage," she said.
"With Abu Dhabi providing the right environment for individuals and companies to thrive, we are thrilled to put forward this grant that will expand clinical research capabilities in the emirate as well as conceptualise unique projects in the capital.
“We look forward to receiving and reading through all entries as well as providing guidance to all applicants. This is an exciting milestone for the healthcare sector that will spread a fine share of healthy competition among science and technology fanatics.”
To offer the grant, DoH has partnered with the emirate's healthcare sector, government and non-government entities in the UAE.
Abu Dhabi aims to become a destination of choice for clinical trials and collaborative healthcare research.
The emirate has ambitions to capture the world's first population-wide genetic library, pioneer connected health and build on previous collaborations with pharmaceutical giants Pfizer and AstraZeneca in medical research.
Last year, G42 Healthcare launched Insights Research Organisation and Solutions, a first-of-its-kind contract research organisation in the UAE to support internationally leading standards of scientific and ethical research, conduct clinical trials and report and develop treatments.
The company was started following the success of the country’s Phase III clinical trials for the development and testing of a Covid-19 vaccine, where 130 nationalities participated.
In March, the capital partnered with Pfizer to train and support 150 Abu Dhabi scientists to conduct clinical research at the level required by multinational companies to produce effective medicines.
Abu Dhabi charts a futuristic path in healthcare - in pictures
Emiratis in the UAE have been urged to voluntarily give an anonymous blood sample to help expand the data collection for the Emirati Genome Project. All photos by Khushnum Bhandari / The National

- Our Leadership
- Terms & Conditions
- Privacy Policy
- Super App Privacy Policy
The first UAE-based Contract Research Organization (CRO) specializing in healthcare research.
IROS is the UAE’s first registered and home-grown contract research organization. IROS was established to promote clinical trials and research with pharma stakeholders to improve patient outcomes, in line with the Abu Dhabi Economic Vision 2030.

Service Line Offerings
- Research Organisation Services
- Project Management
- Medical Writing, Statistics, and Analytics
- Regulatory Affairs
- Bio-statistics
- Data Management
- Insights Services
- Real-World Evidence (RWE) Studies
- Observation Studies
- Patient Engagement Studies
- Completed the worlds 1st and largest Phase III covid-19 vaccine trial.
- 35 projects completed to date (mix of phase II, III and IV trials).
- Undergone and successfully passed vendor audits conducted by US sponsors.
- Successfully registered and qualified as a vendor (to undertake clinical trials) with multi national pharmaceutical companies.
Strong Therapy experience in the following areas:
- Immunology (including Vaccines)
- Cardiovascular

Other Assets

Your destination for world-class complex care. Ranked UAE and GCC’s number one hospital in Newsweek’s 2023 ‘World’s Best Hospitals’ list.

World-class specialty women and children’s hospital in the Emirate of Abu Dhabi.

World’s 3rd Largest Dialysis Provider.

The region’s leading fertility network, with highly specialized and experienced Reproductive Health and IVF experts providing tailored treatment plans i...

Provider of premium specialized outpatient care in the United Arab Emirates and Saudi Arabia.

Multi-specialty hospital combining international standards and the latest innovations with a boutique hospitality experience.

Global leader in diabetes treatment, education, and research.

World-leading

Specialty Clinic & Day Surgery Center.
Committed to bringing world-class diagnostic testing to the UAE and the region.

Leading provider of long-term care and rehabilitation in the Middle East. Pioneer in post-acute care services in the GCC region.

Health screening provider in Abu Dhabi.

Committed to excellence in clinical genetic testing. The state-of-the-art laboratory specializes in advanced disease predisposition diagnostics and prov...
Leading AI-powered healthcare technology company.

Strategic initiative of the Department of Health – Abu Dhabi

- Book appointment
- Hospitals & Clinics
- Specialities
- Patient Portal
- Important notice
Mediclinic International is aware of a fake social media post that is being circulated requesting users to engage with a Medical Feedback Reward Survey. Mediclinic has not circulated any such survey. The social media post has not been published by Mediclinic. Mediclinic advises that people do not engage with the survey or respond. We release all our official communications on our website and our official social media channels .

- Mediclinic Middle East
- Stay Services & Hospitals - Mediclinic Middle East
- Clinics & Hospitals in Dubai & Abu Dhabi - Mediclinic
- Health Knowledge
- Services and Specialities
- Mediclinic Health Packages
- Careers at Mediclinic Middle East
- About Mediclinic Middle East
- Media and News
- Mediclinic Middle East - Referring Doctors
- Mediclinic Middle East - Careers at Mediclinic
- Mediclinic Baby - Prenatal, Maternity & Antenatal Care
- Privacy Statement
- Terms of Use
- Mediclinic Emergency
- Mediclinic Middle East - Health Insurance Partners
- What you should do in an emergency
Clinical Research in The Middle East - Mediclinic
- Mediclinic Middle East Special Offers
- Pharmacy Home Delivery
- Mediclinic Kids
- Mediclinic Middle East - Book an Appointment with Our Doctors
- Ramadan Kareem رمضان كريم
- Mediclinic Precise - Genetic Testing & Profiling
- Mediclinic at Home
- Bourn Hall Fertility Centre
- Ayadi Homecare, أيادي للرعاية الصحية المنزلية
- Quality healthcare services in the comfort of your home
- Patient Privacy Notice
- Health Experience Hub
- Client Experience Hub
- Mediclinic Hospitals and Clinics Ramadan timings - مواعيد عمل مستشفيات وعيادات ميديكلينيك خلال شهر رمضان المبارك
- Mediclinic Middle East International Nursing Conference
- Genetic Health
Mediclinic Middle East is one of the very few private healthcare organisations in the UAE to actively support its clinician’s research endeavours through medical research centres in the UAE. The number of facilities at Mediclinic Middle East provides access to a wide variety of patients and therapeutic areas. MCME has established a Research office to engage research opportunities that can substantially impact patients’ wellbeing.
The Corporate Research Office is dedicated to the following:
- Ensure that all the research projects carried out at MCME are approved by the internal Research and Ethics Committee as well as the local Regulatory Authorities prior to initiation
- Contact point for Clinical Research Organisations and sponsors
- Provide support to the MCME research teams
- Monitor and evaluate ongoing research
- Manage clinical research project budgets
- Provide Good Clinical Practice (GCP) training to MCME Research teams
- Ensure patient safety throughout all clinical trials
MCME Research Brochure
PDF | 1.18 MB
Research Studies

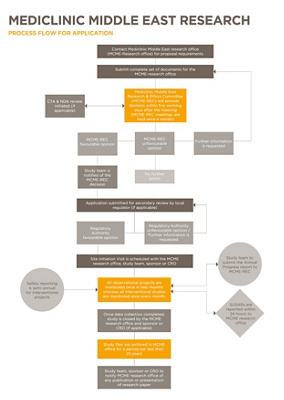
Workflow Process
Mediclinic Middle East Research Office follows specific guidelines to ensure that clinical research is carried out ethically and adds real value to the scientific community.
Our Publications
Dr. Zaka Ullah Khan Senior Corporate Medical Director, Clinical Management Mediclinic Middle East
Dr Rakshinda Mujeeb Research Projects Manager Rakshinda.Mujeeb@mediclinic.ae
Ms. Zineb Katache Research Assistant Zineb.katache@mediclinic.ae
Mediclinic Middle East Research Office Mediclinic Corporate Office, Dubai Production City, Publishing Pavillion, Level 6, PO Box 123812, Dubai UAE. Contact Email: MCME-ResearchOffice@mediclinic.ae

