How much does a Clinical Research Associate make in the United States?
- Clinical Research Associate Salary The average Clinical Research Associate salary in the United States is $119,893 as of March 26, 2024. The range for our most popular Clinical Research Associate positions (listed below) typically falls between $58,136 and $181,651. Keep in mind that salary ranges can vary widely depending on many important factors, including position, education, certifications, additional skills, and the number of years you have spent in your profession. With more online, real-time compensation data than any other website, Salary.com helps you determine your exact pay target.
- Clinical Research Associate II Participates in the design, administration and monitoring of clinical trials. Analyzes and evaluates clinical data gathered during research. Ensures compliance with protocol and overall clinical objectives. Knowledge of FDA regulatory requirements is required. May require ACRP or SOCRA Clinical Research Professional exam completion. Requires a master's degree in science or equivalent. Typically reports to a supervisor or manager. Occasionally directed in several aspects of the work. Gaining exposure to some of the complex tasks within the job function. Typically requires 2-4 years of related experience. View Clinical Research Associate II Salary Alternate Job Titles :Clinical Trails Research Associate II, Clinical Trials Data Analyst II, Clinical Research Associate II Categories : Pharmaceuticals , Biotechnology , Science and Research
- Clinical Research Associate I Participates in the design, administration and monitoring of clinical trials. Analyzes and evaluates clinical data gathered during research. Ensures compliance with protocol and overall clinical objectives. Knowledge of FDA regulatory requirements is required. May require ACRP or SOCRA Clinical Research Professional exam completion. Requires a bachelor's degree in Science or its equivalent. Typically reports to a supervisor or manager. Work is closely managed. Works on projects/matters of limited complexity in a support role. Typically requires 0-2 years of related experience. View Clinical Research Associate I Salary Alternate Job Titles :Clinical Trails Research Associate I, Clinical Trials Data Analyst I, Clinical Research Associate I Categories : Pharmaceuticals , Biotechnology , Entry Level , Science and Research
- Clinical Research Manager Manages the clinical monitoring process and the administration of clinical trials. Supervises CRAs in in-house and on-site monitoring, filing, and clinical trial administration. Oversees adherence to SOPs, Good Clinical Practice and FDA regulations. Helps with the development and implementation of clinical processes, procedures, and programs. May require a master's degree in nursing. May require ACRP or SOCRA Clinical Research Professional exam completion. Typically reports to a director. Manages subordinate staff in the day-to-day performance of their jobs. True first level manager. Ensures that project/department milestones/goals are met and adhering to approved budgets. Has full authority for personnel actions. Typically requires 5 years experience in the related area as an individual contributor. 1-3 years supervisory experience may be required. Extensive knowledge of the function and department processes. View Clinical Research Manager Salary Alternate Job Titles :Clinical Research Programs Manager, Clinical Research Manager Categories : Pharmaceuticals , Biotechnology
- Research and Development Associate I Participates in research and development activities. Utilizes established mathematical and scientific techniques to compile and analyze data. Writes technical reports detailing procedures, outcomes, and observations. Requires a bachelor's degree. Typically reports to a supervisor or manager. Works on projects/matters of limited complexity in a support role. Work is closely managed. Typically requires 0-2 years of related experience. View Research and Development Associate I Salary Alternate Job Titles :R & D Support Associate I, Scientific Research Associate I, Research and Development Associate I Categories : Pharmaceuticals , Biotechnology , Energy and Utilities , Science and Research
- Research and Development Associate IV Participates in research and development activities. Utilizes established mathematical and scientific techniques to compile and analyze data. Writes technical reports detailing procedures, outcomes, and observations. May require a master's degree. Typically reports to a manager or head of a unit/department. Work is highly independent. May assume a team lead role for the work group. A specialist on complex technical and business matters. Typically requires 7+ years of related experience. View Research and Development Associate IV Salary Alternate Job Titles :R & D Support Associate IV, Scientific Research Associate IV, Research and Development Associate IV Categories : Pharmaceuticals , Biotechnology , Energy and Utilities , Science and Research
- Research and Development Associate V Participates in research and development activities. Utilizes established mathematical and scientific techniques to compile and analyze data. Writes technical reports detailing procedures, outcomes, and observations. May require a master's degree. Typically reports to a manager or head of a unit/department. Works autonomously. Goals are generally communicated in "solution" or project goal terms. May provide a leadership role for the work group through knowledge in the area of specialization. Works on advanced, complex technical projects or business issues requiring state of the art technical or industry knowledge. Typically requires 10+ years of related experience. View Research and Development Associate V Salary Alternate Job Titles :R & D Support Associate V, Scientific Research Associate V, Research and Development Associate V Categories : Pharmaceuticals , Biotechnology , Energy and Utilities , Science and Research
- Research and Development Associate II Participates in research and development activities. Utilizes established mathematical and scientific techniques to compile and analyze data. Writes technical reports detailing procedures, outcomes, and observations. Requires a bachelor's degree. Typically reports to a supervisor or manager. Gains exposure to some of the complex tasks within the job function. Occasionally directed in several aspects of the work. Typically requires 2 to 4 years of related experience. View Research and Development Associate II Salary Alternate Job Titles :R & D Support Associate II, Scientific Research Associate II, Research and Development Associate II Categories : Pharmaceuticals , Biotechnology , Energy and Utilities , Science and Research
- Research and Development Associate III Participates in research and development activities. Utilizes established mathematical and scientific techniques to compile and analyze data. Writes technical reports detailing procedures, outcomes, and observations. May require a master's degree. Typically reports to a supervisor or manager. Work is generally independent and collaborative in nature. Contributes to moderately complex aspects of a project. Typically requires 4-7 years of related experience. View Research and Development Associate III Salary Alternate Job Titles :R & D Support Associate III, Scientific Research Associate III, Research and Development Associate III Categories : Pharmaceuticals , Biotechnology , Energy and Utilities , Science and Research
- Scientist - Clinical Research Assumes lead role in various clinical research projects. Proposes, plans, organizes and executes experiments and research. Secures grants and funding for research and is responsible for controlling budgets. Summarizes findings in reports and communicates results. Interacts with other scientists within and outside of the organization. Provides recommendations that influence extensive clinical research activities. Requires an advanced degree. Typically reports to head of a unit/department. Work is generally independent and collaborative in nature. Contributes to moderately complex aspects of a project. Typically requires 4 -7 years of related experience. View Scientist - Clinical Research Salary Alternate Job Titles :Clinical Research Lead Scientist, Senior Clinical Researcher, Scientist - Clinical Research Categories : Science and Research , Biotechnology
- Clinical Research Director Directs and oversees the clinical research function for a healthcare organization. Develops research studies and creates standards and guidelines for clinical research services and programs. Ensures adherence to standard operating procedures, good clinical practice and FDA regulations. Requires an advanced degree. Typically reports to top management. Manages a departmental sub-function within a broader departmental function. Creates functional strategies and specific objectives for the sub-function and develops budgets/policies/procedures to support the functional infrastructure. Deep knowledge of the managed sub-function and solid knowledge of the overall departmental function. Typically requires 5+ years of managerial experience. View Clinical Research Director Salary Alternate Job Titles :Clinical Research Programs Director, Clinical Research Director Categories : Healthcare - Administrative , Science and Research

