Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- For authors
- Browse by collection
- BMJ Journals More You are viewing from: Google Indexer

You are here
- Volume 11, Issue 7
- Management of asthma in childhood: study protocol of a systematic evidence update by the Paediatric Asthma in Real Life (PeARL) Think Tank
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0002-4675-9616 Alexander G Mathioudakis 1 , 2 , 3 ,
- Michael Miligkos 4 ,
- Cristina Boccabella 5 ,
- Gioulinta S Alimani 3 , 6 ,
- Adnan Custovic 7 ,
- A Deschildre 8 ,
- Francine Monique Ducharme 9 ,
- Omer Kalayci 10 ,
- Clare Murray 1 , 2 ,
- Antonio Nieto Garcia 11 ,
- Wanda Phipatanakul 12 ,
- David Price 13 , 14 ,
- Aziz Sheikh 15 ,
- Ioana Octavia Agache 16 ,
- Leonard Bacharier 17 ,
- http://orcid.org/0000-0001-5639-0528 Apostolos Beloukas 6 , 18 ,
- Andrew Bentley 2 , 19 ,
- Matteo Bonini 5 , 20 ,
- Jose A Castro-Rodriguez 21 ,
- Giuseppe De Carlo 22 ,
- Timothy Craig 23 ,
- Zuzana Diamant 24 , 25 , 26 ,
- Wojciech Feleszko 27 ,
- Tim Felton 1 , 2 ,
- James E Gern 28 ,
- Jonathan Grigg 29 ,
- Gunilla Hedlin 30 ,
- Elham M Hossny 31 ,
- Despo Ierodiakonou 32 ,
- Tuomas Jartti 33 ,
- Alan Kaplan 34 ,
- Robert F Lemanske 28 ,
- Peter N Le Souëf 35 ,
- Mika J Mäkelä 36 ,
- Georgios A Mathioudakis 3 ,
- Paolo Matricardi 37 ,
- Marina Mitrogiorgou 38 ,
- Mario Morais-Almeida 39 ,
- Karthik Nagaraju 40 ,
- Effie Papageorgiou 6 ,
- Helena Pité 39 , 41 , 42 ,
- Paulo M C Pitrez 43 ,
- Petr Pohunek 44 ,
- Graham Roberts 45 , 46 , 47 ,
- Ioanna Tsiligianni 32 ,
- Stephen Turner 48 ,
- Susanne Vijverberg 49 ,
- Tonya A Winders 50 ,
- http://orcid.org/0000-0001-5939-812X Gary WK Wong 51 ,
- Paraskevi Xepapadaki 52 ,
- Heather J Zar 53 , 54 ,
- http://orcid.org/0000-0002-4448-3468 Nikolaos G Papadopoulos 1 , 52
- 1 Division of Infection, Immunity and Respiratory Medicine , The University of Manchester , Manchester , UK
- 2 North West Lung Centre, Manchester University NHS Foundation Trust , Manchester , UK
- 3 Athens Breath Centre , Athens , Greece
- 4 First Department of Pediatrics, "Aghia Sofia" Children's Hospital , University of Athens , Athens , Attica , Greece
- 5 Department of Cardiovascular and Thoracic Sciences , Catholic University of the Sacred Heart , Milano , Lombardia , Italy
- 6 Department of Biomedical Sciences , University of West Attica , Egaleo , Attica , Greece
- 7 Department of Paediatrics , Imperial College London , London , UK
- 8 Unité de Pneumologie et Allergologie Pédiatriques, Hôpital Jeanne de Flandre , CHU Lille , Lille , Hauts-de-France , France
- 9 Pediatrics , University of Montreal , Montreal , Quebec , Canada
- 10 Pediatric Allergy and Asthma Unit , Hacettepe Universitesi , Ankara , Turkey
- 11 Pulmonology and Allergy Unity , La Fe University and Polytechnic Hospital , Valencia , Comunidad Valenciana , Spain
- 12 Pediatric Allergy and Immunology , Children's Hospital Boston , Boston , Massachusetts , USA
- 13 Centre of Academic Primary Care , University of Aberdeen , Aberdeen , UK
- 14 Observational and Pragmatic Research Institute , Singapore
- 15 Asthma UK Centre for Applied Research, Usher Institute of Population Health Sciences and Informatics , The University of Edinburgh , Edinburgh , UK
- 16 Allergy and Clinical Immunology , Transylvania University , Brasov , Romania
- 17 Department of Allergy, Immunology, and Pulmonary Medicine , University of Washington , Seattle , Washington , USA
- 18 Institute of Infection and Global Health , University of Liverpool , Liverpool , UK
- 19 Acute Intensive Care Unit , University Hospital of South Manchester NHS Foundation Trust , Manchester , Greater Manchester , UK
- 20 National Heart and Lung Institute (NHLI) , Imperial College London , London , UK
- 21 Department of Pediatrics , Pontifical Universidad Catolica de Chile , Santiago , Chile
- 22 Allergy and Airway Diseases Patient's Associations , European Federation of Pharmaceutical Industries and Associations , Brussels , Belgium
- 23 Allergy, Asthma and Immunology , Penn State University , Hershey , Pennsylvania , USA
- 24 Department of Respiratory Medicine and Allergology, Institute for Clinical Science , Skane University Hospital Lund Hematological Clinic , Lund , Skåne , Sweden
- 25 Department of Respiratory Medicine , First Faculty of Medicine, Charles University and Thomayer Hospital , Prague , Czech Republic
- 26 Department of Clinical Pharmacy & Pharmacology , University of Groningen, University Medical Center of Groningen and QPS-NL , Groningen , Netherlands
- 27 Department of Pediatric Pulmonology and Allergy , Medical University of Warsaw , Warszawa , Poland
- 28 Department of Pediatrics and Medicine , University of Wisconsin School of Medicine and Public Health , Madison , Wisconsin , USA
- 29 Centre for Genomics and Child Health, Blizard Institute , Queen Mary University of London , London , UK
- 30 Department of Women's and Children's Health , Karolinska Institute , Stockholm , Stockholm , Sweden
- 31 Pediatric Allergy and Immunology Unit , Ain Shams University , Cairo , Egypt
- 32 Department of Social Medicine, Faculty of Medicine , University of Crete , Rethimno , Greece
- 33 Department of Paediatrics , University of Turku , Turku , Finland
- 34 Family Physician, Airways Group of Canada , University of Toronto , Toronto , Ontario , Canada
- 35 School of Paediatrics and Child Health , University of Western Australia , Perth , Western Australia , Australia
- 36 Department of Allergy , University of Helsinki , Helsinki , Uusimaa , Finland
- 37 Department of Pediatric Pulmonology, Immunology and Intensive Care Medicine , Charité - University Medicine , Berlin , Germany
- 38 Third Department of Paediatrics , National and Kapodistrian University of Athens School of Health Sciences , Athens , Greece
- 39 Allergy Center , Hospital CUF Descobertas , Lisboa , Portugal
- 40 Allergy & Asthma , VN , Chennai , India
- 41 Allergy Center , CUF Infante Santo Hospital , Lisbon , Portugal
- 42 Chronic Diseases Research Center (CEDOC) , NOVA Medical School / Faculdade de Ciências Médicas, Universidade NOVA de Lisboa , Lisbon , Portugal
- 43 Laboratory of Respiratory Physiology, Infant Center , School of Medicine, Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS) , Porto Alegre , Brazil
- 44 Paediatric Department , Motol University Hospital , Praha , Czech Republic
- 45 The David Hide Asthma and Allergy Research Centre , St Mary's Hospital , Newport Isle of Wight , UK
- 46 Faculty of Medicine, Clinical and Experimental Sciences and Human Development in Health Academic Units , University of Southampton , Southampton , UK
- 47 NIHR Biomedical Research Centre , University Hospital Southampton NHS Foundation Trust , Southampton , UK
- 48 Department of Child Health , University of Aberdeen , Aberdeen , Aberdeen , UK
- 49 Department of Respiratory Medicine and Department of Pediatric Pulmonology , University of Amsterdam , Amsterdam , Netherlands
- 50 Allergy & Asthma , Global Patient Platform , Virginia , Virginia , USA
- 51 Department of Paediatrics, Faculty of Medicine , The Chinese University of Hong Kong , Sha Tin , Hong Kong
- 52 Allergy Department, 2nd Paediatric Clinic , National and Kapodistrian University of Athens , Athens , Attica , Greece
- 53 Department of Paediatrics and Child Health, Red Cross War Memorial Children’s Hospital , University of Cape Town , Rondebosch , Western Cape , South Africa
- 54 Unit on Child and Adolescent Health , Medical Reaserch Council , Cape Town , South Africa
- Correspondence to Professor Nikolaos G Papadopoulos; ngpallergy{at}gmail.com
Introduction Clinical recommendations for childhood asthma are often based on data extrapolated from studies conducted in adults, despite significant differences in mechanisms and response to treatments. The Paediatric Asthma in Real Life (PeARL) Think Tank aspires to develop recommendations based on the best available evidence from studies in children. An overview of systematic reviews (SRs) on paediatric asthma maintenance management and an SR of treatments for acute asthma attacks in children, requiring an emergency presentation with/without hospital admission will be conducted.
Methods and analysis Standard methodology recommended by Cochrane will be followed. Maintenance pharmacotherapy of childhood asthma will be evaluated in an overview of SRs published after 2005 and including clinical trials or real-life studies. For evaluating pharmacotherapy of acute asthma attacks leading to an emergency presentation with/without hospital admission, we opted to conduct de novo synthesis in the absence of adequate up-to-date published SRs. For the SR of acute asthma pharmacotherapy, we will consider eligible SRs, clinical trials or real-life studies without time restrictions. Our evidence updates will be based on broad searches of Pubmed/Medline and the Cochrane Library. We will use A MeaSurement Tool to Assess systematic Reviews, V.2, Cochrane risk of bias 2 and REal Life EVidence AssessmeNt Tool to evaluate the methodological quality of SRs, controlled clinical trials and real-life studies, respectively.
Next, we will further assess interventions for acute severe asthma attacks with positive clinical results in meta-analyses. We will include both controlled clinical trials and observational studies and will assess their quality using the previously mentioned tools. We will employ random effect models for conducting meta-analyses, and Grading of Recommendations Assessment, Development and Evaluation methodology to assess certainty in the body of evidence.
Ethics and dissemination Ethics approval is not required for SRs. Our findings will be published in peer reviewed journals and will inform clinical recommendations being developed by the PeARL Think Tank.
PROSPERO registration numbers CRD42020132990, CRD42020171624.
- paediatrics
- paediatric thoracic medicine
- thoracic medicine
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bmjopen-2020-048338
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Strengths and limitations of this study
Broad evidence syntheses on the management of childhood asthma, with a focus on the differential treatment response according to age and disease phenotypes could reveal clinically exploitable information, that will be used in the development of clinical and research recommendations by Paediatric Asthma in Real Life.
A rigorous methodology that includes thorough evaluation of the literature, appropriate evaluation of the methodological quality of individual studies and—when appropriate—of the body of evidence, and presentation of overall effect estimates.
A prospectively published protocol increases the transparency and allowed for peer-review of the methodology used.
A potential limitation of the overview of systematic reviews (SRs) is that the feasibility of conducting the planned subgroup analyses will depend on whether relevant data have been captured in existing SRs.
Introduction
Having a global prevalence that is anticipated to exceed 400 million children by the year 2025, childhood asthma represents a huge health and socioeconomic burden to patients, their families and the society. 1–3 Despite its diverging mechanisms, triggers, outcomes and response to treatment, childhood asthma is often still approached as an extension of adult asthma. 4 It is underaddressed in clinical guidelines, likely due to unclear diagnosis, limited availability of safety, efficacy and effectiveness data in this population. Clinical recommendations are to a large extent informed by data extrapolated from clinical studies conducted in adults. 2–5
Numerous challenges complicate conducting interventional research studies in children with asthma. Besides the lack of consensus on its definition and diagnostic criteria, childhood asthma is highly heterogeneous and our understanding of different paediatric asthma phenotypes is still limited or contradictory. 6 This is further emphasised by significant variability in disease progression, outcomes and treatment response in children with different phenotypes or ages5, 7 potentially complicating interpretation of trials’ findings. In addition, there are regulatory and ethical constraints in conducting interventional research in children. 8 9 However, this results in the administration of treatments that have not been adequately evaluated in relevant (paediatric) populations, that is, evidently suboptimal.
Paediatric Asthma in Real Life (PeARL), an international Think Tank focusing on paediatric asthma, was initiated in the context of the respiratory effectiveness group, to address this evidence deficit. In a recent international, multistakeholder survey, we have identified and prioritised unmet needs on paediatric asthma. 10 A need for systematic evidence updates focusing on the management of asthma in different age groups emerged. Herein, we present the protocol for a series of systematic evidence updates aiming to summarise direct evidence from clinical studies in children with asthma, evaluating the safety and clinical effectiveness of pharmacological interventions for maintenance management and for the treatment of acute severe asthma attacks, defined as those leading to an emergency presentation with/without hospital admission, in different age groups. Our work will be used to inform clinical recommendations being developed by the PeARL Think Tank. Therefore, we need solid evidence on the efficacy on safety of various interventions. It is considered crucial to incorporate evidence derived from real-life observational studies, which may carry a lower strength of evidence than randomised controlled trials (RCTs), but are available in higher abundance and provide a better representation of clinical practice in real life, where for example, treatment compliance or inhaler technique may be problematic.
Methods and analysis
We will conduct two systematic evidence updates, based on protocols prospectively registered in the PROSPERO register (CRD42020132990, 11 CRD42020171624 12 ). The first will evaluate the safety and clinical effectiveness of pharmacological maintenance treatments for childhood asthma, while the other will focus on the pharmacotherapy of acute severe asthma attacks, defined as those requiring a hospital admission or emergency presentation. We will use standard methodology recommended by the Cochrane Collaboration 13 and will follow the Preferred Reported Items for Systematic Reviews and Meta-Analyses statement. 14
Preliminary searches revealed several RCTs evaluating maintenance pharmacotherapy of childhood asthma, which have already been summarised in high-quality systematic reviews (SRs), some conducted by the Cochrane Collaboration. We identified >40 up-to-date SRs evaluating inhaled corticosteroids (ICS), long-acting beta-2 agonists (LABA), long-acting muscarinic antagonists (LAMA), leukotriene receptor antagonists (LTRA) or biologic therapies, as first line or add-on treatment for asthma in children. As a result, we opted to produce an overview of existing SRs of clinical trials and real-life studies. 15 .
We found less up-to-date SRs on the management of acute severe asthma attacks in children, mainly focusing on short-acting beta-2 agonists (SABA), short acting muscarinic antagonists, oral corticosteroids, aminophylline and magnesium that were recently summarised in a Cochrane Overview of SRs. 16 However, when evaluating the literature, we identified several other pharmacological interventions that are tested in small trials or real-life studies, and while they may show promising early results, they have not been assessed further or introduced in clinical practice guidelines. 17–23 For this reason, we will conduct de novo synthesis of comparative clinical studies of any design aiming to identify any pharmacological intervention that has been tested for acute severe asthma attacks, followed by focused meta-analyses of promising interventions not covered by existing high-quality SRs or clinical practice guidelines.
Overview of SRs evaluating maintenance pharmacotherapy for paediatric asthma
Eligibility criteria.
Eligible studies will comprise SRs and meta-analyses of controlled clinical trials or of real-life studies evaluating maintenance treatments that are broadly used in clinical practice for asthma or recurrent wheeze in children and adolescents, aged up to 18 years. More specifically, we will include SRs comparing any combination of ICS, LABA, LAMA, LTRA, biological therapies (namely omalizumab, mepolizumab, reslizumab, benralizumab or dupilumab), or placebo as monotherapy or add-on maintenance therapy for paediatric asthma. We will accept SRs and meta-analyses evaluating any molecule of the above-mentioned categories, administered at any dose and for a duration of at least 6 weeks. SRs comparing asthma maintenance treatment both in children and adults will be included provided that paediatric data are presented separately. We will only include SRs published between 2005 and December 2020 and reported in the English language. Older SRs are probably outdated and will only be considered in the absence of high-quality, newer SRs.
Outcome measures
The primary outcomes of this overview will be the number of acute attacks requiring the administration of oral corticosteroids or an emergency visit, and the number of acute attacks requiring hospitalisation. Secondary outcomes will include lung function measures, acute attacks irrespective of the severity, symptom scores (including symptom free and rescue medication free days), asthma control, asthma-specific quality of life scores, use of rescue medications, withdrawal rates (overall, due to lack of efficacy or adverse events), adverse events and serious adverse events.
Search strategy and study selection
The electronic databases of Medline/PubMed and Cochrane Library will be systematically searched, using appropriate controlled vocabulary and free search terms to identify relevant SRs (terms describing: childhood asthma, LABA, LAMA, LTRA, ICS, biologics, SRs, detailed search strategy is available in online supplemental appendix ). Databases will be searched from 2006 onwards. Titles and abstracts of all identified manuscripts, and the full texts of potentially relevant manuscripts, will be screened by two investigators independently. We will report the reasons of exclusion of studies that will be excluded after full-text review. Disagreement will be resolved through discussion or adjudication by a third investigator, when necessary.
Supplemental material
Data abstraction.
For each of the included SRs, one investigator will extract the full reference and study identifiers, references of the included trials evaluating paediatric populations, eligibility criteria, predefined outcomes, number and baseline characteristics of the participants and details on the outcomes of interest. A second investigator will cross-check for validity.
Risk of bias assessment
A MeaSurement Tool to Assess systematic Reviews, V.2 (AMSTAR 2) tool will be used to evaluate the methodological quality of all included SRs. 24 25 The AMSTAR 2 tool evaluates 16 domains, focusing on the methodological design, interpretation and potential risk of bias involved in the conduct of a SR. It is considered by the AMSTAR 2 team that seven domains could critically affect the validity of the review, while the remaining domains describe non-critical weaknesses. Critical flaws for an SR include (1) lack of prospective protocol registration, (2) inadequate literature searches, (3) lack of justification of excluding individual studies, (4) of risk of bias evaluation or (5) of risk of bias consideration in interpreting the results, (6) of assessment of presence and likely impact of publication bias and (7) inadequate methodology for conducting meta-analysis. We will consider the results of an SR of high quality, if there is only one or none non-critical weakness, and of moderate quality, if there are more than one non-critical weaknesses. If there are one or more critical weaknesses, then we will consider the confidence low or very low, respectively. Two of the SRs will evaluate the risk of bias independently and disagreement will be resolved through discussion, or adjudication by a third reviewer.
Qualitative synthesis
We will summarise descriptively or in a tabulated format the characteristics of the included SRs and outcomes of interest. When several SRs evaluate the same intervention, we will compare their eligibility criteria, included studies and methodological quality as evaluated by the AMSTAR-2 tool, as well as the pertinent subgroup analyses that are presented. We will present in detail the results of the SR that is most recent, more complete and of high methodological quality. If no single SR fulfil these criteria, we will present in detail more than one SRs. From the remaining SRs, we will present pertinent additional information that may include, such as details about additional outcomes, or additional subgroups.
We will specifically report on the differential effectiveness of the interventions across different maintenance treatment steps (severity), age groups or paediatric asthma phenotypes.
SR of clinical studies evaluating the management of acute severe asthma attacks
Over the past decades, several interventions have been tested for the management of acute severe asthma attacks, such as ketamine or macrolide antibiotics. 17–23 Despite promising early findings, some of these interventions were not further tested in robust, prospective controlled clinical trials. This may partially be due to challenges in conducting experimental clinical studies in children, as previously discussed, particularly during acute, life-threatening conditions.
To identify all evaluated treatments, a two-stage approach will be followed. First, a broad search strategy will be used to identify all pharmacological interventions that have been tested as potential treatments for acute severe asthma attacks. Next, medications that showed positive clinical results, but are not yet thoroughly evaluated in clinical studies and meta-analyses and are therefore not recommended by international asthma guidelines (such as the National Institute for Health and Care Excellence asthma guidelines, the British Thoracic Society and Scottish Intercollegiate Guidelines Network asthma guidelines, the National Asthma Education and Prevention Programme or the Global Strategy for Asthma Management and Prevention document), will be selected and further evaluated in individual meta-analyses. The aim will be to identify novel interventions that could be recommended for use in clinical practice, or might require further evaluation in clinical research studies, to confirm their safety and effectiveness profiles.
Medline/PubMed and the Cochrane Library will be searched, using a broad search strategy, aimed to identify any clinical research studies evaluating the management of acute severe asthma attacks (detailed search strategy is available in online supplemental appendix ).
Any study evaluating pharmacological treatments for acute severe asthma attacks in children and adolescents (<18 years of age) will be included. Any comparative clinical research study, including experimental and observational studies, as well as SRs of such studies will be considered eligible for inclusion. We will only include studies published until May 2021 and reported in the English language, without time restrictions.
Eligible studies will be grouped according to the drug category they evaluate and will be presented narratively. Study design, characteristics and outcomes of interest will be reported descriptively or in a tabulated format. Outcomes of interest are the same for this broad SR and individual medication meta-analyses and are detailed in the next section.
Individual medication meta-analyses
These meta-analyses will further evaluate the safety and clinical effectiveness of individual medications that were assessed by the initial broad SR and were found to be of potential clinical value for the treatment of acute severe asthma attacks. In contrast to most preceding SRs and meta-analyses, we will include data from observational comparative effectiveness (real-life) studies, as well as controlled clinical trials.
For each meta-analysis, eligible studies will comprise controlled clinical trials and observational comparative effectiveness studies comparing the index medication with placebo, no treatment or any active control, as an add-on treatment for acute severe asthma attacks. Index medication will be defined based on the pharmacological action, meaning that molecules targeting the same pharmacological target (eg, salbutamol and terbutaline, both being SABA) will be grouped. Only studies evaluating the management of acute severe asthma attacks, defined as those requiring a hospital admission or emergency presentation, in children and adolescents, aged between 1 and 18 years of age will be included. Studies evaluating both children and adults will be included, provided that paediatric data are reported separately or that we will be able to access these data after requesting them from the investigators. We will only include observational studies that meet the primary criteria of the REal Life EVidence AssessmeNt Tool (RELEVANT) tool (see risk of bias). We will include studies published until May 2021 and reported in the English language.
The primary outcome measures will be (1) treatment success or treatment failure rate evaluated at any time point, within 2 weeks from presentation, (2) serious adverse events and (3) need for asthma related hospitalisation evaluated at any tim epoint within 2 weeks from presentation. Treatment success will be defined as a complete resolution of the symptoms, or an improvement in the clinical signs, symptoms and/or laboratory findings that fulfils specific criteria or thresholds prespecified by the study team. Treatment failure will be defined as a significant deterioration of the patients’ clinical conditions that fulfils specific criteria prespecified by the study team. For example, treatment failure may be defined as the need for paediatric intensive care unit admission, ventilation or death. The definitions of treatment success and treatment failure vary significantly across clinical studies evaluating the management of acute asthma in children; for this reason, meta-analyses will only be conducted in cases they are considered meaningful by the investigators. Need for asthma-related hospitalisation will not be relevant for studies only evaluating hospitalised participants. Secondary outcomes will include (1) mortality, (2) duration of asthma-related hospitalisation, (3) need for intensive care unit admission, (4) duration of intensive care unit stay, (5) re-exacerbation rate, (6) rehospitalisation rate and (7) adverse events. All outcomes will be evaluated at a maximum follow-up of 6 months, as longer-term outcomes are less likely to be directly linked with the index acute event.
Using appropriate controlled vocabulary and free search terms, we will systematically search Medline/PubMed, EMBASE and the Cochrane Library to identify controlled clinical trials and observational comparative effectiveness studies evaluating the safety, efficacy and/or clinical effectiveness of the selected medication (sample search strategies are available in the online appendix). We will also search the WHO International Clinical Trials Registry Platform search portal, the abstract proceedings of the European Respiratory Society, the American Thoracic Society, the Asian Pacific Society of Respirology, the European Academy of Allergy and Clinical Immunology, the American Academy of Allergy, Asthma and Immunology, and the World Allergy Organization, as well as the reference lists of all included studies. All sources will be searched from inception, without language limitations. We will follow standard methodology for screening titles, abstracts and the full text of all identified studies, as described previously.
The full study reference, study identifiers, details on the study design, eligibility criteria, predefined outcomes and potential confounding factors that were considered by the investigators, number and baseline characteristics of participants will be extracted by one investigator and will be cross-checked for validity by a second extractor. Details on the outcomes of interest from all included studies will be extracted by two investigators independently. Conflicts will be resolved through discussion and when needed adjudication by a third investigator.
Risk of bias of individual studies
We will use the second version of the Cochrane risk of bias (RoB2) tool for assessing risk of bias in the included RCTs 26 and the RELEVANT for assessing the risk of bias of observational studies. 27 Risk of bias of each included study will be evaluated by two investigators independently.
The RoB2 tool evaluates the following domains for potential risk of bias: (1) bias arising from the randomisation process, (2) bias due to deviations from intended interventions, (3) bias due to missing outcome data, (4) bias in measurement of the outcome, (5) bias in selection of reported results and (6) any other potential source of bias. High risk of bias in any of these domains will result in an overall judgement of high risk of bias. In the absence of high-risk domains, unclear risk in any domain will lead to an overall judgement of unclear risk. All remaining trials will be considered to be of low risk of bias.
RELEVANT evaluates the quality of observational comparative effectiveness research studies across seven domains, which include background, design, measures, analysis, results, discussion/interpretation and conflicts of interest. Each domain includes primary and secondary items. It is suggested that studies not meeting the primary items of RELEVANT are of very low methodological quality (have ‘fatal flaws’) and should not be used to inform clinical recommendations. Therefore, we will exclude studies not meeting these criteria. We will consider of low risk of bias all studies meeting the secondary criteria of RELEVANT as well, and of high risk of bias studies that do not meet any of the secondary criteria.
For every comparison, we will use funnel plots, Egger’s regression and Begg’s rank tests to evaluate publication bias, if we are able to pool more than 10 studies.
Data synthesis
Data from controlled clinical trials or observational studies will be analysed separately. In addition, studies evaluating different comparators, will be analysed separately. If different doses of the index medication or comparator are evaluated across the included studies, we will consider grouping studies using similar doses, providing that their results are not significantly dissimilar.
For every analysis, I 2 statistic will be used to assess statistical heterogeneity. Substantial heterogeneity (I 2 >50%) will be explored using prespecified subgroup analyses (details in the next section). We will not perform meta-analyses in cases of considerable unresolved heterogeneity (I 2 >75%).
When it is considered meaningful, meta-analyses will be performed using the random-effects model, because we anticipate significant heterogeneity in our data. Results will be presented in the form of relative risk (95% CI) for dichotomous data, mean difference (95% CI) for continuous data and (HR, 95% CI) for time to event data. Meta-analyses will be performed using Review Manager V.5 (RevMan, http://community.cochrane.org/tools/review-production-tools/revman-5 ) and R statistics V.3.4.3 or newer (R Foundation for Statistical Computing, Vienna, Austria).
For dichotomous outcomes, the unit of analysis will preferably be participants, rather than events (ie, number of participants admitted to the intensive care unit, rather than number of admissions per participants).
Sensitivity and subgroup analyses
In sensitivity analyses for all comparisons, we will (1) use fixed effects models, (2) only include studies with low risk of bias, (3) exclude studies reporting limited adherence to the study drugs (<80%) and (4) evaluate separately studies assessing different doses of the index medication, which we may pool in the main analysis.
Subgroup analyses according to participants’ age, asthma phenotypes or, possibly, acute attack phenotypes will also by conducted, depending on data availability. In an additional subgroup analysis, we will evaluate separately trials utilising exploratory versus pragmatic study designs.
Certainty of the body of evidence
Certainty of the body of evidence, for every comparison will be evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology. 28 GRADE assesses the certainty in a body of evidence as high, moderate, low or very low after considering the methodological quality of the included studies, imprecision, inconsistency, indirectness, publication bias, the magnitude of effect, dose response and confounders likely to minimise the effect. All decisions to upgrade or downgrade the quality of evidence will be transparent and justified in evidence profile and summary of evidence tables, in accordance with GRADE guidance. GRADEPro Software (2014; www.gradepro.org ) will be used for the development of these tables.
We will use GRADE methodology to assess the risk of bias associated with missing participant outcome data across the body of the available evidence. 29 GRADE suggests repeating the primary meta-analysis, imputing the most extreme assumptions about the values of the missing data, that the investigators consider plausible. Only if the analyses prove robust to this imputation, the risk of bias due to missing participant outcome data should be deemed low.
The impact that the risk of bias of individual studies and the confidence in the body of the evidence has on the results will be presented.
Ethics and dissemination
Ethical approval is not required for these SRs, since no primary data will be collected.
The findings of these evidence updates will be presented in national and international scientific conferences. They will also be submitted for publication in high-impact peer review journals. Plain English summaries of the final reports will be developed and shared with relevant patient organisations. Moreover, our results will be used to inform clinical recommendations that will be developed by the PeARL Think Tank. We anticipate that the overview of SRs will be completed by the end of 2021 and the remaining SRs by June 2022.
Patient and public involvement
The planned SRs were prioritised through a global, multi-stakeholder survey evaluating research priorities in childhood asthma, conducted by the PeARL Think Tank. 10 Among other stakeholders, this survey included responses from patients, patient caregivers and patient organisations. Moreover, two patient representatives (GDC and TAW) have joined the research group and provided input in this study protocol and they will also provide input throughout the study process.
We report on the methodology of a series of planned systematic evidence updates, aiming to evaluate maintenance management of childhood asthma, and the treatment of acute severe asthma attacks. Their design is informed by preliminary searches and the anticipated data availability. These SRs will be conducted by the PeARL group and will be used to inform clinical recommendations and future research needs. The need for high-quality evidence updates and clinical practice guidelines to improve the management of asthma in children is more urgent now, given the pressure that the unfolding COVID-19 pandemic pose on the healthcare systems, forcing us to reconsider our daily clinical practice. 30 31
Major strengths of our evidence update series are the inclusion of a wide evidence base, including data from RCTs and real-life comparative studies, the prospective design and strong methodology. The methodological quality of all available studies will be scrutinised and will aid the interpretation of our findings. Moreover, we will attempt to evaluate differential therapeutic response of different asthma phenotypes and age groups. We believe this analysis will be revealing, if adequate data is available, but may nevertheless reveal important gaps.
Guided by the available evidence, we will follow different strategies for the evidence updates on maintenance treatment of paediatric asthma and on management of acute severe asthma attacks. In view of the availability of ample published, up-to-date SRs on maintenance pharmacotherapy of childhood asthma, we chose to conduct an overview of SRs. We decided to focus on the most frequently used and thoroughly evaluated drug classes (ICS, LABA, LAMA, LTRA and biological therapies) and we expect to identify good quality data, which would inform clinical practice and research needs. Other, less frequently or experimentally used treatments will need to be evaluated in future studies. A potential limitation of this approach is that we might not be able to capture adequate data regarding the differential effectiveness of interventions across different severity groups, age groups or paediatric asthma phenotypes, if these have not been captured in existing SRs. Moreover, existing SRs may not capture some of the most recent studies, that may have been published after the SRs, although preliminary searches have revealed several very recently update meta-analyses.
The second SR, focusing on the management of acute attacks, will first evaluate a multitude of established and experimental treatments. With regard to the latter, this SR will reveal treatments that have been tested, appeared safe and efficacious and it may be worth to be further evaluated, but will also report on interventions that were tested, but did not appear efficacious, and therefore, further evaluation may not be beneficial. This wide approach would aid the prioritisation of interventions to be further validated in future clinical research studies.
Next, meta-analyses of individual pharmacological interventions will be conducted to further assess the safety and clinical effectiveness of treatments for acute severe asthma attacks that will appear efficacious in our broad SR. In contrast to most previous meta-analyses, that may have been conducted, we will include both controlled clinical trials and observational comparative effectiveness studies. Due to limitations that have already been discussed, few controlled clinical trials are conducted in children. This leads several Cochrane SRs to report low or very low confidence in the body of evidence, due to the lack of data. 32–35 We believe that by incorporating data from observational studies we may be able to conclude more robust results. While observational studies are at a higher risk of bias, we will carefully evaluate this risk using the newly developed, thorough RELEVANT tool and we will discuss potential implications on our findings. The GRADE working groups provides transparent guidance for assessing the certainty in a body of evidence including data from different study designs (controlled clinical trials or observational studies); this guidance will be used for interpreting the findings of our meta-analyes.
Overall, we aim to develop evidence updates on the maintenance treatment of asthma and management of acute severe asthma attacks that will cover all available evidence, carefully considering methodological limitations. These will be used by the PeARL Think Tank for the development of clinical recommendations and to guide future clinical research.
Ethics statements
Patient consent for publication.
Not required.
Acknowledgments
AGM was supported by the National Institute of Health Research Manchester Biomedical Research Centre (NIHR Manchester BRC). We thank Mrs Maria Kritikou for excellent administrative support of the study
- Stanojevic S ,
- Moores G , et al
- Global Initiative for Asthma
- British Thoracic Society
- Scottish Intercollegiate Guidelines Network
- Papadopoulos NG ,
- Čustović A ,
- Cabana MD , et al
- Arakawa H ,
- Carlsen K-H , et al
- Bacharier LB
- Krajinovic M ,
- Chauhan BF , et al
- Turner MA ,
- Catapano M ,
- Hirschfeld S
- Mathioudakis AG ,
- Custovic A ,
- Deschildre A , et al
- Miligkos M ,
- Papadopoulos NG
- Alimani GS ,
- Higgins JPT
- Liberati A ,
- Tetzlaff J , et al
- Thomson D ,
- Russell K ,
- Becker L , et al
- Dalziel SR ,
- Powell CV , et al
- Katsunuma T ,
- Fujisawa T ,
- Maekawa T , et al
- Alshehri M ,
- Almegamesi T ,
- Douglas LC ,
- Esteban-Cruciani N
- Mathioudakis A ,
- Chatzimavridou-Grigoriadou V ,
- Evangelopoulou E , et al
- Robroeks CMHHT ,
- van de Kant KDG ,
- van Vliet D , et al
- Tantichaiyakul P ,
- Preutthipan A
- Reeves BC ,
- Wells G , et al
- Pollock M ,
- Fernandes RM ,
- Sterne JAC ,
- Savović J ,
- Page MJ , et al
- Campbell JD ,
- Papadopoulos NG , et al
- Balshem H ,
- Helfand M ,
- Schünemann HJ , et al
- Guyatt GH ,
- Ebrahim S ,
- Alonso-Coello P , et al
- Custovic A , et al
- Chauhan BF ,
- Ducharme FM
- Normansell R ,
- Mathioudakis AG
- Stovold E , et al
- Knightly R ,
- Hughes R , et al
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
Twitter @mathioudakisag
AGM and MM contributed equally.
Contributors Study conception: AGM and NGP. Study design: AGM, MM and NGP. Preparation of the manuscript: AGM. Critical revision and final approval of the manuscript: AGM, MM, CB, GSA, AC, AD, FMD, OK, CM, ANG, WP, DP, AS, IOA, LB, AB, AB, MB, JAC-R, GDC, TC, ZD, WF, TF, JEG, JG, GH, EMH, DI, TJ, AK, RFL, PNLS, MJM, GAM, PM, MM, MM-A, KN, EP, HP, PMCP, PP, GR, IT, ST, VS, TAW, GWKW, PX, HJZ and NGP.
Funding This work was supported by the Respiratory Effectiveness Group (REG). REG has received support from AstraZeneca, Novartis and Sanofi for continued work on PeARL. (Award/Grant name: PeARL, Award/Grant Number: N/A). This is an investigator initiated study and the funders were not involved in the selection of the topic, or design of these systematic reviews. AGM was supported by the National Institute for Health Research Manchester Biomedical Research Centre (NIHR Manchester BRC).
Competing interests AGM reports grants from Boehringer Ingelheim outside the submitted work. AC reports personal fees from Novartis, Regeneron / Sanofi, Thermo Fisher Scientific, Boehringer Ingelheim and Philips, outside the submitted work. LB reports personal fees from Aerocrine, GlaxoSmithKline, Genentech/Novartis, Merck, DBV Technologies, Teva, Boehringer Ingelheim, AstraZeneca, WebMD/Medscape, Sanofi/Regeneron, Vectura and Circassia outside the submitted work. TC reports grants and personal fees CSL Behring, Dyax, Takeda, BioCryst, Pharming, personal fees from Grifols, grants and non-financial support from GSK, Regeneron, Novartis/Genetech outside the submitted work. AD reports grants and personal fees from Stallergenes Greer, personal fees from Novartis, ALK, TEVA, GSK, MEDA-MYLAN, CHIESI, AImmune, DBV technologies and Astra Zeneca, outside the submitted work. ZD reports personal fees from academic affiliations, ZD acts as Executive and Scientific Medical Director at a phase I/II pharmacological unit (QPS-NL), which performs clinical studies for pharmaceutical companies. ZD reports personal fees from Astrazeneca, ALK, Aquilon, Boehringer Ingelheim, CSL, HAL Allergy, MSD, and Sanofi-Genzyme outside the submitted work. FMD reports grants from Thorasys; personal fees from Jean-Coutu Pharmaceuticals, unrestricted research funds from Novartis Canada, Teva and Trudell Medical, research grants from GlaxoSmithKline and MEDteq in partnership with Thorasys; honorarium for consultancy work from Covis Pharma and Teva; and honorarium as invited speaker from Covis Pharma, Pharmacy Brunet, outside the submitted work. JEG reports grants from NIH/NIAID, personal fees from Regeneron, Ena Theraputics and MedImmune outside the submitted work; personal fees and stock options from Meissa Vaccines Inc outside the submitted work. JG reports personal fees from GSK, Vifor Pharmaceuticals, Novartis, BV Pharma and AstraZeneca outside the submitted work. AK reports personal fees Astra Zeneca, Behring, Boehringer Ingelheim, Covis, GSK, NovoNordisk, Novartis, Griffols, Pfizer, Sanofi, Teva and Trudel, outside the submitted work. RFL reports grants from NIH, non-financial support from GlaxoSmithKline, Boehringer-Ingelheim, Merck, TEVA, American Academy of Allergy, Asthma and Immunology, grants from Clinical and Translational Science Award (NIH), Childhood Origins of ASThma (COAST) grant, AsthmaNet, personal fees from LSU, Elsevier, UpToDate, the University of Kentucky, ThermoFischer, and Food Allergy Research and Education (FARE) Network, outside the submitted work. CM reports personal fees from Novartis, GSK, Astra Zeneca, Thermo Fisher and Boehringer Ingelheim outside the submitted work. NGP reports personal fees from ALK, Novartis, Nutricia, HAL, Menarini/FAES Farma, Sanofi, Mylan/MEDA, Biomay, AstraZeneca, GSK, MSD, ASIT BIOTECH and Boehringer Ingelheim; grants from Gerolymatos International SA and Capricare outside the submitted work. WP reports grants from NIH; grants and personal fees from Genentech/Novartis, Sanofi/Rgeneron; personal fees GSK; non-financial support from Thermo Fisher, Lincoln Diagnostics, Alk Abello, and Monaghen, outside the submitted work. PP reports grants from Astra Zeneca, Chiesi and TEVA; personal fees from Astra Zeneca, TEVA, Novartis, Mundipharma, S&D Pharma, and GlaxoSmithKline outside the submitted work. DP reports grants from AKL Research and Development, British Lung Foundation, Respiratory Effectiveness Group and the UK National Health Service; grants and personal fees from Boehringer Ingelheim, Chiesi, Circassia, Mylan, Mundipharma, Napp, Novartis, Pfizer, Regeneron Pharmaceuticals, Sanofi Genzyme, TEVA, Theravance and Zentiva (Sanofi Generics); personal fees from Cipla, GlaxoSmithKline, Kyorin and Merck; non-financial support from Efficacy and Mechanism Evaluation programme, Health Technology Assessment, outside the submitted work; DP also reports stock/stock options from AKL Research and Development which produces phytopharmaceuticals; and owns 74% of the social enterprise Optimum Patient Care (Australia and UK) and 74% of Observational and Pragmatic Research Institute (Singapore), outside the submitted work. GR reports personal fees from ALK, Allergen Therapeutics, Meda Plus, Merck; and a patent for the use of sublingual immunotherapy to prevent the development of allergy in at-risk infants, outside the submitted work. IT reports personal fees from Novartis, GSK, Boehringer Ingelheim and Astra Zeneca; grants from GSK Hellas, outside the submitted work. PX reports personal fees from Nutricia, Nestle, Friesland, Uriach, Novartis Pharma AG, and GlaxoSmithkline outside the submitted work.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 09 September 2021
Child and caregiver experiences and perceptions of asthma self-management
- Lauren Kelada ORCID: orcid.org/0000-0001-9428-8807 1 , 2 ,
- Charlotte J. Molloy 3 , 4 , 5 ,
- Peter Hibbert 3 , 4 , 5 ,
- Louise K. Wiles 3 , 4 , 5 ,
- Claire Gardner 4 , 6 ,
- Emily Klineberg 7 ,
- Jeffrey Braithwaite ORCID: orcid.org/0000-0003-0296-4957 3 &
- Adam Jaffe 1 , 8 , 9
npj Primary Care Respiratory Medicine volume 31 , Article number: 42 ( 2021 ) Cite this article
4552 Accesses
11 Citations
Metrics details
- Paediatric research
- Patient education
Asthma is the most common chronic condition of childhood. Self-management is integral to good asthma control. This qualitative paper explores how children with asthma and their parents perceive asthma, their experience with asthma, and how they manage symptoms, preventions and medications within and outside the home. We undertook 15 focus groups with 41 school-aged (6–11 years) children with asthma and 38 parents. Parents and their children attended the same focus groups. We used thematic analysis to analyse the transcripts. Our findings show the impact asthma can have on children’s social and emotional wellbeing and highlight how reliant school-aged children are on their parents to effectively manage their asthma. Parents reported being unsure when their child’s symptoms warranted visiting their doctor or hospital. Schools were identified as a source of difficulty regarding asthma management; families reported that children may be self-conscious about their asthma and using their inhaler at school. School policies and teachers’ lack of asthma knowledge were reported to exacerbate children’s reluctance to use their inhaler at school. Our results have implications for the design and implementation of children’s self-management interventions for their asthma, particularly when they are at school and away from their parents.
Similar content being viewed by others
School-based self-management interventions for asthma among primary school children: a systematic review

