Causes and Effects of Obesity Essay
Introduction, laziness as the main cause of obesity, social effects of obesity, effects of obesity: health complications.
Bibliography
Maintaining good body weight is highly recommended by medical doctors as a way of promoting a healthy status of the body. This is to say that there is allowed body weight, which a person is supposed to maintain. Extreme deviations from this weight expose a person to several health complications.
While being underweight is not encouraged, cases of people who are overweight and increasing effects of this condition have raised concerns over the need of addressing the issue of obesity in the society today, where statistics are rising day and night. What is obesity? This refers to a medical condition in which a person’s body has high accumulation of body fat to the level of being fatal or a cause of serious health complications. Additionally, obesity is highly associated with one’s body mass index, abbreviated as BMI.
This denotes the value obtained when a person’s weight in kilograms is divided by the square of their height in meters (Burniat 3). According to medical experts, obesity occurs when the BMI exceeds 30kg/m 2 . While this is the case, people who have a BMI of between 25 and 29 and considered to be overweight. Obesity has a wide-range of negative effects, which may be a threat to the life of a person.
The fist effect of obesity is that it encourages laziness in the society. It is doubtless that obese people find it hard and strenuous to move from one point to the other because of accumulated fats. As a result, most of these people lead a sedentary lifestyle, which is usually characterized by minimal or no movement. In such scenarios, victims prefer being helped doing basic activities, including moving from one point to another.
Moreover, laziness makes one to be inactive and unproductive. For example, a student who is obese may find it hard to attend to his or her homework and class assignments, thus affecting performance. With regard to physical exercises, obese people perceive exercises as punishment, which is not meant for them (Korbonits 265). As a result, they do not accept simple activities like jogging because of their inability to move.
In line with this, obese people cannot participate in games like soccer, athletics, and rugby among others. Based on this sedentary lifestyle, obese people spend a lot of their time watching television, movies, and playing video games, which worsen the situation.
The main effect of obesity is health complications. Research indicates that most of the killer diseases like diabetes, heart diseases, and high blood pressure are largely associated with obesity. In the United States, obesity-related complications cost the nation approximately 150 billion USD and result into 0.3 million premature deaths annually.
When there is increase in body fat, it means that the body requires more nutrients and oxygen to support body tissues (Burniat 223). Since these elements can only be transported by the blood to various parts of the body, the workload of the heart is increased.
This increase in the workload of the heart exerts pressure on blood vessels, leading to high blood pressure. An increase in the heart rate may also be dangerous due to the inability of the body to supply required blood to various parts. Moreover, obesity causes diabetes, especially among adults as the body may become resistant to insulin. This resistance may lead to a high level of blood sugar, which is fatal.
Besides health complications, obesity causes an array of psychological effects, including inferiority complex among victims. Obese people suffer from depression, emanating from negative self-esteem and societal rejection. In some cases, people who become obese lose their friends and may get disapproval from teachers and other personalities (Korbonits 265). This is mainly based on the assumption that people become obese due to lack of self-discipline. In extreme cases, obese people may not be considered for promotion at workplaces, because of the negative perception held against them.
Due to inferiority complex, obese people avoid being in public and prefer being alone. This is because they imagine how the world sees them and may also find it hard being involved in public activities because of their sizes.
This further makes them to consider themselves unattractive based on their deviation from what is considered as the normal body size and shape. Regardless of how obese people are treated, they always believe that they are being undermined because of their body size.
In summary, obesity is a major cause of premature deaths in the United States and around the world. This health condition occurs when there is excess accumulation of body fat, caused by unhealthy lifestyles. Obesity is largely associated with several killer diseases like high blood pressure, diabetes, and diseases of the heart.
These diseases drain world economies since most of them are fatal and expensive to manage. Additionally, obesity promotes sedentary life where victims minimize movement by adopting an inactive lifestyle. Moreover, obese victims suffer psychologically because of societal rejection. In general, obesity has a wide-range of negative effects, which may be a threat to the life of a person.
Burniat, Walter. Child and Adolescent Obesity: Causes and Consequences, Prevention and Management . United Kingdom: Cambridge University Press, 2002. Print.
Korbonits, Márta. Obesity and Metabolism . Switzerland: Karger Publishers, 2008. Print.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2023, October 28). Causes and Effects of Obesity Essay. https://ivypanda.com/essays/effects-of-obesity/
"Causes and Effects of Obesity Essay." IvyPanda , 28 Oct. 2023, ivypanda.com/essays/effects-of-obesity/.
IvyPanda . (2023) 'Causes and Effects of Obesity Essay'. 28 October.
IvyPanda . 2023. "Causes and Effects of Obesity Essay." October 28, 2023. https://ivypanda.com/essays/effects-of-obesity/.
1. IvyPanda . "Causes and Effects of Obesity Essay." October 28, 2023. https://ivypanda.com/essays/effects-of-obesity/.
IvyPanda . "Causes and Effects of Obesity Essay." October 28, 2023. https://ivypanda.com/essays/effects-of-obesity/.
- Workplace Laziness in the Restaurant Business
- Hebrew Teachings on Diligence and Laziness: A Contrast with Wisdom and Folly
- BMI and Stress Levels Among Students in the US
- BMI: Assessment Tools in Adults and Children
- Assessment Tools and Diagnostic Tests: BMI and WC
- Airline Mergers and the Case Between BMI and Lufthansa
- Screening for Obesity
- Public Health. Epidemiology of Obesity
- Recreation Hub as a Way to Combat Sedentary Lifestyle
- Parental Education on Overweight and Obese Children
- Eating Disorders: Assessment & Misconceptions
- Human Digestion
- Definitions of Obesity and Criteria for Diagnosing It
- Obesity Could Be Catching
- White Wines vs. Red Wines
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 27 February 2019

Obesity: global epidemiology and pathogenesis
- Matthias Blüher 1
Nature Reviews Endocrinology volume 15 , pages 288–298 ( 2019 ) Cite this article
55k Accesses
2491 Citations
986 Altmetric
Metrics details
- Epidemiology
- Health policy
- Pathogenesis
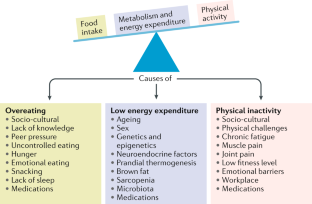
The prevalence of obesity has increased worldwide in the past ~50 years, reaching pandemic levels. Obesity represents a major health challenge because it substantially increases the risk of diseases such as type 2 diabetes mellitus, fatty liver disease, hypertension, myocardial infarction, stroke, dementia, osteoarthritis, obstructive sleep apnoea and several cancers, thereby contributing to a decline in both quality of life and life expectancy. Obesity is also associated with unemployment, social disadvantages and reduced socio-economic productivity, thus increasingly creating an economic burden. Thus far, obesity prevention and treatment strategies — both at the individual and population level — have not been successful in the long term. Lifestyle and behavioural interventions aimed at reducing calorie intake and increasing energy expenditure have limited effectiveness because complex and persistent hormonal, metabolic and neurochemical adaptations defend against weight loss and promote weight regain. Reducing the obesity burden requires approaches that combine individual interventions with changes in the environment and society. Therefore, a better understanding of the remarkable regional differences in obesity prevalence and trends might help to identify societal causes of obesity and provide guidance on which are the most promising intervention strategies.
Obesity prevalence has increased in pandemic dimensions over the past 50 years.
Obesity is a disease that can cause premature disability and death by increasing the risk of cardiometabolic diseases, osteoarthritis, dementia, depression and some types of cancers.
Obesity prevention and treatments frequently fail in the long term (for example, behavioural interventions aiming at reducing energy intake and increasing energy expenditure) or are not available or suitable (bariatric surgery) for the majority of people affected.
Although obesity prevalence increased in every single country in the world, regional differences exist in both obesity prevalence and trends; understanding the drivers of these regional differences might help to provide guidance for the most promising intervention strategies.
Changes in the global food system together with increased sedentary behaviour seem to be the main drivers of the obesity pandemic.
The major challenge is to translate our knowledge of the main causes of increased obesity prevalence into effective actions; such actions might include policy changes that facilitate individual choices for foods that have reduced fat, sugar and salt content.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Obesity and the risk of cardiometabolic diseases
Obesity-induced and weight-loss-induced physiological factors affecting weight regain

The genetics of obesity: from discovery to biology
World Health Organization. Noncommunicable diseases progress monitor, 2017. WHO https://www.who.int/nmh/publications/ncd-progress-monitor-2017/en/ (2017).
Fontaine, K. R., Redden, D. T., Wang, C., Westfall, A. O. & Allison, D. B. Years of life lost due to obesity. JAMA 289 , 187–193 (2003).
PubMed Google Scholar
Berrington de Gonzalez, A. et al. Body-mass index and mortality among 1.46 million white adults. N. Engl. J. Med. 363 , 2211–2219 (2010).
CAS PubMed Google Scholar
Prospective Studies Collaboration. Body-mass index and cause-specific mortality in 900000 adults: collaborative analyses of 57 prospective studies. Lancet 373 , 1083–1096 (2009).
PubMed Central Google Scholar
Woolf, A. D. & Pfleger, B. Burden of major musculoskeletal conditions. Bull. World Health Organ. 81 , 646–656 (2003).
PubMed PubMed Central Google Scholar
Bray, G. A. et al. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 18 , 715–723 (2017).
World Health Organization. Obesity and overweight. WHO https://www.who.int/mediacentre/factsheets/fs311/en/ (2016).
World Health Organization. Political declaration of the high-level meeting of the general assembly on the prevention and control of non-communicable diseases. WHO https://www.who.int/nmh/events/un_ncd_summit2011/political_declaration_en.pdf (2012).
Franco, M. et al. Population-wide weight loss and regain in relation to diabetes burden and cardiovascular mortality in Cuba 1980-2010: repeated cross sectional surveys and ecological comparison of secular trends. BMJ 346 , f1515 (2013).
Swinburn, B. A. et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet 378 , 804–814 (2011).
Yanovski, J. A. Obesity: Trends in underweight and obesity — scale of the problem. Nat. Rev. Endocrinol. 14 , 5–6 (2018).
Heymsfield, S. B. & Wadden, T. A. Mechanisms, pathophysiology, and management of obesity. N. Engl. J. Med. 376 , 254–266 (2017).
Murray, S., Tulloch, A., Gold, M. S. & Avena, N. M. Hormonal and neural mechanisms of food reward, eating behaviour and obesity. Nat. Rev. Endocrinol. 10 , 540–552 (2014).
Farooqi, I. S. Defining the neural basis of appetite and obesity: from genes to behaviour. Clin. Med. 14 , 286–289 (2014).
Google Scholar
Anand, B. K. & Brobeck, J. R. Hypothalamic control of food intake in rats and cats. Yale J. Biol. Med. 24 , 123–140 (1951).
CAS PubMed PubMed Central Google Scholar
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372 , 425–432 (1994).
Coleman, D. L. & Hummel, K. P. Effects of parabiosis of normal with genetically diabetic mice. Am. J. Physiol. 217 , 1298–1304 (1969).
Farooqi, I. S. & O’Rahilly, S. 20 years of leptin: human disorders of leptin action. J. Endocrinol. 223 , T63–T70 (2014).
Börjeson, M. The aetiology of obesity in children. A study of 101 twin pairs. Acta Paediatr. Scand. 65 , 279–287 (1976).
Stunkard, A. J., Harris, J. R., Pedersen, N. L. & McClearn, G. E. The body-mass index of twins who have been reared apart. N. Engl. J. Med. 322 , 1483–1487 (1990).
Montague, C. T. et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387 , 903–908 (1997).
Farooqi, I. S. et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 341 , 879–884 (1999).
Clément, K. et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 392 , 398–401 (1998).
Farooqi, I. S. et al. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J. Clin. Invest. 106 , 271–279 (2000).
Krude, H. et al. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat. Genet. 19 , 155–157 (1998).
Hebebrand, J., Volckmar, A. L., Knoll, N. & Hinney, A. Chipping away the ‘missing heritability’: GIANT steps forward in the molecular elucidation of obesity - but still lots to go. Obes. Facts 3 , 294–303 (2010).
Speliotes, E. K. et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 42 , 937–948 (2010).
Sharma, A. M. & Padwal, R. Obesity is a sign - over-eating is a symptom: an aetiological framework for the assessment and management of obesity. Obes. Rev. 11 , 362–370 (2010).
Berthoud, H. R., Münzberg, H. & Morrison, C. D. Blaming the brain for obesity: integration of hedonic and homeostatic mechanisms. Gastroenterology 152 , 1728–1738 (2017).
Government Office for Science. Foresight. Tackling obesities: future choices – project report. GOV.UK https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/287937/07-1184x-tackling-obesities-future-choices-report.pdf (2007).
World Health Organization. International Statistical Classification of Diseases and Related Health Problems 10th revision. WHO http://apps.who.int/classifications/icd10/browse/2010/en (2010).
Hebebrand, J. et al. A proposal of the European Association for the Study of Obesity to improve the ICD-11 diagnostic criteria for obesity based on the three dimensions. Obes. Facts 10 , 284–307 (2017).
Ramos Salas, X. et al. Addressing weight bias and discrimination: moving beyond raising awareness to creating change. Obes. Rev. 18 , 1323–1335 (2017).
Sharma, A. M. et al. Conceptualizing obesity as a chronic disease: an interview with Dr. Arya Sharma. Adapt. Phys. Activ Q. 35 , 285–292 (2018).
Hebebrand, J. et al. “Eating addiction”, rather than “food addiction”, better captures addictive-like eating behavior. Neurosci. Biobehav. Rev. 47 , 295–306 (2014).
Phelan, S. M. et al. Impact of weight bias and stigma on quality of care and outcomes for patients with obesity. Obes. Rev. 16 , 319–326 (2015).
Kushner, R. F. et al. Obesity coverage on medical licensing examinations in the United States. What is being tested? Teach Learn. Med. 29 , 123–128 (2017).
NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 390 , 2627–2642 (2017).
NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 387 , 1377–1396 (2016).
Organisation for Economic Co-operation and Development. Obesity update 2017. OECD https://www.oecd.org/els/health-systems/Obesity-Update-2017.pdf (2017).
Geserick, M. et al. BMI acceleration in early childhood and risk of sustained obesity. N. Engl. J. Med. 379 , 1303–1312 (2018).
Ezzati, M. & Riboli, E. Behavioral and dietary risk factors for noncommunicable diseases. N. Engl. J. Med. 369 , 954–964 (2013).
Kleinert, S. & Horton, R. Rethinking and reframing obesity. Lancet 385 , 2326–2328 (2015).
Roberto, C. A. et al. Patchy progress on obesity prevention: emerging examples, entrenched barriers, and new thinking. Lancet 385 , 2400–2409 (2015).
Lundborg, P., Nystedt, P. & Lindgren, B. Getting ready for the marriage market? The association between divorce risks and investments in attractive body mass among married Europeans. J. Biosoc. Sci. 39 , 531–544 (2007).
McCabe, M. P. et al. Socio-cultural agents and their impact on body image and body change strategies among adolescents in Fiji, Tonga, Tongans in New Zealand and Australia. Obes. Rev. 12 , 61–67 (2011).
Hayashi, F., Takimoto, H., Yoshita, K. & Yoshiike, N. Perceived body size and desire for thinness of young Japanese women: a population-based survey. Br. J. Nutr. 96 , 1154–1162 (2006).
Hardin, J., McLennan, A. K. & Brewis, A. Body size, body norms and some unintended consequences of obesity intervention in the Pacific islands. Ann. Hum. Biol. 45 , 285–294 (2018).
Monteiro, C. A., Conde, W. L. & Popkin, B. M. Income-specific trends in obesity in Brazil: 1975–2003. Am. J. Public Health 97 , 1808–1812 (2007).
Mariapun, J., Ng, C. W. & Hairi, N. N. The gradual shift of overweight, obesity, and abdominal obesity towards the poor in a multi-ethnic developing country: findings from the Malaysian National Health and Morbidity Surveys. J. Epidemiol. 28 , 279–286 (2018).
Gebrie, A., Alebel, A., Zegeye, A., Tesfaye, B. & Ferede, A. Prevalence and associated factors of overweight/ obesity among children and adolescents in Ethiopia: a systematic review and meta-analysis. BMC Obes. 5 , 19 (2018).
Rokholm, B., Baker, J. L. & Sørensen, T. I. The levelling off of the obesity epidemic since the year 1999 — a review of evidence and perspectives. Obes. Rev. 11 , 835–846 (2010).
Hauner, H. et al. Overweight, obesity and high waist circumference: regional differences in prevalence in primary medical care. Dtsch. Arztebl. Int. 105 , 827–833 (2008).
Myers, C. A. et al. Regional disparities in obesity prevalence in the United States: a spatial regime analysis. Obesity 23 , 481–487 (2015).
Wilkinson, R. G. & Pickett, K. The Spirit Level: Why More Equal Societies Almost Always Do Better 89–102 (Bloomsbury Press London, 2009).
Sarget, M. Why inequality is fatal. Nature 458 , 1109–1110 (2009).
Plachta-Danielzik, S. et al. Determinants of the prevalence and incidence of overweight in children and adolescents. Public Health Nutr. 13 , 1870–1881 (2010).
Bell, A. C., Ge, K. & Popkin, B. M. The road to obesity or the path to prevention: motorized transportation and obesity in China. Obes. Res. 10 , 277–283 (2002).
Ludwig, J. et al. Neighborhoods, obesity, and diabetes — a randomized social experiment. N. Engl. J. Med. 365 , 1509–1519 (2011).
Beyerlein, A., Kusian, D., Ziegler, A. G., Schaffrath-Rosario, A. & von Kries, R. Classification tree analyses reveal limited potential for early targeted prevention against childhood overweight. Obesity 22 , 512–517 (2014).
Reilly, J. J. et al. Early life risk factors for obesity in childhood: cohort study. BMJ 330 , 1357 (2005).
Kopelman, P. G. Obesity as a medical problem. Nature 404 , 635–643 (2000).
CAS Google Scholar
Bouchard, C. et al. The response to long-term overfeeding in identical twins. N. Engl. J. Med. 322 , 1477–1482 (1990).
Sadeghirad, B., Duhaney, T., Motaghipisheh, S., Campbell, N. R. & Johnston, B. C. Influence of unhealthy food and beverage marketing on children’s dietary intake and preference: a systematic review and meta-analysis of randomized trials. Obes. Rev. 17 , 945–959 (2016).
Gilbert-Diamond, D. et al. Television food advertisement exposure and FTO rs9939609 genotype in relation to excess consumption in children. Int. J. Obes. 41 , 23–29 (2017).
Frayling, T. M. et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316 , 889–894 (2007).
Loos, R. J. F. & Yeo, G. S. H. The bigger picture of FTO-the first GWAS-identified obesity gene. Nat. Rev. Endocrinol. 10 , 51–61 (2014).
Wardle, J. et al. Obesity associated genetic variation in FTO is associated with diminished satiety. J. Clin. Endocrinol. Metab. 93 , 3640–3643 (2008).
Tanofsky-Kraff, M. et al. The FTO gene rs9939609 obesity-risk allele and loss of control over eating. Am. J. Clin. Nutr. 90 , 1483–1488 (2009).
Hess, M. E. et al. The fat mass and obesity associated gene (Fto) regulates activity of the dopaminergic midbrain circuitry. Nat. Neurosci. 16 , 1042–1048 (2013).
Fredriksson, R. et al. The obesity gene, FTO, is of ancient origin, up-regulated during food deprivation and expressed in neurons of feeding-related nuclei of the brain. Endocrinology 149 , 2062–2071 (2008).
Cohen, D. A. Neurophysiological pathways to obesity: below awareness and beyond individual control. Diabetes 57 , 1768–1773 (2008).
Richard, D. Cognitive and autonomic determinants of energy homeostasis in obesity. Nat. Rev. Endocrinol. 11 , 489–501 (2015).
Clemmensen, C. et al. Gut-brain cross-talk in metabolic control. Cell 168 , 758–774 (2017).
Timper, K. & Brüning, J. C. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis. Model. Mech. 10 , 679–689 (2017).
Kim, K. S., Seeley, R. J. & Sandoval, D. A. Signalling from the periphery to the brain that regulates energy homeostasis. Nat. Rev. Neurosci. 19 , 185–196 (2018).
Cutler, D. M., Glaeser, E. L. & Shapiro, J. M. Why have Americans become more obese? J. Econ. Perspect. 17 , 93–118 (2003).
Löffler, A. et al. Effects of psychological eating behaviour domains on the association between socio-economic status and BMI. Public Health Nutr. 20 , 2706–2712 (2017).
Chan, R. S. & Woo, J. Prevention of overweight and obesity: how effective is the current public health approach. Int. J. Environ. Res. Public Health 7 , 765–783 (2010).
Hsueh, W. C. et al. Analysis of type 2 diabetes and obesity genetic variants in Mexican Pima Indians: marked allelic differentiation among Amerindians at HLA. Ann. Hum. Genet. 82 , 287–299 (2018).
Schulz, L. O. et al. Effects of traditional and western environments on prevalence of type 2 diabetes in Pima Indians in Mexico and the US. Diabetes Care 29 , 1866–1871 (2006).
Rotimi, C. N. et al. Distribution of anthropometric variables and the prevalence of obesity in populations of west African origin: the International Collaborative Study on Hypertension in Blacks (ICSHIB). Obes. Res. 3 , 95–105 (1995).
Durazo-Arvizu, R. A. et al. Rapid increases in obesity in Jamaica, compared to Nigeria and the United States. BMC Public Health 8 , 133 (2008).
Hu, F. B., Li, T. Y., Colditz, G. A., Willett, W. C. & Manson, J. E. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA 289 , 1785–1791 (2003).
Rissanen, A. M., Heliövaara, M., Knekt, P., Reunanen, A. & Aromaa, A. Determinants of weight gain and overweight in adult Finns. Eur. J. Clin. Nutr. 45 , 419–430 (1991).
Zimmet, P. Z., Arblaster, M. & Thoma, K. The effect of westernization on native populations. Studies on a Micronesian community with a high diabetes prevalence. Aust. NZ J. Med. 8 , 141–146 (1978).
Ulijaszek, S. J. Increasing body size among adult Cook Islanders between 1966 and 1996. Ann. Hum. Biol. 28 , 363–373 (2001).
Snowdon, W. & Thow, A. M. Trade policy and obesity prevention: challenges and innovation in the Pacific Islands. Obes. Rev. 14 , 150–158 (2013).
McLennan, A. K. & Ulijaszek, S. J. Obesity emergence in the Pacific islands: why understanding colonial history and social change is important. Public Health Nutr. 18 , 1499–1505 (2015).
Becker, A. E., Gilman, S. E. & Burwell, R. A. Changes in prevalence of overweight and in body image among Fijian women between 1989 and 1998. Obes. Res. 13 , 110–117 (2005).
Swinburn, B., Sacks, G. & Ravussin, E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am. J. Clin. Nutr. 90 , 1453–1456 (2009).
Swinburn, B. A. et al. Estimating the changes in energy flux that characterize the rise in obesity prevalence. Am. J. Clin. Nutr. 89 , 1723–1728 (2009).
US Department of Agriculture. Food availability (per capita) data system. USDA https://www.ers.usda.gov/data-products/food-availability-per-capita-data-system/ (updated 29 Oct 2018).
Carden, T. J. & Carr, T. P. Food availability of glucose and fat, but not fructose, increased in the U.S. between 1970 and 2009: analysis of the USDA food availability data system. Nutr. J. 12 , 130 (2013).
Hall, K. D., Guo, J., Dore, M. & Chow, C. C. The progressive increase of food waste in America and its environmental impact. PLOS ONE 4 , e7940 (2009).
Scarborough, P. et al. Increased energy intake entirely accounts for increase in body weight in women but not in men in the UK between 1986 and 2000. Br. J. Nutr. 105 , 1399–1404 (2011).
McGinnis, J. M. & Nestle, M. The Surgeon General’s report on nutrition and health: policy implications and implementation strategies. Am. J. Clin. Nutr. 49 , 23–28 (1989).
Krebs-Smith, S. M., Reedy, J. & Bosire, C. Healthfulness of the U.S. food supply: little improvement despite decades of dietary guidance. Am. J. Prev. Med. 38 , 472–477 (2010).
Malik, V. S., Popkin, B. M., Bray, G. A., Després, J. P. & Hu, F. B. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation 121 , 1356–1364 (2010).
Schulze, M. B. et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 292 , 927–934 (2004).
Mozaffarian, D., Hao, T., Rimm, E. B., Willett, W. C. & Hu, F. B. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 364 , 2392–2404 (2011).
Malik, V. S. & Hu, F. B. Sugar-sweetened beverages and health: where does the evidence stand? Am. J. Clin. Nutr. 94 , 1161–1162 (2011).
Qi, Q. et al. Sugar-sweetened beverages and genetic risk of obesity. N. Engl. J. Med. 367 , 1387–1396 (2012).
Heiker, J. T. et al. Identification of genetic loci associated with different responses to high-fat diet-induced obesity in C57BL/6N and C57BL/6J substrains. Physiol. Genomics 46 , 377–384 (2014).
Wahlqvist, M. L. et al. Early-life influences on obesity: from preconception to adolescence. Ann. NY Acad. Sci. 1347 , 1–28 (2015).
Rohde, K. et al. Genetics and epigenetics in obesity. Metabolism . https://doi.org/10.1016/j.metabol.2018.10.007 (2018).
Article PubMed Google Scholar
Panzeri, I. & Pospisilik, J. A. Epigenetic control of variation and stochasticity in metabolic disease. Mol. Metab. 14 , 26–38 (2018).
Ruiz-Hernandez, A. et al. Environmental chemicals and DNA methylation in adults: a systematic review of the epidemiologic evidence. Clin. Epigenet. 7 , 55 (2015).
Quarta, C., Schneider, R. & Tschöp, M. H. Epigenetic ON/OFF switches for obesity. Cell 164 , 341–342 (2016).
Dalgaard, K. et al. Trim28 haploinsufficiency triggers bi-stable epigenetic obesity. Cell 164 , 353–364 (2015).
Michaelides, M. et al. Striatal Rgs4 regulates feeding and susceptibility to diet-induced obesity. Mol. Psychiatry . https://doi.org/10.1038/s41380-018-0120-7 (2018).
Article PubMed PubMed Central Google Scholar
Weihrauch-Blüher, S. et al. Current guidelines for obesity prevention in childhood and adolescence. Obes. Facts 11 , 263–276 (2018).
Nakamura, R. et al. Evaluating the 2014 sugar-sweetened beverage tax in Chile: An observational study in urban areas. PLOS Med. 15 , e1002596 (2018).
Colchero, M. A., Molina, M. & Guerrero-López, C. M. After Mexico implemented a tax, purchases of sugar-sweetened beverages decreased and water increased: difference by place of residence, household composition, and income level. J. Nutr. 147 , 1552–1557 (2017).
Brownell, K. D. & Warner, K. E. The perils of ignoring history: Big Tobacco played dirty and millions died. How similar is Big Food? Milbank Q. 87 , 259–294 (2009).
Mialon, M., Swinburn, B., Allender, S. & Sacks, G. ‘Maximising shareholder value’: a detailed insight into the corporate political activity of the Australian food industry. Aust. NZ J. Public Health 41 , 165–171 (2017).
Peeters, A. Obesity and the future of food policies that promote healthy diets. Nat. Rev. Endocrinol. 14 , 430–437 (2018).
Hawkes, C., Jewell, J. & Allen, K. A food policy package for healthy diets and the prevention of obesity and diet-related non-communicable diseases: the NOURISHING framework. Obes. Rev. 14 (Suppl. 2), 159–168 (2013).
World Health Organisation. Global database on the Implementation of Nutrition Action (GINA). WHO https://www.who.int/nutrition/gina/en/ (2012).
Popkin, B., Monteiro, C. & Swinburn, B. Overview: Bellagio Conference on program and policy options for preventing obesity in the low- and middle-income countries. Obes. Rev. 14 (Suppl. 2), 1–8 (2013).
Download references
Reviewer information
Nature Reviews Endocrinology thanks G. Bray, A. Sharma and H. Toplak for their contribution to the peer review of this work.
Author information
Authors and affiliations.
Department of Medicine, University of Leipzig, Leipzig, Germany
Matthias Blüher
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Matthias Blüher .
Ethics declarations
Competing interests.
The author declares no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Blüher, M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol 15 , 288–298 (2019). https://doi.org/10.1038/s41574-019-0176-8
Download citation
Published : 27 February 2019
Issue Date : May 2019
DOI : https://doi.org/10.1038/s41574-019-0176-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Body composition, lifestyle, and depression: a prospective study in the uk biobank.
BMC Public Health (2024)
Physical activity, gestational weight gain in obese patients with early gestational diabetes and the perinatal outcome – a randomised–controlled trial
- Lukasz Adamczak
- Urszula Mantaj
- Ewa Wender-Ozegowska
BMC Pregnancy and Childbirth (2024)
Effects of N-acetylcysteine on the expressions of UCP1 and factors related to thyroid function in visceral adipose tissue of obese adults: a randomized, double-blind clinical trial
- Mohammad Hassan Sohouli
- Ghazaleh Eslamian
Genes & Nutrition (2024)
What could be the reasons for not losing weight even after following a weight loss program?
- Jyoti Dabas
- S. Shunmukha Priya
- Praveen Budhrani
Journal of Health, Population and Nutrition (2024)
Effect of COVID-19 Pandemic Lockdowns on Body Mass Index of Primary School Children from Different Socioeconomic Backgrounds
- Ludwig Piesch
- Robert Stojan
- Till Utesch
Sports Medicine - Open (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Reference Manager
- Simple TEXT file
People also looked at
Review article, childhood and adolescent obesity: a review.

- 1 Division of Endocrinology, Diabetes and Metabolism, Department of Pediatrics, Medical College of Wisconsin, Milwaukee, WI, United States
- 2 Division of Adolescent Medicine, Department of Pediatrics, Medical College of Wisconsin Affiliated Hospitals, Milwaukee, WI, United States
- 3 Division of Adolescent Medicine, Department of Pediatrics, Medical College of Wisconsin, Milwaukee, WI, United States
Obesity is a complex condition that interweaves biological, developmental, environmental, behavioral, and genetic factors; it is a significant public health problem. The most common cause of obesity throughout childhood and adolescence is an inequity in energy balance; that is, excess caloric intake without appropriate caloric expenditure. Adiposity rebound (AR) in early childhood is a risk factor for obesity in adolescence and adulthood. The increasing prevalence of childhood and adolescent obesity is associated with a rise in comorbidities previously identified in the adult population, such as Type 2 Diabetes Mellitus, Hypertension, Non-alcoholic Fatty Liver disease (NAFLD), Obstructive Sleep Apnea (OSA), and Dyslipidemia. Due to the lack of a single treatment option to address obesity, clinicians have generally relied on counseling dietary changes and exercise. Due to psychosocial issues that may accompany adolescence regarding body habitus, this approach can have negative results. Teens can develop unhealthy eating habits that result in Bulimia Nervosa (BN), Binge- Eating Disorder (BED), or Night eating syndrome (NES). Others can develop Anorexia Nervosa (AN) as they attempt to restrict their diet and overshoot their goal of “being healthy.” To date, lifestyle interventions have shown only modest effects on weight loss. Emerging findings from basic science as well as interventional drug trials utilizing GLP-1 agonists have demonstrated success in effective weight loss in obese adults, adolescents, and pediatric patients. However, there is limited data on the efficacy and safety of other weight-loss medications in children and adolescents. Nearly 6% of adolescents in the United States are severely obese and bariatric surgery as a treatment consideration will be discussed. In summary, this paper will overview the pathophysiology, clinical, and psychological implications, and treatment options available for obese pediatric and adolescent patients.