Clinical Research Associate Salary Estimate United States Nearby Locations...
Clinical research associate job title.
A Clinical Research Associate is responsible for running clinical trials, usually for an organization undertaking pharmacological drug testing. Will ensure that trials adhere to good clinical practice guidelines for monitoring clinical trials.
Salary Details
High demand job locations.
- Boston, MA - $69,183
- San Diego, CA - $97,846
- Philadelphia, PA - $58,054
- New York, NY - $90,654
- Los Angeles, CA - $94,281
Advance your career
- Clinical Research Manager
- Clinical Trial Manager
- Clinical Research Director
Jobs In on Monster
Clinical Research Associate Salary in the US
Clinical research associate - average salary, clinical research associate salary range, clinical research associate - salary differences, clinical research associate - pay by experience level in the united states, how work experience affects the salary of a clinical research associate, gender breakdown, clinical research associate - jobs by location, clinical research associate - related salaries.

Frequently asked questions about the salary of the Clinical Research Associate

For Employers
Salary assessor, executive compensation assessor, nonprofit comparables assessor, our process, global salary calculator, relocation assessor, geographic assessor, occupational assessor, request a quote, learn more about eri's assessor platform.
Try a Free Demo
For Job Seekers
Salary calculator, research salaries, cost of living comparison, research cost of living, get a detailed salary report.
Get Your Customized Pay Report
Why SalaryExpert
Want to learn more about our salary data.
Schedule Time to Learn More
White Papers
Webinar recordings, stay up to date on hr news.
- United States
Clinical Research Associate
$86,960 (usd)/yr, $41.81 (usd) /hr, $1,939 (usd) /yr.
The average clinical research associate gross salary in United States is $86,960 or an equivalent hourly rate of $42. In addition, they earn an average bonus of $1,939. Salary estimates based on salary survey data collected directly from employers and anonymous employees in United States. An entry level clinical research associate (1-3 years of experience) earns an average salary of $62,280. On the other end, a senior level clinical research associate (8+ years of experience) earns an average salary of $107,310.
Data powered by ERI's Salary Expert Database .
This page is a promotion for SalaryExpert’s Assessor Platform and is not intended for professional use.
Professionals should subscribe to SalaryExpert’s Assessor Platform .
ERI’s compensation data are based on salary surveys conducted and researched by ERI. Cost of labor data in the Assessor Series are based on actual housing sales data from commercially available sources, plus rental rates, gasoline prices, consumables, medical care premium costs, property taxes, effective income tax rates, etc.
DO YOU WORK IN HR OR COMPENSATION?
Try our professional compensation software to generate detailed salary and cost of living reports.
$97,680 (USD)
Based on our compensation data, the estimated salary potential for Clinical Research Associate will increase 12 % over 5 years.
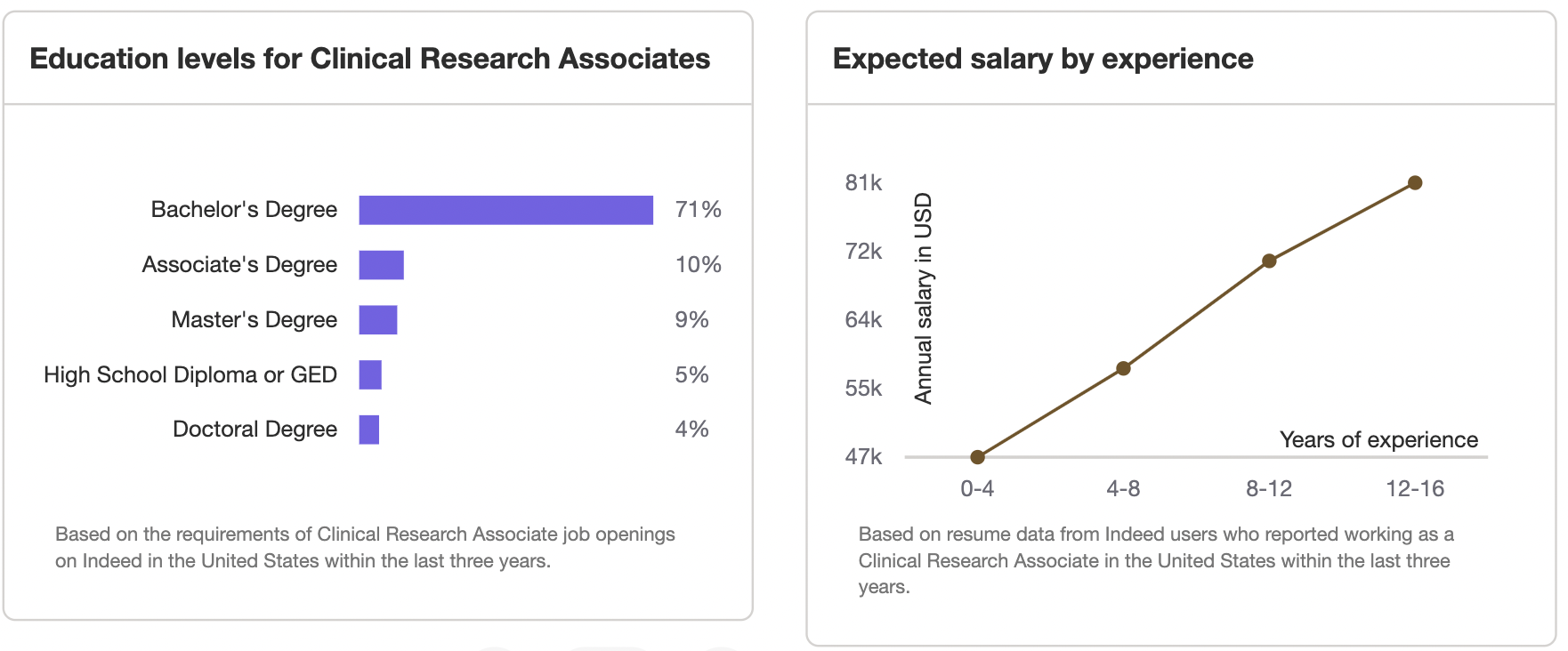
This chart displays the highest level of education for: Clinical Research Associate , the majority at 100% with bachelors.
Typical Field of Study: Community Organization and Advocacy
the United States
Cost of living is calculated based on accumulating the cost of food, transportation, health services, rent, utilities, taxes, and miscellaneous.
The United States of America (USA or U.S.A.), commonly known as the United States (US or U.S.) or America, is a country primarily located in North America. It is a federation of 50 states, a federal capital district (Washington, D.C.), and 326 Indian reservations. Outside the union of states, it asserts sovereignty over five major unincorporated island territories and various uninhabited islands. The country has the world's third-largest land area, second-largest exclusive economic zone, and third...
Are you paid fairly?