Perceptions of childhood asthma and its control among Malays in Malaysia: a qualitative study
A cross-country qualitative analysis of teachers’ perceptions of asthma care in sub-Saharan Africa
Introduction.
Asthma is the most common chronic condition of childhood with approximately 14% of children worldwide experiencing asthma symptoms 1 . In Australia, 1 in 10 children under the age of 15 years has asthma, with the highest prevalence in those aged 5–9 years (13%) 2 . Poorly controlled asthma is commonly observed 3 , related to poorer quality of life among children, and can impose significant burden on families and the health-care system 4 . Children with asthma access health-care services more frequently (416 hospitalisations per 100,000 5 ), experience increased school absenteeism 6 , have sleep disturbance 7 and restriction to everyday activity compared to children without asthma 8 . Caregivers of children with asthma also experience lower quality of life and higher workplace absenteeism than caregivers of children without asthma 6 .
Self-management is integral to good asthma control. Asthma clinical guidelines in Australia and internationally advocate for the inclusion of routine self-management education for patients with asthma 9 , 10 , 11 , 12 . Self-management programmes for children with asthma have important health benefits, including improved lung function, decreased morbidity, fewer days absent from school and reduced visits to emergency departments 13 , 14 . Emerging evidence also shows that apps and other digital media may be effective tools to facilitate asthma self-management, particularly among adolescents and adults 15 , 16 , 17 , 18 . However, further research is needed to assess the appropriateness and desired content of apps for children with asthma and their parents 19 .
Responsibility for management of a child’s asthma predominantly lies with the caregivers. However, it is important for children to begin to learn greater self-management as they commence school and spend increasing amounts of time away from direct parental care 20 , 21 , 22 . This transfer of responsibility for asthma management is likely to align with Piaget’s theory of cognitive development. As children move from the preoperational stage (2–7 years) to the concrete operational stage (7–11 years) of development, they begin to develop logical thinking skills which are necessary for following an asthma management plan 23 . However, school-aged children still lack the abstract thinking and planning skills required for the complex series of decision-making involved in autonomous asthma self-management. Therefore, to understand asthma management among children of this age group, the perspectives of both children and parents should be included.
It is important to understand families’ lived experiences of asthma management, both within and outside the home, given their central role in reducing asthma symptoms, as well as the substantial burden of disease imposed by childhood asthma. The aim of this study was to explore the experiences and perspectives of asthma and current and desired asthma self-management strategies of both children and caregivers, to enable identification of potential areas for improvement 24 .
The current study was part of a larger project to develop an app to help families manage child asthma. The current study focusses on data gathered from focus groups to explore the experiences and perspectives of children with asthma and their caregivers. The study design was qualitative. The consolidated criteria for reporting qualitative research (COREQ) checklist is shown in Supplementary File 1 . This research protocol was approved by the University of New South Wales Human Research Ethics Committee (no: HC15733), with recruitment through schools approved by NSW Department of Education (no. 16/890151), Queensland Department of Education and Training (no: 550/27/1745), and South Australian Department of Education and Child Development (no: DECD CS/16/00066-1.4).
Participants and recruitment
Recruitment of participants (children and their parents) occurred in 2017 across four Australian states: New South Wales (NSW), Queensland (QLD), South Australia (SA) and Victoria (VIC) via e-newsletter circulations, and online or physical noticeboard advertisements through Asthma Australia (the peak national consumer body), schools and Facebook. Inclusion criteria for children–caregiver pairs were children with a medical diagnosis of asthma, aged 6–11 years, proficiency in speaking and understanding English, and without behavioural or intellectual disabilities that might preclude their ability to participate in group discussions or use. Interested families contacted one of the researchers (C.G.) via email and were screened for eligibility. Families were then provided (via email) with a study information sheet and consent form. Signed informed consent was obtained from caregivers prior to partaking in the study, with oral consent obtained from children prior to beginning the focus groups. Participants received an AUD$50 gift voucher as reimbursement for their time at the end of their focus group session.
Study procedures and data collection
Child–caregiver pairs were divided into two groups according to the child’s age—Group A: 6–8 years and Group B: 9–11 years. Research has shown that for children age groupings are a crucial factor in group dynamics and discussion, with a 1–2 year age difference optimal, due to substantial differences by age in style, ability, and level of comprehension 25 . Focus groups comprised no more than six children in the same age group and took place at metropolitan research institutions in participants’ state of residence; no one other than participants and researchers were present.
The focus group incorporated two sessions: an initial group discussion immediately followed by hands-on user testing of asthma self-management apps; each session lasted for a maximum of 60 min (120 min in total). All sessions were audiotaped and transcribed verbatim for later analysis. Field notes were made during and after each Focus Group, with researchers (C.G. and C.J.M.) reaching consensus on the main points and data saturation. This paper reports on the findings of the initial group discussion.
Group discussions were semi-structured and led by a professional focus group facilitator and at least one member of the research team (C.G. and/or C.J.M.) (all females). In total, two professional facilitators were involved in the focus groups (one per session) and each held training in psychology and over 7 years’ experience in social and qualitative research and conducting focus groups with children. One research team member, the professional facilitator, and children with asthma and their caregivers were present at each focus group.
The group discussions were split into four sections: (1) asthma perceptions and feelings; (2) current experience of self-managing asthma; (3) desired asthma self-management strategies; and (4) self-management technology and asthma. To maximally engage the children multiple discussion formats were utilised. Sections one and two were undertaken in a group discussion and small task format, with children and their caregivers together. In sections three and four, caregivers and children were split into separate groups. Caregivers continued in a group discussion format while children completed a drawing or collage activity to design an asthma management “machine” using the prompt “create a machine that takes you from your asthma feeling bad to feeling good”. Drawing and collage modalities were utilised because they can help depict thoughts that are difficult to communicate verbally, especially for children 26 , 27 . These machines were used to prompt discussion rather than being analysed independently.
Focus group question and guides were developed by the authors and facilitators from a literature review and discussions with colleagues with qualitative, behavioural and/or clinical research expertise (Supplementary File 3 ). We used interview schedules drawn from Brown et al. 28 , Laster et al. 29 and Shaw et al. 30 as our key guides. There were also two pilot sessions conducted as per the described methods, with refinements to the focus group guides following each session.
Demographic information was collected via an online caregiver survey, including age, gender, ethnicity (Aboriginal or Torres Strait Islander), asthma profile (including year of diagnosis, diagnosis health-care professional, asthma care plan, number of general practitioner (GP) consults/emergency department (ED) hospital visits/hospital admissions in last 12 months related to asthma, and app usage (general/health or medical/asthma)).
We used thematic analysis of the transcripts to identify meaningful themes from the focus groups. We followed Braun and Clarke’s 31 six-phase method of thematic analysis, using an iterative approach to analysis. We used NVivo Version 12 to code the focus group data. The coding tree is provided in Supplementary File 2 .
Two researchers (L.K. and C.G.) separately read each interview transcript twice and created codes to indicate meaningful passages. Inter-rater reliability ( К = 0.86) between the coders was high 32 and we resolved discrepancies through discussion. The codes were discussed with a third researcher (L.M.) while referring to the original focus groups to ensure accuracy. The first author (L.K.) organised and interpreted the codes into overarching themes which accurately and meaningfully captured the original data, noting convergence and divergence across transcripts. L.K. then reread the focus group transcripts and discussed the themes with CG to finalise the analysis. Children’s machine creations are presented with each theme to help reflect that theme in the children’s words. Quotations (with participant IDs) are presented in-text to represent the themes.
Participants
Over March and April 2017, 41 children and 38 caregivers participated in the focus groups across four Australian states (New South Wales [NSW], Queensland [QLD], South Australia [SA] and Victoria [VIC]), and caregiver surveys were completed for 37 children (Table 1 ). Focus groups ranged from 1 to 6 children per session (M = 2.60; SD = 1.3). An additional 22 families (36.7%; caregiver and child pairs) who had expressed interest in the study but had not yet returned the consent form were lost to follow-up prior to attending the focus groups. Reasons provided included illness, childcare arrangements (for other siblings) and other commitments (e.g., work). Children participating in the study were aged between 6 and 11 years (M = 8.30, SD = 1.6) and 51% were females. All caregivers who participated in the focus groups were females.
Asthma profile
According to the caregiver surveys, none of the participants were newly diagnosed or new to managing asthma, with over half of the participants ( n = 21, 57%) having been diagnosed with asthma for at least 6 years (Table 2 ). Most children (54%) had their asthma diagnosed by a GP, and 95% had a written asthma action plan (Table 2 ). Regarding health service utilisation, over half of children (52%) had more than four GP consults for asthma in the past 12 months, 39% had at least one emergency department presentation for their asthma in the past 12 months and one in six (16%) had an inpatient admission for their asthma in the past 12 months. Approximately three-quarters of children (76%) had used mobile phone apps (with or without parental support); however, less than 1 in 10 had ever used health/medical apps (8%) or those that were asthma specific (5%).
We identified three themes from the data: (1) fear, sadness and frustration associated with asthma; (2) parental responsibility for proactively monitoring triggers, symptoms and medication; and (3) managing asthma at school requiring child communication about symptoms.
Fear, sadness and frustration associated with asthma
Children used positive adjectives to describe how they felt in the absence of their asthma symptoms. Children reported feeling happy, calm, excited, playful and energetic. When they were experiencing asthma symptoms, children reported feeling frustrated and sad, predominately due to missing out on opportunities to play with their friends (Box 1 , Quote 1). One child described feeling frustrated when his symptoms would occur unexpectedly and was left lamenting why he even had asthma at all (Box 1 , Quote 2). Children and parents also reported that the lack of control over asthma symptoms made them feel scared and worried (Box 1 , Quotes 3 and 4).
Children’s machine creations
During the discussions of their machine creations, children expressed that they experienced negative emotions when their asthma symptoms worsened. Children described how they designed their machine creations to reduce their fear and make them happy and laugh. Safety was an important element of children’s creations to making them feel better. For some children, safety involved being removed from asthma triggers (Box 1 , Quote 5). For other children, safety was achieved when their machines would notify parents or doctors about a flare-up of symptoms (Box 1 , Quote 6).
Box 1 Fear, sadness and frustration associated with asthma
Quote 1 : “I feel playful because I’m not sick and I have lots of energy and I feel happy… I feel excited because it’s a better time instead of being all bad and have to sit down instead of playing.” (Child, focus group 7, ages 6–8 years)
Quote 2 : “I just sometimes don’t even know why it happens and I’m like what the heck? What the heck? Why is this happening? Why me?” (Child, focus group 15, ages 6–8 years)
Quote 3 : “When I was in hospital they got a lot of different things I didn’t know and so I just felt scared and frustrated because you keep coughing and it really hurts and you don’t know what to do about it.” (Child, focus group 10, ages 9–11 years)
Quote 4 : “You feel a bit worried that you’re going to get so sick and it is pretty hard to control it even when you have your puffer so you get a bit scared that you are not going to get over it.” (Child, focus group 13, ages 6–8 years)
Quotations from children’s machine creations
Quote 5 : “ Child : That’s a magical bird and that is my human there … and it has asthma because it’s afraid of the [dusty] place and then the bird come and then take it away into the nature place.
Interviewer: And is that nature place not dusty?
Child: Yeah, not dusty.” (Child, machine creations group 1, ages 6–8 years)
Quote 6 : “Child: The cheetah would get you up and run around with you and it can take you to the doctors really quickly.” (Child, machine creations group 13, ages 6–8 years)
Parental responsibility for proactively monitoring triggers, symptoms and medication
Children in the focus groups generally discussed their reactive management of asthma and what they do when their symptoms emerge. Parents, however, were more proactive and discussed preventative behaviours. Accordingly, parents were largely responsible for monitoring triggers, asthma symptoms and medication use. One challenge to children’s preventative medication usage, as described by parents, is that children often did not understand why they had to take their medication when they were not experiencing asthma symptoms (Box 2 , Quote 1).
Children were generally aware of potential triggers for their asthma and the important preventative behaviours they needed to enact, including avoiding dusty places, avoiding allergens, keeping warm, and resting. However, children sometimes reported that they did not adhere to these preventative behaviours unless specifically reminded by their parents. This was true for children of all.
For parents, monitoring their child’s exposure to triggering situations was a challenge (Box 2 , Quote 2).
Similarly, children of all ages were reliant on their parents to monitor symptoms and medication. While children were aware they needed to take their medication, they were generally unaware about the specifics such as dosage and frequency of medications (Box 2 , Quote 3).
During the focus groups, parents and children indicated that children were not yet at a maturity level to be responsible for their own asthma and so parents inhabited this role. Several families indicated that older children were in the process of taking more responsibility for their asthma, particularly for when the child was staying with friends. Yet these children were still reliant on their parents to monitor their asthma at home (Box 2 , Quote 4).
Parents reported various behaviours to monitor their child’s asthma, including mentally tracking symptoms and medication use, writing down symptoms and medication use, and checking the weather forecast to prepare for an asthma attack (Box 2 , Quote 5 and 6). Parents suggested that apps could help them to track their child’s symptoms and medications. Parents emphasised the shared use of apps with their children, and that the purpose of apps should be to better facilitate information sharing between parents and children. Parents also suggested that apps could help their children gain the skills to slowly become more independent in their asthma self-management.
Parents were solely responsible for deciding whether their child’s symptoms were severe enough to warrant seeing a GP or to go to the hospital. Parents typically found this a difficult task and commonly reported feeling unsure about when to escalate their child’s care (Box 2 , Quote 7).
Children explained that their machines creations would sense when they needed their medication and dispense the appropriate dosage (Box 2 , Quote 8). In this sense, children created machines which would take over the responsibility of monitoring their symptoms and medication use. Important to the children, the machine would make this process fun and enjoyable (Box 2 , Quote 9).
Box 2 Parental responsibility for monitoring triggers, symptoms and medication
Quote 1: “When they don’t have asthma it’s like, ‘Why am I doing this?’ And that’s what [my daughter] said the last few days, ‘I don’t feel wheezy.’ And it’s like, ‘We’ve talk about it.’…she knows but at this age it’s, to have to, it’s like ‘take medicine when you feel okay’.
Q: It’s tricky.
F: It’s hard.” (Mother, focus group 14, ages 9–11 years)
Quote 2: “ F : I think controlling outside factors—I like to have the house clean, but my Mother-in-Law doesn’t so going to my Mother-in-Law’s house is stressful for me because I know she’s highly allergic to dust.” (Mother, focus group 1, ages 6–8 years).
Quote 3: “Q: Do you track or record how your asthma’s going at all?
Q: Mum does that?
C: Yeah” (Child, focus group 12, ages 9–11 years)
Quote 4: “When she’s out with her friends … it’s up to her to take that responsibility to use [her inhaler].” (Mother, focus group 12, ages 9–11 years)
Quote 5: “I sometimes make notes on my phone of how often I am giving Ventolin so if we end up in emergency I can say I’ve done this this this and this.” (Mother, focus group 11, ages 6–8 years)
Quote 6: “It depends on how bad it is. Sometimes I do write [symptoms and medications] down so that when we go to the doctor I can tell him exactly what I’ve done when and what time and how bad it was. And sometimes I’ve even recorded his breathing for the doctor so the doctor can hear it.” (Mother, focus group 4, ages 6–8 years)
Quote 7: “In the past she’s just gone downhill very quickly, it’s hard because you don’t want to be every time she gets asthma go to the doctor or hospital or whatever, but at the same time knowing what’s happened previously it’s always in the back of your head what could happen … [do you] just stay home and monitor or do you go to the doctor early and try to nip it in the bud before it exacerbates?” (Mother, focus group 3, ages 6–8 years)
Quote 8: “Child: I have a backpack that [delivers] the medicine…and never stop until they’re better.” (Child, machine creations group 9, ages 6–8 years)
Quote 9: “Child: If you push green [button] the icing cake comes around, if you push the yellow [button] then the magical Maltesers come out…
It would give you normal Ventolin but in a very fun way.” (Child, machine creations group 2, ages 9–11 years)
Managing asthma at school requires child communication about symptoms
Most parents reported that they became aware of flare-ups via observing symptoms in their child, including coughing, wheezing, shortness of breath and low energy. This, however, was sometimes a problem when children were at school and therefore parents could not monitor their child’s symptoms. Only one parent said that their child would actively and consistently communicate their asthma flare-up to their parents and teachers. For the rest of the families, children commonly waited to communicate their symptoms which one parent described as putting them on the “back foot” (Box 3 , Quote 1).
A common issue was that school policy often dictated that inhalers were to be kept locked in the office. Parents were concerned that their child often waited too long to ask for it (Box 3 , Quote 2).
Children rarely reported that their response to experiencing physical asthma symptoms was to tell a teacher. One parent speculated that her daughter was too embarrassed to tell a teacher about an asthma flare-up and did not want to miss out on school activities (Box 3 , Quote 3).
Parents were largely responsible for communicating their children’s asthma needs to their school (Box 3 , Quote 4). Parents described mixed experiences with schools and great variably in response to their child’s asthma between teachers and between schools (Box 3 , Quote 4).
Children described creating machines which would alert their parents when they were beginning to experience symptoms of an asthma flare-up while at school (Box 2 , Quote 5). Children reported that their machine creations may alert their parents or doctors, but no children reported their machine would alert a teacher. This reflects parents’ speculation in the focus groups that children were reluctant to alert their teachers to an asthma flare-up.
Box 3 Managing asthma at school requiring child communication about symptoms
Quote 1: “She doesn’t tell me, not until it’s like beyond - when she should have had a puffer 10 minutes ago she doesn’t say anything until it’s really bad and then you are on back foot trying to help.” (Mother, focus group 11, ages 6–8 years)
Quote 2: “I can tell when he’s got asthma and I give him some Ventolin – he won’t get ask for it. Once I picked him up from school and said ‘how was he?’, ‘Oh fine’ and I took him in a for a lung function and it was 76% and he was crook – terrible” (Mother, focus group 11, ages 6–8 years)
Quote 3: “I think it’s a bit of an embarrassment for [my daughter]. She won’t tell the teacher that her asthma’s bad because I think she thinks that she’s going to miss out on something so …I go in and tell [the teacher].” (Mother, focus group 12, ages 9–11)
Quote 4: “Some people get it, other people don’t, so, trying to get through to them to say, ‘look, he can look really, really well one minute but if he says he needs to go to the office, he needs to go. You are not to keep him in class and just see whether he’s trying to get out of class…it’s from teacher to teacher because I still think some people don’t get it.” (Mother focus group 15, ages 6–8 years)
Quote 5: “The reason why those feathers are there, so then it would – so if I needed extreme help my Mum could - say I was on an excursion and she didn’t know where about’s … she didn’t know if they were on the beach or in the park or something, so then that will connect and it would fly to where I would be and then it would mean that I would be okay because my Mum will be with me.” (Child, machine creation group 1, ages 6–8 years)
This paper examined how school-aged children with asthma and their parents perceive and experience their asthma, and how they manage symptoms, prevention and medications both within and outside the home. We identified the impact asthma can have on children’s social and emotional wellbeing and highlighted how reliant school-aged children are on their parents to effectively manage their asthma. Our results have implications for children’s self-management of their asthma, particularly when they are at school and away from their parents.
Both parents and children of all ages indicated that they were not ready for children to independently self-manage their asthma medications and symptoms. Consistent with previous research, this was particularly the case during acute episodes, when parents were solely responsible for decision-making during acute episodes including whether to escalate care to a health professional 33 . However, families of older children reported that children were in the process of taking more responsibility for their asthma management, which was not reported for the younger children and is consistent with previous research 22 .
These findings echo previous research that found children with chronic illness may lack the cognitive, physical and psychosocial abilities to proactively and autonomously manage their medications 34 . According to Piaget’s Concrete Operational Stage of Cognitive Development, children aged approximately 7–11 years are logical thinkers and have not yet developed the ability to think abstractly and hypothetically 23 . These developmental profiles may explain why children in our study were reactive in managing their asthma, meaning they would begin management once their symptoms appeared. They struggled to understand why it was necessary to take their asthma medication when they were feeling well and were not experiencing active symptoms. Parents, however, used a proactive approach to their child’s asthma management to help prevent symptoms, and were largely responsible for monitoring triggers and medications. Clinical and self-management strategies should consider the complementary and shared roles children and their parents play in managing paediatric asthma, as well as different developmental trajectories across children’s age ranges. Services to assess the self-management knowledge and skills of children and their parents, and provide education and tools to help address identified gaps, are needed.
Despite being solely responsible for escalating their child’s care, parents commonly reported feeling unsure about when their child’s asthma symptoms warranted a visit to the doctor or hospital. These findings support training for parents to be able to detect the early signs of an asthma attack. Nurses and other health professionals are ideally placed to help educate parents about how to recognise worsening asthma symptoms 35 , 36 , 37 , 38 .
Some parents in our study wanted to empower their children to be part of the decision-making process for asthma management. Parents reported that interventions such as phone applications (apps) could have the potential to facilitate information sharing between parents and children and teach children important self-management skills. Apps can support asthma self-management and supplement existing clinical care through real-time monitoring, facilitating information sharing and addressing barriers to self-management (such as forgetting medication) 15 , 16 , 18 . Early evidence shows that apps that assist children to self-manage their asthma can result in improved outcomes such as reduced hospital admissions and missed school attendance 39 . Given the ubiquitousness of mobile usage generally in our sample, with 76% of children having used an app of some description, further studies outlining what works, particularly focussed on children’s developmental cognitive stages and reactive approach to management is warranted.
As well as the practical aspects of asthma management, children were also reliant on their parents for management of their asthma. Children reported negative emotional reactions to their asthma, including sadness, frustration and fear, in line with previous reporting 40 . Consistent with prior research, these reactions were particularly strong when their asthma prevented them from participating in activities with their peers 41 , 42 . Children were also scared by the uncontrollable nature of their symptoms, and in discussions about their machine creations they described the importance of seeking safety from their asthma symptoms by alerting their parents. In confirmation of past research, children with chronic illness soothe their fear by seeking comfort from their parents, particularly their mothers 43 , 44 . Peer interventions could be employed to alleviate some of the negative emotional responses children associate with their asthma, including sadness and frustration.
The challenges of managing children’s asthma at school were also another theme of our findings. Children’s avoidance of appearing different, and lack of understanding by teachers may cause children to avoid taking their medication at school. According to parents, schools would commonly keep children’s asthma inhalers locked in an office. Parents speculated that their children would avoid asking for their asthma medication when they were at school to avoid feeling different. Previous research also shows that children with asthma may ignore early symptoms of an asthma flare-up and avoid taking their asthma medication to prevent feeling “different” to their peers 45 , 46 . Children’s lack of access to their inhalers while at school has commonly been identified as a barrier to appropriate use of their inhaler 46 , 47 , 48 , 49 . Parents also reported that teachers were not always knowledgeable about asthma and their child’s asthma management plan, in agreement with previous research 45 , 46 , 51 Building on existing literature 22 , 51 , 52 , 53 , 54 , future research may seek to more fully assess the perspectives of key stakeholders such as governing councils, school nurses and teachers. This research is needed to design and test solutions, particularly regarding access to children’s inhalers. Interventions to improve children’s self-management of their asthma at school may also aim to reduce the social stigma associated with using inhalers 50 .
A major strength of our study was the inclusion of both parent and child perspectives in the focus groups. Discrepancies have previously been reported between parent-reports and child-reports of child and family health 55 , 56 , so it is important to gain multiple perspectives. The use of the machine creations task allowed us to further facilitate discussion with the children about their perspectives on their asthma management. Some of these perspectives, such as children only alerting parents or doctors and never teachers during an asthma flare-up, were only derived from the discussions around the machine creations task. This may be perceived as a strength of the study as the machine creations gained children’s unique perspectives, or a weakness in that these perspectives were not triangulated with other data sources. Future exploration of the feasibility, acceptability and credibility of this data collection method may be warranted. As to limitations, families who were functioning well may have been more inclined to opt-in to our research. Indeed, 95% of the children in our study had a written asthma management plan, which is unlikely to be representative of the broader population. Given that family functioning is related to children’s asthma management 57 , 58 , this selection bias may have skewed our results and been amplified by the rate of participants who were lost to follow-up. Alternatively, our recruitment e-newsletter and physical notice boards mentioned the importance of good asthma management, and therefore parents who were in some way struggling with their child’s asthma management may have been more inclined to self-select into the study. In addition, no fathers participated in the focus groups, though this may reflect the often gendered approach to asthma care within families; mothers are typically more involved in their child’s asthma management than fathers 59 . Aboriginal and Torres Strait Islander families were underrepresented in the study, with only one participating, and information was not collected regarding other culturally and linguistically diverse (CaLD) backgrounds, meaning we are unable to observe how this may have influenced responses. We also did not gather data regarding socioeconomic status, family income or parent marital status. These factors have all previously been found to influence children’s asthma management, including structural barriers to implementing guideline-based care 57 , 60 , 61 .
This study shows that parents are largely responsible for the practical management of their school-aged children’s asthma at home, particularly during acute attacks. Services to educate parents on how to recognise symptoms of an attack could be beneficial. More research on phone apps that assist children’s self-management of asthma, or joint decision-making with parents, is warranted. These should be designed with children’s cognitive development stages in mind. Parents also provide the primary emotional support to children who may be self-conscious about their asthma and medication and appearing “different” to their peers. Outside of the home, schools can be a source of ongoing difficulties regarding managing their child’s asthma. School policies and teachers’ lack of understanding of asthma can exacerbate children’s reluctance to use their inhaler at school. Greater understanding of safe ways of children accessing their inhalers at schools is needed.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The focus groups guides are provided in Supplementary File C. The interview transcripts are not available due to ethics requirements and potential to identify participants.
Global Asthma Network. The Global Asthma Report 2014 (Global Asthma Network (Gan) Steering Group, 2014).
Australian Institute of Health and Welfare. Australia’s Children . Cat. No. Cws 69 (AIHW, 2020).
Tosca, M. A., Marseglia, G. L. & Ciprandi, G. The Real-World “Control’asma” Study: a nationwide taskforce on asthma control in children and adolescents. Allergol. Immunopathol. 49 , 32–39 (2021).
Article Google Scholar
Bellin, M. H. et al. Stress and quality of life in urban caregivers of children with poorly controlled asthma: a longitudinal analysis. J. Pediatr. Health Care 29 , 536–546 (2015).
Article PubMed PubMed Central Google Scholar
Australian Institue of Health and Welfare. Asthma (AIHW, 2019).
Sullivan, P. et al. School absence and productivity outcomes associated with childhood asthma in the USA. J. Asthma 55 , 161–168 (2018).
Article PubMed Google Scholar
Urrutia-Pereira, M. et al. Sleep disorders in Latin-American children with asthma and/or allergic rhinitis and normal controls. Allergol. Immunopathol. 45 , 145–151 (2017).
Article CAS Google Scholar
Lam, K.-M. et al. Physical activity in school-aged children with asthma in an urban city of Taiwan. Pediatr. Neonatol. 57 , 333–337 (2016).
National Asthma Council. Australian Asthma Handbook. https://www.asthmahandbook.org.au/ (2019).
British Thoracic Society & Scottish Intercollegiate Guidelines Network. British Guideline on the Management of Asthma: A National Clinical Guideline (British Thoracic Society, Scottish Intercollegiate Guidelines Network, 2016).
Global Initiative For Asthma. Global Strategy for Asthma Management and Prevention (Global Initiative For Asthma, 2020).
National Heart Lung and Blood Institute. Asthma Care Diagnosing and Managing Asthma (US Department of Health and Human Services, 2012).
Wolf, F., Guevara, J. P., Grum, C. M., Clark, N. M. & Cates, C. J. Educational interventions for asthma in children. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.Cd000326 (2002).
Guarnaccia, S. et al. Evaluation of a diagnostic therapeutic educational pathway for asthma management in children and adolescents. Front. Pediatr. 8 , 39 (2020).
Ramsey, R. R. et al. A systematic evaluation of asthma management apps examining behavior change techniques. J. Allergy Clin. Immunology Pract. 7 , 2583–2591 (2019).
Davis, S. R. et al. “Kiss Myasthma”: using a participatory design approach to develop a self-management app with young people with asthma. J. Asthma 55 , 1018–1027 (2018).
Article PubMed CAS Google Scholar
Peters, D. et al. Young people’s preferences for an asthma self-management app highlight psychological needs: a participatory study. J. Med. Internet Res. 19 , E113 (2017).
Ramsey, R. R. et al. Systematic review of digital interventions for pediatric asthma management. J Allergy Clin Immunol. Pract. 8 , 1284–1293 (2020).
Iio, M. et al. Beneficial features of a mhealth asthma app for children and caregivers: qualitative study. JMIR Mhealth Uhealth 8 , E18506 (2020).
Brown, N., Gallagher, R., Fowler, C. & Wales, S. The role of parents in managing asthma in middle childhood: an important consideration in chronic care. Collegian 17 , 71–76 (2010).
Mcclure, N. et al. Improving asthma management in the elementary school setting: an education and self-management pilot project. J. Pediatr. Nurs. 42 , 16–20 (2018).
Rehman, N., Morais-Almeida, M. & Wu, A. C. Asthma across childhood: improving adherence to asthma management from early childhood to adolescence. J. Allergy Clin. Immunol. Pract. 8 , 1802–1807.E1801 (2020).
Piaget, J. Child’s Conception of Number: Selected Works Vol. 2 (Routledge, 1952/2013).
Editorial: Deeper understanding. Lancet Psychiatry 6 , 713, https://doi.org/10.1016/S2215-0366(19)30304-9 (2019).
Kennedy, C., Kools, S. & Krueger, R. Methodological considerations in children’s focus groups. Nurs. Res. 50 , 184–187 (2001).
Fargas-Malet, M., Mcsherry, D., Larkin, E. & Robinson, C. Research with children: methodological issues and innovative techniques. J. Early Child. Res. 8 , 175–192 (2010).
Morgan, M., Gibbs, S., Maxwell, K. & Britten, N. Hearing children’s voices: methodological issues in conducting focus groups with children aged 7-11 years. Qual. Res. 2 , 5–20 (2002).
Brown, W. III, Yen, P. Y., Rojas, M. & Schnall, R. Assessment of the Health IT Usability Evaluation Model (Health-Ituem) for evaluating mobile health (Mhealth) technology. J. Biomed. Inf. 46 , 1080–1087 (2013).
Laster, N., Holsey, C. N., Shendell, D. G., Mccarty, F. A. & Celano, M. Barriers to asthma management among urban families: caregiver and child perspectives. J. Asthma 46 , 731–739 (2009).
Shaw, A., Thompson, E. A. & Sharp, D. Complementary therapy use by patients and parents of children with asthma and the implications for nhs care: a qualitative study. BMC Health Serv. Res. 6 , 76 (2006).
Braun, V. & Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 3 , 77–101 (2006).
Landis, J. R. & Koch, G. G. The measurement of observer agreement for categorical data. Biometrics 33 , 159–174 (1977).
Garnett, V., Smith, J. & Ormandy, P. Child-parent shared decision making about asthma management. Nurs. Child. Young People 28 , 16–22 (2016).
Beacham, B. L. & Deatrick, J. A. Health care autonomy in children with chronic conditions: implications for self-care and family management. Nurs. Clin. 48 , 305–317 (2013).
Google Scholar
Still, L. & Dolen, W. K. The perception of asthma severity in children. Curr. Allergy Asthma Rep. 16 , 50 (2016).
National Asthma Council Australia. Australian Asthma Handbook , Version 2.0 (National Asthma Council Australia, 2019).
Yeung, T. Y. et al. Home management of childhood asthma exacerbations. Pulm. Ther. 4 , 149–157 (2018).
Prather, S. L., Foronda, C. L., Kelley, C. N., Nadeau, C. & Prather, K . Barriers and facilitators of asthma management as experienced by African American caregivers of children with asthma: an integrative review. J. Pediatr. Nurs. 55 , 40–74 (2020).
Nkoy, F. L. et al. Ambulatory management of childhood asthma using a novel self-management application. Pediatrics 143 , E20181711 (2019).
Conn, K. M., Fisher, S. G. & Rhee, H. Parent and child independent report of emotional responses to asthma-specific vignettes: the relationship between emotional states, self-management behaviors, and symptoms. J. Pediatr. Nurs. 31 , E83–E90 (2016).
Lambert, V. & Keogh, D. Striving to live a normal life: a review of children and young people’s experience of feeling different when living with a long term condition. J. Pediatr. Nurs. 30 , 63–77 (2015).
Stewart, M., Masuda, J. R., Letourneau, N., Anderson, S. & Mcghan, S. “I want to meet other kids like me”: support needs of children with asthma and allergies. Issues Compr. Pediatr. Nurs. 34 , 62–78 (2011).
Ångström-Brännström, C., Norberg, A. & Jansson, L. Narratives of children with chronic illness about being comforted. J. Pediatr. Nurs. 23 , 310–316 (2008).
Ehrlich, K. B. et al. Secure base representations in children with asthma: links with symptoms, family asthma management, and cytokine regulation. Child Dev. 90 , E718–E728 (2019).
Jonsson, M., Egmar, A.-C., Hallner, E. & Kull, I. experiences of living with asthma—a focus group study with adolescents and parents of children with asthma. J. Asthma 51 , 185–192 (2014).
Naman, J. et al. Student perspectives on asthma management in schools: a mixed-methods study examining experiences, facilitators, and barriers to care. J. Asthma 56 , 1294–1305 (2019).
Penza‐Clyve, S. M., Mansell, C. & Mcquaid, E. L. Why don’t children take their asthma medications? A qualitative analysis of children’s perspectives on adherence. J. Asthma 41 , 189–197 (2004).
Walker, T. J. & Reznik, M. In-school asthma management and physical activity: children’s perspectives. J. Asthma 51 , 808–813 (2014).
Newbould, J., Francis, S.-A. & Smith, F. Young people’s experiences of managing asthma and diabetes at school. Arch. Dis. Child. 92 , 1077–1081 (2007).
Article PubMed PubMed Central CAS Google Scholar
Volerman, A. et al. A mixed-methods study examining inhaler carry and use among children at school. J. Asthma 1–12, https://doi.org/10.1080/02770903.2019.1640729 (2019).
Caruana, M., West, L. M. & Cordina, M. Current asthma management practices by primary school teaching staff: a systematic review. J. Sch. Health 91 , 227–238 (2021).
Hanley Nadeau, E. & Toronto, C. E. Barriers to asthma management for school nurses: an integrative review. J. Sch. Nurs. 32 , 86–98 (2016).
Quaranta, J. E. & Spencer, G. A. Barriers to asthma management as identified by school nurses. J Sch. Nurs. 32 , 365–373 (2016).
Cain, A. & Reznik, M. Asthma management in New York city schools: a classroom teacher perspective. J. Asthma 53 , 744–750 (2016).
Cremeens, J., Eiser, C. & Blades, M. Factors influencing agreement between child self-report and parent proxy-reports on the Pediatric Quality of Life Inventory™ 4.0 (Pedsql™) generic core scales. Health Qual. Life Outcomes 4 , 58 (2006).
Kelada, L., Hasking, P. & Melvin, G. The relationship between nonsuicidal self‐injury and family functioning: adolescent and parent perspectives. J. Marital Fam. Ther. 42 , 536–549 (2016).
Knafl, K. A. et al. Patterns of family management of childhood chronic conditions and their relationship to child and family functioning. J. Pediatr. Nurs. 28 , 523–535 (2013).
Al Ghriwati, N., Winter, M. A., Everhart, R. S. & Fiese, B. H. Family functioning and child asthma severity: a bio-behavioral approach. Fam. Syst. Health 35 , 439–449 (2017).
Friedman, D. et al. Fathers and asthma care: paternal involvement, beliefs, and management skills. J. Pediatr. Psychol. 40 , 768–780 (2015).
Bellin, M. H. et al. Caregiver perception of asthma management of children in the context of poverty. J. Asthma 54 , 162–172 (2017).
Lakhanpaul, M. et al. A qualitative study to identify parents’ perceptions of and barriers to asthma management in children from South Asian and White British families. BMC Pulm. Med. 17 , 1–12 (2017).
Download references
Acknowledgements
This work was supported by an Australian National Health and Medical Research Council Partnership Grant (APP1065898), with contributions by the National Health and Medical Research Council, BUPA Health Foundation, Sydney Children’s Hospitals Network, New South Wales Kids and Families, Children’s Health Queensland, and the South Australian Department of Health. The authors would like to thank all the families who participated in this study. We would also like to thank the facilitators Anne De Silva and Christina Falsone from Hall & Partners Open Mind for their contribution to this study. We thank Lachlan Munro for his contribution to the analysis.
Author information
Authors and affiliations.
School of Women’s and Children’s Health, Faculty of Medicine and Health, UNSW Sydney, Sydney, NSW, Australia
Lauren Kelada & Adam Jaffe
Kids Cancer Centre, Sydney Children’s Hospital, Randwick, NSW, Australia
Lauren Kelada
Australian Institute of Health Innovation, Macquarie University, Sydney, NSW, Australia
Charlotte J. Molloy, Peter Hibbert, Louise K. Wiles & Jeffrey Braithwaite
Australian Centre for Precision Health, Cancer Research Institute, School of Health Sciences, University of South Australia, Adelaide, SA, Australia
Charlotte J. Molloy, Peter Hibbert, Louise K. Wiles & Claire Gardner
South Australian Health and Medical Research Institute, Adelaide, SA, Australia
Charlotte J. Molloy, Peter Hibbert & Louise K. Wiles
Caring Futures Institute, College of Nursing and Health Sciences, Flinders University, Adelaide, SA, Australia
Claire Gardner
Ministry of Health, NSW Health, St Leonards, NSW, Australia
Emily Klineberg
Respiratory Department, Sydney Children’s Hospital, Randwick, NSW, Australia
Aiming for Asthma Improvement in Children, Sydney Children’s Hospital, Randwick, NSW, Australia
You can also search for this author in PubMed Google Scholar
Contributions
The study was conceived by P.H., J.B. and A.J. The research proposal, ethics and site-specific approval applications were written by C.G., L.K.W. and C.J.M., and reviewed by A.J., E.K. and P.H. Focus group guides were developed by C.G. and C.J.M. and reviewed and revised by L.K.W., E.K., A.J. and P.H. Focus groups were conducted by C.G. and C.J.M. and analysed by L.K., C.J.M. and C.G. The first draft of the paper was written by L.K.W. and C.J.M. and reviewed and revised by the rest of the study team. All authors approved the paper for publication.
Corresponding author
Correspondence to Lauren Kelada .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Kelada, L., Molloy, C.J., Hibbert, P. et al. Child and caregiver experiences and perceptions of asthma self-management. npj Prim. Care Respir. Med. 31 , 42 (2021). https://doi.org/10.1038/s41533-021-00253-9
Download citation
Received : 03 November 2020
Accepted : 19 August 2021
Published : 09 September 2021
DOI : https://doi.org/10.1038/s41533-021-00253-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Patient Rep Outcomes
- v.7; 2023 Dec
- PMC10589163
Assessing asthma symptoms in children: qualitative research supporting the development of the Pediatric Asthma Diary—Child (PAD-C) and Pediatric Asthma Diary—Observer (PAD-O)
Helena bradley.
1 Adelphi Values Ltd, Bollington, Cheshire UK
Claire Trennery
2 GlaxoSmithKline, London, UK
Amy M. Jones
Aoife lydon, frances white, rebecca williams-hall, rob arbuckle, erin tomaszewski.
3 AstraZeneca, Gaithersburg, MD USA
Vivian H. Shih
John haughney.
4 NHS Greater Glasgow and Clyde R&I, Queen Elizabeth University Hospital, Glasgow, UK
Amanda Eisen
5 Allergy and Asthma Network, Vienna, VA USA
Tonya Winders
6 Global Allergy and Airways Patient Platform, Vienna, Austria
Stephen Joel Coons
7 Patient-Reported Outcome Consortium, Critical Path Institute, Tucson, AZ USA
Sonya Eremenco
Associated data.
The data described in this article are not publicly available in further detail beyond that provided in the manuscript and the extensive supplementary files.
Pediatric asthma has been identified by regulators, clinicians, clinical trial sponsors, and caregivers as an area in need of novel fit-for-purpose clinical outcome assessments (COAs) developed in accordance with the U.S. Food and Drug Administration’s (FDA’s) regulatory guidance for evaluating clinical benefit in treatment trials. To address this gap, the Patient-Reported Outcome (PRO) Consortium’s Pediatric Asthma Working Group has continued development of 2 COAs to assess asthma signs and symptoms in pediatric asthma clinical trials to support efficacy endpoints: a PRO measure, the Pediatric Asthma Diary—Child ( PAD-C ) for children 8–11 years old (y.o.) and an observer-reported outcome measure, the Pediatric Asthma Diary-Observer ( PAD—O) for caregivers of children 4–11 y.o. This qualitative research aimed to generate evidence regarding the content validity of the PAD-C and PAD-O .
Semi-structured combined concept elicitation and cognitive interviews were conducted with a diverse sample of U.S. participants (15 children 8–11 y.o. and 30 caregivers of children 4–11 y.o.). All children had clinician-diagnosed mild to severe asthma. Interviews explored the experience of pediatric asthma and assessed the understanding and relevance of both measures. Interviews were conducted across 3 iterative rounds to allow for modifications.
Concept elicitation findings demonstrated that the core sign/symptom and impact concepts assessed in the PAD-C (cough, hard to breathe, out of breath, wheezing, chest tightness, and nighttime awakenings/symptoms) and PAD-O (cough, difficulty breathing, short of breath, wheezing, and nighttime awakenings/signs) correspond to those most frequently reported by participants; concept saturation was achieved. All PAD-C and PAD-O instructions and core items were well understood and considered relevant by most participants. Feedback from participants, the Pediatric Asthma Working Group, advisory panel, and FDA supported modifications to the measures, including addition of 1 new item to both measures and removal of 1 caregiver item.
Conclusions
Findings provide strong support for the content validity of both measures. The cross-sectional measurement properties of both measures and their user experience and feasibility in electronic format will be assessed in a future quantitative pilot study with qualitative exit interviews, intended to support the reliability, construct validity, final content, and, ultimately, FDA qualification of the measures.
Supplementary Information
The online version contains supplementary material available at 10.1186/s41687-023-00639-y.
Plain language summary
Pediatric asthma is one of the most common chronic diseases in children. However, there are problems of underdiagnosis, poor disease management, and undertreatment for many pediatric asthma patients, pressuring healthcare systems worldwide. Evaluating asthma symptoms is an important part of the development of treatments for pediatric asthma. However, there are few clinical outcome assessments (COAs) developed in line with regulatory guidance to directly assess symptom severity and evaluate the benefit of new treatments in children with asthma. In this study, we continued the development of the Pediatric Asthma Diary—Child (PAD-C) and the Pediatric Asthma Diary—Observer (PAD-O) , according to regulatory guidance, to assess asthma signs and symptoms in children 4 through 11 years old and address this unmet need. The study aimed to explore the experience of pediatric asthma and assess how well-understood and relevant the measures are. Three rounds of qualitative interviews were conducted with 15 children 8 through 11 years old and 30 caregivers of children 4 through 11 years old with asthma. Results show that both measures are well-understood and assess the relevant and important aspects of pediatric asthma reported by children and caregivers. Findings provide evidence supporting the PAD-C and PAD-O as measures of symptom severity and their future use in pediatric asthma treatment trials. Further research is underway to evaluate their measurement properties and assess the user experience and feasibility of electronic completion, to ultimately support the PAD-C and PAD-O in an ongoing COA qualification process by the United States Food and Drug Administration.
As a chronic inflammatory disease of the airways, pediatric asthma is characterized by recurrent episodes of shortness of breath, wheeze, chest tightness, and cough. These episodes are typically associated with expiratory airflow limitation that may resolve spontaneously or in response to medication [ 1 ]. Pediatric asthma is recognized as the most common chronic disease in children [ 2 , 3 ]; however, prevalence is increasing globally and issues of underdiagnosis, poor disease management, and undertreatment continue to persist [ 4 ]. As a result, pediatric asthma remains a critical area of unmet need and poses a substantial global burden on healthcare systems [ 5 ].
International guidelines issued by the Global Initiative for Asthma (GINA) state that the long-term goals of asthma management are to achieve good symptom control and to minimize future risk of exacerbations, persistent airflow limitation, and side effects of treatment [ 1 ]. The achievement of good symptom control necessitates the assessment of asthma symptoms; however, there are poor correlations between objective measures of asthma severity typically used in clinical trials (e.g., forced expiratory volume in 1 s and peak expiratory flow) and patients’ self-reported experience [ 6 – 8 ]. The assessment of asthma symptoms is a critical component in the development of treatments for pediatric asthma and to ease the burden on children and their families. Therefore, to ensure the patient perspective of asthma is accurately represented and assessed in clinical research, there is a need for novel clinical outcome assessments (COAs) to directly assess symptom severity and evaluate clinical benefit in pediatric asthma populations [ 9 , 10 ].
Symptoms of asthma are most appropriately assessed using patient-reported outcome (PRO) measures, since only persons with asthma can feel and self-report on many symptoms. However, as young children (i.e., ≤ 7 years old [y.o.]) may not be able to reliably self-report symptom experience, pediatric asthma trials can involve the collection of PRO data from older children (i.e., ≥ 8 y.o.) on asthma symptoms and observer-reported outcome (ObsRO) data from parents/caregivers on observable asthma-related signs for younger children [ 9 ]. Although recent efforts to develop COAs in pediatric asthma exist [ 11 ], there is still a lack of fit-for-purpose COAs developed in accordance with United States (U.S.) Food and Drug Administration’s (FDA’s) evidentiary expectations for evaluating clinical benefit in pediatric asthma clinical trials [ 12 ]. During previous interactions between FDA and the PRO Consortium’s Asthma Working Group during qualification of the Asthma Daytime Symptom Diary (ADSD) and the Asthma Nighttime Symptom Diary (ANSD) for adolescent and adult populations [ 13 , 14 ], FDA feedback noted the measurement gap in pediatric populations and requested the development of novel COAs to assess asthma symptoms in a broader range of asthma patients (i.e., < 12 y.o.) in clinical studies.
To address this, Merck Sharpe & Dohme Corporation, a member of Critical Path Institute’s (C-Path’s) PRO Consortium [ 15 ], contributed draft versions of 2 COAs for use in pediatric asthma clinical trials to assess the signs and symptoms of mild to severe persistent asthma: a PRO measure designed for completion by children 8–11 y.o. (originally named the Child Asthma Diary [CAD]); and an ObsRO measure designed for completion by parents/caregivers of children 4–11 y.o. (originally named the Observer Asthma Diary [OAD]) [ 16 ]. Initial development of the measures was informed by multiple stages of qualitative research, including a targeted literature review, input by expert scientific advisors, 3 phases of concept elicitation interviews, and 2 phases of cognitive interviews with the respective target populations. However, initial FDA feedback to Merck raised concerns regarding adequacy of the evidence for the content validity of the CAD and OAD in the planned context of use. As a result, the PRO Consortium’s Pediatric Asthma Working Group embarked on further development of the CAD and OAD, with the intention of submitting for COA qualification by FDA for the assessment of asthma sign and symptom severity in children with asthma (i.e., < 12 y.o.) in pediatric asthma clinical trials [ 17 ]. A reanalysis of Merck’s original qualitative data collected as part of the initial development of the draft CAD and OAD was conducted to address FDA’s feedback. Based on this reanalysis of the original data, the draft CAD and OAD were subsequently modified and renamed the Pediatric Asthma Diary—Child ( PAD-C) and Pediatric Asthma Diary—Observer ( PAD-O) , respectively. FDA accepted the PAD-C and PAD-O into the Drug Development Tool (DDT) COA Qualification Program on June 13, 2017. FDA input has therefore been sought at key points throughout the development and qualification process [ 17 ] and has been outlined throughout this article where applicable.
The PAD-C and PAD-O are intended to be used to derive co-primary or secondary endpoints in pediatric asthma clinical trials to establish clinical benefit and support product-specific labeling claims. This article summarizes the qualitative research conducted to continue the development of the PAD-C and PAD-O and to generate qualitative evidence supporting their content validity in accordance with FDA regulatory guidance [ 10 , 18 , 19 ].
Study design
Figure 1 provides an overview of the qualitative research conducted to support the development of the PAD-C and PAD-O .