Introduction
Obesity is a complex issue that affects children across all age groups ( 1 – 3 ). One-third of children and adolescents in the United States are classified as either overweight or obese. There is no single element causing this epidemic, but obesity is due to complex interactions between biological, developmental, behavioral, genetic, and environmental factors ( 4 ). The role of epigenetics and the gut microbiome, as well as intrauterine and intergenerational effects, have recently emerged as contributing factors to the obesity epidemic ( 5 , 6 ). Other factors including small for gestational age (SGA) status at birth, formula rather than breast feeding in infancy, and early introduction of protein in infant's dietary intake have been reportedly associated with weight gain that can persist later in life ( 6 – 8 ). The rising prevalence of childhood obesity poses a significant public health challenge by increasing the burden of chronic non-communicable diseases ( 1 , 9 ).
Obesity increases the risk of developing early puberty in children ( 10 ), menstrual irregularities in adolescent girls ( 1 , 11 ), sleep disorders such as obstructive sleep apnea (OSA) ( 1 , 12 ), cardiovascular risk factors that include Prediabetes, Type 2 Diabetes, High Cholesterol levels, Hypertension, NAFLD, and Metabolic syndrome ( 1 , 2 ). Additionally, obese children and adolescents can suffer from psychological issues such as depression, anxiety, poor self-esteem, body image and peer relationships, and eating disorders ( 13 , 14 ).
So far, interventions for overweight/obesity prevention have mainly focused on behavioral changes in an individual such as increasing daily physical exercise or improving quality of diet with restricting excess calorie intake ( 1 , 15 , 16 ). However, these efforts have had limited results. In addition to behavioral and dietary recommendations, changes in the community-based environment such as promotion of healthy food choices by taxing unhealthy foods ( 17 ), improving lunch food quality and increasing daily physical activity at school and childcare centers, are extra measures that are needed ( 16 ). These interventions may include a ban on unhealthy food advertisements aimed at children as well as access to playgrounds and green spaces where families can feel their children can safely recreate. Also, this will limit screen time for adolescents as well as younger children.
However, even with the above changes, pharmacotherapy and/or bariatric surgery will likely remain a necessary option for those youth with morbid obesity ( 1 ). This review summarizes our current understanding of the factors associated with obesity, the physiological and psychological effects of obesity on children and adolescents, and intervention strategies that may prevent future concomitant issues.
Definition of Childhood Obesity
Body mass index (BMI) is an inexpensive method to assess body fat and is derived from a formula derived from height and weight in children over 2 years of age ( 1 , 18 , 19 ). Although more sophisticated methods exist that can determine body fat directly, they are costly and not readily available. These methods include measuring skinfold thickness with a caliper, Bioelectrical impedance, Hydro densitometry, Dual-energy X-ray Absorptiometry (DEXA), and Air Displacement Plethysmography ( 2 ).
BMI provides a reasonable estimate of body fat indirectly in the healthy pediatric population and studies have shown that BMI correlates with body fat and future health risks ( 18 ). Unlike in adults, Z-scores or percentiles are used to represent BMI in children and vary with the age and sex of the child. BMI Z-score cut off points of >1.0, >2.0, and >3.0 are recommended by the World Health Organization (WHO) to define at risk of overweight, overweight and obesity, respectively ( 19 ). However, in terms of percentiles, overweight is applied when BMI is >85th percentile <95th percentile, whereas obesity is BMI > 95th percentile ( 20 – 22 ). Although BMI Z-scores can be converted to BMI percentiles, the percentiles need to be rounded and can misclassify some normal-weight children in the under or overweight category ( 19 ). Therefore, to prevent these inaccuracies and for easier understanding, it is recommended that the BMI Z-scores in children should be used in research whereas BMI percentiles are best used in the clinical settings ( 20 ).
As BMI does not directly measure body fat, it is an excellent screening method, but should not be used solely for diagnostic purposes ( 23 ). Using 85th percentile as a cut off point for healthy weight may miss an opportunity to obtain crucial information on diet, physical activity, and family history. Once this information is obtained, it may allow the provider an opportunity to offer appropriate anticipatory guidance to the families.
Pathophysiology of Obesity
The pathophysiology of obesity is complex that results from a combination of individual and societal factors. At the individual level, biological, and physiological factors in the presence of ones' own genetic risk influence eating behaviors and tendency to gain weight ( 1 ). Societal factors include influence of the family, community and socio-economic resources that further shape these behaviors ( Figure 1 ) ( 3 , 24 ).
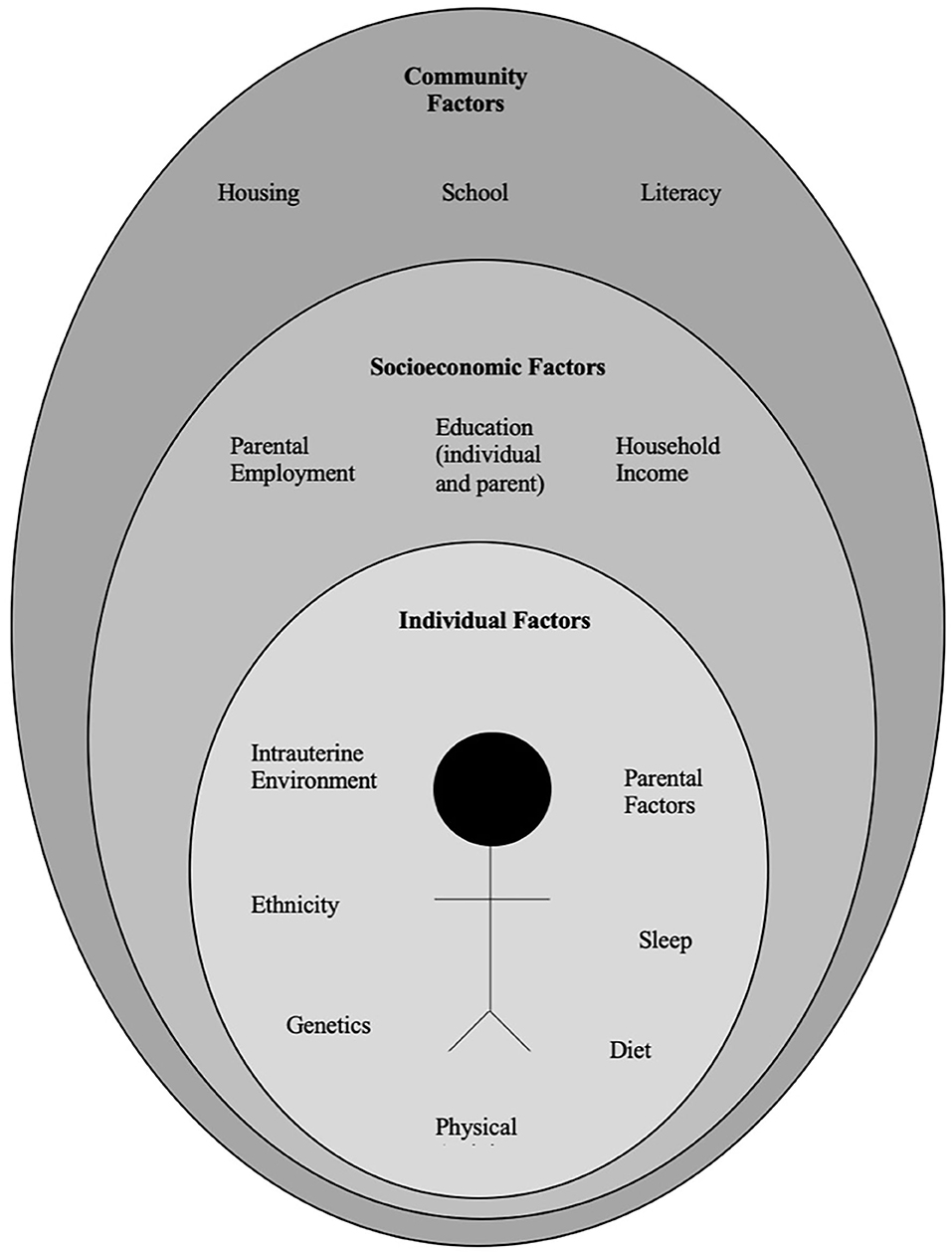
Figure 1 . Multidimensional factors contributing to child and adolescent obesity.
Biological Factors
There is a complex architecture of neural and hormonal regulatory control, the Gut-Brain axis, which plays a significant role in hunger and satiety ( Figure 2 ). Sensory stimulation (smell, sight, and taste), gastrointestinal signals (peptides, neural signals), and circulating hormones further contribute to food intake ( 25 – 27 ).
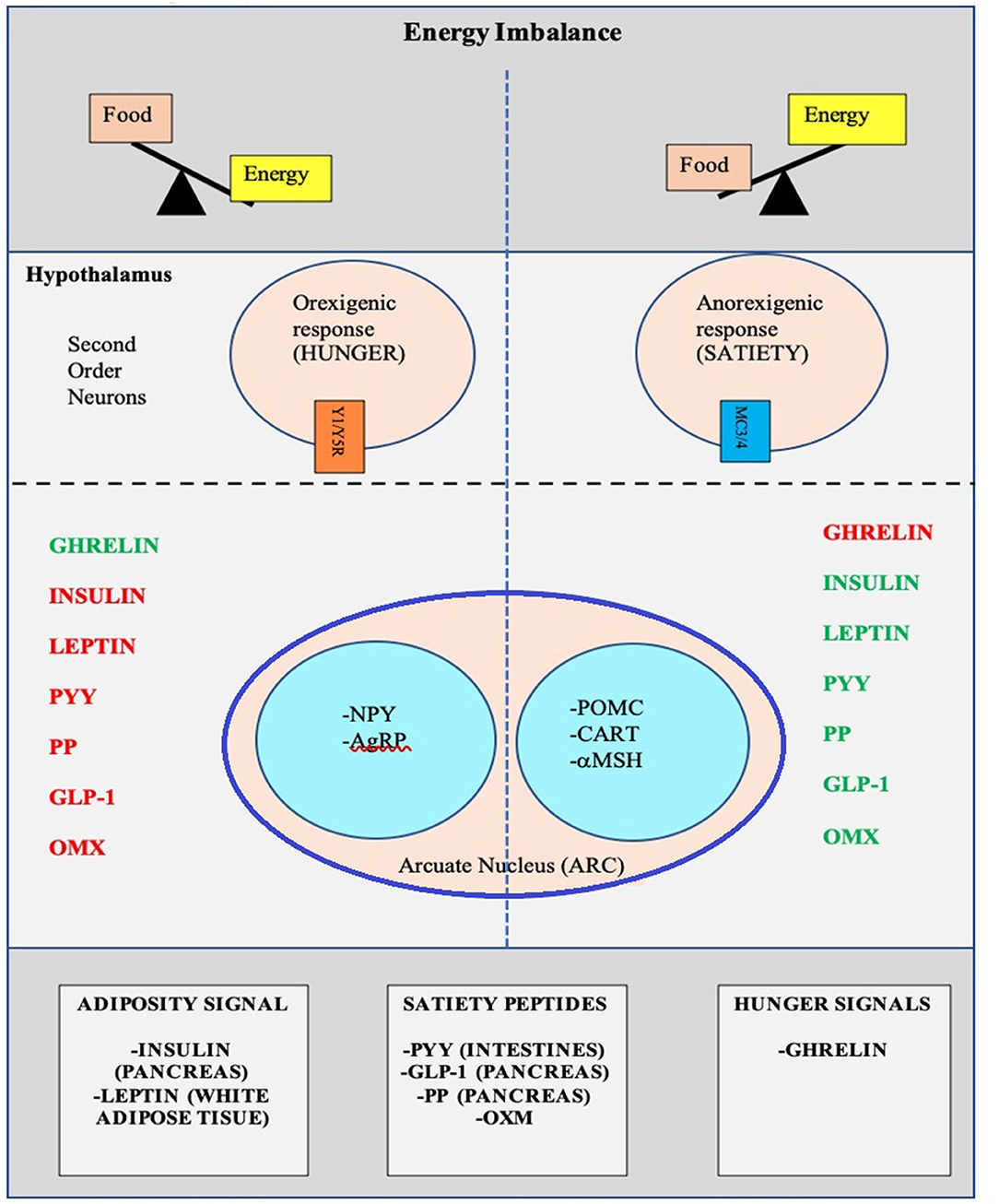
Figure 2 . Pictorial representation of the Hunger-Satiety pathway a and the various hormones b involved in the pathway. a, Y1/Y5R and MC3/4 are second order neuro receptors which are responsible in either the hunger or satiety pathway. Neurons in the ARC include: NPY, Neuropeptide Y; AgRP, Agouti-Related Peptide; POMC, Pro-Opiomelanocortin; CART, Cocaine-and Amphetamine-regulated Transcript; α-MSH, α-Melanocyte Stimulating Hormone. b, PYY, Peptide YY; PP, Pancreatic Polypeptide; GLP-1, Glucagon-Like Peptide- I; OMX, Oxyntomodulin.
The hypothalamus is the crucial region in the brain that regulates appetite and is controlled by key hormones. Ghrelin, a hunger-stimulating (orexigenic) hormone, is mainly released from the stomach. On the other hand, leptin is primarily secreted from adipose tissue and serves as a signal for the brain regarding the body's energy stores and functions as an appetite -suppressing (anorexigenic) hormone. Several other appetite-suppressing (anorexigenic) hormones are released from the pancreas and gut in response to food intake and reach the hypothalamus through the brain-blood barrier (BBB) ( 28 – 32 ). These anorexigenic and orexigenic hormones regulate energy balance by stimulating hunger and satiety by expression of various signaling pathways in the arcuate nucleus (ARC) of the hypothalamus ( Figure 2 ) ( 28 , 33 ). Dysregulation of appetite due to blunted suppression or loss of caloric sensing signals can result in obesity and its morbidities ( 34 ).
Emotional dysfunction due to psychiatric disorders can cause stress and an abnormal sleep-wake cycles. These modifications in biological rhythms can result in increased appetite, mainly due to ghrelin, and can contribute to emotional eating ( 35 ).
Recently, the role of changes in the gut microbiome with increased weight gain through several pathways has been described in literature ( 36 , 37 ). The human gut serves as a host to trillions of microorganisms, referred to as gut microbiota. The dominant gut microbial phyla are Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia, with Firmicutes and Bacteroidetes representing 90% of human gut microbiota ( 5 , 38 ). The microbes in the gut have a symbiotic relationship within their human host and provide a nutrient-rich environment. Gut microbiota can be affected by various factors that include gestational age at birth, mode of infant delivery, type of neonatal and infant feeding, introduction of solid food, feeding practices and external factors like antibiotic use ( 5 , 38 ). Also, the maturation of the bacterial phyla that occurs from birth to adulthood ( 39 ), is influenced by genetics, environment, diet, lifestyle, and gut physiology and stabilizes in adulthood ( 5 , 39 , 40 ). Gut microbiota is unique to each individual and plays a specific role in maintaining structural integrity, and the mucosal barrier of the gut, nutrient metabolism, immune response, and protection against pathogens ( 5 , 37 , 38 ). In addition, the microbiota ferments the indigestible food and synthesizes other essential micronutrients as well as short chain fatty acids (SCFAs') ( 40 , 41 ). Dysbiosis or imbalance of the gut microbiota, in particularly the role of SCFA has been linked with the patho-physiology of obesity ( 36 , 38 , 41 , 42 ). SCFAs' are produced by anaerobic fermentation of dietary fiber and indigestible starch and play a role in mammalian energy metabolism by influencing gut-brain communication axis. Emerging evidence has shown that increased ratio of Firmicutes to Bacteroidetes causes increased energy extraction of calories from diets and is evidenced by increased production of short chain fatty acids (SCFAs') ( 43 – 45 ). However, this relationship is not affirmed yet, as a negative relationship between SCFA levels and obesity has also been reported ( 46 ). Due to the conflicting data, additional randomized control trials are needed to clarify the role of SCFA's in obese and non-obese individuals.
The gut microbiota also has a bidirectional interaction with the liver, and various additional factors such as diet, genetics, and the environment play a key role in this relationship. The Gut- Liver Axis is interconnected at various levels that include the mucus barrier, epithelial barrier, and gut microbiome and are essential to maintain normal homeostasis ( 47 ). Increased intestinal mucosal permeability can disrupt the gut-liver axis, which releases various inflammatory markers, activates an innate immune response in the liver, and results in a spectrum of liver diseases that include hepatic steatosis, non-alcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma (HCC) ( 48 , 49 ).
Other medical conditions, including type 2 Diabetes Mellitus, Metabolic Syndrome, eating disorders as well as psychological conditions such as anxiety and depression are associated with the gut microbiome ( 50 – 53 ).
Genetic Factors
Genetic causes of obesity can either be monogenic or polygenic types. Monogenic obesity is rare, mainly due to mutations in genes within the leptin/melanocortin pathway in the hypothalamus that is essential for the regulation of food intake/satiety, body weight, and energy metabolism ( 54 ). Leptin regulates eating behaviors, the onset of puberty, and T-cell immunity ( 55 ). About 3% of obese children have mutations in the leptin ( LEP ) gene and the leptin receptor (LEPR) and can also present with delayed puberty and immune dysfunction ( 55 , 56 ). Obesity caused by other genetic mutations in the leptin-melanocortin pathway include proopiomelanocortin (POMC) and melanocortin receptor 4 (MC4R), brain-derived neurotrophic factor (BDNF), and the tyrosine kinase receptor B (NTRK2) genes ( 57 , 58 ). Patients with monogenic forms generally present during early childhood (by 2 years old) with severe obesity and abnormal feeding behaviors ( 59 ). Other genetic causes of severe obesity are Prader Willi Syndrome (PWS), Alström syndrome, Bardet Biedl syndrome. Patients with these syndromes present with additional characteristics, including cognitive impairment, dysmorphic features, and organ-specific developmental abnormalities ( 60 ). Individuals who present with obesity, developmental delay, dysmorphic features, and organ dysfunction should receive a genetics referral for further evaluation.
Polygenic obesity is the more common form of obesity, caused by the combined effect of multiple genetic variants. It is the result of the interplay between genetic susceptibility and the environment, also known as the Gene-Environment Interaction (GEI) ( 61 – 64 ). Genome-wide association studies (GWAS) have identified gene variants [single nucleotide polymorphism (SNPs)] for body mass index (BMI) that likely act synergistically to affect body weight ( 65 ). Studies have identified genetic variants in several genes that may contribute to excessive weight gain by increasing hunger and food intake ( 66 – 68 ). When the genotype of an individual confers risk for obesity, exposure to an obesogenic environment may promote a state of energy imbalance due to behaviors that contribute to conserving rather than expending energy ( 69 , 70 ). Research studies have shown that obese individuals have a genetic variation that can influence their actions, such as increased food intake, lack of physical activity, a decreased metabolism, as well as an increased tendency to store body fat ( 63 , 66 , 67 , 69 , 70 ).
Recently the role of epigenetic factors in the development of obesity has emerged ( 71 ). The epigenetic phenomenon may alter gene expression without changing the underlying DNA sequence. In effect, epigenetic changes may result in the addition of chemical tags known as methyl groups, to the individual's chromosomes. This alteration can result in a phenomenon where critical genes are primed to on and off regulate. Complex physiological and psychological adjustment occur during infancy and can thereafter set the stage for health vs. disease. Developmental origins of health and disease (DOHaD) shows that early life environment can impact the risk of chronic diseases later in life due to fetal programming secondary to epigenetic changes ( 72 ). Maternal nutrition during the prenatal or early postnatal period may trigger these epigenetic changes and increase the risk for chronic conditions such as obesity, metabolic and cardiovascular disease due to epigenetic modifications that may persist and cause intergenerational effect on the health children and adults ( 58 , 73 , 74 ). Similarly, adverse childhood experiences (ACE) have been linked to a broad range of negative outcomes through epigenetic mechanisms ( 75 ) and promote unhealthy eating behaviors ( 76 , 77 ). Other factors such as diet, physical activity, environmental and psychosocial stressors can cause epigenetic changes and place an individual at risk for weight gain ( 78 ).
Developmental Factors
Eating behaviors evolve over the first few years of life. Young children learn to eat through their direct experience with food and observing others eating around them ( 79 ). During infancy, feeding defines the relationship of security and trust between a child and the parent. Early childhood eating behaviors shift to more self-directed control due to rapid physical, cognitive, communicative, and social development ( 80 ). Parents or caregivers determine the type of food that is made available to the infant and young child. However, due to economic limitations and parents having decreased time to prepare nutritious meals, consumption of processed and cheaper energy-dense foods have occurred in Western countries. Additionally, feeding practices often include providing large or super-sized portions of palatable foods and encouraging children to finish the complete meal (clean their plate even if they do not choose to), as seen across many cultures ( 81 , 82 ). Also, a segment of parents are overly concerned with dietary intake and may pressurize their child to eat what they perceive as a healthy diet, which can lead to unintended consequences ( 83 ). Parents' excessive restriction of food choices may result in poor self-regulation of energy intake by their child or adolescent. This action may inadvertently promote overconsumption of highly palatable restricted foods when available to the child or adolescent outside of parental control with resultant excessive weight gain ( 84 , 85 ).
During middle childhood, children start achieving greater independence, experience broader social networks, and expand their ability to develop more control over their food choices. Changes that occur in the setting of a new environment such as daycare or school allow exposure to different food options, limited physical activity, and often increased sedentary behaviors associated with school schedules ( 24 ). As the transition to adolescence occurs, physical and psychosocial development significantly affect food choices and eating patterns ( 25 ). During the teenage years, more independence and interaction with peers can impact the selection of fast foods that are calorically dense. Moreover, during the adolescent years, more sedentary behaviors such as video and computer use can limit physical exercise. Adolescence is also a period in development with an enhanced focus on appearance, body weight, and other psychological concerns ( 86 , 87 ).
Environmental Factors
Environmental changes within the past few decades, particularly easy access to high-calorie fast foods, increased consumption of sugary beverages, and sedentary lifestyles, are linked with rising obesity ( 88 ). The easy availability of high caloric fast foods, and super-sized portions, are increasingly common choices as individuals prefer these highly palatable and often less expensive foods over fruits and vegetables ( 89 ). The quality of lunches and snacks served in schools and childcare centers has been an area of debate and concern. Children and adolescents consume one-third to one-half of meals in the above settings. Despite policies in place at schools, encouraging foods, beverages, and snacks that are deemed healthier options, the effectiveness of these policies in improving children's dietary habits or change in obesity rate has not yet been seen ( 90 ). This is likely due to the fact that such policies primarily focus on improving dietary quality but not quantity which can impact the overweight or obese youth ( 91 ). Policies to implement taxes on sugary beverages are in effect in a few states in the US ( 92 ) as sugar and sugary beverages are associated with increased weight gain ( 2 , 3 ). This has resulted in reduction in sales of sugary drinks in these states, but the sales of these types of drinks has risen in neighboring states that did not implement the tax ( 93 ). Due to advancements in technology, children are spending increased time on electronic devices, limiting exercise options. Technology advancement is also disrupting the sleep-wake cycle, causing poor sleeping habits, and altered eating patterns ( 94 ). A study published on Canadian children showed that the access to and night-time use of electronic devices causes decreased sleep duration, resulting in excess body weight, inferior diet quality, and lower physical activity levels ( 95 ).
Infant nutrition has gained significant popularity in relation to causing overweight/obesity and other diseases later in life. Breast feeding is frequently discussed as providing protection against developing overweight/obesity in children ( 8 ). Considerable heterogeneity has been observed in studies and conducting randomized clinical trials between breast feeding vs. formula feeding is not feasible ( 8 ). Children fed with a low protein formula like breast milk are shown to have normal weight gain in early childhood as compared to those that are fed formulas with a high protein load ( 96 ). A recent Canadian childbirth cohort study showed that breast feeding within first year of life was inversely associated with weight gain and increased BMI ( 97 ). The effect was stronger if the child was exclusively breast fed directly vs. expressed breast milk or addition of formula or solid food ( 97 ). Also, due to the concern of poor growth in preterm or SGA infants, additional calories are often given for nutritional support in the form of macronutrient supplements. Most of these infants demonstrate “catch up growth.” In fact, there have been reports that in some children the extra nutritional support can increase the risk for overweight/obesity later in life. The association, however, is inconsistent. Recently a systemic review done on randomized controlled trials comparing the studies done in preterm and SGA infants with feeds with and without macronutrient supplements showed that macronutrient supplements may increase weight and length in toddlers but did not show a significant increase in the BMI during childhood ( 98 ). Increased growth velocity due to early introduction of formula milk and protein in infants' diet, may influence the obesity pathways, and can impact fetal programming for metabolic disease later in life ( 99 ).
General pediatricians caring for children with overweight/obesity, generally recommend endocrine testing as parents often believe that there may be an underlying cause for this condition and urge their primary providers to check for conditions such as thyroid abnormalities. Endocrine etiologies for obesity are rarely identified and patients with underlying endocrine disorders causing excessive weight gain usually are accompanied by attenuated growth patterns, such that a patient continues to gain weight with a decline in linear height ( 100 ). Various endocrine etiologies that one could consider in a patient with excessive weight gain in the setting of slow linear growth: severe hypothyroidism, growth hormone deficiency, and Cushing's disease/syndrome ( 58 , 100 ).
Clinical-Physiology of Pediatric Obesity
It is a well-known fact that early AR(increased BMI) before the age of 5 years is a risk factor for adult obesity, obesity-related comorbidities, and metabolic syndrome ( 101 – 103 ). Typically, body mass index (BMI) declines to a minimum in children before it starts increasing again into adulthood, also known as AR. Usually, AR happens between 5 and 7 years of age, but if it occurs before the age of 5 years is considered early AR. Early AR is a marker for higher risk for obesity-related comorbidities. These obesity-related health comorbidities include cardiovascular risk factors (hypertension, dyslipidemia, prediabetes, and type 2 diabetes), hormonal issues, orthopedic problems, sleep apnea, asthma, and fatty liver disease ( Figure 3 ) ( 9 ).
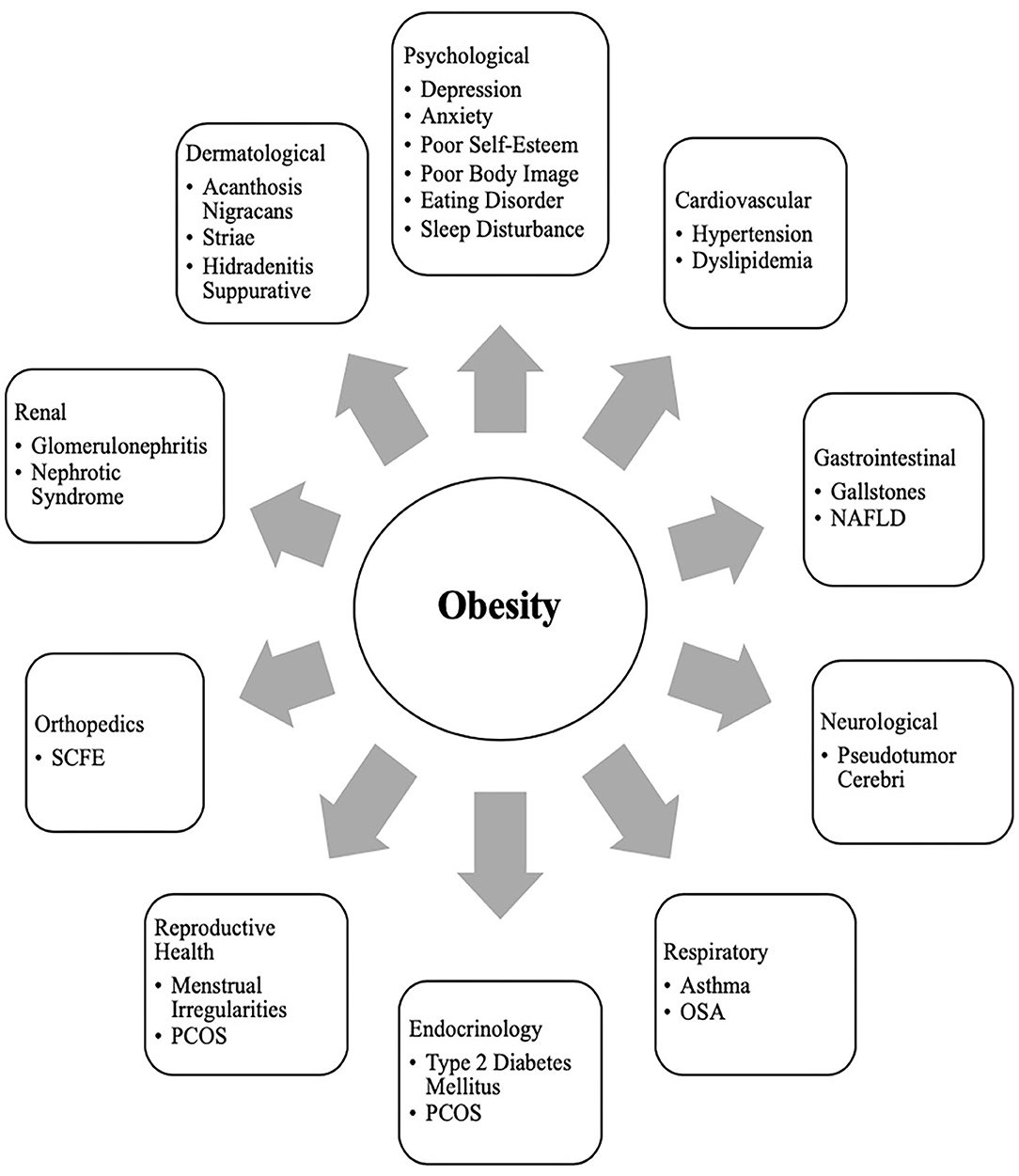
Figure 3 . Obesity related co-morbidities a in children and adolescents. a, NAFLD, Non-Alcoholic Fatty Liver Disease; SCFE, Slipped Capital Femoral Epiphysis; PCOS, Polycystic Ovary Syndrome; OSA, Obstructive Sleep Apnea.
Clinical Comorbidities of Obesity in Children
Growth and puberty.
Excess weight gain in children can influence growth and pubertal development ( 10 ). Childhood obesity can cause prepubertal acceleration of linear growth velocity and advanced bone age in boys and girls ( 104 ). Hyperinsulinemia is a normal physiological state during puberty, but children with obesity can have abnormally high insulin levels ( 105 ). Leptin resistance also occurs in obese individuals who have higher leptin levels produced by their adipose tissue ( 55 , 106 ). The insulin and leptin levels can act on receptors that impact the growth plates with a resultant bone age advancement ( 55 ).