Learn About Our Products
- Scientist Clinical Research
- Associate Clinical Research
How Much Should You Be Paid?
Subscribe to salaryexpert’s emails.
For the latest in HR and compensation news, subscribe to our monthly e-newsletters, blogs, and white papers. Subscribe

Clinical Research Associate Salary - What's the pay for a clinic research associate?
Here's What You Need to Know to Get a Clinical Research Associate Job
What's the pay for a clinic research associate?: $61-$110K
A Clinical Research Associate (or Monitor) is hired either in-house (“the trial site”) or externally (by the sponsor or CRO) to do review clinical trial data and ensure that investigational therapies are tested ethically and scientifically through performing site visits that review files like patient medical notes in order to ensure quality of trial data. The catch 22 of clinical research associate jobs is that the ICH GCP guidelines require both education AND experience in order to work in this role, so you getting your foot in the door is tough. Once you get experience, your education (i.e. certifications or degrees showing understanding of additional responsibilities) can help promotes you quickly through the CRA career ladder.
How to become or get promoted as a clinical research associate?
Having a certification through CCRPS’s accredited Advanced Clinical Research Associate Certification course can help professionals 1) get promoted 2) get a raise 3) improve efficiency 4) get hired as a Clinical Research Associate.
How much does a Clinical Research Associate make in the United States?
The average Clinical Research Associate salary in the United States is $61-110K . Salary ranges can vary widely depending on many important factors, including education, certifications, additional skills, the number of years you have spent in your profession.
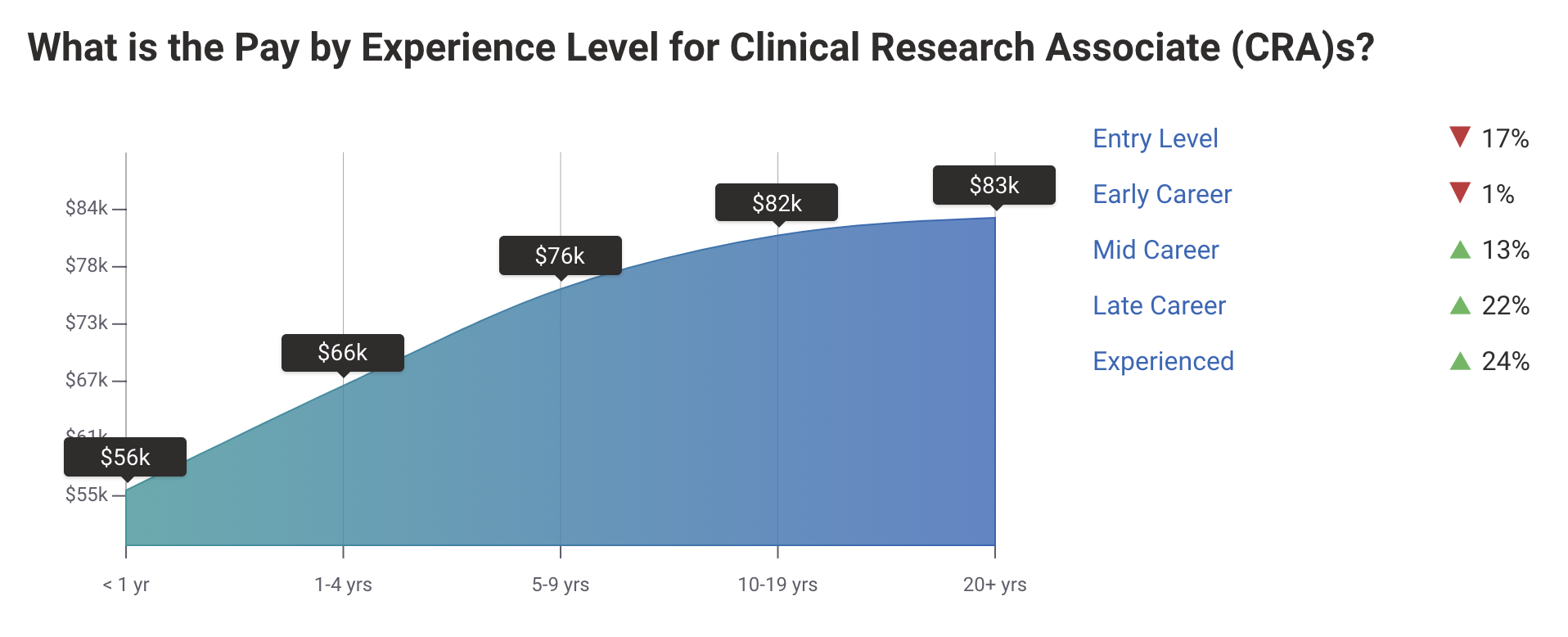
Clinical Research Associate (CRA) Salary
Per Payscale “An entry-level Clinical Research Associate (CRA) with less than 1 year experience can expect to earn an average total compensation (includes tips, bonus, and overtime pay) of $55,588 based on 203 salaries. An early career Clinical Research Associate (CRA) with 1-4 years of experience earns an average total compensation of $66,245 based on 1,118 salaries. A mid-career Clinical Research Associate (CRA) with 5-9 years of experience earns an average total compensation of $76,086 based on 294 salaries. An experienced Clinical Research Associate (CRA) with 10-19 years of experience earns an average total compensation of $81,540 based on 157 salaries. In their late career (20 years and higher), employees earn an average total compensation of $83,342.”
Determine CRA Salary by location using Payscale
CRA Career Progression - Indeed
Salary: Clinical Research Associate
Research Associate Salary resource: Click here to see the CRA Salary Range from 1,800+ employers
Clinical Research Associates Pay: Clinical Research Associate Salary in United States (by city)
Durham, NC - $97K
New York, NY, Irvine, CA, Houston, TX - $95K
Philadelphia, PA - $92K
Atlanta, GA - $84K
Raleigh, NC - $82K
Chicago, IL - $79K
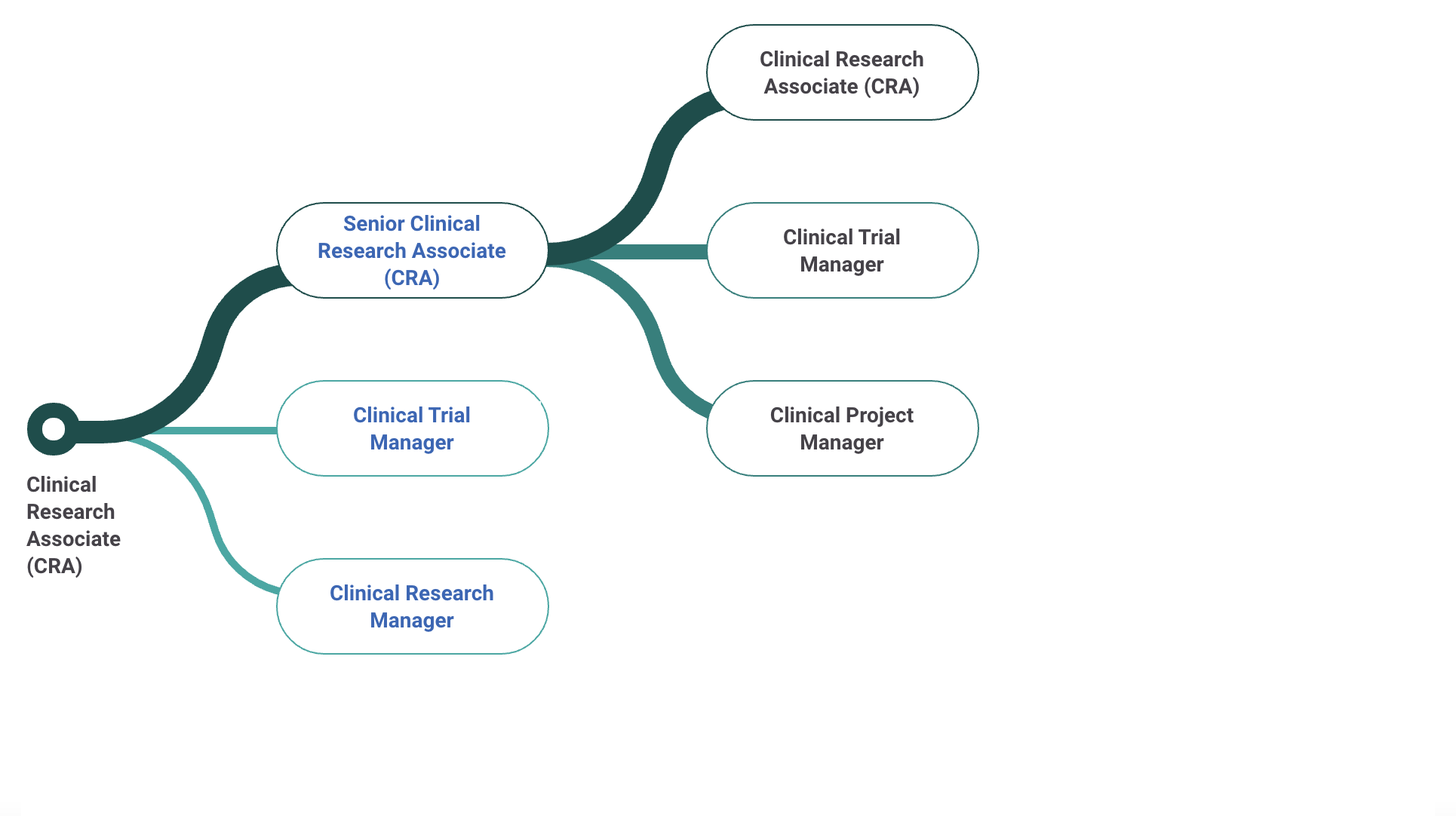
Payscale: CRA Career Pathway
Senior Clinical Research Associate Salary
The average salary of a Senior Senior Clinical Research Associate is ~105K per year ($54/hour, $2k/week, $9k/month). This can range from $81k to $139k.
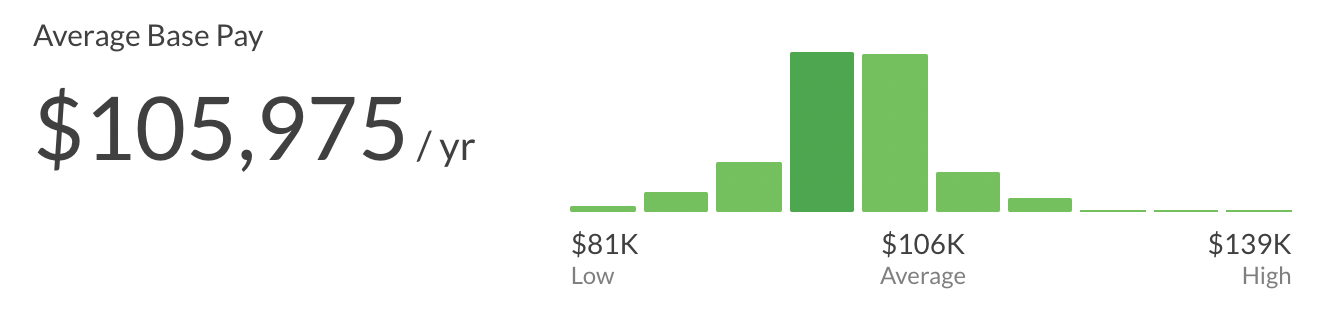
Glassdoor Salary range for Senior CRAs
Starting Salary of a Clinical Research Associate Position is between $60,000 and $65,000
Some employers may prefer hiring entry-level clinical research associates with less experience in clinical research so they can learn their job functions by training under Senior CRAs or CRA IIs.
Clinical Research Associate II Salary is $86,677 / yr
CRA II @ PPD: $84,733 /yr
CRA II @ PRA Health Sciences: $96,017 /yr
CRA II @ IQVIA: $79,412 /yr
CRA II @ ICON: $100,000 /yr
Comment below how much you get paid and what helped you get promoted!
Clinical Research Associate vs Coordinator (CRA vs CRC)
Research jobs near me.
Clinical Research Associate - Home-Based (Southeast)
- Location: United States
- Categories Clinical Monitoring
- __vacancyopjusttionswidget.opt-Business Area__ ICON Strategic Solutions
- __vacancyopjusttionswidget.opt-Remote Working __ Remote
About the role
ICON plc is a world-leading healthcare intelligence and clinical research organization. We’re proud to foster an inclusive environment driving innovation and excellence, and we welcome you to join us on our mission to shape the future of clinical development.
The Clinical Research Associate (CRA) monitors the progress of clinical studies at investigative sites or remotely, ensuring clinical trials are conducted, recorded, and reported in accordance with the protocol, standard operating procedures (SOPs), International Conference on Harmonization Good Clinical Practices (ICH-GCP) and all applicable regulatory requirements.
- Completes onsite and remote monitoring activities in accordance with all ICH-GCP guidelines, applicable regulations, SOPs, and study processes. Activities include qualifying potential investigative sites, initiating clinical trials, maintaining study files, and providing instructions to site personnel and study close out.