Overview of study design
At key points throughout the process, input was obtained from the Pediatric Asthma Working Group, C-Path scientists, the advisory panel (J.K, J.H, A.E, T.W), and FDA’s Qualification Review Team. A translatability assessment was conducted on the measures following each round of interviews to ensure that any modifications to the wording used would be suitable for future translation into other languages.
Initial draft PAD-C and PAD-O
A number of changes occurred to the CAD and OAD to create the modified PAD-C and PAD-O . Prior to the study reported here, the PRO Consortium’s Pediatric Asthma Working Group and Adelphi Values made additional refinements to the PAD-C and PAD-O ahead of inclusion and testing in the qualitative interviews. Changes included rearranging and streamlining the PAD-C and PAD-O training guides and simplifying the terminology used in the instructions and item wording of each measure. This section describes the initial draft PAD-C and PAD-O tested in the Round 1 concept elicitation and cognitive interviews.
The PAD-C and PAD-O are designed to be completed twice daily and include a Morning Diary (completed once daily upon waking up to start the day) to assess nighttime awakenings and nighttime asthma symptom severity and a Bedtime Diary / Evening Diary (completed once daily before going to bed) to assess daytime symptom severity. Both measures include a Training Guide that all participants must read prior to completing the PAD-C or PAD-O to aid understanding of the diaries.
The draft PAD-C (7-item Morning Diary and 12-item Bedtime Diary) has been developed for use in children with asthma 8–11 y.o. to assess self-reported asthma symptom severity. The draft PAD-O (9-item Morning Diary and 12-item Evening Diary) has been developed for use in caregivers of children with asthma 4–11 y.o. to assess caregiver-reported asthma sign/symptom severity. When completing the PAD-O , the caregiver can also consider input from other informants (e.g., the child, siblings, teachers, babysitters, and spouses/partners) regarding observable asthma signs and symptoms.
The PAD-C Bedtime Diary assesses the severity of 5 core asthma symptoms (cough, hard to breathe, out of breath, wheezing, and chest tightness) and the PAD-O Evening Diary assesses the severity of 4 core observable asthma signs and symptoms (cough, difficulty breathing, shortness of breath, and wheezing). Note that chest tightness was not included within the PAD-O as it was found to not be an observable concept that can be reliably reported by caregivers in the previous qualitative research [ 16 ]. Due to difficulty with feasibility of children or caregivers reporting on the severity of individual symptoms during the night, a global assessment of nighttime asthma symptom or sign severity is included within the PAD-C Morning Diary and PAD-O Morning Diary, respectively. The morning diaries also assess presence of nighttime awakenings, which is considered a clinically relevant marker for asthma control and symptom severity [ 1 ]. The asthma sign and symptom concepts included within the PAD-C and PAD-O are assessed in terms of presence (nighttime awakenings), intensity (cough, nighttime asthma symptom severity), or frequency (cough, difficulty breathing, shortness of breath, wheezing, and chest tightness [ PAD-C only]), to provide an assessment of sign and symptom severity which is widely recognized as needed to demonstrate clinical benefit in pediatric asthma treatment trials.
Additional items included in the PAD-C and PAD-O to assess other asthma-relevant measurement concepts are: difficulty falling asleep (Morning Diary), activity limitations (Bedtime Diary / Evening Diary), and rescue medication use for both rescue inhalers and nebulizers (Morning Diary and Bedtime Diary / Evening Diary). Single items designed to assess global daytime asthma symptom severity are included in the Bedtime Diary / Evening Diary only, to support analyses during measure development. In the PAD-O , items are included to capture the sources of information used by caregivers when responding to items for informational purposes only, in addition to 1 item added at FDA’s request assessing whether caregivers check on their child during the night (Morning Diary only).
Items in the PAD-C are answered using a 4- or 5-level verbal rating scales (VRS) with text descriptors for each response option paired with colored boxes of increased shading, or via “Yes/No” response options. Items in the PAD-O are answered using a 5- or 6-level VRS, or via “Yes/No/I don’t know” response options. Number entry fields are also used for the rescue inhaler and nebulizer items in both measures.
Concept elicitation and cognitive interviews
Combined semi-structured concept elicitation and cognitive interviews were conducted across 3 iterative rounds to evaluate modifications made to the PAD-C and PAD-O .
Recruitment
Forty-five participants were targeted for inclusion in the interviews, including 15 children 8–11 y.o., 15 parents/caregivers of children 4–7 y.o., and 15 parents/caregivers of children 8–11 y.o. These subgroups allowed for development and testing of the PAD-C and PAD-O in narrower age groupings to help account for developmental differences in children [ 9 , 20 ]. Participants were recruited from 5 different U.S. locations (Chicago, IL; Baltimore, MD; New Orleans, LA; Pittsburgh, PA; St. Louis, MO) with the assistance of a third-party recruitment agency via referral by general practitioners, pediatricians, and respiratory specialists. Child participants were required to be 8–11 y.o., have a clinician-confirmed diagnosis of asthma as defined by national or international asthma guidelines (i.e., GINA [ 1 ], National Asthma Education and Prevention Program [NAEPP] [ 21 ]) for at least 1 year, have received/filled a prescription for asthma medication in the last year, and have experienced symptoms of asthma in the 3 weeks prior to screening. Caregivers were required to be at least 18 years of age and a parent/caregiver of a child 4–11 y.o. with a clinician-confirmed diagnosis of pediatric asthma, who had received/filled a prescription for asthma medication in the last year and had experienced signs or symptoms of asthma in the 3 weeks prior to screening.
Participants were excluded if they (or their child if a caregiver) had a diagnosis of a condition other than asthma (not including allergies or rhinitis) that affected lung function (e.g., bronchiectasis, chronic sinusitis, cystic fibrosis) or any other significant condition that would impact ability to take part in the study.
Recruitment quotas for the following characteristics were used to ensure a sociodemographically and clinically diverse sample reflective of respondents typically enrolled in pediatric asthma clinical trials: age, sex, ethnicity, race, time since diagnosis, asthma control (i.e., well-controlled and not well-controlled [ 22 ]), exacerbations, and medication use.
Interview procedure
The research was conducted in accordance with the Declaration of Helsinki and ethical approval and oversight were provided by Copernicus Group Independent Review Board (CGIRB), an independent ethical review board in the U.S. (IRB number: 20200606). All data were handled in accordance with Health Insurance Portability and Accountability Act (HIPAA) guidelines and the European General Data Protection Regulation (GDPR) for the security and privacy of health data.
All participants provided written informed consent (or parental permission and participant assent in the case of participants 8–11 y.o.) before their participation in the study. Semi-structured interviews lasting approximately 60 min were conducted by trained qualitative researchers via Microsoft Teams or by telephone. All interviews were audio recorded and transcribed verbatim.
In each round, interviews included an introduction (5 min), concept elicitation (5 min), and cognitive interview (50 min) sections. A brief concept elicitation component was included at the start of the interview, to explore the experience of pediatric asthma and evaluate whether the PAD-C and PAD-O adequately assess the core symptoms reported by participants. Since comprehensive concept elicitation work was completed by Merck during initial development activities, this section was deliberately brief to allocate more time to the cognitive evaluation of the PAD-C and PAD-O . Following concept elicitation, participants were asked to complete a paper version of the PAD-C (children) or PAD-O (caregivers) using a “think aloud” method to vocalize their thoughts as they read each instruction and completed each item. In-depth cognitive interview questions were then used to explore the relevance and understanding of the diary items, instructions, response scales, and recall periods.
A qualitative analysis plan was developed a priori to define the coding process, subgroup analyses, and presentation of results. All interview data were analyzed using qualitative analysis methods and ATLAS.ti software [ 23 ].
Concept saturation, defined as the point at which no new relevant or important information emerges with the collection of more data [ 10 ], was evaluated to ensure that the concepts elicited by participants during the concept elicitation portion of the interview had been fully explored. Saturation analyses were conducted for the child and caregiver samples separately by dividing participants into 3 equal groups according to the chronological order in which they were interviewed. Saturation was said to be achieved if no new concepts emerged within the final group of interviews (i.e., Round 3 interviews).
Sample characteristics
A total of 45 participants were included across 3 rounds of interviews. Fifteen interviews were conducted with children 8–11 y.o. and 30 interviews were conducted with caregivers of children 4–11 y.o. All children had clinician-diagnosed mild to severe asthma.
Table Table1 1 summarizes the sociodemographic and clinical characteristics of children participating or being represented by a caregiver in the qualitative interviews. Sociodemographic characteristics of caregivers are presented in Additional file 1 : Table 1. Overall, the majority of pre-specified recruitment quotas were met or only narrowly missed, and there was good representation of characteristics in both child and caregiver samples for each age group.
Child sociodemographic and clinical characteristics (n = 30)
1 n = 3 children 8–11 years old (y.o.) were represented by caregivers who participated in an interview, but the children were not interviewed themselves
2 All participants’ identified gender was the same as their sex
3 C-ACT = Childhood Asthma Control Test
4 One participant experienced both moderate and severe exacerbations
5 Step-wise categories of medication use are based on GINA guidelines [ 1 ]
Concept elicitation results
The symptoms most frequently reported by children during the concept elicitation section of the interviews correspond to the 5 core symptom concepts assessed in the PAD-C Bedtime Diary; cough, difficulty breathing, and chest tightness were reported by all child participants (n = 15/15, 100%), and shortness of breath and wheezing were reported by almost all (n = 14/15, 93.3%; see Table Table2). 2 ). These symptoms were elicited in each round of interviews and equally across both levels of asthma control (well-controlled and not well-controlled).
Summary of asthma signs/symptoms and impacts reported during concept elicitation in Round 1, 2, and 3 interviews (N = 45)
Participant IDs are presented as follows: participant number, site number, sex (M = Male; F = Female), participant age (y.o. = years old), level of asthma control (WC = Well-controlled; NWC = Not Well-controlled), and participant type (P = pediatric participant; CG = caregiver participant)
Similarly, the signs and symptoms most frequently reported by caregivers during the concept elicitation section of the interviews correspond to the 4 observable signs and symptoms assessed in the PAD-O Evening Diary; cough, difficulty breathing, and wheezing were reported by all caregivers (n = 30/30, 100%), and shortness of breath was reported by most (n = 28/30, 93.3%; see Table Table2). 2 ). Children and caregivers also reported other asthma symptoms including general congestion (n = 3 caregivers), tiredness (n = 3 caregivers), flushed face (n = 2 caregivers), and nasal congestion (n = 2 children).
Children and caregivers discussed how asthma impacted their/their child’s daily life. Impacts on physical activity (n = 15/15 children, 100%; n = 30/30 caregivers, 100%) and sleep (n = 15/15 children, 100%; n = 29/30 caregivers, 96.7%) were reported most frequently by children and caregivers, both of which are assessed by items in the PAD-C and PAD-O . Impacts on social functioning (n = 7/15 children, 46.7%; n = 11/30 caregivers, 36.7%), emotional wellbeing (n = 5/15 children, 33.3%; n = 12/30 caregivers, 40.0%), and school (n = 3/15 children, 20.0%; n = 1/30 caregiver, 3.3%; see Table Table2) 2 ) were also reported by children and caregivers. In terms of asthma treatments, all children (n = 15/15, 100%) and all but 1 caregiver (n = 29/30, 96.7%) reported the use of a rescue inhaler. Nebulizer use (n = 8/15 children, 53.3%; n = 20/30 caregivers, 66.7%) and maintenance inhaler use (n = 5/15 children, 33.3%; n = 18/30 caregivers, 60.0%) were also reported.
Concept saturation was achieved after the first 2 rounds of child interviews, by which point the majority of signs/symptoms and impact domains had been elicited. This included the core symptom concepts assessed by the PAD-C (cough, difficulty breathing, shortness of breath, wheezing, and chest tightness) and PAD-O (cough, difficulty breathing, shortness of breath, and wheezing), as well as impacts on physical activity and sleep (Additional file 1 : Tables 2 and 3, respectively). Note that, nighttime awakenings were spontaneously reported for the first time in the final round of child interviews; however, nighttime awakenings were reported by 13 additional children when probed across the 3 rounds, supporting relevance of the concept to this patient population.
Cognitive interview results for the PAD-C and PAD-O
For the cognitive interviews, the Pediatric Asthma Working Group and Adelphi Values divided each of the measures into core, supplementary, and developmental items. “Core items” assess the severity of key signs, symptoms, and impacts of pediatric asthma intended for inclusion in scoring of the measure. “Supplementary items” assess other optional asthma-relevant concepts intended to supplement the measures when used in clinical trials, and “developmental items” are intended for testing purposes during development of the measures. A distinct sample of 15 participants took part in each round of cognitive interviews; 5 children completed the PAD-C and 10 caregivers completed the PAD-O in each round. Across all 3 rounds of cognitive interviews, PAD-C and PAD-O instructions and items were generally well understood and considered relevant. See Additional file 1 : Figs. 1, 2, 3, 4, 5, 6, 7 and 8—for an overview of understanding and relevance across the 3 rounds of child and caregiver interviews.
The 3 iterative rounds of cognitive interviews supported refinement of the PAD-C and PAD-O, with revisions to the instructions and items implemented after each round, as summarized in Table Table3 3 ( PAD-C ) and Table Table4 4 ( PAD-O ). Modifications made were based on feedback from participants, the Pediatric Asthma Working Group, C-Path scientists, the advisory panel, FDA scientists, and the translatability assessments. Updates were generally applied across both measures where applicable, with the aim of promoting consistency and comprehensiveness.
Summary of key modifications made to the PAD-C following each round of interviews
Summary of key modifications made to the PAD-O following each round of interviews
The majority of instructions and items in the PAD-C and the PAD-O were well understood, and all core items were considered relevant by most participants. Recall period instructions for the Morning Diary and Bedtime Diary / Evening Diary were understood by most children and caregivers asked. Based on interview findings, several modifications were made to the PAD-C and PAD-O training guides and overall measures, including updates to the instructions, item wording, and response options.
The PAD-C and PAD-O instructions and response options were understood by most participants, and all core items were understood and relevant to the majority of participants. Almost all children and caregivers understood the recall period instructions in the Morning Diary and Bedtime Diary / Evening Diary. Despite these results, further modifications were made to the PAD-C and PAD-O training guides and overall measures, including updates to the instructions (to allow for both single and multiple-observer completion [ PAD-O only]), item wording, and response options.
No changes were suggested to the PAD-C and PAD-O core items as all items and response scales were well understood and relevant to the majority of participants. Additional sections in the PAD-O Training Guide relating to single and multiple-observer completion were generally well understood, and half of the caregiver sample (n = 5/10, 50.0%) indicated that they would share completion of the PAD-O with another caregiver (e.g., another parent or grandparent), supporting retention of multiple-observer instructions. Some further modifications were made to the PAD-C and PAD-O training guides and overall measures following Round 3 interviews, including minor updates to the recall period wording within the instructions, item wording, and response scales.
Item finalization
Following completion of the 3 rounds of cognitive interviews, an item finalization meeting was held with the Pediatric Asthma Working Group to discuss findings and confirm the proposed revisions to the PAD-C and PAD-O . The evidence demonstrated that both measures provided sufficient conceptual coverage of the core symptoms in pediatric asthma, and therefore it was agreed that no additional items should be added. All items tested in the Round 3 interviews were retained for both measures, except for 1 caregiver developmental item in the Morning Diary. The revisions made were reviewed and approved by the advisory panel. Following cognitive interviews, the resulting PAD-C consisted of 8 core items, 12 supplementary items, and 1 developmental item (Fig. 2 ); whereas the PAD-O consisted of 7 core items, 12 supplementary items, and 2 developmental items (Fig. 3 ).

