Numbers, Facts and Trends Shaping Your World
Read our research on:
Full Topic List

Regions & Countries
- Publications
- Our Methods
- Short Reads
- Tools & Resources
Read Our Research On:
The Case Against Embryonic Stem Cell Research: An Interview with Yuval Levin
Scientists largely agree that stem cells may hold a key to the treatment, and even cure, of many serious medical conditions. But while the use of adult stem cells is widely accepted, many religious groups and others oppose stem cell research involving the use and destruction of human embryos. At the same time, many scientists say that embryonic stem cell research is necessary to unlock the promise of stem cell therapies since embryonic stem cells can develop into any cell type in the human body.
In late 2007, researchers in the United States and Japan succeeded in reprogramming adult skin cells to act like embryonic stem cells. The new development offers the possibility that the controversy over the use of embryos could end. But many scientists and supporters of embryonic stem cell research caution that this advance has not eliminated the need for embryos, at least for the time being.
Recently, the Pew Forum sat down with Yuval Levin, author of Tyranny of Reason , to discuss the ethical and moral grounds for opposing embryonic stem cell research. Previously, Levin was the executive director of the President’s Council on Bioethics. Currently, he is the Hertog Fellow at the Ethics and Public Policy Center in Washington, D.C., where he also directs the center’s Bioethics and American Democracy program.
A counterargument explaining the case for embryonic stem cell research is made by Jonathan Moreno, a professor at the University of Pennsylvania and a senior fellow at the Center for American Progress in Washington, D.C.
Featuring : Yuval Levin , Hertog Fellow and Director of the Bioethics and American Democracy Program, Ethics and Public Policy Center
Interviewer: David Masci , Senior Research Fellow, Pew Forum on Religion & Public Life
Question & Answer
Recently, researchers in the United States and Japan successfully turned human skin cells into cells that behave like embryonic stem cells. There has been some discussion that this advance makes the moral and ethical debate over embryonic stem cells moot. Do you think that’s an accurate assessment?
I think it’s going to take a while for the ethical debate to catch up with the science. The scientific community has reacted very positively to this advancement, which was made in November 2007. There have been many additional scientific studies published on the topic since then, and it appears increasingly likely that the cells produced using skin cells are the equivalent of embryonic stem cells. So I think that, in time, this probably will be the final chapter of this particular debate about embryonic stem cells, but I don’t think we’re at the end of it quite yet.
Do you agree with Professor James Thomson, who led the American research team that made this breakthrough, when he maintains that this advance does not, for the time being, abrogate the need for embryonic stem cell research?
Part of his argument for continuing to use embryonic stem cells was backward-looking to make the point that researchers wouldn’t have been able to develop this technique if they hadn’t been doing embryonic stem cell research. I think that’s true, although in a certain way it actually vindicates the logic of President Bush’s stem cell policy, which is to allow some work to be done – without creating an incentive for the destruction of further embryos – to advance the basic science in these kinds of directions.
Thomson also argued that there will still be a need to use embryos in the future. I think that’s also a fair argument in the sense that there are always interesting things to learn from different kinds of experiments, but it doesn’t address the ethical issues surrounding the debate. If there were no ethical concerns, then certainly the new development wouldn’t mean embryonic research would become totally useless. But given that there are concerns, the case for destroying embryos does become a lot weaker. For some people, myself included, the ethical concerns are matters of principle and don’t change with new developments.
But for a lot of people, the stem cell debate has always been a matter of balance. People are aware that there are ethical concerns and that there is enormous scientific promise. Now the debate is: Given the ethical questions at stake, is the scientific promise sufficient to make us put the ethical concerns aside and support the research? I think that balance has changed because of this advance, and having an alternative to embryonic stem cell research that achieves the same result will obviously affect the way people think about the ethics of this issue.
That doesn’t mean the scientists no longer have any use for embryonic stem cells or even that they won’t have any use for them. But I do think it means that people are going to change the way they reason about the balance between science and ethics because of this advance.
I know that you believe that human embryos have intrinsic worth. Do you believe that they have the same intrinsic worth as a five-year-old child or a 50-year-old man?
The question of intrinsic worth is complicated. I don’t think it is right to try to determine an embryo’s intrinsic worth by debating when human life begins. The question of when life begins is a biological question, and the answer actually is fairly straightforward: The life of an organism begins at conception. The ethical question, however, is not about when a life begins but whether every life is equal, and that’s a very different question.
I think that the embryonic stem cell debate is ultimately about the question of human equality. The United States has had one answer to that question written in its “birth certificate” – the Declaration of Independence – which states that “all men are created equal.” I think that examining this principle of human equality provides the right answer to this debate, but it is not a simple answer. Human equality doesn’t mean that every person is the same or that every person can even be valued in the same way on every scale. What it means is that our common humanity is something that we all share. And what that means, in turn, is that we can’t treat a human being in certain ways that we might non-human beings.
The protection of human life comes first. And to the extent that the debate is about whether it is acceptable to destroy a living human being for the purpose of science – even for the purpose of helping other human beings – I think that in that sense, the embryo is our equal. That doesn’t mean that I would think of an embryo in the same way that I would think of a three-year-old child, but I would reject a technique that uses either of them for scientific experimentation.
So in other words, even though you would grieve the death of a 50-year-old man more than a five-day-old embryo, on at least the most basic level you believe that they both have the same right to life.
Yes, that’s right. And right to life derives from human equality. The right to life is, in a way, drawn out of the political vocabulary of the Declaration of Independence. And so, to my mind, the argument at the heart of the embryonic stem cell debate is the argument about human equality.
Recently in The New Republic magazine, Harvard psychologist Steven Pinker wrote that conservative bioethicists like yourself consistently predict the worst when looking at developments in biotechnology. He went on to say that had there been a president’s council on cyber-ethics in the 1960s, “no doubt it would have decried the threat of the Internet since it would inexorably lead to 1984 or computers ‘taking over’ like HAL in 2001 .” How do you respond to this suggestion that there always seems to be this sort of chorus of doomsayers every time something new comes along?
To my mind, biotechnology is fundamentally different from past developments in technology because it’s directed to the human person. From the beginning of the scientific revolution, science and technology have tried to allow us to manipulate and shape the world around us for the benefit of man. Now that we’re beginning to manipulate and shape man, the question is: For the benefit of what? In some cases that’s easy to see. Obviously curing disease is more of an “old-fashioned” scientific pursuit. But there are newer scientific developments, such as certain types of human enhancement technologies that raise very complicated questions of how we should judge the ends and the means of technological advancements. That being said, Pinker has a point, in a larger sense – that judging the risks of new technologies is very difficult. In general, I think we ought to give the benefit of the doubt to our ability to use new technologies. I don’t think that we should assume that the worst will happen. But there are specific instances, which are few but very important, when we do need to be cautious.
Let’s shift gears to a question about religion and faith. Obviously there are people of faith on both sides of this debate. In fact, there are conservatives – traditional social conservatives, such as Republican Sen. Orrin Hatch of Utah – who support embryonic stem cell research. But could you explain how the Judeo-Christian and Western moral ethic informs your views on this issue and why you think that God is ultimately on your side?
Well, I don’t know that I think that. My approach to this is not religious. I’m not a particularly religious person and I come at this from more of a liberal democratic concern for human equality and the foundations of our society. That being said, those foundations are not utterly secular, and my understanding of them is not utterly secular. I think that to believe in human equality you do have to have some sense of a transcendent standard by which to make that judgment. In other words, when we talk about equality, what do we mean? Equal in relation to what?
Some people have certainly tried to make a purely secular liberal argument for human equality. While I think it’s very hard to ground a genuine, deep belief in human equality in a worldview that sees nothing above the material, I don’t think that that belief depends on specific theological commitments. To my mind, it’s an American belief more than it is a religious belief.
Certainly I think that President Bush’s commitment to human equality has a lot to do with a particular Christian sense of human worth and human value. But I don’t think that it’s necessary to ground yourself in a particular theological or sectarian preference. I think that this is really about whether we believe in a liberal society, which comes from a belief in human equality. The American left, which for the most part is on the other side of this debate from where I am, has always been the champion of human equality, and I think that it’s a question that they have to really think about.
The Pew Forum and the Pew Research Center for the People & the Press have done polling on this issue over the last six or seven years and have found that Americans generally favor embryonic stem cell research. Why do you think this has happened, and what do you think this trend indicates?
That’s an interesting question. We actually did a poll here at the Ethics and Public Policy Center in February on a similar question, and the lesson I drew from that, and from some other polling that’s been done, is that on the stem cell debate, people are just very confused about the facts, and the trend lines have generally followed the sense that cures are coming. In the end, the issue has been misrepresented as a choice between cures and Christianity, and people increasingly think that curing people like Christopher Reeve is just as much of a human good as protecting an embryo that they can’t even imagine.
But when you dig down into people’s views about stem cell research, you find a great deal of confusion, and when you put the questions in ethical terms, you find small majorities opposing it. When you put the question in medical terms, you find, I think, somewhat larger majorities supporting it. In our poll, we asked the same people a series of questions that basically put the same issue in several different ways, and their responses are total opposites of one another. The fact that the same people come out on the opposite sides of the same issue when it’s put in different ways suggests to me that the issue is very hard to understand – which it is.
Frequently one hears that, ultimately, you can’t stop science or “progress” and that ethical, moral and religious objections inevitably will fall by the wayside when there are clear material gains to be made. Do you think that’s the most likely scenario in this case, assuming the scientific community continues to see a need for embryonic stem cell research?
Well, that’s the big assumption, right? To my mind, the aim of people such as myself has always been to find ways of doing the science without violating the ethics rather than to force a choice between the science and the ethics. If we force that choice, I think it’s more likely that the country would choose science over ethics, and that’s exactly why we have to avoid the choice. I don’t think we should be overconfident in our ability to persuade people to pass up a material benefit for an ethical principle, although I hope that can be done in the stem cell research debate. It certainly has been done in some instances when the principle was more evident and more obvious – such as imposing limits on human subject research.
Again, the aim from my point of view – and from a lot of people on my side of this argument – has been to find ways to advance the science without violating the ethics. That’s the logic of President Bush’s stem cell policy; that’s why people have been pushing for alternatives; that’s why they’re encouraging the development of these latest alternatives – to avoid the choice, not to force the choice. I think that’s the best thing for the country, from everybody’s point of view. You don’t want a situation where you’ve got sort of red-state medicine and blue-state medicine and people believe that the treatment their hospital is giving them is obtained in unethical ways. That would begin to break up the practice of medicine and to affect our attitudes about science – which on the whole has done a tremendous amount of good for society. So I think what everybody should aim for is finding a way to end this potentially very damaging debate rather than force a choice.
This transcript has been edited for clarity, spelling and grammar.
Sign up for our weekly newsletter
Fresh data delivery Saturday mornings
Sign up for The Briefing
Weekly updates on the world of news & information
Most Popular
1615 L St. NW, Suite 800 Washington, DC 20036 USA (+1) 202-419-4300 | Main (+1) 202-857-8562 | Fax (+1) 202-419-4372 | Media Inquiries
Research Topics
- Age & Generations
- Coronavirus (COVID-19)
- Economy & Work
- Family & Relationships
- Gender & LGBTQ
- Immigration & Migration
- International Affairs
- Internet & Technology
- Methodological Research
- News Habits & Media
- Non-U.S. Governments
- Other Topics
- Politics & Policy
- Race & Ethnicity
- Email Newsletters
ABOUT PEW RESEARCH CENTER Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
Copyright 2024 Pew Research Center
Terms & Conditions
Privacy Policy
Cookie Settings
Reprints, Permissions & Use Policy
Pros & Cons of Embryonic Stem Cell Research
- Liberal Voices and Events
- The U. S. Government
- U.S. Foreign Policy
- U.S. Conservative Politics
- Women's Issues
- Civil Liberties
- The Middle East
- Race Relations
- Immigration
- Crime & Punishment
- Canadian Government
- Understanding Types of Government
- M.B.A., California State University, Long Beach
- B.A., Journalism and Nonfiction Writing, University of California, Los Angeles
On March 9, 2009, President Barack Obama lifted, by Executive Order , the Bush administration's eight-year ban on federal funding of embryonic stem cell research .
Remarked the President, "Today... we will bring the change that so many scientists and researchers, doctors and innovators, patients and loved ones have hoped for, and fought for, these past eight years."
In Obama's Remarks on Lifting the Embryonic Stem Cell Research Ban, he also signed a Presidential Memorandum directing the development of a strategy for restoring scientific integrity to government decision-making.
Bush Vetoes
In 2005, H.R. 810, the Stem Cell Research Enhancement Act of 2005, was passed by the Republican-led House in May 2005 by a vote of 238 to 194. The Senate passed the bill in July 2006 by a bipartisan vote of 63 to 37.
President Bush opposed embryonic stem cell research on ideological grounds. He exercised his first presidential veto on July 19, 2006, when he refused to allow H.R. 810 to become law. Congress was unable to muster enough votes to override the veto.
In April 2007, the Democratic-led Senate passed the Stem Cell Research Enhancement Act of 2007 by a vote of 63 to 34. In June 2007, the House passed the legislation by a vote of 247 to 176.
President Bush vetoed the bill on June 20, 2007.
Public Support for Embryonic Stem Cell Research
For years, all polls report that the American public STRONGLY supports federal funding of embryonic stem cell research.
Reported the Washington Post in March 2009 : "In a January Washington Post-ABC News poll, 59 percent of Americans said they supported loosening the current restrictions, with support topping 60 percent among both Democrats and independents. Most Republicans, however, stood in opposition (55 percent opposed; 40 percent in support)."
Despite public perceptions, embryonic stem cell research was legal in the U.S. during the Bush administration: the President had banned the use of federal funds for research. He did not ban private and state research funding, much of which was being conducted by pharmaceutical mega-corporations.
In Fall 2004, California voters approved a $3 billion bond to fund embryonic stem cell research. In contrast, embryonic stem cell research is prohibited in Arkansas, Iowa, North and South Dakota and Michigan.
Developments in Stem Cell Research
In August 2005, Harvard University scientists announced a breakthrough discovery that fuses "blank" embryonic stem cells with adult skin cells, rather than with fertilized embryos, to create all-purpose stem cells viable to treat diseases and disabilities.
This discovery doesn't result in the death of fertilized human embryos and thus would effectively respond to pro-life objections to embryonic stem cell research and therapy.
Harvard researchers warned that it could take up to ten years to perfect this highly promising process.
As South Korea, Great Britain, Japan, Germany, India and other countries rapidly pioneer this new technological frontier, the US is being left farther and farther behind in medical technology. The US is also losing out on billions in new economic opportunities at a time when the country sorely needs new sources of revenues.
Therapeutic cloning is a method to produce stem cell lines that were genetic matches for adults and children.
Steps in therapeutic cloning are:
- An egg is obtained from a human donor.
- The nucleus (DNA) is removed from the egg.
- Skin cells are taken from the patient.
- The nucleus (DNA) is removed from a skin cell.
- A skin cell nucleus is implanted in the egg.
- The reconstructed egg, called a blastocyst, is stimulated with chemicals or electric current.
- In 3 to 5 days, the embryonic stem cells are removed.
- The blastocyst is destroyed.
- Stem cells can be used to generate an organ or tissue that is a genetic match to the skin cell donor.
The first 6 steps are same for reproductive cloning . However, instead of removing stem cells, the blastocyst is implanted in a woman and allowed to gestate to birth. Reproductive cloning is outlawed in most countries.
Before Bush stopped federal research in 2001, a minor amount of embryonic stem cell research was performed by US scientists using embryos created at fertility clinics and donated by couples who no longer needed them. The pending bipartisan Congressional bills all propose using excess fertility clinic embryos.
Stem cells are found in limited quantities in every human body and can be extracted from adult tissue with great effort but without harm. The consensus among researchers has been that adult stem cells are limited in usefulness because they can be used to produce only a few of the 220 types of cells found in the human body. However, evidence has recently emerged that adult cells may be more flexible than previously believed.
Embryonic stem cells are blank cells that have not yet been categorized or programmed by the body and can be prompted to generate any of the 220 human cell types. Embryonic stem cells are extremely flexible.
Embryonic stem cells are thought by most scientists and researchers to hold potential cures for spinal cord injuries, multiple sclerosis, diabetes, Parkinson's disease, cancer, Alzheimer's disease, heart disease, hundreds of rare immune system and genetic disorders and much more.
Scientists see almost infinite value in the use of embryonic stem cell research to understand human development and the growth and treatment of diseases.
Actual cures are many years away, though, since research has not progressed to the point where even one cure has yet been generated by embryonic stem cell research.
Over 100 million Americans suffer from diseases that eventually may be treated more effectively or even cured with embryonic stem cell therapy. Some researchers regard this as the greatest potential for the alleviation of human suffering since the advent of antibiotics.
Many pro-lifers believe that the proper moral and religious course of action is to save existing life through embryonic stem cell therapy.
Some staunch pro-lifers and most pro-life organizations regard the destruction of the blastocyst, which is a laboratory-fertilized human egg, to be the murder of human life. They believe that life begins at conception, and that destruction of this pre-born life is morally unacceptable.
They believe that it is immoral to destroy a few-days-old human embryo, even to save or reduce suffering in existing human life.
Many also believe that insufficient attention been given to explore the potential of adult stem cells, which have already been used to successfully cure many diseases. They also argue that too little attention has been paid to the potential of umbilical cord blood for stem cell research. They also point out that no cures have yet been produced by embryonic stem cell therapy.
At every step of the embryonic stem cell therapy process, decisions are made by scientists, researchers, medical professionals and women who donate eggs...decisions that are fraught with serious ethical and moral implications. Those against embryonic stem cell research argue that funding should be used to greatly expand adult stem research, to circumvent the many moral issues involving the use of human embryos.
Lifting the Ban
Now that President Obama has lifted the federal funding ban for embryonic stem cell research, financial support will soon flow to federal and state agencies to commence the necessary scientific research. The timeline for therapeutic solutions available to all Americans could be years away.
President Obama observed on March 9, 2009, when he lifted the ban:
"Medical miracles do not happen simply by accident. They result from painstaking and costly research, from years of lonely trial and error, much of which never bears fruit, and from a government willing to support that work...
"Ultimately, I cannot guarantee that we will find the treatments and cures we seek. No President can promise that.
"But I can promise that we will seek them -- actively, responsibly, and with the urgency required to make up for lost ground."
- The Obama Administration's Animal Protection Record, 2010-2011
- President Obama's Domestic Agenda
- Pros and Cons of Government Healthcare
- History of the North American Free Trade Agreements
- Pros & Cons of the Death Penalty
- 5 Reasons Why Obama Won the 2008 U.S. Presidential Election
- Biography of Joe Biden, 46th President of the United States
- What Are U.S. Farm Subsidies?
- Congress Members Who Voted Against the 2002 Iraq War
- Biography of Julián Castro, 2020 Presidential Candidate
- The Top 3 Arguments for Gun Control
- Biography of Elizabeth Warren, Senator and Scholar
- Biography of Ross Perot, Third-Party Presidential Candidate
- Top 10 Must-Reads for Liberals
- New Challenges to the Death Penalty
- Alexandria Ocasio-Cortez Biography
- Search Menu
- Advance articles
- Editor's Choice
- ESHRE Pages
- Mini-reviews
- Author Guidelines
- Submission Site
- Reasons to Publish
- Open Access
- Advertising and Corporate Services
- Advertising
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- Journals Career Network
- About Human Reproduction
- About the European Society of Human Reproduction and Embryology
- Editorial Board
- Self-Archiving Policy
- Dispatch Dates
- Contact ESHRE
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Introduction, what are (embryonic) stem cells, potential applications of hes cells and state‐of‐the‐art, ethical exploration, the status of hes cells, instrumental use of embryos, ethics of using surplus ivf embryos as a source of hes cells, therapeutic cloning, conclusions and recommendations, acknowledgements.
- < Previous
Human embryonic stem cells: research, ethics and policy
- Article contents
- Figures & tables
- Supplementary Data
Guido de Wert, Christine Mummery, Human embryonic stem cells: research, ethics and policy, Human Reproduction , Volume 18, Issue 4, April 2003, Pages 672–682, https://doi.org/10.1093/humrep/deg143
- Permissions Icon Permissions
The use of human embryos for research on embryonic stem (ES) cells is currently high on the ethical and political agenda in many countries. Despite the potential benefit of using human ES cells in the treatment of disease, their use remains controversial because of their derivation from early embryos. Here, we address some of the ethical issues surrounding the use of human embryos and human ES cells in the context of state‐of‐the‐art research on the development of stem cell based transplantation therapy.
Human embryonic stem cells (hES cells) are currently discussed not only by the biologists by whom they were discovered but also by the medical profession, media, ethicists, governments and politicians. There are several reasons for this. On the one hand, these ‘super cells’ have a major clinical potential in tissue repair, with their proponents believing that they represent the future relief or cure of a wide range of common disabilities; replacement of defective cells in a patient by transplantation of hES cell‐derived equivalents would restore normal function. On the other hand, the use of hES cells is highly controversial because they are derived from human pre‐implantation embryos. To date, most embryos used for the establishment of hES cell lines have been spare embryos from IVF, but the creation of embryos specifically for deriving hES cells is also under discussion. The most controversial variant of this is the transfer of a somatic cell‐nucleus from a patient to an enucleated oocyte (unfertilized egg) in order to produce hES cells genetically identical to that patient for ‘autologous’ transplantation (so‐called ‘therapeutic’ cloning); this may prevent tissue rejection.
The question ‘Can these cells be isolated and used and, if so, under what conditions and restrictions’ is presently high on the political and ethical agenda, with policies and legislation being formulated in many countries to regulate their derivation. The UK has been the first to pass a law governing the use of human embryos for stem cell research. The European Science Foundation has established a committee to make an inventory of the positions taken by governments of countries within Europe on this issue ( European Science Foundation, 2001 ).
In order to discuss the moral aspects of the isolation and use of hES cells, which is the aim of the present article, it is first essential to understand exactly what these cells are, where they come from, their intended applications and to define the ethical questions to be addressed.
‘Stem cells’ are primitive cells with the capacity to divide and give rise to more identical stem cells or to specialize and form specific cells of somatic tissues. Broadly speaking, two types of stem cell can be distinguished: embryonic stem (ES) cells which can only be derived from pre‐implantation embryos and have a proven ability to form cells of all tissues of the adult organism (termed ‘pluripotent’), and ‘adult’ stem cells, which are found in a variety of tissues in the fetus and after birth and are, under normal conditions, more specialized (‘multipotent’) with an important function in tissue replacement and repair.
hES cells are derived from the so‐called ‘inner cell mass’ of blastocyst stage embryos that develop in culture within 5 days of fertilization of the oocyte ( Thomson et al ., 1998 ; Reubinoff et al ., 2000 ). Although hES cells can form all somatic tissues, they cannot form all of the other ‘extraembryonic’ tissues necessary for complete development, such as the placenta and membranes, so that they cannot give rise to a complete new individual. They are therefore distinct from the ‘totipotent’ fertilized oocyte and blastomere cells deriving from the first cleavage divisions. hES cells are also immortal, expressing high levels of a gene called telomerase, the protein product of which ensures that the telomere ends of the chromosomes are retained at each cell division and the cells do not undergo senescence. The only other cells with proven pluripotency similar to that of ES cells are embryonic germ (EG) cells, which as their name implies, have been derived from ‘primordial germ cells’ that would ultimately form the gametes if the fetus had not been aborted. In humans, hEG cells were first established in culture in 1998, shortly after the first hES cells, from tissue derived from an aborted fetus ( Shamblott et al ., 1998 ). Biologically, hEG cells have many properties in common with hES cells ( Shamblott et al ., 2001 ).
In the adult individual, a variety of tissues have also been found to harbour stem cell populations. Examples include the brain, skeletal muscle, bone marrow and umbilical cord blood, although the heart, by contrast, contains no stem cells after birth (reviewed in McKay 1997 ; Fuchs and Segre, 2000 ; Watt and Hogan, 2000 ; Weissman et al ., 2000 ; Blau et al ., 2001 ; Spradling et al ., 2001 ). These adult stem cells have generally been regarded as having the capacity to form only the cell types of the organ in which they are found, but recently they have been shown to exhibit an unexpected versatility ( Ferrari et al ., 1998 ; Bjornson et al ., 1999 ; Petersen et al ., 1999 ; Pittenger et al ., 1999 ; Brazelton et al ., 2000 ; Clarke et al ., 2000 ; Galli et al ., 2000 ; Lagasse et al ., 2000 ; Mezey et al ., 2000 ; Sanchez‐Ramos et al ., 2000 ; Anderson et al ., 2001 ; Jackson et al ., 2001 ; Orlic et al ., 2001 ). Evidence is strongest in animal experiments, but is increasing in humans, that adult stem cells originating in one germ layer can form a variety of other derivatives of the same germ layer (e.g. bone marrow‐to‐muscle within the mesodermal lineage), as well as transdifferentiate to derivatives of other germ layers (e.g. bone marrow‐to‐brain between the mesodermal and ectodermal lineages). To what extent transdifferentiated cells are immortal or acquire appropriate function in host tissue remains largely to be established but advances in this area are rapid, particularly for multipotent adult progenitor cells (MAPCs) of bone marrow ( Reyes and Verfaillie, 2001 ). Answers to these questions with respect to MAPCs, in particular whether they represent biological equivalents to hES and can likewise be expanded indefinitely whilst retaining their differentiation potential, are currently being addressed ( Jiang et al . 2002 ; Schwartz et al ., 2002 ; Verfaillie, 2002 ; Zhao et al ., 2002 ). For other adult stem cell types, such as those from brain, skin or intestine ( Fuchs and Segre, 2000 ), this may remain unclear for the immediate future. Although the discussion here concerns hES cells and the use of embryos, the scientific state‐of‐the‐art on other types of stem cell is important in the context of the ‘subsidiarity principle’ (see below).
In theory, hES cells could be used for many different purposes ( Keller and Snodgrass, 1999 ). Examples in fundamental research on early human development are the causes of early pregnancy loss, aspects of embryonic ageing and the failure of pregnancy in older women (where genetic defects in the oocyte appear to be important). A second category might be toxicology, more specifically research on possible toxic effects of new drugs on early embryonic cells which are often more sensitive than adult cells (drug screening). The most important potential use of hES cells is, however, clinically in transplantation medicine, where they could be used to develop cell replacement therapies. This, according to most researchers in the field represents the real ‘home run’ and it is the ethics of using embryos in this aspect of medicine that will be discussed here. Examples of diseases caused by the loss, or loss of function, of only one or a limited number of cell types and which could benefit from hES cell‐based therapies include diabetes, Parkinson’s disease, stroke, arthritis, multiple sclerosis, heart failure and spinal cord lesions. Although it is known that hES cells are capable of generating neural, cardiac, skeletal muscle, pancreas and liver cells in teratocarcinomas in vivo in immunodeficient mice as well as in tissue culture, it would be an illusion to consider that cell‐therapies will have widespread application in the short term (i.e. within a couple of years). It is unfortunate that sensational treatment in the media, which implied the generation of whole organs from hES cells, initially left this impression so that the more realistic view emerging is already a disappointment to some patient groups. Nonetheless, a proper scientific evaluation of the therapeutic potential is being carried out in countries that allow the isolation and/or use of existing hES cells. The ethical questions here then also include whether the establishment of new hES cell lines can be justified, in the realisation that eventual therapies, based on either hES or adult stem cells are long‐term perspectives.
There are, at least in theory, various sources of hES cells. In most cases to date, these have been spare IVF embryos, although IVF embryos have been specifically created for the purpose of stem cell isolation ( Lanzendorf et al ., 2001 ). In one variant of ‘embryo creation’, it has even been reported that normally organized blastocysts develop from chimeras of two morphologically non‐viable embryos ( Alikani and Willadsen, 2002 ). The most revolutionary option would be the creation of embryos specifically for the purpose of isolating stem cells via ‘nuclear transfer’ (‘therapeutic cloning’). This option is purported to be the optimal medical use of hES technology since the nuclear DNA of the cells is derived from a somatic cell of a patient to receive the transplant, reducing the chances of tissue rejection (see Barrientos et al ., 1998 ; 2000). It is of note that the oocyte in this case is not fertilized, but receives maternal and paternal genomes from the donor cell nucleus. Since by some definitions an embryo is the result of fertilization of an oocyte by sperm, there is no absolute consensus that nuclear transfer gives rise to an embryo (see below).
The establishment of embryonic cell lines is becoming increasingly efficient, with up to 50% of spare IVF embryos that develop into blastocysts after thawing at the 8‐cell stage reported to yield cell lines. There are reports of efficiencies much lower than 50%, however, the quality of the donated embryos being an important determinant of success. Growth of the cell lines over extended periods and in some cases under defined conditions ( Xu et al ., 2001 ) has also been reported, but the controlled expansion and differentiation to specific cell types is an area where considerable research will be required before cell transplantation becomes clinical practice (for review, see Passier and Mummery, 2003 ). In addition, research will be required on how to deliver cells to the appropriate site in the patient to ensure that they survive, integrate in the host tissue and adopt appropriate function. These are the current scientific challenges that will have to be overcome before cell therapy becomes clinical practice; the problems are common to both hES and adult stem cells. The efficiency of establishing embryonic stem cell lines from nuclear transfer embryos is currently unknown, but expected to be lower than from IVF embryos.
In the following section, the status of hES cells is first considered. The questions of whether it is acceptable to use pre‐implantation embryos as a source of ES cells for research on cell transplantation therapy and if so, whether embryo use should be limited to spare embryos or may also include the creation of embryos via nuclear transfer (‘therapeutic cloning’), are then addressed.
What is the ontological status of hES cells? Should they be considered equivalent to embryos or not? Let us first consider the status of the ‘naked’, isolated inner cell mass (ICM; the source for deriving hES cell lines). The ICM is as it were the ‘essence’ of the pre‐implantation embryo, the precursor of the ‘embryo proper’. The isolated ICM, however, no longer has the potential to develop into a fetus and child, as trophoblast cells, necessary for implantation and nourishment of the embryo, and extra‐embryonic endoderm, are absent. It does not necessarily follow, though, that the isolated ICM is no longer an embryo—we suggest that the whole, isolated ICM could best be qualified as a disabled, ‘non‐viable’ embryo (even though it might, at least in theory, be ‘rescued’ by enveloping the ICM with sufficient trophoblast cells).
What, then, is the status of the individual cells from the ICM once isolated, and the embryonic stem cell lines derived from them? Should we consider these cells/cell lines to be non‐viable embryos too? We would argue that when the cells of the ICM begin to spread and grow in culture, the ICM disintegrates and the non‐viable embryo perishes. Some might argue that hES cells are embryos, because, although hES cells in themselves cannot develop into a human being, they might if they were ‘built into’ a cellular background able to make extra‐embryonic tissues necessary for implantation and nutrition of the embryo. At present this is only possible by ‘embryo reconstruction’ in which the ICM of an existing embryo is replaced by ES cells ( Nagy et al ., 1993 ). Commentators who, against this background, regard hES cells as equivalent to embryos, apparently take recourse to the opinion that any cell from which a human being could in principle be created, even when high technology (micromanipulation) would be required to achieve this, should be regarded as an embryo. An absurd implication of this ‘inclusive’ definition of an embryo is that one should then also regard all somatic cells as equivalent to embryos—after all, a somatic nucleus may become an embryo after nuclear transplantation in an enucleated oocyte. It is therefore unreasonable to regard hES cells as equivalent to embryos.
Research into the development of cell‐replacement therapy requires the instrumental use of pre‐implantation embryos from which hES cells are derived since current technology requires lysis of the trophectoderm and culture of the ICM; the embryo disintegrates and is thus destroyed. As has already been discussed extensively in the embryo‐research debate, considerable differences of opinion exist with regard to the ontological and moral status of the pre‐implantation embryo ( Hursthouse, 1987 ). On one side of the spectrum are the ‘conceptionalist’ view (‘the embryo is a person’) and the ‘strong’ version of the potentiality‐argument (‘because of the potential of the embryo to develop into a person, it ought to be considered as a person’). On the other side of the spectrum we find the view that the embryo (and even the fetus) as a ‘non‐person’ ought not to be attributed any moral status at all. Between these extremes are various intermediates. Here, there is a kind of ‘overlapping consensus’: the embryo has a real, but relatively low moral value. The most important arguments are the moderate version of the potentiality argument (‘the embryo deserves some protection because of its potential to become a person’) and the argument concerning the symbolic value of the embryo (the embryo deserves to be treated with respect because it represents the beginning of human life). Differences of opinion exist on the weight of these arguments (how much protection does the embryo deserve?) and their extent (do they apply to pre‐implantation embryos?). In view of the fact that up to 14 days of development, before the primitive streak develops and three germ layers appear, embryos can split and give rise to twins or two embryos may fuse into one, it may reasonably be argued that at these early stages there is in principle no ontological individuality; this limits the moral value of an embryo.
Pre‐implantation embryos are generally regarded from the ethical point of view as representing a single class, whereas in fact ∼50–60% of these embryos are aneuploid and mostly non‐viable. For non‐viable embryos, the argument of potentiality does not of course apply. Their moral status is thus only based on their symbolic value, which is already low in ‘pre‐individualized’ pre‐implantation embryos. The precise implications of this moral difference for the regulation of the instrumental use of embryos is, however, beyond the scope of the present article.
The view that research with pre‐implantation embryos should be categorically forbidden is based on shaky premises and would be difficult to reconcile with the wide social acceptance of contraceptive intrauterine devices. The dominant view in ethics is that the instrumental use of pre‐implantation embryos, in the light of their relative moral value, can be justified under certain conditions. The international debate focuses on defining these conditions.
Possible objections are connected to the principle of proportionality, the slippery slope argument, and the principle of subsidiarity.
Proportionality
It is generally agreed that research involving embryos should be related to an important goal, sometimes formulated as ‘an important health interest’ (the principle of proportionality). Opinions differ on how this should be interpreted and made operational. In a number of countries, research on pre‐implantation embryos is permitted provided it is related to human reproduction. Internationally, however, such a limitation is being increasingly regarded as too restrictive ( De Wert et al ., 2002 ). The isolation of hES cells for research into cell‐replacement therapies operates as a catalyst for this discussion. It is difficult to argue that research into hES cells is disproportional. If embryos may be used for research into the causes or treatment of infertility, then it is inconsistent to reject research into the possible treatment of serious invalidating diseases as being not sufficiently important. The British Nuffield Council on Bioethics ( Nuffield Council on Bioethics, 2000 ) also saw no reason for making a moral distinction between research into diagnostic methods or reproduction and research into potential cell therapies.
Even if one argued that there is a difference between the two types of research, research on cell therapy would, if anything, be more defensible than research on reproduction. One (in our opinion somewhat dubious) argument is to be found in McGee and Caplan (1999 ); here the suggestion is made that in using embryos for cell therapy, no embryos are actually sacrificed: ‘In the case of embryos already slated to be discarded after IVF, the use of stem cells may actually lend permanence to the embryo. Our point here is that the sacrifice of an early human embryo, whether it involves a human person or not, is not the same as the sacrifice of an adult because life of a 100‐cell embryo is contained in its cells nuclear DNA.’ In other words, the unique characteristic of an embryo is its DNA; by transplanting cells containing this DNA to a new individual, the DNA is preserved and the embryo therefore not sacrificed—a ‘win–win’ situation for both the embryo and cell transplant recipient. The implication is thus that the use of embryos for cell transplantation purposes is ethically preferable to disposing of them or using them in other (‘truly destructive’) types of research. This extreme genetic ‘reductionism’ is highly disputable and not convincing: the fact that embryos are actually sacrificed in research into cell therapy is masked. A second, more convincing, argument, that the instrumental use of embryos is in principle easier to justify for isolation of hES cells than, for example, research directed towards improving IVF, is that it has potentially far wider clinical implications. It therefore, unquestionably meets the proportionality requirement.
Slippery slope
The slippery slope argument can be considered as having two variants, one empirical and the other logical. The empirical version involves a prediction of the future: ‘Acceptance of practice X will inevitably lead to acceptance of (undesirable) practice Y. To prevent Y, X must be banned’. The logical version concerns the presumed logical implications resulting from the moral justification of X: ‘Justification of X automatically implies acceptance of (undesirable) practice Y’. In this context the problem often lies in the lack of precise definition of X: ‘The difficulty in making a conceptual distinction between X and Y that is sharp enough to justify X without at the same time justifying Y, is a reason to disallow X.’ Both versions of the argument play a role in the debate about the isolation of hES cells for research into cell replacement therapy. An example of the logical version is that acceptance of hES cells for the development of stem cell therapy for the treatment of serious disease automatically means there is no argument against acceptance of use, for example, for cosmetic rejuvenation (Nuffield Council on Bioethics, 2000). The main difficulty is, according to these critics, the ‘grey area’ between these two extremes. One answer to this objection is to consider each case individually rather than reject all cases out of hand. One could use the same objection for example against surgery, which can equally be used for serious as well as trivial treatments.
An example of the empirical version of the slippery slope argument is that the use of hES cells for the development of cell therapy would inevitably lead to applications in germ‐line gene therapy and in therapeutic cloning, then ultimately reproductive cloning. This version of the argument is unconvincing too; even if germ line gene therapy and therapeutic cloning would be categorically unacceptable, which is not self‐evident, it does not necessarily follow from this that the use of hES cells for cell‐therapy is unacceptable. The presumed automatism in the empirical version of the slippery slope argument is disputable.
Subsidiarity
A further condition for the instrumental use of embryos is that no suitable alternatives exist that may serve the same goals of the research. This is termed ‘the principle of subsidiarity’. Critics of the use of hES cells claim that at least three such alternatives exist, which have in common that they do not require the instrumental use of embryos: (i) xenotransplantation; (ii) human embryonic germ cells (hEG cells), and (iii) adult stem cells.
The question is not whether these possible alternatives require further research (this is, at least for the latter two, largely undisputed), but whether only these alternatives should be the subject of research. Is a moratorium for isolating hES cells required, or is it preferable to carry out research on the different options, including the use of hES cells, in parallel?
The answer to this question depends on how the principle of subsidiarity ought to be applied. Although the principle of subsidiarity is meant to express concern for the (albeit limited) moral value of the embryo, it is a sign of ethical one‐dimensionality to present every alternative, which does not use embryos, as a priori superior. For the comparative ethical analysis of hES cells from pre‐implantation embryos on the one hand, and the possible alternatives mentioned on the other, a number of relevant aspects should be taken into account. These include: the burdens and/or risks of the different options for the patient and his or her environment; the chance that the alternative options have the same (probably broad) applicability as hES cells from pre‐implantation embryos; and the time‐scale in which clinically useful applications are to be expected.
A basis for initiating a comparative ethical analysis is set out below:
(i) Xenotransplantation is viewed at present as carrying a risk, albeit limited, of cross‐species infections and an accompanying threat to public health. This risk is, at least for the time being, an ethical and safety threshold for clinical trials. Apart from that, the question may be raised from a perspective of animal ethics whether it is reasonable to breed and kill animals in order to produce transplants, when at the same time spare human embryos are available which would otherwise be discarded;
(ii) In principle, the use of hEG cells from primordial germ cells of dead fetuses seems from a moral perspective to be more acceptable than the instrumental use of living pre‐implantation embryos, provided that the decision to abort was not motivated by the use of fetal material for transplantation purposes. To date, however, hEG cells have been difficult to isolate and culture, with only one research group reporting success ( Shamblott et al ., 1998 ; 2001). In addition, research in mice suggests abnormal reprogramming of these cells in culture: chimeric mice generated between mouse (m)EG cells and pre‐implantation embryos develop abnormally while chimeras using mouse (m)ES cells develop as normally as non‐chimeric mice ( Steghaus‐Kovac, 1999 ; Surani, 2001 ). This makes the outcome of eventual clinical application of these cells difficult to predict in terms of health risks for the recipient.
(iii) Analysis of the developmental potential of adult stem cells is a rapidly evolving field of research, particularly in animal model systems. Experiments carried out within the last two years have demonstrated, for example, that bone marrow cells can give rise to nerve cells in mouse brain ( Mezey et al ., 2000 ), neural cells from mouse brain can turn into blood and muscle ( Bjornson et al ., 1999 ; Galli et al ., 2000 ), and even participate in the development of chimeric mouse embryos up to mid‐gestation ( Clarke et al ., 2000 ). Although apparently spectacular in demonstrating that neural stem cells from mice can form most cell types under the appropriate conditions, it is still unclear whether true plasticity in terms of function has been demonstrated or whether the cells simply ‘piggy‐back’ with normal cells during development. Published evidence of ‘plasticity’ in adult human stem cells is more limited, but recent evidence suggests that the MAPCs from bone marrow may represent a breakthrough ( Jiang et al ., 2002 ; Schwartz et al ., 2002 ;). They are accessible. Collection is relatively non‐destructive for surrounding tissue compared, for example, with the collection of neural stem cells from adult brain, although their numbers are low: 1 in 10 8 of these cells exhibit the ability to form populations of nerve, muscle and a number of other cell types and they only become evident after several months of careful culture. Clonal analysis has provided rigorous proof of plasticity: a single haematopoietic stem cell can populate a variety of tissues when injected into lethally irradiated mice ( Krause et al ., 2001 ) or into blastocyst stage embryos to generate chimeric embryos ( Jiang et al ., 2002 ). Nonetheless, there are potential hazards to using cells that have been cultured for long periods for transplantation and although MAPCs seem to have normal chromosomes, it is important to establish that the pathways governing cell proliferation are unperturbed. This is also true for hES cells. However, the powerful performance of mES cells in restoring function in a rat model for Parkinson’s disease ( Kim et al ., 2002 ), has not yet been matched by MAPCs. Bone marrow stem cells have been shown very recently to restore function to some extent in a mouse heart damaged by coronary ligation, an experiment that mimics the conditions of the human heart soon after infarction ( Orlic et al ., 2001 ). Although clinical restoration of function in a damaged organ is usually sought rather longer after the original injury than in these experiments, which were performed before scar tissue had formed, this approach will certainly be worth pursuing. An alternative, non‐invasive, haematopoietic stem cell source is umbilical cord blood. This is used clinically for transplantation as an alternative to bone marrow in patients for whom no bone marrow match is available. Cord blood contains precursors of a number of lineages but its pluripotency, or even multipotency, is far from proven. Nevertheless, the prospect of autologous transplantation of haematopoietic stem cells of bone marrow in the long term makes this an important research area in terms of alternatives to therapeutic cloning (see below).
Although studies with adult stem cells so far have been encouraging, Galli (2000 ), author of the first adult neural stem studies and much cited by advocates of the view that adult stem cells have a proven developmental potency equal to that of ES cells, himself disagrees entirely with this viewpoint (see Editorial, 2000 ). It has even been suggested that the results from adult stem cell research are being misinterpreted for political motives and ‘hints of the versatility of the adult cells have been over interpreted, overplayed and over hyped’ ( Vastag, 2001 ). Opponents of ES cell research are now heralding Verfaillie’s adult stem cells as proof that work on hES cells is no longer needed. However the stem cell research community and Verfaillie herself ( Vastag, 2002 ) have called for more research on both adult and embryonic stem cells. ES cells that can perform as powerfully as those described by Kim et al . (2002 ) in the rat Parkinson model make it far too early in the game for them to be discounted ( Editorial, 2002 ).
The question remains, however, should a moratorium be imposed on isolating hES cells for research in cell therapy in the light of the indisputably promising results from adult stem cell research? The lack of consensus arises largely from disagreement on interpretation of the subsidiarity principle. Against the restrictive viewpoint that research on hES cells may only take place if there is proof that adult stem cells are not optimally useful, there is the more permissive viewpoint that hES cell research may, and indeed should, take place so long it is unclear whether adult stem cells are complete or even partial alternatives.
On the basis of the following arguments, a less restrictive interpretation of the subsidiarity principle is morally justified. ( Stem Cell Research, 2000 ) To begin with, the most optimistic expectation is that only in the long run will adult stem cells prove to have equal plasticity and developmental potential as hES cells (and be as broadly applicable in the clinic), and there is a reasonable chance that this will never turn out to be the case. If hES cells from pre‐implantation embryos have more potential clinical applications in the short term, then the risk of a moratorium is that patients will be deprived of benefit. This in itself is a reason to forgo a moratorium—assuming that the health interests of patients overrule the relative moral value of pre‐implantation embryos. Secondly, the simultaneous development of different research strategies is preferable, considering that research on hES cells will probably contribute to speeding up and optimising clinical applications of adult stem cells. In particular, the stimuli to drive cells in particular directions of differentiation may be common to both cell types, while methods of delivery to damaged tissue are as likely to be common as complementary. A moratorium on hES cell research would remove the driving force behind adult stem cell research.
A final variant on adult stem cell sources concerns the use of embryonal carcinoma (EC) cells, a stem cell population found in tumours (teratocarcinomas) of young adult patients. These cells have properties very similar to hES cells. The results of a phase I (safety) trial using these cells in 11 stroke victims in the USA have recently been published and permission granted by the Food and Drug Administration (FDA) for a phase II trial (effectivity) ( Kondziolka et al ., 2000 ). The patients received neural cells derived from retinoic acid (vitamin A) treatment of teratocarcinoma stem cells. Although the scientific and ethical consensus is that these trials were premature in terms of potential risk of teratocarcinoma development at the transplant site, all patients survived with no obvious detrimental effects, no tumour formation and in two cases a small improvement in symptoms. After two years, the transplanted cells were still detectable by scanning ( Kondziolka et al ., 2000 ). Despite its controversial nature, this trial has nevertheless probably set a precedent for similar trials using neural derivatives of hES, the best controlled differentiation pathway of hES cells at the present time ( Reubinoff et al ., 2001 ; Zhang et al ., 2001 ). Proponents believe that such trials would be feasible even in the short term ( McKay, 1997 ). Neural differentiation of hEC cells is fairly easy to induce reproducibly but most other forms of differentiation are not; even if ultimately regarded as ‘safe’, hEC cells will not replace hES cells in terms of developmental potential and are therefore not regarded as an alternative.
In view of both the only relative moral value of pre‐implantation embryos and the uncertainties and risks of the potential alternative sources for the development of cell therapy, a moratorium for isolating human embryonic stem cells is unjustified.
Before discussing the ethical issues around ‘therapeutic cloning’, the term itself requires consideration. To avoid confusion, it has been proposed that the term ‘cloning’ be reserved for reproductive cloning and that ‘Nuclear transplantation to produce stem cells’ would be better terminology for therapeutic cloning ( NAS report, 2002 ; Vogelstein et al ., 2002 ). Others have pointed out the disadvantage of this alternative term, namely that it masks the fact that an embryo is created for instrumental use. More important in our opinion however, is that the use of the adverb ‘therapeutic’ suggests that hES cell therapy is already a reality: strictu sensu there can only be a question of therapeutic applications once clinical trials have started. In the phase before clinical trials, it is only reasonable to refer to research on nuclear transfer as ‘research cloning’ or ‘nuclear transplantation for fundamental scientific research’, aimed at future applications of therapeutic cloning.
Some consider this technology to be ethically neutral; they claim that the ‘construct’ produced is not a (pre‐implantation) embryo. Qualifications suggested for these constructs include: activated oocyte, ovasome, transnuclear oocyte cell, etc. ( Kiessling, 2001 ; Hansen, 2002 ) However, to restrict the definition of ‘embryo’ to the product of fertilization in the post‐Dolly era is a misleading anachronism. Although the purpose of therapeutic cloning is not the creation of a new individual and it is unlikely that the viability of the constructed product is equivalent to that of an embryo derived from sexual reproduction, it is not correct to say that an embryo has not been created.
The core of the problem is that here human embryos are created solely for instrumental use. Whether or not this can be morally justified—and if so, under what conditions—has already been an issue of debate for years in the context of the development of ‘assisted reproductive technologies’ (ART). Is it acceptable to create embryos for research, and if so, is therapeutic cloning morally acceptable too?
A preliminary question: is it justified to create embryos for research?
Article 18 of the European Convention on Human Rights and Biomedicine forbids the creation of embryos for all research purposes ( Council of Europe, 1996 ). However, this does not close the ethical and political debates in individual EU member states.
In the ‘classical’ normative debate on embryo research, two perspectives can be distinguished: a ‘fetalist’ perspective (focusing on the moral value of the embryo), and a ‘feminist’ perspective (with the interests of women, particularly candidate oocyte donors, playing a central role) ( Raymond, 1987 ). Both perspectives have a different outlook on the question of whether or not there is a decisive moral distinction between research with spare IVF embryos on the one hand, and creating embryos for research on the other. In other words: is the difference between these practices such that the former can be acceptable under specific conditions, and the latter absolutely not?
Fetalist perspective
Instrumentalization of the embryo is sometimes regarded as far greater and fundamentally different when it involves the creation of embryos for research purposes rather than the use of spare embryos. This difference, however, is just gradual. Not only is the embryo used completely instrumentally in both cases, the moral status is also identical. The difference is in the intention at fertilization, which, although a real difference, is relative. It is a misconception to think that in the context of regular IVF treatment every embryo is created as a ‘goal in itself’: the goal is the solution of involuntary childlessness and the loss of some embryos is a calculated risk beforehand.
Feminist perspective
From a feminist perspective, the creation of embryos for research should be evaluated critically in as far as it may require hormone treatment of a woman to obtain oocytes for research purposes: can this be morally justified when it requires unpleasant treatment of the donor with no benefit at all, or even a detrimental outcome, for her own state of health? A first objection is that women themselves become objects of instrumental use. Here, however, an analogy can be made with recruiting healthy research subjects. Relevant considerations concern whether or not the research serves an important goal, whether the burdens and risks to the subjects are proportional, and whether valid informed consent of the research subject/donor is given. The second objection is that the health risks to the women themselves are too high and the degree of discomfort disproportional. Difference of opinion exists, however, also among women, about the disproportionality of hormone treatment. There are, furthermore, several potential alternatives that do not require hormone treatment of healthy women. One involves the in‐vitro maturation (IVM) of immature oocytes after their isolation from dead donors or donors having ovaries removed for other reasons. IVM is successful in cattle and sheep (efficiency ∼40%), although it is, for the moment, much lower in humans.
In conclusion, from both a fetalist and a feminist perspective there is no overriding categorical objection against bringing pre‐implantation embryos into existence for instrumental use. If the research cannot be conducted using spare embryos and its importance for human health is beyond doubt, we believe the creation of embryos specifically for research is morally justified subject to the required oocytes being obtained in a morally sound way.
Ethics of therapeutic cloning
Can therapeutic cloning be morally acceptable? The principle of proportionality, the slippery slope, and the principle of subsidiarity enter the debate again, but in a slightly different way.
It is doubtful whether the principle of proportionality provides a convincing a‐priori objection against therapeutic cloning. If it is considered acceptable to create embryos for research aimed at improving ART (freezing of oocytes; IVM of oocytes, etc…), then it is inconsistent to reject therapeutic cloning beforehand as being disproportional. Maybe even some opponents of creating embryos for the improvement of ART can conditionally accept therapeutic cloning because of the important health interests of patients.
Slippery‐slope
A consequentialist objection (fashioned as a ‘slippery‐slope’ argument) is that therapeutic cloning will inevitably lead to reproductive cloning. This objection is not convincing; if reproductive cloning is categorically unacceptable (the debate on this issue is still ongoing), it is reasonable to prohibit this specific technology, and not to ban other, non‐reproductive, applications of cloning. A second objection that could be raised in this context is that the creation of embryos through cloning for the isolation of stem cells could in the long term be used to justify the initiation of pregnancy from these embryos and their use simply as a vehicle for generating sufficient cells of the required type for transplantation; the pregnancy would be interrupted the moment the appropriate developmental stage was reached ( Lanza et al ., 2002 ). Relevant questions here are: is this a realistic scenario in the human (or just science fiction), would it be unacceptable, and is it unavoidable?
In terms of being a realistic means of generating genetically identical (fetal) tissue for transplantation, it could theoretically be an option, but whether it would actually be useful would depend on the alternatives available at the time transplantation techniques themselves have been perfected to clinical applicability (see below).
In terms of moral acceptability, most people would consider pregnancy‐and‐abortion‐for‐transplantation to be far more difficult to justify than the creation of pre‐implantation embryos for instrumental use in vitro , firstly because of the higher moral status/symbolic value of the fetus, and secondly because of the significantly greater burden of pregnancy‐and‐abortion‐for‐transplantation for women. ( De Wert et al ., 2002 ) Even though many countries do forbid pregnancy‐for‐transplantation, it has been argued that it could be morally justified as a last resort, on the basis that sacrificing a fetus (a potential person) may be justified in order to rescue the life of a person.
Finally, in scrutinising the slippery slope argument, it is important to assess whether instrumental use of pre‐implantation embryos makes pregnancy‐for‐abortion unavoidable. Again, the apparent automatism is disputable: if we reject pregnancy‐for‐abortion as being unacceptable, we can continue its prohibition.
Taking these points for and against together, the slippery slope argument does not provide a convincing basis for banning therapeutic cloning.
Therapeutic cloning can only be morally acceptable if there are no good alternatives. It is important to note that therapeutic cloning strictu sensu is not likely to be short‐term prospect. Apart from unsolved technical difficulties with nuclear transfer itself in human oocytes ( Cibelli et al ., 2002 ), much basic research is still needed to determine whether the differentiation of hES cells can be controlled and sufficient cell numbers generated to be a useful therapy. This research can be done with spare IVF embryos. In this light, creation of embryos for therapeutic cloning is, in our opinion, premature. Although critics of this point of view could use our own argument that delay in the development of research cloning could, just as a moratorium on hES cell isolation and research, have negative consequences for patients, the evidence suggests that further optimization of the technology as such could take place in animals. We believe that the duration of any ‘delay’ in offering therapy to patients would not then be of real significance.
At the same time, research on potential alternatives for therapeutic cloning, which likewise avoid (or at least reduce) the problem of rejection but which do not involve the creation of human embryos for instrumental use, should be stimulated. For the comparative ethical analysis, it is again important to avoid the pitfall of one‐dimensionality. Possible alternative options include: (i) the use of adult cells, both stem cells and differentiated cells; (ii) making optimal use of spare embryos: embryo‐banks and immuno‐tolerance and (iii) the use of entities with an undetermined status: ‘hybrids’ and ‘parthenotes’.
Adult cells
Adult tissue is a potential source of two alternatives: stem cells, which may be induced to transdifferentiate by extracellular signals, and somatic cells (nuclei) which require direct reprogramming signals, for example from an oocyte after nuclear transfer, to adopt a new fate. Both sources will, however, require substantial research to become realistic alternatives. Until it has been shown that adult stem cells at some point re‐express ES cell markers we will never know if transdifferentiation or direct reprogramming are the same or not.
For direct reprogramming of somatic nuclei, new methods may be developed which do not require nuclear transfer to oocyte cytoplasm. Examples of current work in this area include the study of cellular hybrids derived from the fusion of (embryonic) stem cells with somatic or adult stem cells ( Surani, 2001 ; Terada et al ., 2002; Ying et al ., 2002 ). An understanding of the basic mechanisms underlying reprogramming is already being undertaken in mice, cattle and sheep and indeed, the creation of ‘Dolly’ re‐initiated a wave of research in nuclear reprogramming in mammals. The ultimate aim of this research in the context of cell transplantation therapy would be chemically‐induced nuclear re‐programming in the test‐tube to derive the required cell type, obviating the necessity for therapeutic cloning altogether. First evidence that this might be feasible demonstrated direct reprogramming of fibroblasts to neural cells and T‐cells in culture by temporary permeabilization of the fibroblasts to allow them to take up extracts of neural and T‐cells, respectively ( Hakelien et al ., 2002 ). In this sense, therapeutic cloning may be regarded, perhaps, as a temporary option; in the long term it will be replaced by a direct reprogramming alternative.
Research on direct reprogramming of adult somatic nuclei may ultimately require the creation of human embryos for instrumental use. In view of the importance of this research, both in terms of the contribution to the development of cell therapy and the potential ultimately to reduce the instrumental use of human embryos by developing an alternative for therapeutic cloning, this research would no doubt also meet the principle of proportionality.
Optimal use of spare embryos
Various strategies should be considered. Firstly, the generation of a bank of hES cell lines from a wide spectrum of genotypes is required to be able to offer a reasonable tissue match for every patient requiring a cellular transplant. Estimates of the number of independent cell lines that would actually be required for this vary greatly, from a few hundred to several thousand. Such a bank is already being discussed in the UK but could ultimately be established as a European resource. However, even very good tissue matches between donor and recipient require some degree of immunosuppressive therapy, which has long term negative side‐effects for patients, including increased risk of tumorigenesis
Secondly, there should be further development and application of ‘immunotolerance’ methodology. This may be particularly useful in combination with matching from an hES cell bank. The observation that patients receiving bone marrow transplants are more immunotolerant to other tissue transplantation from the same donor have led to the suggestion that immunotolerance may also be induced by initial injection of hES‐derived haematopoietic cells followed by the cell type of interest derived from the same hES cell line ( Kaufman et al ., 2001 ). The transplant may then be tolerated without being genetically identical, and lower doses or no immunosuppressives required. The combination of ‘near match’ with immunotolerance is probably a promising option.
For certain genetically based diseases, autologous transplantation may not always be appropriate since the transplanted tissue will bear the same genetic defect. Immunotolerance hES cell strategies may then be a particularly attractive or the only option. Should the success rates be very high, then attempts to create genetically identical transplantable tissue may become superfluous, not only for these, but for all patients. If, however, it works imperfectly or only for some patients, then therapeutic cloning may well remain an important option for the majority of all other patients.
Creating entities with an undefined status
Various alternative options raise classification problems, as the entities created to obtain cells have an undefined status. Firstly, transplanting the somatic nucleus of a patient into an enucleated animal oocyte. The logic behind this variant of therapeutic cloning is twofold: one, assuming that the ‘units’ thus created are not human embryos because only their nuclear but not mitochondrial DNA is human, advocates of this strategy argue that it circumvents the controversial issue of the instrumental use of human embryos. Two, a technical advantage of this approach would be that plenty of animal oocytes would be available; the feminist objection to creating human embryos for research would, of course, not apply.
It is not yet known whether this is a scientifically realistic option (whether hES cells can be effectively obtained following this approach). Animal research has so far been limited and not generally successful ( Barrientos et al ., 1998 ; 2001); polymorphic interspecies differences in mitochondrial DNA are thought to make such reconstructed zygotes non‐viable or prone to major developmental abnormalities. There are however, unvalidated reports of successful applications of the technique in China. The Donaldson Committee advocated a ban on this approach, but without any argumentation (Stem Cell Research, 2000). However, if this were a realistic option scientifically, then we believe that the issues involved deserve further ethical discussion. The major questions that should be addressed include: is the risk acceptable? As for xenotransplantation, there is also here the risk of cross‐species infection, although this may be extremely small, because the nuclear DNA of the animal, which may harbour viruses, is removed from the oocyte. Is it reasonable to argue that this ‘artificial combination’ should not be considered equivalent to a human embryo? Since the entire nuclear DNA is human, the reconstructed combination should, we think, be regarded as a human embryo. The procedure should thus not be presented as an ‘embryo saving’ variant of therapeutic cloning. However, only further in‐utero research with reconstructed animal embryos, for example embryos created by transplanting the somatic nucleus of a rat into an enucleated mouse oocyte, will provide a more definitive answer. Finally: in‐vitro research may well show that embryos obtained by transplanting a human somatic nucleus into an enucleated animal oocyte are non‐viable (like parthenotes, see below). The moral status of non‐viable pre‐implantation embryos, and more particularly, the question as to whether the conditions for research using non‐viable embryos may be more permissive than the conditions for using viable embryos, needs further debate (see earlier).
A second option may be the generation of parthenogenetic embryos for the isolation of hES cell lines. Here, an unfertilized (haploid) oocyte is treated chemically such that it becomes diploid, with two identical sets of the maternal chromosomes. These uniparental embryos are by definition gynogenetic and never result in viable offspring, because they fail to generate extra‐embryonic tissues. Nevertheless, in mice (see Boediono et al ., 1999 ) and in apes ( Cibelli et al ., 2002 ), parthenotes have been shown to develop to the blastocyst stage and yield cell lines with properties not distinguishable from ES cells derived from fertilized oocytes. However, in view of the fact that some genes are genomically imprinted, such that they are expressed only if inherited via the male germ line, ES cells derived from parthenotes may well be abnormal. First attempts at parthenogenesis in humans have not yielded hES cell lines ( Cibelli et al ., 2002 ). It is important to realise that such hES cell lines, if developed in humans, would only provide a tissue match for the oocyte donor, i.e. women of reproductive age. Although it has been speculated that two sets of male chromosomes could also be used in parthenotes, there is no evidence that this is a real option.
Cibelli and colleagues have referred to parthenogenesis as cloning. Whether this is correct depends on the timing of parthenogenesis: if initiated before the first complete meiotic division, then the procedure amounts to cloning (the same genotype as the female); if after the first meiotic division (ie recombination and loss of half) then it is not cloning. In this light, the experiments of Cibelli et al . (2002) would not qualify as cloning in the strict sense.
Some will certainly argue that the parthenote is not an embryo; parthenogenesis would then be classified as an ‘embryo‐saving’ strategy. As the parthenote undergoes the first divisions normally and is at these stages not distinguishable from embryos derived by normal fertilization, we would argue that it should be regarded as a non‐viable embryo. In the light of its non‐viability, the potentiality argument is not applicable. The moral status of parthenotes may therefore be regarded as very low, lower even than that of normal viable embryos at the same stage (see earlier). Thus, although not an ‘embryo‐saving alternative’, all other things being equal, parthenogenesis may be regarded as ethically preferable to the generation of viable embryos by fertilization or nuclear transfer (for instrumental use). In addressing the question of whether this research is premature given the current lack of proof that human ES cells are clinically useful as a source of transplantable cells, the lower moral status of parthenotes should be taken into account.
Regarding moral judgements as a ‘quasi stable equilibrium’ is particularly appropriate when applied to the ethics of isolating hES cells for research into cell replacement therapy. Stem cell research is highly dynamic, with many questions and ‘unknowns’. New insights into the effectiveness, risks and usefulness of the various alternatives may have immediate consequences for the ethical evaluation of the isolation of hES cells.
The status of the pre‐implantation embryo is the most sensitive and disputed point in the debate on isolation of hES cells for research. The dominant view in ethics, however, is that the moral status of the pre‐implantation embryo is relatively low and that the instrumental use of these embryos can be morally justified under some conditions.
The moral status of non‐viable pre‐implantation embryos is lower than the moral status of viable pre‐implantation embryos. The precise implications of this difference in moral status for the regulation of the instrumental use of embryos need further ethical scrutiny.
Both the principle of proportionality and a permissive interpretation of the principle of subsidiarity, make a moratorium on the isolation of hES cells unjustified.
Parallel research on alternatives is important and requires major support. Research on hES cells can provide an important impetus in this context.
The moral difference between research on surplus embryos and the creation of embryos for research is only gradual. A complete ban on creating embryos for instrumental use in research is morally unjustified.
A categorical ban on research on human therapeutic cloning is not justified, although the creation of embryos by cloning for the isolation of hES cells is, at the present time, premature. The necessary research can currently be carried out using animal embryos and surplus human IVF embryos.
Research into potential alternatives for therapeutic cloning, which does not require human embryos or which requires only the use of spare embryos, should be stimulated.
Banning the transplantation of a human somatic nucleus to an animal oocyte (as a variant of therapeutic cloning) is premature and morally unjustified.
The question whether therapeutic cloning should be allowed, becomes acute if research with spare embryos suggests that usable transplants can be obtained in vitro from hES cells and if the possible alternatives for therapeutic cloning are less promising or need more time for development than is currently expected. In that case, therapeutic cloning can be morally justified on the basis of both the principle of proportionality and the principle of subsidiarity.
We are grateful to Drs K.Lawson and J.Geraedts for comments on the manuscript.
Alikani, M. and Willadsen, S.M. ( 2002 ) Human blastocysts from aggregated mono‐nucleated cells of two or more non‐viable zygote‐derived embryos. Reprod. Biomed. Online , 5 , 56 –58.
Anderson, D.J., Gage, F.H. and Weissman, I.L. ( 2001 ) Can stem cells cross lineage boundaries? Nat. Med. , 7 , 393 –395.
Barrientos, A., Kenyon, L. and Moraes, C.T. ( 1998 ) Human xeno mitochondrial cybrids. Cellular models of mitochondrial complex I deficiency. J. Biol. Chem. , 273 , 14210 –14217.
Barrientos, A., Muller, S., Dey, R., Weinberg, J. and Moraes, C.T. ( 2000 ) Cytochrome c oxidase assembly in primates is sensitive to small evolutionary variations in amino acid sequence. Mol. Biol. Evol. , 17 , 1508 –1519.
Boediono, A., Suzuki, T., Li, L.Y. and Godke, RA. ( 1999 ). Offspring born from chimeras reconstructed from parthenogenetic and in vitro fertilized bovine embryos. Mol. Reprod. Dev. , 53 , 159 –170.
Bjornson, C.R., Rietze, R.L., Reynolds, B.A., Magli, M.C. and Vescovi, A.L. ( 1999 ) Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science , 283 , 534 –537.
Blau, H.M., Brazelton, T.R. and Weimann, J.M. ( 2001 ) The evolving concept of a stem cell. Entity of function? Cell , 105 , 829 –841.
Brazelton, T.R., Rossi, F.M., Keshet, G.I. and Blau, H.M. ( 2000 ) From marrow to brain: expression of neuronal phenotypes in adult mice. Science , 290 , 1775 –1779.
Cibelli, J.B., Grant, K.A., Chapman, K.B., Cunniff, K., Worst, T., Green, H.L., Walker, S.J., Gutin, P.H., Vilner, L., Tabar, V. et al . ( 2002 ) Parthenogenetic stem cells in nonhuman primates. Science , 295 , 819 .
Clarke, D.L., Johansson, C.B., Wilbertz, J., Veress, B., Nilsson, E., Karlstrom, H., Lendahl, U. and Frisen, J. ( 2000 ) Generalized potential of adult neural stem cells. Science , 288 , 1559 –1561.
Council of Europe ( 1996 ) Convention on Human Rights and Biomedicine. Strasburg.
De Wert, G., Berghmans, R., Boer, G.J., Andersen, S., Brambati, B., Carvalho, A.S., Dierickx, K.M., Elliston, S., Nunez, P., Osswald, W. et al . ( 2002 ) Ethical guidance on human embryonic and fetal tissue transplantation: A European overview. Med., Health Care and Philosophy , 5 , 79 –90.
Editorial ( 2002 ) Nature .
European Science Foundation ( 2001 ) Human stem cell research: scientific uncertainties and ethical dilemmas. European Science Foundation Policy Briefing , 14.
Ferrari, G., Cusella‐De Angelis, G., Coletta, M., Paolucci, E., Stornaiuolo, A., Cossu, G. and Mavilio, F. ( 1998 ) Muscle regeneration by bone marrow‐derived myogenic progenitors. Science , 279 , 1528 –1530.
Fuchs, E. and Segre, J.A. ( 2000 ) Stem cells: a new lease on life. Cell , 100 , 143 –155.
Galli, R., Borello, U., Gritti, A., Minasi, M.G., Bjornson, C., Coletta, M., Mora, M., De Angelis, M.G., Fiocco, R., Cossu, G. et al . ( 2000 ) Skeletal myogenic potential of human and mouse neural stem cells. Nat. Neurosci. , 3 , 986 –991.
Hakelien, A.M., Landsverk, H.B., Robl, J.M., Skallhegg, B.S. and Collas, P. ( 2002 ) Reprogramming fibroblasts to express T‐cell functions using cell extracts. Nat. Biotechnol. , 20 , 460 –466.
Hansen, J‐E.S. ( 2002 ) Embryonic stem cell production through therapeutic cloning has fewer ethical problems than stem cell harvest from surplus IVF embryos. J. Med. Ethics , 28 , 86 –88.
Human Fertilisation and Embryology Act ( 1990 ) HFEA, London
Hursthouse, R. ( 1987 ) Beginning lives . Blackwell, Oxford.
Jackson, K.A., Majka, S.M., Wang, H., Pocius, J., Hartley, C.J., Majesky, M.W., Entman, M.L., Michael, L.H., Hirschi, K.K. and Goodell, M.A. ( 2001 ) Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J. Clin. Invest. , 107 , 1395 –1402.
Jiang, Y., Jahagirdar, B.N., Reinhardt, R.L., Schwartz, R.E., Keene, C.D., Ortiz‐Gonzalez, X.R., Reyes, M., Lenvik, T., Lund, T., Blackstad, M. et al . ( 2002 ) Pluripotency of mesenchymal stem cells derived from adult bone marrow. Nature , 418 , 41 –49.
Kaufman, D.S., Hanson, E.T., Lewis, R.L., Auerbach, R. and Thomson, J.A. ( 2001 ) Hematopoietic colony forming cells derived human embryonic stem cells. Proc. Natl Acad. Sci. USA , 98 , 10716 –10721.
Keller, G. and Snodgrass, H.R. ( 1999 ) Human embryonic stem cells: The future is now. Nat. Med. , 5 , 151 –152.
Kiessling, A. ( 2001 ) In the stem‐cell debate, new concepts need new words. Nature , 413 , 453.
Kim, J‐H., Auerbach,J.M., Rodriguez‐Gomez, J.A., Velasco, I.Gavin, D., Lumelsky,N., Lee, S‐H., Nguyen,J., Sanchez‐Pernaute, R., Bankiewicz, K. and McKay, R. ( 2002 ) Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson′s disease. Nature , 418 , 50 ‐56
Kondziolka, D., Wechsler, L., Goldstein, S., Meltzer, C., Thulborn, K.R., Gebel, J., Jannetta, P., DeCesare, S., Elder, E.M., McGrogan, M. et al . ( 2000 ). Transplantation of cultured human neuronal cells for patients with stroke. Neurology , 55 , 565 –569.
Krause, D.S., Theise, N.D., Collector, M.I., Henegariu, O., Hwang, S., Gardner, R., Neutzel, S. and Sharkis, S.J.( 2001 ) Multi‐organ, multi‐lineage engraftment by a single bone marrow‐derived stem cell. Cell , 105 , 369 –377.
Lagasse, E., Connors, H., Al‐Dhalimy, M., Reitsma, M., Dohse, M., Osborne, L., Wang, X., Finegold, M., Weissman, I.L. and Grompe, M. ( 2000 ) Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat. Medicine , 6 , 1229 –1234.
Lanza, RP., Chung, H.Y., Yoo, J.J., Wettstein, P.J., Blackwell, C., Borson, N., Hofmeister, E., Schuch, G., Soker, S., Moraes, C.T. et al .( 2002 ) Generation of histocompatible tissues using nuclear transplantation. Nature Biotech ., advance online publication DOI: 10.1038/nbt703.
Lanzendorf, S.E., Boyd, C.A., Wright, D.L., Muasher, S., Oehninger, S. and Hodgen, G.D. ( 2001 ) Use of human gametes obtained from anonymous donors for the production of human embryonic stem cell lines. Fertil. Steril. , 76 , 132 –137.
McGee, G. and Caplan, A. ( 1999 ) The ethics and politics of small sacrifices in stem cell research. Kennedy Institute of Ethics J. , 9 , 151 –158.
McKay, R. ( 1997 ) Stem cells in the central nervous system. Science , 276 , 66 –71.
Mezey, E., Chandross, K.J., Harta, G., Maki, R.A. and McKercher, S.R. ( 2000 ) Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science , 290 , 1779 –1782.
Nagy, A., Rossant, J., Nagy, R., Abramow‐Newerly, W. and Roder, J.C. ( 1993 ) Derivation of completely cell culture‐derived mice from early‐passage embryonic stem cells. Proc. Natl Acad. Sci. USA , 90 , 8424 –8428.
NAS (2002) Board on Life Sci., National Research Council and Board on Neuroscience and Behavioural Health, Institute of Medicine. Stem cells and future of regenerative medicine. National Academy Press. www.nap.edu/books/0309076307/html .
Nuffield Council on Bioethics ( 2000 ). Stem Cell Therapy: the ethical issues. A discussion paper. April, 2000.
Orlic, D., Kajstura, J., Chimenti, S., Jakoniuk, I., Anderson, S.M., Li, B., Pickel, J., McKay, R., Nadal‐Ginard, B., Bodine, D.M. et al . ( 2001 ) Bone marrow cells regenerate infarcted myocardium. Nature , 401 , 701 –705.
Passier, R. and Mummery, C.L ( 2003 ) The origin and use of embryonic and adult stem cells in differentiation and tissue repair: review in Spotlight Issue on Cell Transplantation and Stem cells in the Cardiovascular system. Cardiovasc. Res. , in press.
Petersen, B.E., Bowen, W.C., Patrene, K.D., Mars ,W.M., Sullivan, A.K., Murase, N., Boggs, S.S., Greenberger, J.S. and Goff, J.P. ( 1999 ) Bone marrow as a potential source of hepatic oval cells. Science , 284 , 1168 –1170.
Pittenger, M.F., Mackay, A.M., Beck, S.C., Jaiswal, R.K., Douglas, R., Mosca, J.D., Moorman, M.A., Simonetti, D.W., Craig, S. and Marshak, D.R. ( 1999 ) Multilineage potential of adult human mesenchymal stem cells. Science , 284 , 143 –147.
Raymond, J.G. ( 1987 ) Fetalists and feminists: they are not the same. In Spallone, P. and Steinberg D.L. (eds), Made to Order: The Myth of Reproductive and Genetic Progress. Pergamon Press, Oxford, pp. 58 –65.
Reubinoff, B.E., Pera, M.F., Fong, C.Y., Trounson, A. and Bongso, A. ( 2000 ) Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nature Biotechnol. , 18 , 399 –404.
Reubinoff, B.E., Itsykson, P., Turetsky, T., Pera, M.F., Reinhartz, E., Itzik, A. and Ben‐Hur, T. ( 2001 ) Neural progenitors from human embryonic stem cells. Nature Biotechnol. , 19 , 1134 –1140.
Reyes, M. and Verfaillie, C.M. ( 2001 ) Characterization of multipotent adult progenitor cells, a subpopulation of mesenchymal stem cells. Ann. N.Y. Acad. Sci. , 938 , 231 –233.
Sanchez‐Ramos, J., Song, S., Cardozo‐Pelaez, F., Hazzi, C., Stedeford, T., Willing, A., Freeman, T.B., Saporta, S., Janssen, W., Patel, N. et al . ( 2000 ) Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp. Neurol. , 164 , 247 –256.
Schwartz, R.E., Reyes, M., Koodie, L., Blacksrad, M., Lund, T., Lenvik, T., Johnson, S., Hu, W‐H. and Verfaillie, C. ( 2002 ) Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte‐like cells. J. Clin. Invest. , 109 , 1291 –302.
Shamblott, M.J., Axelman, J., Wang, S., Bugg, E.M., Littlefield, J.W., Donovan, P.J., Blumenthal, P.D., Huggins, G.R. and Gearhart, J.D. ( 1998 ) Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc. Natl Acad. Sci. USA , 95 , 13726 –13731.
Shamblott, M.J., Axelman, J., Littlefield, J.W., Blumenthal, P.D., Huggins, G.R., Cui Y., Cheng, L. and Gearhart, J.D. ( 2001 ) Human embryonic germ cell derivatives express a broad range of developmentally distinct markers and proliferate extensively in vitro. Proc. Natl Acad. Sci. USA , 98 , 113 –118.
Spradling, A., Drummond‐Barbosa, D. and Kai, T. ( 2001 ) Stem cells find their niche. Nature , 414 , 98 –104.
Steghaus‐Kovac, S. ( 1999 ) Ethical loophole closing up for stem cell researchers. Science , 286 , 31.
Stem Cell Research ( 2000 ) Medical progress with responsibility. A report from the chief medical officer’s expert group reviewing the potential of developments in stem cell research and cell nuclear replacement to benefit human health . Department of Health, UK.
Stem cells show their muscle ( 2000 ). Editorial. Nature Neurosci ., 3 , 961 .
Surani, M.A. ( 2001 ) Re‐programming of genome function through epigenetic inheritance. Nature , 414 , 122 –128.
Terada, N., Hamazaki, T., Oka, M., Hoki, M., Masterlerz, D.M., Nakano, Y., Meyer, E.M., Morel, L., Petersen, B.E. and Scott, E.W. ( 2002 ) Bone marrow cell adopt the phenotype of other cells by spontaneous cell fusion. Nature , 416 , 542 –545.
Thomson, J.A., Itskovitz‐Eldor, J., Shapiro, S.S., Waknitz, M.A., Swiergiel, J.J., Marshall, V.S. and Jones, J.M. ( 1998 ) Embryonic stem cell lines derived from human blastocysts. Science , 282 , 1145 –1147.
Vastag, B. ( 2001 ) Many say adult stem cell reports overplayed. JAMA , 286 , 293 .
Vastag, B. ( 2002 ) New bioethics council offers no recommendations. JAMA , 287 , 2934 –2935.
Verfaillie, CM. ( 2002 ) Hematopoietic stem cells for transplantation. J. Nat. Immunol. , 3 , 314 –317.
Vogelstein, B., Alberts, B. and Shine, K. ( 2002 ) Please don’t call it cloning! Science , 295 , 1237.
Watt, F.M. and Hogan, B.L. ( 2000 ) Out of Eden: stem cells and their niches. Science , 287 , 1427 –1430.
Weissman, I.L. ( 2000 ) Stem cells: units of development, units of regeneration, and units in evolution. Cell , 100 , 157 –168.
Xu, C., Inokuma, M.S., Denham, J., Golds, K., Kundu ,P., Gold, J.D. and Carpenter, M.K. ( 2001 ) Feeder‐free growth of undifferentiated human embryonic stem cells. Nature Biotechnol. , 19 , 971 –974.
Ying, Q.L., Nichols, J., Evans, E.P. and Smith, A.G. ( 2002 ) Changing potency by spontaneous fusion. Nature , 416 , 545 –548.
Zhao, L.R., Duan, W.M., Reyes, M., Keene, C.D., Verfaillie, C.M. and Low, W.C. ( 2002 ). Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp. Neurol. , 174 , 11 –20.
Zhang, S.C., Wernig, M., Duncan, I.D., Brustle, O. and Thomson, J.A.( 2001 ) In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. , 19 , 1129 –1133.
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1460-2350
- Copyright © 2024 European Society of Human Reproduction and Embryology
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Advancements in Human Embryonic Stem Cell Research: Clinical Applications and Ethical Issues
- Review Article
- Open access
- Published: 19 March 2024
- Volume 21 , pages 379–394, ( 2024 )
Cite this article
You have full access to this open access article

