
- Previous Article
- Next Article

Case Presentation
Case study: a patient with uncontrolled type 2 diabetes and complex comorbidities whose diabetes care is managed by an advanced practice nurse.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Geralyn Spollett; Case Study: A Patient With Uncontrolled Type 2 Diabetes and Complex Comorbidities Whose Diabetes Care Is Managed by an Advanced Practice Nurse. Diabetes Spectr 1 January 2003; 16 (1): 32–36. https://doi.org/10.2337/diaspect.16.1.32
Download citation file:
- Ris (Zotero)
- Reference Manager
The specialized role of nursing in the care and education of people with diabetes has been in existence for more than 30 years. Diabetes education carried out by nurses has moved beyond the hospital bedside into a variety of health care settings. Among the disciplines involved in diabetes education, nursing has played a pivotal role in the diabetes team management concept. This was well illustrated in the Diabetes Control and Complications Trial (DCCT) by the effectiveness of nurse managers in coordinating and delivering diabetes self-management education. These nurse managers not only performed administrative tasks crucial to the outcomes of the DCCT, but also participated directly in patient care. 1
The emergence and subsequent growth of advanced practice in nursing during the past 20 years has expanded the direct care component, incorporating aspects of both nursing and medical care while maintaining the teaching and counseling roles. Both the clinical nurse specialist (CNS) and nurse practitioner (NP) models, when applied to chronic disease management, create enhanced patient-provider relationships in which self-care education and counseling is provided within the context of disease state management. Clement 2 commented in a review of diabetes self-management education issues that unless ongoing management is part of an education program, knowledge may increase but most clinical outcomes only minimally improve. Advanced practice nurses by the very nature of their scope of practice effectively combine both education and management into their delivery of care.
Operating beyond the role of educator, advanced practice nurses holistically assess patients’ needs with the understanding of patients’ primary role in the improvement and maintenance of their own health and wellness. In conducting assessments, advanced practice nurses carefully explore patients’ medical history and perform focused physical exams. At the completion of assessments, advanced practice nurses, in conjunction with patients, identify management goals and determine appropriate plans of care. A review of patients’ self-care management skills and application/adaptation to lifestyle is incorporated in initial histories, physical exams, and plans of care.
Many advanced practice nurses (NPs, CNSs, nurse midwives, and nurse anesthetists) may prescribe and adjust medication through prescriptive authority granted to them by their state nursing regulatory body. Currently, all 50 states have some form of prescriptive authority for advanced practice nurses. 3 The ability to prescribe and adjust medication is a valuable asset in caring for individuals with diabetes. It is a crucial component in the care of people with type 1 diabetes, and it becomes increasingly important in the care of patients with type 2 diabetes who have a constellation of comorbidities, all of which must be managed for successful disease outcomes.
Many studies have documented the effectiveness of advanced practice nurses in managing common primary care issues. 4 NP care has been associated with a high level of satisfaction among health services consumers. In diabetes, the role of advanced practice nurses has significantly contributed to improved outcomes in the management of type 2 diabetes, 5 in specialized diabetes foot care programs, 6 in the management of diabetes in pregnancy, 7 and in the care of pediatric type 1 diabetic patients and their parents. 8 , 9 Furthermore, NPs have also been effective providers of diabetes care among disadvantaged urban African-American patients. 10 Primary management of these patients by NPs led to improved metabolic control regardless of whether weight loss was achieved.
The following case study illustrates the clinical role of advanced practice nurses in the management of a patient with type 2 diabetes.
A.B. is a retired 69-year-old man with a 5-year history of type 2 diabetes. Although he was diagnosed in 1997, he had symptoms indicating hyperglycemia for 2 years before diagnosis. He had fasting blood glucose records indicating values of 118–127 mg/dl, which were described to him as indicative of “borderline diabetes.” He also remembered past episodes of nocturia associated with large pasta meals and Italian pastries. At the time of initial diagnosis, he was advised to lose weight (“at least 10 lb.”), but no further action was taken.
Referred by his family physician to the diabetes specialty clinic, A.B. presents with recent weight gain, suboptimal diabetes control, and foot pain. He has been trying to lose weight and increase his exercise for the past 6 months without success. He had been started on glyburide (Diabeta), 2.5 mg every morning, but had stopped taking it because of dizziness, often accompanied by sweating and a feeling of mild agitation, in the late afternoon.
A.B. also takes atorvastatin (Lipitor), 10 mg daily, for hypercholesterolemia (elevated LDL cholesterol, low HDL cholesterol, and elevated triglycerides). He has tolerated this medication and adheres to the daily schedule. During the past 6 months, he has also taken chromium picolinate, gymnema sylvestre, and a “pancreas elixir” in an attempt to improve his diabetes control. He stopped these supplements when he did not see any positive results.
He does not test his blood glucose levels at home and expresses doubt that this procedure would help him improve his diabetes control. “What would knowing the numbers do for me?,” he asks. “The doctor already knows the sugars are high.”
A.B. states that he has “never been sick a day in my life.” He recently sold his business and has become very active in a variety of volunteer organizations. He lives with his wife of 48 years and has two married children. Although both his mother and father had type 2 diabetes, A.B. has limited knowledge regarding diabetes self-care management and states that he does not understand why he has diabetes since he never eats sugar. In the past, his wife has encouraged him to treat his diabetes with herbal remedies and weight-loss supplements, and she frequently scans the Internet for the latest diabetes remedies.
During the past year, A.B. has gained 22 lb. Since retiring, he has been more physically active, playing golf once a week and gardening, but he has been unable to lose more than 2–3 lb. He has never seen a dietitian and has not been instructed in self-monitoring of blood glucose (SMBG).
A.B.’s diet history reveals excessive carbohydrate intake in the form of bread and pasta. His normal dinners consist of 2 cups of cooked pasta with homemade sauce and three to four slices of Italian bread. During the day, he often has “a slice or two” of bread with butter or olive oil. He also eats eight to ten pieces of fresh fruit per day at meals and as snacks. He prefers chicken and fish, but it is usually served with a tomato or cream sauce accompanied by pasta. His wife has offered to make him plain grilled meats, but he finds them “tasteless.” He drinks 8 oz. of red wine with dinner each evening. He stopped smoking more than 10 years ago, he reports, “when the cost of cigarettes topped a buck-fifty.”
The medical documents that A.B. brings to this appointment indicate that his hemoglobin A 1c (A1C) has never been <8%. His blood pressure has been measured at 150/70, 148/92, and 166/88 mmHg on separate occasions during the past year at the local senior center screening clinic. Although he was told that his blood pressure was “up a little,” he was not aware of the need to keep his blood pressure ≤130/80 mmHg for both cardiovascular and renal health. 11
A.B. has never had a foot exam as part of his primary care exams, nor has he been instructed in preventive foot care. However, his medical records also indicate that he has had no surgeries or hospitalizations, his immunizations are up to date, and, in general, he has been remarkably healthy for many years.
Physical Exam
A physical examination reveals the following:
Weight: 178 lb; height: 5′2″; body mass index (BMI): 32.6 kg/m 2
Fasting capillary glucose: 166 mg/dl
Blood pressure: lying, right arm 154/96 mmHg; sitting, right arm 140/90 mmHg
Pulse: 88 bpm; respirations 20 per minute
Eyes: corrective lenses, pupils equal and reactive to light and accommodation, Fundi-clear, no arteriolovenous nicking, no retinopathy
Thyroid: nonpalpable
Lungs: clear to auscultation
Heart: Rate and rhythm regular, no murmurs or gallops
Vascular assessment: no carotid bruits; femoral, popliteal, and dorsalis pedis pulses 2+ bilaterally
Neurological assessment: diminished vibratory sense to the forefoot, absent ankle reflexes, monofilament (5.07 Semmes-Weinstein) felt only above the ankle
Lab Results
Results of laboratory tests (drawn 5 days before the office visit) are as follows:
Glucose (fasting): 178 mg/dl (normal range: 65–109 mg/dl)
Creatinine: 1.0 mg/dl (normal range: 0.5–1.4 mg/dl)
Blood urea nitrogen: 18 mg/dl (normal range: 7–30 mg/dl)
Sodium: 141 mg/dl (normal range: 135–146 mg/dl)
Potassium: 4.3 mg/dl (normal range: 3.5–5.3 mg/dl)
Lipid panel
• Total cholesterol: 162 mg/dl (normal: <200 mg/dl)
• HDL cholesterol: 43 mg/dl (normal: ≥40 mg/dl)
• LDL cholesterol (calculated): 84 mg/dl (normal: <100 mg/dl)
• Triglycerides: 177 mg/dl (normal: <150 mg/dl)
• Cholesterol-to-HDL ratio: 3.8 (normal: <5.0)
AST: 14 IU/l (normal: 0–40 IU/l)
ALT: 19 IU/l (normal: 5–40 IU/l)
Alkaline phosphotase: 56 IU/l (normal: 35–125 IU/l)
A1C: 8.1% (normal: 4–6%)
Urine microalbumin: 45 mg (normal: <30 mg)
Based on A.B.’s medical history, records, physical exam, and lab results, he is assessed as follows:
Uncontrolled type 2 diabetes (A1C >7%)
Obesity (BMI 32.4 kg/m 2 )
Hyperlipidemia (controlled with atorvastatin)
Peripheral neuropathy (distal and symmetrical by exam)
Hypertension (by previous chart data and exam)
Elevated urine microalbumin level
Self-care management/lifestyle deficits
• Limited exercise
• High carbohydrate intake
• No SMBG program
Poor understanding of diabetes
A.B. presented with uncontrolled type 2 diabetes and a complex set of comorbidities, all of which needed treatment. The first task of the NP who provided his care was to select the most pressing health care issues and prioritize his medical care to address them. Although A.B. stated that his need to lose weight was his chief reason for seeking diabetes specialty care, his elevated glucose levels and his hypertension also needed to be addressed at the initial visit.
The patient and his wife agreed that a referral to a dietitian was their first priority. A.B. acknowledged that he had little dietary information to help him achieve weight loss and that his current weight was unhealthy and “embarrassing.” He recognized that his glucose control was affected by large portions of bread and pasta and agreed to start improving dietary control by reducing his portion size by one-third during the week before his dietary consultation. Weight loss would also be an important first step in reducing his blood pressure.
The NP contacted the registered dietitian (RD) by telephone and referred the patient for a medical nutrition therapy assessment with a focus on weight loss and improved diabetes control. A.B.’s appointment was scheduled for the following week. The RD requested that during the intervening week, the patient keep a food journal recording his food intake at meals and snacks. She asked that the patient also try to estimate portion sizes.
Although his physical activity had increased since his retirement, it was fairly sporadic and weather-dependent. After further discussion, he realized that a week or more would often pass without any significant form of exercise and that most of his exercise was seasonal. Whatever weight he had lost during the summer was regained in the winter, when he was again quite sedentary.
A.B.’s wife suggested that the two of them could walk each morning after breakfast. She also felt that a treadmill at home would be the best solution for getting sufficient exercise in inclement weather. After a short discussion about the positive effect exercise can have on glucose control, the patient and his wife agreed to walk 15–20 minutes each day between 9:00 and 10:00 a.m.
A first-line medication for this patient had to be targeted to improving glucose control without contributing to weight gain. Thiazolidinediones (i.e., rosiglitizone [Avandia] or pioglitizone [Actos]) effectively address insulin resistance but have been associated with weight gain. 12 A sulfonylurea or meglitinide (i.e., repaglinide [Prandin]) can reduce postprandial elevations caused by increased carbohydrate intake, but they are also associated with some weight gain. 12 When glyburide was previously prescribed, the patient exhibited signs and symptoms of hypoglycemia (unconfirmed by SMBG). α-Glucosidase inhibitors (i.e., acarbose [Precose]) can help with postprandial hyperglycemia rise by blunting the effect of the entry of carbohydrate-related glucose into the system. However, acarbose requires slow titration, has multiple gastrointestinal (GI) side effects, and reduces A1C by only 0.5–0.9%. 13 Acarbose may be considered as a second-line therapy for A.B. but would not fully address his elevated A1C results. Metformin (Glucophage), which reduces hepatic glucose production and improves insulin resistance, is not associated with hypoglycemia and can lower A1C results by 1%. Although GI side effects can occur, they are usually self-limiting and can be further reduced by slow titration to dose efficacy. 14