PAD-C draft conceptual framework. *Cough currently includes 2 items: cough frequency and cough intensity. Note: “Core Items” are intended for inclusion in the PAD-C scoring algorithm. “Supplementary Items” assess other optional asthma-relevant concepts intended to supplement the PAD-C when used in clinical trials. These items would be scored separately from the PAD-C . “Developmental Items” are intended for testing during PAD-C development.

PAD-O draft conceptual framework. *Cough currently includes 2 items: cough frequency and cough intensity. Note: “Core Items” are intended for inclusion in the PAD-O scoring algorithm. “Supplementary Items” assess other optional asthma-relevant concepts intended to supplement the PAD-O when used in clinical trials. These items would be scored separately from the PAD-O . “Developmental Items” are intended for testing during PAD-O development
Conceptual frameworks
The draft conceptual frameworks for the PAD-C and PAD-O following the 3 rounds of interviews are presented in Figs. 2 and and3, 3 , respectively. These conceptual frameworks will be finalized after the completion of a planned quantitative pilot study with qualitative exit interviews.
Pediatric asthma has been identified by regulators and other relevant stakeholders as an area in need of novel fit-for-purpose COAs for evaluating clinical benefit in pediatric asthma treatment trials. In order to address this unmet measurement need, the PAD-C and PAD-O were accepted into FDA’s DDT COA Qualification Program [ 24 ]. The overall objective of this study was to generate qualitative evidence that the content of these measures effectively assesses the severity of the core signs and symptoms of asthma, achieved via the conduct of combined concept elicitation and cognitive interviews.
Concept elicitation
Concept elicitation findings demonstrated that the core symptom concepts assessed in the current versions of the PAD-C and PAD-O were most frequently reported by participants, providing evidence that these measures assess the most important and relevant signs and symptoms of pediatric asthma. The most frequently reported domains of impacts on daily life were physical activity and sleep impacts (including difficulty falling asleep and nighttime awakenings), both of which are assessed by items in the PAD-C and PAD-O and are considered clinically relevant concepts directly linked to asthma symptoms. The evidence confirms that no core sign or symptom concepts were missing from the PAD-C or PAD-O and the addition of further items is not needed. The findings further substantiate existing literature highlighting the widespread and considerable impact of pediatric asthma and reinforces the need for effective treatments to achieve symptom control and accurate assessments of symptom severity [ 5 , 13 , 25 ].
Cognitive interviews for the PAD-C and PAD-O
Across the 3 rounds of cognitive interviews, instructions and core items in the PAD-C and PAD-O were well understood and considered relevant by most participants, providing qualitative evidence to support their content validity. The iterative rounds of interviews strengthened the measures, with revisions to the instructions and items implemented and tested after each round. Several modifications were made following Round 1 interviews, including updates to item stems, response options, rescue inhaler and/or nebulizer item wording, and the addition of a new item to determine whether a child has a nebulizer for asthma treatment. Following Round 2 interviews, further updates were made to response options including updates to the cough intensity response scale on both the PAD-C and PAD-O, rescue inhaler and/or nebulizer item wording, and additional instructions were added to the PAD-O to allow for single and multiple-observer completion. Findings from Round 3 interviews supported additional changes to the rescue inhaler and/or nebulizer item wording to enhance understanding, and the item assessing whether caregivers check on their child was removed as shown in Table Table4. 4 . This resulted in the current versions of the PAD-C and PAD-O at the time of publication.
Since the initiation of this research in 2016, a new electronic Pediatric Asthma Symptom Diary (ePASD) has been developed for self-completion by children 6–11 y.o., in an attempt to address the existing measurement gap in this population [ 11 ]. However, there are notable advantages of the PAD-C and PAD-O over the ePASD and other existing measures. First, the development of both a PRO measure (the PAD-C for completion by children 8–11 y.o.) and ObsRO measure (the PAD-O for completion by caregivers of children 4–11 y.o.) allows for the assessment of asthma signs and symptoms across a broader range of children with mild to severe asthma, specifically those younger than 6 y.o. Evidence from the qualitative interviews and existing literature demonstrates the importance of assessing symptom severity in children as young as 4 y.o. [ 1 , 26 ], particularly as this often reflects populations included in pediatric asthma clinical trials. As such, there is a critical need for appropriate COAs with adequate evidence of being fit-for-purpose to assess asthma symptom severity in younger age groups, not purely self-reports by older children. Second, there is mixed evidence regarding the age at which a child can independently and reliably self-report, with some doubts around the appropriateness of administering PRO measures to children below the age of 8 y.o. [ 20 , 27 , 28 ]. The PAD-O was developed to avoid these potential issues in younger age groups, as caregivers are more likely to be optimal reporters of observable asthma signs and medication use for children under 8 y.o. [ 16 ]. The PAD-O also offers the unique ability for both single and multiple-observer completion, an addition that was supported by FDA representatives to account for a range of different caregiver and/or living situations that better reflect modern family life and allow for greater inclusivity in future pediatric asthma clinical trials. Finally, an important strength of the PAD-C and PAD-O is the pursuit of qualification as part of FDA’s DDT COA Qualification Program. Qualification ensures both measures have been developed and tested in accordance with FDA expectations and relevant guidance [ 10 , 18 , 19 ], including input from a diverse sample of children and caregivers from the target population with varying sociodemographic (e.g., age, sex, ethnicity, and race) and clinical characteristics (e.g., levels of asthma control, exacerbations, and medication use). This is in addition to involvement from a multidisciplinary team, COA experts, comprising representatives from 2 pharmaceutical firms, C-Path, specialist clinicians involved in the diagnosis and management of children with asthma, patient advocates, and FDA representatives.
Study limitations
Although there was good representation of different sociodemographic and clinical characteristics in the sample, some target quotas were missed. Most notably this included children on medication Step 5 and male caregivers, although, this is likely a reflection of fewer cases of more severe asthma in children and the lack of established Step 5 treatment for children 4–5 y.o. [ 1 ], and the well-documented sex differences in research participation [ 29 , 30 ] and childcare responsibilities [ 31 , 32 ]. Interviews were also conducted with U.S. participants only; however, the cross-cultural suitability of the PAD-C and PAD-O was explored within the translatability assessments, and full translation and cultural adaptation of the measures for other languages will be conducted in future studies.
Additionally, interviews with children 8–11 y.o. were conducted via video call or telephone. Face-to-face interviews were initially proposed as the optimal methodology to build rapport and obtain useful insights from non-verbal cues; however, this was not feasible due to the COVID-19 pandemic and associated public health restrictions when interviews were conducted between October 2020 and July 2021. Nevertheless, research has shown comparability between face-to-face and video or telephone interviews [ 33 , 34 ], and additional steps were taken to mitigate against potential issues with remote interviewing (i.e., color-coding the measures) and to promote engagement throughout the child interviews (i.e., using visual aids and creative tasks).
The results from this qualitative study provide strong support for the content validity of the PAD-C and PAD-O for assessing severity of asthma signs and symptoms in children 4 through 11 y.o. with mild to severe pediatric asthma. The next steps in the development process include the migration of the measures to an electronic mode of data collection and the conduct of a quantitative pilot study with qualitative exit interviews. This continued research will aim to generate further evidence to confirm the cross-sectional measurement properties and evaluate the user experience and feasibility of electronic completion of the PAD-C and PAD-O to support progress towards their qualification in FDA’s COA Qualification Program.
Acknowledgements
Linda Nelsen, an employee of GSK, and Maggie Tabberer, an employee of GSK at the time this research was conducted, provided input and guidance at key stages throughout the research on behalf of the Patient-Reported Outcome Consortium’s Pediatric Asthma Working Group. Maria Mattera, employee of Critical Path Institute and member of the Patient-Reported Outcome Consortium’s Pediatric Asthma Working Group, also provided input at key stages of the research. Asha Lehane, employee at Adelphi Values, contributed to the data analysis and interpretation. Lucy Morgan, employee at Adelphi Values at time of research, contributed to the conception or design of the study, conducted participant interviews and contributed to the data analysis or interpretation. Dr. Jerry Krishnan, a specialist clinician involved in the diagnosis and management of children with asthma, provided expert clinical input at key stages of the research. We thank the members of the U.S. Food and Drug Administration’s Qualification Review Team and other FDA scientists for their feedback during the development of the PAD-C and PAD-O .
Abbreviations
Author contributions.
RA, RW-H, and HB contributed to the conception or design of the study; contributed to the data analysis or interpretation; and were major contributors in writing and/or revising the manuscript. AJ, AL, and FW contributed to the conception or design of the study; conducted participant interviews; contributed substantially to the data analysis or interpretation; and were major contributors in writing and/or revising the manuscript. SJC, SE, CT, ET, and VHS were major contributors to the conception or design of the study, the data analyses strategy and interpretation, and writing and/or revising the manuscript. JH, AE, and TW provided expert input to the conception or design of the study, as well as the data analyses or interpretation. All authors provided critical review and final approval of the publication.
This qualitative study was funded by the U.S. Food and Drug Administration under the Broad Agency Announcement contract 7540119C10135.
Availability of data and material
Declarations.
Ethical approval and oversight were provided by Copernicus Group Independent Review Board (CGIRB), an independent ethical review board in the U.S. (IRB tracking number: 20200606). All caregiver participants provided informed consent and parental permission (if applicable), and all child participants provided informed assent before participating in any study activities.
Dissemination of findings including via publication was included in the consent and parental permission forms signed by caregivers.
Sonya Eremenco and Stephen Joel Coons are employees of Critical Path Institute, which is the copyright holder for the PAD-C and PAD-O on behalf of the PRO Consortium. Rob Arbuckle, Rebecca Williams-Hall, Helena Bradley, Amy Jones, Aoife Lydon, and Frances White are employees of Adelphi Values, a health outcomes agency commissioned to conduct research by companies in the pharmaceuticals industry. Adelphi Values received funding from Critical Path Institute to conduct the research summarized in this article. Claire Trennery was an employee of GSK when the research was completed and is now employed by Adelphi Values. Vivian H Shih was an employee of AstraZeneca when the research was conducted and is now employed by Insmed. Erin Tomaszewski is an employee of AstraZeneca. Dr. John Haughney was a consultant of Adelphi Values on behalf of Critical Path Institute as a scientific advisor and received a fee for his involvement. Amanda Eisen and Tonya Winders were consultants of Adelphi Values on behalf of Critical Path Institute as patient advocacy/caregiver representatives and received a fee for their involvement. Tonya Winders is a paid speaker and advisor to AstraZeneca, Amgen, GSK, Sanofi/Regeneron and Novartis. The authors declare that there are no other competing interests. Critical Path Institute is supported by the Food and Drug Administration (FDA) of the Department of Health and Human Services (HHS) and is 55% funded by the FDA/HHS, totaling $17,612,250, and 45% funded by non-government source(s), totaling $14,203,111. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement by, FDA/HHS or the U.S. Government.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
the Patient-Reported Outcome Consortium’s Pediatric Asthma Working Group: Linda Nelsen , Maggie Tabberer , Maria Mattera , Asha Lehane , Lucy Morgan , and Jerry Krishnan
Disclaimer » Advertising
- HealthyChildren.org

- Previous Article
- Next Article
Introduction
Subjective measures, composite asthma scores, quality of life, objective measures, assessment of lung function, airway hyperresponsiveness, initial consultation, subsequent visits, lead authors, section on allergy and immunology executive committee, 2015–2016, former executive committee members, section on pediatric pulmonology and sleep medicine executive committee, 2015–2016, clinical tools to assess asthma control in children.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
FINANCIAL DISCLOSURE: The authors have indicated they do not have a financial relationship relevant to this article to disclose.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- CME Quiz Close Quiz
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Chitra Dinakar , Bradley E. Chipps , SECTION ON ALLERGY AND IMMUNOLOGY , SECTION ON PEDIATRIC PULMONOLOGY AND SLEEP MEDICINE , Elizabeth C. Matsui , Stuart L. Abramson , Chitra Dinakar , Anne-Marie Irani , Jennifer S. Kim , Todd A. Mahr , Michael Pistiner , Julie Wang , Julie P. Katkin , Kristin N. Van Hook , Lee J. Brooks , Bonnie B. Hudak , Richard M. Kravitz , Shrutim Paranjape , Michael S. Schechter , Girish D. Sharma , Dennis C. Stokes; Clinical Tools to Assess Asthma Control in Children. Pediatrics January 2017; 139 (1): e20163438. 10.1542/peds.2016-3438
Download citation file:
- Ris (Zotero)
- Reference Manager
Asthma affects an estimated 7 million children and causes significant health care and disease burden. The most recent iteration of the National Heart, Lung and Blood Institute asthma guidelines, the Expert Panel Report 3, emphasizes the assessment and monitoring of asthma control in the management of asthma. Asthma control refers to the degree to which the manifestations of asthma are minimized by therapeutic interventions and the goals of therapy are met. Although assessment of asthma severity is used to guide initiation of therapy, monitoring of asthma control helps determine whether therapy should be maintained or adjusted. The nuances of estimation of asthma control include understanding concepts of current impairment and future risk and incorporating their measurement into clinical practice. Impairment is assessed on the basis of frequency and intensity of symptoms, variations in lung function, and limitations of daily activities. “Risk” refers to the likelihood of exacerbations, progressive loss of lung function, or adverse effects from medications. Currently available ambulatory tools to measure asthma control range are subjective measures, such as patient-reported composite asthma control score instruments or objective measures of lung function, airway hyperreactivity, and biomarkers. Because asthma control exhibits short- and long-term variability, health care providers need to be vigilant regarding the fluctuations in the factors that can create discordance between subjective and objective assessment of asthma control. Familiarity with the properties, application, and relative value of these measures will enable health care providers to choose the optimal set of measures that will adhere to national standards of care and ensure delivery of high-quality care customized to their patients.
Guidelines from the National Heart, Lung and Blood Institute for the diagnosis and management of asthma, and the Global Initiative for Asthma Control, revolve around the yardstick of evaluation of the severity of asthma and attainment of control to guide initiation and adjustment of therapy. 1 , 2 Numerous studies have confirmed the inadequacy of asthma control in the United States. 3 , 4
The domains of severity and control can be assessed in terms of impairment (frequency and intensity of symptoms, variations in lung function, and limitations of daily activities) and future risk (likelihood of exacerbations, progressive loss of lung function, or adverse effects from medications). Asthma can be considered to be well controlled if symptoms are present twice a week or less; rescue bronchodilator medication is used twice a week or less; there is no nocturnal or early awakening; there are no limitations of work, school, or exercise; and the peak flow (PEF)/forced expiratory volume in 1 second (FEV 1 ) is normal or at the personal best. Asthma control can be further classified as well controlled, not well controlled, and very poorly controlled as elegantly laid out in the National Heart, Lung and Blood Institute Expert Panel Report 3 (EPR3). 1 Asthma can be considered not well controlled if symptoms are present more than 2 days a week or multiple times on 2 or fewer days per week; rescue bronchodilator medication is used more than 2 days per week; nighttime awakenings are 2 times a month or more; there is some limitation of work, school, or exercise; and the PEF/FEV 1 is 60% to 80% of personal best/predicted, respectively. Asthma is classified as very poorly controlled if symptoms are present throughout the day; rescue bronchodilator medication is used several times per day; nighttime awakenings are more than 1 time a week; there is extreme limitation of work, school, or exercise; and the PEF/FEV 1 is less than 60% of personal best/predicted, respectively.
The keystone of asthma management is the achievement and maintenance of optimal asthma control. However, to date, there is no universally recognized gold standard measure of asthma control that can accurately capture both patient-reported domains of impairment and risk and objective measures of lung function. The tools available in a clinical practice setting can be classified as subjective (“patient reported”) and objective (“physiologic and inflammatory measures”). A judicious combination of measures from each category may be needed to optimally assess asthma control.
Subjective measures of asthma control include (1) detailed history taking, (2) use of composite asthma control scores, and (3) quality-of-life measures (used mainly in research settings).
Assessment of asthma control in the health care provider’s office starts with the history. Detailed information should be sought on patient-centered outcomes (such as asthma exacerbations in the past year and the limitations asthma imposes on the patient’s daily activities including sports and play), sleep disturbance, medication use (both daily controller and reliever medication), adherence to therapy, and comorbidities/factors that may complicate care. 5
Patient-reported composite asthma control score instruments are attempts to capture the multidimensional nature of asthma control in a single numerical value. This enables the degree of asthma control to be compared across encounters. More than 17 composite instruments, each with at least 1 published validated study, are available. 6 These instruments have comparable content and have been designed to measure asthma disease activity over a period of 1 to 4 weeks. Notably, none of them have been validated to assess an acute exacerbation ( Table 1 ). Therefore, from a pediatric emergency medicine perspective, caution should be taken when using composite asthma score instruments during an acute exacerbation, as is typically encountered in the emergency department setting.
Age-Specific Asthma Control Tools and Their Properties
Adapted from Cloutier et al. 6 MCID, minimally clinically important difference.
The commonly used validated tools are the Asthma Control Test (ACT), 7 the Childhood Asthma Control Test C-ACT, 8 and the Asthma Control Questionnaire (ACQ). 9 The ACT contains 5 items, with a recall window of 4 weeks. The C-ACT is for use in children 4 through 11 years of age and consists of 4 pictorial items and 3 verbal items that are scored by the children and parents, respectively. It has been reported that children tend to assess their asthma control to be significantly lower than their parents do. The Asthma Control Questionnaire (ACQ) contains 6 items with a recall window of 1 week, supplemented by percentage of predicted FEV 1 measurement. The Test for Respiratory and Asthma Control in Kids (TRACK) 10 is a 5-question caregiver-completed questionnaire that determines respiratory control in children 0 to 5 years of age with symptoms consistent with asthma. Another less commonly used instrument is the Asthma Therapy Assessment Questionnaire (ATAQ), a 20-item parent-completed questionnaire exploring several domains, with 4 questions relating to symptom control and primarily used in research. 11 , 12
Individual instruments contain 3 to 10 questions, and scoring varies by instrument ( Table 1 ). Four instruments have established cutoff values for uncontrolled versus controlled asthma (ACQ, ACT, C-ACT, and TRACK), and 2 have cutoffs for identifying poorly controlled asthma (ACT and ATAQ). Because these cutoffs have been defined at a population level, they may not be accurate for an individual patient. Tracking the numerical and categorical responses over time for each individual patient may prove to be more helpful than looking at cutoff values alone. For instance, if a patient reports frequent nocturnal awakenings, following the response to that particular question may help individualize attainment of control. The minimal clinically important differences or temporal differences in scores that indicate clinical significance have been determined for a few of the instruments (ACQ, ACT, C-ACT, and TRACK 6 , 13 ; Table 1 ). Three of the instruments (ACQ, ACT, and TRACK) have been validated in Spanish-speaking groups. 14 , – 16 The ACQ and ACT have been validated for use as self-administered instruments in person, at home, by telephone, and by Internet tracking. 6 , 17
Poor asthma control, as measured by the commonly used composite scores, is associated with reduced lung function and elevated exhaled nitric oxide fraction 5 , 18 (discussed later in the article). Studies have shown that changes in these composite scores reflect changes in the overall clinical assessment of asthma control by physicians and the need to step-up therapy. 19 However, a recent study showed that the degree of asthma control, as assessed by these tools, changes over time and shows variable concordance with the risk of exacerbations. 12
Despite being fairly well validated, these scores share drawbacks that limit their usefulness in clinical practice. 6 Although the short recall window facilitates reliable recollection of recent asthma events, it fails to represent the fluctuations in control. Children may be excellently controlled during one season and then have poor control during another. In addition, asthma exacerbations can occur in children with good short-term asthma control. 20 Exacerbations, an important component of the impairment domain of asthma control, are not covered in the ACT, C-ACT, and ACQ but are assessed in the TRACK and the Composite Asthma Severity Index. 21 , 22
A range of pediatric asthma quality-of-life instruments have been developed, encompassing the impact of asthma on children’s or their parents’ lives. 23 The instruments have been validated but are time-intensive to fill out and are therefore not routinely used in clinical practice.
Currently available objective measures of asthma control include (1) assessment of lung function, (2) evaluation of airway hyperresponsiveness, and (3) biomarkers.
The PEF is defined as the highest instantaneous expiratory flow achieved during a maximal forced expiratory maneuver starting at total lung capacity. 24 PEF variability is the degree to which the PEF varies among multiple measurements performed over time ( Table 2 ). The management of acute exacerbations has traditionally been guided by PEF measurements. However, the correlation between PEF and FEV 1 worsens in asthmatic patients with airflow limitation. Also, although reference to normal PEF values is important, the “personal best” value, and the trend of change in individual patients, is of greater value in managing their asthma. 24
Objective Measures of Asthma Control
Adapted from Tepper et al. 24
The advantages of PEF are that it is easier to perform than a spirometric maneuver and it is measurable with a relatively small and inexpensive instrument. Thus, PEF may be suitable for individual testing at home, at school, and in patients who are poor perceivers of their degree of airway obstruction. It may help prevent delayed treatment in underperceivers and excessive use of services in overperceivers.
Many concerns regarding PEF have been described, with the primary ones being that the results are highly variable even when performed well, limiting its utility in the diagnosis and management of asthma. Parents and child should be appropriately trained on use, but there is no gauge of effort, and it gives no information regarding the site of airflow obstruction. It cannot distinguish obstructive from restrictive ventilatory impairment. PEF meters from different manufacturers may show different results, and the “personal best” measurements may change with growth and degree of asthma control. Adherence to PEF monitoring is a challenge 25 and is often the reason it is not widely used in clinical practice. Overall, PEF monitoring alone has not been shown to be more effective than symptom monitoring on influencing asthma outcomes 26 and is no longer recommended. 1
Measurement of spirometric indices of lung function, such as the FEV 1 , forced vital capacity (FVC), and FEV 1 /FVC ratio, are an integral part of the assessment of asthma severity, control, and response to treatment. 1 , 2 They have been shown to be associated with the risk of asthma attacks in children. 27 Children with chronic airway obstruction have been reported to be less likely to perceive dyspnea than those with acute obstruction. 28 The EPR3, therefore, recommends performing office-based spirometry every 1 to 2 years and more frequently if clinically indicated in children 5 years or older with asthma. 1 However, only 20% to 40% of primary care providers use lung function measurements in asymptomatic asthmatic patients, and up to 59% of pediatricians never perform lung function tests. 29
Normal values for spirometry are well established and are based on height, age, sex, and race/ethnicity of the healthy US population. Spirometric measures are highly reproducible within testing sessions in approximately 75% of children older than 5 to 6 years of age. Guidance on performing spirometry in an office setting and coding for asthma visits have been described. 30 The forced expiratory maneuver may be displayed as a flow-volume loop. Guidelines regarding interpretation of the primary measures (FEV 1 , FVC, and the FEV 1 /FVC ratio) are well outlined in the EPR3. 1 , 31 Of note, most automatic interpretations of the spirometry report fail to comment on the FEV 1 /FVC ratio, an important parameter that, in children, is normally 85% predicted or greater. 1 Forced expiratory flow between 25% and 75% of vital capacity (FEF25–75) may reflect obstructive changes that occur in the small airways of children with asthma. However, FEF25–75 is considered to be of secondary importance because it is not specific and is highly variable (effort dependent).
Reduced spirometric measures are associated with symptom severity, reduced quality of life, and poor asthma outcomes. 24 However, individual patients, particularly children, may have misleadingly normal spirometry results, despite frequent or severe symptoms. An analysis of 2728 children between 4 and 18 years of age attending a tertiary care facility showed that the majority of asthmatic children had FEV 1 values within normal ranges. 32
Spirometry, by itself, is not useful in establishing the diagnosis of asthma because airflow limitation may be mild or absent, particularly in children. In other words, if the spirometry result is normal, it does not rule out asthma. Variability of airflow obstruction over time and the response to treatment, when clinically relevant, can aid in the diagnosis and assessment of asthma control.
Although there are organizations that are attempting to integrate spirometry results into the electronic health record with varying degrees of success, the most commonly used approach at this time is to scan the printed spirometry result into the electronic health record.
Prebronchodilator and Postbronchodilator Spirometry (Bronchodilator Reversibility)
Bronchodilator reversibility testing helps determine the presence and magnitude of reversible airflow limitation. 24 Baseline spirometry is performed and repeated after administration of bronchodilator test agents (eg, 15 minutes after 4 inhalations of albuterol). Change in FEV 1 is the most common parameter followed because the value of reversibility in other measurements is less established (eg, FEV 1 /FVC or FEF25–75).
The most widely used definition of “significant” bronchodilator response is that of the American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines for interpretation of spirometry and consists of an improvement in FEV 1 greater than 12% and 200 mL. 33 Other parameters that have been used in children include a 9% to 10% increase in percent predicted FEV 1 . 24
Bronchodilator reversibility testing, although not specific, is useful for confirming the diagnosis of asthma. Increased bronchodilator reversibility correlates with increased asthma severity. Bronchodilator reversibility is diminished in patients with well-controlled asthma as well as those with narrowing or remodeling of the airways. Annual assessment of prebronchodilator and postbronchodilator FEV 1 might help identify children at risk for developing progressive decline in airflow. 34
Recent Advances in Monitoring PEF and Spirometry
Advances in home-based airflow monitoring include the use of electronic, handheld devices with easily downloadable recordings of multiple PEF or FEV 1 point measures with software that facilitates easy use and interpretation. 35 The availability of these instruments for routine clinical use is limited at this time.
Impulse Oscillometry
Impulse oscillometry assesses airflow resistance and bronchodilator response in younger children. Measurement of airway resistance is a direct indicator of airway caliber with increased resistance indicating narrowing of airways. It is used largely as a research tool and is only available in a few centers. 24
A major characteristic of asthma is the variability in bronchial tone in response to a variety of stimuli. Airway hyperresponsiveness (AHR) may be assessed by bronchial provocation tests. Bronchial provocation tests may be performed with agents such as methacholine or stimuli such as physical exercise. 24 , 28 , 36 A positive test result for AHR is indicated by a 20% reduction in FEV 1 after inhalation of a methacholine dose of 8 mg/mL or less. A negative test suggests a diagnosis other than asthma. A reduction in FEV 1 of at least 10% during exercise testing is taken as a sign of exercise-induced bronchoconstriction. These tests take approximately 2 hours and require trained personnel to perform them. In general, evidence does not support the routine assessment of AHR in the clinical management of asthma control. 28
Apart from exhaled nitric oxide measurements, the role and usefulness of noninvasive biomarkers in routine clinical practice for monitoring inflammation in children with asthma is undefined. Sputum eosinophilia, exhaled breath condensates, and urinary leukotrienes are used as tools primarily in research studies. 28 , 37
Exhaled Nitric Oxide
The fractional concentration of nitric oxide in exhaled air (FENO) is a quantitative measure of airway nitric oxide, an endogenously produced gaseous mediator that is an indirect marker of airway inflammation. The joint ATS/ERS guideline for the measurement of FENO is the current standard. 38 , 39 The testing is noninvasive, reproducible, easy to perform in patients (including children), feasible to measure in ambulatory clinical settings, and has no risk to patients. 40 , 41
FENO is generally accepted as a marker of eosinophilic airway inflammation. Individuals with asthma have been reported to have elevated levels of FENO, but because FENO is also related to atopy, elevated levels may be seen in atopic individuals without asthma. Although FENO levels overlap among healthy, atopic, and asthmatic cohorts, in general, the upper value of normal is 25 ppb. It has been suggested that a clinically important decrease of FENO is a change of 20% for values greater than 50 ppb or a change of 10 ppb for values less than 50 ppb. 38 Studies in children suggest that FENO correlates with severity and with asthma control. 42 FENO reduces in a dose-dependent manner with corticosteroid treatment 43 and has been shown to increase with deterioration in asthma control. 44 The value of additional FENO monitoring in children whose asthma is appropriately managed using guideline-based strategies is unproven, 28 , 45 , – 47 and insurance payment for this test varies by geographic location. Nevertheless, some asthma specialists have adopted the use of FENO as an adjunct ambulatory clinical tool for measuring airway inflammation and serial monitoring asthma control in individual patients with difficult-to-control asthma.
Assessing Asthma Control in Children Younger Than 5 Years
In children younger than 5 years, it is recommended that both symptom control and future risk be monitored. 2 The risk domain is assessed by historical review of exacerbations with need for oral steroid. Validated measures to assess asthma control in this age group include the TRACK (0–5 years) and the C-ACT in children (4–11 years) of age.
Children younger than 5 years are typically unable to perform spirometry; hence, confirmation of the diagnosis of asthma is challenging in this age group. Recurrent wheezing occurs in a large proportion of these children, typically with viral infections. A therapeutic trial of regular controller therapy (for 1–3 months) may often be necessary to evaluate response and maintenance of control.
Assessment of risk profiles using tools such as the asthma predictive index (API) may be helpful in predicting the likelihood of recurrent wheezing in school-age children. One study showed that children with a positive API had a fourfold to 10-fold greater chance of developing asthma at 6 through 13 years of age than those with a negative API, and 95% of children with a negative API remained free of asthma. 48 The modified API suggests that the diagnosis of asthma in young children with a history of more than 3 episodes of wheezing is more likely if they meet 1 major or 2 minor criteria. 49 Major criteria include a parent with asthma, physician diagnosis of atopic dermatitis, or sensitization to aeroallergens (positive skin or allergen-specific immunoglobulin E test results). Minor criteria include the presence of food allergies or sensitization to milk, egg, and peanut; blood eosinophil counts greater than 4%; or wheezing apart from colds. 49
Recent advances in measuring lung function, biomarker profiles, adherence, utilization and outcomes data, and development of validated questionnaires have made ongoing assessment and monitoring of asthma control a reality. Following is a schema of suggested measures that may be used in routine ambulatory monitoring of asthma control in clinical practice.
The encounter between patient and health care provider may involve critical and empathetic listening to the patient and accurate elicitation of symptoms as indicators for asthma control, aided by validated asthma control tools such as the C-ACT/ACT. A complete environmental and social history should be obtained to evaluate for triggers. 50
Airway obstruction and AHR can be assessed by measuring prebronchodilator and postbronchodilator FEV 1 . Some specialists may consider evaluation of airway inflammation by using FENO to be useful.
Education and training regarding asthma and its management can be provided, taking into consideration the patient’s personal preference and goals while creating an individualized action plan.
Action strategies can be based on either symptoms or objective criteria, such as by monthly monitoring of the age-specific, validated asthma control instrument, or in individualized circumstances, by daily electronic FEV 1 or conventional peak flow monitoring at home.
Symptom scores with validated control instruments and FEV 1 can be monitored at subsequent visits along with serial health care utilization data to tailor the medication dose to degree of asthma control. The risk domain is validated by a history of systemic steroid prescription, emergency department visits, or hospitalizations.
In individuals whose FENO was elevated at the initial visit and shows variation in response to therapy, repeat FENO monitoring may be considered.
Education regarding asthma triggers, review of inhaler techniques, assessment and reinforcement of adherence, treatment of comorbidities (eg, gastroesophageal reflux, sinusitis, obesity), and encouragement and fortification of the collaborative provider-patient relationship can be provided at each follow-up visit.
The need for continued assessment or reassessment by a pediatric allergist or pulmonologist can be considered when faced with challenges in attaining optimal asthma control.
Information on appropriate coding for the asthma management tools and services provided can be found in the Asthma Coding Fact Sheet at the following link: https://www.aap.org/asthmacodingfactsheets .
Asthma Control Test
Asthma Control Questionnaire
airway hyperresponsiveness
Asthma Therapy Assessment Questionnaire
American Thoracic Society/European Respiratory Society
Childhood Asthma Control
Expert Panel Report 3
fractional exhaled nitric oxide
forced expiratory volume in 1 second
forced expiratory flow between 25% and 75% of vital capacity
ratio of forced expiratory volume in 1 second to forced expiratory volume
forced expiratory volume
Test for Respiratory and Asthma Control in Kids
FUNDING: No external funding.
This document is copyrighted and is property of the American Academy of Pediatrics and its Board of Directors. All authors have filed conflict of interest statements with the American Academy of Pediatrics. Any conflicts have been resolved through a process approved by the Board of Directors. The American Academy of Pediatrics has neither solicited nor accepted any commercial involvement in the development of the content of this publication.
Clinical reports from the American Academy of Pediatrics benefit from expertise and resources of liaisons and internal (AAP) and external reviewers. However, clinical reports from the American Academy of Pediatrics may not reflect the views of the liaisons or the organizations or government agencies that they represent.
The guidance in this report does not indicate an exclusive course of treatment or serve as a standard of medical care. Variations, taking into account individual circumstances, may be appropriate.
All clinical reports from the American Academy of Pediatrics automatically expire 5 years after publication unless reaffirmed, revised, or retired at or before that time.
Chitra Dinakar, MD, FAAP
Bradley Chipps, MD, PhD, FAAP
Elizabeth C. Matsui, MD, MHS, FAAP, Chair
Stuart L. Abramson, MD, PhD, AE-C, FAAP
Anne-Marie Irani, MD, FAAP
Jennifer S. Kim, MD, FAAP
Todd A. Mahr, MD, FAAP, Immediate Past Chair
Michael Pistiner, MD, FAAP
Julie Wang, MD, FAAP
Thomas A. Fleisher, MD, FAAP
Scott H. Sicherer, MD, FAAP
Paul V. Williams, MD, FAAP
Debra L. Burrowes, MHA
Julie P. Katkin, MD, FAAP, Chair
Kristin N. Van Hook, MD, FAAP
Lee J. Brooks, MD, FAAP
Bonnie B. Hudak, MD, FAAP
Richard M. Kravitz, MD, FAAP
Shrutim Paranjape, MD, FAAP
Michael S. Schechter, MD, FAAP, Immediate Past Chair
Girish D. Sharma, MD, FAAP
Dennis C. Stokes, MD, FAAP
Laura Laskosz, MPH
Competing Interests
Re: clinical tools to assess asthma control in children.
It was with great interest that I read the clinical report from Dinakar and Chipps(1) describing the clinical tools to assess asthma control in children in the January issue of Pediatrics. They provide an accurate overview of composite asthma scoring instruments with the exception of the childhood asthma control test (C-ACT) as they failed to include the C-ACT in the listing of instruments that have been validated in Spanish-speaking groups. Several studies have validated the C-ACT in Spanish-speaking populations.(2, 3) This is an important consideration for pediatricians working with Spanish-speaking children with asthma who are considering implementing these control tests in their practice.
Stephen K. de Waal Malefyt, MD, FAAP, AE-C
1. Dinakar C, Chipps BE, Section On A, Immunology, Section On Pediatric P, Sleep M. Clinical Tools to Assess Asthma Control in Children. Pediatrics2016 Dec 26. 2. Perez-Yarza EG, Castro-Rodriguez JA, Villa Asensi JR, Garde Garde J, Hidalgo Bermejo FJ. Validation of a Spanish version of the Childhood Asthma Control Test (Sc-ACT) for use in Spain. Anales de pediatria (Barcelona, Spain : 2003)2015 Aug;83(2):94-103. 3. Rodriguez-Martinez CE, Melo-Rojas A, Restrepo-Gualteros SM, Sossa-Briceno MP, Nino G. Validation of the Spanish version of the childhood asthma control test (cACT) in a population of Hispanic children. J Asthma2014 Oct;51(8):855-62.
Advertising Disclaimer »
Citing articles via
Email alerts.