- Soo Jin Park ORCID: orcid.org/0000-0002-7382-230X 1 ,
- Yoon Young Kim ORCID: orcid.org/0000-0003-0671-519X 1 , 3 ,
- Ji Yeon Han ORCID: orcid.org/0000-0002-1007-3188 1 ,
- Sung Woo Kim ORCID: orcid.org/0000-0003-4689-1323 1 ,
- Hoon Kim ORCID: orcid.org/0000-0002-5623-6368 1 , 2 , 3 &
- Seung-Yup Ku ORCID: orcid.org/0000-0002-6423-854X 1 , 2 , 3
1129 Accesses
Explore all metrics
Background:
The development and use of human embryonic stem cells (hESCs) in regenerative medicine have been revolutionary, offering significant advancements in treating various diseases. These pluripotent cells, derived from early human embryos, are central to modern biomedical research. However, their application is mired in ethical and regulatory complexities related to the use of human embryos.
This review utilized key databases such as ClinicalTrials.gov, EU Clinical Trials Register, PubMed, and Google Scholar to gather recent clinical trials and studies involving hESCs. The focus was on their clinical application in regenerative medicine, emphasizing clinical trials and research directly involving hESCs.
Preclinical studies and clinical trials in various areas like ophthalmology, neurology, endocrinology, and reproductive medicine have demonstrated the versatility of hESCs in regenerative medicine. These studies underscore the potential of hESCs in treating a wide array of conditions. However, the field faces ethical and regulatory challenges, with significant variations in policies and perspectives across different countries.
Conclusion:
The potential of hESCs in regenerative medicine is immense, offering new avenues for treating previously incurable diseases. However, navigating the ethical, legal, and regulatory landscapes is crucial for the continued advancement and responsible application of hESC research in the medical field. Considering both scientific potential and ethical implications, a balanced approach is essential for successfully integrating hESCs into clinical practice.
Similar content being viewed by others

Advances in Pluripotent Stem Cells: History, Mechanisms, Technologies, and Applications

Stem cells: past, present, and future

A Brief Overview of Global Trends in MSC-Based Cell Therapy
Avoid common mistakes on your manuscript.
1 Introduction
The field of stem cell research has undergone a significant transformation with the advent of human embryonic stem cells (hESCs). Since their pioneering isolation in 1998, hESCs have been at the forefront of scientific inquiry due to their unique ability for self-renewal and pluripotency [ 1 , 2 ]. This comprehensive review article delves into the advancements, challenges, and ethical considerations surrounding hESCs and their implications for regenerative medicine.
Over the past two decades, the potential of hESCs to revolutionize the treatment of various diseases has been increasingly recognized [ 3 , 4 ]. Their capacity to differentiate into diverse cell types offers promising prospects for repairing or replacing damaged tissues, especially in conditions where current treatments are limited [ 5 , 6 , 7 , 8 ]. However, the journey of hESC research is not without its complexities. Ethical considerations regarding the use of human embryos have sparked intense debates and have had a profound impact on public perception and the regulatory framework governing hESC research [ 9 , 10 ].
The therapeutic applications of hESCs encompass both systemic and localized approaches, including intravenous or intramuscular injections and surgical implantation, sometimes combined with bioscaffolds [ 11 ]. These strategies are broadly classified into transient dosing for temporary therapeutic effects and permanent implantation for long-term tissue repair and regeneration [ 12 , 13 ]. Despite these advancements, challenges in ensuring consistency in hESC properties across different experimental settings continue to pose hurdles in translating laboratory findings into clinical therapies [ 14 , 15 ].
While induced pluripotent stem cells (iPSCs) have emerged as an alternative, hESCs still hold distinct advantages, particularly in the understanding of genetic diseases and human development [ 16 , 17 ]. Despite the ethical complexities and slower pace of clinical research compared to iPSCs, hESCs remain a crucial tool in biomedical research [ 18 , 19 ]. Their unique position in providing insights into early human development and genetic disorders underscores their invaluable role in medical science [ 17 ].
This review aims to provide an in-depth analysis of the current state of clinical trials involving hESCs, emphasizing their role in regenerative medicine. We explore the evolving landscape of hESC research, highlighting the need for ongoing scientific exploration, ethical deliberation, and regulatory guidance to fully realize the therapeutic potential of hESCs in improving patient care and advancing medical science.
2 Methodology
This narrative review was conducted to assess the clinical applications of hESCs. The primary aim was to gather and analyze data from various sources to understand the current state and advancements in hESC research.
For database search, we utilized ClinicalTrials.gov ( https://clinicaltrials.gov/ ) and EU Clinical Trials Register ( https://www.clinicaltrialsregister.eu/ ) for identifying ongoing and completed clinical trials involving hESCs. Also, we used PubMed and Google Scholar to retrieve published clinical trial reports and peer-reviewed articles on hESCs. Studies and trials were included based on their focus on the clinical application of hESCs. Those not directly involving hESCs or outside the scope of clinical application were excluded. The review primarily targeted articles and trials published or conducted in the last five years to maintain contemporary relevance.
For data extraction and analysis, key information extracted included the study title, indication, participant number, study site, study period, study design, and NCT number. This data was organized systematically to provide a clear overview of the current trends and progress in the field of hESC research in clinical applications.
2.1 Overview of clinical trials in hESC research
Figure 1 displays key aspects of hESC clinical trials included in this review. The first clinical trial registration was in 2002, and the largest number of registered trials were in the United States (19, 40.4%), followed by China (8, 17.0%; Fig. 1 A). By disease category, the largest number of trials were related to ophthalmologic conditions (20, 42.6%), followed by neurologic conditions (10, 21.3%), and clinical studies were mainly conducted on diabetes mellitus (7, 14.9%; Fig. 1 B). Figure 1 C shows the number of trial registrations and the cumulative number of clinical studies by year. There has been a sharp increase since 2012. (Fig. 1 C), and by study design, phase 1 or phase 1/2 designs predominate, accounting for 88% (Fig. 1 D). When looking at studies by a specific disease, dry age-related macular degeneration (AMD) is the most common with 8 (18.2%), followed by type 1 diabetes mellitus (T1DM, 7, 15.9%) and Stargardt Macular Dystrophy (SMD, 5, 11.4%).
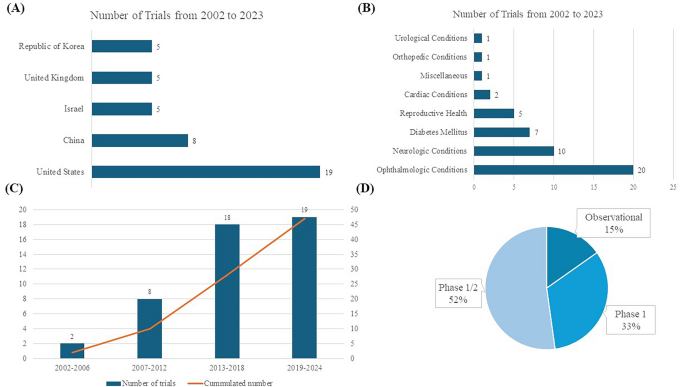
Numbers of trials on human embryonic stem cells ( A ) Global Geographical Distribution of Human Embryonic Stem Cell Clinical Trials ( B ) Distribution of Trials by Disease Category ( C ) Frequency of Trials Across Specific Diseases ( D ) Distribution of Clinical Trials Across Different Phases
2.2 Disease-specific analysis
2.2.1 ophthalmologic diseases.
Retinal degeneration is a significant ophthalmologic disease that affects the eye and vision, including dry AMD, SMD, wet AMD, retinitis pigmentosa (RP), diabetic retinopathy, and myopic macular degeneration, among others [ 20 , 21 , 22 ]. These conditions often lead to severe vision impairment or blindness. Traditional treatments primarily focus on slowing the progression of these diseases but generally fall short of providing substantial visual improvement. For instance, while laser therapy is beneficial in the early stages, there is no established treatment for late-stage dry AMD [ 23 ]. In cases of wet AMD, therapies such as anti-VEGF can be administered through intravitreal infusion (e.g., ranibizumab, bevacizumab, aflibercept, and brolucizumab), yet this disease requires continuous treatment and monitoring due to its chronic nature [ 24 , 25 , 26 , 27 ]. Stem cell therapy, particularly involving retinal pigment epithelium (RPE) degeneration, has emerged as a promising approach in eye diseases [ 28 ]. The RPE is vital for maintaining photoreceptor health and is tasked with recycling photopigments and clearing shed photoreceptor segments [ 29 ]. hESCs have shown significant potential in rescuing photoreceptors and enhancing vision in preclinical macular degeneration models [ 30 ]. One of the initial forays into stem cell therapy using hESCs was directed at treating dry AMD using hESC-derived RPE. Several key factors contributed to this early focus on retinal conditions. Primarily, the unique immune privilege of the eye, reinforced by the blood-ocular barrier, significantly lowers the risk of rejection of transplanted cells—a crucial aspect in the success of any stem cell-based therapy [ 31 , 32 ]. Moreover, the eye's transparency permits the non-invasive tracking of the introduced cells through methods like optical coherence tomography or microperimetry, enabling continuous monitoring and evaluation of the therapy's effectiveness [ 33 ]. The eye's distinct and isolated structure also minimizes the spread of these cells to other body parts, thereby reducing the likelihood of unintended systemic effects [ 34 ]. Furthermore, the absence of synaptic layers in retinal cells aids in their smoother integration [ 29 ]. Lastly, the irreversible progression of many retinal disorders and the absence of adequate existing treatments have necessitated the development of innovative therapeutic strategies, thereby placing retinal ailments at the forefront of hESC research and application.
Dry AMD, a prevalent and progressive ophthalmologic disease affecting elderly patients, is characterized by the degeneration of the RPE layer and impairment of central vision [ 21 ]. The pivotal role of RPE in the pathophysiology of dry AMD makes it a prime target for therapeutic interventions. The potential of stem cells, especially hESCs, in this context, lies in their ability to differentiate into RPE cells, thereby offering the possibility of replacing damaged or degenerated RPE with healthy, functional cells. Preclinical studies in animal models and in vitro experiments have provided substantial evidence supporting the role of stem cells, including hESCs, in treating dry AMD [ 35 , 36 , 37 ].
For example, in Yucatan minipigs, a preclinical study assessed CPCB-RPE1, a hESC-derived retinal pigment epithelium monolayer [ 35 ]. The study successfully placed CPCB-RPE1 implants in the subretinal space without breakage, and histological analysis confirmed the survival of hESC-RPE cells as an intact monolayer for one month [ 35 ]. Another study used differentiated hESC-RPE replacement therapy on albino rabbit eyes induced with NaIO3, employing a 25-gauge transvitreal pars plana vitrectomy (PPV) technique [ 36 ]. Xeno-free hESC-RPE monolayer on a polyester substrate survived and retained functionality for up to four weeks with short-term immunosuppression in a rabbit dry AMD model [ 37 ]. These studies demonstrate the feasibility of generating RPE cells from stem cells and their potential to integrate into the retina, potentially restoring RPE function and rescuing photoreceptors. Also, the critical advantage of hESC-RPE is their reduced risk of uncontrolled proliferation, as they are fully differentiated.
Clinical trials have been conducted to test the safety and feasibility of hESC-derived RPE for dry AMD, as outlined in Table 1 . Dry AMD has been the subject of the most significant number of clinical trials, with studies dating back to 2011 (Table 1 ). The first study involved MA09-hRPE (NCT01344993; NCT01674829; NCT02122159), derived from the MA09 hESC line, a xenograft product with ex vivo exposure to mouse embryonic cells [ 38 ]. Produced by isolating RPE patches when embryoid body formation was confirmed, this treatment was tested in three different dose cohorts (50,000, 100,000, and 150,000 cells) for patients with dry AMD and SMD [ 39 ]. Encouragingly, the study revealed no signs of adverse events like cell proliferation or immune rejection. In addition, the best-corrected visual acuity improved in 10 eyes, and measures related to vision-related quality of life showed enhancements [ 39 ]. In a clinical trial of MA09-hESC-derived RPE cells conducted with an Asian population, which included four participants, there was no evidence of adverse proliferation or tumorigenesis [ 40 ]. Furthermore, one patient experienced improved visual acuity, while the remaining three maintained stable visual acuity throughout the trial [ 40 ]. In the USA, a phase 1/2 clinical study was conducted using CPCB-RPE1, a composite implant consisting of a synthetic parylene substrate and a polarized monolayer of adherent hESC-RPE cells (NCT02590692). This study demonstrated safety and tolerability in legally blind patients with dry AMD [ 41 , 42 ]. However, graft survival remains a significant challenge, influenced by factors like aging of Bruch's membrane, subretinal scarring, para-inflammation, and choroid ischemia [ 33 ].
SMD, a prevalent retinal dystrophy affecting young individuals, is characterized by progressive vision loss, primarily caused by mutations in the ABCA4 gene, which leads to dysfunction of the ABCR protein expressed in retinal photoreceptors [ 43 ]. Currently, there are no established treatments to effectively improve vision in SMD, similar to the situation in dry AMD. Promising outcomes have been observed in preclinical models, including the safe subretinal injection of retinal pigment epithelium (RPE) derived from hESC. This approach was tested in a phase 1 clinical trial in the USA (NCT02941991). The WA-099 hESC line demonstrated the ability to spontaneously differentiate into RPE cells, with subsequent isolation of pigmentation cells. A suspension of these hESC-derived RPE cells, containing 1.0 × 10^6 cells in 0.1 mL, was surgically implanted subretinally in all eyes using a pars plana vitrectomy (PPV) approach [ 44 ]. The study's findings indicated no adverse events during the one-year postoperative follow-up period. Additionally, the treated eyes had no significant improvement in visual acuity [ 44 ]. In China, researchers Li et al. evaluated the Q-CTS-hESC-2 cell line-derived RPE in a 5-year follow-up study on seven patients and reported no significant adverse reactions and some temporary improvements in visual function, though two patients showed a long-term decrease in vision (NCT02749734) [ 45 ]. Sung et al., from the Republic of Korea, reported a 3-year study on Asian patients, also finding no serious adverse events and reporting stable or improved BCVA in some patients (NCT01625559) [ 46 ].
RP is a group of inherited retinal disorders characterized by the progression of vision loss due to photoreceptor degeneration, affecting approximately 1 in 4,000 individuals worldwide [ 47 , 48 ]. A Phase 1/2 clinical trial of RP with monogenic mutations is ongoing (NCT03963154), with interim analysis showing no adverse events in seven patients [ 49 ]. While these studies confirm the long-term safety and tolerability of hESC-RPE cell transplantation, they also highlight the need for further research to improve efficacy, including better patient selection and treatment methodologies, as significant and consistent improvements in visual function are yet to be established.
2.2.2 Neurologic diseases
The utilization of stem cell therapy derived from hESCs in treating neurological disorders is an emerging and promising area of research. As illustrated in Fig. 1 B, neurologic diseases are among the most researched applications in this field. This branch of medical science addresses a diverse spectrum of neurological conditions, including Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), spinal cord injuries (SCI), and multiple sclerosis. These disorders present considerable treatment challenges, largely due to the complexity of the nervous system and the typically permanent nature of neuronal damage involved. Ongoing studies are displayed in Table 2 .
The first-in-patient clinical trial on neurologic disease was conducted on SCI patients [ 50 ]. Oligodendrocyte progenitor cells (LCTOPC1), which are also nomenclature as AST-OPC1 or GRNOPC1, is the world's first hESC-derived therapy, and the phase 1 trial was approved by US-FDA in 2009, and the first patient was enrolled in 2011 (NCT01217008) [ 50 , 51 ]. Recent 10-year follow-up study results on five participants who received intraparenchymal injections of LCTOPC1 showed no serious adverse effects during follow-up, with 80% of patients showing MRI evidence of tissue matrix formation at the injury site [ 51 ]. This pivotal study, leading to a subsequent cervical dose escalation trial (NCT02302157), demonstrated the safety of hESC-derived therapies using LCTOPC1. In the trial, 25 participants with C4-7 spinal injuries received a single dose of 2, 10, or 20 million LCTOPC1 cells and low-dose tacrolimus for 60 days [ 52 ]. Despite some adverse events, including 29 serious ones, the treatment was well tolerated, with MRI scans showing no significant complications, and at a 1-year follow-up, 96% of participants improved by at least one level of neurological function, and 32% improved by two or more levels [ 52 ].
Additionally, research has shown that neural precursor cells marked by polysialic acid-neural cell adhesion molecule (PSA-NCAM), derived from hESC, can enhance neural tissue integrity in a rat stroke model [ 53 ]. Building on these findings, a phase 1/2a clinical trial (NCT04812431) is currently underway to assess the safety and efficacy of PSA-NCAM( +)-NPC for patients with sub-acute C4-C7 level spinal cord injuries. In this trial, the cells will be delivered intrathecally across five sites, and participants will be monitored for one year and five months as part of a follow-up study.
PD is a neurodegenerative disease characterized primarily by the loss of dopaminergic neurons in the substantia nigra, a region of the brain integral to controlling body movement. This loss leads to the classic symptoms of PD, including tremors, rigidity, bradykinesia, and postural instability [ 54 ]. The potential of hESC-based therapies in PD lies in their ability to differentiate into dopaminergic neurons, the type of cell lost in the disease [ 55 ]. The goal of transplanting hESC-derived cells in PD treatment is to replace the depleted neurons and normalize dopamine levels in the brain, which could help alleviate PD symptoms. MSK-DA01, a midbrain dopamine neuron cell derived from hESCs, is currently undergoing a Phase 1 trial in the United States (NCT04802733). A preclinical study on MSK-DA01 demonstrated successful graft survival and improved behavior in rats with 6-hydroxydopamine-induced lesions, a model for PD. Importantly, these studies revealed no adverse effects related to the graft cells and no unexpected cell proliferation outside the brain, indicating a promising safety profile for this innovative therapy [ 56 ].
STEM-PD, another product consisting of dopaminergic neuronal progenitor cells derived from hESCs, has also been evaluated in a preclinical study [ 57 ]. This study showed the precise stereotactic injection of STEM-PD into a pig model and demonstrated effective innervation of the targeted brain regions. Additionally, this intervention led to a reversal of motor deficits in the pig model of Parkinson's disease, demonstrating the potential efficacy of STEM-PD in addressing the symptoms associated with this neurodegenerative disorder [ 57 ]. Presently, STEM-PD is the subject of a phase 1 clinical trial in the United Kingdom, which is in the process of recruiting eight patients, and this trial marks a significant step in evaluating the safety and potential efficacy of STEM-PD in human subjects, specifically targeting the treatment of PD (NCT05635409).
A research team in China successfully derived dopaminergic neurons from hESCs and demonstrated sustained behavioral improvements over two years in a monkey model of PD [ 58 ]. This significant advancement in stem cell research has led to the registration of a Phase 1 clinical trial (NCT03119636). However, the current status of this trial remains unknown.
ALS, a severe neurodegenerative condition, is characterized by the deterioration of both upper and lower motor neurons (MNs), resulting in the progressive paralysis of muscles controlled by these neurons [ 59 ]. While FDA-approved treatments like riluzole have demonstrated some efficacy in prolonging survival, there remains a significant unmet need for more effective ALS therapies [ 60 ]. Recent evidence points to the involvement of astrocytes in the pathogenesis of ALS [ 61 ]. AstroRx®, a novel cell therapy derived from hESCs, has shown promise in addressing this gap, as evidenced by the outcomes of its recent Phase 1/2a clinical trial [ 62 ]. AstroRx®, administered as a single intrathecal injection, was tested in two cohorts of ALS patients—a low-dose and a high-dose group, each consisting of five patients (NCT03482050). The administration of AstroRx® showed a clinically significant impact lasting for three months post-treatment, with particularly notable effects observed in a group of rapid progressors [ 62 ].
NR1, an hESC-derived neural stem cell, is under investigation for chronic ischemic stroke patients who are 6–60 months post-ischemic subcortical mid-cerebral artery stroke (NCT04631406). Six patients underwent transplantation with NR1, and there was a notable improvement in the Mugl-Meyer motor score. Additionally, all six patients exhibited a transient flair signal that resolved within two months, which correlated with neurological recovery [ 63 ].
2.2.3 Diabetes mellitus
Type 1 Diabetes Mellitus (T1DM) commonly manifests in childhood and adolescence and is marked by a chronic autoimmune condition leading to the loss of insulin-producing beta cells in the pancreas [ 64 ]. Unlike Type 2 DM, which often relates to lifestyle and insulin resistance, T1DM is primarily driven by an autoimmune response [ 64 ]. In stem cell therapy for T1DM, two main strategies have emerged: one involves replacing the missing insulin-producing beta cells, while the other focuses on immunomodulation to safeguard existing beta cells from further autoimmune destruction [ 65 ]. Seven registered clinical trials for stem cell-based treatment of T1DM using hESC are summarized in Table 3 .
Schulz and colleagues described the creation of the VC-01 composite product utilizing pancreatic endoderm cells (PEC-01) obtained from CyT49 hESCs with a retrievable semi-permeable encapsulating device drug delivery system [ 66 ]. VC-02, developed in 2017, is an advanced model featuring multiple large pores across the membrane to facilitate vascularization while maintaining immune isolation [ 67 ]. VC-01 was investigated in phase 1/2 trial (NCT02239354; NCT04678557; NCT02939118) and VC-02 was investigated in phase 1/2 trial (NCT03163511). In the phase 1/2 study of the VC-01 product, immunosuppressants were not administered, leading to a host reaction against the implant, ultimately resulting in its destruction, and the study was terminated [ 68 ]. A Phase 1/2 study involving 17 patients with T1DM was carried out following a modification in the VC-02 device. This study demonstrated successful engraftment and insulin release in 63% of the cases, and as early as six months post-implantation, 35.3% of the participants showed positive C-peptide levels. These results indicate the potential of VC-02 as a viable alternative for T1DM treatment. However, it's important to note that some reported adverse events were primarily related to the surgical procedures of implanting or explanting the device and the side effects of immunosuppression therapy [ 69 ]. VCTX210A represents an innovative approach that uses pancreatic endodermal cells (PEC210A) derived from hESC. These cells have been genetically modified using the CRISPR/Cas9 (Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated protein 9) technology. This modification enhances the cells' survival against the patient's immune system, thereby addressing the challenge of graft versus host disease [ 70 ]. Additionally, VX880, a fully differentiated pancreatic islet cell product derived from hESC designed to treat T1DM, is undergoing clinical investigation (NCT04786262). Interim data analysis from this study has yielded positive results, indicating that the treatment successfully restored insulin production in the first two patients enrolled in the trial [ 71 ].
2.2.4 Female reproductive organ and genitourinary disease
The field of female reproductive organ disorders is increasingly looking towards stem cell therapy and cutting-edge biomedical technologies for potential treatments, as shown in Table 4 . Intravenous injection of hESC-derived mesenchymal cells (hESC-MCs) showed restoration of ovarian function induced by the chemotherapeutic agent in a murine model [ 72 , 73 ]. A product, hESC-MC, has been explored by a Chinese research group for treating moderate to severe intrauterine adhesion (NCT04232592). Additionally, a therapy involving hESC-MC product is currently being investigated as a potential treatment for primary ovarian insufficiency (NCT03877471). Additionally, Table 5 showcases the application of hESC-derived mesenchymal stem cell therapy, specifically MR-MVC-01, which is currently under investigation for treating interstitial cystitis, as per the clinical trial registered under NCT04610359.
2.2.5 Cardiovascular disease
In the field of heart failure treatment, the innovative application of human embryonic stem cells (hESCs) offers a promising alternative to conventional therapies. Table 5 also highlights hESC-derived cardiac progenitor cell-based products in treating heart failure and ischemic heart disease, as illustrated in the clinical trials registered under NCT02057900 and NCT05068674. The ESCORT trial (NCT02057900), conducted in France, marked a pioneering venture in employing hESC-derived cardiomyocytes for heart failure treatment, setting a precedent that has been followed by the HECTOR trial (NCT05068674) in the United States, initiated in 2022. The ESCORT trial, focusing on patients with severe ischemic left ventricular dysfunction, demonstrated the feasibility and safety of using hESC-derived cardiovascular progenitor cells, embedded in a fibrin patch, applied to the damaged heart areas during coronary artery bypass surgery [ 74 ]. The results, including the production of a highly purified batch of progenitor cells and significant symptomatic improvements in patients, though with instances of silent alloimmunization, have laid the groundwork for future explorations in this domain. The HECTOR trial in the U.S. is building upon this foundation with a novel approach, utilizing hESC-derived cardiomyocytes (hESC-CMs) to enhance survival and cardiac function in patients with chronic left ventricular dysfunction secondary to myocardial infarction. This phase I dose-escalation pilot study is designed as an initial safety assessment to determine the maximum tolerated dose (MTD) before proceeding to a phase II randomized, double-blinded, placebo-controlled study. Approximately eighteen patients who are scheduled for cardiac catheterization and meet all inclusion/exclusion criteria will participate in this initial phase. The HECTOR trial represents a significant step forward in the application of hESC-CMs in cardiac therapy, with great anticipation for its potential to revolutionize the treatment of heart failure and related conditions.
2.3 Challenges and ethical considerations
As we explore the burgeoning field of hESC research and its clinical applications, it becomes crucial to examine the accompanying ethical and practical challenges thoroughly. While this area of research offers groundbreaking possibilities in treating various diseases, it is intertwined with complex ethical, legal, and social issues, particularly due to the involvement of human embryos.
2.3.1 Derivation of hESC
In the field of hESC research, the ethical implications surrounding the derivation of these cells from embryos are paramount. hESCs are typically harvested from embryos at the blastocyst stage approximately 5–6 days post-fertilization. This stage of development is critical because it leads to the inevitable destruction of the embryo, a primary ethical concern in this field of research [ 19 , 75 , 76 , 77 ].
Due to their pluripotency, the significant potential of hESCs makes them a valuable asset in understanding disease mechanisms, drug testing, and potential regenerative therapies [ 78 ]. Moreover, hESCs are obtained early in induced pluripotent development, making them crucial for studying human developmental processes and various diseases [ 17 ]. They play a vital role, especially when embryos are discarded after positive preimplantation genetic testing (PGT) results, contributing to our understanding of genetic abnormalities and disease ecology [ 17 ].
Regarding the moral status of the embryo, there are varying views. The Catholic perspective often sees life beginning at fertilization, while Judaism and Islam view the blastocyst as having the potential for life but not as fully alive [ 79 , 80 ]. Hinduism and Buddhism do not provide a clear doctrinal definition of life's beginning, adopting a more philosophical and spiritual perspective [ 81 ].
The use of surplus IVF embryos in hESC research is often defended under the principle of proportionality. This approach favors using them for stem cell research due to the broader potential benefits compared to enhancing IVF techniques [ 17 ]. The utilization of embryos with monogenic defects (PGT-M) or aneuploidies (PGT-A) for deriving disease-specific stem cells is seen as a promising avenue for advancing the understanding of specific diseases and developing targeted treatments [ 9 , 17 ].
In summary, hESC research presents a complex ethical landscape. The scientific and medical benefits of hESCs must be balanced against the moral considerations surrounding the use of human embryos, necessitating a nuanced approach to this rapidly evolving field.
2.3.2 Regulatory issues
In the realm of research involving hESCs, regulatory issues play a crucial role, varying significantly across different countries. Obtaining approval from institutional review boards (IRBs) and adhering to regulations set by authoritative bodies are pivotal steps in developing and progressing hESC-related research and development.
Procedures involving the transfer of stem cells are subject to specific regulations. This encompasses the process of transferring stem cell materials, which requires careful adherence to legal and ethical guidelines [ 15 , 82 ]. It's essential to ensure that the transfer agreements are comprehensive, detailing any restrictions and obligations related to using the materials and associated data [ 83 , 84 ]. Such transfers must respect donor rights and comply with the regulatory frameworks of both the donating and receiving entities.
The process of creating stem cell products that are safe for clinical use involves several critical steps. This includes extensive testing for genetic stability and absence of contaminants, ensuring the cells' identity and functionality, and verifying that they meet the stringent safety standards required for clinical application [ 82 ]. These procedures are designed to safeguard patient safety and ensure the efficacy of the stem cell products.
Overall, the development and research involving hESCs must navigate a complex landscape of regulatory requirements. These regulations are in place to ensure the ethical use of human stem cells, the protection of donor rights, and the safety and efficacy of stem cell-based therapies. Compliance with these regulations is not only a legal requirement but also a cornerstone in maintaining the integrity and credibility of stem cell research.
3 Conclusion
The exploration of hESCs over the past two decades has opened new frontiers in medical science, particularly in the fields of regenerative medicine and cell-based therapies. The landmark discovery and subsequent developments have brought immense potential for understanding and treating a wide range of diseases, from genetic disorders to degenerative conditions.
However, the journey of hESC research is intertwined with a plethora of ethical, legal, and regulatory challenges. The ethical considerations, primarily regarding the use of human embryos, highlight the delicate balance between scientific advancement and moral imperatives. Different religious and cultural perspectives on embryo status underline this debate's complexity. As we have seen, approaches to this issue vary significantly worldwide, influencing the regulatory landscape and research in different countries.
The advancements in hESC research also underscore the importance of robust regulatory frameworks and adherence to ethical standards. From acquiring embryonic materials to developing stem cell-based products for clinical use, each step requires careful consideration of ethical guidelines, safety standards, and regulatory compliance. The involvement of IRBs and adherence to international standards and guidelines are critical in ensuring that the research is conducted responsibly and with the utmost respect for human life and dignity.
Looking ahead, the field of hESC research holds immense promise. With continued technological advancements and a deeper understanding of stem cells' capabilities, we stand on the brink of revolutionary medical breakthroughs. However, the path forward must be navigated with a commitment to ethical principles, regulatory compliance, and public engagement. By upholding these standards, the scientific community can ensure that the benefits of hESC research are realized in a manner that respects human values and contributes positively to human health and well-being.
In conclusion, hESC research represents scientific innovation, ethical reflection, and regulatory prudence. As we continue to advance in this field, it is imperative to maintain a balanced approach that fosters scientific discovery while honoring ethical obligations and regulatory requirements. The future of hESC research, promising as it is, depends on our collective ability to navigate these complex and multifaceted challenges.
Data availability statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7.
Article CAS PubMed Google Scholar
Oh SK, Kim HS, Ahn HJ, Seol HW, Kim YY, Park YB, et al. Derivation and characterization of new human embryonic stem cell lines: SNUhES1, SNUhES2, and SNUhES3. Stem Cells. 2005;23:211–9.
Article PubMed Google Scholar
Doğan A. Embryonic stem cells in development and regenerative medicine. Adv Exp Med Biol. 2018;1079:1–15.
Kolios G, Moodley Y. Introduction to stem cells and regenerative medicine. Respiration. 2013;85:3–10.
Jarrige M, Frank E, Herardot E, Martineau S, Darle A, Benabides M, et al. The future of regenerative medicine: cell therapy using pluripotent stem cells and acellular therapies based on extracellular vesicles. Cells. 2021;10:240.
Article CAS PubMed PubMed Central Google Scholar
Kidha DK. Human embryonic stem cell research in transplantation and regenerative medicine: a principlist assessment; 2020.
Kim YY, Ku SY, Liu HC, Cho HJ, Oh SK, Moon SY, et al. Cryopreservation of human embryonic stem cells derived-cardiomyocytes induced by BMP2 in serum-free condition. Rep Sci. 2011;18:252–60.
Kim YY, Min H, Kim H, Choi YM, Liu HC, Ku SY. Differential MicroRNA expression profile of human embryonic stem cell-derived cardiac lineage cells. Tissue Eng Regen Med. 2017;14:163–9.
Douglas T, Savulescu J. Destroying unwanted embryos in research. Talking Point on morality and human embryo research. EMBO Rep. 2009;10:307–12.
Fan R. The ethics of human embryonic stem cell research and the of the family. In: Lee SC, editor. The family, medical decision-making, and biotechnology: Critical reflections on Asian moral perspectives. The MIT Press: Springer; 2007. p. 127–48.
Turksen K. Cell biology and translational medicine. vol. 11. Berlin: Springer; 2021.
Book Google Scholar
Singh MS, Park SS, Albini TA, Canto-Soler MV, Klassen H, MacLaren RE, et al. Retinal stem cell transplantation: balancing safety and potential. Prog Retin Eye Res. 2020;75:100779.
Song MJ, Bharti K. Looking into the future: using induced pluripotent stem cells to build two and three dimensional ocular tissue for cell therapy and disease modeling. Brain Res. 2016;1638:2–14.
Lefkopoulos S. Enhancing reproducibility in human stem cell research. Nat Cell Biol. 2023;25:1237–9.
Ludwig TE, Andrews PW, Barbaric I, Benvenisty N, Bhattacharyya A, Crook JM, et al. ISSCR standards for the use of human stem cells in basic research. Stem Cell Rep. 2023;18:1744–52.
Article CAS Google Scholar
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72.
Salari S, Adashi EY, Keller L, Johnson TRB, Smith GD. Human embryos donated for human embryonic stem cell derivation. Fertil Steril. 2023;119:3–10.
King NM, Perrin J. Ethical issues in stem cell research and therapy. Stem Cell Res Ther. 2014;5:85.
Article PubMed PubMed Central Google Scholar
Banja JD. Ethical considerations in stem cell research on neurologic and orthopedic conditions. PM R. 2015;7:S66-75.
Fleckenstein M, Mitchell P, Freund KB, Sadda S, Holz FG, Brittain C, et al. The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125:369–90.
Chakravarthy U, Bailey CC, Johnston RL, McKibbin M, Khan RS, Mahmood S, et al. Characterizing disease burden and progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125:842–9.
GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9:e144–60.
Guymer RH, Wu Z, Hodgson LAB, Caruso E, Brassington KH, Tindill N, et al. Subthreshold nanosecond laser intervention in age-related macular degeneration: the LEAD randomized controlled clinical trial. Ophthalmology. 2019;126:829–38.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31.
Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36:331–5.
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48.
Pearce I, Amoaku W, Bailey C, Downey L, Gale R, Ghanchi F, et al. The changing landscape for the management of patients with neovascular AMD: brolucizumab in clinical practice. Eye (Lond). 2022;36:1725–34.
George SM, Lu F, Rao M, Leach LL, Gross JM. The retinal pigment epithelium: development, injury responses, and regenerative potential in mammalian and non-mammalian systems. Prog Retin Eye Res. 2021;85:100969.
Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–81.
Sharma A, Jaganathan BG. Stem cell therapy for retinal degeneration: the evidence to date. Biologics. 2021;15:299–306.
PubMed PubMed Central Google Scholar
Idelson M, Alper R, Obolensky A, Yachimovich-Cohen N, Rachmilewitz J, Ejzenberg A, et al. Immunological properties of human embryonic stem cell-derived retinal pigment epithelial cells. Stem Cell Rep. 2018;11:681–95.
Yamasaki S, Sugita S, Horiuchi M, Masuda T, Fujii S, Makabe K, et al. Low immunogenicity and immunosuppressive properties of human ESC- and iPSC-derived retinas. Stem Cell Rep. 2021;16:851–67.
Qiu TG. Transplantation of human embryonic stem cell-derived retinal pigment epithelial cells (MA09-hRPE) in macular degeneration. NPJ Regen Med. 2019;4:19.
Tomita M, Lavik E, Klassen H, Zahir T, Langer R, Young MJ. Biodegradable polymer composite grafts promote the survival and differentiation of retinal progenitor cells. Stem Cells. 2005;23:1579–88.
Koss MJ, Falabella P, Stefanini FR, Pfister M, Thomas BB, Kashani AH, et al. Subretinal implantation of a monolayer of human embryonic stem cell-derived retinal pigment epithelium: a feasibility and safety study in Yucatán minipigs. Graefes Arch Clin Exp Ophthalmol. 2016;254:1553–65.
Petrus-Reurer S, Bartuma H, Aronsson M, Westman S, Lanner F, Kvanta A. Subretinal transplantation of human embryonic stem cell derived-retinal pigment epithelial cells into a large-eyed model of geographic atrophy. J Vis Exp. 2018.
Ilmarinen T, Thieltges F, Hongisto H, Juuti-Uusitalo K, Koistinen A, Kaarniranta K, et al. Survival and functionality of xeno-free human embryonic stem cell-derived retinal pigment epithelial cells on polyester substrate after transplantation in rabbits. Acta Ophthalmol. 2019;97:e688–99.
Schwartz SD, Hubschman J-P, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379:713–20.
Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509–16.
Song WK, Park KM, Kim HJ, Lee JH, Choi J, Chong SY, et al. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: preliminary results in Asian patients. Stem Cell Rep. 2015;4:860–72.
Kashani AH, Lebkowski JS, Rahhal FM, Avery RL, Salehi-Had H, Chen S, et al. One-year follow-up in a phase 1/2a clinical trial of an allogeneic RPE cell bioengineered implant for advanced dry age-related macular degeneration. Transl Vis Sci Technol. 2021;10:13.
Kashani AH, Uang J, Mert M, Rahhal F, Chan C, Avery RL, et al. Surgical method for implantation of a biosynthetic retinal pigment epithelium monolayer for geographic atrophy: experience from a phase 1/2a Study. Ophthalmol Retina. 2020;4:264–73.
Hussain RM, Ciulla TA, Berrocal AM, Gregori NZ, Flynn HW Jr, Lam BL. Stargardt macular dystrophy and evolving therapies. Expert Opin Biol Ther. 2018;18:1049–59.
Brant Fernandes RA, Lojudice FH, Zago Ribeiro L, Santos da Cruz NF, Polizelli MU, Cristovam PC, et al. Transplantation of subretinal stem cell-derived retinal pigment epithelium for Stargardt disease: a phase I clinical trial. Retina. 2023;43:263–74.
Li SY, Liu Y, Wang L, Wang F, Zhao TT, Li QY, et al. A phase I clinical trial of human embryonic stem cell-derived retinal pigment epithelial cells for early-stage Stargardt macular degeneration: 5-years’ follow-up. Cell Prolif. 2021;54:e13100.
Sung Y, Lee MJ, Choi J, Jung SY, Chong SY, Sung JH, et al. Long-term safety and tolerability of subretinal transplantation of embryonic stem cell-derived retinal pigment epithelium in Asian Stargardt disease patients. Br J Ophthalmol. 2021;105:829–37.
Becherucci V, Bacci GM, Marziali E, Sodi A, Bambi F, Caputo R. The new era of therapeutic strategies for the treatment of retinitis pigmentosa: a narrative review of pathomolecular mechanisms for the development of cell-based therapies. Biomedicines. 2023;11:2656.
Lozano B LL, Cervantes A LA. Development of experimental treatments for patients with retinitis pigmentosa. Arch Soc Esp Oftalmol (Engl Ed). 2023;98:646–55.
Monville C, Bertin S, Devisme C, Brazhnikova E, Jaillard C, Walter H, et al. Phase I/II open-label study of implantation into one eye of hESC-derived RPE in patients with retinitis pigmentosa due to monogenic mutation: first safety results. Invest Ophthalmol Vis Sci. 2023;64:3829–929.
Google Scholar
Lebkowski J. GRNOPC1: the world’s first embryonic stem cell-derived therapy. Interview with Jane Lebkowski. Regen Med. 2011;6:11–3.
McKenna SL, Ehsanian R, Liu CY, Steinberg GK, Jones L, Lebkowski JS, et al. Ten-year safety of pluripotent stem cell transplantation in acute thoracic spinal cord injury. J Neurosurg Spine. 2022. https://doi.org/10.3171/2021.12.Spine21622
Fessler RG, Ehsanian R, Liu CY, Steinberg GK, Jones L, Lebkowski JS, et al. A phase 1/2a dose-escalation study of oligodendrocyte progenitor cells in individuals with subacute cervical spinal cord injury. J Neurosurg Spine. 2022;37:812–20.
Kim HS, Choi SM, Yang W, Kim DS, Lee DR, Cho SR, et al. PSA-NCAM(+) neural precursor cells from human embryonic stem cells promote neural tissue integrity and behavioral performance in a rat stroke model. Stem Cell Rev Rep. 2014;10:761–71.
Hayes MT. Parkinson’s Disease and Parkinsonism. Am J Med. 2019;132:802–7.
Sonntag KC, Song B, Lee N, Jung JH, Cha Y, Leblanc P, et al. Pluripotent stem cell-based therapy for Parkinson’s disease: current status and future prospects. Prog Neurobiol. 2018;168:1–20.
Piao J, Zabierowski S, Dubose BN, Hill EJ, Navare M, Claros N, et al. Preclinical efficacy and safety of a human embryonic stem cell-derived midbrain dopamine progenitor product, MSK-DA01. Cell Stem Cell. 2021;28:217-29.e7.
Kirkeby A, Nelander J, Hoban DB, Rogelius N, Bjartmarz H, Storm P, et al. Preclinical quality, safety, and efficacy of a human embryonic stem cell-derived product for the treatment of Parkinson’s disease. STEM-PD Cell Stem Cell. 2023;30:1299-314.e9.
Wang YK, Zhu WW, Wu MH, Wu YH, Liu ZX, Liang LM, et al. Human clinical-grade parthenogenetic ESC-derived dopaminergic neurons recover locomotive defects of nonhuman primate models of Parkinson’s disease. Stem Cell Reports. 2018;11:171–82.
Feldman EL, Goutman SA, Petri S, Mazzini L, Savelieff MG, Shaw PJ, et al. Amyotrophic lateral sclerosis. Lancet. 2022;400:1363–80.
Jaiswal MK. Riluzole and edaravone: a tale of two amyotrophic lateral sclerosis drugs. Med Res Rev. 2019;39:733–48.
Brandebura AN, Paumier A, Onur TS, Allen NJ. Astrocyte contribution to dysfunction, risk and progression in neurodegenerative disorders. Nat Rev Neurosci. 2023;24:23–39.
Gotkine M, Caraco Y, Lerner Y, Blotnick S, Wanounou M, Slutsky SG, et al. Safety and efficacy of first-in-man intrathecal injection of human astrocytes (AstroRx®) in ALS patients: phase I/IIa clinical trial results. J Transl Med. 2023;21:122.
Steinberg GK, Bet A, Williams J, McDonald K, Diaz R, Samos C, et al. First-in-human phase 1/2a study of intracerebral transplantation using embryonic-derived neural stem cells (NR1) for chronic ischemic stroke. Stroke. 2023;54:A147–247.
Article Google Scholar
Acharjee S, Ghosh B, Al-Dhubiab BE, Nair AB. Understanding type 1 diabetes: etiology and models. Can J Diabetes. 2013;37:269–76.
Chen S, Du K, Zou C. Current progress in stem cell therapy for type 1 diabetes mellitus. Stem Cell Res Ther. 2020;11:1–13.
Schulz TC. Concise review: manufacturing of pancreatic endoderm cells for clinical trials in type 1 diabetes. Stem Cells Transl Med. 2015;4:927–31.
Dang HP, Chen H, Dargaville TR, Tuch BE. Cell delivery systems: toward the next generation of cell therapies for type 1 diabetes. J Cell Mol Med. 2022;26:4756–67.
Pullen LC. Stem cell-derived pancreatic progenitor cells have now been transplanted into patients: report from IPITA 2018. Am J Transplant. 2018;18:1581–2.
Shapiro AMJ, Thompson D, Donner TW, Bellin MD, Hsueh W, Pettus J, et al. Insulin expression and C-peptide in type 1 diabetes subjects implanted with stem cell-derived pancreatic endoderm cells in an encapsulation device. Cell Rep Med. 2021;2: 100466.
Ellis CE, Mojibian M, Ida S, Fung VCW, Skovsø S, McIver E, et al. Human A2-CAR T cells reject HLA-A2 + human islets transplanted into mice without inducing graft-versus-host disease. Transplantation. 2023;107:e222–33.
Reichiman TW, Ricordi C, Naji A, Markmann JF, Perkins BA, Wijkstrom M, et al. 836-P: Glucose-dependent insulin production and insulin-independence in type 1 diabetes from stem cell–derived, fully differentiated islet cells—updated data from the VX-880 cinical trial. Diabetes. 2023. https://doi.org/10.2337/db23-836-P
Yoon SY, Yoon JA, Park M, Shin EY, Jung S, Lee JE, et al. Recovery of ovarian function by human embryonic stem cell-derived mesenchymal stem cells in cisplatin-induced premature ovarian failure in mice. Stem Cell Res Ther. 2020;11:255.
Bahrehbar K, Rezazadeh Valojerdi M, Esfandiari F, Fathi R, Hassani SN, Baharvand H. Human embryonic stem cell-derived mesenchymal stem cells improved premature ovarian failure. World J Stem Cells. 2020;12:857–78.
Menasché P, Vanneaux V, Hagège A, Bel A, Cholley B, Parouchev A, et al. Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J Am Coll Cardiol. 2018;71:429–38.
de Wert G, Mummery C. Human embryonic stem cells: research, ethics and policy. Hum Reprod. 2003;18:672–82.
Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, et al. Ethical and safety issues of stem cell-based therapy. Int J Med Sci. 2018;15:36–45.
Lo B, Parham L. Ethical issues in stem cell research. Endocr Rev. 2009;30:204–13.
Liu G, David BT, Trawczynski M, Fessler RG. Advances in pluripotent stem cells: history, mechanisms, technologies, and applications. Stem Cell Rev Rep. 2020;16:3–32.
Walters L. Human embryonic stem cell research: an intercultural perspective. Kennedy Inst Ethics J. 2004;14:3–38.
Neaves W. The status of the human embryo in various religions. Development. 2017;144:2541–3.
Sivaraman MAF, Noor SNM. Human embryonic stem cell research: ethical views of Buddhist, Hindu and Catholic Leaders in Malaysia. Sci Eng Ethics. 2016;22:467–85.
International Society for Stem Cell Research. Standards for human stem Cell use in research. https://www.isscr.org/s/ISSCR_Standards_09_FINAL.pdf.
Bubela T, Guebert J, Mishra A. Use and misuse of material transfer agreements: lessons in proportionality from research, repositories, and litigation. PLoS Biol. 2015;13:e1002060.
Lovell-Badge R, Anthony E, Barker RA, Bubela T, Brivanlou AH, Carpenter M, et al. ISSCR guidelines for stem cell research and clinical translation: the 2021 update. Stem Cell Rep. 2021;16:1398–408.
Download references
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant Number: HI22C1424) and the Grants of the Ministry of ICT Grants and the Ministry of Education, Republic of Korea (2020R1A2C1010293).
Open Access funding enabled and organized by Seoul National University.
Author information
Authors and affiliations.
Department of Obstetrics and Gynecology, Seoul National University Hospital, Seoul, Republic of Korea
Soo Jin Park, Yoon Young Kim, Ji Yeon Han, Sung Woo Kim, Hoon Kim & Seung-Yup Ku
Department of Obstetrics and Gynecology, Seoul National University College of Medicine, 101 Daehak-Ro Jongno-Gu, Seoul, 03080, Republic of Korea
Hoon Kim & Seung-Yup Ku
Institute of Reproductive Medicine and Population, Medical Research Center, Seoul National University, Seoul, Republic of Korea
Yoon Young Kim, Hoon Kim & Seung-Yup Ku
You can also search for this author in PubMed Google Scholar
Contributions
SJP: conceptualization, methodology, formal analysis, resources, data curation, investigations, visualization, Writing—Original Draft, Visualization, project administration, funding acquisition. YYK: methodology, validation, Writing—Review & Editing, Supervision. JYH: methodology, investigation, validation, supervision. SWK: methodology, investigation, validation, supervision. HK: methodology, investigation, validation, supervision. S-YK: conceptualization, methodology, project administration, funding acquisition.
Corresponding author
Correspondence to Seung-Yup Ku .
Ethics declarations
Conflict of interest.
The authors declare nothing to disclose.
Ethical statement
There are no animal experiments carried out for this article.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Park, S.J., Kim, Y.Y., Han, J.Y. et al. Advancements in Human Embryonic Stem Cell Research: Clinical Applications and Ethical Issues. Tissue Eng Regen Med 21 , 379–394 (2024). https://doi.org/10.1007/s13770-024-00627-3
Download citation
Received : 12 December 2023
Revised : 05 January 2024
Accepted : 08 January 2024
Published : 19 March 2024
Issue Date : April 2024
DOI : https://doi.org/10.1007/s13770-024-00627-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Human embryonic stem cells
- Regenerative medicine
- Clinical trials
- Stem cell research
- Find a journal
- Publish with us
- Track your research