- Verifies the protection of study participants by confirming informed consent procedures and protocol have been performed in accordance to applicable regulations.
- Ensures the integrity of clinical data and that the study is conducted in compliance with the approved protocol, GCP, applicable regulations, and SOPs.
- Manages the investigative site staff to facilitate trial deliverables, e.g., subject enrollment, data deliverables.
- Verifies proper management and accountability of Investigational Product (IP).
- Writes and submits reports of investigational site findings and updates applicable tracking systems. Escalates observed deficiencies, issues, and corrective and preventative action plans as appropriate.
- Manages essential documents as required by local regulations and ICH-GCP guidelines before, during, and after a clinical study; assists with resolution of investigational site/data queries.
- Performs key risk assessment and management responsibilities throughout the project, including key risk indicator and site health analysis, site process evaluation, and project escalation.
- Participates in audit preparation and follow-up activities as needed.
- Independently performs a variety of onsite and offsite monitoring visit types.
- Gathers and reviews information for assigned sites and identifies inconsistencies. With limited guidance from project and functional management, assesses risk and escalates as appropriate.
- Assists with non-complex ad hoc, short-term assignments in support of additional studies or departmental initiatives.
- May serve as preceptor, providing training to less experienced clinical team members
- 2 years of experience supporting clinical trials including 1 year of on-site monitoring experience
- In-depth knowledge of the drug development process
- In-depth knowledge and practical utilization of ICH- GCP and applicable regulatory requirements
- Sound knowledge of applicable policies and procedures, SOPs, work instructions and other guidance documents
- Good spoken and written communication skills; good presentation skills
- Strong interpersonal, collaboration and time management skills
- High proficiency with Microsoft Office and company collaboration applications
- Excellent skill in the utilization of applicable clinical systems
- Excellent critical thinking skills
- Excellent organizational skills
- Ability to focus on detail for extended periods of time; high attention to accuracy
- Ability to travel extensively
- Ability to establish and maintain effective working relationships with investigative site staff
- Equivalent combination of education, training and relevant experience may be considered in place of the education and experience stated above. All employees must read, write and speak fluent English and host country language.
What ICON can offer you: Our success depends on the quality of our people. That’s why we’ve made it a priority to build a diverse culture that rewards high performance and nurtures talent. In addition to your competitive salary, ICON offers a range of additional benefits. Our benefits are designed to be competitive within each country and are focused on well-being and work life balance opportunities for you and your family. Our benefits examples include:
- Various annual leave entitlements
- A range of health insurance offerings to suit you and your family’s needs
- Competitive retirement planning offerings to maximise savings and plan with confidence for the years ahead
- Global Employee Assistance Programme, TELUS Health, offering 24-hour access to a global network of over 80,000 independent specialised professionals who are there to support you and your family’s well-being
- Life assurance
- Flexible country-specific optional benefits, including childcare vouchers, bike purchase schemes, discounted gym memberships, subsidised travel passes, health assessments, among others
Visit our careers website to read more about the benefits of working at ICON: https://careers.iconplc.com/benefits At ICON, diversity, inclusion & belonging are fundamental to our culture and values. Our rich diversity makes us more innovative which helps us better serve our people, patients, customers, and our communities. We're proud of our diverse workforce and the work we’ve done to become a more inclusive organisation. We’re dedicated to providing an inclusive and accessible environment for all candidates. ICON is committed to providing a workplace free of discrimination and harassment. All qualified applicants will receive equal consideration for employment without regard to race, color, religion, sex, sexual orientation, gender identity, national origin, disability or protected veteran status. If, because of a medical condition or disability, you need a reasonable accommodation for any part of the application process, or in order to perform the essential functions of a position, please let us know through the form below. https://careers.iconplc.com/reasonable-accommodations Interested in the role, but unsure if you meet all of the requirements? We would encourage you to apply regardless – there’s every chance you’re exactly what we’re looking for here at ICON whether it is for this or other roles.
**Actual compensation will be determined based on factors such as geographic location, work experience, education/training, and skill level.**