Adequate nutrition is essential for the typical timing and tempo of pubertal onset. Excessive weight gain can initiate early puberty, due to altered hormonal parameters ( 10 ). Obese children may present with premature adrenarche, thelarche, or precocious puberty (PP) ( 107 ). The association of early pubertal changes with obesity is consistent in girls, and is well-reported; however, data is sparse in boys ( 108 ). One US study conducted in racially diverse boys showed obese boys had delayed puberty, whereas overweight boys had early puberty as compared to normal-weight boys ( 109 ). Obese girls with PP have high leptin levels ( 110 , 111 ). Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) is a cross-sectional study and suggested an indirect relationship between elevated leptin levels, early puberty, and cardiometabolic and inflammatory markers in obese girls ( 112 ). Additionally, obese girls with premature adrenarche carry a higher risk for developing polycystic ovary syndrome (PCOS) in the future ( 113 , 114 ).
Sleep Disorders
Obesity is an independent risk factor for obstructive sleep apnea (OSA) in children and adolescents ( 12 , 115 ). Children with OSA have less deleterious consequences in terms of cardiovascular stress of metabolic syndrome when compared to adolescents and adults ( 116 , 117 ). In children, abnormal behaviors and neurocognitive dysfunction are the most critical and frequent end-organ morbidities associated with OSA ( 12 ). However, in adolescents, obesity and OSA can independently cause oxidative systemic stress and inflammation ( 118 , 119 ), and when this occurs concurrently, it can result in more severe metabolic dysfunction and cardiovascular outcomes later in life ( 120 ).
Other Comorbidities
Obesity is related to a clinical spectrum of liver abnormalities such as NAFLD ( 121 ); the most important cause of liver disease in children ( 122 – 124 ). NAFLD includes steatosis (increased liver fat without inflammation) and NASH (increased liver fat with inflammation and hepatic injury). While in some adults NAFLD can progress to an end-stage liver disease requiring liver transplant ( 125 , 126 ), the risk of progression during childhood is less well-defined ( 127 ). NAFLD is closely associated with metabolic syndrome including central obesity, insulin resistance, type 2 diabetes, dyslipidemia, and hypertension ( 128 ).
Obese children are also at risk for slipped capital femoral epiphysis (SCFE) ( 129 ), and sedentary lifestyle behaviors may have a negative influence on the brain structure and executive functioning, although the direction of causality is not clear ( 130 , 131 ).
Clinical Comorbidities of Obesity in Adolescents
Menstrual irregularities and pcos.
At the onset of puberty, physiologically, sex steroids can cause appropriate weight gain and body composition changes that should not affect normal menstruation ( 132 , 133 ). However, excessive weight gain in adolescent girls can result in irregular menstrual cycles and puts them at risk for PCOS due to increased androgen levels. Additionally, they can have excessive body hair (hirsutism), polycystic ovaries, and can suffer from distorted body images ( 134 , 135 ). Adolescent girls with PCOS also have an inherent risk for insulin resistance irrespective of their weight. However, weight gain further exacerbates their existing state of insulin resistance and increases the risk for obesity-related comorbidities such as metabolic syndrome, and type 2 diabetes. Although the diagnosis of PCOS can be challenging at this age due to an overlap with predictable pubertal changes, early intervention (appropriate weight loss and use of hormonal methods) can help restore menstrual cyclicity and future concerns related to childbearing ( 11 ).
Metabolic Syndrome and Sleep Disorders
Metabolic syndrome (MS) is a group of cardiovascular risk factors characterized by acanthosis nigricans, prediabetes, hypertension, dyslipidemia, and non-alcoholic steatohepatitis (NASH), that occurs from insulin resistance caused by obesity ( 136 ). Diagnosis of MS in adults requires at least three out of the five risk factors: increased central adiposity, hypertension, hyperglycemia, hypertriglyceridemia, or low HDL level. Definitions to diagnose MS are controversial in younger age groups, and many definitions have been proposed ( 136 ). This is due to the complex physiology of growth and development during puberty, which causes significant overlap between MS and features of normal growth. However, childhood obesity is associated with an inflammatory state even before puberty ( 137 ). In obese children and adolescents, hyperinsulinemia during puberty ( 138 , 139 ) and unhealthy sleep behaviors increase MS's risk and severity ( 140 ). Even though there is no consensus on diagnosis regarding MS in this age group, when dealing with obese children and adolescents, clinicians should screen them for MS risk factors and sleep behaviors and provide recommendations for weight management.
Social Psychology of Pediatric Obesity in Children and Adolescents
Obese children and adolescents may experience psychosocial sequelae, including depression, bullying, social isolation, diminished self-esteem, behavioral problems, dissatisfaction with body image, and reduced quality of life ( 13 , 141 ). Compared with normal-weight counterparts, overweight/obesity is one of the most common reasons children and adolescents are bullied at school ( 142 ). The consequence of stigma, bullying, and teasing related to childhood obesity are pervasive and can have severe implications for emotional and physical health and performance that can persist later in life ( 13 ).
In adolescents, psychological outcomes associated with obesity are multifactorial and have a bidirectional relationship ( Figure 4 ). Obese adolescents due to their physique may have a higher likelihood of psychosocial health issues, including depression, body image/dissatisfaction, lower self-esteem, peer victimization/bullying, and interpersonal relationship difficulties. They may also demonstrate reduced resilience to challenging situations compared to their non-obese/overweight counterparts ( 9 , 143 – 146 ). Body image dissatisfaction has been associated with further weight gain but can also be related to the development of a mental health disorder or an eating disorder (ED) or disorder eating habits (DEH). Mental health disorders such as depression are associated with poor eating habits, a sedentary lifestyle, and altered sleep patterns. ED or DEH that include anorexia nervosa (AN), bulimia nervosa (BN), binge-eating disorder (BED) or night eating syndrome (NES) may be related to an individual's overvaluation of their body shape and weight or can result during the treatment for obesity ( 147 – 150 ). The management of obesity can place a patient at risk of AN if there is a rigid focus on caloric intake or if a patient overcorrects and initiates obsessive self-directed dieting. Healthcare providers who primarily care for obese patients, usually give the advice to diet to lose weight and then maintain it. However, strict dieting (hypocaloric diet), which some patients may later engage in can lead to an eating disorder such as anorexia nervosa ( 151 ). This behavior leads to a poor relationship with food, and therefore, adolescents perseverate on their weight and numbers ( 152 ).
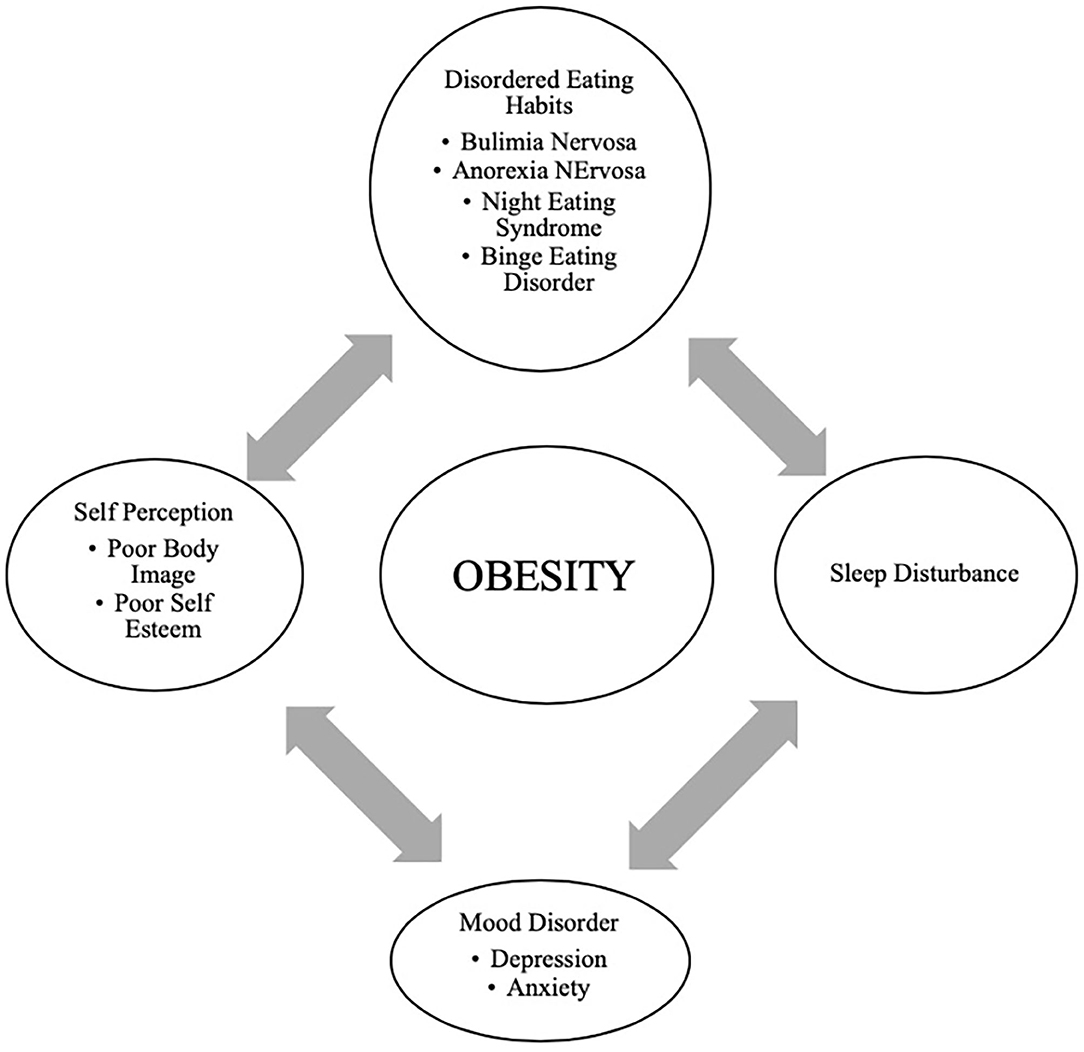
Figure 4 . Bidirectional relationship of different psychological outcomes of obesity.
Providers may not recognize DEHs when a morbidly obese patient loses the same weight as a healthy weight individual ( 149 ). It may appear as a positive result with families and others praising the individual without realizing that this youth may be engaging in destructive behaviors related to weight control. Therefore, it is essential to screen regarding the process of how weight loss was achieved ( 144 , 150 ).
Support and attention to underlying psychological concerns can positively affect treatment, overall well-being, and reduce the risk of adult obesity ( 150 ). The diagram above represents the complexity of the different psychological issues which can impact the clinical care of the obese adolescent.
Eating family meals together can improve overall dietary intake due to enhanced food choices mirrored by parents. It has also may serve as a support to individuals with DEHs if there is less attention to weight and a greater focus on appropriate, sustainable eating habits ( 148 ).
Prevention and Anticipatory Guidance
It is essential to recognize and provide preventive measures for obesity during early childhood and adolescence ( 100 , 153 , 154 ). It is well-established that early AR is a risk factor for adult obesity ( 66 – 68 ). Therefore, health care providers caring for the pediatric population need to focus on measures such as BMI but provide anticipatory guidance regarding nutritional counseling without stigmatizing or judging parents for their children's overweight/obesity ( 155 ). Although health care providers continue to pursue effective strategies to address the obesity epidemic; ironically, they frequently exhibit weight bias and stigmatizing behaviors. Research has demonstrated that the language that health care providers use when discussing a patient's body weight can reinforce stigma, reduce motivation for weight loss, and potentially cause avoidance of routine preventive care ( 155 ). In adolescents, rather than motivating positive changes, stigmatizing language regarding weight may negatively impact a teen and result in binge eating, decreased physical activity, social isolation, avoidance of health care services, and increased weight gain ( 156 , 157 ). Effective provider-patient communication using motivational interviewing techniques are useful to encourage positive behavior changes ( 155 , 158 ).
Anticipatory guidance includes educating the families on healthy eating habits and identifying unhealthy eating practices, encouraging increased activity, limiting sedentary activities such as screen time. Lifestyle behaviors in children and adolescents are influenced by many sectors of our society, including the family ( Figure 1 ) ( 3 , 24 ). Therefore, rather than treating obesity in isolation as an individual problem, it is crucial to approach this problem by focusing on the family unit. Family-based multi-component weight loss behavioral treatment is the gold standard for treating childhood obesity, and it is having been found useful in those between 2 and 6 years old ( 150 , 159 ). Additionally, empowering the parents to play an equal role in developing and implementing an intervention for weight management has shown promising results in improving the rate of obesity by decreasing screen time, promoting healthy eating, and increasing support for children's physical activity ( 160 , 161 ).
When dietary/lifestyle modifications have failed, the next option is a structured weight -management program with a multidisciplinary approach ( 15 ). The best outcomes are associated with an interdisciplinary team comprising a physician, dietician, and psychologist generally 1–2 times a week ( 15 , 162 ). However, this treatment approach is not effective in patients with severe obesity ( 122 ). Although healthier lifestyle recommendations for weight loss are the current cornerstone for obesity management, they often fail. As clinicians can attest, these behavioral and dietary changes are hard to achieve, and all too often is not effective in patients with severe obesity. Failure to maintain substantial weight loss over the long term is due to poor adherence to the prescribed lifestyle changes as well as physiological responses that resist weight loss ( 163 ). American TV hosts a reality show called “The Biggest Loser” that centers on overweight and obese contestants attempting to lose weight for a cash prize. Contestants from “The Biggest Loser” competition, had metabolic adaptation (MA) after drastic weight loss, regained more than they lost weight after 6 years due to a significant slow resting metabolic rate ( 164 ). MA is a physiological response which is a reduced basal metabolic rate seen in individuals who are losing or have lost weight. In MA, the body alters how efficient it is at turning the food eaten into energy; it is a natural defense mechanism against starvation and is a response to caloric restriction. Plasma leptin levels decrease substantially during caloric restriction, suggesting a role of this hormone in the drop of energy expenditure ( 165 ).
Pharmacological Management
The role of pharmacological therapy in the treatment of obesity in children and adolescents is limited.
Orlistat is the only FDA approved medication for weight loss in 12-18-year-olds but has unpleasant side effects ( 166 ). Another medicine, Metformin, has been used in children with signs of insulin resistance, may have some impact on weight, but is not FDA approved ( 167 ). The combination of phentermine/topiramate (Qsymia) has been FDA approved for weight loss in obese individuals 18 years and older. In studies, there has been about 9–10% weight loss over 2 years. However, caution must be taken in females as it can lead to congenital disabilities, especially with use in the first trimester of pregnancy ( 167 ).
GLP-1 agonists have demonstrated great success in effective weight loss and are approved by the FDA for adult obesity ( 168 – 170 ). A randomized control clinical trial recently published showed a significant weight loss in those using liraglutide (3.0 mg)/day plus lifestyle therapy group compared to placebo plus lifestyle therapy in children between the ages of 12–18 years ( 171 ).
Recently during the EASL conference, academic researchers and industry partners presented novel interventions targeting different gut- liver axis levels that include intestinal content, intestinal microbiome, intestinal mucosa, and peritoneal cavity ( 47 ). The focus for these therapeutic interventions within the gut-liver axis was broad and ranged anywhere from newer drugs protecting the intestinal mucus lining, restoring the intestinal barriers and improvement in the gut microbiome. One of the treatment options was Hydrogel technology which was shown to be effective toward weight loss in patients with metabolic syndrome. Hydrogel technology include fibers and high viscosity polysaccharides that absorb water in the stomach and increasing the volume, thereby improving satiety ( 47 ). Also, a clinical trial done in obese pregnant mothers using Docosahexaenoic acid (DHA) showed that the mothers' who got DHA had children with lower adiposity at 2 and 4 years of age ( 172 ). Recently the role of probiotics in combating obesity has emerged. Probiotics are shown to alter the gut microbiome that improves intestinal digestive and absorptive functions of the nutrients. Intervention including probiotics may be a possible solution to manage pediatric obesity ( 173 , 174 ). Additionally, the role of Vitamin E for treating the comorbidities of obesity such as diabetes, hyperlipidemia, NASH, and cardiovascular risk, has been recently described ( 175 , 176 ). Vitamin E is a lipid- soluble compound and contains both tocopherols and tocotrienols. Tocopherols have lipid-soluble antioxidants properties that interact with cellular lipids and protects them from oxidation damage ( 177 ). In metabolic disease, certain crucial pathways are influenced by Vitamin E and some studies have summarized the role of Vitamin E regarding the treatment of obesity, metabolic, and cardiovascular disease ( 178 ). Hence, adequate supplementation of Vitamin E as an appropriate strategy to help in the treatment of the prevention of obesity and its associated comorbidities has been suggested. Nonetheless, some clinical trials have shown contradictory results with Vitamin E supplementation ( 177 ). Although Vitamin E has been recognized as an antioxidant that protects from oxidative damage, however, a full understanding of its mechanism of action is still lacking.
Bariatric Surgery
Bariatric surgery has gained popularity since the early 2000s in the management of severe obesity. If performed earlier, there are better outcomes for reducing weight and resolving obesity-related comorbidities in adults ( 179 – 182 ). Currently, the indication for bariatric in adolescents; those who have a BMI >35 with at least one severe comorbidity (Type 2 Diabetes, severe OSA, pseudotumor cerebri or severe steatohepatitis); or BMI of 40 or more with other comorbidities (hypertension, hyperlipidemia, mild OSA, insulin resistance or glucose intolerance or impaired quality of life due to weight). Before considering bariatric surgery, these patients must have completed most of their linear growth and participated in a structured weight-loss program for 6 months ( 159 , 181 , 183 ). The American Society for Metabolic and Bariatric Surgery (AMBS) outlines the multidisciplinary approach that must be taken before a patient undergoing bariatric surgery. In addition to a qualified bariatric surgeon, the patient must have a pediatrician or provider specialized in adolescent medicine, endocrinology, gastroenterology and nutrition, registered dietician, mental health provider, and exercise specialist ( 181 ). A mental health provider is essential as those with depression due to obesity or vice versa may have persistent mental health needs even after weight loss surgery ( 184 ).
Roux-en-Y Gastric Bypass (RYGB), laparoscopic Sleeve Gastrectomy (LSG), and Gastric Banding are the options available. RYGB and LSG currently approved for children under 18 years of age ( 166 , 181 , 185 ). At present, gastric banding is not an FDA recommended procedure in the US for those under 18y/o. One study showed some improvements in BMI and severity of comorbidities but had multiple repeat surgeries and did not believe a suitable option for obese adolescents ( 186 ).
Compared to LSG, RYGB has better outcomes for excess weight loss and resolution of obesity-related comorbidities as shown in studies and clinical trials ( 183 , 184 , 187 ). Overall, LSG is a safer choice and may be advocated for more often ( 179 – 181 ). The effect on the Gut-Brain axis after Bariatric surgery is still inconclusive, especially in adolescents, as the number of procedures performed is lower than in adults. Those who underwent RYGB had increased fasting and post-prandial PYY and GLP-1, which could have contributed to the rapid weight loss ( 185 ); this effect was seen less often in patients with gastric banding ( 185 ). Another study in adult patients showed higher bile acid (BA) subtype levels and suggested a possible BA's role in the surgical weight loss response after LSG ( 188 ). Adolescents have lower surgical complication rates than their adult counterparts, hence considering bariatric surgery earlier rather than waiting until adulthood has been entertained ( 180 ). Complications after surgery include nutritional imbalance in iron, calcium, Vitamin D, and B12 and should be monitored closely ( 180 , 181 , 185 ). Although 5-year data for gastric bypass in very obese teens is promising, lifetime outcome is still unknown, and the psychosocial factors associated with adolescent adherence post-surgery are also challenging and uncertain.
Obesity in childhood and adolescence is not amenable to a single easily modified factor. Biological, cultural, and environmental factors such as readily available high-density food choices impact youth eating behaviors. Media devices and associated screen time make physical activity a less optimal choice for children and adolescents. This review serves as a reminder that the time for action is now. The need for interventions to change the obesogenic environment by instituting policies around the food industry and in the schools needs to be clarified. In clinical trials GLP-1 agonists are shown to be effective in weight loss in children but are not yet FDA approved. Discovery of therapies to modify the gut microbiota as treatment for overweigh/obesity through use of probiotics or fecal transplantation would be revolutionary. For the present, ongoing clinical research efforts in concert with pharmacotherapeutic and multidisciplinary lifestyle programs hold promise.
Author Contributions
AK, SL, and MJ contributed to the conception and design of the study. All authors contributed to the manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Gurnani M, Birken C, Hamilton. J. Childhood obesity: causes, consequences, and management. Pediatr Clin North Am. (2015) 62:821–40. doi: 10.1016/j.pcl.2015.04.001
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Sahoo K, Sahoo B, Choudhury AK, Sofi NY, Kumar R, Bhadoria. AS. Childhood obesity: causes and consequences. J Family Med Prim Care. (2015) 4:187–92. doi: 10.4103/2249-4863.154628
3. Brown CL, Halvorson EE, Cohen GM, Lazorick S, Skelton JA. Addressing childhood obesity: opportunities for prevention. Pediatr Clin North Am. (2015) 62:1241–61. doi: 10.1016/j.pcl.2015.05.013
4. Qasim A, Turcotte M, de Souza RJ, Samaan MC, Champredon D, Dushoff J, et al. On the origin of obesity: identifying the biological, environmental, and cultural drivers of genetic risk among human populations. Obes Rev. (2018) 19:121–49. doi: 10.1111/obr.12625
5. Rinninella E, Raoul P, Cintoni M, Fransceschi F, Miggiano GAD, Gasbarrini A, et al. What is the healthy gut microbiota composition? a changing ecosystem across age, environment, diet, and diseases. Microorganisms. (2019) 7:14. doi: 10.3390/microorganisms7010014
6. Indrio F, Martini S, Francavilla R, Corvaglia L, Cristofori F, Mastrolia SA, et al. Epigenetic matters: the link between early nutrition, microbiome, and long-term health development. Front Pediatr. (2017) 5:178. doi: 10.3389/fped.2017.00178
7. Marcovecchio ML, Gorman S, Watson LPE, Dunger DB, Beardsall K. Catch-up growth in children born small for gestational age related to body composition and metabolic risk at six years of age in the UK. Horm Res Paediatr. (2020) 93:119–27. doi: 10.1159/000508974
8. Koletzko B, Fishbein M, Lee WS, Moreno L, Mouane N, Mouzaki M, et al. Prevention of childhood obesity: a position paper of the global federation of international societies of paediatric gastroenterology, hepatology nutrition (FISPGHAN). J Pediatr Gastroenterol Nutr. (2020) 70:702–10. doi: 10.1097/MPG.0000000000002708
9. Pulgarón ER. Childhood obesity: a review of increased risk for physical and psychological comorbidities. Clin Ther. (2013) 35:A18–32. doi: 10.1016/j.clinthera.2012.12.014
10. De Leonibus C, Marcovecchio ML, Chiarelli F. Update on statural growth and pubertal development in obese children. Pediatr Rep. (2012) 4:e35. doi: 10.4081/pr.2012.e35
11. Witchel SF, Burghard AC, Tao RH, Oberfield SE. The diagnosis and treatment of PCOS in adolescents. Curr Opin Pediatr . (2019) 31:562–9. doi: 10.1097/MOP.0000000000000778
12. Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics . (2012) 130:e714–55. doi: 10.1542/peds.2012-1672
CrossRef Full Text | Google Scholar
13. Rankin J, Matthews L, Cobley S, Han A, Sanders R, Wiltshire HD, et al. Psychological consequences of childhood obesity: psychiatric comorbidity and prevention. Adolesc Health Med Ther . (2016) 7:125–46. doi: 10.2147/AHMT.S101631
14. Topçu S, Orhon FS, Tayfun M, Uçaktürk SA, Demirel F. Anxiety, depression, and self-esteem levels in obese children: a case-control study. J Pediatr Endocrinol Metabol. (2016) 29:357–61. doi: 10.1515/jpem-2015-0254
15. Katzmarzyk PT, Barlow S, Bouchard C, Catalano PM, Hsia DS, Inge TH, et al. An evolving scientific basis for the prevention and treatment of pediatric obesity. Int J Obes. (2014) 38:887–905. doi: 10.1038/ijo.2014.49
16. Brown T, Moore TH, Hooper L, Gao Y, Zayegh A, Ijaz S, et al. Interventions for preventing obesity in children. Cochrane Database Syst Rev . (2019) 7:CD001871. doi: 10.1002/14651858.CD001871.pub4
17. Smith E, Scarborough P, Rayner M, Briggs ADM. Should we tax unhealthy food and drink? Proc Nutr Soc. (2019) 77:314–20. doi: 10.1017/S0029665117004165
18. Adab P, Pallan M, Whincup PH. Is BMI the best measure of obesity? BMJ. (2018) 360:k 1274. doi: 10.1136/bmj.k1274
19. Anderson LN, Carsley S, Lebovic G, Borkhoff CM, Maguire JL, Parkin PC, et al. Misclassification of child body mass index from cut-points defined by rounded percentiles instead of Z-scores. BMC Res Notes. (2017) 10:639. doi: 10.1186/s13104-017-2983-0
20. Must A, Anderson SE. Body mass index in children and adolescents: consideration for population-based applications. Int J Obes. (2006) 30:590–4. doi: 10.1038/sj.ijo.0803300
21. Flegal KM, Wei R, Ogden C. Weight-for-stature compared with body mass index-for-age growth charts for the United States from the centers for disease control and prevention. Am J Clin Nutr. (2002) 75:761–6.22. doi: 10.1093/ajcn/75.4.761
22. Himes JH, Dietz WH. Guidelines for overweight in adolescent preventive services: recommendations from an expert committee. The expert committee on clinical guidelines for overweight in adolescent preventive services. Am J Clin Nutr. (1994) 59:307–16. doi: 10.1093/ajcn/59.2.307
23. Lazarus R, Baur L, Webb K, Blyth F. Body mass index in screening for adiposity in children and adolescents: systematic evaluation using receiver operating characteristic curves. Am J Clin Nutr. (1996) 63:500–6. doi: 10.1093/ajcn/63.4.500
24. McGinnis JM, Gootman JA. Food Marketing to Children and Youth: Threat or Opportunity? Institute of Medicine of the National Academies. Washington, DC: The National Academies Press. (2006).