Lupus patient Katherine Hammons comforts fellow patient Margaret Laperle, both treated with stem cells from their own bone marrow. Stem cells could launch a new era of regenerative medicine, curing deadly diseases with custom-made tissues and organs.
The Stem Cell Divide
In the beginning, one cell becomes two, and two become four. Being fruitful, they multiply into a ball of many cells, a shimmering sphere of human potential. Scientists have long dreamed of plucking those naive cells from a young human embryo and coaxing them to perform, in sterile isolation, the everyday miracle they perform in wombs: transforming into all the 200 or so kinds of cells that constitute a human body. Liver cells. Brain cells. Skin, bone, and nerve.
The dream is to launch a medical revolution in which ailing organs and tissues might be repaired—not with crude mechanical devices like insulin pumps and titanium joints but with living, homegrown replacements. It would be the dawn of a new era of regenerative medicine, one of the holy grails of modern biology.
Revolutions, alas, are almost always messy. So when James Thomson, a soft-spoken scientist at the University of Wisconsin in Madison, reported in November 1998 that he had succeeded in removing cells from spare embryos at fertility clinics and establishing the world's first human embryonic stem cell line, he and other scientists got a lot more than they bargained for. It was the kind of discovery that under most circumstances would have blossomed into a major federal research enterprise. Instead the discovery was quickly engulfed in the turbulent waters of religion and politics. In church pews, congressional hearing rooms, and finally the Oval Office, people wanted to know: Where were the needed embryos going to come from, and how many would have to be destroyed to treat the millions of patients who might be helped? Before long, countries around the world were embroiled in the debate.
Most alarmed have been people who see embryos as fully vested, vulnerable members of society, and who decry the harvesting of cells from embryos as akin to cannibalism. They warn of a brave new world of "embryo farms" and "cloning mills" for the cultivation of human spare parts. And they argue that scientists can achieve the same results using adult stem cells— immature cells found in bone marrow and other organs in adult human beings, as well as in umbilical cords normally discarded at birth.
Advocates counter that adult stem cells, useful as they may be for some diseases, have thus far proved incapable of producing the full range of cell types that embryonic stem cells can. They point out that fertility clinic freezers worldwide are bulging with thousands of unwanted embryos slated for disposal. Those embryos are each smaller than the period at the end of this sentence. They have no identifying features or hints of a nervous system. If parents agree to donate them, supporters say, it would be unethical not to do so in the quest to cure people of disease.
Few question the medical promise of embryonic stem cells. Consider the biggest United States killer of all: heart disease. Embryonic stem cells can be trained to grow into heart muscle cells that, even in a laboratory dish, clump together and pulse in spooky unison. And when those heart cells have been injected into mice and pigs with heart disease, they've filled in for injured or dead cells and sped recovery. Similar studies have suggested stem cells' potential for conditions such as diabetes and spinal cord injury.
FREE BONUS ISSUE
Critics point to worrisome animal research showing that embryonic stem cells sometimes grow into tumors or morph into unwanted kinds of tissues—possibly forming, for example, dangerous bits of bone in those hearts they are supposedly repairing. But supporters respond that such problems are rare and a lot has recently been learned about how to prevent them.
The arguments go back and forth, but policymakers and governments aren't waiting for answers. Some countries, such as Germany, worried about a slippery slope toward unethical human experimentation, have already prohibited some types of stem cell research. Others, like the U.S., have imposed severe limits on government funding but have left the private sector to do what it wants. Still others, such as the U.K., China, Korea, and Singapore, have set out to become the epicenters of stem cell research, providing money as well as ethical oversight to encourage the field within carefully drawn bounds.
In such varied political climates, scientists around the globe are racing to see which techniques will produce treatments soonest. Their approaches vary, but on one point, all seem to agree: How humanity handles its control over the mysteries of embryo development will say a lot about who we are and what we're becoming.
For more than half of his seven years, Cedric Seldon has been fighting leukemia. Now having run out of options, he is about to become a biomedical pioneer—one of about 600 Americans last year to be treated with an umbilical cord blood transplant.
Cord blood transplants—considered an adult stem cell therapy because the cells come from infants, not embryos—have been performed since 1988. Like bone marrow, which doctors have been transplanting since 1968, cord blood is richly endowed with a kind of stem cell that gives rise to oxygen-carrying red blood cells, disease-fighting white blood cells, and other parts of the blood and immune systems. Unlike a simple blood transfusion, which provides a batch of cells destined to die in a few months, the stem cells found in bone marrow and cord blood can—if all goes well—burrow into a person's bones, settle there for good, and generate fresh blood and immune cells for a lifetime.
Propped on a hospital bed at Duke University Medical Center, Cedric works his thumbs furiously against a pair of joysticks that control a careening vehicle in a Starsky and Hutch video game. "Hang on, Hutch!" older brother Daniel shouts from the bedside, as a nurse, ignoring the screeching tires and gunshots, sorts through a jumble of tubes and hangs a bag of cord blood cells from a chrome pole. Just an hour ago I watched those cells being thawed and spun in a centrifuge—awakening them for the first time since 2001, when they were extracted from the umbilical cord of a newborn and donated by her parents to a cell bank at Duke. The time has come for those cells to prove their reputed mettle.
For days Cedric has endured walloping doses of chemotherapy and radiation in a last-ditch effort to kill every cancer cell in his body. Such powerful therapy has the dangerous side-effect of destroying patients' blood-making stem cells, and so is never applied unless replacement stem cells are available. A search of every bone marrow bank in the country had found no match for Cedric's genetic profile, and it was beginning to look as if he'd run out of time. Then a computer search turned up the frozen cord blood cells at Duke—not a perfect match, but close enough to justify trying.
"Ready?" the nurse asks. Mom and dad, who have spent hours in prayer, nod yes, and a line of crimson wends its way down the tube, bringing the first of about 600 million cells into the boy's body. The video game's sound effects seem to fade behind a muffling curtain of suspense. Although Cedric's balloon-laden room is buoyant with optimism, success is far from certain.
"Grow, cells, grow," Cedric's dad whispers.
His mom's eyes are misty. I ask what she sees when she looks at the cells trickling into her son.
"Life," she says. "It's his rebirth."
It will be a month before tests reveal whether Cedric's new cells have taken root, but in a way he's lucky. All he needs is a new blood supply and immune system, which are relatively easy to re-create. Countless other patients are desperate to regenerate more than that. Diabetics need new insulin-producing cells. Heart attack victims could benefit from new cardiac cells. Paraplegics might even walk again if the nerves in their spinal cords could regrow.
In a brightly lit laboratory halfway across the country from Cedric's hospital room, three teams of scientists at the University of Wisconsin in Madison are learning how to grow the embryonic stem cells that might make such cures possible. Unlike adult stem cells, which appear to have limited repertoires, embryonic stem cells are pluripotent—they can become virtually every kind of human cell. The cells being nurtured here are direct descendants of the ones James Thomson isolated seven years ago.
For years Thomson and his colleagues have been expanding some of those original stem cells into what are called stem cell lines—colonies of millions of pluripotent cells that keep proliferating without differentiating into specific cell types. The scientists have repeatedly moved each cell's offspring to less crowded laboratory dishes, allowing them to divide again and again. And while they worked, the nation struggled to get a handle on the morality of what they were doing.
It took almost two years for President Bill Clinton's administration to devise ethics guidelines and a system for funding the new field. George W. Bush's ascension prevented that plan from going into effect, and all eyes turned to the conservative Texan to see what he would do. On August 9, 2001, Bush announced that federal funds could be used to study embryonic stem cells. But to prevent taxpayers from becoming complicit in the destruction of human embryos, that money could be used only to study the stem cell lines already in the works as of that date—a number that, for practical reasons, has resulted in about two dozen usable lines. Those wishing to work with any of the more than a hundred stem cell lines created after that date can do so only with private funding.
Every month scientists from around the world arrive in Madison to take a three-day course in how to grow those approved cells. To watch what they must go through to keep the cells happy is to appreciate why many feel hobbled by the Bush doctrine. For one thing—and for reasons not fully understood—the surest way to keep these cells alive is to place them on a layer of other cells taken from mouse embryos, a time-consuming requirement. Hunched over lab benches, deftly handling forceps and pipettes with blue latex gloves, each scientist in Madison spends the better half of a day dissecting a pregnant mouse, removing its uterus, and prying loose a string of embryos that look like little red peas in a pod. They then wash them, mash them, tease apart their cells, and get them growing in lab dishes. The result is a hormone-rich carpet of mouse cells upon which a few human embryonic stem cells are finally placed. There they live like pampered pashas.
If their scientist-servants don't feed them fresh liquid nutrients at least once a day, the cells die of starvation. If each colony is not split in half each week, it dies from overcrowding. And if a new layer of mouse cells is not prepared and provided every two weeks, the stem cells grow into weird and useless masses that finally die. By contrast, scientists working with private money have been developing embryonic stem cell lines that are hardier, less demanding, and not dependent on mouse cells. Bypassing the use of mouse cells is not only easier, but it also eliminates the risk that therapeutic stem cells might carry rodent viruses, thereby potentially speeding their approval for testing in humans.
Here in the Madison lab, scientists grumble about how fragile the precious colonies are. "They're hard to get to know," concedes Leann Crandall, one of the course's instructors and a co-author of the 85-page manual on their care and feeding. "But once you get to know them, you love them. You can't help it. They're so great. I see so many good things coming from them."
A few American scientists are finding it is easier to indulge their enthusiasm for stem cells overseas. Scores of new embryonic stem cell lines have now been created outside the U.S., and many countries are aggressively seeking to spur the development of therapies using these cells, raising a delicate question: Can the nation in which embryonic stem cells were discovered maintain its initial research lead?
"I know a lot of people back in the U.S. who would like to move into embryonic stem cell work but who won't because of the political uncertainties," says Stephen Minger, director of the Stem Cell Biology Laboratory at King's College in London, speaking to me in his cramped and cluttered office. "I think the United States is in real danger of being left behind."
Minger could be right. He is one of at least two high-profile stem cell scientists to move from the U.S. to England in the past few years, something less than a brain drain but a signal, perhaps, of bubbling discontent.
The research climate is good here, says Minger. In 2003 his team became the first in the U.K. to grow colonies of human embryonic stem cells, and his nine-person staff is poised to nearly double. He's developing new growth culture systems that won't rely on potentially infectious mouse cells. He's also figuring out how to make stem cells morph into cardiac, neural, pancreatic, and retinal cells and preparing to test those cells in animals. And in stark contrast to how things are done in the U.S., Minger says, he's doing all this with government support—and oversight.
The Human Fertilisation and Embryology Authority (HFEA), the government agency that has long overseen U.K. fertility clinics, is now also regulating the country's embryonic stem cell research. In closed-door meetings a committee of 18 people appointed by the National Health Service considers all requests to conduct research using embryos. The committee includes scientists, ethicists, lawyers, and clergy, but the majority are lay people representing the public.
To an American accustomed to high security and protesters at venues dealing regularly with embryo research, the most striking thing about the HFEA's headquarters in downtown London is its ordinariness. The office, a standard-issue warren of cubicles and metal filing cabinets, is on the second floor of a building that also houses the agency that deals with bankruptcy. I ask Ross Thacker, a research officer at the authority, whether the HFEA is regularly in need of yellow police tape to keep protesters at bay.
"Now that you mention it," he says, "there was a placard holder outside this morning …"
You May Also Like

Swimming just might be the best exercise out there. Here’s why.

Beauty is pain—at least it was in 17th-century Spain

Do you really need 10,000 steps a day? Here’s what the science says.
"… but he was protesting something about the insolvency office."
Thacker politely refrains from criticizing U.S. policy on embryo research, but he clearly takes pride in the orderliness of the British system. The committee has approved about a dozen requests to create stem cell lines in the past 18 months, increasing the number of projects to 35. Most were relatively routine—until a strong-willed fertility doctor named Alison Murdoch decided to ask for permission to do something nobody had done before: create cloned human embryos as sources of stem cells.
As controversial as embryonic stem cell research can be, cloning embryos to produce those stem cells is even thornier. Much of the world became familiar with cloning in 1997, when scientists announced they'd cloned a sheep named Dolly. The process involves creating an animal not from egg and sperm but by placing the nucleus of a cell inside an egg that's had its nucleus removed. It's since been used to replicate mice, rabbits, cats, and cattle, among others.
As in many other countries and a few U.S. states, it's illegal in the U.K. to create cloned human babies (called reproductive cloning), because of concerns that clones may be biologically abnormal and because of ethical issues surrounding the creation of children who would be genetic replicas of their one-and-only "parent."
In 2001 the British Parliament made it legal to create cloned human embryos—as opposed to babies—for use in medical research (called therapeutic cloning). Still, no one on the HFEA was completely comfortable with the idea. The fear was that some rogue scientist would take the work a step further, gestate the embryo in a woman's womb, and make the birth announcement that no one wanted to hear.
But Murdoch, of the University of Newcastle upon Tyne, made a compelling case. If replacement tissues grown from stem cells bore the patient's exact genetic fingerprint, they would be less likely to be rejected by a patient's immune system, she told the committee. And what better way to get such a match than to derive the cells from an embryo cloned from the patient's own DNA? Disease research could also benefit, she said. Imagine an embryo—and its stem cells—cloned from a person with Lou Gehrig's disease, a fatal genetic disorder that affects nerves and muscles. Scientists might learn quite a bit, she argued, by watching how the disease damages nerve and muscle cells grown from those stem cells, and then testing various drugs on them. It's the kind of experiment that could never be done in a person with the disease.
The HFEA deliberated for five months before giving Murdoch permission to make human embryo clones in her lab at the Centre for Life in Newcastle, a sprawling neon-illuminated complex of buildings that strikes a decidedly modern note in the aging industrial hub. But there was a catch: It takes an egg to make a clone. And under the terms of HFEA approval, Murdoch is allowed to use only those eggs being disposed of by the center's fertility clinic after they failed to fertilize when mixed with sperm.
It's not a perfect arrangement, Murdoch says. After all, eggs that have failed to fertilize are almost by definition of poor quality. "They're not brilliant," she says of the eggs. "But the U.K. has decided at the moment that these are the most ethical sort to use. So that's really all we can work with." As of April the group hadn't managed to clone any embryos, despite numerous attempts.
No such obstacle faced Woo-Suk Hwang and his colleagues at Seoul National University in February 2004 when they became the world's first to clone human embryos and extract stem cells from them. The South Korean government allows research on human embryos made from healthy eggs—in this case, donated by 16 women who took egg-ripening hormones.
Cloning is an arduous process that requires great patience and almost always ends in failure as cells burst, tear, or suffer damage to their DNA, but the Koreans are expert cloners, their skills sharpened in the country's state-funded livestock-cloning enterprise. In Hwang's lab alone, technicians produce more than 700 cloned pig or cattle embryos every day, seven days a week, in a quest to produce livestock with precise genetic traits. "There is no holiday in our lab," Hwang told me with a smile.
But there is something else that gives Koreans an edge over other would-be cloners, Hwang says. "As you know, Asian countries use chopsticks, but only the Koreans use steel chopsticks," he explains. "The steel ones are the most difficult to use. Very slippery." I look at him, trying to tell if he's kidding. A lifetime of using steel chopsticks makes Koreans better at manipulating tiny eggs? "This is not simply a joke," he says.
Time will tell whether such skill will be enough to keep Korea in the lead as other countries turn to cloning as a source of stem cells. The competition will be tough. China has pioneered a potentially groundbreaking technique that produces cloned human embryos by mixing human skin cells with the eggs of rabbits, which are more easily obtained than human eggs. A few privately funded researchers in the U.S. are also pursuing therapeutic cloning.
Yet the biggest competition in the international race to develop stem cell therapies may ultimately come from one of the smallest of countries—a tiny nation committed to becoming a stem cell superpower. To find that place, one need only track the migration patterns of top scientists who've been wooed there from the U.S., Australia, even the U.K. Where they've been landing, it turns out, is Singapore.
Amid the scores of small, botanically rich but barely inhabited islands in the South China Sea, Singapore stands out like a post-modern mirage. The towering laboratory buildings of its Biopolis were created in 2001 to jumpstart Singapore's biotechnology industry. Like a scene from a science fiction story, it features futuristic glass-and-metal buildings with names like Matrix, Proteos, and Chromos, connected by skywalks that facilitate exchanges among researchers.
Academic grants, corporate development money, laws that ban reproductive cloning but allow therapeutic cloning, and a science-savvy workforce are among the lures attracting stem cell researchers and entrepreneurs. Even Alan Colman—the renowned cloning expert who was part of the team that created Dolly, the cloned sheep—has taken leave of his home in the U.K. and become the chief executive of ES Cell International, one of a handful of major stem cell research companies blossoming in Singapore's fertile environs.
"You don't have to fly from New York to San Diego to see what's going on in other labs," says Robert Klupacs, the firm's previous CEO. "You just walk across the street. Because Singapore is small, things can happen quickly. And you don't have to go to Congress at every turn."
The company's team of 36, with 15 nationalities represented, has taken advantage of that milieu. It already owns six stem cell lines made from conventional, noncloned embryos that are approved for U.S. federal funding. Now it is perfecting methods of turning those cells into the kind of pancreatic islet cells that diabetics need, as well as into heart muscle cells that could help heart attack patients. The company is developing new, mouse-free culture systems and sterile production facilities to satisfy regulators such as the U.S. Food and Drug Administration. It hopes to begin clinical tests in humans by 2007.
Despite its research-friendly ethos—and its emphasis on entrepreneurial aspects of stem cell science—Singapore doesn't want to be known as the world's "Wild West" of stem cell research. A panel of scientific and humanitarian representatives spent two years devising ethical guidelines, stresses Hwai-Loong Kong, executive director of Singapore's Biomedical Research Council. Even the public was invited to participate, Kong says—an unusual degree of democratic input for the authoritarian island nation. The country's policies represent a "judicious balance," he says, that has earned widespread public support.
Widespread, perhaps, but not universal. After my conversation with Kong, a government official offered me a ride to my next destination. As we approached her parked car, she saw the surprise on my face as I read the bumper sticker on her left rear window: "Embryos—Let Them Live. You Were Once an Embryo Too!"
"I guess this is not completely settled," I said. "No," she replied, choosing not to elaborate.
That bumper sticker made me feel strangely at home. I am an American, after all. And no country has struggled more with the moral implications of embryonic stem cell research than the U.S., with its high church attendance rates and pockets of skepticism for many things scientific. That struggle promises to grow in the months and years ahead. Many in Congress want to ban the cloning of human embryos, even in those states where it is currently legal and being pursued with private funding. Some states have already passed legislation banning various kinds of embryo research. And federally backed scientists are sure to become increasingly frustrated as the handful of cell colonies they're allowed to work with becomes an ever smaller fraction of what's available.
Yet one thing I've noticed while talking to stem cell experts around the world: Whenever I ask who is the best in the field, the answers are inevitably weighted with the names of Americans. The work of U.S. researchers still fills the pages of the best scientific journals. And while federal policy continues to frustrate them, they are finding some support. Following the lead of California, which has committed 300 million dollars a year for embryonic stem cell research for the next decade, several states are pushing initiatives to fund research, bypassing the federal restrictions in hopes of generating well-paying jobs to boost their economies. Moves like those prompt some observers to predict that when all is said and done, it will be an American team that wins the race to create the first FDA-approved embryonic stem cell therapy.
Tom Okarma certainly believes so, and he intends to be that winner. Okarma is president of Geron, the company in Menlo Park, California, that has been at the center of the embryonic stem cell revolution from the beginning. Geron financed James Thomson's discovery of the cells in Wisconsin and has since developed more than a dozen new colonies. It holds key patents on stem cell processes and products. And now it's laying the groundwork for what the company hopes will be the first controlled clinical trials of treatments derived from embryonic stem cells. Moreover, while others look to stem cells from cloned embryos or newer colonies that haven't come into contact with mouse cells, Okarma is looking no further than the very first colonies of human embryonic stem cells ever grown: the ones Thomson nurtured back in 1998. That may seem surprising, he acknowledges, but after all these years, he knows those cells inside out.
"We've shown they're free of human, pig, cow, and mouse viruses, so they're qualified for use in humans," Okarma says at the company's headquarters. Most important, Geron has perfected a system for growing uniform batches of daughter cells from a master batch that resides, like a precious gem, in a locked freezer. The ability to produce a consistent product, batch after batch, just as drug companies do with their pills is what the FDA wants—and it will be the key to success in the emerging marketplace of stem cell therapies, Okarma says. "Why do you think San Francisco sourdough bread is so successful?" he asks. "They've got a reliable sourdough culture, and they stick with it."
Geron scientists can now make eight different cell types from their embryonic lines, Okarma says, including nerve cells, heart cells, pancreatic islet cells, liver cells, and the kind of brain cells that are lost in Parkinson's disease. But what Geron wants most at this point is to develop a treatment for spinal cord injuries.
Okarma clicks on a laptop and shows me a movie of white rats in a cage. "Pay attention to the tail and the two hind legs," he says. Two months before, the rats were subjected to spinal cord procedures that left their rear legs unable to support their weight and their tails dragging along the floor. "That's a permanent injury," he says. He flips to a different movie: white rats again, also two months after injury. But these rats received injections of a specialized nervous system cell grown from human embryonic stem cells. They have only the slightest shuffle in their gait. They hold their tails high. One even stands upright on its rear legs for a few moments.
"It's not perfect," Okarma says. "It's not like we've made a brand new spinal cord." But tests show the nerves are regrowing, he says. He hopes to get FDA permission to start testing the cells in people with spinal cord injuries in 2006.
Those experiments will surely be followed by many others around the world, as teams in China, the U.K., Singapore, and other nations gain greater control over the remarkable energy of stem cells. With any luck the political and ethical issues may even settle down. Many suspect that with a little more looking, new kinds of stem cells may be found in adults that are as versatile as those in embryos.
At least two candidates have already emerged. Catherine Verfaillie, a blood disease specialist at the University of Minnesota, has discovered a strange new kind of bone marrow cell that seems able to do many, and perhaps even all, the same things human embryonic stem cells can do. Researchers at Tufts University announced in February that they had found similar cells. While some scientists have expressed doubts that either kind of cell will prove as useful as embryonic ones, the discoveries have given birth to new hopes that scientists may yet find the perfect adult stem cell hiding in plain sight.
Maybe Cedric Seldon himself will discover them. The stem cells he got in his cord blood transplant did the trick, it turns out. They took root in his marrow faster than in anyone his doctors have seen. "Everyone's saying, 'Oh my God, you're doing so well,' " his mother says.
That makes Cedric part of the world's first generation of regenerated people, a seamless blend of old and new—and, oddly enough, of male and female. His stem cells, remember, came from a girl, and they've been diligently churning out blood cells with two X chromosomes ever since. It's a detail that will not affect his sexual development, which is under the control of his hormones, not his blood. But it's a quirk that could save him, his mother jokes, if he ever commits a crime and leaves a bit of blood behind. The DNA results would be unambiguous, she notes correctly. "They'll be looking for a girl."
Related Topics

4 herbal traditions used every day, all over the world

How do you create your own ‘Blue Zone’? Here are 6 tips
Fungi could be the key to major cancer research breakthroughs

How exercise can help—or hurt—your digestion

Scientists are finally studying women’s bodies. This is what we’re learning.
- Environment
- Perpetual Planet
History & Culture
- History & Culture
- Mind, Body, Wonder
- Paid Content
- Terms of Use
- Privacy Policy
- Your US State Privacy Rights
- Children's Online Privacy Policy
- Interest-Based Ads
- About Nielsen Measurement
- Do Not Sell or Share My Personal Information
- Nat Geo Home
- Attend a Live Event
- Book a Trip
- Inspire Your Kids
- Shop Nat Geo
- Visit the D.C. Museum
- Learn About Our Impact
- Support Our Mission
- Advertise With Us
- Customer Service
- Renew Subscription
- Manage Your Subscription
- Work at Nat Geo
- Sign Up for Our Newsletters
- Contribute to Protect the Planet
Copyright © 1996-2015 National Geographic Society Copyright © 2015-2024 National Geographic Partners, LLC. All rights reserved
- Ground Reports
- 50-Word Edit
- National Interest
- Campus Voice
- Security Code
- Off The Cuff
- Democracy Wall
- Around Town
- PastForward
- In Pictures
- Last Laughs
- ThePrint Essential


New Delhi: Stem cells were meant to be the magic beans that would cure cancer, treat Alzheimer’s, and fix damaged tissue. But after decades of hype around these special human cells that can develop into many different cell types, there are no mind-boggling treatments available to patients. “It continues to be cosmetic, experimental and expensive,” said Dr. Akhil Banerjea, emeritus professor at the National Institute of Immunology during a lecture, Stem Cell Therapeutics: Promises and Future Challenges , at Delhi’s India International Centre.
What was supposed to be a talk on the future avenues of stem cell therapy ended up being a discussion on the history of stem cell research and its—so far—limited success in modern medicine. The ethical and moral complexities over stem cell research have been the centre of much debate with fears of cloning and the ethics of using embryonic stem cells. Banerjea’s lecture was measured, less of a hyperbole and rooted in science. Most of the audience members–there were around 20 people —were doctors and researchers.
Despite not making advancements in precision medicine, stem cell research is still extremely useful to scientists and biologists alike.
The unique factor in stem cells is they can regenerate, and can also transform into any cell type—such as blood, liver, or nerve cells. That is the ‘excitement’ associated with this therapy, which has been around since 1959 when the first bone marrow cell transplant was done in a patient suffering from leukaemia.
Banerjea’s presentation, which the scientific community concurs with, revealed that bone marrow transplant remains the only existing mainstream stem cell treatment. Ethical concerns with stem-cell generation, research barriers and even technical issues about how bodies will accept/reject stem cells are what’s holding back stem-cell therapy from taking over the medical industry.
“However, it is an ongoing field, and who knows what future research will reveal,” he said.
Also read: Human-based tech replacing animal testing in drug development. India must join in
Ethical concerns
As Banerjea delivered his address on the mechanics behind stem cells, and the different kinds of cells, audience members kept returning to their potential. “What about cancer and heart attacks?” asked one young medical student. “What about Alzheimer’s and dementia? Is stem-cell therapy the answer?” piped up another.
Taking a systematic approach, Banerjea first explained how there are different types of stem cells like adult stem cells, which are regenerative stem cells that exist in different organs. They can only produce more cells of that particular type. Then there are embryonic stem cells that are pluripotent—which means they can regenerate and transform into almost every cell type and be used for a wider range of therapies.
“It is with embryonic stem cells that ethical concerns come up,” said Banerjea. Using embryos to harvest stem cells is quite a controversial subject in most countries, especially the United States and it has divided the medical community on the feasibility of using embryos for stem-cell therapy.
In 2006, Dr Shinya Yamanaka’s laboratory came up with a method to convert adult stem cells into pluripotent stem cells—thus eradicating the ethical concern of using embryonic stem cells. It was seen as “breathing new life” into the field of stem-cell therapy. But the potential and promise has yet to become a reality.
Banerjea said that even this advancement was mired in debate, starting from the conversion method itself.
“We introduce four genes to convert an adult stem cell into iPSC (induced pluripotent stem cell). This leads to rapid regeneration of these cells—I mean, you can grow and fill your buckets with these cells,” he said, displaying a picture of pluripotent stem cells he had created using this method during a research stint at Colorado State University.
But this is also where the problem lies—this rapid proliferation of stem cells also poses the risk of it turning into cancer.
A research paper by Yamanaka in 2020 also examined this fallout, the risk of cancer and the bigger problem of immunogenicity—the immune response that foreign cells in the body might evoke. For this reason, Banerjea said that governments across the world look at stem cell research with caution. It’s difficult for most stem-cell therapies to make it to the clinical trial stage.
Despite all his examples to the contrary, Banerjea remains optimistic. Stem-cell therapy remains a landmark medical advancement and has a “bright future” for curing long-term illnesses.
“It has helped cell biologists enormously. Once we understand cell pathways and reactions, we attempt to make cell-based therapies,” said Banerjea.
(Edited by Theres Sudeep)
Subscribe to our channels on YouTube , Telegram & WhatsApp
Support Our Journalism
India needs fair, non-hyphenated and questioning journalism, packed with on-ground reporting. ThePrint – with exceptional reporters, columnists and editors – is doing just that.
Sustaining this needs support from wonderful readers like you.
Whether you live in India or overseas, you can take a paid subscription by clicking here .
- Medical Research
- Stem cell therapy
LEAVE A REPLY Cancel
Save my name, email, and website in this browser for the next time I comment.
Most Popular
Why hc pulled up delhi govt over delay in procuring textbooks — ‘real issue is who takes credit’, verma vs khemka, again — haryana ias officer seeks action against colleague for post on dlf-vadra case, the spy who sold out subhas chandra bose—he worked with britain, germany, ussr, japan, italy.
Required fields are marked *
Copyright © 2024 Printline Media Pvt. Ltd. All rights reserved.
- Terms of Use
- Privacy Policy
Development and application of haploid embryonic stem cells
Affiliations.
- 1 Center for Reproductive Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou University, No. 40 Daxue Road, 450052, Zhengzhou, Henan Province, China. [email protected].
- 2 Center for Reproductive Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou University, No. 40 Daxue Road, 450052, Zhengzhou, Henan Province, China.
- 3 Center for Reproductive Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou University, No. 40 Daxue Road, 450052, Zhengzhou, Henan Province, China. [email protected].
- PMID: 38654389
- PMCID: PMC11040874
- DOI: 10.1186/s13287-024-03727-y
Haploid cells are a kind of cells with only one set of chromosomes. Compared with traditional diploid cells, haploid cells have unique advantages in gene screening and drug-targeted therapy, due to their phenotype being equal to the genotype. Embryonic stem cells are a kind of cells with strong differentiation potential that can differentiate into various types of cells under specific conditions in vitro. Therefore, haploid embryonic stem cells have the characteristics of both haploid cells and embryonic stem cells, which makes them have significant advantages in many aspects, such as reproductive developmental mechanism research, genetic screening, and drug-targeted therapy. Consequently, establishing haploid embryonic stem cell lines is of great significance. This paper reviews the progress of haploid embryonic stem cell research and briefly discusses the applications of haploid embryonic stem cells.
Keywords: Gamete substitution; Genetic screening; Haploid diploidization; Haploid embryonic stem cells; X chromosome inactivation.
© 2024. The Author(s).
Publication types
- Research Support, Non-U.S. Gov't
- Cell Differentiation
- Embryonic Stem Cells* / cytology
- Embryonic Stem Cells* / metabolism
Grants and funding
- 81901476/National Natural Science Foundation of China
- Open access
- Published: 15 April 2024
Human mesenchymal stem cells derived exosomes improve ovarian function in chemotherapy-induced premature ovarian insufficiency mice by inhibiting ferroptosis through Nrf2/GPX4 pathway
- Yuan Zhou 1 na1 ,
- Jinfa Huang 1 na1 ,
- Lingling Zeng 1 ,
- Qian Yang 1 ,
- Fangjuan Bai 1 ,
- Qiqing Mai 1 &
- Kaixian Deng 1
Journal of Ovarian Research volume 17 , Article number: 80 ( 2024 ) Cite this article
457 Accesses
2 Altmetric
Metrics details
Chemotherapy exposure has become a main cause of premature ovarian insufficiency (POI). This study aimed to evaluate the role and molecular mechanism of human umbilical cord mesenchymal stem cell-derived exosomes (hUMSC-Exos) in ovarian function protection after chemotherapy.
hUMSC-Exos were applied to cyclophosphamide-induced premature ovarian insufficiency mice and human ovarian granulosa tumor cells (KGN) to determine their effects on follicular development and granulosa cell apoptosis. Evaluation was done for iron ion and reactive oxygen species (ROS) production, lipid peroxidation levels, and changes in iron death-related molecules (nuclear factor (erythroid-derived 2)-like 2 (Nrf2), Glutathione Peroxidase enzyme 4 (GPX4), and Solute carrier family 7 member 11 cystine glutamate transporter (SLC7A11; xCT)). Furthermore, rescue experiments using an Nrf2 inhibitor were performed to assess the therapeutic effects of hUMSC-Exos on granulosa cells.
hUMSC-Exos promoted ovarian hormone levels and primary follicle development in POI mice and reduced granulosa cell apoptosis. After hUMSC-Exos treatment, the ROS production, free iron ions and lipid peroxidation levels of granulosa cells decreased, and the iron death marker proteins Nrf2, xCT and GPX4 also decreased. Furthermore, the Nrf2 inhibitor ML385 significantly attenuated the effects of hUMSC-Exos on granulosa cells.
hUMSC-Exos inhibit ferroptosis and protect against CTX-induced ovarian damage and granulosa cell apoptosis through the Nrf2/GPX4 signaling pathway, revealing a novel mechanism of hUMSC-Exos in POI therapy.
Cyclophosphamide (CTX) is widely used in clinical treatment of tumors, inhibition of graft rejection after organ transplantation and immunosuppression of autoimmune diseases. However, a large number of women of childbearing age have a serious side effect after using CTX, that is, premature ovarian insufficiency [ 1 ]. Researches showed that up to 40% of women experience POI after chemotherapy, with a risk of lifelong infertility [ 2 ]. This has a profound impact on their quality of life and mental well-being [ 3 ]. Therefore, exploring new strategies to protect against CTX-induced POI is of utmost importance.
Exosomes are small vesicles with a diameter ranging from 30 to 150 nm, capable of carrying diverse bioactive molecules such as proteins, nucleic acids, and lipids. Emerging evidence indicates that hUCMSC-Exos participated in such physiological processes as cell proliferation, differentiation, migration, and apoptosis [ 4 , 5 ]. Therefore, exosomes are considered as a promising therapeutic tool. One study showed that hUCMSC-Exos improved ovarian reserve and hormone levels in POI mice treated with CTX [ 6 ]. In addition, a preclinical study showed that exosomes derived from hUCMSCs enhanced the proliferation of granulosa cells and ovarian cells after CTX treatment, which may be related to a decrease in the accumulation of reactive oxygen species [ 7 ]. Consequently, this intervention partially restored the ovarian phenotype and function.
Iron plays an important role in cellular metabolism and survival, but excessive iron can induce oxidative stress, protein oxidation, and DNA damage, leading to ferroptosis and even cell death [ 8 , 9 , 10 ]. Recent studies have highlighted the significant role of ferroptosis in the treatment of chemotherapy-induced ovarian dysfunction [ 11 , 12 ]. Chemotherapy drugs induce iron accumulation in ovarian tissues, leading to increased oxidative stress and damage to ovarian function [ 13 ]. Furthermore, excess iron can induce apoptosis in ovarian cells, affecting normal ovarian function [ 14 ]. Researches have shown that reducing iron accumulation, such as using iron chelators or iron channel inhibitors, can alleviate oxidative damage in ovarian tissues and protect ovarian function [ 11 ]. Additionally, interventions targeting iron metabolism pathways, iron absorption, storage, and metabolism, have been proved to mitigate the adverse impact of chemotherapy on ovarian function [ 15 ]. Whether hUCMSC-Exos can protect ovarian function by inhibiting ferroptosis remains to be explored.
In summary, this study aims to investigate the role and mechanism of hUCMSC-Exos in treating chemotherapy-induced ovarian dysfunction, specifically focusing on their potential to protect ovarian function by inhibiting ferroptosis.
Materials and methods
Culture and identification of huc-mscs.
hUC-MSCs were obtained from Guangzhou DuDe Biotechnology Co., Ltd. They were cultured in α-MEM medium (Gibco, USA) supplemented with 10% fetal bovine serum from South America (Gibco, USA), 1% penicillin/streptomycin (Gibco, USA), and 0.05% bFGF. The fourth passage hUC-MSCs were selected and identified by flow cytometry. Cell surface markers including CD34 (Elabscience), CD45 (Elabscience), CD73 (Elabscience), CD90 (Biolegend), and CD105 (Endoglin) were used for MSC characterization.
Identification of hUC-MSCs by adipogenic differentiation
The 4th-generation hUC-MSCs with a cell density of 80-90% were seeded into a six-well plate and cultured at 37℃, 5%CO2 and saturated humidity. When the cells reach 100% confluence, add adipogenesis induction medium A; replace it with adipogenesis induction medium B after 3 days of culture, and replace it with A after 24 h of culture. Repeat 4 more cycles. Add solution B and culture for 7 days, changing the medium every 3 days. Control cells grew normally. After 23 days of induction, cells were stained with Oil Red O. The cells were washed with PBS, observed and photographed under an inverted microscope.
Identification of hUC-MSCs by osteogenic differentiation
Pluripotency assay was used to assess the pluripotent ability of MSCs to generate osteoblasts using commercially available differentiation media (StemPro Differentiation Kit, Thermo Fisher Scientific). To this end, different groups of mesenchymal stem cells were cultured in 6-well slides and evaluated histologically. Briefly, cells were cultured in osteogenic differentiation medium for osteogenic differentiation. Change differentiation medium twice weekly. After 21 days, differentiation evaluation and calcium deposition quantification was assessed by alizarin red staining.
Isolation and identification of hUMSC-Exos
The 5th passage of hUC-MSCs, grown to 80–90% confluency, were cultured in α-MEM medium supplemented with 1% exosome-depleted serum and 0.05% bFGF for 48 h. The cell culture supernatant was collected and filtered through a 0.22 μm filter to remove cells, apoptotic bodies, and cellular debris. Sequential centrifugation at 300 g, 2000 g for 10 min, and 10,000 g for 30 min at 4 °C was performed to further eliminate contaminants. Subsequently, ultracentrifugation at 110,000 g for 70 min was carried out to isolate the exosomes. Finally, PBS was used for resuspending the exosomes. Nanoparticle tracking analysis was employed to determine the size of the exosomes, while transmission electron microscopy was utilized for exosome identification.
Establishment of POI mouse model and treatment with hUMSC-Exos
6-week-old C57BL/6 female mice were purchased from Guangdong Yaoke Biotechnology Co., Ltd. (Guangdong, China). The mice were maintained at a temperature of 22 ± 1 °C, a relative humidity of 50 ± 1%, and a 12/12-hour light/dark cycle. Sterilized food and water were provided ad libitum. All animal experiments were conducted in accordance with the guidelines and regulations of Shunde Hospital, Southern Medical University, and the AAALAC and IACUC guidelines.
The mice were divided into three groups: control group, POI group ( n = 10), and POI + Exos group ( n = 10). To establish the POI model, mice received intraperitoneal injections of CTX (50 mg/kg) daily for 14 consecutive days, while the control group received an equivalent volume of PBS. In the POI + Exos group, mice were transplanted with 30 µl of hUMSC-exos (0.015 µg/µl) into each ovary one week after the POI model was established. Meanwhile, mice in the POI group were injected with an equivalent volume of PBS. At the end of the 2nd and 4th week after treatment, five mice from each group were euthanized under anesthesia, and serum and ovarian tissues were collected.
Hematoxylin and eosin staining
The staining procedure for hematoxylin and eosin (H&E) was performed following the established protocol described in previous publications [ 16 ]. Briefly, ovarian tissues fixed with 4% paraformaldehyde (Servicebio, China) were dehydrated, embedded, and sectioned (The maximum transverse section of the ovary wasserially sliced into 4 μm sections). The sections were stained with hematoxylin and eosin. The morphology of each ovarian section was observed and photographed using an inverted microscope (Leica DMI1, Germany). The number of follicles at different stages was counted.
Serum hormone levels determination by ELISA
Serum for hormone measurements was obtained by centrifuging mouse blood at 5000 rpm for 10 min. The levels of anti-Müllerian hormone (AMH), estradiol (E2), and follicle-stimulating hormone (FSH) in serum were determined using ELISA kits (Lengton, China). The optical density (OD) values were read at 450 nm using an ELISA reader.
Culture and treatment of human granular tumor cells
KGN cells (Procell CL-0603) were provided by Wuhan Procell Life Science & Technology Co., Ltd. and cultured in DMEM/F12 medium (Gibco, USA) supplemented with 10% fetal bovine serum (Gibco, USA) and 1% penicillin/streptomycin (Gibco, USA). The cells were divided into four groups: the control group (Nc), the POI group (CTX, 1 mg/mL, POI), the hUMSC-derived exosomes (0.015 µg/µl) treatment group (POI + Exos), and the hUMSC-derived exosomes + NRF2 inhibitor treatment group (ML385, 1 µmol/L, MCE) (POI + Exos + ML385).
EdU staining
Cell proliferation was assessed by EdU staining after both 24 and 48 h of culture in each group. Cells were treated with EdU working solution for 4 h, fixed with 4% paraformaldehyde, and then stained with EdU mixture and DAPI. Cell apoptosis was evaluated using Hoechst staining. After 24 and 48 h of cell culture, cells were incubated with Hoechst/PI staining working solution in the dark for 20 min. Finally, assessment was performed using a fluorescence microscope (Leica DMI8, Germany).
Reactive oxygen species detection
The levels of reactive oxygen species in cells were measured using a ROS detection kit (Beyotime, Shanghai, China) following the manufacturer’s instructions. The probe for intracellular ROS (DCFH-DA) was diluted to a concentration of 10 µmol/L in serum-free medium. KGN cells were then incubated with the diluted DCFH-DA at 37 °C for 30 min. Fluorescence was detected using an inverted fluorescence microscope.
Ferrous ion detection
A fluorescent probe for ferrous ions (FeRhoNox-1) detection kit (MKBio, China) was used to measure Fe2 + levels in cells. FeRhoNox-1 was diluted to a concentration of 5 µM in PBS (prepared fresh) and incubated with KGN cells at 37 °C for 60 min. Fluorescence was detected using an inverted fluorescence microscope.
Cellular lipid peroxidation detection
A lipid peroxidation sensor (BODIPYTM 581/591 C11) (Shanghai BioHub Biotechnology Co., Ltd.) was employed to indicate intracellular lipid peroxidation and antioxidant status. Treated cells were stained with 5 µM BODIPYTM 581/591 C11 at 37 °C for 30 min. Fluorescence images of cells were captured using a fluorescence microscope.
Western blotting
Western blotting was performed using the Multistrip Western blotting protocol as previously described [ 16 ]. The following antibodies were utilized: The exosomes were identified by CD63 (1:1000; Abcam), CD9 (1:1000; Abcam), TSG101 (1:1000; Abcam). The expression of Nrf2/GPX4 pathway was detected by Nrf2 (1:1000;Cell Signaling Technology), xCT (1:1000; Abcam), GPX4 (1:1000; Abcam), and Alpha Tubulin (1:200; Proteintech). After incubation with secondary antibodies at room temperature for 30 min and subsequent washes, the bands were visualized using enhanced chemiluminescence (Abcam, USA).
Statistical analysis
All quantitative data are presented as mean ± standard deviation ( n ≥ 3). Statistical analysis was conducted using GraphPad Prism 9.0 software, and significant differences were evaluated using one-way analysis of variance (ANOVA) and t-tests; p < 0.05 was considered statistically significant.
Characterization of hUC-MSCs and hUMSC-exos
The human umbilical cord mesenchymal stem cells (hUC-MSCs) display spindle-shaped, fibroblast-like morphology. The MSC biomarkers (CD73/90/105) were positively expressed, whereas the haematopoietic and endothelial markers (CD34/45) were negatively expressed (Fig. 1 A). Oil Red O and Alizarin Red staining demonstrated the ability of hUC-MSCs to differentiate into adipocytes and osteoblasts, respectively (Fig. 1 B). Under transmission electron microscopy (TEM), exosomes exhibited the characteristic bilayer vesicle structure (Fig. 1 C). The exosomes isolated from the serum-free culture supernatant of hUMSC-exos were characterised using TEM and Western blotting. Nanoparticle Tracking Analysis (NTA) revealed that hUMSC-exos appeared spherical with a concentration of 1.1 × 10^10 particles/ml and a peak diameter of 128 nm (Fig. 1 D). Bicinchoninic Acid (BCA) protein assay indicated a protein concentration of 2.71 mg/ml in hUMSC-exos. Western blotting confirmed the positive expression of CD63, CD9, and TSG101 in hUMSC-exos (Fig. 1 E).
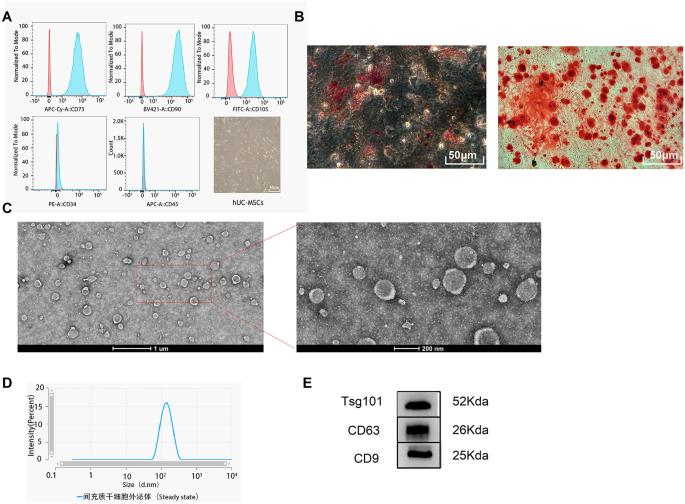
Identification of hUC-MSCs and hUMSC-exos. A Flow cytometry analysis of surface markers’ expression on hUC-MSCs and the morphological characterisation of hUC-MSCs under an optical microscope. B Osteogenic and adipogenic differentiation of hUC-MSCs. C Representative image of hUMSC-exos under Transmission Electron Microscopy (TEM). D Characterisation of hUMSC-exos via Nanoparticle Tracking Analysis (NTA). E Western blot identification of exosomal markers CD63, CD9, and TSG101
hUMSC-exos promote the recovery of ovarian function in mice
Humsc-exos restored the ovarian function of poi mice.
As shown in Fig. 2 A, the trend of body weight changes before and after treatment in the three Premature Ovarian Insufficiency mouse groups was essentially the same, with no significant differences between the groups. However, compared to the control group, the ovarian weight index of the POI group significantly decreased at 2 and 4 weeks post-surgery. hUMSC-exos treatment partly rescued the ovaries from the damage caused by chemotherapy drugs. Four weeks after surgery, the ovarian weight index of the POI + Exos group was significantly higher than that of the POI group (Fig. 2 B). An ELISA test of ovarian function-related hormones showed that the levels of E2 at 2 and 4 weeks post-modelling and AMH at 4 weeks in the POI group were lower than those in the control group ( P < 0.05 for all). hUMSC-exos treatment partially restored ovarian function. Four weeks after hUMSC-exos treatment, the levels of AMH and E2 in the POI + Exos group were higher than those in the POI group, and FSH was lower than in the POI group ( P < 0.05 for all) (Fig. 2 C).
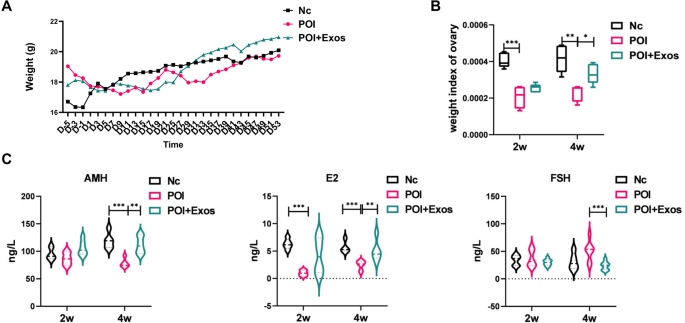
Weight and hormone levels of mice in each group. A Changes in body weight among the three groups of mice (Nc group, POI group, and POI + Exos group). B Comparison of ovarian weight indices at 2 weeks and 4 weeks post-treatment among the three groups (Nc group, POI group, and POI + Exos group). C Comparison of hormone levels among the three groups of mice (Nc group, POI group, and POI + Exos group)
hUMSC-exos reversed CTX-induced reduction in mouse follicle
The results of Hematoxylin and Eosin (HE) staining revealed that under the effects of CTX chemotherapy, mouse ovaries exhibited significant follicular atresia, with a decrease in follicle number that progressed over time (Fig. 3 A). Upon comparing the counts of follicles in each group’s sections, the numbers of primordial, primary, secondary and mature follicles in the POI group were significantly reduced at 2 weeks and 4 weeks, compared to the normal group. At 2 weeks, the numbers of primordial and primary follicles in the POI + Exos group were significantly higher than those in the POI group ( p < 0.05) (Fig. 3 B). At 4 weeks, the number of primary follicles in the POI + Exos group significantly increased compared to the POI group. The number of mature follicles was decreased at the fourth week when compared to the second week in the POI group. While, in the POI + Exos group, the number of mature follicles was increased at the fourth week when compared to the second week (Fig. 3 C). These results suggest that the in-situ transplantation of exosomes promoted the recovery of ovarian function and physiological function in POI mice, primarily with pre-antral follicles observed in the early stage.
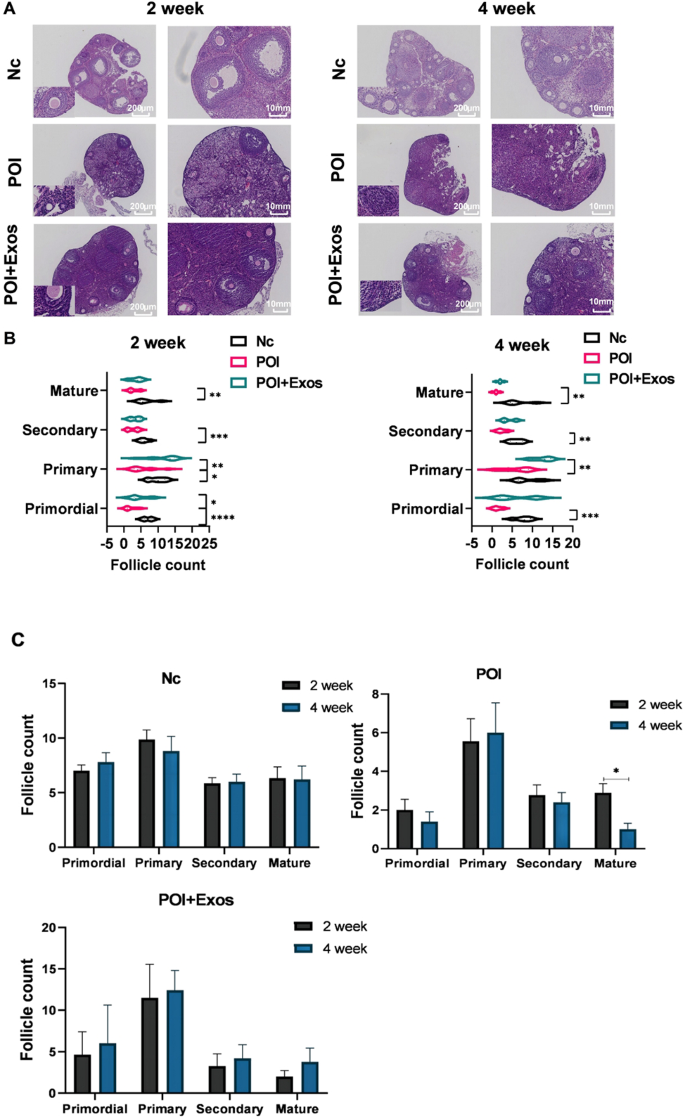
Comparison of follicle numbers among the groups of mice. A Hematoxylin and Eosin (HE) staining results of the ovaries in the three groups (Nc group, POI group, and POI + Exos group) at 2 weeks and 4 weeks (magnification 50×, 100X). B Statistical chart of the numbers of follicles at each stage in the three groups (Nc group, POI group, and POI + Exos group) at 2 weeks and 4 weeks. C Statistical comparison of the number of follicles at different stages between 2 weeks and 4 weeks. (* P < 0.01, ** P < 0.01, *** P < 0.001, n = 5 of each group)

