Revelations on photosystem II, thermoluminescence, and artificial photosynthesis: a retrospective of Govindjee from fundamentals to applications
- Review Article
- Published: 01 November 2023
- Volume 29 , pages 1225–1238, ( 2023 )

Cite this article
- Mathew Veena 1 ,
- P. P. Sameena 2 ,
- Nair G. Sarath 3 ,
- Louis Noble 1 ,
- K. P. Raj Aswathi 1 ,
- M. S. Amritha 1 ,
- Riya Johnson 1 ,
- Joy M. Joel 1 ,
- K. S. Anjitha 1 ,
- Harvey J. M. Hou 4 &
- Jos T. Puthur ORCID: orcid.org/0000-0001-5075-3172 1
327 Accesses
Explore all metrics
Photosynthesis, as one of the most important chemical reactions, has powered our planet for over four billion years on a massive scale. This review summarizes and highlights the major contributions of Govindjee from fundamentals to applications in photosynthesis. His research included primary photochemistry measurements, in the picosecond time scale, in both Photosystem I and II and electron transport leading to NADP reduction, using two light reactions. He was the first to suggest the existence of P 680 , the reaction center of PSII, and to prove that it was not an artefact of Chlorophyll a fluorescence. For most photobiologists, Govindjee is best known for successfully exploiting Chlorophyll a fluorescence to understand the various steps in photosynthesis as well as to predict plant productivity. His contribution in resolving the controversy on minimum number of quanta in favor of 8–12 vs 3–4, needed for the evolution of one molecule of oxygen, is a milestone in the area of photosynthesis research. Furthermore, together with Don DeVault, he is the first to provide the correct theory of thermoluminescence in photosynthetic systems. His research productivity is very high: ~ 600 published articles and total citations above 27,000 with an h-index of 82. He is a recipient of numerous awards and honors including a 2022: Lifetime Achievement Award of the International Society of Photosynthesis Research. We hope that the retrospective of Govindjee described in this work will inspire and stimulate the readers to continue probing the photosynthetic apparatuses with new discoveries and breakthroughs.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Xiong et al. ( 1998 )

Similar content being viewed by others

Stories and photographs of William A. Arnold (1904–2001), a pioneer of photosynthesis and a wonderful friend
Lucinda Choules & Govindjee