Google Scholar
25. Chaudhri OB, Salem V, Murphy KG, Bloom SR. Gastrointestinal satiety signals. Annu Rev Physiol. (2008) 70:239–55. doi: 10.1146/annurev.physiol.70.113006.100506
26. Scaglioni S, De Cosmi V, Ciappolino V, Parazzini F, Brambilla P, Agostoni C. Factors influencing children's eating behaviours. Nutrients. (2018) 10:706. doi: 10.3390/nu10060706
27. Ahima RS, Antwi DA. Brain regulation of appetite and satiety. Endocrinol Metab Clin North Am. (2008) 37:811–23. doi: 10.1016/j.ecl.2008.08.005
28. Niswender KD, Baskin DG, Schwartz MW. Review insulin and its evolving partnership with leptin in the hypothalamic control of energy homeostasis. Trends Endocrinol Metab. (2004) 15:362–9. doi: 10.1016/j.tem.2004.07.009
29. Niswender KD, Schwartz MW. Review insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities. Front Neuroendocrinol. (2003) 24:1–10. doi: 10.1016/S0091-3022(02)00105-X
30. Amitani M, Asakawa A, Amitani H, Inui. A. The role of leptin in the control of insulin-glucose axis. Front Neurosci. (2013) 7:51. doi: 10.3389/fnins.2013.00051
31. Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, Grove KL, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. (2003) 37:649–61. doi: 10.1016/S0896-6273(03)00063-1
32. Buhmann H, le Roux CW, Bueter M. The gut–brain axis in obesity. Best Prac Res Clin Gastroenterol. (2014) 28:559–71. doi: 10.1016/j.bpg.2014.07.003
33. Cone RD. Review anatomy and regulation of the central melanocortin system. Nat Neurosci. (2005) 8:571–8. doi: 10.1038/nn1455
34. Timper K, Brüning JC. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis Model Mech. (2017) 10:679–89. doi: 10.1242/dmm.026609
35. Labarthe A, Fiquet O, Hassouna R, Zizzari P, Lanfumey L, Ramoz N, et al. Ghrelin-derived peptides: a link between appetite/reward, gh axis, and psychiatric disorders? Front Endocrinol. (2014) 5:163. doi: 10.3389/fendo.2014.00163
36. Hills R. D Jr, Pontefract BA, Mishcon HR, Black CA, Sutton SC, Theberge CR. Gut microbiome: profound implications for diet and disease. Nutrients. (2019) 11:1613. doi: 10.3390/nu11071613
37. Torres-Fuentes C, Schellekens H, Dinan TG, Cryan JF. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol Hepatol. (2017) 2:747–56. doi: 10.1016/S2468-1253(17)30147-4
38. Gérard P. Gut microbiota and obesity. Cell Mol Life Sci. (2016) 73:147–62. doi: 10.1007/s00018-015-2061-5
39. Derrien M, Alvarez AS, de Vos WM. The gut microbiota in the first decade of life. Trends Microbiol. (2019) 27:997–1010.40. doi: 10.1016/j.tim.2019.08.001
40. Dao MC, Clément K. Gut microbiota and obesity: concepts relevant to clinical care. Eur J Intern Med . (2018) 48:18–24.41. doi: 10.1016/j.ejim.2017.10.005
41. Kim KN, Yao Y., Ju SY. Short chain fatty acids and fecal microbiota abundance in humans with obesity: a systematic review and meta-analysis. Nutrients. (2019) 11:2512. doi: 10.3390/nu11102512
42. Castaner O, Goday A, Park YM, Lee SH, Magkos F, Shiow STE, et al. The gut microbiome profile in obesity: a systematic review. Int J Endocrinol. (2018) 2018:4095789. doi: 10.1155/2018/4095789
43. Riva A, Borgo F, Lassandro C, Verduci E, Morace G, Borghi E, et al. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in firmicutes populations. Enviroin Microbiol. (2017) 19:95–105. doi: 10.1111/1462-2920.13463
44. Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TMS, Comelli EM. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes . (2014) 4:e121. doi: 10.1038/nutd.2014.23
45. Rahat-Rozenbloom S, Fernandes J, Gloor GB, Wolever TMS. Evidence for greater production of colonic short-chain fatty acids in overweight than lean humans. Int J Obes . (2014) 38:1525–31. doi: 10.1038/ijo.2014.46
46. Barczyńska R, Litwin M, Slizewska K, Szalecki M, Berdowska A, Bandurska K, et al. Bacterial microbiota fatty acids in the faeces of overweight obese children. Pol. J. Microbiol. (2018) 67:339–45. doi: 10.21307/pjm-2018-041
47. Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. (2020) 72:558–77. doi: 10.1016/j.jhep.2019.10.003
48. Yu EL, Golshan S, Harlow KE, Angeles JE, Durelle J, Goyal NP, et al. Prevalence of nonalcoholic fatty liver disease in children with obesity. J Pediatr. (2019) 207:64–70. doi: 10.1016/j.jpeds.2018.11.021
49. Ranucci G, Spagnuolo MI, Iorio R. Obese children with fatty liver: Between reality and disease mongering. World J Gastroenterol. (2017) 23:8277–82. doi: 10.3748/wjg.v23.i47.8277
50. Cox AJ, West NP, Cripps A. W. Obesity, inflammation, and the gut microbiota. Lancet Diabet Endocrinol. (2015) 3:207–15. doi: 10.1016/S2213-8587(14)70134-2
51. Seitz J, Trinh S, Herpertz-Dahlmann B. The microbiome and eating disorders. Psychiatr Clin North Am. . (2019) 42:93–103. doi: 10.1016/j.psc.2018.10.004
52. Deans E. Microbiome and mental health in the modern environment. J Physiol Anthropol. (2016) 36:1. doi: 10.1186/s40101-016-0101-y
53. Peirce JM, Alviña K. The role of inflammation and the gut microbiome in depression and anxiety. J Neurosci Res . (2019) 97:1223–41. doi: 10.1002/jnr.24476
54. Ranadive SA, Vaisse C. Lessons from extreme human obesity: monogenic disorders. Endocrinol Metab Clin North Am. (2008) 37:733–51. doi: 10.1016/j.ecl.2008.07.003
55. Soliman AT, Yasin M, Kassem A. Leptin in pediatrics: a hormone from adipocyte that wheels several functions in children. Indian J Endocrinol Metab . (2012) 16(Suppl. 3):S577–87. doi: 10.4103/2230-8210.105575
56. Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med. (2007) 356:237–47. doi: 10.1056/NEJMoa063988
57. Mutch DM, Clément K. Unraveling the genetics of human obesity. PLoS Genet. (2006) 2:e188. doi: 10.1371/journal.pgen.0020188
58. Crocker MK, Yanovski JA. Pediatric obesity: etiology and treatment. Endocrinol Metab Clin North Am. (2009) 38:525–48. doi: 10.1016/j.ecl.2009.06.007
59. Huvenne H, Dubern B, Clément K, Poitou C. Rare genetic forms of obesity: clinical approach and current treatments in 2016. Obes Facts. (2016) 9:158–73. doi: 10.1159/000445061
60. Stefan M, Nicholls RD. What have rare genetic syndromes taught us about the pathophysiology of the common forms of obesity? Curr Diab Rep. (2004) 4:143–50. doi: 10.1007/s11892-004-0070-0
61. Hetherington MM, Cecil JE. Gene-Environment interactions in obesity. Forum Nutr. (2009) 63:195–203. doi: 10.1159/000264407
62. Reddon H, Guéant JL, Meyre D. The importance of gene-environment interactions in human obesity. Clin Sci. (2016) 130:1571–97. doi: 10.1042/CS20160221
63. Castillo JJ, Orlando RA, Garver WS. Gene-nutrient interactions and susceptibility to human obesity. Genes Nutr. (2017) 12:29. doi: 10.1186/s12263-017-0581-3
64. Heianza Y, Qi L. Gene-Diet interaction and precision nutrition in obesity. Int J Mol Sci. (2017) 18:787. doi: 10.3390/ijms18040787
65. Goodarzi MO. Genetics of obesity: what genetic association studies have taught us about the biology of obesity and its complications. Lancet Diabetes Endocrinol. (2018) 6:223–36. . doi: 10.1016/S2213-8587(17)30200-0
66. Bouchard L, Drapeau V, Provencher V, Lemieux S, Chagnon Y, Rice T, et al. Neuromedin beta: a strong candidate gene linking eating behaviors and susceptibility to obesity. Am J Clin Nutr. (2004) 80:1478–86. . doi: 10.1093/ajcn/80.6.1478
67. Grimm ER, Steinle NI. Genetics of eating behavior: established and emerging concepts. Nutr Rev. (2011) 69:52–60. . doi: 10.1111/j.1753-4887.2010.00361.x
68. van der Klaauw AA, Farooqi IS. The hunger genes: pathways to obesity. Cell. (2015) 161:119–32. . doi: 10.1016/j.cell.2015.03.008
69. Martinez JA. Bodyweight regulation causes of obesity. Proc Nutr Soc. (2000) 59:337–45. Review. doi: 10.1017/S0029665100000380
70. Rask-Andersen M, Karlsson T, Ek WE, Johansson Å. Gene-environment interaction study for BMI reveals interactions between genetic factors and physical activity, alcohol consumption and socioeconomic status. PLoS Genet. (2017) 5:1. doi: 10.1371/journal.pgen.1006977
71. Xulong S, Pengzhou L, Xiangwu Y, Weizheng L, Xianjie Q, Shaihong Z, et al. From genetics and epigenetics to the future of precision treatment for obesity. Gastroenterol Rep. (2017) 5:266–70. doi: 10.1093/gastro/gox033
72. Bianco-Miotto T, Craig JM, Gasser YP, van dijk SJ, Ozanne SE. Epigenetics and DOHaD: from basics to birth and beyond. J Dev Orig Health Dis. (2017) 8:513–9. doi: 10.1017/S2040174417000733
73. van Dijk SJ, Molloy PL, Varinli H, Morrison JL, Muhlhausler BS, Members of EpiSCOPE. Epigenetics and human obesity. Int J Obes . (2015) 39:85–97. doi: 10.1038/ijo.2014.34
74. Li Y. Epigenetic mechanisms link maternal diets and gut microbiome to obesity in the offspring. Front Genet . (2018) 9:342. doi: 10.3389/fgene.2018.00342
75. Kaufman J, Montalvo-Ortiz JL, Holbrook H, O'Loughlin K, Orr C, Kearney C, et al. Adverse childhood experiences, epigenetic measures, and obesity in youth. J Pediatr. (2018) 202:150–6.76. doi: 10.1016/j.jpeds.2018.06.051
76. May Gardner R, Feely A, Layte R, Williams J, McGavock J. Adverse childhood experiences are associated with an increased risk of obesity in early adolescence: a population-based prospective cohort study. Pediatr Res. (2019) 86:522–28. doi: 10.1038/s41390-019-0414-8
77. Cheon BK„ Hong YY. Mere experience of low subjective socioeconomic status stimulates appetite food intake. Proc Natl Acad Sci USA . (2017) 114:72–7. doi: 10.1073/pnas.1607330114
78. Alegría-Torres JA, Baccarelli A, Bollati V. Epigenetics lifestyle. Epigenomics . (2011) 3:267-77. doi: 10.2217/epi.11.22
79. Birch LL, Fisher JO. Development of eating behaviors among children and adolescents. Pediatrics . (2011) 101:539–49.
PubMed Abstract | Google Scholar
80. Birch L, Savage JS, Ventura A. Influences on the development of children's eating behaviours: from infancy to adolescence. Can J Diet Pract Res. (2007) 68:s1–s56.
81. Nielsen SJ, Popkin BM. Patterns and trends in food portion sizes, 1977- 1998. JAMA. (2003) 289:450–53. . doi: 10.1001/jama.289.4.450
82. Munoz KA, Krebs-Smith SM, Ballard-Barbash R, Cleveland LE. Food intakes of US children and adolescents compared with recommendations. Pediatrics. (1997) 100:323–29. doi: 10.1542/peds.100.3.323
83. Fisher JO, Birch LL. Restricting access to palatable foods affects children's behavioral response, food selection, and intake. Am J Clin Nutr. (1999) 69:1264–72. doi: 10.1093/ajcn/69.6.1264
84. Faith MS, Scanlon KS, Birch LL, Francis LA, Sherry B. Parent-child feeding strategies and their relationships to child eating and weight status. Obes Res. (2004) 12:1711–22. . doi: 10.1038/oby.2004.212
85. Smith AD, Sanchez N, Reynolds C, Casamassima M, Verros M, Annameier SK, et al. Associations of parental feeding practices and food reward responsiveness with adolescent stress-eating. Appetite. (2020) 152:104715. doi: 10.1016/j.appet.2020.104715
86. Lowe CJ, Morton JB, Reichelt AC. Adolescent obesity and dietary decision making-a brain-health perspective. Lancet Child Adolesc Health. (2020) 4:388–96. doi: 10.1016/S2352-4642(19)30404-3
87. Goran MI, Treuth MS. Energy expenditure, physical activity, and obesity in children. Pediatr Clin North Am. (2001) 48:931–53. doi: 10.1016/S0031-3955(05)70349-7
88. Romieu I, Dossus L, Barquera S, Blottière HM, Franks PW, Gunter M, et al. Energy balance and obesity: what are the main drivers? Cancer Causes Control. (2017) 28:247–58. doi: 10.1007/s10552-017-0869-z
89. Mattes R, Foster GD. Food environment and obesity. Obesity. (2014) 22:2459–61. doi: 10.1002/oby.20922
90. Ickovics JR, O'Connor Duffany K, Shebl FM, Peters SM, Read MS, Gilstad-Hayden KR, et al. Implementing school-based policies to prevent obesity: cluster randomized trial. Am J Prev Med. (2019) 56:e1–11. doi: 10.1016/j.amepre.2018.08.026
91. Micha R, Karageorgou D, Bakogianni I, Trichia E, Whitsel LP, Story M, et al. Effectiveness of school food environment policies on children's dietary behaviors: A systematic review and meta-analysis. PLoS ONE. ( 2018 ) 13:e0194555. doi: 10.1371/journal.pone.0194555
92. Cawley J, Frisvold D, Hill A, Jones DJ. The impact of the philadelphia beverage tax on purchases and consumption by adults and children. Health Econ. (2019) 67:102225. doi: 10.1016/j.jhealeco.2019.102225
93. John Cawley J, Thow AM, Wen K, Frisvold D. The economics of taxes on sugar-sweetened beverages: a review of the effects on prices, sales, cross-border shopping, and consumption. Annu Rev Nutr. (2019) 39:317–38. doi: 10.1146/annurev-nutr-082018-124603
94. Fuller C, Lehman E, Hicks S, Novick MB. Bedtime use of technology and associated sleep problems in children. Glob Pediatr Health. (2017) 4:2333794X17736972. doi: 10.1177/2333794X17736972
95. Chahal H, Fung C, Kuhle S, Veugelers PJ. Availability and night-time use of electronic entertainment and communication devices are associated with short sleep duration and obesity among Canadian children. Pediatr Obes. (2012) 8:42–51. doi: 10.1111/j.2047-6310.2012.00085.x
96. Minghua T. Protein intake during the first two years of life and its association with growth and risk of overweight. Int J Environ Res Public Health. ( 2018 ) 15:1742. doi: 10.3390/ijerph15081742
97. Azad MB, Vehling L, Chan D, Klopp A, Nickel NC, McGavock JM, et al. Infant feeding and weight gain: separating breast milk from breastfeeding and formula from food. Pediatrics. (2018) 142:e20181092. doi: 10.1542/peds.2018-1092
98. Lin L, Amissah E, Gamble GD, Crowther CA, Harding JE. Impact of macronutrient supplements on later growth of children born preterm or small for gestational age: a systematic review and meta-analysis of randomised and quasirandomised controlled trials. PLoS Med. (2020) 17:e1003122. . doi: 10.1371/journal.pmed.1003122
99. Rzehak P, Oddy WH, Mearin ML, Grote V, Mori TA, Szajewska H, et al. Infant feeding and growth trajectory patterns in childhood and body composition in young adulthood. Am J Clin Nutr. (2017) 106:568–80. doi: 10.3945/ajcn.116.140962
100. Styne DM, Arslanian SA, Connor EL, Farooqi IS, Murad MH, Silverstein JH. Pediatric obesity-assessment, treatment, and prevention: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2017) 102:709–57. doi: 10.1210/jc.2016-2573
101. Whitaker RC, Pepe MS, Wright JA, Seidel KD, Dietz WH. Early adiposity rebound and the risk of adult obesity. Pediatrics . (1998) 101:E5. doi: 10.1542/peds.101.3.e5
102. Geserick M, Vogel M, Gausche R, Lipek T, Spielau U, Keller E, et al. Acceleration of BMI in early childhood and risk of sustained obesity. N Engl J Med. (2018) 379:1303–12. doi: 10.1056/NEJMoa1803527
103. Jabakhanji SB, Boland F, Ward M, Biesma RJ. Body mass index changes in early childhood. Pediatrics. (2018) 202:106–14. doi: 10.1016/j.jpeds.2018.06.049
104. Chung S. Growth and puberty in obese children and implications of body composition. J Obes Metab Syndr. (2017) 26:243–50. doi: 10.7570/jomes.2017.26.4.243
105. Tagi VM, Giannini C, Chiarelli F. Insulin resistance in children. Front Endocrinol. (2019) 10:342. doi: 10.3389/fendo.2019.00342
106. Kelesidis I, Mantzoros CS. Leptin and its emerging role in children and adolescents. Clin Pediatr Endocrinol . (2006) 15:1–14. doi: 10.1297/cpe.15.1
107. Burt Solorzano CM, McCartney CR, Obesity and the pubertal transition in girls and boys. Reproduction . (2010) 140:399–410. doi: 10.1530/REP-10-0119
108. Li W, Liu Q, Deng X, Chen Y, Liu S, Story M. Association between obesity and puberty timing: a systematic review and meta-analysis. Int J Environ Res Public Health. (2017) 14:1266. doi: 10.3390/ijerph14101266
109. Lee JM, Wasserman R, Kaciroti N, Gebremariam A, Steffes J, Dowshen S, et al. Timing of puberty in overweight vs. obese boys. Pediatrics. (2016) 137:e20150164. doi: 10.1542/peds.2015-0164
110. He J, Kang Y, Zheng L. Serum levels of LH, IGF-1 and leptin in girls with idiopathic central precocious puberty (ICPP) and the correlations with the development of ICPP. Minerva Pediatr . (2018). doi: 10.23736/S0026-4946.18.05069-7
111. Kang MJ, Oh YJ, Shim YS, Baek JW, Yang S, Hwang IT. The usefulness of circulating levels of leptin, kisspeptin, and neurokinin B in obese girls with precocious puberty. Gynecol Endocrinol. (2018) 34:627–30. doi: 10.1080/09513590.2017.1423467
112. Rendo-Urteaga T, Ferreira de Moraes AC, Torres-Leal FL, Manios Y, Gottand F, Sjöström M, et al. Leptin and adiposity as mediators on the association between early puberty and several biomarkers in European adolescents: the helena study. J Pediatr Endocrinol Metab. (2018) 31:1221–29. doi: 10.1515/jpem-2018-0120
113. Franks S. Adult polycystic ovary syndrome begins in childhood. Best Pract Res Clin Endocrinol Metab. (2002) 16:263–72. doi: 10.1053/beem.2002.0203
114. Franks S. Polycystic ovary syndrome in adolescents. Int J Obes. (2008) 32:1035–41. doi: 10.1038/ijo.2008.61
115. Jehan S, Zizi F, Pandi-Perumal SR, Wall S, Auguste E, Myers K, et al. Obstructive sleep apnea and obesity: implications for public health. Sleep Med Disord. (2017) 1:00019.
116. Patinkin ZW, Feinn R, Santos M. Metabolic consequences of obstructive sleep apnea in adolescents with obesity: a systematic literature review and meta-analysis. Childhood Obes. (2017) 13:102–10. doi: 10.1089/chi.2016.0248
117. Kaditis A. From obstructive sleep apnea in childhood to cardiovascular disease in adulthood: what is the evidence? Sleep. (2010) 33:1279–80. doi: 10.1093/sleep/33.10.1279
118. Marseglia L, Manti S, D'Angelo G, Nicotera A, Parisi E, Di Rose G, et al. Oxidative stress in obesity: a critical component in human diseases. Int J Mol Sci . (2014) 16:378–400. doi: 10.3390/ijms16010378
119. Eisele HJ, Markart P, Schulz R. Obstructive sleep apnea, oxidative stress, and cardiovascular disease: evidence from human studies. Oxid Med Cell Longev . (2015) 2015:608438. doi: 10.1155/2015/608438
120. Hui W, Slorach C, Guerra V, Parekh RS, Hamilton J, Messiha S, et al. Effect of obstructive sleep apnea on cardiovascular function in obese youth. Am J Cardiol. (2019) 123:341–7. doi: 10.1016/j.amjcard.2018.09.038
121. Matteoni CA, Younossi Z .m., Gramlich T, Boparai N, Liu YC, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. (1999) 1999:116:1413. doi: 10.1016/S0016-5085(99)70506-8
122. Lavine JE, Schwimmer JB. Nonalcoholic fatty liver disease in the pediatric population. Clin Liver Dis. ( 2004 ) 8:549. doi: 10.1016/j.cld.2004.04.010
123. Huang JS, Barlow SE, Quiros-Tejeira RE, Scheimann A, Skelton J, Suskind D, et al. Childhood obesity for pediatric gastroenterologists. J Pediatr Gastroenterol Nutr. (2013) 2013:56:99. doi: 10.1097/MPG.0b013e31826d3c62
124. Anderson EL, Howe LD, Jones HE, Higgins JPT, Lawlor DA, Fraser A. The prevalence of non-alcoholic fatty liver disease in children and adolescents: a systematic review and meta-analysis. PLoS ONE. ( 2015 ) 10:e0140908. doi: 10.1371/journal.pone.0140908
125. Nobili V, Alisi A, Newton KP, Schwimmer JB. Comparison of the phenotype and approach to pediatric vs adult patients with nonalcoholic fatty liver disease. Gastroenterology. (2016) 150:1798–810. doi: 10.1053/j.gastro.2016.03.009
126. Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, et al. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. (2009) 7:234–38. doi: 10.1016/j.cgh.2008.11.005
127. Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angula P. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. (2009) 58:1538. doi: 10.1136/gut.2008.171280
128. Schwimmer JB, Pardee PE, Lavine JE, Blumkin AK, Cook S. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation . (2008) 118:277. doi: 10.1161/CIRCULATIONAHA.107.739920
129. Perry DC, Metcalfe D, Lane S, Turner S. Childhood obesity and slipped capital femoral epiphysis. Pediatrics. (2018) 142:e20181067. doi: 10.1542/peds.2018-1067
130. Zavala-Crichton JP, Esteban-Cornejo I, Solis-Urra P, Mora-Gonzalez J, Cadenas-Sanchez C, Rodriguez-Ayllon M, et al. Association of sedentary behavior with brain structure and intelligence in children with overweight or obesity: Active Brains Project . (2020) 9:1101. doi: 10.3390/jcm9041101
131. Ronan L, Alexander-Bloch A, Fletcher PC. Childhood obesity, cortical structure, and executive function in healthy children. Cereb Cortex. (2019) 30:2519–28. doi: 10.1093/cercor/bhz257
132. Baker ER. Body weight and the initiation of puberty. Clin Obstetr Gynecol. (1985) 28:573–9. doi: 10.1097/00003081-198528030-00013
133. Siervogel RM, Demerath EW, Schubert C, Remsberg KE, Chumlea WM, Sun S, et al. Puberty and body composition. Horm Res. (2003) 60:36–45. doi: 10.1159/000071224
134. Sadeeqa S, Mustafa T, Latif S. Polycystic ovarian syndrome- related depression in adolescent girls. J Pharm Bioallied Sci. (2018) 10:55–9. doi: 10.4103/JPBS.JPBS_1_18
135. Himelein MJ, Thatcher SS. Depression and body image among women with polycystic ovary syndrome. J Health Psychol . (2006) 11:613–25. doi: 10.1177/1359105306065021
136. Magge SN, Goodman E, Armstrong SC. The metabolic syndrome in children and adolescents: shifting the focus to cardiometabolic risk factor clustering. Pediatrics. (2017) 140:e20171603. doi: 10.1542/peds.2017-1603
137. Mauras N, Delgiorno C, Kollman C, Bird K, Morgan M, Sweeten S, et al. Obesity without established comorbidities of the metabolic syndrome is associated with a proinflammatory and prothrombotic state, even before the onset of puberty in children. J Clin Endocrinol Metab. (2010) 95:1060–8. doi: 10.1210/jc.2009-1887
138. Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. (2004) 350:2362–74. doi: 10.1056/NEJMoa031049
139. Erdmann J, Kallabis B, Oppel U, Sypchenko O, Wagenpfeil S, Schusdziarra V. Development of hyperinsulinemia and insulin resistance during the early stage of weight gain. Am J Physiol Endocrinol Metabol. (2008) 294:e568–75. . doi: 10.1152/ajpendo.00560.2007
140. Pulido-Arjona L, Correa-Bautista JE, Agostinis-Sobrinho C, Mota J, Santos R, Correa-Rodrigues M, et al. Role of sleep duration and sleep- related problems in the metabolic syndrome among children and adolescents. Ital J Pediatr. (2018) 44:9. doi: 10.1186/s13052-018-0451-7
141. Harriger JA, Thompson JK. Psychological consequences of obesity: weight bias and body image in overweight and obese youth. Int Rev Psychiatry. (2012) 24:247–53. . doi: 10.3109/09540261.2012.678817
142. Bacchini D, Licenziati MR, Garrasi A, Corciulo N, Driul D, Tanas R, et al. Bullying and victimization in overweight and obese outpatient children and adolescents: an italian multicentric study. PLoS ONE. (2015) 10:e0142715. doi: 10.1371/journal.pone.0142715
143. Loth KA, Watts AW, Berg PVD, Neumark-Sztainer D. Does body satisfaction help or harm overweight teens? A 10-year longitudinal study of the relationship between body satisfaction and body mass index. J Adolesc Health. (2015) 57:559–61. doi: 10.1016/j.jadohealth.2015.07.008
144. Gowey MA, Lim CS, Clifford LM, Janicke DM. Disordered eating and health-related quality of life in overweight and obese children. J Pediatr Psychol. (2014) 39:552–61. doi: 10.1093/jpepsy/jsu012
145. Mannan M, Mamun A, Doi S, Clavarino A. Prospective associations between depression and obesity for adolescent males and females- a systematic review and meta-analysis of longitudinal studies. PLoS ONE. (2016) 11:e0157240. doi: 10.1371/journal.pone.0157240
146. Ruiz LD, Zuelch ML, Dimitratos SM, Scherr RE. Adolescent obesity: diet quality, psychosocial health, and cardiometabolic risk factors. Nutrients. (2019) 12:43. doi: 10.3390/nu12010043
147. Goldschmidt AB, Aspen VP, Sinton MM, Tanofsky-Kraff M, Wilfley DE. Disordered eating attitudes and behaviors in overweight youth. Obesity. (2008) 16:257–64. doi: 10.1038/oby.2007.48
148. Golden NH, Schneider M, Wood C. Preventing obesity and eating disorders in adolescents. Pediatrics. (2016) 138:e1–e12. doi: 10.1542/peds.2016-1649
149. Rastogi R, Rome ES. Restrictive eating disorders in previously overweight adolescents and young adults. Cleve Clin J Med. (2020) 87:165–71. doi: 10.3949/ccjm.87a.19034
150. Hayes JF, Fitzsimmons-Craft EE, Karam AM, Jakubiak JL, Brown ME, Wilfley D. Disordered eating attitudes and behaviors in youth with overweight and obesity: implications for treatment. Curr Obes Rep. (2018) 7:235. doi: 10.1007/s13679-018-0316-9
151. Goldschmidt AB, Wall MM, Loth KA, Neumark-Sztainer D. Risk factors for disordered eating in overweight adolescents and young adults: Table I. J Pediatr Psychol. (2015) 40:1048–55. doi: 10.1093/jpepsy/jsv053
152. Follansbee-Junger K, Janicke DM, Sallinen BJ. The influence of a behavioral weight management program on disordered eating attitudes and behaviors in children with overweight. J Am Diet Assoc. (2010) 110:653–9. doi: 10.1016/j.jada.2010.08.005
153. Blake-Lamb TL, Locks LM, Perkins ME, Woo Baidal JA, Cheng ER, Taveras EM. Interventions for childhood obesity in the first 1,000 days a systematic review. Am J Prev Med. (2016) 50:780–9. doi: 10.1016/j.amepre.2015.11.010
154. McGuire S. Institute of Medicine (IOM). Early childhood obesity prevention policies. Washington, DC: The National Academies Press. Adv Nutr . (2011) 3:56–7. doi: 10.3945/an.111.001347
155. Pont SJ, Puhl R, Cook SR, Slusser W. Stigma experienced by children and adolescents with obesity. Pediatrics. (2017) 140:e20173034. doi: 10.1542/peds.2017-3034
156. Puhl R, Suh Y. Health consequences of weight stigma: implications for obesity prevention and treatment. Curr Obes Rep. (2015) 4:182–90. doi: 10.1007/s13679-015-0153-z
157. Schwimmer JB, Burwinkle TM, Varni JW. Health-related quality of life of severely obese children and adolescents. JAMA. (2003) 289:1813–9. doi: 10.1001/jama.289.14.1813
158. Carcone AI, Jacques-Tiura AJ, Brogan Hartlieb KE, Albrecht T, Martin T. Effective patient-provider communication in pediatric obesity. Pediatr Clin North Am. (2016) 63:525–38. doi: 10.1016/j.pcl.2016.02.002
159. Coppock JH, Ridolfi DR, Hayes JF, Paul MS, Wilfley DE. Current approaches to the management of pediatric overweight and obesity. Curr Treat Options Cardiovasc Med. (2014) 16:343. doi: 10.1007/s11936-014-0343-0
160. Davison KK, Jurkowski JM, Li K, Kranz S, Lawson HA. A childhood obesity intervention developed by families for families: results from a pilot study. Int J Behav Nutr Phys Act. (2013) 10:3. doi: 10.1186/1479-5868-10-3
161. Krystia O, Ambrose T, Darlington G, Ma DWL, Buchholz AC, Haines J. A randomized home- based childhood obesity prevention pilot intervention has favourable effects on parental body composition: preliminary evidence from the guelph family health study. BMC Obes. (2019) 6:10. doi: 10.1186/s40608-019-0231-y
162. Skjåkødegård HF, Danielsen YS, Morken M, Linde SRF, Kolko RP, Balantekin KN, et al. Study protocol: a randomized controlled trial evaluating the effect of family-based behavioral treatment of childhood and adolescent obesity–The FABO-study. BMC Public Health. (2016) 16:1106. doi: 10.1186/s12889-016-3755-9
163. Hall KD, Kahan S. Maintenance of lost weight and long-term management of obesity. Med Clin North Am. (2018) 102:183–97. doi: 10.1016/j.mcna.2017.08.012
164. Hall KD. Diet vs. exercise in “the biggest loser” weight loss competition. Obesity. (2013) 21:957–9. doi: 10.1002/oby.20065
165. Lecoultre V, Ravussin E, Redman LM. The fall in leptin concentration is a major determinant of the metabolic adaptation induced by caloric restriction independently of the changes in leptin circadian rhythms. J Clin Endocrinol Metabol. (2011) 96:E1512–E516. doi: 10.1210/jc.2011-1286
166. Kaur KK, Allahbadia G, Singh M. Childhood obesity: a comprehensive review of epidemiology, aetiopathogenesis and management of this global threat of the 21st century. Acta Sci Paediatr. (2019) 2:56–66. doi: 10.31080/ASPE.2019.02.0132
167. Crimmins NA, Xanthakos SA. Obesity. in Neinstein's Adolescent and Young Adult Health , Guide. Philadelphia, PA: Wolters Kluwer (2016). p. 295–300.
168. Astrup A, Rossner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, et al. Effects of liraglutide in the treatment of obesity: a randomized, double-blind, placebo-controlled study. Lancet. (2009) 374:1606–16. doi: 10.1016/S0140-6736(09)61375-1
169. Monami M, Dicembrini I, Marchionni N, Rotella CM, Mannucci E. Effects of glucagon-like peptide-1 receptor agonists on body weight: a meta-analysis. Exp Diabetes Res. (2012) 2012:672658. doi: 10.1155/2012/672658
170. Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. (2015) 373:11–22 . doi: 10.1056/NEJMoa1411892
171. Kelly AS, Auerbach P, Barrientos-Perez M, Gies I, Hale PM, Marcus C, et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med. (2020) 382:2117–28. doi: 10.1056/NEJMoa1916038
172. Foster BA, Escaname E, Powell T, Larsen B, Siddiqui SK, Menchaca J, et al. Randomized controlled trial of DHA supplementation during pregnancy: child adiposity outcomes. Nutrients . (2017) 9:566. doi: 10.3390/nu9060566
173. Abenavoli L, Scarpellini E, Colica C, Boccuto L, Salehi B, Sharifi-Rad J, et al. Gut microbiota and obesity: a role for probiotics. Nutrients. (2019) 11:2690. doi: 10.3390/nu11112690
174. Vajro P, Mandato C, Veropalumbo C, De Micco I. Probiotics: a possible role in treatment of adult and pediatric nonalcoholic fatty liver disease. Ann Hepatol. (2013) 12:161–63. doi: 10.1016/S1665-2681(19)31401-2
175. Zhao L, Fang X, Marshall M, Chung S. Regulation of obesity and metabolic complications by gamma and delta tocotrienols. Molecules. (2016) 21:344. doi: 10.3390/molecules21030344
176. Wong SK, Chin K-Y, Suhaimi FH, Ahmad F, Ima-Nirwana S. Vitamin E as a potential interventional treatment for metabolic syndrome: evidence from animal and human studies. Front Pharmacol. (2017) 8:444. doi: 10.3389/fphar.2017.00444
177. Galli F, Azzi A, Birringer A, Cook-Mills JM, Eggersdorfer M, Frank J, et al. Vitamin E: Emerging aspects and new directions. Free Radic Biol Med. (2017) 102:16–36. doi: 10.1016/j.freeradbiomed.2016.09.017
178. Galmés S, Serra F, Palou A. Vitamin E metabolic effects and genetic variants: a challenge for precision nutrition in obesity and associated disturbances. Nutrients . (2018) 10:1919. doi: 10.3390/nu10121919
179. Ahn SM. Current issues in bariatric surgery for adolescents with severe obesity: durability, complications, and timing of intervention. J. Obes Metabol Syndrome. (2020) 29:4–11. doi: 10.7570/jomes19073
180. Lamoshi A, Chernoguz A, Harmon CM, Helmrath M. Complications of bariatric surgery in adolescents. Semin Pediatr Surg. (2020) 29:150888. doi: 10.1016/j.sempedsurg.2020.150888
181. Weiss AL, Mooney A, Gonzalvo JP. Bariatric surgery. Adv Pediatr. (2017) 6:269–83. doi: 10.1016/j.yapd.2017.03.005
182. Stanford FC, Mushannen T, Cortez P, Reyes KJC, Lee H, Gee DW, et al. Comparison of short and long-term outcomes of metabolic and bariatric surgery in adolescents and adults. Front Endocrinol. (2020) 11:157. doi: 10.3389/fendo.2020.00157
183. Inge TH, Zeller MH, Jenkins TM, Helmrath M, Brandt ML, Michalsky MP, et al. Perioperative outcomes of adolescents undergoing bariatric surgery: the teen-longitudinal assessment of bariatric surgery (Teen-LABS) study . JAMA Pediatr . (2014) 168:47–53. doi: 10.1001/jamapediatrics.2013.4296
184. Järvholm K, Bruze G, Peltonen M, Marcus C, Flodmark CE, Henfridsson P, et al. 5-year mental health and eating pattern outcomes following bariatric surgery in adolescents: a prospective cohort study. Lancet Child AdolescHealth . (2020) 4:210–9. doi: 10.1016/S2352-4642(20)30024-9
185. Xanthakos SA. Bariatric surgery for extreme adolescent obesity: indications, outcomes, and physiologic effects on the gut–brain axis. Pathophysiology. (2008) 15:135–46. doi: 10.1016/j.pathophys.2008.04.005
186. Zitsman JL, Digiorgi MF, Kopchinski JS, Sysko R, Lynch L, Devlin M, et al. Adolescent Gastric Banding: a five-year longitudinal study in 137 individuals. Surg Obes Relat Dis. (2018) 14. doi: 10.1016/j.soard.2018.09.030
187. Inge TH, Jenkins TM, Xanthakos SA, Dixon JB, Daniels SR, Zeller MH, et al. Long-term outcomes of bariatric surgery in adolescents with severe obesity (FABS-5+). A prospective follow-up analysis. Lancet Diabet Endocrinol . (2017) 5:165–73. doi: 10.1016/S2213-8587(16)30315-1
188. Kindel TL, Krause C, Helm MC, Mcbride CL, Oleynikov D, Thakare R, et al. Increased glycine-amidated hyocholic acid correlates to improved early weight loss after sleeve gastrectomy. Surg Endosc. (2017) 32:805–12. doi: 10.1007/s00464-017-5747-y
Keywords: obesity, childhood, review (article), behavior, adolescent
Citation: Kansra AR, Lakkunarajah S and Jay MS (2021) Childhood and Adolescent Obesity: A Review. Front. Pediatr. 8:581461. doi: 10.3389/fped.2020.581461
Received: 08 July 2020; Accepted: 23 November 2020; Published: 12 January 2021.