hUMSC-exos promoted KGN cell proliferation and alleviated CTX-induced KGN cell death
As depicted in Fig. 4 A and C, the proliferation of KGN cells was significantly inhibited after treatment with CTX for 24 and 48 h ( P < 0.0001). The treatment with hUMSC-exos partly alleviated the inhibition of cell proliferation by CTX. Similarly, the apoptosis of KGN cells significantly increased after treatment with CTX for 24 and 48 h ( P < 0.0001). The treatment with hUMSC-exos partially reduced CTX-induced cell apoptosis (Fig. 4 B and D).
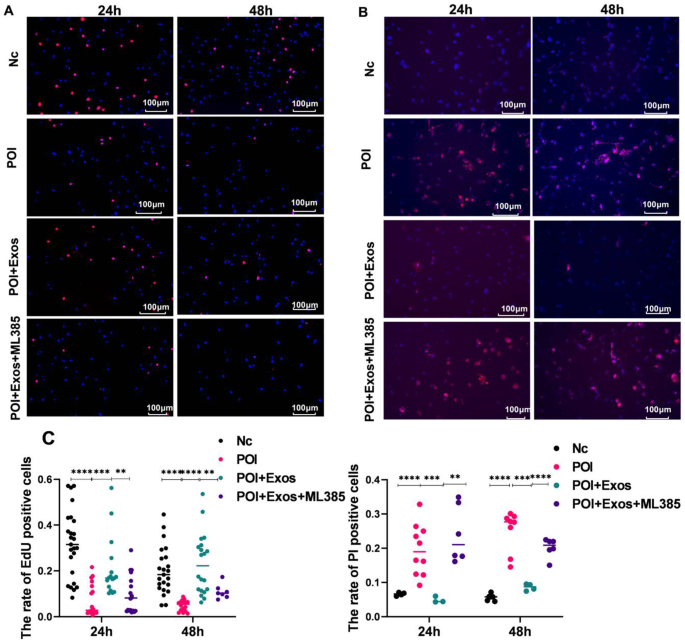
hUMSC-exos alleviated CTX-induced KGN cell apoptosis. A Cell proliferation detected by EDU after co-culturing KGN cells treated with hUMSC-exos and CTX for 24 h and 48 h. (Magnification 100×. Red: EDU. Blue: DAPI.) B KGN cell death detected by Hoechst/PI after co-culturing KGN cells treated with hUMSC-exos and CTX for 24 h and 48 h. (Magnification 100×. Red: DAPI. Blue: Hoechst.) C Statistical results from Fig. 4 A and B. ** P < 0.01, *** P < 0.001, **** P < 0.001
hUMSC-exos inhibit CTX-induced ferroptosis in KGN cells
Compared with the control group, the levels of ROS increased after CTX treatment for 24 h and 48 h ( P < 0.05). Treatment with hUMSC-exos for 24 h and 48 h reduced the production of ROS in KGN cells induced by CTX ( P < 0.05). Moreover, treatment with the Nrf2 inhibitor ML385 reduced the inhibitory effect of hUMSC-exos on ROS in KGN cells ( P < 0.05) (Fig. 5 A and C). As detected by FeRhoNoxTM − 1, the trend of Fe 2+ levels in each group were consistent with that of ROS levels in KGN cells (Fig. 5 B and D).
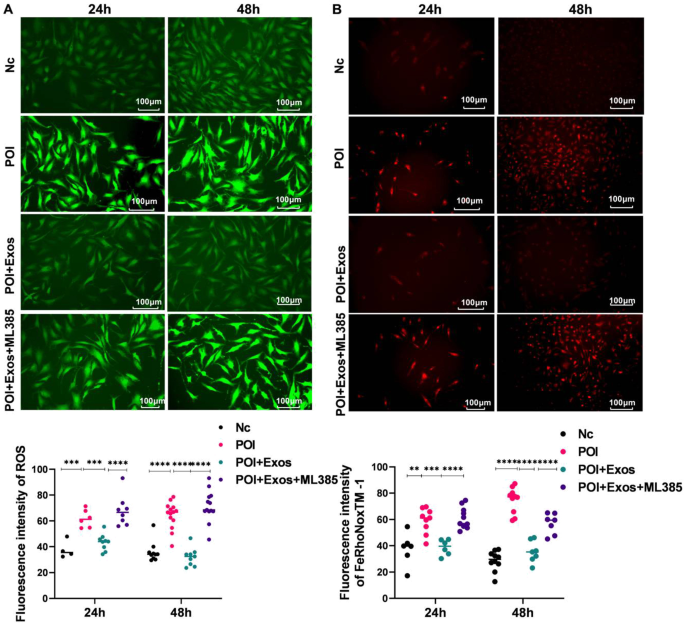
hUMSC-exos alleviated CTX-induced ferroptosis in KGN cells. A Comparison of ROS levels in Nc group, POI group, POI + Exos group, and POI + Exos + ML385 group. B Detection of Fe2 + in Nc group, POI group, POI + Exos group, and POI + Exos + ML385 group by FeRhoNoxTM − 1 staining. C Statistical analysis results of ROS detection and FeRhoNoxTM − 1 staining. (** P < 0.01, *** P < 0.001, **** P < 0.001.)
Similarly, CTX treatment for 24 h and 48 h raised lipid peroxidation levels compared to the control group ( P < 0.05). hUMSC-exos treatment for the same periods reduced these CTX-induced increases ( P < 0.05). Additionally, the Nrf2 inhibitor ML385 mitigated hUMSC-exos’ suppression of lipid peroxidation in KGN cells ( P < 0.05) (Fig. 6 ).
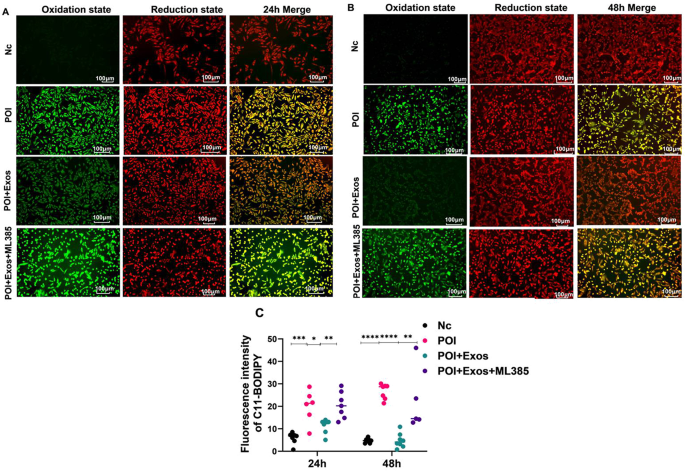
hUMSC-exos alleviated CTX-induced lipid peroxidation in KGN cells. A Lipid peroxidation status in Nc group, POI group, POI + Exos group, and POI + Exos + ML385 group after 24 h of treatment. B Lipid peroxidation status in Nc group, POI group, POI + Exos group, and POI + Exos + ML385 group after 48 h of treatment. C Statistical results of Fig. 6 A and B. (* P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001.)
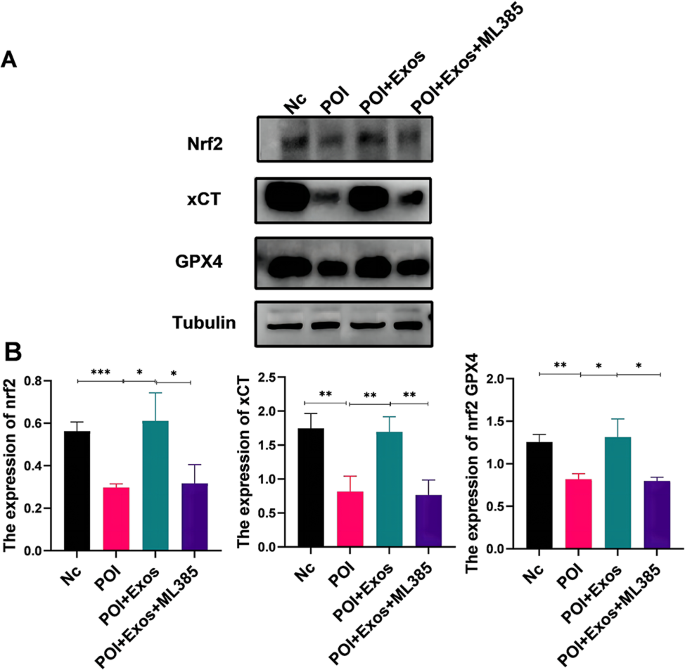
hUMSC-exos Regulate Ferroptosis via the Nrf2/GPX4 Pathway. A Western blotting to examine the protein expression levels of Nrf2, GPX4, and xCT in KGN cells 48 h after CTX, CTX + Exos, and CTX + Exos + ML385 treatments. B Statistical analysis results of Fig. 7 A (* P < 0.05, ** P < 0.01, *** P < 0.001)
hUMSC-exos modulated KGN cell ferroptosis via the Nrf2/GPX4 pathway
Previous studies indicate the Nrf2/GPX4 pathway as a key signal transduction route in ferroptosis. We further examined if hUMSC-exos treatment of POI relates to this pathway. As shown in Fig. 5 A, 48 h post-CTX treatment, KGN cells exhibited markedly lowered Nrf2, xCT, and GPX4 protein expressions ( P < 0.05). Following hUMSC-exos treatment, these protein levels rose ( P < 0.05). The effect of hUMSC-exos was partially counteracted by the Nrf2 inhibitor ML385 ( P < 0.05). These findings suggest that hUMSC-exos mitigate CTX-induced ferroptosis via the Nrf2/GPX4 pathway.
Our findings demonstrated that hUMSC-exos alleviate CTX-induced ovarian damage in mice, enhancing ovarian hormone levels and follicles in vivo. In vitro assay showed hUMSC-exos counteracting CTX-induced granulosa cell apoptosis, promoting cellular proliferation and hormone synthesis. Further investigations revealed hUMSC-exos could mitigate CTX-induced reactive oxygen species, iron deposition, and lipid peroxidation. Mechanistically, hUMSC-exos suppressed CTX-induced ferroptosis via the Nrf2/GPX4 pathway, which was reversed by a Nrf2/GPX4 pathway inhibitor. In essence, our study elucidated the role and mechanism of hUMSC-exos in POI through the regulation of ferroptosis, providing critical insights for advancing therapeutic strategies in this field.
Human mesenchymal stem cell therapy is a novel strategy for retain ovarian function and treat female infertility [ 17 ]. Available hMSCs include human bone marrow-derived mesenchymal stem cells, human adipose tissue-derived stem cells, and human amniotic membrane mesenchymal stem cells [ 18 , 19 ]. However, problems such as embolism, immunogenicity, malignant transformation, poor cell engraftment and post-transplantation survival limit the application of stem cell therapy [ 20 , 21 ]. Notably, exosomes do not express major histocompatibility complex (MHC) I or II [ 22 ], which overcomes all the disadvantages of cell therapy [ 23 ].
Some studies indicated that certain chemicals have a certain toxic effect on the ovaries of females via increasing oxidative stress in the ovaries to trigger cell apoptosis, thereby affecting the survival of ovarian granulosa cells. The redox balance plays a critical role in regulating follicle growth, angiogenesis, and hormone levels. However, when the ROS-antioxidant balance is disturbed, severe oxidative stress (OS) consequences can occur, reducing oocyte quantity and quality. OS mediates microenvironmental changes in genetic material, signaling pathways, and transcription factors, leading to meiotic abnormalities and mitochondrial loss in apoptotic granulosa cells. Therefore, it is likely to improve POI through controlling level OS [ 24 , 25 ]. Our study confirmed the critical role of hUMSC-exos in the regulation of intracellular oxidative stress induced by CTX.
Recent research reveals a pivotal role for exosomes in ovarian function protection, offering a novel direction for the prevention and treatment of ovarian diseases [ 6 , 26 , 27 ]. Exosomes function significantly in cellular communication [ 28 , 29 ], disease progression, and immune regulation, with a particular emphasis on managing oxidative stress. Firstly, exosomes can extracellularly discharge antioxidant proteins, enzymes, and small-molecule antioxidants to eliminate oxidative stress products, thereby safeguarding damaged cells. They are found to encapsulate antioxidant enzymes like Superoxide Dismutase (SOD) and Glutathione Peroxidase (GPX) [ 30 , 31 ]. Under oxidative stress, these enzymes can be transported to distressed cells via exosomes, curtailing oxidative stress levels and preserving key intracellular biomacromolecules. Secondly, exosomes serve a regulatory role in oxidative stress. Stress conditions elevate the production and release of intracellular exosomes. They can both shield damaged cells by disseminating antioxidant proteins and bolster cellular antioxidant capabilities by stimulating the NF-E2 related factor 2 (Nrf2) signalling pathway, thereby advancing the transcription of antioxidant genes [ 32 , 33 ]. Additionally, exosomes can modulate oxidative stress responses by activating ROS production pathways in other cells and adjusting intracellular antioxidant levels, influencing cell survival and metabolism [ 34 ]. Our study shows that human umbilical cord mesenchymal stem cell-derived exosomes (hUMSC-exos) protected granulosa cells by impeding ferroptosis through the Nrf2/GPX4 pathway.
Additionally, our study highlights the potential of exosomes in impeding chemotherapy-induced ovarian toxicity, laying a robust foundation for devising targeted strategies for ovarian protection. Further research could scrutinize the interplay between exosomes and ovarian cells, elucidating their explicit regulatory functions in preserving ovarian functionality.
We recognized the limitations and prospective trajectories of our study. Despite positive indications from our findings, they require further rigorous assessment and validation prior to clinical application. Future studies could enhance the investigation into the biological attributes and operational mechanisms of exosomes, with the aim to pinpoint more accurate and efficacious treatment strategies, thereby broadening the spectrum of choices for managing chemotherapy-induced premature ovarian failure.
Data availability
The authors declare that the data and material of this study are available within the article
Tang L, Wei R, Chen R, Fan G, Zhou J, Qi Z, Wang K, Wei Q, Wei X, Xu X. Establishment and validation of a cholesterol metabolism-related prognostic signature for hepatocellular carcinoma. Comput Struct Biotechnol J. 2022;20:4402–14.
Article CAS PubMed PubMed Central Google Scholar
Hou YM, Yu H, Hao JT, Feng F, An RF. Women with ovarian Cancer and with fertility preservation: a survival analysis using the Surveillance, Epidemiology, and end results database and construction of Nomograms to Predict Cancer-Specific Survival. Front Oncol. 2022;12:860046.
Article PubMed PubMed Central Google Scholar
Cai T, Zhou T, Yuan C, Yu C, Ni F, Sheng Z. Heterogeneity of symptoms and functions among women receiving chemotherapy for breast cancer in China: a multicentre, cross-sectional study. Front Public Health. 2022;10:952710.
Xu C, Zhao J, Li Q, Hou L, Wang Y, Li S, Jiang F, Zhu Z, Tian L. Exosomes derived from three-dimensional cultured human umbilical cord mesenchymal stem cells ameliorate pulmonary fibrosis in a mouse silicosis model. Stem Cell Res Ther. 2020;11(1):503.
Yu Y, Chen M, Guo Q, Shen L, Liu X, Pan J, Zhang Y, Xu T, Zhang D, Wei G. Human umbilical cord mesenchymal stem cell exosome-derived mir-874-3p targeting RIPK1/PGAM5 attenuates kidney tubular epithelial cell damage. Cell Mol Biol Lett. 2023;28(1):12.
Li Z, Zhang M, Zheng J, Tian Y, Zhang H, Tan Y, Li Q, Zhang J, Huang X. Human umbilical cord mesenchymal stem cell-derived exosomes improve ovarian function and proliferation of premature ovarian insufficiency by regulating the Hippo Signaling Pathway. Front Endocrinol. 2021;12:711902.
Article Google Scholar
Ding C, Zhu L, Shen H, Lu J, Zou Q, Huang C, Li H, Huang B. Exosomal miRNA-17-5p derived from human umbilical cord mesenchymal stem cells improves ovarian function in premature ovarian insufficiency by regulating SIRT7. Stem Cells. 2020;38(9):1137–48.
Article CAS PubMed Google Scholar
Gao X, Song Y, Wu J, Lu S, Min X, Liu L, Hu L, Zheng M, Du P, Yu Y et al. Iron-dependent epigenetic modulation promotes pathogenic T cell differentiation in lupus. J Clin Invest 2022, 132(9).
Kim K, Song Y, Oh TJ, Choi SH, Jang HC. Association between Iron Intake and Diabetic Peripheral Neuropathy in Type 2 diabetes: significance of Iron Intake and the ratio between Iron intake and polyunsaturated fatty acids intake. Nutrients 2020, 12(11).
Cui Y, Gutierrez S, Ariai S, Oberg L, Thorn K, Gehrmann U, Cloonan SM, Naessens T, Olsson H. Non-heme iron overload impairs monocyte to macrophage differentiation via mitochondrial oxidative stress. Front Immunol. 2022;13:998059.
Wang F, Liu Y, Ni F, Jin J, Wu Y, Huang Y, Ye X, Shen X, Ying Y, Chen J, et al. BNC1 deficiency-triggered ferroptosis through the NF2-YAP pathway induces primary ovarian insufficiency. Nat Commun. 2022;13(1):5871.
Wang CK, Chen TJ, Tan GYT, Chang FP, Sridharan S, Yu CA, Chang YH, Chen YJ, Cheng LT, Hwang-Verslues WW. MEX3A mediates p53 degradation to suppress ferroptosis and facilitate ovarian Cancer tumorigenesis. Cancer Res. 2023;83(2):251–63.
Wang S, Li X, Li J, Wang A, Li F, Hu H, Long T, Pei X, Li H, Zhong F, et al. Inhibition of cisplatin-induced Acsl4-mediated ferroptosis alleviated ovarian injury. Chem Biol Interact. 2024;387:110825.
Regan SLP, Knight PG, Yovich JL, Leung Y, Arfuso F, Dharmarajan A. Granulosa Cell apoptosis in the ovarian Follicle-A changing view. Front Endocrinol. 2018;9:61.
Escobar-Morreale HF. Iron metabolism and the polycystic ovary syndrome. Trends Endocrinol Metab. 2012;23(10):509–15.
Li Z, Ma D, Peng L, Li Y, Liao Z, Yu T. Compatibility of Achyranthes bidentata components in reducing inflammatory response through arachidonic acid pathway for treatment of Osteoarthritis. Bioengineered. 2022;13(1):1746–57.
Sadeghi S, Mosaffa N, Huang B, Ramezani Tehrani F. Protective role of stem cells in POI: current status and mechanism of action, a review article. Heliyon. 2024;10(1):e23271.
Sen Halicioglu B, Saadat K, Tuglu MI. Adipose-derived mesenchymal stem cell transplantation in Chemotherapy-Induced premature ovarian insufficiency: the role of Connexin and Pannexin. Reprod Sci. 2022;29(4):1316–31.
Kolios G, Moodley Y. Introduction to stem cells and regenerative medicine. Respiration. 2013;85(1):3–10.
Article PubMed Google Scholar
Sharkis SJ, Jones RJ, Civin C, Jang YY. Pluripotent stem cell-based cancer therapy: promise and challenges. Sci Transl Med. 2012;4(127):127ps129.
Yin JQ, Zhu J, Ankrum JA. Manufacturing of primed mesenchymal stromal cells for therapy. Nat Biomed Eng. 2019;3(2):90–104.
Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–72.
Gowen A, Shahjin F, Chand S, Odegaard KE, Yelamanchili SV. Mesenchymal stem cell-derived extracellular vesicles: challenges in clinical applications. Front Cell Dev Biol. 2020;8:149.
Sen Halicioglu B, Saadat K, Tuglu MI. The relationship of 4-vinylcyclohexene diepoxide toxicity with cell death, oxidative stress, and gap junctions in female rat ovaries. Reprod Med Biol. 2021;20(4):543–53.
Shi YQ, Zhu XT, Zhang SN, Ma YF, Han YH, Jiang Y, Zhang YH. Premature ovarian insufficiency: a review on the role of oxidative stress and the application of antioxidants. Front Endocrinol. 2023;14:1172481.
Huang B, Lu J, Ding C, Zou Q, Wang W, Li H. Exosomes derived from human adipose mesenchymal stem cells improve ovary function of premature ovarian insufficiency by targeting SMAD. Stem Cell Res Ther. 2018;9(1):216.
Zhang S, Huang B, Su P, Chang Q, Li P, Song A, Zhao X, Yuan Z, Tan J. Concentrated exosomes from menstrual blood-derived stromal cells improves ovarian activity in a rat model of premature ovarian insufficiency. Stem Cell Res Ther. 2021;12(1):178.
Bano A, Vats R, Yadav P, Bhardwaj R. Exosomics in oral cancer diagnosis, prognosis, and therapeutics - an emergent and imperative non-invasive natural nanoparticle-based approach. Crit Rev Oncol Hematol. 2022;178:103799.
Ozkocak DC, Phan TK, Poon IKH. Translating extracellular vesicle packaging into therapeutic applications. Front Immunol. 2022;13:946422.
Xia C, Dai Z, Jin Y, Chen P. Emerging antioxidant paradigm of mesenchymal stem cell-derived exosome therapy. Front Endocrinol. 2021;12:727272.
Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1–2):317–31.
Xu G, Lu X, Liu S, Zhang Y, Xu S, Ma X, Xia X, Lu F, Zou F, Wang H, et al. MSC-Derived exosomes ameliorate intervertebral disc Degeneration by regulating the Keap1/Nrf2 Axis. Stem Cell Rev Rep. 2023;19(7):2465–80.
Ren P, Qian F, Fu L, He W, He Q, Jin J, Zheng D. Adipose-derived stem cell exosomes regulate Nrf2/Keap1 in diabetic nephropathy by targeting FAM129B. Diabetol Metab Syndr. 2023;15(1):149.
Taniue K, Tanu T, Shimoura Y, Mitsutomi S, Han H, Kakisaka R, Ono Y, Tamamura N, Takahashi K, Wada Y et al. RNA exosome component EXOSC4 amplified in multiple Cancer types is required for the Cancer Cell Survival. Int J Mol Sci 2022, 23(1).
Download references
This work was supported by the Foshan Science and Technology Bureau (2019B1515120082 and 2020001006077).
Author information
Yuan Zhou and Jinfa Huang contributed equally to this work.
Authors and Affiliations
Department of Gynecology, Shunde Hospital, Southern Medical University, Foshan, Guangdong, 528308, China
Yuan Zhou, Jinfa Huang, Lingling Zeng, Qian Yang, Fangjuan Bai, Qiqing Mai & Kaixian Deng
You can also search for this author in PubMed Google Scholar
Contributions
Kaixian Deng conceptualized the study design and supervised the analysis. Yuan Zhou collected the data, conducted the experiment, and wrote the paper. Jinfa Huang performed the statistical analysisand polished the draft. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Kaixian Deng .
Ethics declarations
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Zhou, Y., Huang, J., Zeng, L. et al. Human mesenchymal stem cells derived exosomes improve ovarian function in chemotherapy-induced premature ovarian insufficiency mice by inhibiting ferroptosis through Nrf2/GPX4 pathway. J Ovarian Res 17 , 80 (2024). https://doi.org/10.1186/s13048-024-01403-6
Download citation
Received : 26 December 2023
Accepted : 30 March 2024
Published : 15 April 2024
DOI : https://doi.org/10.1186/s13048-024-01403-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Chemotherapy
- Ovarian dysfunction
- Exosomes derived from stem cells
- Ferroptosis
- Nrf2/GPX4 pathway
Journal of Ovarian Research
ISSN: 1757-2215
- General enquiries: [email protected]
ORIGINAL RESEARCH article
Designer umbilical cord-stem cells induce alveolar wall regeneration in pulmonary disease models.

- 1 Institute of Nano-Life-Systems, Institutes of Innovation for Future Society, Nagoya University, Nagoya, Japan
- 2 Division of Surgical Oncology, Department of Surgery, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan
- 3 Department of Cell Processing and Transfusion, The Institute of Medical Science, The University of Tokyo, Tokyo, Japan
- 4 Department of Thoracic Surgery, Faculty of Medicine, Academic Assembly, University of Toyama, Toyama, Japan
Background: Researchers are focusing on cellular therapy for chronic obstructive pulmonary disease (COPD) using mesenchymal stem cells (MSCs), with human bone marrow-derived MSCs (hBM-MSCs) leading the way. However, BM-MSCs may not be as optimal as therapeutic cells owing to their low growth potential, invasive harvesting, and high expression of aging-related genes with poor differentiation potential. Consequently, umbilical cord-derived MSCs (hUC-MSCs), which have many excellent features as allogeneic heterologous stem cells, have received considerable attention. Allogeneic and heterologous hUC-MSCs appear to be promising owing to their excellent therapeutic properties. However, MSCs cannot remain in the lungs for long periods after intravenous infusion.
Objective: To develop designer hUC-MSCs (dUC-MSCs), which are novel therapeutic cells with modified cell-adhesion properties, to aid COPD treatment.
Methods: dUC-MSCs were cultured on type-I collagen gels and laminin 411, which are extracellular matrices. Mouse models of elastase-induced COPD were treated with hUC-MSCs. Biochemical analysis of the lungs of treated and control animals was performed.
Results: Increased efficiency of vascular induction was found with dUC-MSCs transplanted into COPD mouse models compared with that observed with transplanted hUC-MSCs cultured on plates. The transplanted dUC-MSCs inhibited apoptosis by downregulating pro-inflammatory cytokine production, enhancing adhesion of the extracellular matrix to alveolar tissue via integrin β1, promoting the polarity of M2 macrophages, and contributing to the repair of collapsed alveolar walls by forming smooth muscle fibers. dUC-MSCs inhibited osteoclastogenesis in COPD-induced osteoporosis. hUC-MSCs are a promising cell source and have many advantages over BM-MSCs and adipose tissue-derived MSCs.
Conclusion: We developed novel designer cells that may be involved in anti-inflammatory, homeostatic, injury repair, and disease resistance processes. dUC-MSCs repair and regenerate the alveolar wall by enhancing adhesion to the damaged site. Therefore, they can contribute to the treatment of COPD and systemic diseases such as osteoporosis.
1 Introduction
Researchers are developing cell-based therapies for lung diseases. The life-saving effects of mesenchymal stem cell (MSC) therapy for patients with COVID-19-ARDS have been well documented, and reports on the use of cell-based therapies in preclinical experimental models of chronic obstructive pulmonary disease (COPD) have been published ( 1 , 2 ). Clinical trials exploring the use of allogeneic bone marrow-derived MSCs (BM-MSCs) for the treatment of COPD are underway in the U.S. and India, with documented evidence of the anti-inflammatory effects of BM-MSCs ( 3 , 4 ). However, BM-MSCs are not preferred as therapeutic cells because of their low proliferative potential and susceptibility to invasive infections in recipients of transplanted BM-MSCs. BM-MSCs express human leukocyte antigen (HLA) class II molecules that induce immune reactions and inflammation ( 5 ).
Umbilical cord-derived MSCs (UC-MSCs) are suitable for allogeneic transplantation because they do not express HLA-II and show high immune tolerance ( 6 , 7 ). Umbilical cord tissues can be collected noninvasively and stored in banks to ensure a stable supply of UC-MSCs. UC-MSCs exhibit greater immunosuppression than BM-MSCs due to the secretion of soluble factors (prostaglandin E2 and galectin-1) and their high adherence ability ( 8 ).
Administration of human UC-MSCs (hUC-MSCs) for COPD therapy has been clinically proven to be beneficial ( 9 – 11 ). Paracrine action might underlie the improvement observed following MSC administration, but it does not promote lung reconstruction (repair of alveolar epithelial cells) ( 12 ). Therefore, cells that effectively promote tissue regeneration must be developed. By standardizing the culture conditions for hUC-MSCs to optimize their diverse functions, it is possible to create therapeutic cells for COPD and ARDS treatment that stimulate vascular induction and possess anti-inflammatory properties.
Modulation of hUC-MSCs induces the formation of adhesion structures that connect cells to the extracellular matrix (ECM), whereas cytoskeletal actin modulation promotes osteoblast differentiation and neovascularization ( 13 , 14 ). Interstitial matrix proteins, including collagen and fibrin/fibronectin, serve as key receptors that interact with endothelial cell (EC) surface integrins to activate ECs ( 15 – 18 ). Therefore, the objective of the study was to develop designer hUC-MSCs (dUC-MSCs) by modifying their ECM.
COPD coexists with various systemic diseases including osteoporosis, hyperlipidemia, hypertension, and ischemic heart disease. Here, we focused on osteoporosis, one of the major complications of COPD, and analyzed the contribution of dUC-MSC to osteoclastogenesis in a COPD mouse model. Our findings provide new insights into the immunomodulatory functions of dUC-MSCs used to repair lung injury in COPD mouse models that could be used for the treatment of ARDS.
2 Materials and methods
2.1 cell culture.
hUC-MSCs were provided by Dr. Tokiko Nagamura (Tokyo University, Tokyo, Japan). A two-dimensional collagen gel (Type I-A, Nitta Gelatin Inc. Nitta Gelatin, Tokyo, Japan) culture system was prepared following the manufacturer’s protocol. The collagen gel solution containing hUC-MSCs was poured into a plastic Petri dish, followed by incubation at 37°C to allow the gel to polymerize. Subsequently, hUC-MSCs were seeded onto the polymerized gels.
In this study, umbilical cord MSCs were seeded after coating plastic culture dishes with fibronectin, collagen gel, collagen coat, Laminin 211, Laminin 411, and Laminin 511 ECM. The cells were characterized by seeding umbilical cord MSCs in different culture environments created using these techniques. Normal culture without ECM was described as non-coated.
In addition, plates coated with a mixture of collagen and Laminin 411 were described as collagen coat-L411, in which 1 mL of diluted collagen concentration was mixed with 9 μl of Laminin 411, added to the wells and allowed to stand at room temperature for 1 hour. The collagen was used as collagen coat-L411. Gel-L411 plates were also prepared and used by adding 9 μl of Laminin 411 to 2 mL of dilute collagen solution, mixing, adding to wells, and heating at 37°C for 30 minutes to gelatinize.
The cells from the Gel and Gel-L411 culture environment were lysed from the gel using collagenase and used for gene expression analysis and flow cytometry. Cells from other culture methods were exfoliated using trypsin.
2.2 Animal model of COPD
C57BL/6 (B6) mice were purchased from CLEA Japan Inc. (Tokyo, Japan) and maintained under conventional conditions at the Nagasaki University Animal Center. Induction of emphysema was performed according to a previous protocol established as previously reported ( 19 , 20 ). Briefly, the animals were treated with 3% isoflurane under oxygen anesthesia and challenged with intranasal instillation of 30 μg porcine pancreatic elastase (catalog number 058-05361; FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) in 50 µl of 0.9% saline solution. The animals were administered the dose only once on Day 0. Control animals were administered 50 µl of 0.9% saline solution (vehicle). Lung samples collected from treated and control animals were analyzed (see online Data Supplement for details).
2.3 hUC-MSC administration
The mice were anesthetized (isoflurane: 3% induction and 1% maintenance), and saline solution or cultured hUC-MSCs (3.0 × 10 6 cells, total volume 100 μl) were slowly injected via their tail veins. Lung samples were collected at different time points, and their histology, gene expression profiles, and cell surface proteins were analyzed (see the online Data Supplement for details).
2.4 Statistical analysis
All values are expressed as means ± SD of three independent experiments. Data were analyzed using analysis of variance (ANOVA) followed by the Tukey–Kramer test. The statistical significance of differences between concentrations was set at * P < 0.05 or ** P < 0.01, as indicated.
3.1 Adhesion and activation of MSCs of different origins under ECM culture conditions
After 24 hours of incubation, non-adherent cells were removed by washing with PBS. Cells that remained adherent to the well plate were considered as cells with adhesive ability, and the number of those cells was measured and evaluated using the cell counting kit-8 (CCK-8) assay. The results of CCK-8 assay showed that all types of MSCs exhibited greater attachment to plastic surfaces than human umbilical vein endothelial cells (HUVECs), especially at high cell seeding densities ( Figure 1A ). The ratio of VEGF expression to GAPDH gene expression in each MSCs was analyzed using HUVECs, which are known to promote cellular responses involved in angiogenesis, as a control. The mRNA expression of vascular endothelial growth factor (VEGF), was upregulated in each MSCs group in comparison to HUVEC ( Figure 1B ), indicating that the therapeutic potential of UC-MSCs is comparable to that of hBM-MSCs and hADSC-MSCs. MSCs can secrete the angiogenic factor VEGF, which promotes local angiogenesis, suggesting that MSC-based therapy after tissue injury may increase microvascular density and preserve organ function. For this VEGF gene expression analysis, HUVECs, hBM-MSCs, hADSC-MSCs, and hUC-MSCs were cells cultured on plastic surfaces to which no special coating such as ECM or stimulating factors were added.
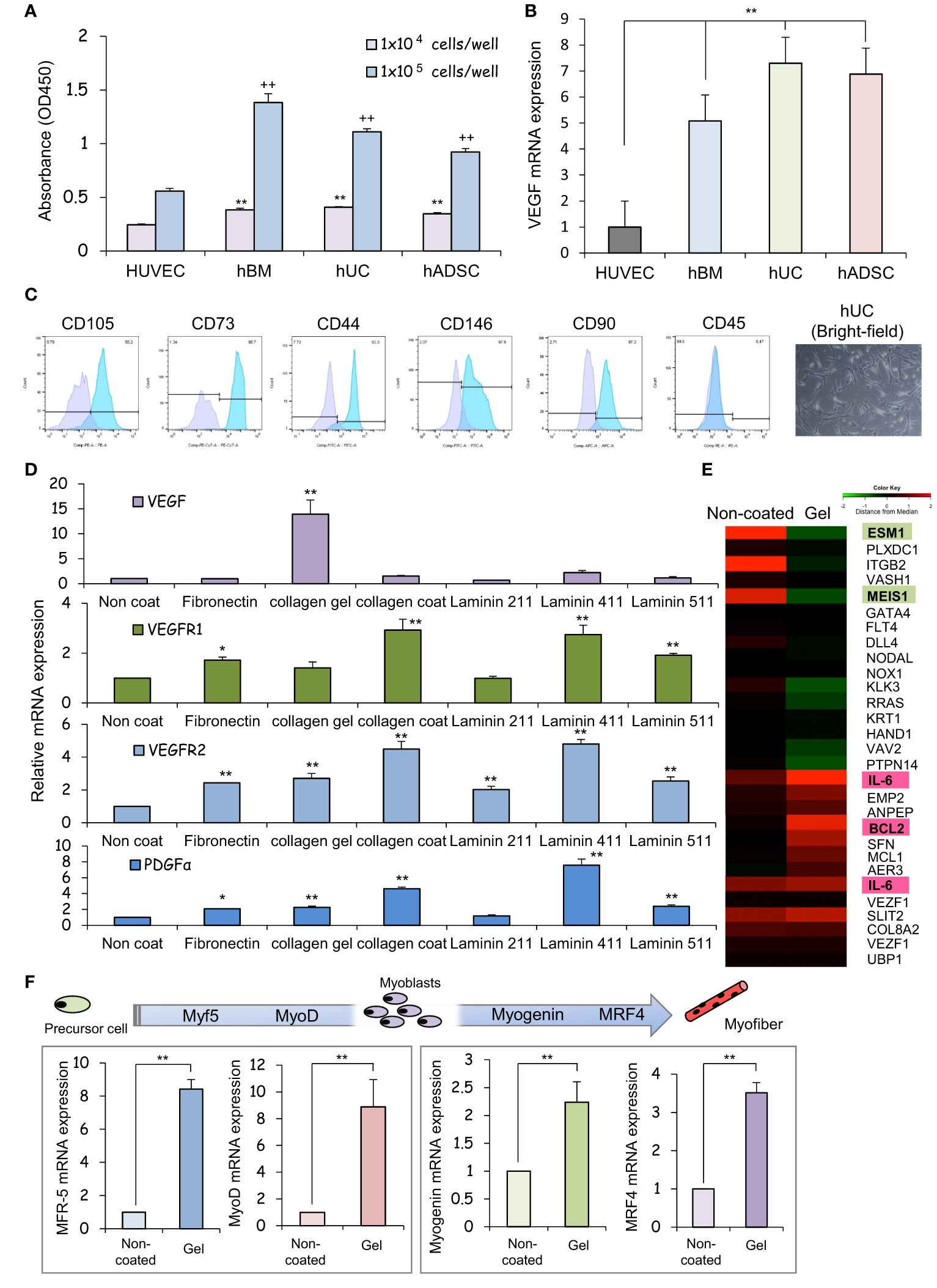
Figure 1 The human umbilical cord is a promising source of mesenchymal stem cells for COPD treatment. (A) Measurement of the viability of HUVECs and hBM-, hUC-, and hADSC-MSCs using CCK-8 assays, and comparison analysis of values at different cell densities. ** p < 0.01; ++ p < 0.01; compared to HUVEC on the same condition. (B) Relative mRNA expression of an angiogenic marker gene, VEGF, after culturing for 1 day; GAPDH was used as a control. ** p < 0.01 compared to HUVEC on the same condition. (C) Flow cytometry of the principal mesenchymal stem cell (MSC) markers. In each diagram, the name of the marker is indicated at the top, the fluorochrome used is indicated at the bottom, and the percentage of positive cells is indicated at the top right. The lower panel presents brightfield images of hUC-MSCs; DAPI was used to label cell nuclei. Scale bar: 20 μm (D) Relative mRNA expression levels of genes for angiogenic factors after culturing for 3 days; GAPDH was used as a control. * p < 0.05; ** p < 0.01; compared to hUC on Non coat culture condition. (E) hUC-MSCs were cultured on different ECM for 6 days; mRNAs were isolated and subjected to mRNA microarray analysis. In the mRNA heatmap, red and green indicate upregulated and downregulated mRNAs, respectively, in cells on type-I collagen gels. (F) Relative mRNA expression levels of genes encoding myogenic regulatory factors after culturing for 3 days; GAPDH was used as a control. ** p < 0.01 compared to hUC on Non coat culture condition.
3.2 Characterization of hUC cultures
The cells isolated from the Wharton’s jelly of the human umbilical cord exhibited a spindle-shaped fibroblast-like morphology and adhered well to the plastic ( Figure 1C ). These cells expressed stem cell-specific transcription factors such as OCT4, NANOG, and SOX2. Flow cytometry analysis revealed up- or downregulated expression of the following surface markers in the sorted cells: CD105-PE, 90.2%; CD73-PE-Cy7, 92.3%; CD146-FITC, 97.9%; CD90-PE, 97.3%, and 0.47% CD45-FITC. These results suggest that our cell cultures exhibited the typical MSC immunophenotype, i.e., CD105 + /CD73 + /CD146 + /CD90 + /CD45 - ( Figure 1C ).
3.3 Effect of ECM on the expression of vasculogenesis- and myoblast differentiation-related mRNAs in hUC-MSCs
We assessed the expression of neovascularization-related factors in hUC-MSCs cultured on different ECM proteins that constitute the basement membrane of alveolar epithelial cells. VEGF expression was higher in collagen I gel-coated wells than in laminin- or fibronectin-coated wells ( Figure 1D ). Coating with laminin 411, but not laminin 211 or laminin 511, promoted PDGFα expression. Since type I collagen gel cultures of hUC-MSCs exhibited vaso-inductive potential, cDNA microarray analysis was performed to evaluate hUC-MSCs’ effects on the regulation of vascular endothelium-related gene expression. A comparison of the microarray data obtained from cells cultured without collagen-1 coating (non-coated) and cells cultured on type I collagen gel revealed differences in the expression of VEGF induction-related genes ( Figure 1E ).
High levels of induction of vascular EC growth factors, IL-6, and BCL2 were found in cells cultured on type-I collagen gels. Additionally, the expression of genes such as endothelial cell-specific molecule 1 ( ESM1 ) and meis homeobox 1 ( MEIS1 ), which negatively regulate cell proliferation, was suppressed ( Figure 1E ). hUC-MSCs cultured on type I collagen gels also exhibited high expression of several genes involved in myofiber differentiation, indicating that these hUC-MSCs may heal injured areas of the airway smooth muscles ( Figure 1F ).
3.4 Effect of ECM combination on vascular endothelial cell markers
The additive effects of different combinations of ECMs on the mRNA, protein, surface marker, vasoinductive marker expression, and morphology of hUC-MSCs attached to these ECMs were evaluated. ECMs containing a collagen coating, collagen gel, and laminin 411 showed increased expression of vasoinductive markers ( Figure 1D ). Although cells cultured on collagen gels (Gel: type I collagen gel) and collagen gels containing mixed substrates (Gel-L411: type I collagen gel and laminin 411) showed greatly enhanced VEGF expression, laminin 411 did not exhibit an additive effect ( Figure 2A ).
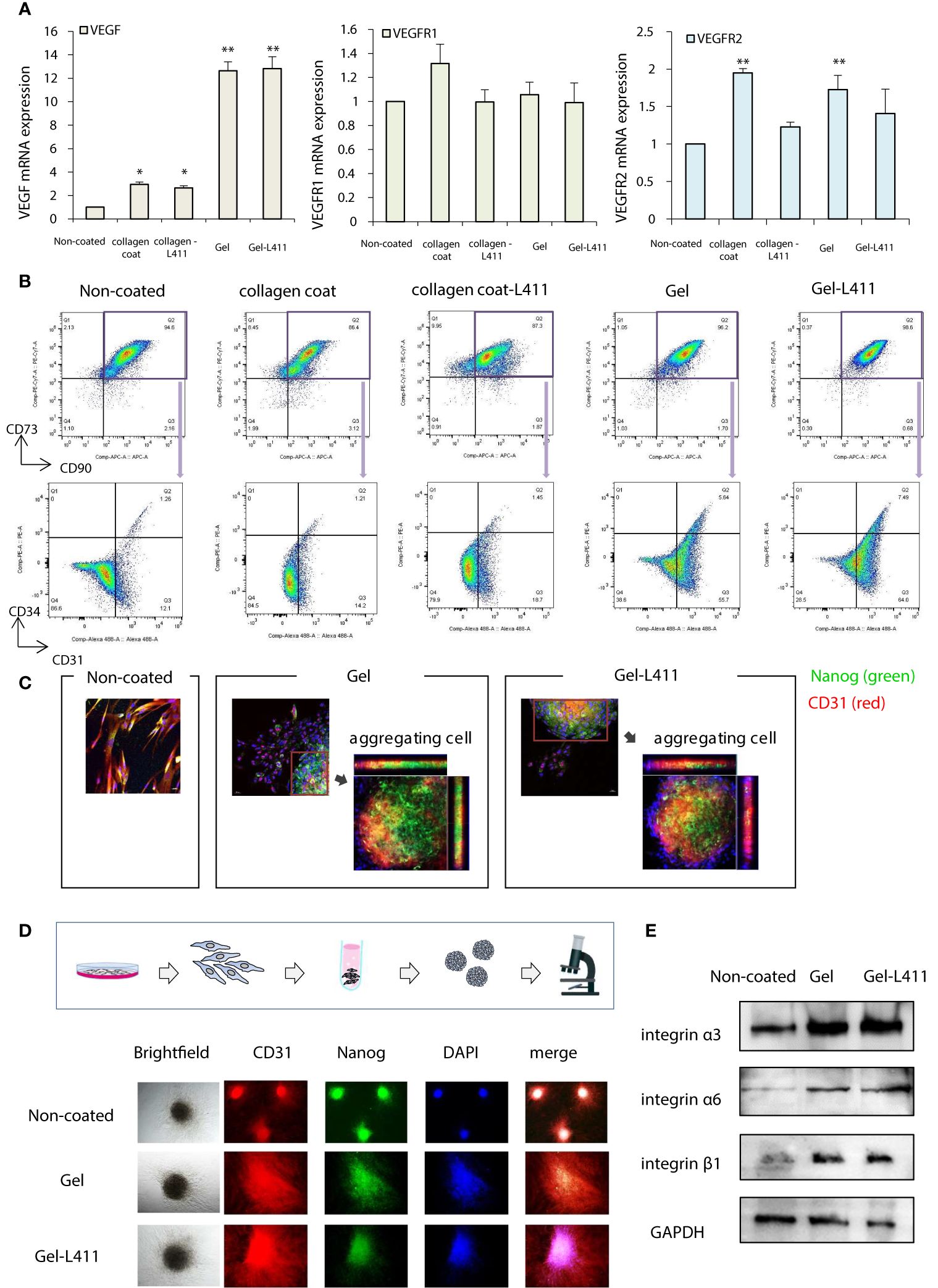
Figure 2 hUC-MSCs cultured on Gel and Gel-L411 induce angiogenesis. (A) hUC-MSCs were cultured on different ECMs for 6 days, and total RNA was isolated. Relative mRNA expression levels of genes encoding angiogenic factors; GAPDH was used as a control. (B) Flow cytometry gating strategy to quantify endothelial progenitor cells. Initially, the mesenchymal fraction was subgated at CD90 and CD73 (upper panel) and was further subgated into bivariate plots of CD31 and CD34 (lower panel). * p < 0.05; ** p < 0.01; compared to hUC on Non coat culture condition. (C) Gel and Gel-411 cells were exfoliated from the ECM, seeded into plates, and cultured for 3 days. Aggregation of these cells was observed in some wells, and the aggregated cells were Nanog (undifferentiated marker)-positive and CD31 (vascular induction marker)-positive. (D) hUC-MSCs removed from the ECM were cultured in 96-well concave microwells for 3 days and formed spheroids. (E) Optical images of hUC-MSC spheroids at different culture conditions and fluorescence microscopy images of CD31 and Nanog.
Flow cytometry was used to characterize the differentiation and maturation processes. Changes in the expression of the cell surface markers, CD31 and CD34, were analyzed in the CD73 + /CD90 + MSC populations (these are the most reliable vascular endothelial progenitor cell markers). High levels of CD31 were observed in cells cultured on Gel and Gel-L411 (approximately 61.4, 71.5, and 13.3% CD31 + cells grown in Gel, Gel-L411, and uncoated-cell culture conditions, respectively). The collagen-coated cultures did not show marked changes in the expression of these markers. The percentage of CD31 + /CD34 + double-positive cells increased from 1.26% in uncoated cells to 5.64% in gel cells and 7.49% in Gel-L411 cells ( Figure 2B ), suggesting that vascular endothelial progenitor cell differentiation was induced in cultures grown in the presence of collagen gel, and cell differentiation was further enhanced by the combination of collagen gel and laminin 411. To observe the effects of ECM on cell morphology, hUC-MSCs were extracted from coated culture dishes, isolated by collagenase digestion, and seeded onto plastic slides. Although spindle-shaped fibroblast-like cells were observed in hUC-MSCs extracted from uncoated cells, hUC-MSCs extracted from Gel or Gel-L411 exhibited a heterogeneous morphology, including small round cells and spindle-shaped cells that showed spontaneous aggregation, suggesting that culturing on collagen gels induced cell-to-cell adhesion of hUC-MSCs. The aggregated cells comprised Nanog-positive undifferentiated MSCs and CD31 + vascular endothelial progenitor cells ( Figure 2C ).
Given these observations, we evaluated whether cell aggregation enhanced the angiogenic potential by inducing spheroid formation ( Figure 2D ). Spheroids formed from uncoated cultured cells exhibited regular, tightly packed aggregates, whereas cells cultured on Gel and Gel-L411 formed loose, irregularly shaped aggregates. Regardless of the culture conditions, none of the hUC-MSC spheroids were positive for Nanog or CD31, indicating that the aggregation of undifferentiated hUC-MSCs promoted differentiation and angiogenesis. Western blot analysis revealed that the expression of the cell adhesion molecule integrin was markedly upregulated in cells cultured on Gel and Gel-L411; however, there was no observable difference in integrin expression in hUC-MSCs cultured in the presence or absence of laminin 411 ( Figure 2E ).
3.5 In vitro effects of hUC-MSCs in lung injury models
Alveolar epithelial progenitor cells (AEpCs) were isolated from adult human lung tissues for in vitro analysis ( Figure 3A ). These progenitor cells express the MSC surface marker CD90 and factors associated with alveolar type II cells, such as the epithelial cell adhesion molecule (EpCAM) and pro-surfactant protein C (pro-SPC). The half-maximal inhibitory concentration (IC 50 ) value of elastase against these AEpCs was 65.063 μg/ml ( Figure 3B ). To evaluate the effect of UC-MSC signaling on the alveolar epithelium, hUC-MSCs and AEpCs were indirectly co-cultured on Transwell ® membranes ( Figure 3C ). qRT-PCR showed significant inhibition of expression of the inflammatory marker, tumor necrosis factor (TNF)-α, and upregulation of expression of the anti-inflammatory marker, interleukin (IL)-10, in hUC-MSCs that were co-cultured with AEpCs.
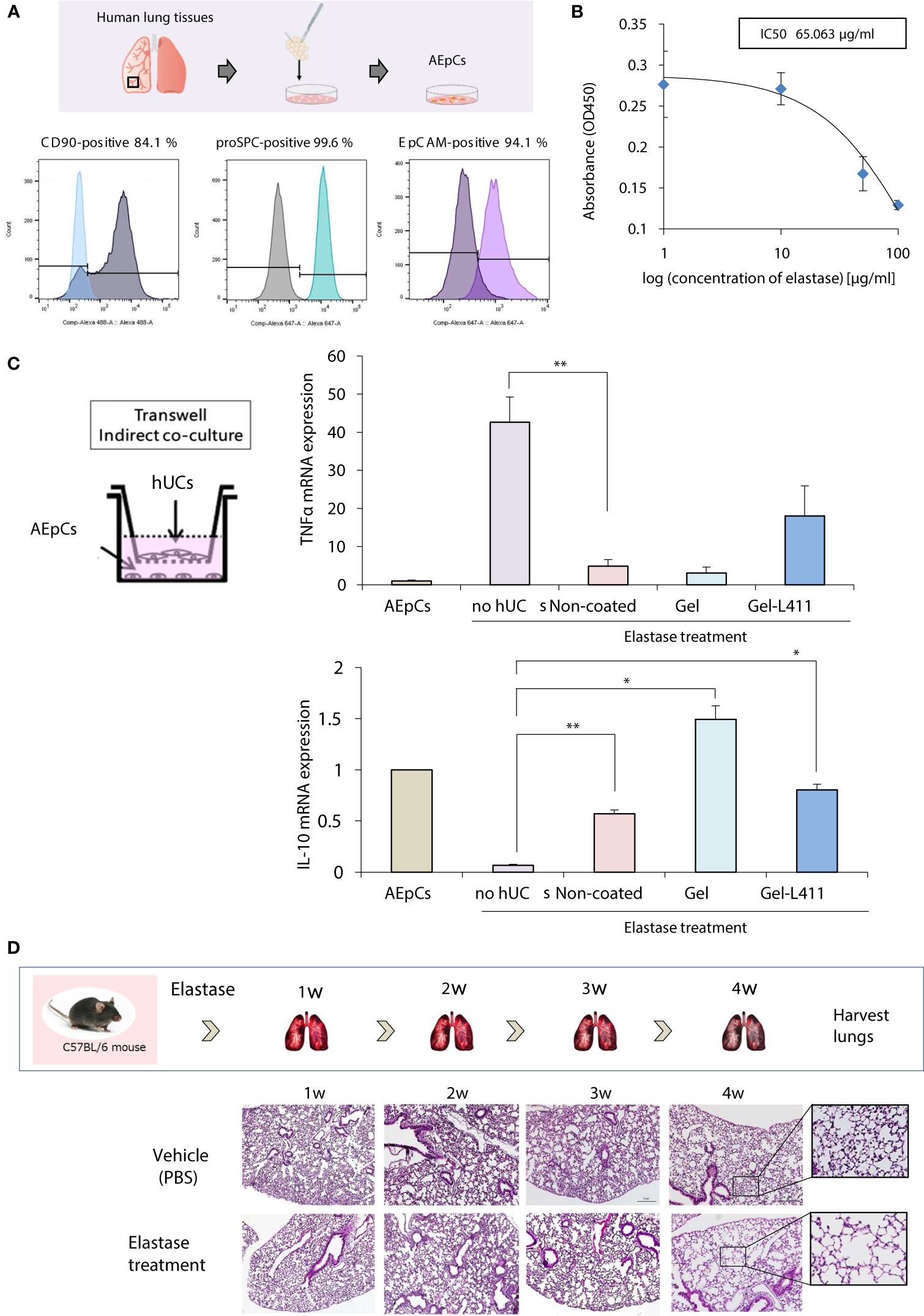
Figure 3 In vitro models of epithelial–mesenchymal crosstalk in the lung and establishment of COPD mouse models. (A) Flow cytometry of the principal mesenchymal stem cell (MSC) markers. In each diagram, the name of the marker is indicated at the top, the fluorochrome used is indicated at the bottom, and the percentage of positive cells is indicated at the top right. (B) Dose-response curves of IC 50 for elastase: AEpCs cells were treated for 24 h with 0, 1, 10, 50 and 100 μM of elastase. (C) Establishment of the AEpC-hUC-MSC co-culture system. The transwell co-culture systems were established in 6-well plates. To assess the effect of co-culture system on AEpCs, we used AEpCs as a control group. Inflammation was evaluated using TNFα and anti-inflammatory effects using IL-10. * p < 0.05; ** p < 0.01; compared to AEpCs on Elastase treatment culture condition. (D) Induction of a COPD-like phenotype in C57BL/6 wild-type mice induced with elastase exposure: representative weekly histopathology based on single intranasal administration of elastase for 4 weeks (n = 5 per group); scale bar: 200 μm.