Photosystem 2 and the oxygen evolving complex: a brief overview
Charles F. Yocum
Evolution of the Z-scheme of photosynthesis: a perspective
Govindjee, Dmitriy Shevela & Lars Olof Björn
Allakhverdiev SI, Shen JR, Edwards GE (2013) Special issues on photosynthesis education honoring Govindjee. Photosynth Res 116:107–110. https://doi.org/10.1007/s11120-013-9913-3
Article CAS PubMed Google Scholar
Arnold W (1949) A calorimetric determination of the quantum yield in photosynthesis. In: Franck J, Loomis WE (eds) Photosynthesis in plants. Iowa State College Press, Ames, pp 273–276
Google Scholar
Arnold W, Sherwood HK (1957) Are chloroplasts semiconductors? Proc Natl Acad Sci USA 43:105–114. https://doi.org/10.1073/pnas.43.1.105
Article CAS PubMed PubMed Central Google Scholar
Balashov S, Ella Imasheva E, Misra S, Masahiro K, Liu S, Govindjee Govindje G, Ebrey TG (2023) Contributions of Rajni Govindjee in the life sciences: celebrating her 88th birthday. Int J Life Sci 12(1):1–14. https://doi.org/10.5958/2319-1198.2023.00001.5
Article Google Scholar
Blankenship RE (2021) Molecular mechanisms of photosynthesis, 3rd edition (Wiley).
Block JE (2022) Life of Govindjee, known as Mister Photosynthesis. J Plant Sci Res. 38(1):1–22. https://doi.org/10.32381/JPSR.2021.38.1.1
Cao J (1992) Effects of amino acid residue substitutions on bicarbonate function in the plastoquinone reductase in cyanobacteria. PhD Dissertation, The University of Illinois at Urbana-Champaign.
Chen S, Strasser RJ, Qiang S, Govindjee G (2012) Tenuazonic acid, a novel natural PSII inhibitor, impacts on photosynthetic activity by occupying the QB-binding site and inhibiting forward electron flow. In: Lu C (ed) Photosynthesis: research for food, fuel and future-15th international conference on photosynthesis, symposium. Zhejiang University Press (China), Springer, pp 453–456. https://doi.org/10.1007/978-3-642-32034-7_93
Demmig-Adams B, Garab G, Adams W, Govindjee G (2014) Non-photochemical quenching and energy dissipation in plants, algae and cyanobacteria, vol 40. Springer, Netherlands, Dordrecht. https://doi.org/10.1007/978-94-017-9032-1
Book Google Scholar
DeVault D, Govindjee G (1990) Photosynthetic glow peaks and their relationship with the free energy changes. Photosynth Res 24:175–181. https://doi.org/10.1007/BF00032597
DeVaunlt D, Govindjee G, Arnold W (1983) Energetics of photosynthetic glow peaks. Proc Natl Acad Sci USA 80:983–987. https://doi.org/10.1073/pnas.80.4.983
Eaton-Rye JJ (2007) Snapshots of the Govindjee lab from the late 1960s to the late 1990s, and beyond. Photosynth Res 94:153–178. https://doi.org/10.1007/s11120-007-9275-9
Article CAS Google Scholar
Eaton-Rye JJ (2013) Govindjee at 80: more than 50 years of free energy for photosynthesis. Photosynth Res 116:111–144. https://doi.org/10.1007/s11120-013-9921-3
Eaton-Rye JJ (2019) Govindjee: a lifetime in photosynthesis. Photosynth Res 139:9–14. https://doi.org/10.1007/s11120-018-0592-y
Eaton-Rye JJ, Govindjee G (1988a) Electron transfer through the quinone acceptor complex of photosystem II after one or two actinic flashes in bicarbonate-depleted spinach thylakoid membranes. Biochim Biophys Acta 935:248–257. https://doi.org/10.1016/0005-2728(88)90221-6
Eaton-Rye JJ, Govindjee G (1988b) Electron transfer through the quinone acceptor complex of photosystem II in bicarbonate-depleted spinach thylakoid membranes as a function of actinic flash number and frequency. Biochim Biophys Acta 935:237–247. https://doi.org/10.1016/0005-2728(88)90220-4
Eaton-Rye JJ (1987) Bicarbonate reversible anionic inhibition of the quinone reductase in photosystem II. Ph.D Dissertation, the University of Illinois at Urbana-Champaign.
Ebrey T (2015) Brighter than the sun: Rajni Govindjee at 80 and her fifty years in photobiology. Photosynth Res 124:1–5. https://doi.org/10.1007/s11120-015-0106-0
Emerson R, Lewis CM (1943) The dependence of the quantum yield of Chlorellaphotosynthesis on wavelength of light. Am J Bot 30(3):165–178. https://doi.org/10.2307/2437236
Emerson R, Chalmers RV, Cederstrand CN (1957) Some factors influencing the long wave limit of photosynthesis. Proc Natl Acad Sci USA 43:133–143. https://doi.org/10.1073/pnas.43.1.133
Govindjee E (2014) 63 Years since Kautsky-chlorophyll-a fluorescence. Aust J Plant Physiol 22(2):131–160. https://doi.org/10.1071/PP9950131
Govindjee G (2023) On the evolution of the concept of two light reactions and two photosystems for oxygenic photosynthesis: a personal perspective. Photosynthetica 61:37–47. https://doi.org/10.32615/ps.2023.006
Govindjee G, Coleman W (1990) How plants make oxygen. Sci Am 262:50–58
Govindjee G, Govindjee R (1974) Primary events in photosynthesis. Sci Am 231:68–82
Govindjee G, Rabinowitch E (1960a) a) Two forms of chlorophyll a in vivo with distinct photochemical function. Science 132:355–356. https://doi.org/10.1126/science.132.3423.355
Govindjee G, Rabinowitch E (1960b) b) Action spectrum of the second Emerson effect. Biophys J 43:73–89. https://doi.org/10.1016/S0006-3495(60)86877-4
Govindjee G, Rabinowitch E (1960c) c) Two forms of chlorophyll a in vivo with distinct photochemical function. Science 132:355–356. https://doi.org/10.1126/science.132.3423.355
Govindjee R, Govindjee G, Hoch G (1962) The Emerson enhancement effect in TPN-photoreduction by spinach chloroplasts. Biochem Biophys Res Commun 9:222–225. https://doi.org/10.1016/0006-291X(62)90062-1
Govindjee G, OvH O, Hoch G (1963) A mass spectroscopic study of the Emerson enhancement effect. Biochim Biophys Acta 75:281–284. https://doi.org/10.1016/0006-3002(63)90611-5
Govindjee R, Govindjee G, Hoch G (1964) Emerson Enhancement Effect in chloroplast reactions. Plant Physiol 39:10–14. https://doi.org/10.1104/pp.39.1.10
Govindjee G, Döring G, Govindjee R (1970) The active chlorophyll a 11 in suspensions of lyophilized and tris-washed chloroplasts. Biochim Biophys Acta 205:303–306. https://doi.org/10.1016/0005-2728(70)90260-4
Govindjee G, Pulles MPJ, Govindjee R, van Gorkom HJ, Duysens LNM (1976) Inhibition of the reoxidation of the secondary electron acceptor of Photosystem II by bicarbonate depletion. Biochim Biophys Acta 449:602–605. https://doi.org/10.1016/0005-2728(76)90173-0
Govindjee G, Wydrzynski T, Marks SB (1978) Manganese and chloride: their roles in photosynthesis. In: Metzner H (ed) Symposium on photosynthetic oxygen evolution. Academic Press, London, pp 321–344
Govindjee G, Koike H, Inoue Y (1985) Thermoluminiscence and oxygen evolution from a thermophilic blue-green alga obtained after single-turnover light flashes. Photochem Photobiol 42:579–585. https://doi.org/10.1111/j.1751-1097.1985.tb01613.x
Govindjee G, Xu C, Schansker G, van Rensen JJS (1997) Chloroacetates as inhibitors of photosystem II: effects on electron acceptor side. J Photochem Photobiol b: Biol 37:107–117. https://doi.org/10.1016/S1011-1344(96)07347-2
Govindjee G, Shevela D, Björn LO (2017) Evolution of the Z-scheme of photosynthesis. Photosynth Res 133:5–15. https://doi.org/10.1007/s11120-016-0333-z
Govindjee G, Papageorgiou GC, Govindjee R (2019) Eugene I. Rabinowitch: A prophet of photosynthesis and of peace in the world. Photosynth Res 141:143–150. https://doi.org/10.1007/s11120-019-00641-w
Govindjee G, Beatty JT, Gest H, Allen JF (2005) Discoveries in Photosynthesis, Adv Photosyn Respir, Springer, Dordrecht, 20
Govindjee G (1960) Effect of combining two wavelengths of light on the photosynthesis of algae, Ph.D. Thesis in Biophysics, University of Illinois at Urbana- Champaign.
Govindjee R (1961) The action spectrum of the hill reaction in whole algal cells and chloroplast suspensions (red drop, second emerson effect and inhibition by extreme red light), Ph.D. Thesis in Biophysics, University of Illinois at Urbana- Champaign.
Hill JF, Govindjee G (2014) The controversy over the minimum quantum requirement for oxygen evolution. Photosynth Res 122:97–112. https://doi.org/10.1007/s11120-014-0014-8
Holub O, Seufferheld MJ, Gohlke C, Govindjee G, Heiss GJ, Clegg RM (2007) Flourescence lifetime imaging microscopy of Chlamydomonas reinhardtii : non-photochemical quenching mutants and the effect of photosynthetic inhibitors on the slow chlorophyll fluorescence transient. J Microsc 226(2):90–120
Hou HJM, Allakhverdiev SI (2023) Photosynthesis: From Plants to Nanomaterials. Elsevier
Hou HJ, Allakhverdiev SI, Najafpour MM, Govindjee, (2014) Current challenges in photosynthesis: from natural to artificial. Front Plant Sci 28(5):232. https://doi.org/10.3389/fpls.2014.00232
Hou HJM, Najafpour MM, Allakhverdiev SI, Govindjee G (2023) Editorial: Current challenges in photosynthesis: from natural to artificial, volume II. Front Plant Sci 5(13):1113693. https://doi.org/10.3389/fpls.2022.1113693
Jajoo A, Guruprasad KN, Bharti S, Mohanty P (2009) International conference “Photosynthesis in the Global Perspective” held in honor of Govindjee, November 27–29, 2008, Indore, India. Photosynth Res 100:49–55. https://doi.org/10.1007/s11120-009-9409-3
Jajoo A, Subramanyam R, Garab G, Allakhverdiev SI (2023) Honoring two stalwarts of photosynthesis research: Eva-Mari Aro and Govindjee. Photosynth Res 27:1–9. https://doi.org/10.1007/s11120-022-00988-7
Kalaji HM, Goltsev V, Bosa K, Allakhverdiev SI, Strasser RJ, Govindjee G (2012) Experimental in-vivo measurements of light emission in plants: a perspective dedicated to David Walker. Photosynth Res 114:69–96. https://doi.org/10.1007/s11120-012-9780-3
Kaur D, Gisriel C, Burnap R, Fromme P, Govindjee G (2020) Gordon research conference 2019: From the biophysics of natural and artificial photosynthesis to bioenergy conversion. Curr Plant Biol 22:100129. https://doi.org/10.1016/j.cpb.2019.100129
Khanna R, Govindjee G, Wydrzynski T (1977) Site of bicarbonate effect in Hill reaction: evidence from the use of artificial electron acceptors and donors. Biochim Biophys Acta 462:208–214. https://doi.org/10.1016/0005-2728(77)90203-1
Khanna R, Wagner R, Junge W, Govindjee G (1980) Effects of CO 2 -depletion on proton uptake and release in thylakoid membranes. FEBS Lett 121:222–224. https://doi.org/10.1016/0014-5793(80)80347-4
Khanna R (1980) Role of bicarbonate and of manganese in photosystem II reactions of photosynthesis. Ph.D. Dissertation, The University of Illinois at Urbana-Champaign.
Krey A, Govindjee G (1964) Fluorescence changes in Porphyridium exposed to green light of different intensity: a new emission band at 693 nm: its significance to photosynthesis. Proc Nat Acad Sci USA 52:1568–1572. https://doi.org/10.1073/pnas.52.6.1568
Laisk A, Nedbal L, Govindjee G (2009) Photosynthesis in silico: understanding complexity from molecules to ecosystems, advances in photosynthesis and respiration, vol 29. Springer, Dordrecht
Laloraya MM, Govindjee G (1955) Effect of tobacco leaf-curl and tobacco mosaic virus on the amino acid content of Nicotiana sp. Nature (london) 175:907–908. https://doi.org/10.1038/175907a0
Magee JL, de Witt TW, Smith EC, Daniels F (1939) A photocalorimeter: the quantum efficiency of photosynthesis in algae. J Am Chem Soc 61:3529–3533. https://doi.org/10.1021/ja01267a089
Manning WM, Stauffer JF, Duggar BM, Daniels F (1938) Quantum efficiency of photosynthesis in Chlorella . J Am Chem Soc 60:266–274. https://doi.org/10.1021/ja01269a013