For Individuals
- Rehabilitation
- Specialized Treatment Programs
For Corporates
- Employees Assistance Program (EAP)
- Smoking Cessation Program
- Stress Debriefing & Critical Incident Stress Management
- Training & Awareness Programs
For Health Professionals
- Continuous Medical Education
- Patient Referral
- Our Clinicians
- Health Education Center
Clinical Research
- Latest ACPN Media Mentions
- Patient Experience
الخط الساخن: 800 ACPN[2276]

- Dr. Taoufik Alsaadi (ACPN, Chair)
- Prof. George Tadros (ACPN, Vice-Chair)
- Mr. Ahmed Mostafa (ACPN)
- Ms. Rana Abounakad (ACPN)
- Dr. Saqib Latif (ACPN)
- Dr. Tarek Shahrour (Sheikh Khalifa Medical City)
- Dr. Ahsan Khandoker (Khalifa University)
- Dr. Khaled Kadry (Maudsley Health Abu Dhabi)
- Dr. Lamya Turkawi (Sheikh Khalifa Medical City)
- Ms. Hajir Elbarrawy (ACPN)
- Ms. Reem Suliman (ACPN)
3. Research Collaborations:
a. Academic and Scientific Research Institutions:
Khalifa University, Abu Dhabi
Zayed University, Abu Dhabi
New York University Abu Dhabi
Harvard Medical School Center for Global Health Delivery – Dubai
Imperial College London Diabetes Centre (ICLDC) – Abu Dhabi
b. Current Pharmaceutical supporters
Biogen, Inc®
4. Guidelines for Proposed Research Studies:
No restrictions on the type of research that can be conducted as long as:
- Goal is justified and implications are identified
- Ethical considerations are met and conduct complies with guidelines of ICH – GCP and DOH Standard Operating Procedures (SOPs)
- Principal investigator meets requirements and has expertise in the study field
Note on clinical trials:
- Products/drugs must be registered with:
- The Ministry of Health Drug Control Department and DOH Medical Products Regulation Section
- Application fees apply
Note: investigational medicinal product is one that has been given marketing authorization but is being investigated for uses other than what is indicated. Does not apply to Phase 1 trials.
5. Criteria for Research:
Proposed studies must meet the following criteria: Benefit: Does the proposed study have clear benefits to the community in which it is conducted? Effectiveness: Will the study be effective in achieving the desired goal? Necessity: Is the study necessary to achieve the goal or is there an alternative that won’t infringe on a competing value? Proportionality: Is the desired goal important enough to justify overriding another principle or value? Least infringement: Is the study designed to minimize infringement on the values that conflict with it? Transparency: Is the Primary Investigator prepared to publicly justify their decision?
6. Ethical Considerations:
Ethics regarding human subjects: Respect for persons: respect autonomous choices; protect those lacking autonomy Obtain informed consent from those capable of giving consent, proxy consent Beneficence: do not harm; maximize benefits, minimize harms Risk/benefit assessment is conducted, clear action if adverse effects arise Justice: fairness in distribution; treat equals equally Recruitment of subjects is fair, vulnerable populations not simply targeted out of convenience
7. Research Ethics Application: What to include
- ACPN ethics review application form ( download form )
- Detailed study protocol ( download form )
- Questionnaires and description of drugs/products that will be used (Arabic and English)
- Subject consent form (Arabic and English)
- Subject information sheet (Arabic and English)
- CVs of all investigators
- Investigators’ certification of Research Ethics Training for Protecting Human Research Participants
8. Application Submission Procedures:
- All ethics proposals are submitted to the IRB coordinator ( [email protected] ) , who reviews the application for completeness and confirms to investigators within five days of its receipt
- The IRB is required to give an ethical opinion on an application within 60 calendar days of the receipt of a valid application. Where the IRB considers that further information is required in order to give an opinion, the IRB may make one request in writing for further information from the applicant. The period of 60 days will be suspended pending receipt of this information
- In the case of a clinical trial involving a medicinal product for somatic cell therapy, the normal statutory time limit for review is extended to 90 days. This may be extended by a further 90 days (i.e. to 180 days in total) where the REC needs to consult a specialist group or Committee about the application
9. Ongoing Clinical Research Studies:
- Observational study to collect information on the safety and drug utilization of Fampyra® – Dr. Taoufik Alsaadi and colleagues
- A Pilot study on the prevalence of depression and anxiety among neurology patients attending ACPN – Dr. Ahmed Mohamed and colleagues
- Narcolepsy in the UAE: A Case series – Dr. Khaldoun Mozahem and colleagues
- Real world retrospective study of effectiveness and safety of Gilenya (Fingolimod®) in relapsing remitting Multiple Sclerosis in the Middle East and North Africa (FINOMENA) – Dr. Taoufik Alsaadi
- Repetitive Transcranial Magnetic Stimulation (rTMS) in the treatment of major depressive disorders: results from the UAE – Ms. Seada Kassie and Dr. Taoufik Alsaadi
- A comparison study of the use of Haptic Handwriting Tool among children with learning difficulties in the UAE – Dr. Samra Tahir and Dr. Mohamad Eid
- Can combined endurance and resistance training slow disease progression in adults with relapsing remitting multiple sclerosis? A pilot study – Ms. Helen Hughes and colleagues
- A cohort study on treatment outcome of anorexia nervosa in the UAE – Ms Carine El Khazen and colleagues
- Investigating the role of sleep-disordered breathing and its treatment on depressive symptoms in the UAE – Dr. Khaldoun Mozahem and colleagues
- Antiepileptic drugs use and major congenital malformation rate: A prospective observational cohort study: The United Arab Emirates – Dr. Taoufik Alsaadi and colleagues
- Patient cost-sharing for ambulatory neuropsychiatric services in Abu Dhabi, UAE – Dr. Yousef Abouallaban and colleagues
- Potential factors impacting health-related quality of life among patients with epilepsy: Results from the United Arab Emirates – Dr. Taoufik Alsaadi and colleagues
- Depression and anxiety as determinants of quality of life in patients with multiple sclerosis: The United Arab Emirates – Dr. Taoufik Alsaadi and colleagues
- Suicidal ideation Is associated with altered variability of fingertip photo-plethysmogram signal in depressed patients – Dr. Yousef Abouallaban and colleagues
- The rapidly changing landscape of multiple sclerosis immunomodulatory therapy a retrospective chart review in The United Arab Emirates – Dr. Taoufik Alsaadi and colleagues
- Impact of working memory and cognitive functioning on specific language impairment (SLI) – Dr. Samra Tahir and colleagues
- Working memory in children with intellectual disability (ID) – Dr. Samra Tahir and colleagues
For more information, please send an email to [email protected] or call +(971-2)-492-6000.
Board Members

Dr. Taoufik Alsaadi

Prof. George Tadros

ACPN offers quality mental health services with utmost care
- Webmail (Office 365)
- Banner Student Registration
- ADERP – [Off Campus]
- ADERP – [On Campus]
- MFA Self-Service
- Future Students
- Prospective Bachelors
- Prospective Masters & PhD
- Equipment Directory
- Business Development
- Procurement & Contracts
- Undergraduate
- Pre-medicine Bridge
- Doctor Of Medicine (M.D.)