3.6 hUC-MSCs cultured on ECM promote lung regeneration and macrophage polarization after elastase treatment in vivo
A preclinical COPD model was used to determine the in vivo effects of human MSC therapy. The COPD model was established by intranasal administration of elastase to mice over 4 weeks to induce emphysema-like changes in the lungs ( Figure 3D ).
Four weeks after elastase administration, hUC-MSCs were administered to the mice, and the lungs were harvested on day 12 for analysis ( Figure 4A ). Alveolar damage was assessed using hematoxylin and eosin (H&E) staining. The total surface area of the alveoli increased after administration of hUC-MSCs, with a significant tissue repair effect, especially in the Gel-L411 cultured cell group ( Figure 4B ). The localization of alpha-smooth muscle actin (α-SMA)-positive cells was examined by immunostaining to confirm the contribution of hUC-MSCs in the formation of vascular smooth muscle cells along the small airways (bronchioles) and alveolar walls of the lungs. Immunofluorescence analysis of the elastase-treated lungs showed that the expression of elastin, a major component of the connective tissue that organizes the alveoli, was suppressed, whereas that of α-SMA increased after hUC-MSC injection ( Figure 4C ). The expression of CD31, also known as platelet/endothelial cell adhesion molecule-1 (PECAM-1, an adhesion molecule that accumulates at adhesion sites between vascular endothelial cells and increases integrin binding activity), was markedly upregulated in the Gel and Gel-L411 cell groups. The expression of the hematopoietic progenitor cell marker, CD34, did not markedly increase ( Figure 4C ). The antioxidant protein heme oxygenase-1 (HO-1) was detected mainly in alveolar type II cells, indicating the co-localization of HO-1 with proSPC ( Figure 4C ).
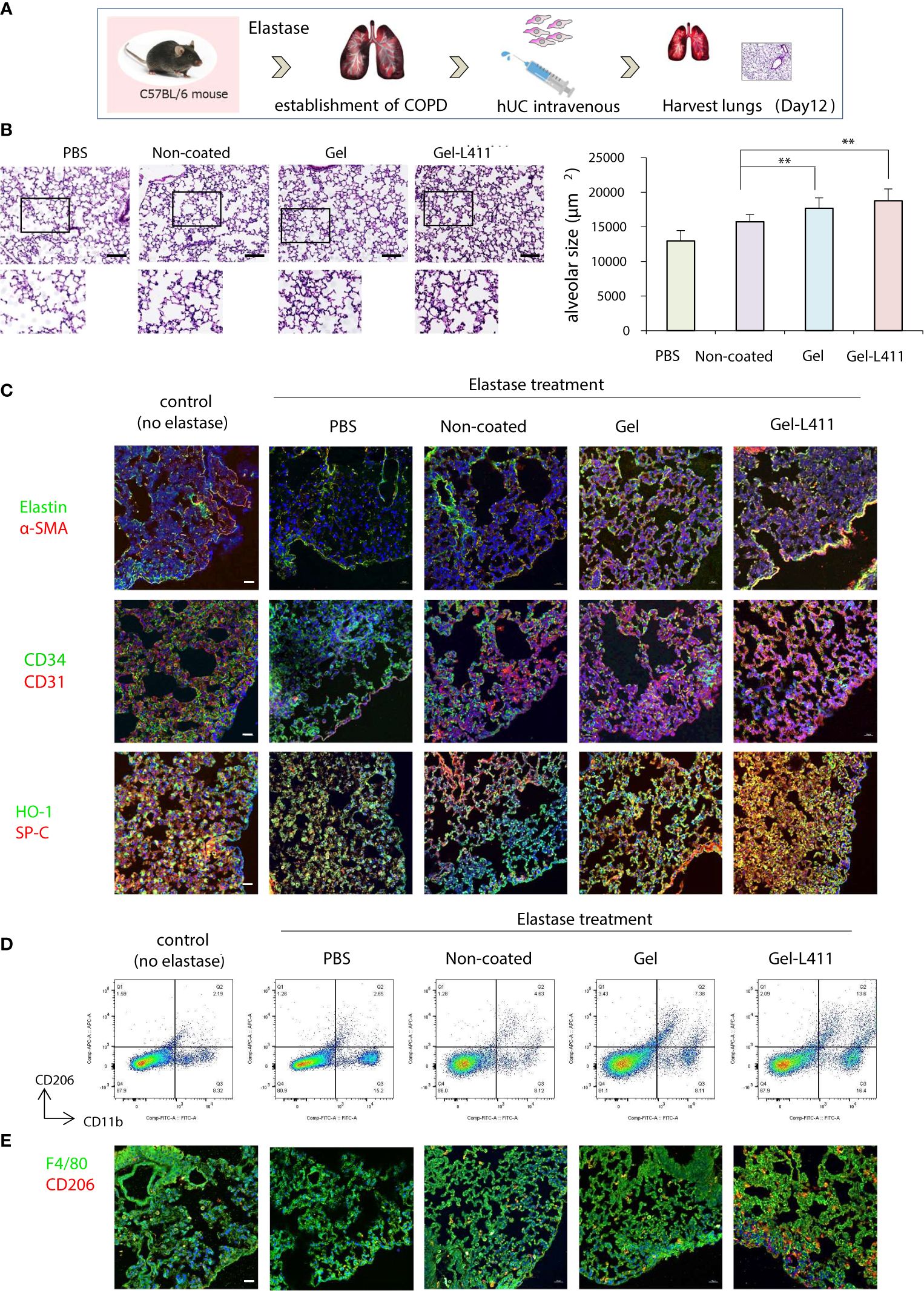
Figure 4 Modified hUC-MSCs exert therapeutic effects on lung tissue in a mouse model of COPD. (A) Schematic representation of the COPD model based on cell administration after intranasal administration of elastase. (B) Histopathological evaluation in the lung tissue of elastase-induced mice after 12 days of treatment (n = 5 per group): H&E staining, scale bar: 200 μm. ** p < 0.01 compared to hUC injection group on Non coat culture condition. (C) Detection of Elastin, α-SMA, CD34, CD31 HO-1, and proSPC-positive cells in UC-MSCs using indirect immunofluorescence and a confocal laser scanning system (n = 5 per group). Magnification, 400×; scale bar: 20 μm. (D) Flow cytometry gating strategy to quantify macrophage cell populations (n = 5 per group). (E) Representative images of immunofluorescence staining of F4/80 (green) and CD206 (red) in hUC-MSCs (n = 5 per group). Magnification 400×.
Alveolar macrophages may play an important role in the pathogenesis of COPD because they express proteases such as matrix metalloproteinases (MMPs) and cathepsins ( 21 ). Therefore, we examined the polarity of macrophages and analyzed the mechanisms underlying alveolar regeneration. hUC-MSCs were co-immunolabeled with CD11b, a monocyte marker, and CD206, an M2 anti-inflammatory macrophage marker, to investigate the phenotypic changes that occur upon the administration of hUC-MSCs to macrophages present in lung tissues. Flow cytometric analysis of isolated lung monocytes revealed an overall increase in CD11b levels in all elastase-treated groups. The hUC injection, especially the injection of Gel-L411 cells, resulted in an increased number of M2 macrophages ( Figure 4D ).
To determine whether the injected hUC-MSCs interacted with macrophages, lungs collected on day 12 were immunostained with antibodies against F4/80 (a pan-macrophage marker) and CD206 ( Figure 4E ). In line with the fluorescence-activated cell sorting (FACS) analysis, we confirmed that the overall polarization of macrophages in lung tissues shifted toward an M2-predominant type after injection of Gel-L411 cells.
3.7 Therapeutic efficacy of administered hUC-MSCs in COPD-related osteoporosis
We examined whether Gel- and Gel-L411-cultured cells, when co-cultured with receptor activator of NF-κB ligand (RANKL)-induced osteoclast progenitors and hUC-MSCs, affected osteoclastogenesis in vitro . Bone marrow from wild-type mice was extracted, seeded in well plates, and cultured in the presence of M-CSF and RANKL to differentiate into mature multinucleated tartrate-resistant acid phosphatase (TRAP)-positive osteoclasts. The contact approach between osteoclasts and hUC-MSCs was evaluated using a non-contact co-culture model of osteoclasts and hUC-MSCs using Transwell (left side of Figure 5A ; insert group) and a direct co-culture model in which hUC-MSCs were added directly to osteoclasts (right side of Figure 5A ; direct group). The group of bone marrow cells to which only M-CSF was added was designated “macrophages” and the group to which M-CSF and RANKL were added was designated “control (no hUCs)”. The hUCs added to osteoclasts in both the insert and direct groups were hUC-MSCs cultured without coating and “non-coated”, “gel” on gel and “Gel-411” on gel with Laminin 411.
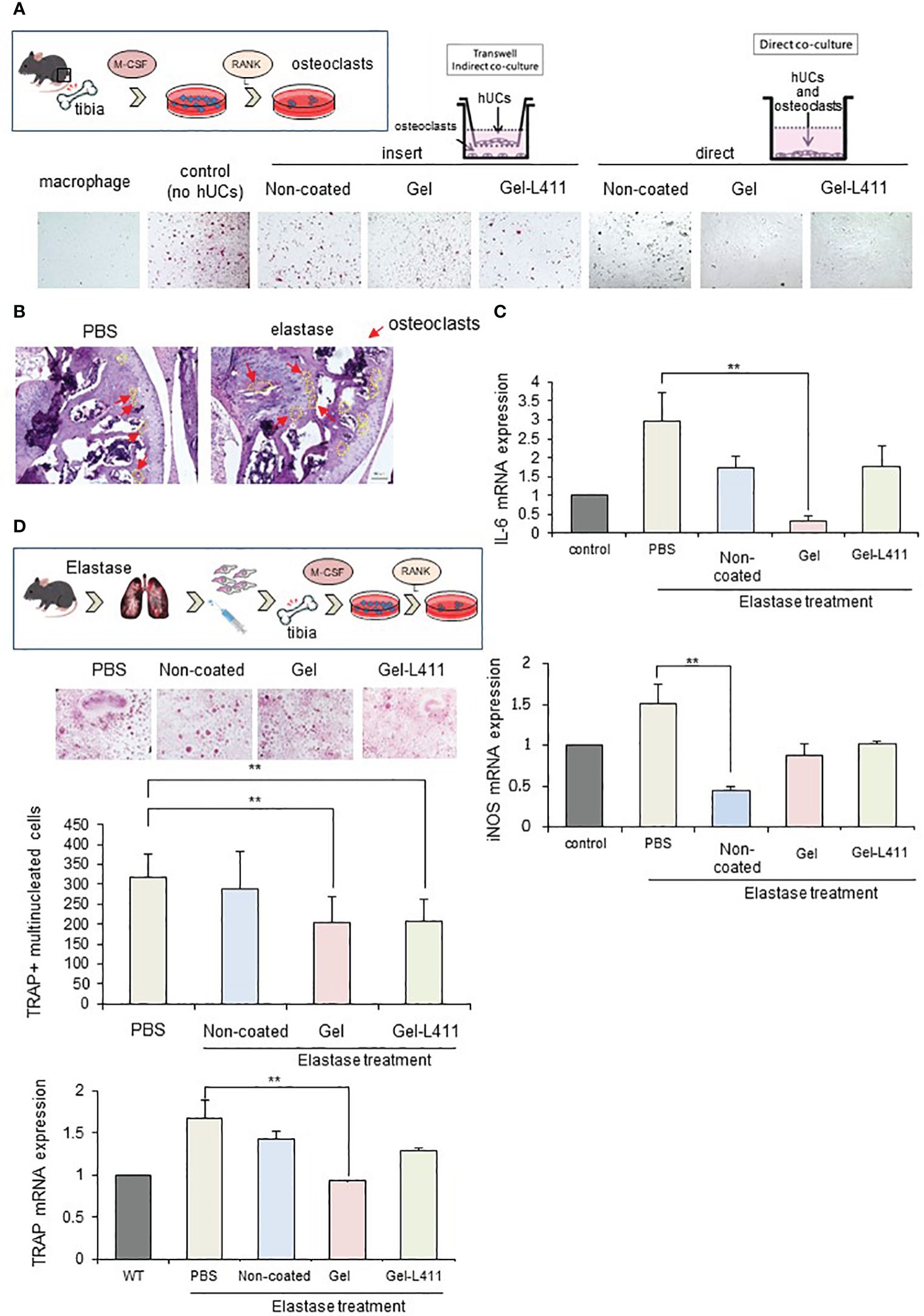
Figure 5 Modified hUC-MSCs exert therapeutic effects on osteoporosis in a mouse model of COPD. (A) Schematic of osteoclast induction from the bone marrow extracted from wild-type mice. To examine the interaction between osteoclast differentiation and modified hUC-MSCs, we established co-culture systems. The left group is a transwell-based non-contact culture, and the right group is a direct culture. Purified TRAP-positive osteoclasts formed in cocultures of hUC-MSCs and bone marrow cells. (B) Schematic of osteoclast induction from the bone marrow extracted from a mouse model of COPD after cell injection. TRAP-positive osteoclast formation induced after bone marrow extraction from mice treated with hUC-MSCs injections. Quantitative analysis showing the number of osteoclasts per well and TRAP mRNA expression. (n = 5 per group) (C) Characterization of gene expression of the inflammatory markers interleukin-6 (IL-6) and inducible nitric oxide synthase (iNOS). ** p < 0.01 compared to hUC injection group on Non coat culture condition. (D) TRAP staining and increased osteoclast formation in the femoral head in COPD-induced osteoporosis. mRNAexpression levels of TRAP were determined by qPCR.
TRAP staining (red) showed that osteoclastogenesis was almost completely inhibited in the Direct assay, indicating that cell-to-cell contact stimulated osteoclastogenesis, which occurs in the presence of soluble mediators ( Figure 5A ).
We also evaluated the morphology and number of osteoclast progenitor cells by culturing bone marrow cells (obtained from the tibiae of the control and experimental mice) in the presence of RANKL and M-CSF for five days. Numerous TRAP-positive multinucleated osteoclasts were observed in the control group. The number of osteoclasts and expression of the mature osteoclast marker, TRAP, in cultures of COPD mouse models were significantly lower in the Gel and Gel-L411 groups than in the control group ( Figure 5B ). Expression of the M1 markers, IL-6 and inducible nitric oxide synthase (iNOS), in bone marrow cells (isolated from experimental mice) decreased in the hUC-MSC-treated group and was suppressed to a greater extent in the Gel group than in the Gel-L411 group ( Figures 5C, D ).
3.8 Gel and Gel-L411 interact with macrophages in the mouse lung after infusion
To investigate the function of hUC-MSCs in vivo , we analyzed their behavior using luciferase-expressing cells (Luc-hUCs). hUC-MSCs almost disappeared 24 h after intravenous administration of Luc-hUCs. Several Luc-hUCs cultured on the Gel or Gel-L411 were observed in the lung samples ( Figure 6A ). To determine whether hUC-MSCs interacted with macrophages in vivo after injection, lung samples were immunolabeled with antibodies against the macrophage marker F4/80. In the Gel and Gel-L411 groups, hUC-MSCs were distributed throughout the lung tissue, accumulated on the outer margins, and co-localized with macrophages. Injection of hUC-MSCs induced the generation of CD206 + M2-type macrophages, while CCR7 + M1-type macrophages were not formed, suggesting that the injection of hUC-MSCs induced an M2 polarization shift in macrophages, resulting in anti-inflammatory effects.
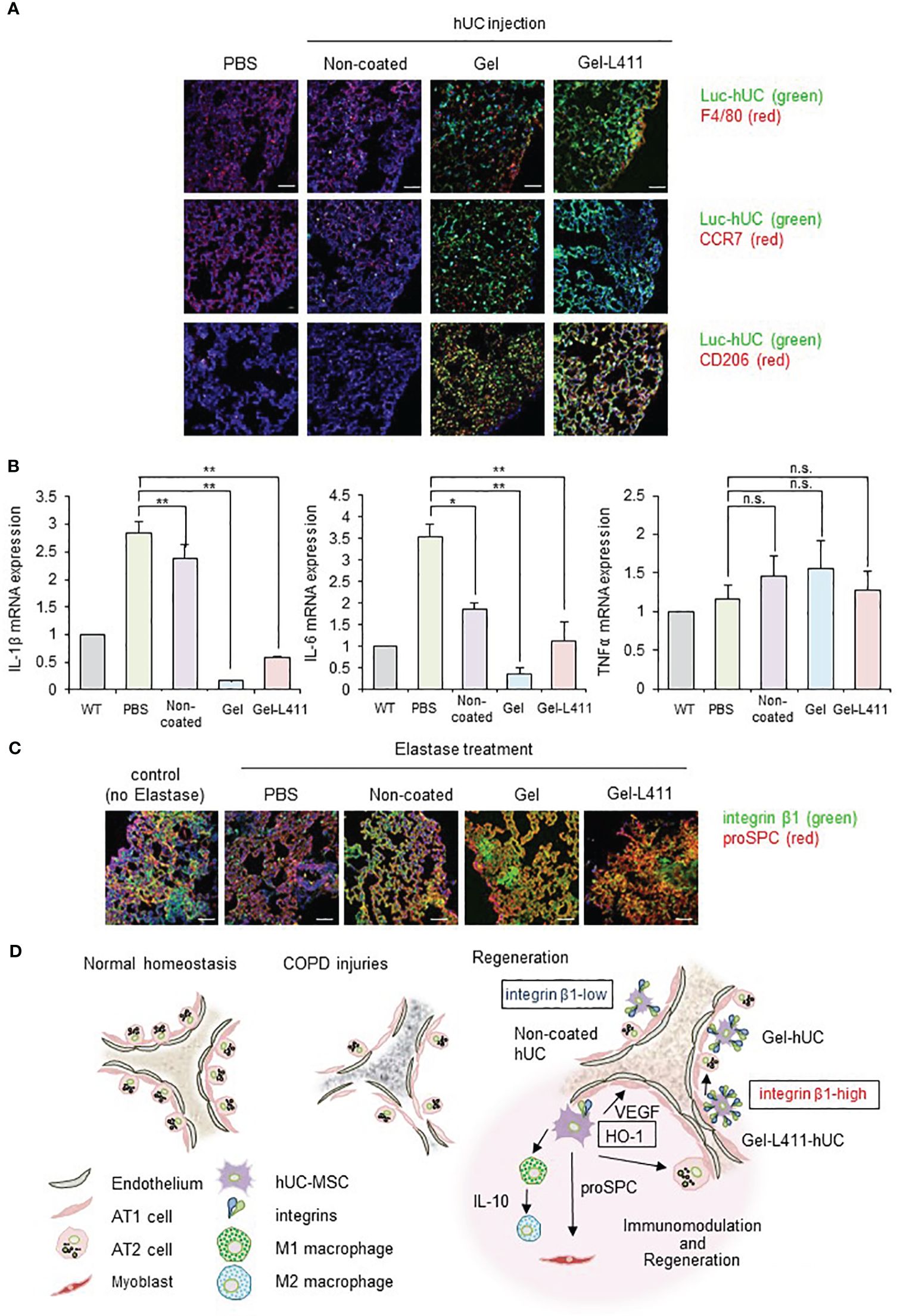
Figure 6 dUC-MSCs promote epithelial spreading and lung regeneration (A) Luciferase-labeled hUC-MSCs were injected, and their biological kinetics were analyzed using fluorescence imaging. Immunostained images of lung tissue collected after 24 h of cell injection into COPD model mice: Luc-hUC (green) and F4/80 (red). Scale bar: 40 μm (B) Gene expression of inflammation markers in RNA extracted from lung tissue 24 h after hUC-MSC injection * p < 0.05, ** p < 0.01, n.s. = Not significant; compared to hUC injection group on Non coat culture condition. (C) Immunostained image of lung tissue using confocal laser microscopy (12 days after cell injection): integrin β1 (green) and proSPC (red). Scale bar: 40 μm. (D) Regeneration of the alveolar epithelium after injury. Elastase-mediated injury results in extensive destruction of all alveolar epithelial cells. Surviving ATII cells are activated and proliferate after injury, restoring the alveolar epithelium. A progenitor cell population that expresses high levels of integrin β1 appears to contribute to alveolar epithelial regeneration.
A noticeable change in TNFα expression was not observed in the lungs 24 h after cell administration ( Figure 6B ) but those of IL-1β and IL-6 were suppressed, indicating that cells cultured on the ECM induced anti-inflammatory effects in the lungs.
3.9 Laminin-411 promotes epithelial spreading
Integrin β1 co-localized with proSPC in the COPD mouse models injected with Gel or Gel-L411 cells ( Figure 6C ). Considering that attached hUC-MSCs are phagocytosed by macrophages, in vitro experiments ( Figures 2C–E ) suggested that integrin β1 may be the key factor that induced the adhesion of hUC-MSCs, leading to alveolus formation in the COPD model. This result is agreement with previously reports that human mesenchymal stem cells cultured on collagen gels differentiate into epithelial cells through the formation of cytokeratin-18 ( 22 ).
4 Discussion
In this study, we developed dUC-MSCs by culturing UC-MSCs on a type I collagen gel and laminin 411. dUC-MSCs were injected via the tail vein of COPD mice, where they were attached to the lung tissue and survived for a long duration. Mice injected with dUC-MSCs showed a greater alleviation of elastase-induced COPD symptoms than those injected with hUC-MSCs. Our experimental data suggest that tissue regeneration occurs because of the binding of dUC-MSCs to the ECM through integrin-mediated cell adhesion mechanisms ( 23 – 26 ). Thus, dUC-MSCs promoted macrophage differentiation through cytokine secretion or direct contact. To the best of our knowledge, this is the first report on the role of modified cells in stimulating lung epithelial cell induction and lung tissue regeneration for COPD treatment. Notably, gene modification was not required to strengthen MSCs. Overall, we developed novel designer cells that may be involved in anti-inflammatory, homeostatic, injury repair, and disease resistance processes.
Research in this field is typically conducted using BM-MSCs or AD-MSCs. MSCs obtained from other sources, such as the umbilical cord and placenta, are also used for therapeutic angiogenesis ( 27 ). Perinatal stem cells (hUC-MSCs) exhibit pluripotency, multipotent tissue maintenance, a high degree of plasticity, and immunomodulatory activity and lack tumorigenicity; they are considered the best sources of allogeneic xenografts ( 28 – 30 ). Furthermore, UC-MSCs secreted VEGFs and uniformly expressed endothelial markers without altering their cellular organization or morphology ( 30 ). We examined VEGF expression in MSCs derived from various tissues and confirmed that UC-MSCs exhibited enhanced VEGF production ( 31 ). hUC-MSCs are a promising cell source and have many advantages over BM-MSCs and AD-MSCs.
hUC-MSCs cultured on collagen I and laminin 411 (Gel-L411 dUC-MSCs) showed the highest expression levels of vascular endothelial markers and myofibroblast progenitor cell markers in vitro and retained their anti-inflammatory effects without undergoing genetic modifications. During the regeneration of the destroyed basement membrane and ECM, MSCs differentiate into myofibroblasts ( 32 ) and pulmonary capillary endothelial cells ( 33 ), and their interaction contributes to angiogenesis and alveolus formation ( Figure 4C ). dUC-MSCs exhibited a higher adhesion capacity than normally cultured hUC-MSCs. ECM interactions and their processes in tissue repair are mediated by integrins, generally through the intracellular signaling molecules, Rho and Rac, which also have synergistic effects on differentiation into the epithelial lineage ( 34 – 38 ). Integrin β1 activity was upregulated in both Gel- and Gel-L411-dUC-MSCs, demonstrating the positive and significant effects of these substrates ( Figure 6D ).
Furthermore, MSCs contribute to changing the phenotype of macrophages from M1 (inflammatory) to M2 (anti-inflammatory). M2 macrophages play important roles in regulating inflammation, angiogenesis, debris removal, and tissue remodeling ( 21 , 39 – 42 ). This was confirmed by flow cytometry ( Figure 4D ), which showed a polarity change toward the M2 macrophage state (along with an increase in macrophage number) in the dUC-treated group, suggesting the involvement of M2 macrophages in tissue repair.
In cell injection experiments using COPD mouse models, dUC-MSCs adhered for a long time, whereas hUC-MSCs adhered for shorter periods in normal cultures. The primary issue in using therapeutic cells to treat lung diseases is the failure of the cells to attach to the target area. In previous clinical trials, two doses of MSCs were administered in one cycle for at least two cycles (four doses in total) (ClinicalTrials.gov; No.: NCT00683722; URL: www.clinicaltrials.gov ). In our study, dUC-MSCs survived for a longer period than normally cultured UC-MSCs in COPD mouse models. Therefore, the use of dUCs may reduce the number of times these therapeutic cells need to be administered.
Systemic inflammation associated with COPD can contribute to the pathogenesis of osteoporosis. Key inflammatory cytokines, such as iNOS and IL-6, interact with RANKL ( 43 ). dUC-MSCs inhibit the formation of abnormal osteoclasts by suppressing the expression of inflammatory mediators. Therefore, dUC-MSCs can be used to treat osteoporosis and other comorbidities caused by inflammatory reactions.
Integrin β1 expression in the lung epithelium is required for airway branching morphogenesis, alveolus formation, and homeostasis ( 44 ). Integrin β1 regulates epithelial cell adhesion and migration, alveolar cell differentiation, and ECM deposition in the alveolar septum ( 45 ). Integrin β1 promoted angiogenesis by stimulating smooth muscle formation by modulating the production of reactive oxygen species ( Figures 4C , 6C ). This appears to be a novel mechanism by which cell–ECM interactions modulate lung inflammation and alveolar septum formation.
However, while culture with gel-form type I collagen activates signal transduction that regulates the induction of differentiation and other factors, it also induces growth suppression. Therefore, for clinical applications, it is necessary to establish a culture environment that allows for mass production while maintaining cellular characteristics; further research is required to confirm this hypothesis. Moreover, dUC-MSCs are not suitable for practical use because they are cultured on gels and require collagenase treatment for cell harvesting. Therefore, we are researching peptides that can mimic collagen-gel culture to secure cell numbers in normal planar culture; accordingly, we have commenced an attempt to mass-produce therapeutic cells with high tissue-repair capacity.
In summary, in this study, we developed therapeutic cells to overcome the nonviability of transplanted cells during cell transplantation therapy. The adhesion properties of dUC-MSCs were modified by binding to cell adhesion molecules, allowing the cells to maintain their adhesive function even after detachment. We focused on elucidating the mechanisms underlying the immune regulation mediated by UC-MSCs and aimed to establish a culture method optimized for the induction of endogenous signaling pathways that could alter cell behavior. This innovative cell therapy method can enable the large-scale production of cell products for transplantation without using any genetic manipulation strategies.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material , further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by Nagasaki University Research Ethics Committee No.22122301. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The animal study was approved by the Nagasaki University Institutional Animal Care and Use Committee guidelines and the ethics committee of Nagasaki University (Approval Number: 2202281776). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
MIw: Writing – original draft, Writing – review & editing, Funding acquisition. TN-I: Formal analysis, Writing – review & editing. RD: Funding acquisition, Writing – review & editing. YT: Funding acquisition, Methodology, Writing – review & editing. MIs: Formal analysis, Funding acquisition, Methodology, Writing – review & editing. HY: Methodology, Writing – review & editing. KM: Writing – review & editing. KT: Writing – review & editing. TN: Writing – review & editing. TT: Funding acquisition, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study received financial support from Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (grant no. 20K10010 to MIw; grant no. 20K0255 to TT; and 21K08907 to MIs) and the Nagasaki University State of the Art Research program (TT).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1384718/full#supplementary-material
1. Xu Z, Huang Y, Zhou J, Deng X, He W, Liu X, et al. Current status of cell-based therapies for COVID-19: evidence from mesenchymal stromal cells in sepsis and ARDS. Front Immunol . (2021) 12:738697. doi: 10.3389/fimmu.2021.738697
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Xiao K, Hou F, Huang X, Li B, Qian ZR, Xie L. Mesenchymal stem cells: current clinical progress in ARDS and COVID-19. Stem Cell Res Ther . (2020) 11:305. doi: 10.1186/s13287-020-01804-6
3. Armitage JD, Tan DBA, Sturm M, Moodley YP. Transcriptional profiling of circulating mononuclear cells from patients with chronic obstructive pulmonary disease receiving mesenchymal stromal cell infusions. Stem Cells Transl Med . (2021) 10:1470–81. doi: 10.1002/sctm.21-0024
4. Cheng S-L, Lin C-H, Yao C-L. Mesenchymal Stem Cell Administration in Patients with Chronic Obstructive Pulmonary Disease: State of the Science. Stem Cells Int . (2017) 2017:1–14. doi: 10.1155/2017/8916570
CrossRef Full Text | Google Scholar
5. Mastrolia I, Foppiani EM, Murgia A, Candini O, Samarelli AV, Grisendi G, et al. Challenges in clinical development of mesenchymal stromal/stem cells: concise review. Stem Cells Transl Med . (2019) 8:1135–48. doi: 10.1002/sctm.19-0044
6. Bocelli-Tyndall C, Zajac P, Di Maggio N, Trella E, Benvenuto F, Iezzi G, et al. Fibroblast growth factor 2 and platelet-derived growth factor, but not platelet lysate, induce proliferation-dependent, functional class II major histocompatibility complex antigen in human mesenchymal stem cells. Arthritis Rheumatol . (2010) 62:3815–25. doi: 10.1002/art.27736
7. Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: their advantages and potential clinical utility. World J Stem Cells . (2014) 6:195–202. doi: 10.4252/wjsc.v6.i2.195
8. Le Thi Bich P, Nguyen Thi H, Dang Ngo Chau H, Phan Van T, Do Q, Dong Khac H, et al. Allogeneic umbilical cord-derived mesenchymal stem cell transplantation for treating chronic obstructive pulmonary disease: a pilot clinical study. Stem Cell Res Ther . (2020) 11:1–4. doi: 10.1186/s13287-020-1583-4
9. Ridzuan N, Zakaria N, Widera D, Sheard J, Morimoto M, Kiyokawa H, et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles ameliorate airway inflammation in a rat model of chronic obstructive pulmonary disease (COPD). Stem Cell Res Ther . (2021) 12:54. doi: 10.1186/s13287-020-02088-6
10. Chen XY, Chen YY, Lin W, Chen CH, Wen YC, Hsiao TC, et al. Therapeutic potential of human umbilical cord-derived mesenchymal stem cells in recovering from murine pulmonary emphysema under cigarette smoke exposure. Front Med (Lausanne) . (2021) 8:713824. doi: 10.3389/fmed.2021.713824
11. Chen Q, Lv L, Zheng C, Pan H, Xu J, Lin J, et al. Human umbilical cord-derived mesenchymal stem cells repair SU5416-injured emphysema by inhibiting apoptosis via rescuing VEGF-VEGFR2-AKT pathway in rats. Int J Stem Cells . (2022) 15:395–404. doi: 10.15283/ijsc21149
12. Jin HJ, Bae YK, Kim M, Kwon SJ, Jeon HB, Choi SJ, et al. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci . (2013) 14:17986–8001. doi: 10.3390/ijms140917986
13. Mayumi I, Yoshinori S, Tokiko N, Izumi A. Actin depolymerization accelerates osteoblastic differentiation of human umbilical cord mesenchymal stem cells [abstract] Vol. Vol. A-28. Kyoto, Japan: TERMIS World Congress (2018). p. a90454.
Google Scholar
14. Madhusoodanan J. Matrix mimics shape cell studies. Nature . (2019) 566:563–5. doi: 10.1038/d41586-019-00681-1
15. Whelan MC, Senger DR. Collagen I initiates endothelial cell morphogenesis by inducing actin polymerization through suppression of cyclic AMP and protein kinase A. J Biol Chem . (2003) 278:327–34. doi: 10.1074/jbc.M207554200
16. Liu Y, Senger DR. Matrix-specific activation of Src and Rho initiates capillary morphogenesis of endothelial cells. FASEB J . (2004) 18:457–68. doi: 10.1096/fj.03-0948com
17. Senger DR. Molecular framework for angiogenesis: A complex web of interactions between extravasated plasma proteins and endothelial cell proteins induced by angiogenic cytokines. Am J Pathol . (1996) 149:1–7.
PubMed Abstract | Google Scholar
18. Davis GE, Bayless KJ. An integrin and Rho GTPase-dependent pinocytic vacuole mechanism controls capillary lumen formation in collagen and fibrin matrices. Microcirculation . (2003) 10:27–44. doi: 10.1080/713773584
19. Wright JL, Cosio M, Churg A. Animal models of chronic obstructive pulmonary disease. Am J Physiol Cell Mol Physiol . (2008) 295:L1–L15. doi: 10.1152/ajplung.90200.2008
20. Río C, Jahn AK, Martin-Medina A, Calvo Bota AM, De Francisco Casado MT, Pont Antona PJ, et al. Mesenchymal stem cells from COPD patients are capable of restoring elastase-induced emphysema in a murine experimental model. Int J Mol Sci . (2023) 24:5813. doi: 10.3390/ijms24065813
21. Finicelli M, Digilio FA, Galderisi U, Peluso G. The emerging role of macrophages in chronic obstructive pulmonary disease: the potential impact of oxidative stress and extracellular vesicle on macrophage polarization and function. Antioxid (Basel) . (2022) 11:464. doi: 10.3390/antiox11030464
22. Takebayashi T, Horii T, Denno H, Nakamachi N, Otomo K, Kitamura S, et al. Human mesenchymal stem cells differentiate to epithelial cells when cultured on thick collagen gel. BioMed Mater Eng . (2013) 23:143–53. doi: 10.3233/BME-120739
23. White SR, Wojcik KR, Gruenert D, Sun S, Dorscheid DR. Airway epithelial cell wound repair mediated by α -Dystroglycan. Am J Respir Cell Mol Biol . (2001) 24:179–86. doi: 10.1165/ajrcmb.24.2.3993
24. Kim HJ, Henke CA, Savik SK, Ingbar DH. Integrin mediation of alveolar epithelial cell migration on fibronectin and type I collagen. Am J Physiol . (1997) 273:L134–41. doi: 10.1152/ajplung.1997.273.1.L134
25. Moore M, Marroquin BA, Gugliotta W, Tse R, White SR. Rho kinase inhibition initiates apoptosis in human airway epithelial cells. Am J Respir Cell Mol Biol . (2004) 30:379–87. doi: 10.1165/rcmb.2003-0019OC
26. Gronthos S, Simmons PJ, Graves SE, Robey PG. Integrin-mediated interactions between human bone marrow stromal precursor cells and the extracellular matrix. Bone . (2001) 28:174–81. doi: 10.1016/S8756-3282(00)00424-5
27. De Kock J, Najar M, Bolleyn J, Al Battah F, Rodrigues RM, Buyl K, et al. Mesoderm-derived stem cells: the link between the transcriptome and their differentiation potential. Stem Cells Dev . (2012) 21:3309–23. doi: 10.1089/scd.2011.0723
28. Gauthaman K, Fong CY, Suganya CA, Subramanian A, Biswas A, Choolani M, et al. Extra-embryonic human Wharton’s jelly stem cells do not induce tumorigenesis, unlike human embryonic stem cells. Reprod BioMed Online . (2012) 24:235–46. doi: 10.1016/j.rbmo.2011.10.007
29. Li X, Bai J, Ji X, Li R, Xuan Y, Wang Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int J Mol Med . (2014) 34:695–704. doi: 10.3892/ijmm.2014.1821
30. Lv F, Lu M, Cheung KM, Leung VY, Zhou G. Intrinsic properties of mesemchymal stem cells from human bone marrow, umbilical cord and umbilical cord blood comparing the different sources of MSC. Curr Stem Cell Res Ther . (2012) 7:389–99. doi: 10.2174/157488812804484611
31. Arutyunyan I, Fatkhudinov T, Kananykhina E, Usman N, Elchaninov A, Makarov A, et al. Role of VEGF-A in angiogenesis promoted by umbilical cord-derived mesenchymal stromal/stem cells: in vitro study. Stem Cell Res Ther . (2016) 7:46. doi: 10.1186/s13287-016-0305-4
32. Sivaraman S, Hedrick J, Ismail S, Slavin C, Rao RR. Generation and characterization of human mesenchymal stem cell-derived smooth muscle cells. Int J Mol Sci . (2021) 22:10335. doi: 10.3390/ijms221910335
33. Hutchings G, Janowicz K, Moncrieff L, Dompe C, Strauss E, Kocherova I, et al. The proliferation and differentiation of adipose-derived stem cells in neovascularization and angiogenesis. Int J Mol Sci . (2020) 21:3790. doi: 10.3390/ijms21113790
34. Legrand C, Gilles C, Zahm JM, Polette M, Buisson AC, Kaplan H, et al. Airway epithelial cell migration dynamics. MMP-9 role in cell-extracellular matrix remodeling. J Cell Biol . (1999) 146:517–29. doi: 10.1083/jcb.146.2.517
35. White SR, Dorscheid DR, Rabe KF, Wojcik KR, Hamann KJ. Role of very late adhesion integrins in mediating repair of human airway epithelial cell monolayers after mechanical injury. Am J Respir Cell Mol Biol . (1999) 20:787–96. doi: 10.1165/ajrcmb.20.4.3318
36. Desai LP, Aryal AM, Ceacareanu B, Hassid A, Waters CM. RhoA and Rac1 are both required for efficient wound closure of airway epithelial cells. Am J Physiol Lung Cell Mol Physiol . (2004) 287:L1134–44. doi: 10.1152/ajplung.00022.2004
37. O’Connor KL, Chen M, Towers LN. Integrin α6β4 cooperates with LPA signaling to stimulate Rac through AKAP-Lbc-mediated RhoA activation. Am J Physiol Cell Physiol . (2012) 302:C605–14. doi: 10.1152/ajpcell.00095.2011
38. Desai LP, Chapman KE, Waters CM. Mechanical stretch decreases migration of alveolar epithelial cells through mechanisms involving Rac1 and Tiam1. Am J Physiol Lung Cell Mol Physiol . (2008) 295:L958–65. doi: 10.1152/ajplung.90218.2008
39. Saradna A, Do DC, Kumar S, Fu QL, Gao P. Macrophage polarization and allergic asthma. Transl Res . (2018) 191:1–14. doi: 10.1016/j.trsl.2017.09.002
40. Zhang L, Wang Y, Wu G, Xiong W, Gu W, Wang CY. Macrophages: friend or foe in idiopathic pulmonary fibrosis? Respir Res . (2018) 19:170. doi: 10.1186/s12931-018-0864-2
41. Gibbings SL, Thomas SM, Atif SM, McCubbrey AL, Desch AN, Danhorn T, et al. Three unique interstitial macrophages in the murine lung at steady state. Am J Respir Cell Mol Biol . (2017) 57:66–76. doi: 10.1165/rcmb.2016-0361OC
42. Arora S, Dev K, Agarwal B, Das P, Syed MA. Macrophages: their role, activation and polarization in pulmonary diseases. Immunobiology . (2018) 223:383–96. doi: 10.1016/j.imbio.2017.11.001
43. Hardy R, Cooper MS. Bone loss in inflammatory disorders. J Endocrinol . (2009) 201:309–20. doi: 10.1677/JOE-08-0568
44. Wu M, Chen L, Qi Y, Ci H, Mou S, Yang J, et al. Human umbilical cord mesenchymal stem cell promotes angiogenesis via integrin β1/ERK1/2/HIF-1α/VEGF-A signaling pathway for off-the-shelf breast tissue engineering. Stem Cell Res Ther . (2022) 13:99. doi: 10.1186/s13287-022-02770-x
45. Plosa EJ, Benjamin JT, Sucre JM, Gulleman PM, Gleaves LA, Han W, et al. β1 integrin regulates adult lung alveolar epithelial cell inflammation. JCI Insight . (2020) 5:e129259. doi: 10.1172/jci.insight.129259
Keywords: cell adhesion, cellular therapy, COPD, macrophage, osteoporosis
Citation: Iwatake M, Nagamura-Inoue T, Doi R, Tanoue Y, Ishii M, Yukawa H, Matsumoto K, Tomoshige K, Nagayasu T and Tsuchiya T (2024) Designer umbilical cord-stem cells induce alveolar wall regeneration in pulmonary disease models. Front. Immunol. 15:1384718. doi: 10.3389/fimmu.2024.1384718
Received: 10 February 2024; Accepted: 02 April 2024; Published: 30 April 2024.
Reviewed by:
Copyright © 2024 Iwatake, Nagamura-Inoue, Doi, Tanoue, Ishii, Yukawa, Matsumoto, Tomoshige, Nagayasu and Tsuchiya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tomoshi Tsuchiya, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Tissue Eng Regen Med
- v.21(3); 2024 Apr
- PMC10987435
Advancements in Human Embryonic Stem Cell Research: Clinical Applications and Ethical Issues
Soo jin park.
1 Department of Obstetrics and Gynecology, Seoul National University Hospital, Seoul, Republic of Korea
Yoon Young Kim
3 Institute of Reproductive Medicine and Population, Medical Research Center, Seoul National University, Seoul, Republic of Korea
Ji Yeon Han
Sung woo kim.
2 Department of Obstetrics and Gynecology, Seoul National University College of Medicine, 101 Daehak-Ro Jongno-Gu, Seoul, 03080 Republic of Korea
Seung-Yup Ku
Associated data.
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Background:
The development and use of human embryonic stem cells (hESCs) in regenerative medicine have been revolutionary, offering significant advancements in treating various diseases. These pluripotent cells, derived from early human embryos, are central to modern biomedical research. However, their application is mired in ethical and regulatory complexities related to the use of human embryos.
This review utilized key databases such as ClinicalTrials.gov, EU Clinical Trials Register, PubMed, and Google Scholar to gather recent clinical trials and studies involving hESCs. The focus was on their clinical application in regenerative medicine, emphasizing clinical trials and research directly involving hESCs.
Preclinical studies and clinical trials in various areas like ophthalmology, neurology, endocrinology, and reproductive medicine have demonstrated the versatility of hESCs in regenerative medicine. These studies underscore the potential of hESCs in treating a wide array of conditions. However, the field faces ethical and regulatory challenges, with significant variations in policies and perspectives across different countries.
Conclusion:
The potential of hESCs in regenerative medicine is immense, offering new avenues for treating previously incurable diseases. However, navigating the ethical, legal, and regulatory landscapes is crucial for the continued advancement and responsible application of hESC research in the medical field. Considering both scientific potential and ethical implications, a balanced approach is essential for successfully integrating hESCs into clinical practice.
Introduction
The field of stem cell research has undergone a significant transformation with the advent of human embryonic stem cells (hESCs). Since their pioneering isolation in 1998, hESCs have been at the forefront of scientific inquiry due to their unique ability for self-renewal and pluripotency [ 1 , 2 ]. This comprehensive review article delves into the advancements, challenges, and ethical considerations surrounding hESCs and their implications for regenerative medicine.
Over the past two decades, the potential of hESCs to revolutionize the treatment of various diseases has been increasingly recognized [ 3 , 4 ]. Their capacity to differentiate into diverse cell types offers promising prospects for repairing or replacing damaged tissues, especially in conditions where current treatments are limited [ 5 – 8 ]. However, the journey of hESC research is not without its complexities. Ethical considerations regarding the use of human embryos have sparked intense debates and have had a profound impact on public perception and the regulatory framework governing hESC research [ 9 , 10 ].
The therapeutic applications of hESCs encompass both systemic and localized approaches, including intravenous or intramuscular injections and surgical implantation, sometimes combined with bioscaffolds [ 11 ]. These strategies are broadly classified into transient dosing for temporary therapeutic effects and permanent implantation for long-term tissue repair and regeneration [ 12 , 13 ]. Despite these advancements, challenges in ensuring consistency in hESC properties across different experimental settings continue to pose hurdles in translating laboratory findings into clinical therapies [ 14 , 15 ].
While induced pluripotent stem cells (iPSCs) have emerged as an alternative, hESCs still hold distinct advantages, particularly in the understanding of genetic diseases and human development [ 16 , 17 ]. Despite the ethical complexities and slower pace of clinical research compared to iPSCs, hESCs remain a crucial tool in biomedical research [ 18 , 19 ]. Their unique position in providing insights into early human development and genetic disorders underscores their invaluable role in medical science [ 17 ].
This review aims to provide an in-depth analysis of the current state of clinical trials involving hESCs, emphasizing their role in regenerative medicine. We explore the evolving landscape of hESC research, highlighting the need for ongoing scientific exploration, ethical deliberation, and regulatory guidance to fully realize the therapeutic potential of hESCs in improving patient care and advancing medical science.
Methodology
This narrative review was conducted to assess the clinical applications of hESCs. The primary aim was to gather and analyze data from various sources to understand the current state and advancements in hESC research.
For database search, we utilized ClinicalTrials.gov ( https://clinicaltrials.gov/ ) and EU Clinical Trials Register ( https://www.clinicaltrialsregister.eu/ ) for identifying ongoing and completed clinical trials involving hESCs. Also, we used PubMed and Google Scholar to retrieve published clinical trial reports and peer-reviewed articles on hESCs. Studies and trials were included based on their focus on the clinical application of hESCs. Those not directly involving hESCs or outside the scope of clinical application were excluded. The review primarily targeted articles and trials published or conducted in the last five years to maintain contemporary relevance.
For data extraction and analysis, key information extracted included the study title, indication, participant number, study site, study period, study design, and NCT number. This data was organized systematically to provide a clear overview of the current trends and progress in the field of hESC research in clinical applications.
Overview of clinical trials in hESC research
Figure 1 displays key aspects of hESC clinical trials included in this review. The first clinical trial registration was in 2002, and the largest number of registered trials were in the United States (19, 40.4%), followed by China (8, 17.0%; Fig. 1 A). By disease category, the largest number of trials were related to ophthalmologic conditions (20, 42.6%), followed by neurologic conditions (10, 21.3%), and clinical studies were mainly conducted on diabetes mellitus (7, 14.9%; Fig. 1 B). Figure 1 C shows the number of trial registrations and the cumulative number of clinical studies by year. There has been a sharp increase since 2012. (Fig. 1 C), and by study design, phase 1 or phase 1/2 designs predominate, accounting for 88% (Fig. 1 D). When looking at studies by a specific disease, dry age-related macular degeneration (AMD) is the most common with 8 (18.2%), followed by type 1 diabetes mellitus (T1DM, 7, 15.9%) and Stargardt Macular Dystrophy (SMD, 5, 11.4%).