Affiliations
- Editorial Board
- Editorial Policies
- Journal Blogs
- Pediatrics On Call
- Online ISSN 1098-4275
- Print ISSN 0031-4005
- Pediatrics Open Science
- Hospital Pediatrics
- Pediatrics in Review
- AAP Grand Rounds
- Latest News
- Pediatric Care Online
- Red Book Online
- Pediatric Patient Education
- AAP Toolkits
- AAP Pediatric Coding Newsletter
First 1,000 Days Knowledge Center
Institutions/librarians, group practices, licensing/permissions, integrations, advertising.
- Privacy Statement | Accessibility Statement | Terms of Use | Support Center | Contact Us
- © Copyright American Academy of Pediatrics
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Current Understanding and Management of Childhood Asthma
Loading... Systematic Review 15 June 2023 Efficacy and safety of subcutaneous immunotherapy in asthmatic children allergic to house dust mite: a meta-analysis and systematic review Chen Zheng , 2 more and Zhimin Chen 2,346 views 2 citations
Original Research 12 June 2023 Effectiveness of sublingual immunotherapy with house dust mite drops in asthmatic children at different ages Tao Ai , 4 more and Li Wang 1,135 views 0 citations
Original Research 23 March 2023 Development and validation of the patient reported outcomes questionnaire of children with asthma in China: A Caregiver's proxy-reported measure Tong Xu , 5 more and Lei Shang 964 views 0 citations
Original Research 06 February 2023 Asthma with recurrent middle lobe syndrome in children: Clinical features and lung function patterns Yong Feng , 4 more and Han Zhang 1,353 views 0 citations
- Open access
- Published: 15 August 2020
Treatment strategies for asthma: reshaping the concept of asthma management
- Alberto Papi 1 , 7 ,
- Francesco Blasi 2 , 3 ,
- Giorgio Walter Canonica 4 ,
- Luca Morandi 1 , 7 ,
- Luca Richeldi 5 &
- Andrea Rossi 6
Allergy, Asthma & Clinical Immunology volume 16 , Article number: 75 ( 2020 ) Cite this article
47k Accesses
55 Citations
4 Altmetric
Metrics details
Asthma is a common chronic disease characterized by episodic or persistent respiratory symptoms and airflow limitation. Asthma treatment is based on a stepwise and control-based approach that involves an iterative cycle of assessment, adjustment of the treatment and review of the response aimed to minimize symptom burden and risk of exacerbations. Anti-inflammatory treatment is the mainstay of asthma management. In this review we will discuss the rationale and barriers to the treatment of asthma that may result in poor outcomes. The benefits of currently available treatments and the possible strategies to overcome the barriers that limit the achievement of asthma control in real-life conditions and how these led to the GINA 2019 guidelines for asthma treatment and prevention will also be discussed.
Asthma, a major global health problem affecting as many as 235 million people worldwide [ 1 ], is a common, non-communicable, and variable chronic disease that can result in episodic or persistent respiratory symptoms (e.g. shortness of breath, wheezing, chest tightness, cough) and airflow limitation, the latter being due to bronchoconstriction, airway wall thickening, and increased mucus.
The pathophysiology of the disease is complex and heterogeneous, involving various host-environment interactions occurring at various scales, from genes to organ [ 2 ].
Asthma is a chronic disease requiring ongoing and comprehensive treatment aimed to reduce the symptom burden (i.e. good symptom control while maintaining normal activity levels), and minimize the risk of adverse events such as exacerbations, fixed airflow limitation and treatment side effects [ 3 , 4 ].
Asthma treatment is based on a stepwise approach. The management of the patient is control-based; that is, it involves an iterative cycle of assessment (e.g. symptoms, risk factors, etc.), adjustment of treatment (i.e. pharmacological, non-pharmacological and treatment of modifiable risk factors) and review of the response (e.g. symptoms, side effects, exacerbations, etc.). Patients’ preferences should be taken into account and effective asthma management should be the result of a partnership between the health care provider and the person with asthma, particularly when considering that patients and clinicians might aim for different goals [ 4 ].
This review will discuss the rationale and barriers to the treatment of asthma, that may result in poor patient outcomes. The benefits of currently available treatments and the possible strategies to overcome the barriers that limit the achievement of asthma control in real-life situations will also be discussed.
The treatment of asthma: where are we? Evolution of a concept
Asthma control medications reduce airway inflammation and help to prevent asthma symptoms; among these, inhaled corticosteroids (ICS) are the mainstay in the treatment of asthma, whereas quick-relief (reliever) or rescue medicines quickly ease symptoms that may arise acutely. Among these, short-acting beta-agonists (SABAs) rapidly reduce airway bronchoconstriction (causing relaxation of airway smooth muscles).
National and international guidelines have recommended SABAs as first-line treatment for patients with mild asthma, since the Global Initiative for Asthma guidelines (GINA) were first published in 1995, adopting an approach aimed to control the symptoms rather than the underlying condition; a SABA has been the recommended rescue medication for rapid symptom relief. This approach stems from the dated idea that asthma symptoms are related to bronchial smooth muscle contraction (bronchoconstriction) rather than a condition concomitantly caused by airway inflammation. In 2019, the GINA guidelines review (GINA 2019) [ 4 ] introduced substantial changes overcoming some of the limitations and “weaknesses” of the previously proposed stepwise approach to adjusting asthma treatment for individual patients. The concept of an anti-inflammatory reliever has been adopted at all degrees of severity as a crucial component in the management of the disease, increasing the efficacy of the treatment while lowering SABA risks associated with patients’ tendency to rely or over-rely on the as-needed medication.
Until 2017, the GINA strategy proposed a pharmacological approach based on a controller treatment (an anti-inflammatory, the pillar of asthma treatment), with a SABA as an additional rescue intervention. The reliever, a short-acting bronc hodilator, was merely an addendum , a medication to be used in case the real treatment (the controller) failed to maintain disease control: SABAs effectively induce rapid symptom relief but are ineffective on the underlying inflammatory process. Based on the requirement to achieve control, the intensity of the controller treatment was related to the severity of the disease, varying from low-dose ICS to combination low-dose ICS/long-acting beta-agonist (LABA), medium-dose ICS/LABA, up to high-dose ICS/LABA, as preferred controller choice, with a SABA as the rescue medication. As a result, milder patients were left without any anti-inflammatory treatment and could only rely on SABA rescue treatment.
Poor adherence to therapy is a major limitation of a treatment strategy based on the early introduction of the regular use of controller therapy [ 5 ]. Indeed, a number of surveys have highlighted a common pattern in the use of inhaled medication [ 6 ], in which treatment is administered only when asthma symptoms occur; in the absence of symptoms, treatment is avoided as patients perceive it as unnecessary. When symptoms worsen, patients prefer to use reliever therapies, which may result in the overuse of SABAs [ 7 ]. Indirect evidence suggests that the overuse of beta-agonists alone is associated with increased risk of death from asthma [ 8 ].
In patients with mild persistent disease, low-dose ICS decreases the risk of severe exacerbations leading to hospitalization and improves asthma control [ 9 ]. When low-dose ICS are ineffective in controlling the disease (Step 3 of the stepwise approach), a combination of low-dose ICS with LABA maintenance was the recommended first-choice treatment, plus as-needed SABA [ 3 , 10 ]. Alternatively, the combination low-dose ICS/LABA (formoterol) was to be used as single maintenance and reliever treatment (SMART). The SMART strategy containing the rapid-acting formoterol was recommended throughout GINA Steps 3 to 5 based on solid clinical-data evidence [ 3 ].
The addition of a LABA to ICS treatment reduces both severe and mild asthma exacerbation rates, as shown in the one-year, randomized, double-blind, parallel-group FACET study [ 11 ]. This study focused on patients with persistent asthma symptoms despite receiving ICS and investigated the efficacy of the addition of formoterol to two dose levels of budesonide (100 and 400 µg bid ) in decreasing the incidence of both severe and mild asthma exacerbations. Adding formoterol decreased the incidence of both severe and mild asthma exacerbations, independent of ICS dose. Severe and mild exacerbation rates were reduced by 26% and 40%, respectively, with the addition of formoterol to the lower dose of budesonide; the corresponding reductions were 63% and 62%, respectively, when formoterol was added to budesonide at the higher dose.
The efficacy of the ICS/LABA combination was confirmed in the post hoc analysis of the FACET study, in which patients were exposed to a combination of formoterol and low-dose budesonide [ 12 ]. However, such high levels of asthma control are not achieved in real life [ 5 ]. An explanation for this is that asthma is a variable condition and this variability might include the exposure of patients to factors which may cause a transient steroid insensitivity in the inflammatory process. This, in turn, may lead to an uncontrolled inflammatory response and to exacerbations, despite optimal controller treatment. A typical example of this mechanism is given by viral infections, the most frequent triggers of asthma exacerbations. Rhinoviruses, the most common viruses found in patients with asthma exacerbations, interfere with the mechanism of action of corticosteroids making the anti-inflammatory treatment transiently ineffective. A transient increase in the anti-inflammatory dose would overcome the trigger-induced anti-inflammatory resistance, avoiding uncontrolled inflammation leading to an exacerbation episode [ 13 , 14 , 15 ].
Indeed, symptoms are associated with worsening inflammation and not only with bronchoconstriction. Romagnoli et al. showed that inflammation, as evidenced by sputum eosinophilia and eosinophilic markers, is associated with symptomatic asthma [ 16 ]. A transient escalation of the ICS dose would prevent loss of control over inflammation and decrease the risk of progression toward an acute episode. In real life, when experiencing a deterioration of asthma control, patients self-treat by substantially increasing their SABA medication (Fig. 1 ); it is only subsequently that they (modestly) increase the maintenance treatment [ 17 ].
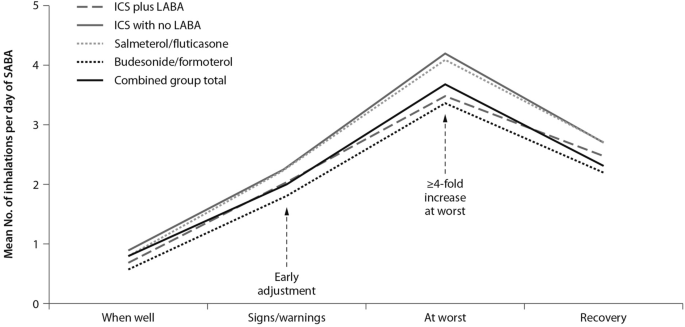
Mean use of SABA at different stages of asthma worsening. Patients have been grouped according to maintenance therapy shown in the legend. From [ 17 ], modified
As bronchodilators, SABAs do not control the underlying inflammation associated with increased symptoms. The “as required” use of SABAs is not the most effective therapeutic option in controlling a worsening of inflammation, as signaled by the occurrence of symptoms; instead, an anti-inflammatory therapy included in the rescue medication along with a rapid-acting bronchodilator could provide both rapid symptom relief and control over the underlying inflammation. Thus, there is a need for a paradigm shift, a new therapeutic approach based on the rescue use of an inhaled rapid-acting beta-agonist combined with an ICS: an anti-inflammatory reliever strategy [ 18 ].
The symptoms of an exacerbation episode, as reported by Tattersfield and colleagues in their extension of the FACET study, increase gradually before the peak of the exacerbation (Fig. 2 ); and the best marker of worsening asthma is the increased use of rescue beta-agonist treatment that follows exactly the pattern of worsening symptomatology [ 19 ]. When an ICS is administered with the rescue bronchodilator, the patient would receive anti-inflammatory therapy when it is required; that is, when the inflammation is uncontrolled, thus increasing the efficiency of the anti-inflammatory treatment.
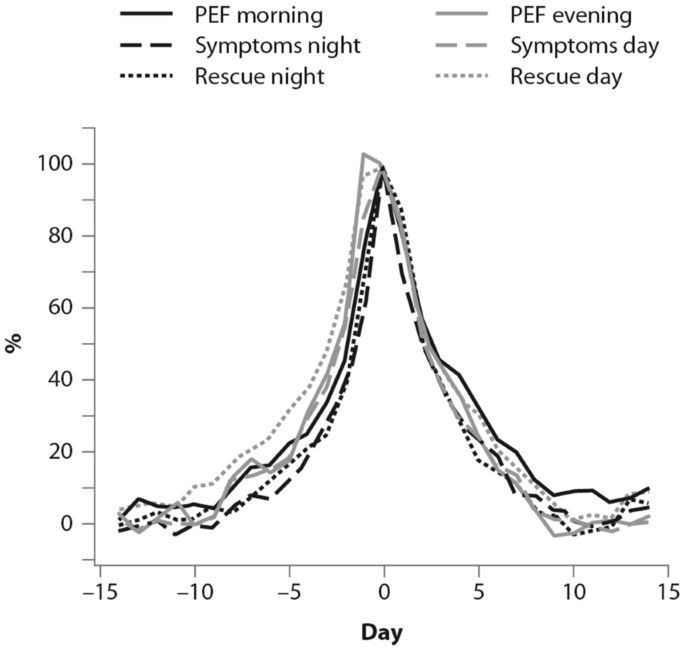
(From [ 19 ])
Percent variation in symptoms, rescue beta-agonist use and peak expiratory flow (PEF) during an exacerbation. In order to allow comparison over time, data have been standardized (Day-14 = 0%; maximum change = 100%)
Barriers and paradoxes of asthma management
A number of barriers and controversies in the pharmacological treatment of asthma have prevented the achievement of effective disease management [ 20 ]. O’Byrne and colleagues described several such controversies in a commentary published in 2017, including: (1) the recommendation in Step 1 of earlier guidelines for SABA bronchodilator use alone, despite asthma being a chronic inflammatory condition; and (2) the autonomy given to patients over perception of need and disease control at Step 1, as opposed to the recommendation of a fixed-dose approach with treatment-step increase, regardless of the level of symptoms [ 20 ]. Other controversies outlined were: (3) a difficulty for patients in understanding the recommendation to minimize SABA use at Step 2 and switch to a fixed-dose ICS regimen, when they perceive SABA use as more effective; (4) apparent conflicting safety messages within the guidelines that patient-administered SABA monotherapy is safe, but patient-administered LABA monotherapy is not; and (5) a discrepancy as to patients’ understanding of “controlled asthma” and their symptom frequency, impact and severity [ 20 ].
Controversies (1) and (2) can both establish an early over-dependence on SABAs. Indeed, asthma patients freely use (and possibly overuse) SABAs as rescue medication. UK registry data have recently suggested SABA overuse or overreliance may be linked to asthma-related deaths: among 165 patients on short-acting relievers at the time of death, 56%, 39%, and 4% had been prescribed > 6, > 12, and > 50 SABA inhalers respectively in the previous year [ 21 ]. Registry studies have shown the number of SABA canisters used per year to be directly related to the risk of death in patients with asthma. Conversely, the number of ICS canisters used per year is inversely related to the rate of death from asthma, when compared with non-users of ICS [ 8 , 22 ]. Furthermore, low-dose ICS used regularly are associated with a decreased risk of asthma death, with discontinuation of these agents possibly detrimental [ 22 ].
Other barriers to asthma pharmacotherapy have included the suggestion that prolonged treatment with LABAs may mask airway inflammation or promote tolerance to their effects. Investigating this, Pauwels and colleagues found that in patients with asthma symptoms that were persistent despite taking inhaled glucocorticoids, the addition of regular treatment with formoterol to budesonide for a 12-month period did not decrease asthma control, and improved asthma symptoms and lung function [ 11 ].
Treatment strategies across all levels of asthma severity
Focusing on risk reduction, the 2014 update of the GINA guidelines recommended as-needed SABA for Step 1 of the stepwise treatment approach, with low-dose ICS maintenance therapy as an alternative approach for long-term anti-inflammatory treatment [ 23 ]. Such a strategy was only supported by the evidence from a post hoc efficacy analysis of the START study in patients with recently diagnosed mild asthma [ 24 ]. The authors showed that low-dose budesonide reduced the decline of lung-function over 3 years and consistently reduced severe exacerbations, regardless of symptom frequency at baseline, even in subjects with symptoms below the then-threshold of eligibility for ICS [ 24 ]. However, as for all post hoc analyses, the study by Reddel and colleagues does not provide conclusive evidence and, even so, their results could have questionable clinical significance for the management of patients with early mild asthma. To be effective, this approach would require patients to be compliant to regular twice-daily ICS for 10 years to have the number of exacerbations reduce by one. In real life, it is highly unlikely that patients with mild asthma would adhere to such a regular regimen [ 25 ].
The 2016 update to the GINA guidelines lowered the threshold for the use of low-dose ICS (GINA Step 2) to two episodes of asthma symptoms per month (in the absence of any supportive evidence for the previous cut-off). The objective was to effectively increase the asthma population eligible to receive regular ICS treatment and reduce the population treated with a SABA only, given the lack of robust evidence of the latter’s efficacy and safety and the fact that asthma is a variable condition characterized by acute exacerbations [ 26 ]. Similarly, UK authorities recommended low-dose ICS treatment in mild asthma, even for patients with suspected asthma, rather than treatment with a SABA alone [ 10 ]. However, these patients are unlikely to have good adherence to the regular use of an ICS. It is well known that poor adherence to treatment is a major problem in asthma management, even for patients with severe asthma. In their prospective study of 2004, Krishnan and colleagues evaluated the adherence to ICS and oral corticosteroids (OCS) in a cohort of patients hospitalized for asthma exacerbations [ 27 ]. The trend in the data showed that adherence to ICS and OCS treatment in patients dropped rapidly to reach nearly 50% within 7 days of hospital discharge, with the rate of OCS discontinuation per day nearly double the rate of ICS discontinuation per day (− 5.2% vs. − 2.7%; p < 0.0001 respectively, Fig. 3 ), thus showing that even after a severe event, patients’ adherence to treatment is suboptimal [ 27 ].

(From [ 27 ])
Use of inhaled (ICS) and oral (OCS) corticosteroids in patients after hospital discharge among high-risk adult patients with asthma. The corticosteroid use was monitored electronically. Error bars represent the standard errors of the measured ICS and OCS use
Guidelines set criteria with the aim of achieving optimal control of asthma; however, the attitude of patients towards asthma management is suboptimal. Partridge and colleagues were the first in 2006 to evaluate the level of asthma control and the attitude of patients towards asthma management. Patients self-managed their condition using their medication as and when they felt the need, and adjusted their treatment by increasing their intake of SABA, aiming for an immediate relief from symptoms [ 17 ]. The authors concluded that the adoption of a patient-centered approach in asthma management could be advantageous to improve asthma control.
The concomitant administration of an as-needed bronchodilator and ICS would provide rapid relief while administering anti-inflammatory therapy. This concept is not new: in the maintenance and reliever approach, patients are treated with ICS/formoterol (fast-acting, long-acting bronchodilator) combinations for both maintenance and reliever therapy. An effective example of this therapeutic approach is provided in the SMILE study in which symptomatic patients with moderate to severe asthma and treated with budesonide/formoterol as maintenance therapy were exposed to three different as-needed options: SABA (terbutaline), rapid-onset LABA (formoterol) and a combination of LABA and ICS (budesonide/formoterol) [ 28 ]. When compared with formoterol, budesonide/formoterol as reliever therapy significantly reduced the risk of severe exacerbations, indicating the efficacy of ICS as rescue medication and the importance of the as-needed use of the anti-inflammatory reliever.
The combination of an ICS and a LABA (budesonide/formoterol) in one inhaler for both maintenance and reliever therapy is even more effective than higher doses of maintenance ICS and LABA, as evidenced by Kuna and colleagues and Bousquet and colleagues (Fig. 4 ) [ 29 , 30 ].
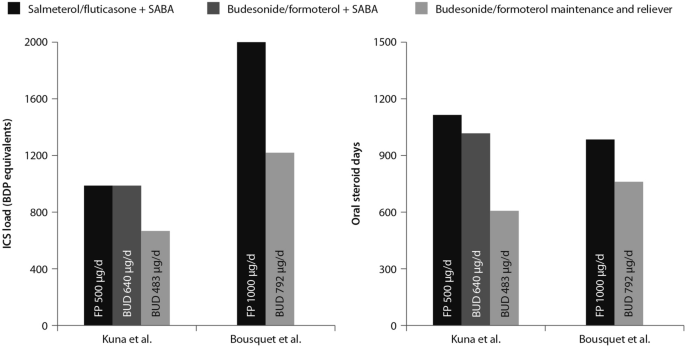
(Data from [ 29 , 30 ])
Comparison between the improvements in daily asthma control resulting from the use of budesonide/formoterol maintenance and reliever therapy vs. higher dose of ICS/LABA + SABAZ and steroid load for the two regimens
The effects of single maintenance and reliever therapy versus ICS with or without LABA (controller therapy) and SABA (reliever therapy) have been recently addressed in the meta-analysis by Sobieraj and colleagues, who analysed 16 randomized clinical trials involving patients with persistent asthma [ 31 ]. The systematic review supported the use of single maintenance and reliever therapy, which reduces the risk of exacerbations requiring systemic corticosteroids and/or hospitalization when compared with various strategies using SABA as rescue medication [ 31 ].
This concept was applied to mild asthma by the BEST study group, who were the first to challenge the regular use of ICS. A pilot study by Papi and colleagues evaluated the efficacy of the symptom-driven use of beclomethasone dipropionate plus albuterol in a single inhaler versus maintenance with inhaled beclomethasone and as-needed albuterol. In this six-month, double-blind, double-dummy, randomized, parallel-group trial, 455 patients with mild asthma were randomized to one of four treatment groups: an as-needed combination therapy of placebo bid plus 250 μg of beclomethasone and 100 μg of albuterol in a single inhaler; an as-needed albuterol combination therapy consisting of placebo bid plus 100 μg of albuterol; regular beclomethasone therapy, comprising beclomethasone 250 μg bid and 100 μg albuterol as needed); and regular combination therapy with beclomethasone 250 μg and albuterol 100 μg in a single inhaler bid plus albuterol 100 μg as needed.
The rescue use of beclomethasone/albuterol in a single inhaler was as efficacious as the regular use of inhaled beclomethasone (250 μg bid ) and it was associated with a lower 6-month cumulative dose of the ICS [ 32 ].
The time to first exacerbation differed significantly among groups ( p = 0.003), with the shortest in the as-needed albuterol and placebo group (Fig. 5 ). Figure 5 also shows equivalence between the as-needed combination therapy and the regular beclomethasone therapy. However, these results were not conclusive since the study was not powered to evaluate the effect of the treatment on exacerbations. In conclusion, as suggested by the study findings, mild asthma patients may require the use of an as-needed ICS and an inhaled bronchodilator rather than a regular treatment with ICS [ 32 ].
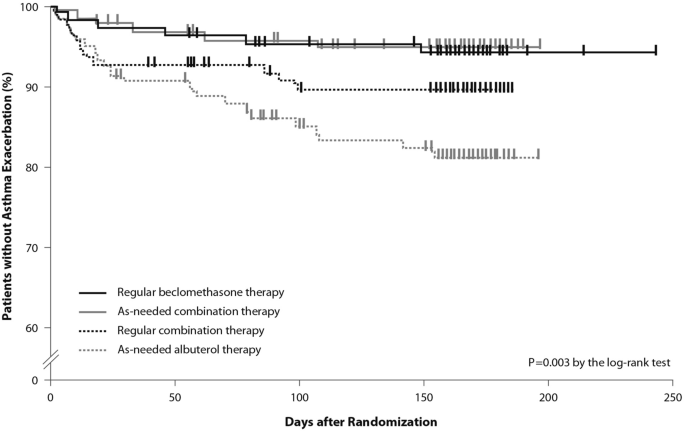
(From [ 32 ])
Kaplan Meier analysis of the time to first exacerbation (modified intention-to-treat population). First asthma exacerbations are shown as thick marks. As-needed albuterol therapy = placebo bid plus 100 μg of albuterol as needed; regular combination therapy = 250 μg of beclomethasone and 100 μg of albuterol in a single inhaler bid plus 100 μg of albuterol as needed; regular beclomethasone therapy = 250 μg of beclomethasone bid and 100 μg of albuterol as needed; as-needed combination therapy = placebo bid plus 250 μg of beclomethasone and 100 μg of albuterol in a single inhaler as needed