Reviewed by:
Copyright © 2021 Kansra, Lakkunarajah and Jay. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alvina R. Kansra, akansra@mcw.edu
This article is part of the Research Topic
Pediatric Obesity: From the Spectrum of Clinical-Physiology, Social-Psychology, and Translational Research
Health Risks

Weight Problems Take a Hefty Toll on Body and Mind
In the old spiritual, “Dem Bones,” each body part is linked to the next one in line: the thigh bone to the knee bone, the knee bone to the leg bone, and so on. But one body “part”-weight-is connected to virtually all of the others. A healthy weight sets the stage for bones, muscles, brain, heart, and others to play their parts smoothly and efficiently for many years.
Excess weight, especially obesity, diminishes almost every aspect of health, from reproductive and respiratory function to memory and mood. Obesity increases the risk of several debilitating, and deadly diseases, including diabetes, heart disease, and some cancers. It does this through a variety of pathways, some as straightforward as the mechanical stress of carrying extra pounds and some involving complex changes in hormones and metabolism. Obesity decreases the quality and length of life, and increases individual, national, and global healthcare costs. The good news, though, is that weight loss can curtail some obesity-related risks. (1) Losing as little as 5 to 10 percent of body weight offers meaningful health benefits to people who are obese, even if they never achieve their “ideal” weight, and even if they only begin to lose weight later in life.
Entire books have been written detailing the effects of obesity on various measures of health. This article briefly summarizes associations between obesity and adult health.
Obesity and Diabetes
The condition most strongly influenced by body weight is type 2 diabetes. In the Nurses’ Health Study, which followed 114,000 middle-age women for 14 years, the risk of developing diabetes was 93 times higher among women who had a body mass index (BMI) of 35 or higher at the start of the study, compared with women with BMIs lower than 22. (2) Weight gain during adulthood also increased diabetes risk, even among women with BMIs in the healthy range. The Health Professionals Follow-Up Study found a similar association in men. (3)
More recently, investigators conducted a systematic review of 89 studies on weight-related diseases and then did a statistical summary, or meta-analysis, of the data. Of the 18 weight-related diseases they studied, diabetes was at the top of the risk list: Compared with men and women in the normal weight range (BMI lower than 25), men with BMIs of 30 or higher had a sevenfold higher risk of developing type 2 diabetes, and women with BMIs of 30 or higher had a 12-fold higher risk. (4)
Fat cells, especially those stored around the waist,secrete hormones and other substances that fire inflammation. Although inflammation is an essential component of the immune system and part of the healing process, inappropriate inflammation causes a variety of health problems. Inflammation can make the body less responsive to insulin and change the way the body metabolizes fats and carbohydrates, leading to higher blood sugar levels and, eventually, to diabetes and its many complications. (5) Several large trials have shown that moderate weight loss can prevent or delay the start of diabetes in people who are at high risk. (6-8)
Obesity and Cardiovascular Disease
Body weight is directly associated with various cardiovascular risk factors. As BMI increases, so do blood pressure, low-density lipoprotein (LDL, or “bad”) cholesterol, triglycerides, blood sugar, and inflammation. These changes translate into increased risk for coronary heart disease, stroke, and cardiovascular death:
- Obesity and Coronary Artery Disease. Numerous studies have demonstrated a direct association between excess body weight and coronary artery disease (CAD). The BMI-CAD Collaboration Investigators conducted a meta-analysis of 21 long-term studies that followed more than 300,000 participants for an average of 16 years. Study participants who were overweight had a 32 percent higher risk of developing CAD, compared with participants who were at a normal weight; those who were obese had an 81 percent higher risk. (9) Although adjustment for blood pressure and cholesterol levels slightly lowered the risk estimates, they remained highly significant for obesity. The investigators estimated that the effect of excess weight on blood pressure and blood cholesterol accounts for only about half of the obesity-related increased risk of coronary heart disease.
- Obesity and Stroke. Ischemic (clot-caused) stroke and coronary artery disease share many of the same disease processes and risk factors. A meta-analysis of 25 prospective cohort studies with 2.3 million participants demonstrated a direct, graded association between excess weight and stroke risk. Overweight increased the risk of ischemic stroke by 22 percent, and obesity increased it by 64 percent. There was no significant relationship between overweight or obesity and hemorrhagic (bleeding-caused) stroke, however. (10) A repeat analysis that statistically accounted for blood pressure, cholesterol, and diabetes weakened the associations, suggesting that these factors mediate the effect of obesity on stroke.
- Obesity and Cardiovascular Death. In a meta-analysis of 26 observational studies that included 390,000 men and women, several racial and ethnic groups, and samples from the U.S. and other countries, obesity was significantly associated with death from CAD and cardiovascular disease. Women with BMIs of 30 or higher had a 62 percent greater risk of dying early from CAD and also had a 53 percent higher risk of dying early from any type of cardiovascular disease, compared with women who had BMIs in the normal range (18.5 to 24.9). Men with BMIs of 30 or higher had similarly elevated risks. (11)
The good news is that weight loss of 5 to 10 percent of body weight can lower blood pressure, LDL cholesterol, and triglycerides, and improve other cardiovascular risk factors. (12-14)
Obesity and Cancer
The association between obesity and cancer is not quite as clear as that for diabetes and cardiovascular disease. This is due in part to the fact that cancer is not a single disease but a collection of individual diseases.

Obesity, Depression, and Quality of Life
The high rates of obesity and depression, and their individual links with cardiovascular disease, have prompted many investigators to explore the relationship between weight and mood. An analysis of 17 cross-sectional studies found that people who were obese were more likely to have depression than people with healthy weights. (17) Since the studies included in the analysis assessed weight and mood only at one point in time, the investigators could not say whether obesity increases the risk of depression or depression increases the risk of obesity. New evidence confirms that the relationship between obesity and depression may be a two-way street: A meta-analysis of 15 long-term studies that followed 58,000 participants for up to 28 years found that people who were obese at the start of the study had a 55 percent higher risk of developing depression by the end of the follow-up period, and people who had depression at the start of the study had a 58 percent higher risk of becoming obese. (18)
Although a biological link between obesity and depression has not yet been definitively identified, possible mechanisms include activation of inflammation, changes in the hypothalamic-pituitary-adrenal axis, insulin resistance, and social or cultural factors.
Studies of the effect of obesity on specific health outcomes such as diabetes or depression provide only a glimpse of the full impact of obesity on health and well-being. Health-related quality of life (HRQoL) integrates the effect of obesity (or any other condition) across physical, psychological, and social functioning. Although HRQoL is a relatively young field of research, a number of studies have evaluated the overall impact of obesity on HRQoL. Among 31 studies in adults, the majority demonstrated that obesity was significantly associated with reduced HRQoL, compared with normal weight. (19) Researchers found a similar association among five HRQoL studies in children and adolescents.
Obesity and Reproduction
Obesity can influence various aspects of reproduction, from sexual activity to conception. Among women, the association between obesity and infertility, primarily ovulatory infertility, is represented by a classic U-shaped curve. In the Nurses’ Health Study, infertility was lowest in women with BMIs between 20 and 24, and increased with lower and higher BMIs. (20) This study suggests that 25 percent of ovulatory infertility in the United States may be attributable to obesity. During pregnancy, obesity increases the risk of early and late miscarriage, gestational diabetes, preeclampsia, and complications during labor and delivery. (21) It also slightly increases the chances of bearing a child with congenital anomalies. (22) One small randomized trial suggests that modest weight loss improves fertility in obese women. (23)
Sexual function may also be affected by obesity. Data from the Health Professionals Follow-Up Study, (26) the National Health and Nutrition Examination Survey (NHANES), (27) and the Massachusetts Male Aging Study (28) indicate that the odds of developing erectile dysfunction increase with increasing BMI. Of note, weight loss appears to be mildly helpful in maintaining erectile function. (29) The effect of obesity on female sexual function is less clear. In a recent French study, obese women were less likely than normal-weight women to report having had a sexual partner in the preceding 12 months, but the prevalence of sexual dysfunction was similar in both groups. (30) In a smaller survey of 118 women, Esposito and colleagues found that obese women had lower scores on the Female Sexual Function Index, with strong correlations between increasing BMI and problems with arousal, lubrication, orgasm, and satisfaction. (31)
Obesity and Lung Function/Respiratory Disease
Excess weight impairs respiratory function via mechanical and metabolic pathways. The accumulation of abdominal fat, for example, may limit the descent of the diaphragm, and in turn, lung expansion, while the accumulation of visceral fat can reduce the flexibility of the chest wall, sap respiratory muscle strength, and narrow airways in the lungs. (32) Cytokines generated by the low-grade inflammatory state that accompanies obesity may also impede lung function.
Asthma and obstructive sleep apnea are two common respiratory diseases that have been linked with obesity. In a meta-analysis of seven prospective studies that included 333,000 subjects, obesity increased the risk of developing asthma in both men and women by 50 percent. (33) Obesity is also a major contributor to obstructive sleep apnea (OSA), which is estimated to affect approximately one in five adults; one in 15 adults has moderate or severe obstructive sleep apnea. This condition is associated with daytime sleepiness, accidents, hypertension, cardiovascular disease, and premature mortality. Between 50 percent and 75 percent of individuals with OSA are obese. (32) Clinical trials suggest that modest weight loss can be helpful when treating sleep apnea. (34, 35)
Obesity, Memory, and Cognitive Function
Alzheimer’s disease and dementia are scourges of populations that enjoy a long life span. In the United States, these diseases affect more than 7.5 million people, most of them over age 65. At 65, the estimated lifetime risk for Alzheimer’s disease is 17.2 percent in women and 9.1 percent in men. (36) Body weight is a potentially modifiable risk factor for Alzheimer’s disease and dementia. A meta-analysis of 10 prospective cohort studies that included almost 42,000 subjects followed for three to 36 years demonstrated a U-shaped association between BMI and Alzheimer’s disease. Compared with being in the normal weight range, being underweight was associated with a 36 percent higher risk of Alzheimer’s disease while being obese was associated with a 42 percent higher risk. (37) The associations were stronger in studies with longer follow-up. A more recent meta-analysis demonstrated a similarly strong association between obesity and Alzheimer’s disease. (38)
Obesity and Musculoskeletal Disorders
Excess weight places mechanical and metabolic strains on bones, muscles, and joints. In the United States, an estimated 46 million adults (about one in five) report doctor-diagnosed arthritis. (1) Osteoarthritis of the knee and hip are both positively associated with obesity, and obese patients account for one-third of all joint replacement operations. (39) Obesity also increases the risk of back pain, lower limb pain, and disability due to musculoskeletal conditions.
Obesity and Other Conditions
A number of additional health outcomes have been linked to excess weight. These include the development of gallstones in men (40) and women, (41) as well as gout, (42, 43) chronic kidney disease, (44) and nonalcoholic fatty liver disease. (25,45)
Obesity and Mortality
Given the adverse consequences of obesity on multiple aspects of health, it makes sense that the condition also shortens survival or increases premature mortality. However, pinning down the contribution of obesity to premature mortality has been fraught with methodological problems and controversy.
Two of the biggest problems that researchers must cope with are reverse causation-low body weight is often the result of chronic disease, rather than being a cause of it-and the effect of smoking. People with BMIs below 25 are a mix of healthy individuals and those who have lost weight due to cancer or some other disease that may or may not have been diagnosed. Smoking also confuses the issue because smokers tend to weigh less than their nonsmoking counterparts. When reverse causation and the adverse effects of smoking aren’t fully accounted for, death rates among lean individuals will be inflated and those among overweight and obese individuals will be diminished. That was a problem with a widely reported study based on data from NHANES, which estimated relatively low numbers of excess obesity-related deaths. (46) A careful critique of using the NHANES data to estimate mortality demonstrated that correcting for statistical biases significantly increased the estimate of excess deaths attributable to obesity. (47)
Findings from larger studies that have more accurately accounted for reverse causation and smoking clearly show that increasing weight increases the risks of dying from cardiovascular disease, cancer, and other causes. In a 14-year study of a million-person cohort, researchers restricted their analyses to initially healthy nonsmokers. The risk of death from all causes, cardiovascular disease, cancer, or other diseases increased as BMI increased above the healthiest range of 23.5 to 24.9 in men and 22.0 to 23.4 in women. (48) A similar association between weight and mortality was observed in another carefully controlled analysis of five prospective cohort studies (49) and a prospective study of more than 500,000 older men and women in the National Institutes of Health/AARP study. (50)
The Bottom Line
Obesity harms virtually every aspect of health, from shortening life and contributing to chronic conditions such as diabetes and cardiovascular disease to interfering with sexual function, breathing, mood, and social interactions. Obesity isn’t necessarily a permanent condition. Diet, exercise, medications and even surgery can lead to weight loss. Yet it is much much harder to lose weight than it is to gain it. Prevention of obesity, beginning at an early age and extending across a lifespan could vastly improve individual and public health, reduce suffering, and save billions of dollars each year in health care costs.
- National Heart, Lung, and Blood Institute. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults . 2002. Accessed January 25, 2012.
- Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med . 1995; 122:4816.
- Koh-Banerjee P, Wang Y, Hu FB, Spiegelman D, Willett WC, Rimm EB. Changes in body weight and body fat distribution as risk factors for clinical diabetes in US men. Am J Epidemiol . 2004; 159:11509.
- Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health . 2009; 9:88.
- Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol . 2009; 6:399409.
- Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med . 2002; 346:393403.
- Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet . 2008; 371:17839.
- Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med . 2001; 344:134350.
- Bogers RP, Bemelmans WJ, Hoogenveen RT, et al. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta-analysis of 21 cohort studies including more than 300,000 persons. Arch Intern Med . 2007; 167:17208.
- Strazzullo P, DElia L, Cairella G, Garbagnati F, Cappuccio FP, Scalfi L. Excess body weight and incidence of stroke: meta-analysis of prospective studies with 2 million participants. Stroke . 2010; 41:e41826.
- McGee DL. Body mass index and mortality: a meta-analysis based on person-level data from twenty-six observational studies. Ann Epidemiol . 2005; 15:8797.
- Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med . 2010; 170:156675.
- Dengo AL, Dennis EA, Orr JS, et al. Arterial destiffening with weight loss in overweight and obese middle-aged and older adults. Hypertension . 2010; 55:85561.
- de las Fuentes L, Waggoner AD, Mohammed BS, et al. Effect of moderate diet-induced weight loss and weight regain on cardiovascular structure and function. J Am Coll Cardiol . 2009; 54:237681.
- American Institute for Cancer Research, World Cancer Research Fund. Food, nutrition, physical activity and the prevention of cancer. Washington, D.C.: American Institute for Cancer Research ; 2007.
- Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA . 2006; 296:193201.
- de Wit L, Luppino F, van Straten A, Penninx B, Zitman F, Cuijpers P. Depression and obesity: a meta-analysis of community-based studies. Psychiatry Res . 2010; 178:2305.
- Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry . 2010; 67:2209.
- Kim D, Kawachi I. Obesity and health-related quality of life. In: Hu FB, ed. Obesity Epidemiology. London: Oxford University Press; 2008:23460.
- Rich-Edwards JW, Spiegelman D, Garland M, et al. Physical activity, body mass index, and ovulatory disorder infertility. Epidemiology . 2002; 13:18490.
- Huda SS, Brodie LE, Sattar N. Obesity in pregnancy: prevalence and metabolic consequences. Semin Fetal Neonatal Med . 2010; 15:706.
- Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA . 2009; 301:63650.
- Clark AM, Ledger W, Galletly C, et al. Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Hum Reprod . 1995; 10:270512.
- Hammoud AO, Wilde N, Gibson M, Parks A, Carrell DT, Meikle AW. Male obesity and alteration in sperm parameters. Fertil Steril . 2008; 90:22225.
- Chavarro JE, Toth TL, Wright DL, Meeker JD, Hauser R. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil Steril . 2010; 93:222231.
- Bacon CG, Mittleman MA, Kawachi I, Giovannucci E, Glasser DB, Rimm EB. A prospective study of risk factors for erectile dysfunction. J Urol . 2006; 176:21721.
- Saigal CS, Wessells H, Pace J, Schonlau M, Wilt TJ. Predictors and prevalence of erectile dysfunction in a racially diverse population. Arch Intern Med . 2006; 166:20712.
- Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts male aging study. J Urol . 2000; 163:4603.
- Wing RR, Rosen RC, Fava JL, et al. Effects of weight loss intervention on erectile function in older men with type 2 diabetes in the Look AHEAD trial. J Sex Med . 2010; 7:15665.
- Bajos N, Wellings K, Laborde C, Moreau C. Sexuality and obesity, a gender perspective: results from French national random probability survey of sexual behaviours. BMJ . 2010; 340:c2573.
- Esposito K, Ciotola M, Giugliano F, et al. Association of body weight with sexual function in women. Int J Impot Res . 2007; 19:353-7.
- McClean KM, Kee F, Young IS, Elborn JS. Obesity and the lung: 1. Epidemiology. Thorax . 2008; 63:64954.
- Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med . 2007; 175:6616.
- Nerfeldt P, Nilsson BY, Mayor L, Udden J, Friberg D. A two-year weight reduction program in obese sleep apnea patients. J Clin Sleep Med . 2010; 6:47986.
- Tuomilehto HP, Seppa JM, Partinen MM, et al. Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med . 2009; 179:3207.
- Alzheimers Association. Alzheimers Facts and Figures. Alzheimers & Dementia . 2010; 6. Accessed January 25, 2012.
- Beydoun MA, Beydoun HA, Wang Y. Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta-analysis. Obes Rev . 2008; 9:204-18
- Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimers disease risk with obesity, diabetes, and related disorders. Biol Psychiatry . 2010; 67:50512.
- Anandacoomarasamy A, Caterson I, Sambrook P, Fransen M, March L. The impact of obesity on the musculoskeletal system. Int J Obes (Lond) . 2008; 32:21122.
- Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Prospective study of abdominal adiposity and gallstone disease in US men. Am J Clin Nutr . 2004; 80:3844.
- Stampfer MJ, Maclure KM, Colditz GA, Manson JE, Willett WC. Risk of symptomatic gallstones in women with severe obesity. Am J Clin Nutr . 1992; 55:6528.
- Bhole V, de Vera M, Rahman MM, Krishnan E, Choi H. Epidemiology of gout in women: Fifty-two-year followup of a prospective cohort. Arthritis Rheum . 2010; 62:106976.
- Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the Health Professionals Follow-up Study. Arch Intern Med . 2005; 165:7428.
- Kopple JD. Obesity and chronic kidney disease. J Ren Nutr . 2010; 20:S2930.
- Tsuneto A, Hida A, Sera N, et al. Fatty liver incidence and predictive variables. Hypertens Res . 2010; 33:63843.
- Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA . 2007; 298:202837.
- Greenberg JA. Correcting biases in estimates of mortality attributable to obesity. Obesity (Silver Spring) . 2006; 14:20719.
- Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW, Jr. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med . 1999; 341:1097105.
- Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB. Annual deaths attributable to obesity in the United States. JAMA . 1999; 282:15308.
- Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med . 2006; 355:76378.

A systematic literature review on obesity: Understanding the causes & consequences of obesity and reviewing various machine learning approaches used to predict obesity
Affiliations.
- 1 Centre for Software Technology and Management, Faculty of Information Science and Technology, Universiti Kebangsaan Malaysia (UKM), Bangi, 43600, Selangor, Malaysia.
- 2 Centre for Software Technology and Management, Faculty of Information Science and Technology, Universiti Kebangsaan Malaysia (UKM), Bangi, 43600, Selangor, Malaysia. Electronic address: [email protected].
- 3 RIADI Laboratory, University of Manouba, Manouba, Tunisia; College of Computer Science and Engineering, Taibah University, Medina, Saudi Arabia.
- 4 Center for Artificial Intelligence Technology, Faculty of Information Science and Technology, Universiti Kebangsaan Malaysia (UKM), Bangi, 43600, Selangor, Malaysia.
- PMID: 34426171
- DOI: 10.1016/j.compbiomed.2021.104754
Obesity is considered a principal public health concern and ranked as the fifth foremost reason for death globally. Overweight and obesity are one of the main lifestyle illnesses that leads to further health concerns and contributes to numerous chronic diseases, including cancers, diabetes, metabolic syndrome, and cardiovascular diseases. The World Health Organization also predicted that 30% of death in the world will be initiated with lifestyle diseases in 2030 and can be stopped through the suitable identification and addressing of associated risk factors and behavioral involvement policies. Thus, detecting and diagnosing obesity as early as possible is crucial. Therefore, the machine learning approach is a promising solution to early predictions of obesity and the risk of overweight because it can offer quick, immediate, and accurate identification of risk factors and condition likelihoods. The present study conducted a systematic literature review to examine obesity research and machine learning techniques for the prevention and treatment of obesity from 2010 to 2020. Accordingly, 93 papers are identified from the review articles as primary studies from an initial pool of over 700 papers addressing obesity. Consequently, this study initially recognized the significant potential factors that influence and cause adult obesity. Next, the main diseases and health consequences of obesity and overweight are investigated. Ultimately, this study recognized the machine learning methods that can be used for the prediction of obesity. Finally, this study seeks to support decision-makers looking to understand the impact of obesity on health in the general population and identify outcomes that can be used to guide health authorities and public health to further mitigate threats and effectively guide obese people globally.
Keywords: Diseases; Machine learning; Obesity; Overweight; Risk factors.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Publication types
- Research Support, Non-U.S. Gov't
- Systematic Review
- Machine Learning
- Metabolic Syndrome*
- Obesity* / epidemiology
- Risk Factors
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Supplements
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 6, Issue 10
- Economic impacts of overweight and obesity: current and future estimates for eight countries
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0002-7486-5176 Adeyemi Okunogbe 1 ,
- Rachel Nugent 2 ,
- Garrison Spencer 2 ,
- Johanna Ralston 3 ,
- John Wilding 3
- 1 Global Health Division , RTI International , Washington , D.C , USA
- 2 Center for Global Noncommunicable Diseases , RTI International , Seattle , Washington , USA
- 3 World Obesity Federation , London , UK
- Correspondence to Dr Adeyemi Okunogbe; aokunogbe{at}rti.org
Background Obesity is a growing public health challenge worldwide with significant health and economic impacts. However, much of what is known about the economic impacts of obesity comes from high-income countries and studies are not readily comparable due to methodological differences. Our objective is to demonstrate a method for estimating current and future national economic impacts of obesity and apply it across a sample of heterogeneous contexts globally.
Methods We estimated economic impacts of overweight and obesity for eight countries using a cost-of-illness approach. Direct and indirect costs of obesity from 2019 to 2060 were estimated from a societal perspective as well as the effect of two hypothetical scenarios of obesity prevalence projections. Country-specific data were sourced from published studies and global databases.
Results In per capita terms, costs of obesity in 2019 ranged from US$17 in India to US$940 in Australia. These economic costs are comparable to 1.8% of gross domestic product (GDP) on average across the eight countries, ranging from 0.8% of GDP in India to 2.4% in Saudi Arabia. By 2060, with no significant changes to the status quo, the economic impacts from obesity are projected to grow to 3.6% of GDP on average ranging from 2.4% of GDP in Spain to 4.9% of GDP in Thailand. Reducing obesity prevalence by 5% from projected levels or keeping it at 2019 levels will translate into an average annual reduction of 5.2% and 13.2% in economic costs, respectively, between 2020 and 2060 across the eight countries.
Conclusion Our findings demonstrate that the economic impacts of obesity are substantial across countries, irrespective of economic or geographical context and will increase over time if current trends continue. These findings strongly point to the need for advocacy to increase awareness of the societal impacts of obesity, and for policy actions to address the systemic roots of obesity.
- health economics
- public health
- health policy
Data availability statement
Data are available on reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bmjgh-2021-006351
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Key questions
What is already known.
Estimates of the economic impacts of obesity as a percentage of gross domestic product (GDP) range from 0.13% in Thailand (Pitayatienanan et al ) to 9.3% in the USA (Milken Institute, 2018) with most estimates for high-income countries.
Most studies use a health system perspective rather than a societal perspective.
Cost of obesity studies vary considerably in the types of outcomes reported, diseases included for the measurement of healthcare costs, age groups included and methods for estimating direct and indirect costs.
Scant evidence is available on the economic impacts of obesity that are comparable across income contexts for policy and advocacy.
What are the new findings?
We estimate obesity costs between 0.8% and 2.4% of gross domestic product (GDP) in 2019 in the eight countries in the study.
Our projections reveal an increasing trend in obesity costs as a percentage of GDP over time, estimated to reach 2.4% of GDP in Spain and up to 4.9% in Thailand in 2060.
The economic impacts of obesity are substantial and reach a similar magnitude in low-income and middle-income countries as in high-income contexts.
Maintaining or reducing the prevalence of obesity can reduce the economic impacts of obesity in the future.
What do the new findings imply?
Quantifying the economic impacts of obesity will help stakeholders understand the importance of addressing obesity through systemic solutions and is a tool for national and international advocates to encourage policy actions.
There is need for a concerted increase in national efforts to combat the global rise in obesity prevalence and overcome the existing policy inertia that has hampered progress on obesity policy implementation.
Introduction
Between 1975 and 2016, the prevalence of obesity increased in every country in the world. 1 Overweight and obesity contribute to numerous noncommunicable diseases (NCDs), including cardiovascular disease, diabetes and cancer. 2 Obesity-related NCDs account for over 5 million deaths globally each year, with over half occurring under the age of 70. 3 The COVID-19 pandemic also revealed obesity as a significant factor in infectious disease morbidity and mortality. 4 Obesity is a complex chronic disease process resulting from the interaction of various factors including genetic susceptibility, high energy-dense nutrition, low physical activity and stress. 2
The multifactorial and chronic nature of overweight and obesity leads to economic impacts for individuals and nations. Most evident are the direct healthcare costs associated with treating obesity-attributable diseases. Individuals living with obesity are significantly more likely to use home healthcare services, have more outpatient visits, be prescribed more medications, be admitted to a hospital and undergo surgery than individuals with lower weight. 5 6 Finally, individuals with obesity experience higher costs of care and longer hospital stays. 7 8
The economic impacts of obesity include indirect costs resulting from lost or reduced productivity and human capital. Studies from multiple countries show that individuals with obesity miss more days of work (absenteeism) than individuals without obesity, and work at less than full capacity when they are at work (presenteeism). 9 Obesity also increases the chances of unemployment and has a negative impact on wages. 10 11 Finally, premature deaths from obesity-attributable diseases imply a loss of potential future contributions to the economy. As with economic studies of other diseases, studying the economic costs of obesity does not imply and should not be misconstrued as meaning that individuals living with obesity create or are responsible for costs or economic losses. Rather, an increasingly obesogenic environment, both directly and through individual epigenetic changes, leads to an increased prevalence of obesity and its associated economic impacts. Although difficult to measure, weight bias also imposes economic and other costs, 12 further underscoring the importance of not blaming individuals experiencing obesity.
Obesity has been shown to have substantial economic impacts in some countries, with estimates of the costs of medical care and reduced productivity ranging from 0.13% of GDP in Thailand to 9.3% in the USA. 13 14 A review of the literature identified 59 studies of economic impacts of obesity published since 2010, a full list of which can be found in the online supplemental appendix . However, most of these studies come from high-income countries, use a healthcare system perspective, and vary considerably in types of outcomes reported, obesity-related diseases included, age groups included, types of costs and methodologies employed in estimating direct and indirect costs.
Supplemental material
This study aims to estimate the current and future economic impacts of obesity using a modelling framework that can be applied to different national contexts around the world and be updated over time. It also assesses the effect of two hypothetical future obesity prevalence scenarios on economic impacts and presents results for eight countries selected to represent a range of geographies and income levels and for which adequate data was available. Cross-country analyses of the economic impacts of obesity are an important way to dispel myths and misunderstandings about the prevalence and causes of obesity, as well as factors that can reduce it. Our review of country studies and methodologies highlights the need to estimate the current and projected economic impacts of obesity across diverse countries in a comparable manner. It is especially important to understand the presence of obesity in low-income and middle-income countries (LMICs) but we found only one cross-country study that included countries that are not high income. 15 Hence, this study seeks to fill that gap. Quantifying the magnitude of economic impacts of obesity helps policy-makers and other stakeholders better understand the scope of the challenge, supports prioritisation and resource allocation efforts, as well as providing a crucial tool for national and international advocates to urge policy-makers to respond with effective policies. 16
We employed a cost-of-illness approach 17 18 to estimate the economic impacts of overweight and obesity in eight countries from a societal perspective in 2019 (baseline) and projected impacts in 2060. This approach translates the adverse effect of obesity into monetary terms, 17 which is useful for understanding the impact of obesity for policy prioritisation and agenda setting. In addition, it is useful for facilitating cross-national comparisons of obesity consequences across different contexts. 19 Overweight is defined as a body mass index (BMI) of 25 kg/m 2 to 29.9 kg/m 2 in adults and obesity as a BMI of 30 kg/m 2 and above; while for children, overweight is defined as weight 1–2 standard deviations (SD) above the median weight and obesity as more than 2 SD above the median. 1 Hereafter, we will use the term ‘obesity’ to refer to both overweight and obesity. The countries in this study—Australia, Brazil, India, Mexico, Saudi Arabia, South Africa, Spain and Thailand—were selected to represent diverse geographic and economic national contexts and based on general data availability. We included 28 obesity-related diseases ( online supplemental appendix 1: table 1 ) from the Global Burden of Disease (GBD) Study with evidence of obesity risk linkages. 3
Under the cost-of-illness approach, the economic impacts of an illness are divided into direct costs and indirect costs ( figure 1 ). In this study, direct costs consist of medical costs and non-medical costs (specifically, the travel and time required to receive care). Indirect costs include economic loss from premature mortality and productivity losses from absenteeism and presenteeism. Other relevant cost components such as long-term disability and early retirement costs 9 were not included as it was not feasible to measure these across countries. Also, in some societies there may be costs associated with weight bias (eg, lower academic achievement, reduced emotional support, reduced likelihood of promotion) and in others there may be a premium associated with obesity 20 ; however, the magnitude and direction of these impacts have not been studied across countries. 12
- Download figure
- Open in new tab
- Download powerpoint
Cost components framework.