Mar T, Govindjee G (1971) Thermoluminescence in spinach chloroplasts and in Chlorella . Biochim Biophys Acta 226:200–203. https://doi.org/10.1016/0005-2728(71)90193-9
Matsubara S, Chen YC, Caliandro R, Govindjee G, Robert Clegg M (2011) Photosystem II fluorescence lifetime imaging in avocado leaves: contributions of the lutein-epoxide and violaxanthin cycles to fluorescence quenching. J Photochem Photobiol b: Biol 104:271–284. https://doi.org/10.1016/j.jphotobiol.2011.01.003
Najafpour MM, Govindjee G (2011) Oxygen evolving complex in photosystem II: better than excellent. Dalton Trans 40(36):9076–9084. https://doi.org/10.1039/C1DT10746A
Najafpour MM, Tabrizi MA, Haghighi B, Govindjee G (2012) A manganese oxide with phenol groups as a promising structural model for water oxidizing complex in Photosystem II: a ‘golden fish.’ Dalton Trans 41(14):3906–3910. https://doi.org/10.1039/C2DT11672C
Najafpour MM, Moghaddam AN, Allakhverdiev SI (2012) Biological water oxidation: lessons from nature. Biochim Biophys Acta Bioenerg 8:1110–1121. https://doi.org/10.1016/j.bbabio.2012.04.002
Nanba O, Satoh N (1987) Isolation of a photosystem II reaction center consisting of D-1 and D-2 polypeptides and cytochrome b-555. Proc Natl Acad Sci USA 84:109–112. https://doi.org/10.1073/pnas.84.1.109
Nickelsen K, Govindjee G (2011) The Maximum quantum yield controversy: otto warburg and the midwest gang. Bern Studies in the History and Philosophy of Science, University of Bern, Switzerland; Institute für Philosophie.
Nonomura A, Kumar A (2022) Celebrating the 2022 lifetime achievement award of the international society of photosynthesis research to Govindjee, who hails from Allahabad. LS-an Int J Life Sci 11(3):153–155. https://doi.org/10.5958/2319-1198.2022.00014.8
Ort DR, Chinnusamy V, Pareek A (2022) Photosynthesis: diving deep into the process in the era of climate change. Plant Physiol Rep 27:539–542. https://doi.org/10.1007/s40502-022-00703-7
Papageorgiou GC, Govindjee G (2004) Chlorophyll a fluorescence: a signature of photosynthesis, advances in photosynthesis and respiration, vol 19. Springer, Dordrecht
Rabinowitch E, Govindjee G (1965) The role of chlorophyll in photosynthesis. Sci Am 213:74–83
Rabinowitch E, Govindjee G, Thomas JB (1960) Inhibition of photosynthesis in some algae by extreme-red light. Science 132(3424):422–422. https://doi.org/10.1126/science.132.3424.422
Rabinowitch E, Govindjee G (1969) Photosynthesis. John Wiley and Sons Inc. New York, p 273
Schansker G, Srivastava A, Govindjee G, Strasser RJ (2003) Characterization of the 820-nm transmission signal paralleling the chlorophyll a fluorescence rise (OJIP) in pea leaves. Funct Plant Biol 30:1–10. https://doi.org/10.1007/s11120-013-9806-5
Seibert M, Mattoo A, Kumar A (2022) A special toast to Govindjee Govindjee on celebrating his 90 th birthday on 24 October 2022. Int J Life Sci 11(3):156–175. https://doi.org/10.5958/2319-1198.2022.00015.X
Shabnam N, Sharmila P, Sharma A, Strasser RJ, Govindjee G, Pardha-Saradhi P (2015) Mitochondrial electron transport protects floating leaves of long leaf pondweed ( Potamogeton nodosus Poir) against photoinhibition: comparison with submerged leaves. Photosynth Res 125:305–319. https://doi.org/10.1007/s11120-014-0051-3
Shevela D, Eaton-Rye JJ, Shen JR, Govindjee G (2012) Photosystem II and unique role of bicarbonate: a historical perspective. Biochim Biophys Acta 1817:1134–1151. https://doi.org/10.1016/j.bbabio.2012.04.003
Shevela D, Bjorn LO, Govindjee G (2018) Photosynthesis: solar energy for life. World Scientific Publishing
Srivastava AK, Strasser RJ, Govindjee G (1995) Polyphasic rise of chlorophyll a fluorescence in herbicide-resistant D1 mutants of Chlamydomonas reinhardtii . Photosynth Res 43:131–141. https://doi.org/10.1007/BF00042970
Stemler A, Govindjee G (1973) Bicarbonate ion as a critical factor in photosnthetic oxygen evolution. Plant Physiol 52:119–123. https://doi.org/10.1104/pp.52.2.119
Stemler AJ (1965) Effect of combining two wavelengths of light on the photosynthesis of algae. Ph.D. Thesis in Biophysics, University of Illinois at Urbana- Champaign.
Stirbet A, Govindjee G (2011) On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: Basics and applications of the OJIP fluorescence transient. J Photochem Photobiol b: Biol 104:236–257. https://doi.org/10.1016/j.jphotobiol.2010.12.010
Stirbet A, Govindjee G (2012) Chlorophyll a fluorescence induction: a personal perspective of the thermal phase, the J-I-P rise. Photosynth Res 113:15–61. https://doi.org/10.1007/s11120-012-9754-5
Stirbet A, Govindjee G (2016) The slow phase of chlorophyll a fluorescence induction in silico: origin of the S-M fluorescence rise. Photosynth Res 130:193–213. https://doi.org/10.1007/s11120-016-0243-0
Stirbet A, Yu RG, Rubin AB, Govindjee G (2014) Modeling chlorophyll a fluorescence transient: relation to photosynthesis. Biochem (moscow) 79:291–323. https://doi.org/10.1134/S0006297914040014
Stirbet A, Lazár D, Papageorgiou GC, Govindjee G (2019) Chlorophyll a fluorescence in cyanobacteria: relation to photosynthesis. In: Mishra AN, Tiwari DN, Rai AN (eds) Cyanobacteria: from basic science to applications, Elsevier Publishers Academic Press, pp 79–130. https://doi.org/10.1016/B978-0-12-814667-5.00005-2
Strasser BJ, Strasser RJ (1995) Measuring fast fluorescence transients to address environmental questions: the JIP test. In: Mathis P (ed) Photosynthesis: from light to Biosphere. Vol. 5. Dordrecht, Kluwer, pp 977–980. https://doi.org/10.1007/978-94-009-0173-5_1142
Tatake VG, Desai TS, Govindjee G, Sane PV (1981) Energy storage states of photosynthetic membranes: activation energies and lifetimes of electrons in the trap states by thermoluminescence method. Photochem Photobiol 33:243–250. https://doi.org/10.1111/j.1751-1097.1981.tb05331.x
Vass I, Govindjee G (1996) Thermoluminescence from the photosynthetic apparatus. Photosynth Res 48:117–126. https://doi.org/10.1007/BF00041002
Warburg O, Krippahl G (1960) Notwendigkeit der Kohlensäure für die Chinon und Ferricyanidreaktionen in grünen Grana. Z. Naturforsch 15b:367–369. https://doi.org/10.1515/znb-1960-0605
Warburg O, Negelein E (1923) Über den Einfluss der Wellenlänge auf den Energieumsatz bei der Kohlensäureassimilation. Zeit Physik Chem 106:191–218. https://doi.org/10.1515/zpch-1923-10614
Wasielewski MR, Johnson DG, Govindjee G, Preston C, Seibert M (1989a) Determination of the primary charge separation rate in photosystem II reaction centers at 15 K. Photosynth Res 22:89–100. https://doi.org/10.1007/BF00114769
Wasielewski MR, Johnson DG, Seibert M, Govindjee G (1989b) Determination of the primary charge separation rate in isolated photosystem II reaction centers with 500 femtosecond time resolution. Proc Natl Acad Sci USA 86:524–548. https://doi.org/10.1073/pnas.86.2.524
Wydrzynski T, Govindjee G (1975) A new site of bicarbonate effect in photosystem II of photosynthesis: evidence from chlorophyll fluorescence transients in spinach chloroplasts. Biochim Biophys Acta 387:403–408. https://doi.org/10.1016/0005-2728(75)90121-8
Wydrzynski TJ, Marks SB, Schmidt PG, Govindjee G, Gutowsky HS (1978) Nuclear magnetic relaxation by the manganese in aqueous suspensions of chloroplasts. Biochemistry 17:2155–2162. https://doi.org/10.1021/bi00604a020
Xiong J, Subramanian S, Govindjee G (1998) A knowledge-based three dimensional model of the photosystem II reaction center of Chlamydomonas reinhardtii . Photosynth Res 56:229–254. https://doi.org/10.1023/A:1006061918025
Xiong J (1996) Experimental and theoretical studies of the photosystem II reaction center: implications for bicarbonate binding and function. PhD Dissertation, The University of Illinois at Urbana-Champaign.
Yates D (2022) Govindjee’s photosynthesis museum. Int J Life Sci 11(2):93–97. https://doi.org/10.5958/2319-1198.2022.00008.2
Yates D (2019) Govindjee’s Photosynthesis Museum. Illinois News Bureau, University of Illinois. https://news.illinois.edu/view/6367/801235
Yusuf MA, Kumar D, Rajwanshi R, Strasser RJ, Tsimilli-Michael M, Govindjee G, Sarin NB (2010) Overexpression of gamma-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: physiological and chlorophyll fluorescence measurements. Biochim Biophys Acta 1797:1428–1438. https://doi.org/10.1016/j.bbabio.2010.02.002
Download references
Acknowledgements
VM, SPP, SGN, NL, ARKP, AMS, RJ, JJ, AKS and JTP thank the University of Calicut for providing all the resources to carry out this work. VM, NL, ARKP, JJ thank the University Grant Commission (UGC) for providing fund in the form of JRF and SRF. AMS and AKS thank the Council of Scientific and Industrial Research, India (CSIR) for providing fund in the form of JRF and SRF. RJ thank Bayers MEDHA Fellowship Program (PHD- 2023-10454) in the form of JRF.We all thank Govindjee for sharing his own articles (Govindjee 2019a, 2023) and for reading this article before its publication.
VM, NL, ARKP, JJ thank the University Grant Commission (UGC) for providing fund in the form of JRF and SRF. AMS and AKS thank the Council of Scientific and Industrial Research, India (CSIR) for providing fund in the form of JRF and SRF. RJ thank Bayers MEDHA Fellowship Program (PHD-2023-10454) for providing fund in the form of JRF.
Author information
Authors and affiliations.
Plant Physiology and Biochemistry Division, Department of Botany, University of Calicut, C. U. Campus, P.O. Malappuram, Kerala, 673635, India
Mathew Veena, Louis Noble, K. P. Raj Aswathi, M. S. Amritha, Riya Johnson, Joy M. Joel, K. S. Anjitha & Jos T. Puthur
Department of Botany, PSMO College, Tirurangadi, Malappuram, Kerala, 676 306, India
P. P. Sameena
Department of Botany, Mar Athanasius College, Kothamangalam College, P.O., Kothamangalam, Kerala, 686 666, India
Nair G. Sarath
Laboratory of Forensic Analysis and Photosynthesis, Department of Physical and Forensic Sciences, Alabama State University, Montgomery, AL, 36104, USA
Harvey J. M. Hou
You can also search for this author in PubMed Google Scholar
Contributions
All the authors declare to have made substantial contributions to the article conception; Writing- original draft preparation: VM, SPP, SGN, NL, ARKP, AMS, RJ, JJ, AKS; Figure setting: ARKP, AMS, VM; Writing- Review and Editing: VM, SPP, JTP and HJMH; Supervision: JTP, HJMH.
Corresponding authors
Correspondence to Harvey J. M. Hou or Jos T. Puthur .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Veena, M., Sameena, P.P., Sarath, N.G. et al. Revelations on photosystem II, thermoluminescence, and artificial photosynthesis: a retrospective of Govindjee from fundamentals to applications. Physiol Mol Biol Plants 29 , 1225–1238 (2023). https://doi.org/10.1007/s12298-023-01373-x
Download citation
Received : 08 June 2023
Revised : 08 June 2023
Accepted : 13 October 2023
Published : 01 November 2023
Issue Date : September 2023
DOI : https://doi.org/10.1007/s12298-023-01373-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Bicarbonate
- Chlorophyll a fluorescence
- Emerson enhancement effect
- Quantum requirement
- Water splitting complex
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- ADVERTISEMENT FEATURE Advertiser retains sole responsibility for the content of this article
A new leaf unfolds in artificial photosynthesis
Produced by