- Research News
- In the Media
- Publications

Two Day Khalifa University SEHA Workshop Launches 2024 Clinical Research Certificate Program
Over 150 SEHA Medical Residents, and Fellows Enrolled in a Clinical Research Training Program, Furthering the Region’s Research Capacity
A two-day workshop marking the inauguration of the Clinical Research Certificate (KU-SEHA CRC) training program, a Khalifa University collaboration with the Abu Dhabi Health Services Company (SEHA) and the Department of Health – Abu Dhabi, was launched at the Main Campus. Over 150 SEHA medical residents and fellows were enrolled in this clinical research training program, furthering the region’s research capacity
The launch ceremony was attended by Professor Sir John O’Reilly, President, Khalifa University, Dr. Ghanem Ali Al Hassani, Group Education Director and Research Director, SEHA. The inaugural event was also attended by Dr. Habiba Al Safar, Dean, College of Medicine and Health Sciences (CMHS), Director of Biotechnology Center, and Professor, Biomedical Engineering and Biotechnology, Dr. Yousof Al Hammadi, Senior Vice-President, Academic and Student Services, Assistant Vice-President, Graduate Studies and Associate Professor Electrical Engineering, and Dr. Waleed Alameri, Assistant Vice-President, Student Affairs and Outreach as well as faculty members and officials from SEHA and DoH – Abu Dhabi
Led by KU-SEHA CRC program director Dr. Abderrahim Oulhaj, Associate Professor, Public Health and Epidemiology, the inaugural workshop addressed the need for research and development (R&D) training in SEHA and the Department of Health (DoH) – Abu Dhabi, while equipping individuals with the necessary skills and knowledge to adapt to the evolving healthcare landscape.
Dr. Habiba Alsafar said: “We are delighted to host this workshop and provide the knowledge and necessary skills to conduct high-quality clinical research studies in the region. The KU-SEHA CRC program stands as a testament to the unwavering commitment of Khalifa University, SEHA, and the Department of Health – Abu Dhabi towards advancing healthcare and research excellence in the UAE. Aligned with the country’s goal to enhance healthcare research capabilities, the program, now in its second year, represents Khalifa University’s consistent collaboration with national and international organizations, marking a significant stride towards the advancement of clinical research and medical education.”
Dr. Oulhaj also outlined the program’s objectives and learning outcomes, the structure of the program, the mode of delivery, placing a strong emphasis on supervision and mentoring to ensure that the course offered personalized guidance throughout the program. Other topics focused on identifying the target population, conducting power and sample size calculations, and creating a statistical analysis plan. Participants learned how to address potential biases in clinical research, including different types of bias and confounding variables, and strategies to mitigate them.
Additionally, the workshop included ways to select the appropriate study design, performing literature search and addressing ethical considerations. Ethics of human experimentation, research integrity, and authorship ethics as well as Good Clinical Practice (GCP) guidelines and the process of submitting research proposals to the SEHA Institutional Review Board (IRB) were also discussed.
Alisha Roy Science Writer 21 February 2024
By Alisha Roy Science Writer
Related Articles

Explore more

- Latest News
- Year of Sustainability
- Abu Dhabi in a Week
- Arts & Culture
- Crown Prince
- Environment
- Government Affairs
- Infrastructure
- Initiatives
Abu Dhabi Media Office - Copyright 2024
Popular search terms
- Mohamed bin Zayed
- Khaled bin Mohamed bin Zayed
- Department of Health – Abu Dhabi and Pfizer collaborate to boost clinical trials capabilities in Abu Dhabi
23 December 2021


DoH and Pfizer will develop specialised training programmes for early-stage clinical trials and drug development programmes The collaboration seeks to train up to 150 qualified clinical researchers within two years including research mentorship opportunities with Pfizer research partners, aiming to result in up to 500 specialized and qualified clinical researchers by 2025 The collaboration contributes to positioning the emirate as an incubator for innovation in life sciences and as a leading health destination in the world
Share this article
- Link Copied
The Department of Health – Abu Dhabi (DoH) , the regulator of the healthcare sector in the emirate, announced a new collaboration with Pfizer, one of the world’s premier biopharmaceutical companies, with the aim to develop national competencies and clinical research capacity in Abu Dhabi. The collaboration reflects the emirate’s vision related to the health and safety of the local community and reinforces Abu Dhabi’s position as a leading research and innovation hub in life science in the region.
In the presence of H.E. Abdulla bin Mohamed Al Hamed, Chairman of DoH and Sean Murphy, Charge d’Affaires at the US Embassy, a Declaration of Collaboration between the two entities was signed by H.E. Dr. Jamal Mohammed Al Kaabi, Undersecretary of DoH and Lindsey Dietschi, Gulf Cluster Lead.
The mutual agreement will focus on developing clinical trials conduct and enhancing research efforts and medical processes in the emirate. Pfizer will support DoH in establishing a training curriculum that’s specialised in advancing the early phase of clinical trials and drug development programmes. The collaboration reflects the emirate's continuous efforts to upgrade the digital health system and foster innovation in healthcare.
Through this collaboration, Pfizer seeks to train up to 150 clinical researchers who will also have an opportunity of research mentorship support by Pfizer research partner within two years supporting DoH aim of reaching 500 qualified clinical researchers by 2025 through various collaborations and initiatives.
H.E. Dr. Jamal Mohammed Al Kaabi, Undersecretary of DoH, said: “Under our wise leadership, DoH is committed to positioning Abu Dhabi as an incubator for innovation in the field of life sciences and as a leading healthcare destination in the world. We do this by providing a legislative environment that attracts and motivates research and innovation in healthcare, enhancing the means of cooperation that join Abu Dhabi with global partners, and continuing to roll out promising clinical trials and research projects.”
Al Kaabi added: “We are excited for our collaboration with Pfizer which focus on conducting safe and efficient early-stage clinical trials based on international guidelines and global best practices. The partnership contributes to developing our expertise and advancing our capabilities in the UAE which positively impact the health and well-being of individuals worldwide.”
Experts will conduct the training courses from Pfizer, academic institutions and other specialised partners. Participants will receive training in technologies and artificial intelligence tools that accelerate the drug discovery process.
Lindsey Dietschi, Gulf Cluster Lead, said: “Abu Dhabi established a robust and sustainable world-class healthcare system that fosters innovation in research and development of processes. We believe in the vision of Abu Dhabi that aims to deliver more medical breakthroughs and ensure healthier communities. We are delighted to have achieved this partnership with the Department of Health – Abu Dhabi in effort to drive innovative research and development in the emirate. We look forward to working together with our partners to help equip local researchers with the skills required to conduct early-stage clinical trials.”
For over 40 years, Pfizer has been working as an innovation leader across the Gulf in partnership with healthcare professionals, communities and governments, to change the lives of millions of people.