We searched PubMed and Google Scholar for peer-reviewed studies and grey literature on the economic impacts of obesity in LMICs, published between 1 January 2010 and December 2019. The PubMed search strategy included MeSH terms for overweight, obesity, world regions and economics, as well as the name of each LMIC (as defined by the World Bank) and synonyms for LMIC status. We identified 59 studies on the economic impacts of obesity, a full list of which can be found in online supplemental appendix 4 . Data for model parameters are from peer-reviewed literature and publicly available global databases (see table 1 ). Data on obesity prevalence and obesity attributable deaths were drawn from the NCD Risk Factor Collaboration (NCD-RisC) study 1 and 2019 GBD Study, 21 respectively. Data on national healthcare expenditure were drawn from the WHO Global Health Expenditure Database. 22 Annual/daily wage data, GDP and employment rates were sourced from the World Bank’s World Development Indicators database. 23 Parameters such as population, life expectancy and background death rates were drawn from the United Nations Population Division. 24 The remaining model parameters—average travel cost; average inpatient and outpatient consultations; hospitalisation days; absenteeism days and presenteeism rate—were sourced from peer-reviewed studies. Where country-specific data were not available, we applied data from another country in the same income group ( table 1 ).
- View inline
Model summary: parameters and data sources
Estimation of direct costs at baseline
Direct costs are typically divided into medical and non-medical costs in the cost-of-illness literature. Direct medical costs measure healthcare goods and services consumed due to obesity-attributable diseases and include curative, rehabilitative and preventative care, ancillary services, and medical goods. 15 To estimate the direct medical cost or obesity-attributable healthcare expenditures, we identified studies from our literature search that reported the proportion of health expenditure attributable to obesity or obesity-attributable fractions (OAF) for a country. We found the OAFs from the Organisation for Economic Co-operation and Development (OECD) report (for the 52 countries in the report) to be the most appropriate because they are based on the GBD study and include the same number of obesity-related diseases as this paper ( online supplemental appendix 1: table 1 ). 15 All eight countries in this study were included in the OECD report except for Thailand. The direct medical costs in 2019 were calculated by multiplying the total health expenditure (THE) of a country in 2019 by the proportion of health expenditure attributed to obesity (OAF).
To estimate OAF for countries not included in the OECD report (Thailand) and for projections, a simple linear regression of OAFs on total obesity prevalence was conducted using the 52 countries in the OECD report. The 52 countries account for about 95% of total health expenditure globally. There is a significant positive association between OAF and obesity prevalence (β=0.094, F=12.92, p=0.001) ( online supplemental appendix 1: figure 1 ). The regression outputs from the estimated equation were used to estimate the OAFs for all countries. We also apply the regression outputs to estimates of projected obesity prevalence to calculate future OAFs as explained subsequently. We used the estimated OAFs from the regression outputs in computing direct medical costs in 2019 for all eight countries instead of the raw values from the OECD report to prevent discordance between current and future direct medical costs and to allow for useful comparisons across years (see the Methods section in online supplemental appendix for more details).
Direct non-medical costs measure the additional costs incurred during the process of seeking care, such as travel to health facilities or doctor appointments, costs incurred by informal caregivers (ICGs), food and lodging during inpatient care, and home modifications. This study includes an estimation of travel expenses and ICG costs only as there are not reliable data about other direct non-medical costs from which to develop cross-country estimates.
Travel costs were estimated separately for inpatient (hospitalisation) and outpatient care. We calculated this by multiplying the average travel cost (ATC) per trip (proxied by country-specific daily average transportation expenditure per capita) by the population with obesity. The formulae for these components are:
ICG costs were estimated for inpatient care and include both travel and time costs during inpatient care. ICG travel cost is calculated the same as inpatient travel cost. ICG time costs denote the income loss of ICGs for time spent tending to a hospitalised family member or friend suffering from an obesity-attributable disease. We calculate this as the average wage lost from the time spent in the hospital.
Estimating indirect costs at baseline
Indirect costs represent the economic loss due to premature mortality and morbidity and includes the following components: economic loss from premature mortality, missed days of work (absenteeism) and reduced productivity while at work (presenteeism). For absenteeism and presenteeism costs, we assume the same employment rates by BMI status. While it is plausible that the population with obesity may have a lower employment rate compared with the population without obesity, the existing evidence is mixed and inconclusive. 26–33
Economic loss from premature mortality is calculated as the number of years of potential life lost by individuals (by age group and sex cohort) who died from obesity multiplied by the economic value of a life year. To quantify the number of years of potential life lost due to obesity, we estimate how many people in each age and sex cohort would have been alive in future years (based on life expectancy) if they had not died from obesity-attributable diseases, while taking into account background death rates from other causes. Background death rates are drawn from country-specific life tables ( table 1 ). 34 We used GDP per capita as a proxy for the economic value of a life year (VLY) to capture economic loss from premature mortality. Other proxies that have been used in estimating cost of premature mortality include wages and the value of a statistical life year (see online supplemental appendix 1: box 1 ). Our choice of GDP per capita is driven by an inclination to value the economic contribution of every individual in the society across the lifecourse irrespective of employment status. This brings an equity lens to how economic contributions are counted. Furthermore, as part of sensitivity analysis for the upper bound of premature mortality cost, we adjust GDP per capita with a GDP multiplier ( online supplemental appendix 1: table 2 ) for the gains in health or life expectancy that would have occurred in the absence of obesity attributable deaths as developed by the Lancet Commission on Investing in Health. 35 The economic cost of obesity-attributable mortality for each sex (S) and age (A) cohort included in the model is calculated as the sum of annual costs from age of death (i=0) to remaining life expectancy when no persons from the cohort would remain alive if they had not died of obesity-attributable diseases, where VLY is the value of a life year proxied by GDP per capita in model death year and People i is the number of people who would have still been alive in year i had they not died of obesity-attributable disease.
For all age and sex cohorts, all future costs are assigned to the year in which death occurred, discounted at a rate of 3% per year to obtain the net present value, and added up to give the total economic cost of obesity-attributable premature mortality.
Productivity losses due to absenteeism occur when employees miss work due to illness or health conditions related to obesity. The calculation for the cost of lost productivity due to excess absenteeism among the working population with obesity is:
where Employed Pop.with Obesity = Employment rate × Working Age Pop. × Obesity Prevalence; Excess Days Absent=Average number of additional days of absenteeism by working population with obesity compared with normal weight working population.
Productivity losses due to excess presenteeism refers to reduced productivity while at work due to obesity-related impairment and disability. The calculation for the cost of lost productivity due to excess presenteeism among the working population with obesity is:
where Employed Pop.with Obesity = Employment rate × Working Age Pop. × Obesity Prevalence; Excess Presenteeism Rate=Rate of reduced productivity among employees with obesity.
Estimating future costs
The projections of obesity’s economic impacts are an extension of the modelling approach used for the current impacts’ estimation. We calculated costs for future years using projected estimates for the different parameters in the model. The index/baseline year for estimating current impacts is 2019, the most recent year with available mortality data from the GBD study. Our projections are therefore from 2020 to 2060. Online supplemental appendix 1: table 3 shows the secondary sources of data for parameters for which existing long-term projections were found. Some parameters such as number of inpatient and outpatient consultations, hospitalisation days, absenteeism days and presenteeism rate were assumed to stay constant. We adjusted travel costs for inflation in the future using GDP Deflator projections. We modelled future estimates for parameters with no existing long-term projections. These are annual wages, obesity prevalence, OAF of health expenditures and obesity-attributable mortality. For future annual wages, we extrapolated historical average annual wage growth to 2060. Future estimates for obesity prevalence, OAF of health expenditures, and obesity-attributable mortality were modelled as described below.
Future obesity prevalence: We used a multivariate autoregression approach to model changes in annual country-level overweight and obesity prevalence from 1975 to 2016 for males and females in two age-groups (less than 20 years and 20 years and above) with data from the NCD-RisC study. 1 This model combines an autoregressive component (past prevalence observations), a differencing step to ensure stationarity and factors that influence prevalence as covariates to predict future obesity prevalence. Our model takes the following form for country i , year t and sex s:
Future OAF of health expenditure: As earlier explained, we obtained estimates of OAFs for 52 countries from the OECD report. 15 We then estimated a simple linear regression of these OAFs on obesity prevalence. The regression outputs (coefficient and intercept) together with projected obesity prevalence estimates were then used to generate future estimates of OAFs.
Future Obesity-attributable mortality: We calculated all-cause mortality (total deaths) from 2020 to 2060 using the annualised rate of change of projections for obesity-related disease mortality from Foreman and colleagues. 37 We then calculated population attributable fraction (PAF) of obesity mortality for each future year by sex, cause and age group. We used the calculated PAF to generate obesity-attributable deaths for projected years by sex, age group and cause. For more details, see online supplemental appendix 1 .
Hypothetical scenarios: In addition to estimating future economic costs based on projected obesity prevalence, we also estimated the impact on the projected economic costs using two scenarios of lower prevalence in the future. Projections are not predictions, hence future obesity prevalence and other model parameters may diverge from projected estimates, which are based largely on historical trends. Therefore, in addition to estimating future economic costs based on projected prevalence, we also developed two hypothetical scenarios for economic impacts of obesity keeping in mind that no country has been able to reduce obesity prevalence 36 and there have been indications of stabilisation in only a handful of countries. 15 The two hypothetical scenarios are: (1) a 5 percentage point reduction in projected obesity prevalence (by sex and age group) for each year and (2) holding obesity prevalence (by sex and age group) constant at 2019 levels.
Currency conversions
All costs are in 2019 constant US dollars. Data for GDP per capita, wages and travel costs were collected in local currency units where possible, adjusted for inflation to 2019 values, and converted to US$ using average annual exchange rates. Purchasing power parity (PPP) costs were also calculated, using PPP conversion factors drawn from the World Bank World Development Indicators Database. 23 38
Current economic impacts of obesity
Total obesity costs per capita range from US$17 in India to US$940 in Australia. Obesity results in an impact comparable to 1.76% of GDP on average across the eight countries, ranging from 0.80% of GDP in India to 2.42% in Saudi Arabia ( figures 2 and 3 ). Table 2 provides a comparison of total costs and costs per capita in US dollars, PPP dollars and as a percentage of GDP.
Total current costs of obesity and costs of obesity per capita expressed in 2019 US$, 2019 PPP US$, and as a percentage of GDP
Total cost of obesity in 2019 in per capita terms (in 2019 US$).
Total cost of obesity in 2019 as a percentage of GDP. GDP, gross domestic product.
Table 3 shows economic impacts by cost components and figure 4 shows total costs by country. Medical costs make up 90% of direct costs on average across all countries. Time cost of informal caregivers constitute more than 90% of direct non-medical costs on average across all countries. The cost of premature mortality constitutes a substantial proportion of indirect costs (about 56%–92%) across all countries. We did a sensitivity analysis which values the life expectancy gains from avoiding premature mortality at a multiple of GDP, following recommendations from the Lancet Commission on Investing in Health. This results in total costs of 2.59% of GDP on average across the eight countries, ranging from 1.70% of GDP in India to 4.16% in South Africa ( table 3 ). These results, which place a higher value on premature mortality costs, represent a 57% increase in total costs on average across the eight countries, ranging from a 19% increase in Australia to a 163% increase in South Africa.
Current Burden Results, 2019 (in 2019 US$)
Current costs of obesity in 2019 (in billions of 2019 US$).
Obesity prevalence, wage and employment data were disaggregated by sex for all eight countries. Economic impacts of obesity are typically higher for males compared with females ( figure 5 ). This difference is driven by obesity prevalence, wages and sex differences in employment.
Total cost of obesity by sex (billions of 2019 constant US$ and as percentage of GDP). GDP, gross domestic product.
Future economic impacts of obesity
Table 4 and figures 6–8 summarise estimated future costs based on projections of model parameters to 2060. Obesity costs across all countries are projected to increase due to rising obesity prevalence, population changes and economic growth. Between 2020 and 2060, obesity costs are projected to double in Spain and increase by 19-fold in India. As a percentage of projected GDP, total costs in 2060 are estimated to be an average of 3.57% across the eight countries, ranging from 2.43% in Spain to 4.88% in Thailand. See online supplemental appendices 2 and 3 for additional figures and tables.
Baseline projections, years 2020, 2030, 2040, 2050 and 2060 (in 2019 US$)
Total costs of obesity per capita (in 2019 US$), 2019–2060.
Total costs of obesity as a per cent of GDP, 2019–2060. GDP, gross domestic product.
Total costs of obesity (in 2019 constant US$ and as a per cent of GDP) and obesity prevalence, 2019–2060. GDP, gross domestic product.
Hypothetical scenarios
Our first hypothetical scenario depicts projected economic impacts if there is a 5 percentage points reduction in obesity prevalence from the projected levels (by sex and age). Using this prevalence scenario with all other projected parameters remaining unchanged, we estimate a slight reduction in obesity’s economic cost trajectory compared with baseline projections. As a percentage of projected GDP, total costs in 2060 will range from 2.32% in Spain to 4.70% in Thailand ( figure 9 ). Compared with baseline projections, this scenario implies an average annual savings of approximately 5.18% across all eight countries between 2021 and 2060 ( table 5 ).
Hypothetical scenario of 5% reduction in obesity prevalence, total costs (in billions of 2019 constant US$), total costs as a per cent of GDP, and obesity prevalence, 2019–2060. GDP, gross domestic product.
Annual cost reductions in hypothetical scenarios 1 and 2 between 2020 and 2060
Our second hypothetical scenario projects obesity’s economic impacts while holding obesity prevalence constant. This is consistent with the WHO NCD Global Monitoring Framework target #7 to halt the rise in obesity. 39 Keeping obesity prevalence at 2019 levels from 2020 to 2060 is equivalent to an average annual reduction in prevalence ranging from 9% to 22% across countries compared with baseline prevalence projections ( table 5 ). As a percentage of projected GDP, total costs in 2060 will range from 1.44% in India to 4.14% in Mexico, translating to average annual savings of 13.18% compared with baseline projection costs ( figure 10 ).
Hypothetical scenario of constant* obesity prevalence, total costs (in billions of 2019 constant US$), total costs as a per cent of GDP and obesity prevalence, 2019–2060. GDP, gross domestic product.
This study uses cost-of-illness methodology to assess the economic impacts of obesity in eight countries from a societal perspective. We estimate obesity costs between 0.80% and 2.42% of GDP in 2019 in the eight countries. To put this into context, annual GDP growth rate in 2019 averaged 1.6% among the eight countries, ranging between −0.12% (Mexico) and 5% (India). 23 It is therefore reasonable to view the economic impact of obesity as a significant hindrance to economic development. However, these estimates are still conservative. Our sensitivity analysis shows that putting a higher value on gains in life expectancy from avoiding premature mortality would yield a higher economic loss from obesity.
While our results are in a comparable range to the most recent multicountry study of obesity’s economic impact (OECD), 15 they are higher than earlier studies in some of the countries as we include the impacts of obesity on more diseases. For example, Pitayatienanan et al estimate healthcare costs (outpatient and inpatient visits only) in Thailand for 13 obesity-related diseases in 2009 as 5.5 billion Thai baht (approximately US$220 million in 2019), compared with our estimate of US$1.3 billion in obesity-attributable healthcare expenditure for 26 obesity-related diseases. In Brazil, Bahia et al estimate the obesity-attributable public healthcare expenditures for 14 diseases in 2010 US$ as 221 million compared with our estimate of US$14 billion in combined public and private obesity-attributable healthcare expenditure for 26 obesity-related diseases. 40 In addition, some of the difference in results can be attributed to a rise in obesity prevalence during the interim (an increase of 11% in Thailand and 8% in Brazil between the periods of these two studies and 2019). 1 With regard to societal impacts more broadly, we also use GDP per capita to proxy the economic value of a life year for premature mortality while some studies use minimum or average wage in each country. 13 35 41 Differing sources, availability and granularity of data could also contribute to differences in cost estimates.
Our findings reveal that the societal impacts of obesity are substantial for countries at different income levels. While high-income countries are known to experience high economic costs from obesity, 15 this study finds that a similar magnitude of impact may be present in LMICs consistent with existing evidence on the double burden of malnutrition. 42 Differences in economic impact across countries are partly explained by differences in obesity prevalence and obesity-attributable mortality. India has the lowest total obesity prevalence, obesity-attributable mortality, and cost per GDP per cent of the eight countries. On the other hand, Saudi Arabia, with the highest cost per GDP per cent, has the highest total obesity prevalence and also has an above-average obesity-attributable mortality among the eight counties. Other factors that drive differences in total costs between countries include the income levels/economic strength (GDP/capita), wage differences, employment rates, national healthcare expenditure and the age distribution of obesity-attributable mortality.
Estimates of obesity’s economic impacts that are limited to only direct healthcare costs underestimate the full economic effect of overweight and obesity. Our findings indicate that indirect costs of obesity account for a larger proportion of total cost (65% on average across countries) compared with direct costs. However, direct medical costs still impose immediate and sometimes unsustainable burdens on health systems. Examination of the variation in costs by sex in 2019 also generally indicate a slightly higher cost for males compared with females reflecting differences in obesity prevalence, wages, and employment, which vary by country.
Our projections reveal an alarming trend across all eight countries as the total costs of obesity in 2019 constant US$ is projected to rise at an average rate of between 1.8% and 6.6% and cost/GDP is projected to rise at an average rate of 0.4%–3.3% from 2019 to 2060. This is partly due to a projected rise in obesity prevalence with an average growth rate ranging from 0.7% to 3.0% in the same period ( online supplemental appendix 3: table A7 ). We project that the prevalence of obesity will increase to about 57% of the population in India and to about 93% of the population in Saudi Arabia in 2060 ( figure 8 ). These estimates are similar to estimates by Kilpi et al who adapted the UK Foresight model to estimate that obesity prevalence will rise to 92% in men and 75% in women by 2050 in Saudi Arabia. 43 In another related study of 10 countries in Latin America, obesity prevalence in 2050 is estimated to increase to 90% of males in Cuba and Panama and to 85% of females in Chile, Cuba, Nicaragua, Panama, Peru and Uruguay. 44
Our hypothetical scenarios demonstrate that economic costs to society can be reduced with lower obesity levels. The scenarios underscore the need to take urgent action to reduce potential economic impacts in the future. This will not be achieved if current levels of underinvestment in treatment and the social determinants of obesity continue. Overall, our findings make the case for a concerted increase in national efforts to combat the global rise in obesity prevalence and overcome the existing policy inertia that has hampered progress on obesity policy implementation. 45 WHO’s ‘best buy’ interventions offer an initial set of cost-effective actions for countries to employ, including community wide public education and awareness for physical activity and taxes on sugar-sweetened beverages, 46 front-of-package labelling and other nutrition profiling schemes. However, many other opportunities to alter the obesogenic environment through food systems, transportation and subsidies have not been widely implemented and evaluated, leaving much room for future study. 16 Efforts to address the economic impacts of obesity must not be left to individuals but focus on altering the complex environmental factors leading to obesity, as well as treatment. The involvement of individuals with obesity in the policy decision-making process and in guiding research is also imperative in achieving equitable allocation and distribution of resources, and for pursuing policies that reduce weight bias. 47
This study has several limitations. To produce comparable estimates across countries, we used data that are available across both data-rich and data-poor geographical contexts. For some of the parameters, such as absenteeism and presenteeism rates associated with obesity, due to data limitations, we assumed the same value for countries in similar income groups which is a simplification as there are important variations in labour market behaviour across countries. In addition, while we attempt to account for indirect costs such as absenteeism and presenteeism, there are other indirect effects such as unemployment, long-term disability and early retirement costs 9 that are difficult to estimate for data-limited country contexts. These are not included in our analyses nor are intangible effects of obesity that are difficult to quantify in monetary terms such as decreased quality of life. 48 Estimates of the value of life across countries, genders and age raise ethical challenges that are not fully resolved in this paper. One challenge is simply differences in access to healthcare among countries which hides some of the impacts of obesity in countries that offer less healthcare for obesity-related diseases. Also, we recognise that differences in labour markets, type of and compensation for work, and what is measured by GDP introduces many inequities across populations. We handle these issues with clear and replicable methodology that allows other data inputs to be selected. Individual country studies are the appropriate place to make adjustments for these differences and we suggest here some of the parameters that should be sourced locally whenever possible. Also, while cost-of-illness studies have played a significant role in public health by supporting advocacy for and formulation of healthcare policies, their usefulness in decision making for prioritisation and resource allocation needs to be augmented by a consideration of both costs and benefits.
Our estimates of future obesity prevalence are based on an assumption that historical and current trends relating obesity to age, sex, and nutrition continue, hence we do not model for unforeseeable changes, such as technology progress that could impact the food environment or medical breakthroughs in obesity treatment or prevention. Our cost projections relied on secondary projections from credible sources. Hence, the assumptions of these sources are necessarily transferred to this study as well. Despite these limitations, this study makes an important contribution in quantifying the comparative economic impacts of obesity across eight countries which can be extended to other countries.
Our findings suggest that there are enormous economic impacts associated with obesity across countries irrespective of geography or income level. There is tremendous variation across countries in the level and impacts of obesity but—as seen in these eight countries—historical and current trends demonstrate that economic costs will rise over time. The COVID-19 pandemic has especially affected people living with obesity, thus further bringing obesity to the attention of national policy makers. The findings of this study will be helpful to further strengthen political commitment for national obesity control efforts in these countries. This is greatly needed to achieve levels of investment commensurate to the economic impact. Future analyses will further extend this methodology to other countries and will estimate the effect of COVID-19 on these results.
Ethics statements
Patient consent for publication.
Not required.
- Abarca-Gómez L ,
- Abdeen ZA ,
- Hamid ZA , et al
- Wilding JPH , et al
- GBD 2019 Risk Factors Collaborators
- Popkin BM ,
- Green WD , et al
- Wolfenstetter SB ,
- Holle R , et al
- Padula WV ,
- Herring AH ,
- Stevens J , et al
- Goettler A ,
- Meyerhoefer C
- Huerta MC ,
- Aurino E , et al
- Russell-Mayhew S ,
- von Ranson K , et al
- Pitayatienanan P ,
- Butchon R ,
- Yothasamut J , et al
- Milken Institute
- ScienceDaily
- World Health Organization
- United Nations Population Division
- Edwards CH ,
- Campos-Vazquez RM ,
- Al-Hanawi MK ,
- Chirwa GC ,
- Kamninga TM
- Félix-Redondo F-J ,
- Lozano-Mera L , et al
- Langley PC ,
- Tornero Molina J ,
- Margarit Ferri C , et al
- Behanan R ,
- Vorster HH , et al
- Jamison DT ,
- Summers LH ,
- Fleming T ,
- Robinson M , et al
- Foreman KJ ,
- Marquez N ,
- Dolgert A , et al
- Turner HC ,
- Tran BX , et al
- Coutinho ESF ,
- Barufaldi LA , et al
- Viscusi WK ,
- Corvalan C ,
- Grummer-Strawn LM
- Musaigner A , et al
- Marsh T , et al
- Swinburn BA ,
- Allender S , et al
- Arthur N , et al
- Cho YG , et al
- Australian Bureau of Statistics
- Key indicators of household expenditure on services and durable goods NSS 72nd round (July 2014-June 2015) , 2016 . Available: https://www.thehinducentre.com/multimedia/archive/02914/Key_Indicators_of__2914425a.pdf
- National Institute of Statistics and Geography (INEGI)
- General Authority for Statistics (GASTAT)
- Instituto Nacional de Estadistica (INE)
- Income-Expenditure Statistics Group
- Social Statistics Division
- National Statistical Office
- Ministry of Digital Economy and Society
- de Menezes Goncalves T , et al
- Paige E , et al
- Espallardo O ,
- Busutil R ,
- Torres A , et al
- Average annual wages . Available: https://stats.oecd.org/viewhtml.aspx?datasetcode=AV_AN_WAGE&lang=en [Accessed 25 Nov 2020 ].
- International Labour Office
- International Labour Organization
- Ordoñez AM ,
- Madalozzo Schieferdecker ME ,
- Cestonaro T , et al
- Keramat SA ,
- Gow J , et al
- Catalina-Romero C ,
- Sanchez Chaparro MA ,
- Valdivielso P , et al
Supplementary materials
- Press release
Handling editor Edwine Barasa
Contributors AO contributed to methodology development, data analysis, interpretation of results and writing of manuscript. RN conceived study and contributed to methodology, interpretation of results and writing of manuscript. GS conducted literature search, data collection, created figures/tables and contributed to interpretation of results and writing of manuscript. JR coconceived the study and provided guidance on scope and interpretation of results. JW reviewed the paper and provided guidance on interpretation of results.
Funding Support for this work came from World Obesity Federation funds which include an unrestricted educational grant from Novo Nordisk. Novo Nordisk was not involved in the conception and design of the study methodology or in the analysis and interpretation of results.
Competing interests AO, RN and GS report the research was supported by a grant from the World Obesity Federation which received an unrestricted grant from Novo Nordisk; JR reports grants from Novo Nordisk, during the conduct of the study; JW reports grants, personal fees and consultancy fees to his institution from AstraZeneca and Novo Nordisk, personal fees and consultancy fees paid to institution from Boehringer Ingelheim, Napp, consultancy fees paid to institution from Astellas, Janssen, Mundipharma, Lilly, Sanofi, Saniona, Rhythm Pharmaceuticals and Wilmington Healthcare, outside the submitted work.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
Health Effects of Overweight and Obesity
Obesity and Excess Weight Increase Risk of Severe Illness; Racial and Ethnic Disparities Persist
Food Assistance and Food Systems Resources
People who have overweight or obesity*, compared to those with healthy weight, are at increased risk for many serious diseases and health conditions. These include: 1,2,3

- All-causes of death (mortality).
- High blood pressure (hypertension).
- High LDL cholesterol, low HDL cholesterol, or high levels of triglycerides (dyslipidemia).
- Type 2 diabetes.
- Coronary heart disease.
- Gallbladder disease.
- Osteoarthritis (a breakdown of cartilage and bone within a joint).
- Sleep apnea and breathing problems.
- Many types of cancer .
- Low quality of life.
- Mental illness such as clinical depression, anxiety, and other mental disorders 4,5.
- Body pain and difficulty with physical functioning 6.
*Overweight is defined as a body mass index (BMI) of 25 or higher. Obesity is defined as a BMI of 30 or higher. See the BMI calculator for people 20 years and older and the BMI calculator for people ages 2 through 19 .
Overweight and Obesity Data, strategies, and initiatives—CDC.
Weight Loss for Good Being overweight brings added risks for people with diabetes—American Diabetes Association.
Clinical Guidelines on the Identification, Evaluation, And Treatment of Overweight And Obesity in Adults [PDF-1.28MB] Health problems associated with overweight and obesity—National Heart, Lung and Blood Institute.
Health Risks of Overweight and Obesity Causes, risk factors, screening, prevention and more—National Heart, Lung and Blood Institute.
Adult Obesity Maps Self-reported US adult obesity prevalence by race, ethnicity, and location.
1 NHLBI. 2013. Managing Overweight and Obesity in Adults: Systematic Evidence Review from the Obesity Expert Panel. [PDF-5.89MB]
2 Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. [PDF-1.25MB]
3 Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5•24 million UK adults. Lancet . 2014 Aug 30;384(9945):755-65.
4 Kasen, Stephanie, et al. “Obesity and psychopathology in women: a three decade prospective study.” International Journal of Obesity 32.3 (2008): 558-566.
5 Luppino, Floriana S., et al. “Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. “ Archives of General Psychiatry 67.3 (2010): 220-229.
6 Roberts, Robert E., et al. “Prospective association between obesity and depression: evidence from the Alameda County Study.” International Journal of Obesity 27.4 (2003): 514-521.
To receive email updates about this topic, enter your email address.

- Physical Activity
- Overweight & Obesity
- Healthy Weight, Nutrition, and Physical Activity
- Breastfeeding
- Micronutrient Malnutrition
- State and Local Programs
- Prevent Type 2 Diabetes
- Prevent Heart Disease
- Healthy Schools – Promoting Healthy Behaviors
- Obesity Among People with Disabilities
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.

It Introduced Ozempic to the World. Now It Must Remake Itself.
Novo Nordisk’s factories work nonstop turning out Ozempic and Wegovy, its blockbuster weight-loss drugs, but the Danish company has far bigger ambitions.
Novo Nordisk is not just trying to make more Ozempic and Wegovy. It wants to prevent obesity. Credit...
Supported by
- Share full article
By Eshe Nelson
Photographs and Video by Charlotte de la Fuente
Eshe Nelson spoke to Novo Nordisk executives at the company’s headquarters in Bagsvaerd, Denmark, and visited its production sites.
- April 20, 2024
Lars Fruergaard Jorgensen has a problem: Too many people want what he’s selling.
Mr. Jorgensen is the chief executive of Novo Nordisk, the Danish drugmaker. Even if the company isn’t quite a household name, the TV jingle for its best-selling drug — “Oh-oh-ohhh, Ozempic!” — might ring in your ears. Across the United States, Novo Nordisk’s diabetes and weight-loss drugs, Ozempic and Wegovy , have soared to celebrity status and helped make the company Europe’s most valuable public firm. It can’t make enough of the drugs.
Mr. Jorgensen’s problem is one many top executives wouldn’t mind, but the success caught him off guard. Last year, when the company was celebrating its centenary, Novo Nordisk’s revenue jumped by a third, to 232 billion Danish kroner, or $33 billion.
“Nobody had forecast this growth — no analyst, nobody in the company,” Mr. Jorgensen said in a recent interview at the company’s headquarters in a suburb of Copenhagen. “Nobody forecast a 100-year-old company would grow more than 30 percent,” he said, seemingly torn between pride and amazement.
For most of its 100 years Novo Nordisk has been focused on the steady business of treating diabetes, one of the world’s most prevalent chronic diseases. Even today, it produces half the world’s insulin. But the development of Ozempic and Wegovy has led to a bigger and bolder ambition to “defeat serious chronic diseases.” That includes treating, and even preventing, obesity, which is linked to other health issues like heart and kidney diseases.
By pursuing a much larger target than diabetes, the company expects to unlock the door to a multibillion-dollar market with nearly a billion potential patients. In the United States alone, more than 40 percent of adults are obese.
And so the Danish drugmaker is undergoing vast changes — it’s getting bigger, more international and closer to the heat of the spotlight. Mr. Jorgensen is trying to ramp up production to meet the huge demand for its weight-loss drugs, stay ahead of competition from Eli Lilly and others and ensure the company’s future so it can meet its lofty goal.
But in all the tumult, there is something executives are trying to hold on to: the company’s longstanding values, codified in the “Novo Nordisk Way.”
Those principles, which include having a “patient-centered business approach,” have helped earn the company a good reputation at home, where it’s considered a place where people are proud to work. But these guideposts are facing pressure as tens of thousands of new employees are hired, lawmakers denounce the drugmaker for its high prices and counterfeit versions of its products make people sick.
The drugmaker’s head office is a homage to its roots: a modern six-story white circular building inspired by the molecular structure of insulin. A staircase spirals around an open atrium. On the top floor, Mr. Jorgensen and the executive team share an open-plan office space.
“Many of us have been here forever,” Mr. Jorgensen, 57, said as a snowstorm gathered strength outside.
He’s worked at Novo Nordisk for more than three decades, and became chief executive in 2017, a turbulent period when the insulin market was under strain: “Three profit warnings in one year, and the share price had tanked by 40 percent,” he recalled.
About a year later, Ozempic hit the market.