After reviewing these options and discussing the need for improved glycemic control, the NP prescribed metformin, 500 mg twice a day. Possible GI side effects and the need to avoid alcohol were of concern to A.B., but he agreed that medication was necessary and that metformin was his best option. The NP advised him to take the medication with food to reduce GI side effects.
The NP also discussed with the patient a titration schedule that increased the dosage to 1,000 mg twice a day over a 4-week period. She wrote out this plan, including a date and time for telephone contact and medication evaluation, and gave it to the patient.
During the visit, A.B. and his wife learned to use a glucose meter that features a simple two-step procedure. The patient agreed to use the meter twice a day, at breakfast and dinner, while the metformin dose was being titrated. He understood the need for glucose readings to guide the choice of medication and to evaluate the effects of his dietary changes, but he felt that it would not be “a forever thing.”
The NP reviewed glycemic goals with the patient and his wife and assisted them in deciding on initial short-term goals for weight loss, exercise, and medication. Glucose monitoring would serve as a guide and assist the patient in modifying his lifestyle.
A.B. drew the line at starting an antihypertensive medication—the angiotensin-converting enzyme (ACE) inhibitor enalapril (Vasotec), 5 mg daily. He stated that one new medication at a time was enough and that “too many medications would make a sick man out of me.” His perception of the state of his health as being represented by the number of medications prescribed for him gave the advanced practice nurse an important insight into the patient’s health belief system. The patient’s wife also believed that a “natural solution” was better than medication for treating blood pressure.
Although the use of an ACE inhibitor was indicated both by the level of hypertension and by the presence of microalbuminuria, the decision to wait until the next office visit to further evaluate the need for antihypertensive medication afforded the patient and his wife time to consider the importance of adding this pharmacotherapy. They were quite willing to read any materials that addressed the prevention of diabetes complications. However, both the patient and his wife voiced a strong desire to focus their energies on changes in food and physical activity. The NP expressed support for their decision. Because A.B. was obese, weight loss would be beneficial for many of his health issues.
Because he has a sedentary lifestyle, is >35 years old, has hypertension and peripheral neuropathy, and is being treated for hypercholestrolemia, the NP performed an electrocardiogram in the office and referred the patient for an exercise tolerance test. 11 In doing this, the NP acknowledged and respected the mutually set goals, but also provided appropriate pre-exercise screening for the patient’s protection and safety.
In her role as diabetes educator, the NP taught A.B. and his wife the importance of foot care, demonstrating to the patient his inability to feel the light touch of the monofilament. She explained that the loss of protective sensation from peripheral neuropathy means that he will need to be more vigilant in checking his feet for any skin lesions caused by poorly fitting footwear worn during exercise.
At the conclusion of the visit, the NP assured A.B. that she would share the plan of care they had developed with his primary care physician, collaborating with him and discussing the findings of any diagnostic tests and procedures. She would also work in partnership with the RD to reinforce medical nutrition therapies and improve his glucose control. In this way, the NP would facilitate the continuity of care and keep vital pathways of communication open.
Advanced practice nurses are ideally suited to play an integral role in the education and medical management of people with diabetes. 15 The combination of clinical skills and expertise in teaching and counseling enhances the delivery of care in a manner that is both cost-reducing and effective. Inherent in the role of advanced practice nurses is the understanding of shared responsibility for health care outcomes. This partnering of nurse with patient not only improves care but strengthens the patient’s role as self-manager.
Geralyn Spollett, MSN, C-ANP, CDE, is associate director and an adult nurse practitioner at the Yale Diabetes Center, Department of Endocrinology and Metabolism, at Yale University in New Haven, Conn. She is an associate editor of Diabetes Spectrum.
Note of disclosure: Ms. Spollett has received honoraria for speaking engagements from Novo Nordisk Pharmaceuticals, Inc., and Aventis and has been a paid consultant for Aventis. Both companies produce products and devices for the treatment of diabetes.
Email alerts
- Advanced Practice Care: Advanced Practice Care in Diabetes: Epilogue
- Advanced Practice Care: Advanced Practice Care in Diabetes: Preface
- Online ISSN 1944-7353
- Print ISSN 1040-9165
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic
RELATED TOPICS
The patient has been treated for hypertension for 10 years, currently with amlodipine 10 mg by mouth daily. She was once told that her cholesterol value was "borderline high" but does not know the value.
She denies symptoms of diabetes, chest pain, shortness of breath, heart disease, stroke, or circulatory problems of the lower extremities.
She estimates her current weight at 165 lbs (75 kg). She thinks she weighed 120 lbs (54 kg) at age 21 years but gained weight with each of her three pregnancies and did not return to her nonpregnant weight after each delivery. She weighed 155 lbs one year ago but gained weight following retirement from her job as an elementary school teacher. No family medical history is available because she was adopted. She does not eat breakfast, has a modest lunch, and consumes most of her calories at supper and in the evening.
On examination, blood pressure is 140/85 mmHg supine and 140/90 mmHg upright with a regular heart rate of 76 beats/minute. She weighs 169 lbs, with a body mass index (BMI) of 30.9 kg/m 2 . Fundoscopic examination reveals no evidence of retinopathy. Vibratory sensation is absent at the great toes, reduced at the medial malleoli, and normal at the tibial tubercles. Light touch sensation is reduced in the feet but intact more proximally. Knee jerks are 2+ bilaterally, but the ankle jerks are absent. The examination is otherwise within normal limits.
- Diabetes Care for Children & Young People
Vol:05 | No:01
Children and young people’s diabetes care: Case study
- 12 Jul 2016
This case study demonstrates the physical and psychological difficulties faced by many young people with type 1 diabetes. Over the year following her diagnosis, Max had a deterioration in glycaemic control despite reporting that little had changed in her management. Detailed assessment revealed a number of psychosocial factors that were preventing her from achieving good control. However, working with her multidisciplinary team, she was able to address these issues and improve her blood glucose levels. This article outlines these issues and the action plan that Max and her diabetes team drew up to overcome them.
Share this article + Add to reading list – Remove from reading list ↓ Download pdf
This case study represents the challenges and issues, both physical and psychological, faced by a young person with type 1 diabetes and the support given by her diabetes multidisciplinary team (MDT). Implications for practice are addressed using current evidence-based research. The names of the child and family have been anonymised to protect their identity.
Case study Max (a pseudonym) is a 17-year-old girl who was diagnosed with type 1 diabetes 4 years ago at the age of 13 years. She and her mother were shocked and upset by the diagnosis, and both felt its management would be too great a task to take on by themselves.
Max is an only child and lives with her mother, a single parent. She attends the local state comprehensive school and is popular with her peer group. Her mother was very involved in her care and diabetes management from the onset. Despite this, her diabetes control deteriorated over time ( Table 1 ). In October 2012, her HbA 1c was 56 mmol/mol (7.3%); however, over the next year, this increased to 84 mmol/mol (9.8%) in July 2013. She found it difficult to count the carbohydrate portions in her food and her injections were hurting much more than when she was first diagnosed. She also expressed a fear of hypoglycaemia and of “looking stupid” in front of her friends.
Max and her MDT discussed treatment options to improve her glycaemic control. She refused insulin pump therapy but agreed to a blood glucose monitor and bolus advisor to assist with her regimen of multiple daily insulin injections (MDI). She is now using the bolus advisor confidently and has had regular one-to-one sessions with a psychologist. She is having fewer hypoglycaemic episodes and her HbA 1c has improved; in January 2016 it was 69 mmol/mol (8.5%) and in April 2016 it was 58 mmol/mol (7.5%).
Discussion Diagnosis Max and her mother were extremely shocked and upset by the diagnosis of type 1 diabetes and the potential severity of the condition and intense management required. Both felt it would be too great a task to take on by themselves.
Kübler-Ross and Kessler (2005) suggested that a diagnosis of diabetes is a life-changing event comparable to the experience of loss, and that children and families will often go through the five stages of grief defined by Kübler-Ross (1970) and outlined in Box 1 . They use this as a coping strategy to enable them to eventually acknowledge the condition. However, many families never reach the fifth stage of acceptance and many will fluctuate between the stages.
Although Max and her mum did accept the diagnosis eventually, at times both of them reverted to the earlier stages of grief. The diabetes MDT supported the family from diagnosis and will continue to support them throughout their time within the paediatric diabetes service, through the transition period with both paediatric and young people’s teams, until discharged to adult diabetes care.
The diabetes MDT was established after the Best Practice Tariff was introduced in 2012. It consists of doctors, nurses, dietitians, a psychologist and a personal assistant. It is well recognised that the MDT needs to work together in close cooperation to achieve good practice, and this can be strengthened by using written protocols, guidelines and targets (Brink, 2010). Logic would suggest that centres with MDTs and the same approaches and treatment regimens would have similar outcomes, yet the Hvidøre Childhood Diabetes Study Group has shown this is not the case (de Beaufort et al, 2013). In terms of glycaemic control, there were notable differences in patient outcomes across 21 diabetes clinics, all of which were committed to MDT-based practice. Although factors such as age, type of insulin regimen and socioeconomic status were shown to have some influence over specific outcomes, they did not explain the apparent differences between these clinics.
Family/social history Max is an only child and lives with her mother, a single parent. East et al (2006) suggested that rapid social change over the past 20 years has seen a marked increase in the number of mother-headed single-parent families. Max attends the local state comprehensive school, where she is generally doing well. She is popular with her peer group. La Greca et al (1995) suggested that peer relationships are important in diabetes management, as children and young people (CYP) may receive considerable emotional support from their friends. However, on occasions, Max’s peer relationships have had a counterproductive effect on her, and she feels she is different from her friends as the only one who has diabetes. This at times affects her self-esteem and impacts her diabetes control.
Max’s mother was very involved in her care and diabetes management from the onset. Anderson and Brackett (2005) suggested that parents typically take on most of the responsibility for management of diabetes when children are young or newly diagnosed.
Deterioration in diabetes control Max’s diabetes control had deteriorated since her diagnosis ( Table 1 ). In October 2012, her HbA 1c was 56 mmol/mol (7.3%), which indicated a good level of diabetes control and a reduced risk of diabetes complications, as suggested by the DCCT (Diabetes Control and Complications Trial; DCCT Research Group, 1994). At her subsequent diabetes clinic appointments up to July 2013, she reported that “nothing had really changed,” except she “didn’t have time to think about her diabetes,” although she felt guilty because she knew she could make herself ill and her mum would get upset. She stated that it was hard counting the carbohydrate portions in her food and her injections were hurting much more than when she was first diagnosed. Her height and weight remained static.
Diabetes care is greatly influenced by psychosocial factors when they obstruct people’s ability to manage their diabetes and achieve good metabolic control. A team-based approach to addressing an individual’s ability to cope is critical (Kent et al, 2010). It is important for healthcare professionals to be aware of how CYP think at the different stages of their development, as their understanding of illness and chronic health conditions is often greater than that of their peers. Jean Piaget (1896–1980) investigated cognitive processes in children, calling them “schemas”. By the time children reach around 12 years of age, they can describe illness in terms of non-functioning or malfunctioning of an internal organ or process. Later in development they can appreciate that a person’s thoughts or feelings can affect the way the body functions, which demonstrates an awareness of psychological factors (Taylor et al, 1999).