Moving forward: a new approach to the management of asthma patients
Nearly a decade after the publication of the BEST study in 2007, the use of this alternative therapeutic strategy was addressed in the SYGMA 1 and SYGMA 2 trials. These double-blind, randomized, parallel-group, 52-week phase III trials evaluated the efficacy of as-needed use of combination formoterol (LABA) and the ICS budesonide as an anti-inflammatory reliever in patients requiring GINA Step 2 treatment, with the current reliever therapy (e.g. as-needed SABA) or with low-dose maintenance ICS (inhaled budesonide bid ) plus as-needed SABA, administered as regular controller therapy [ 33 , 34 ].
The SYGMA 1 trial, which enrolled 3849 patients, aimed to demonstrate the superiority of the as-needed use of the combination budesonide/formoterol over as-needed terbutaline, as measured by the electronically-recorded proportion of weeks with well-controlled asthma [ 34 ]. The more pragmatic SYGMA 2 trial enrolled 4215 patients with the aim to demonstrate that the budesonide/formoterol combination is non-inferior to budesonide plus as-needed terbutaline in reducing the relative rate of annual severe asthma exacerbations [ 33 ]. Both trials met their primary efficacy outcomes. In particular, as-needed budesonide/formoterol was superior to as-needed SABA in controlling asthma symptoms (34.4% versus 31.1%) and preventing exacerbations, achieving a 64% reduction in exacerbations. In both trials, budesonide/formoterol as-needed was similar to budesonide maintenance bid at preventing severe exacerbations, with a substantial reduction of the inhaled steroid load over the study period (83% in the SYGMA 1 trial and 75% in the SYGMA 2 trial). The time to first exacerbation did not differ significantly between the two regimens; however, budesonide/formoterol was superior to SABA in prolonging the time to first severe exacerbation [ 33 , 34 ].
The double-blind, placebo-controlled design of the SYGMA trials does not fully address the advantages of anti-inflammatory reliever strategy in patients who often rely on SABAs for symptom relief, so to what extent the study findings could apply to real-life practice settings was unclear.
These limitations were overcome by the results of the Novel START study, an open-label, randomized, parallel-group, controlled trial designed to reflect real-world practice, which demonstrated the effectiveness in mild asthma of budesonide/formoterol as an anti-inflammatory reliever therapy [ 35 ].
In real-world practice, mild asthma patients are treated with an as-needed SABA reliever or with daily low-dose ICS maintenance therapy plus a SABA reliever. In the Novel START study, 668 patients with mild asthma were randomized to receive either as-needed albuterol 100 µg, two inhalations (SABA reliever as a continuation of the Step 1 treatment according to the 2017 GINA guidelines), budesonide 200 µg (ICS maintenance treatment) plus as-needed albuterol (Step 2 therapy of the GINA 2017 guidelines), or 200 µg/6 µg budesonide/formoterol as anti-inflammatory reliever therapy taken as-needed for a 52-week study period.
In this study, the rate of asthma exacerbations for budesonide/formoterol was lower compared with albuterol (51%) and similar to the twice-daily maintenance budesonide plus albuterol, despite a 52% reduction in the mean steroid dose with the single combination inhaler treatment [ 35 ]. In addition, severe exacerbation rate was lower with budesonide/formoterol as compared with as-needed albuterol and regular twice-daily budesonide. These data support the findings of the SYGMA 1 and 2 trials, highlighting the need for a critical re-examination of current clinical practice. Along with the results of the SYGMA trials, they provide convincing evidence of the advantages of the anti-inflammatory reliever strategy, particularly in real-life settings.
The SYGMA 1, SYGMA 2 and the novel START studies complete the picture of the treatment strategies for asthma at any degree of severity, including mild asthma. A growing body of evidence shows that an anti-inflammatory reliever strategy, when compared with all other strategies with SABA reliever, consistently reduces the rate of exacerbations across all levels of asthma severity (Fig. 6 ) [ 28 , 29 , 34 , 36 , 37 , 38 , 39 ].
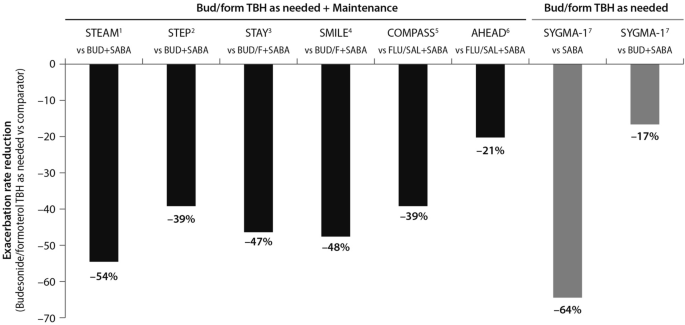
(Data source: [ 39 ])
Risk reduction of severe asthma attack of anti-inflammatory reliever versus SABA across all levels of asthma severity. Bud = budesonide; form = formoterol; TBH = turbohaler. Data from: 1: [ 36 ]; 2: [ 37 ]; 3: [ 38 ]; 4: [ 28 ]; 5: [ 29 ]; 6: [ 30 ]; 7: [ 34 ]
This evidence set the ground (Fig. 7 ) for the release of the 2019 GINA strategy updates. The document provides a consistent approach towards the management of the disease and aims to avoid the overreliance and overuse of SABAs, even in the early course of the disease. The 2019 GINA has introduced key changes in the treatment of mild asthma: for safety reasons, asthmatic adults and adolescents should receive ICS-containing controller treatment instead of the SABA-only treatment, which is no longer recommended.
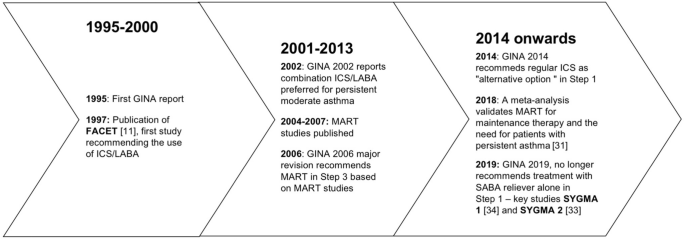
Timeline of key randomized controlled trials and meta-analyses providing the supporting evidence base leading to the Global Initiative for Asthma (GINA) 2019 guidelines. GINA global initiative for asthma, MART maintenance and reliever therapy, SMART single inhaler maintenance and reliever therapy
In Step 1 of the stepwise approach to adjusting asthma treatment, the preferred controller option for patients with fewer than two symptoms/month and no exacerbation risk factors is low-dose ICS/formoterol as needed. This strategy is indirectly supported by the results of the SYGMA 1 study which evaluated the efficacy and safety of budesonide/formoterol as needed, compared with as-needed terbutaline and budesonide bid plus as-needed terbutaline (see above). In patients with mild asthma, the use of an ICS/LABA (budesonide/formoterol) combination as needed provided superior symptom control to as-needed SABA, resulting in a 64% lower rate of exacerbations (p = 0.07) with a lower steroid dose (17% of the budesonide maintenance dose) [ 34 ]. The changes extend to the other controller options as well. In the 2017 GINA guidelines, the preferred treatment was as-needed SABA with the option to consider adding a regular low-dose ICS to the reliever. In order to overcome the poor adherence with the ICS regimen, and with the aim to reduce the risk of severe exacerbations, the 2019 GINA document recommends taking low-dose ICS whenever SABA is taken, with the daily ICS option no longer listed.
Previous studies including the TREXA study in children and adolescents [ 40 ], the BASALT study [ 41 ] and research conducted by the BEST study group [ 32 ] have already added to the evidence that a low-dose ICS with a bronchodilator is an effective strategy for symptom control in patients with mild asthma. A recently published study in African-American children with mild asthma found that the use of as-needed ICS with SABA provides similar asthma control, exacerbation rates and lung function measures at 1 year, compared with daily ICS controller therapy [ 42 ], adding support to TREXA findings that in children with well controlled, mild asthma, ICS used as rescue medication with SABA may be an efficacious step-down strategy [ 40 ].
In Step 2 of the stepwise approach, there are now two preferred controller options: (a) a daily low-dose ICS plus an as-needed SABA; and (b) as-needed low-dose ICS/formoterol. Recommendation (a) is supported by a large body of evidence from randomized controlled trials and observations showing a substantial reduction of exacerbation, hospitalization, and death with regular low-dose ICS [ 7 , 8 , 9 , 24 , 43 ], whereas recommendation (b) stems from evidence on the reduction or non-inferiority for severe exacerbations when as-needed low-dose ICS/formoterol is compared with regular ICS [ 33 , 34 ].
The new GINA document also suggests low-dose ICS is taken whenever SABA is taken, either as separate inhalers or in combination. This recommendation is supported by studies showing reduced exacerbation rates compared with taking a SABA only [ 32 , 40 ], or similar rates compared with regular ICS [ 32 , 40 , 41 ]. Low-dose theophylline, suggested as an alternative controller in the 2017 GINA guidelines, is no longer recommended.
Airway inflammation is present in the majority of patients with asthma, and although patients with mild asthma may have only infrequent symptoms, they face ongoing chronic inflammation of the lower airways and risk acute exacerbations. The GINA 2019 strategy recognizes the importance of reducing the risk of asthma exacerbations, even in patients with mild asthma (Steps 1 and 2) [ 4 ]. In this regard, the new recommendations note that SABA alone for symptomatic treatment is non-protective against severe exacerbation and may actually increase exacerbation risk if used regularly or frequently [ 4 ].
The reluctance by patients to regularly use an ICS controller means they may instead try and manage their asthma symptoms by increasing their SABA reliever use. This can result in SABA overuse and increased prescribing, and increased risk of exacerbations.
As part of the global SABINA (SABA use IN Asthma) observational study programme, a UK study examined primary care records to describe the pattern of SABA and ICS use over a 10-year period in 373,256 patients with mild asthma [ 44 ]. Results showed that year-to-year SABA prescribing was more variable than that of ICS indicating that, in response to fluctuations in asthma symptom control, SABA use was increased in preference to ICS use. Furthermore, more than 33% of patients were prescribed SABA inhalers at a level equivalent to around ≥ 3 puffs per week which, according to GINA, suggests inadequate asthma control.
The problem of SABA overuse is further highlighted by two studies [ 45 , 46 ], also as part of the SABINA programme. These analysed data from 365,324 patients in a Swedish cohort prescribed two medications for obstructive lung disease in any 12-month period (HERA).
The first study identified SABA overuse (defined as ≥ 3 SABA canisters a year) in 30% of patients, irrespective of their ICS use; 21% of patients were collecting 3–5 canisters annually, 7% were collecting 6–10, and 2% more than 11 [ 45 ]. Those patients who were overusing SABA had significantly more asthma exacerbations relative to those using < 3 canisters (20.0 versus 12.5 per 100 patient years; relative risk 1.60, 95% CI 1.57–1.63, p < 0.001). Moreover, patients overusing SABA and whose asthma was more severe (GINA Steps 3 and 4) had greater exacerbation risk compared with overusing patients whose asthma was milder (GINA Steps 1 and 2).
The second study found those patients using three or more SABA reliever canisters a year had an increased all-cause mortality risk relative to patients using fewer SABA canisters: hazard ratios after adjustment were 1.26 (95% CI 1.14–1.39) for 3–5 canisters annually, 1.67 (1.49–1.87) for 6–10 canisters, and 2.35 (2.02–2.72) for > 11 canisters, relative to patients collecting < 3 canisters annually [ 46 ].
The recently published PRACTICAL study lends further support to as-needed low-dose ICS/formoterol as an alternative option to daily low-dose ICS plus as-needed SABA, outlined in Step 2 of the guidelines [ 47 ]. In their one-year, open-label, multicentre, randomized, superiority trial in 890 patients with mild to moderate asthma, Hardy and colleagues found that the rate of severe exacerbations per patient per year (the primary outcome) was lower in patients who received as-needed budesonide/formoterol than in patients who received controller budesonide plus as-needed terbutaline (relative rate 0.69, 95% CI 0.48–1.00; p < 0.05). Indeed, they suggest that of these two treatment options, as-needed low-dose ICS/formoterol may be preferred over controller low-dose ICS plus as-needed SABA for the prevention of severe exacerbations in this patient population.
Step 3 recommendations have been left unchanged from 2017, whereas Step 4 treatment has changed from recommending medium/high-dose ICS/LABA [ 3 ] to medium-dose ICS/LABA; the high-dose recommendation has been escalated to Step 5. Patients who have asthma that remains uncontrolled after Step 4 treatment should be referred for phenotypic assessment with or without add-on therapy.
To summarise, the use of ICS medications is of paramount importance for optimal asthma control. The onset and increase of symptoms are indicative of a worsening inflammation leading to severe exacerbations, the risk of which is reduced by a maintenance plus as-needed ICS/LABA combination therapy. The inhaled ICS/bronchodilator combination is as effective as the regular use of inhaled steroids.
The efficacy of anti-inflammatory reliever therapy (budesonide/formoterol) versus current standard-of-care therapies in mild asthma (e.g. reliever therapy with a SABA as needed and regular maintenance controller therapy plus a SABA as-needed) has been evaluated in two randomized, phase III trials which confirmed that, with respect to as-needed SABA, the anti-inflammatory reliever as needed is superior in controlling asthma and reduces exacerbation rates, exposing the patients to a substantially lower glucocorticoid dose.
Conclusions
A growing body of evidence shows that anti-inflammatory reliever strategy is more effective than other strategies with SABA reliever in controlling asthma and reducing exacerbations across all levels of asthma severity. A budesonide/formoterol therapy exposes asthma patients to a substantially lower glucocorticoid dose while cutting the need for adherence to scheduled therapy.
Availability of data and materials
Not applicable.
Abbreviations
Global Initiative for Asthma
Inhaled corticosteroids
Long-acting beta-agonists
Oral corticosteroids
Short-acting beta-agonists
Single inhaler maintenance and reliever treatment
World Health Organization. Asthma. 2017. https://www.who.int/news-room/fact-sheets/detail/asthma . Accessed 9 April 2019.
Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391(10122):783–800.
Article PubMed Google Scholar
Global Initiative for Asthma. Global strategy for asthma management and prevention, 2017. http://www.ginasthma.org . Accessed 1 June 2019.
Global Initiative for Asthma. Pocket guide for asthma management and prevention. 2019, pp. 1–32. www.ginasthma.org . Accessed 1 June 2019.
Rabe KF, Vermeire PA, Soriano JB, Maier WC. Clinical management of asthma in 1999: the Asthma Insights and Reality in Europe (AIRE) study. Eur Respir J. 2000;16(5):802–7.
Article CAS PubMed Google Scholar
Price D, Fletcher M, Molen V. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med. 2014;24:14009.
Article PubMed Central PubMed Google Scholar
Suissa S, Ernst P, Kezouh A. Regular use of inhaled corticosteroids and the long term prevention of hospitalisation for asthma. Thorax. 2002;57(10):880–4.
Article CAS PubMed Central PubMed Google Scholar
Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. New Engl J Med. 2000;343(5):332–6.
Pauwels RA, Pedersen S, Busse WW, Tan WC, Chen YZ, Ohlsson SV, et al. Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet. 2003;361(9363):1071–6.
Healthcare Improvement Scotland and British Thoracic Society. SIGN 153: British guideline on the management of asthma. 2016. https://www.sign.ac.uk/sign-153-british-guideline-on-the-management-of-asthma.html .
Pauwels RA, Löfdahl CG, Postma DS, Tattersfield AE, O’Byrne P, Barnes PJ, Ullman A. Effect of inhaled formoterol and budesonide on exacerbations of asthma. N Engl J Med. 1997;337(20):1405–11.
O’Byrne PM, Naya IP, Kallen A, Postma DS, Barnes PJ. Increasing doses of inhaled corticosteroids compared to adding long-acting inhaled β2-agonists in achieving asthma control. Chest. 2008;134(6):1192–9.
Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310(6989):1225–9.
Corne JM, Marshall C, Smith S, Schreiber J, Sanderson G, Holgate ST, Johnston SL. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359(9309):831–4.
Papi A, Contoli M, Adcock IM, Bellettato C, Padovani A, Casolari P, et al. Rhinovirus infection causes steroid resistance in airway epithelium through nuclear factor κb and c-Jun N-terminal kinase activation. J Allergy Clin Immunol. 2013;132(5):1075–85.
Romagnoli M, Vachier I, Tarodo de la Fuente P, Meziane H, Chavis C, Bousquet J, et al. Eosinophilic inflammation in sputum of poorly controlled asthmatics. Eur Respir J. 2002;20(6):1370–7.
Partridge MR, van der Molen T, Myrseth SE, Busse WW. Attitudes and actions of asthma patients on regular maintenance therapy: the INSPIRE study. BMC Pulm Med. 2006;6:13.
Papi A, Caramori G, Adcock IM, Barnes PJ. Rescue treatment in asthma. More than as-needed bronchodilation. Chest. 2009;135(6):1628–33.
PubMed Google Scholar
Tattersfield AE, Postma DS, Barnes PJ, Svensson K, Bauer CA, Byrne PM, et al. Exacerbations of asthma: a descriptive study of 425 severe exacerbations The FACET International Study Group. Am J Respir Crit Care Med. 1999;160(2):594–9.
O’Byrne PM, Jenkins C, Bateman ED. The paradoxes of asthma management: time for a new approach? Eur Respir J. 2017. https://doi.org/10.1183/13993003.01103-2017 .
Royal College of Physicians. Why asthma still kills: the National Review of Asthma Deaths (NRAD). Confidential Enquiry report. London: RCP; 2014.
Google Scholar
Suissa S, Ernst P, Boivin JF, Horwitz RI, Habbick B, Cockroft D, et al. A cohort analysis of excess mortality in asthma and the use of inhaled β-agonists. Am J Respir Crit Care Med. 1994;149(3 Pt 1):604–10.
Global Initiative for Asthma. Global strategy for asthma management and prevention, 2014. https://ginasthma.org/wp-content/uploads/2019/01/2014-GINA.pdf .
Reddel HK, Busse WW, Pedersen S, Tan WC, Chen YZ, Jorup C, et al. Should recommendations about starting inhaled corticosteroid treatment for mild asthma be based on symptom frequency: a post hoc efficacy analysis of the START study. Lancet. 2017;389(10065):157–66.
Papi A, Fabbri LM. Management of patients with early mild asthma and infrequent symptoms. Lancet. 2017;389(10065):129–30.
Global Initiative for Asthma. Global strategy for asthma management and prevention, 2016. https://ginasthma.org/wp-content/uploads/2016/04/GINA-Appendix-2016-final.pdf .
Krishnan JA, Riekert KA, McCoy JV, Stewart DY, Schmidt S, Chanmugam A, et al. Corticosteroid use after hospital discharge among high-risk adults with asthma. Am J Respir Crit Care Med. 2004;170(12):1281–5.
Rabe KF, Atienza T, Magyar P, Larsson P, Jorup C, Lalloo UG. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomised controlled, double-blind study. Lancet. 2006;368(9537):744–53.
Kuna P, Peters MJ, Manjra AI, Jorup C, Naya IP, Martínez-Jimenez NE, Buhl R. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. Int J Clin Pract. 2007;61(5):725–36.
Bousquet J, Boulet LP, Peters MJ, Magnussen H, Quiralte J, Martinez-Aguilar NE, Carlsheimer A. Budesonide/formoterol for maintenance and relief in uncontrolled asthma vs. high-dose salmeterol/fluticasone. Respir Med. 2007;101(12):2437–46.
Sobieraj DM, Weeda ER, Nguyen E, Coleman CI, White CM, Lazarus SC, et al. Association of inhaled corticosteroids and long-acting β-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma a systematic review and meta-analysis. JAMA. 2018;319(14):1485–96.
Papi A, Canonica GW, Maestrelli P, Paggiaro P, Olivieri D, Pozzi E, BEST Study Group, et al. Rescue use of beclomethasone and albuterol in a single inhaler for mild asthma. New Engl J Med. 2007;356(20):2040–52.
Bateman ED, Reddel HK, O’Byrne PM, Barnes PJ, Zhong N, Keen C, et al. As-needed budesonide–formoterol versus maintenance budesonide in mild asthma. New Engl J Med. 2018;378(20):1877–87.
O’Byrne PM, FitzGerald JM, Bateman ED, Barnes PJ, Zhong N, Keen C, et al. Inhaled combined budesonide-formoterol as needed in mild asthma. New Engl J Med. 2018;378(20):1865–76.
Beasley R, Holliday M, Reddel HK, Braithwaite I, Ebmeier S, Hancox RJ, Novel START Study Team, et al. Controlled trial of budesonide-formoterol as needed for mild asthma. New Engl J Med. 2019;380(21):2020–30.
Rabe K, Pizzichini E, Ställberg B, Romero S, Balanzat AM, Atienza T, et al. Budesonide/formoterol in a single inhaler for maintenance and relief in mild-to-moderate asthma: a randomized, double-blind trial. Chest. 2006;129(2):246–56.
Scicchitano R, Aalbers R, Ukena D, Manjra A, Fouquert L, Centanni S, et al. Efficacy and safety of budesonide/formoterol single inhaler therapy versus a higher dose of budesonide in moderate to severe asthma. Curr Med Res Opin. 2004;20(9):1403–18.
O’Byrne PM, Bisgaard H, Godard PP, Pistolesi M, Palmqvist M, Zhu Y, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med. 2005;171(2):129–36.
Pavord ID, Beasley R, Agusti A, Anderson GP, Bel E, Brusselle G, et al. After asthma: redefining airways diseases. Lancet. 2017;391(10118):350–400.
Martinez FD, Chinchilli VM, Morgan WJ, Boehmer SJ, Lemanske RF Jr, Mauger DT, et al. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377(9766):650–7.
Calhoun WJ, Ameredes BT, King TS, Icitovic N, Bleecker ER, Castro M, et al. Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial. JAMA. 2012;308(10):987–97.
Sumino K, Bacharier LB, Taylor J, et al. A pragmatic trial of symptom-based inhaled corticosteroid use in African-American children with mild asthma. J Allergy Clin Immunol Pract. 2020;8(176–85):e2.
Byrne PM, Barnes PJ, Rodriguez-Roisin R, Runnerstrom E, Sandstrom T, Svensson K, Tattersfield A. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med. 2001;164(8):1392–7.
Article Google Scholar
Bloom C, Quint J, Cabrera C. SABA and ICS prescriptions among mild asthma patients in UK primary care. Poster presented at the European Respiratory Society International Congress; 2019 Sep 28–Oct 2; Madrid, Spain.
Janson C, Nwaru B, Hasvold P, Wicklund F, Telg G, Ekstrom M. Use of short-acting beta-2 agonists (SABA) and exacerbations in a nationwide Swedish asthma cohort (HERA). Poster presented at the European Respiratory Society International Congress; 2019 Sep 28–Oct 2; Madrid, Spain.
Janson C, Nwaru B, Hasvold P, Wicklund F, Telg G, Ekstrom M. SABA overuse and risk of mortality in a nationwide Swedish asthma cohort (HERA). Late Breaker abstract at the European Respiratory Society International Congress; 2019 Sep 28–Oct 2; Madrid, Spain.
Hardy J, Baggott C, Fingleton J, Reddel HK, Hancox RJ, Harwood M, et al. Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (PRACTICAL): a 52-week, open-label, multicentre, superiority, randomised controlled trial. Lancet. 2019;394(10202):919–28.
Download references
Acknowledgements
The Authors thank Maurizio Tarzia and Gayle Robins, independent medical writers who provided editorial assistance on behalf of Springer Healthcare Communications. The editorial assistance was funded by AstraZeneca.
No funding was received for this study. The editorial assistance was funded by AstraZeneca.
Author information
Authors and affiliations.
Section of Cardiorespiratory and Internal Medicine, Department of Morphology, Surgery and Experimental Medicine, University of Ferrara, Ferrara, Italy
Alberto Papi & Luca Morandi
Internal Medicine Department, Respiratory Unit and Adult Cystic Fibrosis Center, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Italy
Francesco Blasi
Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy
Personalized Medicine Asthma & Allergy Clinic, Humanitas University & Istituto Clinico Humanitas, Milan, Italy
Giorgio Walter Canonica
Università Cattolica del Sacro Cuore, Fondazione Policlinico A. Gemelli IRCCS, Rome, Italy
Luca Richeldi
Respiratory Section, Department of Medicine, University of Verona, Verona, Italy
Andrea Rossi
Respiratory Unit, Emergency Department, University Hospital S. Anna, Via Aldo Moro 8, 44124, Ferrara, Italy
You can also search for this author in PubMed Google Scholar
Contributions
AP, FB, GWC, LM, LR and AR contributed to writing. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Alberto Papi .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
AP reports grants, personal fees, non-financial support and payment for advisory board membership, consultancy, payment for lectures, grants for research, and travel expenses reimbursement from Chiesi, AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Mundipharma and Teva, and personal fees and non-financial support from Menarini, Novartis, Zambon and Sanofi.
FB reports having received in the last three years research grants as well as lecture or advisory board fees from: Alk-Abelló, AstraZeneca, Boehringer Ingelheim, Chiesi, Guidotti, Glaxo Smith Kline, Grifols, Menarini, Novartis, Sanofi, Valeas, Zambon.
GWC reports having received in the last 3 years research grants as well as lecture or advisory board fees from: A. Menarini, Alk-Abelló, AstraZeneca-Medimmune, Boehringer Ingelheim, Chiesi Farmaceutici, Genentech, Guidotti-Malesci, Glaxo Smith Kline, Hal Allergy, Merck Sharp & Dohme, Mundipharma, Novartis, Orion, Sanofi-Aventis, Sanofi Genzyme/Regeneron, Stallergenes-Greers, UCB Pharma, Uriach Pharma, Valeas.
LR Receipt of grants/research supports: Roche, Boehringer Ingelheim.
Receipt of honoraria or consultation fees: Boehringer Ingelheim, Roche, Biogen, FibroGen,
Sanofi-Aventis, Anthera, Promedior, ImmuneWorks, Asahi-Kasei, Bayer, Celgene, RespiVant,
Nitto, Bristol Myers Squibb, Prometic, Pliant Therapeutics, Toray, Global Blood Therapeutics,
Zambon, Veracyte, Acceleron, CSL Behring.
LM and AR reports no conflicts of interest in the last 3 years.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Papi, A., Blasi, F., Canonica, G.W. et al. Treatment strategies for asthma: reshaping the concept of asthma management. Allergy Asthma Clin Immunol 16 , 75 (2020). https://doi.org/10.1186/s13223-020-00472-8
Download citation
Received : 18 March 2020
Accepted : 05 August 2020
Published : 15 August 2020
DOI : https://doi.org/10.1186/s13223-020-00472-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Anti-inflammatory treatment
- Disease control
- Patient outcomes
Allergy, Asthma & Clinical Immunology
ISSN: 1710-1492
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Search by keyword
- Search by citation
Page 1 of 2
Psychological distress and associated factors among asthmatic patients in Southern, Ethiopia, 2021
There is an increased prevalence of psychological distress in adults with asthma. Psychological distress describes unpleasant feelings or emotions that impact the level of functioning. It is a significant exac...
- View Full Text