Department of Health – Abu Dhabi partners with Eli Lilly and World Obesity Federation

Department of Health – Abu Dhabi elevating maternity services in emirate

Department of Health – Abu Dhabi honours healthcare facilities for exceeding Emiratisation targets
Abu Dhabi to host 6th World Health Organization Emergency Medical Teams Global Meeting
Related stories.
-0-323-0-404-crop-aspect-NORTH.jpg?k=8816ebaec1)
Abu Dhabi Emergency, Crises and Disaster Management Team holds meeting to assess readiness of local entities to respond to adverse weather conditions

Abu Dhabi Police, in collaboration with local entities, reaffirms readiness to respond to expected weather conditions in the emirate

DRIFTx underway at Yas Marina Circuit in Abu Dhabi

Daman transfers Abu Dhabi Basic Plan renewal services for domestic workers to TAMM platform
- Terms & Conditions
- Privacy Policy

International Conference on Advancements in Autism Research concludes in Abu Dhabi
Abu Dhabi [ UAE ], April 30 (ANI/WAM): The International Conference on Advancements in Autism Research , themed "Challenges and Solutions," called upon experts, scientists, specialists, practitioners, parents, and early coordination between universities and relevant scientific centres and stressed the importance of enhancing scientific research related to biomarkers for autism spectrum disorder (ASD) at the highest international level to achieve accreditation for research results.
Additionally, it highlighted the importance of academic communication among all relevant faculties to document and exchange experiences and information on the latest research developments related to autism spectrum disorder.
After the conference, participants, including experts and specialists in behavioural modification, biomedical sciences, and related fields, along with representatives from more than 20 countries worldwide, extended their deepest gratitude to Sheikh Khalid bin Zayed Al Nahyan, Chairman of the Board of Trustees of Zayed Higher Organisation for People of Determination (ZHO), for sponsoring the conference for the second consecutive year.
They praised the care and rehabilitation provided to people of determination in the UAE and the programmes offered by the ZHO for these groups through its centres in Abu Dhabi .
The conference, in its twelfth edition, was held under the generous patronage of Sheikh Khalid, organised by the ZHO in collaboration and coordination with ADNOC, Abu Dhabi Health Services Company (SEHA), and Lotus Holistic Abu Dhabi at the Abu Dhabi National Exhibition Centre (ADNEC) in Abu Dhabi .
Abdullah Abdulali Al-Hamidan, the Secretary-General of ZHO, noted that the conference was distinguished by its outcomes and recommendations, which will be implemented in the next phase through the participation of stakeholders who will develop an action plan for their execution.
Speaking on behalf of Al-Hamidan at the closing session, Nafea Al-Hammadi, Executive Director of the Support Services Sector at the ZHO, highlighted the intensive efforts made during the conference days to present and discuss the latest developments in autism research.
This included about 20 sessions, 41 specialised workshops, 91 lectures, and several dialogues with parents and decision-makers, involving over 100 speakers from more than 20 countries specialising in behavioural modification, biomedical sciences, and related fields.
He emphasised that these efforts will greatly enhance awareness and knowledge among all attendees and participants, undoubtedly contributing to improving the level of services provided to individuals with autism spectrum disorder, their families, and caregivers.
The conference called for a survey to enumerate cases of autism at the national level, disclosing multiple cases for utilisation in studies and research. It advocated for the increased use of artificial intelligence in educating children with autism as a means of diagnosis and education.
Moreover, it urged for intensified awareness programmes for all segments of society, identifying early warning signs for newborns to acquaint them with early symptoms of autism spectrum disorder (ASD).
The conference emphasised the necessity of working to protect children with autism and guiding students on how to interact with this group.
It advocated for activating educational institutions to care for integrating children with autism into mainstream schools, providing supportive personnel for educational integration, especially the accompanying teachers, and encouraging educational programmes across various disciplines to work with autistic children.
Furthermore, the conference called for health units to apply preliminary survey forms to identify the most severe cases of autism spectrum disorder and refer them for diagnosis and early intervention services.
It advocated for the formation of representative research groups comprising various specialists willing to collaborate from different countries in the field of autism research and forming teams with integrated specialities. (ANI/WAM)
The conference also recommended the establishment of a laboratory for conducting medical analyses based on scientifically endorsed and published biological indicators in high-impact scientific journals. It stressed the importance of a balanced healthy diet during pregnancy and early years and avoiding caesarean section except in necessary cases.
Participants in the conference called for providing suitable employment opportunities for autistic students to demonstrate their competence according to labour market requirements, promoting talents and hobbies, and showcasing the works of outstanding individuals in various scientific, educational, sports, and artistic fields.
Additionally, they advocated for conducting workshops and lectures for professionals in the autism field on parents' mental health and positive interaction with children, empowering understanding of their needs.
Heba Hegres, the Special Rapporteur on the Rights of Persons with Disabilities at the United Nations, expressed her sincere thanks and appreciation to the UAE . She thanked the ZHO for sponsoring, sustaining, and organising the very important conference. She congratulated the organisation on the decision to prepare and publish the first peer-reviewed and specialised scientific journal on autism, adhering to global scientific and academic standards.
In a press statement, she emphasised the importance of the conference topics regarding the crucial and central role of the family in the lives of individuals with autism and people with disabilities in general, to provide them with support and advocacy without disrupting their independent living. In this context, she expressed her strong belief in this role and her determination to conduct an independent study on this topic. (ANI/WAM)

- Professional
- Healthcare and Medical
- Clinical Research
TOP 10 CLINICAL RESEARCH COURSES IN DUBAI, ABU DHABI, SHARJAH - UAE
Are you looking for Clinical Research courses in the UAE?
Here is a table of Clinical Research classes in the UAE with the duration and cost of the course:
The average course fee of Clinical Research courses in Dubai is 3000 AED for a 1-month Research Training course.
- Online Clinical Research Courses

About Coursetakers.ae
- Courses in USA
- Courses in UK
- Courses in India
- Courses in South Africa
Quick Links
- Upload Your Courses
- How It Works
- Corporate Training
- Delivery and Refund Policy
© 2024 www.coursetakers.ae All Rights Reserved. Terms and Conditions of use | Privacy Policy
Get in touch with all the institutes
- Email Us