Spear (2013) proposed that we can begin to understand how young people with type 1 diabetes think, feel and behave if we consider the cognitive and biological changes that occur during adolescence. Glasper and Richardson (2005) suggested there is now a growing awareness that CYP are able to make their own decisions if given information in an age-appropriate manner. Gillick competence identifies children aged under 16 years as having the capacity to consent to their own treatment if they understand the consequences (NSPCC, 2016).
Butler et al (2007) suggest that adolescence is a time of upheaval when young people have to deal with the influence of peers, school life and developing their own identity, as well as all the physiological changes that occur. Young people with type 1 diabetes have the added responsibility of developing autonomy regarding the self-management of their condition. Hanas (2006) suggests that parents should continue to take part in their child’s diabetes care into adolescence and not hand the responsibility to the young person too early. Snoek and Skinner (2002) suggest that intensive self-management of diabetes is complex and time-consuming, and creates a significant psychosocial burden on children and their families.
There are significant challenges for CYP to engage in effective diabetes self-management. Several of these were identified with Max and her mother:
- Deterioration in diabetes control.
- Difficulty with carbohydrate counting.
- Insulin omission.
- Fear of hypoglycaemia.
- Painful injections.
Action plan An action plan was discussed between Max and the MDT. As she was on an MDI regimen (a long-acting insulin at bedtime and rapid-acting insulin with meals), a bolus advisor/blood glucose monitor was demonstrated and discussed with her and her mum. Max felt she would be able to use this to help eliminate the calculations which, although she was capable of doing them, she often lacked time to do so. With further discussion, Max said she was “scared of getting it wrong and having a hypo”. Insulin pump therapy was discussed but she did not want to “have a device attached to my body because it would remind me all the time that I have diabetes”. Insulin pump therapy is recommended as a treatment option for adults and children over 12 years of age with type 1 diabetes whose HbA 1c levels remain above 69 mmol/mol (8.5%) on MDI therapy despite a high level of care (NICE, 2015a).
The National Service Framework standard 3 (Department of Health, 2001) recommends empowering people with diabetes and encourages them and their carers to gain the knowledge and skills to be partners in decision-making, and giving them more personal control over the day-to-day management of their diabetes, ensuring the best possible quality of life. However, if a diabetes management plan is discussed in partnership with a (Gillick-competent) young person but they elect not to comply with the plan despite full awareness of the implications of their actions, then the diabetes team should support them whilst trying to encourage them to maintain the treatment plan. This can be very difficult and frustrating at times, as a healthcare professional is an advocate for the patient, and promotion of the best interests of the patient is paramount.
Psychology involvement Max was reviewed by the psychologist to assess her psychological health and wellbeing. The psychologist used the Wellbeing in Diabetes questionnaire (available from the Yorkshire and Humber Paediatric Diabetes Network) to assess her and identify an optimal plan of care.
The psychology sessions were focussed on her issues around the following:
- Worry about deterioration in control.
- The consequences of insulin omission.
Max had a series of one-to-one appointments and some joint sessions with the paediatric diabetes specialist nurse and/or dietitian, so this linked into other team members’ specialities.
Carbohydrate counting and use of a bolus advisor The dietitian assessed Max and her mother’s ability to carbohydrate count using a calculator, food diagrams and portion sizes, and both of them were able to demonstrate competency in this task. Garg et al (2008) have shown that the use of automated bolus advisors is safe and effective in reducing postprandial glucose excursions and improving overall glycaemic control. However, this can only be true if the bolus advisor is being used correctly and is confirmed as such by comparing blood glucose and HbA 1c results before and after initiation of the bolus advisor, and observing the patient using the device to ensure it is being used safely and correctly.
Barnard and Parkin (2012) propose that, as long as safety and lifestyle are taken into consideration, advanced technology will benefit CYP, as inaccurate bolus calculation can lead to persistent poor diabetes control. These tools can help with removing the burden of such complex maths and have the potential to significantly improve glycaemic control.
Insulin omission and fear of hypoglycaemia Max also expressed her fear of hypoglycaemia and of “looking stupid” in front of her friends. She admitted to missing some of her injections, especially at school. Wild et al (2007) suggest that a debilitating fear of hypoglycaemia can result in poor adherence to insulin regimens and subsequent poor metabolic control. Crow et al (1998) describe the deliberate omission or reduced administration of insulin, which results in hyperglycaemia and subsequent rapid reduction in body weight. Type 1 diabetes predisposes a person to a high BMI. Adolescent girls and adult women with type 1 diabetes generally have higher BMI values than their peers without the condition (Domargård et al, 1999). Affenito et al (1998) observed that insulin misuse was the most common method of weight control used by young women with type 1 diabetes. However, Max’s weight remained stable and there was no clinical indication that she was missing insulin to lose weight; rather, it was her fear of hypoglycaemia that drove her to omitting insulin at school. With the use of the bolus calculator, she was reassured about her calculations for insulin-to-carbohydrate ratios, but it was reinforced with her that the device would only work efficiently if she used it correctly with each meal.
Painful injections Max also highlighted that her injections were now more painful than when she was first diagnosed, and this was causing her distress each time she had to inject. Injection technique was discussed with her and demonstrated using an injection model, and her injection technique was observed and appeared satisfactory. She was using 5-mm insulin needles and so was switched to 4-mm needles, as recommended by Forum for Injection Technique (2015) guidelines.
Appropriate technique when giving injections is key to optimal blood glucose control; however, evidence suggests that injection technique is often imperfect. Studies by Strauss et al (2002) and Frid et al (2010) revealed disturbing practices in relation to injection technique, with little improvement over the years. Current diabetes guidelines do not include detailed advice on injection technique, and only the guidance on type 2 diabetes in adults (NICE, 2015b) makes any reference to providing education about injectable devices for people with diabetes. However, the older Quality Standard for diabetes in adults (NICE, 2011) recommends a structured programme of education, including injection site selection and care (Diggle, 2014).
Conclusion The issues and concerns this young girl had were identified and addressed by the diabetes MDT. She was assessed by several members of the team, and a credible, evidence-based action plan was put into place to assist her and her mother to manage her diabetes at this difficult time. Max is now using the bolus advisor confidently and having fewer hypoglycaemic episodes, and her HbA 1c has improved. She prefers using the 4-mm injection pen needles, although she remains hesitant when giving injections; she will still not consider insulin pump therapy. Her one-to-one sessions with the psychologist have now ceased, but she is aware she can access a psychologist at clinic on request, or if the MDT assesses that her psychological health has deteriorated.
When a child in a family develops a chronic condition such as type 1 diabetes, effective communication is vitally important to address issues with the family at the earliest stage so that problems can be discussed and, hopefully, resolved before they escalate out of control. Upon reflection, the team could have become more intensely involved at an earlier stage to prevent Max’s diabetes management issues and stop her HbA 1c from reaching such a high level. Furthermore, the new NICE (2015a) guideline has set the target HbA 1c at ≤48 mmol/mol (6.5%), so there is still some work to be done. However, the outcome of this case appears to be favourable at present.
Affenito SG, Rodriguez NR, Backstrand JR et al (1998) Insulin misuse by women with type 1 diabetes mellitus complicated by eating disorders does not favorably change body weight, body composition, or body fat distribution. J Am Diet Assoc 98 : 686–8 Anderson BJ, Brackett J (2005) Diabetes in children. In: Snoek FJ, Skinner TC (eds). Psychology in Diabetes Care (2nd edition). John Wiley & Sons, Chichester Barnard K, Parkin C (2012) Can automated bolus advisors help alleviate the burden of complex maths and lead to optimised diabetes health outcomes? Diabetes Care for Children & Young People 1 : 6–9 Brink SJ (2010) Pediatric and adolescent multidisciplinary diabetes team care. Pediatr Diabetes 11 : 289–91 Butler JM, Skinner M, Gelfand D et al (2007) Maternal parenting style and adjustment in adolescents with type I diabetes. J Pediatr Psychol 32 : 1227–37 Crow SJ, Keel PK, Kendall D (1998) Eating disorders and insulin-dependent diabetes mellitus. Psychosomatics 39 : 233–43 de Beaufort CE, Lange K, Swift PG et al (2013) Metabolic outcomes in young children with type 1 diabetes differ between treatment centers: the Hvidoere Study in Young Children 2009. Pediatr Diabetes 14 : 422–8 Department of Health (2001) National Service Framework: Diabetes . DH, London. Available at: http://bit.ly/18OpAzL (accessed 24.02.16) Diabetes Control and Complications Trial Research Group (1994) Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr 125 : 177–88 Diggle J (2014) Are you FIT for purpose? The importance of getting injection technique right . Journal of Diabetes Nursing 18 : 50–7 Domargård A, Särnblad S, Kroon M et al (1999) Increased prevalence of overweight in adolescent girls with type 1 diabetes mellitus. Acta Paediatr 88 : 1223–8 East L, Jackson D, O’Brien L (2006) Father absence and adolescent development: a review of the literature. J Child Health Care 10 : 283–95 Forum for Injection Technique (2015) The UK Injection Technique Recommendations (3rd edition). Available at: http://bit.ly/1QeZU2E (accessed 24.02.16) Frid A, Hirsch L, Gaspar R et al (2010) The Third Injection Technique Workshop in Athens (TITAN). Diabetes Metab 36 (Suppl 2): 19–29 Garg SK, Bookout TR, McFann KK et al (2008) Improved glycemic control in intensively treated adult subjects with type 1 diabetes using insulin guidance software. Diabetes Technol Ther 10 : 369–75 Glasper EA, Richardson J (2005) A Textbook of Children’s and Young People’s Nursing . Churchill Livingston, London Hanas R (2006) Type 1 Diabetes in Children, Adolescents and Young Adults (3rd edition). Class Publishing, London: 329, 349–50 Kent D, Haas L, Randal D et al (2010) Healthy coping: issues and implications in diabetes education and care. Popul Health Manag 13 : 227–33 Kübler-Ross E (1970) On Death and Dying: What the Dying Have to Teach Doctors, Nurses, Clergy and Their Own Families . Tavistock Publications, London Kübler-Ross E, Kessler D (2005) On Grief and Grieving: Finding the Meaning of Grief Through the Five Stages of Loss . Simon & Schuster UK, London La Greca AM, Auslander WF, Greco P et al (1995) I get by with a little help from my family and friends: adolescents’ support for diabetes care. J Pediatr Psychol 20 : 449–76 NICE (2011) Diabetes in adults (QS6). NICE, London. Available at: www.nice.org.uk/guidance/qs6 (accessed 24.02.16) NICE (2015a) Diabetes (type 1 and type 2) in children and young people: diagnosis and management (NG18). NICE, London. Available at: www.nice.org.uk/guidance/ng18 (accessed 24.02.16) NICE (2015b) Type 2 diabetes in adults: management (NG28). NICE, London. Available at: www.nice.org.uk/guidance/ng28 (accessed 24.02.16) NSPCC (2016) A Child’s Legal Rights: Gillick Competency and Fraser Guidelines . NSPCC, London. Available at: http://bit.ly/1Tj6DcF (accessed 24.02.16) Snoek FJ, Skinner TC (2002) Psychological counselling in problematic diabetes: does it help? Diabet Med 19 : 265–73 Spear LP (2013) Adolescent neurodevelopment. J Adolesc Health 52 (Suppl 2): 7–13 Strauss K, De Gols H, Hannat I et al (2002) A pan-European epidemiologic study of insulin injection technique in patients with diabetes. Practical Diabetes International 19 : 71–76 Taylor J, Müller D, Wattley L, Harris P (1999) The development of children’s understanding. In: Nursing Children: Psychology, Research and Practice . Stanley Thornes, Cheltenham Wild D, von Maltzahn R, Brohan E et al (2007) A critical review of the literature on fear of hypoglycemia in diabetes: implications for diabetes management and patient education. Patient Educ Couns 68 : 10–5
Do youth workers have a role in improving diabetes transition services?
Cgm for children and young people with type 1 diabetes: nice criteria and effects of decision fatigue and alarm fatigue , improving paediatric diabetes in england: areas of focus, delays in accessing continuous glucose monitoring in people with type 1 diabetes, celebrating may ng: the woman behind the obe, fiona campbell awarded an obe for services to paediatric diabetes, diabetes transition: a time to act.