Retrospective assessment of a collaborative digital asthma program for Medicaid-enrolled children in southwest Detroit: reductions in short-acting beta-agonist (SABA) medication use
Real-world evidence for digitally-supported asthma programs among Medicaid-enrolled children remains limited. Using data from a collaborative quality improvement program, we evaluated the impact of a digital i...
Nonadherence to antiasthmatic medications and its predictors among asthmatic patients in public hospitals of Bahir Dar City, North West Ethiopia: using ASK-12 tool
Globally, adequate asthma control is not yet achieved. The main cause of uncontrollability is nonadherence to prescribed medications.
The hen and the egg question in atopic dermatitis: allergy or eczema comes first
Atopic dermatitis (AD) as a chronic inflammatory systemic condition is far more than skin deep. Co-morbidities such as asthma and allergic rhinitis as well as the psychological impact influence seriously the q...
Medication regimen complexity and its impact on medication adherence and asthma control among patients with asthma in Ethiopian referral hospitals
Various studies have found that medication adherence is generally low among patients with asthma, and that the complexity of the regimen may be a potential factor. However, there is no information on the compl...
Monoclonal antibodies targeting small airways: a new perspective for biological therapies in severe asthma
Small airway dysfunction (SAD) in asthma is characterized by the inflammation and narrowing of airways with less of 2 mm in diameter between generations 8 and 23 of the bronchial tree. It is now widely accepte...
Level of asthma control and its determinants among adults living with asthma attending selected public hospitals in northwestern, Ethiopia: using an ordinal logistic regression model
Asthma is a major public health challenge and is characterized by recurrent attacks of breathlessness and wheezing that vary in severity and frequency from person to person. Asthma control is an important meas...
Static lung volumes and diffusion capacity in adults 30 years after being diagnosed with asthma
Long-term follow-up studies of adults with well-characterized asthma are sparse. We aimed to explore static lung volumes and diffusion capacity after 30 + years with asthma.
Over-prescription of short-acting β 2 -agonists and asthma management in the Gulf region: a multicountry observational study
The overuse of short-acting β 2 -agonists (SABA) is associated with poor asthma control. However, data on SABA use in the Gulf region are limited. Herein, we describe SABA prescription practices and clinical outcom...
A serological biomarker of type I collagen degradation is related to a more severe, high neutrophilic, obese asthma subtype
Asthma is a heterogeneous disease; therefore, biomarkers that can assist in the identification of subtypes and direct therapy are highly desirable. Asthma is a chronic inflammatory disease that leads to change...
Adherence to inhalers and associated factors among adult asthma patients: an outpatient-based study in a tertiary hospital of Rajshahi, Bangladesh
Adherence to inhaler medication is an important contributor to optimum asthma control along with adequate pharmacotherapy. The objective of the present study was to assess self-reported adherence levels and to...
The link between atopic dermatitis and asthma- immunological imbalance and beyond
Atopic diseases are multifactorial chronic disturbances which may evolve one into another and have overlapping pathogenetic mechanisms. Atopic dermatitis is in most cases the first step towards the development...
The effects of nebulized ketamine and intravenous magnesium sulfate on corticosteroid resistant asthma exacerbation; a randomized clinical trial
Asthma exacerbation is defined as an acute attack of shortness of breath with more than 25% decrease in morning peak flow compared to the baseline on 2 consecutive days, which requires immediate standard thera...
Determinants of asthma in Ethiopia: age and sex matched case control study with special reference to household fuel exposure and housing characteristics
Asthma is a chronic inflammatory disorder characterized by airway obstruction and hyper-responsiveness. Studies suggest that household fuel exposure and housing characteristics are associated with air way rela...
Feasibility and acceptability of monitoring personal air pollution exposure with sensors for asthma self-management
Exposure to fine particulate matter (PM 2.5 ) increases the risk of asthma exacerbations, and thus, monitoring personal exposure to PM 2.5 may aid in disease self-management. Low-cost, portable air pollution sensors...
Biological therapy for severe asthma
Around 5–10% of the total asthmatic population suffer from severe or uncontrolled asthma, which is associated with increased mortality and hospitalization, increased health care burden and worse quality of lif...
Treatment outcome clustering patterns correspond to discrete asthma phenotypes in children
Despite widely and regularly used therapy asthma in children is not fully controlled. Recognizing the complexity of asthma phenotypes and endotypes imposed the concept of precision medicine in asthma treatment...
Positive change in asthma control using therapeutic patient education in severe uncontrolled asthma: a one-year prospective study
Severe asthma is difficult to control. Therapeutic patient education enables patients to better understand their disease and cope with treatment, but the effect of therapeutic patient education in severe uncon...
Asthma and COVID-19: a dangerous liaison?
The coronavirus disease 2019 (COVID-19) pandemic, caused by the new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), provoked the most striking international public health crisis of our time. COVI...
Knowledge, attitude, and practice towards COVID-19 among chronic disease patients at Aksum Hospital, Northern Ethiopia, 2020: a cross-sectional study
The Coronavirus disease 2019 outbreak is the first reported case in Wuhan, China in December 2019 and suddenly became a major global health concern. Currently, there is no vaccine and treatment have been repor...
Self-reported vs. objectively assessed adherence to inhaled corticosteroids in asthma
Adherence to inhaled corticosteroids (ICS) in asthma is vital for disease control. However, obtaining reliable and clinically useful measures of adherence remains a major challenge. We investigated the associa...
Association between prevalence of obstructive lung disease and obesity: results from The Vermont Diabetes Information System
The association of obesity with the development of obstructive lung disease, namely asthma and/or chronic obstructive pulmonary disease, has been found to be significant in general population studies, and weig...
Changes in quantifiable breathing pattern components predict asthma control: an observational cross-sectional study
Breathing pattern disorders are frequently reported in uncontrolled asthma. At present, this is primarily assessed by questionnaires, which are subjective. Objective measures of breathing pattern components ma...
The role of leukotriene modifying agent treatment in neuropsychiatric events of elderly asthma patients: a nested case control study
In March 2020, the US Food and Drug Administration decided that the dangers related to neuropsychiatric events (NPEs) of montelukast, one of the leukotriene modifying agents (LTMAs), should be communicated thr...
Asthma and stroke: a narrative review
Asthma is a heterogeneous disease, usually characterized by chronic airway inflammation, bronchial reversible obstruction and hyperresponsiveness to direct or indirect stimuli. It is a severe disease causing a...
Comparison of dental caries (DMFT and DMFS indices) between asthmatic patients and control group in Iran: a meta-analysis
The association between caries index, which is diagnosed by Decayed, Missing, and Filled Teeth (DMFT), and asthma has been assessed in several studies, which yielded contradictory results. Meta-analysis is the...
ICS/formoterol in the management of asthma in the clinical practice of pulmonologists: an international survey on GINA strategy
The treatment with short-acting beta-2 agonists (SABA) alone is no longer recommended due to safety issues. Instead, the current Global Initiative for Asthma (GINA) Report recommends the use of the combination...
Sustainability of residential environmental interventions and health outcomes in the elderly
Research has documented that housing conditions can negatively impact the health of residents. Asthma has many known indoor environmental triggers including dust, pests, smoke and mold, as evidenced by the 25 ...
Non-adherence to inhaled medications among adult asthmatic patients in Ethiopia: a systematic review and meta-analysis
Medication non-adherence is one of a common problem in asthma management and it is the main factor for uncontrolled asthma. It can result in poor asthma control, which leads to decreased quality of life, incre...
The outcome of COVID-19 among the geriatric age group in African countries: protocol for a systematic review and meta-analysis
According to the World Health Organization (WHO), the outbreak of coronavirus disease in 2019 (COVID-19) has been declared as a pandemic and public health emergency that infected more than 5 million people wor...
Correction to: A comparison of biologicals in the treatment of adults with severe asthma – real-life experiences
An amendment to this paper has been published and can be accessed via the original article.
The original article was published in Asthma Research and Practice 2020 6 :2
Disease control in patients with asthma and respiratory symptoms (wheezing, cough) during sleep
The Global Initiative for Asthma ( GINA)-defined criteria for asthma control include questions about daytime symptoms, limitation of activity, nocturnal symptoms, need for reliever treatment and patients’ satisfac...
The burden, admission, and outcomes of COVID-19 among asthmatic patients in Africa: protocol for a systematic review and meta-analysis
Coronavirus disease 2019 outbreak is the first reported case in Wuhan, China in December 2019 and suddenly became a major global health concern. According to the European Centre for Disease Prevention and Cont...
The healthcare seeking behaviour of adult patients with asthma at Chitungwiza Central Hospital, Zimbabwe
Although asthma is a serious public health concern in Zimbabwe, there is lack of information regarding the decision to seek for healthcare services among patients. This study aimed to determine the health care...
Continuous versus intermittent short-acting β2-agonists nebulization as first-line therapy in hospitalized children with severe asthma exacerbation: a propensity score matching analysis
Short-acting β2-agonist (SABA) nebulization is commonly prescribed for children hospitalized with severe asthma exacerbation. Either intermittent or continuous delivery has been considered safe and efficient. ...
Patient perceived barriers to exercise and their clinical associations in difficult asthma
Exercise is recommended in guidelines for asthma management and has beneficial effects on symptom control, inflammation and lung function in patients with sub-optimally controlled asthma. Despite this, physica...
Asthma management with breath-triggered inhalers: innovation through design
Asthma affects the lives of hundred million people around the World. Despite notable progresses in disease management, asthma control remains largely insufficient worldwide, influencing patients’ wellbeing and...
A nationwide study of asthma correlates among adolescents in Saudi Arabia
Asthma is a chronic airway inflammation disease that is frequently found in children and adolescents with an increasing prevalence. Several studies are linking its presence to many lifestyle and health correla...
A comparison of biologicals in the treatment of adults with severe asthma – real-life experiences
Anti-IgE (omalizumab) and anti-IL5/IL5R (reslizumab, mepolizumab and benralizumab) treatments are available for severe allergic and eosinophilic asthma. In these patients, studies have shown beneficial effects...
The Correction to this article has been published in Asthma Research and Practice 2020 6 :10
Determinants of Acute Asthma Attack among adult asthmatic patients visiting hospitals of Tigray, Ethiopia, 2019: case control study
Acute asthma attack is one of the most common causes of visits to hospital emergency departments in all age groups of the population and accounts for the greater part of healthcare burden from the disease. Des...
Determinants of non-adherence to inhaled steroids in adult asthmatic patients on follow up in referral hospital, Ethiopia: cross-sectional study
Asthma is one of the major non-communicable diseases worldwide. The prevalence of asthma has continuously increased over the last five decades, resulting in 235 million people suffering from it. One of the mai...
Development of a framework for increasing asthma awareness in Chitungwiza, Zimbabwe
Asthma accounts for significant global morbidity and health-care costs. It is still poorly understood among health professionals and the general population. Consequently, there are significant morbidity and mo...
Epidemiology and utilization of primary health care services in Qatar by asthmatic children 5–12 years old: secondary data analysis 2016–2017
Childhood asthma is a growing clinical problem and a burden on the health care system due to repetitive visits to children’s emergency departments and frequent hospital admissions where it is poorly controlled...
Is asthma in the elderly different? Functional and clinical characteristics of asthma in individuals aged 65 years and older
The prevalence of chronic diseases in the elderly (> 65 years), including asthma, is growing, yet information available on asthma in this population is scarce.
Factors associated with exacerbations among adults with asthma according to electronic health record data
Asthma is a chronic inflammatory lung disease that affects 18.7 million U.S. adults. Electronic health records (EHRs) are a unique source of information that can be leveraged to understand factors associated w...
What is safe enough - asthma in pregnancy - a review of current literature and recommendations
Although asthma is one of the most serious diseases causing complications during pregnancy, half of the women discontinue therapy thus diminishing the control of the disease, mostly due to the inadequate educa...
Biomarkers in asthma: state of the art
Asthma is a heterogenous disease characterized by multiple phenotypes driven by different mechanisms. The implementation of precision medicine in the management of asthma requires the identification of phenoty...
Exhaled biomarkers in childhood asthma: old and new approaches
Asthma is a chronic condition usually characterized by underlying inflammation. The study of asthmatic inflammation is of the utmost importance for both diagnostic and monitoring purposes. The gold standard fo...
Assessment of predictors for acute asthma attack in asthmatic patients visiting an Ethiopian hospital: are the potential factors still a threat?
Recurrent exacerbations in patients with moderate or severe asthma are the major causes of morbidity, mortality and medical expenditure. Identifying predictors of frequent asthma attack might offer the fertile...
Effect of adjusting the combination of budesonide/formoterol on the alleviation of asthma symptoms
The combination of budesonide + formoterol (BFC) offers the advantages of dose adjustment in a single inhaler according to asthma symptoms. We analyzed the relationship between asthma symptoms in terms of peak...
Asthma Research and Practice
ISSN: 2054-7064
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- News & Views
- Asthma in children:...
Asthma in children: environmental factors
- Related content
- Peer review
- P Cullinan ,
- A J N Taylor
Two studies of asthma in children, using identical survey methods and objective assessments separated by a period of 10-15 years, have now been published; each shows an increase in asthmatic symptoms and airway hyper-responsiveness and in seasonal rhinitis. 1 (p 1591). 2 The increased prevalence of asthma has been matched by, and is probably a manifestation of, an increase in sensitisation among children to inhaled allergens, such as those present in house dust, cat fur, and grass pollen. 3
Although genetic factors are important in determining both the propensity to atopic disease and the specificity of the response to protein epitopes, the short period during which the increases in asthma and other allergic diseases have occurred suggests that environmental influences have been mainly responsible. For example, secular trends in the Finnish armed forces show a 20-fold increase in asthma among 18 year old recruits during the past 30 years. 4
Further evidence of important environmental influences comes from the recent observation that sensitisation to common aeroallergens is about twice as common among children in Munich as among genetically similar children in two cities that were formerly in East Germany, Leipzig and Halle. 5 Against this background it is valuable to consider what environmental changes have been common to Western countries in the past 30 to 40 years.
Childhood asthma is predominantly an allergic disease; changes may have occurred in exposure to aeroallergen (quantitatively or qualitatively) or in concurrent exposure to factors that modify the response to allergens. These factors include various respiratory irritants, such as tobacco smoke and possibly air pollutants. Tobacco smokers have an increased risk of sensitisation to agents inhaled at work, including proteins 6 and low molecular weight chemicals. 7 Experiments in animals have shown that sulphur dioxide 8 and ozone 9 increase the risk of both sensitisation and airway responsiveness to inhaled allergens and haptens. Longitudinal studies of workforces exposed to respiratory sensitisers suggest that the highest incidence of sensitisation occurs within two years of new employment, and concomitant exposure to respiratory irritants (cigarette smoking) increases the risk of sensitisation during this period. 7 There may be a “window of vulnerability” in the period after initial exposure to novel allergens during which the effects of exposure and any modifying factors are maximal.
The development of allergy in infancy is probably influenced by factors in fetal life as well as those present after birth such as exposure to inhaled allergens. The level of exposure to house dust mite in infancy is related to the subsequent development of specific sensitisation and asthma, 10 and measurements in Australian homes suggest a secular rise in such exposures, though surprisingly no sensitisation to dust mites. 2 But this mechanism alone would be unlikely to account for the rising rates of sensitisation to other inhaled allergens. The role of infection in early life is being reconsidered in response to observations of an inverse relation between family size and rates in children of skin test sensitivity and associated manifestations of allergic disease including asthma. 11 Childhood exposure to respiratory infections tends to occur earlier in large families, and viral infections may down regulate production of IgE, possibly via (gamma) interferon production through preferential stimulation of TH1 lymphocytes, although this mechanism has yet to be demonstrated.
Which irritant?
The respiratory irritants that have received most attention are cigarette smoke and atmospheric pollutants, particularly motor vehicle exhaust emissions. Infants spend most of their time in the home, and domestic sources of pollution are probably at least as important as external ones. Current evidence for a direct effect of nitrogen dioxide, particulate matter, and other such exposures on the development of allergic sensitisation in children is inconclusive. Any potential modifying role on the response to exposure to aeroallergen has, however, not been formally examined.
Interestingly, the secular rise in asthma in Britain has coincided with a high rate of smoking among young women. Maternal smoking during pregnancy seems to affect infants' lung function independently of postnatal exposure to tobacco smoke 12 ; smoking may also influence cord IgE concentrations. 13 In the Tucson Children's Respiratory Study the proportion of children with episodes of lower respiratory illness during their third year was directly proportional to their cord IgE concentrations, although the relation was inverse during their first year of life. 14 Apart from emphasising that much wheezing in the first three years of life is not asthma, these findings suggest that changes in perinatal exposure to modifiers of the response to aeroallergens may be at least as important as changes in exposures to allergens themselves. The mechanisms behind any such modification, particularly that occurring before birth, however, remain obscure.
The explanation behind the rising incidence of childhood asthma is undeniably complicated and remains poorly understood, but several recently appreciated features are worth emphasising. Wheezing associated with viral infection in the early years of life needs to be clearly distinguished from childhood asthma: influences in both infant and fetal life, and their interactions, are probably important. Changes in exposure to aeroallergens alone seem unlikely to be solely responsible.
- Butland BK ,
- Vaughan-Williams E
- van den Berg RH ,
- Mellis CM ,
- Leeder SR ,
- Woolcock AJ
- Nakagomi T ,
- Tominaga T ,
- Hisamatsu S-I ,
- Haahtela TI ,
- Lindholm H ,
- Borksten F ,
- Koskenvuo K ,
- Laitinen LA
- von Mutius E ,
- Martinez F ,
- Fritzsch C ,
- Nicolai T ,
- Cullinan P ,
- Nieuwenhuijsen M
- Venables K ,
- Stevens JF ,
- Stephens R ,
- Scheinbenbogen C ,
- Biagini R ,
- Moorman W ,
- Bernstein I
- Holgate S ,
- Platts-Mills T ,
- Reitmeir P ,
- Hanrahan J ,
- Tosteson TD ,
- Castile RG ,
- Van Vunakis H ,
- Magnusson C
- Halonen M ,
- Holberg C ,
- Taussig L ,
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
May 20, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Researcher finds mothers live longer as child mortality declines
by Kate Blackwood, Cornell University
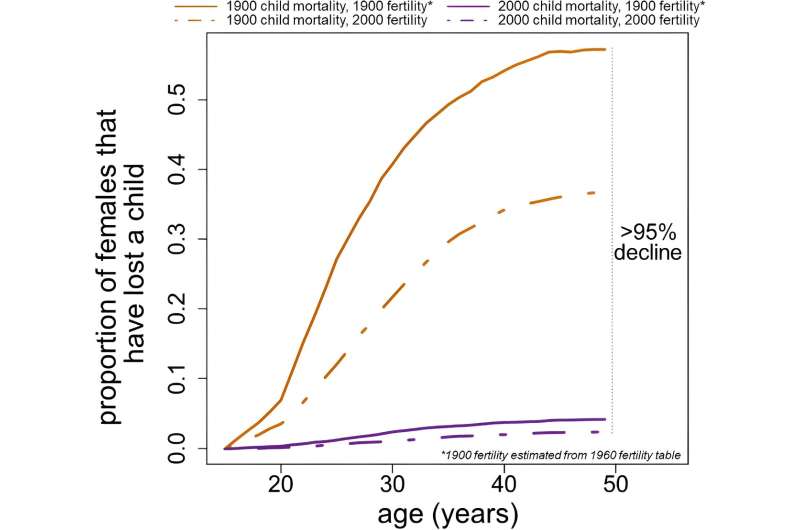
The dramatic decline in childhood mortality during the 20th century has added a full year to women's lives, according to a new study.
"The picture I was building in my mind was to think about what the population of mothers in the U.S. looked like in 1900," said Matthew Zipple, a Klarman Postdoctoral Fellow in neurobiology and behavior in the College of Arts and Sciences and author of "Reducing Childhood Mortality Extends Mothers' Lives," which published May 9 in Scientific Reports .
"It was a population made up of two approximately equal-sized groups: One was mothers who had lost children, and one was mothers who had not," Zipple said. "If we compare that to today, when child loss is mercifully so much less common, nearly all those women who had lost children are shifted into the non-bereaved category."
Several studies find that mothers are more likely to die in the years following the death of a child, Zipple said. This effect does not show up in fathers.
Using mathematical modeling based on Centers for Disease Control and Prevention (CDC) data, he calculated how the absence of bereavement affects the lifespans of present-day mothers in the United States. He estimates that the reduction in maternal bereavement adds, on average, a year to women's lives.
As a doctoral student researching fitness links between mothers and offspring, Zipple found a pattern of maternal death following offspring death in nonhuman primates. In the animals, the effect was attributed to the mothers being in poor condition and less able to care for their offspring.
But in humans, the same sequence of events—offspring death followed by maternal death—has been interpreted differently in literature from studies with a human focus. Instead, epidemiologists and public health researchers conclude that the physical and psychological costs of the trauma of losing a child makes mothers more likely to die.
In the paper, Zipple cites several studies that causally link child death with increased risk of maternal death. The most comprehensive is a study of mothers in Iceland over a 200-year period, spanning a range of health care access and industrialization. It controls for genetics by comparing siblings and shows that bereaved fathers are no more likely than non-bereaved fathers to die in the years following a child's death.
Another study in Sweden shows that mothers are at higher risk of death on and around the anniversary of their child's death than at other times. Common causes of death in bereaved mothers, according to various studies, include heart attack and suicide.
"There's a huge peak of mortality risk immediately in the week surrounding the anniversary," Zipple said. "It's hard to come to a different conclusion than that is the cause, remembering this experience."
Life expectancy for women after age 15 increased by about 16 years between 1900 and 2000, Zipple found from the CDC data he used in the study. His calculation attributes one year, or about 6% of this increase, to the dramatic drop in childhood mortality over the course of the 20th century.
"One of the most awful things one can imagine is losing a child. And we've been able to reduce the frequency of that in our society by more than 95%. That's amazing. That's something to celebrate," Zipple said.
"It's easy to lose sight of progress that happens over the course of a century because it's well beyond any individual's lifetime. But this extension in overall lifespan over the last 100 years has set up human populations and experience to be so much better than they ever have been before."
The study also helps set priorities for improving the future, Zipple said. In many countries, child mortality rates today are similar to those in the U.S. in 1900. Investing in reducing childhood mortality everywhere helps not only the children, but whole communities.
"The child is the core of the community," Zipple said. "Protecting children from mortality has branching positive impacts that start with mothers but probably don't stop there."
Explore further
Feedback to editors
Study finds jaboticaba peel reduces inflammation and controls blood sugar in people with metabolic syndrome
57 minutes ago