ICON and you

ICON history

Career Pathways

Benefits & Rewards

Environmental, Social & Governance

Women in IT

Application process
Once you find a job opening that fits your skills and interests, you can create an account, apply and check back on the progress of your application or apply for other roles. Once your application has been received, a confirmation email will be sent to your email.
Remember: this is our first time meeting you. Read the job description and highlight on your CV those experiences that we should learn about.
One of our experienced recruiters will read your profile and determine if you're fit for the role and the company. We are aware of instances where fake recruitment text messages and emails that can appear to come from ICON have been received by individuals. Always delete suspicious text messages or emails. Never give out personal financial information.
Day in the life

Teaser label
Content type
Publish date
To excel as a Clinical Research Associate (CRA) in a Clinical Research Organization (CRO), you need a combination of education, skills, and the right mindset. Brazil-based CRA II Debora shares her
Brazil-based CRA II Debora Oh shares her tips on how to become a great CRA and provides insight into life at ICON.

Unlocking your Potential: The Benefits of ICON’s EPIC Internship Program Internship programs have become a vital stepping stone for students and young professionals seeking to gain practic
Read more about ICON plc's EPIC (Entry-level Professionals in CRO (Contract Research Organization)) internship program.

How to progress as a Clinical Research AssociateTo thrive as a Clinical Research Associate (CRA), it is imperative to cultivate a multifaceted skill set and demonstrate unwavering commitment to exce
Senior CRA Yemi Moses recounts her development and shares her career ambitions with ICON plc.
Press play to find out more

Similar jobs
Clinical Monitoring
Business Area
ICON Strategic Solutions
Job Categories
Description
A Clinical Research Associate is a professional who contributes to accelerated drug/device/outcomes research through independent monitoring of studies to ensure patient safety and data integrity. You
2024-109513
Expiry date

Remote Working
As a Senior Clinical Research Associate you will be joining the world’s largest & most comprehensive clinical research organisation, powered by healthcare intelligence. You will be partnering with one
2023-103968

As Clinical Research Associate (CRA), you will be joining the world's largest & most comprehensive clinical research organization, powered by healthcare intelligence. A CRA is a professional who cont
2023-103904

Netherlands
ICON plc is a world-leading healthcare intelligence and clinical research organization. We’re proud to foster an inclusive environment driving innovation and excellence, and we welcome you to join us
2024-109461

ICON plc is a world-leading healthcare intelligence and clinical research organisation. From molecule to medicine, we advance clinical research providing outsourced services to pharmaceutical, biotech
2023-104179
2024-109401