Can the involvement of youth workers improve diabetes care for young people transitioning to adult diabetes services?

The impact of decision fatigue and alarm fatigue in children and young people using continuous glucose monitoring

NHSEI National Clinical Lead for Diabetes in Children and Young People, Fulya Mehta, outlines the areas of focus for improving paediatric diabetes care.
16 Nov 2022

NICE guidance urges local trusts to improve processes and advocate for CGM use in children and young people.
Sign up to all DiabetesontheNet journals
- CPD Learning
- Diabetes & Primary Care
- Journal of Diabetes Nursing
- The Diabetic Foot Journal
- Diabetes Digest
Useful information
- Terms and conditions
- Privacy policy
- Editorial policies and ethics

By clicking ‘Subscribe’, you are agreeing that DiabetesontheNet.com are able to email you periodic newsletters. You may unsubscribe from these at any time. Your info is safe with us and we will never sell or trade your details. For information please review our Privacy Policy .
Are you a healthcare professional? This website is for healthcare professionals only. To continue, please confirm that you are a healthcare professional below.
We use cookies responsibly to ensure that we give you the best experience on our website. If you continue without changing your browser settings, we’ll assume that you are happy to receive all cookies on this website. Read about how we use cookies .
- Open access
- Published: 06 April 2024
Assessment of subclinical left ventricular systolic and diastolic dysfunction in patients with type 2 diabetes mellitus under follow-up at Tikur Anbessa specialized hospital, Ethiopia: a case-control study
- Tigist Seleshi 1 ,
- Theodros Alemneh 2 ,
- Dufera Mekonnen 1 ,
- Demu Tesfaye 3 ,
- Sura Markos 4 ,
- Yitagesu Getachew 5 ,
- Konno Taddese 1 &
- Senbeta Guteta 1
BMC Cardiovascular Disorders volume 24 , Article number: 201 ( 2024 ) Cite this article
Metrics details
Individuals with diabetes mellitus are at increased risk of cardiovascular diseases, which in turn are the most common cause of morbidity and mortality in the diabetic population. A peculiar feature of cardiovascular diseases in this population is that they can have significant cardiac disease while remaining asymptomatic. There is a paucity of data regarding subclinical cardiac imaging features among diabetic adults in Africa, particularly in Ethiopia. This study was conducted to compare the magnitude and spectrum of left ventricular systolic and diastolic dysfunction among asymptomatic type 2 diabetic adults versus a normotensive, non-diabetic control group and to evaluate the determinants of left ventricular diastolic and systolic dysfunction.
This was a case-control study conducted at Tikur Anbessa specialized hospital, Addis Ababa, Ethiopia. A standard transthoracic echocardiography was done for all study participants with type 2 diabetes mellitus and their normotensive and non-diabetic controls. Structured questionnaires were used to collect demographic and clinical characteristics and laboratory test results. Statistical analysis was done using the SPSS 25.0 software. The data was summarized using descriptive statistics. Bivariate and multivariate analysis was performed to determine the association between variables and echocardiographic parameters. The strength of statistical association was measured by adjusted odds ratios and 95% confidence intervals, with significant differences taken at p < 0.05.
We analyzed age- and sex-matched 100 participants in the study (diabetic) group and 200 individuals in the control group. Left ventricular systolic and diastolic dysfunction were significantly more prevalent among diabetic adults than their sex and age matched controls. Among diabetic individuals, ages of 60 years and above, dyslipidemia, use of Metformin and Glibenclamide, high serum triglyceride level, presence of neuropathy and use of statins correlated significantly with the presence of left ventricular diastolic dysfunction. Chronic kidney disease and neuropathy were determinants of left ventricular systolic dysfunction.
Left ventricular systolic and diastolic dysfunction were significantly more prevalent among diabetic patients than their sex- and age-matched controls in our study. We recommend early screening for subclinical left ventricular dysfunction, especially in the elderly and in those with chronic kidney disease, dyslipidemia, and microvascular complications such as neuropathy.
Peer Review reports
The prevalence of diabetes is increasing globally at an alarming rate. In 2013, it was projected that 300 million people would be diagnosed with diabetes by the year 2030, but the current prevalence in 2022 has already surpassed this number by 100 million [ 1 ]. In a large-scale survey conducted in Ethiopia in 2015, the prevalence of diabetes and pre-diabetes was determined to be 5.6% and 5.4%, respectively [ 2 ]. Micro- and macrovascular complications of diabetes mellitus are major determinants of morbidity and mortality of patients. Of these, cardiovascular complications account for the majority of the disease burden in relation to diabetes [ 1 , 2 ].
The risk of developing cardiovascular complications and heart failure is by far higher in diabetic patients as compared with non-diabetic ones. Diabetes was found to be associated with an increased risk of heart failure in patients with non-obstructed coronary arteries. The earliest description of diabetic cardiomyopathy was from an autopsy study that evaluated the vascular and myocardial findings of diabetic patients that had glomerulosclerosis. Postmortem study of four patients with no additional risk factors showed cardiomegaly and signs of heart failure with no major coronary obstruction. Based on the findings of intramural arterial thickening and narrowing, micro-angiopathy related to diabetes was considered to be the culprit. This has led to a better understanding of the pathogenesis behind diabetic-induced microvascular and myocardial dysfunction leading to heart failure [ 3 , 4 ].
Both systolic and diastolic dysfunction are observed frequently in diabetic patients. As seen in the Framingham study, the rate of development of heart failure was five times and twice as high in diabetic women and men, respectively, as compared to non-diabetic patients. Among diabetic patients with heart failure, 30% had diastolic dysfunction, considered the earliest sign of heart failure in diabetic individuals [ 5 ]. Diastolic dysfunction is seen to be associated with poor glycemic control. Microvascular dysfunction, renin-angiotensin-aldosterone system imbalance, collagen formation and degradation imbalance, impaired calcium transport, and interstitial accumulation of glycosylation products are all possible mechanisms of diastolic dysfunction in diabetic patients. Diabetes, along with aging, hypertension, and atrial fibrillation, contributes significantly to the pathogenesis and prognosis of diastolic dysfunction [ 6 ].
Various studies have tried to identify the factors associated with diastolic dysfunction in type 2 diabetic patients. An observational study that included about 49,000 patients identified that poor glycemic control was associated with a more severe diastolic dysfunction. Each 1% increase in hemoglobin A1C (HbA1C) was linked to an 8% increase in the risk of heart failure. These findings were also confirmed in a single, small-scale study of type 1 diabetic patients with diabetic neuropathy [ 7 , 8 ]. Small-scale case-control studies from the U.K., Turkey and Australia identified that while systolic function was similar, diastolic function was markedly impaired in diabetic patients. Poor diabetic control, advancing age, treatment with Metformin and a longer duration of diabetes were significantly associated with echocardiographic abnormalities of diastolic dysfunction while the presence of left ventricular dysfunction, and treatment with insulin and angiotensin-converting enzyme inhibitors (ACEIs) were found to be protective [ 9 , 10 , 11 ].
There is a paucity of data regarding the subclinical cardiac imaging features among diabetic adults in Africa, particularly in Ethiopia – the second most populous nation in the continent and one with a high number of its citizens being pre-diabetic or diabetic [ 2 ]. This study was conducted to compare the magnitude and spectrum of left ventricular systolic and diastolic dysfunction among asymptomatic type 2 diabetic adults versus a normotensive, non-diabetic control group and to evaluate the determinants of left ventricular diastolic and systolic dysfunction.
Study setting
This was a case control study conducted from June – October 2022 at Tikur Anbessa Specialized Hospital (TASH), Addis Ababa, Ethiopia.
Study design
This was a case-control study with the source population being all patients with type 2 diabetes mellitus aged 40 years and above and under follow up at the study hospital for at least 6 months and having no prior history of cardiac illness or symptoms. (No prior history of cardiac illness is defined as a patient with no previous diagnosis, treatment or follow up for a cardiac compliant. That was extracted from the diagnosis list on the electronic medical recording and during history).
Diagnosis of Type 2 Diabetes Mellitus: HBA1c>6.5%, RBS>200 mg/dl, FBS> 126mg/dl, on two separate set of tests.
These symptoms include paroxysmal nocturnal dyspnea, orthopnea, dyspnea, chest pain (anginal type), body swelling not attributable to other causes, etc with a combination of symptoms given precedence over solitary symptoms. We enrolled 100 individuals and a further 200 age- and sex-matched controls using a convenience sampling method. The control group were normoglycemic, normotensive surgical patients, aged 40 years and above presenting to the study hospital for non-cardiac complaints.
Patients who were found to have a combination of symptoms that suggest cardiovascular disease were excluded.
Data collection
After obtaining informed consent, structured questionnaires were used to collect demographic and clinical characteristics and laboratory test results from patient interviews and charts. A standard transthoracic echocardiography was also performed on all study participants, using the same equipment (Vivid 9, GE equipped with tissue Doppler imaging and a transducer of 1.5 – 2.5 MHz). Each recorded measurement was the mean of three measurements (Table 1 ). If both left ventricular systolic and diastolic dysfunction were identified in the same participant, then the presence of diastolic dysfunction was not included in the analysis as it could be due to the mere presence of systolic dysfunction even in the absence of diabetes [ 12 ].
Subclinical Diastolic and systolic dysfunction means an Echocardiographic finding without symptoms.
Echocardiographic measurements were taken by blinded operator and the interpretation was done by other members of the team following the standard definitions.
Strain Analysis is demonstrated to have superior sensitivity and specificity for LV systolic dysfunction. The setup to do strain analysis is not available in our country.
Data analysis
Statistical analysis was done using the SPSS 25.0 software. The data were summarized using descriptive statistics. Bivariate and multivariate analysis was performed to determine the association between variables and echocardiographic parameters. The strength of statistical association was measured by adjusted odds ratios and 95% confidence intervals, with significant differences taken at p < 0.05.
Socio-demographic characteristics
The study enrolled 300 individuals: 100 cases and 200 controls. The two groups were sex- and age- matched. The majority of the participants were 50 - 70 years of age (Table 2 ).
The mean duration of diabetes among the study group was 10.9 years. The majority of patients (47%) had a long period since diagnosis of diabetes, defined as more than 10 years with the remaining 23% and 30% of participants having spent 5 years and 5 - 10 years respectively since diagnosis. Various comorbidities were identified in the study group, with hypertension being the most common (47%). The participants in this group were screened for micro- and macrovascular complications of diabetes mellitus, with nephropathy the most commonly identified complication (33%) (Tables 2 , 3 and 4 ).
The study group were on multiple medications, with Metformin being the most commonly used drug (48%) followed by a combination of metformin and sulfonylureas (32%). Only 4 patients were on sodium glucose transport protein 2 inhibitors (SGLT2Is) (one patient on canagliflozin and 3 on dapagliflozin). A total of 48% of patients were taking insulin either in combination with other oral hypoglycemic agents or alone. About 53% of patients were taking ACEis or angiotensin receptor blockers (ARBs). Of these, 45% were on Enalapril, 5% on Losartan and 3% on Candesartan. Nearly 85% of patients were on statins with 88% of those receiving statins were taking Atorvastatin and the remaining12% either on rosuvastatin or simvastatin. The remaining 15% were not taking any type of statin drugs.
Of the various causes of admissions to the hospital among the control group, benign prostatic hyperplasia (BPH) (25%), cholelithiasis (20%), and malignancies (15%) accounted for the majority of admissions. Dyslipidemia was identified as comorbidity in 10% of the control group. All the patients included in the control group had normal fasting blood glucose levels and were normotensive. Their mean ejection fraction was 61.78% (± 4.95%). The remaining laboratory results had normal findings. A comparison of echocardiographic abnormalities of the two groups is shown in Table 5 .
Echocardiographic comparisons
Of the 100 diabetic patients, 11 had a left ventricular ejection fraction < 50% with 89 participants having normal left ventricular systolic function (LVSF) as measured by all parameters. Three had moderate left ventricular systolic dysfunction (LVSD) eight of the patients have a mildly reduced ejection fraction. Three of the patients with mild LVSD had regional wall motion abnormalities (anterior, anteroseptal, and apical wall hypokinesis). Reduced lateral and septal S wave velocities were seen in 51% and 60% of the participants, respectively (Table 5 ).
When compared to the controls, there was a significantly higher prevalence of diastolic dysfunction and systolic dysfunction. The rate of systolic dysfunction in the cases was higher when compared to the control group despite the smaller sample size (OR: 0.13, 95% CI 0.03 – 0.6, p = 0.01). Similarly higher diastolic dysfunction was seen in sub-group analysis of the study group than in the controls (grades I – II). Although there was no difference in grade III diastolic dysfunction between the two groups, it should be noted that its prevalence in both groups is very low (4% in cases and 0.5% in the control group), limiting our ability to compare it among the two groups (Table 6 ).
Determinants of LVSD and LVDD
Among diabetic individuals, ages 60 years and above, dyslipidemia, use of Metformin and Glibenclamide, a high serum triglyceride level, presence of neuropathy and use of statins correlated significantly with the presence of left ventricular diastolic dysfunction. Chronic kidney disease and neuropathy were determinants of left ventricular systolic dysfunction, as seen by reduced left ventricular ejection fraction (Table 7 ).
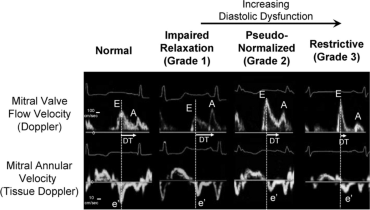
Echo Image [ 26 ]: Representative of diastolic function assessment
Our study showed that left ventricular systolic and diastolic dysfunction were significantly more prevalent among diabetic adults than their sex and age matched controls. Hypertension was the most common comorbidity in our study population (47%). The Framingham study showed that hypertension had an additive adverse effect on the left ventricular volume, relaxation, diastolic function, and systolic function of the left ventricle [ 5 ]. But hypertension was not found to be significantly correlated with left ventricular systolic and diastolic function in our study.