A cell with a solar-to-chemical conversion efficiency of 10.5% yields formate at a cost efficient production rate of 1.2 mole per hour. © Toyota Central R&D Labs., Inc.
In 2021, researchers from Toyota Central R&D Labs developed a large, cost-effective artificial photosynthesis system that produces industrial formate at a solar-to-chemical conversion efficiency (ηSTC) of 10.5% 1 . Researchers from the lab say that, to their knowlege, this ηSTC is a first for a one metre squared cell.
Within the next 10 years, the company aims to establish artificial photosynthesis technology for wide-scale production of useful carbon compounds.
Plant power
Artificial photosynthesis is an emerging technology, attracting interest for its potential to mimic plants by producing industrially useful compounds using CO 2 , water, and sunlight. But the technology is still in its infancy and is unavailable for use on an industrial scale.
This new system is based on the use of metal-complex catalysts to convert CO 2 into carbon compounds at electrical potentials approaching the theoretical lowest limits for the conversion reactions.
The researchers used solar cells connected to electrodes immersed in carbonated water to initiate the redox reactions that produce formate, explains Naohiko Kato, a principal researcher at Toyota Central R&D Labs. This coupling of electrodes with semiconductors and molecular catalysts allowed for CO 2 reduction to occur using minimal energy and relatively inexpensive components.
Advances at the lab have been quick. In March 2021, a 36 centimetre squared cell produced by the company was shown to have a ηSTC of 7.2% 2 . Then in November 2021, the group demonstrated a one metre squared cell with a ηSTC of 10.5%, yielding a formate production rate of 1.2 mole per hour.
Most importantly, Kato emphasizes that cost efficiency has reached the point of realistic discussion of real-world production. “We use inexpensive crystalline silicon solar cells,” he explains. “While other materials have superior conversion efficiency, the material expense makes the cost per watt extremely expensive.”
It’s hoped that this technology is also good for the environment, as it could use the concentrated CO 2 industrial by-products. “Our artificial photosynthesis system now absorbs 100 times more CO 2 compared to cedar forests of the same area,” notes Kato.
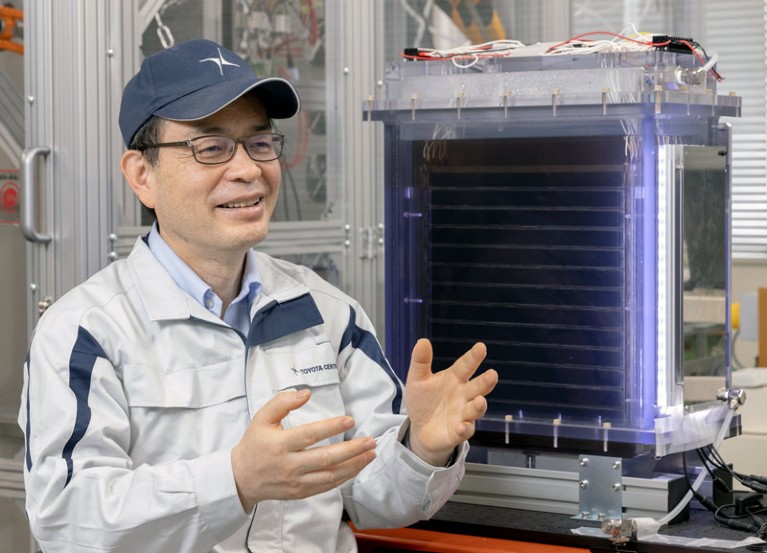
Naohiko Kato is a principal researcher at Toyota Central R&D Labs. © Toyota Central R&D Labs., Inc.
Inspiration from photocatalyst technology
In 2011, Toyota Central R&D Labs established a proof of principle for an artificial photosynthesis systems that produces carbon compounds from water, CO 2 and sunlight, without any other additives 3 .
The system was small, measuring roughly one square centimetre. “The inspiration for this semiconductor/metal-complex catalyst assembly came from a semiconductor photocatalyst technology that we had been supplying to the market for 20 years for odour-eliminating, antibacterial, and antiviral purposes,” explains Takeshi Morikawa, a senior research fellow in Toyota Central R&D Labs.
“Artificial photosynthesis, however, is very challenging,” says Morikawa. Their first one square centimetre system used two photocatalysts immersed in water that, when exposed to sunlight, produced a ηSTC of 0.04%, only one fifth of the ηSTC demonstrated by a plant such as switchgrass. In 2015, however, continued improvements led to the ‘artificial leaf’, a one square centimetre electrode device that achieved a ηSTC of 4.6%, exceeding that of typical plants.