Numbers of trials on human embryonic stem cells ( A ) Global Geographical Distribution of Human Embryonic Stem Cell Clinical Trials ( B ) Distribution of Trials by Disease Category ( C ) Frequency of Trials Across Specific Diseases ( D ) Distribution of Clinical Trials Across Different Phases
Disease-specific analysis
Ophthalmologic diseases.
Retinal degeneration is a significant ophthalmologic disease that affects the eye and vision, including dry AMD, SMD, wet AMD, retinitis pigmentosa (RP), diabetic retinopathy, and myopic macular degeneration, among others [ 20 – 22 ]. These conditions often lead to severe vision impairment or blindness. Traditional treatments primarily focus on slowing the progression of these diseases but generally fall short of providing substantial visual improvement. For instance, while laser therapy is beneficial in the early stages, there is no established treatment for late-stage dry AMD [ 23 ]. In cases of wet AMD, therapies such as anti-VEGF can be administered through intravitreal infusion (e.g., ranibizumab, bevacizumab, aflibercept, and brolucizumab), yet this disease requires continuous treatment and monitoring due to its chronic nature [ 24 – 27 ]. Stem cell therapy, particularly involving retinal pigment epithelium (RPE) degeneration, has emerged as a promising approach in eye diseases [ 28 ]. The RPE is vital for maintaining photoreceptor health and is tasked with recycling photopigments and clearing shed photoreceptor segments [ 29 ]. hESCs have shown significant potential in rescuing photoreceptors and enhancing vision in preclinical macular degeneration models [ 30 ]. One of the initial forays into stem cell therapy using hESCs was directed at treating dry AMD using hESC-derived RPE. Several key factors contributed to this early focus on retinal conditions. Primarily, the unique immune privilege of the eye, reinforced by the blood-ocular barrier, significantly lowers the risk of rejection of transplanted cells—a crucial aspect in the success of any stem cell-based therapy [ 31 , 32 ]. Moreover, the eye's transparency permits the non-invasive tracking of the introduced cells through methods like optical coherence tomography or microperimetry, enabling continuous monitoring and evaluation of the therapy's effectiveness [ 33 ]. The eye's distinct and isolated structure also minimizes the spread of these cells to other body parts, thereby reducing the likelihood of unintended systemic effects [ 34 ]. Furthermore, the absence of synaptic layers in retinal cells aids in their smoother integration [ 29 ]. Lastly, the irreversible progression of many retinal disorders and the absence of adequate existing treatments have necessitated the development of innovative therapeutic strategies, thereby placing retinal ailments at the forefront of hESC research and application.
Dry AMD, a prevalent and progressive ophthalmologic disease affecting elderly patients, is characterized by the degeneration of the RPE layer and impairment of central vision [ 21 ]. The pivotal role of RPE in the pathophysiology of dry AMD makes it a prime target for therapeutic interventions. The potential of stem cells, especially hESCs, in this context, lies in their ability to differentiate into RPE cells, thereby offering the possibility of replacing damaged or degenerated RPE with healthy, functional cells. Preclinical studies in animal models and in vitro experiments have provided substantial evidence supporting the role of stem cells, including hESCs, in treating dry AMD [ 35 – 37 ].
For example, in Yucatan minipigs, a preclinical study assessed CPCB-RPE1, a hESC-derived retinal pigment epithelium monolayer [ 35 ]. The study successfully placed CPCB-RPE1 implants in the subretinal space without breakage, and histological analysis confirmed the survival of hESC-RPE cells as an intact monolayer for one month [ 35 ]. Another study used differentiated hESC-RPE replacement therapy on albino rabbit eyes induced with NaIO3, employing a 25-gauge transvitreal pars plana vitrectomy (PPV) technique [ 36 ]. Xeno-free hESC-RPE monolayer on a polyester substrate survived and retained functionality for up to four weeks with short-term immunosuppression in a rabbit dry AMD model [ 37 ]. These studies demonstrate the feasibility of generating RPE cells from stem cells and their potential to integrate into the retina, potentially restoring RPE function and rescuing photoreceptors. Also, the critical advantage of hESC-RPE is their reduced risk of uncontrolled proliferation, as they are fully differentiated.
Clinical trials have been conducted to test the safety and feasibility of hESC-derived RPE for dry AMD, as outlined in Table 1 . Dry AMD has been the subject of the most significant number of clinical trials, with studies dating back to 2011 (Table 1 ). The first study involved MA09-hRPE ( {"type":"clinical-trial","attrs":{"text":"NCT01344993","term_id":"NCT01344993"}} NCT01344993 ; {"type":"clinical-trial","attrs":{"text":"NCT01674829","term_id":"NCT01674829"}} NCT01674829 ; {"type":"clinical-trial","attrs":{"text":"NCT02122159","term_id":"NCT02122159"}} NCT02122159 ), derived from the MA09 hESC line, a xenograft product with ex vivo exposure to mouse embryonic cells [ 38 ]. Produced by isolating RPE patches when embryoid body formation was confirmed, this treatment was tested in three different dose cohorts (50,000, 100,000, and 150,000 cells) for patients with dry AMD and SMD [ 39 ]. Encouragingly, the study revealed no signs of adverse events like cell proliferation or immune rejection. In addition, the best-corrected visual acuity improved in 10 eyes, and measures related to vision-related quality of life showed enhancements [ 39 ]. In a clinical trial of MA09-hESC-derived RPE cells conducted with an Asian population, which included four participants, there was no evidence of adverse proliferation or tumorigenesis [ 40 ]. Furthermore, one patient experienced improved visual acuity, while the remaining three maintained stable visual acuity throughout the trial [ 40 ]. In the USA, a phase 1/2 clinical study was conducted using CPCB-RPE1, a composite implant consisting of a synthetic parylene substrate and a polarized monolayer of adherent hESC-RPE cells ( {"type":"clinical-trial","attrs":{"text":"NCT02590692","term_id":"NCT02590692"}} NCT02590692 ). This study demonstrated safety and tolerability in legally blind patients with dry AMD [ 41 , 42 ]. However, graft survival remains a significant challenge, influenced by factors like aging of Bruch's membrane, subretinal scarring, para-inflammation, and choroid ischemia [ 33 ].
Table 1
Registered trials of human embryonic stem cells for ophthalmologic disease
AMD: Age-Related Macular Degeneration; ESC: Embryonic Stem Cell; hESC-RPE: Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium; NCT Number: National Clinical Trial Number; RP: Retinitis Pigmentosa; RPE: Retinal Pigment Epithelium; SMD: Stargardt's Macular Dystrophy
SMD, a prevalent retinal dystrophy affecting young individuals, is characterized by progressive vision loss, primarily caused by mutations in the ABCA4 gene, which leads to dysfunction of the ABCR protein expressed in retinal photoreceptors [ 43 ]. Currently, there are no established treatments to effectively improve vision in SMD, similar to the situation in dry AMD. Promising outcomes have been observed in preclinical models, including the safe subretinal injection of retinal pigment epithelium (RPE) derived from hESC. This approach was tested in a phase 1 clinical trial in the USA ( {"type":"clinical-trial","attrs":{"text":"NCT02941991","term_id":"NCT02941991"}} NCT02941991 ). The WA-099 hESC line demonstrated the ability to spontaneously differentiate into RPE cells, with subsequent isolation of pigmentation cells. A suspension of these hESC-derived RPE cells, containing 1.0 × 10^6 cells in 0.1 mL, was surgically implanted subretinally in all eyes using a pars plana vitrectomy (PPV) approach [ 44 ]. The study's findings indicated no adverse events during the one-year postoperative follow-up period. Additionally, the treated eyes had no significant improvement in visual acuity [ 44 ]. In China, researchers Li et al. evaluated the Q-CTS-hESC-2 cell line-derived RPE in a 5-year follow-up study on seven patients and reported no significant adverse reactions and some temporary improvements in visual function, though two patients showed a long-term decrease in vision ( {"type":"clinical-trial","attrs":{"text":"NCT02749734","term_id":"NCT02749734"}} NCT02749734 ) [ 45 ]. Sung et al., from the Republic of Korea, reported a 3-year study on Asian patients, also finding no serious adverse events and reporting stable or improved BCVA in some patients ( {"type":"clinical-trial","attrs":{"text":"NCT01625559","term_id":"NCT01625559"}} NCT01625559 ) [ 46 ].
RP is a group of inherited retinal disorders characterized by the progression of vision loss due to photoreceptor degeneration, affecting approximately 1 in 4,000 individuals worldwide [ 47 , 48 ]. A Phase 1/2 clinical trial of RP with monogenic mutations is ongoing ( {"type":"clinical-trial","attrs":{"text":"NCT03963154","term_id":"NCT03963154"}} NCT03963154 ), with interim analysis showing no adverse events in seven patients [ 49 ]. While these studies confirm the long-term safety and tolerability of hESC-RPE cell transplantation, they also highlight the need for further research to improve efficacy, including better patient selection and treatment methodologies, as significant and consistent improvements in visual function are yet to be established.
Neurologic diseases
The utilization of stem cell therapy derived from hESCs in treating neurological disorders is an emerging and promising area of research. As illustrated in Fig. 1 B, neurologic diseases are among the most researched applications in this field. This branch of medical science addresses a diverse spectrum of neurological conditions, including Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), spinal cord injuries (SCI), and multiple sclerosis. These disorders present considerable treatment challenges, largely due to the complexity of the nervous system and the typically permanent nature of neuronal damage involved. Ongoing studies are displayed in Table 2 .
Table 2
Registered trials of human embryonic stem cells for neurologic disease
ALS: Amyotrophic Lateral Sclerosis; ESC: Embryonic Stem Cell; hESC-NPC: Human Embryonic Stem Cell-Derived Neural Precursor Cells; NCT Number: National Clinical Trial Number; PSA-NCAM( +): Polysialylated Neural Cell Adhesion Molecule Positive Neural Precursor Cells; SCI: Spinal Cord Injury
The first-in-patient clinical trial on neurologic disease was conducted on SCI patients [ 50 ]. Oligodendrocyte progenitor cells (LCTOPC1), which are also nomenclature as AST-OPC1 or GRNOPC1, is the world's first hESC-derived therapy, and the phase 1 trial was approved by US-FDA in 2009, and the first patient was enrolled in 2011 ( {"type":"clinical-trial","attrs":{"text":"NCT01217008","term_id":"NCT01217008"}} NCT01217008 ) [ 50 , 51 ]. Recent 10-year follow-up study results on five participants who received intraparenchymal injections of LCTOPC1 showed no serious adverse effects during follow-up, with 80% of patients showing MRI evidence of tissue matrix formation at the injury site [ 51 ]. This pivotal study, leading to a subsequent cervical dose escalation trial ( {"type":"clinical-trial","attrs":{"text":"NCT02302157","term_id":"NCT02302157"}} NCT02302157 ), demonstrated the safety of hESC-derived therapies using LCTOPC1. In the trial, 25 participants with C4-7 spinal injuries received a single dose of 2, 10, or 20 million LCTOPC1 cells and low-dose tacrolimus for 60 days [ 52 ]. Despite some adverse events, including 29 serious ones, the treatment was well tolerated, with MRI scans showing no significant complications, and at a 1-year follow-up, 96% of participants improved by at least one level of neurological function, and 32% improved by two or more levels [ 52 ].
Additionally, research has shown that neural precursor cells marked by polysialic acid-neural cell adhesion molecule (PSA-NCAM), derived from hESC, can enhance neural tissue integrity in a rat stroke model [ 53 ]. Building on these findings, a phase 1/2a clinical trial ( {"type":"clinical-trial","attrs":{"text":"NCT04812431","term_id":"NCT04812431"}} NCT04812431 ) is currently underway to assess the safety and efficacy of PSA-NCAM( +)-NPC for patients with sub-acute C4-C7 level spinal cord injuries. In this trial, the cells will be delivered intrathecally across five sites, and participants will be monitored for one year and five months as part of a follow-up study.
PD is a neurodegenerative disease characterized primarily by the loss of dopaminergic neurons in the substantia nigra, a region of the brain integral to controlling body movement. This loss leads to the classic symptoms of PD, including tremors, rigidity, bradykinesia, and postural instability [ 54 ]. The potential of hESC-based therapies in PD lies in their ability to differentiate into dopaminergic neurons, the type of cell lost in the disease [ 55 ]. The goal of transplanting hESC-derived cells in PD treatment is to replace the depleted neurons and normalize dopamine levels in the brain, which could help alleviate PD symptoms. MSK-DA01, a midbrain dopamine neuron cell derived from hESCs, is currently undergoing a Phase 1 trial in the United States ( {"type":"clinical-trial","attrs":{"text":"NCT04802733","term_id":"NCT04802733"}} NCT04802733 ). A preclinical study on MSK-DA01 demonstrated successful graft survival and improved behavior in rats with 6-hydroxydopamine-induced lesions, a model for PD. Importantly, these studies revealed no adverse effects related to the graft cells and no unexpected cell proliferation outside the brain, indicating a promising safety profile for this innovative therapy [ 56 ].
STEM-PD, another product consisting of dopaminergic neuronal progenitor cells derived from hESCs, has also been evaluated in a preclinical study [ 57 ]. This study showed the precise stereotactic injection of STEM-PD into a pig model and demonstrated effective innervation of the targeted brain regions. Additionally, this intervention led to a reversal of motor deficits in the pig model of Parkinson's disease, demonstrating the potential efficacy of STEM-PD in addressing the symptoms associated with this neurodegenerative disorder [ 57 ]. Presently, STEM-PD is the subject of a phase 1 clinical trial in the United Kingdom, which is in the process of recruiting eight patients, and this trial marks a significant step in evaluating the safety and potential efficacy of STEM-PD in human subjects, specifically targeting the treatment of PD ( {"type":"clinical-trial","attrs":{"text":"NCT05635409","term_id":"NCT05635409"}} NCT05635409 ).
A research team in China successfully derived dopaminergic neurons from hESCs and demonstrated sustained behavioral improvements over two years in a monkey model of PD [ 58 ]. This significant advancement in stem cell research has led to the registration of a Phase 1 clinical trial ( {"type":"clinical-trial","attrs":{"text":"NCT03119636","term_id":"NCT03119636"}} NCT03119636 ). However, the current status of this trial remains unknown.
ALS, a severe neurodegenerative condition, is characterized by the deterioration of both upper and lower motor neurons (MNs), resulting in the progressive paralysis of muscles controlled by these neurons [ 59 ]. While FDA-approved treatments like riluzole have demonstrated some efficacy in prolonging survival, there remains a significant unmet need for more effective ALS therapies [ 60 ]. Recent evidence points to the involvement of astrocytes in the pathogenesis of ALS [ 61 ]. AstroRx®, a novel cell therapy derived from hESCs, has shown promise in addressing this gap, as evidenced by the outcomes of its recent Phase 1/2a clinical trial [ 62 ]. AstroRx®, administered as a single intrathecal injection, was tested in two cohorts of ALS patients—a low-dose and a high-dose group, each consisting of five patients ( {"type":"clinical-trial","attrs":{"text":"NCT03482050","term_id":"NCT03482050"}} NCT03482050 ). The administration of AstroRx® showed a clinically significant impact lasting for three months post-treatment, with particularly notable effects observed in a group of rapid progressors [ 62 ].
NR1, an hESC-derived neural stem cell, is under investigation for chronic ischemic stroke patients who are 6–60 months post-ischemic subcortical mid-cerebral artery stroke ( {"type":"clinical-trial","attrs":{"text":"NCT04631406","term_id":"NCT04631406"}} NCT04631406 ). Six patients underwent transplantation with NR1, and there was a notable improvement in the Mugl-Meyer motor score. Additionally, all six patients exhibited a transient flair signal that resolved within two months, which correlated with neurological recovery [ 63 ].
Diabetes mellitus
Type 1 Diabetes Mellitus (T1DM) commonly manifests in childhood and adolescence and is marked by a chronic autoimmune condition leading to the loss of insulin-producing beta cells in the pancreas [ 64 ]. Unlike Type 2 DM, which often relates to lifestyle and insulin resistance, T1DM is primarily driven by an autoimmune response [ 64 ]. In stem cell therapy for T1DM, two main strategies have emerged: one involves replacing the missing insulin-producing beta cells, while the other focuses on immunomodulation to safeguard existing beta cells from further autoimmune destruction [ 65 ]. Seven registered clinical trials for stem cell-based treatment of T1DM using hESC are summarized in Table 3 .
Table 3
Registered trials of human embryonic stem cells for diabetes mellitus
ESC: Embryonic Stem Cell; FIH: First-In-Human; NCT Number: National Clinical Trial Number; T1DM: Type 1 Diabetes Mellitus
Schulz and colleagues described the creation of the VC-01 composite product utilizing pancreatic endoderm cells (PEC-01) obtained from CyT49 hESCs with a retrievable semi-permeable encapsulating device drug delivery system [ 66 ]. VC-02, developed in 2017, is an advanced model featuring multiple large pores across the membrane to facilitate vascularization while maintaining immune isolation [ 67 ]. VC-01 was investigated in phase 1/2 trial ( {"type":"clinical-trial","attrs":{"text":"NCT02239354","term_id":"NCT02239354"}} NCT02239354 ; {"type":"clinical-trial","attrs":{"text":"NCT04678557","term_id":"NCT04678557"}} NCT04678557 ; {"type":"clinical-trial","attrs":{"text":"NCT02939118","term_id":"NCT02939118"}} NCT02939118 ) and VC-02 was investigated in phase 1/2 trial ( {"type":"clinical-trial","attrs":{"text":"NCT03163511","term_id":"NCT03163511"}} NCT03163511 ). In the phase 1/2 study of the VC-01 product, immunosuppressants were not administered, leading to a host reaction against the implant, ultimately resulting in its destruction, and the study was terminated [ 68 ]. A Phase 1/2 study involving 17 patients with T1DM was carried out following a modification in the VC-02 device. This study demonstrated successful engraftment and insulin release in 63% of the cases, and as early as six months post-implantation, 35.3% of the participants showed positive C-peptide levels. These results indicate the potential of VC-02 as a viable alternative for T1DM treatment. However, it's important to note that some reported adverse events were primarily related to the surgical procedures of implanting or explanting the device and the side effects of immunosuppression therapy [ 69 ]. VCTX210A represents an innovative approach that uses pancreatic endodermal cells (PEC210A) derived from hESC. These cells have been genetically modified using the CRISPR/Cas9 (Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated protein 9) technology. This modification enhances the cells' survival against the patient's immune system, thereby addressing the challenge of graft versus host disease [ 70 ]. Additionally, VX880, a fully differentiated pancreatic islet cell product derived from hESC designed to treat T1DM, is undergoing clinical investigation ( {"type":"clinical-trial","attrs":{"text":"NCT04786262","term_id":"NCT04786262"}} NCT04786262 ). Interim data analysis from this study has yielded positive results, indicating that the treatment successfully restored insulin production in the first two patients enrolled in the trial [ 71 ].
Female reproductive organ and genitourinary disease
The field of female reproductive organ disorders is increasingly looking towards stem cell therapy and cutting-edge biomedical technologies for potential treatments, as shown in Table 4 . Intravenous injection of hESC-derived mesenchymal cells (hESC-MCs) showed restoration of ovarian function induced by the chemotherapeutic agent in a murine model [ 72 , 73 ]. A product, hESC-MC, has been explored by a Chinese research group for treating moderate to severe intrauterine adhesion ( {"type":"clinical-trial","attrs":{"text":"NCT04232592","term_id":"NCT04232592"}} NCT04232592 ). Additionally, a therapy involving hESC-MC product is currently being investigated as a potential treatment for primary ovarian insufficiency ( {"type":"clinical-trial","attrs":{"text":"NCT03877471","term_id":"NCT03877471"}} NCT03877471 ). Additionally, Table 5 showcases the application of hESC-derived mesenchymal stem cell therapy, specifically MR-MVC-01, which is currently under investigation for treating interstitial cystitis, as per the clinical trial registered under {"type":"clinical-trial","attrs":{"text":"NCT04610359","term_id":"NCT04610359"}} NCT04610359 .
Table 4
Registered trials of human embryonic stem cells for female reproductive organ
BAP-EB: Blastocyst Attachment to a Prepared Endometrium—Embryonic Bodies; ESC: Embryonic Stem Cell; hESC-MC: Human Embryonic Stem Cell-Derived Mesenchymal Cells; hESC-MSC: Human Embryonic Stem Cell-Derived Mesenchymal Stem Cells; IVF: In Vitro Fertilization; MSCs: Mesenchymal Stem Cells; NCT Number: National Clinical Trial Number; NA: Not Applicable
Table 5
Registered trials of human embryonic stem cells for cardiac, urological disease and miscellaneous topics
ESC: Embryonic Stem Cell; hESC: Human Embryonic Stem Cell; hESC-cardiomyocyte: Human Embryonic Stem Cell-Derived Cardiomyocytes; hESC-derived-CD15 + Isl-1 + progenitors: Human Embryonic Stem Cell-Derived CD15 + Isl-1 + Progenitor Cells; hESC-MSC: Human Embryonic Stem Cell-Derived Mesenchymal Stem Cells; MSC: Mesenchymal Stem Cell; NCT Number: National Clinical Trial Number; PGD: Preimplantation Genetic Diagnosis
Cardiovascular disease
In the field of heart failure treatment, the innovative application of human embryonic stem cells (hESCs) offers a promising alternative to conventional therapies. Table Table5 5 also highlights hESC-derived cardiac progenitor cell-based products in treating heart failure and ischemic heart disease, as illustrated in the clinical trials registered under {"type":"clinical-trial","attrs":{"text":"NCT02057900","term_id":"NCT02057900"}} NCT02057900 and {"type":"clinical-trial","attrs":{"text":"NCT05068674","term_id":"NCT05068674"}} NCT05068674 . The ESCORT trial ( {"type":"clinical-trial","attrs":{"text":"NCT02057900","term_id":"NCT02057900"}} NCT02057900 ), conducted in France, marked a pioneering venture in employing hESC-derived cardiomyocytes for heart failure treatment, setting a precedent that has been followed by the HECTOR trial ( {"type":"clinical-trial","attrs":{"text":"NCT05068674","term_id":"NCT05068674"}} NCT05068674 ) in the United States, initiated in 2022. The ESCORT trial, focusing on patients with severe ischemic left ventricular dysfunction, demonstrated the feasibility and safety of using hESC-derived cardiovascular progenitor cells, embedded in a fibrin patch, applied to the damaged heart areas during coronary artery bypass surgery [ 74 ]. The results, including the production of a highly purified batch of progenitor cells and significant symptomatic improvements in patients, though with instances of silent alloimmunization, have laid the groundwork for future explorations in this domain. The HECTOR trial in the U.S. is building upon this foundation with a novel approach, utilizing hESC-derived cardiomyocytes (hESC-CMs) to enhance survival and cardiac function in patients with chronic left ventricular dysfunction secondary to myocardial infarction. This phase I dose-escalation pilot study is designed as an initial safety assessment to determine the maximum tolerated dose (MTD) before proceeding to a phase II randomized, double-blinded, placebo-controlled study. Approximately eighteen patients who are scheduled for cardiac catheterization and meet all inclusion/exclusion criteria will participate in this initial phase. The HECTOR trial represents a significant step forward in the application of hESC-CMs in cardiac therapy, with great anticipation for its potential to revolutionize the treatment of heart failure and related conditions.
Challenges and ethical considerations
As we explore the burgeoning field of hESC research and its clinical applications, it becomes crucial to examine the accompanying ethical and practical challenges thoroughly. While this area of research offers groundbreaking possibilities in treating various diseases, it is intertwined with complex ethical, legal, and social issues, particularly due to the involvement of human embryos.
Derivation of hESC
In the field of hESC research, the ethical implications surrounding the derivation of these cells from embryos are paramount. hESCs are typically harvested from embryos at the blastocyst stage approximately 5–6 days post-fertilization. This stage of development is critical because it leads to the inevitable destruction of the embryo, a primary ethical concern in this field of research [ 19 , 75 – 77 ].
Due to their pluripotency, the significant potential of hESCs makes them a valuable asset in understanding disease mechanisms, drug testing, and potential regenerative therapies [ 78 ]. Moreover, hESCs are obtained early in induced pluripotent development, making them crucial for studying human developmental processes and various diseases [ 17 ]. They play a vital role, especially when embryos are discarded after positive preimplantation genetic testing (PGT) results, contributing to our understanding of genetic abnormalities and disease ecology [ 17 ].
Regarding the moral status of the embryo, there are varying views. The Catholic perspective often sees life beginning at fertilization, while Judaism and Islam view the blastocyst as having the potential for life but not as fully alive [ 79 , 80 ]. Hinduism and Buddhism do not provide a clear doctrinal definition of life's beginning, adopting a more philosophical and spiritual perspective [ 81 ].
The use of surplus IVF embryos in hESC research is often defended under the principle of proportionality. This approach favors using them for stem cell research due to the broader potential benefits compared to enhancing IVF techniques [ 17 ]. The utilization of embryos with monogenic defects (PGT-M) or aneuploidies (PGT-A) for deriving disease-specific stem cells is seen as a promising avenue for advancing the understanding of specific diseases and developing targeted treatments [ 9 , 17 ].
In summary, hESC research presents a complex ethical landscape. The scientific and medical benefits of hESCs must be balanced against the moral considerations surrounding the use of human embryos, necessitating a nuanced approach to this rapidly evolving field.
Regulatory issues
In the realm of research involving hESCs, regulatory issues play a crucial role, varying significantly across different countries. Obtaining approval from institutional review boards (IRBs) and adhering to regulations set by authoritative bodies are pivotal steps in developing and progressing hESC-related research and development.
Procedures involving the transfer of stem cells are subject to specific regulations. This encompasses the process of transferring stem cell materials, which requires careful adherence to legal and ethical guidelines [ 15 , 82 ]. It's essential to ensure that the transfer agreements are comprehensive, detailing any restrictions and obligations related to using the materials and associated data [ 83 , 84 ]. Such transfers must respect donor rights and comply with the regulatory frameworks of both the donating and receiving entities.
The process of creating stem cell products that are safe for clinical use involves several critical steps. This includes extensive testing for genetic stability and absence of contaminants, ensuring the cells' identity and functionality, and verifying that they meet the stringent safety standards required for clinical application [ 82 ]. These procedures are designed to safeguard patient safety and ensure the efficacy of the stem cell products.
Overall, the development and research involving hESCs must navigate a complex landscape of regulatory requirements. These regulations are in place to ensure the ethical use of human stem cells, the protection of donor rights, and the safety and efficacy of stem cell-based therapies. Compliance with these regulations is not only a legal requirement but also a cornerstone in maintaining the integrity and credibility of stem cell research.
The exploration of hESCs over the past two decades has opened new frontiers in medical science, particularly in the fields of regenerative medicine and cell-based therapies. The landmark discovery and subsequent developments have brought immense potential for understanding and treating a wide range of diseases, from genetic disorders to degenerative conditions.
However, the journey of hESC research is intertwined with a plethora of ethical, legal, and regulatory challenges. The ethical considerations, primarily regarding the use of human embryos, highlight the delicate balance between scientific advancement and moral imperatives. Different religious and cultural perspectives on embryo status underline this debate's complexity. As we have seen, approaches to this issue vary significantly worldwide, influencing the regulatory landscape and research in different countries.
The advancements in hESC research also underscore the importance of robust regulatory frameworks and adherence to ethical standards. From acquiring embryonic materials to developing stem cell-based products for clinical use, each step requires careful consideration of ethical guidelines, safety standards, and regulatory compliance. The involvement of IRBs and adherence to international standards and guidelines are critical in ensuring that the research is conducted responsibly and with the utmost respect for human life and dignity.
Looking ahead, the field of hESC research holds immense promise. With continued technological advancements and a deeper understanding of stem cells' capabilities, we stand on the brink of revolutionary medical breakthroughs. However, the path forward must be navigated with a commitment to ethical principles, regulatory compliance, and public engagement. By upholding these standards, the scientific community can ensure that the benefits of hESC research are realized in a manner that respects human values and contributes positively to human health and well-being.
In conclusion, hESC research represents scientific innovation, ethical reflection, and regulatory prudence. As we continue to advance in this field, it is imperative to maintain a balanced approach that fosters scientific discovery while honoring ethical obligations and regulatory requirements. The future of hESC research, promising as it is, depends on our collective ability to navigate these complex and multifaceted challenges.
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant Number: HI22C1424) and the Grants of the Ministry of ICT Grants and the Ministry of Education, Republic of Korea (2020R1A2C1010293).
Authors' contributions
SJP: conceptualization, methodology, formal analysis, resources, data curation, investigations, visualization, Writing—Original Draft, Visualization, project administration, funding acquisition. YYK: methodology, validation, Writing—Review & Editing, Supervision. JYH: methodology, investigation, validation, supervision. SWK: methodology, investigation, validation, supervision. HK: methodology, investigation, validation, supervision. S-YK: conceptualization, methodology, project administration, funding acquisition.
Open Access funding enabled and organized by Seoul National University.
Data availability statement
Declarations.
The authors declare nothing to disclose.
There are no animal experiments carried out for this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 26 April 2024
Induced pluripotent stem cells (iPSCs): molecular mechanisms of induction and applications
- Jonas Cerneckis 1 , 2 ,
- Hongxia Cai 1 &
- Yanhong Shi ORCID: orcid.org/0000-0002-3938-5839 1 , 2
Signal Transduction and Targeted Therapy volume 9 , Article number: 112 ( 2024 ) Cite this article
209 Accesses
6 Altmetric
Metrics details
- Pluripotent stem cells
- Reprogramming
The induced pluripotent stem cell (iPSC) technology has transformed in vitro research and holds great promise to advance regenerative medicine. iPSCs have the capacity for an almost unlimited expansion, are amenable to genetic engineering, and can be differentiated into most somatic cell types. iPSCs have been widely applied to model human development and diseases, perform drug screening, and develop cell therapies. In this review, we outline key developments in the iPSC field and highlight the immense versatility of the iPSC technology for in vitro modeling and therapeutic applications. We begin by discussing the pivotal discoveries that revealed the potential of a somatic cell nucleus for reprogramming and led to successful generation of iPSCs. We consider the molecular mechanisms and dynamics of somatic cell reprogramming as well as the numerous methods available to induce pluripotency. Subsequently, we discuss various iPSC-based cellular models, from mono-cultures of a single cell type to complex three-dimensional organoids, and how these models can be applied to elucidate the mechanisms of human development and diseases. We use examples of neurological disorders, coronavirus disease 2019 (COVID-19), and cancer to highlight the diversity of disease-specific phenotypes that can be modeled using iPSC-derived cells. We also consider how iPSC-derived cellular models can be used in high-throughput drug screening and drug toxicity studies. Finally, we discuss the process of developing autologous and allogeneic iPSC-based cell therapies and their potential to alleviate human diseases.
Similar content being viewed by others
Small-molecule-mediated reprogramming: a silver lining for regenerative medicine
Direct cell reprogramming: approaches, mechanisms and progress
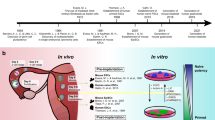
A new era of stem cell and developmental biology: from blastoids to synthetic embryos and beyond
Introduction.
The development of induced pluripotent stem cell (iPSC) technology has opened vast opportunities for in vitro modeling of human biology and for cell therapy applications. 1 , 2 , 3 , 4 , 5 Since the first reports of somatic cell reprogramming into mouse and human iPSCs in 2006 and 2007, respectively, iPSCs have been applied to model human development and diseases in vitro, screen drug candidates, and create cell therapies. 1 , 2 , 3 , 4 , 5 Increasing understanding of the mechanisms that govern iPSC induction has shed light on cell fate decisions, accelerating the development of efficient iPSC derivation methods and protocols for iPSC differentiation into somatic cells. 6 Modeling human biology with iPSCs and iPSC-derived cells is particularly attractive, given the human origin of iPSCs and the ability to derive patient-specific iPSCs with a disease-relevant genetic background. 2 Indeed, iPSC-based cellular models may reveal human-specific phenotypes and molecular mechanisms that do not necessarily manifest in animal models. 7 , 8 , 9 Furthermore, ever increasing complexity of iPSC-based cellular models has resulted in the development of sophisticated human-like tissues, such as organoids, that contain multiple cell types, exhibit primitive human tissue-like architecture and enable modeling of higher order cell-cell interactions. 10 Various iPSC-derived cellular models can be applied to probe disease mechanisms, evaluate drug activity and toxicity, and develop next-generation cell therapies. Given that iPSCs can be genetically modified and differentiated into otherwise inaccessible cell types, autologous and allogeneic cell therapies are being actively developed using the iPSC technology and hold a great promise to provide new approaches for treating complex diseases. 11
In this review, we begin by outlining the historical development of the iPSC technology, including the key discoveries that led to the breakthrough of somatic cell reprogramming to iPSCs in 2006 and 2007. 3 , 4 , 5 Subsequently, we summarize the key molecular and cellular events governing iPSC induction as well as the methods for somatic cell reprogramming to iPSCs. We then discuss the versatile applications of iPSCs, including in vitro modeling of human development and diseases, drug discovery, and cell therapy applications.
Historical overview of somatic cell reprogramming to iPSCs
Today, it is well established that most somatic cells harbor complete genetic information required for the development of an entire organism, whereas phenotypic diversity is achieved by epigenetic mechanisms that define gene expression potential in each cell. 12 , 13 , 14 However, prior to such modern understanding of animal development, various hypotheses to explain how immense physiological complexity of an adult animal could emerge were contemplated. Popular in the 17th and 18th centuries, a theory of preformationism posited that animals would grow from miniature versions of themselves; the imagined homunculi were microscopic preformed human beings that would simply grow into their adult versions. 15 As pioneering work in embryology accumulated and microscopy power improved, preformationism was gradually replaced by the theory of epigenesis, postulating sequential cell differentiation and organ development from an egg. 16 , 17 Yet, it remained unclear how an egg cell could give rise to the breathtaking phenotypic diversity of somatic cells.
In 1892, the German evolutionary biologist August Weismann (1834–1914) proposed the germ plasm theory, also known as the Weismann barrier, postulating that germ cells alone were used to transmit heritable information, whereas acquisition of somatic cell fate involved irreversible modification of heritable information, enabling phenotypic diversity to emerge. 18 The idea of irreversible restriction of a differentiated somatic cell state during development was reiterated by the British developmental biologist Conrad Waddington (1905–1975) in 1957. 19 Waddington proposed a model that would become known as the Waddington’s epigenetic landscape, suggesting that cell differentiation resembled a ball rolling downhill towards a more and more restricted and irreversible state. 19 However, it remained elusive whether somatic cell differentiation truly required irreversible mutational events to occur or whether it could be achieved by some other means, such as by reversible epigenetic mechanisms. 14 A year later, the American geneticist David Nanney (1925–2016) proposed that while the DNA sequence conferred gene expression potential, phenotypic differences in cells sharing the same genome could arise because of gene expression “specificities” regulated by epigenetic systems. 20 Indeed, the reversibility of the mechanisms governing somatic cell specification was demonstrated by the British developmental biologist John Gurdon (b. 1933), who performed somatic cell nuclear transfer (SCNT) experiments (Fig. 1a, b ). 21 , 22 , 23 , 24 , 25 In 1962, using a model of the Xenopus laevis frog, Gurdon demonstrated that a nucleus isolated from a terminally differentiated somatic cell and transplanted into an enucleated egg harbored all the genetic information required to give rise to germline-competent organisms. 21 , 22 , 23 , 24 Therefore, the SCNT experiments revealed that genetic information was preserved during differentiation, whereas phenotypic diversity of somatic cells was likely achieved by reversible epigenetic mechanisms. What kind of epigenetic mechanisms could enable such elaborate yet reversible phenotypic diversity? Among the many layers of epigenetic regulation known today, DNA methylation is a prominent example of stable, yet reversible epigenetic memory acquired along the course of cell fate specification. 26 , 27 , 28 , 29 For a historical review of discovering DNA methylation as a central mechanism of gene expression regulation and maintenance over mitotic divisions, the readers are referred to Tompkins, 2022. 14
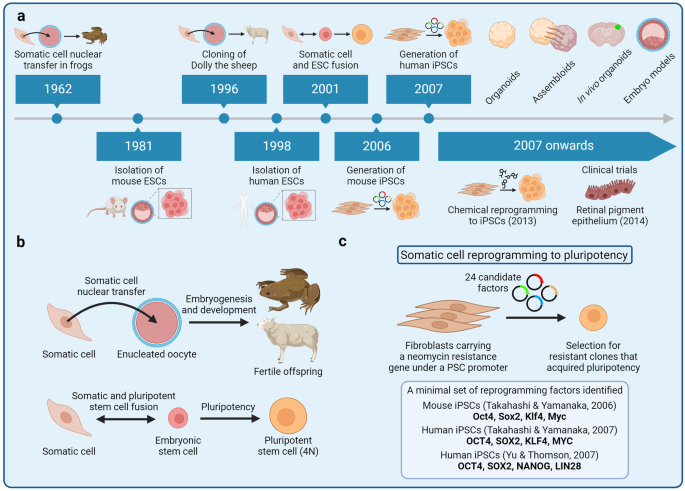
Development of the induced pluripotent stem cell (iPSC) technology. a A timeline of key breakthroughs related to the iPSC technology. b (Top) Somatic cell nuclear transfer (SCNT) experiments were pioneered by John Gurdon in the African clawed frog. Gurdon demonstrated that somatic cells retained all the genetic information necessary to give rise to a germline-competent organism. Successful SCNT in mammals was demonstrated by Keith Campbell, Ian Wilmut, and colleagues who cloned Dolly the sheep. (Bottom) Masako Tada and colleagues demonstrated that pluripotency can also be achieved by fusing a somatic cell with an embryonic stem cell, leading to the formation of a hybrid tetraploid cell. 4N, tetraploid. c The groundbreaking experiments of fibroblast reprogramming to pluripotency were pioneered by Kazutoshi Takahashi and Shinya Yamanaka. The researchers selected 24 factors as candidates for reprogramming and delivered these factors into mouse fibroblasts in various combinations by retroviral transduction. Eventually, Takahashi and Yamanaka identified a combination of 4 reprogramming factors—Oct4, Sox2, Klf4, and Myc—that was sufficient to reprogram mouse fibroblasts into embryonic stem cell-like pluripotent cells, known as iPSCs. Subsequently, Yamanaka and James Thomson independently reprogrammed human fibroblasts into iPSCs in 2007
In 1981, British biologists Martin Evans (b. 1941) and Matthew Kaufman (1942–2013) as well as the American biologist Gail Martin (b. 1944) isolated mouse embryonic stem cells (ESCs) that would serve as a reference point for subsequent somatic cell reprogramming experiments. 30 , 31 Human ESCs were isolated by the American developmental biologist James Thomson (b. 1958) and colleagues in 1998. 32 Cell fusion experiments of mouse 33 and human 34 ESCs with somatic cells revealed the capacity of the resulting heterokaryon for reprogramming to pluripotency, thus reaffirming the notion of cellular plasticity and somatic cell fate reversibility observed by Gurdon (Fig. 1b ). Transdifferentiation experiments by ectopic expression of transcriptions factors further revealed the importance of transcription factors in establishing cell fate; for example, overexpression of the C/EBPα/β transcription factors was found to promote B cell reprogramming into macrophages. 35 , 36 , 37 , 38 With ESCs as a reference point for features of pluripotency and an emerging understanding of how transcription factors orchestrated gene expression, the Japanese stem cell biologist Shinya Yamanaka (b. 1962) together with his postdoctoral fellow Kazutoshi Takahashi designed a series of somatic cell reprogramming experiments that would lead to the breakthrough development of mouse iPSCs in 2006 (Fig. 1c ). 4 Aiming to induce pluripotency in mouse embryonic fibroblasts (MEFs), Takahashi and Yamanaka selected 24 potential reprogramming factors that included transcription factors known to be important for the ESC state and other effectors. The reprogramming factors were cloned into retroviral vectors for MEF transduction, whereas MEFs were engineered to carry β-galactosidase and neomycin resistance encoding genes under a pluripotency-specific promoter of the Fbxo15 gene. Screening different combination of the 24 reprogramming factors, Takahashi and Yamanaka narrowed down the list to four transcription factors that were sufficient to induce pluripotency in MEFs: Oct4, Sox2, Klf4, and Myc (together known as OSKM or Yamanaka factors). 4 Remarkably, these mouse iPSCs resembled the biological potency, gene expression, and the epigenetic landscape of ESCs. 39 A year later, Yamanaka and Thomson independently demonstrated that human fibroblasts could also be reprogrammed into iPSCs; Yamanaka used the same OSKM factors, whereas Thomson used OCT4, SOX2, NANOG, and LIN28. 3 , 5 These combinations of reprogramming factors remain widely used today, whereas Gurdon and Yamanaka were awarded the 2012 Nobel Prize in Physiology or Medicine for their discoveries. Since 2007, various modifications to the original cocktail of reprogramming factors have been developed. For example, small-molecule assisted somatic cell reprogramming was first reported in 2008, 40 , 41 whereas fully chemical reprogramming of murine fibroblasts using seven small-molecule compounds was achieved in 2013. 42
Molecular mechanisms of somatic cell reprogramming to iPSCs
When pluripotent stem cells undergo differentiation into somatic cells, they acquire epigenetic memory and undergo global changes to their chromatin conformation, resulting in inactivation of pluripotency-specific genes and activation of somatic cell-specific genes. 43 Reprogramming of somatic cells back to the pluripotency state involves the erasure of many of these somatic cell signatures; therefore, induction of pluripotency has been proposed to partially resemble the a sequence of developmental events in reverse. 6 , 44 , 45 , 46 Broadly, reprogramming occurs in two phases, early and late. During the early phase, somatic genes are silenced, whereas early pluripotency-associated genes are activated; during the late phase, late pluripotency-associated genes are activated. Early events of reprogramming are largely stochastic, presumably owing to the inefficient access of closed chromatin by OSKM and other transcription factors, whereas late events appear to be more deterministic. 6 Universal aspects of reprogramming, such as two transcriptional waves, are accompanied by somatic cell type-specific reprogramming trajectories and transient events. 47 Overall, reprogramming entails profound remodeling of the chromatin structure and the epigenome as well as changes to almost every aspect of cell biology, including metabolism, cell signaling, intracellular transport, proteostasis, and others. 48 , 49 , 50 , 51 , 52 Given that iPSCs are most often derived from fibroblasts, mesenchymal-to-epithelial transition (MET) is another critical event that occurs during reprogramming. 53
Uncovering the molecular mechanisms of iPSC induction facilitates the development of novel reprogramming approaches and reveals the underlying principles of cell fate transitions and cell fate determination. This knowledge can subsequently be used to design rational strategies for iPSC differentiation towards the desired cell types in an efficient manner. In this section, we focus on the roles of transcription factors as well as chromatin and DNA methylation dynamics in reprogramming.
Transcription factors
OSKM and other transcription factors orchestrate somatic cell reprogramming to pluripotency. 54 , 55 Through concerted action, OSKM expel somatic cell-specific transcription factors from somatic enhancers and activate pluripotency enhancers; silencing of somatic cell-specific enhancers is initiated early in reprogramming, whereas activation of pluripotency-specific enhancers occurs later in reprogramming. 56 , 57 Notably, the chromatin and DNA methylation landscape is restrictive early in reprogramming, requiring pioneering activity of the OSKM factors to access closed chromatin and initiate gene expression. 58 Oct4, Klf4, and Sox2 target partial motifs in the nucleosome-enriched loci, indicating their pioneering activity, 55 whereas Sox2 has even been proposed to be a super pioneer due to its ability to induce DNA demethylation and overcome repressive epigenome. 59 Multiple studies have revealed the dynamics of OSKM binding to DNA and their mode of action. For example, Oct4 dynamics exhibit a hierarchical sequence of events, with Oct4 targeting epigenetically primed states and then maintaining stable DNA occupancy for the duration of reprogramming. 56 Mutagenesis-based analysis of Oct4 protein domains has revealed dynamic DNA and nucleosome binding kinetics and highlights the importance of stable Oct4 interactions with nucleosomes to maintain chromatin accessibility of pluripotency enhancers. 60 Klf4 facilitates topological enhancer-promoter connectivity and organization required for reprogramming to pluripotency, 61 whereas Myc targets open promoter regions to facilitate cell cycle progression. 6 , 62 , 63 Importantly, OSKM closely cooperate with each other to exert global reprogramming of gene expression, which can be illustrated by the concerted action of OSKM to drive MET: Oct4 and Sox2 suppress Snail expression, Klf4 promotes Chd1 expression (encoding E-cadherin), and Myc suppresses the TGFβ signaling axis. 64 In addition to OSKM, multiple other transcription factors play important roles in reprogramming downstream of OSKM and can partially substitute certain OSKM factors. 65 , 66 , 67 , 68 , 69 For example, Klf4 and Sox2 can be substituted by their close homologs, 6 , 70 whereas NKX3-1 or a dominant-negative variant of c-Jun can substitute Oct4. 67 , 71 Notably, certain cell types that endogenously express SKM, such as neural progenitor cells, can be reprogrammed into iPSCs with exogenous expression of Oct4 alone. 72 , 73 , 74 Overall, transcription factors are the drivers of somatic cell reprogramming to pluripotency that coordinate the rewiring of gene expression as well as the remodeling of chromatin and DNA methylation as discussed next.
Chromatin dynamics and histone remodeling
Chromatin remodeling represents another layer of dynamic changes that occur during reprogramming. 44 , 75 , 76 Although pioneer transcription factors can access closed chromatin, the ability of non-pioneer transcription factors to exert gene expression programs requires extensive chromatin remodeling. Given that chromatin becomes progressively restricted during cell differentiation to establish somatic cell-specific gene expression programs, 43 decompaction and remodeling of chromatin is essential for induction of pluripotency. Chromatin remodeling often precedes changes in gene expression and is required for establishing pluripotency-supporting spatial organization of DNA regulatory elements as well as for enabling access of transcription factors to DNA during reprogramming. 45 , 77 Chromatin remodeling occurs in waves as loci enriched for somatic genes transition from open to closed early in reprogramming, whereas loci enriched for OSK motifs transition from closed to open late in reprogramming. 75 , 78
Chromatin dynamics are highly influenced by nucleosome remodeling and histone modifications that modulate chromatin compaction and transcription factor accessibility to DNA. Nucleosome remodeling factors, such as the NuRD complex and the histone chaperone CAF-1, exert context-dependent regulation of gene expression in somatic cells and during induction of pluripotency. 79 , 80 For example, CAF-1 is required for maintaining somatic cell identity, whereas suppression of CAF-1 facilitates chromatin opening at enhancer regions and promotes Sox2-mediated activation of pluripotency genes. 79 Various histone modifiers are also involved in reprogramming; for example, the histone methyltransferase EZH2 is a positive regulator of reprogramming, presumably required to silence somatic cell-specific genes. 81 On the other hand, histone methyltransferase DOT1L is a negative regulator of reprogramming because it maintains permissive chromatin in fibroblast-specific genes associated with the epithelial-to-mesenchymal transition. 81 Changes in global levels of specific histone modifications have also been documented in reprogramming. For example, H3K9 methylation is depleted in iPSCs, and suppression of the H3K9 reader heterochromatin protein Cbx3 promotes fibroblast reprogramming to pluripotency. 82 , 83 Global remodeling of histone modifications can be driven by metabolic reprogramming during the induction of pluripotency. For example, the transcription factor Glis1 targets glycolytic genes to enhance glycolytic flux during reprogramming, leading to increased production of acetyl-CoA and lactate intermediates required for histone acetylation and lactylation at pluripotency genes. 84 Given the roles of histone modifiers in chromatin compaction and reprogramming, small-molecule compounds targeting histone modifiers are often used to promote chromatin decompaction during chemical or transcription factor-mediated reprogramming. For example, the histone deacetylase inhibitor valproic acid as well as the Dot1l inhibitor SGC0946 promote somatic cell reprogramming to pluripotency. 40 , 85 , 86
DNA methylation
Given the critical role of DNA methylation in establishing epigenetic memory during cell differentiation, active remodeling of DNA methylation is another essential part of reprogramming. In development, DNA cytosine methylation is orchestrated by de novo DNA methyltransferases DNMT3A/B that guide DNA methylation at regulatory regions, thus modulating transcription factor accessibility and downstream gene expression. 87 , 88 During reprogramming, such somatic cell-specific DNA methylation patterns are reversed by active DNA demethylation mediated by ten-eleven translocation (Tet) enzymes. 89 , 90 , 91 Indeed, waves of global DNA demethylation during reprogramming result in the loss of DNA methylation at regulatory regions that become enriched for 5-hydroxymethylcytosine (5hmC), an intermediate of Tet-mediated DNA demethylation. 92 , 93 , 94 , 95 These actions of Tet enzymatic activity not only facilitate pluripotency-specific gene expression, but also drive other events required for reprogramming, including MET. 96 Furthermore, Tet enzymes target specific loci to facilitate reprogramming; for example, Tet1 demethylates the endogenous Oct4 locus to reactivate Oct4 expression. 97 , 98 Tet1 can even substitute exogenous Oct4 during reprogramming, indicating a central role for active DNA demethylation in reprogramming to pluripotency. 98 Tet enzymes cooperate with pluripotency-specific transcription factors to reactivate pluripotency-specific genes. For example, Nanog physically interacts with Tet1 and Tet2, whereas cooperative binding of Nanog and Tet1 to loci of pluripotency-specific genes primes their expression during reprogramming. 97 Tet1 activity is also influenced by exogenous vitamin C, indicating that small-molecule compounds can influence active DNA demethylation and epigenetic remodeling during reprogramming. 99 Overall, remodeling of chromatin accessibility and DNA methylation erases somatic cell identity and creates a permissive epigenetic landscape for the pluripotency state during reprogramming.
Population-level dynamics during iPSC induction
The dynamics of cell fate transitions at the population level reveal a stochastic and heterogenous nature of iPSC induction. 76 Somatic cells transition through a continuum of reprogramming intermediates that bifurcate into intermediates that will successfully complete reprogramming and those that will acquire an alternative fate. 100 Most cells do not complete reprogramming, whereas clonal competition leads to the emergence of dominant clones that overtake the culture during reprogramming. 101 Clonal competition is also fueled by the heterogeneity of the starting somatic cell population, the extent of which may be dependent on the somatic cell source. 101 There is a great interest in isolating rare intermediates that complete reprogramming more efficiently than do other cells, so that molecular mechanisms governing productive reprogramming could be elucidated. 102 For example, rare intermediates that exhibit chromatin hyperaccessibility at pluripotency-specific genes and distinct DNA methylation profiles have been isolated based on the presence of pluripotency-specific surface markers. 103 We anticipate that improving high-throughput profiling of gene expression and chromatin accessibility at single cell level will continue to provide new insights into cell fate transitions and reprogramming trajectories during iPSC induction.
Residual somatic cell memory and reprogramming cell source
Although iPSCs resemble primary ESCs in terms of their cellular characteristics and the potential for differentiation into all lineages, limitations associated with reprogramming and persistent features of somatic cell identity render iPSCs distinct. Reprogramming of various somatic cell types reveals persistence of somatic cell transcriptional, DNA methylation, and chromatin accessibility signatures. 104 , 105 , 106 , 107 Incomplete removal of somatic cell-specific epigenetic signatures as well as aberrant de novo DNA methylation associated with reprogramming can affect the status and the differentiation potential of iPSCs. 105 , 107 , 108 Adding small-molecule compounds that target chromatin modifiers to the reprogramming cocktail can facilitate the erasure of the residual chromatin signatures and increase the differentiation potential of iPSCs into alternative lineages. 108 On the other hand, persistence of somatic cell-specific epigenetic signatures can be exploited to enhance iPSC differentiation into the desired cell type by deriving iPSCs from the same somatic cell type. For example, iPSCs derived from pancreatic beta cells retain open chromatin signatures at loci important for beta cell identity; consequently, beta cells can be differentiated more efficiently from beta cell-derived iPSCs as compared to non-beta-cell-derived iPSCs. 104
The cell source used for reprogramming can also influence the heterogeneity and the mutational burden of the resulting iPSCs. iPSCs derived from skin fibroblasts contain common ultraviolet (UV) light-related mutations and exhibit genomic heterogeneity, likely arising from the already heterogenous fibroblast population of the skin. 109 On the contrary, iPSCs derived from peripheral blood mononuclear cells (PBMCs) do not exhibit UV-related damage and may have fewer mutations than do iPSCs derived from skin fibroblasts. Nonetheless, PBMC-derived iPSCs may contain other mutations that are selected for during reprogramming, such as oncogenic mutations in the BCOR gene encoding the BCL-6 corepressor. 109 Age-related heteroplasmic variants of mitochondria can also influence the mitochondrial genetic makeup of iPSCs derived from different donors. 110 Furthermore, spontaneous mutations that arise in the mitochondrial genome during reprogramming could result in the production of novel immunogenic epitopes; new iPSC-specific mitochondrial DNA mutations have been observed in >70% of iPSC lines. 110 , 111 Overall, iPSCs exhibit increased heterogeneity as compared to ESCs due to persistent somatic cell signatures and mutational burden. 112 Such heterogeneity can influence the quality of iPSCs, including their differentiation potential and the immunogenicity of iPSC-derived cellular products, among other features.
Methods of iPSC induction
Since the groundbreaking experiments of fibroblast reprogramming into iPSCs, various approaches to deliver reprogramming factors into somatic cells and induce pluripotency have been developed. 113 , 114 , 115 Viral vectors carrying OSKM expression cassettes are commonly used for reprogramming due to their high efficiency of infection and the capacity to transduce various somatic cell types. 3 , 4 , 5 , 113 , 115 , 116 , 117 , 118 , 119 Viral vectors can be classified as either integrating or non-integrating vectors; lentiviral or retroviral delivery of the reprogramming factors leads to their integration into the genome and thus stable expression for iPSC induction. 3 , 4 , 5 However, viral vector integration into the genome may result in insertional mutagenesis and undesired transgene reactivation beyond the duration of reprogramming. An alternative approach is to use non-integrating viral vectors, such as adenovirus, adeno-associated virus, or Sendai virus. 115 , 119 Non-integrating viral vectors are gradually cleared from proliferating iPSCs, resulting in reprogramming without permanent OSKM integration or disruption of the genome. OSKM factors can also be delivered using non-viral vectors, such as transposons, 120 , 121 episomal plasmids, 122 , 123 mRNA, 124 and others. 115 For example, plasmid-based episomal vectors are commonly used to derive iPSCs for clinical development; reprogramming efficiency when using episomal vectors is comparable to that of Sendai virus-mediated reprogramming, but the cost is much lower. 122 , 123 , 125 Somatic cells can also be reprogrammed into iPSCs without OSKM overexpression. Various combinations of miRNAs can be used to activate the endogenous pluripotency gene networks. 126 , 127 For example, human and mouse iPSCs can be derived by overexpression of miR-200c , miR-302s , and miR-369s . 127 Alternatively, pluripotency can be induced using a cocktail of small-molecule compounds that modulate various signaling pathways and epigenetic modifiers. 128 Small-molecule-based chemical reprogramming is highly attractive due to its simplicity and potential for scalability. 128 , 129 , 130 Combining transcription factors and small-molecule compounds may further accelerate reprogramming. 131 , 132 , 133 Overall, the desired method is often selected based on its efficiency, feasibility, safety, and cost. 115
It should be noted that new insights into the molecular mechanisms of reprogramming using the methods described above are constantly emerging. For example, chemical reprogramming is associated with distinct cell fate transitions and chromatin accessibility dynamics as compared to transcription factor-mediated reprogramming, but it remains unclear if such differences affect the status of the derived iPSCs. 134 , 135 Furthermore, aberrant Oct4 off-target activity has been linked to changes in gene expression and epigenetic profiles that may alter the iPSC differentiation potential. 136 Therefore, newly developed reprogramming methods should be rigorously assessed for their effects on the iPSC status, quality, and differentiation potential.
Applications of iPSCs
Development of the iPSC technology has transformed in vitro research and therapeutic development. 2 , 137 iPSCs can proliferate almost indefinitely and be differentiated into the diversity of human cell types, but with reduced ethical constraints as compared to using human ESCs. 138 , 139 As a result, iPSC-derived cells are widely used for modeling human development and diseases, performing high-throughput drug screening, and developing autologous and allogeneic cell therapies, among other applications. In the rest of the review, we discuss the diverse applications of iPSCs, their key advantages, as well as the limitations that remain to be overcome.
iPSC-derived cellular models
Assembling cellular models of human development and diseases in vitro requires access to large quantities of cells that faithfully recapitulate human biology. Although various primary cell types, such as skin, blood, and cancer cells, can be easily isolated from living donors, other cell types, such as brain and heart cells, are largely unavailable. An alternative approach is to use rodent cells; however, animal models exhibit substantial species divergence and may not recapitulate certain human-specific phenotypes. 7 , 8 , 9 The iPSC technology can be used to overcome both limitations: iPSCs can be readily differentiated into hard-to-access cell types, whereas their human origin and relevant genetic background enable robust modeling of human biology in vitro.
To date, hundreds of protocols to differentiate iPSCs into various cell types have been developed. This is often achieved by mimicking developmental signaling cues in vitro with relevant proteins and small-molecule compounds or by overexpression of cell fate-determining transcription factors to instruct the desired gene expression programs. Certain cell types, such as neurons or cardiomyocytes, can be differentiated with limited resources and training required in about one week. 140 , 141 Other cell types, such as oligodendrocytes or T cells are more difficult to differentiate and require extensive technical expertise. 142 , 143 , 144 For example, differentiation of oligodendrocytes, which arise late in human brain development, involves multiple stages, requires several different media formulations, and can take several months. 143 , 144 , 145 Approaches for uncovering key effectors required for efficient cell differentiation include CRISPR/Cas9-based screens, temporal high-throughput profiling of differentiation trajectories, and comprehensive annotation of transcription factor activity, among others. 146 , 147 , 148 , 149 In-depth understanding of developmental trajectories facilitates rational design of differentiation protocols to derive specific cell types and subtypes. For example, hematopoietic lineage cells can be derived by sequential specification of the mesoderm and the hemogenic endothelium to obtain hematopoietic progenitor cells followed by terminal differentiation of lymphoid and myeloid lineages in the presence of relevant cytokines. 150 , 151 Neural cells can be derived by dual SMAD inhibition that promotes neuroectoderm specification and the emergence of neural progenitor cells (Fig. 2a ). 152 , 153 Furthermore, various morphogens can be applied to instruct regional identity of the differentiating neural cells to obtain specialized cell subtypes; for example, inhibition of the WNT signaling pathways specifies forebrain identity of neural cells. 153 iPSC differentiation can also be considerably accelerated by ectopic expression of cell fate-determining transcription factors. For example, overexpression of six microglia fate-determining transcription factors facilitates rapid differentiation of iPSCs into microglia in as few as 8 days, as compared to several weeks required for microglia differentiation without the use of transcription factors. 154
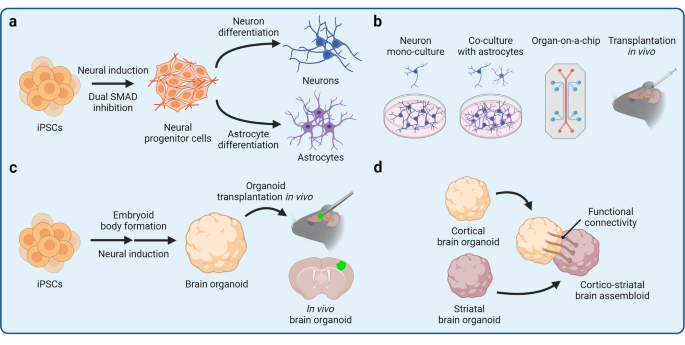
Induced pluripotent stem cell (iPSC)-derived cellular models. The iPSC technology can be applied to derive cellular models of varying complexity, ranging from two-dimensional mono-cultures to three-dimensional multicellular assemblies. Various neural cellular models are shown as an example. a Differentiation of neural progenitor cells (NPCs) from iPSCs is achieved by promoting neuroectoderm specification by dual SMAD inhibition. Subsequently, NPCs can be differentiated into terminal neural lineage cells, such as neurons and astrocytes. b iPSC-derived cells can be maintained in a mono-culture or together with other cell types in a co-culture. Different cell types can also be assembled into an organ-on-a-chip that contains separate compartments and enables modeling of complex tissue architecture. Alternatively, iPSC-derived cells can be transplanted in vivo to expose the cells to a complex tissue environment. c iPSCs can be differentiated into three-dimensional self-organizing organoids that partially resemble endogenous tissue architecture and contain several cells types. Organoids can also be transplanted in vivo to promote their vascularization and maturation. d Different types of organoids can be fused together into assembloids for the study of higher-order tissue interactions, such as long-distance innervation and cell migration
Cellular models of varying complexity can be assembled from iPSC-derived cells (Fig. 2b ). A particular cell type can be studied in mono-culture experiments to evaluate the cellular response to experimental perturbations and uncover cell autonomous molecular mechanisms and phenotypes. Due to its simplicity, mono-culture is also often used to perform high-throughput screens, such as CRISPR/Cas9-based screens, high-content imaging, and drug screening. 155 , 156 , 157 However, the mono-culture environment lacks heterotypic paracrine signaling and cell-cell interactions that are indispensable in vivo. To increase the complexity of iPSC-derived in vitro models, different cell types can be co-cultured together. Co-culture not only enables the study of cell-cell communication, but also promotes cell maturation. For example, co-culturing neurons with astrocytes enhances neuron maturation and survival because astrocytes provide neurotrophic factors required for neuron maintenance. 140 Tri-culture of neurons, astrocytes, and microglia further increases the physiological relevance of the in vitro brain model, enabling complex phenotypes to emerge. 158 , 159 Yet, co-culture experiments still lack the three-dimensional (3D) complexity and organization of human tissues. Remarkably, iPSCs have the capacity to self-organize into 3D tissues, known as organoids, if appropriate differentiation conditions are provided (Fig. 2c ). 10 , 160 , 161 , 162 , 163 , 164 Organoids are often comprised of several cell types and partially recapitulate the complexity of human tissues, enabling the study of context-dependent cell function, organogenesis, and organ-specific diseases. The organoid field has grown extensively in recent years, and dozens of protocols have been developed to derive organoids representing major human organs. 10 , 160 , 161 , 162 , 163 , 164 Importantly, organoids can develop impressive complexity; brain organoids patterned by Sonic hedgehog (SHH) signaling exhibit human-like topographical specification with neocortical, ganglionic eminence, and hypothalamic regions. 165 Kidney organoids contain nephron-like segments, including the Bowman’s capsule, proximal tubules, the loop of Henle, and distal convoluted tubules in a continuous arrangement reflective of the human kidney architecture. 166 Increasing sophistication of organoid differentiation protocols also enables derivation of organoids resembling specific organ regions. For example, exposure of developing neural organoids to various combinations of patterning morphogens yields cortical, 167 , 168 midbrain, 169 , 170 hippocampal, 171 cerebellar, 172 , 173 retinal, 174 , 175 , 176 and other specialized brain organoids. 177 , 178 , 179 , 180 Similarly, fundic and antral gastric organoids recapitulate distinct epithelial lining of the corpus and antrum regions of the stomach, respectively. 181 , 182 Organoid complexity can be further increased by developing multi-lineage organoids or fusing heterotypic organoids to form assembloids (Fig. 2d ). 183 , 184 , 185 For example, multi-lineage neuromuscular organoids contain both neurons and skeletal muscle cells and thus form functional neuromuscular junctions. 186 Similarly, fusing cortical organoids with spinal cord organoids and skeletal muscle spheroids results in the formation of corticofugal projections and innervation of the muscle tissue. 187
An alternative platform to self-organizing organoids is the organ-on-a-chip (OoC), a biomimetic assembly of tissue-relevant cell types into a microfluidics device to recapitulate certain aspects of tissue architecture. 188 , 189 , 190 , 191 , 192 , 193 , 194 OoCs have separate compartments and are constantly perfused, enabling controlled tissue assembly, exposure to shear fluid forces, and separation of culture medium reservoirs. OoCs can be used to model tissue interfaces, such as the blood-brain barrier (BBB) 195 , 196 or the airway epithelium, 197 where compartment separation is critical. Assembling iPSC-derived neural cells and brain microvascular endothelial-like cells (BMECs) into a BBB-on-a-chip yields a BBB model that exhibits in vivo-like transendothelial electrical resistance and restricted permeability. 198 As a result, the BBB-on-a-chip can be perfused with whole human blood at the BMEC interface without inducing toxicity in the neural cell compartment. 198 Microfluidics devices can also be designed to incorporate other functional elements, such as valves to support the mechanical function of cardiac tissue. Fabrication of a microfluidics system with valves has been used to establish an iPSC-derived heart-on-a-chip with unidirectional fluid flow and a closed pressure-volume loop. 199 Heart-on-a-chip devices can record various parameters of cardiac function, including contractile dynamics, active force, tension, and electrical properties of the engineered tissue. 200