In our study, the prevalence of diastolic dysfunction was 82% among the diabetic patients as compared to 30% in the control group. As compared to other studies in low-income settings, our findings showed a higher prevalence. A study conducted in Nigeria with a similar sample size to our study, found that 65% of the diabetic patients had diastolic dysfunction, compared to 3.3% in the control group. This disparity can be explained by the relatively older age of patients included in our study (56 years vs 50.8 years) and having a longer duration of diabetes (10.9 years vs 3.4 years in in Dodiyi-Manuel et al’ study from Nigeria) [ 1 ]. Case-control studies with larger cohorts from the U.S. confirm that diabetic individuals had a significantly higher number of subclinical left ventricular dysfunction [ 17 , 18 , 19 ]. A large study of 751 diabetic adults conducted in Italy discovered a similar higher prevalence of diastolic dysfunction as our study, with approximately 60% of the participants having left ventricular diastolic dysfunction [ 20 ].
We identified a reduced left ventricular ejection fraction in about 11% of the diabetic sub-group of our study. These findings mimicked those of other studies. In a case-control study conducted in Nigeria, 15.6% of participants had an LV ejection fraction of 55%, compared to 4% in the control group. The authors used a cut-off ejection fraction of less than 55%, which may have overestimated the prevalence [ 21 ]. Another study done to evaluate biventricular systolic dysfunction in patients with type 2 diabetes compared 26 cases with 126 control participants. Diabetes showed an independent association with severely decreased biventricular function and an LV ejection fraction of < 30%. In 15.4% of the patients, there was also associated right ventricular dysfunction [ 17 ].
More advanced imaging modalities, such as speckle tracking and strain analysis, show a higher prevalence of subclinical LVSD than 2D echocardiography. The use of dobutamine/exercise stress echocardiography has demonstrated that diabetic patients have a higher prevalence of LV dysfunction [ 22 , 23 , 24 ]. In a study that evaluated longitudinal and radial strain of the left ventricle in 32 diabetic patients and 32 control subjects, there was significantly lower longitudinal strain with preserved LV ejection fraction in type 2 diabetic individuals, which was explained by normal radial strain compensation [ 24 ]. We were not able to do strain analysis because of a lack of access. But we were able to evaluate the lateral and medial S’ wave velocities. Tissue Doppler imaging revealed that 51 - 60% of our study population had significantly lower lateral and septal s-wave velocities. Systolic wave velocity is considered to be a surrogate marker of early left ventricular dysfunction and a measure of longitudinal systolic function. and was found to be associated with LV ejection fraction. A study that evaluated subclinical LVSD and glycemic control in asymptomatic type 2 diabetic patients with preserved LV ejection fraction identified that mean S’ wave velocity was inversely and independently associated with high HgbA1c after adjustment for age, diabetes duration, and body mass index [ 25 ].
Our study also showed that among diabetic individuals, ages 60 years and above, dyslipidemia, use of Metformin and Glibenclamide, high serum triglyceride level, presence of neuropathy and use of statins correlated significantly with the presence of left ventricular diastolic dysfunction. We identified that grade I diastolic dysfunction was significantly associated with ages above 60 years, combined use of metformin and sulfonylurea and a high serum triglyceride level. Dyslipidemia was also associated with both grade I and grade II LVDD. Use of Atorvastatin was significantly associated with Grade II LVDD.
Our findings are similar to most other studies which have concluded that, in particular advanced age and use of Metformin being significant predictors of diastolic dysfunction. A small-scale study of 70 patients showed that serum LDL levels, a high HbA1c level, and the patient's age were significantly associated with LV dysfunction [ 9 ]. While many studies showed factors like duration of diabetes, poor glycemic control as adverse predictors and use of ACEIs as having a protective effect against LVDD and LVSD, such findings could not be replicated in our study [ 7 , 9 , 10 ]. This could be due to the smaller sample size that we enrolled.
Chronic kidney disease and neuropathy were determinants of left ventricular systolic dysfunction in our study. Other studies have confirmed these findings, with additional adverse factors identified being poor glycemic control and advanced age [ 10 , 11 ].
The comparison between the case and control groups in our study showed that diabetes was independently associated with a higher prevalence of left ventricular grade I and II diastolic dysfunction, and a reduced ejection fraction.
The limitations of our study were our relatively smaller sample size and the unavailability of natriuretic peptide tests (brain natriuretic peptide BNP/N-terminal pro b-type natriuretic peptide NT-ProBNP) and advanced imaging techniques like speckle tracking.
Our study showed that left ventricular systolic and diastolic dysfunctions were significantly more prevalent among diabetic patients when compared to normoglycemic, normotensive controls. Among the diabetic sub-group, ages of 60 years and above, dyslipidemia, use of Metformin and Glibenclamide, neuropathy and use of statins predicted the occurrence of left ventricular diastolic dysfunction. Chronic kidney disease and neuropathy were also found to be associated with left ventricular systolic dysfunction. The presence of diabetes and its comorbidities is associated with subclinical left ventricular systolic and diastolic dysfunction. Therefore, similar studies should be done in low-resource settings to devise screening programs aiming to detect subclinical left ventricular dysfunction early, especially in the elderly and in those with predisposing comorbidities.
Availability of data and materials
All study data can be made available from the corresponding author upon a reasonable request.
Abbreviations
Angiotensin converting enzyme inhibitor
Angiotensin receptor blockers
Body Mass Index
Blood pressure
Benign prostatic hyperplasia
Chronic kidney disease
cardiovascular diseases
Chronic obstructive pulmonary disease
ratio- early diastolic flow velocity/late diastolic transmitral flow velocity
ratio of early diastolic mitral inflow velocity to early diastolic mitral annulus velocity
Ejection fraction
Glycosylated hemoglobin level
Human immunodeficiency virus
lateral S wave velocity
low density lipoprotein
Left Ventricle
Left ventricular diastolic dysfunction
Left ventricular ejection fraction
left ventricular hypertrophy
- Left ventricular systolic dysfunction
Mitral annular peak systolic excursion
septal S wave velocity
Sodium glucose transport protein 2 inhibitors
Tricuspid annular peak systolic excursion
Tikur Anbessa Specialized Hospital
Transient ischemic attack
Type 2 Diabetes Mellitus
Dodiyi-Manuel ST, Akpa MR, Odia OJ. Left ventricular dysfunction in normotensive type II diabetic patients in Port Harcourt. Nigeria Vasc Health Risk Manag. 2013;9:529–33. https://doi.org/10.2147/vhrm.s44540 .
Article PubMed Google Scholar
Gebreyes YF, Goshu DY, Geletew TK, Argefa TG, Zemedu TG, Lemu KA, et al. Prevalence of high blood pressure, hyperglycemia, dyslipidemia, metabolic syndrome and their determinants in Ethiopia: Evidences from the National NCDs STEPS Survey, 2015. PLoS One. 2018;13(5):e0194819. https://doi.org/10.1371/journal.pone.0194819 .
Article CAS PubMed PubMed Central Google Scholar
New type of cardiomyopathy associated with diabetic glomerulosclerosis, The AmericanJournal of Cardiology, Volume 30, Issue 6, 1972, Pages 595-602,ISSN 0002-9149, https://doi.org/10.1016/0002-9149(72)90595-4
Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care. 2004;27(8):1879–84. https://doi.org/10.2337/diacare.27.8.1879 .
Galderisi M, Anderson KM, Wilson PW, Levy D. Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (the Framingham Heart Study). Am J Cardiol. 1991;68:85–9. https://doi.org/10.1016/0002-9149(91)90716-x . 10.1097%2FHJH.0b013e32833311691.
Article CAS PubMed Google Scholar
Tsujino T, Kawasaki D, Masuyama T. Left ventricular diastolic dysfunction in diabetic patients: pathophysiology and therapeutic implications. Am J Cardiovasc Drugs. 2006;6(4):219–30. https://doi.org/10.2165/00129784-200606040-00002 .
de Simone G, Devereux RB, Chinali M, Lee ET, Galloway JM, Barac A, et al. Diabetes and incident heart failure in hypertensive and normotensive participants of the Strong Heart Study. J Hypertens. 2010;28(2):353–60. https://doi.org/10.1097%2FHJH.0b013e3283331169 .
Didangelos TP, Arsos GA, Karamitsos DT, Athyros VG, Karatzas ND. Left ventricular systolic and diastolic function in normotensive type 1 diabetic patients with or without autonomic neuropathy: a radionuclide ventriculography study. Diabetes Care. 2003;26(7):1955–60. https://doi.org/10.2337/diacare.26.7.1955 .
Vinereanu D, Nicolaides E, Tweddel AC, Mädler CF, Holst B, Boden LE, Cinteza M, Rees AE, Fraser AG. Subclinical left ventricular dysfunction in asymptomatic patients with Type II diabetes mellitus, related to serum lipids and glycated haemoglobin. Clin Sci (Lond). 2003;105(5):591–9. https://doi.org/10.1042/cs20030168 .
Yazici M, Ozdemir K, Gonen MS, Kayrak M, Ulgen MS, Duzenli MA, et al. Is there any relationship between metabolic parameters and left ventricular functions in type 2 diabetic patients without evident heart disease? Echocardiography. 2008;25(7):675–82. https://doi.org/10.1111/j.1540-8175.2008.00690.x .
Fang ZY, Schull-Meade R, Downey M, Prins J, Marwick TH. Determinants of subclinical diabetic heart disease. Diabetologia. 2005;48(2):394–402. https://doi.org/10.1007/s00125-004-1632-z .
Movahed MR, Milne N. Presence of biventricular dysfunction in patients with type II diabetes mellitus. Congest Heart Fail. 2007;13(2):78-80. https://doi.org/10.1111/j.1527-5299.2007.888138.x .
Armstrong WF & Ryan T. Feigenbaum's Echocardiography (8 th ed.) 2019, Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins.
Chahal NS, Lim TK, Jain P, Chambers JC, Kooner JS, Senior R. Normative reference values for the tissue Doppler imaging parameters of left ventricular function: a population-based study. Eur J Echocardiogr. 2010;11(1):51–6. https://doi.org/10.1093/ejechocard/jep164 .
Hu K, Liu D, Herrmann S, Niemann M, Gaudron PD, Voelker W, et al. Clinical implication of mitral annular plane systolic excursion for patients with cardiovascular disease. Eur Heart J Cardiovasc Imaging. 2013;14(3):205–12. https://doi.org/10.1093/ehjci/jes240 .
Mitter SS, Shah SJ, Thomas JD. A Test in Context: E/A and E/e’ to Assess Diastolic Dysfunction and LV Filling Pressure. J Am Coll Cardiol. 2017;69(11):1451–64. https://doi.org/10.1016/j.jacc.2016.12.037 .
Movahed MR, Milne N. Presence of biventricular dysfunction in patients with type II diabetes mellitus. Congest Heart Fail. 2007;13(2):78–80. https://doi.org/10.1111/j.1527-5299.2007.888138.x .
Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC Jr. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. 2004;27(3):699–703. https://doi.org/10.2337/diacare.27.3.699 .
Barzilay JI, Spiekerman CF, Kuller LH, Burke GL, Bittner V, Gottdiener JS, et al. Cardiovascular Health Study. Prevalence of clinical and isolated subclinical cardiovascular disease in older adults with glucose disorders: the Cardiovascular Health Study. Diabetes Care. 2001;24(7):1233–9. https://doi.org/10.2337/diacare.24.7.1233 .
Cioffi G, Faggiano P, Lucci D, Maggioni AP, Manicardi V, Travaglini A, et al. Left ventricular dysfunction and outcome at two-year follow-up in patients with type 2 diabetes: The DYDA study. Diabetes Research and Clinical Practice. 2013;101(2):236–42. https://doi.org/10.1016/j.diabres.2013.05.010 .
Baba M, Balogun M, Akintomide A, Talle M, Akinwusi P, Abdul H, et al. Left Ventricular Function in Nigerians With Type 2 Diabetes Mellitus With and Without Hypertension. Nigerian Journal of Clinical Medicine. 2013;4(3), https://doi.org/10.4314/njcm.v4i3.5
Fang ZY, Yuda S, Anderson V, Short L, Case C, Marwick TH. Echocardiographic detection of early diabetic myocardial disease. J Am Coll Cardiol. 2003;41(4):611–7. https://doi.org/10.1016/s0735-1097(02)02869-3 .
Fang ZY, Leano R, Marwick TH. Relationship between longitudinal and radial contractility in subclinical diabetic heart disease. Clin Sci (Lond). 2004;106(1):53–60. https://doi.org/10.1042/cs20030153 .
Andersen NH, Poulsen SH, Eiskjaer H, Poulsen PL, Mogensen CE. Decreased left ventricular longitudinal contraction in normotensive and normoalbuminuric patients with Type II diabetes mellitus: a Doppler tissue tracking and strain rate echocardiography study. Clin Sci (Lond). 2003;105(1):59–66. https://doi.org/10.1042/cs20020303 .
Zoppini G, Bergamini C, Bonapace S, Rossi A, Trombetta M, Mantovani A, et al. Association between subclinical left ventricular systolic dysfunction and glycemic control in asymptomatic type 2 diabetic patients with preserved left ventricular function. J Diabetes Complications. 2017;31(6):1035–40. https://doi.org/10.1016/j.jdiacomp.2017.01.021 . ShirleyRubler,JoelDlugash,YusufZiyaYuceoglu,TarikKumral,ArthurWhitleyBranwood,ArthurGrishman.
William C. Little, MD and Jae K. Oh, MD: Echocardiographic Evaluation of Diastolic Function Can Be Used to Guide Clinical Care:circulationaha . https://doi.org/10.1161/circulationaha.109.869602
Download references
Acknowledgments
Not applicable.
Funding was provided for this study by Addis Ababa University. The funder has no role in the study design, data collection and analysis.
Author information
Authors and affiliations.
Department of Internal Medicine, Division of Cardiology, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Tigist Seleshi, Dufera Mekonnen, Konno Taddese & Senbeta Guteta
Department of Internal Medicine, Division of Endocrinology, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Theodros Alemneh
Department of Internal Medicine, Division of Cardiology, Adama Hospital Medical College, Adama, Ethiopia
Demu Tesfaye
Department of Internal Medicine, Division of Cardiology, School of Medicine, Hawassa University, Hawassa, Ethiopia
Sura Markos
Department of Internal Medicine, Division of Cardiology, Yekatit Hospital Medical College, Addis Ababa, Ethiopia
Yitagesu Getachew
You can also search for this author in PubMed Google Scholar
Contributions
T.S and S.G: developed the proposal, obtained the IRB approval and collected data, data analysis and preparation of the manuscript T.A and D.M: editing the proposal, recruiting the cases included in the study from type II diabetic follow-up clinic D.T, S.M, Y.G and K.T. : Data collection. All authors reviewed the manuscript
Corresponding author
Correspondence to Tigist Seleshi .
Ethics declarations
Ethics approval and consent to participate.
This study is approved by the ethical board of Department of Internal Medicine, College of Heath Sciences, Addis Ababa University. A scanned copy of the approval letter will be presented by the corresponding author upon request. Informed written consent was obtained from each study participant in a form written in Amharic and English.( Ethical approval number 10/22).
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Seleshi, T., Alemneh, T., Mekonnen, D. et al. Assessment of subclinical left ventricular systolic and diastolic dysfunction in patients with type 2 diabetes mellitus under follow-up at Tikur Anbessa specialized hospital, Ethiopia: a case-control study. BMC Cardiovasc Disord 24 , 201 (2024). https://doi.org/10.1186/s12872-024-03850-x
Download citation
Received : 14 November 2023
Accepted : 19 March 2024
Published : 06 April 2024
DOI : https://doi.org/10.1186/s12872-024-03850-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Diastolic dysfunction
- Diabetic adults