Study reveals how extremely rare immune cells predict how well treatments work for recurrent hives
2 hours ago

Researchers find connection between PFAS exposure in men and the health of their offspring

Researchers develop new tool for better classification of inherited disease-causing variants

Specialized weight navigation program shows higher use of evidence-based treatments, more weight lost than usual care

Exercise bouts could improve efficacy of cancer drug
3 hours ago
Drug-like inhibitor shows promise in preventing flu

Scientists discover a novel way of activating muscle cells' natural defenses against cancer using magnetic pulses
Cancer drug shows powerful anti-tumor activity in animal models of several different tumor types
4 hours ago

Ultra-processed foods increase cardiometabolic risk in children, study finds
Related stories.

Primate orphans have it tough even before mother dies
Dec 22, 2020
How many mothers have lost a child: A global comparison
Apr 6, 2021

Measuring maternal grief in Africa
Apr 28, 2020

Maternal mental conditions drive climbing death rate in US, according to study
Feb 21, 2024

Severe maternal grief associated with increased risk of heart failure in child

Losing children to foster care endangers mothers' lives
Mar 30, 2018
Recommended for you

Researchers suggest brain scans for babies reduce risk of stroke later in life
9 hours ago

Fluoride exposure during pregnancy linked to increased risk of childhood neurobehavioral problems, study finds

Study sheds light on bacteria associated with pre-term birth

Low-dose iron supplementation has no benefit for breastfed infants, shows study
Study opens the door to designing therapies to improve lung development in growth-restricted fetuses
May 17, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

IMAGES
VIDEO
COMMENTS
While asthma incidence and prevalence are higher in children, morbidity, and mortality are higher in adults. Childhood asthma is more common in boys while adult asthma is more common in women, and the reversal of this sex difference in prevalence occurs around puberty suggesting sex hormones may play a role in the etiology of asthma.
Asthma, a prevalent chronic respiratory condition affecting millions of children globally, presents a significant health challenge. This review critically examines the developmental pathways of asthma in children, focusing on genetic, environmental, and early-life determinants. Specifically, we explore the impact of prenatal and postnatal factors such as maternal smoking, nutrition ...
In a search of PubMed and Web of Science with the terms ("asthma" AND ["control" OR "treatment" OR "management" OR "asthma plan"] AND "income"), for articles in English and Spanish published from July 1, 2011, to July 1, 2021, we retrieved more than 1000 papers; however, none compared asthma treatments or management plans in different age groups or in countries with ...
Strengths and limitations of this study. Broad evidence syntheses on the management of childhood asthma, with a focus on the differential treatment response according to age and disease phenotypes could reveal clinically exploitable information, that will be used in the development of clinical and research recommendations by Paediatric Asthma in Real Life.
Asthma is the most common chronic condition of childhood. Self-management is integral to good asthma control. This qualitative paper explores how children with asthma and their parents perceive ...
1. Introduction. Asthma is one of the most common chronic respiratory diseases. In 2019, an estimated 262 million individuals were affected by asthma, and 461,000 people died as a result of the disease [Citation 1].The prevalence of asthma in European children has been estimated between 5% to over 20% [Citation 2].The presence of airway wall inflammation is a key pathophysiological mechanism ...
Child participants were required to be 8-11 y.o., have a clinician-confirmed diagnosis of asthma as defined by national or international asthma guidelines (i.e., GINA , National Asthma Education and Prevention Program [NAEPP] ) for at least 1 year, have received/filled a prescription for asthma medication in the last year, and have ...
Among the editors' choice studies in this month's issue are three exciting articles. The first, by Cristina Ardura-Garcia et al., 1 reports differences between children at risk of long-term suboptimal asthma control and those at risk of acute asthma attacks. The authors recommend that prediction tools and preventive efforts should differentiate these two asthma outcomes.
The David Hide Asthma and Allergy Research Centre, St. Mary's Hospital, Isle of Wight, UK. Clinical and Experimental Sciences, Faculty of Medicine, University of Southampton, Southampton, UK ... An additional citation screening of relevant articles and the seven identified review papers on childhood asthma prediction tools identified a further ...
With about 1-18% of the population worldwide being affected, wheeze and asthma are the most prevalent chronic respiratory diseases during childhood and adulthood.1 As such, many children each day will enter an outpatient clinic or a paediatrician's office needing diagnostic tests for suspected asthma. If it were only that simple. Individuals with suspected asthma symptoms vary substantially ...
Asthma affects an estimated 7 million children and causes significant health care and disease burden. The most recent iteration of the National Heart, Lung and Blood Institute asthma guidelines, the Expert Panel Report 3, emphasizes the assessment and monitoring of asthma control in the management of asthma. Asthma control refers to the degree to which the manifestations of asthma are ...
Associations between childhood asthma phenotypes and genetic, immunological, ... According to an EAACI position paper in 2019, ... Asthma research produces up to 9000 publications per year and represents one of the most rapidly developing areas. Most of the novel developments of the last year focus in the areas of precision medicine, endotypes ...
Asthma is the most common chronic respiratory disorder in children. Although many papers have been published on this topic, there is still a great need to further understand the disease. The pathophysiology and the long-term prognosis of preschool wheezing are still unclear, which may be due to the heterogeneity of the disorder. In addition, the management of childhood asthma, especially ...
This review provides an overview of existing technologies for the outpatient telemonitoring of pediatric asthma, and specific technologies for preschool children represent a gap in the literature that needs to be specifically addressed in future research. Telemonitoring technologies are rapidly evolving, offering a promising solution for remote monitoring and timely management of asthma acute ...
1 INTRODUCTION. Childhood asthma is highly heterogeneous, with numerous factors contributing towards its development, persistence and severity. 1-3 Despite approximately 80% of asthmatic children developing symptoms (such as wheeze) before the age of six, these clinical symptoms are neither universally present in early life among all future asthmatics nor specific to asthma. 4 With the added ...
Asthma is a common chronic disease characterized by episodic or persistent respiratory symptoms and airflow limitation. Asthma treatment is based on a stepwise and control-based approach that involves an iterative cycle of assessment, adjustment of the treatment and review of the response aimed to minimize symptom burden and risk of exacerbations. Anti-inflammatory treatment is the mainstay of ...
Long-term follow-up studies of adults with well-characterized asthma are sparse. We aimed to explore static lung volumes and diffusion capacity after 30 + years with asthma. Conrad Uldall Becker Schultz, Oliver Djurhuus Tupper and Charlotte Suppli Ulrik. Asthma Research and Practice 2022 8 :4.
Two studies of asthma in children, using identical survey methods and objective assessments separated by a period of 10-15 years, have now been published; each shows an increase in asthmatic symptoms and airway hyper-responsiveness and in seasonal rhinitis.1 (p 1591).2 The increased prevalence of asthma has been matched by, and is probably a manifestation of, an increase in sensitisation among ...
The dramatic decline in childhood mortality during the 20th century has added a full year to women's lives, according to a new study. "The picture I was building in my mind was to think about what ...