Now Novo Nordisk consistently beats investor expectations. Last summer, it eclipsed the French luxury group LVMH Moët Hennessy Louis Vuitton to become Europe’s most valuable company. Its market value exceeds $555 billion.
For those on the sixth floor, who rose through the ranks of a company that concentrated on insulin, the changes are coming quickly.
“Now it’s new patients; a new product presentation; sometimes new molecules,” Mr. Jorgensen said. “It’s a completely different, say, management system and supply chain that’s required.”

The heart of the growth is semaglutide , Novo Nordisk’s synthetic version of a hormone known as glucagon-like peptide 1, or GLP-1, which helps the body regulate blood sugar levels. The patent developed by the company also proved remarkably effective for weight loss. It causes people to feel fuller when they eat and reduces cravings. Physicians say it could revolutionize the way we think about obesity and what we eat; food executives fear the same thing.
Semaglutide revived the fortunes of Novo Nordisk. A couple of decades ago, the company was falling behind international peers, with failed insulin medical trials and too little innovation. And then insulin started drying up as a source of profits, as U.S. lawmakers pushed price caps and drugmakers were forced to pay larger rebates.
Ozempic, the brand name for semaglutide, a weekly injection for Type 2 diabetes patients, has been around for more than six years. But in the last couple of years, there was an explosion in popularity, helped along by heavy advertising, social media videos and intrigue over celebrity use. Elon Musk said he used it , and at the Oscars last year Jimmy Kimmel made a gag about it. TikTok videos tagged Ozempic have more than one billion views, with people documenting their weight loss.

As Ozempic began to take off, Novo Nordisk pushed ahead with Wegovy, which is semaglutide marketed specifically for weight loss. By the time it was approved by the Food and Drug Administration in mid-2021, the Danish company knew it had “something special,” said Camilla Sylvest, the executive vice president for commercial strategy and corporate affairs.
Novo Nordisk leads the pack in obesity treatment, but it now has strong competition from Eli Lilly, which sells a similar drug under the brand names Mounjaro, for diabetes, and Zepbound, for weight loss. Other pharmaceutical companies are clambering to catch up.
By far, most people using Ozempic (two thirds of its sales last year) and Wegovy (nearly all of its sales) are in the United States. That’s partly because drugs tend to be introduced first in the United States.
That means the Danes essentially have Americans to thank for their economic growth. The expansion of the pharmaceutical industry, mostly due to Novo Nordisk, was responsible for all of Denmark’s economic growth last year .
High Prices, Loud Criticism
The cost of these drugs, though, has made Novo Nordisk a target.
“There is no rational reason, other than greed, for Novo Nordisk to charge Americans nearly $1,000 a month for Ozempic,” Senator Bernie Sanders, independent of Vermont, said last month. A frequent critic of high drug prices, he said Canadians paid $155 a month and Germans just $59.
Ozempic could be a “game changer” fighting diabetes and obesity, Mr. Sanders added, but “this outrageously high price has the potential to bankrupt Medicare, the American people and our entire health care system.”
While the U.S. list price for Ozempic is a little under $1,000 a month and about $1,350 for Wegovy, Novo Nordisk says most American patients pay $25 or less for Wegovy. Much of the rest of the cost is shouldered by insurance plans, and some have been overwhelmed. This month, facing ballooning costs, North Carolina quit providing insurance coverage for obesity drugs for state employees. Even Denmark’s national health service won’t subsidize Wegovy, arguing that it isn’t cost effective.
Mr. Jorgensen argues that high rates of obesity lead to enormous medical costs, and that drugs to end obesity ultimately save money. “Health care systems are challenged, with aging populations,” he said. “They’re going to break unless we do something about obesity.”

The Novo Nordisk Way
Although the company’s production facilities operate 24 hours a day, 365 days a year, the limited supply of Ozempic and Wegovy is expected to last for several more years, worrying diabetics while counterfeits are leaking into the market.
Production capacity is a recurring headache. Novo Nordisk has more than 64,000 employees, and traffic jams outside its buildings are common. At the headquarters in Bagsvaerd, arrivals after 9 a.m. might struggle to find a desk.
So Novo Nordisk is in the middle of remaking itself. Cranes and construction workers have descended on its sites as it spends more than $6 billion this year to expand manufacturing, nearly four times the amount it spent just two years ago. The company is buying more production sites and vacuuming up office space in Denmark.
More than 10,000 people were hired last year globally, and the company is becoming more international — specifically American — as it expands research offices in Cambridge, Mass., and buys smaller biotech companies.
Mr. Jorgensen is also trying to transform the mind-set within the company. A couple of years ago, he gathered executives on a retreat for training called NNX, for Novo Nordisk Unknown. The essential question, he said: “What are your own self-limiting beliefs that could trigger you, block you, in actually daring to lead in a different environment?”
Since then, more than 400 managers have been through this program, intended to help them keep up with the company’s sudden growth.
Until drug supplies can better match demand, the company says, it needs to make difficult choices about how to determine who gets what’s available.
Ms. Sylvest says here she is guided by the Novo Nordisk Way, introduced in the late 1990s. It includes 10 principles, like “we are curious and innovate for the benefit of patients and society at large” and “we build and maintain good relations with our stakeholders.”
“One way or the other,” she said, “it always helps us to have these essentials about what’s the right thing to do.”
Novo Nordisk, she added, doesn’t want to just sell where prices are highest — the United States — but expand access internationally for low-income people or those with insufficient insurance, while also keeping existing patients at the top of the list.
Hundreds of Millions of Potential Patients
Until recently, obesity drugs had a dire history, including when Fen-Phen had to be pulled off shelves in the late 1990s for causing serious heart problems.
Obesity was “a therapeutic graveyard,” said Emily Field, a pharmaceuticals analyst at Barclays in London. The drugs either worked well and had bad side effects or led to only middling weight loss, she said.
But the science has changed rapidly, along with public opinion on obesity, which is increasingly understood to be a disease that can be medically treated, rather than a failure of willpower and poor diet.
Novo Nordisk is responsible for some of this changing outlook. Last summer, a five-year study it financed showed that its drugs could reduce the risk of heart attacks, stroke and cardiovascular disease. This is “what really got Novo Nordisk on the radar,” Ms. Field said.
That makes hundreds of millions of people potential patients. The market for obesity medications could grow to $100 billion in the next decade, according to Barclays. So far, Novo Nordisk is treating about 40 million people globally with its diabetes and weight-loss treatments.
The End of Obesity?
The U.S. patents on Ozempic and Wegovy don’t expire until 2032, but already Novo Nordisk is working on new treatments. It’s in advanced development of CagriSema, a weekly injection that is expected to be more effective than Wegovy for shedding weight. Last month, its stock price spiked after early trial results for an oral tablet of another weight-loss treatment.
As the company digs deeper into obesity, which is defined as having a body mass index above 30 , the next question is whether the Danish drugmaker can prevent obesity. Can it predict who is at risk, based on genetics and the data, and treat them first?
Last year, Novo Nordisk established the Transformational Prevention Unit, an internal team looking for ways to predict and prevent obesity.
Not everyone is buying the hype. For more than four years, Jefferies has had a negative “underperform” rating on Novo Nordisk stock. Peter Welford, an analyst at the bank in London, thinks obesity drugs will become common and interchangeable, suffering the same fate as insulin, with higher volumes and pressure on net prices.
“Ultimately, we think Novo Nordisk needs to diversify,” Mr. Welford said. But the bank’s bet that Novo Nordisk’s share price is too high hasn’t worked out so far.
“Clearly we’ve been wrong,” he said.
Eshe Nelson is a reporter based in London, covering economics and business news for The New York Times. More about Eshe Nelson
A Close Look at Weight-Loss Drugs
Misbranded Ozempic: A woman in New York who was using TikTok to sell unauthorized weight-loss drugs, including products labeled Ozempic, is facing charges of smuggling and receiving and distributing misbranded drugs, federal prosecutors said.
Supplement Stores: GNC and the Vitamin Shoppe are redesigning displays and taking other steps to appeal to people who are taking or are interested in drugs like Ozempic and Wegovy.
Senate Investigation: A Senate committee is investigating the prices that Novo Nordisk charges for Ozempic and Wegovy, which are highly effective at treating diabetes and obesity but carry steep price tags.
A Company Remakes Itself: Novo Nordisk’s factories work nonstop turning out Ozempic and Wegovy , but the Danish company has far bigger ambitions.
Transforming a Small Danish Town: In Kalundborg, population under 17,000, Novo Nordisk is making huge investments to increase production of Ozempic and Wegovy.
Ozempic’s Inescapable Jingle: The diabetes drug has become a phenomenon, and “Oh, oh, oh, Ozempic!” — a takeoff of the Pilot song “Magic” — has played a big part in its story.
Advertisement
- Search Menu
- Advance Articles
- Clinical Case Studies
- Journal Club
- Clinical Chemistry Podcasts
- Clinical Trainee Council
- Special Issues
- Clinical Chemistry Guide to Scientific Writing
- Clinical Chemistry Guide to Manuscript Review
- Author Guidelines
- Submission Site
- Self-Archiving Policy
- Call for Papers
- Why Publish?
- About Clinical Chemistry
- Editorial Board
- Advertising & Corporate Services
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Conclusions, 3 nonstandard abbreviations.
- < Previous
Obesity and Cancer: Evidence, Impact, and Future Directions
- Article contents
- Figures & tables
- Supplementary Data
Graham A Colditz, Lindsay L Peterson, Obesity and Cancer: Evidence, Impact, and Future Directions, Clinical Chemistry , Volume 64, Issue 1, 1 January 2018, Pages 154–162, https://doi.org/10.1373/clinchem.2017.277376
- Permissions Icon Permissions
Mounting evidence, particularly from prospective epidemiologic studies but with additional support from animal models and mechanistic studies, supported conclusions in 2016 by the International Agency for Research on Cancer (IARC) in their review of the preventive effects of weight control on cancer risk.
The workgroup concluded that obesity is causally related to cancer at 13 anatomic sites (esophagus: adenocarcinoma; gastric cardia; colon and rectum; liver; gallbladder; pancreas; breast: postmenopausal; uterine endometrial; ovary; kidney: renal cell; meningioma; thyroid; and multiple myeloma). Further, avoiding weight gain and excess body fat will prevent cancer. Evidence on weight loss and reduction in risk of cancer is more limited. Ongoing clinical trials address the benefits of weight loss interventions after diagnosis.
Here, we review the evidence from the 2016 IARC that obesity is causally related to cancer at 13 anatomic sites and identify areas for future research, including the consequences of childhood adiposity, the relation between velocity of weight gain and cancer risk, and improved methods for analysis of life-course adiposity and cancer risk. Refining understanding of mechanisms may further inform prevention strategies.
The increasing burden of cancer due to obesity has been the focus of much epidemiologic research over the past 40 years. Here, we summarize the findings from the 2016 International Agency for Research on Cancer (IARC) 3 and review the current and needed research addressing the role of adiposity/obesity in cancer incidence and address the potential for weight loss to reduce risk. Excess weight or adiposity is a common risk factor for cancer risk incidence, prognosis, and survival, which provides a unique opportunity to address a modifiable risk factor through primary and secondary interventions. Evidence reviewed by the IARC ( 1 , 2 ) and the World Cancer Research Fund (WCRF) ( 3 , 4 ) supports the conclusion that being overweight [body mass index (BMI) 25–29.9 kg/m 2 ] or obese (30+ kg/m 2 ) is a cause of several cancers, including breast, endometrium, esophagus (adenocarcinoma), renal, and colon and rectum ( 1 ). The normal range for BMI defined by the WHO is 18.5–24.9 kg/m 2 . This list of cancers caused by obesity was updated and expanded through the 2016 IARC update on body fatness and cancer. The findings of that committee were confirmed by an umbrella review of 194 metaanalyses of obesity and cancer incidence or cancer mortality ( 5 ).
The IARC committee reviewed human studies, animal studies, and mechanistic models and concluded that the evidence supported causal associations between body fatness and cancer at 13 anatomic sites ( 1 ). Hence, the evidence is sufficient to conclude that avoiding excess body fatness lowers risk of these cancers. For other cancer sites, the committee could not rule out bias and confounding as contributing to the positive associations observed ( 1 ). Additionally, excess weight is also a risk factor for cancer mortality overall ( 6 ). This review summarizes the current evidence on obesity and cancer, building on the comprehensive review of studies undertaken by the IARC committee that addressed studies in humans, animal models of obesity and cancer, and mechanisms for these associations.
Excess body weight is associated with the development of at least 13 different types of cancers, including breast, colon and rectum, endometrial, esophageal (adenocarcinoma), gallbladder, gastric, kidney (renal cell), liver, multiple myeloma, ovary, pancreas, and thyroid ( 1 , 7 ). Here, we summarize the evidence based on the level of evidence and separately the magnitude of the relative risk as previously used in summaries of evidence ( 8 ) ( Table 1 ). Although the specific mechanisms through which obesity increases cancer risk are not entirely clear ( 9 ), multiple potential key pathways and mechanisms have been identified. The impact of body weight on sex hormone levels is thought to play a primary role for cancers such as breast and ovary.
Evidence linking overweight, obesity, and cancer by level of evidence and magnitude of relative risk increase for obesity compared with normal-range body mass index, 2017, as summarized by the IARC work group ( 1 ).
Sufficient evidence indicates that the IARC Handbook Working Group considers that a preventive relationship has been established between the intervention (in this case, the absence of excess body fatness) and the risk of cancer in humans—that is, a preventive association has been observed in studies in which chance, bias, and confounding could be ruled out with confidence. Limited evidence indicates that a reduced risk of cancer is associated with the intervention for which a preventive effect is considered credible by the working group, but chance, bias, or confounding could not be ruled out with confidence. Additional information on the criteria for classification of the evidence is available at http://handbooks.iarc.fr .
Importantly, adult weight is not the sole driver of the obesity and cancer link. Overweight in youth and young adulthood has also been observed to increase risk of many cancers linked to adult weight ( 10 , 11 ). With a global trend towards higher rates of childhood obesity and young adult obesity ( 12 ), the reported links with adiposity from earlier birth cohorts that experienced later adult onset of obesity may portend an even greater burden from obesity and cancer as adults live more of their lives overweight or obese. This observation stresses the importance of reversing the current trend of rising obesity rates in children and young adults.
OBESITY AND CANCER RISK
To summarize current evidence on etiology, we placed greatest emphasis on prospective cohort studies reported as pooled analysis of individual participant data. This approach reduced variation between studies in analytic approaches ( 13 ). Further, for rarer cancers such thyroid, meningioma, and multiple myeloma, it allowed all cohorts to contribute endpoints regardless of whether they have published cohort-specific results or not, hence reducing publication bias that can distort metaanalyses limited to results already in the literature ( 14 ). The Cochrane review group places these individual participant data pooled analyses as the highest level of evidence or “gold standard of systematic reviews” ( 15 ). Accordingly, we report results from these pooled analyses when they are available.
BREAST CANCER
Breast cancer is the most common cancer in US women and is the leading killer of women in midlife. There is ample and consistent evidence that excess weight and weight gain increases the risk of postmenopausal breast cancer (male breast cancer, however, is classified as limited evidence ( 1 )). Renehan et al. ( 7 ), in a metaanalysis of 221 datasets from prospective cohort studies, found a relative risk of postmenopausal breast cancer of 1.12 (95% CI = 1.08–1.16) for every 5 kg/m 2 increase in BMI. The Nurse's Health Study (NHS) analysis, which followed women for up to 46 years, reported that women who had gained 25 kg since age 18 had a relative risk of postmenopausal breast cancer of 1.45 (95% CI = 1.27–1.66), which increased to 1.98 (95% CI = 1.55–2.53) in those who had never taken postmenopausal hormone therapy (HT) ( 9 ). Importantly, in the NHS, weight gain during premenopausal years did not increase risk of premenopausal breast cancer but it had a direct effect on risk of postmenopausal disease ( 16 ). Adipose tissue is the primary source of estrogen among postmenopausal women who do not use HT. Therefore, it is not surprising that the weight-related increases in risk are often higher in women who do not use HT.
Although weight gain across the years increases risk of postmenopausal breast cancer, in premenopausal women, excess weight has consistently been linked to a lower risk of the disease ( 7 ). However, growing evidence suggests that short-term weight gain could increase breast cancer risk in the premenopausal years. A study by Rosner and colleagues ( 17 ) that followed 77000 women for 26 years found that a weight gain of 15 or more pounds (≥6.8 kg) over a 4-year period had increased risk of premenopausal breast cancer of nearly 40% [relative risk (RR) 1.38; 95% CI = 1.13–1.69]. This risk was higher in hormone receptor negative (ER−/PR−) disease (RR 2.06; 95% CI = 1.21–3.51), a type of disease that is more common in premenopausal women. Similar results were reported from the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort ( 18 ). The recent NHS analysis also showed that weight loss from age 18 through adult years is related to significantly lower risk of breast cancer. For women who lost 5 kg or more after age 18, the overall risk of breast cancer was 0.74 (0.61–0.89) compared with women who lost <5 kg. This significant reduction in risk was observed for premenopausal breast cancer and postmenopausal breast cancer ( 16 ).
Interestingly, adiposity in childhood and adolescence is related to lower risk of premenopausal breast cancer ( 19 ). Refining the analysis with a validated measure of childhood adiposity shows that measures from age 10 are inversely related to risk of both pre- and postmenopausal breast cancer ( 20 ) and that these associations persist after controlling for adult weight gain ( 16 ). The reasons for this inverse relationship are unclear and further research in this area is needed. This should not be interpreted as a recommendation regarding childhood adiposity but rather the potential importance of pathways mediating from childhood exposures to lifelong breast cancer risk.
COLORECTAL CANCER
Colon cancer is the third most common cancer among men and women in the US. It is the second most common cause of cancer death in men and third in women. Consistent evidence points to a dose–response relationship between BMI, as well as waist circumference, and increased risk of the disease, with risk generally higher in men than in women. A metaanalysis of 30 prospective studies by Larsson and Wolk ( 21 ) reported that for every 5 kg/m 2 increase in BMI, the risk of colorectal cancer increased by 30% (RR = 1.30; 95% CI = 1.25–1.35) in men and 12% (RR = 1.12; 95% CI = 1.07–1.18) in women. The findings were similar across genders for every 10-cm increase in waist circumference. A pooled analysis by Matsuo et al. ( 22 ) that combined data from 8 large prospective cohort studies also observed a significant trend in colorectal cancer risk with increasing BMI (males, P for trend = <0.001; females, P for trend = 0.032). For each 1-U increase in BMI, the adjusted hazard ratio was 1.03 (95% CI = 1.02–1.04) in men and 1.02 (95% CI = 1.00–1.03) in women.
ENDOMETRIAL CANCER
Endometrial cancer is the most common reproductive cancer in the US, with approximately 60000 women diagnosed with the disease each year. Excess weight is a key risk factor for endometrial cancer. A metaanalysis of 30 prospective studies ( 23 ) found an overall relative risk of 1.54 (95% CI = 1.47–1.61) for every 5 kg/m 2 increase in BMI and a relative risk of 1.27 (95% CI = 1.17–1.39) for every 10-cm increase in waist circumference. In looking at specific types of endometrial cancer, weight was a particularly strong risk factor for type 1 endometrial cancer, which is the most common type and consists largely of endometrial adenocarcinomas. From a pooled analysis of 10 cohort studies and 14 case–control studies, Setiawan and colleagues ( 24 ) reported a relative risk for type 1 endometrial cancer with overweight of 1.45 (95% CI = 1.37–1.53); with obesity class 1 of 2.52 (95% CI = 2.35–2.69); with obesity class 2 of 4.45 (95% CI = 4.05–4.89); and with obesity class 3 of 7.14 (95% CI = 6.33–8.06) compared with normal-weight women.
ESOPHAGEAL CANCER
Evidence from >20 prospective studies and case–control studies indicates a statistically significant positive dose–response relationship between BMI and risk in esophageal cancer ( 7 ). Compared with BMI <25, the relative risk was about 4.6 for those with BMI >40 based on pooled data from 12 studies ( 25 ). The increase in relative risk per 5 kg/m 2 was 1.48 (1.35–1.62) based on dose–response metaanalysis including 9 prospective cohort studies ( 26 ). This association was observed in virtually all studies and in both genders and across regions. Abdominal adiposity has been evaluated in several prospective studies and positive associations with esophageal adenocarcinoma have been observed across several measures (including waist circumference) ( 27 , 28 ). A metaanalysis of 5 prospective cohorts showed a significant increase in risk with increasing waist circumference ( 29 ) consistent with abdominal pressure and reflux ( 30 ) as mechanisms for this association.
KIDNEY CANCER
Evidence from >20 prospective studies and >20 case–control studies indicates a positive dose–response relationship between BMI and risk. This association was observed in virtually all studies, and it was consistent across gender and regions of the world. There is a statistically significant relative risk of about 1.3 for overweight and about 1.8 for obese compared with normal weight as estimated in a metaanalysis of 21 cohort studies ( 31 ).
GASTRIC CARDIA CANCER
Helicobacter pylori is a known cause of gastric cardiac cancer. Studies have shown that H. pylori is more prevalent in obese individuals and that eradication with antibiotics is associated with weight gain ( 32 ) Evidence from >20 prospective studies and case–control studies also indicates a statistically significant positive dose–response relationship between BMI and risk. Compared with normal weight, the relative risk for overweight was found to be about 1.2 and for obesity about 1.8, estimated from metaanalysis of 7 studies ( 33 ). This association was observed in both genders and across regions. In contrast, no association was observed for gastric noncardia cancer.
PANCREATIC CANCER
Evidence from >20 prospective studies and case–control studies indicates a statistically significant positive dose–response relationship between BMI and risk. This association was observed in a large majority of studies and in both genders. Compared with normal weight, the relative risk for overweight was about 1.2 and for obesity about 1.5 estimated from pooled analysis of 14 cohorts ( 34 ). Pancreatic cancer incidence is increasing and is expected to increase by >50% by 2030 ( 35 ). Although certainly not the sole cause, obesity may be one factor contributing to this trend. Importantly, increased risk was also observed for early adult adiposity, almost as strong as mid-life adiposity ( 10 ). This sets the stage for increasing population burden of pancreatic cancer due to obesity as childhood and adolescent prevalence of obesity increases world-wide ( 12 ).
OVARIAN CANCER
Evidence from >10 prospective studies and >20 case–control studies indicates a positive dose–response relationship between BMI and the risk of epithelial ovarian cancer. Among nonusers of HT, overweight women had a relative risk of about 1.1, whereas that for obese women was about 1.2 compared with normal-weight women based on a pooled analysis of 47 studies ( 36 ). There was no association among users of HT.
PROSTATE CANCER
Evidence from >50 prospective studies and >35 new case–control studies suggests a positive association between BMI and increasing risk of prostate cancer mortality, but there was no consistent association between BMI, weight at any age, or weight change and total prostate cancer incidence, aggressive/advanced or nonaggressive/nonadvanced prostate cancer incidence. Some studies of obesity and prostate cancer mortality supported a direct association but bias and confounding cannot be ruled out. This included concern that death certificate classification alone may not be sufficiently accurate. Therefore, fatal prostate cancer is classified as limited evidence ( 1 ). In contrast, however, the WCRF review concluded that consistent evidence supports an association for obesity and aggressive/advanced prostate cancer ( 37 ).
MULTIPLE MYELOMA (MM)
There is substantial evidence from at least 20 prospective studies and several case–control studies and meta or pooled analyses showing positive associations of BMI at baseline and MM mortality. The association appears to be dose-related and begins in the overweight category. From the pooled analysis of 20 cohorts ( 11 ) and compared with men and women with low–normal weight, the relative risk of multiple myeloma mortality was found to be about 1.2 for overweight, about 1.2 for obese class 1, and about 1.5 for obese class 2 or higher. The study of obesity and the progression of monoclonal gammopathy of undetermined significance to MM supported this association being present in African Americans and Caucasians ( 38 ).
Findings from prospective, case–control, and meta or pooled analyses studies suggest a positive association with excess body weight and risk of diffuse large B-cell lymphoma (DLBCL), the most common type of non-Hodgkin lymphoma (NHL), but the results are not fully consistent. The relative risk for overweight was found to be about 1.1, whereas that for obese was about 1.3 compared with normal weight, as estimated by a metaanalysis of 10 cohort studies ( 39 ). Therefore, DLBCL is classified as limited evidence ( 1 ). For Hodgkin lymphoma, cohort studies generally found nonsignificant positive associations for obese compared with normal BMI: the relative risk was approximately 1.4 in a metaanalysis of 5 prospective studies ( 40 ). Findings from case–control studies were largely null.
For total leukemia and myeloid leukemias and for total NHL and B-cell lymphomas as a group, findings for an association between BMI and risk from individual studies and metaanalyses are inconsistent. The inconsistency within the broader categories, such as total leukemia, may be due to heterogeneity among subtypes. There were too few studies of T-cell lymphoma to draw conclusions.
GALLBLADDER CANCER
There is a substantial body of evidence from 10 prospective studies and a metaanalysis of 12 prospective and 8 case–control studies indicating a statistically significant positive dose–response relationship between BMI and risk. The relative risk was found to be about 1.2 for overweight and about 1.6 for obesity estimated in the metaanalysis ( 41 ). For bile duct cancers, the evidence is inconsistent, with few studies separating intrahepatic and extrahepatic bile duct cancers.
THYROID CANCER
There is evidence from >20 prospective studies and 10 case–control studies indicating a positive dose–response relationship between BMI and risk. The relative risk per 5 kg/m 2 of BMI was found to be 1.17 in men and 1.04 in women, both statistically significant, as estimated in a metaanalysis of 22 prospective studies ( 42 ). Risk was significantly increased with increasing adiposity in early adulthood (RR 1.13 per 5 kg/m 2 ) and associations were observed for papillary (the most common type of thyroid cancer), follicular, and anaplastic carcinomas but not for medullary and thyroid carcinomas ( 42 ). The incidence of thyroid cancer is increasing significantly ( 43 ), and although earlier detection certainly plays a large role in this, it is possible that the rise in obesity does as well ( 44 ).
LIVER CANCER (HEPATOCELLULAR CARCINOMA)
There is evidence from over 50 prospective studies and case–control studies that BMI is positively associated with risk of either hepatocellular carcinoma (HCC) or liver cancer overall ( 1 ). This association has been reported in studies from the US, Europe, and Asia. Compared with normal weight, the relative risk for overweight was found to be about 1.2 and for obesity it was about 1.8 in a metaanalysis of 26 prospective studies of general population cohorts ( 45 ). Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease in the world, and it is strongly linked to obesity ( 46 ). NAFLD can lead to cirrhosis and ultimately hepatocellular carcinoma ( 47 ). Its increase in prevalence may be related to the increase in type 2 diabetes and obesity and ultimately the increase in liver cancer incidence ( 48 ). In patients with viral hepatitis (B and C), which are well-known risk factors for hepatocellular carcinoma, obesity may further increase the risk of liver cancer, especially with hepatitis C ( 49 ).
Seven prospective studies and 2 case–control studies showed a consistent positive association with BMI and risk. For glioma, 5 cohort studies and 2 case–control studies, all with only moderate sample sizes, reported inconsistent associations with BMI.
LUNG CANCER AND OTHER SMOKING-ASSOCIATED CANCERS
A large number of prospective and case–control studies show an inverse association between BMI and risk, but studies among nonsmokers generally show no association ( 50 ). Because tobacco smoking is strongly related to lung cancer and reduced weight, residual confounding by tobacco smoking is likely to account for the inverse associations. Thus, the evidence is classified as inadequate to support an association. Other smoking-related cancers including squamous cell carcinoma of the esophagus and urinary bladder also have this classification. Again, residual confounding by tobacco smoking likely accounts for the inverse associations.
EXCESS BODY WEIGHT IN CHILDHOOD AND EARLY ADULTHOOD AND LATER CANCER RISK
Collectively, the emerging findings from studies evaluating relationships between excess body weight in childhood and early adulthood with later cancer risk indicate positive associations with several cancer types known to be associated with excess body weight in middle and later adulthood. Overweight or obesity during childhood and early adulthood was found to be associated with an increased risk of pancreatic cancer [hazard ratio (HR) 1.67 for overweight and HR 2.58 for obese] independent of diabetes ( 10 ). Additionally, it was associated with higher risk of younger age of onset and reduced survival ( 51 ). In women who were overweight in childhood compared with normal-weight children, there was a 28% higher risk of developing colon cancer. The association was weaker in men ( 52 ). Excess body weight in both early and later adulthood is also associated with increased risk of multiple myeloma ( 53 ). The key exception is breast cancers, in which there is some evidence for an inverse association with childhood and early adulthood excess body weight ( 54 ).
IMPACT ON CANCER PROGNOSIS
An increasing number of reports are evaluating the role of obesity and cancer survival and recurrence ( 55 ) and, more specifically, the relationship between BMI at the time of cancer diagnosis and cancer-related mortality. The data are most consistent in breast cancer, in which high BMI has been associated with an increased risk of cancer-related mortality in individual reports and metaanalyses ( 56 ) and in which weight gain after diagnosis is associated with increased mortality ( 57 ). In the NHS of over 5000 women with nonmetastatic breast cancer, weight gain after diagnosis was associated with increased recurrence risk and increased risk of breast cancer-specific mortality (40% increase in recurrence with weight gain of 0.5–2.0 kg/m 2 and 53% for weight gain >2.0 kg/m 2 compared with those who maintained their weight within 0.5 kg/m 2 ; 35% increase in breast cancer-specific mortality for weight gain >0.5kg/m 2 and 64% for weight gain >2.0 kg/m 2 ) ( 58 ). Data are fewer and/or less consistent for other malignancies.
A small number of observational studies in breast cancer have evaluated weight loss and subsequent cancer risk. Findings from these studies are inconsistent, in part reflecting the problem of distinguishing intentional and unintentional weight loss. Two randomized studies evaluated the impact of dietary modification in breast cancer survivors. In the Women's Interventional Nutritional Study, 2400 women with early-stage breast cancer were randomized to low-fat diet intervention or control. The intervention group had an average 6-lb weight loss that was maintained for 5 years, which resulted in a 20% reduction in breast cancer recurrence. In contrast, in the Women's Healthy Eating and Living Study, 3088 women with early-stage breast cancer were randomized to a diet intervention (high fruits and vegetables, low fat, high fiber) or control ( 59 ). The diet was purposely eucaloric, and therefore there was no weight loss in the intervention group. In this study, there was no difference in breast cancer outcome, highlighting the potential need for lifestyle modification to produce weight loss to improve outcomes.
The ongoing Breast Cancer Weight Loss (BWEL) Study (NCT02750826) is recruiting over 3000 overweight or obese women within the first year of breast cancer diagnosis. This randomized phase III clinical trial is evaluating the role of weight loss in the adjuvant treatment of overweight and obese women with early breast cancer. The study has a health education arm (which serves as control) and a weight loss (intervention) arm. The primary outcome is invasive disease-free survival. The results of this should help define the impact of intentional weight loss after a breast cancer diagnosis. Secondary outcomes include changes in biomarkers and patient-reported outcomes such as physical functioning and fatigue. Biomarker outcomes are important to further define the mechanisms of weight and cancer prognosis, and patient-reported outcomes are crucial to understanding the impact of weight loss on outcomes other than disease recurrence, such as depression and body image.