Browse popular job categories below or search all jobs above
Senior Clinical Research Associate Salary in the United States
Senior clinical research associate salary.
How much does a Senior Clinical Research Associate make in the United States? The average Senior Clinical Research Associate salary in the United States is $87,700 as of March 26, 2024, but the salary range typically falls between $76,357 and $100,456 . Salary ranges can vary widely depending on many important factors, including education , certifications, additional skills, the number of years you have spent in your profession. With more online, real-time compensation data than any other website, Salary.com helps you determine your exact pay target.
Clinical Research Associate to CTM
Planet Pharma - Carpinteria, CA
Senior Clinical Trial Management Associate
Bayside Solutions - Fremont, CA
Senior Clinical Research Associate
Research & Development Institute, Inc. - Los Angeles, CA
Senior Clinical Research Coordinator
Barrington James - Los Angeles, CA
Individualize employee pay based on unique job requirements and personal qualifications.
Get the latest market price for benchmark jobs and jobs in your industry.
Analyze the market and your qualifications to negotiate your salary with confidence.
Search thousands of open positions to find your next opportunity.
- View Hourly Wages
- Select State
- Select City
- Choose Similar Job
- Pick Related Category
- View Cost of Living in Major Cities
Review the job openings , similar jobs , level of education , and experience requirements for the Senior Clinical Research Associate job to confirm that it is the job you are seeking.
See user submitted job responsibilities for Senior Clinical Research Associate.
View Job Skills and Competency Data for more than 15,000 Job Titles, 18 Industries, and 26 Job Families.
Our job description management tool- JobArchitect streamlines your job description process. Say goodbye to the hassle of crafting job descriptions.
Search Senior Clinical Research Associate Job Openings
Career path for this job, down a level:, clinical research associate i.
0 - 2 years experience Bachelor's Degree
2 - 4 years experience Master's Degree or MBA
Up a level:
Scientist - clinical research.
4 - 7 years experience JD, MD, PhD or Equivalent
What does a Senior Clinical Research Associate do?
Not the job you're looking for search more salaries here:, are you an hr manager or compensation specialist.
Salary.com's CompAnalyst platform offers:
- Detailed skills and competency reports for specific positions
- Job and employee pricing reports
- Compensation data tools, salary structures, surveys and benchmarks.
Similar Jobs to Senior Clinical Research Associate
Level of education.
- Senior Clinical Research Associate Salaries with a Bachelor's Degree
- Senior Clinical Research Associate Salaries with a Master's Degree or MBA
- Senior Clinical Research Associate Salaries with a JD, MD, PhD or Equivalent
Senior Clinical Research Associate Salary by State
- Connecticut
- District of Columbia
- Massachusetts
- Mississippi
- North Carolina
- North Dakota
- New Hampshire
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- West Virginia
Browse All Pharmaceuticals Jobs by Salary Level
Browse related industries, browse related job categories with senior clinical research associate, understand the total compensation opportunity for a senior clinical research associate, base salary plus other pay elements, average base salary.
Core compensation
Average Total Cash Compensation
Includes base and annual incentives
Discover how your pay is adjusted for skills, experience, and other factors
How much should you be paid.
For a real-time salary target, tell us more about your role in the four categories below.
Your estimated salary based on up-to-date market data and the factors you selected below
Skills associated with Senior Clinical Research Associate: Regulatory Compliance , On-Site , Close , Reporting
Recently searched related titles: Regional Clinical Research Associate , Senior Clinical Research Nurse , Healthcare Informatics Analyst
Learn more about: Compensation Packages , Employee Flight Risk , Gender Pay Gap , Job Openings for This Role
Jobs with a similar salary range to Senior Clinical Research Associate : Real Estate Trainee , Medical Science Liaison Manager , Clinical Research Associates
- Mayo Clinic Careers
- Anesthesiology
- Dermatology
- Emergency Medicine
- Family Medicine
- Internal Medicine
- Lung Transplant
- Psychiatry & Psychology
- Nurse Practitioner & Physician Assistant
- Ambulance Service
- Clinical Labs
- Radiology Imaging
- Clinical Research Coordinator
- Respiratory Care
- Senior Care
- Surgical Services
- Travel Surgical Tech
- Practice Operations
- Administrative Fellowship Program
- Administrative Internship Program
- Career Exploration
- Nurse Residency and Training Program
- Nursing Intern/Extern Programs
- Residencies & Fellowships (Allied Health)
- Residencies & Fellowships (Medical)
- SkillBridge Internship Program
- Training Programs & Internships
- Diversity, Equity & Inclusion
- Employees with Disabilities
You're using Internet Explorer - therefore, some pages or features may not display properly. We recommend switching to a modern browser such as Chrome, Microsoft Edge, or Firefox for a smoother experience.
Search life-changing careers..
Search by Role or Keyword
Enter Location
- United States Applicants
- United Kingdom Applicants
- United Arab Emirate Applicants
- Current Employees
Clinical Data Associate- Research
- Jacksonville, FL
Not ready to apply? Join our talent community
This Hybrid/Remote position supports the Mayo Clinic Research's clinical trials and research studies. You will be part of a larger team that brings innovation and advances to healthcare.
Under the direct supervision of the study team and research leadership, assists in coordinating the details of the study and documentation concerning study protocols, patient scheduling, retrospective & prospective chart work, data collection, data entry, data management, follow-up information, and compliance with federal, state, sponsor, and institutional guidelines.
Overview for the Clinical Data Associate I (CDA I):
- The CDA I provides data management support for clinical trials.
- The CDA I is responsible for data collection in a timely manner by performing data abstraction of clinical and demographic information from the electronic medical record and case report forms into the study specific electronic data capture system, as well as leading and assisting the data team in their roles.
- The CDA I manages the study database as directed.
- The CDA I performs other administrative duties related to the study as assigned including creation of source documents, redaction of protected patient information under HIPAA privacy laws, and sending/faxing query resolutions to industry sponsors or CRO personnel.
- The CDA I is responsible for scheduling clinical research monitor visits, maintaining the monitor visit calendar, and serving as the campus visitor escort to pharmacy, radiology, BAP Lab, and other locations on campus, as needed.
- The CDA I independently prioritizes assignments and completes ongoing tasks following established research regulatory guidelines and best practices, and may assist with general office responsibilities or research related projects.
- CDA I’s perform administrative functions to support work unit and maintain continuing education requirements as required for the position by the completion of Human Subject Protection and Good Clinical Practice (GCP) trainings at initial employment and every 3 years thereafter
During the selection process you may participate in an OnDemand (pre-recorded) interview that you can complete at your convenience. During the OnDemand interview, a question will appear on your screen, and you will have time to consider each question before responding. You will have the opportunity to re-record your answer to each question — Mayo Clinic will only see the final recording. The complete interview will be reviewed by a Mayo Clinic staff member and you will be notified of next steps .
High School Diploma with medical terminology or clinical experience preferred.
Prior data entry, Excel, Outlook mail/calendar and Adobe Acrobat pro experience preferred. Excellent written, oral, and interpersonal communication skills are a must.
A candidate must possess high attention to detail and have demonstrated critical thinking skills.
This position is not eligible for visa sponsorship. Mayo does not participate in OPT Stem.
About our location
Jacksonville, Florida
We would love to connect with you.
Click the button for a list of our upcoming events.
Join Our Talent Community
Sign up, stay connected and get opportunities that match your skills sent right to your inbox
Email Address
Phone Number
Upload Resume/CV (Must be under 1MB) Remove
Job Category* Select One Advanced Practice Providers Business Education Engineering Executive Facilities Support Global Security Housekeeping Information Technology Internship Laboratory Nursing Office Support Patient Care - Other Pharmacy Phlebotomy Physician Post Doctoral Radiology Imaging Research Scientist Surgical Services Therapy
Location Select Location Albert Lea, Minnesota Arcadia, Wisconsin Austin, Minnesota Barron, Wisconsin Bloomer, Wisconsin Caledonia, Minnesota Cannon Falls, Minnesota Chippewa Falls, Wisconsin Decorah, Iowa Duluth, Minnesota Eau Claire, Wisconsin Fairmont, Minnesota Faribault, Minnesota Holmen, Wisconsin Jacksonville, Florida Kasson, Minnesota La Crosse, Wisconsin Lake City, Minnesota London, England Mankato, Minnesota Menomonie, Wisconsin Minneapolis-St. Paul-Bloomington, Minnesota New Prague, Minnesota Onalaska, Wisconsin Osseo, Wisconsin Owatonna, Minnesota Phoenix, Arizona Prairie du Chien, Wisconsin Red Wing, Minnesota Rice Lake, Wisconsin Rochester, Minnesota Saint Cloud, Minnesota Saint James, Minnesota Saint Peter, Minnesota Scottsdale, Arizona Sparta, Wisconsin Waseca, Minnesota Zumbrota, Minnesota
Area of Interest Select One Nursing Research Laboratory Medicine & Pathology Radiology Surgery Pharmacy Physical Medicine & Rehabilitation Facilities Psychiatry & Psychology Licensed Practical Nurse (LPN) Surgical Technician Finance Cardiovascular Medicine Respiratory Therapy General Services Emergency Medicine Neurology Mayo Collaborative Services Environmental Services Ambulance Services Social Work Anesthesiology & Perioperative Medicine Cardiovascular Surgery Family Medicine Gastroenterology & Hepatology Global Security Information Technology International Medical Oncology Orthopedics Radiation Oncology Urology Surgical Assistant Healthcare Technology Management Mayo Clinic Laboratories Office Support Transplant Critical Care Hematology Hospital Internal Medicine Obstetrics & Gynecology Pediatrics Biochemistry & Molecular Biology Ophthalmology Administration Artificial Intelligence & Informatics Community Internal Medicine Desk Operations Linen & Central Services Oncology Senior Care Education Engineering Pulmonary/Sleep Medicine Dermatology Digital Hospice & Palliative Care Otolaryngology (ENT) Patient Scheduling Physiology & Biomedical Engineering Clinical Genomics Clinical Nutrition Development/Philanthropy Endocrinology General Internal Medicine Molecular Medicine Rheumatology Sports Medicine Business Development Cancer Center Comparative Medicine Epidemiology Infectious Diseases Mayo Clinic Platform Cancer Biology Immunology Nephrology & Hypertension Neurologic Surgery Neurosciences Quality Risk Management Travel Volunteer Services Addiction Services Clinical Trials & Biostatistics Communications Dental Specialities Executive Office Health Care Delivery Research Legal Marketing Molecular Pharmacology & Experimental Therapeutics Primary Care Regenerative Biotherapeutics Spiritual Care Center for Individualized Medicine Computational Biology Geriatric Medicine & Gerontology Health Information Management Services Information Security Occupational/Preventative Medicine Pain Medicine Spine Center Women's Health
- Research, Jacksonville, Florida, United States Remove
Confirm Email
By submitting your information, you consent to receive email communication from Mayo Clinic.
Join our talent community.
Join our global talent community to receive alerts when new life-changing opportunities become available.