Takeshi Morikawa’s ‘artifical leaf’ has a solar-to-chemical conversion efficiency of 4.6%. © Toyota Central R&D Labs., Inc.
Interdisciplinary expertise
Kato’s team has been working on increasing the device size. “To make artificial photosynthesis practical in a real-world setting, the device must be large enough for scalable, industrial use,” says Kato, who joined the project after extensive experience working with solar cells.
“Maintaining high efficiency in larger sizes does not come easily. Simply expanding the size of all components would increase the reactive resistance of the electrodes,” he says. “In fact, the conversion efficiency dropped drastically in the initial phase. We had more reduction occuring with oxygen than with CO 2 , leading to an excess of hydrogen and decrease in the amount of formate produced.”
Leveraging the expertise of colleagues in materials and device engineering, they designed a new system that captures energy from the sun with solar cells in lieu of photocatalysts. “Solar cells are superior at converting sunlight to electrons,” explains Kato.
The team aspires to establish technology for an industrial system at a scale of one square metre by the 2030s. “The focus is to improve the system so that it captures and converts high-concentrate CO 2 from industrial emissions,” says Kato.
Globally, 700,000 tonnes of formate are used annually, largely as a preservative in livestock feed and leather processing. It also shows potential as a hydrogen carrier for the new hydrogen economy, says Kato. While other promising liquid carriers, such as ammonia, must be kept at low temperatures (-34°C or above 0.1MPa at room temperature), formate is easier to manage as it maintains a liquid form in ambient conditions.
The production of other chemicals is also in the team’s sights. “This could be a platform for wider artificial photosynthesis,” says Morikawa. “It may be possible to use different catalysts that convert CO 2 and water to other compounds — it’s a matter of how soon we can discover the catalysts.”
1. Kato, N., et al. Solar Fuel Production from CO 2 Using a 1 m-Square-Sized Reactor with a Solar-to-Formate Conversion Efficiency of 10.5%, ACS Sustainable Chem. Eng. 9(48), 16031–16037 (2021).
Doi: 10.1021/acssuschemeng.1c06390
2. Kato, N., et al. A Large-Sized Cell for Solar-Driven CO 2 Conversion with a Solar-to-Formate Conversion Efficiency of 7.2%, Joule 5, 687-705 (2021).
Doi: 10.1016/j.joule.2021.01.002
3. Morikawa, T., et al. Solar-Driven CO 2 Reduction Using a Semiconductor/Molecule Hybrid Photosystem: From Photocatalysts to a Monolithic Artificial Leaf, Accounts of Chemical Research (2021) Articles ASAP.
Doi: 10.1021/acs.accounts.1c00564
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Covering a story? Visit our page for journalists or call (773) 702-8360.