BMC Cardiovascular Disorders
ISSN: 1471-2261
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Biomed Eng Online

A case study of type 2 diabetes self-management
1 Department of Biomedical Engineering, Texas A&M University, College Station, Texas, 77843-3120 USA
This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Associated Data
It has been established that careful diabetes self-management is essential in avoiding chronic complications that compromise health. Disciplined diet control and regular exercise are the keys for the type 2 diabetes self-management. An ability to maintain one's blood glucose at a relatively flat level, not fluctuating wildly with meals and hypoglycemic medical intervention, would be the goal for self-management. Hemoglobin A1c (HbA1c or simply A1c) is a measure of a long-term blood plasma glucose average, a reliable index to reflect one's diabetic condition. A simple regimen that could reduce the elevated A1c levels without altering much of type 2 diabetic patients' daily routine denotes a successful self-management strategy.
A relatively simple model that relates the food impact on blood glucose excursions for type 2 diabetes was studied. Meal is treated as a bolus injection of glucose. Medical intervention of hypoglycaemic drug or injection, if any, is lumped with secreted insulin as a damping factor. Lunch was used for test meals. The recovery period of a blood glucose excursion returning to the pre-prandial level, the maximal reach, and the area under the excursion curve were used to characterize one's ability to regulate glucose metabolism. A case study is presented here to illustrate the possibility of devising an individual-based self-management regimen.
Results of the lunch study for a type 2 diabetic subject indicate that the recovery time of the post-prandial blood glucose level can be adjusted to 4 hours, which is comparable to the typical time interval for non-diabetics: 3 to 4 hours. A moderate lifestyle adjustment of light supper coupled with morning swimming of 20 laps in a 25 m pool for 40 minutes enabled the subject to reduce his A1c level from 6.7 to 6.0 in six months and to maintain this level for the subsequent six months.
Conclusions
The preliminary result of this case study is encouraging. An individual life-style adjustment can be structured from the extracted characteristics of the post-prandial blood glucose excursions. Additional studies are certainly required to draw general applicable guidelines for lifestyle adjustments of type 2 diabetic patients.
It is well established that diabetes can lead to acute and chronic complications, compromising the health and quality of life. Results from various studies [ 1 ] have demonstrated that improved control of blood glucose in type 2 diabetes reduces related complications. Type 2 diabetes results from the metabolic problem that is related to certain tissue resistance to insulin action and to the inability of the pancreas to appropriately regulate the quantity of insulin for glucose metabolism. These metabolic abnormalities lead to the many complications of diabetes. Type 2 diabetes historically occurs predominantly in adults aged 40 and over. A recent trend, however, indicates that children and adolescents of minority ethnic groups, especially in African Americans and American Indians, are increasingly susceptible to type 2 diabetes [ 2 ]. With the prevalence of type 2 diabetes and its associated risk for serious complications, issues related to proactive self-management become an urgent concern.
Dietary management is frequently referred as the cornerstone, or the initial step, in treating of type 2 diabetes mellitus. Foods containing carbohydrates play an important role in the diet. The glycemic Index (GI) ranks foods according to their post-prandial glycemic responses. The GI was introduced more than twenty years ago and has been widely adopted in diabetes management in Australia, New Zealand, Canada, the United Kingdoms, and France [ 3 ]. The World Health Organization states that it is important to consider the GI in constructing a healthful diet because low GI foods help control blood sugar levels by producing minimal fluctuations in blood glucose [ 4 ]. For diabetic patients, choosing low GI foods is particularly important because consumption of high GI foods often results in far more exaggerated glycemic responses, creating a need for drug or insulin therapy [ 3 , 5 ].
Most published GI lists are for single food items only. A GI is a numerical measure of how a carbohydrate would increase one's blood glucose level over a period of two (for normal) or three hours (for diabetic patients) after eating [ 6 , 7 ]. The area of elevated blood glucose level from the baseline (the pre-prandial measure) is expressed as a percent of the area for the same amount of a reference carbohydrate such as a pure glucose or a white bread (usually 50 g) [ 8 , 9 ]. To plan a complete meal using the weighted mean [ 6 ] for various food items is not only tedious, but also impractical.
Diet exchange lists are usually recommended for diabetic patients to use in formulating a sensible meal plan. However, an exchange list is not always convenient to use. Moreover, there is a lack of ethnic diet exchange lists. For a member of an ethnic minority to follow a diet exchange list, he or she must prepare his or her own meal away from the rest of the family. Nutall and Chasuk [ 10 ] have stressed that dietary recommendations for type 2 diabetes should be flexible and highly individualized, yet most of the prepared meal programs and exchange-list diets for diabetes have not had individualization in mind nor are they designed for ethnic minorities.
When diet alone cannot effectively control the type 2 diabetic conditions, medical interventions, such as insulin injections or dispensing hypoglycaemic pills, are usually the next step of managing type 2 diabetes mellitus. Medical interventions notoriously exacerbate the fluctuation of blood glucose excursions. Even with the smallest dosage of hypoglycaemic drug (5 mg glucotrol or glyburide) once in the morning, the subject of this study still experienced frequent acute hypoglycaemias. Besides, his A1c levels hovered around 6.5 levels for many years following his physician's advice of taking 5 mg glucotrol per day. It became obvious that a properly designed drug dispensing regimen was needed to avoid hypoglycaemic bouts and effectively reduce A1c levels.
Fasting blood glucose measurements are not consistent indicators, fluctuating widely from a low of 70 mg/dL to a high of 200 mg/dL (with most frequent range lay between 90 to 150 mg/dL) that were experienced by this type 2 diabetic subject prior to the model-based lifestyle adjustment. Initially, the subject tried to adjust lifestyle based on fasting glucose measurements, but it was not successful. His A1c measurements crept from 6.3 to 6.7 in a year. As glucose binds irreversibly to haemoglobin molecules within red blood cells, the amount of glucose that is bound to haemoglobin is directly tied to the concentration of glucose in the blood. The average life span of erythrocytes is about 120 days [ 11 ], measuring the amount of glucose bound to haemoglobin – by the A1c measurement – can provide an estimate of average blood sugar level during the 3 to 4 months period. It is obvious that A1c is a more reliable indicator than fasting glucose measurements for an effective blood glucose control self-management.
It has been established that exercise can effectively alleviate diabetic conditions. Although no rigorous investigation has been performed here, nor is the focus of this current study, a forty-minute exercise of swimming, or weight lifting, or jogging, or any combination of these, prior to a meal or 3 to 4 hours after a meal, can significantly depress the volunteer's post-prandial blood glucose levels. However, it is impractical to substitute hypoglycemic pills with a multiple daily exercise schedule. A sensible lifestyle adjustment is required to manage the diabetic conditions without altering much of daily routines.
Post-prandial blood glucose excursions (time series) for type 2 diabetes vary widely depending on the variety and the amount of food consumed. It also depends on long and short term physical conditions (exercise routines and stress levels such as insomnia) to a lesser scale. The recovery periods of blood glucose excursions returning to the pre-prandial level (or baseline) for diabetics are generally longer than those for non-diabetics. Although a simple glucose-insulin interaction compartmental model exists [ 12 ], not all the model parameters are readily interpretable. In addition, no case study is given to illustrate its potential applications. Compartmental models can provide first-order approximations that may be sufficient for specific goals. Simple models may not duplicate real phenomena but may reveal enough clues for which alternative approaches or experimental designs may come to light.
A biophysically-based model of impulse-force-generated heavily damped oscillatory system is used here to capture the post-prandial blood glucose characteristics of type 2 diabetes. The model follows the general approach of glucose-insulin interaction model (bolus injection of glucose) with a few modifications, for which parameters can readily be interpreted and a case study is presented for exploring its potential applications. Rather than using single food items for their published GI values, or its cumbersome weighted mean of multiple ingredients in a meal, normally consumed lunch for the subject was used for the test meal. Based on the preliminary results obtained from the model, a moderate lifestyle adjustment was devised for the subject: swimming 20 laps for 40 minutes in a 25 m pool in the morning and dispensing 1/4 of 5 mg glyburide 1/2 to 1 hour before lunch and dinner – that enables him to reduce 10% of his A1c level in six months and maintain the desirable lower level for the subsequent six months.
The subject is a mid-sixty healthy male of 180 lbs with 5'10" frame, leading a productive professional life. He has been diagnosed with type 2 diabetes for more than 30 years. Initially, he was on diet regimen for nearly twenty years and then was instructed by his physician to dispense 5 mg glucotrol once every morning. He experienced frequent acute hypoglycemia that led him to discuss a possible self-managed regimen with his family physician.
Lunch was chosen as the test meal for having sufficient time to take post-prandial measurements. The test meals were 15 sets of lunches that consisted either (1) 10 to 12 oz of steamed rice, stir-fried vegetables with 4 oz canned tuna (or steamed cod), or (2) 10 to 12 oz spaghetti with 6 medium sized meat balls (from Sam's family package). Five sets of data each were collected from: (i) without taking hypoglycemic pills before test meals; (ii) 1/4 size of 5 mg glyburide pills were dispensed pre-prandially right before the meal and (iii) 1/4 size of 5 mg glyburide pills were dispensed pre-prandially an hour before the test meals. One pre- and 8 to 12 post-prandial blood glucose measurements were taken at 30-minute intervals starting at the beginning of a meal (meal is usually consumed in 15 minutes): (i) for 6 hours, (ii) for 5 hours, and (iii) for 4 hours. In addition, for case (iii) two reference measurements were taken with one right before dispensing the pill and one an hour after completion of the 8 post prandial measurements, i.e ., at hour 5, for a total of 11 readings.
The purpose of the first set of measurements was to establish the baseline for this diabetic subject: the recovery period of post-prandial blood glucose excursion without medication. The second and the third sets of the trials were designed to quantitatively measure the hypoglycemic drug effects and the most optimal time frame to administer the pills. Raw data were averaged and the corresponding standard deviations were also calculated for 5 replicates at given times. The averaged data were then used for modeling analysis.
Model formulation
The post-prandial blood glucose excursion can be considered as a hormone regulated resilient system. The food intake is treated as a bolus injection of glucose, and thus the impulse force f ( t ); effects of exercises and hypoglycemic medication are lumped as the damping factor, β . The differential equation of such an oscillatory system, that is used to describe post-prandial blood glucose excursions, can be found in many physics texts:
where x represents blood glucose level over the baseline at time t , ω 0 is the system natural frequency [ 12 ]. The pre-prandial blood glucose levels are generally fluctuating with relatively insignificant magnitudes thus can be approximated as a flat level. If the impulse force f ( t ) takes the form of the Dirac delta function, F δ ( t -0) with F being a food intake dependent parameter, the solution of Eq. (1) is
Parametric estimation
For a given blood glucose excursion, data was taken every 30 minute interval from the time a meal was initially consumed, from which the excursion peak ( MR ), x max , and the corresponding time τ to reach MR can both be estimated. Setting dx / dt = 0 in Eq. (2), the time τ can be expressed as:
Substituting Eq. (3) into Eq.(2), we have
The area under an excursion curve, AUC , can also be obtained:
where T = 2 π / ω is the period of oscillation. The reason for setting the upper integral limit to T /2 is because the damping factor β effectively depresses the glucose excursion levels x near zero for t > T /2, i.e ., it ripples about pre-prandial level. The time T /2 is therefore defined as the recovery period ( RP ). For type 2 diabetic patients who are not in a properly structured regimen, the recovery periods are often longer than 5 hours, by which time the next meal arrives and induces another blood glucose upswing.
Equations (3) – (5) can be used to estimate the three parameters, F , ω and β , from the measurable quantities of τ , x max , and AUC . The procedure is briefly described below:
1. Assign T as twice the roughly estimated recovery period in hours, which can be obtained from the raw data and thus ω = 2 π / T .
4. Fine tune these three parameters by using MATLAB function fminsearch to minimize [ AUC data - AUC ( F , β , ω )] 2 , where AUC data is calculated from the averaged data points by the trapezoidal rule and AUC ( F , β , ω ) is calculated from Eq. (5).
5. These three parameters can further be fine-tuned by fminsearch (sum of squared errors between the averaged data points and the model predicted values).
Two MATLAB user defined functions: GlucoseModel (for No pill and Pill at meal) and GlucoseModel1 (for Pill one hour prior) to estimate these model parameters and calculating the relevant diabetic characteristic measures: τ , x max , AUC are listed in the Additional files 1 and 2 , respectively.
Table Table1 1 lists the fine-tuned values of model parameters: F , ω , β , and those characteristic parameters: RP , τ , x max , and AUC , the latter three are calculated from Eqs. (3) to (5). Also included in Table Table1 1 are the fitting statistics R 2 values that indicate how well model curves fit the data.
Model and characteristic parameters for the post-prandial blood glucose excursion
The parametric value of F is the result of food impact, or the rate of glucose being absorbed into the blood stream. The interpretation of F is rather difficult as the liver acts as a storage compartment for glucose [ 12 ]. Liver regulates blood plasma glucose levels; if it is too high, the excess will be stored in the liver, and the reverse process will take place if the plasma glucose is too low. Although all three model parameters: F , ω , and β are more or less influenced by the liver function, the impact on F deems more pronounced as it has a direct impact on the glucose levels in the blood stream. As the function of the liver is not included in the current model, the estimated F values can only be loosely inferred as a function of insulin level, F increases as hypoglycemic drug depresses the blood glucose levels that in turn increases the absorption rate of glucose into the blood stream as in the case of 1/4 pill taken right before the meal. When the drug is taken an hour before the meal, the liver may have sufficient time to regulate blood glucose levels that additional glucose absorption becomes less intensive.
Ratio of characteristic parameters for the post-prandial blood glucose excursion
No pill trial
Parametric values for no-pill trial reveal that glucose absorption rate is generally slower (low F value) in comparison with the other two cases. The exceedingly long RP of nearly 7 hours is undesirable: as it implies that the next meal time arrives before the blood glucose level could return to the baseline, i.e ., an elevated blood glucose level would be sustained for a prolonged period of time. The high RP and AUC are unmistakably the characteristics for type 2 diabetes. Figure Figure1 1 compares the model and the data with the corresponding standard deviation bars. Model curves are extended for an additional hour beyond the last data point (and in all the figures herewith) to denote the trend of blood glucose excursion.

Post-prandial glucose excursion: no pill trial
1/4 of 5 mg glyburide taken right before the meal
The blood glucose characteristics are significantly improved with a 1/4 size of 5 mg glyburide taken right before lunch. Increased ω and β values translate to significantly lower RP and AUC with virtually unchanged x max . Although the mean RP is less than 5 hours, it is still a bit too long in comparison with the non-diabetics [ 12 ] (~ 4 hours). A higher F value than the one for no-pill trial may partly due to the liver intervention. Figure Figure2 2 compares the model and the data. From the figure one can tell that hypoglycemic drug has an effective delayed effect of about two hours as the rising portion of the model is almost identical to the one for no-pill trial with both x max are about 60, which may be the result of liver function that with initial stimulation of hypoglycemic drug, liver may also release glucose. As the hypoglycemic drug effect persists, the liver ceases to interfere.

Post-prandial glucose excursion: 1/4 pill right before the meal
1/4 of 5 mg glyburide taken an hour before the meal
From the personal experience of the participating subject, the hypoglycemia usually occurs 3 to 4 hours after taking the pill. The trial described in the previous section also reveals that no significant hypoglycemic drug effect is detected in the initial two hours. In order to learn the drug impact on an empty stomach, an additional glucose measurement was made prior to taking the hypoglycemic pill at -1 hour. Another measurement was also taken an hour after the blood glucose excursion returned to the baseline ( i.e ., at hour 5). This is meant to check if the blood glucose would remain near the baseline level. The drop of blood glucose levels between -1 and 0 hours are roughly 10 mg/dL, which can be contributed to the mild liver intervention. No net hypoglycemic drug effect is taking place before the meal as evidenced from the initial rise of the blood excursion curve as shown in Fig. Fig.3 3 (in comparison with Fig. Fig.2), 2 ), where only data between hour 0 and hour 4 were used to generate the model curve. Indeed, all parametric values are improved significantly: both PR and x max are decreased by 20% and their combination that reflected in AUC dropped nearly 35% in comparison to those for pill taken at meal trial as shown in Table Table1. 1 . The food impact parameter F decreased a little from the one for pill at meal trial, which may indicate an hour after dispensing the pill, a quasi-equilibrium state has been reached among the liver function, hypoglycemic drug effects, and the bolus injection of glucose. The system frequency ω increased for more than 25%, which gives a shorter RP that compares favorably with non-diabetics. The drop of damping factor β may be the result of low F , as both τ and x max are already significantly reduced that further strengthening of β becomes unnecessary. The hour 5 measurements confirm that although the model curve shows a decreasing trend, upon returning to the base level the blood glucose excursions practically stabilizes. In addition, the volunteer patient did not experience any hypoglycemia even two to three hours after the final post-prandial measurement.