If you want to know what it's really like at Mayo Clinic, just ask. You'll find that our pride–in where we work, and in what we do–is a common trait. You will also find a lot of inspiring stories about lives changed for the better.
Nurse Residency Program
The Nurse Residency Program (NRP) is for all nurses with less that 12 months of experience, to be completed within the first year. NRP provides a framework for a successful transition to a professional nurse by promoting educational and personal advancement.

As your career evolves, our compensation and benefits packages are designed to change with you — meeting needs now, and anticipating what comes next. We know that when Mayo Clinic takes care of you, you can take better care of our patients.
Equal opportunity
All qualified applicants will receive consideration for employment without regard to race, color, religion, sex, gender identity, sexual orientation, national origin, protected veteran status, or disability status. Learn more about "EEO is the Law." Mayo Clinic participates in E-Verify and may provide the Social Security Administration and, if necessary, the Department of Homeland Security with information from each new employee's Form I-9 to confirm work authorization.
Reasonable accommodations
Mayo Clinic provides reasonable accommodations to individuals with disabilities to increase opportunities and eliminate barriers to employment. If you need a reasonable accommodation in the application process; to access job postings, to apply for a job, for a job interview, for pre-employment testing, or with the onboarding process, please contact HR Connect at 507-266-0440 or 888-266-0440.
Job offers are contingent upon successful completion of a post offer placement assessment including a urine drug screen, immunization review and tuberculin (TB) skin testing, if applicable.
Recruitment Fraud
Learn more about recruitment fraud and job scams
Advertising
Mayo Clinic is a not-for-profit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
Advertising and sponsorship policy | Advertising and sponsorship opportunities
Reprint permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
Any use of this site constitutes your agreement to the Terms and Conditions and Privacy Policy linked below.
Terms and Conditions | Privacy Policy | Notice of Privacy Practices | Notice of Nondiscrimination
© 1998-2024 Mayo Foundation for Medical Education and Research (MFMER). All rights reserved.

Baxter looking for Research Associate, M.Pharm, MSc Apply

Baxter provides a broad portfolio of essential renal and hospital products, including home, acute and in-centre dialysis; sterile IV solutions; infusion systems and devices; parenteral nutrition; surgery products and anesthetics; and pharmacy automation, software and services. The company’s global footprint and the critical nature of its products and services play a key role in expanding access to healthcare in emerging and developed countries.
Post : Research Associate III (Hybrid)
Job Responsibilities • Act as Product Risk Management Owner (PRMO) for Kidney care products • Understand the PRMO risk management process and drive periodic risk reviews in a timely manner to avoid any non compliance • Support the Product Design Owners, Technical leaders, Change Control owners for any updates to the risk files (Design failure mode effect analysis (DFMEA), Process failure mode effect analysis (PFMEA) and other required documents • Support the event based risk review products for the products of interest • Collaborate with the product design owners, technical experts and all the key stakeholders at a global front for implementing the risk management plan for both the Sustaining Organization and New Product Development projects • Organize, plan and execute activities in compliance with current QA/ environmental/ regulations and standards
• Co-ordinate with all the global plants and actively participate in quality reviews to monitor the complaints, process or design related changes and associated failure modes • Organize, plan and follow the execution of product changes, supplier changes and labelling tasks related to life cycle management projects • Ensure good internal and cross-functional communication at a global front and regular status update of projects.
Candidate Profile • Master’s in chemistry or M. Pharm. with at 9+ years of relevant experience • Excellent English verbal and written communication skills • Exposure to medical devices and drug products • Good knowledge of product development stages and life cycle management: development, stability, clinical, registration, process transfer, production, suppliers, customer service • Good knowledge of Design Control documentation and process • Good knowledge of risk management process of drug products and medical devices
• Demonstrated project/program leadership in drug/pharmaceutical products • Working knowledge of international/regional/national regulations and standards • Experience in project management and stakeholder management at a global front • Must be able to able to carry out strategy and vision set by upper management and be able to communicate the vision to more junior associates. • Ability to work independently. Equal Employment Opportunity Baxter is an equal opportunity employer. Baxter evaluates qualified applicants without regard to race, color, religion, gender, national origin, age, sexual orientation, gender identity or expression, disability/handicap status or any other legally protected characteristic.
Additional Information Experience : 9+ years Qualification : MSc, M.Pharm Location : Bengaluru, Karnataka, India Industry Type : Pharma/ Healthcare/ Clinical research Job Category : Regulatory Affairs End Date : 30th April 2024
Apply Online
See All M.Sc Alerts M.Pharm Alerts Ph.D Alerts Bangalore Alerts See All Other Jobs in our Database Subscribe to Pharmatutor Job Alerts by Email
RECOMMENDED POSTS