UChicago Class Visits
Top stories.
- UChicago scientists tap the power of collaboration to make transformative breakthroughs
UChicago announces recipients of Academic Communicators Network awards for 2024
- Donald Whitcomb, renowned scholar of Islamic archaeology, 1944‒2024
Chemists create an 'artificial photosynthesis' system that is 10 times more efficient than existing systems
Uchicago breakthrough creates methane fuel from sun, carbon dioxide and water.
For the past two centuries, humans have relied on fossil fuels for concentrated energy; hundreds of millions of years of photosynthesis packed into a convenient, energy-dense substance. But that supply is finite, and fossil fuel consumption has tremendous negative impact on Earth’s climate.
“The biggest challenge many people don’t realize is that even nature has no solution for the amount of energy we use,” said University of Chicago chemist Wenbin Lin. Not even photosynthesis is that good, he said: “We will have to do better than nature, and that’s scary.”
One possible option scientists are exploring is “artificial photosynthesis”—reworking a plant’s system to make our own kinds of fuels. However, the chemical equipment in a single leaf is incredibly complex, and not so easy to turn to our own purposes.
A Nature Catalysis study from six chemists at the University of Chicago shows an innovative new system for artificial photosynthesis that is more productive than previous artificial systems by an order of magnitude. Unlike regular photosynthesis, which produces carbohydrates from carbon dioxide and water, artificial photosynthesis could produce ethanol, methane, or other fuels.
Though it has a long way to go before it can become a way for you to fuel your car every day, the method gives scientists a new direction to explore—and may be useful in the shorter term for production of other chemicals.
“This is a huge improvement on existing systems, but just as importantly, we were able to lay out a very clear understanding of how this artificial system works at the molecular level, which has not been accomplished before,” said Lin, who is the James Franck Professor of Chemistry at the University of Chicago and senior author of the study.
‘We will need something else’
“Without natural photosynthesis, we would not be here. It made the oxygen we breathe on Earth and it makes the food we eat,” said Lin. “But it will never be efficient enough to supply fuel for us to drive cars; so we will need something else.”
The trouble is that photosynthesis is built to create carbohydrates, which are great for fueling us, but not our cars, which need much more concentrated energy. So researchers looking to create alternates to fossil fuels have to re-engineer the process to create more energy-dense fuels, such as ethanol or methane.
In nature, photosynthesis is performed by several very complex assemblies of proteins and pigments. They take in water and carbon dioxide, break the molecules apart, and rearrange the atoms to make carbohydrates—a long string of hydrogen-oxygen-carbon compounds. Scientists, however, need to rework the reactions to instead produce a different arrangement with just hydrogen surrounding a juicy carbon core—CH4, also known as methane.
This re-engineering is much trickier than it sounds; people have been tinkering with it for decades, trying to get closer to the efficiency of nature.
Lin and his lab team thought that they might try adding something that artificial photosynthesis systems to date haven’t included: amino acids.
The team started with a type of material called a metal-organic framework or MOF, a class of compounds made up of metal ions held together by an organic linking molecules. Then they designed the MOFs as a single layer, in order to provide the maximum surface area for chemical reactions, and submerged everything in a solution that included a cobalt compound to ferry electrons around. Finally, they added amino acids to the MOFs, and experimented to find out which worked best.
They were able to make improvements to both halves of the reaction: the process that breaks apart water and the one that adds electrons and protons to carbon dioxide. In both cases, the amino acids helped the reaction go more efficiently.
Even with the significantly improved performance, however, artificial photosynthesis has a long way to go before it can produce enough fuel to be relevant for widespread use. “Where we are now, it would need to scale up by many orders of magnitude to make an sufficient amount of methane for our consumption,” Lin said.
The breakthrough could also be applied widely to other chemical reactions; you need to make a lot of fuel for it to have an impact, but much smaller quantities of some molecules, such as the starting materials to make pharmaceutical drugs and nylons, among others, could be very useful.
“So many of these fundamental processes are the same,” said Lin. “If you develop good chemistries, they can be plugged into many systems.”
The scientists used resources at the Advanced Photon Source, a synchrotron located at the U.S. Department of Energy’s Argonne National Laboratory, to characterize the materials.
The co-first authors of the paper were Guangxu Lan (PhD’20, now with Peking University), graduate student Yingjie Fan, and Wenjie Shi (Visiting student, now with Tianjin University of Technology. The other authors of the paper were Eric You (BS’20, now a graduate student at MIT) and Samuel Veroneau (BS’20, now a PhD student at Harvard University).
Citation: “ Biomimetic active sites on monolayered metal–organic frameworks for artificial photosynthesis .” Lan et al, Nature Catalysis, Nov. 10, 2022.
Funding: University of Chicago, National Science Foundation, China Scholarship Council
Get more with UChicago News delivered to your inbox.
Recommended

University of Chicago chemists discover a key protein in how…
UChicago scientists invent ultra-thin, minimally-invasive pacemaker…
Related Topics
Latest news, big brains podcast: why we die—and how we can live longer.
Winners of the 2024 UChicago Science as Art competition announced

Meet A UChicagoan
Ecologist tracks how insects can devastate forests—and how to stop them

Big Brains podcast
Big Brains podcast: What dogs are teaching us about aging

Where do breakthrough discoveries and ideas come from?
Explore The Day Tomorrow Began

Department of Race, Diaspora, and Indigeneity
Course on Afrofuturism brings together UChicago students and community members

Education Lab

National study finds in-school, high-dosage tutoring can reverse pandemic-era learning loss
Around uchicago.

Lecture Series
Author and ‘Odyssey’ translator Daniel Mendelsohn to deliver Berlin Family Lectures beginning April 23
Two uchicago scholars elected as 2023 american association for the advancement ….
2024 Guggenheim Fellowships
Profs. Sianne Ngai and Robyn Schiff honored for their innovative literary work

Convocation
Prof. John List named speaker for UChicago’s 2024 Convocation ceremony

The College
Anna Chlumsky, AB’02, named UChicago’s 2024 Class Day speaker
Biological Sciences Division
“You have to be open minded, planning to reinvent yourself every five to seven years.”

Office of Sustainability
Ways to celebrate Earth Month 2024 at the University of Chicago

Artificial photosynthesis can produce food without sunshine
Scientists are developing artificial photosynthesis to help make food production more energy-efficient here on Earth, and one day possibly on Mars
Photosynthesis has evolved in plants for millions of years to turn water, carbon dioxide, and the energy from sunlight into plant biomass and the foods we eat. This process, however, is very inefficient, with only about 1% of the energy found in sunlight ending up in the plant. Scientists at UC Riverside and the University of Delaware have found a way to bypass the need for biological photosynthesis altogether and create food independent of sunlight by using artificial photosynthesis.

The research, published in Nature Food , uses a two-step electrocatalytic process to convert carbon dioxide, electricity, and water into acetate, the form of the main component of vinegar. Food-producing organisms then consume acetate in the dark to grow. Combined with solar panels to generate the electricity to power the electrocatalysis, this hybrid organic-inorganic system could increase the conversion efficiency of sunlight into food, up to 18 times more efficient for some foods.
“With our approach we sought to identify a new way of producing food that could break through the limits normally imposed by biological photosynthesis,” said corresponding author Robert Jinkerson , a UC Riverside assistant professor of chemical and environmental engineering.
In order to integrate all the components of the system together, the output of the electrolyzer was optimized to support the growth of food-producing organisms. Electrolyzers are devices that use electricity to convert raw materials like carbon dioxide into useful molecules and products. The amount of acetate produced was increased while the amount of salt used was decreased, resulting in the highest levels of acetate ever produced in an electrolyzer to date.
“Using a state-of-the-art two-step tandem CO2 electrolysis setup developed in our laboratory, we were able to achieve a high selectivity towards acetate that cannot be accessed through conventional CO2 electrolysis routes,” said corresponding author Feng Jiao at University of Delaware.
Experiments showed that a wide range of food-producing organisms can be grown in the dark directly on the acetate-rich electrolyzer output, including green algae, yeast, and fungal mycelium that produce mushrooms. Producing algae with this technology is approximately fourfold more energy efficient than growing it photosynthetically. Yeast production is about 18-fold more energy efficient than how it is typically cultivated using sugar extracted from corn.
“We were able to grow food-producing organisms without any contributions from biological photosynthesis. Typically, these organisms are cultivated on sugars derived from plants or inputs derived from petroleum—which is a product of biological photosynthesis that took place millions of years ago. This technology is a more efficient method of turning solar energy into food, as compared to food production that relies on biological photosynthesis,” said Elizabeth Hann, a doctoral candidate in the Jinkerson Lab and co-lead author of the study.
The potential for employing this technology to grow crop plants was also investigated. Cowpea, tomato, tobacco, rice, canola, and green pea were all able to utilize carbon from acetate when cultivated in the dark.
“We found that a wide range of crops could take the acetate we provided and build it into the major molecular building blocks an organism needs to grow and thrive. With some breeding and engineering that we are currently working on we might be able to grow crops with acetate as an extra energy source to boost crop yields,” said Marcus Harland-Dunaway, a doctoral candidate in the Jinkerson Lab and co-lead author of the study.
By liberating agriculture from complete dependence on the sun, artificial photosynthesis opens the door to countless possibilities for growing food under the increasingly difficult conditions imposed by anthropogenic climate change. Drought, floods, and reduced land availability would be less of a threat to global food security if crops for humans and animals grew in less resource-intensive, controlled environments. Crops could also be grown in cities and other areas currently unsuitable for agriculture, and even provide food for future space explorers.
“Using artificial photosynthesis approaches to produce food could be a paradigm shift for how we feed people. By increasing the efficiency of food production, less land is needed, lessening the impact agriculture has on the environment. And for agriculture in non-traditional environments, like outer space, the increased energy efficiency could help feed more crew members with less inputs,” said Jinkerson.
This approach to food production was submitted to NASA’s Deep Space Food Challenge where it was a Phase I winner. The Deep Space Food Challenge is an international competition where prizes are awarded to teams to create novel and game-changing food technologies that require minimal inputs and maximize safe, nutritious, and palatable food outputs for long-duration space missions.
“Imagine someday giant vessels growing tomato plants in the dark and on Mars—how much easier would that be for future Martians?” said co-author Martha Orozco-Cárdenas , director of the UC Riverside Plant Transformation Research Center.
Andres Narvaez, Dang Le, and Sean Overa also contributed to the research. The open-access paper, “A hybrid inorganic–biological artificial photosynthesis system for energy-efficient food production,” is available here .
The research was supported by the Translational Research Institute for Space Health (TRISH) through NASA (NNX16AO69A), Foundation for Food and Agriculture Research (FFAR), the Link Foundation, the U.S. National Science Foundation, and the U.S. Department of Energy. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the Foundation for Food and Agriculture Research.
Header photo: Plants are growing in complete darkness in an acetate medium produced in an electrolyzer that replaces biological photosynthesis. (Marcus Harland-Dunaway/UCR)
Media Contacts
Related articles.