iPSC-derived cells and organoids can also be transplanted in vivo to obtain humanized animal models (Fig. 2c ). 201 , 202 , 203 , 204 , 205 In this way, the advantages of iPSC-derived cells, including their human origin and donor-specific genetic background, can be combined with the advantages of animal models, such as their physiological complexity, ability to exhibit cognitive phenotypes, and others. For example, transplantation of iPSC-derived microglia into the mouse brain leads to even distribution of microglia in the brain parenchyma, improved maturation, and long-term survival of microglia. 206 , 207 , 208 , 209 , 210 Similarly, blood vessel organoids form perfusable vascular networks upon transplantation, which is challenging to achieve in vitro. 211 Overall, iPSC-derived cellular models of varying complexity can be generated to address specific hypotheses of cellular function, cell-cell interactions, and tissue-level activity.
Maturation of iPSC-derived cells
Differentiation of iPSCs into various cellular models, especially in mono-culture, occurs with limited exposure of the differentiating cells to a physiologically-relevant tissue microenvironment and at an accelerated rate as compared to cell differentiation in vivo. As a result, iPSC-derived cells are often immature, which is a significant limitation of the iPSC technology to disease modeling and cell therapy applications. Immature cells lack complete functionality of their in vivo counterparts and thus may not reveal important phenotypes when used for disease modeling or be as efficacious as primary cells when used in cell therapy. For example, immature iPSC-derived spinal motor neurons exhibit fetal-like signatures, whereas expression of gene networks relevant to amyotrophic lateral sclerosis (ALS) correlates with motor neuron maturation and aging; these observations suggest that immature iPSC-derived neurons may not fully recapitulate ALS pathology. 212 Therefore, achieving robust maturation of iPSC-derived cells is an important consideration before downstream applications are pursued.
Somatic cells differentiate and mature in the context of their tissue microenvironment that provides signaling cues, metabolites, and cell-cell contacts required for maturation. Reconstituting a physiologically-relevant environment in vitro can thus promote maturation of iPSC-derived cells. For example, artificial extracellular matrix composed of biomimetic nanofibers enhances cortical neuron morphological and functional maturation. 213 Relevant paracrine signaling can also be provided by co-culture experiments, where two or more cell types interact with each other. Co-culture of cardiomyocytes with mesenchymal stem cells promotes myofibril alignment and gap junction formation in cardiomyocytes. 214 Such enhanced cardiomyocyte maturation is partially mediated by mesenchymal stem cell secreted extracellular vesicles, highlighting the importance of paracrine cell-cell interactions that would be challenging to replicate using chemically defined cell culture medium alone. 214 That cell-cell interactions promote maturation of iPSC-derived cells is also evident in 3D in vitro cellular assemblies, including organoids and OoCs that generally exhibit improved maturation over 2D cellular models. For example, incorporating cardiac fibroblasts into spheroids containing cardiomyocytes and epithelial cells leads to cardiomyocyte-fibroblast coupling via gap junctions as well as enhances sarcomere formation and cardiomyocyte eletrophysiological maturation. 215 Similarly, a BBB-on-a-chip exhibits metabolic coupling between neurons and endothelial cells. 216 Organoid maturation can be further improved by transplantation in vivo, leading to organoid vascularization, improved nutrient exchange, and exposure to physiologically-relevant systemic factors. 217 , 218 , 219 , 220 , 221 , 222 , 223 For example, orthotopically transplanted lacrimal gland organoids functionally mature to produce tear-film proteins and resemble primary human tissue. 217
Somatic cells are also exposed to tissue-specific mechanical and environmental conditioning, which may be partially recreated in vitro. Application of mechanical stress to iPSC-derived cardiomyocytes by stretching improves their transcriptional and functional maturation. 224 , 225 Incremental pulsatile stretching also promotes maturation of vascular grafts composed of iPSC-derived smooth muscle cells, leading to increased mechanical strength and minimized dilation of the engineered vessels. 226 Fluid shear stress enhances ciliogenesis and maturation of multiciliated airway cells, whereas cardiomyocyte maturation can be further improved by electrical field conditioning. 197 , 200 , 227 Overall, paracrine signaling and mechanical cues can be readily applied to achieve advanced maturation of iPSC-derived cells.
Ultimately, iPSC-derived cells should faithfully recapitulate the cellular biology and function of their in vivo counterparts to serve as rigorous in vitro models of human development and diseases. Large omics datasets generated from primary human tissues can be used for benchmarking of iPSC-derived cells to determine their maturity and resemblance to primary cells. For example, Shin et al. performed spatial similarity mapping of single-cell transcriptomes of iPSC-derived thalamic organoids and primary human brain tissue, which revealed a strong resemblance of thalamic organoids to the primary thalamus. 228 Therefore, efforts to generate multi-omics datasets of primary tissues, such as the Human Cell Atlas Project, 229 , 230 can provide highly valuable data for iPSC-based studies and serve as a reference point for molecular profiles of functionally mature cells and tissues.
Modeling human development with iPSC-derived cells
Given that iPSCs resemble an ESC-like state after reprogramming, 39 iPSC differentiation into somatic cells or organoids primarily recapitulates embryonic developmental and fetal-like cell states. Therefore, iPSCs are particularly suitable for modeling early human development. Controlled differentiation of iPSCs recapitulates key events of early embryogenesis, such as epiblast lumenogenesis, bipolar embryonic sac formation, and specification of the primitive streak and primordial germ cells. 231 , 232 , 233 , 234 iPSC-derived primordial germ cell-like cells (PGCLCs) exhibit distinct germline-specific transcriptional programs and can be used to study germline development. 232 , 234 Furthermore, differentiation of iPSCs towards presomitic mesoderm recapitulates human somitogenesis and the phenomenon of the segmentation clock. 235 Recently, derivation of post-implantation human embryo models from ESCs has been reported. 236 We anticipate that iPSCs will soon be applied to derive such sophisticated embryo models as well. 237
Although human iPSCs resemble the post-implantation epiblast, they can also be reprogrammed into naïve iPSCs that resemble the pre-implantation epiblast to study human embryogenesis before blastocyst implantation. 238 , 239 , 240 Derivation of naïve human iPSCs from somatic cells was first reported in 2009 and generally requires a combination of transcription factors and small-molecule compounds that modulate various signaling pathways. 240 , 241 , 242 Naïve iPSCs can be used to study X chromosome inactivation, dynamics of transposable element regulation, cell fate transitions, extraembryonic lineage differentiation, and other features and events of pre-implantation embryogenesis. 240 , 243 , 244 Blastoid organoids have been recently developed from naïve iPSCs to study blastocyst development and implantation. 245 In addition to naïve iPSCs, trophoblast stem cells can be derived from iPSCs to model placental development. 246 , 247 , 248
Differentiation of iPSCs into specific cell types reveals the principles of cell type specification and maturation. For example, profiling of dopaminergic neuron differentiation trajectories by single-cell RNA sequencing (scRNA-seq) has indicated an important role for the ASCL1 transcription factor in dopaminergic neuron specification. 249 Differentiation of multiple iPSC lines can also be used to conduct population level analyses, such as the quantitative trait loci (QTL) analysis. 250 In this way, gene regulatory mechanisms that play important roles in development may be uncovered. The organoid platform can be used to study the development of distinct organs. For example, temporal high-throughput profiling of brain organoid differentiation reveals transcriptional and epigenetic regulomes that orchestrate human brain development and regionalization of different brain areas. 251 , 252 Spinal cord organoids recapitulate certain features of neural tube development by undergoing neurulation-like morphogenesis, 253 whereas cardiac organoids co-cultured with epicardial-like cells mimic the envelopment of the myocardium by the epicardium that occurs during heart development. 254 Finally, assembloids enable modeling of multi-tissue interactions that shape developmental programs through paracrine signaling and cell migration. 255 For example, fusing anterior and posterior gut spheroids leads to the emergence of a hepato-biliary-pancreatic anlage-like structure at the interface of the two spheroids in a process that is regulated by retinoic acid signaling. 256 Heterotypic brain assembloids, such as cortico-striatal assembloids, recapitulate interneuron migration that occurs during brain development as well as formation of long-range neuronal projections (Fig. 2d ). 187 , 257 , 258 Overall, modeling development with iPSC-derived cells can provide important insights into human-specific developmental programs and inform cell differentiation approaches for other applications as discussed next.
Modeling human diseases with iPSC-derived cells
The most common application of iPSC-derived cells is disease modeling. 2 , 259 , 260 A key advantage of the iPSC technology for modeling human diseases is that iPSCs can be derived from somatic cells of patients afflicted with a particular disease and carrying causal disease mutations or genetic risk factors. Such iPSCs with a disease-relevant genetic background are subsequently differentiated into the affected cell types that can reveal disease-specific phenotypes. For example, neurons differentiated from iPSCs of patients with familial Alzheimer’s disease recapitulate amyloid β pathology, tau phosphorylation, and other phenotypes observed in Alzheimer’s disease patients. 261 , 262 , 263 Alternatively, disease-relevant mutations can be introduced by CRISPR/Cas9-based gene editing, which enables derivation of isogenic disease models. 264 Isogenic cell lines can be generated by correcting disease-causing mutations in patient-derived iPSCs to obtain a wild-type control iPSC line. 265 The resulting pair of patient-derived iPSCs and corrected control iPSCs shares the same genetic background except for the disease-causing mutation or genetic risk variant. 265 , 266 For example, astrocytes derived from iPSCs of patients with Alexander’s disease reveal disease-specific phenotypes caused by GFAP mutations, whereas isogenic gene-corrected controls exhibit normal cellular function (Fig. 3a ). Similarly, iPSC-derived astrocytes that carry the C variant of the rs11136000 SNP of the CLU gene, a known genetic risk factor for Alzheimer’s disease, but not isogenic SNP-corrected controls, negatively affect oligodendrocyte progenitor cell (OPC) proliferation and myelination. 267 Using isogenic cell lines limits confounding individual-to-individual variation and may increase the statistical power of in vitro experiments. 268 On the other hand, derivation of iPSCs from large cohorts of patients enables genome-wide association studies (GWAS) combined with phenotypic analysis. 269 For example, analysis of iPSC-derived cortical neurons derived from a large cohort of Alzheimer’s disease patients reveals single-nucleotide polymorphisms (SNPs) associated with amyloid β production. Similarly, liver organoids derived from multiple donors reveal pleiotropic SNP interactions associated with non-alcoholic steatohepatitis (NASH). 269 , 270 These iPSC cohorts can also be used to perform high-content screening to rapidly detect and compare disease-relevant pathology as well as evaluate therapeutic candidates. 271 Establishing iPSC biobanks that contain multiple iPSC lines representing different diseases is thus an important goal for advancing iPSC-based disease modeling.
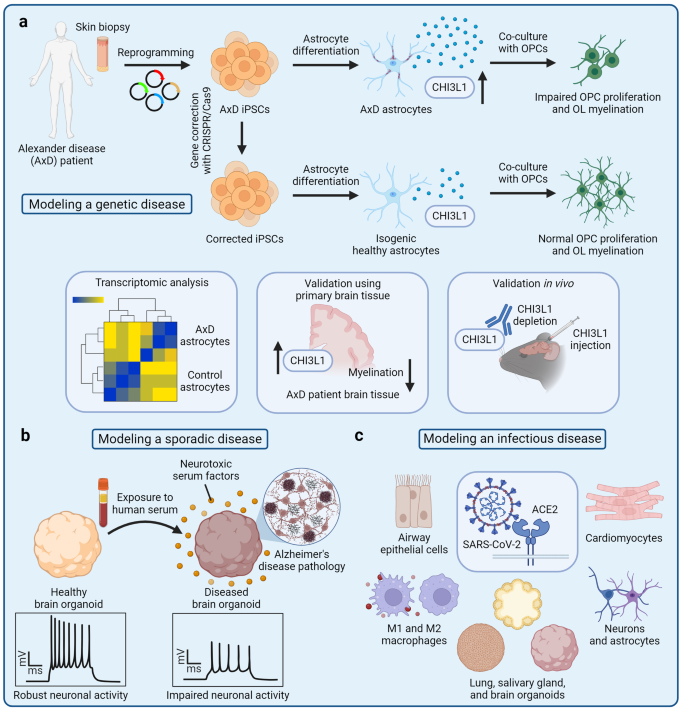
Disease modeling with iPSC-derived cells. a Genetic diseases, such as Alexander disease (AxD), can be modeled using patient-derived iPSCs that carry disease-causing mutations. 144 A tissue biopsy is first taken from a patient with AxD. Somatic cells are reprogrammed into iPSCs, and the GFAP mutations that cause AxD are corrected by gene editing. Patient-derived iPSCs and isogenic corrected controls are then differentiated into astrocytes that express GFAP at high levels. Co-culture of AxD astrocytes with oligodendrocyte progenitor cells (OPCs) reveals impaired OPC proliferation and oligodendrocyte (OL) myelination. Transcriptomic analysis indicates increased expression of the CHI3L1 gene, whereas OPC dysfunction can be partially reversed by CHI3L1 protein depletion. These observations in vitro can be further validated in primary human brain tissues as well as and experiments in vivo. b Sporadic diseases, such as Alzheimer’s disease (AD), can be modeled with patient-derived iPSCs that harbor genetic risk factors; alternatively, iPSC-derived cells can be exposed to non-genetic risk factors to induce disease-relevant pathology. For example, exposure of iPSC-derived brain organoids to human serum mimics the breakdown of the blood-brain barrier and induces AD-like pathology. Brain organoids exposed to neurotoxic serum factors have increased levels of toxic amyloid peptides and hyperphosphorylated tau as well as exhibit impaired neuronal activity. c Infectious diseases, such as COVID-19, can be modeled by exposing iPSC-derived cells and organoids to viral pathogens. iPSC-based models of viral infection can reveal human-specific tropism, mechanisms of entry, and other features of a particular virus
Given the multitude of disease modeling applications using iPSC-derived cells, the breadth of the relevant research could not be covered in a single review article. In the following sections, we consider several diseases that illustrate both the versatility of the iPSC platform as well as the different advantages and limitations of using iPSC-derived disease models. In particular, we discuss iPSC-based modeling of neurodevelopmental, psychiatric, and neurodegenerative diseases that are poorly recapitulated in animal models, require hard-to-access cell types, and can be age-related; cancer initiation that is difficult to study using primary cancer cell models that have already undergone transformation; and COVID-19 that illustrates rapid repurposing of iPSC-based cellular models to study a novel infectious disease during the height of a pandemic.
Modeling neurodevelopmental and psychiatric disorders with iPSC-derived cells
Neurodevelopmental and psychiatric disorders are unique in that their pathogenesis manifests in cognitive changes that can only be studied using animal models that exhibit cognition, whereas in vitro experiments reveal molecular and cellular disease phenotypes only. 272 , 273 However, neurological disorders, especially those that lack clear genetic etiology, cannot be easily recapitulated in animal models due to substantial species divergence and immense complexity of the human brain. 274 , 275 , 276 , 277 These limitations have inevitably hindered scientific discovery and therapeutic development for neurological disorders. Nonetheless, iPSC-based cellular models can provide important insights into the pathogenesis of neurological disorders, whereas state-of-the-art technologies, such as brain organoid transplantation in vivo and machine learning, pave the way for studying complex cognitive phenotypes.
Neural cells derived from iPSCs of patients with neurological disorders exhibit impaired cellular function. 260 , 278 For example, cellular models of schizophrenia reveal aberrant proliferation and migration of neural progenitor cells, dysfunctional arborization of cortical interneurons, and impaired astrocyte glutamate uptake. 279 , 280 , 281 , 282 , 283 Neural progenitor cells derived from iPSCs of patients with the autism spectrum disorder (ASD) exhibit increased proliferation and impaired migration, as well as increased DNA damage and dysregulated chromatin accessibility at the molecular level. 284 , 285 Various assays can be used to assess neuronal network connectivity in cell culture, which is used as a proxy for cognitive dysfunction. Synaptic density can be evaluated by immunostaining, whereas electrophysiology experiments, such as multi-electrode array (MEA)-based assays, can be applied to measure neuronal activity. 286 , 287 , 288 Neuronal cultures derived from iPSCs of patients with schizophrenia exhibit decreased synaptic puncta density, defective glutamatergic synaptic transmission, and molecular phenotypes related to synaptic dysfunction. 289 , 290 On the contrary, neuronal cultures derived from iPSCs of patients with ASD exhibit increased synaptic puncta density and neuronal firing rate, indicating neuronal hyperexcitability. 291 Recently, MEA has also been combined with machine learning to create simulated environments, where neural cell cultures perform complex tasks and undergo synaptic remodeling—an in vitro assay for learning. 292 , 293 It will be interesting to determine whether neurons derived from iPSCs of patients with neurological disorders exhibit impaired synaptic remodeling in such simulated environments.
Neurological disorders can also be modeled with brain organoids that can reveal dysfunctional cell-cell interactions and complex disease phenotypes. 294 , 295 , 296 , 297 For example, brain organoids derived from iPSCs of patients with Down syndrome or ASD exhibit dysregulated proliferation of neural progenitor cells and aberrant production of inhibitory GABAergic interneurons. 298 , 299 An important advantage of using brain organoids for the study of neurological disorders is their complex electrophysiological phenotypes that emerge as a result of improved neuronal maturation and 3D configuration. 300 , 301 For example, cortical-ganglionic eminence assembloids derived from iPSCs of patients with Rett syndrome exhibit neuronal hyperexcitability and epileptiform-like activity characteristic of Rett syndrome. 302 Finally, transplantation of iPSC-derived cells into the rodent brain allows the evaluation of cell behavior in a complex in vivo environment as well as cognitive dysfunction associated with the disease. For example, glial progenitor cells derived from iPSCs of patients with schizophrenia exhibit impaired astrocytic and oligodendrocytic differentiation, premature cell migration into the cortex, and hypomyelination. 303 The chimeric mice also exhibit behavioral deficits, such as excessive anxiety, indicating higher-order neuronal network dysfunction. 303 A powerful approach of iPSC-based modeling of neurological disorders is whole brain organoid transplantation in vivo, which not only creates a complex physiological milieu for the transplanted human cells, but also preserves human cell-specific organoid environment. 218 , 220 , 222 , 223 , 304 , 305 Although neurological disorders have successfully been modeled using brain organoids in vitro, one important limitation of the brain organoid technology is their lack of vascularization, leading to poor nutrient and oxygen exchange, cellular stress, necrosis of the organoid core, and incomplete organoid maturation. 306 Remarkably, brain organoid transplantation in vivo promotes robust organoid vascularization by the host vasculature and substantially improves organoid characteristics, including neuron maturation and microglia survival. 218 , 220 , 222 , 223 , 304 , 305 An in vivo brain organoid model of Timothy syndrome reveals abnormal neuronal morphology and increased frequency of excitatory postsynaptic potentials, whereas a model of ASD indicates microglia activation. 220 , 222 Overall, iPSC-derived cellular models of neurological disorders reveal complex molecular, cellular, and electrophysiological disease-related phenotypes.
Modeling neurodegenerative diseases with iPSC-derived cells
A distinct group of neurological disorders are age-related neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, ALS, and others. 307 , 308 , 309 , 310 In addition to various mutations and genetic risk factors, aging is a strong risk factor for such diseases and is tightly linked to their molecular mechanisms of progression. 311 , 312 , 313 However, iPSC-derived cells are fetal-like and do not naturally exhibit aging-associated phenotypes. 314 , 315 Somatic cell reprogramming to iPSCs is associated with cellular rejuvenation, causing the loss of aging-associated phenotypes, which are not restored upon iPSC differentiation. 316 , 317 The lack of aging-associated phenotypes is a major limitation of iPSC-derived cells for disease modeling. Nonetheless, various iPSC-based models of neurodegenerative diseases have been developed, and methods to study age-related events or induce aging-associated phenotypes are emerging (Fig. 4 ). 306 , 314 , 318 , 319
Modeling aging-associated phenotypes with iPSC-derived cells. One important limitation of using iPSC-derived cells to model human diseases is their fetal-like phenotypes and the lack of aging-associated cellular features. The process of somatic cell reprogramming to iPSCs is associated with a nearly complete erasure of aging-associated epigenetic marks and phenotypes. Therefore, various strategies to induce aging-associated phenotypes in iPSC-derived cells have been developed. a Exposure of iPSC-derived cells to compounds that disrupt cellular homeostasis can be used to induce aging-associated phenotypes, such as mitochondrial stress or cellular senescence. For example, rotenone disrupts electron transfer in mitochondria, leading to an increased production of reactive oxygen species that can cause mitochondrial stress, damage other organelles, and induce cellular senescence. b Aging-associated phenotypes can also be induced by ectopic expression of progerin, a truncated variant of lamin A nuclear lamina protein. Progerin causes the Hutchinson-Gilford progeria syndrome, a disease that manifests as accelerated aging due to the disruption of the nuclear lamina. Ectopic expression of progerin is sufficient to induce senescence- and aging-associated phenotypes in iPSC-derived neurons and other cells. c Aging-associated phenotypes are preserved if target cells are derived by direct transdifferentiation without an iPSC intermediate. Primary fibroblasts can be transdifferentiated into neurons that exhibit aging-associated phenotypes and epigenetic age signatures of the fibroblast donor, and can thus be used to study age-related dysfunction of neural cells
A small proportion of cases of age-related neurodegenerative diseases are familial in nature and are driven by genetic mutations. Such causal mutations are highly penetrant and manifest in clear molecular and cellular phenotypes of iPSC-derived cells. For example, cortical neurons carrying mutations in the PSEN1 gene exhibit amyloid β pathology characteristic of Alzheimer’s disease 262 ; dopaminergic neurons carrying mutations in the SNCA gene exhibit α-synuclein aggregation characteristic of Parkinson’s disease 320 ; and motor neurons carrying mutations in the TDP-43 gene exhibit TDP-43 aggregation characteristic of ALS. 321 However, most cases of neurodegenerative diseases are sporadic and do not have a clear etiology. Various genetic risk factors for sporadic neurodegenerative diseases have been identified through GWAS, and their subtle contributions to disease progression can be modeled with iPSC-derived cells. 266 , 322 , 323 , 324 For example, the E4 variant of the APOE gene is the strongest genetic risk factor for Alzheimer’s disease. 324 , 325 , 326 Accordingly, iPSC-derived APOE4 neurons, astrocytes, oligodendrocytes, and microglia, all exhibit dysregulated cellular homeostasis and function. 266 , 327 , 328 , 329 , 330 , 331 Non-genetic effectors originating from outside the brain also influence progression of neurodegenerative diseases. Such effectors include the peripheral immune system that has recently been implicated in neurodegeneration as well as environmental factors, such as neurotoxins. 332 , 333 , 334 , 335 , 336 For example, co-culture of iPSC-derived dopaminergic neurons with isogenic primary T cells isolated from patients with Parkinson’s disease reveals increased neuronal cell death that is mediated by T cell-secreted IL-17. 336 Furthermore, exposure of iPSC-derived dopaminergic neurons to a neurotoxin 1-methyl-4-phenylpyridinium (MPP + ) leads to increased expression of genes associated with Parkinson’s disease, 334 whereas dopaminergic neurons carrying the A53T mutation in the SNCA gene are more susceptible to environmental pesticides than are normal controls. 335 Finally, population level studies using large cohorts of iPSCs derived from patients with sporadic neurodegeneration may facilitate identification of novel biomarkers for patient stratification and reveal subtle genotype-phenotype relationships. Efforts to create disease-specific iPSC biobanks are underway; for example, hundreds of iPSC lines from patients with ALS have been established as part of the Answer ALS project. 337 , 338 Interestingly, motor neurons derived from iPSCs of patients with sporadic ALS cluster into distinct groups based on their heterogenous phenotypes, illustrating the application of iPSC-derived cellular models to improve patient stratification. 339
The models described above, however, do not incorporate aging-associated disease phenotypes that play a critical role in neurodegenerative diseases. Due to the lack of suitable models, it remains poorly defined how aging interacts with other risk factors to drive neurodegeneration. At the molecular level, aging may be associated with epigenetic erosion and DNA damage that derail homeostatic gene expression programs, resulting in suboptimal cellular phenotypes and cellular senescence. 340 , 341 , 342 , 343 , 344 , 345 In iPSC-derived cells, aging-associated phenotypes, such as mitochondrial dysfunction, can be induced experimentally to mimic age-related cellular dysfunction (Fig. 4a ). For example, iPSC-derived cells can be treated with rotenone that interferes with the mitochondrial electron transport chain, leading to increased production of reactive oxygen species (ROS), mitochondrial damage, and disruption of cellular homeostasis. 346 , 347 , 348 , 349 However, it remains unclear whether disrupting one cellular pathway is sufficient to recapitulate aging or whether it is simply a model of cellular stress. 315 An alternative strategy to induce aging-associated phenotypes is based on overexpression of progerin, a truncated variant of a nuclear lamina intermediate filament lamin A. 317 Progerin is integral in the pathogenesis of Hutchinson-Gilford progeria syndrome (HGPS), a disease that causes premature aging. 350 Remarkably, overexpression of progerin in iPSC-derived dopaminergic neurons induces neurite degeneration, neuromelanin accumulation, and aging-associated gene expression. 317 Although progerin overexpression can induce various cellular phenotypes associated with aging, it should be noted that HGPS is a distinct disease that may not necessarily recapitulate normal human aging and may exhibit HGPS-specific phenotypes that are irrelevant to neurodegenerative diseases. Therefore, substantial efforts have been made to obtain human brain cell models without erasing aging-associated phenotypes of the somatic cells, from which the neural cells are derived. This aim can be achieved by direct transdifferentation of patient-derived fibroblasts into neurons without an iPSC intermediate (Fig. 4b ). 351 , 352 , 353 , 354 , 355 , 356 Fibroblasts can be transdifferentiated into neurons by overexpression of miRNAs or neuron fate-determining transcription factors, such as NGN2 and ASCL1 , combined with a small-molecule treatment. 354 Transdifferentiated neurons retain the epigenetic age and aging-associated phenotypes of the fibroblast donor and can be used to study the impact of aging on the pathogenesis of neurodegenerative diseases. 316 For example, transdifferentiated neurons derived from fibroblasts of elderly patients with Alzheimer’s disease reveal aberrant neuronal phenotypes, such as Warburg-like metabolic transformation, increased post-mitotic senescence, and hypo-mature neuronal identity, that are not observed in fetal-like iPSC-derived neurons. 316 , 357 , 358 Finally, iPSC-derived cellular models can also be used to study age-related events by mimicking various cell non-autonomous conditions associated with aging. For example, breakdown of the BBB may be caused by aging and is a common feature of neurodegenerative diseases, leading to leakage of potentially neurotoxic serum components into the neural tissue. 359 , 360 , 361 , 362 , 363 Mimicking the BBB breakdown by exposure of iPSC-derived brain organoids to human serum induces a rapid onset of Alzheimer’s disease-like pathology, including accumulation of amyloid β and phosphorylated tau as well as impaired neuronal activity (Fig. 3b ). 364 We anticipate that novel approaches to induce aging-associated phenotypes and model age-related events using iPSC-derived cells will provide new insights into both neurodegenerative and other age-related diseases.
Modeling cancer initiation with iPSC-derived cells
Given their proliferative capacity, primary cancer cells derived from tumor biopsies are the most common cellular models for studying tumor cell biology and the response to therapeutic intervention. 365 , 366 , 367 However, primary cancer cells have already undergone transformation, a key event that governs deregulation of cellular homeostasis and leads to cancer initiation. 368 The iPSC technology offers a unique opportunity to study how various somatic mutations and other events rewire molecular and cellular programs of normal cells, so that they are transformed into cancer cells. 369 For example, iPSC-derived neural stem cells carrying an H3.3K27M mutant histone H3.3 variant associated with diffuse intrinsic pontine glioma, a type of a juvenile brain tumor, exhibit aberrant gene expression programs that promote neural stem cell proliferation and stemness. 370 Similarly, colonic organoids derived from iPSCs of patients carrying mutations in the APC gene associated with familial colorectal cancer exhibit elevated activity of the WNT signaling pathway and higher epithelial cell proliferation as compared to wild-type controls. 371 In addition to somatic mutations, environmental factors also play a role in cell transformation. For example, chronic Helicobacter pylori infection is associated with increased incidence of gastric cancer, presumably due to persistent inflammation of the epithelial lining of the stomach. 372 , 373 Injection of H. pylori bacteria into the lumen of iPSC-derived gastric organoids induces a rapid response of epithelial cells, including a twofold increase in cell proliferation. 374 Finally, genetic manipulation of iPSCs and their subsequent differentiation into cancer-relevant cell types can be used to establish cancer evolution models that reflect successive acquisition of somatic mutations and clonal expansion of cancer cells. For example, introducing various driver mutations associated with acute myeloid leukemia into iPSCs followed by differentiation of hematopoietic progenitor cells enables modeling of leukemic transformation from premalignant cell states to transplantable leukemia. 375 High-throughput profiling of gene expression across the continuum of leukemogenesis reveals distinct molecular pathways, such as dysregulated inflammatory signaling, that promote tumorigenesis. 375 Overall, iPSC-based cellular models can provide important insights into molecular and cellular events governing cancer initiation, which may facilitate patient stratification for early screening and cancer prevention.
Modeling COVID-19 with iPSC-derived cells
Modeling viral infection with iPSC-derived cellular models can reveal unique interactions between viruses and human cells (Fig. 3c ). 288 , 376 , 377 , 378 , 379 The COVID-19 pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has prompted the scientific community to rapidly repurpose experimental platforms, so that SARS-CoV-2 cellular tropism, molecular mechanisms of entry, life cycle, and SARS-CoV-2 targeting therapeutics could be investigated. 380 , 381 , 382 Although animal cell lines and models permissive to SARS-CoV-2 have been identified and developed, human iPSC-derived cellular models have the advantage of revealing human-specific SARS-CoV-2 tropism and vulnerabilities. 383 , 384 , 385 , 386 , 387 Therefore, iPSC-based cellular models of SARS-CoV-2 infection have been swiftly applied to study COVID-19, revealing disease-specific phenotypes. 388 For example, SARS-CoV-2 infection of iPSC-derived alveolar epithelial type 2 (AT2) cells cultured at air-liquid interface, a model for respiratory tract infection, induces cytotoxicity and a pro-inflammatory phenotype of AT2 cells. 389 Co-culture of iPSC-derived macrophages and lung epithelial cells reveals a protective role of macrophages against the SARS-CoV-2 infection of epithelial cells; however, M1 and M2 polarized macrophages exhibit different inflammatory responses. 390 Given widespread extrapulmonary manifestations of COVID-19, 391 , 392 , 393 permissiveness of different tissues and organs to SARS-CoV-2 can be evaluated using tissue-specific organoids. 388 , 394 For example, SARS-CoV-2 infects and productively replicates in salivary gland organoids, indicating the potential role for salivary glands as a reservoir of SARS-CoV-2. 221 Similarly, SARS-CoV-2 actively replicates in capillary organoids, which may explain SARS-CoV-2-associated viremia. 395 , 396 SARS-CoV-2 infection also induces cytotoxicity in iPSC-derived cardiomyocytes and cardiospheres, causing myofibrillar disruption, impaired cardiomyocyte beating, and cell death. 397 , 398 , 399 Neurological manifestations of COVID-19 have also been documented. 400 , 401 , 402 SARS-CoV-2 infects iPSC-derived neural progenitor cells, neurons, astrocytes, and brain organoids. 403 , 404 , 405 , 406 SARS-CoV-2 infection of neural tissues leads to increased tau hyperphosphorylation, a hallmark of Alzheimer’s disease, suggesting that SARS-CoV-2 infection may have long-term neurological effects that could contribute to the onset of neurodegeneration. 403 , 407 Interestingly, the susceptibility of iPSC-derived neurons and astrocytes to SARS-CoV-2 infection is dependent on the APOE variant; APOE4 cells exhibit increased susceptibility to SARS-CoV-2 infection as compared to APOE3 cells. 406 Overall, the iPSC technology has been rapidly adapted to investigate human-specific disease phenotypes of COVID-19, providing vital insights into this life-threatening disease.
Drug development using iPSC-derived cells
Various advantages of iPSC-derived cellular models discussed throughout this review are also applicable to drug development applications. 408 Given their human origin, iPSC-derived cells can be used as a preclinical platform to test drug efficacy and toxicity as well as uncover human-specific molecular mechanisms of drug action. Various somatic cell types, including those that are inaccessible from primary sources, can be derived from disease-specific iPSCs that harbor relevant causal mutations or genetic risk variants to assess drug efficacy in the context of a specific genetic background. iPSC-based experiments can also be scaled to perform high-throughput drug screening with thousands of small-molecule candidates. For example, Gu et al. performed a survival screen of 4500 compounds based on the caspase 3/7 activity to identify anti-apoptotic compounds that limited death of endothelial cells derived from iPSCs of patients with pulmonary arterial hypertension. 409 When combined with high-content imaging technologies, drug screening assays can be used to evaluate complex phenotypes, such as changes to cellular morphology or accumulation of disease-associated protein aggregates. 271 , 410 , 411 Park et al. developed a high-throughput drug screening pipeline to evaluate amyloid β and tau pathology in brain organoids derived from iPSCs of patients with Alzheimer’s disease. 271 In particular, the authors used tissue-clearing techniques and high-content imaging to visualize and quantify the burden of amyloid β and phosphorylated tau upon drug treatment. 271 Combining iPSC-based drug screening with computational analyses and machine learning can reveal targetable regulatory nodes associated with a specific disease as well as therapeutic candidates for drug repurposing. 412 Taubes et al. performed an in silico drug repurposing analysis to identify candidates that could reverse APOE4 -associated gene expression signatures in Alzheimer’s disease. 413 Having identified bumetanide as a potential candidate, the authors validated its efficacy in iPSC-derived APOE4 neurons. 413 Furthermore, Theodoris et al. used machine learning to identify small-molecule compounds that could reverse aberrant gene expression associated with haploinsufficiency for the NOTCH1 gene in calcific aortic disease. 414 The authors screened over 1500 predicted candidates using iPSC-derived endothelial cells and identified an inverse agonist of the estrogen-related receptor α (ERRα) as a potent hit. 414
iPSC-derived cellular models can also be used to evaluate drug toxicity, which is a major cause of drug attrition in therapeutic development. 415 , 416 Although preclinical toxicology is based on animal studies, human-specific drug toxicity may not necessarily manifest in animal models, leading to costly drug withdrawals late in the drug development pipeline. Therefore, the iPSC technology can be used as a complementary platform to assess drug toxicity and its human-specific molecular mechanisms. 417 , 418 , 419 For example, drug nephrotoxicity may be evaluated using iPSC-derived podocytes that form the epithelial lining of the kidney glomerulus. 420 A microfluidics-based glomerulus-on-a-chip recapitulates adriamycin-induced podocyte injury and albuminuria. 421 Similarly, iPSC-derived 3D cardiac tissues recapitulate doxorubicin-induced cardiotoxicity, leading to disruption of sarcomeres and cessation of beating. 422 Evaluating drug toxicity using patient-specific iPSCs may also facilitate precision medicine-driven patient stratification based on individual patient susceptibility to particular therapeutics. For example, transcriptomic analysis of a panel of iPSC-derived cardiomyocytes reveals patient-specific cardiomyocyte susceptibility to oxidative stress associated with decreased expression of the NFE2L2 gene. 423 Cardiomyocytes with low NFE2L2 expression are more susceptible to tacrolimus- and rosiglitazone-mediated cardiotoxicity as compared to cardiomyocytes with high NFE2L2 expression. 423 Uncovering the mechanisms of drug toxicity can facilitate the development of novel therapeutic strategies to mitigate such toxicity. Sharma et al. found that exposure of iPSC-derived cardiomyocytes to cardiotoxic tyrosine kinase inhibitors leads to compensatory insulin signaling that may be cardioprotective. 424 Indeed, adding exogenous insulin or IGF1 improves cardiomyocyte viability in the presence of tyrosine kinase inhibitors. 424 Finally, drug toxicity can be elicited by unexpected drug distribution or accumulation in certain human tissues. Drug pharmacokinetics can be assessed in barrier-forming organoids, such as choroid plexus organoids that form fluid-filled cysts and exhibit selective permeability to various drugs. 425 Drug absorption and metabolism by the cytochrome P450 (CYP) family enzymes can be evaluated using iPSC-derived intestinal epithelial cells. 426 Humanized animal models can also reveal human tissue-specific drug pharmacokinetics and accumulation; for example, transplantation of iPSC-derived kidney organoids into athymic rats has been used to evaluate organoid exposure to systemically administered drugs. 427 Overall, the iPSC technology enables complementary evaluation of drug efficacy and toxicity using human-specific models.
iPSC-based cell therapy
Cell therapy has recently emerged as a promising approach to repair or replace damaged tissue as well as engineer immune responses to a disease, such as cancer. 428 , 429 , 430 , 431 , 432 , 433 The success of adoptive chimeric antigen receptor (CAR) T cell therapy to treat acute lymphoblastic leukemia and large B cell lymphoma has paved the way for developing novel cell therapies, including those based on the iPSC technology. 11 , 434 , 435 , 436 Although primary cells, such as T cells, natural killer (NK) cells, and mesenchymal stem cells, can be isolated from a patient and later used as autologous cell therapy, other cell types, such as neurons, cannot be harvested for transplantation. Furthermore, the quality of primary cells may be compromised by a disease or by germline mutations as well as exhibit unwanted heterogeneity. The iPSC technology can be used to overcome these limitations, given that iPSCs can be genetically engineered, clonally expanded, and differentiated into most somatic cell types. 11 Furthermore, iPSC-based cell therapy has fewer ethical constraints as compared to ESC-based cell therapy because iPSCs are derived from somatic cells. 437 , 438 Xenotransplantation experiments serve as a proof of principle that transplanted iPSC-derived cells can mitigate disease-associated tissue dysfunction and restore homeostasis. For example, transplanted human iPSC-derived pancreatic islets secrete insulin and control glycemia in diabetic mice 439 and macaques. 440 Similarly, human iPSC-derived OPCs rescue myelination in myelin-deficient mice upon transplantation, indicating the potential application of OPC-based cell therapy for treating demyelinating white matter disorders. 123 , 145 , 441 These examples indicate that the iPSC technology can be used to derive hard-to-access cell types and restore normal tissue physiology upon transplantation. As a result, various clinical trials using iPSC-derived cellular products to treat human diseases have been initiated (Table 1 ).
Autologous iPSC-based cell therapy
iPSC-based cell therapy can be divided into two categories—autologous and allogeneic (Fig. 5 ). In autologous cell therapy, iPSCs are derived from the same patient who will receive the cell transplant. 442 , 443 , 444 Autologous cell therapy is meant to prevent immune rejection of the transplant by the recipient because the immune system recognizes the transplanted cells as “self” tissue. A tissue biopsy is first collected from the patient who will undergo autologous cell therapy, and the isolated somatic cells are reprogrammed into iPSCs. These iPSCs can then be genetically modified to correct undesired mutations or introduce new gene expression cassettes. For example, if a patient has a monogenic disease that is caused by a germline mutation, gene correction can be performed. After genetic modification, iPSCs are differentiated into the desired cellular product that will be used for transplantation. Extensive quality control of iPSCs and iPSC-derived cells is required to ensure that the cellular product is functional and does not contain any deleterious or tumorigenic mutations. The feasibility of gene correction-based autologous cell therapy has been demonstrated in preclinical animal models. For example, transplantation of hepatocytes derived from gene-corrected iPSCs of a patient with hereditary antithrombin deficiency leads to normalization of antithrombin levels in the plasma of antithrombin-lacking mice, thus mitigating the thrombophilic state. 445 Similarly, transplantation of pancreatic beta cells derived from gene-corrected iPSCs of a patient with monogenic Wolfram syndrome restores normal glucose homeostasis in diabetic mice. 446 A detailed example of preclinical development of iPSC-based autologous cell therapy for Canavan disease, a monogenic neurodevelopmental disorder, is shown in Fig. 6 .
Autologous and allogeneic iPSC-based cell therapy. In autologous cell therapy, somatic cells are collected from the patient who will receive the cell transplant. The isolated somatic cells are reprogrammed into iPSCs, which can then be genetically engineered to correct disease-associated mutations or introduce new gene expression vectors. Modified iPSCs are differentiated into the cellular product that will be transplanted into the patient and rigorously evaluated for quality. In allogeneic cell therapy, iPSCs are taken from a biobank and genetically engineered for immune cloaking. The resulting hypoimmunogenic universal donor iPSCs can be further genetically modified to introduce cell therapy-specific gene expression vectors, such as a chimeric antigen receptor (CAR) expression cassette, and then differentiated into the desired cell type. After rigorous quality assessment, cellular products can be stocked and distributed as off-the-shelf therapeutics for transplantation into multiple recipients. KO, knockout; KI, knockin
Development of iPSC-based autologous cell therapy. Despite the success of adoptive immune cell therapy, multiple other diseases affect cell types that cannot be easily isolated from patients for genetic engineering and transplantation back into the patient. For example, Canavan disease (CD) is a monogenic autosomal recessive neurological disorder caused by mutations in the aspartoacylase ( ASPA) gene. These mutations disrupt ASPA enzymatic activity, leading to the accumulation of N-acetylaspartate (NAA) in the brain and causing spongy degeneration. ASPA enzymatic activity can be restored by transplantation of autologous neural progenitor cells (NPCs) that harbor CRISPR/Cas9-corrected ASPA or ectopically express wild-type ASPA delivered by lentiviral (LV) transduction. a A skin biopsy is obtained from a CD patient, and patient-specific iPSCs are derived from the isolated skin fibroblasts. b iPSCs are genetically engineered to restore wild-type ASPA expression and differentiated into NPCs that will be used for transplantation. c To demonstrate the efficacy of iPSC-derived NPC therapy for CD, preclinical experiments using a CD mouse model (Nur7) can be performed. CD mice exhibit characteristic spongy degeneration with vacuolation, myelin defects, and motor dysfunction. In our studies, 122 , 123 , 441 we transplanted WT-ASPA -NPCs into the corpus callosum (CC), the subcortical region (SC), and the brainstem (BS) by stereotactic injection. We found that WT-ASPA -NPC-transplanted CD mice exhibited increased ASPA activity and reduced NAA levels, increased myelination and reduced vacuolation, and improved motor function. GMP, good manufacturing practice
Allogeneic iPSC-based cell therapy
In allogeneic cell therapy, iPSCs derived from a universal donor are used for transplantation, circumventing the lengthy and costly process of iPSC production from each patient who will receive the cell transplant (Fig. 5 ). 447 , 448 The desired cells can be differentiated, characterized, and stocked in advance, so that the cellular product is available on demand or “off-the-shelf” without the need for in-house manufacturing. However, allogeneic cell therapy poses a risk of immune rejection and graft-versus-host disease, requiring additional “immune cloaking” strategies to evade the host immune system (Fig. 7a ). 449 Commonly used genetic modifications include knockout of the B2M gene, which encodes a component of human leukocyte antigen (HLA, also known as major histocompatibility complex, MHC) class I molecules, to disrupt foreign antigen presentation to cytotoxic CD8 + T cells; knockout of the CIITA gene to disrupt foreign antigen presentation to CD4 + helper T cells; overexpression of the B2M-HLA-E fusion construct to inhibit the “missing-self” response of NK cells; and overexpression of CD47 to provide the “don’t-eat-me” signal to macrophages. 449 A combination of such modifications is often used to evade different immune cell types. For example, Wang et al. engineered hypoimmunogenic universal donor iPSCs by knocking out B2M , CIITA , and PVR (encoding a ligand for NK cell activation) as well as overexpressing B2M-HLA-E . 450 Hu et al. also knocked out B2M and CIITA but instead overexpressed CD47 , having observed that not only macrophages but also most IL-2 stimulated NK cells present the SIRPα receptor of CD47. 451 It should be noted that extensive genetic engineering required for immune cloaking can introduce off-target mutations, whereas prolonged iPSC culture and clonal expansion can lead to accumulation of spontaneous genetic aberrations. In our recent study, we knocked out B 2M and CIITA and took advantage of endogenously expressed CD47 in OPCs, our cell type of interest, to evade the NK response. 441 Therefore, our approach requires two steps of genetic engineering only, reducing the likelihood of undesired mutational events. Having engineered the universal donor cells, their immune evasive properties can be validated in preclinical models. Universal donor cells and primary immune cells from an unrelated donor can be co-cultured together in vitro or co-injected in vivo to evaluate their survival and persistence (Fig. 7b ).
Engineering universal donor cells for allogeneic cell therapy. a Universal donor cells are genetically engineered to prevent the host immune response despite their foreign origin. CD8 + cytotoxic T cells recognize foreign cells via their T cell receptor (TCR) that interacts with human leukocyte antigen (HLA) class I molecules presenting unique antigens. If a foreign antigen is presented by the HLA class I molecules, CD8 + cytotoxic T cells initiate destruction of the encountered cell. Knockout of the β 2 microglobulin ( B2M ) gene is sufficient to disrupt the universal donor cell interaction with CD8 + cytotoxic T cells. However, ablation of HLA class I molecules elicits a “missing-self” response by natural killer (NK) cells, leading to cell lysis. Therefore, B2M knockout is often combined with ectopic expression of HLA-E, which interacts with the inhibitory NK cell receptor NKG2A/CD94 to suppress the missing-self response. Knockout of CIITA disrupts foreign antigen presentation to CD4 + T cells via HLA class II molecules. To prevent macrophage-mediated cell killing, CD47 surface protein can be ectopically expressed in universal donor cells. CD47 interacts with the signal-regulatory protein α (SIRPα) and acts as the “don’t-eat-me” signal to suppress macrophage-mediated phagocytosis. b Hypoimmunogenicity of universal donor cells can be tested by performing in vitro and in vivo cytotoxicity assays, in which universal donor cells are mixed with primary immune cells, such as T cells, derived from an unrelated donor. Universal donor cells exhibit increased survival and stable persistence in the presence of primary immune cells of a mismatched donor, indicating successful immune evasion
An alternative approach to prevent immune rejection of allogeneic cell therapy is to establish HLA-homozygous iPSC haplobanks to match the donor-patient genotypes of the main HLA molecules involved in immune rejection. 452 , 453 , 454 , 455 Several dozens of iPSC lines are sufficient to cover a large proportion of the population by HLA matching. For example, Yoshida et al. established a clinical-grade HLA haplobank of 27 iPSC lines derived from 7 donors, theoretically covering 40% of the Japanese population for HLA-matched iPSCs. 455 Overall, allogeneic cell therapy holds great promise to streamline the production pipeline, but the safety concerns, especially those related to immune rejection, remain to be fully addressed.
Challenges associated with iPSC-based cell therapy
Compared to pharmacological therapy, cell therapy is extremely complex and poses major safety, quality assurance, and logistical challenges, including those specific to iPSC-based therapeutics. 456 , 457 A major concern is the propensity of iPSCs for teratoma formation; it is critical to ensure that undifferentiated iPSCs and stem cell-like intermediates are completely removed from the cellular product that will be transplanted into the patient to prevent tumor formation. 458 Residual iPSCs can be removed from differentiated cell cultures by selective elimination of highly proliferative cells using chemotherapeutic drugs, such as doxorubicin, 459 or by selective elimination of alkaline phosphatase-positive cells using toxic substrates of alkaline phosphatase. 460 Introducing a gene encoding a self-destruction switch can provide an additional safety mechanism to selectively remove transplanted cells if they acquire tumorigenic properties. 461 Such self-destruction systems include inducible activation of apoptosis, expression of enzymes that can convert non-toxic substrates into toxic compounds, and expression of surface receptors that can be targeted by infusion of monoclonal antibodies. 461 As discussed earlier, iPSCs can also exhibit higher intrinsic genetic heterogeneity as compared to ESCs, and acquire mutations during reprogramming, prolonged culture, and gene editing. 111 , 462 , 463 Such mutations may confer tumorigenic potential or lead to the emergence of novel immunogenic epitopes. Therefore, genetic analysis may be required at different stages of iPSC preparation to ensure that the cellular product is free of deleterious mutations.
Incomplete maturation of iPSC-derived cells remains a major hurdle in developing efficacious cell therapies. For example, iPSC-derived CAR T cells are often not as functional as CAR T cells derived from primary T cells, which may limit their tumor cell killing ability and persistence. 431 , 464 Various approaches to improve iPSC differentiation and maturation protocols for cell therapy applications are under active investigation. For example, T cells can be differentiated using hematopoietic or thymic organoids that mimic the in vivo environment of the developing T cells. 465 , 466 , 467 Challenges associated with efficacy of iPSC-based cell therapy for solid tissues include poor transplant engraftment and limited therapeutic response. Systemic infusion of cellular therapeutics may not be sufficient to establish a solid organ graft or may result in off-target engraftment. 456 For example, intrasplenic infusion of iPSC-derived hepatocytes leads to their engraftment into various organs, including the liver, stomach, spleen, and large intestine. 468 Engraftment can be controlled by using biomimetic scaffolds to differentiate cells as structured assemblies, followed by their direct transplantation into the recipient organ. Transplantation of iPSC-derived hepatocytes as a cell sheet generated using a supportive membrane promotes successful liver engraftment with no cells detected in other organs. 468 Biodegradable scaffolds also promote integration and improve functionality of iPSC-derived retinal pigment epithelium patches as compared to epithelial cells cultured and transplanted without a scaffold. 469 Similarly, bio-ink polymers with favorable rheological properties support osteogenic differentiation of iPSC-derived mesenchymal stromal cells and promote repair of cranial defects upon transplantation into a mouse model of cranial injury. 470 Combination therapy can also improve the efficacy of iPSC-based cell therapy via synergistic mechanisms. For example, a combination therapy of iPSC-derived NK cells and anti-PD-1 immunotherapy synergize to kill tumor cells. 471 Similarly, a combination therapy of the neurotrophic factor GDNF and iPSC-derived dopaminergic neurons to treat Parkinson’s disease results in brain-wide dopaminergic neuron innervation in a rat model, whereas transplantation of dopaminergic neurons alone is associated with poor long-distance innervation. 472
Logistics, reproducibility, and the overall cost of iPSC-based cell therapies should also be considered. Logistical challenges include manufacturing and quality assurance of iPSC-based cell therapies. 457 Off-the-shelf iPSC-derived cellular products for allogeneic cell therapy can be generated and distributed in a centralized manner, whereas autologous cell therapies might require hospital-affiliated personnel and facilities to routinely generate cellular products compliant with good manufacturing practices (GMP). 473 Reproducibility and consistency of iPSC-derived cellular products can be improved by automating cell culture with liquid-handling robots, whereas large-scale differentiation of iPSCs can be achieved by using bioreactors. Stirred-tank bioreactors enable the scaling of suspension culture as well as monitoring of cell growth and various biophysical parameters, such as pH. 474 , 475 Automation as well as optimization of iPSC derivation, maintenance, and differentiation protocols can also reduce the overall costs of iPSC-based cell therapies. For example, developing growth factor-free media formulations that do not require costly recombinant proteins could make iPSC maintenance more cost-effective. 476 Although various challenges remain to be overcome, iPSC-based cell therapy holds great promise to restore tissue homeostasis and function in a way that cannot be achieved with pharmacological therapy.
Concluding remarks and future perspectives
Since its development less than two decades ago, the iPSC platform has opened new frontiers for scientific discovery and therapeutic development. The study of somatic cell reprogramming has revealed immense complexity of cellular transformation that occurs during the induction of the pluripotent stem cell state and encompasses both deterministic and stochastic elements. 6 These mechanisms have shed light on the central role of transcription factors in orchestrating gene expression programs, the importance of epigenetic regulation of cell fate, and the cooperative nature of different effectors of reprogramming. With increasing understanding of reprogramming mechanisms, novel methods for efficient and cost-effective derivation of iPSCs continue to emerge. For example, recent reports of fully chemical iPSC derivation methods hold promise for the development of fully defined, scalable, and rapid somatic cell reprogramming protocols. 128 , 129 , 130
As in vitro models of human development, iPSCs and iPSC-derived cells have been used to investigate the principles and mechanisms of cell fate transitions, self-organization, and developmental disorders. Furthermore, iPSC-based cellular models for numerous other diseases, ranging from genetic to sporadic and age-related disorders, enable the study of human-specific disease mechanisms and the testing of potential therapeutic candidates in vitro. 2 Sophisticated cellular models, including organs-on-a-chip, organoids, assembloids, and others, can be used to study higher-order tissue architecture, compartmentalization, and long-range interactions in human development and diseases. 10 , 160 , 163 , 188 , 477 These advanced models of human tissues can also be used to evaluate drug efficacy, toxicity, and pharmacokinetics, thus serving as an additional preclinical platform for drug screening. 408 We anticipate that the complexity and functional maturation of iPSC-derived cells and tissues will continue to improve and will reveal yet unappreciated mechanisms and phenotypes of human biology. For example, emerging methods for brain organoid transplantation and vascularization pave the way for obtaining highly functional and mature human cell-based neural tissues that can integrate into the host circuitry and influence animal behavior. 218 , 220 , 222 Such models enable the study of neuronal network connectivity and its dysfunction in human-specific neurodevelopmental disorders that are challenging to reproduce in preclinical models.
Finally, the promise of the iPSC-based cell therapy has substantially materialized in the past decade, with numerous preclinical studies and early-stage clinical trials being conducted across the spectrum of human diseases (Table 1 ). 11 These efforts are focused on various cancers, for which autologous and allogeneic iPSC-based immune cell therapies are being developed, genetic developmental disorders that require cell transplantation to restore tissue homeostasis, and even sporadic age-related diseases to replace degenerating tissues. Of notable interest are allogeneic cell therapies that utilize universal donor cells engineered to evade immune rejection. 448 Universal donor cells can be prepared, characterized, and stocked in advance, considerably simplifying the manufacturing pipeline and reducing the turnaround time. Although important challenges associated with iPSC-based cell therapy remain to be resolved, the technology holds great promise to alleviate human diseases.
The technological advances that evolve alongside the iPSC technology offer new opportunities to define molecular mechanisms of iPSC induction, optimize protocols of iPSC differentiation into somatic cells, develop sophisticated drug screening platforms, and create efficacious cell therapies. We anticipate that improving technologies, such as microscopy tools, 478 , 479 multiomics, 480 CRISPR/Cas9-based studies of gene and protein function, 481 , 482 , 483 , 484 epigenetic engineering, 485 , 486 , 487 , 488 machine learning algorithms, 489 , 490 , 491 , 492 and others, will provide new insights into the molecular events that govern somatic cell reprogramming to pluripotency and iPSC differentiation into terminal somatic cell types. The study of human development and diseases using iPSC-based models will benefit from enhanced collaboration, including the development of deeply characterized benchmark iPSC lines 493 as well as ethnically diverse iPSC biobanks. 494 Automation of iPSC differentiation into somatic cells and organoids will increase reproducibility of in vitro studies required for rigorous high-throughput applications, including drug screening. 495 Finally, improving iPSC differentiation and maturation protocols will enable derivation of efficacious cellular products for therapeutic development, whereas production of entire iPSC-derived organs may be possible by chimeric organogenesis. 496 , 497 , 498 Overall, the iPSC technology will continue to propel fundamental research and therapeutic development to accelerate scientific discovery and relieve human diseases.
Rowe, R. G. & Daley, G. Q. Induced pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Genet. 20 , 377–388 (2019).
Article CAS PubMed PubMed Central Google Scholar
Shi, Y., Inoue, H., Wu, J. C. & Yamanaka, S. Induced pluripotent stem cell technology: a decade of progress. Nat. Rev. Drug Discov. 16 , 115–130 (2017).
Article CAS PubMed Google Scholar
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131 , 861–872 (2007).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126 , 663–676 (2006).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318 , 1917–1920 (2007).
Takahashi, K. & Yamanaka, S. A decade of transcription factor-mediated reprogramming to pluripotency. Nat. Rev. Mol. Cell Biol. 17 , 183–193 (2016).
Breschi, A., Gingeras, T. R. & Guigo, R. Comparative transcriptomics in human and mouse. Nat. Rev. Genet. 18 , 425–440 (2017).
Gharib, W. H. & Robinson-Rechavi, M. When orthologs diverge between human and mouse. Brief. Bioinform. 12 , 436–441 (2011).
Article PubMed PubMed Central Google Scholar
Lynch, V. J. Use with caution: developmental systems divergence and potential pitfalls of animal models. Yale J. Biol. Med. 82 , 53–66 (2009).
CAS PubMed PubMed Central Google Scholar
Takebe, T. & Wells, J. M. Organoids by design. Science 364 , 956–959 (2019).
Yamanaka, S. Pluripotent Stem Cell-based Cell Therapy- Promise And Challenges. Cell Stem Cell 27 , 523–531 (2020).
Gurdon, J. B. The generation of diversity and pattern in animal development. Cell 68 , 185–199 (1992).
Kiefer, J. C. Epigenetics in development. Dev. Dyn. 236 , 1144–1156 (2007).
Tompkins, J. D. Discovering DNA methylation, the history and future of the writing on DNA. J. Hist. Biol. 55 , 865–887 (2022).
PubMed PubMed Central Google Scholar
Roe, S. A. Matter, life, and generation: eighteen-century embryology and the Haller-Wolff Debate , (Cambridge University Press, 1981).
Kilgour, F. G. William Harvey and his contributions. Circulation 23 , 286–296 (1961).
Aulie, R. P. Caspar Friedrich Wolff and his ‘Theoria generationis’, 1759. J. Hist. Med. Allied Sci. 16 , 124–144 (1961).
Weismann, A. Das Keimplasma; eine Theorie der Vererbung, (Jena, Fischer, 1892).
Waddington, C. H. The Strategy of the Genes; A Discussion of Some Aspects of Theoretical Biology , (Cambridge: Cambridge University Press, 1957).
Nanney, D. L. Epigenetic control systems. Proc. Natl. Acad. Sci. USA 44 , 712–717 (1958).
Gurdon, J. B. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. J. Embryol. Exp. Morphol. 10 , 622–640 (1962).
CAS PubMed Google Scholar
Gurdon, J. B. The transplantation of nuclei between two species of Xenopus. Dev. Biol. 5 , 68–83 (1962).
Gurdon, J. B. Adult frogs derived from the nuclei of single somatic cells. Dev. Biol. 4 , 256–273 (1962).
Gurdon, J. B. Multiple genetically identical frogs. J. Hered. 53 , 5–9 (1962).
Gurdon, J. B., Elsdale, T. R. & Fischberg, M. Sexually mature individuals of Xenopus laevis from the transplantation of single somatic nuclei. Nature 182 , 64–65 (1958).
Jeltsch, A. & Jurkowska, R. Z. New concepts in DNA methylation. Trends Biochem. Sci. 39 , 310–318 (2014).
Riggs, A. D. X inactivation, differentiation, and DNA methylation. Cytogenet. Cell Genet. 14 , 9–25 (1975).
Robertson, K. D. & Wolffe, A. P. DNA methylation in health and disease. Nat. Rev. Genet. 1 , 11–19 (2000).
Schubeler, D. Function and information content of DNA methylation. Nature 517 , 321–326 (2015).
Evans, M. J. & Kaufman, M. H. Establishment in culture of pluripotential cells from mouse embryos. Nature 292 , 154–156 (1981).
Martin, G. R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA 78 , 7634–7638 (1981).
Thomson, J. A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282 , 1145–1147 (1998).
Tada, M., Takahama, Y., Abe, K., Nakatsuji, N. & Tada, T. Nuclear reprogramming of somatic cells by in vitro hybridization with ES cells. Curr. Biol. 11 , 1553–1558 (2001).
Cowan, C. A., Atienza, J., Melton, D. A. & Eggan, K. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science 309 , 1369–1373 (2005).
Davis, R. L., Weintraub, H. & Lassar, A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell 51 , 987–1000 (1987).
Halder, G., Callaerts, P. & Gehring, W. J. Induction of ectopic eyes by targeted expression of the eyeless gene in drosophila. Science 267 , 1788–1792 (1995).
Kulessa, H., Frampton, J. & Graf, T. Gata-1 reprograms Avian Myelomonocytic cell-lines into Eosinophils, Thromboblasts, and Erythroblasts. Gene Dev. 9 , 1250–1262 (1995).
Xie, H., Ye, M., Feng, R. & Graf, T. Stepwise reprogramming of B cells into macrophages. Cell 117 , 663–676 (2004).
Wernig, M. et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 448 , 318–324 (2007).
Huangfu, D. W. et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat. Biotechnol. 26 , 795–797 (2008).
Huangfu, D. et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat. Biotechnol. 26 , 1269–1275 (2008).
Hou, P. et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science 341 , 651–654 (2013).
Zhu, J. et al. Genome-wide chromatin state transitions associated with developmental and environmental cues. Cell 152 , 642–654 (2013).
Apostolou, E. & Hochedlinger, K. Chromatin dynamics during cellular reprogramming. Nature 502 , 462–471 (2013).
Apostolou, E. & Stadtfeld, M. Cellular trajectories and molecular mechanisms of iPSC reprogramming. Curr. Opin. Genet. Dev. 52 , 77–85 (2018).
Cacchiarelli, D. et al. Integrative analyses of human reprogramming reveal dynamic nature of induced pluripotency. Cell 162 , 412–424 (2015).
Nefzger, C. M. et al. Cell type of origin dictates the route to pluripotency. Cell Rep. 21 , 2649–2660 (2017).
Borkent, M. et al. A serial shRNA screen for roadblocks to reprogramming identifies the protein modifier SUMO2. Stem Cell Rep. 6 , 704–716 (2016).
Article CAS Google Scholar
Buckley, S. M. et al. Regulation of Pluripotency and cellular reprogramming by the ubiquitin-proteasome system. Cell Stem Cell 11 , 783–798 (2012).
Qin, H. et al. Systematic identification of barriers to human iPSC generation. Cell 158 , 449–461 (2014).
Simic, M. S. et al. Transient activation of the UPR(ER) is an essential step in the acquisition of pluripotency during reprogramming. Sci. Adv. 5 , eaaw0025 (2019).
Wu, Y. et al. Phospholipid remodeling is critical for stem cell pluripotency by facilitating mesenchymal-to-epithelial transition. Sci. Adv. 5 , eaax7525 (2019).
Pei, D. Q., Shu, X. D., Gassama-Diagne, A. & Thiery, J. P. Mesenchymal-epithelial transition in development and reprogramming. Nat. Cell Biol. 21 , 44–53 (2019).
Soufi, A., Donahue, G. & Zaret, K. S. Facilitators and impediments of the pluripotency reprogramming factors’ initial engagement with the genome. Cell 151 , 994–1004 (2012).
Soufi, A. et al. Pioneer transcription factors target partial DNA Motifs on nucleosomes to initiate reprogramming. Cell 161 , 555–568 (2015).
Chen, J. et al. Hierarchical Oct4 binding in concert with primed epigenetic rearrangements during somatic cell reprogramming. Cell Rep. 14 , 1540–1554 (2016).
Chronis, C. et al. Cooperative binding of transcription factors orchestrates reprogramming. Cell 168 , 442–459.e420 (2017).
Zaret, K. S. & Carroll, J. S. Pioneer transcription factors: establishing competence for gene expression. Gene Dev. 25 , 2227–2241 (2011).
Vanzan, L. et al. High throughput screening identifies SOX2 as a super pioneer factor that inhibits DNA methylation maintenance at its binding sites. Nat. Commun. 12 , 3337 (2021).
Roberts, G. A. et al. Dissecting OCT4 defines the role of nucleosome binding in pluripotency. Nat. Cell Biol. 23 , 834–845 (2021).
Di Giammartino, D. C. et al. KLF4 is involved in the organization and regulation of pluripotency-associated three-dimensional enhancer networks. Nat. Cell Biol. 21 , 1179–1190 (2019).
Rahl, P. B. et al. c-Myc regulates transcriptional pause release. Cell 141 , 432–445 (2010).
Garcia-Gutierrez, L., Delgado, M. D. & Leon, J. MYC oncogene contributions to release of cell cycle brakes. Genes 10 , 244 (2019).
Smith, Z. D., Sindhu, C. & Meissner, A. Molecular features of cellular reprogramming and development. Nat. Rev. Mol. Cell Biol. 17 , 139–154 (2016).
Deng, W., Jacobson, E. C., Collier, A. J. & Plath, K. The transcription factor code in iPSC reprogramming. Curr. Opin. Genet. Dev. 70 , 89–96 (2021).
Hernandez, C. et al. Dppa2/4 facilitate epigenetic remodeling during reprogramming to pluripotency. Cell Stem Cell 23 , 396–411.e398 (2018).
Liu, J. et al. The oncogene c-Jun impedes somatic cell reprogramming. Nat. Cell Biol. 17 , 856–867 (2015).
Markov, G. J. et al. AP-1 is a temporally regulated dual gatekeeper of reprogramming to pluripotency. Proc. Natl. Acad. Sci. USA 118 , e2104841118 (2021).
Silva, J. et al. Nanog is the gateway to the pluripotent ground state. Cell 138 , 722–737 (2009).
Nakagawa, M. et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 26 , 101–106 (2008).
Mai, T. et al. NKX3-1 is required for induced pluripotent stem cell reprogramming and can replace OCT4 in mouse and human iPSC induction. Nat. Cell Biol. 20 , 900–908 (2018).
Kim, J. B. et al. Direct reprogramming of human neural stem cells by OCT4. Nature 461 , 649–653 (2009).
Kim, J. B. et al. Oct4-induced pluripotency in adult neural stem cells. Cell 136 , 411–419 (2009).
Radzisheuskaya, A. & Silva, J. C. Do all roads lead to Oct4? the emerging concepts of induced pluripotency. Trends Cell Biol. 24 , 275–284 (2014).
Li, D. et al. Chromatin accessibility dynamics during iPSC reprogramming. Cell Stem Cell 21 , 819–833.e816 (2017).
Xing, Q. R. et al. Diversification of reprogramming trajectories revealed by parallel single-cell transcriptome and chromatin accessibility sequencing. Sci. Adv. 6 , eaba1190 (2020).
Stadhouders, R. et al. Transcription factors orchestrate dynamic interplay between genome topology and gene regulation during cell reprogramming. Nat. Genet. 50 , 238–249 (2018).
Knaupp, A. S. et al. Transient and permanent reconfiguration of chromatin and transcription factor occupancy drive reprogramming. Cell Stem Cell 21 , 834–845.e836 (2017).