Subscribe with us
Do Not Forget to Verify
(Click on Subscription link in your inbox)

Jobs by Category
Production Jobs
R&D Jobs
F&D Jobs
Sales & Marketing
QC Jobs
Faculty Jobs
Packaging Alerts
Hospital Pharmacist
- Pharma News
- Pharmapedia

IMAGES
VIDEO
COMMENTS
The average salary for a Clinical Research Associate is $80,319 per year in United States. Learn about salaries, benefits, salary satisfaction and where you could earn the most. ... Top companies for Clinical Research Associates in United States. Labcorp. 3.3. 8,193 reviews 19 salaries reported. $119,935 per year. Syneos Health. 3.6. 922 ...
The average salary for a Clinical Research Associate (CRA) is $73,921 in 2024. Visit PayScale to research clinical research associate (cra) salaries by city, experience, skill, employer and more.
The average Clinical Research Associate I salary in the United States is $66,007 as of February 26, 2024, but the range typically falls between $57,991 and $73,867. Salary ranges can vary widely depending on many important factors, including education, certifications, additional skills, the number of years you have spent in your profession.
Most Likely Range. The estimated total pay for a Clinical Research Associate is $99,181 per year in the United States area, with an average salary of $89,174 per year. These numbers represent the median, which is the midpoint of the ranges from our proprietary Total Pay Estimate model and based on salaries collected from our users.
The estimated total pay for a Clinical Research is $125,930 per year in the United States area, with an average salary of $106,962 per year. These numbers represent the median, which is the midpoint of the ranges from our proprietary Total Pay Estimate model and based on salaries collected from our users. The estimated additional pay is $18,969 ...
The average salary for Clinical Research Associate is $94,695 per year in the United States. The average additional cash compensation for a Clinical Research Associate in the United States is $5,764, with a range from $4,323 - $8,070.
The average salary for Clinical Research Associate is US$99,181 per year in the United States. The average additional cash compensation for a Clinical Research Associate in the United States is US$10,007, with a range from US$7,505 - US$14,009. Salaries estimates are based on 5587 salaries submitted anonymously to Glassdoor by Clinical Research ...
The average Clinical Research Associate salary in the United States is $119,597 as of February 26, 2024. The range for our most popular Clinical Research Associate positions (listed below) typically falls between $57,991 and $181,204. Keep in mind that salary ranges can vary widely depending on many important factors, including position, education, certifications, additional skills, and the ...
Find the estimated Clinical Research Associate salary in United States. Compare to Clinical Research Associate national median salary and learn how to boost your worth. ... Clinical Research Associate Salary Estimate $83,797 ...
How much do Clinical Research Associate jobs pay per hour? Average hourly salary for a Clinical Research Associate job in the US is $12.02. Skip to ... As of Mar 29, 2024, the average hourly pay for a Clinical Research Associate in the United States is $41.13 an hour. While ZipRecruiter is seeing hourly wages as high as $69.71 and as low as $12 ...
The average salary for a Clinical Research Associate is $67,560 per year ($5,630 per month), which is $14,070 (+26%) higher than the average salary in the United States. A Clinical Research Associate can expect an average starting salary of $45,680. The highest salaries can exceed $104,590.
The average clinical research associate gross salary in United States is $86,271 or an equivalent hourly rate of $41. In addition, they earn an average bonus of $1,924. Salary estimates based on salary survey data collected directly from employers and anonymous employees in United States. An entry level clinical research associate (1-3 years of ...
Most Likely Range. The estimated total pay for a Clinical Research Associate is $99,137 per year in the United States area, with an average salary of $89,134 per year. These numbers represent the median, which is the midpoint of the ranges from our proprietary Total Pay Estimate model and based on salaries collected from our users.
The average Clinical Research Associate salary in the United States is $79,230 as of March 26, 2024, but the salary range typically falls between $69,150 and $90,801. Salary ranges can vary widely depending on many important factors, including education , certifications, additional skills, the number of years you have spent in your profession.
The average Clinical Research Associate salary in the United States is $61-110K. Salary ranges based on education, certifications, additional skills, the number of years you have spent in your profession. Starting Salary of a Clinical Research Associate Position is between $60,000 and $70,000.
The average salary for a Clinical Research Associate is $81,635 per year in United States. Salaries estimates are based on 5397 salaries submitted anonymously to Glassdoor by a Clinical Research Associate employees in United States.
90%. $117k. The average salary for a Clinical Research Associates is $76,161 in 2024. Base Salary. $48k - $117k. Bonus. $1k - $11k. Profit Sharing.
Boston, MA. Be an early applicant. 7 hours ago. Today's top 9,000+ Clinical Research Associate jobs in United States. Leverage your professional network, and get hired. New Clinical Research ...
36. $28. $36. $32. These charts show the average hourly wage (core compensation), as well as the average total hourly cash compensation for the job of Clinical Research Associate I in the United States. The average hourly rate for Clinical Research Associate I ranges from $28 to $36 with the average hourly pay of $32.
The Clinical Research Associate (CRA) monitors the progress of clinical studies at investigative sites or remotely, ensuring clinical trials are conducted, recorded, and reported in accordance with the protocol, standard operating procedures (SOPs), International Conference on Harmonization Good Clinical Practices (ICH-GCP) and all applicable ...
The average Senior Clinical Research Associate salary in the United States is $87,700 as of March 26, 2024, but the salary range typically falls between $76,357 and $100,456. Salary ranges can vary widely depending on many important factors, including education , certifications, additional skills, the number of years you have spent in your ...
Exemption Status Nonexempt Compensation Detail $19.57 - $27.02 / hour; Education, experience and tenure may be considered along with internal equity when job offers are extended. Benefits Eligible Yes Schedule Full Time Hours/Pay Period 80 Schedule Details Monday-Friday day shift Hours fall between 7:30am-5pm.
3,625 Clinical research associate jobs in United States. Most relevant. Black Hills Regional Eye Institute. 2.7. Clinical Research Coordinator. Rapid City, SD. $33K - $48K (Employer est.) Easy Apply.
Industry Type : Pharma/ Healthcare/ Clinical research Job Category : Regulatory Affairs End Date : 30th April 2024. Apply Online. See All M.Sc Alerts M.Pharm Alerts Ph.D Alerts Bangalore Alerts See All Other Jobs in our Database Subscribe to Pharmatutor Job Alerts by Email
Mount Sinai is hiring a Per Diem Perioperative Scheduling Associate-OR Scheduling Office - Mount Sinai Main Hospital- 1PM-7PM;flex coverage 11:00PM-7:00AM in US. Review all of the job details and apply today!