To find life in the universe, look to deadly Venus

Solving antibiotic and pesticide resistance with infectious worms

CO2 worsens wildfires by helping plants grow
Vaccine breakthrough means no more chasing strains
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Philos Trans R Soc Lond B Biol Sci
- v.369(1640); 2014 Apr 19
Photosynthesis under artificial light: the shift in primary and secondary metabolism
1 Agricultural Institute, Centre for Agricultural Research, Hungarian Academy of Sciences, Martonvásár, Hungary
Parisa Heydarizadeh
2 MicroMar, Mer Molécules Santé, IUML - FR 3473 CNRS, Faculté des Sciences et Techniques, University of Le Mans, Le Mans, France
3 College of Agriculture, Department of Agronomy and Plant Breeding, Isfahan University of Technology, Isfahan 84156-83111, Iran
Benoît Schoefs
Mohammad r. sabzalian.
Providing an adequate quantity and quality of food for the escalating human population under changing climatic conditions is currently a great challenge. In outdoor cultures, sunlight provides energy (through photosynthesis) for photosynthetic organisms. They also use light quality to sense and respond to their environment. To increase the production capacity, controlled growing systems using artificial lighting have been taken into consideration. Recent development of light-emitting diode (LED) technologies presents an enormous potential for improving plant growth and making systems more sustainable. This review uses selected examples to show how LED can mimic natural light to ensure the growth and development of photosynthetic organisms, and how changes in intensity and wavelength can manipulate the plant metabolism with the aim to produce functionalized foods.
1. Introduction
The rising population, climate changes, land use competition for food, feed, fuel and fibre production as well as the increasing demand for valuable natural compounds all reinforce the need for artificial growing systems such as greenhouses, soilless systems and vertical gardening, even in spacecrafts and space stations. Most of these growing systems require the application of additional, at least supplementary, light sources to ensure plant growth. Because these sources are heat dissipaters requiring cooling, artificial systems are frequently at odds with the demand for sustainability in industrial processes. In terms of both economics and sustainability, new lighting technologies such as light-emitting diodes (LEDs) thus were necessary to be developed [ 1 , 2 ]. Above all technological properties, LEDs should be compatible with the photosynthesis and light-signalling requirements of plants, which are tightly linked with the two main characteristics of light: wavelength and fluence.
Being mostly immobile, photosynthetic organisms must adapt to their biotic and abiotic environments that they sense through different types of receptors, including photoreceptors [ 3 ]. The pigment moiety of photoreceptors allows the receptor to extract from the incoming natural white light the specific information related to the intensity of the environmental light constraints. This information is used to develop the adequate response [ 3 ].
Photosynthesis is a photobiochemical process using light energy to produce ATP and NADPH, ultimately consumed in the assembly of carbon atoms in organic molecules. Functionally, photons are harvested by protein–chlorophyll (Chl)–carotenoid complexes (that form the light harvesting antenna of photosystems) and then transferred to the photosystem reaction centre, where electrons are generated; these processes take place in the chloroplast [ 4 ]. If lighting is too weak, photosynthesis cannot work efficiently and etiolation symptoms appear [ 5 ]. However, excessive light generates oxygen radicals and causes photoinhibition. Both phenomena strongly limit primary productivity [ 6 ].
Photosynthetic processes are often modified in plants grown under artificial lighting, because lamps do not usually mimic the spectrum and energy of sunlight. Agronomically, new lighting technologies such as LEDs have the potential to cover fluence and wavelength requirements of plants, while allowing specific wavelengths to be enriched, thus supplying the light quantity and quality essential for different phases of growth. The biomass and metabolic products of cultivated plants can therefore be modified.
This review gives a brief summary of the types of artificial lighting available for growing photosynthetic organisms. The capacity of LEDs to mimic the effects of natural light in terms of energy and information, thus ensuring the growth and development of photosynthetic organisms, and the potential for manipulating the plant metabolism to produce functionalized foods through changes in the intensity and wavelength are also reviewed here using selected examples.
2. Artificial light sources for photosynthesis
Artificial lighting should provide plants with energy and information required for development. For this purpose, fluorescent lamps, particularly those having enhanced blue and red spectra (i.e. cool fluorescent white lamps), are widely used in growth chambers, together with additional light sources to achieve the sustained photosynthetic photon fluence necessary for high productivity [ 1 , 7 ]. However, the spectrum and intensity of fluorescent lights are not stable over a long time (see the comparative information in the electronic supplementary material, table S1).
High intensity discharge (HID) lamps, such as metal halide and high-pressure sodium lamps, have relatively high fluence (max. 200 lumens per watt) and high photosynthetically active radiations (PARs) efficiency (max. 40%), and are typically used in greenhouses and plant growth rooms. The drawbacks including elevated arc to fire energy requirement, the high operational temperature preventing placement close to the canopy and the spectral distribution (high proportion of green–yellow region, significant ultraviolet radiation and altered red : far red ratio), which may shift according to the input power, strongly limit their use and innovation [ 8 ]. Among artificial lighting systems, LEDs present the maximum PAR efficiency (80–100%; see the electronic supplementary material, table S1). LEDs emitting blue, green, yellow, orange, red and far red are available and can be combined to provide either high fluence (over full sunlight, if desired), or special light wavelength characteristics, thanks to their narrow-bandwidth light spectrum [ 9 ]. The high efficiency, low operating temperature and small size enable LEDs to be used in pulsed lighting and be placed close to the leaves in interlighting and intracanopy irradiation [ 7 ]. Their long life expectancy and ease of control make them ideal for greenhouses in use all year round [ 7 ]. The LED technology is predicted to replace fluorescent and HID lamps in horticultural systems and to revolutionize controlled growth environments.
3. Changing light intensity and quality
From the biological point of view, the main questions about LEDs are related to their ability to mimic and enhance the beneficial effects of natural light while avoiding the adverse influence. Below, selected examples are used to provide a short review on useful properties of LED lights in these aspects.
(a) Light-emitting diode light(s) can sustain normal plant growth
Pioneer experiments on plant growth under red LEDs on lettuce were reported by Bula et al . [ 9 ]. Martineau et al. [ 8 ] calculated that the amounts of dry matter per mole of artificial lighting gained by lettuce grown using red (650 nm) LEDs or high-pressure sodium lamps were identical, and Chang et al . [ 10 ] calculated that the maximum photon utilization efficiency for growth of the green alga Chlamydomonas reinhardtii under red LEDs is centred at 674 nm. Lettuce grown under red LEDs presented hypocotyls and cotyledons that were elongated, a phenomenon known to be phytochrome-dependent. Under red LEDs illumination, phytochrome stimulation is especially high as far red light is not provided. Hypocotyl elongation could be prevented by adding at least 15 µmol m –2 s −1 of blue light [ 11 ]. Although a complete demonstration was not provided, one can hypothesize that the supplemented blue light activated cryptochrome, a blue-light photoreceptor that mediates reduction of hypocotyl length [ 12 ].
The efficiency of red (650–665 nm) LEDs on plant growth is easy to understand because these wavelengths perfectly fit with the absorption peak of chlorophylls [ 13 ] and phytochrome, while the supplemented blue light introduced the idea that growth under natural light could be mimicked using blue and red LEDs. In addition to providing a better excitation of the different types of photoreceptors, the blue + red combination allowed a higher photosynthetic activity than that under either monochromatic light [ 14 ]. Some authors attributed this effect to a higher nitrogen content of the blue-light-supplemented plants, whereas others suggested a better stomatal opening, thus providing more CO 2 for photosynthesis. It is well established that stomata opening is controlled by blue-light photoreceptors [ 15 ]. This is possibly reflected in the increase of shoot dry matter with increasing levels of blue light [ 16 ]. The supplementation of blue + red LEDs could also be complemented with green LEDs. Illumination with more than 50% of green LED light causes a reduction in plant growth, whereas treatments containing up to 24% green light enhanced growth for some species [ 17 ]. Recently, LEDs have been successfully tested for their ability to allow the growth of agronomically important crops, fruit and flower plants, and even trees [ 14 , 18 ]. Table 1 shows the parameter changes in selected taxa exposed to different wavelengths of LEDs compared with the other light sources.
Table 1.
The effects of LEDs on plants' growth parameters and metabolism compared with conventional lights: selected examples. HPS, high-pressure sodium; CFL, compact fluorescent light; PPFD, photosynthetic photon flux density; DW, dry weight; FW, fresh weight.
(b) Chloroplast differentiation and de-differentiation
In the absence of light or under deep shade conditions, plants develop etiolation symptoms, such as the absence of Chl, reduced leaf size and hypocotyl elongation [ 5 ]. When the plants are exposed to light, chloroplast differentiation involves the accumulation of proteins, lipids and photosynthetic pigments [ 26 ]. The kinetics of Chl accumulation present a lag phase under white LED light, which is eliminated when plants are grown under blue LED (460–475 nm) but not in red LED light (650–665 nm) [ 27 ]. Interestingly, similar Chl amounts were reached, regardless of the LED colour. In contrast to Chl, red LED-irradiated pea leaves contained higher levels of β-carotene than those grown under blue or white LED light [ 27 ]. The light intensity is also important in Chl synthesis. For instance, Tripathy & Brown [ 28 ] showed that wheat seedlings accumulated Chl under red LED light at 100 µmol m –2 s –1 , but not at 500 µmol m –2 s –1 . This inhibition of Chl accumulation under high fluence red LED light could be avoided by the supplementation of blue light (30 µmol m –2 s –1 ). Although no demonstration of the effect was provided by the authors, the absence of Chl accumulation under high fluence red light could result from a fast photodestruction of the newly formed Chl molecules [ 29 ]. Interestingly, re-etiolation provides adequate conditions for the production of white asparagus, chicory or seakale [ 30 ]. In tea leaves, the re-etiolation increases the content of volatiles (aroma), especially volatile phenylpropanoids/benzenoids and several amino acids, including l -phenylalanine [ 31 ], suggesting the activation of a plastid-located shikimate pathway [ 32 ].
(c) High fluence light-emitting diode triggers production of secondary compounds
Photosynthetic organisms exposed to high light develop short- and long-term response mechanisms to reduce stress effects. Some of these mechanisms are the specific topic of other papers included in this special issue (xanthophyll cycle [ 33 ], non-photochemical quenching [ 34 ], re-oxidation of the reduction equivalents through photorespiration, the malate valve and the action of antioxidants [ 35 ]). This section is dedicated to the metabolic shifts triggered by high light stress. They are used in repairing mechanisms [ 36 ], shielding [ 37 ], reactive oxygen species (ROS) quenching [ 37 ] or the production of storage compounds [ 38 ]. The synthesis of the metabolites takes place in plastids (terpenoids [ 38 ]) or involves them (phenylpropanoids [ 32 ]). Typical examples are medicinal plants and herbs of pharmaceutical importance such as mint ( Mentha sp.) [ 14 ] and jewel orchid ( Anoectohilus sp.) [ 39 ]. However, a decrease in secondary metabolites, flavonoids and phenolics, was also observed with increasing irradiance in the medicinal plant cat's whiskers ( Orthosiphon stamineus ) [ 40 ], indicating that the light irradiance may have negative consequences on secondary metabolite production. In higher plants, it has been documented that depending on species and growing conditions, the secondary metabolites and pigments in the flavonoid family accumulate under photoinhibitory conditions at cell level [ 41 ], although the mechanistic aspects of LED light effects are not well understood.
The high fluence effect of LED light has been studied more in photosynthetic microorganisms, partly because they present huge biotechnological and economic potential (biofuels, pharmaceuticals, food additives and cosmetics) [ 42 ]. For instance, Wang et al . [ 43 ] assessed the economic efficiency of energy converted to biomass in microalga ( Spirulina platensis ) culture under different LED monochromatic lights as grams of biomass per litre per dollar. The data showed that at the light intensity of 1500–3000 µmol m −2 s −1 , red LEDs consumed the least power and yielded the highest economic efficiency when emitted at the same intensity compared with blue LEDs (up to 110 versus lower than 10 g per litre per dollar, respectively). However, such a high fluence is not always requested. For instance, in the green microalga Dunaliella salina , light stress to drive the accumulation of β-carotene was within the range of 170–255 µmol m −2 s −1 using LEDs, whereas 1000 µmol m −2 s −1 photon flux was needed using conventional lights such as fluorescent lamps and high-pressure sodium lamps [ 44 ]. Additional red or blue (470 nm) LED light caused stress whereby the xanthophyll cycle was activated. The additional blue light was less stressful than the red light [ 45 ]. Katsuda et al . [ 46 ] reported that red LED light allowed the growth of the green alga Haematococcus pluvialis , whereas blue LED light enhanced astaxanthin production. More recently, Katsuda et al. [ 47 ] showed that in mixotrophic growing conditions, flashing LED light (8 µmol photon m −2 s −1 ) triggered similar astaxanthin concentration to continuous LED light (12 µmol photon m −2 s −1 ). Such low light requirement suggests the involvement of photoreceptors. A putative transduction mechanism of the blue light signal would involve major carotenoids in D. salina . Signalling of secondary carotenoid synthesis involves chloroplast-generated ROS [ 37 ]. Much more investigation is needed to understand the impact of LED light on primary and secondary metabolism of photosynthetic organisms.
(d) Modification of the metabolism through supplemental monochromatic lighting
The effect of supplemental blue and/or red LED light is not limited to growing and developmental properties. They also increase the antioxidant content of vegetables. For instance, red (658–660 nm) LED light increased the phenolics concentration in lettuce leaves [ 48 ] and the anthocyanin content of red cabbage leaves [ 27 ]. One can therefore imagine designing supplemental LED light treatments as pre- or post-harvesting processes to fashion raw materials. This would provide great commercial and production advantages. For instance, Colquhoun et al . [ 24 ] used LED treatment to modify the synthesis of volatile compounds in flowers and fruits. In tomato, a red LED treatment (668 nm, 50 µmol photon m −2 s −1 ) triggered a significant increase of 2-methyl-butanol and 3-methyl-1-butanol levels, whereas the amount of cis -3-hexanol was reduced when compared with the levels reached with white LED light. Because two of those three compounds are involved in the degree of tomato sweetness [ 49 ], one can hypothesize that the LED treatment will impact the taste of the fruit. The mechanism of action of the monochromatic light has not been studied as yet, but one can assume that the red light affects terpenoid production in the chloroplast through phytochrome. Alternatively, specific ROS production could have the same action as shown in the case of secondary carotenoid synthesis [ 37 ].
4. Photosynthesis in the light of future advances
Food production relies on photosynthesis. Providing sufficient quantity and quality of food for nine billion people as predicted in 2050 is especially challenging under the constraints of global climate change. Controlled-environment agriculture (CEA) technologies, including greenhouse, hydroponics, aquacultures and aeroponic systems, as well as the vertical farming possibilities, provide alternative and complementary sources for crop production, particularly in areas with limited daylight (in northern latitudes) or adverse environmental conditions (droughts, floods, storms and saline soils) or in areas with limited space, such as cities and space stations [ 1 , 7 ].
The advantages of CEA technologies, i.e. elevated crop yield per year (owing to shorter culture period under optimal environmental conditions and cultivation year round), greater growth area per m 2 (large plant density, multi-tier cultivation shelves), efficient nutrient and water use, fewer crop losses and no pesticide application, make them efficient for crop production. In addition, these technologies may produce standard high-quality horticultural products. However, in contrast to outdoor agriculture, closed and indoor plant cultivations rely on novel light sources such as LEDs capable of stimulating plant growth while drastically reducing energy consumption.
LEDs represent an innovative artificial lighting source for plants, both as supplemental or sole-source lighting, not only owing to their intensity, spectral and energy advances (see §2 and the electronic supplementary material, table S1), but also via the possibilities for targeted manipulation of metabolic responses in order to optimize plant productivity and quality. LEDs are now commercially applicable mainly for leafy greens, vegetables, herbs and pot flowers ( table 1 and the electronic supplementary material, table S2). A more complete literature was also presented in the seventh International Symposium on Light in Horticultural Systems, held in Wageningen ( http://www.actahort.org/books/956 ). The application of LEDs also has enormous potential for the processes that generate oxygen and purify water, in algal culture for producing feedstock, pharmaceuticals, fuels or dyes, and in plant tissue cultures for the micropropagation of, for example, strawberry or flowering plants [ 50 , 51 ]. Research on the effects of LEDs on primary and secondary metabolism of plants and on how the direction and mixing of LEDs influence plant responses, coupled with advances in the dynamic modification of light quantity and quality in different phases of growth may contribute to the efficient utilization of LED lighting technologies in plant cultivation in closed environments ( figure 1 ).