- Channels ►
- Outpatient Spine
- Featured Insights
- Specialties ►
- Orthopedics
- Surgery Centers
- Dental / DSO
- Spinal Tech
- Spine Leaders
- Practice Management
- Becker's Healthcare Websites ►
- Dental + DSO
- Behavioral Health
- Physician Leadership
- Newsletters ►
- Spine Review
- Orthopedic Review
- Events ►
- Upcoming Conferences and Events
- 21st Annual Spine, Orthopedic and Pain Management-Driven ASC + The Future of Spine Conference
- Spring Future of Dentistry Roundtable
- 9th Annual Health IT + Digital Health + RCM Meeting: The Future of Business and Clinical Technologies
- Becker's ASC 30th Annual Meeting: The Business and Operations of ASCs
- Fall Future of Dentistry Roundtable
- Fall Payer Issues Roundtable
- 12th Annual CEO + CFO Roundtable
- 15th Annual Meeting
- Call for Speakers
- Exhibiting & Sponsoring
- Virtual Events ►
- Upcoming Virtual Events
- CMO + CNO Virtual Event
- Transform Hospital Operations Virtual Summit
- Infusion Center Operations Virtual Summit
- CEO + CFO Virtual Forum
- Revenue Cycle Management Virtual Event
- Oncology Virtual Forum
- AI + Digital Health Virtual Event
- Digital Innovation + Patient Experience and Marketing Virtual Event
- Dental + DSO Virtual Event
- Human Resources + Talent Virtual Event
- 9th Annual Health IT + Digital Health + RCM Virtual Event
- 12th Annual CEO + CFO Roundtable Virtual Event
- Oncology Virtual Summit
- Past Virtual Events
- Webinars ►
- Upcoming Webinars
- Past Webinars
- Partner Content ►
- Current Partner Content
- Print ►
- Current Issue
- Past issues
- Multimedia ►
- Featured Content
- Career Center
- About Us ►
- Careers at Becker's
- Request Media Kit
- Most Read ►
- Midwest Orthopaedics at Rush adds endoscopic spine surgery
- What spine surgeons expect for the future of value-based care
- Streamlining Acquisition Processes for Surgical Navigation Technology: Introducing the CBYON Eclipse System from MSSI
- NASCAR driver treated at UAB University Hospital following spinal injury
- New spine codes to further clarify back pain
- Federal judge dismisses lawsuit alleging Ohio health system's orthopedic monopoly
- The future of sacroiliac joint fusion
- Spine surgeons on alert for these red flags when joining new groups
- 2 CMS spine proposals to know
- Meet the leaders of the 11 best hospitals in California for spine surgery
- Top 40 Articles ►
- Judge cuts 'shockingly excessive' jury award; new trial sought
- 2 recent orthopedic surgeon suspensions
- Ozempic to have 'bearish' impact on orthopedic, spine practices
- Ozempic's impact on total hip replacements
- How one orthopedic surgeon became one of the richest men in the world
- The orthopedic providers going out of network with Medicare Advantage plans
- Massachusetts orthopedic surgeon convicted of healthcare fraud
- How this orthopedic surgeon predicts Ozempic will disrupt the industry
- Orthopedic surgeon convicted of abuse loses medical license
- Medicare Advantage is getting 'much, much worse' for spine
- Will Ozempic alter spine, orthopedic fields?
- Vanderbilt orthopedic surgeon faces additional misconduct allegations
- Average cost of the 10 most common orthopedic surgeries
- Ozempic could be good for orthopedic surgeons, but experts warn to proceed with caution
- Missouri jury sides with orthopedic surgeon in malpractice suit
- Judge slashes 'shockingly excessive' orthopedic malpractice verdict by $100M
- Texas pain physicians charged in $12M fraud scheme
- Bad news for orthopedics
- 10 worst states for physicians
- 2 Wisconsin hospitals close despite orthopedic group's pushback
- 5 surgeons on the cutting edge of orthopedics
- Bayhealth orthopedic surgeon dies at 49
- NFL linebacker retires at 26 after spine injury
- Medtronic to close 5 manufacturing plants
- Orthopedic surgeon awarded $15M in anti-male bias suit
- WWE star returns to action following spinal fusion
- Spine patient awarded $6.2M in botched surgery case
- 4 WWE stars undergoing spine, orthopedic surgery in 2023
- Louisiana Orthopaedic Specialists founder dies
- How orthopedic surgeon salaries stack up
- New Jersey court vacates neurosurgeons' $24M verdict in hospital privileges dispute
- NFL player plans 2nd career as orthopedic surgeon
- Physician found guilty in spine imaging kickback scheme
- Orthopedic practice to pay $540K+ to resolve billing claims
- Hospital that fired orthopedic leader put on probation
- 2 Louisiana orthopedic practices to merge
- The orthopedic trend that could define 2024
- LA County severs ties with orthopedic surgeon facing misconduct allegations
- The costs behind Elon Musk's Neuralink clinical trials