Post-prandial glucose excursion: 1/4 pill an hour before the meal
This simple impulse-forced model provides a means to shape a self-management regimen for the type 2 diabetic subject: a moderate meal coupled with minimal amount of medical intervention has effectively modulated the blood glucose excursion by reducing its recovery periods and fluctuation amplitudes. Based on the model, the type 2 diabetic subject was able to adjust a lifestyle that include (a) 40 minute swimming in a 25 m pool in the morning, (b) a fruit of mid-size apple or its equivalent and a cup of coffee with cream for breakfast without taking hypoglycaemic pill, (c) moderate lunch with 1/4 size of 5 mg glyburide taken 1/2 to 1 hour before the meal, (d) moderate early dinner, 4 hours prior to bed time, with 1/4 size of 5 mg glyburide taken 1/2 to 1 hour before the meal, (e) snack a mid-size banana, or a small bag (3.5 oz) of peanuts, or 6 crackers when needed in between meals. With this regimen, he was able to reduce his A1c level from 6.7 to 6.0 in 6 months and maintained at this level for the subsequent 6 months. Moreover, he has not had any hypoglycaemic bouts ever since he particitipated in this study more than two years ago.
Elevated blood glucose excursions during the night would boost the A1c levels. To keep a low average fluctuation of blood glucose excursion amplitudes, the evening meal is crucial. In order to avoid hypoglycaemia during the sleep, an early dinner is advised. The subject has been able to keep post-prandial blood glucose levels within 200 mg/dL with the mean fasting reading of 90 ± 20 mg/dL. Occasionally he consumes a can of beer or sugar free deserts. Although no rigorous study has been performed, a forty-minute exercise of swimming, or weight lifting, or jogging, or any combination of these is roughly equivalent to the effect of 1/4 size of 5 mg glyburide. Nonetheless, it is impractical to exercise more than once a day, thus the subject takes 2.5 mg of hypoglycemic pill a day instead. His physician originally prescribed him to take one 5 mg hypoglycemic pill daily. That was more that 10 years ago. The regimen did not work very well as he experienced hypoglycaemic bouts often. This model-based regimen not only reduced A1c level but entirely eliminated hypoglycaemic symptoms. In addition, one fasting blood glucose measurement in the morning is sufficient for him to maintain a healthy daily routine of exercise, consuming meals/snacks and leading a productive life with mental and physical activities.
Lifestyle adjustments are the best regimens for many chronicle ailments such as diabetes, hypertension, high cholesterol levels, etc . Although this model-based self-management regimen for the type 2 diabetic subject is only a case study, it certainly provides a general guideline for an applicable life-style adjustment. Currently not all the model parameters are entirely clear, additional data are required to draw a meaningful general conclusion. A pilot project of testing this regimen on six type 2 diabetic patients in a regional nursing home is proposed for the next phase of study.
Although derived characteristic parameters: RP and AUC (to a lesser degree, τ and x max ), carry clear meaning that can be used to characterize type 2 diabetic subjects from non-diabetics, the implications of model parameters, F , ω and β are not as translucent. With additional data, one may be able to draw plausible conclusions about (a) how F is influenced by food intakes, drug (delaying) effects, and liver (regulatory) functions; and (b) how ω and β behave, whether they are independent of F and of each other, or all three somewhat mutually dependent. Better understanding of these parameters would definitely enhance the self-management for type 2 diabetes.
This model-based lifestyle adjustment has another advantage: it can be used to manage each individual needs. Nutall and Chasuk [ 10 ] have stressed that dietary recommendation for type 2 diabetes should be flexible and highly individualized; most of prepared meal programs and exchange-list diets for diabetes have not had individualization in mind nor are they designed for ethnic minorities. Once we have a comprehensive understanding of these parameters, it is possible to tailor individual lifestyle adjustment accordingly.
For those individuals who are interested in self-managing the type 2 diabetes, the general advice is: avoiding big meals, may snack moderately between meals, eat an early dinner – about 4 hours before bedtime, and exercise regularly. If one is interested in "normal" meal effects on one's post-prandial blood glucose excursion, taking a pre-prandial blood glucose measurement prior to a typical lunch and 8 to 10 post-prandial measurements at half-hour intervals for 5 or more replicates and follow the procedure described here to obtain these characteristic parameters RP , τ , x max , and AUC . Applying a small dosage of medical intervention prior to a meal can keep the blood glucose at a relatively flat level and depress the overnight blood glucose excursion; however, this practice needs the approval from one's family physician and is not recommended here.
Authors' contributions
Sole authorship: data collection/analysis, model building, parameter estimation/interpretation, and the design of life-style adjustment regimen for the participating subject.
Supplementary Material
MATLAB user defined function: GlucoseModel (for No pill and Pill at meal) to estimate model parameters: F , β , ω and to calculate the relevant diabetic characteristic measures: τ , x max , AUC .
MATLAB user defined function: GlucoseModel1 (for Pill one-hour prior) to estimate model parameters: F , β , ω and to calculate the relevant diabetic characteristic measures: τ , x max , AUC .
Acknowledgements
The author wishes to express his appreciation to Ms. Katherine Jakubik for her editing efforts, to Professor Jame B. Bassingthwaighte and two other anonymous reviewers for their critical comments to an earlier version of this manuscript.
- Ratner RE. Type 2 diabetes mellitus: the grand overview. Diabet Med. 1998; 15 :S4–7. doi: 10.1002/(SICI)1096-9136(1998120)15:4+<S4::AID-DIA735>3.3.CO;2-T. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jiwa F. Diabetes in the 1990s – an overview. Stat Bull Metrop Co. 1997; 78 :2–8. [ PubMed ] [ Google Scholar ]
- Brand-Miller J. The Glucose Revolution. Marlowe & Company; 1999. [ Google Scholar ]
- Linder L. What's your number, sweetie? The Washington Post, May 1, HE08. 2001.
- Franz M. In defence of the American Diabetes Association's recommendations on the glycemic index. Nutrition Today. 1999; 34 :78–81. [ Google Scholar ]
- Wolever TMS, Jenkins D, Jenkins AL, Josse RG. The glycemic index: methodology and clinical implication. American Journal of Clinical Nutrition. 1991; 54 :846–854. [ PubMed ] [ Google Scholar ]
- Brand-Miller J. Diets with a low glycemic index: From theory to practice. Nutrition Today. 1999; 34 :64–72. [ Google Scholar ]
- Gannon MC, Nuttall FQ. Factors Affecting Interpretation of Postprandial Glucose and Insulin Areas. Diabetes Care. 1987; 10 :759–763. [ PubMed ] [ Google Scholar ]
- Truswell AS. Glycemic index of foods. Eur J Clin Nutr. 1992; 46 :S91–S101. [ PubMed ] [ Google Scholar ]
- Nuttall FQ, Chasuk RM. Nutrition and the management of type 2 diabetes. Journal of Family Practice. 1998; 47 :S45–53. [ PubMed ] [ Google Scholar ]
- Fournier RL. Basic Transport Phenomena in Biomedical Engineering. Taylor & Francis; 1998. [ Google Scholar ]
- Fisher RJ. Compartmental analysis. In: Enderle J, Blanchard S, Bronzino J, editor. Introduction to Biomedical Engineering. London: Academic Press; 2000. pp. 369–410. [ Google Scholar ]
Ohio State nav bar
The Ohio State University
- BuckeyeLink
- Find People
- Search Ohio State
Patient Education
Patient education (video script).

Welcome to this brief educational video about Type 2 Diabetes, a condition in which blood sugar levels run consistently higher than normal. In order to understand what is not working in Type 2 Diabetes, it is important for us to understand what “normal” is.

When we eat or drink, our body takes that food and drink and breaks it down into nutrients that are absorbed into our bloodstream. This includes sugar, and that is how we end up with elevated blood sugar levels. This is normal right after we eat or drink.
Our body then produces something called insulin. Insulin’s job is take that sugar out of the bloodstream and help cells use it for energy. As insulin does its job and moves the sugar from the bloodstream into the cell, blood sugar levels naturally go down. If our body needs more sugar, during times of intense exercise or when we haven’t eaten for a long time, the liver has stored sugars that it is able to release into the bloodstream. These factors together all help keep our blood sugar level basically even over time.

In Type 2 Diabetes, blood sugars can run higher than normal pretty consistently, and for a number of reasons. One is that cells can start having trouble using the insulin that is there and available. This is called insulin resistance. A second reason is because the body becomes unable to keep with the demand for insulin. It cannot produce enough, and it becomes fatigued in the effort – producing less and less insulin. And the third reason is that the liver can release extra sugar into our system that we don’t even really need.

So why does this matter? Well, the kidneys work extra hard trying to help clear the extra sugar out of our bloodstream, by releasing it into our urine. This can lead to people peeing more frequently, and because they are peeing more frequently, they’ll feel more thirsty in order to prevent dehydration. The kidneys work overtime, and as they work overtime over the period of years, it can lead to eventual kidney disease or even kidney failure.