Trilateral connection of technological and physiological advances for improvement of plant production using LED lighting. CPPS, closed plant production systems; pLED, polymer light-emitting diode; oLED, organic light-emitting diode. (Online version in colour.)
The lighting industry needs to offer energy-efficient, ecologically sustainable lamps adapted to the changing requirements of consumers. LEDs equipped with driver chips could provide the additional benefits of operational flexibility, efficiency, reliability, controllability and intelligence for greenhouse lighting systems. However, the acceptance of solid-state LED lighting in niche applications in horticultural lighting will depend on improvements in conversion efficiency and light output per package of LED light and the cost of lumens per package. It is predicted that horticultural cultivation under controlled environmental conditions (horticulture industry) will expand in the near future, as was presented in the workshop on Challenges in Vertical Farming ( http://challengesinverticalfarming.org/ ). The new technologies provide possibilities for economically efficient consumption of light energy for horticultural cultivation of crops both on Earth and in space in the near future, and may contribute to feeding the growing human population and maintaining outdoor (principally forest) ecosystems and thus to the protection of the Earth.
Acknowledgements
The authors acknowledge their institutes, Hungarian Academy of Sciences, University of Le Mans and Isfahan University of Technology. M.R.S. also thanks the Iranian National Elites Foundation for financial support of work on LED incubator construction and cultivation of horticultural and agronomic crops illuminated with LED lights.
Funding statement
E.D. appreciates partial financial support from TÁMOP (grant no. 4.2.2A-11/1/KONV-2012-0008 ).
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
- We're Hiring!
- Help Center
Artificial Photosynthesis
- Most Cited Papers
- Most Downloaded Papers
- Newest Papers
- Save to Library
- Last »
- Humidity Follow Following
- Biogas as an alternatif energi Follow Following
- Combustion modelling Follow Following
- Electronic Transport In Molecules Follow Following
- Alternative Fuel Follow Following
- Photosynthesis Follow Following
- Electron Beam Lithography Follow Following
- Hydrogen production from renewable material Follow Following
- Biophysics Follow Following
- Self Assembly Follow Following
Enter the email address you signed up with and we'll email you a reset link.
- Academia.edu Publishing
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024

IMAGES
VIDEO
COMMENTS
A porphyrin-based photocatalyst is now shown to produce hydrogen peroxide by an unusual mechanism involving both photoexcited electrons and holes with promising efficiency. Yuanxing Fang. Xinchen ...
ConspectusSunlight is an abundant energy source for a sustainable society. Indeed, photosynthetic organisms harness solar radiation to build the world around us by synthesizing energy-rich compounds from water and CO2. However, numerous energy conversion bottlenecks in the natural system limits the overall efficiency of photosynthesis; the most efficient plants do not exceed solar storage ...
Announcing. Artificial Photosynthesis. We're excited to announce our new journal— Artificial Photosynthesis —a new addition to the ACS journal portfolio. "To ensure sustainable development, the replacement of fossil fuels with renewable energy is essential to address the environmental and ecological problems our world faces.
Artificial photosynthesis systems are proposed as an efficient alternative route to capture CO2 to produce additional food for growing global demand. Here a two-step CO2 electrolyser system was ...
Artificial photosynthesis tries to replicate natural photosynthesis by using sunlight to store energy and is an umbrella term for converting ... We hope you enjoy this particular issue and would like to invite you to submit your papers to the Photosynthesis Research journal in future. Data availability. Data available on request from the authors.
Artificial photosynthesis is a technology with immense potential that aims to emulate the natural photosynthetic process. The process of natural photosynthesis involves the conversion of solar energy into chemical energy, which is stored in organic compounds. ... A research group reported on surface-engineered PtNi-O nanoparticles with enriched ...
In this contribution, we would illustrate recent progresses on molecular systems for artificial photosynthesis by mimicking the nature's strategies. There is another research area of biohybrid systems directly using the part of nature, i.e., bacteria or enzymes, which will not be discussed in this paper. 2 Overview of Photosystem II (PSII)
Artificial photosynthesis is an attractive strategy for converting solar energy into fuels, largely because the Earth receives enough solar energy in one hour to meet humanity's energy needs for ...
Thus, the rate of energy storage averaged over a year by photosynthesis is 100 TW, representing just 0.1 per cent conversion given that solar energy arriving at our planet is at a rate of100 000 TW over the same time period. This energy is mainly stored in wood and fibres of terrestrial trees and plants.
In this Editorial, Guest Editors Holger Dau, Etsuko Fujita, and Licheng Sun introduce the Special Issue of ChemSusChem on "Artificial Photosynthesis for Sustainable Fuels". They discuss the need for non-fossil based fuels, introduce both biological and artificial photosynthesis, and outline various important concepts in artificial photosynthesis, including molecular and solid-state ...
Natural photosynthesis, the process whereby CO. is converted. into carbohydrate and water is oxidized to molecular oxygen, has. supplied the overwhelming majority of stored energy available. on ...
Therefore, research has been carried out to harvest solar energy to produce H 2 by artificial photosynthesis (Lewis & Nocera, 2006; Nocera, 2009). In artificial photosynthesis, the artificial leaf must be able to use sunlight and water to reduce CO 2 and water into H 2 ( Centi & Perathoner, 2010, 2011; Roy, Varghese, Paulose, & Grimes, 2010 ).
In this field of study, Govindjee has co-authored a number of interesting papers, such as literature reviews that highlight the significance of hydrogen production by water splitting complexes, which subsequently aid in artificial photosynthesis (Najafpour et al. 2012b), and research papers on artificial photosynthesis that discusses the ...
Lastly, perspectives on artificial photosynthesis are discussed briefly at the end. ... it is important to note that the overarching research of photosynthesis has broader impacts beyond solar energy storage. How to use the energy delivered by photons instead of heat to enable and/or power chemical reactions is interesting in its own right ...
In 2021, researchers from Toyota Central R&D Labs developed a large, cost-effective artificial photosynthesis system that produces industrial formate at a solar-to-chemical conversion efficiency ...
A Nature Catalysis study from six chemists at the University of Chicago shows an innovative new system for artificial photosynthesis that is more productive than previous artificial systems by an order of ... The co-first authors of the paper were Guangxu Lan (PhD'20, now with Peking University), graduate student Yingjie Fan, and Wenjie Shi ...
Artificial photosynthesis has rapidly developed as an actual field of research, mimicking natural photosynthesis processes in plants or bacteria to produce energy or high-value chemicals. The nanocelluloses are a family of biorenewable materials that can be engineered into nanostructures with favorable properties to serve as a host matrix for encapsulation of photoreactive moieties or cells.
2.2.2 Artificial photosynthesis. In artificial photos ynthesis (the ' artificial leaf'), su nlight is converted dir ectly into solar. fuel, without ma king use of biomass as in the production of ...
Invited and encouraged by the Frontiers Editorial Team, the current Research Topic, "Challenges in photosynthesis: From natural to artificial," Volume II, was organized with the goal to provide an update on natural and artificial photosynthesis from 2014 through 2022. Due to the extremely tight time frame and other unexpected reasons, many ...
The open-access paper, "A hybrid inorganic-biological artificial photosynthesis system for energy-efficient food production," is available here. The research was supported by the Translational Research Institute for Space Health (TRISH) through NASA (NNX16AO69A), Foundation for Food and Agriculture Research (FFAR), the Link Foundation ...
Artificial light sources for photosynthesis. Artificial lighting should provide plants with energy and information required for development. ... Some of these mechanisms are the specific topic of other papers included in this special issue (xanthophyll cycle , ... a review of research at Kennedy Space Center. Acta Hort. 711, 111-119.
Artificial photosynthesis is a technology with immense potential that aims to emulate the natural photosynthetic process. The process of natural photosynthesis involves the conversion of solar energy into chemical energy, which is stored in organic compounds. ... Feature papers represent the most advanced research with significant potential for ...
This work asks how light harvesting in photosynthetic systems can be optimised for economically scalable, sustainable energy production. Hierarchy theory is introduced as a system-analysis and optimisation tool better able to handle multiscale, multiprocess complexities in photosynthetic energetics compared with standard linear-process analysis.