Spine surgeons present at 1st Abu Dhabi 2024 International Brain and Spine Surgery Congress
Dr. Alok Sharan and Dr. Muhammad Abd-El-Barr, along with other surgeons from the US, were recently invited to speak at the 1st ever Abu Dhabi 2024 International Brain and Spine Surgery Congress (ADNeuro24). The conference was organized by Dr. Waseem Aziz, Chief of Neurosurgery at Sheikh Shakhbout Medical City.
Sheikh Shakhbout Medical City is one of the largest hospitals in UAE. Established in 2019 it has been at the forefront of neurosurgical and spine care in the region. The ADNeuro conference brought together surgeons from UAE, Saudi Arabia, Eqypt, China, Japan and the US. There were a variety of topics discussed ranging from updates in the treatment of spine surgery, advances in brain tumor surgery, along with presentations on stroke care. Dr. Sharan presented the results of his Rapid Recovery Spine Surgery protocol, based off the Awake Spinal Fusion procedure. Dr. Muhammad Abd-El-Barr presented information on his newer techniques of minimally invasive spine surgery, including the integration of MRI mapping techniques for surgery. The conference organizers are planning on hosting the next ADNeuro conference in 2025.
Copyright © 2024 Becker's Healthcare. All Rights Reserved. Privacy Policy . Cookie Policy . Linking and Reprinting Policy .
Featured Learning Opportunities
- ASC/Spine Whitepapers
- ASC/Spine Webinars
- Clinical Whitepapers
- Clinical Webinars
- Spine, Orthopedic and Pain Management-Driven ASC + The Future of Spine Conference
- Process Improvement Whitepapers
- Process Improvement Webinars
- Hospital Review Virtual Events
- ASC/Spine Virtual Events
Featured Webinars
Featured whitepapers, most read - spine.
Becker's Websites
Virtual Learning
- Whitepapers
Conferences
- 14th Annual Meeting
- Spring Payer Issues Roundtable
- 1.800.417.2035
- [email protected]
- Today's news
- Reviews and deals
- Climate change
- 2024 election
- Fall allergies
- Health news
- Mental health
- Sexual health
- Family health
- So mini ways
- Unapologetically
- Buying guides
Entertainment
- How to Watch
- My Portfolio
- Latest News
- Stock Market
- Premium News
- Biden Economy
- EV Deep Dive
- Stocks: Most Actives
- Stocks: Gainers
- Stocks: Losers
- Trending Tickers
- World Indices
- US Treasury Bonds
- Top Mutual Funds
- Highest Open Interest
- Highest Implied Volatility
- Stock Comparison
- Advanced Charts
- Currency Converter
- Basic Materials
- Communication Services
- Consumer Cyclical
- Consumer Defensive
- Financial Services
- Industrials
- Real Estate
- Mutual Funds
- Credit cards
- Balance Transfer Cards
- Cash-back Cards
- Rewards Cards
- Travel Cards
- Personal Loans
- Student Loans
- Car Insurance
- Morning Brief
- Market Domination
- Market Domination Overtime
- Opening Bid
- Stocks in Translation
- Lead This Way
- Good Buy or Goodbye?
- Fantasy football
- Pro Pick 'Em
- College Pick 'Em
- Fantasy baseball
- Fantasy hockey
- Fantasy basketball
- Download the app
- Daily fantasy
- Scores and schedules
- GameChannel
- World Baseball Classic
- Premier League
- CONCACAF League
- Champions League
- Motorsports
- Horse racing
- Newsletters
New on Yahoo
- Privacy Dashboard
Yahoo Finance
Napo pharmaceuticals, a jaguar health family company, sponsoring pediatric gastroenterology conference in abu dhabi and panel discussion about microvillus inclusion disease (mvid).
Jaguar's novel plant-based prescription drug crofelemer has been granted Orphan Drug Designation by the FDA and the European Medicines Agency for both MVID and short bowel syndrome (SBS) with intestinal failure
Proof-of-concept studies of crofelemer for MVID and SBS planned for 2024
SAN FRANCISCO, CA / ACCESSWIRE / April 29, 2024 / Jaguar Health, Inc. (Nasdaq:JAGX) today announced that Jaguar family company Napo Pharmaceuticals (Napo) is a Bronze Sponsor of the 10th Annual Elite Ped-GI Congress , which takes place May 2-4, 2024 in Abu Dhabi in the United Arab Emirates (UAE). Additionally, at the conference Napo is sponsoring a panel discussion titled "Masterclass in Congenital Diarrhea" which will focus on microvillus inclusion disease (MVID), an ultra-rare congenital diarrheal disorder. Jaguar's novel plant-based prescription drug crofelemer has been granted Orphan Drug Designation by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for both MVID and short bowel syndrome (SBS) with intestinal failure.
"As previously announced, Jaguar, with strong leadership and participation from Jaguar family companies Napo Pharmaceuticals and Napo Therapeutics, is supporting investigator-initiated proof-of-concept studies of crofelemer for the rare disease indications of MVID and SBS with intestinal failure in the US, EU and Middle East/North Africa (MENA) regions, with results expected in 2024. Additionally, in August 2023 the FDA activated Napo Pharmaceuticals' Investigational New Drug (IND) application for a novel formulation of crofelemer for the treatment of MVID, which allows us to initiate our planned phase 2 trial of crofelemer for treatment of this indication in pediatric MVID patients," said Lisa Conte, Jaguar's president and CEO. "In accordance with the guidelines of specific EU countries, published data from clinical investigations in such rare diseases could support early patient access to crofelemer for these debilitating conditions in those countries."
MVID is a severe infantile disease characterized by diarrhea, malabsorption, and acid/base instability, requiring intensive parenteral support for nutritional and fluid management, and there are currently no approved drug treatments.
Dr. Mohamad Miqdady, a member of Napo's Scientific Advisory Board (SAB), will serve as the moderator of the Napo-sponsored panel discussion. The session panelists include Pravin Chaturvedi, PhD, who is Jaguar's Chief Scientific Officer and Chair of the Napo SAB, as well as Dr. Christos Tzivinikos and Dr. Antonella Diamanti, who are leading experts in the treatment of MVID patients in the UAE and Italy.
Dr. Miqdady is the Division Chief of the Pediatric Gastroenterology, Hepatology & Nutrition Division at Sheikh Khalifa Medical City , a flagship tertiary hospital in the UAE and the largest teaching medical center in Abu Dhabi. He is also an Adjunct Professor at Khalifa University's medical school in Abu Dhabi, and completed his Fellowship in Pediatric Gastroenterology at Baylor College of Medicine and Texas Children's Hospital in Houston.
Dr. Christos Tzivinikos is a Consultant Pediatric Gastroenterologist and Founder of the Pediatric GI Department at Al Jalila Children's Specialty Hospital in Dubai, and an Adjunct Clinical Assistant Professor at Mohammed Bin Rashid University of Medicine and Health Sciences in Dubai.
Dr. Diamanti is Head of the Artificial Nutrition Unit at Bambino Gesù Children's Hospital in Rome, Italy. She has served as Medical Director of the hospital's Gastroenterology Department since 2001.
Dr. Tzivinikos and Dr. Diamanti will be clinical investigators in Napo's planned phase 2 MVID study, titled Evaluation of Safety, Tolerability and Efficacy of Crofelemer Following Multiple Ascending Doses of Crofelemer Powder for Oral Solution in Pediatric Participants with Microvillus Inclusion Disease (MVID) .
About the Elite Ped-GI Congress
This Elite Ped-GI Congress is designed to provide information about high level, clinically significant updates and comprehensive trends relevant to the practice of pediatric gastroenterological, nutrition and liver disorders. The annual event provides a venue for healthcare professionals to share their best practices, to network within groups of interest, and to create and strengthen clinical collaborations. Additional information about the Elite Ped-GI Congress can be viewed here on the event website.
About the Jaguar Health Family of Companies
Jaguar Health, Inc. (Jaguar) is a commercial stage pharmaceuticals company focused on developing novel proprietary prescription medicines sustainably derived from plants from rainforest areas for people and animals with gastrointestinal distress, specifically associated with overactive bowel, which includes symptoms such as chronic debilitating diarrhea, urgency, bowel incontinence, and cramping pain. Jaguar family company Napo Pharmaceuticals (Napo) focuses on developing innovative, patient-centric therapeutic solutions for essential supportive care and the management of neglected side effects across complicated disease states. Napo's goal is to redefine what is possible in supportive care, providing hope and improving outcomes for patients worldwide. Napo's crofelemer drug product candidate is the subject of the OnTarget study, a pivotal Phase 3 clinical trial for preventive treatment of chemotherapy-induced overactive bowel (CIOB) in adults with cancer on targeted therapy. Jaguar family company Napo Therapeutics is an Italian corporation Jaguar established in Milan, Italy in 2021 focused on expanding crofelemer access in Europe and specifically for orphan and/or rare diseases. Jaguar Animal Health is a Jaguar tradename. Magdalena Biosciences, a joint venture formed by Jaguar and Filament Health Corp. that emerged from Jaguar's Entheogen Therapeutics Initiative (ETI), is focused on developing novel prescription medicines derived from plants for mental health indications.
For more information about: Jaguar Health, visit https://jaguar.health Napo Pharmaceuticals, visit www.napopharma.com Napo Therapeutics, visit napotherapeutics.com Magdalena Biosciences, visit magdalenabiosciences.com Visit Jaguar on LinkedIn: https://www.linkedin.com/company/jaguar-health/ Visit Jaguar on X: https://twitter.com/Jaguar_Health Visit Jaguar on Instagram: https://www.instagram.com/jaguarhealthcommunity/
Forward-Looking Statements
Certain statements in this press release constitute "forward-looking statements." These include statements regarding Jaguar's expectation that results from investigator-initiated and IND proof-of-concept studies of crofelemer for MVID and SBS with intestinal failure will be available in 2024, the expectation that, in accordance with the guidelines of specific EU countries, published data from clinical investigations of crofelemer for MVID and SBS could support early patient access to crofelemer for these rare diseases in those countries, and the expectation that Jaguar will conduct a phase 2 clinical study of crofelemer for the treatment of MVID. In some cases, you can identify forward-looking statements by terms such as "may," "will," "should," "expect," "plan," "aim," "anticipate," "could," "intend," "target," "project," "contemplate," "believe," "estimate," "predict," "potential" or "continue" or the negative of these terms or other similar expressions. The forward-looking statements in this release are only predictions. Jaguar has based these forward-looking statements largely on its current expectations and projections about future events. These forward-looking statements speak only as of the date of this release and are subject to a number of risks, uncertainties and assumptions, some of which cannot be predicted or quantified and some of which are beyond Jaguar's control. Except as required by applicable law, Jaguar does not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
Source: Jaguar Health, Inc.
Jaguar-JAGX
SOURCE: Jaguar Health, Inc.
View the original press release on accesswire.com
Rating Action Commentary
Fitch Rates Abu Dhabi Developmental Holding Company's Inaugural GMTN Programme 'AA(EXP)'
Mon 29 Apr, 2024 - 3:32 AM ET
Fitch Ratings - Frankfurt am Main - 29 Apr 2024: Fitch Ratings has assigned Abu Dhabi Developmental Holding Company PJSC's (ADQ) upcoming global medium-term note (GMTN) programme as well as its upcoming USD3 billion dual tranche (five and 10 years) senior unsecured fixed-coupon notes under the programme expected senior unsecured long-term ratings of 'AA(EXP)'.
The assignment of final ratings is contingent on the receipt of documents confirming the information already received.
The GMTN programme allows for borrowings for various tenors and currencies in the form of senior unsecured, unsubordinated debt notes. The expected ratings of the GMTN programme and US dollar-denominated senior unsecured notes are equalised with ADQ's Long Term Issuer Default Rating (IDR).
Key Rating Drivers
The ratings on both the GMTN programme and US dollar-denominated senior unsecured notes are equalised with ADQ's Long Term IDR to reflect that the notes under the programme will represent a direct, unconditional, unsubordinated and unsecured obligation of ADQ and will rank equally with all of its present and other future unsecured and unsubordinated obligations.
The programme has standard clauses such as cross-acceleration, negative pledge, issuer's call option and a change-of-control put option for investors, as well as becoming immediately repayable if ADQ ceases to be more than 50% owned and controlled by Abu Dhabi or by any agency of the government or any entity controlled by the government. English Law is applicable to the programme and its notes.
The net proceeds will be used for general corporate purposes or for any other purpose specified in the applicable final terms.
Derivation Summary
The GMTN programme's and the US dollar-denominated senior unsecured notes' expected ratings are driven by and equalised with ADQ's IDRs since issuance under the programme will remain senior unsecured unsubordinated obligations of the issuer.
RATING SENSITIVITIES
Factors That Could, Individually or Collectively, Lead to Positive Rating Action/Upgrade:
An upgrade of ADQ's Long-Term IDR would be reflected in the GMTN programme's and US dollar-denominated senior unsecured notes' ratings.
Factors That Could, Individually or C ol lectively, Lead to Negative Rating Action/Downgrade:
A downgrade of ADQ's Long- Term IDR would be reflected in the GMTN programme's and US dollar-denominated senior unsecured notes' ratings.
Issuer Profile
ADQ is a strategic long-term investor headquartered in Abu Dhabi contributing to the country's economic diversification away from its hydrocarbons driven revenue source and one of its largest employers. ADQ has more than 85,000 employees in over 25 portfolio companies.
ADQ promotes through its portfolio companies the growth of the key strategic sectors across of Abu Dhabi's economy, namely energy and utilities, healthcare and life sciences, transport and logistics, and food and agriculture; hence contributed to 22% of Abu Dhabi's non-hydrocarbon nominal GDP at end- 2022. Since its inception ADQ's total consolidated assets grew to AED720.5 billion at end-2023 - about 67% of national GDP - from AED182.4billion in 2018.
Date of Relevant Committee
17 April 2024
REFERENCES FOR SUBSTANTIALLY MATERIAL SOURCE CITED AS KEY DRIVER OF RATING
The principal sources of information used in the analysis are described in the Applicable Criteria.
Public Ratings with Credit Linkage to other ratings
ADQ's ratings are credit-linked to Abu Dhabi's ratings.
- senior unsecured
VIEW ADDITIONAL RATING DETAILS
Additional information is available on www.fitchratings.com
PARTICIPATION STATUS
The rated entity (and/or its agents) or, in the case of structured finance, one or more of the transaction parties participated in the rating process except that the following issuer(s), if any, did not participate in the rating process, or provide additional information, beyond the issuer’s available public disclosure.
APPLICABLE CRITERIA
- Government-Related Entities Rating Criteria (pub. 12 Jan 2024)
- Public Policy Revenue-Supported Entities Rating Criteria (pub. 12 Jan 2024) (including rating assumption sensitivity)
ADDITIONAL DISCLOSURES
- Dodd-Frank Rating Information Disclosure Form
- Solicitation Status
- Endorsement Policy
ENDORSEMENT STATUS
- Artificial Intelligence /
- Autonomous Cars
In the first Autonomous Racing League race, the struggle was real
Move slow and be things that break..
By Wes Davis , a weekend editor who covers the latest in tech and entertainment. He has written news, reviews, and more as a tech journalist since 2020.
Share this story
The first race of the Abu Dhabi Autonomous Racing League (A2RL) took place on the Yas Marina Abu Dhabi Grand Prix Formula 1 track today, and I’m pleased to report that a race both began and ended. But the event was not without strife — far from it. During qualifying time trials, the driverless Dallara Super Formula racers outfitted with cameras and software seemed to struggle mightily to complete a full lap.
During the trials, cars randomly juked:
Or turned into walls:
Or just pulled off the track to take a little break:
You get well-acquainted with the interstitial music during these highlights. All praise to the patience and grace of the announcers, who didn’t sigh once that I heard. Instead, they declared things like that these cars are “pushing the boundaries of science.”
When it came time for the actual race , the lead racer, Polimove, spun out on the fourth of eight laps. The second car, Tum, passed it safely, but shortly after that, the event’s officials threw up a yellow flag. And since these are good AI drivers who obey the rules, the two behind Polimove stopped, unwilling to pass the spun-out yellow car. Racers aren’t supposed to pass each other during a caution lap, you see.
About an hour after the first lap of A2RL began, the AI racers completed their eight-lap race. If you must know, Tum won.
These are early days for autonomous racing, and surely things will get better eventually — certainly, they’ve come a long way since Roborace’s first full circuit in 2017. I’m looking forward to the day they’re as good as human racers (if that ever happens ). But for right now, we’re very much still in the “congratulate baby for successfully getting most of its food into its mouth” phase of self-driving racers.
Automatic emergency braking at speeds up to 90mph required under new rule
The drinking fountain button is tragically misunderstood, the apple vision pro’s ebay prices are making me sad, rabbit r1 review: nothing to see here, turns out the rabbit r1 was just an android app all along.
More from this stream Self-driving cars: Google and others map the road to automated vehicles
Orange cones are robot kryptonite., people are afraid of self-driving cars — can the industry change that, uber eats is now delivering burritos via waymo in phoenix., the zoox come out at night..
Abu Dhabi AI Company Presight Takes Majority Stake in Tech Venture AIQ