IMPACT ON PATIENT-REPORTED OUTCOMES
The importance of patient-reported outcomes should be emphasized, and it has been studied extensively. In a metaanalysis of 14 randomized controlled trials including over 700 breast cancer survivors, exercise (a crucial component of weight loss) has been shown to improve not only measures of fitness, such as muscle strength and body composition, but also fatigue, anxiety, depression, self-esteem, and other measures of quality of life in breast cancer survivors ( 60 ). The health, eating, activity, and lifestyle study of over 1000 breast cancer survivors examined the effects of weight, physical activity, and diet on breast cancer prognosis and women completed baseline and follow-up (30 months) assessments. Compared with normal-weight participants, obese participants reported poorer physical functioning. Additionally, compared with those with a weight change of <5%, those with weight gain >5% reported worse physical functioning and vitality ( 61 ). Women who gained weight after breast cancer were also more likely to report fatigue ( 62 ), one of the most common and burdensome long-term effects of cancer.
It is important to note that contrary to common perceptions, weight gain after a breast cancer diagnosis is common ( 63 , 64 ). One study of 535 newly diagnosed breast cancer patients found that 84% gained weight within 12 months of diagnosis ( 63 ). This highlights the need for weight management in this population.
SUSTAINED WEIGHT LOSS AND CANCER RISK: ILLUSTRATIVE EXAMPLES
There is emerging evidence from a large number of morbidly obese patients undergoing bariatric surgery, and with sufficient follow-up, demonstrating that sustained substantial weight loss is associated with reduced subsequent cancer risk ( 65 ), especially for endometrial cancer ( 66 ). However, there are potential methodological problems in the study designs due to confounding by indication and failure to adequately capture the extent of weight reduction after bariatric surgery. A recent study evaluated the influence of intentional weight loss (assessed via self-report) on endometrial cancer in the Women's Health Initiative. After 11 years of follow-up, there were 566 incident endometrial cancer occurrences. Compared with women who had stable weight (defined as ± 5%), women with weight loss had a 29% lower risk of endometrial cancer (HR 0.71, 95% CI 0.54–0.95) ( 67 ).
There has been significant interest and progress in defining the complex mechanisms by which weight affects cancer risk, prognosis, and survival. The most commonly cited mechanisms include systemic alterations in sex hormones, the insulin–glucose pathway, and the direct and indirect effects of changes in other metabolic, inflammatory, and immune-mediated markers ( 68 ). Such alterations can impact known carcinogenic pathways such as mTOR and PI3K and have procarcinogenic effects on the tumor microenvironment. Newer theories are exploring the importance of local effects of fat tissue that may promote tumor development ( 9 ).
The mechanistic link between weight and postmenopausal hormone receptor positive breast cancer has been evaluated and it illustrates how such alterations may affect cancer risk. This link is complex but associated with increased levels of estradiol, testosterone, and DHEAs and reduced levels of sex hormone-binding globulin (SHBG), which has been shown to increase the risk of breast cancer ( 69 ). In addition, obesity leads to insulin resistance, which is associated with increased levels of insulin/insulin-like growth factor that increases the risk of hormone receptor positive breast cancer ( 70 , 71 ). Obesity also alters levels of adipokines (increased leptin, decreased adiponectin) and proinflammatory mediators (TNFα, IL-1, IL-6, PGE 2 ) ( 72 ), which induce breast cancer-associated pathways and aromatase expression ( 73 ), providing an explanation for the increased risk of hormone receptor positive breast cancer and poorer outcomes in obese breast cancer survivors.
GAPS IN KNOWLEDGE AND AREAS FOR FURTHER RESEARCH
Although much is known about obesity and cancer risk and prognosis, much remains unknown and the focus of future research endeavors should strive to inform personalized lifestyle recommendations and ultimately lead to policy reform to support programs to deliver such recommendations.
With the bulk of the evidence showing the link between weight gain and/or excess weight and cancer risk and prognosis, the idea that weight loss reduces cancer incidence and improves cancer prognosis is a reasonable conclusion. In the era of high-level evidence driving clinical recommendations, however, prospective intervention trials with long-term sustained weight loss to confirm and define the magnitude of benefit are needed. Such studies should include not only disease-related endpoints but also quality-of-life assessments and other patient-reported outcomes, assessment of various tools including behavioral medicine techniques to promote compliance and sustainability of lifestyle changes, and collection of biospecimens for correlative studies and identification of novel prognostic biomarkers. The cost-effectiveness of these interventions incorporating the benefits on other chronic diseases and health states must be considered as well to help inform their broader dissemination. Such studies and outcomes are needed to encourage payers to provide coverage for a broad range of weight management services such as exercise and nutrition programs for those at high risk for cancer ( 74 ). The same is true for studies after a cancer diagnosis, such as BWEL, to define the magnitude of benefit in terms of reducing risk of cancer recurrences for those diagnosed with the disease.
Whether lifestyle modification trials should be conducted solely in the overweight and/or obese populations and at what BMI levels the magnitude of benefit may be greatest is unknown. It is also likely that obesity affects the risk of different histologic subtypes of specific cancers to different degrees. Further study into the relative risk reductions of cancer risk and prognosis with weight management by specific baseline BMI and disease histology and other disease characteristics (grade, hormone receptor status, genomic characteristics) could eventually lead to personalized lifestyle recommendations for individuals based on their own individual cancer risk and/or cancer history, a needed but currently lacking component in today's era of personalized medicine.
It is also likely that the velocity of weight gain and duration of excess weight and obesity at different points in one's life-course affect cancer risk to varying degrees. Understanding how weight and weight change at different time points impact cancer risk and prognosis is important for maximizing risk reduction through lifestyle modifications and weight management.
Completing large randomized clinical trials with cancer incidence and cancer recurrence as the primary outcome, however, is not feasible when the goal is to test various methods of weight management and to test such methods in different periods of life and with different types of cancer. Thus, identifying biomarkers that can be validated, that show minimal intraindividual variation over time, that can be easily obtained (for example, through peripheral blood draw), and that can be followed throughout interventions to serve as surrogate endpoints for cancer risk and prognosis is crucial if we are to succeed in personalizing lifestyle modification recommendations. Utilizing such biomarkers potentially would allow smaller trials of shorter duration to be conducted that could assess a wide variety of interventions in many different risk groups and with different tumor types. In addition to metabolic, inflammatory, and immune-mediated markers in serum and plasma, analysis of the gut-microbiome and its role in obesity-related cancer risk is of significant interest and should be included in future studies.
The association of obesity and cancer risk and outcome across race and ethnic groups is not always consistent. Therefore, there is a need to conduct studies in specific races and ethnicities to investigate the possibility of disparities in the effectiveness and impact of various interventions.
Finally, although definitive phase III clinical trials are generally required to support the epidemiologic data and ultimately change practice, there is ample room in the preclinical setting for further study. Such studies may be conducted in conjunction with clinical studies with a translational approach or as independent investigations to guide clinical models. Animal models can be used to evaluate the impact of weight management at different life points and to evaluate the impact of pharmaceuticals on obesity-related markers and pathways, as well as on cancer risk and progression. Such studies are needed to further explore and define the mechanisms of weight management cancer risk and prognosis. The large-scale clinical trials that are needed to move this field forward rely on preclinical and translational studies to guide their design and implementation.
Excess weight affects cancer risk and prognosis to clinically significant but varying degrees based on cancer type. The global epidemic of obesity starting earlier in life and thus the burden of cancer going forward may lead to an underestimation of the impact of weight on cancer risk as summarized here, as the evidence of excess weight and cancer risk comes from cohorts with mid- to later-life onset of overweight and obesity. More consistent approaches to modeling weight gain over life-course and risk of cancer will help refine this understanding and identify where prevention pay-off will be maximized for cancer risk. We should strive for the creation and implementation of individual lifestyle recommendations based on cancer risk and cancer history. Despite important therapeutic advances, cancer remains a deadly disease. Even for cancers such as breast and prostate, in which most patients are cured of their disease, the absolute number of deaths from these cancers is still high. In 2012, it was estimated that 481000 cancers globally were due to overweight or obesity ( 75 ). Improving cancer prognosis is crucial, but reducing cancer incidence through weight management is paramount.
International Agency for Research on Cancer
World Cancer Research Fund
body mass index
Nurse's Health Study
hormone therapy
European Prospective Investigation into Cancer and Nutrition
multiple myeloma
diffuse large B-cell lymphoma
non-Hodgkin lymphoma
hepatocellular carcinoma
nonalcoholic fatty liver disease
Breast Cancer Weight Loss (study).
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors' Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: None declared.
Stock Ownership: None declared.
Honoraria: None declared.
Research Funding: G.A. Colditz, the Foundation for Barnes-Jewish Hospital, St Louis, MO and by NCI U54 CA 155496 and P30 CA091842.
Expert Testimony: None declared.
Patents: None declared.
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or final approval of manuscript.
Lauby-Secretan B , Scoccianti C , Loomis D , Grosse Y , Bianchini F , Straif K , et al. Body fatness and cancer–viewpoint of the IARC Working Group . N Engl J Med 2016 ; 375 : 794 – 8 .
Google Scholar
International Agency for Research on Cancer . Weight control and physical activity . Lyon : International Agency for Research on Cancer ; 2002 . 315 p.
World Cancer Research Fund . Food, nutrition, physical activity, and the prevention of cancer: a global perspective . Washington, DC : AICR ; 2007 .
World Cancer Research Fund . Continuous Update Project. Keeping the science current. Breast Cancer 2010 Report . Washington DC : American Institute for Cancer Research ; 2010 .
Kyrgiou M , Kalliala I , Markozannes G , Gunter MJ , Paraskevaidis E , Gabra H , et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature . BMJ 2017 ; 356 : j477 .
Calle EE , Rodriguez C , Walker-Thurmond K , Thun MJ . Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults . N Engl J Med 2003 ; 348 : 1625 – 38 .
Renehan AG , Tyson M , Egger M , Heller RF , Zwahlen M . Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies . Lancet 2008 ; 371 : 569 – 78 .
Colditz GA , Atwood KA , Emmons K , Monson RR , Willett WC , Trichopoulos D , et al. Harvard report on cancer prevention volume 4: Harvard Cancer Risk Index. Risk Index Working Group, Harvard Center for Cancer Prevention . Cancer Causes Control 2000 ; 11 : 477 – 88 .
Renehan AG , Zwahlen M , Egger M . Adiposity and cancer risk: new mechanistic insights from epidemiology . Nat Rev Cancer 2015 ; 15 : 484 – 98 .
Genkinger JM , Kitahara CM , Bernstein L , Berrington de Gonzalez A , Brotzman M , Elena JW , et al. Central adiposity, obesity during early adulthood, and pancreatic cancer mortality in a pooled analysis of cohort studies . Ann Oncol 2015 ; 26 : 2257 – 66 .
Teras LR , Kitahara CM , Birmann BM , Hartge PA , Wang SS , Robien K , et al. Body size and multiple myeloma mortality: a pooled analysis of 20 prospective studies . Br J Haematol 2014 ; 166 : 667 – 76 .
de Onis M , Blossner M , Borghi E . Global prevalence and trends of overweight and obesity among preschool children . Am J Clin Nutr 2010 ; 92 : 1257 – 64 .
Stewart LA , Tierney JF . To IPD or not to IPD? Advantages and disadvantages of systematic reviews using individual patient data . Eval Health Prof 2002 ; 25 : 76 – 97 .
Arnold M , Renehan AG , Colditz GA . Excess weight as a risk factor common to many cancer sites: words of caution when interpreting meta-analytic evidence . Cancer Epidemiol Biomarkers Prev 2017 ; 26 : 663 – 5 .
Stewart L , Tierney J , Clarke M , on behalf of the Cochrane Individual Patient Data Meta-analysis Methods Group . Chapter 18 Rev of individual patient data . In: Higgins JPT , Green S , editors. Cochrane Handbook for Systematic Rev of Interventions . Chichester, West Sussex, England : John Wiley and Sons, LTD ; 2008 . p. 547 – 59 .
Google Preview
Rosner B , Eliassen AH , Toriola AT , Chen WY , Hankinson SE , Willett WC , et al. Weight and weight changes in early adulthood and later breast cancer risk . Int J Cancer 2017 ; 140 : 2003 – 14 .
Rosner B , Eliassen AH , Toriola AT , Hankinson SE , Willett WC , Natarajan L , et al. Short-term weight gain and breast cancer risk by hormone receptor classification among pre- and postmenopausal women . Breast Cancer Res Treat 2015 ; 150 : 643 – 53 .
Emaus MJ , van Gils CH , Bakker MF , Bisschop CN , Monninkhof EM , Bueno-de-Mesquita HB , et al. Weight change in middle adulthood and breast cancer risk in the EPIC-PANACEA study . Int J Cancer 2014 ; 135 : 2887 – 99 .
Baer HJ , Tworoger SS , Hankinson SE , Willett WC . Body fatness at young ages and risk of breast cancer throughout life . Am J Epidemiol 2010 ; 171 : 1183 – 94 .
Ahlgren M , Melbye M , Wohlfahrt J , Sorensen TI . Growth patterns and the risk of breast cancer in women . N Engl J Med 2004 ; 351 : 1619 – 26 .
Larsson SC , Wolk A . Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies . Am J Clin Nutr 2007 ; 86 : 556 – 65 .
Matsuo K , Mizoue T , Tanaka K , Tsuji I , Sugawara Y , Sasazuki S , et al. Association between body mass index and the colorectal cancer risk in Japan: pooled analysis of population-based cohort studies in Japan . Ann Oncol 2012 ; 23 : 479 – 90 .
Aune D , Navarro Rosenblatt DA , Chan DS , Vingeliene S , Abar L , Vieira AR , et al. Anthropometric factors and endometrial cancer risk: a systematic review and dose-response meta-analysis of prospective studies . Ann Oncol 2015 ; 26 : 1635 – 48 .
Setiawan VW , Yang HP , Pike MC , McCann SE , Yu H , Xiang YB , et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol . 2013 ; 312607 – 18 .
Hoyo C , Cook MB , Kamangar F , Freedman ND , Whiteman DC , Bernstein L , et al. Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: a pooled analysis from the International BEACON Consortium . Int J Epidemiol 2012 ; 41 : 1706 – 18 .
World Cancer Research Fund International/American Institute for Cancer Research . Continuous Update Project Report: Diet, nutrition, physical activity and esophageal cancer . 2016 .
Steffen A , Huerta JM , Weiderpass E , Bueno-de-Mesquita HB , May AM , Siersema PD , et al. General and abdominal obesity and risk of esophageal and gastric adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition . Int J Cancer 2015 ; 137 : 646 – 57 .
O'Doherty MG , Freedman ND , Hollenbeck AR , Schatzkin A , Abnet CC . A prospective cohort study of obesity and risk of oesophageal and gastric adenocarcinoma in the NIH-AARP Diet and Health Study . Gut 2012 ; 61 : 1261 – 8 .
Du X , Hidayat K , Shi BM . Abdominal obesity and gastroesophageal cancer risk: systematic review and meta-analysis of prospective studies . Biosci Rep 2017 ; 37 : pii : BSR20160474 .
Jacobson BC , Somers SC , Fuchs CS , Kelly CP , Camargo CA Jr . Body-mass index and symptoms of gastroesophageal reflux in women . N Engl J Med 2006 ; 354 : 2340 – 8 .
Wang F , Xu Y . Body mass index and risk of renal cell cancer: a dose-response meta-analysis of published cohort studies . Int J Cancer 2014 ; 135 : 1673 – 86 .
Dhurandhar NV , Bailey D , Thomas D . Interaction of obesity and infections . Obes Rev 2015 ; 16 : 1017 – 29 .
Chen Y , Liu L , Wang X , Wang J , Yan Z , Cheng J , et al. Body mass index and risk of gastric cancer: a meta-analysis of a population with more than ten million from 24 prospective studies . Cancer Epidemiol Biomarkers Prev 2013 ; 22 : 1395 – 408 .
Genkinger JM , Spiegelman D , Anderson KE , Bernstein L , van den Brandt PA , Calle EE , et al. A pooled analysis of 14 cohort studies of anthropometric factors and pancreatic cancer risk . Int J Cancer 2011 ; 129 : 1708 – 17 .
Smith BD , Smith GL , Hurria A , Hortobagyi GN , Buchholz TA . Future of cancer incidence in the United States: burdens upon an aging, changing nation . J Clin Oncol 2009 ; 27 : 2758 – 65 .
Collaborative Group on Epidemiological Studies of Ovarian C . Ovarian cancer and body size: individual participant meta-analysis including 25 157 women with ovarian cancer from 47 epidemiological studies . PLoS Med . 2012 ; 9 : e1001200 .
World Cancer Research Fund International/American Institute for Cancer Research . Continuous Update Project Report: Diet, nutrition, physical activity, and prostate cancer . 2014 .
Chang SH , Luo S , Thomas TS , O'Brian KK , Colditz GA , Carlsson NP , Carson KR . Obesity and the transformation of monoclonal gammopathy of undetermined significance to multiple myeloma: a population-based cohort study . J Natl Cancer Inst 2017 ; 109 : pii: djw264 .
Castillo JJ , Ingham RR , Reagan JL , Furman M , Dalia S , Mitri J . Obesity is associated with increased relative risk of diffuse large B-cell lymphoma: a meta-analysis of observational studies . Clin Lymphoma Myeloma Leuk 2014 ; 14 : 122 – 30 .
Larsson SC , Wolk A . Body mass index and risk of non-Hodgkin's and Hodgkin's lymphoma: a meta-analysis of prospective studies . Eur J Cancer 2011 ; 47 : 2422 – 30 .
World Cancer Research Fund International/American Institute for Cancer Research . Continuous Update Project Report: Diet, nutrition, physical activity and gallbladder cancer . 2015 .
Kitahara CM , McCullough ML , Franceschi S , Rinaldi S , Wolk A , Neta G , et al. Anthropometric factors and thyroid cancer risk by histological subtype: pooled analysis of 22 prospective studies . Thyroid 2016 ; 26 : 306 – 18 .
Kilfoy BA , Zheng T , Holford TR , Han X , Ward MH , Sjodin A , et al. International patterns and trends in thyroid cancer incidence, 1973–2002 . Cancer Causes Control 2009 ; 20 : 525 – 31 .
Enewold L , Zhu K , Ron E , Marrogi AJ , Stojadinovic A , Peoples GE , et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005 . Cancer Epidemiol Biomarkers Prev 2009 ; 18 : 784 – 91 .
Chen Y , Wang X , Wang J , Yan Z , Luo J . Excess body weight and the risk of primary liver cancer: an updated meta-analysis of prospective studies . Eur J Cancer 2012 ; 48 : 2137 – 45 .
Milić S , Lulić D , Štimac D . Non-alcoholic fatty liver disease and obesity: Biochemical, metabolic and clinical presentations . World J Gastroenterol 2014 ; 20 : 9330 – 7 .
Yasui K , Hashimoto E , Komorizono Y , Koike K , Arii S , Imai Y , et al. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma . Clin Gastroenterol Hepatol 2011 ; 9 : 428 – 33 ; quiz e50 .
Ryerson AB , Eheman CR , Altekruse SF , Ward JW , Jemal A , Sherman RL , et al. Annual report to the nation on the status of cancer, 1975–2012, featuring the increasing incidence of liver cancer . Cancer 2016 ; 122 : 1312 – 37 .
Ohki T , Tateishi R , Sato T , Masuzaki R , Imamura J , Goto T , et al. Obesity is an independent risk factor for hepatocellular carcinoma development in chronic hepatitis C patients . Clin Gastroenterol Hepatol 2008 ; 6 : 459 – 64 .
Prospective Studies C , Whitlock G , Lewington S , Sherliker P , Clarke R , Emberson J , et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies . Lancet 2009 ; 373 : 1083 – 96 .
Li D , Morris JS , Liu J , Hassan MM , Day RS , Bondy ML , et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer . JAMA 2009 ; 301 : 2553 – 62 .
Zhang X , Wu K , Giovannucci EL , Ma J , Colditz GA , Fuchs CS , et al. Early life body fatness and risk of colorectal cancer in US women and men: results from two large cohort studies . Cancer Epidemiol Biomarkers Prev 2015 ; 24 : 690 – 7 .
Hofmann JN , Moore SC , Lim U , Park Y , Baris D , Hollenbeck AR , et al. Body mass index and physical activity at different ages and risk of multiple myeloma in the NIH-AARP diet and health study . Am J Epidemiol 2013 ; 177 : 776 – 86 .
Demark-Wahnefried W , Platz EA , Ligibel JA , Blair CK , Courneya KS , Meyerhardt JA , et al. The role of obesity in cancer survival and recurrence . Cancer Epidemiol Biomarkers Prev 2012 ; 21 : 1244 – 59 .
Chan DS , Vieira AR , Aune D , Bandera EV , Greenwood DC , McTiernan A , et al. Body mass index and survival in women with breast cancer: systematic literature review and meta-analysis of 82 follow-up studies . Ann Oncol 2014 ; 25 : 1901 – 14 .
Playdon MC , Bracken MB , Sanft TB , Ligibel JA , Harrigan M , Irwin ML . Weight gain after breast cancer diagnosis and all-cause mortality: systematic review and meta-analysis . J Natl Cancer Inst . 2015 ; 107 : djv275 .
Kroenke CH , Chen WY , Rosner B , Holmes MD . Weight, weight gain, and survival after breast cancer diagnosis . J Clin Oncol 2005 ; 23 : 1370 – 8 .
Pierce JP , Natarajan L , Caan BJ , Parker BA , Greenberg ER , Flatt SW , et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women's Healthy Eating and Living (WHEL) randomized trial . JAMA 2007 ; 298 : 289 – 98 .
McNeely ML , Campbell KL , Rowe BH , Klassen TP , Mackey JR , Courneya KS . Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis . CMAJ 2006 ; 175 : 34 – 41 .
Imayama I , Alfano CM , Neuhouser ML , George SM , Wilder Smith A , Baumgartner RN , et al. Weight, inflammation, cancer-related symptoms and health related quality of life among breast cancer survivors . Breast Cancer Res Treat 2013 ; 140 : 159 – 76 .
Demark-Wahnefried W , Campbell K , Hayes SC . Weight management and its role in breast cancer rehabilitation . Cancer 2012 ; 118 ( 8 Suppl ): 2277 – 87 .
Goodwin PJ , Ennis M , Pritchard KI , McCready D , Koo J , Sidlofsky S , et al. Adjuvant treatment and onset of menopause predict weight gain after breast cancer diagnosis . J Clin Oncol 1999 ; 17 : 120 – 9 .
Demark-Wahnefried W , Rimer BK , Winer EP . Weight gain in women diagnosed with breast cancer . J Am Diet Assoc . 1997 ; 97 : 519 – 26 , 29 ; quiz 27–8 .
Casagrande DS , Rosa DD , Umpierre D , Sarmento RA , Rodrigues CG , Schaan BD . Incidence of cancer following bariatric surgery: systematic review and meta-analysis . Obes Surg 2014 ; 24 : 1499 – 509 .
Ward KK , Roncancio AM , Shah NR , Davis MA , Saenz CC , McHale MT , et al. Bariatric surgery decreases the risk of uterine malignancy . Gynecologic oncology 2014 ; 133 : 63 – 6 .
Luo J , Chlebowski RT , Hendryx M , Rohan T , Wactawski-Wende J , Thomson CA , et al. Intentional weight loss and endometrial cancer risk . J Clin Oncol 2017 ; 3 : 1189 – 93 .
Goodwin PJ , Stambolic V . Impact of the obesity epidemic on cancer . Ann Rev Med 2015 ; 66 : 281 – 96 .
Zhang X , Tworoger SS , Eliassen AH , Hankinson SE . Postmenopausal plasma sex hormone levels and breast cancer risk over 20 years of follow-up . Breast Cancer Res Treat 2013 ; 137 : 883 – 92 .
Gunter MJ , Hoover DR , Yu H , Wassertheil-Smoller S , Rohan TE , Manson JE , et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women . J Natl Cancer Inst 2009 ; 101 : 48 – 60 .
Endogenous Hormones and Breast Cancer Collaborative Group , Key TJ , Appleby PN , Reeves GK , Roddam AW . Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies . Lancet Oncol 2010 ; 11 : 530 – 42 .
Olefsky JM , Glass CK . Macrophages, inflammation, and insulin resistance . Ann Rev Physiol 2010 ; 72 : 219 – 46 .
Howe LR , Subbaramaiah K , Hudis CA , Dannenberg AJ . Molecular Pathways: adipose inflammation as a mediator of obesity-associated cancer . Clin Cancer Res 2013 ; 19 : 6074 – 83 .
Chang SH , Stoll CR , Colditz GA . Cost-effectiveness of bariatric surgery: should it be universally available? Maturitas 2011 ; 69 : 230 – 8 .
Arnold M , Pandeya N , Byrnes G , Renehan AG , Stevens GA , Ezzati M , et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study . Lancet Oncol 2015 ; 16 : 36 – 46 .
Email alerts
Citing articles via.
- Recommend to Your Librarian
- Advertising and Corporate Services
- Journals Career Network
Affiliations
- Online ISSN 1530-8561
- Print ISSN 0009-9147
- Copyright © 2024 Association for Diagnostics & Laboratory Medicine
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

COMMENTS
Obesity has become a global epidemic and is one of today's most public health problems worldwide. Obesity poses a major risk for a variety of serious diseases including diabetes mellitus, non-alcoholic liver disease (NAFLD), cardiovascular disease, hypertension and stroke, and certain forms of cancer (Bluher, 2019).Obesity is mainly caused by imbalanced energy intake and expenditure due to a ...
Despite public health efforts, these disorders are on the rise, and their consequences are burgeoning. 1 The Centers for Disease Control and Prevention report that during 2017 to 2018, the prevalence of obesity in the United States was 42.4%, which was increased from the prevalence of 30.5% during 1999 to 2002. 2 Among those afflicted with ...
Introduction. Obesity is an increasing, global public health issue. Patients with obesity are at major risk for developing a range of comorbid conditions, including cardiovascular disease (CVD), gastrointestinal disorders, type 2 diabetes (T2D), joint and muscular disorders, respiratory problems, and psychological issues, which may significantly affect their daily lives as well as increasing ...
Obesity, mortality and BMI. Obesity, as defined by BMI (Table 1), is associated with an increased risk of all-cause mortality, with CVD and malignancy being the most common causes of death. 5 -8 A meta-analysis of 239 prospective studies involving 10.6 million individuals from Asia, Australia, New Zealand, Europe and North America found that all-cause mortality was lowest between a BMI of 20 ...
Besides health complications, obesity causes an array of psychological effects, including inferiority complex among victims. Obese people suffer from depression, emanating from negative self-esteem and societal rejection. In some cases, people who become obese lose their friends and may get disapproval from teachers and other personalities ...
Obesity is defined as when a person has a body mass index [BMI (kg/m 2), dividing a person's weight by the square of their height] greater than or equal to 30, overweight is defined as a BMI of 25.0-29.9. Being overweight or obesity is linked with more deaths than being underweight and is a more common global occurrence than being underweight.
Obesity prevalence among children is >30% in the Cook Islands, Nauru and Palau, with a notable increase over the past few decades. Worldwide prevalence of obesity increased at an alarming rate in ...
Being overweight or obese can have a serious impact on health. Carrying extra fat leads to serious health consequences such as cardiovascular disease (mainly heart disease and stroke), type 2 diabetes, musculoskeletal disorders like osteoarthritis, and some cancers (endometrial, breast and colon). These conditions cause premature death and ...
Obesity is associated with an increased risk of morbidity and mortality; the economic impact of the health care costs associated with obesity is anticipated to have a profound, detrimental effect on the country's economy within the next several decades. A number of psychologists have dedicated their careers to understanding psychos-
Obesity is a complex condition that interweaves biological, developmental, environmental, behavioral, and genetic factors; it is a significant public health problem. The most common cause of obesity throughout childhood and adolescence is an inequity in energy balance; that is, excess caloric intake without appropriate caloric expenditure. Adiposity rebound (AR) in early childhood is a risk ...
Entire books have been written detailing the effects of obesity on various measures of health. This article briefly summarizes associations between obesity and adult health. Obesity and Diabetes. The condition most strongly influenced by body weight is type 2 diabetes. In the Nurses' Health Study, which followed 114,000 middle-age women for ...
A. Khera and T.M. Powell-WileyN Engl J Med 2023;389:2287-2288. Overweight and obesity have reached epidemic proportions, affecting more than 70% of the U.S. adult population and more than 50% of ...
1. Introduction. Obesity is a complex, multifactorial, and largely preventable disease (), affecting, along with overweight, over a third of the world's population today (2,3).If secular trends continue, by 2030 an estimated 38% of the world's adult population will be overweight and another 20% will be obese ().In the USA, the most dire projections based on earlier secular trends point to ...
The present study conducted a systematic literature review to examine obesity research and machine learning techniques for the prevention and treatment of obesity from 2010 to 2020. Accordingly, 93 papers are identified from the review articles as primary studies from an initial pool of over 700 papers addressing obesity.
carbohydrate is a crucial factor in the obesity epidemic. 18 Soft drinks, alcoholic beverages and fast food tend to be calorie rich. In Britain, there has been a signi cant rise in the amount of ...
Accordingly, 93 papers are identified from the review articles as primary studies from an initial pool of over 700 papers addressing obesity. Consequently, this study initially recognized the significant potential factors that influence and cause adult obesity. Next, the main diseases and health consequences of obesity and overweight are ...
This Review describes current knowledge on the epidemiology and causes of child and adolescent obesity, considerations for assessment, and current management approaches. Before the COVID-19 pandemic, obesity prevalence in children and adolescents had plateaued in many high-income countries despite levels of severe obesity having increased. However, in low-income and middle-income countries ...
Abstract. Obesity is a disease with a major negative impact on human health. However, people with obesity may not perceive their weight to be a significant problem and less than half of patients with obesity are advised by their physicians to lose weight. The purpose of this review is to highlight the importance of managing overweight and ...
Background Obesity is a growing public health challenge worldwide with significant health and economic impacts. However, much of what is known about the economic impacts of obesity comes from high-income countries and studies are not readily comparable due to methodological differences. Our objective is to demonstrate a method for estimating current and future national economic impacts of ...
People who have overweight or obesity*, compared to those with healthy weight, are at increased risk for many serious diseases and health conditions. These include: 1,2,3. All-causes of death (mortality). High blood pressure (hypertension). High LDL cholesterol, low HDL cholesterol, or high levels of triglycerides (dyslipidemia). Type 2 diabetes.
INTRODUCTION. The dramatic increase in the prevalence of overweight and obesity in most countries has been of great concern globally. 1-3 This is estimated to be the cause of more than 3.4 million deaths, 4% of Years of Life Lost (YLL), and at least 4% of Disability-Adjusted Life Years (DALYs) all around the word. 2 However, despite the urgency of this problem, there are still some noticeable ...
Obesity was "a therapeutic graveyard," said Emily Field, a pharmaceuticals analyst at Barclays in London. The drugs either worked well and had bad side effects or led to only middling weight ...
The increasing burden of cancer due to obesity has been the focus of much epidemiologic research over the past 40 years. Here, we summarize the findings from the 2016 International Agency for Research on Cancer (IARC) 3 and review the current and needed research addressing the role of adiposity/obesity in cancer incidence and address the potential for weight loss to reduce risk.