Cheloufi, S. et al. The histone chaperone CAF-1 safeguards somatic cell identity. Nature 528 , 218–224 (2015).
dos Santos, R. L. et al. MBD3/NuRD facilitates induction of pluripotency in a context-dependent manner. Cell Stem Cell 15 , 102–110 (2014).
Onder, T. T. et al. Chromatin-modifying enzymes as modulators of reprogramming. Nature 483 , 598–602 (2012).
Chen, J. et al. H3K9 methylation is a barrier during somatic cell reprogramming into iPSCs. Nat. Genet. 45 , 34–42 (2013).
Sridharan, R. et al. Proteomic and genomic approaches reveal critical functions of H3K9 methylation and heterochromatin protein-1gamma in reprogramming to pluripotency. Nat. Cell Biol. 15 , 872–882 (2013).
Li, L. P. et al. Glis1 facilitates induction of pluripotency via an epigenome-metabolome-epigenome signalling cascade (vol 2, pg 882, 2020). Nat. Metab. 2 , 1179–1179 (2020).
Article PubMed Google Scholar
Tran, K. A. et al. Defining reprogramming checkpoints from single-cell analyses of induced pluripotency. Cell Rep. 27 , 1726–1741.e1725 (2019).
Sun, G., Fu, C., Shen, C. & Shi, Y. Histone deacetylases in neural stem cells and induced pluripotent stem cells. J. Biomed. Biotechnol. 2011 , 835968 (2011).
Yin, Y. et al. Impact of cytosine methylation on DNA binding specificities of human transcription factors. Science 356 , eaaj2239 (2017).
Lyko, F. The DNA methyltransferase family: a versatile toolkit for epigenetic regulation. Nat. Rev. Genet. 19 , 81–92 (2018).
Pastor, W. A., Aravind, L. & Rao, A. TETonic shift: biological roles of TET proteins in DNA demethylation and transcription. Nat. Rev. Mol. Cell Biol. 14 , 341–356 (2013).
Piccolo, F. M. & Fisher, A. G. Getting rid of DNA methylation. Trends Cell Biol. 24 , 136–143 (2014).
Rasmussen, K. D. & Helin, K. Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev. 30 , 733–750 (2016).
Caldwell, B. A. et al. Functionally distinct roles for TET-oxidized 5-methylcytosine bases in somatic reprogramming to pluripotency. Mol. Cell 81 , 859–869.e858 (2021).
Doege, C. A. et al. Early-stage epigenetic modification during somatic cell reprogramming by Parp1 and Tet2. Nature 488 , 652–655 (2012).
Sardina, J. L. et al. Transcription factors drive Tet2-mediated enhancer demethylation to reprogram cell fate. Cell Stem Cell 23 , 727–741.e729 (2018).
Zviran, A. et al. Deterministic somatic cell reprogramming involves continuous transcriptional changes governed by Myc and epigenetic-driven modules. Cell Stem Cell 24 , 328–341.e329 (2019).
Hu, X. et al. Tet and TDG mediate DNA demethylation essential for mesenchymal-to-epithelial transition in somatic cell reprogramming. Cell Stem Cell 14 , 512–522 (2014).
Costa, Y. et al. NANOG-dependent function of TET1 and TET2 in establishment of pluripotency. Nature 495 , 370–374 (2013).
Gao, Y. W. et al. Replacement of Oct4 by Tet1 during iPSC induction reveals an important role of DNA Methylation and Hydroxymethylation in reprogramming. Cell Stem Cell 12 , 453–469 (2013).
Chen, J. et al. Vitamin C modulates TET1 function during somatic cell reprogramming. Nat. Genet. 45 , 1504–1509 (2013).
Guo, L. et al. Resolving cell fate decisions during somatic cell reprogramming by single-cell RNA-Seq. Mol. Cell 73 , 815–829.e817 (2019).
Shakiba, N. et al. Cell competition during reprogramming gives rise to dominant clones. Science 364 , eaan0925 (2019).
Francesconi, M. et al. Single cell RNA-seq identifies the origins of heterogeneity in efficient cell transdifferentiation and reprogramming. Elife 8 , e41627 (2019).
Schwarz, B. A. et al. Prospective Isolation of Poised iPSC intermediates reveals principles of cellular reprogramming. Cell Stem Cell 23 , 289–305.e285 (2018).
Bar-Nur, O., Russ, H. A., Efrat, S. & Benvenisty, N. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell 9 , 17–23 (2011).
Kim, K. et al. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat. Biotechnol. 29 , 1117–1119 (2011).
Marchetto, M. C. et al. Transcriptional signature and memory retention of human-induced pluripotent stem cells. PLoS One 4 , e7076 (2009).
Ohi, Y. et al. Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells. Nat. Cell Biol. 13 , 541–549 (2011).
Kim, K. et al. Epigenetic memory in induced pluripotent stem cells. Nature 467 , 285–290 (2010).
Rouhani, F. J. et al. Substantial somatic genomic variation and selection for BCOR mutations in human induced pluripotent stem cells. Nat. Genet. 54 , 1406–1416 (2022).
Wei, W., Gaffney, D. J. & Chinnery, P. F. Cell reprogramming shapes the mitochondrial DNA landscape. Nat. Commun. 12 , 5241 (2021).
Deuse, T. et al. De novo mutations in mitochondrial DNA of iPSCs produce immunogenic neoepitopes in mice and humans. Nat. Biotechnol. 37 , 1137–1144 (2019).
Narsinh, K. H. et al. Single cell transcriptional profiling reveals heterogeneity of human induced pluripotent stem cells. J. Clin. Investig. 121 , 1217–1221 (2011).
Malik, N. & Rao, M. S. A review of the methods for human iPSC derivation. Methods Mol. Biol. 997 , 23–33 (2013).
Manzini, S., Viiri, L. E., Marttila, S. & Aalto-Setala, K. A comparative view on easy to deploy non-integrating methods for patient-specific iPSC production. Stem Cell Rev. Rep. 11 , 900–908 (2015).
Scesa, G., Adami, R. & Bottai, D. iPSC preparation and epigenetic memory: does the tissue origin matter? Cells 10 , 1470 (2021).
Macarthur, C. C. et al. Generation of human-induced pluripotent stem cells by a nonintegrating RNA Sendai virus vector in feeder-free or xeno-free conditions. Stem Cells Int. 2012 , 564612 (2012).
Seki, T. et al. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell 7 , 11–14 (2010).
Zhou, W. B. & Freed, C. R. Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells. Stem Cells 27 , 2667–2674 (2009).
Haridhasapavalan, K. K. et al. An insight into non-integrative gene delivery approaches to generate transgene-free induced pluripotent stem cells. Gene 686 , 146–159 (2019).
Kaji, K. et al. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature 458 , 771–775 (2009).
Woltjen, K. et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature 458 , 766–770 (2009).
Chao, J. et al. Therapeutic development for Canavan disease using patient iPSCs introduced with the wild-type ASPA gene. iScience 25 , 104391 (2022).
Feng, L. et al. Cell-based therapy for canavan disease using human iPSC-Derived NPCs and OPCs. Adv. Sci. 7 , 2002155 (2020).
Warren, L. et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7 , 618–630 (2010).
Wen, W. et al. Enhanced generation of integration-free iPSCs from human adult peripheral blood mononuclear cells with an optimal combination of episomal vectors. Stem Cell Rep. 6 , 873–884 (2016).
Anokye-Danso, F. et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell 8 , 376–388 (2011).
Miyoshi, N. et al. Reprogramming of mouse and human cells to pluripotency using mature MicroRNAs. Cell Stem Cell 8 , 633–638 (2011).
Kim, Y., Jeong, J. & Choi, D. Small-molecule-mediated reprogramming: a silver lining for regenerative medicine. Exp. Mol. Med. 52 , 213–226 (2020).
Liuyang, S. et al. Highly efficient and rapid generation of human pluripotent stem cells by chemical reprogramming. Cell Stem Cell 30 , 450–459.e459 (2023).
Guan, J. et al. Chemical reprogramming of human somatic cells to pluripotent stem cells. Nature 605 , 325–331 (2022).
Li, W. et al. Identification of Oct4-activating compounds that enhance reprogramming efficiency. Proc. Natl. Acad. Sci. USA 109 , 20853–20858 (2012).
Zhu, S. et al. Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell 7 , 651–655 (2010).
Lin, T. et al. A chemical platform for improved induction of human iPSCs. Nat. Methods 6 , 805–808 (2009).
Cao, S. et al. Chromatin accessibility dynamics during chemical induction of pluripotency. Cell Stem Cell 22 , 529–542.e525 (2018).
Zhao, Y. et al. A XEN-like state bridges somatic cells to pluripotency during chemical reprogramming. Cell 163 , 1678–1691 (2015).
Velychko, S. et al. Excluding Oct4 from Yamanaka cocktail unleashes the developmental potential of iPSCs. Cell Stem Cell 25 , 737–753.e734 (2019).
Shi, Y. Induced pluripotent stem cells, new tools for drug discovery and new hope for stem cell therapies. Curr. Mol. Pharm. 2 , 15–18 (2009).
Lo, B. & Parham, L. Ethical issues in stem cell research. Endocr. Rev. 30 , 204–213 (2009).
Robertson, J. A. Human embryonic stem cell research: ethical and legal issues. Nat. Rev. Genet. 2 , 74–78 (2001).
Fernandopulle, M. S. et al. Transcription factor–mediated differentiation of human iPSCs into neurons. Curr. Protoc. Cell Biol. 79 , e51 (2018).
Lin, Y. & Zou, J. Differentiation of cardiomyocytes from human pluripotent stem cells in fully chemically defined conditions. STAR Protoc. 1 , 100015 (2020).
Iriguchi, S. et al. A clinically applicable and scalable method to regenerate T-cells from iPSCs for off-the-shelf T-cell immunotherapy. Nat. Commun. 12 , 430 (2021).
Douvaras, P. & Fossati, V. Generation and isolation of oligodendrocyte progenitor cells from human pluripotent stem cells. Nat. Protoc. 10 , 1143–1154 (2015).
Li, L. et al. GFAP mutations in astrocytes impair oligodendrocyte progenitor proliferation and Myelination in an hiPSC model of alexander disease. Cell Stem Cell 23 , 239–251.e236 (2018).
Wang, S. et al. Human iPSC-derived oligodendrocyte progenitor cells can Myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell 12 , 252–264 (2013).
Hurley, K. et al. Reconstructed single-cell fate trajectories define lineage plasticity windows during differentiation of human PSC-derived distal lung progenitors. Cell Stem Cell 26 , 593–608.e598 (2020).
Joung, J. et al. A transcription factor atlas of directed differentiation. Cell 186 , 209–229.e226 (2023).
Li, Q. V. et al. Genome-scale screens identify JNK-JUN signaling as a barrier for pluripotency exit and endoderm differentiation. Nat. Genet. 51 , 999–1010 (2019).
Washer, S. J. et al. Single-cell transcriptomics defines an improved, validated monoculture protocol for differentiation of human iPSC to microglia. Sci. Rep. 12 , 19454 (2022).
Zheng, H. et al. Generating hematopoietic cells from human pluripotent stem cells: approaches, progress and challenges. Cell Regen. 12 , 31 (2023).
Pratumkaew, P., Issaragrisil, S. & Luanpitpong, S. Induced pluripotent stem cells as a tool for modeling hematologic disorders and as a potential source for cell-based therapies. Cells 10 , 3250 (2021).
Chambers, S. M. et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 27 , 275–280 (2009).
Qi, Y. et al. Combined small-molecule inhibition accelerates the derivation of functional cortical neurons from human pluripotent stem cells. Nat. Biotechnol. 35 , 154–163 (2017).
Drager, N. M. et al. A CRISPRi/a platform in human iPSC-derived microglia uncovers regulators of disease states. Nat. Neurosci. 25 , 1149–1162 (2022).
Leng, K. et al. CRISPRi screens in human iPSC-derived astrocytes elucidate regulators of distinct inflammatory reactive states. Nat. Neurosci. 25 , 1528–1542 (2022).
Tian, R. et al. Genome-wide CRISPRi/a screens in human neurons link lysosomal failure to ferroptosis. Nat. Neurosci. 24 , 1020–1034 (2021).
Tian, R. et al. CRISPR interference-based platform for multimodal genetic screens in human iPSC-derived neurons. Neuron 104 , 239–255.e212 (2019).
Guttikonda, S. R. et al. Fully defined human pluripotent stem cell-derived microglia and tri-culture system model C3 production in Alzheimer’s disease. Nat. Neurosci. 24 , 343–354 (2021).
Park, J. et al. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat. Neurosci. 21 , 941–951 (2018).
Kim, J., Koo, B. K. & Knoblich, J. A. Human organoids: model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 21 , 571–584 (2020).
Schutgens, F. & Clevers, H. Human organoids: tools for understanding biology and treating diseases. Annu Rev. Pathol. 15 , 211–234 (2020).
Hofer, M. & Lutolf, M. P. Engineering organoids. Nat. Rev. Mater. 6 , 402–420 (2021).
Corsini, N. S. & Knoblich, J. A. Human organoids: new strategies and methods for analyzing human development and disease. Cell 185 , 2756–2769 (2022).
Rossi, G., Manfrin, A. & Lutolf, M. P. Progress and potential in organoid research. Nat. Rev. Genet. 19 , 671–687 (2018).
Cederquist, G. Y. et al. Specification of positional identity in forebrain organoids. Nat. Biotechnol. 37 , 436–444 (2019).
Morizane, R. et al. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat. Biotechnol. 33 , 1193–1200 (2015).
Bershteyn, M. et al. Human iPSC-derived cerebral organoids model cellular features of lissencephaly and reveal prolonged mitosis of outer radial Glia. Cell Stem Cell 20 , 435–449.e434 (2017).
Qian, X. et al. Sliced human cortical organoids for modeling distinct cortical layer formation. Cell Stem Cell 26 , 766–781.e769 (2020).
Abbott, J. et al. Generation and characterization of NGLY1 patient-derived midbrain organoids. Front Cell Dev. Biol. 11 , 1039182 (2023).
Sabate-Soler, S. et al. Microglia integration into human midbrain organoids leads to increased neuronal maturation and functionality. Glia 70 , 1267–1288 (2022).
Jacob, F. et al. Human pluripotent stem cell-derived neural cells and brain organoids reveal SARS-CoV-2 neurotropism predominates in choroid plexus epithelium. Cell Stem Cell 27 , 937–950.e939 (2020).
Ballabio, C. et al. Modeling medulloblastoma in vivo and with human cerebellar organoids. Nat. Commun. 11 , 583 (2020).
van Essen, M. J. et al. PTCH1-mutant human cerebellar organoids exhibit altered neural development and recapitulate early medulloblastoma tumorigenesis. Dis. Model Mech . 17 , dmm050323 (2024).
Gabriel, E. et al. Human brain organoids assemble functionally integrated bilateral optic vesicles. Cell Stem Cell 28 , 1740–1757.e1748 (2021).
Gagliardi, G. et al. Characterization and transplantation of CD73-positive photoreceptors isolated from human iPSC-derived retinal organoids. Stem Cell Rep. 11 , 665–680 (2018).
Lane, A. et al. Modeling and rescue of RP2 Retinitis Pigmentosa using iPSC-derived retinal organoids. Stem Cell Rep. 15 , 67–79 (2020).
Del Dosso, A., Urenda, J. P., Nguyen, T. & Quadrato, G. Upgrading the physiological relevance of human brain organoids. Neuron 107 , 1014–1028 (2020).
Di Lullo, E. & Kriegstein, A. R. The use of brain organoids to investigate neural development and disease. Nat. Rev. Neurosci. 18 , 573–584 (2017).
Cerneckis, J. & Shi, Y. Myelin organoids for the study of Alzheimer’s disease. Front. Neurosci. 17 , 1283742 (2023).
Feng, L. et al. Developing a human iPSC-derived three-dimensional myelin spheroid platform for modeling myelin diseases. iScience 26 , 108037 (2023).
Broda, T. R., McCracken, K. W. & Wells, J. M. Generation of human antral and fundic gastric organoids from pluripotent stem cells. Nat. Protoc. 14 , 28–50 (2019).
McCracken, K. W. et al. Wnt/beta-catenin promotes gastric fundus specification in mice and humans. Nature 541 , 182–187 (2017).
Kanton, S. & Pasca, S. P. Human assembloids. Development 149 , dev201120 (2022).
Pasca, S. P. Assembling human brain organoids. Science 363 , 126–127 (2019).
Pasca, S. P. et al. A nomenclature consensus for nervous system organoids and assembloids. Nature 609 , 907–910 (2022).
Martins, J. M. F. et al. Self-organizing 3D human trunk neuromuscular organoids. Cell Stem Cell 26 , 172–186.e176 (2020).
Article Google Scholar
Andersen, J. et al. Generation of functional Human 3D Cortico-Motor Assembloids. Cell 183 , 1913–1929.e1926 (2020).
Leung, C. M. et al. A guide to the organ-on-a-chip. Nat. Rev. Methods Prim. 2 , 33 (2022).
Ma, C., Peng, Y., Li, H. & Chen, W. Organ-on-a-Chip: a new paradigm for drug development. Trends Pharm. Sci. 42 , 119–133 (2021).
Wu, Q. et al. Organ-on-a-chip: recent breakthroughs and future prospects. Biomed. Eng. Online 19 , 1–9 (2020).
Zhang, B. Y., Korolj, A., Lai, B. F. L. & Radisic, M. Advances in organ-on-a-chip engineering. Nat. Rev. Mater. 3 , 257–278 (2018).
Low, L. A., Mummery, C., Berridge, B. R., Austin, C. P. & Tagle, D. A. Organs-on-chips: into the next decade. Nat. Rev. Drug Discov. 20 , 345–361 (2021).
Tavakol, D. N., Fleischer, S. & Vunjak-Novakovic, G. Harnessing organs-on-a-chip to model tissue regeneration. Cell Stem Cell 28 , 993–1015 (2021).
Vunjak-Novakovic, G., Ronaldson-Bouchard, K. & Radisic, M. Organs-on-a-chip models for biological research. Cell 184 , 4597–4611 (2021).
van der Helm, M. W., van der Meer, A. D., Eijkel, J. C., van den Berg, A. & Segerink, L. I. Microfluidic organ-on-chip technology for blood-brain barrier research. Tissue Barriers 4 , e1142493 (2016).
Zakharova, M. et al. Multiplexed blood-brain barrier organ-on-chip. Lab Chip 20 , 3132–3143 (2020).
Sone, N. et al. Multicellular modeling of ciliopathy by combining iPS cells and microfluidic airway-on-a-chip technology. Sci. Transl. Med 13 , eabb1298 (2021).
Vatine, G. D. et al. Human iPSC-derived blood-brain barrier chips enable disease modeling and personalized medicine applications. Cell Stem Cell 24 , 995–1005.e1006 (2019).
Michas, C. et al. Engineering a living cardiac pump on a chip using high-precision fabrication. Sci. Adv. 8 , eabm3791 (2022).
Zhao, Y. et al. A platform for generation of chamber-specific cardiac tissues and disease modeling. Cell 176 , 913–927.e918 (2019).
Shultz, L. D. et al. Humanized mouse models of immunological diseases and precision medicine. Mamm. Genome 30 , 123–142 (2019).
Flahou, C., Morishima, T., Takizawa, H. & Sugimoto, N. Fit-for-all iPSC-derived cell therapies and their evaluation in humanized mice with NK cell immunity. Front. Immunol. 12 , 662360 (2021).
Moquin-Beaudry, G. et al. Autologous humanized mouse models of iPSC-derived tumors enable characterization and modulation of cancer-immune cell interactions. Cell Rep. Methods 2 , 100153 (2022).
Zeleniak, A. et al. De novo construction of T cell compartment in humanized mice engrafted with iPSC-derived thymus organoids. Nat. Methods 19 , 1306–1319 (2022).
Sharma, A., Sances, S., Workman, M. J. & Svendsen, C. N. Multi-lineage human iPSC-derived platforms for disease modeling and drug discovery. Cell Stem Cell 26 , 309–329 (2020).
Abud, E. M. et al. iPSC-derived human microglia-like cells to study neurological diseases. Neuron 94 , 278–293.e279 (2017).
Fattorelli, N. et al. Stem-cell-derived human microglia transplanted into mouse brain to study human disease. Nat. Protoc. 16 , 1013–1033 (2021).
Hasselmann, J. et al. Development of a chimeric model to study and manipulate human microglia in vivo. Neuron 103 , 1016–1033.e1010 (2019).
Svoboda, D. S. et al. Human iPSC-derived microglia assume a primary microglia-like state after transplantation into the neonatal mouse brain. Proc. Natl. Acad. Sci. USA 116 , 25293–25303 (2019).
Xu, R. et al. Human iPSC-derived mature microglia retain their identity and functionally integrate in the chimeric mouse brain. Nat. Commun. 11 , 1577 (2020).
Wimmer, R. A. et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature 565 , 505–510 (2019).
Ho, R. et al. ALS disrupts spinal motor neuron maturation and aging pathways within gene co-expression networks. Nat. Neurosci. 19 , 1256–1267 (2016).
Alvarez, Z. et al. Artificial extracellular matrix scaffolds of mobile molecules enhance maturation of human stem cell-derived neurons. Cell Stem Cell 30 , 219–238.e214 (2023).
Yoshida, S. et al. Maturation of human induced pluripotent stem cell-derived cardiomyocytes by soluble factors from human mesenchymal stem cells. Mol. Ther. 26 , 2681–2695 (2018).
Giacomelli, E. et al. Human-iPSC-derived cardiac stromal cells enhance maturation in 3D cardiac microtissues and reveal non-cardiomyocyte contributions to heart disease. Cell Stem Cell 26 , 862–879.e811 (2020).
Maoz, B. M. et al. A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nat. Biotechnol. 36 , 865–874 (2018).
Hayashi, R. et al. Generation of 3D lacrimal gland organoids from human pluripotent stem cells. Nature 605 , 126–131 (2022).
Mansour, A. A. et al. An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol. 36 , 432–441 (2018).
Munera, J. O. et al. Differentiation of human pluripotent stem cells into colonic organoids via transient activation of BMP signaling. Cell Stem Cell 21 , 51–64.e56 (2017).
Revah, O. et al. Maturation and circuit integration of transplanted human cortical organoids. Nature 610 , 319–326 (2022).
Tanaka, J. et al. Human induced pluripotent stem cell-derived salivary gland organoids model SARS-CoV-2 infection and replication. Nat. Cell Biol. 24 , 1595–1605 (2022).
Schafer, S. T. et al. An in vivo neuroimmune organoid model to study human microglia phenotypes. Cell 186 , 2111–2126.e2120 (2023).
Cerneckis, J. & Shi, Y. Context matters: hPSC-derived microglia thrive in a humanized brain environment in vivo. Cell Stem Cell 30 , 909–910 (2023).
Ronaldson-Bouchard, K. et al. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 556 , 239–243 (2018).
Tu, C. Y., Chao, B. S. & Wu, J. C. Strategies for improving the maturity of human induced pluripotent stem cell-derived cardiomyocytes. Circ. Res. 123 , 512–514 (2018).
Luo, J. et al. Tissue-engineered vascular grafts with advanced mechanical strength from human iPSCs. Cell Stem Cell 26 , 251–261.e258 (2020).
Ronaldson-Bouchard, K. et al. Engineering of human cardiac muscle electromechanically matured to an adult-like phenotype. Nat. Protoc. 14 , 2781–2817 (2019).
Shin, D. et al. Thalamocortical organoids enable in vitro modeling of 22q11.2 microdeletion associated with neuropsychiatric disorders. Cell Stem Cell 31 , 421–432.e428 (2024).
Regev, A. et al. The Human Cell Atlas. Elife 6 , e27041 (2017).
Rozenblatt-Rosen, O., Stubbington, M. J. T., Regev, A. & Teichmann, S. A. The human cell atlas: from vision to reality. Nature 550 , 451–453 (2017).
Zheng, Y. et al. Controlled modelling of human epiblast and amnion development using stem cells. Nature 573 , 421–425 (2019).
Sasaki, K. et al. Robust In vitro induction of human germ cell fate from pluripotent stem cells. Cell Stem Cell 17 , 178–194 (2015).
Hayashi, M., Kawaguchi, T., Durcova-Hills, G. & Imai, H. Generation of germ cells from pluripotent stem cells in mammals. Reprod. Med. Biol. 17 , 107–114 (2018).
Esfahani, S. N. et al. Derivation of human primordial germ cell-like cells in an embryonic-like culture. Nat. Commun. 15 , 167 (2024).
Matsuda, M. et al. Recapitulating the human segmentation clock with pluripotent stem cells. Nature 580 , 124–129 (2020).
Weatherbee, B. A. T. et al. Pluripotent stem cell-derived model of the post-implantation human embryo. Nature 622 , 584–593 (2023).
Zernicka-Goetz, M. The evolution of embryo models. Nat. Methods 20 , 1844–1848 (2023).
Manor, Y. S., Massarwa, R. & Hanna, J. H. Establishing the human naive pluripotent state. Curr. Opin. Genet. Dev. 34 , 35–45 (2015).
Weinberger, L., Ayyash, M., Novershtern, N. & Hanna, J. H. Dynamic stem cell states: naive to primed pluripotency in rodents and humans. Nat. Rev. Mol. Cell Biol. 17 , 155–169 (2016).
Zhou, J., Hu, J., Wang, Y. & Gao, S. Induction and application of human naive pluripotency. Cell Rep. 42 , 112379 (2023).
Giulitti, S. et al. Direct generation of human naive induced pluripotent stem cells from somatic cells in microfluidics. Nat. Cell Biol. 21 , 275–286 (2019).
Li, W. et al. Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell 4 , 16–19 (2009).
Sahakyan, A. et al. Human naive pluripotent stem cells Model X chromosome dampening and X inactivation. Cell Stem Cell 20 , 87–101 (2017).
Theunissen, T. W. et al. Molecular criteria for defining the naive human pluripotent state. Cell Stem Cell 19 , 502–515 (2016).
Kagawa, H. et al. Human blastoids model blastocyst development and implantation. Nature 601 , 600–605 (2022).
Wei, Y. et al. Efficient derivation of human trophoblast stem cells from primed pluripotent stem cells. Sci. Adv. 7 , eabf4416 (2021).
Castel, G. et al. Induction of human trophoblast stem cells from somatic cells and pluripotent stem cells. Cell Rep. 33 , 108419 (2020).
Jang, Y. J., Kim, M., Lee, B. K. & Kim, J. Induction of human trophoblast stem-like cells from primed pluripotent stem cells. Proc. Natl. Acad. Sci. USA 119 , e2115709119 (2022).
Earley, A. M., Burbulla, L. F., Krainc, D. & Awatramani, R. Identification of ASCL1 as a determinant for human iPSC-derived dopaminergic neurons. Sci. Rep. 11 , 22257 (2021).
Jerber, J. et al. Population-scale single-cell RNA-seq profiling across dopaminergic neuron differentiation. Nat. Genet. 53 , 304–312 (2021).
Camp, J. G. et al. Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc. Natl. Acad. Sci. USA 112 , 15672–15677 (2015).
Fleck, J. S. et al. Inferring and perturbing cell fate regulomes in human brain organoids. Nature 621 , 365–372 (2023).
Lee, J. H. et al. Production of human spinal-cord organoids recapitulating neural-tube morphogenesis. Nat. Biomed. Eng. 6 , 435–448 (2022).
Hofbauer, P. et al. Cardioids reveal self-organizing principles of human cardiogenesis. Cell 184 , 3299–3317.e3222 (2021).
Marton, R. M. & Pasca, S. P. Organoid and assembloid technologies for investigating cellular crosstalk in human brain development and disease. Trends Cell Biol. 30 , 133–143 (2020).
Koike, H. et al. Modelling human hepato-biliary-pancreatic organogenesis from the foregut-midgut boundary. Nature 574 , 112–116 (2019).
Miura, Y. et al. Generation of human striatal organoids and cortico-striatal assembloids from human pluripotent stem cells. Nat. Biotechnol. 38 , 1421–1430 (2020).
Xiang, Y. et al. Fusion of regionally specified hPSC-derived organoids models human brain development and interneuron migration. Cell Stem Cell 21 , 383–398.e387 (2017).
Soldner, F. & Jaenisch, R. iPSC disease modeling. Science 338 , 1155–1156 (2012).
Li, L., Chao, J. & Shi, Y. Modeling neurological diseases using iPSC-derived neural cells : iPSC modeling of neurological diseases. Cell Tissue Res. 371 , 143–151 (2018).
Israel, M. A. et al. Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature 482 , 216–220 (2012).
Kwart, D. et al. A large panel of isogenic APP and PSEN1 mutant human iPSC neurons reveals shared endosomal abnormalities mediated by APP beta-CTFs, Not Abeta. Neuron 104 , 256–270.e255 (2019).
Liu, Q. et al. Effect of potent gamma-secretase modulator in human neurons derived from multiple presenilin 1-induced pluripotent stem cell mutant carriers. JAMA Neurol. 71 , 1481–1489 (2014).
Hendriks, D., Clevers, H. & Artegiani, B. CRISPR-Cas tools and their application in genetic engineering of human stem cells and organoids. Cell Stem Cell 27 , 705–731 (2020).
Firth, A. L. et al. Functional gene correction for cystic fibrosis in lung epithelial cells generated from patient iPSCs. Cell Rep. 12 , 1385–1390 (2015).
Lin, Y. T. et al. APOE4 causes widespread molecular and cellular alterations associated with Alzheimer’s disease phenotypes in human iPSC-derived brain cell types. Neuron 98 , 1294–1294 (2018).
Liu, Z. et al. Astrocytic response mediated by the CLU risk allele inhibits OPC proliferation and myelination in a human iPSC model. Cell Rep. 42 , 112841 (2023).
Brunner, J. W. et al. Power and optimal study design in iPSC-based brain disease modelling. Mol. Psychiatry 28 , 1545–1556 (2023).
Kondo, T. et al. Dissection of the polygenic architecture of neuronal Aβ production using a large sample of individual iPSC lines derived from Alzheimer’s disease patients. Nat. Aging 2 , 125–139 (2022).
Kimura, M. et al. En masse organoid phenotyping informs metabolic-associated genetic susceptibility to NASH. Cell 185 , 4216–4232.e4216 (2022).
Park, J. C. et al. A logical network-based drug-screening platform for Alzheimer’s disease representing pathological features of human brain organoids. Nat. Commun. 12 , 280 (2021).
Parenti, I., Rabaneda, L. G., Schoen, H. & Novarino, G. Neurodevelopmental disorders: from genetics to functional pathways. Trends Neurosci. 43 , 608–621 (2020).
Thapar, A., Cooper, M. & Rutter, M. Neurodevelopmental disorders. Lancet Psychiatry 4 , 339–346 (2017).
Fang, R. et al. Conservation and divergence of cortical cell organization in human and mouse revealed by MERFISH. Science 377 , 56–62 (2022).
Hodge, R. D. et al. Conserved cell types with divergent features in human versus mouse cortex. Nature 573 , 61–68 (2019).
Pembroke, W. G., Hartl, C. L. & Geschwind, D. H. Evolutionary conservation and divergence of the human brain transcriptome. Genome Biol. 22 , 1–33 (2021).
Zhu, Y. et al. Spatiotemporal transcriptomic divergence across human and macaque brain development. Science 362 , eaat8077 (2018).
Li, L. & Shi, Y. When glia meet induced pluripotent stem cells (iPSCs). Mol. Cell Neurosci. 109 , 103565 (2020).
Shao, Z. et al. Dysregulated protocadherin-pathway activity as an intrinsic defect in induced pluripotent stem cell-derived cortical interneurons from subjects with schizophrenia. Nat. Neurosci. 22 , 229–242 (2019).
Szabo, A. et al. A human iPSC-astroglia neurodevelopmental model reveals divergent transcriptomic patterns in schizophrenia. Transl. Psychiatry 11 , 554 (2021).
Topol, A. et al. Dysregulation of miRNA-9 in a subset of schizophrenia patient-derived neural progenitor cells. Cell Rep. 15 , 1024–1036 (2016).
Yoon, K. J. et al. Modeling a genetic risk for schizophrenia in iPSCs and mice reveals neural stem cell deficits associated with adherens junctions and polarity. Cell Stem Cell 15 , 79–91 (2014).
Murai, K. et al. The TLX-miR-219 cascade regulates neural stem cell proliferation in neurodevelopment and schizophrenia iPSC model. Nat. Commun. 7 , 10965 (2016).
Schafer, S. T. et al. Pathological priming causes developmental gene network heterochronicity in autistic subject-derived neurons. Nat. Neurosci. 22 , 243–255 (2019).
Wang, M. et al. Increased neural progenitor proliferation in a hiPSC model of autism induces replication stress-associated genome instability. Cell Stem Cell 26 , 221–233.e226 (2020).
Kathuria, A. et al. Synaptic deficits in iPSC-derived cortical interneurons in schizophrenia are mediated by NLGN2 and rescued by N-acetylcysteine. Transl. Psychiatry 9 , 321 (2019).
Kizner, V., Fischer, S. & Naujock, M. Multielectrode Array (MEA)-based detection of spontaneous network activity in human iPSC-derived cortical neurons. Methods Mol. Biol. 1994 , 209–216 (2019).
Sun, G. et al. Modeling human cytomegalovirus-induced microcephaly in human iPSC-derived brain organoids. Cell Rep. Med. 1 , 100002 (2020).
Brennand, K. J. et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature 473 , 221–225 (2011).
Wen, Z. et al. Synaptic dysregulation in a human iPS cell model of mental disorders. Nature 515 , 414–418 (2014).
Zaslavsky, K. et al. SHANK2 mutations associated with autism spectrum disorder cause hyperconnectivity of human neurons. Nat. Neurosci. 22 , 556–564 (2019).
Cai, H. W. et al. Brain organoid reservoir computing for artificial intelligence. Nat. Electron 6 , 1032–1039 (2023).
Kagan, B. J. et al. In vitro neurons learn and exhibit sentience when embodied in a simulated game-world. Neuron 110 , 3952–3969.e3958 (2022).
Chiaradia, I. & Lancaster, M. A. Brain organoids for the study of human neurobiology at the interface of in vitro and in vivo. Nat. Neurosci. 23 , 1496–1508 (2020).
Wang, H. Modeling neurological diseases with human brain organoids. Front. Synaptic Neurosci. 10 , 15 (2018).
Velasco, S., Paulsen, B. & Arlotta, P. 3D brain organoids: studying brain development and disease outside the embryo. Annu Rev. Neurosci. 43 , 375–389 (2020).
Cerneckis, J. & Shi, Y. Modeling brain macrophage biology and neurodegenerative diseases using human iPSC-derived neuroimmune organoids. Front. Cell Neurosci. 17 , 1198715 (2023).
Mariani, J. et al. FOXG1-dependent dysregulation of GABA/Glutamate neuron differentiation in autism spectrum disorders. Cell 162 , 375–390 (2015).
Xu, R. et al. OLIG2 drives abnormal neurodevelopmental phenotypes in human iPSC-based organoid and chimeric mouse models of down syndrome. Cell Stem Cell 24 , 908–926.e908 (2019).
Trujillo, C. A. et al. Complex oscillatory waves emerging from cortical organoids model early human brain network development. Cell Stem Cell 25 , 558–569.e557 (2019).
Passaro, A. P. & Stice, S. L. Electrophysiological analysis of brain organoids: current approaches and advancements. Front. Neurosci. 14 , 622137 (2020).
Samarasinghe, R. A. et al. Identification of neural oscillations and epileptiform changes in human brain organoids. Nat. Neurosci. 24 , 1488–1500 (2021).
Windrem, M. S. et al. Human iPSC glial mouse chimeras reveal glial contributions to schizophrenia. Cell Stem Cell 21 , 195–208.e196 (2017).
Dong, X. et al. Human cerebral organoids establish subcortical projections in the mouse brain after transplantation. Mol. Psychiatry 26 , 2964–2976 (2021).
Wilson, M. N. et al. Multimodal monitoring of human cortical organoids implanted in mice reveal functional connection with visual cortex. Nat. Commun. 13 , 7945 (2022).
Cerneckis, J., Bu, G. & Shi, Y. Pushing the boundaries of brain organoids to study Alzheimer’s disease. Trends Mol. Med . 29 , 659-672 (2023).
Dugger, B. N. & Dickson, D. W. Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 9 , a028035 (2017).
Hardiman, O. et al. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Prim. 3 , 1–19 (2017).
Google Scholar
Kalia, L. V. & Lang, A. E. Parkinson’s disease. Lancet 386 , 896–912 (2015).
Knopman, D. S. et al. Alzheimer disease. Nat. Rev. Dis. Prim. 7 , 33 (2021).
Gonzales, M. M. et al. Biological aging processes underlying cognitive decline and neurodegenerative disease. J. Clin. Investig. 132 , e158453 (2022).
Camandola, S. & Mattson, M. P. Brain metabolism in health, aging, and neurodegeneration. Embo J. 36 , 1474–1492 (2017).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell 186 , 243–278 (2023).
Cornacchia, D. & Studer, L. Back and forth in time: directing age in iPSC-derived lineages. Brain Res. 1656 , 14–26 (2017).
Studer, L., Vera, E. & Cornacchia, D. Programming and reprogramming cellular age in the era of induced pluripotency. Cell Stem Cell 16 , 591–600 (2015).
Mertens, J. et al. Age-dependent instability of mature neuronal fate in induced neurons from Alzheimer’s patients. Cell Stem Cell 28 , 1533–1548.e1536 (2021).
Miller, J. D. et al. Human iPSC-based modeling of late-onset disease via progerin-induced aging. Cell Stem Cell 13 , 691–705 (2013).
Giacomelli, E. et al. Human stem cell models of neurodegeneration: from basic science of amyotrophic lateral sclerosis to clinical translation. Cell Stem Cell 29 , 11–35 (2022).
Okano, H. & Morimoto, S. iPSC-based disease modeling and drug discovery in cardinal neurodegenerative disorders. Cell Stem Cell 29 , 189–208 (2022).
Virdi, G. S. et al. Protein aggregation and calcium dysregulation are hallmarks of familial Parkinson’s disease in midbrain dopaminergic neurons. Npj Parkinsons Dis. 8 , 162 (2022).
Egawa, N. et al. Drug screening for ALS using patient-specific induced pluripotent stem cells. Sci. Transl. Med. 4 , 145ra104 (2012).
Young, J. E. et al. Elucidating molecular phenotypes caused by the SORL1 Alzheimer’s disease genetic risk factor using human induced pluripotent stem cells. Cell Stem Cell 16 , 373–385 (2015).
Wightman, D. P. et al. A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer’s disease. Nat. Genet. 53 , 1276–1282 (2021).
Yamazaki, Y., Zhao, N., Caulfield, T. R., Liu, C. C. & Bu, G. J. Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat. Rev. Neurol. 15 , 501–518 (2019).
Belloy, M. E., Napolioni, V. & Greicius, M. D. A quarter century of APOE and Alzheimer’s disease: progress to date and the path forward. Neuron 101 , 820–838 (2019).
Serrano-Pozo, A., Das, S. & Hyman, B. T. APOE and Alzheimer’s disease: advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 20 , 68–80 (2021).
Sienski, G. et al. APOE4 disrupts intracellular lipid homeostasis in human iPSC-derived glia. Sci. Transl. Med. 13 , eaaz4564 (2021).
Tcw, J. et al. Cholesterol and matrisome pathways dysregulated in astrocytes and microglia. Cell 185 , 2213–2233.e2225 (2022).
Blanchard, J. W. et al. APOE4 impairs myelination via cholesterol dysregulation in oligodendrocytes. Nature 611 , 769–779 (2022).
Murdock, M. H. & Tsai, L. H. Insights into Alzheimer’s disease from single-cell genomic approaches. Nat. Neurosci. 26 , 181–195 (2023).
Victor, M. B. et al. Lipid accumulation induced by APOE4 impairs microglial surveillance of neuronal-network activity. Cell Stem Cell 29 , 1197–1212.e1198 (2022).
Chen, X. et al. Microglia-mediated T cell infiltration drives neurodegeneration in tauopathy. Nature 615 , 668–677 (2023).
Gate, D. et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature 577 , 399–404 (2020).
Krauskopf, J. et al. Transcriptomics analysis of human iPSC-derived dopaminergic neurons reveals a novel model for sporadic Parkinson’s disease. Mol. Psychiatry 27 , 4355–4367 (2022).
Ryan, S. D. et al. Isogenic human iPSC parkinson’s model shows nitrosative stress-induced dysfunction in MEF2-PGC1 alpha transcription. Cell 155 , 1351–1364 (2013).
Sommer, A. et al. Th17 lymphocytes induce neuronal cell death in a human iPSC-based model of Parkinson’s disease. Cell Stem Cell 23 , 123–131.e126 (2018).
Baxi, E. G. et al. Answer ALS, a large-scale resource for sporadic and familial ALS combining clinical and multi-omics data from induced pluripotent cell lines. Nat. Neurosci. 25 , 226–237 (2022).
Workman, M. J. et al. Large-scale differentiation of iPSC-derived motor neurons from ALS and control subjects. Neuron 111 , 1191–1204.e1195 (2023).
Fujimori, K. et al. Modeling sporadic ALS in iPSC-derived motor neurons identifies a potential therapeutic agent. Nat. Med. 24 , 1579–1589 (2018).
Chakrabarti, S. & Mohanakumar, K. P. Aging and neurodegeneration: a tangle of models and mechanisms. Aging Dis. 7 , 111–113 (2016).
Franceschi, C., Garagnani, P., Parini, P., Giuliani, C. & Santoro, A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 14 , 576–590 (2018).
Franceschi, C., Garagnani, P., Vitale, G., Capri, M. & Salvioli, S. Inflammaging and ‘Garb-aging’. Trends Endocrinol. Metab. 28 , 199–212 (2017).
Grimm, A. & Eckert, A. Brain aging and neurodegeneration: from a mitochondrial point of view. J. Neurochem. 143 , 418–431 (2017).
Bertucci, E. M. & Parrott, B. B. Is CpG density the link between epigenetic aging and lifespan? Trends Genet. 36 , 725–727 (2020).
Kosan, C., Heidel, F. H., Godmann, M. & Bierhoff, H. Epigenetic erosion in adult stem cells: drivers and passengers of aging. Cells 7 , 237 (2018).
Little, D. et al. A single cell high content assay detects mitochondrial dysfunction in iPSC-derived neurons with mutations in SNCA. Sci. Rep. 8 , 9033 (2018).
Du, F., Yu, Q., Chen, A., Chen, D. & Yan, S. S. Astrocytes attenuate mitochondrial dysfunctions in human dopaminergic neurons derived from iPSC. Stem Cell Rep. 10 , 366–374 (2018).
Cheng, X. Y. et al. Human iPSCs derived astrocytes rescue rotenone-induced mitochondrial dysfunction and dopaminergic neurodegeneration in vitro by donating functional mitochondria. Transl. Neurodegener. 9 , 1–14 (2020).
Zagoura, D., Canovas-Jorda, D., Pistollato, F., Bremer-Hoffmann, S. & Bal-Price, A. Evaluation of the rotenone-induced activation of the Nrf2 pathway in a neuronal model derived from human induced pluripotent stem cells. Neurochem. Int. 106 , 62–73 (2017).
Benson, E. K., Lee, S. W. & Aaronson, S. A. Role of progerin-induced telomere dysfunction in HGPS premature cellular senescence. J. Cell Sci. 123 , 2605–2612 (2010).
Ambasudhan, R. et al. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell 9 , 113–118 (2011).
Carter, J. L., Halmai, J. & Fink, K. D. The iNs and outs of direct reprogramming to induced neurons. Front. Genome Ed. 2 , 7 (2020).
Drouin-Ouellet, J., Pircs, K., Barker, R. A., Jakobsson, J. & Parmar, M. Direct neuronal reprogramming for disease modeling studies using patient-derived neurons: what have we learned? Front. Neurosci. 11 , 530 (2017).
Mertens, J., Marchetto, M. C., Bardy, C. & Gage, F. H. Evaluating cell reprogramming, differentiation and conversion technologies in neuroscience. Nat. Rev. Neurosci. 17 , 424–437 (2016).
Wang, H., Yang, Y., Liu, J. & Qian, L. Direct cell reprogramming: approaches, mechanisms and progress. Nat. Rev. Mol. Cell Biol. 22 , 410–424 (2021).
Wapinski, O. L. et al. Hierarchical mechanisms for direct reprogramming of fibroblasts to neurons. Cell 155 , 621–635 (2013).
Herdy, J. R. et al. Increased post-mitotic senescence in aged human neurons is a pathological feature of Alzheimer’s disease. Cell Stem Cell 29 , 1637–1652.e1636 (2022).
Traxler, L. et al. Warburg-like metabolic transformation underlies neuronal degeneration in sporadic Alzheimer’s disease. Cell Metab. 34 , 1248–1263.e1246 (2022).
Barisano, G. et al. Blood–brain barrier link to human cognitive impairment and Alzheimer’s disease. Nat. Cardiovasc. Res. 1 , 108–115 (2022).
Knox, E. G., Aburto, M. R., Clarke, G., Cryan, J. F. & O’Driscoll, C. M. The blood-brain barrier in aging and neurodegeneration. Mol. Psychiatr. 27 , 2659–2673 (2022).
Montagne, A. et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85 , 296–302 (2015).
Sweeney, M. D., Sagare, A. P. & Zlokovic, B. V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 14 , 133–150 (2018).
Zlokovic, B. V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 12 , 723–738 (2011).
Chen, X. et al. Modeling sporadic Alzheimer’s disease in human brain organoids under serum exposure. Adv. Sci. 8 , e2101462 (2021).
Mirabelli, P., Coppola, L. & Salvatore, M. Cancer cell lines are useful model systems for medical research. Cancers 11 , 1098 (2019).
Gillet, J. P., Varma, S. & Gottesman, M. M. The clinical relevance of cancer cell lines. J. Natl. Cancer Inst. 105 , 452–458 (2013).
Wilding, J. L. & Bodmer, W. F. Cancer cell lines for drug discovery and development. Cancer Res. 74 , 2377–2384 (2014).
Wijewardhane, N., Dressler, L. & Ciccarelli, F. D. Normal somatic mutations in cancer transformation. Cancer Cell 39 , 125–129 (2021).
Smith, R. C. & Tabar, V. Constructing and deconstructing cancers using human pluripotent stem cells and organoids. Cell Stem Cell 24 , 12–24 (2019).
Haag, D. et al. H3.3-K27M drives neural stem cell-specific gliomagenesis in a human iPSC-derived model. Cancer Cell 39 , 407–422.e413 (2021).
Crespo, M. et al. Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat. Med. 23 , 878–884 (2017).
Ford, A. C., Yuan, Y. & Moayyedi, P. Long-term impact of helicobacter pylori eradication therapy on gastric cancer incidence and mortality in healthy infected individuals: a meta-analysis beyond 10 years of follow-up. Gastroenterology 163 , 754–756.e751 (2022).
Polk, D. B. & Peek, R. M. Jr. Helicobacter pylori: gastric cancer and beyond. Nat. Rev. Cancer 10 , 403–414 (2010).
McCracken, K. W. et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516 , 400–404 (2014).
Wang, T. et al. Sequential CRISPR gene editing in human iPSCs charts the clonal evolution of myeloid leukemia and identifies early disease targets. Cell Stem Cell 28 , 1074–1089.e1077 (2021).
Garcez, P. P. et al. Zika virus impairs growth in human neurospheres and brain organoids. Science 352 , 816–818 (2016).
Scoon, W. A. et al. Ebola virus infection induces a delayed type I IFN response in bystander cells and the shutdown of key liver genes in human iPSC-derived hepatocytes. Stem Cell Rep. 17 , 2286–2302 (2022).
Luo, Y., Zhang, M., Chen, Y., Chen, Y. & Zhu, D. Application of human induced pluripotent stem cell-derived cellular and organoid models for COVID-19 research. Front. Cell Dev. Biol. 9 , 720099 (2021).
Harschnitz, O. & Studer, L. Human stem cell models to study host-virus interactions in the central nervous system. Nat. Rev. Immunol. 21 , 441–453 (2021).
Lamers, M. M. & Haagmans, B. L. SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol 20 , 270–284 (2022).
Cevik, M., Kuppalli, K., Kindrachuk, J. & Peiris, M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ 371 , m3862 (2020).
Harrison, A. G., Lin, T. & Wang, P. Mechanisms of SARS-CoV-2 transmission and pathogenesis. Trends Immunol. 41 , 1100–1115 (2020).
Bestion, E., Halfon, P., Mezouar, S. & Mege, J. L. Cell and animal models for SARS-CoV-2 research. Viruses 14 , 1507 (2022).
Chu, H., Chan, J. F. & Yuen, K. Y. Animal models in SARS-CoV-2 research. Nat. Methods 19 , 392–394 (2022).
Cleary, S. J. et al. Animal models of mechanisms of SARS-CoV-2 infection and COVID-19 pathology. Br. J. Pharm. 177 , 4851–4865 (2020).
Lee, C. Y. & Lowen, A. C. Animal models for SARS-CoV-2. Curr. Opin. Virol. 48 , 73–81 (2021).
Takayama, K. In vitro and animal models for SARS-CoV-2 research. Trends Pharm. Sci. 41 , 513–517 (2020).
Simoneau, C. R. & Ott, M. Modeling multi-organ infection by SARS-CoV-2 using stem cell technology. Cell Stem Cell 27 , 859–868 (2020).
Huang, J. et al. SARS-CoV-2 infection of pluripotent stem cell-derived human lung alveolar Type 2 cells elicits a rapid epithelial-intrinsic inflammatory response. Cell Stem Cell 27 , 962–973.e967 (2020).
Lian, Q. et al. Differential effects of macrophage subtypes on SARS-CoV-2 infection in a human pluripotent stem cell-derived model. Nat. Commun. 13 , 2028 (2022).
Elrobaa, I. H. & New, K. J. COVID-19: pulmonary and extra pulmonary manifestations. Front. Public Health 9 , 711616 (2021).
Gupta, A. et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 26 , 1017–1032 (2020).
Ning, Q. et al. The mechanism underlying extrapulmonary complications of the coronavirus disease 2019 and its therapeutic implication. Signal. Transduct. Target Ther. 7 , 57 (2022).
Chen, K. G., Park, K. & Spence, J. R. Studying SARS-CoV-2 infectivity and therapeutic responses with complex organoids. Nat. Cell Biol. 23 , 822–833 (2021).
Monteil, V. et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 181 , 905–913.e907 (2020).
Wang, W. L. et al. Detection of SARS-CoV-2 in different types of clinical specimens. Jama 323 , 1843–1844 (2020).
Bojkova, D. et al. SARS-CoV-2 infects and induces cytotoxic effects in human cardiomyocytes. Cardiovasc. Res. 116 , 2207–2215 (2020).
Perez-Bermejo, J. A. et al. SARS-CoV-2 infection of human iPSC-derived cardiac cells reflects cytopathic features in hearts of patients with COVID-19. Sci. Transl. Med. 13 , eabf7872 (2021).
Sharma, A. et al. Human iPSC-derived cardiomyocytes are susceptible to SARS-CoV-2 infection. Cell Rep. Med. 1 , 100052 (2020).
Ahmad, I. & Rathore, F. A. Neurological manifestations and complications of COVID-19: a literature review. J. Clin. Neurosci. 77 , 8–12 (2020).
Niazkar, H. R., Zibaee, B., Nasimi, A. & Bahri, N. The neurological manifestations of COVID-19: a review article. Neurol. Sci. 41 , 1667–1671 (2020).
Yassin, A. et al. Neurological manifestations and complications of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. BMC Neurol. 21 , 1–17 (2021).
Ramani, A. et al. SARS-CoV-2 targets neurons of 3D human brain organoids. Embo J. 39 , e106230 (2020).
Zhang, B. Z. et al. SARS-CoV-2 infects human neural progenitor cells and brain organoids. Cell Res. 30 , 928–931 (2020).
Cui, Q. et al. Compound screen identifies the small molecule Q34 as an inhibitor of SARS-CoV-2 infection. iScience 25 , 103684 (2022).
Wang, C. et al. ApoE-isoform-dependent SARS-CoV-2 neurotropism and cellular response. Cell Stem Cell 28 , 331–342.e335 (2021).
Shen, W. B. et al. SARS-CoV-2 invades cognitive centers of the brain and induces Alzheimer’s-like neuropathology. Preprint at BioRxiv (2022).
Kleiman, R. J. & Engle, S. J. Human inducible pluripotent stem cells: Realization of initial promise in drug discovery. Cell Stem Cell 28 , 1507–1515 (2021).
Gu, M. et al. iPSC-endothelial cell phenotypic drug screening and in silico analyses identify tyrphostin-AG1296 for pulmonary arterial hypertension. Sci. Transl. Med. 13 , eaba6480 (2021).
Bray, M. A. et al. Cell Painting, a high-content image-based assay for morphological profiling using multiplexed fluorescent dyes. Nat. Protoc. 11 , 1757–1774 (2016).
Chin, M. Y., Espinosa, J. A., Pohan, G., Markossian, S. & Arkin, M. R. Reimagining dots and dashes: visualizing structure and function of organelles for high-content imaging analysis. Cell Chem. Biol. 28 , 320–337 (2021).
Vamathevan, J. et al. Applications of machine learning in drug discovery and development. Nat. Rev. Drug Discov. 18 , 463–477 (2019).
Taubes, A. et al. Experimental and real-world evidence supporting the computational repurposing of bumetanide for APOE4-related Alzheimer’s disease. Nat. Aging 1 , 932–947 (2021).
Theodoris, C. V. et al. Network-based screen in iPSC-derived cells reveals therapeutic candidate for heart valve disease. Science 371 , eabd0724 (2021).
Pangalos, M. N., Schechter, L. E. & Hurko, O. Drug development for CNS disorders: strategies for balancing risk and reducing attrition. Nat. Rev. Drug Discov. 6 , 521–532 (2007).
Waring, M. J. et al. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat. Rev. Drug Discov. 14 , 475–486 (2015).
Inoue, H. & Yamanaka, S. The use of induced pluripotent stem cells in drug development. Clin. Pharm. Ther. 89 , 655–661 (2011).
Liu, W., Deng, Y., Liu, Y., Gong, W. & Deng, W. Stem cell models for drug discovery and toxicology studies. J. Biochem. Mol. Toxicol. 27 , 17–27 (2013).
Pasteuning-Vuhman, S., de Jongh, R., Timmers, A. & Pasterkamp, R. J. Towards advanced iPSC-based drug development for neurodegenerative disease. Trends Mol. Med. 27 , 263–279 (2021).
Reiser, J. & Sever, S. Podocyte biology and pathogenesis of kidney disease. Annu Rev. Med. 64 , 357–366 (2013).
Musah, S. et al. Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat. Biomed. Eng. 1 , 0069 (2017).
Richards, D. J. et al. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat. Biomed. Eng. 4 , 446–462 (2020).
Matsa, E. et al. Transcriptome profiling of patient-specific human iPSC-cardiomyocytes predicts individual drug safety and efficacy responses in vitro. Cell Stem Cell 19 , 311–325 (2016).
Sharma, A. et al. High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Sci. Transl. Med. 9 , eaaf2584 (2017).
Pellegrini, L. et al. Human CNS barrier-forming organoids with cerebrospinal fluid production. Science 369 , eaaz5626 (2020).
Kwon, O. et al. The development of a functional human small intestinal epithelium model for drug absorption. Sci. Adv. 7 , eabh1586 (2021).
Westerling-Bui, A. D. et al. Transplanted organoids empower human preclinical assessment of drug candidate for the clinic. Sci. Adv. 8 , eabj5633 (2022).
Brown, C. et al. Mesenchymal stem cells: cell therapy and regeneration potential. J. Tissue Eng. Regen. Med. 13 , 1738–1755 (2019).
Chien, K. R. et al. Regenerating the field of cardiovascular cell therapy. Nat. Biotechnol. 37 , 232–237 (2019).
Huang, K., Hu, S. & Cheng, K. A new era of cardiac cell therapy: opportunities and challenges. Adv. Health. Mater. 8 , e1801011 (2019).
Sterner, R. C. & Sterner, R. M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 11 , 69 (2021).
Brown, C. E. & Mackall, C. L. CAR T cell therapy: inroads to response and resistance. Nat. Rev. Immunol. 19 , 73–74 (2019).
Finck, A. V., Blanchard, T., Roselle, C. P., Golinelli, G. & June, C. H. Engineered cellular immunotherapies in cancer and beyond. Nat. Med. 28 , 678–689 (2022).
Bashor, C. J., Hilton, I. B., Bandukwala, H., Smith, D. M. & Veiseh, O. Engineering the next generation of cell-based therapeutics. Nat. Rev. Drug Discov. 21 , 655–675 (2022).
Desgres, M. & Menasche, P. Clinical translation of pluripotent stem cell therapies: challenges and considerations. Cell Stem Cell 25 , 594–606 (2019).
Stevens, K. R. & Murry, C. E. Human pluripotent stem cell-derived engineered tissues: clinical considerations. Cell Stem Cell 22 , 294–297 (2018).
Doss, M. X. & Sachinidis, A. Current challenges of iPSC-based disease modeling and therapeutic implications. Cells 8 , 403 (2019).
Lovell-Badge, R. et al. ISSCR guidelines for stem cell research and clinical translation: the 2021 update. Stem Cell Rep. 16 , 1398–1408 (2021).
Balboa, D. et al. Functional, metabolic and transcriptional maturation of human pancreatic islets derived from stem cells. Nat. Biotechnol. 40 , 1042–1055 (2022).
Du, Y. et al. Human pluripotent stem-cell-derived islets ameliorate diabetes in non-human primates. Nat. Med. 28 , 272–282 (2022).
Feng, L. et al. Developing hypoimmunogenic human iPSC-derived oligodendrocyte progenitor cells as an off-the-shelf cell therapy for myelin disorders. Adv. Sci. 10 , e2206910 (2023).
Madrid, M., Sumen, C., Aivio, S. & Saklayen, N. Autologous induced pluripotent stem cell-based cell therapies: promise, progress, and challenges. Curr. Protoc. 1 , e88 (2021).
Schweitzer, J. S. et al. Personalized iPSC-derived dopamine progenitor cells for Parkinson’s disease. N. Engl. J. Med. 382 , 1926–1932 (2020).
Schweitzer, J. S., Song, B. & Kim, K. S. A step closer to autologous cell therapy for Parkinson’s disease. Cell Stem Cell 28 , 595–597 (2021).
Tang, L. V. et al. Gene editing of human iPSCs rescues thrombophilia in hereditary antithrombin deficiency in mice. Sci. Transl. Med. 14 , eabq3202 (2022).
Maxwell, K. G. et al. Gene-edited human stem cell-derived beta cells from a patient with monogenic diabetes reverse preexisting diabetes in mice. Sci. Transl. Med. 12 , eaax9106 (2020).
Depil, S., Duchateau, P., Grupp, S. A., Mufti, G. & Poirot, L. Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat. Rev. Drug Discov. 19 , 185–199 (2020).
Crow, D. Could iPSCs enable “off-the-shelf” cell therapy? Cell 177 , 1667–1669 (2019).
Lanza, R., Russell, D. W. & Nagy, A. Engineering universal cells that evade immune detection. Nat. Rev. Immunol. 19 , 723–733 (2019).
Wang, B. et al. Generation of hypoimmunogenic T cells from genetically engineered allogeneic human induced pluripotent stem cells. Nat. Biomed. Eng. 5 , 429–440 (2021).
Hu, X. et al. Hypoimmune induced pluripotent stem cells survive long term in fully immunocompetent, allogeneic rhesus macaques. Nat. Biotechnol . 42 , 413–423 (2023).
Alvarez-Palomo, B. et al. Evaluation of the Spanish population coverage of a prospective HLA haplobank of induced pluripotent stem cells. Stem Cell Res Ther. 12 , 233 (2021).
Lee, S. et al. Repurposing the cord blood bank for haplobanking of HLA-Homozygous iPSCs and their usefulness to multiple populations. Stem Cells 36 , 1552–1566 (2018).
Sullivan, S. et al. Haplobanking induced pluripotent stem cells for clinical use. Stem Cell Res. 49 , 102035 (2020).
Yoshida, S. et al. A clinical-grade HLA haplobank of human induced pluripotent stem cells matching approximately 40% of the Japanese population. Med 4 , 51–66.e10 (2023).
Nguyen, P. K., Neofytou, E., Rhee, J.-W. & Wu, J. C. Potential strategies to address the major clinical barriers facing stem cell regenerative therapy for cardiovascular disease: a review. JAMA Cardiol. 1 , 953–962 (2016).
Aijaz, A. et al. Biomanufacturing for clinically advanced cell therapies. Nat. Biomed. Eng. 2 , 362–376 (2018).
Lee, A. S., Tang, C., Rao, M. S., Weissman, I. L. & Wu, J. C. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat. Med. 19 , 998–1004 (2013).
Chour, T. et al. Method for selective ablation of undifferentiated human pluripotent stem cell populations for cell-based therapies. JCI Insight 6 , e142000 (2021).
Kuang, Y. et al. Efficient, selective removal of human pluripotent stem cells via ecto-alkaline phosphatase-mediated aggregation of synthetic peptides. Cell Chem. Biol. 24 , 685–694.e684 (2017).
Jones, B. S., Lamb, L. S., Goldman, F. & Di Stasi, A. Improving the safety of cell therapy products by suicide gene transfer. Front. Pharm. 5 , 254 (2014).
Lund, R. J., Narva, E. & Lahesmaa, R. Genetic and epigenetic stability of human pluripotent stem cells. Nat. Rev. Genet. 13 , 732–744 (2012).
Ma, H. et al. Abnormalities in human pluripotent cells due to reprogramming mechanisms. Nature 511 , 177–183 (2014).
Guo, R. et al. Generation and clinical potential of functional T lymphocytes from gene-edited pluripotent stem cells. Exp. Hematol. Oncol. 11 , 1–17 (2022).
Motazedian, A. et al. Multipotent RAG1+ progenitors emerge directly from haemogenic endothelium in human pluripotent stem cell-derived haematopoietic organoids. Nat. Cell Biol. 22 , 60–73 (2020).
Seet, C. S. et al. Generation of mature T cells from human hematopoietic stem and progenitor cells in artificial thymic organoids. Nat. Methods 14 , 521–530 (2017).
Wang, Z. et al. 3D-organoid culture supports differentiation of human CAR(+) iPSCs into highly functional CAR T cells. Cell Stem Cell 29 , 651–653 (2022).
Nagamoto, Y. et al. Transplantation of a human iPSC-derived hepatocyte sheet increases survival in mice with acute liver failure. J. Hepatol. 64 , 1068–1075 (2016).
Sharma, R. et al. Clinical-grade stem cell-derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs. Sci. Transl. Med. 11 , eaat5580 (2019).
Glaeser, J. D. et al. iPSC-neural crest derived cells embedded in 3D printable bio-ink promote cranial bone defect repair. Sci. Rep. 12 , 18701 (2022).
Cichocki, F. et al. iPSC-derived NK cells maintain high cytotoxicity and enhance in vivo tumor control in concert with T cells and anti-PD-1 therapy. Sci. Transl. Med. 12 , eaaz5618 (2020).
Moriarty, N. et al. A combined cell and gene therapy approach for homotopic reconstruction of midbrain dopamine pathways using human pluripotent stem cells. Cell Stem Cell 29 , 434–448.e435 (2022).
Iancu, E. M. & Kandalaft, L. E. Challenges and advantages of cell therapy manufacturing under good manufacturing practices within the hospital setting. Curr. Opin. Biotechnol. 65 , 233–241 (2020).
Ackermann, M. et al. Continuous human iPSC-macrophage mass production by suspension culture in stirred tank bioreactors. Nat. Protoc. 17 , 513–539 (2022).
Ackermann, M. et al. Bioreactor-based mass production of human iPSC-derived macrophages enables immunotherapies against bacterial airway infections. Nat. Commun. 9 , 5088 (2018).
Yasuda, S. Y. et al. Chemically defined and growth-factor-free culture system for the expansion and derivation of human pluripotent stem cells. Nat. Biomed. Eng. 2 , 173–182 (2018).
Zhao, Z. et al. Organoids. Nat. Rev. Methods Prim. 2 , 94 (2022).
Basu, S. et al. Live-cell three-dimensional single-molecule tracking reveals modulation of enhancer dynamics by NuRD. Nat. Struct. Mol. Biol. 30 , 1628–1639 (2023).
Dodonova, S. O., Zhu, F., Dienemann, C., Taipale, J. & Cramer, P. Nucleosome-bound SOX2 and SOX11 structures elucidate pioneer factor function. Nature 580 , 669–672 (2020).
Wang, J. et al. Phase separation of OCT4 controls TAD reorganization to promote cell fate transitions. Cell Stem Cell 28 , 1868–1883.e1811 (2021).
He, W. et al. De novo identification of essential protein domains from CRISPR-Cas9 tiling-sgRNA knockout screens. Nat. Commun. 10 , 4541 (2019).
Hsu, J. Y. et al. CRISPR-SURF: discovering regulatory elements by deconvolution of CRISPR tiling screen data. Nat. Methods 15 , 992–993 (2018).
Yang, L. et al. High-resolution characterization of gene function using single-cell CRISPR tiling screen. Nat. Commun. 12 , 4063 (2021).
Liu, P., Chen, M., Liu, Y., Qi, L. S. & Ding, S. CRISPR-based chromatin remodeling of the endogenous Oct4 or Sox2 Locus enables reprogramming to pluripotency. Cell Stem Cell 22 , 252–261.e254 (2018).
Baumann, V. et al. Targeted removal of epigenetic barriers during transcriptional reprogramming. Nat. Commun. 10 , 2119 (2019).
Takahashi, Y. et al. Transgenerational inheritance of acquired epigenetic signatures at CpG islands in mice. Cell 186 , 715–731.e719 (2023).
Tompkins, J. et al. Engineering CpG island DNA methylation in pluripotent cells through synthetic CpG-free ssDNA insertion. Cell Rep. Methods 3 , 100465 (2023).
Cerneckis, J., Ming, G. L., Song, H., He, C. & Shi, Y. The rise of epitranscriptomics: recent developments and future directions. Trends Pharm. Sci. 45 , 24–38 (2024).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596 , 583–589 (2021).
Stahl, K., Graziadei, A., Dau, T., Brock, O. & Rappsilber, J. Protein structure prediction with in-cell photo-crosslinking mass spectrometry and deep learning. Nat. Biotechnol . 1−10 (2023).
Greener, J. G., Kandathil, S. M., Moffat, L. & Jones, D. T. A guide to machine learning for biologists. Nat. Rev. Mol. Cell Biol. 23 , 40–55 (2022).
Coronnello, C. & Francipane, M. G. Moving towards induced pluripotent stem cell-based therapies with artificial intelligence and machine learning. Stem Cell Rev. Rep. 18 , 559–569 (2022).
Pantazis, C. B. et al. A reference human induced pluripotent stem cell line for large-scale collaborative studies. Cell Stem Cell 29 , 1685–1702.e1622 (2022).
Bisogno, L. S. et al. Ancestry-dependent gene expression correlates with reprogramming to pluripotency and multiple dynamic biological processes. Sci. Adv. 6 , eabc3851 (2020).
Czerniecki, S. M. et al. High-throughput screening enhances kidney organoid differentiation from human pluripotent stem cells and enables automated multidimensional phenotyping. Cell Stem Cell 22 , 929–940.e924 (2018).
Lu, Y., Zhou, Y., Ju, R. & Chen, J. Human-animal chimeras for autologous organ transplantation: technological advances and future perspectives. Ann. Transl. Med. 7 , 576 (2019).
Takebe, T. et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499 , 481–484 (2013).
Suchy, F., Yamaguchi, T. & Nakauchi, H. iPSC-derived organs in vivo: challenges and promise. Cell Stem Cell 22 , 21–24 (2018).
Download references
Acknowledgements
The authors would like to thank Louise and Herbert Horvitz, the Christopher Family, the Judy and Bernard Briskin Fund, and the Sidell Kagan Foundation for their generosity and forethought. This work was supported by the National Institute on Aging of the National Institutes of Health R01 AG072291 and RF1 AG079307 and the National Institute of Neurological Disorders and Stroke of the National Institutes of Health U01 NS122101 to Y.S. J.C. is a predoctoral scholar in the Stem Cell Biology and Regenerative Medicine Research Training Program of the California Institute for Regenerative Medicine (CIRM). Figures 1 – 7 were created with BioRender.com.
Author information
Authors and affiliations.
Department of Neurodegenerative Diseases, Beckman Research Institute of City of Hope, Duarte, CA, 91010, USA
Jonas Cerneckis, Hongxia Cai & Yanhong Shi
Irell & Manella Graduate School of Biological Sciences, Beckman Research Institute of City of Hope, Duarte, CA, 91010, USA
Jonas Cerneckis & Yanhong Shi
You can also search for this author in PubMed Google Scholar
Contributions
J.C. and Y.S. conceptualized the review article. J.C. drafted the manuscript and prepared the figures. H.C. drafted the table. J.C. revised the manuscript with inputs from H.C. and Y.S. All authors have read and approved the article.
Corresponding author
Correspondence to Yanhong Shi .
Ethics declarations
Competing interests.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Y.S. is the editorial board member of Signal Transduction and Targeted Therapy, but was not involved in the handling of this manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Cerneckis, J., Cai, H. & Shi, Y. Induced pluripotent stem cells (iPSCs): molecular mechanisms of induction and applications. Sig Transduct Target Ther 9 , 112 (2024). https://doi.org/10.1038/s41392-024-01809-0
Download citation
Received : 28 July 2023
Revised : 09 March 2024
Accepted : 17 March 2024
Published : 26 April 2024
DOI : https://doi.org/10.1038/s41392-024-01809-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
VIDEO
COMMENTS
A counterargument explaining the case for embryonic stem cell research is made by Jonathan Moreno, a professor at the University of Pennsylvania and a senior fellow at the Center for American Progress in Washington, D.C. Featuring : Yuval Levin, Hertog Fellow and Director of the Bioethics and American Democracy Program, Ethics and Public Policy ...
An investigation of the economic and ethical arguments made against research with human embryonic stem cells. The British House of Lords voted on January 22, 2001 to ease restrictions on the use of human embryonic stem cells. Researchers in the UK are now allowed to use early stage human embryos for therapeutic purposes, mainly to retrieve stem ...
The ethical implications of stem cell research are often described in terms of risks, side effects, safety, and therapeutic value, which are examples of so-called hard impacts. Hard impacts are typically measurable and quantifiable. To understand the broader spectrum of ethical implications of stem cell research on science and society, it is ...
Since then, research that utilizes human embryonic cells has been a widely debated, controversial ethical issue. Human embryonic cells possess the ability to become stem cells, which are used in medical research due to two significant features. First, they are unspecialized cells, meaning they can undergo cell division and renew themselves even ...
MS: Proponents argue that embryonic stem cell research holds great promise for understanding and curing diabetes, Parkinson's disease, spinal cord injury, and other debilitating conditions. Opponents argue that the research is unethical, because deriving the stem cells destroys the blastocyst, an unimplanted human embryo at the sixth to ...
Those against embryonic stem cell research argue that funding should be used to greatly expand adult stem research, to circumvent the many moral issues involving the use of human embryos. Lifting the Ban . Now that President Obama has lifted the federal funding ban for embryonic stem cell research, financial support will soon flow to federal ...
Most opponents to embryonic stem cell research think that it is wrong to destroy a 2- to 6-day-old embryo, even if it is not destined to start a pregnancy. Others argue that it is immoral not to ...
Many predicted that iPS cells would soon displace embryonic stem cells in the research space, but it didn't happen. The number of ES-cell publications grew rapidly after 2006 and has held pace ...
A U.S. appeals court today upheld the legality of federally funded research on human embryonic stem cells (hESCs)—the latest in a string of wins for the National Institutes of Health (NIH) in a 3-year legal battle with groups that for moral reasons want to block the use of these cells. But although hESC researchers can breathe easy for now, the 27-page decision suggests the battle over hESCs ...
The use of human embryos for research on embryonic stem (ES) cells is currently high on the ethical and political agenda in many countries. Despite the potential benefit of using human ES cells in the treatment of disease, their use remains controversial because of their derivation from early embryos. Here, we address some of the ethical issues ...
The use of human embryonic stem cells to replace damaged cells and tissues promises future hope for the treatment of many diseases. However, many countries now face complex ethical and legal ...
Abstract. This article explores the plausibility of an argument against embryonic stem cell research based on what the regulations already say about research on pregnant women and fetuses. The center of the argument is the notion of vulnerability and whether such a concept is applicable to human embryos. It is argued that such an argument can ...
As embryonic stem cell research is commercialized, the stem cell debate may shift focus from concerns about embryo destruction to concerns about exploitation of the women who donate eggs and embryos for research. Uncomfortable with the polarization of the embryo debate, this paper proposes a more "contemplative" approach than intellectual debate to concerns about exploitation. After ...
This is because they used embryonic stem cells to induce specific gene modifications in mice by isolating the embryonic stem cell from the embryo and implanting it in adult female mice. This will open new horizons for research and therapy. In 1992, the first public and private stem cell bank was established in the United States.
Publication bias in medical journals is detrimental to the free exchange of ideas regarding controversial issues. From 2000 to 2017, a premier publication, the New England Journal of Medicine (NEJM), has shown considerable bias in only publishing articles and editorials highly favorable toward human embryonic stem cell research and abortion, without permitting valid discussion and publication ...
to somatic (adult) stem cells. Thus, advocates believe embryonic stem cell research may aid in developing new, more efficient treatments for severe diseases and ease the pain and suffering of numerous people. However, those that are against embryonic stem cell research believe that the possibility of scientific
The field of stem cell research has undergone a significant transformation with the advent of human embryonic stem cells (hESCs). Since their pioneering isolation in 1998, hESCs have been at the forefront of scientific inquiry due to their unique ability for self-renewal and pluripotency [1, 2].This comprehensive review article delves into the advancements, challenges, and ethical ...
The ethical implications of stem cell research are often discussed in terms of risks, side effects, and safety, which are examples of hard impacts. In this article, Assen and colleagues argue that to understand the broader spectrum of ethical implications of stem cell research on science and society, it is important to recognize the so-called soft impacts.
The Stem Cell Divide. By Rick Weiss. 30 min read. In the beginning, one cell becomes two, and two become four. Being fruitful, they multiply into a ball of many cells, a shimmering sphere of human ...
Human embryonic stem cells (hESCs) are pluripotent, and their derivation sparked new possibilities, from the production of 'spare parts' to treating a plethora of degenerative conditions, the study of early embryonic development, to revolutionizing drug screening and development and broadening the spectrum of human toxicology research.
Early embryonic models were generated by using embryonic stem cells (ESCs) 11, which possess the ability to self-renew and differentiate into a variety of specific cell types of the body.
The ethical and moral complexities over stem cell research have been the centre of much debate with fears of cloning and the ethics of using embryonic stem cells. Banerjea's lecture was measured, less of a hyperbole and rooted in science. Most of the audience members-there were around 20 people —were doctors and researchers.
Haploid cells are a kind of cells with only one set of chromosomes. Compared with traditional diploid cells, haploid cells have unique advantages in gene screening and drug-targeted therapy, due to their phenotype being equal to the genotype. Embryonic stem cells are a kind of cells with strong diff …
Generating brains from two different species via blastocyst complementation enables synchronous development of appropriate interspecies circuits. When host sensory neurons are disabled, donor neurons restore a primal odor-driven food-seeking behavior, showing that one species can sense and respond to the world through the cognate neurons of another.
Chemotherapy exposure has become a main cause of premature ovarian insufficiency (POI). This study aimed to evaluate the role and molecular mechanism of human umbilical cord mesenchymal stem cell-derived exosomes (hUMSC-Exos) in ovarian function protection after chemotherapy. hUMSC-Exos were applied to cyclophosphamide-induced premature ovarian insufficiency mice and human ovarian granulosa ...
Embryonic stem cells are pluripotent cells isolated from the inner cell mass of a blastocyst, the early mammalian embryo that implants into the uterus. Embryonic stem cells self-renew by dividing ...
This article is part of the Research Topic Modified mesenchymal stem/stromal cells as next ... lungs collected on day 12 were immunostained with antibodies against F4/80 ... A, Choolani M, et al. Extra-embryonic human Wharton's jelly stem cells do not induce tumorigenesis, unlike human embryonic stem cells. Reprod BioMed Online. (2012) 24:235 ...
Introduction. The field of stem cell research has undergone a significant transformation with the advent of human embryonic stem cells (hESCs). Since their pioneering isolation in 1998, hESCs have been at the forefront of scientific inquiry due to their unique ability for self-renewal and pluripotency [1, 2].This comprehensive review article delves into the advancements, challenges, and ...
Berberis vulgaris (L.) has remarkable ethnopharmacological properties and is widely used in traditional medicine. The present study investigated B. vulgaris stem bark (Berberidis cortex) by extraction with 50% ethanol. The main secondary metabolites were quantified, resulting in a polyphenols content of 17.6780 ± 3.9320 mg Eq tannic acid/100 g extract, phenolic acids amount of 3.3886 ± 0. ...
The development of induced pluripotent stem cell (iPSC) technology has opened vast opportunities for in vitro modeling of human biology and for cell therapy applications. 1,2,3,4,5 Since the first ...