FILE PHOTO: Presight AI logo is seen near computer motherboard in this illustration taken January 8, 2024. REUTERS/Dado Ruvic/Illustration/File Photo
DUBAI (Reuters) - Artificial intelligence company Presight AI Holding has acquired a majority stake in AIQ, a technology joint venture between Abu Dhabi National Oil Company (ADNOC) and G42, under a new ownership structure announced by the companies on Wednesday.
Under the new arrangement, Presight will hold a 51% stake in AIQ with ADNOC retaining a 49% shareholding, valuing the company at $1.4 billion, a joint company statement said.
Prior to the new structure, AIQ was 60% owned by ADNOC and 40% by G42, another Abu Dhabi advanced technology company.
AIQ uses AI and machine learning to optimise processes, improve planning and increase profitability for ADNOC and the wider oil and gas industry.
War in Israel and Gaza

Sultan Al Jaber, ADNOC's CEO and minister of industry and
advanced technology, will take over as AIQ chairman.
Last year, Reuters reported that ADNOC and G42 were in early discussions about a possible initial public offering (IPO) of AIQ.
(Reporting by Rachna Uppal. Editing by Jane Merriman)
Copyright 2024 Thomson Reuters .
Join the Conversation
Tags: artificial intelligence , United Arab Emirates , Middle East
America 2024

Health News Bulletin
Stay informed on the latest news on health and COVID-19 from the editors at U.S. News & World Report.
Sign in to manage your newsletters »
Sign up to receive the latest updates from U.S News & World Report and our trusted partners and sponsors. By clicking submit, you are agreeing to our Terms and Conditions & Privacy Policy .
You May Also Like
The 10 worst presidents.
U.S. News Staff Feb. 23, 2024

Cartoons on President Donald Trump
Feb. 1, 2017, at 1:24 p.m.