It is possible to have Type 2 Diabetes without these symptoms, however. Other symptoms include fatigue, vision changes, frequent infections, itching, numbness or tingling, weakness, and wounds that do not heal. It is important to share with your care provider any symptoms you are experiencing or any changes to your symptoms, so they can help you create the best plan possible for your treatment. Thank you!
- Case Report
- Open access
- Published: 02 April 2024
A case report: co-occurrence of probable Vogt-Koyanagi-Harada disease and diabetic retinopathy
- Huan Li 1 ,
- Zhiyong Li 1 ,
- Ailing Mao 1 ,
- Ping Dong 1 &
- Wei Wang 1
BMC Ophthalmology volume 24 , Article number: 148 ( 2024 ) Cite this article
78 Accesses
Metrics details
Bilateral retinal detachment and choroidal detachment in a patient are rare occurrences. The presence of bilateral diabetic retinopathy (DR) in such a case is even rarer and complicates the condition.
Case presentation
In this study, we document a case of unconventional VKH. Manifestations in this patient included intense peripheral retinal detachment and choroidal detachment, along with vitreous opacities akin to cotton wool spots, concurrent with DR. The diagnosis was considered as probable VKH with DR. Treatment according to VKH protocols, including high-dose corticosteroids, yielded positive results.
Conclusions
VKH can co-occurrence with DR. VKH manifestations vary, and early, aggressive, and long-term treatment is essential. The complexity of treatment increases with concurrent DR, necessitating the use of immunosuppressants.
Peer Review reports
Bilateral retinal and choroidal detachments in a patient are uncommon. Conditions such as Vogt-Koyanagi-Harada disease (VKH) and sympathetic ophthalmia are common causes. The coexistence of bilateral diabetic retinopathy (DR) is exceedingly rare and adds complexity to the condition.
VKH typically presents with fever and headache in its early stages. Certain patients display symptoms of meningeal irritation including nausea, vomiting, and neck stiffness. These symptoms often escalate to loss of vision in both eyes, widespread chorioretinitis accompanied by serous detachment of the retina, followed by depigmentation of the choroid in advanced stages. During this phase, Fluorescence Fundus Angiography (FFA) reveals numerous initial hyperfluorescent spots, progressing to extensive ‘lake-like’ fluorescein pooling in later stages. Nonetheless, many patients display atypical clinical presentations in real-world scenarios.
A 44-year-old Chinese man presented with a 45-day history of diplopia. Before the onset of his condition, he reported no cold, nausea, dizziness, tinnitus, vomiting, headache, hearing impairment, or hair graying. Local hospital assessments previously identified panuveitis, choroidal detachment, DR, and bilateral macular edema. Macular OCT indicated both eyes had macular neuroepithelium thickening and elevation, along with intercystic low reflex and localized detachment of the neuroepithelium. Despite initiating treatment with corticosteroid eye drops and posterior subtenon injections of corticosteroids, his condition deteriorated progressively. The patient reported no prior trauma or eye surgery. He had managed type 2 diabetes for 15 years and received bilateral total retinal photocoagulation for DR two years earlier. Physical exam revealed BCVA: manual in both eyes, with intraocular pressure at 17 mmHg and 18 mmHg. Exam findings were bilateral conjunctival congestion, atrial flash (+), localized posterior iris synechiae, pupillary margin neovascularization, clear lens, grade II vitreous opacity, and white pompon-like opacities. Right eye fundus examination showed a disc-surrounding neovascular membrane, 360° peripheral retinal and choroidal bulges, dispersed old photocoagulation marks, neovascularization, and superior and inferonasal retinal hemorrhages, extending to the posterior pole. In both eyes, examination revealed the presence of circumferential peripheral retinal and choroidal protrusions. These were accompanied by scattered, pre-existing photocoagulation marks. UBM detected a detachment of the ciliary body in each eye. Utilizing B-mode ultrasonography, vitreous opacity, and detachments in the choroid, and retina were noted for both eyes. The axial length of the right eye is 22.68 mm, and that of the left eye is 22.50 mm.Furthermore, OCT identified a significant bulge in the peripheral retina and a lack of clarity in the macular area. FFA revealed twisted, enlarged retinal veins, pronounced dotted fluorescence, and hemorrhagic fluorescence shading in the retina, with patchy nonperfusion areas (refer to Fig. 1 ). Orbital MRI and liver and kidney function tests were normal. Syphilis, HIV, and T-SPOT tests were negative. Intraocular fluid analysis ruled out microbial infection, with VEGF at 1614.3 pg/mL, BFGF at 478.3 pg/mL, IL-6 at 566 pg/mL, VCAM at 21845.2 pg/mL, and IL-8 at 135.7 pg/mL. HLA-DRB1 results indicated DRB1*04
Eye condition of the patient on admission. a : B ultrasound: vitreous opacity, choroidal detachment and retinal detachment in both eyes. b : Glass white pompon-like cloudy. c : Fundus photography of the right eye. d : Fundus photography of the right eye. e : posterior pole FFA. f : peripheral imeter FFA
He was therefore diagnosed with probable VKH. He was administered intravenous methylprednisolone at 0.5 g per day, gradually transitioning to oral corticosteroid therapy. Subsequently, the patient’s subretinal fluid resolved, and normal choroidal and retinal architecture was restored. Upon discharge, the BCVA was 20/250 in the right eye and 20/500 in the left eye (refer to Fig. 2 ). Post-discharge, despite several adjustments, the patient’s blood glucose levels remained poorly controlled. Consequently, oral cyclosporine at 100 mg per day was added to his regimen. However, the patient experienced general weakness and dizziness, leading to the discontinuation of cyclosporine after 12 days. At the follow-up examination, there was an improvement in retinal and choroidal detachments, with a BCVA of 20/200 in both eyes. Nonetheless, fundus hemorrhage in the right eye had worsened (refer to Fig. 3 ). The patient was then switched to cyclosporine from different manufacturers.
Eye condition of the patient at discharge. a : fundus photography of both eyes. b : macular OCT of the right eye. c : macular OCT of the left eye
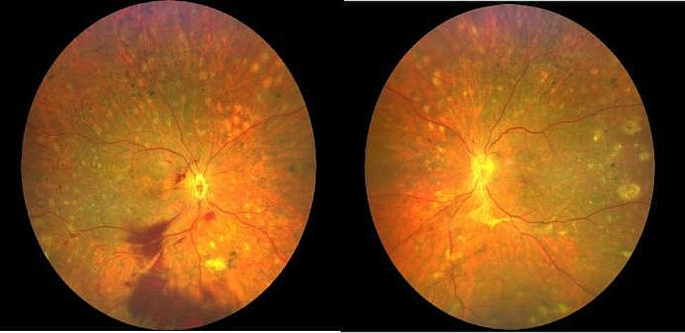
Fundus photography of both eyes at reexamination
Discussion and conclusion
VKH frequently presents with exudative retinal detachment. Other etiologies encompass sympathetic ophthalmia, serpiginous choroiditis, uveitis due to syphilis, sarcoidosis, posterior scleritis, tuberculosis, uveal effusion syndrome, and intraocular lymphoma. Nearly half (47.4%) of VKH cases develop bilateral exudative retinal detachment. Conversely, diagnosed non-VKH patients tend to have a greater incidence of unilateral exudative retinal detachment compared to those with VKH [ 1 – 2 ].
VKH, an autoimmune condition, is marked by bilateral granulomatous uveitis, frequently accompanied by meningeal symptoms, auditory issues, and abnormalities in skin and hair. Most commonly, it affects individuals aged 20 to 50 without major gender differences [ 3 ]. Moorthy’s 1995 classification of VKH outlined four stages: prodromal, acute uveitis, chronic/recovery, and chronic relapse. The disease presents variedly, with patients not necessarily undergoing all four stages. Early, precise diagnosis and treatment are crucial to avert progression to anterior uveal involvement and repeated attacks of granulomatous anterior uveitis. Internationally recognized, the revised VKH diagnostic criteria from the 2001 American Journal of Ophthalmology [ 4 ] divide the disease into three types: complete, incomplete, and probable.
Early VKH diagnosis is contingent on specific criteria: (1) No prior history of penetrating eye trauma or surgery before uveitis development. (2) Absence of signs or laboratory findings pointing to alternative eye disorders. (3) Involvement of both eyes. (4) Either observed diffuse choroiditis and exudative retinal detachment or, lacking overt symptoms, diagnostic imaging such as OCT or ultrasound indicating choroidal thickening, retinal exudative detachment, early hyperfluorescent leakages on FFA, and delayed subretinal fluorescein pooling [ 5 ].
The patient had a bilateral presentation of the condition, with no prior ocular trauma or surgery, and lacked signs of infectious uveitis, systemic rheumatic illness, or any other eye diseases. This case was atypical for VKH due to missing prodromal signs and the unusual occurrence of vitreous opacities similar to cotton wool spots, yet severe exudative retinal detachment was evident initially. The neuroepithelial detachment in the macula observed early on did not suggest macular edema. Peripheral choroidal detachment is a common early finding in VKH [ 6 ]. The widespread periphery of the exudative retinal detachment likely stemmed from prior bilateral retinal photocoagulation, which hindered subretinal fluid build-up in the posterior segment. The extensive detachment limited the efficacy of OCT and FFA diagnostic procedures. Inadequate corticosteroid administration at the initial treatment stage in the local hospital led to uncontrolled inflammation. The patient’s HLA-DRB1 test identified the DRB1*04 allele, a potential genetic indicator for VKH, particularly in the Han Chinese demographic [ 7 – 8 ].
Consequently, we diagnosed the patient with probable VKH disease. Hormonal shock therapy yielded positive results, stabilizing the retinal and choroidal detachments and improving visual acuity. However, the patient’s diabetic retinopathy continued to progress, with increased vitreous blood in the right eye. Some researchers advocate combining immunosuppressants and antimetabolites, using cyclosporine and biologics as first-line treatments for VKH to minimize complications and recurrences.
The concurrent occurrence of VKH and diabetic retinopathy, while uncommon, is a possibility [ 9 ]. Atypical VKH may present with vitreous opacities that resemble cotton wool spots, along with significant retinal and choroidal protrusion and peripheral retinal detachment. In instances of concurrent diabetic retinopathy, it is crucial to rigorously monitor blood glucose levels, considering the potential aggravation of the condition due to inflammation [ 10 ]. Consideration should be given to additional retinal photocoagulation, along with immunosuppressive therapy and, where needed, intravitreal injections of anti-VEGF agents or corticosteroids.
Data availability
All the data were included in the manuscript.
Kinast RM, Solomon SD, Cubillan LD, Hovakimyan A, Acharya N, Cunningham ET. Prevalence and causes of clinically detectable uveitic serous retinal detachment. European Journal of Ophthalmology. 2021;31(6):3093–3098. https://doi.org/10.1177/1120672121991391 .
Article Google Scholar
Deepika S, Craig N,,Grace CL, et al. The prevalence, incidence and risk factors for exudative retinal detachment in Uveitis. Volume 54. Investigative Ophthalmology & Visual Science; 2013. p. 15.
Du L, Kijlstra A, Yang P. Vogt Koyanagi Harada disease: novel insights into pathophysiology, diagnosis and treatment[J]. Prog Retin Eye Res. 2016;52:84–111.
Article CAS PubMed Google Scholar
Read RW, Holland GN, Rao NA, et al. Revised diagnostic criteria for VogtKoyanagiHarada disease: report of an international committee on menclature[J]. Am J Ophthalmol. 2001;131(5):647–52.
Mendes ML, Mayumi VS,,Celso M, et al. Vogt-Koyanagi-Harada disease: review of a rare autoimmune disease targeting antigens of melanocytes [J]. Orphanet J Rare Dis. 2016;11(1):29.
Jia XSJA,,Xuejing C. Unilateral annular choroidal effusions as a rare presentation of Harada’s Disease [J]. RETINAL Cases & Brief Reports, 2022, 26(2).
Masaki T, Akira M,,Jutaro N et al. HLA-DRB1*04:05 is involved in the development of Vogt–koyanagi–Harada disease-like immune-related adverse events in patients receiving immune checkpoint inhibitors[J]. Scientific Reports,2023,13(1)13580.
Anukul N, Pathanapitoon K, Leetrakool et al. HLA-DRB1*04:05 and HLA-DQB1*04:01: alleles potentially Associated with Vogt-Koyanagi-Harada in Northern Thai Patients[J].Ocular immunology and inflammation, 2021, 29(2):260–3.
Ojaimi E, Levy J, Stawell R et al. Vogt-Koyanagi-Harada disease, diabetes mellitus, and psoriasis in a child [J].Ocular immunology and inflammation, 2012, 20(1):56–8.
Abhishek S, Pallavi G,,Singh PT et al. Diabetes, Diabetic Retinopathy, and Inflammatory disorders. [J]. Ocul Immunol Inflamm, 2023, 11–4.
Download references
Acknowledgements
We thank all the patients who participated in this study.
This work was supported by Xingtai Key Research and Development Plan Projects (Project number: 2022ZC232) and S&T Program of Hebei, (Project number: 20577706D).
Author information
Authors and affiliations.
Hebei Provincial Key Laboratory of Ophthalmology, Hebei Provincial Clinical Research Center for Eye Diseases, Hebei Eye Hospital, No.399, Quanbei East Street, 54000, Xingtai, Hebei Province, China
Huan Li, Zhiyong Li, Ailing Mao, Ping Dong & Wei Wang
You can also search for this author in PubMed Google Scholar
Contributions
Huan Li and Wei Wang wrote the main manuscript text.Zhiyong Li, Ailing Mao, and Ping Dong participated in the diagnosis, treatment and review of the patient.All authors reviewed the manuscript.
Corresponding author
Correspondence to Wei Wang .
Ethics declarations
Ethics approval.
This study adhered to the tenets of the Declaration of Helsinki and approved by the Medical Ethics Committee of Hebei Eye Hospital.
Consent for publication
The patient provided written informed consent for the publication of this case. The consent form is available for review by the Editor of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Li, H., Li, Z., Mao, A. et al. A case report: co-occurrence of probable Vogt-Koyanagi-Harada disease and diabetic retinopathy. BMC Ophthalmol 24 , 148 (2024). https://doi.org/10.1186/s12886-024-03410-z
Download citation
Received : 14 January 2024
Accepted : 24 March 2024
Published : 02 April 2024
DOI : https://doi.org/10.1186/s12886-024-03410-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Probable Vogt-Koyanagi-Harada disease
- Diabetes retinopathy
- Exudative retinal detachment
- Choroidal detachment
BMC Ophthalmology
ISSN: 1471-2415
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
The following case study illustrates the clinical role of advanced practice nurses in the management of a patient with type 2 diabetes. Case Presentation A.B. is a retired 69-year-old man with a 5-year history of type 2 diabetes.
PRESENTATION OF CASE. Dr. Max C. Petersen (Medicine): A 34-year-old woman was evaluated in the diabetes clinic of this hospital for hyperglycemia. Eleven years before this presentation, the blood glucose level was 126 mg per deciliter (7.0 mmol per liter) on routine laboratory evaluation, which was performed as part of an annual well visit.
CASE. A 47-year-old woman was found to have hyperglycemia at a health fair when a random blood glucose level was 227 mg/dL (12.6 mmol/L). Several days later, a fasting blood glucose value was 147 mg/dL (8.2 mmol/L). She has no previous history of diabetes, is alarmed by the possibility of having this disorder, and seeks your advice.
Diabetes mellitus currently affects 6.4% or 285 million adults worldwide, and that number is expected to increase to 7.7% or 439 million by 2030. 1 In the United States, the prevalence of diabetes in adults increased from 11.3% in 2010 to 12.3% in 2012. 2 The current type 2 diabetes mellitus (T2DM) epidemic is closely associated with a parallel obesity epidemic, with more than 85% of patients ...
A 59-year-old woman with type 1 diabetes and a 2-year history of cognitive decline presented with obtundation. There was diffuse, symmetric hypointensity in the brain on T2-weighted images and abno...
Case Study: New Onset Type 2 Diabetes Care in a Complex Patient From Inpatient to Self-Management. Elizabeth Buckley, PharmD, CDE, ... Krall J, Donihi A, Hatam M, et al. Nurse education and transition (Neat) model: educating the hospitalized patient with diabetes. Clin Diabetes endocrinol. 2016;2(1). Crossref. PubMed. Google Scholar. 3.
Patient Presentation and History. Chief Complaint: the patient's wife is bringing the patient in after a fall at their home. Presentation: J.S. a 50-year-old African American male who presents with his wife after he fell at home. After the fall, he told his wife "I will be fine, I think my vision just needs checked.".
Glazier et al. conducted a systematic review of 17 studies examining the effectiveness of patient, provider, and health system interventions among patients with type 1 or type 2 diabetes in socially disadvantages populations, defined as those of low socioeconomic status or belonging to an ethnic/racial minority group . Eight of 13 studies ...
Case Study: A Patient With Uncontrolled Type 2 Diabetes and Complex Comorbidities Whose Diabetes Care Is Managed by an Advanced Practice Nurse ... A program was developed for managing diabetic patients within an HMO system that uses physician-supervised diabetes nurse specialists and a computer system to enhance compliance and management of ...
Approximately between 15 and 25% of diabetic patients will present with foot ulcers throughout their life, this being the main cause of non-traumatic amputation worldwide. The overall prevalence rate of this complication is between 1.3 and 4.8%. These are developed by the convergence of several predisposing, triggering and aggravating factors.
Presentation of Case. Dr. Max C. Petersen (Medicine): A 34-year-old woman was evaluated in the diabetes clinic of this hospital for hyperglycemia. Eleven years before this presentation, the blood ...
The three mini-case studies presented with this issue of the journal take you through what to consider in making an accurate diagnosis of type 2 diabetes. The format uses typical clinical scenarios as tools for learning. Information is provided in short sections, with most ending in a question to answer before moving on to the next section.
Just as mentioned in this patient, he is a newly diagnosed diabetes patient who is overweight and he was given metformin 500 mg twice daily. In the study, 29 trials with 5259 participants were included in the analysis, comparing metformin (2007 participants) with sulphonylureas (1167), placebo (702), diet (493), thiazolidinediones (132 ...
[email protected] m. Abstract. Diabetes mellitus, is a grou p of metabolic disorders that leads to high blood glucose level, resul ting in excessive urination, increased thirst, blurred vi ...
Putting Theory Into Practice: A Case Study of Diabetes-Related Behavioral Change Interventions on Chicago's South Side. Monica E. Peek, MD, MPH, 1 Molly J. Ferguson, ... We encouraged the sharing of success stories among diabetes patients within the class to help promote the idea, through personal testimonials, that diabetes is a condition over ...
UK Prospective Diabetes Study (UKPDS) Group (1998) Effect of intensive blood-glucose with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 352: 854-65 Wallentin L et al; PLATO Investigators (2009) Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 361: 1045-57
This case study represents the challenges and issues, both physical and psychological, faced by a young person with type 1 diabetes and the support given by her diabetes multidisciplinary team (MDT). Implications for practice are addressed using current evidence-based research. The names of the child and family have been anonymised to protect ...
Each 1% increase in hemoglobin A1C (HbA1C) was linked to an 8% increase in the risk of heart failure. These findings were also confirmed in a single, small-scale study of type 1 diabetic patients with diabetic neuropathy [7, 8]. Small-scale case-control studies from the U.K., Turkey and Australia identified that while systolic function was ...
The preliminary result of this case study is encouraging. An individual life-style adjustment can be structured from the extracted characteristics of the post-prandial blood glucose excursions. Additional studies are certainly required to draw general applicable guidelines for lifestyle adjustments of type 2 diabetic patients.
Patient Education (Video Script) Welcome to this brief educational video about Type 2 Diabetes, a condition in which blood sugar levels run consistently higher than normal. In order to understand what is not working in Type 2 Diabetes, it is important for us to understand what "normal" is. When we eat or drink, our body takes that food and ...
Background Bilateral retinal detachment and choroidal detachment in a patient are rare occurrences. The presence of bilateral diabetic retinopathy (DR) in such a case is even rarer and complicates the condition. Case presentation In this study, we document a case of unconventional VKH. Manifestations in this patient included intense peripheral retinal detachment and choroidal detachment, along ...