Photos: Obama Behind the Scenes
April 8, 2022

Photos: Who Supports Joe Biden?
March 11, 2020

Job Growth Slows as Unemployment Rises
Tim Smart May 3, 2024

Did Hush Money Fuel Trump’s 2016 Win?
Lauren Camera May 2, 2024

Biden Condemns Unrest on Campuses
Aneeta Mathur-Ashton May 2, 2024

Four More Gag Order Violations

Fed: High Inflation Stalls Rate Cut
Tim Smart May 1, 2024

Congress Comes Down on Campus Protests
Aneeta Mathur-Ashton May 1, 2024


IMAGES
COMMENTS
Clinical Trials Multi-centers Genomics ... The Showcase of Medical Research Publications from Abu Dhabi is a collection of valuable medical research which appeared in peer-reviewed journals from healthcare community within Abu Dhabi's healthcare sector. The Showcase includes scientific literature on a wide range of medical topics.
Cleveland Clinic Abu Dhabi became a designated research facility in 2016, and since then, our Research Department has been conducting studies and clinical trials relating to a wide range of medical conditions. Our aim is to advance the development of new and innovative treatments here in the UAE, with a focus on diseases and conditions that are ...
Cleveland Clinic Abu Dhabi caregivers conduct research studies and clinical trials to develop new healthcare options for a wide variety of medical conditions. The aim of the research programs is to speed up the development of new treatments, and to enhance medical care here in the UAE. All of the research studies carried out at Cleveland Clinic ...
Abu Dhabi has opened a clinical trials centre to further medical research and help to establish the capital as a global hub for medicine. The centre at Sheikh Shakhbout Medical City is a partnership with the Mayo Clinic and will seek cures to diseases after traditional treatments and medicines have failed.. Clinical trials are research studies of medications, vaccines, medical devices ...
The core principles of the Research Center at Sheikh Shakhbout Medical City (SSMC) are based on the principles of Abu Dhabi Health Services Company (SEHA). Defined by high-quality and compassionate patient care delivered in a multispecialty scholarly environment using the most advanced technology, SSMC envisions to become the leading integrated ...
Currently, there are around 400 clinical research trials under way in Abu Dhabi.. Researchers can use the Emirati Genome Programme, which aims to provide preventive and personalised healthcare for the Emirati population, and the Malaffi system that has unified health services and patient information. "We have a wealth of data that makes us confident in understanding what is the impact of ...
Cleveland Clinic Abu Dhabi urges the public to ensure the success of such endeavors by registering as participants in future clinical trials. Dr. Sawsan Abdel-Razig, Chief Academic Officer at Cleveland Clinic Abu Dhabi, said, "Our mission at Cleveland Clinic Abu Dhabi is to deliver world-class care to the UAE community and wider region.
Meanwhile, Dr Asma Al Mannaei, executive director of the research and innovation center at the Department of Health - Abu Dhabi (DoH), noted that currently, there were around 400 clinical ...
Sheikh Shakhbout Medical City (SSMC), one of the UAE's largest hospitals for serious and complex care and a joint-venture partnership between Mayo Clinic and Abu Dhabi Health Services Company (SEHA), launched today one of the most advanced Clinical Trials Units in Abu Dhabi. By conducting research on areas with unmet medical needs, the Unit ...
Abu Dhabi has launched a new programme of funding aimed at supporting research and innovation to fight life-threatening diseases. The emirate's Department of Health said the grant will be offered to those who carry out clinical research projects in the areas of cardio-metabolic and vascular disorders, cancer, Alzheimer's disease, rare diseases, emerging infectious disease and antimicrobial ...
For further information on the trials and eligibility, contact 0564104317. Burjeel Hospital Department of Health - Abu Dhabi Health. As an incubator and hub for life-science research and innovation, Abu Dhabi is set to begin clinical trials to transform the way thalassemia is being treated, with two global clinical trials.
By Mrudvi Bakshi | Jun 19, 2023. Abu Dhabi is home to one of the most advanced clinical trial units, designed to perform world-class research with the aim of translating scientific discoveries into innovative treatments to solve complex and prevalent conditions in the region. The unit belonging to Sheikh Shakbout Medical City (SSMC) is equipped ...
The first UAE-based Contract Research Organization (CRO) specializing in healthcare research. IROS is the UAE's first registered and home-grown contract research organization. IROS was established to promote clinical trials and research with pharma stakeholders to improve patient outcomes, in line with the Abu Dhabi Economic Vision 2030.
About Us. Mediclinic Middle East is one of the very few private healthcare organisations in the UAE to actively support its clinician's research endeavours through medical research centres in the UAE. The number of facilities at Mediclinic Middle East provides access to a wide variety of patients and therapeutic areas.
1. Overview: Clinical Research. Clinical Research at ACPN strives to promote and implement evidence-based clinical practice through well-designed research studies, using sound research methodology. This is in line with the mission of Department of Health (DOH) to promote world class research that improves health and quality of life of both ...
Over 150 SEHA Medical Residents, and Fellows Enrolled in a Clinical Research Training Program, Furthering the Region's Research Capacity . A two-day workshop marking the inauguration of the Clinical Research Certificate (KU-SEHA CRC) training program, a Khalifa University collaboration with the Abu Dhabi Health Services Company (SEHA) and the Department of Health - Abu Dhabi, was launched ...
The Department of Health - Abu Dhabi (DoH), the regulator of the healthcare sector in the emirate, announced a new collaboration with Pfizer, one of the world's premier biopharmaceutical companies, with the aim to develop national competencies and clinical research capacity in Abu Dhabi.The collaboration reflects the emirate's vision related to the health and safety of the local ...
Location: Abu Dhabi, UAE. Job Type: Full Time. Job Level: Experienced (Non-Manager) No.of Positions: 5. Candidate Profile: Master's degree in clinical, para-clinical or Allied health sciences. AHIMA / AAPC Certification - CCS OR CPMA. At least 5 years of hands on experience in coding multiple specialties.
Abu Dhabi [UAE], April 30 (ANI/WAM): The International Conference on Advancements in Autism Research, themed "Challenges and Solutions," called upon experts, scientists, specialists, practitioners ...
4-12 Weeks. -. Gulf Medical University. Ajman. Research Training. 1 Month. 3000 AED. The average course fee of Clinical Research courses in Dubai is 3000 AED for a 1-month Research Training course. Select a City.
Dr. Alok Sharan and Dr. Muhammad Abd-El-Barr, along with other surgeons from the US, were recently invited to speak at the 1st ever Abu Dhabi 2024 International Brain and Spine Surgery Congress (ADNeuro24). The conference was organized by Dr. Waseem Aziz, Chief of Neurosurgery at Sheikh Shakhbout Medical City.
Napo Pharmaceuticals, a Jaguar Health Family Company, Sponsoring Pediatric Gastroenterology Conference in Abu Dhabi and Panel Discussion About Microvillus Inclusion Disease (MVID) Jaguar Health ...
Fitch Ratings - Frankfurt am Main - 29 Apr 2024: Fitch Ratings has assigned Abu Dhabi Developmental Holding Company PJSC's (ADQ) upcoming global medium-term note (GMTN) programme as well as its upcoming USD3 billion dual tranche (five and 10 years) senior unsecured fixed-coupon notes under the programme expected senior unsecured long-term ratings of 'AA(EXP)'.
The first race of the Abu Dhabi Autonomous Racing League (A2RL) took place on the Yas Marina Abu Dhabi Grand Prix Formula 1 track today, and I'm pleased to report that a race both began and ...
Under the new arrangement, Presight will hold a 51% stake in AIQ with ADNOC retaining a 49% shareholding, valuing the company at $1.4 billion, a joint company statement said.